Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 11/74/01. The protocol was agreed in November 2012. The assessment report began editorial review in October 2013 and was accepted for publication in January 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
David Scott has received honoraria within the last 3 years for providing advice to Merck Sharp & Dohme Corp., UCB Pharma and Bristol-Myers Squibb: these values were less than £1000. Additionally, David Scott has received grants from Arthritis Research UK and the National Institute for Health Research in connection with rheumatoid arthritis.
Corrections
-
This article was corrected in November 2016. See Stevenson M, Archer R, Tosh J, Simpson E, Everson-Hock E, Stevens J, et al. Corrigendum: Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess 2016;20(35):611–614. http://dx/doi.org/10.3310/hta20350-c201611
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Stevenson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Aetiology
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterised by progressive, irreversible, joint damage, impaired joint function, and pain and tenderness caused by swelling of the synovial lining of joints and is manifested with increasing disability and reduced quality of life. 1 The primary symptoms are pain, morning stiffness, swelling, tenderness, loss of movement, fatigue and redness of the peripheral joints. 2,3 RA is associated with substantial costs, both direct (associated with drug acquisition and hospitalisation) and indirect (owing to reduced productivity). 4 RA has long been reported as being associated with increased mortality,5,6 particularly due to cardiovascular events. 7
Epidemiology
The initial classification criteria for RA were produced in 1987 by the American College of Rheumatology (ACR). 8 The National Institute for Health and Care Excellence (NICE) Clinical Guideline (CG) 799 provides a summary of the ACR criteria, namely that patients must have at least four of the seven criteria (morning stiffness lasting at least 1 hour; swelling in three or more joints; swelling in hand joints; symmetrical joint swelling; erosions or decalcification on radiograph of hand; rheumatoid nodules; and abnormal serum rheumatoid factor). The first four criteria must have been present for at least a period of 6 weeks. However, in the CG the guideline development group preferred a clinical diagnosis of RA rather than the ACR criteria because ‘an early persistent synovitis where other pathologies have been ruled out needs to treated as if it is RA to try to prevent damage to joints. Identification of persistent synovitis and appropriate early management is more important than whether the disease satisfies classification criteria’, referencing the European League Against Rheumatism (EULAR) recommendations. 10
In 2010 the ACR and EULAR jointly published Rheumatoid Arthritis Classification Criteria, which focused on features at earlier stages of disease that are associated with persistent and/or erosive disease rather than defining the disease by its late-stage features. 11 The classification criteria allocate scores to characteristics of joint involvement, serology, acute-phase reactants and duration of symptoms, to produce a score between 0 and 10, inclusive, with those scoring ≥ 6 and with obvious clinical synovitis being defined as having ‘definite RA’ in the absence of an alternative diagnosis that better explains the synovitis.
Two classifications have dominated the measurement of improvement in RA symptoms: (1) ACR responses;12 and (2) EULAR responses. 13
The initial ACR response was denoted as an ACR20, which required a 20% improvement in tender joint counts; a 20% improvement in swollen joint counts; and a 20% improvement in at least three of the following five ‘core set items’: physician global assessment; patient global assessment; patient pain; self-reported disability (using a validated instrument); and erythrocyte sedimentation rate (ESR)/C-reactive protein (CRP).
The ACR response has been widely adopted in randomised controlled trials (RCTs), although studies have shown that the value can vary between trials owing to the timing of the response. 14 Since the inception of the ACR20 two other response criteria (ACR50 and ACR70) have become more widely used, which are similar to ACR20 and differing only in the level of improvements required to be denoted a responder.
In the UK, monitoring the progression of RA is often undertaken using the Disease Activity Score 28 joints (DAS28). This assesses 28 joints in terms of swelling (SW28) and of tenderness to the touch (TEN28) and also incorporates measures of the ESR and a subjective assessment (SA) on a scale of 0–100 made by the patient regarding disease activity in the previous week.
The equation for calculating DAS28 is as follows:15
The DAS28 can be used to classify both the disease activity of the patient and the level of improvement estimated within the patient.
The EULAR response criteria use the individual change in DAS28 and the level of DAS28 reached to classify trial participants as good, moderate or non-responders. 13 The EULAR response criteria and the ACR20 improvement criteria were found to have reasonable agreement in the same set of clinical trials,16 although van Gestel et al. 16 state that the EULAR response criteria showed better construct and discriminant validity than did ACR20. EULAR response has been reported less frequently in RCTs than ACR responses, although EULAR is much more closely aligned to the treatment continuation rules stipulated by NICE, which require a DAS28 improvement of more than 1.2 to continue treatment. The relationship between change in DAS28 and the level of DAS28 reached with EULAR response is shown in Table 1. Dependent on the initial Disease Activity Score (DAS) score of the patient, this would equate to either a good or moderate EULAR response, as shown in the second column of Table 1.
| DAS28 at end point | Improvement in DAS28 | ||
|---|---|---|---|
| > 1.2 | > 0.6 and ≤ 1.2 | ≤ 0.6 | |
| ≤ 3.2 | Good | Moderate | Non |
| > 3.2 and ≤ 5.1 | Moderate | Moderate | Non |
| > 5.1 | Moderate | Non | Non |
Patients with a DAS28 of ≤ 3.2 are stated as having inactive disease, those with a DAS28 of > 3.2 and ≤ 5.1 are stated as having moderate disease and those with a DAS28 of > 5.1 are stated as having very active disease. 15
A widely used measure of patient disability is the Health Assessment Questionnaire (HAQ). The HAQ is a patient-completed disability assessment17 which has established reliability and validity and has been used in many published RCTs in RA. HAQ scores range from 0 to 3, with higher scores indicating greater disability. The HAQ is a discrete scale with step values of 0.125, resulting in 25 points on the HAQ scale.
Incidence and prevalence
There are an estimated 400,000 people in England and Wales with RA,18 with approximately 10,000 incident cases per year. 19 The disease is more common in females (1.16%) than in males (0.44%),19 with the majority of cases being diagnosed when patients are aged between 40 and 80 years20 and with peak incidence in patients in their seventies. 19 Traditionally, patients have been treated with conventional disease-modifying antirheumatic drugs (cDMARDs), which include methotrexate (MTX), sulfasalazine (SSZ), hydroxychloroquine (HCQ), leflunomide (LEF), and gold injections (GLDs) as well as corticosteroids, analgesics and non-steroidal anti-inflammatory drugs (NSAIDs). However, more recently, a group of drugs have been developed consisting of monoclonal antibodies and soluble receptors that specifically modify the disease process by blocking key protein messenger molecules (such as cytokines) or cells (such as B-lymphocytes). 9 Such drugs have been labelled as biologic disease-modifying antirheumatic drugs (bDMARDs) and form the focus of this report.
Significance for the NHS
Owing to previous NICE technology appraisals (TAs) recommending a number of bDMARDs (see Current service provision), with a potential sequence of three bDMARDs, there has been a considerable increase in expenditure on RA interventions. Given the remit of this research to establish the clinical effectiveness and cost-effectiveness of bDMARDs in advance of cDMARDs for patients with less severe disease (assumed to be those with a DAS28 of between > 3.2 and ≤ 5.1), there is potential for the expenditure to increase further should NICE guidance on these populations be positive. The majority of interventions are provided subcutaneously and would therefore require little additional staff time should there be positive guidance, although this would increase for those drugs which are given intravenously.
Further detailed information on the background of RA can be found within the relatively recent NICE CG. 9 Additional information can also be located in the British Society for Rheumatology guidelines. 21
Current service provision
Clinical guidelines
For people with newly diagnosed RA, NICE CG799 recommends a combination of cDMARDs [including MTX and at least one other disease-modifying anti-rheumatic drug (DMARD) plus short-term glucocorticoids] as first-line treatment, ideally beginning within 3 months of the onset of persistent symptoms. Where combination therapies are not appropriate (e.g. where there are comorbidities or pregnancy), DMARD monotherapy is recommended. Where DMARD monotherapy is used emphasis should be on increasing the dose quickly to obtain best disease control. For the purposes of this assessment the term intensive DMARDs has been used to denote that this is treatment with multiple cDMARDs simultaneously.
Current National Institute for Health and Care Excellence technology appraisal guidance
National Institute for Health and Care Excellence guidance (TA130,22 TA18623 and TA22524) recommends the use of the tumour necrosis factor (TNF) inhibitors etanercept (ETN; Enbrel®, Pfizer), infliximab [IFX; Remicade®, Merck Sharp & Dohme Corp. (MSD)], adalimumab (ADA; Humira®, AbbVie), certolizumab pegol (CTZ; Cimzia®, UCB Pharma) and golimumab (GOL; Simponi®, MSD) in people with RA after the failure of two cDMARDs, including MTX, and who have a DAS28 > 5.1. Terminated NICE guidance (TA224) was unable to issue recommendations for the use of GOL in people with RA that have not been treated with MTX. 25
Technology Appraisal 24726 recommends tocilizumab (TCZ; RoActemra®, Roche) as an alternative to TNF inhibitors in the same circumstances as in TA130,22 that is in patients with a DAS28 > 5.1 after trying two cDMARDs. NICE guidance TA28027 recommends the use of intravenous (i.v.) abatacept (ABT; Orencia®, Bristol-Myers Squibb) in people with RA after the failure of cDMARDs in the same circumstances as TA130; the subcutaneous (s.c.) formulation has not been appraised.
A simplified summary of NICE-recommend bDMARDs is shown in Figure 1. This defines the sequence of treatments that have received positive guidance for patients with a DAS28 of > 5.1. In summary, the typical route would be intensive cDMARDs followed by a bDMARD, followed by rituximab (RTX) plus MTX, then TCZ before returning to cDMARDs.
FIGURE 1.
Summary of the position of bDMARDs within NICE TA recommendations for sequence of treatments for patients with RA and a DAS28 > 5.1. a, If RTX and MTX is contraindicated or withdrawn owing to adverse events then the following can be used: ADA or ETN or IFX or ABT in combination with MTX; ADA or ETN monotherapy TA195,28 TCZ in combination with MTX TA247,26 assuming these have not been used previously in the sequence; b, would not be used if TCZ has been used previously in the sequence.

It is noted that NICE CG79 recommends the use of intensive cDMARDs which have been assumed to be used rather than two cDMARDs used in monotherapy, although this latter option is acceptable.
The National Institute for Health and Care Excellence has also issued guidance (TA195,28 TA22524 and TA24726) on the treatment of RA after the failure of a TNF inhibitor, but such guidance falls outside the scope of this appraisal.
National Institute for Health and Care Excellence criteria for continuing treatment
Each of the NICE TAs states that for patients to continue treatment with a bDMARD there must have been an improvement in DAS28 of at least 1.2 points at 6 months. If this criterion has not been met then treatment should be stopped and the next intervention in the sequence initiated.
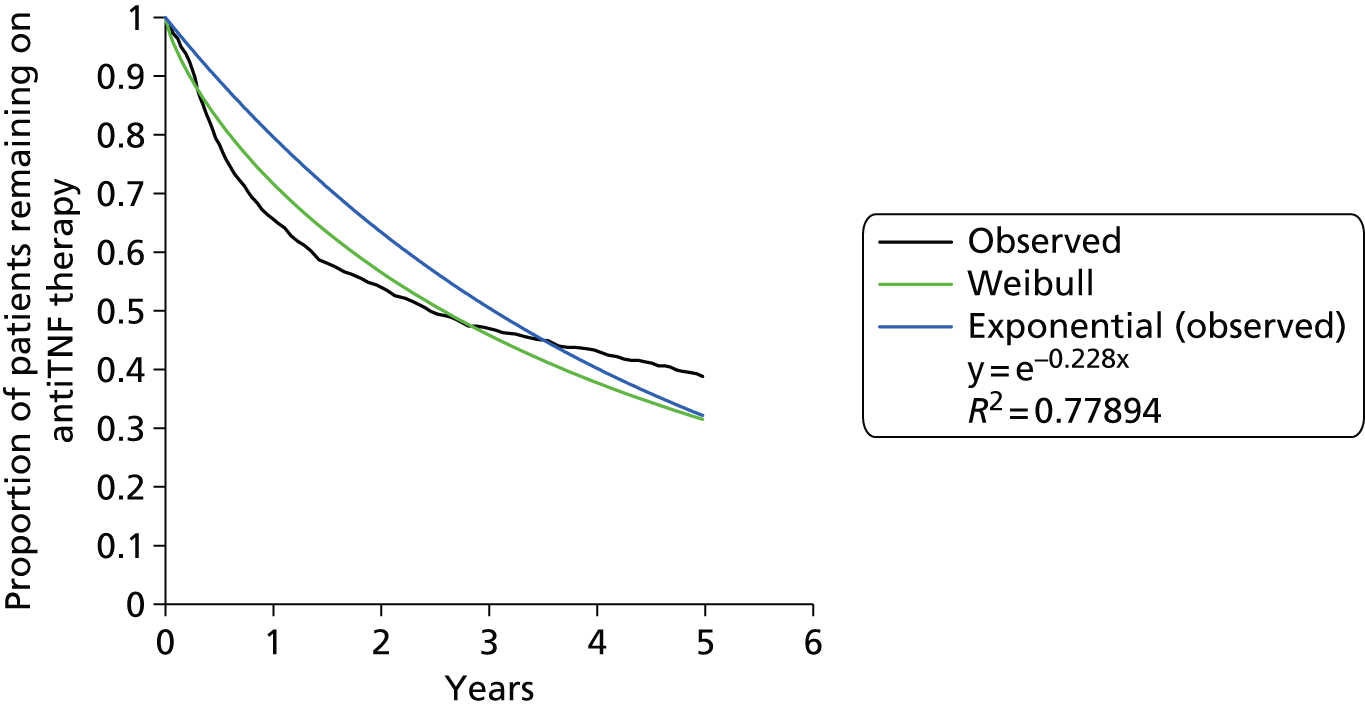
Data were provided by the British Society for Rheumatology Biologics Register (BSRBR) to the Assessment Group and were used to assess the time on first biologic conditional on EULAR response. These indicate that over 25% of patients who had no EULAR response at 6 months were still on treatment at 4.5 years, with the median treatment time being 319 days. This shows that there is not strict adherence to the NICE criteria for continuation of treatment. The majority of patients (94%) had a DAS28 of > 5.1, indicating that the severity criteria stated by NICE were reasonably well adhered to.
Description of the technologies under assessment
Interventions considered in the scope of this report
The scope of the work is to ascertain the clinical effectiveness and cost-effectiveness of seven interventions within three populations that will be detailed subsequently. These interventions are ABT, ADA, CTZ, ETN, GOL, IFX and TCZ. It is noted that ABT can be delivered in two formulations, intravenously and subcutaneously, and that both have been modelled separately. Owing to the large number of interventions these have been initially summarised by mode of action. There then follows a summary of the UK marketing authorisation for each intervention along with a description of administration method. This text is similar to that within the protocol. 29
Mode of action
Adalimumab, ETN, IFX, CTZ and GOL all inhibit the activity of tumour necrosis factor alpha (TNF-α), a pro-inflammatory mediator that is partly responsible for damage to the joints in RA.
Abatacept is a selective modulator of the T-lymphocyte activation pathway. It binds to molecules on the surface of antigen-presenting cells, preventing full activation of the T lymphocytes and interrupting the inflammatory process.
Tocilizumab inhibits the activity of the cytokine interleukin 6, a pro-inflammatory molecule that is also partly responsible for damage to the joints in RA.
Marketing licence and administration method
Abatacept, in combination with MTX, has a UK marketing authorisation for the treatment of moderate to severe active RA in adult patients who responded inadequately to previous therapy with one or more cDMARDs, including MTX or a TNF-α inhibitor. It can be administered by i.v. infusion or by s.c. injection.
Adalimumab, in combination with MTX, has a UK marketing authorisation for the treatment of moderate to severe active RA in adults when the response to cDMARDs, including MTX, has been inadequate and for the treatment of severe, active and progressive RA in adults not previously treated with MTX. ADA can be given as monotherapy in case of intolerance to MTX or when continued treatment with MTX is inappropriate. It is administered subcutaneously.
Certolizumab pegol, in combination with MTX, has a UK marketing authorisation for the treatment of moderate to severe active RA in adult patients when the response to cDMARDs, including MTX, has been inadequate. CTZ can be given as monotherapy in case of intolerance to MTX or when continued treatment with MTX is inappropriate. It is administered subcutaneously.
Etanercept, in combination with MTX, has a UK marketing authorisation for the treatment of moderate to severe active RA in adults when the response to cDMARDs, including MTX (unless contraindicated), has been inadequate, and for the treatment of severe, active and progressive RA in adults not previously treated with MTX. ETN can be given as monotherapy in case of intolerance to MTX or when continued treatment with MTX is inappropriate. It is administered subcutaneously.
Golimumab, in combination with MTX, has a UK marketing authorisation for the treatment of moderate to severe active RA in adult patients when the response to cDMARD therapy, including MTX, has been inadequate, and for the treatment of severe, active and progressive RA in adults not previously treated with MTX. It is administered subcutaneously.
Infliximab, in combination with MTX, has a UK marketing authorisation for the reduction of signs and symptoms as well as the improvement in physical function in adults with active disease when the response to DMARDs, including MTX, has been inadequate. It is also licensed for the treatment of severe, active and progressive RA in adults not previously treated with MTX or other cDMARDs. It is administered by i.v. infusion.
Tocilizumab, in combination with MTX, has a UK marketing authorisation for the treatment of moderate to severe active RA in adult patients who have either responded inadequately, or who were intolerant, to previous therapy with one or more DMARDs or TNF antagonists. In these patients, TCZ can be given as monotherapy in case of intolerance to MTX or where continued treatment with MTX is inappropriate. TCZ is administered by i.v. infusion.
Current usage in the NHS
There is widespread use of the interventions within the NHS. Robust values of the exact breakdown by intervention are not known.
Identification of important subgroups
The current NICE guidance has already identified a subgroup by stating that to receive a bDMARD the patient must have received two cDMARDs and have active RA with a DAS28 in excess of 5.1. The research questions within this report include estimating the cost-effectiveness if the severity criteria were lessened to include patients with a DAS28 of > 3.2; and estimating the cost-effectiveness of using bDMARDs in advance of cDMARDs.
An important clinical subgroup encompasses those patients in whom bDMARDs cannot be given in combination with MTX. The clinical effectiveness and cost-effectiveness of licensed bDMARDs in this population will be estimated in this assessment.
The anticipated costs associated with the interventions
The costs associated with each intervention need to take into account factors, including the acquisition cost of the drug [incorporating any Patient Access Scheme (PAS)]; the average weight of patients with RA for those interventions that are weight based; the administration costs associated with infusions and of district nurses performing s.c. injections; and any loading doses required in the first year.
The acquisition costs and dosing regimens were taken from the British National Formulary (www.bnf.org; accessed June 201330) with details of PASs taken from the manufacturers’ submissions.
The average weights of patients with RA were estimated using data (n = 12,176) from the BSRBR. To be able to be used with all of the weight-based dosing regimens, a large number of categories were required, as detailed in Table 2. From these categories the average cost per dose for those with a weight-based dose can be calculated.
| Weight category (kg) | Number of patients | Percentage of total patients |
|---|---|---|
| 0–30 | 3 | 0.0 |
| 31–33 | 7 | 0.1 |
| 34–35 | 9 | 0.1 |
| 36–45 | 240 | 2.0 |
| 46–50 | 484 | 4.0 |
| 51–60 | 2333 | 19.2 |
| 61–67 | 2115 | 17.4 |
| 68–70 | 949 | 7.8 |
| 71–75 | 1310 | 10.8 |
| 76–85 | 2148 | 17.6 |
| 86–95 | 1351 | 11.1 |
| 96–100 | 412 | 3.4 |
| 101–133 | 734 | 6.0 |
| 134–167 | 67 | 0.6 |
| 168–200 | 14 | 0.1 |
| Total | 12,176 | 100 |
Additional loading doses in the first year were calculated based on the relevant regimen and the administration cost. Table 3 provides a simplified summary of the assumed mean acquisition costs per intervention and can be used to provide indicative rather than exact values. Within the mathematical model described later, timings of costs are explicitly incorporated and also the fact that in some subgroups the distribution of weights may differ from that of the full BSRBR database, a factor also considered within the Assessment Group model.
| Treatment | Dose regimen | Details of PAS if applicable | Cost per cheapest available dose, £ (dose) | Cost per weight-adjusted dosea/standard regimen, £ | Administration costs per treatment, £ | Cost per year (excluding administration costsb), £ | Additional costs in year 1, £ |
|---|---|---|---|---|---|---|---|
| ABT (i.v.) | 500 mg below 60 kg, 750 mg between 60 kg and 100 kg, 1000 mg above 100 kg; 0, 2 and 4 weeks then every 4 weeks thereafter | CiC information has been removed | CiC information has been removed (250 mg) | CiC information has been removed | 154 | CiC information has been removed | CiC information has been removed |
| ABT (s.c.) | 125 mg weekly following loading dose 500 mg below 60 kg, 750 mg between 60 kg and 100 kg, 1000 mg above 100 kg | CiC information has been removed | CiC information has been removed (125 mg) | CiC information has been removed | 3.05 | CiC information has been removed | CiC information has been removed |
| ADA | 40 mg; every other week | N/A | 352.14 (40 mg) | 352.14 | 3.05 | 9234.94 | 0 |
| CTZ | 400 mg per week initially, repeated at weeks 2 and 4 followed by a maintenance dose of 200 mg every 2 weeks | Initial 10 doses free | 357.50 (200 mg) | 357.50 | 3.05 | 9374.30 | –2523.85c |
| ETN | 50 mg; every week | N/A | 178.75 (50 mg) | 178.75 | 3.05 | 9453.60 | 0 |
| GOL | 50 mg below 100 kg, 100 mg above 100 kg; per month | 100 mg dose provided at the same price as the 50 mg dose | 762.97 (50 mg) | 762.97d | 3.05 | 9192.24 | 0 |
| IFXe | 3 mg/kg: 0, 2, 6 then every 8 weeks | N/A | 419.62 (100 mg) | 1110.98 | 154 | 8222.37f | 1820.47 |
| TCZ | 8 mg/kg every 4 weeks | CiC information has been removed | CiC information has been removed (80 mg) | CiC information has been removed | 154 | CiC information has been removed | 0 |
Additional treatments in a sequenced strategy
The nature of RA treatment being sequenced meant that it was necessary for the Assessment Group and the manufacturers to incorporate the costs and effectiveness of RTX into the model as this has positive NICE guidance following the withdrawal of a bDMARD. These will be discussed as applicable.
Chapter 2 Definition of the decision problem
Decision problem
The aim of this assessment was to investigate the clinical effectiveness and cost-effectiveness of ADA, ETN, IFX, CTZ, GOL, TCZ and ABT for the treatment of RA not previously treated with bDMARDs compared with each other and compared with cDMARDs.
Interventions
A detailed description of each of the interventions is provided in Chapter 1, Description of the technologies under assessment. Table 4 summarises the relationship between the market authorisation and the decision problem detailed in Overall aims and objectives of assessment: that is, whether or not the intervention is licensed to be used prior to the initiation of MTX intervention; as a monotherapy (i.e. without needing to be given in combination with MTX); for patients with severe RA; and for patients with moderate to severe RA.
| Intervention | Is the intervention licensed: | |||
|---|---|---|---|---|
| prior to the use of MTX? | as a monotherapy? | for patients with severe RA? | for patients with moderate to severe RA? | |
| ABTa | ✓ | ✓ | ||
| ADA | ✓ | ✓ | ✓ | ✓ |
| CTZ | ✓ | ✓ | ✓ | |
| ETN | ✓ | ✓ | ✓ | ✓ |
| GOL | ✓ | ✓ | ✓ | |
| IFX | ✓ | ✓ | ✓ | |
| TCZ | ✓ | ✓ | ✓ | |
Populations (including subgroups)
The scope issued by NICE defines three distinct populations with RA and includes (1) adults with severe active RA not previously treated with cDMARDs; (2) adults with severe active RA who have been previously treated with cDMARDs but not bDMARDs; and (3) adults with moderate to severe active RA who have been previously treated with cDMARDs only, including MTX (unless contraindicated or inappropriate). Henceforth, these will be referred to as population 1, population 2 and population 3, respectively.
Although the NICE scope did not specify the definition of severe active RA and moderate to severe active RA, the following definition (based on expert clinical advice to the Assessment Group) has been adopted: severe active RA will be defined by a DAS28 of ≥ 5.1 and moderate to severe active RA will be defined as a DAS28 of between 3.2 and 5.1.
As the scope issued by NICE explicitly defined subgroups, no further subgroups will be assessed, with the exception of those patients in whom bDMARD treatment needs to be given as monotherapy. Separate analyses will be conducted for those in whom MTX can be tolerated and in those who can only receive bDMARD monotherapy.
The Assessment Group has chosen to deviate from the scope for population 1 as the definition in the scope stated that MTX needed to have been used previously. Given this definition, the populations were mutually exclusive but not exhaustive, as patients without prior bDMARD treatment who had not received MTX but had instead received an alternative cDMARD would not be allocated to any of the populations. In consultation with NICE and our clinical experts the Assessment Group broadened its interpretation of population 1 to allow previous treatment with any cDMARD.
It is noted that the number of interventions considered in population 1 is fewer than for populations 2 or 3, as only four interventions (ADA, ETN, GOL and IFX) are licensed in this population.
Populations outside the scope of the research
The following groups were explicitly excluded from the research by the scope issued by NICE:
-
the initiation of treatment in patients without active RA
-
patients with a DAS of < 3.2 who had received previous treatment with cDMARDs
-
patients with a DAS of < 5.1 who had not been previously treated with cDMARDs
-
patients who had been previously treated with one or more bDMARDs.
Relevant comparators
The relevant comparators within the final scope differ according to the population considered. The scope stated that tofacitinib (TOF; Xeljanz®, Pfizer; Jakvinus®, Pfizer) would be included if NICE had issued positive guidance prior to the report’s completion, but this did not occur and therefore TOF was not evaluated.
-
For severe active RA not previously treated with MTX or other DMARDs:
-
combination therapy with cDMARDs (including MTX and at least one other DMARD, such as SSZ and LEF as recommended in NICE CG799)
-
the interventions will be compared with each other.
-
-
For severe active RA that has been previously treated with cDMARDs only:
-
management strategies involving further cDMARDs (e.g. SSZ, LEF), NSAIDs and corticosteroids
-
the interventions will be compared with each other.
-
-
For moderate to severe active arthritis that has been previously treated with cDMARDs only:
-
management strategies involving further cDMARDs (e.g. SSZ, LEF), NSAIDs and corticosteroids
-
the interventions will be compared with each other.
-
Outcomes
The outcome measures to be considered include:
-
disease activity
-
physical function
-
joint damage
-
pain
-
mortality
-
fatigue
-
radiological progression
-
extra-articular manifestations of disease
-
adverse effects of treatment
-
health-related quality of life.
Data were also collected on other outcome measures, including disease duration, number of previous cDMARDs and percentage of patients who had received bDMARDs, in case there was sufficient variation in baseline measurements that these could be investigated as treatment effect modifiers within data synthesis.
Overall aims and objectives of assessment
The review aims to:
-
evaluate the clinical effectiveness of each intervention in affecting key outcomes in patients within each of the defined subgroups
-
evaluate the adverse effect profile of each intervention (and comparator)
-
estimate the incremental cost-effectiveness within each of the defined subgroups of each intervention compared with all comparators
-
estimate the overall cost of amending the current provision of interventions in the light of the cost-effectiveness results
-
identify key areas for primary research.
Chapter 3 Assessment of clinical effectiveness
A systematic review of the literature and network meta-analyses (NMAs) were conducted in order to evaluate the clinical effectiveness of ABT, ADA, CTZ, ETN, GOL, IFX and TCZ in the first-line bDMARD treatment of adults with RA.
The systematic review of the evidence was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (www.prisma-statement.org/).
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Methods for reviewing effectiveness
Identification of studies
The aims of the search were to provide as comprehensive a retrieval as possible of clinical effectiveness evidence relating to ABT, ADA, CTZ, ETN, GOL, IFX and TCZ and to identify additional relevant treatments for potential inclusion in the NMA.
Electronic databases
Studies were identified by searching the following electronic databases and research registers:
-
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R) (via Ovid) 1948 to July 2013
-
EMBASE (via Ovid) 1980 to July 2013
-
Cochrane Database of Systematic Reviews (via Wiley Online Library) 1996 to May 2013
-
Cochrane Central Register of Controlled Trials (via Wiley Online Library) 1898 to May 2013
-
Health Technology Assessment (HTA) database (via Wiley Online Library) 1995 to May 2013
-
Database of Abstracts of Review of Effects (via Wiley Online Library) 1995 to May 2013
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCOhost) 1982 to April 2013
-
Toxicology Literature Online to July 2013.
Given the broad scope of interventions to be included in the review and the high volume of potentially relevant studies to be sifted, the keyword searches of electronic resources were undertaken in three stages. No language or date restrictions were applied to any database. Details of keywords strategies are reported in Appendix 1.
Stage 1 was undertaken using keywords relating to the population only (i.e. RA) and did not include keywords relating to the interventions specified in the decision problem. The purpose was to keep the scope of the search broad in order to identify potentially relevant evidence for inclusion in the NMA, in addition to identifying RCTs and systematic reviews of the interventions of interest. For the searches of MEDLINE, EMBASE and CINAHL, methodological filters were added to restrict search results to RCTs and systematic reviews. To maximise the efficiency of the search process at this stage, filters aimed at maximising the precision of search results were applied. 31–35
Stage 2 was undertaken using keywords relating to the population (RA) combined with keywords relating to the interventions of interest (ABT, ADA, CTZ, ETN, GOL, IFX and TCZ) and any interventions identified as potentially allowing indirect comparisons to be made within the NMA. Keyword synonyms relating to the interventions included generic drug names, product names and drug registry numbers. The purpose of stage 2 was to identify RCTs that might not have been retrieved by the ‘high precision’ stage 1 searches. Therefore, RCT search filters aimed at maximising the sensitivity of search results were applied. 33,36 In the first instance, MEDLINE and EMBASE were searched. Given the high volume of references retrieved and the low yield in terms of relevant references identified, it was decided that searches would not be extended to other databases or to other treatments to be potentially included in the NMA.
Stage 3 involved the undertaking of searches for potential supplementary adverse events (AEs) evidence through the combination of keywords relating to the population (RA) with keywords relating to the interventions of interest (ABT, ADA, atacicept, CTZ, ETN, GOL, IFX, RTX, TCZ, TOF). For the searches of MEDLINE and EMBASE, AE filters were applied,37 whereas no filter was required for the Toxicology Literature Online database.
Where possible, and to minimise duplication between search results, the results retrieved by earlier search strategies were excluded from the results retrieved by later search strategies using the ‘not’ Boolean operator. The results retrieved by the MEDLINE and EMBASE high-precision searches (stage 1) were excluded from MEDLINE and EMBASE high-sensitivity searches (stage 2). The results retrieved by the MEDLINE and EMBASE high-precision and high-sensitivity searches (stages 1 and 2) were excluded from the AE searches (stage 3).
Other resources
To identify additional studies, the reference lists of relevant studies (including existing systematic reviews) were checked and a citation search of relevant articles (using the Web of Science Citation Index Expanded and Conference Proceedings Citation Index – Science) was undertaken to identify articles that cite the relevant articles. It was originally intended in the protocol29 that searches be performed to identify ongoing research and unpublished studies using the metaRegister of Current Controlled Trials, the World Health Organization International Clinical Trials Registry Platform, the European Union Clinical Trials Register, the Food and Drug Administration (FDA) and European Medicines Agency websites and the Web of Science Conference Proceedings Citation Index – Science. However, this was not possible within the time scales dictated by the NICE appraisal process. Hand-searching of relevant documents included sponsor submissions to the NICE TA update process, recent systematic reviews and documentation associated with previous relevant NICE TA guidance (TAs 130,22 186,23 224,25 234,38 225,24 24726). Grey literature was also sought using the sources listed in the international grey literature search toolkit produced by the Canadian Agency for Drugs and Technologies in Health. 39
All identified citations from the electronic searches and other resources were imported into and managed using the Reference Manager bibliographic software (version 12.0; Thomson Reuters, Philadelphia, PA, USA).
Inclusion and exclusion criteria
Inclusion and exclusion criteria for the selection of clinical effectiveness and safety evidence were defined according to the decision problem outlined in the NICE scope. 40
The inclusion of potentially relevant articles was undertaken using a two-step process. First, all titles and abstracts were examined for inclusion by one reviewer. Any citations that clearly did not meet the inclusion criteria (e.g. animal studies, studies unrelated to RA) were excluded. Second, full-text articles were initially examined by one reviewer. It was intended in the original protocol that a second reviewer would check approximately 10% of citations. However, because of the very large number of citations identified in the clinical effectiveness searches, this was not possible in the time scales available for this appraisal process. Any uncertainty in the inclusion and exclusion of potential full-text articles was resolved through discussion with the review team. Where agreement could not be reached, expert clinical advice was sought for a final decision.
The relevance of each article for the systematic review was assessed according to the following criteria.
Population
As detailed in Chapter 2, the three populations under consideration in this assessment were:
-
Adults with severe active RA not previously treated with MTX (defined by a DAS of ≥ 5.1). In the original protocol29 this population was defined as ‘adults with severe active RA not previously treated with MTX or other DMARDs (defined by a DAS of ≥ 5.1)’. However, this definition was subsequently modified and broadened by the Assessment Group (in consultation with clinical experts) to include ‘adults with severe active RA not previously treated with MTX’ to permit the inclusion of trial populations relevant to the decision problem which were MTX naive, but may have had some prior experience of other cDMARDs.
-
Adults with severe active RA who had been previously treated with conventional DMARDs only, including MTX (unless contraindicated or inappropriate) (defined by a DAS of ≥ 5.1).
-
Adults with moderate to severe active RA who had been previously treated with conventional DMARDs only, including MTX (unless contraindicated or inappropriate) (defined as a DAS between 3.2 and 5.1).
The following populations were considered outside the appraisal scope and were therefore excluded:
-
patients with a DAS of < 3.2
-
patients with a DAS of < 5.2 who had not been previously treated with MTX
-
patients who had been previously treated with one or more biologic DMARDs.
Interventions
The following interventions were included:
-
For RA not previously treated with MTX:
-
ADA
-
ETN
-
IFX
-
GOL.
-
-
For RA that has been previously treated with conventional DMARDs only:
-
ADA
-
ETN
-
IFX
-
CTZ
-
GOL
-
ABT (i.v. and s.c. preparations)
-
TCZ.
-
The above interventions were assessed in accordance with licensed indications and could be delivered in conjunction with cDMARDs or as monotherapy (as defined in licensed indications).
Comparators
The relevant comparators differed according to the population considered and included the following:
-
For severe active RA not previously treated with MTX:
-
combination therapy with conventional DMARDs (including MTX and at least one other DMARD, such as SSZ and LEF) or DMARD monotherapy with dose escalation
-
biologic interventions compared with each other.
-
-
For severe active RA that has been previously treated with conventional DMARDs only:
-
management strategies involving further conventional DMARDs (e.g. SSZ, LEF), NSAIDs and corticosteroids
-
biologic interventions compared with each other.
-
-
For moderate to severe active RA that has been previously treated with conventional DMARDs only:
-
management strategies involving further conventional DMARDs (e.g. SSZ, LEF), NSAIDs and corticosteroids
-
biologic interventions compared with each other.
-
Outcomes
The outcome measures under consideration included:
-
disease activity (DAS28, ACR and EULAR responses, swollen and tender joint counts and patient and physician global assessments of disease activity)
-
physical function [Health Assessment Questionnaire Disability Index (HAQ-DI), but not modified versions of HAQ]
-
joint damage/radiological progression
-
pain
-
mortality
-
fatigue
-
extra-articular manifestations of disease
-
health-related quality of life
-
adverse effects of treatment.
Study design
The systematic review of clinical effectiveness was based on RCT evidence. It was stated in the protocol29 that, if insufficient data were available from RCTs, observational studies or non-randomised trials may be considered (e.g. for safety evidence). The Assessment Group supplemented the AEs data identified in the included RCTs with safety data from long-term extension (LTE) studies reporting on individual included RCTs. Studies published as abstracts or conference presentations were only included if sufficient details were presented to allow both an appraisal of the methodology and an assessment of the results to be undertaken. Systematic reviews could be used as potential sources of additional references of efficacy evidence.
The following study types were also excluded:
-
animal models
-
preclinical and biological studies
-
narrative reviews, editorials, opinions
-
studies presenting secondary analyses of RCT data or pooled RCT data
-
non-English-language papers.
Data abstraction and critical appraisal strategy
Data relevant to the decision problem were extracted by one reviewer. Data were extracted without blinding to authors or journal. Study arms where intervention treatments were administered in line with licensed indications were extracted; where there was a slight divergence between the regimen used in the RCT and the licensed regimen, this was explicitly highlighted. It was proposed in the original protocol29 that at least 10% of data extraction forms be checked by a reviewer. However, the Assessment Group ensured that all data included in the NMA were double checked by a second reviewer. For data not contributing to the NMA, data were extracted for the following time points: primary end point (for selected efficacy data); latest available controlled RCT end point (for efficacy and safety data); and latest available LTE study end point (for safety data only). The safety data extracted were informed by the Summary of Product Characteristics [available at www.medicines.org.uk/emc/ (accessed 1 April 2014)] and FDA prescribing information for each intervention. 41–47 Graphical data contributing to the NMA were estimated using Engauge software [version 4.1; Mark Mitchell, Los Angeles, CA, USA (2011)] and graphical data not contributing to the NMA were estimated manually by a reviewer. Where multiple publications of the same study were identified, data extraction was undertaken on all relevant associated publications and findings were presented as a single study. Discrepancies were resolved by discussion, with involvement of a third reviewer when necessary.
The methodological quality of each included study was assessed by one reviewer. It was originally intended in the protocol29 that quality assessment would be checked by a second reviewer, but this was not feasible within the time scales available for the appraisal process. The quality assessment of included studies was informed by selected items listed in the NHS Centre for Reviews and Dissemination report48 and Cochrane Risk of Bias tool. 49 Additional quality issues specific to the assessment of RA RCTs (as described by Karsh et al. 50) were also considered during the evaluation of studies.
Methods of data synthesis
The extracted data and quality assessment variables were presented for each study, both in structured tables and as a narrative description.
As the identified evidence base permitted the undertaking of NMAs for the estimation of treatment effects, supplementary meta-analyses were not undertaken. NMAs were conducted to determine efficacy using two different disease activity measures (ACR and EULAR responses).
Methods for the estimation of efficacy using network meta-analysis
Selection of evidence contributing to the network meta-analysis
Evidence considered relevant to the decision problem was selected according to the additional inclusion criteria detailed below.
-
Randomised controlled trials presenting ACR response or EULAR response data at any assessment time point between 22 and 30 weeks. The selection of this time frame and assumption that treatment effects would be broadly comparable across these assessment points was made in conjunction with the clinical advisors to the assessment. This criterion is broadly in line with previous data syntheses summarised by Thorlund et al. :51 9 of the 13 RCTs in the NMA of biologic interventions for RA also employed an assessment time point in the region of 24 weeks/6 months; of the remaining four RCTs, three used 12-week data while one used data obtained between 50 and 55 weeks.
-
Trials with early escape were included only if an appropriate imputation of data as determined by the Assessment Group was employed for dealing with censoring.
-
Randomised controlled trials were not excluded from the base case on the basis of geographical location (a decision made in consultation with clinical advisors).
-
Randomised controlled trials were permitted in the base case where it was not indicated if bDMARDs had been given (and no proportion of bDMARD use was provided), even if trial eligibility did not exclude prior bDMARDs.
-
Trials reporting a small proportion of patients with prior bDMARD experience (≤ 20%) were not included in the base-case analyses but were explored via sensitivity analyses.
Sensitivity analyses were also undertaken to include trials relevant to populations 2 and 3 where the population may not have adequately failed cDMARDs (either there was a sufficient response, MTX treatment duration was too short or a proportion of the population were MTX naive).
Evidence was sought in which bDMARDs not considered as interventions or comparators within the NICE scope were evaluated in head-to-head trials with an included intervention in the first-line treatment of RA. To establish whether or not any such identified data could be used to inform indirect comparisons within the NMA, a review of these interventions against cDMARDs was undertaken. If such trials were found and met the inclusion criteria for the review, then the bDMARD was considered part of the evidence base for the NMA.
A number of assumptions relating to the evidence base were made in conjunction with clinical advisors: (1) It was assumed that all cDMARDs had the same efficacy; (2) it was also assumed that having failed a cDMARD was equivalent to having failed MTX; (3) trials that included the use of immunosuppressants or single intra-articular glucocorticoid were also permitted, assuming that this would not change the efficacy of cDMARDs; and (4) it was assumed that Disease Activity Score 28 C-reactive protein (DAS28-CRP) and Disease Activity Score 28 erythrocyte sedimentation rate (DAS28-ESR) are interchangeable where only one is reported. If both were reported, DAS28-ESR was used as this was reported most regularly (a decision made in consultation with clinical advisors). A systematic review to support assumptions (1) to (3) could not be undertaken within the time scales of the project. This may represent a limitation within the analyses although these assumptions were deemed reasonable by the clinical experts and there was no reason to believe these could cause a systematic bias.
Statistical model for the network meta-analysis
European League Against Rheumatism and ACR outcomes are ordered categorical data. EULAR has three categories (no response, moderate response and good response) and ACR has four categories (no response, ACR20, ACR50 and ACR70). ACRXX represents an improvement of at least XX%; in the analysis, the categories are treated as mutually exclusive so that patients cannot be in more than one category.
The model for the data assumes that the treatment effect is the same irrespective of the category. The likelihood function for the data is described as follows:
-
Let rikj represent the number of patients in arm k of trial i in the mutually exclusive category j = 1,2, . . . J.
The responses rikj will follow a multinomial distribution such that
The parameters in the model are the probabilities, pikj, that a patient in arm k of trial i has a response equivalent to category j.
We use a probit link function to map the probabilities, pikj, onto the real line such that:
so that
In this model, the effect of treatment is to change the probit score of the control arm by δi,bk standard deviations (SDs).
The study-specific treatment effects, δi,bkIk≠1, are assume to arise from a common population distribution with mean treatment effect relative to the reference treatment, which in this analysis is cDMARDs, such that:
We further assume that there is an underlying continuous latent variable which has been categorised by specifying cut-offs, zij, which correspond to the point at which an individual moves from one category to the next in trial i. The model is rewritten as:
The zij can be treated as fixed, which would assume that these points are the same in each trial and each treatment. Alternatively, they can be treated as random in which they are assumed to vary according to the trial but that within a trial they are the same such that:
We used a model in which the zij were treated as being random because this resulted in a much better fit of the model to the data.
In some trials, the reported categories are a subset of the full set of categories so that there is overlap between categories. The multinomial likelihood is rewritten as a series of conditional binomial distributions such that for trial i reporting the number of patients, rikj, in category j = 1, . . ., J – 1, we write:
where
. . .
and
Further details of the model are presented in Dias et al. 52
All analyses were conducted in the freely available software package WinBUGS (MRC Biostatistics Unit, Cambridge, UK).
The model is completed by giving the parameters prior distributions.
When there are sufficient sample data, we can use conventional reference prior distributions and these will have little influence on the posterior results. The reference prior distributions used in the analyses were:
-
trial-specific baselines, µi∼N(0,1000)
-
treatment effects relative to reference treatment, d1t∼N(0,1000)
-
between-study SD of treatment effects, τ∼U(0,2)
-
population cut-offs, υcj=υcj−1+υc′,υc′∼U(0,5)
-
between-study SD of cut-offs, σz2∼U(0,2).
In the case of the analysis of the EULAR data there were relatively few studies and too few to update the between-study SD. Without Bayesian updating, a reference prior distribution that does not represent genuine prior belief will have a significant impact on the results and give posterior distributions that are unlikely to represent genuine posterior beliefs. To allow for this, we used a weakly informative prior distribution for the between-study SD such that τ∼HN(0,0.322).
To estimate the absolute probabilities of being in each category for each treatment, we used a binomial likelihood function for the numbers of patients, rik1 in each study that were classified as ‘no response’ when treated with cDMARDs such that:
We used a probit link function such that:
We assume that the study-specific baselines arise from population of effects such that:
The model was completed by giving the parameters prior distributions such that:
Again, there were relatively few studies providing data on the EULAR outcome so a weakly informative prior distribution was used for the between-study SD such that: τ∼HN(0,0.322).
For the baseline meta-analyses and NMAs, we used a standard burn-in of 100,000 iterations of the Markov chain and retained 25,000 iterations to estimate parameters. In addition, the NMAs exhibited moderately high correlation between successive iterations of the Markov chains so the chains were thinned by retaining every 10th sample.
For EULAR and ACR, analyses were performed according to whether the patient was MTX naive (population 1) or whether patients were MTX experienced (populations 2 and 3). Patients who were MTX naive were also analysed including the Treatment of Early Aggressive Rheumatoid arthritis (TEAR) trial53 and the Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes (TEMPO)54 that included a small proportion of patients who were MTX experienced. In addition, for patients who were MTX experienced, EULAR was analysed according to the main trials and trials that included patients who received prior biologics [with and without the Actemra versus Methotrexate double Blind Investigative Trial In mONotherapy (AMBITION) study55] and ACR was analysed according to the main trials, trials that included patients who received prior biologics (with and without AMBITION55) and trials that included patients who were MTX naive.
We also explored the possibility that duration of disease was a treatment effect modifier. This was done for the main studies that provided ACR data. We did not attempt to adjust EULAR data for duration of disease because of the limited number of studies available. Duration of disease was centred in the model by subtracting the mean duration of disease across studies. Various models could be explored, including having an identical treatment effect modifier for each treatment, a separate treatment effect modifier for each treatment or allowing the treatment effect modifiers to be exchangeable across treatments. Again, because of the limited number of studies available we restricted attention to an exchangeable treatment effect modifier model. The model was completed by giving the common regression parameter a N(0, 1000) prior distribution and the between-treatment SD a U(0, 10) prior distribution. Results are not presented adjusted for duration of disease because the evidence suggested that it was not a treatment effect modifier (deviance information criterion adjusted = 1027.94, deviance information criterion unadjusted 1026.74).
Results
Quantity and quality of research available
Quantity of research available
As a result of the searches described in Methods for reviewing effectiveness, a total of 43,764 citations were identified for the review of clinical effectiveness and safety. This was reduced to 27,464 following deletion of duplicate citations. The study selection process is represented as a PRISMA diagram (Figure 2). A total of 27,334 citations were excluded at title and abstract levels (1606 being non-English-language records). Of the remaining records, a total of 60 studies were included in the review. Studies excluded at the full-text stage are presented (with rationale for exclusion) in Appendix 3.
FIGURE 2.
Flow diagram of study inclusion (adapted from PRISMA).

Randomised controlled trials included in the systematic review of clinical effectiveness and NMAs of ACR and EULAR responses are presented in Table 5 (with MTX-naive and cDMARD-experienced labels denoting trials included in population 1 and populations 2 and 3 respectively).
| Trial name/study | Intervention | Population | Included in NMA? |
|---|---|---|---|
| Abe et al., 200656 | IFX | cDMARD experienced | Not in NMA (14-week RCT) |
| ACT-RAY57 | TCZ | cDMARD experienced | Yes |
| ADACTA58 | ADA, TCZ | cDMARD experienced | Yes |
| ADORE59,60 | ETN | cDMARD experienced | Not in NMA (16-week study) |
| AIM61–65 | ABT | cDMARD experienced | Yes |
| AMPLE66 | ADA, ABT | cDMARD experienced | Yes |
| APPEAL67,68 | ETN | cDMARD experienced | Not in NMA (16-week study) |
| ARMADA69,70 | ADA | cDMARD experienced | Yes |
| ASPIRE71 | IFX | MTX naive | Not in NMA (no ACR/EULAR data at 22–30 weeks) |
| ASSET72 | ABT | cDMARD experienced | Not in NMA (4-month RCT) |
| ASSURE73 | ABT | cDMARD experienced | Not in NMA (no ACR/EULAR data at 22–30 weeks) |
| ATTEST74 | IFX, ABT | cDMARD experienced | Yes |
| ATTRACT75 | IFX | cDMARD experienced | Yes |
| AUGUST II76 | ADA | cDMARD experienced | Yes |
| Bejarano et al., 200877 | ADA | MTX naive | Not in NMA (no ACR/EULAR data at 22–30 weeks) |
| BeST78 | IFX | MTX naive | Yes |
| CERTAIN79 | CTZ | cDMARD experienced | Yes |
| CHANGE80 | ADA | cDMARD experienced | Yes |
| COMET81–83 | ETN | MTX naive | Yes |
| DE01984 | ADA | cDMARD experienced | Yes |
| deFilippis et al., 200685 | ETN, IFX | cDMARD experienced | Yes |
| Durez et al., 200486 | IFX | cDMARD experienced | Not in NMA (14-week study, no valid comparator arm) |
| Durez et al., 2007120 | IFX | MTX naive | Yes |
| ERA87 | ETN | MTX naive | Yes |
| ETN Study 30988,89 | ETN | cDMARD experienced | Yes |
| GO-BEFORE90 | GOL | MTX naive | Yes |
| GO-FORTH91 | GOL | cDMARD experienced | Yes |
| GO-FORWARD92 | GOL | cDMARD experienced | Yes |
| GUEPARD93 | ADA | MTX naive | Not in NMA (no ACR/EULAR data at 22–30 weeks) |
| HIT HARD94 | ADA | MTX naive | Yes |
| IDEA95 | IFX | MTX naive | Not in NMA (no ACR/EULAR data at 22–30 weeks) |
| CREATE IIb96 | ETN | cDMARD experienced | Yes |
| JESMR97 | ETN | cDMARD experienced | Yes |
| Kay et al., 200898 | GOL | cDMARD experienced | Not in NMA [no eligible ACR/EULAR data at 22–30 weeks (owing to PBO group crossover)] |
| Kim et al., 200799 | ADA | cDMARD experienced | Yes |
| Kume et al., 2011100 | ADA, ETN | MTX naive | Not in NMA (early escape at 12 weeks with no imputation for missing data) |
| Lan et al., 2004101 | ETN | cDMARD experienced | Not in NMA (12-week study) |
| LARA102 | ETN | cDMARD experienced | Yes |
| MEASURE103 | TCZ | cDMARD experienced | Not in NMA (no ACR/EULAR data at 22–30 weeks) |
| Moreland et al., 1999104/Mathias et al., 2000105 | ETN | cDMARD experienced | Yes |
| Nishimoto et al., 2004106 | TCZ | cDMARD experienced | Not in NMA (no ACR/EULAR data at 22–30 weeks) |
| OPERA107 | ADA | MTX naive | Not in NMA (no ACR/EULAR data at 22–30 weeks) |
| OPTIMA108 | ADA | MTX naive | Yes |
| PREMIER109 | ADA | MTX naive | Yes |
| Quinn et al., 2005110 | IFX | MTX naive | Not in NMA (no ACR/EULAR data at 22–30 weeks) |
| RACAT111/O’Dell et al., 2013112 | ETN | cDMARD experienced | Yes |
| REALISTIC113 | CTZ | cDMARD experienced | Not in NMA (no biologic-naive ACR/EULAR data at 22–30 weeks) |
| RED-SEA114 | ADA, ETN | cDMARD experienced | Not in NMA (no ACR/EULAR data at 22–30 weeks) |
| SAMURAI115 | TCZ | cDMARD experienced | Yes |
| SATORI116 | TCZ | cDMARD experienced | Yes |
| STAR117 | ADA | cDMARD experienced | Yes |
| START118 | IFX | cDMARD experienced | Yes |
| Swefot119 | IFX | cDMARD experienced | Yes |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| TOWARD121 | TCZ | cDMARD experienced | Yes |
| Van De Putte et al., 2004122 | ADA | cDMARD experienced | Yes |
| Wajdula 2000 (reported in Chen et al., 2006123) | ETN | cDMARD experienced | Not in NMA (12-week study) |
| Weinblatt et al., 1999124 | ETN | cDMARD experienced | Yes |
| Wong et al., 2009125 | IFX | cDMARD experienced | Not in NMA (no ACR/EULAR data at 22–30 weeks) |
| Zhang et al., 2006126 | IFX | cDMARD experienced | Not in NMA (18-week study) |
Sixty RCTs were included in the systematic review of clinical effectiveness. These comprised six trials with head-to-head comparisons of included biologic interventions, [academic-in-confidence (AiC) information has been removed], and 53 trials of biologic interventions compared with placebo (PBO) or cDMARDs.
Methotrexate-naive trial populations are considered separately in the following results section as population 1. For population 1 there were a total of 15 RCTs included in the systematic review (ABT n = 0, ADA n = 6, CTZ n = 0, ETN n = 2, GOL n = 1, IFX n = 5, TCZ n = 0 and head-to-head biologics n = 1). Eight of the MTX-naive trials had data available for the NMA. All these seven trials provided ACR data; however, only one90 contributed EULAR data for analysis. A head-to-head trial of ADA versus ETN was identified but this trial was not eligible for the NMA (due to early escape at 12 weeks with no imputation for missing data). 100
There were 45 trials with cDMARD-experienced populations (considered as populations 2 and 3) (ABT n = 3, ADA n = 7, CTZ n = 2, ETN n = 11, GOL n = 3, IFX n = 7, TCZ n = 6, head-to-head biologics n = 5 and grouped antiTNFs n = 1). Of these, 30 trials had data available for the NMA.
Twelve trials that did not satisfy the inclusion criteria for the systematic review (as outlined in Methods for reviewing effectiveness) were excluded from the systematic review but were used as additional evidence and explored in sensitivity analyses in the NMA (Table 6). These trials contributed ACR and/or EULAR data to sensitivity analyses only. Of these, 10 trials had populations with a small proportion that had received prior biologics (≤ 20%). The other remaining trials were not in the base case because they had populations in which some patients were MTX naive or cDMARD and others were not, or patients were responding to MTX.
| Trial name/study | Intervention | Allocated population | Rationale for ineligibility in systematic review |
|---|---|---|---|
| ACQUIRE127 | ABT | cDMARD experienced | 3.4–6% prior biologics |
| AMBITION55,128 | TCZ | cDMARD experienced | 5–9% prior biologics, mix of MTX naive and prior MTX |
| Yamamoto et al., 2011129 | CTZ | cDMARD experienced | 16% prior biologics |
| LITHE130 | TCZ | cDMARD experienced | 11% prior biologics |
| NCT00254293131 | ABT | cDMARD experienced | 2.6% prior biologics |
| OPTION132 | TCZ | cDMARD experienced | 5–9% prior biologics |
| ORAL Standard133 | ADA, TOF | cDMARD experienced | 10% prior biologics |
| RA0025134 | CTZ | cDMARD experienced | 15% prior biologics |
| RAPID1135 | CTZ | cDMARD experienced | 4% prior biologics |
| RAPID2136 | CTZ | cDMARD experienced | 1.6% prior biologics |
| TEAR53 | ETN | cDMARD experienced and MTX naive | Mix of MTX-naive and prior MTX, some patients (less than 30%) had any prior cDMARD use |
| TEMPO54 | ETN | cDMARD experienced and MTX naive | Mix of MTX-naive, and prior MTX but not inadequate response |
| Kremer et al., 2012137 | TOF | cDMARD experienced | Did not include any bDMARD within the NICE scope |
| van der Heijde et al., 2013138 | TOF | cDMARD experienced | Did not include any bDMARD within the NICE scope |
In addition, two trials providing supplementary network linkages were included in the NMA. These RCTs did not include any of the included interventions as specified in the decision problem, but evaluated TOF versus PBO. 137,138 Both of these trial populations had some prior biologic use (and therefore these trials were considered within the NMA sensitivity analyses).
Quality of research available
The quality of the included RCTs is presented in Table 345 (see Appendix 4) and summarised in Figure 3. There is a reasonably low risk of bias overall among studies included in this review. Items where risk of bias was greatest were those that assessed comparability of groups, blinding and selective reporting. Items generating a large proportion of ‘unclear’ responses (indicating a lack of clarity in reporting) were those relating to generation of allocation sequence, allocation concealment and selective reporting of outcomes. Items with a low risk of bias in a large proportion of trials were comparability at baseline, blinding, analysis by allocated treatment group and most (≥ 80%) participants randomised included in the final analysis. A modified intention-to-treat population was used in around half of trials for efficacy and safety analyses (which was typically based on all randomised patients who received at least one dose of study drug being included in analyses).
FIGURE 3.
Risk-of-bias graph. mITT, modified intention to treat.

Summary of trials and population characteristics
There were some differences between trials in population characteristics, treatment and trial duration. For some trials, intervention and control arms differed in terms of numbers/combinations of concomitant cDMARDs. Some trials allowed physician discretion in other therapies. There was some variation between trials in prior treatment history and disease duration. There was some variation in how early withdrawals were decided, with variation in length of time on allocated treatment.
Trial characteristics
Adults with severe active rheumatoid arthritis not previously treated with methotrexate (population 1)
As discussed in Methods for reviewing effectiveness, trials in which populations were MTX naive but had received some prior treatment with other cDMARDs were considered appropriate for inclusion in population 1. Study characteristics for trials included in population 1 are presented in Tables 345 and 346 (see Appendix 4).
Adults with moderate to severe and severe active rheumatoid arthritis that have been previously treated with cDMARDs (but not bDMARDs) (cDMARD experienced) (populations 2 and 3)
Study characteristics for trials included in populations 2 and 3 are presented in Tables 347–349 (see Appendix 4).
Population characteristics
Adults with severe active rheumatoid arthritis not previously treated with methotrexate (population 1)
Population characteristics for population 1 are presented in Tables 7 and 8.
| Study | Treatment arms | Mean age (years) (SD) | Sex (% female) | Early withdrawal plan reported? | Disease duration (years) (SD) | Mean DAS28 at baseline (SD) – ESR unless stated to be CRP |
|---|---|---|---|---|---|---|
| Kume et al., 2011100 | ADA monotherapy (n = 22) | 63 (17) | 85.7 | Yes | 0.75 (0.42) | ESR 5.34 (1.4) |
| ETN monotherapy (n = 21) | 51 (15) | 85.7 | 0.92 (0.42) | ESR 5.17 (1.5) |
| Trial name/study | Treatment arms | Mean age (years) (SD) | Sex (% female) | Early withdrawal plan reported? | Disease duration (years) (SD) | Mean DAS28 at baseline (SD) – ESR unless stated to be CRP |
|---|---|---|---|---|---|---|
| Bejarano et al., 200877 | PBO + MTX (n = 73) | 47 (9) | 53.4 | Yes | 6.6 | 6.0 (1.5) |
| ADA + MTX (n = 75) | 47 (9) | 58.4 | 7.9 | 5.9 (1.4) | ||
| GUEPARD93 | Initial MTX 12 weeks, then step-up therapya based on DAS28 (n = 32) | 49.3 (15.2) | 81.25 | Yes | 4.4 (3.3–5.1)b months | ESR 6.15 (0.88); CRP 5.85 (0.91) |
| Initial ADA + MTX 12 weeks, then step-upa therapy based on DAS28 (n = 33) | 46.3 (16.3) | 78.79 | 4.4 (3.3–5.1)b months | ESR 6.31 (0.78); CRP 5.80 (0.83) | ||
| HIT HARD94 | MTX + PBO (n = 85) | 52.5 (14.3) | 67.1 | NR | 0.13 (NR) | 6.3 (0.9) |
| ADA + PBO (n = 87) | 47.2 (12.1) | 70.1 | 0.15 (NR) | 6.2 (0.8) | ||
| OPERA107 | MTX + PBO + steroid (n = 91) | 5.42 (28.3, 76.7)c | 69 | Yes | 0.22 (0.12, 0.41)c | CRP 5.6 (3.8, 7.3)c |
| ADA + MTX + steroid (n = 89) | 56.2 (25.8, 77.6)c | 63 | 0.24 (0.12, 0.44)c | CRP 5.5 (3.8, 7.8)c | ||
| OPTIMA108 | MTX + PBO (n = 517) | 50.7 (NR) | 74 | NR | 0.38 (NR) | 6 |
| ADA + MTX (n = 515) | 50.4 (NR) | 74 | 0.30 (NR) | 6 | ||
| PREMIER109 | MTX + PBO (n = 257) | 52.0 (13.1) | 73.9 | Yes | 0.8 (0.9) | 6.3 (0.9) |
| ADA monotherapy + PBO step-up week 16 (n = 274) | 52.1 (13.5) | 77.4 | 0.7 (0.8) | 6.4 (0.9) | ||
| ADA + MTX step-up week 16 (n = 268) | 51.9 (14.0) | 72.0 | 0.7 (0.8) | 6.3 (0.9) | ||
| COMET81,82 | MTX + PBO (n = 268) | 52·3 (0.8) | 73 | NR | Months 9.3 (0.4) | 6.5 (1.0) |
| ETN + MTX (n = 274) | 50·5 (0.9) | 74 | Months 8.8 (0.4) | 6.5 (1.0) | ||
| Bathon and Genovese, 2000139 | MTX + PBO (n = 217) | 49 (13) | 75 | NR | 1 (0.92) | NR |
| ETN + PBO (n = 207) | 50 (13) | 74 | 1 (0.92) | NR | ||
| GO-BEFORE90 | PBO+MTX (n = 160) | 48.6 (12.91) | 83.8 | NR | ≤ 3 years = 72.5%; ≤ 2 years = 61.9%; ≤ 1 year = 45.6% | ESR 6.2 (1.17); CRP 5.6 (1.06) |
| GOL + MTX (n = 159) | 50.9 (11.32) | 84.9 | ≤ 3 years = 73.0%; ≤ 2 years = 64.2%; ≤ 1 year = 50.9% | ESR 6.3 (1.11); CRP 5.7 (1.05) | ||
| ASPIRE71 | PBO i.v. + MTX (n = 298) | 50 (13) | 75 | NR | 0.9 (0.7) | NR |
| IFX + MTX (n = 373) | 51 (12) | 71 | 0.8 (0.7) | NR | ||
| BeST78 | Sequential monotherapy (DAS steered) (n = 126) | 54 (13) | 68 | Yes | 23 weeksd | DAS44 4.5 (0.9) |
| Step-up combination therapy (DAS steered) (n = 121) | 54 (13) | 71 | 26 weeksd | DAS44 4.5 (0.8) | ||
| Initial combination therapy with prednisone (DAS steered) (n = 133) | 55 (14) | 65 | 23 weeksd | DAS44 4.4 (0.9) | ||
| Initial combination therapy with IFX (DAS steered) (n = 128) | 54 (14) | 66 | 23 weeksd | DAS44 4.3 (0.9) | ||
| Durez et al., 2007120 | MTX (n = 14) | 53.8 (15.2) | 71 | NR | 0.45 (0.29) | CRP 5.2 (0.8) |
| MTX + MP (n = 15) | 50.3 (14.2) | 60 | 0.25 (0.33) | 5.3 (1.3) | ||
| IFX + MTX (n = 15) | 50.0 (9.9) | 67 | 0.36 (0.31) | 5.3 (1.1) | ||
| IDEA95 | MP + MTX (n = 112 across both groups) | NR | NR | Yes | NR (described as early RA, 3–12 months symptom duration) | NR |
| IFX 3 mg/kg i.v. at weeks 0, 2, 6, 14, 22 + MTX (IFX dose modifications permitted according to DAS44 from week 26) | NR | NR | NR | |||
| Quinn et al., 2005110 | MTX + PBO (n = 10) | 53.1 (13.7) | 70 | NR | 0.5 (0.31) | 7.0 (0.9) |
| IFX + MTX (n = 10) | 51.3 (9.5) | 60 | 0.62 (0.38) | 6.2 (0.8) |
Adults with moderate to severe and severe active rheumatoid arthritis who have been previously treated with cDMARDs (but not bDMARDs) (cDMARD experienced) (populations 2 and 3)
Population characteristics for populations 2 and 3 are presented in Tables 9 and 10.
| Trial name/study | Treatment arms | Mean age (years) (SD) | Sex (% female) | Early withdrawal plan reported? | Disease duration (years) (SD) | Mean DAS28 at baseline (SD) – ESR unless stated to be CRP |
|---|---|---|---|---|---|---|
| ATTEST74 | PBO + MTX (n = 110) | 49.4 (11.5) | 87.3 | NR | 8.4 (8.6) | ESR 6.8 (1.0) |
| IFX + MTX (n = 165)a | 49.1 (12.0) | 82.4 | 7.3 (6.2) | 6.8 (0.9) | ||
| ABT + MTX (n = 156)b | 49.0 (12.5) | 83.3 | 7.9 (8.5) | 6.9 (1.0) | ||
| AMPLE66 | ABT s.c. (n = 318) | 51.4 | 81.4 | NR | 1.9 | CRP 5.5 |
| ADA (n = 328) | 51.0 | 82.3 | 1.7 | CRP 5.5 | ||
| RED-SEA114 | ADA + cDMARDs (n = 60) | 55.0 | 75 | NR | 7.0 (range 3.3–13.0) | 5.6 |
| ETN50 + cDMARDs (n = 60) | 53.2 | 70 | 5.5 (range 2.0–14.5) | 5.8 | ||
| ADACTA58 | TCZ + PBO (n = 163) | 54.4 (13.0) | 79 | Yes | 7.3 (8.1) | 6.7 (0.9) |
| ADA + PBO (n = 163) | 53.3 (12.4) | 82 | 6.3 (6.9) | 6.8 (0.9) | ||
| deFilippis et al., 200685 | ETN + MTX (n = 16) | 44.7 (14.17) | NR | NR | NR | NR |
| IFX + MTX (n = 16) | 46.79 (10.9) | NR | NR | NR |
| Trial name/study | Treatment arms | Mean age (years) (SD) | Sex (% female) | Early withdrawal plan reported? | Disease duration (years) (SD) | Mean DAS28 at baseline (SD) – ESR unless stated to be CRP |
|---|---|---|---|---|---|---|
| AIM61,62 | MTX + PBO (n = 219) | 50.4 | 81.7 | NR | 8.9 (7.1) | CRP 6.4 (0.1) |
| ABT i.v. + MTX (n = 433) | 51.5 | 77.8 | 8.5 (7.3) | CRP 6.4 (0.08) | ||
| ASSET72 | PBO + MTX (n = 23) | 52.5 (11.5) | 69.6 | NR | 2.4 (1.4) | CRP 5.3 (0.9) |
| ABT i.v. (≈10 mg/kg) + MTX (n = 27) | 51.7 (11.2) | 59.3 | 2.1 (1.5) | CRP 5.3 (1.1) | ||
| ASSURE73 | PBO + cDMARDs (n = 482) | 52.0 (12.1) | 83.7 | NR | 9.5 (9.1) | NR |
| ABT + cDMARDs (n = 959) | 52.2 (11.8) | 83.1 | 9.5 (8.7) | NR | ||
| AUGUST II76 | MTX + PBO (n = 76) | 54 | 84 | NR | 8.4 | 5.8 |
| ADA + MTX (n = 79) | 53 | 81 | 8.8 | 5.8 | ||
| CHANGE80 | PBO (n = 87) | 53.4 | 77 | Yes | 8.4 | NR |
| ADA (n = 91) | 56.9 | 79.1 | 9.9 | NR | ||
| DE01984 | MTX + PBO (n = 200) | 56.1 | 73 | Yes | 10.9 | NR |
| ADA + MTX (n = 207) | 56.1 | 76.3 | 11 | NR | ||
| STAR117 | PBO + cDMARDs (n = 318) | 55.8 | 79.2 | NR | 11.5 | NR |
| ADA + cDMARDs (n = 318) | 55 | 79.6 | 9.3 | NR | ||
| Van De Putte et al., 2004122 | PBO s.c. (n = 110) | 53.5 (13.2) | 77.3 | Yes | 11.6 (9.3) | 7.09 (0.87) |
| ADA monotherapy (n = 113) | 52.7 (13.3) | 79.6 | 10.6 (6.9) | 7.07 (0.86) | ||
| ARMADA69 | MTX + PBO (n = 62) | 56 | 82.3 | Yes | 11.1 | NR |
| ADA + MTX (n = 67) | 57.2 | 74.6 | 12.2 | NR | ||
| Kim et al., 200799 | MTX + PBO rescue week 18 (n = 65) | 49.8 | 85.7 | Yes | 6.9 | NR |
| ADA + MTX (n = 63) | 48.5 | 95.4 | 6.8 | NR | ||
| CERTAIN79 | PBO + cDMARDs (n = 98) | 54.0 (12.4) | 76.5 | Yes | 4.7 (3.3) | ESR 4.47 (0.34) |
| CTZ + DMARDs (n = 96) | 53.6 (11.9) | 84.4 | 4.5 (3.5) | ESR 4.53 (0.43) | ||
| REALISTIC113 | PBO + existing cDMARDs (biologic-naive subgroup) (n = 29) | NR 53.9 (12.7) (overall trial population, n = 212) |
79.7 (overall trial population, n = 212) | NR No (N/A as trial only 12 weeks) |
8.9 (9.1) (overall trial population, n = 212) | DAS28-ESR 6.4 (0.9); DAS28-CRP 5.7 (0.9) (Overall trial population, n = 212) |
| CTZ existing cDMARDs (biologic-naive subgroup) (n = 134) | 55.4 (12.4) (overall trial population, n = 851) | 77.6 (overall trial population, n = 851) | 8.6 (8.8) (overall trial population, n = 851) | DAS28-ESR 6.4 (0.9); DAS28-CRP 5.7 (0.9) (Overall trial population, n = 851) |
||
| ADORE59,60 | ETN (n = 159) | 53 | 79.2 | NR | 10.0 | 6.2 |
| ETN + MTX (n = 155) | 54 | 76.8 | 9.8 | 6.3 | ||
| CREATE IIb96 | DMARD + PBO (n = 65) | 51.5 | 83.1 | NR | 8.2 (7.59) | 6.3 (0.76) |
| ETN50 + DMARD (n = 64) | 51.2 | 85.9 | 7.9 (7.15) | 6.4 (0.85) | ||
| Combe et al., 2006,88 Combe et al., 200989 | SSZ + PBO (n = 50) | 53.3 | 82 | NR | 5.6 | DAS44-ESR 5.0 |
| ETN + PBO (n = 103) | 51.3 | 78.6 | 7.1 | DAS44-ESR 5.1 | ||
| ETN + SSZ (n = 101) | 50.6 | 80.2 | 6.5 | DAS44-ESR 5.2 | ||
| JESMR140 | ETN (n = 74) | 58.1 (12.6) | 87.3 | NR | 10.6 (10.5) | 6.1 |
| ETN + MTX 6–8 mg/week (n = 77) | 56.5 (11.1) | 80.0 | 8.1 (7.7) | 6.0 | ||
| Lan et al., 2004101 | PBO + MTX (n = 29) | 50.79 | 90 | NR | NR (eligibility more than one year) | NR |
| ETN + MTX (n = 29) | 47.55 | 83 | NR | |||
| LARA102 | MTX + DMARD (n = 142) | 48.6 | 90.1 | NR | 9.0 (7.5) | 5.9 |
| ETN50 + MTX (n = 281) | 48.4 | 88.3 | 7.9 (7.0) | 5.9 | ||
| Moreland et al., 1999104 | PBO (n = 800) | 51 | 76 | NR | 12 | NR |
| ETN + PBO (n = 78) | 53 | 74 | 11 | NR | ||
| O’Dell et al., 2013112 | MTX + SSZ + HCQ (n = 178) | 57.8 (13) | 43.4 | Yes | 5.5 (9.3) | 5.8 |
| ETN50 + MTX (n = 175) | 56 (13.2) | 48.9 | 4.9 (8.0) | 5.9 | ||
| Wajdula 2000 (reported in Chen et al., 2006123) | PBO (n = 111) | 53 | NR | N/A (12 week study) | 7.2 | NR |
| ETN (n = 105) | 53 | NR | 7.5 | NR | ||
| Weinblatt et al., 1999124 | MTX + PBO (n = 30) | 53 | 73 | Yes | 13 | NR |
| ETN + MTX (n = 59) | 48 | 90 | 13 | NR | ||
| APPEAL67 | MTX + DMARD (SSZ, HCQ or LEF) (n = 103) | 48.5 (11.3) | 88.4 | NR | 6.9 (8.5) | ESR 6.1 (1.1); CRP 5.34 (1.1) |
| ETN + MTX (n = 197) | 48.4 (12.0) | 91.4 | 6.5 (7.3) | ESR 6.1 (1.1); CRP 5.23 (1.1) | ||
| GO-FORTH91 | PBO + MTX 6–8 mg/week (n = 90) | 51.1 (11.6) | 83.0 | Yes | 8.7 (8.2) | ESR 5.6 (0.99) |
| GOL + MTX 6–8 mg/week (n = 89) | 50.4 (9.9) | 84.9 | 8.8 (8.8) | ESR 5.5 (1.18) | ||
| GO-FORWARD92 | PBO + MTX (n = 133) | Mean (SD) = 51.2 (11.96) 52.0 (42.0–58.0)a |
82.0 (109/133) | Yes | Mean (SD) = 8.62 (7.86) 6.5 (3.1–11.9)a |
CRP 5.458 (4.672–6.093);a ESR 6.111 (5.260–6.574)a |
| GOL + MTX (n = 89) | Mean (SD) = 50.3 (10.98) 52.0 (43.0–57.0)a |
80.9 (72/89) | Mean (SD) = 7.33 (7.83) 4.5 (2.1–9.7)a |
CRP 5.766 (4.628–6.322)a | ||
| Kay et al., 200898 | PBO s.c. + MTX (n = 35) | (46.0–66.0)a | 74.3 | Yes | 5.6 (1.4–10.9)a | CRP 5.8 (5.2–6.4);a ESR 6.3 (5.7–7.0)a |
| GOL + MTX (n = 35) | 57.0 (50.0–64.0)a | 85.7 | 8.2 (4.1–14.3)a | CRP 5.9 (5.5–6.9);a ESR 6.4 (5.6–7.3)a | ||
| Abe et al., 200656 | PBO + MTX (n = 47) | 55.1 (7.6) | 74.5 (35/47) | NR | 7.5 (5.0) | NR |
| IFX + MTX (n = 49) | 55.2 (10.9) | 81.6 (40/49) | 9.1 (7.4) | NR | ||
| ATTRACT75 | PBO + MTX (n = 88) | 51 (19.0–75.0)a | 80 (70/88) | NR | 8.9 (0.8–35.0)b | NR |
| IFX + MTX (n = 86) | 56 (25.0–74.0)a | 81 (70/86) | 8.4 (0.7–45.0)b | NR | ||
| Durez et al., 200486 | Single i.v. infusion of MP (sodium hemisuccinate) at week 0 + MTX (n = 15) | 56 (35–79)b | 73 | NR | 12 (1–24)b | NR |
| IFX + MTX (n = 12) | 48 (34–60)b | 100 | 10 (2–20)b | NR | ||
| START118 | PBO + MTX (n = 363) | 52.0 (44–61)a | 83.2 | Yes | 8.4 (4–15)a | NR |
| IFX + MTX (n = 360) | 53.0 (45–61)a | 80.0 | 7.8 (3–15)a | NR | ||
| Swefot119 | SSZ + HCQ + MTX (n = 130) | 52.9 (13.9) | 78 (101/130) | Yes | 0.525 | 4.79 (1.05) |
| IFX + MTX (n = 128) | 51.1 (13.3) | 76 (97/128) | 0.517 | 4.91 (0.98) | ||
| Wong et al., 2009125 | PBO + MTX (with crossover to open-label IFX at week 24) (n = 9) | 50 (16) | 8/9 | Yes | NR | 6.4 (0.8) |
| IFX + MTX (n = 17) | 48 (12) | 14/17 | NR | 6.2 (0.9) | ||
| Zhang et al., 2006126 | PBO + MTX (n = 86) | 48.9 (8.0) | 84.9 | NR | 8 (6.22) | NR |
| IFX + MTX (n = 87) | 47.9 (10.1) | 85.1 | 7.13 (6.17) | NR | ||
| ACT-RAY57 | TCZ + PBO (n = 277) | 53.6 (11.9) | 78.6 | NR | 8.3 (8.4) | ESR 6.36 (1.00) |
| TCZ + MTX (n = 276) | 53.0 (13.4) | 81.9 | 8.2 (8.0) | ESR 6.33 (0.98) | ||
| MEASURE103 | PBO + MTX (n = 69) | NR | NR | Yes | NR | NR |
| TCZ + MTX (n = 69) | NR | NR | NR | NR | ||
| Nishimoto et al., 2004106 | PBO (n = 53) | 53.0 (31–73)b | 73.6 | NR | 8.4 (0.7–52.7)b | NR |
| TCZ monotherapy (n = 55) | 56.0 (25–74)b | 83.6 | 8.3 (1.3–45.7)b | NR | ||
| SAMURAI115 | cDMARDs (n = 145) | 53.1 | 82 | NR | 124.8 weeks | 6.4 |
| TCZ monotherapy (n = 157) | 52.9 | 79.6 | 114.4 weeks | 6.5 | ||
| SATORI116 | PBO + MTX (n = 64) | 50.8 (12.2) | 48/64 evaluated | NR | 8.7 (7.1) | 6.2 (0.9) |
| TCZ + PBO (n = 61) | 52.6 (10.6) | 90.2 | 8.5 (8.4) | 6.1 (0.9) | ||
| TOWARD121 | PBO + stable cDMARDs (n = 415) | 54 (13) | 84 | Yes | 9.8 (9.1) | 6.6 (1.0) |
| TCZ + stable DMARDs (n = 805) | 53 (13) | 81 | 9.8 (8.8) | 6.7 (1.0) | ||
| TACIT141 | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
Additional population characteristics are outlined in Tables 350–355 (see Appendix 4).
Assessment of effectiveness
Disease activity and physical function
American College of Rheumatology response
One head-to-head RCT in MTX-naive patients was identified in the systematic review. 100 However, no ACR response data were available in this trial. A total of 13 RCTs of biologic versus DMARD(s) or PBO reported ACR response data in MTX-naive patients (six for ADA,77,93,94,107,109,142 two for ETN,81,139 one for GOL90 and four for IFX71,78,110,120) (Table 11). Statistically significant differences in ACR response favouring biologic treatment over comparator were reported for ADA (four studies94,107,109,142), ETN (two studies81,139), GOL (one study90) and IFX (two studies71,120). Seven of the 12 RCTs contributed data to a NMA of ACR response for population 1 (three for ADA,94,109,142 one for ETN,81,139 one for GOL90 and two for IFX78,120).
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Numbers analysed | % achieving ACR20 response | % achieving ACR50 response | % achieving ACR70 response | Data used in NMA? |
|---|---|---|---|---|---|---|---|
| Bejarano et al., 200877 | PBO + MTX | 56 weeks | 73 | 54.8 | 45.2 | 37.5 | No |
| ADA + MTX | 75 | 71.6 | 56.0 | 50.7 | |||
| GUEPARD93 | Initial MTX 12 weeks, then step-up therapy in both groups based on DAS28 | 12 weeks | 32 | 50 | 27 | 19 | No |
| Initial ADA + MTX 12 weeks, then step-up therapy in both groups based on DAS28 | 33 | 84 | 66 | 44 | |||
| GUEPARD93 | Initial MTX 12 weeks, then step-up therapy in both groups based on DAS28 | 52 weeks | 32 | 81 | 68 | 58 | No |
| Initial ADA+MTX 12 weeks, then step-up therapy in both groups based on DAS28 | 33 | 85 | 67 | 42 | |||
| HIT HARD94 | PBO + MTX | 24 weeks | 85 | 67.6 | 48.7 | 26.8 | Yes |
| ADA + MTX | 87 | 79.0 | 63.8 | 48.0a | |||
| OPERA107 | PBO + MTX + steroid | 12 months | 91 | 78 | 63 | 45 | No |
| ADA + MTX + steroid | 89 | 86 | 80a | 65a | |||
| OPTIMA142 | PBO + MTX | 26 weeks | 517 | 57 | 34 | 17 | Yes |
| ADA + MTX | 515 | 70b | 52b | 35b | |||
| PREMIER109 (Supplementary data identified via ClinicalTrials.gov) |
PBO + MTX | 26 weeks | 257 | 61.5 | 40.5 | 22.2 | Yes |
| ADA monotherapy + PBO | 274 | 53.3 | 35.0 | 19.7 | |||
| ADA + MTX | 268 | 68.7 | 58.6 | 42.5 | |||
| PREMIER109 | PBO + MTX | 1 year | 257 | 63 | 46 | 28 | No |
| ADA monotherapy + PBO | 274 | 54a (vs. MTX monotherapy) | 41 | 26 | |||
| ADA + MTX | 268 | 73a (vs. MTX monotherapy), b (vs. ADA monotherapy) | 62b | 46b | |||
| PBO + MTX | 2 years | 257 | 56 | 43 | 28 | No | |
| ADA monotherapy + PBO | 274 | 49 | 37 | 28 | |||
| ADA + MTX | 268 | 69a (vs. MTX monotherapy), b (vs. ADA monotherapy) | 59b | 47b | |||
| COMET81 | PBO + MTX | 24 weeks | 268 | 169 | 102 | 47 | Yes |
| ETN + MTX | 274 | 224 | 167 | 103 | |||
| PBO + MTX | 52 weeks | 268 | 67 | 49 | 28 | No | |
| ETN + MTX | 274 | 86 | 71 | 48b | |||
| COMET82 | MTX in year 1, MTX in year 2 | 2 years (week 104) | 99 | 61 | 46 | 32 | No |
| MTX year 1, ETN + MTX in year 2 | 90 | 81a | 66a | 48a | |||
| ETN + MTX in year 1, ETN + MTX in year 2 | 111 | 86a | 70a | 57b | |||
| ETN + MTX in year 1, ETN in year 2 | 111 | 80 | 64 | 44 | |||
| ERA139 | PBO + MTX | 6 months | 217 | 58.2 | 31.54 | 14.24 | Yes |
| ETN + PBO | 207 | 65.42 | 40.14 | 20.94a | |||
| PBO + MTX | 12 months | 217 | 66c | 44c | 23c | No | |
| ETN + PBO | 207 | 72c | 49c | 26c | |||
| GO-BEFORE90 | PBO + MTX | 24 weeks | 160 | 49.4 | 29.4 | 15.6 | Yes |
| GOL + MTX | 159 | 61.6a | 40.3a | 23.9 | |||
| GO-BEFORE143 | PBO + MTX | 52 weeks | 160 | 63.1 | 40.6 | 24.4 | No |
| GOL + MTX | 159 | 68.6 | 43.4 | 28.3 | |||
| ASPIRE71 | PBO + MTX | 54 weeks | 274 | 53.6 | 32.1 | 21.2 | No |
| IFX + MTX | 351 | 62.4a | 45.6b | 32.5a | |||
| BeST78 | Sequential monotherapy | 6 months | 126 | 49.69 | NR | 15.9 | Yes |
| Step-up combination therapy | 121 | 60.04 | NR | 11.77 | |||
| Initial combination therapy + prednisone | 133 | 70.63 | NR | 26.58 | |||
| Initial combination therapy + IFX | 128 | 74.3 | NR | 31.15 | |||
| Durez et al., 2007120 | MTX | 22 weeks | 14 | 28.13 | 7.69 | 0 | Yes |
| MTX + i.v. MP | N/A | N/A | N/A | N/A | N/A | ||
| IFX + MTX | 22 weeks | 15 | 86.72a | 66.85a | 33.79a | ||
| MTX | 52 weeks | 14 | 46c | 39c | 14c | No | |
| MTX + i.v. MP | 15 | 87c | 67c | 53c | |||
| IFX + MTX | 15 | 80c | 65c | 29c | |||
| Quinn et al., 2005110 | PBO + MTX | 14 weeks | 10 | 20 | 0 | 0 | No |
| IFX + MTX | 10 | 60 | 60 | 60 | |||
| PBO + MTX | 54 weeks | 10 | 60 | 40 | 30 | No | |
| IFX + MTX | 10 | 80 | 80 | 70 |
(NB: in the outcome tables that follow throughout Results, citations are provided where data were extracted from sources additional to the primary publication.)
Four head-to-head RCTs reporting ACR response data in cDMARD-experienced patients were identified (Table 12). Statistically significantly greater proportions of patients achieved ACR20, ACR50 and ACR70 responses in the IFX plus MTX and abatacept i.v. plus MTX treatment groups of the A Trial for Tolerability, Efficacy, and Safety in Treating rheumatoid arthritis (infliximab) (ATTEST) trial,74 when compared against PBO plus MTX. Statistically significant findings were also identified in the ADalimumab ACTemrA (tocilizumab) head-to-head study (ADACTA), whereby greater proportions of patients receiving TCZ monotherapy achieved ACR responses than among patients receiving ADA monotherapy. 58 Thirty-six RCTS evaluating biologic versus DMARD(s) or PBO in cDMARD-experienced patients reported ACR response data (Table 13). Statistically significant findings were reported (four ADA trials,76,84,117,122 one CTZ trial,79 eight ETN trials,89,101,102,104,105,111,124,140 three GOL trials,91,92,98 five IFX trials75,86,118,119,126 and three TCZ trials106,115,121) for ACR response across a range of time points favouring biologic over comparator treatment.
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Numbers analysed | % achieving ACR20 response | % achieving ACR50 response | % achieving ACR70 response | Data used in NMA? |
|---|---|---|---|---|---|---|---|
| ATTEST74 | PBO + MTX | Day 197 | 110 | 41.8 | 20 | 9.1 | Yes |
| IFX + MTX | Day 197 | 165 | 59.4a (vs. PBO) | 37a (vs. PBO) | 24.2a (vs. PBO) | ||
| ABT i.v. + MTX | Day 197 | 156 | 66.7b (vs. PBO) | 40.4b (vs. PBO) | 20.5a (vs. PBO) | ||
| AMPLE66 | ABT s.c. | 28 weeks (197 days) | 328 | 66.13 | 45.7 | 24.19 | Yes |
| ADA | 28 weeks (197 days) | 318 | 64.52 | 42.47 | 22.58 | ||
| AMPLE144 | ABT s.c. | 1 year | 328 | 64.8 | 46.2 | 29.2 | No |
| ADA | 1 year | 318 | 63.4 | 46 | 26.2 | ||
| ADACTA58 | TCZ + s.c. PBO | 24 weeks | 163 | 65.0a | 47.2a | 32.5a | Yes |
| ADA + i.v. PBO | 24 weeks | 162 | 49.4 | 27.8 | 17.9 | ||
| deFilippis et al., 200685 | ETN + MTX | 22 weeks | 15 | 60 | 26 | 7 | Yes |
| IFX + MTX | 22 weeks | 15 | 60 | 33 | 7 | ||
| ETN + MTX | 54 weeks | 15 | 74 | 53 | 7 | No | |
| IFX + MTX | 54 weeks | 15 | 60 | 19 | 20 |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Numbers analysed | % achieving ACR20 response | % achieving ACR50 response | % achieving ACR70 response | Data used in NMA? |
|---|---|---|---|---|---|---|---|
| AIM62 | PBO + MTX | 6 months | 219 | 39.7 | 16.8 | 6.5 | Yes |
| ABT i.v. + MTX | 433 | 67.9 | 39.9 | 19.8 | |||
| PBO + MTX | 12 months | 219 | 39.7 | 18.2 | 6.1 | No | |
| ABT i.v. + MTX | 433 | 73.1 | 48.3 | 28.8 | |||
| AUGUST II76 | PBO + MTX | 26 weeks | 76 | 46 | 15 | 5 | Yes |
| ADA + MTX | 79 | 71b | 38b | 18a | |||
| CHANGE80 | PBO | 24 weeks | 87 | 13.8 | 5.7 | 1.1 | Yes |
| ADA monotherapy | 91 | 44 | 24.2 | 12.1 | |||
| DE01984 | PBO + MTX | 24 weeks | 200 | 29.5 | 9.5 | 2.5 | Yes |
| ADA + MTX | 207 | 63.3 | 39.1 | 20.8 | |||
| PBO + MTX | 52 weeks | 200 | 24.0 | 9.5 | 4.5 | No | |
| ADA + MTX | 207 | 58.9b | 41.5b | 23.2b | |||
| STAR117 | PBO + cDMARDs | 24 weeks | 318 | 34.9 | 11.3 | 3.5 | Yes |
| ADA + cDMARDs | 318 | 52.8a | 28.9a | 14.8a | |||
| Van De Putte et al., 2004122 | PBO s.c. | 26 weeks | 110 | 19.1 | 8.2 | 1.8 | Yes |
| ADA monotherapy | 113 | 46.0b | 22.1a | 12.4a | |||
| ARMADA69 | PBO + MTX | 24 weeks | 62 | 14.5 | 8.1 | 4.8 | Yes |
| ADA + MTX | 67 | 67.2 | 55.2 | 26.9 | |||
| Kim et al., 200799 | PBO + MTX | 24 weeks | 63 | 36.5 | 14.3 | 7.9 | Yes |
| ADA + MTX | 65 | 61.5 | 43.1 | 21.5 | |||
| CERTAIN79 | PBO + cDMARDs | 24 weeks | 98 | 15.3 | 7.1 | 3.1 | Yes |
| CTZ + DMARDs | 96 | 36.5a | 20.8a | 9.4 | |||
| REALISTIC113 | PBO + existing cDMARDs | 12 weeks | 29 | 20.7 | NR | NR | No |
| CTZ + existing cDMARDs | 134 | 54.5 | NR | NR | |||
| ADORE59,60 | ETN monotherapy | 16 weeks | 155 | 71.0 | 41.9 | 17.4 | No |
| ETN + MTX | 152 | 67.1 | 40.1 | 18.4 | |||
| CREATE IIb96,145 | PBO + DMARD | 24 weeks | 65 | 32.3 | 16.9 | 4.6 | Yes |
| ETN50 + DMARD | 64 | 65.6 | 46.9 | 23.4 | |||
| ETN study 30989 | PBO + SSZ | 24 weeks | 50 | 28.0 | 14.0 | 2.0 | Yes |
| ETN + PBO | 103 | 73.8a (vs. SSZ) | 46.6 | 21.4 | |||
| ETN + SSZ | 101 | 74.0a (vs. SSZ, NS vs. ETN + PBO) | 52.0a (vs. SSZ, NS vs. ETN + PBO) | 25.0a (vs. SSZ, NS vs. ETN + PBO) | |||
| PBO + SSZ | 104 weeks | 50 | 34 | 10c | 2c | No | |
| ETN + PBO | 103 | 67a (vs. SSZ) | 45a (vs. SSZ),c | 24a (vs. SSZ),c | |||
| ETN + SSZ | 101 | 77a (vs. SSZ) | 58a (vs. SSZ),c | 27a (vs. SSZ),c | |||
| JESMR140 | ETN monotherapy | 24 weeks | 69 | 63.8 | 47.8 | 26.1 | Yes |
| ETN + MTX | 73 | 90.4b | 64.4 | 38.4 | |||
| ETN monotherapy | 52 weeks | 69 | 63.8 | 43.5 | 29 | No | |
| ETN + MTX | 73 | 86.3b | 76.7b | 50.7a | |||
| Lan et al., 2004101 | PBO + MTX | 12 weeks | 29 | 34 | 10 | 0 | No |
| ETN + MTX | 29 | 90b | 66b | 24 | |||
| LARA102 | MTX + DMARD | 24 weeks | 142 | 50 | 23.2 | 11.3 | Yes |
| ETN50 + MTX | 279 | 83.2b | 62b | 34.8b | |||
| Moreland et al., 1999;104 Mathias et al., 2000105 | PBO | 3 months | 80 | 23 | 8 | 4 | No |
| ETN + PBO | 78 | 62b | 41b | 15a | |||
| PBO | 6 months | 80 | 11 | 5 | 1 | Yes | |
| ETN + PBO | 78 | 59b | 40b | 15b | |||
| RACAT111 | MTX + SSZ + HCQ | 24 weeks | 159 | 55.97 | 25.79 | 5.03 | Yes |
| ETN50 + MTX | 163 | 55.21 | 35.58 | 15.95a | |||
| MTX + SSZ + HCQ In analysis, n = 154 (of whom 39 switched to ETN) |
48 weeks | 154 | 57.4 | 35.5 | 18.1 | No | |
| ETN50 + MTX (n = 175) In analysis, n = 155 (of whom 41 switched to MTX + SSZ + HCQ) |
155 | 65.8 | 42.6 | 26.5 | |||
| Wajdula 2000 (reported in Chen et al., 2006123) | PBO | 12 weeks | 100 | 12 | 5 | 1 | No |
| ETN | 109 | 70 | 34 | 13 | |||
| Weinblatt et al., 1999124 | PBO + MTX | 24 weeks | 30 | 27 | 3 | 0 | Yes |
| ETN + MTX | 59 | 71b | 39b | 15a | |||
| APPEAL67 | MTX + DMARD (SSZ, HCQ or LEF) | 16 weeks | 103 | 58 | 35 | 7 | No |
| ETN + MTX | 197 | 79b | 57b | 19a | |||
| GO-FORTH91 | PBO + MTX | 14 weeks | 88 | 27.3 | 9.1 | 2.3 | No |
| GOL + MTX | 86 | 72.1b | 43.0b | 22.1b | |||
| PBO + MTX | 24 weeks | 88 | 33.0 | 14.8 | 5.7 | Yes | |
| GOL + MTX | 86 | 70.9b | 41.9b | 26.7b | |||
| GO-FORWARD92 | PBO + MTX | 14 weeks | 133 | 33.1 | 9.8 | 3.8 | No |
| GOL + MTX | 89 | 55.1b | 34.8b | 13.5a | |||
| PBO + MTX | 24 weeks | 133 | 27.8 | 13.5 | 5.3 | Yes | |
| GOL + MTX | 89 | 59.6b | 37.1b | 20.2b | |||
| Kay et al., 200898 | PBO + MTX | 16 weeks | 35 | 37.1 | 5.7 | 0 | No |
| GOL + MTX | 35 | 60.0 | 37.1b | 8.6 | |||
| Abe et al., 200656 | PBO + MTX | 14 weeks | 47 | 23.4 | 8.5 | 0 | No |
| IFX + MTX | 49 | 61.2 | 30.6 | 10.2 | |||
| ATTRACT75 | PBO + MTX | 30 weeks | 84 | 20 | 5 | 0 | Yes |
| IFX + MTX | 83 | 50 | 27b | 8a | |||
| Lipsky et al., 2000146 | PBO + MTX | 54 week | 88 | 17 | 8 | 2 | No |
| IFX + MTX | 86 | 42b | 21a | 10a | |||
| Durez et al., 200486 | MP i.v. + MTX | 14 weeks | 12 | 8 | 0 | 0 | No |
| IFX + MTX | 9 | 67a | 44a | 0 | |||
| Swefot119 | SSZ + HCQ + MTX | 12 months after study inclusion [8–9 months (35–39 weeks) after randomisation] | 130 | 28 | 15 | 7 | No |
| IFX + MTX | 128 | 42a | 25a | 12 | |||
| Swefot147 | SSZ + HCQ + MTX | 24 months after study inclusion [20–21 months (87–91 weeks) after randomisation] | 130 | 33 | 22 | 14 | No |
| IFX + MTX | 128 | 40 | 30 | 16 | |||
| START118 | PBO + MTX | 22 weeks | 363 | 25.5 | 9.7 | 4.7 | Yes |
| IFX + MTX | 360 | 58.0b | 32.1b | 14.0b | |||
| Zhang et al., 2006126 | PBO + MTX | 18 weeks | NR (86 randomised) | 48.84 | 25.58 | 13.95 | No |
| IFX + MTX | NR (87 randomised) | 75.86b | 43.68a | 22.99 | |||
| ACT-RAY57 | TCZ + oral PBO | 24 weeks | 276 | 70.3 | 40.2 | 25.4 | Yes |
| TCZ + MTX | 277 | 71.5 | 45.5 | 24.5 | |||
| MEASURE103 | PBO + MTX | 12 weeks | NR | 25 | 6 | 3 | No |
| TCZ + MTX | NR | 51 | 17 | 10 | |||
| Nishimoto et al., 2004106 | PBO | 12 weeks | 53 | 11.3 | 1.9 | 0 | No |
| TCZ | 55 | 78.2b | 40.0b | 16.4a | |||
| SAMURAI115 | cDMARDs | 24 weeks | 145 | 38.67 | 17.64 | 6.86 | Yes |
| TCZ | 157 | 82.06 | 57.27 | 33.82 | |||
| cDMARDs | 52 weeks | 145 | 34 | 13 | 6 | No | |
| TCZ | 157 | 78b | 64b | 44b | |||
| SATORI116 | PBO + MTX | 24 weeks | 64 | 25.0 | 10.9 | 6.3 | Yes |
| TCZ + PBO capsules | 61 | 80.0 | 49.2 | 29.5 | |||
| TOWARD121 | PBO + stable cDMARDs | 24 weeks | 413 | 24.5 | 9 | 2.9 | Yes |
| TCZ + stable DMARDs | 803 | 60.8b | 37.6b | 20.5b |
European League Against Rheumatism response
The only head-to-head trial for MTX-naive patients100 did not report EULAR data. Three MTX-naive trials reported EULAR data, of which two were ADA trials93,107 and one was a GOL trial90 (Table 14). GUÉrir la PolyARthrite rheumotoide Débutante (adalimumab) (GUEPARD)93 reported a significantly better EULAR response for ADA plus MTX than for MTX alone at 12 weeks’ follow-up, but at 1-year follow-up, when both groups had undergone step-up therapy, the groups were responding similarly well. OPtimized treatment algorithm for patients with Early Rheumatoid Arthritis (OPERA)107 reported similar EULAR responses for ADA plus MTX plus steroid and for MTX plus PBO plus steroid at the 1-year follow-up. GOlimumab in active rheumatoid arthritis BEFORE methotrexate therapy (GO-BEFORE),90 at 24 weeks, reported a significantly better EULAR response for GOL plus MTX and for PBO plus MTX, but at the 1-year follow-up the groups were doing similarly well. GO-BEFORE90 contributed EULAR data to the NMA, whereas the others did not report data within 22–30 weeks’ follow-up.
| Trial name/author, year | Treatment arms for which data extraction performed | Assessment time point | Numbers analysed | % achieving no EULAR response | % achieving moderate EULAR response | % achieving good EULAR response | % EULAR responder (moderate/good) | In NMA? |
|---|---|---|---|---|---|---|---|---|
| GUEPARD93 | MTX | Week 12 | 32 | NR | NR | 25 | NR | No |
| ADA + MTX | Week 12 | 33 | NR | NR | 63.6a | NR | No | |
| Initial MTX 12 weeks, then step-up therapy |
Week 52 | 32 | NR | NR | 65.6 | NR | No | |
| Initial ADA + MTX 12 weeks, then step-up therapy |
Week 52 | 33 | NR | NR | 63.6 | NR | No | |
| OPERA107 | MTX + PBO + steroid | 12 months | 91 | 7 | 20 | 74 | 94 | No |
| ADA + MTX + steroid | 12 months | 89 | 7 | 11 | 82 | 93 | No | |
| GO-BEFORE90 | PBO + MTX | 24 weeks | 160 | 38.7 | NR | NR | 61.3 | Yes |
| GOL + MTX | 24 weeks | 159 | 27 | NR | NR | 73a | Yes | |
| GO-BEFORE143 | PBO + MTX | 52 weeks | 160 | 25.6 | NR | NR | 74.4 | No |
| GOL + MTX | 52 weeks | 159 | 19.5 | NR | NR | 80.5 | No |
There were three trials of head-to-head biologics for cDMARD-experienced patients who reported EULAR response data (Table 15). ATTEST74 showed that patients treated with ABT plus MTX or IFX plus MTX responded similarly at 6 months’ follow-up. A Randomised Efficacy and Discontinuation Study of Etanercept versus Adalimumab (RED-SEA)114 reported ADA plus cDMARDs and ETN (50 mg) once a week plus cDMARDs-treated patients responding similarly well at 1-year follow-up. ADACTA58 reported that significantly more TCZ plus PBO-treated patients achieved a good EULAR response than ADA plus PBO-treated patients at 6 months’ follow-up. ADACTA58 and ATTEST74 contributed EULAR data to the NMA, whereas RED-SEA114 did not report data within 22–30 weeks’ follow-up.
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Numbers analysed | % achieving no EULAR response | % achieving moderate EULAR response | % achieving good EULAR response | % EULAR responder (moderate/good) | In NMA? |
|---|---|---|---|---|---|---|---|---|
| ATTEST74 | PBO + MTX | Day 197 | 102 | 45.1 | 44.1 | 10.8 | 54.9 | Yes |
| ABT + MTX | Day 197 | 150 | 23.3 | 56.7 | 20.0 | 76.7 | Yes | |
| IFX + MTX | Day 197 | 156 | 34.0 | 42.9 | 23.1 | 66.0 | Yes | |
| RED-SEA114 | ADA + cDMARDs | 52 weeks | 60 | 40.4 | 33.3 | 26.3 | 59.6 | No |
| ETN50 + cDMARDs | 52 weeks | 60 | 51.5 | 16.7 | 31.7 | 48.4 | No | |
| ADACTA58 | TCZ + PBO | 24 weeks | 163 | 22.1 | 26.4 | 51.5a | 77.9 | Yes |
| ADA + PBO | 24 weeks | 162 | 45.1 | 35.1 | 19.8 | 54.9 | Yes |
Eleven other published trials reported EULAR data for biologics (Table 16). With the exception of CTZ, data were available for all interventions of interest. Two ADA trials reported EULAR data. A Phase II Dose-finding Study of Atacicept in Rheumatoid Arthritis (RA) (AUGUST II)76 reported a significantly better EULAR result for ADA plus MTX than for MTX plus PBO at 6 months. ADA monotherapy had a significantly higher percentage of patients achieving at least moderate EULAR response than a PBO arm. 122 Of four ETN trials, two compared ETN monotherapy with ETN combined with MTX. One of these studies59 found similar EULAR responses for the groups at 16 weeks, whereas the other140 reported significantly better results for combination therapy than for monotherapy at 6 months and 1 year. Latin American Rheumatoid Arthritis study (LARA)102 reported significantly better EULAR response for ETN (50 mg) once a week plus MTX than for MTX in combination with either SSZ or HCQ at 6 months. ETN plus MTX had a similar percentage of participants with good or moderate EULAR response to MTX plus DMARD (SSZ, HCQ or LEF) in the Asia-Pacific Study in Patients to be Treated With Etanercept or an Alternative Listed (APPEAL)67 trial at 16 weeks’ follow-up. GOL plus MTX was significantly better than MTX plus PBO in terms of EULAR response at both 14 and 24 weeks’ follow-up in the golimumab in active rheumatoid arthritis despite methotrexate therapy (GO-FORWARD)92 trial. Swedish pharmacotherapy (Swefot)119 reported IFX plus MTX having significantly better EULAR response than triple therapy with cDMARDs (SSZ + HCQ + MTX) at 1 year, with the difference between groups not significant at 6 months and 2 years. TCZ monotherapy was investigated in two of the three TCZ trials reporting EULAR data. TCZ monotherapy results were similar to TCZ in combination with MTX, in the ACTemra (tocilizumab) RAdiographic studY (ACT-RAY)57 trial at 6 months. TCZ monotherapy treatment had significantly better EULAR responses at 12 weeks compared with PBO. 106 The TOcilizumab in combination With traditional DMARD therapy (TOWARD)121 trial reported significantly better EULAR responses for TCZ in combination with stable cDMARDs than for PBO in combination with stable cDMARDs at 6 months. The following trials contributed EULAR data to the NMA: AUGUST II,76 Van De Putte et al. ,122 Japanese Efficacy and Safety of Etanercept on Active Rheumatoid Arthritis Despite Methotrexate Therapy (JESMR),140 LARA,102 GO-FORWARD,92 Swefot,119 ACT-RAY57 and TOWARD. 121 ADjuvant Oxaliplatin in REctal Cancer (ADORE)59 and APPEAL67 did not have data within 22–30 weeks.
(Academic-in-confidence information has been removed.)
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Numbers analysed | % achieving no EULAR response | % achieving moderate EULAR response | % achieving good EULAR response | % EULAR responder (moderate/good) | In NMA? |
|---|---|---|---|---|---|---|---|---|
| AUGUST II76 | MTX + PBO | 26 weeks | 76 | 41 | NR | NR | 59 | Yes |
| ADA + MTX | 79 | 19 | NR | NR | 81a | Yes | ||
| Van De Putte et al., 2004122 | PBO | 26 weeks | 110 | 73.6 | 22.8 | 3.6 | 26.4 | Yes |
| ADA | 113 | 44.2 | 47.0 | 8.8 | 55.8 | Yes | ||
| ADORE59,60 | ETN | 16 weeks | 156 | 20.0 | NR | NR | 80.0 | No |
| ETN + MTX | 151 | 17.6 | NR | NR | 82.4 | No | ||
| JESMR140 | ETN | 24 weeks | 69 | 29.0 | 37.7 | 33.3 | 71.0 | Yes |
| ETN + MTX 6–8 mg/week | 73 | 4.1b | 43.8c | 52.1c | 95.9 | Yes | ||
| ETN | 52 weeks | 69 | NR | NR | 33.3 | NR | No | |
| ETN + MTX 6–8 mg/week | 73 | NR | NR | 52.1c | NR | No | ||
| LARA102 | MTX + DMARD | 24 weeks | 142 | 35.2 | NR | 12 | 64.8 | Yes |
| ETN50 + MTX | 279 | 8.2 | NR | 47c | 91.8c | Yes | ||
| APPEAL67,68 | MTX + DMARD (SSZ, HCQ or LEF) | 16 weeks | 103 | 26.2 | NR | NR | 73.8 | No |
| ETN + MTX | 197 | 12.2 | NR | NR | 87.8 | No | ||
| GO-FORTH91 | PBO + MTX | 6 months | 84 | 51.2 | 35.7 | 13.1 | 48.8 | Yes |
| GOL + MTX | 81 | 16.0 | 37.0 | 46.9 | 84.0 | Yes | ||
| GO-FORWARD92 | PBO + MTX | 14 weeks | 133 | 55.6 | NR | NR | 44.4 | No |
| GOL + MTX | 89 | 29.2 | NR | NR | 70.8c | No | ||
| PBO + MTX | 24 weeks | 133 | 57.9 | NR | NR | 42.1 | Yes | |
| GOL + MTX | 89 | 28.1 | NR | NR | 71.9c | Yes | ||
| START118 | PBO + MTX | 5.5 months | 332 | 56 | NR | NR | 44 | Yes |
| IFX + MTX | 333 | 25 | NR | NR | 75 | Yes | ||
| Swefot119 | SSZ + HCQ + MTX | 23.8 weeks | 130 | NR | NR | 23.8 | NR | Yes |
| IFX + MTX | 128 | NR | NR | 33.6 | NR | Yes | ||
| SSZ + HCQ + MTX | 12 months after study inclusion [8–9 months (35–39 weeks) after randomisation] | 130 | 51 | NR | 25 | 49 | No | |
| IFX + MTX | 128 | 40 | NR | 39a | 60 | No | ||
| SSZ + HCQ + MTX | 24 months after study inclusion [20–21 months (87–91 weeks) after randomisation] | 130 | 50 | NR | 31 | 50 | No | |
| IFX + MTX | 128 | 41 | NR | 38 | 59 | No | ||
| ACT-RAY57 | TCZ + PBO | 24 weeks | 276 | 13.8 | 34.8 | 51.4 | 86.2 | Yes |
| TCZ + MTX | 277 | 10.5 | 27.8 | 61.7 | 89.5 | Yes | ||
| Nishimoto et al., 2004106 | PBO | 12 weeks | 53 | 81.1 | NR | 0 | 18.9 | No |
| TCZ | 55 | 9.1 | NR | 18.2c | 90.9c | No | ||
| SATORI116 | MTX | 6 months | 64 | 60.3 | 36.5 | 3.2 | 39.7 | Yes |
| TCZ | 61 | 3.4 | 31.1 | 65.5 | 96.6 | Yes | ||
| TOWARD121 | PBO + stable cDMARDs | 24 weeks | 413 | 62.5 | NR | NR | 37.5 | Yes |
| TCZ + stable DMARDs | 803 | 20.3 | NR | NR | 79.7c | Yes | ||
| TACIT141 (AiC) | Intensive DMARDs | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | Yes |
| Grouped biologics | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | Yes |
Disease Activity Score 28 joints
One head-to-head biologics trial in MTX-naive patients reported DAS28 data100 (see Appendix 4, Table 346). At 24 weeks’ follow-up, Kume et al. 100 reported similar mean change from baseline in DAS28-ESR for ADA monotherapy and ETN monotherapy.
Thirteen other trials reported DAS28 mean change or remission data for MTX-naive patient trials, comprising five ADA trials,93,94,107–109 one ETN trial,81 one GOL trial90 and five IFX trials. 71,78,86,95,110 Across all interventions, where reported, mean DAS28 improved slightly in all treatment arms, including control cDMARD arms. Biologic treatment arms reported significantly higher percentage of patients meeting pre-defined DAS28 remission (usually < 2.6), or having significantly more improved DAS28 than baseline, than controls for ADA plus MTX than MTX plus PBO;94,109 ADA plus MTX plus steroid than MTX plus PBO than steroid;107 ETN plus MTX than MTX plus PBO;81 GOL plus MTX than MTX plus PBO at 6 months (not 1-year follow-up);90 IFX plus MTX than MTX plus PBO. 71,110 ADA monotherapy had similar DAS28 results to MTX plus PBO,109 as did IFX plus MTX to MTX plus methylprednisolone (MP). 86,95 Step-up therapy with initial ADA93 or IFX78 did not differ from control groups after 1 year or 6 months respectively. Results are shown in Appendix 4, Table 347.
Four head-to-head trials of cDMARD-experienced patients reported DAS28 results58,66,74,114 (see Appendix 4, Table 348). ABT, ADA, ETN (50 mg) once weekly, IFX and TCZ treatment arms all showed some improvement in DAS28. There were similar levels of DAS28 improvement for ABT plus MTX and IFX plus MTX (both of which were significantly more improved than MTX plus PBO),74 ABT and ADA monotherapies,66 and ADA and ETN (50 mg) once weekly both in combination with cDMARDs. 114 ADACTA58 reported significantly more improvement for TCZ monotherapy than for ADA monotherapy.
Twenty other trials reported DAS28 mean change or remission data for cDMARD-experienced patient trials (see Appendix 4, Table 349), comprising two ABT trials,62,72 one ADA trial,122 two CTZ trials,79,113 five ETN trials,67,96,102,112,140 three GOL trials,91,92,98 two IFX trials118,125 and five TCZ trials. 57,103,115,116,121 Across all interventions, where reported, mean DAS28 improved in all treatment arms, including control cDMARD arms. Biologic treatment arms reported higher percentages of patients meeting pre-defined DAS28 remission (usually < 2.6) than non-biologic control arms with one or two cDMARDs or baseline cDMARDs. There was a significantly higher percentage of patients meeting pre-defined DAS28 remission (usually < 2.6), or having significantly more improved DAS28 than baseline, than controls for ABT plus MTX than MTX plus PBO;62 ADA monotherapy than PBO;122 ETN (50 mg) once weekly plus MTX than MTX plus one other cDMARD;67,102 ETN (50 mg) once weekly plus MTX than MTX plus SSZ plus HCQ at 24 weeks (in an analysis of treatment completers only, although not after 48 weeks with the option to switch therapy);112 GOL plus MTX than MTX plus PBO at 6 months (not 1-year follow-up);91,92,98 IFX plus MTX than MTX plus PBO;118,125 TCZ plus MTX than TCZ monotherapy57 or than MTX plus PBO;103 TCZ monotherapy than cDMARDs,115 although not compared with MTX plus PBO; and116 TCZ plus DMARDs than DMARDs plus PBO. 121 ETN plus MTX performed significantly better than ETN monotherapy,140 although not at 16 weeks’ follow-up. 59
(Academic-in-confidence information has been removed.)
Health Assessment Questionnaire Disability Index
Ten trials reported HAQ-DI change from baseline (see Appendix 4, Table 350). These comprised a head-to-head trial,100 six ADA trials,77,93,94,107–109 two ETN trials81,87 and one GOL trial. 90 There were improvements in HAQ-DI for most treatments, interventions and controls, although there tended to be more improvement for biologics than control arms, although not in all cases. 87
Four head-to-head trials58,66,74,85 reported HAQ-DI change from baseline (see Appendix 4, Table 351). All trial arms improved HAQ-DI. ABT-treated patients achieved similar results to IFX74 and ADA. 66 TCZ monotherapy produced slightly more improvement than ADA monotherapy (significance testing not reported). 58 In a small trial (n = 32), ETN plus MTX produced slightly better HAQ-DI results than IFX plus MTX. 85
Twenty-eight other trials reported HAQ-DI change from baseline for cDMARD-experienced patients (see Appendix 4, Table 352), comprising two ABT trials,62,73 four ADA trials,69,80,84,122 two CTZ trials,79,113 11 ETN trials,59,67,88,96,101,102,104,112,123,124,140,145 two GOL trials,91,92 four IFX trials75,86,118,126 and two TCZ trials. 57,121 Generally, there was some improvement in HAQ-DI for all trial arms, with more improvement for biologics than control arms. (AiC information has been removed.)
Joint counts and assessment of inflammation markers (C-reactive protein and erythrocyte sedimentation rate)
The only head-to-head RCT in MTX-naive patients identified in this review100 did not report any follow-up or change data on joint counts or assessment of inflammation markers. A total of seven RCTs of biologic versus DMARD(s) or PBO reported follow-up or change data on joint counts or assessment of inflammation markers in MTX-naive patients (three for ADA,94,107,108 one for ETN,81 one for GOL90 and two for IFX110,120) (see Appendix 4, Table 364). Statistically significant differences in swollen joint count favouring biologic treatment over comparator were reported for ADA (one study94) and ETN (one study81). Statistically significant differences in tender joint count favouring biologic treatment over comparator were reported for ADA (two studies94,108) and GOL (one study90). Statistically significant differences in CRP response favouring biologic treatment over comparator were reported for ADA (one study108). Statistically significant differences in ESR response were not identified in any trials.
Four head-to-head RCTs reporting data on joint counts and/or assessment of inflammation markers in cDMARD-experienced patients were identified (see Appendix 4, Table 366). Similar improvements were made in swollen joint count, tender joint count and CRP level among patients in the s.c. ABT plus MTX and ADA plus MTX arms of the abatacept vs. adalimumab in biologic-naive RA patients with background MTX (AMPLE) trial. 144 Likewise, swollen joint count, tender joint count and CRP level were not significantly different between patients in the ADA plus cDMARDs and ETN plus cDMARDs arms of the RED-SEA trial. 114 The deFilippis trial85 reported no difference in percentage change between arms for swollen joint count and CRP level but reported significantly greater improvements in tender joint count in the ETN plus MTX arm relative to the IFX versus MTX arm. Finally, similar reductions in swollen joint count and tender joint count were reported for patients in the TCZ plus PBO ADA and ADA plus PBO TCZ arms in the double-dummy ADACTA trial. 58
Twenty RCTs of biologic versus DMARD(s) or PBO reported follow-up or change data on joint counts or assessment of inflammation markers in cDMARD-experienced patients (see Appendix 4, Table 365). Statistically significant differences in swollen joint count favouring biologic treatment over comparator were reported in eight trials [one ADA trial,80 four ETN trials,89,101,104,105,124 one GOL trial,92 one TCZ trial121 and (AiC information has been removed)]. Statistically significant differences in tender joint count favouring biologic treatment over comparator were reported in 11 trials [one ADA trial,80 four ETN trials,101,102,104,105,124 one GOL trial,92 three IFX trials,75,86,125 one TCZ trial121 and (AiC information has been removed)]. Statistically significant differences in CRP response favouring biologic treatment over comparator were reported in six trials (one ADA trial,80 four ETN trials89,101,104,105,124 and one TCZ trial121). Statistically significant differences in ESR response favouring biologic treatment over comparator were reported in seven trials [five ETN trials,59,60,67,68,104,105,124 one TCZ trial121 and (AiC information has been removed)].
Two trials compared biologic monotherapy with biologic combination therapy. A trial of ETN reported significant improvements in swollen joint count, tender joint count and ERS for ETN combined with MTX but not ETN monotherapy and the reverse for CRP,140 whereas a trial of TCZ plus oral PBO versus TCZ combined with MTX found no differences in joint counts or inflammation markers. 57
One trial of biologic and cDMARD combination therapy (ETN + MTX) versus biologic monotherapy140 reported significantly greater improvements in swollen joint count tender joint count and ESR in the combination therapy arm, but significantly greater improvements in CRP in the monotherapy arm. 97 Another trial of biologic and cDMARD combination therapy versus monotherapy (TCZ + MTX vs. TCZ + PBO)57 reported similar changes from baseline in swollen joint count and tender joint count.
Patient and physician global assessments of disease activity
No data were available for this outcome from the single identified head-to-head RCT in MTX-naive patients. 100 Four population 1 trials in MTX-naive patients contributed global assessment evidence (presented in Appendix 4, Table 356), of which two were for ADA,107,108 one for GOL90 and one for IFX. 148 Of these four trials, statistically significant improvements in global assessments of disease activity were reported for one trial favouring GOL plus MTX over PBO and MTX,90 and for one trial148 that favoured initial combination cDMARD therapy plus prednisone and initial combination cDMARD therapy plus IFX over sequential cDMARD monotherapy and step-up combination cDMARD therapy.
Patient and physical global assessment of disease activity data were reported in three head-to-head RCTs of cDMARD-experienced patients66,85,114 (see Appendix 4, Table 357). No statistically significant differences in treatment response were reported.
A total of 23 further RCTs evaluated global assessments of disease activity in four ADA trials,69,70,80,99,122 four ETN trials,89,102,104,105,124 one GOL trial92 and three IFX trials75,86,125 (see Appendix 4, Table 358).
Radiological progression/joint damage
Data were extracted from RCTs where absolute baseline and follow-up, mean change from baseline or proportion change from baseline in joint outcomes were available.
No joint damage/radiological progression data were identified from the single identified head-to-head population 1 trial. 100 Six trials of biologic interventions versus DMARD(s) or PBO in MTX-naive patients reported change in radiographic scores and/or radiographic non-progression (three ADA trials,93,108,109 two ETN trials81,139 and one IFX trial71). Joint outcomes were assessed using a range of radiographic scores,149 and magnetic resonance imaging. Data for radiographic scores are presented in Table 359 (see Appendix 4). Statistically significant results favouring intervention in the reduction of radiological progression were reported for two ADA trials,108,109 one ETN trial139 and one IFX trial. 71 Two trials (one each for ADA108,150 and GOL151) provided joint assessment data as measured by magnetic resonance imaging (both of which reported statistically significant findings favouring biologic treatment; see Appendix 4, Table 360).
One head-to-head trial66 (ADA vs. ABT) (see Appendix 4, Table 361) and eight trials of biologic interventions versus DMARD(s) or PBO in cDMARD-experienced patients reported change in radiographic scores and/or rates of radiographic non-progression (one for ATB,61,62 one for ADA,84 two for ETN,102,111 one for GOL,91 two for IFX146,147 and two for TCZ115,152) (see Appendix 4, Table 362). Statistically significant results indicating reduced radiological progression were reported for one ABT trial,61,62 one ADA trial,84 one ETN trial,102 one GOL trial,91 both IFX trials146,147 and one TCZ trial. 115 Joint outcome data as assessed by magnetic resonance imaging were presented in three trials (one each for ABT,72 GOL153 and IFX120) (see Appendix 4, Table 363), with statistically significant benefits to joint outcomes reported for the GOL trial. 153
Two trials compared biologic monotherapy with biologic combination therapy. A trial of ETN reported significant improvements in erosion score for ETN combined with MTX but not ETN monotherapy,140 whereas a trial of TCZ plus oral PBO versus TCZ combined with MTX found no differences in radiographic progression. 57
Pain
Six trials reported pain visual analogue scale (VAS) score change from baseline (see Appendix 4, Table 364). These comprised three ADA trials,107–109 one ETN trial,81 one GOL trial90 and one IFX trial. 78 There were reductions in pain VAS for most treatments and there were significant benefits for all four biologics compared with controls.
Two head-to-head trials66,85 reported pain VAS change from baseline (see Appendix 4, Table 365). All trial arms reduced pain VAS score. No significant differences were reported between groups.
Twenty-seven other trials reported pain VAS change from baseline for cDMARD-experienced patients (see Appendix 4, Table 366), comprising two ABT trials,62,73 five ADA trials,69,80,84,99,122 one CTZ trial,79 nine ETN trials,59,67,88,101,102,104,112,124,140 one GOL trial,92 two IFX trials75,118 and one TCZ trial. 57 Generally, there was some reduction in pain VAS for all trial arms. ABT had similar reductions compared with control groups. 62,73 There was at least one trial reporting significantly more pain VAS reduction than control for each of ADA, CTZ, ETN, GOL and IFX. In the Rheumatoid Arthritis Comparison of Active Therapies in Methotrexate Suboptimal Responders study (RACAT)112 ETN (50 mg) once weekly plus MTX had similar results to MTX plus SSZ plus HCQ. In the ACT-RAY trial57 TCZ monotherapy had similar results to TCZ plus MTX.
Fatigue
The only head-to-head RCT in MTX-naive patients identified in this review100 did not report any follow-up or change data on fatigue. A total of three RCTs of biologic versus DMARD(s) or PBO reported follow-up or change data on fatigue in MTX-naive patients (two for ADA154,155 and one for ETN83) (see Appendix 4, Tables 377 and 378). Statistically significant differences favouring biologic treatment over comparator were reported for VAS score (one ETN trial83) and Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) score (one ADA trial154). One further ADA trial reported significant differences between ADA and MTX arms at follow-up in a mixed-model repeated measures analysis, but the values appear to be similar. 155
Two head-to-head RCTs reporting data on fatigue in cDMARD-experienced patients were identified (see Appendix 4, Tables 369 and 370). 58,144 Similar improvements were made on fatigue VAS score among patients in the s.c. ABT plus MTX and ADA plus MTX arms of the AMPLE trial144 and on FACIT-F score among patients in the TCZ plus PBO ADA and ADA plus PBO TCZ arms in the ADACTA trial. 58
Six RCTs of biologic versus DMARD(s) or PBO reported follow-up or change data on fatigue data in cDMARD-experienced patients (see Appendix 4, Tables 371 and 372). 63,68–70,79,92,121 A statistically significant difference in VAS fatigue score swollen joint count favouring biologic treatment over comparator was reported in one ABT trial. 63 Statistically significant differences in FACIT-F score favouring biologic treatment over comparator were reported in four trials (one ADA trial,69,70 one ETN trial,68 one GOL trial92 and one TCZ trial121). Mean (SD) change from baseline in the Fatigue Assessment Scale has been reported for the efficacy and safety of CERTolizumab pegol After INcomplete response to DMARDS in RA patients with low to moderate disease activity (CERTAIN) trial79 of 0.1 (SD 2.12) in the PBO arm and –1.2 (SD 2.24) in the CTZ arm at week 24 (ClinicalTrials.gov, NCT00674362) and (AiC information has been removed). 156
Health-related quality of life
The only head-to-head RCT in MTX-naive patients identified in this review100 did not report any follow-up or change data on health-related quality of life. A total of nine RCTs of biologic versus DMARD(s) or PBO reported follow-up or change data on health-related quality of life in MTX-naive patients (four for ADA,77,94,107,155 two for ETN83,157 and three for IFX71,72,110,158) (see Appendix 4, Tables 383–388). Statistically significant differences in Short Form questionnaire-36 items (SF-36) components and domains favouring biologic treatment over comparator were reported for ADA (one study154), ETN (two studies83,157) and IFX (one study78). One further ADA trial reported significant differences between ADA and MTX arms at follow-up in a mixed-model repeated measures analysis, but the values appear to be similar. 155 One study reported a statistically significant difference on the Short Form questionnaire-12 items physical component score for ADA. 107 Statistically significant differences in Rheumatoid Arthritis Quality of Life (RAQoL) score favouring biologic treatment over comparator were reported for ADA (one study77) and IFX (one study110). One further ADA trial reported significant differences on Short Form questionnaire-6 Dimensions (SF-6D) score between ADA and MTX arms at follow-up in a mixed-model repeated measures analysis, but the values appear similar. 155 One study reported a statistically significant difference on European Quality of Life-5 Dimensions (EQ-5D) score for ADA. 107
Three head-to-head RCTs reporting data on health-related quality of life in cDMARD-experienced patients were identified (see Appendix 4, Tables 389–391). Similar improvements were made on SF-36 components and domains scores among patients in the s.c. ABT plus MTX and ADA plus MTX arms of the AMPLE trial144 and among patients in the ABT plus MTX, IFX plus MTX and MTX plus PBO arms of the ATTEST trial. 74 Significantly greater improvements were reported on SF-36 mental component score among patients in the TCZ ( + PBO ADA) arm than in the ADA ( + PBO TCZ) arm in the ADACTA trial. 58 Similar improvements were made on EQ-5D score among patients in the ADA and ETN arms of the RED-SEA trial. 114
Nine RCTs of biologic versus DMARD(s) or PBO reported follow-up or change data on health-related quality of life data in cDMARD-experienced patients (see Appendix 4, Tables 392–397). Statistically significant differences in SF-36 components and domains scores favouring biologic treatment over comparator were reported in six trials (one ABT trial,61,62 one ETN trial,68 one GOL trial,92 two IFX trials86,159 and one TCZ trial121). (AiC information has been removed.) Statistically significant differences in EQ-5D domain scores favouring biologic treatment over comparator were reported in one ETN trial59 and a further ETN trial reported a statistically significant improvement in EuroQol VAS score. 89
Extra-articular manifestations of disease
No included RCTs specifically evaluated the impact of biologic interventions on extra-articular manifestations of RA.
Adverse effects of treatment
Data were extracted relating to discontinuations due to AEs, number of patients experiencing one or more AEs and number of patients experiencing one or more serious AE. Details are presented in Appendix 4, Tables 398–400. Specific AEs of important note as highlighted in the FDA prescribing information for each intervention were extracted from RCTs and associated LTEs of individual included RCTs and tabulated (see Appendix 4, Tables 401–403). These key safety issues identified across the range of interventions included the number of patients experiencing one or more infections, number of patients experiencing one or more serious infections (with pneumonia and reactivation of tuberculosis noted as important safety issues), number of patients experiencing one or more malignancy, and the occurrences of infusion-related or injection-site reactions (as appropriate to the mode of administration for each intervention).
Mortality
Details of number of deaths, cause(s) of death and judgement by study team/adjudicator of whether or not death was potentially attributable to study drug were extracted and have been tabulated (see Appendix 4, Tables 403 and 404).
Additional evidence (trial data not eligible for full systematic review but included to inform network meta-analysis sensitivity analyses for populations 2 and 3)
Study and population characteristics for the trials ineligible for the full systematic review but provided as additional evidence to inform sensitivity analyses are presented in Table 344 (see Appendix 4). Two RCTs137,138 in which TOF was evaluated were included as evidence to supplement the network.
The ACR and EULAR responses in populations 2 and 3 RCTs used in the sensitivity analyses are presented in Tables 17 and 18 respectively.
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Numbers analysed | % achieving ACR20 response | % achieving ACR50 response | % achieving ACR70 response | Data used in NMA? |
|---|---|---|---|---|---|---|---|
| ACQUIRE127 | ABT s.c. + PBO + MTX | 26 weeks | 736 | 74.8 | 50.2 | 25.8 | Yes (SAs) |
| ABT i.v. + PBO + MTX | 26 weeks | 721 | 74.3 | 48.6 | 24.2 | ||
| NCT00254293131 | PBO + MTX | 25.7 weeks | 119 | 35.3 | 11.8 | 1.7 | Yes (SAs) |
| ABT i.v. + MTX | 25.7 weeks | 115 | 60a | 36.5a | 16.5a | ||
| ORAL STANDARD133 | PBO + MTX | 26 weeks | 106 | 28.3 | 12 | 2 | Yes (SAs) |
| TOF5 + MTX | 26 weeks | 196 | 51.5 | 36 | 20 | ||
| TOF10 + MTX | 26 weeks | 196 | 52.6 | 33 | 22.5 | ||
| ADA + MTX | 26 weeks | 199 | 47.2 | 27 | 9.5 | ||
| Yamamoto et al., 2011129 | PBO + MTX | 24 weeks | 77 | 24.7 | 16.9 | 1.3 | Yes (SAs) |
| CTZ + MTX | 24 weeks | 82 | 73.2b | 54.9b | 29.3b | ||
| RA0025134 | PBO + MTX | 24 weeks | 40 | 27.5 | 20 | 2.5 | Yes (SAs) |
| CTZ + MTX | 24 weeks | 81 | 66.7b | 43.2a | 17.3a | ||
| RAPID1135 | PBO + MTX | 24 weeks | 199 | 13.6 | 7.6 | 3 | Yes (SAs) |
| CTZ + MTX | 24 weeks | 393 | 58.8b | 37.1b | 21.4b | ||
| RAPID2136 | PBO + MTX | 24 weeks | 127 | 8.7 | 3.1 | 0.8 | Yes (SAs) |
| CTZ + MTX | 24 weeks | 246 | 57.3b | 32.5b | 15.9a (comparison of ORs from logistic regressions) | ||
| TEAR53 | MTX monotherapy | 24 weeks | 379 | 39.39 | 19 | 3.43 | Yes (SAs) |
| MTX + SSZ + HCQ | 24 weeks | 132 | 55.32 | 31.14 | 8.52 | ||
| ETN50 + MTX | 24 weeks | 244 | 55.7 | 32.3 | 12.04 | ||
| TEMPO54 | MTX monotherapy | 24 weeks | 228 | 74.18 | 41.31 | 15.9 | Yes (SAs) |
| ETN monotherapy | 24 weeks | 223 | 71.58 | 41.31 | 17.98 | ||
| ETN + MTX | 24 weeks | 231 | 82.53 | 60.09 | 36.65 | ||
| LITHE152 | PBO + MTX | 24 weeks | 393 | 27 | 10 | 2 | Yes (SAs) |
| TCZ + MTX | 24 weeks | 398 | 56b | 32b | 13b | ||
| OPTION132 | PBO + MTX | 24 weeks | 204 | 26 | 11 | 2 | Yes (SAs) |
| TCZ + MTX | 24 weeks | 205 | 59b | 44b | 22b | ||
| AMBITION128 | MTX (MTX-experienced subgroup) | 24 weeks | 88 | 47.7 | 30.7 | 15.9 | Yes (SAs) |
| TCZ (MTX-experienced subgroup) | 24 weeks | 89 | 71.9a | 40.4 | 28.1 | ||
| van der Heijde et al., 2013138 | PBO + MTX | 26 weeks | 160 | 25.3 | 8.4 | 1.3 | Yes (SAs) |
| TOF5 + MTX | 26 weeks | 321 | 51.5b (added vs. PBO + MTX) | 32.4b (added vs. PBO + MTX) | 14.6b (added vs. PBO + MTX) | ||
| TOF10 + MTX | 26 weeks | 316 | 61.8b (added vs. PBO + MTX) | 43.7b (added vs. PBO + MTX) | 22.3b (added vs. PBO + MTX) | ||
| Kremer et al., 2012137 | PBO + MTX | 24 weeks | 69 | 24.62 | 23.08 | 19.87 | Yes (SAs) |
| TOF5 + MTX | 24 weeks | 71 | 47.44 | 33.33 | 19.23a (added vs. PBO + MTX) | ||
| TOF10 + MTX | 24 weeks | 74 | 54.49a (added vs. PBO + MTX) | 34.62 | 16.67a (added vs. PBO + MTX) |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Numbers analysed | % achieving no EULAR response | % achieving moderate EULAR response | % achieving good EULAR response | % EULAR responder (moderate/good) | In NMA? |
|---|---|---|---|---|---|---|---|---|
| JRAPID129 | PBO + MTX | 24 weeks | 77 | 70.1 | NR | NR | 29.9 | Yes (SAs) |
| Yamamoto et al., 2011129 | CTZ + MTX | 24 weeks | 82 | 14.6 | NR | NR | 85.4 | Yes (SAs) |
| RAPID1135 | PBO + MTX | 24 weeks | 199 | 72.9 | NR | NR | (AiC information has been removed) | Yes (SAs) |
| RAPID1135 (AiC information has been removed) |
CTZ + MTX | 24 weeks | 393 | 19.1 | NR | NR | (AiC information has been removed) | Yes (SAs) |
| OPTION132 | PBO + MTX | 24 weeks | 205 | 64.9 | 32.2 | 2.9 | 28.8 | Yes (SAs) |
| TCZ + MTX | 24 weeks | 204 | 20.6 | 41.2a | 38.2b | 79.4 | Yes (SAs) |
Network meta-analysis results
For ease of interpretation, a summary of the data used in the NMA is provided (Tables 19–22). As described earlier a number of sensitivity analyses were undertaken to allow the impact of further information, albeit subject to potential biases, including a small proportion of patients with prior bDMARD use, and including studies in which the patients (for populations 2 and 3) have low background MTX use and may not be truly MTX failures. The RCTs have been grouped into those that fit within the Assessment Group base case and those that have prior bDMARD use and/or low background MTX use.
| Trial name/study | Intervention | Mean disease duration (weeks) | Intervention 1 (patients, n) | Intervention 2 (patients, n) | Intervention 3 (patients, n) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | No response | Moderate EULAR | Good EULAR | Total population | No response | Moderate EULAR | Good EULAR | Total population | No response | Moderate EULAR | Good EULAR | Total population | ||
| Base case: full data reported | ||||||||||||||||
| ACT-RAY57 | TCZ + MTX | TCZ | 676 | 29 | 77 | 171 | 277 | 38 | 96 | 142 | 276 | |||||
| ADACTA58 | ADA | TCZ | 354 | 73 | 57 | 32 | 162 | 36 | 43 | 84 | 163 | |||||
| ATTEST74 | cDMARD | ABT i.v. + MTX | IFX + MTX | 405 | 46 | 45 | 11 | 102 | 35 | 85 | 30 | 150 | 53 | 67 | 36 | 156 |
| CERTAIN79 | cDMARD | CTZ + MTX | 239 | 42 | 16 | 11 | 69 | 18 | 32 | 29 | 79 | |||||
| GO-FORTH91 | cDMARD | GOL + MTX | 455 | 43 | 30 | 11 | 84 | 13 | 30 | 38 | 81 | |||||
| JESMR140 | ETN + MTX | ETN | 485 | 3 | 32 | 38 | 73 | 20 | 26 | 23 | 69 | |||||
| LARA102 | Intensive cDMARD | ETN + MTX | 430 | 50 | 75 | 17 | 142 | 23 | 125 | 131 | 279 | |||||
| SATORI116 | cDMARD | TCZ | 447 | 39 | 23 | 2 | 64 | 2 | 19 | 40 | 61 | |||||
| TACIT141 | Intensive cDMARD | Grouped biologicsa + MTX | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | |||||
| Van De Putte et al., 2004122 | ADA | PBO | 577 | 50 | 53 | 10 | 113 | 81 | 25 | 4 | 110 | |||||
| Base case: no response and response (i.e. moderate and good combined) reported | ||||||||||||||||
| AUGUST II76 | cDMARD | ADA + MTX | 447 | 31 | 76 | 15 | 79 | |||||||||
| GO-FORWARD92 | cDMARD | GOL + MTX | 421 | 77 | 133 | 25 | 89 | |||||||||
| START118 | cDMARD | IFX + MTX | 186 | 332 | 83 | 333 | ||||||||||
| TOWARD121 | cDMARD | TCZ + MTX | 510 | 258 | 413 | 163 | 803 | |||||||||
| Base case: good and not good (i.e. moderate and no response combined) reported | ||||||||||||||||
| Swefot119 | Intensive cDMARD | IFX + MTX | 27 | 31 | 130 | 43 | 128 | |||||||||
| Sensitivity analyses: prior bDMARD use for some patients – full data reported | ||||||||||||||||
| OPTION132 | cDMARD | TCZ + MTX | 398 | 133 | 66 | 6 | 205 | 42 | 84 | 78 | 204 | |||||
| Sensitivity analyses: prior biologics – no response and response (i.e. moderate and good combined) reported | ||||||||||||||||
| RAPID1135 | cDMARD | CTZ + MTX | 319 | 145 | 54 | 199 | 75 | 318 | 393 | |||||||
| Yamamoto et al., 2011129 | cDMARD | CTZ + MTX | 296 | 54 | 23 | 77 | 12 | 70 | 82 | |||||||
| Trial name/study | Intervention | Mean disease duration (weeks) | Intervention 1 (patients, n) | Intervention 2 (patients, n) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | No response | ACR20 response | ACR50 response | ACR70 response | Total population | No response | ACR20 response | ACR50 response | ACR70 response | Total population | ||
| Base case: full data reported | |||||||||||||||
| CREATE IIb96 | cDMARD | ETN + MTX | 419 | 44 | 10 | 8 | 3 | 65 | 22 | 12 | 15 | 15 | 64 | ||
| ACT-RAY57 | TCZ + MTX | TCZ | 676 | 79 | 72 | 58 | 68 | 277 | 82 | 83 | 41 | 70 | 276 | ||
| ADACTA58 | ADA | TCZ | 354 | 82 | 35 | 16 | 29 | 162 | 57 | 29 | 24 | 53 | 163 | ||
| AIM61 | cDMARD | ABT i.v. + MTX | 449 | 132 | 50 | 23 | 14 | 219 | 139 | 121 | 87 | 86 | 433 | ||
| AMPLE144 | ADA + MTX | ABT s.c. + MTX | 94 | 117 | 72 | 65 | 74 | 328 | 108 | 65 | 68 | 77 | 318 | ||
| ARMADA69 | cDMARD | ADA + MTX | 607 | 53 | 4 | 2 | 3 | 62 | 22 | 8 | 19 | 18 | 67 | ||
| ATTEST74 | cDMARD | ABT i.v. + MTX | IFX + MTX | 405 | 64 | 24 | 12 | 10 | 110 | 52 | 41 | 31 | 32 | 156 | |
| ATTRACT75 | cDMARD | IFX + MTX | NR | 67 | 13 | 4 | 0 | 84 | 42 | 19 | 16 | 7 | 83 | ||
| AUGUST II76 | cDMARD | ADA + MTX | 447 | 40 | 24 | 8 | 4 | 76 | 23 | 26 | 16 | 14 | 79 | ||
| CERTAIN79 | cDMARD | CTZ + MTX | 239 | 83 | 8 | 4 | 3 | 98 | 61 | 15 | 11 | 9 | 96 | ||
| CHANGE80 | ADA | PBO | 477 | 51 | 18 | 11 | 11 | 91 | 75 | 7 | 4 | 1 | 87 | ||
| deFilippis et al., 200685 | ETN + MTX | IFX + MTX | 7 | 5 | 3 | 1 | 16 | 7 | 4 | 4 | 1 | 16 | |||
| DE01980 | cDMARD | ADA + MTX | 569 | 141 | 40 | 14 | 5 | 200 | 76 | 50 | 38 | 43 | 207 | ||
| ETN study 30989 | cDMARD | ETN + MTX | ETN | 341 | 36 | 7 | 6 | 1 | 50 | 27 | 22 | 27 | 25 | 101 | |
| GO-FORTH91 | cDMARD | GOL + MTX | 455 | 59 | 16 | 8 | 5 | 88 | 25 | 25 | 13 | 23 | 86 | ||
| GO-FORWARD92 | cDMARD | GOL + MTX | 421 | 96 | 19 | 11 | 7 | 133 | 36 | 20 | 15 | 18 | 89 | ||
| JESMR140 | ETN + MTX | ETN | 485 | 7 | 19 | 19 | 28 | 73 | 25 | 11 | 15 | 18 | 69 | ||
| Kim et al., 200799 | cDMARD | ADA + MTX | 356 | 40 | 14 | 4 | 5 | 63 | 25 | 12 | 14 | 14 | 65 | ||
| LARA102 | Intensive cDMARD | ETN + MTX | 430 | 71 | 38 | 17 | 16 | 142 | 47 | 59 | 76 | 97 | 279 | ||
| Mathias et al., 2000105 | ETN | PBO | 598 | 31 | 15 | 20 | 12 | 78 | 71 | 5 | 3 | 1 | 80 | ||
| O’Dell 2013112 | Intensive cDMARD | ETN + MTX | 271 | 70 | 48 | 33 | 8 | 159 | 73 | 32 | 32 | 26 | 163 | ||
| SAMURAI115 | cDMARD | TCZ | 119 | 89 | 30 | 16 | 10 | 145 | 28 | 39 | 37 | 53 | 157 | ||
| SATORI116 | cDMARD | TCZ | 447 | 48 | 5 | 4 | 7 | 64 | 12 | 16 | 13 | 20 | 61 | ||
| STAR117 | cDMARD | ADA + MTX | 541 | 207 | 75 | 25 | 11 | 318 | 150 | 76 | 45 | 47 | 318 | ||
| START118 | cDMARD | IFX | NR | 271 | 57 | 18 | 17 | 363 | 152 | 93 | 65 | 50 | 360 | ||
| TOWARD121 | cDMARD | TCZ | 510 | 312 | 64 | 25 | 12 | 413 | 315 | 186 | 137 | 165 | 803 | ||
| Van De Putte et al., 2004122 | ADA | PBO | 577 | 61 | 27 | 11 | 14 | 113 | 89 | 12 | 7 | 2 | 110 | ||
| Weinblatt et al., 1999124 | cDMARD | ETN + MTX | 676 | 22 | 7 | 1 | 0 | 30 | 17 | 19 | 14 | 9 | 59 | ||
| Sensitivity analyses: prior bDMARD use for some patients – full data reported | |||||||||||||||
| ACQUIRE127 | ABT i.v. + MTX | ABT s.c. + MTX | 398 | 186 | 185 | 176 | 174 | 721 | 185 | 181 | 180 | 190 | 736 | ||
| Kremer et al., 201063 | cDMARD | TOF5 + MTX | TOF10 + MTX | 444 | 52 | 1 | 2 | 14 | 69 | 37 | 10 | 10 | 14 | 71 | |
| LITHE130 | cDMARD | TCZ + MTX | 476 | 287 | 67 | 31 | 8 | 393 | 174 | 96 | 76 | 52 | 398 | ||
| 131 | cDMARD | ABT i.v. + MTX | 483 | 77 | 28 | 12 | 2 | 119 | 46 | 27 | 23 | 19 | 115 | ||
| OPTION132 | cDMARD | TCZ + MTX | 398 | 151 | 31 | 18 | 4 | 204 | 84 | 31 | 45 | 45 | 205 | ||
| RA0025134 | cDMARD | CTZ + MTX | 303 | 29 | 3 | 7 | 1 | 40 | 27 | 19 | 21 | 14 | 81 | ||
| RAPID1135 | cDMARD | CTZ + MTX | 319 | 171 | 12 | 9 | 6 | 199 | 162 | 85 | 62 | 84 | 393 | ||
| RAPID2136 | cDMARD | CTZ + MTX | 308 | 116 | 7 | 3 | 1 | 127 | 105 | 61 | 41 | 39 | 246 | ||
| van der Heijde et al., 2013138 | cDMARD | TOF5 + MTX | TOF10 + MTX | 467 | 120 | 27 | 11 | 2 | 160 | 156 | 61 | 57 | 47 | 321 | |
| Yamamoto et al., 2011129 | cDMARD | CTZ + MTX | 296 | 58 | 6 | 12 | 1 | 77 | 22 | 15 | 21 | 24 | 82 | ||
| Sensitivity analyses: prior biologics – no ACR50 or ACR70 reported | |||||||||||||||
| ORAL STANDARD133 | cDMARD | ADA + MTX | TOF5 + MTX | TOF10 + MTX | 402 | 76 | 30 | 106 | 105 | 94 | N/A | N/A | 199 | ||
| Sensitivity analyses: prior biologics – full data reported and low background MTX use | |||||||||||||||
| AMBITION55 | cDMARD | TCZ | 330 | 46 | 15 | 13 | 14 | 88 | 25 | 28 | 11 | 25 | 89 | ||
| Sensitivity analyses: low background MTX use | |||||||||||||||
| TEAR53 | cDMARD | Intensive cDMARD | ETN + MTX | 18 | 230 | 77 | 59 | 13 | 379 | 59 | 32 | 30 | 11 | 132 | |
| TEMPO54 | cDMARD | ETN + MTX | ETN | 345 | 59 | 75 | 58 | 36 | 228 | 40 | 52 | 54 | 85 | 231 | |
| Trial name | Intervention | Intervention 3 (patients, n) | Intervention 4 (patients, n) | ||||||||||||
| 3 | 4 | No response | ACR20 response | ACR50 response | ACR70 response | Total population | No response | ACR20 response | ACR50 response | ACR70 response | Total population | ||||
| Base case: full data reported | |||||||||||||||
| ATTEST74 | IFX + MTX | 67 | 37 | 21 | 40 | 165 | |||||||||
| ETN study 30989 | ETN | 27 | 28 | 26 | 22 | 103 | |||||||||
| Sensitivity analyses: prior bDMARD use for some patients – full data reported | |||||||||||||||
| Kremer et al., 201063 | TOF10 | 34 | 15 | 13 | 12 | 74 | |||||||||
| van der Heijde et al., 2013138 | TOF10 | 121 | 57 | 68 | 70 | 316 | |||||||||
| Sensitivity analyses: prior biologics – no ACR50 or ACR70 reported | |||||||||||||||
| ORAL STANDARD133 | TOF5 | TOF10 | 95 | 101 | N/A | N/A | 196 | 93 | 103 | N/A | N/A | 196 | |||
| Sensitivity analyses: low background MTX use | |||||||||||||||
| TEAR53 | ETN + MTX | 109 | 57 | 49 | 29 | 244 | |||||||||
| TEMPO54 | ETN | 63 | 68 | 52 | 40 | 223 | |||||||||
Tables 21 and 22 provide data for population 1 using EULAR and ACR criteria respectively. Only one RCT that reported EULAR data met the criteria for inclusion.
| Trial name | Intervention | Mean disease duration (weeks) | Intervention 1 (patients, n) | Intervention 2 (patients, n) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | No response | Moderate EULAR | Good EULAR | Total population | No response | Moderate EULAR | Good EULAR | Total population | ||
| Base case: no response and response (i.e. moderate and good combined) reported | ||||||||||||
| GO-BEFORE90 | cDMARD | GOL + MTX | 166 | 62 | 160 | 43 | 159 | |||||
| Trial name/study | Intervention | Mean disease duration (weeks) | Intervention 1 (patients, n) | Intervention 2 (patients, n) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | No response | ACR20 response | ACR50 response | ACR70 response | Total population | No response | ACR20 response | ACR50 response | ACR70 response | Total population | ||
| Base case: full data reported | |||||||||||||||
| COMET81 | cDMARD | ETN + MTX | 109 | 57 | 55 | 47 | 268 | 50 | 57 | 64 | 103 | 274 | |||
| Durez et al., 2007120 | cDMARD | IFX + MTX | 21 | 10 | 3 | 1 | 0 | 14 | 2 | 3 | 5 | 5 | 15 | ||
| ERA139 | cDMARD | ETN | 52 | 90 | 58 | 38 | 31 | 217 | 65 | 55 | 42 | 45 | 207 | ||
| GO-BEFORE90 | cDMARD | GOL + MTX | 166 | 81 | 32 | 22 | 25 | 160 | 61 | 34 | 26 | 38 | 159 | ||
| HIT HARD94 | cDMARD | ADA + MTX | 7 | 27 | 16 | 19 | 23 | 85 | 20 | 13 | 13 | 41 | 87 | ||
| OPTIMA108 | cDMARD | ADA + MTX | 18 | 222 | 119 | 88 | 88 | 517 | 153 | 93 | 88 | 181 | 515 | ||
| PREMIER109 | cDMARD | ADA + MTX | ADA | 38 | 99 | 54 | 47 | 57 | 257 | 84 | 27 | 43 | 114 | 268 | |
| Base case: data reported only for ACR20 and ACR70 | |||||||||||||||
| BeST78 | cDMARD | IFX + MTX | Intensive cDMARD | Step-up intensive cDMARDa | NR | 63 | 43 | 20 | 126 | 33 | 55 | 40 | 128 | ||
| Sensitivity analyses: low background MTX use | |||||||||||||||
| TEAR53 | cDMARD | Intensive cDMARD | ETN + MTX | 18 | 230 | 77 | 59 | 13 | 379 | 59 | 32 | 30 | 11 | 132 | |
| TEMPO54 | cDMARD | ETN + MTX | ETN | 345 | 59 | 75 | 58 | 36 | 228 | 40 | 52 | 54 | 85 | 231 | |
| Trial name | Intervention | Intervention 3 (patients, n) | Intervention 4 (patients, n) | ||||||||||||
| 3 | 4 | No response | ACR20 response | ACR50 response | ACR70 response | Total population | No response | ACR20 response | ACR50 response | ACR70 response | Total population | ||||
| Base case: full data reported | |||||||||||||||
| PREMIER109 | ADA | 128 | 50 | 42 | 54 | 274 | |||||||||
| Base case: data reported only for ACR20 and ACR70 | |||||||||||||||
| BeST78 | Intensive CDMARD | Step-up intensive cDMARDa | 39 | 59 | 35 | 133 | 48 | 59 | 14 | 121 | |||||
| Sensitivity analyses: low background MTX use | |||||||||||||||
| TEAR53 | ETN + MTX | 109 | 57 | 49 | 29 | 244 | |||||||||
| TEMPO54 | ETN | 63 | 68 | 52 | 40 | 223 | |||||||||
Additionally, the trials with EULAR data have been further subdivided into whether data were reported for all three categories or whether these were aggregated differently, for example only values for response or no response was provided. Data from the tumour necrosis factor inhibitors against combination intensive therapy (TACIT) study141 were provided as AiC.
Tables 19 and 20 provide data for populations 2 and 3 using EULAR and ACR criteria respectively.
In all tables the data have been apportioned so that these are mutually exclusive (i.e. that ACR20 now refers to patients who made an ACR20 response but not an ACR50 response). Typically, the RCTs would include patients with an ACR50 or ACR70 response within the ACR20 category, with the sum of the ACR responses being larger than the total number within the trial arm.
Population 1 (methotrexate naive)
American College of Rheumatology: main trials
A NMA was used to compare the effects of ADA (with and without MTX), ETN (with and without MTX), IFX plus MTX, GOL plus MTX, intensive cDMARDs and step-up intensive cDMARDs relative to cDMARDs on ACR response.
Data were available from eight studies comparing two, three or four interventions. 78,81,86,87,90,94,108,109
Figure 4 presents the network of evidence and Table 23 presents the frequency with which each pair of treatments was compared. There are eight treatment effects to estimate from eight studies. 78,81,86,87,90,94,108,109
FIGURE 4.
American College of Rheumatology (population 1: main trials) – network of evidence. Int, intensive; SU, step-up. Solid green line (two-arm study); dotted blue line (three-arm study); and dotted green line (four-arm study).
| Intervention | cDMARDs | ADA + MTX | ADA | ETN + MTX | ETN | IFX + MTX | GOL + MTX | Intensive cDMARDs | Step-up intensive cDMARDs |
|---|---|---|---|---|---|---|---|---|---|
| cDMARDs | – | 3 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| ADA + MTX | – | – | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| ADA | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 |
| ETN + MTX | – | – | – | – | 0 | 0 | 0 | 0 | 0 |
| ETN | – | – | – | – | – | 0 | 0 | 0 | 0 |
| IFX + MTX | – | – | – | – | – | – | 0 | 1 | 1 |
| GOL + MTX | – | – | – | – | – | – | – | 0 | 0 |
| Intensive cDMARDs | – | – | – | – | – | – | – | – | 1 |
| Step-up intensive cDMARDs | – | – | – | – | – | – | – | – | – |
The probit transformation provides a transformation of data that can only take values between zero and 1 to values that cover the whole real line (i.e. to values between ± ∞). It is used to transform parameters that represent probabilities to a transformed parameter on the real line; treatment effects estimated on the real line usually have better statistical properties than estimates on a restricted scale. The transformation makes use of the standard normal distribution, which has mean zero and variance 1. Parameters representing probabilities can be thought of as being the area under the standard normal distribution from –∞ to some value that represents the transformed value on the probit scale. In the case of EULAR and ACR, parameters represent the probabilities of being in one of several ordered categories. The statistical model includes parameters representing the baseline response (i.e. ‘no response’) for the control arm in each study; a cut-off representing the distance on the standard normal scale between the category boundaries; treatment effect representing the number of SDs on the standard normal scale. Large negative treatment effects represent positive treatment effects (i.e. a smaller proportion of patients in the lower categories).
Figure 5 presents the effects of each intervention relative to cDMARDs on the probit scale, and Figure 6 and Table 24 present the probabilities of treatment rankings. Treatment rankings should be interpreted as in the following example: for cDMARDs there is a 19.6% probability that it is the seventh most efficacious treatment, a 64.8% probability that is the eighth most efficacious treatment and an 11.5% probability that it is the least effective (i.e. ninth) treatment.
FIGURE 5.
American College of Rheumatology (population 1: main trials) – effects of interventions relative to cDMARDs on the probit scale. CrI, credible interval; Int, intensive; SU, step-up.
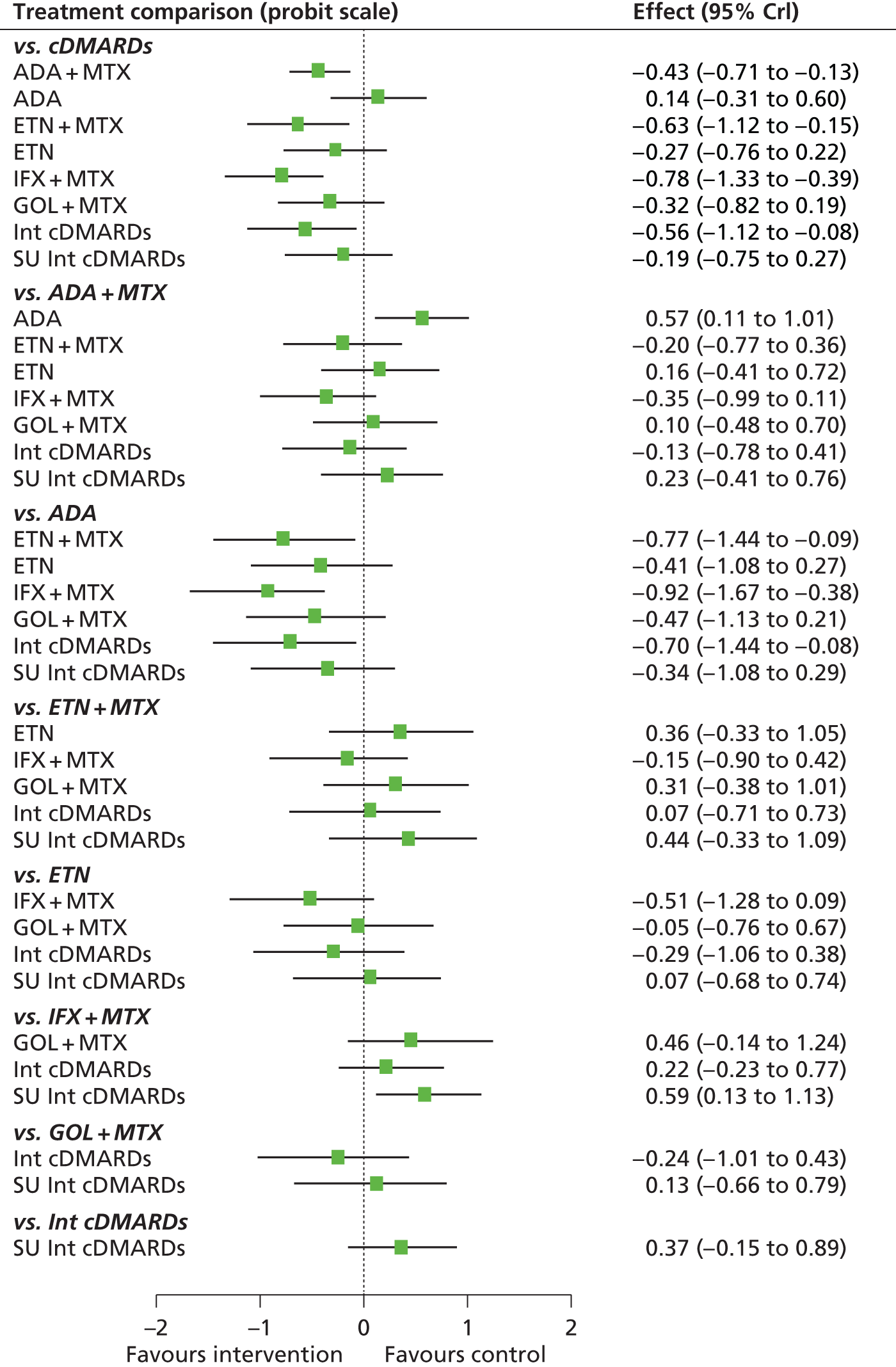
FIGURE 6.
American College of Rheumatology (population 1: main trials) – probability of treatment rankings in terms of efficacy (most efficacious = 1). Int, intensive; SU, step-up.


| Intervention | Rank (mean) | Rank | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| cDMARDs | 7.8 | 0.000 | 0.000 | 0.000 | 0.001 | 0.006 | 0.034 | 0.196 | 0.648 | 0.115 |
| ADA + MTX | 4.2 | 0.013 | 0.057 | 0.180 | 0.378 | 0.234 | 0.101 | 0.031 | 0.005 | 0.001 |
| ADA | 8.5 | 0.001 | 0.001 | 0.004 | 0.007 | 0.013 | 0.029 | 0.066 | 0.135 | 0.744 |
| ETN + MTX | 2.6 | 0.228 | 0.329 | 0.242 | 0.091 | 0.056 | 0.030 | 0.014 | 0.006 | 0.004 |
| ETN | 5.6 | 0.013 | 0.030 | 0.060 | 0.110 | 0.204 | 0.289 | 0.206 | 0.050 | 0.037 |
| IFX + MTX | 1.6 | 0.633 | 0.243 | 0.077 | 0.027 | 0.014 | 0.004 | 0.001 | 0.000 | 0.000 |
| GOL + MTX | 5.2 | 0.021 | 0.048 | 0.093 | 0.156 | 0.235 | 0.229 | 0.145 | 0.042 | 0.030 |
| Intensive cDMARDs | 3.2 | 0.086 | 0.278 | 0.305 | 0.151 | 0.099 | 0.054 | 0.016 | 0.007 | 0.003 |
| Step-up intensive cDMARDs | 6.2 | 0.004 | 0.015 | 0.039 | 0.079 | 0.138 | 0.230 | 0.324 | 0.106 | 0.065 |
The model fitted the data reasonably well, with the total residual deviance, 64.87, close to the total number of data points, 53, included in the analysis. The largest residual deviances were 5.82 from Durez et al. 86 and 4.21 from the BEhandelings STrategie (BeST) study. 78
The between-study SD was estimated to be 0.13 [95% credible interval (CrI) 0.01 to 0.52], which implies mild to moderate heterogeneity between studies in intervention effects.
All interventions except for ADA were associated with beneficial treatment effects relative to cDMARDs with the greatest effect being associated with IFX plus MTX. However, the treatment effects were statistically significant only for ADA plus MTX, ETN plus MTX, IFX plus MTX and intensive cDMARDs at a conventional 5% level. IFX plus MTX (mean rank 1.6; probability of being the best 0.633) was the treatment that was most likely to be the most effective intervention.
A meta-analysis was used to estimate the proportion of patients experiencing an ACR ‘no response’ when treated with cDMARDs.
Data were available from eight studies. 78,81,86,87,90,94,108,109
The model fitted the data reasonably well, with the total residual deviance, 11.74, close to the total number of data points, 8, included in the analysis. The largest residual deviance was 3.35 from Durez et al. 86
The between-study SD was estimated to be 0.14 (95% CrI 0.01 to 0.44), which implies mild heterogeneity between studies in the baseline response.
Table 25 presents the probabilities of achieving at least an ARC20 response, an ACR50 response and an ACR70 response. These are derived by combining the treatment effects estimated from the NMA with the estimate of the cDMARDs ‘no response’ rate.
| Intervention | At least ACR20 (95% CrI) | At least ACR50 (95% CrI) | At least ACR70 (95% CrI) |
|---|---|---|---|
| cDMARDs | 0.564 (0.495 to 0.632) | 0.322 (0.245 to 0.411) | 0.169 (0.116 to 0.237) |
| ADA + MTX | 0.722 (0.600 to 0.820) | 0.486 (0.345 to 0.629) | 0.298 (0.184 to 0.436) |
| ADA | 0.507 (0.323 to 0.692) | 0.272 (0.133 to 0.457) | 0.136 (0.054 to 0.276) |
| ETN + MTX | 0.785 (0.612 to 0.903) | 0.566 (0.360 to 0.754) | 0.370 (0.195 to 0.578) |
| ETN | 0.668 (0.466 to 0.829) | 0.424 (0.235 to 0.632) | 0.246 (0.112 to 0.441) |
| IFX + MTX | 0.828 (0.697 to 0.935) | 0.627 (0.453 to 0.815) | 0.432 (0.268 to 0.656) |
| GOL + MTX | 0.686 (0.481 to 0.844) | 0.445 (0.245 to 0.653) | 0.263 (0.116 to 0.464) |
| Intensive cDMARDs | 0.766 (0.586 to 0.904) | 0.542 (0.339 to 0.754) | 0.348 (0.179 to 0.577) |
| Step-up intensive cDMARDs | 0.639 (0.446 to 0.827) | 0.395 (0.219 to 0.626) | 0.223 (0.101 to 0.432) |
American College of Rheumatology: main trials plus methotrexate experienced
A NMA was used to compare the effects of ADA (with and without MTX), ETN (with and without MTX), IFX plus MTX, GOL plus MTX, intensive cDMARDs and step-up intensive cDMARDs relative to cDMARDs on ACR response.
Data were available from 10 studies comparing two, three or four interventions. 53,54,78,81,90,94,109,120,139,142
Figure 7 presents the network of evidence and Table 26 presents the frequency with which each pair of treatments was compared. There are eight treatment effects to estimate from 10 studies.
FIGURE 7.
American College of Rheumatology (population 1: main trials + MTX experienced) – network of evidence. Int, intensive; SU, step-up. Solid green line (two-arm study); dotted blue line (three-arm study); and dotted green line (four-arm study).

| Intervention | cDMARDs | ADA + MTX | ADA | ETN + MTX | ETN | IFX + MTX | GOL + MTX | Intensive cDMARDs | Step-up intensive cDMARDs |
|---|---|---|---|---|---|---|---|---|---|
| cDMARDs | – | 3 | 1 | 3 | 2 | 2 | 1 | 8 | |
| ADA + MTX | – | – | 1 | ||||||
| ADA | – | – | – | ||||||
| ETN + MTX | – | – | – | – | 1 | 1 | |||
| ETN | – | – | – | – | – | ||||
| IFX + MTX | – | – | – | – | – | – | 1 | ||
| GOL + MTX | – | – | – | – | – | – | – | ||
| Intensive cDMARDs | – | – | – | – | – | – | – | – | |
| Step-up intensive cDMARDs | – | – | – | – | – | – | – | – | – |
Figure 8 presents the effects of each intervention relative to cDMARDs on the probit scale, and Figure 9 and Table 27 presents the probabilities of treatment rankings.
FIGURE 8.
American College of Rheumatology (population 1: main trials + MTX experienced) – effects of interventions relative to cDMARDs on the probit scale. Int, intensive; SU, step-up.
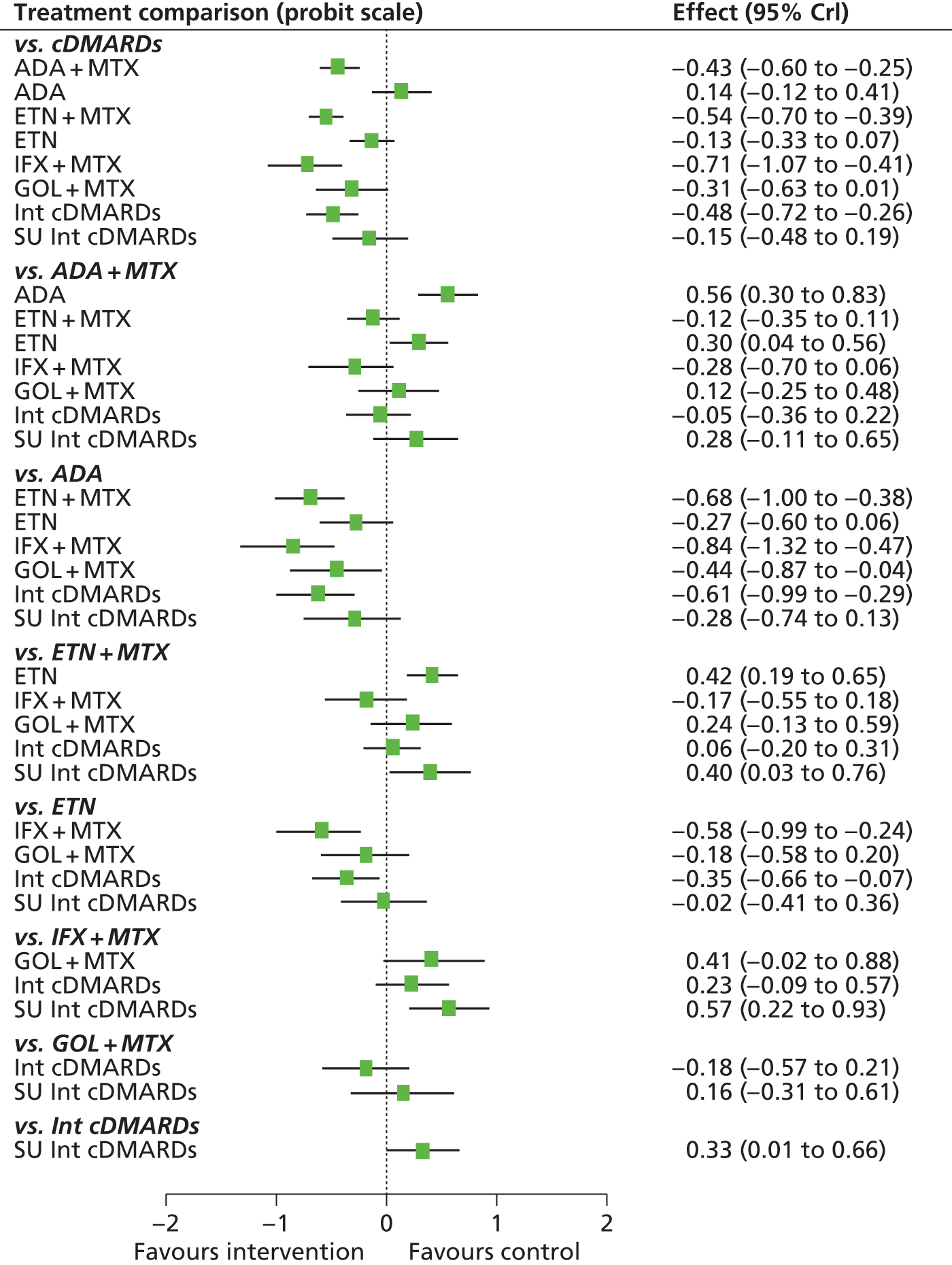
FIGURE 9.
American College of Rheumatology (population1: main trials + MTX experienced) – probability of treatment rankings in terms of efficacy (most efficacious = 1). Int, intensive; SU, step-up.
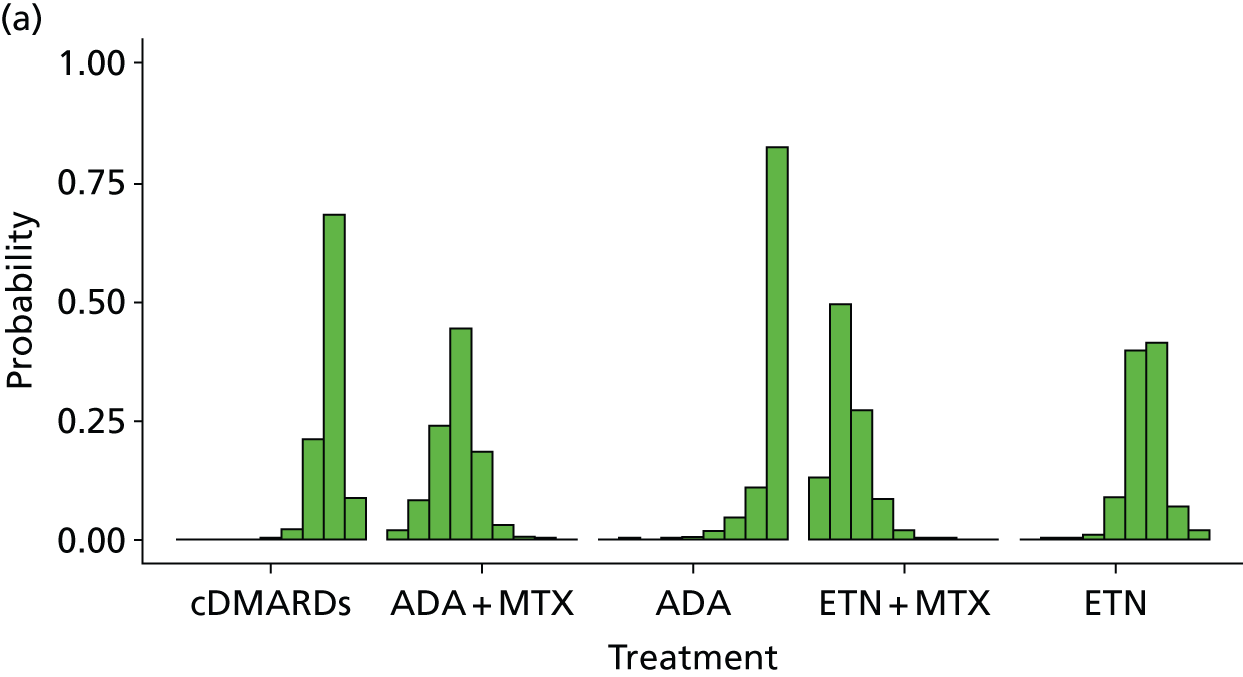

| Intervention | Rank (mean) | Rank | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| cDMARDs | 7.8 | 0.000 | 0.000 | 0.000 | 0.001 | 0.021 | 0.032 | 0.210 | 0.683 | 0.086 |
| ADA + MTX | 3.8 | 0.018 | 0.240 | 0.443 | 0.184 | 0.029 | 0.032 | 0.004 | 0.001 | 0.000 |
| ADA | 8.7 | 0.000 | 0.000 | 0.001 | 0.004 | 0.016 | 0.020 | 0.045 | 0.108 | 0.825 |
| ETN + MTX | 2.4 | 0.130 | 0.271 | 0.083 | 0.020 | 0.002 | 0.008 | 0.000 | 0.000 | 0.000 |
| ETN | 6.5 | 0.000 | 0.002 | 0.009 | 0.089 | 0.398 | 0.075 | 0.413 | 0.068 | 0.020 |
| IFX + MTX | 1.3 | 0.801 | 0.050 | 0.024 | 0.006 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 |
| GOL + MTX | 4.9 | 0.017 | 0.074 | 0.143 | 0.439 | 0.177 | 0.509 | 0.072 | 0.021 | 0.008 |
| Intensive cDMARDs | 3.2 | 0.034 | 0.351 | 0.255 | 0.100 | 0.009 | 0.033 | 0.001 | 0.000 | 0.000 |
| Step-up intensive cDMARDs | 6.4 | 0.000 | 0.012 | 0.042 | 0.157 | 0.346 | 0.290 | 0.255 | 0.119 | 0.062 |
The model fitted the data reasonably well, with the total residual deviance, 84.19, close to the total number of data points, 71, included in the analysis. The largest residual deviances were 5.89 and 3.92 from the Patients REceiving Methotrexate and Infliximab for the treatment of Early Rheumatoid arthritis (PREMIER) study109 and 4.08 from the BeST study. 78
The between-study SD was estimated to be 0.07 (95% CrI 0.00 to 0.26), which implies mild heterogeneity between studies in intervention effects. The addition of the studies including patients who were MTX experienced has reduced the estimate of the between-study SD.
All interventions except for ADA were associated with beneficial treatment effects relative to cDMARDs, with the greatest effect being associated with IFX plus MTX. However, the treatment effects were statistically significant only for ADA plus MTX, ETN plus MTX, IFX plus MTX and intensive cDMARDs at a conventional 5% level. IFX plus MTX (mean rank 1.3; probability of being the best 0.801) was the treatment that was most likely to be the most effective intervention.
A meta-analysis was used to estimate the proportion of patients experiencing an ACR ‘no response’ when treated with cDMARDs.
Data were available from 10 studies. 53,54,78,81,86,87,90,94,108,109
The model fitted the data well, with the total residual deviance, 10.95, close to the total number of data points, 10, included in the analysis.
The between-study SD was estimated to be 0.32 (95% CrI 0.18 to 0.62), which implies mild to moderate heterogeneity between studies in the baseline response.
Table 28 presents the probabilities of achieving at least an ACR20 response, an ACR50 response and an ACR70 response. These are derived by combining the treatment effects estimated from the NMA with the estimate of the cDMARDs ‘no response’ rate.
| Intervention | At least ACR20 (95% CrI) | At least ACR50 (95% CrI) | At least ACR70 (95% CrI) |
|---|---|---|---|
| cDMARDs | 0.559 (0.464 to 0.650) | 0.306 (0.218 to 0.406) | 0.144 (0.090 to 0.216) |
| ADA + MTX | 0.718 (0.613 to 0.806) | 0.468 (0.344 to 0.595) | 0.263 (0.168 to 0.379) |
| ADA | 0.504 (0.356 to 0.640) | 0.259 (0.153 to 0.394) | 0.115 (0.056 to 0.205) |
| ETN + MTX | 0.756 (0.658 to 0.837) | 0.515 (0.391 to 0.637) | 0.302 (0.201 to 0.422) |
| ETN | 0.608 (0.486 to 0.721) | 0.352 (0.236 to 0.482) | 0.174 (0.101 to 0.276) |
| IFX + MTX | 0.805 (0.683 to 0.901) | 0.582 (0.421 to 0.738) | 0.364 (0.224 to 0.533) |
| GOL + MTX | 0.676 (0.525 to 0.805) | 0.420 (0.268 to 0.588) | 0.224 (0.119 to 0.373) |
| Intensive cDMARDs | 0.737 (0.621 to 0.832) | 0.491 (0.355 to 0.630) | 0.282 (0.174 to 0.413) |
| Step-up intensive cDMARDs | 0.616 (0.455 to 0.761) | 0.360 (0.216 to 0.527) | 0.180 (0.089 to 0.313) |
Populations 2 and 3 (methotrexate-experienced populations)
European League Against Rheumatism: main trials
A NMA was used to compare the effects of ABT i.v. plus MTX, ADA (with and without MTX), intensive cDMARDs, ETN (with and without MTX), GOL plus MTX, IFX plus MTX, PBO, TCZ (with and without MTX), the grouped biologics (from the TACIT RCT141) and CTZ plus MTX relative to cDMARDs on EULAR response.
Data were available from 15 studies comparing two or three interventions. 57,58,74,76,79,91,92,102,116,118,119,121,122,140,141
Figure 10 presents the network of evidence and Table 29 presents the frequency with which each pair of treatments was compared. There are 13 treatment effects to estimate from 15 studies. 57,58,74,76,79,91,92,102,116,118,119,121,122,140,141
FIGURE 10.
European League Against Rheumatism (populations 2 and 3: main trials) – network of evidence. Int, intensive; mono, monotherapy. Solid green line (two-arm study); and dotted blue line (three-arm study).
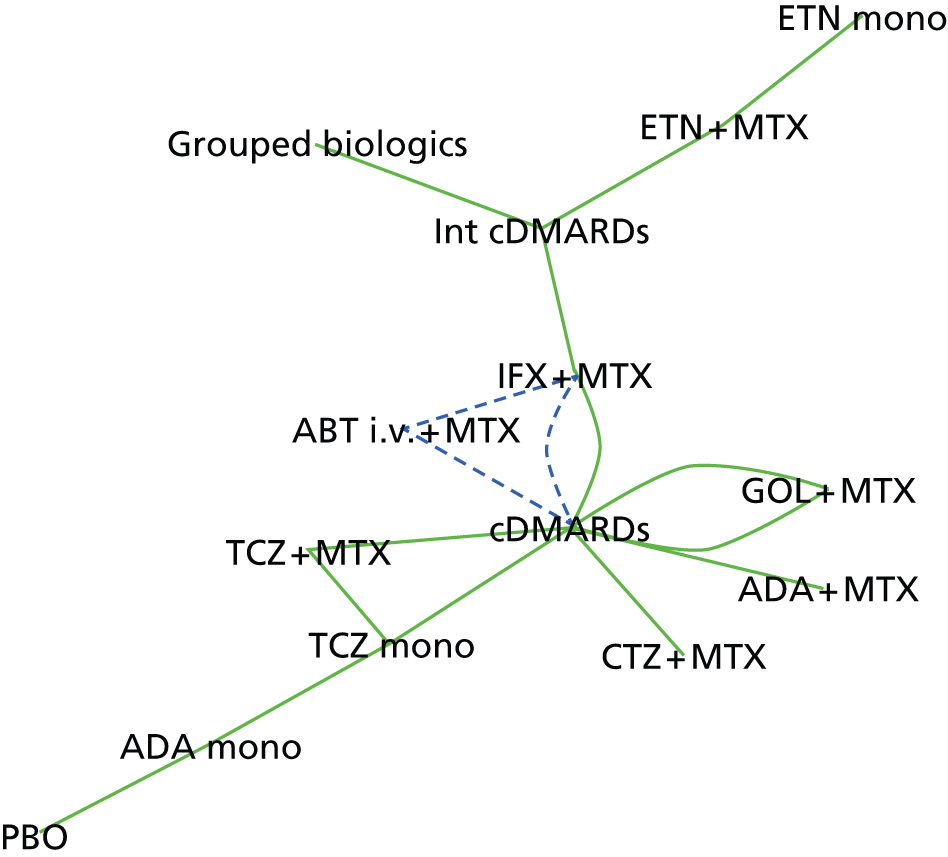
| Intervention | cDMARDs | ABT i.v. + MTX | ADA + MTX | ADA | Intensive cDMARDs | ETN + MTX | ETN | GOL + MTX | IFX + MTX | PBO | TCZ + MTX | TCZ | Grouped biologics | CTZ + MTX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cDMARDs | – | 1 | 1 | 2 | 2 | 1 | 1 | 1 | ||||||
| ABT i.v. + MTX | – | – | 1 | |||||||||||
| ADA + MTX | – | – | – | |||||||||||
| ADA | – | – | – | – | 1 | 1 | ||||||||
| Intensive cDMARDs | – | – | – | – | – | 1 | 1 | 1 | ||||||
| ETN + MTX | – | – | – | – | – | – | 1 | |||||||
| ETN | – | – | – | – | – | – | – | |||||||
| GOL + MTX | – | – | – | – | – | – | – | – | ||||||
| IFX + MTX | – | – | – | – | – | – | – | – | – | |||||
| PBO | – | – | – | – | – | – | – | – | – | – | ||||
| TCZ + MTX | – | – | – | – | – | – | – | – | – | – | – | 1 | ||
| TCZ | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Grouped biologics | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| CTZ + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Figure 11 presents the effects of each intervention relative to cDMARDs on the probit scale and Figure 12 and Table 30 present the probabilities of treatment rankings.
FIGURE 11.
European League Against Rheumatism (populations 2 and 3: main trials) – effects of interventions relative to cDMARDs on the probit scale. Int, intensive.
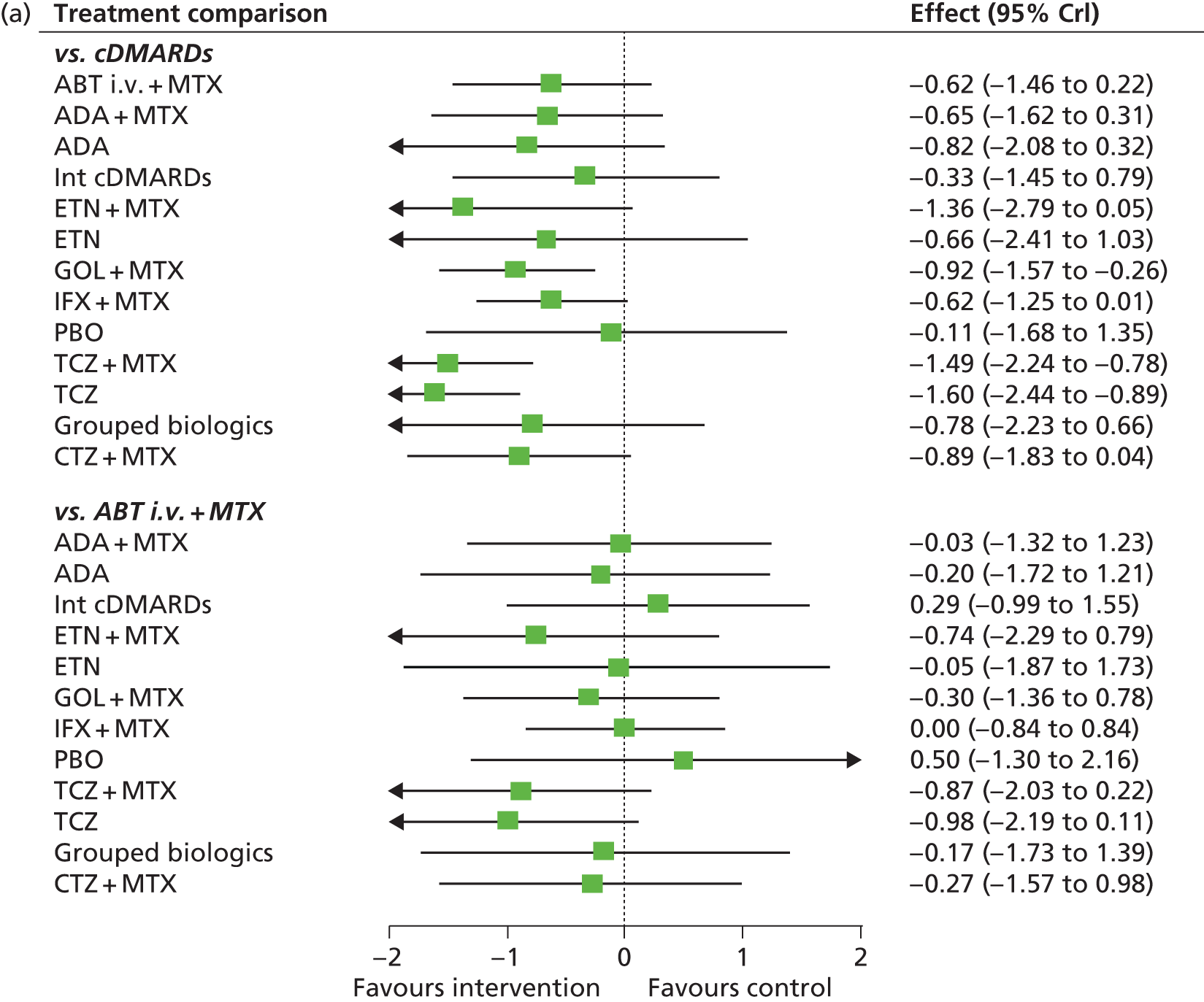


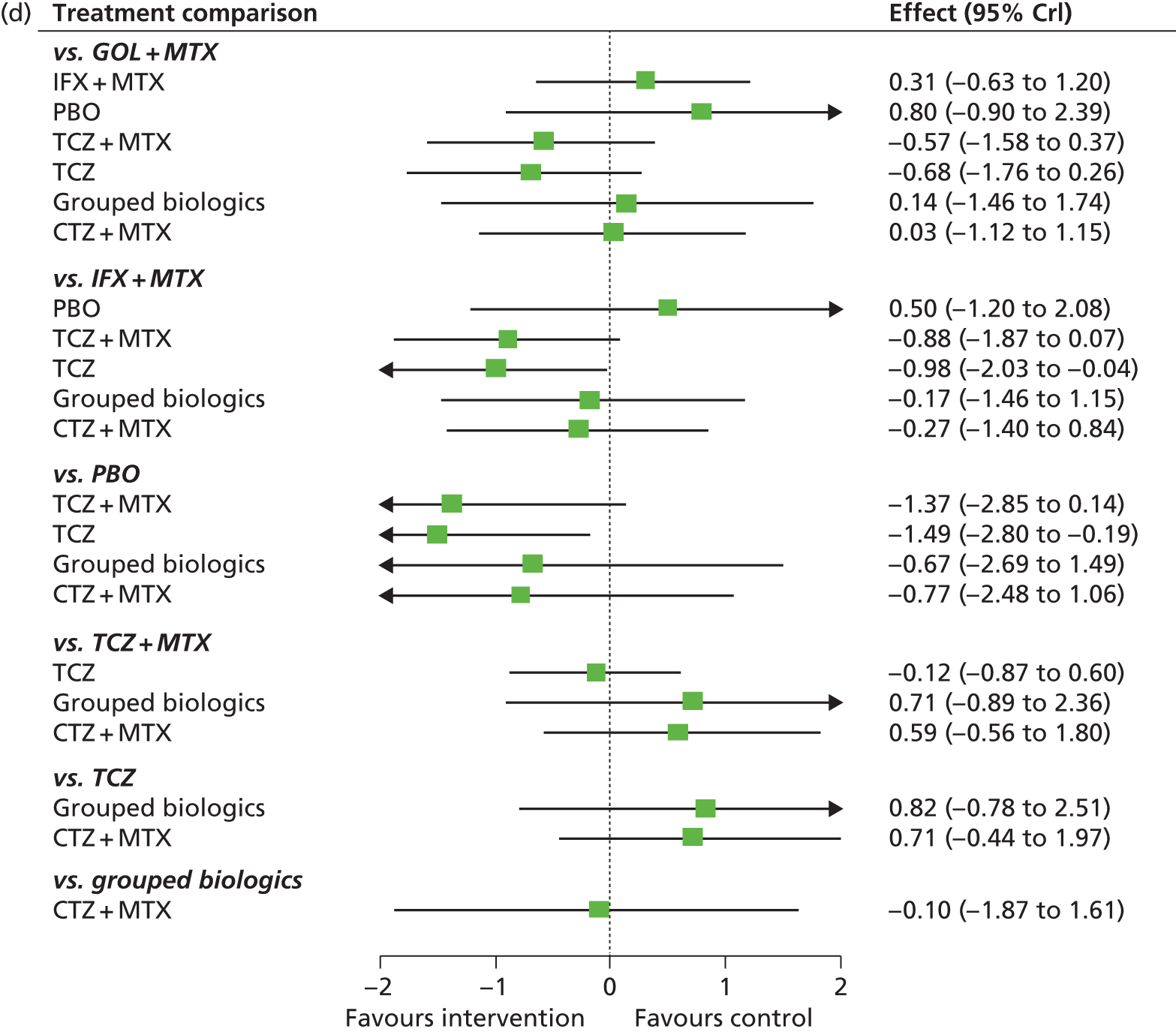
FIGURE 12.
European League Against Rheumatism (populations 2 and 3: main trials) – probability of treatment rankings in terms of efficacy (most efficacious = 1). Int, intensive.

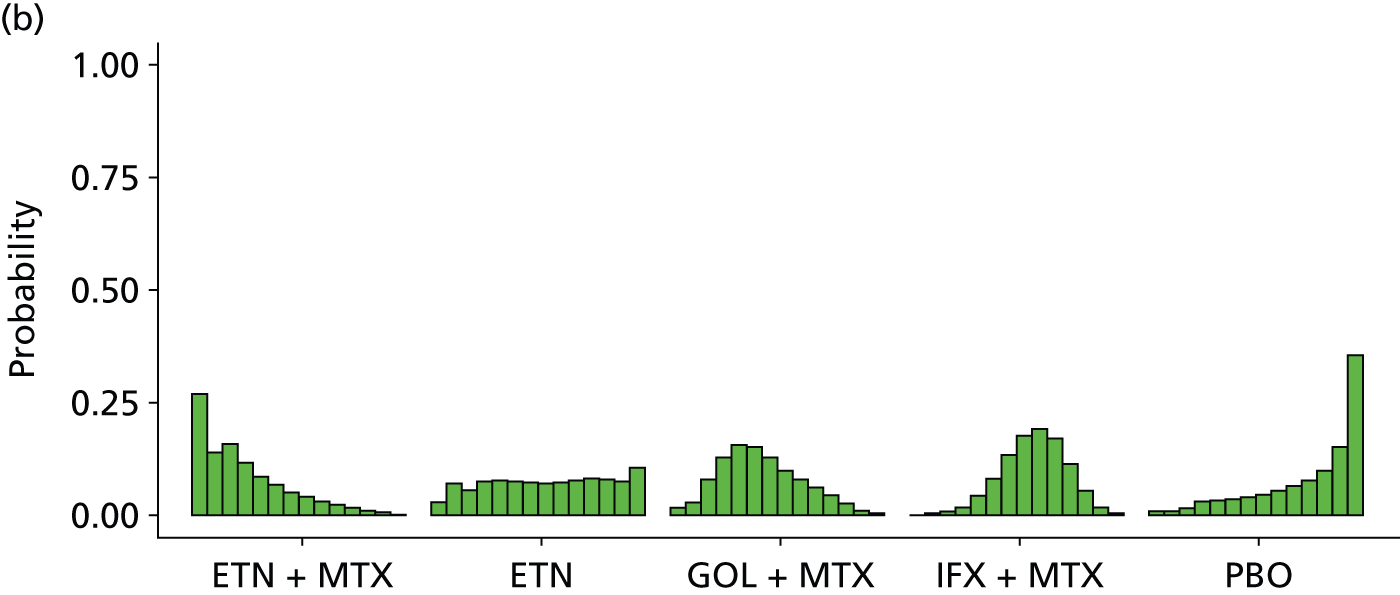
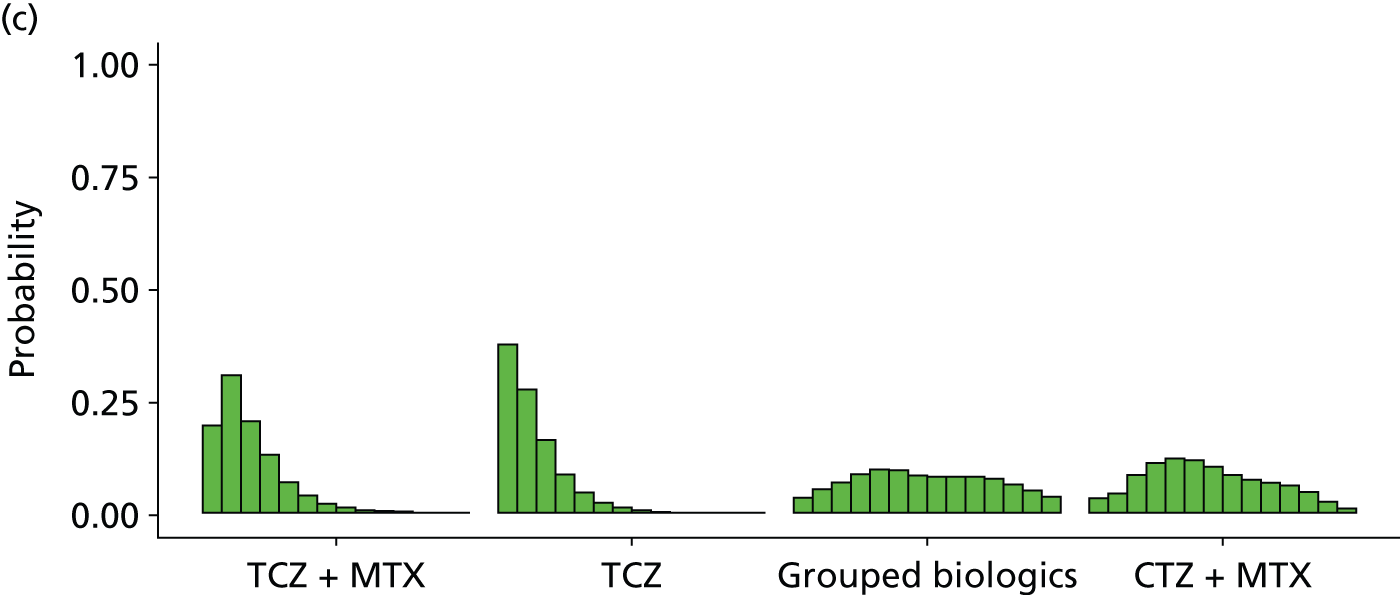
| Intervention | Rank (mean) | Rank | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| cDMARDs | 12.7 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.002 | 0.008 | 0.019 | 0.044 | 0.090 | 0.176 | 0.340 | 0.319 |
| ABT i.v. + MTX | 8.6 | 0.006 | 0.010 | 0.023 | 0.043 | 0.067 | 0.090 | 0.111 | 0.123 | 0.129 | 0.122 | 0.112 | 0.089 | 0.052 | 0.023 |
| ADA + MTX | 8.3 | 0.015 | 0.020 | 0.041 | 0.061 | 0.081 | 0.092 | 0.096 | 0.102 | 0.096 | 0.096 | 0.100 | 0.096 | 0.067 | 0.038 |
| ADA | 7.1 | 0.020 | 0.041 | 0.093 | 0.105 | 0.104 | 0.100 | 0.098 | 0.083 | 0.079 | 0.077 | 0.075 | 0.075 | 0.045 | 0.004 |
| Intensive cDMARDs | 10.7 | 0.000 | 0.001 | 0.006 | 0.014 | 0.020 | 0.037 | 0.050 | 0.066 | 0.085 | 0.114 | 0.153 | 0.187 | 0.165 | 0.104 |
| ETN + MTX | 3.8 | 0.268 | 0.139 | 0.158 | 0.116 | 0.085 | 0.067 | 0.048 | 0.039 | 0.030 | 0.022 | 0.016 | 0.009 | 0.004 | 0.001 |
| ETN | 8.2 | 0.027 | 0.068 | 0.054 | 0.073 | 0.078 | 0.073 | 0.072 | 0.068 | 0.071 | 0.077 | 0.082 | 0.079 | 0.074 | 0.104 |
| GOL + MTX | 6.4 | 0.015 | 0.030 | 0.080 | 0.127 | 0.155 | 0.150 | 0.126 | 0.099 | 0.078 | 0.060 | 0.042 | 0.025 | 0.010 | 0.002 |
| IFX + MTX | 8.6 | 0.000 | 0.002 | 0.007 | 0.016 | 0.042 | 0.080 | 0.134 | 0.175 | 0.191 | 0.168 | 0.113 | 0.053 | 0.016 | 0.003 |
| PBO | 11.2 | 0.006 | 0.008 | 0.013 | 0.028 | 0.030 | 0.033 | 0.040 | 0.045 | 0.053 | 0.064 | 0.075 | 0.098 | 0.151 | 0.354 |
| TCZ + MTX | 3.0 | 0194 | 0.308 | 0.205 | 0.131 | 0.070 | 0.041 | 0.022 | 0.014 | 0.008 | 0.004 | 0.002 | 0.001 | 0.000 | 0.000 |
| TCZ | 2.4 | 0.377 | 0.275 | 0.164 | 0.087 | 0.047 | 0.023 | 0.013 | 0.007 | 0.004 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 |
| Grouped biologics | 7.4 | 0.037 | 0.054 | 0.070 | 0.087 | 0.099 | 0.095 | 0.083 | 0.082 | 0.081 | 0.082 | 0.078 | 0.064 | 0.051 | 0.037 |
| CTZ + MTX | 6.7 | 0.034 | 0.044 | 0.087 | 0.111 | 0.123 | 0.118 | 0.104 | 0.088 | 0.076 | 0.068 | 0.061 | 0.047 | 0.025 | 0.012 |
The model fitted the data well, with the total residual deviance, 59.57, close to the total number of data points, 52, included in the analysis.
The between-study SD was estimated to be 0.38 (95% CrI 0.18 to 0.73), which implies mild to moderate heterogeneity between studies in intervention effects.
All interventions were associated with beneficial treatment effects relative to cDMARDs, with the greatest effects being associated with TCZ, TCZ plus MTX and ETN plus MTX. However, the treatment effects were statistically significant only for GL plus MTX, TCZ and TCZ plus MTX at a conventional 5% level. There was insufficient evidence to differentiate between treatments, although TCZ was ranked highest and was the treatment that was most likely to be the most effective intervention (mean rank 2.4; probability of being the best 0.377).
A meta-analysis was used to estimate the proportion of patients experiencing a EULAR ‘no response’ when treated with cDMARDs.
Data were available from eight studies. 74,76,79,91,92,116,118,121
The model fitted the data well, with the total residual deviance, 8.63, close to the total number of data points, 8, included in the analysis.
The between-study SD was estimated to be 0.18 (95% CrI 0.05 to 0.44), which implies mild heterogeneity between studies in the baseline response.
Table 31 presents the probabilities of achieving at least a moderate and at least a good EULAR response. These are derived by combining the treatment effects estimated from the NMA with the estimate of the cDMARDs ‘no response’ rate.
| Intervention | At least moderate (95% CrI) | At least good (95% CrI) |
|---|---|---|
| cDMARDs | 0.451 (0.384 to 0.520) | 0.094 (0.058 to 0.144) |
| ABT i.v. + MTX | 0.690 (0.358 to 0.913) | 0.242 (0.058 to 0.571) |
| ADA + MTX | 0.700 (0.330 to 0.934) | 0.252 (0.049 to 0.631) |
| ADA | 0.757 (0.328 to 0.975) | 0.311 (0.050 to 0.781) |
| Intensive cDMARDs | 0.581 (0.180 to 0.910) | 0.162 (0.017 to 0.567) |
| ETN + MTX | 0.893 (0.426 to 0.996) | 0.519 (0.082 to 0.931) |
| ETN | 0.706 (0.121 to 0.989) | 0.257 (0.009 to 0.867) |
| GOL + MTX | 0.786 (0.545 to 0.929) | 0.345 (0.134 to 0.620) |
| IFX + MTX | 0.688 (0.436 to 0.874) | 0.241 (0.084 to 0.490) |
| PBO | 0.495 (0.070 to 0.942) | 0.115 (0.004 to 0.648) |
| TCZ + MTX | 0.914 (0.738 to 0.984) | 0.568 (0.283 to 0.833) |
| TCZ | 0.930 (0.770 to 0.990) | 0.613 (0.319 to 0.875) |
| Grouped biologics | 0.746 (0.211 to 0.983) | 0.298 (0.022 to 0.823) |
| CTZ + MTX | 0.779 (0.428 to 0.957) | 0.336 (0.082 to 0.708) |
European League Against Rheumatism: main trials plus prior biologics
A NMA was used to compare the effects of ABT i.v. plus MTX, ADA (with and without MTX), intensive cDMARDs, ETN (with and without MTX), GOL plus MTX, IMFX plus MTX, PBO, TCZ (with and without MTX), the grouped biologics (from the TACIT RCT141) and CTZ plus MTX relative to cDMARDs on EULAR response.
Data were available from 18 studies comparing two or three interventions. 57,58,74,76,79,91,92,102,116,118,119,121,122,132,135,139–141
Figure 13 presents the network of evidence and Table 32 presents the frequency with which each pair of treatments was compared. There are 13 treatment effects to estimate from 18 studies. 57,58,74,76,79,91,92,102,116,118,119,121,122,132,135,139–141
FIGURE 13.
European League Against Rheumatism (populations 2 and 3: main trials + prior biologics) – network of evidence. Int, intensive. Solid green line (two-arm study); and dotted blue line (three-arm study).
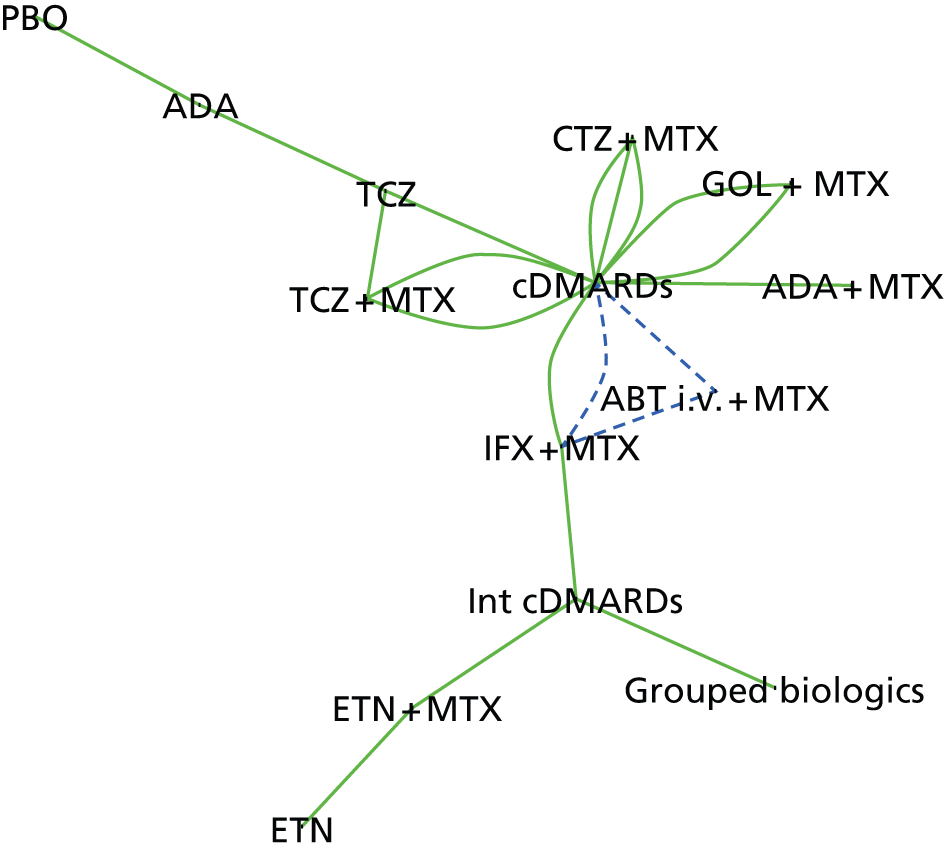
| Intervention | cDMARDs | ABT i.v. + MTX | ADA + MTX | ADA | Intensive cDMARDs | ETN + MTX | ETN | GOL + MTX | IFX + MTX | PBO | TCZ + MTX | TCZ | Grouped biologics | CTZ + MTX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cDMARDs | – | 1 | 1 | 2 | 2 | 2 | 1 | 3 | ||||||
| ABT i.v. + MTX | – | – | 1 | |||||||||||
| ADA + MTX | – | – | – | |||||||||||
| ADA | – | – | – | – | 1 | 1 | ||||||||
| Intensive cDMARDs | – | – | – | – | – | 1 | 1 | 1 | ||||||
| ETN + MTX | – | – | – | – | – | – | 1 | |||||||
| ETN | – | – | – | – | – | – | – | |||||||
| GOL + MTX | – | – | – | – | – | – | – | – | ||||||
| IFX + MTX | – | – | – | – | – | – | – | – | – | |||||
| PBO | – | – | – | – | – | – | – | – | – | – | ||||
| TCZ + MTX | – | – | – | – | – | – | – | – | – | – | – | 1 | ||
| TCZ | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Grouped biologics | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| CTZ + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Figure 14 presents the effects of each intervention relative to cDMARDs on the probit scale and Figure 15 and Table 33 present the probabilities of treatment rankings.
FIGURE 14.
European League Against Rheumatism (populations 2 and 3: main trials + prior biologics) – effects of interventions relative to cDMARDs on the probit scale. Int, intensive.




FIGURE 15.
European League Against Rheumatism (populations 2 and 3: main trials + prior biologics) – probability of treatment rankings in terms of efficacy (most efficacious = 1). Int, intensive.

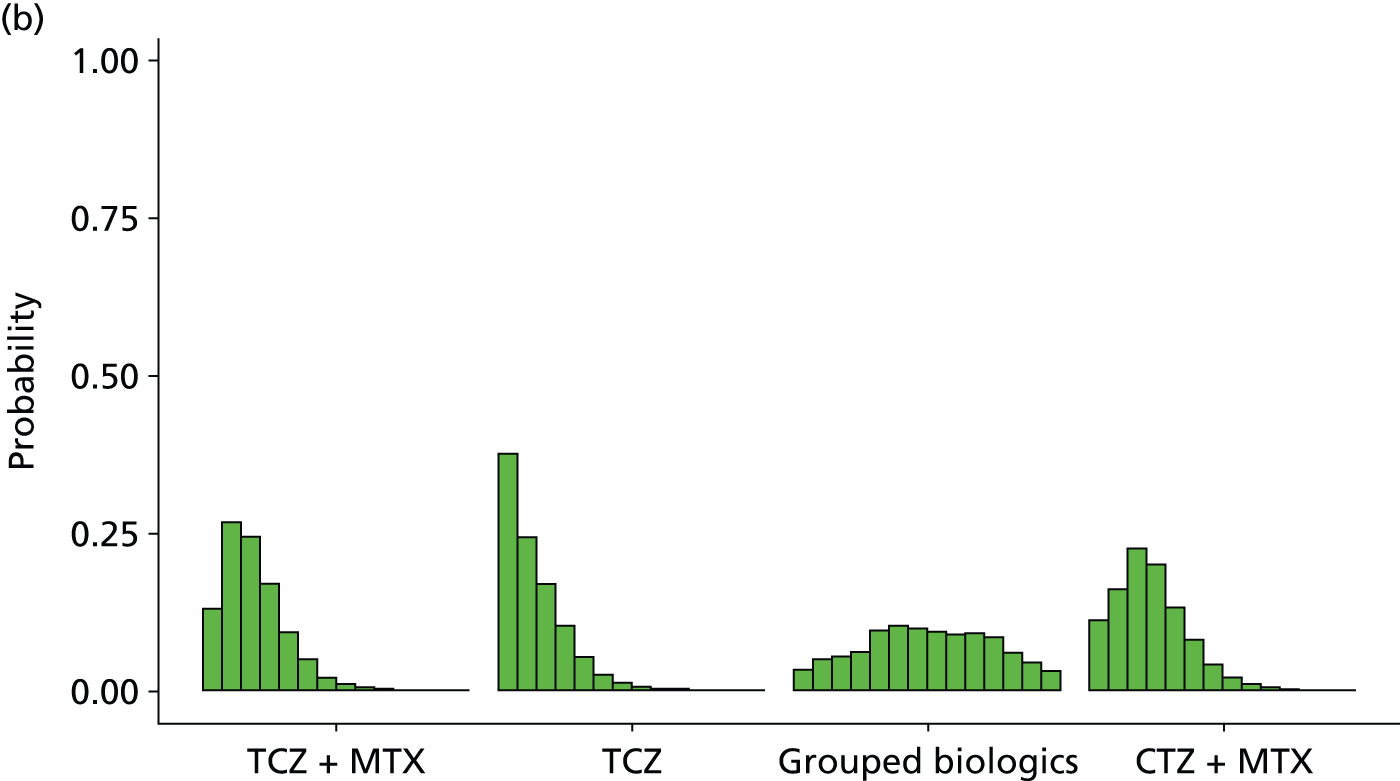

| Intervention | Rank (mean) | Rank | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| cDMARDs | 12.8 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.005 | 0.013 | 0.035 | 0.076 | 0.164 | 0.372 | 0.333 |
| ABT i.v. + MTX | 8.7 | 0.004 | 0.006 | 0.015 | 0.030 | 0.058 | 0.085 | 0.118 | 0.138 | 0.136 | 0.136 | 0.122 | 0.091 | 0.045 | 0.016 |
| ADA + MTX | 8.5 | 0.012 | 0.014 | 0.026 | 0.047 | 0.075 | 0.096 | 0.109 | 0.107 | 0.107 | 0.103 | 0.108 | 0.100 | 0.065 | 0.031 |
| ADA | 7.6 | 0.013 | 0.035 | 0.048 | 0.075 | 0.099 | 0.114 | 0.113 | 0.098 | 0.090 | 0.090 | 0.088 | 0.093 | 0.040 | 0.003 |
| Intensive cDMARDs | 11.0 | 0.000 | 0.001 | 0.003 | 0.007 | 0.011 | 0.020 | 0.039 | 0.059 | 0.085 | 0.123 | 0.172 | 0.215 | 0.168 | 0.096 |
| ETN + MTX | 3.7 | 0.287 | 0.131 | 0.113 | 0.131 | 0.104 | 0.074 | 0.054 | 0.036 | 0.027 | 0.020 | 0.013 | 0.007 | 0.002 | 0.000 |
| ETN | 8.4 | 0.017 | 0.064 | 0.046 | 0.048 | 0.073 | 0.085 | 0.082 | 0.082 | 0.079 | 0.084 | 0.089 | 0.084 | 0.073 | 0.095 |
| GOL + MTX | 6.7 | 0.011 | 0.022 | 0.045 | 0.105 | 0.159 | 0.169 | 0.151 | 0.113 | 0.084 | 0.063 | 0.043 | 0.024 | 0.009 | 0.001 |
| IFX + MTX | 8.9 | 0.000 | 0.000 | 0.002 | 0.008 | 0.025 | 0.065 | 0.121 | 0.185 | 0.218 | 0.185 | 0.118 | 0.057 | 0.015 | 0.001 |
| PBO | 11.7 | 0.003 | 0.004 | 0.008 | 0.012 | 0.018 | 0.027 | 0.034 | 0.045 | 0.050 | 0.059 | 0.079 | 0.103 | 0.166 | 0.392 |
| TCZ + MTX | 3.2 | 0.131 | 0.267 | 0.244 | 0.170 | 0.095 | 0.050 | 0.022 | 0.012 | 0.005 | 0.003 | 0.001 | 0.000 | 0.000 | 0.000 |
| TCZ | 2.4 | 0.377 | 0.243 | 0.169 | 0.103 | 0.054 | 0.026 | 0.013 | 0.006 | 0.004 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 |
| Grouped biologics | 7.5 | 0.033 | 0.050 | 0.055 | 0.063 | 0.095 | 0.105 | 0.101 | 0.094 | 0.091 | 0.091 | 0.085 | 0.061 | 0.044 | 0.032 |
| CTZ + MTX | 3.7 | 0.113 | 0.162 | 0.225 | 0.200 | 0.133 | 0.082 | 0.042 | 0.021 | 0.011 | 0.006 | 0.003 | 0.001 | 0.000 | 0.000 |
The model fitted the data well, with the total residual deviance, 70.90, close to the total number of data points, 60, included in the analysis.
The between-study SD was estimated to be 0.34 (95% CrI 0.17 to 0.62), which implies mild to moderate heterogeneity between studies in intervention effects. The addition of the studies including patients who had received prior biologics resulted in a small reduction in the estimate of the between-study SD.
All interventions were associated with beneficial treatment effects relative to cDMARDs, with the greatest effects being associated with TCZ, TCZ plus MTX, ETN plus MTX and CTZ plus MTX. However, the treatment effects were statistically significant only for ETN plus MTX, GOL plus MTX, IFX plus MTX, TCZ plus MTX, TCZ and CTZ plus MTX at a conventional 5% level. There was insufficient evidence to differentiate between treatments, although TCZ was ranked highest and was the treatment that was most likely to be the most effective intervention (mean rank 2.4; probability of being the best 0.377). The addition of the studies including patients who had received prior biologics had the greatest impact on the estimate of the effect of CTZ plus MTX.
A meta-analysis was used to estimate the proportion of patients experiencing a EULAR ‘no response’ when treated with cDMARDs.
Data were available from 11 studies. 74,76,79,91,92,116,118,121,129,132,135
The model fitted the data well, with the total residual deviance, 11.42, close to the total number of data points, 11, included in the analysis.
The between-study SD was estimated to be 0.24 (95% CrI 0.13 to 0.46), which implies mild heterogeneity between studies in the baseline response.
Table 34 presents the probabilities of achieving at least a moderate and at least a good EULAR response. These are derived by combining the treatment effects estimated from the NMA with the estimate of the cDMARDs ‘no response’ rate.
| Intervention | At least moderate (95% CrI) | At least good (95% CrI) |
|---|---|---|
| cDMARDs | 0.410 (0.344 to 0.479) | 0.077 (0.048 to 0.117) |
| ABT i.v. + MTX | 0.655 (0.356 to 0.878) | 0.212 (0.057 to 0.494) |
| ADA + MTX | 0.664 (0.327 to 0.903) | 0.220 (0.048 to 0.546) |
| ADA | 0.704 (0.321 to 0.948) | 0.254 (0.047 to 0.669) |
| Intensive cDMARDs | 0.539 (0.178 to 0.863) | 0.136 (0.016 to 0.463) |
| ETN + MTX | 0.871 (0.437 to 0.992) | 0.473 (0.085 to 0.886) |
| ETN | 0.670 (0.132 to 0.973) | 0.224 (0.010 to 0.772) |
| GOL + MTX | 0.754 (0.528 to 0.902) | 0.305 (0.126 to 0.545) |
| IFX + MTX | 0.652 (0.424 to 0.832) | 0.210 (0.079 to 0.416) |
| PBO | 0.433 (0.071 to 0.883) | 0.086 (0.004 to 0.500) |
| TCZ + MTX | 0.88 (0.751 to 0.958) | 0.495 (0.293 to 0.710) |
| TCZ | 0.907 (0.752 to 0.979) | 0.550 (0.298 to 0.800) |
| Grouped biologics | 0.711 (0.217 to 0.967) | 0.260 (0.023 to 0.743) |
| CTZ + MTX | 0.864 (0.722 to 0.946) | 0.462 (0.263 to 0.668) |
American College of Rheumatology: main trials
A NMA was used to compare the effects of ABT i.v. plus MTX, ADA (with and without MTX), intensive cDMARDs, ETN (with and without MTX), GOL plus MTX, IFX plus MTX, PBO, TCZ (with and without MTX), CTZ plus MTX and ABT s.c. plus MTX relative to cDMARDs on ACR response.
Data were available from 28 studies comparing two or three interventions. 57,58,62,66,69,70,74–76,79,80,84,85,89,91,92,96,99,102,105,112,115–118,121,122,124,140,160
Figure 16 presents the network of evidence and Table 35 presents the frequency with which each pair of treatments was compared. There were 13 treatment effects to estimate from 28 studies.
FIGURE 16.
American College of Rheumatology (populations 2 and 3: main trials) – network of evidence. Int, intensive; mono, monotherapy. Solid green line (two-arm study); and dotted blue line (three-arm study).

| Intervention | cDMARDs | ABT i.v. + MTX | ADA + MTX | ADA | Intensive cDMARDs | ETN + MTX | ETN | GOL + MTX | IFX + MTX | PBO | TCZ + MTX | TCZ | CTZ + MTX | ABT s.c. + MTX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cDMARDs | – | 2 | 5 | 3 | 1 | 2 | 3 | 1 | 2 | 1 | ||||
| ABT i.v. + MTX | – | – | 1 | |||||||||||
| ADA + MTX | – | – | – | 1 | ||||||||||
| ADA | – | – | – | – | 2 | 1 | ||||||||
| Intensive cDMARDs | – | – | – | – | – | 2 | ||||||||
| ETN + MTX | – | – | – | – | – | – | 2 | 1 | ||||||
| ETN | – | – | – | – | – | – | – | 1 | ||||||
| GOL + MTX | – | – | – | – | – | – | – | – | ||||||
| IFX + MTX | – | – | – | – | – | – | – | – | – | |||||
| PBO | – | – | – | – | – | – | – | – | – | – | ||||
| TCZ + MTX | – | – | – | – | – | – | – | – | – | – | – | 1 | ||
| TCZ | – | – | – | – | – | – | – | – | – | – | – | – | ||
| CTZ + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| ABT s.c. + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Figure 17 presents the effects of each intervention relative to cDMARDs on the probit scale and Figure 18 and Table 36 present the probabilities of treatment rankings.
FIGURE 17.
American College of Rheumatology (populations 2 and 3: main trials) – effects of interventions relative to cDMARDs on the probit scale. Int, intensive.
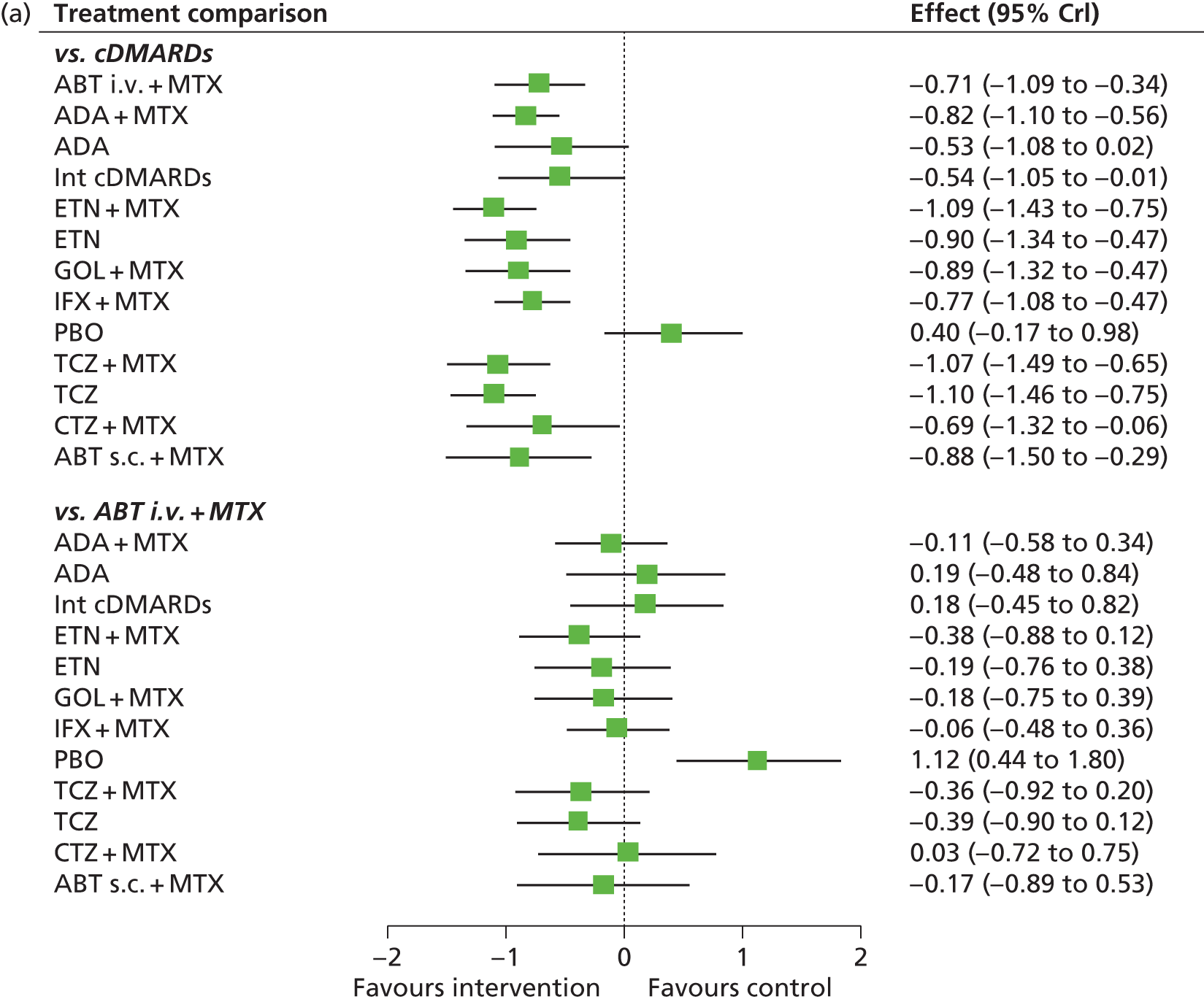
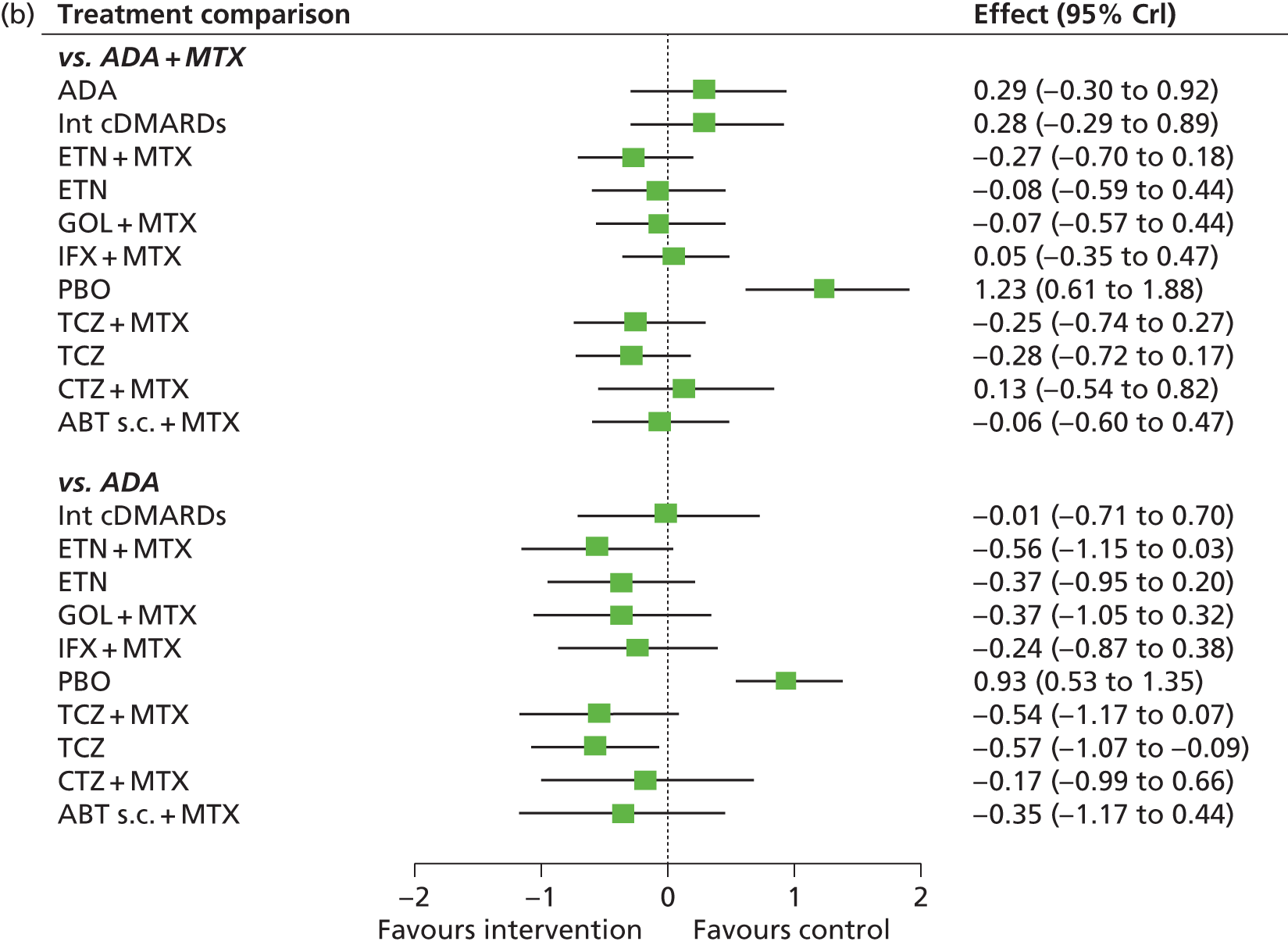
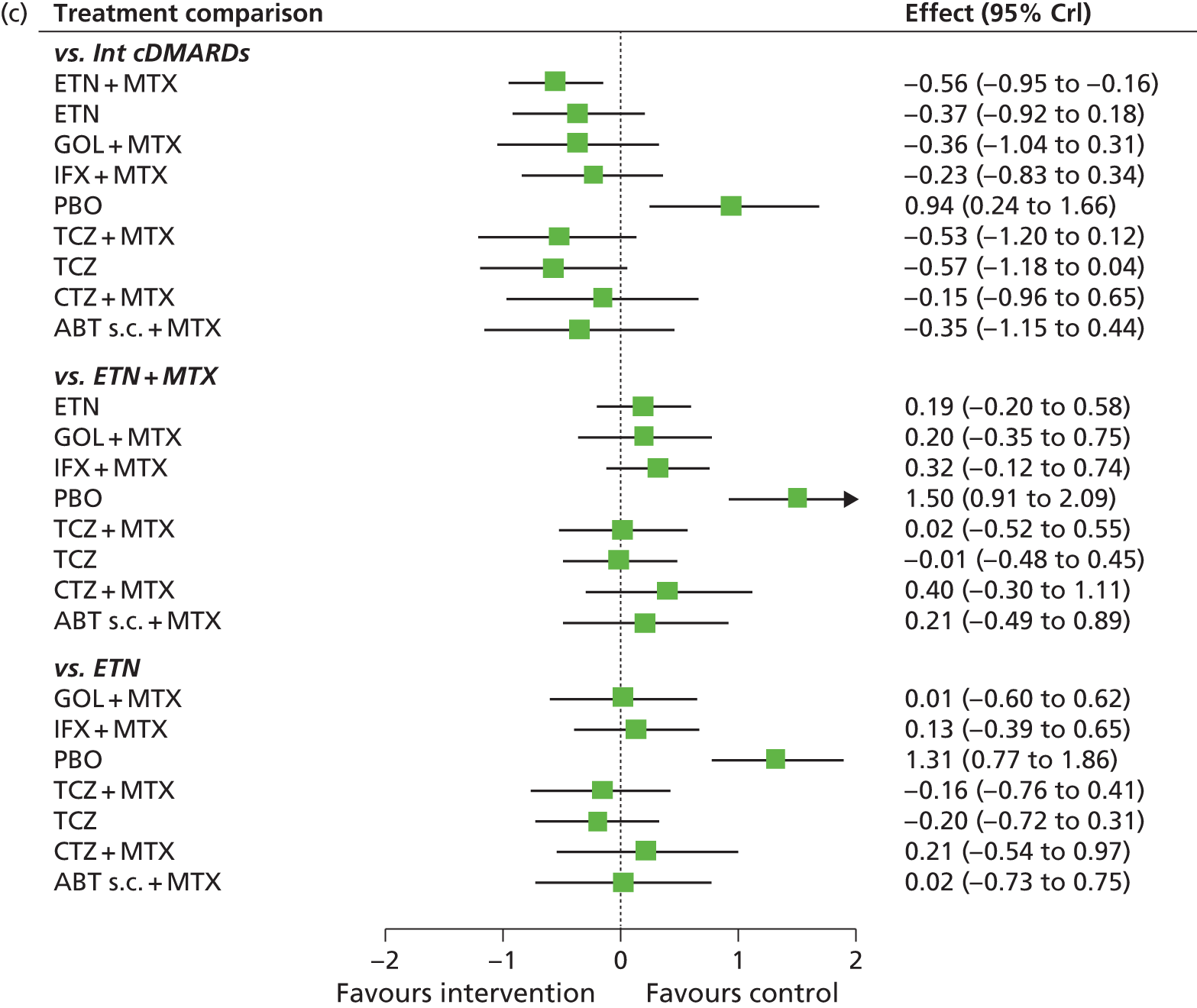

FIGURE 18.
American College of Rheumatology (populations 2 and 3: main trials) – probability of treatment rankings in terms of efficacy (most efficacious = 1). Int, intensive.



| Intervention | Rank (mean) | Rank | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| cDMARDs | 13.0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.063 | 0.859 | 0.075 |
| ABT i.v. + MTX | 8.3 | 0.007 | 0.014 | 0.025 | 0.039 | 0.062 | 0.082 | 0.108 | 0.131 | 0.166 | 0.169 | 0.130 | 0.067 | 0.001 | 0.000 |
| ADA + MTX | 6.8 | 0.004 | 0.019 | 0.040 | 0.078 | 0.122 | 0.168 | 0.181 | 0.164 | 0.117 | 0.071 | 0.029 | 0.006 | 0.000 | 0.000 |
| ADA | 10.1 | 0.003 | 0.005 | 0.011 | 0.017 | 0.029 | 0.038 | 0.049 | 0.058 | 0.085 | 0.136 | 0.230 | 0.312 | 0.027 | 0.000 |
| Intensive cDMARDs | 10.0 | 0.001 | 0.005 | 0.010 | 0.016 | 0.026 | 0.037 | 0.050 | 0.065 | 0.090 | 0.144 | 0.242 | 0.291 | 0.022 | 0.003 |
| ETN + MTX | 3.1 | 0.247 | 0.210 | 0.196 | 0.144 | 0.089 | 0.054 | 0.031 | 0.017 | 0.008 | 0.003 | 0.001 | 0.000 | 0.000 | 0.000 |
| ETN | 5.7 | 0.045 | 0.086 | 0.103 | 0.136 | 0.136 | 0.118 | 0.101 | 0.094 | 0.086 | 0.064 | 0.027 | 0.005 | 0.000 | 0.000 |
| GOL + MTX | 5.8 | 0.067 | 0.079 | 0.098 | 0.113 | 0.124 | 0.118 | 0.103 | 0.094 | 0.076 | 0.065 | 0.042 | 0.019 | 0.000 | 0.000 |
| IFX + MTX | 7.8 | 0.005 | 0.013 | 0.030 | 0.055 | 0.084 | 0.119 | 0.147 | 0.168 | 0.166 | 0.126 | 0.069 | 0.019 | 0.000 | 0.000 |
| PBO | 13.9 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.008 | 0.071 | 0.919 |
| TCZ + MTX | 3.6 | 0.213 | 0.202 | 0.168 | 0.133 | 0.093 | 0.063 | 0.049 | 0.033 | 0.023 | 0.015 | 0.007 | 0.002 | 0.000 | 0.000 |
| TCZ | 3.0 | 0.239 | 0.251 | 0.192 | 0.131 | 0.080 | 0.047 | 0.028 | 0.017 | 0.010 | 0.004 | 0.001 | 0.000 | 0.000 | 0.000 |
| CTZ + MTX | 8.2 | 0.044 | 0.037 | 0.043 | 0.049 | 0.060 | 0.067 | 0.069 | 0.081 | 0.096 | 0.124 | 0.149 | 0.161 | 0.015 | 0.003 |
| ABT s.c. + MTX | 6.0 | 0.125 | 0.081 | 0.083 | 0.090 | 0.095 | 0.089 | 0.083 | 0.079 | 0.077 | 0.078 | 0.069 | 0.047 | 0.003 | 0.001 |
The model fitted the data well, with the total residual deviance, 185.61, close to the total number of data points, 174, included in the analysis. The largest residual deviances were 7.24 and 3.86 from O’Dell et al. ,111 and 4.99 from the Anti-TNF factor Research study program of the Monoclonal antibody ADalimumab (D2E7) in rheumatoid Arthritis (ARMADA) study. 69
The between-study SD was estimated to be 0.24 (95% CrI 0.14 to 0.40), which implies mild heterogeneity between studies in intervention effects.
All interventions except for PBO were associated with beneficial treatment effects relative to cDMARDs with the greatest effects being associated with ETN plus MTX and TCZ (with and without MTX). The treatment effects were statistically significant for all interventions except for ADA and PBO at a conventional 5% level. There was insufficient evidence to differentiate between treatments, although TCZ (mean rank 3.0; probability of being the best 0.234), ETN plus MTX (mean rank 3.1; probability of being the best 0.247) and TCZ plus MTX (mean rank 3.6; probability of being the best 0.213) were the treatments that were most likely to be the most effective interventions.
A meta-analysis was used to estimate the proportion of patients experiencing an ACR ‘no response’ when treated with cDMARDs.
Data were available from 18 studies. 62,69,70,74–76,79,84,89,91,92,96,99,115–118,121,124,160
The model fitted the data well, with the total residual deviance, 18.70, close to the total number of data points, 18, included in the analysis.
The between-study SD was estimated to be 0.23 (95% CrI 0.14 to 0.38), which implies mild heterogeneity between studies in the baseline response.
Table 37 presents the probabilities of achieving at least an ACR20, an ACR50 and an ACR70 response. These are derived by combining the treatment effects estimated from the NMA with the estimate of the cDMARDs ‘no response’ rate.
| Intervention | At least ACR20 (95% CrI) | At least ACR50 (95% CrI) | At least ACR70 (95% CrI) |
|---|---|---|---|
| cDMARDs | 0.298 (0.255 to 0.344) | 0.123 (0.098 to 0.1530 | 0.042 (0.031 to 0.056) |
| ABT i.v. + MTX | 0.573 (0.418 to 0.719) | 0.328 (0.200 to 0.480) | 0.156 (0.079 to 0.268) |
| ADA + MTX | 0.615 (0.500 to 0.726) | 0.368 (0.263 to 0.489) | 0.183 (0.115 to 0.276) |
| ADA | 0.499 (0.286 to 0.712) | 0.264 (0.116 to 0.472) | 0.115 (0.039 to 0.263) |
| Intensive cDMARDs | 0.503 (0.293 to 0.704) | 0.266 (0.120 to 0.462) | 0.117 (0.041 to 0.254) |
| ETN + MTX | 0.713 (0.576 to 0.823) | 0.472 (0.330 to 0.617) | 0.263 (0.157 to 0.394) |
| ETN | 0.645 (0.467 to 0.798) | 0.398 (0.237 to 0.580) | 0.205 (0.100 to 0.359) |
| GOL + MTX | 0.642 (0.469 to 0.793) | 0.395 (0.239 to 0.573) | 0.202 (0.101 to 0.351) |
| IFX + MTX | 0.595 (0.466 to 0.718) | 0.348 (0.236 to 0.479) | 0.169 (0.099 to 0.268) |
| PBO | 0.175 (0.063 to 0.362) | 0.059 (0.015 to 0.163) | 0.016 (0.003 to 0.061) |
| TCZ + MTX | 0.706 (0.542 to 0.837) | 0.464 (0.299 to 0.638) | 0.256 (0.136 to 0.415) |
| TCZ | 0.717 (0.578 to 0.830) | 0.477 (0.332 to 0.627) | 0.266 (0.159 to 0.405) |
| CTZ + MTX | 0.564 (0.314 to 0.785) | 0.319 (0.133 to 0.563) | 0.150 (0.046 to 0.341) |
| ABT s.c. + MTX | 0.638 (0.400 to 0.837) | 0.391 (0.188 to 0.637) | 0.199 (0.073 to 0.415) |
American College of Rheumatology: main trials plus prior biologics with AMBITION
A NMA was used to compare the effects of ABT i.v. plus MTX, ADA (with and without MTX), intensive cDMARDs, ETN (with and without MTX), GOL plus MTX, IFX plus MTX, PBO, TCZ (with and without MTX), CTZ plus MTX, ABT s.c. plus MTX, TOF (5-mg and 10-mg doses) and MTX relative to cDMARDs on ACR response.
Data were available from 40 studies comparing two, three or four interventions. 55,57,58,62,66,69,70,74–76,79,80,84,85,89,91,92,96,99,102,105,112,115–118,121,122,124,127,129–138,140,160
Figure 19 presents the network of evidence and Table 38 presents the frequency with which each pair of treatments was compared. There were 15 treatment effects to estimate from 40 studies. 55,57,58,62,66,69,70,74–76,79,80,84,85,89,91,92,96,99,102,105,112,115–118,121,122,124,127,129–138,140,160
FIGURE 19.
American College of Rheumatology (populations 2 and 3: main trials + prior biologics with AMBITION55) – network of evidence. Int, intensive. Solid green line (two-arm study); dotted blue line (three-arm study); and dotted green line (four-arm study).

| Intervention | cDMARDs | ABT i.v. + MTX | ADA + MTX | ADA | Intensive cDMARDs | ETN + MTX | ETN | GOL + MTX | IFX + MTX | PBO | TCZ + MTX | TCZ | CTZ + MTX | ABA s.c. + MTX | TOF5 + MTX | TOF10 + MTX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cDMARDs | – | 3 | 6 | 3 | 1 | 2 | 3 | 3 | 3 | 5 | 3 | 3 | ||||
| ABT i.v. + MTX | – | – | 1 | 1 | ||||||||||||
| ADA + MTX | – | – | – | 1 | 1 | 1 | ||||||||||
| ADA | – | – | – | – | 2 | 1 | ||||||||||
| Intensive cDMARDs | – | – | – | – | – | 2 | ||||||||||
| ETN + MTX | – | – | – | – | – | – | 2 | 1 | ||||||||
| ETN | – | – | – | – | – | – | – | 1 | ||||||||
| GOL + MTX | – | – | – | – | – | – | – | – | ||||||||
| IFX + MTX | – | – | – | – | – | – | – | – | – | |||||||
| PBO | – | – | – | – | – | – | – | – | – | – | ||||||
| TCZ + MTX | – | – | – | – | – | – | – | – | – | – | – | 1 | ||||
| TCZ | – | – | – | – | – | – | – | – | – | – | – | – | ||||
| CTZ + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| ABT s.c. + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| TOF5 + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3 |
| TOF10 + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Figure 20 presents the effects of each intervention relative to cDMARDs on the probit scale and Figure 21 and Table 39 present the probabilities of treatment rankings.
FIGURE 20.
American College of Rheumatology (populations 2 and 3: main trials + prior biologics with AMBITION55) – effects of interventions relative to cDMARDs on the probit scale. Int, intensive.


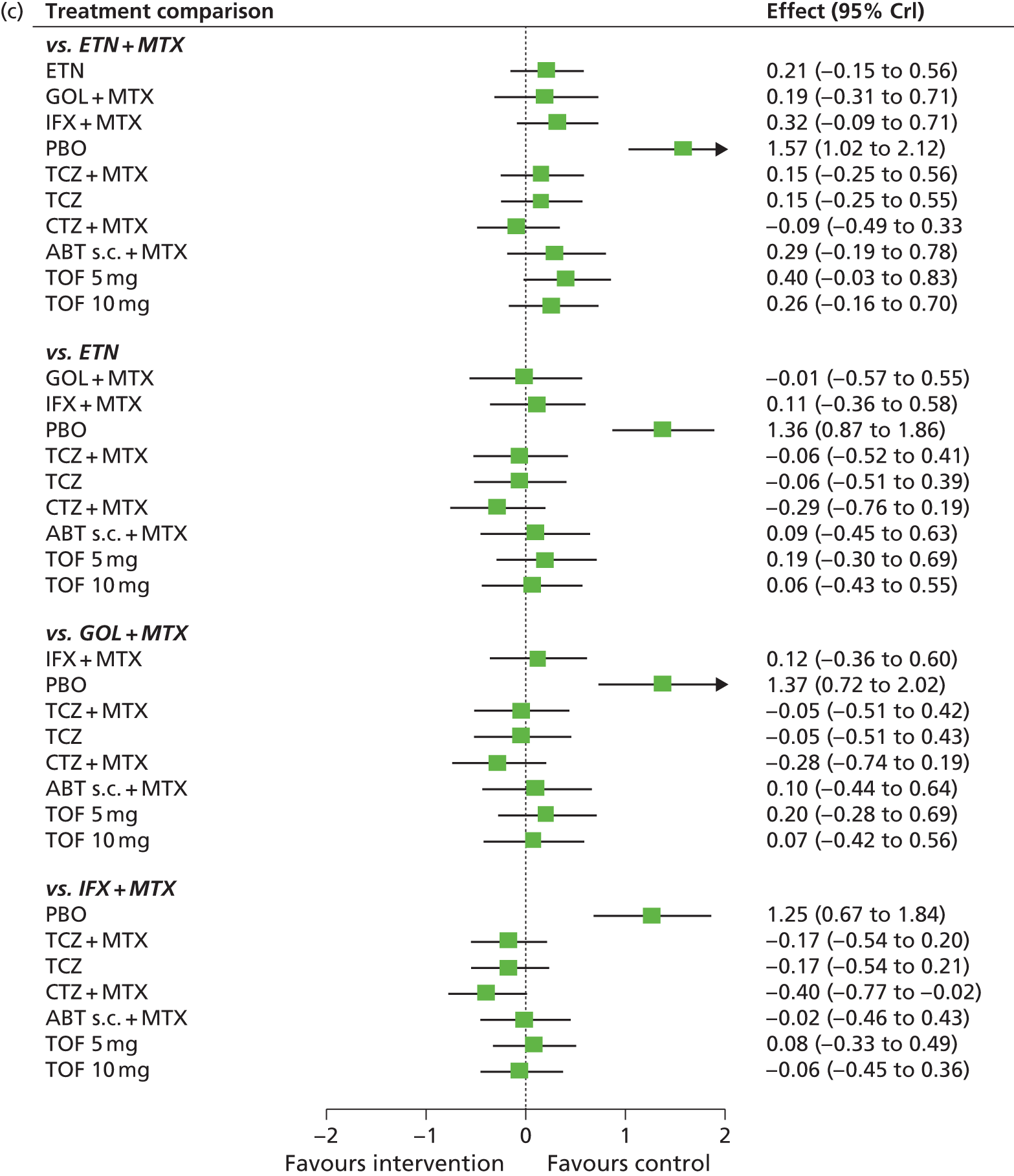
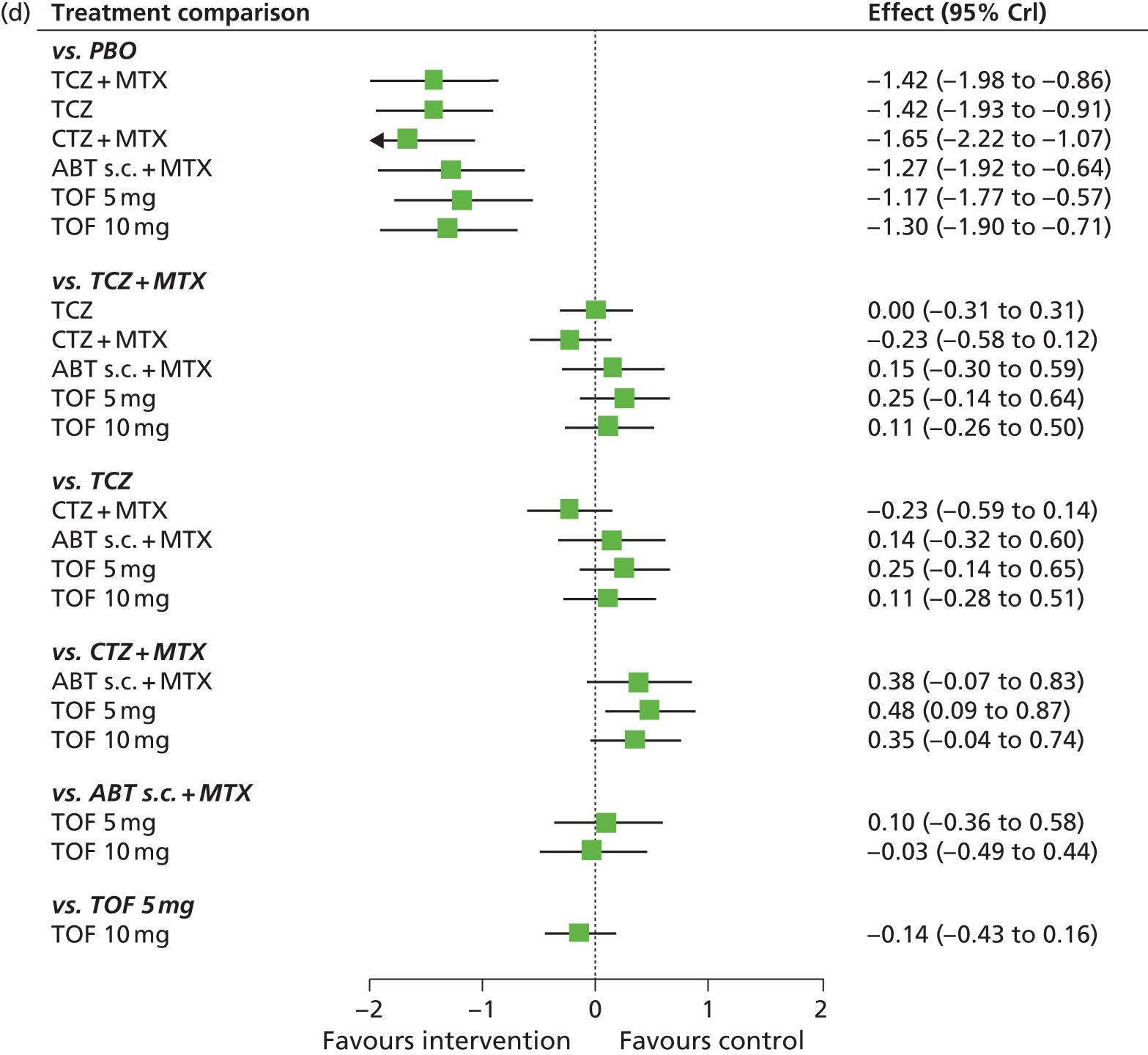
FIGURE 21.
American College of Rheumatology (populations 2 and 3: main trials + prior biologics with AMBITION55) – probability of treatment rankings in terms of efficacy (most efficacious = 1). Int, intensive.
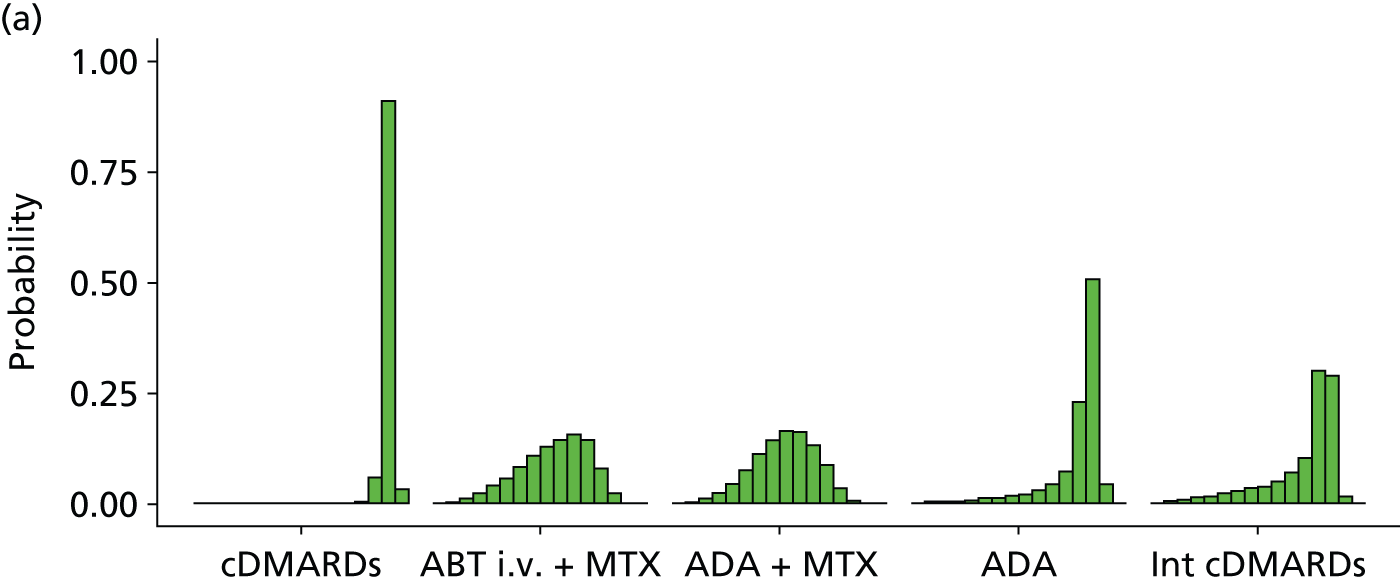
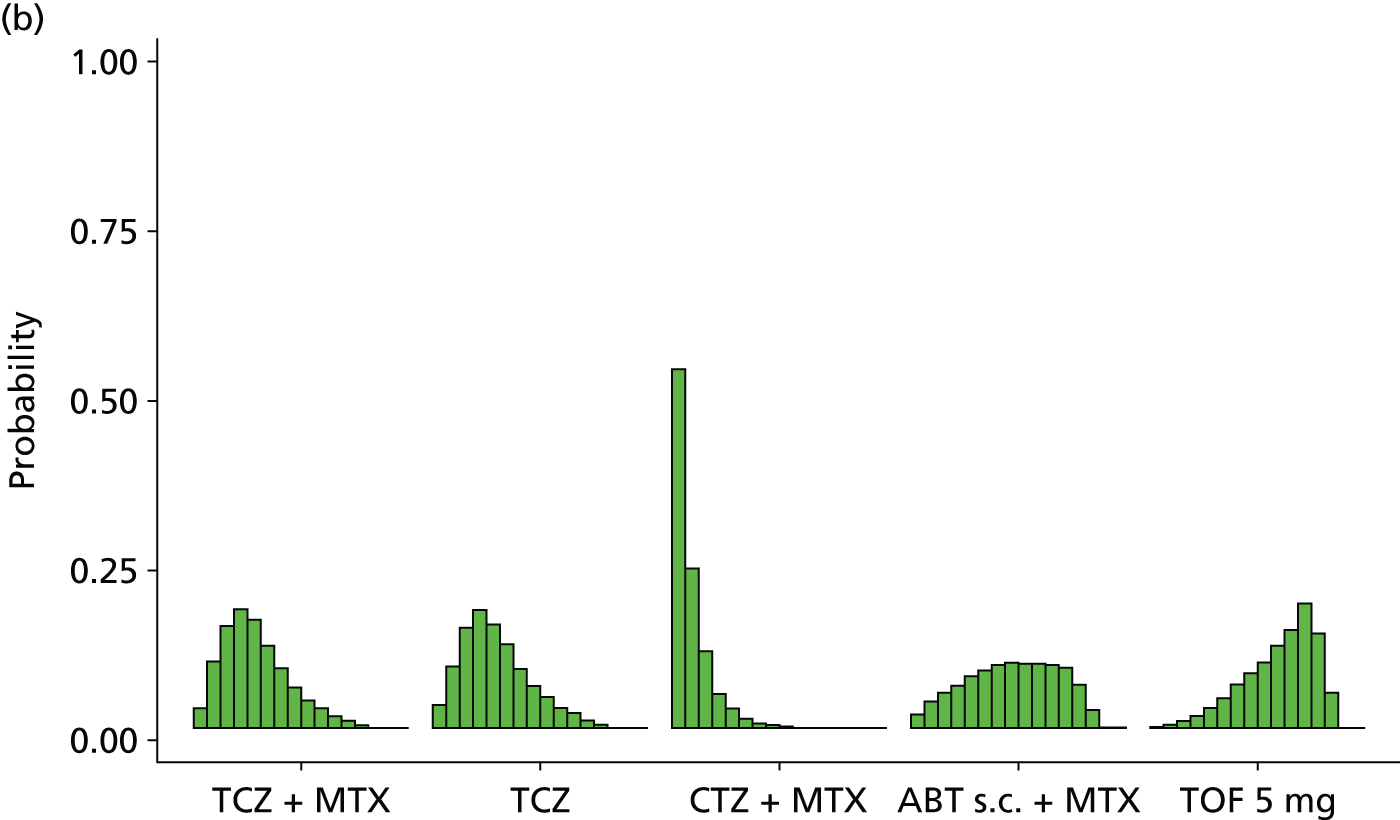
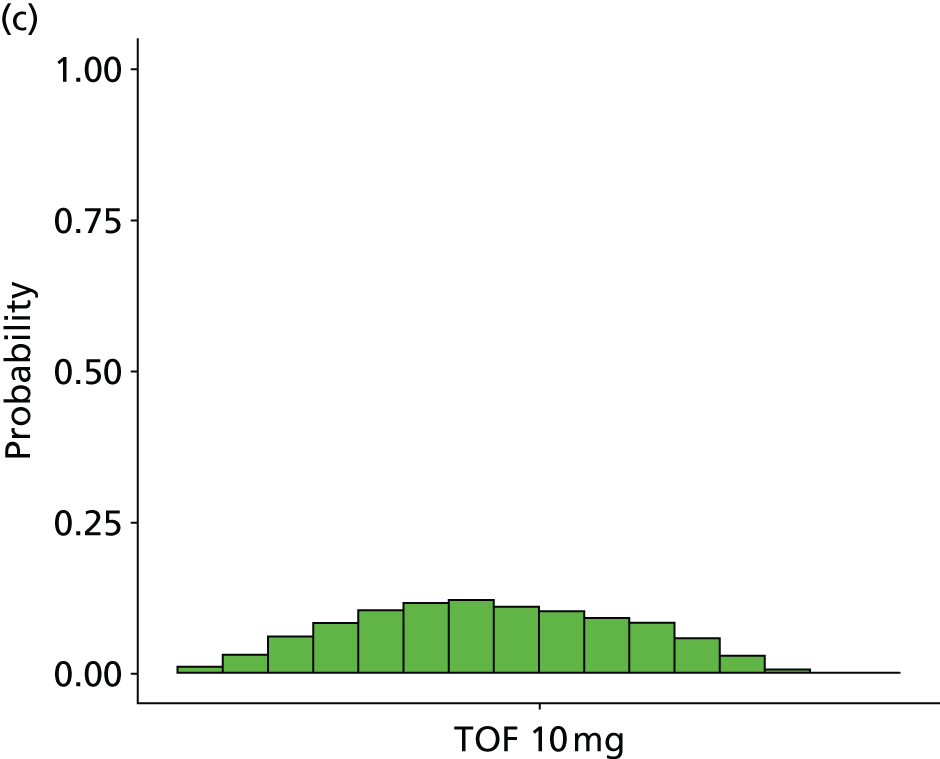
| Intervention | Rank (mean) | Rank | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| cDMARDs | 15.0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.058 | 0.909 | 0.032 |
| ABT i.v. + MTX | 9.5 | 0.001 | 0.004 | 0.013 | 0.024 | 0.039 | 0.057 | 0.082 | 0.107 | 0.129 | 0.144 | 0.154 | 0.145 | 0.078 | 0.023 | 0.000 | 0.000 |
| ADA + MTX | 8.9 | 0.001 | 0.004 | 0.010 | 0.023 | 0.043 | 0.076 | 0.112 | 0.143 | 0.165 | 0.162 | 0.132 | 0.088 | 0.035 | 0.006 | 0.000 | 0.000 |
| ADA | 12.9 | 0.001 | 0.001 | 0.003 | 0.004 | 0.007 | 0.010 | 0.013 | 0.018 | 0.021 | 0.030 | 0.044 | 0.073 | 0.228 | 0.505 | 0.043 | 0.000 |
| Intensive cDMARDs | 11.8 | 0.000 | 0.004 | 0.009 | 0.014 | 0.018 | 0.022 | 0.030 | 0.035 | 0.039 | 0.051 | 0.070 | 0.104 | 0.299 | 0.288 | 0.016 | 0.001 |
| ETN + MTX | 2.9 | 0.263 | 0.295 | 0.165 | 0.103 | 0.067 | 0.042 | 0.026 | 0.017 | 0.011 | 0.006 | 0.004 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 |
| ETN | 6.4 | 0.037 | 0.077 | 0.122 | 0.110 | 0.104 | 0.098 | 0.088 | 0.075 | 0.067 | 0.061 | 0.060 | 0.072 | 0.026 | 0.004 | 0.000 | 0.000 |
| GOL + MTX | 6.3 | 0.063 | 0.096 | 0.107 | 0.105 | 0.102 | 0.096 | 0.083 | 0.070 | 0.064 | 0.056 | 0.056 | 0.054 | 0.035 | 0.014 | 0.000 | 0.000 |
| IFX + MTX | 8.6 | 0.003 | 0.011 | 0.029 | 0.049 | 0.066 | 0.091 | 0.105 | 0.117 | 0.120 | 0.116 | 0.115 | 0.106 | 0.056 | 0.016 | 0.000 | 0.000 |
| PBO | 16.0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.032 | 0.967 |
| TCZ + MTX | 5.1 | 0.030 | 0.100 | 0.153 | 0.179 | 0.162 | 0.123 | 0.089 | 0.061 | 0.041 | 0.030 | 0.018 | 0.010 | 0.003 | 0.001 | 0.000 | 0.000 |
| TCZ | 5.2 | 0.033 | 0.093 | 0.152 | 0.176 | 0.154 | 0.126 | 0.088 | 0.064 | 0.046 | 0.030 | 0.022 | 0.012 | 0.004 | 0.000 | 0.000 | 0.000 |
| CTZ + MTX | 1.9 | 0.538 | 0.239 | 0.115 | 0.051 | 0.029 | 0.014 | 0.007 | 0.004 | 0.002 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| ABT s.c. + MTX | 8.0 | 0.020 | 0.040 | 0.054 | 0.063 | 0.076 | 0.086 | 0.094 | 0.098 | 0.096 | 0.097 | 0.094 | 0.090 | 0.065 | 0.027 | 0.000 | 0.000 |
| TOF5 + MTX | 10.2 | 0.001 | 0.004 | 0.009 | 0.017 | 0.029 | 0.045 | 0.064 | 0.082 | 0.097 | 0.123 | 0.147 | 0.187 | 0.142 | 0.053 | 0.000 | 0.000 |
| TOF10 + MTX | 7.4 | 0.010 | 0.032 | 0.060 | 0.083 | 0.103 | 0.115 | 0.120 | 0.110 | 0.102 | 0.091 | 0.083 | 0.058 | 0.027 | 0.006 | 0.000 | 0.000 |
There was some suggestion that model was not a good fit to all of the data, with the total residual deviance, 291.84, being larger than the total number of data points, 250, included in the analysis. The largest residual deviances, 14.21 and 14.70, were from Kremer et al. ,137 which included patients who received prior biologics, and were from the cDMARDs arm, in which only one patient had an ACR20 response and two patients had an ACR50 response. The next largest residual deviances were 5.92 and 4.04 from the O’Dell et al. study111 and 3.95 from the JESMR study. 140
The between-study SD was estimated to be 0.21 (95% CrI 0.14 to 0.32), which implies mild heterogeneity between studies in intervention effects. The addition of the AMBITION study55 and studies in which patients had received prior biologics reduced the point estimate and the uncertainty in the between study SD.
All interventions except for PBO were associated with beneficial treatment effects relative to cDMARDs with the greatest effects being associated with CTZ plus MTX and ETN plus MTX. The treatment effects were statistically significant for all interventions except for ADA and PBO at a conventional 5% level. There was insufficient evidence to differentiate between treatments, although CTZ plus MTX (mean rank 1.9; probability of being the best 0.538) and ETN plus MTX (mean rank 2.9; probability of being the best 0.263) were the treatments that were most likely to be the most effective interventions. The inclusion of the additional studies has had a small impact on six of the treatment effects. However, the effects of ADA (with and without MTX), TCZ (with and without MTX), ABT s.c. plus MTX and PBO were smaller, and the effect of CTZ plus MTX were larger relative to cDMARDs.
A meta-analysis was used to estimate the proportion of patients experiencing an ACR ‘no response’ when treated with cDMARDs.
Data were available from 29 studies. 55,62,69,70,74–76,79,84,89,91,92,96,99,115–118,121,124,128–138,160
The model fitted the data well, with the total residual deviance, 29.14, close to the total number of data points, 29, included in the analysis.
The between-study SD was estimated to be 0.27 (95% CrI 0.19 to 0.38), which implies mild heterogeneity between studies in the baseline response.
Table 40 presents the probabilities of achieving at least an ACR20, an ACR50 and an ACR70 response. These are derived by combining the treatment effects estimated from the NMA with the estimate of the cDMARDs ‘no response’ rate.
| Intervention | At least ACR20 (95% CrI) | At least ACR50 (95% CrI) | At least ACR70 (95% CrI) |
|---|---|---|---|
| cDMARDs | 0.279 (0.242 to 0.318) | 0.117 (0.095 to 0.142) | 0.038 (0.029 to 0.049) |
| ABT i.v. + MTX | 0.556 (0.444 to 0.664) | 0.321 (0.228 to 0.428) | 0.148 (0.092 to 0.223) |
| ADA + MTX | 0.568 (0.475 to 0.659) | 0.332 (0.252 to 0.424) | 0.155 (0.106 to 0.220) |
| ADA | 0.432 (0.253 to 0.625) | 0.219 (0.102 to 0.387) | 0.088 (0.032 to 0.194) |
| Intensive cDMARDs | 0.475 (0.290 to 0.667) | 0.253 (0.123 to 0.432) | 0.106 (0.041 to 0.226) |
| ETN + MTX | 0.690 (0.563 to 0.800) | 0.457 (0.328 to 0.593) | 0.246 (0.152 to 0.365) |
| ETN | 0.616 (0.452 to 0.761) | 0.378 (0.233 to 0.542) | 0.187 (0.095 to 0.317) |
| GOL + MTX | 0.619 (0.460 to 0.759) | 0.381 (0.240 to 0.540) | 0.189 (0.099 to 0.316) |
| IFX + MTX | 0.572 (0.453 to 0.683) | 0.336 (0.234 to 0.451) | 0.158 (0.096 to 0.241) |
| PBO | 0.143 (0.054 to 0.293) | 0.047 (0.014 to 0.126) | 0.012 (0.003 to 0.042) |
| TCZ + MTX | 0.637 (0.532 to 0.734) | 0.400 (0.299 to 0.508) | 0.202 (0.134 to 0.288) |
| TCZ | 0.636 (0.524 to 0.758) | 0.399 (0.292 to 0.513) | 0.201 (0.130 to 0.292) |
| CTZ + MTX | 0.721 (0.620 to 0.804) | 0.492 (0.381 to 0.599) | 0.274 (0.189 to 0.371) |
| ABT s.c. + MTX | 0.580 (0.428 to 0.723) | 0.344 (0.215 to 0.496) | 0.163 (0.085 to 0.278) |
| TOF5 + MTX | 0.541 (0.413 to 0.660) | 0.308 (0.204 to 0.425) | 0.139 (0.080 to 0.220) |
| TOF10 + MTX | 0.593 (0.469 to 0.708) | 0.356 (0.246 to 0.478) | 0.171 (0.103 to 0.262) |
American College of Rheumatology: main trials plus prior biologics without AMBITION
A NMA was used to compare the effects of ABT i.v. plus MTX, ADA (with and without MTX), intensive cDMARDs, ETN (with and without MTX), GOL plus MTX, IFX plus MTX, PBO, TCZ (with and without MTX), CTZ plus MTX, ABT s.c. plus MTX and TOF plus MTX (5-mg and 10-mg doses) relative to cDMARDs on ACR response.
Data were available from 39 studies comparing two, three or four interventions. 57,58,62,66,69,70,74–76,79,80,84,85,89,91,92,96,99,102,105,112,115–118,121,122,124,127,129–138,140,160
Figure 22 presents the network of evidence and Table 41 presents the frequency with which each pair of treatments was compared. There were 15 treatment effects to estimate from 39 studies.
FIGURE 22.
American College of Rheumatology (populations 2 and 3: main trials + prior biologics without AMBITION55) – network of evidence. Int, intensive. Solid green line (two-arm study); and dotted blue line (three-arm study).
| Intervention | cDMARDs | ABT i.v. + MTX | ADA + MTX | ADA | Intensive cDMARDs | ETN + MTX | ETN | GOL + MTX | IFX + MTX | PBO | TCZ + MTX | TCZ | CTZ + MTX | ABT s.c. + MTX | TOF5 + MTX | TOF10 + MTX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cDMARDs | – | 3 | 6 | 3 | 1 | 2 | 3 | 3 | 2 | 5 | 3 | 3 | ||||
| ABT i.v. + MTX | – | – | 1 | 1 | ||||||||||||
| ADA + MTX | – | – | – | 1 | 1 | 1 | ||||||||||
| ADA | – | – | – | – | 2 | 1 | ||||||||||
| Intensive cDMARDs | – | – | – | – | – | 2 | ||||||||||
| ETN + MTX | – | – | – | – | – | – | 2 | 1 | ||||||||
| ETN | – | – | – | – | – | – | – | 1 | ||||||||
| GOL + MTX | – | – | – | – | – | – | – | – | ||||||||
| IFX + MTX | – | – | – | – | – | – | – | – | – | |||||||
| PBO | – | – | – | – | – | – | – | – | – | – | ||||||
| TCZ + MTX | – | – | – | – | – | – | – | – | – | – | – | 1 | ||||
| TCZ | – | – | – | – | – | – | – | – | – | – | – | – | ||||
| CTZ + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| ABT s.c. + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| TOF5 + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3 |
| TOF10 + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Figure 23 presents the effects of each intervention relative to cDMARDs on the probit scale and Figure 24 and Table 42 present the probabilities of treatment rankings.
FIGURE 23.
American College of Rheumatology (populations 2 and 3: main trials + prior biologics without AMBITION55) – effects of interventions relative to cDMARDs on the probit scale. Int, intensive.
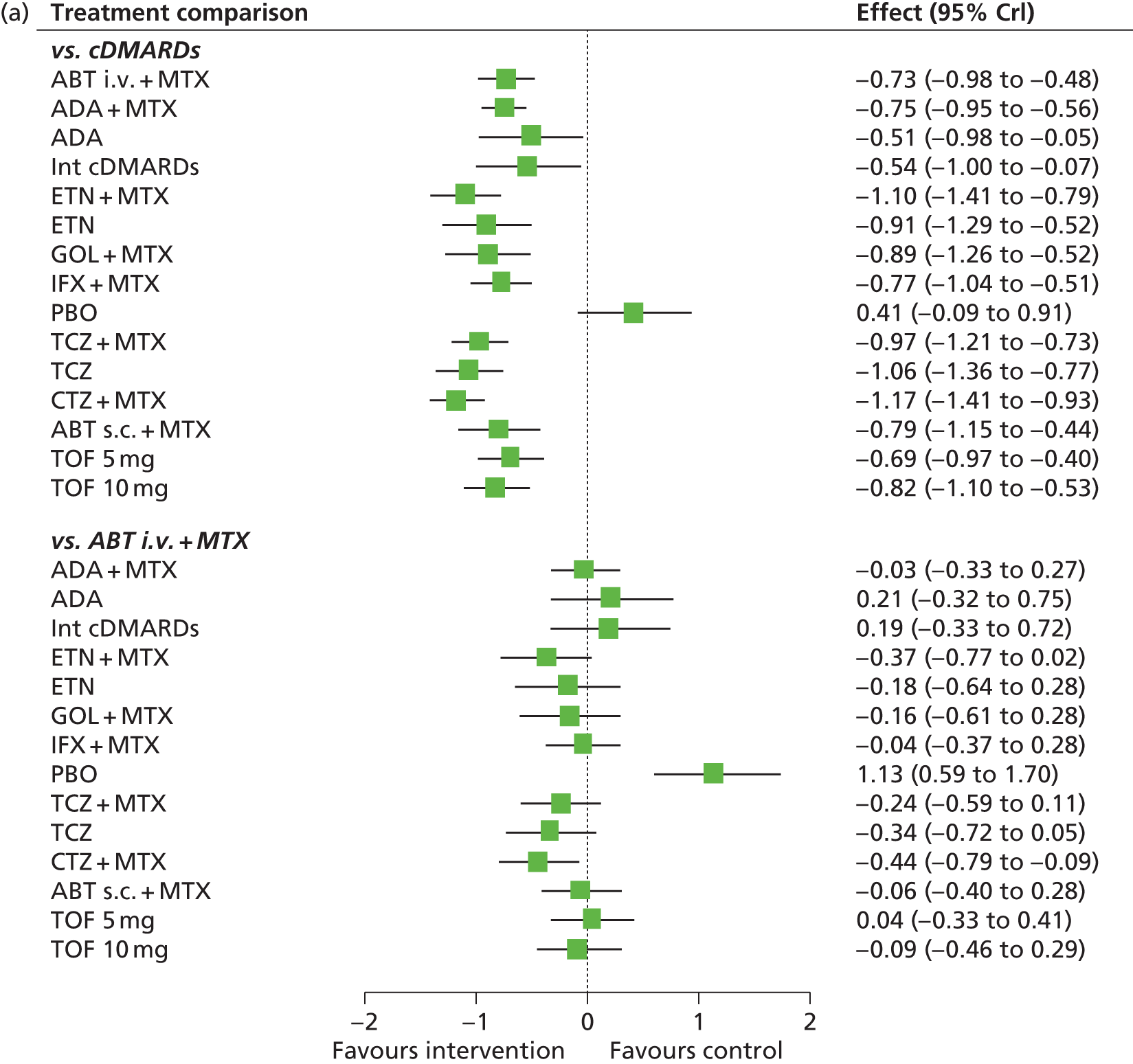

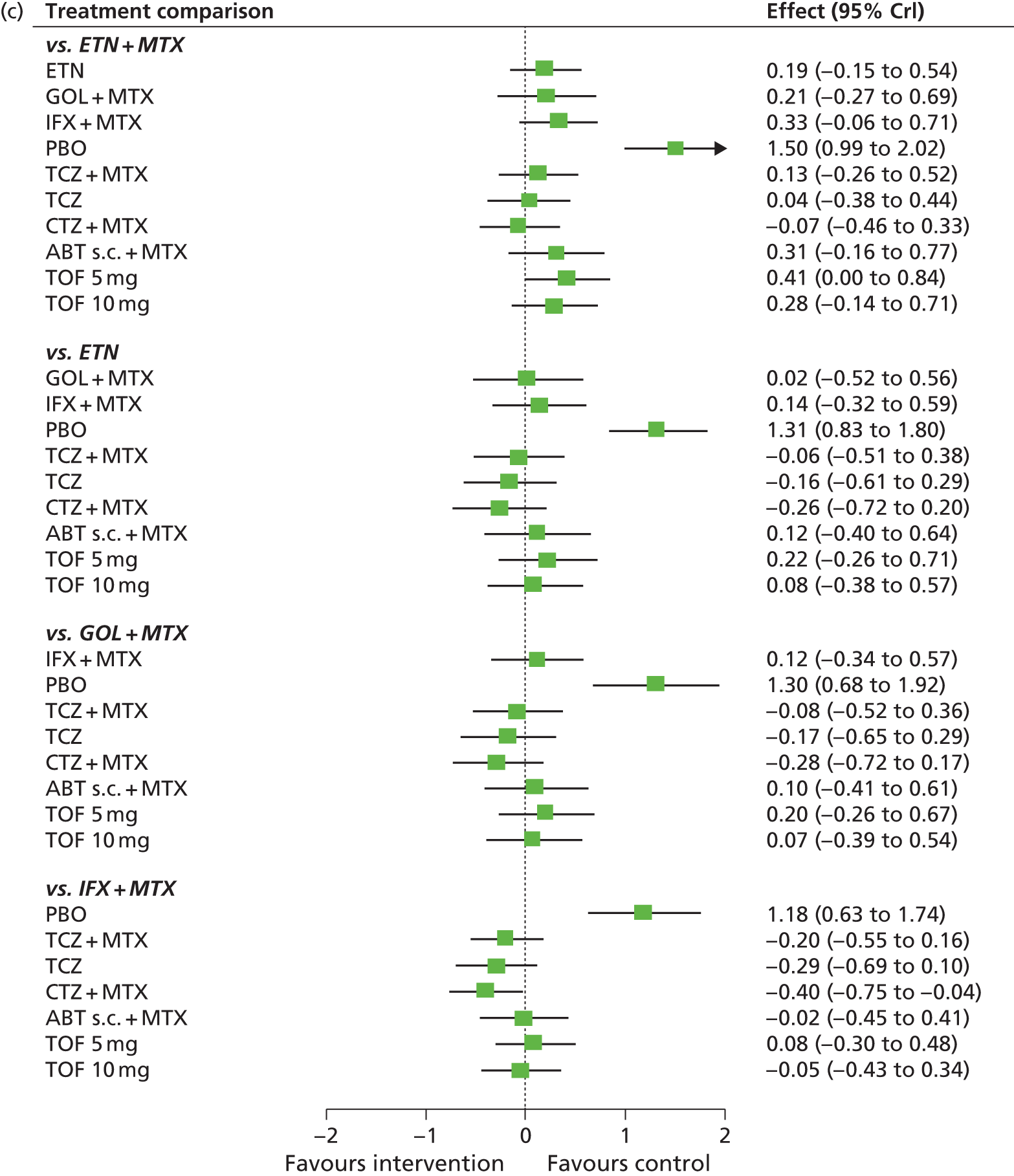
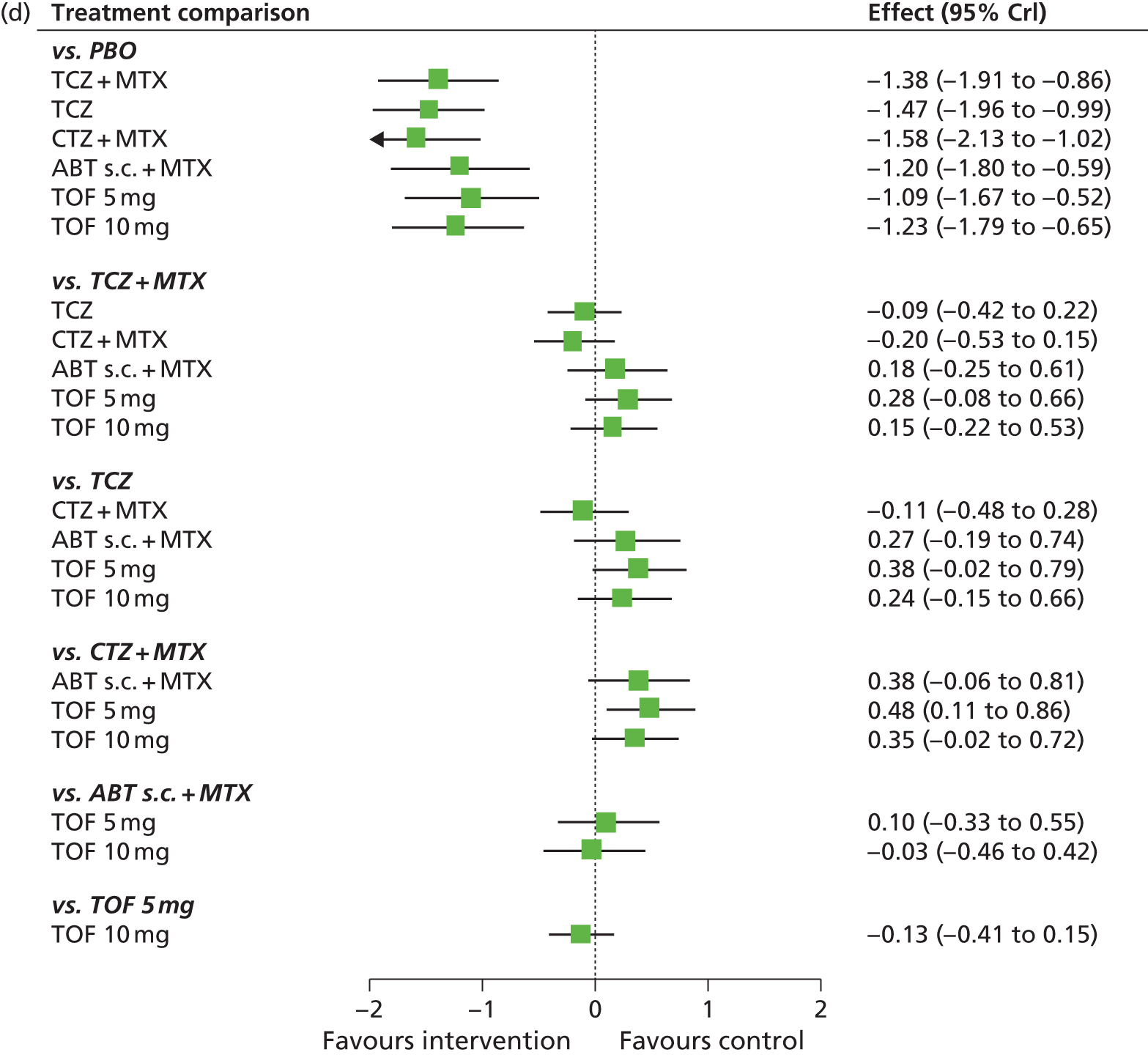
FIGURE 24.
American College of Rheumatology (populations 2 and 3: main trials + prior biologics without AMBITION55) – probability of treatment rankings in terms of efficacy (most efficacious = 1). Int, intensive.

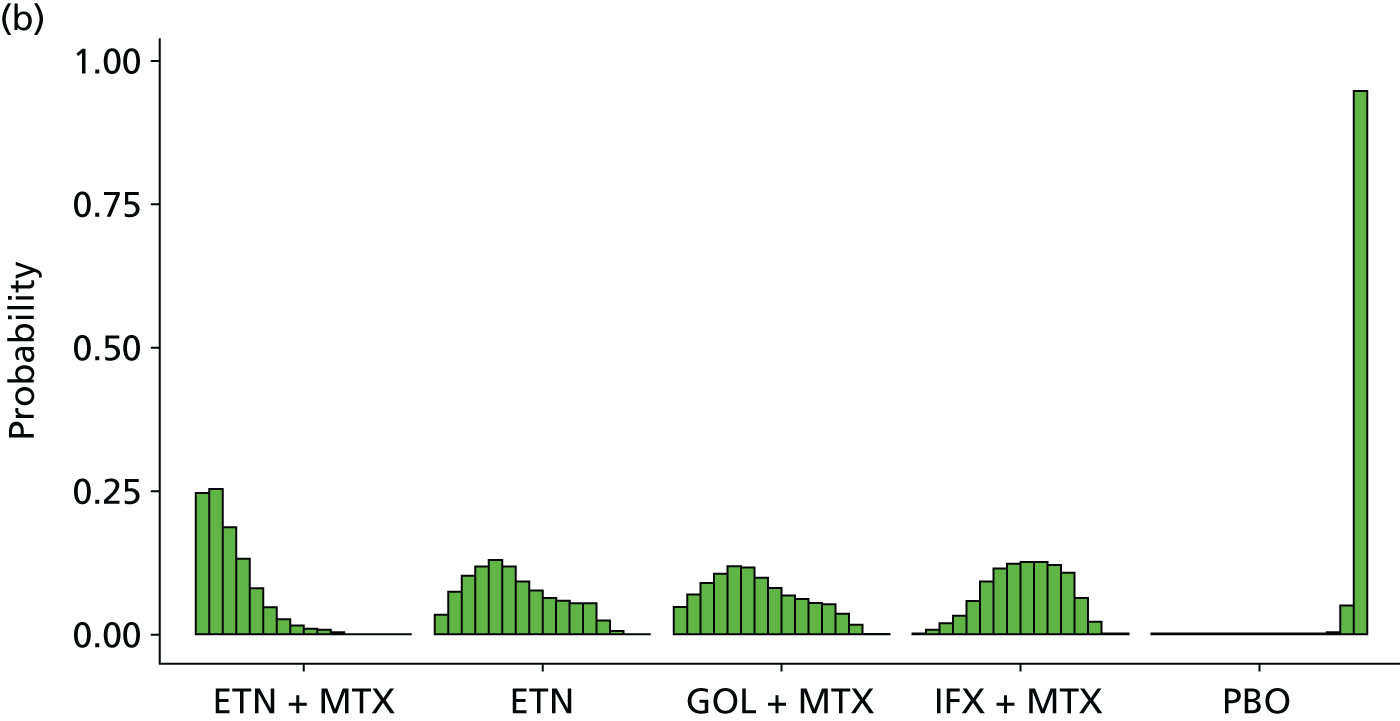
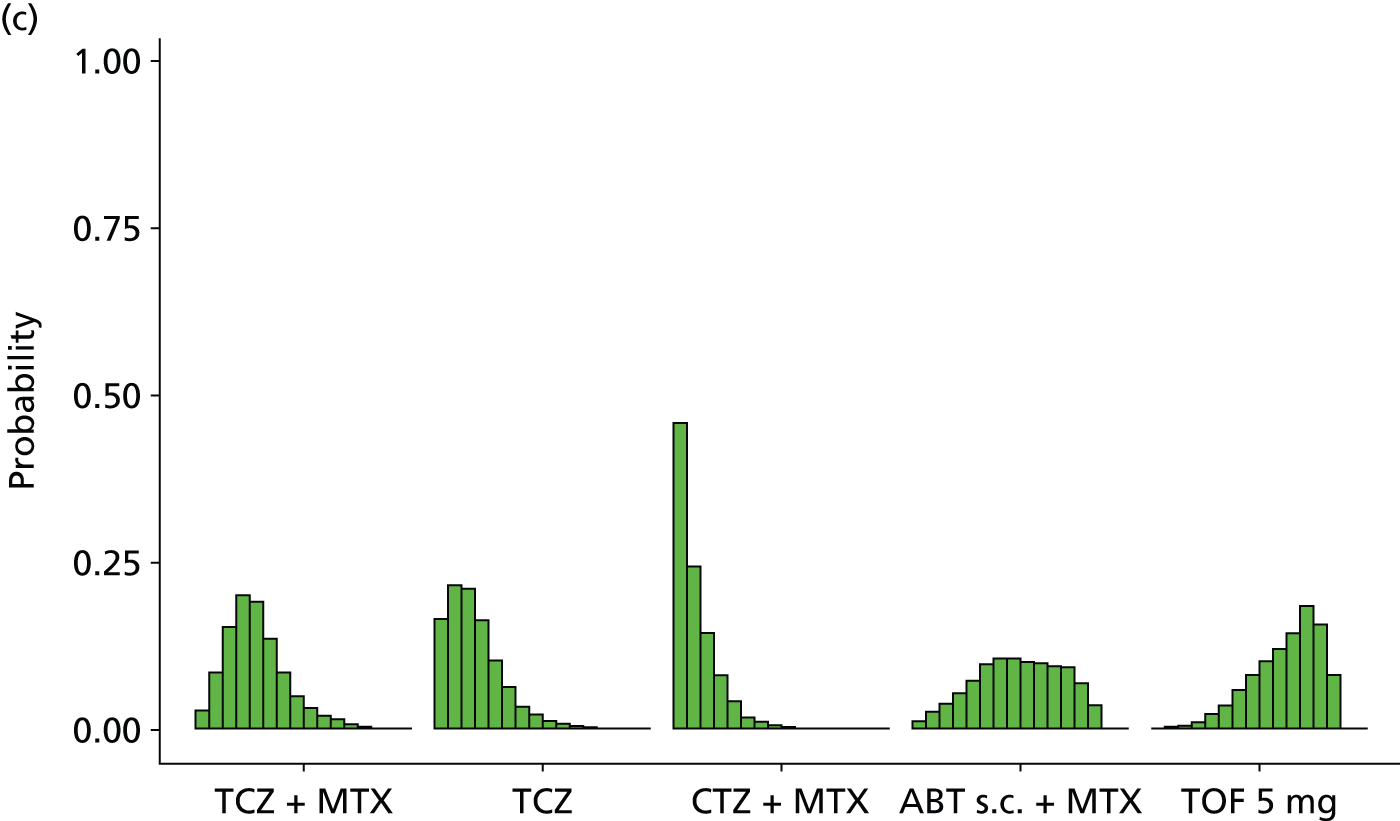
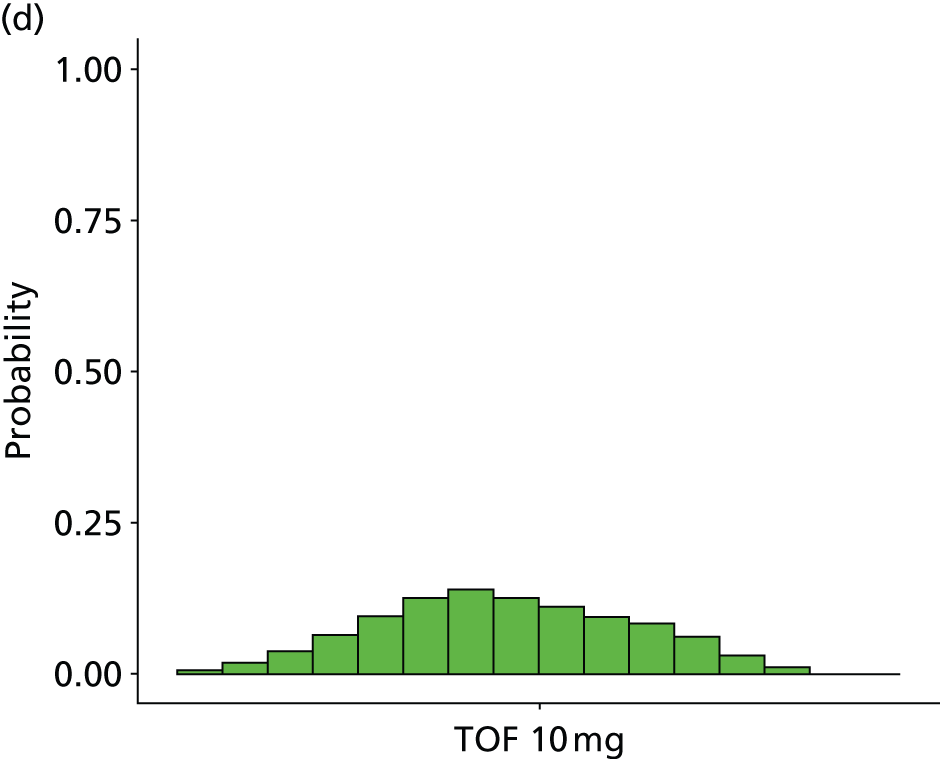
| Intervention | Rank (mean) | Rank | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| cDMARDs | 15.0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.027 | 0.921 | 0.051 |
| ABT i.v. + MTX | 9.7 | 0.000 | 0.002 | 0.007 | 0.015 | 0.030 | 0.051 | 0.079 | 0.109 | 0.133 | 0.146 | 0.158 | 0.148 | 0.089 | 0.032 | 0.000 | 0.000 |
| ADA + MTX | 9.2 | 0.000 | 0.001 | 0.005 | 0.013 | 0.031 | 0.061 | 0.103 | 0.146 | 0.167 | 0.168 | 0.148 | 0.102 | 0.045 | 0.011 | 0.000 | 0.000 |
| ADA | 12.2 | 0.001 | 0.002 | 0.004 | 0.007 | 0.013 | 0.020 | 0.028 | 0.030 | 0.036 | 0.046 | 0.058 | 0.093 | 0.241 | 0.405 | 0.016 | 0.000 |
| Intensive cDMARDs | 12.0 | 0.001 | 0.003 | 0.005 | 0.010 | 0.017 | 0.024 | 0.030 | 0.035 | 0.042 | 0.051 | 0.067 | 0.099 | 0.245 | 0.358 | 0.013 | 0.001 |
| ETN + MTX | 3.0 | 0.246 | 0.253 | 0.187 | 0.130 | 0.080 | 0.047 | 0.025 | 0.013 | 0.009 | 0.007 | 0.003 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 |
| ETN | 6.3 | 0.035 | 0.073 | 0.102 | 0.118 | 0.130 | 0.118 | 0.092 | 0.076 | 0.063 | 0.058 | 0.054 | 0.054 | 0.023 | 0.004 | 0.000 | 0.000 |
| GOL + MTX | 6.6 | 0.046 | 0.068 | 0.088 | 0.104 | 0.117 | 0.115 | 0.098 | 0.080 | 0.068 | 0.060 | 0.053 | 0.051 | 0.035 | 0.016 | 0.000 | 0.000 |
| IFX + MTX | 8.9 | 0.001 | 0.006 | 0.017 | 0.032 | 0.058 | 0.091 | 0.113 | 0.122 | 0.124 | 0.125 | 0.120 | 0.108 | 0.062 | 0.020 | 0.000 | 0.000 |
| PBO | 16.0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.050 | 0.948 |
| TCZ + MTX | 4.9 | 0.028 | 0.085 | 0.154 | 0.200 | 0.191 | 0.136 | 0.084 | 0.049 | 0.031 | 0.019 | 0.013 | 0.007 | 0.002 | 0.000 | 0.000 | 0.000 |
| TCZ | 3.4 | 0.164 | 0.216 | 0.209 | 0.163 | 0.102 | 0.062 | 0.034 | 0.022 | 0.013 | 0.008 | 0.004 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 |
| CTZ + MTX | 2.1 | 0.459 | 0.243 | 0.143 | 0.080 | 0.041 | 0.017 | 0.010 | 0.003 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| ABT s.c. + MTX | 8.3 | 0.012 | 0.026 | 0.037 | 0.054 | 0.073 | 0.096 | 0.106 | 0.107 | 0.100 | 0.097 | 0.095 | 0.093 | 0.068 | 0.036 | 0.000 | 0.000 |
| TOF5 + MTX | 10.5 | 0.000 | 0.002 | 0.004 | 0.011 | 0.021 | 0.036 | 0.060 | 0.081 | 0.101 | 0.120 | 0.144 | 0.184 | 0.156 | 0.080 | 0.000 | 0.000 |
| TOF10 + MTX | 7.7 | 0.006 | 0.019 | 0.038 | 0.064 | 0.095 | 0.125 | 0.139 | 0.125 | 0.111 | 0.094 | 0.083 | 0.060 | 0.031 | 0.010 | 0.000 | 0.000 |
There was some suggestion that model was not a good fit to all of the data, with the total residual deviance, 281.87, being larger than the total number of data points, 244, included in the analysis. The largest residual deviances, 14.8 and 14.21, were from the Kramer et al. study,137 which included patients who received prior biologics, and were from the cDMARDs arm, in which only one patient had an ACR20 response and two patients had an ACR50 response. The next largest residual deviances were 5.76 and 4.23 from the O’Dell et al. study,111 4.08 from the JESMR study140 and 3.86 from the ARMADA study. 69
The between-study SD was estimated to be 0.20 (95% CrI 0.12 to 0.31), which implies mild heterogeneity between studies in intervention effects. The exclusion of the AMBITION study55 had little impact on the estimate of the between-study SD from studies including patients who had received prior biologics.
All interventions except for PBO were associated with beneficial treatment effects relative to cDMARDs, with the greatest effects being associated with CTZ plus MTX, ETN plus MTX and TCZ. The treatment effects were statistically significant for all interventions except for PBO at a conventional 5% level. There was insufficient evidence to differentiate between treatments, although CTZ plus MTX (mean rank 2.1; probability of being the best 0.459) and ETN plus MTX (mean rank 3.0; probability of being the best 0.246) were the treatments that were most likely to be the most effective interventions. The exclusion of the AMBITION study55 has increased the treatment effects for ADA and TCZ (with and without MTX) back towards the effects estimated from the main studies alone, but shrunk the effect of ABT s.c. plus MTX.
A meta-analysis was used to estimate the proportion of patients experiencing an ACR ‘no response’ when treated with cDMARDs.
Data were available from 28 studies. 62,69,70,74–76,79,84,89,91,92,96,99,115–118,121,124,129–138,160
The model fitted the data well, with the total residual deviance, 28.26, close to the total number of data points, 28, included in the analysis.
The between-study SD was estimated to be 0.26 (95% CrI 0.18 to 0.37), which implies mild heterogeneity between studies in the baseline response.
Table 43 presents the probabilities of achieving at least an ACR20, ACR50 and an ACR70 response. These are derived by combining the treatment effects estimated from the NMA with the estimate of the cDMARDs ‘no response’ rate.
| Intervention | At least ACR20 (95% CrI) | At least ACR50 (95% CrI) | At least ACR70 (95% CrI) |
|---|---|---|---|
| cDMARDs | 0.273 (0.238 to 0.311) | 0.114 (0.093 to 0.138) | 0.037 (0.028 to 0.047) |
| ABT i.v. + MTX | 0.550 (0.442 to 0.657) | 0.316 (0.226 to 0.421) | 0.144 (0.090 to 0.217) |
| ADA + MTX | 0.560 (0.472 to 0.648) | 0.325 (0.249 to 0.411) | 0.150 (0.103 to 0.209) |
| ADA | 0.465 (0.284 to 0.651) | 0.244 (0.121 to 0.415) | 0.101 (0.039 to 0.212) |
| Intensive cDMARDs | 0.473 (0.293 to 0.658) | 0.251 (0.125 to 0.422) | 0.105 (0.041 to 0.217) |
| ETN + MTX | 0.689 (0.567 to 0.797) | 0.457 (0.331 to 0.589) | 0.244 (0.153 to 0.360) |
| ETN | 0.619 (0.460 to 0.758) | 0.382 (0.241 to 0.539) | 0.188 (0.098 to 0.314) |
| GOL + MTX | 0.613 (0.461 to 0.748) | 0.375 (0.241 to 0.527) | 0.183 (0.098 to 0.303) |
| IFX + MTX | 0.566 (0.453 to 0.675) | 0.331(0.235 to 0.442) | 0.153 (0.095 to 0.232) |
| PBO | 0.156 (0.064 to 0.307) | 0.053 (0.017 to 0.134) | 0.014 (0.003 to 0.046) |
| TCZ + MTX | 0.643 (0.541 to 0.736) | 0.406 (0.308 to 0.512) | 0.205 (0.139 to 0.290) |
| TCZ | 0.678 (0.561 to 0.781) | 0.443 (0.325 to 0.569) | 0.233 (0.150 to 0.340) |
| CTZ + MTX | 0.714 (0.618 to 0.798) | 0.485 (0.380 to 0.591) | 0.267 (0.186 to 0.362) |
| ABT s.c. + MTX | 0.574 (0.428 to 0.713) | 0.338 (0.215 to 0.484) | 0.158 (0.085 to 0.266) |
| TOF5 + MTX | 0.534 (0.412 to 0.649) | 0.302 (0.204 to 0.413) | 0.135 (0.079 to 0.211) |
| TOF10 + MTX | 0.586 (0.465 to 0.697) | 0.350 (0.243 to 0.466) | 0.166 (0.100 to 0.251) |
American College of Rheumatology: main trials plus randomised controlled trials that have potentially low prior methotrexate exposure
A NMA was used to compare the effects of ABT i.v. plus MTX, ADA (with and without MTX), intensive cDMARDs, ETN (with and without MTX), GOL plus MTX, IFX plus MTX, PBO, TCZ (with and without MTX), CTZ plus MTX and ABT s.c. plus MTX relative to cDMARDs on ACR response.
Data were available from 30 studies comparing two or three interventions. 53,54,57,58,62,66,69,70,74–76,79,80,84,85,89,91,92,96,99,102,105,112,115–118,121,122,124,140,160
Figure 25 presents the network of evidence and Table 44 presents the frequency with which each pair of treatments was compared. There were 13 treatment effects to estimate from 30 studies. 53,54,57,58,62,66,69,70,74–76,79,80,84,85,89,91,92,96,99,102,105,112,115–118,121,122,124,140,160
FIGURE 25.
American College of Rheumatology (populations 2 and 3: main trials + RCTs that have potentially low prior MTX exposure) – network of evidence. Int, intensive. Solid green line (two-arm study); and dotted blue line (three-arm study).

| Intervention | cDMARDs | ABT i.v. + MTX | ADA + MTX | ADA | Intensive cDMARDs | ETN + MTX | ETN | GOL + MTX | IFX + MTX | PBO | TCZ + MTX | TCZ | CTZ + MTX | ABT s.c. + MTX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cDMARDs | – | 2 | 5 | 1 | 5 | 2 | 2 | 3 | 1 | 2 | 1 | |||
| ABT i.v. + MTX | – | – | 1 | |||||||||||
| ADA + MTX | – | – | – | 1 | ||||||||||
| ADA | – | – | – | – | 2 | 1 | ||||||||
| Intensive cDMARDs | – | – | – | – | – | 3 | ||||||||
| ETN + MTX | – | – | – | – | – | – | 3 | 1 | ||||||
| ETN | – | – | – | – | – | – | – | 1 | ||||||
| GOL + MTX | – | – | – | – | – | – | – | – | ||||||
| IFX + MTX | – | – | – | – | – | – | – | – | – | |||||
| PBO | – | – | – | – | – | – | – | – | – | – | ||||
| TCZ + MTX | – | – | – | – | – | – | – | – | – | – | – | 1 | ||
| TCZ | – | – | – | – | – | – | – | – | – | – | – | – | ||
| CTZ + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| ABT s.c. + MTX | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Figure 26 presents the effects of each intervention relative to cDMARDs on the probit scale and Figure 27 and Table 45 present the probabilities of treatment rankings.
FIGURE 26.
American College of Rheumatology (populations 2 and 3: main trials + RCTs that have potentially low prior MTX exposure) – effects of interventions relative to cDMARDs on the probit scale. Int, intensive.
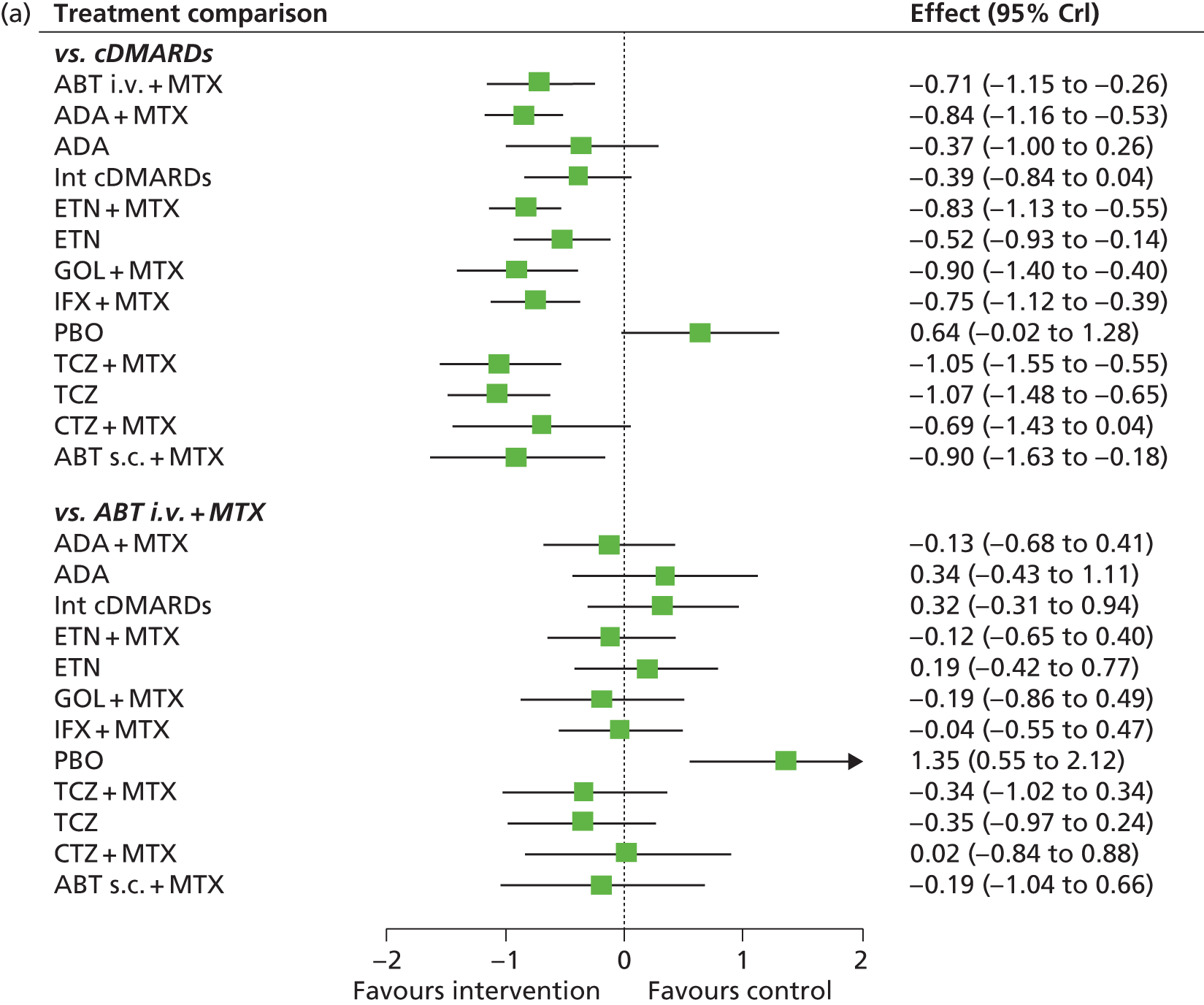

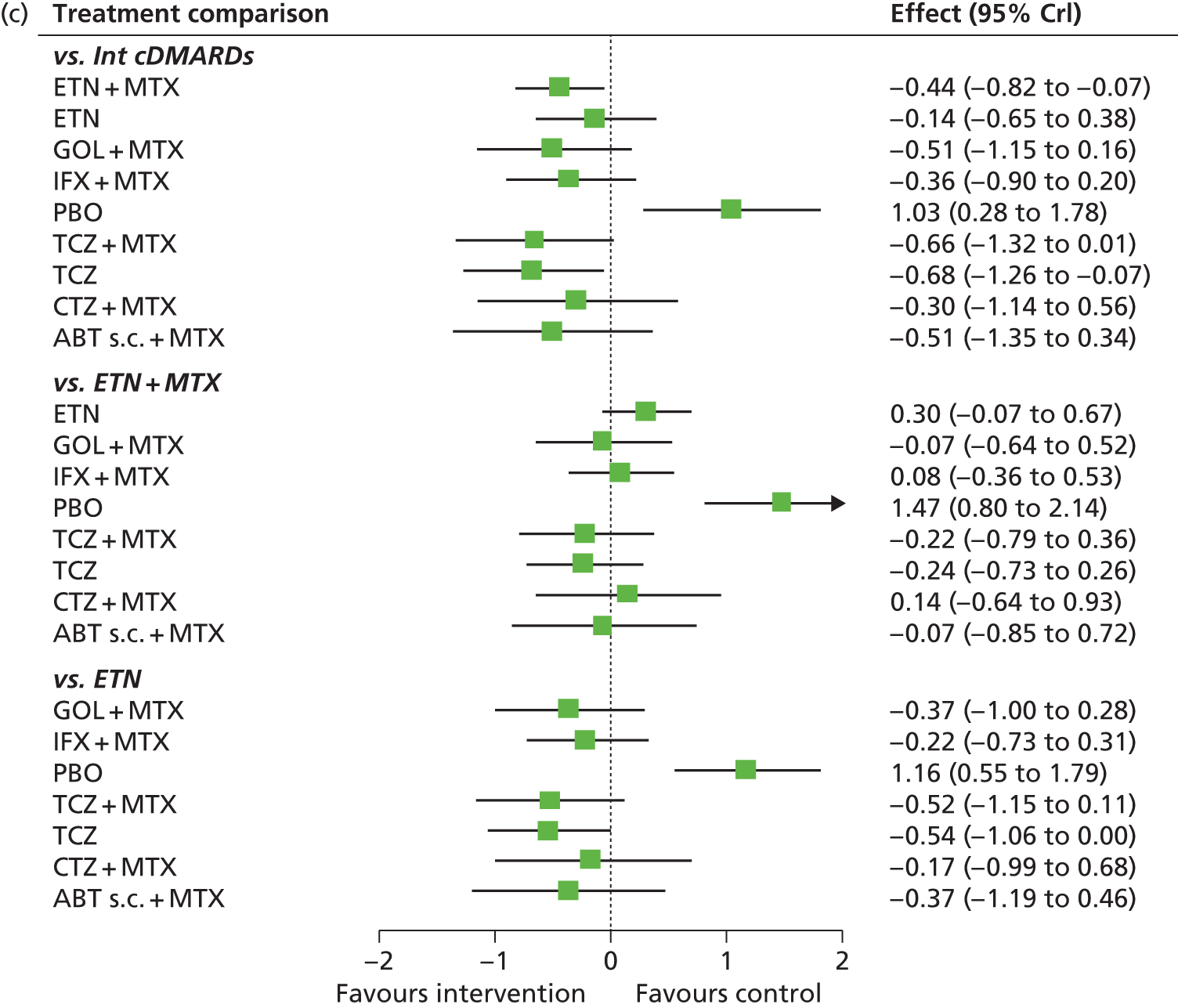
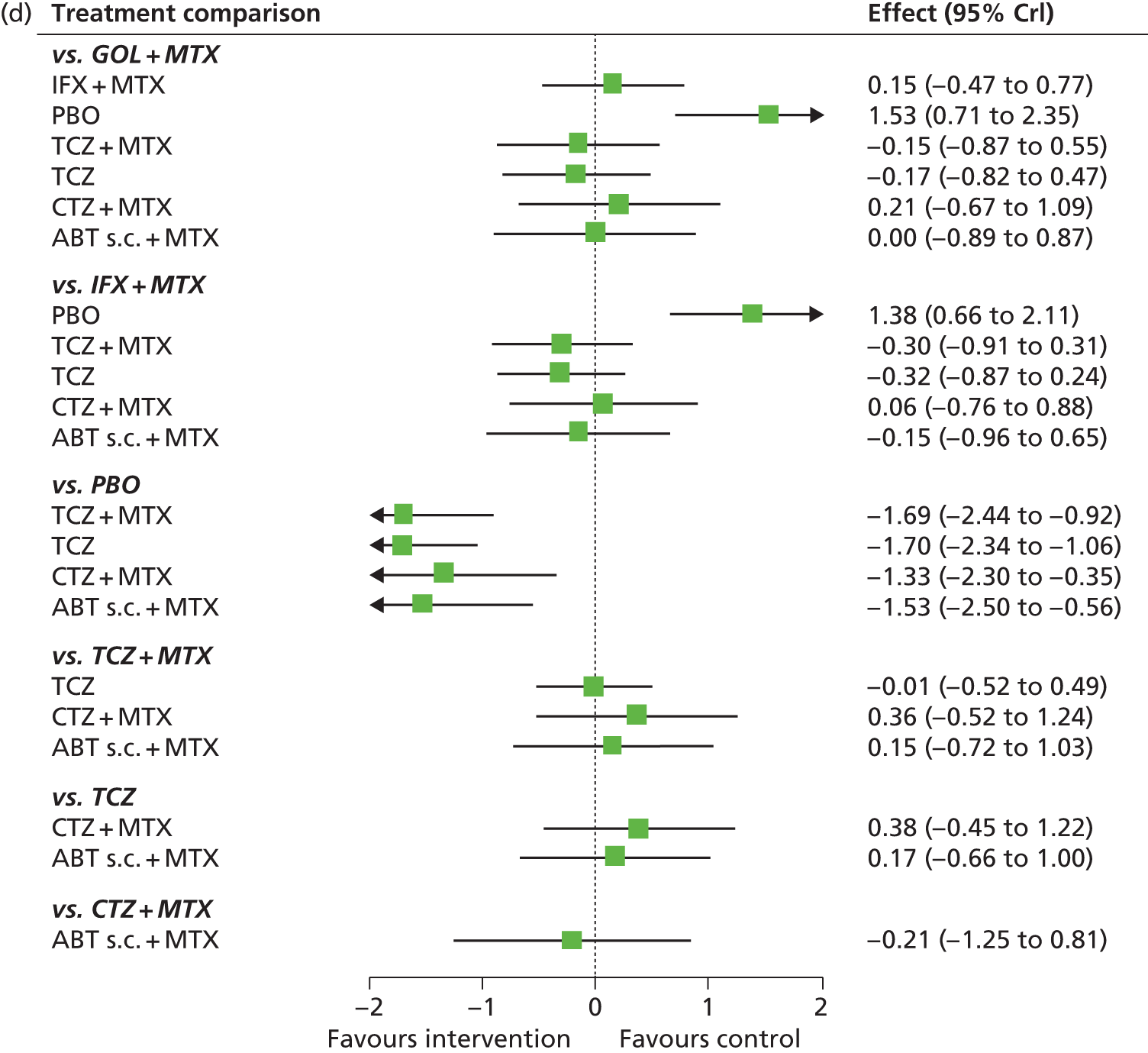
FIGURE 27.
American College of Rheumatology (populations 2 and 3: main trials + RCTs that have potentially low prior MTX exposure) – probability of treatment rankings in terms of efficacy (most efficacious = 1). Int, intensive.



| Intervention | Rank (mean) | Rank | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| cDMARDs | 12.83 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.015 | 0.167 | 0.791 | 0.026 |
| ABT i.v. + MTX | 7.12 | 0.019 | 0.037 | 0.058 | 0.078 | 0.089 | 0.108 | 0.124 | 0.147 | 0.137 | 0.099 | 0.068 | 0.034 | 0.002 | 0.000 |
| ADA + MTX | 5.48 | 0.020 | 0.068 | 0.109 | 0.159 | 0.174 | 0.165 | 0.127 | 0.095 | 0.054 | 0.025 | 0.010 | 0.001 | 0.000 | 0.000 |
| ADA | 10.32 | 0.002 | 0.006 | 0.012 | 0.019 | 0.022 | 0.028 | 0.036 | 0.054 | 0.085 | 0.134 | 0.203 | 0.285 | 0.113 | 0.000 |
| Intensive cDMARDs | 10.47 | 0.001 | 0.002 | 0.002 | 0.006 | 0.011 | 0.015 | 0.029 | 0.053 | 0.100 | 0.183 | 0.281 | 0.284 | 0.031 | 0.002 |
| ETN + MTX | 5.48 | 0.020 | 0.056 | 0.105 | 0.148 | 0.170 | 0.176 | 0.151 | 0.103 | 0.057 | 0.012 | 0.002 | 0.000 | 0.000 | 0.000 |
| ETN | 9.28 | 0.001 | 0.003 | 0.008 | 0.014 | 0.024 | 0.043 | 0.068 | 0.110 | 0.184 | 0.264 | 0.206 | 0.071 | 0.003 | 0.000 |
| GOL + MTX | 4.91 | 0.122 | 0.125 | 0.136 | 0.120 | 0.108 | 0.094 | 0.085 | 0.076 | 0.061 | 0.039 | 0.025 | 0.011 | 0.001 | 0.000 |
| IFX + MTX | 6.60 | 0.017 | 0.034 | 0.061 | 0.088 | 0.116 | 0.133 | 0.158 | 0.157 | 0.119 | 0.072 | 0.034 | 0.011 | 0.000 | 0.000 |
| PBO | 13.96 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.005 | 0.025 | 0.968 |
| TCZ + MTX | 3.28 | 0.269 | 0.224 | 0.157 | 0.102 | 0.072 | 0.055 | 0.047 | 0.032 | 0.023 | 0.012 | 0.006 | 0.002 | 0.000 | 0.000 |
| TCZ | 2.95 | 0.251 | 0.275 | 0.178 | 0.108 | 0.074 | 0.046 | 0.032 | 0.021 | 0.011 | 0.004 | 0.001 | 0.000 | 0.000 | 0.000 |
| CTZ + MTX | 7.18 | 0.082 | 0.066 | 0.068 | 0.065 | 0.061 | 0.066 | 0.074 | 0.085 | 0.104 | 0.101 | 0.103 | 0.094 | 0.027 | 0.003 |
| ABT s.c. + MTX | 5.10 | 0.196 | 0.111 | 0.106 | 0.093 | 0.078 | 0.070 | 0.069 | 0.068 | 0.067 | 0.054 | 0.046 | 0.034 | 0.007 | 0.001 |
The model fitted the data well, with the total residual deviance, 198.62, close to the total number of data points, 192, included in the analysis. The largest residual deviances were 5.999 from the O’Dell et al. study111 and 3.913 from the Safety Trial for rheumatoid Arthritis with Remicade Therapy (START) study. 118
The between-study SD was estimated to be 0.30 (95% CrI 0.20 to 0.46), which implies mild heterogeneity between studies in intervention effects. The addition of the TEAR53 and TEMPO54 studies has increased the variability between treatment effects relative to that estimated from the main studies alone.
All interventions except for PBO were associated with beneficial treatment effects relative to cDMARDs with the greatest effects being associated with TCZ (with and without MTX). The treatment effects were statistically significant for all interventions except for CTZ plus MTX, ADA, intensive cDMARDs and PBO at a conventional 5% level. There was insufficient evidence to differentiate between treatments although TCZ (mean rank 2.95; probability of being the best 0.251) and TCZ plus MTX (mean rank 3.28; probability of being the best 0.269) were the treatments that were most likely to be the most effective interventions.
A meta-analysis was used to estimate the proportion of patients experiencing an ACR ‘no response’ when treated with cDMARDs.
Data were available from 20 studies. 53,54,62,69,70,74–76,79,84,89,91,92,96,99,115–118,121,124,160
The model fitted the data well, with the total residual deviance, 19.53, close to the total number of data points, 20, included in the analysis.
The between-study SD was estimated to be 0.37 (95% CrI 0.26 to 0.55), which implies mild to moderate heterogeneity between studies in the baseline response. The addition of the TEAR53 and TEMPO54 studies has increased the variability between studies in the CDMARDs ‘no response’ rate relative to that estimated from the main studies alone.
Table 46 presents the probabilities of achieving at least an ACR20, an ACR50 and an ACR70. These are derived by combining the treatment effects estimated from the NMA with the estimate of the cDMARDs ‘no response’ rate.
| Intervention | At least ACR20 (95% CrI) | At least ACR50 (95% CrI) | At least ACR70 (95% CrI) |
|---|---|---|---|
| cDMARDs | 0.323 (0.264 to 0.389) | 0.136 (0.102 to 0.180) | 0.046 (0.031 to 0.067) |
| ABT i.v. + MTX | 0.601 (0.410 to 0.767) | 0.351 (0.192 to 0.537) | 0.166 (0.073 to 0.309) |
| ADA + MTX | 0.649 (0.509 to 0.771) | 0.400 (0.268 to 0.542) | 0.199 (0.113 to 0.315) |
| ADA | 0.466 (0.228 to 0.713) | 0.234 (0.083 to 0.472) | 0.095 (0.024 to 0.256) |
| Intensive cDMARDs | 0.473 (0.296 to 0.662) | 0.240 (0.120 to 0.412) | 0.098 (0.039 to 0.209) |
| ETN + MTX | 0.645 (0.515 to 0.765) | 0.396 (0.273 to 0.534) | 0.197 (0.117 to 0.307) |
| ETN | 0.526 (0.360 to 0.695) | 0.284 (0.160 to 0.450) | 0.123 (0.057 to 0.238) |
| GOL + MTX | 0.670 (0.463 to 0.833) | 0.421 (0.232 to 0.629) | 0.216 (0.093 to 0.398) |
| IFX + MTX | 0.614 (0.456 to 0.758) | 0.364 (0.227 to 0.525) | 0.175 (0.090 to 0.300) |
| PBO | 0.136 (0.039 to 0.337) | 0.042 (0.008 to 0.146) | 0.010 (0.001 to 0.050) |
| TCZ + MTX | 0.723 (0.524 to 0.870) | 0.483 (0.280 to 0.689) | 0.264 (0.121 to 0.462) |
| TCZ | 0.729 (0.563 to 0.857) | 0.489 (0.316 to 0.666) | 0.268 (0.142 to 0.437) |
| CTZ + MTX | 0.593 (0.300 to 0.839) | 0.343 (0.122 to 0.637) | 0.160 (0.040 to 0.406) |
| ABT s.c. + MTX | 0.670 (0.383 to 0.883) | 0.422 (0.175 to 0.710) | 0.216 (0.063 to 0.487) |
Discussion of systematic reviewing results
This review differed from other reviews of biologics in RA,123,161–172 in that it included only licensed doses of biologics, was limited to first-line biologics, and considered separately MTX-naive and cDMARD-experienced trials.
Sixty trials met the inclusion criteria for the systematic review of clinical effectiveness and safety evidence. Of these, 38 trials were also used in the NMA56–126,141,160 (eight for population 1 and 30 for populations 2 and 3).
Seven MTX-naive trials78,81–83,87,90,94,108,109 and 24 cDMARD-experienced trials57,58,61–65,68–70,74–76,79,80,84,85,88,89,91,92,96,97,99,102,104,105,111,112,115–119,121,122,124,141,160 (of which four were head-to-head evidence58,66,74,85) were included in the NMA for ACR response. One MTX-naive trial and 15 cDMARD experienced trials were included in the NMA for EULAR data.
In addition, 14 trials (12 trials with interventions of interest53–55,127–136 and two TOF trials137,138) were included in sensitivity analyses for populations 2 and 3 (all 14 with ACR data and 3 with EULAR data). Two of these trials (presenting ACR data only) were used in sensitivity analyses for population 1. 53,54
Many of the trials were of good quality (see Figure 3). They were mostly Phase III trials. Some trials did not report in enough detail to judge randomisation method or allocation concealment, or if all outcomes were reported. Further details regarding study quality are provided in Table 333 (see Appendix 4).
There were several large, multinational, multicentre studies. A few trials were conducted in a single country. For the cDMARD-experienced population, some trial populations may not have had adequate MTX to class as failure. Of particular note, for populations 2 and 3, are the trials that were conducted in Japan only, as some of these trials also utilised low-dose MTX treatment prior to randomisation, potentially impacting on the extent of MTX failure among trial populations and restricting external validity to the UK. Further details regarding geographical location are provided in Tables 335–338 (see Appendix 4). Based on the results shown within the company submissions made by AbbVie173 and MSD,159,174 which did not show a marked difference when Asian studies were excluded, no formal analyses were undertaken removing such studies.
The issues relating to the external validity of RCTs in RA, including (1) the application of strict trial inclusion criteria resulting in narrower study populations relative to RA clinical practice and (2) the limitations of RCTs in general in capturing rare AEs, have been previously discussed and should be borne in mind when considering the generalisability of the trial evidence. 175,176 Some trials had step-up therapy, which in the opinion of our clinical advisors is consistent with real-world practice.
Strengths of this systematic review included the undertaking of a comprehensive search for evidence; the extensive number of RCTs that were identified relating to the decision problem; data were identified for all interventions of interest; there were long-term safety data from LTEs of trials; trials that were not eligible for inclusion in the systematic review or NMA base case (e.g. trials with populations having ≤ 20% prior biologic experience) were explored in sensitivity analyses; and graphical data for the NMA were extracted using Engauge version 4.1.
Limitations of the review included evidence was restricted to English-language publications; ongoing/unpublished trial resources could not be explored owing to the time scales of the assessment; some studies (and consequently some interventions) could not be included in a NMA of EULAR outcome data where this was not reported; and, owing to the extensive variability in the range of available outcome measures reported in trials, it was necessary to prioritise the assessment of the most widely used measures.
Although there was uncertainty in, and overlap between, the effects of treatment on ACR for interventions for patients in population 1, IFX plus MTX was associated with the biggest increase in response rate and this was likely to be the most effective intervention. Other interventions were less effective and appeared to fall into three groups: (1) ETN plus MTX, intensive cDMARDs and ADA plus MTX; (2) GOL plus MTX, ETN and step-up intensive cDMARDs; and (3) CDMARDs and ADA.
Although there was uncertainty in, and overlap between, the effects of treatment on EULAR for interventions in populations 2 and 3 in the main trials, TCZ, TCZ plus MTX and ETN plus MTX were associated with the biggest increase in response rate. Other interventions were less effective and appeared to fall into two groups: (1) GOL plus MTX, CTZ plus MTX, ADA, grouped biologics, ETN, ADA plus MTX, ABT i.v. plus MTX and IFX plus MTX; and (2) intensive cDMARDs, PBO and cDMARDs. The inclusion of the additional studies in which patients received prior biologics resulted in broadly the same groupings, although the effect of CTZ plus MTX was much greater and similar to that for TCZ, TCZ plus MTX and ETN plus MTX.
Although there was uncertainty in, and overlap between, the effects of treatment on ACR for interventions in populations 2 and 3 in the main trials, ETN plus MTX, TCZ and TCZ plus MTX, were associated with the biggest increase in response rate. Other interventions were less effective and appeared to fall into two groups: (1) ETN, GOL plus MTX, ABT s.c. plus MTX, ADA plus MTX, INF plus MTX, CTX plus MTX and ATB i.v. plus MTX; and (2) intensive cDMARDs, ADA and cDMARDs. The inclusion of the additional studies in which patients received prior biologics suggested resulted in a greater estimate of the effect of CTZ plus MTX. Other interventions appeared to give rise to broadly similar response rates.
Other efficacy outcomes
Population 1: methotrexate naive
Where there was step-up therapy with initial biologic or control, the groups were similar after 6 months to a year (i.e. after step-up). Biologic monotherapy was better than PBO, but similar to MTX. Biologic combined with MTX was better than MTX plus PBO.
Populations 2 and 3: conventional disease-modifying antirheumatic drugs experienced
Head-to-head trials indicate similarity of biologics. One exception was the ADACTA trial. 58
This reported greater improvement with TCZ monotherapy than ADA monotherapy for DAS and mental component summary of SF-36 at 24 weeks,58 although this trial had similar results for ADA and TCZ for swollen and tender joint counts, and fatigue. This suggests that the impacts of different biologics on different outcomes may not be straightforward.
Biologics combined with MTX treatment arms reported more improvement than non-biologic control arms with one or two cDMARDs or baseline cDMARDs. Biologics combined with MTX did better than biologic monotherapy, except for TCZ for joint counts and HAQ-DI.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
The Assessment Group conducted a systematic review of published economic evaluations undertaken of the RA interventions being assessed. The objective of this systematic review is to summarise the existing economic evidence for the use of each intervention in patients with RA. The systematic review will assess the strengths and limitations of each specific economic evaluation.
Methods for reviewing existing cost-effectiveness evidence
Systematic searches of online databases were undertaken to identify all published economic evaluations of disease-modifying therapies for RA. To ensure that the systematic search had high sensitivity, the search was developed by applying economic terms to a general disease search for RA and disease-modifying therapies. Database filters to identify economic evaluations were used from the InterTASC Information Specialists’ Sub-Group website [www.york.ac.uk/inst/crd/intertasc/index.htm (accessed 5 July 2013)]. The keywords used for the systematic review are provided in Table 47.
| Population | RA |
|---|---|
| Intervention/comparator | Disease modifying, disease-modifying, DMARD, biologic, therapy, treatment, anti-rheumatic, anti rheumatic, TNF, tumor necrosis factor alpha, tumour necrosis factor alpha, TNF-alpha, TNF inhibitor, TNF blocker, interleukin 1, IL-1, monoclonal antibody, costimulation blocker, interleukin 6, IL-6 |
| Outcomes | Economic, economics, cost, cost-effectiveness, cost-utility, cost-benefit, utility, health related quality of life, quality of life, quality adjusted life year, QALY |
The search strategies used medical subject heading terms, including ‘rheumatoid arthritis’ and ‘economics’ and text string terms, which were combined in the search strategy using Boolean logic. The search strategies were designed to maximise sensitivity (i.e. the identification of all appropriate studies); however, this was at the cost of poor specificity (the rejection of inappropriate studies). This meant the search returned a lot of inappropriate studies and was reliant on hand-sifting, including the removal of economic evaluations of treatments that are not included in this appraisal (RTX, conventional DMARDs, anakinra, etc.).
Systematic searches were conducted in 10 databases provided in Table 48. Reference search was undertaken on all included studies, including any identified reviews of published economic evaluations of disease-modifying therapies for RA.
| Database | Date |
|---|---|
| Bioscience Information Service (all databases) | 1899–February 2013 |
| Cochrane Database of Systematic Reviews | All years–February 2013 |
| Cochrane Database of Methodological Reviews | All years–February 2013 |
| Cochrane Central Register of Controlled Trials | All years–February 2013 |
| Database of Abstracts of Reviews and Effects | All years–February 2013 |
| CINAHL | 1994–February 2013 |
| EMBASE | 1974–February 2013 |
| MEDLINE | 1945–February 2013 |
| NHS Economic Evaluations Database | All years–February 2013 |
| Science Citation Index: Web of Science | 1899–February 2013 |
All database searches were undertaken on 1 February 2013 and no date restriction was applied. No study type or language restrictions were applied to the electronic search. The search strategies were reviewed by an information specialist.
The objective of the systematic search was to identify economic evaluations of ABT, ADA, CTZ, ETN, GOL, IFX and TCZ within populations 1, 2 and 3. The search was irrespective of the decision-making context or the geographical location. The eligibility criteria are presented in Box 1.
Economic evaluation including a comparison of costs and benefits based on outcomes data or undertaken using decision-analytic methods.
Economic evaluations of interventions targeting a change to the natural disease profile of people with RA (i.e. disease-modifying therapies).
Studies reporting costs and health outcomes.
Exclusion criteriaEvaluations of treatments not under review in this appraisal.
Evaluations in patient populations not under review in this appraisal (e.g. sequential biologics).
Partial or non-comparative economic evaluations.
Cost analyses/cost-of-illness/burden-of-illness studies.
Methodological papers that do not report economic and health benefit outcomes.
Commentaries, letters, editorials.
Conference abstracts.
Studies that claim cost-effectiveness, but with no empirical estimation of the costs and effectiveness outcomes.
Economic evaluations of therapies and treatments which do not modify the natural progression of RA.
Non-English language.
The identified studies were appraised using the commonly used and validated Drummond checklist. 177
Results
From the systematic searching of electronic databases, 8281 citations were identified (Figure 28). After excluding 3250 duplicate citations electronically, the remaining 5031 citations were screened by their abstract. Of these, 4913 abstracts did not meet the inclusion criteria and 118 full-text papers were retrieved for a full inspection. A total of 97 papers were excluded for not meeting the inclusion criteria, and nine other studies were identified by reference searches and searching any identified systematic reviews. A total of 30 studies were included in the systematic review.
FIGURE 28.
Quality of reporting of meta-analyses flow diagram.

The studies identified are summarised in Table 49. Twenty-three of the 30 studies (77%) were evaluations of bDMARDs in patients who had already had DMARD therapy previously. Six studies (20%) were in DMARD-naive patients, with one study (3%) in both DMARD-naive and -experienced populations.
| Study | Treatment history | Disease severity | Country (sponsor) | Interventions considered | Form of economic analysis | Model used | Time horizon |
|---|---|---|---|---|---|---|---|
| Bansback et al., 2005178 | Two cDMARDs | Moderate/severe | Sweden (Abbott) | TNF-α with or without MTX vs. cDMARDs | CUA | Individual-level Markov model | Lifetime |
| Barbieri et al., 2005179 | cDMARDs and resistant to MTX | Severe | UK (Schering-Plough) | IFX + MTX vs. MTX | CUA | Markov model | 1 year and lifetime |
| Barton et al., 2004180 | SSZ and MTX | Unclear | UK (HTA) | ETN vs. IFX vs. cDMARD sequence | CUA | Individual sampling model | Lifetime |
| Benucci et al., 2009181 | Two cDMARDs | Moderate/severe | Italy (none reported) | ABT with LEF or MTX vs. ETN with LEF or MTX | CUA | Observational analysis | 2 years |
| Brennan et al., 2004182 | Two cDMARDs | Unclear | UK (Wyeth) | ETN vs. cDMARD sequence | CUA | Individual sampling model | Lifetime |
| Brennan et al., 2007183 | At least two cDMARDs | Active | UK (BSRBR) | TNF-α vs. cDMARDs | CUA | Individual sampling model | Lifetime |
| Chen et al., 2006123 | None (at least for first-line comparators) | Active | UK (HTA) | TNF-α with or without MTX at first line or third line | CUA | Individual sampling model | Lifetime |
| Chiou et al., 2004184 | Unclear | Moderate/severe | USA (none reported) | AKR vs. ETN vs. ADA vs. IFX | CUA | Decision tree | 1 year |
| Choi et al., 2002185 | MTX | Unclear | USA (no funding source) | cDMARD monotherapy and combination vs. bDMARD monotherapy and combination | CEA | Decision tree | 6 months |
| Coyle et al., 2006186 | None | Aggressive | Canada (CCOHTA) | GLD vs. bDMARD monotherapy and combination | CUA | Markov model | 5 years |
| Davies et al., 2009187 | None | Unclear | USA (Abbott) | MTX vs. ADA + MTX vs. ETN vs. IFX + MTX vs. ADA + MTX | CUA | Individual sampling model | Lifetime |
| Diamantopoulos et al., 2012188 | cDMARDs | Moderate/severe | Italy (Roche) | Sequential bDMARD use | CUA | Individual sampling model | lifetime |
| Finckh et al., 2009189 | None | Active | USA (Arthritis Foundation) | Symptomatic therapy vs. MTX vs. bDMARDs | CUA | Individual sampling model | Lifetime |
| Jobanputra et al., 2002172 | SSZ and MTX | Active | UK (HTA) | Adding ETN and IFX into a cDMARD sequence | CUA | Individual sampling model | Lifetime |
| Kobelt et al., 2003190 | cDMARDs including MTX IR | Unclear, ‘advanced’ | Sweden, UK (Schering-Plough) | IFX + MTX vs. MTX | CUA | Markov model | 10 year |
| Kobelt et al., 2004191 | Two cDMARDs including MTX IR | Unclear | Sweden (multiple funders) | TNF-α vs. cDMARDs | CUA | Trial analysis | 1 year |
| Kobelt et al., 2005192 | cDMARDs other than MTX | Severe | Sweden (Wyeth) | ETN vs. MTX vs. ETN + MTX | CUA | Markov model | 5 years/10 years |
| Kobelt et al., 2011193 | None | Severe | Sweden (Wyeth) | ETN + MTX vs. MTX | CUA | Markov model | 10 years |
| Lekander et al., 2010194 | No TNF-αs | Active | Sweden (Schering-Plough) | IFX vs. cDMARDs | CUA | Markov model | 20 years |
| Marra et al., 2007195 | cDMARDs | Active | Canada (none reported) | IFX + MTX vs. MTX | CUA | Markov model | 10 years |
| Nuijten et al., 2001196 | Two cDMARDs | Unclear | The Netherlands (Wyeth) | ETN vs. IFX | CMA | Unclear | 1 year |
| Rubio-Terrés and Dominguez-Gil, 2001197 | cDMARDs (including MTX) | Active | Spain (none reported) | IFX + MTX vs. LEF | CMA | Unclear | 1 year |
| Soini et al., 2012198 | At least one cDMARD | Moderate/severe | Finland (Roche) | ADA vs. ETN vs. TCZ | CUA | Individual sampling model | Lifetime |
| Spalding and Hay, 2006199 | None | Unclear | USA (University of Southern California) | MTX vs. bDMARD monotherapy and combination | CUA | Markov model | Lifetime |
| Tanno et al., 2006200 | Bucillamine | Unclear | Japan (Japanese Government) | Adding ETN to a cDMARD sequence | CUA | Markov model | Lifetime |
| van den Hout et al., 2009201 | None | Active | The Netherlands (multiple funders) | Comparing cDMARD combination vs. IFX combination therapy | CUA | Trial analysis | 2 years |
| Vera-Llonch et al., 2008202 | MTX | Moderate/severe | USA (none reported) | ABT vs. cDMARDs | CUA | Individual sampling model | Lifetime |
| Wailoo et al., 2008203 | No bDMARDs | Unclear | USA (US AHRQ) | ETN vs. ADA vs. AKR vs. IFX | CUA | Individual sampling model | Lifetime |
| Welsing et al., 2004204 | cDMARDs | Active | The Netherlands (none reported) | Usual care vs. LEF vs. TNF-α vs. LEF, TNF-α sequences | CUA | Markov model | 5 years |
| Wong et al., 2002205 | MTX | Active refractory disease | USA (Schering-Plough, NIH) | IFX + MTX vs. MTX | CUA | Markov model | Lifetime |
No studies were identified that evaluated GOL and CTZ, with the majority focusing on ETN, IFX and ADA.
A total of 27 of the 30 studies (90%) were cost–utility analyses and a wide range of model methods and time horizons were adopted.
For ease of reading, the cost-effectiveness results are split into cDMARD-naive (Table 50) and bDMARD-naive (Table 51) populations.
| Drug | Comparator | Study | Price year | Time horizon | Previous treatments | ICER (per QALY gained) |
|---|---|---|---|---|---|---|
| ADA | MTX | Spalding and Hay, 2006199 | 2005 | Lifetime | None | US$64,000 |
| cDMARDs | Chen et al., 2006123 | 2004 | Lifetime | None | £53,000 | |
| ADA + MTX | MTX | Spalding and Hay, 2006199 | 2005 | Lifetime | None | US$195,000 |
| cDMARDs | Davies et al., 2009187 | 2007 | Lifetime | None | US$23,000 | |
| cDMARDs | Chen et al., 2006123 | 2004 | Lifetime | None | £170,000 | |
| ETN | MTX | Spalding and Hay, 2006199 | 2005 | Lifetime | None | US$90,000 |
| cDMARDs | Chen et al., 2006123 | 2004 | Lifetime | None | £49,000 | |
| cDMARDs | Davies et al., 2009187 | 2007 | Lifetime | None | US$28,000 | |
| ETN + MTX | MTX | Kobelt et al., 2011193 | 2008 | 10 years | None | €14,000 |
| cDMARDs | Coyle et al., 2006186 | ? | 5 years | None | Before/after GLD = CA$145,000/CA$126,000 | |
| cDMARDs | Chen et al., 2006123 | 2004 | Lifetime | None | £78,000 | |
| IFX + MTX | MTX | Spalding and Hay, 2006199 | 2005 | Lifetime | None | US$410,000 |
| cDMARDs | Coyle et al., 2006186 | ? | 5 years | None | Before/after GLD = CA$113,000/CA$98,000 | |
| cDMARDs | Davies et al., 2009187 | 2007 | Lifetime | None | US$32,000 | |
| cDMARDs | Chen et al., 2006123 | 2004 | Lifetime | None | £650,000 | |
| Combination cDMARDs | van den Hout et al., 2009201 | 2008 | 2 years | None | €130,000 | |
| TNF-α | cDMARDs | Finckh et al., 2009189 | 2007 | Lifetime | None | Dominated |
| Drug | Comparator | Study | Price year | Time horizon | Previous treatments | ICER (per QALY gained) |
|---|---|---|---|---|---|---|
| ABT i.v. + MTX | MTX | Vera-Llonch et al., 2008202 | 2006 | Lifetime | MTX | US$46,000 |
| ADA | MTX | Bansback et al., 2005178 | 2001 | Lifetime | Two previous cDMARDs | €42,000 |
| cDMARDs | Chen et al., 2006123 | 2004 | Lifetime | Two previous cDMARDs | £35,000–140,000 | |
| Anakinra | Chiou et al., 2004184 | 2003 | 1 year | Unclear | Dominated | |
| Anakinra | Wailoo et al., 2008203 | ? | Lifetime | No bDMARDs | US$143,000 | |
| IFX + MTX | Wailoo et al., 2008203 | ? | Lifetime | No bDMARDs | Dominates | |
| ADA + MTX | MTX | Bansback et al., 2005178 | 2001 | Lifetime | Two previous cDMARDs | €34,000 |
| MTX | Soini et al., 2012198 | 2010 | Lifetime | At least one cDMARD | €21,000 | |
| cDMARDs | Chen et al., 2006123 | 2004 | Lifetime | Two previous cDMARDs | £30,000–64,000 | |
| Anakinra | Chiou 2004184 | 2003 | 1 year | Unclear | Dominated | |
| ETN | MTX | Bansback et al., 2005178 | 2001 | Lifetime | Two previous cDMARDs | €37,000 |
| MTX | Tanno et al., 2006200 | 2005 | Lifetime | Bucillamine | Yen 2.5M | |
| MTX | Kobelt et al., 2005192 | 2004 | 5 years/10 years | cDMARDs other than MTX | 5 years/10 years = €152,000/124,000 | |
| cDMARDs | Chen et al., 2006123 | 2004 | Lifetime | Two previous cDMARDs | £24,000–47,000 | |
| Anakinra | Chiou et al., 2004184 | 2003 | 1 year | Unclear | US$13,000 | |
| IFX + MTX | Nuijten et al., 2001196 | 1999 | 1 year | Two cDMARDs | Dominates | |
| ETN + MTX and cDMARD strategies | Choi et al., 2002185 | 1999 | 6 months | MTX | Extendedly dominated | |
| ETN + MTX | MTX | Bansback et al., 2005178 | 2001 | Lifetime | Two previous cDMARDs | €36,000 |
| MTX | Soini et al., 2012198 | 2010 | Lifetime | At least one cDMARD | €21,000 | |
| MTX | Kobelt et al., 2005192 | 2004 | 5 years/10 years | cDMARDs other than MTX | 5 years/10 years = €55,000/37,000 | |
| cDMARDs | Barton et al., 2004180 | 2000 | Lifetime | SSZ and MTX | £50,000 | |
| cDMARDs | Brennan et al., 2004182 | 2000 | Lifetime | Two cDMARDs | £16,000 | |
| cDMARDs | Jobanputra et al., 2002172 | 2000 | Lifetime | SSZ and MTX | £64,000 | |
| cDMARDs | Chen et al., 2006123 | 2004 | Lifetime | Two previous cDMARDs | £24,000–50,000 | |
| Anakinra | Chiou et al., 2004184 | 2003 | 1 year | Unclear | US$8000 | |
| ADA + MTX | Benucci et al., 2009181 | ? | 2 years | Two cDMARDs | US$25,000 | |
| ADA + MTX | Wailoo et al., 2008203 | ? | Lifetime | No bDMARDs | US$92,000 | |
| IFX + MTX | Wailoo et al., 2008203 | ? | Lifetime | No bDMARDs | Dominates | |
| IFX + MTX | Barton et al., 2004180 | 2000 | Lifetime | SSZ and MTX | £28,000 | |
| IFX + MTX | Jobanputra et al., 2002172 | 2000 | Lifetime | SSZ and MTX | £35,000 | |
| IFX + MTX | Nuijten et al., 2001196 | 1999 | 1 year | Two cDMARDs | Dominates | |
| ETN | Choi et al., 2002185 | 1999 | 6 months | MTX | US$43,000 (per ACR20 response), US$35,000 (per ACR70 response) | |
| IFX + MTX | MTX | Bansback et al., 2005178 | 2001 | Lifetime | Two previous cDMARDs | €48,000 |
| MTX | Barbieri et al., 2005179 | 2000 | 1 year/lifetime | cDMARDs and resistant to MTX | £34,000 (1 year), £24,000 (lifetime) | |
| MTX | Kobelt et al., 2003190 | ? | 10 years | cDMARDs including MTX IR | £22,000 | |
| MTX | Marra et al., 2007195 | 2002 | 10 years | cDMARDs | US$46,000 | |
| MTX | Wong et al., 2002205 | 1998 | Lifetime | MTX | US$307,000 | |
| LEF | Rubio-Terrés et al., 2001197 | 1999 | 1 year | cDMARDs (including MTX) | Dominated (CMA) | |
| cDMARDs | Barton et al., 2004180 | 2000 | Lifetime | SSZ and MTX | £68,000 | |
| cDMARDs | Jobanputra et al., 2002172 | 2000 | Lifetime | SSZ and MTX | £89,000 | |
| cDMARDs | Lekander et al., 2010194 | 2007 | 20 years | No TNF-αs | €23,000 | |
| cDMARDs | Chen et al., 2006123 | 2004 | Lifetime | Two previous cDMARDs | £30,000–140,000 | |
| Anakinra | Chiou et al., 2004184 | 2003 | 1 year | Unclear | Dominated | |
| ADA + MTX | Wailoo et al., 2008203 | ? | Lifetime | No bDMARDs | Dominated | |
| ETN + MTX | Wailoo et al., 2008203 | ? | Lifetime | No bDMARDs | Dominated | |
| TCZ + MTX | ETA + MTX | Diamantopoulos et al., 2012188 | 2009 | Lifetime | cDMARDs | Dominates |
| ADA + MTX | Diamantopoulos et al., 2012188 | 2009 | Lifetime | cDMARDs | Dominates | |
| IFX + MTX | Diamantopoulos et al., 2012188 | 2009 | Lifetime | cDMARDs | €3000 | |
| Add TCZ into first biologic position | Diamantopoulos et al., 2012188 | 2009 | Lifetime | cDMARDs | €17,000 | |
| MTX | Soini et al., 2012198 | 2010 | Lifetime | At least one cDMARD | €19,000 | |
| Grouped bDMARDs | cDMARD | Brennan et al., 2007183 | 2004 | Lifetime | At least two cDMARDs | £24,000 |
| Previous years’ DMARD use | Kobelt et al., 2004191 | 2002 | 1 year | Two cDMARDs including MTX IR | €44,000 | |
| TNF-α | LEF | Welsing et al., 2004204 | ? | 5 years | cDMARDs | €544,000 |
The range of price year, currencies, discount rates and time horizons means that drawing strong conclusions regarding the cost-effectiveness of particular therapies is not possible, and would probably be misleading. In addtition, the complex nature of RA and the range of parameters required to develop a cost-effectiveness model mean that a very detailed review of each study would be required, which was not feasible. In some instances, the price year was not reported, and in a few cases it was not clear if bDMARDs were given with concomitant MTX or if they were a monotherapy. Results in GBP are all above the £30,000 per quality-adjusted life-year (QALY) threshold.
In general, the results in Table 51 suggest that bDMARDs are unlikely to be cost-effective in patients who have not undertaken DMARD therapy.
Like the DMARD-naive population, it is not possible to provide conclusions regarding the cost-effectiveness of individual treatments in the bDMARD-naive population.
Many bDMARDs have incremental cost-effectiveness ratios (ICERs) close to £30,000 per QALY threshold. No one bDMARD consistently seems to be cost-effective compared with any other bDMARD.
Jobanputra et al. ,172 Barton et al. 180 and Chen et al. 123 are HTA reports which informed the development of NICE TA36206 and TA130. 207 Taking the most recent HTA report by Chen et al. ,123 ADA, ADA plus MTX, ETN, ETN plus MTX and IFX plus MTX all have ICERs compared with cDMARDs exceeding £20,000 per QALY, and in many instances above £30,000 per QALY. However, these drugs have since been recommended in certain patient populations. This highlights the sensitivity of cost-effectiveness models to key parameters and modelling assumptions, and careful consideration of all aspects is required to ensure confidence in the final reported ICERs.
Critique of the manufacturers’ submissions
The Assessment Group received submissions for seven interventions. 152,156,159,173,174,208,209 These were from six manufacturers as both GOL and IFX are manufactured by MSD. The submission by Bristol-Myers Squibb evaluated both the i.v. and s.c. formulations of ABT. The length and quality of the submissions varied. For information, Figure 29 details the number of pages within each manufacturer’s submission. In addition, each submission contained a mathematical model.
FIGURE 29.
The number of pages in each submission (including appendices). BMS, Bristol-Myers Squibb.
An initial review of the submissions indicated that there were a multitude of methods employed and that attempting to summarise all seven submissions individually would probably not aid the reader. With this aim, the submissions have been summarised jointly under a number of categories to allow the reader to compare and contrast the methodologies used. This would remove the need for cross-referencing were the reader wanting to know the different assumptions made for a key variable or to quickly compare outputs from the model. Formal evaluation of these models using checklists such as the British Medical Journal (BMJ) or Eddy checklists210,211 was not possible within the time scales of the assessment; however, clear deviances from recommended methods have been outlined in the critique.
Where appropriate, tables and figures will be taken from the manufacturers’ submissions. Minor amendments, such as to the intervention abbreviations, have been made to ensure consistency throughout the report, where possible.
The broad headings chosen were the:
-
decision problem addressed
-
strategies modelled
-
model structure/time cycle
-
time horizon
-
perspective
-
discounting
-
population characteristics
-
the assumed costs of the interventions
-
costs of administration and monitoring
-
comparative treatment efficacy (NMAs)
-
responder criteria
-
HAQ/EQ-5D changes in relation to response levels
-
HAQ trajectory following initial response
-
time to discontinuation of treatment
-
rebound post treatment
-
assumed NHS costs per HAQ band
-
utility related to HAQ
-
assumed costs and disutilities associated with AEs
-
mortality associated with RA
-
cost-effectiveness results
-
cost implications within England and Wales.
Decision problem addressed
Table 52 summarises the decision problems addressed within the manufacturers’ submissions for those drugs that are licensed as monotherapy and for those that cannot. No detailed information is given in the tables which serve as reference only, with subtleties regarding each analysis provided in later sections. Four interventions (ABT i.v., ABT s.c., CTZ and TCZ) are not licensed before the use of MTX. Four interventions (ABT i.v., ABT s.c., GOL and IFX) are not licensed as monotherapy.
| Analysis | Decision problem | Assessment group’s interpretation of the scope | Manufacturer | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AbbVie (ADA) | Bristol-Myers Squibb (ABT) | MSD (GOL) | MSD (IFX) | Pfizer (ETN) | Roche (TCZ) | UCB Pharma (CTZ) | |||
| 1 | Population 2 in combination with MTX | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 2 | Population 3 in combination with MTX | ✓ | ✓ | ✓ | ✓ | ||||
| 3 | Population 1 in combination with MTX | ✓ | ✓ | ✓ | |||||
| 4 | Population 2 monotherapy | ✓ | ✓ | ✓ | ✓ | ||||
| 5 | Population 3 monotherapy | ✓ | ✓ | ✓ | |||||
| 6 | Population 1 monotherapy | ✓ | ✓ | ||||||
| 7 | General RA population who can tolerate MTXa | ✓ | ✓ | ✓ | |||||
| 8 | MTX intolerant or contraindicated RA populationb | ✓ | |||||||
Summary
It is seen that there was considerable variation in the decision problems addressed by the manufacturers with only the submissions by AbbVie and UCB Pharma evaluating all the subgroups within both the scope and the licence of their product.
Strategies modelled
The strategies modelled for each submission have been detailed individually for each manufacturer collated by the analyses numbers provided in Decision problem addressed. These are:
-
population 3 in combination with MTX
-
population 2 in combination with MTX
-
population 1 in combination with MTX
-
population 3 monotherapy
-
population 2 monotherapy
-
population 1 monotherapy
-
general RA population who can receive MTX
-
MTX intolerant or contraindicated RA population.
In summary, most strategies appeared reasonable, although it is noted that there were a few anomalies compared with NICE guidance or intervention licences:
-
MSD (GOL and IFX) and UCB Pharma (CTZ) assumed that TCZ would not be used following RTX.
-
MSD assumed in one strategy that RTX could be used without a bDMARD having been provided previously.
-
Pfizer (ETN) assumed that ABT i.v. would be used third line if TCZ was used first line.
-
Roche (TCZ) assumed a standard sequence of care for those intolerant or contraindicated to MTX that included three lines of bDMARDs, and evaluated only one sequence where TCZ was inserted as the first-line treatment to create four lines of bDMARDs.
-
Importantly, UCB Pharma did not compare with a cDMARD-only option for analyses 1 and 4.
AbbVie
The strategies employed in the AbbVie submission are contained in Tables 53–56. These appear appropriate, although it is noted that ‘rescue’ treatment was not explicitly defined by the manufacturer.
| Treatment number | Sequence number | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| 1 | LEF | ADA + MTX | ETN + MTX | IFX + MTX | CTZ + MTX | GOL + MTX | ABT + MTX | TCZ + MTX |
| 2 | SSZ | RTX + MTX | RTX + MTX | RTX + MTX | RTX + MTX | RTX + MTX | RTX + MTX | RTX + MTX |
| 3 | CYC | TCZ + MTX | TCZ + MTX | TCZ + MTX | TCZ + MTX | TCZ + MTX | TCZ + MTX | LEF |
| 4 | Rescue | LEF | LEF | LEF | LEF | LEF | LEF | SSZ |
| 5 | SSZ | SSZ | SSZ | SSZ | SSZ | SSZ | CYC | |
| 6 | CYC | CYC | CYC | CYC | CYC | CYC | Rescue | |
| 7 | Rescue | Rescue | Rescue | Rescue | Rescue | Rescue | ||
| Treatment number | Sequence number | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 1 | MTX | ADA + MTX | ETN + MTX | IFX + MTX | GOL + MTX | MTX + HCQ |
| 2 | SSZ | RTX + MTX | RTX + MTX | RTX + MTX | RTX + MTX | ADA + MTX |
| 3 | HCQ | TCZ + MTX | TCZ + MTX | TCZ + MTX | TCZ + MTX | RTX + MTX |
| 4 | LEF | LEF | LEF | LEF | LEF | TCZ + MTX |
| 5 | CYC | SSZ | SSZ | SSZ | SSZ | LEF |
| 6 | Rescue | CYC | CYC | CYC | CYC | SSZ |
| 7 | Rescue | Rescue | Rescue | Rescue | CYC | |
| 8 | Rescue | |||||
| Treatment number | Sequence number | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 1 | SSZ + HCQ | ADA | ETN | CTZ | TCZ |
| 2 | LEF | LEF | LEF | LEF | LEF |
| 3 | SSZ | SSZ | SSZ | SSZ | SSZ |
| 4 | CYC | CYC | CYC | CYC | CYC |
| 5 | Rescue | Rescue | Rescue | Rescue | Rescue |
| Treatment number | Sequence number | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 1 | SSZ + HCQ | ADA | ETN | SSZ + HCQ |
| 2 | LEF | LEF | LEF | ADA |
| 3 | SSZ | SSZ | SSZ | LEF |
| 4 | CYC | CYC | CYC | SSZ |
| 5 | Rescue | Rescue | Rescue | CYC |
| 6 | Rescue | |||
Bristol-Myers Squibb
The strategies employed in the Bristol-Myers Squibb submission are contained in Table 57. These appear appropriate.
| Sequences | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | LEF | ABT i.v. + MTX | ABT s.c. + MTX | ADA + MTX | CTZ + MTX | ETN + MTX | GOL + MTX | IFX + MTX | TCZ + MTX |
| 2 | GLD | RTX + MTXa | RTX + MTXa | RTX + MTXa | RTX + MTXa | RTX + MTXa | RTX + MTXa | RTX + MTXa | RTX + MTXa |
| 3 | CYC | TCZ + MTXb | TCZ + MTXb | TCZ + MTXb | TCZ + MTXb | TCZ + MTXb | TCZ + MTXb | TCZ + MTXb | LEF |
| 4 | AZA | LEF | LEF | LEF | LEF | LEF | LEF | GLD | GLD |
| 5 | PC | GLD | GLD | GLD | GLD | GLD | GLD | CYC | CYC |
| 6 | CYC | CYC | CYC | CYC | CYC | CYC | AZA | AZA | |
| 7 | AZA | AZA | AZA | AZA | AZA | AZA | PC | PC | |
| 8 | PC | PC | PC | PC | PC | PC | |||
The analyses assumed that if a patient had an AE within the first 6 months that a randomly sampled (and previously unused) bDMARD would be used instead.
If RTX was contraindicated, then a randomly sampled (and previously unused) bDMARD would be used instead.
From the model structure it appears that if there is a good response to RTX then TCZ would not be used as a third-line treatment option.
Merck Sharp & Dohme Corp.
For brevity, the strategies for GOL and IFX have been discussed jointly as they are identical. The strategies employed in the MSD submissions are contained in Table 58. It is noted that these do not allow TCZ to be used as a third-line biologic as allowed within NICE guidance. MSD assumes that the first- and second-line treatment options have been used prior to the decision point. The Assessment Group comment that the use of RTX in the MTX arm is outside of licence as a bDMARD must have been provided prior to RTX.
| Treatment number | IFX arm | GOL arm | Other biologic DMARD arm | MTX arm |
|---|---|---|---|---|
| 1 | IFX + MTX | GOL + MTX | Biologic DMARD + MTX | MTX |
| 2 | RTX | RTX | RTX | RTX |
| 3 | LEF | LEF | LEF | LEF |
| 4 | GLD | GLD | GLD | GLD |
| 5 | AZA | AZA | AZA | AZA |
| 6 | CYC | CYC | CYC | CYC |
| 7 | PC | PC | PC | PC |
All patients were assumed to have previous lines of MTX and SSZ plus MTX.
The other bDMARDs evaluated were ETN, ADA, CTZ, TCZ, ABT i.v. and ABT s.c.
Pfizer
The strategies employed in the Pfizer submission are contained in Tables 59 and 60. It is noted that the strategy with TCZ first does not follow NICE guidance in that ABT i.v. is used as a third-line treatment.
| Treatment number | Sequences | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 1 | ETN | ABT i.v. | ABT s.c. | CTZ | ADA | IFX | TCZ | GOL | cDMARD | Combination cDMARD |
| 2 | RTX | RTX | RTX | RTX | RTX | RTX | RTX | RTX | RTX | RTX |
| 3 | TCZ | TCZ | TCZ | TCZ | TCZ | TCZ | ABT i.v. | TCZ | TCZ | TCZ |
| 4 | SSZ | SSZ | SSZ | SSZ | SSZ | SSZ | SSZ | SSZ | SSZ | SSZ |
| 5 | LEF | LEF | LEF | LEF | LEF | LEF | LEF | LEF | LEF | LEF |
| 6 | PC | PC | PC | PC | PC | PC | PC | PC | PC | PC |
| Treatment sequences applied by analysis | ||||||||||
| Analysis 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Analysis 2 | ✓ | ✓ | ||||||||
| Analysis 3 | ✓ | ✓ | ✓ | |||||||
| Treatment number | Sequences | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 1 | ETN | ADA | TCZ | TCZ | cDMARD |
| 2 | ADA | ETN | ETN | ADA | ETN |
| 3 | SSZ | SSZ | SSZ | SSZ | SSZ |
| 4 | LEF | LEF | LEF | LEF | LEF |
| 5 | PC | PC | PC | PC | PC |
Roche
Roche evaluated a very limited set of sequences, which consisted of inserting TCZ before a standard sequence of care. This is replicated in Figure 30. Roche evaluated only a sequence of MTX-intolerant or -contraindicated RA population. It is noted that Roche assumes that the standard of care sequence has three lines of bDMARD treatments (followed by palliative care), which is not in accordance with current NICE guidance. Roche evaluated only one sequence in which TCZ was inserted as the first-line treatment to create four lines of bDMARDs.
FIGURE 30.
Strategies modelled by Roche for analysis 8. (a) TCZ sequence; and (b) standard of care sequence.

UCB Pharma
The strategies modelled by UCB Pharma are given in Table 61 and 62. The Assessment Group notes that in the MTX-experienced populations with DAS> 5.1, the continuing use of cDMARDs was not a comparator strategy, which is a serious deviation from the published scope.
| Set-up | Interventions/regimens |
|---|---|
| Comparators | Combination with MTX |
| CTZ | |
| ADA | |
| ETN | |
| GOL | |
| TCZ | |
| IFX | |
| ABT | |
| Monotherapies | |
| CTZ | |
| ADA | |
| ETN | |
| TCZ | |
| Follow-on interventions | RTX + MTX |
| AZA | |
| CYC | |
| GLD | |
| HCQ | |
| LEF | |
| Penicillamine | |
| Palliation |
| Set-up | Parameter |
|---|---|
| Comparators | CTZ + MTX |
| CTZ + cDMARDs | |
| PBO + MTX | |
| PBO + cDMARDs | |
| Follow-on interventions | MTX + SSZ |
| MTX + SSZ + HCQ | |
| MTX + HCQ | |
| MTX + LEF | |
| SSZ + HCQ | |
| CYC | |
| Penicillamine | |
| Palliation |
Model structure/time cycle
This section details the model structure employed by each manufacturer. The two submissions from MSD have been assessed jointly as they have the same structure.
Broad summary
Four individual patient models and two cohort models were submitted. Of the four individual patient-level models, three used discrete event simulation (DES) techniques, which do not need time cycles, with the remainder using a 6-month cycle. Of the two cohort models, one used a 6-month time cycle, whereas the other adopted this after the initial year, with either three cycles of 6, 3 and 3 months in the first year, or 3, 4.5 and 4.5 months depending on the user input. Both cohort models used a half-cycle correction.
Four of the models were constructed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA); one in Arena (©Rockwell Automation, Milwaukee, WI, USA); and one in SIMUL8 (Simul8 Corporation, Boston, MA, USA).
AbbVie
The model is an individual-patient simulation based within Arena run for a cohort of 1000 patients, each with specific baseline characteristics, which are sampled from distributions specified in an Excel input shell. One hundred and fifty replications are done for each analysis to create 150,000 patients per treatment sequence. The overview of the model logic is shown in Figure 31. The model uses a DES approach; thus, there are no time cycles, although all patients are assumed to stay on treatment for 6 months (unless an AE occurs).
FIGURE 31.
The AbbVie model structure.

Bristol-Myers Squibb
Bristol-Myers Squibb reproduced the individual patient model built by Malottki et al. 171 but added first-line biologics to the beginning of the model. This was implemented in SIMUL8 and does not require time cycles. The model logic is shown in Figure 32.
FIGURE 32.
The Bristol-Myers Squibb model structure.

Merck Sharp & Dohme Corp.
A Markov model constructed in Excel was used to estimate the expected costs and QALYs of patients with RA. A time cycle of 6 months was used with half-cycle correction.
The model structure is depicted in Figure 33.
FIGURE 33.
The MSD model structure.
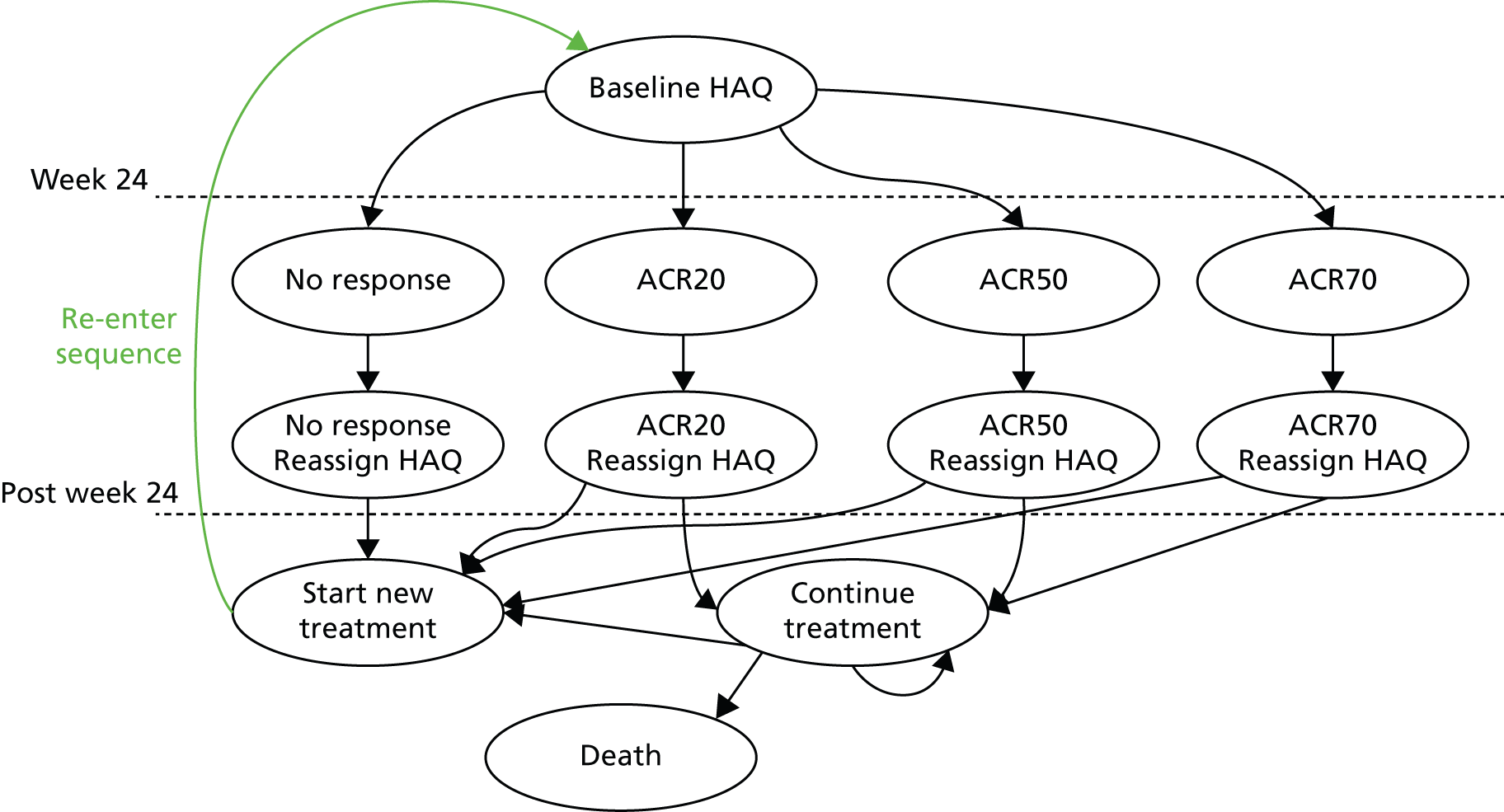
Pfizer
The model was developed in Excel with visual basic for applications and uses a DES approach to model individual patients. As the model uses a DES approach, no time cycles were necessary.
Time on treatment and disease progression are time dependent, whereas modelling the effects of treatment withdrawal and any subsequent rebound effect requires knowledge of patients’ disease status prior to treatment.
The model structure is summarised in Figure 34 and is applicable to each decision problem evaluated.
FIGURE 34.
The Pfizer model structure. (AiC information has been removed.)
Roche
The manufacturer reports that the design of the economic analysis follows guidelines set by the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) Economics Working Group. 212,213
The economic analysis is based on an individual patient model designed in Excel with the use of Visual Basic applications. The model tracks the characteristics of the individuals and maintains a history in particular of a patient’s response to treatment in their assigned drug sequence and change in HAQ score over time.
The model algorithm is presented in Box 2.
Start the simulation.
For patients i = 1, 2, . . . , n, cycles k = 1, 2, . . . , n a random number drawn by a continuous uniform distribution θ ∼ U[0,1], and the relevant risk factor p.
Determine the path of patient i through the model by θi,k ≤ pk
Determine cost ci and utility ui for individual i.
End the simulation.
Estimate the mean cost and utility E[(C, U)] by
a^n=1n∑i=1n(ci,ui).
The model implements a 6-month cycle length, which is in line with timing of available efficacy evidence (ACR data). Patients transition through the model by sequentially moving on to each treatment. Once patients exhaust all treatments in the sequence, they move into palliative care where they remain until death.
UCB Pharma
The cost-effectiveness model is a Markov (cohort health state transition) structure constructed in Excel.
The first model cycle is either 3 or 6 months (12 or 24 weeks), depending on the definition of response selected in the model and reflective of the published clinical guidance [6 months (24 weeks) is used in the base case]. The model allows for clinical response to be measured by either ACR response criteria (developed by the ACR) or EULAR response criteria (developed by the EULAR).
Two further model cycles in the first year are common to both the severe and moderate disease activity populations. Where the first model cycle has been chosen to be 3 months, the subsequent two time steps are each 4.5 months long. Where the first model cycle has been chosen to be 6 months, the subsequent two time steps are each 3 months long. The maximum time step length in the model is 6 months.
At the end of the next and following cycles, patients may remain in the same Markov state, discontinue treatment owing to an AE, discontinue treatment owing to lack of efficacy or intolerance, or die. There are no state transitions other than discontinuation of treatment and death. Discontinuation of treatment was assumed to be the same for all comparators, which was deemed to be a conservative assumption. Transition probabilities were calculated to appropriately reflect the varying length of time steps in the first model year. After the first 12 months, the cycle length is 6 months, reflecting the frequency of monitoring recommended by NICE and the British Society of Rheumatology. A half-cycle correction was employed.
The model structure based on ACR response is depicted in Figure 35 and the model structure based on EULAR response is depicted in Figure 36.
FIGURE 35.
Markov structure: severe disease activity population; model structure based on ACR response presented by UCB Pharma. (AiC information has been removed.)
FIGURE 36.
Markov structure: moderate disease activity population; model structure based on EULAR response presented by UCB Pharma. (AiC information has been removed.)
Time horizon
The time horizon for each model is detailed below. In summary, all models adopted a lifetime, or approximately lifetime, time horizon.
AbbVie
The AbbVie model used a lifetime horizon.
Bristol-Myers Squibb
The Bristol-Myers Squibb model used a lifetime horizon.
Merck Sharp & Dohme Corp.
The MSD model used a time horizon of 45 years, assuming that patients with moderate to severe RA would die at a maximum 95 years and those with severe RA would die at a maximum age of 96 years. Shorter analysis time frames were used in the sensitivity analyses.
Pfizer
The Pfizer model used a lifetime horizon. Shorter analysis timeframes were used in the sensitivity analyses.
Roche
The Bristol-Myers Squibb model used a lifetime horizon.
UCB Pharma
The time horizon in the base-case analysis was an approximation of the lifetime of a patient. UCB Pharma stated that analysis of BSRBR data has revealed an average age of patients starting on TNF inhibitors of 55 years. 214 A time frame of 45 years would assume that patients would die at a maximum age of 100 years. Shorter analysis timeframes were used in the sensitivity analyses.
Perspective
The perspectives adopted in the submissions are detailed below. In summary, all submissions used an NHS and Personal Social Services perspective.
AbbVie
The base-case analysis of the economic evaluation was conducted from a NHS and Personal Social Services perspective. AbbVie note that resource use data related to Personal and Social Services for the management of RA in the UK were not available for costing purposes.
Bristol-Myers Squibb
Although not explicitly stated, the Bristol-Myers Squibb model adopts a NHS and Personal Social Services perspective.
Merck Sharp & Dohme Corp.
The MSD analysis is conducted from the UK NHS perspective. Direct costs included the drug cost, administration cost and heath-care resource use.
Pfizer
The current analysis was conducted from the perspective of the UK NHS and Personal Social Services.
Roche
The Roche submission used an NHS and Personal Social Services perspective.
UCB Pharma
The model takes a payer perspective (i.e. that of the NHS and Personal Social Services), as per NICE guidance, and includes direct medical costs such as hospital care (inpatient and outpatient), primary care and home visits. Sensitivity analyses were conducted using a societal perspective.
Discounting
The discount rates used within the submissions are shown in Table 63. In summary, each submission used the appropriate discount rate in the base-case analysis.
| Manufacturer | Base case | Sensitivity analyses | ||
|---|---|---|---|---|
| Costs | QALYs | Costs | QALYs | |
| AbbVie | 3.5% | 3.5% | 6.0% | 1.5% |
| 1.5% | 1.5% | |||
| Bristol-Myers Squibb | 3.5% | 3.5% | ||
| MSD | 3.5% | 3.5% | 0.0% | 3.5% |
| 3.5% | 0.0% | |||
| 0.0% | 0.0% | |||
| Pfizer | 3.5% | 3.5% | 6.0% | 1.5% |
| Roche | 3.5% | 3.5% | ||
| UCB Pharma | 3.5% | 3.5% | 6.0% | 1.5% |
| 1.5% | 6.0% | |||
| 1.5% | 1.5% | |||
| 6.0% | 6.0% | |||
Population characteristics
The population characteristics for each submission are detailed in this section. In summary, the manufacturers often use drug-specific data from the BSRBR, or from the trials related to their intervention. Typically no comment is made regarding the correlation between parameters with the exception of Pfizer’s model.
AbbVie
The baseline characteristics for patients considered within the AbbVie analyses come from different sources, of which it was stated that wherever possible the source was chosen to reflect the composition of the treated population for RA in the UK. For MTX-experienced patients with moderate disease activity the source was the ReAct study. 215 Data from the BSRBR for this patient population could not be used, because historically patients in the UK have always required a DAS28 of > 5.1 to receive an antiTNF; as such, any patients in the BSRBR with a DAS28 of < 5.1 who received an antiTNF are very select group of patients with non-normal characteristics. For MTX-experienced patients with severe disease activity the source was the BSRBR data. AbbVie report that analysis was undertaken on BSRBR data for ADA from the raw BSRBR. This analysis was presented as AiC data. For MTX-naive patients with severe disease activity the source was the PREMIER trial. 109 The characteristics of patients for each of those populations are outlined in Tables 64–66. No comment is made on the correlation of parameters.
| Patient characteristic | Value (SD) |
|---|---|
| Sex (% female) | 81.4 |
| Age (years) | 54.6 |
| Baseline HAQ-DI | 1.5 (0.65) |
| Disease duration (years) | 10.65 (8.56) |
| Patient characteristic | Value (SD) |
|---|---|
| Sex (% female) | AiC information has been removed |
| Age (years) (males/females) | AiC information has been removed |
| Baseline HAQ-DI (males/females) | AiC information has been removed |
| Disease duration (years) | AiC information has been removed |
| Patient characteristic | Value (SD) |
|---|---|
| Sex (% female) | 75.0 |
| Age (years) (males/females) | 60.8/58.0 |
| Baseline HAQ-DI (males/females) | 1.38 (0.62)/1.58 (0.65) |
| Disease duration (years) | 11.28 (9.07) |
For each subpopulation several sensitivity analyses were conducted, to take into account the effect in the cost-effectiveness estimates of applying the sequences to a fully male or fully female population; a population with average starting age of 55 years or 65 years; a population with average baseline HAQ score of 1.0, 1.5 or 2.0. There is no comment on the correlation assumed between the distributions.
Bristol-Myers Squibb
The Bristol-Myers Squibb patient-level simulation model generates a group of virtual patients, who are assigned individual characteristics, such that each patient has their own sex, age and HAQ score. These values were taken from Chen et al. ,123 and are reproduced in Tables 67 and 68. It is not commented whether or not the age and sex distributions are assumed to be correlated with HAQ distribution.
| Sex | Age (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| 15–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75–84 | Total | |
| Male | 0.9% | 2.5% | 5.4% | 8.3% | 9.0% | 6.8% | 5.1% | 38% |
| Female | 1.5% | 4.0% | 8.8% | 13.7% | 14.7% | 10.9% | 8.4% | 62% |
| Starting HAQ-DI score | 0.125 | 0.25 | 0.375 | 0.5 | 0.625 | 0.75 | 0.875 | 1 | 1.125 | 1.25 | 1.375 | 1.5 | 1.625 | 1.75 | 1.875 | 2 | 2.125 | 2.25 | 2.375 | 2.5 | 2.625 | 2.75 | 2.875 | 3 |
| Patients (%) | 3.1 | 6.7 | 6.7 | 5.8 | 5.3 | 4.9 | 4.8 | 3.1 | 6.7 | 6.7 | 5.8 | 6.3 | 6.6 | 7.0 | 6.9 | 6.2 | 4.7 | 2.7 | 0.9 | 0.1 | 0 | 0 | 0 | 0 |
It is commented that the mean of the assumed duration is a HAQ of 1.22.
Merck Sharp & Dohme Corp.: golimumab
It is reported that the base-case analysis reflects the GO-FORWARD216 population and the subgroup analysis reflects the severe patient group (DAS of > 5.1) from GO-FORWARD. 92 No comment is made on the correlation between parameters.
Merck Sharp & Dohme Corp.: infliximab
It is reported that the base-case analysis reflects the Anti-TNF trial in Rheumatoid Arthritis with Concomitant Therapy (ATTRACT)75 population and the subgroup analysis reflects the severe patient group (DAS28 of > 5.1) from ATTRACT. No comment is made on the correlation between parameters.
Pfizer
Patients used in the Pfizer model are subdivided into three groups: severe DMARD-inadequate responders; moderate to severe inadequate responders; and severe naive patients. The following text is taken largely from the Pfizer submission.
Severe disease-modifying antirheumatic drug-inadequate responders
Characteristics of individual patients in the severe DMARD-inadequate responder population were sampled (with replacement) directly from the baseline ETN BSRBR patient cohort (Table 69). This method has the advantage of maintaining correlation between variables without reliance on strong distributional assumptions, such as multivariate normality, or complex copula-based processes to specify arbitrary marginal distributions. Table 69 presents a summary of the population characteristics assumed within the model for all populations.
| AiC information has been removed) | AiC information has been removed | AiC information has been removed | AiC information has been removed | ||||
|---|---|---|---|---|---|---|---|
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | ||||
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
Moderate to severe disease-modifying antirheumatic drug-inadequate responders
The ETN BSRBR cohort with DAS of ≤ 5.1 was not considered sufficiently generalisable to the moderate to severe population. Patient characteristics for the moderate to severe population were simulated using summary statistics from PRESERVE,217 with the correlation structure taken from the BSRBR (n = 3780). The implicit assumption is that the correlation between variables in these two populations is the same. The population was generated with no restrictions on DAS, and then an acceptance–rejection algorithm was used to redraw characteristics for patients in whom the simulated DAS28 was outside the 3.2–5.1 range or who had a simulated age of < 18 years. This avoided any artificial truncation caused by, for example, assuming all patients simulated with a DAS28 of < 3.2 had a DAS28 = 3.2 and preserved the correlation between variables.
Severe disease-modifying antirheumatic drug-naive patients
Patients within the ETN BSRBR cohort enter the registry within the context of current clinical practice. As current clinical guidance from NICE does not permit the use of bDMARDs before the failure of two conventional DMARDs, the ETN BSRBR cohort does not contain a patient population generalisable to the severe DMARD-naive population. In order to generate this cohort, characteristics were sampled using summary statistics from Combination Of METhotrexate and etabercept in early rheumatoid arthritis (COMET),81 assuming the correlation structure from the ETN BSRBR cohort. The simulation of patients used acceptance/rejection criteria as described for moderate to severe DMARD-inadequate responders in order to ensure all patients had a DAS28 of > 5.1 and were aged ≥ 18 years.
Roche
Roche report that the modelled patient population is consistent with both the drug licence and populations from TCZ and comparator Phase III trials. The population comprises moderate to severe RA patients who have had an inadequate response to one or more cDMARDs, and who are intolerant or contraindicated to MTX.
All baseline characteristics in the model are taken from the Phase IV ADACTA study,58 with the exception of the average patient weight. The average patient weight in the ADACTA study58 was 77 kg, significantly higher than previous estimates for the UK population.
Therefore, Roche used the 70 kg weight previously accepted in NICE TAs (TA130,207 TA19528 and TA24726). The Assessment Group comment that the assumed lower weight assumed by Roche is likely to underestimate the costs of TCZ, as a person weighing 70 kg requires a 400-mg and a 200-mg vial, whereas a person weighing 77 kg would require an additional 80-mg vial.
A summary of the patient characteristic data assumed by Roche is provided in Table 70. No comment is made on the correlation of the parameters.
UCB Pharma
UCB Pharma simulated patients with RA and a moderate or severe disease activity who have had an inadequate response to MTX. The cost-effectiveness of CTZ versus alternative treatments was evaluated separately for the moderate and severe disease activity populations.
Baseline characteristics of the severe RA population and the moderate to severe RA population were based on mean estimates from the CRZ trials, which were assumed to reflect the population eligible for treatment with CTZ in clinical practice (Table 71). Baseline characteristics for the severe disease activity population were based on the pooled estimates from the Rheumatoid Arthritis Prevention of structural Damage (RAPID)1,135 RAPID2136 and FAST4WARD218 studies (including both the CTZ and PBO treatment arms). Baseline characteristics for the moderate disease activity population were based on estimates from the CERTAIN79 study (including both the CTZ and PBO treatment arms). Some data were presented as AiC. No comment is made on the correlation between parameters.
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
|---|---|---|
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
The assumed costs of the interventions
This section details the costs assumed by each manufacturer; administration and monitoring costs are included in a separate section. In summary, the costs seem appropriate apart from the following points: AbbVie does not consider current PASs; Bristol-Myers Squibb and Roche assume that all patients weigh 70 kg, which is likely to underestimate the costs for weight-based dosages (bar GOL); neither Pfizer nor UCB Pharma includes PASs for TCZ or ABT as these are commercial-in-confidence (CiC); MSD does not include the PAS for ABT.
All manufacturers assumed vial wastage for ABT i.v., TCZ and IFX, although Roche discuss that where the appropriate dose is only marginally above that produced by a combination of vials a clinician may opt not to open a new vial.
Both Roche and UCB Pharma assume that it is possible for treatment to be discontinued after 3 months rather than 6 months through lack of efficacy.
AbbVie
The cost of all drugs used in the AbbVie analyses was calculated based on the recommended dosages and vial prices given in the Monthly Index of Medical Specialties 2013. 219 Importantly, the impact to the NHS of PASs on the cost of certain drugs was not taken into account in the analysis. AbbVie cited the NICE Methods Guide,220 which states that PAS are valid until NICE TA review, at which point manufacturers will need to agree a new PAS (even if it remains constant) for the appraisal review. As such, it is not known if all the current PASs in existence will be agreed again by the PAS’s Liaison Unit and this is why they have not been included in the analysis. No sensitivity analyses were conducted using existing PASs. This is unfavourable to CTZ, as the initial 10 doses are provided free; ABT and TCZ, for which AiC discounts are provided; and GOL who provide the 100-mg dose of GOL at the same price as the 50-mg dose.
AbbVie provides detailed breakdown of all conventional DMARDs and biologic treatments and does take patient weight into consideration. ABT s.c. is not considered. The cost per dose for biologic treatments assumed by AbbVie is reproduced in Table 72.
| Treatment | Dose regimen | Cost per dose (£) |
|---|---|---|
| ADA | 40 mg; every other week | 352.14 |
| ETN | 50 mg; every week | 178.75 |
| IFX | 3 mg/kg: at 0, 2 and 6 weeks, then every 8 weeks | 1133.28 |
| ABT | 500 mg if weight is below 60 kg, 750 mg if weight is between 60 kg and 100 kg, 1000 mg if weight is above 100 kg; at 0, 2 and 4 weeks, then every 4 weeks thereafter | 856.27 |
| RTX | 1000 mg followed by 1000 mg 2 weeks later; repeated every 9 months | 1746.30 |
| GOL | 50 mg if weight is below 100 kg, 100 mg if weight is above 100 kg, per month | 832.09 |
| TCZ | 8 mg/kg every 4 weeks | 782.67 |
| CTZ | 400 mg; repeated 2 weeks and 4 weeks after initial injection | 715.00 |
| CTZ | 200 mg; repeated every 2 weeks thereafter | 357.50 |
For interventions that are weight dependent, AbbVie examined the weight distribution of patients enrolled in the BSRBR from the ADA cohort (n = 4364 patients) to determine the most likely average annual drug acquisition cost of TCZ, ABT, INF and GOL in the UK.
Tables 73–76 show the calculations undertaken by AbbVie to establish average cost per dose.
| Possible combinations of TCZ vials (mg) | Total dose (mg) | Lower weight (kg) | Upper weight (kg) | Cost per dose (£) | % patients in BSRBR | Annual cost (£) |
|---|---|---|---|---|---|---|
| 80 + 80 + 80 | 240 | – | 30 | 307.20 | 0.05 | 3993.60 |
| 200 + 80 | 280 | 31 | 35 | 358.40 | 0.18 | 4659.20 |
| 200 + 80 + 80 | 360 | 36 | 45 | 460.80 | 1.67 | 5990.40 |
| 400 | 400 | 46 | 50 | 512.00 | 3.94 | 6656.00 |
| 400 + 80 | 480 | 51 | 60 | 614.40 | 18.42 | 7987.20 |
| 400 + 80 + 80 | 560 | 61 | 70 | 716.80 | 23.97 | 9318.40 |
| 400 + 200 | 600 | 71 | 75 | 768.00 | 11.07 | 9984.00 |
| 400 + 200 + 80 | 680 | 76 | 85 | 870.40 | 17.42 | 11,315.20 |
| 400 + 200 + 80 + 80 | 760 | 86 | 95 | 972.80 | 11.73 | 12,646.40 |
| 400 + 400 | 800 | 96 | – | 1024.00 | 11.55 | 13,312.00 |
| Average cost per dose | 782.67 | |||||
| Average cost per year (13 doses) | 10,174.65 | |||||
| Number of vials | Lower weight (kg) | Upper weight (kg) | Cost per dose (£) | % patients in BSRBR | Annual cost (first year) (£) | Annual cost (second year and beyond) (£) |
|---|---|---|---|---|---|---|
| Two | – | 60 | 604.80 | 24.27 | 8467.20 | 7862.40 |
| Three | 61 | 100 | 907.20 | 68.31 | 12,700.80 | 11,793.60 |
| Four | 36 | 45 | 1209.60 | 7.42 | 16,934.40 | 15,724.80 |
| Average cost per dose | 856.27 | |||||
| Average cost per year (14 doses in the first year, 13 doses for second year and beyond) | 11,987.76 | 11,131.49 | ||||
| Number of vials | Lower weight (kg) | Upper weight (kg) | Cost per dose (£) | % patients in BSRBR | Annual cost (first year) (£) | Annual cost (second year and beyond) (£) |
|---|---|---|---|---|---|---|
| One | – | 33 | 419.62 | 0.14 | 3356.96 | 2727.53 |
| Two | 34 | 66 | 839.24 | 38.13 | 6713.92 | 5455.06 |
| Three | 67 | 99 | 1258.86 | 54.31 | 10,070.88 | 8182.59 |
| Four | 100 | 133 | 1678.48 | 6.58 | 13,427.84 | 10,910.12 |
| Five | 134 | 166 | 2098.10 | 0.64 | 16,784.80 | 13,637.65 |
| Six | 167 | – | 2517.72 | 0.21 | 20,141.76 | 16,365.18 |
| Average cost per dose | 1133.28 | |||||
| Average cost per year (8 doses in the first year, 6.5 doses on average for second year and beyond) | 9066.25 | 7366.33 | ||||
| Number of pens | Lower weight (kg) | Upper weight (kg) | Cost per dose (£) | % patients in BSRBR | Annual cost (£) |
|---|---|---|---|---|---|
| One | – | 100 | 774.58 | 92.58 | 9294.96 |
| Two | 101 | – | 1549.16 | 7.42 | 18,589.92 |
| Average cost per dose | 832.09 | ||||
| Average cost per year (12 doses)a | 11,649.23 | ||||
Bristol-Myers Squibb
Bristol-Myers Squibb estimates the yearly costs of each intervention and additional costs incurred in the first year due to loading doses. Bristol-Myers Squibb assumes that all patients weigh 70 kg, the lack of uncertainty in this value will likely favour those interventions that are weight based, and in particular TCZ. Bristol-Myers Squibb considers PAS in place at the start of the appraisal, two of which, for TCZ and for both ABT formulations, are CiC. The bDMARDs costs assumed by Bristol-Myers Squibb are replicated in Table 77.
| Treatment | Annual cost (£) | Year 1 start-up cost (£) |
|---|---|---|
| ABT i.v. | CiC information has been removed | CiC information has been removed |
| ABT s.c. | CiC information has been removed | (CiC information has been removed) |
| ADA | 9187 | 0 |
| ETN | 9327 | 0 |
| IFX | 8211 | 1259 |
| TCZ | CiC information has been removed | CiC information has been removed |
| GOL | 9156 | 0 |
| CTZ | 9327 | –2503a |
| RTX | 4817 | 0 |
| LEF | 747 | 0 |
| GLD | 135 | 225 |
| CYC | 1685 | 0 |
| AZA | 98 | 0 |
| MTX | 18 | 0 |
Merck Sharp & Dohme Corp.
Merck Sharp & Dohme Corp. has distinguished between the costs in the first 6 months, when loading doses may be needed, and costs in subsequent 6-month cycles. These are replicated in Table 78. The PAS for CTZ and GOL have been applied, but neither the TCZ nor the ABT PAS (which are CiC) is used.
| Intervention | Cost per dose (£) | Number of doses per first 6 months | Number of doses post 6 months | Treatment cost first 6 months (£) | Treatment cost post 6 months (£) |
|---|---|---|---|---|---|
| GOL | 762.97 | 6 | 6 | 4577.82 | 4577.82 |
| ADA | 352.14 | 13 | 13 | 4577.82 | 4577.82 |
| IFXa | 1133.20 | 5 | 3.25 | 5666.00 | 3682.90 |
| ETN | 89.38 | 52 | 52 | 4647.76 | 4647.76 |
| TCZb | 698.32 | 7 | 6.5 | 4888.24 | 4539.08 |
| CTZc | 357.50 | 6 | 13 | 2145.00 | 4647.50 |
| LEF | 1.88 | 205 | 178 | 385.40 | 334.64 |
| GLD | 13.48 | 26 | 26 | 350.48 | 350.48 |
| AZA | 0.07 | 547.5 | 547.5 | 38.33 | 38.33 |
| CYC | 2.14 | 365 | 365 | 781.10 | 781.10 |
| MTX | 0.05 | 78 | 78 | 3.90 | 3.90 |
| ABT i.v.d | 864.92 | 8 | 6.5 | 6919.35 | 5621.97 |
| ABT s.c.e | 302.40 | 26 | 26 | 8727.32 | 7862.40 |
| RTX | 1746.30 | 2 | 1.3 | 3492.60 | 2270.19 |
The costs for weight-based doses were calculated based on the weight distributions of 2775 IFX patients within the BSRBR database to estimate the average number of full vials that are used per patient (or in the case of TCZ the weighted-average cost per patient). These data are shown in Table 79. The Assessment Group notes that the TCZ costs are inaccurate, as a patient weighing between 46 kg and 50 kg would be most inexpensively treated with a 400-mg vial alone, an option not considered.
| Category | 0–33 kg | 34–59 kg | 60–66 kg | 67–100 kg | 101–133 kg | > 134 kg (maximum weight 174 kg) | Total |
|---|---|---|---|---|---|---|---|
| Number in each IFX weight group | 2 | 574 | 465 | 1546 | 176 | 12 | 2775 |
| Percentage in each group | 0.07 | 20.68 | 16.76 | 55.71 | 6.34 | 0.43 | 100 |
| IFX vials per group (3 mg/kg) | 1 | 2 | 2 | 3 | 4 | 6 | – |
| ABT i.v. vials per group | 2 | 2 | 3 | 3 | 4 | 4 | – |
| TCZ vials per group (8 mg/kg) | 200 mg + 80 mg | 400 mg + 80 mg | 400 mg + 400 mg + 80 mg | 400 mg + 400 mg | 400 mg + 400 mg | 400 mg + 400 mg | – |
| Cost per patient per weight group (£) | 358.40 | 614.40 | 716.80 | 1024.00 | 1024.00 | 1024.00 | – |
| Weighted average IFX vials per infusion: 2.70 | |||||||
| Weighted average ABT i.v. vials per infusion: 2.86 | |||||||
| Weighted average TCZ cost per infusion: £887.32 | |||||||
As an example, the calculation for the weighted-average vials of IFX is as follows:
Pfizer
Drug costs in the Pfizer submission were taken from publicly available sources, including PASs for CTZ and GOL. PASs which are not in the public domain, such as those for TCZ, ABT i.v. and ABT s.c., were not included.
For therapies administered based on the individual’s weight, costs were calculated for each patient individually, and vial wastage was permitted.
Palliative care was assumed to consist of a combination of MTX, LEF and ciclosporin. This was assumed to represent a proxy for the cost of treatment in this line of therapy given the heterogeneous nature of treatments that are likely to be given at this stage, in order to try to control disease progression. Costs at this line of therapy are likely to be extremely heterogeneous and no accurate cost estimate was available; however, given that patients reach palliative care after several lines of therapy, potentially taking many years, the effect of discounting will be to make this assumption less influential.
Where applicable [in, for example, the severe DMARD-inadequate responder (monotherapy) population], the cost of the generic ‘cDMARD’ therapy was assumed to have the cost of MTX. Again, the cost was intended to act as a proxy for a generic therapy of this class in the absence of a definitive patient pathway. This is likely to be a conservative estimation given that MTX is the one of the cheapest cDMARDs available. A summary of the drug costs with dosing assumptions is provided in Table 80.
| Treatment | Dosing assumptions | Unit cost (£)a | Unit dose (mg) |
|---|---|---|---|
| ABT i.v. | Body weight ≤ 60 kg, 500 mg; 61–100 kg, 750 mg; > 100 kg, 100 mg; repeated 2 weeks and 4 weeks after initial infusion, then every 4 weeks | 302.40 | 250 |
| ADA | 40 mg every other week | 352.14 | 40 |
| CTZ | 400 mg at 0, 2 and 4 weeks then 200 mg every 2 weeks (PAS 10 for free) | 357.50 | 200 |
| CIC | Maximum of 4 mg/kg daily in two divided doses | 51.50 | 3000 |
| ETN | 25 mg BIW | 89.38 | 25 |
| ABT s.c. | Loading dose by i.v. initially, then first 125-mg s.c. injection given within a day, followed by 125-mg s.c. OW | 302.40b | 125 |
| GOL | 50 mg every 4 weeks | 762.97 | 50 |
| INF | 3 mg/kg at weeks 0, 2 and 6, thereafter every 8 weeks | 419.62 | 100 |
| LEF | Assumed 20 mg OD | 61.36 | 600 |
| MTX | 15 mg OW | 48.44 | 1000 |
| PC | Assumed to be additive combination of MTX, LEF, CIC (oral) | N/A | N/A |
| RTX | 1000 mg repeated 2 weeks after initial infusion = one course; each course 9 months apart | 873.15 | 500 |
| SSZ | 2000 mg/day | 14.83 | 56,000 |
| TCZ | 8 mg/kg every 4 weeks | 102.40 | 80 |
| Combination therapy with cDMARDs | Assumed to be additive combination of MTX and SUL | N/A | N/A |
Roche
The Roche submission considered only the use of TCZ in patients who are intolerant or contraindicated to MTX. It was assumed that all patients weigh 70 kg, although this was altered to 65 kg and 75 kg in sensitivity analyses. Table 81 presents the costs assumed by Roche, although it is noted that this table does not include the PAS for TCZ that is used within the mathematical model. It is commented that it has been assumed that non-responders would be removed from treatment at 3 months, which may underestimate the acquisition costs of treatments.
| Treatment | Dose regimena | Unit costb | Cost for first 6 months (£) | Cost per subsequent cycle (£) | |
|---|---|---|---|---|---|
| Non-responders | Responders | Responders | |||
| ADA | 40 mg every 2 weeks | £352.14 per 40-mg vial | 2289 | 4578 | 4578 |
| CTZ | 200 mg every 2 weeks | £357.50 per 200-mg syringe | 0 | 2324 | 4646 |
| ETA | 50 mg every week | £178.75 per 50-mg syringe | 2324 | 4648 | 4648 |
| TCZ | 8 mg/kg every 4 weeks | £1.28 per mg | 2330 | 4659 | 4659 |
UCB Pharma
The costs of drug acquisition were based on the recommended dosing schedules for treatment multiplied by the unit cost of treatment as reported in the British National Formulary 64. 30 The PASs for CTZ and GOL were included but the CiC PASs for ABT and TCZ were not incorporated.
For i.v. drugs that are administered based on body weight [ATB, INF, TCZ, azathioprine (AZA) and ciclosporin (CYC)], the weight distribution of patients enrolled in either the RAPID1,135 RAPID2136 and FAST4WARD218 trials (severe disease activity population) or the CERTAIN79 study (moderate disease activity population) was applied to estimate the number of vials used.
For drugs that require loading doses or irregular administration, various assumptions were made to estimate the dose received by patients during the first and subsequent 6 months of treatment:
-
For ABT, it was assumed that, during the first 6 months, treatment was administered at weeks 0, 2, 4, 8, 12, 16, 20 and 24, equating to eight administrations. During the subsequent 6 months, it was assumed that administrations occurred at a frequency of every 4 weeks, equating to 6.5 administrations over a 26-week cycle.
-
For INF, similar assumptions were made when estimating dosing, where treatment was administered at weeks 0, 2, 6, 14, and 22 during the first 6 months, and an average of 3.25 administrations during any subsequent 6-month period.
-
For CTZ, treatment was administered at weeks 0, 2 and 4 during the first month of treatment, with further doses administered every 2 weeks on a continuous basis until cessation.
A summary of the acquisition costs assumed by UCB Pharma is provided in Table 82.
| Treatment | Acquisition costs (£) | |
|---|---|---|
| First 6 months | Every 6 months thereafter | |
| Combination treatments with MTX (severe disease activity population) | ||
| CTZ + MTX | 2163 | 4666 |
| ABT i.v. + MTX | 7005 | 5695 |
| IFX + MTX | 5648 | 3677 |
| TCZ + MTX | 6475 | 6475 |
| ADA + MTX | 4596 | 4596 |
| ETN + MTX | 4666 | 4666 |
| GOL + MTX | 4596 | 4596 |
| Monotherapies (severe disease activity population) | ||
| CTZ | 2145 | 4648 |
| TCZ | 6457 | 6457 |
| ADA | 4578 | 4578 |
| ETN | 4648 | 4648 |
| Combination treatments (moderate disease activity population) | ||
| CTZ + MTX | 2163 | 4666 |
| CTZ + cDMARDs | 2255 | 4758 |
| PBO + MTX | 18 | 18 |
| PBO + cDMARDs | 111 | 111 |
Administration and monitoring costs
This section details the administration and monitoring costs assumed within the manufacturers’ submission. Many submissions provide detailed descriptions with multiple tables to support the monitoring costs used. These have been abridged within this summary for brevity. In summary, the monitoring costs are broadly comparable and are unlikely to have a big impact on the conclusions of the cost-effectiveness analyses. The costs of infusion were typically between £100 and £200 per infusion in the submissions, although AbbVie uses a value of £501 per infusion. Some submissions have costs associated with s.c. injections.
It is commented that in a recent NICE review (TA24726) the Appraisal Committee agreed that the value of £154 per infusion was ‘acceptable’. No comment was made on the manufacturer’s assumption that 10% of s.c. injections would require administration by a district nurse.
AbbVie
Administration costs of £501.48 were assumed in the AbbVie submission for each i.v. treatment, using data from NHS reference costs224 and weighting the unit cost per day case admission (91%) and outpatient admission (9%) by activity levels. This assumption is based on the approach used in the NICE guidance for the use of IFX for treatment of adults with psoriasis. 225 An administration cost of £416.12 corresponding to the cost of an outpatient visit was tested in the scenario analysis. 226
Monitoring requirements have been modelled based on UK practice based on share care guidelines and monitoring protocols for rheumatology patients in Bradford teaching hospitals226 (Table 83) and validated by clinical experts prior to the previous NICE submission. Monitoring costs were not applied for ABT, INF, RTX or TCZ to avoid double-counting as 91% of patients are assumed to be admitted as a day case at each administration and the laboratory tests are included in the tariff. The monitoring requirements are, however, presented in Table 84 for completeness.
| Test | Unit cost (£) | MTX/MTX + HCQ + SSZ | SSZ/LEF | CIC | HCQ | ADA/ETN/CTZ/GOL/ monotherapy or combination with MTX | Rescue |
|---|---|---|---|---|---|---|---|
| CXR | 29.33 | 1 | 0 | 0 | 0 | 1 | 0 |
| FBC | 3.39 | 8 | 8 | 9 | 1 | 9 | 0 |
| U&E | 6.36 | 8 | 8 | 9 | 1 | 9 | 0 |
| LFT | 8.91 | 8 | 8 | 9 | 1 | 9 | 0 |
| CRP | 8.49 | 8 | 8 | 9 | 1 | 8 | 0 |
| Urinalysis | 7.84 | 0 | 0 | 1 | 0 | 1 | 0 |
| Mantoux test | 16.34 | 0 | 0 | 0 | 0 | 1 | 0 |
| Hepatitis serology | 7.84 | 0 | 0 | 0 | 0 | 1 | 0 |
| ANA | 8.49 | 0 | 0 | 0 | 0 | 3 | 0 |
| DNA | 8.49 | 0 | 0 | 0 | 0 | 1 | 0 |
| Uric acid | 1.27 | 0 | 0 | 3 | 0 | 0 | 0 |
| Lipids | 3.82 | 0 | 0 | 3 | 0 | 0 | 0 |
| GP visit | 36.36 | 3 | 3 | 3 | 0 | 3 | 0 |
| Outpatient visit | 132.75 | 5 | 5 | 6 | 1 | 6 | 3 |
| Total | 1019.36 | 990.03 | 1173.04 | 159.9 | 1236.75 | 398.25 |
| Test | Unit cost (£) | MTX/LEF, SSZ/MTX + HCQ + SSZ | ADA/ETN/CTZ/GOL/monotherapy or combination | CIC | HCQ | Rescue |
|---|---|---|---|---|---|---|
| CXR | 29.33 | 0 | 0 | 0 | 0 | 0 |
| FBC | 3.39 | 4 | 4 | 4 | 2 | 0 |
| U&E | 6.36 | 4 | 4 | 4 | 2 | 0 |
| LFT | 8.91 | 4 | 4 | 4 | 2 | 0 |
| CRP | 8.49 | 4 | 4 | 4 | 2 | 0 |
| ANA | 8.49 | 0 | 4 | 0 | 0 | 0 |
| Uric acid | 1.27 | 0 | 0 | 4 | 0 | 0 |
| Lipids | 3.82 | 0 | 0 | 4 | 0 | 0 |
| GP visit | 36.36 | 2 | 2 | 2 | 1 | 0 |
| Outpatient visit | 132.75 | 2 | 2 | 2 | 1 | 6 |
| Total | 446.82 | 480.78 | 467.18 | 223.41 | 796.5 |
In the model, costs of monitoring/lab tests required at baseline are applied once the patients start the treatment. Additionally, the scheduled monitoring required in 12 months are applied as a daily cost during the treatment duration.
Monitoring costs at baseline and for the subsequent 12 months are presented in Tables 83 and 84 respectively.
AbbVie report that:
As per the guidelines it was assumed that any monitoring or lab tests in the first three months would be done by a specialist nurse and a shared care arrangement made with general practitioners (GPs) thereafter with routine clinic follow-up on a regular basis. We assumed that a health-care visit was associated with each sequence of laboratory tests. Monitoring subsequently to the first 3 months was assumed to occur at a primary care setting in 60–70% of cases as advised by experts, with the remainder of monitoring being carried out at a hospital. To calculate the distribution of visits the total number of visits beyond the first 3 months was multiplied by 65% and rounded to the closest integer to obtain the number of GP visits. For annual monitoring beyond 6 months, where the number of health-care visits was calculated to be below four, equal distribution between primary and secondary care settings was used to account for regular clinic attendances.
Protocols were not available for GOL; thus, the same monitoring pattern as for ADA was assumed. For combination therapies the maximum requirement for each test from the respective therapies was assumed.
Monitoring costs are set to zero for rescue therapy, apart from an outpatient visit cost every two months as advised by clinical experts. These experts further advised that patients on rescue therapy would be subject to one inpatient admission of approximately three weeks annually. This was not included as additional resource use to avoid double-counting with HAQ-based inpatient and surgery costs. Rescue therapy refers to medical treatment once all active therapies, including traditional DMARDs and biologic treatments, have failed; and is assumed to consist of MTX.
AbbVie acknowledges that monitoring protocols from the British Society of Rheumatology would be more representative of the population modelled, rather than regional guidelines detailed in the Bradford Primary Care Trust protocols. As monitoring patterns from the British Society of Rheumatology229 are not detailed for biologic therapies, the Bradford protocols were used in the base case as all relevant comparators were included, thus allowing for consistent costing of monitoring patterns without the requirement of further assumptions. AbbVie demonstrates the total costs of monitoring for DMARDs between the two sources were reasonably comparable with slightly higher estimates obtained using Bradford protocols. Alternative monitoring patterns from the British Society of Rheumatology, assuming the same monitoring pattern as that of MTX for biologic arms, were tested in scenario analysis. In addition, the sensitivity of monitoring costs was tested by increasing the total monitoring costs for each comparator by 50%.
Bristol-Myers Squibb
Infliximab, ABT i.v. and TCZ are administered as infusions, with s.c. treatments assumed to require visits to a nurse specialist in year 1. 171 Treatment with GLD is assumed to require a visit to a general practitioner (GP) for each dose. Bristol-Myers Squibb assumes that cDMARDs and TCZ require tests before and during treatment. The annual monitoring costs assumed by Bristol-Myers Squibb are shown in Table 85.
| Treatment | Administration costs (£) | Monitoring costs (£) | ||
|---|---|---|---|---|
| Annual cost | Year 1 additional cost | Annual cost | Year 1 additional cost | |
| ABT i.v. | 1777 | 136 | 0 | 0 |
| ABT s.c. | 0 | 283 | 0 | 0 |
| ADA | 0 | 147 | 0 | 0 |
| ETN | 0 | 147 | 0 | 0 |
| IFX | 888 | 136 | 0 | 0 |
| TCZ | 1777 | 0 | 557 | 554 |
| GOL | 0 | 147 | 0 | 0 |
| CTZ | 0 | 147 | 0 | 0 |
| RTX | 188 | 0 | 0 | 0 |
| LEF | 0 | 0 | 854 | 1263 |
| GLD | 516 | 860 | 1710 | 2849 |
| CYC | 0 | 0 | 1671 | 1127 |
| AZA | 0 | 0 | 1709 | 854 |
| MTX | 0 | 0 | 1709 | 570 |
| Palliative care | 545 | 0 | ||
Bristol-Myers Squibb presents a combined intervention acquisition, administration and monitoring cost. All of the bDMARDs are coprescribed with MTX, so all include the annual costs for MTX treatment. The additional year 1 costs for MTX are included only once in the model, as it is assumed that patients move straight onto the next biologic treatment and so do not cease and restart treatment with MTX. These values are replicated in Table 86.
| Treatment | Annual cost (£) | Start-up cost (£) |
|---|---|---|
| ABT i.v. | CiC information has been removed | CiC information has been removed |
| ABT s.c. | CiC information has been removed | CiC information has been removed |
| ADA | 10,913.92 | 147.00 |
| ETN | 11,053.76 | 147.00 |
| IFX | 10,825.87 | 1395.06 |
| TCZ | CiC information has been removed | CiC information has been removed |
| GOL | 10,882.48 | 147.00 |
| CTZ | 11,053.76 | –2355.50a |
| RTX | 6732.08 | 0.00 |
| LEF | 1601.34 | 1408.44 |
| GLD | 2360.40 | 4079.56 |
| CYC | 3356.35 | 1275.33 |
| AZA | 1806.55 | 999.75 |
| Palliative care | 544.80 | 0.00 |
| MTX | 733.48 |
Merck Sharp & Dohme Corp.
Merck Sharp & Dohme Corp. notes that, although many of the TNF-α inhibitors are administered at home, patients are often initially taught how to administer treatment within a hospital. This is calculated as a one-off administration cost.
Merck Sharp & Dohme Corp. reports that the current clinical management of this condition requires patients to have a regular contact with the specialist rheumatology centres in the UK. This was estimated in consultation with two expert clinicians in the UK. Initial resource use estimates were made based on the assumptions made in the Birmingham Rheumatoid Arthritis Model. These were reviewed and validated or changed by the clinical experts. Recent guidelines from the ACR and the British Society for Rheumatology were also reviewed for consistency with our assumptions.
In order to determine the total treatment cost in the model, routine monitoring costs of patients are aggregated. In the UK patient monitoring includes visits to a rheumatologist after 6 months then every 12 months, GP visits every 6 months and a specialist nurse visit every 6 months.
Resource use costs for the UK were sourced from the NHS reference costs (2010–11)224 and the Personal Social Services Research Unit (2011). 228 It is common in the UK for patients to regularly visit a specialist rheumatology nurse more frequently than their rheumatologist. Table 87 presents the unit costs assumed by MSD.
| Health-care resource | Unit cost (£) | Source |
|---|---|---|
| Rheumatologist | 132.07 | NHS reference costs 2010–11 (Consultant Led: Follow up Attendance Non-Admitted Face to Face 410)224 |
| GP | 53.00 | Curtis, 2011, p. 149228 |
| Specialist nurse | 50.00 | Curtis, 2011, p. 144228 |
| Nurse practitioner | 42.00 | Curtis, 2011, p. 146228 |
| FBC | 3.36 | NHS reference costs 2010–11 (NHS Trusts Direct Access: Pathology Services DAP823)224 |
| ESR | 1.26 | NHS reference costs 2010–11 (NHS Trusts Direct Access: Pathology Services DAP841)224 |
| Biochemistry profile | 3.36 | NHS reference costs 2010–11 (NHS Trusts Direct Access: Pathology Services DAP823)224 |
| CRP | 3.36 | NHS reference costs 2010–11 (NHS Trusts Direct Access: Pathology Services DAP823)224 |
| TB test | 1.26 | NHS reference costs 2010–11 (NHS Trusts Direct Access: Pathology Services DAP841)224 |
| Hepatitis B and hepatitis C | 3.36 | NHS reference costs 2010–11 (NHS Trusts Direct Access: Pathology Services DAP823)224 |
| Urinalysis | 1.26 | NHS reference costs 2010–11 (NHS Trusts Direct Access: Pathology Services DAP841)224 |
| Chest X-ray | 29.04 | NHS reference costs 2010–11 (NHS Trusts Outpatient DAPF)224 |
For i.v. drugs (INF, TCZ and ABT i.v.) administration costs are higher and incurred at every administration of treatment. In the UK the cost of infusion is £50 with an additional £59 administration cost. The cost of infusion is assumed equivalent to a visit to a specialist nurse plus an hourly charge for the care of the patient while they are on the ward. MSD assumed that infusion costs can be charged only per whole hour.
In order to account for the difference in cost between initiation of treatment and maintenance treatment, the cost of the first cycle of treatment is aggregated separately to the cost of subsequent cycles of treatment. Table 88 reports the cost of administration treatment included in the model. As this was combined with intervention acquisition costs, these have been included for completeness.
| Intervention | Cost per dose (£) | Number of doses per first 6 months | Number of doses post 6 months | Treatment cost first 6 months (£) | Treatment cost post 6 months (£) | Cost per administration first 6 months (£) | Total cost first 6 months (£) | Total cost post 6 months (£) |
|---|---|---|---|---|---|---|---|---|
| GOL | 762.97 | 6 | 6 | 4577.82 | 4577.82 | 59.00 | 4636.82 | 4577.82 |
| ADA | 352.14 | 13 | 13 | 4577.82 | 4577.82 | 59.00 | 4636.82 | 4577.82 |
| IFXa | 1133.20 | 5 | 3.25 | 5666.00 | 3682.90 | 109.00 | 6211.00 | 4037.15 |
| ETN | 89.38 | 52 | 52 | 4647.76 | 4647.76 | 59.00 | 4706.76 | 4647.76 |
| TCZb | 698.32 | 7 | 6.5 | 4888.24 | 4539.08 | 109.00 | 5651.24 | 5247.58 |
| CTZc | 357.50 | 6 | 13 | 2145.00 | 4647.50 | 59.00 | 2204.00 | 4647.50 |
| LEF | 1.88 | 205 | 178 | 385.40 | 334.64 | 0.00 | 385.40 | 334.64 |
| GLD | 13.48 | 26 | 26 | 350.48 | 350.48 | 0.00 | 350.48 | 350.48 |
| AZA | 0.07 | 547.5 | 547.5 | 38.33 | 38.33 | 0.00 | 38.33 | 38.33 |
| CYC | 2.14 | 365 | 365 | 781.10 | 781.10 | 0.00 | 781.10 | 781.10 |
| MTX | 0.05 | 78 | 78 | 3.90 | 3.90 | 0.00 | 3.90 | 3.90 |
| ABT i.v.d | 864.92 | 8 | 6.5 | 6919.35 | 5621.97 | 109.00 | 7791.35 | 6330.47 |
| ABT s.c.e | 302.40 | 26 | 26 | 8727.32 | 7862.40 | 59.00 | 8895.32 | 7862.40 |
| RTX | 1746.30 | 2 | 1.3 | 3492.60 | 2270.19 | 109.00 | 3710.60 | 2411.89 |
Pfizer
Pfizer reported that the costs associated with pre-treatment monitoring were in accordance with the previous evidence review group models and recent manufacturer’s submission to NICE. These were reported to be then validated at an advisory board. In addition to the costs of tests, an outpatient rheumatology contact (service code 410) was assumed, at a cost of £137. 230 Table 89 provides the unit costs of pre-treatment test while Table 90 summarises the estimated total cost per intervention. Monitoring costs were assumed to be included in the general costs per HAQ band and were thus not included.
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
|---|---|---|---|
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
|---|---|---|
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
The costs of infusion were uplifted by Pfizer from costs presented by Roche in TA198231 to 2011/12 prices using Curtis. 232
The summary of acquisition costs, monitoring and administration costs provided by MSD is replicated in Table 91.
| Treatment | Dosing assumptions | Unit cost (£)a | Unit dose (mg) | Administration costs (£) | Assume vial wastage? | |
|---|---|---|---|---|---|---|
| First administration | Subsequent administration | |||||
| ABT i.v. | Body weight ≤ 60 kg, 500 mg; 61–100 kg, 750 mg; > 100 kg, 100 mg; repeated 2 weeks and 4 weeks after initial infusion, then every 4 weeks | 302.40 | 250 | 151.95b | 151.95b | Yes |
| ADA | 40 mg every other week | 352.14 | 40 | 49.00c | 0.00 | N/A |
| CTZ | 400 mg at 0, 2 and 4 weeks then 200 mg every 2 weeks (PAS 10 for free) | 357.50 | 200 | 49.00c | 0.00 | N/A |
| CYC | Maximum of 4 mg/kg daily in two divided doses | 51.50 | 3000 | 0.00 | 0.00 | N/A |
| ETN | 25 mg BIW | 89.38 | 25 | 49.00c | 0.00 | N/A |
| ABT s.c. | Loading dose by i.v. initially, then first 125 mg s.c. injection given within a day, followed by 125 mg s.c. OW | 302.40d | 125 | 49.00 (of s.c. first administration)e | 0.00 | N/A |
| GOL | 50 mg every 4 weeks | 762.97 | 50 | 49.00c | 0.00 | N/A |
| IFX | 3 mg/kg at weeks 0, 2 and 6, thereafter every 8 weeks | 419.62 | 100 | 151.95b | £151.95b | Yes |
| LEF | Assumed 20 mg OD | 61.36 | 600 | 0.00 | 0.00 | N/A |
| MTX | 15 mg OW | 48.44 | 1000 | 0.00 | 0.00 | N/A |
| PC | Assumed to be additive combination of MTX, LEF, CIC (oral) | N/A | N/A | 0.00 | 0.00 | N/A |
| RTX | 1000 mg repeated 2 weeks after initial infusion = one course; each course 9 months apart | 873.15 | 500 | 441.00f | 441.00f | N/Ag |
| SSZ | 2000 mg/day | 14.83 | 56,000 | 0.00 | 0.00 | N/A |
| TCZ | 8 mg/kg every 4 weeks | 102.40 | 80 | 151.95b | 151.95b | Yes |
| Combination therapy with cDMARD | Assumed to be additive combination of MTX and SUL | N/A | N/A | 0.00 | 0.00 | N/A |
Roche
Table 92 presents administration costs for all the treatments. The model assumes a district nurse will administer 10% of the s.c. injection treatments.
| Treatment | Total cost of administration first 6 months and subsequent cycles (responders) (£) | Assumptions | Source (cost) |
|---|---|---|---|
| ADA | 35.10 | 10% of injections are given by district nurse; cost of district nurse £27.00 | Curtis et al., 2010233 |
| CTZ | 35.10 | 10% of injections are given by district nurse; cost of district nurse £27.00 | Curtis et al., 2010233 |
| ETA | 70.20 | 10% of injections are given by district nurse; cost of district nurse £27.00 | Curtis et al., 2010233 |
| TCZ | 1113.63 | Cost of £171.33 for each infusion given in a cycle (inflated 2000 to 2010) | Barton et al., 2004180 |
The economic model assumes the same schedule of monitoring for all biologics as in the previous NICE submission for TCZ. 231 The cost of TCZ monitoring is assumed to be included in the administration cost: £171.33 per i.v. infusion180 updated to 2009/10 prices. 233
The monitoring cost of ADA, CTZ and ETN is assumed to follow the schedule presented in Table 93. Palliative care is assumed to have only monitoring costs, but a greater number of outpatient follow-up visits in the first cycle and greater resource use in subsequent cycles, resulting in costs of £2589 and subsequent costs of £1287.
| Resource or test | Unit cost (£) | Monitoring frequency per 6 months (first cycle) | Total cost (first cycle: responder) (£) | Frequency of monitoring per 6 months (subsequent cycles) | Total cost (subsequent cycles) (£) | Source |
|---|---|---|---|---|---|---|
| Outpatient visit first attendance | 214.00 | 1 | 214.00 | 0 | 0.00 | Department of Health, 2011234 |
| Outpatient visit follow-up visit | 126.00 | 6 | 756.00 | 3 | 378.00 | Department of Health, 2011234 |
| GP visit | 53.00 | 4 | 212.00 | 3 | 159.00 | Department of Health, 2011234 |
| FBC | 3.00 | 14 | 42.00 | 3 | 9.00 | Department of Health, 2011234 |
| ESR and CRP | 15.41 | 14 | 215.68 | 3 | 46.22 | Barton et al., 2004180 |
| Liver function test | 8.55 | 14 | 119.74 | 3 | 25.66 | Barton et al., 2004180 |
| Urea, electrolytes and creatinine | 8.55 | 14 | 119.74 | 3 | 25.66 | Barton et al., 2004180 |
| Chest radiography | 27.63 | 1 | 27.63 | 0 | 0.00 | Barton et al., 2004180 |
| Total | 1706.79 | 643.53 |
Roche provide a summary table of acquisition, monitoring and administration costs. This is replicated in Table 94.
| Treatment | Total cost: bi-annual (first cycle on treatment, non-responder) (£) | Total cost: bi-annual (first cycle on treatment, responder) (£) | Total cost: bi-annual (subsequent cycles on treatment, responder) (£) |
|---|---|---|---|
| ADA | 3159.85 | 6319.71 | 5256.45 |
| CTZ | 870.94 | 4065.64 | 5326.13 |
| ETN | 3212.24 | 6424.49 | 5361.23 |
| TCZ | 2886.42 | 5772.83 | 5772.83 |
| Palliative care | 2588.79 | 2588.79 | 1287.07 |
UCB Pharma
The monitoring schedule assumed by UCB Pharma is replicated in Table 95. UCB Pharma presents unit costs, but for brevity only the summarised monitoring data, together with drug acquisition costs, are provided in Tables 95 and 96, respectively.
| AiC information has been removed | AiC information has been removed | |||
|---|---|---|---|---|
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| AiC information has been removed | |||||||||
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | |||||||||
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed) | AiC information has been removed |
| AiC information has been removed | |||||||||
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
Comparative treatment efficacy (network meta-analysis)
This section contains the analyses regarding comparative efficacies undertaken by each manufacturer. For consistency, the term NMA has been used even when a manufacturer has denoted the analysis to be a mixed-treatment comparison.
The level of detail in the analyses and in the reporting was very diverse, ranging from the submission by AbbVie, which included a 378-page appendix, to the submission by Roche that consisted of one-page concerning the NMA. The Assessment Group has attempted to capture all key points made by the manufacturer but has had, for brevity reasons, to abridge some analyses. Detailed discussions on the methods used, goodness of fits, consistency checking and convergence have not been incorporated. Similarly, replications of the list of studies that have been used in the NMA by the manufacturers have not been undertaken.
AbbVie
The trials included in AbbVie’s base-case NMA are depicted in Figure 37, which has been reproduced directly from the AbbVie submission. The numbers on the line have been included by AbbVie without a reference, but are believed to represent codes for RCTs; thus, six numbers would indicate six trials informing the direct comparison. Furthermore, AbbVie used different abbreviations from those used in by the Assessment Group. It is commented that there is no cDMARD node, which is assumed to be subsumed within the PBO arm.
FIGURE 37.
The evidence network in AbbVie’s base case. OTT, oral triple therapy.

AbbVie incorporated hurdles within the analyses to eliminate illogical results such as the possibility that a patient may be simulated an ACR50 response, but not an ACR20 response. This was achieved by using parameters such as, of those who have gained an ACR20 response, what proportion achieved an ACR50 response. Within the base case, AbbVie adjusted for baseline risk, prior MTX exposure, prior bDMARD exposure and concomitant standard DMARD. AbbVie report that additional sensitivity analysis controlling for differences in baseline HAQ-DI and disease duration slightly worsened model fit assessed by the deviance information criterion and had little effect on overall results.
AbbVie presents posterior simulated ACR responses for four main groups:
-
MTX-experienced patients who can receive cDMARDs (Figure 38)
-
MTX-experienced patients who receive bDMARD monotherapy (Figure 39)
-
MTX-naive patients who can receive cDMARDs (Figure 40)
-
MTX-naive patients who receive bDMARD monotherapy (Figure 41).
FIGURE 38.
Posterior simulated ACR response for combination therapy in a MTX-experienced population presented by AbbVie.
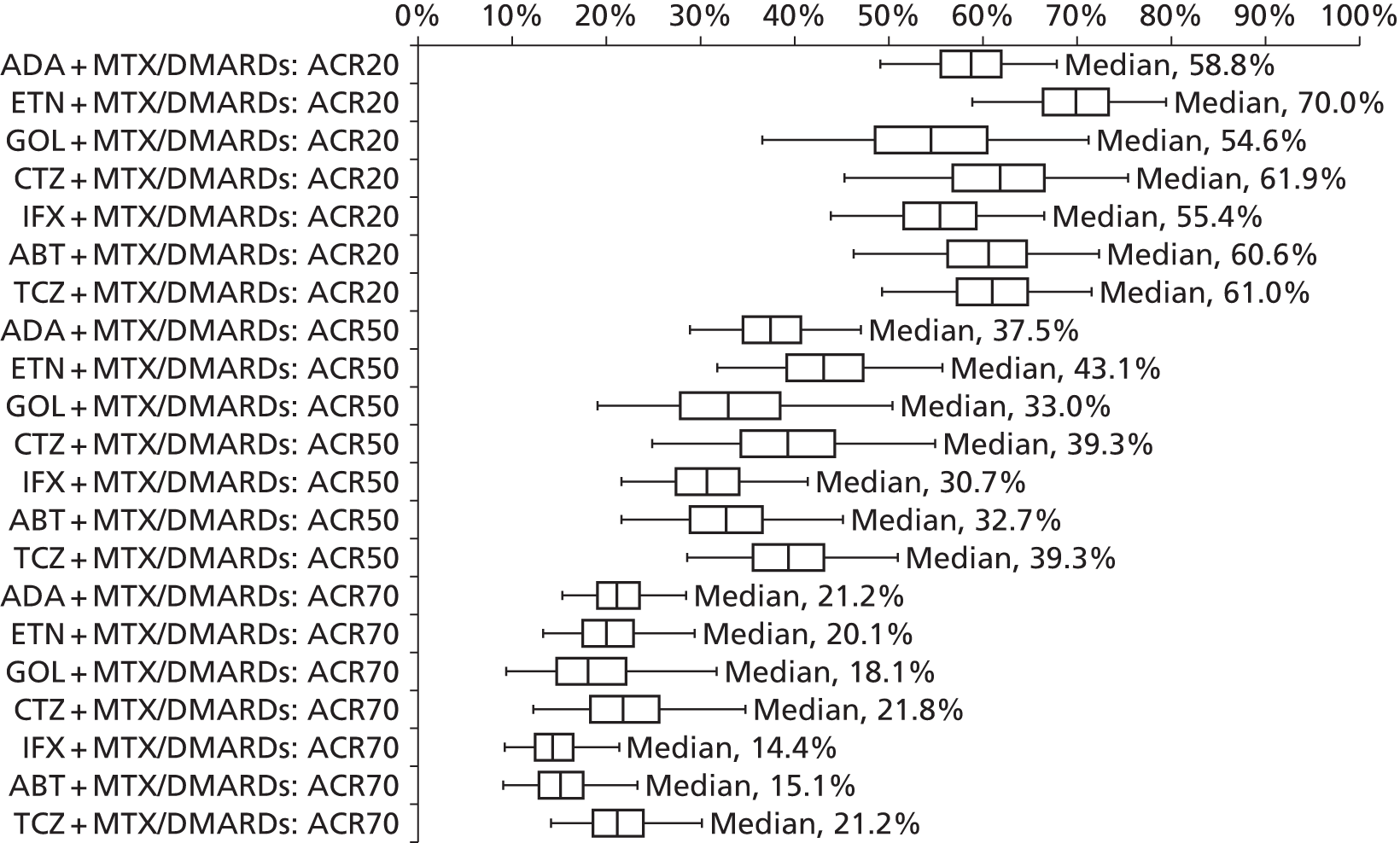
FIGURE 39.
Posterior simulated ACR response for monotherapy in a MTX-experienced population presented by AbbVie.
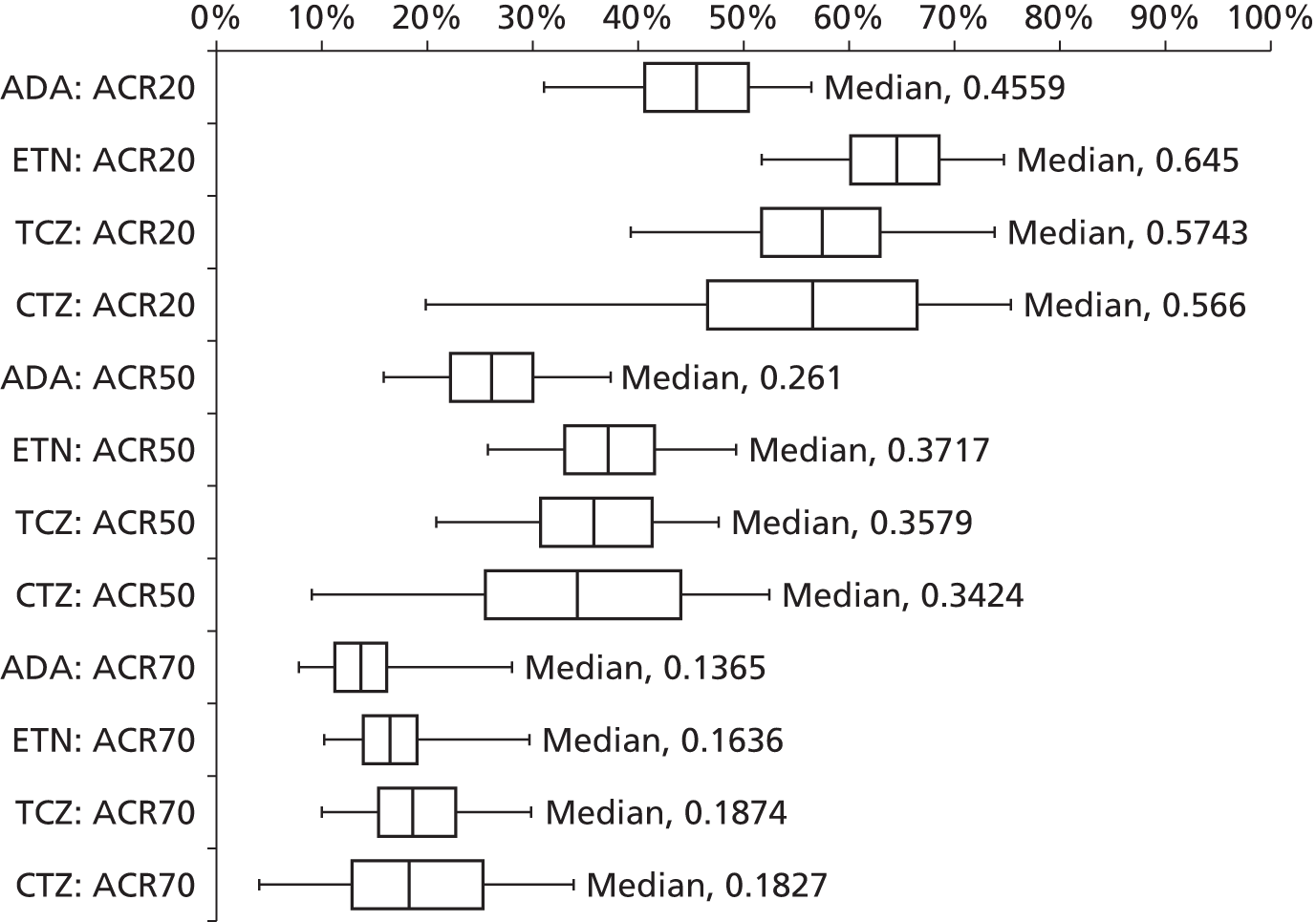
FIGURE 40.
Posterior simulated ACR response for combination therapy in a MTX-naive population presented by AbbVie.
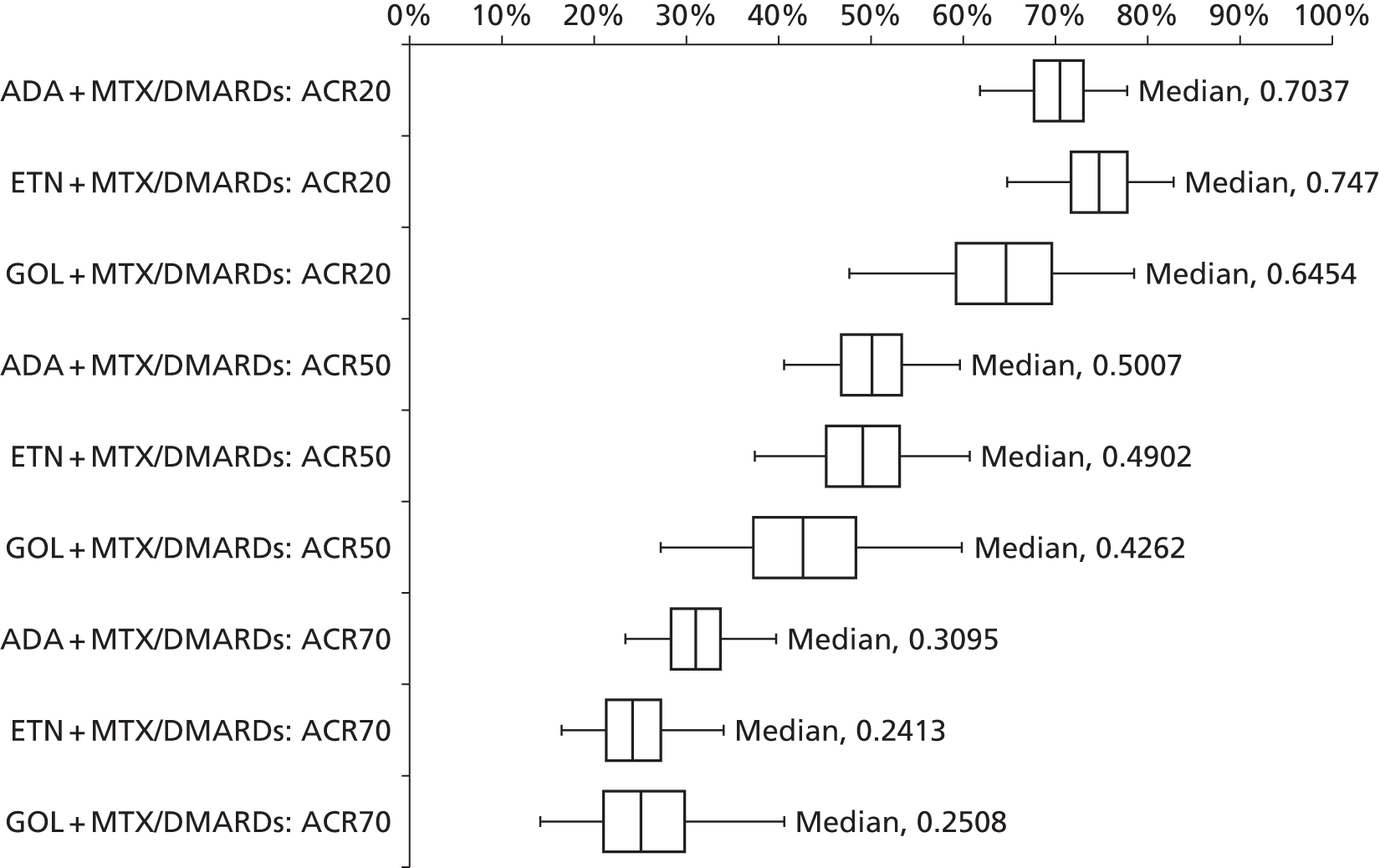
FIGURE 41.
Posterior simulated ACR response for monotherapy in a MTX-naive population presented by AbbVie.

Further analyses (not shown in the Assessment Group summary) investigated a number of sensitivity analyses. These included:
-
The efficacy of TCZ and RTX compared with MTX when used after a bDMARD. These results indicated that the efficacy of TCZ was lower following an initial bDMARD than in people who were bDMARD naive.
-
The inclusion of Asian studies which were shown to favour TCZ monotherapy and slightly favour CTZ.
-
Limiting the data to a 3-month data set. AbbVie comments that, as one would expect, there are lower estimated median response probabilities at higher levels of response, particularly for ACR70, for most treatments including ADA, CTZ, ETN, GOL and TCZ, compared with the ‘6-month’ estimates. The only exceptions are ABT and INF in the MTX-experienced, combination therapy scenario.
AbbVie’s interpretation of the network meta-analysis data
AbbVie states that:
. . . for the MTX-experienced patient population, biologics in combination with MTX or other DMARDs, median posterior simulated ACR20 responses for the 6 month estimates are highest for etanercept and lowest for golimumab. The interquartile ranges are tighter for the three older anti-TNFs, adalimumab, etanercept and infliximab, as well as abatacept than for golimumab and certolizumab. Median posterior simulated ACR50 responses are highest for etanercept and lowest for infliximab, while ACR70 responses are highest for adalimumab and certolizumab and lowest for abatacept and infliximab. Estimated responses get tighter the higher the level of ACR response.
Bristol-Myers Squibb
The inclusion and exclusion criteria for selecting the RCTs to be evaluated in the NMA were not well reported; nor were the time points at which data were extracted, the methods used within the NMA, the assumed properties of the frequentist and Bayesian analyses. Bristol-Myers Squibb provides NMAs of HAQ scores and of DASs. Bristol-Myers Squibb did not report whether or not the frequentist or Bayesian values were used within the analyses. The network for the HAQ scores is shown in Figure 42.
FIGURE 42.
The network of evidence for HAQ scores as supplied by Bristol-Myers Squibb.
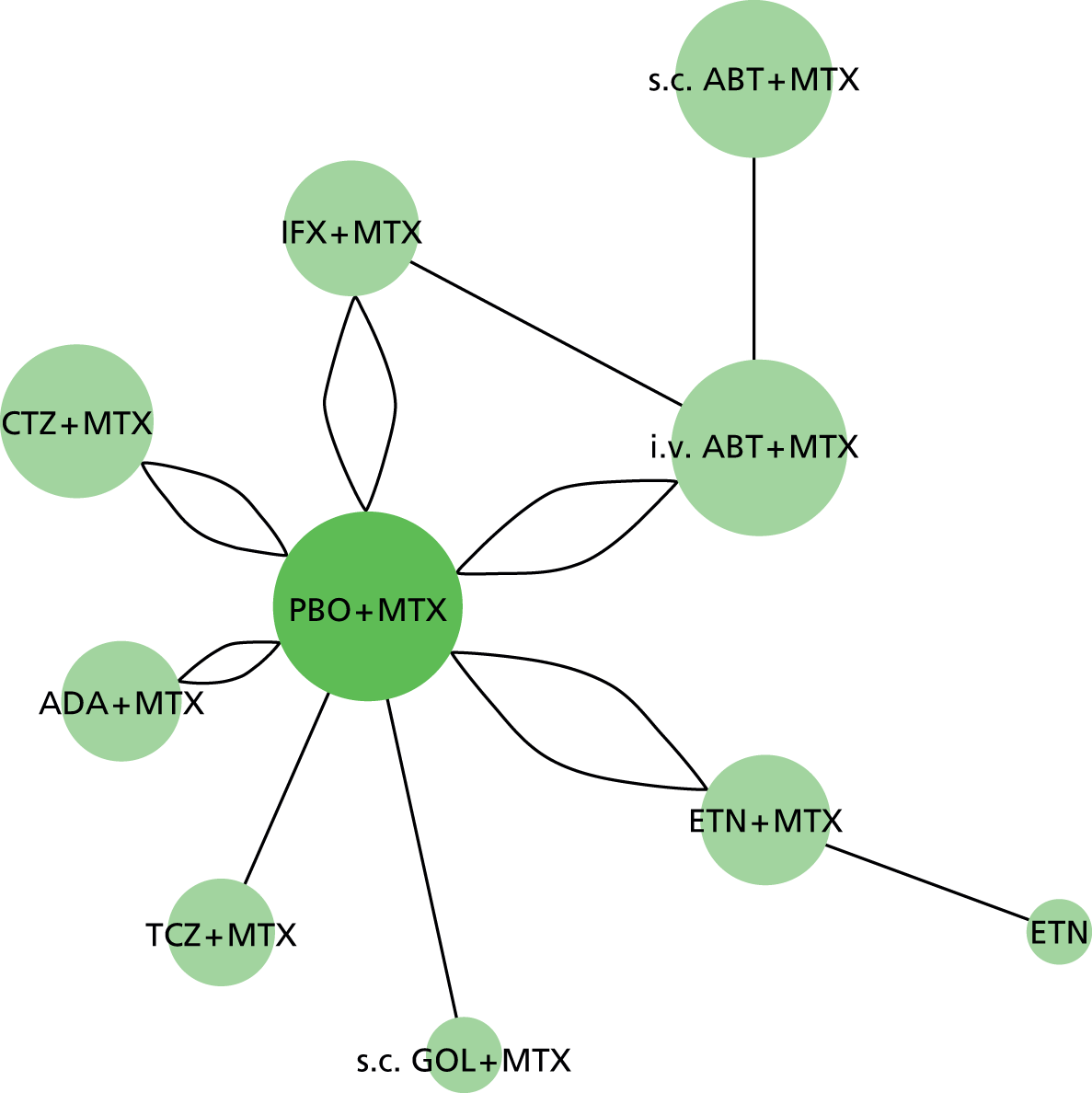
The mean change in HAQ is shown in Figure 43 and absolute mean change is shown in Figure 44.
FIGURE 43.
The mean change in HAQ scores relative to PBO as estimated by Bristol-Myers Squibb.
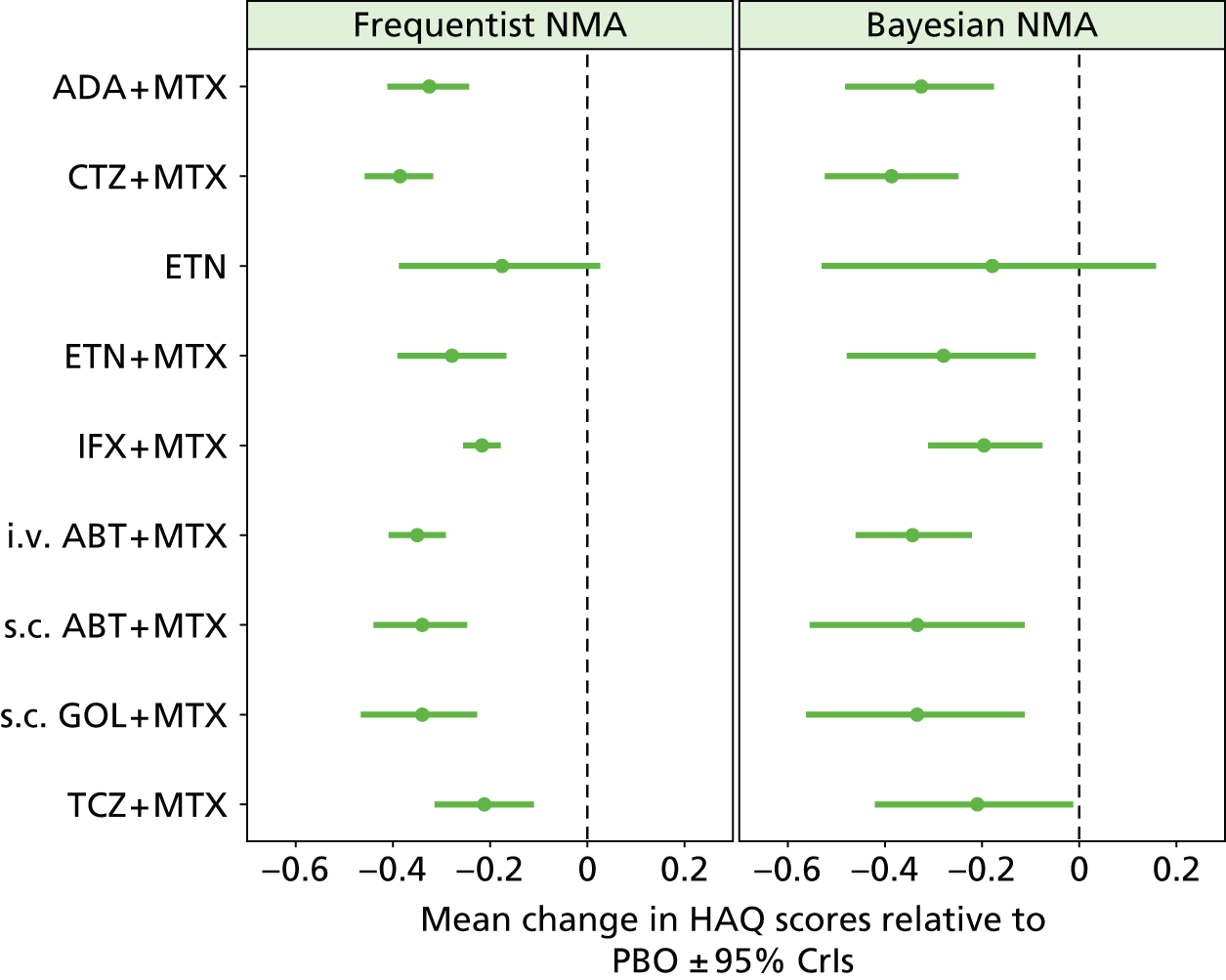
FIGURE 44.
The mean absolute change in HAQ scores as estimated by Bristol-Myers Squibb.

The probability of being the most efficacious treatment is detailed in Figure 45, although the Assessment Group notes that, strictly, it is impossible to quantify the probability of being most efficacious using a frequentist approach.
FIGURE 45.
The probability of being the most efficacious treatment (on HAQ score) as estimated by Bristol-Myers Squibb.
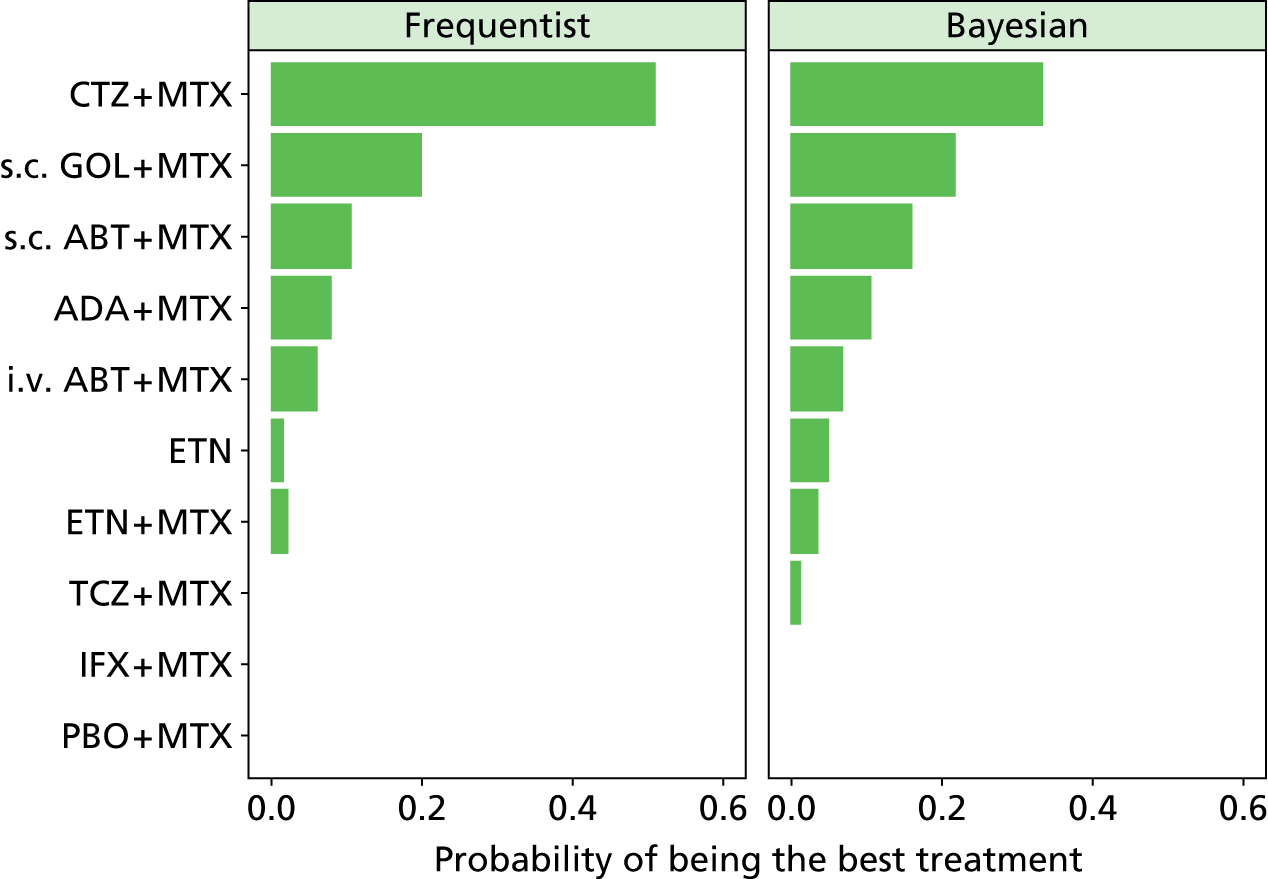
The analysis of DAS by Bristol-Myers Squibb used a linear regression to estimate DASs from HAQ scores where these data were not provided. The assumed relationship is shown in Figure 46. No comment was made on the relationship between change in DAS and change in HAQ scores.
FIGURE 46.
The relationship assumed by Bristol-Myers Squibb between HAQ scores and DASs.
The network assumed in the DAS analyses therefore replicates that for the HAQ analyses (see Figure 43). As with the HAQ analyses, mean changes in DASs, absolute mean changes in DAS and the probability of being the most efficacious treatment are provided. These are shown in Figures 47 and 48.
FIGURE 47.
The mean change in DAS relative to PBO as estimated by Bristol-Myers Squibb.
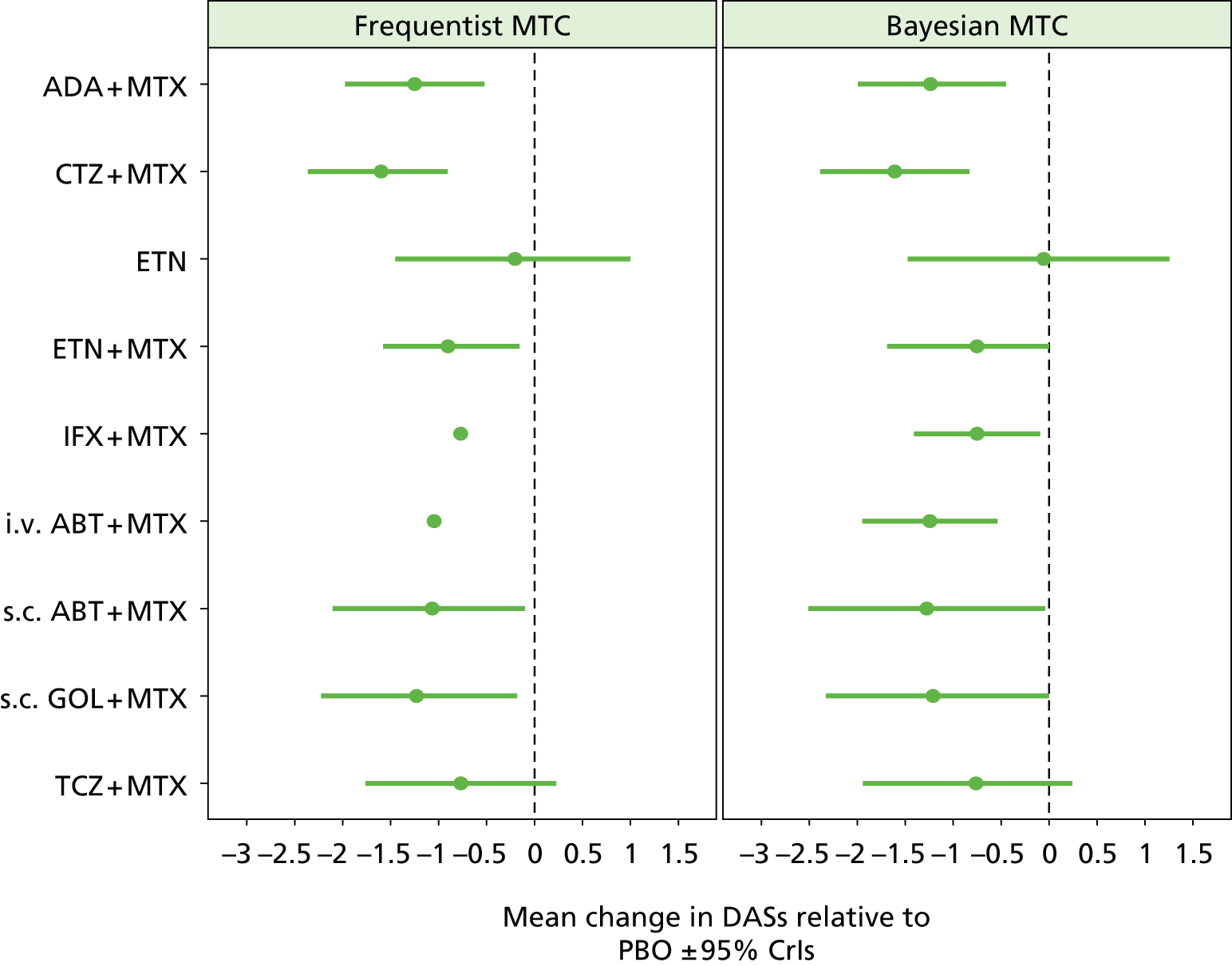
FIGURE 48.
The mean absolute change in DAS as estimated by Bristol-Myers Squibb.
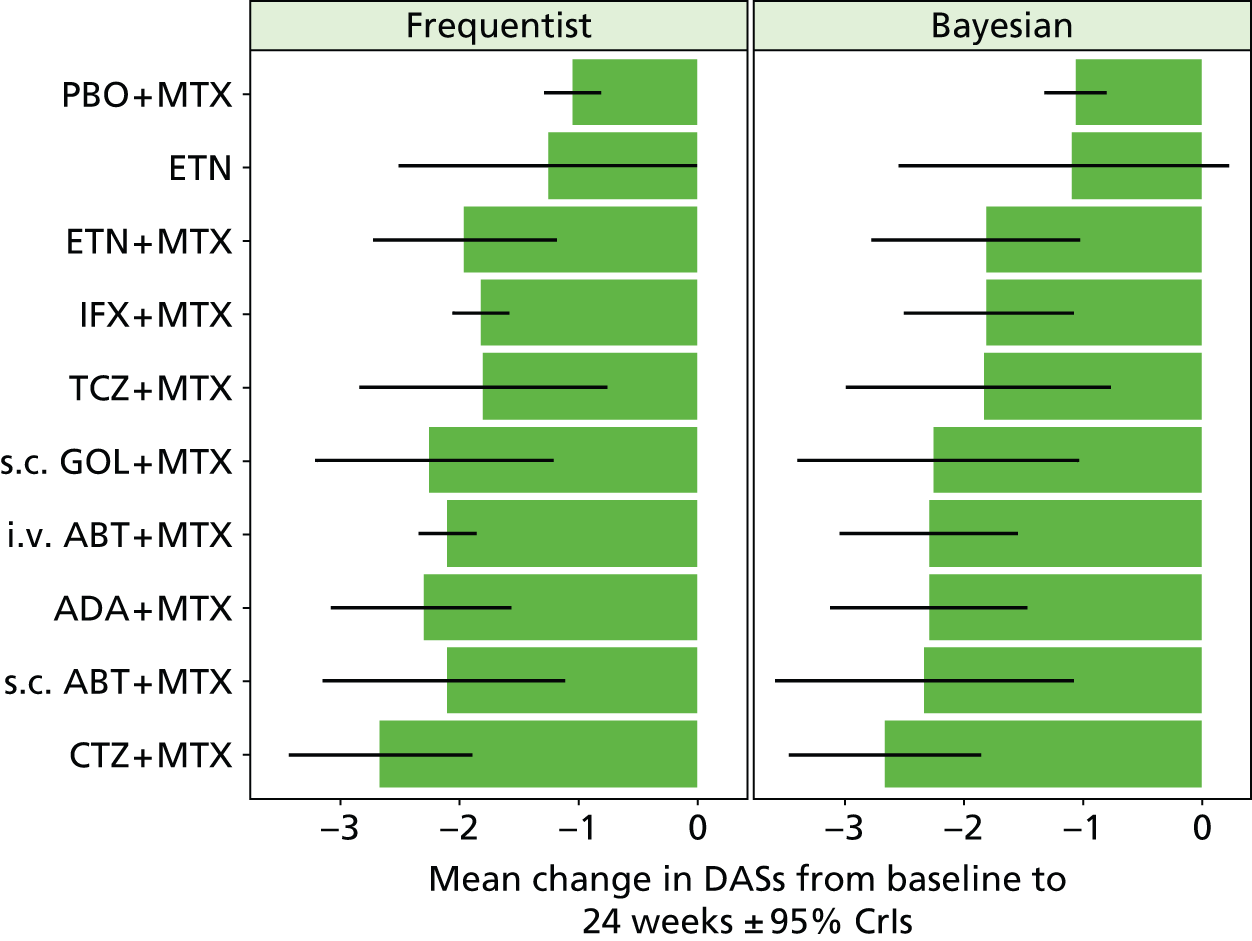
The probability of being the most efficacious treatment is detailed in Figure 49, although the Assessment Group notes that, strictly, it is impossible to quantify the probability of being most efficacious using a frequentist approach.
FIGURE 49.
The probability of being the most efficacious treatment (on DAS) as estimated by Bristol-Myers Squibb.

Bristol-Myers Squibb’s interpretation of the network meta-analysis data
Bristol-Myers Squibb states that:
. . . certolizumab + MTX seems to be the best treatment at reducing both HAQ and DAS scores . . . golimumab + MTX also appears to be an effective treatment in improving QoL, along with etanercept + MTX and s.c. abatacept + MTX
and
Infliximab + MTX and etanercept alone are expected to yield the smallest negative changes in both HAQ and DAS scores other than placebo + MTX.
Merck Sharp & Dohme Corp.
The data used in the NMA conducted by MSD are contained in Tables 16–18 of both the IFX and the GOL submission with the network reproduced in Figure 50. No steps were taken to ensure legitimacy (e.g. that the ACR50 value was lower than the ACR20 example).
FIGURE 50.
The network for DMARD-experienced patients as supplied by MSD.
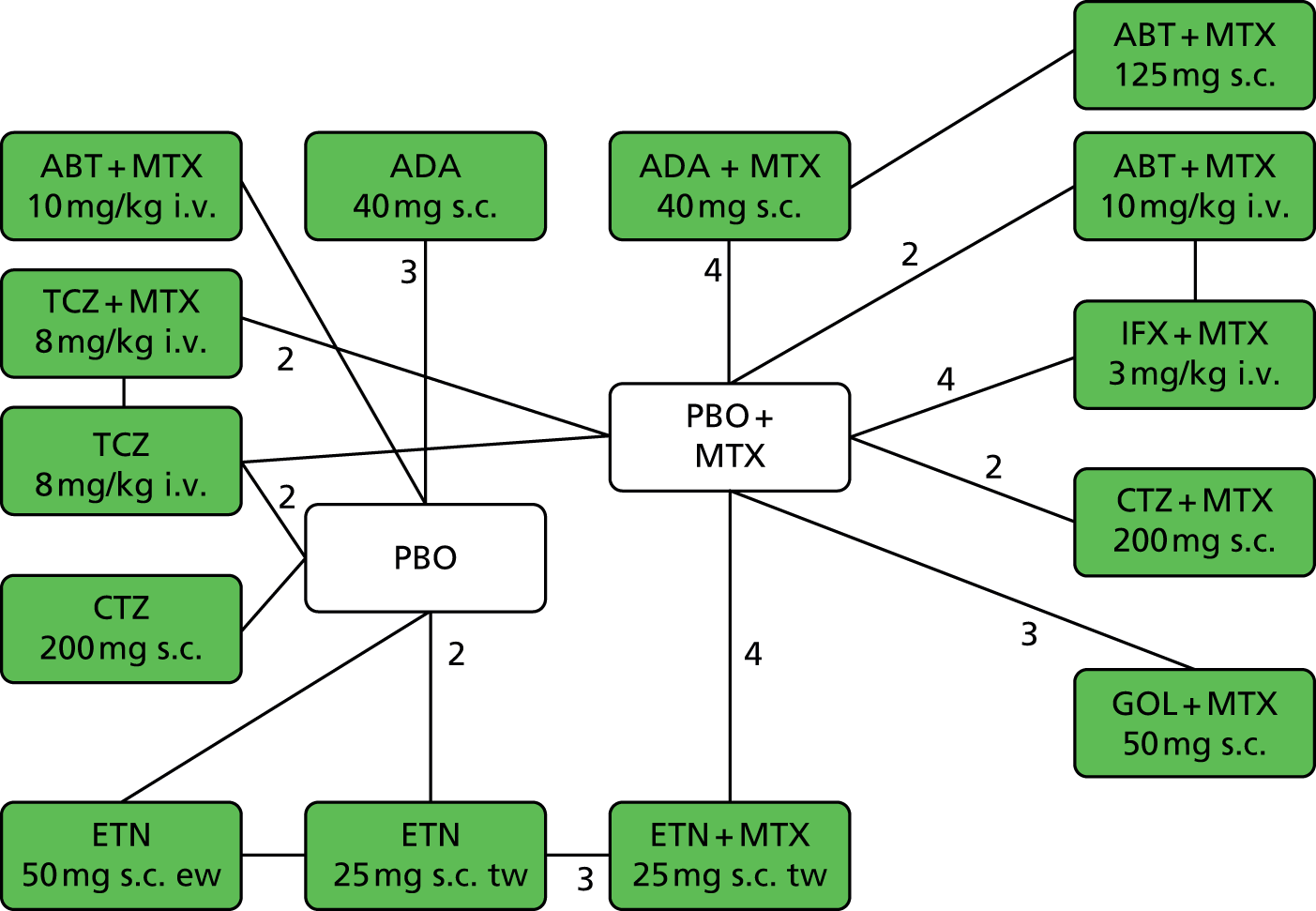
Merck Sharp & Dohme Corp. presents results in terms of the drug that is the focus of the submission (i.e. GOL or IFX). The ACR results for GOL are shown in Figures 51–53, while those for IFX are shown in Figures 54–56.
FIGURE 51.
American College of Rheumatology 20: DMARD-experienced patients at 24 weeks estimated by MSD in the GOL submission.

FIGURE 52.
American College of Rheumatology 50: DMARD-experienced patients at 24 weeks estimated by MSD in the GOL submission.

FIGURE 53.
American College of Rheumatology 70: DMARD-experienced patients at 24 weeks estimated by MSD in the GOL submission.
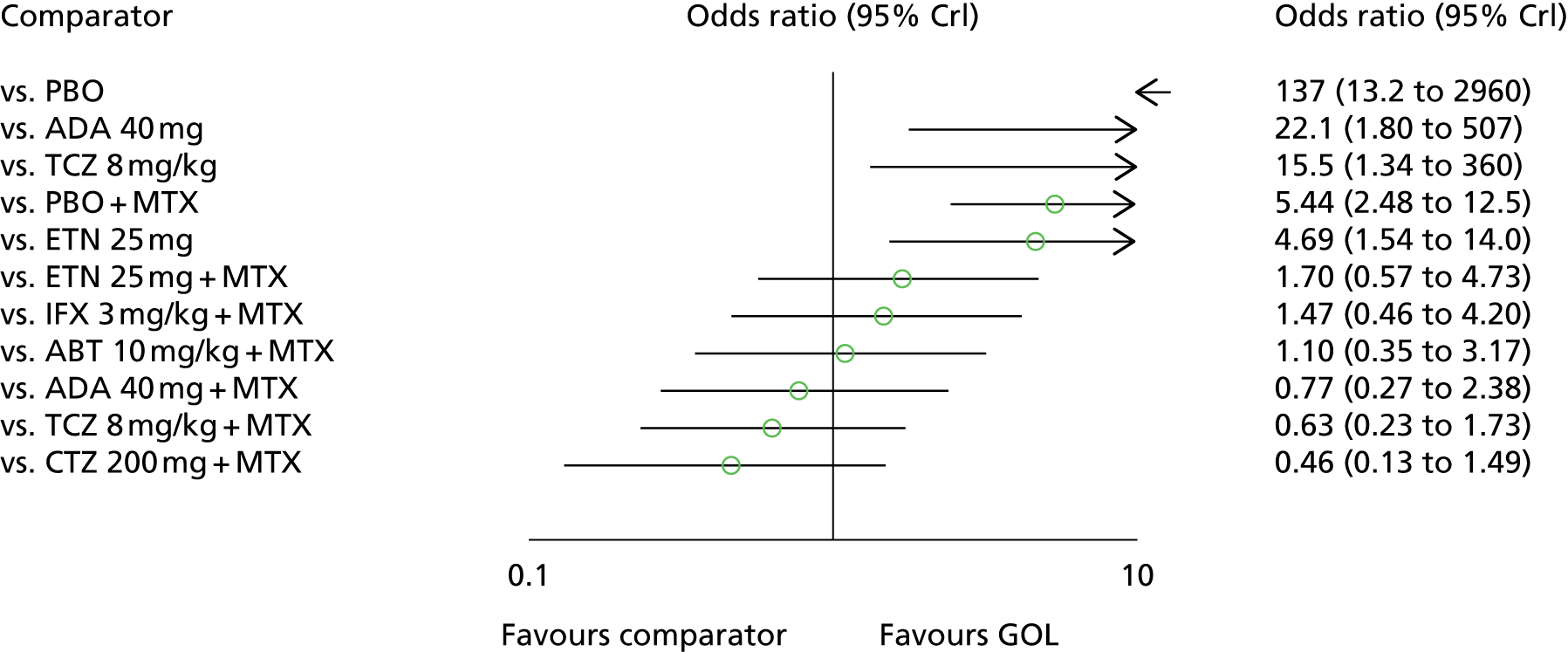
FIGURE 54.
American College of Rheumatology 20: DMARD-experienced patients at 24 weeks estimated by MSD in the IFX submission.
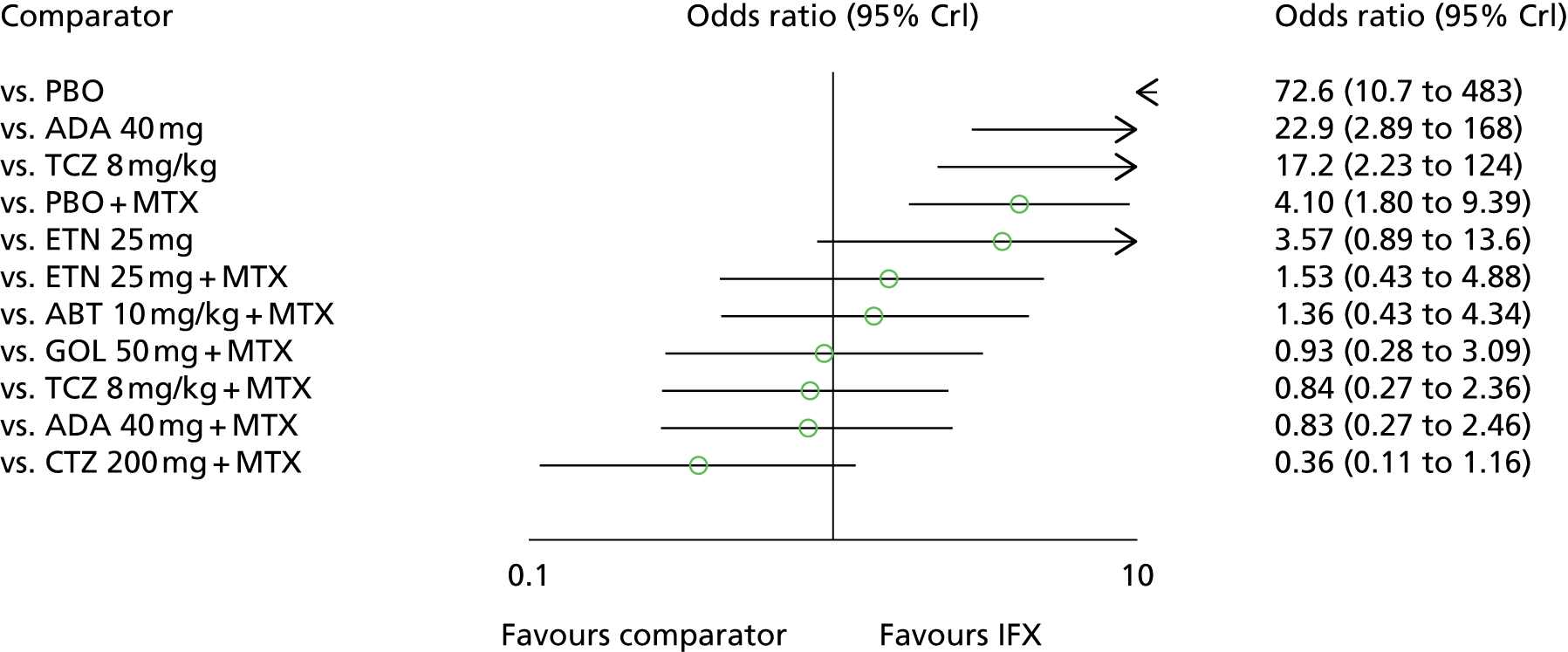
FIGURE 55.
American College of Rheumatology 50: DMARD-experienced patients at 24 weeks estimated by MSD in the IFX submission.
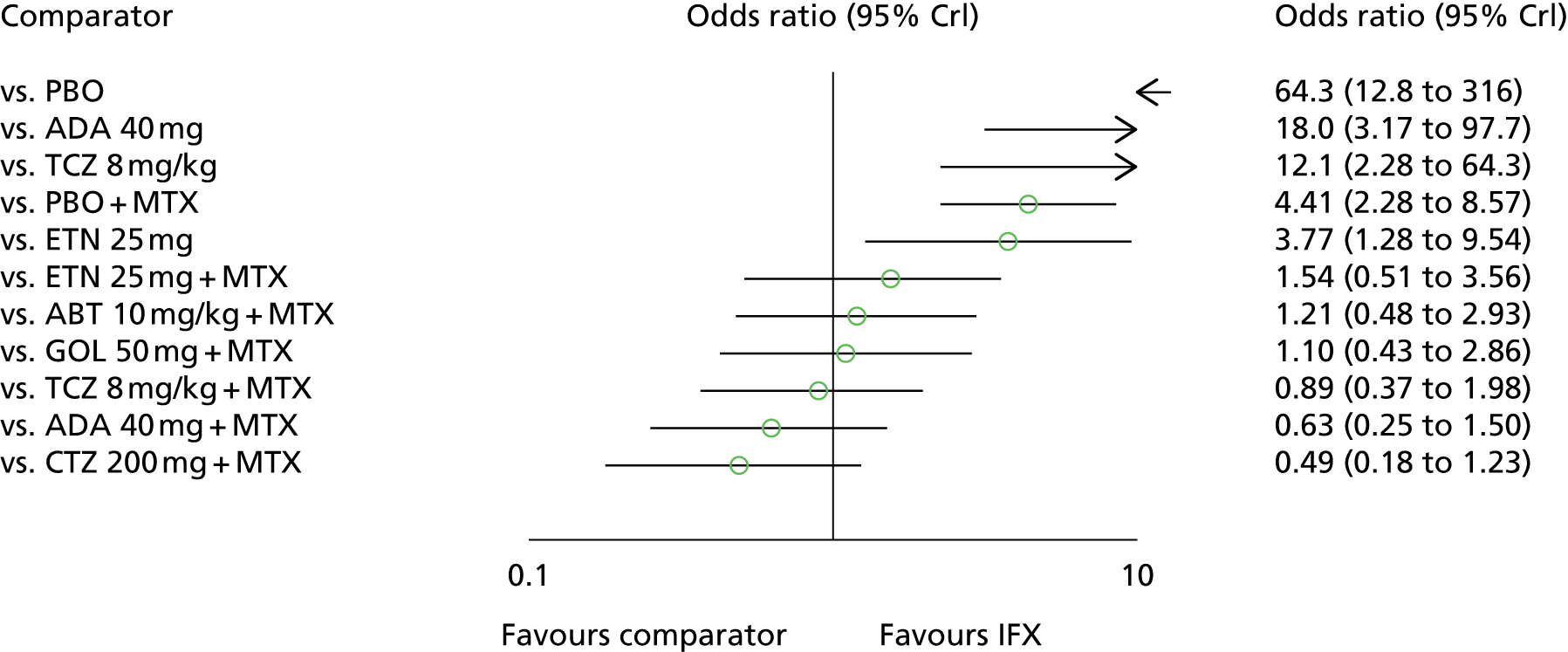
FIGURE 56.
American College of Rheumatology 70: DMARD-experienced patients at 24 weeks estimated by MSD in the IFX submission.

Merck Sharp & Dohme Corp. conducted sensitivity analyses excluding open-label studies as these may have a higher potential for bias. This did not materially affect the ACR20 or ACR50 results, but had a larger (although non-patterned) impact at ACR70.
A second sensitivity analysis was conducted where Asian studies were included (Figure 57 reproduces a figure supplied by MSD and indicates lower background MTX use in these studies): golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis (GO-FORTH),91 Study of Active controlled Monotherapy Used for Rheumatoid Arthritis, an IL-6 Inhibitor (SAMURAI),115 Abe et al. 56 and Kim et al. 99
FIGURE 57.
Comparison of MTX usage (average mg/week) in East Asian vs. non-East Asian studies supplied by MSD. Reference details were not supplied by MSD; therefore, no reference numbers are included.
The exclusion of non-Asian studies did not markedly alter the odds ratios which remain with wide CrIs.
Merck Sharp & Dohme Corp.’s interpretation of the results
Merck Sharp & Dohme Corp. summarises the results of the NMA for GOL and IFX as follows:
-
ACR20: no significant differences were observed between GOL/IFX and other bDMARDs, with the exception of ADA monotherapy and TCZ monotherapy.
-
ACR50: no significant differences were observed between GOL/IFX and other bDMARDs, with the exception of ADA monotherapy, TCZ monotherapy and ETN monotherapy.
-
ACR70: no significant differences were observed between GOL/IFX and other bDMARDs, with the exception of ADA monotherapy, TCZ monotherapy and ETN monotherapy.
In each of the exceptions listed above, GOL and IFX were assumed to be statistically significantly better than the named intervention.
Pfizer
Pfizer undertook three separate NMAs: ACR20/50/70 responses for a severe cDMARD-experienced population; HAQ changes for a severe cDMARD-experienced population; and ACR20/50/70 responses for a severe cDMARD-experienced population who were treated with bDMARD monotherapy. The networks for these NMAs are reproduced in Figures 58–60.
FIGURE 58.
The network diagram for combination therapy, ACR responses in severe DMARD-experienced patients as produced by Pfizer. (AiC information has been removed.)
FIGURE 59.
The network diagram for combination therapy, HAQ changes in severe DMARD-experienced patients as produced by Pfizer. (AiC information has been removed.)
FIGURE 60.
The network diagram for monotherapy, ACR responses in severe DMARD-experienced patients as produced by Pfizer. (AiC information has been removed.)
The results produced by each of these analyses in the base case are provided in Tables 97–99.
No steps were taken to ensure legitimacy (e.g. that the ACR50 value was lower than the ACR20 example).
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
|---|---|---|
| AiC information has been removed | ||
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | ||
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed) |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | ||
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
|---|---|---|
| AiC information has been removed | ||
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
|---|---|---|
| AiC information has been removed | ||
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | ||
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | ||
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
Pfizer’s interpretation of the network meta-analysis results
Pfizer states that for combination therapy in cDMARD-experienced severe RA patients:
ETN was consistently significantly better than ABT IV, ADA and INF for ACR20/50/70 outcomes. Furthermore, with regards to ACR20/70 outcomes ETN was shown to be significantly better than ABT (s.c.), otherwise was similar in efficacy to CZP, GOL, and TOC.
For combination therapy in cDMARD-experienced severe RA patients Pfizer states that:
. . . though all bDMARDs had significantly lower HAQ compared with DMARD control at follow-up, none of the bDMARDs had significantly lower HAQ compared with each other.
For cDMARD-experienced severe RA patients who are treated with monotherapy Pfizer states that:
. . . based on the random-effects network meta-analysis; adalimumab, etanercept and tocilizumab have significantly higher odds of ACR 70 than placebo and etanercept and tocilizumab have significantly higher odds of ACR 50 than placebo but none of the bDMARDs are significantly better than another.
The conclusion made by Pfizer in the executive summary is that:
. . . the network meta-analysis in this submission demonstrated that etanercept is significantly better than adalimumab and infliximab for ACR20/50/70 outcomes. Furthermore, etanercept was shown to be significantly better than abatacept i.v. with regards to ACR20/50/70 outcomes and abatacept subcutaneous for ACR20/70.
Roche
Roche reports that:
. . . the proportion of patients who fall within each response category was informed by a network meta-analysis, performed within a Bayesian framework. This meta-analysis was undertaken to allow indirect comparison of tocilizumab monotherapy with biologics currently recommended by NICE for use as monotherapy in the DMARD-IR [inadequate responder] setting.
Figure 61 reproduces the model setup supplied by Roche. The number of trials informing each ‘link’ in the meta-analysis is indicated next to each line.
FIGURE 61.
The network of studies included in the meta-analysis undertaken by Roche.

The ACR outcomes adjusted within the framework of the NMAs used within the economic model by Roche are presented in Table 100. 235 Unadjusted ACR rates are provided for comparison. The forest plot in Figure 62 was produced by Roche and gives an overview of the uncertainty about each estimate after adjustment in the meta-analysis. 124,218,236
| Treatment | ACR20, % | ACR50, % | ACR70, % |
|---|---|---|---|
| Adjusted values (from NMA) | |||
| ADA | 44 | 22 | 10 |
| CTZ | 44 | 24 | 8 |
| ETN | 53 | 35 | 11 |
| TCZ | 61 | 40 | 19 |
| Unadjusted values | |||
| ADA | 49 | 28 | 18 |
| CTZ | 44 | 23 | 7 |
| ETN | 59 | 40 | 15 |
| TCZ | 65 | 47 | 33 |
FIGURE 62.
Biologic monotherapy ACR responses used in the model submitted by Roche. Figure shows adjusted percentage responses from the NMA with 95% confidence intervals (CIs).

Roche’s interpretation of the network meta-analysis results
Roche state that:
. . . results from the analysis suggest that tocilizumab monotherapy was associated with superior outcomes on ACR20, ACR50 and ACR70 response measures, compared with adalimumab, certolizumab pegol and etanercept monotherapy.
UCB Pharma
UCB Pharma undertook NMAs at both 12 and 24 weeks for each ACR response, and also for DAS28 (ESR) remission and low disease activity (24-week data only). These analyses were undertaken for both bDMARDs in combination with MTX and bDMARD monotherapy [with the exception of DAS28 (ESR) low disease activity]. The results have, however, been marked as AiC.
The results for combination therapy are shown in Figures 63–66. The results for monotherapy are shown in Figures 67–70.
FIGURE 63.
Academic-in-confidence information has been removed.
FIGURE 64.
Academic-in-confidence information has been removed.
FIGURE 65.
Academic-in-confidence information has been removed.
FIGURE 66.
Academic-in-confidence information has been removed.
FIGURE 67.
Academic-in-confidence information has been removed.
FIGURE 68.
Academic-in-confidence information has been removed.
FIGURE 69.
Academic-in-confidence information has been removed.
FIGURE 70.
Academic-in-confidence information has been removed.
UCB Pharma’s interpretation of the results from the network meta-analysis
In the circumstance where a patient can receive MTX, UCB Pharma states that:
The [NMA] conducted showed that certolizumab pegol plus MTX is at least as effective to the other comparators considered in the vast majority of cases. The RR of that certolizumab pegol plus MTX vs. comparators in combination with MTX was greater than one for all outcomes investigated for the majority of cases, which indicated better outcomes in favour of that certolizumab pegol plus MTX. The wide credible intervals noted in most of these cases reflect the minimal differences in relative clinical effect between certolizumab pegol and the comparators considered.
In the circumstance where bDMARD monotherapy is used UCB Pharma states that:
The [NMA] showed that certolizumab pegol was at least as effective to the other monotherapies considered. In the majority of cases, the RR [relative risk] of certolizumab pegol compared to the other monotherapies considered was greater than one, however, no differences were statistically significant.
Responder criteria
This section details the criteria to be designated a responder within the submissions. In summary, five submissions used ACR response as a measure of a responder. Three of these assumed that ACR20 measured at 24 weeks/6 months was the minimal response, one (AbbVie) assumed that an ACR50 response was required, with one (UCB Pharma) allowing an evaluation of ACR20 at either 3 or 6 months. The UCB Pharma submission used a EULAR response of moderate or good (at either 3 or 6 months) in those with moderate to severe disease. The Bristol-Myers Squibb submission assumed a DAS28 reduction of 1.2 at 6 months to designate a responder.
AbbVie
The minimal response required for continuation of treatment after the initial 6-month period is ACR50. The Assessment Group note that the comparative results for AbbVie’s intervention (ADA) appears to perform relatively better using ACR50 than by using ACR20.
Bristol-Myers Squibb
Inadequate treatment is determined by the change in DAS28 – in the base case defined as DAS28 not improved by at least 1.2 by month 6. Patients who discontinue within the first 6 months would then try another first-line biologic.
Merck Sharp & Dohme Corp.
Response is defined as at least an ACR20 response at 24 weeks.
Pfizer
Patients were assumed to discontinue therapy if response (defined as at least an ACR20 response) was not achieved citing previous NICE submissions. 231,237,238
Roche
Response is defined as at least an ACR20 response at 24 weeks.
UCB Pharma
The responder definition in the submission from UCB Pharma is variable owing to the flexibility of the model. For the severe disease activity population a response of at least ACR20 is required to continue treatment. For the moderate disease activity population at least a moderate EULAR response was required. The time at which response was measured could be varied between 3 and 6 months.
Health Assessment Questionnaire/European Quality of Life-5 Dimensions changes in relation to response levels
This section details how the submissions transformed response levels (ACR20, ACR50 and ACR70, and good, moderate and no EULAR response) to changes in HAQ or EQ-5D. In summary, the majority of submissions assessed the associated HAQ score change with response levels from their own data and then assumed that this was applicable to all bDMARDs. All submissions showed that a greater response was associated with a greater HAQ score reduction. UCB Pharma used EQ-5D data recorded within their trials to model the improvement post response. There was not a consistent approach to modelling how the response was assumed to be accumulated. In some cases the response at 6 months was assumed to be experienced throughout the 6-month response period; in others, it was assumed that responses developed linearly or the full effect was applied but a one-off reduction was modelled to assume that the HAQ improvement would not be observed immediately.
AbbVie
AbbVie assumed that the HAQ change by ACR response for all bDMARDs would be the same as for ADA, whereas the changes associated with cDMARDs would be the same as for MTX.
Health Assessment Questionnaire changes are divided into the initial response period (defined as either 12 or 24 weeks) and then from the response period until 52 weeks. The base case assumes a 24-week response period.
Health Assessment Questionnaire changes are assumed to be linear until the response period and linearly between the response period and week 52.
Inputs for the MTX-naive patients were based on the DE013109 trial (AbbVie, data on file) and those for MTX-experienced patients were from the Efficacy and Safety of Adalimumab in Patients With Active Rheumatoid Arthritis Treated Concomitantly With Methotrexate (DE01984) trial (AbbVie, data on file). AbbVie reports that data specific for monotherapy were not available in DE019 trial thus an assumption was made that the relative HAQ changes for monotherapy in MTX-experienced patients were similar to those observed in the MTX-naive patients (i.e. DE013). As sample sizes were deemed insufficient for analysis of relative changes in HAQ by stage or RA (moderate or severe), data were pooled for moderate and severe patients.
Tables 101–103 reproduce the data supplied by AbbVie.
| ACR response | ADA + MTX | MTX | ||||
|---|---|---|---|---|---|---|
| Mean % change | SD | n | Mean % change | SD | n | |
| Baseline to 24 weeks | ||||||
| ACR < 20 | –13.7 | 72.5 | 41 | –5.6 | 57.6 | 88 |
| ACR20 to < 50 | –38.6 | 33.0 | 52 | –31.5 | 33.6 | 41 |
| ACR50 to < 70 | –55.7 | 30.1 | 42 | –55.5 | 30.3 | 14 |
| ACR70 to 100 | –80.0 | 22.5 | 38 | –74.0 | 31.7 | 6 |
| 24–52 weeks | ||||||
| ACR < 20 | 4.7 | 45.4 | 32 | –3.2 | 44.2 | 74 |
| ACR20 to < 50 | –2.1 | 73.5 | 41 | 5.5 | 45.7 | 34 |
| ACR50 to < 70 | –12.8 | 51.7 | 33 | 2.8 | 32.1 | 11 |
| ACR70 to 100 | –40.0 | 48.6 | 17 | –22.9 | 14.7 | 2 |
| ACR response | ADA + MTX | MTX | ||||
|---|---|---|---|---|---|---|
| Mean % change | SD | n | Mean % change | SD | n | |
| Baseline to 24 weeks | ||||||
| ACR < 20 | –30.4 | 43.0 | 36 | –27.9 | 36.2 | 48 |
| ACR20 to < 50 | –53.1 | 38.5 | 41 | –43.3 | 45.2 | 53 |
| ACR50 to < 70 | –61.8 | 31.9 | 51 | –53.7 | 44.2 | 52 |
| ACR70 to 100 | –83.6 | 24.0 | 108 | –82.9 | 22.7 | 62 |
| 24–52 weeks | ||||||
| ACR < 20 | –25.2 | 28.5 | 26 | 10.7 | 104.2 | 35 |
| ACR20 to < 50 | –12.1 | 40.9 | 24 | –4.6 | 58.2 | 42 |
| ACR50 to < 70 | –28.8 | 62.5 | 34 | –11.4 | 47.9 | 43 |
| ACR70 to 100 | –14.5 | 80.2 | 50 | –24.6 | 60.3 | 28 |
| ACR response | ADA | MTX | ||||
|---|---|---|---|---|---|---|
| Mean % change | SD | n | Mean % change | SD | n | |
| Baseline to 24 weeks | ||||||
| ACR < 20 | –18.7 | 43.6 | 70 | –27.9 | 36.2 | 48 |
| ACR20 to < 50 | –45.8 | 33.8 | 50 | –43.3 | 45.2 | 53 |
| ACR50 to < 70 | –68.0 | 26.8 | 48 | –53.7 | 44.2 | 52 |
| ACR70 to 100 | –83.2 | 23.7 | 52 | –82.9 | 22.7 | 62 |
| 24–52 weeks | ||||||
| ACR < 20 | –10.1 | 41.9 | 50 | 10.7 | 104.2 | 35 |
| ACR20 to < 50 | 22.2 | 112.3 | 38 | –4.6 | 58.2 | 42 |
| ACR50 to < 70 | 31.1 | 135.8 | 35 | –11.4 | 47.9 | 43 |
| ACR70 to 100 | 54.0 | 199.7 | 22 | –24.6 | 60.3 | 28 |
Bristol-Myers Squibb
Bristol-Myers Squibb provides a table that details the assumed reduction in HAQ. This is reproduced in Table 104. The Assessment Group comments that it has been assumed that the HAQ reduction for cDMARDs used after bDMARDs was halved; however, the data for bDMARDs used after an initial bDMARD appear to generally perform better than the same bDMARD used first line.
| Treatment | HAQ (reduction) change from baseline, mean | HAQ change from baseline, SE | Source |
|---|---|---|---|
| First-line biologics | |||
| ABT i.v. | 0.344 | 0.063 | Bristol-Myers Squibb NMA (2013)208 |
| ABT s.c. | 0.332 | 0.112 | |
| ADA | 0.326 | 0.077 | |
| ETN | 0.279 | 0.097 | |
| IFX | 0.199 | 0.063 | |
| TCZ | 0.213 | 0.100 | |
| GOL | 0.333 | 0.112 | |
| CTZ | 0.386 | 0.069 | |
| Second-line biologics | |||
| ABT i.v. | 0.5 | 0.05 | Malottki et al., 2011171 |
| ADA | 0.48 | 0.048 | Malottki et al., 2011171 |
| ETN | 0.35 | 0.035 | Malottki et al., 2011171 |
| IFX | 0.35 | 0.035 | Malottki et al., 2011171 |
| TCZ | 0.39 | 0.039 | Strand et al., 2012239 |
| GOL | 0.25 | 0.025 | Smolen et al., 2009240 |
| RTX | 0.4 | 0.04 | Malottki et al., 2011171 |
| DMARDs | |||
| LEF | 0.24 | 0.024 | Chen et al., 2006123 – halved |
| GLD | 0.2 | 0.02 | Chen et al., 2006123 – halved |
| CYC | 0.2 | 0.02 | Chen et al., 2006123 – halved |
| AZA | 0.1 | 0.01 | Chen et al., 2006123 – halved |
Bristol-Myers Squibb reports that as the improvement in HAQ-DI score on starting each treatment would actually be more gradual than a sudden decrease, ‘start and end effects’ are applied as a one-off deduction in QALYs on starting and ending each treatment. This deduction is equal to 20% of the increase in quality of life. No justification for this value was provided.
Merck Sharp & Dohme Corp.
Merck Sharp & Dohme Corp. presents EQ-5D data for patients dependent on their health state (non-responder; ACR20; ACR50; ACR70). These values have been calculated with the HAQ score being transformed to a utility using the equation of Hurst et al. 241 Substantially different values are provided for the GOL submission and for the IFX submission, with these data being assumed to apply to all interventions in the relevant submission. MSD does not comment on this discrepancy.
Golimumab data
Table 105 provides data on the assumed utility for each health state. These data have been taken from GO-FORWARD216 and GO-FORTH91 for the DMARD-experienced population and from GO-FORWARD216 for the severe subgroup. These values have been calculated by the HAQ score being used within the Hurst et al. mapping. 241
| Health state | DMARD experienced | DMARD-experienced severe subgroup (DAS ≥ 5.1) (GO-FORWARD216) |
|---|---|---|
| Baseline | 0.401 | 0.355 |
| GOL-treated non-responder | 0.461 | 0.362 |
| GOL-treated ACR20 | 0.581 | 0.636 |
| GOL-treated ACR50 | 0.638 | 0.689 |
| GOL-treated ACR70 | 0.787 | 0.790 |
Infliximab data
Table 106 provides data on the assumed utility for each health state. These data have been taken from START118 and ATTRACT75 for the DMARD-experienced population and from ATTRACT75 for the severe subgroup. These values have been calculated by the HAQ score being used within the Hurst et al. mapping. 241
| Health state | DMARD experienced | DMARD-experienced severe subgroup (DAS28 > 5.1) (ATTRACT75) |
|---|---|---|
| Baseline | 0.282 | 0.271 |
| IFX-treated non-responder | 0.307 | 0.290 |
| IFX-treated ACR20 | 0.462 | 0.452 |
| IFX-treated ACR50 | 0.568 | 0.554 |
| IFX-treated ACR70 | 0.684 | 0.660 |
Pfizer
Pfizer presents the HAQ improvement associated with each of four response levels: no ACR response; ACR20; ACR50; and ACR70. Pfizer states that following a systematic review only one reference allowed separate estimates to be made for cDMARD-inadequate responders and bDMARD-inadequate responders. 231
This source permitted the estimation of HAQ change associated with each ACR response category separately for both cDMARD-inadequate responders (first line within a treatment sequence) and bDMARD-inadequate responders (second and subsequent lines within a treatment sequence). Table 107 presents the estimates of HAQ improvement used in cDMARD-inadequate responders and bDMARD-inadequate responders. Pfizer notes that this approach may lead to further uncertainty in the model due to the extra mapping function, so a comparison using available HAQ data from the NMA was undertaken as a sensitivity analysis.
| ACR response | cDMARD-IR | bDMARD-IR | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| No response | 0.136 | 0.017 | 0.098 | 0.022 |
| ACR20 | 0.443 | 0.018 | 0.405 | 0.034 |
| ACR50 | 0.668 | 0.026 | 0.670 | 0.058 |
| ACR70 | 0.923 | 0.032 | 0.949 | 0.064 |
Roche
The Roche analysis assumes that response to treatment has an impact on disease severity (as measured by individual HAQ score). Data from ADACTA236 were analysed to estimate the relationship between ACR response and individual HAQ score for the first 24 weeks. The data from the first 24 weeks of the study suggest that the higher the observed ACR response the greater the drop in HAQ score. Table 108 presents the individual HAQ score drop per ACR response and the corresponding standard errors (SEs).
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
|---|---|---|---|
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| AiC information has been removed | AiC information has been removed | AiC information has been removed |
For every response to a new treatment, the model applies the corresponding HAQ score reduction to every simulated individual during the first cycle on treatment (first 6 months). The relationship between ACR response and initial HAQ drop is assumed to be conditional only to ACR response; it is applied universally to all interventions.
UCB Pharma
UCB Pharma recorded EQ-5D data within the RAPID trials135,136 which were used for patients with severe RA and within the CERTAIN79 study for those will moderate to severe RA. These are detailed in Table 109, although the data for CERTAIN79 were marked as AiC.
| Severe RA population | Moderate to severe RA population | ||
|---|---|---|---|
| No response | 0.062 | AiC information has been removed | AiC information has been removed |
| ACR20 | 0.173 | ||
| ACR50 | 0.238 | ||
| ACR70 | 0.358 | ||
The data for the severe population were calculated using a regression analysis of EQ-5D versus ACR in RAPID trials;135,136 no further information was provided.
The data for the severe population were calculated using a regression analysis of (AiC information has been removed).
Health Assessment Questionnaire trajectory following initial response
This section details the HAQ trajectory post the initial response. In summary, the majority of submissions use data from previous NICE appraisals although the Assessment Group comments that the evidence base for these values is very limited. Given that HAQ progression is linked in the majority of models to utility, disease costs and mortality, any inaccuracies in the projected HAQ trajectories could have a marked impact on the results.
AbbVie
AbbVie reports that, in line with current NICE guidance on the use of ADA, ETN and IFX for the treatment of RA,207 the model assumes different levels of HAQ progression for patients receiving antiTNF therapy, cDMARD therapy and non-responders after 1 year. The assumption on long-term HAQ-DI progression while on biological therapy is based on the results of a variety of long-term studies on ADA and ETN. 110,242,243 Two sensitivity analyses were undertaken changing the HAQ progression while on bDMARDs to 0.030 and the HAQ progression on cDMARDs to 0.030. These data are shown in Table 110.
| Intervention | Base case | HAQ-DI progression | |
|---|---|---|---|
| Scenario 1 | Scenario 2 | ||
| Biologic therapy | 0.000 | 0.030 | 0.000 |
| cDMARD | 0.045 | 0.045 | 0.030 |
| Non-responders | 0.060 | 0.060 | 0.060 |
Bristol-Myers Squibb
Bristol-Myers Squibb assumes that the HAQ score increases (clinically worsens) gradually over time while the patient is receiving treatment with DMARDs or palliative care. This is modelled as an increase of 0.125 every 2.7 years on DMARDs and of 0.125 every 2 years on palliative care. It is assumed that patients on bDMARDs have a constant HAQ. These assumptions are based on Malottki et al. 171
Merck Sharp & Dohme Corp.
In the MSD model the HAQ score declines at a rate of 0.045 per year if a patient is receiving cDMARDs. Patients receiving palliative care have an assumed HAQ progression of 0.06 per year. The model assumes that bDMARD treatment halts disease progression and thus the HAQ progression per year is 0.00. This assumption is aligned with comments from the NICE TA TA130,207 which states that it is ‘appropriate to primarily examine the estimates of cost-effectiveness based on the assumption of no HAQ progression while on TNF-α inhibitor therapy, while acknowledging the effects on the estimates of incorporating different assumptions of HAQ progression’ and assumes the same holds true for the other bDMARDs.
Pfizer
Pfizer assumes an annual HAQ progression rate of 0.00 for bDMARDs, 0.046 for cDMARDs and 0.06 per year for palliative care, citing that these values have been used in previous NICE appraisals.
Different rates of HAQ progression were explored as sensitivity analyses in both moderate to severe and severe naive populations.
Scenario analysis within the moderate to severe population uses rates of progression observed within PRESERVE217 period 2, weeks 36–88. Rates of progression in period 2 of PRESERVE217 were greater for MTX than those used in previous economic evaluations. Rates of HAQ for ETN plus MTX initially increase in the first 4 weeks after randomisation, but then stabilise from week 40 to week 88, suggesting little or no further HAQ progression over this period. HAQ change from weeks 36, 40 and 56 to week 88 for both ETN plus MTX and MTX alone has been included in the sensitivity analyses.
Scenario analysis within the severe naive population uses rates of progression from period 2 of COMET81 (weeks 52–104). (CiC information has been removed.)
A further scenario analysis within the all populations uses rates of progression (0.031 for cDMARDs and 0.0102 for bDMARDs) observed by Scott et al. 244
Roche
Roche reports that there is a dearth of evidence on the changes a patient’s condition undergoes while on treatment. Moreover, there are no available data from the Roche clinical trials (ACT-RAY213 and ADACTA57,236) following the first 24 weeks (first cycle).
For these reasons Roche states that its model uses evidence in previous submissions to NICE. The model assumes no HAQ score progression for all treatments while patients continue responding. For patients in palliative care, a per-cycle HAQ score progression (worsening) of 0.03 is assumed. These data are shown in Table 111.
UCB Pharma
In the UCB Pharma model it was assumed that HAQ would decrease at a rate of 0.1913 per annum while on treatment, but increase by 0.048 per annum when a second-line bDMARD was used. However, it appears that there are typographical errors within the model as the 6-month response on bDMARDs was half that of the 3-month response, and the changes at 3 months and 6 months for follow-up biologics were equal. For patients on palliative care or cDMARDs, HAQ progression was assumed to be 0.06 per annum. UCB Pharma cites previous NICE guidance for these figures, except the HAQ change on first-line treatment, which was calculated from data on file.
Time to discontinuation of treatment
This section details the methods used by the manufacturers to determine when a patient discontinued treatment. In summary, a multitude of methods were used by the manufacturers.
AbbVie
Time-to-treatment discontinuation curves from Edwards et al. 245 (based on General Practice Research Database data) were used to model overall withdrawal (due to any reasons) while on cDMARDs. AbbVie states that these curves, although somewhat dated, have been judged as representative of withdrawal patterns from non-bDMARDs today by a practising UK rheumatologist, although it was indicated that withdrawal due to HCQ was not expected to be so low. Assumptions were made for combination DMARDs not examined by Edwards et al. 245 that time on treatment would be similar to time on treatment with MTX.
The digitised curves (reading in 90+ points from each curve) were used to create mock patient-level data, following the method of Hoyle and Henley246 when number of patients at risk was available (antiTNFs), and Tierney et al. 247 when number of patients at risk is unavailable (DMARDs). Parametric survival models were estimated using SAS (SAS Institute Inc., Cary, NC, USA) [and Stata (StataCorp LP, College Station, TX, USA) for Gompertz], and provided parameter estimates and variance–covariance matrices. For the time-to-treatment discontinuation data the exponential, Weibull, Gompertz, log-normal, log-logistic and gamma survival models were estimated. The gamma model was estimated only for information purposes, as the Arena model submitted by AbbVie cannot generate samples from it. The fits of the curves were compared visually, as well as using the Akaike information criterion and Bayesian information criterion.
Curves for MTX, SSZ and HCQ in the Edwards et al. 245 study were fitted best by the log-normal function and these were, therefore, used for modelling time on treatment. The fitted curves to the data are shown in Table 112. The correlation between the parameters was not provided in the report.
| Treatment | Lambda | Gamma | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| MTX | 2.1163 | 0.0531 | 2.8986 | 0.0472 |
| MTX + HCQa | 2.1163 | 0.0531 | 2.8986 | 0.0472 |
| SSZ + HCQa | 2.1163 | 0.0531 | 2.8986 | 0.0472 |
| LEFa | 2.1163 | 0.0531 | 2.8986 | 0.0472 |
| HCQ | 0.4165 | 0.0802 | 2.1706 | 0.0674 |
| SSZ | 0.6336 | 0.0303 | 2.4548 | 0.0259 |
| CYCb | 0.6336 | 0.0303 | 2.4548 | 0.0259 |
AbbVie states that:
. . . for anti-TNFs, separate withdrawal curves by reason either through adverse or lack of efficacy are presented in the published literature. Modelling these two reasons separately allows more flexibility in modelling the time on treatment and corresponds to the new treat to target paradigm; for patients on non-biologic DMARDs, they would be evaluated monthly and could start dropping off immediately, while for those on biologics, patients would have to stay on the drug for at least three to six months for the assessment of response. 248
Patients on biologics are subjected to risk of withdrawal due to AEs immediately after start of therapy based on analysis of BSRBR data presented in Soliman et al. 249 The same withdrawal pattern was assumed applicable for all biologic therapies including antiTNFs due to lack of data on the newer biologics not included in BSRBR, the lack of recent comparative data across antiTNFs in BSRBR, and conflicting comparative withdrawal evidence about the antiTNFs in the international literature. 250,251 Biologic monotherapy was assumed to have a higher withdrawal rate due to AEs (evidenced by a recent BSRBR-based analysis, Soliman et al. 249).
AbbVie comments that although the Cochrane review found evidence of differences among clinical trials of biologics, various design elements (e.g. mandatory and optional early escape in some but not all trials) make it difficult to compare withdrawal and to generalise trial results for long-term withdrawal patterns.
The Gompertz model fitted best in the AbbVie analyses for the AE-specific withdrawal data from BSRBR for all antiTNFs presented by Soliman et al. 249 It assumes that after approximately 9 years on biologic treatment, there would be no further withdrawals due specifically to AEs (i.e. all long-term withdrawals are due to lack of efficacy). This was consistent with the experience of a UK practising clinician consulted by AbbVie. AbbVie stated that since the Gompertz survival model is a proportional hazard model, published reason-specific adjusted hazard ratios (HRs) in the same study for the antiTNF monotherapy versus antiTNF combination therapy with MTX have been applied to obtain monotherapy withdrawal curves. 249 The paper did not present reason-specific Kaplan–Meier curves for antiTNFs as monotherapy versus antiTNF plus MTX specifically. The assumption used was that overall the antiTNF AE withdrawal curve is identical to the combination therapy AE withdrawal curve. This assumption is supported by data from the study in which similar proportions of patients discontinued the treatment due to AEs at year 5, this was shown between those receiving antiTNFs in combination with MTX and the overall antiTNF cohort (28% vs. 29%, see Table 2 in Soliman et al. 249). In addition, the Kaplan–Meier curves of the observed overall persistence between these two groups run very close to each other (Figure 71). Parameter estimates for modelling of withdrawals due to AEs for biologics are shown in Soliman et al. 249
FIGURE 71.
Kaplan–Meier estimates of the observed persistence with all antiTNFs and with the combination therapy of antiTNFs and MTX in BSRBR.

Table 113 provides data on withdrawals from bDMARD therapy due to AEs. The correlation between the parameters was not provided in the report.
| Treatment | Lambda | Gamma | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Combination with MTX | –1.5164 | 0.0308 | –0.6247 | –0.0005 |
| Monotherapy | –1.1311a | 0.0308 | –0.6247 | –0.0005 |
Data on withdrawal due to lack of efficacy have been presented for overall antiTNF groups by the same study. 249 This curve starts sloping downwards at around 3 months, and the slope is very flat (i.e. there is no evidence of a stopping rule being applied despite clinical guidance on stopping patients on biologic therapy if adequate response is not observed at 6 months). 248
In the AbbVie base case, the model applies a stopping rule based on response rates; all those without an ACR50 or ACR20 (in a sensitivity analysis) response would be stopped at a given time (i.e. 12 or 24 weeks). AbbVie state:
. . . therefore, the initial part of the withdrawal curve due to lack of efficacy from BSRBR is ignored. The differences in response rates would result in differential withdrawal due to lack of efficacy on biologics, including monotherapy versus combination therapy (i.e., with MTX); no additional adjustment would be applied. Beyond the time point of response assessment, the lack of efficacy curves from BSRBR would be applied to allow for further drop out due to lack of efficacy. In other words, the model predicts a time to withdrawal due to lack of efficacy for all patients in the simulation when each treatment is initiated. If the time predicted is earlier than the stopping rule (i.e., 12 or 24 weeks), it is ignored. If it is later than the stopping rule, and the patient is a responder not stopping treatment at e.g., 12 or 24 weeks, they would be withdrawn at that time.
For withdrawal beyond the non-responder withdrawal (i.e. at 12 or 24 weeks), the same curve is applied across all biologics.
Because the flat initial part of the withdrawal due to loss of efficacy curve is flat, AbbVie report that no survival model provided a good fit to the overall data. However, the fit was much improved when the flat part of the curve for the initial 3.337 months was removed from the data. The best fit for the truncated data was provided by the log-normal function. Time to withdrawal due to lack of efficacy predicted from these parameters was added back by 3.337 months in the simulation. Table 114 provides the parameter estimates given by AbbVie. The correlation between the parameters was not provided in the report.
| Treatment | Lambda | Gamma | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Biologics | 3.1171 | 0.0643 | 3.0225 | 0.0512 |
Bristol-Myers Squibb
The probabilities of AEs assumed by Bristol-Myers Squibb are shown in Table 115. The source for these data appears to be a NMA of AEs undertaken within the Bristol-Myers Squibb submission. As with the NMA for comparative efficacy the reporting of the NMA assumptions is lacking.
| Treatment | At month 6/week 24: probability of AE |
|---|---|
| ABT i.v. | 0.023 |
| ABT s.c. | 0.016 |
| ADA | 0.041 |
| ETN | 0.030 |
| IFX | 0.086 |
| TCZ | 0.041 |
| GOL | 0.020 |
| CTZ | 0.096 |
For all first-line biologic treatments, if an AE had not been simulated then time on treatment is sampled from a Weibull distribution with shape parameter 0.71 and scale parameter 7.06, giving a mean time on treatment 4.21 years (Bristol-Myers Squibb’s submission document to NICE for TA23438).
Bristol-Myers Squibb assumes that the probability of having an AE on RTX is 3.54%, as 17 of 480 patients discontinued due to AEs in the REFLEX study. 252 If the patient does not discontinue treatment with RTX at 6 months, their long-term time on RTX is sampled from a Weibull distribution with shape 0.474 and scale 5.1. 171
Malottki et al. 171 considered i.v. ABT, ADA, ETN, IFX and RTX, so Bristol-Myers Squibb state that it was necessary to find inputs for s.c. ABT, GOL and TCZ. s.c. ABT was assumed to have the same efficacy and safety profile as i.v. ABT. The early withdrawal inputs for GOL and TCZ came from the GO-AFTER study253 and the RADIATE study254 respectively. GOL is an antiTNF, so the long-term time on treatment is assumed to be the same as that of the other antiTNFs (ADA, ETN and IFX) as reported by Malottki et al. 171 TCZ is not an antiTNF, but, in the absence of data, the long-term time on treatment is assumed to be the same as that of the antiTNFs. Inputs for short- and long-term time on treatment are shown in Tables 116 and 117 respectively.
| Treatment | Parameter | Point estimate (%) |
|---|---|---|
| ADA | Probability of withdrawal at 12 weeks | 9.9 |
| Proportion of the discontinuations at 12 weeks that are due to ineffectiveness | 56.2 | |
| ETN | Probability of withdrawal at 13 weeks | 5.2 |
| Proportion of the discontinuations at 13 weeks that are due to ineffectiveness | 16.7 | |
| IFX | Probability of withdrawal at 16 weeks | 23 |
| Proportion of the discontinuations at 16 weeks that are due to ineffectiveness | 66.7 | |
| ABT | Probability of withdrawal at 24 weeks | 13.6 |
| Proportion of the discontinuations at 24 weeks that are due to ineffectiveness | 25.7 | |
| TCZ | Probability of withdrawal at 24 weeks | 14.7 |
| Proportion of the discontinuations at 24 weeks that are due to ineffectiveness | 64.5 | |
| GOL | Probability of withdrawal at 24 weeks | 12.4 |
| Proportion of the discontinuations at 24 weeks that are due to ineffectiveness | 72.0 |
| Treatment | Weibull shape parameter | Weibull scale parameter | Mean (years) |
|---|---|---|---|
| ADA | 0.701 | 3.21 | 4.06 |
| ETN | 0.701 | 3.21 | 4.06 |
| IFX | 0.701 | 3.21 | 4.06 |
| ABT | 0.81 | 5.49 | 6.17 |
| TCZ | 0.701 | 3.21 | 4.06 |
| GOL | 0.701 | 3.21 | 4.06 |
Third-line TCZ use was assumed to have the same rate of AEs and time to withdrawal as second-line TCZ treatment.
For cDMARDs, Bristol-Myers Squibb used data reported by Malottki et al. 171 These data are reproduced in Tables 118 and 119.
| Treatment | Parameter | Point estimate (%) |
|---|---|---|
| LEF | Probability of withdrawal at 6 weeks | 13 |
| Probability of withdrawal at 6–24 weeks | 30 | |
| Proportion of the discontinuations at 24 weeks that are due to ineffectiveness | 33.2 | |
| GLD | Probability of withdrawal at 6 weeks | 14 |
| Probability of withdrawal at 6–24 weeks | 27.1 | |
| Proportion of the discontinuations at 24 weeks that are due to ineffectiveness | 66.7 | |
| CYC | Probability of withdrawal at 6 weeks | 8 |
| Probability of withdrawal at 6–24 weeks | 24 | |
| Proportion of the discontinuations at 24 weeks that are due to ineffectiveness | 50 | |
| AZA | Probability of withdrawal at 6 weeks | 15 |
| Probability of withdrawal at 6–24 weeks | 25 | |
| Proportion of the discontinuations at 24 weeks that are due to ineffectiveness | 50 |
| Treatment | Alpha Weibull parameter | Beta Weibull parameter | Mean (years) |
|---|---|---|---|
| LEF | 1 | 5.98 | 5.98 |
| GLD | 0.48 | 1.81 | 3.91 |
| CYC | 0.5 | 4.35 | 8.70 |
| AZA | 0.39 | 4.35 | 15.53 |
Merck Sharp & Dohme Corp.
Merck Sharp & Dohme Corp. states that no studies with sufficient follow-up were identified for GOL, ADA, CTZ, TCZ or ABT and thus these were all set equivalent to IFX. This is stated to be a very conservative assumption for GOL given that the drop-out rate after 52 weeks of GOL (50 mg) is very low in the GO-FORWARD clinical trial,216 only 6% at week 52. The long-term drop-out rates for the other bDMARDs from clinical trials were stated to be more aligned with the evidence available for IFX. Keystone et al. 84 report comparable drop-out rates at week 52 with those observed in a 52-week trial for IFX.
A summary of the probability of discontinuation due to long-term loss of efficacy parameters used by MSD is shown in Table 120. The probability of remaining on treatment at a given month (x) was estimated from Equation 17:
| Long-term discontinuation due to loss of efficacy | |||
|---|---|---|---|
| Treatment | Lamda | Gamma | Mean (years) |
| GOL | 0.103 | 0.532 | 9 |
| ADA | 0.103 | 0.532 | 9 |
| IFX | 0.103 | 0.532 | 9 |
| ETN | 0.027 | 0.738 | 12 |
| CTZ | 0.103 | 0.532 | 9 |
| TCZ | 0.103 | 0.532 | 9 |
| ABT i.v. | 0.103 | 0.532 | 9 |
| ABT s.c. | 0.103 | 0.532 | 9 |
| MTX | 0.091 | 0.438 | 20 |
Pfizer
Pfizer used 5-year data from the ETN cohort of the BSRBR to estimate treatment cessation. This was selected because it represented the most appropriate long-term evidence available. Calculations in the ETN cohort were made separately for combination and monotherapy patients. Severe disease status (relative to moderate to severe disease status) was included within the analysis as a covariate, allowing separate estimates of treatment cessation for both severe and moderate to severe populations.
Although Pfizer acknowledges the limitations of the use of the ETN BSRBR cohort in the moderate to severe population, in the absence of any long-term data in this population these estimates were considered the best available. It is hypothesised that such patients may be at greater risk of progression than a more representative moderate to severe population, and therefore treatment cessation may be overestimated within this cohort. In the absence of data in the severe DMARD-naive patient population, treatment discontinuation was assumed to be equivalent to that of the severe DMARD-inadequate responder combination therapy population.
Parametric survival curves were fitted to the data with the log-logistic distribution found to provide the best fit to data based on the AIC. 255 Figure 72 presents the estimated cumulative hazard of treatment cessation versus the observed treatment cessation for the ETN BSRBR cohort, both combination and monotherapy, although these are marked as CiC.
FIGURE 72.
Commercial-in-confidence information has been removed.
Data for treatment discontinuation were not accessible for comparator therapies from the BSRBR. Therefore, an observational study by Hetland et al. 250 was selected that presented Kaplan–Meier curves for all-cause treatment cessation for ETN, IFX and ADA from the DANBIO registry,256 which was considered the most similar to the UK population from registries identified in a Pfizer systematic review. Curves were digitised using Engauge Digitizer and a pseudo-patient-level data set was created for all three therapies. 246,257,258 These data sets were used to fit log-logistic parametric survival models that provided relative treatment effects for both IFX and ADA versus ETN (Figure 73).
FIGURE 73.
The fitted log-logistic survival distributions estimated by Pfizer. (AiC information has been removed.)
These relative effects were applied to the baseline estimates for ETN from the BSRBR in order to generate time-on-treatment estimates for IFX and ADA.
In the absence of long-term data for other therapies, the relative effect for ADA was assumed by Pfizer to apply to CTZ and GOL, on the basis that they are also monoclonal antibodies. TCZ, i.v. ABT, s.c. ABT and RTX were conservatively assumed to share the same time on treatment as ETN. A scenario analysis was performed by Pfizer in which there was assumed to be no difference in treatment cessation between bDMARDs.
A cDMARD curve was also generated from the BSRBR control cohort, and this was used for all cDMARDs. Severe disease status (relative to moderate to severe disease status) was also included within the analysis as a covariate. Figure 74 presents the time on treatment assumptions graphically for the severe DMARD-inadequate responder combination therapy population.
FIGURE 74.
Commercial-in-confidence information has been removed.
As Pfizer believes it is difficult to appreciate differences in treatment cessation across all therapies within Figure 74, the same data are presented as a conditional inference tree in Figure 75. A conditional inference tree performs univariate partitioning of the simulated times to treatment cessation by using a significance test procedure in order to identify differences between time on treatment by therapy. Differences in treatment cessation are identified where partitioning occurs. There are four resulting patterns of ‘times’ based on the assumptions described previously: IFX; cDMARD; those based on that of ADA (CTZ and GOL); and those based on that of ETN (ABT i.v., ABT s.c., TCZ and RTX).
FIGURE 75.
Conditional inference tree of first-line treatment cessation, showing patterns of treatment cessation within the economic model, (left to right) shortest to longest times presented by Pfizer. (AiC information has been removed.)
The resulting treatment cessation curves for the model first-line therapy were adjusted by Pfizer to reflect the increased risk of cessation in subsequent lines of therapy. The (log) time ratio for second- versus first-line therapy was estimated as –0.365 using the same methodology of patient-level data set generation as described above, with data taken from DANBIO. 256 This effect was applied in all subsequent lines of therapy and to all therapies (including cDMARDs). Figure 75 presents a comparison of original data and model output. Note that the model output here does not include the effects of the treatment discontinuation rule. The model by default actually models time to start of next therapy (rather than end of current therapy); in order to provide a representative comparison, the time between cessation of RTX therapy and the start of the next therapy was ignored in the generation of Figure 76. The model was able to recreate the effects of second- and subsequent-line treatment cessation accurately.
FIGURE 76.
Treatment cessation in second and subsequent lines estimated by Pfizer. (AiC information has been removed.)
Treatment cessation data used in the model are presented in Table 121. Times were generated stochastically for each patient using a random number combined with the inverse survival distributions. 180
| CiC information has been removed | ||||
|---|---|---|---|---|
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | ||||
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | ||||
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Relative treatment effects from log-logistic survival model (vs. ETN)a | ||||
| Parameter | Coefficient | |||
| ADA vs. ETN | –0.412b | |||
| IFX vs. ETN | –0.905 | |||
| Relative treatment effects from log-logistic survival model (vs. ETN) | ||||
| Parameter | Coefficient | |||
| Subsequent lines vs. first-line use | –0.365 | |||
Roche
The Roche model assumes that all patients receive each treatment for a minimum of one cycle, until response is evaluated. This is consistent both with previous evidence submissions and with the available efficacy evidence. At 6 months, patients will continue on their first therapy, providing they achieved a response greater than or equal to ACR20. Therapy is stopped for a non-responding patient and they move on to the next drug.
Soliman et al. 249 published an analysis of treatment duration using BSRBR data (large cohort with n = 10,396). A proportion of these patients do not receive any concomitant DMARD treatment (32.1%, n = 3339) and this fact was used in the economic analysis as a basis for estimating the withdrawal risk of patients receiving biologic monotherapy.
Roche provided a Kaplan–Meier curve showing treatment persistence with antiTNF. A Weibull and an exponential model were explored to derive a discontinuation rate from the Kaplan–Meier curve. Both models appear to overestimate discontinuation. Roche assumed that the steep rate of discontinuation in the first 2 years reflects the ‘non-responders’, whereas the flat rate after 2.5 years reflects the ‘good responders’. Roche fitted an exponential distribution to the Kaplan–Meier curve after the first 2.5 years and used that as the probability of discontinuation from treatment for patients with initial response: annual rate of 0.098 (R2 = 0.99), 6-month probability of 0.05. The figure provided by Roche illustrating the fits is reproduced in Figure 77.
An adjustment to these curves is based on data from Anderson et al. ,259 a study that explores predicting factors of response to treatment in RA. The study suggests that disease duration is one of the most important factors predicting response. Anderson analysed data from randomised control trials of drugs or devices in RA and found that the disease duration effect on odds of response was 0.98 per extra year of disease duration. This is not included in the base case but has been tested in the sensitivity analysis.
UCB Pharma
UCB Pharma presents data on the risk of treatment discontinuation due to AEs explicitly and due to all causes. The discontinuation due to AEs was denoted AiC.
(Academic-in-confidence information has been removed.)
For all discontinuations the time spent on treatment was based on values from a study including over 2300 patients treated with a TNF-α inhibitor over 9 years. 251 Results from this study showed that the median time on treatment with a TNF-α inhibitor was 37 months (3.08 years). The same treatment duration was assumed for all biologics.
Rebound post treatment
All interventions
Following the cessation of treatment a patient’s HAQ score is updated to reflect the loss of HAQ improvement on the previous line of therapy. MSD, Pfizer, Roche and UCB Pharma conduct sensitivity analyses around this assumption. UCB Pharma assumes that the loss of efficacy from the previous treatment and the gain in efficacy from the subsequent treatment happen simultaneously.
Assumed NHS costs per Health Assessment Questionnaire band
The hospital costs assumed to be associated with HAQ score in each model are reported in this section. In summary, a number of different sources are used (the data have been graphed in Figure 78). The data from MSD have been omitted as these are based on a more complex formula incorporating factors such as age, disease duration and previous number of DMARDs and cannot be easily summarised. Pfizer and UCB Pharma purport to use the same source and the reason for the slight discrepancy is unclear.
FIGURE 78.
A summation of the hospital costs (annual) assumed associated with each HAQ band.

AbbVie
AbbVie reports that patients with more severe symptoms of joint disease are more likely to be hospitalised and may require surgical procedures such as joint replacement. Disease-related hospital costs were estimated based on the Norfolk Arthritis Register (NOAR) database260 and multiplied by NHS reference costs. 261 The resource use for HAQ costs assumed by AbbVie are given in Table 122.
| HAQ band | Total cost (£) |
|---|---|
| 0.0 to < 0.5 | 167.41 |
| 0.5 to < 1.0 | 102.54 |
| 1.0 to < 1.5 | 364.68 |
| 1.5 to < 2.0 | 523.68 |
| 2.0 to < 2.5 | 1246.26 |
| 2.5 to < 3.0 | 2687.97 |
Bristol-Myers Squibb
Bristol-Myers Squibb assumes a cost per unit HAQ score, to incorporate costs for hospitalisation and joint replacement based on Malottki et al. 171 This was inflated to £1245 per HAQ unit score to reflect 2011/12 prices. 232
Merck Sharp & Dohme Corp.
Data from Brennan et al. 183 were used to estimate the number of hospitalisations within the UK for every cycle of the model dependent on a number of characteristics, including TNF-α inhibitor treatment, which is used as a proxy for bDMARD treatment. The coefficients reported in Brennan et al. 183 are reproduced in Table 123. Costs of an inpatient day were estimated from NHS reference costs 2010–11 (non-elective inpatient PA34B) with a mean of £517. 261
| Independent variable | Coefficient |
|---|---|
| Intercept | 0.2351 |
| Utility at baseline | –0.5467 |
| Age (years) | 0.0078 |
| Disease duration | 0.0075 |
| Previous number of DMARDs | 0.0648 |
| AntiTNF | –0.062 |
Pfizer
Direct annual costs of medical resource use, stratified by HAQ score, were uplifted232 to 2011/12 prices from estimates provided by Kobelt et al. 262 derived from a UK observational database [the Early Rheumatoid Arthritis Study (ERAS)263]. Pfizer considered these data to be the most appropriate because it involved a multifaceted approach from the perspective of the NHS. Approaches to estimating costs in other identified sources were more restrictive in the items included. For example, Brennan et al. 183 included only inpatient and monitoring costs. The costs assumed by Pfizer are provided in Table 124.
| AiC information has been removed | AiC information has been removed |
|---|---|
| AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed |
These costs encompassed a broad range of resource use including hospitalisations, surgical interventions, outpatient visits, medication and drug monitoring. The analysis did not include the costs of lost productivity, which have been used previously,228 which do not meet the NICE reference case. 225 Alternative cost scenarios were considered in scenario analysis, including those used by Malottki et al. 171
Roche
It is assumed that patients often require inpatient care associated with RA in addition to the NHS resources utilised for drug administration and routine patient monitoring. Inpatient costs were calculated using the NOAR database. Inpatient hospitalisation was grouped by six HAQ score bands and is shown in Table 125.
| HAQ band at registration | Patients in band, n | Patients with inpatient stay | Number of days in hospital in the following 12 months | ||||
|---|---|---|---|---|---|---|---|
| n | % | Mean | Median | IQR | Range | ||
| 0.0 < HAQ score < 0.5 | 326 | 7 | 0.02 | 0.26 | 0 | 0–0 | 0–26 |
| 0.6 < HAQ score < 1.0 | 800 | 16 | 0.02 | 0.13 | 0 | 0–0 | 0–21 |
| 1.1 < HAQ score < 1.5 | 386 | 11 | 0.03 | 0.51 | 0 | 0–0 | 0–83 |
| 1.6 < HAQ score < 2.0 | 229 | 12 | 0.05 | 0.72 | 0 | 0–0 | 0–25 |
| 2.1 < HAQ score < 2.6 | 127 | 25 | 0.13 | 1.86 | 0 | 0–0 | 0–48 |
| 2.6 < HAQ score < 3.0 | 148 | 31 | 0.21 | 4.16 | 0 | 0–0 | 0–50 |
The method to incorporate resource utilisation in this analysis follows Kobelt et al. 264,265
Each HAQ score category was assigned an inpatient cost of £240.00 per day which is multiplied with the utilisation factor corresponding to each HAQ score category. The resulting inpatient resource utilisation values used in the analysis is summarised in Table 126. Note, the Assessment Group has altered a typographical error in the last column (which read £62.40) and has changed the term per cycle (which is 6 months in the Roche model) to annual costs.
| HAQ scores | 0< 0.5 | 0.6< 1 | 1.1< 1.5 | 1.6< 2.0 | 2.1< 2.5 | 2.6< 3.0 |
| Inpatient cost per year (£) | 62.40 | 31.20 | 122.40 | 172.80 | 446.40 | 998.40 |
UCB Pharma
Additional costs by HAQ-DI category, used by UCB Pharma, were taken from a study by Kobelt et al. 262 In this study, a cohort of 916 patients in the UK were followed up for a mean of 7.8 years. Costs included the use of health-care resources (direct) and loss of work capacity (indirect). Regression analyses were performed according to patients’ HAQ-DI categories. Values were stated to be converted to British pounds (£), although it is unclear why this was necessary given a UK cohort and inflated to a cost year of 2012. 232 The costs are applied at each cycle within the model, based on the HAQ score of each health state at each time point. Only direct costs were included in the base-case analysis, although the indirect costs were taken into account in a sensitivity analysis. The Assessment Group noted a slight discrepancy between the numbers reported by UCB Pharma and those used in the model. These are reported in Table 127.
| HAQ category | Direct costs (used in base case) (£) | Direct values used in the model (£) | Total costs including indirect costs (used in sensitivity analyses) (£) |
|---|---|---|---|
| < 0.6 | 1102 | 1082 | 1212 |
| 0.6–1.1 | 2827 | 2777 | 5000 |
| 1.1–1.6 | 1876 | 1842 | 4902 |
| 1.6–2.1 | 2769 | 2719 | 7388 |
| 2.1–2.6 | 3051 | 2996 | 10,105 |
| ≥ 2.6 | 2419 | 2376 | 9781 |
Utility related to the Health Assessment Questionnaire
This section details the utility values used in the models and a summary of the studies used in the submissions. Figure 79 provides a graphical estimation of the relationship between HAQ and utility assumed in the manufacturers’ models. Data from UCB Pharma are not shown, as UCB Pharma uses EQ-5D data collected in the trial for ACR and EULAR categories and base utility around response categories.
FIGURE 79.
The relationship between HAQ and utility assumed in the manufacturers’ models.
AbbVie
The utility values used in the base-case analysis by AbbVie were calculated using an equation reported within a poster266 that maps between HAQ and EQ-5D, according to the UK-specific EQ-5D tariff derived by Dolan. 267
Both linear and non-linear equations for mapping HAQ to EQ-5D were presented. Using the linear utility mapping equation it is not possible for patients to achieve a negative utility, whereas the non-linear utility mapping equation relates a HAQ-DI score greater than approximately 2.7 to an EQ-5D score of < 0.
Several studies examining quality of life in patients with RA indicate that severe RA health states can be associated with negative utility values, indicating that the non-linear mapping equation more accurately represents the relationship between HAQ and quality of life in patients with very severe RA and functional impairment. 268–271 This is supported by Ducournau et al. ,266 who report that the inclusion of a non-linear term resulted in an improved fit, and that the non-linear term was a significant coefficient. Previous analyses have also suggested a non-linear relationship between HAQ-DI and utility in RA patients. 272
The main report provides no details whatsoever on issues required to judge the appropriateness or otherwise of the statistical models. No details of how uncertainty in the estimates was propagated in the model, if at all, are provided. No details are provided either on the data used to estimate the relationship, or the performance of the models in that data set. The appendix reports an additional model from the same data set that also includes age as a covariate, though the coefficient is quite small. No details are given as to why this was not used.
The provided poster of the Ducournau et al. reference266 gives little additional detail. The overall numbers of patients reported in the trials are reported but no details on the numbers of observations used in the statistical analyses are provided.
The quadratic mapping equation was therefore selected for the base-case analysis whereas the linear mapping equation was examined in sensitivity analyses.
The model used to calculate utility values in the base-case analysis is:
In order to investigate the impact of the quadratic term on the results of the cost-effectiveness analysis, a sensitivity analysis was conducted using the linear regression model reported by Ducournau et al. 266
The linear regression model used in the sensitivity analysis was:
Bristol-Myers Squibb
The HAQ score is converted into a utility value using the mapping algorithm used by Malottki et al. 171 (see Equation 18).
The report does not state whether or not the parameter uncertainty in this regression was taken into account (e.g. by using the variances/covariances) or if the error terms were also included in order to reflect the additional heterogeneity in the patient-level sample. Bristol-Myers Squibb consider a sensitivity analysis that uses an alternative linear regression from Malottki et al. ,171 which excludes the quadratic term.
Malottki et al. 171 report this regression as ‘Birmingham analysis of data set from Hurst’. 241 Only CIs on the coefficients are reported, not the covariances. Hurst et al. 241 is a study from 1997 of 233 RA patients. Note that in their regression work they also find that both pain and HAQ score are significant predictors of EQ-5D. No detail of model fit is provided.
Merck Sharp & Dohme Corp.
The quality-of-life equations used in the MSD submission are provided in Table 128 with reference to Chen et al. 123 It is not clear if the uncertainty, and covariance in the estimated coefficients, was considered in sensitivity analysis.
| Parameter | Regression estimate | SE |
|---|---|---|
| Constant | 0.862 | 0.034 |
| Coefficient for HAQ score | –0.327 | 0.0201 |
Pfizer
The primary analysis in all populations used the algorithm derived by Malottki et al. 171 (see Equation 18).
Pfizer undertook a systematic review of mapping studies in RA (section 2.3.3.2.2). Many studies were discarded because the studies were conducted using patients from a non-UK patient population.
The Assessment Group comment that there is no requirement in the NICE methods guide (either version 2008273 or 2013274) for patients to be selected from the UK, nor is there any obvious theoretical reason why this should be the case. The guide requires that the valuations of health states described by these patients are drawn from the UK, and in RA this would be appropriately achieved by using the UK tariff of the EQ-5D instrument.
The use of this criterion in their selection of studies is therefore misguided.
Three studies remain in Pfizer’s table 50: Hurst et al. 241 (and the subsequent fitting of a quadratic equation to the same data in Malottki et al. 171), Bansback et al. 275 and Hernandez-Alava et al. 276 The submission uses the Malottki et al. 171 equation as the base case and the original Hurst et al. 241 regression in scenario analysis. Pfizer‘s table 50 provides their rationale for discarding the Bansback et al. 275 and Hernandez-Alava et al. 276 studies. Further details are given for each of these studies below but some key points require addressing here:
The reporting of the characteristics of these three studies is misleading:
-
Bansback et al. 275 is discarded on the basis that it includes both UK and Canadian patients. However, it is clearly stated that the UK tariff is applied to the EQ-5D analysis and therefore the criticism is misguided.
-
Hurst et al. 241 is claimed to have ‘Relevant summary statistics reported’ whereas Hernandez-Alava et al. 276 is ‘The sample of the statistical analysis is not clearly stated’. In fact, the sample of patients is fully described in the accompanying clinical trial paper referred to in the manuscript. Critical to the selection of an appropriate statistical model is the distributional characteristics of the dependent variable – this is not reported in Hurst et al. 241
-
Doubt is cast on the Hernandez-Alava et al. 276 results as the patients are defined as having early RA at baseline which may not be generalisable to more established disease. However, Hurst et al. 241 comprises a mixed population of both early- and late-stage disease, there is a clear relationship between patient degree of functional severity and disease duration (table I), but there is no statistically significant relationship between duration and EQ-5D (table V) and nor does it feature in any of the regression analyses (though the study may be too small to detect any effect). It is therefore difficult to see how the same criticism of the relevance of the Hernandez-Alava et al. 276 paper to the current decision problem does not also apply to the Hurst et al. 241 analysis.
-
The most important issue is stated as VAS pain is not estimated over time, therefore not supporting the current model approach. For clarity, the Hernandez-Alava et al. 276 work did include pain score as a separate covariate alongside HAQ because a much more powerful model results (this was also found by Hurst et al. 241). It is the Pfizer cost-effectiveness model that does not consider pain and therefore was considered incapable of using the results, though, of course, a HAQ based model could be adapted to also include the assessment of pain.
Roche
The method to assign utility weights to simulated patients and to derive QALY outcomes in the model is the same as used in our TCZ and MTX combination therapy NICE submission. 231 The analysis uses a mechanism of mapping utility from patient HAQ score. This technique is also similar to previously published cost–utility studies and reimbursement submissions of biologic treatments in RA. 178,182 A description of the methods is presented in the appendix.
The base-case analysis uses a quadratic equation to map HAQ to utility:
The estimates come from two Phase III trials [tOcilimumab Pivotal Trial In methotrexate inadequate respONders (OPTION)132 and tocilizumab safety and the prevention of structural joint damage methotrexate and sulfasalazine combination trial (LITHE)130]. The numbers within the analyses are not reported, nor is any information on the distribution of the data. Only p-values are given for the estimated coefficients: no SEs or CIs. There is no information that allows one to judge the fit of the model to the actual data. Roche compared HAQ and HAQ2 models, and one with age (not age2). Roche found that the age coefficient was very small (surprisingly and not consistent with most other findings that EQ-5D is strongly related to age) so dropped these analyses.
The model with HAQ2 is selected because it has a better fit, but this is not assessed using any kind of penalised likelihood test. In fact, their chi-squared test is equivalent to the p-value on the HAQ2 coefficient and not appropriate for comparing models. This is important because adding an additional covariate will improve fit, but it is not good practice to simply improve fit by adding covariates: this risks losing generalisability.
In the sensitivity analysis three alternatives are tested, though it is not reported where they have come from, except the last which is based on Hernandez-Alava et al. ;276 however, the uncertainty in the coefficients was not used.
UCB Pharma
UCB Pharma has a different model structure from the others in that they are basing it predominantly around response categories within a Markov framework.
This is done in several steps.
Critically, in the severe disease population:
-
Initial response is defined in terms of ACR category and a mean EQ-5D improvement estimated from a linear regression using trial data from the RAPID RCTs. 135,136 No information on key statistics such as model fit or the data sample was provided, making it impossible to judge appropriateness or otherwise. It was unclear how probabilistic sensitivity analysis (PSA) implemented nor how additional covariates were selected or used.
-
Continued improvement in HAQ is converted to EQ-5D score from Bansback et al. 277
In the moderate disease population:
-
Initial response is defined in terms of EULAR category. Regression analysis is used to estimate EQ-5D change by EULAR category based on data from the CERTAIN study. 79 No details are given. Different estimates are made according to the treatment strategy (i.e. this is not assumed to be a relationship that is independent of treatment).
-
The same Bansback et al. 277 estimate is then used for other elements of the model.
Summary of studies used in submissions
Hurst et al. 241 and Malottki et al. 171 are used as the base case by Bristol-Myers Squibb, MSD and Pfizer, and used in sensitivity analysis by TCZ.
Hurst et al. 241 recruited 233 patients with RA from Scottish RA outpatient departments. They also aimed to recruit more severe patients from inpatients and via GPs and residential care. They failed to recruit the desired numbers of patients into functional severity class 4. The paper reports 3-month follow-up data and compares them with baseline data. There is no combined analysis.
The paper does not display the distribution of HAQ or EQ-5D tariff score.
Linear regression was used to estimate EQ-5D as a function of HAQ and other covariates, with stepwise regression used to select variables.
The reported model for EQ-5D at 3 months includes HAQ, HAQ mood score, pain VAS, disease activity and ESR.
The simple linear model that uses only HAQ as an explanatory variable is not reported in the Hurst et al. 241 paper but is reported in Chen et al. ,123 who were supplied with the Hurst et al. 241 data set. They report no details about the sample used (whether or not this was identical to that reported in the paper), its spread, how repeated observations were dealt with, the distribution of the explanatory variable and its range, how the model performed in terms of fit, bias, predictions outside the feasible range. No details of the uncertainty in the estimated coefficients are provided by Chen et al. 123 Malottki et al. 171 is an update from the same group and they similarly report no details on any relevant information required to make a judgement as to the appropriateness or otherwise of the statistical model. The only change made is the addition of a quadratic term.
The assumed costs and disutilities associated with adverse events
The assumptions regarding AEs within each submission are detailed in this section. In summary, only two of the six manufacturers explicitly included the costs of serous AEs within the submission. These were AbbVie (£4568 per episode) and Pfizer (£1497 per episode), with Pfizer only examining this within a sensitivity analysis.
Only Pfizer included disutility associated with a serious AE, assuming a disutility of 0.156 for a period of 28 days, equating to approximately a 0.012 QALY loss.
Data on the rates of AEs are summarised in Time to discontinuation of treatment.
AbbVie
AbbVie takes into account serious infections within its model, citing the important consequences arising in terms of resource utilisation following serious infection. It was assumed that mild or moderate AEs had minimal impact on a patient’s quality of life and have minimal cost implications. The baseline annual risk of serious infections under treatment with non-bDMARDs was extracted from a prospective observational study using BSRBR278 data and assumed to be the same for all non-bDMARDs.
Baseline values for cDMARDs were extracted from BSRBR data, the risk of serious infections for biologic treatments being adjusted through risk parameters derived from a meta-analysis of safety parameters from clinical studies of biologics used in majority in RA.
Risk of serious infections under treatment with biologics was derived using odds ratios of serious infections of biologics versus control treatment derived from a systematic review and meta-analysis of 160 randomised clinical trials by the Cochrane collaboration (erroneously referenced as Hetland et al. 250). Although the meta-analysis includes trials of biologics in indications other than RA (but excluding human immunodeficiency virus), the majority of trials have been conducted in RA, and AEs are considered to happen irrespective of indication.
To calculate the risks of serious infections under treatment of biologics, the baseline risk for DMARDs was converted to odds, the odds for each respective biologic were calculated using the odds ratios, which were subsequently converted to risks. Serious infection risks employed in the base-case analyses as well as odds ratios employed to estimate these are displayed in Table 129. The Assessment Group comment that the odds ratios shown in Table 129 do not match Figure 4 in the most recent version of Singh et al. 165
| Treatment | Risk | Odds ratioa |
|---|---|---|
| DMARDs (MTX, MTX + HCQ, SSZ + HCQ, LEF, SSZ, CYC, HCQ) | 0.031493b | Reference treatment |
| ABA (± MTX) | 0.018198 | 0.57 |
| ADA (± MTX) | 0.035140 | 1.12 |
| ETA (± MTX) | 0.033320 | 1.08 |
| INF (± MTX) | 0.045027 | 3.51 |
| RTX (± MTX) | 0.030578 | 1.06 |
| GOL (± MTX) | 0.040259 | 1.29 |
| TCZ (± MTX) | 0.048867 | 1.45 |
| CTZ (± MTX) | 0.102444 | 0.97 |
A sensitivity analysis was conducted setting the risk of AEs for ETN, ADA and IFX to 0.03767, 0.04075 and 0.04075, respectively (higher), based on the Galloway et al. BSRBR data. 278 Data are not available for other biologics from this BSRBR analysis.
The cost of serious infections was assumed to be equal to NHS reference cost HD23A (Inflammatory Spine, Joint or Connective Tissue Disorders, with Major CC) and was assumed to be £4568.38 per episode of care corresponding to the elective spell tariff of inflammatory spine, joint or connective tissue disorders with major complications. The mean length of stay corresponding to the elective spell tariff was 8.2 days, which was comparable with the median of 7 days suggested by Galloway et al. 278 used to derive baseline AE risks. Despite commenting on the effect on patients on serious infections no disutility associated with serious AEs were used.
Bristol-Myers Squibb
The probabilities of AEs used within the Bristol-Myers Squibb model are shown in Table 130. The source for these data was not provided in the submission. AEs only result in discontinuation of present treatment. There are no cost implications, nor explicit utility implications.
| Treatment | At month 6/week 24: probability of AE |
|---|---|
| ABT i.v. | 0.023 |
| ABT s.c. | 0.016 |
| ADA | 0.041 |
| ETN | 0.030 |
| IFX | 0.086 |
| TCZ | 0.041 |
| GOL | 0.020 |
| CTZ | 0.096 |
Merck Sharp & Dohme Corp.
Adverse events are incorporated into the model based on the proportion of patients who discontinue treatment due to AEs in the first 24 weeks (Figure 80).
FIGURE 80.
Odds ratio of discontinuations due to AEs in cDMARD-experienced patients assumed by MSD.

Adverse events are assumed to be class related; therefore, the costs and utility outcomes are assumed to be equivalent between the bDMARDs. This rate does not appear to be tabulated in the submission. No costs or disutility associated with AEs are included in the MSD model, although MSD comments that it is possible that AE disutility associated with RA treatment was already incorporated into the mapping equation from HAQ to utility.
Pfizer
Pfizer’s base case did not model AEs, with the manufacturer noting that several manufacturers’ submissions for NICE appraisals (for RA) have not modelled AEs. 231,237,238
A scenario analysis including serious infections was performed. The medical resource use estimates derived from data presented by Kobelt et al. 262 contain costs of hospitalisations, and therefore AEs were not concluded within the primary analysis in order to avoid any ‘double counting’ of these costs. 226 Serious infections were selected for the model as opposed to, for example, serious AEs, as health-related quality of life consequences associated with infection in alternative populations have been well documented. 279 Following a serious infection, the Summary of Product Characteristics for all bDMARDs stipulates treatment cessation, which is not the case for other serious AEs. Pfizer argues that the treatment of other AEs is unlikely to utilise a significant amount of medical resources or costs to the NHS.
Pfizer performed a NMA to estimate HRs of serious infection versus cDMARDs. These HRs were applied to the risk of serious infection for MTX,280 estimated from NMA, to provide the cumulative probability of serious infection and are replicated in Table 131. GOL and IFX were assumed to have the same rate of serious infection as ADA as all have a similar mode of action. RTX was assumed to have the same rate of serious infection as TCZ as both are intravenously administered treatments.
| AiC information has been removed | |||
|---|---|---|---|
| AiC information has been removed | |||
| AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
Cost of adverse events
Within the AEs scenario analysis, the cost of serious infection was assumed to be £1497 based on relevant NHS costs, weighted by inpatient activity. 230 Relevant Healthcare Resource Group codes were identified based on Lekander et al. 194 Conservatively, the without complications and contraindications Healthcare Resource Group costs were used. The costs of serious infection assumed by Pfizer in a sensitivity analysis are provided in Table 132.
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
|---|---|---|---|
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | ||
Serious infections were assumed to persist for 28 days and confer a disutility of 0.156 during that time. 279
Roche
The economic model does not assume a difference in AEs between biologic treatments and assumes that neither associated costs nor utility decreases associated with AEs.
UCB Pharma
The costs and outcomes associated with AEs were not included within the UCB Pharma model as it was assumed that all biologic therapies had similar safety profiles.
UCB Pharma comments on the robustness of Cochrane collaboration review of the AEs of biologics regarding the AEs of CTZ. 281 This comment is marked AiC.
(Academic-in-confidence information has been removed.)
Mortality associated with rheumatoid arthritis
The assumptions regarding the effect of RA (and HAQ score) on mortality are detailed for each submission.
In summary, there is no consensus of the most appropriate approach, although four submissions assume that the relative risk of mortality per HAQ score can be determined from a paper by Wolfe et al. 282
These data (as will be detailed in the methodology used by the Assessment Group) are dated and have been superseded. Furthermore, these data do not indicate whether or not the mortality risk is reversible following treatment that reduces a patient’s HAQ.
Two submissions have assumed standardised mortality rate for patients with RA that is assumed independent of HAQ. Pfizer has commented that the impact of mortality on cost-effectiveness ratios has been shown to be marginal owing to discounting.
AbbVie
The submitted model includes general population mortality rates based on UK life tables. However, mortality rates are assumed to be affected by HAQ score. The effect of HAQ on mortality was expressed as a HR of 1.33 per unit increase in HAQ score for both males and females taken from Wolfe et al. 282 Sensitivity analysis varied the HR using values 1.00 and 1.88.
To implement this, general population mortality risks (2009) were derived by fitting a Gompertz function to the data from sex-specific UK life tables. The Gompertz function describes the exponential increase in mortality rates with increasing age in the absence of high rates of age-independent mortality.
Table 133 provides the assumed Gompertz fit to standard mortality data.
| Sex | Mean | SE | Correlation |
|---|---|---|---|
| Females | |||
| Lamda | –10.688847 | 0.05353145 | –0.92256954 |
| Gamma | 0.0951409 | 0.00077774 | |
| Males | |||
| Lamda | –9.6568365 | 0.05960999 | –0.92256954 |
| Gamma | 0.08567803 | 0.00086605 | |
The effect of HAQ on mortality was expressed as a HR of 1.33 per unit increase in HAQ score for males and females. 19 Two major assumptions are made:
-
the HR was assumed to be linear in the HAQ
-
a change in the HAQ has an immediate effect on the expected mortality (i.e. not only the baseline HAQ).
AbbVie presents illustrative curves for mortality dependent on HAQ scores, which are reproduced in Figures 81 and 82.
FIGURE 81.
An illustrative mortality survival curve presented by AbbVie for males.
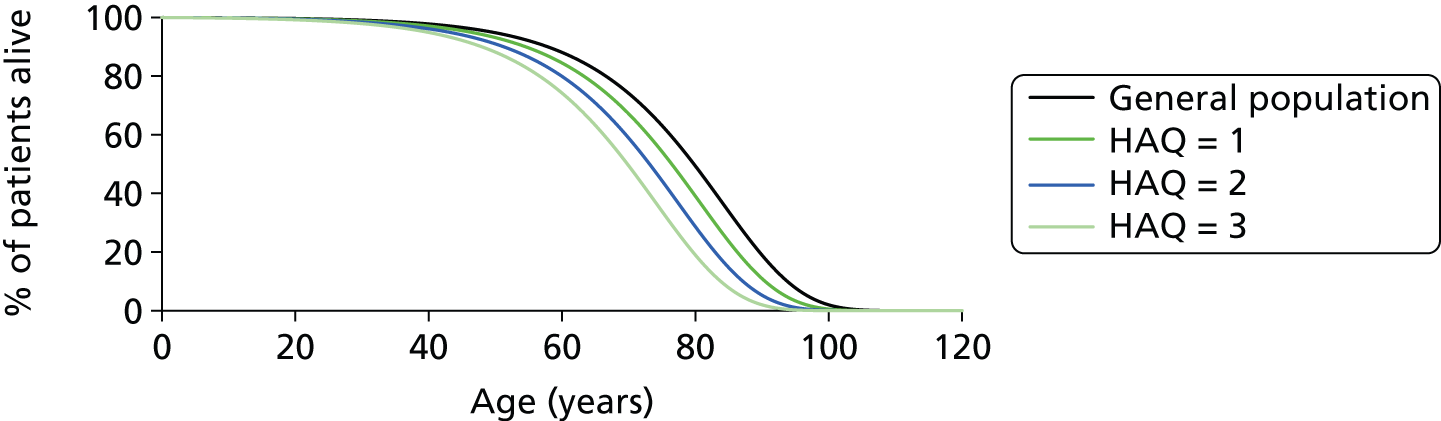
FIGURE 82.
An illustrative mortality survival curve presented by AbbVie for females.
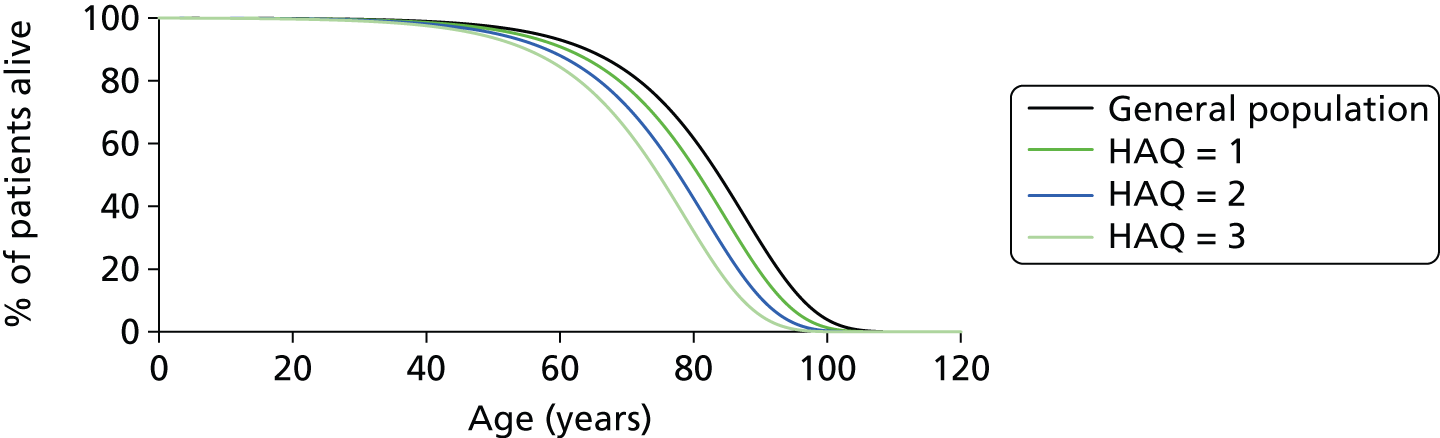
The Assessment Group comments that no goodness-of-fit values for the Gompertz model compared with the life table data were presented.
Bristol-Myers Squibb
The expected age at which a patient dies is based on age, sex and HAQ score, and is recalculated every time the HAQ score changes. Once the age of the patient exceeds their assigned ‘age at death’, the patient dies. The age at death is calculated using conditional probabilities, as follows, replicating the methodology used by Barton et al. 180
Let a and b be the sex-specific survival probabilities for ages x and y, respectively, for a member of the general population. The probability p that a patient of age x will survive to the age y is p=ba.
However, it is assumed that there is an increased risk of death for patients with RA, modelled as a HAQ mortality ratio of 1.33 per unit HAQ. 282 Therefore, the probability p that a patient of age x will survive to the age y is p=(ba)1.33×HAQ. This can be rearranged to give b=a×p11.33×HAQ.
The model looks up the survival probability for the current age of the patient for a and uses a random number between 0 and 1 for p. The age at death is then calculated by looking up the age with the corresponding survival probability closest to b.
Merck Sharp & Dohme Corp.
National life tables for the UK283 were used to obtain age-dependent mortality rates. Furthermore, the proportions of males and females recruited in the IFX trials were used to estimate a weighted-average mortality risk by sex. The mortality rates taken from national life tables were annual rates. They were adjusted to the model cycle length rate using Equation 22:
The cycle rates were transformed into transition probabilities using Equation 23:
A standardised mortality ratio of 1.65 is used in the model, although not referenced in the report. This value is not HAQ dependent.
Pfizer
Pfizer identifies a number of economic evaluations that have assumed either a general risk of mortality associated with RA that is independent of disease severity measures183,192,194,202,238,284,285 or have expressed mortality as dependent on functional status (typically as expressed by HAQ). 171,180,193,198,231,237,286,287
The Pfizer model adopts the former approach, assuming an age–sex-specific standardised mortality ratio from Brennan et al. ,183 who report age- and sex-specific standardised mortality ratios for a UK population.
This approach avoids the implicit assumption that mortality rates would differ between treatment sequences, but Pfizer reports that evidence suggests that this approach may be conservative. 288,289
However, Pfizer also notes that assumptions on mortality have little impact on the cost-effectiveness ratios owing to discounting citing both NICE TA130207 and Vera-Llonch et al. 202
Pfizer comments that the original data used to estimate the function relating HAQ to mortality are now nearly 20 years old and from a non-UK population. 282 Therefore, the standardised mortality ratios used by Brennan et al. 183 were applied to life tables for England and Wales. 283 These values are replicated in Table 134.
| Age (years) | Male | Female |
|---|---|---|
| 0–24 | 2.0 | 2.0 |
| 25–64 | 1.6 | 1.8 |
| 65–101 | 1.3 | 1.5 |
Roche
The probability of death used within the Roche model is based on an adjusted life table provided by the Office for National Statistics. 290 A RA risk multiplier related to each simulated individual’s HAQ score is applied at each cycle based on work by Wolfe et al. ,282 who studied the relationship between HAQ score and early mortality. Wolfe et al. 282 concluded that a relative risk of 1.33 (95% CI 1.099 to 1.610) was associated with each HAQ score point increase. The formula for converting this finding into an adjusted mortality risk (1.33 HAQ) was derived from Barton et al. 180
UCB Pharma
The probability of all-cause mortality was derived from age- and sex-specific mortality rates for the general population from the Government Actuary Department, adjusted by HAQ-DI score. The base-case estimate of relative risk of death of 1.330 per HAQ-DI unit (95% CI 1.099 to 1.610) was taken from a 35-year cohort study of 3501 RA patients in Canada. 282 The starting mortality rate in cycle 1 was adjusted to the age and sex distribution of the model population and further adjustment was made in each model cycle to represent the increased risk of death as patients became older.
Examination of the UCB Pharma model suggests that an exponential distribution is fitted to the life table data and then a relative risk is applied. The exponential fits performed by the Assessment Group are shown in Figure 83 for females and Figure 84 for males. It is seen that the R2 value is in excess of 0.99.
FIGURE 83.
The general mortality rate for females assumed by UCB Pharma, with an exponential fit to these data points. (AiC information has been removed.)

FIGURE 84.
The general mortality rate for males assumed by UCB Pharma, with an exponential fit to these data points. (AiC information has been removed.)
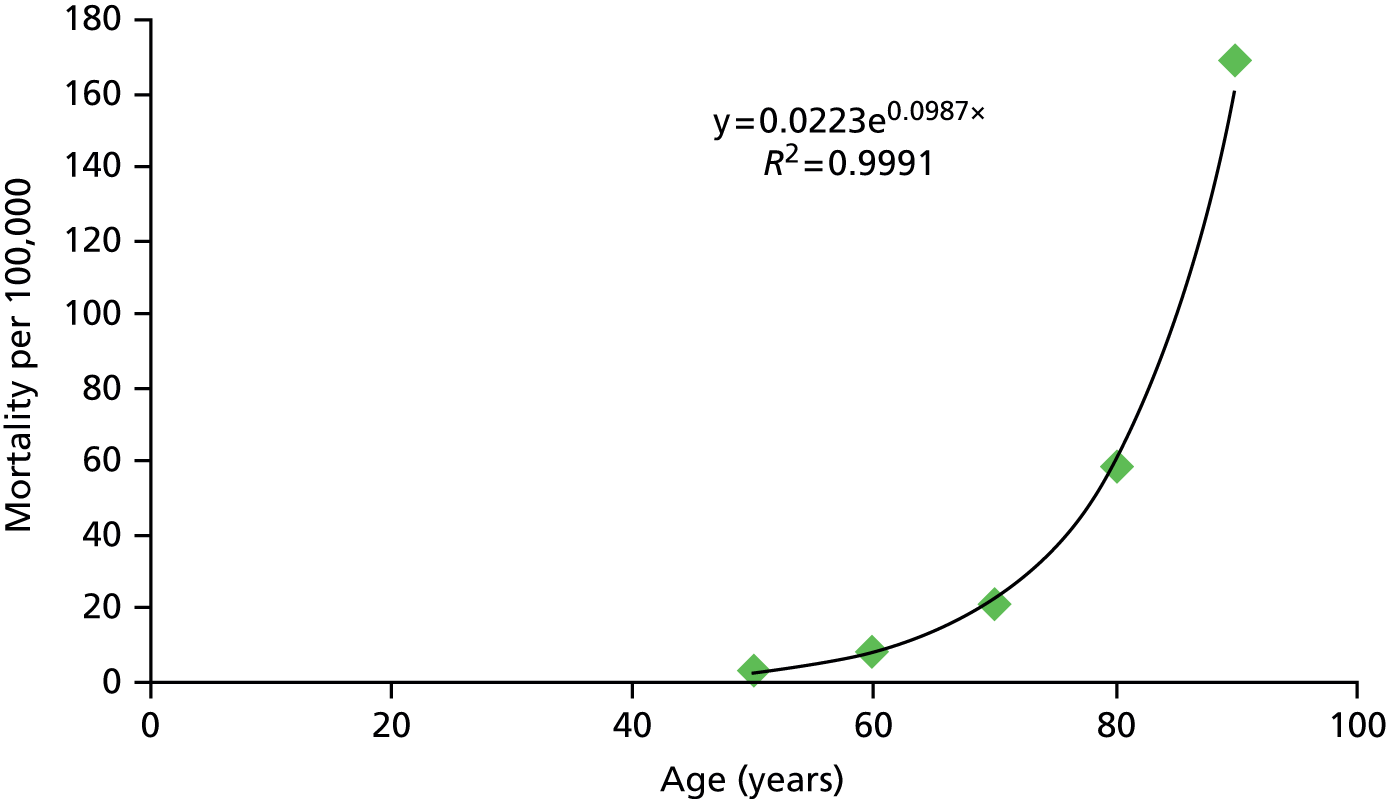
Cost-effectiveness results within the manufacturers’ submission
This section details the cost-effectiveness results reported by the manufacturers within their base cases for each of the analyses undertaken. Typically a large number of sensitivity analyses and descriptive features, such as cost-effectiveness acceptability curves (CEACs), cost-effectiveness planes and scatterplots are presented by the manufacturers. The Assessment Group has selected and reported the key information for brevity reasons, but has endeavoured to report the salient conclusions.
Within the section the following terminology has been used to aid understanding (analyses 1–6 represent the decision problems within the NICE scope):
-
analysis 1: population 2 in combination with MTX
-
analysis 2: population 3 in combination with MTX
-
analysis 3: population 1 in combination with MTX
-
analysis 4: population 2 monotherapy
-
analysis 5: population 3 monotherapy
-
analysis 6: population 1 monotherapy
-
analysis 7: general RA population who can receive MTX
-
analysis 8: MTX-intolerant or -contraindicated RA population.
Table 135 provides a summary of each manufacturer’s interpretation of the cost-effectiveness analyses for their product. Where a manufacturer did not undertake an analysis the cell is blank; otherwise the Assessment Group’s conclusion of the manufacturers’ interpretation of the cost-effectiveness is shown. Three manufacturers (AbbVie, Bristol-Myers Squibb and MSD) have stated that the bDMARDs have similar cost-effectiveness ratios and should be analysed jointly; Pfizer and UCB Pharma make preferential statements about their interventions, whereas Roche has conducted an analysis that consists only of adding TCZ as a monotherapy as first line before a non-NICE-recommended sequence. There are few clear patterns exhibited in Table 135, except that all manufacturers believe their product is cost-effective in analysis 1 and all bar UCB Pharma believe their interventions are cost-effective in analysis 2. It is commented that the analysis 1 undertaken by UCB Pharma omitted a comparison against a cDMARD-only strategy. Given that the remaining manufacturers often commented that the ICERs between populations 2 and 3 were similar, it is possible that UCB Pharma would have estimated bDMARDs not to be cost-effective in population 3 were the correct comparison to be made.
| Analysis | Decision problem | Scope | Manufacturer | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AbbVie (ADA) | Bristol-Myers Squibb (ABT i.v.; ABT s.c.) | MSD (GOL) | MSD (IFX) | Pfizer (ETN) | Roche (TCZ) | UCB Pharma (CTZ) | |||
| 1 | Population 2 in combination with MTX | ✓ | CE (group) | CE (group) | CE (group) | Most CE | Most CE | ||
| 2 | Population 3 in combination with MTX | ✓ | CE (group) | CE (sole) | Not CE | ||||
| 3 | Population 1 in combination with MTX | ✓ | Not CE | Not CE | |||||
| 4 | Population 2 monotherapy | ✓ | Not CE | Most CE | Most CE | ||||
| 5 | Population 3 monotherapy | ✓ | Not CE | Not CE | |||||
| 6 | Population 1 monotherapy | ✓ | Not CE | ||||||
| 7 | General RA population who can tolerate MTXa | CE (group) | CE (group) | CE (group) | |||||
| 8 | MTX-intolerant or -contraindicated RA populationb | CE (sole) | |||||||
These results will be affected by the consideration (or not) of PASs, which are in place for ABT i.v., ABT s.c., CTZ, GOL and TCZ. AbbVie does not consider current PASs. None of MSD, Pfizer and UCB Pharma include PASs for TCZ or ABT as these are CiC. Bristol-Myers Squibb and Roche use PASs for all relevant drugs in their analyses.
Data have been reproduced from a manufacturer’s submission. In some cases it was not possible to align the abbreviations used with those used by the Assessment Group.
AbbVie
The Assessment Group notes that ABT s.c. has not been included in the Abbvie submission, that the responder criterion is ACR50 and that the PASs in place for some interventions have not been included.
Despite performing PSAs, AbbVie presents deterministic results in the base-case tables. The sequence numbers shown in the AbbVie results are aligned with those reported in AbbVie.
The incremental cost-effectiveness analyses are shown in Table 136 for analysis 1 and Table 137 for analysis 2. CEACs from the probabilistic analyses are provided in Figure 85 for analysis 1 and Figure 86 for analysis 2.
| Sequence | Technology | Total | Incremental | ICER | |||
|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | vs. DMARDs (£) | Incremental (£) | ||
| 1 | cDMARDs | 36,636 | 1.747 | ||||
| 8 | TCZ + MTX | 94,128 | 4.433 | 57,492 | 2.686 | 21,405 | Extendedly dominated |
| 4 | IFX + MTX | 97,366 | 4.981 | 60,731 | 3.234 | 18,781 | Dominated |
| 7 | ABT + MTX | 116,143 | 5.036 | 79,508 | 3.289 | 24,172 | Dominated |
| 6 | GOL + MTX | 95,754 | 5.107 | 59,118 | 3.360 | 17,594 | Dominated |
| 2 | ADA + MTX | 94,618 | 5.230 | 57,983 | 3.483 | 16,650 | Extendedly dominated |
| 5 | CTZ + MTX | 97,091 | 5.288 | 60,455 | 3.541 | 17,071 | Dominated |
| 3 | ETN + MTX | 96,785 | 5.377 | 60,149 | 3.630 | 16,571 | 16,571 |
| Sequence | Technology | Total | Incremental | ICER | |||
|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | vs. DMARDs (£) | Incremental (£) | ||
| 1 | cDMARDs | 36,521 | 3.510 | ||||
| 8 | TCZ + MTX | 99,402 | 6.128 | 62,882 | 2.619 | 24,014 | Extendedly dominated |
| 4 | IFX + MTX | 103,092 | 6.680 | 66,571 | 3.170 | 21,000 | Dominated |
| 7 | ABT + MTX | 123,455 | 6.735 | 86,935 | 3.226 | 26,952 | Dominated |
| 6 | GOL + MTX | 101,605 | 6.799 | 65,084 | 3.290 | 19,784 | Dominated |
| 2 | ADA + MTX | 100,495 | 6.914 | 63,974 | 3.404 | 18,792 | Extendedly dominated |
| 5 | CTZ + MTX | 103,093 | 6.974 | 66,572 | 3.464 | 19,217 | Dominated |
| 3 | ETN + MTX | 103,015 | 7.061 | 66,494 | 3.552 | 18,721 | 18,721 |
FIGURE 85.
Cost-effectiveness acceptability curve for analysis 1 provided by AbbVie.

FIGURE 86.
Cost-effectiveness acceptability curve for analysis 2 provided by AbbVie.
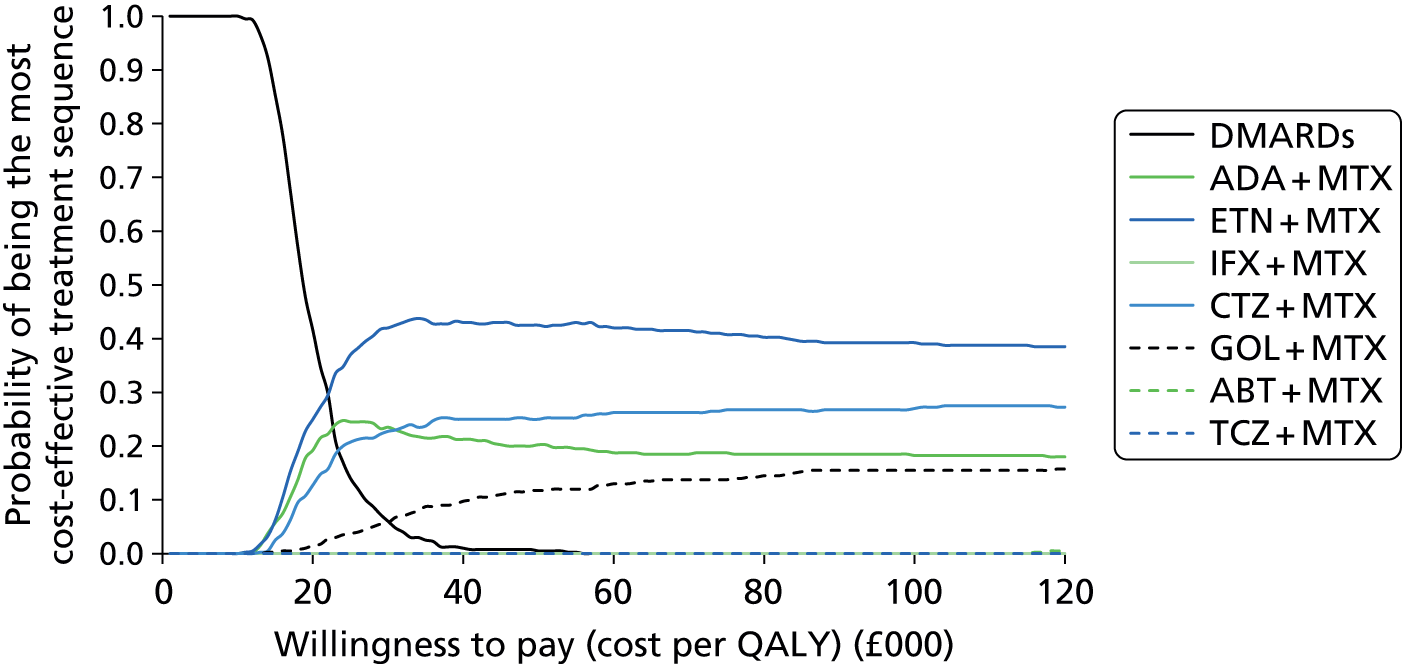
The incremental cost-effectiveness analyses for analysis 3 are shown in Table 138 with the CEACs from the probabilistic analyses provided in Figure 87.
| Sequence | Technology | Total | Incremental | ICER | |||
|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | vs. DMARDs (£) | Incremental (£) | ||
| 1 | MTX | 27,076 | 5.104 | ||||
| 6 | MTX + HCQ | 64,908 | 7.162 | 37,832 | 2.058 | 18,381 | 18,381 |
| 5 | GOL + MTX | 107,556 | 7.539 | 80,479 | 2.436 | 33,044 | Dominated |
| 3 | ETN + MTX | 107,172 | 7.709 | 80,096 | 2.605 | 30,742 | Dominated |
| 4 | IFX + MTX | 113,598 | 7.721 | 86,522 | 2.618 | 33,055 | Dominated |
| 2 | ADA + MTX | 107,097 | 7.765 | 80,021 | 2.661 | 30,071 | 69,971 |
FIGURE 87.
Cost-effectiveness acceptability curve for analysis 3 provided by AbbVie.

The incremental cost-effectiveness analyses are shown in Table 139 for analysis 4 and Table 140 for analysis 5. CEACs from the probabilistic analyses are provided in Figure 88 for analysis 4 and Figure 89 for analysis 5.
| Sequence | Technology | Total | Incremental | ICER | |||
|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | vs. DMARDs (£) | Incremental (£) | ||
| 1 | cDMARDs | 29,905 | 2.686 | ||||
| 2 | ADA | 51,019 | 3.278 | 21,114 | 0.592 | 35,641 | Extendedly dominated |
| 5 | TCZ | 75,098 | 3.573 | 45,193 | 0.887 | 50,972 | Dominated |
| 4 | CTZ | 57,245 | 3.579 | 27,341 | 0.893 | 30,609 | Dominated |
| 3 | ETN | 56,556 | 3.594 | 26,651 | 0.908 | 29,338 | 29,338 |
FIGURE 88.
Cost-effectiveness acceptability curve for analysis 4 provided by AbbVie.
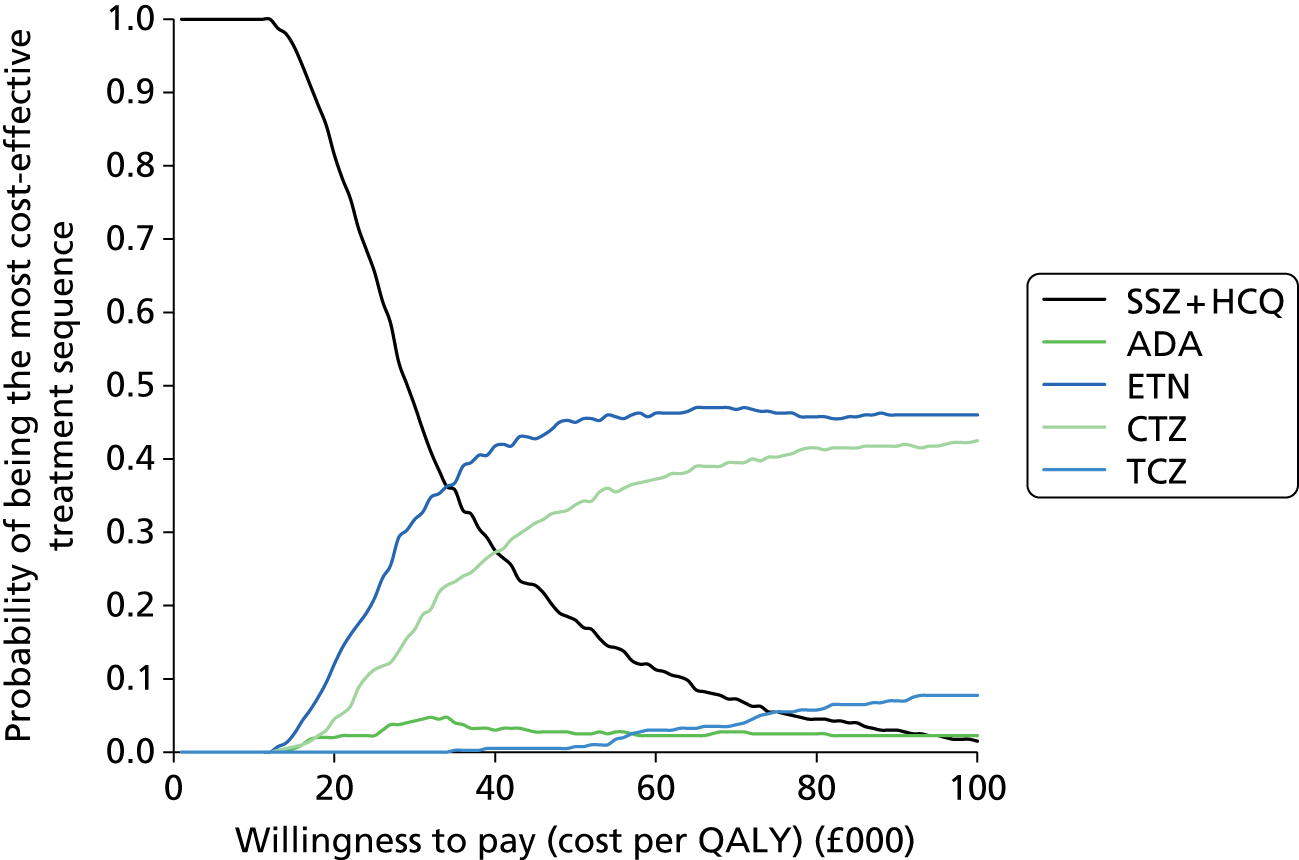
| Sequence | Technology | Total | Incremental | ICER | |||
|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | vs. DMARDs (£) | Incremental (£) | ||
| 1 | cDMARDs | 30,113 | 4.319 | ||||
| 2 | ADA | 53,107 | 4.907 | 22,994 | 0.588 | 39,083 | Extendedly dominated |
| 5 | TCZ | 79,158 | 5.197 | 49,045 | 0.878 | 55,844 | Dominated |
| 4 | CTZ | 59,905 | 5.200 | 29,792 | 0.882 | 33,791 | Dominated |
| 3 | ETN | 59,272 | 5.222 | 29,159 | 0.903 | 32,276 | 32,276 |
FIGURE 89.
Cost-effectiveness acceptability curve for analysis 5 provided by AbbVie.

The incremental cost-effectiveness analyses for analysis 6 are shown in Table 141 with the CEACs from the probabilistic analyses provided in Figure 90.
| Sequence | Technology | Total | Incremental | ICER | |||
|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | vs. DMARDs (£) | Incremental (£) | ||
| 1 | cDMARDs | 29,629 | 5.122 | ||||
| 2 | ADA | 60,778 | 5.156 | 31,149 | 0.034 | 918,015 | Dominated |
| 3 | ETN | 63,859 | 5.293 | 34,230 | 0.170 | 201,097 | Dominated |
| 4 | SSZ + HCQ (followed by ADA) | 41,703 | 5.774 | 12,074 | 0.651 | 18,540 | 18,540 |
FIGURE 90.
Cost-effectiveness acceptability curve for analysis 6 provided by AbbVie.
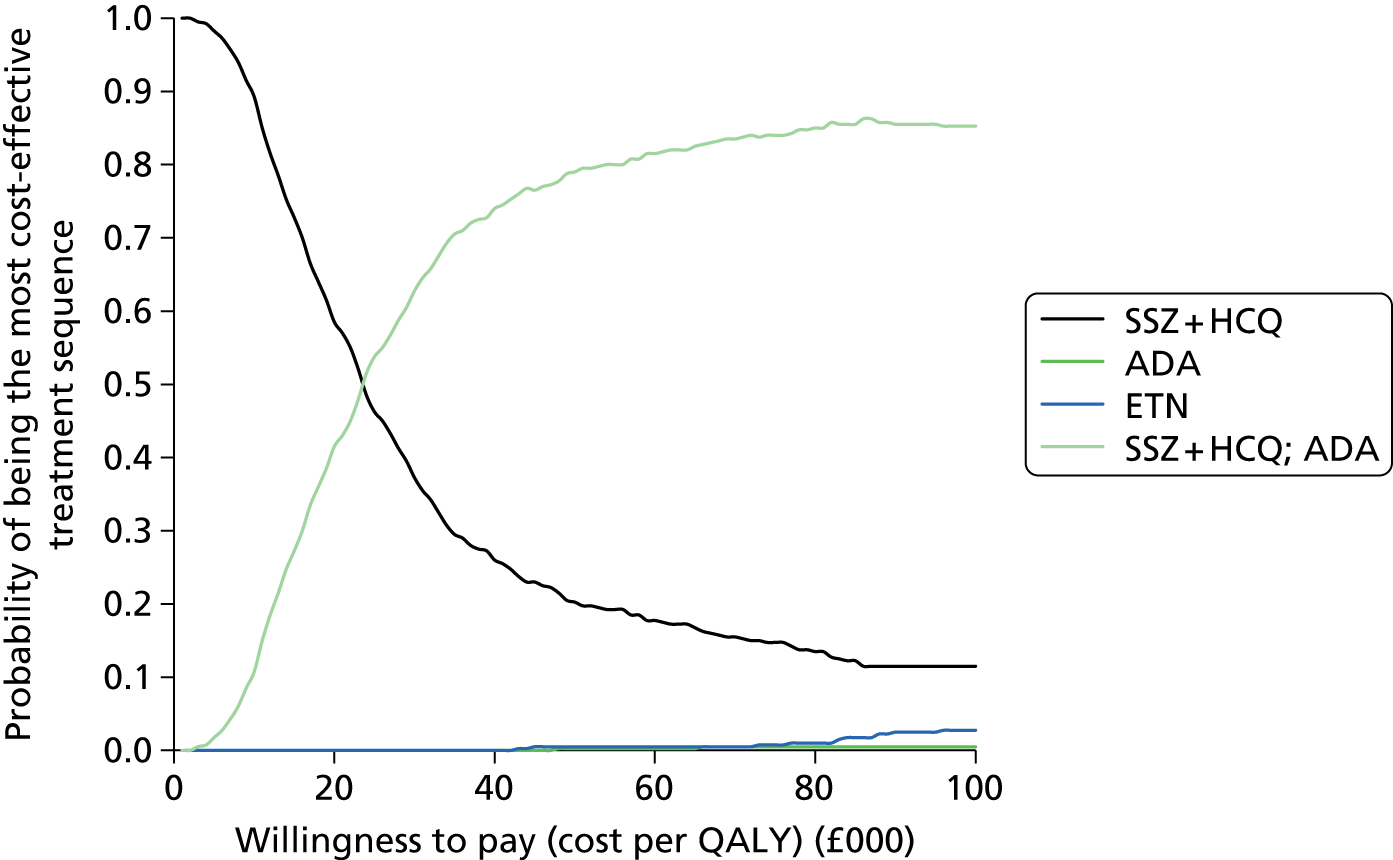
AbbVie’s interpretation of its cost-effectiveness results
AbbVie states that:
The main results from the cost-utility model are:
In the MTX-experienced patient population with severe disease activity (DAS28 > 5.1), ADA in combination with MTX is considered cost-effective, with a lifetime incremental cost per quality-adjusted life year (QALY) gained with respect to conventional DMARDs of £16,650. This is very similar to the estimated cost per QALY of ETN (£16,571) and certolizumab (£17,071), both taken in combination with MTX.
In the MTX-experienced patient population with moderate disease activity (3.2 < DAS28 ≤ 5.1), ADA in combination with MTX is considered cost-effective, with a lifetime incremental cost per quality-adjusted life year (QALY) gained with respect to conventional DMARDs of £18,792. This is very similar to the estimated cost per QALY of ETN (£18,721) certolizumab (£19,217) and GOL (£19,784), all taken in combination with MTX.
AbbVies conclude that its ‘submission demonstrates that ADA in combination with MTX represents a clinical and cost-effective option for the treatment of RA patients with moderate and severe disease activity, for the NHS in the UK’.
Bristol-Myers Squibb
The submission by Bristol-Myers Squibb evaluated the use of bDMARDs only in combination with MTX. The submission did not distinguish between patients with severe and moderate to severe RA, but evaluated these groups together. This did not meet the requirements of the scope and has been denoted as analysis 7.
Bristol-Myers Squibb presents the disaggregated incremental costs and QALYs for the deterministic scenario, but not for the probabilistic values where only the ICER (and CI around the ICER) are provided. The Assessment Group notes that the ICERs are lower for the probabilistic analyses than for the deterministic analyses.
The probabilistic ICERs detailed by Bristol-Myers Squibb are shown in Table 142. These data are marked CiC. Figure 91 shows the CEAC generated by Bristol-Myers Squibb.
| Initial treatment | ICER vs. DMARDs | ||
|---|---|---|---|
| Mean | 95% CI lower bound | 95% CI upper bound | |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| ADA + MTX | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| ETN + MTX | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| IFX + MTX | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| GOL + MTX | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | CiC information has been removed |
FIGURE 91.
Commercial-in-confidence information has been removed.
Bristol-Myers Squibb’s interpretation of its cost-effectiveness results
Bristol-Myers Squibb concludes that:
. . . the results demonstrate that all of the biologics have similar ICERs when compared to DMARDs. The ICERs remain similar in scenario analyses (except when PASs are not considered). This, coupled with the overlap in the probabilistic sensitivity analysis demonstrates considerable uncertainty as to which treatment is the most cost-effective option.
Merck Sharp & Dohme Corp.
The two submissions (one for GOL and one for IFX) from MSD will be detailed individually in terms of the cost-effectiveness results. It is commented that for both submissions only analyses 1 and 7 were undertaken. Analysis 7 does not meet the NICE scope as it combines RA patients with moderate to severe and severe disease.
The Assessment Group notes that MSD makes no comment on the discrepant absolute QALY values in the submission (in the region of 8 for the GOL submission and in the region of 6 for the IFX report).
Golimumab
The incremental analysis for analysis 1 within the GOL submission is reproduced in Table 143. Note that an additional column has been added to correctly calculate the incremental analysis.
| Technologies | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) vs. baseline (MTX) | MSD’s incremental analysis (£) | Assessment Group’s incremental analysis (£) |
|---|---|---|---|---|---|---|---|
| MTX | 56,036 | 6.425 | – | – | – | – | – |
| GOL + MTX | 89,270 | 8.007 | 33,234 | 1.582 | 21,013 | N/A | 21,013 |
The incremental analysis for analysis 7 within the GOL submission is reproduced in Table 144. Note that an additional column has been added to correctly calculate the incremental analysis. The CEAC for analysis 7 is shown in Figure 92.
| Technologies | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) vs. baseline (MTX) | MSD’s incremental analysis (£) | Assessment Group’s incremental analysis (£) |
|---|---|---|---|---|---|---|---|
| MTX | 56,382 | 6.706 | – | – | – | – | – |
| IFX + MTX | 88,326 | 8.207 | 31,944 | 1.501 | 21,278 | 21,278 | Extendedly dominated |
| ETN + MTX | 91,025 | 8.068 | 2699 | –0.139 | 25,429 | Dominated | Dominated |
| GOL + MTX | 92,130 | 8.307 | 1105 | 0.238 | 22,331 | 4631 | Extendedly dominated |
| ADA + MTX | 93,892 | 8.512 | 1762 | 0.205 | 20,769 | 8589 | Extendedly dominated |
| CTZ + MTX | 97,469 | 8.890 | 3577 | 0.377 | 18,817 | 9476 | 18,817 |
| TCZ + MTX | 100,702 | 8.495 | 3233 | –0.395 | 24,774 | Dominated | Dominated |
| ABT i.v. + MTX | 105,102 | 8.100 | 4400 | –0.395 | 34,953 | Dominated | Dominated |
| ABT s.c. + MTX | 118,036 | 8.100 | 12,934 | 0.000 | 44,232 | Dominated | Dominated |
FIGURE 92.
Cost-effectiveness acceptability curve for all bDMARDs for analysis 7 within the MSD GOL submission.
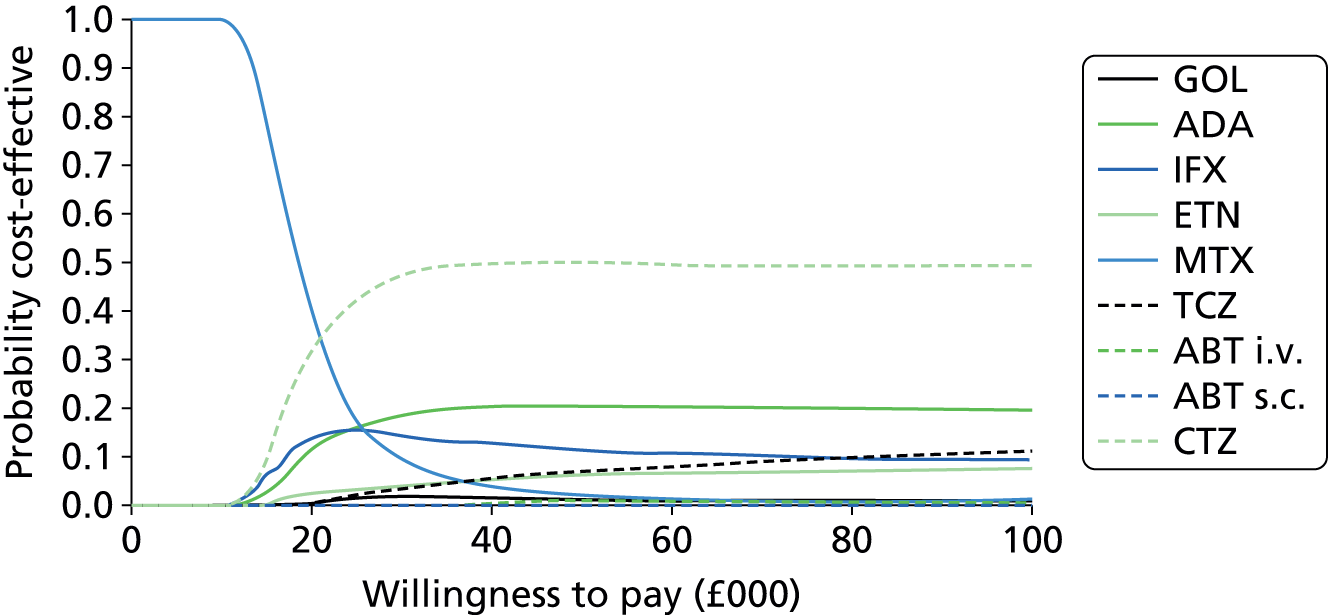
Infliximab
The incremental analysis for analysis 1 within the IFX submission is reproduced in Table 145. Note that an additional column has been added to correctly calculate the incremental analysis.
| Technologies | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) vs. baseline (MTX) | MSD’s incremental analysis (£) | Assessment Group’s incremental analysis |
|---|---|---|---|---|---|---|---|
| MTX | 58,181 | 4.504 | – | – | – | – | – |
| IFX + MTX | 84,007 | 5.539 | 25,827 | 1.034 | 24,968 | N/A | 24,968 |
The incremental analysis for analysis 7 within the IFX submission is reproduced in Table 146. Note that an additional column has been added to correctly calculate the incremental analysis. The CEAC for analysis 7 is shown in Figure 93.
FIGURE 93.
Cost-effectiveness acceptability curve for all bDMARDs for analysis 7 within the MSD IFX submission.
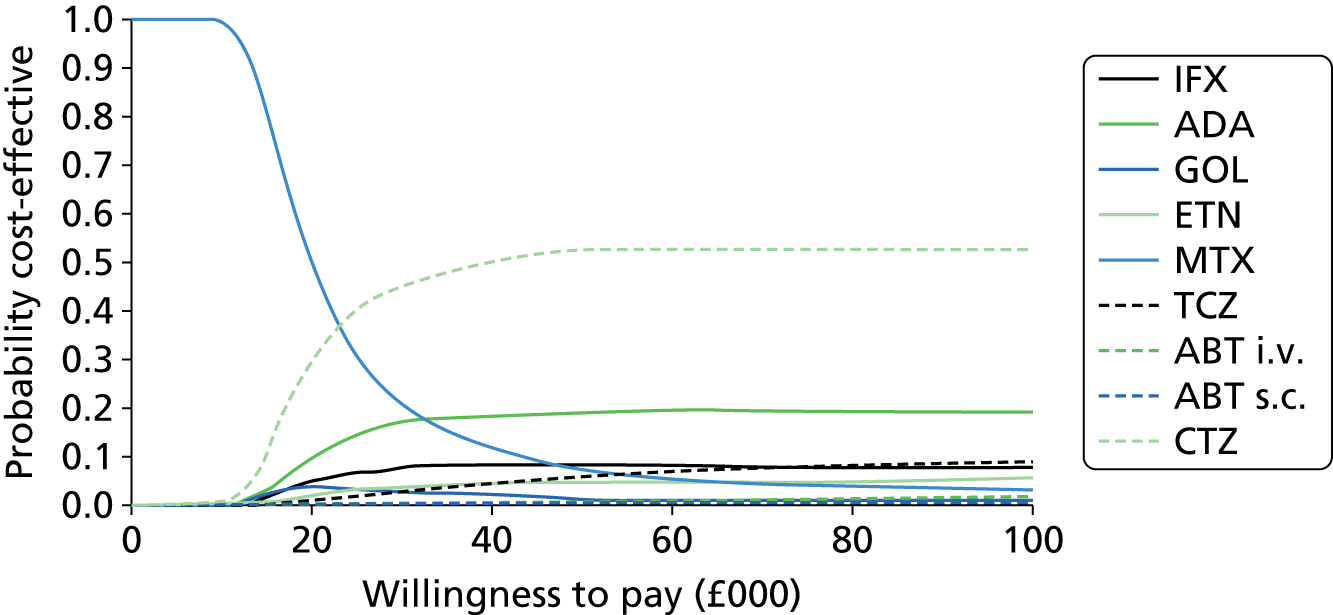
| Technologies | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) vs. baseline (MTX) | Incremental analysis (£) | Assessment Group’s incremental analysis |
|---|---|---|---|---|---|---|---|
| MTX | 57,376 | 4.791 | – | – | – | – | – |
| IFX + MTX | 83,887 | 5.845 | 26,511 | 1.054 | 25,144 | 25,144 | Extendedly dominated |
| ETN + MTX | 84,947 | 5.678 | 1059 | –0.167 | 31,065 | Dominated | Dominated |
| GOL + MTX | 87,027 | 5.909 | 2080 | 0.231 | 26,512 | 9010 | Extendedly dominated |
| ADA + MTX | 88,750 | 6.117 | 1723 | 0.207 | 23,663 | 8305 | Extendedly dominated |
| CTZ + MTX | 93,696 | 6.519 | 4946 | 0.403 | 21,011 | 12,281 | 21,011 |
| TCZ + MTX | 94,777 | 6.065 | 1080 | –0.454 | 29,339 | Dominated | Dominated |
| ABT i.v. + MTX | 97,346 | 5.710 | 2570 | –0.355 | 43,455 | Dominated | Dominated |
| ABT s.c. + MTX | 108,181 | 5.710 | 10,834 | 0.000 | 55,234 | Dominated | Dominated |
Merck Sharp & Dohme Corp.’s interpretation of the cost-effectiveness results in both its golimumab and infliximab submissions
Merck Sharp & Dohme Corp. states:
These results indicate that golimumab/infliximab is a cost-effective treatment option for patients with moderate to severe RA who have had an inadequate response to conventional DMARDs. Due to differences in trial populations and design, using ICERs to ‘rank’ technologies should be approached with caution and we believe that the indirect comparison results indicate a class effect as no significant differences were identified between technologies. A casing [sic] point for this would be the placebo arm dropout in the certolizumab trials which would have acted to inflate the efficacy results for this technology.
Merck Sharp & Dohme Corp. additionally states that:
Compared to other published studies in literature our DMARD experienced results indicate similar ICERs for TNFα inhibitors compared to palliation. Our model derives many assumptions from the Birmingham Rheumatoid Arthritis Model and thus the ICERs are in a similar range of those approved in recent NICE appraisals.
It can be seen that the ICER for GOL/IFX in the severe only subgroup (DAS > 5.1) is similar to the ICER derived for the moderate–severe population and as such GOL/IFX can be considered cost-effective in both populations and should not be limited only to the treatment of patients with severe disease.’
Pfizer
Pfizer sent an addendum to the Assessment Group after detecting minor errors within their mathematical model. These errors affected only scenarios where patients were ineligible for RTX plus MTX, which are not summarised in this section.
Pfizer undertook analyses 1–4. The results from these analyses are reproduced in Tables 147–150, with the CEACs reproduced in Figures 94–97.
| Strategy | Costs (£) | QALYs | vs. cDMARD | vs. next less costly | ICER (£) | ||
|---|---|---|---|---|---|---|---|
| Incremental costs (£) | Incremental QALYs | Incremental costs (£) | Incremental QALYs | ||||
| cDMARDs | 111,612 | 2.638 | |||||
| IFX + MTX | 130,090 | 3.240 | 18,478 | 0.602 | 18,478 | 0.602 | Extendedly dominated |
| ADA + MTX | 133,121 | 3.395 | 21,509 | 0.756 | 3031 | 0.154 | Extendedly dominated |
| CTZ + MTX | 135,304 | 3.768 | 23,692 | 1.130 | 2183 | 0.374 | Extendedly dominated |
| GOL + MTX | 136,452 | 3.470 | 24,840 | 0.832 | 1148 | –0.298 | Dominated |
| ETN + MTX | 140,686 | 4.055 | 29,074 | 1.417 | 4233 | 0.585 | 20,520 |
| ABT i.v. + MTX | 151,963 | 3.513 | 40,351 | 0.875 | 11,277 | –0.542 | Dominated |
| TCZ + MTX | 153,442 | 3.704 | 41,830 | 1.066 | 1479 | 0.191 | Dominated |
| ABT s.c. + MTX | 162,064 | 3.530 | 50,452 | 0.891 | 8622 | –0.174 | Dominated |
| Strategy | Costs (£) | QALYs | vs. cDMARD | vs. next less costly | ICER (£) | ||
|---|---|---|---|---|---|---|---|
| Incremental costs (£) | Incremental QALYs | Incremental costs (£) | Incremental QALYs | ||||
| cDMARD | 128,305 | 8.493 | |||||
| ETN + MTX | 159,730 | 9.764 | 31,425 | 1.271 | 31,425 | 1.271 | 24,727 |
| Strategy | Costs (£) | QALYs | vs. comb cDMARD | vs. next less costly | ICER (£) | ||
|---|---|---|---|---|---|---|---|
| Incremental costs (£) | Incremental QALYs | Incremental costs (£) | Incremental QALYs | ||||
| cDMARDa | 108,488 | 4.754 | |||||
| cDMARD | 112,462 | 4.615 | 3974 | –0.139 | 3974 | –0.139 | Dominated |
| ETN + MTX | 150,095 | 5.965 | 41,607 | 1.210 | 37,633 | 1.350 | 34,373 |
| Strategy | Costs (£) | QALYs | vs. ADA | vs. next less costly | ICER (£) | ||
|---|---|---|---|---|---|---|---|
| Incremental costs (£) | Incremental QALYs | Incremental costs (£) | Incremental QALYs | ||||
| cDMARD | 79,837 | 1.570 | |||||
| ADA | 95,474 | 2.083 | 15,637 | 0.513 | 15,637 | 0.513 | Dominated |
| ETN | 98,143 | 2.265 | 18,306 | 0.695 | 2669 | 0.182 | 26,335 |
| TCZ2 | 115,782 | 2.642 | 35,945 | 1.071 | 17,639 | 0.376 | Extendedly dominated |
| TCZ1a | 122,013 | 2.963 | 42,176 | 1.393 | 6231 | 0.321 | 34,227 |
FIGURE 94.
Cost-effectiveness acceptability curve for analysis 1 within the Pfizer submission. (AiC information has been removed.)
FIGURE 95.
Cost-effectiveness acceptability curve for analysis 2 within the Pfizer submission. (AiC information has been removed.)
FIGURE 96.
Cost-effectiveness acceptability curve for analysis 3 within the Pfizer submission. (AiC information has been removed.)
FIGURE 97.
Cost-effectiveness acceptability curve for analysis 4 within the Pfizer submission. (AiC information has been removed.)
Pfizer’s interpretation of its cost-effectiveness results
Pfizer states that:
. . . the primary analysis demonstrated that, based on current NICE sequential guidance and comparisons made within the analysis, a strategy in which ETN is provided after the failure of two conventional DMARDs is the most cost-effective treatment strategy at a cost-effectiveness threshold of £30,000 per QALY in the Severe DMARD-IR [inadequate responder] combination therapy, Severe DMARD-IR monotherapy and Moderate to Severe populations. The results in a Severe-DMARD-IR population appear to be consistent with previously economic evaluations conducted from a UK perspective identified in the economic SR, when limited or no HAQ progression has been assumed for bDMARDs.
In the Severe Naïve population, the ETN strategy had an ICER of £34,373 versus combination DMARD strategy. This result appears to be different from a previous economic evaluation conducted from a UK perspective, which suggested ETN + MTX may be cost effective at a £30,000 threshold when no HAQ progression is assumed for ETN + MTX. 123 Difference in the economic evaluations results are likely to be partially explained by difference in discount rates used, as if the alternative discount rates used in Chen et al, 2006123 are implemented, then ETN + MTX does becomes a cost effective strategy at £30,000.
Pfizer reports that the secondary analyses, which were not shown in this summary, that used strategies with alternative second-line therapies and additional comparator strategies were:
. . . unable to change the conclusions of the primary analyses. The exception was the inclusion of an alternative 2nd line therapy in the Severe DMARD-IR [inadequate responder] combination therapy population; in this analysis ETN became the optimal strategy at a cost-effectiveness threshold of £20,000 per QALY.
Roche
The Roche submission evaluated a subpopulation not defined in the scope as a MTX-intolerant or -contraindicated RA population, which was in essence analyses 4 and 5 analysed jointly. This was denoted analysis 8.
Roche’s base case evaluated only adding TCZ as the first-line treatment to an existing sequence. The Assessment Group comments that the existing sequence is not recommended by NICE, as three bDMARDs were assumed, and also that sequences of treatment should have been evaluated. For these reasons the results presented by Roche should be treated with caution.
The probabilistic results are shown in Table 151. The CEAC is shown in Figure 98.
| Model output | Standard of care | TCZ strategy | Incremental results | ICER (£ per QALY) |
|---|---|---|---|---|
| Total QALYs | 8.477 | 9.328 | 0.8503 | |
| Total cost (£) | 123,390 | 135,736 | 12,346 | 14,520 |
FIGURE 98.
The CEAC produced by Roche for analysis 8.
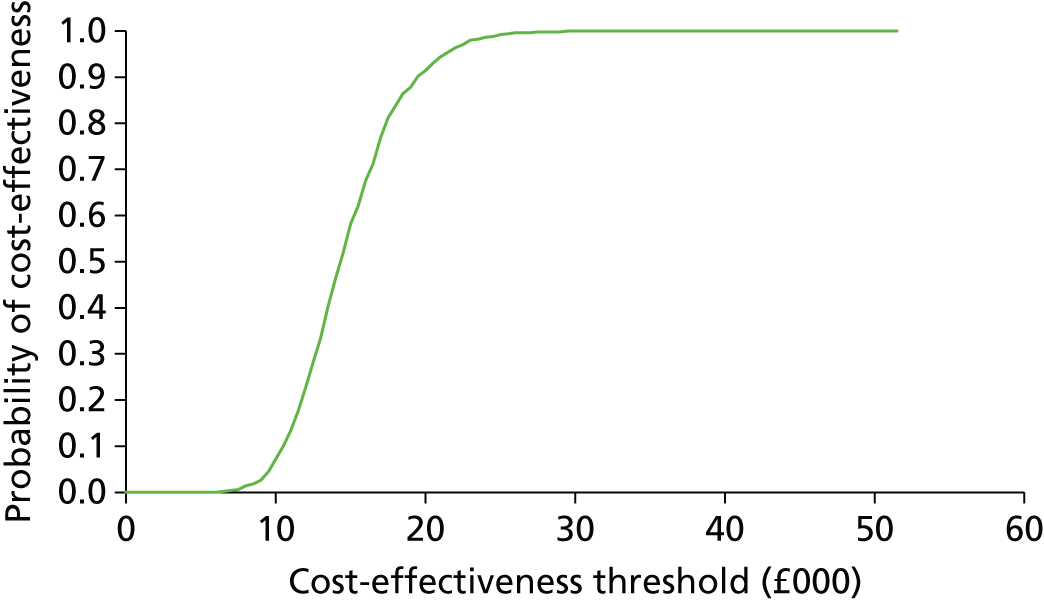
Roche’s interpretation of its cost-effectiveness evidence
Roche states that:
. . . the cost-effectiveness analysis results suggest that the use of first line tocilizumab for DMARD-IR [inadequate responder] RA patients who are intolerant or unsuited to MTX represents a cost-effective use of resources within the NHS. Overall, the results are robust to changes in cost and clinical parameters within the economic model, and moreover the ICERs remain cost-effective across a range of alternative methods of comparison (comparing sequences, comparing individual biologics with one another, comparing biologics to palliation alone).
UCB Pharma
UCB Pharma presented analyses for the populations in the scope for which CTZ was licensed. These are analyses 1, 2, 4 and 5. The Assessment Group comments that this analysis omits a fundamental comparison, which is that of bDMARDs versus cDMARDs. It is unclear whether or not the model submitted by UCB Pharma would estimate whether or not bDMARDs are cost-effective given that the remaining submissions comment that the ICER for population 2 is generally similar to that for population 3, and that UCB Pharma estimates that CYZ is not cost-effective in population 3.
The base-case results for analysis 1 are given in Table 152, with the CEAC reproduced in Figure 99.
| Therapy | Mean costs (£) | Difference in costs (CTZ vs. treatment) (£) | Mean QALYs | Difference in QALYs (CTZ vs. treatment) | ICER (CTZ vs. treatment) (£) | Incremental values | Probability of cost-effectiveness at WTP of £20,000/QALY (%) |
|---|---|---|---|---|---|---|---|
| Combination therapies | |||||||
| GOL + MTX | 126,900 | 929 | 7.092 | 0.193 | £4,822 | Optimal at WTP threshold < £4822 | 0 |
| CTZ + MTX | 127,829 | – | 7.284 | – | – | Optimal at WTP threshold > £4822 | 100 |
| ADA + MTX | 128,267 | –437 | 7.175 | 0.109 | CTZ dominates | CTZ dominates | 0 |
| IFX + MTX | 128,542 | –713 | 7.024 | 0.260 | CTZ dominates | CTZ dominates | 0 |
| ETN + MTX | 128,623 | –793 | 7.184 | 0.100 | CTZ dominates | CTZ dominates | 0 |
| TCZ + MTX | 139,532 | –11,703 | 7.106 | 0.179 | CTZ dominates | CTZ dominates | 0 |
| ABT + MTX | 143,982 | –16,152 | 7.008 | 0.276 | CTZ dominates | CTZ dominates | 0 |
FIGURE 99.
Base-case CEAC for analysis 1 produced by UCB Pharma. (AiC information has been removed.)
The results for analyses 2 and 5 were combined in Table 153. No CEACs for these analyses were provided.
| Therapy | Mean costs (£) | Difference in costs (CTZ vs. PBO) (£) | Mean QALYs | Difference in QALYs (CTZ vs. PBO) | ICER (CTZ vs. PBO) (£) | Probability of cost-effectiveness at WTP of £20,000/QALY (%) |
|---|---|---|---|---|---|---|
| Combination cDMARDs therapies: analysis 2 | ||||||
| PBO + cDMARD | 90,241 | – | 8.760 | – | – | 100 |
| CTZ + cDMARD | 120,217 | 29,976 | 9.387 | 0.627 | 47,821 | 0 |
| Combination MTX therapies: analysis 5 | ||||||
| PBO + MTX | 89,801 | – | 8.726 | – | – | 100 |
| CTZ + MTX | 116,603 | 26,802 | 9.270 | 0.544 | 49,226 | 0 |
UCB Pharma’s base-case results for analysis 4 are provided in Table 154, with the CEAC shown in Figure 100.
| Therapy | Mean costs (£) | Difference in costs (CTZ vs. treatment) (£) | Mean QALYs | Difference in QALYs (CTZ vs. treatment) | ICER (CTZ vs. treatment) (£) | Incremental values | Probability of cost-effectiveness at WTP of £20,000/QALY (%) |
|---|---|---|---|---|---|---|---|
| Monotherapies | |||||||
| ADA | 121,595 | 3019 | 6.846 | 0.315 | 9587 | Optimal at WTP threshold < £9587 | 0 |
| CTZ | 124,614 | – | 7.161 | – | – | Optimal at WTP threshold > £9587 and < £962,778 | 100 |
| ETN | 127,185 | –2571 | 7.163 | –0.003 | £962,778 (ETN vs. CZP) | Optimal at WTP threshold > £962,778 | 0 |
| TCZ | 138,971 | –14,357 | 7.086 | 0.075 | CTZ dominates | Extended dominance by CTZ and ADA | 0 |
FIGURE 100.
Base-case CEAC for analysis 4 produced by UCB Pharma. (AiC information has been removed.)
UCB Pharma’s interpretation of its cost-effectiveness evidence
UCB Pharma states that:
. . . the base case analysis of the severe disease activity population indicated that certolizumab pegol has the highest probability of being cost-effective of all the combination therapies and monotherapies considered, at all willingness-to-pay thresholds between £10,000 and £100,000 per QALY. At £20,000 per QALY, CZP in combination with MTX or as monotherapy is the most cost-effective treatment with a probability of 100%.
Budget impact
This section details the budget impact analyses undertaken by the manufacturers. No comment will be made on the Bristol-Myers Squibb, MSD or Roche submissions as these did not include budget impact analyses. For brevity, only summary figures for the base case will be provided rather than the methods used in the calculations. In summary, each submission predicted that the expenditure on RA interventions would probably increase owing to the increased population that would be eligible if a positive recommendation was issued for the moderate to severe RA population.
AbbVie
Table 155 reproduces the budget impact estimated by AbbVie assuming ADA was used for all eligible patients. The initial year is inflated owing to treating all incident cases.
| Parameter | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Incremental annual budget impact for RA patients with moderate and severe disease activity (£) | 258,556,867 | 149,487,523 | 153,870,726 | 158,282,136 | 162,723,747 |
Pfizer
Pfizer’s summarised results of the number of patients requiring treatment each year is reproduced in Table 156.
| Category | 2014 | 2015 | 2016 |
|---|---|---|---|
| Prevalence | 58,050 | 58,526 | 58,993 |
| Incidence | 1714 | 1729 | 1742 |
| Total | 59,764 | 60,254a | 60,735 |
UCB Pharma
UCB Pharma states that:
It was estimated that the current use of the recommended biological therapy for the severe disease activity population would result in a budget impact of £225 million in 2013, rising to £234 million in 2017. A sensitivity analysis assuming an increased CZP use compared to the base case led to budgetary savings of £2.6 million over 5 years.
Independent economic assessment
Description of the Assessment Group’s model
None of the models submitted by the manufacturers replicated the clinical reality within England and Wales to the satisfaction of the Assessment Group. Primarily, this is because the majority of models assumed that the efficacy of the intervention was based on improvements in ACR, whereas NICE guidance has defined stopping rules where an intervention is stopped unless a DAS28 reduction of 1.2 points22 is achieved. The criterion of achieving a 1.2-point reduction in DAS is associated with a good or moderate EULAR response.
Furthermore, clinicians in the UK predominantly measure EULAR, rather than ACR responses. The use of EULAR is recommended by the British Society for Rheumatology and British Health Professionals in Rheumatology, which consider the EULAR response to be an evidence-based and validated measure of response to treatment. 291
For these reasons the Assessment Group constructed a model in which the assessment of treatment response was based on EULAR response at 6 months. This also alleviates the need for assumptions to be made by decision-makers regarding the proportion of patients who remain on treatment following each category of ACR response.
Two of the submissions, those by Bristol-Myers Squibb286 and UCB Pharma,237 did attempt to model reductions in DAS28; however, neither was considered fully appropriate. The model by Bristol-Myers Squibb did not assess all of the questions within the decision problem, had minimal information on the NMA performed and additionally was written in SIMUL8 (a DES software which is not included in the list of current NICE-recommended packages and thus this platform could not be used by the AG). The model by UCB Pharma was a Markov cohort model that treated all patients as homogenous and would not have the flexibility desired for employing patient-level covariates to represent the heterogeneity of patient outcomes.
The description of the Assessment Group’s model is conducted using the same heading as employed when describing the manufacturers’ models, bar the cost-effectiveness results and cost implications headings that form separate sections of this report. Where appropriate, reasons why the Assessment Group has taken a different approach to the manufacturers will be provided.
The Assessment Group was granted access to data provided by the BSRBR and also from the ERAS and the US National Data Bank for Rheumatic Diseases (NDB), which were used to assess key model parameters and correlations. Specific systematic reviews were undertaken for specific parameters and when these produced relevant information the papers identified are discussed. Contact was also made with key researchers in the field to identify pertinent and/or ongoing research with preliminary findings in the public domain.
The decision problem addressed
The Assessment Group has undertaken evaluations of all the subpopulations defined in the scope, which equate to the defined analyses 1–6. The Assessment Group deviated from the scope for population 1; this was deemed necessary as the defined populations were not exhaustive and did not specify into which population a patient who had received cDMARDs but not MTX would fall. On clinical advice such patients were assumed to be MTX naive. The decision problem addressed by the Assessment Group matches that undertaken by AbbVie and UCB Pharma (for the populations for whom CTZ is licensed).
The strategies modelled
This Assessment Group model considers strategies of sequencing treatments but acknowledges that, owing to the scope, NICE can make recommendations only on the first-line use of bDMARDs. Therefore, this report will assume that NICE guidance after the first biologic treatment is routinely followed. This means that RTX with MTX will be used after failure of the first bDMARD should a patient be able to take MTX and following this a patient receives TCZ and MTX if not previously received.
For simplicity, it was assumed that it would be known whether or not a patient required monotherapy at the time of the first bDMARD initiation based on their experience of cDMARDs and also that any patient who could tolerate MTX could also receive RTX. This would not be correct when analysing population 1, adults with severe active RA not previously treated with cDMARDs, but is likely to be of limited impact as (1) it would be apparent only if bDMARDs were recommended in advance of intensive cDMARDs, and (2) the effect would be dampened as each treatment sequence would have to replace RTX with a bDMARD that is licenced for use in monotherapy and any impact would be relatively equal across all strategies.
Although the Assessment Group model can incorporate sequences of up to seven treatments, for simplicity it was decided that modelling large number of cDMARDs would not be overly informative. The rationale for this is that there are insufficient data on the effectiveness of cDMARDs after either bDMARDs or multiple cDMARDs. For this reason, once a patient had received intensive cDMARD therapy and/or the allotted bDMARDs within the sequence, patients were assumed to have one further cDMARD (typically MTX, but an alternative cDMARD if MTX was not suitable) before moving to ‘non-biologic therapy’ (NBT), which was a term defined to encompass a selection of treatments that clinicians may feel is appropriate for individual patients. It was assumed that NBT would be associated with no initial EULAR response, unlike MTX where the results from the NMA indicated that MTX had a significant EULAR response.
This description is in line with the data on HAQ progression that were presented by Norton et al. 292,293 Given that this assumption applies to all strategies, the contraction of a cDMARD sequence to NBT is unlikely to influence the results and should allow an easier interpretation of the results.
For populations 2 and 3, it was assumed that all patients would have previously received intensive cDMARD therapy prior to the first bDMARD and thus this intervention was not explicitly modelled.
It is acknowledged that these represent simplified pathways and that for individuals there may be alternative strategies, but the Assessment Group and their clinical advisors feel that these are fairly representative and these are also relatively in line with the typical strategies presented by the manufacturers.
Table 157 provides the broad strategies that were deemed appropriate by the Assessment Group for consideration in patients who could receive MTX.
| Population | Strategy |
|---|---|
| 1 | MTX → intensive cDMARDs → NBT |
| MTX → intensive cDMARDs → bDMARDa + MTX → RTX + MTX → TCZ + MTX → MTX → NBT | |
| MTX → intensive cDMARDs → TCZ + MTX → RTX + MTX → MTX → NBT | |
| bDMARDb + MTX → RTX + MTX → TCZ + MTX → MTX → intensive cDMARDs → NBT | |
| 2 and 3 | MTX → NBT |
| bDMARDa + MTX → RTX + MTX → TCZ + MTX → MTX → NBT | |
| TCZ + MTX → RTX + MTX → MTX → NBT |
Table 158 provides the broad strategies that were deemed appropriate by the Assessment Group for consideration in patients who could not receive MTX.
| Population | Strategy |
|---|---|
| 1 | cDMARD → intensive cDMARDs → NBT |
| bDMARDa → bDMARDb → cDMARD → intensive cDMARDs → NBT | |
| 2 and 3 | cDMARDs → NBT |
| bDMARD → bDMARDb → cDMARD → NBT |
The broad strategies were distilled into the following strategies which were evaluated (Tables 159–162). The Assessment Group believes that these provide representative results. These strategies are not significantly different from those of the manufacturers bar the exclusion of named cDMARDs at the end of the sequence. Given the large uncertainty in the efficacy of the cDMARDs in post bDMARD or post intensive cDMARDs the inclusion of specific interventions may be introducing spurious accuracy.
| Strategy | First-line treatment | Second-line treatment | Third-line treatment | Fourth-line treatment | Fifth-line treatment |
|---|---|---|---|---|---|
| Strategy 1 | MTX | NBT | |||
| Strategy 2 | ABT i.v. + MTX | RTX + MTX | TCZ + MTX | MTX | NBT |
| Strategy 3 | ABT s.c. + MTX | RTX + MTX | TCZ + MTX | MTX | NBT |
| Strategy 4 | ADA + MTX | RTX + MTX | TCZ + MTX | MTX | NBT |
| Strategy 5 | CTZ + MTX | RTX + MTX | TCZ + MTX | MTX | NBT |
| Strategy 6 | ETN + MTX | RTX + MTX | TCZ + MTX | MTX | NBT |
| Strategy 7 | GOL + MTX | RTX + MTX | TCZ + MTX | MTX | NBT |
| Strategy 8 | IFX + MTX | RTX + MTX | TCZ + MTX | MTX | NBT |
| Strategy 9 | TCZ + MTX | RTX + MTX | MTX | NBT |
| Strategy | First-line treatment | Second-line treatment | Third-line treatment | Fourth-line treatment | Fifth-line treatment |
|---|---|---|---|---|---|
| Strategy 1 | SSZ | NBT | |||
| Strategy 2 | ADA | ETN | SSZ | NBT | |
| Strategy 3 | CTZ | ETN | SSZ | NBT | |
| Strategy 4 | ETN | ADA | SSZ | NBT | |
| Strategy 5 | TCZ | ETN | SSZ | NBT |
| Strategy | First-line treatment | Second-line treatment | Third-line treatment | Fourth-line treatment | Fifth-line treatment | Sixth-line treatment | Seventh-line treatment |
|---|---|---|---|---|---|---|---|
| Strategy 1 | MTX | Intensive cDMARDs | NBT | ||||
| Strategy 2 | ETN + MTX | RTX + MTX | TCZ + MTX | MTX | Intensive cDMARDs | NBT |
| Strategy | First-line treatment | Second-line treatment | Third-line treatment | Fourth-line treatment | Fifth-line treatment | Sixth-line treatment |
|---|---|---|---|---|---|---|
| Strategy 1 | SSZ | NBT | ||||
| Strategy 2 | ETN | ADA | NBT |
For population 1, in order to ease interpretation of results the analyses have been conducted assuming that ETN is generalisable in terms of costs and QALYs to all other bDMARDs. This assumption is given some support by the results for populations 2 and 3 presented in Results. The cost-effectiveness of a bDMARD first option compared with an option of bDMARD use after initial intensive cDMARD use is the analysis presented for populations 2 and 3.
Model structure/time cycle
A simplified schematic of the Assessment Group’s model is shown in Figure 101. The model is individual patient based, written in Microsoft Excel and uses a DES approach. Therefore, a time cycle was not employed. The model allows only legitimate HAQ scores (the 25 points defined in the 0–3 range) with time to a change in HAQ score being a competing risk. The advantage of using discrete HAQ scores means that if some outputs (such as costs, utility or risk of mortality) are assumed, related by HAQ, there is no need to be continually updating the output as a HAQ score is assumed to linearly progress between legitimate HAQ points.
FIGURE 101.
Conceptual simplified schematic of the modelling process.
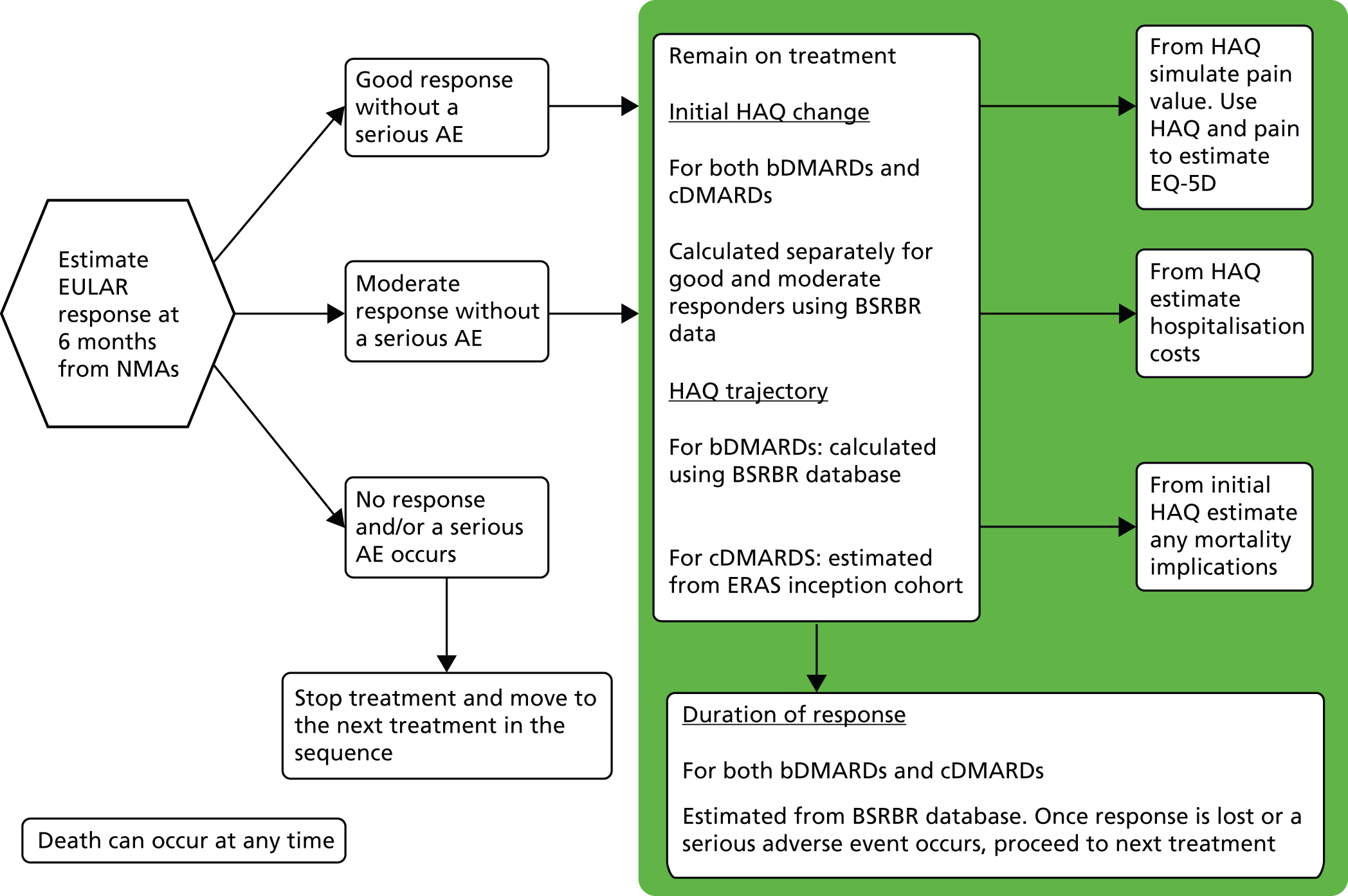
The Assessment Group model differs substantially from that of the manufacturers, as it is EULAR based and uses large databases for population of key parameters such as the initial HAQ changes conditional on EULAR response, and HAQ trajectory based on EULAR response.
Time horizon
The Assessment Group model employs a lifetime patient horizon, but assumes that no patient will live beyond 101 years. This is similar to the approaches undertaken in the manufacturer’s submission.
Perspective
The Assessment Group model employs a direct NHS and Personal Social Services perspective, which is in line with that adopted by the manufacturers.
Discounting
The Assessment Group model used discount rates of 3.5% per annum for both costs and benefits as recommended within both the 2013 NICE methods guide274 and the 2008 methods guide. 220 Sensitivity analyses were undertaken assuming values of 6.0% for costs and 1.5% for benefits.
Population characteristics
The Assessment Group samples patients who are MTX experienced from the BSRBR, which allows correlation to be maintained between the following characteristics: age; sex; disease duration; DAS; previous DMARDs; HAQ; and weight. Individual patients were resampled until the patient met the criteria for the population being analysed. This approach significantly increased the running times for those patients with a DAS between 3.2 and 5.1, as these represented a minority of patients in the BSRBR and required considerable resampling.
Having sampled the patient’s characteristics, the HAQ score is set at a legitimate value. As an example, suppose that a non-legitimate HAQ of 1.600 was simulated. Sampling the probabilities of the bordering legitimate HAQ scores in inverse relation to their distance from 1.6 (20% chance of being 1.5 and 80% chance of being 1.625) would retain the mean value but allow legitimate HAQ scores. Thus, in this example we would simulate 80% of patients having a HAQ score of 1.625 with the remaining patients having a HAQ of 1.5 rather than 100% having a HAQ of 1.600.
The Assessment Group populated patients’ characteristics based on the BSRBR, whereas a number of manufacturers have used the patient characteristics from their pivotal trials to populate their mathematical models. The advantage of the Assessment Group’s approach is that it is a much larger data set (7250 patients), it is representative of people treated in England and Wales and the correlation structure between parameters is maintained. A disadvantage is that the data set for moderate to severe RA patients is much smaller, with approximately 500 patients, although this is not small relative to the numbers of patients within the RCTs.
For patients who are MTX naive it was deemed that the BSRBR database was not an appropriate data source, as this would contain a very small number of such patients. Both AbbVie and Pfizer presented population characteristics for MTX-naive patients with a DAS > 5.1. Of the two estimates, that of Pfizer based on the COMET trial81 was deemed more appropriate, as the disease duration was of 1 year, compared with 11.28 years reported by AbbVie (citing Breedveld et al. 109), which was thought to be a long period without having experienced MTX. The estimate from Pfizer had a greater HAQ at baseline (1.70 compared with 1.38) and were on average younger (a mean age of 51.4 years compared with 60 years).
Costs of the interventions
These costs are similar to those used by the manufacturers; however, there are two comments worth noting: (1) that the Assessment Group takes all PASs into consideration whereas the majority of manufacturers do not; and (2) that a number of manufacturers have assumed a fixed weight per person, which can underestimate the costs of weight-based interventions. The intensive cDMARDs strategy was costed as triple cDMARD plus prednisolone therapy. This is consistent with the intensive cDMARD therapy provided in the TICORA study. 294 The treatment included MTX (20 mg weekly), HCQ (6.5 mg/kg daily), SSZ (3 g daily) and prednisolone (oral, 7.5 mg daily). The total treatment cost in the response period is £3365.32, with a regular monthly treatment cost of £491.34.
An additional treatment option is listed in Tables 161 and 162 that is not an intervention within the NICE scope: RTX plus MTX.
The costs of other drugs used within the sequence (RTX and the costs of cDMARDs) are provided in Table 163.
| Treatment | Dose regimen | Cost per cheapest dose (£)a | Cost of first 6 months (£)b | Subsequent annual treatment cost (£)b |
|---|---|---|---|---|
| RTX | 2000 mg every 9 months | 3492.60 (2000 mg) | 3492.60 | 4656.80c |
| HCQ | 6.5 mg/kg per day (maximum 400 mg per day) | 0.17 (400 mg) | 31.35d | 62.70d |
| MTX | 7.5 mg per week escalated by 2.5 mg per week up to 20 mg per week | 0.80 (20 mg) | 19.32 | 41.57 |
| Prednisolone | 7.5 mg per day | 1.07 (7.5 mg) | 196.25 | 392.50 |
| SSZ | 500 mg per day escalated by 500 mg per week up to 3000 mg per day | 0.79 (3000 mg) | 131.38 | 290.17 |
| Intensive combination DMARD therapye | HCQ + MTX + prednisolone + SSZ (doses as per monotherapy treatments) | N/A | 378.31 | 786.94 |
| Palliative care/rescue therapy | N/Ae | Assumed 60 per monthf | 360 | 720 |
Costs of administration and monitoring
The administration costs of infusions were taken from TA247,26 in which the final appraisal determination stated that ‘the manufacturer’s revised estimate of £154 was acceptable’. This estimate (of 60 minutes infusion time) was also applied to ABT and IFX in the absence of a robust relationship between costs and infusion times. This assumption may be favourable to IFX and unfavourable to ABT, as the recommended infusion times are at least 2 hours and 30 minutes respectively. The final appraisal determination for TA24726 did not comment on the assumption that 10% of s.c. injections would be performed by district nurses and the Assessment Group has assumed that these were also thought acceptable. This resulted in an average administration cost per s.c. injection of £2.61. Neither of the administration costs has been inflated as they were relatively recent and there is uncertainty in the direction of costs in the current economic climate. The value used by the Assessment Group is in broad agreement with the majority of manufacturers.
The assumed monitoring costs are provided in Table 164. These are assumed equal for MTX and bDMARDs. It is possible that the estimate of one hospital outpatient appointment per month may be an overestimate. However, because, on clinical advice, this was assumed, in both the bDMARD and the cDMARD arms, and there was no benefit on mortality assumed, there would be no impact on the cost-effectiveness ratio were this value to be changed to a different value.
| Monitoring component | FBC | ESR | BCP | CXR | Urinalysis | Hospital outpatient attendance | Total cost (£) |
|---|---|---|---|---|---|---|---|
| Assumed cost (£) | 3a | 3a | 3a | 33a | 0.09b | 128b | |
| MTX monitoring: before treatment initiation | 1 | 1 | 1 | 1 | 0 | 1 | 170 |
| MTX monitoring: first 6 months of treatment | 10 | 0 | 10 | 0 | 0 | 10 | 1700 |
| Monthly monitoring cost | 1 | 0 | 1 | 0 | 0 | 1 | 134 |
Comparative treatment efficacy (network meta-analysis)
The NMA undertaken by the Assessment Group has been detailed in Chapter 3, Network meta-analysis results. For information graphical depictions of the estimated proportions of EULAR response are provided in Figures 102 and 103 for EULAR and in Figures 104–109 for ACR mapped to EULAR. It is stressed that these figures do not reflect the considerable uncertainty in the values and reflect mean estimates only.
FIGURE 102.
Estimated mean EULAR responses in cDMARD-experienced patients (main analyses). Int, intensive.
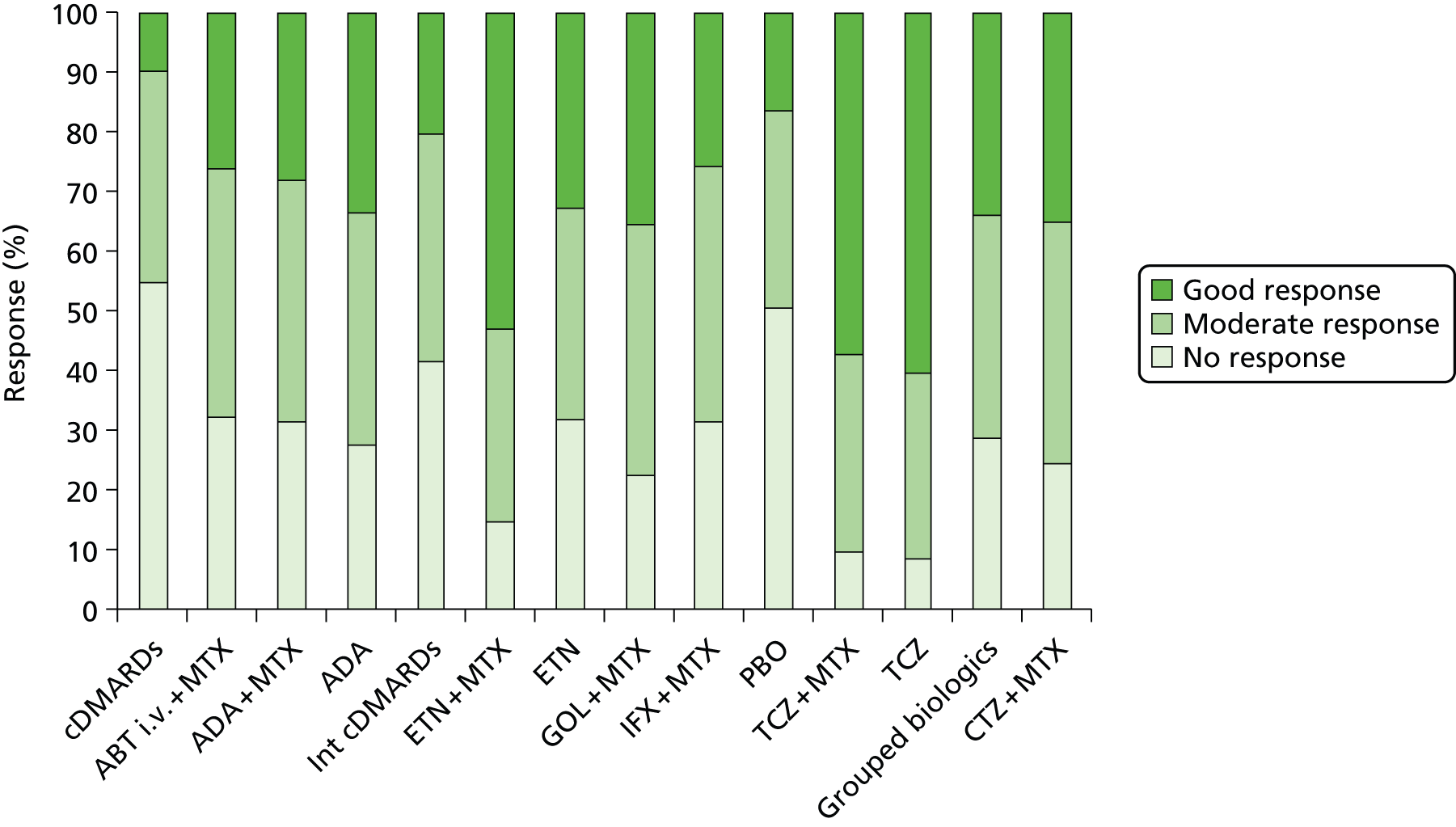
FIGURE 103.
Estimated mean EULAR responses in cDMARD-experienced patients (main analyses + RCTs with a small level of bDMARD use). Int, intensive.

FIGURE 104.
Estimated mean EULAR response in cDMARD-experienced mapped patients from ACR trials (main analyses). Int, intensive.

FIGURE 105.
Estimated mean EULAR response in cDMARD-experienced mapped patients from ACR trials (main analyses + RCTs with a small level of bDMARD use). Int, intensive.
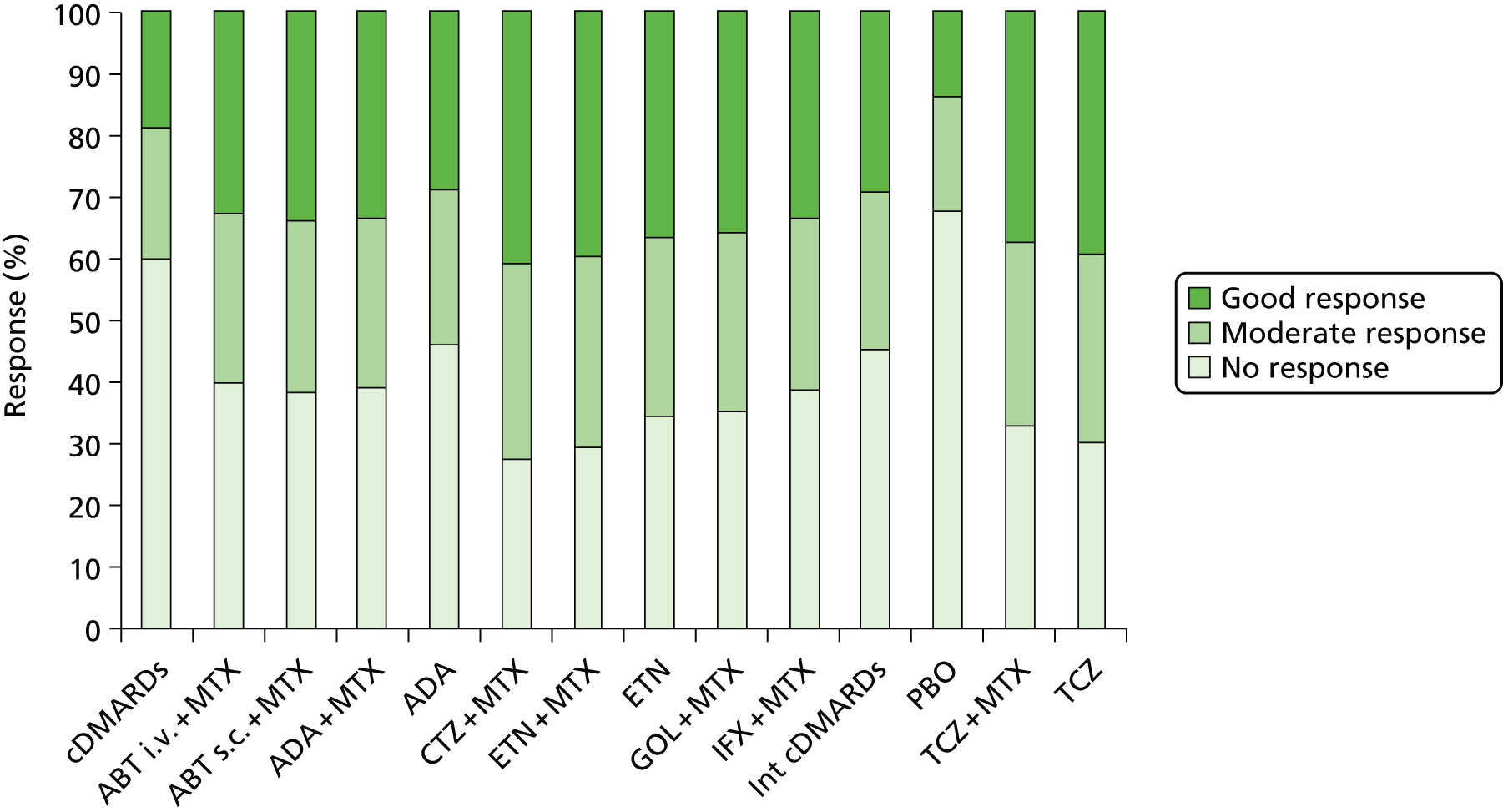
FIGURE 106.
Estimated mean EULAR response in cDMARD-experienced patients mapped from ACR trials (main analyses + RCTs with a small level of bDMARD use and also allowing a trial with low MTX background use). Int, intensive.

FIGURE 107.
Estimated mean EULAR response in cDMARD-experienced patients mapped from ACR trials (main analyses + RCTs with low MTX background use). Int, intensive.
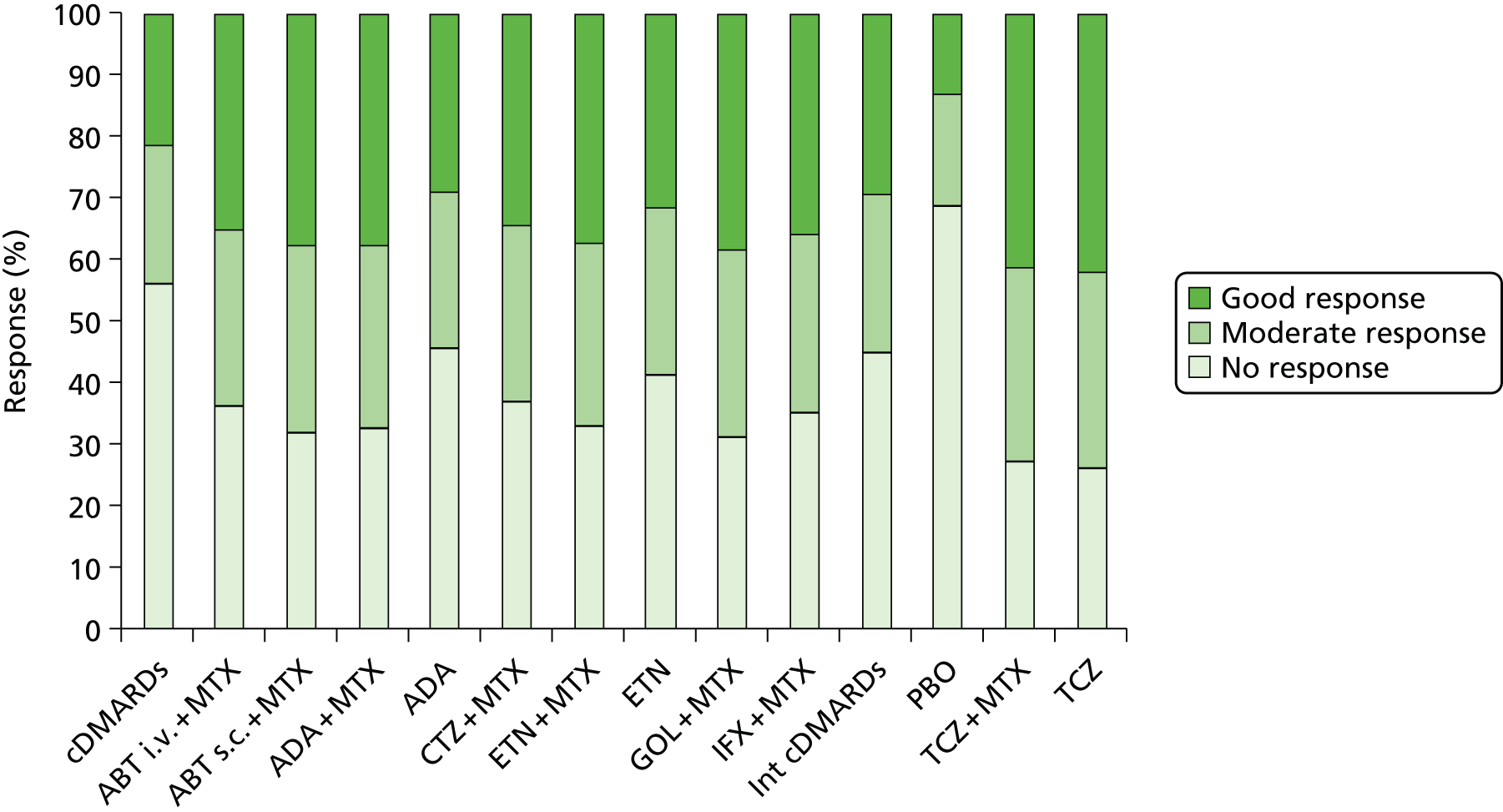
FIGURE 108.
Estimated mean EULAR response in cDMARD-experienced patients mapped from ACR trials in cDMARD-naive patients. Int, intensive.
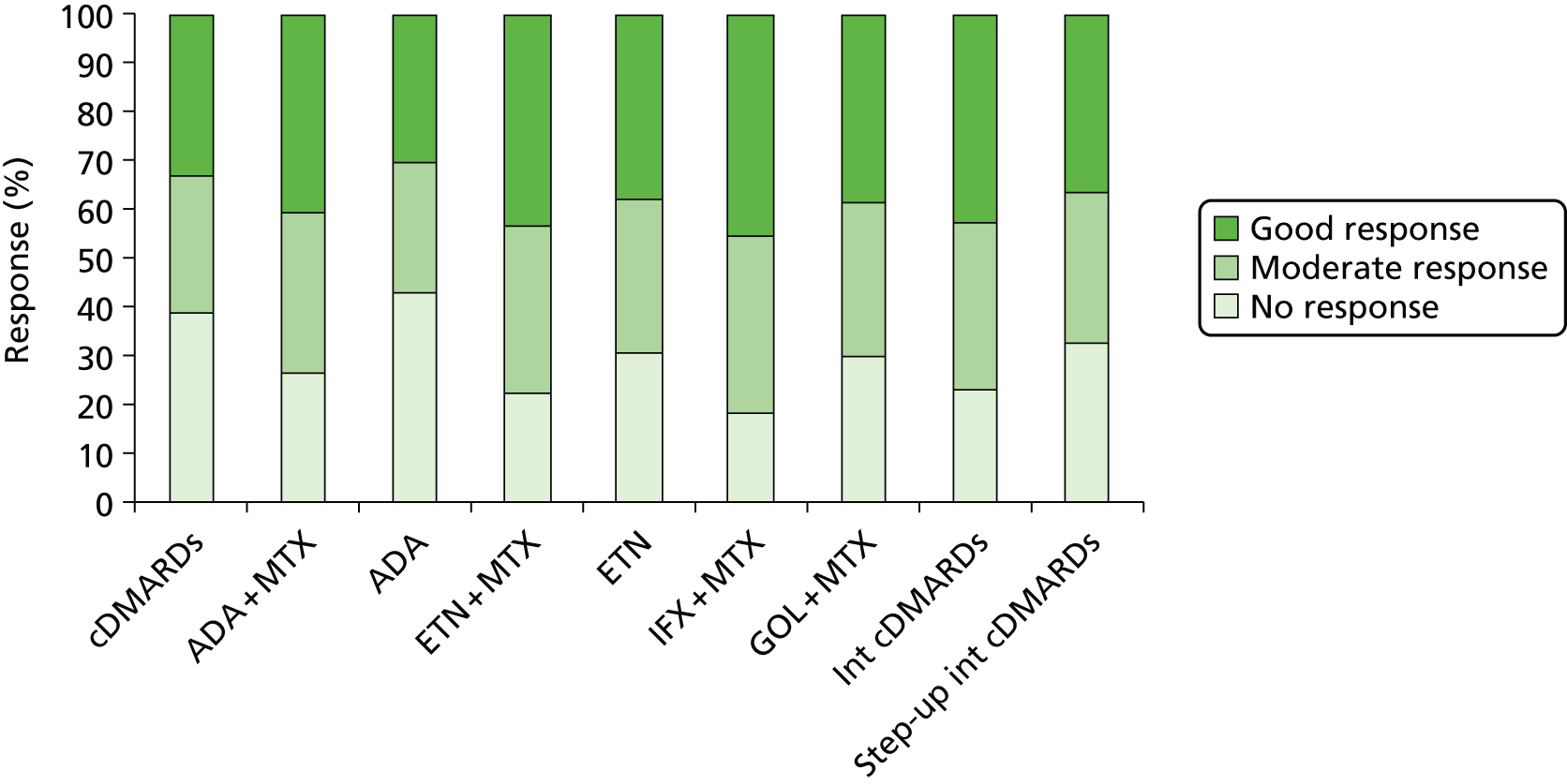
FIGURE 109.
Estimated mean EULAR response in cDMARD-experienced patients mapped from ACR trials in cDMARD-naive patients including RCTs with a proportion of cDMARD-experienced patients. Int, intensive.

The Assessment Group model reflects current NICE guidance and UK practice by simulating patient response in terms of EULAR categories (none, moderate, good). However, the evidence on clinical effectiveness does not universally report EULAR responses, with ACR categories widely used. In order to inform the evidence synthesis and to be able to make use of the entirety of the evidence base in the most informed and efficient manner, we sought evidence of the relationship between these response categories using individual patient-level data.
The Veterans Affairs Rheumatoid Arthritis (VARA) registry provided such estimates to the Assessment Group as AiC. VARA is a multicentre, US database of veterans aged ≥ 19 years (Table 165).
| Patient category | Less | ACR20 | ACR50 | ACR70 | Total |
|---|---|---|---|---|---|
| EULAR ESR, all patients | |||||
| None | 755 | 4 | 2 | 0 | 759 |
| Moderate | 136 | 27 | 2 | 2 | 163 |
| Good | 57 | 26 | 10 | 2 | 83 |
| EULAR ESR, severe active | |||||
| None | 72 | 2 | 0 | 0 | 74 |
| Moderate | 33 | 19 | 0 | 0 | 52 |
| Good | 3 | 9 | 5 | 1 | 12 |
Analyses were undertaken (1) using both versions of EULAR response (CRP based and ESR based) and (2) for all patients and just those with DA28 > 5.1 at baseline. There was great similarity between the CRP- and ESR-based measures. Table 165 reports ESR-based values, which were used in the economic model as it is this measure that was reported most regularly in the relevant RCTs.
By assuming that the relationships shown in Table 165 were correct it was possible to use data taken from the NMA of ACR by mapping these onto EULAR data and subsequently using the same procedures as for the Assessment Group model.
The following assumptions have been made regarding the efficacy of RTX based on work by Malottki et al. 171 Table 46 in Malottki et al. 171 reports that in terms of ACR20, ACR50, ACR70 and withdrawal for any reason that the indirect comparison of RTX versus ABT either favoured RTX, albeit with wide CIs, or there was no difference. Given these data, the efficacy of RTX was assumed equal to i.v. ABT.
There are no marked differences between the results produced by the Assessment Group and the combined evidence presented by the manufacturers.
Responder criteria
The Assessment Group model is based on EULAR response category (good/moderate/none) in order to reflect current NICE guidance on biologic therapies in RA and to align more closely to UK clinical practice in terms of the assessment of response to therapies. The estimated probability of each EULAR response has been taken from the NMAs conducted by the Assessment Group. This allowed analyses to be conducted purely on EULAR data or estimated based on ACR responses in order to encompass a wider evidence base. This differs from the majority of submissions, which assumed that ACR responses would be used to determine whether or not patients were responders (i.e. there is an implicit stopping rule associated with ACR and its relationship to EULAR criteria that underpins these models, though this is not explicitly stated).
Health Assessment Questionnaire/European Quality of Life-5 Dimensions changes in relation to response levels
For each simulated individual the model allocates a change in HAQ from baseline, dependent on the individual’s EULAR response. Different sources for these values were considered, including the option of allocating different values for those on bDMARDs compared with those on cDMARDs. However, in the base case we used the same values for both cDMARDs and bDMARDs.
In the base case we used values modelled from the BSRBR. We assumed zero change for non-responders, a HAQ reduction of 0.317 (SE 0.048) for moderate responders and 0.672 (SE 0.112) for good responders (Table 166). These values were obtained from modelling data from the BSRBR and equate to predictions for a person with the characteristics equivalent to the mean of the overall sample. Full details of the approach are provided in Health Assessment Questionnaire trajectory following initial response because the method estimates both 6-month and subsequent HAQ changes in a single statistical approach.
| EULAR response | Mean | SE |
|---|---|---|
| None | –0.000 | – |
| Moderate | –0.317 | 0.048 |
| Good | –0.672 | 0.112 |
bDMARDs
For patients with the mean characteristics of the actual sample of EULAR moderate responders in the BSRBR, the statistical model predicts a change from 2.08 to 1.79 (a change of 0.29). The mean change in the raw data for this group is from 2.08 to 1.75 (a change of 0.33). For patients with the mean characteristics of the actual sample of EULAR good responders the statistical model predicts a change from 1.81 to 1.27 (a change of 0.54). The mean change in the raw data for this group from 1.81 to 1.26 (a change of 0.55). These data are provided to demonstrate the fact that the observed and predicted values are extremely similar. As the patients sampled in the cost-effectiveness model have the mean characteristics of the entire BSRBR bDMARDs-treated cohort and there is no mechanism to link those characteristics to the probability of EULAR response it is appropriate to use the values in Table 166. This could favour bDMARDs as the HAQ change associated with a good EULAR response is higher.
The statistical model that estimates HAQ change at 6 months and beyond, conditional on EULAR response category, is designed to do so at the individual patient level. However, as the School of Health and Related Research (ScHARR) model is not a true patient-level model in the sense that many of the functions in fact are programmed to estimate the average course of a patient, and because using this statistical model at the patient level substantially increased computational run time, we instead used the mean 6-month HAQ improvement for all patients. This was calculated by setting all characteristics at their mean values and assuming that the model error and mean random effect were both set to zero.
The statistical model estimating initial response is calculated at the individual patient level; however, as the data for cDMARDs were only at the aggregate level, aggregate data for bDMARDs were used. Without this adaptation the results would be unfavourable to bDMARDs as individual patients could be predicted to have a HAQ increase despite a good EULAR response, and when this is combined with the non-linear mapping of HAQ to utility such patients would have a disproportionate weight when calculating the average QALYs.
cDMARDs
In the base-case model the same values were applied for cDMARDs as for bDMARDs.
In addition, the mean HAQ improvements for patients on cDMARDs according to their EULAR response between baseline and 6 months was calculated from the ERAS data set. These data are shown in Table 167 for all patients between baseline and 6 months later.
| HAQ | ||||||
|---|---|---|---|---|---|---|
| Mean | SE | z-value | p-value | LCL | UCL | |
| EULAR response baseline > 6-month visits | ||||||
| None | –0.050 | 0.025 | –2.03 | 0.043 | –0.098 | –0.002 |
| Moderate | –0.509 | 0.035 | –14.67 | 0.000 | –0.577 | –0.441 |
| Good | –0.650 | 0.043 | –15.10 | 0.000 | –0.735 | –0.566 |
It is seen that the average HAQ improvement for both moderate and good EULAR responses were markedly larger than that for no EULAR response and are relatively close to each other. Given the degree of uncertainty surrounding these mean values, it was possible in some instances that the HAQ improvement for those with a moderate EULAR response was greater than those with a good EULAR response.
The use of the modelled data for the entire BSRBR cohort for all treatments and for both those with moderate and those with severe active disease has the advantage of avoiding this potential anomaly, it reduces the running time of the model, and it provides results that are closely aligned to those observed in the BSRBR and ERAS data sets. For EULAR moderate responders the value we used (average HAR improvement 0.32) is close to that observed for moderate responders in the BSRBR (average HAR improvement 0.33). This is a smaller improvement in HAQ than observed it the ERAS data set (0.51). For EULAR good responders the value used (average HAQ improvement 0.67) was closer to the ERAS values (average HAR improvement 0.65) and significantly higher than the values seen for good responders in the BSRBR (average HAR improvement 0.55). The choice of values therefore is likely to be favourable to the cost-effectiveness of bDMARDs in the base case.
The methods used by the Assessment Group differ from those used by the majority of the manufacturers, which assume that the relationship between HAQ and ACR response observed within their key trials is applicable to all interventions. These assumptions use a relatively small sample size and may be subject to variability, as observed in the two MSD submissions where the assumed HAQ changes per ACR level are markedly different. Additionally, the patients recruited to RCTs may not be representative of those patients who will be treated: this could influence the relation between the absolute change in HAQ and HAQ at baseline.
Health Assessment Questionnaire trajectory following initial response
This section has been divided into two subsections: one relating to bDMARDs and one relating to cDMARDs.
In addition to the values assumed by the Assessment Group in our base case, sensitivity analyses were run using values considered within previous NICE TAs. These assumed that the HAQ trajectory on biologics is flat, 0.045 per annum while on cDMARDs and 0.06 per annum while on ‘palliative care’ (which equated to NBT in the Assessment Group model).
bDMARDs
The BSRBR database was used in order to estimate the trajectory of HAQ. The BSRBR database measures HAQ at 6-month intervals for all registered patients for a maximum of 3 years. The evolution of HAQ while a patient remains on a biologic therapy was estimated as a function of a patient’s baseline characteristics and 6-month EULAR response category.
The patient data were restricted to those patients who had a full set of baseline characteristics, including HAQ and at least two other recorded measurements of HAQ while on a biologic therapy. The only bDMARDs for which there was sufficient follow-up time were deemed to be ETN, IFX and ADA.
There are 10,186 such patients in the data set, of whom 2417 are EULAR good responders, 5492 are EULAR moderate responders and 2277 are EULAR non-responders (of whom a quarter had treatment longer than 4 years’ duration). Figure 110 shows the average HAQ in the sample by EULAR response. It is seen that HAQ decreases in the first 6 months after starting on a biologic therapy (with the level of decrease greater as the level of EULAR response increases) and levels off towards the end of the 3-years observation period. For good responders there is a degree of loss of initial 6-month HAQ improvement in subsequent periods. It is important to note that there is an imbalance between the three groups of responders. For example, it can be seen that ‘good’ EULAR responders have a lower baseline HAQ than ‘moderate’ or non-responders.
FIGURE 110.
Mean HAQ by EULAR response category for those receiving bDMARDs.
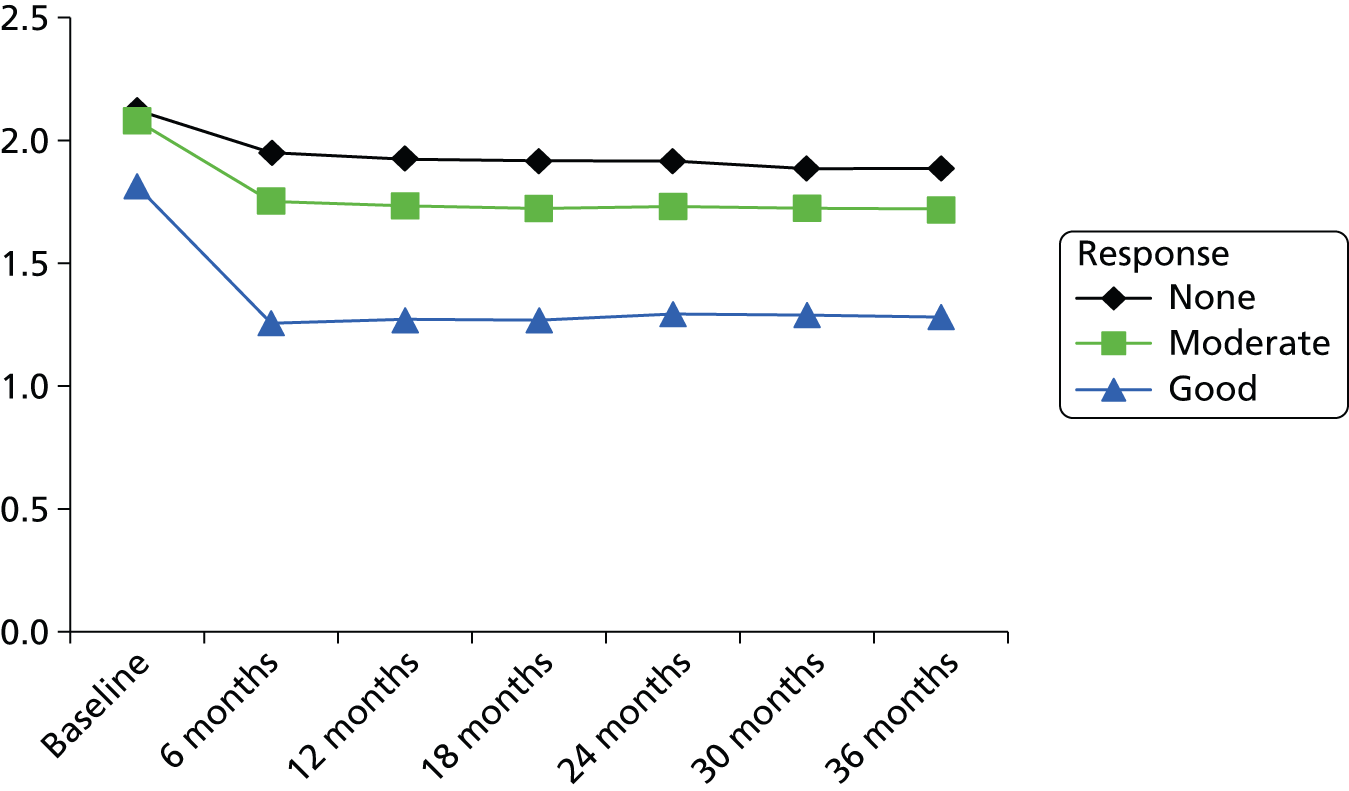
Statistical analyses have been undertaken for those patients who have a good or moderate EULAR response. No formal analysis was conducted for those patients who had no EULAR response as they are assumed to have treatment stopped after 6 months in accordance with NICE guidance within the cost-effectiveness analyses.
An ‘autoregressive latent trajectory model’296 was fitted separately for moderate and good responders. The model uses baseline characteristics, including baseline HAQ, to estimate both initial HAQ response (6 months) and the longer-term progression of HAQ in a single statistical model. The model incorporates a random intercept and a random slope from a growth model that captures the fixed and random effects of the latent growth trajectories over time. It also includes an autoregressive structure representing any time-specific influences between the repeated measures of HAQ over time. The model can be written as follows:
where yit denotes HAQ for patient i at time t for t = 1, . . ., 6 (where t = 1 corresponds to 6 months after starting biologic, t = 2 corresponds to 12 months after, etc.); η0i and η1i are a random intercept and a random slope respectively; w′i is a time invariant, individual specific vector of baseline covariates; xt are the time scores of a non-linear trend where, for identification purposes, we set the first one to zero (x1 = 0) and the last one, 30 months later, to 3 (x6 = 3) and freely estimate the remaining time scores (x2, . . ., x5). If a linear trend can appropriately describe the data the estimated time scores should follow the sequence 0.6, 1.2, 1.8, 2.4 for successive periods t = 2, . . ., 5. The εit are mean zero normal disturbances with time varying variances equal to σεt2, they are independent over time and uncorrelated with the ui’s. The ui’s are mean zero, normally distributed, time invariant individual random terms with a full covariance matrix and potentially correlated with εi0. The parameters γ0,α0,α1 and the vectors of parameters γ1,β0,β1 are fixed over time whereas ρt is a time-varying parameter.
Health Assessment Questionnaire at baseline is treated as predetermined. Baseline covariates, w′i, include age; sex; disease duration (in months); DAS28; and number of previous DMARDs. The continuous baseline covariates are centred on their overall sample means (Table 168). In addition, the covariate age is divided by 10 in the model to avoid convergence problems due to scaling differences. This is for ease of interpretation of the estimated parameters but does not change the model in any way.
| Covariate | All sample (sample mean n = 10,186) | Moderate responders (sample mean n = 5492) | Good responders (sample mean n = 2417) |
|---|---|---|---|
| Age (years) | 56.096 | 56.854 | 53.815 |
| Female (%) | 0.763 | 0.781 | 0.700 |
| Disease duration (months) | 159.444 | 160.188 | 155.544 |
| DAS | 6.551 | 6.763 | 6.281 |
| Number of previous DMARDs | 3.898 | 3.937 | 3.645 |
We estimate the model using maximum likelihood with robust SEs using a sandwich estimator to guard against non-normality. Initially a joint model for the three groups (good EULAR response, moderate EULAR response and no EULAR response) was estimated to try to maximise informative data. However, it was found that no restrictions across groups could be imposed and thus the final models had to be estimated conditional on EULAR response to therapy at 6 months. Table 169 shows the estimated parameters of the models for moderate and good responders.
| Covariates | Moderate | Good | |||
|---|---|---|---|---|---|
| x 2 | 0.159 | (0.397) | 1.649 | (1.531) | |
| x 3 | 1.634*** | (0.314) | 2.515*** | (4.395) | |
| x 4 | 2.732*** | (0.351) | 3.260*** | (12.639) | |
| x 5 | 3.249*** | (0.415) | 2.810*** | (6.998) | |
| Random intercept (η0i) | Intercept | 1.365*** | 0.05 | 1.233*** | 0.112 |
| (Age – mean age)/10 | 0.088*** | 0.008 | 0.147*** | 0.014 | |
| Female | 0.161*** | 0.021 | 0.145*** | 0.035 | |
| Disease duration (months) – mean disease duration | 0.006*** | 0.001 | 0.013*** | 0.002 | |
| DAS – mean DAS | 0.097*** | 0.010 | 0.091*** | 0.021 | |
| Number of previous DMARDs – mean number of previous DMARDs | 0.044*** | 0.005 | 0.106*** | 0.013 | |
| Random slope (η1i) | Intercept | 0.043 | 0.03 | –0.091** | 0.042 |
| (Age – mean age)/10 | 0.009*** | 0.003 | –0.009* | 0.005 | |
| Female | 0.009* | 0.006 | 0.003 | 0.008 | |
| Disease duration (months) – mean disease duration | 0.000 | 0.000 | –0.001*** | 0.000 | |
| DAS – mean DAS | 0.003 | 0.003 | –0.011* | 0.006 | |
| Number of previous DMARDs – mean number of previous DMARDs | 0.004** | 0.002 | –0.007* | 0.004 | |
| HAQ at baseline | Intercept | 1.915*** | 0.015 | 1.797*** | 0.023 |
| (Age – mean age)/10 | 0.052*** | 0.006 | 0.069*** | 0.010 | |
| Female | 0.155*** | 0.017 | 0.139*** | 0.027 | |
| Disease duration (months) – mean disease duration | 0.004*** | 0.001 | 0.006*** | 0.001 | |
| DAS – mean DAS | 0.179*** | 0.007 | 0.158*** | 0.013 | |
| Number of previous DMARDs – mean number of previous DMARDs | 0.033*** | 0.004 | 0.076*** | 0.008 | |
| ρ1 | 0.111*** | 0.025 | 0.007 | 0.058 | |
| ρ2 | 0.117*** | 0.034 | 0.129** | 0.052 | |
| ρ3 | 0.069*** | 0.021 | 0.182*** | 0.046 | |
| ρ4 | 0.040 | 0.033 | 0.246*** | 0.055 | |
| ρ5 | 0.019 | 0.047 | 0.216*** | 0.041 | |
| ρ6 | 0.026 | 0.040 | 0.225*** | 0.052 | |
| Cov | HAQ0 – η0i | 0.171*** | 0.008 | 0.241*** | 0.022 |
| HAQ0 – η1i | 0.005 | 0.004 | –0.018** | 0.008 | |
| η0i – η1i | 0.005 | 0.006 | –0.039** | 0.019 | |
| Var(η0i) | 0.259 | 0.017 | 0.431 | 0.067 | |
| Var(η1i) | 0.004 | 0.001 | 0.009 | 0.005 | |
| Var | Eps0 | 0.245*** | (0.006) | 0.335*** | (0.010) |
| Eps1 | 0.069*** | (0.008) | 0.039 | (0.041) | |
| Eps2 | 0.050*** | (0.003) | 0.074*** | (0.011) | |
| Eps3 | 0.058*** | (0.005) | 0.073*** | (0.007) | |
| Eps4 | 0.044*** | (0.004) | 0.072*** | (0.010) | |
| Eps5 | 0.047*** | (0.007) | 0.060*** | (0.008) | |
| Eps6 | 0.053*** | (0.005) | 0.065* | (0.010) | |
The autoregressive latent trajectory model fits better than either the autoregressive model or the growth model on its own. Restrictions are tested using the Satorra and Bentler297 scaled difference chi-squared test.
As discussed above, the model provided estimates very close to the observed data in terms of 6-month HAQ changes. The cost-effectiveness model used estimates of the 6-month HAQ change for a patient with mean characteristics of the overall sample, baseline HAQ of 2.03, with all error terms set to zero and conditional on EULAR response category. This resulted in estimates of 0.317 (SE 0.048) for moderate responders and 0.672 (SE 0.112) for good responders.
cDMARDs
The cost-effectiveness model simulates, for each patient, the progression of HAQ for the period that patient remains on non-biologic DMARDs. This could be (a) for patients on the cDMARD (comparator) element of the simulation model, or (b) for patients on the bDMARD strategy at the point when they withdraw from the biologic therapy.
Previously, Norton et al. 292 estimated HAQ progression in patients not receiving bDMARDs using data from patients recruited to the ERAS inception cohort study. This is a large, UK-based cohort that has long-term follow-up. In the Norton et al. study,292 observations relate to patients recruited between 1986 and 1998 (n = 1460), followed for up to 10 years. A growth mixture model approach was taken to the analysis of the data. In the published paper, four classes were identified. Full details of the statistical methods are provided in the Norton et al. paper,292 including details of the process for selecting the optimal number of latent classes. These findings have since been corroborated in the NOAR data set with follow-up to 15 years and the Early Rheumatoid Arthritis Network data set. 293 Although the concern in the cost-effectiveness analysis is to estimate the expected change in HAQ over time, not with the latent classes per se, the latent class analysis provides a more flexible and appropriate method of modelling HAQ change over time. It allows the incorporation of patient characteristics as predictors of HAQ progression in a more appropriate manner. Importantly, it also provides a reflection of how the rate of HAQ progression changes over time and places no restriction on this being a simple linear progression. This is likely to be a more appropriate reflection of a chronic disease, the use of different treatments (including drugs and surgical interventions) at different points in the care pathway which influence that progression and the nature of the HAQ scale itself. The use of a simple annual progression rate for all patients at all time points does none of these things.
A modified analysis based on the published Norton et al. study292 was performed so that additional patient descriptors, including those used to define patients within the cost-effectiveness model, were used as covariates within the statistical model. Importantly, these were used as explanatory variables for group membership. In this way, the expected HAQ at any point for a patient with a given set of baseline characteristics can be estimated. The model is formally:
where c is the class and the probabilities of class membership are estimated using a multinomial logit model:
where z contains a series of factors as covariates within the model that were originally considered in separate analyses in Norton et al. 298 plus additional factors relevant to our decision model. Specifically, the model used for the analysis in this report includes age at disease onset; sex; deprivation level; disease duration; rheumatoid factor positive at baseline; fulfilment of ACR criteria for RA at baseline; baseline DAS; failed two DMARDs; and DAS response achieved at 6 months.
The four classes used in the assessment are shown in Figure 111. Probabilities in this case relate to the ERAS population as a whole. For the cost-effectiveness populations, covariate adjustment was used to estimate relevant class probabilities.
FIGURE 111.
Academic-in-confidence information has been removed.
The plots show that there are clearly identifiable separate groups in terms of HAQ progression. Three classes exhibit a J-shaped curve and the fourth shows a general worsening over time. In all cases, the rate of worsening over time decreases. This is contrary to the typical assumptions of DMARD worsening incorporated into cost-effectiveness models, which are assumed to be linear. The use of the growth model also avoids the prediction that large proportions of patients progress to the worst HAQ state (3) before death. This is contrary to the pattern seen in the ERAS, Early Rheumatoid Arthritis Network and NOAR observational data sets both in and beyond. For example, in the US NDB just 1% of observations exceed a HAQ of 2.5. 299 Although there may be reasons why observational data sets like this do not fully represent patients with such extreme levels of functional disability (e.g. that self-completed surveys are not returned), it is unlikely that these are substantially biased.
There are limitations with this approach: ERAS is an inception cohort with follow-up of patients up to 15 years and we therefore cannot be sure what happens beyond that time. Covariates refer to baseline characteristics in the ERAS data set and, while many of these are set, this baseline does not match all the uses of the data in the cost-effectiveness analysis. It should be noted, however, that many of the limitations that are pertinent to the ERAS analysis are similarly applicable, often to a greater degree, in the studies that underpin the mean HAQ progression rates that are typically used in cost-effectiveness analyses of drug therapies in RA.
To implement the results of the statistical model in the cost-effectiveness analysis, a number of choices were made:
-
Rather than use the model predictions for absolute HAQ values, we used the model to predict change in HAQ. This ensured consistency with the baseline sampled HAQ value, the degree of improvement modelled at 6 months based on the EULAR response seen in clinical trials and the simulated HAQ scores for patients treated with bDMARDs.
-
The output provided to us [from the software package Mplus version 7 (Muthén & Muthén, Los Angeles, CA, USA)] reports parameter estimates to three decimal places. This is not sufficient and results in some very large fluctuations in the predicted HAQ, particularly at times exceeding 10 years from the start of treatment (this is because there is a cubic term in the model that requires a much greater degree of precision). Instead we used the values for each class reported in Figure 111. The model for this analysis differs from that underpinning Figure 111 only in that there are more variables entering as explanatory variables for class membership. The trajectories within the four classes are unaffected.
-
Not all explanatory variables that appear in the statistical model are relevant to the way that the cost-effectiveness model defines individuals: deprivation level, rheumatoid factor positive at baseline, fulfilment of ACR criteria for RA. We therefore set deprivation level and RF factor positive at the means for the ERAS cohort (0.49 and 0.73 respectively). We set ACR criteria of RA to 1.
-
The HAQ trajectory for the ERAS cohort includes the initial period where patients with early RA start on cDMARDs and, in many cases, experience improvement in their disease. As this period is modelled separately in the ScHARR model we incorporated values from year 2 onwards only, as this is the point where initial treatment benefits appear to have been lost for all latent classes.
-
Where extrapolation was used beyond the period for which data were available (i.e. beyond year 15), we assumed zero HAQ progression, as this is the rate of progression predicted by the statistical model, for all classes. This also ensures that the cost-effectiveness model did not simulate counter intuitive results, whereby HAQ improves for patients on cDMARDs but not for patients on bDMARDs. Additionally, it should be noted that it is at these long extrapolations beyond 10 years where there is evidence that the model may underpredict HAQ worsening, even within the period covered by the data. In ERAS there appears to be continued worsening of HAQ in the observed data, though NOAR does not exhibit this characteristic.
-
For those patients simulated to follow bDMARD therapy who then return to cDMARDs after the sequence of biologic drugs has been exhausted, we again take each class from year 2 of the modelled data. Patient covariates are taken from the current position in the model rather than from baseline characteristics.
Overall, for patients population simulated in the cost-effectiveness model for group 2 (those that have failed two previous DMARDs and have active disease), there is a lower probability of being in the lowest class 1 (13% vs. 22% in the overall ERAS cohort), a higher probability of class 2 (36% vs. 33%) and class 3 (38% vs. 29%), and a lower probability of being in class 4 (12% vs. 16%). Thus, the cohort of patients simulated within the cost-effectiveness analysis are concentrated more in the latent classes that exhibit rapid HAQ progression than in the overall ERAS cohort.
The methods used by the Assessment Group differ from those used by the manufacturers, which typically assume within their base cases that HAQ progression on bDMARDs is zero and that HAQ progression on cDMARDs is at the rate of 0.045 per annum.
As seen in Figure 110, the assumption that there is no HAQ progression while on bDMARDs appears, in the short term, to be supported by the 3-year follow-up data from the BSRBR. However, the assumed progression on cDMARDs is not compatible with that seen in Figure 111, and lacks face validity as this leads to predictions that most patients reach the ceiling value of HAQ prior to death.
It should also be noted that the use of an annual worsening in HAQ of 0.045 entirely lacks any empirical support. Chen et al. 123 are the source of this value; they state:
In the base case, the following assumptions were made concerning HAQ increases over time. It was assumed that patients remaining on TNF inhibitors experience a worsening (increase) in HAQ equivalent to the general population. Based on the study by Krishnan and colleagues, this was set a progression of 0.03 per year. . . It was assumed that TNF inhibitors halve the general worsening in HAQ, so that patients on palliation have a progression rate of 0.06 per year . . . For conventional DMARDs, an intermediate progression rate of 0.045 per year was assumed . . . These assumptions were varied in sensitivity analysis.
Chen et al. , p.100123
Calculating an accurate HAQ progression can be challenging as historical data on past trends may be only a weak predictor of future trajectories; and there are no data on patients who are inadequately treated. In addition, HAQ alone may not encompass all utility impacts of RA that can be caused by flares.
The Assessment Group identified three papers that provided detail on HAQ trajectory while patients were receiving cDMARDs. 243,300,301 The search was not systematic and it is possible that papers were not identified. Key elements of these trials have been tabulated (Table 170). It is also not known whether or not the use of current cDMARDs would be associated with a lower HAQ trajectory.
| Study | Number of patients analysed | cDMARDs | Mean follow-up (years) | Average HAQ progression per annum |
|---|---|---|---|---|
| Plant et al., 2005300 | 421 | HCQ, sodium aurothiomalate, auranofin and penicillamine | 5 | 0.08 (from years 1 to 5) |
| Symmons et al., 2005301 | 466 | Intensive cDMARD treatment | 3 | 0.06 |
| Munro et al., 1998243 | 440 | i.m. GLD | 5 | 0.05 (from years 2 to 5) |
The clinical advisors within the Assessment Group stated that observational studies of RA populations generally show a HAQ progression substantially < 0.05 per year, but caution that these often cover the spectrum of RA patients and would contain patients who would not have received bDMARDs. This point is highlighted in McWilliams et al. 302
In order to provide an insight into the impact of assumed HAQ trajectory while on cDMARDs the Assessment Group has undertaken scenario analyses using the values of 0.045 for cDMARDs and 0.06 for palliative care in addition to using the models derived from the ERAS database.
There appears to be little long-term evidence to support the value used by the manufacturers; in contrast the values used by the Assessment group have come from a large, prospective, observational database that has been corroborated in a separate database. Assuming a linear HAQ progression does not take into account the impact of surgery that may halt HAQ progression, the costs of which are currently assumed to be incurred without benefit.
Time to discontinuation on treatment
The duration of treatment on the first biologic for adult RA patients was estimated using the BSRBR database, which records the dates on which therapies are initiated and ended. Separate analyses were undertaken for those patients obtaining good and moderate EULAR responses at 6 months. Patients classed as non-responders at 6 months are assumed to be withdrawn from therapy in the Assessment Group model (as in current NICE guidance,22,24,26,27,237 which requires an improvement in DAS28 of at least 1.2 at this time point for treatment to be maintained). This allows patients who have been withdrawn prior to 6 months to be included in the analysis, though there is a risk that their response category recorded at 6 months is in fact related to having switched to some other therapy.
A range of parametric survival models (Weibull, exponential, Gompertz, log-logistic, log-normal, gamma and Weibull frailty models) were considered. The best-fitting model, in terms of both the Akaike information criterion and the Bayesian information criterion, was that based on the gamma distribution. The following covariates were included: age, sex, disease duration at baseline, DAS; number of previous DMARDs and HAQ at baseline. We included all covariates, even if insignificant, but considered alternative specifications (such as squared and log-terms) in order to identify our preferred model, guided by AIC/BIC.
Establishing separate covariates for the individual biologic therapies within this appraisal was considered. As GOL, ABT, TCZ and CTZ comprised < 1% of the observations, and had follow-up durations of much shorter duration, these were excluded leaving only IFX, ETN and ADA. Although the duration of treatment for those on ETN and ADA was significantly shorter than for IFX, this is likely to be due to the times at which therapies became available in the UK. Owing to this potential confounding and the lack of data for a number of treatments, separate terms for individual therapies in the cost-effectiveness analysis were not adopted.
Two plots comparing the duration on treatment estimated by the models with those observed in the BSRBR database are shown in Figure 112. These are divided into those patients with moderate or good EULAR response, and are constrained to only those patients who would be eligible for biologics under current NICE guidance. Patients who met the NICE criteria were the overwhelming majority and constituted 7250 of the 7743 patients (94%).
FIGURE 112.
Plots of the estimated data from the statistical models compared with the observed data. (a) Moderate responders (NICE eligible); and (b) good responders (NICE eligible).
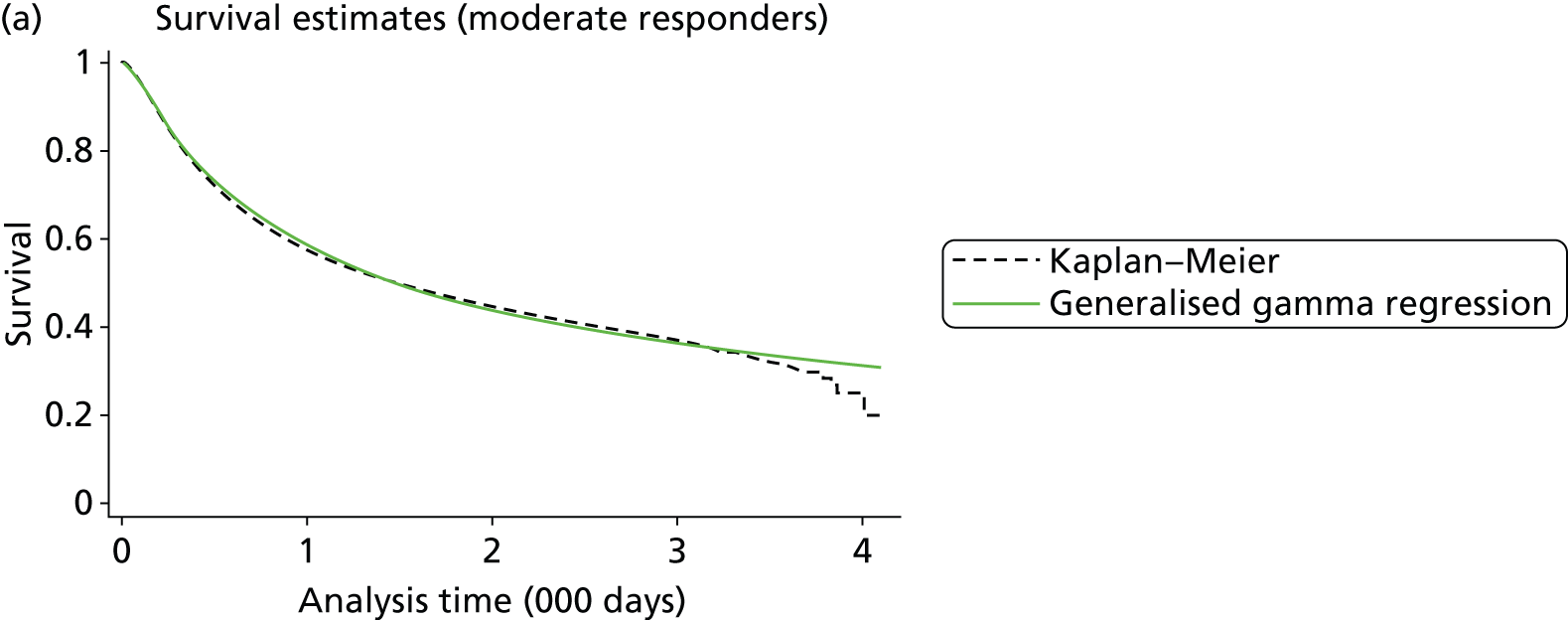

Given the paucity of data on bDMARDs used before cDMARDs an assumption was required regarding the duration of treatment if bDMARDs were used before cDMARDs. It was assumed that the duration would be unaffected by whether or not cDMARDs were used prior to bDMARDs.
There were also few data on the duration of response for patients receiving cDMARDs. Based on the assumption that cDMARDs are not likely to be more toxic than biologics used in combination with a cDMARD, it was assumed that the survival duration for each EULAR response category for bDMARDs would be applicable for cDMARDs.
It was assumed that patients would not switch to a subsequent treatment within 6 months of initiating a treatment; this assumes that any AE would be monitored before changing treatment at 6 months.
The method used by the Assessment Group differs from those of the manufacturers but it is commented that there was diversity in the methods used by the manufacturers with no clear consensus reached. One flaw in the approach taken by manufacturers is that the discontinuation rates had frequently not been conditional on EULAR response and thus the average time on treatment would be decreased by those patients without a response who typically stay on treatment for 1 year, despite the current NICE stopping criteria.
In summary, the Assessment Group does not believe any of the methods assumed by the manufacturers represents a significantly better method than that used by the Assessment Group and there is a reason to believe that the approach taken by the Assessment Group is the preferred method.
Rebound post treatment
The change in a patient’s HAQ when treatment has failed to be efficacious or is stopped owing to an AE is not known with certainty. The Assessment Group has assumed that following cessation of treatment the initial HAQ improvement experienced on treatment initiation would be lost. The resultant HAQ would be assumed for the subsequent 6 months when the next treatment in the sequence is trialled.
This is similar to assumptions made within the manufacturers’ models.
Assumed NHS costs per Health Assessment Questionnaire band
A brief review of the recent literature regarding the costs associated with active RA and in particular HAQ score identified few data that were not identified collectively within the manufacturers’ submissions. The only information of note was a poster by Bansback et al. 303 which, using Canadian data, concluded that ‘the study finds no signal after three years that biologic therapies in patients with RA have led to overall cost offsets from related treatment costs’. Possible explanations that were proffered were falling resource utilisation in general, potentially due to more aggressive use of cDMARDs, have given a false impression that biologics are causally associated with resource utilisation; that cost offsets occur beyond 3 years; and that the model is mis-specified and estimates remain biased.
Although these results are noted the Assessment Group believes it is plausible that there could be an increase in hospitalisation costs as HAQ increases. Having reviewed the hospital costs within the manufacturers’ submissions the Assessment Group decided to use that reported by AbbVie for the base case, which were among the lowest of those presented and were relatively flat until the patient had severe HAQ scores (defined as HAQ scores of ≥ 2.125). These values were derived from data taken from the NOAR database on impatient days and joint replacements260,261 and were multiplied by NHS reference costs. The values assumed in the Assessment Group base case are depicted in Figure 113.
FIGURE 113.
The assumed relationship between annual hospitalisation costs and HAQ score in the Assessment Group model.
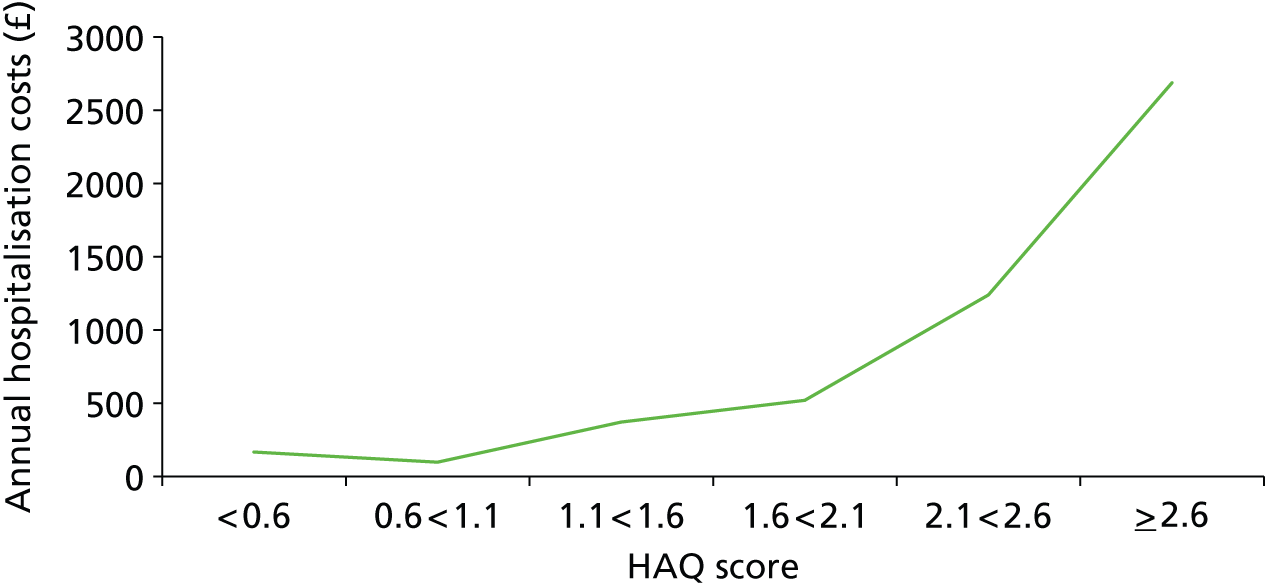
Utility related to Health Assessment Questionnaire
The NICE methods guide states that mapping is an acceptable method for estimating EQ-5D from clinical outcome measures in the absence of direct evidence, but that the statistical properties of the model ‘should be fully described, its choice justified, and it should be adequately demonstrated how well the function fits the data’ (pp. 39–40). 227 UCB Pharma (CTZ) provided data on the changes in EQ-5D in the initial 6-month period but these were marked AiC.
Hernandez-Alava et al. 299,304 report the results of fitting a bespoke mixture model to data from patients with RA from a US observational database comprising in excess of 100,000 observations. Full details of the data set, the statistical model and its performance (in comparative and absolute terms) are provided in the manuscripts.
The set of models reported include HAQ, HAQ2, pain, age, age2 and sex as explanatory variables. These were included because models performed substantially better when they are included. Most previous analyses have excluded pain. However, a substantially better estimate of EQ-5D is obtained by the inclusion of pain alongside HAQ than via HAQ alone. This is to be expected, as the domains covered by the HAQ instrument are very similar to the domains of usual activities, mobility and self-care in the EQ-5D. The dimension of ‘pain’ attracts the highest weights in the EQ-5D UK scoring regression. The fact that pain enters as a separate covariate in the Hernandez-Alava et al. 299,304 model is because HAQ and pain are not perfectly correlated. It is therefore important to include pain as an explanatory variable in estimating EQ-5D.
This does not mean that the cost-effectiveness model needs to be both HAQ and pain based, or that separate HAQ and pain treatment effects need to be estimated for therapies. There are alternative methods by which the relationship between HAQ and pain can be incorporated in to the cost-effectiveness model without the requirement for additional complexity, rather than reverting to poorer methods of explaining EQ-5D.
The Assessment Group uses a two-step process for estimating EQ-5D values from HAQ values: the first step simulates the expected pain score associated with HAQ; the second step estimates EQ-5D based on both HAQ value and pain score.
Step 1: simulating the expected pain score associated with Health Assessment Questionnaire
The estimation of EQ-5D utility scores is substantially more accurate when based on HAQ and pain than on HAQ alone as detailed in Hernandez-Alava et al. 276 In order to incorporate the published statistical models that estimate this relationship, pain is independently predicted from the simulated HAQ score for each patient within the model. Although this assumes that all treatments affect pain proportionate to their effect on HAQ score, this is also the assumption implicit in all models that exclude pain.
Health Assessment Questionnaire and pain are not related in a simple linear fashion as shown in data from the NDB and data from ERAS (Figure 114), which incorporate 100,398 observations for the NDB and 13,357 from ERAS.
FIGURE 114.
The relationship between HAQ score and pain value. (a) NDB; and (b) ERAS.


Data from the NDB are used to populate the mathematical model, with the mean pain score (and its variance) being estimated for each feasible HAQ score.
Step 2: estimating European Quality of Life-5 Dimensions based on both Health Assessment Questionnaire value and pain scores
It is well recognised that simple linear regression models are inappropriate for estimating EQ-5D values as a function of clinical outcomes. This is because the assumption of conditional normality does not hold for an outcomes measure that is limited above by full health (1), at the worst health state (–0.594) and that is typically bi- or tri-modal within this range. This theoretical assertion is supported by empirical findings across a broad range of disease areas305 and within RA from two separate large data sets that span the full spectrum of disease. 276,299 Linear models lead to biased estimates of EQ-5D. They estimate higher EQ-5D scores for patients in severe health states and lower EQ-5D scores for those patients in less severe health states. The net effect is an undervaluation of the cost-effectiveness of effective therapies. This has been shown to be of a substantial magnitude in RA with ICERs varying by up to 20%. 299
In this report an alternative method is undertaken, based on mixture models that use an underlying distribution that is bespoke to the EQ-5D UK instrument. This has been reported in Hernandez-Alava et al. 299 The model was estimated using data from the US NDB. A total of 103,867 observations were included in the total data set from 16,011 patients. The size of the data set dwarfs that which is typical of most ‘mapping’ studies and provides a good exemplar in which to test competing methods because patients spanned the full range of HAQ, pain and EQ-5D values.
The preferred model comprised four components, each of which includes HAQ and HAQ2, pain, age and age2 as explanatory variables. HAQ, pain and pain2 enter the model as predictors of component membership. The model fits substantially better than linear regression or response mapping approaches, does not generate non-feasible values or suffer from systematic bias in the estimates. Full coefficient values are reported in the associated publications. We used the full covariance matrix to incorporate parameter uncertainty into the cost-effectiveness model when running PSAs. These data can be obtained online (http://rheumatology.oxfordjournals.org/content/suppl/2013/01/20/kes400.DC1 – accessed July 2013304).
The Assessment Group believes that its method is more appropriate than those used by the manufacturers. All of the studies used in the base-case manufacturers’ submissions are based on linear regression models with insufficient information on which to judge the appropriateness of the statistical models being used and with far fewer patients than used to derive the relationship between HAQ, pain and utility used by the Assessment Group.
The Assessment Group reports that there are further studies that could have been used to inform the manufacturers’ submissions that report on the relationship between health utilities, HAQ and other covariates. These are briefly summarised:
-
Hawthorne et al. 306 used UK EQ-5D data from 139 patients with RA recruited in Australia in a linear regression with HAQ as the only covariate.
-
Lindgren et al. 284 used Swedish registry data from 1787 patients and used the UK EQ-5D tariff to estimate EQ-5D as a function of HAQ, DAS and age.
-
Marra et al. 195 report UK tariff EQ-5D as a function of HAQ and age (n = 317) from a sample of Canadian patients with RA.
-
Kobelt et al. 262,264 report mean EQ-5D scores by HAQ category using Swedish registry data (n = 116) in the former paper and a combination of Swedish and UK patients in the latter (n = 210). For illustrative purposes only, we fitted simple linear models to these reported mean values.
Compared with these studies, the models used as the base case for the entire set of manufacturer submissions171,241,266,275 have a greater assumed impact on utility than the remaining studies particularly where HAQ exceeds 2, which is the case for a sizeable proportion of cDMARD-treated patients given the assumptions used in many of the cost-effectiveness models regarding HAQ progression over time while on cDMARDs (Figure 115).
FIGURE 115.
A comparison of published relationships between utility and HAQ.
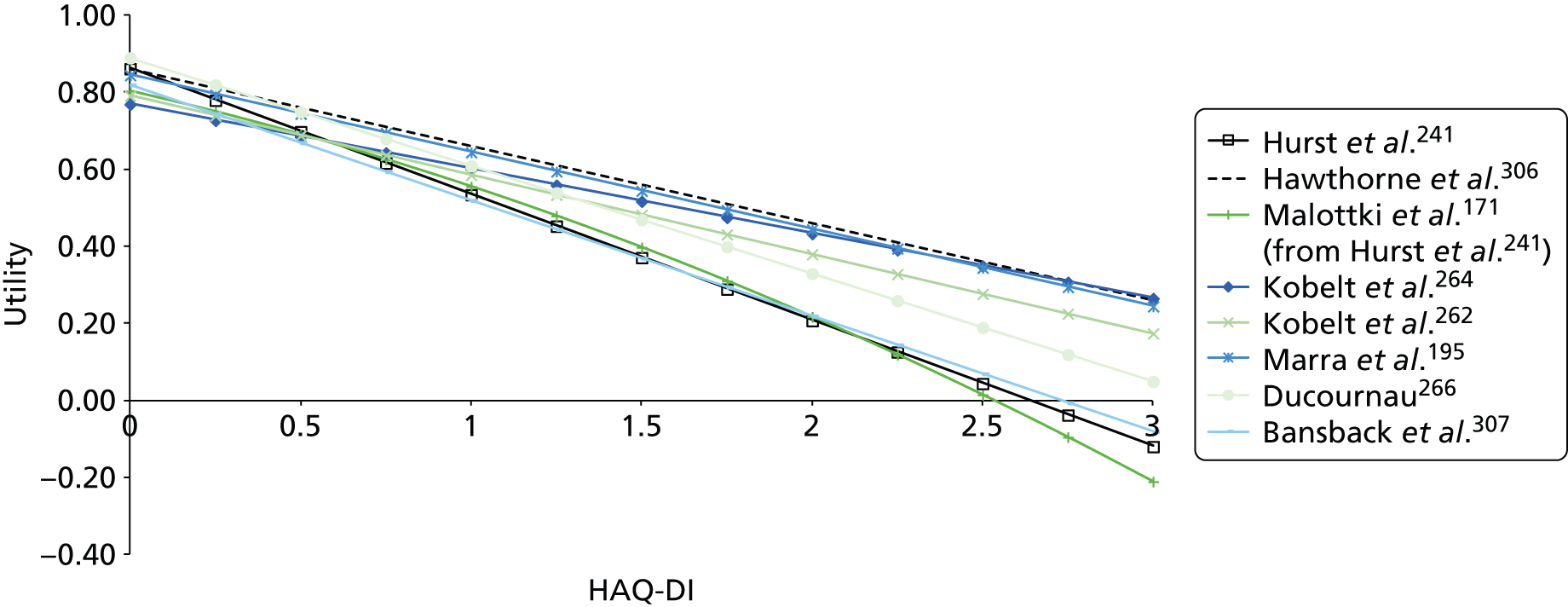
In a sensitivity analysis the equation mapping HAQ to utility described in Malottki et al. 171 was used. Additionally, using the relationship between HAQ and pain taken from the ERAS rather than that from the NDB was evaluated.
The assumed costs and disutilities associated with adverse events
The Assessment Group took a simplistic view regarding AEs.
It was assumed that only serious infections would carry a significant cost and disutility burden and limited the AEs within the model to serious infections alone. A review of the adverse effects of biologics165 indicated that serious infections were observed in 35 per 1000 patients (95% CI 27 to 46). Singh et al. 281 reported the rate of serious infections in people on cDMARDs to be 26 per 1000 patients (no CI reported), implying that an additional 9 per 1000 patients would sustain a serious infection when using a bDMARD. It was assumed that the rate of serious infection was independent of the bDMARDs used. The Assessment Group accepted arguments presented as AiC by UCB Pharma (the manufacturer of CTZ) that there were different exposure durations between CTZ and PBO in the CTZ RCTs and that the increased risk of serious infections reported by Singh et al. 281 for CTZ should be treated with caution.
The costs (£1479 per episode) and undiscounted QALY loss associated with serious infections (a loss in utility of 0.156 for 28 days) were both taken from the Pfizer submission. 209 Based on the assumed increased rate of serious infection it was assumed that a bDMARD strategy would incur an additional £13.31 and a QALY loss of 0.0001 per typical patient treated. These values were increased 100-fold in sensitivity analyses to assess the impact of events that may be too infrequent to be observed in RCTs, but may become apparent when large numbers of patients are treated.
The majority of submissions excluded AEs from the model, although Pfizer included both costs and disutility in a sensitivity analysis and AbbVie included costs alone within the base case.
Mortality associated with rheumatoid arthritis
The link between RA and early mortality has been long documented with a seminal paper being that of Wolfe et al. 282 published in 1994. A meta-analysis by Naz and Symmons5 incorporating 15 studies involving > 300 subjects and published between 1993 and 2006 indicated a range in the standardised mortality ratio of between 1.01 and 2.70. Dadoun et al. 6 undertook a meta-analysis of studies reporting mortality rates in RA and reported a meta- standardised mortality ratio of 1.47 (95% CI 1.19 to 1.83) from eight studies although the level of heterogeneity was high with an I2 statistic of 93.47.
However, few data have been published on the relationship between change in HAQ and change in expected mortality, which is the key relationship that is required if there is to be proof that an increase in HAQ score is associated with an increase in mortality. Following a literature review, a paper by Michaud et al. 308 published in 2012 was identified that aimed to establish the relationship between change in HAQ and mortality. Their conclusions were that ‘changes in the PCS [SF36 physical component summary score] and HAQ did not contribute substantially to predictive value over and above the baseline values of these variables’. As such, the Assessment Group assumed that only the baseline HAQ score was important for predicting mortality and the HRs detailed in Table 171 were applied. It is noted that as initial HAQ increases then the HRs also increases. It was assumed that these HRs were independent of time.
| Initial HAQ category | HR (95% CI) |
|---|---|
| 0.000 | 1 referent |
| 0.125–0.375 | 1.4 (1.1 to 1.8) |
| 0.500–0.875 | 1.5 (1.2 to 1.9) |
| 1.000–1.375 | 1.8 (1.4 to 2.2) |
| 1.500–1.875 | 2.7 (2.2 to 3.5) |
| 2.000–2.375 | 4.0 (3.1 to 5.2) |
| 2.500–3.000 | 5.5 (3.9 to 7.7) |
The CIs for each HAQ category overlap with the neighbouring category. In order to preserve monotonicity for the HRs, quantile matching was assumed when drawing the HR for each category for each PSA iteration. The patient was assumed to die mid-way through their final year.
The Assessment Group method straddles those of the manufacturers in that it applies a fixed HR for mortality but selects this HR based on the initial HAQ category of the patient, with those with a worse HAQ dying sooner on average. This contrasts with the methods used of applying a non-HAQ-related HR, and allowing mortality to be determined by current HAQ score. The Assessment Group comments that the data source used to determine their method is much more recent than those used by the manufacturers.
Calculation of the appropriate number of patients to run when generating results
Analyses were undertaken to assess the number of patients required to be simulated in order that stable results were produced; although these analyses were conducted on an earlier version of the model, it is believed the conclusions in terms of number of patients required are generalisable. The strategies compared were strategies 1 and 6 in Table 159, which started with MTX, and ETN and MTX respectively. It was demonstrated (Figure 116) that beyond 20,000 simulated patients the change in cost per QALY was small, being < £500 from a base of approximately £62,000. Therefore, 20,000 patients were simulated for all analyses involving patients with severe RA who could receive MTX. It is commented that the cost per QALY between active interventions is likely to require greater numbers of patients for stability, but running greater numbers of patients was not possible within the time constraints of the project.
FIGURE 116.
Evaluating the number of patients required in analyses involving patients with severe RA who could receive MTX.

For patients with moderate RA the computational time required was significantly greater as patients were resampled until the DAS criterion of between 3.1 and 5.2 was met, meaning that large numbers of simulated patients were discarded. This led to the results for this group to be taken from 2000 patients. As such, only 2000 patients were simulated and it is unclear whether or not a stable cost per QALY had been reached (Figure 117): the potential error, however, was not deemed to be excessive and appeared to be between £1000 and £2000 on the cost per QALY value.
For a cDMARD-naive population with severe RA, 20,000 patients were run, at which value the results had appeared to stabilise (see Figure 117).
FIGURE 117.
Evaluating the number of patients required in analyses involving patients with moderate RA who could receive MTX.

For the population who had severe RA and were bDMARD naive the numbers of patients required to be simulated to generate stable results were investigated (Figure 118). Analyses were conducted assuming 20,000 patients at which value the results appeared relatively stable.
FIGURE 118.
Discounted cost per QALY of a bDMARD strategy compared with a non-bDMARD strategy in a cDMARD-naive population.
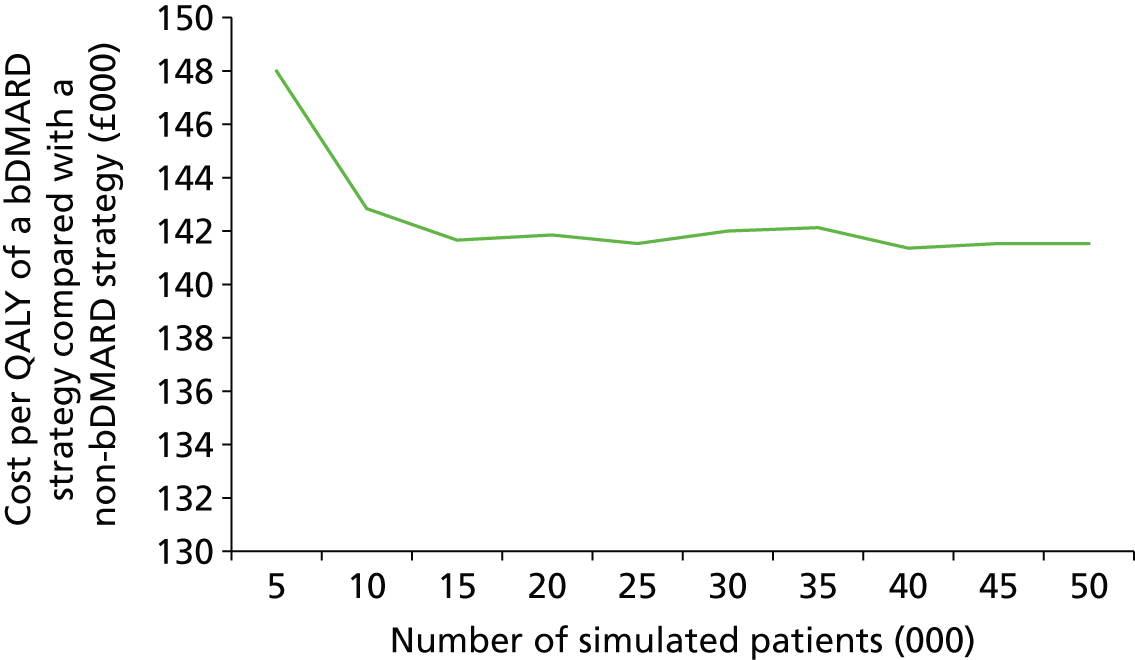
The large computational time required meant that the simulated patient numbers were reduced further in the PSA. For severe patients 100 Monte Carlo samples of 2000 patients were conducted and 100 Monte Carlo samples of 200 patients for the moderate group. Although there are fewer patients simulated the expectation of the results are likely to be robust as O’Hagan et al. 309 proved that the most efficient method of generating the expectation of cost-effectiveness would be to generate only one patient per PSA iteration. The greater numbers used in our PSA was to facilitate the generation of CEACs.
For both the moderate and the severe RA populations the computational time required for a deterministic analysis was approaching 90 minutes. For the probabilistic analyses the number of simulated patients was reduced by 90% (i.e. 1000 for severe patients and 100 for moderate patients) and 100 probabilistic samples were evaluated, representing approximately 15 hours of computational time.
Results
A summary of the analyses undertaken is provided in Table 172. These are all 24 combinations of factors shown excluding those combining EULAR response in MTX-naive patients as the only data available were for an intervention (GOL) unlicensed in this population. Each analysis had further sensitivity analyses conducted assessing the impact of using a different RCT evidence base, a different mapping of HAQ to utility, an increase in the effects of serious AEs and a different assumed relationship between HAQ and pain.
| Population | Treatment provided | Response measure | HAQ trajectory on cDMARDs |
|---|---|---|---|
| Population 3 (severe MTX experienced) | In combination with MTX | EULAR | Taken from the ERAS database |
| Population 2 (moderate to severe MTX experienced) | As monotherapy | ACR (then mapped to EULAR) | Using previous NICE appraisal values |
| Population 1 (severe MTX naive) |
Owing to the number of results presented, the Assessment Group decided that a summary table, providing indicative results, would aid the reader. As will be seen, there is little difference in the estimated cost-effectiveness of the bDMARDs, with the exception of TCZ, which differs as it cannot be used after RTX if it was used as the first bDMARD. As such, the median ICERs for all bDMARDs in populations 2 and 3 are presented in Tables 173 and 174. The median was selected as a method of detailing the cost-effectiveness of an average bDMARD. The ICERs for population 1 are provided in Tables 175 and 176. No results are presented for a model based on EULAR data for population 1, as there was only one RCT identified that did not include intensive cDMARDs, which are the recommended treatment. The results provided use ACR transformed to EULAR data, but as is seen this approach produced similar cost per QALY results to the models which used EULAR data in populations 2 and 3.
| Population | Response measure | Assumed HAQ progression | Base case + | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No change (£) | RCTs with small percentage of bDMARD prior use, adequate MTX history (£) | RCTs with small percentage of bDMARD prior use (irrespective of MTX history) (£) | Trials with inadequate MTX history (£) | Malottki et al.171 mapping of HAQ to utility (£) | Discount rates (6% costs, 1.5% QALYs) (£) | Impact of AEs assumed to be 100-fold higher (£) | Relationship between HAQ and pain taken from ERAS (£) | PSA (£) | |||
| 2 (severe MTX experienced) | EULAR | ERAS | 41,600 | 41,200 | No data | No data | 34,700 | 27,700 | 42,400 | 51,100 | 41,300 |
| Linear | 37,900 | 36,300 | No data | No data | 32,400 | 22,300 | 38,300 | 46,300 | 37,600 | ||
| ACR | ERAS | 41,500 | 41,400 | 41,000 | 41,900 | 34,800 | 28,200 | 42,400 | 50,000 | 42,500 | |
| Linear | 35,500 | 35,100 | 35,700 | 36,400 | 30,900 | 21,400 | 35,600 | 43,700 | 35,900 | ||
| 3 (moderate MTX experienced) | EULAR | ERAS | 51,000 | 51,900 | No data | No data | 36,000 | 32,700 | 52,200 | 58,100 | 52,000 |
| Linear | 37,500 | 36,600 | No data | No data | 31,300 | 21,800 | 39,300 | 48,300 | 35,800 | ||
| ACR | ERAS | 51,300 | 52,200 | 52,100 | 51,300 | 37,500 | 33,000 | 52,100 | 59,400 | 51,600 | |
| Linear | 38,000 | 36,700 | 38,000 | 39,200 | 30,000 | 21,800 | 39,100 | 46,700 | 38,400 | ||
| Population | Response measure | Assumed HAQ progression | Base case + | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No change (£) | RCTs with small percentage of bDMARD prior use, adequate MTX history (£) | RCTs with small percentage of bDMARD prior use (irrespective of MTX history) (£) | Trials with inadequate MTX history (£) | Malottki et al.171 mapping of HAQ to utility (£) | Discount rates (6% costs, 1.5% QALYs) (£) | Impact of AEs assumed to be 100-fold higher (£) | Relationship between HAQ and pain taken from ERAS (£) | PSA (£) | |||
| 2 (severe MTX experienced) | EULAR | ERAS | 48,300 | 49,000 | No data | No data | 40,230 | 32,700 | 48,900 | 59,000 | 48,200 |
| Linear | 39,600 | 38,000 | No data | No data | 34,800 | 24,800 | 40,200 | 49,200 | 39,100 | ||
| ACR | ERAS | 49,700 | 49,500 | 49,500 | 50,000 | 41,400 | 33,700 | 50,100 | 60,400 | 50,100 | |
| Linear | 38,500 | 37,300 | 37,200 | 37,200 | 34,100 | 23,600 | 39,300 | 47,800 | 38,800 | ||
| 3 (moderate MTX experienced) | EULAR | ERAS | 58,800 | 55,900 | No data | No data | 41,900 | 37,500 | 59,400 | 66,100 | 59,700 |
| Linear | 41,400 | 39,300 | No data | No data | 32,800 | 23,900 | 41,600 | 49,700 | 41,700 | ||
| ACR | ERAS | 57,800 | 59,100 | 62,400 | 61,900 | 44,900 | 39,600 | 61,800 | 67,300 | 60,300 | |
| Linear | 38,800 | 38,500 | 38,000 | 37,200 | 31,100 | 23,800 | 40,500 | 47,100 | 39,600 | ||
| Population | Response measure | Assumed HAQ progression | Base case + | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No change (£) | RCTs with small percentage of MTX prior use (£) | Malottki et al.171 mapping of HAQ to utility (£) | Discount rates (6% costs, 1.5% QALYs) (£) | Impact of AEs assumed to be 100-fold higher (£) | Relationship between HAQ and pain taken from ERAS (£) | PSA (£) | |||
| Population 1 (severe MTX naive) | ACR mapped to EULAR | ERAS | 68,300 | 68,200 | 50,500 | 42,600 | 71,100 | 79,500 | 66,100 |
| Linear | 58,300 | 58,100 | 44,700 | 33,600 | 59,800 | 68,300 | 60,500 | ||
| Population | Response measure | Assumed HAQ progression | Base case + | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No change (£) | RCTs with small percentage of MTX prior use (£) | Malottki et al.171 mapping of HAQ to utility (£) | Discount rates (6% costs, 1.5% QALYs) (£) | Impact of AEs assumed to be 100-fold higher (£) | Relationship between HAQ and pain taken from ERAS (£) | PSA (£) | |||
| Population 1 (severe MTX naive) | ACR mapped to EULAR | ERAS | 77,500 | 78,400 | 57,800 | 50,400 | 79,900 | 89,900 | 76,200 |
| Linear | 63,200 | 63,300 | 49,300 | 37,400 | 64,400 | 74,100 | 61,300 | ||
Fully incremental results follow the summary tables. However, these may be misleading when between-bDMARD comparisons are made, as the ICERs compared with the cDMARD-alone strategy are relatively similar, and there is considerable uncertainty in efficacy data. Interventions labelled as dominated may be only slightly more expensive and marginally less effective than a comparator. This cannot be seen in the results as owing to the CiC PASs both discounted costs and discounted QALYs are marked CiC. CEACs are presented; however, CEACs show only the probability of being optimal and inferences regarding relative cost-effectiveness should be made with caution.
European League Against Rheumatism response measure: Early Rheumatoid Arthritis Study cDMARD Health Assessment Questionnaire progression and a severe, methotrexate-experienced, rheumatoid arthritis population
The base-case results for this population and those from sensitivity analyses are provided in Tables 177–183. The CEAC for the base case is shown in Figure 119.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 41,647 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 39,142 | 39,142 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 39,884 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 41,015 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 42,194 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 42,087 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 42,014 | 74,290 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £39,000–43,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 41,194 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed) | CiC information has been removed | 38,771 | 38,771 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 39,246 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 41,497 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 41,700 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 40,467 | 60,158 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 41,748 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 34,734 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 32,613 | 32,613 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 33,193 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 34,158 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 35,234 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 34,912 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 34,984 | 61,719 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 28,495 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 25,618 | 25,618 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 26,007 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 26,875 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 27,750 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 27,682 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 27,670 | 50,770 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 42,426 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed) | CiC information has been removed | 40,059 | 40,059 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 40,490 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 42,797 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 41,756 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 42,759 | Dominated |
| ETN + MTX | CiC information has been removed) | CiC information has been removed | 42,719 | 72,481 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 50,985 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 47,972 | 47,972 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 48,393 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 51,133 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 49,945 | Extendedly dominated |
| GOL + MTX | CiC information has been removed) | CiC information has been removed | 51,058 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 51,019 | 83,942 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 41,305 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 38,904 | 38,904 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 39,376 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 40,505 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 41,710 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 41,617 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 41,691 | 73,145 |
FIGURE 119.
The CEAC when using EULAR data directly: ERAS cDMARD HAQ progression and a severe, MTX-experienced, RA population.

It is seen that at a willingness to pay of £30,000 the MTX strategy has a very high probability of being optimal (see Figure 119).
European League Against Rheumatism response measure: linear cDMARD Health Assessment Questionnaire progression and a severe, methotrexate-experienced, rheumatism arthritis population
The base-case results for this population and those from sensitivity analyses are provided in Tables 184–190. The CEAC for the base case is shown in Figure 120.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 35,872 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 35,794 | 34,247 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 36,176 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 38,463 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 37,867 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 38,689 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 39,068 | 83,446 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £35,000–40,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 33,795 | 33,795 |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 33,896 | 35,682 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 34,473 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 36,589 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 36,800 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 36,292 | 69,464 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 37,377 | 616,967 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 30,635 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 30,412 | 30,412 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 31,067 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 33,066 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 32,382 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 33,160 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 33,193 | 67,129 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 22,212 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 21,057 | 21,057 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 21,470 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 22,479 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 22,998 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 23,178 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 23,476 | 32,884 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 35,890 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 35,421 | 35,421 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 36,303 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 38,543 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 37,866 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 38,608 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 39,067 | 87,843 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 44,112 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 43,866 | 43,866 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 44,533 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 47,199 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 46,305 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 47,439 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 47,830 | 99,048 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 38,152 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 34,843 | 34,843 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 35,425 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 36,644 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 37,583 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 37,779 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 38,355 | 86,917 |
FIGURE 120.
The CEAC using EULAR data directly and assuming linear CDMARD HAQ progression.
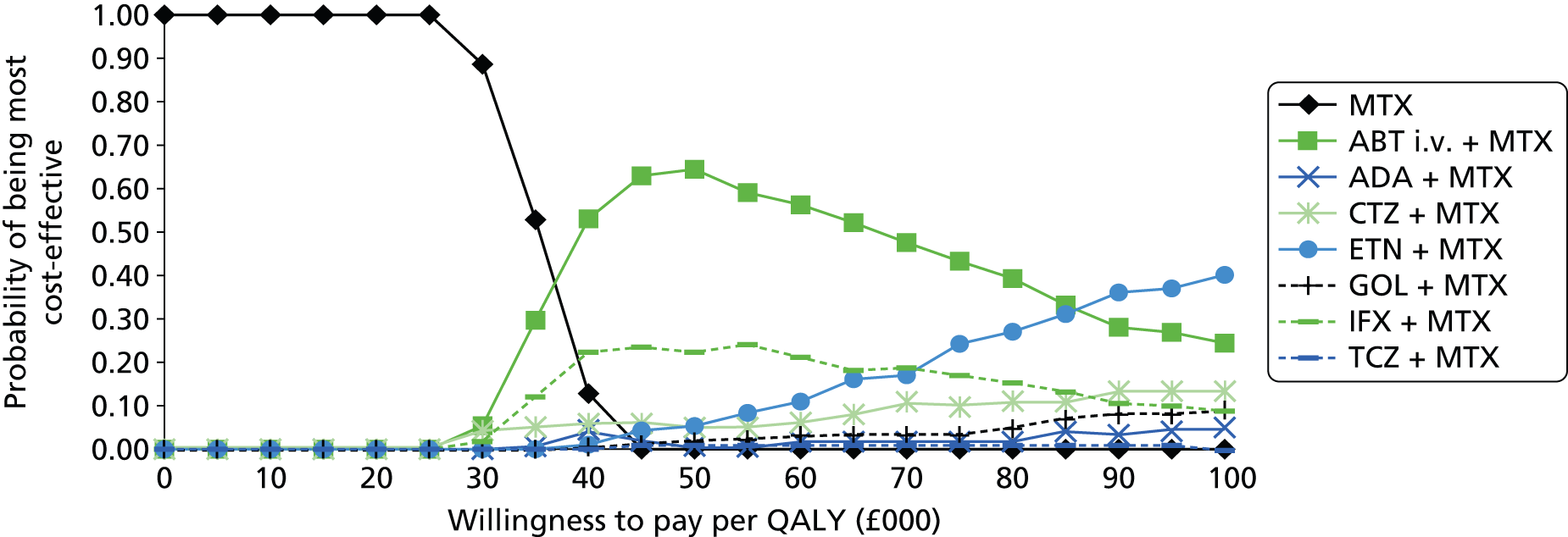
It is seen that at a willingness to pay of £30,000 per QALY the MTX strategy has a high probability of being optimal.
American College of Rheumatology response measure: Early Rheumatoid Arthritis Study cDMARD Health Assessment Questionnaire progression and a severe, methotrexate-experienced, rheumatoid arthritis population
The base-case results for this population and those from sensitivity analyses are provided in Tables 191–199. The CEAC for the base case is shown in Figure 121.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 41,453 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 38,468 | 38,468 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 38,503 | 43,937 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 39,924 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 41,314 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 41,611 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 41,567 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 42,494 | 201,284 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £38,000–43,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 41,396 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 38,743 | 38,743 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 38,844 | 50,533 |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 41,468 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 41,892 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 41,943 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 41,144 | 105,558 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 42,894 | 1,526,573 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 40,977 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 38,171 | 38,171 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 38,446 | Extendedly dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 40,945 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 41,263 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 41,104 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 40,690 | 104,017 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 42,404 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 42,440 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 38,774 | 38,774 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 39,223 | 150,385 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 40,750 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 41,827 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 41,879 | 211,049 |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 42,060 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 42,857 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 34,810 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 32,233 | 32,233 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 32,497 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 33,681 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 34,606 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 34,976 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 34,751 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 35,581 | 125,993 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 29,441 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 26,238 | 26,238 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 26,295 | 30,514 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 27,266 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 28,264 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 28,197 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 28,300 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 28,947 | 127,884 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 42,766 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 39,148 | 39,148 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 39,550 | 88,576 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 41,482 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 42,350 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 42,441 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 42,849 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 43,656 | 223,921 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 50,025 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 46,468 | 46,468 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 47,302 | 127,526 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 48,910 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 50,522 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 50,490 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 50,581 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 51,744 | 212,575 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 42,537 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 39,355 | 39,355 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 39,803 | 107,673 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 41,317 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 42,334 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 42,599 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 42,551 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 43,632 | 187,586 |
FIGURE 121.
The CEAC when using ACR data mapped to EULAR data: ERAS cDMARD HAQ progression and a severe, MTX-experienced, RA population.

It is seen that at a willingness to pay of £30,000 the MTX strategy has a very high probability of being optimal.
American College of Rheumatology response measure: linear Health Assessment Questionnaire progression and a severe, methotrexate-experienced, rheumatoid arthritis population
The base-case results for this population and those from sensitivity analyses are provided in Tables 200–208. The CEAC for the base case is shown in Figure 122.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 33,099 | 33,099 |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 33,660 | 38,771 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 34,348 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 35,518 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 36,794 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 36,878 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 36,701 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 38,078 | 213,466 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £33,000–39,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 31,190 | 31,190 |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 32,511 | 43,343 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 32,570 | 82,908 |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 35,233 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 35,142 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 35,470 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 35,107 | 132,855 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 36,633 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 31,647 | 31,647 |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 32,806 | 42,791 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 33,283 | 91,638 |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 35,721 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 35,859 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 35,977 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 35,736 | 150,620 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 37,174 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 34,228 | 34,228 |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 34,493 | 36,870 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 34,870 | 258,927 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 36,362 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 37,610 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 37,938 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 37,693 | 261,545 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 38,550 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 29,087 | 29,087 |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 29,472 | 32,461 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 29,940 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 30,948 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 31,900 | Extendedly dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 31,970 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 32,190 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 33,104 | 176,415 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 20,847 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 20,379 | 20,379 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 20,739 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 21,424 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 22,309 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 22,486 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 22,485 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 23,184 | 199,830 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 33,139 | 33,139 |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 33,923 | 40,436 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 34,236 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 35,603 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 36,703 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 36,861 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 36,819 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 38,160 | 180,120 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 41,248 | 41,248 |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 41,523 | 43,865 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 41,926 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 43,663 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 45,232 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 45,383 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 45,326 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 46,770 | 142,639 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 33,537 | 33,537 |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 34,047 | 38,505 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 34,504 | 148,650 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 35,803 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 36,957 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 37,163 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 37,139 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 38,328 | 244,601 |
FIGURE 122.
The CEAC using ACR data mapped to EULAR data and assuming linear CDMARD HAQ progression.
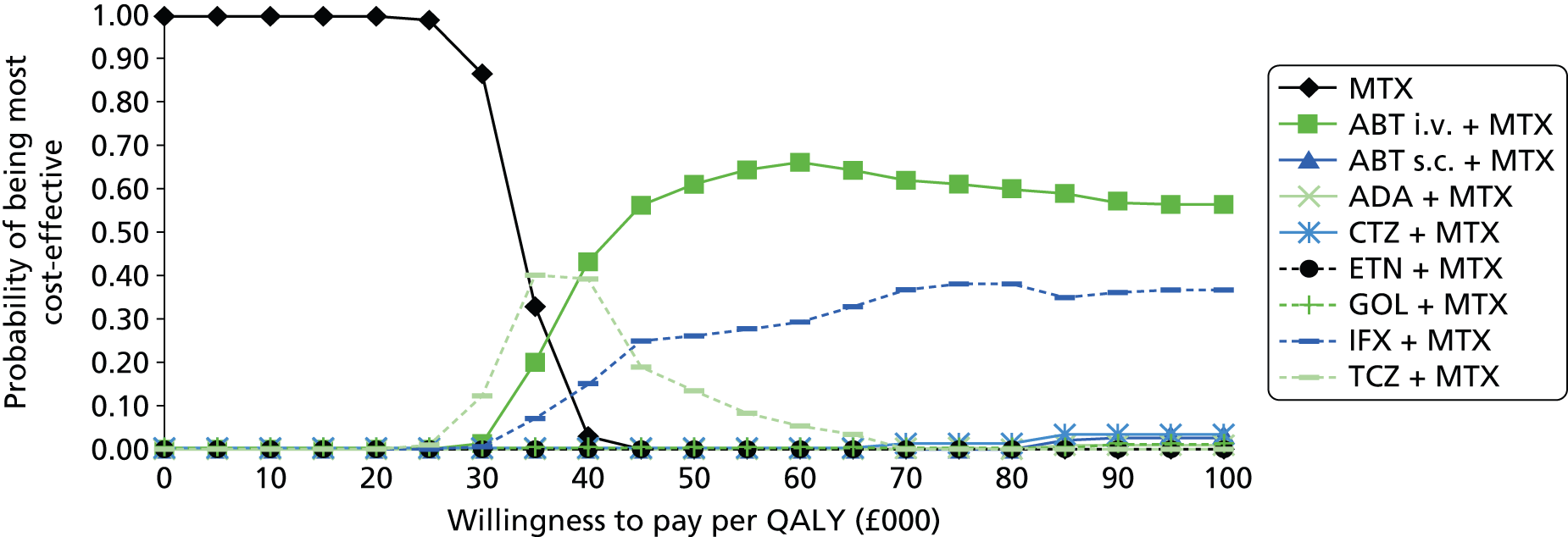
It is seen that at a willingness to pay of £30,000 per QALY the MTX strategy has a relatively high probability of being optimal.
European League Against Rheumatism response measure: Early Rheumatoid Arthritis Study cDMARD Health Assessment Questionnaire progression and a moderate, methotrexate-experienced, rheumatoid arthritis population
The base-case results for this population and those from sensitivity analyses are provided in Tables 209–215. The CEAC for the base case is shown in Figure 123.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 51,559 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 47,533 | 47,533 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 48,424 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 51,472 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 50,467 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 51,111 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 51,436 | 95,714 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £48,000–53,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 52,480 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 48,494 | 48,494 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 49,409 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 52,827 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 51,858 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 51,782 | 94,534 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 52,861 | 108,335 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 36,290 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 34,666 | Extendedly dominated |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 34,147 | 34,147 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 36,848 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 36,272 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 36,573 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 36,834 | 69,318 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 33,821 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 30,491 | 30,491 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 30,880 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 32,723 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 32,079 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 32,788 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 32,801 | 57,940 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 52,423 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 48,186 | 48,186 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 49,231 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 52,157 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 51,015 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 52,813 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 52,762 | 117,717 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 57,687 | Extendedly dominated |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 56,810 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 55,790 | 55,790 |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 58,483 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 58,078 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 59,136 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 59,475 | 88,312 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 52,032 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 48,816 | 48,816 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 49,071 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 50,891 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 52,093 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 52,203 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 52,275 | 89,540 |
FIGURE 123.
The CEAC when using EULAR data directly: ERAS cDMARD HAQ progression and a moderate, MTX-experienced, RA population.

It is seen that at a willingness to pay of £30,000 the MTX strategy has a very high probability of being optimal.
European League Against Rheumatism response measure: linear cDMARD Health Assessment Questionnaire progression and a moderate, methotrexate-experienced, rheumatoid arthritis population
The base-case results for this population and those from sensitivity analyses are provided in Tables 216–222. The CEAC for the base case is shown in Figure 124.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 37,769 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 36,815 | 36,815 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 37,270 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 39,702 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 39,468 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 40,379 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 41,265 | 110,772 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £36,000–42,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 39,018 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 39,012 | 39,012 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 39,767 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 41,515 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 42,197 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 42,137 | 71,973 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 43,054 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 30,439 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 29,571 | 29,571 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 29,942 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 32,260 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 31,257 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 32,137 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 32,313 | 64,372 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 20,043 | 20,043 |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 21,297 | Dominated |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 20,370 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 21,972 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 21,758 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 22,216 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 22,936 | 80,582 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 36,912 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 36,224 | 36,224 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 37,115 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 39,254 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 39,587 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 40,239 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 41,128 | 125,849 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 46,453 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 45,674 | 45,674 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 45,886 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 48,343 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 49,405 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 49,113 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 50,252 | 115,803 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 38,152 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 34,843 | 34,843 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 35,425 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 36,644 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 37,583 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 37,779 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 38,355 | 86,917 |
FIGURE 124.
The CEAC using EULAR data directly and assuming linear CDMARD HAQ progression and a moderate, MTX-experienced, RA population.
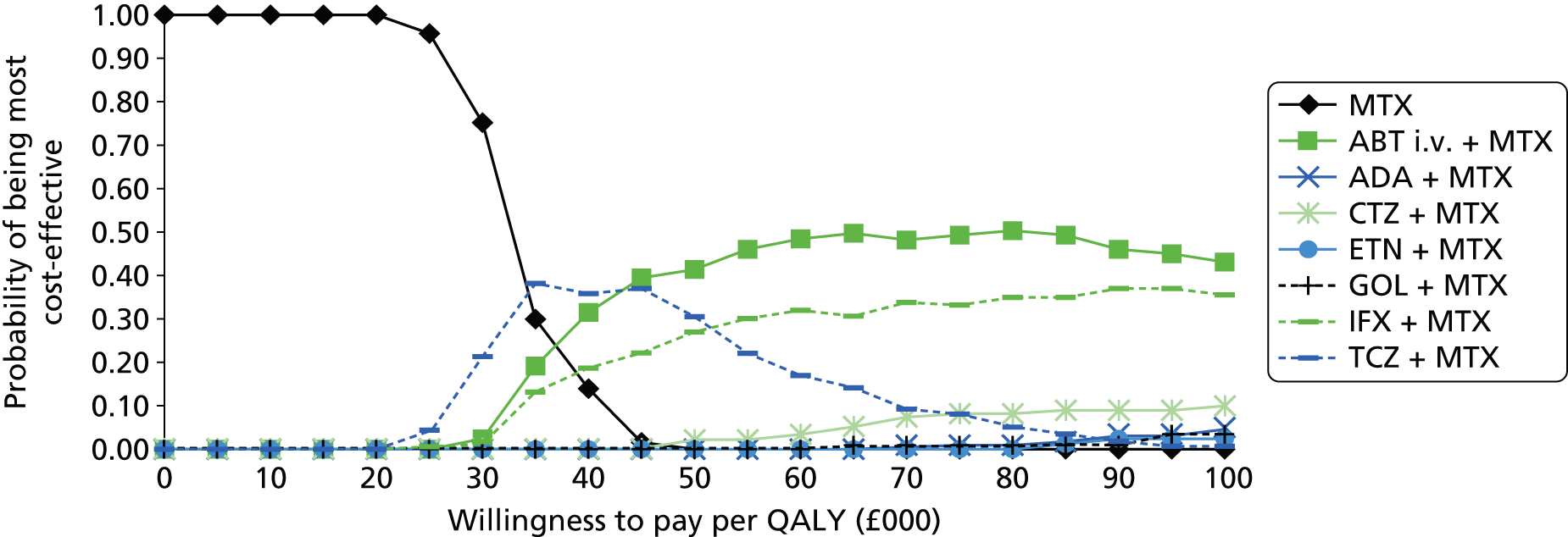
It is seen that at a willingness to pay of £30,000 per QALY the MTX strategy has the highest probability of being optimal.
American College of Rheumatology response measure: Early Rheumatoid Arthritis Study cDMARD Health Assessment Questionnaire progression and a moderate, methotrexate-experienced, rheumatoid arthritis population
The base-case results for this population and those from sensitivity analyses are provided in Tables 223–230.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 52,410 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 47,833 | 47,833 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 48,474 | 101,458 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 50,044 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 51,625 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 51,573 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 51,341 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 53,006 | 468,878 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £47,000–54,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 52,779 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 47,839 | 47,839 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 49,646 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 52,111 | Extendedly dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 52,771 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 52,489 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 52,215 | 167,643 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 53,866 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 53,650 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 46,985 | 46,985 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 49,149 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 51,016 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 52,073 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 52,375 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 51,296 | 145,070 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 53,588 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 52,464 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 47,673 | 47,673 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 47,685 | 48,465 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 51,273 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 51,471 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 51,230 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 51,540 | 399,034 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 53,193 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 37,766 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 34,586 | 34,586 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 34,852 | 64,571 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 36,358 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 37,506 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 37,229 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 37,535 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 38,247 | 242,769 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 33,852 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 30,721 | 30,721 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 31,023 | Extendedly dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 32,074 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 33,016 | Extendedly dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 32,807 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 33,300 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 33,712 | 97,679 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 52,982 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 48,644 | 48,644 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 48,818 | 90,480 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 51,151 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 52,012 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 52,104 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 52,992 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 53,614 | 183,170 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 59,499 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 54,356 | 54,356 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 54,514 | 67,602 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 58,334 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 59,480 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 59,394 | Extendedly dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 59,107 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 60,912 | 372,652 |
Probabilistic results using American College of Rheumatology data mapped to European League Against Rheumatism data and assuming Early Rheumatoid Arthritis Study cDMARD Health Assessment Questionnaire progression and a moderate, methotrexate-experienced, rheumatoid arthritis population
The probablistic base-case results for this population and those from sensitivity analyses are provided in Table 231. The CEAC for the base case is shown in Figure 125.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 51,651 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 47,658 | 47,658 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 48,260 | 110,763 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 50,444 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 51,566 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 51,674 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 51,638 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 53,133 | 247,395 |
FIGURE 125.
The CEAC when using ACR data mapped to EULAR data: ERAS cDMARD HAQ progression and a moderate, MTX-experienced, RA population.
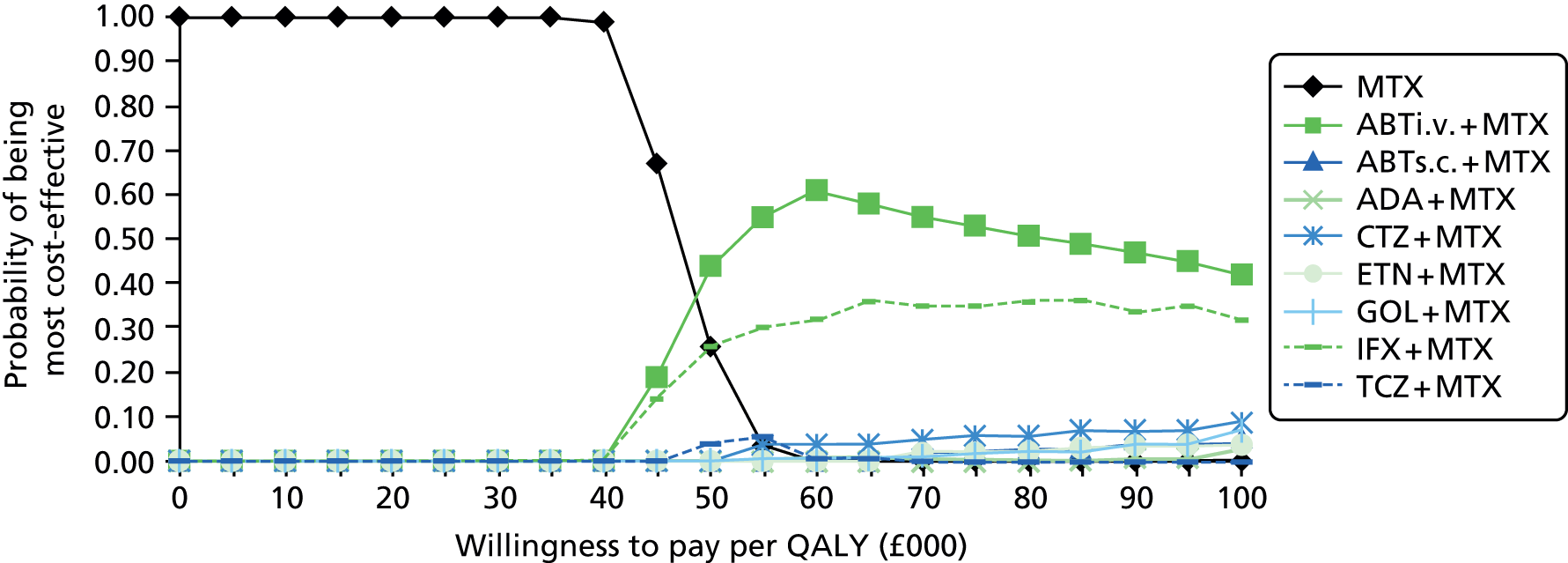
It is seen that at a willingness to pay of £30,000 the MTX strategy has a very high probability of being optimal.
American College of Rheumatology response measure: linear cDMARD Health Assessment Questionnaire progression and a moderate, methotrexate-experienced, rheumatoid arthritis population
The base-case results for this population and those from sensitivity analyses are provided in Tables 232–239.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 36,576 | 36,576 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 36,916 | 39,965 |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 37,372 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 38,039 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 39,847 | Extendedly dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 40,035 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 39,785 | 264,074 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 40,893 | Dominated |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £36,000–41,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 33,337 | 33,337 |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 34,568 | Extendedly dominated |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 34,413 | 43,931 |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 36,691 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 37,177 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 37,111 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 37,387 | 275,630 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 39,123 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 39,495 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 34,536 | 17,203 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 37,952 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 37,623 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 37,909 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 41,375 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 37,765 | 129,483 |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 42,666 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 40,094 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 35,589 | 35,589 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 39,245 | Dominated |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 37,348 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 38,788 | 807,662 |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 39,151 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 42,274 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 42,085 | Dominated |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 32,540 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 27,857 | 27,857 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 28,284 | 112,265 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 28,972 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 30,258 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 29,952 | 216,351 |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 30,289 | Dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 31,287 | 6,775,191 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 20,467 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 20,373 | 20,373 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 21,397 | 144,174 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 21,831 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 22,822 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 22,899 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 23,129 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 23,524 | 264,012 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 38,589 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 34,339 | 34,339 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 35,366 | 153,812 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 35,983 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 37,069 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 37,360 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 37,359 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 38,510 | 239,256 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 48,514 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 42,655 | 42,655 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 43,444 | 198,638 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 45,289 | Dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 46,863 | Dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 46,719 | Extendedly dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 47,094 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 48,243 | 387,730 |
Probabilistic results using American College of Rheumatology data mapped to European League Against Rheumatism data and assuming linear cDMARD Health Assessment Questionnaire progression
The probabilistic base-case results for this population and those from sensitivity analyses are provided in Table 240. The CEAC for the base case is shown in Figure 126.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| MTX | CiC information has been removed | CiC information has been removed | – | – |
| TCZ + MTX | CiC information has been removed | CiC information has been removed | 40,241 | Extendedly dominated |
| ABT i.v. + MTX | CiC information has been removed | CiC information has been removed | 35,088 | 35,088 |
| IFX + MTX | CiC information has been removed | CiC information has been removed | 35,318 | 70,967 |
| CTZ + MTX | CiC information has been removed | CiC information has been removed | 36,970 | Dominated |
| ABT s.c. + MTX | CiC information has been removed | CiC information has been removed | 38,183 | Extendedly dominated |
| GOL + MTX | CiC information has been removed | CiC information has been removed | 38,412 | Extendedly dominated |
| ADA + MTX | CiC information has been removed | CiC information has been removed | 38,369 | Extendedly dominated |
| ETN + MTX | CiC information has been removed | CiC information has been removed | 39,754 | 340,953 |
FIGURE 126.
The CEAC using ACR data mapped to EULAR data and assuming linear CDMARD HAQ progression and a moderate, MTX-experienced, RA population.

It is seen at a willingness to pay of £30,000 per QALY that the MTX strategy has a very high likelihood of being optimal.
European League Against Rheumatism response measure: Early Rheumatoid Arthritis Study cDMARD Health Assessment Questionnaire progression and a severe, methotrexate-experienced, rheumatoid arthritis population treated with monotherapy
The base-case results for this population and those from sensitivity analyses are provided in Tables 241–247. The CEAC for the base case is shown in Figure 127.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 48,306 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 48,528 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 46,327 | 46,327 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £46,000–49,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 49,001 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 49,084 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 46,660 | 46,660 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 40,230 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 40,890 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 38,369 | 38,369 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 32,747 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 33,110 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 31,064 | 31,064 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 48,869 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 48,917 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 46,797 | 46,797 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 58,955 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 59,351 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 55,361 | 55,361 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 48,192 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 48,392 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 46,040 | 46,040 |
FIGURE 127.
The CEAC when using EULAR data directly: ERAS cDMARD HAQ progression and a severe, MTX-experienced, RA population treated with monotherapy.
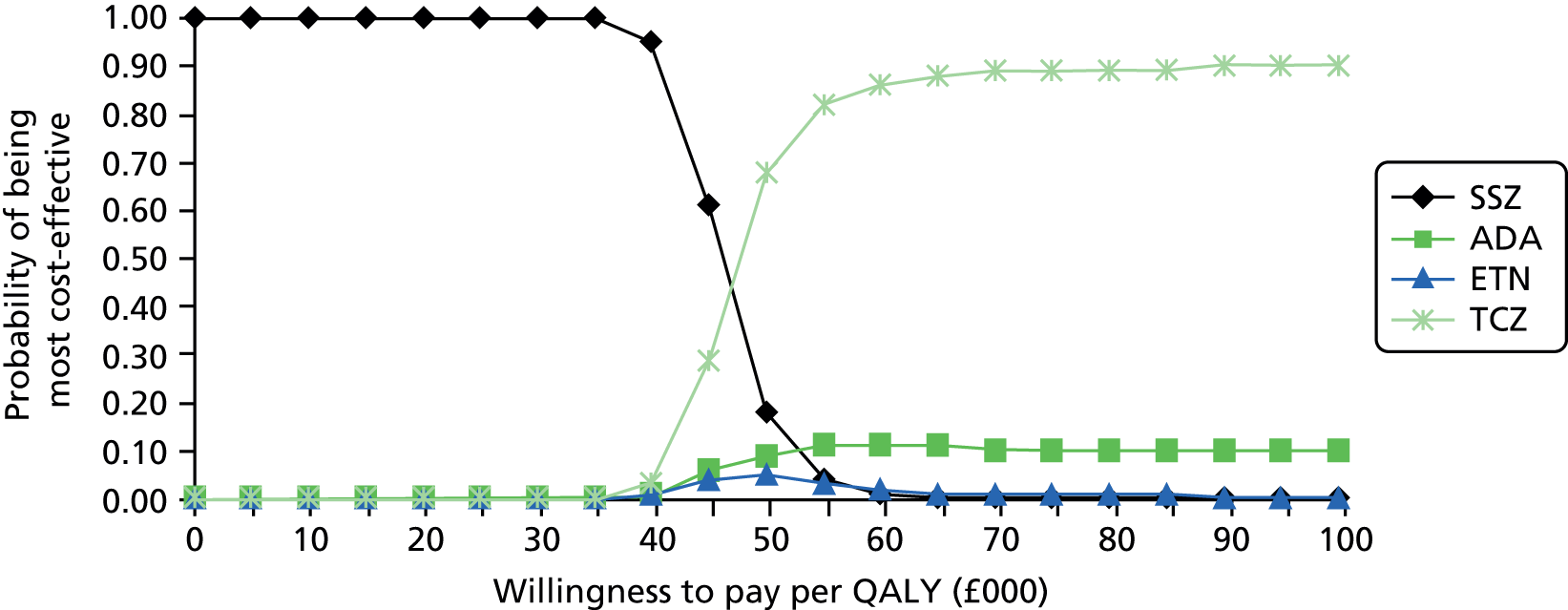
It is seen that at a willingness to pay of £30,000 the SSZ strategy has a very high probability of being optimal.
European League Against Rheumatism response measure: linear cDMARD Health Assessment Questionnaire progression and a severe, methotrexate-experienced, rheumatoid arthritis population treated with monotherapy
The base-case results for this population and those from sensitivity analyses are provided in Tables 248–254. The CEAC for the base case is shown in Figure 128.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 39,171 | 39,171 |
| ETN | CiC information has been removed | CiC information has been removed | 39,637 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 39,654 | 43,846 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £39,000–40,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 37,799 | 37,799 |
| ETN | CiC information has been removed | CiC information has been removed | 37,975 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 38,558 | 44,689 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 34,836 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 34,997 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 34,565 | 34,565 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 24,632 | 24,632 |
| ETN | CiC information has been removed | CiC information has been removed | 24,789 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 24,770 | 26,079 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 40,074 | 40,074 |
| ETN | CiC information has been removed | CiC information has been removed | 40,207 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 40,337 | 42,432 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 49,152 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 49,716 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 49,145 | 49,145 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 38,806 | 38,806 |
| ETN | CiC information has been removed | CiC information has been removed | 39,132 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 39,115 | 41,651 |
FIGURE 128.
The CEAC when using EULAR data directly: linear cDMARD HAQ progression and a severe, MTX-experienced, RA population treated with monotherapy.

It is seen that at a willingness to pay of £30,000 the SSZ strategy has a very high probability of being optimal.
American College of Rheumatology response measure: Early Rheumatoid Arthritis Study cDMARD Health Assessment Questionnaire progression and a severe, methotrexate-experienced, rheumatoid arthritis population treated with monotherapy
The base-case results for this population and those from sensitivity analyses are provided in Tables 255–266. The CEAC for the base case is shown in Figure 129.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 49,707 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 49,808 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 49,584 | 49,584 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £49,000–50,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 49,550 | Extendedly dominated |
| ADA | CiC information has been removed | CiC information has been removed | 49,484 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 49,394 | 49,394 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 49,473 | Extendedly dominated |
| ADA | CiC information has been removed | CiC information has been removed | 49,953 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 49,169 | 49,169 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 49,983 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 50,384 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 49,546 | 49,546 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 41,558 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 41,409 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 41,007 | 41,007 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 41,558 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 41,409 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 41,007 | 41,007 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 50,272 | Extendedly dominated |
| ADA | CiC information has been removed | CiC information has been removed | 50,135 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 49,575 | 49,575 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 60,418 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 60,704 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 60,386 | 60,386 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 50,272 | Extendedly dominated |
| ADA | CiC information has been removed | CiC information has been removed | 50,135 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 49,575 | 49,899 |
FIGURE 129.
The CEAC when mapping EULAR data from ACR data: ERAS cDMARD HAQ progression and a severe, MTX-experienced, RA population treated with monotherapy.
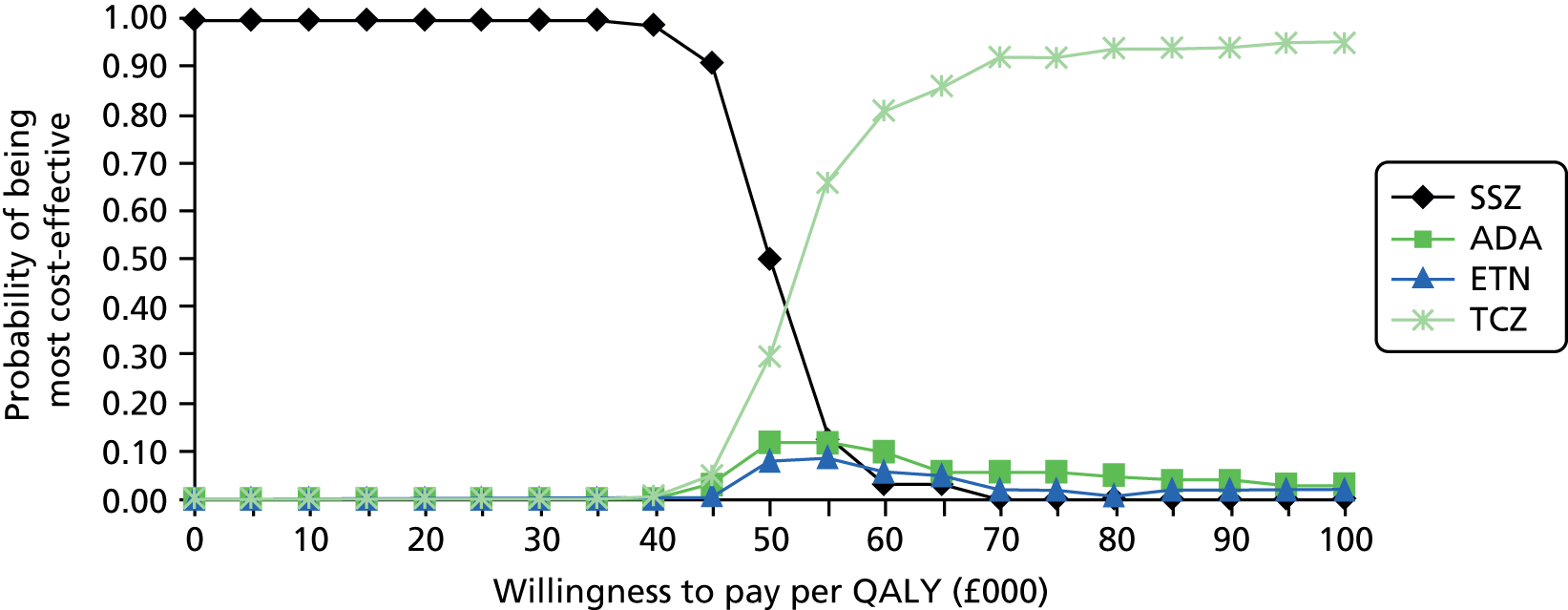
It is seen that at a willingness to pay of £30,000 the SSZ strategy has a very high probability of being optimal.
American College of Rheumatology response measure: linear cDMARD Health Assessment Questionnaire progression and a severe, methotrexate-experienced, rheumatoid arthritis population treated with monotherapy
The base-case results for this population and those from sensitivity analyses are provided in Tables 264–272. The CEAC for the base case is shown in Figure 130.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 38,501 | 38,501 |
| ETN | CiC information has been removed | CiC information has been removed | 38,547 | 49,828 |
| TCZ | CiC information has been removed | CiC information has been removed | 40,049 | 70,054 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £38,000–41,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 37,261 | Extendedly dominated |
| ADA | CiC information has been removed | CiC information has been removed | 37,343 | 37,261 |
| TCZ | CiC information has been removed | CiC information has been removed | 38,835 | 66,329 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 37,185 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 37,087 | 37,087 |
| TCZ | CiC information has been removed | CiC information has been removed | 38,562 | 67,396 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 36,796 | 36,796 |
| ADA | CiC information has been removed | CiC information has been removed | 37,204 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 38,432 | 59,568 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 34,042 | 34,042 |
| ETN | CiC information has been removed | CiC information has been removed | 34,055 | 49,928 |
| TCZ | CiC information has been removed | CiC information has been removed | 35,280 | 55,140 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 23,591 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 23,537 | 23,537 |
| TCZ | CiC information has been removed | CiC information has been removed | 24,343 | 39,745 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 39,173 | 39,173 |
| ETN | CiC information has been removed | CiC information has been removed | 39,257 | 59,684 |
| TCZ | CiC information has been removed | CiC information has been removed | 40,674 | 65,518 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 47,732 | 47,732 |
| ETN | CiC information has been removed | CiC information has been removed | 47,801 | 73,402 |
| TCZ | CiC information has been removed | CiC information has been removed | 49,552 | 78,345 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 38,765 | 38,765 |
| ADA | CiC information has been removed | CiC information has been removed | 38,766 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 40,229 | 70,551 |
FIGURE 130.
The CEAC when mapping EULAR data from ACR data: linear cDMARD HAQ progression and a severe, MTX-experienced, RA population treated with monotherapy.
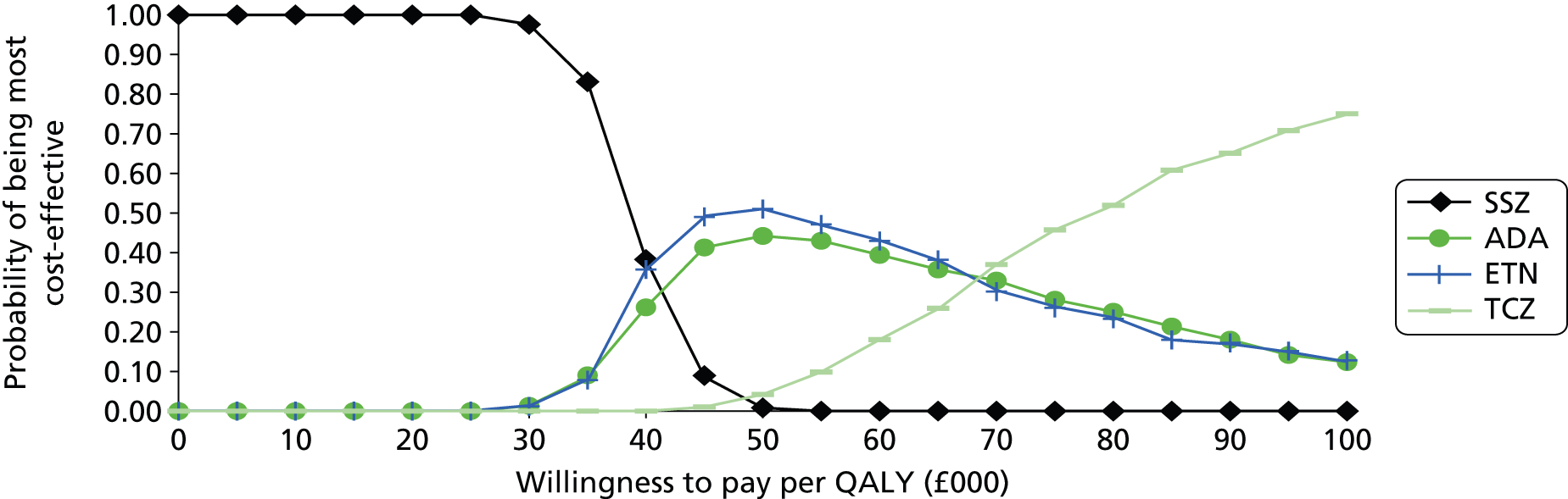
It is seen that at a willingness to pay of £30,000 the SSZ strategy and the TCZ strategy have reasonably high probabilities of being optimal.
European League Against Rheumatism response measure: Early Rheumatoid Arthritis Study cDMARD Health Assessment Questionnaire progression and a moderate, methotrexate-experienced, rheumatoid arthritis population treated with monotherapy
The base-case results for this population and those from sensitivity analyses are provided in Tables 273–279. The CEAC for the base case is shown in Figure 131.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 59,036 | Extendedly dominated |
| CTZ | CiC information has been removed | CiC information has been removed | 58,798 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 58,673 | 58,673 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £58,000–60,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 55,934 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 57,588 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 55,364 | 55,364 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 42,028 | Extendedly dominated |
| ADA | CiC information has been removed | CiC information has been removed | 41,852 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 40,433 | 40,433 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 39,090 | Extendedly dominated |
| ADA | CiC information has been removed | CiC information has been removed | 37,482 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 35,952 | 35,952 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 59,928 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 59,443 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 58,291 | 58,291 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 67,022 | Extendedly dominated |
| ADA | CiC information has been removed | CiC information has been removed | 66,067 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 63,456 | 63,456 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 59,657 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 59,719 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 57,809 | 57,809 |
FIGURE 131.
The CEAC when using EULAR data directly: ERAS cDMARD HAQ progression and a moderate, MTX-experienced, RA population treated with monotherapy.
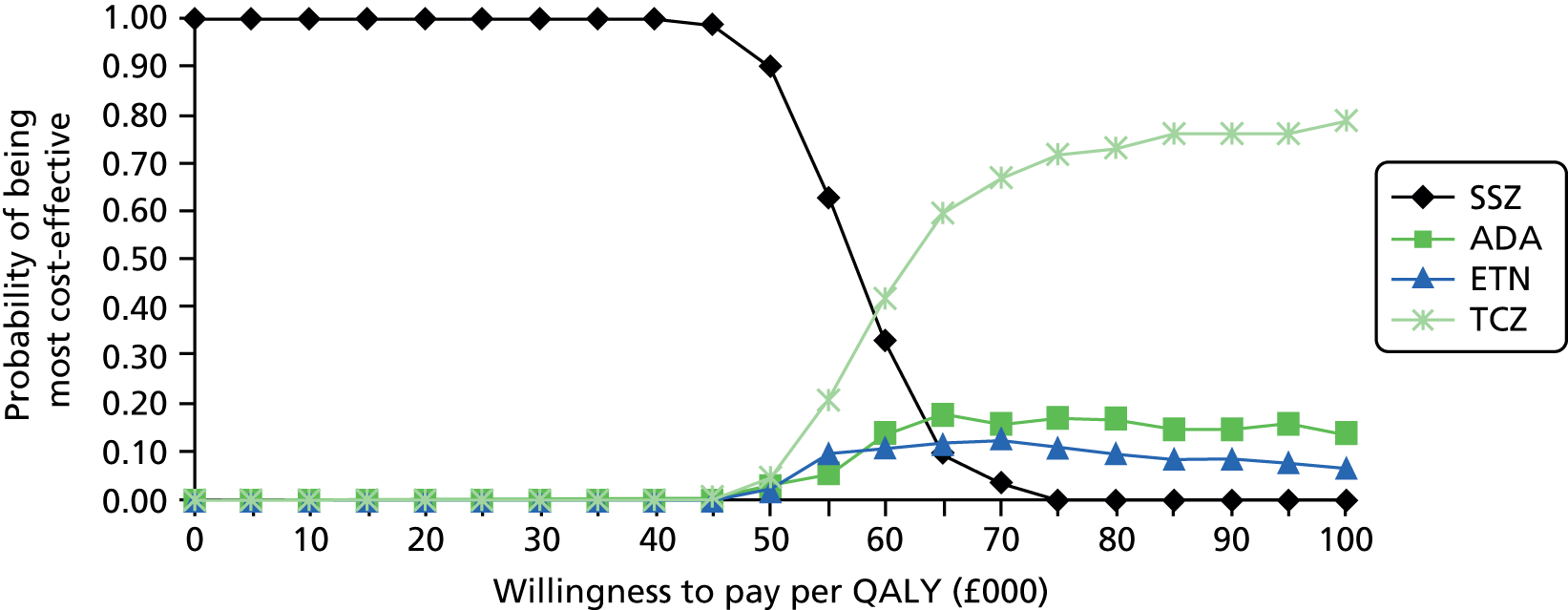
It is seen that at a willingness to pay of £30,000 the SSZ strategy has a very high probability of being optimal.
European League Against Rheumatism response measure: linear cDMARD Health Assessment Questionnaire progression and a moderate, methotrexate-experienced, rheumatoid arthritis population treated with monotherapy
The base-case results for this population and those from sensitivity analyses are provided in Tables 280–286. The CEAC for the base case is shown in Figure 132.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 41,190 | 41,190 |
| ETN | CiC information has been removed | CiC information has been removed | 41,385 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 42,465 | 54,415 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £41,000–43,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 38,937 | 38,937 |
| ETN | CiC information has been removed | CiC information has been removed | 39,300 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 41,020 | 61,560 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 32,767 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 32,872 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 32,465 | 29,207 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 23,671 | 23,671 |
| ETN | CiC information has been removed | CiC information has been removed | 23,869 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 24,469 | 32,344 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 41,580 | 41,580 |
| ETN | CiC information has been removed | CiC information has been removed | 41,621 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 43,376 | 60,121 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 48,834 | 48,834 |
| ETN | CiC information has been removed | CiC information has been removed | 49,661 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 50,440 | 65,894 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 41,263 | 41,263 |
| ETN | CiC information has been removed | CiC information has been removed | 41,683 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 43,062 | 62,038 |
FIGURE 132.
The CEAC when using EULAR data directly: linear cDMARD HAQ progression and a severe, MTX-experienced, RA population treated with monotherapy.

It is seen that at a willingness to pay of £30,000 the SSZ strategy has a high probability of being optimal.
American College of Rheumatology response measure: Early Rheumatoid Arthritis Study cDMARD Health Assessment Questionnaire progression and a moderate, methotrexate-experienced, rheumatoid arthritis population treated with monotherapy
The base-case results for this population and those from sensitivity analyses are provided in Tables 287–295. The CEAC for the base case is shown in Figure 133.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 58,981 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 57,264 | 57,264 |
| TCZ | CiC information has been removed | CiC information has been removed | 57,786 | 62,823 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £57,000–59,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 58,346 | 58,346 |
| ADA | CiC information has been removed | CiC information has been removed | 59,145 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 59,533 | 77,492 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 62,630 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 62,393 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 62,210 | 62,210 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 63,131 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 61,889 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 61,577 | 61,577 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 44,867 | Extendedly dominated |
| ADA | CiC information has been removed | CiC information has been removed | 44,934 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 44,729 | 44,729 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 40,068 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 39,282 | 39,282 |
| TCZ | CiC information has been removed | CiC information has been removed | 39,575 | 44,275 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 61,794 | Extendedly dominated |
| ADA | CiC information has been removed | CiC information has been removed | 61,928 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 60,755 | 60,755 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 67,208 | 67,208 |
| ETN | CiC information has been removed | CiC information has been removed | 67,659 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 67,318 | 68,411 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ETN | CiC information has been removed | CiC information has been removed | 60,034 | 60,034 |
| ADA | CiC information has been removed | CiC information has been removed | 60,659 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 60,269 | 62,566 |
FIGURE 133.
The CEAC when mapping EULAR data from ACR data: ERAS cDMARD HAQ progression and a moderate, MTX-experienced, RA population treated with monotherapy.

It is seen that at a willingness to pay of £30,000 the SSZ strategy has a very high probability of being optimal.
American College of Rheumatology response measure: linear cDMARD Health Assessment Questionnaire progression and a moderate, methotrexate-experienced, rheumatoid arthritis population treated with monotherapy
The base-case results for this population and those from sensitivity analyses are provided in Tables 296–304. The CEAC for the base case is shown in Figure 134.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 38,751 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 38,469 | 38,469 |
| TCZ | CiC information has been removed | CiC information has been removed | 40,644 | 94,949 |
It is seen that the ICERs of all the bDMARDs compared with the MTX strategy are in the region of £38,000–41,000.
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 38,956 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 38,547 | 38,547 |
| TCZ | CiC information has been removed | CiC information has been removed | 41,321 | 107,580 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 38,264 | 38,264 |
| ETN | CiC information has been removed | CiC information has been removed | 38,545 | Extendedly dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 40,304 | 76,254 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 37,025 | 37,025 |
| ETN | CiC information has been removed | CiC information has been removed | 37,217 | 50,652 |
| TCZ | CiC information has been removed | CiC information has been removed | 40,508 | 85,586 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 30,914 | 30,914 |
| ETN | CiC information has been removed | CiC information has been removed | 31,053 | 49,620 |
| TCZ | CiC information has been removed | CiC information has been removed | 32,431 | 56,717 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 23,654 | Extendedly dominated |
| ETN | CiC information has been removed | CiC information has been removed | 23,766 | 23,766 |
| TCZ | CiC information has been removed | CiC information has been removed | 24,878 | 47,829 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 39,857 | 39,857 |
| ETN | CiC information has been removed | CiC information has been removed | 40,477 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 42,660 | 147,373 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 46,973 | 46,973 |
| ETN | CiC information has been removed | CiC information has been removed | 47,107 | Dominated |
| TCZ | CiC information has been removed | CiC information has been removed | 49,606 | 95,878 |
| First intervention in the strategy | Discounted costs | Discounted QALYs | CPQ compared with MTX strategy (£) | Incremental CPQ (£) |
|---|---|---|---|---|
| SSZ | CiC information has been removed | CiC information has been removed | – | – |
| ADA | CiC information has been removed | CiC information has been removed | 39,460 | 39,460 |
| ETN | CiC information has been removed | CiC information has been removed | 39,550 | 59,914 |
| TCZ | CiC information has been removed | CiC information has been removed | 41,843 | 84,981 |
FIGURE 134.
The CEAC when mapping EULAR data from ACR data: linear cDMARD HAQ progression and a moderate, MTX-experienced, RA population treated with monotherapy
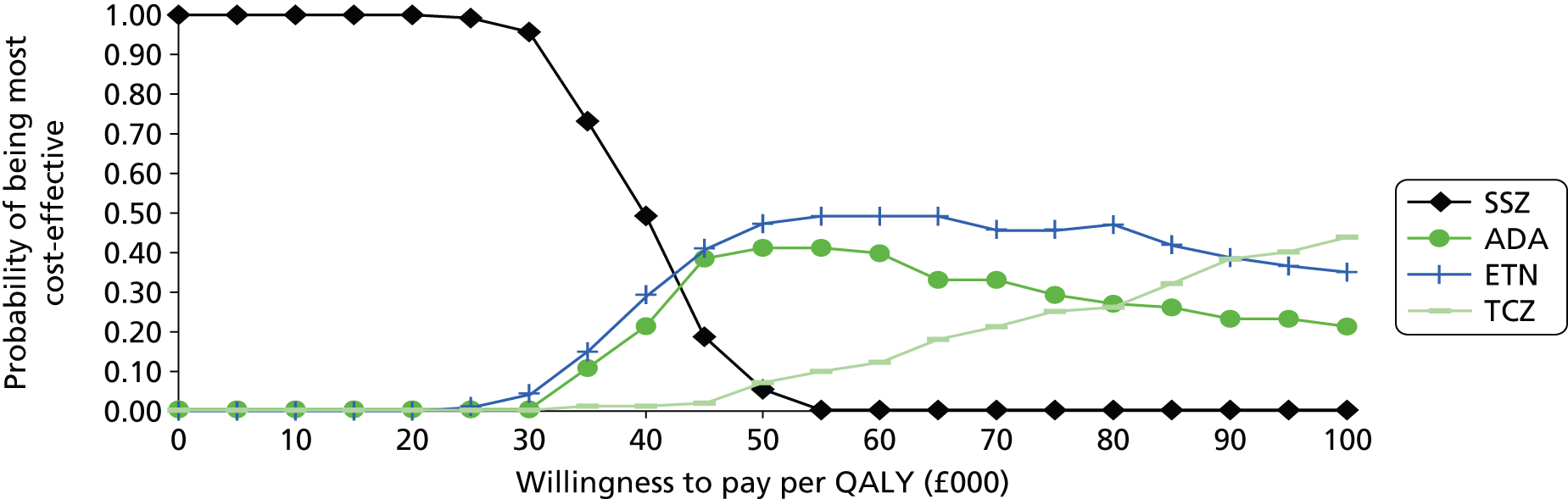
It is seen that at a willingness to pay of £30,000 the SSZ strategy has the highest probability of being optimal.
Response measure American College of Rheumatology: Early Rheumatoid Arthritis Study cDMARD Health Assessment Questionnaire progression and a severe, methotrexate-naive, rheumatoid arthritis population
The base-case results for this population and those from sensitivity analyses are provided in Tables 305–311. The CEAC for the base case is shown in Figure 135.
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 68,277 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 68,152 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 50,504 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 42,587 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 71,147 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 79,535 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 66,091 |
FIGURE 135.
The CEAC when mapping EULAR data from ACR data: ERAS cDMARD HAQ progression and a severe, MTX-naive, RA population treated. Int, intensive.

It is seen that at a willingness to pay of £30,000 that MTX/intensive cDMARDs/NBT strategy has a very high probability of being optimal.
Response measure American College of Rheumatology: linear cDMARD Health Assessment Questionnaire progression and a severe, methotrexate-naive, rheumatoid arthritis population
The base-case results for this population and those from sensitivity analyses are provided in Tables 312–318. The CEAC for the base case is shown in Figure 136.
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 58,290 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 58,065 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 44,694 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 33,580 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 59,839 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 68,258 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/MTX/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 60,484 |
FIGURE 136.
The CEAC when mapping EULAR data from ACR data: linear cDMARD HAQ progression and a severe, MTX-naive, RA population. Int, intensive.
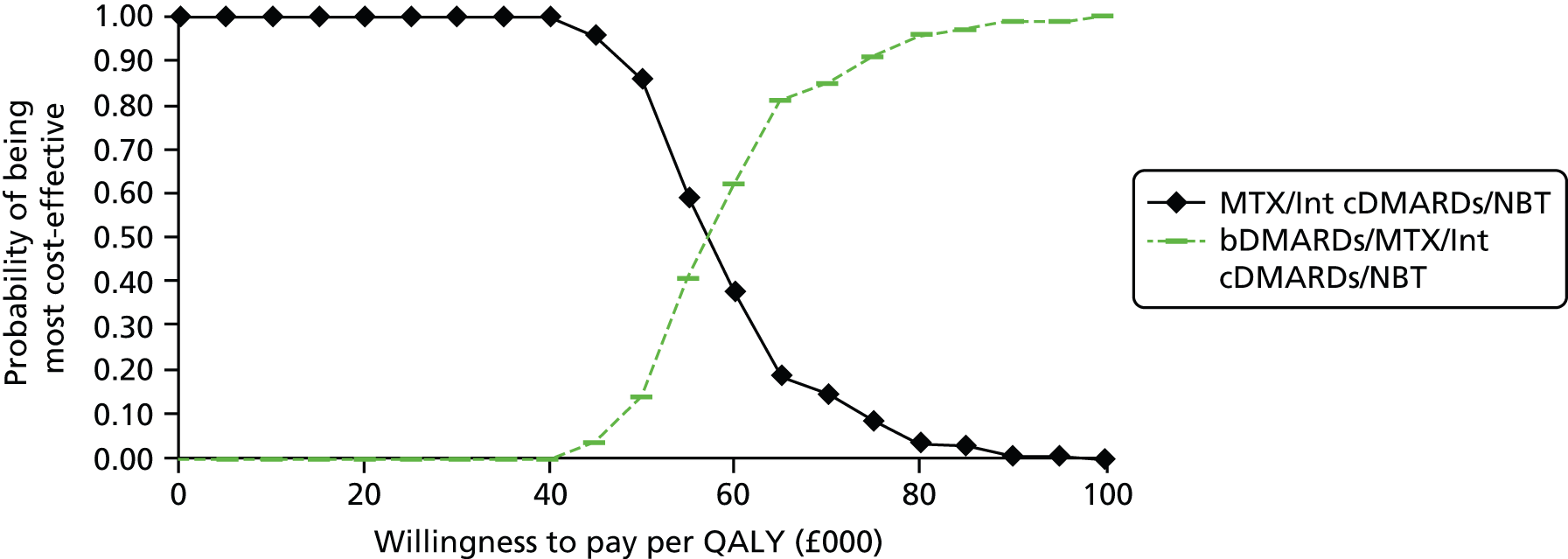
It is seen that at a willingness to pay of £30,000 the MTX/NBT strategy has a very high probability of being optimal.
Response measure American College of Rheumatology: Early Rheumatoid Arthritis Study cDMARD Health Assessment Questionnaire progression and a severe, MTX-naive, rheumatoid arthritis population treated with monotherapy
The base-case results for this population and those from sensitivity analyses are provided in Tables 319–325. The CEAC for the base case is shown in Figure 137.
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 68,277 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 68,152 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 50,504 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 42,587 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 71,147 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 79,535 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 66,091 |
FIGURE 137.
The CEAC when mapping EULAR data from ACR data: ERAS cDMARD HAQ progression and a severe, MTX-naive, RA population treated with monotherapy. Int, intensive.

Response measure American College of Rheumatology: linear cDMARD Health Assessment Questionnaire progression and a severe, methotrexate-naive, rheumatoid arthritis population treated with monotherapy
The base-case results for this population and those from sensitivity analyses are provided in Tables 326–332. The CEAC for the base case is shown in Figure 138.
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 58,290 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 58,065 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 44,694 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 33,580 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 59,839 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 68,258 |
| Strategy | Discounted costs | Discounted QALYs | Incremental CPQ (£) |
|---|---|---|---|
| SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | – |
| bDMARDs/SSZ/intensive cDMARDs/NBT | CiC information has been removed | CiC information has been removed | 60,484 |
FIGURE 138.
The CEAC when mapping EULAR data from ACR data: linear cDMARD HAQ progression and a severe, MTX-naive, RA population. Int, intensive.
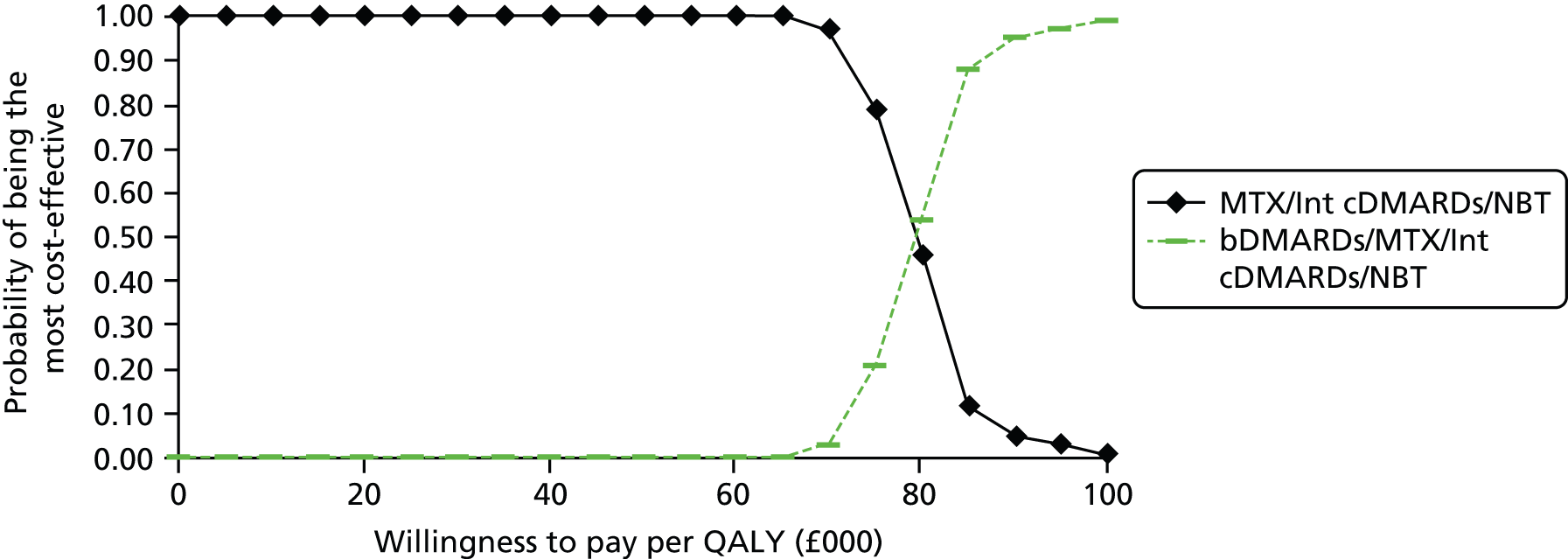
Interpretation of the results
Methotrexate-experienced rheumatoid arthritis patients
It is seen that the results are particularly sensitive to the assumptions made regarding the progression of HAQ while on cDMARDs, particularly for those with moderate RA and for those who cannot receive MTX.
The base-case analyses undertaken by the Assessment Group estimate the HAQ progression while on cDMARDs to be that produced by a statistical analysis of the ERAS database, which contains a large number of patients diagnosed with RA with a 15-year follow-up. This results in ICERs for the bDMARDs typically > £40,000 per QALY when compared with a cDMARD-alone option for patients with severe disease who can receive MTX. This value rises to £50,000 per QALY when patients with moderate disease are evaluated.
In contrast, the manufacturers typically used a linear HAQ progression that has been used in previous NICE appraisals;26,28,171,207 when the Assessment Group used the same assumptions the ICERs were typically in the region of £30,000–40,000 per QALY for both patients with severe or moderate disease.
The most appropriate HAQ progression to assume is discussed in Health Assessment Questionnaire trajectory following initial response. The Assessment Group believes that the progression calculated from ERAS data is likely to be more plausible, although may underestimate HAQ progression as it may contain patients who would not receive bDMARDs.
Altering the discount rate to that used in the initial appraisals of bDMARDs (6% per annum for costs and 1.5% per annum for QALYs) noticeably reduces the ICERs; using the relationship between HAQ and pain from a different data source noticeably increases the ICERs. The ICERs for severe RA patients were typically lower than for moderate RA patients, although the difference was smaller when a linear HAQ progression was used.
The results between EULAR-only data and EULAR mapped from ACR were comparable, which is reassuring given the wider evidence base reporting ACR data.
The ICERs for those patients who receive monotherapy are higher than for those who can be treated with MTX, increasing to approximately £48,000 per QALY gained for patients with severe RA, approximately £59,000 using the ERAS HAQ progression, and increasing to approximately £39,000 for both groups when using the linear HAQ progression.
Methotrexate-naive rheumatoid arthritis patients
The ICERs associated with treating with bDMARDs prior to MTX are over £60,000 per QALY gained for patients regardless of whether or not the patient can receive MTX or whether the assumed HAQ progression was linear or that estimated from the ERAS data set.
In addition, these ICERs are expected to be highly favourable to bDMARDs, as it is assumed that the HAQ progressions associated with single cDMARDs (either linear or non-linear) would also apply to intensive cDMARDs, which, according to the clinical advisors to the Assessment Group, is becoming widespread. If, as expected, the HAQ progression on intensive cDMARDs was lower than that assumed within the analyses then the ICERs would increase, potentially very considerably.
Discussion
Summary of key results
See Chapter 6, Statement of principal findings.
Comparison of the Assessment Group results and those produced by the companies
The base-case ICERs estimated by the Assessment Group are considerably higher than those estimated by the companies, whose values for patients who have severe RA and can receive MTX typically lie between £20,000 and £30,000 per QALY gained. Contrastingly, the base-case value produced by the Assessment Group was £37,000.
The discrepancy is initially reduced if the value produced by the Assessment Group when using the linear model and the Malottki et al. 171 HAQ to utility mapping is used. This evaluation is more in line with the analyses conducted by the companies and in this scenario the median ICER in the Assessment Group model fell to £32,400.
On investigation, it was seen that the reduction in HAQ assumed with a positive response to treatment was typically assumed to be greater in the submissions from the companies (see Health Assessment Questionnaire/European Quality of Life-5 Dimensions changes in relation to response levels) than that seen in the BSRBR database and used by the Assessment Group. To illustrate this, if the HAQ reduction values assumed by AbbVie were applied, a patient with an initial HAQ of 2.0 would, following an ACR50–70 response, be assumed to have a HAQ of 0.77 (a reduction of 1.23); contrastingly, the BSRBR data show that on average the HAQ reduction following a good response is 0.67, resulting in a HAQ of 1.33. The use of greater HAQ reductions for bDMARDs will have the effect of reducing the ICER, as HAQ is linked to utility and to disease-related cost. It is noted that the HAQ changes assumed by the companies are typically taken from relatively small RCTs whereas the Assessment Group has used a large UK database.
Additionally, not all submissions have assumed that HAQ score will affect mortality. The approach taken within the Assessment Group model is that people with greater HAQ score on model entry will die, on average, earlier than those with lower HAQ scores. If such an assumption was not applied, this would be expected to have the impact of reducing the ICER, owing to the greater number of years the most severe patients lived, with both low utility and high disease-related costs and the likely difference in HAQ progression between those on cDMARDs and those on bDMARDs.
These reasons lead the Assessment Group to believe that there is no large unexplainable difference between its model and those submitted by the companies. Although other differences exist, for example the Assessment Group model is EULAR based, whereas the majority of company models are ACR based, it is not clear that such differences would represent a systematic difference in the comparative ICERs.
Generalisability of results
There is no reason to believe that the results detailed in this report are not generalisable to the English and Welsh populations.
Strengths and limitations of analysis
A strength of this report is that a systematic review of RCTs for bDMARDs in bDMARD-naive patients has been conducted. The primary outcome measures are EULAR or ACR response at 6 months and a formal NMA has been conducted to assess relative effectiveness. Different analyses have been undertaken to assess the impact of including RCTs with a small proportion of patients with prior bDMARD use, and/or including RCTs when patients may not have received adequate prior MTX treatment.
A major strength of the analyses presented is that the Assessment Group has constructed a EULAR-based model that is much more appropriate to practice in England and Wales than previous ACR-based models. Estimates of ICERs for both EULAR data only and when mapping ACR data to EULAR data indicate that the conclusions were not altered by restricting the selection of RCTs to only those that reported EULAR data.
An additional strength is that large observational databases were used to generate data on parameters such as HAQ change conditional on EULAR response and HAQ progression while on cDMARDs. This is preferable to data taken from relatively small RCTs of limited follow-up.
The model has known limitations. The plausible reduced efficacy of treatments when used subsequent to other treatments has not been formally incorporated. It is expected that this omission will favour bDMARDs. Additionally, the effects of non-adherence to NICE guidelines (as shown in the BSRBR) have not formally been incorporated; it is expected that, were this included, then the ICERs for bDMARDs compared with cDMARDs would increase and disfavour bDMARDs.
Lost productivity has not been included in the model, which could favour bDMARDs if it were included. However, an estimation of any lost productivity gains associated with technologies not funded because of the purchase of bDMARDs would be required to produce a definitive conclusion on the effect on the ICER.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Beyond potential impact on expenditure there is unlikely to be any major implications for the NHS as the interventions are largely s.c. and self-administered. The implications for expenditure are uncertain as it will be heavily dependent on the guidance produced by NICE.
Chapter 6 Discussion
Statement of principal findings
Although there was uncertainty in, and overlap between, the effects of treatment on ACR for interventions for patients in population 1, IFX plus MTX was associated with the biggest increase in response rate and this was likely to be the most effective intervention. Other interventions were less effective and appeared to fall into three groups: intensive cDMARDs and ADA plus MTX; ETN, GOL plus MTX and step-up combination cDMARDs; ADA and cDMARDs.
Although there was uncertainty in, and overlap between, the effects of treatment on EULAR for interventions in populations 2 and 3 in the main trials, ETN plus MTX and TCZ plus MTX were associated with the biggest increase in response rate. Other interventions were less effective and appeared to fall into two groups: (1) TCZ, GOL plus MTX, ADA plus MTX, ABT i.v. plus MTX and grouped biologics; and (2) ETN, IFX plus MTX, ADA and intensive cDMARDs. The inclusion of the additional studies in which patients received prior biologics resulted in broadly the same groupings, although CTZ plus MTX was associated with an even bigger response than ETN plus MTX and TCZ plus MTX.
Although there was uncertainty in, and overlap between, the effects of treatment on ACR for interventions in populations 2 and 3 in the main trials, ETN plus MTX, TCZ and TCZ plus MTX were associated with the biggest increase in response rate. Other interventions were less effective and appeared to fall into two groups: (1) ETN, GOL plus MTX, ABT s.c. plus MTX, ADA plus MTX, IFX plus MTX and ABT i.v. plus MTX; and (2) CTZ plus MTX, intensive cDMARDs and ADA. The inclusion of the additional studies in which patients received prior biologics suggested that CTZ plus MTX and ETN plus MTX resulted in the highest response rates. Other interventions appeared to give rise to broadly similar and slightly smaller response rates except for intensive cDMARDs and ADA which are associated with even smaller response rates.
The Assessment Group believes the ICERs for bDMARDs used in MTX-experienced patients with severe RA is credibly > £40,000 per QALY gained when compared with a cDMARD-alone strategy. These values are higher (£50,000) for moderate RA patients, higher for patients who cannot receive MTX and higher when bDMARDs were used before cDMARDs (£60,000), although this last estimate is likely to be favourable to bDMARDs owing to the assumption of HAQ progression while on intensive cDMARDs.
These estimates are lower if a different assumption, used in previous NICE appraisals regarding HAQ progression on cDMARDs, is adopted; however, the Assessment Group found few data to support this historic assumption.
The analyses have assumed that the discontinuation rule specified by NICE has been strictly adhered to: data from the BSRBR show that this is not the case. If such non-adherence continues the ICERs will be considerably higher than those presented. Analysis of the impact has not been undertaken owing to the possibility of back-calculation of CiC discounts offered through PASs.
Strengths and limitations of the assessment
A strength of this report is that a systematic review of RCTs for bDMARDs in bDMARD-naive patients has been conducted. The primary outcome measures are EULAR or ACR response at 6 months and a formal NMA has been conducted to assess relative efficacy. Different analyses have been undertaken to assess the impact of including RCTs with a small proportion on patients with prior bDMARD use, and/or including RCTs when patients may not have received adequate prior MTX treatment.
A major strength of the analyses presented is that the Assessment Group has constructed a EULAR-based model that is much more appropriate to practice in England and Wales than previous ACR-based models. Estimates of ICERs both for EULAR data only and when mapping ACR data to EULAR data indicate that the conclusions were not altered by restricting the selection of RCTs to those that reported EULAR data.
An additional strength is that large observational databases were used to generate data on parameters such as HAQ change conditional on EULAR response and HAQ progression while on cDMARDs. This is preferable to data taken from relatively small RCTs with limited follow-up.
The model has known limitations. The plausible reduced efficacy of treatments when used subsequent to other treatments has not been formally incorporated. It is expected that this omission will favour bDMARDs. Additionally, the effects of non-adherence to NICE guidelines (as shown in the BSRBR) have not formally been incorporated; it is expected that were this included then the ICERs for bDMARDs compared with cDMARDs would increase and disfavour bDMARDs.
Lost productivity has not been included in the model, although the impact of lost productivity in those interventions displaced due to purchasing bDMARDs is unknown.
Uncertainties
A key uncertainty relating to the cost-effectiveness results is related to the HAQ progression while on cDMARDs. This has been shown to have a large influence on the results, particularly for patients with moderate RA and those who cannot receive MTX. The relationship between HAQ and pain can also greatly influence the ICER, as is currently uncertain, with two large observational databases providing different estimated relationships.
Chapter 7 Conclusions
Implications for service provision
The implications for service provision are unclear and would be dependent on the final guidance issued by NICE. The majority of interventions are administered subcutaneously by the patient or family member, although it is possible that requirements for infusions or for district nurse time are affected conditional on the final guidance.
Suggested research priorities
In order to provide a more accurate estimate of the cost-effectiveness of bDMARDs the following research priorities are suggested by the Assessment Group. These aim to establish:
-
the evaluation of the long-term HAQ trajectory while on cDMARDs
-
the relationship between HAQ and utility
-
the relationship between HAQ and hospital costs consumed
-
the relationship between HAQ and pain
-
the relative efficacy of bDMARDs assessed through head-to-head RCTs, although it is acknowledged that this is unlikely to occur owing to the large-scale, costly RCTs that would be required
-
the relative efficacy of bDMARDs when used after a previous bDMARD and/or RTX compared with bDMARD naive
-
the relative efficacy of cDMARDs when used after a bDMARD and/or RTX compared with bDMARD naive
-
whether or not bDMARDs could be stopped once a patient has achieved a stated target (e.g. remission).
Acknowledgements
The authors wish to thank the BSRBR for providing access to their data and expert advice on how to use it, in particular Rebecca Davies, Xuejuan Fan, Kath Watson and Kimme Hyrich; Sam Norton for providing data and expert analyses from the ERAS data set; the VARA database for providing access to their data, and Kaleb Michaid for performing analyses on those data. The authors wish to thank Alan Brennan, Louise Preston and Colin Angus for advice and help throughout the project.
The authors would also like to thank Gill Rooney and Andrea Shippam for providing administrative support, help in preparing and formatting the report. Gill Rooney also assisted with digitising curves from published papers.
Contributions of authors
Matt Stevenson led the project and was involved in all aspects of the project.
Rachel Archer led the systematic review along with Emma Simpson and Emma Everson-Hock.
Jon Tosh constructed the mathematical model and undertook the review of economic evaluations.
John Stevens undertook the NMA.
Monica Hernandez-Alava, along with Allan Wailoo, formulated statistical models based on these data.
Suzy Paisley and Kath Dickinson formulated and ran the search strategies.
David Scott and Adam Young provided clinical advice.
Allan Wailoo provided advice throughout the project, liaised with registry holders, commented on elements of the manufacturer submissions and provided a detailed account of the utility mapping models.
About the School of Health and Related Research
The ScHARR is one of the nine departments that constitute the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research, and the application of health economics and decision science to the development of health services and the improvement of public health.
The ScHARR Technology Assessment Group synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the National institute for Health Research HTA programme on behalf of a range of policy-makers, including NICE. ScHARR Technology Assessment Group is part of a wider collaboration of a number of units from other regions including Health Economics Research Unit and Health Services Research Unit, University of Aberdeen; Southampton HTA Centre, University of Southampton; Liverpool Reviews and Implementation Group, University of Liverpool; Peninsular Technology Assessment Group, University of Exeter; the NHS Centre for Reviews and Dissemination, University of York; Warwick Evidence, University of Warwick; the BMJ Technology Assessment Group, BMJ Evidence Centre; and Kleijnen Systematic Reviews Ltd.
Data sharing statement
Data can be obtained from the corresponding author subject to them being non-confidential.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Scott DL, Steer S. The course of established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:943-67. http://dx.doi.org/10.1016/j.berh.2007.05.006.
- Pincus T, Fuchs HA, Callahan LF, Nance EP, Kaye JJ. Early radiographic joint space narrowing and erosion and later malalignment in rheumatoid arthritis: a longitudinal analysis. J Rheumatol 1998;25:636-40.
- Drossaers-Bakker KW, Kroon HM, Zwinderman AH, Breedveld FC, Hazes JM. Radiographic damage of large joints in long-term rheumatoid arthritis and its relation to function. Rheumatology 2000;39:998-1003. http://dx.doi.org/10.1093/rheumatology/39.9.998.
- Allaire S, Wolfe F, Niu J, LaValley MP, Zhang B, Reisine S. Current risk factors for work disability associated with rheumatoid arthritis: recent data from a US national cohort. Arthritis Rheum 2009;61:321-8. http://dx.doi.org/10.1002/art.24281.
- Naz SM, Symmons DP. Mortality in established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:871-83. http://dx.doi.org/10.1016/j.berh.2007.05.003.
- Dadoun S, Zeboulon-Ktorza N, Combescure C, Elhai M, Rozenberg S, Gossec L, et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Joint Bone Spine 2013;80:29-33. http://dx.doi.org/10.1016/j.jbspin.2012.02.005.
- Meune C, Touze E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology 2009;48:1309-13. http://dx.doi.org/10.1093/rheumatology/kep252.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315-24. http://dx.doi.org/10.1002/art.1780310302.
- Rheumatoid Arthritis: National Clinical Guideline for Management and Treatment in Adults. London: Royal College of Physicians; 2009.
- Combe B, Landewe R, Lukas C, Bolosiu HD, Breedveld F, Dougados M, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66:34-45. http://dx.doi.org/10.1136/ard.2005.044354.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580-8. http://dx.doi.org/10.1136/ard.2010.138461.
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727-35. http://dx.doi.org/10.1002/art.1780380602.
- van Gestel AM, Prevoo ML, van‘t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 1996;39:34-40. http://dx.doi.org/10.1002/art.1780390105.
- Felson DT. Assessing the efficacy and safety of rheumatic disease treatments: obstacles and proposed solutions. Arthritis Rheum 2003;48:1781-7. http://dx.doi.org/10.1002/art.11087.
- Prevoo M, van’t Hof M, Kuper H, Van Leeuwen M, van de Putte L, van Riel P. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44-8. http://dx.doi.org/10.1002/art.1780380107.
- van Gestel AM, Anderson JJ, van Riel PL, Boers M, Haagsma CJ, Rich B, et al. ACR and EULAR improvement criteria have comparable validity in rheumatoid arthritis trials. American College of Rheumatology European League of Associations for Rheumatology. J Rheumatol 1999;26:705-11.
- Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ). Clin Exp Rheumatol 2005;23:S14-18.
- Symmons D, Turner G, Webb R, Asten P, Barrett EM, Lunt M, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology 2002;41:793-800. http://dx.doi.org/10.1093/rheumatology/41.7.793.
- Symmons DP, Barrett EM, Bankhead CR, Scott DG, Silman AJ. The incidence of rheumatoid arthritis in the United Kingdom: results from the Norfolk Arthritis Register. Br J Rheumatol 1994;33:735-9. http://dx.doi.org/10.1093/rheumatology/33.8.735.
- Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 2005;4:130-6. http://dx.doi.org/10.1016/j.autrev.2004.09.002.
- Lugmani R, Hennell S, Estrach C, Birrell F, Bosworth A, Davenport G, et al. British Society for Rheumatology and British Health Professionals in Rheumatology Guideline for the management of rheumatoid arthritis (the first 2 years). Rheumatology 2006;45:1167-9. http://dx.doi.org/10.1093/rheumatology/kel215a.
- National Institute for Health and Care Excellence . Adalimumab, Etanercept and Infliximab for the Treatment of Rheumatoid Arthritis 2007. www.nice.org.uk/guidance/TA130 (accessed 30 July 2013).
- National Institute for Health and Care Excellence . Certolizumab Pegol for the Treatment of Rheumatoid Arthritis 2010. www.nice.org.uk/guidance/TA186 (accessed 2 August 2013).
- National Institute for Health and Care Excellence . Golimumab for the Treatment of Rheumatoid Arthritis After the Failure of Previous Disease-Modifying Anti-Rheumatic Drugs 2011. www.nice.org.uk/guidance/TA225 (accessed 30 July 2013).
- National Institute for Health and Care Excellence . Rheumatoid Arthritis (Methotrexate-Naïve) – Golimumab (Terminated Appraisal): Guidance 2011. www.nice.org.uk/guidance/TA224 (accessed 30 July 2013).
- National Institute for Health and Care Excellence . Tocilizumab for the Treatment of Rheumatoid Arthritis (Rapid Review of Technology Appraisal Guidance 198) 2012. www.nice.org.uk/guidance/ta247 (accessed 31 July 2013).
- National Institute for Health and Care Excellence . Abatacept for Treating Rheumatoid Arthritis After the Failure of Conventional Disease-Modifying Anti-Rheumatic Drugs (Rapid Review of Technology Appraisal Guidance 234) 2013. www.nice.org.uk/guidance/TA280 (accessed 2 August 2013).
- National Institute for Health and Care Excellence . Adalimumab, Etanercept, Infliximab, Rituximab and Abatacept for the Treatment of Rheumatoid Arthritis After the Failure of a TNF Inhibitor 2010. www.nice.org.uk/guidance/ta195 (accessed 2 August 2013).
- NICE Protocol – Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Tocilizumab and Abatacept for the Treatment of Rheumatoid Arthritis not Previously Treated with Disease – Modifying Anti-Rheumatic Drugs and After the Failure of Conventional Disease-Modifying Anti-Rheumatic Drugs Only (Review of Technology Appraisal Guidance 130, 186, 224, 234 and a Part Review of Technology Appraisal Guidance 225 and 247). Sheffield: University of Sheffield; 2012.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2012.
- Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Were SR. Hedges Team . Optimal search strategies for retrieving scientifically strong studies of treatment from MEDLINE: analytical survey. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38446.498542.8F.
- Montori VM, Wilczynski NL, Morgan D, Haynes RB. Optimal search strategies for retrieving systematic reviews from MEDLINE: analytical survey. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38336.804167.47.
- Wong SS, Wilczynski NL, Haynes RB. Optimal CINAHL search strategies for identifying therapy studies and review articles. J Nurs Scholarsh 2006;38:194-9. http://dx.doi.org/10.1111/j.1547-5069.2006.00100.x.
- Wilczynski NL, Haynes RB. EMBASE search strategies achieved high sensitivity and specificity for retrieving methodologically sound systematic reviews. J Clin Epidemiol 2007;60:29-33. http://dx.doi.org/10.1016/j.jclinepi.2006.04.001.
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc 2006;94:41-7.
- Lefebvre C, Manheimer E, Glanville J, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. n.d.
- Golder S, Mcintosh HM, Duffy S, Glanville J. Developing efficient search strategies to identify reports of adverse effects in MEDLINE and EMBASE. Health Info Libr J 2006;23:3-12. http://dx.doi.org/10.1111/j.1471-1842.2006.00634.x.
- National Institute for Health and Care Excellence . Abatacept for the Treatment of Rheumatoid Arthritis After the Failure of Conventional Disease Modifying Anti-Rheumatic Drugs 2011. www.nice.org.uk/guidance/ta234 (accessed 5 July 2013).
- Grey Matters: A Practical Search Tool for Evidence-Based Medicine. Ottawa, ON: CADTH; 2013.
- National Institute for Health and Care Excellence . Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Abatacept and Tocilizumab for the Treatment of Rheumatoid Arthritis (Review of TA Guidance 130, 186, 224, 234 and Part Review of TA Guidance 225 and 247) Final Scope 2012. www.nice.org.uk/guidance/TA375/documents/rheumatoid-arthritis-adalimumab-etanercept-infliximab-certolizumab-pegol-golimumab-abatacept-and-tocilizumab-review-final-scope-2 (accessed 5 July 2013).
- Electronic Medicines Compendium . Summary of Product Characteristics – Cimzia (Certolizumab) 2013. www.medicines.org.uk/emc/medicine/22323/SPC/Cimzia+200+mg+solution+for+injection/ (accessed 5 July 2013).
- Electronic Medicines Compendium . Summary of Product Characteristics – Enbrel (Etanercept) 2012. www.medicines.org.uk/emc/searchresults.aspx?term=Enbrel&searchtype=QuickSearch (accessed 5 July 2013).
- Electronic Medicines Compendium . Summary of Product Characteristics – HUMIRA (Adalimumab) 2013. www.medicines.org.uk/emc/medicine/21201/SPC/Humira+Pre-filled+Pen%2c+Pre-filled+Syringe+and+Vial/ (accessed 5 July 2013).
- Electronic Medicines Compendium . Summary of Product Characteristics – Orencia® (Abatacept) 2012. www.medicines.org.uk/emc/medicine/27216/SPC/ORENCIA+125+mg+solution+for+injection+(pre-filled+syringe)/ (accessed 5 July 2013).
- Electronic Medicines Compendium . Summary of Product Characteristics – Remicade (Infliximab) 2013. www.medicines.org.uk/emc/medicine/3236/SPC/Remicade+100mg+powder+for+concentrate+for+solution+for+infusion/ (accessed 5 July 2013).
- Electronic Medicines Compendium . Summary of Product Characteristics – Simponi (Golimumab) 2013. www.medicines.org.uk/emc/medicine/23766/SPC/Simponi+50+mg+solution+for+injection/ (accessed 5 July 2013).
- Electronic Medicines Compendium . Summary of Product Characteristics. RoActemra (Tocilizumab) 2013. www.medicines.org.uk/emc/medicine/22311/SPC/RoActemra+20mg+ml+Concentrate+for+Solution+for+Infusion/ (accessed 5 July 2013).
- Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care. York: University of York; 2009.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
- Karsh J, Keystone EC, Haraoui B, Thorne JC, Pope JE, Bykerk VP, et al. Canadian recommendations for clinical trials of pharmacologic interventions in rheumatoid arthritis: inclusion criteria and study design. J Rheumatol 2011;38:2095-104. http://dx.doi.org/10.3899/jrheum.110188.
- Thorlund K, Druyts E, Avina-Zubieta JA, Wu P, Mills EJ. Why the findings of published multiple treatment comparison meta-analyses of biologic treatments for rheumatoid arthritis are different: an overview of recurrent methodological shortcomings. Ann Rheum Dis 2012;72:1524-35. http://dx.doi.org/10.1136/annrheumdis-2012-201574.
- Dias S, Welton N, Sutton A, Ades A. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. London: NICE; 2011.
- Moreland LW, O’Dell JR, Paulus HE, Curtis JR, Bathon JM, Clair EW, et al. A randomised comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheum 2012;64:2824-35. http://dx.doi.org/10.1002/art.34498.
- Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675-81. http://dx.doi.org/10.1016/S0140-6736(04)15640-7.
- Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 2010;69:88-96. http://dx.doi.org/10.1136/ard.2008.105197.
- Abe T, Takeuchi T, Miyasaka N, Hashimoto H, Kondo H, Ichikawa Y, et al. A multicenter, double-blind, randomised, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. J Rheumatol 2006;33:37-44.
- Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan PG, Mola EM, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis 2013;72:43-50. http://dx.doi.org/10.1136/annrheumdis-2011-201282.
- Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 2013;381:1541-50. http://dx.doi.org/10.1016/S0140-6736(13)60250-0.
- van Riel PL, Taggart AJ, Sany J, Gaubitz M, Nab HW, Pedersen R, et al. Efficacy and safety of combination etanercept and methotrexate versus etanercept alone in patients with rheumatoid arthritis with an inadequate response to methotrexate: the ADORE study. Ann Rheum Dis 2006;65:1478-83. http://dx.doi.org/10.1136/ard.2005.043299.
- van Riel PLCM, Freundlich B, MacPeek D, Pedersen R, Foehl JR, Singh A. Patient-reported health outcomes in a trial of etanercept monotherapy versus combination therapy with etanercept and methotrexate for rheumatoid arthritis: the ADORE trial. Ann Rheum Dis 2008;67:1104-10. http://dx.doi.org/10.1136/ard.2006.068585.
- Russell AS, Wallenstein GV, Li T, Martin MC, MacLean R, Blaisdell B, et al. Abatacept improves both the physical and mental health of patients with rheumatoid arthritis who have inadequate response to methotrexate treatment. Ann Rheum Dis 2007;66:189-94. http://dx.doi.org/10.1136/ard.2006.057018.
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomised trial. Ann Intern Med 2006;144:865-76. http://dx.doi.org/10.7326/0003-4819-144-12-200606200-00003.
- Kremer J, Russell AS, Westhovens R, Teng J, Rosenblatt L, Emery P. Sustained and clinically meaningful improvements in both day-and night-time aspects of HRQoL are observed with abatacept treatment in patients with rheumatoid arthritis (RA) and previous inadequate response to MTX: 5-year data from the AIM trial. Arthritis Rheum 2010;62.
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Results of a two-year followup study of patients with rheumatoid arthritis who received a combination of abatacept and methotrexate. Arthritis Rheum 2008;58:953-63. http://dx.doi.org/10.1002/art.23397.
- Kremer JM, Russell AS, Emery P, Abud-Mendoza C, Szechinski J, Westhovens R, et al. Long-term safety, efficacy and inhibition of radiographic progression with abatacept treatment in patients with rheumatoid arthritis and an inadequate response to methotrexate: 3-year results from the AIM trial. Ann Rheum Dis 2011;70:1826-30. http://dx.doi.org/10.1136/ard.2010.139345.
- Fleischmann R, Schiff MH, Weinblatt ME, Maldonado MA, Massarotti EM, Yazici Y. Effects of subcutaneous abatacept or adalimumab on remission and associated changes in physical function and radiographic outcomes: one year results from the ample (abatacept versus adalimumab comparison in biologic-naive RA subjects with background methotrexate) trial. Arthritis Rheum 2012;64.
- Kim H-Y, Hsu P-N, Barba M, Sulaiman W, Robertson D, Vlahos B, et al. Randomised comparison of etanercept with usual therapy in an Asian population with active rheumatoid arthritis: the APPEAL trial. Int J Rheum Dis 2012;15:188-96. http://dx.doi.org/10.1111/j.1756-185X.2011.01680.x.
- Bae SC, Gun SC, Mok CC, Khandker R, Nab HW, Koenig AS, et al. Improved health outcomes with Etanercept versus usual DMARD therapy in an Asian population with established rheumatoid arthritis. BMC Musculoskelet Disord 2013;14. http://dx.doi.org/10.1186/1471-2474-14-13.
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumour necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003;48:35-4. http://dx.doi.org/10.1002/art.10697.
- Weinblatt ME, Keystone EC, Furst DE, Kavanaugh AF, Chartash EK, Segurado OG. Long term efficacy and safety of adalimumab plus methotrexate in patients with rheumatoid arthritis: ARMADA 4 year extended study. Ann Rheum Dis 2006;65:753-9. http://dx.doi.org/10.1136/ard.2005.044404.
- St Clair EW, van der Heijde DMFM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomised, controlled trial. Arthritis Rheum 2004;50:3432-43. http://dx.doi.org/10.1002/art.20568.
- Conaghan P, Durez P, Alten RE, Burmester GR, Tak PP, Klareskog L, et al. Impact of intravenous abatacept on synovitis, osteitis and structural damage in patients with rheumatoid arthritis and an inadequate response to methotrexate: the ASSET randomised controlled trial. Ann Rheum Dis 2013;72:1287-94. http://dx.doi.org/10.1136/annrheumdis-2012-201611.
- Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: a one-year randomised, placebo-controlled study. Arthritis Rheum 2006;54:2807-16. http://dx.doi.org/10.1002/art.22070.
- Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 2008;67:1096-103. http://dx.doi.org/10.1136/ard.2007.080002.
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet 1999;354:1932-9. http://dx.doi.org/10.1016/S0140-6736(99)05246-0.
- van Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomised, placebo-controlled trial. Arthritis Rheum 2011;63:1782-92. http://dx.doi.org/10.1002/art.30372.
- Bejarano V, Quinn M, Conaghan PG, Reece R, Keenan A-M, Walker D, et al. Effect of the early use of the anti-tumour necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritis. Arthrit Care Res 2008;59:1467-74. http://dx.doi.org/10.1002/art.24106.
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van ZD, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeST study): a randomised, controlled trial. Arthritis Rheum 2008;52:3381-90. http://dx.doi.org/10.1002/art.21405.
- Smolen J, Emery P, Ferraccioli G. Efficacy and safety of certolizumab pegol after incomplete response to DMARDS in RA patients with low moderate disease activity: results from certain, a phase IIIb study. Ann Rheum Dis 2011;70. http://dx.doi.org/10.1136/annrheumdis-2013-204632.
- Miyasaka N. Clinical investigation in highly disease-affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Mod Rheumatol 2008;18:252-62. http://dx.doi.org/10.3109/s10165-008-0045-0.
- Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375-82. http://dx.doi.org/10.1016/S0140-6736(08)61000-4.
- Emery P, Breedveld F, van der Heijde D, Ferraccioli G, Dougados M, Robertson D, et al. Two-year clinical and radiographic results with combination etanercept-methotrexate therapy versus monotherapy in early rheumatoid arthritis: a two-year, double-blind, randomised study. Arthritis Rheum 2010;62:674-82. http://dx.doi.org/10.1002/art.27268.
- Kekow J, Moots RJ, Emery P, Durez P, Koenig A, Singh A, et al. Patient-reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Ann Rheum Dis 2010;69:222-5. http://dx.doi.org/10.1136/ard.2008.102509.
- Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumour necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomised, placebo-controlled, 52-week trial. Arthritis Rheum 2004;50:1400-11. http://dx.doi.org/10.1002/art.20217.
- deFilippis L, Caliri A, Anghelone S, Scibilia G, Lo Gullo R, Bagnato G. Improving outcomes in tumour necrosis factor a treatment: comparison of the efficacy of the tumour necrosis factor a blocking agents etanercept and infliximab in patients with active rheumatoid arthritis. Panminerva Med 2006;48:129-35.
- Durez P, Nzeusseu TA, Lauwerys BR, Manicourt DH, Verschueren P, Westhovens R, et al. A randomised comparative study of the short term clinical and biological effects of intravenous pulse methylprednisolone and infliximab in patients with active rheumatoid arthritis despite methotrexate treatment. Ann Rheum Dis 2004;63:1069-74. http://dx.doi.org/10.1136/ard.2003.012914.
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000;343:1586-93. http://dx.doi.org/10.1056/NEJM200011303432201.
- Combe B, Codreanu C, Fiocco U, Gaubitz M, Geusens PP, Kvien TK, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis 2006;65:1357-62. http://dx.doi.org/10.1136/ard.2005.049650.
- Combe B, Codreanu C, Fiocco U, Gaubitz M, Geusens PP, Kvien TK, et al. Efficacy, safety and patient-reported outcomes of combination etanercept and sulfasalazine versus etanercept alone in patients with rheumatoid arthritis: a double-blind randomised 2-year study. Ann Rheum Dis 2009;68:1146-52. http://dx.doi.org/10.1136/ard.2007.087106.
- Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, et al. Golimumab, a human anti-tumour necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomised, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum 2009;60:2272-83. http://dx.doi.org/10.1002/art.24638.
- Tanaka Y, Harigai M, Takeuchi T, Yamanaka H, Ishiguro N, Yamamoto K, et al. Golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis: results of the GO-FORTH study. Ann Rheum Dis 2012;71:817-24. http://dx.doi.org/10.1136/ard.2011.200317.
- Keystone E, Genovese MC, Klareskog L, Hsia EC, Hall S, Miranda PC, et al. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52-week results of the GO-FORWARD study. Ann Rheum Dis 2010;69:1129-35. http://dx.doi.org/10.1136/ard.2009.116319.
- Soubrier M, Puechal X, Sibilia J, Mariette X, Meyer O, Combe B, et al. Evaluation of two strategies (initial methotrexate monotherapy vs its combination with adalimumab) in management of early active rheumatoid arthritis: data from the GUEPARD trial. Rheumatology 2009;48:1429-34. http://dx.doi.org/10.1093/rheumatology/kep261.
- Detert J, Bastian H, Listing J, Weis A, Wassenberg S, Liebhaber A, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis 2013;72:844-50. http://dx.doi.org/10.1136/annrheumdis-2012-201612.
- Nam JL, Villeneuve E, Conaghan PG, Hensor E, Keen HI, Amarasena R, et al. A preliminary report of remission induction with two therapeutic strategies with infliximab or high dose intravenous steroids for the treatment of rheumatoid arthritis. Arthritis Rheum 2011;63.
- Keystone EC, Wang MM, Layton M, Hollis S, McInnes IB. Study Team . Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann Rheum Dis 2012;71:1630-5. http://dx.doi.org/10.1136/annrheumdis-2011-143578.
- Kameda H, Ueki Y, Saito K, Nagaoka S, Hidaka T, Atsumi T, et al. Etanercept (ETN) with methotrexate (MTX) is better than ETN monotherapy in patients with active rheumatoid arthritis despite MTX therapy: a randomised trial. Mod Rheumatol 2010;20:531-8. http://dx.doi.org/10.3109/s10165-010-0324-4.
- Kay J, Matteson EL, Dasgupta B, Nash P, Durez P, Hall S, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomised, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum 2008;58:964-75. http://dx.doi.org/10.1002/art.23383.
- Kim H-Y, Lee S-K, Song YW, Yoo D-H, Koh E-M, Yoo B, et al. A randomised, double-blind, placebo-controlled, phase III study of the human anti-tumour necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with methotrexate. Int J Rheum Dis 2007;10:9-16.
- Kume K, Amano K, Yamada S, Hatta K, Ohta H, Kuwaba N. Tocilizumab monotherapy reduces arterial stiffness as effectively as etanercept or adalimumab monotherapy in rheumatoid arthritis: an open-label randomised controlled trial. J Rheumatol 2011;38:2169-71. http://dx.doi.org/10.3899/jrheum.110340.
- Lan J-L, Chou S-J, Chen D-Y, Chen Y-H, Hsieh T-Y, Young J. A comparative study of etanercept plus methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis: a 12-week, double-blind, randomised, placebo-controlled study. J Formos Med Assoc 2004;103:618-23.
- Machado D, Guzman R, Xavier R, Simon J, Ferdousi T, Pedersen R, et al. Combination Etanercept and Methotrexate Therapy Provides Better Outcomes Than Standard DMARD and Methotrexate Therapy in Rheumatoid Arthritis: Results From a Study in the Latin America Region n.d.
- Mcinnes I, Lee JS, Wu W, Giles JT, Bathon JM, Salmon JE, et al. MEASURE: a translational, randomised, placebo (PBO)-controlled study to evaluate the effects of tocilizumab (TCZ) on parameters of lipids and inflammation. Ann Rheum Dis 2011;70.
- Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis: a randomised, controlled trial. Ann Intern Med 1999;130:478-86. http://dx.doi.org/10.7326/0003-4819-130-6-199903160-00004.
- Mathias SD, Colwell HH, Miller DP, Moreland LW, Buatti M, Wanke L. Health-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin Ther 2000;22:128-39. http://dx.doi.org/10.1016/S0149-2918(00)87984-9.
- Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Treatment of rheumatoid arthritis with humanised anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2004;50:1761-9. http://dx.doi.org/10.1002/art.20303.
- Hørslev-Petersen K. Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA study: an investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled trial. Ann Rheum Dis 2014;73:654-61. http://dx.doi.org/10.1136/annrheumdis-2012-202735.
- Kavanaugh A, Fleischmann RM, Emery P, Kupper H, Redden L, Guerette B, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis 2013;72:64-71. http://dx.doi.org/10.1136/annrheumdis-2011-201247.
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: a multicenter, randomised, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26-37. http://dx.doi.org/10.1002/art.21519.
- Quinn MA, Conaghan PG, O’Connor PJ, Karim Z, Greenstein A, Brown A, et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomised, double-blind, placebo-controlled trial. Arthritis Rheum 2005;52:27-35. http://dx.doi.org/10.1002/art.20712.
- O’Dell JR, Mikuls TR, Taylor T, Ahluwalia V, Brophy M, Warren S, et al. Rheumatoid arthritis comparison of active therapies in methotrexate suboptimal responders: validation of the strategy of conventional disease modifying anti-rheumatic drugs before biologicals. Arthritis Rheum 2012;64.
- O’Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, Warren SR, et al. Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med 2013;369:307-18. http://dx.doi.org/10.1056/NEJMoa1303006.
- Weinblatt ME, Fleischmann R, van Vollenhoven R, Emery P, Huizinga TWJ, Goldermann R, et al. Certolizumab pegol in patients with active rheumatoid arthritis aligned with NICE guidance for anti-TNF therapy: post-HOC analyses of the realistic phase IIIB randomised controlled study. Rheumatology 2012;51.
- Jobanputra P, Maggs F, Deeming A, Carruthers D, Rankin E, Jordan AC, et al. A randomised efficacy and discontinuation study of etanercept versus adalimumab (RED SEA) for rheumatoid arthritis: a pragmatic, unblinded, non-inferiority study of first TNF inhibitor use: outcomes over 2 years. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2012-001395.
- Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an X ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis 2007;66:1162-7. http://dx.doi.org/10.1136/ard.2006.068064.
- Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol 2009;19:12-9. http://dx.doi.org/10.3109/s10165-008-0125-1.
- Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti-tumour necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of star (safety trial of adalimumab in rheumatoid arthritis). J Rheumatol 2003;30:2563-71.
- Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomised, placebo-controlled trial. Arthritis Rheum 2006;54:1075-86. http://dx.doi.org/10.1002/art.21734.
- van Vollenhoven RF, Ernestam S, Geborek P, Petersson IF, Coster L, Waltbrand E, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet 2009;374:459-66. http://dx.doi.org/10.1016/S0140-6736(09)60944-2.
- Durez P, Malghem J, Toukap AN, Depresseux G, Lauwerys BR, Westhovens R, et al. Treatment of early rheumatoid arthritis: a randomised magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis Rheum 2007;56:3919-27. http://dx.doi.org/10.1002/art.23055.
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968-80. http://dx.doi.org/10.1002/art.23940.
- Van De Putte LBA, Atkins C, Malaise M, Sany J, Russell AS, Van Riel PLCM, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis 2004;63:508-16. http://dx.doi.org/10.1136/ard.2003.013052.
- Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10420.
- Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumour necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340:253-9. http://dx.doi.org/10.1056/NEJM199901283400401.
- Wong M, Oakley SP, Young L, Jiang BY, Wierzbicki A, Panayi G, et al. Infliximab improves vascular stiffness in patients with rheumatoid arthritis. Ann Rheum Dis 2009;68:1277-84. http://dx.doi.org/10.1136/ard.2007.086157.
- Zhang F-C, Hou Y, Huang F, Wu D-H, Bao C-D, Ni L-Q, et al. Infliximab versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a preliminary study from China. Int J Rheum Dis 2006;9:127-30. http://dx.doi.org/10.1111/j.1479-8077.2006.00186.x.
- Genovese MC, Covarrubias A, Leon G, Mysler E, Keiserman M, Valente R, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum 2011;63:2854-64. http://dx.doi.org/10.1002/art.30463.
- Jones G. The AMBITION trial: tocilizumab monotherapy for rheumatoid arthritis. Expert Rev Clin Immunol 2010;6:189-95. http://dx.doi.org/10.1586/eci.10.2.
- Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and Safety of Certolizumab Pegol Plus Methotrexate in Japanese Rheumatoid Arthritis Patients With an Inadequate Response to Methotrexate n.d.
- Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland A-M, Vernon E, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomised placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011;63:609-21. http://dx.doi.org/10.1002/art.30158.
- Kremer JM, Dougados M, Emery P, Durez P, Sibilia J, Shergy W, et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve-month results of a phase IIb, double-blind, randomised, placebo-controlled trial. Arthritis Rheum 2005;52:2263-71. http://dx.doi.org/10.1002/art.21201.
- Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987-97. http://dx.doi.org/10.1016/S0140-6736(08)60453-5.
- van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Meijide JAG, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508-19. http://dx.doi.org/10.1056/NEJMoa1112072.
- Kang Y, Park W, Park Y, Choe J, Bae S-C. Efficacy and safety of certolizumab pegol (CZP) with concomitant methotrexate (MTX) in Korean rheumatoid arthritis (RA) patients (PTS) with an inadequate response to MTX. Ann Rheum Dis 2012;71. http://dx.doi.org/10.1136/annrheumdis-2012-eular.501.
- Keystone E, van der Heijde D, Mason J, Landewe R, van Vollenhoven R, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomised, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 2008;58:3319-29. http://dx.doi.org/10.1002/art.23964.
- Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009;68:797-804. http://dx.doi.org/10.1136/ard.2008.101659.
- Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012;64:970-81. http://dx.doi.org/10.1002/art.33419.
- van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four month phase III randomised radiographic study. Arthritis Rheum 2013;65:559-70. http://dx.doi.org/10.1002/art.37816.
- Bathon JM, Genovese MC. The Early Rheumatoid Arthritis (ERA) trial comparing the efficacy and safety of etanercept and methotrexate. Clin Exp Rheumatol 2003;21:S195-7.
- Kameda H, Kanbe K, Sato E, Ueki Y, Saito K, Nagaoka S, et al. Continuation of methotrexate resulted in better clinical and radiographic outcomes than discontinuation upon starting etanercept in patients with rheumatoid arthritis: 52-week results from the JESMR study. J Rheumatol 2011;38:1585-92. http://dx.doi.org/10.3899/jrheum.110014.
- Scott DL, Ibrahim F, Farewell V, O’Keeffe AG, Ma M, Walker D, et al. Randomised controlled trial of tumour-necrosis-factor inhibitors against combination intensive therapy with conventional disease modifying anti-rheumatic drugs in established rheumatoid arthritis: the TACIT trial. Health Technol Assess 2013;18. http://dx.doi.org/10.3310/hta18660.
- Smolen J, Fleischmann R, Guerette B. Adalimumab plus methotrexate vs. methotrexate monotherapy for early rheumatoid arthritis: 26-week results (first phase) from the 78-week optima study. Ann Rheum Dis 2010;69. http://dx.doi.org/10.1136/annrheumdis-2011-201247.
- Fleischmann R, Emery P. Long-term efficacy and safety of golimumab, a human anti-TNF alpha monoclonal antibody, in methotrexate-naïve rheumatoid arthritis patients: results from the go-before study. Ann Rheum Dis 2010;69.
- Weinblatt ME, Schiff M, Valente R, van der Heijde D, Citera G, Zhao C, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis. Arthritis Rheum 2013;65:28-3. http://dx.doi.org/10.1002/art.37711.
- AstraZeneca . A Randomised, Double-Blind (with Open Comparator Etanercept Limb), Placebo-Controlled, Phase IIb, Multicentre Study to Evaluate the Efficacy of 4 Doses of AZD9056 Administered for 6 Months on the Signs and Symptoms of Rheumatoid Arthritis in Patients With Active Disease Receiving Background Methotrexate or Sulphasalazine 2009. www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical-trials/resources/pdf/10775953 (accessed 8 August 2013).
- Lipsky PE, van der Heijde DMFM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med 2000;343:1594-602. http://dx.doi.org/10.1056/NEJM200011303432202.
- van Vollenhoven RF, Geborek P, Forslind K, Albertsson K, Ernestam S, Petersson IF, et al. Conventional combination treatment versus biological treatment in methotrexate-refractory early rheumatoid arthritis: 2 year follow-up of the randomised, non-blinded, parallel-group Swefot trial. Lancet 2012;379:1712-20. http://dx.doi.org/10.1016/S0140-6736(12)60027-0.
- van der Kooij SM, de Vries-Bouwstra JK, Goekoop-Ruiterman YP, Ewals JA, Han KH, Hazes JM, et al. Patient-reported outcomes in a randomised trial comparing four different treatment strategies in recent-onset rheumatoid arthritis. Arthritis Rheum 2009;61:4-12. http://dx.doi.org/10.1002/art.24367.
- Boini S, Guillemin F. Radiographic scoring methods as outcome measures in rheumatoid arthritis: properties and advantages. Ann Rheum Dis 2001;60:817-27.
- Peterfy C, Haraoui B, Durez P, Patra K, Kupper H. Decreased Incidence of Synovitis, Osteitis, and Erosion in Early RA Patients Treated With Adalimumab Plus Methotrexate Compared to Those With Methotrexate Alone: High-Field MRI Analysis from OPTIMA n.d.
- Ostergaard M, Emery P, Conaghan PG, Fleischmann R, Hsia EC, Xu W, et al. Significant improvement in synovitis, osteitis, and bone erosion following golimumab and methotrexate combination therapy as compared with methotrexate alone: a magnetic resonance imaging study of 318 methotrexate-naive rheumatoid arthritis patients. Arthritis Rheum 2011;63:3712-22. http://dx.doi.org/10.1002/art.30592.
- Tocilizumab (RoActemra) for the Treatment of Rheumatoid Arthritis 2013.
- Conaghan PG, Emery P, Ostergaard M, Keystone EC, Genovese MC, Hsia EC, et al. Assessment by MRI of inflammation and damage in rheumatoid arthritis patients with methotrexate inadequate response receiving golimumab: results of the GO-FORWARD trial. Ann Rheum Dis 2011;70:1968-74. http://dx.doi.org/10.1136/ard.2010.146068.
- Emery P, Kavanaugh AF, Smolen J, Cifaldi MA, Chaves L, Guerette B, et al. Combination Therapy With Adalimumab Methotrexate Significantly Improved Work Ability, Physical Function, Fatigue, and Other Patient-Reported Outcomes in Early Rheumatoid Arthritis: Results From a 26-Week Analysis n.d.
- Strand V, Rentz AM, Cifaldi MA, Chen N, Roy S, Revicki D. Health-related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomised multicenter study. J Rheumatol 2012;39:63-72. http://dx.doi.org/10.3899/jrheum.101161.
- Multiple Technology Appraisal . ID537. Certolizumab Pegol for the Treatment of Rheumatoid Arthritis That Has Previously Been Treated With Conventional Disease-Modifying Anti-Rheumatic Drugs 2013.
- Kosinski M, Kujawski SC, Martin R, Wanke LA, Buatti MC, Ware J, et al. Health-related quality of life in early rheumatoid arthritis: impact of disease and treatment response. Am J Manag Care 2002;8:231-40.
- Allaart CF, Breedveld FC, Dijkmans BAC. Treatment of recent-onset rheumatoid arthritis: lessons from the BeSt study. J Rheumatol 2007;34:S25-33.
- National Institute for Health and Care Excellence . Adalimumab, Etanercept, Infliximab, Certolizumab, Golimumab, Abatacept, and Tocilizumab for the Treatment of Rheumatoid Arthritis (Review of TA Guidance 130, 186, 224, 234, and Part Review of TA Guidance 225 and 247) [ID537] 2013.
- Kremer JM, Weinblatt ME, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Jackson CG, et al. Etanercept added to background methotrexate therapy in patients with rheumatoid arthritis: continued observations. Arthritis Rheum 2003;48:1493-9. http://dx.doi.org/10.1002/art.11142.
- Graudal N, Jurgens G. Similar effects of disease-modifying antirheumatic drugs, glucocorticoids, and biologic agents on radiographic progression in rheumatoid arthritis: meta-analysis of 70 randomised placebo-controlled or drug-controlled studies, including 112 comparisons. Arthritis Rheum 2010;62:2852-63. http://dx.doi.org/10.1002/art.27592.
- Singh JA, Beg S, Lopez-Olivo MA. Tocilizumab for rheumatoid arthritis. Cochrane Database Syst Rev 2010;7. http://dx.doi.org/10.1002/14651858.cd008331.
- Singh JA, Noorbaloochi S, Singh G. Golimumab for rheumatoid arthritis. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd008341.
- Ruiz Garcia V, Jobanputra P, Burls A, Cabello JB, Galvez Munoz JG, Saiz Cuenca ES, et al. Certolizumab pegol (CDP870) for rheumatoid arthritis in adults. Cochrane Database Syst Rev 2011;2. http://dx.doi.org/10.1002/14651858.cd007649.pub2.
- Singh JA, Wells GA, Christensen R, Tanjong GE, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011;2. http://dx.doi.org/10.1002/14651858.cd008794.pub2.
- Blumenauer B, Judd M, Wells G, Burls A, Cranney A, Hochberg M, et al. Infliximab for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev 2002;3. http://dx.doi.org/10.1002/14651858.cd003785.
- Navarro-Sarabia F, Ariza-Ariza R, Hernandez-Cruz B, Villanueva I. Adalimumab for treating rheumatoid arthritis. Cochrane Database Syst Rev 2005;3. http://dx.doi.org/10.1002/14651858.cd005113.pub2.
- Blumenauer B, Judd M, Cranney A, Burls A, Coyle D, Hochberg M, et al. Etanercept for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev 2003;4. http://dx.doi.org/10.1002/14651858.cd004525.
- Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopez-Olivo MA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database Syst Rev 2009;4. http://dx.doi.org/10.1002/14651858.cd007848.
- Connock M, Tubeuf S, Malottki K, Uthman A, Round J, Bayliss S, et al. Certolizumab pegol (CIMZIA (R)) for the treatment of rheumatoid arthritis. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14suppl2/01.
- Malottki K, Barton P, Tsourapas A, Uthman AO, Liu Z, Routh K, et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15140.
- Jobanputra P, Barton P, Bryan S, Burls A. The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess 2002;6. http://dx.doi.org/10.3310/hta6210.
- National Institute for Health and Care Excellence . Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Tocilizumab and Abatacept for the Treatment of Rheumatoid Arthritis Not Previously Treated With Disease-Modifying Anti-Rheumatic Drugs and After the Failure of Conventional Disease-Modifying Anti-Rheumatic Drugs Only 2013.
- National Institute for Health and Care Excellence . Adalimumab, Etanercept, Infliximab, Certolizumab, Golimumab, Abatacept, and Tocilizumab for the Treatment of Rheumatoid Arthritis (Review of TA Guidance 130, 186, 224, 234, and Part Review of TA Guidance 225 and 247) [ID537] 2013.
- Zink A, Strangfeld A, Schneider M, Herzer P, Hierse F, Stoyanova-Scholz M, et al. Effectiveness of tumour necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomised clinical trials. Arthritis Rheum 2006;54:3399-407. http://dx.doi.org/10.1002/art.22193.
- Pincus T, Sokka T. Should contemporary rheumatoid arthritis clinical trials be more like standard patient care and vice versa?. Ann Rheum Dis 2004;63:32-9. http://dx.doi.org/10.1136/ard.2004.028415.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Bansback NJ, Brennan A, Ghatnekar O. Cost-effectiveness of adalimumab in the treatment of patients with moderate to severe rheumatoid arthritis in Sweden. Ann Rheum Dis 2005;64:995-1002. http://dx.doi.org/10.1136/ard.2004.027565.
- Barbieri M, Wong JB, Drummond M. The cost-effectiveness of infliximab for severe treatment-resistant rheumatoid arthritis in the UK. Pharmacoeconomics 2005;23. http://dx.doi.org/10.2165/00019053-200523060-00007.
- Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8110.
- Benucci M, Gobbi FL, Sabadini L, Saviola G, Baiardi P, Manfredi M. The economic burden of biological therapy in rheumatoid arthritis in clinical practice: cost-effectiveness analysis of subcutaneous anti-TNF alpha treatment in Italian patients. Int J Immunopathol Pharmacol 2009;22:1147-52.
- Brennan A, Bansback N, Reynolds A, Conway P. Modelling the cost-effectiveness of etanercept in adults with rheumatoid arthritis in the UK. Rheumatology 2004;43:62-7. http://dx.doi.org/10.1093/rheumatology/keg451.
- Brennan A, Bansback N, Nixon R, Madan J, Harrison M, Watson K, et al. Modelling the cost-effectiveness of TNF-alpha antagonists in the management of rheumatoid arthritis: results from the British Society for Rheumatology Biologics Registry. Rheumatology 2007;46:1345-54. http://dx.doi.org/10.1093/rheumatology/kem115.
- Chiou C-F, Choi J, Reyes CM. Cost-effectiveness analysis of biological treatments for rheumatoid arthritis. Expert Rev Pharmacoecon Outcomes Res 2004;4:307-15. http://dx.doi.org/10.1586/14737167.4.3.307.
- Choi HK, Seeger JD, Kuntz KM. A cost-effectiveness analysis of treatment options for methotrexate-naive rheumatoid arthritis. J Rheumatol 2002;29:1156-65.
- Coyle D, Judd M, Blumenauer B, Cranney A, Maetzel A, Tugwell P, et al. Infliximab and Etanercept in Patients With Rheumatoid Arthritis: A Systematic Review and Economic Evaluation. Ottawa, ON: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); 2006.
- Davies A, Cifaldi MA, Segurado OG, Weisman MH. Cost-effectiveness of sequential therapy with tumour necrosis factor antagonists in early rheumatoid arthritis. J Rheumatol 2009;36:16-2. http://dx.doi.org/10.3899/jrheum.080257.
- Diamantopoulos A, Benucci M, Capri S, Berger W, Wintfeld N, Giuliani G, et al. Economic evaluation of tocilizumab combination in the treatment of moderate-to-severe rheumatoid arthritis in Italy. J Med Econ 2012;15:576-85. http://dx.doi.org/10.3111/13696998.2012.665110.
- Finckh A, Bansback N, Marra CA, Anis AH, Michaud K, Lubin S, et al. Treatment of very early rheumatoid arthritis with symptomatic therapy, disease-modifying antirheumatic drugs, or biologic agents a cost-effectiveness analysis. Ann Intern Med 2009;151:612-21. http://dx.doi.org/10.7326/0003-4819-151-9-200911030-00006.
- Kobelt G, Jonsson L, Young A, Eberhardt K. The cost-effectiveness of infliximab (Remicade®) in the treatment of rheumatoid arthritis in Sweden and the United Kingdom based on the ATTRACT study. Rheumatology 2003;42:326-35. http://dx.doi.org/10.1093/rheumatology/keg107.
- Kobelt G, Eberhardt K, Geborek P. TNF inhibitors in the treatment of rheumatoid arthritis in clinical practice: costs and outcomes in a follow up study of patients with RA treated with etanercept or infliximab in southern Sweden. Ann Rheum Dis 2004;63:4-10. http://dx.doi.org/10.1136/ard.2003.010629.
- Kobelt G, Lindgren P, Singh A, Klareskog L. Cost-effectiveness of etanercept (enbrel) in combination with methotrexate in the treatment of active rheumatoid arthritis based on the TEMPO trial. Ann Rheum Dis 2005;64:1174-9. http://dx.doi.org/10.1136/ard.2004.032789.
- Kobelt G, Lekander I, Lang A, Raffeiner B, Botsios C, Geborek P. Cost-effectiveness of etanercept treatment in early active rheumatoid arthritis followed by dose adjustment. Int J Technol Assess Health Care 2011;27:193-200. http://dx.doi.org/10.1017/S0266462311000195.
- Lekander I, Borgstrom F, Svarvar P, Ljung T, Carli C, van Vollenhoven RF. Cost-effectiveness of real-world infliximab use in patients with rheumatoid arthritis in Sweden. Int J Technol Assess Health Care 2010;26:54-61. http://dx.doi.org/10.1017/S0266462309990596.
- Marra CA, Marion SA, Guh DP, Najafzadeh M, Wolfe F, Esdaile JM, et al. Not all ‘quality-adjusted life-years’ are equal. J Clin Epidemiol 2007;60:616-24. http://dx.doi.org/10.1016/j.jclinepi.2006.09.006.
- Nuijten MJC, Engelfriet P, Duijn K, Bruijn G, Wierz D, Koopmanschap M. A cost-cost study comparing etanercept with infliximab in rheumatoid arthritis. Pharmacoeconomics 2001;19:1051-64. http://dx.doi.org/10.2165/00019053-200119100-00006.
- Rubio-Terrés C, Dominguez-Gil A. Pharmacoeconomic analysis of the treatment of rheumatoid arthritis with leflunomide in comparison with the combination of infliximab and methotrexate. J Med Econ 2001;4. http://dx.doi.org/10.3111/200104019034.
- Soini EJ, Hallinen TA, Puolakka K, Vihervaara V, Kauppi MJ. Cost-effectiveness of adalimumab, etanercept, and tocilizumab as first-line treatments for moderate-to-severe rheumatoid arthritis. J Med Econ 2012;15:340-51. http://dx.doi.org/10.3111/13696998.2011.649327.
- Spalding JR, Hay J. Cost-effectiveness of tumour necrosis factor-alpha inhibitors as first-line agents in rheumatoid arthritis. Pharmacoeconomics 2006;24:1221-32. http://dx.doi.org/10.2165/00019053-200624120-00006.
- Tanno M, Nakamura I, Ito K, Tanaka H, Ohta H, Kobayashi M, et al. Modelling and cost-effectiveness analysis of etanercept in adults with rheumatoid arthritis in Japan: a preliminary analysis. Mod Rheumatol 2006;16:77-84. http://dx.doi.org/10.3109/s10165-006-0461-y.
- van den Hout WB, Goekoop-Ruiterman YP, Allaart CF, de Vries-Bouwstra JK, Hazes JM, Kerstens PJ, et al. Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 2009;61:291-9. http://dx.doi.org/10.1002/art.24169.
- Vera-Llonch M, Massarotti E, Wolfe F, Shadick N, Westhovens R, Sofrygin O, et al. Cost-effectiveness of abatacept in patients with moderately to severely active rheumatoid arthritis and inadequate response to methotrexate. Rheumatology 2008;47:535-41. http://dx.doi.org/10.1093/rheumatology/ken007.
- Wailoo AJ, Bansback N, Brennan A, Michaud K, Nixon RM, Wolfe F. Biologic drugs for rheumatoid arthritis in the Medicare program: a cost-effectiveness analysis. Arthritis Rheum 2008;58:939-46. http://dx.doi.org/10.1002/art.23374.
- Welsing PMJ, Severens JL, Hartman M, Van Riel PLCM, Laan RFJM. Modelling the 5-year cost-effectiveness of treatment strategies including tumour necrosis factor-blocking agents and leflunomide for treating rheumatoid arthritis in the Netherlands. Arthritis Rheum 2004;51:964-73. http://dx.doi.org/10.1002/art.20843.
- Wong JB, Singh G, Kavanaugh A. Estimating the cost-effectiveness of 54 weeks of infliximab for rheumatoid arthritis. Am J Med 2002;113:400-8. http://dx.doi.org/10.1016/S0002-9343(02)01243-3.
- National Institute for Health and Care Excellence . The Clinical Effectiveness and Cost Effectiveness of Etanercept and Infliximab for Rheumatoid Arthritis and Juvenile Poly-Articular Idiopathic Arthritis (TA36) 2002. www.nice.org.uk/guidance/ta36 (accessed 5 July 2013).
- National Institute for Health and Care Excellence . Adalimumab, Etanercept and Infliximab for the Treatment of Rheumatoid Arthritis n.d. www.nice.org.uk/guidance/ta130 (accessed 3 August 2013).
- National Institute for Health and Care Excellence . Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Tocilizumab and Abatacept for the Treatment of Rheumatoid Arthritis Not Previously Treated With Disease-Modifying Anti-Rheumatic Drugs and After the Failure of Conventional Disease-Modifying Anti-Rheumatic Drugs Only (Review of Technology Appraisal Guidance 130, 186, 224, 234 and a Part Review of Technology Appraisal Guidance 225 and 247) 2013.
- National Institute for Health and Care Excellence . Etanercept for the Treatment of Rheumatoid Arthritis. (Review of TA Guidance 130, 186, 224, 234 and Part Review of TA Guidance 225 and 247). Multiple Technology Appraisal (MTA) 2013.
- Drummond M, Jefferson T. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Eddy D, Mosteller F. Assessing Medical Technologies. Washington, DC: National Academy Press; 1985.
- Gabriel S, Drummond M, Maetzel A, Boers M, Coyle D, Welch V, et al. Omeract 6 economics working group report: a proposal for a reference case for economic evaluation in rheumatoid arthritis. J Rheumatol 2003;30:886-90.
- Maetzel A, Tugwell P, Boers M, Guillemin F, Coyle D, Drummond M, et al. Economic evaluation of programs or interventions in the management of rheumatoid arthritis: defining a consensus-based reference case. J Rheumatol 2003;30:891-6.
- Hyrich K, Symmons D, Watson K, Silman A. British Society for Rheumatology Biologics Register . Baseline comorbidity levels in biologic and standard DMARD treated patients with rheumatoid arthritis: results from a national patient register. Ann Rheum Dis 2006;65:895-8. http://dx.doi.org/10.1136/ard.2005.043158.
- Burmester GR, Mariette X, Montecucco C, Monteagudo-Saez I, Malaise M, Tzioufas AG, et al. Adalimumab alone and in combination with disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis in clinical practice: the Research in Active Rheumatoid Arthritis (ReAct) trial. Ann Rheum Dis 2007;66:732-9. http://dx.doi.org/10.1136/ard.2006.066761.
- Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, et al. Golimumab, a human antibody to tumour necrosis factor (alpha) given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD study. Ann Rheum Dis 2009;68:789-96. http://dx.doi.org/10.1136/ard.2008.099010.
- Smolen JS, Nash P, Durez P, Hall S, Ilivanova E, Irazoque-Palazuelos F, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013;381:918-29. http://dx.doi.org/10.1016/S0140-6736(12)61811-X.
- Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis 2009;68:805-11. http://dx.doi.org/10.1136/ard.2008.099291.
- Monthly Index of Medical Specialties 2013. www.mims.co.uk (accessed 1 February 2013).
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2008 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed 20 July 2013).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2012.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com/mc/bnf/current/index.htm (accessed 1 April 2013).
- Electronic Medicines Compendium . Tocilizumab Summary of Product Characteristics 2011. www.medicines.org.uk/EMC/browsedocuments.aspx (accessed February 2013).
- NHS . National Schedule of Reference Costs Year: 2010–11 NHS Trusts and PCTS Combined HRG Data 2013. https://data.gov.uk/dataset/nhs-reference-costs-2010-11 (accessed 30 July 2013).
- National Institute for Health and Care Excellence . Costing Template for Infliximab for the Treatment of Adults With Psoriasis (Guidance TA134) 2008. www.nice.org.uk/guidance/ta134/resources (accessed 31 July 2013).
- Shared Care Guidelines and Monitoring Protocols for Rheumatology Patients on Disease Modifying Drugs (Including Biologic Therapies). Bradford: Bradford Teaching Hospitals NHS Trust & Bradford and Airedale teaching Primary Care Trust; 2010.
- National Institute for Health and Care Excellence . Costing Template: Tuberculosis: Clinical Diagnosis and Management of Tuberculosis and Measures for Its Prevention and Control; Clinical Guideline 33 2013. www.nice.org.uk/guidance/cg117/resources (accessed 31 July 2013).
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2011.
- Chakravarty K, McDonald H, Pullar T, Taggart A, Chalmers R, Oliver S, et al. BSR/BHPR guideline for disease-modifying anti-rheumatic drug (DMARD) therapy in consultation with the British Association of Dermatologists. Rheumatology 2008;47:924-5. http://dx.doi.org/10.1093/rheumatology/kel216a.
- Department of Health . NHS Trusts and PCTs Combined Reference Cost Schedules 2010–11 2012. https://data.gov.uk/dataset/nhs-reference-costs-2010-11/resource/91f6f5fd-c185-4bf0-8e56-718a4d769b24 (accessed 31 July 2013).
- Roche . Achieving Clinical Excellence in the Treatment of Moderate to Severe Rheumatoid Arthritis. RoActemra® (Tocilizumab) NICE STA Submission 2009.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Department of Health . NHS Reference Costs 2009–2010 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123459 (accessed 1 April 2013).
- Harland D, Gibbons C, Diamantopoulos A, Pang H, Huertas D, Dejonckheere F. Tocilizumab in patients where methotrexate is considered inappropriate – a cost-utility model for the UK. Value Health 2012;15:A277-575. http://dx.doi.org/10.1016/j.jval.2012.08.1403.
- Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Klearman M, et al. Tocilizumab (TCZ) monotherapy is superior to adalimumab (ADA) monotherapy in reducing disease activity in patients with rheumatoid arthritis (RA): 24-week data from the phase 4 ADACTA trial. Ann Rheum Dis 2012;71. http://dx.doi.org/10.1136/annrheumdis-2012-eular.1963.
- UCB Pharma . Certolizumab Pegol (CIMZIA®) for the Treatment of Rheumatoid Arthritis 2009.
- Schering-Plough Ltd . Golimumab for the Treatment of Rheumatoid Arthritis After Failure of Previous Disease-Modifying Antirheumatic Drugs 2010.
- Strand V, Burmester GR, Ogale S, Devenport J, John A, Emery P. Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomised controlled radiate study. Rheumatology 2012;51:1860-9. http://dx.doi.org/10.1093/rheumatology/kes131.
- Smolen JS, Kay J, Doyle MK, Landewe R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 2009;374:210-21. http://dx.doi.org/10.1016/S0140-6736(09)60506-7.
- Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Rheumatology 1997;36:551-9. http://dx.doi.org/10.1093/rheumatology/36.5.551.
- van der Heijde D, Landewe R, van Vollenhoven R, Fatenejad S, Klareskog L. Level of radiographic damage and radiographic progression are determinants of physical function: a longitudinal analysis of the TEMPO trial. Ann Rheum Dis 2008;67:1267-70. http://dx.doi.org/10.1136/ard.2007.081331.
- Munro R, Hampson R, McEntegart A, Thompson E, Modhok R, Capell H. Improved functional outcome in patients with early RA treated with intramuscular gold: results of a five year prospective study. Ann Rheum Dis 1998;57:88-93. http://dx.doi.org/10.1136/ard.57.2.88.
- Scott DL, Pugner K, Kaarela K, Doyle DV, Woolf A, Holmes J, et al. The links between joint damage and disability in rheumatoid arthritis. Rheumatology 2000;39:122-32. http://dx.doi.org/10.1093/rheumatology/39.2.122.
- Edwards CJ, Arden NK, Fisher D, Saperia JC, Reading I, van Staa TP, et al. The changing use of disease-modifying anti-rheumatic drugs in individuals with rheumatoid arthritis from the United Kingdom General Practice Research Database. Rheumatology 2005;44:1394-8. http://dx.doi.org/10.1093/rheumatology/kei024.
- Hoyle M, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol 2011;11. http://dx.doi.org/10.1186/1471-2288-11-139.
- Tierney J, Stewart L, Ghersi D, Burdett S, Sydes M. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-16.
- National Clinical Guideline for Rheumatoid Arthritis. London: Royal College of Physicians; 2008.
- Soliman MM, Ashcroft DM, Watson KD, Lunt M, Symmons DPM, Hyrich KL. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2011;70:583-9. http://dx.doi.org/10.1136/ard.2010.139774.
- Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum 2010;62:22-3. http://dx.doi.org/10.1002/art.27227.
- Du Pan SM, Dehler S, Ciurea A, Ziswiler HR, Gabay C, Finckh A. Comparison of drug retention rates and causes of drug discontinuation between anti-tumour necrosis factor agents in rheumatoid arthritis. Arthritis Rheum 2009;61:560-8. http://dx.doi.org/10.1002/art.24463.
- Keystone EC, Cohen SB, Emery P, Kremer JM, Dougados M, Loveless JE, et al. Multiple courses of rituximab produce sustained clinical and radiographic efficacy and safety in patients with rheumatoid arthritis and an inadequate response to 1 or more tumour necrosis factor inhibitors: 5-year data from the REFLEX Study. J Rheumatol 2012;39:2238-46. http://dx.doi.org/10.3899/jrheum.120573.
- Smolen JS, Kay J, Landewe RBM, Matteson EL, Gaylis N, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis who have previous experience with tumour necrosis factor inhibitors: results of a long-term extension of the randomised, double-blind, placebo-controlled GO-AFTER study through week 160. Ann Rheum Dis 2012;71:1671-9. http://dx.doi.org/10.1136/annrheumdis-2011-200956.
- Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008;67:1516-23. http://dx.doi.org/10.1136/ard.2008.092932.
- Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control 1974;19. http://dx.doi.org/10.1109/TAC.1974.1100705.
- Hjardem E, Ostergaard M, Podenphant J, Tarp U, Andersen LS, Bing J, et al. Do rheumatoid arthritis patients in clinical practice benefit from switching from infliximab to a second tumour necrosis factor alpha inhibitor?. Ann Rheum Dis 2007;66:1184-9. http://dx.doi.org/10.1136/ard.2006.054742.
- Guyot P, Ades A, Ouwens M, Welton N. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol 2012;12. http://dx.doi.org/10.1186/1471-2288-12-9.
- Trueman D, Livings C, Mildred M. PRM81 Methods of obtaining evidence from published survival data for use in decision analytic models. Value Health 2012;15. http://dx.doi.org/10.1016/j.jval.2012.08.1544.
- Anderson J, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum 2000;43:22-9. http://dx.doi.org/10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9.
- Wiles NJ, Symmons D. Resource Use Within the Norfolk Arthritis Register (NOAR) Cohort During the First Five Years of Disease. Report for Roche. London: NICE; 2005.
- Department of Health . National Schedule of Reference Costs Year: 2010–11 – NHS Trusts and PCTs Combined HRG Data 2013. www.gov.uk/government/publications/2010-11-reference-costs-publication (accessed 2 August 2013).
- Kobelt G, Jonsson L, Lindgren P, Young A, Eberhardt K. Modelling the progression of rheumatoid arthritis: a two-country model to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheum 2002;46:2310-19. http://dx.doi.org/10.1002/art.10471.
- Young A, Dixey J, Cox N, Davies P, Devlin J, Emery P, et al. How does functional disability in early rheumatoid arthritis (RA) affect patients and their lives? Results of 5 years of follow-up in 732 patients from the Early RA Study (ERAS). Rheumatology 2000;39:603-11. http://dx.doi.org/10.1093/rheumatology/39.6.603.
- Kobelt G, Eberhardt K, Jonsson L, Jonsson B. Economic consequences of the progression of rheumatoid arthritis in Sweden. Arthritis Rheum 1999;42:347-56. http://dx.doi.org/10.1002/1529-0131(199902)42:2<347::AID-ANR18>3.0.CO;2-P.
- Kobelt G, Lindgren P, Lindroth Y, Jacobson L, Eberhardt K. Modelling the effect of function and disease activity on costs and quality of life in rheumatoid arthritis. Rheumatology 2005;44:1169-75. http://dx.doi.org/10.1093/rheumatology/keh703.
- Ducournau P, Keilhorn A, Wintfield N. Comparison of Linear and Non-Linear Utility Mapping Between HAQ and EQ-5D Using Pooled Data from the Tolicizumab Trials OPTION and LITHE 2009.
- Dolan P. Modelling Valuations for EuroQol Health States. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Russell AS, Conner-Spady B, Mintz A, Maksymowych WP. The responsiveness of generic health status measures as assessed in patients with rheumatoid arthritis receiving infliximab. J Rheumatol 2003;30:941-7.
- Wolfe F, Hawley D. Measurement of the quality of life in rheumatic disorders using the EuroQol. Rheumatology 1997;36:786-93. http://dx.doi.org/10.1093/rheumatology/36.7.786.
- Conner-Spady B, Suarez-Almazor ME. Variation in the estimation of quality-adjusted life-years by different preference-based instruments. Med Care 2003;41:791-80. http://dx.doi.org/10.1097/00005650-200307000-00003.
- Marra C, Woolcott J, Kopec J, Shojania K, Offer R, Brazier J. A comparison of generic, indirect utility measures (the HUI2, HUI3, SF-6D, and the EQ5D) and disease specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Soc Sci Med 2005;60:1571-82. http://dx.doi.org/10.1016/j.socscimed.2004.08.034.
- Boggs R, Sengupta N, Ashraf T. Estimating health utility from a physical function assessment in rheumatoid arthritis (RA) patients treated with adalimumab. Value Health 2002;5. http://dx.doi.org/10.1016/S1098-3015(10)61216-8.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisals 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed 2 August 2013).
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisals 2013. www.nice.org.uk/article/pmg9/chapter/foreword (accessed 2 August 2013).
- Bansback N, Marra C, Tsuchiya A, Anis A, Guh D, Hammond T, et al. Using the health assessment questionnaire to estimate preference-based single indices in patients with rheumatoid arthritis. Arthritis Rheum 2007;57:963-71. http://dx.doi.org/10.1002/art.22885.
- Hernandez-Alava M, Wailoo AJ, Ara R. Tails from the Peak District: adjusted limited dependent variable mixture models of EQ-5D questionnaire health state utility values. Value Health 2012;15:550-61. http://dx.doi.org/10.1016/j.jval.2011.12.014.
- Bansback N, Young A, Brennan A, Dixey J. A prognostic model for functional outcome in early rheumatoid arthritis. J Rheumatol 2006;33:1503-10.
- Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Fu B, Ustianowski AP, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: Updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology 2011;50:124-31. http://dx.doi.org/10.1093/rheumatology/keq242.
- Oppong R, Kaambwa B, Nuttall J, Hood K, Smith R, Coast J. The impact of using different tariffs to value EQ-5D health state descriptions: an example from a study of acute cough/lower respiratory tract infections in seven countries. Eur J Health Econ n.d.;14:197-209. http://dx.doi.org/10.1007/s10198-011-0360-9.
- Dixon W, Symmons D, Lunt M, Watson K, Hyrich K, Silman A. Serious infection following anti-tumour necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum 2007;56:2896-904. http://dx.doi.org/10.1002/art.22808.
- Singh JA, Wells GA, Christensen R, Tanjong GE, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2012;2. http://dx.doi.org/10.1136/annrheumdis-2012-eular.3407.
- Wolfe F, Mitchell D, Sibley J, Fries J, Bloch D, Williams C, et al. The mortality of rheumatoid arthritis. Arthritis Rheum 1994;37:481-94. http://dx.doi.org/10.1002/art.1780370408.
- Office for National Statistics . Interim Life Tables: 2008–2010 n.d. www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2008-2010/index.html (accessed 30 January 2013).
- Lindgren P, Geborek P, Kobelt G. Modelling the cost-effectiveness of treatment of rheumatoid arthritis with rituximab using registry data from Southern Sweden. Int J Technol Assess Health Care 2009;25:181-9. http://dx.doi.org/10.1017/S0266462309090230.
- Merkesdal S, Kirchhoff T, Wolka D, Ladinek G, Kielhorn A, Rubbert-Roth A. Cost-effectiveness analysis of rituximab treatment in patients in Germany with rheumatoid arthritis after etanercept-failure. Eur J Health Econ 2010;11:95-104. http://dx.doi.org/10.1007/s10198-009-0205-y.
- Bristol-Myers Squibb . Abatacept for the Treatment of Rheumatoid Arthritis After the Failure of Conventional Disease-Modifying Anti-Rheumatic Drugs 2010.
- Wu B, Wilson A, Wang FF, Wang SL, Wallace DJ, Weisman MH, et al. Cost-effectiveness of different treatment strategies in the treatment of patients with moderate to severe rheumatoid arthritis in China. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0047373.
- Pincus T, Furer V, Keystone E, Yazici Y, Bergman MJ, Luijtens K. RAPID3 (Routine Assessment of Patient Index Data 3) severity categories and response criteria: Similar results to DAS28 (Disease Activity Score) and CDAI (Clinical Disease Activity Index) in the RAPID 1 (Rheumatoid Arthritis Prevention of Structural Damage) clinical trial of certolizumab pegol. Arthritis Rheum 2011;63:1142-9. http://dx.doi.org/10.1002/acr.20481.
- Yelin E, Trupin L, Wong B, Rush S. The impact of functional status and change in functional status on mortality over 18 years among persons with rheumatoid arthritis. J Rheumatol 2002;29:1851-7.
- Office for National Statistics . Interim Life Tables, United Kingdom, 1980–82 to 2008–10 2010. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-223324 (accessed 1 April 2013).
- Deighton C, Hyrich K, Ding T, Ledingham J, Lunt M, Kiely P, et al. BSR and BHPR rheumatoid arthritis guidelines on eligibility criteria for the first biological therapy. Rheumatology 2010;49:1197-9. http://dx.doi.org/10.1093/rheumatology/keq006a.
- Norton S, Sacker A, Dixey D, Done J, Williams P, Young A. Trajectories of functional limitation in early rheumatoid arthritis and their association with mortality. Rheumatology 2013;52:2016-24. http://dx.doi.org/10.1093/rheumatology/ket253.
- Norton S, Fu B, Verstappen S, Symmons D, Lunt M, Davies R, et al. Common Trajectories of HAQ Disability Progression Over 15-years in the Early Rheumatoid Arthritis Study and the Norfolk Arthritis Register. Berlin: EULAR; 2012.
- Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263-9. http://dx.doi.org/10.1016/S0140-6736(04)16676-2.
- Department of Health . NHS Reference Costs: Financial Year 2011 to 2012 n.d. www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (accessed 1 April 2013).
- Bollen K, Curran P. Autoregressive latent trajectory (ALT) models. A synthesis of two traditions. Sociol Method Res 2004;32:336-83. http://dx.doi.org/10.1177/0049124103260222.
- Satorra A, Bentler PM. A scaled difference chisquare test statistic for moment structure analysis. Psychometrika 2001;66:507-14. http://dx.doi.org/10.1007/BF02296192.
- Norton S, Verstappen SMM, Symmons DP, Lunt M, Davies R, Scott DL, et al. Common trajectories of HAQ disability progression over 15-years in the early rheumatoid arthritis study and the Norfolk arthritis register. Ann Rheum Dis 2012;71. http://dx.doi.org/10.1136/annrheumdis-2012-eular.3060.
- Hernandez-Alava M, Wailoo A, Wolfe F, Michaud K. A comparison of direct and indirect methods for the estimation of health utilities from clinical outcomes. Med Decis Making 2013;34:919-30. http://dx.doi.org/10.1177/0272989X13500720.
- Plant MJ, O’Sullivan MM, Lewis PA, Camilleri JP, Coles EC, Jessop JD. What factors influence functional ability in patients with rheumatoid arthritis. Do they alter over time?. Rheumatology 2005;44:1181-5. http://dx.doi.org/10.1093/rheumatology/keh707.
- Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott D. The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9340.
- McWilliams DF, Kiely P, Young A, Walsh D. Baseline factors predicting change from the initial DMARD treatment during the first 2 years of rheumatoid arthritis: experience in the ERAN inception cohort. BMC Musculoskelet Disord 2013;14. http://dx.doi.org/10.1186/1471-2474-14-153.
- Bansback N, Fu EHY, Sun H, Guh D, Zhang W, Lacaille D, et al. Impact of Biologics on Healthcare Utilisation in Patients With Rheumatoid Arthritis: An Instrumental Variable Approach n.d.
- Hernandez-Alava M, Wailoo A, Wolfe F, Michaud K. The relationship between EQ-5D, HAQ and pain in patients with rheumatoid arthritis. Rheumatology 2013;52:944-50. http://dx.doi.org/10.1093/rheumatology/kes400.
- Brazier JE, Yang YL, Tsuchiya A, Rowen DL. A review of studies mapping (or cross walking) non-preference based measures of health to generic preference-based measures. Eur J Health Econ 2010;11:215-25. http://dx.doi.org/10.1007/s10198-009-0168-z.
- Hawthorne G, Buchbinder R, Defina J. Functional Status and Health Related Quality of Life Assessment in Patients with Rheumatoid Arthritis (Working Paper 116). Melbourne, VIC: Monash University Centre for Health Program Evaluation; 2000.
- Bansback NJ, Ara R, Barkham N, Brennan A, Fraser AD, Conway P, et al. Estimating the cost and health status consequences of treatment with TNF antagonists in patients with psoriahc arthritis. Rheumatology 2006;45:1029-38. http://dx.doi.org/10.1093/rheumatology/kel147.
- Michaud K, Vera-Llonch M, Oster G. Mortality risk by functional status and health-related quality of life in patients with rheumatoid arthritis. J Rheumatol 2012;39:54-9. http://dx.doi.org/10.3899/jrheum.110491.
- O’Hagan A, Stevenson M, Madan J. Monte Carlo probabilistic sensitivity analysis for patient level simulation models: efficient estimation of mean and variance using ANOVA. Health Econ 2007;16:1009-23. http://dx.doi.org/10.1002/hec.1199.
- Heijde D, Breedveld FC, Kavanaugh A, Keystone EC, Landewé R, Patra K, et al. Disease activity, physical function, and radiographic progression after longterm therapy with adalimumab plus methotrexate: 5-year results of PREMIER. J Rheumatol 2010;37:2237-46. http://dx.doi.org/10.3899/jrheum.100208.
- Emery P, Genovese MC, van Vollenhoven R, Sharp JT, Patra K, Sasso EH. Less radiographic progression with adalimumab plus methotrexate versus methotrexate monotherapy across the spectrum of clinical response in early rheumatoid arthritis. J Rheumatol 2009;36:1429-41. http://dx.doi.org/10.3899/jrheum.081018.
- Haugeberg G, Conaghan PG, Quinn M, Emery P. Bone loss in patients with active early rheumatoid arthritis: infliximab and methotrexate compared with methotrexate treatment alone. Explorative analysis from a 12-month randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2009;68:1898-901. http://dx.doi.org/10.1136/ard.2008.106484.
- Bejarano V, Conaghan PG, Quinn MA, Saleem B, Emery P. Benefits 8 years after a remission induction regime with an infliximab and methotrexate combination in early rheumatoid arthritis. Rheumatology 2010;49:1971-4. http://dx.doi.org/10.1093/rheumatology/keq194.
- Fleischmann R, Weinblatt ME, Schiff MH, Khanna D, Furst D, Maldonado MA. Changes in patient reported outcomes in response to subcutaneous abatacept or adalimumab in rheumatoid arthritis: results from the ample (abatacept versus adalimumab comparison in biologic naive RA subjects with background methotrexate) trial. Arthritis Rheum 2012;64.
- Emery P, Smolen JS, Samborski W, Berenbaum F, Davies O, Ambrugeat J, et al. Efficacy and safety of certolizumab pegol after incomplete response to DMARDs in rheumatoid arthritis patients with low moderate disease activity: results from CERTAIN, a Phase IIIB study. Rheumatology 2012;51.
- Kameda H, Kurasawa T, Nagasawa H, Amano K, Takeuchi T. The addition of another disease-modifying anti-rheumatic drug, bucillamine, to methotrexate in place of infliximab improves the rate of infliximab-free sustained remission. Int J Rheum Dis 2010;13. http://dx.doi.org/10.3109/14397595.2013.844886.
- Hidaka T, Suzuki K, Matsuki Y, Takamizawa-Matsumoto M, Kataharada K, Ishizuka T, et al. Filtration leukocytapheresis therapy in rheumatoid arthritis: a randomised, double-blind, placebo-controlled trial. Arthritis Rheum 1999;42:431-7. http://dx.doi.org/10.1002/1529-0131(199904)42:3<431::AID-ANR6>3.0.CO;2-2.
- Kremer JM, Westhovens R, Leon M, Di GE, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med 2003;349:1907-15. http://dx.doi.org/10.1056/NEJMoa035075.
- Jobanputra P, Maggs F, Deeming A, Carruthers D, Rankin E, Jordan A, et al. Randomized efficacy and discontinuation study of etanercept versus adalimumab (red sea): a pragmatic, open-label, non-inferiority study of first TNF inhibitor use in rheumatoid arthritis: 1-year data. Rheumatology 2012;51.
- Genovese MC, Han C, Keystone EC, Hsia EC, Buchanan J, Gathany T, et al. Effect of golimumab on patient-reported outcomes in rheumatoid arthritis: results from the GO-FORWARD study. J Rheumatol 2012;39:1185-91. http://dx.doi.org/10.3899/jrheum.111195.
- Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum 2002;46:1443-50. http://dx.doi.org/10.1002/art.10308.
- Nishimoto N, Takagi N. Assessment of the validity of the 28-joint disease activity score using erythrocyte sedimentation rate (DAS28-ESR) as a disease activity index of rheumatoid arthritis in the efficacy evaluation of 24-week treatment with tocilizumab: subanalysis of the SATORI study. Mod Rheumatol 2010;20:539-47. http://dx.doi.org/10.3109/s10165-010-0328-0.
- van der Heide D. How to read radiographs according to the Sharp/van der Heijde method. Rheumatology 2000;27:261-3.
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with and methotrexate. Arthritis Rheum 2004;50:1051-65. http://dx.doi.org/10.1002/art.20159.
- Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis 2009;68:1580-4. http://dx.doi.org/10.1136/ard.2008.092866.
Appendix 1 Search strategies
Search strategies for systematic review of clinical effectiveness
Stage 1 clinical effectiveness searches
Stage 1 searches identified trials and systematic reviews using, where appropriate, high precision search filters. Searches were undertaken in December 2012. MEDLINE and EMBASE searches were updated in May 2013. In addition to the searches detailed below, evidence was sought through hand-searching, citation and grey literature searching and through consultation of clinical trials registers. Further details are given in the main report.
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R) (via Ovid)
Date range searched: 1948 to July 2013.
Search undertaken December 2012, updated May 2013.
-
exp Arthritis, Rheumatoid/
-
rheumatoid arthritis.tw.
-
1 or 2
-
randomized controlled trial.pt.
-
randomized controlled trial.mp.
-
4 or 5
-
3 and 6
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R) (via Ovid)
Date range searched: 1948 to July 2013.
Search undertaken December 2012, updated May 2013.
-
exp Arthritis, Rheumatoid/
-
rheumatoid arthritis.tw.
-
1 or 2
-
medline.tw.
-
systematic review.tw.
-
meta analysis.pt.
-
4 or 5 or 6
-
3 and 7
EMBASE (via Ovid)
Date range searched: 1980 to July 2013.
Search undertaken December 2012, updated May 2013.
-
exp rheumatoid arthritis/
-
rheumatoid arthritis.tw.
-
1 or 2
-
double blind:.mp.
-
placebo:.tw.
-
blind:.tw.
-
4 or 5 or 6
-
3 and 7
EMBASE (via Ovid)
Date range searched: 1980 to July 2013.
Search undertaken December 2012, updated May 2013.
-
exp rheumatoid arthritis/
-
rheumatoid arthritis.tw.
-
1 or 2
-
meta-analysis.tw.
-
systematic review.tw.
-
4 or 5
-
3 and 6
Cochrane Database of Systematic Reviews (Wiley Online Library) (1996 to May 2013); Cochrane Central Register of Controlled Trials (via Wiley Online Library) (1898 to May 2013)
Search undertaken December 2012.
“rheumatoid arthritis” OR explode Arthritis, Rheumatoid
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost)
Date range searched: 1982 to April 2013.
Search undertaken December 2012.
-
Explode Rheumatoid Arthritis (MH)
-
Rheumatoid arthritis (TX)
-
1 or 2
-
Randomized (TX)
-
Treatment outcomes (MH)
-
Clinical trial (PT)
-
4 or 5 or 6
-
3 and 7
Web of Science (via ISI Web of Knowledge)
Date range searched: 1900 to present.
Search undertaken December 2012.
Rheumatoid arthritis (topic) AND (randomi?ed NEAR trial*) (topic)
Web of Science (via ISI Web of Knowledge)
Date range searched: 1900 to present.
Search undertaken December 2012.
Rheumatoid arthritis (topic) AND (systematic review* OR meta-analys?s) (topic)
Centre for Reviews and Dissemination Database of Abstracts of Reviews of Effectiveness and Health Technology Assessment
Date range searched: 1995 to 2013.
Search undertaken December 2012.
Rheumatoid arthritis (all fields)
Stage 2 clinical effectiveness searches
Stage 2 searches identified trials using high sensitivity search filters. Searches were undertaken in April 2013.
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R) (via Ovid)
Date range searched: 1948 to July 2013.
Search undertaken April 2013.
-
adalimumab.af.
-
humira.af.
-
d 2e7.af.
-
d2e7.af.
-
331731-18-1.rn.
-
etanercept.af.
-
enbrel.af.
-
185243-69-0.rn.
-
infliximab.af.
-
remicade.af.
-
170277-31-3.rn.
-
ta650.af.
-
ta 650.af.
-
certolizumab pegol.af.
-
cimzia.af.
-
cdp870.af.
-
428863-50-7.rn.
-
1132819-27-2.rn.
-
czp.af.
-
abatacept.af.
-
orencia.af.
-
213252-14-3.af.
-
332348-12-6.af.
-
bms188667.af.
-
bms 188667.af.
-
ctla4ig.af.
-
ctla 4ig.af.
-
golimumab.af.
-
cnto148.af.
-
cnto 148.af.
-
simponi.af.
-
476181-74-5.af.
-
tocilizumab.af.
-
atlizumab.af.
-
actemra.af.
-
roactemra.af.
-
375823-41-9.af.
-
tofacitinib.af.
-
xeljanz.af.
-
tasocitinib.af.
-
cp690550.af.
-
cp 690550.af.
-
540737-29-9.af.
-
rituximab.af.
-
rituxan.af.
-
mabthera.af.
-
174722-31-7.rn.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47
-
rheumatoid arthritis.tw.
-
exp Arthritis, Rheumatoid/
-
49 or 50
-
48 and 51
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
53 or 54 or 55 or 56 or 57 or 58 or 59 or 60
-
exp animals/ not humans.sh.
-
61 not 62
-
52 and 63
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R) (via Ovid)
Date range searched: 1948 to July 2013.
Search undertaken April 2013.
-
atacicept.af.
-
845264-92-8.rn.
-
unii-k3d9a0icq3.af.
-
uniik3d9a0icq3.af.
-
taci-fc5.af.
-
tacifc5.af.
-
taci-ig.af.
-
taciig.af.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
-
rheumatoid arthritis.tw.
-
exp Arthritis, Rheumatoid/
-
10 or 11
-
9 and 12
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
14 or 15 or 16 or 17 or 18 or 19 or 20 or 21
-
exp animals/ not humans.sh.
-
22 not 23
-
13 and 24
EMBASE (via Ovid)
Date range searched: 1980 to July 2013.
Search undertaken April 2013.
-
adalimumab.af.
-
humira.af.
-
d 2e7.af.
-
d2e7.af.
-
331731-18-1.af.
-
etanercept.af.
-
enbrel.af.
-
185243-69-0.af.
-
infliximab.af.
-
remicade.af.
-
170277-31-3.af.
-
ta650.af.
-
ta 650.af.
-
certolizumab pegol.af.
-
cimzia.af.
-
cdp870.af.
-
cdp 870.af.
-
428863-50-7.af.
-
1132819-27-2.af.
-
czp.af.
-
abatacept.af.
-
orencia.af.
-
213252-14-3.af.
-
332348-12-6.af.
-
bms188667.af.
-
bms 188667.af.
-
ctla4ig.af.
-
ctla 4ig.af.
-
golimumab.af.
-
cnto148.af.
-
cnto 148.af.
-
simponi.af.
-
476181-74-5.af.
-
tocilizumab.af.
-
atlizumab.af.
-
actemra.af.
-
roactemra.af.
-
375823-41-9.af.
-
tofacitinib.af.
-
xeljanz.af.
-
tasocitinib.af.
-
cp690550.af.
-
cp 690550.af.
-
540737-29-9.af.
-
rituximab.af.
-
rituxan.af.
-
mabthera.af.
-
174722-31-7.af.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48
-
exp rheumatoid arthritis/
-
rheumatoid arthritis.tw.
-
50 or 51
-
49 and 52
-
random$.tw.
-
clinical trial$.mp.
-
exp health care quality/
-
54 or 55 or 56
-
53 and 57
EMBASE (via Ovid)
Date range searched: 1980 to July 2013.
Search undertaken April 2013.
-
atacicept.af.
-
845264 92 8.af.
-
unii-k3d9a0icq3.af.
-
uniik3d9a0icq3.af.
-
taci-fc5.af.
-
tacifc5.af.
-
taci-ig.af.
-
taciig.af.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
-
exp rheumatoid arthritis/
-
rheumatoid arthritis.tw.
-
10 or 11
-
9 and 12
-
random$.tw.
-
clinical trial$.mp.
-
exp health care quality/
-
14 or 15 or 16
-
13 and 17
Stage 3 clinical effectiveness searches
Stage 3 searches identified studies of adverse events. Searches were undertaken in July 2013. In addition to the searches detailed below evidence was sought through consultation of the National Library of Medicine (NLM) TOXLINE resource and through the website of the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R) (via Ovid)
Date range searched: 1948 to July 2013.
Search undertaken July 2013.
-
adalimumab.af.
-
humira.af.
-
d 2e7.af.
-
d2e7.af.
-
331731-18-1.rn.
-
etanercept.af.
-
enbrel.af.
-
185243-69-0.rn.
-
infliximab.af.
-
remicade.af.
-
170277-31-3.rn.
-
ta650.af.
-
ta 650.af.
-
certolizumab pegol.af.
-
cimzia.af.
-
cdp870.af.
-
428863-50-7.rn.
-
1132819-27-2.rn.
-
czp.af.
-
abatacept.af.
-
orencia.af.
-
213252-14-3.af.
-
332348-12-6.af.
-
bms188667.af.
-
bms 188667.af.
-
ctla4ig.af.
-
ctla 4ig.af.
-
golimumab.af.
-
cnto148.af.
-
cnto 148.af.
-
simponi.af.
-
476181-74-5.af.
-
tocilizumab.af.
-
atlizumab.af.
-
actemra.af.
-
roactemra.af.
-
375823-41-9.af.
-
tofacitinib.af.
-
xeljanz.af.
-
tasocitinib.af.
-
cp690550.af.
-
cp 690550.af.
-
540737-29-9.af.
-
rituximab.af.
-
rituxan.af.
-
mabthera.af.
-
174722-31-7.rn.
-
atacicept.af.
-
845264-92-8.rn.
-
unii-k3d9a0icq3.af.
-
uniik3d9a0icq3.af.
-
taci-fc5.af.
-
tacifc5.af.
-
taci-ig.af.
-
taciig.af.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55
-
rheumatoid arthritis.tw.
-
exp Arthritis, Rheumatoid/
-
57 or 58
-
56 and 59
-
(ae or co or de).fs.
-
(safe or safety or side effect* or undesirable effect* or treatment emergent or tolerability or toxicity or adrs or (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes))).tw.
-
61 or 62
-
60 and 63
EMBASE (via Ovid)
Date range searched: 1980 to July 2013.
Search undertaken July 2013.
-
adalimumab.af.
-
humira.af.
-
d 2e7.af.
-
d2e7.af.
-
331731-18-1.af.
-
etanercept.af.
-
enbrel.af.
-
185243-69-0.af.
-
infliximab.af.
-
remicade.af.
-
170277-31-3.af.
-
ta650.af.
-
ta 650.af.
-
certolizumab pegol.af.
-
cimzia.af.
-
cdp870.af.
-
cdp 870.af.
-
428863-50-7.af.
-
1132819-27-2.af.
-
czp.af.
-
abatacept.af.
-
orencia.af.
-
213252-14-3.af.
-
332348-12-6.af.
-
bms188667.af.
-
bms 188667.af.
-
ctla4ig.af.
-
ctla 4ig.af.
-
golimumab.af.
-
cnto148.af.
-
cnto 148.af.
-
simponi.af.
-
476181-74-5.af.
-
tocilizumab.af.
-
atlizumab.af.
-
actemra.af.
-
roactemra.af.
-
375823-41-9.af.
-
tofacitinib.af.
-
xeljanz.af.
-
tasocitinib.af.
-
cp690550.af.
-
cp 690550.af.
-
540737-29-9.af.
-
rituximab.af.
-
rituxan.af.
-
mabthera.af.
-
174722-31-7.af.
-
atacicept.af.
-
845264 92 8.af.
-
unii-k3d9a0icq3.af.
-
uniik3d9a0icq3.af.
-
taci-fc5.af.
-
tacifc5.af.
-
taci-ig.af.
-
taciig.af.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56
-
exp rheumatoid arthritis/
-
rheumatoid arthritis.tw.
-
58 or 59
-
57 and 60
-
(safe or safety or side effect* or undesirable effect* or treatment emergent or tolerability or toxicity or adrs or (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes))).tw.
-
61 and 62
Appendix 2 Quality assessment summary of findings
| Trial name/study | Intervention | Population | NMA (Y/N) | Was the method used to generate the allocation sequence to treatment groups adequate? (Y/N/U) | Was the allocation of treatment concealed adequately? (Y/N/U) | Were the treatment groups comparable at baseline? (Y/N/U/N/A) | Were patients and study personnel blinded to treatment? (Y/N/U) | Were participants analysed in their allocated treatment groups? (Y/N/U) | Were all randomised patients included in efficacy analyses? (Y/N/U/mITT/N/A) | Were all randomised patients included in safety analyses? (Y/N/U/mITT/N/A) | Were at least 80% of participants originally randomised included in the final analysis? (Y/N/U) | Free of evidence of selective reporting of outcomes? (Y/N/U) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abe et al., 200656 | IFX | 2/3 | N | U | U | Y | Y | U | mITT | mITT | Y | U |
| ACT-RAY57 | TCZ | 2/3 | Y | Y | Y | Y | Y | Y | mITT | mITT | Y | N |
| ADACTA58 | ADA, TCZ | 2/3 | Y | Y | Y | Y | Y | Y | mITT | mITT | Y | N |
| ADORE59 | ETN | 2/3 | N | U | U | U | N | Y | mITT | mITT | Y | U |
| AIM61 | ABT | 2/3 | Y | Y | Y | Y | Y | Y | mITT | mITT | Y | Y |
| AMPLE66 | ADA, ABT | 2/3 | Y | U | U | Y | N | Y | mITT | mITT | Y | Y |
| APPEAL68 | ETN | 2/3 | N | U | U | Y | N | Y | mITT | mITT | Y | N |
| ARMADA69 | ADA | 2/3 | Y | U | U | Y | Y | Y | Y | Y | Y | U |
| ASPIRE71 | IFX | 1 | N | Y | Y | Y | Y | Y | N | mITT | Y | U |
| ASSET72 | ABT | 2/3 | N | Y | Y | N | Y | Y | mITT | mITT | Y | N |
| ASSURE73 | ABT | 2/3 | N | U | U | Y | Y | Y | mITT | mITT | Y | N |
| ATTEST74 | IFX, ABT | 2/3 | Y | U | U | Y | Y | Y | mITT | mITT | U | N |
| ATTRACT75 | IFX | 2/3 | Y | Y | Y | N | Y | Y | U | Y | Y | U |
| AUGUST II76 | ADA | 2/3 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y |
| Bejarano et al., 200877 | ADA | 1 | N | Y | Y | Y | Y | Y | Y | Y | Y | N |
| BeST78 | IFX | 1 | Y | Y | N | Y | N | Y | U | U | Y | U |
| CERTAIN79 | CTZ | 2/3 | Y | U | U | U | Y | Y | U | U | Y | Y |
| CHANGE80 | ADA | 2/3 | Y | U | U | Y | Y | Y | Y | Y | Y | U |
| COMET81 | ETN | 1 | N | Y | Y | Y | Y | Y | mITT | mITT | Y | Y |
| DE01984 | ADA | 2/3 | Y | U | U | Y | Y | Y | Y | Y | Y | Y |
| deFilippis et al., 200685 | ETN, IFX | 2/3 | Y | U | U | Y | N | Y | N | N | Y | U |
| Durez et al., 200486 | IFX | 2/3 | N | U | U | N | N | Y | U | U | U | U |
| Durez et al., 2007120 | IFX | 1 | Y | U | U | N | U | U | U | U | Y | Y |
| ERA139 | ETN | 1 | Y | U | U | Y | Y | Y | mITT | mITT | Y | U |
| ETN study 30989 | ETN | 2/3 | Y | U | U | Y | Y | Y | mITT | mITT | Y | Y |
| GO-BEFORE90 | GOL | 1 | Y | Y | Y | Y | Y | Y | Y | mITT | Y | N |
| GO-FORTH91 | GOL | 2/3 | Y | U | U | Y | Y | Y | mITT | mITT | Y | Y |
| GO-FORWARD92 | GOL | 2/3 | Y | Y | Y | Y | Y | Y | Y | mITT | Y | N |
| GUEPARD93 | ADA | 1 | N | U | U | Y | N | Y | mITT | Y | Y | U |
| HIT HARD94 | ADA | 1 | Y | U | U | N | Y | Y | mITT | mITT | Y | U |
| IDEA95 | IFX | 1 | N | U | U | U | U | U | U | N/A | U | U |
| CREATE IIb96 | ETN | 2/3 | Y | U | U | Y | N | Y | Y | Y | Y | N |
| JESMR140 | ETN | 2/3 | Y | U | U | N | N | Y | mITT | mITT | Y | Y |
| Kay et al., 200898 | GOL | 2/3 | N | U | U | N | Y | Y | Y | mITT | Y | N |
| Kim et al., 200799 | ADA | 2/3 | Y | U | U | Y | Y | Y | mITT | Y | Y | U |
| Kume et al., 2011100 | ADA, ETN | 1 | N | U | U | Y | N | Y | N | N/A | Y | N |
| Lan et al., 2004101 | ETN | 2/3 | N | U | U | Y | Y | Y | mITT | mITT | Y | U |
| LARA102 | ETN | 2/3 | Y | U | U | Y | N | Y | mITT | Y | Y | U |
| MEASURE103 | TCZ | 2/3 | N | U | U | U | Y | U | U | N/A | U | U |
| Moreland et al., 1999104 | ETN | 2/3 | Y | Y | Y | Y | Y | Y | mITT | mITT | Y | U |
| Nishimoto et al., 2004106 | TCZ | 2/3 | N | U | U | Y | Y | Y | Y | Y | Y | U |
| OPERA107 | ADA | 1 | N | Y | Y | Y | Y | Y | mITT | mITT | Y | U |
| OPTIMA108 | ADA | 1 | Y | Y | Y | Y | Y | Y | mITT | mITT | Y | Y |
| PREMIER109 | ADA | 1 | Y | U | U | N | Y | Y | mITT | mITT | Y | U |
| Quinn et al., 2005110 | IFX | 1 | N | U | U | Y | Y | Y | U | U | Y | U |
| RACAT111 | ETN | 2/3 | Y | Y | Y | Y | Y | Y | N | N | Y | Y |
| REALISTIC113 | CTZ | 2/3 | N | Y | Y | Y | U | Y | Y | mITT | Y | N |
| RED-SEA114 | ADA, ETN | 2/3 | N | Y | N | Y | N | Y | mITT | mITT | Y | Y |
| SAMURAI115 | TCZ | 2/3 | Y | U | Y | Y | N | Y | mITT | mITT | Y | U |
| SATORI116 | TCZ | 2/3 | Y | Y | Y | Y | Y | Y | mITT | mITT | Y | N |
| STAR117 | ADA | 2/3 | Y | U | U | Y | Y | Y | mITT | mITT | Y | U |
| START118 | IFX | 2/3 | Y | U | U | Y | Y | Y | mITT | N | Y | U |
| Swefot119 | IFX | 2/3 | Y | Y | Y | Y | N | Y | Y | Y | Y | N |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| TOWARD121 | TCZ | 2/3 | Y | U | U | Y | Y | Y | mITT | mITT | Y | U |
| Van De Putte et al., 2004122 | ADA | 2/3 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U |
| Wajdula 2000 (reported in Chen et al., 2006123) | ETN | 2/3 | N | U | U | Y | Y | U | U | U | Y | U |
| Weinblatt et al., 1999124 | ETN | 2/3 | Y | U | U | Y | Y | Y | Y | Y | Y | U |
| Wong et al., 2009125 | IFX | 2/3 | N | U | U | Y | Y | Y | U | N/A | U | U |
| Zhang et al., 2006126 | IFX | 2/3 | N | U | U | N | Y | U | U | U | U | U |
Appendix 3 Excluded studies
| Study | Rationale for exclusion |
|---|---|
| ADJUST Emery P, Durez P, Dougados M, Legerton CW, Becker JC, Vratsanos G, et al. Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial). Ann Rheum Dis 2010;69:510–16. [Erratum published in Ann Rheum Dis 2011;70:1519] |
Population: DMARD naive but moderate–severe (ABT) |
| AGREE Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis 2009;68:1870–7 |
Population: MTX naive (not licensed for this population) (ABT) |
| ALLOW Kaine J, Gladstein G, Strusberg I, Robles M, Louw I, Gujrathi S, et al. Evaluation of abatacept administered subcutaneously in adults with active rheumatoid arthritis: impact of withdrawal and reintroduction on immunogenicity, efficacy and safety (Phase IIIb ALLOW study). Ann Rheum Dis 2012;71:38–44 |
Population: prior biologics (open-label run-in phase) (ABT) |
| ARRIVE Schiff M, Pritchard C, Huffstutter JE, Rodriguez-Valverde V, Durez P, Zhou X, et al. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: the ARRIVE trial. Ann Rheum Dis 2009;68:1708–14 |
Population: previous use of antiTNF therapy in all (ABT) |
| ATTAIN Genovese MC, Becker J-C, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumour necrosis factor alpha inhibition. N Engl J Med 2005;353:1114–23 |
Population: previous use of antiTNF therapy in all (ABT) |
| ATTUNE Keystone EC, Kremer JM, Russell A, Box J, Abud-Mendoza C, Elizondo MG, et al. Abatacept in subjects who switch from intravenous to subcutaneous therapy: results from the phase IIIb ATTUNE study. Ann Rheum Dis 2012;71:857–61 |
Study design: not a RCT. LTE of AIM61–65 and ATTAIN trials (ABT) |
| TAMARA Burmester GR, Feist E, Kellner H, Braun J, Iking-Konert C, Rubbert-Roth A. Effectiveness and safety of the interleukin 6-receptor antagonist tocilizumab after 4 and 24 weeks in patients with active rheumatoid arthritis: the first phase IIIb real-life study (TAMARA). Ann Rheum Dis 2011;70:755–9 |
Not a RCT (single-arm study) (TCZ) |
| ACT-SURE Bykerk VP, Ostor AJ, Alvaro-Gracia J, Pavelka K, Ivorra JA, Graninger W, et al. Tocilizumab in patients with active rheumatoid arthritis and inadequate responses to DMARDs and/or TNF inhibitors: a large, open-label study close to clinical practice. Ann Rheum Dis 2012;71:1950–4 |
Not a RCT (TCZ) |
| C87014 Choy E, McKenna F, Vencovsky J, Valente R, Goel N, Vanlunen B, et al. Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology 2012;51:1226–34 |
Intervention (not licensed dose) (CTZ) |
| CanACT Haraoui B, Cividino A, Stewart J, Guerette B, Keystone EC. Safety and effectiveness of adalimumab in a clinical setting that reflects Canadian standard of care for the treatment of rheumatoid arthritis (RA): results from the CanACT study. BMC Musculoskelet Disord 2011;12:261 |
Not a RCT (ADA) |
| Chen HA, Lin KC, Chen CH, Liao HT, Wang HP, Chang HN, et al. The effect of etanercept on anti-cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis. Ann Rheum Dis 2006;65:35–9 | Study investigating serum levels of anticyclic citrullinated peptide antibodies and rheumatoid factor: excluded outcomes (ETN) |
| Chen D-Y, Chou S-J, Hsieh T-Y, Chen Y-H, Chen H-H, Hsieh C-W, et al. Randomised, double-blind, placebo-controlled, comparative study of human anti-TNF antibody adalimumab in combination with methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis. J Formos Med Assoc 2009;108:310–19 | Participants on MTX, unclear if had inadequate response, 12-week study, n = 47 (ADA) |
| Choy EH, Hazleman B, Smith M, Moss K, Lisi L, Scott DG, et al. Efficacy of a novel PEGylated humanised anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomised, dose-escalating trial. Rheumatology 2002;41:1133–7 | Intervention: not licensed dose (CTZ) |
| Choy EH, Isenberg DA, Garrood T, Farrow S, Ioannou Y, Bird H, et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomised, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum 2002;46:3143–50 | Not in line with licensed indications |
| DART Moots RJ, Haraoui B, Matucci-Cerinic M, Van Riel PLCM, Kekow J, Schaeverbeke T, et al. Differences in biologic dose-escalation, non-biologic and steroid intensification among three anti-TNF agents: evidence from clinical practice. Clin Exp Rheumatol 2011;29:26–34 |
Not a RCT (ADA, ETN, IFX) |
| Doseflex Furst D, Shaikh S, Greenwald M, Bennett B, Staelens F. Evaluation of two dosing regimens of certolizumab pegol for maintenance of clinical response in patients with active rheumatoid arthritis: primary results from doseflex, a phase IIIB study. Ann Rheum Dis 2012;71:513 |
Population: prior biologics (open-label run-in) (CTZ) |
| Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 1994;344:1105–10 | Not in line with licensed indications (IFX) |
| RADIATE Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008;67:1516–23 |
Biologic-experienced population (outside appraisal scope) (TCZ) |
| FAST4WARD Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis 2009;68:805–11 |
Intervention: not licensed dose (CTZ) |
| Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 2012;64:617–29 | Approximately 10% of participants had prior biologics, fewer than 22 weeks of ADA treatment (10 weeks ADA then switch to TOF), so not included as additional evidence |
| OPPOSITE Furst DE, Gaylis N, Bray V, Olech E, Yocum D, Ritter J, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis 2007;66:893–9 |
Biologic-experienced population (outside appraisal scope) (IFX) |
| Genovese MC, Sebba A, Rubbert-Roth A, Scali JJ, Alten R, Kremer JM, et al. Long-term safety of tocilizumab in patients with rheumatoid arthritis and a mean treatment duration of 3.7 years. Arthritis Rheum 2012;64:1640 | Pooled data excluded |
| Genovese MC, Cohen S, Moreland L, Lium D, Robbins S, Newmark R, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum 2004;50:1412–19 | Comparators unlicensed as ETN in combination with anakinra |
| Hall S, Fleischmann R. Tocilizumab inhibits radiological progression and improves physical function in rheumatoid arthritis (RA) patients at 2 years with increasing clinical efficacy over time. Intern Med J 2010;40:13 | Insufficient details on data-analyses and no useable pre-withdrawal data (TCZ) |
| HIKARI (NCT00791921) Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and safety of certolizumab pegol without methotrexate co-administration in Japanese patients with active rheumatoid arthritis. Arthritis and Rheumatism Conference: Annual Scientific Meeting of the American College of Rheumatology and Association of Rheumatology Health Professionals, Chicago, IL, 4–9 November 2011 |
Study design: no separate 6-month data for those with concomitant cDMARDs and monotherapy (CTZ) |
| RESTART Ingham M, Tang L, Decktor D, Bolce R, Wang J. Benefits in patient reported outcomes supporting a ‘treat to target’ paradigm for infliximab-treated RA patients previously inadequately responsive to prior anti-TNF treatment. Value Health 2012;15:A42–3 |
All patients received IFX prior to randomisation to range of IFX doses (not comparable with other trial populations at baseline) (IFX) |
| Johnsen AK, Schiff MH, Mease PJ, Moreland LW, Maier AL, Coblyn JS, et al. Comparison of 2 doses of etanercept (50 vs 100 mg) in active rheumatoid arthritis: a randomised double blind study. J Rheumatol 2006;33:659–64 | Comparator unlicensed dose (ETN) |
| Kaufman J, Seel S, Roske A-E. Comparison of tocilizumab and TNF inhibitor therapy in rheumatoid arthritis. Arthritis Rheum 2011;63:S1271 | Not a RCT (TCZ) |
| Kavanaugh A, St Clair EW, McCune WJ, Braakman T, Lipsky P. Chimeric anti-tumour necrosis factor-alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapy. J Rheumatol 2000;27:841–50 | Not in line with licensed indications (IFX) |
| Kellner H, Kellner W. Tocilizumab improves in rheumatoid arthritis patients with longstanding but still active disease the clinical disease activity (DAS28) and ameliorates MRI findings within the first three months of therapy. Arthritis Rheum 2011;63:S951 | Pre-treatment with biologics (TCZ) |
| Keystone EC, Schiff MH, Kremer JM, Kafka S, Lovy M, DeVries T, et al. Once-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomised, double-blind, placebo-controlled Trial. Arthritis Rheum 2004;50:353–63 | Cannot distinguish results between monotherapy and combination therapy, half of participants in each of three treatment arms given MTX, half not, 8-week RCT stage of 16-week study (ETN) |
| Khraishi et al. Long-term efficacy of tocilizumab (TCZ) in patients with rheumatoid arthritis (RA). Ann Rheum Dis 2011;70:472 | Pooled data excluded (TCZ) |
| Kume K, Amano K, Yamada S, Hatta K. Tocilizumab improves arterial stiffness compared with abatacept in patients with TNF blockers-resistant active rheumatoid arthritis. An open label randomised controlled trial. Arthritis Rheum 2011;63:S147 | All had prior biologics (TCZ) |
| Kume K, Amano K, Yamada S, Hatta K. Tocilizumab monotherapy improves bone mineral density as well as TNF blockers plus methotrexate with methotrexate-resistant active rheumatoid arthritis: an open-label randomised clinical trial. T-BONE trial. Arthritis Rheum 2011;63:S396 | No useable scope outcome data (TCZ) |
| NEO-RACo Leirisalo-Repo M, Kautiainen H, Laasonen L, Korpela M, Kauppi MJ, Kaipiainen-Seppanen O, et al. Infliximab for 6 months added on combination therapy in early rheumatoid arthritis: 2-year results from an investigator-initiated, randomised, double-blind, placebo-controlled study (the NEO-RACo Study). Ann Rheum Dis 2013;72:851–7 |
Dosing interval in induction phase not in line with licensed indications (IFX) |
| Lim M, Park S-H, Shim S, Baek H, Yoo D-H. A double-blind, placebo-controlled, multicenter trial of tocilizumab in moderate to severe active RA patients with inadequate response to methotrexate in Korean population. Ann Rheum Dis 2012;71:670 | Insufficient description of statistical analyses in conference abstract to permit critical appraisal and handling of data (TCZ) |
| Lisbona MP, Maymo J, Perich J, Almirall M, Perez-Garcia C, Carbonell J. Etanercept reduces synovitis as measured by magnetic resonance imaging in patients with active rheumatoid arthritis after only 6 weeks. J Rheumatol 2008;35:394–7 Lisbona MP, Maymo J, Perich J, Almirall M, Carbonell J. Rapid reduction in tenosynovitis of the wrist and fingers evaluated by MRI in patients with rheumatoid arthritis after treatment with etanercept. Ann Rheum Dis 2010;69:1117–1122 |
Treatment of tendosynovitis in RA, mostly excluded outcomes, 6-week study (ETN) |
| Lorenz HM, Antoni C, Valerius T, Repp R, Grunke M, Schwerdtner N, et al. In vivo blockade of TNF-alpha by intravenous infusion of a chimeric monoclonal TNF-alpha antibody in patients with rheumatoid arthritis. Short term cellular and molecular effects. J Immunol 1996;156:1646–53 | Not in line with licensed indications (IFX) |
| Lorenz HM, Grunke M, Hieronymus T, Antoni C, Nusslein H, Schaible TF, et al. In vivo blockade of tumour necrosis factor-alpha in patients with rheumatoid arthritis: longterm effects after repeated infusion of chimeric monoclonal antibody cA2. J Rheumatol 2000;27:304–10 | Not in line with licensed indications (IFX) |
| Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumour necrosis factor monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998;41:1552–63 | Not in line with licensed indications (IFX) |
| CHARISMA Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double-blind randomised controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006;54:2817–29 |
Low levels of prior biologics and no ACR–EULAR response data at weeks 22–30 for NMA (week 16 data only) (TCZ) |
| Nakashima Y, Kondo M, Harada H, Horiuchi T, Ishinishi T, Jojima H, et al. Clinical evaluation of tocilizumab for patients with active rheumatoid arthritis refractory to anti-TNF biologics: tocilizumab in combination with methotrexate. Mod Rheumatol 2010;20:343–52 | Not a RCT (TCZ) |
| Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ. Randomised phase 2 trial of anti-tumour necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. Am J Clin Nutr 2006;84:1463–72 | Treatment of cachexia (ETN) |
| Markatseli TE, Alamanos Y, Saougou I, Voulgari PV, Drosos AA. Survival of TNF-alpha antagonists in rheumatoid arthritis: a long-term study. Clin Exp Rheumatol 2012;30:31–8 | Not a RCT (TNF inhibitors) |
| Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL, et al. Treatment of rheumatoid arthritis with a recombinant human tumour necrosis factor receptor (p75)–Fc fusion protein. N Engl J Med 1997;337:141–7 | Unlicensed dose (ETN) |
| Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol 2010;20:222–32 | Pooled data excluded |
| Pavelka K, Jarosova K, Suchy D, Senolt L, Chroust K, Dusek L, et al. Increasing the infliximab dose in rheumatoid arthritis patients: a randomised, double blind study failed to confirm its efficacy. Ann Rheum Dis 2009;68:1285–9 | All patients received prior biologics (IFX) |
| Perkins DJ, St Clair EW, Misukonis MA, Weinberg JB. Reduction of NOS2 overexpression in rheumatoid arthritis patients treated with anti-tumour necrosis factor alpha monoclonal antibody (cA2). Arthritis Rheum 1998;41:2205–10 | Not in line with licensed indications (IFX) |
| PRESERVE Smolen JS, Nash P, Durez P, Hall S, Ilivanova E, Irazoque-Palazuelos F, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013;381:918–29 |
All participants on ETN, before randomisation |
| PRIZE Etanercept for the Treatment of Rheumatoid Arthritis. (Review of TA Guidance 130, 186, 224, 234 and Part Review of TA Guidance 225 and 247). Multiple Technology Appraisal (MTA). Pfizer Submission. 2013 |
All participants on ETN, before randomisation |
| ReACT Bombardieri S, Ruiz AA, Fardellone P, Geusens P, McKenna F, Unnebrink K, et al. Effectiveness of adalimumab for rheumatoid arthritis in patients with a history of TNF-antagonist therapy in clinical practice. Rheumatology 2007;46:1191–99 |
Not a RCT, prior biologics (ADA) |
| Roux CH, Breuil V, Valerio L, Amoretti N, Brocq O, Albert C, et al. Etanercept compared to intraarticular corticosteroid injection in rheumatoid arthritis: double-blind, randomised pilot study. J Rheumatol 2011;38:1009–11 | Comparator steroid only (ETN) |
| Smeets TJ, Kraan MC, van Loon ME, Tak PP. Tumour necrosis factor alpha blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum 2003;48:2155–62 | No scope outcomes |
| GO-AFTER Smolen JS, Kay J, Doyle MK, Landewe R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 2009;374:210–21 |
Biologic-experienced population (outside appraisal scope) (GOL) |
| STREAM van Eijk IC, Nielen MMJ, van der Horst-Bruinsma I, Tijhuis GJ, Boers M, Dijkmans BAC, et al. Aggressive therapy in patients with early arthritis results in similar outcome compared with conventional care: the STREAM randomised trial. Rheumatology 2012;51:686–94 |
Participants did not have to have diagnosis of RA to be eligible for trial, DAS < 3.2 (ADA) |
| Takeuchi T, Matsubara T, Nitobe T, Suematsu E, Ohta S, Honjo S, et al. Phase II dose–response study of abatacept in Japanese patients with active rheumatoid arthritis with an inadequate response to methotrexate. Mod Rheumatol 2013;23:226–35 | Population: prior biologics (ABT) |
| RISING Takeuchi T, Miyasaka N, Inoue K, Abe T, Koike T. Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study. Mod Rheumatol 2009;19:478–87 |
All patients received IFX prior to randomisation to range of IFX doses (not comparable with other trial populations at baseline) (IFX) |
| GO-MONO Takeuchi T, Harigai M, Tanaka Y, Yamanaka H, Ishiguro N, Yamamoto K, et al. Golimumab monotherapy in Japanese patients with active rheumatoid arthritis despite prior treatment with disease-modifying antirheumatic drugs: results of the phase 2/3, multicentre, randomised, double-blind, placebo-controlled GO-MONO study through 24 weeks. Ann Rheum Dis 2013;72:1488–95 |
Not in line with licensed indications (monotherapy) (GOL) |
| Tam LS, Shang Q, Li EK, Wang S, Li RJ, Lee KL, et al. Infliximab is associated with improvement in arterial stiffness in patients with early rheumatoid arthritis – a randomised trial. J Rheumatol 2012;39:2267–75 | Insufficient description of cDMARD treatment history (and no ACR/EULAR data at 22–30 weeks) (IFX) |
| TAME Greenwald MW, Shergy WJ, Kaine JL, Sweetser MT, Gilder K, Linnik MD. Evaluation of the safety of rituximab in combination with a tumour necrosis factor inhibitor and methotrexate in patients with active rheumatoid arthritis: results from a randomised controlled trial. Arthritis Rheum 2011;63:622–32 |
Comparator RTX |
| Taylor PC, Steuer A, Gruber J, Cosgrove DO, Blomley MJK, Marsters PA, et al. Comparison of ultrasonographic assessment of synovitis and joint vascularity with radiographic evaluation in a randomised, placebo-controlled study of infliximab therapy in early rheumatoid arthritis. Arthritis Rheum 2004;50:1107–16 | Not in line with licensed indications (IFX) |
| Van De Putte LBA, Rau R, Breedveld FC, Kalden JR, Malaise MG, Van Riel PLCM, et al. Efficacy and safety of the fully human anti-tumour necrosis factor alpha monoclonal antibody adalimumab (D2E7) in DMARD refractory patients with rheumatoid arthritis: a 12 week, phase II study. Ann Rheum Dis 2003;62:1168–77 | Unlicensed dose (ADA) |
| van Vollenhoven R, Ducournau P, Wintfeld N, Berger W, Alten R. Health assessment questionnaire-disability index (HAQ-DI) scores in patients with rheumatoid arthritis (RA) treated with tocilizumab plus conventional anti-rheumatic drugs. Value Health 2009;12:A434 | Pooled data excluded (TCZ) |
| Weinblatt ME, Schiff MH, Ruderman EM, Bingham CO III, Li J, Louie J, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomised, double-blind, active drug-controlled study. Arthritis Rheum 2008;58:1921–30 | Unlicensed dose (ETN), all prior inadequate response to etanercept |
| ACT-STAR Weinblatt ME, Kremer J, Cush J, Rigby W, Teng LL, Devenport J, et al. Tocilizumab as monotherapy or in combination with nonbiologic DMARDs: 24-week results of an open-label, clinical practice study (ACT-STAR). Arthritis Care Res 2013;65:362–71 |
High proportion of prior biologic use (outside appraisal scope) (TCZ) |
| Westhovens R, Cole JC, Li T, Martin M, MacLean R, Lin P, et al. Improved health-related quality of life for rheumatoid arthritis patients treated with abatacept who have inadequate response to anti-TNF therapy in a double-blind, placebo-controlled, multicentre randomised clinical trial. Rheumatology 2006;45:1238–46 | Population: inadequate response to antiTNF therapy (ABT) |
| Westhovens R, Houssiau F, Joly J, Everitt DE, Zhu Y, Sisco D, et al. A phase I study assessing the safety, clinical response, and pharmacokinetics of an experimental infliximab formulation for subcutaneous or intramuscular administration in patients with rheumatoid arthritis. J Rheumatol 2006;33:847–53 | Not in line with licensed indications (IFX) |
| GO-FURTHER Westhovens R, Weinblatt ME, Han C, Gathany T, Kim L, Mack M, et al. Fatigue is an independent variable predicting physical function and DAS-28 remission for patients with rheumatoid arthritis treated with intravenously administered golimumab: results from a phase 3, placebo controlled clinical trial. Value Health 2012;15:A42 |
Unlicensed dose (i.v. administration) (GOL) |
| REACTION Yamanaka H, Tanaka Y, Inoue E, Hoshi D, Momohara S, Hanami K, et al. Efficacy and tolerability of tocilizumab in rheumatoid arthritis patients seen in daily clinical practice in Japan: results from a retrospective study (REACTION study). Mod Rheumatol 2011;21:122–33 |
Not a RCT (TCZ) |
| ROSE Yazici Y, Curtis JR, Ince A, Baraf H, Malamet RL, Teng LL, et al. Efficacy of tocilizumab in patients with moderate to severe active rheumatoid arthritis and a previous inadequate response to disease-modifying antirheumatic drugs: the ROSE study. Ann Rheum Dis 2012;71:198–205 |
High proportion of prior biologic use (outside appraisal scope) (TCZ) |
Appendix 4 Additional data relating to the included randomised controlled trials
| Trial name/study | Trial design (RCT, phase, LTE) | Treatment arms for which data extraction performed (number of patients randomised per treatment arm) | MTX dose during study (where applicable) (mg/week) | Concomitant treatments | Duration of RCT phase | Primary outcome | Early withdrawal plan reported? | Geographical location | Funding source | Primary and supplementary publication details [author, year, publication type (e.g. full text, abstract)] |
|---|---|---|---|---|---|---|---|---|---|---|
| Kume et al., 2011100 | RCT (open label) | ADA monotherapy (n = 22 randomised) | N/A | NR | 24 weeks | Change in cardio-ankle vascular index | All patients with worsening disease activity (DAS28-ESR > 5.1 or change from baseline of DAS28-ESR > 1 at week 12 were allowed to leave the group, by clinician’s judgement | Japan | NR | Kume et al., 2011100 full text |
| Kume et al., 2011100 | ETN monotherapy (n = 21 randomised) | N/A | NR |
| Trial name/study | Trial design (RCT, phase, LTE) | Treatment arms for which data extraction performed (number of patients randomised per treatment arm) | MTX dose during study (where applicable) | Concomitant treatments | Duration of RCT phase | Primary outcome | Early withdrawal plan reported? | Geographical location | Funding source | Primary and supplementary publication details [author, year, publication type (e.g. full text, abstract)] |
|---|---|---|---|---|---|---|---|---|---|---|
| Bejarano et al., 200877 | Multicentre, RCT | PBO + MTX (n = 73) | MTX dosage increased from 7.5 mg/week to 25 mg/week by week 12 in the presence of remaining synovitis | Folate was administered according to regionally agreed Guidelines (5 mg six times/week) Stable doses of anti-inflammatory drugs, analgesics and prednisolone (up to 10 mg/day) were maintained in order for study treatment effect to be assessed without confounders. Swollen joints were permitted to be treated during the study with intra-articular injections of MP (up to 80 mg over the course of the study) |
56 weeks | Job loss of any cause and/or imminent job loss at or after week 16 | Rules for participant withdrawal included job loss, imminent job loss and AEs (at the discretion of the physician). Physicians could withdraw patients due to an unacceptably high disease activity | UK | Abbott Laboratories | Bejarano et al., 200877 full-text article in peer-reviewed journal |
| Bejarano et al., 200877 | ADA + MTX (n = 75) | |||||||||
| GUEPARD/Soubrier et al., 199993 | RCT, prospective, unblinded | Initial MTX for 12 weeks, then step-up therapy in both groups based on DAS28 (n = 32) Treatment adjusted every 3 months on the basis DAS28 If the patient did not achieve a low disease activity (DAS28 ≤ 3.2), the treating physician adjusted therapy by proceeding to the next step in the allocated treatment group Initial monotherapy started with MTX (0.3 mg/kg/week, maximum of 20 mg/week, without escalating dose regimen). In the event of remission (DAS28 < 2.6 for at least 6 months), MTX was tapered (2.5 mg/month) to a maintenance dose of 7.5 mg/week. If disease activity flared after tapering of MTX, the initial dose of MTX was reintroduced. Subsequent steps for patients with an insufficient response at week 12 or thereafter were MTX and ADA (40 mg every other week), MTX and ADA (40 mg/week), MTX and ETN (25 mg twice a week) and MTX and LEF |
12 weeks MTX 0.3 mg/kg/week, maximum of 20 mg/week, without escalating dose regimen (then step-up) | Patients were allowed to continue concomitant treatment with corticosteroids initiated before but not after inclusion (maximum daily dose of 10 mg of oral prednisone) and to take NSAIDs and simple analgesics. A single intra-articular steroid injection was allowed during the trial. All patients received folic acid (20 mg 72 hours after MTX therapy) | 1 year | The proportion of patients in low disease activity at week 12 for whom antiTNF was not introduced or reintroduced at 1 year | Step-up therapy part of intervention groups | France | Supported by a grant from the French Society of Rheumatology and the ADA treatment was provided free of charge by Abbott France | Soubrier et al., 199993 full-text article in peer-reviewed journal |
| GUEPARD93 | Initial ADA + MTX ADA 40 mg s.c. every other week 12 weeks, then step-up therapy in both groups based on DAS28 (n = 33) Treatment adjusted every 3 months on the basis DAS28 If the patient did not achieve a low disease activity (DAS28 ≤ 3.2), the treating physician adjusted therapy by proceeding to the next step in the allocated treatment group If the DAS28 was < 3.2 at week 12, ADA was stopped. In the event of remission (DAS28 < 2.6 for at least 6 months), MTX was tapered (2.5 mg/month) to a maintenance dose of 7.5 mg/week. If disease activity flared after tapering of MTX, the initial dose of MTX was reintroduced. In the event of relapse, patients restarted ADA 40 mg every other week for 12 weeks. If the DAS28 was > 3.2 after 12 weeks, ADA was stopped. In the event of inefficacy (DAS28 > 3.2 after 12 weeks of treatment), ADA was increased (40 mg/week) for 12 weeks. After 12 weeks of effective therapy, ADA was decreased (40 mg every other week) for 12 weeks and stopped if successful. In the event of ailure on ADA 40 mg/week, ETN (25 mg twice a week) was initiated for 12 weeks. If effective, ETN was stopped and started again for 12 weeks if relapse occurred. If ETN failed, LEF was initiated. If the treatment was unsuccessful after the initial 12 weeks, the same regimen was applied according to the protocol indicated above |
12 weeks MTX 0.3 mg/kg/week, maximum of 20 mg/week, without escalating dose regimen (then step-up) | ||||||||
| HIT HARD94 | RCT | MTX + PBO (n = 85 randomised) | 15 mg/week | Folic acid 10 mg/week, stable dose of ≤ 10 mg/day prednisone or equivalent permitted | 24 weeks | DAS28 at week 48 | No | Germany | German Federal Ministry of Education and Research (ADA provided by Abbott under unconditional scientific grant) | Detert et al., 201394 full-text paper |
| HIT HARD94 | ADA + PBO (n = 87 randomised) | N/A | ||||||||
| OPERA/Horslev-Petersen 2013107 | RCT | MTX + PBO + steroid (n = 91 randomised) | Dose escalated from 7.5 mg/week at baseline to 15 mg/week at 1 month and 20 mg/week after 2 months (or highest tolerated dose) | Folic acid (5–10 mg/week) and oral calcium with vitamin D (1000 mg calcium + 800 IU vitamin D daily). Alendronate (70 mg/week) initiated at baseline and mild analgesics (but not NSAIDs, muscle relaxants or other analgesics) were permitted | 12 months | Proportion of patients in each group that had achieved low disease activity (DAS28-CRP < 3) at 12 months | Treatment escalation – HCQ or SSZ given at 3 months if DAS28-CRP ≥ 3.2 and ≥ 1 swollen joint or 4 mg of triamcinolone had been given monthly for 3 consecutive months. If low disease activity not achieved by 6 months patient treated as a non-responder, excluded and open-label biologics (not ADA) prescribed | Denmark | Abbott Laboratories, Denmark (who also provided free ADA and PBO). Triamcinolone supplied by Meda Pharmaceuticals, Denmark | Horslev-Petersen 2013107 full-text paper |
| OPERA/Horslev-Petersen 2013107 | ADA + MTX + steroid (n = 89 randomised) | |||||||||
| OPTIMA108 | RCT (Phase IV) | MTX + PBO (n = 517 randomised) | Titrated to 20 mg/week by week 8 | NSAIDs (79%), corticosteroids (46%) | 26 weeks | Composite of DAS28(CRP) < 3.2 at week 78 and no radiographic progression from baseline to week 78 | No | North and South America, Europe, Africa, New Zealand and Australia | Abbott Laboratories | Kavanaugh et al., 2013108 full-text paper Peterfy et al., 2010150 abstract Emery et al., 2011154 abstract Smolen et al., 2010142 abstract |
| OPTIMA108 | ADA + MTX (n = 515 randomised) | NSAIDs (78%), corticosteroids (41%) | ||||||||
| PREMIER109 | RCT | MTX + PBO (n = 257 randomised) | 7.5 mg/week for first 4 weeks, increased to 15 mg/week at weeks 4–8 if tolerated and to 20 mg/week at week 9 | Folic acid, 5–10 mg/week | 2 years | ACR50 response and mean change from baseline in modified total Sharp score | Dose escalation (frequency) of ADA or PBO for those not achieving ACR20 response at week 16 or later | Australia, Europe and North America | Abbott Laboratories | Breedveld et al., 2006109 full-text paper Heijde et al., 2010310 full-text paper Emery et al., 2009311 full-text paper Strand et al., 2012155 full-text paper |
| PREMIER109 | ADA monotherapy + PBO step-up week 16 (n = 274 randomised) | N/A | ||||||||
| PREMIER109 | ADA + MTX step-up week 16 (n = 268 randomised) | 7.5 mg/week for first 4 weeks, increased to 15 mg/week at weeks 4–8 if tolerated and to 20 mg/week at week 9 | ||||||||
| COMET81–83 Emery et al., 2010,82 Kekow et al., 201083 (NCT00195494) |
Prospective double-blind multicentre RCT | MTX + PBO (n = 268) First period comprised two randomised groups: (a) MTX monotherapy in year 1 followed by combination (ETN + MTX) treatment in year 2 (n = 90 at start of period 2) (b) MTX monotherapy in year 1 followed by continued MTX monotherapy in year 2 (n = 99 at start of period 2) |
Starting at 7.5 mg once a week. In patients with tender or swollen joints, the dose was titrated up over 8 weeks to a maximum of 20 mg a week | Stable doses of oral corticosteroids (≤ 10 mg per day of prednisone or an equivalent agent) or a single NSAID were permitted if started at least 4 weeks before baseline and kept constant throughout the first 24 weeks of the study | 52 weeks | Coprimary end points were the proportion of patients achieving remission (DAS28 < 2.6) at week 52 and the change in van der Heijde modified total Sharp score (modified total Sharp score; joint erosion score plus JSN score) from baseline to week 52 | NR | Europe, Latin America, Asia and Australia | Wyeth Research | Emery et al. 200881 full-text article in peer-reviewed journal |
| COMET81–83 | ETN + MTX (n = 274) First period comprised two randomised groups: (a) combination ETM + MTX treatment in year 1 followed by continued combination treatment in year 2 (n = 111 at start of period 2) (b) combination treatment in year 1 followed by ETN alone in year 2 (n = 111 at start of period 2) |
Starting at 7.5 mg once a week. In patients with tender or swollen joints, the dose was titrated up over 8 weeks to a maximum of 20 mg a week | ||||||||
| ERA139 | RCT | MTX + PBO (n = 217 randomised) | Initial dose of 7.5 mg/week escalated to 15 mg/week at week 4 and 20 mg/week at week 8. One 5-mg reduction permitted | Folic acid (1 mg/day) | 12 months | Overall response during the first 6 months | No | NR | Immunex | Bathon et al., 200087 full-text paper Bathon and Genovese, 2003139 full-text paper Kosinski et al., 2002157 full-text paper |
| ERA/Bathon and Genovese, 2003139 multicentre | ETN + PBO (n = 207 randomised) | |||||||||
| GO-BEFORE90 (EudraCT database no. 2004–003295–10) |
RCT (Phase III, double blind) | PBO + MTX (n = 160) | 19.1 mg/week (SD = 2.7 mg/week) (week 23) | NSAIDs, other analgesics for RA and oral corticosteroids (≤ 10 mg of prednisone/day or equivalent) permitted if doses stable for ≥ 2 weeks before initiation of study agent and during treatment | 52 weeks | Co-primary end points: ACR50 response at week 24 Change from baseline in modified Sharp/van der Heijde score at week 52 |
No | Multicentre, multinational [90 sites across Europe/Australia/New Zealand (n = 34), Asia (n = 25), North American (n = 2) and Latin America (n = 10)] | Centocor Research and Development and Schering-Plough Research Institute) | Emery et al., 200990 full publication |
| GO-BEFORE90 | GOL 50 mg s.c. every 4 weeks + MTX (n = 159) | 19.2 mg/week (SD = 2.35 mg/week) (week 23) | ||||||||
| ASPIRE71 | RCT (Phase III, double blind) | PBO + MTX (n = 298 randomised) | MTX started at 7.5 mg/week and increased (2.5 mg/week every 1–2 weeks) to 15 mg/week by week 4 and 20 mg/week by week 8. MTX dose could be adjusted in case of intolerance | Oral corticosteroids (≤ 10 mg/day of prednisone or equivalent) and NSAIDs maintained at baseline doses. Other DMARDs not allowed during study | 54 weeks | For radiographic progression of joint damage: change from baseline to week 54 in van der Heijde modification of total Sharp score For physical function: change from baseline in HAQ scores averaged over weeks 30–54 |
No | Multicentre, multinational (122 sites in North America and Europe) | Centocor | St Clair et al. 200471 full publication |
| ASPIRE71 | IFX i.v. 3 mg/kg at weeks 0, 2 and 6 and every 8 weeks thereafter + MTX (n = 373 randomised) | |||||||||
| BeST78 | RCT (phase NR, open label) | Sequential monotherapy (n = 126 randomised) | DAS-steered step-up strategies for all four treatment groups | Concomitant treatment with NSAIDs and intra-articular injections with corticosteroids permitted | 3 years | HAQ and modified Sharp/van der Heijde score | No (DAS-steered step-up strategies for all four treatment groups) | Multicentre, the Netherlands | Dutch College of Health Insurances; Schering-Plough | Goekoop-Ruiterman et al. 200878 full publication |
| BeST78 | Step-up combination therapy (n = 121 randomised) | |||||||||
| BeST78 | Initial combination therapy with prednisone (n = 133 randomised) | |||||||||
| BeST78 | Initial combination therapy with IFX (n = 128 randomised) | |||||||||
| Durez et al., 2007120 (NCT00396747) |
RCT (Phase IV, single blind) | MTX (n = 14 randomised) | All patients received MTX at dosage ranging from 7.5 mg/week (baseline) to 20 mg/week (week 14) | Patients receiving NSAIDs required to be receiving stable doses (remaining unchanged during study), i.e. steroids not permitted. Introduction of oral glucocorticosteroids of other DMARDs not permitted | 12 months | Evaluation of magnetic resonance imaging scores over time | No | Belgium | Schering-Plough | Durez et al., 2007120 full publication |
| Durez et al., 2007120 | MTX + i.v. methylprednisolone 1 g at weeks 0, 2 and 6 and then every 8 weeks thereafter (n = 15 randomised) | |||||||||
| Durez et al., 2007120 | IFX 3 mg/kg i.v. at weeks 0, 2, 6, 14, 22, 30, 38 and 46 + MTX (n = 15 randomised) | |||||||||
| IDEA95 | RCT (phase NR, double blind to week 26) | Methylprednisolone 250 mg i.v. at week 0, PBO i.v. at weeks 2, 6, 14 and 22 + MTX Numbers randomised NR (n = 112 patients included across both groups) |
IFX 3 mg/kg at weeks 0, 2, 6, 14, 22 + MTX 10 mg weekly increasing to 20 mg by week 6 with IFX dose modification depending on DAS44 from week 26 | NR | 78 weeks | NR | Step-up from week 26 if DAS > 2.4. Other biologics permitted from week 26 (no further details) (data extracted to week 26) | Multicentre (no further details) | NR | Nam et al., 201195 conference abstract |
| IDEA95 | IFX 3 mg/kg i.v. at weeks 0, 2, 6, 14 and 22 + MTX (IFX dose modifications permitted according to DAS44 from week 26) Numbers randomised NR (n = 112 patients included across both groups) |
|||||||||
| Quinn et al., 2005110 | RCT | MTX + PBO (n = 10 randomised) | 7.5 mg/week with escalation up to 15 mg/week by week 14. Increments up to 25 mg/week titrated against evidence of active disease | Folic acid 5 mg twice a week | 54 weeks | Comparison of magnetic resonance imaging-measured synovitis at week 14 between groups | No | NR | Arthritis Research Campaign | Quinn et al., 2005110 full-text paper Haugeberg et al., 2009312 full-text paper Bejarano et al., 2010,313 full-text paper 200877 |
| Quinn et al., 2005110 | IFX 3 mg/kg + MTX (n = 10 randomised) |
| Trial name/study (NCT/sponsor number) | Trial design (RCT, phase, LTE) | Treatment arms for which data extraction performed (number of patients randomised per treatment arm) | MTX dose during study (where applicable) | Concomitant treatments | Duration of RCT phase | Primary outcome | Early withdrawal plan reported? | Geographical location | Funding source | Primary and supplementary publication details [author, year, publication type (e.g. full, abstract)] |
|---|---|---|---|---|---|---|---|---|---|---|
| ATTEST74 (NCT00095147) |
RCT (Phase III, double blind) | PBO + MTX (with blinded crossover to ABT at day 198) (n = 110 randomised) | No MTX dose adjustments permitted except due to AEs MTX dose could be altered (to < 25 mg/week) between days 198–365 |
Permitted days 1–197: oral corticosteroids (≤ 10 mg/day of prednisone or equivalent) (stable ≥ 25/28 days prior to randomisation), and/or stable NSAIDs and analgesics. Days 198–365 dose of oral corticosteroids could be modified (≤ 10 mg/day of prednisone or equivalent), HCQ, SSZ, GLD or AZA also permitted | PBO-controlled phase to day 197 | DAS28-ESR ABT vs. PBO at 6 months (not powered with superiority or non-inferiority design to compare two active arms) | No | Multinational, multicentre (86 sites) | Bristol-Myers Squibb, USA | Schiff et al., 200874 full publication |
| ATTEST74 | IFX 3 mg/kg i.v. administered on days 1 (i.e. week 0), 15 (i.e. week 2), 43 (i.e. week 6) and 85 (i.e. week 12) and every 56 days (i.e. 8 weeks) thereafter (NB: licensed dose 3 mg/kg i.v. at weeks 0, 2, 6 and every 8 weeks thereafter, adjustments in dosage and frequency of administration permitted after week 12 in license) + MTX (n = 165 randomised) | |||||||||
| ATTEST74 | ABT dosed according to weight: patients weighing < 60 kg, 60–100 kg or > 100 kg received 500 mg, 750 mg or 1000 mg of ABT respectively. ABT administered i.v. on days 1, 15 and 29 and every 28 days thereafter, up to and including day 337 + MTX (n = 156 randomised) | |||||||||
| AMPLE66 | RCT (non-inferiority) | ABT s.c. + MTX (n = 318) | 15–25 mg/week (or ≥ 7.5 mg/week in patients intolerant to higher doses) 17.5 (6.35) mg/week at baseline |
Predisone (mean dose 6.6 mg/day) Corticosteroids (50.9%) SFZ (3.1%) HCQ (13.2%) |
2 years (first 12 months’ data just published) | ACR20 response at 1 year | No | North and South America | Bristol-Myers Squibb | Weinblatt et al., 2013144 full-text paper Fleischmann et al., 2012314 |
| AMPLE144 | ADA + MTX (n = 328) | 15–25 mg/week (or ≥ 7.5 mg/week in patients intolerant to higher doses) 17.3 (6.16) mg/week at baseline |
Predisone (mean dose 6.4 mg/day) Corticosteroids (50.3%) SFZ (3.4%) HCQ (10.7%) |
|||||||
| RED-SEA114 EU Clinical Trials Register 2006–006275–21/GB |
Pragmatic, randomised, parallel group, multicentre, unblinded and non-inferiority trial | ADA + cDMARDs (n = 60) | 66.7% patients on MTX, median dose 20 mg/week) | There were no constraints on changes in the dose of MTX, use of other DMARDs including previously untried agents, or on use of oral, parenteral or intra-articular corticosteroids once patients were included in the study Other DMARDs: AZA 1 (1.7%) HCQ 12 (20%) LEF 5 (8.3%) Penicillamine 1 (1.7%) SSZ 13 (21.7%) |
52 weeks | Proportion of patients continuing treatment after 52 weeks | Yes | UK | Sponsorship of University Hospital Birmingham NHS Foundation Trust, part supported by a grant from the Queen Elizabeth Hospital Birmingham Charity | Jobanputra et al., 2012114 full-text article in peer-reviewed journal |
| RED-SEA114 | ETN50 + cDMARDs (n = 60) | 66.7% patients on MTX, median dose 17.5 mg/week) | Other DMARDs: AZA 1 (1.7%) HCQ 1 (1.7%) LEF 8 (13.3%) Penicillamine 0 SSZ 8 (13.3%) |
|||||||
| ADACTA58 (NCT01119859) |
RCT (Phase IV, double blind) | TCZ 8 mg/kg i.v. every 4 weeks + s.c. PBO ADA (n = 163 randomised) | N/A | All DMARDs washed out before baseline (all ≥ 2 weeks, LEF ≥ 12 weeks or after standard washout) | 24 weeks | Mean change from baseline in DAS28 at 24 weeks | Yes | Multicentre, multinational | Roche | Gabay et al., 201358 full publication |
| ADACTA58 | ADA + PBO (n = 163 randomised) | N/A | ||||||||
| deFilippis et al., 200685 | RCT | ETN + MTX (n = 16) | Between 10 mg/week and 12.5 mg/week | Prednisone (maximum dosage 10 mg/day) | 54 weeks | ACR20, ACR50, ACR70 and HAQ improvement | No | Sicily | NR | deFilippis et al., 200685 full-text paper |
| deFilippis et al., 200685 | IFX + MTX (n = 16) | Between 10 mg/week and 12.5 mg/week |
| Trial name/study (NCT/sponsor number) | Trial design (RCT, phase, LTE) | Treatment arms for which data extraction performed (number of patients randomised per treatment arm) | MTX dose during study (where applicable) | Concomitant treatments | Duration of RCT phase | Primary outcome | Early withdrawal plan reported? | Geographical location | Funding source | Primary and supplementary publication details [author, year, publication type (e.g. full, abstract)] |
|---|---|---|---|---|---|---|---|---|---|---|
| AIM61,62 (NCT00048568) |
Randomised, double-blind, PBO-controlled trial Confirmatory Phase III |
MTX + PBO (n = 219) | 15.7 (SD 3.5) mg/week | Patients were permitted to continue taking oral corticosteroids, provided that the prescribed dose was reduced to the equivalent of 10 mg of prednisone daily for 28 days | 12 months | Health-related quality of life | NR | USA and Europe (including UK) | Bristol-Myers Squibb | Russell et al., 200761 Kremer et al., 200662 |
| AIM61 | ABT i.v. + MTX (n = 433) | 16.1 (SD 3.6) mg/week | ||||||||
| ASSET72 | RCT (Phase IIIb) | PBO + MTX (n = 23 randomised) | 10–25 mg/week, mean dose at baseline 17.3 (SD 4.2) mg/week | MTX (100%), oral and/or injectable corticosteroids (60.9%), low-dose oral corticosteroids (52.2%), NSAIDs (87.0%) | 4 months | Reduction in wrist synovitis score from mean magnetic resonance imaging scores at baseline and month 4 | No | Europe | Bristol-Myers Squibb | Conaghan et al., 201372 full-text paper |
| ASSET72 | ABT i.v. (≈ 10 mg/kg) + MTX (n = 27 randomised) | 10–25 mg/week, mean dose at baseline 16.9 (SD 4.6) mg/week | MTX (100%), oral and/or injectable corticosteroids (70.4%), low-dose oral corticosteroids (59.3%), NSAIDs (81.5%) | |||||||
| ASSURE73 | RCT | PBO + cDMARDs (n = 482 treated) | NR | MTX, HCQ, chloroquine, SSZ, LEF, GLD, AZA (ETN, IFX, ADA) | 1 year | Safety | No | NR | Bristol-Myers Squibb | Weinblatt et al., 200673 full-text paper |
| ASSURE73 | ABT + cDMARDs (n = 959 treated) | NR | MTX, HCQ, chloroquine, SSZ, LEF, GLD, AZA (ETN, IFX, ADA) | |||||||
| AUGUST II76 (NCT00595413) |
Phase II, randomised, PBO-controlled trial | MTX + PBO (n = 76) | NR | Allowed steroids unless prednisone dosage > 10 mg/day (or equivalent) or change in steroid or NSAID dosing regimen ≤ 28 days before study day 1 | 25 weeks | Proportion of patients with 20% improvement in disease severity according to the ACR criteria, as assessed using the CRP level (ACR20-CRP) | NR | Europe and USA | Merck Serono, Geneva, Switzerland and EMD Serono, Rockland, MA, which are affiliates of Merck KGaA, Darmstadt, Germany | van Vollenhoven et al., 201176 full-text article in peer-reviewed journal |
| AUGUST II76 | ADA + MTX (n = 79) | NR | ||||||||
| CHANGE/Miyasaka, 200880 | Phase II/III, multicenter, double-blind, PBO-controlled trial | PBO (n = 87) | N/A | Steroids allowed | 24 weeks | ACR20 response rate at week 24 | Patients who experienced an increase in disease activity or who had less than 10% reduction in tender joint counts and swollen joint counts compared with baseline after at least 8 weeks of treatment stopped study therapy with ADA/PBO and were switched to an open-label rescue treatment that could include higher doses of steroids, NSAIDs, or cDMARDs | Japan | Abbott, Osaka, Japan, and Eisai, Tokyo, Japan | Miyasaka, 200880 full-text article in peer-reviewed journal |
| CHANGE80 | ADA monotherapy (n = 91) | N/A | ||||||||
| DE019/Keystone et al., 200484 (NCT00195702) |
Phase III, multicenter, double-blind, PBO-controlled study | MTX + PBO (n = 200) | 16.7 (4.1) mg/week | Doses and routes of administration of concomitant RA therapies, such as MTX, corticosteroids and NSAIDs were kept constant throughout the study Oral corticosteroids, if used previously, were allowed at a maximum prednisone-dose equivalent of 10 mg/day |
52 weeks | Radiographic progression at week 52 (total Sharp score by a modified method), clinical response at week 24 [improvements of at least 20% in the ACR core criteria (ACR20)], and physical function at week 52 (disability index of the HAQ) | At week 16 or thereafter, patients who were not achieving an ACR20 response (improvements of at least 20% in the ACR core criteria) were allowed to receive ‘rescue’ treatment with a traditional DMARD at the discretion of their treating physician | USA and Canada | Abbott Laboratories, Abbott Park, IL | Keystone et al., 200484 full-text article in peer-reviewed journal |
| DE01984 | ADA + MTX (n = 207) | 16.7 (SD 4.5) weekly dose mg/kg | ||||||||
| STAR/Furst et al., 2003117 | Randomised, double-blind, PBO-controlled trial | PBO + cDMARDs (n = 318) | Number of traditional DMARDs: 0 DMARDs, n = 48 (15.1%) 1 DMARD, n = 172 (54.1%) 2 DMARDs, n = 84 (26.4%) 3+ DMARDs, n = 14 (4.4%) Mean number of DMARDs 1.2 |
Patients continued to receive their baseline doses of standard antirheumatic therapy, which could include traditional DMARD, low-dose corticosteroids (prednisone equivalent dose ≤ 10 mg/day), NSAID, and/or analgesics. Treatment with traditional DMARDs permitted during the study included chloroquine, HCQ, LEF, MTX, parenteral GLD, oral GLD, SSZ, or any combination of these. Doses of traditional DMARDs, corticosteroids, NSAID, and/or analgesics must have been stable for at least 28 days before screening | 24 weeks | Frequencies of AEs, serious AEs, severe or life-threatening AEs, AEs leading to withdrawal, infection, or serious infection | NR | USA | Abbott Laboratories, Abbott Park, IL | Furst et al., 2003117 full-text article in peer-reviewed journal |
| STAR117 | ADA + cDMARDs (n = 318) | Number of traditional DMARDs: 0 DMARDs, n = 57 (17.9%) 1 DMARD, n = 184 (57.9%) 2 DMARDs, n = 66 (20.8%) 3+ DMARDs, n = 11 (3.5%) Mean number of DMARDs 1.1 |
||||||||
| Van De Putte et al., 2004122 | RCT (Phase III, double blind) | PBO s.c. (n = 110 randomised) | NR | Use of NSAIDs and oral corticosteroids before study permitted at stable doses (up to 10 mg/day of prednisolone or equivalent). Analgesics permitted (not within 12 hours of study visits) | 26 weeks | ACR20 response at week 26 | Yes (ADA or PBO patients with increased inflammatory synovitis or < 10% improvement in tender joint counts and swollen joint counts after > 8 weeks treatment could enter rescue arm, during which study drug could be discontinued and doses of NSAIDs/corticosteroids increased/other DMARDs initiated at physician’s discretion) | Multicentre, multinational (Europe, Canada, Australia) | Abbott | Van De Putte et al., 2004122 full publication |
| Van De Putte et al., 2004122 | ADA monotherapy (n = 113 randomised) | NR | ||||||||
| ARMADA69,70 | Randomised, double-blind, PBO-controlled trial Phase II/III |
MTX + PBO (n = 62) | 16.5 (SD 5.0) mg/week | Salicylates, NSAIDs and corticosteroids (maximum daily dose of 10 mg of oral prednisone or equivalent) Folic acid or leucovorin was permitted |
24 week | ACR criteria for 20% improvement (ACR20) at 24 weeks | Patients who failed to meet or to maintain an ACR20 response but had received study drug (ADA or PBO) for at least 16 weeks were eligible to remain in the study or to roll over to an open-label continuation study with ADA | USA and Canada | Abbott Laboratories and Knoll Pharmaceuticals | Weinblatt et al., 200369 full-text article in peer-reviewed journal |
| ARMADA69 | ADA + MTX (n = 67) | 16.4 (SD 4.1 mg/week | ||||||||
| Kim et al., 200799 | Phase III, randomised, double-blind, PBO-controlled study | MTX + PBO rescue week18 (n = 65) | 16.3 (3.4) mg/week | NR | 24 weeks | 20% improvement in the ACR response criteria (ACR20) at week 24 | Beginning at week 18, patients with documented non-response could discontinue their double-blind study medication and switch to rescue therapy with open-label ADA 40 mg s.c. every other week | Republic of Korea | Abbott Laboratories, Abbott Park, IL | Kim et al., 200799 full-text article in peer-reviewed journal |
| Kim et al., 200799 | ADA + MTX (n = 63) | 16.6 (3.3) mg/week | ||||||||
| CERTAIN79 (NCT00674362) |
RCT (Phase IIIb) | PBO + cDMARDs (n = 98 randomised) | N/A | Existing cDMARDs | 52 weeks | % patients in Clinical Disease Activity Index remission (≤ 2.8) | Patients in Clinical Disease Activity Index remission at weeks 20 and 24 stopped CTZ and were monitored to week 52 | NR | UCB | Smolen et al., 201179 abstract Emery et al., 2012315 abstract |
| CERTAIN79 | CTZ 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks + DMARDs (n = 96 randomised) | N/A | Existing cDMARDs | |||||||
| REALISTIC113 | RCT (Phase III) | PBO + existing cDMARDs | N/A | MTX, LEF, SSZ, chlorquine, HCQ, AZA, GLD, steroids, NSAIDs | 12 weeks | ACR20 at 12 weeks | N/A (12-week study) | USA, Canada and Europe | UCB | Weinblatt et al., 2012113 abstract |
| REALISTIC113 | CTZ 400 mg weeks 0, 2, 4 then 200 mg every 2 weeks + existing cDMARDs | MTX, LEF, SSZ, chlorquine, HCQ, AZA, GLD, steroids, NSAIDs | ||||||||
| ADORE59,60 | Prospective, 16 week, randomised, open-label, parallel group, outpatient study | ETN monotherapy (n = 160) (n = 159 received treatment and provided data) | N/A | NSAIDs and corticosteroids allowed | 16 weeks | The primary efficacy measure was the proportion of evaluable patients in each treatment group who achieved an improvement of > 1.2 units in DAS28 from baseline to week 16 | NR | 60 centres in eight countries (Denmark, Finland, France, Germany, the Netherlands, Turkey, UK and Spain) | Wyeth Research | van Riel et al., 200659 full-text article in peer-reviewed journal van Riel et al., 200860 full-text article in peer-reviewed journal |
| ADORE59 | ETN + MTX (n = 155) | MTX (≥ 12.5 mg/week orally or by injection) Median 15 mg/week |
||||||||
| CREATE IIb96 [D1520C00001; NCT00520572 (Phase IIa and IIb trials)] |
Phase IIb study was a randomised, double-blind, PBO controlled, parallel-group multicentre trial (with an open-label ETN treatment group) to evaluate the efficacy of four doses of AZD9056 administered for 6 months on background MTX or sulphasalazine | DMARD + PBO (n = 65) | Patients were required to have received MTX for ≥ 6 months (the dose must have been stable between 5 and 25 mg/week for ≥ 6 weeks) or sulphasalazine for ≥ 16 weeks (at a stable dose of 0.5–3 g/day for ≥ 6 weeks) prior to randomisation | Concurrent treatment with stable doses of NSAIDs and/or prednisone (maximum 10 mg daily) was allowed throughout the study | 6 months | The proportion of patients meeting ACR 20% response criteria (ACR20) at 6 months (based on 28 joint counts) | NR | Canada and UK | AstraZeneca | Keystone et al., 201296 full-text article in peer-reviewed journal |
| CREATE IIb96 | ETN50 + DMARD (n = 64) Note either MTX across both arms (89.8%) or SSZ (9.7%) used as DMARD (not both) |
|||||||||
| ETN study 30989 | Randomised, double-blind, controlled trial | SSZ + PBO (n = 50) | SSZ dose (g/day), mean (SD) 2.1 (0.4) | Patients were permitted stable doses of oral corticosteroids (10 mg/day of prednisone or equivalent), one NSAID, simple analgesics with no anti-inflammatory action or daily doses of aspirin (300 mg) during the study | 2 years | Percentage of patients achieving > 20% improvement as assessed by the ACR20 response at week 24 | NR | Europe (including UK), Australia, USA | Wyeth Research, Collegeville, PA | Combe et al., 200688 full-text article in peer-reviewed journal Combe et al., 200989 full-text article in peer-reviewed journal |
| ETN study 30989 | ETN + PBO (n = 103) | N/A | ||||||||
| ETN study 30989 | ETN + SSZ (n = 101) | SSZ dose (g/day), mean (SD) 2.1 (0.5) | ||||||||
| JESMR140 | RCT (Phase IV) | ETN monotherapy (n = 74 randomised) | 7.0 (1.4) mg/week | Folic acid (37.7%), corticosteroids (46.4%) | 52 weeks | Good EULAR response and ACR50 response at week 24 | No | Japan | Japanese Ministry of Health, Labour and Welfare | Kameda et al., 2010316 full-text paper Kameda et al., 2011140 full-text paper |
| JESMR140 | ETN + MTX 6–8 mg/week (n = 77 randomised) | 7.4 (1.1) mg/week | Folic acid (52.1%), corticosteroids (60.3%) | |||||||
| Lan et al., 2004101 | RCT, double blind | PBO + MTX (n = 29) | 12.5–20 mg/week | NSAIDs, aspirin and corticosteroids were allowed | 12 weeks | Reduction of tender and swollen joint counts by 20% (ACR20), 50%, 70% at 12 weeks | NR | Taiwan | Wyeth-Ayerst Ltd, Taiwan branch | Lan et al., 2004101 full-text article in peer-reviewed journal |
| Lan et al. 2004101 | ETN + MTX (n = 29) | |||||||||
| LARA102 (NCT00848354) |
Randomised, open-label, active-comparator study Phase IV |
MTX + DMARD (n = 142) | 14.4 (3.9) mg/week | NR | 24 weeks | Proportion of subjects achieving ACR50 criteria at week 24 | NR | Latin American region (Argentina, Chile, Colombia, Mexico, Panama) | Wyeth | Machado et al., 2012102 conference abstract |
| LARA102 | ETN50 + MTX (n = 281) | 14.1 (3.8) mg/week | ||||||||
| Moreland et al., 1999;104 Mathias et al., 2000105 | Confirmatory, Phase III, randomised, double-blind, PBO-controlled trial | PBO (n = 80) | N/A | Corticosteroids and NSAIDs allowed | 6 months | 20% and 50% improvement ACR, at 3 months and 6 months | NR | USA | Immunex Corp, Seattle, WA | Moreland et al., 1999104 full-text article in peer-reviewed journal Mathias et al., 2000105 full-text article in peer-reviewed journal |
| Moreland et al., 1999;104 Mathias et al., 2000105 | ETN + PBO (n = 78) | N/A | ||||||||
| RACAT;111 O’Dell et al., 2013112 (NCT00405275) |
Randomised, double-blind, PBO-controlled, non-inferiority trial | MTX + SSZ + HCQ (n = 178) Potential to switch groups at week 24 |
19.5 (5.0) mg/week | Participants continued to receive non-steroidal anti-inflammatory agents and prednisone (≤ 10 mg/day) at stable doses | 48 weeks | The originally proposed primary outcome was the difference in the proportion of participants who had a DAS28 of ≤ 3.2 at week 48. In response to unexpectedly low enrolment, the protocol was amended in October 2008 to change the primary outcome from a binary outcome to a continuous outcome in order to increase the power of the study | Part of study design – if the score on the DAS28 decreased (indicating improvement) by 1.2 or more by 24 weeks, the initial therapy was continued. If the score on the DAS28 decreased by < 1.2, the participant was switched to the alternative regimen | USA and Canada | Supported by the Cooperative Studies Program, Department of Veterans Affairs Office of Research and Development and the Canadian Institutes for Health Research and by an interagency agreement with the National Institutes of Health–American Recovery and Reinvestment Act | O’Dell et al., 2013112 full-text article in peer-reviewed journal O’Dell et al., 2012111 conference abstract |
| RACAT111 | ETN50 + MTX (n = 175) Potential to switch groups at week 24 |
19.7 (4.5) mg/week | ||||||||
| Wajdula 2000 (reported in Chen et al., 2006123) European Etanercept Investigators Group protocol 0881A1–300-EU |
RCT, multicentre, double blind | PBO (n = 105) | 12 weeks | Change from baseline in the number of swollen and painful joints at 3 months | N/A (12-week study) | Europe, multicentre | Information taken from a published HTA report that had access to manufacturer trial reports Chen et al., 2006123 |
|||
| Wajdula 2000 (reported in Chen et al., 2006123) | ETN (n = 111) | |||||||||
| Weinblatt et al., 1999124 | RCT, double blind | MTX + PBO (n = 30) | Stable dose 12.5–25 mg/week | NSAIDs and corticosteroids allowed | 24 weeks | ACR criteria for a 20% improvement in measures of disease activity (ACR20) at 24 weeks | Condition not described; patients who received intra-articular injections of corticosteroids during the study were counted as having or not having a response according to their overall evaluation | Multicentre, USA | Supported by Immunex | Weinblatt et al., 1999124 full-text article in peer-reviewed journal Kremer et al., 2003160 full-text article in peer-reviewed journal |
| Weinblatt et al., 1999124 | ETN + MTX (n = 59) | |||||||||
| APPEAL67,68 | Open-label, active-comparator, parallel-design, multicentre RCT | MTX + DMARD (SSZ, HCQ or LEF) (n = 103) | 6.9 (8.5) mg/week | NSAIDs or corticosteroids were allowed, but not multiple NSAIDs, and any increase in dosage of baseline NSAID or corticosteroid | 16 weeks | ACR response area under the curve over 16 weeks | NR | Asia – Pacific region | Wyeth | Kim et al., 201267 full-text article in peer-reviewed journal Bae et al., 201368 full-text article in peer-reviewed journal |
| APPEAL68 | ETN + MTX (n = 197) | 6.5 (7.3) mg/week | ||||||||
| GO-FORTH91 | RCT (Phase II/III) | PBO every 4 weeks + MTX 6–8 mg/week (n = 90 randomised) | NR | Concurrent NSAIDs, analgesic and oral corticosteroids (≤ 10 mg of prednisolone/day or equivalent) allowed with stable doses ≥ 2 weeks prior to and during the study | 24 weeks | ACR20 response at week 14 | Patients with < 20% improvement from baseline in tender joint counts and swollen joint counts at week 14 could enter double-blind early escape where the dose was increased (or added in PBO arm) | Japan | Centocor Research & Development Inc., Janssen Pharmaceuticals K.K. and Mitsubishi Tanabe Pharmaceutical Corporation | Tanaka et al., 201291 full-text paper |
| GO-FORTH91 | GOL 50 mg s.c. every 4 weeks + MTX 6–8 mg/week (n = 89 randomised) | NR | ||||||||
| GO-FORWARD92 (NCT00264550) |
RCT (Phase III, double blind) | PBO s.c. every 4 weeks + MTX (n = 133 randomised) | Mean (SD) 17.0 (2.75) mg/week Median 15.0 (IQR 15.0–20.0) |
Patients receiving NSAIDs or other analgesics for RA required to have been taking stable dose for at least 2 weeks before first dose of study agent Patents receiving oral corticosteroids required to have been taking stable dose equivalent to 10 mg/day or less of prenisone for at least 2 weeks before first dose of study drug |
Double-blind, PBO-controlled phase to week 24 and open-label extension up to 5 years | Two co primary end points: proportion of patients achieving ACR20 response at week 14 and improvement from baseline in HAQ-DI score at week 24 | Yes | Multinational, multicentre (60 sites over 12 countries) | Centocor | Keystone et al., 2009216 full publication, results to week 24 Keystone et al., 201092 full publication, results to week 52 |
| GO-FORWARD92 | GOL 50 mg s.c. every 4 weeks + MTX (n = 89 randomised) | Mean (SD) 17.4 (3.00) mg/week Median 15.0 (IQR 15.0–20.0) mg/week |
||||||||
| Kay et al., 200898 (NCT00207714) |
RCT (Phase II, double blind) | PBO s.c. + MTX (n = 35 randomised) | All patients continued to receive stable doses of MTX (at least 10 mg/week) through end of study | Oral corticosteroids permitted at stable pre-study dosage not exceeding equivalent 10 mg prednisone per day Commercially available NSAIDs permitted at stable pre-study dose Folic acid at stable dosage of at least 5 mg every week for at least 4 weeks before first study drug dose |
52 weeks | Proportion of patients meeting ACR 20% improvement criteria (achieving an ACR20 response) at week 16 | Yes | Multicentre [40 study sites, geographical location(s) not stated] | Centocor | Kay et al., 200898 full publication |
| Kay et al., 200898 | GOL 50 mg s.c. every 4 weeks + MTX (n = 35 randomised) | |||||||||
| Abe et al., 200656 | RCT (phase NR, double blind) | PBO + MTX (n randomised NR, 47 patients received one or more infusions) | 7.4 (SD = 2.2) mg/week | Patients taking NSAIDs, folic acid or corticosteroids (10 mg/day or less of prednisolone equivalent) required to have received stable dose for at least 4 weeks before study entry | 14 weeks | ACR20 response at week 14 | No | Multicentre, Japan | NR | Abe et al., 200656 full publication |
| Abe et al., 200656 | IFX 3 mg/kg i.v. at weeks 0, 2 and 6 + MTX (n randomised NR, 49 patients received one or more infusions) |
7.1 (SD = 1.9) mg/week | ||||||||
| ATTRACT75 | RCT (Phase III, double blind) | PBO i.v. + MTX (n = 88 randomised) | Median 15 (IQR 12.5–17.5) mg/week | Patients receiving oral corticosteroids (10 mg/kg or less of prednisone equivalent) or NSAIDs required to have stable dose for at least 4 weeks before screening (and must not have received either drug for at least 4 weeks before screening) Patients received baseline dose of MTX or corticosteroids during study |
54-week PBO-controlled RCT with LTE to 102 weeks | ACR20 response at week 30 without requiring a surgical joint procedure, initiation of new antirheumatic drugs or increased in antirheumatic drugs ACR20 response at week 30 |
No | Multicentre, multinational | Centocor | Maini et al., 199975 full publication |
| ATTRACT75 | IFX 3 mg/kg i.v. at weeks 0, 2 and 6 and every 8 weeks thereafter + MTX (n = 86 randomised) | Median 15 (IQR 12.5–17.5) mg/week | ||||||||
| Durez et al., 200486 | RCT (Phase NR, open label) | Single i.v. infusion of 1 g of MP (sodium hemisuccinate) at week 0 + MTX (n = 14 randomised) | Median 12.5 (range 10–15) mg/week | Oral glucocorticoid doses remained unaltered during study. Intra-articular steroids not permitted. Introduction of new NSAID or DMARD not permitted | 14 weeks | NR | No | Belgium | Schering-Plough | Durez et al., 200486 full publication |
| Durez et al., 200486 | IFX 3 mg/kg at weeks 0, 2 and 6 + MTX (n = 12 randomised | Median 15 (range 10–15) mg/week | ||||||||
| START118 | RCT | PBO + MTX (n = 363 randomised) | Median 15.0 (IQR 10–15) mg/week | MTX only (70.0%), MTX + one DMARD (25.3%), MTX + two DMARDs (4.4%), NSAIDs (39.4%), corticosteroids (59.2%), narcotics/opioid analgesics (6.1%) | 1 year (22 weeks before dose escalation commenced) | Occurrence of a serious infection within 22 weeks of initiating therapy | No, but dose escalation from 22 weeks if < 20% improvement in swollen joint counts and tender joint counts or ≥ 50% discontinuation in improvement in combined swollen joint counts and tender joint counts | NR | Centocor Research and Development Inc. | Westhovens et al., 2006118 full-text paper |
| START118 | IFX 3 mg/kg + MTX (n = 360 randomised) | Median 15.0 (IQR 10–18) mg/week | MTX only (70.8%), MTX + one DMARD (24.4%), MTX + two DMARDs (4.7%), NSAIDs (43.3%), corticosteroids (59.2%), narcotics/opioid analgesics (5.8%) | |||||||
| Swefot119 (WHO database number CT20080004) |
RCT (Phase NR, open label) | SSZ (1000 mg twice daily orally) + HCQ (400 mg daily orally) + MTX (with optional increase to SSZ 1500 mg twice daily if ineffective and cDMARD adjustment in event of toxicity with potential switch to CYC (five switched to CYC, included in primary analyses) (n = 130) | Up to 20 mg/week | If patients were receiving glucocorticoids, dose was required to be stable for at least 4 weeks at no more than 10 mg daily of prednisolone (or equivalent) | 2 years | EULAR good response at 12 months | Dose adjustments permitted (see left) | Multicentre (15 rheumatology units), Sweden | Swedish Rheumatism Association Schering-Plough |
van Vollenhoven et al., 2009,119 van Vollenhoven et al., 2012147 full publication |
| Swefot119 | IFX 3 mg/kg i.v. at weeks 0, 2, 6 and every 8 weeks thereafter with optional increase to IFX every 6 weeks thereafter (in event of toxicity, optional switch to ETN 50 mg weekly) (five switched to ETN, included in primary analyses) + MTX (n = 128) | |||||||||
| Wong et al., 2009125 | RCT (Phase NR, double blind) | PBO + MTX (with crossover to open-label IFX at week 24) (n = 9) | NR | All antirheumatic medications kept stable for at least 4 weeks before and during study (unless dose alterations were clinically indicated) | 56 weeks | Vascular ultrasound assessments at weeks 24 and 56 | Yes (PBO patients could escape to open-label IFX at week 16) | UK | Centocor Pty Ltd Arthritis Foundation of Australia |
Wong et al., 2009125 full publication |
| Wong et al., 2009125 | IFX 3 mg/kg at weeks 0, 2, 6 and 8 weeks thereafter + MTX (n = 17) | |||||||||
| Zhang et al., 2006126 | RCT (Phase NR, double blind) | PBO i.v. + MTX (n = 86) | Stable dose of MTX continued during study | Glucocorticosteroid dose required to be stable for 4 weeks before screening and dosage not permitted to exceed 10 mg/day of prednisone or equivalent | 18 weeks | NR | No | Multicentre (five centres), China | Zhang et al., 2006126 full publication | |
| Zhang et al., 2006126 | IFX 3 mg/kg i.v. at weeks 0, 2, 6 and 14 + MTX (n = 87) | |||||||||
| ACT-RAY57 (NCT00810199) |
RCT (Phase III, double blind) | TCZ 8 mg/kg i.v. every 4 weeks + oral PBO (n = 277 randomised) | Patients received mean weekly doses of MTX/PBO ranging from: TCZ 8 mg/kg i.v. every 4 weeks + oral PBO = 15.8–16.3 mg/week; TCZ 8 mg/kg i.v. every 4 weeks + MTX = 15.2–15.9 mg/week | Oral corticosteroids (≤ 10 mg/day of prednisone or equivalent) and NSAIDs permitted if doses had been stable for at least 25 of 28 days before start of study agent | 2 years | % patients in remission according to DAS28-ESR (DAS28 < 2.6) at week 24 | No | NR | Roche | Dougados et al., 201357 full publication |
| ACT-RAY57 | TCZ 8 mg/kg i.v. every 4 weeks + MTX (n = 276) | |||||||||
| MEASURE103 | RCT (Phase NR, double blind) | PBO + MTX (n = 69 randomised) | NR | NR | 24 weeks double-blind phase of 2-year study | NR | Yes (27 patients in PBO arm entered early escape treatment with open-label TCZ at week 16) | UK, USA, Canada | Lead author: grant/research support from Roche | Mcinnes et al., 2011103 conference abstract |
| MEASURE103 | TCZ 8 mg/kg i.v. every 4 weeks + MTX (n = 69 randomised) | NR | ||||||||
| Nishimoto et al., 2004106 | RCT (phase NR, double blind) | PBO i.v. every 4 weeks (n = 53 randomised) | N/A | Stable prednisolone (≤ 10 mg/day) and NSAIDs permitted at stable doses No parenteral and/or intra-articular corticosteroids permitted during 4-week washout period before initiation of study agent and during study period |
3 months | ACR20 at week 12 | No | Multicentre, Japan | Chugai Pharmaceutical, Japan | Nishimoto et al., 2004106 full publication |
| Nishimoto et al., 2004106 | TCZ 8 mg/kg i.v. every 4 weeks (n = 55 randomised) | N/A | ||||||||
| SAMURAI115 | Multicentre, X-ray reader-blinded, randomised, controlled trial Phase III |
cDMARDs disease activity (n = 145) | 8.0 (± 2.1) mg/week 123 patients (85%) received MTX: 81 (56%) received a combination of MTX and DMARDs, 42 (29%) received MTX monotherapy and 20 (14%) received DMARDs and/or immunosuppressants other than MTX, besides corticosteroids |
For the conventional DMARD group, the dose, type and combination of DMARDs and/or immunosuppressants, except for antiTNF agents and LEF, could be varied according to disease activity at the discretion of the treating physician | 52 weeks | Progression of structural joint damage | NR | Japan | Chugai Pharmaceutical, Tokyo, Japan | Nishimoto et al., 2007115 full-text article in peer-reviewed journal |
| SAMURAI115 | TCZ i.v. (n = 157) | N/A | Both groups – oral corticosteroids (10 mg of prednisolone per day) were allowed, but the dosage could not be increased during the study Use of one NSAID, including switching to another NSAID, was allowed |
|||||||
| SATORI116 (NCT00144521) |
RCT (Phase III, double blind) | PBO + MTX (n = 64) | 8 mg/week (maximum permitted dose in Japan) | Oral corticosteroids permitted at ≤ 10 mg/day of prednisolone (as worded) (dose increase not permitted) Intra-articular corticosteroid injections (one joint maximum at one treatment) and hyaluronate preparations permitted Use of one NSAID permitted (switching to another NSAID allowed) DMARDs, i.v. or i.m. corticosteroids, plasmapharesis and surgical treatment not allowed |
Double-blind controlled phase to week 24 | ACR20 response at week 24 | No | Single country, multicentre (25 sites across Japan) | Chugai Pharmaceutical, Japan | Nishimoto et al., 2009116 full publication |
| SATORI116 | TCZ 8 mg/kg i.v. every 4 weeks + PBO capsules (n = 61) | |||||||||
| TOWARD121 | RCT (Phase III, double blind) | PBO i.v. every 4 weeks + stable cDMARDs (n = 415 randomised) | 14.7 mg/week | Oral glucocorticoids (≤ 10 mg/day of prednisone or equivalent) and NSAIDs/COX-2 inhibitors permitted if doses stable for ≥ 6 weeks | 24 weeks | ACR20 at week 24 | Yes (early escape at week 16 for patients failing to achieve > 20% improvement in both swollen joint counts and tender joint counts consisting of adjustment of background DMARD dosage and/or a different DMARD and/or intra-articular/oral glucocorticoids) | Multinational (18 countries), multicentre | Roche | Genovese et al., 2008121 full publication |
| TOWARD121 | TCZ 8 mg/kg i.v. every 4 weeks + stable DMARDs (n = 805 randomised) | 15.0 mg/week | ||||||||
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Trial name/study (NCT/sponsor number) | Treatment arms for which data extraction performed (number of patients randomised per treatment arm) | MTX dose during study (where applicable) | Concomitant treatments | Duration of RCT phase | Primary outcome | Early withdrawal plan reported? | Geographical location | Funding source | Primary and supplementary publication details [author, year, publication type (e.g. full, abstract)] |
|---|---|---|---|---|---|---|---|---|---|
| ACQUIRE127 | ABT s.c. + PBO i.v. + MTX (n = 736) | ≥ 15 mg/week [mean at baseline 16.3 (SD 3.6) mg/week] | Corticosteroids (oral and/or injectable): 72.1%, mean (SD) dose 4.8 (4.5) mg/day | 6 months | ACR20 (% patients achieving response) at 6 months | No | NR | Bristol-Myers Squibb | Genovese et al., 2011127 full-text paper |
| ACQUIRE127 | ABT i.v. + PBO s.c. + MTX (n = 721) | ≥ 15 mg/week [mean at baseline 16.5 (SD 3.8) mg/week] | Corticosteroids (oral and/or injectable): 74.6%, mean (SD) dose 5.2 (6.9) mg/day | ||||||
| NCT00254293131 | PBO + MTX (n = 119 randomised) | 10–30 mg/week, mean 15.8 (SD 4.1) mg/week | Addition of another DMARD (HCQ, SSZ, GLD, AZA) and/or adjustment in corticosteroids equivalent to ≤ 10 mg/day of prednisone were permitted. Use of the above not reported | 12 months | ACR20 response at 6 months | No | Multicentre | Bristol-Myers Squibb | Kremer et al., 2005131 full-text paper Kremer et al., 2003318 full-text paper |
| NCT00254293131 | ABT i.v. (≈ 10 mg/kg) + MTX (n = 115 randomised) | 10–30 mg/week, mean 15.0 (SD 4.4) mg/week | |||||||
| ORAL STANDARD133 (NCT00853385) |
MTX + PBO (n = 108) | 7.5–25 mg of MTX weekly, all groups | Glucocorticoids and lipid-lowering medication allowed | 12 months | 20% improvement at month 6 in ACR20; the change from baseline to month 3 in the score on the HAQ-DI (which ranges from 0 to 3, with higher scores indicating greater disability); and the percentage of patients at month 6 who had a DAS28 based on the ESR | Patients in the PBO group who did not have a 20% reduction in the number of swollen and tender joints after 3 months (considered as not having had a response) were randomly assigned to either 5 mg or 10 mg of TOF | Europe, USA, Korea, Latin America | Supported by Pfizer | van Vollenhoven et al., 2012133 full-text article in peer-reviewed journal |
| ORAL STANDARD133 | TOF5 + MTX (n = 204) | ||||||||
| ORAL STANDARD133 | TOF10 + MTX (n = 201) | ||||||||
| ORAL STANDARD133 | ADA + MTX (n = 204) | ||||||||
| JRAPID/Yamamoto et al., 2011129 (NCT00791999) |
PBO + MTX every 2 weeks (n = 77 patients randomised) | NR | MTX | 24 weeks | ACR20 response at week 12 | Early escape at week 16 for patients who failed to achieve ACR20 response at both weeks 12 and 14 | Japan, multicentre | NR | Yamamoto et al., 2011129 conference abstract |
| JRAPID129 | CTZ 200 mg + MTX every 2 weeks (n = 82 patients randomised) | NR | MTX | ||||||
| RA0025134 | PBO + MTX (n = 40 randomised) | 10–20 mg/week | MTX | 24 weeks | ACR20 response at week 24 | Patients with no ACR20 response at both weeks 12 and 14 were withdrawn | Korea | NR | Kang et al., 2012134 abstract |
| RA0025134 | CTZ 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks + MTX (n = 81 randomised) | 10–20 mg/week | MTX | ||||||
| RAPID1135 | PBO + MTX (n = 199 randomised) | 13.4 mg/week | MTX, oral corticosteroids (≤ 10 mg/day of prednisone or equivalent with stable dose from 4 weeks prior to baseline), NSAIDs/COX-2 inhibitors and analgesics | 52 weeks | ACR20 response rate at week 24 and mean change form baseline in modified total Sharp score at week 52 | Early escape at week 16 for patients who failed to achieve ACR20 response at both weeks 12 and 14 | NR | UCB | Keystone et al., 2008135 full-text paper |
| RAPID1135 | CTZ 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks + MTX (n = 393 randomised) | 13.6 mg/week | |||||||
| RAPID2136 | PBO + MTX (n = 127 randomised) | 12.2 mg/week | MTX | 24 weeks | ACR20 response at week 24 | Early escape at week 16 for patients who failed to achieve ACR20 response at both weeks 12 and 14 | International | UCB | Smolen et al., 2009136 full-text paper |
| RAPID2136 | CTZ 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks + MTX (n = 246 randomised) | 12.5 mg/week | MTX | ||||||
| TEAR53 (SA mixed population) (NCT00259610) |
MTX monotherapy (ST) (n = 124) ST = step-up from MTX to triple DMARD therapy (MTX + SSZ plus HCQ) |
MTX, which was escalated to a dosage of 20 mg/week or to a lower dosage if treatment resulted in no active tender/painful or swollen joints by week 12 | For those receiving corticosteroids, the dosage (up to 10 mg/day of prednisone) had to be stable for at least 2 weeks prior to screening; for those receiving NSAIDs, the dosage had to be stable for at least 1 week prior to screening folic acid at a dosage of 1 mg per day | 102 weeks | An observed-group analysis of DAS28-ESR values from week 48 to week 102 | Step-up therapy part of study design | USA | Supported by Amgen through a grant to the University of Alabama at Birmingham, AL. The study drugs were provided by Amgen (ETN and PBO), Barr Pharmaceuticals (MTX) and Pharmacia (SSZ and PBO). The initial phases of the study were supported by the National Institute for Health (planning grant 1-R34-AR-055122 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases | Moreland et al., 201253 full-text article in peer-reviewed journal |
| TEAR53 (SA mixed population) | MTX monotherapy (SE) (n = 255) SU = step-up from MTX to MTX + ETN |
||||||||
| TEAR53 (SA mixed population) | MTX + SSZ + HCQ (n = 132) | ||||||||
| TEAR53 (SA mixed population) | ETN50 + MTX (n = 244) | ||||||||
| TEMPO54 | MTX monotherapy (n = 228) | MTX dose [median 10 (IQR 7.5–15.0) mg/week] | NSAIDs and corticosteroids allowed 5-mg folic acid supplement twice a week |
52 weeks | Numeric index of the ACR response area under the curve (AUC) over the first 24 weeks | NR | Europe, Australia, USA | Wyeth Research | Klarekskog et al., 200454 full-text article in peer-reviewed journal |
| TEMPO54 | ETN monotherapy (n = 223) | MTX dose [median 10 (IQR 7.5–13.8) mg/week] | |||||||
| TEMPO54 | ETN + MTX (n = 231) | MTX dose [median 10 (IQR 7.5–15.0) mg/week] | |||||||
| AMBITION55 (NCT00109408) |
MTX alone (n = 284 randomised) | 7.5–20 mg/week | Oral glucocorticoids (≤ 10 mg/day of prednisone or equivalent) and NSAIDs permitted if dose stable for ≥ 6 weeks | 24 weeks | ACR20 at week 24 | No | Multicentre, multinational | Roche | Jones et al., 201055 full publication |
| AMBITION55 | TCZ 8 mg/kg i.v. every 4 weeks (n = 288 randomised) | N/A | |||||||
| LITHE130 (NCT00106535) |
PBO i.v. every 4 weeks + MTX (n = 393 randomised) | Patients received stable dose of MTX 10–25 mg/week Mean 15.0 (SD 4.2) mg/week |
Oral corticosteroids (≤ 10 mg/day prednisone or equivalent) and NSAIDs permitted if doses had been stable for ≥ 6 weeks before study entry | 52 weeks | Co-primary end points at week 52: change from baseline in total Genant-modified Sharp score and area under the curve for change from baseline in HAQ-DI | Yes Rescue therapy at week 16 for patients not achieving ≥ 20% improvement in tender joint count and swollen joint count. PBO group received TCZ 4 mg/kg + steroids. TCZ 8 mg/kg group received TCZ 8 mg/kg + steroids. If < 20% improvement persisted after three doses of blinded first-step rescue therapy, patients received second-step rescue of TCZ 8 mg/kg. If still no response, treatment discontinued |
Multicentre, multinational (14 countries) | Roche | Kremer et al., 2011130 full publication |
| LITHE130 | TCZ 8 mg/kg i.v. every 4 weeks + MTX (n = 398 randomised) |
Mean 15.4 (SD 10.6) mg/week | |||||||
| OPTION132 | PBO i.v. every 4 weeks + MTX (n = 204 randomised) | 14.8 (4.2) mg/week | Oral glucocorticoids (≤ 10 mg/day of prednisone or equivalent) and NSAIDs permitted if doses stable for ≥ 6 weeks before study entry | 24 weeks | ACR20 at week 24 | Yes Patients not achieving ≥ 20% improvement in both swollen joint count and tender joint count by week 16 eligible for rescue therapy with TCZ 8 mg/kg and steroids if necessary or increase in oral corticosteroid dose (maximum 10 mg/day) |
Multicentre (73 centres), multinational (17 countries) | Roche, Chugai Pharmaceutical | Smolen et al., 2008132 full publication |
| OPTION132 | TCZ 8 mg/kg i.v. every 4 weeks + MTX (n = 205 randomised) |
14.5 (4.4) mg/week |
| Trial name/study | Treatment arms for which data extraction performed | Ethnicity (where reported) | Rheumatoid factor (% positive) | Prior DMARD treatment history (brief description, including definition of active RA despite previous treatment, where relevant) | % receiving NSAIDs at baseline | % receiving steroids at baseline |
|---|---|---|---|---|---|---|
| Kume et al., 2011100 | ADA monotherapy | NR | 85.8 | No prior treatment with MTX or biologics. Dosage of all DMARDs had to be stable for ≥ 8 weeks prior to enrolment | NR | NR |
| Kume et al., 2011100 | ETN monotherapy | NR | 88.6 | No prior treatment with MTX or biologics. Dosage of all DMARDs had to be stable for ≥ 8 weeks prior to enrolment | NR | NR |
| Trial name/study | Treatment arms for which data extraction performed | Ethnicity (where reported) | Rheumatoid factor (% positive) | Prior DMARD treatment history (brief description, including definition of active RA despite previous treatment, where relevant) | % receiving NSAIDs at baseline | % receiving steroids at baseline |
|---|---|---|---|---|---|---|
| Bejarano et al., 200877 | PBO + MTX (n = 73) | NR | 95 | MTX naive; mean 0.2 prior cDMARDs | NR | NR |
| Bejarano et al., 200877 | ADA + MTX (n = 75) | NR | 96 | MTX naive; mean 0.2 prior cDMARDs | NR | NR |
| GUEPARD93 | Initial MTX 12 weeks, then step-up therapy in both groups based on DAS28 (n = 32) | NR | 77.4 | MTX naive; no prior biologics | NR | 31.3 |
| GUEPARD93 | Initial ADA + MTX 12 weeks, then step-up therapy in both groups based on DAS28 (n = 33) | NR | 70.0 | MTX naive; no prior biologics | NR | 30.3 |
| HIT HARD94 | MTX + PBO | NR | 69.4 | Required to be DMARD naive, mean number of prior DMARDs was 0 | NR | NR |
| HIT HARD94 | ADA + PBO | NR | 63.2 | Required to be DMARD naive, mean number of prior DMARDs was 0 | NR | NR |
| OPERA107 | MTX + PBO + steroid | NR | 74 | Active RA by ACR (1987) revised criteria. Excluded if had glucocorticoids within the last 4 weeks or previous DMARD therapy | NR | NR |
| OPERA107 | ADA + MTX + steroid | NR | 70 | Active RA by ACR (1987) revised criteria. Excluded if had glucocorticoids within the last 4 weeks or previous DMARD therapy | NR | NR |
| OPTIMA108 | MTX + PBO | 90% white | 89 | Patients were excluded if they had received prior MTX, more than two synthetic DMARDs or biologics | 79 | 46 |
| OPTIMA108 | ADA + MTX | 89% white | 87 | Patients were excluded if they had received prior MTX, more than two synthetic DMARDs or biologics | 78 | 41 |
| PREMIER109 | MTX + PBO | 94.4% white | 84.0 | Required to be MTX naive (and no previous treatment with cyclophosphamide, CYC, AZA or more than two other DMARDs). 31.5% had prior DMARD experience | N/A | 35.4 |
| PREMIER109 | ADA monotherapy + PBO step up week 16 | 93.5% white | 83.5 | Required to be MTX naive (and no previous treatment with cyclophosphamide, CYC, AZA or more than two other DMARDs). 33.2% had prior DMARD experience | N/A | 36.5 |
| PREMIER109 | ADA + MTX step-up week 16 | 93.6% white | 85.1 | Required to be MTX naive (and no previous treatment with cyclophosphamide, CYC, AZA or more than two other DMARDs). 32.5% had prior DMARD experience | N/A | 35.8 |
| COMET81–83 | MTX + PBO (n = 268) | White 88% | NR | MTX naive; % having prior cDMARDs = 24% | 76 | 50 |
| COMET81 | ETN + MTX (n = 274) | White 87% | NR | MTX naive; % having prior cDMARDs = 18% | 72 | 49 |
| ERA/Bathon and Genovese, 2003139 (multicentre) | MTX + PBO | 88% Caucasian | 89 | Required to be MTX naive. 46% of patients had prior DMARDs, mean number of DMARDs = 0.6 (SD 0.7) | 80 | 41 |
| ERA/Bathon and Genovese, 2003139 (multicentre) | ETN + PBO | 86% Caucasian | 87 | Required to be MTX naive. 40% of patients had prior DMARDs, mean number of DMARDs = 0.5 (SD 0.7) | 86 | 39 |
| GO-BEFORE90 | PBO + MTX | White = 71.3%; black = 3.8%; Asian = 15.6%; other (no further details) = 9.4% | NR | MTX-naive patients. Patients had not received more than three weekly doses of oral MTX as RA treatment. Patients who had previously received IFX, ETN, ADA, RTX, natalizumab or cytotoxic agents excluded. Patients receiving anakinra could participate 4 weeks after receiving last dose. Patients receiving alefacept or efalizumab could participate 3 months after last dose Previous DMARDs = 83/160 (51.9%) HCQ = 26/160 (16.3%) SSZ = 51/160 (31.9) LEF = 12/160 (7.5%) Other DMARDs (no further details) = 26 (16.3%) Anakinra = 0/0 (0.0%) Immunosuppresive agents = 3/160 (1.9%) |
95.6 | 68.1 |
| GO-BEFORE90 | GOL + MTX | White = 74.8%; black = 0.6%; Asian = 18.9%; other (no further details) = 5.7% | NR | Previous DMARDs = 80/159 (50.3%) HCQ = 33/159 (20.8%) SSZ = 36/159 (22.6%) LEF = 13/159 (8.2%) Other DMARDs (no further details) = 29/159 (18.2%) Anakinra = 0 (0.0%) Immunosuppressive agents = 2/159 (1.3%) |
98.1 | 69.8 |
| ASPIRE71 | PBO + MTX | NR | 71 | Patients had persistent synovitis ≥ 3 months and ≤ 3 years, ≥ 10 swollen joints, and ≥ 12 tender joints All patients were MTX naive. 65–71% were DMARD naive Patients were excluded if any prior treatment with MTX (had to be three or fewer pre-study doses), had received other DMARDs within 4 weeks of entry (or LEF within past 6 months), or had been treated with IFX, ETN, ADA or other antiTNF agent 65% DMARD naive |
82 | 38 |
| ASPIRE71 | IFX i.v. 3 mg/kg at weeks 0, 2 and 6 and every 8 weeks thereafter + MTX | NR | 71 | 71% DMARD naive | 85 | 37 |
| BeST78 | Sequential monotherapy (DAS steered) | NR | 67 | Patients had active disease with ≥ 6 of 66 swollen joints, ≥ 6 of 68 tender joints and ESR ≥ 28 mm/hour or global health score of ≥ 20 mm (0–100 VAS) Exclusion criteria included previous treatment with DMARDs other than antimalarials. (HCQ and chloroquine = antimalarials) Previous antimalarial therapy = 7% |
NR | NR |
| BeST78 | Step-up combination therapy (DAS steered) | NR | 64 | Previous antimalarial therapy = 11% | NR | NR |
| BeST78 | Initial combination therapy with prednisone (DAS steered) | NR | 65 | Previous antimalarial therapy = 8% | NR | NR |
| BeST78 | Initial combination therapy with IFX (DAS steered) | NR | 64 | Previous antimalarial therapy = 9% | NR | NR |
| Durez et al., 2007120 | MTX | NR | 64 | MTX-naive population. Patients had not been previously treated with MTX. Exclusion criteria included previous treatment with two or more DMARDs (no further details), MTX or i.v. MP | NR | NR |
| Durez et al., 2007120 | MTX + i.v. MP | NR | 100 | NR | NR | |
| Durez et al., 2007120 | IFX 3 mg/kg i.v. at weeks 0, 2, 6, 14, 22, 30, 38, 46 + MTX | NR | 67 | NR | NR | |
| IDEA95 | MP 250 mg i.v. at week 0, PBO i.v. at weeks 2, 6, 14, 22 + MTX | NR | NR | Patients described as DMARD naive (no further details) | NR | NR |
| IDEA95 | IFX 3 mg/kg i.v. at weeks 0, 2, 6, 14, 22 + MTX (IFX dose modifications permitted according to DAS44 from week 26) | NR | NR | NR | NR | |
| Quinn et al., 2005110 | MTX + PBO | NR | 60 | No prior treatment with DMARDs or oral corticosteroids | NR | NR |
| Quinn et al., 2005110 | IFX + MTX | NR | 70 | No prior treatment with DMARDs or oral corticosteroids | NR | NR |
| Trial name/study | Treatment arms for which data extraction performed | Ethnicity (where reported) | Rheumatoid factor (% positive) | Prior DMARD treatment history (brief description, including definition of active RA despite previous treatment, where relevant) | % receiving NSAIDs at baseline | % receiving steroids at baseline |
|---|---|---|---|---|---|---|
| ATTEST74 | PBO + MTX | 76.4% Caucasian | 77.3 | MTX ≥ 15 mg/week for ≥ 3 months (stable for ≥ 28 days) and washed out all DMARDs (at least 28 days prior) except for MTX. No prior ABT or antiTNF therapy permitted MTX = 110/110 (100%) Dose, mg/week = 16.6 (SD 3.7) Duration, months = 23.7 (SD 25.6) |
84.5 | 70.0 |
| ATTEST74 (NCT00095147) |
IFX 3 mg/kg i.v. administered on days 1 (i.e. week 0), 15 (i.e. week 2), 43 (i.e. week 6) and 85 (i.e. week 12) and every 56 days (i.e. 8 weeks) thereafter (NB: licensed dose 3 mg/kg i.v. at weeks 0, 2, 6 and every 8 weeks thereafter, adjustments in dosage and frequency of administration permitted after week 12 in license) + MTX | 80.6% Caucasian | 84.8 | MTX = 164/165 (99.4%) Dose, mg/week = 16.3 (SD 3.6) Duration, months = 23.6 (SD 26.8) |
86.1 | 71.5 |
| ATTEST74 (NCT00095147) |
ABT dosed according to weight: patients weighing < 60 kg, 60–100 kg, or > 100 kg received 500 mg, 750 mg or 1000 mg of ABT respectively. ABT administered i.v. on days 1, 15 and 29 and every 28 days thereafter, up to and including day 337 (156 randomised) + MTX | 80.8% Caucasian | 87.2 | MTX = 156/156 (100%) Dose, mg/week = 16.5 (SD 3.7) Duration, months = 18.3 (SD 20.0) |
85.3 | 75.6 |
| AMPLE66 | ABT s.c. | 80.8% Caucasian | 75.5 | Inadequate response to MTX, no prior bDMARDs. Concomitant medication included SSZ (3.1%) and HCQ (13.2%) | NR | 50.9 |
| AMPLE66 | ADA | 78.0% Caucasian | 77.4 | Inadequate response to MTX, no prior bDMARDs. Concomitant medication included SSZ (3.4%) and HCQ (10.7%) | NR | 50.3 |
| RED-SEA114 | ADA + cDMARDs (n = 60) | NR | 91.7 | 100% prior MTX | 58.3% | On oral prednisolone 33.3% |
| RED-SEA114 | ETN50 + cDMARDs (n = 60) | NR | 85 | 100% prior MTX | 43.3% | On oral prednisolone 45% |
| ADACTA58 | TCZ + PBO | NR | 75 | Patients with RA of at least 6 months duration and DAS28 > 5.1 who were MTX intolerant or for whom continued treatment with MTX was considered ineffective or inappropriate Mean number of previous DMARDs = 2.0 (SD 1.1) Stopped taking MTX < 2 months before baseline = 99/163 (61%) |
NR | 55 |
| ADACTA58 | ADA + PBO | NR | 73 | Mean number of previous DMARDs = 2.0 (SD 1.1) Stopped taking MTX < 2 months before baseline = 102/162 (63%) |
NR | 57 |
| deFilippis et al., 200685 | ETN + MTX | NR | NR | Non-responder to DMARDs for > 6 months (no further detail reported). All receiving a stable dose of concomitant MTX in 3 months before entering the study | NR | NR |
| deFilippis et al., 200685 | IFX + MTX | NR | NR | Non-responder to DMARDs for > 6 months (no further detail reported). All receiving a stable dose of concomitant MTX in 3 months before entering the study |
| Trial name/study | Treatment arms for which data extraction performed | Ethnicity (where reported) | Rheumatoid factor (% positive) | Prior DMARD treatment history (brief description, including definition of active RA despite previous treatment, where relevant) | % receiving NSAIDs at baseline | % receiving steroids at baseline |
|---|---|---|---|---|---|---|
| AIM61,62 | MTX + PBO n = 219 |
88.1% white | 78.5 | 100% prior MTX; 8.7% prior cDMARDs other than MTX | 82.6 | 68.5 |
| AIM61 | ABT i.v. + MTX (n = 433) | 87.5% white | 81.8 | 100% prior MTX; 12.2% prior cDMARDs other than MTX | 85.5 | 72.1 |
| ASSET72 | PBO + MTX | 82.6% Caucasian | 82.6 | Non-response to MTX (≥ 15 mg/week or a maximum tolerated dose of ≥ 10 mg/week for ≥ 3 months prior to day 1) | 87.0 | 60.9 |
| ASSET72 | ABT i.v. (≈ 10 mg/kg) + MTX | 96.3% Caucasian | 55.6 | Non-response to MTX (≥ 15 mg/week or a maximum tolerated dose of ≥ 10 mg/week for ≥ 3 months prior to day 1) | 81.5 | 70.4 |
| ASSURE73 | PBO + cDMARDs | 83.3% white | NR | Active disease (functional classes I, II, III, IV ACR) despite one or more biologic and/or NBT, stable dose for ≥ 28 days before trial (split analyses, only nonbiologic extracted) | NR | 73.7 (concomitant) |
| ASSURE73 | ABT + cDMARDs | 83.9% white | NR | Active disease (functional classes I, II, III, IV ACR) despite one or more biologic and/or NBT, stable dose for ≥ 28 days before trial (split analyses, only nonbiologic extracted) | NR | 71.6 (concomitant) |
| AUGUST II76 | MTX + PBO (n = 76) | 83 | 100% prior MTX | NR | 59 | |
| AUGUST II76 | ADA + MTX (n = 79) | 81 | 100% prior MTX | NR | 66 | |
| CHANGE80 | PBO (n = 87) | NR | 86.2 | 87.2% prior MTX (91.5% two or more DMARDs across all arms) | NR | NR |
| CHANGE80 | ADA monotherapy (n = 91) | NR | 90.8 | 87.2% prior MTX | NR | NR |
| DE01984 | MTX + PBO (n = 200) | 83.0% white | 89.5 | 100% prior MTX; mean 2.4 prior cDMARDs including MTX | NR | 49.5 |
| DE01984 | ADA + MTX (n = 207) | 83.6% white | 81.6 | 100% prior MTX; mean 2.4 prior cDMARDs including MTX | NR | Across two ADA arms, 44.9% |
| STAR117 | PBO + cDMARDs (n = 318) | 85.8% white | 62.3 | Mean 1.2 prior cDMARDs | 63.8 | 54.4 |
| STAR117 | ADA + cDMARDs (n = 318) | 89.0% white | 63.4 | Mean 112 prior cDMARDs | 62.3 | 50.9 |
| Van De Putte et al., 2004122 | PBO s.c. | NR | 81.8 | Previous treatment with at least one DMARD had failed, with patients having active RA defined as ≥ 12 tender joints (0–68 scale), ≥ 10 swollen joints (0–66 scale), and either ESR ≥ 28 mm/first hour or CRP ≥ 20 mg/l Patients excluded if had received investigational small molecule drug or biological agent within 2 months or 6 months before screening respectively 4-week washout period required for patients taking cDMARDs at time of recruitment Number of cDMARDs = 3.6 (SD 1.8) |
83.6 | 67.3 |
| Van De Putte et al., 2004122 | ADA monotherapy | NR | 79.6 | Number of cDMARDs = 3.8 (SD 1.8) | 82.3 | 68.1 |
| ARMADA69,70 | MTX + PBO (n = 62) | NR | Rheumatoid factor, IU/litre mean = 321.2 (SD 518.2) | 100% prior MTX; mean 3.0 prior cDMARDs including MTX | NR | 58.1 |
| ARMADA69 | ADA + MTX (n = 67) | NR | Rheumatoid factor, IU/litre mean = 269.3 (SD 390.0) | 100% prior MTX; mean 2.9 prior cDMARDs including MTX | NR | Across all ADA dose arms, 46.4% |
| Kim et al., 200799 | MTX + PBO rescue week 18 (n = 65) | NR | 82.5 | 100% prior MTX; 79.3% used two or three cDMARDs | NR | NR |
| Kim et al., 200799 | ADA + MTX (n = 63) | NR | 76.9 | 100% prior MTX; 86.2% used two or three cDMARDs | NR | NR |
| CERTAIN79 | PBO + cDMARDs | NR | 67.3 | Inclusion criteria of using cDMARD therapy for ≥ 6 months (and < 10 years). No prior antiTNF use. (AiC information has been removed) | NR | NR |
| CERTAIN79 | CTZ 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks + DMARDs | NR | 74.0 | Inclusion criteria of using cDMARD therapy for ≥ 6 months (and < 10 years). No prior antiTNF use. (AiC information has been removed) | NR | NR |
| REALISTIC113 | PBO + existing cDMARDs | NR | NR overall trial population, 76.5 | Inadequate response to one or more DMARDs. Post-hoc analysis of those with DAS28 > 5.1 at baseline, two or more prior cDMARDs and antiTNF naive | NR | NR |
| REALISTIC113 | CTZ 400 mg at weeks 0, 2, 4 then 200 mg every 2 weeks + existing cDMARDs | NR | NR overall trial population, 73.9 | Inadequate response to one or more DMARD. Post-hoc analysis of those with DAS28 > 5.1 at baseline, two or more prior cDMARDs and antiTNF naive | NR | NR |
| ADORE59,60 | ETN monotherapy (n = 159) | White 158 (99.4%); black 0 (0%); Asian 1 (0.6%) | 70.9 | 100% prior MTX; mean 2.2 other prior DMARDs | 74.2 | 51.6 |
| ADORE59 | ETN + MTX (n = 155) | White 153 (98.7%); black 2 (1.3%); Asian 0 (0%) | 69.5 | 100% prior MTX; mean 2.3 other prior DMARDs | 81.3 | 56.8 |
| CREATE IIb96 | DMARD + PBO (n = 65) | NR | 81.5 | 100% prior MTX or SSZ | NR | NR |
| CREATE IIb96 | ETN50 + DMARD (n = 64) | NR | 85.9 | 100% prior MTX or SSZ | NR | NR |
| ETN Study 309/Combe et al., 2006,88 Combe et al., 200989 | SSZ + PBO (n = 50) | NR | NR | 100% prior SSZ; 58% prior cDMARDs other than SSZ | NR | 40 |
| ETN Study 309/Combe et al., 2006,88 Combe et al., 200989 | ETN + PBO (n = 103) | NR | NR | 100% prior SSZ; 69.9% prior cDMARDs other than SSZ | NR | 59.2 |
| ETN Study 309/Combe et al., 2006,88 Combe et al., 200989 | ETN + SSZ (n = 101) | NR | NR | 100% prior SSZ; 58.4% prior cDMARDs other than SSZ | NR | 44.6 |
| JESMR140 | ETN 25 mg every 2 weeks monotherapy | NR | 91.5 | Non-response to MTX (6–8 mg/week). No prior biologics | NR | 46.4 |
| JESMR140 | ETN 25 mg every 2 weeks + MTX 6–8 mg/week | NR | 86.7 | Non-response to MTX (6–8 mg/week). No prior biologics | NR | 60.3 |
| Lan et al., 2004101 | PBO + MTX (n = 29) | NR | NR | 100% prior MTX | NR | NR |
| Lan et al., 2004101 | ETN + MTX (n = 29) | 100% prior MTX | NR | NR | ||
| LARA102 | MTX + DMARD (n = 142) | White, 65 (45.8%) Mestizos, 34 (23.9%) African-Latin American, 23 (16.2%) Other, 20 (14.1%) |
83.8 | 100% prior MTX | NR | NR |
| LARA102 | ETN50 + MTX (n = 281) | White, 134 (47.7%) Mestizos, n (%) 60 (21.4%) African-Latin American, 39 (13.9%) Other, 48 (17.1%) |
86.1 | 100% prior MTX | ||
| Moreland et al., 1999104 | PBO (n = 80) | 89% white | 79 | 90% prior MTX; mean three prior cDMARDs including MTX | 84 | 58 |
| Moreland et al., 1999104 | ETN + PBO (n = 78) | 94% white | 79 | 87% prior MTX; mean 3.3 prior cDMARDs including MTX | 67 | 81 |
| RACAT111 | MTX + SSZ + HCQ (n = 178) | 90.4% white | 65.7 | 100% prior MTX | NR | 47.2 |
| RACAT111 | ETN50 + MTX (n = 175) | 83.4% white | 67.2 | 100% prior MTX | 49.7 | |
| Wajdula 2000 (reported in Chen et al., 2006123) | PBO (n = 111) | Mean 3.5 prior cDMARDs; failed to respond to at least one DMARD | 85 | 71 | ||
| Wajdula 2000 (reported in Chen et al., 2006123) | ETN (n = 105) | Mean 3.6 prior cDMARDs; failed to respond to at least one DMARD | 86 | 70 | ||
| Weinblatt et al., 1999124 | MTX + PBO, (n = 30) | White 83% | 90 | 100% prior MTX | 80 | 70 |
| Weinblatt et al., 1999124 | ETN 25 mg twice weekly + MTX (n = 59) | White 76% | 84 | 100% prior MTX | 75 | 53 |
| APPEAL67,68 | MTX + DMARD (SSZ, HCQ or LEF) (n = 103) | NR | NR | 100% prior MTX; 30.1% also other cDMARD(s) | NR | NR |
| APPEAL68 | ETN 25 mg twice weekly (licensed dose) + MTX (n = 197) | NR | NR | 100% prior MTX; 24.4% also other cDMARD(s) | NR | NR |
| GO-FORTH91 | PBO every 4 weeks + MTX 6–8 mg/week | NR | NR | All patients had received MTX > 6 mg/week for ≥ 3 months prior to the start of the study. Other prior DMARDs and biologics not reported | NR | NR |
| GO-FORTH91 | GOL 50 mg s.c. every 4 weeks + MTX 6–8 mg/week | NR | NR | All patients had received MTX > 6 mg/week for ≥ 3 months prior to the start of the study. Other prior DMARDs and biologics not reported | NR | NR |
| GO-FORWARD92 | PBO s.c. every 4 weeks + MTX | NR | 81.2 | Patients had to have been on stable MTX dose of 15 mg/week or greater but 25 mg/week or less during the 4-week period immediately preceding screening. Must have tolerated at least 15 mg/week for at least 3 months before screening. Patients had active RA defined as ≥ 4 of 66 swollen joints, ≥ 4 of 68 tender joints and at least two of following criteria: CRP ≥ 1.5 mg/dl or ESR ≥ 28 mm/hour Median MTX dose (mg/week) = 15.0 (IQR 15.0–20.0) Duration of previous MTX use (years): < 1 = 33 (24.8%) ≥ 1 to < 3 = 30 (22.6%) ≥ 3 = 68 (51.1%) Patients with previous use of DMARD other than MTX = 94 (70.7%) (Any previous use of any antiTNF agent, RTX, natalizumab or cytotoxic agents excluded patients from trial participation. In addition, patients should not have taken anakinra; DMARDs other than MTX; or i.v., i.m. or intra-articular corticosteroids within 4 weeks before first dose of study drug or alefacept or efalizumab within 3 months of first dose of study drug) |
85.7 | 65.4 |
| GO-FORWARD92 | GOL 50 mg s.c. every 4 weeks + MTX | NR | 86.5 (77/89) | Median MTX dose (mg/week) = 15.0 (IQR 15.0–20.0) Duration of previous MTX use (years): < 1 = 20 (22.5%) ≥ 1 to < 3 = 32 (36.0%) ≥ 3 = 37 (41.6%) Patients with previous use of DMARD other than MTX = 70 (78.7%) |
86.5 | 75.3 |
| Kay et al., 200898 (NCT00207714) |
PBO s.c. + MTX | NR | NR | All patients treated with MTX at dosage of at least 10 mg/week for ≥ 3 months and at stable dosage for ≥ 4 weeks before receiving first dose of study drug Patients had active RA defined as ≥ 6 swollen joints, ≥ 6 tender joints and at least two of the following three criteria: CRP ≥ 1.5 mg/dl, ESR ≥ 28 mm/hour or morning stiffness of ≥ 30 minutes |
NR | NR |
| Kay et al., 200898 | GOL 50 mg s.c. every 4 weeks + MTX | NR | NR | NR | NR | |
| Abe et al., 200656 | PBO + MTX | Japanese patients | NR | Eligible patients had received MTX treatment for more than 3 months, with a stable MTX dosage at 6 mg/week or more during the last 4 weeks Patients had active RA defined as ≥ 6 of 68 tender joints, ≥ 6 of 66 swollen joints, and at least two of the following: morning stiffness ≥ 45 minutes, ESR ≥ 28 mm/hour, or CRP ≥ 2 mg/dl. Patients not permitted to use DMARD, immunosuppressive drugs other than MTX, or intra-articular, i.m., i.v. or epidural corticosteroids |
95.7 | 89.4 |
| Abe et al., 200656 | IFX 3 mg/kg i.v. at weeks 0, 2 and 6 + MTX | NR | 89.8 | 85.7 | ||
| ATTRACT75 | PBO i.v. + MTX | White 78/88 (89) | 77 | Patients had been receiving MTX for at least 3 months with no break in treatment of more than 2 weeks during that period. MTX dose required to have been stable at ≥ 12.5 mg/week for at least 4 weeks before screening Patients were excluded if they had used a DMARD other than MTX or received intra-articular/IM/IV corticosteroids in 4 weeks before screening; received any other agent to reduce TNF Mean number (SD) of previous DMARDs (excluding MTX) = 2.5 (1.4) |
72 | 64 |
| ATTRACT75 | IFX 3 mg/kg i.v. at weeks 0, 2 and 6 and every 8 weeks thereafter + MTX | White 80/86 (93%) | 84 | Mean number of previous DMARDs (excluding MTX) = 2.8 (SD 1.5) | 79 | 63 |
| Durez et al., 200486 | Single i.v. infusion of MP (sodium hemisuccinate) at week 0 + MTX | NR | 87 | Eligible patients had received 15 mg/week MTX treatment (10 mg when tolerance poor) Previous treatment with i.v. MP pulse and/or antiTNF agents excluded patients from participation By randomisation, patients had received: MTX (100%), SSZ (85%), GLD salts (79%), HCQ (61%), CYC (58%), D-penicillamine (42%), AZA (30%) and LEF (18%) (authors stated no differences between i.v. MP and IFX arms, no data presented) Previous DMARDs, median 3 (range 1–7) |
NR | NR |
| Durez et al., 200486 | IFX 3 mg/kg at weeks 0, 2 and 6 + MTX | NR | 67 | Previous DMARDs, median 3 (range 2–6) | NR | NR |
| START118 | PBO + MTX | NR | 80.7 | All patients had been receiving MTX for at least 6 months prior to randomisation and were permitted to receive stable doses of the following: chloroquine, AZA, penicillamine, oral/i.m. GLD, HCQ, SSZ, LEF, CYC, oral corticosteroids, NSAIDs. No prior biologics allowed | 39.4 | 59 |
| START118 | IFX 3 mg/kg + MTX | NR | 82.8 | All patients had been receiving MTX for at least 6 months prior to randomisation and were permitted to receive stable doses of the following: chloroquine, AZA, penicillamine, oral/i.m. GLD, HCQ, SSZ, LEF, CYC, oral corticosteroids, NSAIDs. No prior biologics allowed | 43.3 | 59.2 |
| Swefot119 | SSZ (1000 mg twice daily orally) + HCQ (400 mg daily orally) + MTX | NR | 65 | Patients with early RA (with no previous treatment with DMARDs) were administered MTX (up to 20 mg/week). After 3–4 months, patients who had not achieved low disease activity (having DAS28 > 3), but were able to tolerate MRX were randomised to treatment arms | NR | 8 |
| Swefot119 | IFX 3 mg/kg i.v. at weeks 0, 2, 6 and every 8 weeks thereafter + MTX | NR | 69 | NR | 6 | |
| Wong et al., 2009125 | PBO + MTX (with crossover to open-label IFX at week 24) | NR | 7/8 | Eligible patients had failed on two DMARDs including MTX. All patients had been receiving MTX (≤ 25 mg/week) | NR | NR |
| Wong et al., 2009125 | IFX 3 mg/kg at weeks 0, 2, 6 and 8 weeks thereafter + MTX | NR | 7/16 | NR | NR | |
| Zhang et al., 2006126 | PBO i.v. + MTX | Chinese patients | NR | Patients had been treated with MTX for at least 3 months at a stable dose (7.5–20 mg/week) for at least 4 weeks Patients who began treatment with other DMARDs within 4 weeks before screening were ineligible. Treatment with other antiTNF agents within 3 months of study entry was not permitted 64.0% had previously used drug other than MTX (no other details) |
NR | NR |
| Zhang et al., 2006126 | IFX 3 mg/kg i.v. at weeks 0, 2, 6 and 14 + MTX | NR | 55.2% had previously used drug other than MTX (no other details) | NR | NR | |
| ACT-RAY57 (NCT00810199) |
TCZ 8 mg/kg i.v. every 4 weeks + oral PBO (n = 277 randomised) | NR | NR | Subjects had been receiving MTX for at least 12 weeks with stable dose of at least 15 mg/week for at least 6 weeks before starting study treatment Patients were excluded if had any previous use of biological agents as well as any cDMARD drug treatment other than MTX during the month (3 months for LEF) preceding baseline visit Mean MTX dose, mg/week = 16.2 (SD 4.1) Number of prior DMARDs (including MTX before study entry), mean = 1.9 (SD 1.0) |
NR | 49.1 |
| ACT-RAY57 | TCZ 8 mg/kg i.v. every 4 weeks + MTX | NR | NR | Mean MTX dose, mg/week = 16.0 (SD 4.4) Number of prior DMARDs (including MTX before study entry), mean = 1.9 (SD 1.1) |
NR | 48.9 |
| MEASURE103 | PBO + MTX | NR | NR | Patients were described as MTX inadequate responders | NR | NR |
| MEASURE103 | TCZ 8 mg/kg i.v. every 4 weeks + MTX | NR | NR | NR | NR | |
| Nishimoto et al., 2004106 | PBO i.v. every 4 weeks | NR | NR | Eligible patients had been treated unsuccessfully (due to lack of efficacy) with one or more DMARD or immunosuppressant Active RA defined as ≥ 6 swollen joints, ≥ tender joints and one of the following two criteria: ESR ≥ 30 mm/hour or CRP > 1.0 mg/dl No DMARDs permitted during 4-week washout period before initiation of study agent and during study period Number of failed DMARDs, median = 5 (range 1–10) |
NR | NR |
| Nishimoto et al., 2004106 | TCZ 8 mg/kg i.v. every 4 weeks | NR | NR | Number of failed DMARDs, median = 5 (range 1–11) | NR | NR |
| SAMURAI115 | cDMARDs disease activity (n = 145) | NR | NR | 67% prior MTX | NR | NR |
| SAMURAI115 | TCZ i.v. (n = 157) | NR | NR | 73% prior MTX | NR | NR |
| SATORI116 (NCT00144521) |
PBO i.v. every 4 weeks + MTX | NR | NR | Mean number of failed DMARDs = 3.6 (range 1–8) All candidates were treated with MTX 8 mg/week for at least 8 weeks until enrolment. Inadequate response to MTX defined as presence of active disease (as above) Patients not permitted to receive prior antiTNF agents or LEF (within 12 weeks prior to first dose). Patients not permitted to receive DMARDs other than MTX or immunosuppressants (within 2 weeks prior to first dose) |
NR | NR |
| SATORI116 | TCZ 8 mg/kg i.v. every 4 weeks + PBO capsule | NR | NR | Mean number of failed DMARDs = 3.3 (range 1–8) | NR | NR |
| TOWARD121 | PBO i.v. every 4 weeks + stable cDMARDs | 72% white; 10% Asian; 8% American Indian/Native Alaskan; 7% black; 3% other | NR | Eligible patients had received stable doses of permitted DMARDs (MTX, chloroquine, HCQ, parenteral GLD, SSZ, AZA, and LEF) for ≥ 8 weeks before study entry Patients unsuccessfully treated with an antiTNF agent or any cell-depleting therapy were excluded Medication at baseline (%): MTX = 73.9 Chloroquine/HCQ = 19.8 SSZ = 14.3 LEF = 15.5 Parenteral GLD = 0.7 AZA = 2.2 Number of background DMARDs at baseline (%): One = 75 Two or more = 24 None = 1 |
77.1 | 54.6 |
| TOWARD121 | TCZ 8 mg/kg i.v. every 4 weeks + stable DMARDs | 72% white; 9% Asian; 10% American Indian/Native Alaskan; 4% black; 3% other | NR | Medication at baseline (%): MTX = 75.8 Chloroquine/HCQ = 20.6 SSZ = 13.1 LEF = 12.1 Parenteral GLD = 0.2 AZA = 2.2 Number of background DMARDs at baseline (%): One = 77 Two or more = 22 None = 1 |
71.4 | 51.2 |
| TACIT141 | Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| TACIT141 | TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Trial name/study | Treatment arms for which data extraction performed | Mean age (years, SD) | Sex (% female) | Early withdrawal plan reported? | Disease duration (years, SD) | Mean DAS28 at baseline (SD) (ESR or CRP where stated) |
|---|---|---|---|---|---|---|
| ACQUIRE127 | ABT + PBO + MTX (n = 736) | 49.9 (13.2) | 84.4 | NR | 7.6 (8.1) | 6.23 (0.85) CRP |
| ACQUIRE127 | ABT + PBO + MTX (n = 721) | 50.1 (12.6) | 80.4 | 7.7 (7.8) | 6.20 (0.8) DAS 28-CRP | |
| NCT00254293131 | PBO + MTX (n = 119) | 54.7 (NR), range 23–80 | 66 | NR | 8.9 (8.3) | 5.5 (0.87) CRP |
| NCT00254293131 | ABT i.v. (≈ 10 mg/kg) + MTX (n = 115) | 55.8 (NR), range 17–83 | 75 | 9.7 (9.8) | 5.5 (0.6) CRP | |
| ORAL STANDARD133 | MTX + PBO (n = 108) | 53.7 | 75.9 | Yes | 7.9 | 6.5 ESR; 5.5CRP |
| ORAL STANDARD133 | TOF5 + MTX (n = 204) | 53.0 | 85.3 | 7.6 | 6.6 ESR; 5.4 CRP | |
| ORAL STANDARD133 | TOF10 + MTX (n = 201) | 52.9 | 83.6 | 7.4 | 6.5 ESR; 5.4 CRP | |
| ORAL STANDARD133 | ADA + MTX (n = 204) | 52.5 | 79.4 | 8.1 | 6.4 ESR; 5.3 CRP | |
| JRAPID129 | MTX + PBO (n = 77) | 51.9 (11.1) | 85.7 | Yes | 5.8 (4.1) | 6.5 (0.9) ESR |
| JRAPID129 | CTZ 200 mg every 2 weeks + MTX (n = 82) | 50.6 (11.4) | 84.1 | 5.6 (4.2) | 6.2 (0.8) ESR | |
| RA0025134 | PBO + MTX (n = 40) | 51.6 (11.7) | 88.9 | Yes | 6.5 (4.2) | 7.33 (1.09) ESR |
| RA0025134 | CTZ + MTX (n = 81) | 50.8 (11.1) | 87.5 | 5.5 (4.6) | 7.46 (1.29) ESR | |
| RAPID1135 | PBO + MTX (n = 199) | 52.2 (11.2) | 83.9 | Yes | 6.2 (4.4) | 7.0 (0.9) ESR |
| RAPID1135 | CTZ + MTX (n = 393) | 51.4 (11.6) | 82.4 | 6.1 (4.2) | 6.9 (0.8) ESR | |
| RAPID2136 | PBO + MTX (n = 127) | 51.5 (11.8) | 84.3 | Yes | 5.6 (3.9) | 6.83 (0.87) ESR |
| RAPID2136 | CTZ + MTX (n = 246) | 52.2 (11.1) | 83.7 | 6.1 (4.1) | 6.85 (0.84) ESR | |
| TEAR53 | MTX monotherapy (ST) (n = 124) ST = step-up from MTX to triple DMARD therapy (MTX + SSZ + HCQ) |
49.3 | 70.2 | Yes | 0.38 | 5.8 ESR |
| TEAR53 | MTX monotherapy (SE) (n = 255) SE = step-up from MTX to MTX + ETN |
48.6 | 69 | 0.24 | 5.8 ESR | |
| TEAR53 | MTX + SSZ + HCQ (n = 132) | 48.8 | 76.5 | 0.34 | 5.8 ESR | |
| TEAR53 | ETN50 + MTX (n = 244) | 50.7 | 74.2 | 0.29 | 5.8 ESR | |
| TEMPO54 | MTX monotherapy (n = 228) | 53.0 | 79 | NR | 6.8 (5.5) | 5.5 (1.2) |
| TEMPO54 | ETN monotherapy (n = 223) | 53.2 | 77 | 6.3 (5.1) | 5.7 (1.1) | |
| TEMPO54 | ETN + MTX (n = 231) | 52.5 | 74 | 6.8 (5.4) | 5.5 (1.2) | |
| AMBITION55 (ITT baseline covariate data presented) |
MTX (n = 284) (Note that data are presented for the whole trial population as data for the MTX-experienced subgroup was not reported) |
50.0 (12.9) | 79 | NR | 6.2 (7.8) | 6.8 (0.9) |
| AMBITION55 | TCZ monotherapy (n = 288) | 50.7 (13.1) | 83 | 6.4 (7.9) | 6.8 (1.0) | |
| LITHE130 | PBO + MTX (n = 393) | 51.3 (12.4) | 83 | Yes | Mean = 9.0 (range 0.5–44.3) | 6.5 (1.0) |
| LITHE130 | TCZ + MTX (n = 398) | 53.4 (11.7) | 82 | Mean = 9.3 (range 0.6–48.8) | 6.6 (1.0) | |
| OPTION132 | PBO + MTX (n = 204) | 50.6 (12.1) | 78 | NR | 7.8 (7.2) | 6.8 (0.9) |
| OPTION132 | TCZ + MTX (n = 205) | 50.8 (11.8) | 85 | 7.5 (7.3) | 6.8 (0.9) |
| Trial name/author, year | Treatment arms for which data extraction performed | Ethnicity (where reported) | Rheumatoid factor (% positive) | Prior DMARD treatment history (brief description, including definition of active RA despite previous treatment, where relevant) | n (%) receiving NSAIDs at baseline | n (%) receiving steroids at baseline |
|---|---|---|---|---|---|---|
| ACQUIRE127 | ABT s.c. + PBO i.v. + MTX | 74.7% Caucasian | 84.8 | Inadequate response to ≥ 3 months of MTX at ≥ 15 mg/week. Prior biologics in 4.3% of the sample | NR | NR |
| ACQUIRE127 | ABT i.v. + PBO s.c. + MTX | 74.5% Caucasian | 85.9 | Inadequate response to ≥ 3 months of MTX at ≥ 15 mg/week. Prior biologics in 6.0% of the sample | NR | NR |
| NCT00254293131 | PBO + MTX | 87% white | NR | 99.2% prior MTX, 21.0% other prior DMARDs, 2.6% prior antiTNF | NR | 67.2 |
| NCT00254293131 | ABT i.v. (≈ 10 mg/kg) + MTX | 87% white | NR | 99.1% prior MTX, 16.5% other prior DMARDs, 2.6% prior antiTNF | NR | 60.0 |
| ORAL STANDARD133 | MTX + PBO | Region of origin: North America 28.7%; Latin America 4.7%; Europe 49%; other 18.5% | 66.3 | 100% prior MTX, 54.7% other prior cDMARDs, 8.3% prior TNF inhibitor | NR | 66.7 |
| ORAL STANDARD133 | TOF5 + MTX | Region of origin: North America 24.5%; Latin America 3.9; Europe 53.9%; other 17.6% | 66.8 | 100% prior MTX, 53.4% other prior cDMARDs, 5.9% prior TNF inhibitor | NR | 61.8 |
| ORAL STANDARD133 | TOF10 + MTX | Region of origin: North America 24.9%; Latin America 1.5%; Europe 55.7%; other 17.9% | 66.2 | 100% prior MTX, 57.2% other prior cDMARDs, 7.0% prior TNF inhibitor | NR | 64.2 |
| ORAL STANDARD133 | ADA + MTX | Region of origin: North America 25.5%; Latin America 2.9%; Europe 53.9%; other 17.6% | 68.2 | 100% prior MTX, 55.9% other prior cDMARDs, 7.8% prior TNF inhibitor | NR | 61.3 |
| JRAPID/Yamamoto et al., 2011129 (NCT00791999) |
MTX + PBO | NR | 85.7 | Inadequate response to MTX. 19.5% had prior TNF inhibitors | NR | NR |
| JRAPID129 | CTZ 200 mg every 2 weeks + MTX | NR | 86.6 | Inadequate response to MTX. 13.4% had prior TNF inhibitors | NR | NR |
| RA0025134 | PBO + MTX | NR | NR | Inadequate response to MTX. Study MTX dose 10–20 mg (min.–max.). Prior TNF inhibitors in 13.6% | NR | NR |
| RA0025134 | CTZ 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks + MTX | NR | NR | Inadequate response to MTX. Study MTX dose 10–20 mg (min.–max.). Prior TNF inhibitors in 17.5% | NR | NR |
| RAPID1135 | PBO + MTX | NR | 82.8 | Patients were required to receive MTX for ≥ 6 months with a stable dosage of ≥ 10 mg/week for ≥ 2 months prior to baseline. No biologics within 6 months of baseline (or within 3 months for ETN/AKR) and/or no previous biologics that resulted in severe hypersensitivity or anaphylactic reaction, or response failure to antiTNF agent. Mean (SD) of 1.4 (one previous DMARD). Prior TNF inhibitors in 3.5% | NR | NR |
| RAPID1135 | CTZ 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks + MTX | NR | 79.6 | Patients were required to receive MTX for ≥ 6 months with a stable dosage of ≥ 10 mg/week for ≥ 2 months prior to baseline. No biologics within 6 months of baseline (or within 3 months for ETN/ANA) and/or no previous biologics that resulted in severe hypersensitivity or anaphylactic reaction, or response failure to antiTNF agent. Mean (SD) of 1.3 (one previous DMARD). Prior TNF inhibitors in 2.8% | NR | NR |
| RAPID2136 | PBO + MTX | NR | 78.2 | Patients were required to receive MTX for ≥ 6 months with a stable dosage of ≥ 10 mg/week for ≥ 2 months prior to baseline. No biologics within 6 months of baseline (or within 3 months for ETN/ANA) and/or no previous biologics that resulted in severe hypersensitivity or anaphylactic reaction, or response failure to antiTNF agent. Mean (SD) of 1.2 (one previous DMARD excluding MTX). Prior antiTNF use in 1.6% patients | NR | 59.8 |
| RAPID2136 | CTZ 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks + MTX | NR | 77.5 | Patients were required to receive MTX for ≥ 6 months with a stable dosage of ≥ 10 mg/week for ≥ 2 months prior to baseline. No biologics within 6 months of baseline (or within 3 months for ETN/ANA) and/or no previous biologics that resulted in severe hypersensitivity or anaphylactic reaction, or response failure to antiTNF agent. Mean (SD) of 1.2 (one previous DMARD excluding MTX). Prior antiTNF use in 1.6% patients | NR | 55.3 |
| TEAR53 | MTX monotherapy (ST) (n = 124) ST = step-up from MTX to triple DMARD therapy (MTX + SSZ + HCQ) |
White 85.5%; African American 11.3%; Hispanic 8.1% | 87.1 | 14.5% prior MTX, 0% prior biologics | NR | 33.1 |
| TEAR53 | MTX monotherapy (SE) (n = 255) SE = step-up from MTX to MTX + ETN |
White 78.4%; African American 11.4%; Hispanic 12.6% | 91 | 20% prior MTX, 0.8% prior biologics | NR | 43.5 |
| TEAR53 | MTX + SSZ + HCQ (n = 132) | White 81.1%; African American 8.3%; Hispanic 12.9% | 91.7 | 20.5% prior MTX, 0% prior biologics | NR | 43.9 |
| TEAR53 | ETN50 + MTX (n = 244) | White 77.1%; African American 12.7%; Hispanic 10.7% | 88.5 | 24.6% prior MTX, 0.8% prior biologics | NR | 43.0 |
| TEMPO54 | MTX monotherapy (n = 228) | NR | 71 | 42% prior MTX, mean 2.3 prior cDMARDs including MTX | 86 | 64 |
| TEMPO54 | ETN monotherapy (n = 223) | NR | 75 | 42% prior MTX, mean 2.3 prior cDMARDs including MTX | 88 | 57 |
| TEMPO54 | ETN + MTX (n = 231) | NR | 76 | 44% prior MTX, mean 2.3 prior cDMARDs including MTX | 88 | 62 |
| AMBITION55 (ITT baseline covariate data presented) |
MTX alone | NR | NR | Patients excluded if they had been unsuccessfully treated with an antiTNF agent, had received MTX in the 6 months before randomisation or discontinued MTX due to clinically important adverse effects or lack of efficacy. Patients who had temporarily discontinued MTX owing to side effects or desire to become pregnant and those who discontinued antiTNF agents for reasons other than efficacy (e.g. treatment cost, side effects) could participate in study Patients had active RA defined as ≥ 6 of 66 swollen joints, ≥ 8 of 68 tender joints and CRP ≥ 1 mg/dl or ESR ≥ 28 mm/hour MTX naive = 67% Number previous DMARDs/antiTNF agents, mean = 1.1 (SD 1.4) Previous use of antiTNF agents = 7.4% (per-protocol) |
NR | 47 |
| AMBITION55 | TCZ 8 mg/kg i.v. every 4 weeks | NR | NR | MTX naive = 67% Number previous DMARDs/antiTNF agents, mean = 1.2 (SD 1.3) Previous use of antiTNF agents = 8.3% (per protocol) |
NR | 48 |
| LITHE130 | PBO i.v. every 4 weeks + MTX | NR | 82 | Eligible patients who had inadequate responses to MTX [despite receiving MTX for ≥ 12 weeks before baseline (stable dose of 10–25 mg/week for ≥ 8 weeks)], with active RA defined as ≥ 6 swollen joints, ≥ 8 tender joints, and either CRP ≥ 1 mg/dl or ESR ≥ 28 mm/hour, and had ≥ radiographically confirmed joint erosion All other DMARDs or biological agents were discontinued before study entry (LEF for ≥ 12 weeks, IFX or ADA for ≥ 8 weeks and ETN for ≥ 2 weeks) Additional exclusion criteria: failure to respond to antiTNF treatment Number of previous DMARDs/antiTNFs, mean = 1.6 (SD 1.5) % with past use of DMARDs = 71.2% % with past use of antiTNF agents = 11.5% |
NR | 70 |
| LITHE130 | TCZ 8 mg/kg i.v. every 4 weeks + MTX | NR | 83 | Number of previous DMARDs/antiTNFs, mean = 1.6 (SD 1.4) % with past use of DMARDs = 75.4% % with past use of antiTNF agents = 10.8% |
NR | 62 |
| OPTION132 | PBO i.v. every 4 weeks + MTX | NR | 71 | Eligible patients had experienced an inadequate response to MTX, with active RA defined as ≥ 6 swollen joints, ≥ 8 tender joints and CRP ≥ 10 mg/l or ESR ≥ 28 mm/hour. Patients had received MTX for ≥ 12 weeks before study entry (with a stable dose of 10–25 mg/week for ≥ 8 weeks) All other DMARDs were discontinued prior to study entry (LEF for ≥ 12 weeks, AKR for ≥ 1 week, ETN for ≥ 2 weeks, and IFX or ADA for ≥ 8 weeks) Patients excluded if they had previous unsuccessful antiTNF treatment (discontinuations owing to cost or injection discomfort not excluded) Number of previous DMARDs (not including MTX) = 1.7 (SD 1.5) Previous antiTNF treatment = 19/204 (9%) |
68 | 54 |
| OPTION132 | TCZ 8 mg/kg i.v. every 4 weeks + MTX | NR | 83 | Number of previous DMARDs (not including MTX) = 1.5 (SD 1.4) Previous antiTNF treatment = 11/205 (5%) |
66 | 55 |
| Study | Treatment arms for which data extraction performed | Assessment time point | DAS28-ESR or DAS28-CRP where stated | n analysed | Mean DAS28 at baseline (SD) | DAS28 mean change from baseline (SD) |
|---|---|---|---|---|---|---|
| Kume et al., 2011100 | ADA monotherapy | 24 weeks | DAS28-ESR | 19 | 5.34 (1.4) (n = 21) | –2.12 (0.38) |
| Kume et al., 2011100 | ETN monotherapy | 24 weeks | DAS28-ESR | 20 | 5.17 (1.5) (n = 21) | –2.84 (0.42) |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | DAS28-ESR or DAS28-CRP where stated | n analysed | Mean DAS28 at baseline (SD) | Mean DAS28 at follow-up (SD) | DAS28 mean change from baseline (SD) | % achieving DAS28 remission (defined threshold) |
|---|---|---|---|---|---|---|---|---|
| GUEPARD93 | Initial MTX | Week 12 | NR | 32 | DAS28 (ESR) 6.15 (0.88) DAS28 (CRP) 5.85 (0.9) |
NR | NR | DAS < 2.6, 12.5% |
| GUEPARD93 | Initial ADA + MTX | Week 12 | NR | 33 | DAS28 (ESR) 6.31 (0.78) DAS28 (CRP) 5.80 (0.8) |
NR | NR | DAS < 2.6, 36.4% |
| GUEPARD93 | Initial MTX 12 weeks, then step-up therapy in both groups based on DAS28 |
Week 52 | NR | 32 | NR | NR | NR | DAS < 2.6, 59.4% |
| GUEPARD93 | Initial ADA + MTX 12 weeks, then step-up therapy in both groups based on DAS28 |
Week 52 | NR | 33 | NR | NR | NR | DAS < 2.6, 39.4% |
| HIT HARD94,173 | MTX + PBO | 24 weeks (study RCT end point) | DAS28-ESR | 85 | 6.3 (0.9) | 3.6 (1.4) | –2.7 (NR) | 29.5 (< 2.6) |
| HIT HARD94 | ADA + MTX | 24 weeks (study RCT end point) | DAS28-ESR | 87 | 6.2 (0.8) | 3.0 (1.2)a | –3.2 (NR) | 47.9a (< 2.6) |
| OPERA107 | MTX + PBO + steroid | 12 months (primary end point and study RCT end point) | DAS28-CRP | 91 | 5.6 (3.8, 7.0)b | 2.6 (1.7, 4.7)b | –3.0 (NR) | 49 (< 2.6) |
| OPERA107 | ADA + MTX + steroid | 12 months (primary end point and study RCT end point) | DAS28-CRP | 89 | 5.5 (3.8, 7.8)b | 2.0 (1.7, 5.0)a,b | –3.5 (NR) | 74c (< 2.6) |
| OPTIMA108 | MTX + PBO | 26 weeks (study RCT end point) | DAS28-CRP | 517 | 6.0 (1.0) | 4.1 (n = 505) | –1.9 (NR) | 17 (< 2.6) |
| OPTIMA108 | ADA + MTX | 26 weeks (study RCT end point) | DAS28-CRP | 515 | 6.0 (1.0) | 3.3a (n = 499) | –2.7 (NR) | 34 (< 2.6)c |
| PREMIER109 | MTX + PBO | 1 year (primary end point) | NR | 257 | 6.3 (0.9) | NR | NR | 21 (< 2.6) |
| PREMIER109 | ADA monotherapy + PBO step-up week 16 | 1 year (primary end point) | NR | 274 | 6.4 (0.9) | NR | NR | 23 (< 2.6) |
| PREMIER109 | ADA + MTX step-up week 16 | 1 year (primary end point) | NR | 268 | 6.3 (0.9) | NR | NR | 43 (< 2.6)c (vs. MTX vs. ADA) |
| PREMIER109 | MTX + PBO | 2 years (study RCT end point) | NR | 257 | 6.3 (0.9) | NR | NR | 25 (< 2.6) |
| PREMIER109 | ADA monotherapy + PBO step-up week 16 | 2 years (study RCT end point) | NR | 274 | 6.4 (0.9) | NR | NR | 25 (< 2.6) |
| PREMIER109 | ADA + MTX step-up week 16 | 2 years (study RCT end point) | NR | 268 | 6.3 (0.9) | NR | NR | 49 (< 2.6)c (vs. MTX vs. ADA) |
| COMET81 | MTX + PBO (n = 268) | 52 weeks | NR | 263 | 6.5 (1.0) | NR | NR | DAS28 < 2.6, 28% |
| COMET81 | ETN + MTX (n = 274) | 52 weeks | NR | 265 | 6.5 (1.0) | NR | NR | DAS28 < 2.6, 50%c |
| COMET82 | MTX in year 1, MTX in year 2 n = 99 at start of period 2 |
2 years | NR | 130 | NR | NR | NR | 22% |
| COMET81 | MTX in year 1, ETN + MTX in year 2 n = 90 at start of period 2 |
2 years | NR | 133 | NR | NR | NR | 36%a vs. group given MTX both years |
| COMET81 | ETN + MTX in year 1, ETN + MTX in year 2 n = 111 at start of period 2 |
2 years | NR | 131 | NR | NR | NR | 45%c vs. group given MTX both years |
| COMET81 | ETN + MTX in year 1, ETN in year 2 n = 111 at start of period 2 |
2 years | NR | 134 | NR | NR | NR | 37%a vs. group given MTX both years |
| GO-BEFORE90 | PBO + MTX | 24 weeks | DAS28-ESR | 160 | DAS28-ESR 6.2 (1.17) | NR | NR | DAS28-ESR 11 |
| GO-BEFORE90 | GOL 50 mg s.c. every 4 weeks + MTX | 24 weeks | 159 | DAS28-ESR 6.3 (1.1) | NR | NR | 25c | |
| GO-BEFORE143 | PBO + MTX | 52 weeks | DAS28-CRP | 160 | DAS28-ESR 6.2 (1.17) | NR | NR | 38.8 |
| GO-BEFORE90 | GOL 50 mg s.c. every 4 weeks + MTX | 52 weeks | 159 | DAS28-ESR 6.3 (1.1) | NR | NR | 45 | |
| ASPIRE71 | PBO i.v. + MTX | 54 weeks | NR | 235 | 6.7 (1) | 4.6 (1.8) | –2.1 (NR) | Defined as DAS28 < 2.6, 15.0% |
| ASPIRE71 | IFX i.v. 3 mg/kg at weeks 0, 2 and 6 and every 8 weeks thereafter + MTX | 54 weeks | NR | 294 | 6.6 (1) | 4.0 (1.8)c | –2.6 (NR) | 21.2 |
| BeST78 | Sequential monotherapy (DAS steered) | 6 months | DAS44 | 126 | DAS44 4.5 (0.9) | 3 | –1.5 (NR) | NR |
| BeST78 | Step–up combination therapy (DAS steered) | 6 months | DAS44 | 121 | DAS44 4.5 (0.8) | 3 | –1.5 (NR) | NR |
| BeST78 | Initial combination therapy with prednisone (DAS steered) | 6 months | DAS44 | 133 | DAS44 4.4 (0.9) | 2.2 | –2.2 (NR) | NR |
| BeST78 | Initial combination therapy with IFX (DAS steered) | 6 months | DAS44 | 128 | DAS44 4.3 (0.9) | 2.2 | –2.1 (NR) | NR |
| Durez et al., 2007120 | MTX | 52 weeks (study RCT end point) | DAS28-CRP | 14 | 5.2 (0.8) | 3.26 (1.3) | –1.94 (NR) | NR |
| Durez et al., 2007120 | MTX + i.v. MP | 52 weeks (study RCT end point) | DAS28-CRP | 15 | 5.3 (1) | 2.77 (1.09) | –2.53 (NR) | NR |
| Durez et al., 2007120 | IFX 3 mg/kg i.v. at weeks 0, 2, 6, 14, 22, 30, 46 + MTX | 52 weeks (study RCT end point) | DAS28-CRP | 15 | 5.3 (1) | 2.79 (0.77) | –2.51 (NR) | NR |
| IDEA95 | MP 250 mg i.v. at week 0, PBO i.v. at weeks 2, 6, 14, 22 + MTX | 26 weeks | NR | 56 | NR | NR | NR | DAS (assumed DAS44 < 1.6), 44.6% |
| IDEA95 | IFX 3 mg/kg i.v. at weeks 0, 2, 6, 14, 22 + MTX (IFX dose modifications permitted according to DAS44 from week 26) | 26 weeks | NR | 54 | NR | NR | NR | 33.3 |
| Quinn et al., 2005110 | MTX + PBO | 14 weeks (primary end point) | Not stated | 10 | 7.0 (0.9) | 6.0 (4.9–6.8)d,e | –1.0 (NR) | NR |
| Quinn et al., 2005110 | IFX 3 mg/kg + MTX | 14 weeks (primary end point) | Not stated | 10 | 6.2 (0.8) | 2.9 (2.3–3.8)a,d,e | –3.3 (NR) | NR |
| Quinn et al., 2005110 | MTX + PBO | 54 weeks (study RCT end point) | Not stated | 10 | 7.0 (0.9) | 4.6 (3.1–5.1)d,e | –2.4 (NR) | NR |
| Quinn et al., 2005110 | IFX 3 mg/kg + MTX | 54 weeks (study RCT end point) | Not stated | 10 | 6.2 (0.8) | 2.7 (2.0–3.5)d,e | –3.5 (NR) | NR |
| Trial name | Treatment arms for which data extraction performed | Assessment time point | DAS28-ESR or DAS28-CRP where stated | n analysed | Mean DAS28 at baseline (SD) | Mean DAS28 at follow-up (SD) | DAS28 mean change from baseline (SD) | % achieving DAS28 remission (defined threshold) |
|---|---|---|---|---|---|---|---|---|
| ATTEST74 | PBO + MTX | Day 197 | DAS28-ESR | 110 | 6.8 (1.0) | 5.32 (NR) | –1.48 | Defined as DAS28-ESR < 2.6, 2.9% |
| ATTEST74 | IFX + MTX | Day 197 | DAS28-ESR | 165 | 6.8 (0.9) | 4.55 (NR) | –2.25a | 12.8 |
| ATTEST74 | ABT + MTX | Day 197 | DAS28-ESR | 156 | 6.9 (1.0) | 4.37 (NR) | –2.53a | 11.3 |
| AMPLE66 | ABT s.c. | 1 year (primary end point) | DAS28-CRP | 318 | 5.5 (1) | 3.188 | –2.30 (0.08) | 43.3 (< 2.6) |
| AMPLE66 | ADA | 1 year (primary end point) | DAS28-CRP | 328 | 5.5 (1) | 3.188 | –2.27 (0.08) | 41.9 (< 2.6) |
| RED-SEA114 | ADA + cDMARDs (n = 60) | 24 weeks | DAS28-CRP | 60 | 5.6 (0.9) | 4.16 (NR) | –1.44 (NR) | NR |
| RED-SEA114 | ETN50 + cDMARDs (n = 60) | 24 weeks | DAS28-CRP | 60 | 5.8 (0.9) | 4.04 (NR) | –1.76 (NR) | NR |
| RED-SEA114,319 | ADA + cDMARDs (n = 60) | 12 months | DAS28-CRP | 60 | Median 5.8 (5.1–6.1)b | 4.4 (3.1–5.0)b | Median –1.4 Mean –1.54 (1.47) |
NR |
| RED-SEA114 | ETN50 + cDMARDs (n = 60) | 12 months | DAS28-CRP | 60 | Median 5.7 (5.0–6.5)b | 4.6 (3.5–5.6)b | Median –1.1 Mean –1.34 (1.3) |
NR |
| ADACTA58 | TCZ 8 mg/kg i.v. every 4 weeks + s.c. PBO ADA | 24 weeks | DAS28-ESR | 163 | 6.7 (0.9) | 3.4 (NR) | –3.3 | (DAS28 < 2.6) 65/163 (39.9%) |
| ADACTA58 | ADA 40 mg s.c. every 2 weeks + i.v. PBO TCZ | 24 weeks | DAS28-ESR | 162 | 6.8 (0.9) | 5.0 (NR) | –1.8a | 17/162 (10.5%)a |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | DAS28-ESR or DAS28-CRP where stated | n analysed | Mean DAS28 at baseline (SD) | Mean DAS28 at follow-up (SD) | DAS28 mean change from baseline (SD) | % change from baseline | % achieving DAS28 remission (defined threshold) |
|---|---|---|---|---|---|---|---|---|---|
| AIM61,62 | MTX + PBO (n = 219) | 12 months | CRP | 219 | 6.4 (0.1) CRP | NR | NR | NR | DAS28 < 3.2, 9.9% DAS28 < 2.6, 1.9% |
| AIM61 | ABT i.v. + MTX (n = 433) | 12 months | CRP | 433 | 6.4 (0.08) CRP | NR | NR | NR | DAS28 < 3.2, 42.5% DAS28 < 2.6, 23.8%a |
| ASSET72 | PBO + MTX | 4 months (primary end point and study RCT end point) | DAS28-CRP | 22 | 5.3 (0.9) | 4.75 (NR) | –0.55 (95% CI –0.95 to –0.16) | NR | 0.0 (< 2.6) |
| ASSET72 | ABT i.v. (≈ 10 mg/kg) + MTX | 4 months (primary end point and study RCT end point) | DAS28-CRP | 26 | 5.3 (1) | 3.62 (NR) | –1.68 (95% CI –2.15 to –1.2) | NR | 15.4 (< 2.6) |
| Van De Putte et al., 2004122 | PBO s.c. | 26 weeks | NR | 110 | 7.1 (0.9) | 6.4 (NR) | –0.7 (1.3) | –9.1 | NR |
| Van De Putte et al., 2004122 | ADA 40 mg s.c. every other week monotherapy | 26 weeks | NR | 113 | 7.1 (0.8) | 5.4 (NR) | –1.7 (1.6) | –23.8b | NR |
| CERTAIN79 | PBO + cDMARDs | 24 weeks (primary end point and study RCT end point) | DAS28-ESR | 98 | 4.47 (0.3) | 4.5 | –0.07 (1.20) | NR | 5.5 (< 2.6) |
| CERTAIN79 | CTZ 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks + DMARDs | 24 weeks (primary end point and study RCT end point) | DAS28-ESR | 96 | 4.53 (0.4) | 3.38 | –1.12 (1.06) | NR | 26.1 (< 2.6) |
| REALISTIC113 | PBO + existing cDMARDs | 12 weeks | DAS28-CRP DAS28-ESR |
29 | NR | NR | –0.80c –0.80c |
NR | NR |
| REALISTIC113 | CTZ 400 mg weeks 0, 2, 4 then 200 mg every 2 weeks + existing cDMARDs | 12 weeks | DAS28-CRP DAS28-ESR |
134 | NR | NR | –1.88c –1.94c |
NR | NR |
| ADORE59,60 | ETN monotherapy (n = 159) | 16 weeks | ESR | 156 | 6.2 ESR | 4.25 (NR) | 1.95 | NR | DAS28 (4) < 2.6, 14.6%d DAS28 (3) < 2.6, 15.2%e |
| ADORE59 | ETN + MTX (n = 155) | 16 weeks | ESR | 151 | 6.3 ESR | 4.1 (NR) | 2.20 | NR | DAS28 (4) < 2.6, 17.3% DAS28 (3) < 2.6, 15.1% |
| CREATE IIb96 | DMARD + PBO | 24 weeks | NR | 65 | 6.3 (0.76) | 5.3 (NR) | –1 (1.2) | NR | NR |
| CREATE IIb96 | ETN50 + DMARD | 24 weeks | NR | 64 | 6.4 (0.85) | 4.1 (NR) | –2.3 (1.38) | NR | NR |
| JESMR140 | ETN 25 mg every 2 weeks monotherapy | 24 weeks (primary end point) | Not stated | 69 | 6.1 (95% CI 5.9 to 6.2) | 4.1 (95% CI 3.8 to 4.5) | –2.0 (NR) | NR | 10.1 (< 2.6) |
| JESMR140 | ETN 25 mg every 2 weeks + MTX 6–8 mg/week | 24 weeks (primary end point) | Not stated | 73 | 6.0 (95% CI 5.8 to 6.2) | 3.3 (95% CI 3.0 to 3.5)b | –2.7 (NR) | NR | 27.4a (< 2.6) |
| JESMR140 | ETN 25 mg every 2 weeks monotherapy | 52 weeks (primary end point) | Not stated | 69 | 6.1 (0.9) | 4.2 (1.5) | –1.9 (NR) | NR | 18.8 (< 2.6) |
| JESMR140 | ETN 25 mg every 2 weeks + MTX 6–8 mg/week | 52 weeks (primary end point) | Not stated | 73 | 6.0 (1.0) | 3.0 (1.0)b | 3.0 (NR) | NR | 35.6b (< 2.6) |
| LARA102 | MTX + DMARD | 24 weeks | ESR | 142 | 5.9 (0.7) | NR | NR | NR | DAS < 2.6, 5/142 (3.5%) DAS < 3.2, 12.0% |
| LARA102 | ETN50 + MTX | 24 weeks | 279 | 5.9 (0.6) | NR | NR | NR | DAS < 2.6, 70/279 (25.1%)b DAS < 3.2, 47.0%b |
|
| RACAT111 | MTX + SSZ + HCQ | 24 weeks | CRP | 157 | 5.8 (0.9) | 4.1 (NR) | –1.79 (1.20) | NR | DAS28 ≤ 2.6, 12.7% DAS28 ≤ 3.2, 24.8% |
| RACAT111 | ETN50 + MTX | 24 weeks | 161 | 5.9 (0.9) | 3.8 (NR) | –2.06 (1.35) | NR | DAS28 ≤ 2.6, 21.7%a DAS28 ≤ 3.2, 34.8%a |
|
| RACAT111 | MTX + SSZ + HCQ (n = 178) In analysis n = 154 (of whom 39 switched to ETN) |
48 weeks | CRP | 154 | NR | NR | –2.12 (1.28) | NR | DAS28 ≤ 2.6, 20.8% DAS28 ≤ 3.2, 37.0% |
| RACAT111 | ETN50 + MTX (n = 175) In analysis n = 155 (of whom 41 switched to MTX + SSZ + HCQ) |
48 weeks | 155 | NR | NR | –2.29 (1.30) | NR | DAS28 ≤ 2.6, 25.2% DAS28 ≤ 3.2, 41.9% |
|
| APPEAL67,68 | MTX + DMARD (SSZ, HCQ or LEF) | 16 weeks (primary end point and study RCT end point) | DAS28-ESR DAS28-CRP |
103 | 6.1 (1.1) 5.34 (1.1) |
4.4 3.7 |
–1.7 (NR) –1.64 (NR) |
27.5 31.0 |
7.8 (< 0.26) 21.4 (< 0.26) |
| APPEAL68 | ETN 25 mg twice weekly (licensed dose) + MTX | 16 weeks (primary end point and study RCT end point) | DAS28-ESR DAS28-CRP |
197 | 6.1 (1.1) 5.23 (1.1) |
3.8b 3.1b |
–2.3 (NR) –2.13 (NR) |
38.3b 40.3b |
15.7 (< 0.26) 41.6b (< 0.26) |
| GO-FORTH91 | PBO every 4 weeks + MTX 6–8 mg/week | 14 weeks (primary end point) | DAS28-ESR | 88 | 5.6 (0.99) | 5.17 (NR) | –0.43 (1.20) | NR | 3.4 (< 2.6) |
| GO-FORTH91 | GOL 50 mg s.c. every 4 weeks + MTX 6–8 mg/week | 14 weeks (primary end point) | DAS28-ESR | 86 | 5.5 (1.18) | 3.52 (NR) | –1.98 (1.25)b | NR | 31.4b (< 2.6) |
| GO-FORTH91 | PBO every 4 weeks + MTX 6–8 mg/week | 24 weeks (study RCT end point) | DAS28-ESR | 88 | 5.6 (0.99) | 5.0 (NR) | –0.60 (1.38) | NR | 6.8 (< 2.6) |
| GO-FORTH91 | GOL 50 mg s.c. every 4 weeks + MTX 6–8 mg/week | 24 weeks (study RCT end point) | DAS28-ESR | 86 | 5.5 (1.18) | 3.45 (NR) | –2.05 (1.23)b | NR | 34.9b (< 2.6) |
| GO-FORWARD92 | PBO s.c. every 4 weeks + MTX | 14 weeks | DAS28-CRP DAS28-ESR |
133 | DAS28-CRP 5.458 (4.672–6.09)f DAS28-ESR 6.111 (5.260–6.57)f |
NR | NR | NR | 1.5 |
| GO-FORWARD92 | GOL 50 mg s.c. every 4 weeks + MTX | 14 weeks | DAS28-CRP DAS28-ESR |
89 | DAS28-CRP 5.766 (4.628–6.32)f DAS28-ESR 6.105 (5.366–6.940)f |
NR | NR | NR | 15.7b |
| GO-FORWARD92 | PBO s.c. every 4 weeks + MTX | 24 weeks | DAS28-CRP DAS28-ESR |
133 | 5.458 (4.672–6.09)f 6.111 (5.260–6.57)f |
NR | NR | NR | 6.0 |
| GO-FORWARD92 | GOL 50 mg s.c. every 4 weeks + MTX | 24 weeks | DAS28-CRP DAS28-ESR |
89 | 5.766 (4.628–6.32)f 6.105 (5.366–6.940)f |
NR | NR | NR | 20.2 |
| Kay et al., 200898 | PBO s.c. + MTX | 16 weeks | Both measures reported | 35 | DAS28-CRP 5.8 (5.2–6.0)f DAS28-ESR 6.3 (5.7–7.0)f |
4.8f | DAS28-CRP –1.0 (1.0) –1.0 (–1.8 to –0.0)f DAS28-ESR –1.0 (1.1) –1.0 (–2.0 to 0.0)f |
NR | DAS28-CRP 0 (DAS28 < 2.6) DAS28-ESR 0 (DAS28 < 2.6) |
| Kay et al., 200898 | GOL 50 mg s.c. every 4 weeks + MTX | 16 weeks | Both measures reported | 35 | DAS28-CRP 5.9 (5.5–6.9)f DAS28-ESR 6.4 (5.6–7.3)f |
3.9f | DAS28-CRP –2.0 (1.3) –2.0 (–2.6, –1.5)c DAS28-ESR –2.1 (1.4) –2.2 (–2.8, –1.5)c,b |
NR | DAS28-CRP 11a,f (DAS28 < 2.6) DAS28-ESR 5.7 (DAS28 < 2.6) |
| START118 | PBO + MTX | 22 weeks (primary end point and study RCT end point) | Not stated | 363 | NR | 4.4 (1.40) | NR | NR | 14 (< 2.6) |
| START118 | IFX 3 mg/kg + MTX | 22 weeks (primary end point and study RCT end point) | Not stated | 360 | NR | 3.5 (1.4)b | NR | NR | 31b (< 2.6) |
| Wong et al., 2009125 | PBO + MTX (with crossover for PBO group to open-label IFX at week 2) | Week 16 | NR | NR | 6.4 (0.8) | 6.7 | 0.3 (NR) | NR | NR |
| Wong et al., 2009125 | IFX 3 mg/kg at weeks 0, 2, 6 and 8 weeks thereafter + MTX | Week 16 | NR | NR | 6.2 (0.9) | 4.4b | –1.8 (NR) | NR | NR |
| ACT-RAY57 | TCZ + oral PBO | 24 weeks | DAS28-ESR | 267 | 6.36 (1.00) | 3.15 (NR) | –3.21 (1.3) | NR | 34.8 |
| ACT-RAY57 | TCZ + MTX | 24 weeks | DAS28-ESR | 277 | 6.33 (0.98) | 2.9 (NR) | –3.43 (1.3)a | NR | 40.4% (p = 0.19 for absolute difference of 5.65%, 95% CI –2.41% to 13.71%) |
| ACT-RAY57 | TCZ + oral PBO | 52 weeks | NR | NR | 6.36 (1.00) | 2.62 (NR) | –3.74 | NR | 36.6 |
| ACT-RAY57 | TCZ + MTX | 52 weeks | NR | NR | 6.33 (0.98) | 2.67 (NR) | –3.66 | NR | 45.5a |
| MEASURE103 | PBO + MTX | 12 weeks | NR | NR | NR | NR | –0.8 | NR | NR |
| MEASURE103 | TCZ + MTX | 12 weeks | NR | NR | NR | NR | –2.7 | NR | NR |
| SAMURAI115 | cDMARDs disease activity | 24 weeks | NR | 145 | 6.4 (0.9) | 5.91 (NR) | –0.49 (NR) | NR | NR |
| SAMURAI115 | TCZ monotherapy | 24 weeks | NR | 157 | 6.5 (0.8) | 2.75 (NR) | –3.75 (NR) | NR | NR |
| SAMURAI115 | cDMARDs disease activity | 52 weeks | NR | 145 | 6.4 (0.9) | NR | NR | NR | DAS28< 2.6, 3% |
| SAMURAI115 | TCZ monotherapy | 52 weeks | NR | 157 | 6.5 (0.8) | NR | NR | NR | DAS28< 2.6, 59%b |
| SATORI116 | PBO i.v. every 4 weeks + MTX | 24 weeks | NR | 64 | 6.2 (0.9) | 5.13 (NR) | –1.07 (NR) | NR | NR |
| SATORI116 | TCZ 8 mg/kg i.v. every 4 weeks + PBO capsules | 24 weeks | NR | 61 | 6.1 (0.9) | 2.86 (NR) | –3.24 (NR) | NR | NR |
| TOWARD121 | PBO i.v. every 4 weeks + stable cDMARDs (n = 415 randomised) | 24 weeks | NR | 413 | 6.6 (1.0) | 5.44 (NR) | –1.16 | NR | DAS28 < 2.6, 3% |
| TOWARD121 | TCZ 8 mg/kg i.v. every 4 weeks + stable DMARDs (n = 805 randomised) | 24 weeks | NR | 803 | 6.7 (1.0) | 3.53 (NR) | –3.17b | NR | 30%b |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) | (AiC information has been removed) |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | n analysed | Mean HAQ-DI score at baseline (range 0–3) (SD) | Mean HAQ-DI score at follow-up (range 0–3) (SD) | HAQ-DI mean change from baseline (SD) | % change from baseline |
|---|---|---|---|---|---|---|---|
| Bejarano et al., 200877 | MTX + PBO | Week 56 | 73 | 1.3 (0.6) | 0.9 (NR) | –0.4 (0.7) | |
| Bejarano et al., 200877 | ADA + MTX | Week 56 | 75 | 1.3 (0.6) | 0.6 (NR) | –0.7 (0.6) | |
| GUEPARD93 | Initial MTX | Week 12 | 32 | 1.41 (0.74) | 0.9 (NR) | –0.51 (95% CI –0.30 to –0.72) | NR |
| GUEPARD93 | Initial ADA + MTX | Week 12 | 33 | 1.69 (0.59) | 0.87 (NR) | –0.82 (95% CI –0.52 to –1.11) | NR |
| GUEPARD93 | Initial MTX 12 weeks, then step-up therapy in both groups based on DAS28 |
Week 52 | 32 | NR | NR | –0.93 (95% CI –0.69 to –1.17) | NR |
| GUEPARD93 | Initial ADA + MTX 12 weeks, then step-up therapy in both groups based on DAS28 |
Week 52 | 33 | NR | NR | –1.02 (95% CI –0.81 to –1.24) | NR |
| HIT HARD94 | MTX + PBO | 24 weeks (study RCT end point) | 85 | 1.3 (0.6) | 0.72 (0.6) | –0.58 (NR) | NR |
| HIT HARD94 | ADA + MTX | 24 weeks (study RCT end point) | 87 | 1.4 (0.6) | 0.49 (0.6) | –0.91 (NR) | NR |
| OPERA107 | MTX + PBO + steroid | 12 months (primary end point and study RCT end point) | 91 | 1.00 (0.25, 2.31)a | 0.13 (0, 1.5)a | –0.63 (–0.82, 0.38)a | NR |
| OPERA107 | ADA + MTX + steroid | 12 months (primary end point and study RCT end point) | 89 | 1.13 (0.17, 2.58)a | 0.25 (0, 1.44)a | –0.88 (–2.46, 0.13)a | NR |
| OPTIMA108 | MTX + PBO | 26 weeks (study RCT end point) | 517 | 1.6 (0.65) | 0.9 | –0.66 (0.73) (n = 512) | NR |
| OPTIMA108 | ADA + MTX | 26 weeks (study RCT end point) | 515 | 1.61 (0.69) | 0.7 | –0.89 (0.74) (n = 512) | NR |
| PREMIER109 | MTX + PBO | 1 year (primary end point) | 256 | 1.5 (0.7) | 0.7 (0.6) | –0.8 (0.6) | NR |
| PREMIER109 | ADA monotherapy + PBO step-up week 16 | 1 year (primary end point) | 272 | 1.6 (0.6) | 0.8 (0.6) | –0.8 (0.7) | NR |
| PREMIER109 | ADA + MTX step-up week 16 | 1 year (primary end point) | 266 | 1.5 (0.6) | 0.5 (0.5) | –1.1 (0.6) | NR |
| PREMIER109 | MTX + PBO | 2 years (study RCT end point) | 256 | 1.5 (0.7) | 0.5 (0.6) | –0.9 (0.6) | NR |
| PREMIER109 | ADA monotherapy + PBO step-up week 16 | 2 years (study RCT end point) | 272 | 1.6 (0.6) | 0.6 (0.6) | –0.9 (0.6) | NR |
| PREMIER109 | ADA + MTX step-up week 16 | 2 years (study RCT end point) | 266 | 1.5 (0.6) | 0.3 (0.5) | –1.0 (0.7) | NR |
| COMET81 | MTX + PBO | Week 52 | 263 | 1.64 (0.65) | 0.92 (0.74) | –0.72 | NR |
| COMET81 | ETN + MTX | Week 52 | 265 | 1.70 (0.68) | 0.68 (0.71) | –1.02b | NR |
| COMET82 | MTX in year 1, MTX in year 2 n = 99 at start of period 2 |
From week 52 to week 104 | 99 | NR | NR | Non-significant change from baseline | NR |
| COMET81 | MTX in year 1, ETN + MTX in year 2 n = 90 at start of period 2 |
From week 52 to week 104 | 90 | NR | NR | 0.17 (0.42)b | NR |
| COMET81–83 | ETN + MTX in year 1, ETN + MTX in year 2 n = 111 at start of period 2 |
From week 52 to week 104 | 111 | NR | NR | Non-significant change from baseline | NR |
| COMET81 | ETN + MTX in year 1, ETN in year 2 n = 111 at start of period 2 |
From week 52 to week 104 | 111 | NR | NR | Non-significant change from baseline | NR |
| ERA/Bathon and Genovese, 2003139 (multicentre) | MTX + PBO | 12 months (study RCT end point) | 217 | NR | NR | –0.76 (SE 0.05) | NR |
| ERA/Bathon and Genovese, 2003139 (multicentre) | ETN 25 mg every 2 weeks + PBO | 12 months (study RCT end point) | 207 | NR | NR | –0.73 (SE 0.05) | NR |
| GO-BEFORE90 | PBO + MTX | 24 weeks | 160 | 1.5 (0.64) | NR | NR | 36.95 |
| GO-BEFORE90 | GOL 50 mg s.c. every 4 weeks + MTX | 24 weeks | 159 | 1.5 (0.66) | NR | NR | 43.65 |
| Kume et al., 2011100 | ADA | 6 months | 22 | NR | NR | –0.69 (0.11) | NR |
| Kume et al., 2011100 | ETN | 6 months | 21 | NR | NR | –0.68 (0.09) | NR |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | n analysed | Mean HAQ-DI score at baseline (range 0–3) (SD) | Mean HAQ-DI score at follow-up (range 0–3) (SD) | HAQ-DI mean change from baseline (SD) | % change from baseline |
|---|---|---|---|---|---|---|---|
| ATTEST74 | PBO + MTX | Day 197 | 110 | 1.8 (0.7) | NR | NR | % achieving ≥ 0.3 improvement from baseline = 40.9 |
| ATTEST74 | IFX + MTX | Day 197 | 165 | 1.7 (0.7) | NR | NR | % achieving ≥ 0.3 improvement from baseline = 58.8a (vs. PBO + MTX) |
| ATTEST74 | ABT + MTX | Day 197 | 156 | 1.8 (0.6) | NR | NR | % achieving ≥ 0.3 improvement from baseline = 61.5a (vs. PBO + MTX) |
| AMPLE66 | ABT s.c. | 1 year (primary end point) | 318 | 1.5 (0.7) | NR | NR | 41.7 |
| AMPLE144 | ADA | 1 year (primary end point) | 328 | 1.5 (0.7) | NR | NR | 38.7 |
| ADACTA58 | TCZ 8 mg/kg i.v. every 4 weeks + s.c. PBO ADA | 24 weeks | 163 | 1.6 (0.6) | 0.9 (NR) | –0.7 | NR |
| ADACTA58 | ADA 40 mg s.c. every 2 weeks + i.v. PBO TCZ | 24 weeks | 162 | 1.7 (0.6) | 1.2 (NR) | –0.5 | NR |
| deFilippis et al., 200685 | ETN + MTX | 22 weeks | 16 | 1.89 (0.65) | NR | NR | –17.5 |
| deFilippis et al., 200685 | IFX + MTX | 22 weeks | 16 | 1.67 (0.68) | NR | NR | –16.2 |
| deFilippis et al., 200685 | ETN + MTX | 54 weeks | 16 | 1.89 (0.65) | NR | NR | –32.3 |
| deFilippis et al., 200685 | IFX + MTX | 54 weeks | 16 | 1.67 (0.68) | NR | NR | –21.6 |
| Trial name/author, year | Treatment arms for which data extraction performed | Assessment time point | n analysed | Mean HAQ-DI score at baseline (range 0–3) (SD) | Mean HAQ-DI score at follow-up (range 0–3) (SD) | HAQ-DI mean change from baseline (SD) | % change from baseline |
|---|---|---|---|---|---|---|---|
| AIM61,62 | MTX + PBO | 12 months | 219 | 1.7 (0.6) | (Estimate from graph 1.3) | Adjusted –0.50 (0.05) | NR |
| AIM61 | ABT i.v. + MTX | 12 months | 433 | 1.7 (0.7) | (Estimate from graph 1.05) | Adjusted –0.68 (0.03) | NR |
| ASSURE73 | PBO + cDMARDs | 1 year (primary end point and study RCT end point) | 413 | 1.5 (0.7) (n = 418) | 1.24 (NR) | –0.26 | 9 |
| ASSURE73 | ABT + cDMARDs | 1 year (primary end point and study RCT end point) | 845 | 1.5 (0.6) (n = 856) | 1.03 (NR) | –0.47 | 30 |
| CHANGE80 | PBO | 24 weeks | 87 | 1.4 (0.7) | 1.3 (NR) | 0.1 (0.6) | NR |
| CHANGE80 | ADA monotherapy | 24 weeks | 91 | 1.6 (0.7) | 1.4 (NR) | –0.2 (0.6) | NR |
| DE01984 | MTX + PBO (n = 200) | 24 weeks | 200 | 1.48 (0.59) | 1.24 (NR) | –0.24 (0.52) | –16.2 |
| DE01980 | ADA + MTX (n = 207) | 24 weeks | 207 | 1.45 (0.63) | 0.89 (NR) | –0.56 (0.52) | –38.6 |
| DE01980 | MTX + PBO (n = 200) | 52 weeks | 200 | 1.48 (0.59) | 1.23 (NR) | –0.25 (0.56) | –16.9 |
| DE01980 | ADA + MTX (n = 207) | 52 weeks | 207 | 1.45 (0.63) | 0.86 (NR) | –0.59 (0.57) | –40.7 |
| Van De Putte et al., 2004122 | PBO s.c. | 26 weeks | 110 | 1.88 (0.64) | 1.81 (NR) | –0.07 (0.49) | 1.8 |
| Van De Putte et al., 2004122 | ADA monotherapy | 26 weeks | 113 | 1.83 (0.59) | 1.45 (NR) | –0.38 (0.60) | –21.3a |
| ARMADA69,70 | MTX + PBO | 24 weeks | 62 | 1.64 (0.63) | 1.37 (NR) | –0.27 (0.57) | –16.5 |
| ARMADA69 | ADA +MTX | 24 weeks | 67 | 1.55 (0.61) | 0.93 (NR) | –0.62 (0.63) | –40.0a |
| CERTAIN79 | PBO + cDMARDs (biologic-naive subgroup) | 24 weeks (primary end point and study RCT end point) | 98 | 1.11 (0.62) | 1.05 (NR) | –0.06 | NR |
| CERTAIN79 | CTZ 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks + DMARDs (biologic-naive subgroup) | 24 weeks (primary end point and study RCT end point) | 96 | 1.04 (0.60) | 0.79 (NR) | –0.25 | NR |
| REALISTIC113 | PBO + existing cDMARDs | 12 weeks | 29 | NR | NR | –0.10b | NR |
| REALISTIC113 | CTZ 400 mg weeks 0, 2, 4 then 200 mg every 2 weeks + existing cDMARDs | 12 weeks | 134 | NR | NR | –0.48b | NR |
| ADORE59,60 | ETN monotherapy (n = 159) | 16 weeks | 142 | 1.6 | 1.01 (NR) | –0.59 (0.69) | NR |
| ADORE59 | ETN + MTX (n = 155) | 16 weeks | 141 | 1.7 | 1.11 (NR) | –0.59 (0.58) | NR |
| ETN Study 30988,89 | SSZ + PBO (n = 50) | 24 weeks | 50 | 1.6 (0.5) | 1.5 (NR) | –0.1 (NR) | 9.2 |
| ETN Study 30988,89 | ETN + PBO (n = 103) | 24 weeks | 103 | 1.7 (0.6) | 1.1 (NR) | –0.6 (NR) | 35.3a vs. SSZ |
| ETN Study 30988,89 | ETN + SSZ (n = 101) | 24 weeks | 101 | 1.6 (0.6) | 1.0 (NR)c vs. SSZ Non-significant vs. ETN + PBO |
–0.6 (NR) | 40.2c vs. SSZ Non-significant vs. ETN + PBO |
| ETN Study 30988,89 | SSZ + PBO (n = 50) | 104 weeks | 50 | 1.6 (0.5) | (Estimate from graph 1.6) | (Estimate from graph 0) (NR) | NR |
| ETN Study 30988,89 | ETN + PBO (n = 103) | 104 weeks | 103 | 1.7 (0.6) | (Estimate from graph 1.1)a vs. SSZ | (Estimate from graph 0.6) (NR) | NR |
| ETN Study 30988,89 | ETN + SSZ (n = 101) | 104 weeks | 101 | 1.6 (0.6) | (Estimate from graph 0.9)a vs. SSZ | (Estimate from graph 0.7) (NR) | NR |
| JESMR140 | ETN 25 mg every 2 weeks monotherapy | 24 weeks (primary end point) | 69 | 1.3 (0.8) | 0.9 (0.8) | –0.4 (NR) | NR |
| JESMR140 | ETN 25 mg every 2 weeks + MTX 6–8 mg/week | 24 weeks (primary end point) | 73 | 1.2 (0.7) | 0.7 (0.6) | –0.5 (NR) | NR |
| JESMR140 | ETN 25 mg every 2 weeks monotherapy | 52 weeks (primary end point) | 69 | 1.3 (0.8) | 0.9 (0.7) | –0.4 (NR) | NR |
| JESMR140 | ETN 25 mg every 2 weeks + MTX 6–8 mg/week | 52 weeks (primary end point) | 73 | 1.2 (0.7) | 0.6 (0.6) | –0.6 (NR) | NR |
| Lan et al., 2004101 | PBO + MTX | 12 weeks (primary end point and study RCT end point) | 29 | 1.23 | 0.99 | –0.24 | NR |
| Lan et al., 2004101 | ETN 25 mg twice weekly + MTX | 12 weeks (primary end point and study RCT end point) | 29 | 0.99 | 0.34 | –0.65 | NR |
| LARA102 | MTX + DMARD | 24 weeks | 142 | 1.6 (0.7) | NR | Adjusted –0.5 (SE 0.1) | NR |
| LARA102 | ETN50 + MTX | 24 weeks | 279 | 1.6 (0.7) | NR | Adjusted –0.9 (SE < 0.1)a | NR |
| Moreland et al., 1999104 | PBO | 6 months | 80 | 1.66 (0.06) | 1.54 (NR) | –0.12 | NR |
| Moreland et al., 1999104 | ETN + PBO | 6 months | 78 | 1.63 (0.06) | 1.04 (NR) | –0.59c | NR |
| RACAT111 | MTX + SSZ + HCQ (n = 178) | 24 weeks | 155 | 4 (0.8) | 0.97 (0.85) | –0.44 (0.77) | NR |
| RACAT111 | ETN50 + MTX (n = 175) | 24 weeks | 160 | 1.5 (0.8) | 0.98 (0.87) | –0.51 (0.84) | NR |
| RACAT111 | MTX + SSZ + HCQ (n = 178 randomised) In analysis n = 155 (of whom 39 switched to ETN) |
48 weeks | 155 | 1.4 (0.8) | 0.93 (0.85) | –0.46 (0.82) | NR |
| RACAT111 | ETN50 + MTX (n = 175 randomised) In analysis n = 155 (of whom 41 switched to MTX + SSZ + HCQ) |
48 weeks | 155 | 1.5 (0.8) | 0.83 (0.81) | –0.64 (0.78) | NR |
| Wajdula 2000 (reported in Chen et al., 2006123) | PBO | 12 weeks | 81 | 1.8 | 0.1 (NR) | 1.70 (0.60) | NR |
| Wajdula 2000 (reported in Chen et al., 2006123) | ETN | 12 weeks | 99 | 1.9 | 0.6 (NR) | 1.30 (0.60) | NR |
| Weinblatt et al., 1999124 | MTX + PBO | 24 weeks | 30 | 1.5d | 1.1d | –0.4 (NR) | NR |
| Weinblatt et al., 1999124 | ETN + MTX | 24 weeks | 59 | 1.5d | 0.8a,d | –0.7 (NR) | NR |
| APPEAL67,68 | MTX + DMARD (SSZ, HCQ or LEF) | 16 weeks (primary end point and study RCT end point) | 100 | 1.4 (0.7) | 0.9 | –0.5 (NR) | 38.3 |
| APPEAL68 | ETN 25 mg twice weekly (licensed dose) + MTX | 16 weeks (primary end point and study RCT end point) | 193 | 1.4 (0.7) | 0.7 | –0.7 (NR) | 49.4 |
| CREATE IIb96(ClinicalTrials.gov) | PBO + DMARD | 24 weeks | 65 | 1.4 (0.59) | 1.1 (NR) | –0.3 (0.46) | NR |
| CREATE IIb96 | ETN50 + DMARD | 24 weeks | 64 | 1.5 (0.68) | 0.9 (NR) | –0.6 (0.66) | NR |
| GO-FORTH91 | PBO every 4 weeks + MTX 6–8 mg/week | 14 weeks (primary end point) | 88 | 1.0 (0.68) | 0.93 (NR) | 0.07 (0.49) | NR |
| GO-FORTH91 | GOL 50 mg s.c. every 4 weeks + MTX 6–8 mg/week | 14 weeks (primary end point) | 86 | 1.0 (0.61) | 0.68 (NR) | 0.32 (0.40) | NR |
| GO-FORTH91 | PBO every 4 weeks + MTX 6–8 mg/week | 24 weeks (study RCT end point) | 88 | 1.0 (0.68) | 0.97 (NR) | 0.03 (0.58) | NR |
| GO-FORTH91 | GOL 50 mg s.c. every 4 weeks + MTX 6–8 mg/week | 24 weeks (study RCT end point) | 86 | 1.0 (0.61) | 0.67 (NR) | 0.33 (0.42) | NR |
| GO-FORWARD320 | PBO s.c. every 4 weeks + MTX | 14 weeks | 133 | 1.3 (0.7) 1.250 (0.750–1.750)d |
1.14 (NR) | –0.16 (0.49) –0.13 (–0.38 to 0.13)d |
NR |
| GO-FORWARD92 | GOL 50 mg s.c. every 4 weeks + MTX | 14 weeks | 89 | 1.4 (0.7) 1.375 (1.000–1.875)d |
NR | –0.42 (0.50) (p < 0.001 vs. PBO) –0.38 (–0.75 to – 0.13)d (a vs. PBO + MTX) |
NR |
| GO-FORWARD92 | PBO s.c. every 4 weeks + MTX | 24 weeks | 133 | 1.3 (0.7) (0.750–1.750)d |
NR | –0.13 (0.58) –0.13 (–0.38 to 0.13)d |
NR |
| GO-FORWARD92 | GOL 50 mg s.c. every 4 weeks + MTX | 24 weeks | 89 | 1.4 (0.7) (1.000–1.875)d |
NR | –0.47 (0.55) (p < 0.001 vs. PBO) –0.38 (–0.75 to – 0.13)d (a vs. PBO + MTX) |
NR |
| ATTRACT75 | PBO i.v. + MTX | 30 weeks | 88 | HAQ (0–3) 1.8 (1.3–2.1)d | HAQ (0–3) 1.5 (1.0–2.0)d | –0.3 (NR) | –3 |
| ATTRACT75 | IFX 3 mg/kg i.v. at weeks 0, 2 and 6 and every 8 weeks thereafter | 30 weeks | 86 | HAQ (0–3) 1.8 (1.4–2.3)d | HAQ (0–3) 1.5 (0.9–2.1)d | –0.3 (NR) | –13 (p = 0.167) |
| ATTRACT159 | PBO i.v. + MTX | 54 week | 68 | 1.8 (1.3–2.1)d | 1.8 (NR) | HAQ change 0d (range 0.0–2.2) | % achieving HAQ change ≥ 0.25 = 43 |
| ATTRACT75 | IFX 3 mg/kg i.v. at weeks 0, 2 and 6 and every 8 weeks thereafter | 54 week | 77 | HAQ 1.8 (1.4–2.3)d | 1.4 (NR) | HAQ –0.4d (range 0.0–1.9) | % achieving HAQ change ≥ 0.25 = 69a |
| Durez et al., 200486 | Single i.v. infusion of 1-g MP at week 0 + MTX (n = 15 randomised) | 14 weeks | NR | HAQ 1.5 (0.75–2.13)d | 1.55 | 0.05 (NR) | NR |
| Durez et al., 200486 | IFX 3 mg/kg at weeks 0, 2 and 6 + MTX (n = 12 randomised) | 14 weeks | NR | HAQ 1.3 (0.75–2)d | 0.95c | –0.35 (NR) | NR |
| START118 | PBO + MTX | 22 weeks (primary end point and study RCT end point) | 363 | 1.5 (1–2)d | 1.39 (NR) | –0.11 | NR |
| START118 | IFX 3 mg/kg + MTX | 22 weeks (primary end point and study RCT end point) | 360 | 1.5 (1–2)d | 1.11 (NR) | –0.39 | NR |
| Zhang et al., 2006126 | PBO i.v. + MTX (n = 86 randomised, n = 71 completed) | 18 weeks | NR | NR | NR | HAQ score decreased by 0.45 (unclear whether or not mean value reported) | NR |
| Zhang et al., 2006126 | IFX 3 mg/kg i.v. at weeks 0, 2, 6 and 14 + MTX (n = 87 randomised, n = 78 completed) | 18 weeks | NR | NR | NR | HAQ score decreased by 0.76 (unclear whether or not mean value reported)a | NR |
| ACT-RAY57 | TCZ 8 mg/kg i.v. every 4 weeks + oral PBO | 24 weeks | 276 | 1.48 (0.60) | NR | –0.54 | NR |
| ACT-RAY57 | TCZ 8 mg/kg i.v. every 4 weeks + MTX | 24 weeks | 277 | 1.46 (0.66) | NR | –0.56 | NR |
| TOWARD121 | PBO i.v. every 4 weeks + stable cDMARDs (n = 415 randomised) | 24 weeks | 413 | 1.5 (0.6) | 1.3 (NR) | –0.2 | % achieving ≥ 0.3 change from baseline = 34 |
| TOWARD121 | TCZ 8 mg/kg i.v. every 4 weeks + stable DMARDs (n = 805 randomised) | 24 weeks | 803 | 1.5 (0.6) | 1.0 (NR) | –0.5a | % achieving ≥ 0.3 change from baseline = 60 |
| TACIT141 | Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | NR |
| TACIT141 | TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | NR |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Mean swollen joint count at baseline (SD) (scale) | Mean swollen joint count at follow-up (SD) (scale) | Swollen joint count % change from baseline | Mean tender joint count at baseline (SD) (scale) | Mean tender joint count at follow-up (SD) (scale) | Tender joint count % change from baseline | CRP level at baseline (mean) (mg/dl) | CRP level at follow-up (mean) (mg/dl) | ESR level at baseline (mean) (mm/hour) | ESR level at follow-up (mean) (mm/hour) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIT HARD94 | MTX + PBO | 24 weeks | 10.7 (4.5) (0–28 scale) | 3.6 (4.9) (0–28 scale) | NR | 13.1 (5.9) (0–28 scale) | 5.0 (6) (0–28 scale) | NR | 17 (7–34)a | 7.1 (8.1) | 36 (29–55)a | 18.7 (14.2) |
| ADA + MTX | 24 weeks | 10.2 (5.0) (0–28 scale) | 1.4 (2.2)b (0–28 scale) | NR | 13.0 (6.5) (0–28 scale) | 3.2 (4.8)c (0–28 scale) | NR | 12 (6–37)a | 5.7 (10.3) | 33 (29–45)a | 16.1 (13.3) | |
| OPERA107 | MTX + PBO + steroid | 12 months | 11 (3, 31)d | 0 (0, 3)d | NR | 16 (6, 34)d | 0 (0, 9)d | NR | 15 (7, 109)d | 7 (7, 44)d | NR | NR |
| ADA + MTX + steroid | 12 months | 10 (3, 33)d | 0 (0, 6)d | NR | 15 (5, 38)d | 0 (0, 13)d | NR | 15 (7, 133)d | 7 (7, 21)d | NR | NR | |
| OPTIMA108 | MTX + PBO | 26 weeks | 12 (5.8) (0–28 scale) 18 (11) (0–66 scale) |
5.8 (0–28 scale) | NR | 16 (6.7) (0–28 scale) 27 (15) (0–68 scale) |
7.6 (0–28 scale) | NR | 30 (33) | 11.7 | NR | NR |
| ADA + MTX | 26 weeks | 13 (5.8) (0–28 scale) 18 (11) (0–66 scale) |
3.6 (0–28 scale) | NR | 16 (6.6) (0–28 scale) 29 (15) (0–68) |
5.3c (0–28 scale) | NR | 27 (32) | 7.1c | NR | NR | |
| PREMIER311 | MTX + PBO | 1 year | 22.1 (11.7) (0–66 scale) | NR | NR | 32.3 (14.3) (0–68 scale) | NR | NR | 4.0 (4.0) | NR | NR | NR |
| ADA monotherapy + PBO step-up week 16 | 1 year | 21.8 (10.5) (0–66 scale) | NR | NR | 31.8 (13.6) (0–68 scale) | NR | NR | 4.1 (3.9) | NR | NR | NR | |
| ADA + MTX step-up week 16 | 1 year | 21.1 (11.2) (0–66 scale) | NR | NR | 30.7 (14.2)(0–68 scale) | NR | NR | 3.9 (4.2) | NR | NR | NR | |
| PREMIER 311 | MTX + PBO | 2 years | 22.1 (11.7) (0–66 scale) | NR | NR | 32.3 (14.3) (0–68 scale) | NR | NR | 4.0 (4.0) | NR | NR | NR |
| ADA monotherapy + PBO step-up week 16 | 2 years | 21.8 (10.5) (0–66 scale) | NR | NR | 31.8 (13.6) (0–68 scale) | NR | NR | 4.1 (3.9) | NR | NR | NR | |
| ADA + MTX step-up week 16 | 2 years | 21.1 (11.2) (0–66 scale) | NR | NR | 30.7 (14.2) (0–68 scale) | NR | NR | 3.9 (4.2) | NR | NR | NR | |
| COMET82 | MTX + PBO (n = 268) | 52 weeks | Mean DAS28 swollen joint count 12.3 | 4.3 | 65% improvement | NR | NR | NR | NR | NR | NR | NR |
| ETN + MTX (n = 274) | 12.4 | 1.8 | 85% improvementc | NR | NR | NR | NR | NR | NR | NR | ||
| COMET82 | MTX in year 1, MTX in year 2 n = 99 at start of period 2 |
From week 52 to week 104 | 2.4 | 2.9 | NR | NR | NR | NR | NR | NR | NR | NR |
| MTX in year 1, ETN + MTX in year 2 n = 90 at start of period 2 |
2.6 | 1.3b (vs. MTX year 1 and MTX year 2) | NR | NR | NR | NR | NR | NR | NR | NR | ||
| COMET81 | ETN + MTX in year 1, ETN + MTX in year 2 n = 111 at start of period 2 |
1.7 | 1.3 | NR | NR | NR | NR | NR | NR | NR | NR | |
| ETN + MTX in year 1, ETN in year 2 n = 111 at start of period 2 |
1.1 | 1.7 | NR | NR | NR | NR | NR | NR | NR | NR | ||
| ERA321 | MTX + PBO | 6 months | 24 (11.9) | NR | NR | 30 (16.1) | NR | NR | 3.7 | NR | NR | NR |
| ETN 25 mg every 2 weeks + PBO | 6 months | 24 (11.9) | NR | NR | 31 (15.8) | NR | NR | 3.3 | NR | NR | NR | |
| ERA321 | MTX + PBO | 12 months | 24 (11.9) | NR | NR | 30 (16.1) | NR | NR | NR | NR | NR | NR |
| ETN 25 mg every 2 weeks + PBO | 12 months | 24 (11.9) | NR | NR | 31 (15.8) | NR | NR | NR | NR | NR | NR | |
| GO-BEFORE90 | PBO + MTX | 24 weeks | (0–66) 14.9 (10.01) |
NR | 66.7a | (0–68) 27.3 (16.16) |
NR | 57.1a | 2.6 (3.28) | NR | NR | NR |
| GOL 50 mg s.c. every 4 weeks + MTX | 24 weeks | 16.0 (9.98) | NR | 75.6a | 29.2 (17.05) | NR | 67.2a,c | 2.4 (3.02) | NR | NR | NR | |
| Durez et al., 2007120 | MTX | 52 weeks (study RCT end point) | 10.3 (5.5) | NR | NR | 11.6 (7.5) | NR | NR | 2.5 (3.5) 7 (3–121)a |
2.5 (1–31)a | NR | NR |
| MTX + i.v. MP | 52 weeks (study RCT end point) | 12.4 (7.6) | NR | NR | 13.2 (9.1) | NR | NR | 4.7 (5.1) 32 (3–213)a |
7.5 (1–27)a | NR | NR | |
| IFX 3 mg/kg i.v. at weeks 0, 2, 6, 14, 22, 30 and 46 | 52 weeks (study RCT end point) | 12.5 (5.4) | NR | NR | 15.9 (8.0) | NR | NR | 4.8 (5.2) 19 (1–29)a |
3.5 (1–29)a | NR | NR | |
| Quinn et al., 2005110 | MTX + PBO | 14 weeks | NR | NR | NR | NR | NR | NR | 37 (38.8) | 41e | NR | NR |
| IFX 3 mg/kg + MTX | 14 weeks | NR | NR | NR | NR | NR | NR | 47 (27.9) | 7e | NR | NR | |
| Quinn et al., 2005110 | MTX + PBO | 54 weeks | NR | NR | NR | NR | NR | NR | 37 (38.8) | 10e | NR | NR |
| IFX 3 mg/kg + MTX | 54 weeks | NR | NR | NR | NR | NR | NR | 47 (27.9) | 8e | NR | NR |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Mean swollen joint count at baseline (SD) (scale) | Mean swollen joint count at follow-up (SD) (scale) | Swollen joint count % change from baseline | Mean tender joint count at baseline (SD) (scale) | Mean tender joint count at follow-up (SD) (scale) | Tender joint count % change from baseline | CRP level at baseline (mean) (mg/dl) | CRP level at follow-up (mean) (mg/dl) | ESR level at baseline (mean) (mm/hour) | ESR level at follow-up (mean) (mm/hour) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMPLE66 | ABT s.c. + MTX | 1 year | 15.8 (9.8) (0–66 scale) | NR | 70.9 improved | 25.4 (15.3) (0–68 scale) | NR | 59.8 improved | 1.6 (2.1) | 0.80 (1.13) | NR | NR |
| ADA + MTX | 1 year | 15.9 (10.0) (0–66 scale) | NR | 68.2 improved | 26.3 (15.8) (0–68 scale) | NR | 61.4 improved | 1.5 (2.8) | 0.65 (1.21) | NR | NR | |
| RED-SEA114 | ADA + cDMARDs (n = 60) | 12 months | (Scale 0–28) 9 (5–12)a |
4 (1–6)a | (Scale 0–28) 14 (9–20)a |
5 (1–14)a | 10 (5–22)a | 6 (3–14)a | ||||
| ETN50 + cDMARDs (n = 60) | (Scale 0–28) 9 (6–13)a |
5 (2–11)a | (Scale 0–28) 14 (8–20)a |
8 (4–14)a | 12.5 (5–31)a | 9 (3–14)a | ||||||
| deFilippis et al., 200685 | ETN + MTX | 54 weeks | 16.87 (7.31) | Conflicting data | 49.5 | 22.40 (8.10) | Conflicting data | –61.3b | NR | NR | 35.47 (20.31) | Conflicting data |
| IFX + MTX | 54 weeks | 14.73 (5.04) | Conflicting data | 45.3 | 20.93 (9.97) | Conflicting data | –24.33 | NR | NR | 38 (26.28) | Conflicting data | |
| ADACTA58 | TCZ 8 mg/kg i.v. every 4 weeks + s.c. PBO ADA | 24 weeks | (0–28 scale) 11.3 (5.3) |
(0–66 scale) 6.7 (10.7) |
NR | (0–28 scale) 15.9 (6.7) |
(0–68 scale) 12.7 (SD NR) |
NR | N/A | NR | N/A | NR |
| ADA 40 mg s.c. every 2 weeks + i.v. PBO TCZ | 24 weeks | 12.4 (5.4) | 8.6 (10.5) | NR | 16.5 (7.0) | 16.8 (16.2) | NR | N/A | NR | N/A | NR |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Mean swollen joint count at baseline (SD) (scale) | Mean swollen joint count at follow-up (SD) (scale) | Swollen joint count % change from baseline | Mean tender joint count at baseline (SD) (scale) | Mean tender joint count at follow-up (SD) (scale) | Tender joint count, n % change from baseline | CRP level at baseline (mean) (mg/dl) | CRP level at follow-up (mean) (mg/dl) | ESR level at baseline (mean) (mm/hour) | ESR level at follow-up (mean) (mm/hour) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIM62 | MTX + PBO (n = 219) | 12 months | (Scale unclear) 22.1 (8.8) |
NR | Adjusted mean change –11.5 (0.54) | (Scale unclear) 32.3 (13.6) |
NR | Adjusted mean change –16.3 (0.85) | 28 (25) | Adjusted mean change –8.2 (1.4) | NR | NR |
| ABT i.v. + MTX (n = 433) | 21.4 (8.8) | NR | Adjusted mean change –16.1 (0.35) | 31.0 (13.2) | NR | Adjusted mean change –22.5 (0.55) | 33 (31) | Adjusted mean change –18.3 (0.9) | NR | NR | ||
| ASSET72 | PBO + MTX | 4 months | 8.5 (4.1) (Scale NR) |
NR | NR | 13.3 (7.2) (Scale NR) |
NR | NR | 16.6 (16.8) | NR | NR | NR |
| ABT i.v. (≈ 10 mg/kg) + MTX | 4 months | 11.3 (6.6) (Scale NR) |
NR | NR | 12.9 (7.1) (Scale NR) |
NR | NR | 13.6 (17.4) | NR | NR | NR | |
| CHANGE80 | PBO (n = 87) | 24 weeks | (Scale unclear) 19.3 (7) |
NR | Mean change –1.8 (7.4) | (Scale unclear) 23.7 (8.8) |
NR | Mean change –0.5 (10.9) | 5.9 (3.3) | Mean change 0.1 (3.2) | NR | NR |
| ADA monotherapy (n = 91) | 19.1 (7.3) | NR | Mean change –8.2 (8.8)a | 24.4 (10.7) | NR | Mean change –10.7 (12.3)a | 6.5 (4.4) | Mean change –1.6 (4.1)a | NR | NR | ||
| ADORE59,60 | ETN monotherapy (n = 159) n = 156 at 16 weeks |
16 weeks | NR | NR | NR | NR | NR | NR | NR | 33.2 | 26.4 | |
| ETN + MTX (n = 155) n = 151 at 16 weeks |
NR | NR | NR | NR | NR | NR | NR | 36.7 | 20.8b | |||
| ETN study 30989 | SSZ + PBO (n = 50) | 24 weeks | (Scale unclear) | NR | 38.5% improvement | Painful joints (scale unclear) | NR | Painful joints 22.7% improvement | NR | 32.9c | NR | 0.2d |
| ETN + PBO (n = 103) | NR | 68.7% improvement | NR | 65.4% improvement | NR | 69.9a (vs. SSZ)c | NR | 37.6a (vs. SSZ)d | ||||
| ETN + SSZ (n = 101) | NR | 70.1%a vs. SSZ improvement | NR | 62.0% improvement | NR | 66.7a (vs. SSZ)c | NR | 43.0a (vs. SSZ)d | ||||
| JESMR140 | ETN 25 mg every 2 weeks monotherapy | 24 weeks | 12.4 (6.1) (0–66 scale) |
4.3 (5.2) | NR | 15.0 (9.4) (0–68 scale) |
4.5 (8.0) | NR | 2.5 (2.5) | 1.2 (1.7) | 59.7 (28.4) | 41.6 (25.4) |
| ETN 25 mg every 2 weeks + MTX 6–8 mg/week | 24 weeks | 12.5 (6.5) (0–66 scale) |
3.0 (3.8) | NR | 15.1 (8.1) (0–68 scale) |
2.4 (3.9) | NR | 3.0 (3.2) | 0.6 (1.0)a | 59.5 (26.5) | 29.9 (23.3)a | |
| JESMR140 | ETN 25 mg every 2 weeks monotherapy | 52 weeks | 12.4 (6.1) (0–66 scale) |
4.0 (4.4) | NR | 15.0 (9.4) (0–68 scale) |
4.3 (5.3) | NR | 2.5 (2.5) | 1.3 (1.6) | 59.7 (28.4) | 43.7 (27.0) |
| ETN 25 mg every 2 weeks + MTX 6–8 mg/week | 52 weeks | 12.5 (6.5) (0–66 scale) |
1.8 (2.3)a | NR | 15.1 (8.1) (0–68 scale) |
2.1 (2.8)a | NR | 3.0 (3.2) | 3.0 (3.2)b | 59.5 (26.5) | 28.9 (23.8)b | |
| Lan et al., 2004101 | PBO + MTX | 12 weeks | 14.45 (0–28 scale) | 10.59 (0–28 scale) | 27 | 16.00 (0–28 scale) | 13.55 (0–28 scale) | 15 | 1.83 | 1.38 | NR | NR |
| ETN 25 mg twice weekly + MTX | 12 weeks | 13.21 (0–28 scale) | 4.66a (0–28 scale) | 65 | 14.03 (0–28 scale) | 7.03a (0–28 scale) | 50 | 1.65 | 0.39a | NR | NR | |
| LARA102 | MTX + DMARD (n = 142) | 24 weeks | (Scale unclear) 19.3 (10.1) |
NR | –8.6 (0.6)e | (Scale unclear) 26.2 (12.3) |
NR | –12.8 (0.8)e | NR | NR | NR | NR |
| ETN50 + MTX (n = 281) n = 279 at week 24 |
18.2 (8.4) | NR | –15.1 (0.4)a,e | 25.1 (11.9) | NR | –19.8 (0.6)a,e | NR | NR | NR | NR | ||
| Moreland et al., 1999104 | PBO (n = 80) | 6 months | (Scale 0–68) 25 |
NR | 7% (worsening) | (Scale 0–71) 35 |
NR | –6% | 4.1 | 207% worsed | 39 | 18% worsed |
| ETN + PBO (n = 78) | 25 | NR | –47b | 33 | NR | –56%a | 4.7 | 31% improvedb,d | 35 | 18% improveda,d | ||
| RACAT111 | MTX + SSZ + HCQ (n = 178; not all analysed) | 24 weeks | (Scale 0–28) 11.12 (5.26) |
5.32 (4.73) | NR | (Scale 0–28) 13.39 (6.62) |
5.87 (5.96) | NR | NR | NR | 27.39 (21.03) | 20.38 (16.73) Change 0.97 (0.85) |
| ETN50 + MTX (n = 175; not all analysed) | (Scale 0–28) 11.34 (5.22) |
4.76 (5.14) | NR | (Scale 0–28) 13.39 (6.39) |
5.94 (6.85) | NR | NR | NR | 29.80 (23.51) | 19.01 (17.89) Change 0.98 (0.87) |
||
| RACAT111 | MTX + SSZ+ HCQ (n = 178; not all analysed, some switched) | 48 weeks (n = 310 both groups) | NR | NR | 3.93 (4.19) | NR | 4.64 (5.61) | NR | NR | NR | NR | 18.88 (15.35) |
| ETN50 + MTX (n = 175; not all analysed, some switched) | NR | NR | 3.50 (3.87) | NR | 4.61 (6.10) | NR | NR | NR | NR | 19.76 (18.30) | ||
| Weinblatt et al., 1999124 | MTX + PBO, (n = 30) | 24 weeks | (Scale 0–68) 17f |
16f | NR | (Scale 0–71) 28c |
17f | NR | 2.6f | 1.6f | 36f | 30f |
| ETN 25 mg twice weekly + MTX (n = 59) | 20f | 6b,f | NR | 28f | 7b,f | NR | 2.2c | 0.5b,f | 25c | 15a,f | ||
| APPEAL67,68 | MTX + DMARD (SSZ, HCQ or LEF) | 16 weeks | NR | NR | NR | NR | NR | NR | 2.06 (2.48) calculated from 20.6 (24.8) | 9.8 (52.2) | 54.80 (28.2) | 40.4 (26.2) |
| ETN 25 mg twice weekly (licensed dose) + MTX | 16 weeks | NR | NR | NR | NR | NR | NR | 1.70 (2.10) calculated from 17.0 (21.0) | 7.9 (53.3) | 57.7 (33.0) | 34.4 (40.4)a | |
| GO-FORTH91 | PBO every 4 weeks + MTX 6–8 mg/week | 14 weeks | 11.4 (6.58) (0–66 scale) |
NR | NR | 13.2 (7.83) (0–68 scale) |
NR | NR | NR | NR | NR | NR |
| GOL 50 mg s.c. every 4 weeks + MTX 6–8 mg/week | 14 weeks | 11.8 (6.72) (0–66 scale) |
NR | NR | 13.1 (8.38) (0–68 scale) |
NR | NR | NR | NR | NR | NR | |
| GO-FORTH91 | PBO every 4 weeks + MTX 6–8 mg/week | 24 weeks | 11.4 (6.58) (0–66 scale) |
NR | NR | 13.2 (7.83) (0–68 scale) |
NR | NR | NR | NR | NR | NR |
| GOL 50 mg s.c. every 4 weeks + MTX 6–8 mg/week | 24 weeks | 11.8 (6.72) (0–66 scale) |
NR | NR | 13.1 (8.38) (0–68 scale) |
NR | NR | NR | NR | NR | NR | |
| GO-FORWARD92 | PBO s.c. every 4 weeks + MTX | Week 14 | 12.0 (8.0–19.0)f (0–66 scale) |
NR | 37.5 (0.0–71.4)f As reported |
21.0 (14.0–34.0)f (0–68 scale) |
NR | 30.0 (–12.1 to 66.7)f As reported |
0.8 (0.3–2.0)f | NR | NR | NR |
| GOL 50 mg s.c. every 4 weeks + MTX | Week 14 | 13.0 (8.0–22.0)f (0–66 scale) |
NR | 62.1 (28.6–84.6)b,f As reported |
26.0 (16.0–39.0)f (0–68 scale) |
NR | 59.5 (24.0–77.8)a,f As reported |
1.00 (0.40–2.80)f | NR | NR | NR | |
| GO-FORWARD92 | PBO s.c. every 4 weeks + MTX | Week 24 | 12.0 (8.0–19.0)f | NR | 32.1 (–9.1 to 71.4)f As reported |
21.0 (14.0–34.0)f As reported |
NR | 20.9 (–13.3 to 64.3)f | NR | NR | NR | NR |
| GOL 50 mg s.c. every 4 weeks + MTX | Week 24 | 13.0 (8.0–22.0)f | NR | 72.1 (24.0–92.3)b,f As reported |
26.0 (16.0–39.0)f As reported |
NR | 61.6 (18.7–85.4)f | NR | NR | NR | NR | |
| ATTRACT75 | PBO i.v. + MTX | 30 weeks | (0–66) 19 (13–28)f |
13 (8–26)f | –20 | (0–68) 24 (16–48)f |
16 (7–33)f | –26 | 3.0 (1.2–5.7)f | 2.3 (0.7–5.1)f (–9% change) | NR | NR |
| IFX 3 mg/kg i.v. at weeks 0, 2 and 6 and every 8 weeks thereafter | 30 weeks | (0–66) 19 (13–30)f |
9 (4–18)b,f | –52b | (0–68) 32 (16–46)f |
12 (3–21)a,f | –59a | 3.1 (1.3–5.3)f | 0.8 (0.4–2.3)b,f (–60% change)b |
NR | NR | |
| ATTRACT146 | PBO i.v. + MTX | 54 week | (0–66) 19 (13–28)f |
NR | 13 (61)g As reported |
(0–68) 24 (16–48)f |
NR | 23 (63)g As reported |
N/A | NR | NR | NR |
| IFX 3 mg/kg i.v. at weeks 0, 2 and 6 and every 8 weeks thereafter | 54 week | (0–66) 19 (13–30)f |
NR | 37 (62)b,g As reported |
(0–68) 32 (16–46)f |
NR | 49 (52)b,g As reported |
N/A | NR | NR | NR | |
| Durez et al., 200486 | Single i.v. infusion of 1-g MP at week 0 + MTX (n = 15 randomised) | 14 weeks | 22 (7–38)h (0–66 scale) |
22 | NR | 24 (7–38)h (0–68 scale) |
20 | NR | 1.9h | 2.0 | NR | NR |
| IFX 3 mg/kg at weeks 0, 2 and 6 + MTX (n = 12 randomised) | 14 weeks | 16 (8–27)h (0–66 scale) |
7b | NR | 20 (6–44)h (0–68 scale) |
8a | NR | 1.3h | 0.9 | NR | NR | |
| START118 | PBO + MTX | 22 weeks | 15 (10–21)f (0–66 scale) |
NR | NR | 22 (15–32)f (0–68 scale) |
NR | NR | 1.2 (1–3)f | NR | NR | NR |
| IFX 3 mg/kg + MTX | 22 weeks | 15 (11–21)f (0–66 scale) |
NR | NR | 22 (15–31)f (0–68 scale) |
NR | NR | 1.6 (1–3)f | NR | NR | NR | |
| Wong et al., 2009125 | PBO + MTX (with crossover for PBO group to open-label IFX at week 24) | Week 16 | (0–28 scale) 12 (5) |
12 | NR | (0–28 scale) 15 (7) |
16 | NR | 3.0 | 22 | 40 (24) | 37 |
| IFX 3 mg/kg at weeks 0, 2, 6 and 8 weeks thereafter + MTX | Week 16 | 10 (5) | 4 | NR | 14 (7) | 8a | NR | 3.2 | 12 | 39 (26) | 26 | |
| ACT-RAY57 | TCZ 8 mg/kg i.v. every 4 weeks + oral PBO | Week 24 | 15.3 (10.2) (Scale NR) |
NR | –11.75 (9.45)g | 26.6 (15.2) (Scale NR) |
NR | –17.00 (13.64)i | NR | NR | NR | NR |
| TCZ 8 mg/kg i.v. every 4 weeks + MTX | Week 24 | 14.4 (8.9) (Scale NR) |
NR | –11.33 (8.04)g | 25.8 (13.9) (Scale NR) |
NR | –17.25 (13.35)g | NR | NR | NR | NR | |
| SATORI322 | PBO i.v. every 4 weeks + MTX | 24 weeks | (0–28) 12i | (0–28) 9i | NR | (0–28) 10.5i | (0–28) 7i | NR | 2.6i | 7i | 50i | 45i |
| TCZ 8 mg/kg i.v. every 4 weeks + PBO capsules | 24 weeks | (0–28) 10i |
(0–28) 4.5i | NR | (0–28) 10i | (0–28) 2i | NR | 3.0i | 2i | 50i | 11i | |
| TOWARD121 | PBO i.v. every 4 weeks + stable cDMARDs (n = 415 randomised) | 24 weeks | (0–68) 18.7 (10.8) |
13.8 | NR | (0–66) 29.1 (14.8) |
20.6 | NR | 2.6 (4.7) | 2.33 | 49.2 (28.3) | 44.5 |
| TCZ 8 mg/kg i.v. every 4 weeks + stable DMARDs (n = 805 randomised) | 24 weeks | 19.7 (11.6) | 9.4b | NR | 30.1 (16.0) | 14.4b | NR | 2.6 (3.2) | 0.4b | 48.2 (27.5) | 12.6b | |
| TACIT141 | Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Patient’s global assessment of disease activity at baselinea | Patient’s global assessment of disease activity at follow-upa | % change from baseline | Evaluator’s global assessment of disease activity at baselinea | Evaluator’s global assessment of disease activity at follow-upa | % change from baseline |
|---|---|---|---|---|---|---|---|---|
| OPERA107 | PBO + MTX + steroid | 12 months | 65 (17–96)b | 18 (0–69)b | NR | 51 (22–86)b | 4 (0–33)b | NR |
| ADA + MTX + steroid | 12 months | 70 (12–100)b | 10 (0–54)b | NR | 57 (22–86)b | 1 (0–59)b | NR | |
| OPTIMA108 | PBO + MTX | 26 weeks | 63 (22) | 35.1 | NR | 62 (18) | 28.9 | NR |
| ADA + MTX | 26 weeks | 64 (23) | 26.4 | NR | 63 (18) | 21.3 | NR | |
| GO-BEFORE90 | PBO + MTX | 24 weeks | (0–10 scale) 5.9 (2.32) |
NR | –36.70 | (0–10 scale) 6.0 (1.72) |
NR | –63.00 |
| GOL + MTX | 24 weeks | (0–10 scale) 6.1 (2.21) |
NR | –49.55c | (0–10 scale) 6.2 (1.63) |
NR | –66.70 | |
| BeST148 | Sequential monotherapy | 6 months | 59.2 | NR | Mean change from baseline = –22.3 | NR | NR | NR |
| Step-up combination therapy | 6 months | 59.4 | NR | Mean change from baseline = –28.0 | NR | NR | NR | |
| Initial combination therapy + prednisone | 6 months | 59.5 | NR | Mean change from baseline = –32.0c (for sequential monotherapy vs. initial combination + prednisone and initial combination + MTX) | NR | NR | NR | |
| Initial combination therapy + IFX | 6 months | 61.8 | NR | Mean change from baseline = –35.9c (for sequential monotherapy vs. initial combination + prednisone and initial combination + MTX) | NR | NR | NR |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Patient’s global assessment of disease activity at baseline, mean (SD)a | Patient’s global assessment of disease activity at follow-up, mean (SD)a | % change from baseline | Evaluator’s global assessment of disease activity at baseline, mean (SD)a | Evaluator’s global assessment of disease activity at follow-upa | % change from baseline |
|---|---|---|---|---|---|---|---|---|
| AMPLE66 | ABT s.c. | 12 months | 61.1 (22.1) | NR | 46.1 (as reported) | 58.8 (18.6) | NR | 68.5 (as reported) |
| ADA | 12 months | 61.5 (22.5) | NR | 41.2 (as reported) | 58.8 (18.9) | NR | 63.0 (as reported) | |
| bRED-SEA114 | ADA + cDMARDs | 12 months | 70 (50–82) | 49 (20–65) | NR | NR | NR | NR |
| ETN50 + cDMARDs | 12 months | 70 (54–80) | 50 (27–71) | NR | NR | NR | NR | |
| deFilippis et al., 200685 | ETN + MTX | 22 weeks | 64.33 (18.89) | NR | 34.8 (as reported) | 58.33 (14.60) | NR | 38.3 (as reported) |
| IFX + MTX | 22 weeks | 69.33 (16.57) | NR | 21.4 (as reported) | 60.67 (12.0) | NR | 35.6 (as reported) | |
| deFilippis et al., 200685 | ETN + MTX | 54 weeks | 64.33 (18.89) | 74.88 | 50.6 (as reported) | 58.33 (14.60) | 77.05 | 41.8 (as reported) |
| IFX + MTX | 54 weeks | 69.33 (16.57) | 86.91 | 22.2 (as reported) | 60.67 (12.0) | 83.31 | 43.6 (as reported) |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Patient’s global assessment of disease activity at baseline, mean (SD)a | Patient’s global assessment of disease activity at follow-up, mean (SD)a | % change from baseline, mean (SD) | Evaluator’s global assessment of disease activity at baseline, mean (SD)a | Evaluator’s global assessment of disease activity at follow-upa | % change from baseline, mean (SD) |
|---|---|---|---|---|---|---|---|---|
| AIM62 | PBO + MTX | 12 months | 62.8 (21.6) | NR | Adjusted mean change –24.2 (1.72) | 67.4 (17.0) | NR | Adjusted mean change –34.3 (1.44) |
| ABT i.v. + MTX | 12 months | 62.7 (21.2) | NR | Adjusted mean change –35.8 (1.12) | 68.0 (16.0) | NR | Adjusted mean change –49.1 (0.93) | |
| ASSURE73 | PBO + cDMARDs | 1 year | 61.3 (20.1) | NR | 20 | 58.3 (17.5) | NR | 37 |
| ABT + cDMARDs | 1 year | 60.6 (19.7) | NR | 41 | 57.8 (17.4) | NR | 56 | |
| CHANGE80 | PBO | 24 weeks | 64.6 (22.9) | NR | Mean change 2.6 (23.5) | 74.1 (15.6) | NR | Mean change –8.0 (21.8) |
| ADA monotherapy | 24 weeks | 71.2 (19.2) | NR | Mean change –19.9 (31.0)b | 76.2 (14.7) | NR | Mean change –30.3 (24.8)b | |
| DE01984 | PBO + MTX | 52 weeks | 54.3 (22.9) | NR | –20.1 | 61.3 (17.3) | NR | –31.8 |
| ADA + MTX | 52 weeks | 52.7 (21.0) | NR | –52.2 | 62.0 (16.7) | NR | –63.5 | |
| Van De Putte et al., 2004122 | PBO | 26 weeks | 71.8 (19.9) | NR | –7.9 | 68.5 (18.2) | NR | –12.9 |
| ADA monotherapy | 26 weeks | 72.6 (19.3) | NR | –38.9c | 67.3 (16.6) | NR | –38.8c | |
| ARMADA69,70 | PBO + MTX | 24 weeks | 58.0 (23.2) | NR | –14.7 | 58.9 (15.3) | NR | –11.6 |
| ADA + MTX | 24 weeks | 56.9 (21.1) | NR | –52.4c | 58.7 (15.8) | NR | –53.0c | |
| Kim et al., 200799 | PBO + MTX | 24 weeks | 63.2 (20.44) | NR | Mean change –10.7 (24.85) | 64.0 (13.61) | NR | Mean change –9.6 (26.47) |
| ADA + MTX | 24 weeks | 59.7 (17.19) | NR | Mean change –23.7 (26.54)b | 63.7 (15.16) | NR | Mean change –29.2 (27.48)c | |
| ADORE59 | ETN monotherapy | 16 weeks | (0–10 scale) 6.6 |
NR | (0–10 scale) Mean change from baseline –2.78 (2.60) |
NR | NR | NR |
| ETN + MTX | 16 weeks | (0–10 scale) 6.6 |
NR | (0–10 scale) Mean change from baseline –2.95 (2.59) |
NR | NR | NR | |
| ETN study 30989 | PBO + SSZ | 24 weeks | NR | NR | (0–10 scale) 13.6 |
NR | NR | (0–10 scale) 16.0 |
| ETN + PBO | 24 weeks | NR | NR | 50.5c vs. SSZ | NR | NR | 59.5c vs. SSZ | |
| ETN + SSZ | 24 weeks | NR | NR | 53.5c vs. SSZ, NS vs. ETN + PBO | NR | NR | 62.0c vs. SSZ, NS vs. ETN + PBO | |
| JESMR140 | ETN monotherapy | 24 weeks | 62.5 (20.5) | 31.5 (28.4) | NR | 58.2 (21.5) | NR | NR |
| ETN + MTX | 24 weeks | 53.7 (23.7) | 21.6 (18.8) | NR | 58.2 (19.3) | NR | NR | |
| JESMR140 | ETN monotherapy | 52 weeks | 62.5 (20.5) | 27.4 (25.1) | NR | NR | NR | NR |
| ETN + MTX | 52 weeks | 53.7 (23.7) | 21.3 (19.4) | NR | NR | NR | NR | |
| Lan et al., 2004101 | PBO + MTX | 12 weeks | 69.7 | 61.4 | NR | 79.7 | 54.2 | NR |
| ETN + MTX | 12 weeks | 66.2 | 37.9 | NR | 75.2 | 22.8 | NR | |
| LARA102 | MTX + DMARD | 24 weeks | (1–10 scale) 7.1 (1.9) |
NR | (1–10 scale) Adjusted mean change –2.3 (SE 0.2) |
(1–10 scale) 6.7 (1.6) |
NR | (1–10 scale) Adjusted mean change –2.4 (SE 0.2) |
| ETN50 + MTX | 24 weeks | (1–10 scale) 7.1 (2.0) |
NR | (1–10 scale) Adjusted mean change –3.9 (SE 0.2)c |
(scale 1–10) 6.7 (1.6) |
NR | (1–10 scale) Adjusted mean change –4.8 (SE0.1)c |
|
| Moreland et al., 1999,104 Mathias et al., 2000105 | PBO | 6 months | (0–10 scale) 6.9 |
NR | 3 (worse) | (0–10 scale) 6.9 |
NR | 2 (improved) |
| ETN + PBO | 6 months | (0–10 scale) 7.0 |
NR | 6 (improved) between groupsc | (0–10 scale) 6.9 |
NR | 44 (improved) between groupsc | |
| RACAT111 | MTX + SSZ + HCQ (n = 178; not all analysed) | 24 weeks | (scale 0–10) 5.43 (2.20) |
3.51 (2.19) | NR | (scale 0–100) 60.14 (22.98) |
35.70 (22.18) | NR |
| ETN50 + MTX (n = 175; not all analysed) | 5.63 (1.95) | 3.18 (2.32) | NR | 61.06 (20.01) | 35.35 (24.43) | NR | ||
| RACAT111 | MTX + SSZ + HCQ (n = 178; not all analysed, some switched) | 48 weeks (n = 310 both groups) | (scale 0–10) 5.43 (2.20) |
3.01 (2.33) | NR | (scale 0–100) 60.14 (22.98) |
32.87 (25.07) | NR |
| ETN50 + MTX (n = 175; not all analysed, some switched) | 5.63 (1.95) | 2.98 (2.38) | NR | 61.06 (20.01) | 30.77 (23.05) | NR | ||
| APPEAL67,68 | MTX + DMARD (SSZ, HCQ or LEF) | 16 weeks | 6.5 (1.8) | 4.5 | 30.6 | 6.6 (1.8) | 3.6 | 45.0 |
| ETN + MTX | 16 weeks | 6.7 (2.0) | 3.3 | 50.8 | 6.6 (1.7) | 2.5 | 62.1 | |
| Weinblatt et al., 1999124 | PBO + MTX | 24 weeks | (0–10 scale) 6.0d |
(0–10 scale) 4.0d |
NR | (0–10 scale) 6.5d |
(0–10 scale) 4.0d |
NR |
| ETN + MTX | 24 weeks | (0–10 scale) 6.0d |
(0–10 scale) 2.0b,d |
NR | (0–10 scale) 6.0d |
(0–10 scale) 2.0b,d |
NR | |
| GO-FORWARD92 | PBO + MTX | Week 14 | (0–10 scale) 5.30 (3.70–7.20)d | NR | –14.6 (+10.8, –50.0)d | (0–10 scale) 5.65 (4.30–6.85)d | NR | –34.9 (+2.4 to –64.6)d |
| GOL + MTX | Week 14 | (0–10 scale) 6.00 (3.80–7.90)d |
NR | –45.3 (–16.7 to –76.9)c,d | (0–10 scale) 6.10 (5.10–7.10)d | NR | –54.5 (–35.2 to –72.9)c,d | |
| GO-FORWARD92 | PBO + MTX | Week 24 | (0–10 scale) 5.30 (3.70–7.20)d | NR | –17.3 (+16.3 to –46.0)d | (0–10 scale) 5.65 (4.30–6.85)d | NR | –39.1 (–1.3 to –67.3)d |
| GOL + MTX | Week 24 | (0–10 scale) 6.0 (3.8–7.9)d |
NR | –47.9 (–17.0 to –76.1)c,d | (0–10 scale) 6.1 (5.1– 7.1)d | NR | –61.7 (–38.7 to –82.1)c,d | |
| ATTRACT75 | PBO + MTX | 30 weeks | (0–10 scale) 6.2 (4.3–8.1)d |
(0–10 scale) 5.5 (3.1–7.5)d |
–7 | (0–10 scale) 6.5 (5.2–7.4)d |
(0–10 scale) 5.0 (3.0–7.0)d |
–13 |
| IFX + MTX | 30 weeks | (0–10 scale) 6.6 (4.9–7.8)d |
(0–10 scale) 3.6 (1.8–6.7)d |
–23b | (0–10 scale) 6.1 (4.8–7.1)d |
(0–10 scale) 2.6 (1.5–5.2)d |
–53c | |
| Durez et al., 200486 | MP + MTX | 14 weeks | 63 (19–100)d | 50e | NR | 58 (18–83)d | 59e | NR |
| IFX + MTX | 14 weeks | 52 (15–80)d | 42e | NR | 43 (14–85)d | 16c,e | NR | |
| Wong et al., 2009125 | PBO + MTX | Week 16 | 70 (25) | 68e | NR | NR | NR | NR |
| IFX + MTX | Week 16 | 68 (15) | 32b,e | NR | NR | NR | NR | |
| ACT-RAY57 | TCZ + oral PBO | Week 24 | NR | NR | Mean change –32.4 (SD 24.34) | NR | NR | Mean change –38.5 (SD 21.65) |
| TCZ + MTX | Week 24 | NR | NR | Mean change –34.3 (SD 25.68) | NR | NR | Mean change –40.7 (SD 19.55) | |
| SATORI322 | PBO + MTX | 24 weeks | 57e | 47e | NR | 60e | 47e | NR |
| TCZ + PBO capsules | 24 weeks | 60e | 28e | NR | 63e | 22e | NR | |
| TACIT141 | Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Trial name | Scoring system applied | Treatment arms for which data extraction performed | Assessment point | Mean (SD) change from baseline in total score | Mean (SD) change from baseline in erosion score | Mean (SD) change from baseline in JSN score | Radiographic non-progression |
|---|---|---|---|---|---|---|---|
| GUEPARD93 | van der Heijde-modified Sharp score data323 | Initial MTX 12 weeks, then step-up therapy in both groups based on DAS28 (n = 29) | 52 weeks | (Score range of 0–448) 1.8 (4.7) |
NR | NR | % patients with no radiographic progression = 55 (16/29) |
| Initial ADA + MTX 12 weeks, then step-up therapy in both groups based on DAS28 (n = 27) | 52 weeks | 1.9 (4) | NR | NR | % patients with no radiographic progression = 59 (16/27) | ||
| OPTIMA108 | van der Heijde-modified total Sharp score | PBO + MTX (n = 517; n = 514 analysed for Δ modified total Sharp score) | 26 weeks | 0.96 (NR) | 0.48 (NR) | 0.48 (SD NR) | (Δ modified total Sharp score ≤ 0.5), 72% |
| ADA + MTX (n = 515; n = 508 analysed for Δ modified total Sharp score) | 26 weeks | 0.15a (NR) | 0.10a (NR) | 0.05a (SD NR) | 87%a | ||
| PREMIER109 | van der Heijde-modified total Sharp score | PBO + MTX (n = 257) | 1 year | (0–398, with higher scores indicating greater progression) 5.7 worse |
(Scale NR, higher scores indicate worse erosion) 3.7 worse |
(Scale NR, higher scores indicate worse JSN) 2.0 worse |
(Change in modified total Sharp score ≤ 0.5 from baseline), 37% |
| ADA monotherapy + PBO (n = 274) | 1 year | 3.0 worse | 1.7 worse | 1.3 worse | 51%b (vs. MTX monotherapy) | ||
| ADA + MTX (n = 268) | 1 year | 1.3 worsea (vs. MTX monotherapy vs. ADA monotherapy) | 0.8 worsea (vs. MTX monotherapy vs. ADA monotherapy) | 0.5 worse | 64%b (vs. MTX monotherapy vs. ADA monotherapy) | ||
| PREMIER109 | van der Heijde-modified total Sharp score | PBO + MTX (n = 257) | 2 years | 10.4 worse | 6.4 worse | 4.0 worse | 34% |
| ADA monotherapy + PBO (n = 274) | 2 years | 5.5 worse | 3.0 worse | 2.6 worse | 45%b (vs. MTX monotherapy) | ||
| ADA + MTX (n = 268) | 2 years | 1.9 worsea (vs. MTX monotherapy vs. ADA monotherapy) | 1.0 worsea (vs. MTX monotherapy vs. ADA monotherapy) | 0.9 worse | 61%b (vs. MTX monotherapy vs. ADA monotherapy) | ||
| COMET81–83 | van der Heijde-modified total Sharp score | PBO + MTX (n = 230) | 52 weeks | 2.44 (95% CI 1.45 to 3.43) | NR | NR | (Defined as modified total Sharp score of ≤ 0.5) 135/230 (59%) |
| ETN + MTX (n = 246) | 52 weeks | 0.27 (95% CI –0.13 to 0.68) | NR | NR | 196/246 (80%) | ||
| ERA139 | Total modified Sharp score | PBO + MTX (n = 217) |
6 months | [modified total Sharp score 0 (no damage) to 398 (severe joint destruction) scale] 1.06 (worse) |
[Erosion score 0 (no new erosion) to 230 (new erosion, worsening of erosion)] 0.65 (worse)c |
[JSN score 0 (no narrowing) to 168 (complete loss of joint space)] 0.35 (worse)c |
NR |
| ETN + PBO (n = 207) | 6 months | 0.57a (worse) | 0.25b,c (worse) | 0.2 (worse) | NR | ||
| ERA139 | Total modified Sharp score | PBO + MTX (n = 217) | 12 months | 1.59 (worse) | 1.0 (worse)c | 0.55 (worse)c | NR |
| ETN + PBO (n = 207) | 12 months | 1.00 (worse) | 0.45b,c (worse) | 0.55 (worse) | NR | ||
| ASPIRE71 | van der Heijde-modified Sharp score data | PBO + MTX (n = 282 for total score, n = 226 for erosion and JSN scores) | 54 weeks | (Scale 0–48, higher score = more joint damage) 3.7 (9.6) 0.43d (0.0, 4.5) |
(Scale 0–280) 3.0 (7.8) 0.3d (0.0, 3.8) |
(Scale 0–168) 0.6 (2.1) 0.0d (0.0, 0.4) |
NR |
| IFX + MTX (n = 359 for total score, n = 306 for erosion and JSN scores) |
54 weeks | 0.4 (5.8) 0.0d (–0.8, 1.3)a |
0.3 (4.9) 0.0d (–0.8, 1.3)a |
0.1 (1.6) 0.0d (0.0, 0.0)a |
NR |
| Trial name/study | Treatment arms for which data extraction performed | Assessment point | Mean change from baseline in synovitis (SD) | Mean change from baseline in erosions (SD) | Mean change from baseline in osteitis (SD) |
|---|---|---|---|---|---|
| OPTIMA108/Peterfy et al., 2010150 | PBO + MTX (n = 32) | 26 weeks | OMERACT-RAMRIS scoring system. Progression or improvement of magnetic resonance imaging scores defined as positive or negative change from baseline ≥ smallest detectable change respectively –2.0 (improved) % patients showing progression = 6 % patients showing improvement = 44 |
OMERACT-RAMRIS scoring system 1.4 (worse) % patients showing progression = 38 % patients showing improvement = 9 |
OMERACT-RAMRIS scoring system 0.0 % patients showing progression = 13 % patients showing improvement = 9 |
| ADA + MTX (n = 27) | 26 weeks | –3.6 (improved) % patients showing progression = 0 % patients showing improvement = 74a |
–0.8 (improved) % patients showing progression = 4a % patients showing improvement = 22 |
–4.0 (improved) % patients showing progression = 0 % patients showing improvement = 30 |
|
| GO-BEFORE151 | PBO + MTX (synovitis n = 81 wrists + MCP joint, n = 82 wrist joints only, osteitis and erosion n = 82) | 24 weeks | [RAMRIS scores (higher RAMRIS scores = more severe inflammation/damage)] Wrist + MCP joints (range 0–21) Mean = –1.04 (3.04) Median = –1.00 (IQR –1.63 to 0.00) Wrist joints only (range 0–9) Mean = –0.74 (1.86) Median = –0.50 (IQR –1.00 to 1.00) |
(RAMRIS scores; (range 0–230) –0.24 (6.39) 0.00b (IQR 0.00 to 0.50) |
[RAMRIS scores oedema (osteitis) (range 0–69)] –0.32 (4.66) 0.00b (IQR –1.50 to 1.00) |
| GOL + MTX (synovitis n = 77 wrists + MCP joint, n = 78 wrist joints only, osteitis and erosion n = 78) | 24 weeks | Wrist + MCP joints (range 0–21) –2.21 (3.10) –1.50 (IQR –3.50 to –0.33)a,b Wrist joints only (range 0–9) –1.29 (1.67) –1.00 (IQR –2.50 to 0.00)a,b |
(Range 0–230) –0.65 (5.98) 0.00 (–0.58, 0.00)a,b |
Oedema (osteitis) (range 0–69) –2.47 (4.08) –1.00 (–3.00, 0.00)a,b |
| Trial name | Scoring system applied | Treatment arms for which data extraction performed | Assessment point | Mean (SD) change from baseline in total score | Mean (SD) change from baseline in erosion score | Mean (SD) change from baseline in JSN score | Radiographic non-progression |
|---|---|---|---|---|---|---|---|
| AMPLE66 | Modified Sharp/van der Heijde scoring system | ABT s.c. (n = 318, 91.1% assessed for radiographic non-progression) | 1 year | (Scale 0–448, direction NR) 0.58 (3.22) |
(Scale and direction NR) 0.29 (1.84) |
(Scale and direction NR) 0.28 (1.92) |
(Change from baseline in total score ≤ smallest detectable change at cut-off 2.8) 84.8% |
| ADA (n = 328, 88.1% assessed for radiographic non-progression) | 1 year | 0.38 (5) | –0.01 (2.83) | 0.39 (2.50) | 88.6% |
| Trial name | Scoring system applied | Treatment arms for which data extraction performed | Assessment point | Mean (SD) change from baseline in total score | Mean (SD) change from baseline in erosion score | Mean (SD) change from baseline in JSN score | Radiographic non-progression |
|---|---|---|---|---|---|---|---|
| AIM61,62 | Total Genant-modified Sharp score | PBO + MTX (n = 195) | 1 year | 2.32 (NR) 0.53 (0.0, 2.5)a |
1.14 (NR) 0.27 (0.0, 1.3)a,b |
1.18 (NR) 0.0 (0.0, 1.0)a,b |
NR |
| ABT i.v. + MTX (n = 391) | 1 year | 1.21 (NR) 0.25 (0.0, 1.8)a,b |
0.63 (NR) 0.0 (0.0, 1.0)a,b |
0.58 (NR) 0.0 (0.0, 0.5)a,b |
NR | ||
| DE01984 | Total Sharp score | PBO + MTX (n = 200) | 52 weeks | 2.7 (6.8) | 1.6 (4.4) | 1.0 (3.0) % patients with improvement or no change in JSN = 52.2 |
NR |
| ADA + MTX (n = 207) | 52 weeks | 0.1 (4.8)c | 0.0 (2.8)c | 0.1 (2.3)b% patients with improvement or no change in JSN = 68.5b | NR | ||
| JESMR140 | van der Heijde-modified Sharp score | ETN monotherapy (n = 71) | 24 weeks | (0–448, positive score indicates progression) 2.57 (NR) |
(Scale NR, positive value indicates progression) 1.16 (NR) |
(Scale NR, positive value indicates progression) 1.42 (NR) |
NR |
| ETN + MTX (n = 76) | 24 weeks | 0.34 (NR) | –0.02 (NR) | 0.37 (NR) | NR | ||
| JESMR140 | van der Heijde-modified Sharp score | ETN monotherapy (n = 71) | 52 weeks | 3.6 (NR) | 1.87 (NR) | 1.78 (NR) | No radiographic progression to week 52 (change ≤ 0.5) = 39.6% No clinically significant radiographic progression to week 52 (≤ smallest detectable change) = 58.5% |
| ETN + MTX (n = 76) | 52 weeks | 0.8 (NR) | –0.15 (NR)b | 1.01 (NR) | No radiographic progression to week 52 (change ≤ 0.5) = 57.4% No clinically significant radiographic progression to week 52 (≤ smallest detectable change) = 67.6% |
||
| LARA102 | Modified total Sharp score | MTX + DMARD (n = 119) | 24 weeks | Adjusted mean change = 1.4 (SE 0.5) | Adjusted mean change = 1.1 (SE 0.3) | Adjusted mean change = 0.2 (SE 0.3) | % patients with change ≤ 0 = 68.1 |
| ETN + MTX (n = 247) | 24 weeks | Adjusted mean change = 0.4 (SE 0.4)b | Adjusted mean change = 0.4 (SE 0.2)b | Adjusted mean change = –0.1 (SE 0.2) | % patients with change ≤ 0 = 75.3 | ||
| RACAT111 | van der Heijde-modified Sharp score | MTX + SSZ + HCQ (n = 158) | 24 weeks | 0.42 (1.91) | 0.23 (1.32) | 0.19 (1.25) | NR |
| ETN50 + MTX (n = 160) | 24 weeks | 0.003 (0.62) | –0.03 (0.44) | 0.03 (2.47) | NR | ||
| RACAT111 | van der Heijde-modified Sharp score | MTX + SSZ + HCQ (n = 151) | 48 weeks | 0.54 (1.93) | 0.29 (1.35) | 0.25 (1.18) | NR |
| ETN50 + MTX (n = 153) | 48 weeks | 0.29 (3.32) | 0.08 (1.48) | 0.21 (2.09) | NR | ||
| GO-FORTH91 | van der Heijde-modified Sharp score | PBO + MTX (n = 88) | 24 weeks | Scale NR, positive value indicates greater progression 2.51 (5.52) |
Scale NR, positive value indicates greater progression 1.66 (3.73) (n = 84) |
Scale NR, positive value indicates greater progression 0.83 (2.31) (n = 84) |
(No increase in total van der Heijde Sharp score, i.e. change from baseline to week 24 < 0) 44/88 (50.0%) |
| GOL + MTX (n = 66) | 24 weeks | 1.05 (3.71)b | 0.54 (1.62)b (n = 81) |
0.71 (2.91) (n = 81) |
51/86 (59.3%) | ||
| ATTRACT146 | van der Heijde-modified Sharp score | PBO + MTX (n = 64) | 54 weeks | (Total scores range 0–440, higher scores indicating more joint damage) 7.0 (10.3) |
(Erosion scores range 0–280) 4.0 (7.9) |
(JSN scores range 0–160) 2.9 (4.2) |
Major progression (% patients) = 31 Improvement (% patients) = 14 |
| IFX + MTX (n = 71) | 54 weeks | 1.3 (6.0)c | 0.2 (2.9)c | 1.1 (4.4)c | Major progression (% patients) = 8c Improvement (% patients) = 44c | ||
| Swefot147 | van der Heijde-modified Sharp score | SSZ + HCQ + MTX (n = 109) | 24 months from baseline (i.e. 20–21 months post-randomisation) | Treatment difference = 3.23 (95% CI 0.14 to 6.32)b | Treatment difference = 1.53 (95% CI –0.03 to 3.09)b | Treatment difference = 1.66 (95% CI –0.14 to 3.46)b | NR |
| IFX + MTX (n = 106) | 24 months from baseline (i.e. 20–21 months post-randomisation) | NR | |||||
| ACT-RAY57 | Total Genant-modified Sharp score | TCZ + oral PBO (n = 276) | 24 weeks | 0.22 (1.11) | 0.11 (0.63) | 0.11 (0.70) | % patients with no radiographic progression (change in score ≤ 0) = 58.7 |
| TCZ + MTX (n = 277) | 24 weeks | 0.08 (1.88) | –0.01 (0.78) | 0.08 (1.48) | % patients with no radiographic progression (change in score ≤ 0) = 65.3 | ||
| ACT-RAY152 | Total Genant-modified Sharp score | TCZ + oral PBO (n = 276) | 52 weeks | 0.63 (NR) | NR | NR | % patients with no radiographic progression (change in score ≤ 0) = 57.6 |
| TCZ + MTX (n = 276) | 52 weeks | 0.40 (NR) | NR | NR | % patients with no radiographic progression (change in score ≤ 0) = 67.5 | ||
| SAMURAI115 | Modified total Sharp score (no further detail) | cDMARDs (n = 143) | 52 weeks | Mean 6.1 (95% CI 4.2 to 8.0) | Mean 3.2 (95% CI 2.1 to 4.3) | Mean 2.9 (95% CI 2.0 to 3.8) | [Change from baseline in total Sharp score (0.5)] 39% |
| TCZ (n = 157) | 52 weeks | 2.3 (95% CI 1.5 to 3.2)b | Mean 0.9 (95% CI 0.3 to 1.4)c | Mean 1.5 (95% CI 0.9 to 2.1)c | 56%b |
| Trial name/study | Treatment arms for which data extraction performed | Assessment point | Mean change from baseline in synovitis | Mean change from baseline in erosions | Mean change from baseline in osteitis |
|---|---|---|---|---|---|
| ASSET72 | PBO + MTX (n = 23) | 4 months | (OMERACT-RAMRIS scores) Adjusted mean change (wrists) = 0.38 (SE 0.27) |
(OMERACT-RAMRIS scores) Adjusted mean change (wrist and hand) = 0.95 (SE 0.45) |
(OMERACT-RAMRIS scores) Adjusted mean change (wrist and hand) = 1.54 (SE 0.90) |
| ABT i.v. + MTX (n = 25) | 4 months | Adjusted mean change (wrists) = –0.31 (SE 0.26) | Adjusted mean change (wrist and hand) = 0.45 (SE 0.43) | Adjusted mean change (wrist and hand) = –1.94 (SE 0.86) | |
| GO-FORWARD153 | PBO + MTX (n = 72) | 24 weeks | RAMRIS synovitis (wrist + MCP) –0.38 (2.66) –0.50 (IQR –1.45 to 1.00)a RAMRIS synovitis (wrist) 0.08 (1.51) 0.00 (IQR –1.00 to 1.00)a |
RAMRIS bone erosion score –0.47 (3.40) 0.00 (IQR –0.50 to 0.00)a |
RAMRIS bone oedema (osteitis) score 0.71 (7.54) 0.00 (IQR –0.50 to 0.50)a |
| GOL + MTX (n = 47) | 24 weeks | RAMRIS synovitis (wrist + MCP) –1.85 (2.28) –1.75 (–3.00, –0.50)a,b RAMRIS synovitis (wrist) –1.13 (1.61) 1.00 (IQR –2.00 to 0.00)a,b |
RAMRIS bone erosion score –1.08 (4.35) 0.00 (–0.50, 0.00)a |
RAMRIS bone oedema (osteitis) score Mean = –2.58 (SD 4.75) –0.50 (IQR –4.09 to 0.00)a,b |
|
| Durez et al., 2007120 | MTX (n = 14) | 52 weeks | [OMERACT-RAMRIS scores. Global synovitis score ranged from 0 (absence of synovitis) to 66 (severe synovitis)] (Mean change NR) Score at baseline = 21 (15, 33)c,d Score at follow-up = 20 (12, 24)c,d |
[OMERACT-RAMRIS scores. 0 (no erosion) to 300 (100% bone eroded)] (Mean change NR) Score at baseline = 12 (8, 25)c,d Score at follow-up = 14 (9, 32)c,d |
(OMERACT-RAMRIS scores) (Mean change NR) Score at baseline = 13 (10, 31)d Score at follow-up = 13 (5, 21)d |
| MTX + i.v. MP (n = 15 randomised) | 52 weeks | Score at baseline = 29 (17, 33)c,d Score at follow-up = 14 (7, 29)c,d |
Score at baseline = 5 (3, 23)c,d Score at follow-up = 13 (5, 41)c,d |
Score at baseline = 22 (7, 40)c,d Score at follow-up = 12 (6, 38)c,d |
|
| IFX + MTX (n = 15 randomised) | 52 weeks | Score at baseline = 25 (15, 29)c,d Score at follow-up = 10 (6, 12)c,d |
Score at baseline = 9 (5, 11)c,d Score at follow-up = 11 (6, 21)c,d |
Score at baseline = 25 (12, 32)c,d Score at follow-up = 11 (7, 16)c,d |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | n analysed | Mean pain VAS score at baseline, 0–100 (SD) | Mean pain VAS score at follow-up, 0–100 (SD) | Pain VAS mean change from baseline (SD) | Mean % change from baseline |
|---|---|---|---|---|---|---|---|
| OPERA107 | MTX + PBO + steroid | 12 months (primary end point and study RCT end point) | 91 | 58 (13–92)a,b | 20 (0–71)a | NR | NR |
| OPERA107 | ADA + MTX + steroid | 12 months (primary end point and study RCT end point) | 89 | 63 (13–98)a,b | 7 (0–64)a,c | NR | NR |
| OPTIMA154 | MTX + PBO | 26 weeks (study RCT end point) | 517 | 65 (21) | 49.4 (NR) | –37.9 (28.61) (n = 513) | NR |
| OPTIMA108 | ADA + MTX | 26 weeks (study RCT end point) | 515 | 65 (21) | 36.1 (NR) | –28.9 (26.61) (n = 513) | NR |
| PREMIER109 | MTX + PBO | 1 year (primary end point) | 256 | 59.6 (24.3) | 23.4 (16.1) | –36.2 (NR) | NR |
| PREMIER109 | ADA monotherapy + PBO step-up week 16 | 1 year (primary end point) | 273 | 64.6 (23.6) | 26.6 (17.1) | –38.0 (NR) | NR |
| PREMIER109 | ADA + MTX step-up week 16 | 1 year (primary end point) | 265 | 62.5 (21.3) | 16.8 (15.7)b,d (vs. MTX) | –45.7 (NR) | NR |
| PREMIER109 | MTX + PBO | 2 years (study RCT end point) | 256 | 59.6 (24.3) | 12.5 (15.8) | –47.1 (NR) | NR |
| PREMIER109 | ADA monotherapy + PBO step up-week 16 | 2 years (study RCT end point) | 273 | 64.6 (23.6) | 19.6 (16.6) | –45.0 (NR) | NR |
| PREMIER109 | ADA + MTX step-up week 16 | 2 years (study RCT end point) | 265 | 62.5 (21.3) | 9.6 (14.9)b,d (vs. MTX) | –52.9 (NR) | NR |
| COMET83 | MTX + PBO | Week 52 | 263 | 65.1 (20.8) | 33.7 (27.5) | –31.4 | NR |
| COMET81 | ETN + MTX | Week 52 | 265 | 66.0 (21.4) | 24.1(24.2) | –41.9b | NR |
| GO-BEFORE90 | PBO + MTX | 24 weeks | 160 | (0–10 scale); 6.3 (2.12) | NR | NR | 44.35a,e |
| GO-BEFORE90 | GOL 50 mg s.c. every 4 weeks + MTX | 24 weeks | 159 | (0–10 scale); 6.4 (2.11) | NR | NR | 52.15a,c,e |
| BeST148 | Sequential monotherapy (DAS steered) | 6 months | NR | 53.1 (NR) | 35.7 (NR) | –17.4 | NR |
| BeST78 | Step–up combination therapy (DAS steered) | 6 months | NR | 53.4 (NR) | 27.9 (NR) | –25.5 | NR |
| BeST78 | Initial combination therapy with prednisone (DAS steered) | 6 months | NR | 54.1 (NR) | 23.8 (NR) | –30.3c | NR |
| BeST78 | Initial combination therapy with IFX (DAS steered) | 6 months | NR | 54.1 (NR) | 23.9 (NR) | –30.2c | NR |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | n analysed | Mean pain VAS score at baseline, 0–100 (SD) | Mean pain VAS score at follow-up, 0–100 (SD) | Pain VAS mean change from baseline (SD) | Mean % change from baseline |
|---|---|---|---|---|---|---|---|
| AMPLE66 | ABT s.c. | 1 year (primary end point) | 318 | 63.1 (22.3) | NR | NR | 53 |
| AMPLE144 | ADA | 1 year (primary end point) | 328 | 65.5 (21.8) | NR | NR | 39.2 |
| deFilippis et al., 200685 | ETN + MTX | 22 weeks | 15 | 60.67 (16.57) | NR | NR | 28.6 |
| deFilippis et al., 200685 | IFX + MTX | 22 weeks | 15 | 70.10 (14.14) | NR | NR | 22 |
| deFilippis et al., 200685 | ETN + MTX | 54 weeks | 15 | 60.67 (16.57) | 77.54 | 16.87 (NR) | 43.06 |
| deFilippis et al., 200685 | IFX + MTX | 54 weeks | 15 | 70.10 (14.14) | 87.75 | 17.65 (NR) | 21.1 |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | n analysed | Mean Pain VAS score at baseline, 0–100 (SD) | Mean Pain VAS score at follow-up, 0–100 (SD) | Pain VAS mean change from baseline (SD) | Mean % change from baseline |
|---|---|---|---|---|---|---|---|
| AIM61,62 | MTX + PBO | 12 months | 219 | 65.9 (20.6) | NR | Adjusted –24.2 (1.72) | NR |
| AIM61 | ABT i.v. + MTX | 12 months | 433 | 63.3 (21.1) | NR | Adjusted –35.8 (1.12) | NR |
| ASSURE73 | PBO + cDMARDs | 1 year (primary end point and study RCT end point) | 413 | 61.3 (20.8) (n = 418) | NR | NR | 18 |
| ASSURE73 | ABT + cDMARDs | 1 year (primary end point and study RCT end point) | 845 | 61.1 (20.4) (n = 856) | NR | NR | 37 |
| CHANGE80 | PBO (n = 87) | 24 weeks | 87 | 62.7 (22.8) | 66.2 (NR) | 3.5 (25.4) | NR |
| CHANGE80 | ADA monotherapy (n = 91) | 24 weeks | 91 | 68.1 (21) | 50.7 (NR) | –17.4 (27.9)a | NR |
| DE01984 | MTX + PBO (n = 200) | 52 weeks | 200 | 56.3 (22.9) | 45.1 (NR) | –11.2 (27.7) | –19.9 |
| DE01984 | ADA + MTX (n = 207) | 52 weeks | 207 | 55.9 (20.4) | 26.5 (NR) | –29.4 (26.4) | –52.6 |
| Van De Putte et al., 2004122 | PBO s.c. | 26 weeks | 110 | 70.2 (18.1) | 59.2 (NR) | –11.0 (26.7) | –11.4 |
| Van De Putte et al., 2004122 | ADA 40 mg s.c. every other week monotherapy | 26 weeks | 113 | 70.3 (19.9) | 42.7 (NR) | –27.6 (31.1) b (vs. PBO) | –37.7 b (vs. PBO) |
| ARMADA69,70 | MTX + PBO (n = 62) | 24 weeks | 62 | 57.2 (21) | 48.6 (NR) | –8.6 (22.5) | –15.0 |
| ARMADA69 | ADA + MTX (n = 67) | 24 weeks | 67 | 53 (22) | 27.9 (NR) | –25.1 (33.1) | –47.2b |
| Kim et al., 200799 | MTX + PBO rescue week18 (n = 63) | 24 weeks | 63 | 59.4 (18.6) | 52.1 (NR) | –7.3 (27.5) | NR |
| Kim et al., 200799 | ADA + MTX (n = 65; n = 64 at 24 weeks) | 24 weeks | 64 | 57.6 (18.2) | 33.9 (NR) | –23.7 (22.86)b | NR |
| CERTAIN156 | PBO + cDMARDs | 24 weeks (primary end point and study RCT end point) | 98 | NR | NR | (AiC information has been removed) | NR |
| CERTAIN79 | CTZ 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks + DMARDs | 24 weeks (primary end point and study RCT end point) | 96 | NR | NR | (AiC information has been removed) | NR |
| ADORE59,60 | ETN monotherapy (n = 159) | 16 weeks | 140 | 62.7 | 33.3 (NR) | –29.40 (25.09) | NR |
| ADORE59 | ETN + MTX (n = 155) | 16 weeks | 135 | 63.3 | 33.37 (NR) | –29.93 (27.25) | NR |
| ETN Study 309/Combe et al., 2006,88 Combe et al., 200989 | SSZ + PBO (n = 50) | 24 weeks | 50 | 58.8 (20) | NR | NR | 13.3 |
| ETN Study 309/Combe et al., 2006,88 Combe et al., 200989 | ETN + PBO (n = 103) | 24 weeks | 103 | 62.6 (21.7) | NR | NR | 55.6b vs. SSZ |
| ETN Study 309/Combe et al., 200688,89 | ETN + SSZ (n = 101) | 24 weeks | 101 | 58.5 (20.7) | NR | NR | 53.9b vs. SSZ Non-significant vs. ETN + PBO |
| JESMR140 | ETN 25 mg every 2 weeks monotherapy | 24 weeks (primary end point) | 69 | NR | NR | NR | NR |
| JESMR140 | ETN 25 mg every 2 weeks + MTX 6–8 mg/week | 24 weeks (primary end point) | 73 | NR | NR | NR | NR |
| Lan et al., 2004101 | PBO + MTX | 12 weeks (primary end point and study RCT end point) | 29 | 57.52 | 57.59 | 0.07 (NR) | 0.05 |
| Lan et al., 2004101 | ETN + MTX | 12 weeks (primary end point and study RCT end point) | 29 | 55.21 | 31.66a | –23.55 (NR) | 43 |
| Moreland et al., 1999104 | PBO | 6 months | 80 | (0–10 scale) 6.5 |
NR | NR | 22 (worse) |
| Moreland et al., 1999104 | ETN + PBO | 6 months | 78 | (0–10 scale) 6.7 |
NR | NR | –53 (improved)b |
| RACAT111 | MTX + SSZ + HCQ (n = 178; not all analysed) | 24 weeks | 319 both groups | 5.64 (2.21) | 3.64 (2.38) | –2.0 (NR) | NR |
| RACAT111 | ETN50 + MTX (n = 175; not all analysed) | 24 weeks | 5.88 (1.99) | 3.56 (2.53) | –2.32 (NR) | NR | |
| RACAT111 | MTX + SSZ + HCQ (n = 178 randomised) In analysis n = 155 (of whom 39 switched to ETN) |
48 weeks | 155 | NR | 3.22 (2.37) | NR | NR |
| RACAT111 | ETN50 + MTX (n = 175 randomised) In analysis n = 155 (of whom 41 switched to MTX + SSZ + HCQ) |
48 weeks | 155 | NR | 3.17 (2.58) | NR | NR |
| Weinblatt et al. 1999124 | MTX + PBO | 24 weeks | 30 | (0–10 scale) 5.6c |
(0–10 scale) 4.4c |
–1.2 (NR) | NR |
| Weinblatt et al. 1999124 | ETN + MTX (n = 59) | 24 weeks | 59 | (0–10 scale) 5.0c |
(0–10 scale) 1.8b,c |
–3.2 (NR) | NR |
| APPEAL67,68 | MTX + DMARD (SSZ, HCQ or LEF) | 16 weeks (primary end point and study RCT end point) | 103 | 60.8 (19.2) | 38.6 | –22.2 (NR) | 36.5 |
| APPEAL68 | ETN 25 mg twice weekly (licensed dose) + MTX | 16 weeks (primary end point and study RCT end point) | 197 | 62.5 (23.4) | 28.5b | –34.0 (NR) | 54.4b |
| GO-FORWARD92 | PBO s.c. every 4 weeks + MTX | Week 14 | 133 | (0–10 scale) 5.70 (3.60, 7.50)c | NR | NR | 17.6 (–8.1, 40.0)c |
| GO-FORWARD92 | GOL 50 mg s.c. every 4 weeks + MTX | Week 14 | 89 | (0–10 scale) 6.10 (4.70, 7.70)c | NR | NR | 55.0 (17.0, 76.5)b,c |
| GO FORWARD92 | PBO s.c. every 4 weeks + MTX | Week 24 | 133 | (0–10 scale) 5.70 (3.60, 7.50)c | NR | NR | 15.4 (–16.4, 41.6)c |
| GO FORWARD92 | GOL 50 mg s.c. every 4 weeks + MTX | Week 24 | 89 | (0–10 scale) 6.10 (4.70, 7.70)c | NR | NR | 50.4 (16.3, 83.3)b.c |
| ATTRACT75 | PBO i.v. + MTX | 30 weeks | 88 | (0–10 scale) 6.7 (5.0, 8.0)c |
(0–10 scale) 5.9 (3.3, 7.4)c |
–0.8 (NR) | –6d |
| ATTRACT75 | IFX 3 mg/kg i.v. at weeks 0, 2 and 6 and every 8 weeks thereafter | 30 weeks | 86 | (0–10 scale) 7.0 (5.6, 8.1)c |
(0–10 scale) 3.8 (2.3, 6.9)c |
–3.2 (NR) | –33a,d |
| START118 | PBO + MTX | 22 weeks (primary end point and study RCT end point) | 363 | (0–10 scale) 5.9 (5–7)e |
NR | NR | NR |
| START118 | IFX 3 mg/kg + MTX | 22 weeks (primary end point and study RCT end point) | 360 | (0–10 scale) 6.1 (5–8)e |
NR | NR | NR |
| ACT-RAY57 | TCZ 8 mg/kg i.v. every 4 weeks + oral PBO | Week 24 | 276 | NR | NR | –29.8 (24.92) | NR |
| ACT-RAY57 | TCZ 8 mg/kg i.v. every 4 weeks + MTX | Week 24 | 277 | NR | NR | –29.3 (26.64) | NR |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean score at baseline | Mean score at follow-up | Mean change from baseline |
|---|---|---|---|---|---|
| COMET83 | MTX | 52 weeks | NR | NR | –19.7 |
| ETN + MTX | 52 weeks | NR | NR | –29.6a |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean (SD) score at baseline | Mean (SD) score at follow-up | Mean (SD) change from baseline | % change from baseline |
|---|---|---|---|---|---|---|
| OPTIMA154 | MTX + PBO | 26 weeks | NR | NR | 8.3 (11.12) | NR |
| ADA + MTX | 26 weeks | NR | NR | 10.5 (11.82)a | NR | |
| PREMIER155 | MTX + PBO | 1 year | 29.0 (11.1) | 40.0 (8.10) | 11.0 (NR) | NR |
| ADA monotherapy + PBO step-up week 16 | 1 year | 26.2 (11.3)a,b (vs. MTX) | 38.6 (8.0) | 12.4 (NR) | NR | |
| ADA + MTX step-up week 16 | 1 year | 28.4 (11.7) | 41.1 (8.2)b,c (vs. MTX) | 12.7 (NR) | NR | |
| PREMIER155 | MTX + PBO | 2 years | 29.0 (11.1) | 42.5 (8.1) | 13.5 (NR) | NR |
| ADA monotherapy + PBO step-up week 16 | 2 years | 26.2 (11.3)a,b (vs. MTX) | 40.8 (8.1) | 14.6 (NR) | NR | |
| ADA + MTX step-up week 16 | 2 years | 28.4 (11.7) | 43.0 (8.1)b,c (vs. MTX) | 14.6 (NR) | NR |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean score at baseline | Mean score at follow-up | Mean change from baseline |
|---|---|---|---|---|---|
| AMPLE144 | ABT s.c. + MTX | 1 year | NR | NR | –23.2 |
| ADA + MTX | 1 year | NR | NR | –23.2 |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean score at baseline | Mean score at follow-up | Mean change from baseline | % change from baseline |
|---|---|---|---|---|---|---|
| ADACTA58 | TCZ 8 mg/kg i.v. every 4 weeks + s.c. PBO ADA | 24 weeks | NR | NR | 8.9a | NR |
| ADA 40 mg s.c. every 2 weeks + i.v. PBO TCZ | 24 weeks | NR | NR | 11.4a | NR |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean score at baseline | Mean score at follow-up | Mean change from baseline |
|---|---|---|---|---|---|
| AIM63 | MTX + PBO | 1 year | 63.5 | 40.9 (NR) | –22.6 |
| ABT + PBO | 1 year | 65.3 | 37.3 (NR) | –28.0a |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean (SD) score at baseline | Mean (SD) score at follow-up | Mean (SD) change from baseline | % change from baseline |
|---|---|---|---|---|---|---|
| ARMADA69,70 | MTX + PBO | 24 weeks | NR | NR | 3.0 improvement | NR |
| ADA + MTX | 24 weeks | NR | NR | 8.5a improvement | NR | |
| APPEAL68 | MTX + DMARD (SSZ, HCQ or LEF) | 16 weeks | 30.1 | 33.2 | 3.1 (NR) | 10.4 |
| ETN + MTX | 16 weeks | 28.1 | 36.2a | 8.1 (NR) | 28.0a | |
| GO-FORWARD92 | PBO + MTX | Week 24 | 28.7 (10.5) | 30.86 (NR) | 2.16 (9.53) | NR |
| GOL 50 mg + MTX | Week 24 | 26.6 (11.0) | 33.9 (NR) | 7.30 (8.65)b | NR | |
| TOWARD121 | PBO + cDMARDs | 24 weeks | NR | NR | 3.6 | NR |
| TCZ 8 mg/kg i.v. + DMARDs | 24 weeks | NR | NR | 8.0b | NR |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean (SD) PCS at baseline | Mean (SD) PCS at follow-up | Mean (SD) change from baseline in PCS | Mean (SD) MCS at baseline | Mean (SD) MCS at follow-up | Mean (SD) change from baseline in MCS | Mean (SD) ASHI score at baseline | Mean (SD) ASHI at follow-up | Mean (SD) change from baseline in ASHI score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HIT HARD94 | MTX + PBO | 24 weeks | 31.7 (8.3) | 39.8 (9.9) | 8.1 (NR) | 45.2 (10.2) | 48.9 (8.8) | 3.7 (NR) | NR | NR | NR |
| ADA + MTX | 24 weeks | 28.3 (7.7)a | 44.0 (11.1)b | 15.7 (NR) | 46.7 (9.9) | 48.8 (9.8) | 2.1 (NR) | NR | NR | NR | |
| PREMIER155 | MTX + PBO | 1 year | 32.2 (7.9) | 43.5 (8.1) | 11.3 (NR) | 43.5 (12.4) | 51.3 (8.5) | 7.8 (NR) | NR | NR | NR |
| ADA monotherapy + PBO step-up week 16 | 1 year | 30.7 (7.4) | 42.5 (7.9) | 11.8 (NR) | 42.6 (12.1) | 49.1 (8.2)a,c | 6.5 (NR) | NR | NR | NR | |
| ADA + MTX step-up week 16 | 1 year | 31.7 (7.8) | 46.6 (8.2)b,c (vs. MTX) | 14.9 (NR) | 44.1 (12.5) | 50.7 (8.7) | 6.6 (NR) | NR | NR | NR | |
| PREMIER155 | MTX + PBO | 2 years | 32.2 (7.9) | 45.9 (7.8) | 13.7 (NR) | 43.5 (12.4) | 52.4 (8.4) | 8.9 (NR) | NR | NR | NR |
| ADA monotherapy + PBO step-up week 16 | 2 years | 30.7 (7.4) | 44.7 (8.0) | 14 (NR) | 42.6 (12.1) | 49.8 (8.1)a,c (vs. MTX) | 7.2 (NR) | NR | NR | NR | |
| ADA + MTX step-up week 16 | 2 years | 31.7 (7.8) | 48.8 (8.3)b,c (vs. MTX) | 17.1 (NR) | 44.1 (12.5) | 51.8 (8.8) | 7.7 (NR) | NR | NR | NR | |
| COMET83 | MTX | 52 weeks | NR | NR | 10.7 | NR | NR | 6.1 | NR | NR | NR |
| ETN + MTX | 52 weeks | NR | NR | 13.7a | NR | NR | 6.8 | NR | NR | NR | |
| ERA157 | MTX + PBO | 52 weeks | NR | NR | 9.6 (0.8)d | NR | NR | 4.1 (0.8)d | NR | NR | 8.1 (1.0)d |
| ETN 25 mg every 2 weeks + PBO | 52 weeks | NR | NR | 10.7 (0.8)d | NR | NR | 3.6 (0.8)d | NR | NR | 8.2 (1.0)a,d | |
| ASPIRE71 | PBO i.v. + MTX | 54 weeks | NR | NR | 10.1 (11.4) | NR | NR | NR | NR | NR | NR |
| IFX i.v. + MTX | 54 weeks | NR | NR | 11.7 (11.6) | NR | NR | NR | NR | NR | NR | |
| BeST78 | Sequential monotherapy | 6 months | NR | NR | 8.0b (vs. combination + prednisone and combination + IFX) | NR | NR | 3.1 | NR | NR | NR |
| Step-up combination therapy | 6 months | NR | NR | 8.5b (vs. combination + prednisone and combination + IFX) | NR | NR | 3.5 | NR | NR | NR | |
| Initial combination therapy with prednisone | 6 months | NR | NR | 12.5 | NR | NR | 1.2 | NR | NR | NR | |
| Initial combination therapy with IFX | 6 months | NR | NR | 12.4 | NR | NR | 4.1 | NR | NR | NR |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean (SD) PF score at baseline | Mean (SD) PF score at follow-up | Mean (SD) RP score at baseline | Mean (SD) RP score at follow-up | Mean (SD) BP score at baseline | Mean (SD) BP score at follow-up | Mean (SD) GH score at baseline | Mean (SD) GH score at follow-up | Mean (SD) VT score at baseline | Mean (SD) VT score at follow-up | Mean (SD) SF score at baseline | Mean (SD) SF score at follow-up | Mean (SD) RE score at baseline | Mean (SD) RE score at follow-up | Mean (SD) MH score at baseline | Mean (SD) MH score at follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PREMIER155 | MTX + PBO | 1 year | 31.5 (10.3) | 41.8 (9.7) | 32.6 (8.4) | 44.1 (8.9) | 32.7 (7.7) | 46.5 (7.3) | 40.5 (9.1) | 46.4 (8.2) | 40.6 (9.7) | 51.8 (8.7) | 38.1 (12.2) | 47.9 (7.8) | 36.7 (13.8) | 46.2 (8.6) | 42.6 (12.1) | 50.0 (9.0) |
| ADA monotherapy + PBO step-up week 16 | 1 year | 29.1 (9.5) | 40.5 (9.0) | 32.5 (8.1) | 43.3 (8.0) | 31.6 (7.8) | 44.9 (6.9)a,b | 39.8 (9.6) | 45.4 (7.9)a,b | 39.2 (9.4) | 49.6 (8.3)a,b | 35.2 (12.2) | 45.9 (7.4)a,b | 37.5 (13.9) | 44.5 (7.9)a,b | 41.4 (11.9) | 48.0 (8.7) | |
| ADA + MTX step-up week 16 | 1 year | 30.2 (10.0) | 44.7 (9.2)b,c | 33.1 (8.8) | 46.6 (8.2)b,c | 32.5 (7.1) | 49.7 (7.3)b,c | 40.9 (10.0) | 48.2 (8.2) | 40.0 (10.0) | 52.9 (8.8)a,b | 38.3 (12.0) | 48.7 (7.4) | 38.4 (14.1) | 47.3 (8.1) | 42.1 (12.2) | 49.9 (8.8) | |
| PREMIER155 | MTX + PBO | 2 years | 31.5 (10.3) | 44.3 (9.3) | 32.6 (8.4) | 46.5 (8.6) | 32.7 (7.7) | 48.8 (7.1) | 40.5 (9.1) | 47.2 (8.2) | 40.6 (9.7) | 53.7 (8.5) | 38.1 (12.2) | 49.2 (7.6) | 36.7 (13.8) | 48.1 (8.0) | 42.6 (12.1) | 51.1 (9.3) |
| ADA monotherapy + PBO step-up week 16 | 2 years | 29.1 (9.5) | 43.0 (9.1) | 32.5 (8.1) | 45.5. (8.0) | 31.6 (7.8) | 47.1 (6.9)a,b | 39.8 (9.6) | 46.7 (8.1)a,b | 39.2 (9.4) | 51.4 (8.4)a,b | 35.2 (12.2) | 48.0 (7.6)a,b | 37.5 (13.9) | 45.8 (7.9)a,b | 41,4 (11.9) | 49.2 (8.7) | |
| ADA + MTX step-up week 16 | 2 years | 30.2 (10.0) | 46.9 (9.2)b,c | 33.1 (8.8) | 48.8 (8.2)b,c | 32.5 (7.1) | 51.8 (7.2)b,c | 40.9 (10.0) | 49.5 (8.3) | 40.0 (10.0) | 54.7 (9.0)a,b | 38.3 (12.0) | 49.9 (7.4) | 38.4 (14.1) | 49.1 (7.8) | 42.1 (12.2) | 51.1 (8.7) |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean (SD) change from baseline in PF score | Mean (SD) change from baseline in RP score | Mean (SD) change from baseline in BP score | Mean (SD) change from baseline in GH score | Mean (SD) change from baseline in VT score | Mean (SD) change from baseline in SF score | Mean (SD) change from baseline in RE score | Mean (SD) change from baseline in MH score |
|---|---|---|---|---|---|---|---|---|---|---|
| ERA157 | MTX + PBO | 52 weeks | 10.4 (0.8) | 9.9 (0.9) | 10.1 (0.7) | 3.4 (0.7) | 6.8 (0.8) | 8.1 (0.9) | 4.7 (1.0) | 5.8 (0.8) |
| ETN 25 mg every 2 weeks + PBO | 52 weeks | 9.7 (0.8) | 10.8 (0.9) | 10.5 (0.8) | 4.5 (0.7) | 7.9 (0.8) | 8.4 (0.9) | 4.0 (1.1) | 4.4 (0.8) |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean (SD) PCS at baseline (0–100) | Mean (SD) PCS at follow-up (0–100) | Mean (SD) change from baseline in PCS (0–100) | Mean (SD) MCS at baseline (0–100) | Mean (SD) MCS at follow-up (0–100) | Mean (SD) change from baseline in MCS (0–100) |
|---|---|---|---|---|---|---|---|---|
| OPERA107 | MTX + PBO + steroid | 12 months | 31.7 (19.3, 44.5)a | 43.3 (26.1, 55.8)a | 10.6 (–11.26, 22.7)a | 46.7 (25.7, 60.1)a | 54.8 (40.4, 65.7)a | 4.3 (–9.3, 27.4)a |
| ADA + MTX + steroid | 12 months | 30.9 (13.1, 50.6)a | 49.2 (29.9, 56.6)a,b | 13.2 (–2.3, 33.0)a,b | 47.0 (28.6, 60.6)a | 55.7 (35.8, 62.6)a | 5.5 (–8.5, 20.1)a |
| Trial name/study | Treatment arms for which data extraction performed | Assessment point | Mean (SD) SF-6D score at baseline | Mean (SD) SF-6D score at follow-up | Mean (SD) change from baseline in RAQoL score | % change from baseline in RAQoL score |
|---|---|---|---|---|---|---|
| Bejarano et al., 200877 | PBO + MTX | 56 weeks | NR | NR | –4.7 (8.4) | NR |
| ADA + MTX | 56 weeks | NR | NR | –7.6 (7.4)a | NR | |
| PREMIER155 | MTX + PBO | 1 year | 0.56 (0.11) | 0.72 (0.14) | NR | NR |
| ADA monotherapy + PBO step-up week 16 | 1 year | 0.54 (0.11) | 0.70 (0.14)a,b | NR | NR | |
| ADA + MTX step-up week 16 | 1 year | 0.45 (0.11) | 0.75 (0.13)a,b | NR | NR | |
| PREMIER155 | MTX + PBO | 2 years | 0.56 (0.11) | 0.73 (0.14) | NR | NR |
| ADA monotherapy + PBO step-up week 16 | 2 years | 0.54 (0.11) | 0.70 (0.13)a,b | NR | NR | |
| ADA + MTX step-up week 16 | 2 years | 0.45 (0.11) | 0.76 (0.14)a,b | NR | NR | |
| Quinn et al., 2005110 | MTX + PBO | 14 weeks | NR | NR | NR | 7b (worse) |
| IFX 3 mg/kg + MTX | 14 weeks | NR | NR | NR | –74a,b (improved) | |
| Quinn et al., 2005110 | MTX + PBO | 54 weeks | NR | NR | NR | 0b |
| IFX 3 mg/kg + MTX | 54 weeks | NR | NR | NR | –82a,b (improved) |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean EQ-5D score at baseline (0–1) | Mean EQ-5D score at follow-up (0–1) | Mean (SD) change from baseline in EQ-5D score (0–1) |
|---|---|---|---|---|---|
| OPERA107 | MTX + PBO + steroid | 12 months | 0.64 (0.22, 0.80)a | 0.78 (0.49, 1.00)a | 0.20 (–0.06, 0.56)a |
| ADA + MTX + steroid | 12 months | 0.61 (0.17, 0.80)a | 0.82 (0.38, 1.00)a,b | 0.22 (–0.05, 0.67)a | |
| BeST158 | Sequential monotherapy | 6 months | 0.5c | 0.65c | –0.15 (NR) |
| Step-up combination therapy | 6 months | 0.5c | 0.6c | –0.1 (NR) | |
| Initial combination therapy with prednisone | 6 months | 0.5c | 0.75c | –0.25 (NR) | |
| Initial combination therapy with IFX | 6 months | 0.5c | 0.8c | –0.03 (NR) |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean PCS at baseline | Mean PCS at follow-up | Mean change from baseline in PCS | Mean MCS at baseline | Mean MCS at follow-up | Mean change from baseline in MCS |
|---|---|---|---|---|---|---|---|---|
| ATTEST74 | PBO + MTX | Day 197 | NR | NR | 4a | NR | NR | 1a |
| IFX + MTX | Day 197 | NR | NR | 7a | NR | NR | 4a | |
| ABT + MTX | Day 197 | NR | NR | 8a | NR | NR | 5a | |
| AMPLE144 | ABT s.c. + MTX | 1 year | NR | NR | 9.37 | NR | NR | 3.92 |
| ADA + MTX | 1 year | NR | NR | 8.84 | NR | NR | 3.62 | |
| ADACTA58 | TCZ 8 mg/kg i.v. every 4 weeks + s.c. PBO ADA | 24 weeks | NR | NR | 9.2 | NR | NR | 7.9 |
| ADA 40 mg s.c. every 2 weeks + i.v. PBO TCZ | 24 weeks | NR | NR | 7.6 | NR | NR | 5.0b |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean change from baseline in PF score | Mean change from baseline in RP score | Mean change from baseline in BP score | Mean change from baseline in GH score | Mean change from baseline in VT score | Mean change from baseline in SF score | Mean change from baseline in RE score | Mean change from baseline in MH score |
|---|---|---|---|---|---|---|---|---|---|---|
| AMPLE66 | ABT s.c. + MTX | 1 year | 7.92 | 8.87 | 10.67 | 5.44 | 5.84 | 7.33 | 6 | 4.21 |
| ADA + MTX | 1 year | 7.81 | 7.91 | 10.65 | 5.26 | 5.51 | 6.5 | 5.84 | 3.86 |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean EQ-5D score at baseline (0–1) | Mean (SD) EQ-5D score at follow-up (0–1) | Mean (SD) change from baseline in EQ-5D score (0–1) |
|---|---|---|---|---|---|
| RED-SEA114 | ADA (n = 60) | 12 months | 0.52 (0.06–0.66)a | 0.59 (0.52–0.69) | 0.07 (NR) |
| ETN (n = 60) | 12 months | 0.52 (0.06–0.69)a | 0.59 (0.24–0.73) | 0.07 (NR) |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean (SD) PCS at baseline | Mean (SD) PCS at follow-up | Mean (SD) change from baseline in PCS | % change from baseline in PCS | Mean (SD) MCS at baseline | Mean (SD) MCS at follow-up | Mean (SD) change from baseline in MCS | Mean % change from baseline in MCS |
|---|---|---|---|---|---|---|---|---|---|---|
| AIM61,62 | MTX + PBO | 1 year | 30.7 (7.5) | 35a | 4.3 (NR) | NR | 40.8 (11.2) | 46a | 5.2 (NR) | NR |
| ABT + PBO | 1 year | 30.6 (7.3) | 40a,b | 9.4 (NR) | NR | 41.8 (11.4) | 49a,c | 7.2 (NR) | NR | |
| CERTAIN79 ClinicalTrials.gov (NCT00674362) |
PBO + cDMARDs | 24 weeks | NR | NR | 1.7 (5.6) | NR | NR | NR | 0.5 (9.6) | NR |
| CTZ + cDMARDs | 24 weeks | NR | NR | 6.0 (7.50) | NR | NR | NR | 4.0 (9.77) | NR | |
| APPEAL68 | MTX + DMARD (SSZ, HCQ or LEF) | 16 weeks | 30.1 | 37.3 | 7.2 (NR) | 22.8 improvement | 42.4 | 47.8 | 5.4 (NR) | 13.3 improvement |
| ETN + MTX | 16 weeks | 30.5 | 40.4b | 9.9 (NR) | 31.4b improvement | 42.9 | 50.2c | 7.3 (NR) | 17.5c improvement | |
| GO-FORWARD92 | PBO + MTX | Week 24 | NR | NR | 2.54 (8.06) improvement | NR | NR | NR | 0.75 (9.68) improvement | NR |
| GOL 50 mg + MTX | Week 24 | NR | NR | 8.28 (8.33)b improvement | NR | NR | NR | 1.83 (10.87) improvement | NR | |
| ATTRACT159 | PBO i.v. + MTX | 54 week | NR | NR | NR | NR | NR | NR | NR | 9 improvement |
| IFX i.v. monotherapy | 54 week | NR | NR | NR | NR | NR | NR | NR | 34b improvement | |
| TOWARD121 | PBO + cDMARDs | 24 weeks | NR | NR | 4.1 improvement | NR | NR | NR | 2.3 improvement | NR |
| TCZ 8 mg/kg i.v. + DMARDs | 24 weeks | NR | NR | 8.9b improvement | NR | NR | NR | 5.3b improvement | NR | |
| TACIT141 | Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | NR |
| TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | NR |
| Trial name/study | Treatment arms for which data extraction performed | Assessment point | Mean (SD) PF score at baseline | Mean (SD) PF score at follow-up | Mean (SD) RP score at baseline | Mean (SD) RP score at follow-up | Mean (SD) BP score at baseline | Mean (SD) BP score at follow-up | Mean (SD) GH score at baseline | Mean (SD) GH score at follow-up | Mean (SD) VT score at baseline | Mean (SD) VT score at follow-up | Mean (SD) SF score at baseline | Mean (SD) SF score at follow-up | Mean (SD) RE score at baseline | Mean (SD) RE score at follow-up | Mean (SD) MH score at baseline | Mean (SD) MH score at follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Durez et al., 200486 | MP + MTX | 14 weeks | 27 (26) | 24 (26) | 13 (28) | 35 (41) | 26 (16) | 32 (24) | 26 (19) | 29 (22) | 27 (20) | 29 (22) | 44 (16) | 40 (25) | 22 (39) | 39 (47) | 45 (21) | 45 (22) |
| IFX + MTX | 14 weeks | 36 (22) | 55 (23)a | 42 (48) | 45 (42) | 35 (23) | 52 (16) | 40 (16) | 50 (16)a | 31 (25) | 45 (20) | 53 (30) | 66 (22)a | 58 (47) | 67 (42) | 52 (25) | 60 (23) | |
| TACIT141 | Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean (SD) change from baseline in PF score | Mean (SD) change from baseline in RP score | Mean (SD) change from baseline in BP score | Mean (SD) change from baseline in GH score | Mean (SD) change from baseline in VT score | Mean (SD) change from baseline in SF score | Mean (SD) change from baseline in RE score | Mean (SD) change from baseline in MH score |
|---|---|---|---|---|---|---|---|---|---|---|
| CERTAIN79 ClinicalTrials.gov (NCT00674362) |
PBO + cDMARDs | 24 weeks | 0.4 (8.90) | 1.7 (7.81) | 2.8 (8.50) | 0.9 (8.06) | 0.6 (8.41) | 0.8 (8.89) | –0.2 (12.33) | 1.2 (7.72) |
| CTZ + cDMARDs | 24 weeks | 5.1 (7.36) | 4.7 (9.77) | 8.0 (8.70) | 5.0 (7.59) | 6.4 (8.74) | 4.3 (10.21) | 3.2 (13.74) | 5.2 (8.43) | |
| TACIT141 | Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean EQ-5D score at baseline (0–1) | Mean (SD) EQ-5D score at follow-up (0–1) | Mean (SD) change from baseline in EQ-5D score (0–1) | Mean (SD) change from baseline in EQ-5D VAS (0–100) |
|---|---|---|---|---|---|---|
| ADORE59,60 | ETN monotherapy | 16 weeks | NR | NR | 0.1883 (0.33) | 19.76 (27.24) |
| ETN + MTX | 16 weeks | NR | NR | 0.2399 (0.32) | 21.00 (26.61) | |
| TACIT141 | Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean (SD) change from baseline in usual activities (0–1) | Mean (SD) change from baseline in self-care (0–1) | Mean (SD) change from baseline in pain/discomfort (0–1) | Mean (SD) change from baseline in mobility (0–1) | Mean (SD) change from baseline in anxiety/depression (0–1) |
|---|---|---|---|---|---|---|---|
| ADORE59 | ETN monotherapy | 16 weeks | 0.3077 (0.61) | 0.1731 (0.55) | 0.3718 (0.62) | 0.3077 (0.50) | 0.2323 (0.59) |
| ETN + MTX | 16 weeks | 0.2867 (0.55) | 0.3533 (0.55)a | 0.4400 (0.65) | 0.2318 (0.52) | 0.24 (0.65) |
| Trial name | Treatment arms for which data extraction performed | Assessment point | Mean (SD) baseline score | Mean % change |
|---|---|---|---|---|
| ETN study 30989 | SSZ + PBO | 24 weeks | 44.6 (19.0) | 20.1 |
| ETN + PBO | 24 weeks | 45.5 (21.3) | 64.6a (vs. SSZ) | |
| ETN + SSZ | 24 weeks | 43.1 (22.4) | 67.6a (vs. SSZ) |
| Trial name/author, year | Treatment arms for which data extraction performed | RCT/LTE phase | Assessment time point | Discontinuation due to AE(s), n/N (%) | Number of patients experiencing one or more AE, n/N (%) | Number of patients experiencing one or more serious AE, n/N (%) |
|---|---|---|---|---|---|---|
| GUEPARD93 | Initial MTX 12 weeks, then step-up therapy in both groups based on DAS28 | RCT | 52 weeks | NR | NR | 5/32 (16) Five patients were hospitalised for the following reasons: one for vasculitis with revision of diagnosis to Sharp syndrome (week 6), one for hepatitis secondary to MTX (week 4), one for a hip prosthesis operation (week 12), one for weight loss (week 36) and one for haemopthysis (week 32) |
| Initial ADA + MTX 12 weeks, then step-up therapy in both groups based on DAS28 | RCT | 52 weeks | NR | NR | 5/33 (15) One had hepatitis (week 6), the other had MTX pneumonia (week 6) and the last had acoustic neuroma (week 10) plus two malignancy |
|
| HIT HARD94 | PBO + MTX | RCT | 24 weeks | 4/85 (4.7) | NR | NR |
| ADA + MTX | RCT | 24 weeks | 2/87 (2.3) | NR | NR | |
| HIT HARD94 | PBO + MTX for 24 weeks followed by MTX for 24 weeks | LTE | 48 weeks | 7/85 (8.2) | NR | 22/85 (25.9) Four serious infections (two urosepsis, one pneumonia), one stroke, one diplopia, one paraesthesia, three cardiac disorders (one bypass surgery, one claudication, one myocarditis), one reactive depression, three solid malignant tumours (one prostate, two cervix), one peripheral artery angioplasty, one shoulder impingment syndrome, one prolapsed lumbar disc, one fracture, three arthritis flare, one nephrolithiasis |
| ADA + MTX for 24 weeks followed by OL MTX for 24 weeks | LTE | 48 weeks | 4/87 (4.6) | NR | 12/87 (13.8) Three serious infections (one bronchitis, two abscess), one concussion, one syncope, one benign neoplasm (prostate), one subileus, one gastric haemorrhage, one varicose veins, one vasculitis, one coxarthrosis, one fracture |
|
| OPERA107 | PBO + MTX + steroid | RCT | 12 months | 1/91 (1.1) | NR | 10/91 (11.0) Two malignancies (one urothelial carcinoma, one basocellular carcinoma), three serious infections (one pneumonia, one bronchitis, one dental abscess), two fivefold increased serum alanine aminotransferase, one disease exacerbation, one leucopoenia, one polyneuropathia, one peptic ulcer, one coronary bypass, one hip fracture, one coxarthrosis One patient who terminated due to non-compliance at 6 months died due to pneumonia 4 months later |
| ADA + MTX + steroid | RCT | 12 months | 2/89 (2.2) | NR | 14/89 (15.7) Three malignancies (one small cell lung carcinoma, one myelodysplastic syndrome, one basocellular carcinoma), three serious infections (one empyema, one pneumonia, one bronchitis), one suspected but unconfirmed infectious arthritis, one local s.c. atrophy, one blurred vision, one acute myocardial infarction, one tachicardia, one gonarthrosis |
|
| OPTIMA108 | PBO + MTX | RCT | 26 weeks | 16/517 (3) | NR Infections in 36.4% |
NR Six serious infections |
| ADA + MTX | RCT | 26 weeks | 21/515 (4) | NR | NR Two malignancies (one malignant melanoma in situ, one squamous cell carcinoma), 13 serious infections, one case of lupus-like syndrome, no lymphoma or demyelinating disease |
|
| PREMIER109 | PBO + MTX | RCT | 2 years | 19/257 (7.4) | 245/257 (95.3) | Seven serious infections (two pneumonia, one septic arthritis, one sinusitis, one abscess, one bacteraemia, one parotitis), four malignancies (lymphoma, melanoma, prostate, breast) |
| ADA monotherapy + PBO | RCT | 2 years | 26/274 (9.5) | 262/274 (95.6) | Three serious infections (one pneumonia, one cellulitis, one septic arthritis), one lupus-like reaction, four malignancies (breast, colon, multiple myeloma, metastatic cancer with unknown primary site) | |
| ADA + MTX | RCT | 2 years | 32/268 (11.9) | 262/268 (97.8) | Nine serious infections [three pulmonary infections (including one plaural tuberculosis)], one sinus infection, one wound infection, one septic arthritis, one infected hygroma, one cellulitis, one urinary tract infection), two malignancies (one ovarian, one prostate) | |
| PREMIER109 | PBO + MTX to OL ADA monotherapy | LTE | 5 years | 7.7% | NR | 2/497 (0.4) During open-label period: 3.3 serious infections per 100 person-years Two TB, one lymphoma, one non-melanoma skin cancer, three breast cancer, two bladder cancer, one malignant melanoma, one tongue neoplasm, one pancreatic neoplasm, one lung cancer, one gastric cancer, one colon cancer. No lupus-like syndrome or demyelinating disease |
| ADA monotherapy + PBO to OL ADA monotherapy | LTE | 5 years | 10.7% | NR | ||
| ADA + MTX to OL ADA monotherapy | LTE | 5 years | 14.2% | NR | ||
| COMET81–83 | PBO + MTX | RCT period 1, 52 weeks | 52 weeks | 34/268 (12.7) | 246/268 (91.8) | 34/268 (12.7) % NR if less than 1% Cardiac, two Eye, one Gastrointestinal, four General and administration site, one Infection, eight (3%) Injury, poisoning, and procedural complications, four Laboratory values, one Musculoskeletal and connective tissue, nine (3%) Nervous system, one Psychiatric, one Renal and urinary, one Respiratory, thoracic and mediastinal, one Surgical and medical procedures, two Vascular, two Malignancy, four |
| ETN + MTX | RCT period 1, 52 weeks | 52 weeks | 28/274 (10.2) | 247/274 (90.2) | 33/274 (12.0) Cardiac, two Ear and labyrinth, one Gastrointestinal, one General and administration site, two Hepatobiliary, three Infection, five (2%) Injury, poisoning and procedural complications, three Laboratory values, one Metabolic and nutritional, two Musculoskeletal and connective tissue, four Nervous system, four Psychiatric, one Renal and urinary, one Respiratory, thoracic and mediastinal, three Skin and s.c. tissue, one Surgical and medical procedures, one Vascular, one Malignancy, four |
|
| COMET/Emery 201082 | MTX in year 1, MTX in year 2 | RCT period 2 | Weeks 52–104 | NR | 79/99 (79.8) | 12/99 (12.1) One death Three malignancies Remainder serious infections |
| MTX year 1, ETN + MTX in year 2 | RCT period 2 | Weeks 52–104 | NR | 71/90 (78.9) | 11/90 (12.2) Five malignancies Remainder serious infections |
|
| ETN + MTX in year 1, ETN + MTX in year 2 | RCT period 2 | Weeks 52–104 | NR | 91/111 (82.0) | 8/111 (7.2) Serious infections |
|
| ETN + MTX in year 1, ETN in year 2 | RCT period 2 | Weeks 52–104 | NR | 89/111 (80.2) | 10/111 (9.0) One malignancy Rest serious infections |
|
| ERA139 | PBO + MTX | RCT | 12 months | 22/217 (10) | NR | NR Two malignancies (bladder cancer, colon cancer) Infections requiring hospitalisation/i.v. antibiotics in < 3% |
| ETN + PBO | RCT | 12 months | 10/207 (5) | NR | NR Three malignancies (carcinoid lung cancer, Hodgkin’s disease and prostate cancer) Infections requiring hospitalisation/i.v. antibiotics in < 3% |
|
| ERA139 | PBO + MTX | LTE | 2 years | 27/217 (12) | NR | NR Nine patients had infections requiring hospitalisation/i.v. antibiotics Three malignancies |
| ETN + PBO | LTE | 2 years | 15/207 (7.2) | NR | NR Seven patients had infections requiring hospitalisation/i.v. antibiotics Four malignancies |
|
| GO-BEFORE90 | PBO + MTX | RCT | 24 weeks | 2/160 (1.3) | 116/160 (72.5) | 11/160 (6.9) (NR for extracted treatment arm) |
| GOL + MTX | RCT | 24 weeks | 6/158 (3.8) | 129/158 (81.6) | 10/185 (5.4) (NR for extracted treatment arm) | |
| GO-BEFORE143 | PBO + MTX | LTE | Week 104 | NR | NR | N/A |
| GOL + MTX | LTE | Week 104 | NR | NR | 13.7% (NR) | |
| ASPIRE71 | PBO + MTX | RCT | 54 weeks | 9/298 (3.0) | NR | 2/291 (0.7) (myocardial infarction) |
| IFX + MTX | RCT | 54 weeks | 34/373 (9.1) | NR | 16/372 (4.3) (pneumonia, myocardial infarction, asthma, three TB, two infusion reactions) | |
| Durez et al., 2007120 | MTX | RCT | 52 weeks | 0/14 | 0/14 | 0/14 |
| MP + MTX | RCT | 52 weeks | 0/15 | 0/15 | 0/15 | |
| IFX + MTX | RCT | 52 weeks | 1/15 (6.7) | 1/15 (6.7) | 1/15 (6.7) One case of MTX-related pneumonitis |
|
| Quinn et al., 2005110 | PBO + MTX | LTE | 104 weeks | 0/10 | NR | NR |
| IFX + MTX | LTE | 104 weeks | 1/10 (10) | NR | NR One cutaneous vasculitis (after single injection; withdrawn) |
| Trial name/study | Treatment arms for which data extraction performed | RCT/LTE phase | Assessment time point | Discontinuation due to AE(s), n/N (%) | Number of patients experiencing one or more AE, n/N (%) | Number of patients experiencing one or more serious AE, n/N (%) |
|---|---|---|---|---|---|---|
| ATTEST74 | PBO + MTX | RCT | Day 197 | 1/110 (0.9) | 92/110 (83.6) | 13/110 (11.8) (type NR) |
| IFX + MTX | RCT | Day 197 | 8/165 (4.8) | 140/165 (84.8) | 19/165 (11.5) (type NR) | |
| ABT + MTX | RCT | Day 197 | 2/156 (1.3) | 129/156 (82.7) | 8/156 (5.1) (type NR) | |
| ATTEST74 | (1) PBO + MTX | RCT | Day 365 | – | – | – |
| (2) IFX + MTX | RCT | Day 365 | 12/165 (7.3) | 154/165 (93.3) | 30/165 (18.2) (type NR) | |
| (3) ABT + MTX | RCT | Day 365 | 5/156 (3.2) | 139/156 (89.1) | 15/156 (9.6) (type NR) | |
| AMPLE66 | ABT s.c. | RCT | 1 year | 11/318 (3.5) | 280/318 (88.1) | 32/318 (10.1) Seven serious infections (three pneumonia, two urinary tract infection, one gastroenteritis, one helicobacter gastritis), five malignancies (two squamous cell carcinoma of skin, one lymphoma, one prostate cancer, one squamous cell carcinoma of lung), one psoriasis, one erythema nodosum, one leukocytoclastic vasculitis, two Raynaud’s phenomenon, one cutaneous lymphocytic vasculitis, one episcleritis, one Sjögren’s syndrome |
| ADA | RCT | 1 year | 20/328 (6.1) | 283/328 (86.3) | 30/328 (9.1) Nine serious infections (two pneumonia, three bacterial arthritis, one chest wall abscess, one diverticulitis, one meningitis, one staphylococcal bursitis), four malignancies (two basal cell carcinoma, one small cell lung cancer, one transitional cell carcinoma), one psoriasis, one erythema nodosum, one Raynaud’s phenomenon, one antidouble-stranded DNA seropositivity |
|
| RED-SEA114 | ADA + cDMARDs | RCT | 12 months | 10/60 (16.7) | NR | 6/60 (10) There were two deaths, both occurring in patients allocated ADA and resulting from ischaemic heart disease, one occurred a week after drug withdrawal Other events possibly related to therapy were acute cholecystitis (ADA) One ovarian cancer |
| ETN50 + cDMARDs | RCT | 12 months | 12/60 (20) | NR | 7/60 (11.7) One diagnosed with heart failure 2 weeks after drug withdrawal: an event believed to be possibly related to the treatment events possibly related to therapy A patient hospitalised with chest symptoms One acute myeloid leukaemia, group not specified Other serious AEs included hospitalisation for a ruptured popliteal cyst; chest symptoms; syncope; suspected femoral fracture; angioedema and urticaria; stillbirth from pregnancy while on treatment and cellulitis |
|
| ADACTA58 | TCZ + s.c. PBO | RCT | 24 weeks | 9/163 (5.5) | 133/162 (82.1) | 19/162 (11.7) (including infections, two myocardial infarction/acute coronary syndrome, one stroke, one cancer) |
| ADA + i.v. PBO | RCT | 24 weeks | 10/163 (6.1) | 134/162 (82.7) | 16/162 (9.9) (including infections, two myocardial infarction/acute coronary syndrome, one stroke, one cancer, one hypersensitivity reaction) |
| Trial name/author, year | Treatment arms for which data extraction performed | RCT/LTE phase | Assessment time point | Discontinuation due to AE(s), n/N (%) | Number of patients experiencing one or more AE, n/N (%) | Number of patients experiencing one or more serious AE, n/N (%) |
|---|---|---|---|---|---|---|
| AIM61,62 | PBO + MTX | RCT | 12 months | 1.8 | 184/219 (84.0) | 26/219 (11.9) Related to study drug, one (0.5%) Discontinuations due to serious AEs, three (1.4%) Musculoskeletal and connective tissue disorders, 10 (4.6%) Infections, five (2.3%) Nervous system disorders, four (1.8%) Cardiac disorders), two (0.9%) Neoplasms (benign, malignant and unspecified), two (0.9%) |
| ABT i.v. + MTX | RCT | 12 months | 4.2 | 378/433 (87.3) | 65/433 (15.0) Related to study drug, 15 (3.5%) Discontinuations due to serious AEs, 10 (2.3%) Musculoskeletal and connective tissue disorders, 20 (4.6%) Infections, 17 (3.9%) Nervous system disorders, six (1.4%) Cardiac disorders, four (0.9%) Neoplasms (benign, malignant and unspecified), four (0.9%) |
|
| AIM64 | ABT i.v. + MTX 2 years or MTX + PBO 1 year then ABT i.v. + MTX 1 year | LTE | 2 years | 38/593 (6.4) | 550/593 (92.7) | 149/593 (25.1) Excluding worsening of arthritis, the most frequent serious AEs were osteoarthritis, pneumonia, basal cell carcinoma and chest pain, all of which occurred in > 0.5% of patients during the cumulative study period |
| AIM65 | ABT i.v. + MTX 2 years or MTX + PBO 1 year then ABT i.v. + MTX 1 year | LTE | 3 years | 55/593 | 569/593 (96) | NR |
| ASSET72 | PBO + MTX | RCT | 4 months | 0/23 | 14/23 (60.9) | 2/23 (8.7) One atrial fibrillation, one study drug overdose |
| ABT i.v. + MTX | RCT | 4 months | 0/27 | 20/27 (74.1) | 0/27 | |
| ASSET72 | ABT i.v. + MTX | LTE | 1 year | 0/49 | 41/49 (83.7) | 6/49 (12.2) One pneumonia, one hyperthyroidism and post-operative wound infection (in same patient), one study drug overdose and coronary artery disease (in same patient), one chronic anaemia, one worsening of RA, one depression |
| AUGUST II76 | PBO + MTX | 38-week follow-up of 26-week RCT treatment | 38 weeks | 2/76 (2.6) | 38/76 (50) | NR |
| ADA + MTX | 38-week follow-up of 26-week RCT treatment | 38 weeks | 2/79 (2.5) | 50/79 (63) | NR | |
| CHANGE80 | PBO | RCT | 24 weeks | 4/87 (4.6) | 71/87 (81.6) | 8/87 (9.2) |
| ADA monotherapy | RCT | 24 weeks | 12/91 (13.2) | 90/91 (98.9) | 17/91 (18.7) One death Others not specified |
|
| DE01984 | PBO + MTX | RCT | 52 weeks | NR | 181/200 (90.5) | NR Two malignancies Others not specified |
| ADA + MTX | RCT | 52 weeks | NR | NR | NR | |
| ASSURE73 | PBO + cDMARDs | RCT | 1 year | 18/418 (4.3) | 360/418 (86.1) | 51/418 (12.2) Seven serious infections, 16 neoplasms and the following serious infections: four respiratory, one dermatological, one urinary, one gastrointestinal, one gynaecologic, two opportunistic |
| ABT + cDMARDs | RCT | 1 year | 43/856 (5.0) | 768/856 (89.7) | 100/856 (11.7) 22 serious infections, 27 neoplasms and the following serious infections: nine respiratory, five dermatological, four urinary, two gastrointestinal) |
|
| STAR117 | PBO + cDMARDs | RCT | 24 weeks | 8/318 (2.5) (Of which one considered non-treatment related) |
263/318 (82.7) | 22/318 (6.9) Six serious infections Others not specified Severe or life-threatening AEs, 49/318 (15.4) |
| ADA + cDMARDs (n = 318) | RCT | 24 weeks | 9/318 (2.8) | 275/318 (86.5) | 17/318 (5.3) Four serious infections One death One malignancy Others not specified Severe or life-threatening AEs, 38/318 (11.9) |
|
| Van De Putte et al., 2004122 | PBO s.c. | RCT | 26 weeks | 1/110 (0.9) | 105/110 (95.5) | 16/110 (14.5) |
| ADA monotherapy | RCT | 26 weeks | 6/113 (5.3) | NR | 13/113 (11.5) | |
| ARMADA69,70 | PBO + MTX | RCT | 24 weeks | 2/62 (3.2) | NR | NR |
| ADA + MTX | RCT | 24 weeks | 0/67 (0) | NR | NR | |
| Kim et al., 200799 | PBO + MTX | RCT | 24 weeks | NR | 82.5% (Possibly related to study drug 28.6%) |
6/63 (9.5) NR |
| ADA + MTX | RCT | 24 weeks | NR | 84.6% (Possibly related to study drug 26.2%) |
7/65 (10.8) The number of serious AEs reported was comparable between the ADA group and the PBO group. Three of the seven serious AEs reported in the ADA group were of infectious aetiology (two pneumonia and one disseminated tuberculosis), one was a complication due to the serious AE of pneumonia (acute respiratory distress syndrome), and the other was vasovagal attack One death in the ADA treatment group |
|
| CERTAIN79 | PBO + cDMARDs | RCT | 24 weeks | NR | NR (67.3) | NR (7.1) Serious infections in 1.0% |
| CTZ + DMARDs | RCT | 24 weeks | NR | NR (68.8) | NR (5.2) Serious infections in 5.2% |
|
| ADORE59,60 | ETN monotherapy | RCT | 16 weeks | 13/159 (8.2) | 100/159 (62.9) | 8/159 (5.0) |
| ADORE59 | ETN + MTX | RCT | 16 weeks | 9/155 (5.8) | 109/155 (70.3) | 7/155 (4.5) |
| CREATE IIb96 | DMARD + PBO (n = 65) | RCT | 24 weeks | 9.2% | NR | NR |
| ETN50 + DMARD (n = 64) | RCT | 24 weeks | 3.1% | NR | NR | |
| ETN study 30989 | SSZ + PBO | RCT | 24 weeks | Due to serious AE, 1/50 | NR Non-infectious AEs, 29/50 (58) |
NR Non-infectious serious AEs, 1/50 (2) |
| ETN + PBO | RCT | 24 weeks | Due to serious AE, 1/103 | NR Non-infectious AEs, 74/103 (71.8) |
NR Non-infectious serious AEs, 3/103 (2.9) |
|
| ETN + SSZ | RCT | 24 weeks | Due to serious AE, 1/101 | NR Non-infectious AEs, 72/101 (71.3) |
NR Non-infectious serious AEs, 5/101 (5) |
|
| ETN study 30989 | SSZ + PBO | RCT | 2 years | NR | NR Non-infectious AEs 32/50 (64) Infectious AEs 21/50 (42.0) 32 |
2/50 (4) |
| ETN + PBO | RCT | 2 years | NR | NR Non-infectious AEs 90/103 (87.4) Infectious AEs 76/103 (73.8) |
27/103 (26.2) | |
| ETN + SSZ | RCT | 2 years | NR | NR Non-infectious AEs 80/101 (79.2) Infectious AEs 60/101 (59.4) |
23/101 (22.8) | |
| JESMR140 | ETN monotherapy | RCT | 52 weeks | 4/71 (5.6) | NR | 2/71 (2.8) Two bone fractures |
| ETN + MTX | RCT | 52 weeks | 1/76 (13.1) | NR | 7/76 (9.2) Three bone fractures, one congestive heart failure, one cellulitis (in same patient as one of the fractures), one herpes zoster, one brain, one mammary carcinoma |
|
| Lan et al., 2004101 | PBO + MTX | RCT | 12 weeks | 1/29 (3.4) | NR | NR One bronchiolitis obliterans |
| ETN + MTX | RCT | 12 weeks | 1/29 (3.4) | NR Most frequently occurring AEs in line with summary of product characteristics |
NR One viral pneumonia |
|
| LARA102 | MTX + DMARD | RCT | 24 weeks | NR | 97/142 (68.3) | 2/142 (1.4) Specific AEs NR |
| ETN50 + MTX | RCT | 24 weeks | NR | 193/281 (68.7) | 10/281 (3.6) Specific AEs NR |
|
| RACAT111 | MTX + SSZ + HCQ On treatment analysis, n = 222 (some patients exposed to both treatments throughout trial) |
RCT including crossover | 48 weeks | 12/222 (5.4) | 170/222 (76.6) | 25/222 (11.3) (some patients counted in more than one event) Cardiac disorders, four Gastrointestinal disorders, five Infections and infestations, four Renal and urinary disorders, one Respiratory, thoracic and mediastinal disorders, four Surgical and medical procedures, three Vascular disorders, three Other (events occurring fewer than three times), nine |
| ETN50 + MTX On treatment analysis, n = 219 (some patients exposed to both treatments throughout trial) |
RCT including crossover | 48 weeks | 5/219 (2.3) | 165/219 (75.3) | 26/219 (11.9) (some patients counted in more than one event) Cardiac disorders, one Gastrointestinal disorders, four Infections and infestations, 12 Renal and urinary disorders, three Respiratory, thoracic and mediastinal disorders, one Surgical and medical procedures, five Vascular disorders, four Other (events occurring fewer than three times), nine |
|
| Weinblatt et al., 1999124 | PBO + MTX | RCT | 24 weeks | 1/30 (3.3) | NR | NR |
| ETN + MTX | RCT | 24 weeks | 2/59 (3.4) | NR | NR | |
| APPEAL67,68 | MTX + DMARD (SSZ, HCQ or LEF) | RCT | 16 weeks | 8/103 (7.8) | 79/103 (77) | 3/103 (3) One infection/infestation, two increased alanine aminotransferase |
| ETN + MTX | RCT | 16 weeks | 3/197 (1.5) | 134/197 (68) | 6/197 (3) One cardiac disorder, one gastrointestinal disorder, one general disorder, three infections and infestations, two poisoning and procedural complications |
|
| GO-FORTH91 | PBO + MTX | RCT | 24 weeks | NR | 67/88 (76.1) | 1/88 (1.1) One intervertebral disc protrusion |
| GOL + MTX | RCT | 24 weeks | NR | 70/86 (81.4) | 2/86 (2.3) One ileus, one bone neoplasm (borderline or low malignancy potential) |
|
| GO-FORWARD92 | PBO + MTX | RCT | 24 weeks | 5/133 (3.8) | 89/134 (66.4) | 5/134 (3.7) (type NR) |
| GOL + MTX | RCT | 24 weeks | 2/89 (2.2) | 87/212 (41.0) | 9/212 (4.2) (type NR) | |
| GO-FORWARD92 | PBO + MTX | RCT | 52 weeks | 8/133 (6.0) | 98/133 (73.7) | 6/133 (4.5) (type NR) |
| GOL + MTX | RCT | 52 weeks | 7/212 (3.3) | 167/212 (78.8) | 17/212 (8.0) (type NR) | |
| Kay et al., 200898 | IFX + MTX (PBO group crossed over to IFX at week 20) | RCT | 52 weeks | 3/25 (12.0) | 16/25 (64.0) | 3/25 (12.0) (type NR) |
| GOL + MTX | 52 weeks | 4/37 (10.8) | 34/37 (91.9) | 7/37 (18.9) (type NR) | ||
| Abe et al., 200656 | PBO + MTX | RCT | 14 weeks | 1/47 (2.1) | NR | 1/47 (2.1) (type NR for extracted arm) |
| IFX + MTX | RCT | 14 weeks | 1/49 (2.0) | NR | 0 | |
| Abe et al., 200656 | PBO group crossover to IFX | LTE | To week 36 of LTE | 9/41 (22.0) | NR | 6/41 (14.6) (type NR for extracted arm) |
| IFX + MTX | LTE | To week 36 of LTE | 4/49 (8.2) | NR | 2/49 (4.1) (type NR for extracted arm) | |
| ATTRACT146 | PBO + MTX | RCT | 54 weeks | 7/88 (8.0) | 94% | 18/86 (20.9) (type NR) |
| IFX + MTX | RCT | 54 weeks | 5/86 (5.8) | NR | 10/88 (11.4) (type NR) | |
| ATTRACT324 | PBO + MTX | LTE | 102 weeks | NR | NR | 28/NR (33) (type NR) |
| IFX + MTX | LTE | 102 weeks | NR | NR | 29/NR (33) (type NR) | |
| START118 | PBO + MTX | RCT | 22 weeks | 5/361 (1.4) | 239/361 (66.2) | 27/361 (7.5) One fever, one osteoarthritis, four RA |
| IFX + MTX | RCT | 22 weeks | 0/360 | 251/360 (69.7) | 28/360 (7.8) Two pneumonia, one cellulitis, one chest pain, two osteoarthritis, one cardiac failure, one myocardial infarction, two uterine fibroid, one RA |
|
| START118 | IFX + MTX | LTE | 54 weeks | NR | 211/244 (86.5) | 39/244 (16.0) Five pneumonia, one active TB, one abscess, two pyelonephritis |
| Swefot119 | SSZ + HCQ + MTX | RCT | 24 months | 22/130 (16.9) | NR/130 (45) | Serious AEs = one (1) (generalised symptoms) |
| IFX + MTX | RCT | 24 months | 19/128 (14.8) | NR/128 (38) | Serious AEs = two (2) (persistent fever and generalised symptoms) | |
| Zhang et al., 2006126 | PBO + MTX | RCT | 18 weeks | 4/86 (4.7) | 48/86 (55.8) | NR |
| IFX + MTX | RCT | 18 weeks | 6/87 (6.9) | 57/87 (65.5) | NR | |
| ACT-RAY152 | TCZ + oral PBO | RCT | 52 weeks | NR | 228/276 (82.6) | 26/276 (9.4) |
| TCZ + MTX | RCT | 52 weeks | NR | 277/277 (81.9)a | 24/277 (8.7) | |
| Nishimoto et al., 2004106 | PBO i.v. | RCT | 12 weeks | 4/53 (7.5) | NR/53 (56) | 2/53 (3.8) (type NR) |
| TCZ | RCT | 12 weeks | 2/55 (3.6) | NR/55 (51) | NR for TCZ 8 mg/kg | |
| STREAM/Nishimoto et al., 2009325 (LTE of Nishimoto et al., 2004106) | PBO i.v. | LTE | To year 5 | – | – | – |
| TCZ | LTE | To year 5 | 32/143 (22.4) | NR | 77/143 (53.8) [including joint surgery n = 20 (most common), pneumonia n = 9, herpes zoster n = 7, tendon rupture n = 5, humerus fracture n = 4, acute bronchitis n = 2) | |
| SAMURAI115 | cDMARDs | RCT | 52 weeks | 5/145 (3.4) | 119/145 (82) | NR/145 (13) Only serious infections listed, not other serious AEs Eight serious infections were reported: three (2.1%) patients with gastroenteritis, two (1.4%) with pneumonia and one (0.7%) each with upper respiratory tract infection, herpes zoster and sepsis |
| TCZ | RCT | 52 weeks | 17/157 (10.8) | 140/157 (89) | NR/157 (18) Only serious infections listed, not other serious AEs 12 serious infections were reported: three (1.9%) patients with pneumonia, two (1.3%) with upper respiratory tract infection, two (1.3%) with cellulitis, one (0.6%) each with gastroenteritis, herpes zoster, herpes simplex, perianal abscess and an unidentified infection |
|
| SATORI116 | PBO i.v. + MTX | RCT | 24 weeks | 3/64 (4.7) | 46/64 (71.9) (104 AEs) | 3/64 (4.7) (one pneumonia, one spinal compression fracture, one femoral neck fracture) |
| TCZ + PBO capsules | RCT | 24 weeks | 2/61 (3.3) | 56/61 (91.8) (211 AEs) | 4/61 (6.6) (one pneumonia, one infectious arthritis, one colonic polyp, one headache) | |
| TOWARD121 | PBO + stable cDMARDs | RCT | 24 weeks | 8/414 (1.9) | 253/414 (61.1) | 18/414 (4.3) [Related serious AE = 6 (1.4) type NR] |
| TCZ + stable DMARDs | RCT | 24 weeks | 31/802 (3.9) | 584/802 (72.8) | 54/802 (6.7) [related serious AE = 23 (2.9) type NR] |
|
| TACIT141 | (1) Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| (2) TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| TACIT141 | (1) Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| (2) TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Trial name/study | Treatment arms for which data extraction performed | RCT/LTE phase | Assessment time point | Number of patients experiencing one or more infection, n/N (%) | Number of patients experiencing one or more serious infection, n/N (%) | Number of patients experiencing any infection requiring antibiotics, n/N (%) | Number of patients experiencing one or more malignancy, n/N (%) | Number of patients experiencing one or more injection-site reaction (s.c. administration), n/N (%) | Number of patients experiencing one or more infusion-related reaction (i.v. administration), n/N (%) |
|---|---|---|---|---|---|---|---|---|---|
| GUEPARD93 | Initial MTX 12 weeks, then step-up therapy in both groups based on DAS28 | RCT | 52 weeks | NR | 1/32 (3) | NR | 0 | NR | N/A |
| Initial ADA + MTX 12 weeks, then step-up therapy in both groups based on DAS28 | RCT | 52 weeks | NR | 2/33 (6) | NR | 2/33 (6) | NR | N/A | |
| HIT HARD94 | MTX + PBO for 24 weeks followed by OL MTX for 24 weeks | LTE | 48 weeks | 10/85 (11.8) | 4/85 (4.7) | NR | 3/85 (3.5) | 4/85 (4.7) | NR |
| ADA + MTX for 24 weeks followed by OL MTX for 24 weeks | LTE | 48 weeks | 16/87 (18.4) | 3/87 (3.4) | NR | 0/87 | 14/87 (16.1) | NR | |
| OPERA107 | PBO + MTX + steroid | RCT | 12 months | NR | 3/91 (3.3) | NR | 2/91 (2.2) | NR | NR |
| ADA + MTX + steroid | RCT | 12 months | NR | 3/89 (3.4) | NR | 3/89 (3.4) | NR | NR | |
| PREMIER109 | ADA (all patients who received ≥ 1 dose) | LTE | 5 years | NR | NR; 3.3 events per 100 patient-years | NR | 11/497 (2.2) | NR | NR |
| COMET81 | PBO + MTX | RCT period 1, 52 weeks | 52 weeks | 8/268 (3.0) | NR | NR | 4/268 (1.5) | NR | NR |
| ETN + MTX | RCT period 1, 52 weeks | 52 weeks | 5/274 (1.8) | NR | NR | 4/274 (1.5) | NR | NR | |
| COMET82 | MTX in year 1, MTX in year 2 | RCT period 2 | Weeks 52–104 | NR | 2/99 (2.0) | NR | 3/99 (3.0) | NR | NR |
| COMET81 | MTX in year 1, ETN + MTX in year 2 | RCT period 2 | Weeks 52–104 | NR | 1/90 (1.1) | NR | 5/90 (5.6) | NR | NR |
| COMET81 | ETN + MTX in year 1, ETN + MTX in year 2 | RCT period 2 | Weeks 52–104 | NR | 1/111 (0.9) | NR | 0 (0) | NR | NR |
| COMET81 | ETN + MTX in year 1, ETN in year 2 | RCT period 2 | Weeks 52–104 | NR | 2/111 (1.8) | NR | 1/111 (0.9) | NR | NR |
| ERA139 | PBO + MTX | RCT | 12 months | NR | NR | < 3% | 2/217 (0.9) | 16/217 (7.4) | NR |
| ETN + PBO | RCT | 12 months | NR | NR | < 3% | 3/207 (1.4) | 77/207 (37.2) | NR | |
| ERA139 | PBO + MTX | LTE | 2 years | NR | 9/217 (4.1) | NR | 3/217 (1.4) | 19/217 (8.8) | NR |
| ETN + PBO | LTE | 2 years | NR | 7/207 (3.4) | NR | 4/207 (1.9) | 81/207 (39.1) | NR | |
| GO-BEFORE90 | PBO + MTX | RCT | 24 weeks | 52/160 (32.5) | 3/160 (1.9) | NR | 2/160 (1.3) | 3/160 (1.9) | N/A |
| GOL + MTX | RCT | 24 weeks | 54/158 (34.2) | 2/158 (1.3) | NR | 1/158 (0.6) | 7/158 (4.4) | N/A | |
| GO-BEFORE143 | PBO + MTX | LTE | Week 104 | NR | NR | NR | Two (no n provided, assumed n = 160) | NR | NR |
| GOL + MTX | LTE | Week 104 | NR | 5.5% | NR | Six (no n provided, assumed n = 158) | NR | NR | |
| ASPIRE71 | PBO i.v. + MTX | RCT | 54 weeks | NR | 21/372 (5.6) | NR | 0 | N/A | 20/291 (6.9) |
| IFX + MTX | RCT | 54 weeks | NR | PBO i.v. + MTX = 6/291 (2.1) IFX + MTX = 21/372 (5.6)a |
NR | 0 | N/A | 79/372 (21.2) (two classed as serious) | |
| Durez et al., 2007120 | MTX | RCT | 52 weeks | 14/14 (100) | 0/14 | NR | NR | NR | NR |
| MTX + MP | RCT | 52 weeks | 12/15 (80) | 0/15 | NR | NR | NR | NR | |
| IFX + MTX | RCT | 52 weeks | 12/15 (80) | 1/15 (6.7) | NR | NR | NR | NR | |
| Quinn et al., 2005110 | PBO + MTX | LTE | 104 weeks | NR | NR | NR | NR | NR | 0/10 |
| IFX + MTX | LTE | 104 weeks | NR | NR | NR | NR | NR | 1/10 (10) |
| Trial name/author, year | Treatment arms for which data extraction performed | RCT/LTE phase | Assessment time point | Number of patients experiencing one or more infection, n/N (%) | Number of patients experiencing one or more serious infection, n/N (%) | Number of patients experiencing any infection requiring antibiotics, n/N (%) | Number of patients experiencing one or more malignancy, n/N (%) | Number of patients experiencing one or more injection-site reaction (s.c. administration), n/N (%) | Number of patients experiencing one or more infusion-related reaction (i.v. administration), n/N (%) |
|---|---|---|---|---|---|---|---|---|---|
| ATTEST74 | PBO + MTX | RCT | Day 197 | NR | 3/110 (2.7) | NR | 1/110 (0.9) | N/A | 10.0% |
| IFX + MTX | RCT | Day 197 | NR | 7/165 (4.2) | NR | 2/165 (1.2) | N/A | 18.2% | |
| ABT + MTX | RCT | Day 197 | NR | 2/156 (1.3) | NR | 1/156 (0.6) | N/A | 5.1% | |
| ATTEST74 | PBO + MTX | RCT | Day 365 | – | – | – | – | – | – |
| IFX + MTX | RCT | Day 365 | NR | 14/165 (8.5) | NR | 2/165 (1.2) | N/A | 41/165 (24.8) | |
| ABT + MTX | RCT | Day 365 | NR | 3/156 (1.9) | NR | 1/156 (0.6) | N/A | 11/156 (7.1) | |
| AMPLE66 | ABT s.c. | RCT | 2 years | 63.2% | 7/318 (2.2) | NR | 5/318 (1.6) | 12/318 (3.8) | N/A |
| ADA | RCT | 2 years | 61.3% | 9/328 (2.7) | NR | 4/328 (1.2) | 30/328 (9.1) | N/A | |
| RED-SEA114 | ADA + cDMARDs | RCT | 12 months | NR | NR | NR | 1/60 (1.7) | 9/60 (15) | N/A |
| ETN50 +cDMARDs | RCT | 12 months | NR | NR | NR | 1/60 (1.7) | 19/60 (31.7) | N/A | |
| ADACTA58 | TCZ + s.c. PBO | RCT | 24 weeks | 77/162 (47.5) | 5/162 (3.1) | NR | 1/162 | N/A | NR |
| ADA + i.v. PBO | RCT | 24 weeks | 68/162 (42.0) | 5/162 (3.1) | NR | 1/162 | NR | N/A | |
| ADACTA58 | TCZ | LTE | To year 5 | NR | 25/143 (17.5) (pneumonia, herpes zoster, acute bronchitis, pyelonephritis) | NR | 4/143 (2.8) (bladder cancer, breast cancer, large intestine carcinoma, intraductal papilloma) | N/A | NR |
| Trial name/study | Treatment arms for which data extraction performed | RCT/LTE phase | Assessment time point | Number of patients experiencing one or more infection, n/N (%) | Number of patients experiencing one or more serious infection, n/N (%) | Number of patients experiencing any infection requiring antibiotics, n/N (%) | Number of patients experiencing one or more malignancy, n/N (%) | Number of patients experiencing one or more injection-site reaction (s.c. administration), n/N (%) | Number of patients experiencing one or more infusion-related reaction (i.v. administration), n/N (%) |
|---|---|---|---|---|---|---|---|---|---|
| AIM62 | PBO + MTX | RCT | 12 months | NR | 5/219 (2.3) | NR | NR | NR | Acute infusional AEs 37/219 (16.9) |
| ABT i.v. + MTX | RCT | 12 months | NR | 17/433 (3.9) | NR | NR | NR | Acute infusional AEs 38/433 (8.8) |
|
| AIM64 | ABT i.v. + MTX 2 years or MTX + PBO 1 year then ABT i.v. + MTX 1 year | LTE | 2 years | 400/593 (67.5) | 43/593 (7.3) | NR | NR | NR | NR |
| ASSET72 | PBO + MTX | RCT | 4 months | 6/23 (26.1) | 0/23 (0) | NR | 0/23 (0) | N/A | Acute infusion events: 4/23 (17.4) Peri-infusional events: 5/23 (21.7) |
| ABT i.v. + MTX | RCT | 4 months | 10/27 (37.0) | 0/27 (0) | NR | 0/27 (0) | N/A | Acute infusion events: 0/27 Peri-infusional events: 4/27 (14.8) |
|
| ASSET72 | ABT i.v. + MTX (OLE) | LTE | 1 year | 26/49 (53.1) | 1/49 (2.0) | NR | 0/49 (0) | N/A | Acute infusion events: 2/49 (4.1) Peri-infusional events: 6/49 (12.2) |
| ASSURE73 | PBO + cDMARDs | RCT | 1 year | 224/418 (53.6) | 7/418 (1.7) | NR | NR | NR | NR |
| ABT + cDMARDs | RCT | 1 year | 470/856 (54.9) | 22/856 (2.6) | NR | NR | NR | NR | |
| AUGUST II76 | PBO + MTX | 38 week follow-up of 26-week RCT treatment | 38 weeks | NR | 1/76 (1.3) | NR | NR | NR | N/A |
| ADA + MTX | 38 week follow-up of 26-week RCT treatment | 38 weeks | NR | 3/79 (3.8) | NR | NR | NR | N/A | |
| CHANGE80 | PBO | RCT | 24 weeks | 32/87 (36.8) | 1/87 (1.1) | NR | 2/87 (2.3) | 2/87 (2.3) | NR |
| ADA monotherapy | RCT | 24 weeks | 41/91 (45.1) | 6/91 (6.6) | NR | 0 (0) | 28/91 (30.8) | NR | |
| DE01984 | PBO + MTX | RCT | 52 weeks | Upper respiratory tract infection, 13.5% Infection, 4.5% |
NR | NR | 0 | 48/200 (24%) | NR |
| ADA + MTX | RCT | 52 weeks | Upper respiratory tract infection, 19.8% Infection, 7.2% |
NR | NR | Across both ADA groups, four ADA-treated patients developed non-skin cancers, including non-Hodgkin’s lymphoma, adenocarcinoma, testicular seminoma, and breast cancer (not stated which ADA group) | 54/207 (26%) | NR | |
| STAR117 | PBO + cDMARDs | RCT | 24 weeks | 157/318 (49.4) | 6/318 (1.9) | NR | 0 | 37 (11.6%) | N/A |
| ADA + cDMARDs | RCT | 24 weeks | 166 (52.2%) | 4 (1.3%) | NR | 1/318 (0.3) | 62 (19.5%)a | N/A | |
| Van De Putte et al., 2004122 | PBO s.c. | RCT | 26 weeks | NR | NR | NR | 1/110 (0.9) | 1/110 (0.9) | N/A |
| ADA monotherapy | RCT | 26 weeks | NR | NR | NR | 4/434 (0.9) (of all four ADA groups) | 11/113 (9.7) | N/A | |
| ARMADA69,70 | PBO + MTX (n = 62) | RCT | 24 weeks | Any infection, NR Upper respiratory tract infection, 9.7% |
NR | NR | NR | Pain, 3.2% Reaction, 0% |
N/A |
| ADA + MTX (n = 67) | RCT | 24 weeks | Any infection, NR Upper respiratory tract infection, 14.9% |
NR | NR | NR | Pain, 10.4% Reaction, 1.5% |
N/A | |
| Kim et al., 200799 | PBO + MTX | RCT | 24 weeks | 22/63 (34.9) | NR | NR | 0 | NR | NR |
| ADA + MTX | RCT | 24 weeks | 24/65 (36.9) | NR | NR | 0 | NR | NR | |
| CERTAIN79 | PBO + cDMARDs | RCT | 24 weeks | NR | NR (1.0) | NR | NR | NR | NR |
| CTZ + DMARDs | RCT | 24 weeks | NR | NR (2.1) | NR | NR | NR | NR | |
| ADORE59,60 | ETN monotherapy | RCT | 16 weeks | 39/159 (24.5) | 2/159 (1.3) | NR | NR | NR | NR |
| ETN + MTX | RCT | 16 weeks | 50/155 (32.3) | 1/155 (0.6) | NR | NR | NR | NR | |
| CREATE IIb96 | PBO + DMARD | RCT | 24 weeks | NR | 0/65 | NR | NR | NR | NR |
| ETN50 + DMARD | RCT | 24 weeks | NR | 0/64 | NR | NR | NR | NR | |
| ETN study 30989 | SSZ + PBO | RCT | 24 weeks | 13/50 (26) | 0 | NR | 0 | 1/50 (2) | NR |
| ETN + PBO | RCT | 24 weeks | 47/103 (45.6)a (vs. SSZ) | 2/103 (1.9) | NR | 2/103 (1.9) | 33/103 (32.0)a (vs. SSZ) | NR | |
| ETN + SSZ | RCT | 24 weeks | 31/101 (30.7)a (vs. SSZ vs. ETN + PBO) | 0 | NR | 0 | 16/101 (15.8)a (vs. SSZ vs. ETN + PBO) | NR | |
| ETN30989 | SSZ + PBO | RCT | 2 years | 21/50 (42.0) | NR | NR | NR | 2/50 (4.0) | NR |
| ETN + PBO | RCT | 2 years | 76/103 (73.8)a (vs. SSZ) | NR | NR | NR | 34/103 (33.0)a (vs. SSZ) | NR | |
| ETN + SSZ | RCT | 2 years | 60/101 (59.4)a (vs. ETN + PBO) | NR | NR | NR | 21/101 (20.8)a (vs. SSZ) | NR | |
| JESMR140 | ETN monotherapy | RCT | 52 weeks | 19/71 (26.8) | 0/71 | NR | NR | 13/71 (18.3) | NR |
| ETN + MTX | RCT | 52 weeks | 21/76 (27.6) | 2/76 (2.6) | NR | NR | 7/76 (9.2) | NR | |
| Lan et al., 2004101 | PBO + MTX | RCT | 12 weeks | NR | NR | NR | NR | 0/29 | NR |
| ETN + MTX | RCT | 12 weeks | NR | NR | NR | NR | 1/29 (3.4) | NR | |
| LARA102 | MTX + DMARD | RCT | 24 weeks | 31/142 (21.8) | 0 | NR | NR | NR | NR |
| ETN50 + MTX | RCT | 24 weeks | 107/281 (38.1)a | 5/281 (1.8) | NR | NR | NR | NR | |
| Moreland et al., 1999104 | PBO | RCT | 6 months | NR | NR | NR | NR | 10/80 (13) | NR |
| ETN + PBO | RCT | 6 months | NR | NR | NR | NR | 38/78 (49)b | NR | |
| RACAT111 | MTX + SSZ + HCQ On treatment analysis, n = 222 (some patients exposed to both treatments throughout trial) |
RCT including crossover | 48 weeks | 56/222 (25.2) | 4/222 (1.8) | NR | NR | NR | NR |
| ETN50 + MTX On treatment analysis, n = 219 (some patients exposed to both treatments throughout trial) |
RCT including crossover | 48 weeks | 82/219 (37.4) | 9/219 (4.1) | NR | NR | NR | NR | |
| Wajdula 2000 (reported in Chen et al., 2006123) | PBO | RCT | 12 weeks | NR | NR | NR | 1/105 (1.0) | NR | NR |
| ETN | RCT | 12 weeks | NR | NR | NR | 0/111 | NR | NR | |
| Weinblatt et al., 1999124 | PBO + MTX | RCT | 24 weeks | 19/30 (63) | NR | NR | NR | 21/30 (7) | NR |
| ETN + MTX | RCT | 24 weeks | 30/59 (51) | NR | NR | NR | 25/59 (42) | NR | |
| Kremer et al., 2003160 (LTE of Weinblatt et al., 1999124) | ETN + MTX or MTX + PBO followed by ETN + MTX (n = 79) | LTE | 3-year LTE | NR | 4/79 (5.1) required hospitalisation | NR | 3/79 (3.8) | NR | NR |
| GO-FORTH91 | PBO + MTX | RCT | 24 weeks | 39/88 (44.3) | 0/88 | NR | 0/88 | 7/88 (8.0) | N/A |
| GOL + MTX | RCT | 24 weeks | 36/86 (41.9) | 0/86 | NR | 0/86 | 8/86 (9.3) | N/A | |
| GO-FORWARD92 | PBO + MTX | RCT | 24 weeks | 37/134 (27.6) | 1/134 (0.7) (Urinary tract infection) |
NR | 1/134 (0.7) (Basal cell cancer) |
4/134 (3.0) | N/A |
| GOL + MTX | RCT | 24 weeks | 34/212 (16.0) | 2/212 (0.9) (One cellulitis, one s.c. abscess) |
NR | 0 | 5/212 (2.4) | N/A | |
| GO-FORWARD92 | PBO + MTX | RCT | 52 weeks | 42/133 (31.6) | 1/133 (0.8) | NR | 2/133 (1.5) | 4/133 (3.0) | N/A |
| GOL + MTX | RCT | 52 weeks | 98/212 (46.2) | 4/212 (1.9) | NR | 3/212 (1.4) | 10/212 (4.7) | N/A | |
| Kay et al., 200898 | IFX + MTX (PBO group crossed over to IFX at week 20) | RCT | 52 weeks | 9/25 (36.0) | 1/25 (4.0) | NR | 0/25 | N/A | NR |
| GOL + MTX | RCT | 52 weeks | 23/37 (62.2) | 1/37 (2.7) | NR | 1/37 (2.7) | 6/37 (16.2) | N/A | |
| Abe et al., 200656 | PBO + MTX | RCT | 14 weeks | 17/47 (36.2) | NR (Pneumonia = 0) |
NR | NR | N/A | 17/47 (36.2) |
| IFX + MTX | RCT | 14 weeks | 22/49 (44.9) | NR [Pneumonia = 1 (2.0)] |
NR | NR | N/A | 23/49 (46.9) | |
| Abe et al., 200656 | PBO group crossover to IFX | LTE | To week 36 of LTE | 22/41 (53.7) | NR | NR | NR | N/A | 17/41 (41.5) |
| IFX + MTX | LTE | To week 36 of LTE | 31/49 (63.3) | NR | NR | NR | N/A | 33/49 (67.3) | |
| ATTRACT75/Lipsky et al., 2000146 | PBO i.v. + MTX | RCT | 54 weeks | NR | 7/86 (8.1) | 35% | 0 | N/A | Serious infusion reactions = 0 |
| IFX + MTX | RCT | 54 weeks | NR | 2/88 (2.3) | NR | 0 | N/A | 0 | |
| ATTRACT75/Maini et al., 2004324 | PBO i.v. + MTX | LTE | 102 weeks | NR | 11/NR (13) | NR | 1/NR (1) | N/A | Serious infusion reactions = 0 |
| IFX + MTX | LTE | 102 weeks | NR | 10/NR (11) | NR | 1/NR (1) | N/A | Serious infusion reactions = 0 | |
| Durez et al., 200486 | MP + MTX | RCT | 14 weeks (n unclear) | NR | 0/NR | NR | NR | N/A | 0/NR |
| IFX + MTX | 14 weeks (n unclear) | NR | 0/NR | NR | NR | N/A | 0/NR | ||
| START118 | PBO + MTX | RCT | 22 weeks | 38/361 (10.5) (Upper respiratory tract infection) |
6/361 (1.7) | 0/361 | Serious infusion reactions: 1/361 (0.3) | ||
| IFX + MTX | RCT | 22 weeks | 35/360 (9.7) (Upper respiratory tract infection) |
6/360 (1.7) | 2/360 (0.6) | Serious infusion reactions = 0 | |||
| START118 | IFX + MTX (not dose escalated) | LTE | 54 weeks | 119/244 (49) | 11/244 (4.5) | 1/244 (0.4) | Serious infusion reactions: 2/244 (0.8) | ||
| Swefot119 | SSZ + HCQ + MTX | RCT | 24 months | AEs = 1/130 (0.8) | NR | NR | AEs = 0 | NR | NR |
| IFX + MTX | RCT | 24 months | AEs = 8/128 (6.3) | NR | NR | AEs = 1/128 (0.8) | NR | NR | |
| ACT-RAY152 | TCZ + oral PBO | RCT | 52 weeks | NR | 9/276 (3.3) | NR | NR | N/A | NR |
| TCZ + MTX | RCT | 52 weeks | NR | 10/277 (3.6) | NR | NR | N/A | NR | |
| Nishimoto et al., 2004106 | PBO | RCT | 12 weeks | NR | NR | NR | NR | N/A | 15% |
| TCZ | RCT | 12 weeks | NR | NR | NR | NR | N/A | 16% | |
| STREAM325 (LTE of Nishimoto et al., 2004106) | PBO | LTE | To year 5 | – | – | – | – | – | – |
| TCZ | LTE | To year 5 | NR | 25/143 (17.5) | NR | 4/143 (2.8) (Bladder cancer, breast cancer, large intestine carcinoma, intraductal papilloma) |
N/A | NR | |
| SAMURAI115 | cDMARDs | RCT | 52 weeks | NR | 8/145 (5.5) | NR | 0/145 | NR | N/A |
| TCZ | RCT | 52 weeks | NR | 12/157 (7.6) | NR | 3/157 (1.9) | NR | 11/157 (7.0) | |
| SATORI116 | PBO + MTX | RCT | 24 weeks | NR | NR | NR | NR | N/A | NR |
| TCZ + PBO capsules | RCT | 24 weeks | NR | NR | NR | NR | N/A | 7/61 (11.5) | |
| TOWARD121 | PBO i.v. + stable cDMARDs | RCT | 24 weeks | 131/414 (31.6) | 8/414 (1.9) | NR | NR | N/A | NR |
| TCZ + stable DMARDs | RCT | 24 weeks | 300/802 (37.4) | 22/802 (2.7) | NR | NR | N/A | NR | |
| TACIT141 | Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Deaths, n/N (%) | Cause of death | Considered by investigators/adjudicators to be related to study drug? |
|---|---|---|---|---|---|
| GUEPARD93 | Initial MTX 12 weeks, then step-up therapy in both groups based on DAS28 | 1 year | 0/32 | N/A | N/A |
| Initial ADA + MTX 12 weeks, then step-up therapy in both groups based on DAS28 RACAT | 1 year | 0/33 | N/A | N/A | |
| HIT HARD94 | MTX + PBO for 24 weeks followed by OL MTX for 24 weeks | 48 weeks | 0/85 | N/A | N/A |
| ADA + MTX for 24 weeks followed by OL MTX for 24 weeks | 48 weeks | 0/87 | N/A | N/A | |
| OPERA107 | MTX + PBO + steroid | 12 months | 1/91 (1.1) | Pneumonia 4 months after terminating the study | NR |
| ADA + MTX + steroid | 12 months | 0/89 | N/A | N/A | |
| PREMIER109 | MTX + PBO | 2 years | 1/257 (0.4) | Pneumonia | NR |
| ADA monotherapy + PBO | 2 years | 4/274 (1.5) | One chronic obstructive pulmonary disease/pulmonary disease and pulmonary hypertension sudden death; one metastatic liver cancer (unknown primary); one metastatic colon cancer; one liver failure (pre-existing cirrhosis) | NR | |
| ADA + MTX | 2 years | 1/268 (0.4) | Ovarian cancer | NR | |
| PREMIER109 | MTX + PBO to OL ADA monotherapy | 5 years LTE | NR (0.6) | NR | NR |
| ADA monotherapy + PBO to OL ADA monotherapy | 5 years LTE | NR (0.6) | NR | NR | |
| ADA + MTX to OL ADA monotherapy | 5 years LTE | NR (1.1) | NR | NR | |
| COMET83 | MTX in year 1, MTX in year 2 | 2 years | 1/99 | Pneumonia and adenocarcinoma of the lungs with metastasis | NR |
| MTX in year 1, ETN + MTX in year 2 | 2 years | 0 | N/A | N/A | |
| ETN + MTX in year 1, ETN + MTX in year 2 | 2 years | 0 | N/A | N/A | |
| ETN + MTX in year 1, ETN in year 2 | 2 years | 1/111 | Pneumonia | NR | |
| ERA139 | MTX + PBO | 12 months | 0/217 (0) | N/A | N/A |
| ETN + PBO | 12 months | 1/207 (0.5) | Non-infectious complications resulting from dissection of a pre-existing aortic aneurysm | NR | |
| ERA139 | MTX + PBO | 2 years | 0/217 (0) | N/A | N/A |
| ETN + PBO | 2 years | 1/207 (0.5) | See above | N/A | |
| GO-BEFORE143 | PBO + MTX | RCT 24 weeks | 0 | N/A | N/A |
| GOL + MTX | RCT 24 weeks | 1 | Suicide | NR | |
| GO-BEFORE143 | PBO + MTX | LTE 104 weeks | 0 | N/A | N/A |
| GOL + MTX | LTE 104 weeks | 4/159 (2.5) | One hypoglycaemic coma, one lung cancer, one septic shock, one probable non-small cell lung cancer | NR | |
| ASPIRE71 | PBO i.v. + MTX | RCT 54 weeks | 2 | One due to respiratory failure attributed to MTX-related drug toxicity, one due to upper gastrointestinal bleed | NR |
| IFX + MTX | RCT 54 weeks | 1 | Cardiac arrest | NR | |
| Durez et al., 2007120 | MTX | 52 weeks | 0/14 | N/A | N/A |
| MTX + MP | 52 weeks | 0/15 | N/A | N/A | |
| IFX + MTX | 52 weeks | 0/15 | N/A | N/A |
| Trial name | Treatment arms for which data extraction performed | Assessment time point | Deaths, n/N | Cause of death | Considered by investigators/adjudicators to be related to study drug? |
|---|---|---|---|---|---|
| ATTEST74 | PBO + MTX | RCT day 197 | 0 | N/A | N/A |
| IFX + MTX | RCT day 197 | 1/165 | Cerebrovascular accident | NR | |
| ABT + MTX | RCT day 197 | 1/156 | Fibrosarcoma | NR | |
| ATTEST74 | PBO + MTX | RCT day 365 | No further deaths | N/A | N/A |
| IFX + MTX | RCT day 365 | One additional death | Patient with peritoneal tuberculosis, death due to septic shock following surgery | NR | |
| ABT + MTX | RCT day 365 | No further deaths | N/A | N/A | |
| AMPLE66 | ABT s.c. | 1 year | 1/318 | Sudden cardiac arrest | No |
| ADA | 1 year | 0/328 | N/A | N/A | |
| RED-SEA114 | ADA + cDMARDs | 12 months | 2/60 | Both ischaemic heart disease | NR |
| ETN50 + cDMARDs | 12 months | 0/60 | N/A | N/A | |
| ADACTA58 | TCZ + oral PBO | 24 weeks | 2/162 | One sudden death, one illicit drug overdose | Overdose considered by study team unrelated to study drug. Sudden death considered by study team to be possibly related to study drug (unautopsied) |
| ADA + i.v. PBO | 24 weeks | 0 | N/A | N/A |
| Trial name/study | Treatment arms for which data extraction performed | Assessment time point | Deaths, n/N | Cause of death | Considered by investigators/adjudicators to be related to study drug? |
|---|---|---|---|---|---|
| AIM61,62 | MTX + PBO | 12 months | 1/219 | Pneumonia, sepsis, and multiorgan failure | NR |
| ABT i.v. + MTX | 12 months | 1/433 | History of tuberculosis, asbestos exposure and pulmonary fibrosis, died of bronchopneumonia, pulmonary aspergillosis, and septicaemia | NR | |
| AIM65 | ABT i.v. + MTX 2 years or MTX + PBO 1 year then ABT i.v. + MTX 1 year | LTE 3 years | 9/593 during LTE | Myocardial ischaemia with postprocedural complications, lobar pneumonia, lung cancer, pneumonia/sepsis, malignant melanoma, aortic aneurysm, three cases of cardiac arrest | NR |
| ASSET72 | PBO + MTX | 4 months | 0/23 | N/A | N/A |
| ABT i.v. + MTX | 4 months | 0/27 | N/A | N/A | |
| ASSET72 | ABT i.v. + MTX | 1-year LTE | 0/49 | N/A | N/A |
| ASSURE73 | PBO + cDMARDs | 1 year | 4/418 (1.0%) | Congestive heart failure, cardiopulmonary arrest, cardiac arrest, pneumonia | Three no, one possibly |
| ABT + cDMARDs | 1 year | 5/856 (0.6%) | Hypertensive heart disease, coronary atherosclerosis/acute ischaemic cardiopathy, central atherosclerosis/advanced coronary atherosclerosis with focal stenosis, cardiac arrest | Four no, one cannot tell (unautopsied) | |
| AUGUST II76 | MTX + PBO | 38-week follow-up of 26-week RCT treatment | 0/76 | N/A | N/A |
| ADA + MTX | 38-week follow-up of 26-week RCT treatment | 0/79 | N/A | N/A | |
| CHANGE80 | PBO | 24 weeks | 0/87 | N/A | N/A |
| ADA monotherapy | 24 weeks | 1/91 (1.1%) | Interstitial lung disease and lung infection | Considered possibly related to treatment | |
| DE01984 | MTX + PBO | 52 weeks | 0/200 | N/A | N/A |
| ADA + MTX | 52 weeks | 2/207 | One related to multiple fractures and one related to urosepsis | NR | |
| STAR117 | PBO + cDMARDs | 24 weeks | 0/318 | N/A | N/A |
| ADA + cDMARDs | 24 weeks | 1/318 (0.3%) | Secondary streptococcal A superinfection | NR | |
| Van De Putte et al., 2004122 | PBO s.c. | 26 weeks | 1 | Complications of bowel obstruction | All stated by authors to be unrelated or unlikely to be related to study drug |
| ADA monotherapy | 26 weeks | Three in ADA group (dose not specified) | Metastatic adenocarcinoma, cholangiocarcinoma, and myocardial infarction | ||
| ARMADA69 | MTX + PBO | 24 weeks | 0/62 | N/A | N/A |
| ADA + MTX | 24 weeks | 0/67 | N/A | N/A | |
| ARMADA70 | ADA + MTX | 4 years LTE | 6/262 | Congestive heart failure, acute myocardial insufficiency, aortic aneurysm previously treated surgically, cerebrovascular accident, intracranial haemorrhage, acute kidney failure | NR |
| Kim et al., 200799 | MTX + PBO | 24 weeks | 0/63 | N/A | N/A |
| ADA + MTX | 1/65 | Acute respiratory distress syndrome | NR | ||
| ADORE59,60 | ETN monotherapy | 16 weeks | 0/159 | N/A | N/A |
| ADORE59 | ETN + MTX | 16 weeks | 3/155 | Cardiac arrhythmia that occurred 1 month after the patient discontinued study drugs, second due to cardiac arrest and third due to massive cerebral haemorrhage | All considered to be unrelated to study drugs by the investigator |
| CREATE IIb96 | DMARD + PBO | 24 weeks | 0/65 | N/A | N/A |
| ETN50 + DMARD | 24 weeks | 0/64 | N/A | N/A | |
| ETN study 30989 | SSZ + PBO | 24 weeks | 0/50 | N/A | N/A |
| ETN + PBO | 24 weeks | 0/103 | N/A | N/A | |
| ETN + SSZ | 24 weeks | 0/101 | N/A | N/A | |
| RACAT111 | MTX + SSZ + HCQ On treatment analysis, n = 222 (some patients exposed to both treatments throughout trial) |
48 weeks | 0/222 | N/A | N/A |
| ETN50 + MTX On treatment analysis, n = 219 (some patients exposed to both treatments throughout trial) |
48 weeks | n = 1 (0.5%) Originally randomised and received MTX + SSZ + HCQ, switched to ETN50 + MTX |
Pneumonia | NR | |
| Weinblatt et al. 1999160 | PBO + MTX | 24 weeks (and 30 days after treatment) | 0/30 | N/A | N/A |
| Weinblatt et al. 1999160 | ETN 25 mg twice weekly + MTX | 24 weeks (and 30 days after treatment) | 0/59 | N/A | N/A |
| Weinblatt et al. 1999160 | ETN + MTX or MTX + PBO followed by ETN + MTX | 3-year LTE | 1/79 | Myocardial infarction | NR |
| APPEAL67,68 | MTX + DMARD (SSZ, HCQ or LEF) | 16 weeks | 0/103 | N/A | N/A |
| ETN + MTX | 16 weeks (study RCT end point) | 1/197 (0.5%) | Gastrointestinal haemorrhage thought to be result of NSAID therapy following accidental fall and pelvic fracture | No | |
| GO-FORTH91 | PBO + MTX | 24 weeks | 0/88 | N/A | N/A |
| GOL + MTX | 24 weeks | 0/86 | N/A | N/A | |
| GO-FORWARD92 | PBO + MTX | 24 weeks | 0/133 | N/A | N/A |
| GOL + MTX | 24 weeks | 0/89 One death in unlicensed GOL 100 mg every 4 weeks arm (ileus, aspiration pneumonia and death from sepsis) |
N/A | N/A | |
| Kay et al., 200898 | PBO + MTX (crossover to IFX + MTX at week 20) | 52 weeks | 0/35 | N/A | N/A |
| GOL + MTX | 52 weeks | 0/35 | N/A | N/A | |
| Abe et al., 200656 | PBO + MTX | 14 weeks | 0/47 | N/A | N/A |
| IFX + MTX | 14 weeks | 0/49 [two deaths but not in 3 mg/kg extracted dose (both due to pneumonia)] | NR | NR | |
| Abe et al., 200656 | PBO group crossover to IFX | To week 36 of LTE | N/A | N/A | N/A |
| IFX + MTX | To week 36 of LTE | 0/129 | N/A | N/A | |
| ATTRACT324 | PBO + MTX | LTE 102 weeks | 4/88 (4.5%) | Left ventricle rupture resulting in cardiopulmonary arrest, intestinal gangrene, arrhythmia and cardiopulmonary failure | Judged to be unrelated to study drug |
| IFX + MTX | LTE 102 weeks | 3/86 (3.5%) | Not reported separately for extracted arm | NR | |
| START118 | PBO + MTX | 22 weeks | 1/361 | Septic shock | NR |
| IFX + MTX | 22 weeks | 0/360 | N/A | N/A | |
| Swefot147 | SSZ + HCQ + MTX | 24 months | 0/130 | N/A | N/A |
| IFX + MTX | 24 months | 1/128 (0.8%) | Complications of acute myeloid leukaemia | NR | |
| ACT-RAY57 | TCZ + oral PBO | To week 52 | 2/276 (0.7%) | Causes of death in four patients: sepsis, septic shock preceded by scrotal abscess, skin necrosis, acute renal failure and congestive heart failure, myocardial infarction, and sepsis with meningitis | NR |
| TCZ + MTX | To week 52 | 2/277 (0.7%) | NR | NR | |
| Nishimoto et al., 2004106 | PBO | 12 weeks | 0/53 | N/A | N/A |
| TCZ | 12 weeks | 1/55 (1.8%) | Due to reactivation of chronic Epstein–Barr virus and consequent haemophagocytosis syndrome 61 days after single dose of TCZ 8 mg/kg i.v. | NR | |
| TOWARD121 | PBO i.v. + stable cDMARDs | 24 weeks | 2/413 (0.5%) | Pneumonia, intestinal obstruction | NR |
| TCZ + stable DMARDs | 24 weeks | 2/803 (0.3%) | Haemorrhagic stroke, postprocedural complications from triple coronary artery bypass graft | NR | |
| TACIT141 | Combination cDMARDs | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| TACIT141 | Combination cDMARDs | AiC information has been removed | AiC information has been removed) | AiC information has been removed | AiC information has been removed |
| TNF inhibitor + DMARD | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
List of abbreviations
- ABT
- abatacept
- ACR
- American College of Rheumatology
- ACT-RAY
- ACTemra (tocilizumab) RAdiographic studY
- ADA
- adalimumab
- ADACTA
- ADalimumab ACTemrA (tocilizumab) head-to-head study
- ADORE
- ADjuvant Oxaliplatin in REctal Cancer
- AE
- adverse event
- AiC
- academic-in-confidence
- AMBITION
- Actemra versus Methotrexate double Blind Investigative Trial In mONotherapy
- AMPLE
- abatacept vs. adalimumab in biologic naive RA patients with background MTX
- APPEAL
- Asia-Pacific Study in Patients to be Treated With Etanercept or an Alternative Listed
- ARMADA
- Anti-TNF factor Research study program of the Monoclonal antibody ADalimumab (D2E7) in rheumatoid Arthritis
- ATTEST
- A Trial for Tolerability, Efficacy, and Safety in Treating rheumatoid arthritis (infliximab)
- ATTRACT
- Anti-TNF trial in Rheumatoid Arthritis with Concomitant Therapy
- AUGUST II
- A Phase II Dose-finding Study of Atacicept in Rheumatoid Arthritis (RA)
- AZA
- azathioprine
- bDMARD
- biologic disease-modifying antirheumatic drug
- BeST
- BEhandelings STrategie
- BMJ
- British Medical Journal
- BSRBR
- British Society for Rheumatology Biologics Register
- cDMARD
- conventional disease-modifying antirheumatic drug
- CEAC
- cost-effectiveness acceptability curve
- CERTAIN
- efficacy and safety of CERTolizumab pegol After INcomplete response to DMARDS in RA patients with low to moderate disease activity
- CG
- clinical guideline
- CI
- confidence interval
- CiC
- commercial-in-confidence
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- COMET
- Combination Of METhotrexate and etabercept in early rheumatoid arthritis
- CrI
- credible interval
- CRP
- C-reactive protein
- CTZ
- certolizumab pegol
- CYC
- ciclosporin
- DAS
- Disease Activity Score
- DAS28
- Disease Activity Score 28 joints
- DAS28-CRP
- Disease Activity Score 28 C-reactive protein
- DAS28-ESR
- Disease Activity Score 28 erythrocyte sedimentation rate
- DE019
- Efficacy and Safety of Adalimumab in Patients With Active Rheumatoid Arthritis Treated Concomitantly With Methotrexate
- DES
- discrete event simulation
- DMARD
- disease-modifying antirheumatic drug
- EQ-5D
- European Quality of Life-5 Dimensions
- ERAS
- Early Rheumatoid Arthritis Study
- ESR
- erythrocyte sedimentation rate
- ETN
- etanercept
- EULAR
- European League Against Rheumatism
- FACIT-F
- Functional Assessment of Chronic Illness Therapy – Fatigue
- FDA
- Food and Drug Administration
- GLD
- gold injection
- GO-BEFORE
- GOlimumab in active rheumatoid arthritis BEFORE methotrexate therapy
- GO-FORTH
- golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis
- GO-FORWARD
- golimumab in active rheumatoid arthritis despite methotrexate therapy
- GOL
- golimumab
- GP
- general practitioner
- GUEPARD
- GUÉrir la PolyARthrite rheumotoide Débutante (adalimumab)
- HAQ
- Health Assessment Questionnaire
- HAQ-DI
- Health Assessment Questionnaire Disability Index
- HCQ
- hydroxychloroquine
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IFX
- infliximab
- i.v.
- intravenous
- JESMR
- Japanese Efficacy and Safety of Etanercept on Active Rheumatoid Arthritis Despite Methotrexate Therapy
- LARA
- Latin American Rheumatoid Arthritis study
- LEF
- leflunomide
- LITHE
- tocilizumab safety and the prevention of structural joint damage methotrexate and sulfasalazine combination trial
- LTE
- long-term extension
- MP
- methylprednisolone
- MSD
- Merck Sharp & Dohme Corp.
- MTX
- methotrexate
- NBT
- non-biologic therapy
- NDB
- National Data Bank for Rheumatic Diseases
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NOAR
- Norfolk Arthritis Register
- NSAID
- non-steroidal anti-inflammatory drug
- OMERACT
- Outcome Measures in Rheumatoid Arthritis Clinical Trials
- OPERA
- OPtimized treatment algorithm for patients with Early Rheumatoid Arthritis
- OPTION
- tOcilimumab Pivotal Trial In methotrexate inadequate respONders
- PAS
- Patient Access Scheme
- PBO
- placebo
- PREMIER
- Patients REceiving Methotrexate and Infliximab for the treatment of Early Rheumatoid arthritis
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RA
- rheumatoid arthritis
- RACAT
- Rheumatoid Arthritis Comparison of Active Therapies in Methotrexate Suboptimal Responders study
- RAPID
- Rheumatoid Arthritis Prevention of structural Damage
- RAQoL
- Rheumatoid Arthritis Quality of Life
- RCT
- randomised controlled trial
- RED-SEA
- a Randomised Efficacy and Discontinuation Study of Etanercept versus Adalimumab
- RTX
- rituximab
- SAMURAI
- Study of Active controlled Monotherapy Used for Rheumatoid Arthritis, an IL-6 Inhibitor
- s.c.
- subcutaneous
- ScHARR
- School of Health and Related Research
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SSZ
- sulfasalazine
- START
- Safety Trial for rheumatoid Arthritis with Remicade Therapy
- Swefot
- Swedish pharmacotherapy
- TA
- technology appraisal
- TACIT
- tumour necrosis factor inhibitors against combination intensive therapy
- TCZ
- tocilizumab
- TEAR
- Treatment of Early Aggressive Rheumatoid arthritis
- TEMPO
- Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes
- TNF
- tumour necrosis factor
- TNF-α
- tumour necrosis factor alpha
- TOF
- tofacitinib
- TOWARD
- TOcilizumab in combination With traditional DMARD therapy
- VARA
- Veterans Affairs Rheumatoid Arthritis
- VAS
- visual analogue scale
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed commercial-in-confidence and/or academic-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence and/or academic-in-confidence data removed and replaced by the statement ‘commercial-in-confidence and/or academic-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.