Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/63/03. The contractual start date was in August 2012. The draft report began editorial review in March 2015 and was accepted for publication in November 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Dr Fortnum and Professor Taylor were co-authors on the previous Health Technology Assessment (HTA) publication reporting evaluation of the school entry hearing screen [Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al. Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen. Health Technol Assess 2007;11(32)]. Professor Taylor is chairperson of the National Institute for Health Research (NIHR) Health Services and Delivery Research researcher-led panel, March 2014–February 2016 (appointment extended to February 2018), and a member from 2013. He is also a member of NIHR Priority Research Advisory Methodology Group (PRAMG), August 2015–present, is a core member of NIHR HTA Themed Call Board, 2012–present and is a member of the core group of methodological experts for the NIHR Programme Grants for Applied Research programme, 2013–present.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Fortnum et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and main questions
Childhood hearing impairment and screening
Identification of permanent hearing impairment at the earliest possible age is crucial to maximise the development of speech and language and contribute to the best opportunities for educational achievement and quality of life. 1 Approximately 1 in every 1000 children born in the UK has a permanent bilateral hearing impairment of > 40 dB (average across four frequencies: 0.5, 1, 2 and 4 kHz) and a further 0.6 per 1000 has a unilateral impairment. 2 This equates to 800 children per year born with a permanent bilateral hearing impairment (moderate or greater) and 500 with a unilateral impairment. The introduction of the highly sensitive and specific Universal Newborn Hearing Screening (UNHS) programme has led to the identification of the vast majority of children born with a hearing impairment who undergo the screen. 3,4 However, not all children who will ultimately have a hearing impairment are identifiable at birth. Data published in 20015 reported an adjusted prevalence of permanent hearing impairment of > 40 dB (average of 0.5, 1, 2 and 4 kHz) at age 3 years of 1.07 per 1000 and a prevalence for children aged 9–15 years of 2.05 per 1000. Thus, because of acquisition, progression or late onset of hearing impairment and/or geographical movement of families, there remain a significant number of children to be identified with a permanent hearing impairment after the newborn period. The onset of hearing impairment in children after the newborn period can occur at any time, which means there is no optimum time for a further universal hearing screen. The universal distraction hearing test, established in the UK in the 1950s and undertaken by health visitors at around 8 months of age, was abandoned following the introduction of UNHS, based on a lack of robust implementation and a low yield of cases. 6,7 Identification of hearing impairment in children in the time between the newborn period and school entry is achieved through parental and professional awareness and a close follow-up of children who pass the neonatal screen but are considered to be at risk. 8 A universal hearing screen when children start school, the school entry screening (SES) programme, was established in 1955 and remains in place in many parts of the UK. It is considered as a ‘back-stop’ screen to identify children as part of a ‘captive population’ at school entry. Note: SES always refers to the hearing screening in this report.
A number of studies and reviews2,9–12 have explored evidence for the value of the SES programme but without clear conclusions. Research has shown that the number of children identified by this screen around age 5 years (the yield) has decreased following the introduction of UNHS, and widespread development of a system that is responsive to professional and parental concerns at any age. 8,12 The SES programme is no longer universally applied. Bamford et al. 12 reported in 2007 that one in eight services had stopped offering the screen by 2005 and it is more likely that others have stopped since 2005 than that services have reinstated the screen. There are no guidelines on standard methodology nationally and procedures are variable. However, despite a lack of evidence of its value, many support its continuation as a ‘back-stop’ to identify an acknowledged small number of children who would otherwise not be identified with the consequent effect on their development of speech and language.
Childhood hearing impairment can be permanent (usually sensorineural although permanent conductive impairment also occurs) and will not improve, or transient (also referred to as conductive) and will usually fluctuate and can get better.
A sensorineural impairment occurs when the inner ear (cochlea) or auditory nerve does not function properly; this can be caused by many factors. As mentioned, the majority of children with sensorineural impairments will have been identified by the newborn screening programme. However, there is the possibility that a child has a progressive impairment that, at birth, was minimal and therefore enabled them to pass the newborn screen. It is also possible for a child to develop a sensorineural impairment. Sensorineural impairments are permanent and require management to ensure that the child has access to language; management is usually to fit children with hearing aids or cochlear implants.
Many more children will have a transient hearing impairment at some point in their childhood than will have a permanent impairment. A conductive loss occurs when there is a problem with the outer or middle ear, such as impacted wax in the ear canal or a build up of fluid in the middle ear [otitis media with effusion (OME), which usually is associated with colds and respiratory tract infections]. Conductive impairments are usually temporary, unless there is a malformation of the outer or middle ear, which can lead to a permanent impairment. Prevalence is greatest in the first year of life and again at around age 4–5 years. For children < 6 years, 80% will experience OME. 13,14 Most episodes of OME get better in a couple of months but a small proportion (3–4%) can be persistent and severe and lead to problems with behaviour, communication and progress at school. It is therefore important to identify those children with an ongoing problem, as their hearing impairment needs management to ensure that they can access spoken language clearly. The recommended management options are initially to watch and wait. Owing to the fact that the majority of these transient impairments will resolve spontaneously, most children will be observed for 3 months before any active management takes place, then the usual options are surgical insertion of grommets if the child’s hearing impairment meets national recognised criteria,15 or for them to be fitted with a hearing aid.
Some children with permanent sensorineural impairments may also have an additional transient conductive impairment that would require management.
Screening tests for hearing impairment identify any child who has a hearing impairment. The tests do not discriminate between a permanent impairment and a transient impairment. They are also able to identify a hearing impairment in one ear or in both ears. Thus, all children with a hearing impairment, whether permanent or transient, bilateral or unilateral, should be identified by the screening test if it is 100% sensitive.
Testing children between the ages of 4 and 6 years can be difficult. Most tests require the engagement of the child in a simple task, such as raising their hand or putting a ball on a stick. Some children may find it difficult to maintain their attention throughout the test and this can give rise to spurious results. It is important that the screener/tester has experience in working with children to enable them to identify when their attention could be affecting the results of the test and be able to change their own behaviour accordingly to engage with the child. At any age it is possible that some children may not be able to co-operate with the testing because of specific learning or behavioural needs.
The pure-tone screen (PTS) (Amplivox, Eynsham, UK) test involves placing headphones over the child’s ears and then presenting pure tones across the key frequencies for speech understanding (0.5, 1, 2 and 4 kHz). The child needs to indicate by a simple action that they have heard a sound. The screen works on the basis that a child needs to hear two out of three presentations of each frequency at 20 dB hearing level (HL) in each ear to pass the screen.
The HearCheck (HC) screener (Siemens, Frimley, UK) is placed over the child’s ear and an automatic sequence of pure tones is played once at each of three levels at each of the frequencies 1 kHz (55 dB, 35 dB and 20 dB) and 3 kHz (75 dB, 55 dB, 35 dB), which is six tones in total. The child needs to indicate, usually by raising their hand that they have heard each tone.
Any child identified by a screening test as having a possible hearing impairment should be referred to audiology services, but many children at school entry have a transient conductive impairment and hence it is the case that many children referred will ultimately be found to have no permanent impairment.
The commonly used screening method of the PTS can identify all types of impairment. Specifically including tympanometry would indicate a problem with the middle ear, but only by including both air and bone conduction thresholds from pure-tone audiometry (PTA), masked where necessary, could a conductive impairment be indicated.
This is important because there are conflicting opinions on the target group of children to be identified by SES. Should it be designed to only identify children with a permanent impairment for whom intervention is a priority, or should it be designed to identify any impairment to ensure every child is assessed appropriately and intervention provided for all who would benefit, regardless of the permanence of the condition? For this report, in both the analyses of diagnostic accuracy in Chapter 3 and in the analyses of yield in Chapter 5, we have considered identification of any type of impairment as the outcome for assessing the screening tests or the screening programme as a whole.
Previous Health Technology Assessment study
A previous National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme-commissioned study to evaluate SES by Bamford et al. 12 reported a survey of current practice, longitudinal data on yield, a systematic review of effectiveness of SES (which included a systematic review of the diagnostic accuracy of screening tests) and an economic model estimating cost-effectiveness. The 2007 HTA report12 concluded that there was insufficient good-quality data on which to base a decision about the value of SES following the introduction of UNHS. The executive summary of the report is included as Appendix 1.
However, the 2007 study did report longitudinal data from a single district in London which indicated a small but significant number of children with a permanent hearing impairment first identified via SES in that particular population,8 and national survey data which reported examples of children not identified by other methods.
One of the recommendations of the 2007 HTA report12 was the need for trials to compare the effectiveness and cost-effectiveness of alternative approaches to the identification of a post-newborn screen for permanent hearing impairment. Studies concerned with the relative accuracy (in terms of sensitivity and specificity) of alternative screening tests are difficult to compare and are often flawed by differing referral criteria and differing case definitions. The 2007 HTA report12 identified 25 publications reporting studies of alternative screens or tests for screening at school entry. These data indicate that, using full PTA as the reference standard, the PTS test appears to have high sensitivity and high specificity for minimal, mild and greater hearing impairments – better than alternative tests for which evidence was identified. Spoken word tests were reported with acceptable levels of sensitivity and specificity but are variable in their implementation. Otoacoustic emissions (OAEs), tympanometry, acoustic reflectometry, parental questionnaires and otoscopy were reported with either variable or poor sensitivity and specificity. Only one study, published in 1980,16 has compared screening with no screening and the results were inconclusive.
Assessment of cost-effectiveness
In order to best provide a service for the identification of permanent childhood hearing impairment while making best use of scarce NHS resources, it is important to gather robust evidence to support particular cost-effective implementations of service delivery at times relevant to the aetiology of hearing impairment and the child’s development. There is no question that screening for hearing impairment at birth is efficient and cost-effective,3 but the value of any further universal screen remains uncertain. Aside from the minimal and very weak evidence for the effectiveness and cost-effectiveness of different implementations of SES reported by the 2007 HTA report12 we are not aware of literature on the resource implications of different implementations or different technologies. A version of the HC screener has been evaluated as a screening tool in children in only one published paper,17 but it did not report any resource use.
Study aims and objectives
The two overarching aims of this project were to evaluate the diagnostic accuracy of hearing screening tests and to assess the cost-effectiveness of screening for hearing impairment at school entry. The specific research objectives of this project were:
-
To determine and compare the diagnostic accuracy of two screening methods used to identify hearing impairment at or around school entry. These are the widely established PTS (which is applied using headphones) and the HC screener, a hand-held PTS.
-
To investigate the impact of a potential false-negative result.
-
To update the 2007 HTA report12 systematic review of diagnostic accuracy of tests used for SES.
-
To assess the yield, referral age and route through assessment to intervention for childhood hearing impairment and measure the costs of referrals for a service that employs a routine SES programme and for a service that does not.
-
To determine the impact, both psychological and economic, on the child and the family of the child being referred for further assessment following the school entry hearing screen (both true and false positives).
-
To determine the resource costs in implementing either of the two alternative screening methods in primary schools and to elicit the views of the school nurses implementing the screening tests.
-
To refine an existing SES economic model (from the 2007 HTA report12) and to assess the cost-effectiveness of SES.
-
To provide estimates of the yield and nature of hearing impairment detected in a system with no SES system; the yield, consequences and costs of screen-positive individuals in a SES system; and the costs of setting up a SES system.
This study thus addressed the question of whether or not there should be a screening programme to identify permanent hearing impairment in children when they start primary school. It assessed if the cost of such a screen is appropriate for the outcomes achieved, that is, the number of children identified by this method compared with a system with no screen, which is responsive to parental or professional concern, along with comparisons of diagnostic accuracy of two different ways of doing the screen. Based on the findings, we make recommendations to contribute to decisions regarding the continued implementation of SES and the form that implementation should take.
Structure of the project and the report
The project comprised four primary studies, a questionnaire survey and two systematic reviews. The planned participant flow for the four studies is illustrated in Figure 1.
FIGURE 1.
Planned participant flow.

Chapter 2 reports results from a systematic review undertaken to update the 2007 HTA report12 review of diagnostic accuracy of tests used for SES.
Chapter 3 reports results from the study that assessed the diagnostic accuracy of two methods of screening for the identification of hearing impairment for children aged 4–6 years. The PTS (four frequencies and one level) was compared with the HC screener (two frequencies and three levels) with respect to sensitivity and specificity.
Chapter 4 reports results from a systematic review of the issues around false negatives in screening for hearing impairment, and from the diagnostic accuracy study described above.
Chapter 5 reports and compares outcomes, including yield and age at referral, for an area where SES is in place (Nottingham) and an area where there has been no SES since 1997 (Cambridge).
Chapter 6 reports results from a questionnaire survey of parents of children referred to audiology services in Nottingham from SES. Data on parent experience are reported.
Chapter 7 reports results of a study of the practical implementation of the two screening tests in a primary school environment.
Chapter 8 reports results from the cost-effectiveness model of SES. Data from Chapters 3, 5, 6 and 7 informed the parameters in the model.
Finally, Chapter 9 summarises the discussion points from each part of the project and makes conclusions and recommendations.
Patient and public involvement
We acknowledge the importance of the involvement of members of the public in all health research. This project evaluated a screening system for children and input from parents to the development and interpretation of the research question was very important. Recruiting such a person proved challenging, as parents have childcare responsibilities and/or employment considerations, which mean they have little time to spare and commit to a 2.5-year project. Having advertised and discussed the project with several parents, most of whom were unable to commit the time, we recruited the parent of a child who had experienced conductive hearing impairment during his early school years. This parent became a full member of the research team (JW).
He provided comments on research literature for parents, including the questionnaire used for the survey of parents of children referred to audiology services from SES. He also contributed to the development of methodology, offering advice on how to deal with lower-than-expected recruitment. He attended all the meetings of the research team, either in person or via the telephone, and contributed to discussions of the findings, presentation of results and development of recommendations. The project addressed various issues concerned with the identification of hearing impairment in children, and we recognise that individual parents may have experience of only one part of the service. Nonetheless, they bring the lay perspective to research design and, as an individual, represent other parents. To access input from a wider range of parents we recruited a representative from the National Deaf Children’s Society to the project steering group. Based on the guidelines issued by INVOLVE (the organisation funded by NIHR to support public involvement in NHS, public health and social care research) we provided reimbursement to the parent joining the research team for their input of an average of 1 day per month to advise the design and attend research meetings. In addition all travel costs were reimbursed.
Chapter 2 Update of the diagnostic accuracy systematic review
Introduction
This chapter presents an update of the systematic review of the diagnostic test accuracy undertaken in the 2007 HTA report. 12 That report identified 25 publications reporting studies of alternative screens or tests for screening at school entry, showing that, using full PTA as the reference standard, the PTS test appears to have high sensitivity and high specificity for minimal, mild and greater hearing impairments – better than alternative tests for which evidence was identified. Other tests (spoken word tests, OAEs, tympanometry, acoustic reflectometry, parental questionnaires and otoscopy) had either variable or poor sensitivity and specificity. Using additional evidence published since the 2007 HTA report, the aim of this update was to produce an updated summary of diagnostic test accuracy.
Objectives
To update the 2007 HTA report systematic review of diagnostic accuracy of tests used for SES. This work summarises the literature that has been published since the previous review and draws together the evidence from the previous review and the updated review.
Methods
Search strategy
The search strategy used and published in the 2007 HTA report on SES12 was reviewed and updated by an information specialist in the Peninsula Technology Assessment Group and re-run to identify studies published in the period January 2005 (search cut-off date of 2007 report, May 2005) to July 2014 (see Appendix 2). The following electronic databases were searched: The Cochrane Library (via Wiley) (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects), MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid), EMBASE (via Ovid), Cumulative Index to Nursing and Allied Health Literature (via Ovid), PsycINFO (via Ovid), Science Citation Index (via Web of Science), Education Resources Information Center and ongoing trial databases (National Research Register, ClinicalTrials.gov and Research Findings Register).
Inclusion/exclusion criteria
The inclusion and exclusion criteria were identical to those of the 2007 HTA report12 unless stated otherwise:
-
Study design: we included all primary diagnostic accuracy studies regardless of their specific design.
-
Population: we included children 4–6 years old. We excluded studies with a discrete age range that was completely outside our criteria (e.g. 1–3 years or 7–10 years). If a study included 4- to 6-year-old children but the age range was too wide (e.g. 6–19 years) and the results for different age categories were not reported separately, the study was also excluded from the review. We included, however, studies that at least partially covered but slightly exceeded the 4–6 years age range (e.g. 5–10 years) provided they met all other inclusion criteria. Whenever relevant and possible, the age of the included children is noted in the list of excluded full-text studies (see Appendix 2).
-
Screening tests or programmes: studies that evaluated one or more of the following hearing screening tests were included: sweep PTA, single-frequency PTA, transient-evoked otoacoustic emissions (TEOAE), distortion product otoacoustic emissions, questionnaires, otoadmittance tests, tympanometry, reflectometry and speech audiometry. (Note: sweep PTA and PTS are alternative terms for the same procedure. Where publications have used the term sweep PTA this description has been maintained.) Tests had to have been undertaken in either a primary school or the community [e.g. community clinic, family home or general practitioner (GP) surgery]. This could include hearing screening as a component of a multifaceted screen, such as a school entry medical examination.
-
Comparator: no hearing screening or hearing screening based on different tests or test protocols. We did not exclude studies without a comparator (studies that evaluated a single screening test) as long as they measured the performance of the evaluated test against an acceptable reference standard.
-
Reference standard: we included all studies that assessed the accuracy of the evaluated test(s) against a reference standard that included PTA.
-
Outcomes: the 2007 HTA review12 had a wider scope and also included studies that reported (1) the screen performance, that is, uptake (the number of children who actually received the screen) and yield (the number of cases identified); and (2) screen effectiveness, that is, language skills, health-related quality of life, communication skills, social interaction and educational performance. The current update focused on diagnostic accuracy only and included studies that reported the diagnostic accuracy of the evaluated test(s), regardless of whether or not the reported data were sufficient to reconstruct two-by-two table(s).
-
Language: no language restrictions were applied to the search and selection of studies.
Selection of studies and data extraction
After removing the duplicates, all records identified by the electronic searches were screened independently by two reviewers (HF and ZZ) at title and abstract level. Full-text copies were obtained for all publications identified as potentially relevant by at least one of the reviewers. Their suitability for inclusion in the review was assessed by one reviewer (ZZ) and checked by a second reviewer (CH) against the criteria specified above. Data were extracted from included studies by one reviewer (ZZ), entered into an Excel 2010 (Microsoft Corporation, Redmond, WA, USA) spreadsheet and checked by a second reviewer (CH). Disagreements in the selection of studies and data extraction were resolved through discussion. Data were extracted from studies published in a language other than English with the help of a translator.
Assessment of the methodological quality
Although a new version of the quality assessment of diagnostic accuracy studies (QUADAS) tool used in the 2007 HTA review12 is now available, the difference between the two versions is mainly in the structure and process of customising the tool and does not concern the contents of the actual checklist. 18,19 Therefore, we assessed the methodological quality of the included studies using the original QUADAS checklist from the 2007 HTA review. This allowed for a direct comparison between the quality of the studies included in the 2007 report and in the current update. However, we provided more specific definitions of some QUADAS items that were not explicitly defined in the original checklist (see Appendix 2).
Statistical analysis and data synthesis
Whenever possible, two-by-two tables were used to report the numbers of true-positive, false-positive, false-negative and true-negative results. The sensitivity and specificity of the index test (the test under evaluation) were calculated using The Cochrane Collaboration’s Review Manager (RevMan) software version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). The same software was used to create forest plots of the paired sensitivity and specificity estimates and to plot the true-positive rate (sensitivity) versus the false-positive rate (1 - specificity) in receiver operating characteristic (ROC) space. Heterogeneity was investigated visually by examining the forest plots and the ROC plot, and a decision was made whether or not to pool the results across studies. As standard funnel plots and tests for publication bias are not recommended in systematic reviews of diagnostic accuracy studies, we did not investigate publication bias. 20
Results
Search results and selection of studies
The electronic searches returned 892 papers after removal of duplicates. Screening at title or/and abstract level led to 39 citations being identified as potentially relevant. A further seven citations were found through hand-searching.
We obtained full-text copies of 45 of these studies and failed to obtain one. 21 After full-text evaluation 10 studies were selected for inclusion in the review. 17,22–30 Figure 2 details the selection process while the reasons for exclusion of full-text papers are given in Appendix 2.
FIGURE 2.
Flow diagram of the selection process.

Characteristics of included studies
The characteristics of the included studies are summarised in Tables 1 and 2. Briefly, 10 primary studies were included in this update. 17,22–30 One was published before 200524 but was included here as it had been missed by the original 2007 HTA report. 12 The other nine studies were published in the period 2005–2014. 17,22,23,25–30 Four of them were conducted in China22,23,27,30 and one in each of the following countries: Brazil,25 Greece,29 Japan,26 Kenya,24 the Philippines17 and the USA. 28
| Source and country | Sampling and inclusion criteria | Exclusion criteria | Number of children (% male) | Mean age in years, (SD) or range |
|---|---|---|---|---|
| Bu et al.,22 2005, China | Convenience sample of children grade 1–6 studying at one primary school | No exclusion criteria (except for a lack of consent) | 317 (49.5) | 9.43 (1.79) |
| Georgalas et al.,29 2008, Greece | Convenience sample of primary school children (6–12 years old) in one geographical area | No exclusion criteria (except for a lack of consent) | 86 (52.0) | 8.7 (2.0) |
| Gloria-Cruz et al.,17 2013, Philippines | Convenience sample of grade one elementary school children from three metropolitan schools | No exclusion criteria (except for a lack of consent) | 418 (56.9) | 8.6 (n/a) |
| Li et al.,23 2009, China | Convenience sample of children grade 1–6 studying at one rural school | No exclusion criteria (except for a lack of consent) | 154 (n/a) | 9.3 (1.7) |
| McPherson et al.,30 2010, China | Convenience sample of children 6–8 years old from a mainstream urban primary school | No exclusion criteria (except for a lack of consent) but no children with known cognitive or hearing impairments took part | 80 (62.5) | 7.04 (0.74) |
| Newton et al.,24 2001, Kenya | Convenience sample of children attending nursery schools and child health clinics in districts with audiologically trained ENT officers | No exclusion criteria | 735 (49.1) | 5.2 (n/a); range 2.21–7.5 |
| Samelli et al.,25 2011, Brazil | Convenience sample of children 2–10 years old living in an underserved metropolitan area with no UNHS | None specified | 214 (59.3) | Range 2–10 |
| Soares et al.,26 2014, Japan | Convenience sample of children 3–5 years old from a mainstream kindergarten (unclear if children from the nearby special school for hearing impaired children were also included in this age group) | Children unable to lie down still for several minutes (unable to complete procedure); lack of consent | 115 (n/a) | Range 3–5 |
| Wu et al., 2014,27 China | Random sample (5%, randomisation procedure not described) of 6288 eligible children enrolled from 41 kindergartens from a district area with a total of 106 kindergartens | Children who refused to participate or had learning disabilities | 312 (52.2) | 5.06 (0.72) |
| Yin et al., 2009,28 USA | Convenience sample of children 2–6 years old who are socioeconomically at risk (14% special education students) attending preschools in a large, urban, metropolitan school district | No exclusion criteria specified (except for the lack of consent) but no children previously identified with hearing impairment took part | 135 (n/a) | Range 2–6 |
| Source and country | Index test(s) | Setting (index test) | Test administrator (index test) | Cut-off (index test) | Reference standard | Setting (reference standard) | Test administrator (reference standard) | Definition of hearing impairment |
|---|---|---|---|---|---|---|---|---|
| Bu et al., 2005,22 China | Questionnaire (CHQS) | Home | Parents/carers | No prespecified cut-off | Otoscopy, tympanometry and PTA | Same as TEOAE | Otolaryngologists, audiologists and nurses | Tympanogram type ‘B’ or an average threshold across four frequencies (0.5, 1, 2 and 4 kHz) > 20 dB HL in either ear |
| TEOAE (Madsen Celesta 503 connected to a laptop) | School; quiet but not sound-treated room during normal attendance hours; ambient noise monitored | Otolaryngologists, audiologists and nurses | Pass: SNR values (an average of 1.5–4 kHz) of at least 3 dB and whole-wave reproducibility of at least 50% | |||||
| Georgalas et al.,29 2008, Greece | TEOAE (ILO 92 recorder). (Also, in combination with tympanometry but results not reported) | School; partially sound-proofed rooms | Otolaryngologists | Pass: if the TEOAE spectrum was recorded at least 3 dB above the noise floor and halfway across the frequency bands of 2–3 kHz and 3–4 kHz | Otoscopy, tympanometry and PTA | Same as for the index test | Same as for the index test | Average threshold > 25 dB across 0.5, 1, 2 and 4 kHz in either ear (> 30 dB also used but no full data reported) |
| Gloria-Cruz et al.,17 2013, Philippines | Audiometry (Siemens HC Navigator) | School; quiet but not sound-treated room; ambient noise monitored | Not specified but probably an audiologist | Pass: green light; refer: yellow or red light (separate results for red and yellow were also reported) | PTA | Same as for the index test | Audiologist | > 40 dB at 0.5, 1, 2 or 4 kHz in either ear |
| Li et al.23 2009, China | Questionnaire (CHQS-II) | Home | Parents/carers | No prespecified cut-off | Otoscopy, tympanometry and PTA | School; quiet but not sound-treated room during normal attendance hours; ambient noise monitored | Medical doctors and audiologists, under the supervision of an otolaryngologist | Evidence of OME or ‘B’-type tympanogram or threshold > 40 dB at 0.5, 1, 2 or 4 kHz in either ear |
| McPherson et al.,30 2010, China | Audiometry (Home Audiometer Software version 1.83) | School; quiet but not sound-treated room during non-attendance days; ambient noise monitored | Speech and hearing science undergraduates who had received 12 hours’ training | Refer: > 40 dB at 0.5, 1, 2 or 4 kHz in either ear; results also reported after excluding 0.5 kHz data | PTA | Same as for the index test | Same as for the index test (test administrators randomly assigned each time) | > 40 dB HL at 0.5, 1, 2 or 4 kHz in either ear |
| Newton et al.,24 2001, Kenya | Questionnaire | Home, nursery, child health clinic | Three modes: (1) a nursery teacher interviewing parents; (2) parents/carers; (3) a community nurse interviewing parents | Refer: one or more answers suggesting hearing impairment or uncertainty (question 5 excluded from analysis) | ENT examination including otoscopy and PTA | Quietest possible room (not sound treated); ambient noise measured whenever possible | ENT officers trained to perform PTA | Average threshold (0.5, 1 and 4 kHz) > 40 dB bilaterally |
| Samelli et al.,25 2011, Brazil | Questionnaire | Home, kindergarten, school or health unit | Evaluators (unspecified) | Refer: score ≥ 6 (ROC optimised); two additional cut-offs established to distinguish between conductive and sensorineural hearing impairment | Otoscopy, tympanometry and PTA | Sound-attenuated testing room | Evaluators (unspecified) | > 15 dB HL (0.25–8 kHz), tympanogram type B, C, As, or Ad and/or an absence of acoustic reflexes with one or both ears |
| Soares et al.,26 2014, Japan | AABR (MB11 BERA-phone®, MAICO Diagnostic GmbH, Berlin, Germany) | Kindergarten; quiet room (no further details); ambient noise monitored | Audiologist | Pass: a ‘pass’ line on the device’s graphic screen; refer: when 180 seconds elapsed without achieving pass line | PTA (preceded by ENT examination) | Same as for the index test | Audiologist from the institution in which the children belonged | > 25 dB HL at frequencies between 0.5 and 4 kHz |
| Wu et al.,27 2014, China | Audiometry (Smart Hearing) | School; quiet but not sound-treated room; ambient noise measured | Screening personnel (unspecified) who had some training | Refer: > 30 dB HL at 1, 2 and 4 kHz in either ear (plus children who failed the guidance stage) | Otoscopy, tympanometry, PTA (standard or play) and distortion product OAEs | Children’s Hearing and Speech Centre | Specialists at the centre (unspecified) | Hearing impairment: mild (26–40 dB HL), moderate (41–60 dB HL), severe (61–80 dB HL), or profound (> 80 dB HL) based on the average value of threshold at 0.5, 1, 2 and 4 kHz |
| Yin et al.,28 2009, USA | TEOAE (Otodynamics Echo Port ILO 288) | Preschool; quiet but not sound-treated room | Nurses and a paediatrician who had 1 hour of ‘hands-on’ training | Pass: automatically indicated when a TEOAE response was obtained for three of five frequency range with TEOAE being 5 dB above noise floor Refer: no TEOAE present or did not pass the required number of frequencies |
PTA | Unspecified | School audiologists | No response at any frequency (1, 2, 4 kHz) to 25 dB HL pure tone |
Although the majority of the included children were at or around school entry age, there was marked variability in terms of age range. Children’s age ranged from 225,28 to 13 years23 with the mean age ranging from 5.1 years [standard deviation (SD) 0.7 years]27 to 9.4 years (SD 1.8 years)22 (three studies25–27 did not report mean age and only gave age range as an inclusion criterion). The studies were relatively balanced in terms of participants’ gender, the proportion of males ranging from 48.4%26 to 62.5%30 (two papers23,28 did not report details). Apart from lack of consent, most studies did not specify any other exclusion criteria. One study26 excluded children who were unable to lie down still for several minutes, which was a necessary requirement for completing the screen procedure; one study27 excluded children with learning disabilities and two studies26,30 commented that no children with known cognitive or hearing impairments were included but it was unclear if this had been a prespecified exclusion criterion. Seven studies17,22–25,27,30 were conducted in countries without established UNHS.
Studies evaluated the performance of questionnaires (n = 4), audiometry (n = 3), TEOAE (n = 3) and automated auditory brainstem response (AABR) (n = 1). AABR is usually used to screen infants and was not listed in the initial inclusion criteria. However, we decided to include the study, as it evaluated, probably for the first time, the use of AABR in SES. Two studies22,23 evaluated different versions of the Chinese Hearing Questionnaire for School Children (CHQS). Only one study22 compared directly the performance of two different tests, a questionnaire and TEOAE, and one study29 evaluated a combination of TEOAE and tympanometry but did not report the results as adding tympanometry had not improved performance.
Questionnaires were completed by parents or carers in two studies,22,23 either by parents/carers or by teachers or community nurses interviewing parents (three different testing conditions) in one study24 and by unspecified evaluators in one study. 25 All other index tests were performed either by audiologists, otolaryngologists or other professional staff who had some preliminary training. With the exception of questionnaires, all other tests were performed at school or a community health centre in a quiet but not sound-treated room and the ambient noise level was monitored.
All studies except those evaluating questionnaires used a prespecified positivity threshold to define ‘pass’ and ‘refer’ outcomes. Data-driven selection of a threshold to achieve optimal performance (best sensitivity and/or best specificity) could lead to overly optimistic diagnostic accuracy estimates and the test is likely to perform worse when the same threshold is used in an independent sample of patients. 31
The reference standard was a combination of otoscopy, tympanometry and audiometry in four studies,22,23,25,29 otoscopy, tympanometry, audiometry and OAEs in one study,27 ear, nose and throat (ENT) examination including audiometry in two studies,24,26 and audiometry only in three studies. 17,28,30 The tests were performed by audiologists and otolaryngologists, usually in quiet but not sound-treated rooms with monitored ambient noise.
The mean prevalence of the target condition across all studies that reported outcomes at the level of the individual was 10.8% (SD 12.6%) and ranged from 0.7%28 to 46.7%. 25 The prevalence in the only study that reported ear-level outcomes was 4.8%. 17 These numbers should, however, be treated with caution, as the studies varied in their pass/refer criteria and included convenience samples drawn from populations likely to be different in terms of prevalence and spectrum of hearing impairments.
Methodological quality of included studies
The definitions of the assessment criteria are given in Appendix 2 and the results from the methodological quality assessment are summarised in Table 3. All studies had a prospective cross-sectional single-gate design. ‘Single-gate’ is a term introduced by Rutjes et al. 32 to describe diagnostic accuracy studies in which a single sample drawn from the target population receives the index test and the reference standard to allow calculation of the test’s sensitivity and specificity. In contrast, in ‘two-gate’ designs sensitivity and specificity are calculated separately based on two different samples of participants, that is, one with and one without the target condition. The two-gate design is prone to spectrum bias as the mix of participants in the two samples combined is unlikely to be representative of the target population. 32 Therefore, studies using a single-gate design are considered to be of better methodological quality.
| Study | Representative spectrum? | Selection criteria described? | Acceptable reference standard? | Acceptable time between tests? | Whole/random sample received the reference standard? | Same reference standard? | Reference standard and index test independent? | Index test described? | Reference standard described? | Blinded interpretation of index test? | Blinded interpretation of reference standard? | Same clinical data available as in practice? | Uninterpretable/intermediate results reported? | Withdrawals explained | Total score (number of ‘yes’) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study 1 | Bu et al.,22 2005 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not clear | Not clear | Not clear | Yes | Yes | 10 | Study 1 |
| Study 2 | Georgalas et al.,29 2008 | No | No | Yes | Not clear | Yes | Yes | Yes | Yes | Yes | Not clear | Not clear | Not clear | Yes | Yes | 8 | Study 2 |
| Study 3 | Gloria-Cruz et al.,17 2013 | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not clear | Yes | Yes | 11 | Study 3 |
| Study 4 | Li et al.,23 2009 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not clear | Yes | Yes | Yes | 12 | Study 4 |
| Study 5 | McPherson et al.,30 2010 | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not clear | Not clear | 10 | Study 5 |
| Study 6 | Newton et al.,24 2001 | No | Yes | No | Not clear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 | Study 6 |
| Study 7 | Samelli et al.,25 2011 | No | No | Yes | Not clear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not clear | Not clear | 9 | Study 7 |
| Study 8 | Soares et al.,26 2014 | No | No | No | Not clear | Yes | Yes | Yes | Yes | Yes | Not clear | Not clear | Not clear | Yes | Yes | 7 | Study 8 |
| Study 9 | Wu et al.,27 2014 | No | Yes | Yes | Not clear | Yes | Yes | Yes | Yes | Yes | Yes | Not clear | Yes | Yes | Yes | 11 | Study 9 |
| Study 10 | Yin et al.,28 2009 | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 | Study 10 |
Although all included studies had a single-gate design, none of them included a representative sample of a relevant target population defined for the purpose of this review as 4- to 6-year-old children (± 1 year) at or around school entry stage who have no high-risk features (such as Down syndrome, cytomegalovirus infection or meningitis) and have been tested and found to have no hearing impairment at birth. Therefore, the studies are likely to suffer from a selection bias and to have limited applicability to the UK context because of the following methodological issues. First, some studies included children younger or older than the defined 4–6 years age range, which reflects, to some extent, the fact that school entry age varies across countries. Second, seven of the studies17,22–25,27,30 were conducted in countries without established UNHS, which is likely to impact on the prevalence and spectrum of hearing impairments in the included children. Third, most of the studies included small self-selected samples recruited from a single locality and, therefore, may not be representative, even when drawn from a relevant target population.
The execution of the index test(s) and the reference standard were reported in sufficient detail in the majority of the studies. In five studies,17,24,26,28,30 however, the reference standard was suboptimal and did not meet the quality criterion ‘PTA + tympanometry’. The time between the performance of the index test and the reference standard was < 1 month in four studies,17,22,23,30 was > 1 month in one study28 and was not reported in five studies. 24–27,29 Blinding of the index test evaluators to the results of the reference standard was not reported in three studies22,26,29 and blinding of the reference standard evaluators to the results of the index test was not reported in five studies. 22,23,26,27,29 Those criteria that were consistently met across the studies were questions 5–9 (see Appendix 2) concerning the application of the same reference standard to the whole or random sample, regardless of the index test result (verification bias); the independence of the reference standard from the index test (incorporation bias) and the description of index test and reference standard in sufficient detail to allow replication. According to the criteria for calculating a total quality score published in the 2007 HTA report,12 three studies25,26,29 were of ‘moderate’ quality (total score 7–9) and the remaining seven studies17,22–24,27,28,30 were of ‘good’ quality (total score of > 9).
Test accuracy
Given the significant heterogeneity in the study characteristics and the reported test accuracy estimates (see Table 2 and Figure 3) we considered quantitative synthesis inappropriate and, instead, summarised the performance of different test types in tables and figures (Figures 3 and 4, and Table 4).
FIGURE 3.
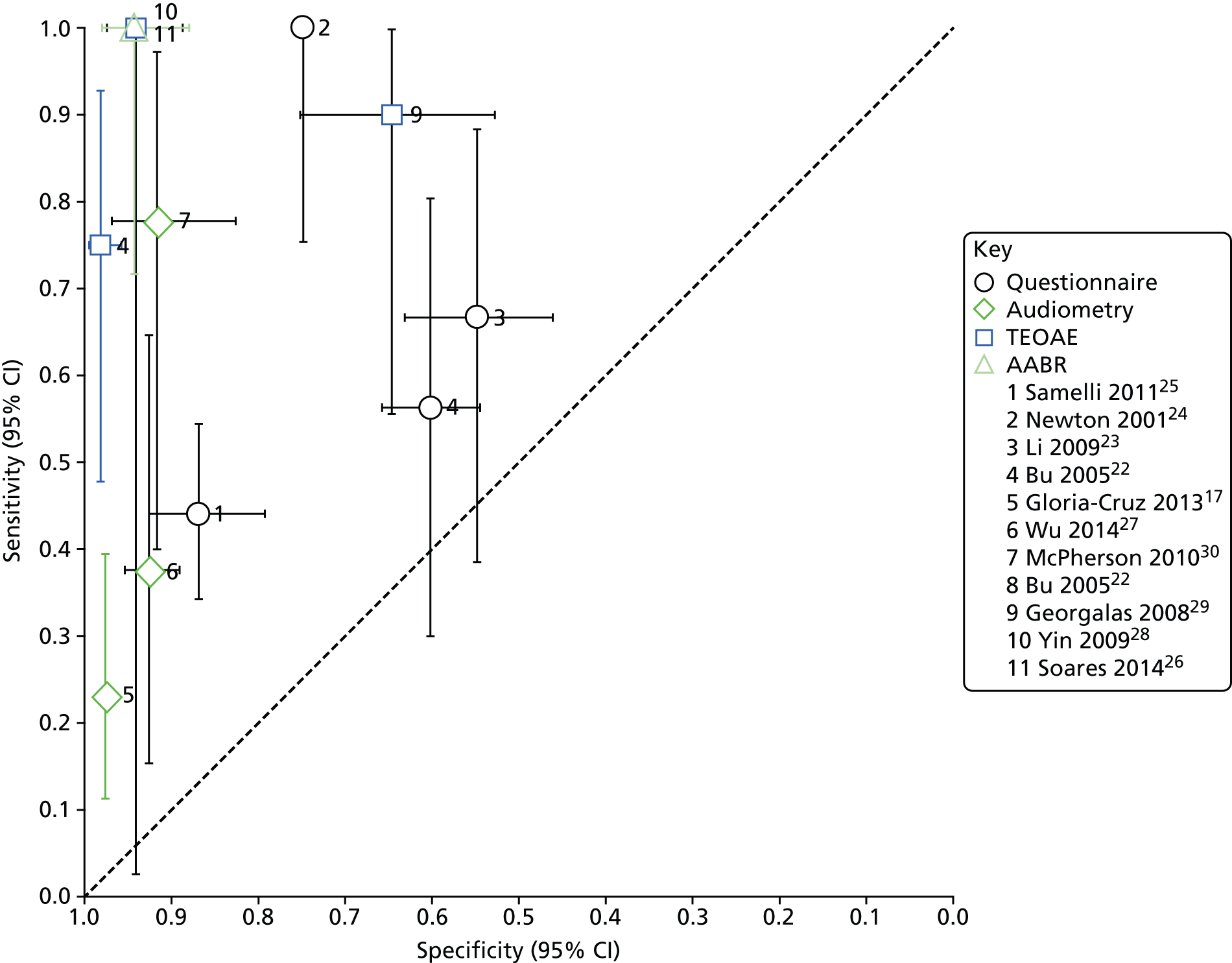
Forest plot of sensitivity and specificity of different types of hearing screening tests. Please note that the results for Bu et al. 22 are based on reading off the ROC plot in the paper and do not refer to a particular positivity threshold; the results for Li et al. 23 are based on ‘1+’ answers suggesting hearing impairment; and the results for McPherson et al. 30 are based on the data set that did not include results from 0.5 kHz. CI, confidence interval; FN, false negative; FP, false positive; TN, true negative; TP, true positive.
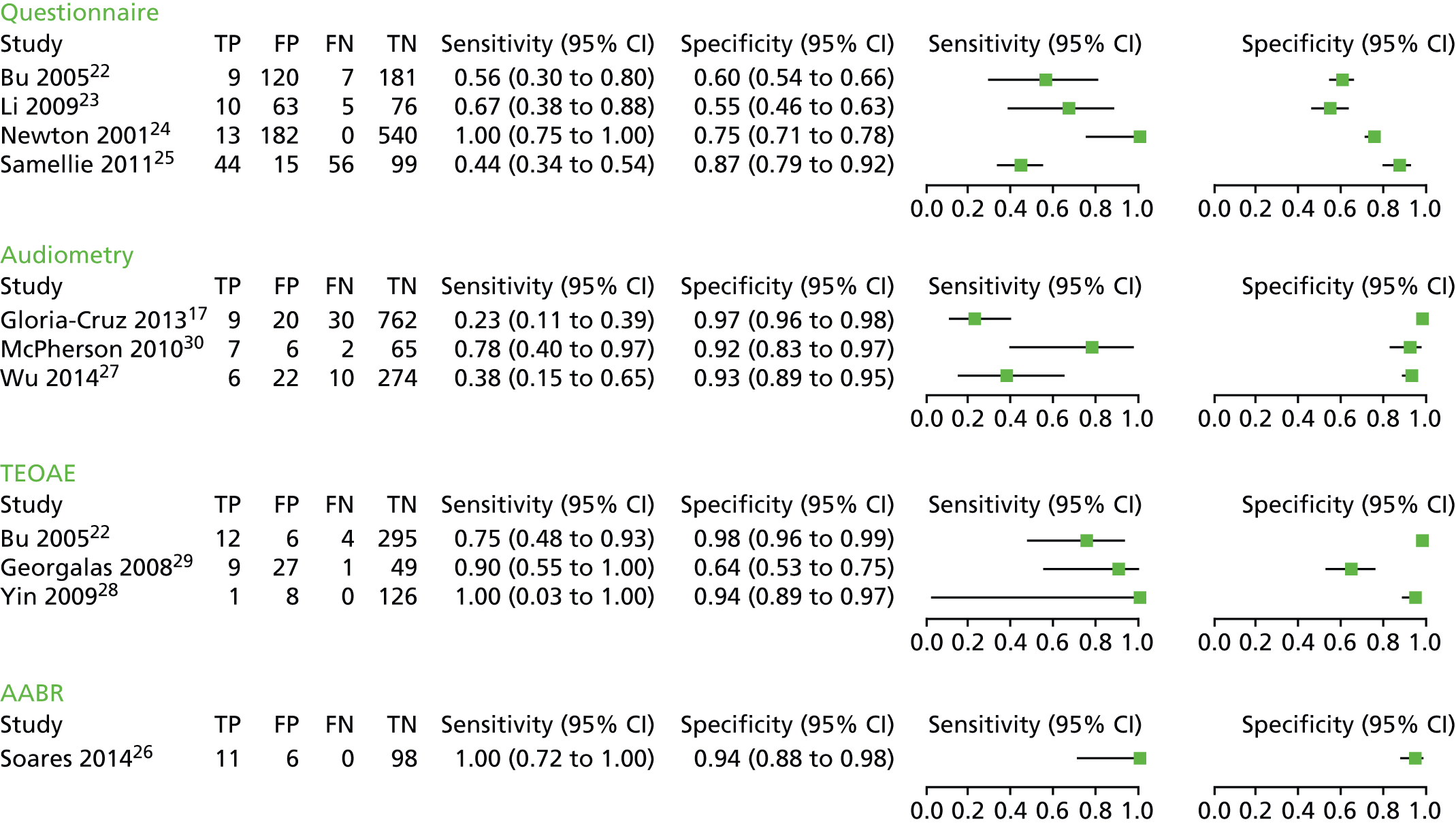
FIGURE 4.
Summary ROC plot of different hearing screening tests. Please note that the results for Bu et al. 22 are based on reading off the ROC plot in the paper and do not refer to a particular positivity threshold; the results for Li et al. 23 are based on ‘1+’ answers suggesting hearing impairment; and the results for McPherson et al. 30 are based on the data set that did not include results from 0.5 kHz. CI, confidence interval.
| Study and country | Index test cut-off | Total number | TP | FP | FN | TN | Prevalence % | Sensitivity % (95% CI) | Specificity % (95% CI) | Withdrawals and uninterpretable results |
|---|---|---|---|---|---|---|---|---|---|---|
| Questionnaire | ||||||||||
| Bu et al.,22 2005, China | Single questions | 317 | n/a | n/a | n/a | n/a | 5.05 | Range 7–42 | Range 76–99 | None reported; response rate to questionnaire was 61% |
| Unspecified cut-off (based on the ROC plot reported in the paper) | 317 | 9 | 120 | 7 | 181 | 5.05 | 56 (30 to 80) | 60 (54 to 66) | As above | |
| Li et al.23 2009, China | Scores of ≥ 1 to ≥ 5 | 154 | n/a | n/a | n/a | n/a | 9.74 | Range 0–67 | Range 55–100 | None reported; questionnaire’s response rate was 100% (after being distributed via students randomly selected by teachers) |
| Score of ≥ 1 | 154 | 10 | 63 | 5 | 76 | 9.74 | 0.67 (0.38 to 0.88) | 0.55 (0.46 to 0.63) | As above | |
| Newton et al.,24 2001, Kenya | Score of ≥ 1 (question 5 excluded) | 735 | 13 | 182 | 0 | 540 | 1.77 | 100 (75 to 100) | 75 (71 to 78) | Response rate to questionnaire was 88%; PTA could not be obtained for 22 children who were excluded from analysis |
| Samelli et al.,25 2011, Brazil | Score of ≥ 6 | 214 | 44 | 15 | 56 | 99 | 46.72 | 44 (34 to 54) | 87 (79 to 92) | None reported; response rate also not reported |
| Audiometry | ||||||||||
| Gloria-Cruz et al.,17 2013, Philippines | Pass: green; refer: yellow/red | 821 (ears) | 9 | 20 | 30 | 762 | 4.75 (ears) | 23 (11 to 39) | 97 (96 to 98) | 73% coverage of the entire population under study: 7 out of 418 children excluded as not available for testing; one child had only one ear tested |
| McPherson et al.,30 2010, China | > 40 dB any frequency | 80 | 10 | 35 | 0 | 35 | 12.5 | 100 (69 to 100) | 50 (38 to 62) | None reported; coverage of entitled children also not reported |
| > 40 dB, 0.5 kHz excluded | 80 | 7 | 6 | 2 | 65 | 11.25 | 78 (40 to 97) | 92 (83 to 97) | ||
| Wu et al.,27 2014, China | > 30 dB at 1, 2 or 4 kHz | 312 | 6 | 22 | 10 | 274 | 5.13 | 38 (15 to 65) | 93 (89 to 95) | 76 children refused to take part; eight children with learning disabilities were excluded |
| TEOAE | ||||||||||
| Bu et al.,22 2005, China | See Table 2 | 317 | 12 | 6 | 4 | 295 | 5.05 | 75 (48 to 93) | 98 (96 to 99) | As above |
| Georgalas et al.,29 2008, Greece | See Table 2 | 86 | 9 | 27 | 1 | 49 | 11.63 | 90 (55 to 100) | 64 (53 to 75) | 196 students enrolled but only 86 received PTA owing to financial constraints (selection criteria not clear) |
| Yin et al.,28 2009, USA | See Table 2 | 135 | 1 | 8 | 0 | 126 | 74 | 100 (3 to 100) | 94 (89 to 97) | 142 students enrolled in the diagnostic cohort of whom seven were excluded (two special education students refused TEOAE, three non-special education refused or were unable to do PTA, two students could not be tested using TEOAE owing to complete cerumen impaction) |
| AABR | ||||||||||
| Soares et al.,26 2014, Japan | n/a | 115 | 11 | 6 | 0 | 98 | 9.57 | 100 (72 to 100) | 94 (88 to 98) | 17 individuals (34 ears) excluded from the sample owing to failure to get good impedance or accurate PTA; and five ears (from the 163 included individuals) were also excluded |
Parental questionnaires
Four studies22–25 reported the diagnostic accuracy of questionnaires, two of which evaluated different versions of the same tool (CHQS). 22,23 All questionnaires had been independently validated and the authors reported satisfactory reliability. None of the studies used a prespecified positivity threshold, which means that the reported diagnostic accuracy might be exaggerated. The two studies evaluating CHQS reported results from a range of different scores23 or a range of sensitivity and specificity values obtained for different individual questions and combinations. 22 The results included in the forest plot and the ROC curve plot (see Figures 3 and 4) for these two studies correspond to positivity thresholds that resulted in the best overall performance: a total score of > 1 for the study by Li et al. ,23 and sensitivity of 56% and specificity of 60% read-off the ROC plot in the paper for the study by Bu et al. 22
Across all studies, sensitivity ranged from 44% to 100% and specificity from 55% to 87% (see Figure 3). The ROC plot in Figure 4 clearly shows that the performance of the questionnaires was poor, with three of the studies,22,23,25 close to the line of no effect (the dotted diagonal line), which indicates accuracy no better than expected due to chance. Only the study conducted by Newton et al. 24 showed relatively good overall accuracy with excellent sensitivity (100%) and moderate specificity (75%). However, the definition of hearing impairment in this study was bilateral hearing impairment of > 40 dB and three of the four children with unilateral hearing impairment of > 40 dB were missed by the questionnaire. Including these children in the analysis brings the sensitivity down to 82% while specificity remains the same (75%).
Audiometry
The diagnostic accuracy of audiometry was evaluated in three studies: two evaluated computer-based audiometers27,30 and one a hand-held device. 17 Sensitivity ranged from 23% to 78% and specificity from 92% to 97% (see Figure 3). McPherson et al. 30 reported two sets of results – before and after excluding the data for 0.5 kHz – which they considered problematic owing to possible interference from ambient noise. With the 0.5 kHz data included, sensitivity was 100% and specificity was 50%; excluding 0.5 kHz data led to a marked decrease in sensitivity (78%) and increase in specificity (92%). The forest plot and the ROC plot (see Figures 3 and 4) show that, based on the studies included here, portable audiometry tools have much better and more consistent specificity, but variable sensitivity, noting, however, that there is a considerable difference between the technologies evaluated, particularly between the computer-based audiometers and the hand-held HC screening device.
Transient-evoked otoacoustic emissions
Three studies22,28,29 evaluated the accuracy of TEOAE, one of which compared its performance with that of a questionnaire (see Figures 3 and 4). 22 Sensitivity ranged from 75% to 100% and specificity from 64% to 98%. However, the sensitivity estimate reported by Yin et al. 28 had a very wide confidence interval (CI) (3% to 100%), indicating considerable statistical uncertainty. The comparative study found that the diagnostic accuracy of TEOAE was superior to that of the questionnaire with sensitivity of 75% versus 56% and specificity of 98% versus 60%. The sensitivity estimates of the two tests, however, had overlapping CIs, suggesting that the result might be due to chance rather than real superiority.
Automated auditory brainstem response
Only one study26 evaluated the diagnostic accuracy of AABR as a screening test for school entry children. This study was conducted in Japan, included children 3–5 years old and the evaluated device, MB11 BERA-phone® (MAICO Diagnostic GmbH, Berlin, Germany), was operated by an audiologist. The study had the lowest quality score of all included studies (see Table 3) because of a failure to provide a clear description of the selection process and to report important aspects of the study design, such as time between index test and reference standard, blinding and availability of clinical data to test administrators. The test showed very high performance with 100% sensitivity and 94% specificity but the sensitivity estimate had a relatively wide 95% CI (72% to 100%) owing to a small sample size and a low event rate.
Discussion
Summary of the findings from the 2007 Health Technology Assessment report
The 2007 HTA report12 included 25 primary studies reporting the performance of a wide range of hearing screening tests, including parental questionnaires, impedance audiometry/tympanometry, spoken word tests, otoscopy, audiometry, TEOAE and other tests and combinations of tests. The overall conclusion was that evidence was of unacceptable variability in terms of methodological quality and study characteristics and, as a result, drawing strong conclusions about the performance of different tests was not possible.
With those caveats taken into account and including only the subset of studies that used PTA as a reference standard, the findings from the 2007 HTA report suggested that: sweep PTA had high sensitivity and specificity; spoken word tests had acceptable levels of sensitivity and specificity; TEOAE had high specificity but somewhat lower sensitivity; tympanometry and acoustic reflectometry had variable sensitivity and specificity; parental questionnaires and otoscopy had poor sensitivity and specificity; and there was insufficient evidence to comment on the accuracy of combinations of tests (p. 48).
Combining the results from the 2007 Health Technology Assessment report and the current update
The studies identified in the current update provide additional test accuracy data for the following three categories of tests evaluated in the original HTA review: parental questionnaires, sweep PTA tests and TEOAE. Below we discuss the combined evidence from the two sets of studies for each category of tests.
Parental questionnaires
In the 2007 HTA report12 three studies33–35 examined the accuracy of parental questionnaires and reported sensitivities ranging from 34% to 71% and specificities ranging from 52% to 95%. In the update we included four studies22–25 evaluating questionnaires that reported sensitivities and specificities in the range of 44–100% and 55–87%, respectively (Table 5). As none of them used a prespecified positivity threshold, the reported test accuracy estimates are likely to be overoptimistic and the performance of the evaluated questionnaires worse when applied in practice. The study by Newton et al. 24 reported very high sensitivity but the definition of hearing impairment was very restrictive and may not be appropriate for most circumstances. Taking into account the methodological limitations of the studies and the marked heterogeneity in their results, the combined evidence from the original 2007 HTA report and the current update suggest that, on the whole, parental questionnaires have poor diagnostic accuracy and may not be suitable for mass screening, especially in the context of established UNHS and sensitised educational and health-care systems.
| Report | Study | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| Current update | Bu et al., 200522 | 56 (30 to 80) | 60 (54 to 66) |
| Li et al., 200923 | 67 (38 to 88) | 55 (46 to 63) | |
| Newton et al., 200124 | 100 (75 to 100) | 75 (71 to 78) | |
| Samelli et al., 201125 | 44 (34 to 54) | 87 (79 to 92) | |
| 2007 HTA report12 | Gomes and Lichtig, 200533 | 71 (n/a) | 64 (n/a) |
| Olusanya, 200134 | 34 (n/a) | 95 (n/a) | |
| Hammond et al., 199735 | 56 (n/a) | 52 (n/a) |
Audiometry-based tests
Five evaluations reported in four studies36–39 included in the original 2007 HTA report and three17,27,30 in the current update evaluated the accuracy of audiometry-based tests. The original five studies, all assessing pure-tone sweep devices, reported sensitivities ranging from 86% to 100% and specificities from 65% to 99%. The three studies in the update reported sensitivities in the range of 23% to 78% and specificities 92% to 97% (Table 6). McPherson et al. ,30 achieved high specificity (92%) only after the results from the 0.5 kHz frequency were excluded from the analysis. This was a post-hoc decision made to reduce the interference from background noise. With this caveat noted, the new studies reported higher and more consistent specificity but lower and widely varying sensitivity estimates compared with the original studies. When combined, the results from the two sets of studies are more difficult to interpret, as there is a marked heterogeneity in both sensitivity and specificity. However, given the gap of > 10 years between the two sets of studies and the technical differences between the evaluated devices, it might be more reasonable to interpret the results from the new studies separately instead of combining them with those from the 2007 HTA review. 12
| Report | Study | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| Current update | Gloria-Cruz et al., 201317 | 23 (11 to 39) | 97 (96 to 98) |
| McPherson et al., 201030 | 78 (40 to 97)a | 92 (83 to 97) | |
| Wu et al., 201427 | 38 (15 to 65) | 93 (89 to 95) | |
| 2007 HTA report12 | Sabo et al., 200036 | 87 (n/a) | 80 (n/a) |
| Orlando and Frank, 198737 | Range 82–100b | Range 65–90b | |
| Orlando and Frank, 198737 | Range 91–100b | Range 97–98b | |
| FitzZaland and Zink, 198438 | 93 (n/a) | 99 (n/a) | |
| Holtby et al., 199739 | 86 (n/a) | 70 (n/a) |
Transient-evoked otoacoustic emissions
Only two of the studies included in the original 2007 HTA report12 evaluated the accuracy of TEOAE. 36,40 The first reported sensitivity of 63% and specificity of 91%, and the second one reported sensitivities ranging from 67% to 100% and specificities from 80% to 98% depending on the ‘refer’ criterion used. In comparison, three studies22,28,29 were included in the current update, reporting sensitivities from 75% to 100% and specificities from 64% to 98%. Table 7 illustrates the heterogeneity in the results. Although the new studies reported higher sensitivities, the CIs were very wide, indicating considerable statistical uncertainty. With the exception of Georgalas et al. 29 the specificity estimates across all studies were higher and more consistent, suggesting a relatively low false-positive rate. We could not identify obvious reasons for the low specificity reported by Georgalas et al. 29 However, this was a small, poorly reported study with considerable methodological limitations (total quality score = 8) and its results should be interpreted with caution.
| Report | Study | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| Current update | Bu et al., 200522 | 75 (48 to 93) | 98 (96 to 99) |
| Georgalas et al., 200829 | 90 (55 to 100) | 64 (53 to 75) | |
| Yin et al., 200928 | 100 (3 to 100) | 94 (89 to 97) | |
| 2007 HTA report12 | Sabo et al., 200036 | 63 (n/a) | 91 (n/a) |
| Nozza et al., 199740 | 67–100 (n/a) | 80–98 (n/a) |
Other tests
None of the studies included in the original HTA report assessed the accuracy of AABR and none of the studies included in the update assessed the accuracy of spoken word tests, otoscopy, the audiometric Rinne test and reflectometry. Although one study29 evaluated the accuracy of tympanometry in combination with TEOAE it reported results only for TEOAE as adding tympanometry had failed to improve performance.
Conclusion
This updated review confirms the conclusion from the 2007 HTA report12 that, because of a marked variability in the design, methodological quality and the results of the existing studies, it is not possible to draw strong conclusions about the performance of individual test types for use in SES.
Moreover, there were significant differences in the technical characteristics of some of the evaluated devices even when they belonged to the same screening modality (e.g. in the audiometry-based category there were two computer-based devices, one of which involves joysticks, etc., to help children respond, whereas the third one was a hand-held device). This is not surprising given that the studies included in the 2007 HTA and the current update span a period of 30 years. Interpreting their results together, even in the context of a narrative synthesis, requires careful consideration of the differences between the evaluated tests, which, in some cases, might be too great to justify such an approach. Combining the results from more modern devices with those from older ones and making general conclusions based on all the studies included in a particular category may be inappropriate. The performance of currently available tests is of interest to policy-makers.
With these caveats in mind, the findings from the current update, interpreted in the context of the 2007 HTA report,12 could be summarised as follows:
-
We were able to identify a limited number of studies that provided additional diagnostic accuracy data for only the following categories of hearing screening tests: parental questionnaires, audiometry-based tests, TEOAE and AABR. No studies evaluating AABR were included in the 2007 HTA report. 12
-
Questionnaires had the poorest diagnostic accuracy compared with all other tests. The only study that directly compared a questionnaire with another test (TEOAE) supported this finding. 22
-
Audiometry-based tests had high specificity but variable sensitivity.
-
Studies evaluating TEOAE reported variable sensitivity with wide CIs, whereas specificity estimates were relatively high and more consistent (with the exception of one study). 29
-
The study evaluating AABR reported high sensitivity and specificity.
The majority of the studies were conducted in countries without an established UNHS system and with variable health-care arrangements and, therefore, the reported results may have limited applicability to the UK context characterised with a well-established UNHS system, sensitised educational system and highly accessible and responsive health care.
Chapter 3 Diagnostic accuracy of the pure-tone screen and HearCheck screener for identifying hearing impairment in school children
Introduction
In the survey of practice reported in the 2007 HTA report12 the test used for the hearing screen was in all cases the PTS but there was a wide variety of implementations of this, with different frequencies, pass criteria and retest protocols. Studies concerned with the relative accuracy (in terms of sensitivity and specificity) of alternative screening tests are difficult to compare and often flawed by their use of different referral criteria and case definitions. As reviewed in Chapter 2, the 2007 HTA report12 identified 25 publications reporting comparative trials of alternative screens or tests for screening at school entry. Most studies were undertaken in populations where the prevalence of undetected hearing impairment was considerably greater than that likely to be encountered in a system where a UNHS programme has been introduced. These published data indicate that, using full PTA as the reference standard, the PTS test appears to have high sensitivity and high specificity for minimal, mild and greater hearing impairments; better than alternative tests for which evidence was identified. Spoken word tests were reported with acceptable levels of sensitivity and specificity but are variable in their implementation. OAEs, tympanometry, acoustic reflectometry, parental questionnaires and otoscopy were reported, with either variable or poor accuracy.
A new device, the HC screener, came onto the market in 2005 as a tool for screening for hearing impairment in adults in a general practice setting. It is hand held and has an automatic presentation of a series of tones at two frequencies and three levels: 1 kHz at 55, 35 and 20 dB HL, and 3 kHz at 75, 55 and 35 dB HL. It has potential to be a quicker test in the school setting but it has not previously been assessed as a tool for screening in children.
Objectives
In this chapter we use a two-gate case–control design32 to:
-
estimate the diagnostic accuracy of the PTS and HC tests for discriminating between children with a hearing impairment (of any type) and children with no hearing impairment, using PTA results as the reference standard
-
compare the diagnostic accuracy between the PTS and HC methods.
Measures of diagnostic accuracy are reported at the level of the ear and at the level of the child. From the outset we considered the ear-level analysis to be primary to the objectives addressed in this chapter because it directly addresses the question of the accuracy of the tests for discriminating between hearing and impaired ears. The child-level accuracy estimates are more relevant, however, for informing the refinement of the existing economic model of the benefits of a SES programme, reported in Chapter 8.
Methods
Design
This study used a directly comparative two-gate (‘case–control’) design,32 with separate sources (gates) used to recruit those with the target condition (hearing impairment – cases) and those without (no hearing impairment – controls). Case children were recruited through audiology services in England whereas the control children were recruited largely from Nottinghamshire primary schools. The implications of this are discussed later in this chapter (see Discussion).
Recruitment
Cases were children aged 4–6 years recruited between February 2013 and August 2014 who were identified by collaborating audiology services [in Nottingham, Sheffield, Leicester (City and County), Chesterfield, Derby, Mansfield, Lincoln, Birmingham, Huntingdon, Bradford, Rotherham and Doncaster] and who had permanent sensorineural or conductive hearing impairment averaged across the four frequencies 0.5, 1, 2 and 4 kHz, either bilaterally (average of 20–60 dB HL) or unilaterally (any level ≥ 20 dB HL). The senior paediatric audiologist in each centre drew up a list of all children meeting the definition criteria. Audiologists were given stamped envelopes to address and post to potential participants and agreed together with the researchers when to send the letters. Each identified family was sent a letter on behalf of the research team inviting them to take part in the study, together with information about the study. Children were not invited to take part if they were unwell such that their illness would affect the results of the tests or if the responsible audiologist felt it would be inappropriate or cause added unnecessary burden (e.g. seriously or terminally ill family member). Parents willing to take part replied directly to the research team. Eligible children for whom agreement was provided to take part were invited to undergo the two screening tests, either in their own homes, or at Nottingham Hearing Biomedical Research Unit (NHBRU), depending on their preference. Researchers sent holding letters if necessary for children < 4 years old, explaining that an appointment would be made for them after their fourth birthday. The reference standard definition of hearing impairment was based on PTA results, thus case children were also excluded if there was no record of a PTA in the previous 12 months or planned for the following 3 months, and if the family was unwilling to travel to their local service or to Nottingham to undergo the assessment.
Control children, defined as having no previously identified hearing impairment, were recruited from the Foundation Year and Year 1 of schools in the Nottingham area, between February 2013 and June 2014. The study researchers provided a letter of invitation and information pack for the school to distribute to all parents of children in the aforementioned year groups; invitation methods were agreed with the school. Children for whom agreement to take part was provided were invited to undergo the two screening tests and a PTA at NHBRU. Children needed to have the PTA measured in a soundproofed room, and hence the option to have the screening test at home was not possible.
To help ensure that the cases and controls were representative of the sources from which they were drawn, the audiologists and researchers were advised to not just pick those whom they thought might be easy to test. For all children the invitation pack contained an invitation letter, a one-page summary information sheet, a pictorial information sheet for the children and a pre-paid return envelope. Full participant information sheets were sent to the parent (either via e-mail or post) once an appointment was made (see Appendix 3) (the full sheet was originally included in the invitation pack, but a revision was made in December 2013, to be less overwhelming for the parent and to save paper).
Parents of all children involved were offered the opportunity to receive a short summary of the findings at the end of the study. A £20 book token (revised from £10 after 7 months of recruitment in an attempt to improve recruitment) was given to each child as a thank you for his/her time and inconvenience. Travel expenses to NHBRU were reimbursed in line with University of Nottingham standard policy. Schools that participated in the recruitment of control children were entered into a prize draw with a chance to win £100.
Assessment
Once a reply slip was received, the researcher contacted the parent (via telephone, e-mail or letter) to make an appointment either at NHBRU (case and control children) or at their home (case children only). They were also asked about access issues or translator needs. Parents were usually reminded about the appointment by telephone or e-mail on the day prior to the appointment. After case children had their appointment, the researcher phoned the audiologist, asking them to post their most recent PTA results to NHBRU. This could be a PTA measured in the previous 12 months or one that is scheduled to be measured in the following 3 months.
For all appointments the researcher checked that the parent had all the study information, explained what would happen at the appointment and how that fitted into the project, and answered any questions they might have. The consent form was explained and written consent obtained. The researcher emphasised that participation was voluntary and consent regarding the child’s participation in the project could be withdrawn at any time without penalty or affecting the quality or quantity of the child’s future medical care, or loss of benefits to which the child and his/her family was otherwise entitled. It was explained that in the event of withdrawal, their data collected so far could not be erased and that consent would be sought to use the data in the final analyses where appropriate. The informed consent form was signed and dated by the parent or legal guardian and researcher before the child entered the study – usually at the start of the screening appointment. Copies of the consent form were kept by the parent or legal guardian, the researchers, and, for case children only, in the child’s hospital records (see Appendix 3).
Data collection was planned prospectively in advance of administering the tests and reference standard to the children. Background details [visit date, school for control children, hospital for case children, date of birth, postcode, background sound level, gender, ethnicity, medical conditions on the day (including respiratory infections)] were recorded on the case report form (CRF) and testing was then administered.
The audiometer (used for the PTS testing for all children and for the PTA for control children) and HC device were checked each day, by listening to the tones at the testing frequencies, to ensure they were audible. Valid calibration was undertaken in the previous 12 months for the audiometer and sound level meter and in the previous 3 years for the HC screener according to manufacturers’ recommendations. Headphones were cleaned with a disinfectant wipe before each child was tested and new disposable cups were used for the HC screener. Items likely to cause noise (e.g. mobile phones, washing machines, televisions) were turned off where possible, and the level of background noise was measured with a sound level meter. Although, for control children, the door of the room in which the two screening tests were conducted was left open, and sometimes siblings were present, the ambient noise conditions were much quieter than rooms that are usually available in schools for conducting the hearing screen. For case children, where the screens were conducted at home, attempts were made to minimise the noise levels.
The researchers were trained in administering the PTA and PTS tests by the audiologists in the Children’s Hearing Assessment Centre (CHAC) in Nottingham, using a mixture of observation, practice with children and feedback. Further familiarisation with the equipment and procedures was gained by testing staff from the research department.
The order of administering the two screening tests and which researcher undertook them were each determined by separate lists based on computer-generated simple (unrestricted) random numbers. Each test was administered to both ears. For case children, one researcher performed the PTS and another researcher performed the HC screen. For control children, one researcher carried out both the screening tests and then another researcher performed the PTA measurement. An effort was made to blind the second researcher to the results of the first test(s) by asking them to leave the room. The PTA result for case children obtained from their audiologist (measured within 12 months before or 3 months after the study visit) was examined only after the result of the screening tests were known.
Screening tests
Pure-tone screen
A traditional PTS tests across four frequencies (0.5, 1, 2 and 4 kHz), which cover the majority of the frequencies contained in speech, was undertaken. The researcher was positioned to ensure that they had a clear view of the child without giving any visual cues throughout the test. The child was instructed to place a ball onto a frame every time they heard a sound, even if the sound was quiet. Hearing aids, glasses, hairbands and earrings were removed where relevant. The headphones were placed over the child’s ears, adjusting the fit if necessary. A familiarisation tone (1 kHz at 60 dB HL) was presented as a practice to ensure that the child had understood the instructions. Up to three tones were presented at a single level (20 dB HL) for each of the frequencies in the order 1 kHz, 2 kHz, 4 kHz and 0.5 kHz. Each tone was held for 2–3 seconds, with staggered pauses between tones to reduce expectation. For each frequency and ear, at least two out of three responses to a tone was considered to be a pass at that frequency. One response from three presentations or zero responses from two presentations of the tone was considered to be a refer result. If the first two presentations were both passed at a given frequency or both referred at a given frequency the tone was not presented a third time. On the CRF a tick was used to indicate response (i.e. child placed a ball on the frame) and a cross for no response. Failure to hear at any frequency for a given ear was considered an overall refer result for that ear. All four frequencies were tested in one ear before changing to the other ear. For children who did not appear to hear any of the tones, the 20 dB HL tones were interspersed with sounds of greater intensity to check attention.
HearCheck screener
The HC41 screener (Figure 5) automatically generates six tones in total – one tone for each combination of frequency and level: 1 kHz at 55, 35 and 20 dB HL and 3 kHz at 75, 55 and 35 dB HL. The tasks and test were explained, in particular that there was one tone much louder than the others. Children were asked to raise a hand when they heard a tone. The response method for the HC screen had to be quick to perform as there was only a very short automated gap between the tones, and was also chosen to be different from that for the PTS to keep the child’s interest. For some children a different response method (e.g. tap the table) was used if they did not seem able to raise their hand for whatever reason. The child was asked to remove hearing aids, and also glasses and earrings if necessary for a good fit. A disposable cardboard ear cover was put onto the HC screener for each ear. The HC screener was held against the ear to be tested, often holding the child’s head still with the free hand. The button was pressed and the first three tones were allowed to play for the first frequency. The button was then pressed again for the remaining three tones for the second frequency. The procedure was repeated on the other ear. A tick was recorded on the CRF for a response, and a cross for no response. The refer criterion for each ear is anything less than all six tones being responded to.
FIGURE 5.
The HC screener.
Pure-tone audiometry reference standard
The PTA used to define the reference standard was administered in the study to all children recruited as controls. For control children, PTA was carried out in NHBRU at the same session as the screening tests were administered, using the audiometer in a soundproofed booth with the child sat facing away from the equipment. The parent was able to watch through an observation window. Instructions were given and then the headphones were put on. For familiarisation, 1 kHz tones at 60 dB HL were presented in one ear with further explanation provided if needed. Testing followed standard British Society of Audiology recommended procedure42 without otoscopic examination or masking, for air conduction only. The testing tones were administered in the order 1, 2, 4, 8, 0.5 and 0.25 kHz for each ear. Sounds were presented for approximately 2 seconds with variable pauses between them. Each frequency was first presented at 40 dB HL. If not heard then the intensity was increased to 60 or 80 dB if needed. If still not heard then the equipment and the child’s concentration were checked. If the participant heard the tone, then intensity was decreased by 10 dB HL, then if not heard it was increased by 5 dB HL. This stepwise approach was repeated until a threshold was found (defined as the quietest sound heard twice as the intensity was increased) for each frequency. The procedure was repeated in the second ear. The child indicated hearing the tone using the response button connected to the audiometer. If the child did not understand the use of the response button, then stacking bricks were occasionally used. The results were then printed out (only possible if the response button was used) and the audiometer results were transcribed into the CRF. The child identifier (ID) was written on the print out and attached to the CRF.
If a child decided that they did not want to continue with the testing during an assessment session, for whatever reason, he/she could be withdrawn from the study.
As the children recruited as controls had no previously identified hearing impairment, it was expected that they would pass the PTA. However, if the child was found to have a threshold of ≥ 30 dB HL at any frequency then, regardless of screening results, their parents were asked if they would like a referral to CHAC for further audiological testing. If such a referral was made this was indicated on the CRF and patient details were obtained. A letter and leaflet about the referral were given to the parents with an explanation that they would be contacted in the next 2–3 weeks with an appointment time. A copy of the CRF was passed to CHAC who made the appointment.
For children recruited as cases, a suitable PTA (administered between 12 months before and 3 months after the appointment) was obtained from the audiologist after the appointment and the data were added to the CRF. The original PTA result (with name, hospital number and other IDs removed and study ID added) was attached to the CRF.
Data collection
Each page of the CRF for each child was identified with the participant identity code, date of birth and partial postcode. The researchers entered the data from the CRFs into the study database. Copies of the CRFs were then sent to Peninsula Clinical Trials Unit (PenCTU) for second data entry and consistency checks. Emerging queries were noted to the Nottingham researchers and corrected where appropriate. Recruitment logs (for children nominally recruited as cases and controls, audiologist and school), the password-protected appointment spreadsheet and progress documents were completed as appropriate. Consent forms, reply slips and CRFs were filed in a secure place at NHBRU and then archived in a secure facility.
Sample size calculation
The target sample size was 80 hearing impaired (HI) case children and 160 not hearing impaired (NHI) control children. Eighty HI children is a large enough sample to estimate a sensitivity of 80% with a margin of error of 10.4% based on the lower bound of the 95% CI; 160 NHI children is large enough to estimate a specificity of 80% with a margin of error of 7.0%. The margin of error for these estimates provides plausible ranges of values within which to test the sensitivity of the results from the economic model to our assumptions about screening accuracy (see Chapter 8).
Statistical analysis
The characteristics of the children were summarised separately for those nominally recruited as case children (i.e. with impairment) and those nominally recruited as control children (without impairment), using mean and SD for age, and numbers and percentages for gender and ethnicity. For case children, the time interval in weeks between measurement of the PTA reference standard and administration of the screening tests was summarised using the mean with SD and median with interquartile range (IQR).
The criteria used to define recruitment of case children were based on information available from the PTA. The children who were recruited as controls had no known hearing impairment. However, it was possible for children nominally recruited as cases to pass some elements of the reference standard and for children nominally recruited as controls to receive a refer result from the reference standard. Evaluation of the diagnostic accuracy of the screening tests was based on using the PTA reference standard, and not the nominal recruitment status of the children (i.e. it was not based on the ‘gate’ through which they were recruited). We classified those who were referred by PTA as HI and those who passed PTA as hearing (or NHI), regardless of whether they were nominally recruited as cases or controls. The discordance between nominal case–control status and the PTA reference standard classification is summarised later.
The accuracy of each of the screening test results was assessed in relation to the PTA reference standard classification based on analyses at the level of individual ear and at the level of individual child. From the outset we considered the ear-level analysis to be primary to the objectives addressed in this chapter because it directly addresses the question of the accuracy of the tests for discriminating between hearing and impaired ears. We also present child-level analyses, however, because the resulting accuracy estimates from these are most relevant for the economic model reported in Chapter 8. For the child-level analyses hearing was considered impaired (HI) if they had at least one impaired ear on the PTA reference standard and considered to have been referred by a given screening test if at least one of their ears was referred by the test.
When examining the relationship between the screening test results and the reference standard, separate sets of analyses were carried out for two different definitions of hearing impairment status. Under the primary definition, impairment was considered present when the PTA reference standard threshold was ≥ 30 dB on at least one of the four frequencies examined (0.5 kHz, 1 kHz, 2 kHz and 4 kHz) and considered absent when the reference threshold was < 30 dB on all four frequencies. Secondary analyses were carried out under the alternative definition where impairment was considered present when the mean PTA threshold across the four frequencies was ≥ 30 dB and considered absent when the mean threshold was < 30 dB. The primary definition is the stricter of the two.
Separate sets of analyses relating the screening test result and the reference standard classification were also conducted based on the inclusion of different subsets of impaired ears (or children). In the primary analysis all HI ears (or children) were included regardless of whether nominally recruited as a case child or as a control child. In two further sets of secondary analyses we included only impaired ears (or children) for those nominally recruited as cases and then included only impaired ears (or children) for those nominally recruited as controls.
Cross-tabulation was used to report the test results (refer vs. pass) for the PTS and HC screen in relation to each other, separately for the groups of ears (or children) that were classified on the PTA reference standard as impaired (to compare sensitivity between tests), hearing (to compare specificity between tests) and missing. Sensitivity, specificity, positive likelihood ratio and negative likelihood ratio were reported for the PTS and HC screening tests for ear-level and child-level analyses and all scenarios defined by the above definitions of impairment. Sensitivity is the percentage of impaired ears (or children) that are referred by the test; specificity is the percentage of hearing ears (or children) that pass the test; positive likelihood ratio is the ratio of the percentages that are referred by the screening test between the impaired and hearing groups; and negative likelihood ratio is the ratio of the percentages that pass the screening test between the impaired and hearing groups. For the ear-level analyses, correlation between the results for ears of a given child was accounted for when reporting CIs for sensitivity and specificity by fitting null logistic regression models (i.e. with no covariates) to the binary test result outcome with information sandwich (‘robust’) estimates of standard error, with the resulting log odds and 95% CI converted to the proportions (percentage) scale. When estimating sensitivity the model was fitted using only impaired ears and when estimating specificity using only hearing ears. The test result outcome was coded 1 for refer and 0 for pass when estimating sensitivity and vice versa when estimating specificity.
We report the absolute difference in percentages between the PTS and HC screen for both sensitivity and specificity with 95% CIs and McNemar’s test p-value [using the mcc command in Stata software (version 13.1, StataCorp LP, College Station, TX, USA)]. The calculation of the CI and p-value recognise the pairing of the PTS and HC test results within ear (or within child). This was done for ear-level and child-level analyses and all scenarios defined by the above definitions of impairment. Unlike when estimating the accuracy of each test, when comparing accuracy between tests for the ear-level analysis there was no need to allow for the correlation between results related to ears from the same children because the estimated difference in sensitivity (and specificity) between the PTS and HC tests is based on information within ears. An alternative analysis in which the data structure was reshaped so that there were four rows per child (PTS result for the left ear, HC result for the left ear, PTS result for the right ear and HC result for the right ear) and logistic regression models fitted to the test result outcome (coded 1 for pass and 0 for refer) on the predictor test type (coded 1 for PTS and 0 for HC) to estimate the difference between test types in accuracy, taking account of the correlation of test results for ears that belong to the same child, provided identical CIs and p-values to those reported here.
Missing data
To be included in the main ear-level analysis the ear needed to have provided data on both the screening tests and provided hearing-level data on all four frequencies presented under the PTA reference standard. It was possible for a given child, therefore, to provide only one ear that was used in the ear-level analyses. To be included in the child-level analysis the child had to provide full data on the PTS and HC tests and the PTA reference standard for both ears. Analyses were carried out using Stata statistical software.
Results
Participants
Nominal case children were recruited from 14 centres. The original eight centres in the East Midlands were: Nottingham, Leicester City, Leicester County, Sheffield, Derby, Lincoln, Mansfield and Chesterfield. Owing to recruitment difficulties a further six centres were added: Bradford, Rotherham, Huntingdon, Birmingham Children’s Hospital, Birmingham Heartlands Hospital and Doncaster. From 379 invitations sent by the audiologists, a total of 86 replies were received, a response rate of 23%. Eight children were not eligible, being outside the required age range. We were unable to contact one of the initial respondents, and we were unable to see a further two children due to researcher illness just before the close of recruitment. We recruited and tested the remaining 75 children. See the flow diagram (Figure 6) below. Details of recruitment by centre are given in Table 8.
FIGURE 6.
Numbers of case children in the diagnostic accuracy study.
| Audiology clinic | Number of invitations sent | Date posted to audiologist | Number of replies received | Ineligible (wrong age) | Eligible children not seen | Number tested | Child did not complete the tests | |
|---|---|---|---|---|---|---|---|---|
| Nottingham | Original posting | 39 | 8 February 2013 | 17 | 3 | 0 | 14 | 1 |
| Reminders | 27 | April 2014 | ||||||
| Newly identifieda | 0 | N/A | ||||||
| Leicester County | Original posting | 8 | 6 February 2013 | 2 | 0 | 0 | 2 | 0 |
| Reminders | 4 | 15 December 2013 | ||||||
| Newly identified | 0 | 15 December 2013 | ||||||
| Sheffield | Original posting | 47 | 5 March 2013 | 7 | 1 | 0 | 6 | 0 |
| Reminders | 9 | 6 January 2013 | ||||||
| Newly identified | 1 | 6 January 13 | ||||||
| Derby | Original posting | 35 | May 2013 | 13 | 1 | 1 | 11 | 0 |
| Reminders | N/A | January 2014 | ||||||
| Newly identified | 21 | January 2014 | ||||||
| Lincoln | Original posting | 18 | June 2013 | 9 | 0 | 0 | 9 | 1 |
| Reminders | 8 | 24 March 2014 | ||||||
| Newly identified | 7 | 24 March 2014 | ||||||
| Leicester City | Original posting | 16 | June 2013 | 7 | 0 | 0 | 7 | 0 |
| Reminders | 8 | April 2014 | ||||||
| Newly identified | 28 | April 2014 | ||||||
| Mansfield | Original posting | 20 | July 2013 | 5 | 0 | 0 | 5 | 1 |
| Reminders | 14 | March 2014 | ||||||
| Newly identified | 4 | March 2014 | ||||||
| Chesterfield | Original posting | 16 | July 2013 | 5 | 2 | 0 | 3 | 0 |
| Reminders | 14 | January 2014 | ||||||
| Newly identified | 6 | January 2014 | ||||||
| Bradford | Original posting | 37 | 8 January 2014 | 7 | 1 | 1 | 5 | 0 |
| Reminders | 30 | March 2014 | ||||||
| Newly identified | 0 | March 2014 | ||||||
| Rotherham | Original posting | 17 | February 2014 | 2 | 0 | 0 | 2 | 0 |
| Reminders | 15 | June 2014 | ||||||
| Newly identified | 0 | N/A | ||||||
| Birmingham (Heartlands) | Original posting | 18 | 28 January 2014 | 5 | 0 | 0 | 5 | 0 |
| Reminders | 13 | March 2014 | ||||||
| Newly identified | 0 | March 2014 | ||||||
| Huntingdon | Original posting | 8 | 16 January 2014 | 2 | 0 | 0 | 2 | 0 |
| Reminders | 6 | June 2014 | ||||||
| Newly identified | 0 | N/A | ||||||
| Birmingham (Children’s Hospital) | Original posting | 24 | 12 February 2014 | 3 | 0 | 0 | 3 | 0 |
| Reminders | 21 | June 2014 | ||||||
| Newly identified | 0 | N/A | ||||||
| Doncaster | Original posting | 9 | 26 March 2014 | 2 | 0 | 1 | 1 | 1 |
| Reminders | 7 | July 2014 | ||||||
| Newly identified | 0 | N/A | ||||||
| Total | 379 | 86 | 8 | 3 | 75 | 3 | ||
Nominal control children were recruited from 51 of the 164 schools in the Nottingham area that were invited by post to take part (response rate of 31% at school level). Twenty-three of the participating schools agreed to take part only after a follow-up telephone call to discuss the information received.
The 51 schools, between them, gave information packs to the parents of 2787 children, of whom 291 (10.4%; median reply rate per school of 9.1%, IQR 4.6–14.9%, range 0–28.6%) replied, confirming they would like to participate. Seven of the schools that agreed to take part did not recruit any children. An additional 11 siblings of children who attended the appointment but who did not receive the invitation were in the correct age range and parents agreed for them to take part, bringing the number of children initially indicating that they wanted to participate to 302. Eight of these children were subsequently found to be ineligible for the study (one was too old, six already had hearing problems identified and one replied after recruitment closed), 11 changed their minds about taking part and we were unable to see 43 either because we could not make an appointment (mostly not contactable) or because they did not attend the arranged appointment.
The remaining 240 children were recruited as nominal controls and seen for study appointments (Figure 7).
FIGURE 7.
Numbers of control children in the diagnostic accuracy study.
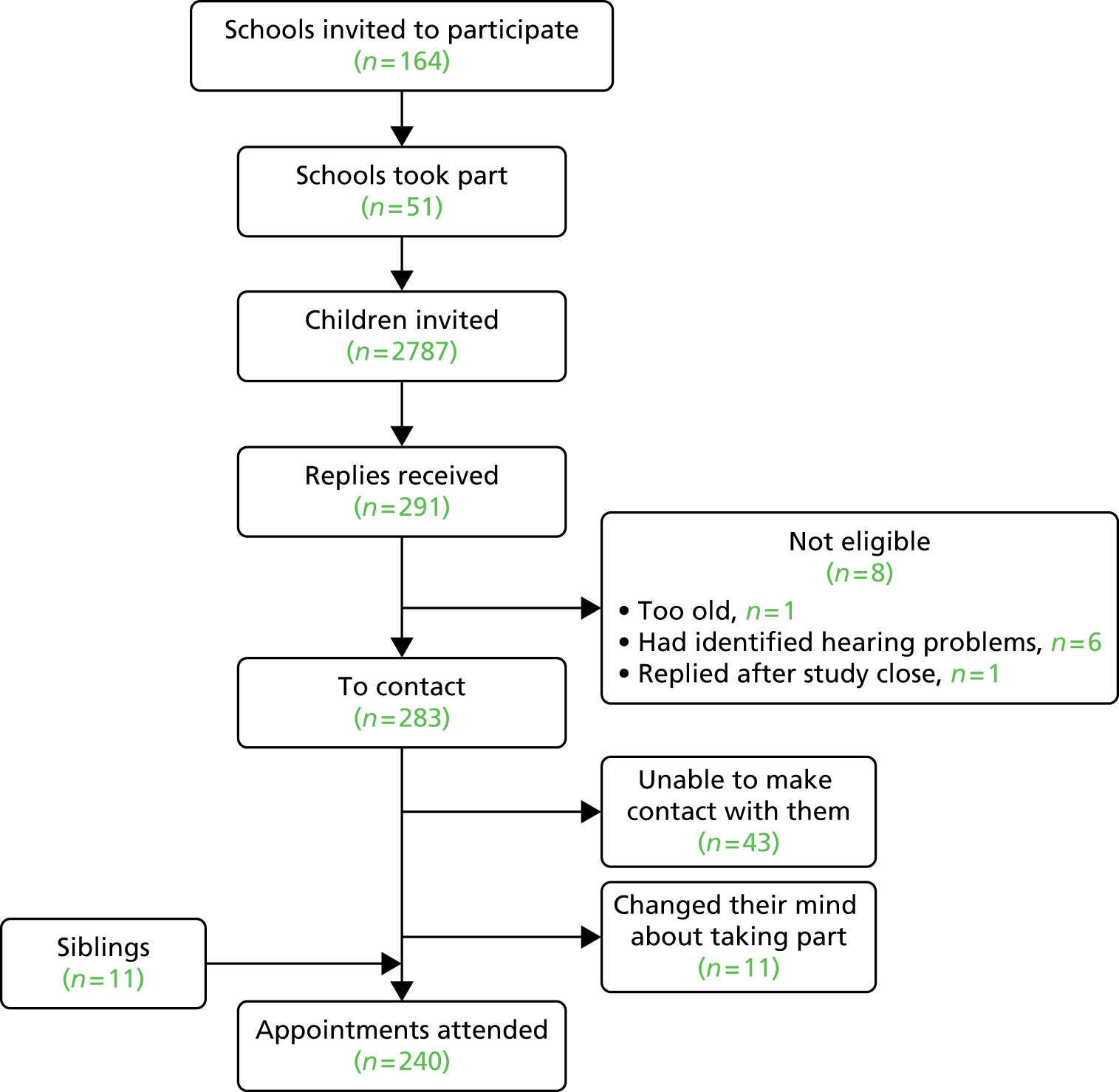
Demographic characteristics
Table 9 summarises the demographic characteristics of children who attended appointments (315) by whether they were nominally recruited as cases (75 children) or controls (240 children). The groups were similar with respect to gender and age. Four-fifths of each group was categorised as white on ethnicity. The nominal case group had a higher percentage of Asians (15% vs. 4%) and a lower percentage of mixed ethnicity children (1% vs. 9%) than the nominal control group.
| Characteristic | Recruited as case child (n = 75) | Recruited as control child (n = 240) |
|---|---|---|
| Male, n (%) | 38 (51) | 117 (49) |
| Age (years), mean (SD, range) | 5.4 (0.9, 3.9–7.0) | 5.4 (0.6; 4.0–6.9) |
| Ethnicity | ||
| White, n (%) | 61 (81) | 189 (79) |
| Black, n (%) | 2 (3) | 14 (6) |
| Asian, n (%) | 11 (15) | 10 (4) |
| Mixed, n (%) | 1 (1) | 22 (9) |
| Other, n (%) | 0 (0) | 5 (2) |
Time interval between screening test and reference standard for children nominally recruited as cases
Table 10 summarises the time interval between when the screening tests were administered and when the PTA reference standard was measured for the nominal cases, reporting the difference in weeks. Separate rows summarise the time interval for the 65 nominal cases for which reference standard data were available before the tests were administered, and for the 10 nominal cases who completed the screening tests before the reference standard. The absolute time interval is summarised for all 75 nominal cases. The median absolute time interval was 16 weeks. The reference standard was completed within the criterion period of 12 months (52 weeks) before to 3 months (13 weeks) after the screening tests for 73 children. For each of the remaining two children a reference standard measure was available prior to the 52-week cut-off (65 and 63 weeks before the screening test) and another was available for each at 24 weeks after the screening test. The two measures for each child showed very similar results and indicated a stable hearing impairment by both definitions and the children were therefore included in the analyses. As described in the methods section the children nominally recruited as controls completed the tests and the reference standard on the same day.
| Time interval in weeks | n | Mean (SD) | Median (IQR) | Range |
|---|---|---|---|---|
| Reference standard performed first | 65 | 19.3 (14.2) | 17.3 (7.4–24.4) | 0.7–63.3 |
| Screening test performed first | 10 | 8.9 (7.0) | 9.5 (1.6–11.9) | 1.1–24.1 |
| Absolute interval for all children | 75 | 17.9 (13.9) | 16.3 (6.9–24.1) | 0.7–63.3 |
Missing data and indeterminate results
Of the 630 recruited ears, 600 (95.2%) provided full data on the PTS and HC tests and scores for all four frequencies of the PTA reference standard and were included in the main analyses. Sixty-two (82.7%) of the 75 children recruited as cases and 233 (97.1%) of the 240 children recruited as controls (total = 295/315, 93.7%), provided full data on the tests and reference standard. There were no indeterminate screening test or PTA results. For children recruited as cases it was usually the PTA that was part missing; however, one child refused to do the HC test, another child refused to do the PTS test and one child had no physical ear on one side. For those recruited as controls, PTA and PTS results were missing for two participants due to equipment failure. There were a further two children with autism who were unable to complete the tests and the remainder were children who refused to do or complete other tests mainly because of lack of concentration or finding the headphones too tight.
Concordance between nominal case–control status and pure-tone audiometry reference standard classification
A unique feature of the two-gate (‘case–control’) design used for this accuracy study is that the criteria used to recruit children as cases and controls were not the same as the reference standard classification (using the PTA) that was used to define presence of the target condition when evaluating the screening tests. In other words the nominal target condition status is not necessarily consistent with the status based on application of the reference standard. For children nominally recruited as cases, hearing status was based on audiology notes. The criterion for inclusion was a bilateral impairment up to an average of 60 dB HL or a unilateral impairment of any level. Consequently, although nominally recruited as case or control children, some of the case children had no hearing impairment when assessed on the PTA reference standard and some of the control children were categorised as having impairment in the child-level analyses. Furthermore, and of particular relevance to the fact that the primary analyses are conducted at the level of the ear, there were case children for whom the PTA reference standard defined them as having the target condition in one ear but not the other (i.e. had unilateral impairment). There were, therefore, ears that were categorised and analysed based on the reference standard as HI that belonged to children nominally recruited as controls and ears that were categorised and analysed as NHI that belonged to children nominally recruited as cases.
Figure 8 indicates the number of ears belonging to participants nominally recruited as cases and controls that under the reference standard PTA were classified as HI, classified as NHI or for which there were insufficient data to classify the ear (i.e. PTA data were missing for that ear). The figure is based on the PTA reference standard definition of impairment as having a HL of ≥ 30 dB on at least one of the four frequencies (the primary definition). Twenty-six of the 150 ears of children nominally recruited as cases were classified on the reference standard as hearing and 48 of the 480 ears of children nominally recruited as controls were classified as impaired. Similar data are summarised at the level of child in Figure 9. Two of the 75 children who were nominally recruited as cases were classified on the reference standard as hearing. The pure-tone audiograms provided by the child’s local audiology service did not match the inclusion criteria but the children were tested within the study because, as per the protocol, the research team did not access the PTA until after the screening tests were undertaken. Of the 240 children who were nominally recruited as controls 37 were classified as impaired. We suggest that parents who had concerns about their child’s hearing were more likely to take up the invitation to take part.
FIGURE 8.
Nominal recruitment status at the ear level and the reference standard classification by or of hearing impairment status based on a PTA score of ≥ 30 dB on at least one of the four frequencies.

FIGURE 9.
Nominal recruitment status at the child level and the reference standard classification by or of hearing impairment status based on a PTA score of ≥ 30 dB on at least one of the four frequencies for at least one ear.
Accuracy of the pure-tone screen and HearCheck screener for identifying hearing impairment at the level of the ear
Figures 10 and 11 present flow charts that describe the number of impaired ears (based on a PTA score of ≥ 30 dB on at least one of the four frequencies) and hearing ears that passed and referred on the PTS and HC tests, respectively. Table 11 summarises the relationship between the PTS test results and the HC test results separately for impaired ears, hearing ears and ears for which information on the reference standard was missing. The figures highlighted in green indicate the numbers that were used in the calculation of sensitivity and specificity. The relationship between the PTS and HC results is summarised for the other five scenarios (see Tables 47–51, Appendix 4).
FIGURE 10.
Pure-tone screen results at the ear level by hearing impairment status based on a PTA score of ≥ 30 dB on at least one of the four frequencies.

FIGURE 11.
HearCheck screener test results at the ear level by hearing impairment status based on a PTA score of ≥ 30 dB on at least one of the four frequencies.
| PTS test results | |||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | Total | ||
| Impaired ears (by reference standard) | |||||
| HC test results | Refer | 136 | 2 | 1 | 139 |
| Pass | 10 | 7 | 0 | 17 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 146 | 9 | 1 | 156 | |
| Hearing ears (by reference standard) | |||||
| HC test results | Refer | 34 | 26 | 0 | 60 |
| Pass | 45 | 340 | 0 | 385 | |
| Missing | 0 | 1 | 0 | 1 | |
| Total | 79 | 367 | 0 | 446 | |
| Missing ears (no reference standard) | |||||
| HC test results | Refer | 13 | 2 | 1 | 16 |
| Pass | 3 | 2 | 1 | 6 | |
| Missing | 2 | 0 | 4 | 6 | |
| Total | 18 | 4 | 6 | 28 | |
Table 12 reports the sensitivity and specificity of the screening tests for the ear level. The sensitivity was 94.2% for the PTS and 89.0% for the HC screen. The 95% CIs for sensitivity indicate that we can be fairly certain that the true sensitivity is no lower than 89% for the PTS but could be as low as 83% for the HC screen. The McNemar’s test result (p = 0.02) indicates evidence that the true sensitivity is greater for the PTS than for the HC screener. The difference of 5% in sensitivity implies that for every 20 impaired ears tested the PTS would correctly identify an extra ear as having a hearing problem compared with using the HC screener. The corresponding values for specificity were 82.2% for the PTS and 86.5% for the HC screener, with evidence provided by McNemar’s test that the true specificity is higher for the HC screener than the PTS (p = 0.02).
| Measure | PTS | HC | Difference in accuracy (PTS – HC) | |
|---|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | p-value | |
| Sensitivity | 94.2% (89.0% to 97.0%) | 89.0% (82.9% to 93.1%) | 5.2% (0.2% to 10.1%) | 0.02 |
| Specificity | 82.2% (77.7% to 86.0%) | 86.5% (82.5% to 90.0%) | –4.3% (–8.2% to –0.4%) | 0.02 |
| Positive likelihood ratio | 5.31 | 6.60 | ||
| Negative likelihood ratio | 0.07 | 0.13 | ||
Table 13 reports the sensitivity and specificity for each combination of definition of impairment (PTA score of ≥ 30 dB on at least one frequency vs. a mean PTA score of ≥ 30 dB across all four frequencies) and subset of impaired ears used to calculate sensitivity (all ears, ears of children nominally recruited as cases and ears of children nominally recruited as controls). The results for the primary analysis for which the reference standard is a PTA score of ≥ 30 dB on at least one frequency and all ears are used to calculate sensitivity is shown on the top row. The sensitivity is generally higher (especially for the HC screener) when impairment is defined based on average HL across the four frequencies. This might be expected, as the primary definition of impairment is more stringent and thus more likely to result in less severe impaired ears being included in the impaired group. For the same reason the specificity is lower for both tests when impairment status is based on average HL across the frequencies presented under the PTA. Restricting impaired ears in the analysis to only those belonging to children recruited as cases results in increased sensitivity relative to inclusion of all ears. Again, this would be expected as ears of such children would be expected to have more severe hearing loss. Restricting impaired ears in the analysis to only those belonging to children recruited as controls results in lower sensitivity. Although only a sensitivity analysis, this latter result is notable because the impaired ears of children nominally recruited as controls may be more representative of the spectrum of impairment in the type of child that the screen would predominantly want to identify in a school-based setting (i.e. children with less severe hearing impairment).
| Reference standard | Subset of impaired ears | PTS | HC | ||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | ||
| PTA score of ≥ 30 dB on at least one frequency | All (primary analysis) | 94.2% | 82.2% | 89.0% | 86.5% |
| Nominal cases | 99.1% | 82.2% | 97.2% | 86.5% | |
| Nominal controls | 83.3% | 82.2% | 70.8% | 86.5% | |
| Average PTA score of ≥ 30 dB across frequencies | All | 95.7% | 76.4% | 94.8% | 81.8% |
| Nominal cases | 98.9% | 76.4% | 97.8% | 81.8% | |
| Nominal controls | 84.0% | 76.4% | 84.0% | 81.8% | |
Accuracy of the pure-tone screen and HearCheck screener for identifying hearing impairment at the level of the child
Figures 12 and 13 contain flow charts that describe the number of hearing-impaired children (defined based on a PTA score of ≥ 30 dB on at least one of the four frequencies in either ear) and hearing children who passed and referred on the PTS and HC tests, respectively. Table 14 summarises the relationship between the PTS test results and the HC test results for hearing-impaired children, hearing children and children who did not provide full data on the reference standard. The numbers highlighted in green were used to calculate sensitivity and specificity. The relationship between the PTS and HC results is summarised for the other five scenarios (see Tables 52–56, Appendix 4). Table 15 reports the sensitivity and specificity of the screening tests at child level. The sensitivity estimates (95.9% for the PTS and 88.7% for the HC screener) were similar to those reported for the ear-level analyses. The McNemar’s test (p = 0.02) indicates that the true sensitivity is greater for the PTS than for the HC screener. The difference of 7% in sensitivity implies that for every 14 impaired children tested the PTS would correctly identify an extra child as having a hearing problem compared with using the HC screener. Specificity at the child level was higher for the HC screener than for the PTS (83.8% vs. 79.8%) but there was little evidence of a true difference (p = 0.18).
FIGURE 12.
Pure-tone screen test results at the child level by hearing impairment status based on a PTA score of ≥ 30 dB on at least one of the four frequencies in at least one ear.

FIGURE 13.
HearCheck test results at the child level by hearing impairment status based on a PTA score of ≥ 30 dB on at least one of the four frequencies in at least one ear.

| PTS test results | Total | ||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | |||
| Impaired (by reference standard) | |||||
| HC test results | Refer | 85 | 1 | 0 | 86 |
| Pass | 8 | 3 | 0 | 11 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 93 | 4 | 0 | 97 | |
| Hearing (by reference standard) | |||||
| HC test results | Refer | 18 | 14 | 0 | 32 |
| Pass | 22 | 144 | 0 | 166 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 40 | 158 | 0 | 198 | |
| Missing (no ears reference standard) | |||||
| HC test results | Refer | 14 | 0 | 1 | 15 |
| Pass | 1 | 0 | 0 | 1 | |
| Missing | 1 | 0 | 3 | 4 | |
| Total | 16 | 0 | 4 | 20 | |
| Measure | PTS | HC | Difference in accuracy (PTS – HC) | |
|---|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | p-value | |
| Sensitivity | 95.9% (89.8% to 98.9%) | 88.7% (80.6% to 94.2%) | 7.2% (0.3% to 14.1%) | 0.02 |
| Specificity | 79.8% (73.5% to 85.2%) | 83.8% (78.0% to 88.7%) | –4.0% (–10.5% to 2.3%) | 0.18 |
| Positive likelihood ratio | 4.75 | 5.49 | ||
| Negative likelihood ratio | 0.05 | 0.14 | ||
Table 16 reports the sensitivity and specificity for each combination of definition of impairment (PTA score of ≥ 30 dB on at least one frequency vs. an average PTA score of ≥ 30 dB across all four frequencies) and subset of hearing-impaired children used to calculate sensitivity (all children, children nominally recruited as cases and children nominally recruited as controls). The results for the primary analysis in which the reference standard is a PTA score of ≥ 30 dB on at least one frequency for at least one ear and all impaired children are used to calculate sensitivity are shown on the top row. The pattern of results is similar to the ear-level analyses. The use of less stringent passing criteria for the reference standard (average PTA score of ≥ 30 dB across the four frequencies presented) generally results in higher sensitivity and markedly lower specificity. Restricting the sample to impaired children who were nominally recruited as cases resulted in higher sensitivity and restricting the sample to impaired children who were nominally recruited as controls resulted in lower sensitivity.
| Reference standard | Subset of impaired children | PTS | HC | ||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | ||
| PTA score of ≥ 30 dB on at least one frequency | All (primary analysis) | 95.9% | 79.8% | 88.7% | 83.8% |
| Nominal cases | 98.3% | 79.8% | 98.3% | 83.8% | |
| Nominal controls | 91.9% | 79.8% | 73.0% | 83.8% | |
| Average PTA score of ≥ 30 dB across frequencies | All | 97.3% | 72.7% | 93.3% | 78.2% |
| Nominal cases | 98.1% | 72.7% | 98.1% | 78.2% | |
| Nominal controls | 95.2% | 72.7% | 81.0% | 78.2% | |
Discussion
In this chapter we have estimated and compared the diagnostic accuracy of the PTS and HC tests for discriminating between children with and without hearing impairment (sensorineural, permanent conductive or transient) basing our reference standard on the results of PTA. Our primary definition of hearing impairment was a threshold of ≥ 30 dB on at least one of the four frequencies presented for the PTA assessment. Diagnostic accuracy was primarily assessed at the level of ear, as this chapter is principally concerned with differentiating impaired ears from hearing ears, but child-level analyses were also reported, as those estimates are most relevant for the economic analyses reported in Chapter 8. Our primary analysis included ears and children regardless of whether they had nominally been recruited as cases or controls. The inclusion of all ears/children is appropriate for assessing the extent to which the screening tests can differentiate the presence of hearing impairment from no hearing impairment across all children. However, the secondary analyses that include only impaired ears/children who were nominally recruited as controls may provide estimates of accuracy that are more pertinent for assessing the value of such tests in the context of a SES programme, where we are looking to identify children with previously unsuspected impairment.
Confidence intervals for the accuracy measures at ear level indicate that the sensitivity is likely to be no lower than 89% for the PTS and 83% for the HC screener, and that the specificity is no lower than 78% for the PTS and 83% for the HC screener. There was evidence at the 5% level that the sensitivity is greater for the PTS than the HC screener and that the specificity is greater for the HC screener than for the PTS. Considering only the results presented in this chapter and not taking into account other issues considered in the cost-effectiveness analyses presented later in this report (see Chapter 8), the value of the SES tests rests on the minimum accuracy level that makes them worthwhile in practice. The choice between the screening methods hinges on the importance of the test being sensitive relative to the importance of it being specific – in other words whether false negatives (children with a hearing impairment who pass the screening tests) are a more important problem than false positives (children with no hearing impairment referred by the screening tests). Reducing the false-negative fraction (i.e. increasing sensitivity) is more salient because, ultimately, the priority is identifying impaired children rather than confirming the status of children without impairment. On this basis one might lean towards the PTS as the ‘better’ test. It should be noted that the additional impaired children who are identified as a result of the increased sensitivity are likely to be at the less severe end of impairment and that there are therefore diminishing returns in improved identification.
Another consideration in the interpretation of the accuracy estimates is to take account of the effect of the prevalence of hearing impairment. This is particularly important in diagnostic case–control studies where the study prevalence of hearing loss is artificially determined (Table 17). For this we have assumed a prevalence of hearing loss typical of the pre and early school period as identified for the purposes of the health economic model in Chapter 8 – 47 in every 10,000 children, which we have rounded up to 50 in every 10,000 children to simplify the 2 x 2 table. We applied this prevalence to the primary analysis accuracy estimates at child level for a PTA score of ≥ 30 dB on at least one frequency, PTS 95.9% sensitivity and 79.8% specificity, and HC screener 88.7% sensitivity and 83.8% specificity.
| Test result | PTS analysis | HC analysis | ||||
|---|---|---|---|---|---|---|
| Reference standard result | Reference standard result | |||||
| Refer | Pass | Total | Refer | Pass | Total | |
| Refer | 48 | 2010 | 2058 | 44 | 1612 | 1656 |
| Pass | 2 | 7940 | 7942 | 6 | 8338 | 8344 |
| Total | 50 | 9950 | 10,000 | 50 | 9950 | 10,000 |
This allows quantification of the benefit of the PTS relative to the HC screener arising from improved sensitivity in context, with four additional true positives identified for every 10,000 children screened – the number needed to screen to achieve one additional true positive is 2500. It also emphasises that where the prevalence of hearing impairment is low, the effect of changes in specificity is amplified. The specificity obtained for the screening tests suggests that considerable numbers of false-positive tests [referrals for a diagnostic evaluation with an audiologist (DEA) who turn out not to have hearing impairment] will be generated – 1612 in the case of the HC screener and 2010 in the case of the PTS. This might argue that specificity should be the metric that decides whether the PTS or the HC screener is preferable. However, as is discussed further in Chapter 8, the actual number of referrals observed in a health-care system with SES in place is considerably less than would be predicted considering just the specificity of the screening test in isolation (see Table 21).
Analyses directly comparing the results of the PTS and HC screener revealed some discordance in the pass/refer classification, that is, some children who passed the PTS were referred by the HC screener, and vice versa. This suggests that the tests may be making different types of errors and that the best aspects of both tests could be combined into a new or hybrid test that is more accurate than either of the individual existing methods. As well as using different methods, they also have a basic difference in that PTS tests four frequencies at one level and the operator has the option to repeat tones if he/she feels that the child, for instance, has lost concentration whereas the HC tests two frequencies at three levels and is automated. Each frequency is presented only once to the child and there is some regularity to the presentation of tones such that the next tone could be anticipated. The only tested frequency that the two tests have in common is 1 kHz. The PTS is applied under headphones, which means that the ear not being tested has some barrier to any sound being presented to the test ear. The HC screener is applied through a close-fitting cardboard cup to one ear but the other ear has no sound protection and might hear the sound applied to the test ear.
Strengths and limitations
The study has a number of strengths. It was carefully designed and conducted, and data analysis was rigorous and included a number of secondary (sensitivity) analyses across different definitions of impairment. A sample size calculation was conducted a priori and the recruited sample yielded narrower CIs (and thus less uncertainty) for the accuracy estimates than the estimates from the economic model in the 2007 report. 12
A weakness of the study is that it estimated and compared the diagnostic accuracy between the PTS and HC screener methods using a two-gate (‘case–control’) design. 32 Under this approach those with the target condition (HI) and those without (NHI) are sampled from separate sources. The impaired children were largely identified via audiology services and the hearing children were largely recruited through schools. The two-gate design contrasts with the single-gate (or cohort) design where both those with and without the target condition are sampled from a single source without any knowledge of their target condition status. The more traditional single-gate design would have been preferable, but the challenge with this approach is that, because the prevalence of hearing impairment is so low, a large number of children would need to be recruited to the study in order to ensure that there is a sufficient number with the target condition. Fewer than 1 in 2000 children screened at school entry will have sensorineural hearing impairment8 and 160,000 school children would need to be approached and tested in order to identify the target of 80 cases originally proposed in this study. The anticipated size of the single-gate design test accuracy study challenges both its feasibility and value for money in this context. This is not an issue for the two-gate case–control design used here. The two-gate design, however, is known to be more prone to biased estimates of accuracy largely resulting from the fact that those with and without the target condition are sampled from separate sources rather than drawn from a single defined population. This is problematic when estimating the accuracy of each test but is a less salient issue when comparing accuracy between hearing screening tests since any biases operating will be experienced equally for each test assessed. With respect to differential accuracy, conclusions on whether one hearing screen is better than the other should remain robust.
The criteria used to recruit children in each gate of the design were not used as the reference standard to categorise the children according to impairment status. A consequence of this is that some of the participants who were nominally recruited as controls were categorised as having a hearing impairment on the PTA reference standard. Cases were recruited as having a bilateral or unilateral hearing impairment and hence some ears were categorised as hearing on the PTA reference standard. In this event, this was a strength of the study as the HI group included children with established hearing impairment as well as children with no previously identified hearing impairment. The latter group may have levels of impairment severity that are more representative of the type of children who one might seek to identify with hearing impairment using a school entry hearing screen. The recruitment of such children enabled us to carry out sensitivity analyses based on whether impaired children were known cases (and therefore had a permanent hearing impairment) or were not previously identified with a problem (and therefore probably included children with transient hearing impairment).
A higher-than-anticipated number of children nominally recruited as controls were shown to have a hearing impairment on PTA measurement. We suggest this may be caused by a bias towards participation in those families who had some concerns about their child’s hearing and therefore saw the study as a means by which their child could be tested, even though Nottingham operates an open referral system offering an appointment without a referral from a GP.
For the children nominally recruited as controls (which made up most of the hearing group) the screening tests and the reference standard were administered on the same day. The children nominally recruited as cases (mostly children in the HI group), however, generally had the reference standard administered at a different time to the test; up to 62 weeks before the test and up to 24 weeks after the test. The HL of children with permanent hearing impairment, however, is likely to be stable or worsening and it is not possible for children who have permanent sensorineural hearing impairment at one point to not have that hearing impairment later on.
Results in comparison with other studies
Several studies assessing the accuracy of the PTS were identified in the 2007 HTA report12 (see Table 6) and no accuracy studies of the PTS were identified in the update review. The sensitivity from these earlier studies ranged from 82% to 100% and specificity from 65% to 99%. Thus, the accuracy obtained in this study, sensitivity 94.2% (95% CI 89.0% to 97.0%) and specificity 82.2% (95% CI 77.7% to 86.0%) is consistent with the previous findings, and shows no evidence of the overestimation of accuracy, which is theoretically predicted by using a case–control methodology. This provides reassurance that the accuracy estimate obtained for the HC screener, particularly the relative accuracy of the HC screener against the PTS, is also robust, as relative accuracy is less likely to be vulnerable to biases associated with study design. This is helpful as an additional accuracy study was identified for a HC device relative to reference standard of PTA in the update review. Gloria-Cruz et al. 17 estimated sensitivity as 23% (95% CI 11% to 39%) and specificity as 97% (95% CI 96% to 98%). The study was conducted in the Philippines in a convenience sample of Grade 1 elementary school children from three metropolitan schools with a mean age of 7.6 years. The prevalence of hearing loss was approximately 5% (39/821). The circumstances are thus very different from our consideration of SES in the context of the UK, which is likely to explain the marked difference in accuracy between the estimates. Additionally, the devices were not identical in each study. The device used by Gloria-Cruz et al. 17 (HC navigator) used three frequencies rather than two and a higher threshold to define hearing impairment, which would decrease sensitivity and increase specificity.
As well as impacting on the validity of our accuracy estimates, comparison with other studies also impacted on our decision-making about the choice of accuracy estimates to be used in the base case for the health economic evaluation. In the current protocol the 2007 HTA study estimates were identified as the source of estimates for our base-case analysis. This decision seems to be justified both in terms of the internal validity of the estimates and their applicability relative to alternative sources of accuracy data identified.
Chapter 4 False-negative results from screening tests
Introduction
An important issue to be addressed in any research on population identification is how many people with the target condition are not identified, that is, the false negatives from any test, screen or awareness programme. Such data are expensive to collect, involving whole population follow-up and only realistic in longitudinal whole-cohort studies.
In this particular screening scenario, true and false positives are relatively easy to confirm. A child who is referred by the screen and is subsequently found to have a hearing impairment is a true positive and a child who is referred by the screen but is subsequently found to have no hearing impairment is a false positive.
It is also possible for a child to pass the screen and be therefore a true or false negative. A true negative is a child who passes the screen and is found to have no hearing impairment. A false negative would be a child who passes the screen when they in fact did have a hearing impairment.
Delayed identification in children who pass the hearing screen but who do have a hearing impairment (false negatives) is a concern. The challenge is how to identify false negatives. As children who pass the screen are not routinely followed up, only by evaluating every child in the SES programme at a diagnostic centre could one assess the numbers who are true negatives and those who are false negatives.
With the numbers involved this is clearly not feasible. Instead noting children who are referred for audiological assessment at any time after they have undergone and passed the screen could derive an estimate of the false negatives. However, even if such children passed the screen and are subsequently found to have a hearing impairment there can never be any certainty that the hearing impairment was present on the day the child was screened. If it is a sensorineural impairment, it could have been acquired or have progressed beyond a level identifiable by the screen. If it is a transient conductive impairment, by its very nature, it is very likely not to have been present at the time of screening. Only a permanent conductive impairment caused by anatomical pathology could be presumed to have been present on both occasions.
One way to address these issues is through a review of the literature, looking specifically for robust reports of data that explore the impact on the family and the child’s development and education, of an unidentified or late-identified hearing impairment. However, such studies are likely to be rare for the reasons outlined above.
In addition, from the data collected in the diagnostic accuracy study (see Chapter 3) we can report the number of false negatives, that is, children who passed either of the screening tests and were found to be HI by the reference standard (see Chapter 5). This was a departure from our original protocol, added at the suggestion of our study steering group.
The key issue to be addressed by the review and the data is the size of any reduction or increase in the number of children identified late in a system with and without SES, and how much benefit is likely to accrue through their earlier identification and management.
Objectives
-
To review literature on the impact of false-negative results from screening tests.
-
To describe children with false-negative screening results in the diagnostic accuracy study.
Methods
The following electronic databases were searched from inception to May 2014: The Cochrane Library (via Wiley), MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid), EMBASE (via Ovid), PsycINFO (via Ovid) and Science Citation Index (via Web of Science). Using the search terms ‘false negative’ and ‘hearing’ identified a total of 173 titles. Using broad inclusion criteria of reference to hearing screening, sensitivity, specificity and/or false negatives, two of the authors (HF and CH) reviewed the titles and selected 26 papers for further consideration of abstracts. Applying the same criteria to the abstracts, 13 papers were identified for full review.
From an additional search using the terms ‘negligence’ and ‘hearing’ we identified 88 titles of which three abstracts were identified for full review, one of which had previously been included. We therefore sought 15 papers3,7,8,26,43–53 for full review. All but one45 (published in 1981) were published between 1996 and 2014.
Data collected for children participating in the diagnostic accuracy study (see Chapter 3) who passed the screening tests but had a refer result on the reference standard were extracted and tabulated.
Results
Literature review
No studies were identified that reported false-negative data for the school entry hearing screen. Four of the studies reported comparisons of two different screening tests without reference standards,44–47 two of the studies (identified from the search on negligence) reported no cases owing to missed diagnosis of hearing impairment,48,49 and three included general discussion of the value of hearing screens beyond the neonatal period. 8,50,51 These nine studies were not reviewed further.
Geal-Dor et al. 52 reviewed the results of neonatal screening at birth and behavioural screening at 7–9 months for 1545 children in Israel. They identified 58 children who had passed both screening tests or had passed the only screening test they underwent who were later referred for audiological assessment between the ages of 1 month and 4 years. Thirty-four had age-appropriate thresholds. Of these, 18 were discharged and 16 belonged to high-risk groups and were followed up. All of the remaining 24 children had a conductive impairment. No cases of permanent hearing impairment were identified.
In a further sample of 49 children identified with permanent hearing impairment, the results of the two screening tests were reviewed. Although eight children passed one screen and were referred by the other, only one child passed both screening tests, a conservative false-negative rate of 2% (1/49).
In an 8-year follow-up of the cohort of 21,279 babies enrolled in the Wessex (UK) trial of neonatal screening,3 two of the 31 children identified by the age of 8–10 years with a permanent bilateral hearing impairment ≥ 40 dB had passed the neonatal screen (false-negative rate 6%). Of the 28,172 children who did not undergo neonatal screening, 35 children were identified with a permanent bilateral hearing impairment ≥ 40 dB and six of these had passed the distraction screening test at 7–9 months (false-negative rate 17%). The authors note that seven children (who had failed early screens) had evidence of progression of hearing impairment and speculate that the eight children who passed the screens might also have a progressive impairment, implying that the negative results may not have been false negatives.
Lo et al. 53 compared a parental questionnaire with PTA results in 6- to 7-year-old Chinese children in Hong Kong. They report that parents were able to identify only 20% of the cases of OME (no permanent hearing impairments were identified).
A birth cohort of 49,335 children in Australia with no risk factors for hearing impairment at birth underwent the distraction test at 7–9 months and were followed up at age 6 years. 7 Of 45,078 children who passed the distraction test eight were later identified to have a bilateral hearing impairment > 40 dB HL and fitted with hearing aids. The authors report a false-negative rate of 0.02% (8/45,078). If we assume those who failed the distraction test had a hearing impairment, the true false-negative rate would be 0.19% (8/4257 + 8); however, this assumption cannot be validated.
A study of college students (> 18 years) in the USA43 noted that the false-negative rate was higher when the reference standard included all frequencies rather than the frequencies at which the screening was performed. However, they also note that the major reason for this was the presence of hearing impairment at high frequency (6 kHz) in these young adults, possibly caused by leisure noise. This is an important issue and is particularly relevant for this project when evaluating the HC screener (1 and 3 kHz) against gold standard PTA at 0.5, 1, 2 and 4 kHz.
Soares et al. 26 compared the results of a new AABR screener against PTA in children and young adults aged from 3 to 22 years in Japan. For the pre-school children aged 3–5 years the false-negative rate was zero but increased to 3% (3/108) for ages 6–17 years and 12% (2/17) for 18- to 22-year-olds.
Diagnostic accuracy study (see Chapter 3)
For control children in the diagnostic accuracy study, if a screening test (either PTS or HC) was passed but the PTA result indicated a hearing impairment, the result of the screening test is defined as a false-negative result. Sixteen ears of 16 children met this definition. Each ear underwent two screening tests, so the 16 ears underwent 32 screening tests. Of these, 22 tests were passed (false negatives) and 10 tests gave a refer results (true positives).
Of the 22 false-negative test results, eight passed only the HC, two passed only the PTS and six passed both screening tests (Figure 14).
FIGURE 14.
False negatives from diagnostic accuracy study (PTA average criterion: average of four frequencies is ≥ 30 dB. PTA at least one criterion: at least one of four frequencies is ≥ 30 dB).

Of the six ears that passed both screening tests, all had a PTA score of ≥ 30 dB on at least one of the four frequencies, 0.5, 1, 2, or 4 kHz, and three of those had a PTA score of ≥ 30 dB averaged across the four frequencies.
Of the eight ears that passed the HC but referred on the PTS, all had a PTA score of ≥ 30 dB on at least one of the four frequencies, 0.5, 1, 2, or 4 kHz, and two of those had a PTA score of ≥ 30 dB averaged across the four frequencies.
Of the two ears that passed the PTS but referred on the HC, both had a PTA score of ≥ 30 dB on at least one of the four frequencies, 0.5, 1, 2, or 4 kHz, and one of those had a PTA score of ≥ 30 dB averaged across the four frequencies.
All children for whom there was a refer result on the PTA (regardless of screening test results) were offered referral for further assessment. Of the 16 children with false-negative screening test results on one ear, one child was not referred because the result was considered to be because of a lack of concentration and one did not attend the appointment. Of the remaining 14, 10 were found to have no hearing impairment and discharged, presumably because a transient hearing impairment had resolved. Three children who passed the HC test but referred on the PTS test were found to have mild impairments and the one child who passed both screening tests was found to have a mild impairment.
Discussion
We were unable to identify studies that reported false-negative data from the school entry hearing screen. Across five different screening settings (neonatal at birth, distraction test at 8 months, parent questionnaire at 6–7 years, AABR at age 3–22 years, and the PTS at age 18–49 years), false-negative rates varied from 0.2% to 20%. All of the studies included in this review note the difficulty in assessing false negatives across evaluations separated in time for a condition that can fluctuate (transient conductive impairments) or progress or is of later onset (sensorineural impairments). The presence of a hearing impairment at a later time in an individual who passed a screening test at an earlier time can never be determined with certainty to be a false-negative result of the screen.
For the children nominally recruited as controls in the diagnostic accuracy study, the screening tests and the reference standard were conducted on the same day and the false-negative results could be considered to be more certain. The results of the further assessment which was conducted some weeks later indicate that most of the impairments identified by the reference standard on the day of the screening tests were transient and improved before the child attended the later appointment.
Owing to the lack of robust published data on false-negative results from SES, we did not take the results of this review further in terms of the economic modelling (see Chapter 8).
Chapter 5 Comparison of a site with a school hearing screening programme (Nottingham) with a site without a school hearing screening programme (Cambridge)
Introduction
One alternative to the standard SES programme would be to not have a programme at all. The systematic review in the 2007 HTA report12 described one poor quality study that compared screening with no screening, but the results were inconclusive. 16 The updated systematic review reported here in Chapter 2 failed to identify any further studies.
In 2005, one in eight of all services in the UK responsible for implementing a universal school entry hearing screen were no longer doing so. 12 Approximately half of those services ran no screen and the remaining half offered a targeted screen for children identified as most at risk. The reasons given for not running a universal screen were based mainly on practical issues, including lack of resources, rather than any research-based evidence. There is no evidence whether implementing a screen at school entry leads to a heightened awareness and hence no failure to identify hearing impairment in children, or whether it results in children, who would otherwise be identified by the screen, slipping through the net and ultimately either never being identified or being identified much later in their school career.
In this chapter we compare two areas of the UK, one with a standard SES programme (Nottingham) and one without such a programme (Cambridge).
Objectives
-
To compare children referred for investigation of suspected hearing impairment in a geographical area that applies routine SES (Nottingham) with a service with no routine SES (Cambridge) with respect to the number of referrals, the age at referral, the source of referral, the route through assessment to intervention, the number of children ultimately identified to have a hearing impairment (yield) and the nature of hearing impairment identified.
Methods
Routine data were analysed to compare data on referrals between a site with SES (Nottingham) and a site without SES (Cambridge).
Background to study sites
Site with a school screen (Nottingham)
The CHAC is a third-tier service and is part of Nottingham Audiology Services within Nottingham University Hospitals NHS Trust. There is no separate second-tier audiology service for children within Nottingham, therefore, all children referred are seen within the same service. CHAC provides services for all children up to the age of 16 years (19 years if in special education) within the catchment area of Nottingham City, Rushcliffe, Erewash, Ashfield, Broxtowe and Gedling. It also accepts referrals from surrounding areas for specialised services. The service is led by a band 8a Clinical Scientist (CB) and has six whole-time equivalent (WTE) audiological staff ranging from band 6 to 8a. CHAC has an open referral policy and therefore accepts referrals from parents as well as from all professionals. It works closely alongside the ENT Department at Nottingham University Hospitals and children move between the two services depending on their needs. The service has around 3400 new referrals a year with a total number of 5900 patient appointments on average.
The service assesses children’s hearing according to national and local protocols and provides counselling and advice to parents with any concerns regarding their child’s hearing. If a child is identified as having a transient conductive impairment, they are referred onto ENT as appropriate after monitoring. For those children identified as having a permanent impairment and those children with a transient conductive impairment whose parent(s) choose not to have surgical management, the service provides hearing aids and ongoing habilitation (Figure 15).
FIGURE 15.
Pathway for Nottingham patients. CHI, conductive hearing impairment.
All schools within the Nottingham City and County authorities carry out the school entry hearing screen as part of the school entry health screen.
Site with no school screen (Cambridge)
The Cambridge service is a second-tier service provided by Cambridge Community Paediatric Audiology Service, which is part of the Cambridgeshire Community Services (CCS) NHS Trust. It receives approximately 1800 new referrals per year with a total of approximately 2400 appointments annually. The service provides second-tier hearing assessment for children aged 7 months to 16 years (19 years if in special education) and information and education to carers and professionals. The catchment area covers children living in, attending school in or with a GP in Cambridge City, or South and East Cambridgeshire. The service is provided by two community paediatricians (total 1.3 WTE, one of which is clinical lead for the service), one WTE band 7 lead clinical scientist (Audiology) (JM), members from the Addenbrooke’s Paediatric Audiological Team who provide sessional coverage, and administrative support provided by members of the Clinical Support Team attached to Children’s Services within CCS.
The service is provided within family-friendly environments located in the community. A community paediatrician and an audiologist usually run each clinic by working together, using their own areas of expertise, to look at the whole child.
Referrals are made to the service from GPs, child and family nurse team/health visitors, speech and language therapists, paediatricians, education, social services, and the Newborn Hearing Screening Programme (NHSP). When a chronic or permanent hearing impairment is identified the service aim is to facilitate further assessment and management within third-tier services, that is, the tertiary audiology service at Addenbrooke’s Hospital, the ENT department at Addenbrooke’s Hospital, and speech and language therapy.
The role of the service is to assess children’s hearing, according to national and local protocols, for children referred due to carer or professional concern. It also includes assessment of children who have been identified to be ‘at risk’ by the NHSP despite clear responses on the screen. The main condition seen in the second tier is transient conductive hearing impairment associated with middle ear effusion or ‘glue ear’. The second tier provides carers and professionals with information and education regarding this type of hearing impairment and good strategies for supporting the child. If the middle ear effusion or conductive hearing impairment is persistent there are agreed written protocols for referring these children to the ENT Department at Addenbrooke’s Hospital. If a child is identified as having a sensorineural (unilateral or bilateral) hearing impairment, the child will be referred to the Tertiary Paediatric Audiology Service at Addenbrooke’s Hospital for diagnostic assessment and management; this includes any child where it has not been possible to exclude a sensorineural hearing impairment. Referrals to the second tier are often made in order to exclude a hearing impairment as a contributing factor to educational, behavioural and/or speech and language concerns. This includes children who may go on to be identified as being on the autistic spectrum. A diagrammatic representation of the pathway of care for Cambridge is shown in Figure 16.
FIGURE 16.
Pathway for Cambridge patients. CPAS, Community Paediatric Audiology Service; CHI, conductive hearing impairment; CUHFT, Cambridge University Hospitals Foundation Trust (third-tier hospital).
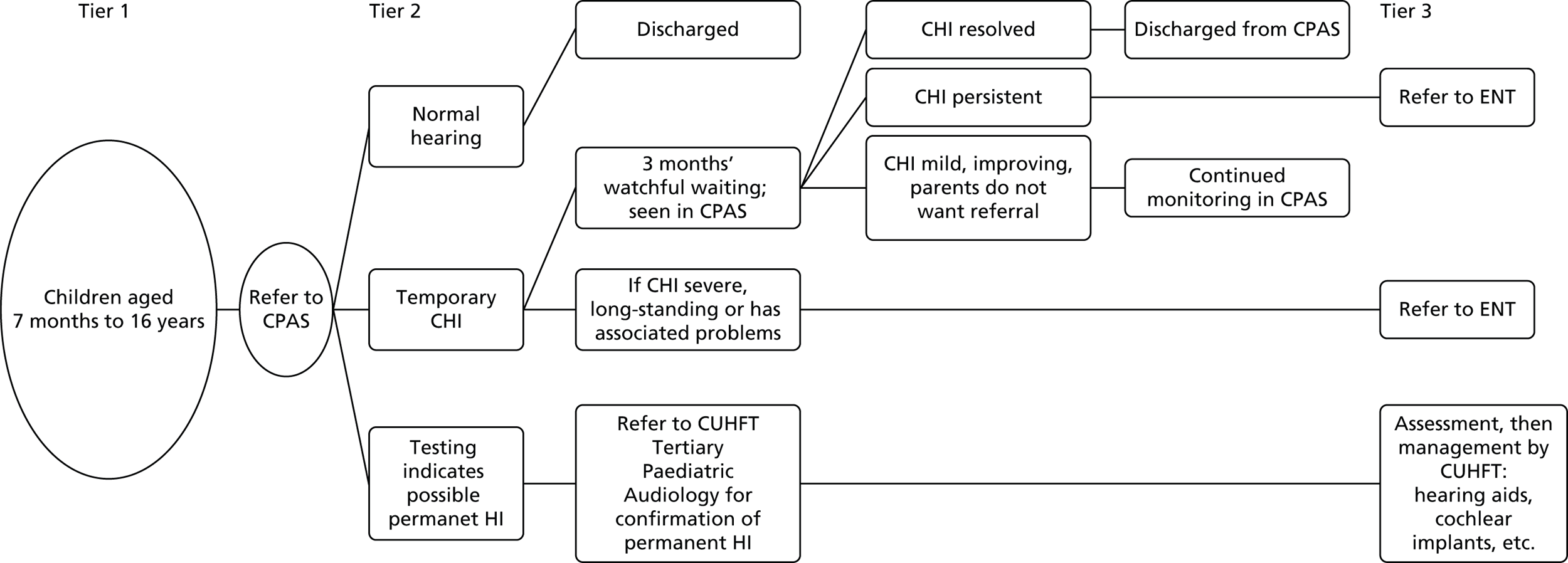
There has been no SES in Cambridge City, South and East Cambridgeshire since 1997, when the health visitor distraction testing was abolished in this area. Reasons for stopping included cost, variability of practice and lack of strong guidance from the Department of Health on what should be provided for SES and how it should be implemented. Community Paediatric Audiology in CCS was set up in 1997 with a strong campaign to first-tier services (GP, speech and language therapists, etc.) informing them that there would be no routine hearing tests in Cambridgeshire and emphasising the importance of referring to second-tier services if there are parental and professional concerns. This involved letters to all GP practices and health visiting teams, and speech therapy services outlining the changes in provision of routine hearing tests, and posters in GP practices and speech therapy clinics, highlighting the presenting symptoms of glue ear.
Data collection
Data were collected for children aged between 3 years and 6 years 364 days who were referred to Nottingham paediatric audiology or Cambridge audiology services by any source other than the UNHS. All referrals between 1 September 2012 and 30 June 2014 were included. Data on follow-up appointments that took place up to 30 September 2014 were included.
We originally proposed to explore retrospective data collected in Cambridge from 2007 to 2012. However, as those data were not collected with the objectives of the research in mind, much information was missing and it was decided that analyses would not add constructively to the project.
The collaborating audiologists for the areas of Nottingham (site with SES) and Cambridge City, and South and East Cambridgeshire (site without SES) collected data on referrals. Further data were collected via questionnaire from parents of children referred from the SES programme to the Nottingham service (see Chapter 6). Prospective data were processed using a database built by PenCTU.
Audiologists entered data from patient notes. In Nottingham, the waiting list co-ordinator identified eligible children, and audiologists within the service with permission to access patient records entered the data. In Cambridge assistance was also provided by one of the Nottingham researchers. Data were entered for the complete care pathway, from referral through to discharge (or 30 September 2014, whichever occurred first) including: date of birth; postcode; date of referral; location of clinic; referral source (GP, health visitor, school screen, etc.) date of appointment(s); type of assessment (e.g. visual reinforcement audiometry, play audiometry in the soundfield, PTA); result of assessment [normal bilateral, normal soundfield (better ear) thresholds, unilateral sensorineural, bilateral sensorineural, unilateral conductive, bilateral conductive, mixed bilateral, incomplete]; tympanometry results; hearing thresholds/minimal response levels; probable cause of impairment (if known); end of care (yes/no); and outcome [discharge, referral to ENT, referred for diagnostic confirmation (Cambridge only), hearing aids fitted (Nottingham only)].
Each child’s records were accessed by audiologists authorised to look at them, hence consent was not required from individual patients. Anonymised data were entered onto the database. These procedures received ethical approval. Referral data were checked and corrected for implausible values. Staff time to undertake a planned second data extraction check of 10% was severely restricted by the pressure of service delivery. Undertaking this data check was not possible with the staff resources available without causing a significant delay to the study reporting. Copies of the questionnaires were sent to PenCTU for second data entry and double entry data checking.
Statistical analysis
The yield, rate of referral and age at referral were compared between the site with a SES programme (Nottingham) and the site without a SES programme (Cambridge).
Yield was defined as the number of children between their third and seventh birthdays identified as having hearing impairment whose date of referral was between 1 September 2012 and 30 June 2014 (the study period) per 1000 person-years at risk. Hearing impairment included transient conductive and permanent sensorineural or conductive hearing impairments. The rate of referral was defined as number of referrals for suspected hearing impairment in the same period per 1000 person-years at risk. Some referrals resulted in more than one appointment. Children for whom the outcome of the last appointment was further referral or hearing aid were considered to have hearing impairment and included in the numerator in the calculations for yield; children discharged at the last appointment were considered to have no hearing impairment. In order to calculate the denominator for yield and the referral rate, the population size in each site was obtained from the Office for National Statistics mid-2013 estimates54 of the population who were aged 3, 4, 5 or 6 years in the study sites. The Nottingham site included referrals from the local authorities of Nottingham, Erewash, Ashfield, Broxtowe, Gedling and Rushcliffe; the Cambridge site included Cambridge, East Cambridgeshire and South Cambridgeshire. The number of person-years observed was calculated by multiplying the population size by the number of days during the study period (668 days) and dividing by the mean number of days in a year (365.25 days). The two sites were compared with respect to yield and referral rate using the rate ratio, reported with 95% CI and p-value.
The t-test was used to compare the mean age at referral between the Nottingham and Cambridge sites (1) for all initial referrals; and (2) for confirmed HI cases only.
We report the percentage of referrals that resulted in the identification of HI cases for: (1) Nottingham referrals that were via a school screen; (2) Nottingham referrals that were via any other source (e.g. GP, speech therapist); and (3) Cambridge referrals.
Finally, we report the median (IQR) level of hearing impairment in dB at each of four frequencies (0.5, 1, 2 and 4 kHz) in each ear and the source of referral (using numbers and percentages) for both Nottingham and Cambridge. These variables were summarised for all referrals and then for the subset of referrals that resulted in the identification of children with impaired hearing.
Analyses were carried out using Stata statistical software.
Results
Referral rate and yield
There were 1702 referrals in Nottingham (21.9 referrals per 1000 person-years) and 1108 in Cambridge (34.4 referrals per 1000 person-years); the referral rate in Nottingham was two-thirds that of Cambridge (rate ratio 0.64, 95% CI 0.59 to 0.69; p < 0.001) (Table 18). Hearing impairment was confirmed in 195 children in Nottingham (yield of 2.51 cases per 1000 person-years) and 98 children in Cambridge (3.04 cases per 1000 person-years). There was little evidence that the yield is different between Nottingham and Cambridge (rate ratio 0.82, 95% CI 0.64 to 1.06; p = 0.12). Confirmed hearing loss cases made up 17.0% of referred children in Nottingham (25.2% of children who were referred via SES and 14.9% of children referred via other sources) and 10.6% of referred children in Cambridge (Table 19).
| Outcome | Nottingham estimate | Cambridge estimate | Nottingham relative to Cambridge | ||
|---|---|---|---|---|---|
| Rate ratio | 95% CI | p-value | |||
| Number of referred children per 1000 children per year | 21.9 | 34.4 | 0.64 | 0.59 to 0.69 | < 0.001 |
| Number of confirmed cases (yield) per 1000 children per year | 2.51 | 3.04 | 0.82 | 0.64 to 1.06 | 0.12 |
| Site/referral source | % (n/N) | 95% CI |
|---|---|---|
| Nottingham – referred via school screen | 25.2% (60/238) | 19.8% to 31.2% |
| Nottingham – referred via other source | 14.9% (135/907) | 12.6% to 17.4% |
| Cambridge | 10.6% (98/923) | 8.7% to 12.8% |
The mean age of referral was 4.7 years for both the Nottingham and Cambridge sites, but the mean age at referral for children who were subsequently confirmed as HI was higher in Nottingham than Cambridge (5.0 years vs. 4.5 years; mean difference 0.47 years, 95% CI 0.24 to 0.70 years; p < 0.001) (Table 20).
| Participants | Nottingham | Cambridge | Difference (Nottingham – Cambridge) | ||||
|---|---|---|---|---|---|---|---|
| n | Mean, years (SD) | n | Mean, years (SD) | Mean, years | 95% CI, years | p-value | |
| All referred children | 1702 | 4.70 (1.01) | 1108 | 4.66 (1.08) | 0.04 | –0.04 to 0.11 | 0.37 |
| Confirmed cases only | 195 | 4.97 (0.96) | 98 | 4.51 (0.94) | 0.47 | 0.24 to 0.70 | < 0.001 |
The characteristics are summarised in Table 21 for all referred children and in Table 22 for children confirmed as HI cases, separately for each of the Nottingham and Cambridge sites. In Nottingham 21.5% of all referrals and 30.8% of the confirmed HI cases were originally referred via SES. Other key sources of referral in Nottingham were ENT consultants (23.6% of confirmed cases), parents (11.8% of confirmed cases), GPs (10.8% of confirmed cases) and health visitors (10.8% of confirmed cases). In the Cambridge site the key sources of referral for confirmed HI cases were GPs (64.3%), health visitors (21.4%) and speech therapist (12.2%).
| Characteristic/summary | Nottingham | Cambridge |
|---|---|---|
| Number of children referred | 1702 | 1108 |
| Number of confirmed cases (yield) | 195 | 98 |
| Base population | 42,553 | 17624 |
| Person-years at risk for base population | 77,825 | 32,232 |
| Children referred per 1000 person-years at risk | 21.9 | 34.4 |
| Yield per 1000 person-years at risk | 2.51 | 3.04 |
| Age at referral in years, mean (SD) | 4.7 (1.0) | 4.7 (1.1) |
| Age at referral at last birthday | ||
| 3 years, n (%) | 508 (29.8) | 381 (34.4) |
| 4 years, n (%) | 496 (29.1) | 316 (28.5) |
| 5 years, n (%) | 492 (28.9) | 259 (23.4) |
| 6 years, n (%) | 206 (12.1) | 152 (13.7) |
| Level of hearing impairment in dB HLa | ||
| Left ear (0.5 kHz), median (IQR) | 15 (15–25) | 15 (10–20) |
| Left ear (1 kHz), median (IQR) | 15 (10–20) | 15 (10–20) |
| Left ear (2 kHz), median (IQR) | 15 (10–20) | 10 (10–20) |
| Left ear (4 kHz), median (IQR) | 15 (10–20) | 10 (10–20) |
| Left ear (average), median (IQR) | 15 (11.3–20) | 13.1 (10–20) |
| Right ear (0.5 kHz), median (IQR) | 15 (15–25) | 20 (10–20) |
| Right ear (1 kHz), median (IQR) | 15 (10–20) | 15 (10–20) |
| Right ear (2 kHz), median (IQR) | 10 (10–20) | 10 (10–20) |
| Right ear (4 kHz), median (IQR) | 15 (10–20) | 10 (10–20) |
| Right ear (average), median (IQR) | 15 (10–20) | 12.5 (10–20) |
| Source of referral | ||
| GP, n (%) | 186 (10.9) | 458 (41.3) |
| Health visitor, n (%) | 387 (22.7) | 269 (24.3) |
| School screen, n (%) | 366 (21.5) | n/a |
| Speech therapist, n (%) | 147 (8.6) | 278 (25.1) |
| Paediatrician, n (%) | 137 (8.0) | 35 (3.2) |
| Parent, n (%) | 211 (12.4) | 46 (4.2) |
| Education, n (%) | 0 (0) | 21 (1.9) |
| School nurse, n (%) | 53 (3.1) | 0 (0) |
| Community nursery nurse, n (%) | 14 (0.8) | 0 (0) |
| ENT consultant, n (%) | 139 (8.2) | 0 (0) |
| Other consultant, n (%) | 28 (1.6) | 0 (0) |
| NHSP, n (%) | 0 (0) | 1 (0.1) |
| Referrals from diagnostic accuracy study, n (%) | 34 (2.0) | 0 (0) |
| Outcome of referral | ||
| Discharge, n (%) | 950 (83.0) | 825 (89.4) |
| Further referral, n (%) | 149 (13.0) | 97 (10.5) |
| Hearing aid, n (%) | 46 (4.0) | 1 (0.1) |
| Missing, n (not reached end of care) | 557b | 185 |
| Characteristic/summary | Nottingham | Cambridge |
|---|---|---|
| Confirmed cases | ||
| Age at referral in years, mean (SD) | 5.0 (1.0) | 4.5 (0.9) |
| Age at referral at last birthday | ||
| 3 years, n (%) | 35 (17.9) | 32 (32.7) |
| 4 years, n (%) | 59 (30.3) | 38 (38.8) |
| 5 years, n (%) | 70 (35.9) | 22 (22.4) |
| 6 years, n (%) | 31 (15.9) | 6 (6.1) |
| Level of hearing impairment in dB HLa | ||
| Left ear (0.5 kHz), median (IQR) | 40 (30–45) | 30 (25–45) |
| Left ear (1 kHz), median (IQR) | 35 (25–40) | 30 (25–40) |
| Left ear (2 kHz), median (IQR) | 30 (20–35) | 25 (15–35) |
| Left ear (4 kHz), median (IQR) | 35 (25–45) | 35 (22.5–40) |
| Left ear (average), median (IQR) | 35 (26.3–41.3) | 31.3 (22.5–38.8) |
| Right ear (0.5 kHz), median (IQR) | 35 (25–45) | 35 (25–40) |
| Right ear (1 kHz), median (IQR) | 35 (25–40) | 35 (25–40) |
| Right ear (2 kHz), median (IQR) | 30 (20–35) | 25 (15–40) |
| Right ear (4 kHz), median (IQR) | 35 (25–45) | 32.5 (20–40) |
| Right ear (average), median (IQR) | 32.5 (22.5–40) | 31.3 (23.8–37.5) |
| Source of referral | ||
| GP, n (%) | 21 (10.8) | 63 (64.3) |
| Health visitor, n (%) | 21 (10.8) | 21 (21.4) |
| School screen, n (%) | 60 (30.8) | n/a |
| Speech therapist, n (%) | 7 (3.6) | 12 (12.2) |
| Paediatrician | 4 (2.1) | 0 (0) |
| Parent, n (%) | 23 (11.8) | 2 (2.0) |
| Education, n (%) | 0 (0) | 0 (0.0) |
| School nurse, n (%) | 9 (4.6) | 0 (0) |
| Community nursery nurse, n (%) | 0 (0) | 0 (0) |
| ENT consultant, n (%) | 46 (23.6) | 0 (0) |
| Other consultant, n (%) | 2 (1.0) | 0 (0) |
| Referrals from diagnostic accuracy study, n (%) | 2 (1.0) | 0 (0) |
| Type of hearing impairment | ||
| ‘Normal’ binaural, n (%)b | 7 (3.6) | 2 (2.0) |
| Conductive impairment (bilateral), n (%) | 138 (70.8) | 70 (71.4) |
| Conductive impairment (unilateral), n (%) | 40 (20.5) | 20 (20.4) |
| Sensorineural impairment (bilateral), n (%) | 1 (0.5) | 2 (2.0) |
| Sensorineural impairment (unilateral), n (%) | 5 (2.6) | 1 (1.0) |
| Mixed impairment (unilateral), n (%) | 0 (0) | 2 (2.0) |
| Incomplete, n (%) | 4 (2.1) | 1 (1.0) |
Discussion
It might be expected that adding a screen to a system would result in a greater number of referrals. However, the observational comparison of two sites, one with SES (Nottingham) and one without SES (Cambridge), showed evidence that the rate of referral for hearing problems is lower when SES is present. The referral rate was 36% lower in Nottingham relative to Cambridge (rate ratio 0.64; p < 0.001) and the CI for the rate ratio indicates the true rate is at least 31% lower when there is SES.
In the SES site, one-third of children subsequently confirmed as cases were initially referred via SES. There was little evidence (p = 0.12) that the yield of confirmed cases is altered by SES; the estimated rate of confirmed cases was 18% lower in Nottingham relative to Cambridge but it is plausible within the bounds of the 95% CI that the true yield rate is the same in areas with and without SES. The CI, however, does indicate that it is unlikely that SES areas truly have a markedly higher yield rate. A higher proportion of referred children were subsequently confirmed to be HI in the area with SES (17.0% vs. 10.6%).
The mean age of referral was nearly identical (4.7 years) between the two sites when looking at all referrals. When focusing solely on children who were subsequently confirmed to have a hearing impairment, however, there was strong evidence that the children in the site with a screen were older at referral (5.0 years vs. 4.5 years; p < 0.001). One possibility is that, for children in this age range, parents/professionals in Cambridge seek referral when concerns are raised whereas in the area with SES, if concerns are raised around age 4 years, parents and professionals are aware that SES is coming up later that year and wait.
The CIs for the mean age difference indicates that the true mean age at referral in areas with a screen could plausibly be anywhere between 3 and 8 months greater than areas without one. Delay in identification of a hearing impairment has the potential to adversely affect development1 but further research would be needed to evaluate the extent of the impact for children at school age.
There are also differences in the audiology services operated in the two areas. In Nottingham there is no second-tier audiology service and all referred children are seen within the same service. It has an open referral policy, which includes referrals from parents. It works closely with the ENT department and children move between the two services dependent on their needs. A health professional concerned that a child might have OME and hearing loss has the option to refer for a DEA or to ENT; ENT might then refer for a DEA if appropriate.
The second-tier service in Cambridge accepts referrals from health and education professionals. It provides assessment for children and onward referral for those children who may require hearing aids or surgical management. A health professional with a child with possible OME and hearing loss knows that there is a well-staffed intermediate (DEA) service which can effectively sieve referrals and send those needing ENT examination to ENT departments and those requiring hearing aids to third-tier audiology. Thus GPs will be likely to refer to second-tier audiology and referrals from ENT departments to second-tier audiology will be highly unlikely.
The very different numbers of children referred from different routes are likely to be a function of these different systems in the two areas. Parents can directly refer to the CHAC in Nottingham but in Cambridge they would have to go via a GP (or other professional). GPs in Cambridge would know that they have a second-tier community service which acts as a filter, so would have a tendency to refer there rather than to ENT or third-tier audiology. Hence referrals from ENT to second-tier audiology would be rare in Cambridge, but from ENT to the CHAC would be expected to be higher, as they are. Provision of hearing aids as an outcome is clear in the CHAC, but second-tier audiology services in Cambridge do not provide hearing aids.
Strengths and limitations
Our study had a number of strengths. Data collection in both sites was comprehensive and actively monitored by a senior member of the clinical staff with responsibility for audiological services in each of the two areas. Both were members of the research team. An electronic database used in both sites was developed by staff of PenCTU to standardise data collection.
However, our study design of an observational comparison of two areas [one that operates a SES programme (Nottingham) and the other that does not operate a SES programme (Cambridge)] is subject to major methodological limitations, in spite of our best attempts to choose two sites that were similar to each other. We acknowledge that there may be epidemiological and social differences between the two geographical areas that are likely to confound our findings. Reassuringly, population estimates indicate the proportion of children aged < 16 years to be similar in Nottinghamshire (18.7%) and in Cambridgeshire (18.5%). 54 However, the index of socioeconomic deprivation indicates the city of Nottingham (rank 17) to be more deprived on a range of measures than the Cambridge district (rank 188). 55 Given the lack of availability of child-level data, we were unable to adjust our analyses to take account of these and other potential confounders. Furthermore, given that we consider only two geographical areas, our results may not be considered as generalisable.
Both sites mainly receive referrals from a defined geographical region but also accept referrals outwith that area. Equally, some children within the defined area may be referred elsewhere; the numbers are estimated to be few by the responsible audiologists. The referral catchment areas also do not exactly match the areas defined by the Office for National Statistics for the population estimates used and there may, therefore, be some minor imprecision in the population denominators used in the analyses.
Chapter 6 Exploring the impact on the child and family of a child being referred by the school hearing screen: findings from a questionnaire survey
Introduction
Any analysis of the cost-effectiveness of the screening process needs to consider the costs not only to the health and education services, but also to the families of children referred by the screen. Some of these children will be true positives (i.e. they have a confirmed hearing impairment) and many more will be false positives (identified by the screen but found to have no hearing impairment). Information to contribute to the cost-effectiveness model might include details of the amount of time and travel spent by families in attending the screen follow-up process but also an estimate of any anxiety caused. Our literature searches identified no prior information on the impact and costs of SES referral.
Objectives
-
To determine the impact, both psychological and economic, for the child and the family of the child being referred for further assessment following the SES (both true and false positives).
-
To collect cost data to inform the economic model.
Methods
Study population
Questionnaires (see Appendix 5) were distributed to parents of children referred to Nottingham CHAC from the school entry hearing screen between 1 September 2012 and 30 June 2014 (age range 4–6 years).
Questionnaire
The questionnaire captured data on aspects of the family’s experience of the child being referred for a hearing assessment. Questions included how they found out about the screen and further testing, parent opinion about having a school hearing screen, the time taken to attend appointments, the cost of travel to appointments, and impact on work, school and social activities of attending clinic appointments. Data on gender, ethnicity and school attended were also requested. The level of anxiety experienced by the parent on finding out that their child needed further testing and again when they attended the appointment was rated by the parent on a scale from 0 (not at all anxious) to 10 (extremely anxious). There was also the possibility of an option to follow-up responses by telephone, with those who gave their contact details. No follow-up was carried out as it was not considered that it would add anything to the data already collected on the questionnaire, especially taking into account the bias of recall over a long time period and the small data set.
Data collection
Questionnaires plus pre-paid return envelopes were sent by the audiologist to the parents of all children referred to the CHAC by the school screen, once the end of care (discharge, hearing aid provision or referral to ENT) had been reached, whether or not they had a hearing impairment. The audiologist kept a list of questionnaires sent and which participant ID these related to. Parents who chose to take part returned the completed questionnaire to the researchers at NHBRU. These returns could be anonymous but questionnaire responders could also choose to be entered into a prize draw to win vouchers of their choice to a value of £50, or to agree to a possible follow-up interview and hence supply contact details.
Nottingham researchers entered the data into the study database and copies of questionnaires (excluding the contact details page) were sent to PenCTU for second data entry and validation. A tracking log of the ID numbers of questionnaires returned was kept locally by researchers at NHBRU. A reminder and repeat questionnaire was sent after 3 months to non-responders identified from the list held by the audiologist.
Consent for the data collected in this study was implied by return of a completed questionnaire. It was explained to the parents completing the questionnaire that entry into the project was entirely voluntary and that the management and care of their child would not be affected by their decision on whether or not to take part.
Data analysis
The quantitative data were summarised using means with SDs or medians with IQRs.
To give structure to the qualitative data obtained from the questionnaires, a thematic analysis was carried out. Open comments from parents were assessed using a template analysis in which identified themes from each of the questionnaires were selected and ordered. Responses to the following questions were analysed using the template: (1) What are the good things about your child having their hearing checked at school?; (2) What are the not so good things about your child having their hearing checked at school?; and (3) Any further comments?
A five-step framework for analysing the questionnaire responses was used: familiarisation; identification of themes; indexing; charting; and interpretation. 56
-
Familiarisation: each questionnaire was read through six times to get a feel for the data.
-
Identification of themes: the broad research question was to explore what parents thought about the SES tests. Using an inductive approach, parents’ opinions were categorised into main themes that emerged across all the questionnaires. The researchers made notes on relevant points raised under each category. This process was repeated for all questionnaires and common themes were included in the final template. Ambiguous statements were not included as they were open to interpretation.
-
Indexing: subthemes based on similarities between all responses within the main identified categories were explored. Indexing involved generating more precise descriptions of the themes to make the analysis reader-friendly.
-
Charting: a template was drawn up for each category, theme and descriptor.
-
Interpretation: illustrative quotes were identified to provide the reader with examples of the parents’ experiences.
Results
Questionnaires were sent to all 246 parents whose child was referred to the CHAC from the school entry hearing screen in the relevant period, and who reached end of care. Completed questionnaires were received from 60 parents by the end of the data collection period. No data were collected on the reasons for non-return.
Quantitative data
Of the 60 children for whom questionnaires were returned, 32 (53%) were female, 44 (73%) were white, 3 (5%) were mixed ethnicity, 10 (17%) were Asian, 1 (2%) was black/African/Caribbean and 2 (3%) were of other ethnic group.
Forty-five (75%) parents knew that their child was having their hearing checked at school. Parents found out that their child needed further testing by means of a letter either taken home (n = 25) or sent in the post (n = 9); by telephone (n = 13) or by other means (n = 13) [already noticed at home (n = 5), told by school (n = 4), told by school nurse (n = 2), told by doctor (n = 1), do not know (n = 1)].
Parental anxiety was scored on a 0–10 scale where 0 indicated ‘not anxious at all’ and 10 indicated ‘extremely anxious’. The mean level of anxiety reported by the parent(s) on finding out that their child needed further testing was 5.3 (SD 2.1), and the median was 5 (IQR 4–7). At the clinic appointment the mean parental anxiety had reduced to 4.7 (SD 2.4); the median was 5 (IQR 3–6). Frequencies of anxiety are given in Table 23.
| Anxiety level | Anxiety on finding out, n (%) | Anxiety at clinic appointment, n (%) |
|---|---|---|
| 0 (not at all anxious) | 1 (2%) | 2 (3%) |
| 1 | 3 (5%) | 5 (8%) |
| 2 | 2 (3%) | 5 (8%) |
| 3 | 5 (8%) | 8 (13%) |
| 4 | 5 (8%) | 6 (10%) |
| 5 | 16 (27%) | 14 (23%) |
| 6 | 10 (17%) | 7 (12%) |
| 7 | 10 (17%) | 5 (8%) |
| 8 | 6 (10%) | 5 (8%) |
| 9 | 0 (0%) | 1 (2%) |
| 10 (extremely anxious) | 2 (3%) | 2 (3%) |
The same anxiety level on both occasions was reported by 36 out of 60 respondents (60%); 18 out of 60 (30%) reported feeling less anxious at the appointment and 6 out of 60 (10%) reported more anxiety at the appointment.
Most parents [51 out of 60 (85%)], strongly agreed that ‘children should have their hearing checked at school’, six (10%) agreed, one (2%) had no opinion, no one (0%) disagreed and one (2%) strongly disagreed (one missing).
Of the 60 questionnaires returned, parents reported that 25 children had only one appointment, 16 children had two appointments in total, nine had three appointments, five had four appointments, and five had five appointments. Altogether the 60 children had 129 appointments.
Time taken to get to an appointment was < 15 minutes for 24.8% (32/129) of appointments, about half an hour for 59.7% (n = 77) of appointments, about 1 hour for 14.0% (n = 18) of appointments and 1–2 hours for 1.6% (n = 2) of appointments. The length of appointment was < 30 minutes for 33.3% (43/129) of appointments, 30–60 minutes for 44.2% (n = 57) of appointments, about an hour for 13.2% (n = 17) of appointments, and 1–2 hours for 7.0% (n = 9) of appointments. Data on length of appointment were missing for three appointments.
For the journey to an appointment, 20.9% (27/129) included a bus or tram ride, 72.9% (n = 94) of journeys used a car, 5.4% (n = 7) included a taxi ride and one walked (0.8%). The median (IQR) total cost per appointment was £4.88 (£3.40–£8.80).
Parents were required to take either all or part of a day off work for 45.0% (58/129) of appointments; 29.5% (n = 38) of appointments were with parents who did not work and 25.6% (n = 33) of appointments did not require the parent to take time off work. The child was required to miss school for all or part of the day for 72.9% (n = 94) of appointments.
Twelve out of the 60 (20%) children missed other activities, including ballet and music lessons but eight of these referred to missing school. Thirteen (21.7%) of the 60 parents missed other activities, although eight referred to work – probably a misunderstanding of the questions. Eight of 60 (13%) families said appointments caused problems for other family members by requiring additional childcare for other children.
Qualitative data
Table 24 lists the themes that emerged from the data and provides illustrative quotes.
| Main themes | Subthemes | Description | Example quotes | Respondent ID number |
|---|---|---|---|---|
| What are the good things about your child having their hearing checked at school? | ||||
| A1. Ease of testing | i. Convenience | Refers to the benefits to parents of the hearing test being carried out at school during school hours | . . . in normal school hours | 1 |
| . . . without parents having to be proactive | 1 | |||
| Don’t need to take time from school to attend appointment | 16 | |||
| Easier than trying to attend appointments | 19 | |||
| It is local . . . | 37 | |||
| . . . don’t need to worry about . . . taking your child out of school to have their hearing tested | 39 | |||
| ii. Familiarisation | Refers to how comfortable the child is having the hearing test in a location and with classmates that are familiar to them | . . . feels normal to child as everyone has it done | 1 | |
| Child not . . . upset as they are in a familiar environment | 9 | |||
| Secure, familiar environment | 14 | |||
| No anxiety for the child | 34 | |||
| Child sees their peers having the same test | 38 | |||
| All children tested together takes away. . . fear/anxiety | 42 | |||
| A2. Identification of hearing impairment | i. Importance | Refers to how important it is to parents to have any hearing impairments in their children identified | . . . they need to hear well in order to follow lessons | 4 |
| . . . the child may become disengaged with school/learning if their hearing is impaired | 18 | |||
| . . . notifies the school so they become aware of [the] hearing issues . . . sooner | 24 | |||
| It can rule out many other things | 33 | |||
| [A] lack of hearing can have huge effects on child’s ability and confidence . . . | 40 | |||
| ii. Early detection | Refers to how the hearing test can enable potential hearing impairments to be picked up and treated quickly | Any problems will be dealt with much quicker | 2 | |
| . . . any problems . . . can be investigated at an early stage | 3 | |||
| . . . parents can be alerted immediately | 37 | |||
| . . . know early on that there is a problem and it can be sorted out | 51 | |||
| They can be referred straight away for further tests | 53 | |||
| iii. Acknowledgement | Refers to how a referral from the hearing screen at school supported suspicions that parent may already have had | [My] concern was acknowledged | 10 | |
| Confirmation from another source that there was a concern | 11 | |||
| For us, having our school nurse check our daughter’s hearing got her an appointment to get a proper check | 23 | |||
| . . . backed up suspicions I already had regarding my daughter’s hearing . . . and made me feel it was worthwhile going back to the GP . . . | 50 | |||
| iv. Otherwise missed | Refers to recognition from parents that any potential hearing problems may have been missed without the school hearing test | . . . children of a young age are easily distracted and hearing problems won’t be picked up readily as this [not hearing] would be considered the norm (as in my son’s case) | 6 | |
| . . . not noticed by home | 8 | |||
| Could identify problems which are otherwise missed | 15 | |||
| Often parents . . . dismiss things and . . . wouldn’t follow up on possible concerns | 41 | |||
| What are the not so good things about your child having their hearing checked at school? | ||||
| B1. Communication with parents | i. Lack of information | Refers to parents being unaware of the screening test | Parents not informed about how the test is done or how accurate the test is | 1 |
| I didn’t know he was having this done | 54 | |||
| I didn’t know my child was having a test on that day until she came home and told me she had one | 47 | |||
| ii. Poor correspondence | Refers to the dissatisfaction parents had with the correspondence after their child had been referred by the school hearing test | The letter was very vague – it said she required further testing and it wasn’t until I spoke to the school nurse that I established what the test had clinically shown. It was probably designed not to panic parents but I would have preferred to have been told | 50 | |
| . . . unreliable test results, parents get informed there may be a problem when there isn’t | 15 | |||
| Got letter from school to say he would be retested but no date. The retest happened a long time after first test . . . | 15 | |||
| B2. Parental absence | – | Refers to parents opinions about not being present while the school hearing test was undertaken | Can’t talk to the person carrying out the test about the results | 38 |
| Not being present to ask questions if test failed | 12 | |||
| A parent is not around for when something (problem) arises | 36 | |||
| I would have preferred to be present for checks | 16 | |||
| Parents don’t have the opportunity to be there | 30 | |||
| Could provoke anxiety in child (no parent accompanying) | 28 | |||
| Possibly the child may feel more comfortable with their parent being present | 35 | |||
| B3. Problems with the test | B3i. Distractions | Refers to testing children whilst they are at school may result in them being distracted from the test | . . . may be distracted | 3 |
| Could be distracted if friends nearby or . . . at end of day and child not concentrating | 9 | |||
| B3ii. Noise | Refers to testing children within noisy school environments rather than in sound proofed conditions | The school is not the place to have a proper hearing test as they don’t have a quiet room or the equipment to do so | 23 | |
| Very noisy environment | 26 | |||
| Not in a sound proof[ed] room | 44 | |||
| Any further comments? | ||||
| C1. Age of the child | – | Refers to hearing tests being conducted at pre-school age (earlier than the current SES programme) | My child had her pre-school check missed. This is why it was done at school | 13 |
| I agree children need a hearing test early and school is the only option currently. Would be better to be done before entering school at a proper hearing unit | 22 | |||
| As a parent I think a child’s hearing should also be checked as part of a pre-school check at the doctor’s | 23 | |||
| C2. Satisfaction | i. With the follow-up service | Refers to positive comments on the service received after the school hearing test | The staff of . . . was really good and made my daughter feel comfortable and less worried about the tests | 56 |
| I found the . . . centre brilliant and they put my mind at ease straight away | 54 | |||
| Overall I’m very happy with the service we received. Everyone was really nice to my daughter and she wasn’t worried about the appointments | 47 | |||
| We have been happy with the whole process. The initial communication from the school test was informative and prompt. The tests were thoroughly explained to our child & to us, as were the results both immediately after the tests & via post a week later . . . | 48 | |||
| ii. Overall | Refers to summaries about the school hearing screening test | I think the pros outweigh the cons | 26 | |
| School test cannot replace a proper hearing test as they cannot recreate that acoustic free environment but it can act as a good indicator of any problem area | 55 | |||
Discussion
Main findings
The consequences for costs and the views of parents concerning referral for diagnostic evaluation were assessed by means of a questionnaire sent to parents of children referred to the Nottingham audiological service by the SES programme. The response rate was 24.4% (60/246). It is possible that those who did not respond are neutral, that is, they had no strong opinion with respect to the subjects in the questionnaire, but this cannot be assumed. If an adverse experience engendered non-response, then the low response rate could conceal an unsuspected level of ‘harm’ associated with SES.
Of the respondents, the majority had more than one appointment. Some of those with multiple appointments may be individuals in whom hearing impairment was confirmed (true positives), but others will be those in whom no hearing impairment was identified (false positives).
The mean parental anxiety score on finding out about the referral appointment was 5.3 on a 0–10 scale, with 0 indicating ‘not at all anxious’ and 10 ‘extremely anxious’. The mean score was 4.7 at the clinic appointment. Severe anxiety, indicated by scores of 10, was registered by only two (3%) parents. Thus, although there was some anxiety associated with referral in those who responded, it appeared moderate, albeit sustained during the waiting period.
Costs were associated with attending the referral appointment in the respondents in terms of time, travel and activities foregone. The median travel cost per appointment was £4.88, travel times to get to the appointment was about half an hour in 60% of appointments, and appointment times were 30–60 minutes in 44% of appointments. Activities foregone were generally work in the case of parents (45% of appointments involved missing work) and school in the case of children (73% of appointments involved missing school).
Despite the inconvenience, virtually all respondents were supportive of SES; 85% of parents strongly agreeing that ‘children should have their hearing checked at school’. Supportive statements and overall satisfaction were also evident in the open comments. These identified areas where care needs to be maintained in delivering SES, particularly attention to communication, thereby minimising concerns about the absence of parents at the initial test but also the potential for noise and other distractions in schools to reduce the value of hearing tests.
Strengths and weaknesses
The study was prospectively designed and carried out in accordance with a protocol without deviation. The most important challenge to the validity of the findings was the low response rate, with only 24% of parents to whom questionnaires were sent out providing responses. This was despite a small incentive for taking part and a reminder being offered. This may have been in part because of the length of time between the child being referred and receiving the questionnaire which was sent out after the child reached the end of care. However, for the specific purpose that this component of the clinical studies was designed, we do not believe this is a major shortcoming, but the conclusions drawn should be viewed in the light of a low and potentially biased response rate.
Children referred from SES may truly have a hearing impairment (true positives), may not have a hearing impairment, or have a hearing impairment at the time of screening which later resolves (false positives). We failed to identify information in the literature on what the consequences of false positives might be in a SES programme (see Chapter 4). As far as the original 2007 HTA report12 was concerned, the potential for disutility associated with false positives was not considered, almost certainly because there was no evidence to inform any assumptions. In this respect it would be useful to gauge whether there were likely to be substantial consequences associated with false positives, indicating that they might have a major impact on overall effectiveness and cost-effectiveness. However, as parents were given the option to return the questionnaires anonymously we were not able to collect data on outcomes for all those who responded. In Chapter 5 we report that there were 366 referrals from the SES to the Nottingham service, 60 (16.4%) of whom were confirmed cases. Nearly 90% of confirmed cases had a conductive impairment. We can assume, therefore, that the majority of the questionnaires were returned by the parents of children who did not have a permanent impairment (false positives).
There are clearly some adverse consequences of referral, but these appear to be small, judged externally or by the effect on parental satisfaction. Furthermore, it is reasonable to assume that the effects in non-respondents are unlikely to be greater than those observed in the respondents, so we evaluate that the adverse consequences of referral, particularly for those found not to have hearing impairment, are likely to be no greater on average than those observed in the respondents.
Results in the context of other studies
We emphasise that this is the first attempt to quantify the effect of the referral process in SES on parents and children. Inevitably, there is still much uncertainty, but we would argue that the uncertainty has been reduced. How the results of this study were considered in the economic model of this report is discussed in more detail in Chapter 8.
We explored the option to formally quantify the impact on health-related quality of life, particularly for children, through the Health Utilities Index (HUI) instrument but this was not pursued, both because the instrument is not responsive to specific issues related to hearing and on the grounds of cost of using the instrument under licence, a factor amplified by the need to repeat the assessment. Given that the response rate emerged as being problematic in the actual study, it is likely that an attempt to use a more formal instrument like the HUI may have further compromised the response rate.
Chapter 7 Observations of the practical implementation of screening tests for hearing in schools
Introduction
Aside from the minimal and very weak evidence for the effectiveness of different implementations of SES reported in the 2007 HTA report,12 we were unable to identify any literature on the practical implications of different implementations or different screen technologies. One paper17 evaluated the accuracy of the HC navigator device in children with a mean age of 6.8 years in schools in the Philippines and reported low sensitivity when used in the school setting where there is significant ambient noise. The use of the PTS has not been formally evaluated in terms of practicality.
Objectives
-
To determine the time resource in implementing either of the two alternative screening methods (PTS and HC screener) in primary schools.
-
To elicit the views of the school nurses implementing the screening tests.
Methods
This was a prospective observational cohort study. A researcher from the project team observed school nurses while they conducted hearing screening using two methods: the standard PTS and the HC screener.
The primary end point was the mean cost per child of implementing each of the two test technologies based on time taken to do each test. Secondary end points included a pass or refer for each test, total time of session, school demographics, nurse opinion on ease of use, how much the nurses would want to use that screener in the future, plus other comments.
The school nurse team was approached by a member of the research team and asked for support with the study. School nurses who delivered hearing screening and were happy to be involved drew up a list of schools in which they routinely screened children for hearing impairment. The research team made contact directly with the head teachers of these identified schools, initially by letter, to gain approval for the researcher to access the school to observe the school nurse. Visits were conducted in all three terms of the school year, in order to cover a range of school conditions and the effects of seasonal infections.
Information sheets were distributed to the school via the school nurses (see Appendix 6). They were given to parents of children together with information about the school health screen, following the usual process of securing informed consent for the hearing screen. It was explained that, in addition to the routinely used PTS, an extra test (HC) would be carried out by the school nurse and why. Anyone not consenting to either the standard school screen or our extra screen replied to the school nurse, withdrawing their consent via an ‘opt-out’ system. Only those giving consent to both tests were included in the study, implied by no reply to the contrary.
Tests during SES were observed in primary schools in the Nottingham area. The number of schools was chosen to ensure resources were measured for at least 180 child screens, representing a range of catchment populations. Children in the Foundation year or Year 1 (age 4–6 years) were included if they had the usual parental consent for the school screen (following protocols and guidelines for parental consent normally administered by the service) and had not opted out of the SES study.
Data collection took place in the school year from October 2013 to June 2014.
Before the first school session, the researcher met with the nurse to explain and demonstrate how to use the HC screener.
In each school all children > 5 years old in Foundation and Year 1 classes were screened by the school nurse. This meant it was necessary for the school nurse and the observing researcher to attend each school more than once. All children in the appropriate classes who did not opt out were screened using both technologies (routine PTS and HC screener), unless the school nurse chose not to perform both tests (for instance because of a lack of attention or nervousness of the child). The order of the tests was randomised according to computer-generated lists provided by members of the research team in Exeter, but if the school nurse felt that the child might have found it difficult to complete two tests, the PTS was performed first to increase the likelihood of completing the routine screening data.
The HC screening method was used as explained in Chapter 3. The nurse indicated ‘pass’ or ‘refer’ to the researcher. The researcher recorded this, and the time taken for the screen, on the CRF (see Appendix 6) (note: on the CRF a refer outcome is entered as a fail). Nurses did not use HC data to inform their decision on whether a child should be referred for further assessment by audiology services.
The nurse performed the PTS method according to usual practice. The nurse indicated to the researcher whether or not the child had passed the screen. The researcher recorded the time taken for the screen (not including explanation). Data were recorded on the CRF. If the result of the PTS was to refer the child or it was unclear, the child was rescreened on another day and, if necessary, referred onward as appropriate according to usual practice.
The start and end time of the session were recorded on the CRF, along with date, school name and postcode, number of pupils on roll, number of pupils eligible for free school meals (a marker of deprivation), and the name of the nurse and the researcher. Comments were also made about the conditions for the screen (e.g. room, noise levels, disturbances, number of children seen and any difficulties during testing). The researcher informed the nurse which screen was to be conducted first for each child according to the randomisation scheme. The total time of the session included time to take children to and from classrooms, break time, screen explanation time, other screens (e.g. vision tests) and interruptions. The CRF included details of what ‘other’ times were included in the session.
If, during an assessment session, a particular child became upset, uncomfortable, or uncooperative it was up to the school nurse to decide whether or not they continued the session.
Finally, the school nurses were asked on a scale of 0–10 (0 being low) how they would rate each screening test on ease of use, accuracy and how much they would want to use it in the future.
Each participant was allocated a participant number, but to maintain anonymity and to work with the school nurse’s system, no record was kept of which child the number applied to. The original CRFs were kept securely at NHBRU. Photocopies of all CRFs were sent to PenCTU for second data entry and checks.
The schools involved received a short summary of the findings at the end of the study.
Sample size
It was anticipated that a sample size of four schools would provide a convenience sample of about 180 children. The study size was not formally calculated.
Analysis
The mean and median time taken to complete the screening tests are presented for: (1) all children, (2) children for whom the PTS was administered first and (3) children for whom the HC screen was administered first. The mean time was compared between the PTS and the HC screener using linear regression models. As the distribution of time to complete the test was skewed, bias-corrected accelerated bootstrap CIs were constructed for the mean difference between the PTS and HC tests.
Results
The three school nurses covering Nottingham East (Carlton, Hucknall, Arnold and Calverton) agreed to do the extra screening. In the catchment areas of the three nurses, seven of the 34 schools covered were willing to take part.
Twenty-two observational sessions were conducted in the seven schools in the Nottingham East area through the school year 2013–14. The parents of four children at the sessions attended did not give consent for the study. Of the children for whom consent was given, 191 were observed, 184 of whom completed both tests; three did not complete either test, the other four did not complete the HC screen. Data were analysed only where the test produced a pass or refer classification. For the remaining participants either the test was not done at all or was incomplete.
Children were seen in groups of between two and five, depending on distance to the classroom and occasionally how disruptive the children were. Sometimes, if the classroom was close by, children were allowed to return to class once they had finished. The total session time was recorded, and included time spent collecting children, administration of other tests (vision) and activities (brushing teeth) and occasionally measurement of height and weight, or retests of previous screens.
Of the 188 PTS tests, 40 (21.3%) were referred. Of the 184 HC tests 71 (38.6%) were referred.
Time taken
The mean/median times taken for each screening test were similar for the two tests, at around 1.4 minutes per test, but the range of test times was wider for the PTS (to be expected as the test was not automated). The test time did not appear to vary with the order of the tests. The CI for the mean difference across all screens indicates that the PTS is unlikely to be more than 5 seconds quicker and unlikely to be more than 9 seconds longer on average to administer than the HC screen. That the CI includes zero indicates that it is plausible that there is no difference between the PTS and HC screener in mean time taken (Table 25).
| Test | n | Mean (SD) | Median (IQR) | Range | Mean difference (PTS – HC) | |
|---|---|---|---|---|---|---|
| Across all occasions | ||||||
| PTS | 188 | 1.39 (0.67) | 1.24 (1.05–1.55) | 0.63–7.5 | 0.002 | 95% CI –0.08 to 0.14a |
| HC | 184 | 1.39 (0.24) | 1.33 (1.27–1.58) | 1.03–3.47 | ||
| When the PTS is administered first | ||||||
| PTS | 105 | 1.37 (0.42) | 1.28 (1.08–1.57) | 0.73–3.22 | –0.007 | 95% CI –0.08 to 0.09a |
| HC | 102 | 1.38 (0.18) | 1.33 (1.28–1.45) | 1.12–2.45 | ||
| When the HC is administered first | ||||||
| PTS | 83 | 1.42 (0.89) | 1.2 (1.03–1.50) | 0.63–7.5 | 0.013 | 95% CI –0.13 to 0.33a |
| HC | 82 | 1.41 (0.30) | 1.33 (1.25–1.45) | 1.03–3.47 | ||
Observations of researchers and nurses
The researchers observed that schools were often unsuitable for testing hearing because they were too noisy and a suitable alternative room was often not available. On some occasions the nurse had to give up with the hearing tests and return on a different day.
The nurses suggested advantages and disadvantages of each test (Table 26)
| PTS | HC |
|---|---|
| Advantages | |
| Provides a full audiogram if needed | Lightweight |
| Can turn it up in noisy situations | Portable |
| Can turn it up to check a child’s understanding | Hygienic |
| The headphones help block background noise | No need for mains electrical socket |
| No need for headphones | |
| Disadvantages | |
| Headphones can be tight | Cannot pause to check understanding |
| Headphones disliked by some children | Cannot pause to wait for background noise to stop |
| Needs to be plugged into a socket | Cannot vary timing of presentation so some children anticipate when to put up their hand (particularly if they see their peers doing the test before them) |
| Cannot repeat a particular tone | |
| Only plays six tones – and only two frequencies are tested | |
| The tone at 20 dB is very quiet in a school situation and a lot of children miss it | |
| Younger children found this more difficult to understand | |
| No time between tones to give praise or encouragement | |
| The cups can fall off | |
| The equipment can get in the way of the child’s hand going up | |
The three nurses scored each of the tests on a total of 20 occasions for 185 children (no tests at one session because no suitable room available; hearing testing abandoned at another session, after three children tested, owing to noise). All nurses scored all tests as 5 or above for ease of use, accuracy and future use. The mean, median and range of scores are shown in Table 27. The PTS test scores higher than the HC test on all measures but in terms of ease of use that might have been because the nurses were more familiar with using the PTS. They also rated accuracy more highly for the PTS than the HC screener. When asked how much they would want to use a test in the future they again rated the PTS higher, but commented that they could see the HC screener as being useful as a back-up in some situations.
| Attribute | PTS | HC | ||||
|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | |
| Ease of use | 8.75 | 9 | 7–10 | 8.40 | 8 | 6–10 |
| Accuracy | 8.70 | 9 | 7–10 | 6.45 | 6 | 6–9 |
| Future use | 9.10 | 10 | 7–10 | 6.30 | 8 | 5–8 |
For two of the nurses the scores all either decreased or stayed the same over time. For the third nurse the HC screener scores stayed the same and the PTS scores increased for ease of use and future use and decreased for accuracy over time.
Discussion
In order to assess the practical issues associated with using the PTS or HC screener as the screening tests in a SES programme, careful observation of screening 184 children in seven schools in the Nottingham SES programme was undertaken. Three nurses covered 22 sessions. Each child had both tests applied, with the test given first being randomly determined.
The average time taken to implement each screening test was nearly identical at about 1.4 minutes, noting that the variability in time was greater for the PTS than for the HC screener. The difference in average time taken to conduct the test was not statistically significant and this was not affected by which test was the first to be used.
Nurses slightly preferred the PTS, but acknowledged that the HC screener could still prove a useful backup for children who refused to wear headphones or when there was no electrical socket available to run the PTS test. The observations about potentially noisy school environments raise the possibility that the accuracy of screening tests may be overestimated in the quieter research environment experienced in diagnostic accuracy studies, including our own.
Strengths and weaknesses
The study was prospective and undertaken in accordance with a protocol without deviation.
The target number of participants was achieved. The observations were carried out in familiar environments for the children allowing assessment of the operation of the screening tests without being compromised by the children feeling anxious – an issue that may have hindered the testing of control children at NHBRU in the diagnostic accuracy study.
Testing took place in a number of different schools throughout the school year, enabling the capture of seasonal changes that might affect hearing through colds, illness or hay fever.
The observations captured data on a range of children from different backgrounds. Several city schools were observed, enabling examination of how the hearing screens by school nurses might be affected by school size, behavioural challenges, support from teaching staff and school facilities, such as test room conditions.
We note the limitation that feedback on the two screening tests and the observations of testing involved only three nurses. However, all nurses undergo the same training and follow a set protocol while screening and therefore procedural differences should not affect test performance. With regard to verbal feedback provided by the nurses on the two tests, all three demonstrated inter-rater reliability, finding similar strengths and weaknesses for both the PTS and HC tests. Gaining opinion from a wider nursing community is unlikely to affect the conclusions drawn.
Results in the context of other studies
As far as we are aware this is the first study to systematically examine the practical issues associated with applying tests that might be used in SES with children in real-life circumstances.
Chapter 8 Modelling cost-effectiveness of school entry hearing screening
Introduction
Ultimately, the value of a new approach to health care must be judged on the degree to which additional benefits that might arise match the amount of additional resource that would be required to bring about the new approach. This is equally required of SES and in the forerunner to this HTA report,12 health economic modelling was employed to this end. The health economists on that research team conducted a decision-analytic model after finding that there was little relevant health economic literature and reported it in 2007. The report concluded that SES was potentially cost-effective but subject to considerable uncertainty. The main source of this uncertainty was test accuracy estimates (Professor Linda Davies, University of Manchester, 2011, personal communication). One purpose of this project was thus to improve the estimates of cost-effectiveness by incorporating more precise parameter estimates (particularly test accuracy) into the existing health economic model, accepting that re-examination and redevelopment might be required too, which turned out to be the case as indicated in the methods that follow. The inter-relationship between the studies reported in Chapters 3–7 and the economic modelling as originally envisaged is shown diagrammatically in Figure 17. The other major change from the original health economic model was to be more specific about the methods of screening in the current report. The PTS and HC tests were the two methods evaluated.
FIGURE 17.
Decision tree representation of SES from original bid.
Objectives
The overall aim of this chapter is to compare the cost-effectiveness of the PTS and HC tests as methods of SES for hearing impairment and to compare the cost-effectiveness of SES for hearing impairment relative to no screening.
Specific objectives were to:
-
update the existing hearing screening model from the 2007 HTA report12
-
incorporate data from the studies on referrals, costs and diagnostic accuracy (see Chapters 3–7)
-
estimate the health-related quality of life, costs and utilities of SES compared with no screening and of the PTS versus HC screen, with comparisons based on cost per quality-adjusted life-year (QALY) gained.
Methods
Overall approach
Our approach was designed to estimate the cost-effectiveness of SES primarily from a health-care perspective but to consider other costs where data were available. In this report this was limited to consideration of transport costs associated with families attending diagnostic evaluation; thus the perspective adopted in this report was that of the NHS and the family. The study was designed to capture the costs and benefits of the two different methods of SES in order to inform the policy decision regarding the appropriate use of SES. Since SES is likely to have an impact on the costs and outcomes of all types of hearing impairment, the analysis is concerned with the identification of children with sensorineural or permanent conductive (SNPC) hearing impairment or transitory hearing impairment not diagnosed during the newborn hearing screen or the first 3 years of life (the final pre-school year which is the starting point for the economic model).
There are three key considerations that inform whether or not screening is cost-effective relative to no screening. First, there is the issue of whether or not screening at school entry improves the timeliness with which children are referred to diagnostic evaluation and, if indicated, to management, thus generating a more prolonged improvement in quality of life than would otherwise occur. Second, cost-effectiveness will depend on whether or not the diagnostic accuracy of the test makes a difference in the time at which children are identified, referred and managed. If a test has a high level of false negatives, children who should have been picked up by the screening test will experience a delay in their diagnosis and management. Third, to the extent that there are false positives (either from screening or other modes of identification), there is a potential associated negative impact on the child and family of stress and lost time in school or work, as well as additional unnecessary costs to the health system.
The following sections outline the key components of our modelling approach. This includes the rationale and important assumptions required to estimate the cost-effectiveness of the PTS and HC tests and provides some background to the evolution of the modelling approach since the 2007 HTA report. 12
Decision-analytic structure
The screening question was addressed using a decision-analytical approach to evaluate the cost-effectiveness of school entry hearing screening programmes. The model estimates the costs and consequences of a hypothetical cohort of 10,000 children with a given prevalence of hearing impairment receiving the PTS, HC testing or no screening. The basic structure is presented in Figure 18. Figure 19 shows the route followed from screening to diagnosis of hearing impairment while Figures 20 and 21 illustrate the management follow-up for transitory and SNPC hearing impairment, respectively. As the decision tree representation does not explicitly capture the time element in the model, this aspect is detailed in the following sections.
FIGURE 18.
Basic decision tree structure.
FIGURE 19.
Decision tree: screening arm.

FIGURE 20.
Transitory hearing impairment.

FIGURE 21.
Sensorineural or permanent conductive hearing impairment.

Initial model development
Although a version of the original model from the 2007 HTA report12 [built in TreeAge Pro 2005 (TreeAge Software Inc., Williamstown, MA, USA)] was provided for the current research, the updated model was developed in Microsoft Excel to provide flexibility in the modelling approach. Part of the initial work on this project was to simplify the decision tree model developed for the 2007 HTA report. 12 Based on consultations with the Project Steering Group, a flexible modelling approach was adopted which would retain the logic of the original model and could be represented by a decision tree format, but also capture the way in which hearing impairment is identified over a period of time. This is relevant not only when SES is unavailable and hearing impairment is identified exclusively by other means, but also in the presence of screening, when these other routes of identification may also be important. This chapter describes the initial development of the model in anticipation of the results of the clinical studies described elsewhere in this report (see Chapters 3, 5–7) and subsequent to the data becoming available, at which point we were able to generate final estimates of cost-effectiveness for the new technology (HC screener) and the existing technology (PTS) compared with no screening. For the two screening tests, the structure of the evaluation is identical.
Based on information in the 2007 HTA report12 and correspondence with the lead modeller (Professor Linda Davies), we reconstructed the model used to generate the cost-effectiveness results presented in that report. Before updating the model, as specified in the original protocol, the modelling approach was reviewed to ensure that it was still fit for purpose. The alternative of a more detailed simulation model was considered but it was felt that it would add minimal benefit in terms of answering the research question. It was concluded that a Markov model or discrete-event simulation would not greatly improve the representation of screening while adding to the complexity of the modelling by increasing its data requirements (e.g. the use of transition probabilities). At the same time, this would have presented a considerable challenge in terms of data availability and would have introduced additional parameter uncertainty. As part of the validation of the initial model we developed, we were able to reproduce, within reasonable limits, the original base-case results from the 2007 HTA report.
This initial model was populated with the parameter values used in the original modelling exercise conducted for the 2007 HTA report12 and was driven by the diagnostic accuracy of the screening tests. For the screening arm of the model, this generated a number of referrals for suspected hearing impairment in the first year (true positives and false positives). As Figure 19 indicates, children who are referred by the screening test (positive result) are sent for a DEA for a definitive assessment (DEA being assumed to be 100% accurate). It was then assumed that all remaining cases of hearing impairment (false-negative results of the screen) would be identified over the following 2 years (i.e. up to age 7) by referral for a DEA as a result of concern by parents, teachers or other professionals. An assumption was required about the timing of these additional cases. In the absence of other evidence, they were evenly spread over the subsequent 2 years of the model. Similarly, an assumption was required about the rate of identification in the no screening arm. Under no screening, it is assumed that those with a suspected hearing impairment (on the basis of concern by parents, teachers or other professionals) are referred for a DEA. The background rate of identification was set so as to generate an even flow of diagnosed cases over a 3-year period.
Those referred by the screen or referred for a DEA on the basis of the concerns of parents, health professionals, teachers and others (in the presence or absence of screening) will either be found not to have a hearing impairment (false positives) or will be confirmed as having transitory or SNPC hearing impairment in either one or both ears (true positives). Management strategies received by children with hearing impairment include active observation, non-surgical and surgical interventions, with the type of intervention offered varying depending on the severity of hearing impairment.
Model development incorporating new clinical data
Although the basic model structure illustrated in Figure 18 has remained essentially unchanged in the current version of the model, there have been substantial revisions compared with the model described above to take account of the clinical observations reported in previous chapters. The present model has been informed by the two-gate (‘case–control’) study investigating the diagnostic accuracy of the two screening methods (see Chapter 3), a comparison of a site with a SES programme (Nottingham) and a site without a SES programme (Cambridge) (see Chapter 5), a study exploring the impact of referral for diagnostic evaluation on parents and children (see Chapter 6) and data on practical implementation in schools (see Chapter 7).
For both transitory and SNPC hearing impairment, the model takes account of the benefits of management for those found to have mild, moderate or severe hearing impairment. The analysis runs for a 4-year time period, starting in the year before school entry. By the end of the 4-year time period, evidence on referrals from the studies undertaken in Nottingham and Cambridge indicates that all cases of hearing impairment are likely to have been diagnosed regardless of whether children at age 4 years are screened or not screened. In the absence of screening, identification of hearing problems occurs as a result of the concerns of parents, teachers or other professionals, sources which can also generate referrals when a screening programme is in place. As discussed in the following sections, the results of the model are primarily driven by the total numbers of referrals with and without screening, and the numbers referred for a DEA in each year.
Given the key influence of referrals on the results of the analysis, the analysis has explored the effect of varying the number and pattern of referrals over time. This analysis has been reported as a threshold analysis following the presentation of the base-case results. A probabilistic sensitivity analysis (PSA) was not carried out, as it was not considered that probability distributions could usefully be attributed to the numbers of referrals or their timing based on referrals data drawn from two areas. It is argued here that PSA may not always be the most informative technique for exploring parameter uncertainty, as it can result in attention being focused on those variables for which probability distributions are most easily attributed rather than those that have the greatest influence on the results. In this case, while it is recognised that there will be variability in the numbers and rate of referrals with and without hearing screening, this uncertainty is particularly difficult to quantify. Threshold analysis was therefore performed on these variables.
Total referrals and cases of hearing impairment
Table 28 presents the key items of data obtained from the clinical studies that were used in obtaining estimates of the prevalence of hearing impairment, numbers of referrals and results of the screening tests.
| Parameter | Parameter value | Source |
|---|---|---|
| Prevalence of hearing impairment | 45.8 per 10,000 children | Confirmed cases in Nottingham as a proportion of base population (see Chapter 5) |
| Referrals for a DEA | 628.7 per 10,000 children | Referrals in Cambridge as a proportion of base population (see Chapter 5) |
| Diagnostic sensitivity | PTS: 95.9%; HC: 88.7% | Diagnostic case–control study (see Table 16) |
The total number of cases of hearing impairment to be identified (with or without screening) is based on the prevalence of hearing impairment using the number of confirmed cases of hearing impairment in Nottingham (n = 195) as a proportion of the base population (N = 42,553). This gives a prevalence of approximately 46 cases in a population of 10,000. These cases were divided into SNPC and transitory cases of hearing impairment in accordance with the assumption used in the 2007 HTA report. 12 In the base case, the number of referrals has been assumed to be the same in both screening and non-screening arms of the model and is given by the number of children referred in Cambridge (n = 1108) as a proportion of the base population (N = 17,624), or around 6.3%. This is equivalent to 629 referrals in a population of 10,000. Using the rate of referrals observed in Nottingham does not have a material impact on the cost-effectiveness results. The estimates of test sensitivity are the child-level estimates reported in the diagnostic accuracy study. The implications of these data in terms of screening results (true positive, false positives, true negatives and false negatives) are presented in Table 29.
| Category | PTS | HC |
|---|---|---|
| True positives | 43.9 | 40.6 |
| False positives | 582.9 | 582.9 |
| True negatives | 9371.3 | 9371.3 |
| False negatives | 1.9 | 5.2 |
The three groups of most interest are the true positives, false positives and false negatives since no further intervention is required in the true-negative group. True positives and false negatives are generated by the observed diagnostic sensitivity of the two tests, given the prevalence of hearing impairment. Total referrals are made up of true positives, false positives and false negatives, the last of these groups being referred by means other than the screening test so that all cases of hearing impairment are ultimately identified.
Owing to the constraints imposed by the numbers of referrals and prevalence of hearing impairment, the resulting number of false positives implies a substantially higher test specificity (the probability of a child without hearing impairment testing negative) than that found in the new diagnostic accuracy data from Nottingham (Table 30). Moreover, these constraints mean that the implied specificity is the same under either screening method in the base case and cannot be varied between the PTS and HC screener.
| Variable | Sensitivity – observed and modelleda | Specificity – observedb | Specificity – modelledc |
|---|---|---|---|
| PTS | 95.9% | 79.8% | 94.1% |
| HC | 88.7% | 83.8% | 94.1% |
However, it was possible to explore the impact of varying the numbers of false-positive referrals between the screening and no screening options by varying the total number of referrals. This is a particularly relevant area of uncertainty to consider as, in the base case, total referrals are assumed to be the same with and without screening, whereas evidence from the service comparison study suggests that the rate of referrals may be higher in the absence of screening than when a screening programme is in place.
Although the significantly higher referral rate in Cambridge than Nottingham (34.4 vs. 21.9 per 1000 children per year) does not lead to a significantly higher yield of confirmed cases in Cambridge (3.04 vs. 2.51 per 1000 children per year), the possibility that a screening programme will reduce the number of referrals needs to be considered. Given the differences between the characteristics of the populations in the two areas, it is unclear what the difference in referral rates might be in any given area in the presence or absence of screening. Nevertheless, a higher rate of referrals in the no screening arm could result in a substantial increase in costs relative to the screening option (given the unit cost of diagnostic evaluation relative to screening) and thus have an important influence on the cost-effectiveness of screening. We therefore vary the referral rate between screening and no screening arms of the model in sensitivity analysis.
Distribution of referrals over time
In the current model, the previous assumptions about the identification of hearing impairment over time have been superseded by data on referrals obtained from the screening area (Nottingham) and the no screening area (Cambridge). Table 31 gives the distribution of referrals by age at last birthday in Cambridge and Nottingham. Referrals at age 3 years are taken to apply to the pre-school entry year (year 1 of the model) while referrals at ages 4–6 years are taken to apply to the first 3 years of school (years 2–4 of the model). In addition to the distribution over time, the model also takes account of the different sources of referral in the presence and absence of screening, based on the available data (Table 32). It is worth noting the importance in the screening area of sources of referral other than screening.
| Age at last birthday (years) | Model year | Total referrals (non-screening area) (%) | Cumulative | Total referrals (screening area) (%) | Cumulative |
|---|---|---|---|---|---|
| 3 | 1 | 34.4% | 34.4% | 29.9% | 29.9% |
| 4 (school entry year) | 2 | 28.5% | 62.9% | 29.1% | 59.0% |
| 5 | 3 | 23.4% | 86.3% | 28.9% | 87.9% |
| 6 | 4 | 13.7% | 100% | 12.1% | 100% |
| Type of referral | No screening (%) | Screening (%) |
|---|---|---|
| GP | 41.3 | 10.9 |
| Health visitor | 24.3 | 22.7 |
| School screen | 0 | 21.5 |
| Speech therapist | 25.1 | 8.6 |
| Paediatrician | 3.2 | 8.1 |
| Parent | 4.2 | 12.4 |
| Education | 1.9 | 0.0 |
| School nurse | 0 | 3.1 |
| Community nursery nurse | 0 | 0.8 |
| ENT consultant | 0 | 8.2 |
| Other consultant | 0 | 1.7 |
| Referrals from diagnostic accuracy study | 0 | 2.0 |
Management of hearing impairment
Once cases of hearing impairment are confirmed by a DEA, their management will depend on the type of hearing impairment and its severity. The available management options are essentially unchanged from the 2007 HTA report. 12 We simply note here that management of hearing impairment results in an improvement in quality of life, which translates into QALYs. While the total QALY difference between screening and no screening will include the QALYs experienced by those with no hearing impairment, we exclude these from our results, as we assume that the characteristics of the modelled populations are the same in respects other than their exposure to type of screening (or no screening). The reported QALYs in screening and no screening groups represent the QALYs accruing only to those with managed hearing impairment who thereby experience an improvement in quality of life. The assumptions underlying the QALY calculations and the other parameters in the model are reported in the following sections.
Sources of parameter inputs
Values of input parameters for the model are based on the original HTA model, the literature, the observational studies described in greater detail elsewhere in this report (see Chapters 3, 5–7) and standard reference sources. A limited literature review was carried out to update health state utilities and costs. For probabilities of different severities of hearing impairment and utilisation of management options, the model used the data on which the original HTA report12 was based as the steering group felt that these were still relevant.
An overview of sources for the main broad categories of data input is given in Table 33.
| Type of parameter | HTA 2007 parameter source | This HTA parameter source | Updated | Note |
|---|---|---|---|---|
| Discount rate (costs and QALYs) | UK Treasury green book | UK Treasury green book57 | No | Deemed to be still valid and follows the NICE Reference Case58 |
| Prevalence of deafness | Literature and calculation | New clinical studies | Yes | |
| Probabilities of types of hearing impairment | Study, literature and calculation | HTA 2007 report and calculation | Yes | |
| Probabilities of management types | Study, literature and calculation | HTA 2007 report and calculation | Yes | |
| Utility values | Literature | Literature, HTA 2007 report and new clinical studies | Yes | Updated where new data were available from systematic search |
| Costs of screening | NHS Reference Costs | Clinical studies and NHS reference costs59,60 | Yes | |
| Costs of management | NHS Reference Costs | New clinical studies and NHS reference costs | Yes | |
| Diagnostic accuracy estimates | Literature | New clinical studies (sensitivity and specificity) | Yes |
Reviews of the literature
A short literature review was undertaken to search for any updates to the data required by the model that were not expected to be included in the results of the clinical studies. These were utility values of hearing impairment, the prevalence of hearing impairment (using data from the NHSP) and costs of management.
The scope for searches was publications since 2007, to capture new research published since the 2007 HTA report. 12 Search ranges were from January 2007 to July 2014 and were carried out on 10 July 2014.
The following databases were searched:
-
MEDLINE
-
EconLit
-
SocINDEX
-
PsycINFO.
Searches were also run through Google Scholar.
The following charity and support group websites were checked for useful publications (grey literature) that may not show up in searches of conventional databases:
-
Action on Hearing Loss
-
National Children’s Bureau
-
National Deaf Children’s Society.
Referrals
The clinical studies have, to date, reported on samples of 1108 children referred to audiology services in the no screening area (Cambridge) and 1702 children referred in the screening area (Nottingham).
Number of children with hearing impairment
Determining the number of children who need to be identified is a critical assumption within the model and the majority of the analysis relies on this calculation. The original HTA report12 on which this analysis draws assumed that the prevalence of hearing impairment was 78 of 1000 children. This was split into approximately 3.5 of 1000 children with SNPC hearing impairment and 74.5 of 1000 children with transitory hearing impairment through the probabilities that populate the model. The SNPC number is supported by research used, and subsequently published, for the 2007 HTA report,12 which gives a figure of 3.65 of 1000 children with SNPC hearing impairment. 8,12
However, although this figure is likely to be an accurate representation of the total prevalence of SNPC hearing impairment in the child population, it is also likely to be a sizeable overestimate of the numbers of children who have SNPC hearing impairment yet to be diagnosed at the age of 3 years (prior to the start of the model). This is because cases of hearing impairment in children are generally identified either through the NHSP or by parents before school age. Moreover, while our main interest is in children with SNPC hearing impairment, the vast majority of the children who are detected as a result of screening will have transitory hearing impairment.
The prevalence of both transitory and SNPC hearing impairment in the modelled population has been estimated on the basis of the numbers with a confirmed diagnosis of hearing impairment as a proportion of the base population in the two areas studied. Based on last appointment, we obtain estimates of 46 per 10,000 children in Nottingham (195/42,553) or 56 per 10,000 children in Cambridge (98/17,624). The former has been used for the purposes of the model and divided between transitory and SNPC hearing impairment in the ratio 96% : 4% as applied in the 2007 HTA report. 12 This gives approximately 2 children per 10,000 with undiagnosed SNPC hearing impairment and 44 with undiagnosed transitory hearing impairment.
Diagnostic accuracy
The sensitivity of the two screening tests used in the model was based on the child-level analysis of the case–control study using all children whether nominally recruited as cases (HI) or controls (NHI), where hearing impairment was defined as a PTA score of ≥ 30 dB on at least one of the four frequencies. Estimates of diagnostic accuracy have been reported (see Table 30).
Both screening methods generate a small number of false-negative cases, and, in these instances, the children should be picked up via other referral methods such as through GPs or speech therapists. Differences in sensitivity between the two screening methods will give rise to different numbers of false negatives which have been distributed over time in the model in the manner illustrated in Table 34. The distribution draws on the distribution of total referrals but adjusts for false negatives of the screening tests occurring only in year 2 onwards (rather than all 4 years of the model).
| Parameter | PTS value | HC value | Total false negatives (%) |
|---|---|---|---|
| Incorrectly have negative test, will be identified in subsequent years | 1.89 | 5.20 | – |
| Incorrectly pass screening test but are identified in first year | N/A, as screening test run in second year | N/A, as screening test run in second year | – |
| Incorrectly pass and are identified in second year | 0.78 | 2.16 | 42% |
| Incorrectly pass and are identified in third year | 0.78 | 2.14 | 41% |
| Incorrectly pass and are identified in fourth year | 0.33 | 0.90 | 17% |
Probabilities of hearing impairment by severity
Table 35 reports the proportions of cases of transitory and SNPC hearing impairment that are unilateral or bilateral and the distributions of unilateral and bilateral SNPC hearing impairment by severity. The source of these probabilities is the 2007 HTA report. 12
| Type of hearing impairment | Probability |
|---|---|
| Transitory hearing impairment | |
| Unilateral | 0.56 |
| Bilateral | 0.44 |
| SNPC hearing impairment is unilateral | |
| Unilateral | 0.60 |
| Bilateral | 0.40 |
| Unilateral SNPC | |
| Minimal severity | 0.58 |
| Mild or moderate | 0.20 |
| Severe or profound | 0.22 |
| Bilateral SNPC | |
| Minimal severity | 0.20 |
| Mild | 0.36 |
| Moderate | 0.23 |
| Severe | 0.10 |
| Profound | 0.11 |
Management probabilities
Probabilities of each management type for unilateral and bilateral transitory hearing impairment and the different severities of unilateral and bilateral SNPC hearing impairment were sourced from the 2007 HTA report12 and are listed in Table 36.
| Type of hearing impairment | Type of management | Probability |
|---|---|---|
| Unilateral transitory | Active observation | 0.935 |
| Non-surgical | 0.001 | |
| Surgical | 0.064 | |
| Bilateral transitory | Active observation | 0.74 |
| Non-surgical | 0.05 | |
| Surgical | 0.21 | |
| Unilateral SNPC minimal | No intervention | 0.99 |
| Non-surgical | 0.01 | |
| Unilateral SNPC mild or moderate | No intervention | 0.49 |
| Non-surgical | 0.51 | |
| Unilateral SNPC severe or profound | Non-surgical | 0.05 |
| Surgical | 0.95 | |
| Bilateral SNPC minimal | No intervention | 0.96 |
| Non-surgical | 0.04 | |
| Bilateral SNPC mild | No intervention | 0.7 |
| Non-surgical | 0.3 | |
| Bilateral SNPC moderate | Non-surgical | 0.7 |
| Surgical | 0.3 | |
| Bilateral SNPC severe | Non-surgical | 0.9 |
| Surgical | 0.1 | |
| Bilateral SNPC profound | Non-surgical | 0.5 |
| Surgical | 0.5 |
Resource use and costs
The following sections report the sources underlying the calculation of costs over the 4-year modelling period. The cost base year is 2012–13. Costs in the first year are undiscounted and, in subsequent years, are discounted at an annual rate of 3.5%. This is the rate recommended by the UK Treasury Green Book,57 based on the rate at which individuals discount future consumption over present consumption.
Screening costs
All children in the screening arm incur the costs of screening. Children who are referred by the test also incur the cost of a DEA and the subsequent cost of management is incurred by children who are referred by the screening test and are diagnosed with hearing impairment by a DEA.
The length of time to perform the test was collected (see Chapter 7). A mean duration of 1.4 minutes (measured to be the same for both methods) and an hourly rate of £73 per hour from the relevant 2012–13 Reference Costs60 (N05OGS – School-Based Children’s Health Other Services – Group Single Professional) give a staff cost associated with the screening tests of £1.69. This is reported in Table 37 while unit costs for other sources by which hearing impairment can be identified are presented in Table 38. The cost per child of screening was calculated by dividing the total costs (total costs of screening tests and total capital costs) by the total cohort of children screened over 5 years (10,000). The cost of each diagnostic evaluation was £150 (NHS Reference Costs 2012–1360).
| Parameter | Value | Source | Comment |
|---|---|---|---|
| PTS | |||
| School-Based Children’s Health Other Services – Group Single Professional (1 hour) | £73.00 | NHS Reference Costs 2012–1360 | |
| Average duration of screening test | 1.39 minutes | Clinical study | |
| Screening cost per child | £1.69 | Calculation | |
| Cost of device | £898.80 | Clinical study | Equipment suppliers |
| Printer | £228.00 | Clinical study | Equipment suppliers |
| Battery | £102.00 | Clinical study | Equipment suppliers |
| Calibration (per year) | £170.88 | Clinical study | Calibration needed every 12 months |
| Medicated alcohol-free wipes | £0.032 | www.firstaid4less.co.uk/12888_-Infection-Control/Wipes/Multi-Purpose-Wipes/Bioguard-Wipes.html | 200 wipes per pack |
| HC | |||
| School-Based Children’s Health Other Services – Group Single Professional (1 hour) | £73.00 | NHS Reference Costs 2012–1360 | |
| Cost of device | £139.20 | Clinical study | Cost calculated with 20% VAT |
| Cost of ear cup | £0.72 | Calculation based on information from clinical study | One to two ear cups used per child. Average of 1.5 used to calculate cost |
| Calibration cost | £70.01 | Calculation based on information from clinical study | Between £50–100 (mid-point used) Calibration needed after 3 years (cost discounted) |
| Cost of AAA battery | £0.81 | www.amazon.co.uk | RRP for pack of eight is £6.49 |
| Outcome pad | £5.60 | Clinical study | |
| Method of identification | Unit cost | Source |
|---|---|---|
| GP | £45.00 | Curtis 201459 |
| Health visitor | £20.33 | Curtis 201459 |
| Speech therapist | £90.00 | Curtis 201459 |
| Paediatrician | £289.00 | Curtis 201459 |
| Parent | £13.14 | Survey data: 60 children incurred costs of £788.64 |
| Education | £14.10 | Calculation (based on average teacher salary of £22,000, working 40 hours a week 39 weeks per year) |
| School nurse | £27.00 | Curtis 201459 |
| Community nursery nurse | £95.00 | Curtis 201459 |
| ENT consultant | £289.00 | Curtis 201459 |
| Other consultant | £289.00 | Curtis 201459 |
Cost of travelling to appointments
Sixty respondents to a questionnaire asking about travel costs to attend screening appointments in Nottingham reported a total of 129 appointments and expenditure of £788.64, or £13.14 per child.
Unit costs of the pure-tone screen and HearCheck screener
Unit costs of each method of screening are presented in Table 37. Where costs extend beyond 1 year, these have been discounted accordingly in the model. Equipment suppliers have provided the costs of devices and consumables. Although the device costs for the PTS are higher than those for the HC screener (£898.80 vs. £139.20), the total costs for the HC screener are higher, primarily owing to the costs of ear cups. Table 38 reports the unit costs associated with other means by which hearing impairment is identified.
Management costs
Management costs were compiled from NHS Reference Costs for 2012–13,60 representing national unit costs, and are reported in Table 39. Where cost estimates were available for unilateral impairment only, these were multiplied by two to give the corresponding costs associated with bilateral impairment. This is a conservative assumption to account for the costs of providing and maintaining hearing aids for two ears. In the case of surgery, expert opinion suggests that this approach may overestimate costs. No follow-up costs or postoperation observation (active observation) were taken into account with surgical interventions. As with NHS reference costs generally, figures relating to paediatric services or the under 18 years age group were used where available. However, for hearing aids, the reference costs are not broken down for children and adults separately.
| Item | Unit cost | Source |
|---|---|---|
| Costs of conductive hearing impairment | ||
| Cost of ENT consultant appointment | £95 | NHS Reference Costs 2012–1360 |
| Cost of grommet operation (surgical intervention) | £2325.61 | NHS Reference Costs 2012–1360 |
| Cost of SNPC hearing impairment | ||
| Cost of ENT consultant appointment | £95 | NHS Reference Costs 2012–1360 |
| Cost of hearing aid assessment | £65 | NHS Reference Costs 2012–1360 |
| Cost of hearing aid fit: first visit | £65 | NHS Reference Costs 2012–1360 |
| Cost of hearing aid fit: follow-up visit | £54 | NHS Reference Costs 2012–1360 |
| Cost of hearing aid repair | £26 | NHS Reference Costs 2012–1360 |
| Cost of cochlear implant: unilateral | £20,148.16 | NHS Reference Costs 2012–1360 |
| Cost of cochlear implant: bilateral | £35,653.73 | NHS Reference Costs 2012–1360 |
| Cost of standard hearing aid | £77 | NHS Reference Costs 2012–1360 |
| Cost of digital hearing aid | £85 | NHS Reference Costs 2012–1360 |
| Cost of bone anchored hearing aid: fixture | £3077.35 | NHS Reference Costs 2012–1360 |
| Cost of bone anchored hearing aid: fit | £7997.51 | NHS Reference Costs 2012–1360 |
Utilities
A search to update the utilities from the 2007 HTA model12 was undertaken and the results were used to inform the updated parameter sheet (Table 40). Where possible, a distinction was made between unilateral and bilateral hearing impairment; otherwise, the utility associated with hearing impairment was determined primarily by its severity. In the first year (the pre-screening year), we assume that children entering the model do so evenly over the course of the year. Total utilities in the first year are therefore reduced by 50% (in undiscounted terms) compared with subsequent years. QALYs have been calculated only for children with a managed hearing impairment who consequently achieve a quality-of-life improvement. As QALYs accruing to children without hearing impairment do not affect the incremental cost-effectiveness ratio (ICER), they have been excluded from the calculations. Limited updating of the parameter values used in the 2007 HTA report was possible. For example, utilities for cochlear implants came from Summerfield et al. ,62 and utilities for grommet surgery came from Bisonni et al. 63 These studies provided intervention-specific utility data to supplement the evidence on utility by severity of hearing impairment.
| Health state | Utility | Source |
|---|---|---|
| Utility of 1 year with minimal hearing impairment | 1.000 | Modelling assumption |
| Utility of 1 year with mild hearing impairment | 1.000 | Modelling assumption |
| Utility of 1 year with conductive hearing impairment (unilateral and bilateral) | 0.677 | Modelling assumption |
| Utility of 1 year with moderate hearing impairment | 0.677 | Barton et al., 200461 |
| Utility of 1 year with severe hearing impairment | 0.616 | Barton et al., 200461 |
| Utility of 1 year with profound hearing impairment | 0.353 | Barton et al., 200461 |
| Utility of 1 year with mild or moderate hearing impairment | 0.8385 | Average (mild and moderate) |
| Utility of 1 year with severe or profound hearing impairment | 0.485 | Average (severe and profound) |
| Utility of 1 year with active observation | 1.000 | Modelling assumption |
| Utility of 1 year with hearing aid (non-surgical) | 1.000 | Modelling assumption |
| Utility of bilateral cochlear implant | 0.965 | Summerfield et al., 200262 |
| Utility of unilateral cochlear implant | 0.934 | |
| Utility of 1 year with grommet surgery | 0.995 | Bisonni et al., 199163 |
Modelling the potential costs and consequences of false-positive results
Chapter 6 presents the outcomes of a questionnaire given to parents whose children were referred from SES for a DEA appointment. Survey responses were obtained in respect of 60 children over 129 appointments.
The questionnaire asked a range of questions concerning parents’ views of the screening programme and the impact on themselves and their children. The travel costs associated with screening and diagnostic visits were based on this survey. One impact that is commonly discussed in the context of screening programmes, although not straightforward to value in monetary terms, is the anxiety associated with false-positive results. This was an issue addressed by the questionnaire.
Anxiety
Parents were asked to rate their anxiety level on a scale from 0 to 10 as a result of finding out that their child needed further testing. Approximately 60% of respondents listed their anxiety as ≤ 5 out of 10, with only one parent rating their anxiety > 8 out of 10. Between finding out that their child needed further testing and attending the clinic visit, mean parental anxiety score fell slightly, from a score of 5.3 to a score of 4.7. The survey was felt to provide insufficiently compelling evidence to make an adjustment to the model, either by incorporating anxiety into the QALY or as a monetary disbenefit. On the basis of current evidence, it is unknown whether or not there is a health impact on parents of further hearing tests that would outweigh the benefits gained by children receiving a correct diagnosis; this is an issue on which further research may shed some light.
Results
Number of referrals to diagnostic evaluation and numbers diagnosed with hearing impairment
Tables 41 and 42 show the number of children referred for a DEA and the numbers diagnosed with hearing impairment in each model arm over the 4 years of the model. Under the base case, a hypothetical population of 10,000 children has been used, of which the number referred for diagnostic evaluation in the counterfactual and the two intervention arms is 629, and the number with hearing impairment is approximately 46. The numbers referred in each year are determined by the data on referrals at different ages in the screening and no screening sites and have an important bearing on the cost-effectiveness of screening compared with no screening.
| Year | No screening | PTS | HC | |||
|---|---|---|---|---|---|---|
| Referred each year | Cumulative | Referred each year | Cumulative | Referred each year | Cumulative | |
| 1 | 216.3 | 216.3 | 188.0 | 188.0 | 188.0 | 188.0 |
| 2 | 179.2 | 395.5 | 181.8 | 369.8 | 179.9 | 367.9 |
| 3 | 147.1 | 542.6 | 182.5 | 552.3 | 183.8 | 551.7 |
| 4 | 86.1 | 628.7 | 76.4 | 628.7 | 77.0 | 628.7 |
| Year | No screening | PTS | HC | |||
|---|---|---|---|---|---|---|
| Diagnosed each year | Cumulative | Diagnosed each year | Cumulative | Diagnosed each year | Cumulative | |
| 1 | 15.3 | 15.3 | 14.3 | 14.3 | 14.3 | 14.3 |
| 2 | 13.2 | 28.5 | 12.5 | 26.8 | 10.6 | 24.9 |
| 3 | 10.6 | 39.1 | 12.8 | 39.6 | 14.1 | 39.0 |
| 4 | 6.8 | 45.9 | 6.2 | 45.8 | 6.8 | 45.8 |
School entry hearing screening versus no screening: costs and quality-adjusted life-years
Tables 43 and 44 present the undiscounted and discounted costs broken down into the costs of identification (whether by screening or other means), diagnostic evaluation and management over 4 years for each arm of the model.
| Category | No screening | PTS | HC |
|---|---|---|---|
| Screening and identification | £34,902 | £63,786 | £69,699 |
| Diagnostic evaluation | £102,564 | £102,564 | £102,564 |
| Management | £52,194 | £52,194 | £52,194 |
| Total | £189,660 | £218,544 | £224,457 |
| Category | No screening | PTS | HC |
|---|---|---|---|
| Screening and identification | £33,553 | £61,224 | £66,929 |
| Diagnostic evaluation | £98,602 | £98,360 | £98,346 |
| Management | £50,177 | £50,054 | £50,047 |
| Total | £182,332 | £209,638 | £215,322 |
Diagnosis and management of hearing impairment has a positive impact on children’s quality of life as measured using the QALY. The figure for QALYs gained outlined in Table 45 is an estimate of the QALYs gained from children being diagnosed and managed (children moving from hearing impairment to no hearing impairment/managed hearing impairment) over the 4 years of the model. The QALYs accruing to children with no hearing impairment are not included here. The results show that, in the base case, not having a screening programme results in more QALYs than either the PTS or HC screen, which is associated with the lowest QALY gain of the three options.
| QALYs | No screening | PTS | HC |
|---|---|---|---|
| Undiscounted | 40.93 | 39.91 | 39.86 |
| Discounted | 35.90 | 35.21 | 35.16 |
In order to determine the cost-effectiveness of each method of screening compared with no screening, the ICER needs to be calculated. The ICER presents the ratio of the marginal gain of the intervention over the counterfactual in terms of both costs and benefits. It is calculated as:
Table 46 presents incremental costs and QALYs for the two screening approaches relative to no screening and for the PTS compared with the HC. In the base case, it is not appropriate to report an ICER, as no screening dominates (is more effective and less costly than) either screening approach. In the context of our research question, the results indicate that SES is not cost-effective compared with no screening.
| Cost-effectiveness values | PTS vs. no screening | HC vs. no screening | HC vs. PTS |
|---|---|---|---|
| Total costs | £23,171 | £28,840 | £5,669 |
| QALYs | –0.68 | –0.74 | –0.06 |
| Incremental cost per QALY ratio | No screening dominates | No screening dominates | PTS dominates |
Sensitivity analysis
The key data from the clinical studies that influence the magnitude and direction of the cost-effectiveness results primarily relate to the number and timing of referrals with and without a SES programme. While there is uncertainty about the applicability of the findings from Nottingham and Cambridge to a more general assessment of the costs and benefits of a screening programme as opposed to no screening programme, it is difficult to put boundaries on this uncertainty. This is in part because of the sociodemographic differences between the populations in Cambridge (the counterfactual) and Nottingham (screening site), and to the differences in service configuration between the two sites. A factor of particular significance is the difference in the rates of referrals between the two areas (the question of whether a screening site has more or fewer referrals than a non-screening site was an explicit objective of the study). In the light of clinical data suggesting that the referral rate in Cambridge is higher than that in Nottingham, the impact of a higher referral rate in the absence of screening was felt worthy of exploration. Increasing the referral rate when no screening programme exists increases the costs of this option while leaving QALY benefits unchanged, as these depend on the timing rather than the number of referrals. Increasing referrals sufficiently will render no screening more costly than screening and enable the trade-off between costs and benefits to be investigated.
In order to determine whether an intervention is cost-effective, the National Institutes of Health and Care Excellence (NICE) recommends comparing the ICER with a benchmark value of between £20,000 and £30,000 per QALY gained. 58 Technologies with an ICER of < £20,000 are generally considered to be cost-effective while, for those with an ICER > £20,000, reference needs to be made to other factors when considering value for money (and the case needs to be made increasingly strongly in relation to these factors when the ICER is > £30,000). Using £30,000 per QALY as the cut-off point for cost-effectiveness, the referral rate in the absence of screening would need to increase by ≥ 36% for no screening to cease being cost-effective relative to the PTS. This gives an upper limit on the extent to which referrals can be increased in the absence of screening without this option becoming excessively costly relative to its QALY benefits.
We are also interested in the circumstances under which screening becomes more effective than no screening. Leaving the baseline level of referrals unchanged, we investigated the extent to which referrals would need to be brought forward with screening compared with no screening. As we lacked clear bounds to place around the proportions of children referred at different ages, we again conducted a threshold analysis. It was found that the benefits of the PTS test would be increased sufficiently for the ICER to fall below £30,000 per QALY gained compared with no screening if the proportion of children referred in the first year of school (the screening year) increased by 5.9 percentage points or more.
Compared with the distribution and total numbers of referrals, other variables had relatively little impact on the conclusions of the analysis. For example, raising the sensitivity of the screening tests to 100% increased the QALY benefits under the PTS and HC but not sufficiently to make screening more effective than no screening. Altering the prevalence of hearing impairment had no impact on the results.
Discussion
Based on a hypothetical population of 10,000 children, it has been calculated that 629 children will be identified and referred for diagnostic evaluation, of which 46 children will be identified as having hearing impairment in the screening and no screening scenarios. The summary of results for this population is as follows:
-
The cost of screening ranges between £1.93 (PTS) and £2.49 (HC screener) per child. The total discounted costs associated with the no screening arm are estimated to be £182,333, which consists of the costs of identification, referral, diagnostic evaluation (including travel costs) and management. The screening arm is more costly, with total discounted incremental costs ranging between £27,304 (PTS) and £32,990 (HC screener).
-
The discounted QALY gain associated with children being treated for hearing impairment in the no screening arm is 35.9 over 4 years. In the base case, QALYs generated in the absence of screening are greater than those generated in the presence of the PTS (35.21) or HC screen (35.16).
-
No screening dominates both screening methods in the base case.
When considering the relative cost-effectiveness of each screening method, the PTS test is less costly (£5686) and is more effective (incremental QALY gain of 0.06) than the HC screen, rendering it dominant over the HC screener.
These conclusions appear to be robust in various sensitivity analyses. The notable exception is where SES is associated with fewer referrals for a DEA, implying a reduction in false-positive cases relative to no screening, which suggest SES could be the cost-effective (if less effective) option. To be more effective than no screening, referrals need to be expedited relative to the base case.
Discussion of key assumptions
Several key modelling assumptions require further discussion. Good use of assumptions is crucial to economic modelling. Done correctly, assumptions simplify the modelling approach, allowing for targeted models to be built using the best quality of data. A model with large parameter demands often has to make compromises and assumptions that can end up weakening the model.
One of the main assumptions in the model concerns the rate of referral to diagnostic evaluation. For the base-case scenario, it has been assumed that the number of referrals in the screening and non-screening areas is the same. From the comparative data presented in Chapter 5, the rate of referral in Cambridge (area without SES) was higher than that of Nottingham (area with SES). However, after careful consideration of the issue, these comparative data were not used for the base case, as Cambridge may not be reflective of all non-screening areas. Assuming that the rates of referral would be higher when no screening programme is in place makes an implicit assumption that other methods of referral, such as referral via GP, health visitors and speech therapists have low specificity. That is, it assumes that these other methods require a higher number of referrals than are required with a screening programme to identify the same number of cases of hearing impairment when, in practice, it is not known what the true increase in rate of referrals for non-SES areas relative to SES areas would be.
In the base case, screening is more costly than no screening. However, if referrals are increased in the no screening option, there is a point when no screening becomes more costly than screening. If referrals are increased further, the no screening option will eventually become too costly to justify its additional QALY benefits over screening relative to conventional cost-effectiveness benchmarks such as the £30,000 figure used by NICE. Sensitivity analysis considered the ‘tipping point’ in terms of referral numbers under the no screening option at which no screening would no longer be cost-effective. It was found that the cost per QALY ratio of no screening relative to the PTS test increased to £30,000 if the rate of referrals was 36% higher in the absence of screening compared with the PTS. In this case, screening is the cost-effective (if less effective) option.
An alternative way in which screening could become cost-effective is if, despite being more costly than no screening (as in the base case), it was also more effective. This could come about if screening was associated with more rapid detection of cases of hearing impairment than no screening. A threshold analysis on the pattern of referrals suggested that, under screening, referrals would need to increase by around 5.9 percentage points in the screening year (increasing the proportion of referrals taking place either in the pre-screening year or the screening year from 59% to 64.9%) in order to reduce the cost per QALY of the PTS relative to no screening to £30,000. While there are grounds for believing that the number of referrals is likely to be higher without screening, the potential for screening to achieve timelier referral and management of HI children is less clear.
Strengths and weaknesses
The model developed has a number of features that support the validity of its results.
It builds on an existing model (2007 HTA report12), which allowed a systematic consideration of areas where the original model could be improved and incorporates these into the updated model. This was greatly assisted through the involvement of the architect of the original model who contributed to the project as a consultant (Professor Linda Davies).
The model was conducted by an experienced multidisciplinary team of researchers who had been involved in the development of economic models, and models concerning hearing impairment in particular, prior to this project.
The model also drew on the experience of the project steering group, both in terms of content knowledge to advise on the design of the model and the input of a health economics specialist who fed back on the model design and early results.
The model was underpinned by a protocol outlining the key features and limiting the opportunity for results to be data driven. In the event, changes were made to the structure of the model so that it could capture aspects of the impact of SES that were not originally anticipated. These changes have been fully documented relative to the original model plan, so reducing the opportunity for bias.
The model was conducted in parallel with a series of clinical studies that were designed to improve information on key parameters where high levels of uncertainty had been identified in the 2007 HTA report model (see Chapters 3, 5–7). 12
There were also some limitations. The greatest limitation was that, despite attempts to reduce uncertainty, the data collection in the accompanying clinical studies was unable to overcome this uncertainty completely. For instance, the study designs for accuracy were chosen on the basis of feasibility and used a diagnostic case–control study, which is known to exaggerate accuracy, particularly where the controls are healthy subjects. Similarly, a randomised comparison between SES and non-SES areas would have been desirable to assess the impact of SES on referrals and yield. Instead we had to employ an observational two-centre comparative study design subject to major potential limitations, including confounding and lack of generalisability. In retrospect we could have invested research in quantifying the accuracy of processes used to refer children for a DEA where SES was not in place, but the importance of this emerged only when the results of the clinical studies were reported.
The inevitable lack of availability of the results of the clinical studies until late in the research programme limited the time available to perform sensitivity analyses in the economic model. These were thus prioritised in consultation with the research and steering group and we remain confident that all key aspects have been covered and that the cost-effectiveness findings remain robust.
Findings in comparison with other health economic evaluations
The 2007 HTA report12 identified virtually no health economics literature on SES, and the update search for this report identified no new health economics literature since the 2007 report. Thus the main point of comparison for the new economic model is the economic model from the 2007 HTA report. 12
The most important difference between the two reports is a change in view about the likely cost-effectiveness of SES from possibly cost-effective (albeit with considerable uncertainty in 2007) to probably not cost-effective in this report. This change is primarily because of the use of observations on the timing of referrals as the basis for the updated model. The 1-year results from the 2007 report, showing a favourable cost-effectiveness ratio for screening, are consistent with a substantial advantage for screening in terms of the timeliness of referrals compared with no screening. This is also implied by the assumptions used in initial attempts to replicate the 2007 results. In comparison, the observational data incorporated into the model on which the findings presented here are based suggest that screening does not result in a more rapid rate of referral and that other methods used in the absence of screening may be more effective in this regard.
Chapter 9 Conclusions and recommendations
The overarching aims of this project were to evaluate the diagnostic accuracy of hearing screening tests and the cost-effectiveness of screening for hearing impairment at school entry in the UK.
Summary of findings
Systematic review of diagnostic accuracy (see Chapter 2)
The updated review of diagnostic accuracy studies confirms the conclusion from the 2007 HTA report12 that research to date demonstrates significant variability in the design, methodological quality, and results. Robust conclusions about the performance of individual test types for use in SES cannot be drawn. Summarising the review reported in the 2007 HTA report and this update we conclude that:
-
Parental questionnaires had the poorest diagnostic accuracy compared with all other tests.
-
The findings from the new audiometry-based studies evaluating computer-based devices and the HC screener reported higher and more consistent specificity but lower and widely varying sensitivity estimates compared with the sweep PTA studies included in the original report.
-
Studies evaluating TEOAE reported variable sensitivity with wide CIs, while specificity estimates were relatively high and more consistent.
-
The study evaluating AABR reported high sensitivity and specificity.
The review included studies from countries with and without an established UNHS system and with very different systems of health-care delivery. The generalisability of the findings to other situations, including the UK NHS system, is likely to be limited.
Diagnostic accuracy study (see Chapter 3)
The findings of our diagnostic accuracy study indicate that the PTS and HC devices have a high level of sensitivity (PTS ≥ 89%, HC ≥ 83%) and an acceptably high level of specificity (PTS ≥ 78%, HC ≥ 83%) for identifying hearing impairment at the level of the ear. The PTS test has greater sensitivity than the HC screener and the HC screener has greater specificity than the PTS.
These conclusions appear robust, with the child-level analyses indicating similar levels of sensitivity and specificity for the screening tests to those seen for the ear-level analyses and for all different definitions of impairment.
Assessment of false negatives (see Chapter 4)
Assessment of false-negative rates is challenging for screening evaluations of a condition that may fluctuate, progress or be of later onset. From our review of the existing literature and data from the diagnostic accuracy study, we are unable to quantify the effect of false-negative results from the PTS or HC screening, but were able to confirm that the rate was extremely low. Of the 16 ears of children in our diagnostic study (total N = 630) which passed one or both of the screening tests but were referred by the PTA measure, only four were confirmed to have a hearing impairment at diagnostic evaluation and all were mild (4/630, 0.63%).
Comparison of school entry hearing screening and non-school entry hearing screening services (see Chapter 5)
There was strong evidence that the rate of referral for hearing problems is lower when a SES programme is present. The referral rate was 36% lower in Nottingham (SES) relative to Cambridge (no SES) (rate ratio 0.64, 95% CI 0.59 to 0.69; p < 0.001).
There was little evidence that the yield of confirmed cases differs between areas with and without a SES programme but a higher proportion of referred children were subsequently confirmed to be HI in the area with a SES programme (17.0% in Nottingham vs. 10.6% in Cambridge).
The mean age of referral was nearly identical between the two sites when looking at all referrals but for children who were subsequently confirmed as having a hearing impairment there was strong evidence that children in sites with a screen are older at referral (mean age difference 0.47 years, 95% CI 0.24 to 0.70 years; p < 0.001).
It appears that the site with a SES programme is referring fewer children for suspected hearing problems than the site with no SES programme, but there was little evidence that the yield of children with confirmed hearing impairment was different. Children with confirmed hearing impairment are older at referral in the SES site.
Survey of parents (see Chapter 6)
We found from our survey of parents of children referred by the SES programme in Nottingham that the consequences of the referral process for parents and children, including false positives, are minor. They certainly do not appear to be sufficient to undermine parental views about the value of SES. However, it should be noted that the referral process, including the possibility of false positives, occurs both in a system with SES and one relying on ad-hoc referral based on concern alone and hence the data on anxiety and costs could apply to both service implementations. The difference for parents whose child is referred by the SES programme is that they may have had no concerns prior to the screening test.
Practical implementation (see Chapter 7)
We demonstrated minimal differences between the PTS and HC tests in terms of time taken to conduct each examination and practical issues. Testing covered a range of schools throughout the school year and thus we suggest the findings might be generalisable beyond the Nottingham schools.
Cost-effectiveness of school entry hearing screening (see Chapter 8)
Our economic modelling showed that SES is unlikely to be cost-effective and, using base-case assumptions, including the assumption that the number of referrals is the same in the presence or absence of screening, is dominated by a no screening strategy. This is consistent with the observed results of the clinical studies (see Chapter 5), which suggest that cases of hearing impairment are identified in similar numbers but at a younger average age in the absence of SES.
Two situations where SES might be cost-effective were identified. In the first situation, SES may be considered cost-effective if there are fewer referrals associated with SES or, conversely, if there are more referrals without screening. This assumption might be supported by the observation from our clinical study (see Chapter 5) that referral rates (and by assumption, potential false positives) were less in the site where SES had been in place for many years. However, in order for this to be the case the reduction in referrals would need to be attributable to SES and there is considerable uncertainty about this. The model is also sensitive to a second set of assumptions in which referrals occur more quickly with screening than is observed from our study comparing SES and non-SES sites.
Discussion
The underlying rationale of this research project was to revisit an earlier attempt to assess the clinical effectiveness and cost-effectiveness of SES in the context of the UK NHS, and to seek to improve the quality of evidence on key pieces of information about which there was great uncertainty in the 2007 HTA report. 12 These key uncertainties included (1) the accuracy of the screening tests which might be used; (2) the feasibility of using such tests, particularly in terms of the time required to do them; (3) the consequences to children and parents of being referred for a DEA; and (4) most importantly, the difference in number of referrals and yield which might arise where SES is implemented. We have integrated the findings from our clinical studies designed to address these key uncertainties into an updated version of the economic model used in the 2007 HTA report. 12 The overall conclusions for this report should be drawn from our updated overarching cost-effectiveness analysis.
A good example of the need to adopt this more integrated approach to the interpretation of the results is the test accuracy study for the PTS and HC screener (see Chapter 3). Although it is clear that the accuracy of both tests, particularly their sensitivity, may look acceptable for the purposes of screening in isolation, it is only when these results are put in the context of our economic model and the screening test considered in the context of the wider health-care system as part of a programme of care, that the question of whether or not the tests are accurate enough can be truly answered. Integrating the various clinical studies of this project, our economic modelling shows that SES is unlikely to be cost-effective and, using base-case assumptions, is dominated by a no screening strategy.
There are, however, some aspects of the individual clinical studies that deserve highlighting in addition. We examined the diagnostic accuracy of two devices, but it might be argued that these were not the optimal devices to assess, and that other devices would perform better, leading to improved effectiveness and cost-effectiveness of SES. The relatively good accuracy achieved by the chosen devices suggests otherwise. Even though there may be devices that achieve greater accuracy, sensitivity analysis on the cost-effectiveness results suggests that even an assumption of 100% sensitivity would not give a cost-effective result. The differences between the tests could be of value in terms of guiding technology for developing improved hearing screening devices. Considering some combination of the two tests might be appropriate, but this was not evaluated here. The updated systematic review does, however, emphasise that technology is constantly evolving with new devices continually emerging and improved evaluations of existing devices being produced.
Two further issues arise when considering diagnostic accuracy. The first relates to considerations of sensitivity and specificity. Sensitivity measures the extent to which the screening test correctly identifies children with a hearing impairment (true positives). Specificity measures the extent to which the screening test correctly identifies children who do not have a hearing impairment (true negatives). Evaluation of the screening tests is influenced by the balance between the two. A high false-negative rate (low sensitivity, i.e. children with a hearing impairment who pass the screening test) is probably a more important problem than a high false-positive rate (low specificity, i.e. children with no hearing impairment referred for a DEA by the screening test). Thus, the desirable balance is for high sensitivity over high specificity.
The second issue concerns the definition of hearing impairment that the screening tests aim to identify and that definition concerns both degree and type. In terms of degree, the 2007 HTA report12 found that there was considerable variation around the UK in the frequencies used by different services (not all used 0.5, 1, 2, and 4 kHz) and the referral level (20, 30 or 35 dB). The diagnostic accuracy study reported here used the 20 dB level as a referral criterion for the PTS test (as used in several SES programmes) and ≥ 30 dB (PTA) to indicate a hearing impairment.
A more important issue of definition concerns the type of hearing impairment the screening test is designed to identify. Sensorineural hearing impairments are permanent and have implications for the child at whatever level they occur, although the greater the level of impairment, the greater the consequences tend to be. Conductive hearing impairments may be permanent, often associated with anatomical causes, but are usually transient. Such impairments are often caused by OME (glue ear), a very prevalent condition in children. When considering long-term effects for the child, management of permanent impairments is critical but transient impairments can be important in the shorter term, particularly if the glue ear is persistent, and so do warrant management.
One crucial question is ‘What should a screening test of hearing be aiming to identify and hence how should the sensitivity and specificity be assessed?’. Hearing screening tests as they are currently used cannot distinguish between sensorineural and conductive impairments so all children with a hearing impairment will be referred by the screening test. There is an argument that any assessment of cost-effectiveness of a hearing screening programme should consider only permanent impairments and consider the identification of transient losses as an unavoidable by-product. A key point here is that if the programme is not cost-effective when transient impairments are included (and hence more children are identified), a situation based on only identification of permanent impairments is unlikely ever to be cost-effective because identifying and managing transient impairment is no longer counted as a benefit. For the diagnostic accuracy study we have considered a positive result as any impairment, since that is what the screening tests are designed to identify. We have analysed this by ear (to assess the screening tests) and by child (to assess the screening programme).
Ascertaining and capturing the impact of false negatives is a major challenge in any assessment of any screening programme. False negatives arising from hearing impairment, which would be invisible even if the best diagnostic test (PTA) was applied to all children, need to be distinguished from hearing impairment that is overlooked because a screening test is used. Concerning the former, the evidence is that this is likely to be small in amount and minor in nature. In practice false-negative cases are most likely to arise where children are not exposed to the general surveillance of the health care and educational system that might prompt an ad-hoc referral. In contrast, the false negatives arising because a screening test is used instead of a definitive test are measurable and reflected in the screening test’s sensitivity. It is again noted that the sensitivity of the PTS and HC screener is good with few false negatives and that where hearing impairment was missed, the nature of the hearing impairment was minor.
The comparison between a site with a SES programme and a site that has not provided a SES programme for a number of years is novel and timely given the number of sites that are actively reviewing all their audiological services. However, any service without a SES programme needs to be very responsive and backed up with information strategies for parents and professionals.
One issue not raised thus far is the availability of the population to be screened. Children who undergo hearing screening at school age could be considered to be a captive audience. Their parents usually have the option to opt out of the screen but the default situation is that children will be screened. In an area with no SES programme, children are referred only if concern is expressed. For this reason in the SES system it could be argued that fewer children with true hearing impairment might be missed (fewer false negatives). For both systems the rate of parents not attending for a DEA with their children is about 15%.
The survey of parents provided measures of anxiety and qualitative data on views of the service as a whole. We did not quantify the costs of that anxiety which might have informed any disutility associated with false positives. False positives would be particularly important if it was thought that screening does lead to a reduction in referrals because, if there was serious disutility, this would amplify the effect of reducing false positives.
This project is unique in that it observed the screening devices being used by regular personnel under usual situations. The data collected on time taken for each test have contributed to the economic model. Perhaps more notable were the anecdotal observations of the researchers and nurses although views from only three nurses using two devices were sought. Confirming the situation reported in the 2007 report,12 the rooms and facilities offered for screening in many schools are not ideal. Account was not always taken of the requirement for a relatively quiet environment. The presence of children waiting to be screened while another child is screened allows them to become bored and impatient as well as enabling them to see how the system works and what they have to do. This raises the issue of how well accuracy studies that are carried out in rather more optimal conditions with individual children (including our own diagnostic accuracy study), can truly reflect performance in the real-world situation. In assessing the practical implementation of the tests the views of those administering the test are important.
There are strengths and weaknesses of this programme of research that need to be taken into account in drawing an overall conclusion. The strengths and limitations of the various individual studies of this project have been identified in the context of the contributory chapters of this report. The key overarching strength in this project is that it was planned and conducted in a prospective manner specifically to address the key uncertainties of the 2007 HTA report12 and thereby provide an improved understanding of the clinical effectiveness and cost-effectiveness of SES, particularly in the context of the UK NHS. The main limitation is that, although uncertainty has been reduced, it has not been eliminated and these continuing areas of uncertainty are highlighted below to inform the suggestions for further research. The most important uncertainty remains the estimates of the number of referrals and yield attributable to SES. The evidence collected here to inform this comes from the observational study comparing an established SES site (Nottingham) with a site where SES has not been employed for many years (Cambridge). While its prospective design limits some of the biases associated with this comparison, major threats to its validity remain, in particular selection bias and confounding. In other words, differences between the areas of Nottingham and Cambridge (other than the provision of SES), such as population characteristics and the nature of the other parts of the health-care system contributing to identification and management of hearing impairment in children, may explain the observed differences in referral rate and yield. While a more experimental design, such as a cluster randomised controlled trial, may overcome these biases and therefore provide a higher level of evidence, this needs to be weighed against the ethical and logistical challenges and costs of such a study.
Overall conclusions and recommendations
In the context of the UK NHS, and similar health-care systems, SES using tests such as the PTS test is unlikely to be effective in increasing the number of cases of hearing impairment identified or lowering the average age at which these cases are identified. SES is also unlikely to be cost-effective when judged against the benchmarks normally used by NICE, relative to a system entirely reliant on ad-hoc referral when a suspicion of hearing impairment is raised.
Implications for practice
Although our finding that the lack of cost-effectiveness of SES may be considered as a reason to withdraw SES where it is currently being practised, we would highlight aspects of the results that suggest caution. First, we have not completely excluded the possibility that SES is cost-effective and we have shown that there are at least two scenarios in which it may be cost-effective. Second, our conclusions are highly dependent on findings in the two specific areas (Nottingham and Cambridge) that were used here, and may not be generalisable to other areas. Third, the cost-effectiveness of SES depends on how effective (or ineffective) the ‘no SES system’ is. This, in turn, is highly dependent on the effectiveness of ad-hoc identification and referral for a DEA, which is not only largely unknown, but likely to be variable. It seems plausible that SES might have greater potential to be cost-effective where ad-hoc identification and referral is less well developed than in a system where it is well established. If withdrawal of the SES service is to be considered it needs to be carefully managed to ensure that the ad-hoc referral system is working effectively. Health professionals, school and nursery staff, and parents who would then be responsible for referral of children about whom there were concerns in the school entry year might need to be reminded to be more vigilant for signs of hearing impairment.
Implications for research
There are opportunities for further research.
We have highlighted the continuing evolution of evidence on the accuracy of tests for screening for hearing impairment in children of school entry age, and think that it would be useful to do this in an ongoing manner, particularly for the general value of the information to health-care workers testing hearing in childhood. Cochrane now includes reviews of test accuracy, and so would be an ideal vehicle for such an ongoing systematic review. We thus suggest that even though it might not directly impinge on a decision about SES, systematic reviews of the accuracy of devices that might be used to measure hearing in children at around school entry age should continue to be pursued, as this performance will undoubtedly influence the performance of any ad-hoc referral system prompted by suspicion of hearing loss.
Our economic model has highlighted the need for careful consideration of the alternatives to SES. It is not appropriate to consider a comparison with SES where there is absolutely no screening/identification activity. Although less well described and potentially subject to variation, the alternative to SES in the UK is a system in which parents, or those involved in the care of children, raise concerns. Where the concerns are substantiated this is followed by referral from a health-care professional, such as a GP, for a DEA. This system will also be taking place where SES is provided, not just in the years before or after school entry, but also in the year of SES. This is an important general finding from this work. Success in identifying hearing impairment in children in the UK is heavily dependent on the effectiveness and cost-effectiveness of the ad-hoc system of referral by a wide range of agents (parents, education, social services and health care) for formal evaluation on suspicion of hearing impairment. This appears to have been very little explored. Thus, in the future, characterising and then measuring the cost-effectiveness of different approaches to the ad-hoc system, with a view to optimising it should receive as much, if not greater priority, than further evaluation of SES.
A related issue is the process by which concern, or referral from SES, is converted into DEAs. It is clear from this project that there is considerable additional triage as the actual number of referrals for a DEA in Nottingham (a service with a SES programme) is much less than the number of referrals that would be expected from the false-positive rates seen in the accuracy estimates (see Chapter 3). This may, in part, be because of the fact that, in the real-world implementation of the SES, a child who is referred by a screening test will be re-tested some weeks later before an onward referral for a DEA is made. In this respect, more research on what determines programme specificity (as opposed to test specificity) would be useful.
Understanding why the referral rate in Nottingham was less than in Cambridge (a service without a SES programme) and whether or not this is related to the presence of SES would be of specific interest. Whether or not the difference and cause of any differences is generalisable to other areas would be the wider objective of further research. Further observational studies similar to our comparison between Nottingham and Cambridge could be undertaken, albeit recognising the difficulty of matching the geographical areas.
Further research to better quantify the impact of referral, particularly with respect to anxiety, and whether or not all referrals are affected to the same degree as respondents in our study may be required, particularly if it appears that overall effectiveness and cost-effectiveness could be critically dependent on the costs and disutilities experienced by those testing false positive. We note also that our research has not explored children’s perspectives on testing for hearing impairment and so this could be an important target for further research.
Expanding the research on implementation to more sites could contribute further information on differences in provision and further opinion from nurses applying the tests within those different systems.
If withdrawal of SES is contemplated in particular settings, this could be used as an opportunity for further data collection. Particularly where the pattern of referrals and cases was known over many years in the run up to withdrawal, any change in pattern of referrals/cases could be very useful evidence confirming the lack of effectiveness and cost-effectiveness of SES, or challenging it. More formally if SES cessation is being contemplated in many areas, a randomised trial of withdrawal of SES programmes could be designed using referrals and hearing impairment cases identified as outcomes.
Acknowledgements
We would like to thank the following, without whom this project could not have taken place.
The participating families and schools.
The steering committee for their contributions to the project and in particular the chairperson, Professor John Bamford, for his advice on the final report.
Linda Davies (University of Manchester) and Jon Deeks (University of Birmingham) who provided comments on the cost-effectiveness and test accuracy components of the study respectively and received a small consultancy payment for this feedback.
The school nurses who carried out the extra screening for us: Natalie Campbell, Rachel Hodgkinson and Shelley Whiteley, and Mary Barks and Ann Allardice for helping to co-ordinate the practical implementation study.
Elliot Carter, Brendan Lane and Paul Williams from PenCTU who developed the data entry websites and databases. Brian Wainman and Mark Warner from PenCTU for entering data and assisting with the data management tasks.
Former project team member Vasilis Nikolaou.
Ketevan Rtveladze, and Tracey Jhita (Optimity Matrix) for developing the model.
Nicola Huxley and Tristan Snowshill (PenTAG, University of Exeter Medical School) for checking the model.
Venessa Vas for helping to complete the screening in the diagnostic accuracy study when Sam Errington left the team.
The audiologists who extracted and entered data, and sent out questionnaires for the survey: Karenbir Basi, Jeff Davis, Gemma Hopkins and Emily Balmer.
The audiologists at each centre for sending out invitations for the diagnostic accuracy study.
Simon Briscoe (Exeter) for reviewing the search strategy.
Heather Fortnum is funded by the University of Nottingham and supported by the NIHR NHBRU.
Mara Ozolins and Sam Errington are supported by a grant from the NIHR Health HTA programme (10/63/03).
Obioha C Ukoumunne, Rod S Taylor and Zhivko Zhelev are supported by the NIHR Collaboration for Leadership in Applied Health Research and Care South West Peninsula at the Royal Devon and Exeter NHS Foundation Trust.
Chris Hyde is supported by the University of Exeter.
Claire Benton is funded by Nottingham University Hospitals NHS Trust.
Joanne Moody is funded by CCS NHS Trust and supported by Addenbrookes Hospital, Cambridge University Hospitals Foundation Trust.
Membership of the steering committee
John Bamford (Chairperson and Honorary Professor of Audiology) University of Manchester.
John Fitzgerald (Consultant Clinical Scientist and Head of Audiological Services) Norfolk and Norwich University Hospital.
Vicki Kirwin (Audiology Specialist) The National Deaf Children’s Society.
Kevin Munro (Professor of Audiology) University of Manchester and Honorary Consultant Clinical Scientist, Central Manchester University Hospitals NHS Foundation Trust.
Kate Northstone (Senior Research Fellow) School of Social and Community Medicine, University of Bristol (to March 2013).
Karen Smith (Principal Statistician) Department of Health Sciences, University of Leicester (from April 2013).
Eldon Spackman (Research Fellow) Centre for Health Economics, University of York.
Observers
Heather Fortnum (Chief Investigator), Associate Professor and Reader, NIHR NHBRU, University of Nottingham.
Maria Koufali (deputised by Stacey Arland), Deputy Director, Research and Innovation, Nottingham University Hospitals.
Angela Shone (Sponsor), Head of Research Governance, Research and Graduate Services, University of Nottingham.
Obi Ukoumunne (Project Statistician), Associate Professor in Medical Statistics, University of Exeter.
Contributions of authors
Dr Heather Fortnum (Associate Professor and Reader, hearing research) conceived the study, was principal investigator for the study, wrote the original protocol, contributed to revisions of design and conduct of the study, oversaw data collection and analyses, was responsible for the first draft of Chapters 1, 4, 6, 7 and 9 and contributed to the whole manuscript.
Dr Obioha C Ukoumunne (Associate Professor, statistics) was a co-applicant, contributed to revisions of design and conduct of the study, is the project statistician, analysed all data for the project, was responsible for Chapters 3 and 5 and contributed to the whole manuscript.
Professor Chris Hyde (Professor, public health and epidemiology) was a co-applicant, contributed to revisions of design and conduct of the study, developed and supervised the analyses of the economic model, was responsible for Chapter 8 and contributed to the whole manuscript. He took over the role of corresponding author on the retirement of the principal investigator, Heather Fortnum, in 2015.
Professor Rod S Taylor (Professor, health services research) was a co-applicant, contributed to revisions of design and conduct of the study, and contributed to this manuscript.
Ms Mara Ozolins (Research Fellow, hearing research) contributed to revisions of design and conduct of the study, wrote study documents and meeting minutes, collected data for the diagnostic accuracy and practical implementation studies, monitored the budget, produced progress reports for the HTA and contributed to the whole manuscript.
Ms Sam Errington (Research Associate, hearing research) contributed to revisions of design and conduct of the study, wrote study documents and meeting minutes, collected data for the diagnostic accuracy and practical implementation studies, obtained ethical approvals, ran search strategies for systematic reviews and contributed to this manuscript.
Dr Zhivko Zhelev (Research Fellow, systematic reviews) conducted and wrote up the systematic review (see Chapter 2).
Mr Clive Pritchard (Principal Economist) managed the changes to the economic model and contributed to Chapter 8.
Ms Claire Benton [Clinical Scientist (audiology), paediatric audiology] was a co-applicant, contributed to revisions of design and conduct of the study, invited case children for the diagnostic accuracy study, collected data for the comparison study and contributed to this manuscript.
Ms Joanne Moody [Clinical Scientist (audiology), paediatric audiology] was a co-applicant, contributed to revisions of design and conduct of the study, collected data for the comparison study and contributed to this manuscript.
Mrs Laura Cocking (PenCTU Senior Data Manager) advised on and co-ordinated database design and development, data validation and data export, and contributed to this manuscript.
Mr Julian Watson (Parent Representative) contributed to design and conduct of the study, and contributed to this manuscript.
Ms Sarah Roberts (Economist) made the changes to the economic model and contributed to Chapter 8.
Data sharing statement
Data are available via the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Yoshinaga-Itano C, Coulter D, Thomson V. The Colorado Newborn Hearing Screening Project: effects on speech and language development for children with hearing loss. J Perinatol 2000;20:S132-7. http://dx.doi.org/10.1038/sj.jp.7200438.
- Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S. A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment. Health Technol Assess 1997;1.
- Kennedy C, McCann D, Campbell MJ, Kimm L, Thornton R. Universal newborn screening for permanent childhood hearing impairment: an 8-year follow-up of a controlled trial. Lancet 2005;366:660-2. http://dx.doi.org/10.1016/S0140-6736(05)67138-3.
- van Dommelen P, van Straaten HL, Verkerk PH. Ten-year quality assurance of the nationwide hearing screening programme in Dutch neonatal intensive care units. Acta Paediatr 2011;100:1097-103. http://dx.doi.org/10.1111/j.1651-2227.2011.02230.x.
- Fortnum HM, Summerfield AQ, Marshall DH, Davis AC, Bamford JM. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. BMJ 2001;323:536-40. http://dx.doi.org/10.1136/bmj.323.7312.536.
- Bamford J, Uus K, Davis A. Screening for hearing loss in childhood: issues, evidence and current approaches in the UK. J Med Screen 2005;12:119-24. http://dx.doi.org/10.1258/0969141054855256.
- Russ SA, Poulakis Z, Wake M, Barker M, Rickards F, Jarman FC, et al. The distraction test: the last word?. J Paediatr Child Health 2005;41:197-200. http://dx.doi.org/10.1111/j.1440-1754.2005.00587.x.
- Watkin PM, Baldwin M. Identifying deafness in early childhood: requirements after the newborn hearing screen. Arch Dis Child 2011;96:62-6. http://dx.doi.org/10.1136/adc.2010.185819.
- Stewart-Brown S, Haslum MN. Screening for hearing loss in childhood: a study of national practice. Br Med J (Clin Res Ed) 1987;294:1386-8. http://dx.doi.org/10.1136/bmj.294.6584.1386.
- Haggard M. Research and Development of Effective Services for Hearing-Impaired People. London: Nuffield Provincial Hospitals Trust; 1993.
- Fonseca S, Forsyth H, Neary W. School hearing screening programme in the UK: practice and performance. Arch Dis Child 2005;90:154-6. http://dx.doi.org/10.1136/adc.2003.046979.
- Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al. Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11320.
- Williamson I. Otitis media with effusion in children. BMJ Clin Evid 2011;2011.
- Midgley EJ, Dewey C, Pryce K, Maw AR. The frequency of otitis media with effusion in British pre-school children: a guide for treatment. ALSPAC Study Team. Clin Otolaryngol Allied Sci 2000;25:485-91. http://dx.doi.org/10.1046/j.1365-2273.2000.00360.x.
- Surgical Management of Otitis Media with Effusion in Children. London: RCOG Press; 2008.
- Feldman W, Milner RA, Sackett B, Gilbert S. Effects of preschool screening for vision and hearing on prevalence of vision and hearing problems 6–12 months later. Lancet 1980;2:1014-16. http://dx.doi.org/10.1016/S0140-6736(80)92167-4.
- Gloria-Cruz TLI, Carrillo JRC, Chan AL, Chiong CM, Llanes EGDV, Plete JR, et al. The accuracy of the hearing screener device as a hearing screening tool in the school setting for first graders aged 5–10 years. Otolaryngology 2013;134. http://dx.doi.org/10.4172/2161-119x.1000134.
- Whiting P, Rutjes A, Reitsma J, Bossuyt P, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3. http://dx.doi.org/10.1186/1471-2288-3-25.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93. http://dx.doi.org/10.1016/j.jclinepi.2005.01.016.
- El-Naggar M, Hashlamoun M. Paediatric hearing assessment and screening clinic at Fujairah: analysis of the results of the first 6 months of clinic practice. Emirates Med J 2005;23:15-20.
- Bu X, Li X, Driscoll C. The Chinese hearing questionnaire for school children. J Am Acad Audiol 2005;16:687-97. http://dx.doi.org/10.3766/jaaa.16.9.6.
- Li X, Driscoll C, Culbert N. Investigating the performance of a questionnaire for hearing screening of schoolchildren in China. Aust NZ J Audiol 2009;31:45-52. http://dx.doi.org/10.1375/audi.31.1.45.
- Newton VE, Macharia I, Mugwe P, Ototo B, Kan SW. Evaluation of the use of a questionnaire to detect hearing loss in Kenyan pre-school children. Int J Pediatr Otorhinolaryngol 2001;57:229-34. http://dx.doi.org/10.1016/S0165-5876(00)00453-5.
- Samelli AG, Rabelo CM, Vespasiano AP. Development and analysis of a low-cost screening tool to identify and classify hearing loss in children: a proposal for developing countries. Clinics (Sao Paulo) 2011;66:1943-8.
- Soares M, Nakazawa M, Ishikawa K, Sato T, Honda K. Hearing screening for Japanese children and young adults using the automated auditory brainstem response. Auris Nasus Larynx 2014;41:17-21. http://dx.doi.org/10.1016/j.anl.2013.08.001.
- Wu W, Lu J, Li Y, Kam AC, Fai Tong MC, Huang Z, et al. A new hearing screening system for preschool children. Int J Pediatr Otorhinolaryngol 2014;78:290-5. http://dx.doi.org/10.1016/j.ijporl.2013.11.026.
- Yin L, Bottrell C, Clarke N, Shacks J, Poulsen MK. Otoacoustic emissions: a valid, efficient first-line hearing screen for preschool children. J Sch Health 2009;79:147-52. http://dx.doi.org/10.1111/j.1746-1561.2009.00383.x.
- Georgalas C, Xenellis J, Davilis D, Tzangaroulakis A, Ferekidis E. Screening for hearing loss and middle-ear effusion in school-age children, using transient evoked otoacoustic emissions: a feasibility study. J Laryngol Otol 2008;122:1299-304. http://dx.doi.org/10.1017/S0022215108002156.
- McPherson B, Law MMS, Wong MSM. Hearing screening for school children: comparison of low-cost, computer-based and conventional audiometry. Child Care Health Dev 2010;36:323-31. http://dx.doi.org/10.1111/j.1365-2214.2010.01079.x.
- Leeflang MM, Moons KG, Reitsma JB, Zwinderman AH. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values: mechanisms, magnitude, and solutions. Clin Chem 2008;54:729-37. http://dx.doi.org/10.1373/clinchem.2007.096032.
- Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM. Case–control and two-gate designs in diagnostic accuracy studies. Clin Chem 2005;51:1335-41. http://dx.doi.org/10.1373/clinchem.2005.048595.
- Gomes M, Lichtig I. Evaluation of the use of a questionnaire by non-specialists to detect hearing loss in preschool Brazilian children. Int J Rehabil Res 2005;28:171-4. http://dx.doi.org/10.1097/00004356-200506000-00012.
- Olusanya B. Early detection of hearing impairment in a developing country: what options?. Audiology 2001;40:141-7. http://dx.doi.org/10.3109/00206090109073109.
- Hammond PD, Gold MS, Wigg NR, Volkmer RE. Preschool hearing screening: evaluation of a parental questionnaire. J Paediatr Child Health 1997;33:528-30. http://dx.doi.org/10.1111/j.1440-1754.1997.tb01664.x.
- Sabo MP, Winston R, Macias JD. Comparison of pure tone and transient otoacoustic emissions screening in a grade school population. Am J Otol 2000;21:88-91. http://dx.doi.org/10.1016/S0196-0709(00)80080-0.
- Orlando MS, Frank T. Audiometer and AudioScope hearing screening compared with threshold test in young children. J Pediatr 1987;110:261-3. http://dx.doi.org/10.1016/S0022-3476(87)80168-3.
- FitzZaland RE, Zink GD. A comparative study of hearing screening procedures. Ear Hear 1984;5:205-10. http://dx.doi.org/10.1097/00003446-198407000-00005.
- Holtby I, Forster DP, Kumar U. Pure tone audiometry and impedance screening of school entrant children by nurses: evaluation in a practical setting. J Epidemiol Community Health 1997;51:711-15. http://dx.doi.org/10.1136/jech.51.6.711.
- Nozza RJ, Sabo DL, Mandel EM. A role for otoacoustic emissions in screening for hearing impairment and middle ear disorders in school-age children. Ear Hear 1997;18:227-39. http://dx.doi.org/10.1097/00003446-199706000-00006.
- Siemens . HearCheck Screener Userguide 2012. www.connevans.info/image/connevans/38shearcheck.pdf (accessed 24 March 2015).
- British Sociery of Audiology . Pure-Tone Air-Conduction And Bone-Conduction Threshold Audiometry With And Without Masking 2012. www.thebsa.org.uk/wp-content/uploads/2014/04/BSA_RP_PTA_FINAL_24Sept11_MinorAmend06Feb12.pdf (accessed 24 March 2015).
- Taylor EJ, Emanuel DC. Assessment of the efficacy of a hearing screening program for college students. J Am Acad Audiol 2013;24:607-15. http://dx.doi.org/10.3766/jaaa.24.7.9.
- Sideris I, Glattke TJ. A comparison of two methods of hearing screening in the preschool population. J Comm Disord 2006;39:391-40. http://dx.doi.org/10.1016/j.jcomdis.2005.11.006.
- Bennett M, Mowat L. Validity of impedance measurements and referral criteria in school hearing screening programmes. Br J Audiol 1981;15:147-50. http://dx.doi.org/10.3109/03005368109081431.
- Grill E, Hessel F, Siebert U, Schnell-Inderst P, Kunze S, Nickisch A, et al. Comparing the clinical effectiveness of different new-born hearing screening strategies. A decision analysis. BMC Public Health 2005;5. http://dx.doi.org/10.1186/1471-2458-5-12.
- Olusanya BO, Bamigboye BA. Is discordance in TEOAE and AABR outcomes predictable in newborns?. Int J Pediatr Otorhinolaryngol 2010;74:1303-9. http://dx.doi.org/10.1016/j.ijporl.2010.08.010.
- Mathew R, Asimacopoulos E, Valentine P. Toward safer practice in otology: a report on 15 years of clinical negligence claims. Laryngoscope 2011;121:2214-9. http://dx.doi.org/10.1002/lary.22136.
- Reilly BK, Horn GM, Sewell RK. Hearing loss resulting in malpractice litigation: what physicians need to know. Laryngoscope 2013;123:112-17. http://dx.doi.org/10.1002/lary.23608.
- Mason S, Davis A, Wood S, Farnsworth A. Field sensitivity of targeted neonatal hearing screening using the Nottingham ABR Screener. Ear Hear 1998;19:91-102. http://dx.doi.org/10.1097/00003446-199804000-00001.
- Lutman ME, Davis AC, Fortnum HM, Wood S. Field sensitivity of targeted neonatal hearing screening by transient-evoked otoacoustic emissions. Ear Hear 1997;18:265-76. http://dx.doi.org/10.1097/00003446-199708000-00001.
- Geal-Dor M, Adelman C, Levi H, Zentner G, Stein-Zamir C. Comparison of two hearing screening programs in the same population: oto-acoustic emissions (OAE) screening in newborns and behavioral screening when infants. Int J Pediatr Otorhinolaryngol 2010;74:1351-5. http://dx.doi.org/10.1016/j.ijporl.2010.08.023.
- Lo PS, Tong MC, Wong EM, van Hasselt CA. Parental suspicion of hearing loss in children with otitis media with effusion. Eur J Pediatr 2006;165:851-7. http://dx.doi.org/10.1007/s00431-006-0181-5.
- Office of National Statistics (ONS) . Mid-2013 Estimates of the Population n.d. www.ons.gov.uk/ons/rel/pop-estimate/population-estimates-for-uk--england-and-wales--scotland-and-northern-ireland/2013/index.html (accessed 24 March 2015).
- Government Department for communities and local government . English Indices of Deprivation 2010 2011. www.gov.uk/government/statistics/english-indices-of-deprivation-2010 (accessed 24 March 2015).
- Ritchie J, Spencer L, Burgess BA. Analysing Qualitative Data. London: Routledge; 1994.
- HM Treasury . The Green Book. Appraisal and Evaluation in Central Government 2003. www.gov.uk/government/uploads/system/uploads/attachment_data/file/220541/green_book_complete.pdf (accessed 24 March 2015).
- National Institute for Health and Care Excellence . Developing NICE Guidelines: The Manual 2014. www.nice.org.uk/article/pmg20/resources/non-guidance-developing-nice-guidelines-the-manual-pdf (accessed 24 March 2015).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU; 2014.
- Reference Costs 2012–2013. London: Department of Health; 2013.
- Barton GR, Bankart J, Davis AC, Summerfield QA. Comparing utility scores before and after hearing-aid provision: results according to the EQ-5D, HUI3 and SF-6D. Appl Health Econ Health Policy 2004;3:103-5. http://dx.doi.org/10.2165/00148365-200403020-00006.
- Summerfield AQ, Marshall DH, Barton GR, Bloor KE. A cost-utility scenario analysis of bilateral cochlear implantation. Arch Otolaryngol Head Neck Surg 2002;128:1255-62. http://dx.doi.org/10.1001/archotol.128.11.1255.
- Bisonni RS, Lawler FH, Pierce L. Recurrent otitis media: a cost-utility analysis of simulated treatment using tympanostomy tubes vs antibiotic prophylaxis. Fam Pract Res J 1991;11:371-8.
Appendix 1 Executive summary from 2007 report12
This text is reproduced from Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al. Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen. Health Technol Assess 2007;11(32) under the Non-Commercial Government Licence for public sector information.
Background
The ability to hear is important, particularly during children’s formal education. Hearing impairment is amenable to intervention and hence a screening programme when children begin their school careers has potential value. School entry hearing screening (SES) has been implemented throughout the UK since the 1950s. There is evidence of mixed practice and uncertainty about the value of the screen. In addition, recent changes in childhood hearing screening policy (abandonment of a screen at 8 months and introduction of universal newborn screening) have implications for identification of children with hearing impairment at school entry.
Objectives
This report aimed to determine answers to the following three questions:
-
What is current practice for the SES in the UK?
-
What is known about the accuracy of alternative screening tests and the effectiveness of interventions?
-
What is known about costs, and what is the likely cost-effectiveness of the SES?
Methods
A national postal questionnaire survey was addressed to all leads for the SES in the UK, considering current practice in terms of implementation, protocols, target population and performance data. Primary data from cohort studies in one area of London were examined. A systematic review of alternative SES tests, test performance and impact on outcomes was carried out. Finally, a review of published studies on costs, plus economic modelling of current and alternative programmes was prepared.
Results
The evidence from the national survey of current practice is that:
-
the SES is in place in most areas of England, Wales and Scotland; just over 10% of respondents have abandoned the screen; others are awaiting guidance in the light of the national implementation of newborn hearing screening
-
coverage of the SES is variable, but is often over 90% for children in state schools
-
referral rates are variable, with a median of about 8%
-
the test used for the screen is the pure-tone sweep test but with wide variation in implementation, with differing frequencies, pass criteria and retest protocols; written examples of protocols were often poor and ambiguous
-
there is no national approach to data collection, audit and quality assurance, and there are variable approaches at local level
-
the screen is performed in less than ideal test conditions
-
resources are often limited and this has an impact on the quality of the screen.
The evidence from the primary cohort studies is that:
-
the prevalence of permanent childhood hearing impairment continues to increase through infancy
-
of the 3.47 in 1000 children with a permanent hearing impairment at school screen age, 1.89 in 1000 required identification after the newborn screen
-
the introduction of newborn hearing screening is likely to reduce significantly the yield of SES for permanent bilateral and unilateral hearing impairments; yield had fallen from about 1.11 in 1000 before newborn screening to about 0.34 in 1000 for cohorts that had had newborn screening, of which only 0.07 in 1000 were unilateral impairments
-
just under 20% of permanent moderate or greater bilateral, mild bilateral and unilateral impairments, known to services as 6-year-olds or older, remained to be identified around the time of school entry.
The evidence from the systematic review of the alternative tests and of the effectiveness of interventions is that:
-
no good-quality published comparative trials of alternative screens or tests for school entry hearing screening were identified
-
studies concerned with the relative accuracy of alternative tests are difficult to compare and often flawed by differing referral criteria and case definitions; with full pure-tone audiometry as the reference test, the pure-tone sweep test appears to have high sensitivity and high specificity for minimal, mild and greater hearing impairments, better than alternative tests for which evidence was identified
-
there is insufficient evidence to draw any conclusions about possible harm of the screen
-
there were no published studies identified that examined the possible effects of SES on longer-term outcomes.
The evidence from the cost-effectiveness study is that:
-
no good-quality published economic evaluations of SES were identified
-
a universal SES based on pure-tone sweep tests was associated with higher costs and slightly higher quality-adjusted life-years (QALYs) compared with no screen and other screen alternatives; the ICER for such a screen is around £2500 per QALY gained; the range of expected costs, QALYs and net benefits was broad, indicating a considerable degree of uncertainty
-
targeted screening could be more cost-effective than universal SES
-
lack of primary data and the wide limits for variables in the modelling mean that any conclusions must be considered indicative and exploratory only.
A national screening programme for permanent hearing impairment at school entry meets all but three of the criteria for a screening programme, but at least six criteria are not met for screening for transient hearing impairment.
Conclusions
The lack of good-quality evidence in this area remains a serious problem. Services should improve quality and audit screen performance for identification of previously unknown permanent hearing impairment, pending evidence-based policy decisions based on the research recommendations.
Recommendations for research
Further research is highlighted in the following areas:
-
evaluation of an agreed national protocol for services delivering the SES to make future studies and audits of screen performance more directly comparable
-
development and evaluation of systems for data monitoring so that robust data on screen performance are available
-
determination with greater certainty of the prevalence of congenital unilateral hearing impairment, and permanent mild and minimal hearing impairment at school entry, that could be identified by a suitable quality-assured screen protocol
-
a comparison of the effectiveness, efficacy and efficiency of alternative approaches (reactive services, formal surveillance, targeted screening and universal screening at school entry age) to the identification of permanent hearing impairment post newborn screen
-
controlled studies of the effectiveness of hearing screening and subsequent interventions for later outcomes in children with permanent mild, minimal and unilateral hearing impairment identified at school entry
-
determination of the distribution of detection thresholds for pure tones in the population at school entry.
Appendix 2 Systematic review
Search strategies: accuracy of diagnostic tests
Ovid MEDLINE(R)
Date range of search: January 2005 to July 2014.
Date of search: 10 July 2014.
Search strategy
1 audiometry.mp. or audiometry/ or exp
audiometry, pure-tone/
2 exp otoacoustic emissions, spontaneous/ or
otoacoustic emission$.mp.
3 exp acoustic impedance tests/ or acoustic
impedance.mp.
4 exp hearing tests/is, mt [instrumentation,
methods]
5 hearing test$.mp.
6 sweep audio.mp.
7 sweep test$.mp.
8 (hearing adj2 questionnaire$).mp.
9 cmedhq.mp.
10 conventional audiometry.mp.
11 conditioned play audiometry.mp.
12 cpa.mp.
13 exp audiometry, evoked response/
14 audiologic$ assessment$.mp.
15 acoustic intermittance.tw.
16 tympanometry.mp.
17 otoscopy.mp. or exp otoscopy/ or exp
diagnostic techniques, otological/
18 otological exam$.mp.
19 acoustic reflex test$.mp.
20 teoae.mp.
21 dpoae.mp.
22 (impedance adj screening).mp.
23 (impedance adj method$).mp.
24 fixed frequency audio.mp.
25 (speech adj2 noise).mp.
26 reflectometry.mp.
27 acoustic impedance.mp.
28 or/1-27
29 exp hearing loss, sensorineural/pc, di
30 exp hearing disorders/di, pc
31 exp otitis media/pc, di
32 exp hearing loss, high-frequency/pc, di
33 exp hearing loss/di, pc
34 hearing impairment$.mp.
35 exp hearing loss, conductive/di, pc
36 (hearing adj3 screen$).mp.
37 exp “sensitivity and specificity”/
38 exp predictive value of tests/
39 (diagnos$ adj2 accura$).mp.
40 or/37-39
41 exp child, preschool/ or school entry.mp.
42 exp child development/
43 early detect$.mp.
44 infant school$.mp.
45 exp schools, nursery/ or exp nurseries/ or exp
child day care centers/ or kindergarten$.mp.
46 nursery school$.mp.
47 or/41-46
48 screen$.mp. or exp mass screening/
49 (school entry adj3 (screen$ or exam$)).mp.
50 (medical exam$ adj2 school$).mp.
51 or/48-50
52 28 and 40
53 47 or 51
54 or/29-36
55 52 and 53
56 40 and 54
57 53 and 56
58 55 or 57
EMBASE
Date range search from: January 2005 to July 2014.
Date of search: 10 July 2014.
Search strategy
1 exp pure tone audiometry/ or exp audiometry/
or audiometry.mp.
2 otoacoustic emission$.mp. or exp spontaneous
otoacoustic emission/ or exp otoacoustic
emission/
3 acoustic impedance.mp. or exp acoustic
impedance/
4 hearing test$.mp.
5 exp hearing test/
6 sweep audio.mp.
7 sweep test$.mp.
8 (hearing adj2 questionnaire$).mp.
9 cmedhq.mp.
10 ((conventional or conditioned play) adj
audiometry).mp.
11 cpa.mp.
12 exp evoked response audiometry/
13 (audiologic$ adj assessment$).mp.
14 (acoustic adj intermittance).mp.
15 tympanometry.mp. or exp tympanometry/
16 otoscopy.mp. or exp otoscopy/
17 (otological adj2 technique$).mp.
18 (otological adj2 (exam$ or technique$)).mp.
19 teoae.mp.
20 dpoae.mp. or exp distortion product
otoacoustic emission/
21 (impedance adj (screen$ or method$)).mp.
22 fixed frequency audio.mp.
23 (speech adj2 noise).mp.
24 reflectometry.mp. or exp reflectometry/
25 or/1-24
26 hearing loss/pc, di [prevention, diagnosis]
27 hearing disorder/pc, di [prevention, diagnosis]
28 otitis media/pc, di [prevention, diagnosis]
29 hearing impair$.mp.
30 hearing impairment/pc, di [prevention,
diagnosis]
31 (hearing adj3 screen$).mp.
32 exp “sensitivity and specificity”/
33 (predictive adj2 test$).mp.
34 (diagnos$ adj2 accura$).mp.
35 or/32-34
36 or/26-31
37 25 and 35
38 school entry.mp.
39 pre-school.mp.
40 child/
41 exp child development/
42 early detect$.mp.
43 infant school$.mp.
44 nursery school$.mp. or exp nursery school/
45 exp child care/ or child day care.mp.
46 kindergarten$.mp. or exp kindergarten/
47 or/38-46
48 screen$.mp.
49 exp mass screening/
50 (school entry adj3 (screen$ or exam$)).mp.
51 (medical exam$ adj2 school$).mp.
52 or/48-49
53 or/50-51
54 25 and 35 and 47
55 35 and 36 and 47
56 54 or 55
57 or/52-53
58 47 or 57
59 35 and 36 and 58
60 56 or 59
Cumulative Index to Nursing and Allied Health Literature
Date range searched from: January 2005 to July 2014.
Date of search: 10 July 2014.
Search strategy
1 audiometry.mp. or exp audiometry, evoked
response/ or exp audiometry/ or exp
audiometry, pure-tone/
2 otoacoustic emission$.mp. or exp otoacoustic
emissions, spontaneous/
3 exp acoustic impedance tests/ or acoustic
impedance.mp.
4 hearing test$.mp. or exp hearing tests/
5 sweep test$.mp.
6 sweep audio.mp.
7 (hearing adj2 questionnaire$).mp.
8 cmedhq.mp.
9 (conventional adj2 audiometry).mp.
10 (conditioned adj2 audiometry).mp.
11 cpa.mp.
12 evoked response.mp. or exp evoked
potentials/
13 (audiologic$ adj assessment$).mp.
14 (acoustic adj intermittance).mp.
15 tympanometry.mp.
16 otoscopy.mp.
17 (otological adj2 technique$).mp.
18 (otological adj2 exam$).mp.
19 teoae.mp.
20 dpoae.mp. or exp otoacoustic emissions,
evoked/
21 (impedance adj (screen$ or method$)).mp.
22 fixed frequency audio.mp.
23 (speech adj2 noise).mp.
24 reflectometry.mp.
25 or/1-24
26 hearing disorders/di, pc
27 hearing impair$.mp.
28 exp hearing screening/
29 (hear$ adj2 screen$).mp.
30 or/26-29
31 exp “sensitivity and specificity”/
32 exp “predictive value of tests”/
33 (predictive adj2 test$).mp.
34 (diagnos$ adj2 accura$).mp.
35 or/31-34
36 school entry.mp.
37 exp child, preschool/ or pre-school.mp.
38 exp child development/
39 early detect$.mp.
40 infant school$.mp. or exp infant development/
41 nursery school$.mp. or exp schools, nursery/
42 child day care.mp. or exp child day care/
43 kindergarten$.mp.
44 or/36-43
45 screen$.mp.
46 exp hearing screening/
47 exp school admissions/
48 (school entry adj2 (screen$ or exam$)).mp.
49 (medical exam$ adj2 school$).mp.
50 or/45-46
51 or/47-49
52 25 and 35 and 44
53 30 and 35 and 44
54 52 or 53
55 50 or 51 or 44
56 30 and 35 and 55
57 54 or 56
PsycINFO
Date range searched: January 2005 to July 2014.
Date of search: 10 July 2014.
Search strategy
1 exp bone conduction audiometry/ or exp
audiometry/ or audiometry.mp.
2 otoacoustic emission$.mp.
3 acoustic impedance.mp.
4 hearing test$.mp.
5 sweep audio.mp.
6 sweep test$.mp.
7 (hearing adj2 questionnaire$).mp.
8 cmedhq.tw.
9 cpa.mp.
10 evoked response audiometry.mp.
11 audiologic$ assessment$.mp.
12 acoustic intermittance.tw.
13 tympanometry.mp.
14 otoscopy.mp.
15 (otological adj2 diagnos$).mp.
16 otological exam$.mp.
17 acoustic reflex test$.mp.
18 teoae.mp.
19 dpoae.mp.
20 (impedance adj screening).mp.
21 (impedance adj method$).mp.
22 fixed frequency audio.mp.
23 (speech adj2 noise).mp.
24 reflectometry.mp.
25 acoustic impedance.mp.
26 or/1-25
27 (sensitivity adj2 specificity).mp.
28 (predictive value adj2 test$).mp.
29 (diagnos$ adj2 accurac$).mp.
30 or/27-29
31 ((hearing loss$ or hearing disorder$ or
hearing impair$ or otitis media) adj3
(diagnos$ or screen$)).mp.
32 30 or 31
33 26 and 32
34 child$.mp. or exp child day care/
35 exp early childhood development/ or exp
preschool education/ or exp preschool
students/ or pre-school.mp. or exp nursery
schools/
36 kindergarten$.mp. or exp kindergartens/
37 nursery school$.mp.
38 exp elementary school students/ or infant
school$.mp.
39 exp early intervention/ or early detect$.mp.
40 or/34-39
41 33 and 40
Education Resources Information Center (Cambridge Scientific Abstracts)
Date range searched: January 2005 to July 2014.
Search date: 10 July 2014.
Search strategy
(hearing or otitis) and (diagnos* or screen* or
test*) and (school*or nurser* or infant*) and
(accur* or predictive or sensitiv*)
Science Citation Index (Web of Knowledge)
Date range searched: January 2005 to July 2014.
Search date: 10 July 2014.
Search strategy
(Hearing or otitis or deaf*) and (screen* or test*
or diagnos*) and (accura* or predictive or
sensitive) and (pre-school or infant* or nurser* or school*
Full-text studies excluded from the review and reasons for exclusion
Anon. The EarCheck Middle Ear Monitor for detection of middle ear effusion in children. Med Lett Drugs Ther 2008;50:55–6. (Not a diagnostic accuracy study, description of device.)
Ahn JH, Lee HS, Kim YJ, Yoon TH, Chung JW. Comparing pure-tone audiometry and auditory steady state response for the measurement of hearing loss. Otolaryngol Head Neck Surg 2007;136:966–71. (Mean age around 20 years.)
Arnold L, Boyle P, Canning D. Development of a paediatric audiovisual speech test in noise. Cochlear Implants Int 2010;11(Suppl. 1):244–8. (Not a screening study, cochlear implant users.)
Bagatto MP, Brown CL, Moodie ST, Scollie SD. External validation of the LittlEARS(R) Auditory Questionnaire with English-speaking families of Canadian children with normal hearing. Int J Pediatr Otorhinolaryngol 2011;75:815–7. (Not a diagnostic accuracy study, children younger than 2 years.)
Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al. Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen. Health Technol Assess 2007;11(32). (Systematic review, 2007 HTA report.)
Bhatia P, Mintz S, Hecht BF, Deavenport A, Kuo AA. Early identification of young children with hearing loss in federally qualified health centers. J Dev Behav Pediatr 2013;34:15–21. (Not a diagnostic accuracy study, children 0–3 years old.)
Blomgren K, Haapkyla J, Pitkaranta A. Tympanometry by nurses--can allocation of tasks be optimised? Int J Pediatr Otorhinolaryngol 2007;71:7–10. (Evaluate performance of nurses, aim is not screening but testing effectiveness of training.)
Bristow K, Fortnum H, Fonseca S, Bamford J. United Kingdom school-entry hearing screening: current practice. Arch Dis Child 2008;93:232–5. (Not a diagnostic accuracy study, survey included in the original HTA report.)
Chen G, Fu S, Luo S, Zhang W, Yang G. Screening of delayed-onset hearing loss in preschool children in the mid-south of China. Int J Audiol 2013;52:568–71. (Only patients with positive result on the index test received the reference standard.)
Dale OT, McCann LJ, Thio D, Wells SC, Drysdale AJ. The use of transient evoked otoacoustic emissions as a hearing screen following grommet insertion. J Laryngol Otol 2011;125:692–5. (Screening after grommet insertion, children 3–16 years old.)
Demorest ME, Wark DJ, Erdman SA. Development of the screening test for hearing problems. Am J Audiol 2011;20:100–10. (Screening tool for communication problems and psychological adjustment to hearing impairment.)
Dille M, Glattke TJ, Earl BR. Comparison of transient evoked otoacoustic emissions and distortion product otoacoustic emissions when screening hearing in preschool children in a community setting. Int J Pediatr Otorhinolaryngol 2007;71:1789–95. (Children’s age ranged from 4 months to 4 years, most of them younger than 3 years; focus on agreement between tests, no 2 x 2 data reported.)
Eiserman WD, Hartel DM, Shisler L, Buhrmann J, White KR, Foust T. Using otoacoustic emissions to screen for hearing loss in early childhood care settings. Int J Pediatr Otorhinolaryngol 2008;72:475–82. (Children younger than 3 years.)
Eiserman WD, Shisler L. Identifying hearing loss in young children: technology replaces the Bell. Zero Three (J) 2010;30:24–8. (Review paper.)
El-Naggar M, Hashlamoun M. Paediatric hearing assessment and screening clinic at Fujairah: analysis of the results of the first 6 months of clinic practice. Emirates Med J 2005;23:15–20. (Unable to obtain an abstract or full text.)
Fasunla AJ, Adeosun AA, Afolabi AO, Nwaorgu OG. Usefulness of behavioral test of hearing as a rapid public health screening tool for infants. J Pediatr Neurol 2011;9:29–33. (Infants aged ≤ 12 months.)
Gierek T, Gwozdz-Jezierska M, Slaska-Kaspera A. [The evaluation of efficaciency of the ‘Slysze’ screening test on the base of the results of hearing examinations in schoolchildren in Silesia in 2002 year]. Otolaryngologia Polska 2007;61:707–12. (Children aged ≥ 7 years or older.)
Glasziou P. Review: self report of hearing loss and the whispered voice test are useful for screening for hearing impairment. Evid Based Med 2006;11:116. (Systematic review, adolescents over 16 years old.)
Halloran DR, Hardin JM, Wall TC. Validity of pure-tone hearing screening at well-child visits. Arch Pediatr Adolesc Med 2009;163:158–63. (Participants’ age ranged from 3 to 19 years, no separate results for different age groups are reported.)
Kemper AR. Primary care hearing screening is of limited utility. J Pediatr 2009;155:448–9. (Summary of Halloran 2009.)
Khoza-Shangase K, Kassner L. Automated screening audiometry in the digital age: exploring Uhear and its use in a resource-stricken developing country. Int J Technol Assess Health Care 2013;29:42–7. (Children aged between 8 and 10 years.)
Kiese-Himmel C, Kruse E. [Who first suspects a hearing loss in infancy and childhood?] HNO 2005;53:810–16. (Not a diagnostic accuracy study, a survey of who and when first suspected hearing impairment.)
Liao W-H, Lien C-F, Young S-T. The hearing scale test for hearing screening of school-age children. Int J Pediatr Otorhinolaryngol 2010;74:760–4. (Children aged 9–10 years.)
Liu C, Xing G, Xu X, Chen Z, Zhou H, Wang D, et al. [The application of improved CHQS for mass epidemiology study on hearing impairment]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2010;24:19–20. (Participants aged ≥ 7 years, adults as well as children.)
Lo AH, McPherson B. Hearing screening for school children: utility of noise-cancelling headphones. BMC Ear, Nose and Throat Disord 2013;13:6. (Not a data study, compares performance of different earphones, children between 6 and 8 years old.)
Margolis RH, Glasberg BR, Creeke S, Moore BC. AMTAS: automated method for testing auditory sensitivity: validation studies. Int J Audiol 2010;49:185–94. (No 2 × 2 data provided for the screening test, only for the tool designed to identify poor quality audiograms.)
Munoz K, Caballero A, White K. Effectiveness of questionnaires for screening hearing of school-age children: a comprehensive literature review. Int J Audiol 2014;53:910–4. (Systematic review.)
Parving A, Sørup Sørensen M, Christensen B, Davis A. Evaluation of a hearing screener. Audiolog Med 2008;6:115–9. (Participants between 20 and 86 years old.)
Perez MC, Gaya JA, Savio G, Perera M, Ponce de Leon M, Sanchez M. A 25-year review of Cuba’s screening program for early detection of hearing loss. MEDICC review 2009;11:21–8. (Review paper.)
Sideris I, Glattke TJ. A comparison of two methods of hearing screening in the preschool population. J Commun Disord 2006;39:391–401. (Compares if PTA and TEOAE have the same referral rates and reasons for referral but no 2 x 2 data reported, children’s age ranged from 2 years and 1 month to 5 years and 10 months.)
Sliwa L, Hatzopoulos S, Kochanek K, Pilka A, Senderski A, Skarzynski PH. A comparison of audiometric and objective methods in hearing screening of school children. A preliminary study. Int J Pediatr Otorhinolaryngol 2011;75:483–8. (Children between 10.9 and 14.9 years old.)
Stearn N, Swanepoel de W. AJTCAM/African Networks on Ethnomedicines. Afr J Tradit Complement Altern Med 2006;4:205–10. (Participants between 15 and 19 years old.)
Swanepoel DW. Early detection of infant hearing loss in South Africa. S Afr Med J 2009;99:158–9. (Review paper.)
Szudek J, Ostevik A, Dziegielewski P, Robinson-Anagor J, Gomaa N, Hodgetts B, et al. Can Uhear me now? Validation of an iPod-based hearing loss screening test. J Otolaryngol Head Neck Surg 2012;41(Suppl. 1):S78–84. (Adult participants.)
Taylor EJ, Emanuel DC. Assessment of the efficacy of a hearing screening program for college students. J Am Acad Audiol 2013;24:607–15. (Adults between 18 and 49 years old.)
Walker AN, G. The role of the school entry hearing screen in identifying childhood hearing impairment in reception age children. [Only children with positive screen (unspecified) received the reference standard.]
List of criteria used in the methodological quality assessment
| 1. Was the spectrum of participants representative of the population who will receive the test in practice? The spectrum was considered representative if the study included children 4–6 (± 1) years old at or around school entry stage who had been tested and found to have no hearing impairment at birth. |
| 2. Were the selection criteria clearly described? |
| 3. Is the reference standard likely to correctly classify the target condition? The reference standard was considered able to correctly classify the target condition if it included a combination of PTA and tympanometry performed in a suitable setting (quite room with ambient noise monitored) by a qualified professional. |
| 4. Is the time period between reference standard and index test short enough to be reasonable? The time period between the performance of the index test and the reference standard was considered reasonable if it was < 4 weeks. |
| 5. Did the whole sample or a random selection of the sample receive verification using a reference standard? |
| 6. Did patients receive the same reference standard regardless of the index test result? |
| 7. Was the reference standard independent of the index test? (i.e. the index test did not form part of the reference standard) |
| 8. Was the execution of the index test described in sufficient detail to permit replication of the test? |
| 9. Was the execution of the reference standard described in sufficient detail to permit its replication? |
| 10. Were the index test results interpreted without knowledge of the results of the reference standard? |
| 11. Were the reference standard results interpreted without knowledge of the results of the index test? |
| 12. Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? Testers usually have no prior information about children’s hearing, so any information that may affect the interpretation of the index test was considered inappropriate. |
| 13. Were uninterpretable/intermediate test results reported? |
| 14. Were withdrawals explained? |
Appendix 3 Diagnostic accuracy study: information
Case record form – cases p. 140.
Case record form – controls p. 144.
Participant information sheet – cases p. 147.
Participant summary information sheet – cases p. 150.
Children’s pictorial information sheet – cases p. 152.
Participant information sheet – controls p. 153.
Participant summary information sheet – controls p. 156.
Children’s information sheet – controls p. 157.
Appendix 4 The relationship between the pure-tone screen and HearCheck screener results
| PTS test results | |||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | Total | ||
| Impaired (by reference standard) | |||||
| HC test results | Refer | 104 | 0 | 1 | 105 |
| Pass | 2 | 1 | 0 | 3 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 106 | 1 | 1 | 108 | |
| Hearing (by reference standard) | |||||
| HC test results | Refer | 34 | 26 | 0 | 60 |
| Pass | 45 | 340 | 0 | 385 | |
| Missing | 0 | 1 | 0 | 1 | |
| Total | 79 | 367 | 0 | 446 | |
| Missing (no reference standard) | |||||
| HC test results | Refer | 13 | 2 | 1 | 16 |
| Pass | 3 | 2 | 1 | 6 | |
| Missing | 2 | 0 | 4 | 6 | |
| Total | 18 | 4 | 6 | 28 | |
| PTS test results | |||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | Total | ||
| Impaired (by reference standard) | |||||
| HC test results | Refer | 32 | 2 | 0 | 34 |
| Pass | 8 | 6 | 0 | 14 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 40 | 8 | 0 | 48 | |
| Hearing (by reference standard) | |||||
| HC test results | Refer | 34 | 26 | 0 | 60 |
| Pass | 45 | 340 | 0 | 385 | |
| Missing | 0 | 1 | 0 | 1 | |
| Total | 79 | 367 | 0 | 446 | |
| Missing (no reference standard) | |||||
| HC test results | Refer | 13 | 2 | 1 | 16 |
| Pass | 3 | 2 | 1 | 6 | |
| Missing | 2 | 0 | 4 | 6 | |
| Total | 18 | 4 | 6 | 28 | |
| PTS test results | |||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | Total | ||
| Impaired (by reference standard) | |||||
| HC test results | Refer | 109 | 1 | 1 | 111 |
| Pass | 2 | 4 | 0 | 6 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 111 | 5 | 1 | 117 | |
| Hearing (by reference standard) | |||||
| HC test results | Refer | 61 | 27 | 0 | 88 |
| Pass | 53 | 343 | 0 | 396 | |
| Missing | 0 | 1 | 0 | 1 | |
| Total | 114 | 371 | 0 | 485 | |
| Missing (no reference standard) | |||||
| HC test results | Refer | 13 | 2 | 1 | 16 |
| Pass | 3 | 2 | 1 | 6 | |
| Missing | 2 | 0 | 4 | 6 | |
| Total | 18 | 4 | 6 | 28 | |
| PTS test results | |||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | Total | ||
| Impaired (by reference standard) | |||||
| HC test results | Refer | 89 | 0 | 1 | 90 |
| Pass | 1 | 1 | 0 | 2 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 90 | 1 | 1 | 92 | |
| Hearing (by reference standard) | |||||
| HC test results | Refer | 61 | 27 | 0 | 88 |
| Pass | 53 | 343 | 0 | 396 | |
| Missing | 0 | 1 | 0 | 1 | |
| Total | 114 | 371 | 0 | 485 | |
| Missing (no reference standard) | |||||
| HC test results | Refer | 13 | 2 | 1 | 16 |
| Pass | 3 | 2 | 1 | 6 | |
| Missing | 2 | 0 | 4 | 6 | |
| Total | 18 | 4 | 6 | 28 | |
| PTS test results | |||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | Total | ||
| Impaired (by reference standard) | |||||
| HC test results | Refer | 20 | 1 | 0 | 21 |
| Pass | 1 | 3 | 0 | 4 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 21 | 4 | 0 | 25 | |
| Hearing (by reference standard) | |||||
| HC test results | Refer | 61 | 27 | 0 | 88 |
| Pass | 53 | 343 | 0 | 396 | |
| Missing | 0 | 1 | 0 | 1 | |
| Total | 114 | 371 | 0 | 485 | |
| Missing (no reference standard) | |||||
| HC test results | Refer | 13 | 2 | 1 | 16 |
| Pass | 3 | 2 | 1 | 6 | |
| Missing | 2 | 0 | 4 | 6 | |
| Total | 18 | 4 | 6 | 28 | |
| PTS test results | |||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | Total | ||
| Impaired (by reference standard) | |||||
| HC test results | Refer | 59 | 0 | 0 | 59 |
| Pass | 0 | 1 | 0 | 1 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 59 | 1 | 0 | 60 | |
| Hearing (by reference standard) | |||||
| HC test results | Refer | 18 | 14 | 0 | 32 |
| Pass | 22 | 144 | 0 | 166 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 40 | 158 | 0 | 198 | |
| Missing (no reference standard) | |||||
| HC test results | Refer | 14 | 0 | 1 | 15 |
| Pass | 1 | 0 | 0 | 1 | |
| Missing | 1 | 0 | 3 | 4 | |
| Total | 16 | 0 | 4 | 20 | |
| PTS test results | |||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | Total | ||
| Impaired (by reference standard) | |||||
| HC test results | Refer | 26 | 1 | 0 | 27 |
| Pass | 8 | 2 | 0 | 10 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 34 | 3 | 0 | 37 | |
| Hearing (by reference standard) | |||||
| HC test results | Refer | 18 | 14 | 0 | 32 |
| Pass | 22 | 144 | 0 | 166 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 40 | 158 | 0 | 198 | |
| Missing (no reference standard) | |||||
| HC test results | Refer | 14 | 0 | 1 | 15 |
| Pass | 1 | 0 | 0 | 1 | |
| Missing | 1 | 0 | 3 | 4 | |
| Total | 16 | 0 | 4 | 20 | |
| PTS test results | |||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | Total | ||
| Impaired (by reference standard) | |||||
| HC test results | Refer | 70 | 0 | 0 | 70 |
| Pass | 3 | 2 | 0 | 5 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 73 | 2 | 0 | 75 | |
| Hearing (by reference standard) | |||||
| HC test results | Refer | 33 | 15 | 0 | 48 |
| Pass | 27 | 145 | 0 | 172 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 60 | 160 | 0 | 220 | |
| Missing (no reference standard) | |||||
| HC test results | Refer | 14 | 0 | 1 | 15 |
| Pass | 1 | 0 | 0 | 1 | |
| Missing | 1 | 0 | 3 | 4 | |
| Total | 16 | 0 | 4 | 20 | |
| PTS test results | |||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | Total | ||
| Impaired (by reference standard) | |||||
| HC test results | Refer | 53 | 0 | 0 | 53 |
| Pass | 0 | 1 | 0 | 1 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 53 | 1 | 0 | 54 | |
| Hearing (by reference standard) | |||||
| HC test results | Refer | 33 | 15 | 0 | 48 |
| Pass | 27 | 145 | 0 | 172 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 60 | 160 | 0 | 220 | |
| Missing (no reference standard) | |||||
| HC test results | Refer | 14 | 0 | 1 | 15 |
| Pass | 1 | 0 | 0 | 1 | |
| Missing | 1 | 0 | 3 | 4 | |
| Total | 16 | 0 | 4 | 20 | |
| PTS test results | |||||
|---|---|---|---|---|---|
| Refer | Pass | Missing | Total | ||
| Impaired (by reference standard) | |||||
| HC test results | Refer | 17 | 0 | 0 | 17 |
| Pass | 3 | 1 | 0 | 4 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 20 | 1 | 0 | 21 | |
| Hearing (by reference standard) | |||||
| HC test results | Refer | 33 | 15 | 0 | 48 |
| Pass | 27 | 145 | 0 | 172 | |
| Missing | 0 | 0 | 0 | 0 | |
| Total | 60 | 160 | 0 | 220 | |
| Missing (no reference standard) | |||||
| HC test results | Refer | 14 | 0 | 1 | 15 |
| Pass | 1 | 0 | 0 | 1 | |
| Missing | 1 | 0 | 3 | 4 | |
| Total | 16 | 0 | 4 | 20 | |
Appendix 5 Parent questionnaire
Appendix 6 Practical implementation of screening tests for hearing in schools: information
Participant information sheet
Case record form
List of abbreviations
- AABR
- automated auditory brainstem response
- CCS
- Cambridgeshire Community Services
- CHAC
- Children’s Hearing Assessment Centre
- CHQS
- Chinese Hearing Questionnaire for School Children
- CI
- confidence interval
- CRF
- case report form
- DEA
- diagnostic evaluation with an audiologist
- ENT
- ear, nose and throat
- GP
- general practitioner
- HC
- HearCheck
- HI
- hearing impaired
- HL
- hearing level
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ID
- identifier
- IQR
- interquartile range
- NHBRU
- Nottingham Hearing Biomedical Research Unit
- NHI
- not hearing impaired
- NHSP
- Newborn Hearing Screening Programme
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OAE
- otoacoustic emission
- OME
- otitis media with effusion
- PenCTU
- Peninsula Clinical Trials Unit
- PSA
- probabilistic sensitivity analysis
- PTA
- pure-tone audiometry
- PTS
- pure-tone screen
- QALY
- quality-adjusted life-year
- QUADAS
- quality assessment of diagnostic accuracy studies
- ROC
- receiver operating characteristic
- SD
- standard deviation
- SES
- school entry screen(ing)
- SNPC
- sensorineural or permanent conductive
- TEOAE
- transient-evoked otoacoustic emission
- UNHS
- Universal Newborn Hearing Screening
- WTE
- whole-time equivalent