Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/178/01. The contractual start date was in March 2014. The draft report began editorial review in March 2015 and was accepted for publication in September 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Aileen Clarke is Professor of Public Health and Health Services Research, Warwick Medical School, University of Warwick, UK, and the Warwick Medical School receive payment for this work. Aileen Clarke is a member of the National Institute for Health Research (NIHR) Health Technology Assessment and Efficacy and Mechanism Evaluation editorial board. Ngianga-Bakwin Kandala and Aileen Clarke are also supported by the NIHR Collaboration for Leadership in Applied Health Research and Care West Midlands at University Hospitals Birmingham NHS Foundation Trust.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Auguste et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Overview
Tuberculosis (TB) is a major cause of morbidity and mortality globally. Nearly one-third of the world’s population is infected with Mycobacterium tuberculosis (MTB) [(Zopf 1883) Lehmann and Neumann 1896]; TB has an annual incidence of 9 million new cases and each year causes 2 million deaths annually worldwide. TB ranks as the second leading cause of death from an infectious disease. 1–3
In the UK, the prevalence of TB steadily decreased until the mid-1980s but has started to rise over the last 20 years, especially in ethnic minorities born in places with a high TB prevalence. 4,5 Between 1998 and 2009, annual TB notifications in the UK rose by 44%, from 6167 to 8900 cases. 4,6 Since 2005, this rate has remained high, leading to projections that in 2 years there will be more TB cases in the UK than in the USA,7 thereby posing a major public health challenge. The re-emergence has been largely driven by recently arriving immigrants in whom latent infection has been reactivated or who have acquired new infection as a result of their maintaining links with high-prevalence countries.
Aetiology and pathology of tuberculosis
Tuberculosis infection is transmitted to a healthy person through the air by inhaling respiratory fluids/sputum droplets containing MTB discharged by a person with active TB. The infected sputum droplets can dry and form into droplet nuclei, which can float in the air for a long period of time and penetrate the host. 8 TB can be transmitted through other routes including ingestion (e.g. from drinking unpasteurised cow’s milk)9 and inoculation (e.g. Prosector’s wart), although such cases are rare in the UK.
Once the bacteria are inhaled, the droplet nuclei travel through the mouth or nasal passages to the upper respiratory tract, bronchi and finally the alveoli of the lungs. The bacteria grow slowly and multiply in the alveoli over several weeks. Sometimes a small number of tubercle bacilli enter the bloodstream and spread throughout the body such as to the bones, lymph nodes or brain. 8 In > 80% of cases the immune system kills and removes the bacteria from the body. 10 If the immune system does not kill the bacteria, macrophages within the immune system ingest and surround the tubercle bacilli within 2–8 weeks. The cells form a barrier shell that keeps the bacteria suppressed and under control, resulting in latent tuberculosis infection (LTBI). Individuals with LTBI do not exhibit any clinical, radiological or bacteriological evidence of the pathogen. They are not infectious and may remain asymptomatic. 11 However, the latent infection may reactivate later in life, causing the individual to develop symptoms and become infectious. It has been estimated that people with LTBI are at 5–10% risk for developing active TB during their lifetime. 12,13 Therefore, this large pool of LTBI is an important reservoir of infection. 8,12
If the immune system cannot keep the bacteria suppressed or the barrier fails later, the bacilli begin to multiply and the individual develops active TB disease. Individuals who have active TB are infectious and each can spread MTB to up to 10–15 close contacts within a year. 14 The pathogen affects primarily the lungs (pulmonary TB) but can also involve other organs of the body (extrapulmonary TB). In the UK in 2012, pulmonary TB accounted for about 53% of all TB cases. 5
The period between infection and first signs of illness (incubation period) varies between 8 weeks and decades. The greatest chance of progressing to disease is within the first 2 years after infection, when approximately 50% of the 5–10% lifetime risk occurs. 15 The risk of infection and progression to active TB disease depends mostly on the host’s immune function as well as on the duration and proximity of exposure to a source afflicted with active MTB. 16 Therefore, certain population groups have a higher lifetime risk of developing TB. These vulnerable groups with low immunity and/or high exposure include long-term care facility workers, people born in or coming from countries with a high prevalence of TB, infants, children, those infected with human immunodeficiency virus (HIV), people with close contacts suspected of having active TB or those living in confined facilities (e.g. prison, homeless shelters). 5 These groups are particularly important as a reservoir of latent infection that could reactivate, and explain the trends observed for TB in the UK. 17
Active tuberculosis
When infection with MTB becomes active TB disease, the symptoms that occur are non-specific and depend on the site of TB infection. 18,19 Common signs and symptoms of active pulmonary TB may include a chronic cough for weeks or months accompanied by the coughing up of blood or blood-stricken mucus, pain in the chest, weight loss, intermittent fever and/or night sweats, poor appetite, chills, weakness or fatigue, and listlessness. 1,18,20 The clinical diagnosis of TB is based on TB-characteristic clinical signs and symptoms, chest radiography and microscopy of tissue biopsy or sputum samples. A definitive diagnosis of TB, however, is made through the identification of MTB in clinical samples (e.g. pus, tissue biopsy, sputum) using culture. 21,22 TB is difficult to culture and it takes several weeks to obtain a definitive result.
Tuberculosis is a curable disease; however, treatment is long and requires adherence, even through the side effects of treatment. 23 In the UK, most MTB infections are sensitive to the antibiotics used. 10 The routine management of active pulmonary TB includes a combination of antibiotics (e.g. isoniazid, rifampicin, pyrazinamide and ethambutol) given over 6 months. 18 Although patients start to feel better after 2 months of treatment and are not infectious any longer, it is vital that they complete their treatment. 24,25 This ensures that the TB bacteria are completely killed off, preventing the return of symptoms and the risk of bacteria becoming drug resistant. Treatment of drug-resistant forms of TB is less effective, requires longer than 6 months and causes greater side effects. 10,26
Measurement of latent tuberculosis infection
Unfortunately, there is no diagnostic gold standard for the identification of individuals with LTBI. Instead, the available screening tests for LTBI provide an indirect assessment of the presence of LTBI by relying on a host’s immunological response to TB antigens. 27 In addition, none of the available LTBI tests can accurately differentiate between people with LTBI and people with active TB. 11
There are two types of commercially available tests used to identify LTBI in the UK: (1) the tuberculin skin test (TST) and (2) the interferon gamma (IFN-γ) release assays (IGRAs). 5 Until recently, the TST (introduced by Mantoux in 1907) has been the only standard test used for the identification of LTBI. 13 The administration of the TST involves an intradermal injection of purified protein derivative (PPD) in the forearm. The immune response (i.e. delayed hypersensitivity caused by T cells) to the TST is determined 48–72 hours after the injection by measuring the transverse diameter (in mm) of skin induration. 13,16 There is no international agreement on cut-off values for the definition of a positive tuberculin reaction. 12 The choice among commonly used cut-off values (e.g. a diameter of induration of ≥ 5 mm, ≥ 10 mm or ≥ 15 mm) depends on an individual’s risk factor profile for TB. Usually, a lower cut-off value of ≥ 5 mm is used for individuals at higher risk of TB (e.g. patients with organ transplants, immunocompromised patients, patients with HIV infection and those who have had recent contacts with an active TB patient) and a higher cut-off value of ≥ 10 mm is applied for individuals at lower risk of TB (e.g. high-risk racial minorities, children, recently arrived immigrants from high-prevalence countries and patients with diabetes, malignancies or renal failure). 16 The administration of the TST is relatively cheap and does not require a laboratory, but it does require a skilled operator.
Interferon gamma release assays have been recently developed as alternative screening tests for LTBI. There are two types of IGRA: QuantiFERON®-TB Gold-in-Tube (QFT-GIT) [old version: QuantiFERON®-TB Gold (QFT-G)] (Cellestis/Qiagen, Carnegie, Australia) and T-SPOT. TB (Oxford Immunotec, Abingdon, UK). Both tests are commercially available in the UK. The QFT test is a whole-blood test based on an enzyme-linked immunosorbent assay whereas the T-SPOT. TB test uses peripheral blood mononuclear cells and is based on an enzyme-linked immunospot (ELISPOT) assay. 11 Both tests measure the cluster of differentiation 4 (CD4) cell-released IFN-γ response to MTB-specific antigens [early secretion antigen target 6 (ESAT-6), culture filtrate protein 10 (CFP-10) and tb7.7] in in vitro blood samples. 12,13,16
Treatment of latent tuberculosis infection
The aim of LTBI treatment is to prevent MTB bacteria from developing into active TB disease. Before treatment, all individuals found to have LTBI need to be tested for active TB. For individuals in whom active TB is ruled out, the prophylactic treatment of choice is isoniazid. For adults and children, the treatment should be given for between 3 and 6 months depending on the treatment regime. For individuals affected by HIV, treatment is given for 6 months. Rifampicin given for 4 months is the second-line treatment that can be used as an alternative in individuals who are resistant to isoniazid or at high risk of side effects from isoniazid. 16
Incidence, prevalence and epidemiology
All forms of active TB are legally notifiable by the physician making or suspecting the diagnosis under the Public Health (Control of Disease) Act 198428 in England and Wales. It first became a statutory requirement to notify TB cases in 1913. Known as the Notifications of Infectious Diseases system, it continues to play a valuable role in the surveillance of TB; however, the information collected is limited and trends within subgroups of the population cannot be monitored. 29
In 1999, the Enhanced Tuberculosis Surveillance system was established to collect more detailed information on annual TB cases, including patient age, sex, ethnic group, country of birth, site of disease, NHS region and treatment outcomes. It has been reported that the Enhanced Tuberculosis Surveillance system reflects the true incidence of TB better than the Notifications of Infectious Diseases system as many measures are used to ensure that quality standards are met annually, thereby providing a corrected analysis of TB cases. 30 In 2012, completeness of data was 100% for mandatory fields and approximately 91% across other key fields for England and 89% for Wales. 5 This system provides the most comprehensive, timely and accurate information on active TB incidence in the UK29 and is therefore robust.
There is no national system that collects data for LTBI. For this reason there are no robust data for LTBI, although we can predict that for every person with active TB there are likely to be several with undiagnosed LTBI. Therefore, it seems reasonable to extrapolate from active TB and make the assumption that LTBI will follow a similar epidemiological pattern.
The rates of active TB peaked during the early 1900s with an annual incidence rate of approximately 320 per 100,000. The rate declined dramatically until at least 1987 to as low as 10.1 per 100,000 population per year. However, since the 1980s, the incidence rate began reversing and has reached highs of between 13.6 and 14.4 per 100,000 since 2005. 5 The most recent figures in 2012 report a total of 8751 active TB cases across the UK, giving an incidence rate of 13.9 per 100,000. 5 The burden of TB is highest in England, where in 2012 there were 8130 cases of active TB, a rate of 15.2 per 100,000; in Wales there were 136 active TB cases, a rate of 4.4 per 100,000. 5 Between 2010 and 2011, a total of 436 people died of TB in the UK. 5
Place of birth and ethnic minorities
The re-emergence of TB has been attributed to international migration, as recently arriving migrants have accounted for the majority of TB cases since 2000. In 2011 and 2012, foreign-born individuals accounted for 73% of reported TB cases. 5 It has been reported that there has been a 98% increase in the number of TB cases in individuals born overseas. 4,6,31 The rate of TB among the non-UK-born population is 80 per 100,000, which is almost 20 times the rate in the UK-born population. Almost half of the patients born outside the UK were diagnosed within 5 years of coming to the UK, with another 30% diagnosed within 2 years. 5 In total, 60% of foreign-born patients originated from South Asia, followed by 22% from sub-Saharan Africa. With respect to country of origin of foreign-born patients, the highest proportions are from India (31%), Pakistan (18%) and Somalia (6%). Similarly, a higher proportion of non-UK-born patients (> 50%) than UK-born patients (31%) present with extrapulmonary TB. 32
Among UK-born individuals, the highest rate of TB is found in ethnic minority groups. The largest proportions of cases are found in those of Indian (27%, 2296/8525), white (21%, 1814/8525) and Pakistani (17%, 1418/8525) ethnic origin. The highest rates of TB per 100,000 population are found in Indian, Pakistani and black ethnic groups (155, 132 and 97 per 100,000, respectively). 5 It has been indicated that recently arriving immigrants and ethnic minorities are vulnerable as a result of reactivation of latent infection once in the country or acquiring new infection as a result of their maintaining links with high-prevalence countries (e.g. they may visit rural Pakistan or they may have relatives from high-prevalence areas visit them). 33 In addition, having diabetes increases the likelihood of reactivation of TB, and diabetes is more common in individuals from South-East Asia, including the ethnic groups highlighted above. 34
Geographical difference
Since the establishment of the enhanced TB surveillance system, it has become clear that there is a drastic regional variation in the burden of TB. Active TB is highly concentrated in large cities, with London consistently accounting for the highest rates and sharpest increases since the early 1990s. In 2012, London accounted for almost 40% of all TB cases, with an annual rate of 41.8 per 100,000. London has the highest TB rate among all high-income European countries. 35,36 London is followed by the West Midlands, which accounts for 12% of the burden and has an annual rate of 19.3 per 100,000. 5 Both London and the West Midlands have high rates of immigration. 37
Within London there is great variation between boroughs. Twelve of the 33 local authorities have an annual incidence rate of 40 per 100,000. The boroughs with the highest annual incidence rates of TB are Newham (122 per 100,000) and Brent (100 per 100,000). However, other boroughs, such as Havering and Richmond-upon-Thames, have an annual incidence rate of < 10 per 100,000. 38 Similar to regional variation, borough variation within London may reflect demographic characteristics as Newham and Brent have some of the highest rates of immigrants and ethnic minorities. 39
A similar picture is seen in Birmingham. Annual incidence rates for Birmingham as a whole fluctuated between 33.7 and 44.8 cases per 100,000 between 2009 and 2013. In the fourth quarter of 2013, Sandwell and West Birmingham Clinical Commissioning Group had an annual incidence rate of 49.6 per 100,000 [95% confidence interval (CI) 43.5 per 100,000 to 56.4 per 100,000] whereas in Solihull it was 1.9 per 100,000 (95% CI 0.5 per 100,000 to 4.9 per 100,000). Again, this reflects the ethnic make-up of the areas [Helen Bagnall, Public Health England (PHE), West Midlands, May 2014, personal communication].
Age differences
The majority of patients with TB (60%) are aged between 15 and 44 years, followed by patients aged 45–64 years (21%) and patients aged ≥ 65 years (14%). The groups with the lowest rates of TB are those aged 5–14 years (3%) and those aged < 5 years (2%). Although children have a low burden of overall TB cases, once TB is transmitted to them they are more likely than adult hosts to develop active TB. Most cases in those aged 0–14 years are in the UK-born population from black African, Pakistani and white ethnic groups. 5
Immunosuppression and tuberculosis
In addition to young children, the risk of progression from LTBI to active TB is higher in people coinfected with HIV, patients immunocompromised because of comorbidity (e.g. diabetes, malignancy, renal disease) and/or people with long-term use of immunosuppressant medications [e.g. corticosteroids, tumour necrosis factor alpha (TNF-α) antagonists]. 11,16,40 Coinfection with HIV and TB has been internationally well documented. 41–43 In the UK there has been a decrease in the number of coinfected HIV–TB cases, from 9% of TB cases in 2003/4 to 3.6% of TB cases in 2013. 5 This has been in line with the general downward trends in HIV and TB in migrants from sub-Saharan Africa. 32
Social risk factors
There are defined social factors that contribute to the burden of TB in the UK. These social risk factors include homelessness (2.4%), a history of imprisonment (2.8%), and drug (2.8%) and alcohol (3.2%) misuse. 5 It is indicated that approximately 7.7% of TB cases present with at least one of these risk factors. These social risk factors are more common in UK-born (13.4%) than foreign-born (5.4%) cases. Within UK-born cases, almost half with at least one risk factor (46%) are from the white ethnic group. 5
Impact of the health problem
Significance for patients
For the 5–10% of patients who develop active TB, those with pulmonary TB can suffer extreme pain from the symptoms for weeks to months. 44 Similarly, extrapulmonary TB can result in serious complications for the bones, brain, liver, kidneys and heart. 44 Tissue damage can be permanent if TB is not treated early. 45 As a result of tissue damage, active TB can be fatal. In addition to the impact on physical functioning, active TB can also have psychosocial impacts, in particular from the isolation experienced during the treatment of TB. This can include anxiety, depression, disorientation, feelings of loss of control and mood swings. 46,47 A diagnosis of TB can also bring related stigma through which individuals face social and economic consequences. 48
Treatment of active TB causes many side effects depending on the regimen prescribed. Some symptoms are mild but other side effects can be serious and potentially life-threatening. These can include loss of appetite, nausea, vomiting, jaundice, fever, abdominal pain, lower chest pain or heartburn, skin rash, bleeding gums and nose, blurred vision, ringing sounds, hearing loss, peripheral neuropathy and hepatotoxicity. 16 Individuals on antiretroviral treatment for HIV infection may suffer more side effects with certain TB drugs. These side effects cause poor adherence to treatment. If treatment is incomplete, active TB is more likely to be complex and drug-resistant and result in the need for treatments with greater side effects. 16,49 To avoid the consequences of the disease and the side effects of treatment, it would be easier for patients to undergo LTBI treatment and prevent active disease.
However, the treatment of LTBI uses the same medication, with the same side effects, albeit usually for a shorter period of time. Adherence to treatment is likely to be a factor as taking medicines when you feel well is much harder than taking them when you feel unwell.
Significance for the NHS
The impact of TB as a health problem is extensive. As TB possesses the capacity to spread through the air to practically anyone it is a serious public health threat, although, in practice, infection beyond family members or close contacts is unusual. TB is on the increase in the UK and is decreasing in the USA. It has been estimated that in 2–5 years the burden of TB in the UK will be higher than that in the whole of the USA. 7 Furthermore, drug-resistant TB is increasing in the UK, which means that transmission of drug-resistant strains of TB may continue to increase and complicate the fight against TB.
The health-care costs associated with active TB include the costs of diagnosing and treating pulmonary TB, extrapulmonary TB, multidrug-resistant TB and extensively drug-resistant TB. In the UK, the normal cost of treating a case of active TB is £5000 but the cost of treating multidrug-resistant TB is between £50,000 and £70,000 and the cost of treating extensively drug-resistant TB can be up to £100,000. 35,50 Using 2012 figures, we have estimated that annually TB treatment could cost approximately £50M. Given that LTBI represents a reservoir of potential TB disease, it is important to identify and, if appropriate, treat people with LTBI to reduce the spread and burden of TB disease. 13,18
Current service provision
Management of latent tuberculosis infection
The goal of screening for LTBI is to identify individuals who are at high risk of developing active TB who would potentially benefit from prophylactic treatment. In the UK, LTBI screening is recommended for contacts of patients diagnosed with active TB and recently arrived migrants. Contacts include household contacts defined as those who share a bedroom, kitchen, bathroom or sitting room with the index active TB case, as well as boyfriends or girlfriends and frequent visitors to the home. Workplace associates in close proximity to a patient for extended periods may be judged to be household contacts; however, the majority of workplace contacts are not screened. Casual contacts should be assessed only if the index case is particularly infectious or the contact case is at increased risk from infection. Nevertheless, all contacts should be offered information and advice about TB. Similar risk assessments take place in schools, nurseries, institutions such as prisons and hospitals, and for aircraft passengers, leading to screening of those perceived to be at risk. 10,51
Active case finding is recommended for migrants who have recently arrived in the UK from countries with a TB incidence of ≥ 40 per 100,000. 10 Identification of new migrants is recommended from port of arrival reports, new registrations with primary care, entry to education and links with statutory or voluntary groups working with new migrants. Health-care professionals responsible for new migrant screening are advised to co-ordinate a programme to detect and treat active and latent TB, provide the bacillus Calmette–Guérin (BCG) vaccination when appropriate and provide relevant referrals and information. Commissioners, NHS employees and providers of TB services, and other statutory and voluntary organisations, are particularly advised to identify and manage TB in hard-to-reach groups such as the homeless, substance misusers, prisoners and vulnerable migrants. 52
A simplified care pathway for LTBI screening derived from the National Collaborating Centre for Chronic Conditions10,51 is presented in Figure 1 and further details about testing strategies for people being screened for LTBI are provided in Box 1.
-
Generally, individuals are tested for LTBI using TST (Mantoux), IGRA, both or a dual strategy of TST followed by IGRA. If the results are positive, individuals are assessed for active TB; if the results are positive they are treated for active TB and if they are negative they are then treated for LTBI. If the results for LTBI are negative the individual is offered a BCG vaccination if aged < 16 years or aged 16–35 years and from sub-Saharan Africa or from an area with an incidence of > 500 per 100,000. Individuals are given information and advice about TB. However, different testing and treatment pathways are recommended for different populations, including different age groups, new migrants and immunocompromised individuals. 10,51
-
TST is recommended for contacts aged > 5 years for the diagnosis of LTBI. IGRA is recommended for individuals whose TST shows positive results (≥ 5 mm diameter for those who have not been vaccinated with BCG and ≥ 15 mm diameter for those who have been vaccinated) or in people for whom TST would be less reliable, such as BCG-vaccinated people. Individuals with a positive IGRA or inconclusive TST are referred to specialist TB care. For contacts who are aged 2–5 years, a TST should be offered as the initial diagnostic test and, if the result if positive, taking BCG history into account, they should be referred to a TB specialist to exclude the possibility of active disease and to determine treatment, depending on the result. If the result of the TST is negative but the child is a contact of a person with sputum smear-positive disease, then an IGRA should be offered after 6 weeks alongside a repeat TST to increase sensitivity. 10,51
-
For child contacts of a person with sputum smear-positive disease aged 4 weeks to 2 years who have not been vaccinated, isoniazid should be started and a TST should be performed. If the TST is reported as positive, the child should be assessed for active TB and if active TB is excluded the child should then be offered full treatment for LTBI. If the TST is negative (< 5-mm induration), isoniazid should be continued for 6 weeks after which a repeat TST and IGRA should be performed. If repeat tests are negative, isoniazid should be stopped and BCG offered, whereas if either is positive active TB should be assessed and, if excluded, treatment for LTBI should be considered. For child contacts of a person with sputum smear-positive disease aged 4 weeks to 2 years who have been vaccinated, a TST should be performed and any positive results (≥ 15 mm) should be assessed for active TB. If active TB is excluded then a regimen of either 3 months of rifampicin and isoniazid or 6 months of isoniazid should be given. If the TST is negative (< 15 mm) it should be performed again with an IGRA after 6 weeks. If both tests are negative no further action is needed. If either is positive, active TB has to be excluded and treatment for LTBI followed. 10,51
-
To diagnose LTBI in recently arriving migrants from high-incidence countries, for children aged 5–15 years a TST should be offered and if positive an IGRA should be performed. For individuals aged 16–35 years, either an IGRA alone or in a dual strategy with a TST should be offered. For those aged > 35 years, the individual risks and benefits of treatment should be considered before testing. For children aged < 5 years, a TST should be offered and if the initial test is positive taking BCG history into account then active TB disease should be excluded and LTBI treatment considered. 10,51
-
Regarding those who are immunocompromised, children should be referred to a TB specialist. For people with HIV infection and a CD4 count of < 200 cells/mm3 or between 200 and 500 cells/mm3, an IGRA should be offered with a concurrent TST. If either is positive, active TB should be ruled out before LTBI treatment is given. For other people who are immunocompromised, an IGRA should be offered alone or with a TST. 10,51
-
Once active TB has been excluded by chest radiography and examination, individuals should be offered treatment. Individuals aged ≥ 35 years without HIV infection should be assessed further and counselled about treatment because of the increasing risk of hepatotoxicity from medication. For those aged 16–35 years and not known to have HIV infection, treatment should include either 6 months of isoniazid or 3 months of rifampicin and isoniazid. 10,51
-
Neonates who have been in close contact with people who have sputum smear-positive TB and who have not received at least 2 weeks of antiTB drug treatment should be started on isoniazid for 3 months and then a TST performed after 3 months of treatment. If the TST is positive, active TB should be assessed and, if found negative for active TB, isoniazid should be continued for a total of 6 months. If the TST is negative it should be repeated with an IGRA and if both are negative isoniazid should be stopped and BCG vaccination performed. In children aged > 2 years, 3 months of rifampicin and isoniazid or 6 months of isoniazid should be given.
Current service cost
Estimates for the costs of diagnosing and treating LTBI have been provided by the National Institute for Health and Care Excellence (NICE) (Table 1). These costs are based on NICE guidelines from 200651 and the partial update from 2011. 10 The costs shown include the unit costs of the disposables, the time to administer and read tests and the costs of collecting a blood sample per patient for the tests, calculated in 2011. The cost of chemoprophylaxis includes the cost of drugs, active TB tests, consultations and nurse visits, which were calculated in 2006. BCG costs are also from 2006. Compared with the cost of treating active TB (≥ £5000), diagnosing and treating LTBI per patient is less costly.
| Description | Test type | Unit cost (£) |
|---|---|---|
| Cost of TST | – | 16.42 |
| Cost of IGRA | – | 30.34 |
| Household and other close contacts aged ≥ 5 years | TST | 16.42 |
| New entrants from high-incidence countries | ||
| Children aged < 5 years | TST | 16.42 |
| Children aged 5–15 years | TST | 16.42 |
| Adults aged 16–34 years | IGRA or dual | 30.34 or 46.76 |
| People aged > 35 years | Consider individual risk | |
| Household contacts aged 2–5 years | TST | 16.42 |
| IGRA if contact with a sputum smear-positive person and TST is negative | 30.34 | |
| Contacts aged ≥ 5 years – outbreak | IGRA | 30.34 |
| Immunocompromised HIV CD4 count < 200 cells/mm3 | IGRA with concurrent TST | 46.76 |
| Immunocompromised HIV CD4 count 200–500 cells/mm3 | IGRA test or | 30.34 |
| IGRA with concurrent TST | 46.76 | |
| Cost of complete chemoprophylaxis treatment | – | 483.74 |
| BCG vaccination | – | 11.71 |
Variation in services and/or uncertainty about best practice
Limitations of latent tuberculosis infection screening tests
The main limitation of the TST is its inability to distinguish between reactions caused by MTB and those caused by BCG vaccination or non-tuberculous mycobacteria (NTM). 11 The BCG vaccination is routinely used in countries with a high TB prevalence to prevent the spread of TB infection in infants and young children. The use of the TST test in such areas results in high false-positive rates. The boosting phenomenon, which occurs after repeated TSTs, may also lead to false positives, thereby limiting the specificity of the test. The TST has limited sensitivity when used in certain subpopulations (e.g. people with active TB, immunocompromised patients, the elderly and people with HIV infection, malnutrition or renal failure). The above-mentioned limitations are compounded by issues related to the interpretation of test results, which may independently influence false-positive and false-negative rates of the TST (e.g. different cut-off values, PPD dose). 12,13,16 Two health visits are required for the completion of the TST, which results in missed diagnoses in 10% of cases. 53 Measurement of the TST is also dependent on interobserver variability, which therefore requires adequate training to reduce variability. 54,55
Because the antigens in the IGRA tests are not present in the BCG vaccination and most NTM, the IGRAs are less influenced by previous BCG vaccinations and are less susceptible to false-positive NTM reactions, leading to higher specificity of these tests compared with the TST. 56 IGRAs also have the advantage of requiring a single patient visit rather than the sequential two-step testing required with the TST. Automated testing also means increasing the objectivity in the interpretation of test results. Finally, there is no influence from the boosting effect and so repeat screening is feasible. 57 The IGRAs, however, have their own limitations: specifically, they are more costly and labour intensive than the TST. Moreover, care in blood sampling is required and the time available for blood sample storage and analysis is restricted to 8–12 hours after collection. 12
Diagnostic accuracy of latent tuberculosis infection tests
Since the introduction of IGRAs evidence on estimating and comparing the performance of the TST and IGRAs in people with LTBI has emerged; however, this assessment has been hampered by the absence of a gold standard for the diagnosis of LTBI, which would allow direct calculation of sensitivity and specificity for both types of tests. 11,12,18,40,57–59 Most studies have, instead, determined associations [e.g. diagnostic odds ratios (DORs) and other regression-based effect measures] between test results (i.e. TST or IGRAs) and surrogate measures of LTBI such as duration/proximity of exposure to a person with active TB or risk of development of active TB, or progression from LTBI to active TB [e.g. sensitivity, DORs, positive predictive values (PPVs) and negative predictive values (NPVs), incidence rate ratios, cumulative incidence ratios (CIRs)]. 18,58,60 Some studies have assessed and compared the specificity of these tests in people at very low risk for MTB infection (e.g. healthy individuals, residents of low-incidence countries)57 or compared sensitivity in culture-confirmed individuals with active TB (taken as a surrogate reference standard for LTBI). 40,57,59 Using suboptimal reference standards for diagnostic accuracy testing can lead to an overestimation or underestimation of the true accuracy of a test. The degree of concordance (inter-rater or intrarater agreement, kappa statistic) and discordance between the results of the two tests (IGRAs and TST) has also been used. In general, both pooled sensitivity and specificity values of the IGRAs and the TST were similarly high in people who were not vaccinated with BCG (> 90%); however, the pooled specificity of the TST in BCG-vaccinated populations was much lower than that of IGRAs (about 56% vs. 96%). 11,53,57 In contrast, prospective longitudinal studies showed that neither the IGRAs nor the TST had a high prognostic value in predicting the risk of progression to active TB. 11,18
Treatment of latent tuberculosis infection
Once patients are diagnosed with LTBI using any of the available tests, there are claims of low adherence to chemotherapy treatment. 61 As a result of low adherence, an alternative therapy recommended in the USA62 has been implemented in some hospitals in the UK. It includes a new combination of isoniazid and a long-acting rifampicin called rifapentine given weekly for 12 weeks. Each of the 12 doses is directly observed being taken by a treatment supervisor. After LTBI is confirmed and active TB excluded, individuals are assessed for suitability for the rifapentine/isoniazid regimen. 61 Suitability is based on certain criteria including normal renal and liver function, aged ≥ 16 years, not pregnant, HIV-infected patients not on antiretroviral treatment, agreeable to direct observations and direct observations are feasible. If suitable, the regimen is prescribed and a TB specialist nurse sets up the direct observations. If it is not suitable other latent TB treatment is offered. This combination has been found to be as effective as the 9-month daily isoniazid regime used in the USA, with higher completion rates as only 12 doses are needed. 61
Relevant national guidelines including National Service Frameworks
The latest guidelines on the diagnosis, management and prevention of TB are available from NICE. There is a 2006 clinical guideline51 on the clinical diagnosis and management of TB and measures for its prevention and control, with a partial update in 2011,10 as well as 2012 public health guidance52 to identify and manage TB among hard-to-reach groups. The Department of Health has also published guidelines for the planning, commissioning and delivery of TB services,63 guidelines for testing health-care workers,64 a wider action plan for stopping TB in England65 and guidance for the prevention and control of HIV-related and drug-resistant TB. 66 Finally, the British Thoracic Society has published guidelines on the prevention, risk assessment and management of TB in adult patients with chronic kidney disease67 and in patients due to start antiTNF-α treatment,68 management of air travel passengers69 and management of opportunist mycobacterial infections. 70
Description of the technology under assessment
Summary of the intervention
As noted earlier, screening for LTBI is crucial to curb the re-emergence of TB as the majority of TB cases consist of latent TB that has been reactivated. 71 Testing and treating high-risk individuals for LTBI would not only prevent active TB illness for the individual but also would reduce the transmission of TB, thus reducing the pool of infection. 72
There is much interest in using IGRAs to identify individuals at high risk of LTBI because of the advantages that they have over the traditional TST, particularly that they require only one visit and that previous BCG status does not interfere with the results. For IGRAs to replace the TST in the current care pathway, they would have to show improved cost-effectiveness relative to the TST, although in the absence of a gold standard this is difficult. 73 Otherwise, IGRAs may have to be used as complementary tests to the TST, as is currently recommended in the national guidelines. 10
The results of an IGRA test depend on local arrangements but can be available within 1 week. 74 The TST takes 2–3 days as individuals must return to have the test read. 13,16 In combination, therefore, both tests take several days to be completed. IGRA testing comes at a higher cost than the TST and shifts the costs and labour from the clinic to the laboratory. 75 Both the TST and IGRAs require specific equipment either for administering the injection or taking a blood sample. In addition, IGRA testing requires advanced laboratory facilities. 75 Skilled personnel are needed to administer both tests and, in the case of the TST, are needed to read the result, whereas for IGRA testing laboratory personnel are needed to process the result. 73 In both cases patients follow a common pathway, with nurses providing patients with the result, following up patients for testing of active TB and offering treatment and advice. 10 IGRAs can be used in similar settings to the TST as long as there is access to a laboratory and pathways are negotiated so that samples can be analysed within 12 hours. 46
Screening tests for latent tuberculosis infection in special subgroups at risk
It has been suggested that screening tests applied to presumably healthy populations or those at low risk for progression to active TB may not be justified given the potential harms from unnecessary treatment. 16,76 It is also not feasible or cost-effective to universally screen the population as the administrative and clinical costs outweigh the benefits of identifying TB cases. 46 The benefits of screening for LTBI using these tests are likely to be maximal in individuals at high risk of contracting MTB (e.g. those recently arrived from countries with a high TB incidence, close contacts of those with active TB) and those with suspected LTBI who are at high risk of progression to active TB and complications associated with the infection (e.g. immunocompromised patients, young children). As these subgroups are at higher risk of developing active TB, it is of public health importance to identify LTBI in them.
Studies comparing the TST and IGRAs for detecting LTBI in children have mostly demonstrated better specificity for IGRAs than the TST. 59 Sensitivity has been shown to be comparable between the TST and the IGRAs but to vary considerably between studies. Both specificity and sensitivity depend on an implied association between LTBI and exposure to TB (as a proxy for true-positive LTBI). The comparative evidence in immunocompromised people has been too scarce to draw definitive conclusions. One systematic review showed suboptimal but comparable performance between the TST and the IGRAs for identifying LTBI in HIV-infected patients. 40 In general, based on limited data, the accuracy indices for the TST and IGRAs in the subgroups of children and immunocompromised people have been shown to be suboptimal. However, the absence of a gold standard, small samples, indeterminate test results and heterogeneity between the studies make adequate comparisons between tests difficult. 11,16
One study has compared the TST and the two IGRAs (QFT-GIT and T-SPOT. TB) for detecting LTBI in migrants to the UK. 77 However, comparison of the tests was carried out only by evaluating the positive results of each, concordance between the tests and the factors associated with positivity. Yields of the test were computed at different incidence thresholds and the cost-effectiveness of the tests was estimated. The authors found that the TST was positive in 30.3% (53/175) of individuals who completed screening, QFT-GIT was positive in 16.6% (38/229) of individuals and T-SPOT. TB was positive in 22.5% (36/160) of individuals. The higher rate for the TST could be a result of the effect of BCG vaccination. Although NICE recommends that recently arriving migrants from countries with a TB incidence of ≥ 40 per 100,000 should be screened, the study found that this would require 97–99% of the cohort to be screened and would identify 98–100% of cases of LTBI whereas screening migrants from countries with an incidence of 150 per 100,000 would identify 49–71% of cases of LTBI but would require screening of only half of the cohort. The most cost-effective option was to screen recently arriving migrants from countries with a TB incidence of > 250 per 100,000 with one QFT-GIT test (£21,565.3 per case prevented) but, as this would miss many cases, screening recently arriving migrants from countries with a TB incidence of 150 per 100,000 was recommended as it was only slightly less cost-effective (£31,867 per case prevented) and would prevent an additional 7.8 cases of TB. This was confirmed in a previous study assessing groups of new migrants in the UK who should be screened for LTBI. 6 Despite these findings it is difficult to draw firm conclusions about the accuracy of identifying LTBI in immigrants as no reference test was used for LTBI when comparing the tests.
New evidence is needed to determine the best approaches for identifying LTBI in all three groups of people (children, immunocompromised individuals and recently arrived immigrants from high-incidence countries). This will help in deciding whether IGRAs should replace or complement the TST and, if so, in which circumstances. There is an ongoing large multicentre cohort study assessing the efficacy and cost-effectiveness of IGRAs compared with the TST for predicting active TB in recently arrived migrants in the UK from high-incidence countries (> 40 per 100,000) and people who have been in contact with TB cases. In total, 10,000 participants (aged ≥ 16 years) will be recruited from 12 hospitals and general practitioner (GP) surgeries and followed up for 24 months; the results from this study will be available in 2017. 78
Current usage in the NHS
The UK National Screening Committee decided that TB screening should be organised locally rather than as a national programme. 79 Therefore, the implementation of NICE guidelines on LTBI testing using the TST and IGRAs has been very ad hoc across the NHS. In London, for example, it is reported that it has not been fully implemented and that current practice is not effective in detecting LTBI. 50
More recently, in March 2014, a tri-borough Joint Strategic Needs Assessment (JSNA) report80 stated that ‘However, GP screening has to date been inconsistent and no clear assessment and patient pathway exists for latent TB’. Leicester, Leicestershire and Rutland’s Tuberculosis Summary Needs Assessment from December 201381 mentions expanding numbers of cases of LTBI from IGRA testing but calls for a more systematic testing process for testing new entrants to make an impact on active TB cases. In addition, Kirklees’s JSNA82 mentions exploring funding to develop IGRA testing and Manchester City Council JSNA83 reports needing to improve LTBI screening.
Commissioners are currently looking at models for local service provision. This is in line with the suggested approach of TB control boards in the recent PHE consultation document Collaborative Tuberculosis Strategy for England 2014 to 2019. 7 There is not one agreed service model and PHE has recently sponsored several pilot projects, which are ongoing at present, looking at the feasibility of screening in different settings. These include the identification of eligible individuals from GP practice lists followed by an invitation for screening at the GP surgery by IGRA (Dr Huda Mohammed, PHE, West Midlands, 12 May 2014, personal communication) and a more innovative approach in which screening for LTBI was carried out using an IGRA at a college of further education among self-selected individuals taking part in English for Speakers of Other Languages classes following a campaign of education. 84 Neither of these studies has reported yet but they are expected to show positive result rates of between 17% and 20% (Dr Huda Mohammed, personal communication).
It is difficult to know how many GPs are identifying new entrants and organising testing for them or how many new entrants are contacting TB services directly for testing. The websites of several community TB85 teams list testing new entrants for LTBI as part of their remit and give a contact number or e-mail address. The Birmingham and Solihull Tuberculosis Service86 has a full page on its website with eligibility criteria for screening, whereas the Liverpool Community Health NHS Trust Tuberculosis Service87 excludes testing of new entrants who are students.
Taking the Coventry and Warwickshire area as a case study, the Meridian Practice in Coventry, a specialist service that cares for refugees and asylum seekers, offers IGRA testing to all registered patients (Najeeb Wai, practice manager, Meridian Practice, 8 July 2014, personal communication). The Coventry and Warwickshire Tuberculosis Service reports that it ‘indirectly tr[ies] to identify high TB risk individuals other than identified contacts and offer screening’ (Debbie Crisp, lead TB nurse specialist and primary care services for the Arden Community TB Service, 9 July 2014, personal communication). Apart from supporting the work at the Meridian Practice, it also supports the Warwickshire programme for looked-after children, which has an established TB screening programme incorporated into its medical review, and has plans to discuss the programme with the Coventry team. In addition, the Coventry and Warwickshire Partnership Trust commenced a TB screening programme for HIV-infected individuals in July 2013 and supports the LTBI treatment programme.
In summary, it is difficult to know how much awareness there is for LTBI screening in the primary care setting in the NHS. Pathways are not widely available, if they exist at all. Secondary care specialist services are more aware, but do not employ standard criteria for testing. There is great variability within the system. There is a clear need for new evidence to provide information on the most appropriate strategies available for identifying LTBI in the three subgroups of interest: children, immunocompromised individuals and recently arrived immigrants from high-incidence countries. This evidence will aid in the decision-making process on whether IGRAs should be used as a replacement or as an adjunct to the TST for the diagnosis of LTBI in these populations.
The next chapter discusses the decision problem and outlines the key clinical questions and objectives of this work.
Chapter 2 Definition of the decision problem
Tuberculosis is a major cause of morbidity and mortality worldwide. The timely identification and prophylactic treatment of people with LTBI is of public health and clinical importance. Unfortunately, there is no diagnostic gold standard for the identification of individuals with LTBI who would benefit from such prophylactic treatment. Instead, the available screening tests provide indirect and imperfect assessment of the presence of LTBI. There are two types of tests used to identify LTBI in the UK: (1) the TST and (2) IGRAs.
In light of new evidence since 2009, this systematic review aimed to compare the clinical effectiveness and cost-effectiveness of screening tests for LTBI (IGRAs and TST) in children, people who are immunocompromised or at risk from immunosuppression and recent arrivals from countries with a high incidence of TB. To do this we updated the searches since 2009 to identify relevant evidence and incorporate both pre- and post-2009 evidence into the analysis. This review also attempted to determine the most cost-effective approach for identifying LTBI.
The key clinical questions to be considered were:
-
Which diagnostic strategy is most clinically effective and cost-effective in accurately identifying LTBI in children?
-
Which diagnostic strategy is most clinically effective and cost-effective in accurately identifying LTBI in people who are immunocompromised or at risk of immunosuppression?
-
Which diagnostic strategy is most clinically effective and cost-effective in accurately identifying LTBI in people who are recent arrivals from countries with a high incidence of TB?
Chapter 3 Clinical effectiveness review methods
A protocol to which we adhered was developed for undertaking this systematic review of the clinical effectiveness literature. The presentation of our systematic review is in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Identification and selection of studies
Search strategy for clinical effectiveness
Scoping searches were undertaken to inform the development of the overall search strategy. An iterative procedure was used, with input from the searches and studies included in NICE clinical guideline 117 (CG117)10 and methods manuals. 88,89 The bibliographic database search strategies focused on the diagnosis of LTBI using IGRAs compared with other methods and were limited to articles in English that had been added to databases since searches for the equivalent questions in CG11710 were run (7–14 December 2009; see Appendix 1). The searches automatically picked up comparisons in performance between IGRAs and TSTs and therefore it was not necessary to search independently for comparator technologies (e.g. TSTs). The search strategies used in the major databases are provided in Appendix 2. Bibliographic database searches were undertaken on 9 and 10 April 2014 and were updated on 2 December 2014 using the same strategies. Supplementary searches were undertaken between 10 June 2014 and 5 August 2014 (see Appendix 2 for exact dates).
The search strategy included the following main elements:
-
searching of electronic bibliographic databases
-
contact with experts in the field
-
scrutiny of references of included studies and relevant systematic reviews
-
screening of manufacturers’ and other relevant websites.
The bibliographic databases searched were MEDLINE (Ovid); MEDLINE In-Process & Other Non-Indexed Citations (Ovid); EMBASE (Ovid); The Cochrane Library incorporating the Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects and Health Technology Assessment database (Wiley); Science Citation Index and Conference Proceedings Citation Index (Web of Science); and Medion. ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) were searched for ongoing and recently completed trials.
Specific conference proceedings selected with input from a clinical expert were checked for the last 5 years. The online resources of relevant organisations were also searched. Further details of these searches are provided in Appendix 2.
Citation searches of included studies were undertaken using the Web of Science and Scopus citation search facilities. The reference lists of included studies and relevant systematic reviews were checked. Included papers were checked for errata using PubMed. Identified references were downloaded to bibliographic management software (EndNote X7; Thomson Reuters, CA, USA).
Inclusion and exclusion of studies
Inclusion criteria
Primary studies evaluating and comparing the head-to-head effectiveness of commercially available approaches/tests used for identifying people with LTBI:
-
IGRAs, for example:
-
QFT-GIT (old version: QFT-G)
-
T-SPOT. TB.
-
-
TST (i.e. Mantoux test).
Head-to-head studies involving a direct comparison between an IGRA and TST only were included.
Type and language of publication
-
Full-text reports published in English.
-
Abstracts (only if they were companion publications to full-text included studies).
Study design
-
Longitudinal studies (randomised controlled trials, retrospective or prospective cohort studies).
-
Cross-sectional or case–control studies.
Population
-
Children (both sexes, aged < 18 years, immunocompetent) (research question 1).
-
People (both sexes, any age) who were immunocompromised or at risk from immunosuppression (e.g. transplant recipients or those with HIV infection, renal disease, diabetes, liver disease, haematological disease, cancer or autoimmune disease or those who were on or about to start antiTNF-α treatment, steroids or ciclosporins) (research question 2).
-
People (both sexes, any age, immunocompetent) who had recently arrived from regions with a high incidence/prevalence of TB (countries/territories with an estimated incidence rate of ≥ 40 per 100,000, e.g. those in Africa, Central/South America, Eastern Europe and Asia) (research question 3).
Intervention
-
Two IGRAs (one- or two-step testing):
-
QFT-GIT (old version: QFT-G)
-
T-SPOT. TB.
-
Comparator
-
TST (Mantoux test) alone or plus IGRA (one- or two-step testing).
Construct validity measures (as a proxy for outcomes)
-
Progression to active TB.
-
Exposure to MTB defined by proximity, duration, geographical location or dose–response gradient.
-
People at low risk of MTB infection or healthy populations.
Exclusion criteria
-
Studies not comparing IGRAs with the TST with regard to the prespecified construct validity (i.e. incidence of TB, exposure to MTB defined by proximity, duration, geographical location, dose–response gradient).
-
Studies not comparing the accuracy of tests (IGRAs with TSTs) in a head-to-head comparison to identify people with LTBI.
-
Studies (involving children, recently arrived immigrants or immunocompromised people) not reporting subgroup data separately for each relevant population.
-
Studies comparing the IGRAs with each other (e.g. QFT-G-IT vs. T-SPOT. TB) in identifying people with LTBI.
-
Studies applying non-commercial IGRAs, in-house IGRAs, older-generation IGRAs [e.g. PPD-based first-generation QuantiFERON-TB (QFT)] or tests unavailable in the UK.
-
Studies assessing the effects of TB treatment on IGRA/TST test results.
-
Studies evaluating and/or comparing the reproducibility (test and retest) of tests for identifying LTBI.
-
Studies not focusing specifically on LTBI [e.g. studies in which the presence of blood culture-positive TB (active TB) was used to estimate sensitivity – ‘active TB’ is assumed as the reference standard for the ‘true presence of LTBI’; however, given that active TB and LTBI are two clinically and immunologically distinct forms of TB, this assumption is problematic].
-
Studies using serial testing (e.g. health-care staff/students, military personnel or prisoners) of IGRAs (or TST) to detect LTBI.
-
Studies focusing on a specific biomarker (e.g. IFN-γ-inducible protein 10).
-
Systematic/narrative reviews, meta-analyses, case reports, case series, abstracts (see Type and language of publication), commentaries, letters or editorials.
Review outcomes
Diagnostic accuracy measures
-
Measures of association between test (IGRAs, TST) results and construct validity – I [i.e. prognostic value of tests in predicting the development/risk of active TB (sensitivity, specificity, false-negative and false-positive rates, PPVs and NPVs, incidence density rate ratios (IDRRs), CIRs].
-
Measures of association between test (IGRAs, TST) results and construct validity – II {i.e. exposure status/level with regard to MTB defined by proximity, length of time and type of contact and including the dose–response gradient if applicable [sensitivity, specificity, false-negative and false-positive rates, DORs, regression-based odds ratios (ORs) of test positivity]}.
-
Measures of association between test (IGRAs, TST) results and other construct(s) of validity – III [e.g. people at low risk for LTBI, e.g. healthy people, residents of low-incidence countries (specificity and false-positive rate)].
Measures of concordance and discordance
-
Agreement (inter-rater, intrarater) (kappa statistic, 95% CI).
-
Concordance between tests (%, 95% CI).
-
Discordance between tests (%, 95% CI).
Other outcomes
-
Dependence of test positivity (IGRAs, TST) on previous BCG vaccination.
-
Adverse events.
-
Likelihood of an indeterminate result.
-
Health-related quality of life.
Study selection strategy
Two independent reviewers screened all identified bibliographic records on title/abstract (screening level I) using a prespecified and piloted questionnaire form. Full-text reports of all potentially relevant records passing screening level I were then retrieved and independently reviewed using the same study eligibility criteria (screening level II). Any disagreements over inclusion/exclusion were resolved by discussion between two reviewers or by recourse to a third-party reviewer.
Data extraction strategy
Two reviewers independently extracted relevant data using an a priori defined pre-piloted data extraction sheet (see Appendix 3). Data extracted were cross-checked and any disagreements were resolved by discussion or by recourse to a third-party reviewer. Data extracted included information on the study [e.g. author, country, publication year, design, setting, sample size, follow-up duration, risk of bias (ROB) items such as blinding or incomplete outcome data], participants (e.g. age, sex, study eligibility criteria, comorbidities, BCG vaccination status/time, immune status), intervention/comparator tests (type of test/assay used for identification of LTBI, definition of positivity/negativity thresholds/cut-off values for each test, methods of laboratory analysis used for the derivation of test results, repeat testing), construct validity (e.g. definition of exposure to MTB in terms of proximity, length of time and/or type of contact; incidence of progression to active TB; timing of exposure to MTB/incidence of active TB; definition of low-risk populations; type of summary effect measures).
For individual studies, 2 × 2 contingency tables were constructed by cross-tabulating test results (separately for IGRAs and TST) with construct validity responses in relation to exposure level or incidence of progression to active TB. The proportions of subjects with positive and negative test results were extracted. For each test, all summary parameters of interest (see the list of outcomes) with corresponding measures of variability (95% CIs, p-values) were ascertained or calculated, if reported data permitted. A value of 0.5 was imputed for incidence studies with zero events for one of the compared tests to allow the calculation of CIRs and their ratios (R-CIRs). The R-CIRs were rendered indeterminate in the case of zero events in the 2 × 2 tables of both tests compared (no imputation was carried out). All relevant summary parameters were entered into the data extraction sheets and evidence and summary tables. Calculated parameters were marked as ‘calculated’.
Study quality assessment
The methodological quality of the incidence and exposure studies included in the current review was assessed using the Quality in Prognosis Studies (QUIPS) tool90 and a modified tool reported by Dinnes et al. ,44 respectively (see Appendix 4).
The QUIPS tool90 (also referred to as the ‘Methodology checklist: prognostic studies’, developed by Hayden et al. ,90 in the NICE Guidelines Manual89) was used to assess studies reporting the diagnostic performance/validation of tests (e.g. sensitivity, specificity, incidence density rate/CIRs, PPVs/NPVs, DORs, regression-based ORs). The QUIPS tool assesses the ROB in the six domains of patient selection/participation, study sample attrition, index test measurement, outcome/construct validity measurement, confounding and statistical analysis/outcome reporting. According to responses to prompting items, each of the six domains is rated as high, moderate or low ROB. The overall summary ROB rating for each study is then derived based on the domain-specific ROB ratings.
We used a modified tool reported by Dinnes et al. 44 to assess the quality of retrospective/cross-sectional studies reporting associations between test results and exposures. The QUIPS tool is not directly applicable to assessing the quality of retrospective/cross-sectional studies of association between test results and exposure because of the non-prognostic nature of their design (exposure is ascertained retrospectively, which is then correlated with test results). Appendix 4 outlines the criteria used to appraise these exposure studies. Each study was assessed for blinding of test results from exposure, description of index test and threshold (TST and IGRA), definition/description of exposure, completeness of verification of exposure and sample attrition. Each study was then awarded an overall quality score defined as:
-
low quality: studies with 0–2 satisfied (yes response) quality features
-
moderate quality: studies with three satisfied (yes response) quality features
-
high quality: studies with 4–5 satisfied (yes response) quality features.
Study quality was assessed independently by two reviewers (PS and KF). Any disagreements were resolved by discussion or by a third reviewer. The evidence across studies was summarised qualitatively using the overall ROB ratings (low, moderate, high).
Data synthesis and analysis
Given the absence of a gold standard for diagnosing LTBI, the performance of tests was compared using alternative methodologies that rely on validation of test results against predetermined validity constructs (i.e. proxies for a reference standard). Thus, our analyses focused on the following recommended approaches: (1) we evaluated and compared predictive values of IGRAs and the TST in relation to construct validity I (i.e. progression rate to active TB); (2) we evaluated and compared the degree of association/correlation of IGRA and TST results with construct validity II (i.e. exposure to MTB defined by proximity, duration or dose–response gradient); (3) we estimated and compared the specificity (or false positives) of IGRAs and the TST in relation to construct validity III (i.e. people at low risk of MTB or healthy populations); and (4) we measured the degree of concordance/discordance between IGRA and TST results. 44,91–94
For each index test (TST, IGRAs), if data permitted (either directly reported or, if not reported, calculated if possible), relevant statistical parameters of diagnostic test accuracy are presented per individual study. For statistics measuring agreement/disagreement between two tests, values for concordant (both tests positive or negative) and discordant (one test negative, the other test positive or vice versa) test results are presented or calculated if data permitted. Moreover, when possible, the likelihood of indeterminate test results was calculated.
The performance of the tests (in terms of diagnostic accuracy and concordance) was compared (e.g. IGRA vs. TST) using sensitivity, specificity, PPVs/NPVs, ratio of diagnostic odds ratios (R-DOR), ratio of incidence density rate ratios (R-IDRR) (or CIRs), regression-based ORs, kappa statistics, per cent discordance and likelihood of indeterminate test results. Note that, as there is no gold standard for the diagnosis of LTBI, specificity and sensitivity does not have the same meaning as in the conventional paradigm (i.e. against a gold standard) but reflects the performance of tests in relation to predetermined proxy constructs of validity (i.e. past exposure to TB or future progression to active TB).
The association between BCG vaccination and test performance in terms of specificity was explored by comparing false-positive rates (or odds of false positivity) of the TST and IGRAs in both BCG-vaccinated and unvaccinated individuals (i.e. dependence of false-positive rates on BCG vaccination status).
Summary measures of effectiveness (e.g. sensitivity, specificity, DOR, R-DOR, R-CIR) were pooled when deemed appropriate and feasible (based on the absence of clinical/methodological heterogeneity, the same cut-off values of a test or the absence of a test threshold effect on the DOR) using univariate95 and/or bivariate random-effects meta-analysis models. 19 The presence of heterogeneity across studies was determined using visual inspection of forest plots (of individual study ORs and R-DOR estimates and degree of overlap across 95% CIs) and chi-squared tests (two tailed, p ≤ 0.10). 96,97 A series of subgroup and sensitivity analyses (see below) was undertaken to explore potential reasons for statistical heterogeneity, if present. When pooling was not feasible, because of a lack of sufficient data or important clinical/statistical heterogeneity across studies (e.g. significant test threshold effect),98 the findings from individual studies were summarised qualitatively.
Data synthesis for the summary outcome measures is presented in evidence/summary tables and text overall and/or stratified by demographic characteristics (e.g. age), TST thresholds (≥ 5 mm, ≥ 10 mm, ≥ 15 mm), intervention (T-SPOT. TB vs. QFT) and prevalence/burden of TB in country of origin (high burden vs. low burden). 1 In addition, for people who were immunocompromised or at risk from immunosuppression (research question 2), when possible outcomes have been stratified by type of immunosuppression, use of immunosuppressive drugs (e.g. steroids, antiTNF-α treatment, antirheumatic drugs) and comorbidity condition (e.g. HIV infection, renal disease, diabetes, liver disease, haematological disease, cancer, autoimmune disease, transplant recipients).
It was planned to conduct subgroup analysis according to BCG vaccination status, TST threshold (≥ 5 mm, ≥ 10 mm, ≥ 15 mm) and prevalence of TB in country of origin, if data permitted.
Calculations were performed using Meta-DiSc version 1.4 (Unit of Clinical Biostatistics, Ramón y Cajal Hospital, Madrid, Spain)99 and Stata version 14 (StataCorp LP, College Station, TX, USA). 100
Overall quality of evidence
There is no formally accepted and validated approach for the assessment of the overall quality of evidence that would be appropriate to the type of evidence synthesised in this review. Work on the formulation of this approach is still ongoing [Grading of Recommendations Assessment, Development and Evaluation Working Group; see www.gradeworkinggroup.org (accessed 15 December 2015)]. 101
Derivation of summary measures of diagnostic accuracy
We used Bayesian meta-analysis to derive the sensitivity and specificity for various testing strategies for LTBI in the various population subcategories. The methods and results for this are reported in Chapter 6 [see Performance of screening texts (sensitivity and specificity)].
Chapter 4 Clinical effectiveness results
Number of studies identified
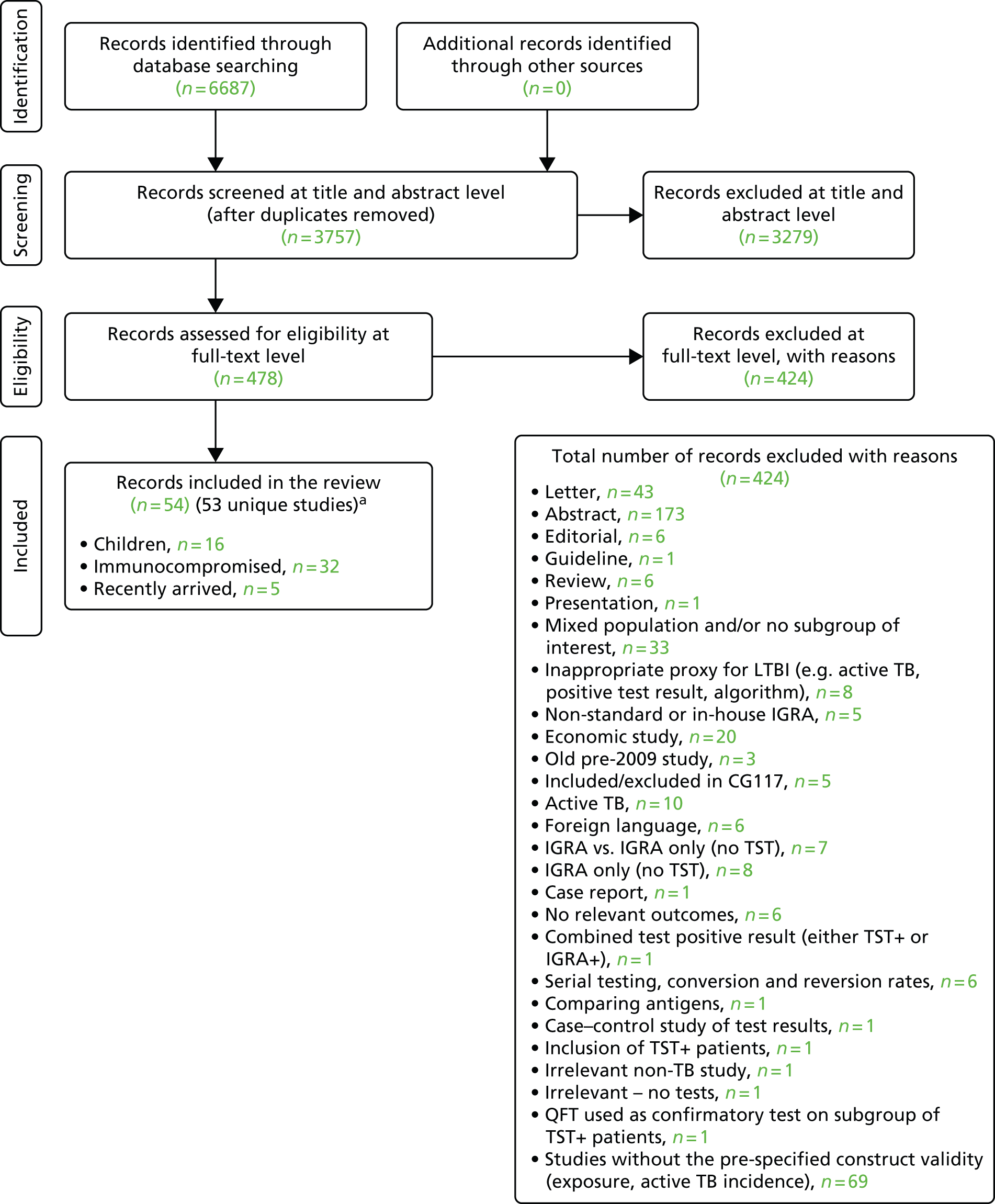
A total of 6687 bibliographic records were identified through electronic database searches. After removing duplicates, 3757 records were screened for inclusion. On the basis of title/abstract, 3279 records were excluded. The remaining 478 records were included for full-text screening. A further 424 records were excluded at the full-text stage. The remaining 54 records102–155 (53 unique studies) were considered relevant to the review since the previous NICE clinical guidance work in 2011 (CG117). 10 One study by Rutherford et al. 110,111 was presented in two publications. In addition, 37 studies156–192 from CG11710 were included in the current evidence synthesis (see Appendix 5). The study flow and the reasons for exclusion are shown in Figure 2 and Appendix 6. A search for ongoing trials was undertaken in different databases (Clinical Trials.gov, WHO ICTRP) up to August 2014. A total of 50 ongoing trials were identified, of which 30 were excluded; the reasons for exclusion are presented in Appendix 7. Twenty ongoing trials were therefore considered relevant for inclusion in our review (see Appendix 8).
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses study flow diagram of studies identified since 2011. a, An additional 37 studies were included from CG117. 10
Description of included studies and synthesis
In the following sections we describe the baseline characteristics and study quality for the incidence and exposure studies of the three populations of interest: (1) children, (2) immunocompromised individuals and (3) those recently arrived from countries with a high TB incidence. Full data-extraction sheets including baseline characteristics for all recently identified studies since CG11710 are provided in Appendix 9. For each of the three populations we present the synthesis of the evidence in terms of the comparative performance of tests (diagnostic accuracy indices for identifying LTBI) and between-test concordance, discordance and agreement. Appendix 10 provides the incidence rates of TB for each included study since CG117. 10
Children and adolescents
Description of baseline characteristics
This section included 27 studies (28 publications102–113,148,150–152,154,156–166) in children and adolescents, of which 11 studies156–166 had already been reviewed in CG11710 (see Appendix 5). Our searches identified 16 additional studies (in 17 publications102–113,148,150–152,154), five102–104,150,152 of which investigated the incidence of active TB following testing for LTBI (incidence studies) and 11 of which (in 12 publications105–113,148,151,154) investigated levels of exposure in relation to LTBI test outcomes (exposure studies). Two publications110,111 reported data on the same population and were therefore considered as one study. See Appendix 9 for the full data-extraction sheets for all new included studies.
Incidence studies
Three102,104,152 of the five incidence studies included close contacts of TB cases and one study150 included only TST-positive (≥ 15 mm) children with no history of close contact with a TB case. Mahomed et al. 103 recruited low-risk high-school students in a high TB burden country, of whom 25% had current or past household contact with TB. Four studies were carried out in countries with TB vaccination: South Africa,104 Iran,103 Turkey150 and South Korea. 152 One study102 was carried out in Germany, in which only 35.7% of participants were BCG vaccinated. Four studies102–104,152 investigated the agreement of a QFT test with the TST. Four studies compared QFT-GIT with the TST in community settings102,103,150,152 whereas Noorbakhsh et al. 104 investigated the agreement between QFT-G and TST (≥ 10 mm) in a hospital setting. Follow-up to confirm active TB across the five studies ranged from 1 year104 to 3.8–4 years. 102,103 Table 2 provides further details on these studies.
| Study ID, country (burden) | Study aim, setting, design, follow-up duration and funding source | Method(s) of diagnosis of active TB | Inclusion/exclusion criteria | Type and positivity threshold(s) of tests compared | Characteristics of study participants at baseline | Number of recruited and excluded study participants | Comments |
|---|---|---|---|---|---|---|---|
| Diel 2011,102 Germany (low) | Aim: to compare QFT-GIT with the TST in close contacts of patients with TB and evaluate progression to active TB for up to 4 years Setting: community-based contact study Design: prospective cohort study Follow-up: 2–4 years Funding source: NR (none of the authors had a financial relationship with a commercial entity that had an interest in the subject of this manuscript) |
CXR (and CT), identification of AFB in sputum samples by bronchoscopy or lavage of gastric secretions, conventional culture of MTB, nucleic acid amplification assays and/or histopathology, assessment of preceding clinical suspicion of TB. In culture-negative cases, and given a CXR consistent with TB, subsequent clinical and radiographic response to multidrug therapy over an appropriate time course (1–3 months) was considered sufficient to confirm the diagnosis of TB | Inclusion criteria: close contacts of smear-positive and subsequently culture-confirmed source MTB index cases; aggregate exposure time of the contact in the 3 months before the diagnosis of the respective index case (presumed period of infectiousness) > 40 hours indoors with shared air Exclusion criteria: contacts with an exposure time of < 40 hours to the source |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA: IFN-γ ≥ 0.35 IU/ml; TST: induration of > 5 mm or > 10 mm |
Mean (SD) age: 10.4 (4.3) years Female, n (%): NR Race/ethnicity, n (%): NR Geographical origin, n (%): Germany 84 (66.7) BCG vaccination, n (%): 45 (35.7) History of antiTB treatment, n (%): NR Total incidence of active TB, n/N (%): 6/104 (5.7) Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): NR |
Total number of recruited patients: 141; total number of excluded patients: 15 | Assessors of the TST were blinded to the QFT results and vice versa. Induration was read by trained and well-experienced public health nurses. If there was a borderline result (e.g. 5 mm exactly), a second reading was performed by a different nurse to verify the result. If there was disagreement, a third nurse read the TST and the consensus result was used |
| Mahomed 2011,103 South Africa (high) | Aim: to compare the predictive value of a baseline TST with that of the QFT-GIT for subsequent microbiologically confirmed TB disease among adolescents Setting: high school (TB vaccine trial site in the town of Worcester and surrounding villages; high burden of TB) Design: longitudinal cohort study Follow-up: 3.8 years Funding source: Aeras Global TB Vaccine Foundation with some support from the Gates Grand Challenge 6 and Gates Grand Challenge 12 grants for QFT testing |
Two sputum samples for smear microscopy on two separate occasions. If any single sputum sample was smear positive, a mycobacterial culture, chest radiography, and HIV test were performed | Inclusion criteria: adolescents aged 12–18 years Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA: IFN-γ ≥ 0.35 IU/ml; TST: induration of ≥ 5 mm |
Mean (range or SD) age: NR Female, n (%): 2842 (54.2) Race/ethnicity, n (%): black 995 (19.0); mixed race: 3839 (73.2); Indian/white: 410 (7.8) BCG vaccination, n (%): yes 4917 (93.8); unknown 281 (5.4) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): 52 (1.0) Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: 6363; total number of excluded patients: 1119 | People with a recent household contact, TB-related symptoms, a positive TST of ≥ 10-mm induration or a positive QFT were referred for two sputum smears. If the results of either or both were sputum positive for AFB, the sputum samples were cultured and chest radiography and a HIV test were undertaken |
| Metin Timur 2014,150 Turkey (intermediate) | Aim: to compare QFT-GIT and TST for the diagnosis of LTBI in children who have been vaccinated with the BCG vaccine Setting: community based Design: prospective cohort study Follow-up: 3 years as an outpatient with 3-month intervals between assessments Funding source: NR |
Active TB disease was defined as both TST and QFT-GIT test positive in a child who had symptoms of TB disease and/or abnormal findings on chest radiography or CT, or proven MTB culture, polymerase chain reaction or histopathological examination | Inclusion criteria: children with positive TST results, children without a history of contact with a TB case, active TB case in the household not detected through family screening, no medical reason for immunosuppression, diagnosed with TB disease without a contact with an active TB case Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT) and TST Cut-off values/thresholds: IGRA: NR; TST: induration of ≥ 15 mm |
Mean (SD) age: 94.8 (51.9) months Female, n (%): 33 (40.7) Race/ethnicity, n (%): NR BCG vaccination, n (%): one BCG scar 69 (85.2); two BCG scars 12 (14.8) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): none Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): acute appendicitis 1 (1.2) Type of during-study treatment, n (%): no treatment n = 69 children with TST+/QFT– results; isoniazid n = 8 children with TST+/QFT+ results but no symptoms (assumed with LTBI); isoniazid, rifampicin and pyrazinamide n = 4 children with TST+/QFT+ results with symptoms (with TB) |
Total number of recruited patients: NR; total number of excluded patients: NR | |
| Noorbakhsh 2011,104 Iran (intermediate) | Aim: to detect the agreement between TST and QFT-G in young household contacts of cases of proven active pulmonary TB in a BCG-vaccinated population in Tehran, Iran, and to compare subjects progressing to TB with non-progressive subjects Setting: pulmonary and infectious diseases department of Rasul Hospital in Tehran Design: cross-sectional study Follow-up: 1 year Funding source: Research Centre of Paediatric Infectious Diseases, Iran University of Medical Sciences |
Person diagnosed by an internist in the pulmonary and infectious ward of Rasul Hospital. The index cases were confirmed by positive culture for MTB or sputum smear-positive TB | Inclusion criteria: all young (age < 20 years) close or household contacts of people (any person who had lived with the index case for > 3 months) with confirmed active pulmonary TB and previous BCG vaccination received at birth. The subjects were invited to the research centre for clinical and laboratory follow-up Exclusion criteria: household contacts were excluded if they had been treated for TB in the past year or had a known immunodeficiency status according to history or clinical signs (malignancy, corticosteroid therapy, HIV infection, etc.) |
Type of tests: IGRA (QFT-G), TST Cut-off values/thresholds: IGRA: NR; TST: induration of ≥ 10 mm |
Mean (range or SD) age: NR Female, n (%): 34 (57.6) Race/ethnicity, n (%): NR BCG vaccination, n (%): NR History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): 10 (16.9) Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: NR; total number of excluded patients: NR | |
| Song 2014,152 South Korea (high) | Study aim: to determine the agreement between QFT-GIT and TST, and identify the relationships between the results of these tests and the development of active TB in middle- and high-school students in close contact with TB patients in South Korea Setting: community based Design: prospective cohort study Follow-up: 24 months Funding source: Research of Korea Centers for Disease Control and Prevention |
NR | Inclusion criteria: close contacts of identified smear-positive TB cases with normal chest radiography aged 11–19 years Exclusion criteria: participants showing abnormal findings on simple chest radiography, taken immunosuppressive agents or anticancer drugs previously and been treated with antiTB drugs or chemoprophylaxis previously |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA: IFN-γ 0.35 IU/ml; TST induration of ≥ 10 mm or ≥ 15 mm |
Mean (SD) age: 15.1 (1.3) years Female, n (%): 1356 (45.5) Race/ethnicity, n (%): NR BCG vaccination, n (%): 1818 (61.0) History of antiTB treatment, n (%): NR Total incidence of active TB, n/N (%): 23/2982 (0.77) Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n/N (%): 5/215 (2.32) (isoniazid) |
Total number of recruited patients: 3202; total number of excluded patients: 220 | To eliminate the possibility of false-positive IGRA results due to PPD reagents, blood samples were collected before PPD injection |
Exposure studies
Eleven studies (in 12 publications105–113,148,151,154) compared one or more QFT tests with the TST test in children and adolescents by relating test results to previous levels of exposure (exposure studies). Five studies were carried out in countries with a high TB incidence [Gambia,105 South Africa,107,108 Indonesia (one study in two publications)110,111 and Thailand154], two studies were carried out in countries with TB of intermediate incidence (Mexico148 and Brazil151) and four studies were carried out in low-incidence countries (USA,106,112 Croatia109 and Greece113).
The mean and/or median age of the recruited children was reported in eight106–109,112,148,151,154 of the 11 studies. The populations in the studies by Pavic et al. 109 and Perez-Porcuna et al. 151 had a mean age of < 4 years. The studies by Laniado-Laborın et al. 148 and Tieu et al. 154 included children whose mean age was about 8 years. Cruz et al. 106 and Kasambira et al. 107 recruited children with a median age of 8.6 and 6 years, respectively. Mahomed et al. 108 and Talbot et al. 112 investigated adolescents with an age range of 12–18 years and a median age of 20 years, respectively. The reported proportion of females was just above 50% in the majority of studies105–108,112,148,151,154 and was 40% in one study. 109 Eight studies compared QFT-GIT with the TST ≥ 5 mm107,108,148 or the TST ≥ 10 mm. 109–111,151,154 The T-SPOT. TB test was compared with the TST (≥ 10 mm or ≥ 15 mm) in three studies. 106,112,154 Adetifa et al. 105 compared three tests [QFT-GIT, T-SPOT. TB and TST (≥ 10 mm)] whereas Tsolia et al. 113 compared QFT-GIT with TST at two different thresholds (≥ 5 mm and ≥ 10 mm).
Exposure to TB was defined as household contacts in one study108 and was further categorised by four studies to include sleep proximity105 (same room/different room), time spent with contact107,109 (≥ 40 hours in closed rooms; < 6 hours per day or > 7 hours per day, respectively) or both110,111 (different room/same room/same bed and < 2 hours per day or 2–8 hours per day or > 8 hours per day). One study described exposure only as contact with a source case,106 another study described it in terms of country of birth, residence and extended visit to a high-incidence country,112 and a further study distinguished exposure as either non-household but regular contact or household contact. 113 Three studies used a TB contact score151,154 or duration of exposure to the TB index case. 148,151,154
Studies were either community based105,107,108,112,151,154 or hospital based. 106,109–111,113,148 The level of BCG vaccination was high in six studies,107–109,148,151,154 medium in a further three studies,105,106,110,111 low in one study112 and not reported in another study. 113 Table 3 provides further details on these studies.
| Study ID, country (burden) | Study aim, setting, design and funding source | Definition of construct validity (i.e. LTBI exposure-based proxy) | Inclusion/exclusion criteria | Type and positivity threshold(s) of tests compared | Characteristics of study participants at baseline | Number of recruited and excluded study participants | Comments |
|---|---|---|---|---|---|---|---|
| Adetifa 2010,105 Gambia (high) | Aim: to compare T-SPOT.TB, QFT-GIT and TST for the diagnosis of LTBI in Gambian childhood contacts of TB patients Setting: community based Design: retrospective cohort/cross-sectional study Funding source: Medical Research Council (MRC) laboratories, UK |
Sleep proximity: non-exposed: different house (reference group); exposed 1: same house, different room; exposed 2: same house, same room | Inclusion criteria: household contacts (< 16 years) of newly diagnosed TB index cases Exclusion criteria: history of treatment for active TB, TB diagnosis within 1 month of recruitment |
Type of tests: IGRAs (T-SPOT.TB, QFT-GIT), TST Cut-off values/thresholds: IGRA (T-SPOT.TB): ≥ 6 spots in either the ESAT-6 or CFP-10 panel after subtracting the number of spots in the negative control panel; IGRA (QFT-GIT): IFN-γ ≥ 0.35 IU/ml; TST: induration of ≥ 10 mm |
Mean (range or SD) age: NR Female, n (%): 145 (51) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n/N (%): 127/199 (59.1) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): HIV positive 3 (1.1) Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: 285; total number of excluded patients: NR | |
| Cruz 2011,106 USA (low) | Study aim: to compare the performance of T-SPOT.TB with that of TST in children with different epidemiological risk factors for TB Setting: paediatric TB clinics Design: retrospective cohort/cross-sectional study Funding source: Cellestis Ltd, Oxford Immunotec, Inc. |
Non-exposed: no contact with an identifiable source case; exposed 1: contact with an identifiable source case | Inclusion criteria: children (aged 1 month to 18 years) with LTBI or TB disease and children uninfected with TB Exclusion criteria: children on any TB medication for ≥ 2 months were not eligible for enrolment |
Type of tests: IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (T-SPOT.TB): ≥ 8 spots; TST: induration of ≥ 15 mm |
Median (range) age: 8.6 years (1 month to 18 years) Female, n (%): 9451 Race/ethnicity, n (%): Hispanic 115 (62.5), non-Hispanic black 36 (19.6), non-Hispanic white 19 (10.3), Asian 6 (3) Geographical origin, n (%): low-prevalence regions (US/UK) 121 (65.7) BCG vaccination, n (%): 68 (37) History of antiTB treatment, n (%): Total incidence of active TB, n (%): none Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: NR; total number of excluded patients: NR | Borderline results (5–7 spots) were excluded from concordance analyses but were analysed separately. A subgroup analysis was performed for specimens with six to seven spots because these specimens are sometimes considered positive internationally |
| Kasambira 2011,107 South Africa (high) | Aim: to determine and compare the prevalence of MTB infection as assessed by TST and QFT-GIT, and to assess agreement between the two test methods and identify factors associated with various patterns of test results Setting: community based Design: retrospective cohort/cross-sectional study (with limited follow-up of 6 months) Funding source: United States Agency for International Development |
Adult index case type of TB diagnosis: non-exposed: smear-positive TB; exposed 1: smear-negative, culture-positive TB; exposed 2: clinical TB Adult index case smear grade: non-exposed: negative; exposed 1: scanty; exposed 2: 1+; exposed 3: 2+; exposed 4: 3+ Exposure to index case during the day: non-exposed: minority of day (< 6 hours); exposed: majority of day (> 7 hours) |
Inclusion criteria: children aged 6–16 years whose parents and guardians were TB index cases aged ≥ 18 years, with a diagnosis of pulmonary TB within the preceding 3 months, willingness to have their child undergo study testing and provision of informed consent Exclusion criteria: previous diagnosis or treatment of active or latent TB |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: definition of positive test: IGRA (QFT-GIT): NR; TST: induration of ≥ 5 mm |
Median (IQR) age: 6 (3–9) years Female, n (%): 141 (52) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 257 (95) History of antiTB treatment, n (%): none Total incidence of active TB, n (%): NR Chest radiography (yes/no): NR Clinical examination (yes/no): yes Morbidity, n (%): HIV 14 (5) Comorbidity, n (%): NA Type of during-study treatment, n (%): active TB treatment 37 (19), LTBI treatment 19 (10) |
Total number of recruited patients: NR; total number of excluded patients: NR | |
| Laniado-Laborın 2014,148 Mexico (intermediate) | Aim: to compare the prevalence of LTBI between paediatric contacts of drug-resistant cases and drug-susceptible cases Setting: TB clinic Design: cross-sectional/retrospective cohort study Funding source: NR |
Non-exposed: NR; exposed: exposure to source, hours per day exposure, number of cohabitants, number of rooms | Inclusion criteria: family contacts of culture-proven cases aged ≤ 16 years Exclusion criteria: subjects with a history of TB, a previous diagnosis of LTBI or the administration of a TST in the past year |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): IFN-γ ≥ 0.35 IU/ml; TST: induration of ≥ 5 mm |
Mean (SD) age: drug susceptible 7.79 (4.28) years; drug resistant 7.36 (4.46) years Female, n/N (%): 86/173 (50.0) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 164 (95) History of antiTB treatment, n (%): none Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n/N (%): 77/173 (44.5) contacts of multidrug-susceptible index cases were treated for LTBI with isoniazid or rifampicin; 96/173 (55.5) contacts of multidrug-resistant cases did not receive treatment for LTBI |
Total number of recruited patients: NR; total number of excluded patients: NR | |
| Mahomed 2011,108 South Africa (high) | Aim: to determine the prevalence of and predictive factors associated with LTBI in adolescents Setting: high school Design: retrospective cohort/cross-sectional study Funding source: Aeras Global TB Vaccine Foundation and the Gates Grand Challenge 6 and Gates Grand Challenge 12 grants for QuantiFERON testing |
Non-exposed: no current or previous TB household contact; exposed: current or previous TB household contact | Inclusion criteria: all adolescents aged 12–18 years Exclusion criteria: diagnosed with active TB |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): IFN-γ ≥ 0.35 IU/ml TST: induration of ≥ 5mm |
Age range: 12–18 years Female, n (%): 2842 (54.2) Race/ethnicity, n (%): Indian/white 410 (7.8); mixed race 3839 (73.2); black 995 (19.0) Geographical origin, n (%): NR BCG vaccination, n (%): no 46 (0.9); yes 4917 (93.8); unknown 281 (5.4) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): no Clinical examination (yes/no): no Morbidity, n (%): NR Comorbidity, n (%): chronic allergy-related condition, e.g. asthma, hay fever, eczema yes 53 (1.0); no 5191 (99.0) Type of during-study treatment, n (%): NR |
Total number of recruited patients: 6363 enrolled, 5244 enrolled for analysis Total number of excluded patients: 13 because of indeterminate QFT results, 639 because TST was not performed with past TB, 22 because TST was not performed with current TB, 22 because diagnosed with active TB |
|
| Pavic 2011,109 Croatia (low) | Aim: to evaluate an IGRA for the diagnosis of LTBI in BCG-vaccinated children up to 5 years of age with documented exposure to active TB Setting: children’s hospital and general hospital Design: retrospective cohort/cross-sectional study Funding source: none |
Non-exposed: distant contact was defined as occasional or unclear exposure time or < 40 hours during the presumed period of infectiousness; exposed: close contact was defined as household contact with aggregate exposure to a patient with active TB of ≥ 40 hours in closed rooms | Inclusion criteria: paediatric patients aged ≤ 5 years with documented exposure (close or distant contact) to a case of active TB Exclusion criteria: children aged > 5 years, immunocompromised children, inadequate blood sampling and diagnosis of active TB |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): IFN-γ ≥ 0.35 IU/ml as recommended by the manufacturer; TST: induration of ≥ 10 mm |
Mean age: 29 ± 16 months Female, n (%): 57 (40.1) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 142 (100) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): NR Morbidity, n (%): NR Comorbidity, n (%): pneumonia 1 (0.7) Type of during-study treatment, n (%): NR |
Total number of recruited patients: 142; total number of excluded patients: 1 | Blood samples for QFT-GIT were drawn under standardised conditions in hospital on the same day as the TST was carried out. The test was considered indeterminate if the value of the positive control was < 0.5 IU/ml and/or the nil negative control was > 8 IU/l |
| Perez-Porcuna 2014,151 Brazil (intermediate) | Aim: to evaluate the response of the QFT-GIT and TST tests in young children with recent exposure to an index case Setting: community based Design: cross-sectional/retrospective study Funding source: Brazilian National Council of Scientific and Technological and Development, Foundation for Research Support of the State of Amazonas and University of Barcelona. Cellestis Ltd donated QFT kits |
Time of exposure to the index case: non-exposed: NR; exposed: number of months (continuous scale covariate) MTB contact (MTC) score 0–15: non-exposed: NR; exposed: MTC score (continuous scale covariate) was composed of infectivity of the index case (0–4), the duration of exposure in hours per day (0–4), the relationship to the index case (0–4) and the type of exposure (0–3) |
Inclusion criteria: children aged 0–6 years with contact with an adult symptomatic TB index case within the last 12 months Exclusion criteria: children receiving treatment or prophylaxis for TB |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): IFN-γ ≥ 0.35 IU/ml; TST: induration of ≥ 10 mm |
Mean (range) age: 46 (28.0–64.5) months Female, n (%): 74 (54.8) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 118 (90.8) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: 140; total number of excluded patients: 3 | Experienced laboratory technicians who were unaware of the data of the study subjects |
| Rutherford 2012,110,111 Indonesia (high) | Aim: to quantify MTB infection in children living with a smear-positive adult TB case and identify risk factors for TST and QFT-GIT positivity Setting: outpatient-based clinic Design: retrospective cohort/cross-sectional study Funding source: NR |
Characteristics of TB case smear positivity: non-exposed: scanty and 1+; exposed 1: 2+; exposed 2: 3+ Relationship to child: non-exposed: other; exposed 1: uncle; exposed 2: parent Sleeping proximity to child: non-exposed: different room; exposed 1: same room; exposed 2: same bed Time spent with child (number of hours per day): non-exposed: < 2; exposed 1: 2–8; exposed 2: > 8 |
Inclusion criteria: child contacts living for > 3 months with newly diagnosed TB cases (index cases) who were smear and CXR positive Exclusion criteria: child contacts who had received a diagnosis of TB disease within the past year or who were aged < 6 months |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): NR; TST: induration of ≥ 10 mm |
Median (IQR) age: 58 (31–81) months Female, n (%): 152 (50.7) Race/ethnicity, n (%): Sudanese 284 (93.7); other 19 (6.3) Geographical origin, n (%): NR BCG vaccination, n (%): with scar 221 (73.2); unknown BCG status 30 (9.9) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): yes [children who were symptomatic and test negative (on either IGRA or TST) were referred to the children’s clinic for further assessment according to clinic policy] Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: 320; total number of excluded patients: 16 | |
| Talbot 2012,112 USA (low) | Aim: to test the specificity of the TST and T-SPOT.TB assay among students at low risk for TB exposure Setting: college health setting Design: retrospective cohort/cross-sectional study Funding source: Oxford Immunotec, Inc. |
Non-exposed: low TB exposure risk group; exposed: non-low TB exposure risk [any history of exposure to TB through country of birth, residence or visits of > 3 weeks to high TB burden areas (> 40 cases per 100,000 population) or occupational exposure] | Inclusion criteria: students with a history of exposure to TB Exclusion criteria: NR |
Type of tests: IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (T-SPOT.TB): 5–7 spots borderline and results with a low mitogen response or a high nil control response are indeterminate TST: induration of > 15 mm for students with no risk factors for TB exposure |
Median (range) age: 20 (17–47) years Female, n (%): 97 (53.9) Race/ethnicity, n (%): US born 165 (91.7); white 135 (75) Geographical origin, n (%): NR BCG vaccination, n (%): 7 (3.9) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): NR Clinical examination (yes/no): NR Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: 184; total number of excluded patients: 4 | |
| Tieu 2014,154 Thailand (high) | Aim: to compare the performances of the IGRAs (T-SPOT.TB, QFT-GIT) and TST at two different cut-off thresholds (10 mm and 15 mm) in Thai children who had a recent exposure to an adult index case with TB Setting: community based Design: cross-sectional/retrospective cohort study Funding source: investigator-initiated research grant from Tibotec REACH Initiative |
TB contact score (range 6–19): non-exposed: TB contact score 8–10; exposed 1: TB contact score 11–12; exposed 2: TB contact score 13–14; exposed 3: TB contact score 15–16 TB contact score (range 6–19): non-exposed: TB contact score 8–12; exposed: TB contact score ≥ 13 Relationship to TB index case: non-exposed: relative other contact in household with TB; exposed 1: second caregiver in household with TB; exposed 2: primary caregiver in household with TB Duration of average contact per day with TB index case: non-exposed: 0–7 hours; exposed: ≥ 8 hours Duration of contact with TB index case in last 12 months: non-exposed: 0–7 months; exposed: > 7 months Index TB case history: non-exposed: sputum acid-fast smear negative; exposed: sputum acid-fast smear positive |
Inclusion criteria: children between the ages of 2 months and 16 years with recent exposure (defined as having lived with and/or having had close contact with) to adults with active pulmonary TB (confirmed by positive AFB stain, polymerase chain reaction test for TB or TB culture), with or without extrapulmonary TB manifestations Exclusion criteria: children’s caregivers refused study participation, were receiving antiTB medications for TB disease (including isoniazid for latent TB) or had recently been diagnosed with active TB |
Type of tests: IGRA (T-SPOT.TB, QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT, T-SPOT.TB): NR TST: induration of 10 mm or ≥ 15 mm |
Mean (SD) age: 7.6 (4.3) years Female, n (%): 67 (49.3) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 132 (96.4) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): none (for TB exposed) |
Total number of recruited patients: 137 (TB exposed); total number of excluded patients: NR | Study investigators, site co-ordinators, and clinicians were blinded to the results of the IGRAs until completion of enrolment and the 9-month follow-up |
| Tsolia 2010,113 Greece (low) | Aim: to evaluate and compare the performance of the QFT-GIT assay and the TST among children with active TB or possible LTBI in a low-endemic country Setting: TB clinic Design: retrospective cohort/cross-sectional study Funding source: Bienmoyo Foundation |
Contact with an adult TB: non-exposed: non-household occasional contact; exposed 1: non-household regular contact; exposed 2: household contact | Inclusion criteria: adolescents aged ≤ 15 years Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): NR TST: induration of ≥ 10 mm for BCG-immunised children, ≥ 5 mm for non-BCG-immunised children |
Mean (range or SD) age: NR Female, n (%): NR Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): NR History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: 295; total number of excluded patients: 9 because of refusal, lost specimen and sample processing delay | Indeterminate results on the QFT-GIT were excluded from the analysis |
Study quality
Incidence of active tuberculosis
Of the five102–104,150,152 newly identified active TB incidence studies in children, three102,103,152 were rated as having a moderate ROB and two104,150 were rated as having a high ROB. Most studies had a moderate ROB for the item misclassification of individuals in relation to construct validity groups. The studies also failed to provide information on prognostic factor and outcome measurement. Table 4 provides further details.
| Study ID (burden) | Study design | Study participation (risk of selection bias) | Study attrition (risk of selection bias) | Prognostic factor measurement (risk of exposure measurement bias) | Outcome/construct measurement (ROB in misclassification of individuals in relation to construct validity groups) | Study confounding (ROB from confounding) | Statistical analysis and reporting (ROB from analysis and selective reporting) | Total ROB (high, moderate, low) |
|---|---|---|---|---|---|---|---|---|
| Diel 2011102 (low) | Low | Low | Low | Moderate | Moderate | Low | Low | Moderate |
| Mahomed 2011103 (high) | Low | Moderate | Moderate | Moderate | Moderate | High | Low | Moderate |
| Metin Timur 2014150 (intermediate) | Low | High | High | Moderate | Moderate | High | High | High |
| Noorbakhsh 2011104 (intermediate) | Moderate | High | High | High | Moderate | High | High | High |
| Song 2014152 (high) | Low | Low | Moderate | Low | High | Moderate | Low | Moderate |
Exposure levels
The majority of the 11 included exposure studies in children105–113,148,151,154 identified since the publication of CG11710 were rated as being of low quality, with only three109,151,154 studies rated as being of high quality. One study was of moderate quality. 148 Table 5 provides further details.
| Study ID (burden) | Recruitment of subjects [consecutive (yes), arbitrary or unreported (no)] | Blinding of test results from exposure [blinded (yes), not blinded or unreported (no)] | Description of index test and threshold [adequate (yes), inadequate or unreported (no)] | Definition and description of exposure [adequate (yes), inadequate or unreported (no)] | Sample attrition [adequate (yes),a inadequate or unreported (no)] | Overall quality score of satisfactory featuresb |
|---|---|---|---|---|---|---|
| Adetifa 2010105 (high) | No | No | Yes | Yes | No | Low |
| Cruz 2011106 (low) | No | No | No | No | Yes | Low |
| Kasambira 2011107 (high) | No | No | No | Yes | Yes | Low |
| Laniado-Laborın 2014148 (intermediate) | Yes | Yes | Yes | No | No | Moderate |
| Mahomed 2011108 (high) | No | No | No | No | No | Low |
| Pavic 2011109 (low) | Yes | No | Yes | Yes | Yes | High |
| Perez-Porcuna 2014151 (intermediate) | Yes | Yes | Yes | Yes | No | High |
| Rutherford 2012110,111 (high) | No | No | No | Yes | Yes | Low |
| Talbot 2012112 (low) | No | No | Yes | No | No | Low |
| Tieu 2014154 (high) | Yes | Yes | No | Yes | Yes | High |
| Tsolia 2010113 (low) | Yes | No | No | No | Yes | Low |
Comparative performance of tests (diagnostic accuracy indices for identifying latent tuberculosis infection): children
Incidence of active tuberculosis
Ratios of cumulative incidence ratios
This analysis included seven studies: two studies161,162 reviewed in CG11710 (see Appendix 5) and five more recent studies, three published in 2011102–104 and two published in 2014150,152 (see Appendix 9). For three150,161,162 of the studies, R-CIRs could not be calculated because none of the children developed active TB. The R-CIRs in the remaining four studies102–104,152 were pooled (Table 6), with one analysis comparing QFT-GIT with TST 5 mm and the other comparing QFT-GIT with TST 10 mm (they were pooled separately because TST performance differs according to its threshold). The pooled estimates indicated that there was no significant difference in performance between QFT-GIT and TST 5 mm (pooled R-CIR 1.12, 95% CI 0.72 to 1.75) (Figure 3),102,103 whereas QFT-GIT was better than TST 10 mm in identifying/predicting LTBI (pooled R-CIR 4.33, 95% CI 1.32 to 14.23) (Figure 4). 102,104,152
| Study ID, country (burden) | Test results | Test diagnostic accuracy (95% CI) (%) | Development of active TB | |||
|---|---|---|---|---|---|---|
| Cumulative incidence (%), CIR, IDR, IDRR (95% CI) | R-CIR, R-IDRR (95% CI), IGRA vs. TST (by threshold) | |||||
| IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | |||
| Diel 2011,102 Germany (low) | Number of test results: QFT-GIT 106; T-SPOT.TB NA; TST 106 Test (+/–): QFT-GIT 23/83; T-SPOT.TB NA; TST ≥ 5 mm 40/66; TST ≥ 10 mm 20/86 Number of indeterminate results: QFT-GIT NR; T-SPOT.TB NA; TST NR Number lost to follow-up: NR |
QFT-GIT: SN 100 (60.97 to 100); SP 84.69 (76.27 to 90.5); PPV 28.57 (13.81 to 49.96); NPV 100 (95.58 to 100) | TST ≥ 5 mm: SN 100 (60.97 to 100); SP 65.31 (55.47 to 73.99); PPV 15.00 (7.06 to 29.07); NPV 100 (94.34 to 100) TST ≥ 10 mm: SN 66.67 (30.00 to 90.32); SP 63.27 (53.39 to 72.14); PPV 10.00 (3.96 to 23.05); NPV 96.88 (89.3 to 99.14) |
QFT-GIT: CI (+) 28.57 (13.81 to 49.96); CI (–) 1.20 (0.03 to 6.53); CIR 23.7 (2.57 to 110.3) | TST ≥ 5 mm: CI (+) 15.00 (7.06 to 29.07); CI (–) 1.55 (0.04 to 8.4); CIR 9.6 (1.08 to 448.2) TST ≥ 10 mm: CI (+) 10.00 (3.95 to 23.05); CI (–): 3.12 (0.22 to 11.33); CIR 3.20 (0.61 to 16.67) |
R-CIR (QFT-GIT vs. TST ≥ 5 mm): 2.47 (0.40 to 15.12) R-CIR (QFT-GIT vs. TST ≥ 10 mm): 7.41 (2.06 to 26.57) |
| Mahomed 2011,103 South Africa (high) | Number of test results: QFT-GIT 5244; T-SPOT.TB NA; TST 5244 Test (+/–): QFT-GIT 2669/2575; T-SPOT.TB NA; TST ≥ 5 mm 2894/2350 Number of indeterminate results: QFT-GIT NR; T-SPOT.TB NA; TST NR Number lost to follow-up: 18% |
QFT-GIT: SN 75.00 (61.79 to 84.77); SP 49.35 (47.99 to 50.71); PPV 1.46 (1.07 to 1.99); NPV 99.50 (99.14 to 99.7) | TST ≥ 5 mm: SN 76.92 (63.87 to 86.28); SP 45.03 (43.68 to 46.39); PPV 1.38 (1.02 to 1.88); NPV 99.49 (99.11 to 99.71) | QFT-GIT: CI (+) 1.46 (1.07 to 1.99); CI (–) 0.50 (0.28 to 0.87); CIR 2.89 (1.55 to 5.40); IDR (+) 0.64 (0.45 to 0.87) per 100 person-years; IDR (–) 0.22 (0.12 to 0.38) per 100 person-years; IDRR 2.92 (1.58 to 5.67) | TST ≥ 5 mm: CI (+) 1.38 (1.02 to 1.87); CI (–) 0.51 (0.28 to 0.90); CIR 2.71 (1.42 to 5.14); IDR (+) 0.60 (0.43 to 0.82) per 100 person-years; IDR (–) 0.22 (0.11 to 0.39) per 100 person-years; IDRR 2.73 (1.45 to 5.42) | R-CIR (QFT-GIT vs. TST ≥ 5 mm): 1.07 (0.68 to 1.68); R-IDRR (QFT-GIT vs. TST ≥ 5 mm): 1.07 (0.67 to 1.71) |
| Metin Timur 2014,150 Turkey (intermediate) | Number of test results: QFT-GIT 81; T-SPOT.TB NA; TST 81 Test (+/–): QFT-GIT 12/69; T-SPOT.TB NA; TST ≥ 15 mm 81/0 Number of indeterminate results: QFT-GIT 0; T-SPOT.TB NA; TST 0 Number lost to follow-up: NR |
QFT-GIT: SN NA; SP 100 (NR); PPV NA; NPV 100 (NR) | TST ≥ 15 mm: SN NA; SP 0.0 (NR); PPV 0.0 (NR); NPV NA | QFT-GIT: CI (+) NA; CI (–) 0.0 (NR); CIR NA | TST ≥ 15 mm: CI (+) 0.0 (NR); CI (–) NA; CIR: NA | R-CIR (QFT-GIT vs. TST ≥ 15 mm): NA |
| Noorbakhsh 2011,104 Iran (intermediate) | Number of test results: QFT-G 59; T-SPOT.TB NA; TST 58 Test (+/–): QFT-G 18/41; T-SPOT.TB NA; TST ≥ 10 mm 8/50 Number of indeterminate results: QFT-G NR; T-SPOT.TB NA; TST 1 Number lost to follow-up: NR |
QFT-G: SN 100 (72.25 to 100); SP 83.67 (70.96 to 91.49); PPV 55.56 (33.72 to 75.44); NPV 100 (91.43 to 100) | TST ≥ 10 mm: SN 30.00 (10.78 to 60.32); SP 89.58 (77.83 to 95.47); PPV 37.50 (13.68 to 69.43); NPV 86.00 (73.81 to 93.05) | QFT-G: CI (+) 55.56 (33.72 to 75.44); CI (–) 2.41 (0.06 to 12.9); CIR 22.78 (2.75 to 101.1) | TST ≥ 10 mm: CI (+) 37.5 (13.49 to 69.62); CI (–) 14.00 (6.63 to 26.50); CIR 2.68 (0.86 to 8.27) | R-CIR (QFT-G vs. TST ≥ 10 mm): 8.50 (2.87 to 25.17) |
| Song 2014,152 South Korea (high) | Number of test results: QFT-GIT 2966; T-SPOT.TB NA; TST 2982 Test (+/–): QFT-GIT 317/2649; T-SPOT.TB NA; TST ≥ 10 mm 663/2319; TST ≥ 15 mm 231/2751 Number of indeterminate results: QFT-GIT 16; T-SPOT.TB NA; TST 0 Number lost to follow-up: NR |
QFT-GIT: SN 47.83 (29.24 to 67.04); SP 89.6 (88.45 to 90.65); PPV 3.47 (1.94 to 6.10); NPV 99.55 (99.21 to 99.74) | TST ≥ 10 mm: SN 56.52 (36.81 to 74.37); SP 78.03 (76.51 to 79.49); PPV 1.96 (1.14 to 3.32); NPV 99.57 (99.21 to 99.77) TST ≥ 15 mm: SN 56.52 (36.81 to 74.37); SP 92.63 (91.64 to 93.52); PPV 5.62 (3.31 to 9.38); NPV 99.64 (99.33 to 99.80) |
QFT-GIT: CI (+) 3.47 (1.87 to 6.17); CI (–) 0.45 (0.24 to 0.79); CIR 7.66 (3.41 to 17.21); OR 7.90 (3.46 to 18.06) | TST ≥ 10 mm: CI (+) 1.96 (1.11 to 3.36); CI (–) 0.43 (0.22 to 0.80); CIR 4.55 (2.00 to 10.32) ; OR 4.62 (2.02 to 10.58) TST ≥ 15 mm: CI (+) 5.62 (3.23 to 9.47); CI (–) 0.36 (0.18 to 0.67); CIR 15.48 (6.86 to 34.92); OR 16.35 (7.08 to 37.71) |
R-CIR (QFT-GIT vs. TST ≥ 10 mm): 1.68 (0.94 to 3.03); R-OR (QFT-GIT vs. TST ≥ 10 mm): 1.71 (0.94 to 3.11) R-CIR (QFT-GIT vs. TST ≥ 15 mm): 0.49 (0.28 to 0.89); R-OR (QFT-GIT vs. TST ≥ 15 mm): 0.48 (0.27 to 0.88) |
FIGURE 3.
Pooled R-CIR (QFT-GIT vs. TST 5 mm) in children and adolescents. df, degrees of freedom; IV, inverse variance; SE, standard error.
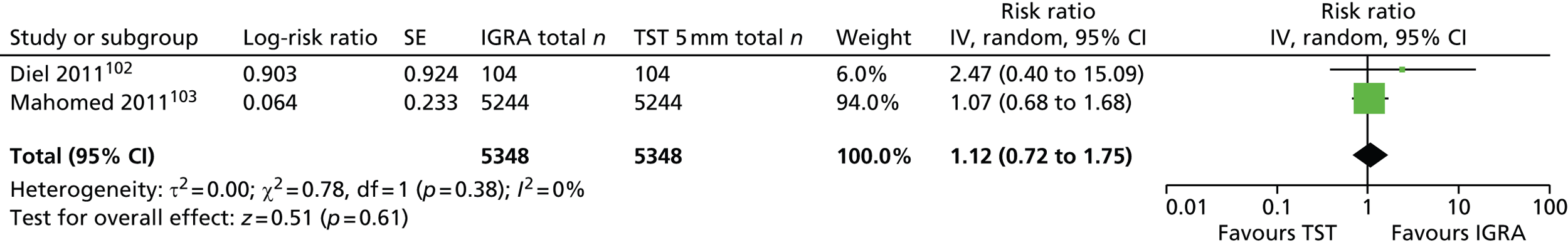
FIGURE 4.
Pooled R-CIR (QFT-GIT vs. TST 10 mm) in children and adolescents. df, degrees of freedom; IV, inverse variance; SE, standard error.
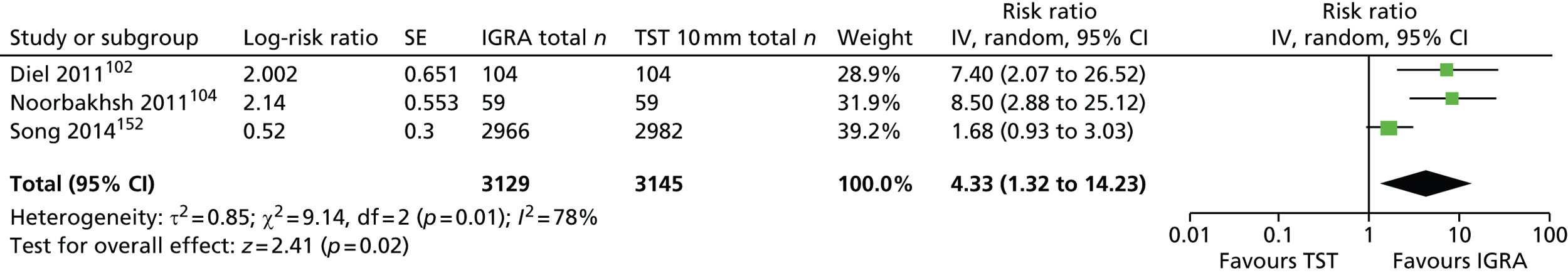
Sensitivity and specificity
There was wide variability in the sensitivity and specificity of IGRAs (QFT-GIT/G) and the TST (5 mm or 10 mm) across newly identified studies. 102–104,150,152 TST sensitivity was higher at 5 mm than at 10 mm/15 mm and, vice versa, specificity was better at 10 mm/15 mm than at 5 mm. IGRAs (QFT-GIT/G) demonstrated a similar sensitivity (range 48–100%) to that of TST 5 mm (sensitivity range 57–100%) and slightly better specificity (range 49–90%) than that of TST 5 mm (range 45–65%). Although the sensitivities of the IGRAs and TST 5 mm were higher than those for TST 10 mm/15 mm (range 30–56%), the corresponding specificities of these tests were lower than those for TST 10 mm/15 mm (range 63–93%). Forest plots of sensitivities and specificities were generated and because of high unexplained heterogeneity (not explained by IGRA type and TST threshold, different methods for diagnosing active TB), no meta-analysis could be performed (Figures 5–8).
FIGURE 5.
Forest plot of sensitivity based on incidence of active TB (QFT-GIT/G) in children and adolescents. df, degrees of freedom.
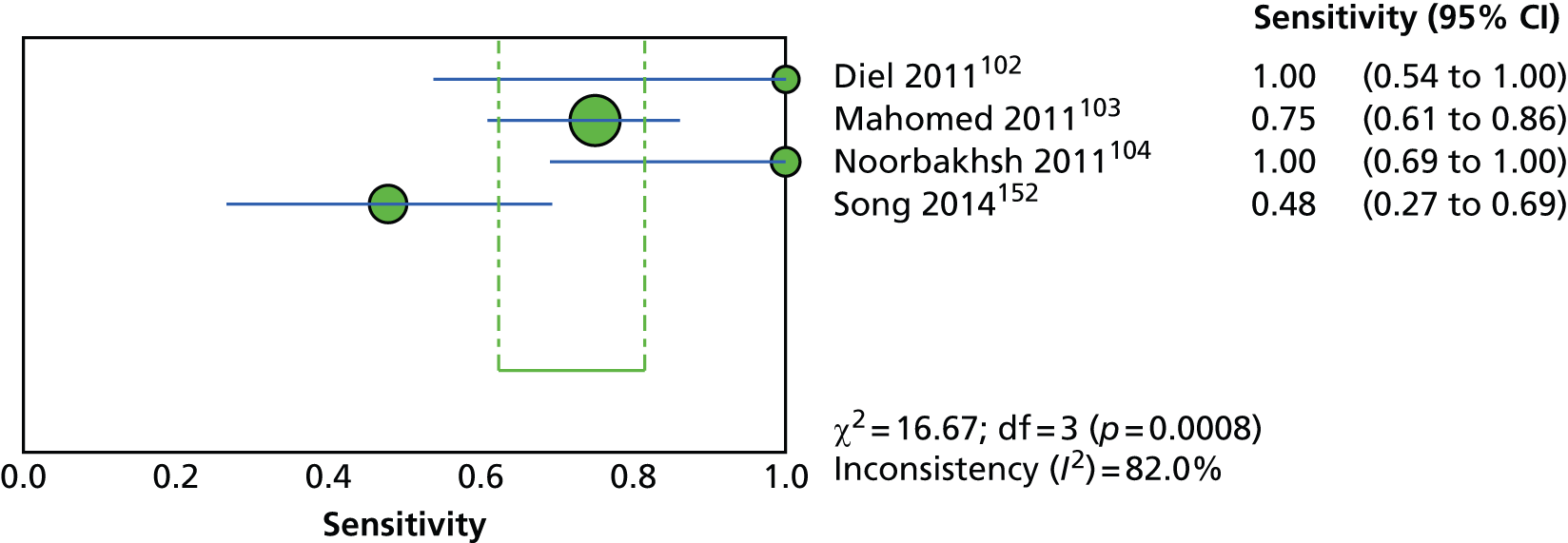
FIGURE 6.
Forest plot of sensitivity based on incidence of active TB (TST) in children and adolescents. df, degrees of freedom.

FIGURE 7.
Forest plot of specificity based on incidence of active TB (QFT-GIT/G) in children and adolescents. df, degrees of freedom.

FIGURE 8.
Forest plot of specificity based on incidence of active TB (TST) in children and adolescents. df, degrees of freedom.

Exposure levels
Ratios of diagnostic odds ratios
This analysis included 17 studies: six studies156,157,160,162–164 from CG11710 (see Appendix 5) and 11 studies105–113,148,151,154 from the updated review (see Appendix 9). The association between the screening test results and the risk of LTBI/exposure level measured using the R-DORs (IGRA vs. TST) in individual studies ranged from 0.27105 to 11.01113 (Table 7).
| Study ID, country (burden) | Test results | Test diagnostic accuracy (95% CI) (%) | Construct validity (i.e. LTBI exposure-based proxy) | |||
|---|---|---|---|---|---|---|
| DOR (95% CI) (vs. non-exposed; reference group) | R-DOR (95% CI), IGRA vs. TST (by threshold) | |||||
| IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | |||
| Adetifa 2010,105 Gambia (high) | Number of test results: QFT-GIT 215; T-SPOT.TB 215; TST 215 Test (+/–): QFT-GIT 72/143; T-SPOT.TB 71/144; TST ≥ 10 mm 57/158 Number of indeterminate results: QFT-GIT/G 2; T-SPOT.TB 0; TST 0 |
QFT-GIT: Same house/different room vs. different house: SN NR; SP NR; PPV NR; NPV NR Same house/same room vs. different house: SN NR; SP NR; PPV NR; NPV NR T-SPOT.TB: Same house/different room vs. different house: SN NR; SP NR; PPV NR; NPV NR Same house/same room vs. different house: SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 10 mm: Same house/different room vs. different house: SN NR; SP NR; PPV NR; NPV NR Same house/same room vs. different house: SN NR; SP NR; PPV NR; NPV NR |
QFT-GIT: Same house/different room vs. different house: DOR 1.20 (0.60 to 2.60); DORa 1.50 (0.70 to 3.10) Same house/same room vs. different house: DOR 3.20 (1.20 to 9.10); DORa 4.00 (1.40 to 11.40) T-SPOT.TB: Same house/different room vs. different house: DOR 2.00 (0.80 to 5.10); DORa 2.60 (0.90 to 7.10) Same house/same room vs. different house: DOR 5.30 (1.50 to 18.50); DORa 6.60 (1.70 to 25.20) |
TST ≥ 10 mm: Same house/different room vs. different house: DOR 2.40 (1.00 to 5.80) DORa: 2.90 (1.30 to 6.70) Same house/same room vs. different house: DOR 10.10 (3.20 to 32.10); DORa 15.00 (4.70 to 47.20) T-SPOT.TB: Same house/different room vs. different house: DOR 2.40 (1.00 to 5.80); DORa 2.90 (1.30 to 6.70) Same house/same room vs. different house: DOR 10.10 (3.20 to 32.10); DORa 15.00 (4.70 to 42.20) |
QFT-GIT vs. TST ≥ 10 mm: Same house/different room: R-DOR 0.58 (0.28 to 0.90); R-DORa 0.52 (0.29 to 0.91) Same house/same room: R-DOR 0.32 (0.14 to 0.69); R-DORa 0.27 (0.12 to 0.59) T-SPOT vs. TST ≥ 10 mm: Same house/different room: R-DOR 0.83 (0.43 to 1.60); R-DORa 0.90 (0.46 to 1.76) Same house/same room: R-DOR 0.52 (0.22 to 1.25); R-DORa 0.44 (0.18 to 1.09) |
| Cruz 2011,106 USA (low) | Number of test results: T-SPOT.TB 163; TST 163 Test (+/–): T-SPOT.TB 94/69; TST ≥ 15 mm 94/69 Number of indeterminate results: T-SPOT.TB 22; TST 22 |
T-SPOT.TB: Contact with an identifiable source case vs. no such contact: SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 15 mm: Contact with an identifiable source case vs. no such contact: SN NR; SP NR; PPV NR; NPV NR |
T-SPOT.TB: Contact with an identifiable source case vs. no such contact: DOR NR; DORa 4.41 (1.78 to 10.94) |
TST ≥ 15 mm Contact with an identifiable source case vs. no such contact: DOR NR; DORa 0.48 (0.26 to 0.91) |
T-SPOT vs. TST ≥ 15 mm Contact with an identifiable source case: R-DOR NR; R-DORa 9.19 (5.23 to 16.3) |
| Kasambira 2011,107 South Africa (high) | Number of test results: QFT-GIT 251; TST 254 Test (+/–): QFT-GIT 79/172; TST ≥ 5 mm 71/183 Number of indeterminate results: QFT-GIT 19; TST 16 |
QFT-GIT: Exposure to index case during the majority of the day (> 7 hours) vs. minority of the day (< 6 hours): SN 29.87 (23.2 to 37.52); SP 71.68 (62.77 to 79.17); PPV 58.97 (47.89 to 69.22); NPV 42.86 (36.01 to 49.99) |
TST ≥ 5 mm: Exposure to index case during the majority of the day (> 7 hours) vs. minority of the day (< 6 hours): SN 29.79 (22.86 to 37.79); SP 73.64 (64.71 to 80.97); PPV 59.15 (47.54 to 69.83); NPV 45.00 (37.91 to 52.30) |
QFT-GIT: Exposure to index case during the majority of the day (> 7 hours) vs. minority of the day (< 6 hours): DOR 1.10 (0.63 to 1.80); DORa 1.30 (0.69 to 2.30) Adult index case smear grade (vs. negative): scanty: DOR 0.30 (0.05 to 1.60), DORa NR; 1+: DOR 1.50 (0.70 to 3.60), DORa 5.50 (0.89 to 34.70); 2+: DOR 1.50 (0.50 to 4.90), DORa 8.70 (1.20 to 62.00); 3+: DOR 3.20 (1.40 to 7.40), DORa 11.40 (1.80 to 72.00) |
TST ≥ 5 mm: Exposure to index case during the majority of the day (> 7 hours) vs. minority of the day (< 6 hours): DOR 1.20 (0.67 to 2.10); DORa 1.10 (0.58 to 2.10) Adult index case smear grade (vs. negative): scanty: DOR NR, DORa NR; 1+: DOR 2.81 (1.20 to 6.70), DORa 7.90 (1.50 to 41.00); 2+: DOR 2.90 (0.80 to 10.60), DORa 15.70 (2.60 to 92.0); 3+: DOR 4.10 (1.50 to 11.10), DORa 11.70 (2.20 to 62.00) |
QFT-GIT vs. TST ≥ 5 mm: Exposure to index case during the majority of the day (> 7 hours): R-DOR 0.92 (0.62 to 1.36); R-DORa 1.18 (0.75 to 1.85) Adult index case smear grade (3+): R-DOR 0.78 (0.40 to 1.52); R-DORa 0.97 (0.27 to 3.47) |
| Laniado-Laborin 2014,148 Mexico (intermediate) | Number of test results: QFT-GIT 172; TST 172 Test (+/–): QFT-GIT 71/101; TST ≥ 5 mm 136/36 Number of indeterminate results: QFT-GIT 1; TST 1 |
QFT-GIT: Exposure to source, hours per day exposure, number of cohabitants, number of rooms: SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 5 mm: Exposure to source, hours per day exposure, number of cohabitants, number of rooms: SN NR; SP NR; PPV NR; NPV NR |
QFT-GIT : Exposure to source: DORa 0.91 (0.57 to 1.45) Hours per day exposure: DORa 1.03 (0.96 to 1.10) Number of cohabitants: DORa 0.91 (0.79 to 1.05) Number of rooms: DORa 1.12 (0.77 to 1.61) |
TST ≥ 5 mm: Exposure to source: NR (p = NR) Hours per day exposure: NR (p = NR) Number of cohabitants: NR (p = NR) Number of rooms: NR (p = NR) |
QFT-GIT vs. TST ≥ 5 mm: R-DORa NR |
| Mahomed 2011,108 South Africa (high) | Number of test results: QFT-GIT 5244; TST 5244 Test (+/–): QFT-GIT 2669/2562; TST ≥ 5 mm 2894/2350 Number of indeterminate results: QFT-GIT 13; TST 0 |
QFT-GIT: Current or previous TB household contact vs. no such contact: SN 66.67 (64.09 to 69.15); SP 54.32 (52.75 to 55.88); PPV 33.27 (31.51 to 35.08); NPV 82.67 (81.16 to 84.09) |
TST ≥ 5 mm: Current or previous TB household contact vs. no such contact: SN 71.32 (68.83 to 73.69); SP 50.31 (48.74 to 51.87); PPV 32.83 (31.14 to 34.56); NPV 83.74 (82.2 to 85.18) |
QFT-GIT: Current or previous TB household contact vs. no such contact: DOR 2.40 (2.11 to 2.74); DORa 1.90 (1.70 to 2.20) |
TST ≥ 5 mm: Current or previous TB household contact vs. no such contact: DOR 2.52 (2.20 to 2.88); DORa 2.00 (1.70 to 2.30) |
QFT-GIT vs. TST ≥ 5 mm: Current or previous TB household contact: R-DOR 0.94 (0.86 to 1.04); R-DORa 0.95 (0.86 to 1.05) |
| Pavic 2011,109 Croatia (low) | Number of test results: QFT-GIT 141; TST 142 Test (+/–): QFT-GIT 18/123; TST ≥ 10 mm 24/118 Number of indeterminate results: QFT-GIT 1; TST 0 |
QFT-GIT: Close contact (household contact with aggregate exposure to a patient with active TB of ≥ 40 hours in closed rooms) vs. distant contact (occasional or unclear exposure time or < 40 hours of exposure during the presumed period of infectiousness): SN 19.54 (12.57 to 29.08); SP 98.15 (90.23 to 99.67); PPV 94.44 (74.24 to 99.01); NPV 43.09 (34.68 to 51.92) |
TST ≥ 10 mm: Close contact (household contact with aggregate exposure to a patient with active TB of ≥ 40 hours in closed rooms) vs. distant contact (occasional or unclear exposure time or < 40 hours of exposure during the presumed period of infectiousness): SN 26.44 (18.31 to 36.56); SP 98.18 (90.39 to 99.68); PPV 95.83 (79.76 to 99.26); NPV 45.76 (37.05 to 54.74) |
QFT-GIT: Close contact (household contact with aggregate exposure to a patient with active TB of ≥ 40 hours in closed rooms) vs. distant contact (occasional or unclear exposure time or < 40 hours of exposure during the presumed period of infectiousness): DOR 12.87 (1.66 to 99.80); DORa NR |
TST ≥ 10 mm: Close contact (household contact with aggregate exposure to a patient with active TB of ≥ 40 hours in closed rooms) vs. distant contact (occasional or unclear exposure time or < 40 hours of exposure during the presumed period of infectiousness): DOR 19.41 (2.53 to 148.40); DORa NR |
QFT-GIT vs. TST ≥ 10 mm: Close contact (household contact with aggregate exposure to a patient with active TB of ≥ 40 hours in closed rooms): R-DOR 0.66 (0.15 to 2.89); R-DORa NR |
| Perez-Porcuna 2014,151 Brazil (intermediate) | Number of test results: QFT-GIT 116; TST 135 Test (+/–): QFT-GIT 36/80; TST ≥ 10 mm 47/88 Number of indeterminate results: QFT-GIT 19; TST 0 |
QFT-GIT: Time of exposure to the index case (number of months): SN NR; SP NR; PPV NR; NPV NR MTC score 0–15: SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 10 mm: Time of exposure to the index case (number of months): SN NR; SP NR; PPV NR; NPV NR MTC score 0–15: SN NR; SP NR; PPV NR; NPV NR |
QFT-GIT: Time of exposure to the index case (number of months): DOR NR (p = 0.024); DORa NR (p = 0.537) MTC score 0–15: DOR NR (p = 0.021); DORa 1.16 (1.01 to 1.33; p = 0.035) |
TST ≥ 10 mm: Time of exposure to the index case (number of months): DOR NR (p < 0.001); DORa 1.15 (1.04 to 1.27; p = 0.009) MTC score 0–15: DOR NR (p < 0.001); DORa 1.29 (1.08 to 1.54; p = 0.005) |
QFT-GIT vs. TST ≥ 10 mm: Time of exposure to the index case (number of months): R-DOR NR; R-DORa NR MTC score 0–15: R-DOR NR; R-DORa 0.90 (0.80 to 1.01) |
| Rutherford 2012,110,111 Indonesia (high) | Number of test results: QFT-GIT 290; TST 302 Test (+/–): QFT-GIT 152/138; TST ≥ 10 mm 145/157 Number of indeterminate results: QFT-GIT 14; TST 2 |
QFT-GIT: Characteristics of TB case smear positivity (3+ vs. scanty/1+): SN 62.5 (53.58 to 70.65); SP 59.6 (49.75 to 68.73); PPV 65.22 (56.15 to 73.3); NPV 56.73 (47.14 to 65.85) Relationship to child (parent vs. other): SN 61.19 (54.59 to 67.4); SP 77.27 (63.01 to 87.16); PPV 93.06 (87.69 to 96.18); NPV 28.57 (21.22 to 37.26) Sleeping proximity to child (same bed vs. different room): SN 59.24 (51.42 to 66.61); SP 59.05 (49.48 to 67.97); PPV 68.38 (60.15 to 75.6); NPV 49.21 (40.63 to 57.83) Time spent with child (number of hours per day: > 8 vs. < 2): SN 52.00 (44.06 to 59.85); SP 42.55 (29.51 to 56.72); PPV 74.29 (65.17 to 81.68); NPV 21.74 (14.54 to 31.21) |
TST ≥ 10 mm: Characteristics of TB case smear positivity (3+ vs. scanty/1+): SN 61.9 (53.19 to 69.91); SP 68.27 (58.81 to 76.43); PPV 70.27 (61.21 to 77.98); NPV 59.66 (50.68 to 68.04) Relationship to child (parent vs. other): SN 55.9 (49.42 to 62.18); SP 90.71); PPV 94.12 (88.82 to 96.99); NPV 26.81 (20.12 to 34.76) Sleeping proximity to child (same bed vs. different room): SN 51.52 (43.94 to 59.02); SP 56.88 (47.51 to 65.79); PPV 64.39 (55.92 to 72.05); NPV 43.66 (35.78 to 51.88) Time spent with child (number of hours per day: > 8 vs. < 2): SN 47.47 (39.83 to 55.22); SP 41.67 (28.85 to 55.72); PPV 72.82 (63.52 to 80.47); NPV 19.42 (12.94 to 28.1) |
QFT-GIT: Characteristics of TB case smear positivity (2+ vs. scanty/1+): DOR 1.56 (0.78 to 3.11); DORa NR Characteristics of TB case smear positivity (3+ vs. scanty/1+): DOR 2.43 (1.21 to 4.86); DORa 2.28 (1.06 to 4.90) Relationship to child (aunt/uncle vs. other): R-DOR 1.51 (0.44 to 5.17); R-DORa NR Relationship to child (parent vs. other): R-DOR 5.61 (2.40 to 13.12); R-DORa 4.30 (1.48 to 12.45) Sleeping proximity to child (same room vs. different room): R-DOR 1.87 (0.70 to 5.02); R-DORa NR Sleeping proximity to child (same bed vs. different room): R-DOR 2.01 (1.12 to 3.61); R-DORa 1.45 (0.70 to 2.99) Time spent with child (number of hours per day: 2–8 vs. < 2): R-DOR 0.78 (0.33 to 1.80); R-DORa NR Time spent with child (number of hours per day: > 8 vs. < 2): R-DOR 0.83 (0.38 to 1.79); R-DORa NR |
TST ≥ 10 mm: Characteristics of TB case smear positivity (2+ vs. scanty/1+): DOR 1.80 (0.89 to 3.63); DORa NR Characteristics of TB case smear positivity (3+ vs. scanty/1+): DOR 3.35 (1.81 to 6.21); DORa 2.93 (1.59 to 5.39) Relationship to child (aunt/uncle vs. other): R-DOR 2.31 (0.77 to 6.79); R-DORa NR Relationship to child (parent vs. other): R-DOR 5.85 (2.56 to 13.38); R-DORa 7.04 (2.23 to 22.28) Sleeping proximity to child (same room vs. different room): R-DOR 1.21 (0.41 to 3.53); R-DORa NR Sleeping proximity to child (same bed vs. different room): R-DOR 1.35 (0.79 to 2.32); R-DORa NR Time spent with child (number of hours per day: 2–8 vs. < 2): R-DOR 0.55 (0.24 to 1.24); R-DORa NR Time spent with child (number of hours per day: > 8 vs. < 2): R-DOR 0.64 (0.31 to 1.36); R-DORa NR |
QFT-GIT vs. TST ≥ 10 mm: Characteristics of TB case smear positivity (3+): R-DOR 0.73 (0.45 to 1.17); R-DORa 0.78 (0.47 to 1.28) Relationship to child (parent vs. other): R-DOR 0.96 (0.52 to 1.61); R-DORa 0.78 (0.47 to 1.28) Sleeping proximity to child (same bed): R-DOR 1.47 (1.05 to 2.16); R-DORa NR Time spent with child (> 8 hours per day): R-DOR 1.30 (0.75 to 2.24); R-DORa NR |
| Talbot 2012,112 USA (low) | Number of test results: T-SPOT.TB 143; TST 143 Test (+/–): T-SPOT.TB 5/138; TST ≥ 15 mm 6/137 Number of indeterminate results: T-SPOT.TB 15; TST 22 |
T-SPOT.TB: Non-low TB exposure risk group vs. low TB exposure risk group: SN NR; SP 100 (97.00 to 100); PPV NR; NPV NR |
TST ≥ 15 mm: Non-low TB exposure risk group vs. low TB exposure risk group: SN NR; SP 98.39 (94.31 to 99.56); PPV NR; NPV NR |
T-SPOT.TB: Non-low TB exposure risk group vs. low TB exposure risk group: DOR NR; DORa NR |
TST ≥ 15 mm: Non-low TB exposure risk group vs. low TB exposure risk group: DOR NR; DORa NR |
T-SPOT.TB vs. TST ≥ 15 mm: Non-low TB exposure risk group vs. low TB exposure risk group: R-DOR NR; R-DORa NR |
| Tieu 2014,154 Thailand (high) | Number of test results: QFT-GIT 136; T-SPOT.TB 136; TST 136 Test (+/–): QFT-GIT 40/96; T-SPOT.TB 36/100; TST ≥ 10 mm 88/48; TST ≥ 15 mm 48/88 Number of indeterminate results: QFT-GIT 0; T-SPOT.TB 0; TST 0 |
QFT-GIT, T-SPOT.TB: TB contact score: SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 10 mm, TST ≥ 15 mm: TB contact score: SN NR; SP NR; PPV NR; NPV NR |
QFT-GIT: TB contact score (≥ 13 vs. 8–12): DOR 4.04 (1.81 to 8.99); DORa 1.98 (0.64 to 6.11) T-SPOT.TB: TB contact score (≥ 13 vs. 8–12): DOR 3.50 (1.57 to 7.81); DORa 3.15 (1.35 to 7.34) |
TST ≥ 10 mm : TB contact score (≥ 13 vs. 8–12): DOR 2.59 (1.28 to 5.23); DORa 2.21 (0.99 to 4.98) TST ≥ 15 mm: TB contact score (≥ 13 vs. 8–12): DOR 2.19 (1.09 to 4.43); DORa 0.83 (0.35 to 1.99) |
QFT-GIT vs. TST ≥ 10 mm: TB contact score (≥ 13 vs. 8–12): R-DOR 1.56 (0.91 to 2.69); R-DORa 0.90 (0.44 to 1.82) QFT-GIT vs. TST ≥ 15 mm: TB contact score (≥ 13 vs. 8–12): R-DOR 1.84 (1.07 to 3.18); R-DORa 2.39 (1.15 to 4.93) T-SPOT.TB vs. TST ≥ 10 mm: TB contact score (≥ 13 vs. 8–12): R-DOR 1.35 (0.78 to 2.33); R-DORa 1.43 (0.78 to 2.59) T-SPOT.TB vs. TST ≥ 15 mm: TB contact score (≥ 13 vs. 8–12): R-DOR 1.60 (0.93 to 2.75); R-DORa 3.80 (2.04 to 7.05) |
| Tsolia 2010,113 Greece (low) | Number of test results: QFT-GIT 95; TST 99 Test (+/–): QFT-GIT 32/63; TST ≥ 5 mm 55/44 Number of indeterminate results: QFT-GIT 4; TST 0 |
QFT-GIT: Contact with an adult TB case (non-household regular vs. non-household occasional): SN 33.33 (18.64 to 52.18); SP 90.91 (62.26 to 98.38); PPV 90.00 (59.58 to 98.21); NPV 35.71 (20.71 to 54.17) Contact with an adult TB case (household vs. non-household occasional): SN 38.6 (27.06 to 51.57); SP 90.91 (62.26 to 98.38); PPV 95.65 (79.01 to 99.23); NPV 22.22 (12.54 to 36.27) |
TST ≥ 5 mm: Contact with an adult TB case (non-household regular vs. non-household occasional): SN 64.29 (45.83 to 79.29); SP 36.36 (15.17 to 64.62); PPV 72.00 (52.42 to 85.72); NPV 28.57 (11.72 to 54.65) Contact with an adult TB case (household vs. non-household occasional): SN 50.00 (37.73 to 62.27); SP 36.36 (15.17 to 64.62); PPV 81.08 (65.79 to 90.52); NPV 11.76 (4.67 to 26.62) |
QFT-GIT: Contact with an adult TB case (non-household regular vs. non-household occasional): DOR 5.00 (0.55 to 45.39); DORa NR Contact with an adult TB case (household vs. non-household occasional): DOR 6.28 (0.75 to 52.56); DORa NR |
TST ≥ 5 mm: Contact with an adult TB case (non-household regular vs. non-household occasional): DOR 1.03 (0.24 to 4.39); DORa NR Contact with an adult TB case (household vs. non-household occasional): DOR 0.57 (0.15 to 2.15); DORa NR |
QFT-GIT vs. TST ≥ 5 mm: Contact with an adult TB case (non-household regular): R-DOR 4.85 (1.26 to 18.69); R-DORa NR Contact with an adult TB (household regular): R-DOR 11.02 (3.07 to 39.60); R-DORa NR |
The updated meta-analysis included 14 studies: six studies156,157,160,162–164 from CG11710 (see Appendix 5) and eight more recent studies105–111,113,154 published from 2009 onwards (see Appendix 9). One study112 did not provide sufficient information to calculate the R-DOR and therefore this study could not be included in the meta-analysis. In a random-effects meta-analysis of the 14 studies,105–111,113,154,156,157,160,162–164 of which two studies106,160 used T-SPOT. TB and the remaining 12 studies used QFT-GIT [or QuantiFERON-Gold (QFT-G)], the pooled R-DOR showed a significantly stronger association for the IGRAs than for the TST in relation to a risk of LTBI/exposure level (pooled R-DOR 1.98, 95% CI 1.19 to 3.28; I2 = 89%) (Figure 9).
FIGURE 9.
Pooled R-DOR of IGRA vs. TST based on high- and low-risk exposure in children and adolescents. df, degrees of freedom; IV, inverse variance; SE, standard error.

Heterogeneity was high (I2 = 89%) and the sources of heterogeneity were explored through subgroup analyses with regard to burden of TB incidence, IGRA type, TST threshold and study setting. The simultaneous meta-analytical stratification by IGRA type (QFT-GIT/G and T-SPOT. TB) and TST threshold (5 mm, 10–15 mm) (Figures 10–12) as well as study setting (community-based contact and hospital-based studies) (Figures 13 and 14) did not help to explain the presence of heterogeneity (i.e. heterogeneity persisted in these analyses). The study by Adetifa et al. 105 displayed a very aberrant result (see Figure 9; R-DOR 0.27, 95% CI 0.12 to 0.59) indicating a significant superiority of TST (10 mm) over IGRA (QFT-GIT), which could not be readily explained. The report did not provide the raw data needed for the calculation and verification of the correctness of the reported DORs for the IGRA and TST. The authors explained this finding by the delayed presentation of TB cases (mean time 9 weeks) with early reversion of the IGRA and about 30% of TB cases in the Gambia being infected with Mycobacterium africanum (Castets et al. 1969), which has a reduced response to ESAT-6.
FIGURE 10.
Pooled R-DOR of QFT vs. TST 5 mm based on high- and low-risk exposure in children and adolescents. df, degrees of freedom; IV, inverse variance; SE, standard error.
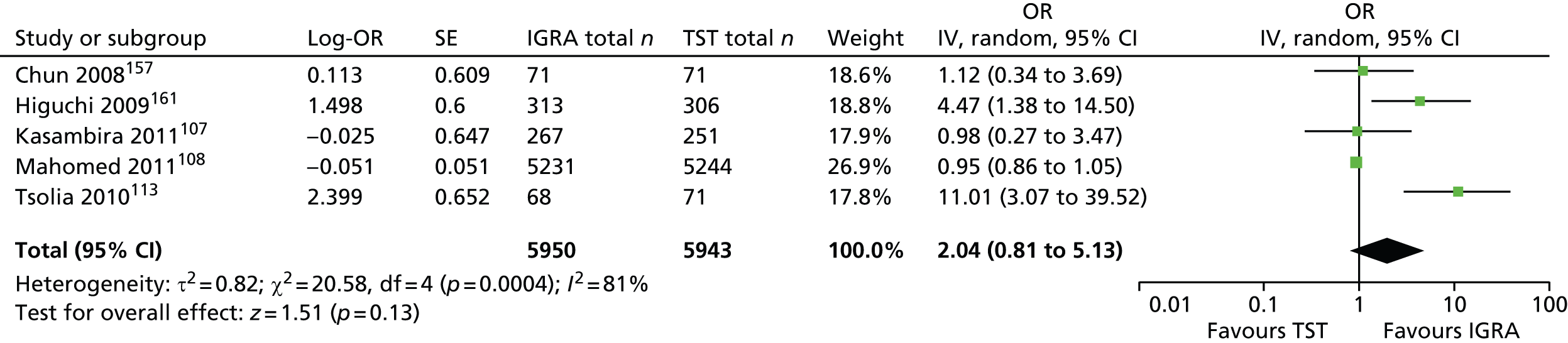
FIGURE 11.
Pooled R-DOR of QFT vs. TST 10–15 mm based on high- and low-risk exposure in children and adolescents. df, degrees of freedom; IV, inverse variance; SE, standard error.

FIGURE 12.
Pooled R-DOR of T-SPOT. TB vs. TST 10–15 mm based on high- and low-risk exposure in children and adolescents. df, degrees of freedom; IV, inverse variance; SE, standard error.
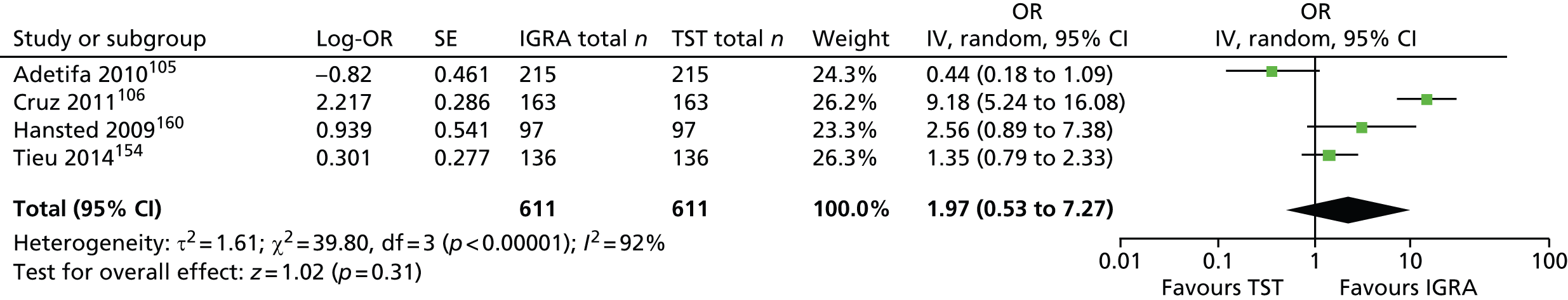
FIGURE 13.
Pooled R-DOR of IGRA vs. TST based on high- and low-risk exposure in children and adolescents: community-based contact studies only. df, degrees of freedom; IV, inverse variance; SE, standard error.

FIGURE 14.
Pooled R-DOR of IGRA vs. TST based on high- and low-risk exposure in children and adolescents: hospital-based studies only. df, degrees of freedom; IV, inverse variance; SE, standard error.
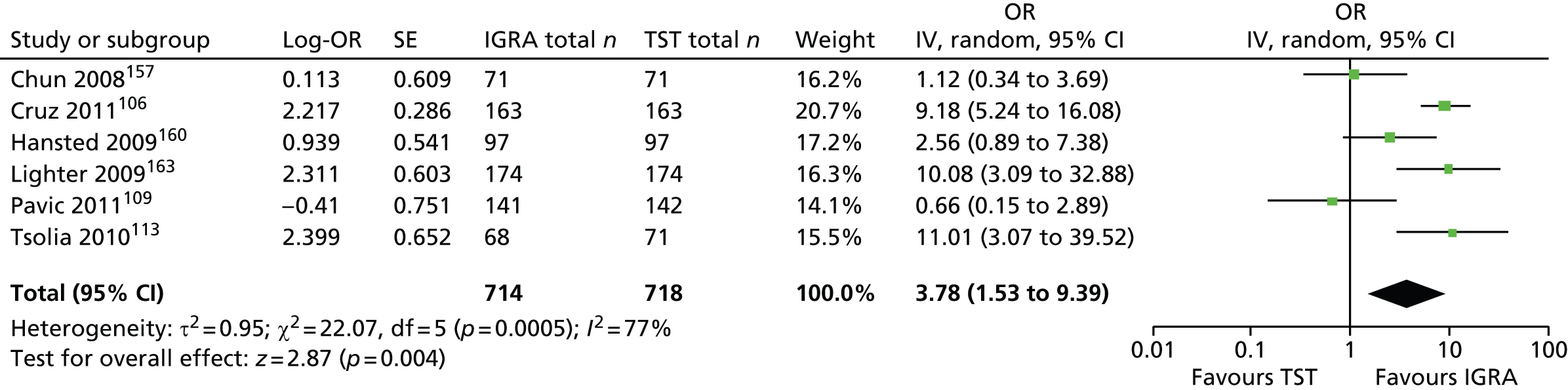
However, the subgroup analysis by country of burden explained some (but not all) of the observed heterogeneity and revealed an interesting trend, showing no difference between IGRAs and the TST in identifying LTBI across studies conducted in countries of high TB burden (pooled R-DOR 1.13, 95% CI 0.78 to 1.65; I2 = 71%) (Figure 15).
FIGURE 15.
Pooled R-DOR of IGRA vs. TST based on high- and low-risk exposure in children and adolescents: studies conducted in high-burden countries. df, degrees of freedom; IV, inverse variance; SE, standard error.
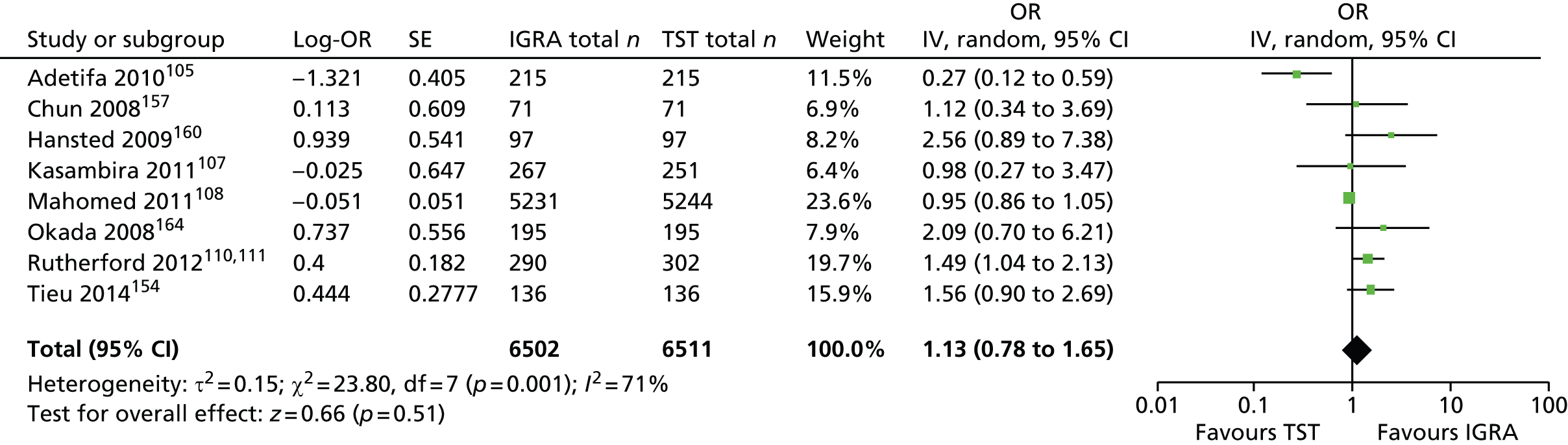
In contrast, IGRAs were significantly superior to the TST in identifying LTBI in the settings of low TB burden (pooled R-DOR 4.74, 95% CI 2.15 to 10.44; I2 = 67%) (Figure 16).
FIGURE 16.
Pooled R-DOR of IGRA vs. TST based on high- and low-risk exposure in children and adolescents: studies conducted in low-burden countries. df, degrees of freedom; IV, inverse variance; SE, standard error.

In five studies, trends for exposure gradient (across more than two ordinal exposure groups) for IGRAs and the TST were explored with respect to sleeping proximity (same house/same room, same house/different room, different house),105,110,111 adult index case type of TB diagnosis,107 adult index case smear grade (negative, scanty, 1+, 2+, 3+),107,110,111 duration of exposure to index case (time spent with child),107,110,111,154 relationship to index case (parent, aunt/uncle, other),110,111,154 TB contact score (score-based categories)154 and type of contact (household, non-household regular, occasional). 113 In general, for both IGRAs and the TST there was an increasing trend in DOR across the exposure groups. In two studies this trend was absent for both tests in relation to duration of exposure to the index case110,111 and for the TST in relation to type of contact. 113 See Appendix 9 for full extraction sheets.
Sensitivity and specificity
In this analysis, six105,106,112,148,151,154 of the included 11 recent studies105–113,148,151,154 failed to provide sufficient information for calculating both sensitivity and specificity. There was wide variability in the sensitivity and specificity of the IGRAs (QFT-GIT/G) and TST (5 mm or 10 mm), with overlapping values across the five remaining studies107–111,113 (Figures 17–24).
FIGURE 17.
Forest plot of sensitivity based on exposure groups (QFT-GIT) in children and adolescents. df, degrees of freedom.
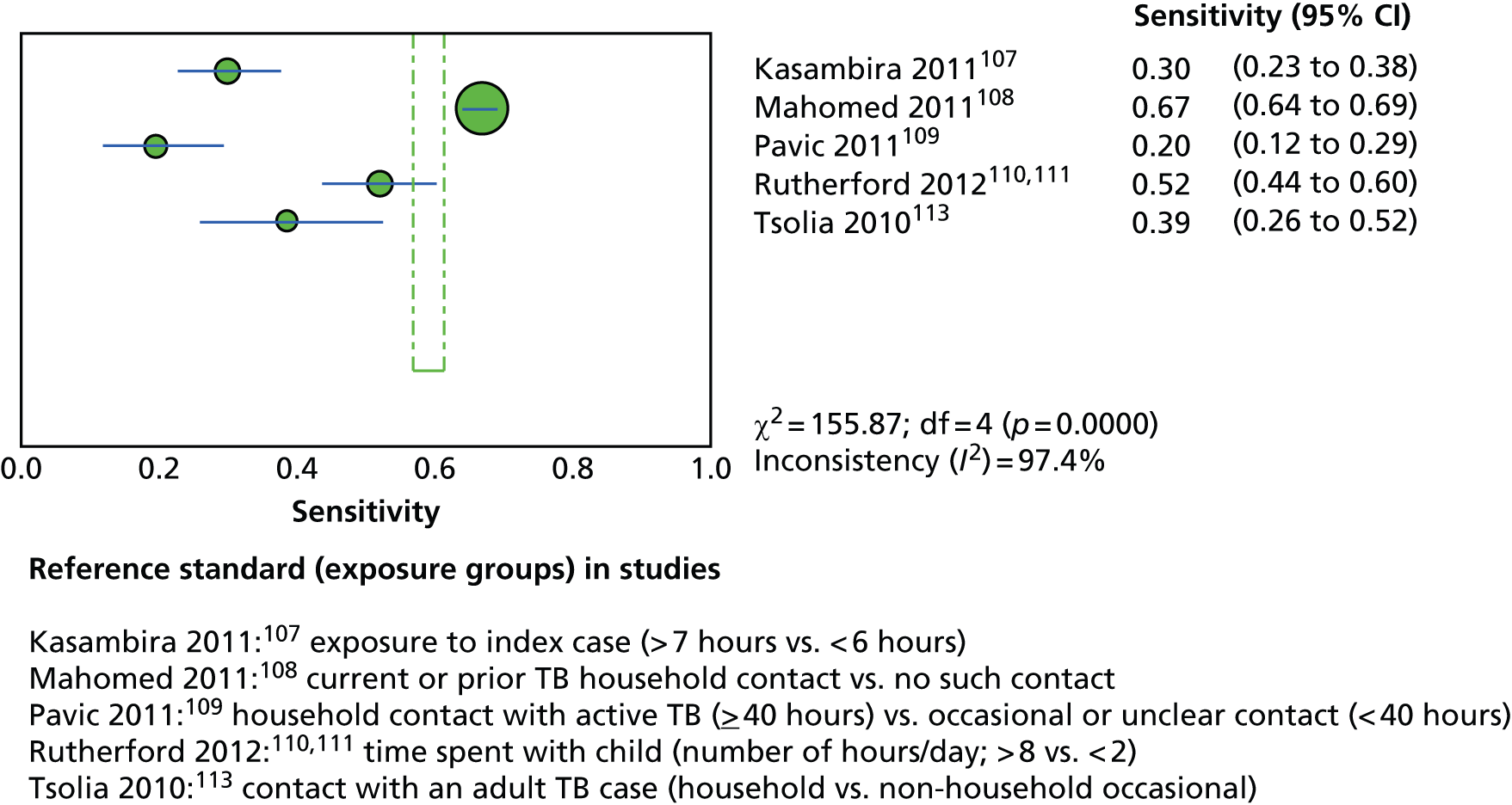
FIGURE 18.
Forest plot of sensitivity based on exposure groups (TST) in children and adolescents. df, degrees of freedom.

FIGURE 19.
Forest plot of sensitivity based on exposure groups (TST 5 mm) in children and adolescents. df, degrees of freedom.

FIGURE 20.
Forest plot of sensitivity based on exposure groups (TST 10 mm) in children and adolescents. df, degrees of freedom.
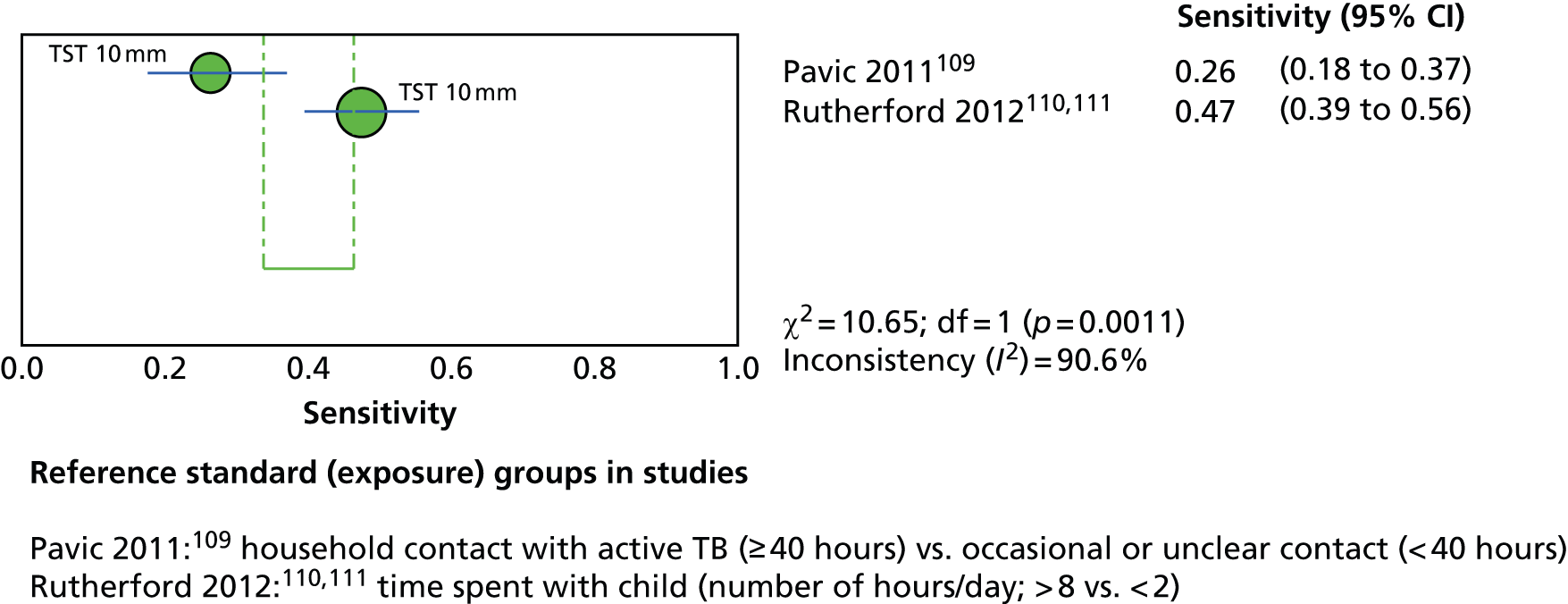
FIGURE 21.
Forest plot of specificity based on exposure groups (QFT-GIT) in children and adolescents. df, degrees of freedom.

FIGURE 22.
Forest plot of specificity based on exposure groups (TST) in children and adolescents. df, degrees of freedom.

FIGURE 23.
Forest plot of specificity based on exposure groups (TST 5 mm) in children and adolescents. df, degrees of freedom.

FIGURE 24.
Forest plot of specificity based on exposure groups (TST 10 mm) in children and adolescents. df, degrees of freedom.
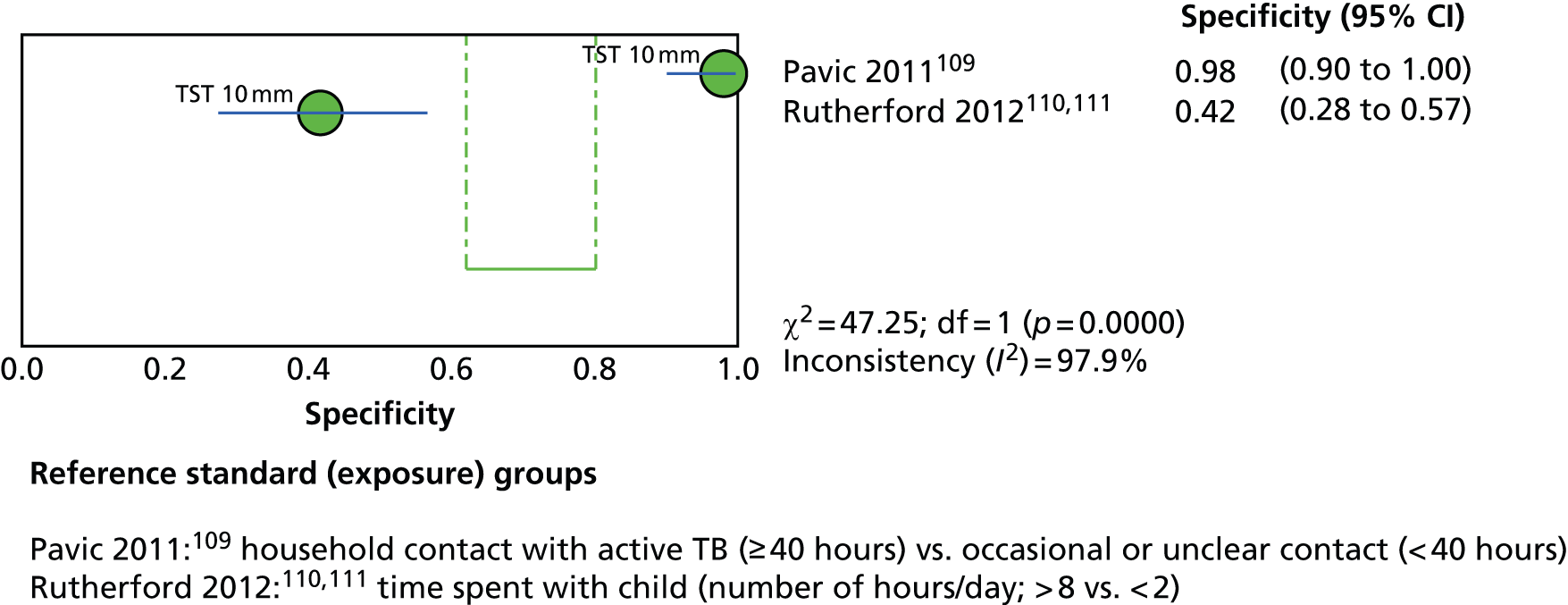
Both the QFT-GIT/G and TST (5 mm or 10 mm) demonstrated better specificity (range 36–98%) than sensitivity (range 20–71%). There was no clear numerical pattern indicating the superiority of the IGRA over the TST (or vice versa) with respect to sensitivity and specificity. Forest plots of sensitivities and specificities showed a great extent of heterogeneity that was not explained by IGRA type and/or TST threshold and therefore a meta-analysis was not performed.
Influence of bacillus Calmette–Guérin vaccination status on test positivity
In this analysis, four109,112,148,154 of the included 11 recent studies105–113,148,151,154 did not report any information needed to determine whether or not BCG vaccination status influenced the odds of test positivity differentially for the IGRAs and TST. Of the seven remaining studies reporting this evidence, only three106,108,113 demonstrated significantly increased ORs for TST positivity in relation to BCG vaccination status (range of ORs 1.16–20.34). The odds of test positivity for IGRAs across the seven studies were not significantly different between the BCG-vaccinated group and the non-vaccinated group (Table 8). One study with a relatively large sample size and narrow CIs demonstrated more conclusively that BCG vaccination status was associated with an increased odds of test positivity for TST (OR 1.16, 95% CI 1.0 to 1.33) but not for IGRA (OR 0.99, 95% CI 0.86 to 1.12). 108
| Study ID, country (burden) | Sample size, n | Type of IGRA/TST induration threshold | Association between test positivity and BCG vaccination status: OR (95% CI) | |
|---|---|---|---|---|
| Crude/unadjusted | Adjusted | |||
| Adetifa 2010,105 Gambia (low) | 199 | QFT-GIT | 1.10 (0.60 to 2.00) | NR |
| 199 | T-SPOT.TB | 1.10 (0.61 to 2.09) | NR | |
| 199 | TST 10 mm | 0.89 (0.50 to 1.70) | NR | |
| Cruz 2011,106 USA (low) | NR | T-SPOT.TB | 0.69 (0.37 to 1.31) | NR |
| NR | TST 15 mm | 4.32 (1.02 to 18.35) | NR | |
| Kasambira 2011,107 South Africa (high) | 262 | QFT-GIT | 0.62 (0.08 to 4.76) | 0.83 (0.08 to 8.33) |
| 247 | TST 5 mm | 0.38 (0.05 to 2.85) | 0.52 (0.06 to 4.00) | |
| Laniado-Laborın 2014,148 Mexico (intermediate) | 172 | QFT-GIT | NR | NR |
| 172 | TST 5 mm | NR | NR | |
| Mahomed 2011,108 South Africa (high) | 3554 | QFT-GIT | 0.99 (0.86 to 1.12) | NR |
| 3554 | TST 5 mm | 1.16 (1.00 to 1.33) | NR | |
| Pavic 2011,109 Croatia (low) | NR | QFT-GIT | NR | NR |
| NR | TST 10 mm | NR | NR | |
| Perez-Porcuna 2014,151 Brazil (intermediate) | 116 | QFT-GIT | 3.89 (0.46 to 32.33) | NR |
| 135 | TST 10 mm | 1.85 (0.36 to 9.36) | NR | |
| Rutherford 2012,110,111 Indonesia (high) | 260 | QFT-GIT | 0.51 (0.26 to 1.00) | 0.60 (0.26 to 1.38) |
| 272 | TST 10 mm | 0.68 (0.35 to 1.35) | NR | |
| Talbot 2012,112 USA (low) | NR | T-SPOT.TB | NR | NR |
| NR | TST 15 mm | NR | NR | |
| Tieu 2014,154 Thailand (high) | 136 | QFT-GIT | NR | NR |
| 136 | TST 10 mm | NR | NR | |
| 136 | T-SPOT.TB | NR | NR | |
| 136 | TST 15 mm | NR | NR | |
| Tsolia 2010,113 Greece (low) | NR | QFT-GIT | 0.19 (0.06 to 0.60) | NR |
| NR | TST 5 mm | 20.34 (5.60 to 73.89) | NR | |
Between-test concordance, discordance and agreement
This section included five studies156–159,164 reviewed in CG11710 (see Appendix 5) and 16 more recent studies102–113,148,150–152,154 (see Appendix 9). The agreement kappa statistic was not available for four studies. 102,104,106,150 There was a wide variation in the kappa statistic across the remaining studies, ranging from 0.13113 to 0.91113 (Table 9). In the post-2009 studies,103,105,107–113,148,151,152,154 the ranges of the kappa statistic according to specific TST threshold and IGRA type were as follows: QFT-GIT compared with TST 5 mm – range 0.27–0.91; QFT-GIT compared with TST 10 mm – range 0.13–0.64; and T-SPOT. TB compared with TST 10 mm – range 0.53–0.71. According to one study, both between-test per cent concordance and the kappa statistic were lower among participants with a BCG vaccination history (concordance 46.5%, kappa 0.16) than among those without such history (concordance 96.20%, kappa 0.91). 113
| Study ID, country (burden) | Sample size, total or by subgroup, n | Type of IGRA vs. TST induration threshold | Concordance (95% CI) (%) | Discordance (95% CI) (%) | Agreement kappa (95% CI) |
|---|---|---|---|---|---|
| Adetifa 2010,105 Gambia (low) | 217 | QFT-GIT vs. TST 10 mm | 80.00 (74.15 to 84.80) | 20.00 (15.2 to 25.85) | 0.52 (0.39 to 0.65) |
| 215 | T-SPOT.TB vs. TST 10 mm | 80.47 (74.65 to 85.21) | 19.53 (14.79 to 25.35) | 0.53 (0.40 to 0.66) | |
| Cruz 2011,106 USA (low) | NR | T-SPOT.TB vs. TST 15 mm | NR | NR | NR |
| Diel 2011,102 Germany (low) | NR | QFT-GIT vs. TST 5/10 mm | NR | NR | NR |
| Kasambira 2011,107 South Africa (high) | 254 | QFT-GIT vs. TST 5 mm | 86.86 (81.96 to 90.59) | 13.14 (9.41 to 18.04) | 0.68 (0.56 to 0.81) |
| 254 | QFT-GIT vs. TST 10 mm | 85.59 (80.54 to 89.5) | 14.41 (10.5 to 19.46) | 0.64 (0.51 to 0.76) | |
| Laniado-Laborın 2014,148 Mexico (intermediate) | 172 | QFT-GIT vs. TST 5 mm | 59.88 (52.42 to 66.92) | 40.12 (33.08 to 47.58) | 0.27 (0.17 to 0.38) |
| Mahomed 2011,108 South Africa (high) | NR | QFT-GIT vs. TST 5 mm | 84.8 (NR) | NR | 0.70 (0.68 to 0.71) |
| NR | QFT-GIT vs. TST 10 mm | 81.4 (NR) | NR | 0.63 (0.61 to 0.65) | |
| NR | QFT-GIT vs. TST 15 mm | 64.3 (NR) | NR | 0.30 (0.27 to 0.32) | |
| Mahomed 2011,103 South Africa (high) | 5244 | QFT-GIT vs. TST 5 mm | 84.80 (83.80 to 85.75) | 15.20 (14.25 to 16.20) | 0.69 (0.66 to 0.72) |
| Metin Timur 2014,150 Turkey (intermediate) | 81 | QFT-GIT vs. TST 15 mm | NR | NR | NR |
| Noorbakhsh 2011,104 Iran (intermediate) | NR | QFT-GIT vs. TST 10 mm | NR | NR | NR |
| Pavic 2011,109 Croatia (low) | 141 | QFT-GIT vs. TST 10 mm | 89.36 (83.19 to 93.45) | 10.64 (6.554 to 16.81) | 0.59 (0.42 to 0.75) |
| Perez-Porcuna 2014,151 Brazil (intermediate) | 116 | QFT-GIT vs. TST 10 mm | 71.55 (62.75 to 78.97) | 28.44 (21.03 to 37.25) | 0.35 (0.16 to 0.53) |
| Rutherford 2012,110,111 Indonesia (high) | 292 | QFT-GIT vs. TST 10 mm | 80.48 (75.55 to 84.62) | 19.52 (15.38 to 24.45) | 0.61 (0.49 to 0.72) |
| Song 2014,152 South Korea (high) | 2982 | QFT-GIT vs. TST 10 mm | 82.6 (81.2 to 83.92) | 17.4 (16.08 to 18.80) | 0.38 (0.34 to 0.42) |
| 2982 | QFT-GIT vs. TST 15 mm | 92.52 (91.51 to 93.41) | 7.48 (6.59 to 8.48) | 0.55 (0.50 to 0.61) | |
| Talbot 2012,112 USA (low) | 143 | T-SPOT.TB vs. TST 15 mm | 97.9 (94.01 to 99.28) | 2.01 (0.72 to 5.99) | 0.71 (0.55 to 0.88) |
| Tieu 2014,154 Thailand (high) | 131 | QFT-GIT vs. TST 10 mm | 59.54 (50.98 to 67.56) | 40.46 (32.44 to 49.02) | 0.29 (0.18 to 0.40) |
| 131 | QFT-GIT vs. TST 15 mm | 79.39 (71.67 to 85.43) | 20.61 (14.57 to 28.33) | 0.53 (0.38 to 0.69) | |
| 131 | T-SPOT.TB vs. TST 10 mm | 55.73 (47.18 to 63.95) | 44.27 (36.05 to 52.82) | 0.23 (0.12 to 0.34) | |
| 131 | T-SPOT.TB vs. TST 15 mm | 78.63 (70.84 to 84.78) | 21.37 (15.22 to 29.16) | 0.51 (0.35 to 0.66) | |
| Tsolia 2010,113 Greece (low) | 99 | QFT-GIT vs. TST NR | 71.58 (61.81 to 79.67) | 28.42 (20.33 to 38.19) | 0.45 (0.27 to 0.63) |
| 43 with BCG historya | QFT-GIT vs. TST 10 mm | 46.50 (NR) | NR | 0.13 (p = 0.06) | |
| 52 no BCG historya | QFT-GIT vs. TST 5 mm | 96.20 (NR) | NR | 0.91 (p = 0.06) |
Summary of studies in children and adolescents
Although there is a limited amount of evidence, the three prospective studies suggested no significant difference between QFT-GIT and TST 5 mm (pooled R-CIR 1.12, 95% CI 0.72 to 1.75). QFT-GIT performed significantly better than TST 10 mm in identifying LTBI or predicting risk of active TB (pooled R-CIR 4.33, 95% CI 1.32 to 14.23). In five newly identified prospective studies investigating the incidence of active TB, there was a wide variability in sensitivity and specificity of IGRAs (QFT-GIT/G) and TST (5 mm or 10 mm). Because of high unexplained heterogeneity (not explained by IGRA type and TST threshold, similar diagnostic methods of active TB), no meta-analysis could be performed. IGRAs (QFT-GIT/G) demonstrated similar sensitivity (range 48–100%) and slightly better specificity (range 49–90%) than TST 5 mm (sensitivity range 57–100%; specificity range 45–65%). Although the sensitivities of the IGRAs and TST 5 mm were higher than that for TST 10 mm/15 mm (range 30–56%), the corresponding specificities of these tests were lower than that of TST 10 mm/15 mm (range 63–93%).
The updated meta-analysis of 14 studies showed a significantly stronger association for IGRAs than for the TST in relation to risk of LTBI/exposure level (pooled R-DOR 1.98, 95% CI 1.19 to 3.28; I2 = 89%). The subgroup analysis by country of burden explained some (but not all) of the observed heterogeneity and revealed a trend showing no difference between the IGRAs and the TST in identifying LTBI across studies conducted in countries of high TB burden (pooled R-DOR 1.13, 95% CI 0.78 to 1.65; I2 = 71). In contrast, IGRAs were significantly superior to the TST in identifying LTBI in the settings of low TB burden (pooled R-DOR 4.74, 95% CI 2.15 to 10.44; I2 = 67%). In five studies both tests revealed strong associations of increasing strength across the exposure gradient for most exposures (sleeping proximity, adult index case type of TB diagnosis, adult index case smear grade, TB contact score and relationship to index case).
There was limited evidence of whether or not BCG vaccination status influenced the odds of test positivity differentially for IGRAs and TST. Out of seven studies reporting relevant data, only three demonstrated a significantly increased OR for TST positivity in relation to BCG vaccination status (range of ORs 1.16–20.34). The odds of test positivity for IGRAs across six studies were not significantly different between the BCG vaccinated and the non-vaccinated groups. One large study showed that there was a statistically significant association between BCG vaccination status and an increased odds of test positivity for TST (OR 1.16, 95% CI 1.0 to 1.33) but not for IGRA (OR 0.99, 95% CI 0.86 to 1.12).
There was a wide variation in the kappa statistic across 17 studies (five studies from CG11710 and 12 more recent studies), ranging from 0.13 to 0.91. In the post-2009 studies,103,105,107–113,148,151,152,154 the ranges of the kappa statistic according to specific TST threshold and IGRA type were as follows: QFT-GIT vs. TST 5 mm – range 0.27–0.91 mm; QFT-GIT vs. TST 10 mm – range 0.13–0.64 mm; and T-SPOT. TB vs. TST 10 mm – range 0.53–0.71 mm.
Immunocompromised people
Description of baseline characteristics: qualitative synthesis in text and tables
This section included 48 studies. 114–142,149,153,155,167–182 Our searches identified 32 studies114–142,149,153,155 in immunocompromised patients, of which eight investigated the incidence of active TB following testing for LTBI (incidence studies) and 24120–142,153 investigated levels of exposure in relationship to LTBI test outcomes (exposure studies). An additional 16 studies167–182 in immunocompromised patients were identified in CG117. 10
Incidence studies
Eight studies114–119,149,155 compared an IGRA test with the TST in immunocompromised people. Reasons for immunodeficiency (condition and procedure) varied across studies. We identified the following subpopulations: (1) HIV patients, (2) haematopoietic stem cell transplantation candidates or recipients, (3) post-kidney transplantation patients, (4) patients undergoing haemodialysis in end-stage renal disease (ESRD) and (5) patients with immune-mediated inflammatory disease before antiTNF-α therapy. The included studies are described below according to these subpopulations. Table 10 provides further details on these studies.
| Study ID, country (burden) | Study aim, setting, design, follow-up duration and funding source | Method(s) of diagnosis of active TB | Inclusion/exclusion criteria | Type and positivity threshold(s) of tests compared | Characteristics of study participants at baseline | Number of recruited and excluded study participants | Comments |
|---|---|---|---|---|---|---|---|
| HIV infection | |||||||
| Elzi 2011,114 Switzerland (Low) | Aim: to evaluate the sensitivity of T-SPOT.TB in comparison to TST in identifying HIV-infected individuals with LTBI Setting: community-based cohort Design: retrospective case-only study (no control group) Follow-up: 2 years Funding source: grants/honoraria received from private manufacturers (Abbott, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Roche, Janssen, Pfizer) |
NR | Inclusion criteria: NR Exclusion criteria: NR |
Type of tests: IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (T-SPOT.TB): ≥ 6 spots in either or both of panel A and panel B – when the positive control was < 20 spots or the negative control was ≥ 10 spots, the test was scored as indeterminate; TST: induration of ≥ 5 mm |
Median (IQR) age: 33 (31–42) years Female, n/N (%): 20/64 (31) Race/ethnicity, n/N (%): white 29/64 (45.3) Geographical origin, n/N (%): NR BCG vaccination, n/N (%): NR History of antiTB treatment, n/N (%): NR Total incidence of active TB, n/N (%): NR Chest radiography (yes/no): NR Clinical examination (yes/no): NR Morbidity, n/N (%): HIV Co-morbidity, n/N (%): NR |
Total number of recruited patients: 64; total number of excluded patients: 0 – however, the total number of patients with valid results for both IGRA and TST was 44 | T-SPOT.TB was retrospectively performed using frozen viable lymphocytes of HIV-infected individuals stored within 6 months before culture-confirmed TB occurred. This retrospective case-only study does not allow an estimate of the incidence of active TB in test-positive and test-negative groups from baseline (no denominators provided) |
| Haematopoietic stem cell transplantation candidates | |||||||
| Moon 2013,115 South Korea (high) | Aim: to compare QFT-GIT with the TST in HCT candidates for detecting LTBI Setting: Asan Medical Center, Seoul Design: prospective cohort study Follow-up: median (IQR) 0.8 (0.1–2.6) years Funding source: Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology (MEST) (grant 2010–0005898) |
NR | Inclusion criteria: all adult patients admitted for HCT Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): according to manufacturer; TST: induration of ≥ 5mm |
Mean (range) age: 47 (35–55) Female, n (%): 107 (44) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 201 (82) History of antiTB treatment, n (%): 10 (4) Total incidence of active TB, n (%): 2 (0.80) Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): acute myelogenous leukaemia 72 (30), acute lymphoblastic leukaemia 28 (11), chronic myelogenous leukaemia 4 (2), aplastic anaemia 17 (7), myelodysplastic syndrome 19 (8), non-Hodgkin’s lymphoma 58 (24), Hodgkin’s lymphoma 3 (1), multiple myeloma 38 (16), plasmacytoma 2 (1), others 3 (1) Comorbidity, n (%): diabetes mellitus 25 (10), hypertension 38 (16), chronic kidney disease 21 (9), ESRD with dialysis 1 (0.4), hepatitis 16 (7), HIV infection 0 (0), non-haematological malignancy 9 (4) Type of during-study treatment, n (%): ciclosporin 71 (29), ciclosporin–MTX 65 (27), ciclosporin–corticosteroid 8 (3), corticosteroid therapy 111 (46) |
Total number of recruited patients: NR; total number of excluded patients: 52 patients died and 2 were lost to follow-up | Blood samples were collected before performing the TST to avoid a possible boosting effect of the TST on the QFT-GIT test. The laboratory technicians did not know the results of the TST |
| Haematopoietic stem cell transplantation recipients | |||||||
| Lee 2014,149 South Korea (high) | Aim: to test the hypothesis that HCT recipients who are QFT-TB positive develop active TB more frequently than QFT-TB-negative or indeterminate patients and to evaluate if the QFT-TB assay can predict active TB development in HCT recipients without any clinical risk factors for LTBI Setting: tertiary hospital based Design: prospective cohort study Follow-up: median (IQR) 1.3 (0.6–2.3) years Funding source: supported by a grant from the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning |
Chest radiography, a sputum AFB smear and CT scan (pulmonary TB) | Inclusion criteria: adult patients admitted for allogeneic HCT Exclusion criteria: history of close contact with active TB, history of untreated or inadequately treated TB, radiographic evidence of old TB, refused informed consent, presence of active TB, presence of skin disease that precluded the TST (between January 2010 and December 2011) and paediatric HCT candidates (aged < 16 years) |
Type of tests: IGRA (QFT-GIT) and TST Cut-off values/thresholds: QFT-GIT: NR; TST: induration of ≥ 5 mm or ≥ 10 mm |
Mean (SD) age: 42.3 (13.8) years Female, n (%): 183 (46.8) Race/ethnicity, n (%): Asian 409 (100) Geographical origin, n (%): NR BCG vaccination, n (%): 353 (90.7) History of antiTB treatment, n (%): 0 Total incidence of active TB, n/N (%): 8/391 (2.04) Chest radiography (yes/no): yes Clinical examination (yes/no): NR Morbidity, n (%): HCT recipients Comorbidity, n (%): acute or chronic graft-versus-host disease 151 (38.6); diabetes mellitus 32 (8.2); liver cirrhosis 4 (1.0); solid organ transplant 2 (0.5); HIV 0 |
Total number of recruited patients: 409; total number of excluded patients: 18 | |
| Post-kidney transplantation | |||||||
| Kim 2011,116 South Korea (high) | Aim: to assess whether an ELISPOT assay is capable of predicting active TB development in KT recipients with negative TST results and without LTBI risk factors Setting: tertiary-care hospital Design: prospective cohort study Follow-up: median (IQR) 14 (8–19) months Funding source: basic Science Research Program through National Research Foundation funded by the Ministry of Education, Science and Technology (MEST) (grant 2008-E00136) |
Symptoms/signs, sputum AFB smear and a CT scan | Inclusion criteria: KT patients aged ≥ 16 years with TST (< 10 mm) and without LTBI risk factors (history of close contact with TB case, abnormal chest radiography, history of untreated or inadequately treated TB, newly infected) Exclusion criteria: refusal of informed consent, presence of active TB, presence of skin disease that precluded TST, paediatric renal transplant candidates (aged < 16 years), TB risk factors and presence of any contraindication for KT (e.g. malignancy) |
Type of tests: IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (T-SPOT.TB): NR; TST: induration of ≥ 10 mm 48–72 hours after injection and in accordance with Korea Centers for Disease Control and Prevention guidelines |
Age range: 40.4–46.0 years Female, n (%): 126 (46.3) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 215 (79.0) History of anti-TB treatment, n (%): 0 Total incidence of active, n/N (%): 4/272 (1.47) (incidence rate 0.83 per person-years, 95% CI 0.23 to 2.12 per person-years) Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): glomerulonephritis 72 (26.5), hypertension 65 (23.9), diabetes mellitus 48 (17.6), unknown 58 (21.3), polycystic kidney 12 (4.4), other 11 (4.0) Comorbidity, n (%): NR |
Total number of recruited patients: 324; total number of excluded patients: 52 – the total number of patients with valid results for both IGRA and TST was 242 | The development of TB after KT was observed by attending surgeons, nephrologists and infectious disease specialists blind to the results of the ELISPOT assays, to avoid verification bias |
| Haemodialysis in ESRD | |||||||
| Anibarro 2012,117 Spain (low) | Aim: to compare IGRA with TST in patients with ESRD after a TB outbreak at a dialysis centre Setting: outbreak investigation Design: prospective cohort study Follow-up: 18 months Funding source: University of Vigo and Sudoefeder (IMMUNONET-SOE1/P1/E014) |
Microscopic examination of sputum and sputum culture | Inclusion criteria: all patients who attended the dialysis unit while index case was on duty Exclusion criteria: patients who had had a previous positive TST test |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): 0.35 IU/ml; TST: induration of ≥ 5 mm (a second test was performed 5 days later if the first TST was < 5 mm) |
Mean (SD) age: 62 (16.8) years Female, n (%): 21 (40.4) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 7 (13.5) History of anti-TB treatment, n (%): NR Total incidence of active TB, n (%): 0 Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): ESRD 58 (100) Comorbidity, n (%): diabetes mellitus 8 (15.4) |
Total number of recruited patients: 58; total number of excluded patients: 6 | The second test was performed 5 days after the initial test but it is not stated when the result of the second test was read |
| Lee 2009,118 Taiwan (high) | Aim: to compare QFT-G, T-SPOT.TB and TST in terms of their ability to diagnose LTBI in ESRD patients and to determine the prevalence of LTBI in ESRD patients compared with healthy control subjects, the risk factors for QFT-G and TST positivity and the predictive value of a positive QFT-G, T-SPOT.TB or TST for active TB disease over a 2-year period Setting: NR Study design: prospective, matched, double-cohort study Follow up: 2-year follow-up Funding source: National Health Research Institutes, Department of Health, Executive Yuan, Republic of China (NHRI-CN-CL-094-PP13) and Kaohsiung Veterans General Hospital, Kaohsuing, Taiwan (VGHKS95–012) |
Asymptomatic cases were diagnosed by chest radiography and symptomatic cases were diagnosed with a sputum TB smear, culture and chest radiography | Inclusion criteria: patients with ESRD Exclusion criteria: NR |
Type of tests: IGRA (QFT-G, T-SPOT.TB), TST (two step) Cut-off values/thresholds: IGRA (QFT-G): according to analysis software, available for download from the Cellestis Ltd website; IGRA (T-SPOT.TB): NR; TST: ≥ 10-mm induration for ESRD patients and BCG-unvaccinated individuals, ≥ 15-mm induration for BCG-vaccinated, healthy individuals |
Mean (range) age: 53.8 (34.4–77.7) years Female, n (%): 24 (37.5) Race/ethnicity, n (%): NR Geographical origin, n (%): Kaohsiung BCG vaccination, n (%): 53 (82.8) History of anti-TB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): NR Morbidity, n (%): ESRD Comorbidity, n (%): NR |
Total number of recruited patients: 64; total number of excluded patients: 0 | |
| Sherkat 2014,155 Iran (intermediate) | Aim: to compare the IGRA (T-SPOT.TB) and TST in the detection of LTBI in KT candidates and evaluate the agreement between the two tests Setting: hospital based Design: prospective cohort study Follow-up: 21 months (follow-up included 9 months of prophylactic treatment and 12 months post transplantation) Funding source: none |
NR | Inclusion criteria: candidates for KT Exclusion criteria: active TB, history of previous TB or isoniazid prophylactic treatment, refusal to continue prophylactic treatment, symptoms of isoniazid-induced hepatitis or drug reaction |
Type of tests: IGRA (T-SPOT.TB), TST Cut-off values/thresholds: T-SPOT.TB: NR; TST: induration of ≥ 10 mm |
Mean (SD) age: 44 (15.5) years Female, n (%): 15 (66) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 12 (27.3) History of anti-TB treatment, n (%): 0 Total incidence of active TB, n/N (%): 1/44 (2.27) Chest radiography (yes/no): NR Clinical examination (yes/no): yes Morbidity, n (%): ESRD Comorbidity, n (%): dialysis 30 (68.2), hypertension 10 (22.7), diabetes 10 (22.7), obstructive uropathy 6 (13.6), polycystic kidney 6 (13.6), other renal etiologies 17 (38.6), other 3 (6.8) |
Total number of recruited patients: NR; total number of excluded patients: NR | |
| Immune-mediated inflammatory diseases before antiTNF-α therapy | |||||||
| Chang 2011,119 South Korea (high) | Aim: to evaluate the usefulness of IGRA for the diagnosis of LTBI in arthritis patients who received TNF antagonists in South Korea Setting: hospital based Design: prospective cohort study Follow-up: 18 months (median) Funding source: IN-SUNG Foundation for Medical Research (CA98051) |
Medical history (current symptoms, previous history of treatment for TB and recent history of contact with a case of active TB) and TST (according to the recommendation of the Korea Food and Drug Administration) | Inclusion criteria: patients with inflammatory arthritis including rheumatoid arthritis and ankylosing spondylitis who visited the facility to evaluate LTBI before starting TNF antagonists Exclusion criteria: active TB |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): ≥ 0.35 IU/ml; TST: induration of ≥ 10 mm after 48–72 hours |
Median age: 39 years Female, n (%): 44 (41) Race/ethnicity, n (%): Asian Geographical origin, n (%): NR BCG vaccination, n (%): 63 (59) History of anti-TB treatment, n (%): 4 (3.8) Total incidence of active TB, n (%): 1 (0.9%) (patient had active TB at recruitment and was excluded from the study) Chest radiography (yes/no): NR Clinical examination (yes/no): yes Morbidity, n (%): rheumatoid arthritis 46 (43), ankylosing spondylitis 61 (57) Comorbidity, n (%): NR |
Total number of recruited patients: 108; total number of excluded patients: 1 | Both the TST and QFT-GIT were performed on the same day as the screening examination in all patients before initiating TNF antagonists |
One study compared the T-SPOT. TB with the TST (≥ 5 mm) in a retrospective case study of HIV patients (31.1% female) with a median age of 33 years. 114 The study was carried out in a community setting in Switzerland with a follow up of 2 years. The proportion of BCG-vaccinated participants was not reported.
Moon et al. 115 compared QFT-GIT with TST (≥ 5 mm) in haematopoietic stem cell transplantation candidates in a prospective cohort study in a hospital setting in South Korea. The mean age of patients was 47 years and 44% were female. The median (interquartile range) follow-up time to assess patients for active TB was 0.8 (0.1–2.6) years. BCG vaccination was high at 82%. Another study by Lee et al. 149 compared QFT-GIT with TST (≥ 5 mm or ≥ 10 mm) in haematopoietic stem cell transplant recipients who were followed up for a median of 1.3 years. The patients’ mean age was 42.3 years, 47% were female and 91% had a BCG immunisation scar.
Post-kidney transplantation patients were investigated by Kim et al. 116 in a prospective cohort study comparing T-SPOT. TB with TST (≥ 10 mm). The setting was a tertiary care hospital in South Korea. The age range reported was 40–46 years, 46% of the participants were female and 79% were BCG vaccinated. Patients were followed up for a median of 14 months.
Three studies117,118,155 investigated IGRA and TST in haemodialysis patients with ESRD. Tests compared were QFT-GIT and TST (≥ 5 mm),117 T-SPOT. TB and TST (≥ 10 mm),155 and QFT-G, T-SPOT. TB and TST (two step; ≥ 10 mm). 118 Anibarro et al. 117 undertook a prospective cohort study in a Spanish dialysis unit following a TB outbreak in the dialysis centre. Lee et al. 118 carried out a prospective, matched cohort study in Taiwan. The setting was unreported. The mean age and proportion of female patients was 62 years and 40% in the study by Anibarro et al. ,117 44 years and 66% in the study by Sherkat et al. 155 and 54 years and 38% in the study by Lee et al. 118 The follow-up across the three studies ranged from 1.5 years117 to 2 years. 118 The proportion of BCG-vaccinated patients was low (13.5%) in the study by Anibarro et al. ,117 intermediate (27.3%) in the study by Sherkat et al. 155 and high (82.8%) in the study by Lee et al. 118
Chang et al. 119 compared QFT-GIT with TST (≥ 10 mm) in a prospective cohort study in patients with immune-mediated inflammatory diseases investigated for LTBI before treatment with antiTNF-α. The study setting was a hospital in South Korea. Patients were followed up for a median of 18 months. The median age of patients was 39 years, 41% were female and 59% were BCG vaccinated.
Exposure studies
Twenty-four newly identified studies120–142,153 compared an IGRA test with the TST in immunocompromised people, relating test outcome to previous level of exposure. All studies within this group were therefore classed as having either a retrospective cohort or a cross-sectional design. Reasons for immunodeficiency (condition and procedure) varied across studies. We identified the following subpopulations: (1) HIV patients, (2) solid organ transplantation candidates, (3) post-kidney transplantation patients, (4) patients on haemodialysis for ESRD, (5) patients with immune-mediated inflammatory diseases before antiTNF-α therapy, (6) patients with hepatitis C and (7) lupus erythematosus patients. The included studies are described below according to these subpopulations. Table 11 provides further details on these studies.
| Study ID, country (burden) | Study aim, setting, design and funding source | Definition of construct validity (i.e. LTBI exposure-based proxy) | Inclusion/exclusion criteria | Type and positivity threshold(s) of tests compared | Characteristics of study participants at baseline | Nos of recruited and excluded study participants | Comments |
|---|---|---|---|---|---|---|---|
| HIV infection | |||||||
| Chkhartishvili 2013,125 Georgia (high) | Aim: to assess the performance of two commercially available IGRAs (QFT-GIT and T-SPOT.TB) compared with that of the TST for the diagnosis of LTBI in HIV-infected patients and to identify risk factors for LTBI in an effort to improve TB prevention and care among HIV patients Setting: national referral institution for HIV diagnosis, treatment and care Design: retrospective/cross-sectional study Funding source: the US Civilian Research and Development Foundation award; the National Institutes of Health Fogarty International Center through the Emory AIDS International Training and Research Program award and the Emory–Georgia Tuberculosis Research Training Program award |
Non-exposed: no household member treated for TB; exposed 1: household member treated for TB; exposed 2: NA | Inclusion criteria: age ≥ 18 years, confirmed HIV infection and ability to provide written informed consent Exclusion criteria: patients with a history of active TB disease |
Type of tests: IGRA (QFT-GIT), IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (QFT-GIT): IFN-γ response to TB antigens minus the negative control was ≥ 0.35 IU/ml and also > 25% of the negative control, indeterminate if either the negative control was > 8 IU/ml or the positive control was < 0.5 IU/ml; IGRA (T-SPOT.TB): six or more spot-forming cells or twice the nil control, indeterminate if the nil control spot count was > 10 spot-forming cells or if the reading in the positive control was < 20 spot-forming cells; TST: induraton of ≥ 5 mm |
Median (range) age: 38.0 (32.8–43.8) years Female, n (%): 81 (33.75) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 219 (94%) History of anti-TB treatment, n (%): NR Total incidence of active TB, n (%): NA Chest radiography (yes/no): NR Clinical examination (yes/no): NR Morbidity, n (%): HIV infection Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: NR; total number of excluded patients: NR | Blood was drawn for the IGRAs prior to placement of the TST |
| Mutsvangwa 2010,136 Zimbabwe (high) | Aim: to test for LTBI using T-SPOT.TB and TST, correlate test results with TB exposure in household contacts of TB cases and assess the impact of HIV co-infection on test results in these contacts Setting: NR Design: retrospective cohort/cross-sectional study Funding source: the Wellcome Trust |
Non-exposed: contact of index control (no TB); exposed 1: contact of index TB case; exposed 2: NA | Inclusion criteria: all consenting individuals over the age of 10 years living with the TB cases (index case household contacts) and those living with control subjects (no TB); TB cases were sampled from factories in Harare and control samples were sampled randomly from the same factories Exclusion criteria: NR |
Type of tests: IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (T-SPOT.TB): NR; TST: induration of ≥ 10 mm (if < 10 mm second TST after 7–14 days) |
Mean (range or SD) age: NR Female, n (%): 65 (89.0) Race/ethnicity, n (%): NR Geographical origin, n (%): sub-Saharan Africa BCG vaccination, n (%): 63 (86.0) History of anti-TB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): NR Clinical examination (yes/no): NR Morbidity, n (%): HIV infection Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: NR; total number of excluded patients: NR | Persons performing and reading the assays were blind to all personal identifiers and TST results |
| Souza 2014,153 Brazil (intermediate) | Aim: to evaluate the added value of QFT-GIT over the TST for detecting LTBI among those living with HIV/AIDS and to explore the factors associated with a positive QFT-GIT and with discordant QFT-GIT/TST results Setting: outpatient clinics Design: retrospective cohort/cross-sectional study Funding source: Fundação de Apoio ā Pesquisa do Distrito Federal |
Non-exposed: no history of contact with index case; exposed: history of contact with index case | Inclusion criteria: people with HIV/AIDS aged > 17 years who had not had a TST in the previous 5 weeks Exclusion criteria: patients with a history of other immunosuppressive conditions (severe AIDS-related opportunistic infections, acute viral infections, those undergoing any vaccination in the previous 2 months and those using immunosuppressive drugs), patients with present or past active TB and those with a history of a previous positive TST |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): ≥ 0.35 UI/ml; TST: induration of ≥ 5 mm |
Median (IQR) age: 40 (32–46) years Female, n (%): 85 (28.3) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 228 (76.0) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NA Chest radiography (yes/no): NR Clinical examination (yes/no): NR Morbidity, n (%): HIV/AIDS 300 (100) Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: NR; total number of excluded patients: NR | |
| SOT candidates | |||||||
| Ahmadinejad 2013,120 Iran (intermediate) | Aim: to compare the QFT and TST in the diagnosis of LTBI in SOT candidates (kidney, liver, lung) Setting: tertiary care teaching hospital Design: cross-sectional/retrospective cohort study Funding source: Tehran University of Medical Sciences and Health Services grant |
Non-exposed: no history of exposure to active TB; exposed 1: exposure history to active TB; exposed 2: NA | Inclusion criteria: SOT candidates referred to the transplant clinic Exclusion criteria: failure to return to the clinic to read the results of the TST within 5 days of the initial intradermal injection or unwillingness to continue the study at any stage |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): NR; TST: induration of ≥ 10 mm |
Mean (SD) age: 39.9 (12.7) years Female, n (%): 76 (46.3) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 151 (92.1) History of antiTB treatment, n/N (%): 1/164 (0.6) Total incidence of active TB, n/N (%): 1/164 (0.6) Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): ESRD 64 (39.0), chronic hepatic failure 97 (59.2), pulmonary failure 3 (1.8) Comorbidity, n (%): NA Type of during-study treatment, n (%): patients with positive TST received chemoprophylaxis with 300 mg of isoniazid for 9 months; immunosuppressive medication 24 (14.6) |
Total number of recruited patients: 187; total number of excluded patients: 23 (dropouts) | For prevention of potential boosting effect of TST on QFT, blood sampling and PPD injection were carried out simultaneously for all patients |
| Casas 2011,124 Spain (low) | Aim: to compare the performance of the TST and the QFT-GIT test in detecting LTBI in patients with ESLD requiring LT Setting: hospital based Study design: retrospective/cross-sectional study Funding source: grants from the Spanish Ministry for Health and Consumer Affairs and the Carlos III Health Institute through the Fund for Health Investigations (PI070810, 2007–2010) and from the Carlos III Health Institute and Spanish Federation for Rare Diseases through the Spanish Network for Research in Infectious Diseases; research grant from the University of Barcelona |
Non-exposed: no risk factors for TB; exposed 1: risk factors for TB (previous contact with TB, abnormal chest radiography, birth or prolonged residence in a country with a high TB burden, alcoholism, drug abuse, a previous stay in prison and involvement with health care); exposed 2: NA | Inclusion criteria: all patients with ESLD being considered for LT were invited to participate in the study Exclusion criteria: patients aged < 18 years, patients with a previous history of TB, patients who had recently been tested with the TST and patients with a known immunosuppressive condition |
Type of tests: IGRA (QFT-GIT), TST (two step) Cut-off values/thresholds: IGRA (QFT-GIT): ≥ 0.35 IU/ml (the MTB-specific antigen tube minus the nil tube), indeterminate < 0.5 IU/ml (the mitogen tube minus the nil tube) or > 8.0 IU/ml (the nil tube) (plasma samples with indeterminate results were retested); TST: induration of ≥ 5 mm at 48–72 hours in accordance with the national transplant guidelines |
Mean (SD) age: 56.4 (7.6) years Female, n (%): 23 (24.2) Race/ethnicity, n (%): Spanish 89 (93.7) Geographical origin, n (%): born or residing in a country with a high TB burden 6 (6.3) BCG vaccination, n (%): 30 (31.6) History of antiTB treatment, n (%): 0 Total incidence of active TB, n (%): NA Chest radiography (yes/no): yes Clinical examination (yes/no): NR Morbidity, n (%): cirrhosis 52 (54.7), hepatocellular carcinoma 35 (36.8), other hepatopathies 8 (8.4) Comorbidity, n (%): diabetes mellitus 28 (29.5), chronic pulmonary obstructive disease 3 (3.2), renal failure 12 (12.6) Type of during-study treatment, n (%): NR |
Total number of recruited patients: 110; total number of excluded patients: 15 (previous TB infection, HIV infection, dropouts, antiTNF-α agents, incomplete IGRA results) | |
| Kim 2010,130 South Korea (high) | Aim: to compare the results of T-SPOT.TB with those of the TST in renal transplant candidates before transplantation in a country with an intermediate TB burdena Setting: clinic based Design: retrospective/cross-sectional study Funding source: Korea Research Foundation |
Non-exposed: no LTBI group; exposed 1: (i) close contact with a person with TB within the last year, (ii) abnormal chest radiography, (iii) a history of untreated or inadequately treated TB or (iv) newly acquired infection (recent conversion of the TST to positive status); exposed 2: NA | Inclusion criteria: kidney transplant adult candidates before transplantation Exclusion criteria: if abnormal chest radiograph findings were observed, a sputum acid-fast bacilli smear and a CT scan were performed to rule out active pulmonary TB |
Type of tests: IGRA (T-SPOT.TB), TST (≥ 5 mm), TST (≥ 10 mm) Cut-off values/thresholds: IGRA (T-SPOT.TB): as recommended by manufacturer; TST: induration of ≥ 10 mm 48–72 hours after injection |
Mean (range or SD) age: NR Female, n (%): NR Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 163 (78.0) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): ESRD Comorbidity, n (%): NR Type of during-study treatment, n (%): isoniazid for 9 months immediately after renal transplantation 5 (19) |
Total number of recruited patients: 213; total number of excluded patients: 4 (n = 1 refusal, n = 1 active TB, n = 2 cancer) | All blood samples were collected before the TST to avoid the possible boosting effect of the TST on the ELISPOT assay |
| Kim 2013,131 South Korea (high) | Aim: to compare the results of the TST and QFT-GIT as methods for screening for LTBI and determine the agreement between the TST and QFT-GIT in renal transplant candidates before transplantation in a country with an intermediate TB burdena Setting: clinic based Study design: retrospective/cross-sectional study Funding source: grant from the Korean Health Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea |
Non-exposed: no LTBI group; exposed 1: (i) patients with a history of LTBI or active TB, (ii) patients with abnormal chest radiography findings consistent with previously healed TB and (iii) patients with a history of close contact with active pulmonary TB patients within the past year; exposed 2: NA | Inclusion criteria: kidney transplant adult candidates before transplantation Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): IFN-γ response of TB antigen minus that of the nil tube ≥ 0.35 IU/ml and ≥ 25% of the negative control value; TST: induration of ≥ 10 mm after 48–72 hours |
Mean (range) age: 47 (20–69) years Female, n (%): 55 (43.6) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 115 (91.3) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): ESRD/haemodialysis 100 (79.4), peritoneal dialysis 12 (9.5), no dialysis 14 (11.1) Comorbidity, n (%): hypertension 60 (47.6), diabetes mellitus 31 (24.6) Type of during-study treatment, n (%): NR |
Total number of recruited patients: NR; total number of excluded patients: NR | |
| Post-kidney transplantation | |||||||
| Hadaya 2013,128 Switzerland (low) | Aim: to compare the diagnostic performance of the TST and two IGRAs (T-SPOT.TB and QFT-GIT) in renal transplant recipients under stable immunosuppression Setting: Geneva University Hospital Design: retrospective cohort/cross-sectional study Funding source: Ligue Pulmonaire Genevoise (a non-profit organisation) |
Non-exposed: no risk for LTBI; exposed 1: risk for LTBI [chest radiography suggestive of previous infection (calcified granuloma or adenopathy, suggestive fibrotic scars) and/or close contact with TB patient]; exposed 2: NA | Inclusion criteria: aged > 18 years, able to provide informed consent, had a renal transplant at least 12 months before inclusion and having stable immunosuppression Exclusion criteria: treatment for acute rejection within the preceding 3 months and signs or symptoms of acute infection |
Type of tests: IGRA (QFT-GIT), IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (QFT-GIT): according to manufacturer; IGRA (T-SPOT.TB): according to manufacturer; TST: ≥ 5 mm transverse diameter, measured 48–72 hours after injection |
Mean (SD) age: 59.0 (13.2) years Female, n (%): 84 (42.0) Race/ethnicity, n (%): NR Geographical origin, n (%): high incidence of TB in country of origin 24 (12.0) BCG vaccination, n (%): 155 (77.5) History of antiTB treatment, n (%): active therapy 9 (4.5), LTBI treatment 12 (6.0) Total incidence of active TB, n (%): NA Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): renal transplant recipients Comorbidity, n (%): NR Type of during-study treatment, n (%): prednisone 88 (44.0), tacrolimus 127 (63.5), ciclosporin 41 (20.5), mycophenolate mofetil 159 (79.5), azathioprine 17 (8.5), sirolimus 12 (6.0) |
Total number of recruited patients: 205; total number of excluded patients: 5 (indeterminate IGRAs) | Blood sampling for the two IGRAs was performed simultaneously |
| Kim 2013,132 South Korea (high) | Aim: to compare the QFT-GIT with the TST for screening for LTBI in kidney transplant recipients Setting: NR Design: retrospective cohort/cross-sectional study (with prospective part) Funding source: Korea Health Care Technology R&D project, Ministry for Health, Welfare and Family Affairs, Republic of Korea |
Non-exposed: NR; exposed 1: history of treated TB; exposed 2: abnormal chest radiography | Inclusion criteria: kidney transplant recipients Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): ≥ 0.35 IU/ml and ≥ 25% in the presence of TB-specific antigen minus that of the nil tube; TST: induration of ≥ 10 mm at 48–72 hours after injection |
Mean age: 44.7 ± 11.5 years Female, n (%): 41 (38) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): NR History of antiTB treatment, n (%): 3 (2.8) Total incidence of active TB, n (%): 1 (0.9) Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): glomerulonephritis 19 (17.4); hypertensive nephrosclerosis 11 (10.1); diabetes mellitus 31 (28.4); unknown 34 (31.2); polycystic kidney disease 2 (1.8); other 12 (11.0) Type of during-study treatment, n (%): NR |
Total number of recruited patients: 109; total number of excluded patients: 4 with indeterminate QFT-GIT results (excluded for analysis) | |
| Haemodialysis in patients with ESRD | |||||||
| Al Jahdali 2013,121 Saudi Arabia (low) | Aim: to compare the performance of the QTF-GIT test and the TST for detecting LTBI among haemodialysis patients and to investigate the agreement between these two tests in the detection of TB infection in a population showing an intermediate TB prevalence Setting: outpatient haemodialysis unit, hospital based Design: retrospective cohort/cross-sectional study Funding source: none |
Non-exposed: no high likelihood of LTBI; exposed 1: high likelihood of LTBI (contact with TB case, abnormal chest radiography, diabetes mellitus, immunosuppressant in the last 12 months, failed kidney transplant or BMI ≤ 20 kg/m2); exposed 2: NA | Inclusion criteria: haemodialysis patients Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): ≥ 0.35 IU/ml for the relationship [(IFN-γ in the TB antigen tube)−(IFN-γ in the negative control tube)] – if the IFN-γ level was < 0.35 IU/ml in the TB antigen tube and the mitogen control was positive (≥ 0.5 IU/ml), the test was recorded as negative; TST: induration of ≥ 10 mm for LTBI – for results < 10 mm a second TST was carried out within 3–6 weeks (positive if either the first or second test showed a response of ≥ 10 mm) |
Mean (SD) age: 58.42 (17.65) years Female, n (%): 103 (51.5) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 28 (14.0) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NA Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): haemodialysis patients Comorbidity, n (%): diabetic nephropathy 127 (63.5), kidney transplant failed 21 (10.5), NR 52 (26.0) Type of during-study treatment, n (%): immunosuppressant in the last 12 months 2 (1.0) |
Total number of recruited patients: 215; total number of excluded patients: 15 (active TB) | IGRA blood was collected before the administration of the TST |
| Ates 2009,122 Turkey (intermediate) | Aim: to assess the efficacy of the QTF-GIT test for the detection of LTBI and determine the degree of agreement between the TST and QTF-GIT in haemodialysis patients Setting: outpatient haemodialysis hospital centres Design: retrospective cohort/cross-sectional study Funding source: grant from the University of Dicle |
Non-exposed: no TB exposure; exposed 1: TB exposure; exposed 2: NA | Inclusion criteria: haemodialysis patients aged ≥ 18 years Exclusion criteria: patients diagnosed with active TB and receiving treatment for the last 12 months, those taking immunosuppressive medicine or those aged < 18 years |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): according to the QTF-GIT analysis software; TST: induration of ≥ 10 mm |
Mean (SD) age: 51.9 (16.2) years Female, n (%): 137 (50.0) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 134 (48.72) History of antiTB treatment, n (%): 17 (7.4%) Total incidence of active TB, n (%): NA Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): haemodialysis Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: 290; total number of excluded patients: 15 (rejected tests, improper blood sampling and unsuccessful phlebotomy) | Observers were blinded to the results of the TST |
| Chung 2010,126 South Korea (high) | Aim: to compare two IGRAs (QFT-GIT and T-SPOT.TB) simultaneously with the TST for diagnostic efficacy for LTBI in Korea, an intermediate TB burden countrya Setting: medical centre Design: retrospective cohort/cross-sectional study Funding source: Gil Medical Centre |
Non-exposed: low risk; exposed 1: high-risk group for LTBI consisting of patients with a history of close contact with TB patients, old TB lesions on chest radiography or a history of TB infection; exposed 2: NA | Inclusion criteria: haemodialysis patients with ESRD Exclusion criteria: patients who had taken empirical antiTB medications and patients taking antiTB medication for active TB infection |
Type of tests: IGRA (QFT-GIT), IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (QFT-GIT): performed according to manufacturer’s instructions; IGRA (T-SPOT.TB): as previously described; TST: induration of ≥ 10 mm (mean value of two measurements) |
Mean (SD) age: 54.1 (14.4) years Female, n (%): 71 (42.5) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 111 (67.3) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NA Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): ESRD because of diabetes mellitus 67 (40.1), hypertension 18 (10.8), glomerulonephritis 12 (7.2), other 11 (6.6), unknown 59 (35.3) Comorbidity, n (%): history of cancer 12 (7.2), cardiac disease 46 (27.5), cerebrovascular accident 13 (7.8), history of TB infection 21 (12.6) Type of during-study treatment, n (%): immunosuppressant medication 9 (5.4) |
Total number of recruited patients: NR; total number of excluded patients: NR | |
| Seyhan 2010,139 Turkey (intermediate) | Aim: to compare the results of the QFT-G with those of the TST for detecting LTBI in haemodialysis patients Setting: NR Design: retrospective cohort/cross-sectional study Funding source: none |
(1) History of active TB – non-exposed: no previous history of active TB; exposed 1: previous history of active TB (2) Contact of the patient with TB – non-exposed: no previous contact of the patient with TB cases; exposed 1: previous contact of the patient with TB cases (details of any contact with a person having TB, individuals who had household contact with or who had worked in the same rooms as patients with smear-positive pulmonary TB, elapsed time after the contact) (3) Chest radiograph changes – non-exposed: no chest radiography changes consistent with old TB; exposed 1: chest radiography changes consistent with old TB |
Inclusion criteria: haemodialysis patients Exclusion criteria: suspicion of active TB infection, use of immunosuppressive drugs and other known immunodeficiency status (HIV infection, malignancy) |
Type of tests: IGRA (QFT-G), TST Cut-off values/thresholds: IGRA (QFT-G): IFN-γ ≥ 0.35 IU/ml in the TB antigen tube minus the negative control tube; TST: induration of ≥ 10 mm |
Mean age: 56.2 ± 15.3 years Female, n (%): 53 (53) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 72 (72) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): NR Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: NR; total number of excluded patients: NR | Blood was collected before TST placement. Those with an initial induration of < 10 mm were administered a second TST 1 week later to cause a potential booster response. Results from the two-step testing were used in all further analyses |
| Immune-mediated inflammatory diseases before antiTNF-α therapy | |||||||
| Casas 2011,123 Spain (low) | Aim: to assess the prevalence of LTBI obtained by the whole blood-based QFT-GIT and the TST in patients with immune-mediated inflammatory diseases and to determine if QFT-GIT performs in the same way as in healthy people Setting: outpatient clinics Design: retrospective cohort/cross-sectional study Funding source: the first author received a research grant from the University of Barcelona (October 2006–January 2010). This study was supported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III-FEDER, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008) |
Non-exposed: no risk factors for TB infection; exposed 1: risk factors for TB infection (birth or residence for ≥ 6 months in a high TB incidence country, TB contact, previous prison stay, intravenous drug abuse, health-care worker, abnormal chest radiography and history of past TB); exposed 2: NA | Inclusion criteria: patients with immune-mediated inflammatory diseases before antiTNF-α therapy Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA QFT-GIT): according to manufacturer, indeterminate results were retested; TST: induration of ≥ 5 mm at 48–72 hours |
Mean (SD) age: 49.1 (12.9) years Female, n (%): 109 (50.9) Race/ethnicity, n (%): NR Geographical origin, n (%): born in a high TB incidence country 16 (7.5) BCG vaccination, n (%): 56 (26.2) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NA Chest radiography (yes/no): NR Clinical examination (yes/no): NR Morbidity, n (%): rheumatoid arthritis 91 (42.5), cutaneous psoriasis 57 (26.6), spondyloarthropathies 29 (13.6), psoriatic arthropathy 21 (9.8), IBD 14 (6.5), other 2 (0.9) Comorbidity, n (%): NR Type of during-study treatment, n (%): immunosuppressive treatment 163 (76.2), corticosteroids 91 (42.5), methotrexate 91 (42.5), leflunomide 36 (16.8), ciclosporin A 22 (10.3), azathioprine/efalizumab 13 (6.1) |
Total number of recruited patients: 323; total number of excluded patients: 9 (n = 2 no immune-mediated inflammatory disease, n = 7 problems with QFT-GIT plasma sample storage) | |
| Costantino 2013,127 France (low) | Aim: to compare TST and IGRA results in screening for LTBI in a large population of patients with chronic inflammatory arthritis requiring biological treatment and to investigate predictive factors of the results of these two tests, with special attention paid to indeterminate IGRA results Setting: rheumatology department of Nancy University Hospital Design: retrospective cohort/cross-sectional study Funding source: NR |
Non-exposed: no CRFs of LTBI; exposed 1: CRFs of LTBI (history of active TB treated before 1970 or not treated for at least 6 months including 2 months with a combination of rifampicin and pyrazinamide, close contact with a patient with active TB and chest radiography suggestive of previous TB infection); exposed 2: NA | Inclusion criteria: patients with rheumatoid arthritis and spondyloarthritis requiring TNF antagonists Exclusion criteria: patients with previous antiTB chemoprophylaxis |
Type of tests: IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (T-SPOT.TB): ≥ 6 spots, indeterminate if the negative control spot count yielded > 10 spots or if the positive control spot count yielded < 20 spots; TST: induration of ≥ 5 mm |
Mean (range) age: 51.0 (39.0–59.0) years Female, n (%): 321 (57.0) Race/ethnicity, n (%): NR Geographical origin, n (%): birth in endemic zone of TB 52 (9.2) BCG vaccination, n (%): 439 (78.0) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NA Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): rheumatoid arthritis 293 (52.0), spondyloarthritis 270 (48.0) Comorbidity, n (%): NR Type of during-study treatment, n (%): DMARDs 277 (49.2), corticosteroids 254 (45.1), NSAIDs 255 (45.4) |
Total number of recruited patients: NR; total number of excluded patients: NR | To avoid any potential boosting effect of the TST on the IGRA results, all T-SPOT.TB assays were performed before initiating the TST |
| Hsia 2012,129 USA (low) | Aim: to evaluate the performance of an IGRA compared with the standard TST as a screening tool for LTBI prior to the initiation of antiTNF therapy in patients with autoimmune inflammatory diseases Setting: NR Design: retrospective cohort/cross-sectional study Funding source: Johnson & Johnson, honoraria from Genentech, Pfizer, Celgene, Corrona, Amgen, Bristol-Myers Squibb and Janssen |
Non-exposed: North America; exposed 1: Western Europe; exposed 2: Asia; exposed 3: Eastern Europe; exposed 4: Latin America | Inclusion criteria: no history of latent/active TB prior to screening (except in the GO-AFTER trial, which allowed the inclusion of patients with a history of latent TB who had been treated within the last 3 years) and having no signs or symptoms of active TB or no recent close contact with anyone with active TB. All patients were required to have a chest radiograph, obtained within 3 months before the first dose of study agent, that showed no evidence of active TB or old inactive TB Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): according to manufacturer; TST: according to the local country guidelines for defining an immunosuppressed host or induration of ≥ 5 mm |
Mean (SD) age: 48.58 (12.6) years Female, n (%): 1515 (65.7) Race/ethnicity, n (%): NR Geographical origin, n (%): North America 962 (41.8), Western Europe 440 (19.1), Eastern Europe 432 (18.8), Latin America 203 (8.8), Asia 266 (11.6) BCG vaccination, n (%): 788 (34.2) History of antiTB treatment, n (%): 317 (13.8) Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): rheumatoid arthritis 1542 (67.0), psoriatic arthritis 405 (17.6), ankylosing spondylitis 356 (15.5) Comorbidity, n (%): NR Type of during-study treatment, n (%): methotrexate 571 (24.8), corticosteroids 1000 (43.4) |
Total number of recruited patients: 2303; total number of recruited patients: NR | |
| Kleinert 2012,133 Germany (low) | Aim: to compare the utility of IGRAs and the TST in LTBI screening in a large cohort of patients with rheumatic diseases receiving immunosuppressive therapy Setting: hospital based Design: retrospective cohort study Funding source: Abbott, Pfizer, Roche and Wyeth, Chugai, Cellestis, Oxford Immunotec, Pharmore and Roche |
Non-exposed: none of the CRFs present; exposed 1: a CRF defined as the presence of at least one of these three risk factors: (i) history of previous TB, (ii) close contact with a patient with TB or (iii) chest radiography suggestive of LTBI; exposed 2: NA | Inclusion criteria: patients with rheumatic diseases Exclusion criteria: NR |
Type of tests: IGRA (QFT-G), IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (QFT-G): NR; IGRA (T-SPOT.TB): ≥ 6 spots; TST: induration of ≥ 5 mm |
Mean age range: 50.8–59.5 years Female, n (%): 937 (61.3) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 204 (13.3) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NA Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): rheumatoid arthritis 852 (55.7), ankylosing spondylitis 294 (19.2), psoriatic arthritis 215 (14.0), undifferentiated spondyloarthropathy 92 (6.0), various other rheumatological disorders 76 (5.0) Comorbidity, n (%): NR Type of during-study treatment, n (%): immunosuppressive therapy (not specified) |
Total number of recruited patients: NR; total number of excluded patients: none | All patients received one type of IGRA, either T-SPOT.TB or QFT-G, depending on what was available in the corresponding laboratory |
| Laffitte 2009,134 Switzerland (low) | Study aim: to determine the frequency of LTBI in a population of patients with psoriasis before antiTNF treatment, compare the TST with T-SPOT.TB for detecting LTBI evaluate the tolerance and effectiveness of treatment for LTBI for those on antiTNF therapy Setting: hospital based Study design: retrospective cohort/cross-sectional study Funding source: NR |
Non-exposed: no probable LTBI; exposed 1: probable LTBI defined as having a history of definite exposure to a case of active TB and/or chest radiography suggestive of previous TB infection (granulomas, calcified adenopathy) and/or originating from a high-incidence country (defined as > 40 cases in 100,000 per year); exposed 2: NA | Inclusion criteria: patients with moderate to severe psoriasis qualifying for antiTNF-α therapy Exclusion criteria: NR |
Type of tests: IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (T-SPOT.TB): NR; TST: induration of ≥ 5 mm or ≥ 10 mm |
Mean (range) age: 48 (17–74) years Female, n (%): 15 (30) Race/ethnicity, n (%): NR Geographical origin, n (%): high TB incidence in country of origin 10 (20) BCG vaccination, n (%): 45 (90) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): 0 Chest radiography (yes/no): yes Clinical examination (yes/no): NR Morbidity, n (%): psoriasis Comorbidity, n (%): NR Type of during-study treatment, n (%): 12 patients treated for LTBI (9 with rifampicin and 3 with isoniazid) before antiTNF therapy |
Total number of recruited patients: NR; total number of excluded patients: NR | |
| Maritsi 2011,135 UK (low) | Aim: to describe the findings of the QFT-GIT test when applied to a paediatric rheumatology population and to assess the efficacy of this test compared with the methods previously used for the exclusion of TB infection prior to starting antiTNF-α treatment Setting: Paediatric Rheumatology Centre Design: retrospective case study Funding source: None |
Non-exposed: low-risk group; exposed 1: high-risk group [TB risk evaluation was performed using the questionnaire formulated by the US Pediatric Tuberculosis Collaborative Group (2004)193]; exposed 2: NA | Inclusion criteria: children on infliximab since 2007 Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): NR; TST: NR |
Median (range) age: 8.9 (1.5–13) years Female, n (%): 12 (52.1) Race/ethnicity, n (%): Caucasian (55), Afro-Caribbean (19), Asian (26) Geographical origin, n (%): NR BCG vaccination, n (%): 5 (22) History of antiTB treatment, n (%): 5 (22) Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): no Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): methotrexate 5 (22), infliximab 23 (100) |
Total number of recruited patients: 27; total number of excluded patients: 4 (no record of the QFT test) | Authors suggested that the results for the QFT-GIT are reported as positive, negative and indeterminate |
| Papay 2011,137 Austria (low) | Aim: to evaluate the impact of immune-modulatory treatment on results from the TST and IGRA in IBD patients before starting therapy with a biological agent Setting: outpatient clinic Design: retrospective cohort/cross-sectional study Funding source: NR |
Non-exposed: NR; exposed 1: from a high-prevalence country; exposed 2: history of contact with active TB; exposed 3: chest radiography indicative of LTBI | Inclusion criteria: IBD patients Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): ≥ 0.35 IU/ml; TST: people with immunomodulators – induration of ≥ 5 mm, people with IBD – induration of > 10 mm |
Mean age at screening: 36.6 ± 11.3 years Female, n (%): 107 (51.4) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): all subjects underwent BCG vaccination during childhood History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): medically confirmed active TB 1 (0.5) Chest radiography (yes/no): yes Clinical examination (yes/no): NR Morbidity, n (%): Crohn’s disease 152 (73.1), ulcerative colitis 56 (26.9) Comorbidity, n (%): NR Type of during-study treatment, n (%): immunotherapy |
Total number of recruited patients: 208; total number of excluded patients: NR | |
| Ramos 2013,138 Spain (low) | Aim: to evaluate the performance of QFT-GIT compared with the TST for the diagnosis of LTBI in patients with immune-mediated inflammatory disease before TNF-α antagonist therapy and evaluate the impact of immunosuppressive therapy on QFT-GIT and TST performance in different immune-mediated inflammatory diseases Setting: outpatient infectious diseases clinic of a university hospital Design: retrospective cohort/cross-sectional study Funding source: grants from Conselleria de Sanidad (051/2007) and FIS (PI08/90778) |
Non-exposed: not born in a TB-endemic area/no contact with TB patients; exposed 1: born in a TB-endemic area/contact with TB patients; exposed 2: NA | Inclusion criteria: all adult (aged ≥ 15 years) candidates for antiTNF-a therapy who attended the clinic Exclusion criteria: NR |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): ≥ 0.35 IU/ml, indeterminate if negative control ≥ 8.0 IU/ml or positive control < 0.5 IU/ml or if IFN-γ level ≥ 0.10 IU/ml but < 0.35 IU/ml; TST: induration of > 5 mm |
Median (range) age: 52 (16–82) years Female, n (%): 73 (47.7) Race/ethnicity, n (%): NR Geographical origin, n (%): born in a TB-endemic area 8 (5.2) BCG vaccination, n (%): 29 (19) History of antiTB treatment, n (%): 5 (3.3) Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): NR Morbidity, n (%): rheumatoid arthritis 53 (43.6), psoriasis/psoriatic arthritis 45 (29.4), IBD 25 (16.3), spondyloarthropathy 22 (14.4), severe hidradenitis 3 (2.0), systemic lupus erythematosus 2 (1.3), polymyositis 1 (0.6), sarcoidosis 1 (0.6), mixed connective tissue disease 1 (0.6) Comorbidity, n (%): NR Type of during-study treatment, n (%): immunosuppressive drug 91 (59.5) [methotrexate 57 (37.3), corticosteroids 28 (18.3), leflunomide 21 (13.7), azathioprine 19 (12.4), ciclosporin 6 (3.9)] |
Total number of recruited patients: NR; total number of excluded patients: NR | The QFT assay and TST were performed simultaneously in a blinded fashion |
| Vassilopoulos 2011,142 Greece (low) | Aim: to compare the latest IGRAs (QFT-GIT and T-SPOT.TB) and the TST for LTBI diagnosis in rheumatic patients starting antiTNF treatment Setting: outpatient rheumatology clinic of Hippokration General Hospital Design: retrospective cohort study/cross-sectional study Funding source: supported in part by research grants from the Hellenic Society for Rheumatology and the Special Account for Research Grants, National and Kapodistrian University of Athens, Athens, Greece |
(1) History of TB contact – non-exposed: no history of previous TB contact; exposed 1: history of previous TB contact (2) Chest radiography – non-exposed: chest radiography without signs suggestive of old TB; exposed 1: chest radiography suggestive of old TB (3) Risk factor for TB – non-exposed: no risk factor for TB; exposed 1: any risk factor for TB (≥ 1 risk factor) including aged > 50 years, chest radiography suggestive of old/healed TB, contact with a person with TB and birth or residence in a country with a high TB prevalence (non-Greek nationality) |
Inclusion criteria: patients with various rheumatic diseases who were seen at the outpatient rheumatology clinic of Hippokration General Hospital and were scheduled for antiTNF treatment Exclusion criteria: patients with active TB, a history of treatment with antiTB agents including isoniazid for LTBI or a history of previous treatment with antiTNF agents or other biological agents |
Type of tests: IGRA (QFT-GIT), IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRAs: NR; TST: induration of ≥ 5 mm |
Mean age: 52 ± 16 years Female, n (%): 90 (58) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): 81 (76) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): NR Morbidity, n (%): NR Comorbidity, n (%): 15 (21.4) Type of during-study treatment, n (%): immunosuppressive therapy (DMARDs/steroids) 98 (63) [DMARDs 80 (52), steroids 66 (43)] |
Total number of recruited patients: 157; total number of excluded patients: 2 (indeterminate QFT-GIT results from the analysis: spondyloarthropathy related to ulcerative colitis on high-dose methylprednisolone) | The blood draw for both IGRAs was performed just prior to TST application to avoid potential interference with the IGRA results |
| Hepatitis C | |||||||
| Shen 2012,140 China (high) | Aim: to evaluate the diagnostic value of ELISPOT measuring interferon-γ in hepatitis C patients with LTBI Setting: university hospital Design: retrospective study Funding source: none |
Non-exposed: no history of TB exposure and no clinical symptoms (n = 39); exposed 1: history of exposure to TB (suspected of having TB but no symptoms, n = 31); exposed 2: NA | Inclusion criteria: hepatitis patients: TB exposure group – patients who had a history of exposure to TB without a clinical diagnosis of TB and with obvious clinical symptoms; non-TB exposure group – patients who had no history of exposure to TB and no clinical symptoms; TB group – patients who were clinically diagnosed with TB and who had apparent clinical symptoms Exclusion criteria: NR |
Type of tests: IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (T-SPOT.TB): NR; TST: induration of ≥ 5 mm |
Mean age: TB exposure group (n = 40) 42.9 ± 18.6; no TB exposure group (n = 39) 37.8 ± 17.6 Female, n (%): TB exposure group 37 (47); no TB exposure group 17 (45) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): NR History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): hepatitis C Comorbidity, n (%): heart disease, diabetes mellitus, liver cirrhosis, solid tumour, chronic renal failure Type of during-study treatment, n (%): NR |
Total number of recruited patients: NR; total number of excluded patients: NR | |
| Lupus erythematosus | |||||||
| Takeda 2011,141 Japan (low) | Study aim: to evaluate whether QFT-2G (QFT-G) is useful in detecting LTBI in systemic lupus erythematosus patients Setting: hospital based Design: retrospective cohort/cross-sectional study Funding source: NR |
Non-exposed: without risk of LTBI; exposed 1: with risk factors for LTBI [history of household TB contact, chest radiography suggestive of previous TB (nodules, fibrotic scars, calcified granulomas, basal thickening), history of active TB)]; exposed 2: NA | Inclusion criteria: systemic lupus erythematosus patients and those with non-systemic lupus erythematosus connective tissue disease Exclusion criteria: NR |
Type of tests: IGRA (QFT-2G), TST Cut-off values/thresholds: IGRA (QFT-2G): ≥ 0.35 IU/ml; TST: induration of ≥ 10 mm |
Mean (SD) age: 38.3 (15.2) years Female, n (%): 58 (81.7) Race/ethnicity, n (%): NR Geographical origin, n (%): NR BCG vaccination, n (%): NR History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NA Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): systemic lupus erythematosus Comorbidity, n (%): NR Type of during-study treatment, n (%): corticosteroids 37 (52.1), immunosuppressive drugs 19 (26.8), prednisolone pulse therapy 2 (2.8), NSAIDs or no therapy 13 (18.3) |
Total number of recruited patients: NR; total number of excluded patients: NR | |
Three studies125,136,153 assessed the test performance of different IGRA tests compared with that of the TST in patients with HIV. Chkhartishvili et al. 125 compared QFT-GIT and T-SPOT. TB with TST (≥ 5 mm) in HIV patients recruited from a national referral centre for HIV in Georgia, with the non-exposed group having no household member treated for TB and the exposed group having a household member treated for active TB. Mutsvangwa et al. 136 compared T-SPOT. TB with TST at the ≥ 10 mm cut-off value in HIV-positive household contacts of TB cases identified in a factory in Zimbabwe. The non-exposed control group consisted of contacts of factory workers without TB. Souza et al. 153 compared QFT-GIT with TST (≥ 5 mm) in adults living with HIV and/or acquired immune deficiency syndrome (AIDS) in outpatient sexually transmitted disease public clinics in a low TB incidence urban area (11.1 per 100,000 inhabitants). The rate of BCG vaccination across the three studies ranged from 76%153 to 94%125 and the proportion of women ranged from 28%153 to 89%. 136 The median age was reported for only two studies and ranged from 38125 to 40 years. 153
Four studies compared either QFT-GIT120,124,131 or T-SPOT. TB130 with TST at the cut-off level of ≥ 5 mm,124 ≥ 10mm120,131 or both130 in solid organ transplantation candidates. All four studies were hospital based. Two studies were undertaken in South Korea,130,131 one in Iran120 and one in Spain. 124 The mean age was 39.9 years,120 47 years,131 56.4 years124 or not reported. 130 The proportion of women was close to 50% in two studies120,131 and < 25% in one study. 124 One study did not report sex. 130 BCG vaccination was high in studies from Korea (78%130 and 91%131) as well as in the study from Iran (91%)120 but low in the Spanish study (31.6%). 124 Exposure to TB was universally defined as a history of (close) contact with active TB. Two studies also included newly acquired TB130 or a history of active TB130,131 as a risk factor for LTBI. The non-exposed groups consisted of participants without contact with or at a low risk of LTBI.
Hadaya et al. 128 and Kim et al. 132 compared one or more IGRA tests with the TST in patients post-kidney transplantation. Hadaya et al. 128 compared QFT-GIT, T-SPOT.TB and TST (≥ 5 mm) in the setting of a Swiss hospital and Kim et al. 132 compared QFT-GIT with TST (≥ 10 mm) in South Korean kidney transplant recipients. Exposure was defined as close contact with a TB patient or previous TB according to (1) chest radiography128 or (2) a history of treated TB or abnormal chest radiography. 132
Four studies121,122,126,139 investigated the agreement between IGRA and TST tests in patients on haemodialysis for ESRD. Three studies121,122,126 compared QFT-GIT with TST (≥ 10 mm) and one study139 compared QFT-G with TST (≥ 10 mm). Chung et al. 126 additionally investigated the T-SPOT. TB. Three studies121,122,126 reported the setting to be hospital based whereas one study139 did not report the study setting. The rate of BCG vaccination of the study participants was low in the study from Saudi Arabia (14%)121 and intermediate in the two studies from Turkey (49%122 and 72%139) and the study from South Korea (67%). 126 The mean age of study participants was similar across all four studies (58,121 52,122 54126 and 56139 years) and the sex distribution within the studies was balanced (52%,121 50%,122 43%126 and 53%139 female). Exposure to TB was not well defined. Three studies121,122,126 described exposure as (close) contact with a TB case whereas one study139 specified the contact as household contact or working in the same room with the TB case. History of active TB was included as a risk factor in the exposure group in two studies. 126,139 The comparison group included people who were at low risk of LTBI.
Patients with immune-mediated inflammatory diseases before antiTNF-α treatment were recruited in nine studies123,127,129,133–135,137,138,142 comparing IGRA with TST tests. The combination of tests investigated varied greatly among the studies. Three studies123,129,138 compared QFT-GIT with TST (≥ 5 mm) with one study142 additionally including the T-SPOT. TB. One study135 compared the TST with QFT-GIT but did not provide the threshold for a positive TST test, one study137 compared QFT-GIT with the TST test at two different thresholds (≥ 5 mm and ≥ 10 mm) for different subgroups of patients, one study133 compared QFT-G with the T-SPOT. TB and TST (≥ 5 mm), and two studies compared the T-SPOT. TB with the TST at only the ≥ 5 mm threshold127 or at two different thresholds (≥ 5 mm and ≥ 10 mm). 134 All studies were undertaken in low TB incidence countries in either Europe123,127,133–135,137,138,142 or the USA129 and all studies were hospital based. BCG vaccination was low in studies undertaken in Spain (26%123 and 19%138), the USA (34%),129 Germany (13%)133 and the UK (22%)135 but was higher in studies from France (78%)127 and Greece (76%),142 and considerably higher in studies from Switzerland (90%)134 and Austria (100%). 137 Male and female participants were generally well balanced in the studies, with two possible exceptions: the study by Laffitte et al. 134 recruited a population with only 30% women and in the study by Hsia et al. 129 66% of the participants were women. One study135 investigated children with a median age of 8.9 years whereas the participants’ mean age in the remaining studies ranged from 37 years137 to 52 years. 142 Exposure to TB was not well defined in any of the studies. High risk of LTBI was described as a history of contact with a TB case in the majority of studies. 123,127,133–135,137,138,142 Additional risk factors reported were origin or residence in a high-incidence country129,134,137,138,142 and a history of active TB. 123,127,133 The non-exposed group was generally described as having no history of TB contact.
Shen et al. 140 compared a T-SPOT. TB test with the TST (≥ 5 mm) in hepatitis C patients in a university hospital in China. The mean age of participants was 40 years and 47% were women. BCG vaccination was not reported in this study and exposure was loosely defined as a history of exposure compared with no exposure to TB.
Takeda et al. 141 evaluated the agreement between the QFT-2G (QFT-G) and the TST (≥ 10 mm) in a hospital in Japan in patients with lupus erythematosus. The mean age of participants was 38 years and 82% were women. BCG vaccination of participants was not reported in this study and exposure to TB was defined as a household TB contact. This was combined with other LTBI risk factors and compared with a group without LTBI risk factors.
Study quality
Incidence of active tuberculosis
Of the eight included incidence studies114–119,149,155 concerning immunocompromised patients identified since the publication of CG117,10 one 116 had a low ROB rating, three115,117,149 had a moderate ROB rating and four114,118,119,155 had a high ROB rating. Potential ROB because of confounding was noted in five studies. 114,117–119,155 Overall, in most of the studies the study design, study attrition, statistical analysis and reporting was appropriate. Table 12 provides further details of the ROB assessment.
| Study ID (burden) | Study design | Study participation (risk of selection bias) | Study attrition (risk of selection bias) | Prognostic factor measurement (risk of exposure measurement bias) | Outcome/construct measurement (ROB in misclassification of individuals in relation to construct validity groups) | Study confounding (ROB from confounding) | Statistical analysis and reporting (ROB from analysis and selective reporting) | Total ROB (high, moderate, low) |
|---|---|---|---|---|---|---|---|---|
| Anibarro 2012117 (low) | Low | Low | Low | Moderate | Moderate | High | Low | Moderate |
| Chang 2011119 (high) | Low | Moderate | Low | Moderate | High | High | Low | High |
| Elzi 2011114 (low) | High | High | Low | Low | Moderate | High | Low | High |
| Kim 2011116 (high) | Low | Low | Low | Low | Low | Moderate | Low | Low |
| Lee 2009118 (high) | Low | High | Low | Low | Moderate | High | Low | High |
| Lee 2014149 (high) | Low | High | Moderate | Moderate | Moderate | Low | Low | Moderate |
| Moon 2013115 (high) | Low | Moderate | Low | Moderate | Moderate | Moderate | Low | Moderate |
| Sherkat 2014155 (intermediate) | Low | High | High | Moderate | High | High | Moderate | High |
Exposure levels
Of the 24 included exposure studies120–142,153 concerning immunocompromised patients identified since the publication of CG117,10 19 studies120,122–126,128–136,140–142,153 were identified as being of low quality and the remaining five studies121,127,137–139 were rated as being of moderate quality. However, all studies failed to blind the test results from exposure and only two studies126,139 provided an adequate description of exposure. Table 13 provides further details of the ROB assessment.
| Study ID (burden) | Recruitment of subjects [consecutive (yes), arbitrary or unreported (no)] | Blinding of test results from exposure [blinded (yes), not blinded or unreported (no)] | Description of index test and threshold [adequate (yes), inadequate or unreported (no)] | Definition and description of exposure [adequate (yes), inadequate or unreported (no)] | Sample attrition [adequate (yes),a inadequate or unreported (no)] | Overall quality score of satisfactory featuresb |
|---|---|---|---|---|---|---|
| Ahmadinejad 2013120 (intermediate) | Yes | No | No | No | No | Low |
| Al Jahdali 2013121 (low) | Yes | No | Yes | No | Yes | Moderate |
| Ates 2009122 (intermediate) | No | No | No | No | No | Low |
| Casas 2011123 (low) | No | No | No | No | Yes | Low |
| Casas 2011124 (low) | Yes | No | Yes | No | No | Low |
| Chkhartishvili 2013125 (high) | No | No | Yes | No | Yes | Low |
| Chung 2010126 (high) | No | No | No | Yes | Yes | Low |
| Costantino 2013127 (low) | Yes | No | Yes | No | Yes | Moderate |
| Hadaya 2013128 (low) | No | No | No | No | Yes | Low |
| Hsia 2012129 (low) | No | No | No | No | Yes | Low |
| Kim 2010130 (high) | Yes | No | No | No | Yes | Low |
| Kim 2013131 (high) | No | No | Yes | No | Yes | Low |
| Kim 2013132 (high) | No | No | Yes | No | No | Low |
| Kleinert 2012133 (low) | No | No | No | No | Yes | Low |
| Laffitte 2009134 (low) | Yes | No | No | No | Yes | Low |
| Maritsi 2011135 (low) | Yes | No | No | No | No | Low |
| Mutsvangwa 2010136 (high) | No | No | No | No | Yes | Low |
| Papay 2011137 (low) | Yes | No | Yes | No | Yes | Moderate |
| Ramos, 2013138 (low) | Yes | No | Yes | No | Yes | Moderate |
| Seyhan 2010139 (intermediate) | No | No | Yes | Yes | Yes | Moderate |
| Shen 2012140 (high) | No | No | Yes | No | Yes | Low |
| Souza 2014153 (intermediate) | Yes | Yes | No | No | No | Low |
| Takeda 2011141 (low) | No | No | Yes | No | Yes | Low |
| Vassilopoulos 2011142 (low) | Yes | No | No | No | Yes | Low |
Comparative performance of tests (diagnostic accuracy indices for identifying latent tuberculosis infection)
Incidence of active tuberculosis
Ratios of cumulative incidence ratios
This section included eight newly identified studies (Table 14). 114–119,149,155 Of these, R-CIRs were not available for four studies114,116,117,119 because of missing incidence data for one or both compared tests. Of the remaining four studies, R-CIRs in three studies comparing IGRAs (QFT-G/GIT or T-SPOT. TB) with the TST in haematopoietic stem cell transplant candidates115 and haemodialysis ESRD patients118,155 were not statistically significant, rendering these results inconclusive (wide 95% CIs). Only one study,149 which was conducted in haematopoietic stem cell transplant recipients, showed that QFT-GIT performed significantly better than the TST (at ≥ 5 mm or ≥ 10 mm) in identifying people with LTBI (TST at ≥ 5 mm: R-CIR 9.71, 95% CI 1.71 to 55.15; TST at ≥ 10 mm: R-CIR 5.85, 95% CI 1.05 to 32.70). A meta-analysis of R-CIRs could not be performed because of differences in the study populations and tests used.
| Study ID, country (burden) | Test results | Test diagnostic accuracy (95% CI) (%) | Development of active TB | |||
|---|---|---|---|---|---|---|
| Cumulative incidence (%), CIR, IDR, IDRR (95% CI) | R-CIR, R-IDRR (95% CI), IGRA vs. TST (by threshold) | |||||
| IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | |||
| Anibarro 2012,117 Spain (low) | Number of test results: QFT-GIT 52; TST 52 Test (+/–): QFT-GIT 18/34; TST ≥ 5 mm 11/41 Number of indeterminate results: QFT-GIT 0; TST 0 Number lost to follow-up: 4 |
QFT-GIT: SN NR; SP NR; PPV NR; NPV 100 (89.28 to 100) | TST ≥ 5 mm: SN NR; SP NR; PPV NR; NPV 100 (89.28 to 100) | QFT-GIT: CI (+) NR; CI (–) 0/32 (0.00); CIR NR; IDR (+) NR; IDR (–) NR; IDRR NR | TST ≥ 5 mm: CI (+) NR; CI (–) 0/32 (0.00); CIR NR; IDR (+) NR; IDR (–) NR; IDRR NR | R-CIR (QFT-GIT vs. TST ≥ 5 mm) NR; R-IDRR (QFT-GIT vs. TST ≥ 5 mm) NR |
| Chang 2011,119 South Korea (high) | Number of test results: QFT-GIT 100; TST 107 Test (+/–): QFT-GIT 36/64; TST ≥ 10 mm 36/71 Number of indeterminate results: QFT-GIT 7; TST 0 Number lost to follow-up: 0 |
QFT-GIT: SN NR; SP 100 (94.8 to 100); PPV NR; NPV 100 (94.8 to 100) | TST ≥ 10 mm: SN NR; SP 77.14 (66.05 to 85.41); PPV 0/16 (CI 0.0); NPV 100 (93.4 to 100) | QFT-GIT: CI (+) NR; CI (–) 0/64 (0.00); CIR NR; IDR (+) NR; IDR (–) NR; IDRR NR | TST ≥ 10 mm: CI (+) 0/16 (0.00); CI (–) 0/54 (0.00); CIR NR; IDR (+) NR; IDR (–) NR; IDRR NR | R-CIR (QFT-GIT vs. TST ≥ 10 mm) NR; R-IDRR (QFT-GIT vs. TST ≥ 10 mm) NR |
| Elzi 2011,114 Switzerland (low) | Number of test results: T-SPOT.TB 43; TST 44 Test (+/–): T-SPOT.TB 25/18; TST ≥ 5 mm 22/22 Number of indeterminate results: T-SPOT.TB 21; TST 0 Number lost to follow-up: NR |
T-SPOT.TB: SN 58.14 (43.33 to 71.62); SP NR; PPV NR; NPV NR T-SPOT.TB and TST ≥ 5 mm: SN 65.91 (51.14 to 78.12); SP NR; PPV NR; NPV NR |
TST ≥ 5 mm: SN 50.00 (35.83 to 64.17); SP NR; PPV NR; NPV NR | T-SPOT.TB: CI (+) NR; CI (–) NR; CIR NR; IDR (+) NR; IDR (–) NR; IDRR NR T-SPOT.TB and TST ≥ 5 mm: CI (+) NR; CI (–) NR; CIR NR; IDR (+) NR; IDR (–) NR; IDRR NR |
TST ≥ 5 mm: CI (+) NR; CI (–) NR; CIR NR; IDR (+) NR; IDR (–) NR; IDRR NR | R-CIR (T-SPOT.TB vs. TST ≥ 5 mm) NR; R-IDRR (T-SPOT.TB vs. TST ≥ 5 mm) NR; R-CIR (T-SPOT.TB and TST ≥ 5 mm vs. TST ≥ 5 mm) NR; R-IDRR (T-SPOT.TB and TST ≥ 5 mm vs. TST ≥ 5 mm) NR |
| Kim 2011,116 South Korea (high) | Number of test results: T-SPOT.TB 242; TST 272 Test (+/–): T-SPOT.TB 71/171; TST ≥ 10 mm 0/272 Number of indeterminate results: T-SPOT.TB 30; TST 0 Number lost to follow-up: 2 |
T-SPOT.TB: SN 100 (51.01 to 100.00); SP 71.84 (65.82 to 77.18); PPV 5.63 (2.21 to 13.61); NPV 100 (97.80 to 100) | TST ≥ 10 mm: SN NR; SP NR; PPV NR; NPV 98.53 (96.28 to 99.43) | T-SPOT.TB: CI (+) 5.63 (2.21 to 13.61); CI (–) 0/171 (0.0); CIR NR; IDR (+) 3.28 per 100 person-years (0.89 to 8.39 per 100 person-years); IDR (–) 0.00 per 100 person-years (NR); IDR difference: 3.3 per 100 person-years (1.3 to 5.3 per 100 person-years) | TST ≥ 10 mm: CI (+) NR; CI (–) 1.47 (0.43 to 3.85); CIR NR; IDR (+) NR; IDR (–) 0.83 per 100 person-years (0.23 to 2.12 per 100 person-years); IDRR NR | R-CIR (T-SPOT.TB vs. TST ≥ 10 mm) NR; R-IDRR (T-SPOT.TB vs. TST ≥ 10 mm) NR |
| Lee 2009,118 Taiwan (high) | Number of test results: QFT-G 30; T-SPOT.TB 32; TST 32 Test (+/–): QFT-G 12/18; T-SPOT.TB 15/17; TST ≥ 10 mm 20/12 Number of indeterminate results: QFT-G 2; T-SPOT.TB 0; TST 0 Number lost to follow-up: 0 |
QFT-G: SN 100 (20.65 to 100); SP 60.00 (44.00 to 77.31); PPV 8.33 (1.49 to 35.39); NPV 100 (82.41 to 100) T-SPOT.TB: SN 0.00 (0.00 to 65.76); SP 50.00 (33.15 to 66.85); PPV 0.00 (0.00 to 20.39); NPV 88.24 (65.66 to 96.71) |
TST ≥ 10 mm (two step): SN 50.00 (9.45 to 90.55); SP 36.67 (21.87 to 54.49); PPV 5.00 (0.89 to 23.61); NPV 100 (74.12 to 100) | QFT-G: CI (+) 8.33 (1.49 to 35.39); CI (–) 5.56 (5.40 to 27.29); CIR 1.55 (0.02 to 124.2); IDR (+) 3.40 per 100 person-years (NR); IDR (–) NR; IDRR NR T-SPOT.TB: CI (+) 6.67 (0.17 to 31.9); CI (–) 11.76 (2.03 to 35.59); CIR 0.57 (0.01 to 12.1); IDR (+) NR; IDR (–) NR; IDRR NR |
TST ≥ 10 mm (two step): CI (+) 5.00 (0.89 to 23.61); CI (–) 9.09 (0.23 to 41.3); CIR 0.55 (0.01 to 47.06); IDR (+) NR; IDR (–) NR; IDRR NR | R-CIR [QFT-G vs. TST ≥ 10 mm (two step)] 2.82 (0.13 to 62.64); R-IDRR [QFT-G vs. TST ≥ 10 mm (two step)] NR; R-CIR [T-SPOT.TB vs. TST ≥ 10 mm (two step)] 1.04 (0.06 to 17.34); R-IDRR [T-SPOT.TB vs. TST ≥ 10 mm (two step)] NR |
| Lee 2014,149 South Korea (high) | Number of test results: QFT-GIT 159; TST 169 Test (+/–): QFT-GIT 26/133; TST ≥ 5 mm 19/150 TST ≥ 10 mm (12/157) Number of indeterminate results: QFT-GIT 10; TST 0 Number lost to follow-up: 0 |
QFT-GIT: SN 60.00 (23.07 to 88.24); SP 85.06 (78.59 to 89.84); PPV 11.54 (4.00 to 28.98); NPV 98.5 (94.68 to 99.59) | TST ≥ 5 mm: SN 0.0 (0.0 to 43.45); SP 88.41 (82.61 to 92.46); PPV 0.0 (0.0 to 16.82); NPV 96.67 (92.43 to 98.57) TST ≥ 10 mm: SN 0.0 (0.0 to 43.45); SP 92.68 (87.65 to 95.77); PPV 0.0 (0.0 to 24.25); NPV 96.82 (92.76 to 98.63) |
QFT-GIT: CI (+) 11.54 (3.17 to 29.80); CI (–) 1.50 (0.07 to 5.66); CIR 7.67 (1.34 to 43.67); IDR (+) 5.43 per 100 person-years (1.12 to 15.88 per 100 person-years); IDR (–) 0.80 per 100 person-years (0.10 to 2.88 per 100 person-years); IDRR 6.78 per 100 person-years (NR) | TST ≥ 5 mm: CI (+) 2.63 (0.0 to 23.22); CI (–) 3.33 (1.22 to 7.77); CIR 0.79 (0.04 to 13.89); IDR (+) 0 per 100 person-years (0.00 to 8.41 per 100 person-years); IDR (–) 1.79 per 100 person-years (0.58 to 4.18 per 100 person-years); IDRR 0 per 100 person-years (NR) TST ≥ 10 mm: CI (+) 4.16 (0.0 to 33.00); CI (–) 3.18 (1.16 to 7.43); CIR 1.31 (0.07 to 22.55); IDR (+) 0.0 per 100 person-years (0.0 to 14.93 per 100 person-years); IDR (–) NR; IDRR NR |
R-CIR (QFT-GIT vs. TST ≥ 5 mm) 9.71 (1.71 to 55.15); R-IDRR (QFT-GIT vs. TST ≥ 5 mm) NR; R-CIR (QFT-GIT vs. TST ≥ 10 mm) 5.85 (1.05 to 32.70); R-IDRR (QFT-GIT vs. TST ≥ 10 mm) NR |
| Moon 2013,115 South Korea (high) | Number of test results: QFT-GIT 210; TST 244 Test (+/–): QFT-GIT 40/170; TST ≥ 5 mm 39/205 Number of indeterminate results: QFT-GIT 34; TST 0 Number lost to follow-up: 2 |
QFT-GIT: SN 50.00 (9.45 to 90.55); SP 81.25 (75.4 to 85.97); PPV 2.50 (0.44 to 12.88); NPV 99.41 (96.74 to 99.9) | TST ≥ 5 mm: SN 0.00 (0.00 to 65.76); SP 83.88 (78.73 to 87.98); PPV 0.00 (0.00 to 8.96); NPV 99.02 (96.51 to 99.73) | QFT-GIT: CI (+) 2.50 (0.44 to 12.88); CI (–) 0.58 (0.00 to 3.59); CIR 4.25 (0.27 to 66.49); IDR (+) 2.80 per 100 person-years (0.07 to 15.81 per 100 person-years); IDR (–) NR; IDRR NR | TST ≥ 5 mm: CI (+) 2.56 (0.06 to 13.5); CI (–) 0.97 (0.03 to 3.71); CIR 2.63 (0.04 to 51.4); IDR (+) 0 per 100 person-years (0.00 to 8.00 per 100 person-years); IDR (–) NR; IDRR NR | R-CIR (QFT-GIT vs. TST ≥ 5 mm) 1.62 (0.16 to 16.18); R-IDRR (QFT-GIT vs. TST ≥ 5 mm) 1.62 (0.16 to 16.18) |
| Sherkat 2014,155 Iran (intermediate) | Number of test results: T-SPOT.TB 44; TST 44 Test (+/–): T-SPOT.TB 6/38; TST ≥ 10 mm 8/36 Number of indeterminate results: T-SPOT.TB NR; TST NR Number lost to follow-up: 1 |
T-SPOT.TB: SN 100 (20.65 to 100); SP 88.37 (75.52 to 94.93); PPV 16.67 (3.00 to 56.35); NPV 100 (90.82 to 100) | TST ≥ 10 mm: SN 100 (20.65 to 100); SP 83.72 (70.03 to 91.88); PPV 12.5 (2.24 to 47.09); NPV 100 (90.36 to 100) | T-SPOT.TB: CI (+) 16.67 (3.00 to 56.35); CI (–) 1.31 (0.00 to 12.86); CIR 12.67 (0.47 to 337.8) | TST ≥ 10 mm: CI (+) 12.5 (0.11 to 47.09); CI (–) 1.39 (0.00 to 13.49); CIR 9.00 (0.33 to 245.7) | R-CIR (T-SPOT.TB vs. TST ≥ 10 mm) 1.41 (0.13 to 15.20) |
Sensitivity and specificity
This section included eight newly identified studies. 114–119,149,155 The study by Anibarro et al. 117 did not report test performance parameters of sensitivity and specificity. Across the remaining seven studies there was wide variability and the absence of a clear pattern in the estimates of sensitivity (IGRA/TST range 0–100%) (Figures 25 and 26) and specificity (IGRAs range 50–88%; TST range 37–93%) (Figures 27 and 28). Some or all of this variation was the result of zero count events (unstable estimates) and underlying differences in study populations/conditions and TST thresholds. No meta-analysis was performed given the observed heterogeneity.
FIGURE 25.
Forest plot of sensitivity based on incidence of active TB (IGRA) in immunocompromised patients. df, degrees of freedom; KT, kidney transplant.

FIGURE 26.
Forest plot of sensitivity based on incidence of active TB (TST) in immunocompromised patients. df, degrees of freedom; KT, kidney transplant.
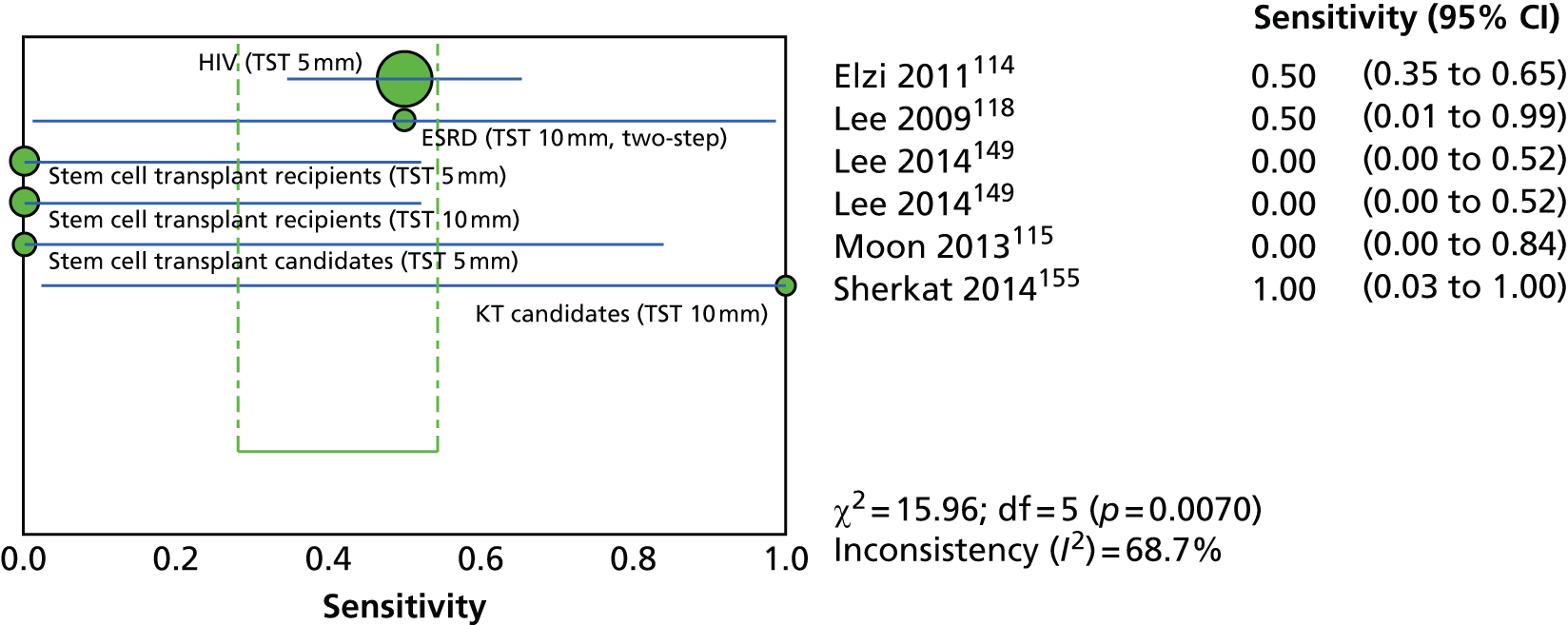
FIGURE 27.
Forest plot of specificity based on incidence of active TB (IGRA) in immunocompromised patients. df, degrees of freedom; KT, kidney transplant.
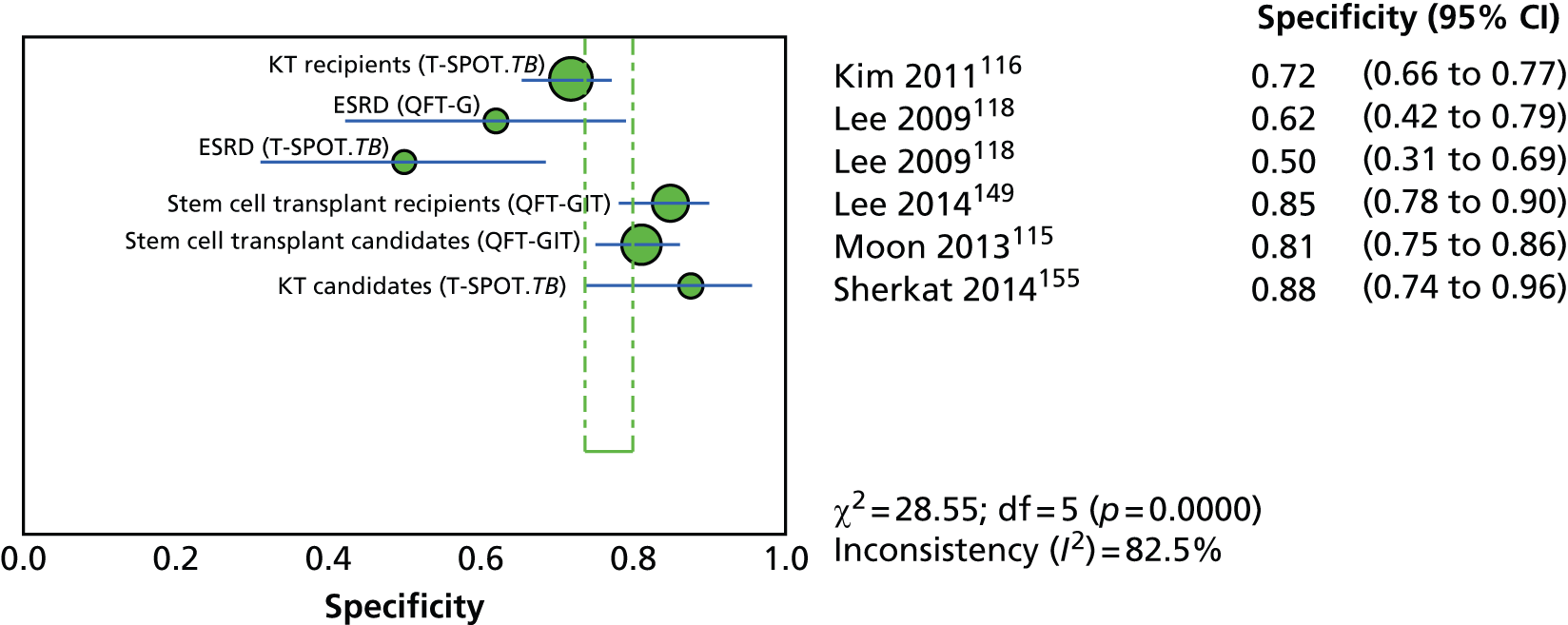
FIGURE 28.
Forest plot of specificity based on incidence of active TB (TST) in immunocompromised patients. AS, ankylosing spondylitis; df, degrees of freedom; KT, kidney transplant; RA, rheumatoid arthritis.
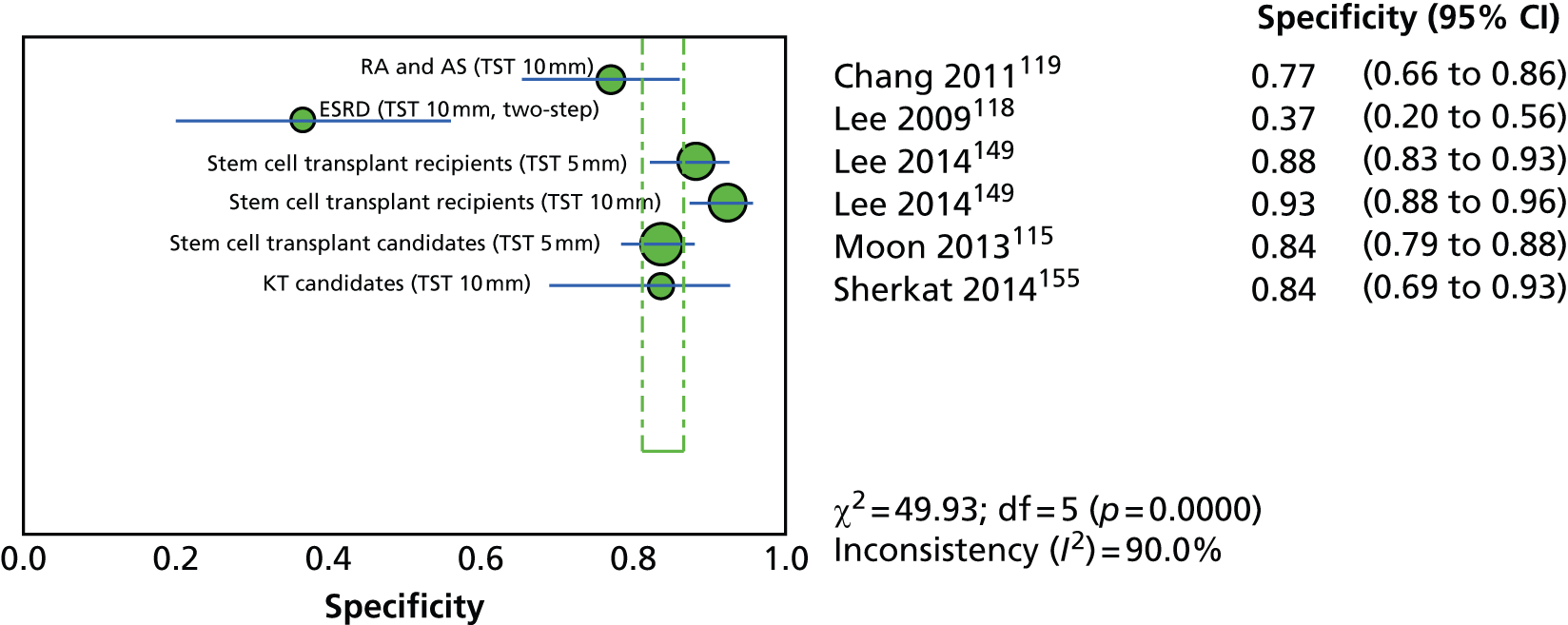
Exposure levels
Ratios of diagnostic odds ratios
This section included 26 studies: two studies174,180 from CG11710 and 24 more recent studies120–142,153 (Table 15). The association between the screening test results and the risk of LTBI/exposure measured using the R-DOR (IGRA vs. TST) in individual studies ranged from 0.07131 to 8.45. 140 R-DORs for three studies120,132,135 could not be estimated because of missing data.
| Study ID, country (burden) | Test results | Test diagnostic accuracy (95% CI) (%) | Construct validity (i.e. LTBI exposure-based proxy) | |||
|---|---|---|---|---|---|---|
| DOR (95% CI) (vs. non-exposed; reference group) | R-DOR (95% CI), IGRA vs. TST (by threshold) | |||||
| IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | |||
| Ahmadinejad 2013,120 Iran (intermediate) | Number of test results: QFT-GIT 159; TST 164 Test (+/–): QFT-GIT 33/126; TST ≥ 10 mm 26/138 Number of indeterminate results: QFT-GIT 5; TST 0 |
QFT-GIT: Exposure history to active TB vs. no such history: SN 0.00; SP 78.57 (71.44 to 84.32); PPV 0.00; NPV 96.03 (91.05 to 98.29) |
TST ≥ 10 mm: Exposure history to active TB vs. no such history: SN 0.00; SP: 83.65 (77.12 to 88.59); PPV 0.00; NPV 96.38 (91.8 to 98.44) |
QFT-GIT: Exposure history to active TB vs. no such history: DOR 0.00; DORa NR |
TST ≥ 10 mm: Exposure history to active TB vs. no such history: DOR 0.00; DORa NR |
QFT-GIT vs. TST ≥ 10 mm: Exposure history to active TB vs. no such history: R-DOR NR; R-DORa NR |
| Al Jahdali 2013,121 Saudi Arabia (low) | Number of test results: QFT-GIT 200; TST 200 Test (+/–): QFT-GIT 65/135; TST ≥ 10 mm 26/174 Number of indeterminate results: QFT-GIT NR; TST NR |
QFT-GIT: High likelihood of LTBI vs. no high likelihood of LTBI: SN 33.12 (26.00 to 41.00); SP 69.57 (55.19 to 80.92); PPV 78.46 (67.03 to 86.71); NPV 23.70 (17.32 to 31.54) |
TST ≥ 10 mm (two step): High likelihood of LTBI vs. no high likelihood of LTBI: SN 12.34 (8.04 to 18.47); SP 84.78 (71.78 to 92.43); PPV 73.08 (53.92 to 86.3); NPV 22.41 (16.85 to 29.17) |
QFT-GIT: High likelihood of LTBI vs. no high likelihood of LTBI: DOR 1.13 (0.55 to 2.31); DORa NR |
TST ≥ 10 mm (two step): High likelihood of LTBI vs. no high likelihood of LTBI: DOR 0.78 (0.31 to 2.00); DORa NR |
QFT-GIT vs. TST ≥ 10 mm (two step): High likelihood of LTBI vs. no high likelihood of LTBI: R-DOR 1.45 (0.79 to 2.64); R-DORa NR |
| Ates 2009,122 Turkey (intermediate) | Number of test results: QFT-GIT 246; TST 259 Test (+/–): QFT-GIT 115/131; TST ≥ 10 mm 92/167 Number of indeterminate results: QFT-GIT 29; TST 16 |
QFT-GIT: TB exposure vs. no TB exposure: SN 58.82 (36.01 to 78.39); SP 54.15 (47.68 to 60.48); PPV 8.69 (4.79 to 15.27); NPV 94.66 (89.38 to 97.39) |
TST ≥ 10 mm: TB exposure vs. no TB exposure: SN 29.41 (13.28 to 53.13); SP 64.05 (57.83 to 69.83); PPV 5.43 (2.34 to 12.10); NPV 92.81 (87.86 to 95.84) |
QFT-GIT: TB exposure vs. no TB exposure: DOR 1.68 (0.62 to 4.58); DORa 1.30 (0.43 to 3.91) |
TST ≥ 10 mm: TB exposure vs. no TB exposure: DOR 0.74 (0.25 to 2.17); DORa 0.49 (0.17 to 1.45) |
QFT-GIT vs. TST ≥ 10 mm: TB exposure vs. no TB exposure: R-DOR 2.27 (1.07 to 4.81); R-DORa 2.65 (1.21 to 5.82) |
| Casas 2011,123 Spain (low) | Number of test results: QFT-GIT 214; TST 214 Test (+/–): QFT-GIT 45/157; TST ≥ 5 mm 52/162 Number of indeterminate results: QFT-GIT 12; TST 0 |
QFT-GIT: Risk factors for TB infection vs. no risk factors for TB infection: SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 5 mm: Risk factors for TB infection vs. no risk factors for TB infection: SN: NR; SP NR; PPV NR; NPV NR |
QFT-GIT: Risk factors for TB infection vs. no risk factors for TB infection: DOR 2.50 (1.20 to 5.10); DORa 2.90 (1.30 to 6.30) |
TST ≥ 5 mm: Risk factors for TB infection vs. no risk factors for TB infection: DOR 2.80 (1.40 to 5.50); DORa 2.90 (1.40 to 6.00) |
QFT-GIT vs. TST ≥ 5 mm: Risk factors for TB infection vs. no risk factors for TB infection: R-OR 0.89 (0.54 to 1.48); R-ORa 1.00 (0.58 to 1.73) |
| Casas 2011,124 Spain (low) | Number of test results: QFT-GIT 95; TST 95 Test (+/–): QFT-GIT 42/51; TST ≥ 5 mm 44/51 Number of indeterminate results: QFT-GIT 2; TST 0 |
QFT-GIT: Risk factors for TB infection vs. no risk factors for TB infection: SN 45.00 (33.09 to 57.51); SP 57.14 (40.86 to 72.02); PPV 64.29 (49.17 to 77.01); NPV 37.74 (25.94 to 51.19) |
TST ≥ 5 mm (two step): Risk factors for TB infection vs. no risk factors for TB infection: SN 50.00 (37.73 to 62.27); SP 60.00 (43.57 to 74.45); PPV 68.18 (53.44 to 80.00); NPV 41.18 (28.75 to 54.83) |
QFT-GIT: Risk factors for TB infection vs. no risk factors for TB infection: DOR 1.66 (0.66 to 3.33); DORa 1.50 (0.50 to 4.10) |
TST ≥ 5 mm (two step): Risk factors for TB infection vs. no risk factors for TB infection: DOR 1.25 (0.50 to 2.50); DORa 1.80 (0.60 to 5.10) |
QFT-GIT vs. TST ≥ 5 mm (two step): Risk factors for TB infection vs. no risk factors for TB infection: R-DOR 1.33 (0.74 to 2.38); R-DORa 0.83 (0.39 to 1.79) |
| Chkhartishvili 2013,125 Georgia (high) | Number of test results: QFT-GIT 237; T-SPOT.TB 218; TST 236 Test (+/–): QFT-GIT 70/167; T-SPOT.TB 56/162; TST ≥ 5 mm 41/195 Number of indeterminate results: QFT-GIT 3; T-SPOT.TB 22; TST 4 |
QFT-GIT: Household member treated for TB vs. no household member treated for TB: SN NR; SP NR; PPV NR; NPV NR T-SPOT.TB: Household member treated for TB vs. no household member treated for TB: SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 5 mm: Household member treated for TB vs. no household member treated for TB: SN NR; SP NR; PPV NR; NPV NR |
QFT-GIT: Household member treated for TB vs. no household member treated for TB DOR 0.43 (0.09 to 1.97); DORa NR T-SPOT.TB: Household member treated for TB vs. no household member treated for TB: DOR 1.48 (0.44 to 5.00); DORa NR |
TST ≥ 5 mm: Household member treated for TB vs. no household member treated for TB: DOR 1.48 (0.39 to 5.62); DORa NR |
QFT-GIT vs. TST ≥ 5 mm: Household member treated for TB vs. no household member treated for TB: R-DOR 0.29 (0.10 to 0.82); R-DORa NR T-SPOT.TB vs. TST ≥ 5 mm Household member treated for TB vs. no household member treated for TB: R-DOR 1.00 (0.40 to 2.51); R-DORa NR |
| Chung 2010,126 South Korea (high) | Number of test results: QFT-G 146; T-SPOT.TB 146; TST 146 Test (+/–): QFT-G 56/90; T-SPOT.TB 83/63; TST ≥ 10 mm 32/114 Number of indeterminate results: QFT-G NR; T-SPOT.TB NR; TST NR |
QFT-GIT: High risk for LTBI vs. low risk for LTBI: SN 52.94 (30.96 to 73.84); SP 63.57 (54.98 to 71.37); PPV 16.07 (8.69 to 27.81); NPV 91.11 (83.43 to 95.43) T-SPOT.TB: High risk for LTBI vs. low risk for LTBI: SN 47.06 (26.16 to 69.04); SP 41.86 (33.70 to 50.49); PPV 9.64 (4.96 to 17.88); NPV 85.71 (75.03 to 92.30) |
TST ≥ 10 mm: High risk for LTBI vs. low risk for LTBI: SN 11.76 (3.28 to 34.34); SP 76.74 (68.75 to 83.20); PPV 6.25 (1.73 to 20.15); NPV 86.84 (79.42 to 91.86) |
QFT-GIT: High risk for LTBI vs. low risk for LTBI: DOR 1.96 (0.71 to 5.43); DORa NR T-SPOT.TB: High risk for LTBI vs. low risk for LTBI: DOR 0.64 (0.23 to 1.76); DORa NR |
TST ≥ 10 mm: High risk for LTBI vs. low risk for LTBI: DOR 0.44 (0.09 to 2.03); DORa NR |
QFT-G vs. TST ≥ 10 mm: High risk for LTBI vs. low risk for LTBI: R-DOR 4.45 (1.72 to 11.51); R-DORa NR T-SPOT.TB vs. TST ≥ 10 mm High risk for LTBI vs. low risk for LTBI: R-DOR 1.45 (0.56 to 3.76); R-DORa NR |
| Costantino 2013,127 France (low) | Number of test results: T-SPOT.TB 475; TST 514 Test (+/–): T-SPOT.TB 122/353; TST ≥ 5 mm 196/318 Number of indeterminate results: T-SPOT.TB 88; TST 49 |
T-SPOT.TB: Conventional risk factors for LTBI vs. no risk factors for LTBI: SN 47.92 (34.47 to 61.67); SP 76.81 (72.58 to 80.57); PPV 18.85 (12.9 to 26.70); NPV 92.92 (89.75 to 95.16) |
TST ≥ 5 mm: Conventional risk factors for LTBI vs. no risk factors for LTBI: SN 63.27 (49.27 to 75.34); SP 64.52 (60.06 to 68.73); PPV 15.82 (11.37 to 21.58); NPV 94.34 (91.23 to 96.39) |
T-SPOT.TB: Conventional risk factors for LTBI vs. no risk factors for LTBI: DOR 3.05 (1.65 to 5.60); DORa 2.70 (1.49 to 4.89) |
TST ≥ 5 mm: Conventional risk factors for LTBI vs. no risk factors for LTBI: DOR 3.13 (1.70 to 5.77); DORa 1.95 (1.13 to 3.36) |
T-SPOT.TB vs. TST ≥ 5 mm: Conventional risk factors for LTBI vs. no risk factors for LTBI: R-DOR 0.97 (0.63 to 1.51); R-DORa 1.38 (0.92 to 2.09) |
| Hadaya 2013,128 Switzerland (low) | Number of test results: QFT-GIT 202; T-SPOT.TB 203; TST 200 Test (+/–): QFT-GIT 47/155; T-SPOT.TB 41/162; TST ≥ 5 mm 9/191 Number of indeterminate results: QFT-GIT 3; T-SPOT.TB 2; TST 0 |
QFT-GIT: Risk for LTBI vs. no risk for LTBI: SN 33.30 (19.60 to 49.50); SP 80.10 (72.90 to 86.20); PPV NR; NPV 81.10 (73.80 to 87.00) T-SPOT.TB: Risk for LTBI vs. no risk for LTBI: SN 33.30 (19.60 to 49.50); SP 85.50 (78.90 to 90.70); PPV NR; NPV 81.90 (75.00 to 87.60) |
TST ≥ 5 mm: Risk for LTBI vs. no risk for LTBI: SN 7.10 (1.50 to 19.50); SP 95.50 (90.80 to 98.20); PPV NR; NPV 78.40 (71.70 to 84.20) |
QFT-GIT: Risk for LTBI vs. no risk for LTBI: DOR 2.01 (1.25 to 2.76); DORa NR T-SPOT.TB: Risk for LTBI vs. no risk for LTBI: DOR 3.02 (1.36 to 6.71); DORa NR |
TST ≥ 5 mm: Risk for LTBI vs. no risk for LTBI: DOR 1.73 (0.41 to 7.24); DORa NR |
QFT-GIT vs. TST ≥ 5 mm: Risk for LTBI vs. no risk for LTBI: R-DOR 1.16 (0.51 to 2.66); R-DORa NR T-SPOT.TB vs. TST ≥ 5 mm: Risk for LTBI vs. no risk for LTBI: R-DOR 1.75 (0.76 to 4.04); R-DORa NR |
| Hsia 2012,129 USA (low) | Number of test results: QFT-GIT 2241; TST 2282 Test (+/–): QFT-GIT 160/2081; TST ≥ 5 mm 215/2067 Number of indeterminate results: QFT-GIT 41; TST 0 |
QFT-GIT: Geographical study location: SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 5 mm: Geographical study location: SN NR; SP NR; PPV NR; NPV NR |
QFT-GIT: Western Europe vs. North America: DOR NR; DORa 3.41 (1.99 to 5.83) Latin America vs. North America: DOR NR; DORa 3.43 (1.64 to 7.19) Eastern Europe vs. North America: DOR NR; DORa 3.58 (1.93 to 6.63) Asia vs. North America: DOR NR; DORa 8.48 (4.78 to 15.03) |
TST ≥ 5 mm: Western Europe vs. North America: DOR NR; DORa 2.10 (1.30 to 3.38) Latin America vs. North America: DOR NR; DORa 1.56 (0.80 to 3.05) Eastern Europe vs. North America: DOR NR; DORa 0.95 (0.53 to 1.70) Asia vs. North America: DOR NR; DORa 7.47 (4.61 to 12.08) |
QFT-GIT vs. TST ≥ 5 mm: Western Europe vs. North America: R-DOR NR; R-DORa 1.62 (1.13 to 2.34) Latin America vs. North America: R-DOR NR; R-DORa 2.20 (1.32 to 3.66) Eastern Europe vs. North America: R-DOR NR; R-DORa 3.77 (2.44 to 5.81) Asia vs. North America: R-DOR NR; R-DORa 1.14 (0.77 to 1.66) |
| Kim 2010,130 South Korea (low) | Number of test results: T-SPOT.TB 184; TST ≥ 5 mm 209; TST ≥ 10 mm 209 Test (+/–): T-SPOT.TB 65/119; TST ≥ 5 mm 47/162; TST ≥ 10 mm 21/188 Number of indeterminate results: T-SPOT.TB 25; TST ≥ 5 mm 0; TST ≥ 10 mm 0 |
T-SPOT.TB: Risk group for LTBI vs. no risk group for LTBI: SN 52.63 (31.71 to 72.67); SP 66.67 (59.17 to 73.41); PPV 15.38 (8.57 to 26.06); NPV 92.44 (86.25 to 95.97) |
TST ≥ 5 mm: Risk group for LTBI vs. no risk group for LTBI: SN 36.36 (19.73 to 57.05); SP 79.14 (72.76 to 84.35); PPV 17.02 (8.88 to 30.14); NPV 91.36 (86.02 to 94.78) TST ≥ 10 mm: Risk group for LTBI vs. no risk group for LTBI: SN 18.18 (7.31 to 38.52); SP 90.91 (85.92 to 94.25); PPV 19.05 (7.66 to 40.00); NPV 90.43 (85.37 to 93.86) |
T-SPOT.TB: Risk group for LTBI vs. no risk group for LTBI: DOR 2.35 (0.90 to 6.12); DORa 2.38 (0.87 to 6.52) |
TST ≥ 5 mm: Risk group for LTBI vs. no risk group for LTBI: DOR 2.17 (0.85 to 5.54); DORa 2.11 (0.82 to 5.46) TST ≥ 10 mm: Risk group for LTBI vs. no risk group for LTBI: DOR 2.22 (0.67 to 7.32); DORa 2.12 (0.60 to 7.49) |
T-SPOT.TB vs. TST ≥ 5 mm: Risk group for LTBI vs. no risk group for LTBI: R-DOR 1.02 (0.52 to 2.03); R-DORa 1.08 (0.55 to 2.15) T-SPOT.TB vs. TST ≥ 10 mm: Risk group for LTBI vs. no risk group for LTBI: R-DOR 1.00 (0.46 to 2.19); R-DORa 1.06 (0.48 to 2.31) |
| Kim 2013,131 South Korea (high) | Number of test results: QFT-GIT 120; TST 119 Test (+/–): QFT-GIT 53/67; TST ≥ 10 mm 35/91 Number of indeterminate results: QFT-GIT 6; TST 7 |
QFT-GIT: Risk group for LTBI vs. no risk group for LTBI: SN 73.33 (48.05 to 89.1); SP 60.00 (50.44 to 68.86); PPV 20.75 (12.00 to 33.46); NPV 94.03 (85.63 to 97.65) |
TST ≥ 10 mm: Risk group for LTBI vs. no risk group for LTBI: SN 86.67 (62.12 to 96.26); SP 90.38 (83.2 to 94.69); PPV 56.52 (36.81 to 74.37); NPV 97.92 (92.72 to 99.43) |
QFT-GIT: Risk group for LTBI vs. no risk group for LTBI: DOR 4.13 (1.23 to 13.82); DORa 4.62 (1.15 to 18.64) |
TST ≥ 10 mm: Risk group for LTBI vs. no risk group for LTBI: DOR 61.1 (12.03 to 310.4); DORa NR |
QFT-GIT vs. TST ≥ 10 mm: Risk group for LTBI vs. no risk group for LTBI: R-DOR 0.07 (0.02 to 0.19); R-DORa NR |
| Kim 2013,132 South Korea (high) | Number of test results: QFT-GIT 102; TST 93 Test (+/–): QFT-GIT 21/81; TST ≥ 10 mm 12/81 Number of indeterminate results: QFT-GIT 4; TST 0 |
QFT-GIT: History of treated TB vs. no such history: SN 100 (34.24 to 100); SP 81.32 (72.10 to 88.00); PPV 10.53 (2.93 to 31.39); NPV 100 (95.06 to 100) Abnormal chest radiograph vs. no abnormal chest radiograph: SN 75.00 (30.06 to 95.44); SP 82.02 (72.77 to 88.62); PPV 15.79 (5.52 to 37.57); NPV 98.65 (92.73 to 99.76) |
TST ≥ 10 mm: History of treated TB vs. no such history: SN NR; SP NR; PPV NR; NPV NR Abnormal chest radiograph vs. no abnormal chest radiograph: SN NR; SP NR; PPV NR; NPV NR |
QFT-GIT: History of treated TB vs. no such history: DOR NR; DORa 9.21 (NR) Abnormal chest radiograph vs. no abnormal chest radiograph: DOR 13.69 (1.33 to 140.30); DORa 27.95 (1.22 to 636.62) |
TST ≥ 10 mm: History of treated TB vs. no such history: DOR NR; DORa NR (NS) Abnormal chest radiograph vs. no abnormal chest radiograph: DOR NR; DORa NR (NS) |
QFT-GIT vs. TST ≥ 10 mm: History of treated TB vs. no such history: R-DOR NR; R-DORa NR Abnormal chest radiograph vs. no abnormal chest radiograph: R-DOR NR; R-DORa NR |
| Kleinert 2012,133 Germany (low) | Number of test results: QFT-G 685; T-SPOT.TB 844; TST 1529 Test (+/–): QFT-G 50/635; T-SPOT.TB 70/774; TST ≥ 5 mm 173/1356 Number of indeterminate results: QFT-G + T-SPOT.TB 80; TST NR |
QFT-G: Presence of CRF vs. absence of CRF: SN 16.67 (9.02 to 28.74); SP 93.5 (91.3 to 95.17); PPV 18.00 (9.77 to 30.8); NPV 92.91 (90.65 to 94.66) T-SPOT.TB: Presence of CRF vs. absence of CRF: SN 35.29 (25.00 to 47.16); SP 94.07 (92.18 to 95.53); PPV 34.29 (24.25 to 45.96); NPV 94.32 (92.45 to 95.74) |
TST ≥ 5 mm: Presence of CRF vs. absence of CRF: SN 39.34 (31.13 to 48.21); SP 91.12 (89.52 to 92.49); PPV 27.75 (21.61 to 34.85); NPV 94.54 (93.2 to 95.63) |
QFT-G: Presence of CRF vs. absence of CRF: DOR 2.88 (1.31 to 6.29); DORa 2.63 (1.15 to 5.98) T-SPOT.TB: Presence of CRF vs. absence of CRF: DOR 8.65 (4.84 to 15.46); DORa 8.74 (4.83 to 15.82) |
TST ≥ 5 mm: Presence of CRF vs. absence of CRF: DOR 6.65 (4.42 to 9.99); DORa 6.20 (4.08 to 9.44) |
QFT-G vs. TST ≥ 10 mm: Presence of CRF vs. absence of CRF: R-DOR 0.43 (0.28 to 0.68); R-DORa 0.42 (0.26 to 0.68) T-SPOT.TB vs. TST ≥ 10 mm: Presence of CRF vs. absence of CRF: R-DOR 1.30 (0.91 to 1.87); R-DORa 1.41 (0.97 to 2.04) |
| Laffitte 2009,134 Switzerland (low) | Number of test results: T-SPOT.TB 50; TST ≥ 5 mm 50; TST ≥ 10 mm 50 Test (+/–): T-SPOT.TB 10/40; TST ≥ 5 mm 20/30; TST ≥ 10 mm 18/32 Number of indeterminate results: T-SPOT.TB NR; TST ≥ 5 mm NR; TST ≥ 10 mm NR |
T-SPOT.TB: Probable LTBI vs. no probable LTBI: SN 36.36 (19.73 to 57.05); SP 92.86 (77.35 to 98.02); PPV 80.00 (49.02 to 94.33); NPV 65.00 (49.51 to 77.87) |
TST ≥ 5 mm: Probable LTBI vs. no probable LTBI: SN 50.00 (30.72 to 69.28); SP 67.86 (49.34 to 82.07); PPV 55.00 (34.21 to 74.18); NPV 63.33 (45.51 to 78.13) TST ≥ 10 mm: Probable LTBI vs. no probable LTBI: SN 54.55 (34.66 to 73.08); SP 78.57 (60.46 to 89.79); PPV 66.67 (43.75 to 83.72); NPV 68.75 (51.43 to 82.05) |
T-SPOT.TB: Probable LTBI vs. no probable LTBI: DOR 7.43 (1.38 to 39.90); DORa NR |
TST ≥ 5 mm: Probable LTBI vs. no probable LTBI: DOR 3.00 (0.93 to 9.70); DORa NR TST ≥ 10 mm: Probable LTBI vs. no probable LTBI: DOR 2.08 (0.64 to 6.73); DORa NR |
T-SPOT.TB vs. TST ≥ 5 mm: Probable LTBI vs. no probable LTBI: R-DOR 3.52 (1.25 to 9.96); R-DORa NR T-SPOT.TB vs. TST ≥ 10 mm: Probable LTBI vs. no probable LTBI: R-DOR 1.69 (0.58 to 4.89); R-DORa NR |
| Maritsi 2011,135 UK (low) | Number of test results: QFT-GIT 23; TST 14 Test (+/–): QFT-GIT 1/20; TST ≥ NR mm 0/14 Number of indeterminate results: QFT-GIT 2; TST 0 |
QFT-GIT: High-risk group vs. low-risk group: SN 33.33 (6.15 to 79.23); SP 100 (82.41 to 100); PPV 100 (20.65 to 100); NPV 90.00 (69.9 to 97.21) |
TST ≥ NR mm: High-risk group vs. low-risk group: SN 0.00 (0.00 to 56.15); SP 100 (74.12 to 100); PPV NR; NPV 78.57 (52.41 to 92.43) |
QFT-GIT: High-risk group vs. low-risk group: DOR NR; DORa NR |
TST ≥ NR mm: High-risk group vs. low-risk group: DOR NR; DORa NR |
QFT-GIT vs. TST ≥ NR mm: High-risk group vs. low-risk group: R-DOR NR; R-DORa NR |
| Mutsvangwa 2010,136 Zimbabwe (high) | Number of test results: T-SPOT.TB 73; TST 73 Test (+/–): T-SPOT.TB 22/51; TST ≥ 10 mm 33/40 Number of indeterminate results: T-SPOT.TB NR; TST NR |
T-SPOT.TB: Contact of index TB case vs. contact of index control: SN 34.55 (23.36 to 47.75); SP 83.33 (60.78 to 94.16); PPV 86.36 (66.66 to 95.25); NPV 29.41 (18.71 to 43.0) Smear status of index case (smear –ve, culture +ve vs. smear –ve, culture –ve): SN NR; SP NR; PPV NR; NPV NR Smear status of index case (smear +ve, culture +ve vs. smear –ve, culture –ve): SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 10 mm (two step): Contact of index TB case vs. contact of index control: SN 49.09 (36.38 to 61.92); SP 66.67 (43.75 to 83.72); PPV 81.82 (65.61 to 91.39); NPV 30.00 (18.07 to 45.43) Smear status of index case (smear –ve, culture +ve vs. smear –ve, culture –ve): SN NR; SP NR; PPV NR; NPV NR Smear status of index case (smear +ve, culture +ve vs. smear –ve, culture –ve): SN NR; SP NR; PPV NR; NPV NR |
T-SPOT.TB: Contact of index TB case vs. contact of index control: DOR 2.64 (0.67 to 10.27); DORa NR Smear status of index case (smear –ve, culture +ve vs. smear –ve, culture –ve): DOR 1.60 (0.20 to 12.69); DORa 1.87 (0.22 to 16.16) Smear status of index case (smear +ve, culture +ve vs. smear –ve, culture –ve): DOR 4.80 (1.05 to 21.91); DORa 5.36 (1.11 to 25.93) |
TST ≥ 10 mm (two step): Contact of index TB case vs. contact of index control: DOR 1.93 (0.63 to 5.87); DORa NR Smear status of index case (smear –ve, culture +ve vs. smear –ve, culture –ve): DOR 1.50 (0.24 to 9.46); DORa 1.09 (0.13 to 9.42) Smear status of index case (smear +ve, culture +ve vs. smear –ve, culture –ve): DOR 3.50 (0.88 to 13.93); DORa 3.43 (0.76 to 15.52) |
T-SPOT.TB vs. TST ≥ 10 mm (two step): Contact of index TB case vs. contact of index: R-DOR 1.37 (0.56 to 3.36); R-DORa NR Smear status of index case (smear –ve, culture +ve vs. smear –ve, culture –ve): R-DOR 1.07 (0.26 to 4.39); R-DORa 1.72 (0.36 to 8.06) Smear status of index case (smear +ve, culture +ve vs. smear –ve, culture –ve): R-DOR 1.37 (0.48 to 3.91); R-DORa 1.56 (0.51 to 4.76) |
| Papay 2011,137 Austria (low) | Number of test results: QFT-GIT 192; TST 192 Test (+/–): QFT-GIT 15/177; TST ≥ 5 mm 26/166 Number of indeterminate results: QFT-GIT 0; TST 0 |
QFT-GIT: Presence of risk factors vs. absence of risk factors: SN 13.85 (7.45 to 24.27); SP 95.28 (90.08 to 97.82); PPV 60.00 (35.75 to 80.18); NPV 68.36 (61.18 to 74.76) Origin from a high-incidence country vs. origin from a low-incidence country: SN 14.29 (5.69 to 31.49); SP 93.29 (88.39 to 96.21); PPV 26.67 (10.9 to 51.95); NPV 86.44 (80.62 to 90.72) History of contact with index case vs. no history of contact: SN 20.00 (5.668 to 50.98); SP 92.86 (88.16 to 95.78); PPV 13.33 (3.736 to 37.88); NPV 95.48 (91.34 to 97.69) |
TST ≥ 5 mm: Presence of risk factors vs. absence of risk factors: SN 21.74 (13.64 to 32.82); SP 92.09 (86.38 to 95.52); PPV 57.69 (38.95 to 74.46); NPV 70.33 (63.33 to 76.49) Origin from a high-incidence country vs. origin from a low-incidence country: SN 37.93 (22.69 to 56); SP 91.62 (86.64 to 94.86); PPV 42.31 (25.54 to 61.05); NPV 90.11 (84.91 to 93.65) History of contact with index case vs. no history of contact: SN 36.36 (15.17 to 64.62); SP 88.83 (83.67 to 92.51); PPV: 15.38 (6.15 to 33.53); NPV: 96.15 (92.27 to 98.12) |
QFT-GIT: Presence of risk factors vs. absence of risk factors: DOR 3.24 (1.10 to 9.54); DORa NR Origin from a high-incidence country vs. origin from a low-incidence country: DOR 2.32 (0.68 to 7.87); DORa NR History of contact with index case vs. no history of contact: DOR 3.25 (0.62 to 16.91); DORa NR |
TST ≥ 5 mm: Presence of risk factors vs. absence of risk factors: DOR 3.23 (1.39 to 7.49); DORa NR Origin from a high-incidence country vs. origin from a low-incidence country: DOR 6.68 (2.67 to 16.73); DORa NR History of contact with index case vs. no history of contact: DOR 4.54 (1.23 to 16.78); DORa NR |
QFT-GIT vs. TST ≥ 5 mm: Presence of risk factors vs. absence of risk factors: R-DOR 1.00 (0.50 to 2.02); R-DORa NR Origin from a high-incidence country vs. origin from a low-incidence country: R-DOR 0.35 (0.16 to 0.76); R-DORa NR History of contact with index case vs. no history of contact: R-DOR 0.72 (0.24 to 2.10); R-DORa NR |
| Ramos 2013,138 Spain (low) | Number of test results: QFT-GIT 153; TST 153 Test (+/–): QFT-GIT 15/137; TST ≥ 5 mm 43/110 Number of indeterminate results: QFT-GIT 1; T-SPOT.TB 0; TST 0 |
QFT-GIT: Contact of index TB case vs. contact of index control: SN 42.86 (15.82 to 74.95); SP 91.72 (86.09 to 95.20); PPV 20.00 (7.04 to 45.19); NPV 97.08 (92.73 to 98.86) Born in an endemic country vs. not born in an endemic country: SN 50.00 (21.52 to 78.48); SP 92.36 (86.84 to 95.68); PPV 26.67 (10.90 to 51.95); NPV 97.08 (92.73 to 98.86) |
TST ≥ 5 mm: Contact of index TB case vs. contact of index control: SN 57.14 (25.05 to 84.18); SP 73.29 (65.58 to 79.8); PPV 9.30 (3.67 to 21.6); NPV 97.27 (92.29 to 99.07) Born in an endemic country vs. not born in an endemic country: SN 50.00 (21.52 to 78.48); SP 73.1 (65.36 to 79.66); PPV 9.30 (3.67 to 21.60); NPV 96.36 (91.02 to 98.58) |
QFT-GIT: Contact of index TB case vs. contact of index control: DOR 8.31 (1.66 to 41.56); DORa NR Born in an endemic country vs. not born in an endemic country: DOR 12.09 (2.65 to 55.07); DORa NR |
TST ≥ 5 mm: Contact of index TB case vs. contact of index control: DOR 3.66 (0.78 to 17.08); DORa NR Born in an endemic country vs. not born in an endemic country: DOR 2.72 (0.65 to 11.40); DORa NR |
QFT-GIT vs. TST ≥ 5 mm: Contact of index TB case vs. contact of index control: R-DOR 2.27 (0.73 to 7.08); R-DORa NR Born in an endemic country vs. not born in an endemic country: R-DOR 4.44 (1.53 to 12.89); R-DORa NR |
| Seyhan 2010,139 Turkey (intermediate) | Number of test results: QFT-GIT 100; TST 100 Test (+/–): QFT-GIT 43/57; TST ≥ 10 mm 34/66 Number of indeterminate results: QFT-GIT NR; TST 0 |
QFT-GIT: Previous contact with an index case vs. no contact: SN 76.92 (49.74 to 91.82); SP 62.07 (51.57 to 71.55); PPV 23.26 (13.15 to 37.74); NPV 94.74 CI (85.63 to 98.19) Previous TB disease vs. no previous disease: SN 75.0 (40.93 to 92.85); SP 59.78 (49.57 to 69.22); PPV 13.95 (6.556 to 27.26); NPV 96.49 (88.08 to 99.03) |
TST ≥ 10 mm: Previous contact with an index case vs. no contact: SN 46.15 (23.21 to 70.86); SP 67.82 (57.43 to 76.7); PPV 17.65 (8.349 to 33.51); NPV 89.39 (79.69 to 94.77) Previous TB disease vs. no previous disease: SN 37.5 (13.68 to 69.43); SP 66.3 (56.17 to 75.14); PPV 8.824 (3.047 to 22.96); NPV 92.42 (83.46 to 96.72) |
QFT-GIT: Previous contact with an index case vs. no contact: DOR 5.45 (1.40 to 21.27); DORa NR Previous TB disease vs. no previous disease: DOR 4.46 (0.85 to 23.31); DORa NR |
TST ≥ 10 mm: Previous contact with an index case vs. no contact: DOR 1.81 (0.55 to 5.87); DORa NR Previous TB disease vs. no previous disease: DOR 1.18 (0.26 to 5.26); DORa NR |
QFT-GIT vs. TST ≥ 10 mm: Previous contact with an index case vs. no contact: R-DOR 3.01 (1.20 to 7.56); R-DORa NR Previous TB disease vs. no previous disease: R-DOR 3.78 (1.21 to 11.83); R-DORa NR |
| Shen 2012,140 China (high) | Number of test results: T-SPOT.TB 70; TST 70 Test (+/–): T-SPOT.TB 26/44; TST ≥ 5 mm 34/36 Number of indeterminate results: T-SPOT.TB 0; TST 0 |
T-SPOT.TB: Suspected TB disease vs. no suspected TB: SN 70.97 (53.41 to 83.90); SP 89.74 (76.42 to 95.94); PPV 84.62 (66.47 to 93.85); NPV 79.55 (65.5 to 88.85) |
TST ≥ 5 mm: Suspected TB disease vs. no suspected TB: SN 61.29 (43.82 to 76.27); SP 61.54 (45.9 to 75.11); PPV 55.88 (39.45 to 71.12); NPV 66.67 (50.33 to 79.79) |
T-SPOT.TB: Suspected TB disease vs. no suspected TB: DOR 21.39 (5.87 to 77.93); DORa NR |
TST ≥ 5 mm: Suspected TB disease vs. no suspected TB: DOR 2.53 (0.96 to 6.67); DORa NR |
T-SPOT.TB vs. TST ≥ 5 mm: Suspected TB disease vs. no suspected TB: R-DOR 8.45 (3.71 to 19.28); R-DORa NR |
| Souza 2014,153 Brazil (intermediate) | Number of test results: QFT-GIT 299; TST 300 Test (+/–): QFT-GIT 14/285; TST ≥ 5 mm 10/290 Number of indeterminate results: QFT-GIT 1; TST 0 |
QFT-GIT: History of contact with index case vs. no history of contact with index case: SN 0.0 (0.00 to 9.89); SP 94.96 (91.57 to 97.03); PPV 0.0 (0.00 to 22.81); NPV 87.5 (83.11 to 90.87) |
TST ≥ 5 mm: History of contact with index case vs. no history of contact with index case: SN 2.86 (0.50 to 14.53); SP 96.91 (94.02 to 98.43); PPV 11.11 (1.99 to 43.5); NPV 88.07 (83.79 to 91.34) |
QFT-GIT: History of contact with index case vs. no history of contact with index case: DOR 0.50 (0.06 to 4.24); DORa NR |
TST ≥ 5 mm: History of contact with index case vs. no history of contact with index case: DOR 0.93 (0.11 to 7.61); DORa 1.21 (0.13 to 11.16) |
QFT-GIT vs. TST ≥ 5 mm: History of contact with index case vs. no history of contact with index case: R-DOR 0.54 (0.12 to 2.49); R-DORa NR |
| Takeda 2011,141 Japan (low) | Number of test results: QFT-GIT 71; TST 43 Test (+/–): QFT-GIT 2/46; TST ≥ 10 mm 3/40 Number of indeterminate results: QFT-GIT 23; T-SPOT.TB NR; TST 0 |
QFT-GIT: Risk of LTBI vs. no risk of LTBI: SN 11.11 (10 to 32.80); SP 100.00 (88.65 to 100.00); PPV 100.00 (34.24 to 100.00); NPV 65.22 (53.45 to 75.38) |
TST ≥ 10 mm: Risk of LTBI vs. no risk of LTBI: SN 7.14 (1.27 to 31.47); SP 93.10 (78.04 to 98.09); PPV 33.33(6.15 to 79.23); NPV 67.50; (52.02 to 79.92) |
QFT-GIT: Risk of LTBI vs. no risk of LTBI: DOR 3.75 (0.31 to 44.6); DORa NR |
TST ≥ 10 mm: Risk of LTBI vs. no risk of LTBI: DOR 1.04 (0.08 to 12.53); DORa NR |
QFT-GIT vs. TST ≥ 10 mm: Risk of LTBI vs. no risk of LTBI: R-DOR 3.61 (0.59 to 21.99); R-DORa NR |
| Vassilopoulos 2011,142 Greece (low) | Number of test results: QFT-GIT 157; T-SPOT.TB 157; TST 157 Test (+/–): QFT-GIT 32/123; T-SPOT.TB 39/116; TST ≥ 5 mm 58/97 Number of indeterminate results: QFT-GIT 2; T-SPOT.TB 2; TST 2 |
T-SPOT.TB: TB exposure vs. no exposure: SN 25.00 (11.19 to 46.87); SP 74.81 (66.88 to 81.38); PPV 12.82 (5.60 to 26.71); NPV 87.07 (79.76 to 92.00) QFT-GIT: TB exposure vs. no exposure: SN 15.00 (5.23 to 36.04); SP 78.52 (70.85 to 84.61); PPV 9.37 (3.24 to 24.22); NPV 86.18 (78.98 to 91.19) |
TST ≥ 5 mm: TB exposure vs. no exposure: SN 50.00 (29.93 to 70.07); SP 64.44, (56.07 to 72.02); PPV 17.24 (9.64 to 28.91); NPV 89.69 (82.05 to 94.3) |
T-SPOT.TB: TB exposure vs. no exposure: DOR 0.99 (0.33 to 2.92); DORa NR QFT-GIT: TB exposure vs. no exposure: DOR 0.64 (0.17 to 2.35); DORa NR |
TST ≥ 5 mm: TB exposure vs. no exposure: DOR 1.81 (0.70 to 4.66); DORa NR |
T-SPOT.TB vs. TST ≥ 5 mm: TB exposure vs. no exposure: R-DOR 0.55 (0.26 to 1.14); R-DORa NR QFT-GIT vs. TST ≥ 5 mm: TB exposure vs. no exposure: R-DOR 0.35 (0.15 to 0.81); R-DORa NR |
The forest plot analysis of R-DORs from the remaining 21 studies is stratified according to specific conditions/procedures (HIV infection, solid organ transplantation candidates, post-kidney transplantation, haemodialysis – ESRD, immune-mediated inflammatory diseases before antiTNF-α therapy, hepatitis C and lupus erythematosus) (Figure 29). There was a significant amount of heterogeneity across all subgroups of participants except for those with haemodialysis in whom IGRA (QFT-GIT) was more strongly associated with exposure groups than TST 10 mm (pooled R-DOR 2.53, 95% CI 1.48 to 4.34; I2 = 40%). Similarly, in participants with hepatitis C, IGRA (T-SPOT. TB) outperformed TST 5 mm in detecting LTBI (R-DOR 8.45, 95% CI 3.71 to 19.24).
FIGURE 29.
Pooled R-DOR of IGRAs vs. TST in all studies based on high- and low-risk exposure in immunocompromised patients. df, degrees of freedom; IMID, immune-mediated inflammatory disease; IV, inverse variance; SE, standard error.
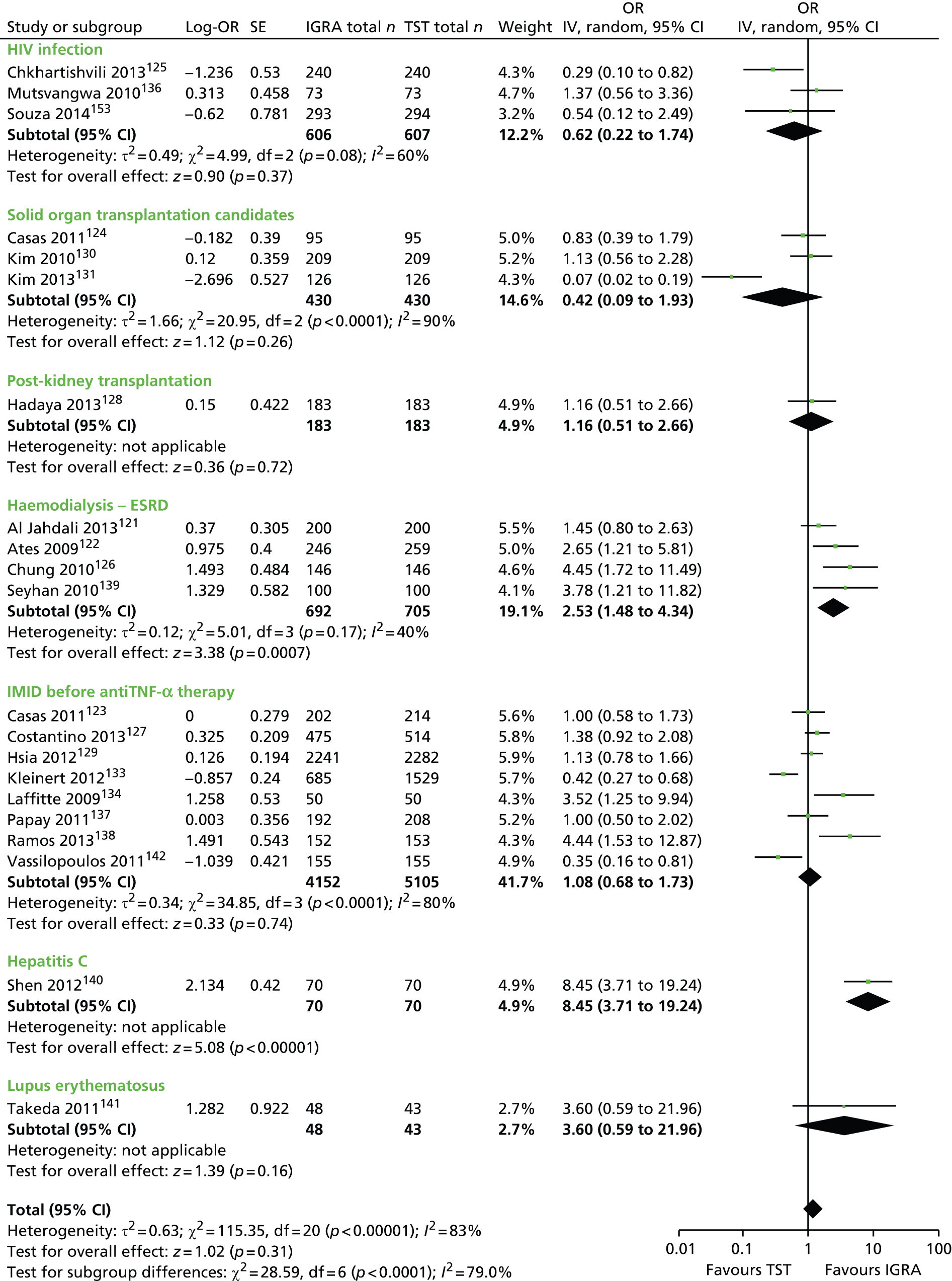
Within-subgroup heterogeneity by IGRA type (QFT-GIT, T-SPOT. TB) and TST threshold (5 mm, 10 mm, 15 mm) could not be examined for most subgroups because of sparse data. The underlying differences in the definition/measurement of exposure and differential performance of tests across the disease spectrum may have additionally contributed to the non-uniformity observed in the R-DOR estimates (Figures 30–33). For example, for participants with immune-mediated inflammatory diseases before antiTNF-α therapy, the non-uniformity persisted even after accounting for the type of IGRA (QFT-GIT) and TST threshold (5 mm) (pooled R-DOR 0.90, 95% CI 0.52 to 1.54; I2 = 80%; see Figure 30). However, the stratification by IGRA type and TST threshold revealed that the TST 5 mm was better than the IGRA (QFT-GIT) at detecting LTBI in participants with HIV infection (pooled R-DOR 0.35, 95% CI 0.15 to 0.83; I2 = 0%; see Figure 30). Based on the results from two studies of solid organ transplantation candidates, there was no significant difference between the performance of IGRAs (T-SPOT. TB130 and QFT-GIT124) and the TST 5 mm in relation to the identification of LTBI (see Figures 30, 32 and 33). In contrast, in another study of solid organ transplantation candidates,131 the TST 10 mm outperformed QFT-GIT (R-DOR 0.07, 95% CI 0.02 to 0.19; see Figure 31). In two studies, the performance of QFT-GIT did not significantly differ from that of the TST among participants with lupus erythematosus (QFT-GIT vs. TST 10 mm: R-DOR 3.60, 95% CI 0.59 to 21.96; see Figure 31)141 and kidney transplant recipients (QFT-GIT vs. TST 5 mm: R-DOR 1.16, 95% CI 0.51 to 2.66; see Figure 30). 128
FIGURE 30.
Pooled R-DOR of QFT-GIT/G vs. TST 5 mm based on high- and low-risk exposure in immunocompromised patients. df, degrees of freedom; IMID, immune-mediated inflammatory disease; IV, inverse variance; SE, standard error.
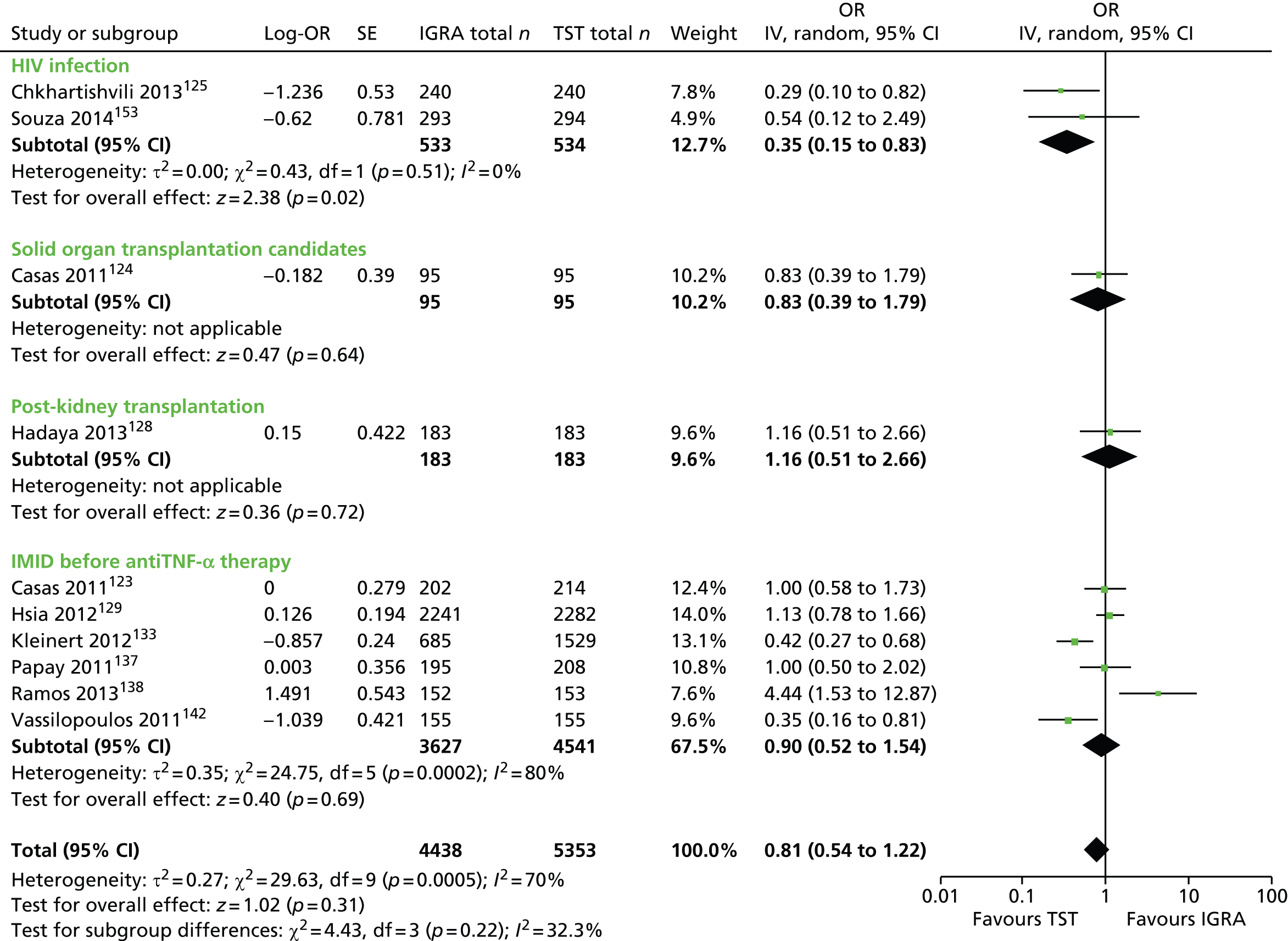
FIGURE 31.
Pooled R-DOR of QFT-GIT/G vs. TST 10 mm based on high- and low-risk exposure in immunocompromised patients. df, degrees of freedom; IV, inverse variance; SE, standard error.
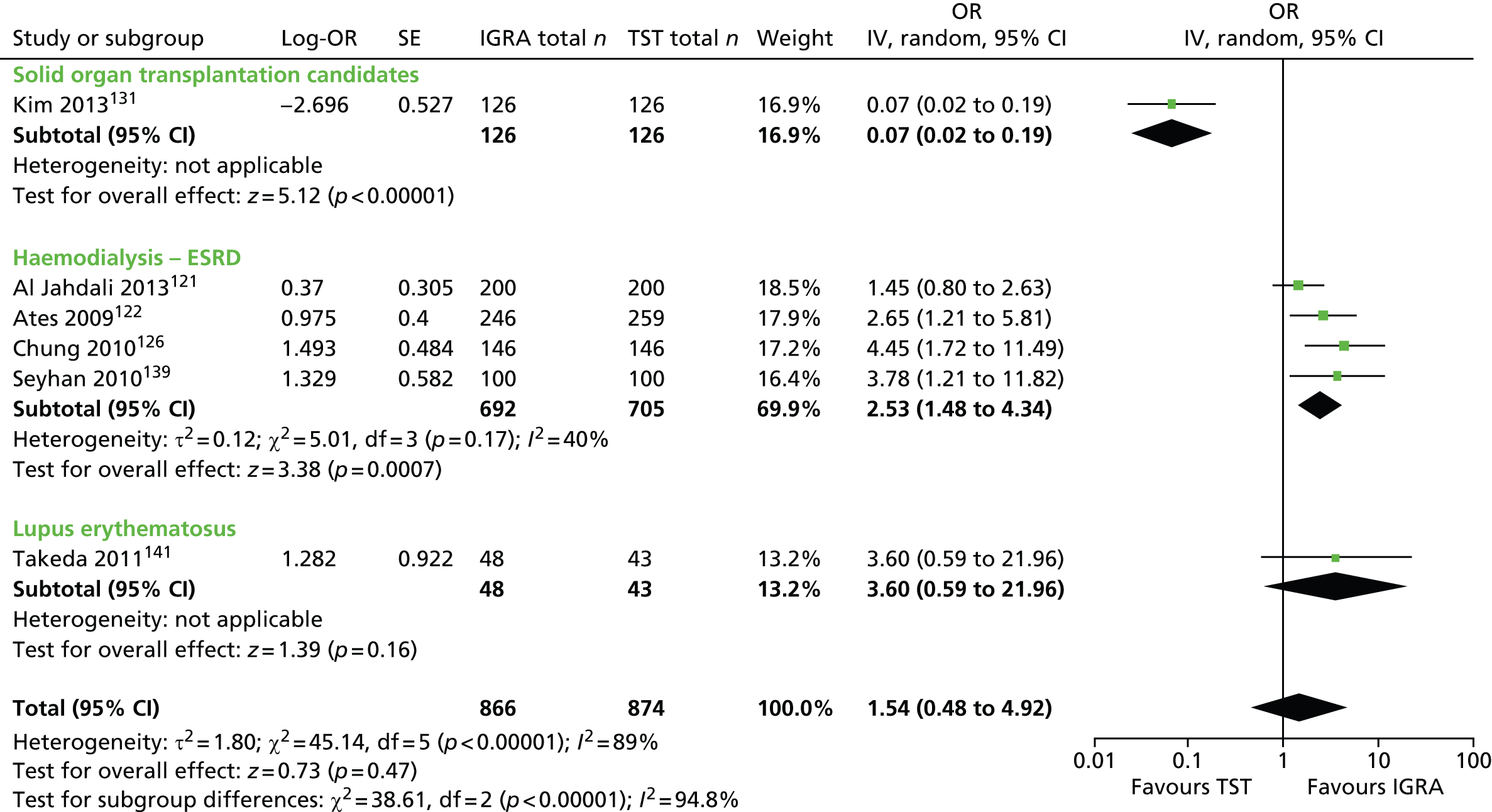
FIGURE 32.
Pooled R-DOR of T-SPOT. TB vs. TST 5 mm based on high- and low-risk exposure in immunocompromised patients. df, degrees of freedom; IMID, immune-mediated inflammatory disease; IV, inverse variance; SE, standard error.

FIGURE 33.
Pooled R-DOR of T-SPOT. TB vs. TST 10 mm based on high- and low-risk exposure in immunocompromised patients. df, degrees of freedom; IMID, immune-mediated inflammatory disease; IV, inverse variance; SE, standard error.
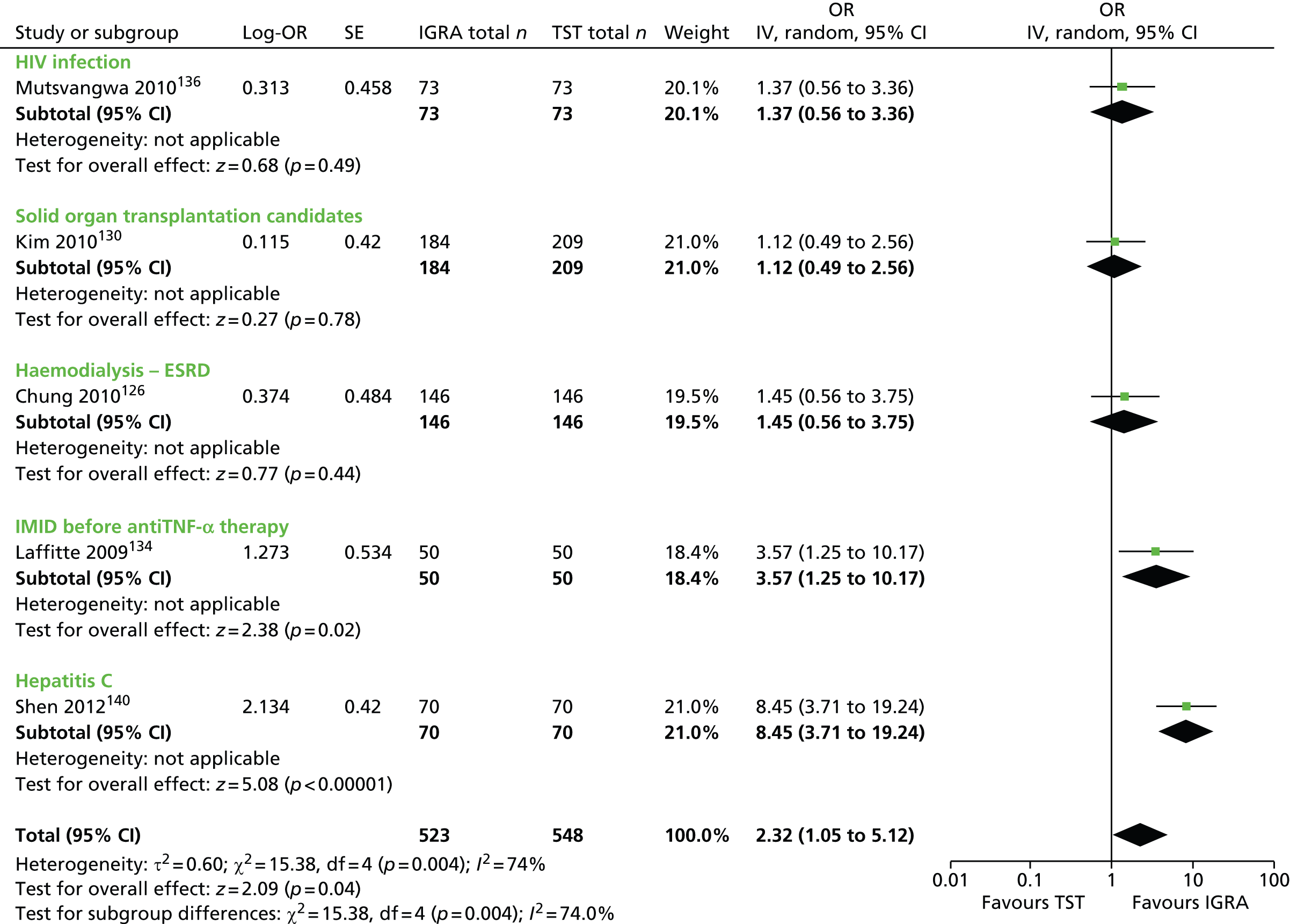
Sensitivity and specificity
This section incorporates 24 newly identified studies120–142,153 (see Table 15). Three studies123,125,129 did not report sensitivity and specificity parameters for both IGRA and TST and one study132 reported them only for TST. The forest plots for the remaining 21 studies displayed a wide variability in sensitivity (IGRA range 0–75%; TST 5 mm range 0–61%; TST 10 mm range 0–87%) and specificity (IGRA range 57–100%; TST 5 mm range 62–96%; TST 10 mm range 64–93%). The heterogeneity persisted even after stratifying the estimates by type of IGRA (QFT-GIT, T-SPOT. TB) and TST threshold (5 mm, 10 mm). Of the two IGRAs, QFT-GIT/G demonstrated markedly wider variation in the estimates of specificity and sensitivity than T-SPOT. TB. In general, for both the IGRAs and the TST, specificity tended to be greater than sensitivity (Figures 34–41). The absence of any clear pattern in the distribution of sensitivity and specificity values reflects the underlying between-study differences in study populations/conditions and settings and variation in exposure definitions and measurement. In light of the observed heterogeneity, no meta-analysis was undertaken.
FIGURE 34.
Forest plot of sensitivity based on exposure groups (QFT-GIT/G) in immunocompromised patients. df, degrees of freedom; HC, hepatitis C; IMID, immune-mediated inflammatory disease; LE, lupus erythematosus; PKT, post-kidney transplantation; SOTC, solid organ transplantation candidate.
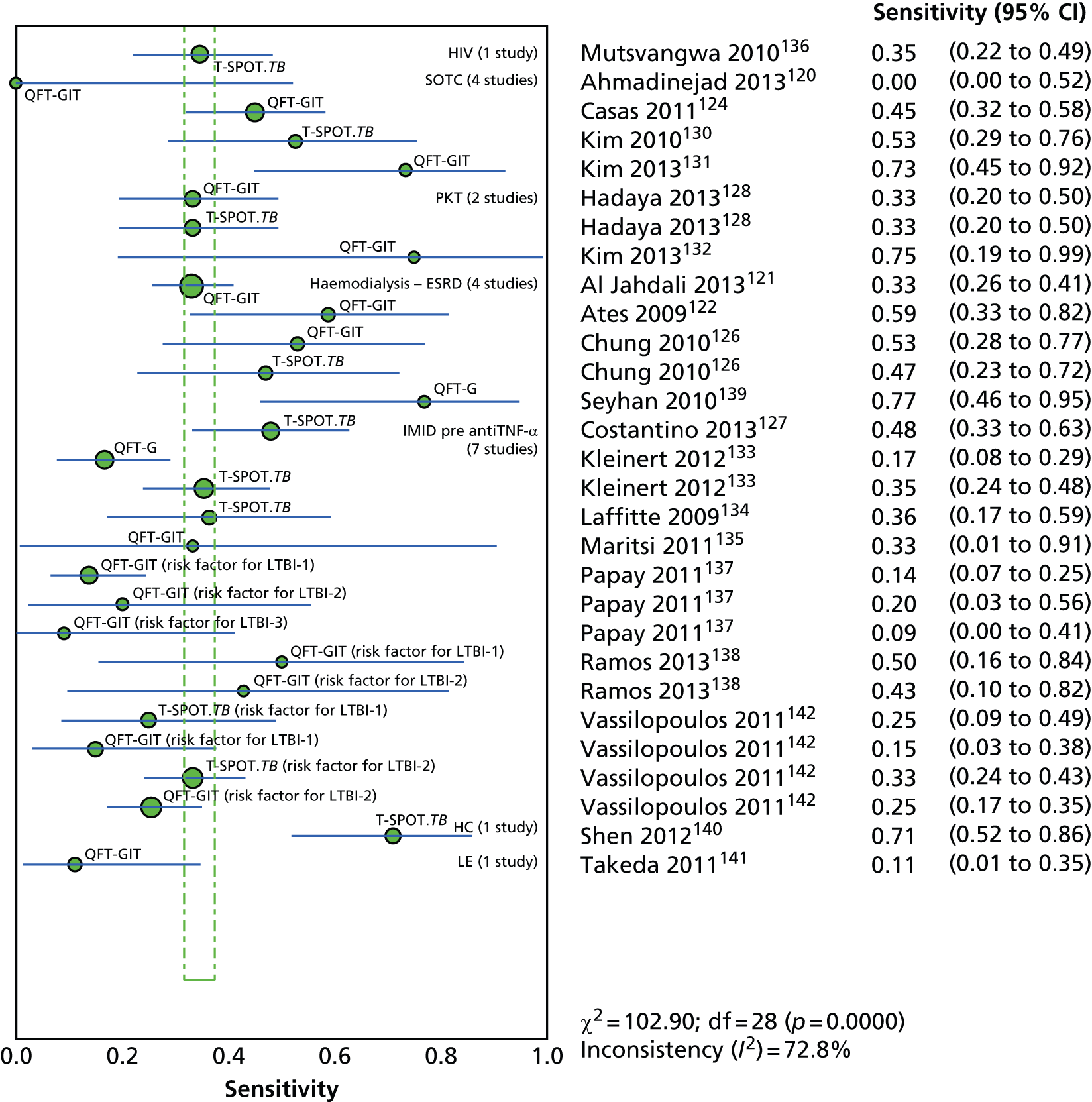
FIGURE 35.
Forest plot of sensitivity based on exposure groups (T-SPOT. TB) in immunocompromised patients. df, degrees of freedom; HC, hepatitis C; IMID, immune-mediated inflammatory disease; PKT, post-kidney transplantation; SOTC, solid organ transplantation candidate.
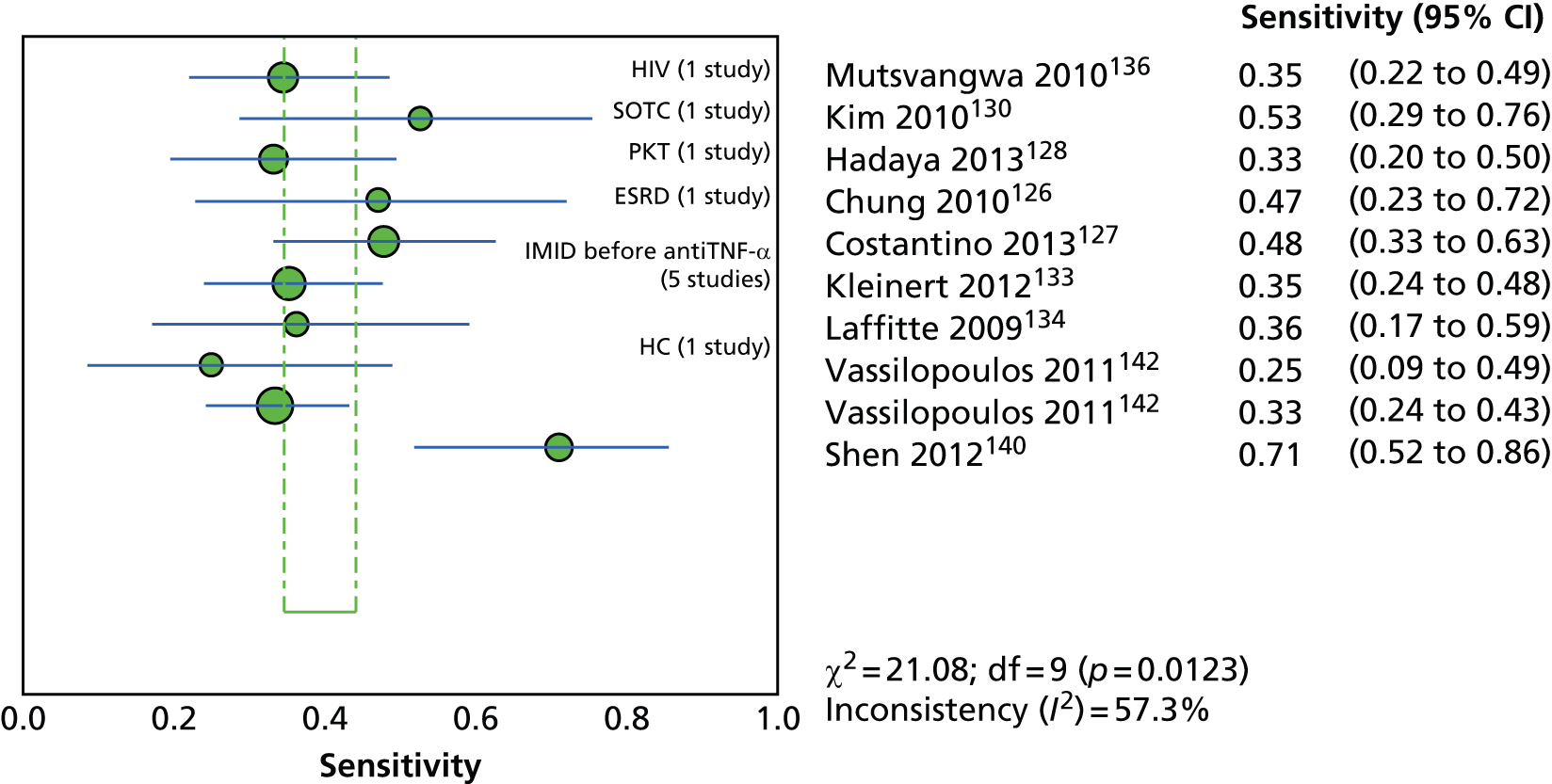
FIGURE 36.
Forest plot of sensitivity based on exposure groups (TST 5 mm) in immunocompromised patients. df, degrees of freedom; IMID, immune-mediated inflammatory disease; LE, lupus erythematosus; PKT, post-kidney transplantation; SOTC, solid organ transplantation candidate.

FIGURE 37.
Forest plot of sensitivity based on exposure groups (TST 10 mm) in immunocompromised patients. df, degrees of freedom; IMID, immune-mediated inflammatory disease; LE, lupus erythematosus; SOTC, solid organ transplantation candidate.
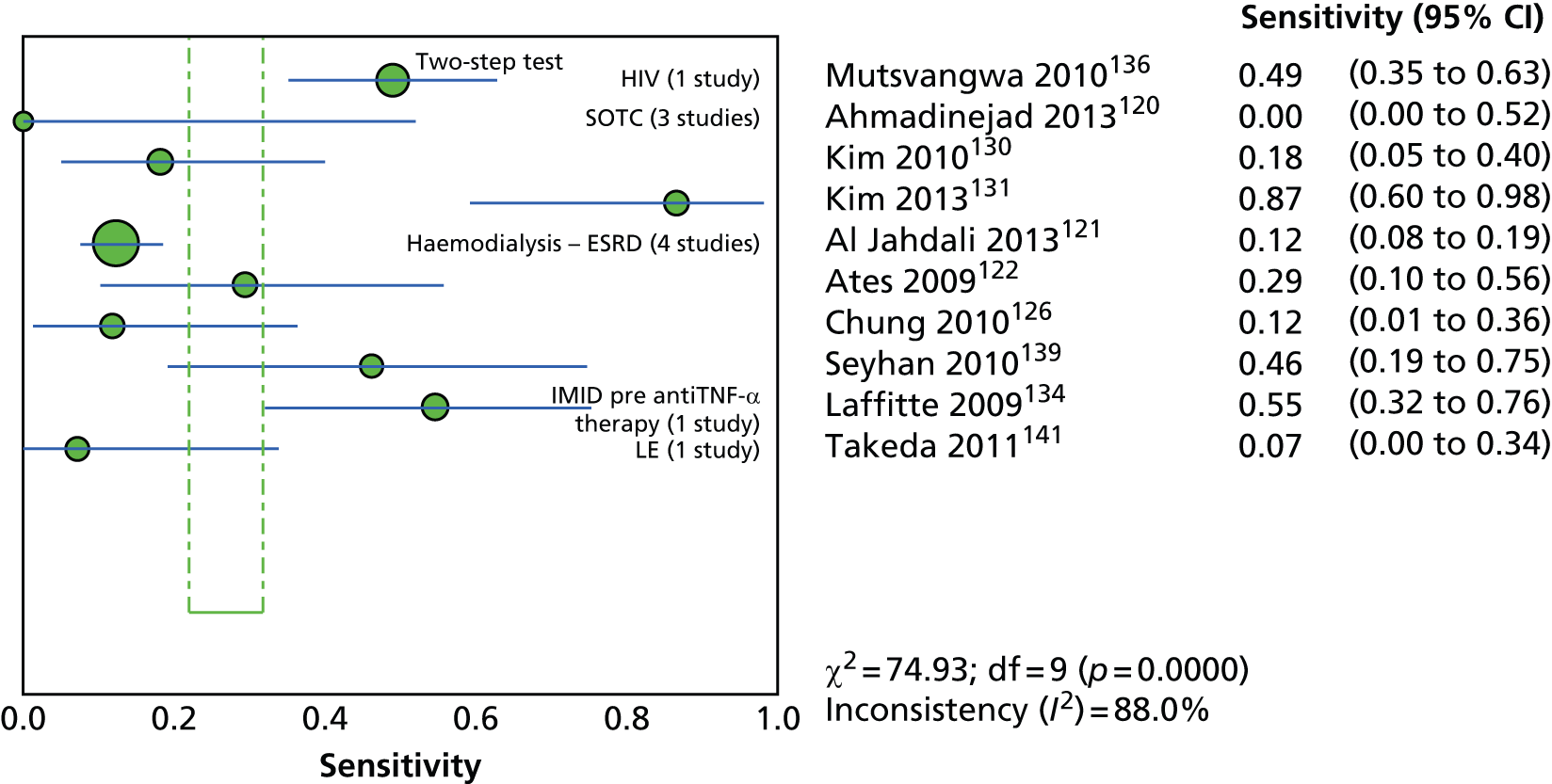
FIGURE 38.
Forest plot of specificity based on exposure groups (QFT-GIT/G) in immunocompromised patients. df, degrees of freedom; HC, hepatitis C; IMID, immune-mediated inflammatory disease; LE, lupus erythematosus; PKT, post-kidney transplantation; SOTC, solid organ transplantation candidate.

FIGURE 39.
Forest plot of specificity based on exposure groups (T-SPOT. TB) in immunocompromised patients. df, degrees of freedom; HC, hepatitis C; IMID, immune-mediated inflammatory disease; PKT, post-kidney transplantation; SOTC, solid organ transplantation candidate.

FIGURE 40.
Forest plot of specificity based on exposure groups (TST 5 mm) in immunocompromised patients. df, degrees of freedom; HC, hepatitis C; IMID, immune-mediated inflammatory disease; PKT, post-kidney transplantation; SOTC, solid organ transplantation candidate.
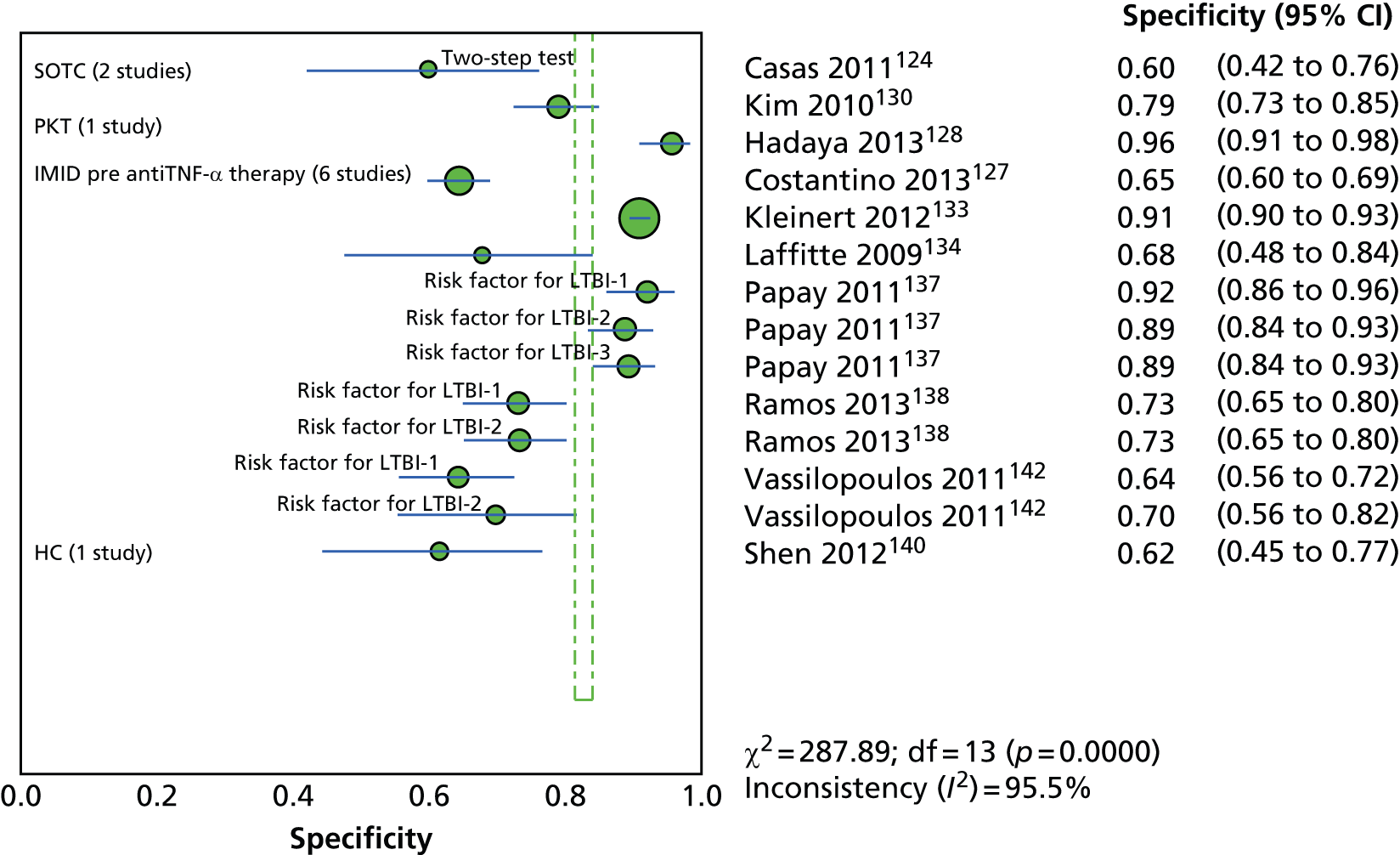
FIGURE 41.
Forest plot of specificity based on exposure groups (TST 10 mm) in immunocompromised patients. df, degrees of freedom; IMID, immune-mediated inflammatory disease; LE, lupus erythematosus; SOTC, solid organ transplantation candidate.
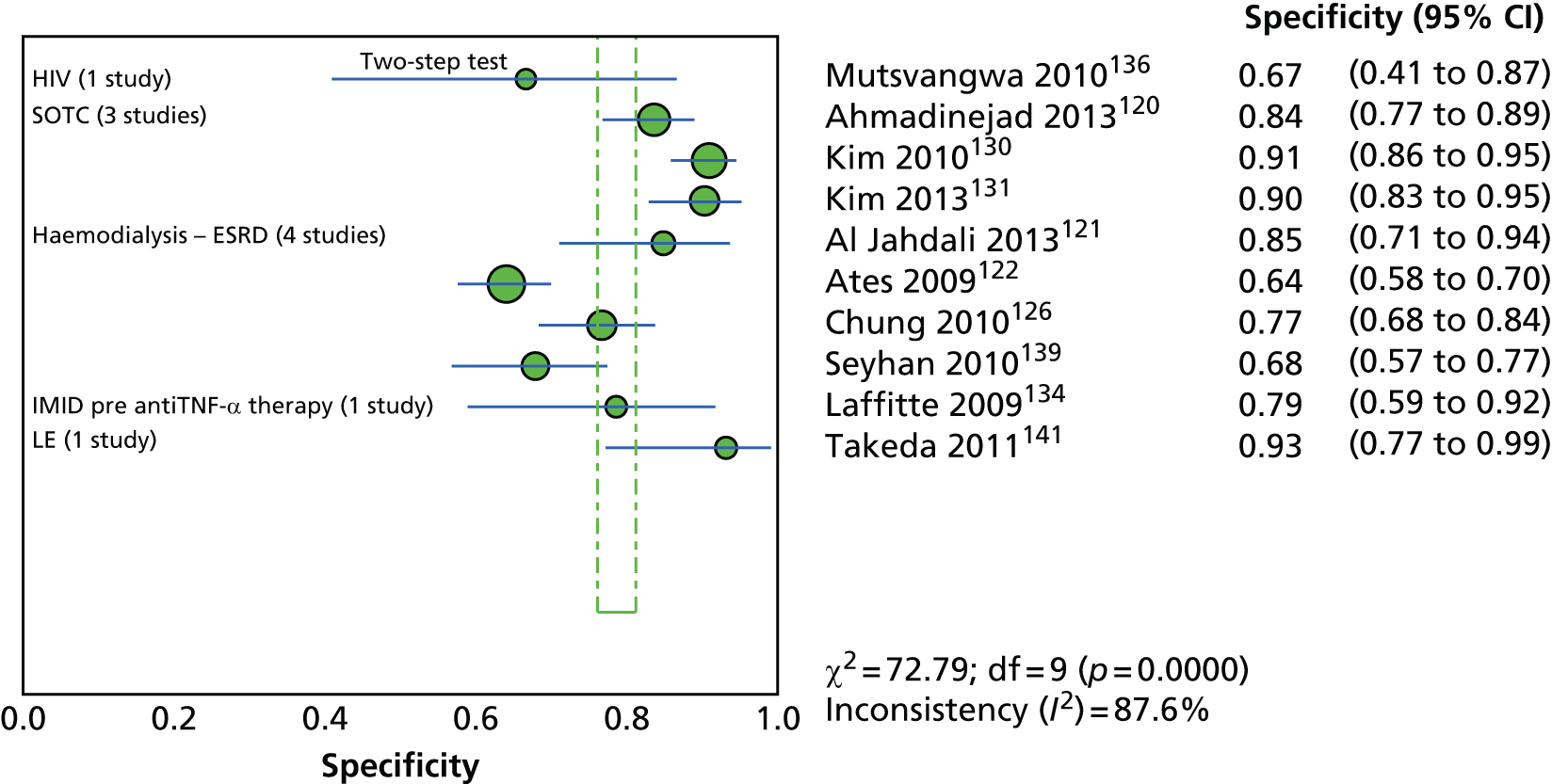
Influence of bacillus Calmette–Guérin vaccination status on test positivity
Of the 24 newly identified studies included in this section,120–142,153 only 14120,122–125,127,129–131,133,134,138,139,142 reported on the association between test positivity and BCG vaccination status. Overall, there was no evidence indicating a differential effect of BCG vaccination status on IGRA and TST positivity. 120,122–125,130,131,133,134,137–142 In other words, the odds of test positivity for the IGRA and TST were not significantly different between the BCG vaccinated and the non-vaccinated groups (Table 16). Only one study139 demonstrated a significantly increased OR for TST 10 mm positivity (OR 4.28, 95% CI 1.35 to 13.64) as opposed to a non-significant OR for the IGRA (OR 1.89, 95% CI 0.75 to 4.73) in relation to BCG vaccination status.
| Study ID, country (burden) | Sample size, n | Type of IGRA/TST induration threshold | Association between test positivity and BCG vaccination status: OR (95% CI) | |
|---|---|---|---|---|
| Crude/unadjusted | Adjusted | |||
| Ahmadinejad 2013,120 Iran (intermediate) | 159 | QFT-GIT | 0.38 (0.11 to 1.24) | NR |
| 164 | TST 10 mm | 0.60 (0.15 to 2.34) | NR | |
| Al Jahdali 2013,121 Saudi Arabia (low) | NA | QFT-GIT | NR | NR |
| NA | TST 10 mm (two step) | NR | NR | |
| Ates 2009,122 Turkey (intermediate) | 246 | QFT-GIT | 1.13 (0.68 to 1.86) | 1.14 (0.68 to 1.92) |
| 259 | TST 10 mm | 0.85 (0.51 to 1.43) | 0.87 (0.50 to 1.51) | |
| Casas 2011,123 Spain (low) | 214 | QFT-GIT | 1.20 (0.50 to 3.20) | NR |
| 214 | TST 5 mm | 1.70 (0.90 to 3.40) | 1.50 (0.70 to 3.40) | |
| Casas 2011,124 Spain (low) | 95 | QFT-GIT | 0.62 (0.26 to 1.42) | NR |
| 95 | TST 5 mm (two step) | 0.83 (0.35 to 2.00) | NR | |
| Chkhartishvili 2013,125 Georgia (high) | 240 | QFT-GIT | 1.41 (0.38 to 5.29) | NR |
| 240 | T-SPOT.TB | 1.78 (0.38 to 8.28) | NR | |
| 240 | TST 5 mm | 2.55 (0.32 to 20.18) | NR | |
| Chung 2010,126 South Korea (high) | 146 | QFT-GIT | NR | NR |
| 146 | T-SPOT.TB | NR | NR | |
| 146 | TST 10 mm | NR | NR | |
| Costantino 2013,127 France (low) | 563 | T-SPOT.TB | NR | 0.39 (0.24 to 0.62) |
| 563 | TST 5 mm | NR | NR (p = 0.11, NS) | |
| Hadaya 2013,128 Switzerland (low) | 183 | QFT-GIT | NR | NR |
| 183 | T-SPOT.TB | NR | NR | |
| 183 | TST 5 mm | NR | NR | |
| Hsia 2012,129 USA (low) | 2029 | QFT-GIT | NR | 1.00 (0.66 to 1.51) |
| 2029 | TST 5 mm | NR | 2.47 (1.71 to 3.55) | |
| Kim 2010,130 South Korea (high) | 184 | T-SPOT.TB | 0.69 (0.36 to 1.34) | NR |
| 209 | TST 5 mm | 1.25 (0.55 to 2.82) | NR | |
| 209 | TST 10 mm | 0.89 (0.31 to 2.58) | NR | |
| Kim 2013,131 South Korea (high) | 120 | QFT-GIT | 1.94 (0.48 to 7.91) | 2.32 (0.50 to 10.66) |
| 119 | TST 10 mm | 2.56 (0.31 to 21.06) | 3.32 (0.38 to 28.97) | |
| Kim 2013,132 South Korea (high) | 93 | QFT-GIT | NR | NR |
| 93 | TST 10 mm | NR | NR | |
| Kleinert 2012,133 Germany (low) | 685 | QFT-G | NR | 0.43 (0.17 to 1.10) |
| 844 | T-SPOT.TB | NR | 1.07 (0.47 to 2.43) | |
| 1529 | TST 5 mm | 3.17 (2.19 to 4.58) | 2.95 (2.00 to 4.35) | |
| Laffitte 2009,134 Switzerland (low) | 50 | T-SPOT.TB | 1.00 (0.01 to 10.07) | NR |
| 50 | TST 5 mm | 2.92 (0.30 to 28.29) | NR | |
| 50 | TST 10 mm | 2.43 (0.25 to 23.57) | NR | |
| Maritsi 2011135 UK (low) | NR | QFT-GIT | NR | NR |
| NR | TST NR mm | NR | NR | |
| Mutsvangwa 2010,136 Zimbabwe (high) | NR | T-SPOT.TB | NR | NR |
| NR | TST 10 mm (two step) | NR | NR | |
| Papay 2011,137 Austria (low) | 192 | QFT-GIT | NR | NR |
| 192 | TST 5 mm | NR | NR | |
| Ramos 2013,138 Spain (low) | 153 | QFT-GIT | NR | 5.10 (1.50 to 17.50) |
| 153 | TST 5 mm | NR | 2.40 (1.01 to 5.80) | |
| Seyhan 2010,139 Turkey (intermediate) | 100 | QFT-G | NR | NR |
| 100 | TST 10 mm | NR | 4.10 (1.30 to 13.90) | |
| Shen 2012,140 China (high) | 70 | T-SPOT.TB | NR | NR |
| 70 | TST 5 mm | NR | NR | |
| Souza 2014,153 Brazil (intermediate) | 299 | QFT-GIT | NR | NR |
| 300 | TST 5 mm | NR | NR | |
| Takeda 2011,141 Japan (low) | 71 | QFT-2G (QFT-G) | NR | NR |
| 43 | TST 10 mm | NR | NR | |
| Vassilopoulos 2011,142 Greece (low) | 157 | T-SPOT.TB | 0.75 (NR; p = 0.45) | 0.51 (NR; p = 0.17) |
| 157 | TST | 1.36 (NR; p = 0.39) | 1.43 (NR; p = 0.34) | |
| 157 | QFT-GIT | 1.14 (NR; p = 0.76) | 1.05 (NR; p = 0.90) | |
Between-test concordance, discordance and agreement
This section included 16 studies167–182 reviewed in CG11710 (see Appendix 5) and 32 more recent studies114–142,149,153,155 reviewed in this update (see Appendix 9). Overall, nine studies114,125,136,153,167,170–172,181 were conducted in people with HIV infection, three studies115,149,175 in people with haematological disorders, four studies120,124,130,131 in solid organ transplantation candidates, three studies116,128,132 in people who had undergone kidney transplantation, seven studies117,118,121,122,126,139,155 in people with ESRD/haemodialysis, one study140 in those with hepatitis C, one study141 in those with lupus erythematosus and 18 studies119,123,127,129,133–135,137,138,142,168,169,174,176,178–180,182 in patients with immune-mediated inflammatory diseases before antiTNF-α therapy (rheumatoid arthritis, rheumatic or inflammatory diseases). The remaining two studies looked at patients with chronic liver173 and mixed (HIV infection with liver transplantation)177 conditions.
The data on between-test concordance, discordance and agreement from the 32 more recent studies are presented in Table 17. Six116,126,133,135,140,141 of the 32 studies did not report these data (see Table 17). Overall, the per cent concordance and kappa ranges between QFT-GIT and TST according to each condition were as follows: HIV infection – concordance 75–96%, kappa 0.29–0.48; haematological disorders – concordance 70.6–80%, kappa 0.09–0.16; solid organ transplantation candidates – concordance 65–80%, kappa 0.19–0.57; post-kidney transplantation – concordance 80%, kappa 0.09–0.27; ESRD/haemodialysis – concordance 60–86.4%, kappa 0.21–0.49; and immune-mediated inflammatory diseases before antiTNF-α therapy – concordance 60–93%, kappa 0.08–0.56 (see Table 17).
| Study ID, country (burden) | Sample size, total or by subgroup, n | Type of IGRA vs. TST induration threshold | Concordance (95% CI) (%) | Discordance (95% CI) (%) | Agreement kappa (95% CI) |
|---|---|---|---|---|---|
| HIV infection | |||||
| Chkhartishvili 2013,125 Georgia (high) | 233 | QFT-GIT vs. TST 5 mm | 74.25 (68.27 to 79.44) | 25.75 (20.56 to 31.73) | 0.29 (0.16 to 0.42) |
| 217 | T-SPOT.TB vs. TST 5 mm | 75.12 (68.96 to 80.4) | 24.88 (19.6 to 31.04) | 0.22 (0.07 to 0.29) | |
| Elzi 2011,114 Switzerland (low) | 32 | T-SPOT.TB vs. TST 5 mm | 56.25 (39.33 to 71.83) | 43.75 (28.17 to 60.67) | 0.12 (–0.22 to 0.46) |
| Mutsvangwa 2010,136 Zimbabwe (high) | Total | T-SPOT.TB vs. TST 10 mm (two step) | NR | NR | NR |
| 55 TB index case contacts | T-SPOT.TB vs. TST 10 mm (two step) | 70.91 (57.86 to 81.23) | 29.09 (18.77 to 42.14) | 0.41 (0.16 to 0.66) | |
| 18 control index contacts | T-SPOT.TB vs. TST 10 mm (two step) | 72.22 (49.13 to 87.5) | 27.78 (12.5 to 50.87) | 0.28 (–0.13 to 0.70) | |
| Souza 2014,153 Brazil (intermediate) | 299 | QFT-GIT vs. TST 5 mm | 96.00 (93.12 to 97.69) | 4.01 (2.31 to 6.88) | 0.48 (0.37 to 0.59) |
| Haematopoietic stem cell transplantation candidates | |||||
| Lee 2014,149 South Korea (high) | 159 | QFT-GIT vs. TST 5 mm | 79.87 (72.97 to 85.37) | 20.13 (14.63 to 27.03) | 0.16 (0.01 to 0.31) |
| 159 | QFT-GIT vs. TST 10 mm | NR | NR | NR | |
| Moon 2013,115 South Korea (high) | 210 | QFT-GIT vs. TST 5 mm | 73.81 (67.47 to 79.29) | 26.19 (20.71 to 32.53) | 0.09 (–0.04 to 0.22) |
| 210 | QFT-GIT vs. TST 10 mm | 78.57 (72.53 to 83.58) | 21.43 (16.42 to 27.47) | 0.15 (0.02 to 0.27) | |
| 176 with BCG history | QFT-GIT vs. TST 5 mm | 74.43 (67.51 to 80.31) | 25.57 (19.69 to 32.49) | 0.13 (–0.02 to 0.27) | |
| 34 with no BCG history | QFT-GIT vs. TST 5 mm | 70.59 (53.83 to 83.17) | 29.41 (16.83 to 46.17) | –0.10 (–0.35 to 0.14) | |
| Solid organ transplantation candidates | |||||
| Ahmadinejad 2013,120 Iran (intermediate) | 159 | QFT-GIT vs. TST 10 mm | 79.87 (72.97 to 85.37) | 20.13 (14.63 to 27.03) | 0.32 (0.17 to 0.47) |
| Casas 2011,124 Spain (low) | 95 | QFT-GIT vs. TST 5 mm (two step) | 78.95 (69.71 to 85.94) | 36.36 (24.93 to 49.58) | 0.57 (0.37 to 0.77) |
| Kim 2010,130 South Korea (high) | 184 total | T-SPOT.TB vs. TST 10 mm | 71.2 (64.27 to 77.25) | 28.8 (22.75 to 35.73) | 0.23 (0.12 to 0.34) |
| 145 BCG vaccinated | T-SPOT.TB vs. TST 10 mm | 70.34 (62.46 to 77.18) | 29.66 (22.82 to 37.54) | 0.19 (0.06 to 0.31) | |
| Kim 2013,131 South Korea (high) | 119 | QFT-G vs. TST 10 mm | 65.49 (56.34 to 73.61) | 34.51 (26.39 to 43.66) | 0.26 (0.10 to 0.41) |
| Post-kidney transplantation | |||||
| Hadaya 2013,128 Switzerland (low) | 200 | QFT-GIT vs. TST 5 mm | NR | NR | 0.11 (p = 0.010) |
| 200 | T-SPOT.TB vs. TST 5 mm | NR | NR | 0.09 (p = 0.034) | |
| Kim 2011,116 South Korea (high) | NR | NR | NR | NR | NR |
| Kim 2013,132 South Korea (high) | 93 | QFT-G vs. TST 10 mm | 79.57 (70.28 to 86.51) | 20.43 (13.49 to 29.72) | 0.27 (0.07 to 0.46) |
| Haemodialysis – ESRD | |||||
| Al Jahdali 2013,121 Saudi Arabia (low) | 200 | QFT-GIT vs. TST 10 mm (two step) | 75.50 (69.10 to 80.94) | 24.50 (19.06 to 30.90) | 0.34 (0.22 to 0.45) |
| Anibarro 2012,117 Spain (low) | 52 | QFT-GIT vs. TST 5 mm | 71.15 (57.73 to 81.67) | 28.85 (18.33 to 42.27) | 0.21 (0.04 to 0.37) |
| 52 | QFT-GIT vs. TST 5 mm (two step) | 78.85 (65.97 to 87.76) | 21.15 (12.24 to 34.03) | 0.49 (0.22 to 0.74) | |
| Ates 2009,122 Turkey (intermediate) | 230 | QFT-GIT vs. TST 10 mm | 67.83 (61.54 to 73.53) | 32.17 (26.47 to 38.46) | 0.34 (0.21 to 0.47) |
| Chung 2010,126 South Korea (high) | 146 | QFT-G vs. TST 10 mm | NR | NR | NR |
| 146 | T-SPOT.TB vs. TST 10 mm | NR | NR | NR | |
| Lee 2009,118 Taiwan (high) | 32 | QFT-G vs. TST 10 mm (two step) | 60.00 (NR) | 40.00 (NR) | 0.25 (–0.06 to 0.56) |
| 32 | T-SPOT.TB vs. TST 10 mm (two step) | 65.60 (NR) | 34.40 (NR) | 0.32 (–0.01 to 0.65) | |
| Seyhan 2010,139 Turkey (intermediate) | 100 | QFT-GIT vs.TST 10 mm | 65.00 (55.25 to 73.64) | 35.00 (26.36 to 44.75) | 0.27 (0.07 to 0.46) |
| Sherkat 2014,155 Iran (intermediate) | 44 | T-SPOT.TB vs. TST 10 mm | 86.36 (73.29 to 93.6) | 13.64 (6.40 to 26.71) | 0.49 (0.20 to 0.78) |
| Immune-mediated inflammatory diseases before antiTNF-α therapy | |||||
| Casas 2011,123 Spain (low) | 202 | QFT-GIT vs.TST 5 mm | 84.16 (78.49 to 88.55) | 15.84 (11.45 to 21.51) | 0.56 (0.42 to 0.70) |
| Chang 2011,119 South Korea (high) | 100 | QFT-GIT vs. TST 10 mm | 67.0 (57.31 to 75.44) | 33.0 (24.56 to 42.69) | 0.26 (0.07 to 0.45) |
| 42 RA sample | QFT-GIT vs. TST 10 mm | 76.20 (61.47 to 86.52) | 23.80 (13.48 to 38.53) | 0.46 (0.21 to 0.72) | |
| 58 AS sample | QFT-GIT vs. TST 10 mm | 60.34 (47.49 to 71.91) | 39.66 (28.09 to 52.51) | 0.14 (–0.10 to 0.39) | |
| Costantino 2013,127 France (low) | 444 total | T-SPOT.TB vs. TST 5 mm | 62.84 (58.25 to 67.2) | 37.16 (32.8 to 41.75) | 0.16 (0.07 to 0.25) |
| NR BCG vaccinated | T-SPOT.TB vs. TST 5 mm | NR | NR | 0.15 (NR) | |
| NR BCG non-vaccinated | T-SPOT.TB vs. TST 5 mm | NR | NR | 0.22 (NR) | |
| Hsia 2012,129 USA (low) | 2282 total | QFT-GIT vs. TST 5 mm | NR | NR | 0.22 (0.15 to 0.27) |
| 781 BCG vaccinated | QFT-GIT vs. TST 5 mm | 82.84 (80.04 to 85.32) | 17.16 (14.68 to 19.96) | 0.20 (0.13 to 0.27) | |
| 1248 BCG non-vaccinated | QFT-GIT vs. TST 5 mm | 93.11 (91.57 to 94.39) | 6.89 (5.61 to 8.43) | 0.32 (0.26 to 0.37) | |
| Kleinert 2012,133 Germany (low) | 685 | QFT-G vs. TST 5 mm | NR | NR | NR |
| 844 | T-SPOT.TB vs. TST 5 mm | NR | NR | NR | |
| Laffitte 2009,134 Switzerland (low) | 50 | T-SPOT.TB vs. TST 5 mm | 72.00 (58.33 to 82.53) | 28.00 (17.47 to 41.67) | 0.36 (0.12 to 0.61) |
| Maritsi 2011,135 South Africa (high) | NR | QFT-G vs. TST NR mm | NR | NR | NR |
| Papay 2011,137 Austria (low) | 192 | QFT-GIT vs. TST 5 mm | 84.90 (79.15 to 89.27) | 15.10 (10.73 to 20.85) | 0.21 (0.07 to 0.34) |
| Ramos 2013,138 Spain (low) | 90 | QFT-GIT vs. TST 5 mm | 75.56 (65.75 to 83.27) | 24.44 (16.73 to 34.25) | 0.08 (–0.05 to 0.22) |
| Vassilopolous 2011,142 Greece (low) | 155 | QFT-GIT vs. TST 5 mm | 63.87 (56.06 to 71.01) | 36.13 (28.99 to 43.94) | 0.15 (0.01 to 0.29) |
| 155 | T-SPOT.TB vs. TST 5 mm | 71.0 (63.38 to 77.54) | 29.03 (22.46 to 36.62) | 0.34 (0.17 to 0.50) | |
| Hepatitis C | |||||
| Shen 2012,140 China (high) | 70 | T-SPOT.TB vs. TST 5 mm | NR | NR | NR |
| Lupus erythematosus | |||||
| Takeda 2011,141 Japan (low) | NR | QFT-GIT vs. TST 10 mm | NR | NR | NR |
Four studies115,127,129,130 reported between-test agreement parameters by BCG vaccination status, three127,129,130 of which showed a lower per cent concordance and kappa values for BCG-vaccinated participants than for non-vaccinated participants (see Table 17).
Indeterminate test results
This section included three studies170,171,181 reviewed in CG11710 (see Appendix 5) and 32 more recent studies (see previous section) (see Appendix 9). Of the recent studies, six121,126,133,134,136,155 did not report this outcome.
The proportions of indeterminate results according to each condition and type of IGRA test were follows: HIV infection – QFT-GIT 0.30–17.87%, T-SPOT. TB 32.80%;114,125,153,170,171,181 haematological disorders – QFT-GIT 6.00–13.93%;115,149 solid organ transplantation candidates – QFT-GIT 2.11–4.76%, T-SPOT. TB 11.96%;120,124,130,131 post-kidney transplantation – QFT-GIT 1.64–4.30%, T-SPOT. TB 11%;116,128,132 ESRD/haemodialysis – QFT-GIT 0–10.55%, T-SPOT. TB 0%;117,118,122,139 immune-mediated inflammatory diseases before antiTNF-α therapy – QFT-GIT 0–7.69%, T-SPOT. TB 0–15.63%;119,123,127,129,137,138,142 hepatitis C – T-SPOT. TB 0%;140 and lupus erythematosus – QFT-GIT 32.39%. 141
Summary of studies in immunocompromised patients
This section included 48 studies: 16 studies reviewed in CG11710 (see Appendix 5) and 32 more recent studies published from 2009 onwards (see Appendix 9). The studies were stratified and analysed according to the following subgroups: HIV infection, solid organ transplantation candidates, post-kidney transplantation, haemodialysis (ESRD), immune-mediated inflammatory diseases before antiTNF-α therapy, hepatitis C and lupus erythematosus. The majority of the more recent studies were rated as being at moderate/high ROB (incidence studies) or of moderate/low methodological quality (exposure studies).
Only two of eight studies reported sufficient data to calculate R-CIRs to compare the performance of IGRAs and the TST in predicting the incidence of active TB. The R-CIR estimates in both studies were non-significant with very wide CIs, thereby rendering their interpretation inconclusive. These studies were not combined because the TST was used with different thresholds and one study used a two-step TST.
Across the 32 newly identified studies there was wide variability and the absence of a clear pattern in the estimates of sensitivity and specificity. In general, for both the IGRAs and TST, specificity tended to be greater than sensitivity. Some or all of the observed variation was the result of zero count events (unstable estimates), underlying differences in study populations/conditions and settings, and variation in exposure definitions and measurement and TST thresholds. The heterogeneity persisted even after stratifying the estimates by type of IGRA (QFT-GIT, T-SPOT. TB) and TST threshold (5 mm, 10 mm). In light of the observed heterogeneity, no meta-analysis was undertaken.
The association between the screening test results and the risk of LTBI/exposure level was measured using the R-DOR (IGRA vs. TST) in individual studies and ranged from 0.07 to 8.45. The forest plot analysis of R-DORs included 21 studies and revealed a significant amount of heterogeneity across all subgroups of participants except for those undergoing haemodialysis, in whom the IGRA (QFT-GIT) was more strongly associated with exposure groups than the TST 10 mm (pooled R-DOR 2.53, 95% CI 1.48 to 4.34). Similarly, in participants with hepatitis C, the IGRA (T-SPOT. TB) outperformed the TST 5 mm in detecting LTBI (R-DOR 8.45, 95% CI 3.71 to 19.24). In people with HIV/AIDS, the TST 10 mm performed significantly better than QFT-GIT (pooled R-DOR 0.35, 95% CI 0.15 to 0.83). For the remaining subgroups (lupus erythematosus, solid organ transplantation candidates, kidney transplant recipients), the performance of QFT-GIT did not significantly differ from that of the TST (wide 95% CIs and inconclusive results). For most subgroups the within-subgroup heterogeneity by IGRA type (QFT-GIT, T-SPOT. TB) and TST threshold (5 mm, 10 mm, 15 mm) could not be examined because of sparse data.
Overall, there was no evidence indicating a differential effect of BCG vaccination status on IGRA and TST positivity in the 14 newly identified studies reporting the association between test positivity and BCG vaccination status. Only one study demonstrated a significantly increased OR for TST 10 mm positivity (OR 4.28, 95% CI 1.35 to 13.64) as opposed to the non-significant OR for IGRA (OR 1.89, 95% CI 0.75 to 4.73) in relation to BCG vaccination status.
Overall, the per cent concordance and kappa ranges between QFT-GIT and TST according to each condition were as follows: HIV – concordance 75–96%, kappa 0.29–0.48; haematological disorders – concordance 70.6–80%, kappa 0.09–0.16; solid organ transplantation candidates – concordance 65–80%, kappa 0.19–0.57; post-kidney transplantation – concordance 80%; kappa 0.09–0.27); ESRD/haemodialysis – concordance 60–86.4%, kappa 0.21–0.49; and immune-mediated inflammatory diseases before antiTNF-α therapy – concordance 60–93%, kappa 0.08–0.56. Three studies reported between-test agreement parameters by BCG vaccination status, which showed a lower per cent concordance and kappa values for BCG-vaccinated participants than for non-vaccinated participants.
Recent arrivals from countries with a high incidence of tuberculosis
Description of baseline characteristics
This section included 15 studies in total. 143–147,166,183–191 Our searches identified five studies143–147 in individuals who had recently arrived from mainly high TB incidence countries: two143,144 investigated the incidence of active TB following testing for LTBI (incidence studies) and three145–147 investigated levels of exposure in relationship to LTBI test outcomes (exposure studies). An additional 10 studies166,183–191 in recently arrived immigrants were identified in CG117. 10 Details of the additional studies included from CG11710 can be found in Appendix 5.
Incidence studies
Two studies143,144 investigated the agreement of a QFT test with the TST in individuals recently arrived from high TB incidence countries, one143 from Norway and the other144 from the Netherlands. Both studies used a prospective cohort design and were community based. Follow-up ranged from 23 to 32 months in the study by Harstad et al. 143 whereas Kik et al. 144 followed up participants for 24 months.
Harstad et al. 143 compared the QFT-GIT and TST with cut-off values of ≥ 6 mm and ≥ 15mm, whereas Kik et al. 144 compared the QFT-GIT, T-SPOT. TB and TST with cut-off values of ≥ 10 mm and ≥ 15 mm. Around 25%143 and 43%144 of patients in the studies were female. Kik et al. 144 included people who were aged 16–45 years and Harstad et al. 143 included people aged > 18 years. In the study by Kik et al. 144 approximately 8% of the study population originated from Europe/North America, 8% from South America, 36% from Asia, 29% from African countries other than sub-Saharan countries and 17% from sub-Saharan Africa, with 1.5% of participants being of unknown geographical origin. In this study the proportion of patients who had received a BCG vaccination was high at 81%. 144 In the study by Harstad et al. ,143 13% of participants were from Europe, 42% from Africa, a further 42% from Asia and 3% from other countries. BCG vaccination was not reported in this study. Table 18 provides further details on these studies.
| Study ID, country (burden) | Study aim, setting, design, follow-up duration and funding source | Method(s) of diagnosis of active TB | Inclusion/exclusion criteria | Type and positivity threshold(s) of tests compared | Characteristics of study participants at baseline | Numbers of recruited and excluded study participants | Comments |
|---|---|---|---|---|---|---|---|
| Harstad 2010,143 Norway (low) | Aim: to compare PPV and NPV between QFT-GIT and the TST in asylum seekers in Norway Setting: community based Design: prospective cohort study Follow-up: 23–32 months Funding source: Norwegian Health Association; regional Health Authorities |
NR | Inclusion criteria: asylum seekers aged ≥ 18 years Exclusion criteria: active TB |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA: NR; TST: induration of ≥ 6 mm and ≥ 15 mm |
Age range: 18–34 years n = 587, 35–49 years n = 201 and ≥ 50 years n = 35 Female, n (%): 206 (25.0) Race/ethnicity, n (%): NR Geographical origin, n (%): Europe 103 (12.5), Africa 347 (42.0), Asia 346 (42.0), other 27 (3.3) BCG vaccination, n (%): NR History of antiTB treatment, n (%): NR Total incidence of active TB, n/N (%): 9/823 (1.1) Chest radiography (yes/no): yes Clinical examination (yes/no): NR Morbidity, n (%): NA Comorbidity, n (%): NA |
Total number of recruited patients: NR; total number of excluded patients: NR | |
| Kik 2010,144 Netherlands (low) | Aim: to assess PPV and NPV, sensitivity and specificity for TB disease of QFT-GIT, T-SPOT.TB and TST in immigrant individuals in the Netherlands who were recently exposed to infectious pulmonary TB patients Setting: community based Design: prospective cohort study Follow-up: 24 months Funding source: unrestricted grants from the Netherlands Organisation for Health Research and Development |
Contacts diagnosed with TB ≥ 3 months after the diagnosis of the index patient were considered to be incident cases whereas TB cases diagnosed < 3 months after the diagnosis of the index patient were considered to be coprevalent and were excluded from the analysis. The diagnosis of TB disease was based on chest radiography, symptoms, smear and/or culture results | Inclusion criteria: close contacts (aged ≥ 16 years and born in a TB-endemic country) of sputum smear-positive pulmonary TB patients who tested positive on the TST (≥ 5 mm) Exclusion criteria: contacts with known conditions associated with an increased risk of progression to disease (including diabetes and HIV infection) and individuals who were given preventative treatment |
Type of tests: IGRA (QFT-GIT), IGRA (T-SPOT.TB), TST Cut-off values/thresholds: IGRA (QFT-GIT): two-tube format positive test was defined as ≥ 0.35 IU/ml; IGRA (T-SPOT.TB): according to the manufacturer; TST: induration of ≥ 10 mm and ≥ 15 mm |
Age range: 16–24 years n = 53 (15.6%), 25–34 years n = 80 (23.6%), 35–44 years n = 115 (33.9%) and ≥ 45 years n = 91 (26.8%) Female, n (%): 147 (43.4) Race/ethnicity, n (%): NR Geographical origin, n (%): Europe/North America 27 (8.0), South America 27 (8.0), Asia 123 (36.3), other Africa 98 (28.9), sub-Saharan Africa 59 (17.4), unknown 5 (1.5) BCG vaccination, n (%): 274 (80.8) History of antiTB treatment, n (%): 0 Total incidence of active TB, n/N (%): 9/339 (2.65) Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): NR |
Total number of recruited patients: 433; total number of excluded patients: 91 (furthermore, five contacts were excluded in the secondary analysis as their follow-up started 12 months before 1 August 2008) |
Exposure studies
Three studies145–147 compared an IGRA test with the TST test in recent arrivals from countries with a high incidence of TB, relating test outcome to previous level of exposure. All studies within this group were therefore classed as having either a retrospective cohort or a cross-sectional design. The tests compared were the QFT-GIT and TST (≥ 10 mm),145–147 with Lucas et al. 145 also testing the T-SPOT. TB. The studies were undertaken in community settings in Australia145 and Italy. 146,147 Lucas et al. 145 studied children with a mean age of 7.5 years from Africa (78%) and Asia (22%), with the exposed group having definite or suspected household TB contact and the unexposed group having no contact. BCG vaccination in this cohort was 69%. Participants in the Italian studies were young adults of whom 56% were female in the study by Orlando et al. 146 but only 4% were female in the study by Saracino et al. 147 Immigrants arrived from Latin America (50%), Eastern Europe (27%), Africa (16%) and Asia (7%) in the study by Orlando et al. 146 and from Africa (48%), Eastern Mediterranean countries (47%), Europe (3%) and South-East Asia (2%) in the study by Saracino et al. 147 Orlando et al. 146 reported an overall very low rate of BCG vaccination (6%), whereas the study by Saracino et al. 147 did not report BCG vaccination of participants. 147 Both studies defined exposure groups by geographical area of origin and the level of TB burden147 or TB prevalence146 in the country of origin. In addition, Orlando et al. 146 specified a third exposed group as contacts of TB cases and compared this with an unexposed group without TB contact. Table 19 provides further details on these studies.
| Study ID, country (burden) | Study aim, setting, design and funding source | Definition of construct validity (i.e. LTBI exposure-based proxy) | Inclusion/exclusion criteria | Type and positivity threshold(s) of tests compared | Characteristics of study participants at baseline | Numbers of recruited and excluded study participants | Comments |
|---|---|---|---|---|---|---|---|
| Lucas 2010,145 Australia (low) | Aim: to compare IGRAs and the TST for the diagnosis of LTBI in recently resettled refugee children Setting: community based Design: retrospective cohort/cross-sectional study Funding source: Oxford Immunotec |
Household TB contact: non-exposed: none; exposed 1: definite/suspected; exposed 2: NA | Inclusion criteria: children aged from 5 months to 16 years from refugee families attending the Migrant Health Unit Exclusion criteria: NR |
Type of tests: IGRA (T-SPOT.TB), IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (T-SPOT.TB): NR; IGRA (QFT-GIT): NR; TST: induration of ≥ 10 mm given that all children originated from high-prevalence countries or ≥ 15 mm if children were aged < 5 years and had received a BCG vaccination [5 mm was subtracted from these cut-off values for children at increased risk for TB infection (such as household contacts) and for those aged > 1 year] |
Mean (range) age: 7.5 (2.8–11.9) years Female, n (%): 260 (49.6) Race/ethnicity, n (%): NR Geographical origin, n (%): African 411 (78.4), Asian 113 (21.56) BCG vaccination, n (%): 361 (69.0) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NR Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): malaria 486 (92.7), hepatitis B 356 (68.0), hepatitis C 492 (94.0), schistosomiasis 431 (82.2) Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: 524; total number of excluded patients: NR | NA |
| Orlando 2010,146 Italy (low) | Aim: to compare the efficiency and efficacy of the TST and QFT-GIT for the detection of LTBI in recent immigrants from highly endemic countries Setting: community based (outpatient ward) Design: retrospective cohort/cross-sectional study Funding source: Provincia di Milano, Assessorato alle Politiche Sociali |
(1) Continent: non-exposed: Africa (reference group); exposed 1: Asia; exposed 2: East Europe; exposed 3: Latin America (2) TB prevalence in country of origin: non-exposed: < 50 per 100,000 population (reference group); exposed 1: 50–200 per 100,000 population; exposed 2: > 200 per 100,000 population (3) Contact with TB patient: non-exposed: no (reference group); exposed 1: yes |
Inclusion criteria: NR Exclusion criteria: active TB |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): positive if the IFN-γ value after stimulation with TB antigen minus the value in the nil control was ≥ 0.35 UI/ml; TST: induration of ≥ 10 mm |
Median (IQR) age: 35.3 (27.7–44.5) years Female, n (%): 630 (55.7) Race/ethnicity, n (%): NR Geographical origin, n (%): Latin America 562 (49.73), Eastern Europe 308 (27.26), Africa 181 (16.02), Asia 79 (6.99) BCG vaccination, n (%): 72 (6.37), unknown 46 (4.07) History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NA Chest radiography (yes/no): yes Clinical examination (yes/no): yes Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): treatment for LTBI was offered to 57 of the 79 eligible patients according to standard guidelines |
Total number of recruited patients: NR; total number of excluded patients: NR | NA |
| Saracino 2009,147 Italy (low) | Aim: to evaluate the agreement between the QFT-GIT and the TST for LTBI screening in a population of recent immigrants to Italy from high-incidence countries Setting: community based Design: retrospective cohort/cross-sectional study Funding source: NR |
(1) Born in a country with a TB burden (n cases per 100,000): non-exposed: NR; exposed 1: 30–100; exposed 2: 101–200; exposed 3: > 301 (2) Region of origin: non-exposed: NR; exposed 1: African; exposed 2: Eastern Mediterranean; exposed 3: European; exposed 4: South-East Asian |
Inclusion criteria: recent (< 2 months) immigrants to Italy Exclusion criteria: active TB, HIV infection |
Type of tests: IGRA (QFT-GIT), TST Cut-off values/thresholds: IGRA (QFT-GIT): positive if the IFN-γ level was above the cut-off test value (≥ 0.35 IU/ml); TST: induration of ≥ 10 mm after 72 hours (≥ 5 mm and ≥ 15 mm were used for comparison) |
Mean (SD) age: 27.1 (6.2) years Female, n (%): 11 (4) Race/ethnicity, n (%): NR Geographical origin, n (%): African 135 (48.4), Eastern Mediterranean 131 (46.95), European 7 (2.5), South-East Asian 6 (2.2) BCG vaccination, n (%): NR History of antiTB treatment, n (%): NR Total incidence of active TB, n (%): NA Chest radiography (yes/no): yes Clinical examination (yes/no): NR Morbidity, n (%): NR Comorbidity, n (%): NR Type of during-study treatment, n (%): NR |
Total number of recruited patients: NR; total number of excluded patients: NR | NA |
Study quality
Incidence of active tuberculosis
Only one144 of the studies provided an adequate description about study design, study participants, study attrition, statistical analysis and reporting; this study was judged to have a low ROB. The other study143 was judged as being at high ROB because of selection bias, confounding and selective reporting of results. Table 20 provides further details.
| Study ID (burden) | Study design | Study participation (risk of selection bias) | Study attrition (risk of selection bias) | Prognostic factor measurement (risk of exposure measurement bias) | Outcome/construct measurement (ROB in misclassification of individuals in relation to construct validity groups) | Study confounding (ROB from confounding) | Statistical analysis and reporting (ROB from analysis and selective reporting) | Total ROB (high, moderate, low) |
|---|---|---|---|---|---|---|---|---|
| Harstad 2010143 (low) | Low | High | Low | High | Moderate | High | High | High |
| Kik 2010144 (low) | Low | Low | Low | Low | Low | Low | Low | Low |
Exposure levels
All three145–147 of the exposure studies identified since the publication of CG11710 concerning recent arrivals from countries with a high incidence of TB were rated as being of low quality. There was a lack of blinding of test results from exposure, inadequate descriptions of exposure and inadequate reporting of sample attrition. Table 21 provides further details.
| Study ID (burden) | Recruitment of subjects [consecutive (yes), arbitrary or unreported (no)] | Blinding of test results from exposure [blinded (yes), not blinded or unreported (no)] | Description of index test and threshold [adequate (yes), inadequate or unreported (no)] | Definition and description of exposure [adequate (yes), inadequate or unreported (no)] | Sample attrition [adequate (yes),a inadequate or unreported (no)] | Overall quality score of satisfactory featuresb |
|---|---|---|---|---|---|---|
| Lucas 2010145 (low) | Yes | No | No | No | No | Low quality |
| Orlando 2010146 (low) | Yes | No | Yes | No | No | Low quality |
| Saracino 2009147 (low) | No | No | Yes | No | No | Low quality |
Comparative performance of tests (diagnostic accuracy indices for identifying latent tuberculosis infection)
Incidence of active tuberculosis
Ratios of cumulative incidence ratios
This section included two studies143,144 that followed up participants for the development of active TB. Both studies correlated IGRA (QFT-GIT;143 QFT-GIT and T-SPOT. TB144) and TST results with the cumulative incidence of active TB. The resulting CIRs for QFT-GIT were not significantly different from those for TST 5 mm (R-CIR 2.55, 95% CI 0.57 to 11.40)143 and TST 10 mm (R-CIR 0.87, 95% CI 0.17 to 4.56)144 (Table 22). Similarly, in the study by Kik et al. ,144 the R-CIR for T-SPOT. TB vs. TST 15 mm was not significant (R-CIR 0.37, 95% CI 0.10 to 1.41).
| Study ID, country (burden) | Test results | Test diagnostic accuracy in % (95% CI) | Development of active TB | |||
|---|---|---|---|---|---|---|
| Cumulative incidence (%), CIR, IDR, IDRR (95% CI) | R-CIR, R-IDRR (95% CI), IGRA vs. TST (by threshold) | |||||
| IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | |||
| Harstad 2010,143 Norway (low) | Number of test results: QFT-GIT 823; TST 823 Test (+/–): QFT-GIT 246/577; TST ≥ 6 mm 426/395; TST ≥ 15 mm 128/693 Number of indeterminate results: QFT-GIT NR; TST NR Number lost to follow-up: NR |
QFT-GIT/G: SN 88.89 (56.5 to 98.01); SP 71.46 (68.25 to 74.47); PPV 3.36 (1.71 to 6.49); NPV 99.83 (99.02 to 99.97) | TST ≥ 6 mm: SN 88.89 (56.5 to 98.01); SP 49.19 (45.74 to 52.65); PPV 1.92 (0.98 to 3.75); NPV 99.75 (98.58 to 99.96) TST ≥ 15 mm: SN 33.33 (12.06 to 64.58); SP 85.32 (82.71 to 87.60); PPV 2.48 (0.84 to 7.03); NPV 99.13 (98.12 to 99.6) |
QFT-GIT: CI (+) 3.36 (1.71 to 6.49); CI (–) 0.17 (0.00 to 1.08); CIR 19.39 (2.43 to 154.2); IDR (+) NR; IDR (–) NR; IDRR NR | TST ≥ 6 mm: CI (+) 1.92 (0.98 to 3.75); CI (–) 0.25 (0.00 to 1.57); CIR 7.61 (0.95 to 60.59); IDR (+) NR; IDR (–) NR; IDRR NR TST ≥ 15 mm: CI (+) 2.48 (0.84 to 7.03); CI (–) 0.86 (0.35 to 1.92); CIR: 2.86 (0.725 to 11.28); IDR (+) NR; IDR (–) NR; IDRR NR |
R-CIR (QFT-GIT vs. TST ≥ 6 mm) 2.55 (0.57 to 11.40); R-IDRR (QFT-GIT vs. TST ≥ 6 mm) NR R-CIR (QFT-GIT vs. TST ≥ 15 mm) 0.38 (0.11 to 1.34); (QFT-GIT vs. TST ≥ 15 mm) NR |
| Kik 2010,144 the Netherlands (low) | Number of test results: QFT-GIT 339; T-SPOT.TB 339; TST: 339 Test (+/–): QFT-GIT 178/149; T-SPOT.TB 181/118; TST ≥ 10 mm 288/51; TST ≥ 15 mm 184/138 Number of indeterminate results: QFT-GIT 12; T-SPOT.TB 40; TST ≥ 10 mm 0; TST ≥ 15 mm 0 Number lost to follow-up: NR |
QFT-GIT: SN 62.50 (30.57 to 86.32); SP 45.77 (40.38 to 51.25); PPV 2.80 (1.20 to 6.40); NPV 98.0 (94.20 to 99.31) T-SPOT.TB: SN 5.00 (40.93 to 92.85); SP 39.86 (34.4 to 45.58); PPV 3.31 (1.52 to 7.04); NPV 98.31 (94.03 to 99.53) |
TST ≥ 10 mm: SN 100.00 (70.08 to 100.00); SP 15.45 (11.95 to 19.75); PPV 3.12 (1.65 to 5.83); NPV 100.00 (93.00 to 100.00) TST ≥ 15 mm: SN 87.5 (52.91 to 97.76); SP 43.63 (38.25 to 49.16); PPV 3.80 (1.85 to 7.64); NPV 99.28 (96.01 to 99.87) |
QFT-GIT: CI (+) 2.80 (1.20 to 6.40); CI (–) 2.00 (0.42 to 6.02); CIR 1.39 (0.34 to 5.74); IDR (+) NR; IDR (–) NR; IDRR NR T-SPOT.TB: CI (+) 3.31 (1.52 to 7.04); CI (–) 1.69 (0.08 to 6.35); CIR 1.95 (0.40 to 9.52); IDR (+) NR; IDR (–) NR; IDRR NR |
TST ≥ 10 mm: CI (+) 3.12 (1.65 to 5.83); CI (–) 1.96 (0.05 to 10.4); CIR 1.59 (0.21 to 71.2); IDR (+) NR; IDR (–) NR; IDRR NR TST ≥ 15 mm: CI (+) 3.80 (1.85 to 7.64); CI (–) 0.72 (0.00 to 4.39); CIR 5.25 (0.65 to 42.17); IDR (+) NR; IDR (–) NR; IDRR NR |
R-CIR (QFT-GIT vs. TST ≥ 10 mm) 0.87 (0.17 to 4.56); R-IDRR (QFT-GIT vs. TST ≥ 10 mm) NR R-CIR (T-SPOT.TB vs. TST ≥ 15 mm) 0.37 (0.10 to 1.41); R-IDRR (T-SPOT.TB vs. TST ≥ 15 mm) NR |
The pooled estimate of the R-CIR across the two studies indicated no significant difference between QFT-GIT and TST (5 mm or 10 mm) (pooled R-CIR 1.57, 95% CI 0.52 to 4.76) (Figure 42).
FIGURE 42.
Pooled R-CIR of QFT-GIT vs. TST (5 mm or 10 mm) based on high- and low-risk exposure in recent arrivals from countries with a high incidence of TB. df, degrees of freedom; IV, inverse variance; SE, standard error.
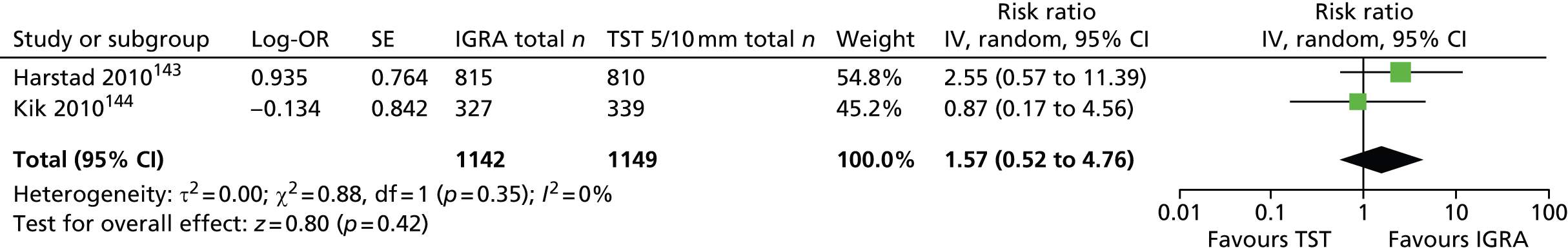
Sensitivity and specificity
This section included two newly identified studies. 143,144 There was homogeneity in the sensitivity of both QFT-GIT (pooled sensitivity 76%, 95% CI 50% to 93%; I2 = 40.7%) and TST 5 mm/10 mm (pooled sensitivity 94%, 95% CI 73% to 100%; I2 = 30.8%). In contrast, specificity estimates for QFT-GIT (71% and 46%; I2 = 98.4%) and TST (49% and 15%; I2 = 99.2%) were heterogeneous and these estimates could not be pooled (Figures 43–46). In summary, QFT-GIT demonstrated greater specificity values (range 46–71%) than TST (range 15–49%) but lower sensitivity (pooled estimate 76%) than TST (pooled estimate 94%). One study144 showed that TST 15 mm performed better than T-SPOT. TB in terms of both sensitivity (87% vs. 75%) and specificity (44% vs. 40%).
FIGURE 43.
Forest plot of sensitivity based on incidence of active TB (QFT-GIT) in recent arrivals from countries with a high incidence of TB. df, degrees of freedom.

FIGURE 44.
Forest plot of sensitivity based on incidence of active TB (TST) in recent arrivals from countries with a high incidence of TB. df, degrees of freedom.

FIGURE 45.
Forest plot of specificity based on incidence of active TB (QFT-GIT) in recent arrivals from countries with a high incidence of TB. df, degrees of freedom.
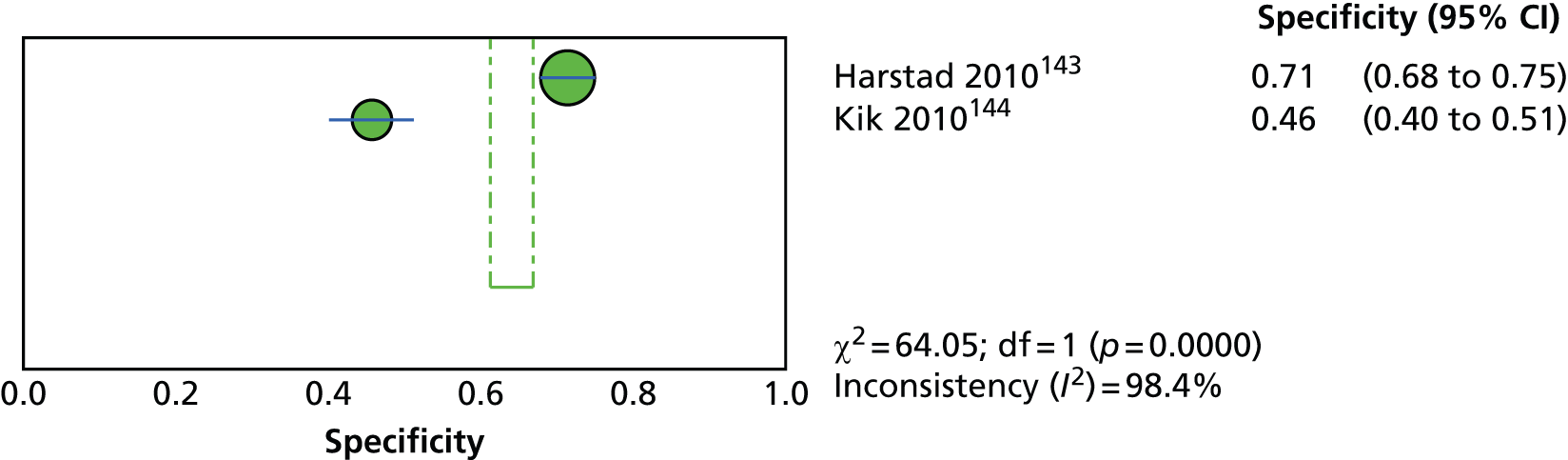
FIGURE 46.
Forest plot of specificity based on incidence of active TB (TST) in recent arrivals from countries with a high incidence of TB. df, degrees of freedom.
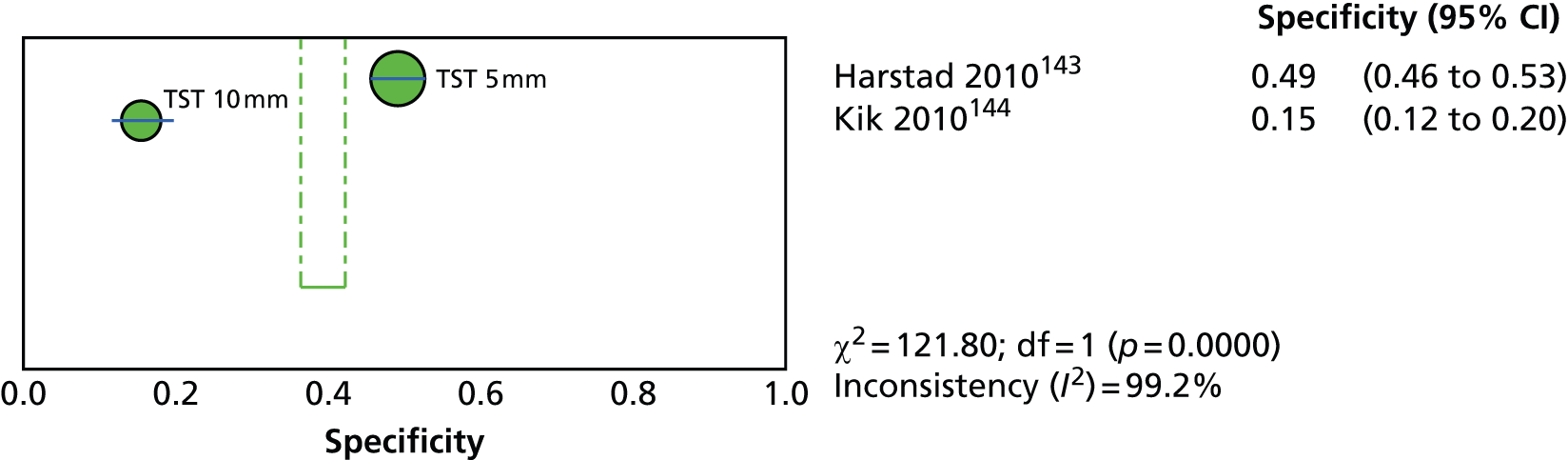
Exposure levels
Ratios of diagnostic odds ratios
Seven166,185,186,188–191 of the 10 studies reviewed in CG11710 (see Appendix 5) found significant strong associations between exposure and positive test results, presented as DORs for both IGRA and TST (5 mm, 10 mm, 15 mm) across exposure gradient groups defined as place of birth, racial group and country prevalence. The estimates of R-DORs comparing IGRA with TST across these studies ranged from 0.14191 to 0.98. 188 As CG11710 did not provide the 95% CIs around these estimates, it is not clear what the predictive performance of IGRA relative to TST is in terms of identifying LTBI. With regard to the studies identified in the present review, one study146 showed that IGRA compared with TST was more strongly correlated with the exposure groups of geographical origin (Latin America/East Europe vs. Africa; R-DOR 1.42) and TB prevalence (> 200/50–200 per 100,000 vs. < 50 per 100,000; R-DOR range 1.88–1.91), but this correlation across the two tests was similar for contact with TB case (R-DOR 1.13, 95% CI 0.85 to 1.49). In two other studies145,147 the comparisons of IGRA and TST in relation to exposure to TB (R-DOR 0.60, 95% CI 0.32 to 1.12) and birth in TB burden country (R-DOR 1.00, 95% CI 0.60 to 1.66) were not statistically significant (Table 23).
| Study ID, country (burden) | Test results | Test diagnostic accuracy (95% CI) (%) | Construct validity (i.e. LTBI exposure-based proxy) | |||
|---|---|---|---|---|---|---|
| DOR (95% CI) (vs. non-exposed; reference group) | R-DOR (95% CI), IGRA vs. TST (by threshold) | |||||
| IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | IGRA: QFT-GIT/G and/or T-SPOT.TB | TST (by threshold) | |||
| Lucas 2010,145 Australia (low) | Number of test results: QFT-GIT 460; T-SPOT.TB 420; TST 304 Test (+/–): QFT-GIT 45/345; T-SPOT.TB 38/374; TST ≥ 10 mm 54/250 Number of indeterminate results: QFT-GIT 70; T-SPOT.TB 8; TST 0 Number lost to follow-up: 37 |
QFT-GIT: High exposure level vs. low exposure level: SN NR; SP NR; PPV NR; NPV NR T-SPOT.TB High exposure level vs. low exposure level: SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 10 mm High exposure level vs. low exposure level: SN NR; SP NR; PPV NR; NPV NR T-SPOT.TB High exposure level vs. low exposure level: SN NR; SP NR; PPV NR; NPV NR |
QFT-GIT: High exposure level vs. low exposure level: DOR 2.40 (1.00 to 5.80); DORa NR T-SPOT.TB: High exposure level vs. low exposure level: DOR 2.50 (0.90 to 6.50); DORa NR |
TST ≥ 10 mm: High exposure level vs. low exposure level: DOR 4.00 (1.70 to 9.50); DORa NR T-SPOT.TB: High exposure level vs. low exposure level: DOR 4.00 (1.70 to 9.50); DORa NR |
QFT-GIT vs. TST ≥ 10 mm: High exposure level vs. low exposure level: Low: R-DOR 0.60 (0.32 to 1.12); R-DORa NR T-SPOT.TB vs. TST ≥ 10 mm: High exposure level vs. low exposure level: Low: R-DOR 0.63 (0.32 to 1.22); R-DORa NR |
| Orlando 2010,146 Italy (low) | Number of test results: QFT-GIT 1130; TST 1129 Test (+/–): QFT-GIT 337/778; TST ≥ 10 mm 407/492 Number of indeterminate results: QFT-GIT 15; TST 0 Number lost to follow-up: TST 230 (dropouts) |
QFT-GIT: Asian continent vs. African continent: SN NR; SP NR; PPV NR; NPV NR Latin America vs. Africa: SN NR; SP NR; PPV NR; NPV NR TB prevalence (number per 100,000): 50–200 vs. < 50: SN NR; SP NR; PPV NR; NPV NR TB prevalence (number per 100,000): ≥ 200 vs. < 50: SN NR; SP NR; PPV NR; NPV NR Contact with TB case vs. no contact: SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 10 mm: Asian continent vs. African continent: SN NR; SP NR; PPV NR; NPV NR Latin America vs. Africa: SN NR; SP NR; PPV NR; NPV NR TB prevalence (number per 100,000): 50–200 vs. < 50: SN NR; SP NR; PPV NR; NPV NR TB prevalence (number per 100,000): ≥ 200 vs. < 50: SN NR; SP NR; PPV NR; NPV NR Contact with TB case vs. no contact: SN NR; SP NR; PPV NR; NPV NR |
QFT-GIT: Asian continent vs. African continent: DOR 1.61 (0.90 to 2.88); DORa 1.07 (0.52 to 2.23) Latin America vs. Africa: DOR 1.46 (0.99 to 2.16); DORa 0.81 (0.46 to 1.42) TB prevalence (number per 100,000): 50–200 vs. < 50: DOR 1.76 (1.10 to 2.80); DORa 1.34 (0.72 to 2.49) TB prevalence (number per 100,000): ≥ 200 vs. < 50: DOR 2.31 (1.48 to 3.61); DORa 2.72 (1.70 to 5.02) Contact with TB case vs. no contact: DOR 2.54 (1.82 to 3.54); DORa 2.11 (1.47 to 3.03) |
TST ≥ 10 mm: Asian continent vs. African continent: DOR 0.91 (0.50 to 1.64); DORa 0.72 (0.34 to 1.53) Latin America vs. Africa: DOR 0.86 (0.59 to 1.26); DORa 0.57 (0.33 to 1.00) TB prevalence (number per 100,000): 50–200 vs. < 50: DOR 0.66 (0.44 to 1.01); DORa 0.70 (0.39 to 1.25) TB prevalence (number per 100,000): ≥ 200 vs. < 50: DOR 0.99 (0.66 to 1.48); DORa 1.45 (0.80 to 2.62) Contact with TB case vs. no contact: DOR 1.87 (1.30 to 2.69); DORa 1.87 (1.24 to 2.80) |
QFT-GIT vs. TST ≥ 10 mm: Asian continent vs. African continent: R-DOR 1.77 (1.16 to 2.70); R-DORa 1.49 (0.87 to 2.53) Latin America vs. Africa: R-DOR 1.70 (1.29 to 2.24); R-DORa 1.42 (0.95 to 2.24) TB prevalence (Number per 100,000): 50–200 vs. < 50: R-DOR 2.67 (1.94 to 3.67); R-DORa 1.91 (1.24 to 2.95) TB prevalence (Number per 100,000): ≥ 200 vs. < 50: R-DOR 2.33 (1.72 to 3.17); DORa 1.88 (1.25 to 2.83) Contact with TB case vs. no contact: R-DOR 1.36 (1.06 to 1.75); R-DORa 1.13 (0.85 to 1.49) |
| Saracino 2009,147 Australia (low) | Number of test results: QFT-GIT 452; TST 452 Test (+/–): QFT-GIT 107/172; TST ≥ 10 mm 72/207 Number of indeterminate results: QFT-GIT 173; TST 173 Number lost to follow-up: QFT-GIT 169; TST 169 |
QFT-GIT: Region of origin vs. region of origin: SN NR; SP NR; PPV NR; NPV NR |
TST ≥ 10 mm: Region of origin vs. region of origin: SN NR; SP NR; PPV NR; NPV NR |
QFT-GIT: Region of origin vs. region of origin: DOR NR; DORa NR |
TST ≥ 10 mm: Region of origin vs. region of origin: DOR NR; DORa NR |
QFT-GIT vs. TST ≥ 10 mm: Region of origin vs. region of origin: R-DOR NR; R-DORa NR |
Based on the meta-analysis of the three studies,145–147 the pooled R-DOR for the IGRA (QFT-GIT) compared with TST 10 mm (contact with TB case, exposure to TB, birth in TB burden country) (R-DOR 0.96, 95% CI 0.69 to 1.33) was not statistically significant, suggesting that there is no evidence that IGRA performs better than TST in identifying LTBI in this population (Figure 47).
FIGURE 47.
Pooled R-DOR for IGRA vs. TST 10 mm based on high- and low-risk exposure in recent arrivals from countries with a high incidence of TB. df, degrees of freedom; IV, inverse variance; SE, standard error.
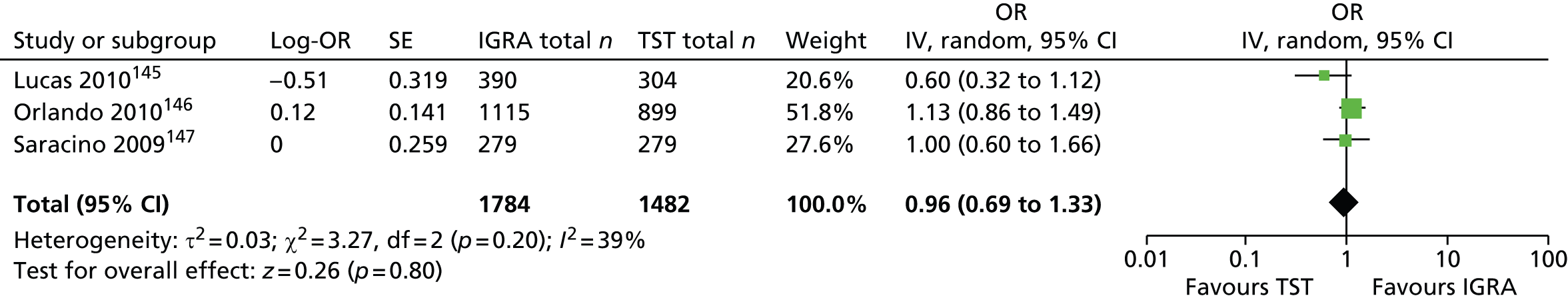
Sensitivity, specificity, positive predictive value and negative predictive value
None of the three studies reported these parameters and there was not sufficient information to derive 2 × 2 table cell counts to calculate sensitivity and specificity values.
Influence of bacillus Calmette–Guérin vaccination status on test positivity
Of the three newly identified studies,145–147 only one145 reported the association between test positivity and BCG vaccination status. Given the study results, there was no evidence indicating a differential effect of BCG vaccination status on IGRA (QFT, T-SPOT. TB) and TST positivity. Namely, the odds of test positivity for QFT-GIT (OR 1.70, 95% CI 0.80 to 3.60), T-SPOT. TB (OR 1.80, 95% CI 0.80 to 4.00) and TST (OR 1.70, 95% CI 0.80 to 3.50) were not significantly different between the BCG-vaccinated group and the non-vaccinated group (Table 24).
| Study ID, country (burden) | Sample size, n | Type of IGRA/TST induration threshold | Association between test positivity and BCG vaccination status: OR (95% CI) | |
|---|---|---|---|---|
| Crude/unadjusted | Adjusted | |||
| Lucas 2010,145 Australia (low) | 420 | QFT-GIT | 1.70 (0.80 to 3.60) | NR |
| 460 | T-SPOT.TB | 1.80 (0.80 to 4.00) | NR | |
| 304 | TST ≥ 10 mm | 1.70 (0.80 to 3.50) | NR | |
| Orlando 2010,146 Italy (low) | 1130 | QFT-GIT | NR | NR |
| 1129 | TST ≥ 10 mm | NR | NR | |
| Saracino 2009,147 Australia (low) | 452 | QFT-GIT | NR | NR |
| 452 | TST ≥ 10 mm | NR | NR | |
Between-test concordance, discordance and agreement
This relevant evidence was reported for nine CG11710 studies166,183–188,190,191 (see Appendix 5) and three newly identified studies145–147 (see Appendix 9). In overall samples, the per cent concordance between the IGRA and the TST 10 mm ranged from 63.6%188 to 84.2%. 190 The corresponding concordance between the IGRA and the TST 5 mm was similar and ranged from 60.7%188 to 90%. 191 The kappa values between the IGRA and the TST (regardless of TST threshold and BCG vaccination status) ranged from 0.08 to 0.68,188 with most values being < 0.45. Both concordance and kappa were greater among BCG-unvaccinated (or total sample) than among vaccinated-only groups146,166,183–186,188,190 (Table 25; see Appendix 5 for CG11710 studies).
| Study ID, country (burden) | Sample size, total or by subgroup, n | Type of IGRA vs. TST induration threshold | Concordance (95% CI) (%) | Discordance (95% CI) (%) | Agreement kappa (95% CI) |
|---|---|---|---|---|---|
| Lucas 2010,145 Australia (low) | NR | T-SPOT.TB vs. TST 10 mm | NR | NR | 0.45 (0.38 to 0.53) |
| NR | QFT-GIT vs. TST 10 mm | NR | NR | 0.46 (0.39 to 0.53) | |
| Orlando 2010,146 Italy (low) | 887 | QFT-GIT vs. TST 10 mm | 70.46 (67.32 to 73.43) | 29.53 (NR) | 0.38 (NR) |
| 56 BCG vaccinated | QFT-GIT vs. TST 10 mm | 66.07 (52.09 to 77.84) | 33.92 (NR) | 0.35 (NR) | |
| 789 unvaccinated | QFT-GIT vs. TST 10 mm | 71.36 (68.04 to 74.46) | 28.64 (NR) | 0.40 (NR) | |
| Saracino 2009,147 Australia (low) | 279 total | QFT-GIT vs. TST 10 mm | 70.97 (65.39 to 75.98) | 29.03 (24.02 to 34.61) | 0.35 (0.23 to 0.46) |
| Harstad 2010,143 Norway (low) | 823 | QFT-GIT vs. TST 10 mm | NR | NR | NR |
| 823 | QFT-GIT vs. TST 15 mm | NR | NR | NR | |
| Kik 2010,144 the Netherlands (low) | 433 | QFT-GIT vs. TST 10 mm | NR | NR | NR |
Summary of studies on recent arrivals from countries with a high incidence of tuberculosis
Two studies that correlated IGRA (QFT-GIT and T-SPOT. TB) and TST results with cumulative incidence of active TB showed no significant difference in CIRs for QFT-GIT compared with TST 5 mm (R-CIR 2.55, 95% CI 0.57 to 11.40) and QFT-GIT compared with TST 10 mm (R-CIR 0.87, 95% CI 0.17 to 4.56). The pooled estimate of R-CIRs across the two studies was not significant (pooled R-CIR 1.57, 95% CI 0.52 to 4.76). Based on two studies, the QFT-GIT demonstrated greater specificity values (range 46–71%) than the TST (range 15–49%) but lower sensitivity (pooled estimate 76%) than the TST (pooled estimate 94%). One study showed TST 15 mm to have performed better than T-SPOT. TB in terms of both sensitivity (87% vs. 75%) and specificity (44% vs. 40%).
Seven of the 10 studies reviewed in CG117 found significant strong associations presented as DORs for both the IGRA and the TST (5 mm, 10 mm, 15 mm) across exposure gradient groups defined as place of birth, racial group and country prevalence. However, the R-DORs comparing IGRA with TST across these studies ranged from 0.14 to 0.98. As CG11710 did not provide the 95% CIs, it is not clear what the predictive performance of IGRA relative to TST was in terms of identifying LTBI. Based on the meta-analysis of the three more recent studies, the pooled R-DOR for IGRA (QFT-GIT) compared with TST 10 mm (contact with TB case, exposure to TB, birth in TB burden country) was not statistically significant, suggesting that the IGRA does not perform better than the TST in identifying LTBI.
Given the results from one study, there was no evidence indicating a differential effect of BCG vaccination on IGRA (QFT-GIT, T.SPOT. TB) and TST positivity. The odds of test positivity for the QFT-GIT (OR 1.70, 95% CI 0.80 to 3.60), T.SPOT. TB (OR 1.80, 95% CI 0.80 to 4.00) and TST (OR 1.70, 95% CI 0.80 to 3.50) were not significantly different between the BCG-vaccinated group and the non-vaccinated group.
Based on nine CG11710 and three newly identified studies, the overall per cent concordance between the IGRA and the TST 10 mm ranged from 63.6% to 84.2%. The corresponding concordance between the IGRA and the TST 5 mm was similar (range 60.7–90%). Most kappa values between the IGRA and the TST (regardless of TST threshold and BCG vaccination status) were < 0.45. Both concordance and kappa were greater among BCG-unvaccinated groups.
Overall summary of results
We identified 53 studies published since the previous NICE clinical guidance work in 2011 (CG117). 10 ROB was assessed for 15 studies that evaluated the incidence of active TB and methodological quality was assessed for the remaining 38 studies, which correlated test results with previous TB exposure. Seven of the 15 incidence studies were identified as having a high ROB, six as having a moderate ROB and two as having a low ROB. All had important drawbacks with regard to design, methods and reporting. Of the 38 exposure studies, 29 were generally of lower quality, six were of moderate quality and three were of high quality.
Children and adolescents
Although the limited evidence in children and adolescents showed no significant difference in test accuracy between QFT-GIT and TST 5 mm (pooled R-CIR 1.12, 95% CI 0.72 to 1.75), QFT-GIT performed significantly better than TST 10 mm in predicting risk of active TB (pooled R-CIR 4.33, 95% CI 1.32 to 14.23). The IGRA (QFT-GIT/G) demonstrated a similar sensitivity to (range 48–100%) and a slightly better specificity (range 49–90%) than TST 5 mm (sensitivity range 57–100%; specificity range 45–65%). Although the sensitivities of IGRA and TST 5 mm were higher than those for TST 10 mm (range 30–56%), the corresponding specificities of these tests were lower than those for TST 10 mm (range 63–93%). Evidence from exposure studies suggested the superiority of IGRAs over TST in identifying LTBI in the low TB burden setting (pooled R-DOR 4.74, 95% CI to 2.15 to 10.44) compared with high TB burden settings (pooled R-DOR 1.13, 95% CI to 0.78 to 1.65).
Immunocompromised people
In terms of LTBI diagnosis, IGRAs (QFT-GIT or T.SPOT. TB) performed better than TST 5 mm/10 mm in people receiving haemodialysis (pooled R-DOR 2.53, 95% CI 1.48 to 4.34) and people with hepatitis C (R-DOR 8.45, 95% CI 3.71 to 19.24). In contrast, for patients with HIV/AIDS, TST 10 mm performed significantly better than QFT-GIT (pooled R-DOR 0.35, 95% CI 0.15 to 0.83). The comparative evidence on the performance of the IGRAs and TST for the remaining subgroups (e.g. those with lupus erythematosus, solid organ transplantation candidates, kidney transplant recipients) was inconclusive because of the high level of uncertainty around the effect estimates.
Recent arrivals from countries with a high incidence of tuberculosis
Overall, based on studies of incidence, there was no significant difference between the performance of QFT-GIT and TST 5 mm/10 mm in identifying LTBI among newly arrived people from high TB burden countries (pooled R-CIR 1.57, 95% CI 0.52 to 4.76). Similarly, there was no significant difference between T.SPOT. TB and TST 10 mm in predicting LTBI (R-CIR 0.37, 95% CI 0.10 to 1.41). Likewise, the pooled result showed no significant difference between QFT-GIT and TST 10 mm for the association with previous TB exposure (pooled R-DOR 0.96, 95% CI 0.69 to 1.33).
The studies identified in this review were highly heterogeneous in terms of types of tests for LTBI, TST cut-off levels, study settings and definitions of constructs for previous TB exposure for defining LTBI. Previous exposure to TB was highly variable and ill-defined, lacking a description of duration and proximity of contact to index TB cases. Overall, although the number of studies identified was substantial, extensive heterogeneity across many potential test performance modifier factors (e.g. study methodology, test administration, study populations and exposure-based construct definitions) precluded a more meaningful subgroup analysis because of the scarcity of evidence for each subgroup.
Chapter 5 Systematic review of economic evaluation studies
Identification and selection of studies
Search methods for cost-effectiveness
A comprehensive search of the health-care literature for published economic evaluations, cost studies and utility studies was performed. The purpose of this search was to identify existing cost-effectiveness models and model designs, and also to identify studies that reported costs and health-related quality-of-life data for use in generating cost per quality-adjusted life-years (QALYs).
The main cost-effectiveness search was developed and conducted as part of the wider systematic review that aimed to compare both the clinical effectiveness and the cost-effectiveness of screening tests (IGRAs and TST) for LTBI in high-risk groups: children, immunocompromised people or those at risk from immunosuppression, and people recently arriving from countries with a high incidence of active TB. The bibliographic database search strategies for the main cost-effectiveness search were the same as those used for the clinical effectiveness review and focused on the diagnosis of LTBI using IGRAs compared with other methods. Searches were limited to articles in English and articles that had been added to the databases since the health economics searches for the equivalent questions in CG11710 were carried out (5–6 January 2010; see Appendix 1). These searches automatically picked up comparisons between IGRAs and TSTs and therefore it was not necessary to search independently for comparator technologies (e.g. TSTs). The searches were not restricted by study type and therefore an economics search filter was not required. The search strategies are provided in Appendix 2. Details of the databases and other sources searched are provided in Chapter 3 (see Identification and selection of studies). Additional databases searched for cost-effectiveness studies were:
-
Research Papers in Economics
-
Cost-Effectiveness Analysis Registry
-
Health Economic Evaluations Database (Wiley).
A separate search in MEDLINE was performed to identify existing cost-effectiveness model designs for LTBI. The search strategy is available in Appendix 2.
Inclusion and exclusion of relevant studies
To be included in the review, the following inclusion criteria were applied:
-
Population:
-
Children (both sexes, aged < 18 years, immunocompetent) (research question 1).
-
People (both sexes, any age) who are immunocompromised or at risk from immunosuppression (e.g. transplant recipients or those with HIV infection, renal disease, diabetes, liver disease, haematological disease, cancer or autoimmune disease or who are on or about to start antiTNF-α treatment, steroids or ciclosporins) (research question 2).
-
People (both sexes, any age, immunocompetent) who have recently arrived from regions with a high incidence/prevalence of TB (countries/territories with an estimated incidence rate of ≥ 40 cases per 100,000, e.g. those in Africa, Central/South America, Eastern Europe and Asia) (research question 3).
-
-
Intervention: IGRAs (QFT-G, QFT-GIT and T-SPOT. TB)
-
Comparator: TST (Mantoux method)
-
Outcome measures:
-
The main outcome measure was the cost per QALY.
-
Other outcomes such as correct diagnosis of LTBI and cost per active TB case prevented were also considered.
-
-
Study design: studies including a formal economic evaluation involving direct comparison between IGRAs (QFT-G, QFT-GIT or T-SPOT. TB) and the TST and including a decision-analytic model in identifying people with LTBI
-
Type and language of publication:
-
Full-text reports published in English.
-
Abstracts (only if companion publications to full-text included studies).
-
Two reviewers (PA and AT) reviewed the titles and abstracts of the citations retrieved from the initial database searches. Full texts of potentially relevant articles were read and those that were considered model-based economic evaluations were reviewed.
Data extraction
Data extraction was conducted by one reviewer (PA) and further cross-checked by a second reviewer (AT). Any disagreements were resolved by discussion or by recourse to a third-party reviewer. Data were extracted on study details (title, author and year of study), baseline characteristics (population, intervention, comparator and outcomes), methods (study perspective, time horizon, discount rate, measure of effectiveness, current assumptions and analytical methods), results (study parameters, base-case and sensitivity analysis results), discussion (study findings, limitations of the models and generalisability) and ‘other’ (source of funding and conflicts of interests). The completed data extraction sheets are presented in Appendix 11.
Quality assessment
The economic evaluations were appraised against a framework for best practice for reporting economic evaluation studies developed by the Consolidated Health Economic Reporting Standards (CHEERS) task force. 194 The CHEERS assessment tool consists of six dimensions: title and abstract, introduction, methods, results, discussion and other. Under these dimensions, a series of questions check whether or not the criteria have been clearly reported (see Appendix 12). Additionally, the models were critically appraised against a framework for best practice for reporting decision-analytical models developed by Phillips et al. 195 The Phillips et al. 195 quality assessment tool includes two main dimensions: structure of the model and data used to parameterise the model. Under these dimensions several questions assess whether or not the criteria have been clearly reported (see Appendix 13).
Study quality was assessed by one reviewer (PA) and cross-checked by a second reviewer (AT). Any disagreements were resolved by discussion or by recourse to a third-party reviewer.
Data synthesis
Information extracted from the included studies was summarised and tabulated. The findings from individual studies are compared narratively and recommendations for the future modelling of LTBI are discussed.
Results
The electronic database searches and searches of other sources identified 5959 records (Figure 48). After removing duplicates, 3057 records were screened for inclusion. On the basis of title and abstract, 3032 records were excluded and the remaining 25 records were included for full-text screening. A further 15 articles were excluded at the full-text stage, with the reasons for exclusion shown in Figure 48 (see Appendix 14 for a list of excluded studies), leaving 10 studies10,77,196–203 that included a decision-analytical model to estimate the cost-effectiveness of IGRAs compared with the TST in diagnosing people who are at high risk of LTBI.
FIGURE 48.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses study flow diagram.

Summary of the general approaches to modelling latent tuberculosis infection
The general modelling approaches used for the diagnosis of LTBI are presented in the following sections by population of interest and in Table 26.
| Study ID, country | Aim of the study | Study characteristics (study design, perspective, setting) | Intervention | Outcome measure(s) | Model type | Health states | Results (base-case and sensitivity analysis) |
|---|---|---|---|---|---|---|---|
| Children | |||||||
| Kowada 2012,197 Japan | To assess the cost-effectiveness of school-based TB screening using QFT-GIT vs. TST and CXR | Cost-effectiveness analysis, societal perspective, setting not reported | QFT-GIT | Cost per QALY | Decision tree structure to model the short-term events followed by a Markov modelling structure | Healthy, LTBI, TB and dead | QFT-GIT was less costly and more effective than TST |
| Mandalakas 2013,203 South Africa | To estimate the health and economic outcomes of five TB screening strategies | Cost-effectiveness analysis, third-party payer and societal perspectives | IGRA (QFT, T-SPOT.TB) | Cost per LYS | Decision tree structure to model the short-term events followed by a Markov modelling structure | LTBI health state and could progress to no infection, initial infection, subsequent infection from future exposures, pulmonary TB, disseminated TB, TB death and death from other causes | In the 0–2 years cohort, the no testing strategy dominated other strategies. In the 3–5 years cohort, the TST negative followed by IGRA was the most effective with a reported ICER of approximately US$233,000 per LYS vs. no testing |
| Immunocompromised people | |||||||
| Kowada 2010,196 Japan | To assess the cost-effectiveness of QFT-GIT vs. TST for TB screening of RA patients prior to initiation of TNF-α antagonist therapy | Cost-effectiveness analysis, societal perspective, setting not reported | QFT-GIT | Cost per QALY | Decision tree model with Markov nodes | No LTBI, LTBI, TB and death | QFT-GIT was less costly and more effective than TST. At society’s WTP per QALY, the QFT-GIT testing strategy had a 100% probability of being cost-effective compared with the TST strategy |
| Kowada 2013,198 Japan | To assess the cost-effectiveness of QFT-GIT vs. TST and CXR for TB screening of haemodialysis patients | Cost-effectiveness analysis, societal perspective, setting not reported | QFT-GIT | Cost per QALY | Decision tree model with Markov nodes | Maintenance dialysis with no disorder, maintenance dialysis with LTBI, maintenance dialysis with TB and death | QFT-GIT was dominant compared with the TST testing strategy. Results from the sensitivity analysis showed that the base-case results were sensitive to the BCG vaccination rate. At all WTP thresholds, the QFT-GIT testing strategy had a 100% probability of being cost-effective compared with the TST testing strategy |
| Kowada 2014,199 Japan | To assess the cost-effectiveness for TB screening of high-risk HIV-positive pregnant women of using IGRAs vs. TST in low-incidence countries | Cost-effectiveness analysis, health service perspective, low incidence of TB country but setting not reported | (1) TST alone, (2) QFT alone, (3) T-SPOT.TB, (4) TST followed by QFT and (5) TST followed by T-SPOT.TB | Cost per QALY | Decision tree model with Markov nodes | Non-LTBI and non-TB, LTBI, non-MDR TB, MDR TB and death | Base-case results showed that T-SPOT.TB is less costly and more effective than other strategies. Sensitivity analysis showed that cost-effectiveness was sensitive to the sensitivity of T-SPOT.TB, the sensitivity of QFT, the specificity of T-SPOT.TB and the specificity of QFT in close contacts |
| Laskin 2013,200 USA | To determine the most cost-effective LTBI screening strategy before long-term steroid therapy in a child with new-onset idiopathic nephrotic syndrome | Cost-effectiveness analysis, societal perspective, setting not reported | IGRAs | Cost per QALY | Decision tree structure to model the short-term events followed by a Markov modelling structure | Well, LTBI, TB, nephrotic relapse and dead for the longer-term events | Base-case results showed that IGRA was less costly and produced moderately more QALYs than universal TST |
| Linas 2011,201 USA | To estimate the cost-effectiveness of LTBI screening using the TST and IGRAs | Cost-effectiveness analysis, health service, setting not reported | IGRAs and TST | Number needed to screen to prevent one case of active TB, life expectancy, quality-adjusted life expectancy | Markov model | LTBI with INH, LTBI no INH, isoniazid-related hepatitis, < 6 months’ INH, 6–8 months’ INH, 9 months’ INH, active TB, post active TB and death | Base-case results showed that, in people who are taking immunosuppressive medication, neither TST nor IGRA screening was cost-effective compared with the no screening strategy. Similar results were reported for people with ESRD |
| Swaminath 2013,202 USA | To compare the performance of TST and QFT-G screening of LTBI among immunosuppressed IBD patients based on prevalence, mortality risk from reactivation TB and costs | Cost-effectiveness, health-care payer, setting not reported | QFT-G | Cost per false-negative case of LTBI avoided, cost per TB death avoided, cost per reactivation TB avoided (this can be derived from the information provided) | Decision tree model | True positive, true negative, false positive, false negative, hepatitis, survive/death hepatitis | Base-case results showed that QFT-G dominated the TST strategy. Additionally, the use of QFT-G would avoid 30 false-negative cases, 4.92 TB reactivations and 1.4 deaths compared with the TST strategy |
| Recent arrivals from countries with a high incidence of TB | |||||||
| CG117 2011,10 UK | To compare the costs and effects of four strategies of testing for people suspected of having LTBI in England and Wales | Cost-effectiveness analysis, NHS and Personal Social Services | (1) TST, (2) IGRA, (3) TST followed by IGRA for people with positive TST and (4) no test (to inform and advise only) | Cost per QALY | Decision tree model | Test results, treatment for LTBI, treatment for TB | Results showed that TST positive followed by IGRA and IGRA testing strategies were associated with ICERs of < £30,000 per QALY compared with no testing. The results from the sensitivity analyses showed that varying the cost of an IGRA (from £50 to £60) changed the direction of the cost-effectiveness results |
| Pareek 2013,77 UK | To assess the cost-effectiveness of LTBI screening using different screening modalities at different incidence thresholds in a primary care setting, with and without CXR screening on arrival at port of entry | Cost-effectiveness analysis, NHS, primary care setting | (1) T-SPOT.TB alone, (2) QFT-GIT alone, (3) TST plus confirmatory T-SPOT.TB (if TST positive), and (4) TST plus confirmatory QFT-GIT (if TST positive) | Cost per case of active TB avoided | Decision tree model | The illustrative modelling structure was presented in a supplementary web appendix but unfortunately, these structures were illegible | Results showed that screening of recently arrived immigrants from countries of origin with a moderate (not defined) TB incidence is likely to be cost-effective for the use of one-step IGRA testing compared with other screening strategies |
Children
Kowada197
Kowada197 estimated the cost-effectiveness of QFT-GIT compared with the TST and chest radiography for the diagnosis of LTBI in children. The author developed a decision tree structure with Markov nodes to demonstrate the clinical pathway that children would undergo for the diagnosis and treatment of LTBI. The model started with a hypothetical cohort of children receiving one of three diagnostic strategies (QFT-GIT alone, TST alone or chest radiography). The model structure continued with children being in the LTBI/initial active TB or no LTBI health state, characterised by the prevalence of the disease. On positive test results, children received chest radiography to confirm initial active TB. Children who received a negative result on chest radiography were treated for LTBI. Children who adhered to LTBI treatment could develop isoniazid-induced hepatotoxicity. For the state transition model, children entered the model at the no LTBI health state and could remain or progress over time to LTBI, TB or death. Data required to populate the model were obtained from published sources. Estimates of sensitivity and specificity of tests in this population were obtained from a meta-analysis of developed-country studies. Cost data from published sources were adjusted to 2009 Japanese yen and converted to US dollars. The analysis was conducted from the societal perspective and the base-case results were expressed as an incremental cost-effectiveness ratio (ICER) based on the outcome of cost per QALY gained. Kowada197 conducted one- and two-way sensitivity analyses and populated with data to run the model probabilistically to represent the uncertainty in key model input parameters. The base-case results demonstrated that the QFT-GIT-alone strategy was less costly and more effective than the TST-alone strategy.
Mandalakas et al.203
Mandalakas et al. 203 used a decision tree structure with Markov nodes to estimate the health and economic outcomes of five screening strategies for the diagnosis of MTB infection in young household contacts with an index case. The model started with a cohort of children aged < 5 years who received one of five diagnostic strategies (no test, TST alone, IGRA alone, TST positive followed by IGRA and TST negative followed by IGRA) and continued with children being in the LTBI/initial active TB or no LTBI/no initial TB health state, characterised by the prevalence of the disease. Children with positive test results were eligible for treatment for LTBI and could either accept or refuse treatment. For the Markov model, children entered the model at the LTBI health state and could progress to no infection, initial infection, subsequent infection from future exposures, pulmonary TB, disseminated TB, TB death and death from other causes. The analysis was conducted from the third-party payer and societal perspectives, and the base-case results were reported in terms of an ICER based on the outcome of cost per life-year saved. Base-case results indicate that for those aged 0–2 years the no testing strategy was the dominant strategy whereas for those aged 3–5 years an IGRA following a negative TST was the most effective strategy but was not cost-effective compared with no testing. The authors conducted one-way sensitivity analyses to determine the impact of data uncertainties on the results.
Immunocompromised people
Kowada196
Kowada196 used a decision tree structure with Markov nodes to assess the cost-effectiveness of using QFT-GIT alone compared with TST alone to diagnose LTBI in patients with rheumatoid arthritis. The model simulated a pathway for a hypothetical cohort of people with rheumatoid arthritis being screened for LTBI and cost-effectiveness was estimated over a lifetime horizon. The model started with a cohort of people aged 40 years who received either diagnostic strategy and continued with people being in the LTBI/initial active TB or no LTBI/no initial TB health state, characterised by the prevalence of the disease. People with positive or negative results on the TST or positive QFT-GIT results received chest radiography to detect active TB. If active TB was detected they received treatment for active TB, whereas if active TB was not detected they received treatment for LTBI. Here, the author assumed that chest radiography to diagnose initial active TB was 100% sensitive and specific. People who adhered to LTBI treatment were at risk of developing isoniazid-induced hepatotoxicity. Kowada196 presented an illustrative Markov structure to depict the transitions that could occur between health states. From the structure, people could enter the model from the no LTBI, LTBI or TB health state.
The information required to populate the model was obtained from published sources. However, the author did not comment on/discuss the sources of prevalence of LTBI in this population. Information on the sensitivity and specificity of the tests was obtained from secondary sources and a meta-analysis. All costs included in the model were reported in 2009 Japanese yen and converted to US dollars using the same price year. The primary outcome measure of effectiveness was QALYs gained over a lifetime horizon; however, the author did not elaborate on the descriptive tools used to value these health states. All costs and benefits were discounted at 3% per annum. The analysis was conducted from the societal perspective and results were presented in terms of an ICER expressed as cost per QALYs gained. Kowada196 conducted one-way and two-way sensitivity analyses by changing key model input parameters to determine the impact on the deterministic results. Additionally, a probabilistic sensitivity analysis (PSA) was undertaken, but the distributions and the cost-effectiveness acceptability curve (CEAC) were not presented. The author demonstrated that QFT-GIT alone was the most cost-effective strategy for the diagnosis of LTBI in people undergoing haemodialysis. The results from the sensitivity analyses showed that the base-case results were robust to changes in model input parameters. Results from the probabilistic analysis showed that IGRA was the preferred option, with a 100% probability of being cost-effective compared with TST at society’s willingness to pay of US$50,000 per QALY.
Kowada198
In this study Kowada198 used a decision tree structure with Markov nodes to assess the costs and effects of using QFT-GIT alone, TST alone and chest radiography alone to diagnose LTBI in patients undergoing haemodialysis. The model simulated a pathway for a hypothetical cohort of people with haemodialysis being screened and cost-effectiveness was estimated over a lifetime horizon. The model started with a cohort of people who received one of the three diagnostic tests. People with positive results on the TST or QFT-GIT received chest radiography to detect active TB. If active TB was detected they received treatment for active TB, whereas if active TB was not detected they received treatment for LTBI. The author assumed that chest radiography to diagnose initial active TB was 100% sensitive and specific. People who adhered to LTBI treatment were at risk of developing isoniazid-induced hepatitis. Kowada198 did not present the illustrative Markov structure but described the clinical health states; however, no further comment was made on how people progressed through these health states. The information required to populate the model was obtained from published sources. The author conducted a review of the literature but did not state whether or not the accuracy of the tests was derived from a meta-analysis. The primary outcome measure of effectiveness was QALYs gained; however, the author did not elaborate on the descriptive tools used to value these health states. The analysis was conducted from the societal perspective and the results were presented in terms of an ICER expressed as cost per QALYs gained. Kowada198 conducted one-way and two-way sensitivity analyses by changing key model input parameters to determine the impact on the deterministic results. Additionally, PSA was undertaken but the distributions and the CEAC were not presented. The author demonstrated that QFT-GIT alone was the most cost-effective strategy for the diagnosis of LTBI in haemodialysis patients.
Kowada 2014199
Kowada used a decision tree structure with Markov nodes to estimate the cost-effectiveness of IGRAs compared with TST for TB screening in high-risk HIV-positive pregnant women in countries with a low incidence (< 24 cases per 100,000) of TB. The model simulated the pathway for four cohorts (BCG vaccinated during pregnancy, non-BCG vaccinated during pregnancy, BCG vaccinated in the post-partum period and non-BCG vaccinated in the post-partum period) separately and cost-effectiveness was estimated over a 30-year time horizon. The starting point of the model was a hypothetical cohort of women aged 20 years who received one of five (TST alone, QFT-G alone, T-SPOT. TB alone, TST positive followed by QFT or TST positive followed by T-SPOT. TB) testing strategies. A result was considered positive on TST if the induration was ≥ 5 mm and ≥ 10 mm in those who were non-BCG vaccinated and BCG vaccinated respectively. Women who had positive results on the TST-, QFT-G- or T-SPOT. TB-alone strategies received chest radiography to diagnose initial active TB. On the combination strategies, women who received a positive result on TST further received QFT-G or T-SPOT. TB and, if the result was positive, received chest radiography to detect initial active TB and received treatment for LTBI/TB. Women who adhered to LTBI treatment were at risk of developing isoniazid-induced hepatotoxicity and were treated accordingly. In the Markov structure, the author considered five health states (non-LTBI and non-TB, LTBI, non-multidrug-resistant TB, multidrug-resistant TB and dead) that women could enter based on proportions from the decision tree and showed the transitions between these health states.
Data required to populate the models were obtained from published sources. The analysis was conducted from the public health payer perspective and results were presented in terms of ICERs expressed as cost per QALYs gained. All costs included in the model were reported in 2012 Japanese yen and converted to US dollars using the same price year. The primary outcome measure of effectiveness was QALYs gained over a 30-year time horizon. All costs and benefits were discounted at 3% per annum. Kowada199 conducted PSA and one- and two-way sensitivity analyses by changing key model input parameters to determine the impact on the base-case results. The base-case results showed that the TST positive followed by QFT-G strategy was the most cost-effective strategy for the diagnosis of LTBI in occasional screening of HIV-positive pregnant women who were non-BCG vaccinated during pregnancy. Similar results were demonstrated in the other hypothetical cohorts. Results from the PSA showed that the TST followed by QFT-G strategy was the preferred option, with a 100% probability of being cost-effective at all of society’s willingness-to-pay levels per QALY. The results from the sensitivity analyses showed that the base-case results were sensitive to changes in the sensitivity of T-SPOT. TB and the sensitivity of QFT-G in occasional screening of non-BCG vaccinated pregnant women.
Laskin et al.200
Laskin et al. 200 used a decision tree structure with Markov nodes to determine the most cost-effective screening strategy for children with new-onset idiopathic nephrotic syndrome. The decision tree component of the model represented the pathway that children would undertake in a 6-month time period before they entered into the Markov model. Here, the longer-term events were simulated over a lifetime horizon with 3-month cycle lengths. The starting point of the model was a hypothetical cohort with new onset nephrotic syndrome. Children who received a positive test result were treated for LTBI and were at risk of developing hepatitis. The starting points of the Markov model were derived from the proportions of children with negative TST/IGRA results, children in whom LTBI treatment was successful and those in whom LTBI treatment had failed. The authors assumed that effective LTBI treatment provided long-term protection against LTBI/TB. Data required to populate the model were obtained from published sources. The analyses were conducted from the societal perspective applying an annual discount rate of 3% on costs and benefits. Indirect costs incurred in the analysis included travel time and loss of productivity. Base-case results showed that the no-screen strategy was least costly and more effective than other strategies. However, the results from this study should be interpreted with caution because the discounted and undiscounted costs were similar. The results from the sensitivity analysis showed that the results were robust when indirect medical costs were excluded from the analysis. The results were sensitive to changes in the prevalence of LTBI in this population, with the questionnaire followed by the IGRA screening strategy being the most cost-effective strategy at a prevalence of > 4.9%. The results from the probabilistic analysis showed that, at a prevalence of 1.1%, no screening was the preferred screening option compared with IGRA, but the authors did not state the willingness-to-pay value used.
Linas et al. 2011201
Linas et al. 201 constructed a decision tree structure with Markov nodes to estimate the cost-effectiveness of using TST compared with IGRAs for the diagnosis of LTBI in various populations. The model begins with a hypothetical cohort of people who received one of three diagnostic strategies (TST alone, IGRA alone or no screening). The model continued with people characterised by their disease status (LTBI/no LTBI). People with a positive IGRA or TST result received treatment for LTBI. The decision tree structure was used to inform on the proportion of people who started in the Markov model structure. The Markov structure started with people in the LTBI with isoniazid treatment state, the LTBI with no treatment state or the active TB health state, and showed the transitions between these health states. People who received treatment for LTBI were at risk of developing isoniazid-induced hepatitis.
Data required to populate the model were obtained from published sources. All costs included were obtained from published sources and presented in 2011 US dollars. The primary outcome was cost per QALY gained over a lifetime horizon. Utility values estimated were based on the Short Form questionnaire-36 items and European Quality of Life-5 Dimensions descriptive systems. The analysis was conducted from the health service perspective and all costs and benefits were discounted at 3% per annum. The authors further conducted one- and two-way sensitivity analyses around the key model input parameters. Results from the analysis showed that, in the HIV-infected cohort, screening with IGRA alone was marginally more costly and effective than the no screening option, with an ICER of $12,800. For people who were on immunosuppressive medication, the reported ICER for TST screening compared with no screening was $129,000. Sensitivity analyses showed that increasing the mean age of the population to 65 years and screening with TST remained cost-effective in people living with HIV infection. The base-case results were sensitive to changes in the estimates of health-related quality of life for people who received treatment for active TB. Screening with TST or IGRA resulted in ICERs that were > $100,000 for people with diabetes or end-stage renal disease.
Swaminath et al.202
Swaminath et al. 202 used a decision tree structure to estimate the costs and benefits of using QFT-G alone compared with TST alone for the diagnosis of LTBI in people with inflammatory bowel disease. The model simulated a cohort of people with moderate to severe active Crohn’s disease being treated with immunosuppressive medication. The starting point of the model was a cohort of people who received one of two tests. The structure started from disease status (LTBI/no LTBI) followed by test results. On positive test results people received treatment for LTBI and could further develop isoniazid-induced hepatitis and either survived or died from this event. People who were false negative could have reactivated TB and could survive or die from this event. People who were false positive received treatment and could further develop isoniazid-induced hepatitis. The authors suggested that people with indeterminate results on the QFT-G would immediately receive a second QFT-G test. However, this pathway was not shown in the decision tree structure. Data required to populate the model were obtained from secondary sources. The prevalence of LTBI in this population was obtained from the WHO. The sensitivity and specificity of tests were derived based on information obtained from a few sources and not from a literature review. The analysis was conducted from the health payer perspective and the results were presented in terms of the costs of false-negative cases avoided, TB reactivations and deaths avoided. The authors conducted one-way sensitivity analyses around key model input parameters. They suggested that QFT-G was less costly and more effective than the TST in this population.
Recent arrivals from countries with a high incidence of tuberculosis
Pareek et al.77
Pareek et al. 77 used a decision tree structure to simulate the costs and benefits of using T-SPOT. TB alone, QFT-GIT alone, TST plus confirmatory T-SPOT. TB (if TST positive) or TST plus confirmatory QFT-GIT (if TST positive) for screening immigrants for LTBI. The illustrative model structure presented by the authors in the supplementary appendix was illegible and hence further comment on/appraisal of the structure/pathways could not be made. The authors suggested that immigrants who were symptomatic at initial screening or who had a positive IGRA/TST result were referred for chest radiography and further clinical assessment. Immigrants with a positive IGRA and/or positive TST result and a normal chest radiograph without any symptoms suggestive of active TB were considered to have LTBI. For a positive TST test, cut-offs of ≥ 6 mm and ≥ 15 mm were used for BCG-unvaccinated and BCG-vaccinated participants, respectively. Additionally, the authors used a non-stratified cut-off of ≥ 10 mm to suggest a positive TST. The data required to populate the model were obtained from an observational study undertaken by the authors and from published sources. To be included in the observational study, participants had to be recently arrived (within the last 5 years) immigrants to the UK, aged ≥ 16 years (with symptoms of TB) or from a country with a TB incidence of ≥ 40 per 100,000 (asymptomatic). Information on the prevalence of LTBI was derived from immigrants aged ≤ 35 years who had been tested with the three screening tests. Cost data from published sources were inflated to 2010 prices using the Consumer Prices Index. The analysis was undertaken from the UK NHS perspective in a primary care setting. The outcome measures included in the analyses were the number of cases of active TB avoided and the number of LTBI cases needed to be treated to prevent one case of active TB, over a 20-year time horizon. The results were presented as cost per active TB cases avoided. Both costs and benefits were discounted at 3.5% per annum. Pareek et al. 77 conducted sensitivity analyses on key model input parameters (prevalence of LTBI, progression rate from LTBI to active TB, specificity, proportion of immigrants accepting and adhering to LTBI treatment). The base-case results showed that the screening strategy of no port-of-entry chest radiography and screening with one-step QFT-GIT was cost-effective with an ICER of £21,570 per case of active TB avoided for immigrants whose country of origin had an incidence of TB of 250 per 100,000. For immigrants whose country of origin had an incidence of TB of ≤ 150 per 100,000, the strategy was not cost-effective (at a willingness to pay of £30,000 per QALY). Results from the sensitivity analyses showed that varying the prevalence and the progression rate from LTBI to active TB increased the cost-effectiveness of the one-step QFT-GIT strategy. Reducing the specificity of the test resulted in the one-step T-SPOT. TB becoming the most cost-effective strategy. Reducing the proportion of people accepting and adhering to LTBI treatment led to higher cost-effectiveness estimates.
National Collaborating Centre for Chronic Conditions, Centre for Clinical Practice at the National Institute for Health and Care Excellence10
The authors of CG11710 used a decision tree structure to compare the costs and effects of four testing strategies [TST alone, IGRA alone, TST followed by IGRA and no test (to provide information and advice only)] for the diagnosis of LTBI in immigrants from countries with a high prevalence of active TB. The model started with a cohort of recently arrived immigrants who received one of the four testing strategies. In the TST-/IGRA-alone strategies, people who received a positive test result were treated for LTBI. Conversely, a proportion of people who had negative test results were given the BCG vaccination. In the combination strategy, people who tested positive on the TST received a QFT test. Immigrants who had a positive QFT result were treated for LTBI and, of those with a negative result, a proportion were given a BCG vaccination. The end point of the model was the proportion of people developing TB having received a BCG vaccination or treatment for LTBI. Data required to populate the model were obtained from published sources. Sensitivity of the tests was based on two publications and average values were used as estimates. Costs included in the model were those related to the UK NHS and Personal Social Services. All costs were presented in UK pounds sterling in 2008/9 prices. Costs obtained from published sources were inflated using the Hospital and Community Health Services pay and price index. The results showed that a positive TST result followed by IGRA and the IGRA-alone testing strategy were associated with ICERs of < £30,000 per QALY compared with the no-testing strategy. The results from the sensitivity analyses showed that varying the cost of an IGRA (from £50 to £60) changed the direction of the cost-effectiveness results.
Characteristics of the included studies
The characteristics of the models included in these evaluations are summarised in Table 26. All of the included studies used an economic model to determine the cost-effectiveness of various strategies for the diagnosis of LTBI. Four196–199 of the economic evaluations were conducted in Japan, three200–202 in the USA, two10,77 in the UK and one203 in South Africa. Three studies196–198 compared QFT-GIT only with TST only, two studies200,201 compared an IGRA with TST but did not indicate the type of IGRA being used, one study202 compared QFT-G only with TST only and four studies10,77,199,203 compared various testing strategies (TST alone, QFT alone, QFT-GIT alone, T-SPOT. TB alone, TST followed by QFT and TST followed by T-SPOT. TB, TST negative followed by IGRA) for the diagnosis of LTBI. Two197,203 economic evaluations were conducted in children, six196,198–202 evaluations were conducted in the immunocompromised population and two10,77 were conducted in the recently arrived population.
Most of the decision-analytical models196–200,203 used for the analyses were decision tree structures with Markov nodes; three studies10,77,202 used a decision tree structure alone and one study201 used a Markov model alone to show diagnostic strategies for detecting LTBI and progression over time to active TB. The health states included in the models represented those that people would experience while being screened for LTBI. In the models with a cohort of children, the health states included healthy, LTBI, TB and dead. There was some variation in the health states used for the immunocompromised population; this may be because of the presence of various diseases/conditions when trying to assess which diagnostic strategy is cost-effective for the diagnosis of LTBI. In the models with a cohort of recently arrived immigrants, the health states included test results, treatment for LTBI and treatment for TB. One of the model structures was illegible in this population.
Model time horizons ranged from 1 year to a lifetime. In the models with children, the time horizon was a lifetime (up to 80 years) with cycle lengths of 6 months203 and 1 year. 197 In the models with immunocompromised cohorts, the time horizons ranged from 1 year to a lifetime, with 3-month or 1-year cycle lengths, and in the models with a recently arrived cohort, the time horizons ranged from 15 years to 20 years, with annual cycle lengths. The authors stated that the time horizons chosen were long enough to measure the costs and benefits of the diagnostic strategies.
Resource use and costs included in the economic analyses depended on the perspective taken. All studies clearly stated the perspective or viewpoint from which the analysis was undertaken. Five studies10,77,199,201,202 conducted their analyses from the UK NHS or other national health payer perspective and the remaining five studies196–198,200,203 conducted their analyses from the societal perspective. The five models10,77,199,201,202 that presented results based on the health payer perspective included direct costs related to the health service (costs of diagnostic tests, chest radiography and sputum examinations, treatment for LTBI/active TB and treatment for isoniazid-induced hepatotoxicity). Of the five models196–198,200,203 that presented results based on the societal perspective, three models196–198 did not include indirect costs or loss of productivity.
Six10,196–200 studies reported their results in terms of cost per QALY only, three studies77,202,203 reported their results in terms of cost per life-year saved, cost per false-negative case of LTBI avoided, cost per TB death avoided, cost per reactivation TB case avoided or cost per TB case avoided, and in one study201 the outcomes were based on the number needed to screen to prevent one case of active TB, life expectancy and QALYs gained. Of the studies that reported results in terms of QALYs, utility values were obtained from published sources to derive QALY estimates. These studies referenced the original source of the utility values but did not elaborate on which descriptive system was used to values these health states. From the base-case results reported in these studies, the consensus was that IGRAs were less costly and more effective than other strategies.
Because of the uncertainty around key model input parameters and assumptions made in the models, all authors conducted sensitivity analyses. Five studies10,77,201–203 conducted deterministic (one- and two-way) sensitivity analyses alone. The remaining studies196–200 conducted both deterministic sensitivity analyses and PSAs. Sensitivity analyses were conducted around changing the prevalence of LTBI, test accuracies (sensitivity and specificity) of diagnostic tests, the costs of the IGRAs, return rates for TST and the progression rate from LTBI to active TB.
This review was used to inform model development for the diagnosis of LTBI in three populations. In the following section we provide an appraisal of the modelling structures, the data used to parameterise the models and the handling of uncertainty. We also consider relevant issues when deriving key model input parameters (prevalence, sensitivity/specificity of diagnostic tests and combination strategies).
Quality assessment of the modelling methods
We present a summary of the reporting quality of the studies included in the current review assessed against the Philips et al. 195 checklist in Appendix 13.
Structure
The structures of the models included in this review were generally of good quality. In accordance with best practice for developing model structures, studies clearly stated their decision problem and the perspective of the analysis, the objectives of the model, which were consistent with the decision problem, and the structures which represented the clinical pathway people that would follow while being screened for LTBI. However, there were some structural issues noticed. Three studies196–198 conducted their analyses from the societal perspective but did not include indirect costs or loss of productivity in the analyses. Studies generally stated the location of the analyses but not the setting and this may have an impact on the generalisability of the results. Illustrative model structures were also presented in the majority of the studies but in one study77 the model structure was illegible. All studies clearly stated and justified their time horizon and cycle lengths.
All authors justified their choice of model structure, which represented coherent pathways of LTBI disease and its treatment. Six models10,196–200 used decision tree structures with Markov nodes for their analyses, three studies77,202,203 used decision tree structures alone and one study201 used a Markov model alone. Of the studies identified, six10,198–201,203 modelled from the test result first followed by LTBI diagnosis, whereas four77,196,197,202 modelled from LTBI diagnosis followed by the test result. One study10 included a proportion of people returning to have their TST result read. One study202 included a proportion of people with indeterminate test results on an IGRA and assumed that they would receive a second IGRA immediately (not shown in the decision tree). All studies included chest radiography to confirm whether or not active TB was present. All studies also included treatment for LTBI and TB. As a result of adhering to LTBI treatment, all studies included a proportion of people developing isoniazid-induced hepatotoxicity but they did not include any other adverse events from adhering to TB treatment. Studies that included a Markov model196–200,203 generally used similar health states (no LTBI, LTBI, active TB, reinfection, disseminated TB and dead) to show the possible transitions over time.
Key model input parameters
The methods used to identify relevant information to populate the models were satisfactory in most studies. Studies stated that a literature review was undertaken but did not specify the purpose/aim of the review, that is, to search the literature to inform on the data inputs and/or to inform on the model structure or model design. All studies provided references for their model inputs but they were not clear on the choices between data sources or the quality of information used in the models. This may have been a result of a paucity of information in the literature.
In the four models77,196,197,202 that started from known disease status, information required at this point was the prevalence of LTBI in the population. Most studies used secondary sources to obtain a point estimate or to derive an estimate of the prevalence of LTBI but they did not elaborate on what the prevalence represented (prevalence of LTBI in contact tracing, prevalence of LTBI based on occasional screening in the population of interest or prevalence of LTBI that would develop to active TB). Additionally, studies that used multiple sources were not transparent on the methods used to derive an estimate of the prevalence of LTBI.
Test characteristics of the TST and IGRAs were required for all of the models. In most studies10,77,197,199–203 a literature review was carried out and estimates of sensitivity and specificity were derived based on sources identified. Most studies10,77,197,199–201 elaborated on the methods used to derive sensitivity and specificity. These methods included calculating an estimate based on an average of sensitivity (and specificity) obtained from the literature, obtaining estimates from sources that conducted a meta-analysis or using Bayesian statistics to calculate an estimate of sensitivity and specificity based on confirmed TB cases. The study77 that used Bayesian statistics acknowledged that there is no gold standard test available for the diagnosis of LTBI in these populations and provided equations used to derive sensitivity and specificity. Studies that included a combination strategy,10,199 for example TST positive followed by IGRA, did not elaborate on the methods used to derive the sensitivity and specificity of a test conditional on an initial positive/negative result.
All costs required for the models were justified and referenced. Costs obtained from published literature were inflated using the appropriate indices. All authors clearly stated the unit costs used in the models, but some authors196,198–200 did not elaborate on the resources used to estimate the unit costs, especially for the treatment of LTBI/active TB. All authors stated the perspective of the analyses, but in some studies196–198 the costs included did not reflect the viewpoint/perspective of the analyses. All authors, when necessary, discounted costs and benefits using the appropriate rates.
In the models that reported their results in terms of QALYs,10,196–201 authors provided the references used to obtain the utility weights. However, the majority of the authors196–200 did not elaborate on the descriptive tools/measures used to value these health states in these populations. Additionally, authors did not elaborate whether or not the sources of utility information used were relevant to their population of interest.
Uncertainty and assumptions
Uncertainty is unavoidable in economic modelling. Briggs and Gray204 and Philips et al. 195 have outlined methods to handle the four main types of uncertainty (methodological, structural, parameter and generalisability). All of the models attempted to address uncertainty, but none of these studies addressed all types of uncertainty. All of the studies undertook univariate or multivariate sensitivity analysis on key model input parameters. Four studies196–199 also undertook PSA for joint uncertainty in model parameters to assess the impact on the base-case results.
To have a workable model structure to conduct these analyses, all studies except that by Kowada199 clearly stated the simplifying assumptions of their models. In general, these assumptions outlined in the studies appeared to be feasible but were strong in some cases. One study77 assumed that testing with an IGRA would not lead to an indeterminate result whereas in CG11710 the authors assumed that treatment of LTBI/TB was adhered to by the population and that it would not lead to any adverse events.
Conclusion
The evidence base described here offers insight on the decision-analytic models available to determine the cost-effectiveness of an IGRA compared with the TST for the diagnosis of LTBI in children, immunocompromised people and people from countries with a high incidence of active TB. We identified 10 model-based economic evaluations across these three populations. The majority of these models included immunocompromised or immunosuppressed populations, with the evidence available for the other two populations being sparse. The majority of the models used decision tree structures with Markov nodes to simulate a cohort of people being tested for LTBI.
We appraised these models against frameworks on best practice for reporting an economic evaluation and economic modelling. In general, all models performed well in terms of defining the decision problem, including the study perspective, outlining the choice of comparators, presenting an illustrative model structure and providing a clear outline of the assumptions. These models all add to the existing literature but are subject to limitations. First, the majority of the studies indicated the location of the study but did not state the setting of the analysis and this may limit the generalisability of the results. Second, the majority of the studies used QALYs as the outcome measure and referenced the source of the utility values. However, the authors did not provide commentary on the descriptive tools used to value these health states. Third, the perspective of the analysis was stated in all studies; however, some of the resource use and costs reported did not reflect the viewpoint of the analysis. Fourth, the majority of the studies were transparent with regard to the methods used to identify information to populate the models, but it was unclear if any quality assessment of the information was undertaken. Finally, all models explored uncertainty around key model input parameters by undertaking one- and two-way sensitivity analyses but no attempt was made to explore the other types of uncertainty: methodological, structural or generalisability. Other concerns relate to the derivation of prevalence, test accuracy and transition probabilities; most studies did not elaborate on these statistical/pre-model analyses.
In Chapter 6 we outline the development of a de novo model that includes two stages to inform on the cost-effectiveness of various strategies for the diagnosis of LTBI in our populations of interest.
Chapter 6 Health economics methods and results
Objective
The objective of the economic evaluation was to compare the cost-effectiveness of various screening strategies for the diagnosis of LTBI in immunocompetent children, people who are immunocompromised or at risk of immunosuppression and people who are recent arrivals from countries with a high incidence of active TB.
Currently in the UK, the following strategies are recommended to diagnose people with LTBI:10
-
Children. Offer a Mantoux test to children aged 2–15 years. If positive, follow up with an IGRA.
-
Immunocompromised. For people who are HIV negative, offer an IGRA alone or an IGRA with a concurrent Mantoux test. If either test is positive perform a clinical assessment to exclude active TB and treat.
-
Recently arrived immigrants. Offer an IGRA alone or a dual strategy for people aged 16–35 years. If either test is positive, refer to a TB specialist to exclude active TB and treat.
-
General population. Offer an IGRA alone or IGRA testing for people whose Mantoux test shows positive results.
Developing the model structure
To assess the cost-effectiveness of various strategies for the diagnosis of LTBI, we developed an economic model using R (version 3.1.1; The R Foundation for Statistical Computing, Vienna, Austria).
The model was developed with clinical input and represents, as far as possible, the clinical pathways that people would take while being screened for LTBI. The model structure for the child population is presented in Figure 49. The model was structured in two stages: diagnosis of LTBI and disease progression to active TB. The first stage of the model represents the clinical pathway that people would take in a 1-year time period before entering the infectious disease model. For this stage we used a decision tree structure for the diagnosis of LTBI. Four diagnostic strategies were examined in the model for each population:
-
TST alone
-
IGRA alone
-
combinations of TST and IGRA
-
simultaneous testing.
FIGURE 49.
Decision tree structure for the child population.
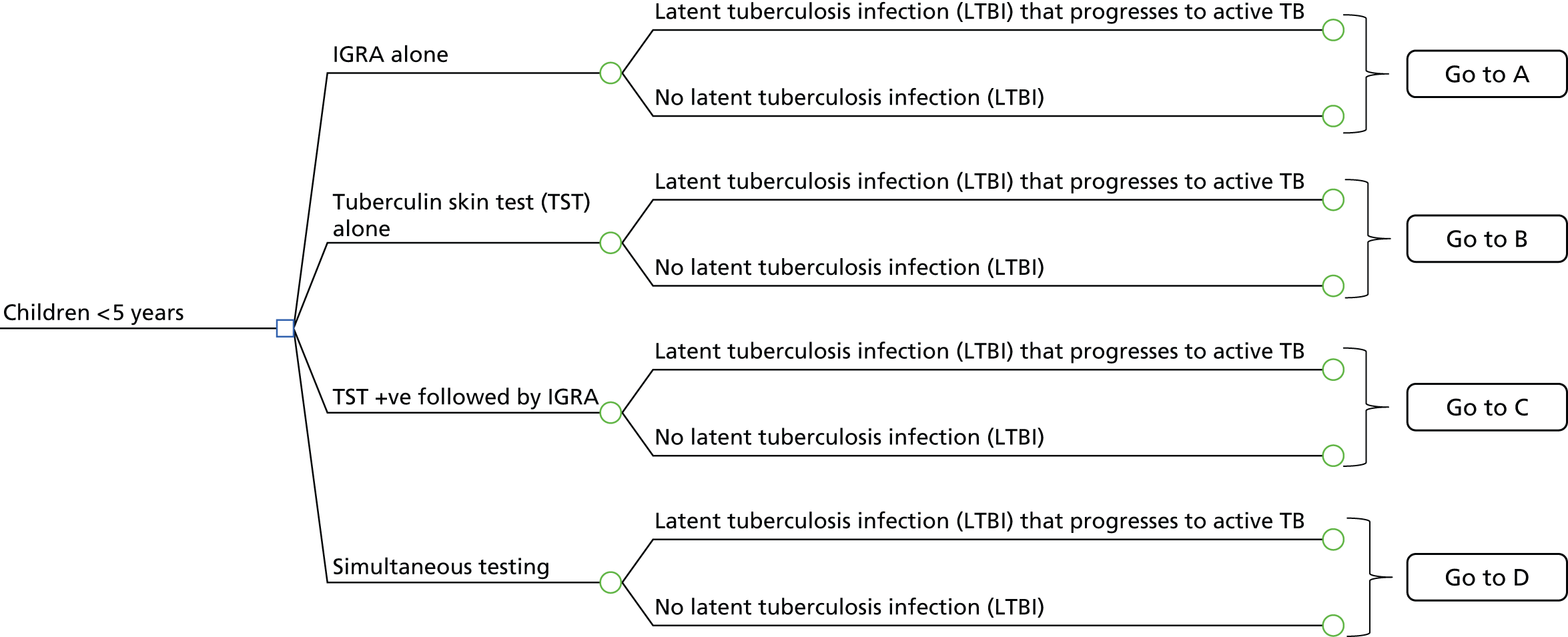
These strategies being compared were derived based on the strategies outlined in CG11710 and with input from the TB Guideline Development Group. The model begins with people receiving one of these diagnostic strategies (see Figure 49). The branches to the right of the decision node (square symbol) represent the strategies being compared (see Figures 50–54 for the child population and Appendix 15, Figures 60–74, for all other populations). People begin in one of the possible health states to the right of the chance nodes (circle symbols). The decision tree is modelled from individuals who have LTBI that progresses to active TB/no LTBI, followed by the probability of test results. However, in clinical practice the test result is known before LTBI is diagnosed. Modelling the test result first followed by disease category or vice versa makes no mathematical difference in terms of the expected values calculated for each diagnostic strategy. 205 In the following sections we describe each strategy in detail.
Tuberculin skin test-alone strategy (Figure 50)
When screening with a TST, an individual may or may not return to have the test results interpreted (TST not read). Adults with positive TST results (induration ≥ 5 mm/10 mm) are assessed for initial active TB by chest radiography and sputum examination. Children with positive TST results are assessed for active TB by chest radiography and, if that is positive, a gastric lavage procedure. Those who have a positive result on chest radiography and sputum examination are treated for active TB. We assumed here that chest radiography and sputum examination are 100% accurate at diagnosing people who have initial active TB. People who adhere to TB treatment in the immunocompromised or recently arrived population may develop hepatitis and can survive or die from this adverse event. In the model with a cohort of children, we assumed that they would not develop hepatitis because it is a rare adverse event in this population. 203 People who have a negative result on chest radiography and sputum examination (LTBI) can either accept or refuse to be treated for LTBI. Those who accept LTBI treatment may adhere/not adhere to treatment. If the TST is not read or the TST results are negative, the individual is not followed up.
FIGURE 50.
Pathway for the TST-alone diagnostic strategy in children. –ve, negative; +ve, positive; CXR, chest radiography.

Interferon gamma release assay-alone strategy (Figure 51)
When screening with an IGRA alone, an individual may have a determinate or an indeterminate result. Adults with a determinate result and who are IGRA positive are assessed for initial active TB by chest radiography and sputum examination. Children with positive IGRA results are assessed for active TB by chest radiography and, if that is positive, a gastric lavage procedure. Those who have a positive result on chest radiography and sputum examination are treated for active TB. Those who have a negative result on chest radiography and sputum examination (LTBI) can either accept or refuse to be treated for LTBI. People who accept LTBI treatment can adhere or not adhere to treatment. People with an indeterminate IGRA result receive a second IGRA test, which is the same as the initial IGRA test. If the IGRA result is negative or both IGRA tests are indeterminate, the individual is not followed up.
FIGURE 51.
Pathway for the IGRA-alone diagnostic strategy in children. –ve, negative; +ve, positive; CXR, chest radiography.

Combined strategy (Figure 52)
For children and the recently arrived population, those who had their TST results interpreted, and whose results are positive, receive an IGRA test. Children with determinate, positive IGRA results receive chest radiography and, if positive, the gastric lavage procedure before a sputum examination for the assessment of active TB. Children with negative chest radiography/sputum examination results are either treated or not treated for LTBI. Children with indeterminate results receive a second IGRA test, which is the same as the initial IGRA test. If the TST is not read or the TST is negative, the individual is not followed up. Recent arrivals with determinate, positive IGRA results are assessed for active TB by chest radiography and sputum examination. If there is a positive result on chest radiography and sputum examination, they are treated for active TB. Those who have a negative result on chest radiography and sputum examination (LTBI) can either accept or refuse to be treated for LTBI. If people accept LTBI treatment, they may adhere/not adhere to treatment. People with an indeterminate IGRA result receive a second IGRA test, which is the same as the initial IGRA test. These people follow similar pathways to those who received one IGRA test. At most, people will receive two IGRA tests. If the TST result is not read, the TST result is negative, the IGRA result is negative or both IGRA tests are indeterminate, the individual is not followed up.
FIGURE 52.
Pathway for the diagnostic strategy TST positive followed by IGRA in children. –ve, negative; +ve, positive; CXR, chest radiography.

Conversely, in the immunocompromised group, people receive an IGRA test first. Those who have a positive result on the IGRA test receive chest radiography and sputum examination to detect initial active TB. Those with a positive result on chest radiography and sputum examination are treated for active TB. Those who have a negative result can accept or refuse treatment for LTBI. People who have accepted and adhered to LTBI treatment may develop hepatitis and can survive or die from this adverse event.
Individuals with a negative IGRA result undergo a TST test. People here follow similar pathways as those who received the TST-alone strategy. Those with an indeterminate IGRA result receive a second IGRA test, which is the same as the initial IGRA test. These people follow similar pathways to those who received one IGRA test. At most, people will receive two IGRA tests. If the IGRA result is negative, both IGRA tests are indeterminate, the TST result is negative or the TST result has not been read, the individual is not followed up.
Simultaneous testing strategy (Figures 53 and 54)
When screening with an IGRA and a TST, people can have a combination of test results: a determinate result on the IGRA and the TST read, a determinate result on the IGRA and the TST not read, an indeterminate result on the IGRA and the TST read or an indeterminate result on the IGRA and the TST not read. Children with a positive result on either test receive chest radiography and, if positive, the gastric lavage procedure and sputum examination to detect initial active TB. For the other populations, those with a positive result on either test receive chest radiography and, if positive, a sputum examination to detect active TB. If the IGRA result is indeterminate and the TST is not read, the individual is not followed up.
FIGURE 53.
Decision tree structure for the child population receiving the simultaneous testing strategy. –ve, negative; +ve, positive.
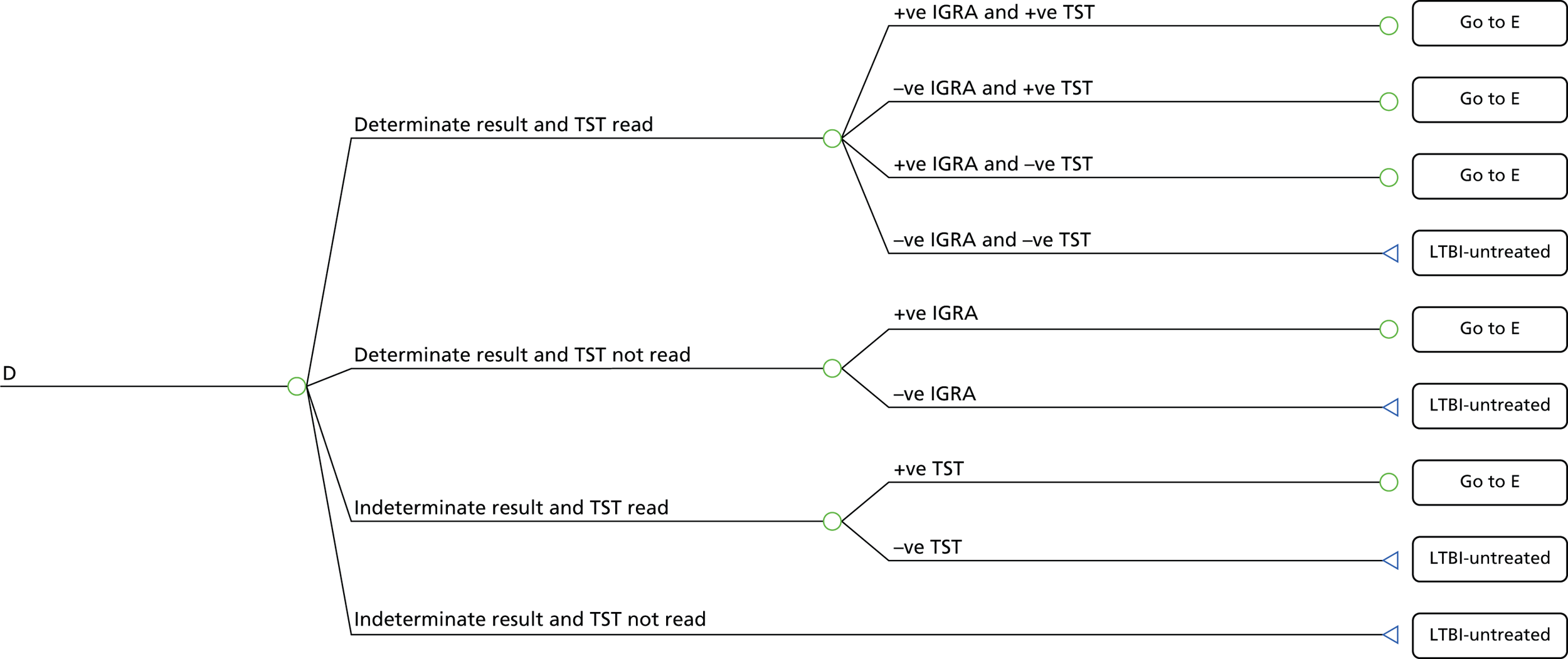
FIGURE 54.
Pathway for the simultaneous testing strategy in children. –ve, negative; +ve, positive; CXR, chest radiography.

Stage 2 of the model is a disease progression model looking at progression between no TB/LTBI, LTBI that will progress to active TB and active TB, as well as secondary infections in other individuals caused by people with active TB. The basic model structure is shown in Figure 55. This structure is the same for people who are/are not being treated for LTBI/active TB, although the transmission probabilities are different in each of these cases. The outputs of the decision tree are used to determine the proportions of people who start in each state, specifically:
-
active TB
-
LTBI – treated for LTBI
-
LTBI – untreated
-
no TB/LTBI – treated for LTBI
-
no TB/LTBI – untreated.
FIGURE 55.
Dynamic transmission model.
The model used was a discrete event simulation, modelling individual patients, built using R (version 3.1.1). An initial simulation, starting with an identical cohort of 500,000 individuals in each arm, was run using the mean values of each parameter. To account for parameter uncertainty, we also ran a Monte Carlo simulation, consisting of 2000 different sampled parameter sets, each run on a starting sample of 100,000 individuals. An individual’s event risks at any time point are determined by their age, TB status and current treatment and remain constant until one of these factors changes.
People who begin the model with LTBI and who are not treated will develop active TB at a later point (from the definition of LTBI in our model as LTBI that progresses to active TB). The mean delay between the diagnostic test and progression to active TB was estimated from the systematic review, with individual activation times simulated assuming a constant activation rate over time. People who begin the model with LTBI and who are treated for LTBI have a certain probability of not developing active TB in the future (the effectiveness of the treatment – assumed to be 6 months of isoniazid), with activation times for those whose treatment is unsuccessful sampled as above.
Age-specific all-cause mortality rates were taken from UK-specific data in the Human Mortality Database206 and applied to all individuals in the model. Age-specific utilities for individuals without TB were calculated using data from the Health Survey for England. 207 When an individual develops active TB, they have an immediate, age-specific probability of death, over that of all-cause mortality. Recovery rates from active TB were calculated from the mean length of an active TB episode, assuming a constant probability of recovery over time. Individuals with resolved TB have an annual probability of relapse, with subsequent activations having the same probability as the initial episode.
For each TB activation (primary or relapse), individuals generate a certain number of secondary cases of LTBI that will progress to active TB, sampled from a Poisson distribution. These cases are assumed to occur in the general population; hence, the age of the secondarily infected individuals was simulated from the average age distribution of active TB cases in the UK. These secondary cases were assumed to be identical (in terms of probability of death, average length of active TB episode, utility loss, number of secondary cases generated) to similarly aged individuals in the initial population. We did not simulate secondary cases of LTBI that do not progress to active TB as we have also not considered these in our initial population.
As the model is run, any new cases of LTBI generated are included in the disease progression model from that time forward. Costs and QALYs are accrued by individuals according to the length of time that they spend in each state of the model. Unlike a traditional economic model, it is not possible to continue running the simulation until all individuals have died, as there is a continuous stream of new individuals being added as a result of new infections. Consequently, the simulation was run for 100 years, with discounting meaning that any results over a longer time horizon than this are unlikely to make a meaningful difference to the outcome. The parameters for the discrete event simulation are presented in Table 27 for the child population and in Tables 64 and 65 (see Appendix 16) for the immunocompromised and recently arrived populations respectively.
| Variable | Base-case value | Range for SA | PSA distribution | Source |
|---|---|---|---|---|
| Probabilities | ||||
| Prevalence of LTBI | 0.0288 | 0.0206–0.0384 | a | Derived from the current clinical effectiveness study |
| Sensitivity TST (≥ 5 mm) | 0.7280 | 0.6059–0.7294 | a | |
| Specificity TST (< 5 mm) | 0.4903 | 0.4796–0.5008 | a | |
| Sensitivity TST (≥ 10 mm) | 0.5351 | 0.3821–0.6769 | a | |
| Specificity TST (< 10 mm) | 0.7481 | 0.3434–0.7618 | a | |
| Sensitivity QFT-GIT | 0.6884 | 0.5856–0.7820 | a | |
| Specificity QFT-GIT | 0.6103 | 0.6030–0.6176 | a | |
| Sensitivity T-SPOT.TB | 0.500 | 0.0245–0.9764 | a | |
| Specificity T-SPOT.TB | 0.7758 | 0.6738–0.8640 | a | |
| Sensitivity of QFT-GIT conditional on positive TST (LTBI arm) | 0.6775 | 0.4674–0.9233 | a | |
| Specificity of QFT-GIT conditional on positive TST (no LTBI arm) | 0.3213 | 0.3073–0.3353 | a | |
| Sensitivity of QFT-GIT conditional on negative TST (LTBI arm) | 0.7031 | 0.1122–0.9921 | a | |
| Specificity of QFT-GIT conditional on negative TST (no LTBI arm) | 0.9108 | 0.9013–0.9200 | a | |
| Sensitivity of CXR for diagnosing active TB | 0.7800 | Not reported | Not varied | Kumar et al.208 |
| Specificity of CXR for diagnosing active TB | 0.5100 | Not reported | Not varied | Kumar et al.208 |
| Determinate QFT-GIT | 0.97 | – | Beta(873,27) | Derived from Laskin et al.200 |
| Determinate T-SPOT.TB | 0.97 | – | Beta(873,27) | Derived from Laskin et al.200 |
| TST read | 0.9400 | 0.6–1.00 | Beta(164,10.5) | Pareek et al.77 |
| Initial active TB | 0.00001 | – | Not varied | Laskin et al.200 |
| TB treatment adherence | 1.0000 | – | Not varied | Pareek et al.77 |
| Accepting LTBI treatment | 0.9400 | 0.50–1.00 | Beta(141,9) | CG11710 |
| Adherence to LTBI treatment | 0.8000 | 0.50–0.90 | Beta(41,10) | Kowada198 |
| Isoniazid-induced hepatitis after TB treatment | 0.0040 | 0.001–0.010 | Beta(2.7,664) | Assumption |
| Death from isoniazid-induced hepatitis | 0.00002 | 0.00001–0.0001 | Beta(0.5,25125) | Pooran et al.209 |
| Transmission model parameters | ||||
| Proportion still infected post LTBI treatment | 0.345 | – | Log-normal(–1.065,0.842) | White and Jit210 |
| Average number of secondary cases from one index case | 0.2 | 0.1–0.3 | Log-normal(–1.609,0.354) | Pareek et al.6 |
| Average delay from infection to activation (secondary cases) | 2.88 | – | Log-normal(1.058,0.333) | Okuonghae211 |
| Annualised reactivation rate from resolved TB | 0.013 | 0.004–0.025 | Beta(7,513) | Oxlade et al.212 |
| Case fatality rate for active TB (0–4 years) | 0.0477 | – | Beta(628,12543) | Crofts et al.213 |
| Case fatality rate for active TB (5–14 years) | 0.0034 | – | Beta(1,290) | Crofts et al.213 |
| Case fatality rate for active TB (15–44 years) | 0.0018 | – | Beta(1,564) | Crofts et al.213 |
| Case fatality rate for active TB (45–64 years) | 0.0476 | – | Beta(125,2500) | Crofts et al.213 |
| Case fatality rate for active TB (65+ years) | 0.1755 | – | Beta(413,1940) | Crofts et al.213 |
| Resource use and costs (£) | ||||
| TST | 17.48 | – | Not varied | Pooran et al.209 |
| QFT-GIT | 48.73 | – | Not varied | Pooran et al.209 |
| T-SPOT.TB | 59.57 | – | Not varied | Pooran et al.209 |
| CXR | 35.00 | – | Not varied | NHS reference costs 2012/13214 |
| Gastric lavage procedure | 916.00 | – | Not varied | NHS reference costs 2012/13214 |
| Sputum examination | 7.00 | – | Not varied | NHS reference costs 2012/13214 |
| Cost of adherence to active TB treatment | 5461.12 | – | Gamma(10.41,524.6) | Bothamley et al.215 |
| Cost of non-adherence to active TB treatment | 910.19 | – | Not varied | Assumption |
| Cost of adherence to LTBI treatment | 677.07 | – | Uniform(511.69,842.45) | NHS drug tariff216 |
| Cost of non-adherence to LTBI treatment | 112.85 | – | Uniform(85.24,140.41) | Assumption |
| Treatment of isoniazid-induced hepatitis | 389.51 | – | Gamma(7.13,55.64) | Pareek et al.77 |
| Utility decrements | ||||
| Active TB (while on treatment) | 0.15b | Not reported | Gamma(11.2,0.0134) | Derived from Kowada197 |
| Treatment for LTBI | 0.001 | – | Uniform (0,0.002) | Derived from Kowada197 |
| Other | ||||
| Discount rate per annum (costs and QALYs) | 3.5% | |||
Model assumptions
A number of assumptions were required to develop a workable model structure to enable the analyses to be undertaken:
-
We assumed that our population is similar to the population in the clinical effectiveness studies, but excluding studies with populations with a high incidence of active TB.
-
People being assessed for initial active TB undergo chest radiography and, if positive, receive a sputum examination.
-
Children being assessed for initial active TB undergo chest radiography and, if positive, undergo a gastric lavage procedure.
-
The sputum examination is 100% accurate when diagnosing initial active TB.
-
Individuals with a second indeterminate result on the IGRA test are at the same risk of developing active TB as those with a false-negative result.
-
People who have been diagnosed with initial TB accept treatment.
-
People who do not adhere to LTBI treatment take medication for 1 month.
-
People who do not adhere to LTBI treatment are not at risk of developing isoniazid-induced hepatotoxicity.
-
People who do not adhere to active TB treatment take medication for 1 month.
-
Children are not at risk of developing hepatitis as a result of treatment for active TB or LTBI.
-
No health loss is experienced by people with LTBI who do not progress to active TB.
Data required for the model
The model was populated with clinical information from the current clinical effectiveness review and supplemented with information from secondary sources. Information required to parameterise the model included prevalence, sensitivity and specificity, adverse events, resource use, and costs and utilities. We acknowledge here that there is no gold standard test for a LTBI diagnosis. Hence, we have used clinical information from studies in this review that reported information on confirmed cases of active TB (the proportion of untreated individuals who go on to develop active TB at a later date).
All of the data available for the child population were based on studies in which there was previous contact with an index case. We therefore restricted our analysis to this population both because of the lack of data and because it was thought unlikely that a general screening programme for all children, irrespective of contact, would ever be introduced.
Prevalence
In this analysis, prevalence was defined as the proportion of people who have LTBI that will progress to active TB, assuming that they are not treated. We derived estimates for this LTBI prevalence criterion based on empirical data from the three cohorts separately. We used WinBUGS software (version 1.4.3; MRC Biostatistics Unit, Cambridge, UK) to conduct Bayesian Markov chain Monte Carlo (MCMC) simulation to derive the prevalence of LTBI in each cohort using the following formula:
Rearranging the above equation for prevalence of LTBI:
To avoid overestimating the prevalence of LTBI that progresses to active TB, we excluded studies with populations with a high incidence (≥ 40 cases per 100,000) of active TB. For the recently arrived population, we derived the prevalence from all studies on recent arrivals found in the clinical effectiveness review, which included people with LTBI who progressed to active TB.
Performance of screening tests (sensitivity and specificity)
The sensitivities and specificities of the various strategies were derived based on information obtained from longitudinal studies in people who received testing and developed active TB. Therefore, our calculated sensitivities and specificities represent the sensitivities and specificities of detecting people with LTBI that will progress to active TB, not the sensitivities and specificities of detecting LTBI in general. Bayesian MCMC was used to derive posterior distributions for test performance assuming weakly informative priors to derive the sensitivity and specificity of the diagnostic tests by population. Estimates for sensitivity and specificity were derived for TST (≥ 5 mm), TST (≥ 10 mm), QFT-GIT and T-SPOT. TB.
To synthesise the clinical evidence in WinBUGS, there were three main components of the model: the statistical model, priors and data. Appendix 17 provides the WinBUGS code for the child population.
Statistical model
In our models we have used distributions to represent the unknown variables in the model. For the evidence synthesis for children, immunocompromised people and recent arrivals we used the binomial distribution to derive the sensitivities and specificities of TST, QFT-G, QFT-GIT and T-SPOT. TB. We chose the binomial distribution because we were interested in the probability p of the number of successes (people with positive/negative results that progressed to active TB) from n number of longitudinal studies.
First, we were interested in the probability ppos of the number of positive test results from n longitudinal studies and, second, we were interested in the probability papos of the number of positive results that progressed to active TB from n number of positive test results. Likewise, we were interested in the probability paneg of a negative result progressing to active TB.
Logical expressions were built into the model to represent the relationship between the probability of a positive result, the prevalence of LTBI, test sensitivity and test specificity (see Appendix 17).
We initially explored both fixed- and random-effects models. However, for two of the populations (children and immunocompromised people) the random-effects models did not converge (most likely because in a number of studies either no individuals or only a very small number of individuals progressed to active TB). Hence, for consistency, we used the fixed-effects model for the three populations.
Priors
We stated in the WinBUGS model the prior distribution to be used. We chose the uniform distribution because all possible combinations of positive and negative test results have an equal a priori probability of occurring. In our WinBUGS code we added a logic expression to inform the model that the sensitivity of TST (≥ 5 mm) is greater than the sensitivity of TST (≥ 10 mm), which is greater than the sensitivity of TST (≥ 15 mm). Likewise, the specificity of TST (< 5 mm) is lower than the specificity of TST (< 10 mm), which is lower than the specificity of TST (< 15 mm). We included this logic expression because the TST is a single test with various cut-off thresholds for a positive result and, by definition, TST (≥ 5 mm) would be more sensitive and less specific than TST (≥ 10/15 mm).
Data
Observed data from longitudinal studies identified in the clinical effectiveness review were entered into the model in a list format. Data included the number of people being tested, the number of people with positive results, the number of people with positive results who were untreated and who developed active TB and the number of people with negative results who developed active TB. Tables 67–72 (see Appendix 18) show the information obtained from the clinical effectiveness studies. The term ‘not applicable’ was used to represent any missing values. After compiling the model, we specified distributions from which to sample initial values for the model.
To obtain accurate posterior probabilities we used 60,000 simulations; a burn-in period of 30,000 simulations was used. Output from the remaining 30,000 simulations represented the posterior mean, along with its posterior standard deviation, posterior median and 95% credible intervals. Convergence of the model was assessed using a visual inspection of the sample trace plots (see Appendix 17).
The results of the meta-analysis are presented in Table 28. The sensitivity and specificity of TST (≥ 5 mm) for the diagnosis of LTBI in children were estimated at 72.80% and 49.03%, respectively. In the immunocompromised group we derived estimates of 32.42% and 74.22% for the sensitivity and specificity of TST (≥ 5 mm), respectively. In the recently arrived immigrants group we derived estimates of 93.56% and 50.11% for the sensitivity and specificity of TST (≥ 5 mm), respectively. In the models we have not stratified by BCG status and hence we used a cut-off of ≥ 5 mm to define a positive TST.
| Test | Sensitivity (95% credible interval) (%) | Specificity (95% credible interval) (%) |
|---|---|---|
| Children | ||
| TST (≥ 5 mm) | 72.80 (60.59 to 72.94) | 49.03 (47.96 to 50.08) |
| TST (≥ 10 mm) | 53.51 (38.21 to 67.69) | 74.81 (34.34 to 76.18) |
| QFT-GIT | 68.84 (58.56 to 78.20) | 61.03 (60.30 to 61.76) |
| T-SPOT.TB | 50.00 (2.45 to 97.64) | 77.58 (67.38 to 86.40) |
| Immunocompromised people | ||
| TST (≥ 5 mm) | 32.42 (11.19 to 58.48) | 74.22 (72.88 to 75.57) |
| TST (≥ 10 mm) | 16.82 (2.52 to 38.99) | 83.97 (78.99 to 88.31) |
| QFT-GIT | 55.48 (24.73 to 83.73) | 82.27 (80.52 to 83.96) |
| T-SPOT.TB | 66.65 (35.17 to 91.44) | 68.46 (63.46 to 73.37) |
| Recent arrivals from countries with a high incidence of TB | ||
| TST (≥ 5 mm) | 93.56 (77.86 to 99.77) | 50.11 (47.90 to 52.29) |
| QFT-GIT | 59.15 (35.84 to 81.42) | 79.29 (77.80 to 80.73) |
| T-SPOT.TB | 70.01 (39.78 to 92.42) | 39.92 (34.39 to 45.54) |
Similar methods were used to derive the sensitivity and specificity of TST (≥ 10 mm) in these populations. The sensitivity and specificity of QFT-GIT for the diagnosis of LTBI in children were estimated at 68.84% and 61.03%, respectively. In the immunocompromised group we derived estimates of 55.48% and 82.27% for sensitivity and specificity, respectively, and in the recently arrived group we derived estimates of 59.15% and 79.29% for sensitivity and specificity, respectively. In the models we used QFT-GIT values as the base-case values for the analysis because the majority of the studies compared QFT-GIT with the TST.
Resource use and costs
The resource use and costs included were those directly incurred by the NHS. Costs for diagnostic tests, chest radiography, gastric lavage, sputum examination, treatment of LTBI/TB and isoniazid-induced hepatitis were all included in the analysis. Societal costs (indirect costs, loss of productivity or cost of death) were not included. The unit costs are presented in Table 27. The majority of the cost information used in the analyses was obtained from secondary sources. The costs for QFT-GIT (testing kit, consumables, processing and phlebotomy) and the TST (disposables, administration and reading) were obtained from Pooran et al. 209 Estimated costs for chest radiography, the gastric lavage procedure and sputum examination were obtained from NHS reference costs 2012/13. 214 Estimated costs for the treatment of LTBI were obtained from the NHS drug tariff 2015216 and in consultation with a clinical expert (see Appendix 16). Costs for the treatment of TB were obtained from Bothamley et al. 215 (see Appendix 16). Management of LTBI included further blood tests (full blood count and liver function tests), doctor and nurse outpatient visits, and treatment with 300 mg of isoniazid daily for 6 months. Estimated costs for treating isoniazid-induced hepatitis were obtained from Pareek et al. 77 All costs were adjusted to 2012/13 prices using the Hospital and Community Health Services pay and price index217 and discounted at a rate of 3.5% per annum, as recommended by NICE. 91
Outcomes
Two different outcome measures were used in the analysis, QALYs and diagnostic error avoided. To calculate QALYs, age-related utility weights for the general population were obtained from the Health Survey for England207 and the utility decrement of 0.15 for people who received treatment for active TB was derived from the published literature. 197 With respect to the diagnostic error avoided, we did not require any effectiveness information; the true-positive and true-negative cases were given the value of 1 and we reserved the value of 0 for an error (false positives and false negatives) in the diagnosis.
Analysis
The models were constructed to assess the cost-effectiveness of various strategies for the diagnosis of LTBI in three populations (children, immunocompromised people and recently arrived immigrants). The models estimated the mean costs and effects associated with each diagnostic strategy. For children, we began with a hypothetical cohort of children aged 5 years, whereas for the recently arrived and immunocompromised populations the starting distributions were representative of the UK recent arrival and UK general populations respectively. 218 The analysis was undertaken from a NHS perspective in a primary care setting and outcomes were reported as ICERs, expressed in terms of cost per diagnostic error avoided and cost per QALY gained. Because using QALYs allows trade-offs between the harms of false negatives and the harms of false positives, which are treated as equal in a cost per error avoided analysis, our primary conclusions are drawn from the ICERs expressed as cost per QALY gained. Univariate sensitivity analyses and PSAs were undertaken to assess the impact of the uncertainty of model input parameters.
Probabilistic sensitivity analysis
A PSA was undertaken to determine the joint uncertainty in the key model input parameters of prevalence, sensitivity and specificity, and expected QALYs. We undertook the PSA based on an outcome of cost per QALY only. In the PSA, each model parameter is assigned a distribution, reflecting the amount and pattern of its variation, and cost-effectiveness results are calculated by simultaneously selecting random values from each distribution. In total, 2000 sets of parameters were simulated, each of which was run on a starting cohort of 100,000 individuals. Because of the considerable heterogeneity of the studies included in our meta-analysis, the results from the PSA, which explicitly includes the impact of that uncertainty, were considered to provide more plausible estimates of costs and outcomes than our single simulation based on mean parameter values. Therefore, costs and outcomes used to produce ICERs were calculated as the means of the costs and outcomes in each of the 2000 PSA simulations. The distributions used in the PSA are presented in Table 27. We also calculated the probability that each strategy is the most cost-effective at a willingness to pay of £20,000 per QALY.
Results of the cost-effectiveness modelling
The results of the cost-effectiveness modelling of various strategies for the diagnosis of LTBI in the three populations, based on the outcomes of cost per diagnostic error avoided and cost per QALY gained, are presented in the following sections.
Model 1: children
The results from the 250,000 patient simulations, based on the mean value of each parameter, are presented in Tables 29 and 30. Table 29 shows the mean per patient cost (including both the initial cohort and subsequent secondary cases) for each of the six strategies as well as a breakdown of the total cost into diagnosis, LTBI treatment, active TB and hepatitis costs. Table 30 shows the incidence rates of active TB in the initial cohort, the numbers of secondary infections, mean life-years and mean QALYs for each of the strategies.
| Strategy | Mean cost (£) | Mean diagnosis cost (£) | Mean LTBI cost (£) | Mean active TB cost (£) | Mean hepatitis cost (£) |
|---|---|---|---|---|---|
| TST (≥ 5 mm) | 362.47 | 58.28 | 192.57 | 111.55 | 0.07 |
| TST (≥ 10 mm) | 298.42 | 48.02 | 119.89 | 130.42 | 0.09 |
| QFT-GIT | 357.38 | 83.61 | 160.22 | 113.48 | 0.07 |
| T-SPOT.TB | 328.97 | 80.90 | 113.21 | 134.76 | 0.10 |
| TST (≥ 5 mm) positive then QFT-GIT | 360.47 | 83.16 | 134.23 | 142.98 | 0.10 |
| TST (≥ 5 mm) negative then QFT-GIT | 389.24 | 114.98 | 196.17 | 78.03 | 0.06 |
| Strategy | Mean QALYs (discounted) | Mean life-years (discounted) | Number of active TB cases (initial cohort) | Number of active TB cases (secondary) |
|---|---|---|---|---|
| TST (≥ 5 mm) | 23.095 | 27.036 | 4722 | 1133 |
| TST (≥ 10 mm) | 23.090 | 27.035 | 5521 | 1332 |
| QFT-GIT | 23.093 | 27.036 | 4804 | 1149 |
| T-SPOT.TB | 23.091 | 27.036 | 5620 | 1349 |
| TST (≥ 5 mm) positive then QFT-GIT | 23.091 | 27.036 | 5653 | 1367 |
| TST (≥ 5 mm) negative then QFT-GIT | 23.097 | 27.037 | 4150 | 996 |
The primary results, based on the 2000 Monte Carlo simulations, are presented in Tables 31 (diagnostic accuracy) and 32 (QALYs). Considering diagnostic accuracy, the TST (≥ 10 mm)-alone strategy dominated the TST (≥ 5 mm) negative followed by QFT-GIT, TST (≥ 5 mm), QFT-GIT and TST (≥ 5 mm) positive followed by QFT-GIT strategies. The TST (≥ 10 mm) strategy had a mean cost of approximately £272 with a corresponding diagnostic error of 0.2449 compared with a mean cost of approximately £306 and a diagnostic error of 0.2322 for the T-SPOT. TB-alone strategy. The ICER for T-SPOT. TB compared with TST (≥ 10 mm) indicates the additional cost required to avoid one diagnostic error. The results for the simultaneous testing strategy and the TST (≥ 10 mm) followed by QFT-GIT are not presented because these results were dominated by the sequential testing strategy and TST (≥ 5 mm) followed by QFT-GIT, respectively.
| Strategy | Mean costa (£) | Incremental cost (£) | False positives | False negatives | Effectiveness (diagnostic error)a | Incremental diagnostic error | ICER (£) |
|---|---|---|---|---|---|---|---|
| TST (≥ 5 mm) negative followed by QFT-GIT | 361.42 | NA | 0.5032 | 0.0040 | 0.5072 | NA | Dominated |
| TST (≥ 5 mm) | 339.26 | –22.16 | 0.4654 | 0.0084 | 0.4740 | –0.0332 | Dominated |
| QFT-GIT | 324.07 | –15.19 | 0.3790 | 0.0091 | 0.3880 | –0.0860 | Dominated |
| TST (≥ 5 mm) positive followed by QFT-GIT | 324.12 | 0.05 | 0.3040 | 0.0154 | 0.3194 | –0.0686 | Dominated |
| TST (≥ 10 mm) | 271.66 | –52.46 | 0.2307 | 0.0142 | 0.2449 | –0.0745 | NA |
| T-SPOT.TB | 306.09 | 34.43 | 0.2172 | 0.0150 | 0.2322 | –0.0127 | 2711.02 |
| Strategy | Mean costa (£) | Incremental cost (£) | Mean QALYsa | Incremental QALYs | ICER (£) | Probability most cost-effectiveb |
|---|---|---|---|---|---|---|
| TST (≥ 10 mm) | 300.21 | NA | 23.088 | NA | NA | 0.032 |
| T-SPOT.TB | 332.46 | 32.25 | 23.091 | 0.003 | Extendedly dominated | 0.122 |
| TST (≥ 5 mm) positive followed by QFT-GIT | 366.45 | 33.99 | 23.092 | 0.001 | Dominated | 0.045 |
| QFT-GIT | 361.03 | –5.42 | 23.095 | 0.002 | 8249 (vs. TST ≥ 10 mm) | 0.210 |
| TST (≥ 5 mm) | 371.14 | 10.09 | 23.096 | 0.001 | 11,255 (vs. QFT-GIT) | 0.269 |
| TST (≥ 5 mm) negative followed by QFT-GIT | 393.03 | 21.89 | 23.097 | 0.001 | 18,871 | 0.322 |
The QALY outcomes of the Monte Carlo simulations showed that the TST (≥ 10 mm) diagnostic strategy alone was the least costly strategy and the TST (≥ 5 mm) negative followed by QFT-GIT was the most effective strategy for the diagnosis of LTBI in this population. The QFT-GIT-alone diagnostic strategy had a mean cost of £361 with corresponding QALYs of 23.095, whereas the TST (≥ 5 mm)-alone strategy had a mean cost of £371 and 23.096 QALYs. The ICER of £11,255 indicates the additional cost required to gain an extra QALY. In terms of the joint uncertainty in the expected mean costs and QALYs, the results show that TST (≥ 5 mm) negative followed by QFT-GIT is the most cost-effective strategy at a willingness to pay of £20,000 per QALY in 32% of the simulations followed by TST (≥ 5 mm) (27%) and QFT-GIT (21%).
The results of the univariate sensitivity analyses are presented in Table 33. In each scenario we present costs and QALYs for each of the three most effective strategies [QFT-GIT, TST (≥ 5 mm) and TST (≥ 5 mm) negative followed by QFT-GIT]. We also show which of the three strategies was the most cost-effective in each scenario, assuming a willingness to pay of £20,000 per QALY. In the majority of scenarios, as in the base case, the TST (≥ 5 mm) negative followed by QFT-GIT strategy was the most cost-effective strategy. However, decreases in prevalence, the sensitivity of the TST, the effectiveness of LTBI treatment or the disutility associated with active TB, as well as increases in the sensitivity of QFT-GIT, all led to QFT-GIT being the most cost-effective option. Conversely, decreases in the sensitivity of QFT-GIT led to the TST (≥ 5 mm) being selected as the most cost-effective option.
| Parameter varied | Value | Cost (QFT-GIT) (£) | QALYs (QFT-GIT) | Cost (TST ≥ 5 mm) (£) | QALYs (TST ≥ 5 mm) | Cost (TST ≥ 5 mm negative then QFT-GIT) (£) | QALYs (TST ≥ 5 mm negative then QFT-GIT) | Most cost-effective strategy (WTP £20,000 per QALY) |
|---|---|---|---|---|---|---|---|---|
| Base case | 361.03 | 23.095 | 371.17 | 23.096 | 393.03 | 23.097 | TST (≥ 5 mm) negative then QFT-GIT | |
| Prevalence | 0.0206 | 329.42 | 23.104 | 336.83 | 23.104 | 363.87 | 23.105 | QFT-GIT |
| 0.0384 | 397.36 | 23.087 | 406.60 | 23.091 | 422.86 | 23.093 | TST (≥ 5 mm) negative then QFT-GIT | |
| Sensitivity: IGRAs | QFT-GIT 0.5856, QFT-GIT following negative TST 0.1122 | 368.16 | 23.089 | 363.76 | 23.096 | 397.13 | 23.095 | TST (≥ 5 mm) |
| QFT-GIT 0.7820, QFT-GIT following negative TST 0.9921 | 369.69 | 23.100 | 357.12 | 23.096 | 388.54 | 32.099 | QFT-GIT | |
| Specificity: IGRAs | QFT-GIT 0.6030, QFT-GIT following negative TST 0.9013 | 368.46 | 23.095 | 363.76 | 23.096 | 393.43 | 23.097 | TST (≥ 5 mm) negative then QFT-GIT |
| QFT-GIT 0.6176, QFT-GIT following negative TST 0.9200 | 354.02 | 23.095 | 379.48 | 23.096 | 393.98 | 23.097 | TST (≥ 5 mm) negative then QFT-GIT | |
| Sensitivity: TST ≥ 5mm | 0.6059 | 361.03 | 23.095 | 379.54 | 23.095 | 395.48 | 23.096 | QFT-GIT |
| 0.7294 | 361.03 | 23.095 | 368.47 | 36.098 | 392.62 | 23.099 | TST (≥ 5 mm) negative then QFT-GIT | |
| Specificity: TST ≥ 5mm | 0.4796 | 361.03 | 23.095 | 374.27 | 23.096 | 395.75 | 23.097 | QFT-GIT |
| 0.5008 | 361.03 | 23.095 | 361.28 | 23.096 | 383.20 | 23.097 | TST (≥ 5 mm) negative then QFT-GIT | |
| Effectiveness of LTBI treatment (proportion of cases of active TB prevented) | 0.392 | 384.94 | 23.092 | 395.23 | 23.093 | 420.81 | 23.093 | QFT-GIT |
| 0.805 | 349.73 | 32.097 | 358.29 | 23.099 | 377.78 | 23.100 | TST (≥ 5 mm) negative then QFT-GIT | |
| Cost of LTBI treatment (£0) | 511.69 | 321.89 | 23.095 | 324.13 | 23.096 | 345.11 | 23.097 | TST (≥ 5 mm) negative then QFT-GIT |
| 842.45 | 400.17 | 23.095 | 418.21 | 23.096 | 440.95 | 23.097 | TST (≥ 5 mm) negative then QFT-GIT | |
| Cost of active TB treatment (£0) | 2664.38 | 302.91 | 23.095 | 314.25 | 23.096 | 343.07 | 23.097 | TST (≥ 5 mm) |
| 9244.44 | 419.15 | 23.095 | 428.09 | 23.096 | 432.99 | 23.097 | TST (≥ 5 mm) negative then QFT-GIT | |
| Utility decrement – active TB | 0.75 | 361.03 | 23.090 | 371.17 | 23.091 | 393.03 | 23.092 | TST (≥ 5 mm) negative then QFT-GIT |
| 0.95 | 361.03 | 23.099 | 371.17 | 23.099 | 393.03 | 23.100 | QFT-GIT | |
| Number of secondary TB cases per index case | 0 | 324.07 | 23.105 | 339.26 | 23.105 | 361.42 | 23.106 | QFT-GIT |
Finally, Figure 56 presents CEACs for each of the same three strategies, showing the proportion of simulations in which each has the highest net benefit at different willingness-to-pay thresholds.
FIGURE 56.
Cost-effectiveness acceptability curves for the child population, showing the proportion of simulations in which each strategy is the most cost-effective at different willingness-to-pay thresholds.

Model 2: immunocompromised people
The results from our 250,000 patient simulations, based on the mean value of each parameter, are presented in Tables 34 and 35. Table 34 shows the mean per patient cost (including both the initial cohort and subsequent secondary cases) for each of the six strategies as well as a breakdown of the total cost into diagnosis, LTBI treatment, active TB and hepatitis costs. Table 35 shows the incidence rates of active TB in the initial cohort, the numbers of secondary infections, mean life-years and mean QALYs for each of the strategies.
| Strategy | Mean cost (£) | Mean diagnosis cost (£) | Mean LTBI cost (£) | Mean active TB cost (£) | Mean hepatitis cost (£) |
|---|---|---|---|---|---|
| TST (≥ 5 mm) | 272.79 | 28.59 | 127.86 | 116.00 | 0.35 |
| TST (≥ 10 mm) | 266.96 | 24.35 | 88.91 | 153.50 | 0.20 |
| QFT-GIT | 252.93 | 58.67 | 97.50 | 96.52 | 0.24 |
| T-SPOT.TB | 287.83 | 61.04 | 134.28 | 92.10 | 0.41 |
| QFT-GIT positive then TST (≥ 5 mm) | 286.49 | 67.91 | 63.95 | 154.51 | 0.12 |
| QFT-GIT negative then TST (≥ 5 mm) | 315.00 | 79.99 | 145.50 | 89.08 | 0.43 |
| Strategy | Mean QALYs (discounted) | Mean life-years (discounted) | Number of active TB cases (initial cohort) | Number of active TB cases (secondary) |
|---|---|---|---|---|
| TST (≥ 5 mm) | 15.527 | 33.018 | 4826 | 1158 |
| TST (≥ 10 mm) | 15.526 | 33.017 | 5228 | 1251 |
| QFT-GIT | 15.532 | 33.018 | 4086 | 987 |
| T-SPOT.TB | 15.532 | 33.018 | 3772 | 902 |
| QFT-GIT positive then TST (≥ 5 mm) | 15.526 | 33.017 | 5271 | 1254 |
| QFT-GIT negative then TST (≥ 5 mm) | 15.534 | 33.018 | 3671 | 886 |
The primary results, based on the 2000 Monte Carlo simulations, are presented in Tables 36 (diagnostic accuracy) and 37 (QALYs). Considering diagnostic accuracy, QFT-GIT dominated the QFT-GIT negative followed by TST (≥ 5 mm), T-SPOT. TB and TST (≥ 5 mm) strategies. The TST (≥ 10 mm) strategy had a mean cost of approximately £236 with a corresponding diagnostic error of 0.1641 whereas the QFT-GIT positive followed by TST (≥ 5 mm) strategy had a mean cost of approximately £253 and a diagnostic error of 0.1047. The ICER of £297 per diagnostic error avoided for the QFT-GIT positive followed by TST (≥ 5 mm) strategy compared with the TST (≥ 10 mm) strategy shows the additional cost required to avoid a diagnostic error. We have not presented the results for the simultaneous testing strategies because these strategies were dominated by the equivalent sequential strategies.
| Strategy | Mean costa (£) | Incremental cost (£) | False positives | False negatives | Effectiveness (diagnostic error)a | Incremental diagnostic error | ICER (£) |
|---|---|---|---|---|---|---|---|
| QFT-GIT negative then TST (≥ 5 mm) | 287.77 | NA | 0.3100 | 0.0066 | 0.3166 | NA | Dominated |
| T-SPOT.TB | 252.01 | –35.76 | 0.3080 | 0.0072 | 0.3152 | –0.0018 | Dominated |
| TST (≥ 5 mm) | 249.33 | –2.68 | 0.2371 | 0.0155 | 0.2526 | –0.0626 | Dominated |
| QFT-GIT | 234.41 | –14.92 | 0.1734 | 0.0084 | 0.1814 | –0.0712 | NA |
| TST (≥ 10 mm) | 236.11 | 1.70 | 0.1474 | 0.0167 | 0.1641 | –0.0173 | 98.27 (vs. QFT-GIT) |
| QFT-GIT positive then TST (≥ 5 mm) | 253.77 | 17.66 | 0.0876 | 0.0171 | 0.1047 | –0.0594 | 297.31 (vs. TST ≥ 10 mm) |
| Strategy | Mean costa (£) | Incremental cost (£) | Mean QALYsa | Incremental QALYs | ICER (£) | Probability most cost-effectiveb |
|---|---|---|---|---|---|---|
| TST (≥ 10 mm) | 269.42 | NA | 15.516 | NA | Dominated | 0.046 |
| QFT-GIT positive then TST (≥ 5 mm) | 289.31 | 19.89 | 15.516 | 0.000 | Dominated | 0.052 |
| TST (≥ 5 mm) | 276.01 | –13.30 | 15.517 | 0.001 | Dominated | 0.067 |
| QFT-GIT | 258.61 | –17.40 | 15.523 | 0.006 | NA | 0.187 |
| T-SPOT.TB | 280.90 | 12.29 | 15.524 | 0.001 | 10,402.63 (vs. QFT-GIT) | 0.249 |
| QFT-GIT negative then TST (≥ 5 mm) | 318.26 | 37.36 | 15.526 | 0.002 | 18,746.01 (vs. T-SPOT.TB) | 0.399 |
The QALY outcomes of our Monte Carlo simulations showed that TST (≥ 10 mm), QFT-GIT positive followed by TST (≥ 5 mm) and TST (≥ 5 mm) were dominated by the QFT-GIT-alone strategy, which had a mean cost of £259 with corresponding QALYs of 15.523. The ICER reported for the T-SPOT. TB-alone strategy shows the additional cost required to gain 1 extra QALY compared with the QFT-GIT strategy. At a willingness to pay of £20,000 per QALY, the QFT-GIT negative followed by TST (≥ 5 mm) had the highest net benefit in the largest proportion of simulations (40%), followed by the T-SPOT. TB (25%) and QFT-GIT-alone (19%) strategies. All other strategies had the largest net benefit in < 7% of the simulations.
The results of the univariate sensitivity analyses are presented in Table 38. In each scenario we present costs and QALYs for each of the three strategies that were not strictly dominated by another strategy in the primary results. We also show which of the three strategies was the most cost-effective in each scenario, assuming a willingness to pay of £20,000 per QALY. In the scenarios in which the importance of test sensitivity was equal to or higher than that in the base case, the QFT-GIT negative followed by TST (≥ 5 mm) strategy was consistently the most cost-effective strategy at a willingness to pay of £20,000 per QALY. In the scenarios in which the relative importance of test specificity was increased (by decreasing LTBI prevalence, decreasing the effectiveness of LTBI treatment, increasing the cost of LTBI treatment, decreasing the cost of active TB or ignoring the impact of secondary TB cases), QFT-GIT often became the most cost-effective strategy.
| Parameter varied | Value | Cost (QFT-GIT) (£) | QALYs (QFT-GIT) | Cost (T-SPOT.TB) (£) | QALYs (T-SPOT.TB) | Cost (QFT-GIT negative then TST ≥ 5 mm) (£) | QALYs (QFT-GIT negative then TST ≥ 5 mm) | Most cost-effective strategy (WTP £20,000 per QALY) |
|---|---|---|---|---|---|---|---|---|
| Base case | 258.61 | 15.523 | 280.90 | 15.524 | 318.26 | 15.526 | QFT-GIT negative then TST (≥ 5 mm) | |
| Prevalence | 0.0152 | 228.77 | 15.537 | 258.47 | 15.537 | 293.19 | 15.539 | QFT-GIT |
| 0.0306 | 301.73 | 15.508 | 315.09 | 15.510 | 355.47 | 15.513 | QFT-GIT negative then TST (≥ 5 mm) | |
| Sensitivity: IGRAs | QFT-GIT 0.2473, T-SPOT.TB 0.3517 | 275.95 | 15.516 | 295.74 | 15.517 | 330.35 | 15.522 | QFT-GIT negative then TST (≥ 5 mm) |
| QFT-GIT 0.8373, T-SPOT.TB 0.9144 | 243.54 | 15.529 | 271.36 | 15.530 | 308.81 | 15.531 | QFT-GIT | |
| Specificity: IGRAs | QFT-GIT 0.8052, T-SPOT.TB 0.6346 | 268.55 | 15.523 | 305.26 | 15.524 | 324.82 | 15.526 | QFT-GIT negative then TST (≥ 5 mm) |
| QFT-GIT 0.8396, T-SPOT.TB 0.7331 | 247.43 | 15.523 | 268.69 | 15.524 | 312.34 | 15.526 | QFT-GIT | |
| Sensitivity: TST ≥ 5 mm | TST following negative IGRA 0.0121 | 258.61 | 15.523 | 280.90 | 15.524 | 321.89 | 15.526 | QFT-GIT negative then TST (≥ 5 mm) |
| TST following negative IGRA 0.7989 | 258.61 | 15.523 | 280.90 | 15.524 | 314.87 | 15.526 | QFT-GIT negative then TST (≥ 5 mm) | |
| Specificity: TST ≥ 5 mm | TST following negative IGRA 0.3909 | 258.61 | 15.523 | 280.90 | 15.524 | 342.16 | 15.526 | T-SPOT.TB |
| TST following negative IGRA 0.4993 | 258.61 | 15.523 | 280.90 | 15.524 | 291.20 | 15.526 | QFT-GIT negative then TST (≥ 5 mm) | |
| Effectiveness of LTBI treatment (proportion of cases of active TB prevented) | 0.392 | 272.49 | 15.518 | 294.85 | 15.519 | 334.58 | 15.521 | QFT-GIT |
| 0.805 | 249.77 | 15.528 | 273.12 | 15.530 | 309.56 | 15.534 | QFT-GIT negative then TST (≥ 5 mm) | |
| Cost of LTBI treatment (£) | 511.69 | 235.90 | 15.523 | 249.62 | 15.524 | 284.37 | 15.526 | QFT-GIT negative then TST (≥ 5 mm) |
| 842.45 | 281.32 | 15.523 | 312.18 | 15.524 | 352.15 | 15.526 | QFT-GIT | |
| Cost of active TB treatment (£) | 2664.38 | 207.18 | 15.523 | 233.73 | 15.524 | 272.64 | 15.526 | QFT-GIT |
| 9244.44 | 323.48 | 15.523 | 344.70 | 15.524 | 379.97 | 15.526 | QFT-GIT negative then TST (≥ 5 mm) | |
| Utility decrement – active TB | 0.75 | 258.61 | 15.520 | 280.90 | 15.522 | 318.26 | 15.524 | QFT-GIT negative then TST (≥ 5 mm) |
| 0.95 | 258.61 | 15.526 | 280.90 | 15.526 | 318.26 | 15.528 | QFT-GIT negative then TST (≥ 5 mm) | |
| Number of secondary TB cases per index case | 0 | 234.41 | 15.536 | 252.01 | 15.536 | 287.77 | 15.38 | QFT-GIT |
Finally, Figure 57 presents CEACs for each of the three non-dominated treatment strategies, showing the proportion of simulations in which each has the highest net benefit at different willingness-to-pay thresholds.
FIGURE 57.
Cost-effectiveness acceptability curves for the immunocompromised population, showing the proportion of simulations in which each strategy is the most cost-effective at different willingness-to-pay thresholds.

Model 3: recent arrivals from countries with a high incidence of tuberculosis
The results from our 250,000 patient simulations, based on the mean value of each parameter, are presented in Tables 39 and 40. Table 39 shows the mean per patient cost (including both the initial cohort and subsequent secondary cases) for each of the six strategies as well as a breakdown of the total cost into diagnosis, LTBI treatment, active TB and hepatitis costs. Table 40 shows the incidence rates of active TB in the initial cohort, the numbers of secondary infections, mean life-years and mean QALYs for each of the strategies.
| Strategy | Mean cost (£) | Mean diagnosis cost (£) | Mean LTBI cost (£) | Mean active TB cost (£) | Mean hepatitis cost (£) |
|---|---|---|---|---|---|
| TST (≥ 5 mm) | 310.00 | 34.19 | 203.04 | 72.09 | 0.68 |
| QFT-GIT | 295.11 | 57.72 | 114.42 | 122.50 | 0.47 |
| T-SPOT.TB | 432.95 | 77.45 | 259.89 | 94.74 | 0.86 |
| TST (≥ 5 mm) positive then QFT-GIT | 310.83 | 78.88 | 101.04 | 130.07 | 0.84 |
| TST (≥ 5 mm) negative then QFT-GIT | 363.64 | 74.15 | 219.87 | 68.91 | 0.72 |
| Strategy | Mean QALYs (discounted) | Mean life-years (discounted) | Number of active TB cases (initial cohort) | Number of active TB cases (secondary) |
|---|---|---|---|---|
| TST (≥ 5 mm) | 19.929 | 24.160 | 2883 | 705 |
| QFT-GIT | 19.924 | 24.158 | 4329 | 1041 |
| T-SPOT.TB | 19.922 | 24.158 | 4289 | 998 |
| TST (≥ 5 mm) positive then QFT-GIT | 19.915 | 24.157 | 4522 | 1091 |
| TST (≥ 5 mm) negative then QFT-GIT | 19.931 | 24.160 | 2756 | 660 |
The primary results, based on the 2000 Monte Carlo simulations, are presented in Tables 41 (diagnostic accuracy) and 42 (QALYs). Considering diagnostic accuracy, the QFT-GIT-alone strategy was the least costly strategy and the TST (≥ 5 mm) positive followed by QFT-GIT strategy was the most effective. The QFT-GIT strategy had a mean cost of approximately £266 with a corresponding diagnostic error of 0.2113, whereas the TST (≥ 5 mm) positive followed by QFT-GIT strategy had a mean cost of approximately £277 and a diagnostic error of 0.1955. The ICER for the TST (≥ 5 mm) positive followed by QFT-GIT strategy compared with the QFT-GIT-alone strategy shows an additional cost of £692 to avoid one diagnostic error. We have not presented the results for the simultaneous testing strategies because these strategies were dominated by the equivalent sequential strategies.
| Strategy | Mean costa (£) | Incremental cost (£) | False positives | False negatives | Effectiveness (diagnostic error)a | Incremental diagnostic error | ICER (£) |
|---|---|---|---|---|---|---|---|
| T-SPOT.TB | 374.60 | NA | 0.5669 | 0.0071 | 0.5740 | NA | Dominated |
| TST (≥ 5 mm) negative then QFT-GIT | 325.81 | –48.79 | 0.4680 | 0.0016 | 0.4696 | –0.1044 | Dominated |
| TST (≥ 5 mm) | 277.46 | –48.35 | 0.4566 | 0.0025 | 0.4391 | –0.0305 | Dominated |
| QFT-GIT | 265.87 | –11.59 | 0.2015 | 0.0098 | 0.2113 | –0.2278 | NA |
| TST (≥ 5 mm) positive then QFT-GIT | 276.80 | 10.93 | 0.1846 | 0.0109 | 0.1955 | –0.0158 | 691.77 |
| Strategy | Mean costa (£) | Incremental cost (£) | Mean QALYsa | Incremental QALYs | ICER (£) | Probability most cost-effectiveb |
|---|---|---|---|---|---|---|
| TST (≥ 5 mm) positive then QFT-GIT | 300.10 | NA | 19.909 | NA | Dominated | 0.032 |
| T-SPOT.TB | 400.12 | 100.02 | 19.915 | 0.006 | Dominated | 0.042 |
| QFT-GIT | 291.13 | –108.99 | 19.917 | 0.002 | NA | 0.177 |
| TST (≥ 5 mm) | 298.75 | 7.62 | 19.922 | 0.005 | 1524 (vs. QFT-GIT) | 0.469 |
| TST (≥ 5 mm) negative then QFT-GIT | 353.47 | 54.72 | 19.923 | 0.001 | 58,720 (vs. TST ≥ 5 mm) | 0.280 |
The QALY outcomes of our Monte Carlo simulations showed that the QFT-GIT strategy dominated the TST (≥ 5 mm) positive followed by QFT-GIT and T-SPOT. TB strategies. TST (≥ 5 mm) had a mean cost of £299 with corresponding QALYs of 19.922. TST (≥ 5 mm) negative followed by QFT-GIT was more expensive than the TST (≥ 5 mm) strategy, with corresponding QALYs of 19.923 and an ICER of £58,720 compared with TST (≥ 5 mm). At a willingness to pay of £20,000 per QALY, the TST (≥ 5 mm) strategy had the highest net benefit in the largest proportion of simulation (47%) followed by the TST (≥ 5 mm) negative then QFT-GIT strategy (28%) and the QFT-GIT-alone strategy (18%). All other strategies had the largest net benefit in < 5% of the simulations.
The results of the univariate sensitivity analyses are presented in Table 43. In each scenario we present costs and QALYs for the three strategies that were not strictly dominated by another strategy in the primary results. We also show which of the three strategies was the most cost-effective in each scenario, assuming a willingness to pay of £20,000 per QALY. In the majority of scenarios, as in our base case, the TST (≥ 5 mm)-alone strategy was the most cost-effective strategy. However, a decrease in the prevalence of LTBI, increase in the sensitivity of QFT-GIT and decrease in the sensitivity of the TST all led to strategies involving QFT-GIT becoming the most cost-effective.
| Parameter varied | Value | Cost (QFT-GIT) (£) | QALYs (QFT-GIT) | Cost (TST ≥ 5 mm) (£) | QALYs (TST ≥ 5 mm) | Cost (TST ≥ 5 mm negative then QFT-GIT) (£) | QALYs (TST ≥ 5 mm negative then QFT-GIT) | Most cost-effective strategy (WTP £20,000 per QALY) |
|---|---|---|---|---|---|---|---|---|
| Base case | 291.13 | 19.917 | 298.75 | 19.922 | 353.47 | 19.923 | TST (≥ 5 mm) | |
| Prevalence | 0.0150 | 250.19 | 19.930 | 271.80 | 19.931 | 326.65 | 19.932 | QFT-GIT |
| 0.0345 | 342.56 | 19.904 | 331.53 | 19.910 | 389.21 | 19.912 | TST (≥ 5 mm) | |
| Sensitivity: IGRAs | QFT-GIT 0.3584, QFT-GIT following negative TST 0.0225 | 309.31 | 19.913 | 298.75 | 19.922 | 354.82 | 19.922 | TST (≥ 5 mm) |
| QFT-GIT 0.8172, QFT-GIT following negative TST 0.9724 | 271.22 | 19.921 | 298.75 | 19.922 | 353.18 | 19.923 | QFT-GIT | |
| Specificity: IGRAs | QFT-GIT 0.7780, QFT-GIT following negative TST 0.9555 | 299.23 | 19.917 | 298.75 | 19.922 | 355.66 | 19.923 | TST (≥ 5 mm) |
| QFT-GIT 0.8073, QFT-GIT following negative TST 0.9893 | 283.62 | 19.918 | 298.75 | 19.922 | 349.92 | 19.923 | TST (≥ 5 mm) | |
| Sensitivity: TST ≥ 5 mm | 0.7786 | 291.13 | 19.917 | 303.86 | 19.920 | 354.48 | 19.922 | TST (≥ 5 mm) negative then QFT-GIT |
| 0.9977 | 291.13 | 19.917 | 297.08 | 19.924 | 352.08 | 19.924 | TST (≥ 5 mm) | |
| Specificity: TST ≥ 5 mm | 0.4790 | 291.13 | 19.917 | 311.44 | 19.922 | 363.91 | 19.923 | TST (≥ 5 mm) |
| 0.5229 | 291.13 | 19.917 | 288.84 | 19.922 | 344.32 | 19.923 | TST (≥ 5 mm) | |
| Effectiveness of LTBI treatment | 0.392 | 302.35 | 19.915 | 311.22 | 19.918 | 369.71 | 19.919 | TST (≥ 5 mm) |
| 0.805 | 283.73 | 19.919 | 279.48 | 19.925 | 334.96 | 19.926 | TST (≥ 5 mm) | |
| Cost of LTBI treatment (£) | 511.69 | 264.48 | 19.917 | 251.46 | 19.922 | 302.26 | 19.923 | TST (≥ 5 mm) |
| 842.45 | 317.78 | 19.917 | 346.04 | 19.922 | 404.68 | 19.923 | TST (≥ 5 mm) | |
| Cost of active TB treatment (£) | 2664.38 | 228.40 | 19.917 | 261.83 | 19.922 | 318.18 | 19.923 | TST (≥ 5 mm) |
| 9244.44 | 375.99 | 19.917 | 348.69 | 19.922 | 401.21 | 19.923 | TST (≥ 5 mm) | |
| Utility decrement – active TB | 0.75 | 291.13 | 19.911 | 298.75 | 19.917 | 353.47 | 19.918 | TST (≥ 5 mm) |
| 0.95 | 291.13 | 19.923 | 298.75 | 19.926 | 353.47 | 19.927 | TST (≥ 5 mm) | |
| Number of secondary TB cases per index case | 0 | 265.87 | 19.928 | 277.46 | 19.931 | 325.81 | 19.932 | TST (≥ 5 mm) |
Finally, Figure 58 presents CEACs for each of the three non-dominated treatment strategies, showing the proportion of simulations in which each has the highest net benefit at different willingness-to-pay thresholds.
FIGURE 58.
Cost-effectiveness acceptability curves for the recently arrived population, showing the proportion of simulations in which each strategy is the most cost-effective at different willingness-to-pay thresholds.

Exploring sensitivity and specificity
Clearly, key drivers of differences between the models are sensitivity and specificity. To illustrate the impact that these parameters have on the outputs of our models, Figure 59 shows graphs of sensitivity and specificity plotted against costs, QALYs and net monetary benefit (at £20,000 per QALY) for each of the six strategies that were simulated in the child population.
FIGURE 59.
Sensitivity and specificity plotted against costs, QALYs and net monetary benefit (NMB) (at £20,000 per QALY) for each of the six strategies in the child population. (a) Sensitivity against QALYs; (b) specificity against QALYs; (c) sensitivity against costs; (d) specificity against costs; (e) sensitivity against NMB; and (f) specificity against NMB. NMB, net monetary benefit.
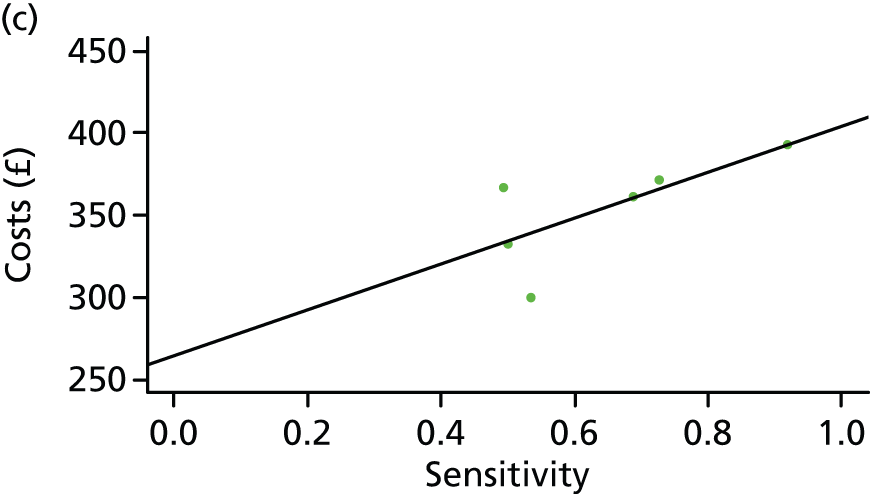



These graphs show, at first sight, the counterintuitive result that increased specificity is associated with lower QALYs and lower net monetary benefit whereas higher sensitivity is associated with higher costs. This is because of the high levels of correlation between sensitivity and specificity (specifically, higher sensitivity is associated with lower specificity) in the strategies that were simulated. Therefore, both sets of graphs are in fact showing the same result, namely that, as sensitivity increases and specificity decreases, this leads to higher QALYs, higher costs and, on balance, a higher net monetary benefit.
To try and remove the effect of this sensitivity/specificity correlation, instead of using the different strategies we used the outputs of the PSA simulations for one of these strategies. This gave us 2000 realisations of sensitivity, specificity, costs and QALYs and, as each of these sensitivity/specificity pairs was a sample from the posterior distribution of our MCMC, we would expect lower correlations between sensitivity and specificity than when comparing between different strategies. We then ran a linear regression model for costs and QALYs, with sensitivity and specificity as the predictor variables. The results of this regression model are shown in Table 44.
| Parameter | Costs (£) | QALYs |
|---|---|---|
| Intercept | 578.72 | 23.080 |
| Sensitivity | –0.99 | 0.00015 |
| Specificity | –2.60 | 0.00001 |
In this model, in which we jointly estimated the impact of both sensitivity and specificity on outcomes, the results are much more intuitive. Increases in both sensitivity and specificity lead to increases in QALYs and decreases in costs, with increases in sensitivity providing the largest QALY gains and increases in specificity the largest cost reductions. It should be noted that the output data from the PSA simulation very likely do not conform to the necessary assumptions (linearity, additivity, etc.) for linear regression and the models contain a lot of noise because of the impact of varying other parameters and so the actual values of these parameters should be treated with extreme caution. Nevertheless, they do give an indicative picture of what the key drivers of difference between the models are.
Discussion and conclusion
The results based on the outcome of cost per diagnostic error avoided showed that, in the child population, the TST (≥ 10 mm) strategy dominated all strategies except for the T-SPOT. TB strategy alone. T-SPOT. TB compared with TST (≥ 10 mm) was more effective but more expensive, with an ICER of approximately £2711 per diagnostic error avoided. A breakdown of effectiveness showed that the T-SPOT. TB strategy resulted in fewer false-positive cases (0.2172) than the TST (≥ 10 mm) strategy (0.2307), but a larger number of false-negative cases (0.0150 vs. 0.0142). If the T-SPOT. TB strategy were to be used in this population to diagnose LTBI that progresses to active TB, this would lead to a slight reduction in the number of children being overtreated for LTBI. In the immunocompromised population, QFT-GIT dominated QFT-GIT negative followed by TST, T-SPOT. TB and TST (≥ 5 mm) in terms of diagnostic errors avoided. The results showed that QFT-GIT resulted in fewer false positives and fewer false negatives than the other strategies. The use of TST (≥ 10 mm) in this population was more effective, with overall diagnostic errors avoided of 0.1641. A breakdown of this effectiveness showed that TST (≥ 10 mm) resulted in fewer false-positive but more false-negative results. Likewise, the combination strategy QFT-GIT positive followed by TST (≥ 5 mm) produced fewer false-positive results but more false-negative results. In the population of recent arrivals from countries with a high incidence of TB, QFT-GIT dominated the T-SPOT. TB, TST (≥ 5 mm) negative followed by QFT-GIT and TST (≥ 5 mm) strategies. TST (≥ 5 mm) positive followed by QFT-GIT had an ICER of £692 per diagnostic error avoided compared with QFT-GIT, with more false negatives and fewer false positives.
The cost per QALY outcomes are summarised in terms of the probability of each strategy being the most cost-effective (at a given threshold). We used a willingness-to-pay threshold of £20,000 per QALY, a standard threshold that is used in the UK. The results in the child population showed that TST (≥ 5 mm) is marginally more effective than the QFT-GIT-alone strategy, with an ICER of approximately £11,255 per QALY, and has a 27% probability of being the most cost-effective strategy at a willingness to pay of £20,000 per QALY. The most effective strategy was TST (≥ 5 mm) negative followed by QFT-GIT, which was the most cost-effective strategy in 32% of the simulations. The results in the immunocompromised population showed that QFT-GIT negative followed by TST (≥ 5 mm) was the most effective strategy, with an ICER of approximately £18,746 compared with T-SPOT. TB, and is the most cost-effective strategy in 40% of the simulations. In the population of recent arrivals, TST (≥ 5 mm) dominated the TST (≥ 5 mm) positive followed by QFT-GIT, T-SPOT. TB and QFT-GIT strategies, and had a probability of 47% of being cost-effective at a willingness to pay of £20,000 per QALY.
Based on the current clinical evidence on people with LTBI without treatment that progressed to active TB as well as expert opinion used to develop the model structures, the results demonstrate that TST (≥ 5 mm) was slightly more cost-effective than QFT-GIT in the child population. In the immunocompromised population the results based on cost per QALY showed that QFT-GIT negative followed by TST (≥ 5 mm) was the most cost-effective strategy. In the recent arrivals population the results based on cost per QALY showed that TST (≥ 5 mm) dominated the TST (≥ 5 mm) positive followed by QFT-GIT, T-SPOT. TB and QFT-GIT-alone strategies.
Chapter 7 Discussion
The purpose of the current review was to compare the clinical effectiveness and cost-effectiveness of new screening tests for LTBI (IGRAs vs. TST) in children, people who are immunocompromised or at risk from immunosuppression and recent arrivals from countries with a high incidence of TB. We aimed to address the following questions:
-
Which diagnostic strategy is most clinically effective and cost-effective in accurately identifying LTBI in children?
-
Which diagnostic strategy is most clinically effective and cost-effective in accurately identifying LTBI in people who are immunocompromised or at risk of immunosuppression?
-
Which diagnostic strategy is most clinically effective and cost-effective in accurately identifying LTBI in people who are recent arrivals from countries with a high incidence of TB?
In this chapter, the principal findings of the clinical effectiveness and cost-effectiveness reviews and economic evaluation are interpreted alongside an assessment of the strengths and limitations of the review and the individual studies. Areas of uncertainty, implications for further research and implications for practice are highlighted.
Main findings
Clinical effectiveness review
There is no gold standard for the accurate diagnosis of LTBI. The existing screening tests for LTBI (IGRAs and TST) provide indirect assessment of the presence of LTBI by relying on a host’s immunological response to TB antigens. The evaluation of the comparative effectiveness of IGRAs and TST in accurately identifying LTBI has been a challenging task because of the absence of a gold standard for direct estimation of the screening tests’ accuracy indices (i.e. sensitivity and specificity) and the tests’ own limitations. 11–13,16,27,56,57 To address this issue, many studies have tried to estimate and compare the measures of association between the test results (i.e. TST and/or IGRAs) and constructs of validity for LTBI (e.g. duration/proximity of exposure to a person with active TB, risk of development of active TB). 11,18,58,60
This review identified and appraised a large amount of evidence (53 new studies since CG11710 and 37 studies from CG11710) comparing IGRAs with TST for identifying LTBI in children, immunocompromised people and recently arrived immigrants from countries with a high TB incidence. Overall, the limited evidence from prospective studies in children showed no significant difference in the performance of QFT-GIT and TST 5 mm in predicting LTBI. However, QFT-GIT was significantly better than TST 10 mm in predicting LTBI. In children, IGRAs (QFT-GIT/G) demonstrated similar sensitivity to and slightly better specificity than those of TST 5 mm. Moreover, IGRAs tended to have a greater sensitivity but lower specificity than those of TST 10 mm/15 mm. As the predictive value of a test is a function of its sensitivity, the greater predictive ability of IGRAs than of TST 10 mm in predicting LTBI (as a proxy for developing active TB) could be explained by the better sensitivity of the former. Based on the exposure studies in children, IGRAs outperformed the TST in identifying LTBI in the setting of low TB burden but not in the setting of high TB burden. This finding is consistent with a growing body of evidence showing reduced sensitivity and specificity of IGRAs in high compared with low TB burden areas, the former represented mostly by developing countries where BCG vaccination is given at birth. 44,59,219–221 This heterogeneity in test performance could be explained by a higher frequency of exposure to MTB, different transmission dynamics, malnutrition, comorbidity, people coinfected with HIV, exposure to NTMs and helminthic infection in high TB burden settings. 105,220,221 Moreover, in high TB burden settings (mostly developing countries), the specificity of the TST is not greatly reduced because BCG vaccination is given mostly at birth without repeating it. In contrast, in some low TB burden settings (e.g. developed countries), BCG vaccination with booster shots may be offered after infancy, which is known to compromise TST specificity. 220
Evidence comparing IGRAs with TST in predicting the incidence of active TB in immunocompromised people was insufficient and inconclusive. The forest plot of 21 exposure-based studies showed a large variation in the performance of IGRAs compared with the TST across different clinical subgroups. In general, QFT-GIT and T-SPOT. TB performed better than TST 5 mm/10 mm in identifying LTBI among people undergoing haemodialysis and in those with hepatitis C. In contrast, in patients with HIV/AIDS, QFT-GIT was significantly worse than TST 10 mm at identifying LTBI. One explanation of this finding could be the reduced sensitivity of IGRA to detect LTBI because of CD4+ T-lymphocyte depletion in those with HIV-induced immunosuppression, leading to a high proportion of indeterminate IGRA results. Interestingly, it is not clear whether or not QFT-GIT and TST are differentially affected by CD4 depletion. 40,220,222,223 Evidence on the comparative performance of IGRAs and TST in people with lupus erythematosus, those with immune-mediated inflammatory diseases before antiTNF-α therapy, solid organ transplantation candidates and kidney transplant recipients was inconclusive because of the high level of uncertainty around the statistically non-significant effect estimates. The agreement between IGRAs and the TST in immunocompromised people was low.
There was no significant difference in the performance of the IGRAs and TST in identifying LTBI among recently arrived people from countries with a high TB burden. QFT-GIT demonstrated greater specificity but lower sensitivity than the TST. Similarly, there was no evidence indicating a differential effect of BCG vaccination on IGRA (QFT, T-SPOT. TB) and TST positivity. Limited evidence indicated that both concordance and kappa were greater among BCG-unvaccinated people (or among people who have/have not been vaccinated) than among BCG-vaccinated people.
In general, the degree of agreement (measured by the kappa statistic) between the IGRAs and the TST across the three subgroups of children, immunocompromised people and those recently arrived from high TB burden areas was low. Several studies indicated better between-test (IGRAs vs. TST) concordance and agreement in unvaccinated than in BCG-vaccinated people. The higher rates of discordance between the IGRAs and the TST in BCG-vaccinated populations could be explained by the TST having reduced specificity (i.e. a higher false-positive rate) because of its cross-reactivity with antigens that are common to both MTB and the BCG vaccine. 219 Overall, there was no clear and convincing evidence indicating a differential effect of BCG vaccination on IGRA and TST positivity. The evidence, if reported, was conflicting and inconclusive, with most studies indicating non-significant differences in the odds of test positivity (with great uncertainties) for the IGRAs and TST between BCG-vaccinated and BCG non-vaccinated people.
Cost-effectiveness review
Ten studies10,77,196–203 reported evidence on decision-analytical models to determine the cost-effectiveness of IGRAs compared with TST for the diagnosis of LTBI in the three populations of interest. The majority of these models were in the immunocompromised population. These results highlight that there is a paucity of evidence available for children and recently arrived populations. The majority of the models used decision tree structures with Markov nodes to simulate a cohort of people being tested for LTBI.
We appraised these models against frameworks for best practice for reporting model-based economic evaluations. All performed well in terms of defining the decision problem, including the study perspective, outlining the choice of comparators, presenting an illustrative model structure and providing a clear outline of the assumptions. These models all add insight to the existing literature but were subject to some limitations. First, the majority of the studies stated the location of the study but not the setting of the analysis and this may limit the generalisability of the results. Second, the majority of the studies used QALYs as the outcome measure but did not elaborate on the descriptive tool used to value health states. Third, the perspective of the analysis was stated in all studies but the resource use and costs reported did not reflect the viewpoint of the analysis in some studies. Finally, all models explored uncertainty around key model input parameters but no attempt was made to explore methodological, generalisability or structural uncertainty. Other concerns relate to the derivation of prevalence, test accuracy and transition probabilities; most studies did not elaborate on these statistical/pre-model analyses.
Economic evaluation
In the child population, the TST negative followed by QFT-GIT strategy had the lowest proportion of false-negative results and the T-SPOT. TB strategy had the lowest proportions of false-positive results and overall errors. TST (≥ 10 mm) was the strategy with the lowest overall cost whereas TST (≥ 5 mm) negative followed by QFT-GIT produced the highest QALYs, was the most cost-effective at a willingness to pay of £20,000 per QALY and had the highest probability of being the most cost-effective strategy.
In the immunocompromised population, the QFT-GIT negative followed by TST (≥ 5 mm) strategy had the lowest proportion of false-negative results and the QFT-GIT positive followed by TST (≥ 5 mm) strategy had the lowest proportions of false-positive results and overall errors. QFT-GIT was the strategy with the lowest overall cost whereas the QFT-GIT negative followed by TST (≥ 5 mm) strategy produced the highest QALYs, was the most cost-effective at a willingness to pay of £20,000 per QALY and had the highest probability of being the most cost-effective strategy.
In the recently arrived population, the TST (≥ 5 mm) negative followed by QFT-GIT strategy had the lowest proportion of false-negative results and the TST (≥ 5 mm) positive followed by QFT-GIT strategy had the lowest proportions of false-positive results and overall errors. QFT-GIT was the strategy with the lowest overall cost, the TST (≥ 5 mm) negative followed by QFT-GIT strategy produced the highest QALYs and the TST (≥ 5 mm) strategy was the most cost-effective at a willingness to pay of £20,000 per QALY and had the highest probability of being the most cost-effective strategy.
Current findings compared with those from other systematic reviews
In general, our findings agreed with those from three other systematic reviews59,91,221 in showing that IGRAs have improved specificity and a greater ability to predict LTBI relative to the TST in the setting of low (but not high) TB burden in children. All three previous reviews also highlight the lack or insufficient amount of evidence and heterogeneity in estimates, methodology and clinical characteristics across the studies that were reviewed.
The findings of this review could not be directly compared with those of several previously published systematic reviews for the following reasons: (1) our review results were stratified by children, immunocompromised people and those recently arrived from high TB burden countries, whereas other reviews18,44,57,58,219,224 did not analyse these three populations; (2) unlike other studies40,219,222 we did not use prevalent culture-positive active TB as a proxy for LTBI; (3) one review224 included in-house IGRAs, which we did not; (4) one review222 compared QFT-GIT with T-SPOT. TB only; and (5) two reviews225,226 reported no relevant outcomes.
Current results compared with those from other cost-effectiveness studies
When comparing our model with others from the literature, it is important to note that our definitions of sensitivity and specificity are not the same as those used in most studies. In the absence of a gold standard we have used LTBI that progresses to active TB, rather than any LTBI, as in previously published papers, and hence the numbers derived for sensitivity and specificity are not comparable. In addition, most of the previously published papers did not include sequential testing as a possible strategy and so we have to restrict our comparisons to the results for the TST- and IGRA-alone strategies only.
In the immunocompromised population, previous studies196,198,200,202 indicated that, when using a single test, IGRAs were preferable to TST, a conclusion that our results concur with. In the child population our results agree with those of Mandalakas et al. 203 in that the TST (≥ 5 mm) negative followed by IGRA strategy was the most effective; however, they disagree with those of Kowada197 who found QFT-GIT to be more cost-effective than the TST, the opposite of our conclusion. Finally, in the recently arrived population, Pareek et al. 77 found QFT-GIT to be more cost-effective than the TST whereas we found the reverse, with the TST (≥ 5 mm) strategy being the most cost-effective strategy.
The reasons for these differences, other than those that always apply (different populations modelled, different parameter values used, etc.), can be found in the different underlying structures of the models. First, Kowada197 considered only primary cases of TB and not secondary infections. From our univariate sensitivity analyses in the child population we see that, when we set our secondary infection rate to zero, we also find the QFT-GIT strategy to be the most cost-effective strategy. When comparing IGRAs with the TST in the recently arrived population, Pareek et al. 77 used indurations of 10 mm and 6/15 mm (stratified by BCG status). Our results for the recently arrived population are based on an induration of 5 mm, a value not modelled in the Pareek et al. 77 study, and therefore differences in conclusions may be explained by these different thresholds used.
It is important to note that our model is designed to evaluate only which is the most cost-effective diganostic strategy, conditional on a decision having been made to test. It does not say anything about whether or not testing itself, compared with no testing, is cost-effective and should be undertaken in these populations. Research addressing this question (testing/no testing) has recently been published. 210 This model and our model were built to address fundamentally different questions, in different populations, and hence the results obtained from them cannot be directly compared. In particular, the inclusion criteria for studies in the two reviews were entirely different (our criteria included only studies on TSTs vs. IGRAs whereas their criteria included only studies on treatment vs. no treatment) and hence papers included in one review will have been specifically excluded from the other.
Considering parameter inputs to the models, identical parameter values were used for the effectiveness of LTBI treatment and case fatality rates for active TB, with very similar values used for the costs of active TB, differing by only 2%. The costs of managing hepatitis differed more substantially (by around £200), but as isoniazid-induced hepatitis contributed only a small fraction to the costs in our model this is unlikely to make a major impact. As progression to active TB was calculated using different methods in the two models, it is not possible to compare the input parameters directly. However, by restricting the comparison to a subsample of the full population that can be extracted from both models, we can compare the number of active TB cases that each predicts. In particular, for a sample of patients aged 51–65 years with a positive TST result, the White and Jit210 model predicts 2091 active TB cases per 100,000 in treated patients and 5928 active TB cases per 100,000 untreated patients. Our model, in contrast, predicts 1736 cases per 100,000 treated patients and 5372 cases per 100,000 untreated patients. These differences are most likely explained simply by the different data used to populate the two models. However, if the incidence rate used in the White and Jit210 study is believed to be more accurate, this would have the effect of increasing the prevalance of LTBI in our starting population, the net effect of which can be explored from our univariate sensitivity analyses.
Strengths and limitations of the evidence
The assessment, comparison and interpretation of the clinical effectiveness of the existing tests in identifying LTBI is hampered by the absence of a gold standard for diagnosing LTBI. The evidence relied mostly on indirect measures of association derived between the test results (i.e. TST and/or IGRAs) and constructs of validity for LTBI (e.g. duration/proximity of exposure to a person with active TB, risk of development of active TB). Moreover, the existing commercially available screening tests for LTBI are imperfect in that they measure a host’s immunological response to TB antigens, which may be affected by a number of factors other than LTBI and which differ from study to study (such as previous BCG vaccination, inter-/intrarater variability in interpretation of test results, boosting, conversion, reversion, different cut-offs for test positivity, assay manufacturing, pre-analytical processing and/or incubation delay). Thus, the findings of this review warrant a cautious interpretation.
Although we appraised and summarised a large amount of evidence, much of it was inconclusive because of unexplained heterogeneity in the effect estimates, poor reporting, missing data and great uncertainty around the effect estimates for the association between test results and the constructs of validity for LTBI. One of the difficulties in the assessment and interpretation of test performance (IGRAs vs. TST) in correctly detecting LTBI is the inconsistent use of definitions for high compared with low risk for LTBI (i.e. construct of validity). The heterogeneity in the measures of association between test results and previous exposure to TB observed even at within-study level could be the result of inadequate definition of the construct of validity for LTBI (e.g. previous exposure definition may not represent the true presence of LTBI), exposure misclassification (e.g. not all people exposed to a TB case will become infected) or both. Furthermore, some but not all of the observed heterogeneity in the parameters of test performance (e.g. sensitivity, specificity, DORs, between-test agreement) could be explained by study setting, type of population, type of test and the outcome characteristics. Some heterogeneity, especially with regard to the sensitivity and specificity estimates derived from previous TB exposure-based categories, could not be explained, thereby rendering some of our findings inconclusive. These factors were compounded by the scarcity of evidence in analyses stratified by population, type of IGRA test and TST threshold.
Another concern in interpreting the evidence relates to the ROB and methodological quality of the individual studies. In general, most studies were rated as being at high or moderate ROB (incidence studies) or of low methodological quality (exposure studies). Apart from the issues highlighted above, various sources of bias may have independently distorted the review findings and their interpretation. For example, results from the studies that we reviewed may have been biased because of diagnostic review bias (i.e. lack of blinding or knowledge of the IGRA/TST results influencing the ascertainment of exposure status or diagnosis of incident active TB), selection bias (i.e. study sample distorted with respect to previous TB exposure or disease spectrum because of an inadequate sampling frame, inadequate participant recruitment, non-participation and exclusions at study baseline), partial verification bias (i.e. incomplete outcome data assessment because of indeterminate IGRA results, missing TB exposure data, withdrawals and/or losses to follow-up) and incorporation bias (i.e. incorporation of IGRA/TST results as a criterion for the diagnosis of LTBI or incident active TB). 18,44,90,227
Although the results from the incidence studies merit more credibility given their prospective design and standard and uniform ascertainment of the outcome (i.e. diagnosis of incident active TB), this evidence was scarce, the studies included small sample sizes and their follow-up was not long enough to document and evaluate the predictive ability of the tests more reliably. Moreover, the use of incident case of active TB as the validity construct for the presence of LTBI may also lead to misclassification as not all LTBI cases will develop into active TB or some seemingly incident cases of active TB (assumed to have developed from LTBI) may actually be people with newly acquired TB infection (prevalent active TB cases).
One of the limitations of this review was that we excluded non-English-language publications, which might have led to language-related bias in the estimates. However, none of the six excluded studies (see Appendix 6) (two in Turkish, two in Chinese, one in Spanish and one in Persian) would have been eligible for inclusion in the clinical part of this review as three included mixed samples not stratified by the subgroups of interest, one was a cost-effectiveness study and two did not use the LTBI constructs, such as previous exposure group or incidence of progression to active TB. Therefore, we believe that these language-based exclusions would not have had any impact on our findings.
Strengths and limitations of the current reviews and economic evaluation
We undertook a systematic review to identify all relevant studies providing evidence on the clinical effectiveness of IGRAs compared with the TST for identifying LTBI in the prespecified populations. The main strengths of this review were the application of systematic comprehensive searches, study screening, data extraction, use of relevant quality/ROB assessment tools for different study designs and the stratified analyses (by children, immunocompromised people and those recently arrived from high TB burden countries, subgroups defined by clinical condition, type of IGRA, TST threshold, high vs. low TB burden area and study setting). Our review, unlike other systematic reviews,40,219,222 avoided including studies that used invalid constructs for LTBI, such as culture-confirmed active TB. Instead, this review focused on studies that defined the construct of LTBI either through the incidence of active TB or through study participants’ previous exposure to index TB cases (e.g. risk categories defined by exposure proximity, duration and/or relationship to index TB case).
Our economic evaluation analyses are based on test accuracy data obtained from the current clinical effectiveness review, which represents the best available information on the accuracy of tests for LTBI that progresses to active TB. Our analyses represent the work of a multidisciplinary team, which includes input from clinical experts to develop the model structure. Additionally, considerable efforts were made to identify the most appropriate model input parameters to be used in the decision-analytic model.
The main limitation of the clinical effectiveness review is that full additional data extraction and quality assessment were not undertaken for studies included in CG117. 10 Moreover, because of a lack of relevant reported evidence, it was not possible to evaluate the effectiveness of the two-step testing procedure (using both IGRAs and TST) for identifying people with LTBI. Another limitation was our inability to stratify the study findings by BCG vaccination status; the individual study publications failed to report their results separately for vaccinated and non-vaccinated populations; even though this may have been an important distinguishing feature in the effectiveness of the different tests. The proportions of people vaccinated with BCG varied considerably in the included studies such that it was not possible to dichotomise populations into, for example, vaccinated and non-vaccinated. In any case, further stratification by BCG status was not feasible because of the scarcity of the data. With regard to the economic evaluation, we applied a unit cost for people being tested with the TST. The unit cost included the costs of the test consumables, administering the test and reading the result. We applied this cost both to people who had their TST result read and to those who did not have their result read. This had the effect of inflating the cost of an unread TST result. In addition, the model took into account the need for two clinic visits for the TST; however, it did not take into account the need for skilled operators and the wide intraobserver variability in interpretation. IGRAs require one visit, need fewer skilled personnel for interpretation and have less reliance on observer interpretation. Second, to our knowledge there are no systematic reviews on the accuracy of chest radiography for identifying people who have active TB. In our model we used the sensitivity and specificity from the study by Kumar et al. 208 for the accuracy of chest radiography for identifying the presence/absence of active TB in our three populations. This may over/underestimate the diagnostic accuracy of chest radiography in these populations. Third, detailed resource use information with regard to treatment for LTBI was unavailable in the literature. We therefore estimated resource use for LTBI treatment using input from our clinical advisors and this may have resulted in either over- or underestimation of resource use.
Chapter 8 Conclusion
The review draws attention to the clinical effectiveness evidence published since CG117. 10 The research adds to the existing literature but highlights the poor quality of the evidence. Surprisingly, the results show that the two different generations of tests are broadly equivalent, although the results vary in a number of different settings and subgroups. The limitations in the evidence (e.g. absence of a gold standard for LTBI diagnosis, ROB in individual studies, scarcity of evidence, test administration/interpretation, variation in the exposure-based definitions of the LTBI construct, limitations of the screening tests) and heterogeneity in IGRA performance relative to TST performance limit the applicability of the review findings. Generally, the findings from population-based setting studies conducted in countries of low TB burden would be more applicable to the UK’s routine general practice of LTBI screening. The findings of this review underscore the variability of test performance across clinical conditions within the immunocompromised population, thereby limiting the applicability of test results from one subgroup (e.g. those with HIV infection or rheumatoid arthritis) to another (e.g. those with hepatitis C or lupus erythematosus).
The review of the cost-effectiveness evidence enabled the identification of previously published methodology before we developed our model structure to determine the cost-effectiveness of IGRAs compared with TST for the diagnosis of LTBI. These models offer insight and, in general, performed well against the frameworks on best practice for reporting a model-based economic evaluation, but were subjected to some limitations. Areas of concern included the perspective of the analysis, the handling of uncertainty in the models, the derivation of prevalence, test accuracy and transition probabilities; most studies did not elaborate on these statistical/pre-model analyses.
In the population of children who had had contact with an index case, the results based on the outcome of cost per diagnostic error avoided showed that the TST (≥ 10 mm) strategy dominated all strategies except for the T-SPOT. TB strategy alone. T-SPOT. TB compared with TST (≥ 10 mm) was more effective but more expensive, with an ICER of approximately £2710 per diagnostic error avoided. The TST (≥ 5 mm) strategy was slightly more effective than the QFT-GIT-alone strategy, with an ICER of approximately £11,260 per QALY, and had a 26.9% probability of being cost-effective at a willingness to pay of £20,000 per QALY.
In the immunocompromised population, QFT-GIT dominated QFT-GIT negative followed by TST (≥ 5 mm), T-SPOT. TB and TST (≥ 5 mm) strategies in terms of diagnostic errors avoided. The QFT-GIT positive followed by TST (≥ 5 mm) strategy was the most effective strategy. The results in terms of cost per QALY showed that the QFT-GIT negative followed by TST (≥ 5 mm) strategy was the most effective strategy, with an ICER of approximately £18,750 compared with T-SPOT. TB, and had a 40% probability of being cost-effective at a willingness to pay of £20,000.
In the recent arrivals from countries with a high incidence of TB, the QFT-GIT strategy dominated all strategies except for TST (≥ 5 mm) positive followed by QFT-GIT. The TST (≥ 5 mm) positive followed by QFT-GIT strategy was more costly and resulted in more diagnostic errors avoided, with an ICER of approximately £690 compared with the QFT-GIT-alone strategy. The results in terms of cost per QALY showed that QFT-GIT dominated the T-SPOT. TB and TST (≥ 5 mm) positive followed by QFT-GIT strategies and had an 18% probability of being cost-effective at a willingness to pay of £20,000 per QALY. The TST (≥ 5 mm) strategy had the highest probability (47%) of being cost-effective at a willingness to pay of £20,000.
Implications for service provision and local commissioning
The results of the health economic analysis show which diagnostic strategy is likely to be the most cost-effective for the diagnosis of LTBI that progresses to active TB.
Our results do not show whether or not screening compared with no screening is likely to be cost-effective nor do they demonstrate which IGRA (e.g. QFT-GIT vs. T-SPOT. TB) is more cost-effective.
Our findings should be interpreted by clinicians, commissioners and policy makers with caution because of the limited evidence, the lack of a gold standard diagnostic test and the assumptions made. Clinicians should be mindful of the variation in performance of the different testing strategies among different populations.
Suggested research priorities
A key priority is to conduct research in populations from both high and low TB burden countries to explore and confirm whether the inconsistent performance of IGRAs is real or whether it represents a chance finding. The natural history of the condition needs to be clarified. Prospective population-based studies with an adequate sample size and follow-up should be conducted in people at high risk for TB. These studies should employ standard diagnostic methodology and criteria for ascertaining incident cases of active TB. Research is also needed to clarify the role of serial as opposed to single cross-sectional testing in light of the comparative effectiveness of IGRAs and the TST for the diagnosis of LTBI; future studies need to evaluate the utility of two-step compared with single testing to maximise both sensitivity and specificity for identifying people with LTBI. Although strain and infectivity data have not been used in the present analyses because they were not available, they are relevant to future research.
Consensus-based standard criteria or a multivariable risk prediction model for the construct of LTBI should be developed. This would provide a standard set of all of the component exposures to classify people into high or low risk for LTBI. This would improve retrospective or cross-sectional studies of previous TB exposure by facilitating standardised definitions across different studies and would allow for more objective comparisons between IGRAs and the TST in terms of detecting LTBI in subgroups of interest.
There is very little evidence on the roles of IGRAs and the TST for the diagnosis of LTBI in different clinical subgroups of immunocompromised people (e.g. those with HIV infection or hepatitis C, solid organ transplant recipients, those with rheumatoid arthritis) and future research could be directed at providing this. Finally, more efforts need to be directed at identifying new more accurate markers of LTBI.
Acknowledgements
We would like to thank our clinical and methodological advisors for their consideration and support during the development and preparation of this review: Dr Martin Dedicoat, Professor Jeremy Hawker, Professor Ajit Lalvani and Professor Graham Medley.
We thank Dr Mark Jit and Dr Peter White for providing information from their model (ahead of publication) to allow comparison of health economic results.
We also thank the members of the NICE Guideline Development Group, with particular thanks to Andrew Hayward, Ibrahim Abubakar, Michael Eisenhut, Gabriel Rogers, Chris Gibbons, Hugh McGuire, Margaret Derry and Michael Heath – together they provided continuous clinical and health economic support.
Contributions of authors
Peter Auguste conducted the clinical effectiveness systematic review (screening and retrieving papers, assessing papers against the inclusion criteria, appraising the quality of papers and abstracting information from papers for synthesis), the cost-effectiveness systematic review and the health economic analysis, and wrote the discussion section.
Alexander Tsertsvadze wrote the background section, conducted the clinical effectiveness systematic review (screening and retrieving papers, assessing papers against the inclusion criteria, appraising the quality of papers and abstracting information from papers for synthesis) and the clinical effectiveness analysis, wrote the clinical effectiveness results section, conducted the cost-effectiveness systematic review and wrote the discussion section.
Joshua Pink conducted the health economic analysis and wrote the discussion section.
Rachel Court developed the search strategy and undertook the searches.
Farah Seedat wrote the background section.
Tara Gurung conducted the clinical effectiveness systematic review (screening and retrieving papers, assessing papers against the inclusion criteria, appraising the quality of papers and abstracting information from papers for synthesis).
Karoline Freeman conducted the clinical effectiveness systematic review (screening and retrieving papers, assessing papers against the inclusion criteria, appraising the quality of papers and abstracting information from papers for synthesis) and wrote the clinical effectiveness results section.
Sian Taylor-Phillips provided support for the clinical effectiveness analysis.
Clare Walker wrote the background section and the discussion.
Jason Madan provided support for the cost-effectiveness analysis.
Ngianga-Bakwin Kandala provided support for the clinical effectiveness analysis.
Aileen Clarke wrote the discussion section and provided clinical and methodological input.
Paul Sutcliffe co-ordinated the review, conducted the clinical effectiveness systematic review (screening and retrieving papers, assessing papers against the inclusion criteria, appraising the quality of papers and abstracting information from papers for synthesis) and wrote the discussion section.
All authors were involved in writing draft and final versions of the report.
Data sharing statement
All data in this report were already in the public domain; therefore, there are no data to share.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Global Tuberculosis Report 2013. Geneva: World Health Organization; 2013.
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 1999;282:677-86. http://dx.doi.org/10.1001/jama.282.7.677.
- Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull World Health Organ 1994;72:213-20.
- Anderson L, Moore J, Kruijshaar M, Pedrazzoli D, Bradshaw L, Crofts J, et al. Tuberculosis in the UK: Report on Tuberculosis Surveillance and Control in the UK 2010. London: Health Protection Agency; 2010.
- Pedrazzoli D. Tuberculosis in the UK: Annual Report on Tuberculosis Surveillance in the UK, 2013. London: Public Health England; 2013.
- Pareek M, Watson JP, Ormerod LP, Kon OM, Woltmann G, White PJ, et al. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis 2011;11:435-44. http://dx.doi.org/10.1016/S1473-3099(11)70069-X.
- Public Health England . Collaborative Tuberculosis Strategy for England 2014 to 2019: For Consultation 2014. www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317140970182 (accessed 1 July 2015).
- Core Curriculum on Tuberculosis: What the Clinician Should Know. Atlanta, GA: Centers for Disease Control and Prevention; 2013.
- Doran P, Carson J, Costello E, More SJ. An outbreak of tuberculosis affecting cattle and people on an Irish dairy farm, following the consumption of raw milk. Ir Vet J 2009;62. http://dx.doi.org/10.1186/2046-0481-62-6-390.
- Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. London: NICE; 2011.
- Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014;27:3-20. http://dx.doi.org/10.1128/CMR.00034-13.
- Al-Orainey IO. Diagnosis of latent tuberculosis: can we do better?. Ann Thorac Med 2009;4:5-9. http://dx.doi.org/10.4103/1817-1737.44778.
- Herrera V, Perry S, Parsonnet J, Banaei N. Clinical application and limitations of interferon-gamma release assays for the diagnosis of latent tuberculosis infection. Clin Infect Dis 2011;52:1031-7. http://dx.doi.org/10.1093/cid/cir068.
- World Health Organization . Tuberculosis: Factsheet 104 2014. www.who.int/mediacentre/factsheets/fs104/en/ (accessed 8 January 2015).
- Taylor ZNC, Blumberg HM. Infectious Diseases Society of America . Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep 2005;54:1-81.
- Hauck FR, Neese BH, Panchal AS, El-Amin W. Identification and management of latent tuberculosis infection. Am Fam Physician 2009;79:879-86.
- Bakhshi SS, Hawker J, Ali S. The epidemiology of tuberculosis by ethnic group in Birmingham and its implications for future trends in tuberculosis in the UK. Ethn Health 1997;2:147-53. http://dx.doi.org/10.1080/13557858.1997.9961823.
- Rangaka MX, Wilkinson KA, Glynn JR, Ling D, Menzies D, Mwansa-Kambafwile J, et al. Predictive value of interferon-gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:45-5. http://dx.doi.org/10.1016/S1473-3099(11)70210-9.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. http://dx.doi.org/10.1016/j.jclinepi.2005.02.022.
- Core Curriculum on Tuberculosis: What the Clinician Should Know. Atlanta, GA: Centers for Disease Control and Prevention; 2013.
- Cunningham J, Perkins M, Mwaluko G, Wringe A, Todd J, Glynn JR, et al. Diagnostics for tuberculosis: global demand and market potential. Lancet 2007;369:612-13.
- Kumar V, Abbas AK, Fausto N, Mitchell RN. Robbins Basic Pathology. Philadelphia, PA: Saunders Elsevier; 2007.
- Tuberculosis: What You Need To Know. Washington, DC: US Department of Agriculture, Food Safety and Inspection Service; 1997.
- Chaulk C, Kazandjian V. Directly observed therapy for treatment completion of pulmonary tuberculosis: consensus statement of the Public Health Tuberculosis Guidelines Panel. JAMA 1998;279:943-8. http://dx.doi.org/10.1001/jama.279.12.943.
- Chaulet P. Treatment of Tuberculosis: Case Holding until Cure. Geneva: World Health Organization; 1983.
- GBC Health . Drug-Resistant TB: Why It Matters 2011. www.gbchealth.org/system/documents/category_1/7/GBCHealth%20Issue%20Brief_Drug-Resistant%20TB.pdf?1311656059 (accessed 8 January 2015).
- American Thoracic Society . Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-95. http://dx.doi.org/10.1164/ajrccm.161.4.16141.
- Public Health (Control of Disease) Act 1984. London: The Stationery Office; 1984.
- Public Health England . UK Tuberculosis (TB) Surveillance Data 2014. www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Tuberculosis/TBUKSurveillanceData/ (accessed 1 July 2014).
- Health Protection Agency . Statutory notifications of tuberculosis. Commun Dis Rep CDR Wkly 2004;14.
- EuroTB . Total TB Cases and TB Notification Rates, 1995–2006, WHO European Region 2006. www.eurotb.org/slides/2008/TBCases_Rates1995–2006.pdf (accessed 27 February 2014).
- Migrant Health: Infectious Diseases in Non-UK Born Populations in England, Wales, and Northern Ireland. A Baseline Report – 2006. London: Health Protection Agency Centre for Infections; 2006.
- McCarthy OR. Asian immigrant tuberculosis – the effect of visiting Asia. Br J Dis Chest 1984;78:248-53. http://dx.doi.org/10.1016/0007-0971(84)90136-0.
- Moore-Gillon J. Diabetes and tuberculosis: a gathering storm?. Thorax 2010;65:571-2. http://dx.doi.org/10.1136/thx.2009.134247.
- The Lancet. The ongoing problem of tuberculosis in the UK . Lancet 2013;381. http://dx.doi.org/10.1016/S0140-6736(13)60910-1.
- Kirby T. Tuberculosis rates unacceptably high in UK cities. Lancet Infect Dis 2013;13:836-7. http://dx.doi.org/10.1016/S1473-3099(13)70276-7.
- Rienzo C, Vargas-Silva C. Migrants in the UK Overview 2013. www.migrationobservatory.ox.ac.uk/briefings/migrants-uk-overview (accessed 1 July 2014).
- Anderson C, Anderson S, Kanagarajah S, Hamblion E. Tuberculosis in London: Annual Review (2011 Data). London: Health Protection Agency, Regional Epidemiology Unit; 2012.
- Krausova A, Vargas-Silva C. London: Census Profile 2013. www.migrationobservatory.ox.ac.uk/briefings/london-census-profile (accessed 1 July 2014).
- Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 2011;56:230-8. http://dx.doi.org/10.1097/QAI.0b013e31820b07ab.
- Pawlowski A, Jansson M, Sköld M, Rottenberg M, Källenius G. Tuberculosis and HIV co-infection. PLOS Pathog 2012;8. http://dx.doi.org/10.1371/journal.ppat.1002464.
- Sharma S, Mohan A, Kadhiravan T. HIV–TB co-infection: epidemiology, diagnosis and management. Indian J Med Res 2005;121:550-67.
- McShane H. Co-infection with HIV and TB: double trouble. Int J STD AIDS 2005;16:95-101. http://dx.doi.org/10.1258/0956462053057576.
- Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11030.
- Vyas JM. Pulmonary Tuberculosis 2013. www.nlm.nih.gov/medlineplus/ency/article/000077.htm (accessed 1 July 2015).
- Marra CA, Marra F, Cox VC, Palepu A, Fitzgerald JM. Factors influencing quality of life in patients with active tuberculosis. Health Qual Life Outcomes 2004;2. http://dx.doi.org/10.1186/1477-7525-2-58.
- Thorn P. Overcoming Tuberculosis: A Handbook for Patients. Geneva: Stop TB Partnership; 2007.
- Courtwright A, Turner AN. Tuberculosis and stigmatization: pathways and interventions. Public Health Rep 2010;125:34-42.
- The Facts about Tuberculosis. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Treatment; 1995.
- Case for Change: TB Services in London. London: London Health Programmes; 2011.
- Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. London: NICE; 2006.
- Identifying and Managing Tuberculosis among Hard-to-Reach Groups. London: NICE; 2012.
- Diel R, Nienhaus A, Lange C, Schaberg T. Cost-optimisation of screening for latent tuberculosis in close contacts. Eur Respir J 2006;28:35-44. http://dx.doi.org/10.1183/09031936.06.00011806.
- Chaparas SD, Vandiviere HM, Melvin I, Koch G, Becker C. Tuberculin test. Variability with the Mantoux procedure. Am Rev Respir Dis 1985;132:175-7.
- Perez-Stable EJ, Slutkin G. A demonstration of lack of variability among six tuberculin skin test readers. Am J Public Health 1985;75:1341-3. http://dx.doi.org/10.2105/AJPH.75.11.1341.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000;356:1099-104. http://dx.doi.org/10.1016/S0140-6736(00)02742-2.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007;146:340-54. http://dx.doi.org/10.7326/0003-4819-146-5-200703060-00006.
- Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, Kampmann B, et al. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 2011;37:88-99. http://dx.doi.org/10.1183/09031936.00115110.
- Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, Hatherill M, Moyo S, Hanekom W, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J 2011;30:694-700. http://dx.doi.org/10.1097/INF.0b013e318214b915.
- Girardi E, Angeletti C, Puro V, Sorrentino R, Magnavita N, Vincenti D, et al. Estimating diagnostic accuracy of tests for latent tuberculosis infection without a gold standard among healthcare workers. Euro Surveill 2009;14.
- Ahitan M, Smith G. Latent Tuberculosis Treatment with Rifapentine and Isoniazid by Directly Observed Therapy. Birmingham: Heart of England NHS Foundation Trust; 2013.
- Centers for Disease Control and Prevention . Recommendations for use of an isoniazid–rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011;60:1650-3.
- Tuberculosis Prevention and Treatment: A Toolkit for Planning, Commissioning and Delivering High-Quality Services in England. London: Department of Health; 2007.
- Health Clearance for Tuberculosis, Hepatitis B, Hepatitis C and HIV: New Healthcare Workers. London: Department of Health; 2007.
- Stopping Tuberculosis in England: An Action Plan from the Chief Medical Officer. London: Department of Health; 2004.
- The Interdepartmental Working Group on Tuberculosis: The Prevention and Control of Tuberculosis in the United Kingdom. London: Department of Health; 1998.
- Milburn H, Ashman N, Davies P, Doffman S, Drobniewski F, Khoo S, et al. British Thoracic Society Standards of Care Committee and Joint Tuberculosis Committee . Guidelines for the prevention and management of Mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. Thorax 2010;65:557-70. http://dx.doi.org/10.1136/thx.2009.133173.
- British Thoracic Society Standards of Care Committee . BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005;60:800-5. http://dx.doi.org/10.1136/thx.2005.046797.
- British Thoracic Society Standards of Care Committee . Managing passengers with respiratory disease planning air travel: British Thoracic Society recommendations. Thorax 2002;57:289-304.
- Subcommittee of the Joint Tuberculosis Committee of the British Thoracic Society . Management of opportunist mycobacterial infections: Joint Tuberculosis Committee guidelines 1999. Thorax 2000;55:210-18. http://dx.doi.org/10.1136/thorax.55.3.210.
- Model of Care: TB Services in London. London: London Health Programmes, National Health Service; 2011.
- Menzies D, Al Jahdali H, Al Otaibi B. Recent developments in treatment of latent tuberculosis infection. Indian J Med Res 2011;133:257-66.
- Trajman A, Steffen RE, Menzies D. Interferon-gamma release assays versus tuberculin skin testing for the diagnosis of latent tuberculosis infection: an overview of the evidence. Pulm Med 2013;2013:601-737. http://dx.doi.org/10.1155/2013/601737.
- Latent TB Testing and Treatment for Migrants: A Practical Guide for Commissioners and Practitioners. London: Public Health England; 2015.
- Kawamura LM. IGRAs in public health practice: economic issues. Int J Tuberc Lung Dis 2010;14:S60-3.
- Landry J, Menzies D. Preventive chemotherapy. Where has it got us? Where to go next?. Int J Tuberc Lung Dis 2008;12:1352-64.
- Pareek M, Bond M, Shorey J, Seneviratne S, Guy M, White P, et al. Community-based evaluation of immigrant tuberculosis screening using interferon gamma release assays and tuberculin skin testing: observational study and economic analysis. Thorax 2013;68:230-9. http://dx.doi.org/10.1136/thoraxjnl-2011-201542.
- National Institute for Health Research . Prognostic Value of Interferon Gamma Release Assays in Predicting Active Tuberculosis Among Individuals With, or at Risk Of, Latent Tuberculosis Infection 2010. www.nets.nihr.ac.uk/projects/hta/086801 (accessed 15 January 2015).
- Public Health England . Tuberculosis Screening 2014. www.gov.uk/guidance/tuberculosis-screening (accessed 10 February 2016).
- Junghans C, Anya I, Brodie C, Carter S. Tuberculosis (TB): An Epidemiological Analysis and Review of Service Provision in Hammersmith and Fulham, Kensington and Chelsea, and Westminster. Tri-Borough Joint Strategic Needs Assessment Report 2014 2014. www.jsna.info/sites/default/files/Tuberculosis%20%28TB%29%20JSNA%202014.pdf (accessed 4 August 2014).
- Theaker S. Tuberculosis Summary Needs Assessment. Leicester, Leicestershire and Rutland 2013. www.leicester.gov.uk/EasySiteWeb/GatewayLink.aspx?alId = 222528 (accessed 8 July 2014).
- Kirklees Joint Strategic Needs Assessment . Infectious Disease and Human Immunodeficiency Virus (HIV) 2014. www.kirklees.gov.uk/you-kmc/partners/health/jsna/pdf/KirkleesJSNAInfectiousdiseaseandHIV.pdf (accessed 4 August 2014).
- Manchester City Council Joint Strategic Needs Assessment . Tuberculosis (TB) 2014. www.manchester.gov.uk/info/500230/joint_strategic_needs_assessment/6110/tuberculosis_tb (accessed 4 August 2014).
- Dedicoat M. Birmingham and Solihull Tuberculosis Service: South and City Colleage Birmingham Tackles TB 2014. http://tuberculosis.heartofengland.nhs.uk/south-city-college-birmingham-tackles-tb/ (accessed 4 August 2014).
- Newcastle upon Tyne Hospitals NHS Foundation Trust . TB Service n.d. www.newcastle-hospitals.org.uk/services/NHCH_services_community-nursing_tb.aspx (accessed 29 February 2016).
- Birmingham and Solihull TB services . Tuberculosis. n.d. www.heartofengland.nhs.uk/tuberculosis/ (accessed 10 February 2016).
- Liverpool Community Health NHS Trust . Tuberculosis (TB) Service 2014 n.d. www.liverpoolcommunityhealth.nhs.uk/health-services/nursing-services/tb-service.htm (accessed 10 February 2016).
- Diagnostics Assessment Programme Manual. London: NICE; 2011.
- Diagnostic Assessments Programme Manual. London: NICE; 2012.
- Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280-6. http://dx.doi.org/10.7326/0003-4819-158-4-201302190-00009.
- Ling DI, Zwerling AA, Steingart KR, Pai M. Immune-based diagnostics for TB in children: what is the evidence?. Paediatr Respir Rev 2011;12:9-15. http://dx.doi.org/10.1016/j.prrv.2010.09.009.
- Reitsma JB, Rutjes AW, Khan KS, Coomarasamy A, Bossuyt PM. A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J Clin Epidemiol 2009;62:797-806. http://dx.doi.org/10.1016/j.jclinepi.2009.02.005.
- Trikalinos TA, Balion CM. Chapter 9: options for summarizing medical test performance in the absence of a ‘gold standard’. J Gen Intern Med 2012;27:67-75. http://dx.doi.org/10.1007/s11606-012-2031-7.
- Rutjes AW, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PM. Evaluation of diagnostic tests when there is no gold standard. A review of methods. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11500.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. http://dx.doi.org/10.1016/0197-2456(86)90046-2.
- Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001;323:157-62. http://dx.doi.org/10.1136/bmj.323.7305.157.
- Deville WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2002;2. http://dx.doi.org/10.1186/1471-2288-2-9.
- Irwig L, Macaskill P, Glasziou P, Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol 1995;48:119-30. http://dx.doi.org/10.1016/0895-4356(94)00099-C.
- Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6. http://dx.doi.org/10.1186/1471-2288-6-31.
- Harbord RM, Whiting P. metandi: Meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J 2009;9:211-29.
- Huguet A, Hayden JA, Stinson J, McGrath PJ, Chambers CT, Tougas ME, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Syst Rev 2013;2. http://dx.doi.org/10.1186/2046-4053-2-71.
- Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. Negative and positive predictive value of a whole-blood interferon-γ release assay for developing active tuberculosis: an update. Am J Respir Crit Care Med 2011;183:88-95. http://dx.doi.org/10.1164/rccm.201006-0974OC.
- Mahomed H, Hawkridge T, Verver S, Abrahams D, Geiter L, Hatherill M, et al. The tuberculin skin test versus QuantiFERON TB Gold in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0017984.
- Noorbakhsh S, Mousavi J, Barati M, Shamshiri AR, Shekarabi M, Tabatabaei A, et al. Evaluation of an interferon-gamma release assay in young contacts of active tuberculosis cases. East Mediterr Health J 2011;17:714-18.
- Adetifa IM, Ota MO, Jeffries DJ, Hammond A, Lugos MD, Donkor S, et al. Commercial interferon gamma release assays compared to the tuberculin skin test for diagnosis of latent Mycobacterium tuberculosis infection in childhood contacts in the Gambia. Pediatr Infect Dis J 2010;29:439-43. http://dx.doi.org/10.1097/INF.0b013e3181cb45da.
- Cruz AT, Geltemeyer AM, Starke JR, Flores JA, Graviss EA, Smith KC. Comparing the tuberculin skin test and T-SPOT.TB blood test in children. Pediatrics 2011;127:e31-8. http://dx.doi.org/10.1542/peds.2010-1725.
- Kasambira TS, Shah M, Adrian PV, Holshouser M, Madhi SA, Chaisson RE, et al. QuantiFERON-TB Gold In-Tube for the detection of Mycobacterium tuberculosis infection in children with household tuberculosis contact. Int J Tuberc Lung Dis 2011;15:628-34. http://dx.doi.org/10.5588/ijtld.10.0555.
- Mahomed H, Hawkridge T, Verver S, Geiter L, Hatherill M, Abrahams DA, et al. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis 2011;15:331-6.
- Pavic I, Topic RZ, Raos M, Aberle N, Dodig S. Interferon-release assay for the diagnosis of latent tuberculosis in children younger than 5 years of age. Pediatr Infect Dis J 2011;30:866-70. http://dx.doi.org/10.1097/INF.0b013e318220c52a.
- Rutherford ME, Hill PC, Maharani W, Apriani L, Sampurno H, van Crevel R, et al. Risk factors for Mycobacterium tuberculosis infection in Indonesian children living with a sputum smear-positive case. Int J Tuberc Lung Dis 2012;16:1594-9. http://dx.doi.org/10.5588/ijtld.12.0389.
- Rutherford ME, Nataprawira M, Yulita I, Apriani L, Maharani W, van Crevel R, et al. QuantiFERON-TB Gold In-Tube assay vs. tuberculin skin test in Indonesian children living with a tuberculosis case. Int J Tuberc Lung Dis 2012;16:496-502. http://dx.doi.org/10.5588/ijtld.11.0491.
- Talbot EA, Harland D, Wieland-Alter W, Burrer S, Adams LV. Specificity of the tuberculin skin test and the T-SPOT.TB assay among students in a low-tuberculosis incidence setting. J Am Coll Health 2012;60:94-6. http://dx.doi.org/10.1080/07448481.2011.580029.
- Tsolia MN, Mavrikou M, Critselis E, Papadopoulos NG, Makrinioti H, Spyridis NP, et al. Whole blood interferon-release assay is a useful tool for the diagnosis of tuberculosis infection particularly among Bacille Calmette–Guérin-vaccinated children. Pediatr Infect Dis J 2010;29:1137-40. http://dx.doi.org/10.1097/INF.0b013e3181ebfe8a.
- Elzi L, Steffen I, Furrer H, Fehr J, Cavassini M, Hirschel B, et al. Improved sensitivity of an interferon-gamma release assay (T-SPOT.TB) in combination with tuberculin skin test for the diagnosis of latent tuberculosis in the presence of HIV co-infection. BMC Infect Dis 2011;11. http://dx.doi.org/10.1186/1471-2334-11-319.
- Moon SM, Lee SO, Choi SH, Kim YS, Woo JH, Yoon DH, et al. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection prior to hematopoietic stem cell transplantation. Transpl Infect Dis 2013;15:104-9. http://dx.doi.org/10.1111/j.1399-3062.2012.00765.x.
- Kim SH, Lee SO, Park JB, Park IA, Park SJ, Yun SC, et al. A prospective longitudinal study evaluating the usefulness of a T-cell-based assay for latent tuberculosis infection in kidney transplant recipients. Am J Transplant 2011;11:1927-35. http://dx.doi.org/10.1111/j.1600-6143.2011.03625.x.
- Anibarro L, Trigo M, Feijoo D, Rios M, Palomares L, Pena A, et al. Value of the tuberculin skin testing and of an interferon-gamma release assay in haemodialysis patients after exposure to M. tuberculosis. BMC Infect Dis 2012;12. http://dx.doi.org/10.1186/1471-2334-12-195.
- Lee SSJ, Chou KJ, Su IJ, Chen YS, Fang HC, Huang TS, et al. High prevalence of latent tuberculosis infection in patients in end-stage renal disease on hemodialysis: comparison of quantiFERON-TB GOLD, ELISPOT, and tuberculin skin test. Infection 2009;37:96-102. http://dx.doi.org/10.1007/s15010-008-8082-3.
- Chang B, Park HY, Jeon K, Ahn JK, Cha HS, Koh EM, et al. Interferon-γ release assay in the diagnosis of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. Clin Rheumatol 2011;30:1535-41. http://dx.doi.org/10.1007/s10067-011-1771-9.
- Ahmadinejad Z, Azmoudeh Ardalan F, Razzaqi M, Davoudi S, Jafarian A. QuantiFERON-TB Gold In-Tube test for diagnosis of latent tuberculosis (TB) infection in solid organ transplant candidates: a single-center study in an area endemic for TB. Transpl Infect Dis 2013;15:90-5. http://dx.doi.org/10.1111/tid.12027.
- Al Jahdali H, Ahmed AE, Balkhy HH, Baharoon S, Al Hejaili FF, Hajeer A, et al. Comparison of the tuberculin skin test and Quanti-FERON-TB Gold In-Tube (QFT-G) test for the diagnosis of latent tuberculosis infection in dialysis patients. J Infect Public Health 2013;6:166-72. http://dx.doi.org/10.1016/j.jiph.2013.02.002.
- Ates G, Ozekinci T, Yildiz T, Danis R. Comparison of interferon-gamma release assay versus tuberculin skin test for latent tuberculosis screening in hemodialysis patients. Biotechnol Biotechnol Equip 2009;23:1242-6. http://dx.doi.org/10.1080/13102818.2009.10817646.
- Casas S, Andreu A, Juanola X, Bordas X, Alcaide F, Moure R, et al. Diagnosis of tuberculosis infection by tuberculin skin test and a whole-blood interferon-release assay in patients considered for anti-tumor necrosis factor-alpha therapy. Diagn Microbiol Infect Dis 2011;71:57-65. http://dx.doi.org/10.1016/j.diagmicrobio.2010.12.020.
- Casas S, Muñoz L, Moure R, Castellote J, Guerra MR, Gonzalez L, et al. Comparison of the 2-step tuberculin skin test and the quantiFERON-TB Gold In-Tube Test for the screening of tuberculosis infection before liver transplantation. Liver Transpl 2011;17:1205-11. http://dx.doi.org/10.1002/lt.22375.
- Chkhartishvili N, Kempker RR, Dvali N, Abashidze L, Sharavdze L, Gabunia P, et al. Poor agreement between interferon-gamma release assays and the tuberculin skin test among HIV-infected individuals in the country of Georgia. BMC Infect Dis 2013;13. http://dx.doi.org/10.1186/1471-2334-13-513.
- Chung WK, Zheng ZL, Sung JY, Kim S, Lee HH, Choi SJ, et al. Validity of interferon-release assays for the diagnosis of latent tuberculosis in haemodialysis patients. Clin Microbiol Infect 2010;16:960-5. http://dx.doi.org/10.1111/j.1469-0691.2009.02949.x.
- Costantino F, de Carvalho Bittencourt M, Rat AC, Loeuille D, Dintinger H, Bene MC, et al. Screening for latent tuberculosis infection in patients with chronic inflammatory arthritis: discrepancies between tuberculin skin test and interferon-release assay results. J Rheumatol 2013;40:1986-93. http://dx.doi.org/10.3899/jrheum.130303.
- Hadaya K, Bridevaux PO, Roux-Lombard P, Delort A, Saudan P, Martin PY, et al. Contribution of interferon-release assays (IGRAs) to the diagnosis of latent tuberculosis infection after renal transplantation. Transplantation 2013;95:1485-90. http://dx.doi.org/10.1097/TP.0b013e3182907073.
- Hsia EC, Schluger N, Cush JJ, Chaisson RE, Matteson EL, Xu S, et al. Interferon-release assay versus tuberculin skin test prior to treatment with golimumab, a human anti-tumor necrosis factor antibody, in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis. Arthritis Rheum 2012;64:2068-77. http://dx.doi.org/10.1002/art.34382.
- Kim SH, Lee SO, Park IA, Park SJ, Choi SH, Kim YS, et al. Diagnostic usefulness of a T cell-based assay for latent tuberculosis infection in kidney transplant candidates before transplantation. Transpl Infect Dis 2010;12:113-19. http://dx.doi.org/10.1111/j.1399-3062.2010.00495.x.
- Kim SY, Jung GS, Kim SK, Chang J, Kim MS, Kim YS, et al. Comparison of the tuberculin skin test and interferon-release assay for the diagnosis of latent tuberculosis infection before kidney transplantation. Infection 2013;41:103-10. http://dx.doi.org/10.1007/s15010-012-0291-0.
- Kim JS, Cho JH, Park GY, Kang YJ, Kwon O, Choi JY, et al. Comparison of QuantiFERON-TB Gold with tuberculin skin test for detection of latent tuberculosis infection before kidney transplantation. Transplant Proc 2013;45:2899-902. http://dx.doi.org/10.1016/j.transproceed.2013.08.059.
- Kleinert S, Tony HP, Krueger K, Detert J, Mielke F, Rockwitz K, et al. Screening for latent tuberculosis infection: performance of tuberculin skin test and interferon-release assays under real-life conditions. Ann Rheum Dis 2012;71:1791-5. http://dx.doi.org/10.1136/annrheumdis-2011-200941.
- Laffitte E, Janssens JP, Roux-Lombard P, Thielen AM, Barde C, Marazza G, et al. Tuberculosis screening in patients with psoriasis before antitumour necrosis factor therapy: comparison of an interferon-gamma release assay vs. tuberculin skin test. Br J Dermatol 2009;161:797-800. http://dx.doi.org/10.1111/j.1365-2133.2009.09331.x.
- Maritsi D, Al-Obadi M, Brogan PA, Eleftheriou D, Dixon GL. The performance of Quantiferon TB Gold In-Tube as a screening tool in paediatric rheumatology prior to initiation of infliximab: a single centre’s experience. ISRN Rheumatol 2011;2011. http://dx.doi.org/10.5402/2011/505171.
- Mutsvangwa J, Millington KA, Chaka K, Mavhudzi T, Cheung YB, Mason PR, et al. Identifying recent Mycobacterium tuberculosis transmission in the setting of high HIV and TB burden. Thorax 2010;65:315-20. http://dx.doi.org/10.1136/thx.2009.124891.
- Papay P, Eser A, Winkler S, Frantal S, Primas C, Miehsler W, et al. Factors impacting the results of interferon-release assay and tuberculin skin test in routine screening for latent tuberculosis in patients with inflammatory bowel diseases. Inflamm Bowel Dis 2011;17:84-90. http://dx.doi.org/10.1002/ibd.21427.
- Ramos JM, Masia M, Rodriguez JC, Lopez C, Padilla S, Robledano C, et al. Negative effect of immunosuppressive therapy in the performance of the QuantiFERON Gold In-Tube test in patients with immune-mediated inflammatory diseases. Clin Exp Med 2013;13:177-86. http://dx.doi.org/10.1007/s10238-012-0192-7.
- Seyhan EC, Sokucu S, Altin S, Gunluoglu G, Trablus S, Yilmaz D, et al. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection in hemodialysis patients. Transpl Infect Dis 2010;12:98-105. http://dx.doi.org/10.1111/j.1399-3062.2009.00469.x.
- Shen KS, Zhang KY, Wang F, Yu L, Zhang SQ, Zhao WJ, et al. Diagnostic value of an IFN-gamma ELISPOT assay to detect latent tuberculosis infection in hepatitis C patients. Afr J Microbiol Res 2012;6:1736-41.
- Takeda N, Nojima T, Terao C, Yukawa N, Kawabata D, Ohmura K, et al. Interferon-gamma release assay for diagnosing Mycobacterium tuberculosis infections in patients with systemic lupus erythematosus. Lupus 2011;20:792-800. http://dx.doi.org/10.1177/0961203310397966.
- Vassilopoulos D, Tsikrika S, Hatzara C, Podia V, Kandili A, Stamoulis N, et al. Comparison of two gamma interferon release assays and tuberculin skin testing for tuberculosis screening in a cohort of patients with rheumatic diseases starting anti-tumor necrosis factor therapy. Clin Vaccine Immunol 2011;18:2102-8. http://dx.doi.org/10.1128/CVI.05299-11.
- Harstad I, Winje BA, Heldal E, Oftung F, Jacobsen GW. Predictive values of QuantiFERON-TB Gold testing in screening for tuberculosis disease in asylum seekers. Int J Tuberc Lung Dis 2010;14:1209-11.
- Kik SV, Franken WP, Mensen M, Cobelens FG, Kamphorst M, Arend SM, et al. Predictive value for progression to tuberculosis by IGRA and TST in immigrant contacts. Eur Respir J 2010;35:1346-53. http://dx.doi.org/10.1183/09031936.00098509.
- Lucas M, Nicol P, McKinnon E, Whidborne R, Lucas A, Thambiran A, et al. A prospective large-scale study of methods for the detection of latent Mycobacterium tuberculosis infection in refugee children. Thorax 2010;65:442-8. http://dx.doi.org/10.1136/thx.2009.127555.
- Orlando G, Merli S, Cordier L, Mazza F, Casazza G, Villa AM, et al. Interferon-gamma releasing assay versus tuberculin skin testing for latent tuberculosis infection in targeted screening programs for high risk immigrants. Infection 2010;38:195-204. http://dx.doi.org/10.1007/s15010-010-0015-2.
- Saracino A, Scotto G, Fornabaio C, Martinelli D, Faleo G, Cibelli D, et al. QuantiFERON-TB Gold In-Tube test (QFT-GIT) for the screening of latent tuberculosis in recent immigrants to Italy. New Microbiol 2009;32:369-76.
- Laniado-Laborin R, Cazares-Adame R, Volker-Soberanes ML, Del Portillo-Mustieles C, Villa-Rosas C, Oceguera-Palao L, et al. Latent tuberculous infection prevalence among paediatric contacts of drug-resistant and drug-susceptible cases. Int J Tuberc Lung Dis 2014;18:515-19. http://dx.doi.org/10.5588/ijtld.13.0840.
- Lee YM, Lee SO, Choi SH, Kim YS, Woo JH, Kim DY, et al. A prospective longitudinal study evaluating the usefulness of the interferon-gamma releasing assay for predicting active tuberculosis in allogeneic hematopoietic stem cell transplant recipients. J Infect 2014;69:165-73. http://dx.doi.org/10.1016/j.jinf.2014.02.019.
- Metin Timur O, Tanir G, Oz FN, Bayhan GI, Aydin Teke T, Tuygun N. Comparison of QuantiFERON-TB Gold In-Tube test with tuberculin skin test in children who had no contact with active tuberculosis case. Tuberk Toraks 2014;62:116-21. http://dx.doi.org/10.5578/tt.3552.
- Perez-Porcuna TM, Ascaso C, Malheiro A, Abellana R, Martins M, Sardinha JFJ, et al. Mycobacterium tuberculosis infection in young children: analyzing the performance of the diagnostic tests. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0097992.
- Song SE, Yang J, Lee KS, Kim H, Kim YM, Kim S, et al. Comparison of the tuberculin skin test and interferon gamma release assay for the screening of tuberculosis in adolescents in close contact with tuberculosis TB patients. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0100267.
- Souza JMO, Evangelista MDSN, Trajman A. Added value of quantiFERON TB-Gold In-Tube for detecting latent tuberculosis infection among persons living with HIV/AIDS. Biomed Res Int 2014;2014. http://dx.doi.org/10.1155/2014/294963.
- Tieu HV, Suntarattiwong P, Puthanakit T, Chotpitayasunondh T, Chokephaibulkit K, Sirivichayakul S, et al. Comparing interferon-gamma release assays to tuberculin skin test in Thai children with tuberculosis exposure. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0105003.
- Sherkat R, Yaran M, Shoaie P, Mortazavi M, Shahidi S, Hamidi H, et al. Concordance of the tuberculin skin test and T-SPOT®.TB test results in kidney transplant candidates. J Res Med Sci 2014;19:26-9.
- Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med 2004;170:65-9. http://dx.doi.org/10.1164/rccm.200402-232OC.
- Chun JK, Kim CK, Kim HS, Jung GY, Lee TJ, Kim KH, et al. The role of a whole blood interferon-gamma assay for the detection of latent tuberculosis infection in bacille Calmette–Guérin vaccinated children. Diagn Microbiol Infect Dis 2008;62:389-94. http://dx.doi.org/10.1016/j.diagmicrobio.2008.08.022.
- Connell TG, Curtis N, Ranganathan SC, Buttery JP. Performance of a whole blood interferon gamma assay for detecting latent infection with Mycobacterium tuberculosis in children. Thorax 2006;61:616-20. http://dx.doi.org/10.1136/thx.2005.048033.
- Connell TG, Ritz N, Paxton GA, Buttery JP, Curtis N, Ranganathan SC. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLOS ONE 2008;3. http://dx.doi.org/10.1371/journal.pone.0002624.
- Hansted E, Andriuskeviciene A, Sakalauskas R, Kevalas R, Sitkauskiene B. T-cell-based diagnosis of tuberculosis infection in children in Lithuania: a country of high incidence despite a high coverage with bacille Calmette–Guérin vaccination. BMC Pulm Med 2009;9. http://dx.doi.org/10.1186/1471-2466-9-41.
- Higuchi K, Kondo S, Wada M, Hayashi S, Ootsuka G, Sakamoto N, et al. Contact investigation in a primary school using a whole blood interferon-gamma assay. J Infect 2009;58:352-7. http://dx.doi.org/10.1016/j.jinf.2009.02.019.
- Higuchi K, Harada N, Mori T, Sekiya Y. Use of QuantiFERON-TB Gold to investigate tuberculosis contacts in a high school. Respirology 2007;12:88-92. http://dx.doi.org/10.1111/j.1440-1843.2006.01000.x.
- Lighter J, Rigaud M, Eduardo R, Peng CH, Pollack H. Latent tuberculosis diagnosis in children by using the QuantiFERON-TB Gold In-Tube test. Pediatrics 2009;123:30-7. http://dx.doi.org/10.1542/peds.2007-3618.
- Okada K, Mao TE, Mori T, Miura T, Sugiyama T, Yoshiyama T, et al. Performance of an interferon-gamma release assay for diagnosing latent tuberculosis infection in children. Epidemiol Infect 2008;136:1179-87. http://dx.doi.org/10.1017/s0950268807009831.
- Tsiouris SJ, Coetzee D, Toro PL, Austin J, Stein Z, El-Sadr W. Sensitivity analysis and potential uses of a novel gamma interferon release assay for diagnosis of tuberculosis. J Clin Microbiol 2006;44:2844-50. http://dx.doi.org/10.1128/JCM.02411-05.
- Winje BA, Oftung F, Korsvold GE, Mannsaker T, Jeppesen AS, Harstad I, et al. Screening for tuberculosis infection among newly arrived asylum seekers: comparison of QuantiFERONTB Gold with tuberculin skin test. BMC Infect Dis 2008;8. http://dx.doi.org/10.1186/1471-2334-8-65.
- Balcells ME, Perez CM, Chanqueo L, Lasso M, Villanueva M, Espinoza M, et al. A comparative study of two different methods for the detection of latent tuberculosis in HIV-positive individuals in Chile. Int J Infect Dis 2008;12:645-52. http://dx.doi.org/10.1016/j.ijid.2008.03.005.
- Bartalesi F, Vicidomini S, Goletti D, Fiorelli C, Fiori G, Melchiorre D, et al. QuantiFERON-TB Gold and the TST are both useful for latent tuberculosis infection screening in autoimmune diseases. Eur Respir J 2009;33:586-93. http://dx.doi.org/10.1183/09031936.00107608.
- Cobanoglu N, Ozcelik U, Kalyoncu U, Ozen S, Kiraz S, Gurcan N, et al. Interferon-gamma assays for the diagnosis of tuberculosis infection before using tumour necrosis factor-alpha blockers. Int J Tuberc Lung Dis 2007;11:1177-82.
- Jones S, de Gijsel D, Wallach FR, Gurtman AC, Shi Q, Sacks H. Utility of QuantiFERON-TB Gold In-Tube testing for latent TB infection in HIV-infected individuals. Int J Tuberc Lung Dis 2007;11:1190-5.
- Luetkemeyer AF, Charlebois ED, Flores LL, Bangsberg DR, Deeks SG, Martin JN, et al. Comparison of an interferon-gamma release assay with tuberculin skin testing in HIV-infected individuals. Am J Respir Crit Care Med 2007;175:737-42. http://dx.doi.org/10.1164/rccm.200608-1088OC.
- Mandalakas AM, Hesseling AC, Chegou NN, Kirchner HL, Zhu X, Marais BJ, et al. High level of discordant IGRA results in HIV-infected adults and children. Int J Tuberc Lung Dis 2008;12:417-23.
- Manuel O, Humar A, Preiksaitis J, Doucette K, Shokoples S, Peleg AY, et al. Comparison of quantiferon-TB gold with tuberculin skin test for detecting latent tuberculosis infection prior to liver transplantation. Am J Transplant 2007;7:2797-801. http://dx.doi.org/10.1111/j.1600-6143.2007.02011.x.
- Matulis G, Juni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis 2008;67:84-90. http://dx.doi.org/10.1136/ard.2007.070789.
- Piana F, Codecasa LR, Cavallerio P, Ferrarese M, Migliori GB, Barbarano L, et al. Use of a T-cell-based test for detection of tuberculosis infection among immunocompromised patients. Eur Respir J 2006;28:31-4. http://dx.doi.org/10.1183/09031936.06.00110205.
- Ponce de Leon D, Acevedo-Vasquez E, Alvizuri S, Gutierrez C, Cucho M, Alfaro J, et al. Comparison of an interferon-gamma assay with tuberculin skin testing for detection of tuberculosis (TB) infection in patients with rheumatoid arthritis in a TB-endemic population. J Rheumatol 2008;35:776-81.
- Richeldi L, Losi M, D’Amico R, Luppi M, Ferrari A, Mussini C, et al. Performance of tests for latent tuberculosis in different groups of immunocompromised patients. Chest 2009;136:198-204. http://dx.doi.org/10.1378/chest.08-2575.
- Schoepfer AM, Flogerzi B, Fallegger S, Schaffer T, Mueller S, Nicod L, et al. Comparison of interferon-gamma release assay versus tuberculin skin test for tuberculosis screening in inflammatory bowel disease. Am J Gastroenterol 2008;103:2799-806. http://dx.doi.org/10.1111/j.1572-0241.2008.02050.x.
- Shovman O, Anouk M, Vinnitsky N, Arad U, Paran D, Litinsky I, et al. QuantiFERON-TB Gold in the identification of latent tuberculosis infection in rheumatoid arthritis: a pilot study. Int J Tuberc Lung Dis 2009;13:1427-32.
- Soborg B, Ruhwald M, Hetland ML, Jacobsen S, Andersen AB, Milman N, et al. Comparison of screening procedures for Mycobacterium tuberculosis infection among patients with inflammatory diseases. J Rheumatol 2009;36:1876-84. http://dx.doi.org/10.3899/jrheum.081292.
- Talati NJ, Seybold U, Humphrey B, Aina A, Tapia J, Weinfurter P, et al. Poor concordance between interferon-gamma release assays and tuberculin skin tests in diagnosis of latent tuberculosis infection among HIV-infected individuals. BMC Infect Dis 2009;9. http://dx.doi.org/10.1186/1471-2334-9-15.
- Vassilopoulos D, Stamoulis N, Hadziyannis E, Archimandritis AJ. Usefulness of enzyme-linked immunospot assay (ELISPOT) compared to tuberculin skin testing for latent tuberculosis screening in rheumatic patients scheduled for anti-tumor necrosis factor treatment. J Rheumatol 2008;35:1271-6.
- Brodie D, Lederer DJ, Gallardo JS, Trivedi SH, Burzynski JN, Schluger NW. Use of an interferon-gamma release assay to diagnose latent tuberculosis infection in foreign-born patients. Chest 2008;133:869-74. http://dx.doi.org/10.1378/chest.07-1815.
- Carvalho AC, Pezzoli MC, El-Hamad I, Arce P, Bigoni S, Scarcella C, et al. QuantiFERON-TB Gold test in the identification of latent tuberculosis infection in immigrants. J Infect 2007;55:164-8. http://dx.doi.org/10.1016/j.jinf.2007.02.004.
- Diel R, Nienhaus A, Lange C, Meywald-Walter K, Forssbohm M, Schaberg T. Tuberculosis contact investigation with a new, specific blood test in a low-incidence population containing a high proportion of BCG-vaccinated persons. Respir Res 2006;7. http://dx.doi.org/10.1186/1465-9921-7-77.
- Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med 2008;177:1164-70. http://dx.doi.org/10.1164/rccm.200711-1613OC.
- Franken WP, Timmermans JF, Prins C, Slootman EJ, Dreverman J, Bruins H, et al. Comparison of Mantoux and QuantiFERON TB Gold tests for diagnosis of latent tuberculosis infection in army personnel. Clin Vaccine Immunol 2007;14:477-80. http://dx.doi.org/10.1128/CVI.00463-06.
- Janssens JP, Roux-Lombard P, Perneger T, Metzger M, Vivien R, Rochat T. Contribution of a IFN-gamma assay in contact tracing for tuberculosis in a low-incidence, high immigration area. Swiss Med Wkly 2008;138:585-93. http://dx.doi.org/2008/39/smw-12243.
- Kik SV, Franken WP, Arend SM, Mensen M, Cobelens FG, Kamphorst M, et al. Interferon-gamma release assays in immigrant contacts and effect of remote exposure to Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2009;13:820-8.
- Nienhaus A, Schablon A, Bacle CL, Siano B, Diel R. Evaluation of the interferon-gamma release assay in healthcare workers. Int Arch Occup Environ Health 2008;81:295-300. http://dx.doi.org/10.1007/s00420-007-0212-1.
- Porsa E, Cheng L, Seale MM, Delclos GL, Ma X, Reich R, et al. Comparison of a new ESAT-6/CFP-10 peptide-based gamma interferon assay and a tuberculin skin test for tuberculosis screening in a moderate-risk population. Clin Vaccine Immunol 2006;13:53-8. http://dx.doi.org/10.1128/CVI.13.1.53-58.2006.
- Winje BA, Oftung F, Korsvold GE, Mannsaker T, Ly IN, Harstad I, et al. School based screening for tuberculosis infection in Norway: comparison of positive tuberculin skin test with interferon-gamma release assay. BMC Infect Dis 2008;8. http://dx.doi.org/10.1186/1471-2334-8-140.
- Saiman L, Colson P. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics 2004;114:1175-201.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ 2013;14:367-72. http://dx.doi.org/10.1007/s10198-013-0471-6.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Kowada A. Cost effectiveness of interferon-gamma release assay for tuberculosis screening of rheumatoid arthritis patients prior to initiation of tumor necrosis factor-alpha antagonist therapy. Mol Diagn Ther 2010;14:367-73. http://dx.doi.org/10.1007/BF03256394.
- Kowada A. Cost effectiveness of interferon-gamma release assay for school-based tuberculosis screening. Mol Diagn Ther 2012;16:181-90. http://dx.doi.org/10.1007/BF03262207.
- Kowada A. Cost effectiveness of the interferon-release assay for tuberculosis screening of hemodialysis patients. Nephrol Dial Transplant 2013;28:682-8. http://dx.doi.org/10.1093/ndt/gfs479.
- Kowada A. Cost effectiveness of interferon-release assay for TB screening of HIV positive pregnant women in low TB incidence countries. J Infect 2014;68:32-4. http://dx.doi.org/10.1016/j.jinf.2013.08.009.
- Laskin BL, Goebel J, Starke JR, Schauer DP, Eckman MH. Cost-effectiveness of latent tuberculosis screening before steroid therapy for idiopathic nephrotic syndrome in children. Am J Kidney Dis 2013;61:22-3. http://dx.doi.org/10.1053/j.ajkd.2012.06.004.
- Linas BP, Wong AY, Freedberg KA, Horsburgh CR. Priorities for screening and treatment of latent tuberculosis infection in the United States. Am J Respir Crit Care Med 2011;184:590-601. http://dx.doi.org/10.1164/rccm.201101-0181OC.
- Swaminath A, Bhadelia N, Wang YC. Cost-effectiveness of QuantiFERON testing before initiation of biological therapy in inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2444-9. http://dx.doi.org/10.1097/MIB.0b013e31829f008f.
- Mandalakas AM, Hesseling AC, Gie RP, Schaaf HS, Marais BJ, Sinanovic E. Modelling the cost-effectiveness of strategies to prevent tuberculosis in child contacts in a high-burden setting. Thorax 2013;68:247-55. http://dx.doi.org/10.1136/thoraxjnl-2011-200933.
- Briggs AH, Gray AM. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess 1999;3.
- Hunink M, Glasziou P. Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge: Cambridge University Press; 2001.
- The Human Mortality Database n.d. www.mortality.org/ (accessed 6 February 2015).
- Health and Social Care Information Centre . Health Survey for England – 2012 2013. www.hscic.gov.uk/catalogue/PUB13218 (accessed 2 November 2014).
- Kumar N, Bhargava SK, Agrawal CS, George K, Karki P, Baral D. Chest radiographs and their reliability in the diagnosis of tuberculosis. JNMA J Nepal Med Assoc 2005;44:138-42.
- Pooran A, Booth H, Miller RF, Scott G, Badri M, Huggett JF, et al. Different screening strategies (single or dual) for the diagnosis of suspected latent tuberculosis: a cost effectiveness analysis. BMC Pulm Med 2010;10. http://dx.doi.org/10.1186/1471-2466-10-7.
- White P, Jit M. What is the Cost-Effectiveness of Latent Tuberculosis Infection (LTBI) Treatment with Different Regimens?. London: Imperial College Consultants; 2015.
- Okuonghae D. A mathematical model of tuberculosis transmission with heterogeneity in disease susceptibility and progression under a treatment regimen for infectious cases. Appl Math Model 2013;37:6786-808. http://dx.doi.org/10.1016/j.apm.2013.01.039.
- Oxlade O, Schwartzman K, Benedetti A, Pai M, Heymann J, Menzies D. Developing a tuberculosis transmission model that accounts for changes in population health. Med Decis Making 2011;31:53-68. http://dx.doi.org/10.1177/0272989X10369001.
- Crofts JP, Pebody R, Grant A, Watson JM, Abubakar I. Estimating tuberculosis case mortality in England and Wales, 2001–2002. Int J Tuberc Lung Dis 2008;12:308-13.
- NHS Reference Costs 2012 to 2013. London: Department of Health; 2013.
- Bothamley GH, Rowan JP, Griffiths CJ, Beeks M, McDonald M, Beasley E, et al. Screening for tuberculosis: the port of arrival scheme compared with screening in general practice and the homeless. Thorax 2002;57:45-9. http://dx.doi.org/10.1136/thorax.57.1.45.
- NHS Business Services Authority . NHS Electronic Drug Tariff: February 2015 2015. www.ppa.org.uk/edt/February_2015/mindex.htm (accessed 19 February 2015).
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Office for National Statistics . Population and Migration Data Catalogue 2014. www.ons.gov.uk/ons/guide-method/compendiums/compendium-of-uk-statistics/population-and-migration/data-catalogue/index.html (accessed 5 February 2014).
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008;149:177-84. http://dx.doi.org/10.7326/0003-4819-149-3-200808050-00241.
- Dheda K, Van Zyl Smit R, Badri M, Pai M. T-cell interferon-release assays for the rapid immunodiagnosis of tuberculosis: clinical utility in high-burden vs. low-burden settings. Curr Opin Pulm Med 2009;15:188-200. http://dx.doi.org/10.1097/MCP.0b013e32832a0adc.
- Mandalakas AM, Detjen AK, Hesseling AC, Benedetti A, Menzies D. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis 2011;15:1018-32. http://dx.doi.org/10.5588/ijtld.10.0631.
- Santin M, Munoz L, Rigau D. Interferon-release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0032482.
- Sarrazin H, Wilkinson KA, Andersson J, Rangaka MX, Radler L, van Veen K, et al. Association between tuberculin skin test reactivity, the memory CD4 cell subset, and circulating FoxP3-expressing cells in HIV-infected persons. J Infect Dis 2009;199:702-10. http://dx.doi.org/10.1086/596735.
- Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon-release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest 2012;142:63-75. http://dx.doi.org/10.1378/chest.11-3157.
- Song GG, Bae SC, Lee YH. Interferon-gamma release assays versus tuberculin skin testing in patients with rheumatoid arthritis. Int J Rheum Dis 2013;16:279-83. http://dx.doi.org/10.1111/1756-185X.12098.
- Shahidi N, Fu YT, Qian H, Bressler B. Performance of interferon-gamma release assays in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2012;18:2034-42. http://dx.doi.org/10.1002/ibd.22901.
- Schmidt RL, Factor RE. Understanding sources of bias in diagnostic accuracy studies. Arch Pathol Lab Med 2013;137:558-65. http://dx.doi.org/10.5858/arpa.2012-0198-RA.
- Public Health England . World Health Organization (WHO) Estimates of Tuberculosis Incidence by Rate, 2012 (Sorted by Rate 2014. www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1195733837507 (accessed 1 July 2014).
- Heldal E, Kuyvenhoven JV, Wares F, Migliori GB, Ditiu L, Fernandez de la Hoz K, et al. Diagnosis and treatment of tuberculosis in undocumented migrants in low or intermediate incidence countries. Int J Tuberc Lung Dis 2008;12:878-88.
- Mor Z, Migliori GB, Althomsons SP, Loddenkemper R, Trnka L, Iademarco MF. Comparison of tuberculosis surveillance systems in low incidence industrialised countries. Eur Respir J 2008;32:1616-24.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
Appendix 1 Search strategies and results from 2011
Main searches
Diagnosis of latent tuberculosis infection using interferon gamma release assays based on M. tuberculosis-specific antigens
Databases were searched to answer questions relating to the diagnosis of LTBI using IGRAs based on M. tuberculosis-specific antigens (ESAT-6, CFP 10 and TB7.7), including the following commercially available assays:
-
QFT-GIT
-
QFT-G
-
T-SPOT. TB.
The diagnostic utility of these assays, alone or in combination with a tuberculin skin test, was compared with that of the TST alone.
The database searches were undertaken between 7 and 14 December 2009. The databases searched were:
-
EMBASE (Ovid)
-
MEDLINE (Ovid)
-
MEDLINE In-Process & Other Non-Indexed Citations (Ovid)
-
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost)
-
Database of Abstracts of Reviews of Effects (CRD)
-
Health Technology Assessment database (CRD)
-
The Cochrane Library (Wiley)
-
Cochrane Register of Diagnostic Test Accuracy Studies (Wiley)
-
Medion
-
Aggressive Research Intelligence Facility.
The MEDLINE search strategy is presented below. It was translated for use in the databases listed above.
Ovid MEDLINE 1950 to November Week 3 2009
-
(laten* adj3 (tb* or tubercul*)).tw.
-
ltb*.tw.
-
Tuberculosis, Pulmonary/
-
Tuberculosis/
-
Mycobacterium tuberculosis/
-
or/1-5 (123029)
-
IGRA*.tw.
-
IGT*.tw.
-
(interferon adj3 gamma adj3 (release* or test* or assay*)).tw.
-
((y-interferon or interferon-y) adj3 (release* or assay* or test*)).tw.
-
(quantiferon adj3 gold*).tw.
-
(quantiferon adj3 (in tube or test*)).tw.
-
QFT*.tw.
-
t spot*.tw.
-
Interferon-gamma/
-
(enzyme* adj3 link* adj3 immunosorbent adj3 (test* or assay*)).tw.
-
ELISA*.tw.
-
(ELISPOT* or (enzyme* adj3 link* adj3 immunospot)).tw.
-
(ESAT6* or ESAT-6* or ESAT 6*).tw.
-
(early adj3 secret* adj3 antigen adj3 target-6).tw.
-
(CFP10* or (culture adj3 filtrate adj3 protein-10)).tw.
-
“TB7.7”.tw.
-
Fluorospot*.tw.
-
“region of difference”.tw.
-
Enzyme-Linked Immunosorbent Assay/
-
or/7-25
-
6 and 26
-
mass screening/
-
(screen* adj3 (program* or mass or population* or disease*)).tw.
-
28 or 29
-
30 and 6
-
27 or 31
-
Animals/ not Humans/
-
32 not 33
-
limit 34 to english language
Health economics
The following sources were searched to identify economic evaluations and quality-of-life data relating to IGRAs for LTBI:
-
Health Economics Evaluations Database (Wiley)
-
NHS Economic Evaluation Database (NHS EED) (Wiley and CRD website)
-
EMBASE (Ovid)
-
MEDLINE (Ovid)
-
MEDLINE In-Process & Other Non-Indexed Citations (Ovid)
The searches were undertaken on 5 and 6 January 2009.
The MEDLINE search strategy is presented below. It was translated for use in other databases.
Ovid MEDLINE 1950 to December Week 4 2009
-
(laten* adj3 (tb* or tubercul*)).tw.
-
ltb*.tw.
-
Tuberculosis, Pulmonary/
-
Tuberculosis/
-
Mycobacterium tuberculosis/
-
or/1-5
-
IGRA*.tw.
-
IGT*.tw.
-
(interferon adj3 gamma adj3 (release* or test* or assay*)).tw.
-
((y-interferon or interferon-y) adj3 (release* or assay* or test*)).tw.
-
(quantiferon adj3 gold*).tw.
-
(quantiferon adj3 (in tube or test*)).tw.
-
QFT*.tw.
-
t spot*.tw.
-
Interferon-gamma/
-
(enzyme* adj3 link* adj3 immunosorbent adj3 (test* or assay*)).tw.
-
ELISA*.tw.
-
(ELISPOT* or (enzyme* adj3 link* adj3 immunospot)).tw.
-
(ESAT6* or ESAT-6* or ESAT 6*).tw.
-
(early adj3 secret* adj3 antigen adj3 target-6).tw.
-
(CFP10* or (culture adj3 filtrate adj3 protein-10)).tw.
-
“TB7.7”.tw.
-
Fluorospot*.tw.
-
“region of difference”.tw.
-
Enzyme-Linked Immunosorbent Assay/ [Double click to insert footer here] 23 of 315
-
or/7-25
-
6 and 26
-
mass screening/
-
(screen* adj3 (program* or mass or population* or disease*)).tw.
-
28 or 29
-
30 and 6
-
27 or 31
-
Animals/ not Humans/
-
32 not 33
-
limit 34 to english language
-
Economics/
-
exp “Costs and Cost Analysis”/
-
Economics, Dental/
-
exp Economics, Hospital/
-
exp Economics, Medical/
-
Economics, Nursing/
-
Economics, Pharmaceutical/
-
Budgets/
-
exp Models, Economic/
-
Markov Chains/
-
Monte Carlo Method/
-
Decision Trees/
-
econom$.tw.
-
cba.tw.
-
cea.tw.
-
cua.tw.
-
markov$.tw.
-
(monte adj carlo).tw.
-
(decision adj2 (tree$ or analys$)).tw.
-
(cost or costs or costing$ or costly or costed).tw.
-
(price$ or pricing$).tw.
-
budget$.tw.
-
expenditure$.tw.
-
(value adj2 (money or monetary)).tw.
-
(pharmacoeconomic$ or (pharmaco adj economic$)).tw.
-
or/36-60
-
“Quality of Life”/
-
quality of life.tw.
-
“Value of Life”/
-
Quality-Adjusted Life Years/
-
quality adjusted life.tw.
-
(qaly$ or qald$ or qale$ or qtime$).tw.
-
disability adjusted life.tw. (571)
-
daly$.tw.
-
Health Status Indicators/
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw.
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw.
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw.
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw.
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw.
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(qol or hql or hqol or hrqol).tw.
-
(hye or hyes).tw.
-
health$ year$ equivalent$.tw.
-
utilit$.tw.
-
(hui or hui1 or hui2 or hui3).tw.
-
disutili$.tw.
-
rosser.tw.
-
quality of wellbeing.tw.
-
quality of well-being.tw.
-
qwb.tw.
-
willingness to pay.tw.
-
standard gamble$.tw.
-
time trade off.tw.
-
time tradeoff.tw.
-
tto.tw.
-
or/62-91
-
61 or 92
-
35 and 93
Appendix 2 Search strategies and results 2014
The objective of the search strategies was to identify literature on the diagnosis of LTBI using IGRAs compared with other methods. The following sources were searched: Ovid MEDLINE, Ovid MEDLINE In-Process & Other Non-Indexed Citations, The Cochrane Library via Wiley, Science Citation Index Expanded (SCI-EXPANDED), Conference Proceedings Citation Index – Science (CPCI-S), Medion, ClinicalTrials.gov, WHO ICTRP, conferences and websites.
The bibliographic database searches were undertaken on 9 and 10 April 2014 and were updated on 2 December 2014 using the same strategies. Supplementary searches were undertaken between 10 June and 5 August 2014.
MEDLINE
Update search
Ovid MEDLINE 1946 to November Week 3 2014, searched 2 December 2014.
The search in Table 45 was rerun with the following limit:
Line 24 = limit 23 to ed=20140312-20141202 (222)
Total = 1288 + 222 = 1510.
| Search term | Number of hits | |
|---|---|---|
| 1 | (laten* adj3 (tb* or tubercul*)).tw. | 2701 |
| 2 | ltb*.tw. | 6939 |
| 3 | tubercul*.tw. | 158,617 |
| 4 | Tuberculosis/ | 51,049 |
| 5 | Latent Tuberculosis/ | 866 |
| 6 | Tuberculosis, Pulmonary/ | 63,874 |
| 7 | Mycobacterium tuberculosis/ | 35,401 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 195,420 |
| 9 | quantiferon*.tw. | 819 |
| 10 | QFT*.tw. | 557 |
| 11 | t spot*.tw. | 261 |
| 12 | exp Enzyme-Linked Immunosorbent Assay/ | 122,317 |
| 13 | Interferon-gamma Release Tests/ | 377 |
| 14 | ((interferon* or IFN*) adj3 gamma* adj3 (release* or test* or assay*)).tw. | 3856 |
| 15 | ((y-interferon or interferon-y) adj3 (release* or test* or assay*)).tw. | 7 |
| 16 | IGRA*.tw. | 448 |
| 17 | 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 | 126,234 |
| 18 | 8 and 17 | 3840 |
| 19 | Latent Tuberculosis/di | 576 |
| 20 | 18 or 19 | 4061 |
| 21 | Animals/ not Humans/ | 3,812,070 |
| 22 | 20 not 21 | 3480 |
| 23 | limit 22 to english language | 3014 |
| 24 | limit 23 to ed=20091207-20140409 | 1288 |
MEDLINE In-Process & Other Non-Indexed Citations
Update search
Ovid MEDLINE In-Process & Other Non-Indexed Citations 1 December 2014, searched 2 December 2014.
The search in Table 46 was rerun with the following limit:
Line 15 = limit 14 to ed=20140312-20141202 (19)
Total = 263 + 19 = 282.
| Search term | Number of hits | |
|---|---|---|
| 1 | (laten* adj3 (tb* or tubercul*)).tw. | 312 |
| 2 | ltb*.tw. | 340 |
| 3 | tubercul*.tw. | 10,405 |
| 4 | 1 or 2 or 3 | 10,625 |
| 5 | quantiferon*.tw. | 121 |
| 6 | QFT*.tw. | 83 |
| 7 | t spot*.tw. | 42 |
| 8 | (enzyme* adj3 link* adj3 (immunosorbent or immunospot) adj3 (test* or assay*)).tw. | 3522 |
| 9 | ((interferon* or IFN*) adj3 gamma* adj3 (release* or test* or assay*)).tw. | 148 |
| 10 | ((y-interferon or interferon-y) adj3 (release* or test* or assay*)).tw. | 1 |
| 11 | IGRA*.tw. | 102 |
| 12 | 5 or 6 or 7 or 8 or 9 or 10 or 11 | 3778 |
| 13 | 4 and 12 | 281 |
| 14 | limit 13 to english language | 263 |
EMBASE
Update search
EMBASE 1980 to 2014 Week 48, searched 2 December 2014.
The search in Table 47 was rerun with the following limits:
Line 25 = imit 24 to dd=20140409-20141202 (364)
Line 26 = limit 24 to em=201414-201448 (387)
Line 27 = 25 or 26 (387)
Total = 2483 + 387 = 2870.
| Search term | Number of hits | |
|---|---|---|
| 1 | (laten* adj3 (tb* or tubercul*)).tw. | 3880 |
| 2 | ltb*.tw. | 8397 |
| 3 | tubercul*.tw. | 175,055 |
| 4 | tuberculosis/ | 87,819 |
| 5 | latent tuberculosis/ | 1696 |
| 6 | lung tuberculosis/ | 62,789 |
| 7 | Mycobacterium tuberculosis/ | 47,234 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 227,447 |
| 9 | quantiferon*.tw. | 1477 |
| 10 | QFT*.tw. | 871 |
| 11 | t spot*.tw. | 442 |
| 12 | enzyme linked immunospot assay/ | 5911 |
| 13 | *enzyme linked immunosorbent assay/ | 14,220 |
| 14 | exp interferon gamma release assay/ | 1062 |
| 15 | ((interferon* or IFN*) adj3 gamma* adj3 (release* or test* or assay*)).tw. | 1925 |
| 16 | ((y-interferon or interferon-y) adj3 (release* or test* or assay*)).tw. | 12 |
| 17 | IGRA*.tw. | 841 |
| 18 | 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 | 23,387 |
| 19 | 8 and 18 | 3410 |
| 20 | latent tuberculosis/di | 573 |
| 21 | 19 or 20 | 3619 |
| 22 | animal/ not human/ | 1,176,853 |
| 23 | 21 not 22 | 3556 |
| 24 | limit 23 to english language | 3171 |
| 25 | limit 24 to dd=20091207-20140409 | 2280 |
| 26 | limit 24 to em=200900-201414 | 2482 |
| 27 | 25 or 26 | 2483 |
The Cochrane Library
All results 108: Cochrane reviews 0, other reviews 19, trials 53, methods studies 0, technology assessments 6, economic evaluations 30, Cochrane Groups 0.
Update search
The Cochrane Library via Wiley, searched 2 December 2014.
The search in Table 48 was rerun with the following limit:
Line 21 = #18 or #19 Publication Year from 2014 to 2014 (11)
All results 11: Cochrane reviews 0, other reviews 3, trials 7, methods studies 0, technology assessments 0, economic evaluations 1, Cochrane Groups 0.
Total = 108 + 11 = 119.
| Search term | Number of hits | |
|---|---|---|
| #1 | (laten* near/3 (tb* or tubercul*)):ti,ab,kw | 186 |
| #2 | ltb*:ti,ab,kw | 270 |
| #3 | tubercul*:ti,ab,kw | 3404 |
| #4 | MeSH descriptor: [Tuberculosis] this term only | 598 |
| #5 | MeSH descriptor: [Latent Tuberculosis] this term only | 53 |
| #6 | MeSH descriptor: [Tuberculosis, Pulmonary] this term only | 824 |
| #7 | MeSH descriptor: [Mycobacterium tuberculosis] this term only | 306 |
| #8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 | 3632 |
| #9 | quantiferon*:ti,ab,kw | 44 |
| #10 | QFT*:ti,ab,kw | 22 |
| #11 | t next spot*:ti,ab,kw | 15 |
| #12 | MeSH descriptor: [Enzyme-Linked Immunosorbent Assay] explode all trees | 2107 |
| #13 | MeSH descriptor: [Interferon-gamma Release Tests] this term only | 31 |
| #14 | ((interferon* or IFN*) near/3 gamma* near/3 (release* or test* or assay*)):ti,ab,kw | 164 |
| #15 | ((y-interferon or interferon-y) near/3 (release* or test* or assay*)):ti,ab,kw | 0 |
| #16 | IGRA*:ti,ab,kw | 22 |
| #17 | #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 | 2260 |
| #18 | #8 and #17 | 145 |
| #19 | MeSH descriptor: [Latent Tuberculosis] this term only and with qualifier(s): [Diagnosis - DI] | 31 |
| #20 | #18 or #19 | 154 |
| #21 | #18 or #19 Publication Date from 2009 to 2014 | 108 |
Science Citation Index Expanded and Conference Proceedings Citation Index – Science
Update search
Science Citation Index Expanded (SCI-EXPANDED) 1970 to present and Conference Proceedings Citation Index – Science (CPCI-S) 1990 to present via Web of Knowledge, searched 2 December 2014.
The search in Table 49 was rerun with the following limit:
Timespan = 2014
#14 = 277
Total = 3314 + 277 = 3591.
| Search term | Number of hits | |
|---|---|---|
| #14 | (#13) AND LANGUAGE: (English) Indexes=SCI-EXPANDED, CPCI-S Timespan=2009-2014 | 1608 |
| #13 | #4 and #12 Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 3139 |
| #12 | #5 or #6 or #7 or #8 or #9 or #10 or #11 Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 63,467 |
| #11 | TS=IGRA* Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 601 |
| #10 | TS=((y-interferon or interferon-y) NEAR/3 (release* or test* or assay*)) Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 5 |
| #9 | TS=((interferon* or IFN*) NEAR/3 gamma* NEAR/3 (release* or test* or assay*)) Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 5812 |
| #8 | TS=(enzyme* NEAR/3 link* NEAR/3 (immunosorbent or immunospot) NEAR/3 (test* or assay*)) Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 56,262 |
| #7 | TS=((t-spot*) OR (t NEAR/1 spot*)) Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 464 |
| #6 | TS=QFT* Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 1894 |
| #5 | TS=quantiferon* Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 949 |
| #4 | #1 or #2 or #3 Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 108,863 |
| #3 | TS=tubercul* Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 103,332 |
| #2 | TS=ltb*Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 6278 |
| #1 | TS=(laten* NEAR/3 (tb or tubercul*)) Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | 3314 |
Medion
Searched 10 June 2014.
Search 1
Searched in subset of Medion – systematic reviews of diagnostic studies.
Signssymp - selected:
-
divers, other, general,
-
Laboratory tests
Abstract:
-
Tuberculosis
Total: 33.
Search 2
Searched in subset of Medion – systematic reviews of diagnostic studies.
Signssymp - selected:
-
divers, other, general,
-
Laboratory tests
Abstract:
-
tb
Total: 37.
Both searches
-
Total of both searches after duplicates removed: 47.
-
Saved to Microsoft Word® 2010 (Microsoft Corporation, Redmond, WA, USA) and removed 19 pre-2009 reviews, leaving 28.
-
Checked against results of other database searching in EndNote and removed 11 duplicates.
-
Total unique records: 17.
World Health Organization International Clinical Trials Registry Platform
Searched 5 August 2014.
Advanced search:
(quantiferon* or QFT* or t-spot* or interferon* or IFN* or gamma* or y-interferon or interferon-y or IGRA*) in Title
AND
(tuberculosis or latent tb) in Condition
Total: 10.
ClinicalTrials.gov
Searched 5 August 2014.
(quantiferon* OR QFT* OR t-spot* OR interferon* OR IFN* OR gamma* OR y-interferon OR interferon-y OR IGRA*) AND (tuberculosis or “latent tb”)
Excluded unknown status.
Total: 41.
Conferences
Specific conference proceedings, selected with input from a clinical expert (Professor Jeremy Hawker, Public Health England, 29 April 2014), were searched on 24 and 25 June 2014 for the last 5 years:
-
European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE) [http://ecdc.europa.eu/en/ESCAIDE/about_ESCAIDE/Pages/previous_conferences.aspx (accessed 25 June 2014)]
-
PHE Annual Conference (previously Health Protection Agency Annual Conference) (www.phe-events.org.uk/hpa/frontend/reg/thome.csp?pageID=117557&eventID=286&eventID=286)
-
5 Nations Health Protection Conference [http://5nations.org.uk/?page_id=44 (accessed 13 January 2016)]
-
Federation of Infection Societies Annual Conference [http://fis-infection.org.uk/ (accessed 13 January 2016), e.g. www.actiononinfection.com/abstracts-and-poster-walk/ (accessed 25 June 2014)]
-
British Thoracic Society [www.brit-thoracic.org.uk/bts-learning-hub/bts-summer-and-winter-meetings/summer-meeting-2014/ (accessed 13 January 2016)]
-
Annual Conferences of the Union North America Region [www.bc.lung.ca/association_and_services/union.html (accessed 25 June 2014)].
Websites
Websites of specific organisations, selected with input from a clinical expert (Professor Jeremy Hawker), were checked for relevant literature on 25 June 2014:
-
PHE (including old Health Protection Agency site) [www.gov.uk/government/organisations/public-health-england (accessed 13 January 2016) and www.hpa.org.uk/ (accessed 25 June 2014)].
-
Centers for Disease Control and Prevention (Atlanta) [www.cdc.gov/ (accessed 13 January 2016)].
-
European Centre for Disease Prevention and Control [www.ecdc.europa.eu/en/Pages/home.aspx and www.ecdc.europa.eu/en/activities/diseaseprogrammes/programme_tuberculosis/Pages/index.aspx (accessed 25 June 2014)].
-
World Health Organization [www.who.int/en/ (accessed 13 January 2013) and http://dosei.who.int/uhtbin/cgisirsi/tXRt5oo9vL/245820007/60/86/X (accessed 25 June 2014)].
-
British Thoracic Society [(www.brit-thoracic.org.uk/ (accessed 13 January 2016)].
-
Cellestis (manufacturer of QFT-G and QFT-GIT) [www.cellestis.com/ (accessed 13 January 2016)].
-
Oxford Immunotec (manufacturer of T-SPOT. TB) [www.oxfordimmunotec.com/ (accessed 13 January 2016)].
Appendix 3 Data extraction table for included primary study reports
Appendix 4 Quality assessment and risk of bias
| Study ID (burden) | Recruitment of subjects [consecutive (yes), arbitrary or unreported (no)] | Blinding of test results from exposure [blinded (yes), not blinded or unreported (no)] | Description of index test and threshold [adequate (yes), inadequate or unreported (no)] | Definition and description of exposure [adequate (yes), inadequate or unreported (no)] | Sample attrition [adequate (yes),a inadequate or unreported (no)] | Overall quality score of satisfactory featuresb |
|---|---|---|---|---|---|---|
| Study ID (first author, year, ref. ID): | |||||
|---|---|---|---|---|---|
| Reviewer 1: | |||||
| Reviewer 2: | |||||
| Domain of bias | Question | Issues to consider for judging overall rating of ROB | Comments (if issue not satisfied) | Rating (yes, partial, no, unsure) | ROB (high, moderate, low) |
| Study design | Prospective (yes/no)? | Prospective (low ROB), cross-sectional (moderate ROB), case–control (high ROB) | |||
| Study participation (risk of selection bias) | Does the study sample adequately represent the population of interest? How likely is it that the relationship between the test result and the outcome is different for participants and eligible non-participants? | The source population is adequately described | |||
| The sampling frame and recruitment are adequately described | |||||
| The period and place of recruitment are adequately described | |||||
| Inclusion and exclusion criteria are adequately described | |||||
| The baseline study sample is adequately described | |||||
| Adequate participation in the study by eligible individuals | |||||
| Participants were consecutively enrolled | |||||
| Study attrition (risk of selection bias) | Do the study data available (participants not lost to follow-up) adequately represent the study sample? How likely is it that the relationship between the test results and the outcome is different for completing and non-completing participants? | The response rate (i.e. proportion of study sample completing the study and providing outcome data) is adequate | |||
| Attempts to collect information on participants who dropped out are described | |||||
| Reasons for loss to follow-up are provided | |||||
| Participants lost to follow-up are adequately described for key characteristics | |||||
| There are no important differences between key characteristics and outcomes between participants who completed the study and those who did not | |||||
| Prognostic factor measurement (risk of exposure measurement bias) | Was the test measured in a similar way for all participants? How likely is it that the measurement or knowledge of the outcome influenced the test results? | A clear definition or description of the test is provided (e.g. type, assay, threshold for positivity and method of measurement) | |||
| The method of test conduct was adequate and test results were ascertained adequately (e.g. raters were blinded to outcomes in relation to construct validity, previous test ratings, clinical or other characteristics not intended to be a part of the test) | |||||
| Test thresholds used are appropriate | |||||
| The method and setting of the test measurement are the same for all study participants | |||||
| An adequate proportion of the study sample has complete data of the test results | |||||
| Appropriate methods of imputation are used for missing data on test results | |||||
| Outcome/construct measurement (ROB in misclassification of individuals in relation to construct validity groups) | Was the outcome of interest (i.e. exposure to MTB, incidence of active TB, definition of low-risk population) measured in a similar way for all participants? How likely is differential measurement of the outcome (e.g. outcome measurement related to the test results)? | A clear definition of outcomes is provided, including duration of follow-up and level and extent of the outcome construct | |||
| The method of outcome measurement used is valid and reliable to limit misclassification bias (e.g. blinded measurement, adequate methods of outcome/construct ascertainment – exposure proximity plus duration considered) | |||||
| The method and setting of outcome/construct measurement are the same for all study participants | |||||
| Study confounding (ROB related to confounding) | Were important potential confounding factors appropriately accounted for? How likely is bias because of confounding? | All important confounders, including treatments (key variables in the conceptual mode) are defined and measured | |||
| All important confounders are accounted for at the design and/or analysis stage | |||||
| Statistical analysis and reporting (ROB related to the analysis and selective reporting) | Was the statistical analysis appropriate and were all primary outcomes reported? How likely is bias related to the statistical analysis and presentation of the results? | There is sufficient presentation of data to assess the adequacy of the analysis | |||
| The strategy for model building (i.e. inclusion of variables in the statistical model) is appropriate and is based on a conceptual framework or model | |||||
| The selected statistical model is adequate for the design of the study | |||||
| There is no selective reporting of results | |||||
| Total ROB (high, medium, low) | |||||
| Domain of bias | Definitions for ROB ratings | ||
|---|---|---|---|
| High ROB | Moderate ROB | Low ROB | |
| Study design | Case–control study | Cross-sectional study | Prospective cohort study |
| Study participation | The relationship between the test results and the construct/outcome is very likely to be different for participants and eligible non-participants | The relationship between the test results and the outcome may be different for participants and eligible non-participants | The relationship between the test results and the outcome is unlikely to be different for participants and eligible non-participants |
| Study attrition | The relationship between the test results and the construct/outcome is very likely to be different for completing and non-completing participants | The relationship between the test results and the construct/outcome may be different for completing and non-completing participants | The relationship between the test results and the outcome is unlikely to be different for completing and non-completing participants |
| Prognostic factor measurement | The measurement of the test is very likely to be different for different levels of the outcome/construct of interest | The measurement of the test may be different for different levels of the outcome/construct of interest | The measurement of the test is unlikely to be different for different levels of the outcome/construct of interest |
| Outcome measurement/construct | The measurement of the outcome/construct is very likely to be different related to the baseline level of the test | The measurement of the outcome/construct may be different related to the baseline level of the test | The measurement of the outcome/construct is unlikely to be different related to the baseline level of the test |
| Study confounding | The observed association between the test and the outcome/construct is very likely to be distorted by another factor related to prognostic factor and outcome | The observed association between the test and the outcome/construct may be distorted by another factor related to prognostic factor and outcome | The observed association between the test and the outcome/construct is unlikely to be distorted by another factor related to prognostic factor and outcome |
| Statistical analysis and reporting | The reported results are very likely to be spurious or biased related to analysis or reporting | The reported results may be spurious or biased related to analysis or reporting | The reported results are unlikely to be spurious or biased related to analysis or reporting |
Appendix 5 Studies included in the clinical effectiveness review 201110
Tables reproduced with permission from NICE CG117. 10
Children
| Bibliography reference (Ref ID) | Study type/country of study/origin of participants/BCG vaccination | Number/age/patient characteristics | Exposure status/contact/gradient | Type of test | Reference standard | Sensitivity and specificity modified measure of effect | Positive and negative predictive values | Source of funding | Additional comments |
|---|---|---|---|---|---|---|---|---|---|
| Brock 2004156 | Observational. Done in Denmark on Danish School population | 125 mean age of 17 years. 85 not BCG vaccinated. Subjects nearest contact case also 17 asked to participate | Stratified by high and low exposure. High exposure contained individuals with close contact to the index case either through household, school class or local choir that index case regularly attended. Low exposure comprised 40 students from two other classes at the school with no connection to the index case | IGRA (QFT-G) | TST PPD RT23 (2 tuberculin units were used) | Determined concordance between the tests in both levels of exposure. And also in both BCG and non-BCG vaccinated individuals. Overall kappa = 0.866 | Not determined | Not reported | Study demonstrated that IGRA is similar in performance to TST in detecting LTBI in young non-BCG vaccinated individuals |
| Chun 2008157 | Observational conducted in South Korea | Aged up to 15 years. Patients visiting a children’s hospital. All children but one had been BCG vaccinated | Divided into four groups according to contact status
|
IGRA (QFT-G) | TST PPD RT23 (2 tuberculin units were used) | Close contacts: kappa 0.19 for 5 mm and 0.529 for 10 mm. (B) Kappa 0.378 for 10 mm. A significantly higher rate of positive QFT-G results was evident for the close contact group. 8/42, 19% as compared with the control group 3 subjects 1/65, 1.5% p < 0.05. Majority of indeterminate QFT-G results were from group 4 who were suffering from medical conditions that could be associated with impaired immune function at the time of testing | Not determined | Not reported | Authors found that in children with no exposure to TB, the QFT-G was positive in only one of the 65 children, although all of them were positive by the TST at 5 mm and 64.6% at 10 mm. They also found that there was a significant relationship between higher responses to mitogen-positive control and increasing age of the children |
| Connell 2006158 | Observational study. Australia. Some children born in high prevalence countries 52% | Children aged < 18 years with a high risk of latent TB infection | Contact with high risk as defined by siblings or parents recently diagnosed with TB disease, clinical suspicion of TB disease and those recently immigrated from high prevalence of TB | IGRA (QFT-G) 0.35 IU/ml positive response | TST PPD 10 IU of tuberculin. Positive if 15 mm in individuals with evidence of prior BCG, > 5 mm in TB contacts regardless of BCG and > 10 mm for all others | Concordance between TST and IGRA poor overall k = 0.3. 70% of TST positives were negative by IGRA. 65% of TST positives had a known TB contact | Not determined | John Burge Trust, VIC, Australia | Recommended further studies to clarify predictive values |
| Connell 2008159 | Observational study. Australia/Australia and some born in high prevalence countries. 52% BCG vaccinated | 96 children from 6 months of age to 19 years. Children who were at risk of latent TB or with suspected TB infection were eligible for inclusion. At risk was defined as a recent TB contact and/or recent immigration from a country of high prevalence of TB | 38 participants had LTBI TST positive with no additional symptoms. 49 patients TST negative with no confirmation of active TB Contacts were either household or non-household |
IGRA (QFT-G), T-SPOT.TB | TST PPD 10 IU of tuberculin. Positive > 10 mm | Out of 100 patients, 38 were TST positive of which 16 were household contacts, 6 non-household contacts and 6 had no known contacts to active TB. 49 were TST negative, of which 10 were household contacts, 1 non-household contact and 38 had no known contacts with active TB | Authors conclude the need for longitudinal studies for determination of predictive values | Not reported | Interesting how latent and uninfected participants were defined. LTBI: those who were TST positive but with no other symptoms and chest radiograph not suggestive of TB. Uninfected: defined as a well-child with negative TST or child with symptoms potentially suggestive of TB but in whom investigations for TB were negative or a child with an alternative diagnosis and complete recovery in the absence of specific TB treatment |
| Hansted 2009160 | Observational study done in Lithuania. All participants were BCG vaccinated | 10–17 year olds | Study subjects who had been in contact with a case of infectious TB were divided into three groups. (1) Culture confirmed; (2) high-risk group; those living with a family member with infectious TB or having contact with such a person at school. Those in this group were free from symptoms. (3) Low risk; those who have no identifiable risk of TB(no known risk of contact with TB patient, no symptoms and no complaints | IGRA (TSPOT.TB) | TST Mantoux test SSI PPD RT-23, 2TU positive if > 10 mm | 60% high-risk TST positive. 17.8% IGRA positive Calculated RR 3.375. For the low risk 65.4% were TST positive while 9.6% were IGRA positive. Calculated RR 6.8. The total number of discordant results was 54 out of 97 subjects in both high-risk and low-risk populations. Out of 61 TST positive patients 51 were IGRA negative |
Not recorded | No records of funding | Authors conclude that identifying latent TB in children using this method is useful, especially in countries like Lithuania which have a high incidence of TB despite a high coverage with BCG vaccination |
| Higuchi 2007162 | Observational prospective. Japan. Japanese students all BCG vaccinated | 349 aged 15–16 years. Patients were all male and previously BCG vaccinated. They attended the same high school as a student diagnosed with active TB | Students stratified into two groups those with close contact (sharing of classes with index case; 210) and those with limited contact (not attending classes with the index case; 139) | QFT-G. Considered positive when > 0.35 IU/ml | TST (defined standard test dose of tuberculin PPD equivalent to 2.5 tuberculin units). Erythema used instead of induration. An erythema of > 30 mm considered positive for a BCG vaccinated individual | The distribution of TST responses in both close and limited contacts was similar. (p = 0.20) | Follow up of 91 students with positive TST but negative QFT-G showed no signs of active TB after 3.5 years of follow up | Ministry of Health Labour and Welfare Japan | Partial verification only patients with positive TST were tested with QFT-G. Authors suggest that similar positive rates of TST in both strata of exposed groups suggest limited transmission of MTB |
| Higuchi 2009161 | Prospective observational study Japan. Participants from Japan BCG vaccination done | 313 participants between the ages of 8–12 years. In a Japanese school | Participants were exposed to an index case in the school. Close contact participants were those who had daily contact (at 90 hours contact). Casual participants: total of < 18 hours | IGRA (QFT-G) 0.35 IU/ml positive response | TST 0.1 ml [PPD NIPPON (BCG Manufacturing Tokyo Japan)] Equivalent to 3 TU PPD-S | QFT-G positivity in close contacts 9.8% as compared with 1.8% in casual contacts (p = 0.02). TST (5 mm) positivity in close contacts 52.6% as compared with 67.2% (p = 0.078). TST (10 mm) 34.2% compared with 28.7% (p = 0.488) | Not recorded. No child with negative QFT result developed active TB after 3 years. 3 out of 298 QFT negatives had a positive after 1 year | Not recorded | Authors suggest that QFT has the same performance characteristics in 8–12 year olds as adults. Suggestion of testing contacts 3 months after the end of exposure as an appropriate and sensitive approach |
| Lighter 2009163 | Observational prospective | 253 children aged < 18 years (mean age 9 years). Age stratified as follows < 24 months, 24–59 months, 60 months. Recruited from the well child clinic, paediatric chest clinic and paediatric inpatient ward. 42% were female. 72 received a single vaccination, 59 had visible BCG scars | Level of exposure graded as minimal (No known risk), low/moderate risk factors [(birth in or travel to a disease-endemic region and/or living with a household member with specific risks (emigrating from a disease-endemic region, having HIV, or having a history of imprisonment, homelessness, or intravenous drug use)]. High (known direct contact with TB index case) | QFT-G. Considered positive when > 0.35 IU/ml and > 25% than nil control value | TST (Mantoux technique). Considered positive with induration of > 10 mm | Proportion of QFT-G positive results for children with increasing gradients of M tuberculosis exposure Minimal –0% of TST+and –ve Low/moderate 6% of TST –ve and 19% TST+ were QFT-G+. High 0% of TST –ve and 100% of TST+ case were QFT-G+ |
Not determined | Pott's memorial foundation and the Thrasher Research Fund | Cut off of 0.35 IU/ml not validated especially for very young children who produce on average less IFN-8 than school-aged children and adults |
| Okada 2008164 | Observational/ Japan | They used 161 index cases and 217 contacts aged ≤ 5 years | Contacts stratified by varying risk of infection as classified by smear and culture result of index cases. (A) Smear –ve with positive or negative culture. (B) Smear positive grade 1+ including scanty smear. (C) Smear positive grade 2+. (D) Smear positive grade 3+ | IGRA (QFT-G) 0.35 IU/ml positive response | TST 0.1 ml [PPD NIPPON (BCG Manufacturing Tokyo Japan)] Equivalent to 2.5 TU PPD-S | Measured concordance rates and kappa values by smear positivity of index cases and by age of children. Concordance 0.87, 0.906, 0.837, 0.893 and 0.877 overall, kappa 0.308, 0.711, 0.536, 0.774 and 0.626 overall. Also measured multivariate ORs for positive results for both TST and QFT-G. The following covariates were analysed. Sex, age, BCG scar, period from final contact and smear positivity | Not determined | Japan International Cooperation Agency | Smear positivity of index cases was the most important factor for positivity of both TST and QFT-G |
| Tsiouris 2006165 | Observational/United States/South Africa | 1741. Mean age of 5–15 years | Participants grouped according to the status of contact they were living with. (A) Current case of active TB in the household. (B). Past case of active TB. (C) Current and past case of active TB | IGRA (QFT-G) | TST PPD RT23 (2 tuberculin units were used) | Univariate analysis showed the likelihood of having a positive IGRA increased with increasing age (p = 0.011) as did having a TST > 10 mm. Overall agreement increased with increasing cut-off of TST 0.52, 0.56 and 0.62 for 5 mm, 10 mm and 15 mm, respectively | Not determined | Aeras Global TB vaccine foundation | IGRA performed well without indeterminate results. The inability to obtain adequate blood specimen from 16.7% of participants is a drawback which is likely to be true of any whole-blood based paediatric test |
| Winje 2008192 | Cross sectional study/Norway/determined by presence of scar | 14–15 year olds | Factors associated with latent TB investigated include. Origin, sex, exposure to TB, travel history. Children grouped into western born, second generation and first generation | IGRA (QFT-G) 0.35 IU/ml positive | TST PPD RT23 (2 tuberculin units were used) | 9% of 511 TST positive children were IGRA positive. They determined adjusted ORs for a positive IGRA for origin of child and exposure. 0.9 (0.3–2.4) and 3.3 (1.6–6.2) for second generation and first generation, respectively, as compared with Western born. 2.9 (1.1–7.6) comparing exposure with non-exposure of TB | Not determined | Division of infectious disease control at the Norwegian Institute of Public Health | The authors conclude that factors other than TB infection are widely contributing to positive TST results in this group and indicate the improved IGRA specificity for latent TB |
Immunocompromised people
| Bibliography (Ref ID) | Number of participants. Type of study/country of origin. Immunocompromised condition/medicines. Risk factors. Characteristics | Reference test | Index test | Specificity and sensitivity or modified measure of effect/measures of agreement | Positive and negative predictive values | Source of funding | Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balcells 2008167 | Observational study of individuals from Chile. HIV positive patients. Mean CD4 count 393/µl (range 100–977 µl) 116 mean age 38.8 years (range 21–71 years). Older age, history of previous TB disease, previous known exposure to a case of active pulmonary TB, health-care workers or individuals working with homeless people, residence in prison | TST (Mantoux method. 2 TU) dose of PPD RT23) | IGRA (QFT) | Correlation between TST and IGRA results in HIV-positive individuals IGRA+IGRA–TotalTST+9211TST–89098Total1792109 They also performed univariate analysis for a positive LTBI test depending on several factors TB risk factors |
IGRA+ | IGRA– | Total | TST+ | 9 | 2 | 11 | TST– | 8 | 90 | 98 | Total | 17 | 92 | 109 | Not determined | Supported by a grant from the Department of the Pontificia, University of Chile. IGRA were supplied at reduced price by Cellestis | Authors observed that, multivariate analysis confirmed that past TB was independently associated with a positive TST (p = 0.016) as well as a higher CD4 count (p = 0.044). For IGRA past TB was the only factors significantly associated with a positive result. (p = 0.041) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| IGRA+ | IGRA– | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST+ | 9 | 2 | 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST– | 8 | 90 | 98 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 17 | 92 | 109 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bartalesi 2009168 | 398 participants with rheumatic diseases requiring the use of biological drugs in Italy. Participants were treated with systemic corticosteroids, conventional DMARDs, and TNF-α inhibitors. Risk factors associated with LTBI included birth or residence in high prevalence area, close contact to patients with sputum-positive TB | TST (5 units PPD) | IGRA (QFT) | Overall results IGRA+IGRA–TotalTST+393574TST–13306319Total52341393 Also presented ORs adjusting for the association of risk factors for TB infection and IGRA and TST positivity Number of risksIGRA+TST+ORp-valueORp-value01113.3< 0.052.57< 0.05> 25.71< 0.055.35< 0.05 |
IGRA+ | IGRA– | Total | TST+ | 39 | 35 | 74 | TST– | 13 | 306 | 319 | Total | 52 | 341 | 393 | Number of risks | IGRA+ | TST+ | OR | p-value | OR | p-value | 0 | 1 | 1 | 1 | 3.3 | < 0.05 | 2.57 | < 0.05 | > 2 | 5.71 | < 0.05 | 5.35 | < 0.05 | Not determined | Not recorded | Until further data are available on the implication of discordant TST/IGRA results, a strategy of simultaneous TST and IGRA testing in populations with low prevalence of BCG vaccination should maximise the sensitivity of LTBI diagnosis | ||||||||||||||||||||||||||||||
| IGRA+ | IGRA– | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST+ | 39 | 35 | 74 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST– | 13 | 306 | 319 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 52 | 341 | 393 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Number of risks | IGRA+ | TST+ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OR | p-value | OR | p-value | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0 | 1 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | 3.3 | < 0.05 | 2.57 | < 0.05 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| > 2 | 5.71 | < 0.05 | 5.35 | < 0.05 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cobanoglu 2007169 | 106 divided into groups 1 and 2. Group 1 (38 healthy individuals), group 2 (68 patients with chronic inflammatory diseases). 87% of these patients were on immunosuppressive medications such as methotrexate, methylprednisolone, prednisolone. The study was conducted in the University Faculty of Medicine in Ankara, Turkey | TST 0.1 ml (5TU) of PPD | IGRA (QFT) | Results stratified by age to adjust for supposed BCG effect < 25 years (57 participants) Group 1 9/25 discordant results All TST+ IGRA– Group 2 17/32 discordant results 16 (TST+ IGRA–) 1 (TST– IGRA+) > 25 years (40 participants) Group 1 4/11 discordant results 3 (TST+ IGRA–) 1 (TST– IGRA+) Group 2 13/29 discordant results All 13 (TST+ IGRA–) 9 had IGRA indeterminate results of whom 7 were immunocompromised |
Not determined | Not recorded | Authors say study should be accepted as a basis for the design of future studies that will be helpful for physicians to decide whether or not the IGRA is more sensitive than TST to detect LTBI before the use of TNF-α blockers | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jones 2007170 | 207 HIV-infected individuals with a mean age of 47 years. 52% were male. They were also stratified according to CD4 count < 100, 19; 101–199, 24; 200–499, 88; > 500, 70. Study conducted in Mount Sinai Medical Centre in New York, New York, NY, USA | TST 0.1 ml (5 TU PPD) | IGRA (QFT) | Overall concordance between IGRA and TST results IndIGRA–IGRA+TotalTST–101726188TST+08513Total1018011201Ind, indeterminate. |
Ind | IGRA– | IGRA+ | Total | TST– | 10 | 172 | 6 | 188 | TST+ | 0 | 8 | 5 | 13 | Total | 10 | 180 | 11 | 201 | Ind, indeterminate. | Not determined | QuantiFERON kits donated by Cellestis | IGRA is able to distinguish between indeterminate tests and those that are truly negative. In contrast, a negative TST does not differentiate between individuals who are anergic and those who might have a truly negative TST | |||||||||||||||||||||||||||||||||||||||||||||
| Ind | IGRA– | IGRA+ | Total | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST– | 10 | 172 | 6 | 188 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST+ | 0 | 8 | 5 | 13 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 10 | 180 | 11 | 201 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ind, indeterminate. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Luetkemeyer 2007171 | 294 HIV-infected patients sampled from two cohorts based in the United States. 55% of participants had lived or worked in homeless shelter, prison, hospital, or a drug rehabilitation unit or were born in a country with high TB incidence, or had had contact with an active TB case | TST (5TU PPD) | IGRA (QFT) | 196 participants with both TST and IGRA results valid had the following overall result TST+TST–TotalIG+81119IG–10167177Total18178196 Results were also stratified by CD4 count CD4+ STRATA (cells/mm3)< 100100–350> 350TotalIG+061925IG–26101127254IG(I)54615Total31111152294TST+071219TST–217689186Total2183101205 |
TST+ | TST– | Total | IG+ | 8 | 11 | 19 | IG– | 10 | 167 | 177 | Total | 18 | 178 | 196 | CD4+ STRATA (cells/mm3) | < 100 | 100–350 | > 350 | Total | IG+ | 0 | 6 | 19 | 25 | IG– | 26 | 101 | 127 | 254 | IG(I) | 5 | 4 | 6 | 15 | Total | 31 | 111 | 152 | 294 | TST+ | 0 | 7 | 12 | 19 | TST– | 21 | 76 | 89 | 186 | Total | 21 | 83 | 101 | 205 | Not determined | Not recorded | Authors noted that until further data are available on the implication of discordant TST and IGRA results, a strategy of simultaneous TST and QFT testing where feasible would maximize potential LTBI diagnoses in HIV-infected patients | ||||||||||
| TST+ | TST– | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG+ | 8 | 11 | 19 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG– | 10 | 167 | 177 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 18 | 178 | 196 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CD4+ STRATA (cells/mm3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 100 | 100–350 | > 350 | Total | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG+ | 0 | 6 | 19 | 25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG– | 26 | 101 | 127 | 254 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG(I) | 5 | 4 | 6 | 15 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 31 | 111 | 152 | 294 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST+ | 0 | 7 | 12 | 19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST– | 21 | 76 | 89 | 186 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 21 | 83 | 101 | 205 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mandalakas 2008172 | 43 HIV-infected participants were enrolled in this study. 23 children and 20 adults. The mean age of adults was 18.7 years, the mean for children was 4.4 years. Study was conducted in South Africa | TST (2 TU 0.1 ml PPD RT23) | IGRA (QFT and T.SPOT.TB) | Discordant results for TST and IGRAs TSPOT+ TST–TSPOT– TST+All29.710.8Children39.113.0Adults14.37.1 QFT+ TST–QFT– TST+All026.9Children025.0Adults028.6 |
TSPOT+ TST– | TSPOT– TST+ | All | 29.7 | 10.8 | Children | 39.1 | 13.0 | Adults | 14.3 | 7.1 | QFT+ TST– | QFT– TST+ | All | 0 | 26.9 | Children | 0 | 25.0 | Adults | 0 | 28.6 | Not determined | Funded by Bill and Melinda Gates Foundation | Authors commented that no indeterminate results were observed in children with a CD4 count higher than adults. Adults with indeterminate results tended to have low CD4 counts and negative TST results | |||||||||||||||||||||||||||||||||||||||||||
| TSPOT+ TST– | TSPOT– TST+ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All | 29.7 | 10.8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Children | 39.1 | 13.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adults | 14.3 | 7.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| QFT+ TST– | QFT– TST+ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All | 0 | 26.9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Children | 0 | 25.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adults | 0 | 28.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Manuel 2006173 | 153 patients with chronic liver disease who were candidates for liver transplant. Patients had various risk factors such as contact with active TB patient, born or stay in country with high prevalence of TB. Study was conducted in a preliver transplant clinic in Canada | TST | IGRA (QFT) | Overall results 5 mm cut-off TST+TST–TotalIGRA+25934IGRA–1295107Total37104141 10 mm cut-off TST+TST–TotalIGRA+181634IGRA–998107Total27114141 Indeterminate IGRA result 12/153 = 7.8% |
TST+ | TST– | Total | IGRA+ | 25 | 9 | 34 | IGRA– | 12 | 95 | 107 | Total | 37 | 104 | 141 | TST+ | TST– | Total | IGRA+ | 18 | 16 | 34 | IGRA– | 9 | 98 | 107 | Total | 27 | 114 | 141 | Not determined | Test kits provided by Cellestis Ltd | Authors conclude that study demonstrates that IGRA and TST performed similarly for the diagnosis of LTBI in a population with end-stage liver disease | |||||||||||||||||||||||||||||||||||
| TST+ | TST– | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IGRA+ | 25 | 9 | 34 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IGRA– | 12 | 95 | 107 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 37 | 104 | 141 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST+ | TST– | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IGRA+ | 18 | 16 | 34 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IGRA– | 9 | 98 | 107 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 27 | 114 | 141 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Matulis 2008174 | 142 participants of which 126 received immunosuppressive therapy. 50% were female. AntiTNF, DMARDS and corticosteroids were the medicines they received. The mean age was 48 years. Study was conducted in a University Hospital in Berne Switzerland | TST (2 TU 0.1 ml PPD RT23) | IGRA (QFT) | Overall results TST+TST–UnTotalIG+105217IG–346023117Indeterminate2428Total466927142 Multivariate analysis were presented as ORs Corticosteroid treatment (yes, no) OR IGRA = 1.11 (0.30–4.14) OR TST = 0.74 (0.32–1.72) DMARDs treatment (yes, no) OR IGRA = 2.34 (0.52–10.6) OR TST = 0.75 (0.32–1.77) TNF-α inhibitors OR IGRA = 0.19 (0.05–0.76) |
TST+ | TST– | Un | Total | IG+ | 10 | 5 | 2 | 17 | IG– | 34 | 60 | 23 | 117 | Indeterminate | 2 | 4 | 2 | 8 | Total | 46 | 69 | 27 | 142 | Not determined | Study funded by Swiss commission for Rheumatic Disease and the Swiss National Science Foundation | They did a multivariate analysis which did not include analysis for the participants which had two or more immunosuppressant medications | |||||||||||||||||||||||||||||||||||||||||
| TST+ | TST– | Un | Total | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG+ | 10 | 5 | 2 | 17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG– | 34 | 60 | 23 | 117 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indeterminate | 2 | 4 | 2 | 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 46 | 69 | 27 | 142 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Piana 2007175 | 138 immunosuppressed haematology patients in Italy. All patients were identified as nosocomial contacts of a case of smear positive TB. No information on graded exposure. Study was conducted in a chemotherapy unit in Italy | TST 0.1 ml (5 TU) of Siebert PPD | IGRA (T-SPOT.TB) | Overall result IGRA+IGRA–IndIns T cellTotalTST+2130024TST–34575298No res681116Total616863138Ind, indeterminate; Ins, insufficient; No res, no result. Results also stratified by pathological WBC count Pathological (< 4.3 × 103 or > 10.8 × 103 WBC/mm–3) IGRA 44.3% +ve TST 14.5% +ve Non-pathological IGRA 44.6% +ve TST 25.9+ve |
IGRA+ | IGRA– | Ind | Ins T cell | Total | TST+ | 21 | 3 | 0 | 0 | 24 | TST– | 34 | 57 | 5 | 2 | 98 | No res | 6 | 8 | 1 | 1 | 16 | Total | 61 | 68 | 6 | 3 | 138 | Ind, indeterminate; Ins, insufficient; No res, no result. | Not determined | T-SPOT.TB kits provided by Oxford Immunotech | It was important to determine whether or not the higher apparent prevalence of infection found with IGRA was due to the TST being falsely negative due to anergy, or to the IGRA being falsely positive in a number of patients | |||||||||||||||||||||||||||||||||||
| IGRA+ | IGRA– | Ind | Ins T cell | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST+ | 21 | 3 | 0 | 0 | 24 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST– | 34 | 57 | 5 | 2 | 98 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No res | 6 | 8 | 1 | 1 | 16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 61 | 68 | 6 | 3 | 138 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ind, indeterminate; Ins, insufficient; No res, no result. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ponce de Leon 2008176 | Cross sectional study conducted in Peru. 106 rheumatoid arthritis patients, of whom 73% were receiving methotrexate and 91% were receiving prednisolone at a dose of < 10 mg daily. They also recruited 97 controls | TST (Mantoux method. 2 TU dose of PPD RT23) | IGRA (QFT) | Overall results showing TST and IGRA results of immunosuppressed patients and controls RA patientsTST+TST–TotalIG+212445IG–65056Total2774101RA, rheumatoid arthritis. ControlTST+TST–TotalIG+50555IG–112738Total613293 |
RA patients | TST+ | TST– | Total | IG+ | 21 | 24 | 45 | IG– | 6 | 50 | 56 | Total | 27 | 74 | 101 | RA, rheumatoid arthritis. | Control | TST+ | TST– | Total | IG+ | 50 | 5 | 55 | IG– | 11 | 27 | 38 | Total | 61 | 32 | 93 | Not determined | Not recorded | Authors concede that a limitation of the study was the lack of a gold standard method for diagnosing LTBI. They attempted to compensate for this by evaluating both diagnostic tests in RA patients and matched controls. Data indicate that IGRA is more accurate than the TST in RA patients but cannot determine absolute sensitivity of both tests | ||||||||||||||||||||||||||||||||
| RA patients | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST+ | TST– | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG+ | 21 | 24 | 45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG– | 6 | 50 | 56 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 27 | 74 | 101 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RA, rheumatoid arthritis. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Control | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST+ | TST– | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG+ | 50 | 5 | 55 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG– | 11 | 27 | 38 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 61 | 32 | 93 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Richeldi 2009177 | 369 participants who were prospectively enrolled into the following immunosuppressed groups. Liver transplantation candidates, chronically HIV-infected patients and patients with haematological malignancies. Study participants were evaluated in a referral centre in Italy. Only about 3.6% patients were BCG vaccinated | TST (5 IU PPD) | IGRA (T-SPOT.TB) and (QFT) | Overall results LTCHIVHM12011695TST+20610TST–10011085TSP+32425TSP–8711269TSP.I101QFT+28517QFT–8010473QFT.I1275HM, haematologic ,malignancies; LTC, liver transplantation candidates; QFT.I, indeterminate result; TSP.I indeterminate result. |
LTC | HIV | HM | 120 | 116 | 95 | TST+ | 20 | 6 | 10 | TST– | 100 | 110 | 85 | TSP+ | 32 | 4 | 25 | TSP– | 87 | 112 | 69 | TSP.I | 1 | 0 | 1 | QFT+ | 28 | 5 | 17 | QFT– | 80 | 104 | 73 | QFT.I | 12 | 7 | 5 | HM, haematologic ,malignancies; LTC, liver transplantation candidates; QFT.I, indeterminate result; TSP.I indeterminate result. | Not determined | Not recorded | Study shows that the performance of IGRA, both in terms of rates of positive results and in diagnostic agreement varies greatly across different categories of patients who are at increased risk of TB reactivation. Because of the importance of targeting such high-risk groups, for effective TB control, we advise caution when interpreting the results of IGRA among immunosuppressed patients | ||||||||||||||||||||||||||
| LTC | HIV | HM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 120 | 116 | 95 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST+ | 20 | 6 | 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST– | 100 | 110 | 85 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TSP+ | 32 | 4 | 25 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TSP– | 87 | 112 | 69 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TSP.I | 1 | 0 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| QFT+ | 28 | 5 | 17 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| QFT– | 80 | 104 | 73 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| QFT.I | 12 | 7 | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HM, haematologic ,malignancies; LTC, liver transplantation candidates; QFT.I, indeterminate result; TSP.I indeterminate result. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schoepfer 2008178 | 212 participants consisting of 114 Crohn’s disease, 44 ulcerative colitis 10 indeterminate colitis and 44 controls. Study was conducted in Switzerland | TST (2 TU 0.1 ml PPD RT23) | IGRA (QFT) | Overall results DiagnosisnBCGIGRA+TST+IBD168+ve12/11827/118–ve2/503/50Control44+ve3/3317/33–ve1/112/11IBD, inflammatory bowel disease. |
Diagnosis | n | BCG | IGRA+ | TST+ | IBD | 168 | +ve | 12/118 | 27/118 | –ve | 2/50 | 3/50 | Control | 44 | +ve | 3/33 | 17/33 | –ve | 1/11 | 2/11 | IBD, inflammatory bowel disease. | Not determined | Not recorded | Authors concluded that the application of TST for detecting LTBI is limited in RA patients by the frequent presence of anergy. Combined IGRA assay and TST can aid in detecting LTBI in RA patients receiving adalimumab therapy | |||||||||||||||||||||||||||||||||||||||||||
| Diagnosis | n | BCG | IGRA+ | TST+ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IBD | 168 | +ve | 12/118 | 27/118 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| –ve | 2/50 | 3/50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Control | 44 | +ve | 3/33 | 17/33 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| –ve | 1/11 | 2/11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IBD, inflammatory bowel disease. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shovman 2009179 | Study performed in Israel. 35 rheumatoid arthritis patients and 15 controls | TST (2 TU 0.1 ml PPD RT23) | IGRA (QFT) | Overall results TST results as percentage+ve–veAnergyRA451737Control15778RA, rheumatoid arthritis. IGRA results by percentage+ve–veIndeterminateRA11.46028.6Control13870RA, rheumatoid arthritis. |
TST results as percentage | +ve | –ve | Anergy | RA | 45 | 17 | 37 | Control | 15 | 7 | 78 | RA, rheumatoid arthritis. | IGRA results by percentage | +ve | –ve | Indeterminate | RA | 11.4 | 60 | 28.6 | Control | 13 | 87 | 0 | RA, rheumatoid arthritis. | Not determined | Not recorded | The authors commented that the high rate of indeterminate results reduces the clinical utility of IGRA and questions its use in the diagnosis of LTBI in rheumatoid arthritis patients | |||||||||||||||||||||||||||||||||||||||
| TST results as percentage | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| +ve | –ve | Anergy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RA | 45 | 17 | 37 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Control | 15 | 7 | 78 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RA, rheumatoid arthritis. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IGRA results by percentage | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| +ve | –ve | Indeterminate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RA | 11.4 | 60 | 28.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Control | 13 | 87 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RA, rheumatoid arthritis. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Soborg 2009180 | 302 patients with inflammatory disease were included. 153 had rheumatoid arthritis, 40 spondyloarthropathies, 51 sarcoidosis and 58 participants presenting with other conditions such as psoriatic arthritis. Patients either received DMARDs or corticosteroid treatment. The study was conducted in the rheumatology department of the Heart centre in Copenhagen, Denmark | TST (2 TU 0.1 ml PPD RT23) | IGRA (QFT) | Results presented as risk ratios which determined the associations between factors relevant to TB infection and test reactivity to either IGRA or TST Corticosteroid treatment (yes, no) RR IGRA = 0.5 (0.1–1.6) RR TST = 0.4 (0.1–1.0) DMARDs treatment (yes, no) RR IGRA = 0.7 (0.3–1.7) RR TST = 1.3 (0.7–2.3) CD4 count (< 500 > 500) RR IGRA = 1 (0.2–3.2) RR TST = 1.5 (0.7–3.3) Danish GuidelineTST–TST+IGRA–18036IGRA +99 US GuidelineTST–TST+IGRA–15957IGRA+99 |
Danish Guideline | TST– | TST+ | IGRA– | 180 | 36 | IGRA + | 9 | 9 | US Guideline | TST– | TST+ | IGRA– | 159 | 57 | IGRA+ | 9 | 9 | Not recorded | Not recorded | Interesting that authors stated that study was not designed to address the question of disease progression, as protocol recommended prophylactic treatment to test-positive patients | |||||||||||||||||||||||||||||||||||||||||||||||
| Danish Guideline | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST– | TST+ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IGRA– | 180 | 36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IGRA + | 9 | 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| US Guideline | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TST– | TST+ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IGRA– | 159 | 57 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IGRA+ | 9 | 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Talati 2009181 | 336 HIV-positive patients of mean age of 42 years. Patients had a past medication history of LTBI, diabetes mellitus, chronic renal insufficiency, history of malignancy, anytime smoker and intravenous drug use. Study done in the USA | TST 0.1 ml (5 TU) of Siebert PPD | IGRA (TSPOT.TB AND QFT) | Reported a CD4 count of < 200 as associated with an indeterminate result for both IGRAs OR = 3.6 (1.9,6.8) | Not determined | Partly supported by Centers for Disease Control and Prevention (CDC) | Authors commented that given the results of the study and the limited data currently available it was unclear if IGRAs can be used alone for the diagnosis of LTBI in HIV-infected individuals | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vassilopoulos 2008182 | Observational study Some were on DMARD and various other immunosuppressive medicines such as steroids 70 participants with various rheumatic diseases with a mean age of 60 years. The study was conducted in an outpatients rheumatology clinic in Athens, Greece |
TST (Mantoux method 2 TU dose of PPD RT23) | IGRA (T-SPOT.TB) | Overall results showing discordant and concordant results between tests TST+TST–TotalIG+12416IG–153954Total274370 |
TST+ | TST– | Total | IG+ | 12 | 4 | 16 | IG– | 15 | 39 | 54 | Total | 27 | 43 | 70 | Not determined | Not recorded | Authors concluded that at this point based on the available data, replacement of the TST by the TSPOT cannot definitely be recommended. More data examining the tests cost, feasibility and reproducibility as well as the outcome of antiTNF treated rheumatic patients with discordant TST/TSPOT results are needed before recommendations can be made | ||||||||||||||||||||||||||||||||||||||||||||||||||
| TST+ | TST– | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG+ | 12 | 4 | 16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IG– | 15 | 39 | 54 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 27 | 43 | 70 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Recent arrivals from countries with a high incidence of tuberculosis
| Bibliographic reference (Ref ID) | Study type | Number of participants | Prevalence/incidence | Country of study/origin of participants | Participant characteristics | Type of test | Reference standard | Sensitivity and specificity/modified measure of effect | Positive/negative predictive values or modified | Source of funding | Additional comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brodie 2008183 | Prospective | 123 | Not specifically recorded | United States/does not mention countries of origin of immigrants | Patients > 5 years. Study group were those who had had contact with active TB patients and controls were those who had not had any contact. A lot of the patients were recent immigrants with a high rate of BCG vaccination | IGRA (ESAT-6 and CFP-10) | TST | Overall agreement between TSPOT.TB and TST was 64% and the kappa value was 0.33 (0.19–0.48). For BCG-vaccinated people it was 56% (43–68%) and 0.22 (0.06–0.37) respectively. In non-vaccinated people it was 82% (68–96%) and 0.64 (0.38–0.91) | Yes | Oxford Immunotech | Does not mention how they determined either those with ATB or LTBI. Used contact status as surrogate for LTBI and used that as gold standard. Does not give indication of prevalence or incidence of countries of origin of immigrants |
| Carvalho 2007184 | Observational cross-sectional/retrospective | 130 | Immigrants from countries with at least an incidence of 50 per 100,000 | Italy/sub-Saharan Africa, Northern Africa, Eastern Europe, Asia, Latin America | 32 female 98 male. Median age 28 years (range 19–50 years). Immigrants from high-incidence countries within the last 5 years | IGRA (QFTBG) threshold level 0.35 IU/ml | TST (threshold 10 mm) | Association of discordance/concordance between two tests and BCG scar, sex, age, race, previous TB contact. Overall agreement was 71% k coefficient = 0.37. 100% agreement between TST and IGRA for induration below 10 mm | No data | Lombardia Region grant no 286/98 | BCG vaccination independently negatively associated with discordance between tests. 0.28 (0.1–0.77) p = 0.01. BCG scar not always good indicator of BCG vaccination. Overall k coefficient = 0.37. 100% agreement between TST and IGRA for induration below 10 mm |
| Diel 2008186 | Observational prospective study | 1794 | Incidence of TB in Hamburg, Germany reported to be 10.8 per 100,000 | Germany/noted as ‘foreign born’ but cases progressing to TB documented as from Turkey, Angola | Close contacts of sputum-smear positive cases with at least 40 hours exposure in a closed room. Age range between 0 and 60 years, with most (87.5%) falling between the 16–50 range. 28% were migrants from 29 different countries | IGRA (ESAT-6, CFP-10) (QFTGinTube) | TST (threshold 5 mm and 10 mm) | Overall kappa statistics 0.276 and 0.119 and 0.616 for BCG vaccinated and non BCG, respectively. For the concordance the values were 69.2%, 44.2% and 90.7% respectively. OR for a positive test if foreign born adjusted for BCG vaccination, age and exposure time were determined as follows. TST 5 mm 5.81 (3.6–9.1), 10 mm 5.2 (3.2–8.4), QFT 2.28 (1.3–3.9) | Not determined | No declared sponsor | Specific countries of origin of migrants not mentioned |
| Diel 2006185 | Observational prospective study | 311 | TB incidence rate in Hamburg 12 per 100,000. Immigrants from countries with incidence of at least 20 per 100,000 | Germany/25 different countries including former Soviet Union and Turkey | Close contacts of sputum-smear positive cases. Contacts with < 40 hours contact time were excluded. Mean age 28.5 years Previous BCG vaccination 157 (50.8%), foreign/German (27.1%/72.9) | IGRA (ESAT-6, CFP-10) (QFTGinTube) | TST 5 mm = 137/309 TST (28/137 positive by IGRA) 10 mm = 64/309 15 mm = 25/309 | Overall kappa statistics 0.2, 95% CI 0.14 to 0.23. Concordant results 197/309 (63.8%). Positive result 169/172 (98.2%). Negative result 28/137 (20.4%). Concordance for 5 mm between BCG vaccination 38.9% k = 0.08 (0.026–0.08). Not vaccinated 89.5% k = 0.58 (0.4–0.68) for 10 mm, 77.1% k = 0.35 (0.24–0.35) for no BCG, and 94.1% k = 0.68 (0.46–0.81) for BCG. For TST (5 mm) OR = 5.4, TST (10 mm) 7.3 and 4.7 QFT | No data | No sponsor | For QFT-only origin is an independent predictor of a positive test result. For TST BCG vaccination also acts an independent predictor. Study does not mention how the specific countries or how recent migrants had been in the country |
| Franken 2007187 | Prospective cross-sectional study | 909 | Range from < 10, 10–49, 50–99, 100–199 > 200) per 100,000 | Netherlands/Bosnia Kyrgystan Iraq and Afghanistan | Army personnel who had returned from mission (738) in high-incidence countries compared with new recruits (171) who had not been on mission | IGRA QFGinTube (ESAT-6 CFP-10, TB7.7) | TST (threshold 10 mm and 15 mm) | Discordance and concordance between tests. Overall concordance and kappa values were determined to be 82% and 0.19 respectively for 10 mm cut-off and 92.3% and 0.24 respectively for 15 mm TST cut-off | No data | Study not clear with regard to the definition of LTBI | |
| Janssens 2008188 | Observational prospective study | 295 | TB incidence 20 per 100,000 in Geneva. Incidence in countries from which immigrants originated between (50 – > 100) per 100,000 | Switzerland/countries not specified but categorised by incidence | Mean age 40 years (range 16–83 years). Foreign born 73.9% (218). Contacts were exposed to cavitary TB 105 (35.6%), non-cavitary TB 168 (56.9%) and pulmonary TB 22 (7.5%) | IGRA (ESAT-6, CFP-10) (T-SPOT.TB) | TST induration 5 mm 173 (58.6%), 10 mm 148 (50.2%), 61 mm (20.7%) | Overall concordant results showed 60.7% TST 5 mm, 63.6% 10 mm, 63.9% 15 mm. Kappa values were 0.24, 0.27 and 0.19 respectively. BCG Non-vaccinated subjects concordant results were 78.4%, 76.5% and 78.4% respectively while kappa values were 0.47, 0.41 and 0.28 for 5 mm, 10 mm and 15 mm respectively when comparing with IGRA. aOR for sex, BCG and incidence in country of origin (< 50 per 100,000 is used as baseline) showed these variables were independent predictors of a positive result 2.07 (1.22–3.51), 2.98 (1.39–6.41) 3.67 (1.40–1.90) respectively for TST 5 mm. Only incidence in country of origin showed the significant association with a positive result for TST 10 mm 2.22 (1.15–4.27) and 3.84 (1.61–9.20) for 50–99 per 100,000 and > 100 per 100,000 respectively. < 50 per 100,000 was baseline. For IGRA, age by 10-year increments and incidence in country of origin were the independent predictors of a positive result. 1.30 (1.06–1.6) for age and 2.17 (1.13–4.15) and 2.62 (1.18–5.82), respectively, for two categories of incidence | Not determined | Ligue Pulmonaire Genevoise | Countries of origin of foreign-born nationals not listed. Not very specific of exclusion of positive results if any of chest radiography. In the analysis they did not mention if they adjusted for immunocompromised individuals. They were only 6%. The TB incidence of Geneva from where they recruited was 20 per 100,000. They did not use that as the baseline value in calculations |
| Kik 2009189 | Observational retrospective study | 821 | Not specifically recorded | Netherlands/South America, Asia, sub-Saharan Africa | Participants aged > 16 years. Close contacts of sputum smear positive TB patients. Foreign born and second generation immigrants | IGRA (QET-GIT, TSPOT.TB) (ESAT-6, CFP-10, TB7.7) | TST (threshold 5 mm 10 mm and 15 mm) | Associations between test results and remote exposure, defined as birth outside Europe and North America. Attributable fraction to particular risk factors calculated. Overall kappa values TST 15 mm 0.418 for QFT and 0.379 for TSPOT.TB. For 10 mm they were 0.198 and 0.190 respectively. Agreement values were 71.3% and 69.9% for QFT and TSPOT.TB respectively for 15 mm. For 10 mm they were 62.1% and 64.9% respectively. The continent of birth was the only variable which was independently associated with a positive result for TST 10 mm, p-value for trend 0.031. Both QFT and TSPOT.TB also showed a positive result independently associated with continent of birth and age | No data | Netherlands Organisation for Health Research and Development | Partial verification was performed on those with TST > 5 mm. Possibility of inclusion of patients with past active TB infections. Vague about the level of contact. Does not indicate duration of contact with infected individuals. Does not mention what they did with positive or negative CXRs. They do not mention how deduced LTBI |
| Nienhaus 2008190 | Observational cross-sectional/retrospective | 1040 | Incidence of TB in Germany reported to be < 6 per 100,000 and > 20 per 100,000 in countries from where the immigrants originated | Germany/Germany Turkey, Eastern Europe and Africa | Study population 1040 healthy individuals. Mean age of 31.6 years 61.8% female, 25.4% foreign born, 43.4% had previous BCG vaccination. 41.8% HCW | IGRA (QFTBG) threshold level 0.35 IU/ml positive result 100/1033 | TST (threshold 5 mm 311/1033 (30.1%) 10 mm = 191/1033 (18.5%) 15 mm = 69/1033 (6.7%) | Agreement 5 mm 74.8%, 10 mm 84.2%, 15 mm 89.8%. Kappa statistics 5 mm (0.26) 10 mm (0.37) 15 mm (0.33.) BCG vaccinated 5 mm (0.12) 10 mm (0.28) 15 mm (0.34). No vaccination 5 mm (0.5) 10 mm (0.54) 15 mm (0.3) aOR for positive TST (10 mm) for foreign birthplace was 4.6 (3.21–6.53) as compared with German birth, for QFT it was 2.6 (1.71–4.09) | No data | No sponsor reported | Although study states the population consisted of health persons they have said nothing to rule out symptomless TB by chest radiography. TST at 10 mm could possibly be confounded by sex, foreign birthplace and BCG vaccination. QFT could be confounded by age and foreign birthplace. TST+/QFT- discordance is associated with foreign birthplace. Authors explain that such discordance might be explained by resolved or old TB infections that are detected by TST and not QFT |
| Porsa 2006191 | Cross-sectional/observational | 474 | TB prevalence in United States < 10 per 100,000 of foreign born the prevalence reported 25–300 per 100,000 | United States/Mexico, Jamaica, Nicaragua, Ecuador, El Salvador, Honduras, the Philippines and Brazil | Adult inmates > 18 years. 114 female, 295 male. 370 born in the United States, 39 foreign born. 344 patients had prior incarceration. There was a mix of Caucasian African-American and Hispanic ethnicities | IGRA (ESAT-6 and CFP-10) (QFG-In-Tube) | TST induration 10 mm | Kappa statistics for discordance and concordance between TST and QFGT. Adjusted ORs calculated to determine which factors including ethnicity, old age, foreign birth and prior incarceration were more associated with discordance | Not determined | Health Resources and Services Administration Bureau of health professions Grant. Kits provided by Cellestis | On logistic regression African-American ethnicity only variable associated with positive results for both assays. Mentioned that positive IGRA indicates more recent and ongoing infection while positive TST indicates a remote infection in the past. Hence sensitivity appeared better in TSTs than IGRAs |
| Winje 2008166 | Observational cross-sectional/retrospective | 1000 | TB incidence rate in Norway 6.3 per 100,000 | Norway/Iraq, Somalia, Russia, Iran, Eritrea, Afghanistan, sub-Saharan Africa | Asylum seekers. At least 18 years of age. 75.1% male and 24.9% female | IGRA (ESAT-6 and CFP-10) (QFG-In-Tube) | TST (threshold 6 mm) 460/912 (50.4%) 10 mm 311/921 (34.1%) 15 mm (15.5%) | Agreement 72% for 6 mm, 79% 10 mm, 78% 15 mm. Kappa 6 mm 0.43 (0.37–0.49), 10 mm 0.51 (0.45–0.57), 15 mm 0.39 (0.32–0.47), statistics 0.43 (0.37–0.49). aOR continent of origin with Asia as baseline for TST 15 mm 3.8 and 3.3 for QFT | Not determined | Definite prevalence or incidence not recorded for countries of origin. For QFT, BCG vaccination and sex were not independent predictors of a positive result while country of origin, age group and level of exposure independently predicted a positive test. For TST 15 mm the variables which independently predicted a positive result were sex, country of origin and level of exposure |
Appendix 6 List of studies excluded from the clinical effectiveness review with reasons for exclusion (n = 424)
MEDLINE
| Number | Author ID | Details | Reason(s) for exclusion |
|---|---|---|---|
| 1 | Abud-Mendoza C | Should tuberculin skin test be positive to give latent tuberculosis treatment before tumor necrosis factor-alpha inhibitors in selected patients in developing countries? J Rheumatol 2010;37:672–3; author reply 673 | Letter |
| 2 | Abu-Taleb AM | Interferon-gamma release assay for detection of latent tuberculosis infection in casual and close contacts of tuberculosis cases. East Mediterr Health J 2011;17:749–53 | Mixed population and/or no subgroup of interest |
| 3 | Ahmadinejad Z | Diagnosis of latent tuberculosis infection in candidates for kidney transplantation (comparison of two tests). Acta Med Iran 2012;50:305–10 | No construct validity |
| 4 | Altet-Gomez N | Diagnosing TB infection in children: analysis of discordances using in vitro tests and the tuberculin skin test. Eur Respir J 2011;37:1166–74 | Results for TST and IGRA were combined |
| 5 | American College Health Association | Tuberculosis screening and targeted testing of college and university students. J Am Coll Health 2011;59:670–7 | Guideline |
| 6 | Andrisani G | Comparison of Quantiferon-TB Gold versus tuberculin skin test for tuberculosis screening in inflammatory bowel disease patients. J Gastrointestin Liver Dis 2013;22:21–5 | No construct validity |
| 7 | Anibarro L | Tuberculin skin test and interferon-γ release assay show better correlation after the tuberculin ‘window period’ in tuberculosis contacts. Scand J Infect Dis 2011;43:424–9 | Mixed population and/or no subgroup of interest |
| 8 | Anonymous | Proceedings of the Second Global Symposium on Interferon-Gamma Release Assays. Dubrovnik, Croatia, May 30–1 June 2009. Int J Tuberc Lung Dis 2010;14(Suppl. 1):3–70 | Abstract |
| 9 | Baboolal S | Comparison of the QuantiFERON-TB Gold assay and tuberculin skin test to detect latent tuberculosis infection among target groups in Trinidad and Tobago. Pan Am J Public Health 2010;28:36–42 | Inappropriate proxy for LTBI |
| 10 | Basu Roy R | Identifying predictors of interferon-γ release assay results in pediatric latent tuberculosis: a protective role of bacillus Calmette–Guérin?: a pTB-NET collaborative study. Am J Respir Crit Care Med 2012;186:378–84 | No construct validity |
| 11 | Belard E | Prednisolone treatment affects the performance of the QuantiFERON gold in-tube test and the tuberculin skin test in patients with autoimmune disorders screened for latent tuberculosis infection. Inflamm Bowel Dis 2011;17:2340–9 | No construct validity |
| 12 | Bergot E | Observational study of QuantiFERON-TB gold in-tube assay in tuberculosis contacts in a low incidence area. PLOS ONE 2012;7:e43520 | Mixed population and/or no subgroup of interest |
| 13 | Bienek DR | Evaluation of an interferon-gamma release assay, T-SPOT.TB, in a population with a low prevalence of tuberculosis. Int J Tuberc Lung Dis 2009;13:1416–21 | Mixed population and/or no subgroup of interest |
| 14 | Bottger EC | Interferon-γ release assays and the risk of developing active tuberculosis. Am J Respir Crit Care Med 2012;185:786–7; author reply 787 | Letter |
| 15 | Bua A | Tuberculin skin test and QuantiFERON in children. New Microbiol 2013;36:153–6 | No construct validity |
| 16 | Camlar SA | Performance of tuberculin skin test and interferon gamma assay for the diagnosis of latent tuberculosis infection in juvenile idiopathic arthritis. Clin Rheumatol 2011;30:1189–93 | No construct validity |
| 17 | Campainha S | Negative predictive value of TST and IGRA in anti-TNF treated patients. Eur Respir J 2012;40:790–1 | Letter |
| 18 | Carvalho AC | Contact investigation based on serial interferon-gamma release assays (IGRA) in children from the hematology-oncology ward after exposure to a patient with pulmonary tuberculosis. Infection 2013;41:827–31 | IGRA vs. IGRA only (no TST) |
| 19 | Cassone A | High rate of Quantiferon positive and tuberculin negative tests in infants born at a large Italian university hospital in 2011: a cautionary hypothesis. Pathog Glob Health 2012;106:8–11 | Review |
| 20 | Cheallaigh CN | Interferon gamma release assays for the diagnosis of latent TB infection in HIV-infected individuals in a low TB burden country. PLOS ONE 2013;8:e53330 | No construct validity |
| 21 | Chou CH | Comparison of 2 interferon-gamma assays and Roche Cobas Amplicor Mycobacterium tuberculosis assay for rapid diagnosis of tuberculosis among patients with suspected tuberculosis in Taiwan. J Microbiol Immunol Infect 2009;42:251–7 | IGRA vs. IGRA only (no TST) |
| 22 | Chung WK | Serial testing of interferon-gamma-release assays for the diagnosis of latent tuberculosis in hemodialysis patients. J Infect 2010;61:144–9 | Serial testing, conversion and reversion rates |
| 23 | Connell DW | A comparison between interferon gamma release assays and the tuberculin skin test in the contact tracing of patients with chronic kidney disease. Thorax 2011;66:729–30; author reply 730 | Letter |
| 24 | Connell TG | Indeterminate interferon-gamma release assay results in children. Pediatr Infect Dis J 2010;29:285–6 | Letter |
| 25 | Critselis E | The effect of age on whole blood interferon-gamma release assay response among children investigated for latent tuberculosis infection. J Pediatr 2012;161:632–8 | No construct validity |
| 26 | Dagnew AF | Diagnosis of latent tuberculosis infection in healthy young adults in a country with high tuberculosis burden and BCG vaccination at birth. BMC Res Notes 2012;5:415 | Mixed population and/or no subgroup of interest |
| 27 | Davies MA | Detection of tuberculosis in HIV-infected children using an enzyme-linked immunospot assay. AIDS 2009;23:961–9 | Active TB |
| 28 | de Andrade Lima E | Evaluation of an IFN-gamma assay in the diagnosis of latent tuberculosis in patients with psoriasis in a highly endemic setting. Acta Derm Venereol 2011;91:694–7 | No construct validity |
| 29 | de Kantor IN | Diagnosis of latent tuberculosis infection in BCG-vaccinated subjects in China. Int J Tuberc Lung Dis 2011;15:1560–1; author reply 1561 | Letter |
| 30 | Del Tedesco E | Does anti-TNF therapy influence the performance of Mycobacterium tuberculosis antigen-specific interferon-gamma assays? A French multicenter experience. Inflamm Bowel Dis 2011;17:1824 | Letter |
| 31 | Denholm JT | Immigration screening for latent tuberculosis infection. Med J Aus 2013;198:524 | Letter |
| 32 | Denholm JT | Immigration screening for latent tuberculosis infection. Med J Aus 2013;199:654 | Letter |
| 33 | Deuffic-Burban S | Cost-effectiveness of QuantiFERON-TB test vs. tuberculin skin test in the diagnosis of latent tuberculosis infection. Int J Tuberc Lung Dis 2010;14:471–81 | Economic study |
| 34 | Diel R | Enhanced cost–benefit analysis of strategies for LTBI screening and INH chemoprevention in Germany. Respir Med 2009;103:1838–53 | Economic study |
| 35 | Dilektasli AG | Is the T-cell-based interferon-gamma releasing assay feasible for diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country? Jpn J Infect Dis 2010;63:433–6 | Mixed population and/or no subgroup of interest |
| 36 | Doberne D | Preanalytical delay reduces sensitivity of QuantiFERON-TB gold in-tube assay for detection of latent tuberculosis infection. J Clin Microbiol 2011;49:3061–4 | No relevant outcomes; population ineligible |
| 37 | Dowdy DW | Tests for latent tuberculosis infection and isoniazid preventive therapy. Lancet Infect Dis 2012;12:827–8 | Letter |
| 38 | Dyrhol-Riise AM | Diagnosis and follow-up of treatment of latent tuberculosis; the utility of the QuantiFERON-TB Gold In-tube assay in outpatients from a tuberculosis low-endemic country. BMC Infect Dis 2010;10:57 | Mixed population and/or no subgroup of interest |
| 39 | Garcia-Elorriaga G | Interferon in patients with HIV/AIDS and suspicion or latent tuberculosis infection. Asian Pac J Trop Med 2013;6:135–8 | No construct validity |
| 40 | Garcia-Gasalla M | Use of Quantiferon-TB-Gold in Tube test for detecting latent tuberculosis in patients considered as candidates for anti-TNF therapy in routine clinical practice. Enferm Infecc Microbiol Clin 2013;31:76–81 | No construct validity |
| 41 | Garcovich S | Clinical applicability of Quantiferon-TB-Gold testing in psoriasis patients during long-term anti-TNF-alpha treatment: a prospective, observational study. J Eur Acad Dermatol Venereol 2012;26:1572–6 | No construct validity |
| 42 | Gautam M | Tuberculosis infection in the indigenous elderly white UK population: a study of IGRAs. Int J Tuberc Lung Dis 2012;16:564 | Letter |
| 43 | Gilham L | Tuberculosis screening before biologics – T-SPOT for all? J Rheumatol 2011;38:179 | Letter |
| 44 | Girlanda S | ELISPOT-IFN-gamma assay instead of tuberculin skin test for detecting latent Mycobacterium tuberculosis infection in rheumatic patients candidate to anti-TNF-alpha treatment. Clin Rheumatol 2010;29:1135–41 | Non-standard or in-house IGRA |
| 45 | Gogus F | Comparison of tuberculin skin test and QuantiFERON-TB gold in tube test in patients with chronic inflammatory diseases living in a tuberculosis endemic population. Clin Exp Med 2010;10:173–7 | No construct validity |
| 46 | Gonzalez-Salazar F | Snapshot of Quantiferon TB gold testing in Northern Mexico. Tuberculosis 2011;91(Suppl. 1):34–7 | Mixed population and/or no subgroup of interest |
| 47 | Goujon C | Diagnosis of latent tuberculosis infection (LTBI) before anti-TNF-alpha treatment – the tuberculin skin test is useful. Eur J Dermatol 2012;22:701–2 | Case report |
| 48 | Grare M | QuantiFERON-TB Gold In-Tube as help for the diagnosis of tuberculosis in a French pediatric hospital. Diagn Microbiol Infect Dis 2010;66:366–72 | No construct validity |
| 49 | Greveson K | Yield and cost effectiveness of mycobacterial infection detection using a simple IGRA-based protocol in UK subjects with inflammatory bowel disease suitable for anti-TNFalpha therapy. J Crohns Colitis 2013;7:412–18 | IGRA only (no TST) |
| 50 | Griffin DW | Immigration screening for latent tuberculosis infection. Med J Aus 2013;199:654 | Editorial |
| 51 | Grinsdale JA | Programmatic impact of using QuantiFERON-TB Gold in routine contact investigation activities. Int J Tuberc Lung Dis 2011;15:1614–20 | Mixed population and/or no subgroup of interest |
| 52 | Gupta D | Interferon gamma release assay (QuantiFERON-TB Gold In Tube) in patients of sarcoidosis from a population with high prevalence of tuberculosis infection. Sarcoidosis Vasc Diffuse Lung Dis 2011;28:95–101 | Active TB |
| 53 | Hanta I | Detection of latent tuberculosis infection in rheumatologic diseases before anti-TNFalpha therapy: tuberculin skin test versus IFN-γ assay. Rheumatol Int 2012;32:3599–603 | No construct validity |
| 54 | Hardy AB | Cost-effectiveness of the NICE guidelines for screening for latent tuberculosis infection: the QuantiFERON-TB Gold IGRA alone is more cost-effective for immigrants from high burden countries. Thorax 2010;65:178–80 | Economic study |
| 55 | Hatemi G | Quantiferon-TB Gold in tube assay for the screening of tuberculosis before and during treatment with tumor necrosis factor alpha antagonists. Arthritis Res Ther 2012;14:R147 | No construct validity |
| 56 | He D | High incidence of tuberculosis infection in rheumatic diseases and impact for chemoprophylactic prevention of tuberculosis activation during biologics therapy. Clin Vaccine Immunol 2013;20:842–7 | IGRA only (no TST) |
| 57 | Helwig U | Corticosteroids and immunosuppressive therapy influence the result of QuantiFERON TB Gold testing in inflammatory bowel disease patients. J Crohns Colitis 2012;6:419–24 | IGRA only (no TST) |
| 58 | Hernandez-Garduno E | The positive predictive value of T-SPOT.TB and tuberculin skin test in patients with silicosis. Am J Respir Crit Care Med 2011;183:277; author reply 277–8 | Letter |
| 59 | Hernandez-Garduno E | An update: the predictive value of QuantiFERON-TB-Gold In-Tube assay and the tuberculin skin test. Am J Respir Crit Care Med 2011;183:414; author reply 414–15 | Letter |
| 60 | Hernandez-Garduno E | The predictive value of interferon-γ release assays and tuberculin skin test: what about those not vaccinated with Bacillus Calmette–Guérin? Chest 2013;143:1514–15 | Letter |
| 61 | Higuchi K | Comparison of specificities between two interferon-gamma release assays in Japan. Int J Tuberc Lung Dis 2012;16:1190–2 | IGRA vs. IGRA only (no TST) |
| 62 | Hill PC | Surprisingly high specificity of the PPD skin test for M. tuberculosis infection from recent exposure in the Gambia. PLOS ONE 2006;1:e68 | Old pre-2009 study |
| 63 | Hoffmann M | Assessment of an Interferon-gamma release assay for the diagnosis of latent tuberculosis infection in haemodialysis patient. Swiss Med Wkly 2010;140:286–92 | No construct validity |
| 64 | Huang YW | Latent tuberculosis infection among close contacts of multidrug-resistant tuberculosis patients in central Taiwan. Int J Tuberc Lung Dis 2010;14:1430–5 | Mixed population and/or no subgroup of interest |
| 65 | Inanc N | Agreement between Quantiferon-TB gold test and tuberculin skin test in the identification of latent tuberculosis infection in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol 2009;36:2675–81 | Included in CG11710 and hence excluded from our search |
| 66 | Ingram PR | Latent tuberculosis infection in travelers: is there a role for screening using interferon-gamma release assays? J Travel Med 2009;16:352–6 | Review |
| 67 | Jacobs S | The tuberculin skin test is unreliable in school children BCG-vaccinated in infancy and at low risk of tuberculosis infection. Pediatr Infect Dis J 2011;30:754–8 | No relevant outcomes of interest; only children with positive TST result were given QFT-GIT |
| 68 | Jeong YJ | Positive tuberculin skin test or interferon-gamma release assay in patients with radiographic lesion suggesting old healed tuberculosis. J Korean Med Sci 2012;27:761–6 | Mixed population and/or no subgroup of interest |
| 69 | Jo KW | Poor correlation between tuberculin skin tests and interferon-γ assays in close contacts of patients with multidrug-resistant tuberculosis. Respirology 2012;17:1125–30 | Mixed population and/or no subgroup of interest |
| 70 | Katsenos S | Use of interferon-gamma release assay for latent tuberculosis infection screening in older adults exposed to tuberculosis in a nursing home. J Am Geriatr Soc 2011;59:858–62 | Mixed population and/or no subgroup of interest |
| 71 | Kawamura LM | Interferon-γ release assays for prediction of tuberculosis. Lancet Infect Dis 2012;12:584 | Letter |
| 72 | Kim EY | Performance of the tuberculin skin test and interferon-gamma release assay for detection of tuberculosis infection in immunocompromised patients in a BCG-vaccinated population. BMC Infect Dis 2009;9:207 | No construct validity |
| 73 | Kim JH | Factors influencing discrepancies between the QuantiFERON-TB gold in tube test and the tuberculin skin test in Korean patients with rheumatic diseases. Semin Arthritis Rheum 2013;42:424–32 | No construct validity |
| 74 | Klein M | Quantiferon TB Gold and tuberculin skin tests for the detection of latent tuberculosis infection in patients treated with tumour necrosis factor alpha blocking agents. Clin Exp Rheumatol 2013;31:111–17 | No construct validity |
| 75 | Kleinert S | Comparison of two interferon-gamma release assays and tuberculin skin test for detecting latent tuberculosis in patients with immune-mediated inflammatory diseases. Ann Rheumatic Dis 2010;69:782–4 | Letter |
| 76 | Kowada A | Cost effectiveness of interferon-gamma release assay for tuberculosis screening of rheumatoid arthritis patients prior to initiation of tumor necrosis factor-alpha antagonist therapy. Mol Diagn Ther 2010;14:367–73 | Economic study |
| 77 | Kowada A | Cost effectiveness of interferon-gamma release assay for school-based tuberculosis screening. Mol Diagn Ther 2012;16:181–90 | Economic study |
| 78 | Kowada A | Cost effectiveness of the interferon-γ release assay for tuberculosis screening of hemodialysis patients. Nephrol Dial Transplant 2013;28:682–8 | Economic study |
| 79 | Kwakernaak AJ | A comparison of an interferon-gamma release assay and tuberculin skin test in refractory inflammatory disease patients screened for latent tuberculosis prior to the initiation of a first tumor necrosis factor alpha inhibitor. Clin Rheumatol 2011;30:505–10 | No construct validity |
| 80 | Lange B | Indeterminate results of a tuberculosis-specific interferon-gamma release assay in immunocompromised patients. Eur Respir J 2010;35:1179–82 | Letter |
| 81 | Laskin BL | Cost-effectiveness of latent tuberculosis screening before steroid therapy for idiopathic nephrotic syndrome in children. Am J Kidney Dis 2013;61:22–32 | Economic study |
| 82 | Latorre I | IFN-γ response on T-cell based assays in HIV-infected patients for detection of tuberculosis infection. BMC Infect Dis 2010;10:348 | No construct validity |
| 83 | Lee SS | High prevalence of latent tuberculosis infection in dialysis patients using the interferon-gamma release assay and tuberculin skin test. Clin J Am Soc Nephrol 2010;5:1451–7 | No construct validity |
| 84 | Legesse M | Community–based cross-sectional survey of latent tuberculosis infection in Afar pastoralists, Ethiopia, using QuantiFERON-TB Gold In-Tube and tuberculin skin test. BMC Infect Dis 2011;11:89 | Mixed population and/or no subgroup of interest |
| 85 | Legesse M | Association of the level of IFN-γ produced by T cells in response to Mycobacterium tuberculosis-specific antigens with the size of skin test indurations among individuals with latent tuberculosis in a highly tuberculosis-endemic setting. Int Immunol 2012;24:71–8 | Mixed population and/or no subgroup of interest |
| 86 | Leung CC | Tests for prediction of active tuberculosis. Lancet Infect Dis 2012;12:6–8 | Editorial |
| 87 | Lienhardt C | Evaluation of the prognostic value of IFN-gamma release assay and tuberculin skin test in household contacts of infectious tuberculosis cases in Senegal. PLOS ONE 2010;5:e10508. [Erratum published in PLOS ONE 2010;5(12)] | Mixed population and/or no subgroup of interest |
| 88 | Lighter-Fisher J | Performance of an interferon-gamma release assay to diagnose latent tuberculosis infection during pregnancy. Obstet Gynecol 2012;119:1088–95. [Erratum published in Obstet Gynecol 2012;120:399] | Mixed population and/or no subgroup of interest |
| 89 | Linas BP | Priorities for screening and treatment of latent tuberculosis infection in the United States. Am J Respir Crit Care Med 2011;184:590–601 | Economic study |
| 90 | Losi M | Tuberculosis infection in foreign-born children: a screening survey based on skin and blood testing. Int J Tuberc Lung Dis 2011;15:1182–4 | No construct validity |
| 91 | Maden E | Evaluation of performance of quantiferon assay and tuberculin skin test in end stage renal disease patients receiving hemodialysis. New Microbiol 2011;34:351–6 | No construct validity |
| 92 | Maeda T | Usefulness and limitations of QuantiFERON-TB Gold in Japanese rheumatoid arthritis patients: proposal to decrease the lower cutoff level for assessing latent tuberculosis infection. Mod Rheumatol 2010;20:18–23 | Inappropriate proxy for LTBI; definition includes previous active TB |
| 93 | Maeda T | Comparison of QuantiFERON-TB Gold and the tuberculin skin test for detecting previous tuberculosis infection evaluated by chest CT findings in Japanese rheumatoid arthritis patients. J Infect Chemother 2011;17:842–8 | No construct validity |
| 94 | Mahan CS | Concordance of a positive tuberculin skin test and an interferon gamma release assay in bacille Calmette–Guérin vaccinated persons. Int J Tuberc Lung Dis 2011;15:174–8 | Mixed population and/or no subgroup of interest |
| 95 | Mancuso JD | Cost-effectiveness analysis of targeted and sequential screening strategies for latent tuberculosis. Int J Tuberc Lung Dis 2011;15:1223–30 | Economic study |
| 96 | Mandalakas AM | Can we accurately diagnose tuberculosis infection in children? Pediatr Infect Dis J 2011;30:817–18 | Letter |
| 97 | Mandalakas AM | Is screening immigrants for latent tuberculosis cost-effective? Lancet Infect Dis 2011;11:418–19 | Editorial |
| 98 | Mandalakas AM | Modelling the cost-effectiveness of strategies to prevent tuberculosis in child contacts in a high-burden setting. Thorax 2013;68:247–55 | Economic study |
| 99 | Mandalakas AM | Detecting tuberculosis infection in HIV-infected children: a study of diagnostic accuracy, confounding and interaction. Pediatr Infect Dis J 2013;32:e111–18 | No construct validity; two samples on exposure (HIV positive and HIV negative) were included together |
| 100 | Mariette X | Influence of replacing tuberculin skin test with ex vivo interferon release assays on decision to administer prophylactic antituberculosis antibiotics before anti-TNF therapy. Ann Rheum Dis 2012;71:1783–90 | No construct validity |
| 101 | Marques CD | Evaluation of an interferon gamma assay in the diagnosis of latent tuberculosis infection in patients with rheumatoid arthritis. Rheumatol Int 2009;30:57–62 | Inappropriate proxy for LTBI |
| 102 | Martin J | Comparison of interferon-γ release assays and conventional screening tests before tumour necrosis factor-α blockade in patients with inflammatory arthritis. Ann Rheum Dis 2010;69:181–5 | IGRA vs. IGRA only (no TST) |
| 103 | Martyn-Simmons CL | Evaluating the use of the interferon-γ response to Mycobacterium tuberculosis-specific antigens in patients with psoriasis prior to antitumour necrosis factor-α therapy: a prospective head-to-head cross-sectional study. Br J Dermatol 2013;168:1012–18 | No construct validity |
| 104 | Mendez-Echevarria A | Interferon-γ release assay for the diagnosis of tuberculosis in children. Arch Dis Childhood 2012;97:514–16 | No construct validity; only for IGRA |
| 105 | Milman N | Quantiferon test for tuberculosis screening in sarcoidosis patients. Scand J Infect Dis 2011;43:728–35 | IGRA only (no TST) |
| 106 | Minguez S | Interferon-gamma release assays in the detection of latent tuberculosis infection in patients with inflammatory arthritis scheduled for anti-tumour necrosis factor treatment. Clin Rheumatol 2012;31:785–94 | No construct validity |
| 107 | Molicotti P | Performance of QuantiFERON TB in a student population at low risk of tuberculosis. J Infect Develop Countries 2012;6:100–1 | Letter |
| 108 | Moran Mendoza O | Interferon-γ release assays for the diagnosis of latent Mycobacterium tuberculosis infection. Eur Respir J 2011;38:1237–8; author reply 1238–9 | Letter |
| 109 | Moyo S | Tuberculin skin test and QuantiFERON assay in young children investigated for tuberculosis in South Africa. Int J Tuberc Lung Dis 2011;15:1176–81 | Active TB |
| 110 | Mrozek N | Tuberculosis screening before biologic therapy. Comment about the article entitled ‘role for interferon-gamma release assays in latent tuberculosis screening before TNF-alpha antagonist therapy’ by Liote H et al. Joint Bone Spine 2011;78:655–6; author reply 656–7 | Letter |
| 111 | Murakami S | Screening of tuberculosis by interferon-gamma assay before biologic therapy for rheumatoid arthritis. Tuberculosis 2009;89:136–41 | Case–control study of test results |
| 112 | Nellore A | Screening strategies for tuberculosis in children with kidney disease: what is cost-effective? Am J Kidney Dis 2013;61:3–5 | Letter |
| 113 | Nguyen MQ | What are the differences between the tuberculin skin test and the QuantiFERON-TB Gold test? J Occupational Environ Med 2012;54:1177–8 | Editorial |
| 114 | Nkurunungi G | Determining Mycobacterium tuberculosis infection among BCG-immunised Ugandan children by T-SPOT.TB and tuberculin skin testing. PLOS ONE 2012;7:e47340 | No construct validity |
| 115 | Ohnishi T | Comparison of QuantiFERON-TB Gold and the tuberculin skin test for the detection of previous tuberculosis infection evaluated by chest CT findings in Japanese rheumatoid arthritis patients. J Infect Chemother 2011;17:849–50 | Letter |
| 116 | Oni T | Smoking, BCG and employment and the risk of tuberculosis infection in HIV-infected persons in South Africa. PLOS ONE 2012;7:e47072 | Non-standard or in-house IGRA |
| 117 | Onur H | Comparison of quantiferon test with tuberculin skin test for the detection of tuberculosis infection in children. Inflammation 2012;35:1518–24 | Inappropriate proxy for LTBI |
| 118 | Ormerod LP | Further evidence supporting programmatic screening for, and treatment of latent TB Infection (LTBI) in new entrants to the UK from high TB prevalence countries. Thorax 2013;68:201 | Letter |
| 119 | Pareek M | Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis 2011;11:435–44 | Economic study |
| 120 | Pareek M | Community-based evaluation of immigrant tuberculosis screening using interferon release assays and tuberculin skin testing: observational study and economic analysis. Thorax 2013;68:230–9 | Economic study |
| 121 | Pattnaik S | Agreement between skin testing and QuantiFERON-TB Gold In-Tube assay (QFT-TB) in detecting latent tuberculosis infection among household contacts in India. Indian J Tuberc 2012;59:214–18 | No construct validity |
| 122 | Petrescu L | Tuberculin skin test, interferon-gamma assay, and T cells subpopulations in hemodialysis patients. J Ren Nutr 2010;20(Suppl. 5):109–17 | No construct validity |
| 123 | Pooran A | Different screening strategies (single or dual) for the diagnosis of suspected latent tuberculosis: a cost effectiveness analysis. BMC Pulm Med 2010;10:7 | Economic study |
| 124 | Qumseya BJ | QuantiFERON TB gold testing for tuberculosis screening in an inflammatory bowel disease cohort in the United States. Inflamm Bowel Dis 2011;17:77–83 | No construct validity |
| 125 | Ramos JM | Contribution of interferon gamma release assays testing to the diagnosis of latent tuberculosis infection in HIV-infected patients: a comparison of QuantiFERON-TB Gold In Tube, T-SPOT.TB and tuberculin skin test. BMC Infect Dis 2012;12:169 | Inappropriate proxy for LTBI |
| 126 | Riazi S | Rapid diagnosis of Mycobacterium tuberculosis infection in children using interferon-gamma release assays (IGRAs). Allergy Asthma Proc 2012;33:217–26 | Active TB |
| 127 | Ringrose JS | Detecting latent tuberculosis infection during anti-tumor necrosis factor therapy. Clin Exp Rheumatol 2011;29:790–4 | No relevant outcomes |
| 128 | Santin M | Detection of latent tuberculosis by the tuberculin skin test and a whole-blood interferon-γ release assay, and the development of active tuberculosis in HIV-seropositive persons. Diagn Microbiol Infect Dis 2011;69:59–65 | Mixed population and/or no subgroup of interest for construct validity |
| 129 | Sattah MV | Interferon-gamma release assay T-SPOT.TB and HIV-related tuberculosis. Int J Tuberc Lung Dis 2012;16:281–2 | Letter |
| 130 | Sayarlioglu H | QuantiFERON-TB Gold test for screening latent tuberculosis infection in hemodialysis patients. Tuberkuloz ve Toraks 2011;59:105–10 | No construct validity |
| 131 | Schneider WJ | QuantiFERON-TB testing for latent tuberculosis infection in low-prevalence countries: making the most of an imperfect process. Infect Control Hosp Epidemiol 2011;32:1055 | Letter |
| 132 | Serrano-Escobedo CJ | Performance of tuberculin skin test compared to QFT-IT to detect latent TB among high-risk contacts in Mexico. Arch Med Res 2013;44:242–8 | Mixed population and/or no subgroup of interest |
| 133 | Seshadri C | Low sensitivity of T-cell based detection of tuberculosis among HIV co-infected Tanzanian in-patients. East Afr Med J 2008;85:442–9 | Old pre-2009 study |
| 134 | Setiawati L | Effect of BCG vaccination and non-tuberculous Mycobacterium infection on interferon gamma specific assay and a tuberculin skin test among children with a tuberculosis contact in Surabaya, Indonesia. Southeast Asian J Trop Med Public Health 2011;42:1460–8 | No construct validity |
| 135 | Shah M | Programmatic impact of QuantiFERON-TB Gold In-Tube implementation on latent tuberculosis diagnosis and treatment in a public health clinic. PLOS ONE 2012;7:e36551 | Mixed population and/or no subgroup of interest |
| 136 | Shah M | QuantiFERON-TB gold in-tube implementation for latent tuberculosis diagnosis in a public health clinic: a cost-effectiveness analysis. BMC Infect Dis 2012;12:360 | Economic study |
| 137 | Shanaube K | Risk factors associated with positive QuantiFERON-TB Gold In-Tube and tuberculin skin tests results in Zambia and South Africa. PLOS ONE 2011;6:e18206 | Mixed population and/or no subgroup of interest |
| 138 | Shovman O | QuantiFERON-TB Gold in the identification of latent tuberculosis infection in rheumatoid arthritis: a pilot study. Int J Tuberc Lung Dis 2009;13:1427–32 | Included in CG11710 and hence excluded from our search |
| 139 | Simsek H | Comparison of tuberculin skin testing and T-SPOT.TB for diagnosis of latent and active tuberculosis. Jpn J Infect Dis 2010;63:99–102 | Mixed population and/or no subgroup of interest |
| 140 | Singanayagam A | Evaluation of screening methods for identification of patients with chronic rheumatological disease requiring tuberculosis chemoprophylaxis prior to commencement of TNF-alpha antagonist therapy. Thorax 2013;68:955–61 | Inappropriate proxy for LTBI |
| 141 | Song Q | Evaluation of a new interferon-gamma release assay and comparison to tuberculin skin test during a tuberculosis outbreak. Int J Infect Dis 2012;16:e522–6 | Non-standard or in-house IGRA |
| 142 | Song S | Performance of confirmatory interferon-γ release assays in school TB outbreaks. Chest 2012;141:983–8 | QFT used as confirmatory test on subgroup of TST-positive patients |
| 143 | Soysal A | Diagnosing latent tuberculosis infection in haemodialysis patients: T-cell based assay (T-SPOT.TB) or tuberculin skin test? Nephrol Dial Transplant 2012;27:1645–50 | No construct validity |
| 144 | Starke JR | Interferon-γ release assays for the diagnosis of tuberculosis infection in children. J Pediatr 2012;161:581–2 | Letter |
| 145 | Stefan DC | Interferon-gamma release assays for the detection of Mycobacterium tuberculosis infection in children with cancer. Int J Tuberc Lung Dis 2010;14:689–94 | No construct validity |
| 146 | Steffen RE | Cost-effectiveness of Quantiferon-TB Gold-in-Tube versus tuberculin skin testing for contact screening and treatment of latent tuberculosis infection in Brazil. PLOS ONE 2013;8:e59546 | Economic study |
| 147 | Sultan B | Comparison of two interferon-gamma release assays (QuantiFERON-TB Gold In-Tube and T-SPOT.TB) in testing for latent tuberculosis infection among HIV-infected adults. Int J STD AIDS 2013;24:775–9 | IGRA vs. IGRA only (no TST) |
| 148 | Talati NJ | Diagnosis of latent tuberculosis infection among HIV discordant partners using interferon gamma release assays. BMC Infect Dis 2011;11:264. | No construct validity |
| 149 | Tannus Silva DG | Latent tuberculosis in rheumatoid arthritis: evaluating cellular response and high-resolution computed tomography. Arch Bronconeumol 2012;48:144–9 | No construct validity |
| 150 | Tebruegge M | Interferon-γ release assays for the diagnosis of tuberculosis in children. Arch Dis Childhood 2013;98:239–40 | Letter |
| 151 | Theodoropoulos N | Use of the QuantiFERON-TB Gold interferon-gamma release assay for screening transplant candidates: a single-center retrospective study. Transplant Infect Dis 2012;14:1–8 | IGRA only (historical TST) |
| 152 | Thomas B | Concordance between tuberculin skin test and interferon-γ assay and interferon-γ response to mitogen in pediatric tuberculosis contacts. Pediatr Pulmonol 2011;46:1225–32 | No construct validity |
| 153 | Thomas TA | Malnutrition and helminth infection affect performance of an interferon gamma-release assay. Pediatrics 2010;126:e1522–9 | No construct validity |
| 154 | Uluk T | Evaluation of an interferon-gamma release assay in children with suspected tuberculosis in Papua New Guinea. Pediatr Infect Dis J 2013;32:187–9 | No construct validity |
| 155 | Wassie L | Parasitic infection may be associated with discordant responses to QuantiFERON and tuberculin skin test in apparently healthy children and adolescents in a tuberculosis endemic setting, Ethiopia. BMC Infect Dis 2013;13:265 | No construct validity |
| 156 | Weinfurter P | Predictors of discordant tuberculin skin test and QuantiFERON-TB Gold In-Tube results in various high-risk groups. Int J Tuberc Lung Dis 2011;15:1056–61 | No construct validity |
| 157 | Wolf T | Tuberculosis skin test, but not interferon-γ-releasing assays is affected by BCG vaccination in HIV patients. J Infect 2013;66:376–80 | No construct validity |
| 158 | Xie X | A T-cell-based enzyme-linked immunospot assay for tuberculosis screening in Chinese patients with rheumatic diseases receiving infliximab therapy. Clin Exp Med 2011;11:155–61 | No construct validity |
| 159 | Yilmaz N | Comparison of QuantiFERON-TB Gold test and tuberculin skin test for the identification of latent Mycobacterium tuberculosis infection in lupus patients. Lupus 2012;21:491–5 | No construct validity |
| 160 | Zhao J | Low agreement between the T-SPOT.TB assay and the tuberculin skin test among college students in China. Int J Tuberc Lung Dis 2011;15:134–6 | No construct validity |
MEDLINE In-Process & Other Non-Indexed Citations
| Number | Author ID | Details | Reason(s) for exclusion |
|---|---|---|---|
| 161 | No authors listed | Interferon-gamma release assays for diagnosis of latent tuberculosis infection: evidence in immune-mediated inflammatory disorders: erratum. Curr Opin Rheumatol 2011;23:504 | Letter |
| 162 | No authors listed | Society for Adolescent Health and Medicine Annual Meeting: Impact of Trauma on Teens: Building the Safety Net Conference Proceedings. J Adolesc Health 2012;50(Suppl. 2) | Irrelevant |
| 163 | No authors listed | 40th Annual Conference Abstracts, APIC 2013. Am J Infect Control 2013;41(Suppl. 6) | Abstract |
| 164 | No authors listed | World Tuberculosis Day Symposium 2012. Tuberculosis 2013;93(1) | Abstract |
| 165 | Abdel-Nabi EA | QuantiFERON vs. tuberculin testing in detection of latent tuberculous infection among chronic renal failure patients. Egypt J Chest Dis Tuberc 2014;63:161–5 | No construct validity |
| 166 | Abdel-Samea SA | Comparative study between using QuantiFERON and tuberculin skin test in diagnosis of Mycobacterium tuberculosis infection. Egypt J Chest Dis Tuberc 2013;62:137–43 | Mixed population and/or no subgroup of interest |
| 167 | Abraham B | Monitoring and management of latent tuberculosis in IBD patients on antiTNF therapy: a case series. Am J Gastroenterol 2013;108:S521–2 | Abstract |
| 168 | Aggarwal P | Performance of an interferon-gamma release assay to diagnose latent tuberculosis infection during pregnancy. Obstet Gynecol 2012;120:398; author reply 398 | Letter |
| 169 | Ahmad M | False-positive QuantiFERON Gold tests. Chest 2010;138:84A | Abstract |
| 170 | Ahmadinejad Z | Evaluation of QuantiFERON-gold (tuberculin skin test) for the identification of latent tuberculosis infection in would-be transplant recipient patients referring to an Iranian transplant clinic from September 2007 to December 2008. Clin Microbiol Infect 2010;16:S542 | Abstract |
| 171 | Akpaka PE | Evaluation of cost and methods for detecting latent tuberculosis infection among target individual groups in Trinidad and Tobago. Int J Infect Dis 2010;14:e148 | Abstract |
| 172 | Alberte-Castineiras A | Discordant QuantiFERON-TB Gold In-Tube and tuberculin skin test results in various high-risk groups. Clin Microbiol Infect 2012;18:548 | Abstract |
| 173 | Andrisani G | Tubercolosis screening in Italian patients affected by inflammatory bowel disease: comparison of QuantiFERON-TB Gold versus tuberculin skin test. Digest Liver Dis 2010;42:S181–2 | Abstract |
| 174 | Arias M | Performance of two interferon-gamma release assays (T-SPOT.TB and QuantiFERON-TB Gold in Tube) increase diagnostic yield of tuberculin skin testing for detection of latent tuberculosis in patients with inflammatory bowel disease. Gastroenterology 2011;1:S691 | Abstract |
| 175 | Atanassova A | Screening for tuberculosis in patients candidates for anti-TNF terapy in IBD. J Gastroenterol Hepatol 2013;28:141 | Abstract |
| 176 | Awan S | Can Quanti-FERON-TB replace TST (Mantoux) as a screening tool prior to (biologics) anti-TNF therapy. Irish J Med Sci 2012;181:S75 | Abstract |
| 177 | Bakir M | Use of T cell-based diagnosis of tuberculosis infection to optimize interpretation of tuberculin skin testing for child tuberculosis contacts. Clin Infect Dis 2009;48:302–12 | Inappropriate proxy for LTBI |
| 178 | Behar SM | Use of the T-SPOT.TB assay to detect latent tuberculosis infection among rheumatic disease patients on immunosuppressive therapy. J Rheumatol 2009;36:546–51 | Inclusion of TST-positive patients |
| 179 | Belard E | Effects of corticosteroid treatment on the performance of QuantiFERON Gold in-Tube test in the screening of latent tuberculosis infection. Gastroenterology 2010;1:S523 | Abstract |
| 180 | Bergamini BM | Performance of commercial blood tests for the diagnosis of latent tuberculosis infection in children and adolescents. Pediatrics 2009;123:e419–24 | IGRA vs. IGRA only (no TST) |
| 181 | Berry MPR | Systems biology approaches characterise the host response to tuberculosis. Thorax 2009;64:A10 | Abstract |
| 182 | Bianchi L | Interferon-gamma release assay improves the diagnosis of tuberculosis in children. Pediatr Infect Dis J 2009;28:510–14 | No construct validity |
| 183 | Blandinieres A | Deficient IFN-gamma response to Mycobacterium tuberculosis antigens in infants improved since 1 year of age. Immunology 2012;137:727–8 | Abstract |
| 184 | Blandinieres A | QuantiFERON to diagnose infection by Mycobacterium tuberculosis: performance in infants and older children. J Infect 2013;67:391–8 | No construct validity |
| 185 | Borkowska D | Interferon-gamma assays T-SPOT.TB for the diagnosis of latent tuberculosis infection. Pneumonol Alergol Pol 2011;79:264–71 | Mixed population and/or no subgroup of interest |
| 186 | Borra H | Reliability of tuberculosis screening tests in patients receiving tumor necrosis factor antagonist therapy in a United States rheumatology clinic. Arthritis Rheum 2009;60:137 | Abstract |
| 187 | Bortlik M | Usefulness of the QuantiFERON TB Gold test in assessing the necessity for TB prophylaxis in IBD patients treated with biologicals. Gastroenterology 2009;1:A197 | Abstract |
| 188 | Bottger EC | Interferon-gamma release assays and the risk of developing active tuberculosis. Am J Resp Crit Care Med 2012;185:786–7 | Abstract |
| 189 | Brebner J | Questionable utility of T-SPOT testing in a TB exposure incident on a clinical haematology unit. Thorax 2010;65:A103 | Abstract |
| 190 | Bruzzese E | Gamma interferon release assays for diagnosis of tuberculosis infection in immune-compromised children in a country in which the prevalence of tuberculosis is low. J Clin Microbiol 2009;47:2355–7 | Letter |
| 191 | Bua A | Epidemic of tuberculosis in a high school in Northern Sardinia. Int J Mycobacteriol 2012;1:161–3 | No relevant outcomes |
| 192 | Bumbacea D | New immunodiagnostic tests for latent and active tuberculosis. Rev Romana Med Laborator 2011;19:267–78 | Abstract |
| 193 | Buonsenso D | Evaluation of a mathematical model proposed to predict the diagnosis of tuberculosis in children with cervical lymph node enlargement. Int J Pediatr Otorhinolaryngol 2012;76:1068–70 | Letter |
| 194 | Buonsenso D | Pediatric tuberculosis in two tertiary hospitals in Rome: a 20-year retrospective study. Arch Dis Childhood 2012;97:A11–12 | Abstract |
| 195 | Burgos JL | Targeted screening and treatment for latent tuberculosis infection using QuantiFERON-TB Gold is cost-effective in Mexico. Int J Tuberc Lung Dis 2009;13:962–8 | Economic study |
| 196 | Cagan Appak Y | Comparison of tuberculin skin testing and in vitro interferon-gamma release assay test for diagnosis of latent tuberculosis in children. J Med Sci 2013;33:1402–7 | Foreign language (Turkish) |
| 197 | Cagatay T | The role of IGRA tests and tuberculin test for determination of latent tuberculosis in TNF-alpha antagonist users (candidates) [TNF-alpha antagonisti kullanacak hastalarda latent tuberkulozun belirlenmesinde IGRA testleri (quantiFERON-elispot) ve ppd’nin yeri]. Turk Dermatoloji Dergisi 2012;6:62–4 | Foreign language (Turkish) |
| 198 | Capocci S | Screening for latent TB in HIV: are NICE and BHIVA guidance effective? Thorax 2011;66:A21–2 | Abstract |
| 199 | Capocci S | Is testing for latent tuberculosis infection in an UK HIV clinic cost effective? HIV Med 2012;13:44 | Abstract |
| 200 | Casas S | Diagnosis of tuberculosis infection in patients awaiting transplantation. Clin Microbiol Infect 2010;16:S73 | Abstract |
| 201 | Castaneda-Hernandez DM | Importance of the use of interferon-gamma release assays in the epidemiological surveillance of tuberculosis Rev Med Chile 2012;140:128–9 | Abstract |
| 202 | Cetin EA | QuantiFERON-TB gold test may be more advantageous than tuberculin skin test for screening latent tuberculosis infection in psychiatry clinics. Balkan Med J 2012;29:115–16 | Abstract |
| 203 | Chang B | Interferon-gamma assay in the diagnosis of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. Am J Resp Crit Care Med 2010;181:A4773 | Abstract |
| 204 | Chawla H | Use of the interferon-gamma release assay blood test to confirm latent tuberculosis infection in tuberculin skin test-positive immIGRAnts: Our experience at a Connecticut pulmonary clinic. Am J Resp Crit Care Med 2010;181:A4774 | Abstract |
| 205 | Chen JW | Evaluation of a T-cell-based enzyme-linked immunospot assay for monitoring tuberculosis in patients with rheumatic diseases receiving Infliximab therapy. Int J Rheum Dis 2010;13:87 | Abstract |
| 206 | Chen QF | Interferon-γ release assays screening for latent tuberculosis screening: a cost-effectiveness analysis. Chinese J Evid Based Med 2011;11:768–74 | Foreign language (Chinese) |
| 207 | Chun JK | The role of a whole blood interferon-γ releasing assay for the tracing of tuberculosis infection in bacilli Calmette Guérin vaccinated children. Int J Infect Dis 2010;14:e312 | Abstract |
| 208 | Clark BJ | Detection of latent tuberculosis infection in patients with end stage renal disease: interferon-gamma release assays versus tuberculin skin test. Am J Resp Crit Care Med 2009;179:A5926 | Abstract |
| 209 | Connell DW | Comparison between interferon-gamma release assays and the tuberculin skin test in the diagnosis of tuberculosis in patients with renal disease. Thorax 2009;64:A108 | Abstract |
| 210 | Connell T | Interferon-γ release assays for the diagnosis of tuberculosis. Pediatr Infect Dis J 2009;28:758–9 | Abstract |
| 211 | Costantino F | High level of disease activity in chronic inflammatory rheumatisms increases the rate of indeterminate interferon-gamma-release assay results for latent tuberculosis infection detection. Arthritis Rheum 2010;62:768 | Abstract |
| 212 | Davarpanah MA | Association between PPD and QuantiFERON Gold TB test in TB infection and disease among HIV-infected individuals in Southern Iran. Iran Red Crescent Med J 2009;11:71–5 | Included/excluded in CG11710 |
| 213 | De Francisco R | Interferon-gamma release assays (T-SPOT.TB and QuantiFERON-TB GOLD in Tube) versus tuberculin skin testing for detection of latent tuberculosis in patients with inflammatory bowel disease. J Crohns Colitis 2011;5:S52–3 | Abstract |
| 214 | De Leon DP | Comparison of IGRAs with TST for the detection of LTBI in RA patients in a TB endemic population. Int J Tuberc Lung Dis 2010;14(Suppl. 1):40–1 | Abstract |
| 215 | Delgado Naranjo J | Comparative performance of QuantiFERON®-TB Gold IT versus tuberculin skin test among contact investigations for latent tuberculosis infection. Med Clín 2011;137:289–96 | Foreign language (Spanish) |
| 216 | Del Tedesco E | Interferon gamma release assay (IGRA) and/or tuberculin skin test (TST) in inflammatory bowel disease population: discordance and performance. Best strategy for detecting tuberculosis. Gastroenterology 2010;1:S672–3 | Abstract |
| 217 | Demkow U | Interferon gamma based tests as a new tool in diagnosis of latent tuberculosis. Pneumonol Alergol Pol 2011;79:261–3 | Editorial |
| 218 | Denholm JT | Diagnosis and management of latent tuberculosis infection. Med Today 2010;11:72–6 | Review |
| 219 | Diel R | The predictive value of interferon-gamma release assays and tuberculin skin test: what about those not vaccinated with bacillus Calmette–Guérin? Response. Chest 2013;143:1515–16 | Abstract |
| 220 | Dominguez J | Role of the T-cell interferon-gamma release assays in preventing reactivation of latent tuberculosis infection in immunosuppressed patients in treatment with anti-TNF agents. J Crohns Colitis 2008;2:250–4 | Old pre-2009 study |
| 221 | Eather G | Comparison of tuberculin skin test with an interferon-gamma release assay (IGRA) in screening for latent tuberculosis infection in a low prevalence population. Respirology 2012;17:17 | Abstract |
| 222 | Eisenhut M | Performance of tuberculin skin test measured against interferon gamma release assay as reference standard in children. Tuberc Res Treat 2014;2014:413459 | Review |
| 223 | Elzi L | Low sensitivity of an Interferon-gamma releasing assay (Elispot-TB™) for the diagnosis of latent tuberculosis in HIV-infected individuals. Swiss Med Wkly 2009;139:39S | Abstract |
| 224 | Erkens CG | Added value of interferon-gamma release assays in screening for tuberculous infection in the Netherlands. Int J Tuberc Lung Dis 2014;18:413–20 | Mixed population and/or no subgroup of interest |
| 225 | Evans LC | IFN-gamma release assays improve detection of latent tuberculosis infection in tuberculin-anergic candidates for anti-TNF-alpha blockade. Am J Resp Crit Care Med 2009;179:A5927 | Non-standard or in-house IGRA |
| 226 | Fernandez S | Use of interferon-gamma release assay (IGRA) and tuberculin skin test (TST) for tuberculosis screening in patients candidates for anti-TNF terapy in inflammatory bowel disease (IBD). J Crohns Colitis 2013;7:S58 | Abstract |
| 227 | Ferrara G | Interferon-gamma-release assays detect recent tuberculosis re-infection in elderly contacts. Int J Immunopathol Pharmacol 2009;22:669–77 | Mixed population and/or no subgroup of interest |
| 228 | Fontana R | Diagnosis and management of latent tuberculosis identified by the QuantiFERON assay in liver transplant patients. Am J Transpl 2010;10:97 | Abstract |
| 229 | Francois C | Cost effectiveness analysis of strategies using new immunological diagnostic tests of latent tuberculosis infection before anti-TNF therapy. Ann Rheum Dis 2013;72:A538 | Abstract |
| 230 | Gao KK | Comparison of detection performances between two kits for mycobacterium tuberculosis infection. J Shanghai Jiaotong Uni Med Sci 2011;31:1440–3 | Foreign language (Chinese) |
| 231 | Gao Y | Evaluation of latent Mycobacterium tuberculosis infection screening using TSPOT.TB assay and TST in IMID patients prior to initiation of anti-TNF alpha therapy. Int J Infect Dis 2011;15:S103 | Abstract |
| 232 | Garcia-Garcia JM | Comparison of tuberculin skin test and QuantiFERON-TB-Gold in Tube in the diagnosis of latent tuberculosis infection (LTBI) in a prospective community study of contacts. Am J Resp Crit Care Med 2010;181:A1789 | Abstract |
| 233 | Garcia-Pedrazuela M | Evaluation of the use of QuantiFERON TB-Gold in the routine setting at a university hospital, 2007–2011. Clin Microbiol Infect 2012;18:544 | Abstract |
| 234 | Garfein RS | Latent tuberculosis among persons at risk for infection with HIV, Tijuana, Mexico. Emerg Infect Dis 2010;16:757–63 | IGRA only (no TST) |
| 235 | Gomes CMF | Clinical performance of 4 methods for detecting latent tuberculosis infection (LTbI) in patients with active chronic inflammatory arthritis taking TNFalpha blockers. Arthritis Rheum 2013;65:S1063 | Abstract |
| 236 | Gonzalez-Diaz V | Efficiency of interferon-γ release assay for screening for latent tuberculosis in patients with systemic lupus erythematosus. Lupus 2013;22:48 | Abstract |
| 237 | Goto N | How do we manage kidney transplant recipients with latent tuberculosis infection (LTBI)? Am J Transpl 2010;10:426 | Abstract |
| 238 | Gray JM | Improvement in latent tuberculosis testing of HIV patients after switching from the tuberculin skin test to QuantiFERON-TB Gold-In-Tube. Am J Resp Crit Care Med 2010;183:A1197 | Abstract |
| 239 | Greveson K | Comparative cost-effectiveness of IGRA to detect latent TB infection in UK inflammatory bowel disease patients initiating anti-TNFalpha agents. Thorax 2012;67:A87–8 | Abstract |
| 240 | Guidi L | Screening inflammatory bowel disease patients for latent tubercolosis in Italy: comparison of QuantiFERON-TB Gold versus tuberculin skin test. Gastroenterology 2010;1:S526 | Abstract |
| 241 | Hadaya K | Contribution of interferon gamma release assays to the diagnosis of latent tuberculosis infection after renal transplantation. Respiration 2013;85:590 | Abstract |
| 242 | Hanjiu W | Application of the T-SPOT.TB assay to identify tuberculosis infection in children. Acta Med Mediterr 2013;29:443–6 | No relevant outcomes |
| 243 | Hashemi Shahri M | To compare the performance of Quanti-FERON with the tuberculin skin test for identifying latent tuberculosis infection. Iran J Epidemiol 2012;7:57–65 | Foreign language (Persian) |
| 244 | Hasunuma T | New tools for old diseases comparison of positive reaction of tuberculin skin test and QuantiFERON among healthy adult males in Japan. Basic Clin Pharmacol Toxicol 2010;107:321–2. | Abstract |
| 245 | Hayes, Inc. | Interferon-Gamma Release Assays for Tuberculosis. Lansdale, PA: Hayes, Inc.; 2012 | Abstract |
| 246 | Hesseling AC | The predictive value of the ELIspot-based interferon-γ-release assay for tuberculosis disease. Ann Intern Med 2009;150:428–9 | Letter |
| 247 | Hesseling AC | Highly discordant T cell responses in individuals with recent exposure to household tuberculosis. Thorax 2009;64:840–6 | No construct validity |
| 248 | Higuchi K | Comparison of performance in two diagnostic methods for tuberculosis infection. Med Microbiol Immunol 2009;198:33–7 | IGRA vs. IGRA only (no TST) |
| 249 | Hofland R | Should screening for latent tuberculosis infection be repeated after travel to tuberculosis endemic areas in patients treated with TNF-alpha inhibitor therapy? Gastroenterology 2012;1:S249 | Abstract |
| 250 | Horsburgh CR | Latent tuberculosis infection in the United States. N Engl J Med 2011;364:1441–8 | Editorial |
| 251 | Horvat RT | From the arm to the test tube: laboratory’s new role in tuberculosis testing. Clin Microbiol News 2012;34:117–25 | Review |
| 252 | Hosker HSR | How useful are interferon-gamma release assays in cases of suspected tuberculosis? Thorax 2009;64:A108 | Abstract |
| 253 | Hsia EC | QuantiFERON-TB Gold In-Tube test versus tuberculin skin test across RA, PsA, and AS patients prior to treatment with golimumab, a human anti-TNF antibody. Arthritis Rheum 2010;62:908 | Abstract |
| 254 | Iqbal AZ | Cost-effectiveness of Using QuantiFERON Gold (QFT-G)® versus tuberculin skin test (TST) among US and foreign born populations at a public health department clinic with a low prevalence of tuberculosis. Public Health Nurs 2014;31:144–52 | Economic study |
| 255 | Jackson C | Diabetes and latent tuberculosis infection: nested case–control study within the PREDICT cohort. Thorax 2013;68:A31–2 | Abstract |
| 256 | James PM | The performance of QuantiFERON-TB Gold in-Tube (QFT-IT) test compared to tuberculin skin test (TST) in detecting latent tuberculosis infection (LTBI) in the presence of HIV coinfection in a high TB-burden area with BCG-vaccinated population. J Int Assoc Provid AIDS Care 2014;13:47–55 | No construct validity |
| 257 | Jan Wu YJ | Different cut off of tuberculin skin test in latent tuberculosis screening in systemic lupus erythematosus, rheumatoid arthritis and ankylosing spondylitis with an intermediate tuberculosis burden population. Ann Rheum Dis 2013;72:A963–4 | Abstract |
| 258 | Jegal Y | The significance of whole blood interferon-gamma assay in patients with history of pulmonary tuberculosis. Am J Resp Crit Care Med 2009;179:A4098 | Abstract |
| 259 | Jeong JC | Utility of QuantiFERON-TB assay for prediction of tuberculosis development in kidney transplant patients in an intermediate-tuberculosis-burden country: lack of evidence for enhanced prediction for short-term tuberculosis development. Transplant Proc 2014;46:583–7 | IGRA only (no TST) for construct validity |
| 260 | Jeong Y | Prevalence of latent tuberculosis infection in patients with radiographic lesions suggesting old healed tuberculosis. Am J Resp Crit Care Med 2011;183:A1190 | Abstract |
| 261 | Jordan N | QuantiFERON Gold screening for latent tuberculosis: cost comparison with mantoux testing. Rheumatology 2009;48:i70 | Abstract |
| 262 | Jung YJ | The ‘either test positive’ strategy for latent tuberculous infection before anti-tumour necrosis factor treatment. Int J Tuberc Lung Dis 2014;18:428–34 | No construct validity |
| 263 | Kadavath S | Effectiveness of combining tuberculin skin test and interferon gamma release assays as a screening strategy for detecting latent tuberculosis infection in high risk patients with systemic lupus erythematosus. Ann Rheum Dis 2013;72:478 | Abstract |
| 264 | Kalyoncu U | Comparison of QuantiFERON-TB test and TST in routine practice during anti-TNF treatment. Ann Rheum Dis 2013;72:231 | Abstract |
| 265 | Karabela S | QuantiFERON TB Gold assay in tuberculosis diagnosis in patients from areas with high and low TB incidence. Clin Microbiol Infect 2009;15:S669–70 | Abstract |
| 266 | Karabela S | QuantiFERON TB Gold in Tube assay for TB diagnosis: a two-year experience. Clin Microbiol Infect 2009;15:S392–3 | Abstract |
| 267 | Karimi A | Discrepancy between whole blood interferon gamma assay and tuberculin skin test for diagnosis of latent TB infection in BCG vaccinated children. IUBMB Life 2009;61:314 | Abstract |
| 268 | Kariminia A | Comparison of QuantiFERON TB-G-test to TST for detecting latent tuberculosis infection in a high-incidence area containing BCG-vaccinated population. J Eval Clin Pract 2009;15:148–51 | Mixed population and/or no subgroup of interest |
| 269 | Kashyap RS | Latent TB infection diagnosis in population exposed to TB subjects in close and poor ventilated high TB endemic zone in India. PLOS ONE 2014;9:e89524 | Abstract |
| 270 | Kawamura LM | IGRAs in public health practice: economic issues. Int J Tuberc Lung Dis 2010;14(Suppl. 1):60–3 | Economic study |
| 271 | Kenney A | Physicians inconsistently perform tuberculosis screening in inflammatory bowel disease patients managed with anti-TNF agents. Inflamm Bowel Dis 2011;17:S45 | Abstract |
| 272 | Ketenci A | Comparison of whole blood gamma interferon assay and tuberculin skin testing in tuberculosis contacts. Respirology 2013;18:71 | Abstract |
| 273 | Khalilzadeh S | Role of QuantiFERON-TB test in detection of children infected with mycobacterium tuberculosis. Tanaffos 2010;9:22–7 | No construct validity |
| 274 | Kiet G | Comparing T-SPOT and tuberculosis skin testing screening methods for treatment acceptance and cost efficacy. J Adolesc Health 2012;1:S68 | Abstract |
| 275 | Kik S | Predictive value of IGRAs. Enfermedades Emergentes 2009;11:178–9 | Abstract |
| 276 | Kim C | Comparison of QuantiFERON-TB gold with tuberculin skin test for detecting latent tuberculosis infection before kidney transplantation. Am J Transpl 2013;13:341 | Abstract |
| 277 | Kim EY | Performance of tuberculosis skin test and inferferon-γ release assay for detection of tuberculosis infection in immunocompromised patients in an intermediate burden country. Respirology 2009;14:A188 | Abstract |
| 278 | Kim H | Clinical usefulness of ELISPOT assay for diagnosis of tuberculosis in a Korean population. Clin Microbiol Infect 2010;16:S545 | Abstract |
| 279 | Kim JH | Evaluation of the usefulness of interferon-gamma release assays and tuberculin skin test for detection of latent mycobacterium tuberculosis infection in Korean rheumatic patients with biologic agents. Arthritis Rheum 2013;65:S560 | Abstract |
| 280 | Kleinert S | Screening for latent tuberculosis infection (LTBI) performance of tuberculous skin test and interferon-gamma release assays (IGRA) under real life conditions. Arthritis Rheum 2010;62:782 | Abstract |
| 281 | Kowada A | Cost effectiveness of IGRAs assays in Japan. Int J Tuberc Lung Dis 2010;14(Suppl. 1):59–60 | Abstract |
| 282 | Kowada A | Cost effectiveness of interferon-gamma release assay for TB screening of HIV positive pregnant women in low TB incidence countries. J Infect 2014;68:32–42 | Abstract |
| 283 | Kruczak K | QuantiFERON-TBGIT (QFT) vs TST in diagnosis of latent tuberculosis (TB) infection (LTBI) in vaccinated population in Poland. Am J Resp Crit Care Med 2009;179:A5925 | Abstract |
| 284 | Kruczak K | QuantiFERON-TBGIT (QFT) vs tuberculosis skin test (TST) in diagnosis of latent tuberculosis (Tb) infection (LTBI) in patients treated by oral glucocorticosteroids. Am J Resp Crit Care Med 2010;181:A4779 | Abstract |
| 285 | Kumar P | Basic and clinical immunology – 3019. Gamma interferon release assay for diagnosis of latent tuberculosis – comparison with TB skin test. World Allergy Organ J 2013;6(Suppl. 1):P195 | Abstract |
| 286 | Lagrange PH | A toolbox for tuberculosis (TB) diagnosis: an Indian multicentric study (2006–2008). Evaluation of QuantiFERON-TB Gold in Tube for TB diagnosis. PLOS ONE 2013;8:e73579. | Active TB |
| 287 | Lee J | Comparison of quantiferon-TB gold assay with tuberculin skin test in Korean children. J Allergy Clin Immunol 2010;1:AB152 | Abstract |
| 288 | Lee JH | Poor agreement between QuantiFERON-TB Gold test and tuberculin skin test results for the diagnosis of latent tuberculosis infection in rheumatoid arthritis patients and healthy controls. Korean J Intern Med 2014;29:76–84 | No construct validity |
| 289 | Lee SJ | Risk factors for latent tuberculosis infection in close contacts of active tuberculosis patients in South Korea. Respirology 2012;17:130 | Abstract |
| 290 | Lee SSJ | Positive predictive value of interferon-gamma release assay for incident active tuberculosis in HIV-infected persons. Retrovirology 2012;9(Suppl. 1):P133 | Abstract |
| 291 | Lee SSJ | Prediction of the risk of active tuberculosis in HIV-infection with an interferon-gamma release assay. Lancet 2013;382:16 | Abstract |
| 292 | Lee T | Diagnosis of latent tuberculosis infection by using the QuantiFERON-TB Gold in-Tube test in children whose household contact has contagious pulmonary tuberculosis disease. Int J Infect Dis 2010;14:e307 | Abstract |
| 293 | Leung CC | Evaluation of the T SPOT-TB test in the targeted screening of close contacts of smear-positive tuberculosis patients. Am J Resp Crit Care Med 2011;183:A1186 | Abstract |
| 294 | Lim S | Risk of latent tuberculosis infection following exposure to active tuberculosis in at-risk children with rheumatic diseases. Intern Med J 2011;41:32 | Abstract |
| 295 | Lim SC | Risk of latent tuberculosis in at-risk children with rheumatic diseases. Pediatr Rheumatol 2011;9(Suppl. 1):P218 | Abstract |
| 296 | Linas BP | The cost effectiveness of tuberculin skin test and interferon gamma release assay screening for latent tuberculosis infection in the US. Am J Resp Crit Care Med 2011;183:A6337 | Abstract |
| 297 | Lindemann M | Diagnosis of tuberculosis infection in patients awaiting liver transplantation. Hum Immunol 2009;70:24–8 | No relevant outcomes |
| 298 | Ling DI | Interferon-gamma release assay for pediatric tuberculosis: does it impact diagnostic and treatment decisions? Am J Resp Crit Care Med 2010;181:A4776 | Abstract |
| 299 | Liza MI | Anti-TNFa therapy and tuberculosis risk: a two-centre experience. Int J Rheum Dis 2010;13:90 | Abstract |
| 300 | Lombardi G | Tuberculosis infection in young children: a screening based on skin and blood testing. Clin Microbiol Infect 2012;18:548 | Abstract |
| 301 | Lopez Y | Correlation between the response to Mycobacterium tuberculosis antigens and the tuberculin skin test in patients with rheumatoid arthritis in Colombia. Biomedica 2013;33:226–32 | No construct validity |
| 302 | Losi M | Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in patients with rheumatic diseases. Clin Microbiol Infect 2010;16:S543 | Abstract |
| 303 | Losi M | Role of the QFT-IT assay for the diagnosis of latent tuberculosis infection among adult immigrants. Am J Resp Crit Care Med 2011;183:A1194 | Abstract |
| 304 | Machado A Jr | Analysis of discordance between the tuberculin skin test and the interferon-gamma release assay. Int J Tuberc Lung Dis 2009;13:446–53 | Mixed population and/or no subgroup of interest |
| 305 | Machado J | Diagnosis of latent tuberculosis in patients with inflammatory bowel disease: prospective comparison between tuberculin skin test and IGRA test QuantiFERON TB-Gold. J Crohns Colitis 2011;5:S64 | Abstract |
| 306 | Mahomed H | Predictive value of the TST vs. QuantiFERON-TB Gold in-Tube in adolescents in a high-burden setting. Int J Tuberc Lung Dis 2010;14(Suppl. 1):48–9 | Presentation |
| 307 | Manosa M | Current incidence of active tuberculosis in IBD patients treated with anti-TNF agents: still room for improvement. J Crohns Colitis 2013;7:e499–500 | Letter |
| 308 | Mardani M | Performance of QuantiFERON TB Gold test compared with the tuberculin skin test for detecting latent tuberculosis infection in lung and heart transplant candidates. Exp Clin Transpl 2014;12:129–32 | No construct validity |
| 309 | Mardani M | Accuracy of QuantiFERON-TB Gold test versus tuberculin skin test to detect latent tuberculosis infection in HIV-positive individuals in Iran. Clin Microbiol Infect 2009;15:S391–2 | Abstract |
| 310 | Mardani M | Performance of QuantiFERON-TB Gold test compared to tuberculin skin test in detecting latent tuberculosis infection in HIV-positive individuals in Iran. Ann Thorac Med 2010;5:43–6 | No construct validity |
| 311 | Mariette X | Before beginning anti-TNF, a better targeted screening and a twice decrease frequency of latent tuberculosis (TB) with IFN gamma release assays (IGRA) compared with tuberculin skin test. Results in 396 patients from the ETAT study. Arthritis Rheum 2010;62:376 | Abstract |
| 312 | Maritsi D | Assessment of quantiferon as a screening tool prior to initiation of infliximab: a single centre’s perspective. Clin Exp Rheumatol 2011;29:402–3 | Abstract |
| 313 | Marra F | Cost-effectiveness of a new interferon-based blood assay, QuantiFERON-TB Gold, in screening tuberculosis contacts. Int J Tuberc Lung Dis 2008;12:1414–24 | Economic study |
| 314 | Martinez-Morillo M | Interferon-γ release assays in rheumatic patients: baseline study and in the course of anti-tumor necrosis factor-α agents. Arthritis Rheum 2011;63(Suppl. 10):1941 | Abstract |
| 315 | Martinez-Morillo M | Interferon-gamma release assays in rheumatic patients: baseline study and in the course of anti-tumor necrosis factor-alpha agents. Ann Rheum Dis 2014;71(Suppl. 3):277 | Abstract |
| 316 | Mateo L | Usefulness of in vitro interferon-release assays (IGRAS) for diagnosis of latent tuberculosis infection in rheumatic patients scheduled for anti-TNF-treatment. Arthritis Rheum 2009;60:996 | Abstract |
| 317 | Matsubara J | Indeterminate and positivity rates of a commercially available enzyme-linked immunospot (ELISPOT) blood test in at-risk groups for tuberculosis infection. Am J Infect Control 2010;38:e48–9 | Abstract |
| 318 | Matsubara J | Indeterminate and positivity rates of the T-SPOT.TB test in at-risk individuals screened for tuberculosis infection. Am J Resp Crit Care Med 2010;181:A6831 | Abstract |
| 319 | Mehta B | Combining tuberculin skin test and interferon gamma release assays for latent tuberculosis infection screening may be necessary for the exclusion of latent tuberculosis in a high risk individuals with rheumatoid arthritis. Arthritis Rheum 2011;63(Suppl. 10):1190 | Abstract |
| 320 | Mehta B | A proposed effective strategy to screening latent TB infection in RA patients. Ann Rheum Dis 2013;71(Suppl. 3):169 | Abstract |
| 321 | Melath S | Screening for latent TB in patients with rheumatic disorders prior to biologic agents in a ‘high-risk’ TB population: comparison of two interferon gamma release assays. Rheumatol Int 2014;34:149–50 | Abstract |
| 322 | Mendes MA | Contact screening in tuberculosis: can we identify those with higher risk? Eur Respir J 2013;41:758–60 | Mixed population and/or no subgroup of interest |
| 323 | Mendoza OM | Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection. Eur Respir J 2011;38:1237–8 | Letter |
| 324 | Meyssonnier V | Performance of Quantiferon® for the diagnosis of TB. Med Mal Infect 2012;42:579–84 | Active TB |
| 325 | Milburn H | A comparison between interferon gamma release assays and the tuberculin skin test in the contact tracing of patients with chronic kidney disease response. Thorax 2011;66:730 | Letter |
| 326 | Miller RF | Comparison of two interferon-gamma release assays (Quantiferon-TB Gold in-Tube and T-SPOT.TB) in screening for latent tuberculosis infection (LTBI) among HIV-infected adults attending an inner London HIV clinic. Thorax 2011;66:A72–3 | Abstract |
| 327 | Ministro P | Diagnosis of latent tuberculosis in patients with inflammatory bowel disease: prospective comparison between tuberculin skin test and interferon gamma release assay (IGRA) test. Gastroenterology 2011;140:S776 | Abstract |
| 328 | Mittal C | QuantiFERON TB gold testing for latent tuberculosis is more frequently indeterminate in patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:S62 | Abstract |
| 329 | Mount C | Mantoux or gamma interferon (IGRA) – which test is best in children? Thorax 2011;66:A138–9 | Abstract |
| 330 | Mulder C | Predictive value of the tuberculin skin test among newly arriving immigrants. PLOS ONE 2013;8 e60130 | IGRA only (no TST) |
| 331 | Munoz L | Prevention of tuberculosis associated with tumour necrosis factor antagonists. An 8-year observational cohort study. Clin Microbiol Infect 2012;18:33 | Abstract |
| 332 | Neira-Munoz E | Extensive transmission of mycobacterium tuberculosis among children on a school bus. Pediatr Infect Dis J 2008;27:836–7 | Abstract |
| 333 | Ni Cheallaigh C | Sensitivity, specificity and inter-test agreement of interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals with advanced immunodeficiency. Clin Microbiol Infect 2010;16:S72 | Abstract |
| 334 | Nicol MP | Comparison of T-SPOT.TB assay and tuberculin skin test for the evaluation of young children at high risk for tuberculosis in a community setting. Pediatrics 2009;123:38–43 | Inappropriate proxy for LTBI |
| 335 | Noorbakhsh S | Evaluation of the agreement between Quantiferon-TB assay and tuberculin skin test in TB infected cases: Tehran, Iran. Int J Infect Dis 2012;16:e288 | Abstract |
| 336 | Novak S | Tuberculosis among patients treated with anti TNF inhibitors prior and after the use of Quantiferon test. Clin Exp Rheumatol 2009;27:710 | Abstract |
| 337 | O’Flynn E | Quantiferon testing in mantoux negative patients commencing anti-TNF therapy identifies additional at risk patients. Irish J Med Sci 2012;181:S58 | Abstract |
| 338 | Ong SY | How good are we at screening for infections prior to anti-TNF-alpha therapy? J Gastroenterol Hepatol 2012;27:113–14 | Abstract |
| 339 | Oon H | The interferon-gamma release assay: experience from a tertiary dermatology center in the tropics. J Am Acad Dermatol 2013;68:AB135 | Abstract |
| 340 | Ortakayla M | Concordance of the interferon-γ release assay (IGRA) and the tuberculin skin test (TST) for the screening of tuberculosis infection in the inflammatory rheumatic disease (IRD) population. Chest 2012;142(Suppl. 4):211A | Abstract |
| 341 | Ozbek S | Detection of latent tuberculosis infection in rheumatologic diseases before anti-TNFalpha therapy: tuberculin skin test versus IFN-γ assay. Arthritis Rheum 2013;65:S577 | Abstract |
| 342 | Ozen Alahdab Y | Interferon-gamma release assay or tuberculin skin test in inflammatory bowel disease patients – which is reliable. J Crohns Colitis 2011;5:S53 | Abstract |
| 343 | Painter JA | Tuberculosis screening by tuberculosis skin test or QuantiFERON-TB Gold In-Tube assay among an immigrant population with a high prevalence of tuberculosis and BCG vaccination. PLOS ONE 2013;8:e82727 | Active TB |
| 344 | Paluch-Oles J | Identification of latent tuberculosis infection in rheumatic patients under consideration for treatment with anti-TNF-alpha agents. Arch Med Sci 2013;9:112–17 | No construct validity |
| 345 | Papay P | Immunosuppressive (IS) therapy impacts the results of QuantiFERON and tuberculin skin test in routine screening for latent tuberculosis (LTB) in patients with inflamm bowel diseases (IBD). Gastroenterol 2009;1:A195. | Abstract |
| 346 | Pareek M | Modelling the health impact and cost-effectiveness of screening new entrants to the UK for latent tuberculosis infection. J Infect 2009;59:S442 | Abstract |
| 347 | Pareek M | Community-based evaluation of immigrant TB screening using interferon gamma release assays and tuberculin skin testing: yields and cost-effectiveness. Thorax 2011;66:A20 | Abstract |
| 348 | Patel D | Screening for latent tuberculosis in patients starting anti-TNF therapy. Rheumatol 2012;51:iii175 | Abstract |
| 349 | Pease E | Does the dual-testing strategy under-diagnose latent TB infection in HIV-infected individuals? A 1-year experience in a TB high-incidence area in the UK. HIV Med 2013;14:69 | Abstract |
| 350 | Perez-Escolano E | Comparison of an interferon-gamma release assay with tuberculin skin test for the diagnosis of tuberculosis infection in a contact investigation. Clin Microbiol Infect 2009;15:S392 | Abstract |
| 351 | Perez-Escolano E | Comparison of QuantiFERON TB Gold with tuberculin skin test for the diagnosis of tuberculosis infection in risk groups. Clin Microbiol Infect 2010;16:S542–3 | Abstract |
| 352 | Pesola GR | Quantiferon gold in tube latent tuberculosis testing in low risk healthy adults. Am J Resp Crit Care Med 2011;183:A4884 | Abstract |
| 353 | Pullar ND | Low prevalence of positive interferon-gamma tests in HIV-positive long-term immigrants in Norway. Int J Tuberc Lung Dis 2014;18:180–7 | No construct validity |
| 354 | Punal Rioboo J | Interferon-Gamma Release Assays (IGRAs) for Diagnosis of Latent Tuberculosis Infection and Active Tuberculosis. Santiago de Compostela: Galician Agency for Health Technology Assessment (AVALIA-T); 2010 | Abstract |
| 355 | Qin LL | T-SPOT.TB for detection of tuberculosis infection among hematological malignancy patients and hematopoietic stem cell transplant recipients. Asian Pac J Cancer Prev 2013;14:7415–19 | No construct validity |
| 356 | Richeldi L | Prior tuberculin skin testing does not boost QuantiFERON-TB results in paediatric contacts. Eur Respir J 2008;32:524–5 | Letter |
| 357 | Rotar Z | Performance of a two-step latent tuberculosis screening algorithm in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis prior to treatment with tumor necrosis alpha inhibitors: prospective observational data from the Biorx.Si registry. Arthritis Rheum 2013;65:S578 | Abstract |
| 358 | Sauzullo I | Detection of M. tuberculosis infection by interferon-gamma release assays: a comparative study in HIV-infected patients and in immunosuppressed candidates for anti-TNF-alpha therapy. HIV Med 2009;10:155–6 | Abstract |
| 359 | Sauzullo I | Usefulness of interferon-gamma release assays for latent tuberculosis screening in patients candidate for TNF-α therapy. Clin Microbiol Infect 2010;16:S72 | Abstract |
| 360 | Schichter-Konfino V | Interferon-γ-release assay prevents unnecessary tuberculosis therapy in individuals with positive tuberculin skin test. J Allergy Clin Immunol 2014;133:AB244 | Abstract |
| 361 | Seagar AL | Assessment of the use of the Quantiferon-TB gold in-tube assay for the diagnosis of TB infection in Lothian, Scotland. Clin Microbiol Infect 2010;16:S544 | Abstract |
| 362 | Sester M | Head-to-head analysis of M. tuberculosis interferon-γ release assays (IGRAs) and skin-testing in immunocompromised patients: interim analysis of a European multicenter TBNET study. Am J Transpl 2011;11:115 | Abstract |
| 363 | Shakak AO | Latent tuberculosis infections (LTBI): tuberculin skin test and whole blood IFN-gamma as surrogate markers in developing countries. Clin Chem Lab Med 2011;49:S541 | Abstract |
| 364 | Sharma N | ELISPOT as a predictor for development of TB in children with TB contact. Thorax 2009;64:320 | Abstract |
| 365 | Soborg B | Comparison of screening procedures for LTBI among patients with inflammatory diseases. Int J Tuberc Lung Dis 2010;14(Suppl. 1):38–40 | Included/excluded in CG11710 |
| 366 | Stavri HR | Prospective comparison of two brands of tuberculin skin tests and Quantiferon-TB Gold in-Tube assay performances for tuberculosis infection in hospitalized children. Medica 2010;5:271–6 | Active TB |
| 367 | Swaminath A | Quantiferon testing is superior to tuberculosis skin test (TST) in identifying latent TB in immunosuppressed patients with inflammatory bowel disease: a decision analysis. Am J Gastroenterol 2012;107:S688 | Abstract |
| 368 | Swaminath A | Cost-effectiveness of QuantiFERON testing before initiation of biological therapy in inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2444–9 | Economic study |
| 369 | Tavast E | IGRA tests perform similarly to TST but cause no adverse reactions: pediatric experience in Finland. BMC Res Notes 2009;2:9 | Active TB |
| 370 | Tavast E | Immunosuppression adversely affects TST but not IGRAs in patients with psoriasis or inflammatory musculoskeletal diseases. Int J Rheumatol 2012;2012:381929 | Non-standard or in-house IGRA |
| 371 | Triverio PA | Interferon-gamma release assays versus tuberculin skin testing for detection of latent tuberculosis in chronic haemodialysis patients. Nephrol Dial Transpl 2009;24:1952–6 | Included/excluded in CG11710 |
| 372 | Van Zyl-Smit RN | Immunodiagnosis of latent TB in HIV-infected persons in a high burden setting. Am J Resp Crit Care Med 2011;183:A4885 | Abstract |
| 373 | Vassilopoulos D | Comparison of two interferon-gamma release assays to tuberculin skin testing for latent tuberculosis screening in rheumatic patients starting anti-TNF treatment. Arthritis Rheum 2009;60:1907 | Abstract |
| 374 | Velizarova SA | To what extent T-SPOT.TB could be used in the diagnosis of tuberculosis in children exposed to TB infection? Eur J Immunol 2009;39:S217 | Abstract |
| 375 | Vortia E | Use of the QuantiFERON-TB Gold in-Tube test for latent tuberculosis screening in children with inflammatory bowel disease treated with infliximab. Gastroenterology 2013;144:S887 | Abstract |
| 376 | Wang H | Clinical value of a whole blood interferon-γ release assay for the diagnosis of Mycobacterium tuberculosis infection during antitubercular treatment. Exp Ther Med 2013;6:455–8 | Active TB |
| 377 | Wiwanitkit V | QuantiFERON-TB Gold test versus tuberculin skin test. Ann Thorac Med 2010;5:119 | Abstract |
| 378 | Wollman J | The effect of the severity of psoriasis on screening for latent tuberculosis: a comparison study between psoriasis and rheumatoid arthritis patients. Ann Rheum Dis 2013;71(Suppl. 3):692 | Abstract |
| 379 | Wong SH | Tuberculosis screening with interferon-gamma release assay in inflammatory bowel disease in a tuberculosis-endemic population. Gastroenterology 2013;144:S418 | Abstract |
| 380 | Yilmaz N | Comparison of QuantiFERON-TB Gold test and tuberculin skin test for identification of latent Mycobacterium tuberculosis infection in lupus patients. Arthritis Rheum 2009;60:286 | Abstract |
| 381 | Zapantis E | What is the optimal screening test to detect latent tuberculosis infection in high risk patients with systemic lupus erythematosus? Findings from a US inner city high-risk SLE cohort. Lupus 2013;22:61 | Abstract |
| 382 | Zelinkova Z | Effectiveness of the screening for latent tuberculosis in inflammatory bowel disease patients with previous BCG vaccination. Gastroenterology 2013;144:S413–14 | Abstract |
| 383 | Zlnay M | The risk of tuberculosis in patients with ankylosing spondylitis during anti-TNF therapy: data from national database in Slovakia. Ann Rheum Dis 2013;72(Suppl. 3):513 | Abstract |
December 2014 update
| Number | Author ID | Details | Reason(s) for exclusion |
|---|---|---|---|
| 384 | Al-Taweel T | A pilot study of optimal screening for latent tuberculosis in patients with inflammatory bowel disease. Gastroenterology 2014;1:S–582 | Abstract |
| 385 | Al Wakeel JS | The use of QuantiFERON TB Gold in-Tube test in screening latent and active tuberculosis among saudi dialysis patients. Nephrol Dial Transpl 2014;29:iii477–8 | Abstract |
| 386 | Arenas Miras MDM | Diagnosis of latent tuberculosis in patients with systemic lupus erythematosus: T.SPOT.TB versus tuberculin skin test. Biomed Res Int 2014;2014:291031 | No construct validity; immunocompromised: concordance information |
| 387 | Arstikyte I | The value of the Quantiferon TB Gold In-Tube test in the identification of latent tuberculosis in rheumatic patients before treatment with TNF-alpha blockers in Vilnius University Hospital Santariskiu Clinics. Scand J Rheumatol 2014;43:44–5 | Abstract |
| 388 | Belknap R | Interferon-gamma release assays. Clin Lab Med 2014;34:337–49 | Review |
| 389 | Bennett A | Does the tuberculin skin test increase the detection of TB infection when screening HIV positive patients? Three years’ experience in a district general hospital. Thorax 2014;69:A209 | Abstract |
| 390 | Calzada-Hernandez J | PReS-FINAL-2265: tuberculosis in pediatric patients who are receiving anti-TNF agents. Pediatr Rheumat 2013;11(Suppl. 2):P255 | Abstract |
| 391 | Chuke SO | Tuberculin skin tests versus interferon-gamma release assays in tuberculosis screening among immigrant visa applicants. Tuberc Res Treat Print 2014;2014:217969 | No construct validity; recently arrived: concordance information |
| 392 | Cruz AT | Relationship between tuberculin skin test (TST) size and interferon gamma release assay (IGRA) result: when should clinicians obtain IGRAs in children with positive TSTs? Clin Pediatr 2014;53:1196–9 | No construct validity for LTBI (prior TB is not a construct of LTBI); study aim was to compare the tests in predicting chest radiography result suggesting the presence of MTB |
| 393 | Duman N | Screening for latent tuberculosis infection in psoriasis and psoriatic arthritis patients in a tuberculosis-endemic country: a comparison of the Quantiferon-TB Gold In-Tube test and tuberculin skin test. Int J Dermatol 2014;53:1286–292 | No construct validity |
| 394 | Elfrink F | Screening travellers to high-endemic countries for infection with Mycobacterium tuberculosis using interferon gamma release assay; a prospective study. BMC Infect Dis 2014;14:515 | Repeat testing |
| 395 | Elmahdy MMGF | Tuberculin skin test and QuantiFERON test for detection of latent Mycobacterium tuberculosis. Int J Infect Dis 2014;21:349 | Abstract |
| 396 | Golovics PA | Is the tuberculin skin test alone accurate in moderate-to-severe BCG vaccinated patients with inflammatory bowel disease to test for latent tuberculosis? J Crohns Colitis 2014;8:S144 | Abstract |
| 397 | Islam S | Tuberculin skin test and QuantiFERON performance, and testing of populations at low risk for tuberculosis infection. Clin Infect Dis 2014;59:1187–8 | Letter to the editor |
| 398 | Jenum S | The frequencies of IFNγ+IL2+TNFα+ PPD-specific CD4+CD45RO+ T-cells correlate with the magnitude of the QuantiFERON Gold In-Tube response in a prospective study of healthy Indian adolescents. PLOS ONE 2014;9:e101224 | Comparing antigens |
| 399 | Julian AN | Diagnosis of tuberculosis infection in pediatric patients treated with inhibitors of the tumour necrosis factor alpha. A multicenter national study comparing tuberculin skin test and igra tests. Pediatr Rheumatol 2014;12:P282 | Abstract |
| 400 | Marquez C | Tuberculosis infection in early childhood in Uganda and the influence of HIV exposure. Topics Antiviral Med 2014;22:47–8 | Abstract |
| 401 | Mathad JS | Effect of HIV on latent TB screening of pregnant women in Pune, India. Topics Antiviral Med 2014;22:425–6 | Abstract |
| 402 | McMullen SE | Performance of QuantiFERON-TB Gold and tuberculin skin test relative to subjects’ risk of exposure to tuberculosis. Clin Infect Dis 2014;58:1260–6 | Population aged > 18 years |
| 403 | Mendy A | Higher specificity of tuberculin skin test compared with QuantiFERON-TB Gold for detection of exposure to Mycobacterium tuberculosis. Clin Infect Dis 2014;59:1188–9 | Letter to editor |
| 404 | Nassiri AA | Re: Interferon-gamma release assay agreement with tuberculin skin test in pretransplant screening for latent tuberculosis in a high-prevalence country. Iran J Kidney Dis 2014;8:432–3 | Letter |
| 405 | O’Flynn E | Performance and benefits of replacing Mantoux test with QuantiFERON in screening for latent TB in patients prior to anti-TNF therapy. Ann Rheum Dis 2014;73(Suppl. 2):2–1247 | Abstract |
| 406 | O’Flynn E | Performance and benefits of replacing Mantoux test with QuantiFERON in screening for latent TB in patients prior to anti TNF therapy. Irish J Med Sci 2014;183:S105 | Abstract |
| 407 | Opris D | Is tuberculosis screening sufficient for preventing TB reactivation in biologic treated patients? Ann Rheum Dis 2014;73:497 | Abstract |
| 408 | O’Shea MK | Tuberculin skin testing and treatment modulates interferon-gamma release assay results for latent tuberculosis in migrants. PLOS ONE 2014;9:e97366 | Military recruits |
| 409 | Panchal RK | The effectiveness of primary care based risk stratification for targeted latent tuberculosis infection screening in recent immigrants to the UK: a retrospective cohort study. Thorax 2014;69:354–62 | No comparison between IGRA and TST |
| 410 | Pease E | Does the dual testing strategy under-diagnose latent tuberculosis infection in UK HIV-infected individuals?: a one year experience in a tuberculosis high incidence area. Int J STD AIDS 2013;24:56 | Poster |
| 411 | Prignano F | Latent tuberculosis infection in psoriasis and other dermatological immunomediated diseases: a combined approach by QuantiFERON-TB Gold and tuberculin skin tests. Int J Dermatol 2014;53:e372–4 | Letter |
| 412 | Rose W | QuantiFERON Gold-in-Tube assay for TB screening in HIV infected children: influence of quantitative values. BMC Infect Dis 2014;14:516 | Repeat testing, proportion of people had self-read TST results |
| 413 | Sanchez Riera L | QuantiFERON-TB more useful than tuberculin skin test for latent tuberculosis screening: a hospital experience. Ann Rheum Dis 2014;73:950–1 | Abstract |
| 414 | Santoro-Lopes G | Screening for latent tuberculosis infection in low-incidence areas. Am J Transpl 2014;14:1709 | Letter to the editor |
| 415 | Savaj S | Interferon-gamma release assay agreement with tuberculin skin test in pretransplant screening for latent tuberculosis in a high-prevalence country. Iranian J Kidney Dis 2014;8:329–32 | No construct validity |
| 416 | Scholman T | Analysis of agreement between IGRAs and tuberculin skin-testing by the use of PPD as the same antigen. Transplantation 2014;90:540 | Abstract |
| 417 | Senturk T | Comparison of diagnostic test for latent tuberculosis infection. Int J Rheum Dis 2014;17:103 | Abstract |
| 418 | Shokrollahi MR | Diagnosis of latent tuberculosis in individuals with recent exposure: tuberculin skin test versus interferon-gamma release assay. Br J Biomed Sci 2014;71:125–6 | Not population of interest |
| 419 | Soare A | Preventing active tuberculosis in rheumatoid arthritis patients receiving TNF inhibitors: TB screening at baseline is not enough. Ann Rheum Dis 2014;73:325 | Abstract |
| 420 | Sztajnbok F | PReS-FINAL-2054: latent tuberculosis infection in patients with juvenile idiopathic arthritis undergoing methotrexate therapy: a longitudinal study with TST and ELISPOT. Pediatr Rheumatol Online J 2013;11(Suppl. 2):P67 | Repeat testing at 3 and 12 months |
| 421 | Sztajnbok F | Tuberculin skin test and ELISPOT/T.SPOT.TB in children and adolescents with juvenile idiopathic arthritis. Pediatr Rheumatol 2014;12:17 | Repeat testing at 3 and 12 months |
| 422 | Verhagen LM | Agreement between QuantiFERON-TB Gold in-Tube and the tuberculin skin test and predictors of positive test results in Warao Amerindian pediatric tuberculosis contacts. BMC Infect Dis 2014;14:383 | Repeat testing |
| 423 | Zelinkova Z | Screening for latent tuberculosis is effective but does not fully protect against tuberculosis reactivation during anti-TNF treatment in areas with high background incidence of tuberculosis. J Crohns Colitis 2014;8:S212 | Abstract |
| 424 | Zelinkova Z | Screening for latent tuberculosis is effective but does not fully protect against tuberculosis reactivation during anti-TNF treatment in areas with high background incidence of tuberculosis. Gastroenterology 2014;1:S585 | Abstract |
Appendix 7 ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform list of excluded ongoing studies (n = 30)
| Number | Study title | Recruitment status | URLa | Reason(s) for exclusion |
|---|---|---|---|---|
| 1 | Screening for latent tuberculosis infection (LTBI) in US army recruits | Active, not recruiting | http://ClinicalTrials.gov/show/NCT00804713 | Army recruits |
| 2 | Diagnosis of tuberculosis infection in health care workers using ex-vivo interferon-gamma assay | Completed | http://ClinicalTrials.gov/show/NCT01007396 | Health-care workers, active TB |
| 3 | Comparison of the Quantiferon®-TB GOLD (in Tube) assay with tuberculin skin testing for detecting latent tuberculosis infection in patients with chronic liver disease being evaluated for or awaiting liver transplantation | Withdrawn | http://ClinicalTrials.gov/show/NCT00424684 | Withdrawn |
| 4 | Surveillance and follow-up for latent tuberculosis infection and risk of developing active tuberculosis in patients receiving long-term dialysis | Completed | http://ClinicalTrials.gov/show/NCT01311999 | No comparison between IGRAs and TST |
| 5 | QuantiFERON®-TB Gold in-Tube for the diagnosis of tuberculosis infection in contact tracing study (OPTIMIST) | Active, not recruiting | http://ClinicalTrials.gov/show/NCT01223534 | No subgroup of interest |
| 6 | QuantiFERON for detection of latent tuberculosis in healthcare workers | Completed | http://ClinicalTrials.gov/show/NCT00797836 | Health-care workers |
| 7 | Is tuberculin skin testing effective in screening for latent tuberculosis (TB) in elderly residents of nursing homes? | Completed | http://ClinicalTrials.gov/show/NCT00756808 | No subgroup of interest |
| 8 | QuantiFERON Gold test for detecting tuberculosis (TB) infection in HIV/AIDS patients in South Africa | Recruiting | http://ClinicalTrials.gov/show/NCT02119130 | Active TB |
| 9 | Diagnosis and treatment of co-infection with human immunodeficiency virus/latent tuberculosis infection (HIV/TBL) | Active, not recruiting | http://ClinicalTrials.gov/show/NCT01875952 | No comparison between IGRAs and TST |
| 10 | The role of IGRA in screening and monitoring for TB during anti TNF therapy in patients with IMID | Recruiting | http://ClinicalTrials.gov/show/NCT02135289 | No comparison between IGRAs and TST |
| 11 | Immune response to Mycobacterium tuberculosis infection | Completed | http://ClinicalTrials.gov/show/NCT00257907 | Active TB |
| 12 | Performance of IGRAs for TB infection diagnosis in elderly (IGRage) | Recruiting | http://ClinicalTrials.gov/show/NCT01895582 | Active TB |
| 13 | Monthly follow up of interferon gamma releasing assay (IGRA) among health-care workers treating tuberculosis (TB) patients | Completed | http://ClinicalTrials.gov/show/NCT01121068 | Health-care workers |
| 14 | Vitamin A supplementation for modulation of Mycobacterium tuberculosis immune responses in latent tuberculosis | Withdrawn | http://ClinicalTrials.gov/show/NCT00558480 | Withdrawn |
| 15 | Diagnosis of latent tuberculosis (TB) infection in health care workers using TST and whole blood interferon-γ assay | Completed | http://ClinicalTrials.gov/show/NCT00962793 | Health-care workers |
| 16 | Latent tuberculosis infection in bone marrow transplant recipients | Completed | http://ClinicalTrials.gov/show/NCT01021124 | No comparison between IGRAs and TST |
| 17 | Conversion rate of (TST) tuberculin skin test and QuantiFERON-TB Gold in Tube assay in health care workers | Completed | http://ClinicalTrials.gov/show/NCT01376843 | Health-care workers |
| 18 | Determining risk in latent tuberculosis | Terminated | http://ClinicalTrials.gov/show/NCT01571739 | Study terminated |
| 19 | Treatment of latent tuberculosis infection with isoniazid | Completed | http://ClinicalTrials.gov/show/NCT00293228 | Focus on the effect of treatment |
| 20 | Effects of vitamin D supplementation on antimycobacterial immunity | Completed | http://ClinicalTrials.gov/show/NCT00157066 | Focus on the effect of treatment |
| 21 | A Phase I/IIa safety and immunogenicity of AERAS-456 in HIV-negative adults with and without latent tuberculosis infection (C-035–456) | Completed | http://ClinicalTrials.gov/show/NCT01865487 | Comparing antigen and placebo |
| 22 | Isoniazid (INH) treatment based on ELISPOT assay | Completed | http://ClinicalTrials.gov/show/NCT01087190 | Focus on the effect of treatment |
| 23 | A safety and immunogenicity trial with an adjuvanted TB subunit vaccine (Ag85B-ESAT-6 + IC31) (THYB-03) | Completed | http://ClinicalTrials.gov/show/NCT01049282 | Comparing antigens |
| 24 | IFN-gamma-releasing assay based approach in patients with suspected tuberculous peritonitis | Recruiting | http://ClinicalTrials.gov/show/NCT02175134 | Diagnosis of tuberculous peritonitis |
| 25 | A Phase III contact tracing trial comparing the diagnostic performance of C-Tb to QuantiFERON®-TB Gold in-Tube, in combination with a double blind randomized split body safety assessment of C-Tb versus 2 TU tuberculin PPD RT23 SSI | Authorised | www.clinicaltrialsregister.eu/ctr-search/trial/2011-005617-36/ES | Active TB |
| 26 | Ensayo clínico de dos estrategias para la toma de decisiones terapéuticas en el estudo de contactos de tuberculosis: estrategia estándar, basada en la prueba de la tuberculina (PT) sola frente a la combinación de PT y QuantiFERON-TB-Gold in-Tube | Authorised | www.clinicaltrialsregister.eu/ctr-search/trial/2009-017430-49/ES | Not English language |
| 27 | Interferon-gamma release assays in tuberculosis (TB) – HIV co-infected children | Recruiting | http://ClinicalTrials.gov/show/NCT00604617 | Active TB |
| 28 | Screening for latent tuberculosis in healthcare workers with QuantiFERON-Gold assay: a cost-effectiveness analysis | Recruiting | http://ClinicalTrials.gov/show/NCT00449345 | Health-care workers and economic analysis |
| 29 | Use TST and QFT-RD1 test to monitor the tuberculous infection in patients, close contact people and health care workers | Recruiting | http://ClinicalTrials.gov/show/NCT00311220 | Health-care workers |
| 30 | Diagnosis of active tuberculosis by ELISPOT | Recruiting | http://ClinicalTrials.gov/show/NCT00174083 | Active TB |
Appendix 8 Included ongoing trials comparing interferon gamma release assays with the tuberculin skin test (n = 20)
| Number | Study title | Recruitment status | URL |
|---|---|---|---|
| 1 | Interferon gamma release assays (IGRA) testing versus tuberculin skin test in renal transplant recipients | Completed | http://ClinicalTrials.gov/show/NCT01608685 |
| 2 | Latent tuberculosis in second generation immigrants from high risk countries compare to low-risk young Israeli adults | Not yet recruiting | http://ClinicalTrials.gov/show/NCT02073669 |
| 3 | Evaluation of 2 interferon γ assays in the diagnosis of latent tuberculosis in HIV-infected patients (ANRS EP 40 QUANTI SPOT) | Completed | http://ClinicalTrials.gov/show/NCT00647205 |
| 4 | The usefulness of interferon-γ release assays and tuberculin skin test for detection of latent tuberculosis infection | Unknown | http://ClinicalTrials.gov/show/NCT01685905 |
| 5 | Use of a gamma-IFN assay in contact tracing for tuberculosis in a low-incidence, high immigration area | Completed | http://ClinicalTrials.gov/show/NCT00557765 |
| 6 | Detection of latent tuberculosis in haemodialysis patients | Completed | http://ClinicalTrials.gov/show/NCT00695734 |
| 7 | Improving latent tuberculosis (TB) diagnosis in Thai children (TB Px) | Completed | http://ClinicalTrials.gov/show/NCT00947609 |
| 8 | Is tuberculin skin testing effective in screening for latent tuberculosis in patients with HIV? | Completed | http://ClinicalTrials.gov/show/NCT00763295 |
| 9 | Prevalence of latent tuberculosis (TB) infection diagnosed by interferon-gamma release assay and tuberculin skin tests in patients with old healed TB | Completed | http://ClinicalTrials.gov/show/NCT01099098 |
| 10 | T cell interferon-gamma release assay (TIGRA) in immunocompromised individuals (TBNET-TIPS) | Completed | http://ClinicalTrials.gov/show/NCT00707317 |
| 11 | A study on changes in IFN-gamma levels following anti-TNF treatment in patients undergoing serial QuantiFERON-TB Gold in-Tube | Completed | http://ClinicalTrials.gov/show/NCT01475409 |
| 12 | Medical and economical impact of IGRAs diagnosis of latent tuberculosis in HIV-infected patients | Completed | http://ClinicalTrials.gov/show/NCT00805272 |
| 13 | Comparison of Quantiferon-TB Gold assay with tuberculin skin testing in patients with chronic liver disease | Completed | http://ClinicalTrials.gov/show/NCT00402402 |
| 14 | Tuberculosis (TB) screening for the diagnosis of latent TB in immunocompromised populations | Completed | http://ClinicalTrials.gov/show/NCT00134342 |
| 15 | Impact of new immunological diagnosis tests of latent tuberculosis before anti TNF therapy | Completed | http://ClinicalTrials.gov/show/NCT00811343 |
| 16 | Latent tuberculosis infection in cancer patients | Completed | http://ClinicalTrials.gov/show/NCT00507754 |
| 17 | Latent tuberculosis infection in renal transplant recipients | Completed | http://ClinicalTrials.gov/show/NCT00682045 |
| 18 | Prognostic value of interferon gamma release assays in predicting active tuberculosis among individuals with, or at risk of, latent tuberculosis infection (PREDICT) | Not yet recruiting | http://clinicaltrials.gov/show/NCT01162265 |
| 19 | Comparison of the tuberculin skin test (TST) and QuantiFERON®-TB Gold Test (QFT-G) in patients with rheumatoid arthritis being considered for anti-TNF-alpha therapy | Unknown | http://clinicaltrials.gov/show/NCT00925249 |
| 20 | Quantiferon-TB Gold in the assessment of latent TB in patients candidate to treatment or treated with TNFα antagonists | Unknown | http://clinicaltrials.gov/show/NCT00491933 |
Appendix 9 Data extraction tables for included clinical effectiveness studies
Appendix 10 Included studies and incidence of tuberculosis228
| Study ID, country | Categorya | Estimated rate per 100,000 population |
|---|---|---|
| Studies in children and adolescents: incidence studies | ||
| Diel 2011,102 Germany | Low incidence | 5.6 |
| Mahomed 2011,103 South Africa | High incidence | 1003 |
| Metin Timur 2014,150 Turkey | Intermediate incidence | 22 |
| Noorbakhsh 2011,104 Iran | Intermediate incidence | 21 |
| Song 2014,152 South Korea | High incidence | 409 |
| Studies in children and adolescents: exposure studies | ||
| Adetifa 2010,105 Gambia | High incidence | 284 |
| Cruz 2011,106 USA | Low incidence | 3.6 |
| Kasambira 2011,107 South Africa | High incidence | 1003 |
| Laniado-Laborın 2014,148 Mexico | Intermediate incidence | 23 |
| Mahomed 2011,108 South Africa | High incidence | 1003 |
| Pavic 2011,109 Croatia | Low incidence | 14 |
| Perez-Porcuna 2014,151 Brazil | High incidence | 46 |
| Rutherford 2012,110,111 Indonesia | High incidence | 185 |
| Talbot 2012,112 USA | Low incidence | 3.6 |
| Tieu 2014,154 Thailand | High incidence | 119 |
| Tsolia 2010,113 Greece | Low incidence | 4.5 |
| Studies in immunocompromised people: incidence studies | ||
| Anibarro 2012,117 Spain | Low incidence | 14 |
| Chang 2011,119 South Korea | High incidence | 409 |
| Elzi 2011,114 Switzerland | Low incidence | 6 |
| Kim 2011,116 South Korea | High incidence | 409 |
| Lee 2009,118 Taiwan | High incidence | 73 |
| Lee 2014,149 South Korea | High incidence | 409 |
| Moon 2013,115 South Korea | High incidence | 409 |
| Sherkat 2014,155 Iran | Intermediate incidence | 21 |
| Studies in immunocompromised people: exposure studies | ||
| Ahmadinejad 2013,120 Iran | Intermediate incidence | 21 |
| Al Jahdali 2013,121 Saudi Arabia | Low incidence | 15 |
| Ates 2009,122 Turkey | Intermediate incidence | 22 |
| Casas 2011,123 Spain | Low incidence | 14 |
| Casas 2011,124 Spain | Low incidence | 14 |
| Chkhartishvili 2013,125 Georgia | High incidence | 116 |
| Chung 2010,126 South Korea | High incidence | 409 |
| Costantino 2013,127 France | Low incidence | 8.2 |
| Hadaya 2013,128 Switzerland | Low incidence | 6 |
| Hsia 2012,129 USA | Low incidence | 3.6 |
| Kim 2010,130 South Korea | High incidence | 409 |
| Kim 2013,131 South Korea | High incidence | 409 |
| Kim 2013,132 South Korea | High incidence | 409 |
| Kleinert 2012,133 Germany | Low incidence | 5.6 |
| Laffitte 2009,134 Switzerland | Low incidence | 6 |
| Maritsi 2011,135 UK | Low incidence | 15 |
| Mutsvangwa 2010,136 Zimbabwe | High incidence | 562 |
| Papay 2011,137 Austria | Low incidence | 7.9 |
| Ramos 2013,138 Spain | Low incidence | 14 |
| Seyhan 2010,139 Turkey | Intermediate incidence | 22 |
| Shen 2012,140 China | High incidence | 83 |
| Souza 2014,153 Brazil | High incidence | 46 |
| Takeda 2011,141 Japan | Low incidence | 19 |
| Vassilopoulos 2011,142 Greece | Low incidence | 4.5 |
| Studies in people recently arrived from high burden TB countries: incidence studies | ||
| Harstad 2010,143 Norway | Low incidence | 7.5 |
| Kik 2010,144 the Netherlands | Low incidence | 6.3 |
| Studies in people recently arrived from high burden TB countries: exposure studies | ||
| Lucas 2010,145 Australia | Low incidence | 6.5 |
| Orlando 2010,146 Italy | Low incidence | 6.7 |
| Saracino 2009,147 Italy | Low incidence | 6.7 |
Appendix 11 Data extraction tables for included cost-effectiveness studies
Appendix 12 Critical appraisal of the economic evaluation using the Consolidated Health Economic Reporting Standards checklist
| Assessment | Kowada 2010196 | Kowada 2012197 | Kowada 2013198 | Kowada 2014199 | Laskin 2013200 | Linas 2011201 | Mandalakas 2013203 | CG11710 | Pareek 201377 | Swaminath 2013202 |
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Abstract | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Introduction | ||||||||||
| Background and objectives | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Methods | ||||||||||
| Target population and subgroups | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Setting and location | UNC | UNC | UNC | UNC | UNC | UNC | Y | Y | Y | Y |
| Study perspective | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Comparators | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Time horizon | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Discount rate | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Choice of health outcomes | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Measurement of effectiveness | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Measurement and valuation of preference-based outcomes | N | N | N | N | N | Y | NA | N | Y | Y |
| Estimating resources and costs | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Currency, price date and conversion | Y | Y | Y | Y | Y | Y | Y | Y | Y | UNC |
| Choice of model | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Assumptions | Y | Y | Y | UNC | Y | Y | Y | Y | Y | Y |
| Analytical methods | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Results | ||||||||||
| Study parameters | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Incremental costs and outcomes | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Characterising uncertainty | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Discussion | ||||||||||
| Study findings | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Limitations | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Generalisability | Y | Y | UNC | Y | UNC | UNC | UNC | Y | Y | N |
| Other | ||||||||||
| Source of funding | Y | Y | UNC | Y | Y | Y | Y | Y | Y | Y |
| Conflicts of interest | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Appendix 13 Critical appraisal of the economic models using an adapted Philips et al.195 checklist
| Criteria | Kowada 2010196 | Kowada 2012197 | Kowada 2013198 | Kowada 2014199 | Laskin 2013200 | Linas 2011201 | Mandalakas 2013203 | CG11710 | Pareek 201377 | Swaminath 2013202 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure | |||||||||||
| 1 | Is there a clear statement of the decision problem? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2 | Is the objective of the model specified and consistent with the stated decision problem? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 3 | Is the primary decision maker specified? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 4 | Is the perspective of the model stated clearly? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 5 | Are the model inputs consistent with the stated perspective? | N | N | N | Y | Y | Y | Y | Y | Y | Y |
| 6 | Has the scope of the model been stated and justified? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 7 | Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 8 | Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Y | Y | Y | Y | Y | Y | Y | Y | UNC | Y |
| 9 | Are the sources of the data used to develop the structure of the model specified? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 10 | Are the causal relationships described by the model structure justified appropriately? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 11 | Are the structural assumptions transparent and justified? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 12 | Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| 13 | Is there a clear definition of the options under evaluation? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 14 | Have all feasible and practical options been evaluated? | Y | Y | Y | Y | Y | Y | Y | Y | Y | N |
| 15 | Is there justification for the exclusion of feasible options? | NA | NA | NA | NA | NA | NA | NA | NA | NA | N |
| 16 | Is the chosen model type appropriate given the decision problem and specified casual relationships within the model? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 17 | Is the time horizon of the model sufficient to reflect all important differences between the options? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 18 | Are the time horizon of the model and the duration of treatment described and justified? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 19 | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 20 | Is the cycle length defined and justified in terms of the natural history of disease? | Y | Y | Y | Y | Y | NA | Y | NA | NA | NA |
| Data | |||||||||||
| 21 | Are the data identification methods transparent and appropriate given the objectives of the model? | UNC | Y | UNC | Y | Y | Y | Y | Y | Y | Y |
| 22 | Where choices have been made between data sources are these justified appropriately? | UNC | UNC | UNC | UNC | UNC | UNC | UNC | UNC | UNC | UNC |
| 23 | Has particular attention been paid to identifying data for the important parameters of the model? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 24 | Has the quality of the data been assessed appropriately? | UNC | UNC | UNC | UNC | UNC | UNC | UNC | UNC | UNC | UNC |
| 25 | Where expert opinion has been used are the methods described and justified? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 26 | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 27 | Is the choice of baseline data described and justified? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 28 | Are transition probabilities calculated appropriately? | Y | Y | Y | Y | Y | NA | Y | NA | Y | NA |
| 29 | Has a half-cycle correction been applied to both costs and outcomes? | N | N | N | N | N | N | N | N | N | N |
| 30 | If not, has the omission been justified? | N | N | N | N | N | N | N | N | N | N |
| 31 | If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | NA | NA | NA | NA | NA | NA | UNC | NA | NA | NA |
| 32 | Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 33 | Have alternative extrapolation assumptions been explored through sensitivity analysis? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 34 | Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 35 | Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 36 | Are the costs incorporated into the model justified? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 37 | Has the source for all costs been described? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 38 | Have discount rates been described and justified given the target decision maker? | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA |
| 39 | Are the utilities incorporated into the model appropriate? | Y | Y | Y | Y | Y | Y | NA | Y | NA | NA |
| 40 | Is the source of utility weights referenced? | Y | Y | Y | Y | Y | Y | NA | Y | NA | NA |
| 41 | Are the methods of derivation for the utility weights justified? | UNC | UNC | UNC | UNC | UNC | Y | NA | UNC | NA | NA |
| 42 | Have all data incorporated into the model been described and referenced in sufficient detail? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 43 | Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 44 | Is the process of data incorporation transparent? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 45 | If data have been incorporated as distributions, has the choice of distributions for each parameter been described and justified? | N | N | N | N | Y | NA | NA | NA | NA | NA |
| 46 | If data have been incorporated as distributions, is it clear that second order uncertainty is reflected? | UNC | UNC | UNC | UNC | Y | NA | NA | NA | NA | NA |
| 47 | Have the four principal types of uncertainty been addressed? | N | N | N | N | N | N | N | N | N | N |
| 48 | If not, has the omission of particular forms of uncertainty been justified? | N | N | N | N | N | N | N | N | N | N |
| 49 | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | N | N | N | Y | NA | N | N | N | Y | N |
| 50 | Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | N | N | N | N | N | N | N | N | N | N |
| 51 | Has heterogeneity been dealt with by running the model separately for different subgroups? | Y | Y | Y | Y | Y | N | Y | N | Y | NA |
| 52 | Are the methods of assessment of parameter uncertainty appropriate? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 53 | If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | Y | Y | Y | Y | Y | Y | UNC | Y | Y | Y |
| 54 | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | UNC | UNC | UNC | UNC | UNC | UNC | UNC | Y | UNC | UNC |
| 55 | Are any counterintuitive results from the model explained and justified? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 56 | If the model has been calibrated against independent data, have any differences been explained and justified? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 57 | Have the results been compared with those of previous models and any differences in results explained? | Y | Y | Y | NA | Y | N | Y | N | Y | N |
Appendix 14 List of studies excluded from the cost-effectiveness review with reasons for exclusion (n = 15)
| Number | Author ID | Details | Reason(s) for exclusion |
|---|---|---|---|
| 1 | Burgos JL | Targeted screening and treatment for latent tuberculosis infection using QuantiFERON-TB Gold is cost-effective in Mexico. Int J Tuberc Lung Dis 2009;13:962–8 | No comparator |
| 2 | Deuffic-Burban | Cost-effectiveness of QuantiFERON-TB test vs. tuberculin skin test in the diagnosis of latent tuberculosis infection. Int J Tuberc Lung Dis 2010;14:471–81 | Close contacts |
| 3 | Diel R | Enhanced cost–benefit analysis of strategies for LTBI screening and INH chemoprevention in Germany. Respir Med 2009;103:1838–53 | Cost analysis |
| 4 | Hardy AB | Cost-effectiveness of the NICE guidelines for screening for latent tuberculosis infection: the QuantiFERON-TB Gold IGRA alone is more cost-effective for immigrants from high burden countries. Thorax 2010;65:178–80 | No economic model |
| 5 | Iqbal AZ | Cost-effectiveness of using QuantiFERON Gold (QFT-G) versus tuberculin skin test (TST) among US and foreign born populations at a public health department clinic with a low prevalence of tuberculosis. Public Health Nurs 2014;31:144–52 | No economic model |
| 6 | Jit M | Dedicated outreach service for hard to reach patients with tuberculosis in London: observational study and economic evaluation. BMJ 2011;343:d5376 | Active TB |
| 7 | Kawamura LM | IGRAs in public health practice: economic issues. Int J Tuberc Lung Dis 2010;14(Suppl. 1):60–3 | Letter to editor |
| 8 | Langley I | Modelling the impacts of new diagnostic tools for tuberculosis in developing countries to enhance policy decisions. Health Care Manag Sci 2012;15:239–53 | Active TB |
| 9 | Mancuso JD | Cost-effectiveness analysis of targeted and sequential screening strategies for latent tuberculosis. Int J Tuberc Lung Dis 2011;15:1223–30 | Military recruits |
| 10 | Pareek M | Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis 2011;11:435–44 | No comparator |
| 11 | Pooran A | Different screening strategies (single or dual) for the diagnosis of suspected latent tuberculosis: a cost-effectiveness analysis. BMC Pulm Med 2010;10:7 | Close contacts |
| 12 | Shah M | QuantiFERON-TB Gold in-Tube implementation for latent tuberculosis diagnosis in a public health clinic: a cost-effectiveness analysis. BMC Infect Dis 2012;12:360 | TST-positive referrals |
| 13 | Steffen RE | Cost-effectiveness of QuantiFERON-TB Gold-in-Tube versus tuberculin skin testing for contact screening and treatment of latent tuberculosis infection in Brazil. PLOS ONE 2013;8:e59546 | Immunocompetent close contacts |
| 14 | van der Have M | Optimizing screening for tuberculosis and hepatitis B prior to starting tumor necrosis factor-alpha inhibitors in Crohn’s disease. Dig Dis Sci 2014;59:554–63 | Intervention not of interest |
| 15 | Verma G | Tuberculosis screening for long-term care: a cost-effectiveness analysis. Int J Tuberc Lung Dis 2013;17:1170–7 | Compared screening strategies (no screening, LTBI screening and active TB screening) |
Appendix 15 Illustrative structures for the immunocompromised population, recent arrivals from countries with a high incidence of active tuberculosis and the general population
Immunocompromised or people at risk of immunosuppression
FIGURE 60.
Decision tree pathway for the immunocompromised population. –ve, negative.
FIGURE 61.
Pathway for the IGRA-alone diagnostic strategy in the immunocompromised population. –ve, negative; +ve, positive; CXR, chest radiography.

FIGURE 62.
Pathway for the TST-alone diagnostic strategy in the immunocompromised population. –ve, negative; +ve, positive; CXR, chest radiography.
FIGURE 63.
Pathway for the diagnostic strategy IGRA negative followed by TST in the immunocompromised population. –ve, negative; +ve, positive; CXR, chest radiography.

FIGURE 64.
Pathway for the diagnostic strategy IGRA and TST in the immunocompromised population. –ve, negative; +ve, positive.
Recent arrivals from countries with a high incidence of active tuberculosis
FIGURE 65.
Decision tree structure for recent arrivals from countries with a high incidence of active TB. +ve, positive.

FIGURE 66.
Pathway for the IGRA-alone diagnostic strategy in recently arrived population. –ve, negative; +ve, positive; CXR, chest radiography.
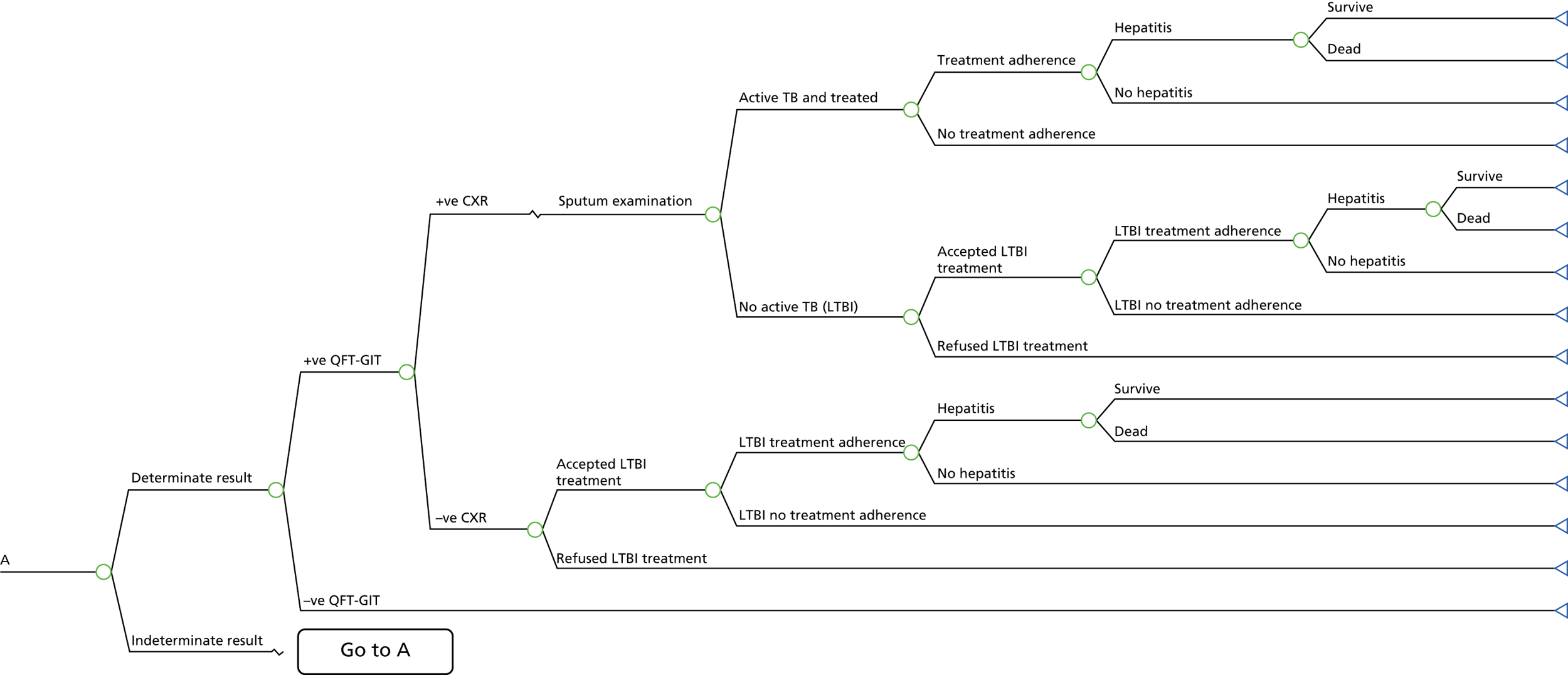
FIGURE 67.
Pathway for the TST-alone diagnostic strategy in the recently arrived population. –ve, negative; +ve, positive; CXR, chest radiography.

FIGURE 68.
Pathway for the diagnostic strategy of TST positive followed by IGRA in the recently arrived population. –ve, negative; +ve, positive; CXR, chest radiography.
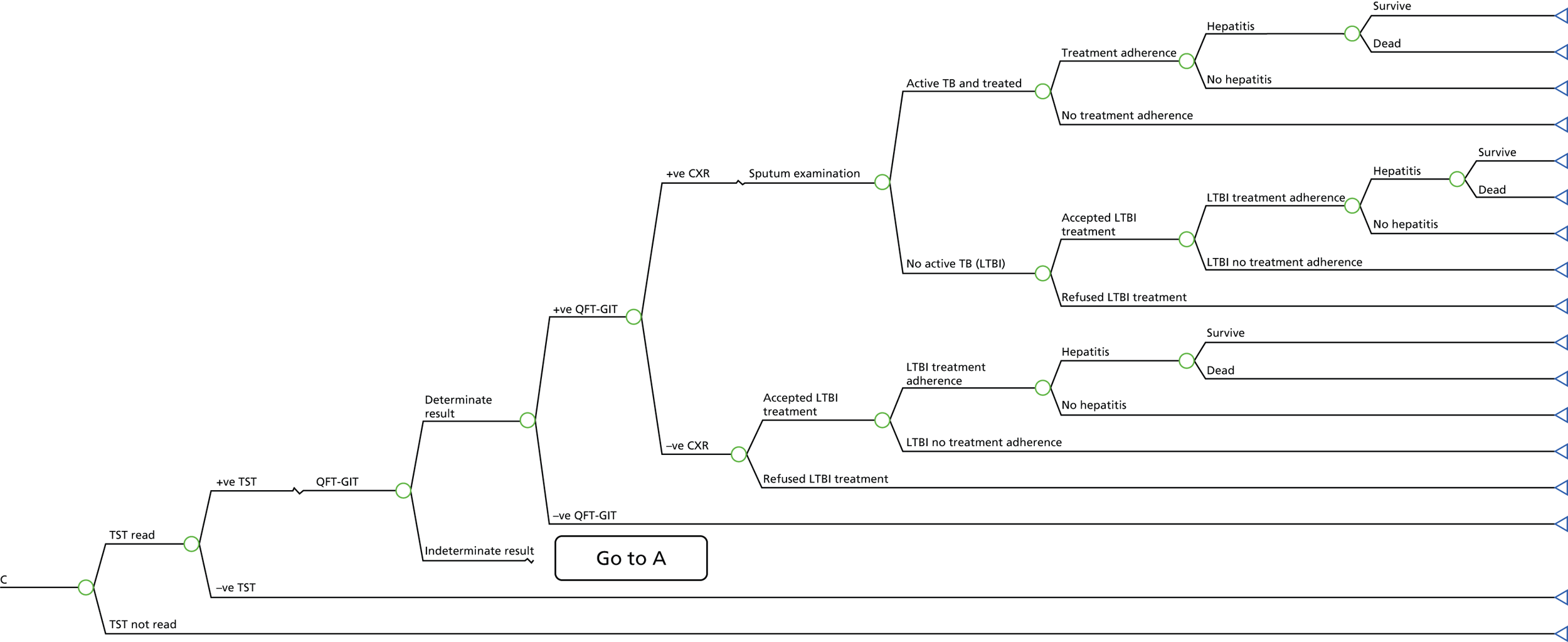
FIGURE 69.
Pathway for the diagnostic strategy of IGRA and TST in the recently arrived population. –ve, negative; +ve, positive.

General population
FIGURE 70.
Decision tree structure for the general population. +ve, positive.

FIGURE 71.
Pathway for the IGRA-alone diagnostic strategy in the general population. –ve, negative; +ve, positive; CXR, chest radiography.
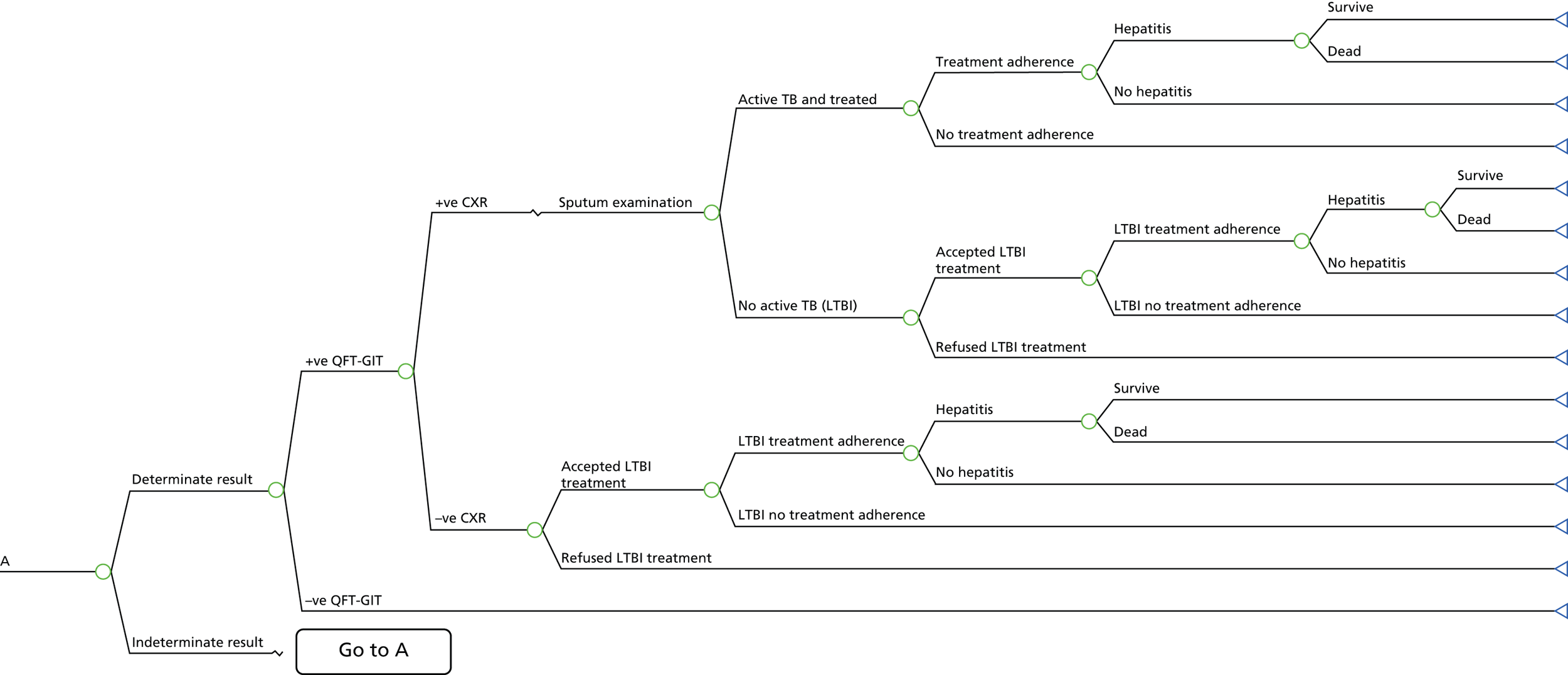
FIGURE 72.
Pathway for the TST-alone diagnostic strategy in the general population. –ve, negative; +ve, positive; CXR, chest radiography.

FIGURE 73.
Pathway for the diagnostic strategy of TST positive followed by IGRA in the general population. –ve, negative; +ve, positive; CXR, chest radiography.

FIGURE 74.
Pathway for the diagnostic strategy of IGRA and TST in the general population. –ve, negative; +ve, positive.

Appendix 16 Resources used to derive unit costs for the treatment of latent tuberculosis infection and tuberculosis and model input parameters
| Resource use | Quantity | Description | Unit costs (£, 2013) | Source |
|---|---|---|---|---|
| Investigations | ||||
| Full blood count | 2 | DAPS08 – Phlebotomy | 4 | Assumptions and consultation with clinical expert on the number of full blood counts, liver function tests and outpatient visits (NICE TB Guideline Development Group, 12 January 2015, personal communication); NHS reference costs 2012/13;214 Curtis217 |
| Liver function test | 4 | DAPS08 – Phlebotomy | 4 | |
| Outpatient visits | 2 visits | Weighted average of all outpatient procedures | 135 | |
| Nurse contact (in clinic)a,b | 3 visits | 15 minutes | 12.25 | Assumption and consultation with clinical expert; Curtis217 |
| Drug treatment | ||||
| Isoniazid | 18 packs (28 × 100-mg tablets per pack) | 6 months of isoniazidc | 19.24 | NHS electronic drug tariff216 |
| Estimated cost per person for treatment of LTBI | 677.07 (6H) | |||
| Resource use | Quantity | Description | Unit costs (£, 2013) | Source |
|---|---|---|---|---|
| Investigations | ||||
| Chest radiography | 3 | DAPF – Direct access plain film | 28 | NHS reference costs 2012/13214 |
| Sputum examination | 6 | DAPS07 – Microbiology | 7 | |
| Full blood count | 2 | DAPS08 – Phlebotomy | 4 | |
| Liver function test | 8 | DAPS08 – Phlebotomy | 4 | |
| Inpatient stay | 7.28 days | DZ14E – Pulmonary, Pleural or Other Tuberculosis, with CC Score 0–1 | 492 | Bothamley et al.215 |
| Outpatient visits | 8 visits | Weighted average of all outpatient procedures | 135 | |
| Drug treatment | ||||
| Ethambutol | 6 packs | 1200 mg daily for 2 months | 256.44 | BNF231 |
| Pyrazinamide | 8 packs | 2 g daily for 2 months | 250.80 | BNF231 |
| Rifinah® (300 mg/150 mg) (Sanofi) (isoniazid 150 mg, rifampicin 300 mg) | 6 packs | Two tablets daily for 6 months | 126.12 | BNF231 |
| Estimated cost for treatment of active TB per person | 5461.12 | |||
| Variable | Base-case value | Range for SA | PSA distribution | Source |
|---|---|---|---|---|
| Probabilities | ||||
| Prevalence of LTBI | 0.0222 | 0.0152–0.0306 | a | Derived from the current clinical effectiveness study |
| Sensitivity TST (≥ 5 mm) | 0.3242 | 0.1119–0.5848 | a | |
| Specificity TST (< 5 mm) | 0.7422 | 0.7288–0.7557 | a | |
| Sensitivity TST (≥ 10 mm) | 0.1682 | 0.0252–0.3899 | a | |
| Specificity TST (< 10 mm) | 0.8397 | 0.7899–0.8831 | a | |
| Sensitivity QFT-GIT | 0.5548 | 0.2473–0.8373 | a | |
| Specificity QFT-GIT | 0.8227 | 0.8052–0.8396 | a | |
| Sensitivity T-SPOT.TB | 0.6665 | 0.3517–0.9144 | a | |
| Specificity T-SPOT.TB | 0.6846 | 0.6346–0.7331 | a | |
| Sensitivity of TST conditional on negative QFT-GIT (LTBI arm) | 0.2775 | 0.0121–0.7989 | Not varied | |
| Specificity of TST conditional on negative QFT-GIT (no LTBI arm) | 0.4465 | 0.3909–0.4993 | Not varied | |
| Sensitivity of TST conditional on positive QFT-GIT (LTBI arm) | 0.4206 | 0.0023–0.3891 | Not varied | |
| Specificity of TST conditional on positive QFT-GIT (no LTBI arm) | 0.8058 | 0.00006–0.8058 | Not varied | |
| Determinate QFT-GIT | 0.97 | – | Beta(873,27) | Derived from Laskin et al.200 |
| Determinate T-SPOT.TB | 0.97 | – | Beta(873,27) | Derived from Laskin et al.200 |
| TST read | 0.9400 | 0.6–1.00 | Beta(164,10.5) | Pareek et al.77 |
| Initial active TB | 0.00001 | – | Not varied | Laskin et al.200 |
| TB treatment adherence | 1.0000 | – | Not varied | Pareek et al.77 |
| Accepting LTBI treatment | 0.9400 | 0.50–1.00 | Beta(141,9) | CG11710 |
| Adherence to LTBI treatment | 0.8000 | 0.50–0.90 | Beta(41,10) | Kowada198 |
| Isoniazid-induced hepatitis after TB treatment | 0.0040 | 0.001–0.010 | Beta(2.7,664) | Assumption |
| Isoniazid-induced hepatitis after LTBI treatment | 0.0040 | 0.001–0.010 | Beta(2.7,664) | Laskin et al.200 |
| Death from isoniazid-induced hepatitis | 0.00002 | 0.00001–0.0001 | Beta(0.5,25125) | Pooran et al.209 |
| Transmission model parameters | ||||
| Proportion still infected post LTBI treatment | 0.345 | – | Log-normal(–1.065,0.842) | White and Jit210 |
| Average number of secondary cases from one index case | 0.2 | 0.1–0.3 | Log-normal(–1.609,0.354) | Pareek et al.6 |
| Average delay from infection to activation | 2.88 | – | Log-normal(1.058,0.333) | Okuonghae et al.211 |
| Annualised reactivation rate from resolved TB | 0.013 | 0.004–0.025 | Beta(7,513) | Oxlade et al.212 |
| Case fatality rate for active TB (0–4 years) | 0.0477 | – | Beta(628,12543) | Crofts et al.213 |
| Case fatality rate for active TB (5–14 years) | 0.0034 | – | Beta(1,290) | Crofts et al.213 |
| Case fatality rate for active TB (15–44 years) | 0.0018 | – | Beta(1,564) | Crofts et al.213 |
| Case fatality rate for active TB (45–64 years) | 0.0476 | – | Beta(125,2500) | Crofts et al.213 |
| Case fatality rate for active TB (≥ 65 years) | 0.1755 | – | Beta(413,1940) | Crofts et al.213 |
| Resource use and costs (£) | ||||
| TST | 17.48 | Not varied | Pooran et al.209 | |
| QFT-GIT | 48.73 | Not varied | Pooran et al.209 | |
| T-SPOT.TB | 59.57 | Not varied | Pooran et al.209 | |
| Chest radiography | 35.00 | Not varied | NHS reference costs 2012/13214 | |
| Sputum examination | 7.00 | Not varied | NHS reference costs 2012/13214 | |
| Adherence to active TB treatment | 5461.12 | Gamma(10.41,524.6) | Bothamley et al.215 | |
| Cost of non-adherence to active TB treatment | 910.19 | Not varied | Assumption | |
| Adherence to LTBI treatmentb | 677.07 | Uniform(511.69,842.45) | NHS electronic drug tariff216 | |
| Cost of non-adherence to LTBI treatment | 112.85 | Uniform(85.24,140.41) | Assumption | |
| Treatment of isoniazid-induced hepatitis | 389.51 | Gamma(7.13,55.64) | Pareek et al.77 | |
| Utility decrements | ||||
| Active TB (while on treatment) | 0.15c | Not reported | Gamma(11.2,0.0134) | Derived from Kowada197 |
| Treatment for LTBI | 0.0010 | Not reported | Uniform(0,0.002) | |
| Other | ||||
| Discount rate per annum (costs and QALYs) | 3.5% | |||
| Variable | Base-case value | Range for SA | PSA distribution | Source |
|---|---|---|---|---|
| Probabilities | ||||
| Prevalence of LTBI | 0.0237 | 0.0150–0.0345 | a | Derived from the current clinical effectiveness study |
| Sensitivity TST (≥ 5 mm) | 0.9356 | 0.7786–0.9977 | a | |
| Specificity TST (< 5 mm) | 0.5011 | 0.4790–0.5229 | a | |
| Sensitivity TST (≥ 10 mm) | 0.5915 | 0.3584–0.8172 | a | |
| Specificity TST (< 10 mm) | 0.7929 | 0.7780–0.8073 | a | |
| Sensitivity T-SPOT.TB | 0.7001 | 0.3978–0.9242 | a | |
| Specificity T-SPOT.TB | 0.3992 | 0.3439–0.4554 | a | |
| Sensitivity of QFT-GIT conditional on negative TST (LTBI arm) | 0.6009 | 0.3465–0.8514 | a | |
| Specificity of QFT-GIT conditional on positive TST (no LTBI arm) | 0.6102 | 0.5775–0.6421 | a | |
| Sensitivity of QFT-GIT conditional on negative TST (LTBI arm) | 0.4807 | 0.0225–0.9724 | a | |
| Specificity of QFT-GIT conditional on negative TST (no LTBI arm) | 0.9746 | 0.9555–0.9893 | a | |
| Sensitivity of CXR for diagnosing active TB | 0.7800 | Not reported | Not varied | Kumar et al.208 |
| Specificity of CXR for diagnosing active TB | 0.5100 | Not reported | Not varied | Kumar et al.208 |
| Determinate QFT-GIT | 0.97 | – | Beta(873,27) | Derived from Laskin et al.200 |
| Determinate T-SPOT.TB | 0.97 | – | Beta(873,27) | Derived from Laskin et al.200 |
| TST read | 0.9400 | 0.6–1.00 | Beta(164,10.5) | Pareek et al.77 |
| Initial active TB | 0.00001 | – | Not varied | Laskin et al.200 |
| TB treatment adherence | 1.0000 | – | Not varied | Pareek et al.77 |
| Accepting LTBI treatment | 0.9400 | 0.50–1.00 | Beta(141,9) | CG11710 |
| Adherence to LTBI treatment | 0.8000 | 0.50–0.90 | Beta(41,10) | Kowada198 |
| Isoniazid-induced hepatitis after TB treatment | 0.0040 | 0.001–0.010 | Beta(2.7,664) | Assumption |
| Isoniazid-induced hepatitis after LTBI treatment | 0.0040 | 0.001–0.010 | Beta(2.7,664) | Laskin et al.200 |
| Death from isoniazid-induced hepatitis | 0.00002 | 0.00001–0.0001 | Beta(0.5,25125) | Pooran et al.209 |
| Transmission model parameters | ||||
| Proportion still infected post LTBI treatment | 0.345 | – | Log-normal(–1.065,0.842) | White and Jit210 |
| Average number of secondary cases from one index case | 0.2 | 0.1–0.3 | Log-normal(–1.609,0.354) | Pareek et al.6 |
| Average delay from infection to activation | 2.88 | – | Log-normal(1.058,0.333) | Okuonghae et al.211 |
| Annualised reactivation rate from resolved TB | 0.013 | 0.004–0.025 | Beta(7,513) | Oxlade et al.212 |
| Case fatality rate for active TB (0–4 years) | 0.0477 | – | Beta(628,12543) | Crofts et al.213 |
| Case fatality rate for active TB (5–14 years) | 0.0034 | – | Beta(1,290) | Crofts et al.213 |
| Case fatality rate for active TB (15–44 years) | 0.0018 | – | Beta(1,564) | Crofts et al.213 |
| Case fatality rate for active TB (45–64 years) | 0.0476 | – | Beta(125,2500) | Crofts et al.213 |
| Case fatality rate for active TB (≥ 65 years) | 0.1755 | – | Beta(413,1940) | Crofts et al.213 |
| Resource use and costs | ||||
| TST | 17.48 | – | NA | Pooran et al.209 |
| QFT-GIT | 48.73 | – | NA | Pooran et al.209 |
| T-SPOT.TB | 59.57 | – | NA | Pooran et al.209 |
| CXR | 35.00 | – | NA | NHS reference costs 2012/13214 |
| Sputum examination | 7.00 | – | NA | NHS reference costs 2012/13214 |
| Cost of adherence to active TB treatment | 5461.12 | – | Gamma(10.41,524.6) | Bothamley et al.215 |
| Cost of non-adherence to active TB treatment | 910.19 | – | Not varied | Assumption |
| Adherence to LTBI treatment | 677.07 | – | Uniform(511.69,842.45) | NHS electronic drug tariff216 |
| Cost of non-adherence to LTBI treatment | 112.85 | – | Gamma(85.24,140.41) | Assumption |
| Treatment of INH-induced hepatitis | 389.51 | – | Gamma(7.13,55.64) | Pareek et al.77 |
| Utility decrements | ||||
| Active TB (while on treatment) | 0.15b | Not reported | Gamma(11.2,0.0134) | Derived from Kowada197 |
| Treatment for LTBI | 0.001 | Not reported | Uniform(0,0.002) | |
| Other | ||||
| Discount rate per annum (costs and QALYs) | 3.5% | |||
Appendix 17 WinBUGS code
In this appendix we report on the WinBUGS code used in the evidence synthesis for the child population. The WinBUGS codes used for the immunocompromised and recently arrived populations are very similar but use different sample data. Table 66 shows the variables and descriptions used in the models.
| Variable name | Description |
|---|---|
| Prev | Prevalence |
| pposQFTG | Probability of a positive QFT-G result |
| sensQFTG | Sensitivity of QFT-G |
| specQFTG | Specificity of QFT-G |
| ATBposQFTG | Number of active TB cases given a positive result on QFT-G |
| pATBposQFTG | Probability of active TB given a positive result on QFT-G |
| ATBnegQFTG | Number of active TB cases given a negative result on QFT-G |
| pATBnegQFTG | Probability of active TB given a negative result on QFT-G |
| pposQFTGIT | Probability of a positive QFT-GIT result |
| sensQFTGIT | Sensitivity of QFT-GIT |
| specQFTGIT | Specificity of QFT-GIT |
| ATBposQFTGIT | Number of active TB cases given a positive result on QFT-GIT |
| pATBposQFTGIT | Probability of active TB given a positive result on QFT-GIT |
| ATBnegQFTGIT | Number of active TB cases given a negative result on QFT-GIT |
| pATBnegQFTGIT | Probability of active TB given a negative result on QFT-GIT |
| pposTSPOTTB | Probability of a positive T-SPOT.TB result |
| sensTSPOTTB | Sensitivity of T-SPOT.TB |
| specTSPOTTB | Specificity of T-SPOT.TB |
| ATBposTSPOTTB | Number of active TB cases given a positive result on T-SPOT.TB |
| pATBposTSPOTTB | Probability of active TB given a positive result on T-SPOT.TB |
| ATBnegTSPOTTB | Number of active TB cases given a negative result on T-SPOT.TB |
| pATBnegTSPOTTB | Probability of active TB given a negative result on T-SPOT.TB |
| pposTST5 | Probability of a positive TST5 result |
| sensTST5 | Sensitivity of TST5 |
| specTST5 | Specificity of TST5 |
| ATBposTST5 | Number of active TB cases given a positive result on TST5 |
| pATBposTST5 | Probability of active TB given a positive result on TST5 |
| ATBnegTST5 | Number of active TB cases given a negative result on TST5 |
| pATBnegTST5 | Probability of active TB given a negative result on TST5 |
| pposTST10 | Probability of a positive TST10 result |
| sensTST10 | Sensitivity of TST10 |
| specTST10 | Specificity of TST10 |
| ATBposTST10 | Number of active TB cases given a positive result on TST10 |
| pATBposTST10 | Probability of active TB given a positive result on TST10 |
| ATBnegTST10 | Number of active TB cases given a negative result on TST10 |
| pATBnegTST10 | Probability of active TB given a negative result on TST10 |
| pposTST15 | Probability of a positive TST15 result |
| sensTST15 | Sensitivity of TST15 |
| specTST15 | Specificity of TST15 |
| ATBposTST15 | Number of active TB cases given a positive result on TST15 |
| pATBposTST15 | Probability of active TB given a positive result on TST15 |
| ATBnegTST15 | Number of active TB cases given a negative result on TST15 |
| pATBnegTST15 | Probability of active TB given a negative result on TST15 |
| TST5QFTGIT | Probability of positive QFT-GIT following a positive result on TST5 |
| TST10QFTGIT | Probability of positive QFT-GIT following a positive result on TST10 |
WinBUGS code used in the child population
model{
for (study in 1:Nstudy){
prev[study] <- mprev
#Binomial link between the number of positive results and probability of a positive result
rplusTST10[study] ∼dbin(pposTST10[study],Npats[study,1])
rminusTST10[study] <- Npats[study,1] - rplusTST10[study]
pposTST10[study] <- prev[study]*sensTST10 + (1-prev[study])*(1-specTST10)
ATBposTST10[study]∼dbin(pATBposTST10[study],rplusTST10[study])
pATBposTST10[study] <- prev[study]*sensTST10/pposTST10[study]
ATBnegTST10[study]∼dbin(pATBnegTST10[study],rminusTST10[study])
pATBnegTST10[study] <- prev[study]*(1-sensTST10)/(prev[study]*(1-sensTST10)+specTST10*(1-prev[study]))
rplusTST10IT[study] ∼dbin(pposTST10IT[study],Npats[study,2])
rminusTST10IT[study] <- Npats[study,2] - rplusTST10IT[study]
pposTST10IT[study] <- prev[study]*sensTST10IT + (1-prev[study])*(1-specTST10IT)
ATBposTST10IT[study]∼dbin(pATBposTST10IT[study],rplusTST10IT[study])
pATBposTST10IT[study] <- prev[study]*sensTST10IT/pposTST10IT[study]
ATBnegTST10IT[study]∼dbin(pATBnegTST10IT[study],rminusTST10IT[study])
pATBnegTST10IT[study] <- prev[study]*(1-sensTST10IT)/(prev[study]*(1-sensTST10IT)+specTST10IT*(1-prev[study]))
rplusTSPOTTB[study] ∼dbin(pposTSPOTTB[study],Npats[study,3])
rminusTSPOTTB[study] <- Npats[study,3] - rplusTSPOTTB[study]
pposTSPOTTB[study] <- prev[study]*sensTSPOTTB + (1-prev[study])*(1-specTSPOTTB)
ATBposTSPOTTB[study]∼dbin(pATBposTSPOTTB[study],rplusTSPOTTB[study])
pATBposTSPOTTB[study] <- prev[study]*sensTSPOTTB/pposTSPOTTB[study]
ATBnegTSPOTTB[study]∼dbin(pATBnegTSPOTTB[study],rminusTSPOTTB[study])
pATBnegTSPOTTB[study] <- prev[study]*(1-sensTSPOTTB)/(prev[study]*(1-sensTSPOTTB)+specTSPOTTB*(1-prev[study]))
rplusTST10[study] ∼ dbin(pposTST10[study],Npats[study,4])
rminusTST10[study] <- Npats[study,4] - rplusTST10[study]
pposTST10[study] <- prev[study]*sensTST10 + (1-prev[study])*(1-specTST10)
ATBposTST10[study]∼dbin(pATBposTST10[study],rplusTST10[study])
pATBposTST10[study] <- prev[study]*sensTST10/pposTST10[study]
ATBnegTST10[study]∼dbin(pATBnegTST10[study],rminusTST10[study])
pATBnegTST10[study] <- prev[study]*(1-sensTST10)/(prev[study]*(1-sensTST10)+specTST10*(1-prev[study]))
rplusTST10[study] ∼dbin(pposTST10[study],Npats[study,5])
rminusTST10[study] <- Npats[study,5] - rplusTST10[study]
pposTST10[study] <- prev[study]*sensTST10 + (1-prev[study])*(1-specTST10)
ATBposTST10[study]∼dbin(pATBposTST10[study],rplusTST10[study])
pATBposTST10[study] <- prev[study]*sensTST10/pposTST10[study]
ATBnegTST10[study]∼dbin(pATBnegTST10[study],rminusTST10[study])
pATBnegTST10[study] <- prev[study]*(1-sensTST10)/(prev[study]*(1-sensTST10)+specTST10*(1-prev[study]))
rplusTST15[study] ∼dbin(pposTST15[study],Npats[study,6])
rminusTST15[study] <- Npats[study,6] - rplusTST15[study]
pposTST15[study] <- prev[study]*sensTST15 + (1-prev[study])*(1-specTST15)
ATBposTST15[study]∼dbin(pATBposTST15[study],rplusTST15[study])
pATBposTST15[study] <- prev[study]*sensTST15/pposTST15[study]
ATBnegTST15[study]∼dbin(pATBnegTST15[study],rminusTST15[study])
pATBnegTST15[study] <- prev[study]*(1-sensTST15)/(prev[study]*(1-sensTST15)+specTST15*(1-prev[study]))
}
for (i in 1:N.cs){
rplusTST10TST10IT[i]∼dbin(pplusTST10TST10IT[i],rplusTST10[cs.index[i]])
pplusTST10TST10IT[i] <-prev[cs.index[i]]*sensTST10*cpos.sensTST10IT5+((1-specTST10)*(1-prev[cs.index[i]])*(1-cpos.specTST10IT5))/pposTST10[cs.index[i]]
rnegTST10TST10IT[i]∼dbin(pnegTST10TST10IT[i],rminusTST10[cs.index[i]])
pnegTST10TST10IT[i] <-((1-prev[cs.index[i]])*specTST10*cneg.specTST10IT5+(1-sensTST10)*prev[cs.index[i]]*(1-cneg.sensTST10IT5))/((1-prev[cs.index[i]])*specTST10+prev[cs.index[i]]*(1-sensTST10))
}
for (i in 1:N.cs2){
rplusTST10TST10IT[i]∼dbin(pplusTST10TST10IT[i],rplusTST10[cs2.index[i]])
pplusTST10TST10IT[i] <-prev[cs2.index[i]]*sensTST10*cpos.sensTST10IT10+((1-specTST10)*(1-prev[cs2.index[i]])*(1-cpos.specTST10IT10))/pposTST10[cs2.index[i]]
rnegTST10TST10IT[i]∼dbin(pnegTST10TST10IT[i],rminusTST10[cs2.index[i]])
pnegTST10TST10IT[i] <-((1-prev[cs2.index[i]])*specTST10*cneg.specTST10IT10+(1-sensTST10)*prev[cs2.index[i]]*(1-cneg.sensTST10IT10))/((1-prev[cs2.index[i]])*specTST10+prev[cs2.index[i]]*(1-sensTST10))
}
sensTST10IT <- cpos.sensTST10IT5*sensTST10 + cneg.sensTST10IT5*(1-sensTST10)
specTST10IT <- cpos.specTST10IT5*(1-specTST10) + cneg.specTST10IT5*(specTST10)
#Prior at baseline
sensTST10∼dunif(0,1)
specTST10∼dunif(0,1)
logit(sensTST10)<-logit(sensTST10)-dsens510
dsens510∼dunif(0,5)
logit(specTST10)<-logit(specTST10)+dspec510
dspec510∼dunif(0,5)
sensTST15∼dunif(0,1)
specTST15∼dunif(0,1)
sensTST10∼dunif(0,1)
specTST10∼dunif(0,1)
sensTSPOTTB∼dunif(0,1)
specTSPOTTB∼dunif(0,1)
cpos.sensTST10IT5∼dunif(0,1)
cpos.specTST10IT5∼dunif(0,1)
cneg.sensTST10IT5∼dunif(0,1)
cneg.specTST10IT5∼dunif(0,1)
cpos.sensTST10IT10∼dunif(0,1)
cpos.specTST10IT10∼dunif(0,1)
cneg.sensTST10IT10∼dunif(0,1)
cneg.specTST10IT10∼dunif(0,1)
mprev ∼ dbeta(1,1)
}
#Sample data from the clinical evidence
list(Nstudy=13,Npats=structure(.Data=c(84,84,73,84,84,84,306,306,306,306,306,306,104,104,104,104,104,104,5244,5244,5244,5244,5244,5244,59,59,59,59,59,59,69,69,69,69,69,69,204,204,204,204,204,204,195,195,195,195,195,195,184,184,184,184,184,184,1073,1073,1073,1073,1073,1073,104,104,104,104,104,104,50,50,50,50,50,50,2982,2966,2982,2982,2982,2982),.Dim=c(13,6)),N.cs=6,cs.index=c(1,4,6,9,10,11),N.cs2=4,cs2.index=c(7,8,12,13), rplusTST10=c(NA,6,NA,NA,18,NA,NA,NA,NA,NA,NA,NA,NA),rplusTST10IT=c(20,NA,21,2669,NA,10,31,33,61,331,21,30,317),rplusTSPOTTB=c(16,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA), rplusTST10=c(38,200,40,2894,NA,42,NA,NA,84,645,27,NA,NA),rplusTST10=c(NA,90,40,NA,8,NA,115,47,NA,NA,NA,32,663),rplusTST15=c(NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,231),ATBposTST10=c(NA,0,NA,NA,10,NA,NA,NA,NA,NA,NA,NA,NA),ATBposTST10IT=c(NA,NA,6,39,NA,NA,NA,NA,NA,NA,NA,NA,11),ATBposTSPOTTB=c(NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA),ATBposTST10=c(NA,0,6,40,NA,NA,NA,NA,NA,NA,NA,NA,NA),ATBposTST10=c(NA,0,4,NA,3,NA,NA,NA,NA,NA,NA,NA,13),ATBposTST15=c(NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,13),ATBnegTST10=c(NA,0,NA,NA,0,NA,NA,NA,NA,NA,NA,NA,NA),ATBnegTST10IT=c(NA,NA,0,13,NA,NA,NA,NA,NA,NA,NA,NA,12),ATBnegTSPOTTB=c(NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA),ATBnegTST10=c(NA,0,0,12,NA,NA,NA,NA,NA,NA,NA,NA,NA), ATBnegTST10=c(NA,0,2,NA,7,NA,NA,NA,NA,NA,NA,NA,10),ATBnegTST15=c(NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,NA,10),rplusTST10TST10IT=c(18,2383,10,51,266,19),rnegTST10TST10IT=c(44,2064,27,90,363,75), rplusTST10TST10IT=c(27,28,30,231),rnegTST10TST10IT=c(85,143,18,2219))
#Sample initial values
list(dsens510=0.5,dspec510=0.5)
The robustness of the model was assessed by examining the convergence diagnostics for evidence of when the simulation appeared to mix. This was examined based on visual inspection of the sample trace plots. A burn-in period of 30,000 simulations was used followed by a further 30,000 simulations.
FIGURE 75.
Sample traces of chains for sensitivity of TST (≥ 5 mm) where convergence/mixing looks reasonable.

FIGURE 76.
Sample traces of chains for specificity of TST (< 5 mm) where convergence/mixing looks reasonable.
FIGURE 77.
Sample traces of chains for sensitivity of TST (≥ 10 mm) where convergence/mixing looks reasonable.
FIGURE 78.
Sample traces of chains for specificity of TST (< 10 mm) where convergence/mixing looks reasonable.
FIGURE 79.
Sample traces of chains for sensitivity of QFT-GIT where convergence/mixing looks reasonable.
FIGURE 80.
Sample traces of chains for specificity of QFT-GIT where convergence/mixing looks reasonable.
Appendix 18 Information required to derive the diagnostic accuracy of various testing strategies by population
Children and adolescents
| Test | Total tested | Number of positives | Number of positives who developed active TB | Length of follow-up (years) | Source |
|---|---|---|---|---|---|
| QFT-G | 306 | 6 | 0 | 3 | Higuchi et al.161 |
| TST (≥ 5 mm) | 306 | 200 | 0 | ||
| TST (≥ 10 mm) | 306 | 90 | 0 | ||
| QFT-GIT | 104 | 21 | 6 | 2–4 | Diel et al.102 |
| TST (≥ 5 mm) | 104 | 40 | 6 | ||
| TST (≥ 10 mm) | 104 | 40 | 4 | ||
| QFT-GIT | 5244 | 2669 | 39 | 3.8 | Mahomed et al.103 |
| TST (≥ 5 mm) | 5244 | 2894 | 40 | ||
| QFT-G | 59 | 18 | 10 | 1 | Noorbakhsh et al.104 |
| TST (≥ 10 mm) | 59 | 8 | 3 | ||
| QFT-GIT | 2966 | 317 | 11 | 2 | Song et al.152 |
| TST (≥ 10 mm) | 2982 | 663 | 13 | ||
| TST (≥ 15 mm) | 2982 | 231 | 13 |
| Test | Total tested | Number of positives | Number of positives who developed active TB | Length of follow-up (years) | Source |
|---|---|---|---|---|---|
| QFT-G | 306 | 300 | 0 | 3 | Higuchi et al.161 |
| TST (< 5 mm) | 306 | 106 | 0 | ||
| TST (< 10 mm) | 306 | 216 | 0 | ||
| QFT-GIT | 104 | 83 | 0 | 2–4 | Diel et al.102 |
| TST (< 5 mm) | 104 | 64 | 0 | ||
| TST (< 10 mm) | 104 | 64 | 2 | ||
| QFT-GIT | 5244 | 2575 | 13 | 3.8 | Mahomed et al.103 |
| TST (< 5 mm) | 5244 | 2350 | 12 | ||
| QFT-G | 59 | 41 | 0 | 1 | Noorbakhsh et al.104 |
| TST (< 10 mm) | 59 | 50 | 7 | ||
| QFT-GIT | 2966 | 2649 | 12 | 2 | Song et al.152 |
| TST (< 10 mm) | 2982 | 2319 | 10 | ||
| TST (< 15 mm) | 2982 | 2751 | 10 |
Immunocompromised people
| Test | Total tested | Number of positives | Number of positives who developed active TB | Length of follow-up (years) | Source |
|---|---|---|---|---|---|
| T-SPOT.TB | 265 | 89 | 4 | 1.17 (median) | Kim et al.116 |
| TST (≥ 5 mm) | 288 | 26 | 1 | ||
| QFT-G | 30 | 12 | 1 | 2 | Lee et al.118 |
| T-SPOT.TB | 32 | 15 | 0 | ||
| TST (≥ 10 mm) | 32 | 20 | 1 | ||
| QFT-GIT | 210 | 40 | 1 | 0.8 (median) | Moon et al.115 |
| TST (≥ 5 mm) | 244 | 39 | 0 | ||
| QFT-GIT | 159 | 26 | 3 | 1.3 (median) | Lee et al.149 |
| TST (≥ 10 mm) | 169 | 19 | 0 | ||
| TST (≥ 15 mm) | 169 | 12 | 0 | ||
| T-SPOT.TB | 44 | 6 | 1 | 1.75 | Sherkat et al.155 |
| TST (≥ 10 mm) | 44 | 8 | 1 |
| Test | Total tested | Number of positives | Number of negatives that developed active TB | Length of follow-up (years) | Source |
|---|---|---|---|---|---|
| T-SPOT.TB | 265 | 176 | 0 | 1.17 (median) | Kim et al.116 |
| TST (< 5 mm) | 288 | 262 | 3 | ||
| QFT-G | 30 | 18 | 0 | 2 | Lee et al.118 |
| T-SPOT.TB | 32 | 17 | 2 | ||
| TST (< 10 mm) | 32 | 12 | 1 | ||
| QFT-GIT | 210 | 170 | 1 | 0.8 (median) | Moon et al.115 |
| TST (< 5 mm) | 244 | 205 | 2 | ||
| QFT-GIT | 159 | 133 | 2 | 1.3 (median) | Lee et al.149 |
| TST (≤ 10 mm) | 169 | 150 | 5 | ||
| TST (≤ 15 mm) | 169 | 157 | 5 | ||
| T-SPOT.TB | 44 | 38 | 0 | 1.75 | Sherkat et al.155 |
| TST (≤ 10 mm) | 44 | 36 | 0 |
Glossary
- Acid-fast bacilli
- Bacteria that, having been stained with a dye, retain their colour in acid alcohol. Used as a technique for the microscopic detection of mycobacteria.
- Active tuberculosis
- Infection with mycobacteria of the Mycobacterium tuberculosis (MTB) [(Zopf 1883) Lehmann and Neumann 1896] complex in which mycobacteria are growing and causing symptoms and signs of disease. This is distinct from latent tuberculosis, in which mycobacteria are present, and may be dormant, but are not causing disease. The symptoms of disease include weakness, weight loss, fever, no appetite, chills and sweating at night. Other symptoms of tuberculosis disease depend on where in the body the bacteria are growing. If tuberculosis is in the lungs (pulmonary tuberculosis), the symptoms may include a cough, pain in the chest and coughing up blood [source: www.hpa.org.uk (accessed 12 December 2015)].
- Adherence
- Refers to the patient’s ability or choice to adhere to a treatment regimen. Also see Concordance.
- Algorithm (in guidelines)
- A flow chart of the clinical decision pathway described in the guideline in which decision points are represented with boxes, linked by arrows.
- Atypical mycobacteria
- Mycobacteria other than those of the Mycobacterium tuberculosis complex.
- Bacillus Calmette–Guérin vaccine
- A vaccine for tuberculosis named after the French scientists Calmette and Guerin [source: www.hpa.org.uk (accessed 12 December 2015)].
- Bias
- Deviation of results from the truth because of systematic error(s) in the methods used.
- Cochrane review
- A systematic review of the evidence from randomised controlled trials relating to a particular health problem or health-care intervention, produced by the Cochrane Collaboration. Available electronically as part of The Cochrane Library.
- Cohort study
- A retrospective or prospective follow-up study. Groups of individuals to be followed up are defined on the basis of the presence or absence of exposure to a suspected risk factor or intervention. A cohort study can be comparative, in which case two or more groups are selected on the basis of differences in their exposure to the agent of interest.
- Compliance
- The extent to which a patient complies with a recommended treatment regimen. In recent years, use of the term ‘compliance’ has been discouraged because of its connotations of patient subservience (see Concordance and Adherence).
- Concordance
- The percentage of agreement between two tests.
- Confidence interval
- A range of values that contains the true value for the population with a stated ‘confidence’ (conventionally 95%). The interval is calculated from sample data and generally straddles the sample estimate. The 95% confidence value means that, if the study, and the method used to calculate the interval, is repeated many times, 95% of the calculated intervals will actually contain the true value.
- Contact (domestic, close, casual and workplace)
- A person who has spent time with a person with infectious tuberculosis [source: www.hpa.org.uk (accessed 12 December 2015)].
- Cost-effectiveness analysis
- An economic study design in which the consequences of different interventions are measured using a single outcome, usually in natural units (e.g. life-years gained, deaths avoided, heart attacks avoided, cases detected). Alternative interventions are then compared in terms of cost per unit of effectiveness.
- Cost–utility analysis
- A form of cost-effectiveness analysis in which the units of effectiveness are quality-adjusted life-years.
- Culture
- The process of growing tuberculosis bacteria from sputum or other samples for identification and diagnosis.
- Discordance
- The percentage of disagreement between two tests.
- Heterogeneity
- Variability or differences between studies in the estimates of effects (when the results or estimates from individual studies appear to have a different magnitude, if not a different sign or direction).
- High-incidence country
- Following the widely used threshold, any country with an incidence of tuberculosis that is ≥ 40 cases per 100,000 population per year. A similar definition is made for areas within countries and may be used to decide on the local need for vaccination, for instance for neonatal bacillus Calmette–Guérin vaccination.
- Immunocompromised
- Refers to an individual who has a significantly impaired immune system. This may be caused by prolonged steroid use, tumour necrosis factor alpha antagonists, antirejection therapy, immunosuppression-causing medication or comorbid states that affect the immune system, for example human immunodeficiency virus infection, chronic renal disease, many haematological and solid cancers, and diabetes.
- Infectious tuberculosis
- Active sputum smear-positive pulmonary tuberculosis, that is, with acid-fast bacilli visible on microscopy. Active tuberculosis affecting other parts of the respiratory tract or oral cavity, although rare, is also considered infectious.
- Interferon gamma test
- A blood test used to diagnose latent tuberculosis (which may be used as an alternative, or an addition, to tuberculin skin tests) based on detecting the response of white blood cells to tuberculosis antigens.
- Latent tuberculosis
- Infection with mycobacteria of the Mycobacterium tuberculosis complex in which the bacteria are alive but are not currently causing active disease. Also known as latent tuberculosis infection.
- Mantoux test
- A type of tuberculin skin test in which tuberculin is injected intracutaneously. The injection site is examined for signs of an immune response after 2–3 days.
- Multidrug-resistant tuberculosis
- Tuberculosis resistant to isoniazid and rifampicin, with or without any other resistance.
- Mycobacterium tuberculosis complex
- The related mycobacterial species Mycobacterium tuberculosis, Mycobacterium bovis [(Hale et al. 1962) Askaa and Erno 1976] and Mycobacterium africanum [Castets et al. 1969], which can cause tuberculosis in humans.
- Skin test
- See Tuberculin skin test.
- Smear positive
- See Sputum smear positive.
- Specificity (of a test)
- The proportion of individuals classified as negative by the gold (or reference) standard who are correctly identified by the study test.
- Sputum
- Mucus expelled from the bronchi and lungs by coughing (or retrieved from gastric washings). Sputum is examined for tuberculosis bacteria by microscopic examination of a stained smear; part of the sputum can also be used for culture.
- Sputum smear positive (‘smear positive’)
- Respiratory tuberculosis in which mycobacteria (‘acid-fast bacilli’) have been seen in a stained smear of sputum examined under a microscope [source: www.hpa.org.uk (accessed 12 December 2015)].
- Weighted contact score
- The weighted contact score represents a weight based on the relationship (e.g. primary caregiver, secondary caregiver, relative or non-household contact) between the tuberculosis index case and an individual, the type (e.g. sleeps in the same house or lives in the same house) and duration (e.g. 0–3 hours or 4–7 hours of contact per day) of exposure to the index case and the infectivity (sputum acid-fast bacilli positivity) of the index case (Tieu HV, Suntarattiwong P, Puthanakit T, Chotpitayasunondh T, Chokephaibulkit K, Sirivichayakul S, et al. Comparing interferon-gamma release assays to tuberculin skin test in Thai children with tuberculosis exposure. PLOS ONE 2014;9:e105003).
List of abbreviations
- AIDS
- acquired immunodeficiency syndrome
- BCG
- bacillus Calmette–Guérin
- CD4
- cluster of differentiation 4
- CEAC
- cost-effectiveness acceptability curve
- CFP-10
- culture filtrate protein 10
- CG
- clinical guideline
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CIR
- cumulative incidence ratio
- DOR
- diagnostic odds ratio
- ELISPOT
- enzyme-linked immunospot
- ESAT-6
- early secretion antigen target 6
- ESRD
- end-stage renal disease
- GP
- general practitioner
- HIV
- human immunodeficiency virus
- ICER
- incremental cost-effectiveness ratio
- IDRR
- incidence density rate ratio
- IFN-γ
- interferon gamma
- IGRA
- interferon gamma release assay
- JSNA
- Joint Strategic Needs Assessment
- LTBI
- latent tuberculosis infection
- MCMC
- Markov chain Monte Carlo
- MTB
- Mycobacterium tuberculosis
- NICE
- National Institute for Health and Care Excellence
- NPV
- negative predictive value
- NTM
- non-tuberculous mycobacteria
- OR
- odds ratio
- PHE
- Public Health England
- PPD
- purified protein derivative
- PPV
- positive predictive value
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QFT
- QuantiFERON-TB
- QFT-G
- QuantiFERON®-TB Gold
- QFT-GIT
- QuantiFERON®-Gold-in-Tube
- QUIPS
- Quality in Prognosis Studies
- R-CIR
- ratio of cumulative incidence ratios
- R-DOR
- ratio of diagnostic odds ratios
- R-IDRR
- ratio of incidence density rate ratios
- ROB
- risk of bias
- TB
- tuberculosis
- TNF-α
- tumour necrosis factor alpha
- TST
- tuberculin skin test
- WHO ICTRP
- World Health Organization International Clinical Trials Registry Platform
