Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 12/51/01. The protocol was agreed in November 2013. The assessment report began editorial review in July 2014 and was accepted for publication in July 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Archer et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Ulcerative colitis (UC) is recognised as the most common form of inflammatory bowel disease (IBD) in the UK. The incidence of UC is approximately 10 per 100,000 population per year, while the prevalence of the disease is approximately 240 per 100,000 population. 1 This is typical for countries with a Westernised lifestyle. 2 Peak incidence is between 15 and 25 years of age, with a potential second peak between 55 and 65 years. 1 The majority (approximately 80%) of incident cases are reported to be of mild or moderate severity. An estimated 132,600 people in England and Wales have been diagnosed with UC,1 which is distinct from Crohn’s disease (CD) – the other principal form of IBD. 2
Ulcerative colitis is a chronic disease of unknown cause. It is understood that pathogenesis may result from a change in the colonic environment of a genetically susceptible person and the condition is genetically heterogeneous, having a large number of implicated genes. 2,3 Genetic screening is therefore not currently indicated for UC;2 however, appendectomy and smoking have been linked with a reduced risk and severity of UC. 2
Inflammation in UC typically occurs in the colon and rectum. Disease may be limited to the rectum (proctitis), may be left sided or distal, or may be extensive (pancolitis). 3 Symptoms include the development of bloody diarrhoea with or without mucus, abdominal pain, weight loss, fatigue and an urgent need to defecate. Extraintestinal manifestations may occur in 10–30% of patients on the skin, eyes, mouth, joints or liver. 2,4 Symptoms may vary according to the degree and severity of bowel inflammation. 1,2 Acute severe exacerbations of UC are characterised by the development of systemic signs of disease (e.g. high temperature, tachycardia, anaemia, etc.) and require admission to hospital for urgent monitoring and treatment. 3
Diagnosis of UC is made by medical history, endoscopy and biopsy following the exclusion of potential infectious causes by stool examination. 5 These techniques permit the evaluation of relevant histological features and enable the differentiation of UC from other conditions such as CD. 2 For example, inflammation is characteristically restricted to the mucosal layer of the colon. 2 Diagnostic investigations also enable a determination of disease severity and there is evidence to indicate that severity of disease may be associated with younger age at diagnosis. 6,7 Based on the findings of diagnostic investigations, appropriate treatment can then be identified.
Colectomy by definition removes the source of inflammation in UC and is therefore associated with the relief of UC symptoms but is associated with a range of complications. 2,8 Pharmacological treatments for UC do not offer the possibility of cure and the disease course follows a relapsing–remitting pattern. The aim of clinical management is to induce and maintain disease remission and to avoid potential complications and the necessity for surgical intervention. 9 Selection of the appropriate therapy to induce remission of UC is determined by a number of factors, including severity and extent of disease. Evidence on prognosis indicates that, in the first decade, remission occurs in most patients and the rate of colectomy after diagnosis is low. 10 Otherwise, reported rates of colectomy among patients with UC are in the region of approximately 5% and 20%11,12 however this is an area of considerable uncertainty (some studies in selected populations have reported markedly higher colectomy rates, e.g. Gustavsson et al. 13). A range of factors have been suggested as potentially influencing the risk of relapse, including age (and age at first relapse), sex, smoking status and number of previous relapses. 12
Impact of health problem
Significance for patients
Complications of UC, depending on the severity and duration of the disease and age at onset, include severe bleeding and toxic megacolon, extraintestinal manifestations and osteoporosis. 2 Dysplasia and bowel cancer may also develop. A meta-analysis by Jess et al. 14 demonstrated that UC is not associated with an increase in overall mortality. UC can have a substantial impact on the health-related quality of life (HRQoL) of patients on account of the young age of disease onset for some patients, the severity of symptoms and the likelihood of relapse. 8,15–17 The risk of relapse and disease flares is increased by poor adherence to medication regimens. 18,19 Relapse and flares can be unpredictable and require further treatment, thus affecting patients’ HRQoL, their ability to perform daily activities (including work) and lead to increases in health-care costs. 8,19,20
Significance for the NHS
The burden of UC for the NHS is substantial, particularly with respect to those patients who suffer from poor disease control. A study of the costs of IBD (UC and CD) to the NHS reported in 2004 found that, compared with quiescent cases of IBD, disease relapse was associated with a two- to threefold increase in costs for non-hospitalised cases and a 20-fold increase in costs for hospitalised cases. 21
Truelove and Witts’ severity index23
The Truelove and Witts’ severity index describes the frequency of diarrhoea and whether or not systemic features of illness, such as high temperature, tachycardia and anaemia, are present or absent in patients (Table 1). When the disease is active, patients are categorised as having mild, moderate or severe disease.
| Disease classification | Clinical features |
|---|---|
| Severe disease | Diarrhoea frequency > 6 stools per 24 hours with blood |
| Temperature > 37.5 °C | |
| Tachycardia > 90 b.p.m. | |
| Anaemia (< 75% of normal value) | |
| Erythrocyte sedimentation rate > 30 mm per hour | |
| Moderate disease | Values ranging between mild and severe |
| Mild disease | Diarrhoea < 4 stools per 24 hours, intermittently or non-bloody |
| No fever | |
| No tachycardia | |
| Normal haemoglobin | |
| Erythrocyte sedimentation rate ≤ 30 mm per hour |
Mayo score
The Mayo score assesses patients’ disease in relation to four components: (1) stool frequency; (2) rectal bleeding; (3) endoscopic findings; and (4) physician’s global assessment24 (Table 2). Full Mayo scores range from 0 to 12 points, with scoring increasing with disease severity. The partial Mayo score, which comprises the non-endoscopic elements of the full Mayo score (i.e. stool frequency, rectal bleeding and physician’s global assessment), has been reported to have reasonable correlation with the full Mayo score (Spearman’s correlation coefficient ρ = 0.70). Partial Mayo scores range from 0 to 9 points. 27
| Mayo score features | |
|---|---|
| Stool frequency | |
| 0 | Normal stool frequency for patient |
| 1 | 1–2 stools more than usual |
| 2 | 3–4 stools more than usual |
| 3 | ≥ 5 stools more than usual |
| Rectal bleeding | |
| 0 | No blood |
| 1 | Streaks of blood < 50% of time with stool |
| 2 | Obvious blood most of time with stool |
| 3 | Blood alone passed |
| Endoscopic findingsa | |
| 0 | Normal/inactive disease |
| 1 | Mild disease (erythema, decreased vascular pattern, mild friability) |
| 2 | Moderate disease (marked erythema, lack of vascular pattern, friability, erosions) |
| 3 | Erosions |
| Physician’s global assessment | |
| 0 | Normal |
| 1 | Mild |
| 2 | Moderate |
| 3 | Severe |
Paediatric Ulcerative Colitis Activity Index
The PUCAI was developed with the aim of providing a non-invasive assessment instrument for use in paediatric practice and is based on measures of abdominal pain, rectal bleeding, stool consistency, stool frequency, nocturnal stools and activity level (Table 3). The tool has been described as showing good correlation with physician’s global assessment (Pearson’s r = 0.91; p < 0.001), full Mayo scores (r = 0.95; p < 0.001) and endoscopic subscores (r = 0.77; p < 0.001). 25
| Variable | Points scored |
|---|---|
| Abdominal pain | |
| Absent | 0 |
| Able to be ignored | 5 |
| Not able to be ignored | 10 |
| Rectal bleeding | |
| None | 0 |
| Small amount (< 50%) of stools | 10 |
| Small amount with most stools | 20 |
| Large amount (> 50%) of stools | 30 |
| Stool consistency | |
| Formed | 0 |
| Partially formed | 5 |
| Completely loose | 10 |
| Stool frequency (in 24 hours) | |
| 0–2 | 0 |
| 3–5 | 5 |
| 6–8 | 10 |
| ≥ 9 | 15 |
| Nocturnal stools | |
| Absent | 0 |
| Present | 10 |
| Activity level | |
| No limitations | 0 |
| Occasional limitations | 5 |
| Severe limitations | 10 |
Current service provision
Clinical guidelines
As outlined in National Institute for Health and Care Excellence (NICE) Clinical Guideline 166,1 conventional treatment options for moderately to severely active (non-systemic) UC include the use of oral or topical aminosalicylates, corticosteroids and/or immunosuppressants. Recommended conventional treatment options can vary according to the extent and location of colitis. Colectomy may be considered in the event of inadequate control of symptoms and/or poor HRQoL on conventional drug treatment.
Current NICE Technology Appraisal Guidance
Three NICE Technology Appraisals have previously been undertaken. 28–30 Infliximab (IFX) [Remicade®, Merck Sharp & Dohme Ltd (MSD)] was not previously recommended by NICE for the treatment of ‘subacute’ manifestations of moderately to severely active UC (NICE Technology Appraisal Guidance 140). 28 NICE Technology Appraisal 262 was terminated as no evidence submission was provided by the manufacturer. 29 NICE Technology Appraisal Guidance 163 recommended the use of IFX as an option for the treatment of acute exacerbations of severely active UC only in patients for whom ciclosporin is contraindicated or clinically inappropriate. 30
Current service cost
Cohen et al. 31 reports estimates of the direct and indirect costs of UC within the USA and Europe based on a systematic review of published cost studies. Cohen reports estimated annual per-patient direct medical costs of UC of between €8949 and €10,395 in Europe (2008 currency values). The study authors note that hospitalisations associated with UC accounted for 41–55% of direct medical costs. Indirect costs are also reported to be substantial, accounting for between 54% and 68% of total costs in Europe. The total economic burden of UC in Europe was estimated to be in the range of €12.5B to €29.1B.
Variation in services and uncertainty about best practice
The optimal treatment duration using IFX, adalimumab (ADA) (Humira®, AbbVie) and golimumab (GOL; Simponi®, MSD) is not yet known. The safety and efficacy of the readministration of interventions following an interruption of treatment has not been fully established. Furthermore, the maintenance of clinical remission following the withdrawal of biological treatment in responding patients is also unclear. There is no randomised controlled trial (RCT) evidence for the efficacy and safety of switching to a second biological intervention in patients who are primary or secondary non-responders, or in patients who are intolerant to a first biological intervention.
Current treatment pathway
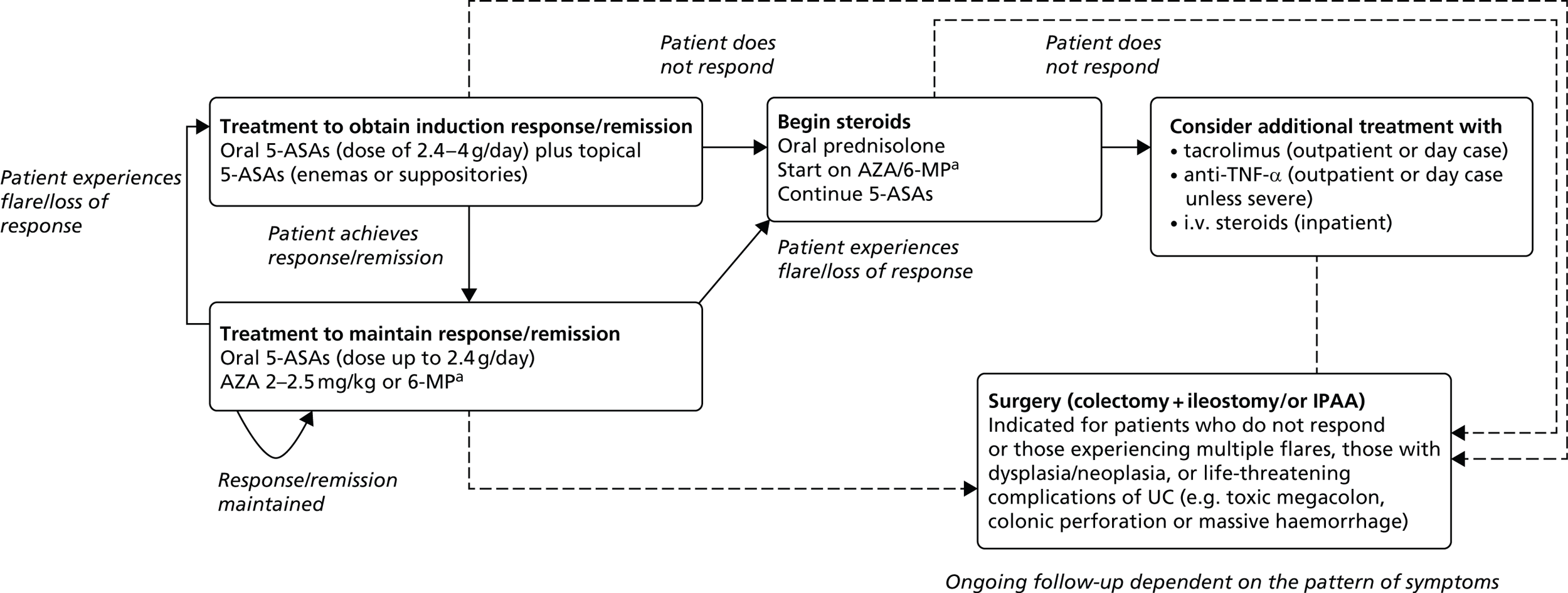
There does not exist a universally agreed pathway for the second-line treatment of patients with moderate to severe UC. Treatments received by patients may be influenced by the severity of symptoms, the extent and location of inflammation, clinical advice and individual patient choice. Treatments may include a combination of aminosalicylates [5-aminosalicylates (5-ASAs) – sulfasalazine, mesalazine/mesalamine, balsalazide and olsalazine], corticosteroids (beclomethasone, budesonide, hydrocortisone or prednisolone), and thiopurines [6-mercaptopurine (6-MP) or azathioprine (AZA)], calcineurin inhibitors and surgical intervention (colectomy). The care of people with UC is usually shared between primary care and specialist gastroenterology units working in collaboration with specialist colorectal surgical units. 1 Figure 1 presents a simplified pathway of the main types of treatments used for the management of patients with moderately to severely active UC who have had an inadequate response to conventional therapy including corticosteroids and 6-MP or AZA, or who are intolerant to, or have medical contraindications against, such therapies.
FIGURE 1.
Treatment pathway for moderate to severe UC. Anti-TNF-α; anti-tumour necrosis factor alpha; IPAA, ileal pouch anal anastomosis; i.v. intravenous. a, steroids (oral prednisolone) are indicated for inducing response/remission. AZA and 6-MP are indicated as maintenance treatments in patients with two or more flares requiring systemic steroids, for whom it is not possible to taper steroids, or following acute severe attack. AZA and 6-MP would be started at the same time as oral prednisolone.
Induction and maintenance of response
Current medical treatments for UC are principally concerned with treating active disease to address symptoms of urgency, frequency of defecation and rectal bleeding to improve the patient’s HRQoL and, thereafter, to maintain remission. 1 Treatment usually follows an escalation approach whereby additional drugs are added in order to induce and subsequently maintain response/remission. Initially, patients would most likely be treated using oral and topical 5-ASAs to induce a response. Most commonly, oral 5-ASA treatment involves high-dose oral mesalazine (usually 2.4–4.8 g/day depending on the particular product used). A dose of up to 2.4 g/day of mesalazine is used for maintenance. It is very likely that topical 5-ASAs (enemas or suppositories) would also be used during induction; the use of topical 5-ASAs is time-limited (usually a maximum of 4 weeks) and their efficacy is dependent on the extent of disease and severity of symptoms. If the patient does not respond or achieves but subsequently loses a response, or is contraindicated to or unable to tolerate 5-ASAs, treatment is likely to involve the use of oral corticosteroids and immunomodulators. Oral corticosteroids (most likely prednisolone) would be used as a short-term therapy with the intention of inducing a response; however, corticosteroids are not used as a maintenance treatment. Prednisolone is typically given at a dose of 40 mg/day, with the aim of the dose being tapered by 5 mg each week (8 weeks of treatment until the dose is zero). Treatment using immunomodulators, most commonly AZA and less commonly 6-MP, would be started at the same time as oral corticosteroids. These are indicated for maintenance rather than induction of response; hence patients may receive them on a long-term basis. Patients would likely remain on oral 5-ASA treatment continuously as they may confer other benefits in avoiding cancer, although evidence is conflicting in this respect. 32 If the patient does not respond to corticosteroids, it is likely that the patient would be considered for treatment using tacrolimus, intravenous (i.v.) steroids or anti-tumour necrosis factor alpha (anti-TNF-α) therapy.
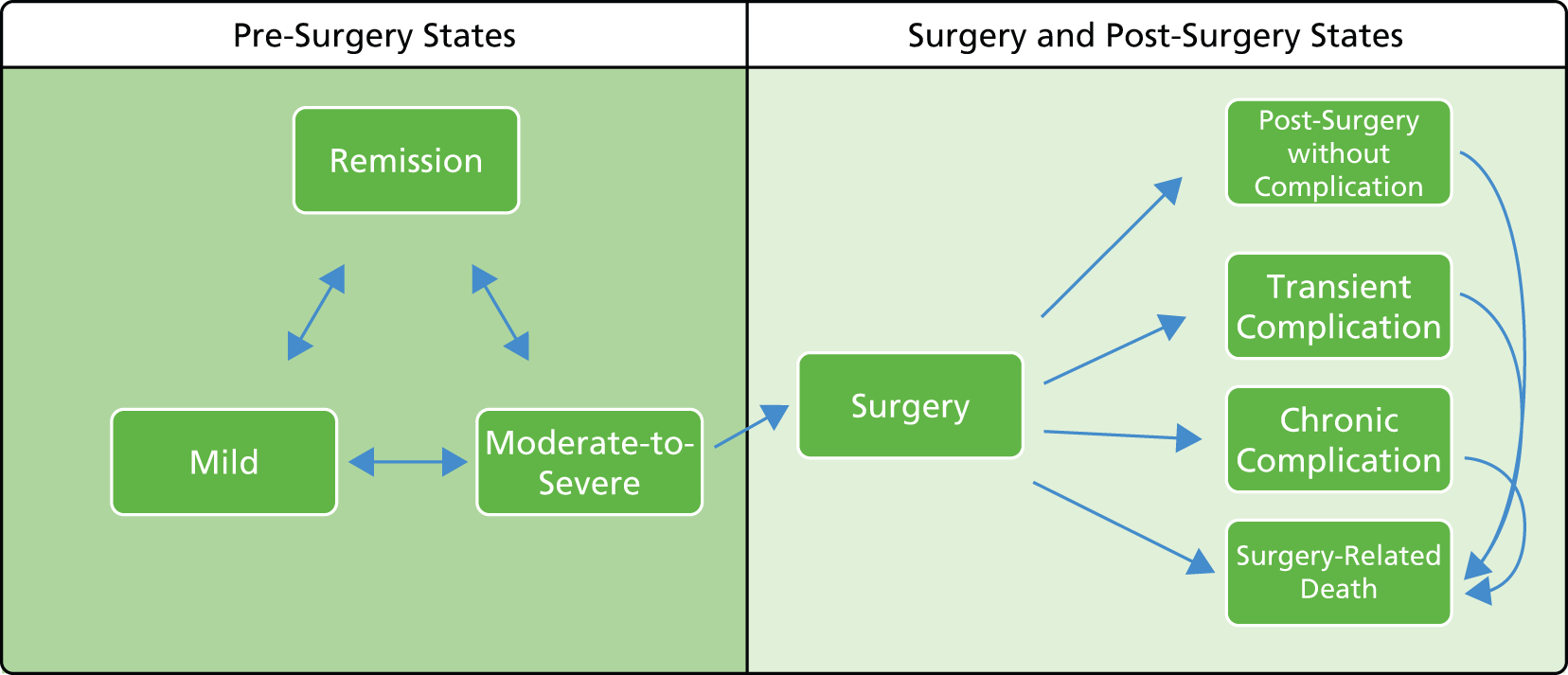
Surgery may be required in emergency scenarios (e.g. in cases of acute severe/fulminant UC) but within the moderately to severely active population, surgery is most likely to be elected by the individual patient. Emergency surgery may be required to ameliorate life-threatening complications of UC, such as toxic megacolon, colonic perforation and massive haemorrhage; it should be noted that surgery might also be used prophylactically to avoid the onset of these complications. More commonly, surgery is elective and is undertaken for severe disease characterised by prior treatment failures and/or frequent UC flares. In some cases, surgery may also be indicated owing to the increased risk of colorectal cancer associated with long-standing UC and may also be driven by the identification of pre-malignant dysplasia or malignant neoplasia. Colectomy is associated with post-operative morbidity and a risk of death. Among others, complications of surgery may include infertility, transient and chronic pouchitis, wound infections, wound dehiscence and small bowel obstruction. 1
Patients with less severe disease may be managed either in primary or secondary care. For patients with left-sided or extensive UC, follow-up is likely to take place in an outpatient setting, with appointments every 3–12 months depending on the pattern of flares. Follow-up may be consultant-led or IBD nurse-led, but will usually involve a combination of both.
Description of technology under assessment
Interventions considered in the scope of this report
Three interventions are considered for the adult population (IFX, ADA and GOL). Only IFX is licensed for use in children and adolescents. Two biosimilars (Remsima®, Celltrion Healthcare, and Inflectra®, Hospira) are also considered as part of the evidence base for IFX. Interventions are assessed in line with licensed indications, as described in the respective Summary of Product Characteristics (SmPCs) for each intervention. 33–35 The interventions under assessment are licensed for the treatment of rheumatoid arthritis (RA), adult CD (IFX and ADA only), paediatric CD (IFX and ADA only), adult UC, paediatric UC (IFX only), ankylosing spondylitis (AS), psoriatic arthritis and psoriasis (IFX and ADA only). 33–35
Mode of action
Infliximab, ADA and GOL are monoclonal antibodies that inhibit the activity of TNF-α, a key component in the inflammation process.
Marketing licence and administration method
Infliximab
Infliximab has a UK marketing authorisation for the treatment of moderately to severely active UC in adults who have had an inadequate response to conventional therapy including corticosteroids and mercaptopurine or AZA, or who are intolerant to, or have medical contraindications against, such therapies. 33 IFX also has a UK marketing authorisation for the treatment of severely active UC in children and adolescents aged 6 to 17 years, who have had an inadequate response to conventional therapy including corticosteroids and mercaptopurine or AZA, or who are intolerant to or have medical contraindications against such therapies. 33
Infliximab for the treatment of UC is administered by i.v. infusion at a dosage of 5 mg/kg followed by additional 5-mg/kg infusion doses at 2 and 6 weeks after the initial infusion, then every 8 weeks thereafter. 33 The SmPC states that other concomitant therapies (e.g. corticosteroids and immunosuppressants) should be optimised during IFX therapy. 33 IFX is typically administered intravenously over a 2-hour period as an outpatient or day-case appointment. As IFX treatment is associated with the development of acute infusion reactions, all patients receiving IFX are required to be observed, in a setting where emergency equipment is available, during the infusion for 1–2 hours post infusion for safety. Patients may receive pre-infusion treatment with, for example, an antihistamine, hydrocortisone and/or paracetamol. Contraindications to IFX treatment include a history of hypersensitivity to IFX or other murine proteins, the presence of tuberculosis (TB) or other severe infections such as sepsis, abscesses, and opportunistic infections, and moderate or severe heart failure. Furthermore, women of childbearing potential must use adequate contraception and continue use for at least 6 months after last receipt of IFX treatment.
Biosimilar versions of IFX (Remsima and Inflectra) are licensed for the same indications as Remicade. The therapeutic indications (including the wording of the licensed indication), dosage and method of administration for Remsima and Inflectra are identical to those for IFX (Remicade).
Adalimumab
Adalimumab has a UK marketing authorisation for the treatment of moderately to severely active UC in adults who have had an inadequate response to conventional therapy including corticosteroids and mercaptopurine or AZA, or who are intolerant to, or have medical contraindications against, such therapies. 34 ADA for the treatment of UC is administered subcutaneously according to an induction dose regimen of 160 mg at week 0 and 80 mg at week 2 followed by a recommended maintenance dosage of 40 mg every other week (increased to 40 mg every week if clinical response is insufficient). 34 Following physician advice, appropriate training and medical follow-up if required, patients may self-inject with ADA. The SmPC states that other concomitant therapies (e.g. corticosteroids and immunosuppressants) should be optimised during ADA therapy. 34 Contraindications to ADA treatment include hypersensitivity to the active substance, the presence of active TB or other severe infections such as sepsis and opportunistic infections, and moderate to severe heart failure [New York Heart Association (NYHA) class III/IV]. The administration of ADA during pregnancy is not recommended.
Golimumab
Golimumab has a UK marketing authorisation for the treatment of moderately to severely active UC in adults who have had an inadequate response to conventional therapy, including corticosteroids and mercaptopurine or AZA, or who are intolerant to, or have medical contraindications against, such therapies. 35
Golimumab for the treatment of UC is administered subcutaneously according to body weight. Patients with body weight < 80 kg receive an initial dose of 200 mg, followed by 100 mg at week 2, then 50 mg every 4 weeks thereafter. Patients with body weight ≥ 80 kg receive an initial dose of 200 mg, followed by 100 mg at week 2, then 100 mg every 4 weeks thereafter. 35 Following physician advice and adequate training, patients may self-inject with GOL. Contraindications to GOL include hypersensitivity to the active substance, the presence of active TB or other severe infections such as sepsis, opportunistic infections, and moderate or severe heart failure (NYHA class III/IV). The use of GOL during pregnancy is not recommended.
Criteria for continuing treatment
The SmPC for each intervention describes the use of stopping rules for treatment in non-responders. 33–35
The SmPC for IFX states that clinical response should typically be achieved within 14 weeks of treatment (i.e. three doses) and that continued therapy should be carefully reconsidered in patients who show no evidence of therapeutic benefit within 14 weeks. The SmPC also indicates that, for paediatric patients, there is no evidence to support the further use of IFX in patients who do not respond within the first 8 weeks of treatment.
For ADA, the SmPC states that clinical response should be reached within 2–8 weeks of treatment and that treatment should not be continued in patients who fail to respond within this time frame.
The SmPC for GOL states that clinical response is expected to be achieved within 12–14 weeks of treatment (i.e. after four doses) and that continued therapy should be reconsidered in patients who do not experience therapeutic benefit within this time period.
The SmPCs for each intervention also refer to the requirement to monitor patients closely for infections and to discontinue treatment in patients who develop a serious infection or sepsis.
Current usage in the NHS
Infliximab is currently recommended by NICE as an option for the treatment of acute exacerbations of severely active UC, only in patients in whom ciclosporin is contraindicated or clinically inappropriate. ADA and GOL do not have recommendations from NICE for use in the treatment of UC. The Assessment Group has received clinical advice to suggest that IFX, and to a lesser degree ADA, are currently used for the treatment of moderate to severe UC in some larger centres in England and Wales.
Identification of important subgroups
The only subgroup pre-specified in the final NICE scope36 relates to duration of disease.
Anticipated costs associated with interventions
Table 4 summarises the costs associated with the interventions based on their list prices. 37
| Drug | Unit type and dose | Price per unit |
|---|---|---|
| IFX | Powder for reconstitution, 100-mg vial | £419.62 |
| ADA | 40-mg pre-filled pen or pre-filled syringe, 40-mg/0.8-ml vial | £352.14 |
| GOL | 50-mg pre-filled pen or pre-filled syringe | £762.97 |
| 100-mg pre-filled pen | £1525.94 |
Chapter 2 Definition of the decision problem
Decision problem
The aim of this assessment is to evaluate the clinical effectiveness and cost-effectiveness of IFX, ADA and GOL for the treatment of patients with moderately to severely active UC after the failure of conventional therapy.
Interventions
Three interventions are considered within this assessment: IFX (Remicade), ADA (Humira) and GOL (Simponi). These interventions are described in detail in Chapter 1, Description of technology under assessment. Biosimilar versions of IFX (Remsima and Inflectra) are also licensed for the same indications and are considered as part of the evidence base for IFX within this assessment report.
Populations (including subgroups)
The assessment considers the following two populations:
-
Adults aged ≥ 18 years with moderately to severely active UC who have had an inadequate response to conventional therapy including corticosteroids and mercaptopurine or AZA, or who are intolerant to, or have medical contraindications against, such therapies.
As referred to in the final NICE scope,36 severity of disease in adults would be defined according to the modified Truelove and Witts’ severity index (as described in NICE Clinical Guideline 166). 1
The following interventions are indicated for use in adults:
-
ADA
-
IFX
-
GOL.
-
-
Children and adolescents aged 6–17 years (inclusive) with severely active UC, who have had an inadequate response to conventional therapy including corticosteroids and mercaptopurine or AZA, or who are intolerant to, or have medical contraindications against, such therapies.
As described in NICE Clinical Guideline 166,1 severity of UC in children and adolescents was to be assessed using the PUCAI. 25
The following intervention is indicated for use in children and adolescents:
-
IFX.
-
The final NICE scope36 highlighted duration of disease as a potential subgroup of interest; this is examined according to the availability of evidence.
Populations outside of the scope of the appraisal
The following groups were considered to be beyond the scope of the appraisal and, therefore, are not considered in this assessment report:
-
children with mildly or moderately active UC (as defined by the PUCAI measure)
-
adults with mildly active UC (as defined by the modified Truelove and Witts’ criteria)
-
adults and children with acute severe (systemic) UC.
Relevant comparators
The interventions are compared against each other. Other relevant comparators include standard clinical management options, which (as described in the final NICE scope36) could include a combination of aminosalicylates (sulfasalazine, mesalazine, balsalazide or olsalazine), corticosteroids (beclomethasone, budesonide, hydrocortisone or prednisolone), thiopurines (mercaptopurine or AZA), calcineurin inhibitors or elective surgical intervention.
Emergency surgical intervention is not considered as a comparator in this assessment as acute severe UC was stated in the final scope as being beyond the remit of the appraisal.
Outcomes
The outcome measures to be considered included:
-
mortality
-
measures of disease activity
-
rates of and duration of response, relapse and remission
-
rates of hospitalisation
-
rates of surgical intervention (both elective and emergency)
-
time to surgical intervention (both elective and emergency)
-
adverse events (AEs) of treatment (including leakage and infections following surgery)
-
HRQoL.
Following discussions during the NICE appraisal scoping process, data relating to mucosal healing were not considered eligible for this assessment.
Overall aims and objectives of assessment
This assessment addresses the question ‘what is the clinical effectiveness and cost-effectiveness of IFX, ADA and GOL for the treatment of patients with moderately to severely active UC after the failure of conventional therapy as compared against each other and standard clinical management?’.
More specifically, the objectives of the assessment are:
-
to evaluate the clinical effectiveness of each intervention
-
to examine the effect of disease duration on the clinical effectiveness of each intervention (subject to the availability of evidence)
-
to evaluate the adverse effect profile of each intervention
-
to evaluate the incremental cost-effectiveness of each intervention compared (1) against each other and (2) against all comparators (including medical and surgical options)
-
to estimate the expected net budget impact associated with implementing each intervention
-
to identify key areas in which future research may be valuable.
Chapter 3 Assessment of clinical effectiveness
A systematic review of the literature including network meta-analyses (NMAs) was conducted in order to evaluate the clinical effectiveness and safety of IFX, ADA and GOL in the treatment of moderately to severely active UC after the failure of conventional therapy.
The systematic review of clinical effectiveness was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 38
Methods for reviewing clinical effectiveness
The protocol for this review is registered with PROSPERO (CRD42013006883). 39
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Identification of studies
A comprehensive search was undertaken to systematically identify literature relating to the clinical effectiveness and safety of IFX, ADA and GOL for treating moderately to severely active UC after the failure of conventional therapy. The search strategy comprised the following main elements:
-
searching of electronic databases
-
hand-searching bibliographies of retrieved papers, key journals and conference proceedings
-
contact with experts in the field.
The following electronic databases were searched from inception for published trials and systematic reviews:
-
MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations: via Ovid – 1946 to December 2013.
-
EMBASE: via Ovid – 1974 to December 2013.
-
Cochrane Library: via Wiley Interscience
-
Cochrane Database of Systematic Reviews (CDSR) – 1996 to December 2013
-
Database of Abstracts of Reviews of Effects (DARE) – 1995 to December 2013
-
Cochrane Central Register of Controlled Trials (CCRT) – 1995 to December 2013
-
Cochrane Methodology Register – 1904 to December 2013
-
Health Technology Assessment (HTA) database – 1995 to December 2013.
-
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL): via EBSCOhost – 1982 to December 2013.
-
Web of Science Citation Index: via Web of Knowledge – 1900 to December 2013.
-
Conference Proceedings Citation Index: via Web of Knowledge – 1990 to December 2013.
-
Bioscience Information Service (BIOSIS) Previews: via Web of Knowledge – 1969 to December 2013.
The MEDLINE search strategy is presented in Appendix 1. The search strategy combined free text and medical subject headings (MeSHs) or thesaurus terms relating to UC, with free text and MeSHs or thesaurus terms relating to IFX, ADA or GOL combined with highly sensitive filters to retrieve RCTs and systematic reviews. Search terms for IFX biosimilars were also included. The search strategy was translated across all databases. No date or language restrictions were applied. Literature searches were conducted during December 2013. References were collected in a bibliographic management database and duplicates were removed.
Searches were undertaken to identify unpublished studies (nearing or at completion) relevant to the decision problem within the following research registers:
-
ClinicalTrials.gov (searched December 2013)
-
UK Clinical Research Network Portfolio database (searched December 2013)
-
World Health Organization International Clinical Trials Registry Platform (searched March 2014).
Proceedings of the following conferences were searched from 2009 to 2014 (when possible) for recent research:
-
Congress of Crohn’s and Colitis Conference, European Crohn’s and Colitis Organisation (ECCO)
-
Digestive Disease Week
-
Gut (British Society of Gastroenterology).
Key journals were identified using the PubMed PubReMiner facility and electronic tables of contents were searched from March 2013 to February 2014 for the following journals:
-
Inflammatory Bowel Diseases
-
Alimentary Pharmacology & Therapeutics
-
Gastroenterology
-
Journal of Crohn’s and Colitis
-
American Journal of Gastroenterology.
Citation searches were performed on included studies in Web of Science in March 2014.
Manufacturers’ submissions received by NICE, as well as any relevant systematic reviews, were also hand-searched in order to identify any further potentially relevant clinical trials.
Inclusion and exclusion criteria
Inclusion and exclusion criteria were based on the final NICE scope36 and were applied as described below.
Study selection
The selection of eligible articles was undertaken using a two-stage process. First, in order to assess agreement in the sifting approach between systematic reviewers, a check for consistency was conducted in the early stages of the sifting process. The reviewers (RA and MMSJ) double-sifted a total of 940 titles and abstracts. Kappa statistics of 0.888 and 1.000 were obtained, indicating very high strength of agreement.
All remaining titles and abstracts were examined for inclusion by one reviewer (either RJA or MMSJ sifted 50% of total citations at title and abstract level). Any citations that clearly did not meet the inclusion criteria (e.g. animal studies, studies unrelated to UC) were excluded. During the second stage of the sifting process, full-text articles were examined for inclusion by one reviewer (RJA or MMSJ). Any uncertainty in the eligibility of potentially relevant full-text articles was resolved through discussion. Trials retrieved for full-paper screening which were subsequently excluded were tabulated (see Appendix 2) together with justification for their exclusion.
Inclusion criteria
Studies were included in the review if they met the inclusion criteria outlined below.
Interventions
Any of the following interventions were included:
-
For adults (defined by the Assessment Group as aged ≥ 18 years):
-
ADA
-
IFX
-
GOL.
-
-
For children and adolescents aged 6–17 years (inclusive):
-
IFX.
-
Biosimilar versions of IFX (Remsima and Inflectra) are also licensed for the same indications as Remicade and have been considered as part of the evidence base for IFX within this assessment.
Studies in which the interventions were assessed in line with licensed indications were included in the systematic review.
Populations
-
Adults aged ≥ 18 years with moderately to severely active (non-systemic) UC (defined as patients with moderately active disease according to the modified Truelove and Witts’ criteria23) whose disease has responded inadequately to conventional therapy including corticosteroids and mercaptopurine or AZA, or who are intolerant of, or have medical contraindications to, such therapies.
-
Children and adolescents aged 6–17 years with severely active (non-systemic) UC (as classified by the PUCAI measure25) whose disease has responded inadequately to conventional therapy including corticosteroids and mercaptopurine or AZA, or who are intolerant to, or have medical contraindications to, such therapies.
Comparators
Relevant comparators included in the final NICE scope were (1) interventions as defined in the protocol for this assessment (i.e. IFX, ADA or GOL compared with each other) and (2) standard clinical management, which may include a combination of aminosalicylates (sulfasalazine, mesalazine/mesalamine, balsalazide or olsalazine), corticosteroids (beclomethasone, budesonide, hydrocortisone or prednisolone), thiopurines (mercaptopurine or AZA), calcineurin inhibitors or elective surgical intervention.
It should be noted that although calcineurin inhibitors (tacrolimus and ciclosporin) were included as potential comparators in the final NICE scope, these options were excluded from the assessment for two reasons:
-
They are treatments typically reserved for patients with acute severe disease. This differs to the population within the final NICE scope. Studies were specifically excluded if they related to these patients (see Exclusion criteria).
-
There are no direct data comparing biologicals versus calcineurin inhibitors for the population under investigation. Altogether, there are very limited data on the efficacy of either ciclosporin or tacrolimus in this indication.
Outcomes
Eligible outcomes for consideration were:
-
mortality
-
measures of disease activity
-
rates of and duration of response, relapse and remission
-
rates of hospitalisation
-
rates of surgical intervention (both elective and emergency)
-
time to surgical intervention (both elective and emergency)
-
AEs of treatment (including leakage and infections following surgery)
-
HRQoL.
Following discussions during the NICE appraisal scoping process, data relating to mucosal healing were not considered eligible for this assessment.
Study design
Randomised controlled trials were eligible for inclusion in the systematic review of clinical effectiveness. Long-term extension studies associated with included RCTs were also included in the review.
Studies published as abstracts or conference presentations were eligible for inclusion only if sufficient details were presented to allow an assessment of the trial methodology and results to be undertaken.
Exclusion criteria
The following types of studies were excluded from the review:
-
Studies that included adults with mildly active UC (as defined by the modified Truelove and Witts’ criteria23), for which no separate data were reported for patients with moderate to severe UC.
-
Studies that included children with mildly or moderately active UC (as defined by the PUCAI measure25).
-
Studies that included adults with (acute) severely active UC as defined by the modified Truelove and Witts’ criteria23 (representing patients who are systemically ill and are, therefore, beyond the remit of this appraisal).
-
Studies that included adults, adolescents or children with acute severe UC, whose disease is systemic as shown by tachycardia, fever, anaemia or a raised erythrocyte sedimentation rate (representing patients who are excluded as they are outside the remit of this appraisal).
-
Studies that included patients with acute severe UC previously hospitalised and treated with i.v. steroids (representing patients in a potentially life-threatening medical emergency and excluded as they are outside the remit of this appraisal).
-
Studies that included patients with IBD other than UC (e.g. CD) for which data were not reported separately for UC patients.
-
Studies that interventions were not administered in accordance with licensed indications.
-
Systematic reviews and clinical guidelines (selected systematic reviews identified by the clinical effectiveness searches were used as sources of references).
-
Studies that were published only in languages other than English.
-
Studies based on animal models.
-
Pre-clinical and biological studies.
-
Narrative reviews, editorials and commentaries.
-
Reports published as abstracts or conference presentations only, for which insufficient details were reported to allow an assessment of study quality or results.
Data abstraction strategy
Data relevant to the decision problem were extracted by one reviewer (RA or MMSJ). Data were extracted without blinding to authors or journal. A data extraction form was developed and piloted on two included trials before slight revisions and final use on all included trials. Data relating to study arms in which the intervention treatments were administered in line with their licensed indications were extracted; data relating to the unlicensed use of the interventions were not extracted. All extracted data were double-checked by a second reviewer (MMSJ or CC). The safety data extracted were informed by the SmPCs for each product [available from www.medicines.org.uk/emc/ (accessed 26 May 2014)]. 33–35 The key safety issues included such items as the number of patients experiencing infections, number of patients experiencing serious infections, number of patients experiencing malignancy and the occurrences of infusion-related or injection site reactions (as appropriate to the mode of administration for each intervention). Study results that were presented only in graphical format were digitised and estimated using Engauge software version 4.1 [http://sourceforge.net/projects/digitizer/files/Engauge%20Digitizer/digitizer-4.1/ (accessed April 2014)]. When multiple publications of the same study were identified, data extraction was undertaken on all relevant associated publications and findings were presented together with reference to their published source.
Critical appraisal strategy
The methodological quality of each included study was assessed by one reviewer (RJA or MMSJ). The quality of included studies was assessed using the Cochrane Risk of Bias Tool. 40 This tool addresses specific domains, namely sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective outcome reporting. RCTs were classified as being at ‘high risk’ of attrition bias where drop-out in any treatment arm was ≥ 10%. 41 The Assessment Group requested the trial protocols for all included trials from the manufacturers of the products included in this appraisal. These were received for some trials and were used alongside clinical study reports (CSRs) provided by the manufacturer for some trials and outcomes listed in ClinicalTrials.gov records in order to inform the selective reporting domain of the Cochrane Risk of Bias Tool. All quality assessment findings were double-checked by a second reviewer (RJA or MMSJ).
Methods of data synthesis
The extracted data were presented for each study, both in structured tables and as a narrative description.
Methods for the estimation of efficacy using network meta-analysis
Network meta-analysis methods are described in full on page 69.
Supplementary meta-analyses
When considered appropriate, secondary outcomes of interest were analysed using classical meta-analysis methods. Meta-analysis was undertaken using Cochrane Review Manager software (version 5.2; The Cochrane Collaboration, Copenhagen, Denmark). Outcomes reported as continuous data were estimated using a mean difference with 95% confidence intervals (CIs). Dichotomous outcomes were estimated as risk ratios (RRs) with associated 95% CIs. When RCTs reported AEs in sufficient detail, these were analysed as dichotomous data. Clinical heterogeneity across RCTs (the degree to which RCTs appear different in terms of participants, intervention type and duration and outcome type) was considered prior to data pooling. Random-effects models were applied and effect estimates, estimated in Review Manager as z-values, were considered statistically significant at a cut-off point of p < 0.05.
Results
Quantity of research available
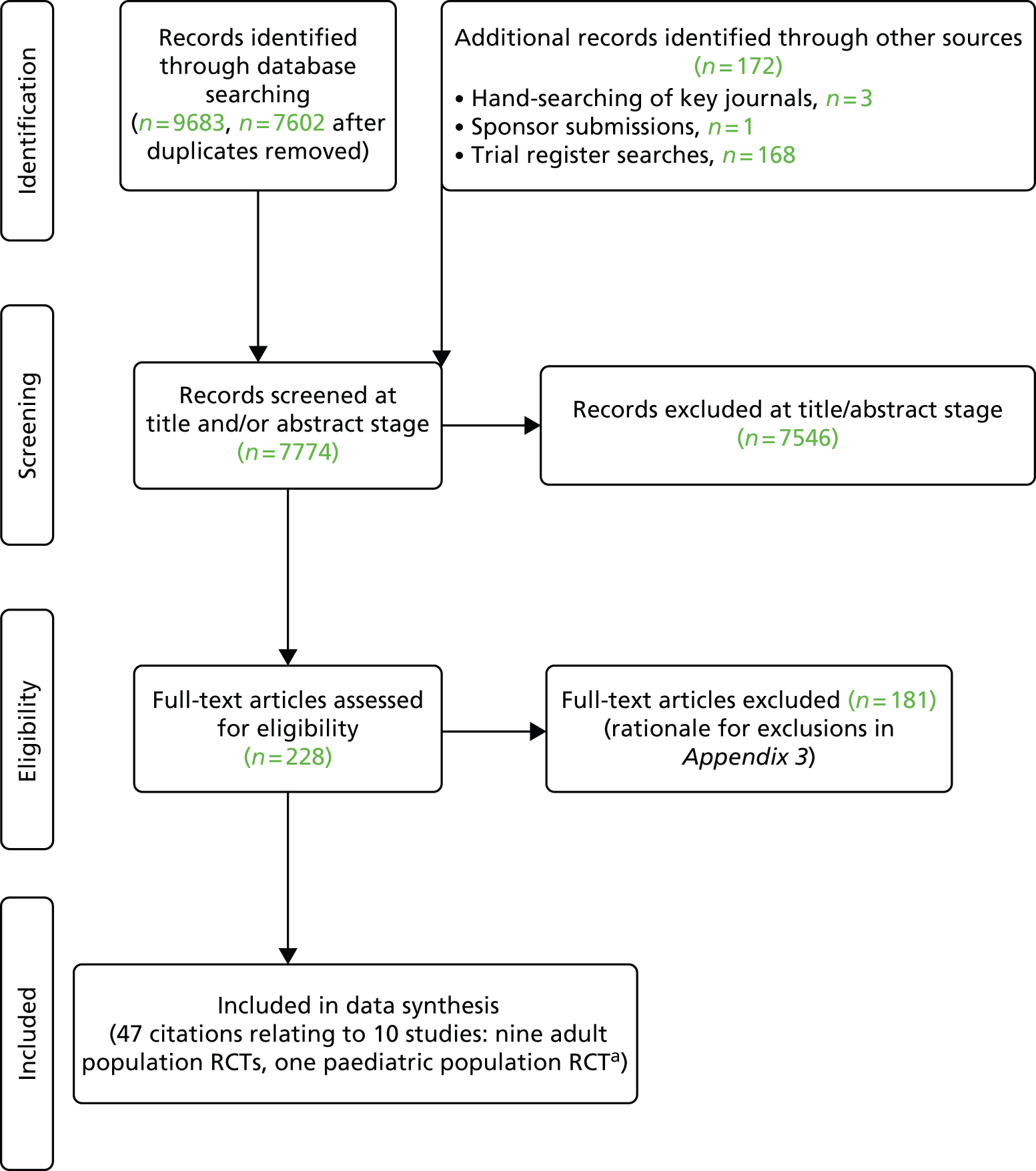
The searches described in Identification of studies yielded 7774 potentially relevant citations (7602 from searches of electronic databases after removal of duplicates), three from hand-searching of key journals, one from sponsor submissions and 168 from trial register searches). Of these records, 7546 were excluded at the title and abstract stage. Full texts of 228 studies were obtained for scrutiny and of these, 181 citations were excluded (it was not possible to obtain nine studies and hence these were excluded; see Appendix 2).
No additional eligible trials that were completed or nearing completion were identified through the trial register searches. Trial NCT01551290 [a study of IFX vs. placebo (PBO) in Chinese subjects by Xian-Janssen Pharmaceutical Ltd42] was stated to be ongoing with an estimated completion date of November 2014. Trial NCT01863771 (a study of GOL maintenance treatment vs. PBO in Japanese patients by Janssen Pharmaceutical43) was recruiting as of February 2014. As such, neither trial was judged to be completed or nearing completion.
A total of 47 citations relating to 10 RCTs were included in the review. 44–52 The search process is summarised in the form of a PRISMA diagram in Figure 2.
FIGURE 2.
Flow diagram of study inclusion (adapted from PRISMA). 53 a, Not including sponsor submissions and European Public Assessment Reports.
European Public Assessment Reports (EPARs) were available for all included interventions; however, associated Food and Drug Administration (FDA) reports for interventions could not be identified from the FDA website (www.fda.gov/drugs/).
Summary of study and population characteristics of included trials
Study characteristics
The available comparisons between licensed doses of interventions and PBO are tabulated within the adult population RCTs in Table 5. The trial design characteristics of the included trials are outlined in Tables 6 and 7. The outcome measures pre-specified in the final NICE scope36 and protocol were all addressed by the included trial evidence, with the exception of rates of relapse. As stated in Methods for reviewing clinical effectiveness, data relating to mucosal healing were not eligible for this assessment.
| Trial | Licensed treatment comparisons |
|---|---|
| ULTRA144 | PBO; 160 mg/80 mg of ADA (licensed induction dose) |
| ULTRA245 | PBO; 160 mg of ADA at week 0, 80 mg at week 2 and then 40 mg every other week (licensed maintenance dose) beginning at week 4 |
| Suzuki et al.46 | PBO; 160 mg/80 mg of ADA (licensed induction dose) |
| PURSUIT-SC47 | PBO; 200 mg/100 mg of GOL (licensed induction dose) |
| PURSUIT-Maintenance48 | PBO; 50 mg of GOL; 100 mg of GOL (licensed maintenance doses) |
| ACT149 | PBO; 5 mg/kg of IFX |
| ACT249 | PBO; 5 mg/kg of IFX |
| Probert et al.50 | PBO; 5 mg/kg of IFX |
| UC-SUCCESS51 | No PBO; 5 mg/kg of IFX; AZA; IFX5 mg/kg/AZA |
| Trial identifier (NCT number), primary publication details | Trial design | Inclusion/exclusion criteria | Geographical location of study sites | Treatment groups and numbers randomised | Open label escape allowance | Primary outcome | Assessment of induction | Assessment of maintenance | Study sponsor |
|---|---|---|---|---|---|---|---|---|---|
| ADA | |||||||||
| ULTRA1 (NCT00385736, M06–826), Reinisch et al., 201144 | Multicentre, randomised, double-blind, PBO-controlled trial. Phase III | Inclusion: adult ambulatory patients, moderately to severely active UC, Mayo score of 6–12 points with endoscopy subscore of 2–3, despite concurrent and stable treatment with oral CSs and/or immunomodulators. Concurrent therapy not required if failed to respond/could not tolerate previous CS/immunomodulator treatment. Exclusion: ulcerative proctitis, previous receipt of anti-TNF agent or biological agent, receipt of i.v. CSs within 14 days prior to screening/during screening; receipt of ciclosporin, tacrolimus, mycophenolate mofetil, or methotrexate within 60 days of baseline | USA, Puerto Rico, Canada, Western Europe, and Eastern Europe. 94 study centres: (USA, 34; Puerto Rico, 3; Canada, 5; Western Europe, 32; and Eastern Europe, 20) | Original study protocol: ADA 160 mg/80 mg s.c. = 93 randomised, PBO s.c. = 93 randomised Protocol amended to include ADA 80 mg/40 mg group ADA 160 mg/80 mg s.c. = 130 randomised ADA 80 mg/40 mg s.c. = 130 randomised PBO s.c. = 130 randomised ADA 160 mg/80 mg group received ADA 160 mg at week 0, ADA 80 mg at week 2, ADA 40 mg at weeks 4 and 6 ADA 80 mg/40 mg group received ADA 80 mg at week 0, ADA 40 mg at week 2 and ADA 40 mg at weeks 4 and 6 PBO group received same number of s.c. injections as patients in ADA treatment groups |
No | Clinical remission (Mayo score of ≤ 2 with no individual subscore of > 1) at week 8 assessed in the ITT-A3 (amendment) population | Week 8. Included in NMA? Yes | NA. Included in NMA? No (8-week study) | Abbott |
| ULTRA2(NCT00408629, M06–827), Sandborn et al., 201245 | Multicentre, randomised, double-blind, PBO-controlled trial. Phase III | Inclusion: adults with moderately to severely active UC for ≥ 3 months and Mayo score of 6–12 points (endoscopy subscore of ≥ 2), despite concurrent therapy with steroids and/or AZA or 6-MP. Exclusion: previous treatment with ADA; receipt of i.v. CSs, i.v. CSs within 2 weeks of screening; receipt of ciclosporin, tacrolimus, or mycophenolate mofetil within 1 month of baseline; or receipt of any investigational agent within 30 days/five half-lives before baseline | North America, Europe, Australia, New Zealand and Israel. 103 study centres | PBO n = 260 (14 excluded owing to site non-compliance) ADA 160 mg/80 mg/40 mg n = 258 (10 excluded owing to site non-compliance) Patients received ADA s.c. 160 mg at week 0, 80 mg at week 2 and 40 mg EOW from week 4 or matching PBO and followed through week 52 |
Yes. Patients with inadequate response permitted to switch to open-label ADA (40 mg EOW) from week 12. Patients with inadequate response at two visits on open-label ADA 40 mg EOW permitted to escalate to 40 mg EW. Data handled using non-responder imputation methods | Proportion of patients achieving clinical remission at week 8 and proportion of patients achieving clinical remission at week 52 | Week 8 included in NMA? Yes | Weeks 32 and 52 included in NMA? Yes | Abbott |
| ULTRA3(M10–223), Reinisch et al., 201354 | Long-term, single-arm, open-label extension study including patients from ULTRA1 and ULTRA2 (currently ongoing) | Inclusion: patients in both studies who completed the 52-week visit had the option of enrolling in the extension study (M10–223). Exclusion: patients not responding to weekly ADA from Study M06–826 or M06–827 | See ULTRA1 and ULTRA2 | Patients continued to receive open-label ADA (EOW or EW dosing permitted). ADA 40 mg EOW or EW (n = 588) | Yes. Patients who had inadequate response or responded and then experienced disease flare eligible for ADA dose increase to 40 mg EW (no earlier than the week 12 visit) or the week 2 visit if already receiving open-label ADA | NR | NA | Evaluation of ADA maintenance regimens | Abbott |
| Suzuki et al., 2014(NCT00853099, M10–447), Suzuki et al., 201446 | Multicentre, randomised, double-blind, PBO-controlled trial. Phase II/III | Inclusion: Japanese patients ≥ 15 years, moderately to severely active UC, Mayo score of 6–12 points with endoscopy subscore of ≥ 2 despite concurrent treatment with stable doses of oral CSs and/or immunomodulators. Patients previously treated with CSs or immunomodulators during past 5 years and had failed to respond or who could not tolerate treatment eligible. Exclusion: patients with prior treatment with anti-TNF therapies or other biologicals, discontinuation of oral CSs ≤ 2 weeks before baseline; receipt of CS injection, ciclosporin, tacrolimus, or mycophenolate mofetil ≤ 4 weeks before baseline | Japan. 65 study centres | PBO n = 96 ADA 160 mg/80 mg = 90 randomised ADA 80 mg/40 mg = 87 randomised Patients received s.c. ADA 160 mg at week, 80 mg at week 2 and 40 mg EOW from week 4, or ADA 80 mg at week 0, 40 mg at week 2 and 40 mg EOW from week 4 or PBO |
Yes. Patients with inadequate response to study drug or flare at or after week 8 permitted to enter rescue arm with 4 weeks of blinded ADA (either 160 mg initially and 80 mg 2 weeks later for PBO group, or 40 mg initially and 2 weeks later for patients in either ADA group) followed by open-label ADA 40 mg EOW (with option to escalate to 80 mg EOW if inadequate response/flare ≥ 8 weeks later. Data handled using non-responder imputation | NR | Week 8. Included in NMA? Sensitivity analysis only (on basis of exclusively Japanese population and population eligibility age patients ≥ 15 years) | Weeks 32 and 52. Included in NMA? Sensitivity analysis only (on basis of exclusively Japanese population and population eligibility age patients ≥ 15 years) | Abbott |
| GOL | |||||||||
| PURSUIT-SC(NCT00487539)(programme of UC Research Studies Utilising an Investigational Treatment – Subcutaneous), Sandborn et al., 201447 | Multicentre, randomised, double-blind, PBO-controlled trial. Integrated Phase II and Phase III trial | Inclusion: patients with moderate to severe UC, Mayo score of 6–12 points, with endoscopic subscore of ≥ 2, inadequate response to/failed to tolerate one or more of following: oral 5-ASA, oral CSs, AZA, and/or 6-MP; or CS dependent. Exclusion: patients with colitis limited to 20 cm of colon; patients with earlier use of: biological anti-TNF agent(s) natalizumab or other agents targeting alpha-4 integrin, B-cell depleting agents (rituximab), or T-cell depleting agents (alemtuzumab, visilizumab) within 12 months of first study drug dose (or continued B- or T-cell depletion > 12 months after completing treatment with lymphocyte-depleting agents); oral CSs at dose > 40 mg prednisone or equivalent per day; receipt of ciclosporin, tacrolimus, sirolimus, or mycophenolate mofetil within 8 weeks before first study agent infection | Eastern Europe, North America, Asia Pacific, South Africa, Western Europe, Israel. 217 sites (Eastern Europe 400 patients, North America 278 patients, Asia Pacific and South Africa 204 patients, and Western Europe and Israel 183 patients) | Phase II PBO = 42 plus 31 enrolled while Phase II data being analysed. Phase II GOL 200 mg/100 mg = 42 plus 31 enrolled while Phase II data being analysed. Phase III PBO = 258. Phase III GOL 200 mg/100 mg = 258. Patients received s.c. GOL or PBO at weeks 0 and 2 | No | Phase III primary end point was clinical response at week 6 | Week 6. Included in NMA? Yes | NA. Included in NMA? No (6-week study) | Janssen Research & Development |
| PURSUIT-Maintenance (NCT00488631), Sandborn et al., 201448 | Randomised-withdrawal, PBO-controlled, double-blind multicentre trial. Patients who responded to GOL induction therapy (n = 464) randomised at baseline visit in 1 : 1 : 1 ratio to SC PBO, GOL 50 mg or GOL 100 mg. Phase III | Inclusion: patients had completed one of two GOL induction studies (PURSUIT-SC or PURSUIT-IV). Patients eligible for induction studies had moderate to severe UC and Mayo score of 6–12 points with endoscopic subscore of ≥ 2. Patients had inadequate response to/had failed to tolerate one or more of following: oral 5-ASA, oral CSs, immunosuppressives (AZA or 6-MP) or were CS-dependent. Exclusion: patients with isolated proctitis excluded from induction studies | Eastern Europe, North America, Asia Pacific and South Africa, and Western Europe and Israel. 251 sites across Eastern Europe (477 patients), North America (323 patients), Asia Pacific and South Africa (237 patients), and Western Europe and Israel (191 patients) | PBO randomised = 156 GOL 50 mg = 154 randomised GOL 100 mg = 154 randomised Randomised patients received s.c. PBO, GOL 50 mg or GOL 100 mg every 4 weeks through week 52 (efficacy analysis population). PBO-induction responders and PBO- or GOL-induction non-responders eligible but not randomised. PBO induction responders received PBO every 4 weeks through week 52. GOL-induction or PBO-induction non-responders received GOL 100 mg every 4 weeks through week 12, assessed at week 16 and discontinued from study if disease activity unimproved. Non-randomised patients included in demographic, pharmacokinetic and safety summaries only |
Yes. Induction responders who lost clinical response permitted to modify treatment. PBO group to GOL 100 mg every 4 weeks, GOL 50 mg rerandomised to GOL 50 mg or GOL 100 mg every 4 weeks and GOL 100 mg rerandomised to GOL 100 mg or GOL 200 mg (GOL 200 mg dose subsequently discontinued and patients on GOL 200 mg decreased to GOL 100 mg). Proportions of subjects who underwent dose adjustments were 33.8% in the GOL 50 mg group, 28.5% in the GOL 100 mg group and 48.7% in the PBO group | Clinical response maintained through week 54 among GOL induction responders | NA. Included in NMA? No (maintenance trial only) | Weeks 30 and 54. Included in NMA? Yes | Janssen Research & Development |
| IFX | |||||||||
| ACT1(NCT00096655), Rutgeerts et al., 200549 | Multicentre, randomised, double-blind, PBO-controlled trial. Phase III | Active UC with Mayo score of 6–12 points and moderate to severe active disease on sigmoidoscopy despite concurrent treatment with CSs alone or in combination with AZA or 6-MP included. Patients with diagnosis of indeterminate colitis, CD or clinical findings suggestive of CD; positive tuberculin skin tests; previously exposed to IFX or any other anti-TNF agent excluded | 62 sites. Geographical locations NR | PBO:121 randomised IFX 5 mg/kg:121 randomised IFX 10 mg/kg:121 randomised Received agent at weeks 0, 2, 6, 14, 22, 30, 38 and 46 |
No | Clinical response at week 8 | Week 8. Included in NMA? Yes | Weeks 30 and 54. Included in NMA? Yes | Schering-Plough |
| ACT2(NCT00036439), Rutgeerts et al., 200549 | Multicentre, randomised, double-blind, PBO-controlled trial. Phase III | Patients needed only have failed 5-ASA as a minimum. Active UC with a Mayo score of 6–12 points and endoscopy subscore of 2–3. Concurrent treatment with at least oral CSs, immunosuppressants or 5-ASAs. Patients could enrol if they failed to tolerate or respond to CSs, AZA, 6-MP or 5-ASA | 55 sites. Geographical locations NR | PBO:123 randomised IFX 5 mg/kg:121 randomised IFX 10 mg/kg:120 randomised Received agent at weeks 0, 2, 6, 14, 22, 30, 38 and 46: Received agent at weeks 0, 2, 6, 14, 22, 30, 38 and 46 |
No | Clinical response at week 8 | Week 8. Included in NMA? Yes | Week 30. Included in NMA? Yes | Schering-Plough |
| ACT1 and ACT2 extension studies, Reinisch et al., 201255 | Long-terms extension studies (open label) of ACT Phase III trials. Study design identical for ACT1 and ACT2 extension studies | Inclusion: patient eligibility described for ACT studies. Patients who (in opinion of investigator) could benefit from continued treatment eligible to enter extension study after completing main study treatment and assessments through weeks 46 and 54 (ACT1) or weeks 22 and 30 (ACT2). Exclusion: patients who received experimental medication to treat UC after completion of main study ineligible | See ACT1 and ACT2 | 229 randomised patients in IFX group entering extension studies. Participating patients continued to receive blinded treatment to which they had been randomised. Sites unblinded to treatment after week 54 ACT1 and extension week 24 in ACT2 analyses completed (and PBO patients discontinued at this point and not included in analyses) | Yes. Patients receiving IFX 10 mg/kg permitted to lower dose to 5 mg/kg. Patients losing response while receiving IFX 5 mg/kg permitted to raise dose to 10 mg/kg | NR | NA | Evaluation of long-term IFX maintenance to week 152 | As for ACT1 and ACT2 |
| Probert et al., 2003 (NCT number NR), Probert et al., 200350 | Multicentre, randomised, double-blind, PBO-controlled trial. Phase NR | Inclusion: patients with UCSS of ≥ 6 and a sigmoidoscopy score of ≥ 2 on Baron scale, failed to respond to conventional treatment with glucocorticoids. Exclusion: patients who had received ciclosporin, any therapeutic agent used to directly reduce TNF, or any investigational drug within 3 months of enrolment, as well as those who had recently commenced treatment (within the last 3 months) with 6-MP or AZA | UK and Germany. Four study centres | PBO = 20 randomised IFX 5 mg/kg = 23 Patients received i.v. IFX 5 mg/kg or PBO at week 0 and second identical infusion at week 2. At week 6, all patients with continued active UC offered open-label IFX 10 mg/kg |
Yes, from week 6 only (all patients with continued active UC offered open-label IFX 10 mg/kg) | Clinical remission at week 6 | Week 6 (clinical remission only; no clinical response data available for week 6). Included in NMA? No (excluded from NMA as definition of clinical remission inconsistent with other trials (i.e. total Mayo score of ≤ 2 points but does not specify no individual subscore of > 1 as in other trials) | NA. Included in NMA? No (no maintenance time points) | Schering-Plough and BMBF Competence Network (Germany) |
| UC-SUCCESS (NCT00537316), Panacionne et al., 201451 | Multicentre, randomised, double-blind (double-dummy), PBO-controlled trial. Phase III | Inclusion: patients with moderate to severe UC defined as Mayo scores of 6–8 and 9–12 points, respectively. Patients responded inadequately to course of CSs± mesalamine within past 12 weeks. Patients required to be either AZA-naive or free from AZA treatment for at least 3 months before enrolment. Prohibited medications at study entry included methotrexate and calcineurin inhibitors (tacrolimus, ciclosporin) | 62 study centre. Geographical locations NR | AZA = 80 randomised IFX = 79 randomised IFX/AZA = 80 randomised Patients received i.v. IFX 5 mg/kg at weeks 0, 2, 6 and 14 + oral once daily PBO capsules, or oral AZA 2.5 mg/kg daily + PBO i.v. on IFX schedule or combination therapy with both drugs |
Yes. Non-responders to AZA at week 8 had IFX rescue infusions at weeks 8, 10 and 14 while continuing AZA. Non-responders considered treatment failures | CS-free remission at week 16 | Week 8. Included in NMA? No (borderline inclusion and partial Mayo response only at week 8) | 16 weeks. Included in NMA? No (16 weeks of treatment only) | Schering-Plough |
| Trial identifier (NCT number), primary publication details | Trial design | Inclusion/exclusion criteria | Geographical location of study sites | Treatment groups and numbers randomised | Open label escape allowance? | Primary outcome | Assessment of induction | Assessment of maintenance | Study sponsor |
|---|---|---|---|---|---|---|---|---|---|
| IFX | |||||||||
| Hyams (NCT00336492, C0168T72), Hyams et al., 201252 | Randomised, Multicentre, open-label study. Phase III | Inclusion: patients aged 6–17 years old, with moderately to severely active UC, Mayo score of 6–12 points and endoscopy subscore of ≥ 2), failed to respond to adequate treatment/experienced medical complications/adverse effects from 5-ASAs immunomodulators (6-MP/AZA) or oral/i.v. CSs. Exclusion: patients with acute severe extensive UC and those who previously used other investigational drugs or any TNF antagonist | USA, the Netherlands, Canada and Belgium. 23 study centres | All patients received induction regimen of IFX 5 mg/kg at weeks 0, 2 and 6.45 patients who achieved clinical response at week 8 (primary endpoint) randomised to receive: IFX 5 mg/kg q8w = 22. IFX 5 mg/kg q12w = 23 | Yes. Patients losing response during maintenance eligible to increase IFX dose and/or frequency to set regimens. IFX 5 mg/kg q8w group to 10 mg/kg q8w. IFX 5 mg/kg q12w group to 10 mg/kg q8w, with those losing response between 8 and 12 weeks after previous infusion to 5 mg/kg q8w | Clinical response at week 8 | All patients received induction with IFX 5 mg/kg at week 0, 2 and 6 and clinical response and clinical remission assessed at week 8 (no PBO control for induction) | Week 54 (no PBO control for maintenance) | Janssen Research & Development |
Population: adults aged ≥ 18 years with moderately to severely active ulcerative colitis who have had an inadequate response to conventional therapy including corticosteroids and mercaptopurine or azathioprine, or who are intolerant to, or have medical contraindications against, such therapies
A total of nine relevant RCTs were identified which were performed in adult populations. Four RCTs evaluated the use of IFX [Active Ulcerative Colitis Trial (ACT)1,49 ACT2,49 Probert et al. 50 and UC-SUCCESS51], three RCTs were of ADA [Ulcerative colitis Long-Term Remission and maintenance with Adalimumab treatment of moderate to severe ulcerative colitis (ULTRA)1,44 ULTRA2,45 and Suzuki et al. , 201446] and two RCTs were of GOL [Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment (PURSUIT)-SC,47 PURSUIT-Maintenance48]. Four of these RCTs (ACT1,49 ACT2,49 ULTRA1,44 and ULTRA245) had long-term open-label extension studies associated with them (ACT1 and ACT2 extension studies,55 ULTRA354) that were also included as part of the evidence for these interventions. All of the included RCTs for adults were undertaken against a comparator of PBO, with the exception of UC-SUCCESS51 which assessed the use of IFX against active comparators of AZA and combination IFX/AZA. No head-to-head RCTs comparing interventions of interest against each other were identified for adults. All RCTs were Phase III (if stated), with the exception of Suzuki et al. 46 (Phase II/III) and PURSUIT-SC47 (Phase II/III). If stated, all included adult population trials were powered for the primary end points of clinical remission (ULTRA1,44 ULTRA2,45 Probert et al. ,50 UC-SUCCESS51) or clinical response (ACT1,49 ACT2,49 PURSUIT-SC,47 PURSUIT-Maintenance48). Where the geographical location(s) of study sites were reported, all trials were multicentre, international studies, with the exception of Probert et al. ,50 which was performed in the UK and Germany, and Suzuki et al. ,46 which was conducted exclusively in Japan. All trials were at least partly industry funded.
Eight trials included time points for the assessment of the use of interventions in achieving induction of clinical response or remission, of which four assessed IFX (ACT1,49 ACT2,49 Probert et al. 50 and UC-SUCCESS51), three assessed ADA (ULTRA1,44 ULTRA245 and Suzuki et al. 46) and one assessed GOL (PURSUIT-SC47). Six trials reported outcomes at time points for the evaluation of the use of interventions in the maintenance of clinical response or remission, consisting of three IFX trials (ACT1,49 ACT249 and UC-SUCCESS51), two ADA trials (ULTRA245 and Suzuki et al. 46) and one GOL trial (PURSUIT-Maintenance48).
None of the included RCTs applied Truelove and Witts’ disease severity criteria23 in their eligibility criteria (as referred to in the final NICE scope for this appraisal36 and as specified in the protocol). All included trials applied the Mayo score [except Probert et al. 50 for which the score was specified simply as Ulcerative Colitis Symptom Score (UCSS)] to classify the disease severity of potential participants (note: the UCSS was confirmed to be equivalent to Mayo score by Professor C Probert, University of Liverpool, 2014, personal communication). The included trials required a Mayo score of 6–12 points (with evidence of endoscopic disease) for participant eligibility. Mayo scores of 6–12 points were described in the included trial literature as moderate to severe disease and were also subsequently confirmed following clinical advice as representing moderate to severe disease (note: ad-hoc searches were performed to attempt to identify evidence relating to the relationship between the Truelove and Witts’ and Mayo disease severity indices; however, no evidence published in full text in English could be identified). Included trials required a varying range of prior use of conventional therapy for eligibility, as described in Tables 6 and 7. The UC-SUCCESS trial,51 which specified patients to be either AZA-naive or free from AZA treatment for at least 3 months before enrolment, was a borderline inclusion in the clinical effectiveness systematic review because the wording of the population in the scope and the licensed indications required prior use of AZA or 6-MP. However, as the trial reported the use of a stated (albeit low) proportion of prior immunosuppressant use, this trial was included in the clinical effectiveness systematic review for completeness. However, this trial was not eligible for subsequent inclusion in meta-analyses or NMAs. Suzuki et al. 46 included Japanese patients aged ≥ 15 years (ADA is not licensed in the paediatric population), but the mean ages of participants across treatment arms at baseline was 41.3–42.5 years.
The Core Outcome Measures in Effectiveness Trials (COMET) initiative,56 which promotes the use of core outcome sets in clinical trials, referenced the work by Cooney et al. 22 in classifying the use of outcome measures in UC clinical trials. Although acknowledging the very broad range of available disease severity/activity measures available, all adult population trials included in the assessment were consistent in their utilisation of the Mayo score as a measure of clinical response and/or remission. The included trial by Probert et al. 50 applied the UCSS in the evaluation of clinical remission at induction. This score is equivalent to the full Mayo score: the components of the UCSS are consistent with the elements assessed within the Mayo score (i.e. stool frequency, rectal bleeding, sigmoidoscopic appearance and physician’s global assessment) and also is referenced using the citation quoted for the Mayo score. 24 None of the included studies utilised the modified Truelove and Witts’ criteria23 (as referred to in the NICE appraisal scope36 and as specified in the assessment protocol) in their outcome assessments.
As recommended in the Committee for Human Medicinal Products (CHMP) guideline57 on the development of new medicinal products for the treatment of UC patients with confirmed UC were eligible for the included trials. Severity of disease was defined by clinical and endoscopic evaluation, as recommended in the CHMP guideline. Although the interventions of interest in this assessment were developed for the treatment of patients not responding/intolerant to previous immunomodulatory therapy, the Assessment Group did not consider that adequate definitions of inadequate response/intolerance were included in trials, as recommended by the CHMP guideline. The guideline recommended that, for refractory populations, a minimum duration and dose of previous baseline medication should be defined, but this was not the case in the included trials. In addition, intolerance was not defined by minimum criteria of severity in the trials. In terms of study duration, it was recommended that induction studies should be 8–12 weeks, but could be shorter based on the pharmacodynamic properties of the study drug. All induction trials assessed efficacy at 8 weeks, with the exception of the PURSUIT-SC GOL trial,47 which was a 6-week study. All maintenance studies were at least 1 year in length, as recommended in the CHMP guideline.
Adalimumab
ULTRA144 was a multicentre Phase III RCT in adults undertaken across the USA, Puerto Rico, Canada, Western Europe, and Eastern Europe. In the original protocol, 186 participants were randomised and in the amended protocol 390 were randomised (130 per group including PBO). Length of treatment was 12 weeks in the original protocol and 8 weeks in the amendment, and outcomes were reported at week 8. ULTRA245 was a multicentre Phase III RCT in adults undertaken across North America, Europe, Australia, New Zealand and Israel. A total of 518 patients entered the study, of which 258 were randomised to 160 mg/80 mg/40 mg of ADA and 260 were randomised to PBO. Outcomes were reported at week 8 and week 52. Suzuki et al. 46 was a 52-week Phase II/III trial in Japanese adults in which 274 participants were randomised to three treatment groups, including PBO. Outcomes were reported at 8 weeks and 52 weeks. The two induction ADA groups (one licenced dose and the other unlicensed) were combined as one active treatment group for outcomes at 52 weeks. ULTRA354 was the 156-week open-label extension study to ULTRA144 and ULTRA2. 45
Golimumab
PURSUIT-SC47 was a Phase II/III multicentre RCT in adults reporting outcomes at week 6. The trial was performed across 217 sites (Eastern Europe, 400 patients; North America, 278 patients; Asia Pacific and South Africa, 204 patients; and Western Europe and Israel, 183 patients). This was a dose-ranging study with 169 patients randomised to four groups, including PBO. PURSUIT-Maintenance48 was a Phase III RCT in adults across 251 sites (Eastern Europe, 477 patients; North America, 323 patients; Asia Pacific and South Africa, 237 patients; and Western Europe and Israel, 191 patients). Out of the 1228 patients who enrolled in PURSUIT, 464 were randomised to receive PBO (n = 156), 50 mg of GOL (n = 154) or 100 mg of GOL (n = 154). A total of 764 patients were not randomised: 129 were PBO non-responders, 230 were PBO induction non-responders and 405 were GOL induction non-responders.
Infliximab
ACT149 was a multicentre Phase III RCT conducted across 62 sites. A total of 364 adult patients were randomly assigned to licensed and unlicensed induction doses or PBO. ACT2 was a multicentre Phase III RCT across 55 sites. A total of 364 adult patients were randomly assigned to licensed and unlicensed maintenance doses or PBO. Probert et al. 50 was a RCT undertaken across four centres in the UK and Germany; 43 adult participants were randomised to IFX or PBO and outcomes assessed at week 6. UC-SUCCESS51 was a multicentre RCT undertaken in adults. A total of 239 participants were randomised to IFX, AZA or combination therapy (with no PBO group included). Outcomes were assessed at weeks 8 and 16.
Population: children and adolescents aged 6–17 years (inclusive) with severely active ulcerative colitis who have had an inadequate response to conventional therapy including corticosteroids and mercaptopurine or azathioprine, or who are intolerant to, or have medical contraindications against, such therapies
A single Phase III open-label RCT was identified for the paediatric population (T7252) which evaluated the use of IFX in maintenance therapy. All patients received the licensed IFX induction regimen before being randomised to one of two IFX maintenance regimens. Outcomes were reported at week 30 and week 54. No PBO-controlled or head-to-head RCTs were identified for children and young people. The absence of a PBO or non-IFX control group in the included RCT made it difficult to consider the effectiveness of IFX in paediatric patients compared with conventional UC therapies. This industry-funded trial had the primary end point of clinical response and was conducted in the USA, the Netherlands, Canada and Belgium. Eligible patients were 6–17 years of age with moderately to severely active UC and a Mayo score of 6–12 points with endoscopic evidence of disease. Therefore, although IFX is licensed in this age group with severe disease only (as reflected in the scope population), this trial was included in consideration of limited paediatric RCT evidence.
Quality of included evidence
All of the included trials were considered to be at low risk of selection bias as all trials reported an appropriate method for generating the randomisation sequence. Likewise, the majority of trials reported adequate information that allocation was concealed and were considered to be at low risk of bias for this domain. This was with the exception of two trials in which there was no information reported to make a judgement. These trials were therefore classified as being at unclear risk of bias. 46,52 Eight out of the 10 trials (see Figure 3) were considered to be at low risk of performance bias because there was reporting to indicate that participants and personnel were blinded to participants’ treatment allocation. Two trials were considered at unclear risk of bias for this domain; one because there was no clear statement in the trial report52 and one because the treatment regimen differed for non-responders at week 8 in AZA arm which could break the blinding. 51 Blinding of the outcome assessment was reported by five trials,44,45,47,48,50 all of which were considered at low risk of bias for this domain. The remaining five trials included no statement in the trial report and were considered at unclear risk for this domain (ACT1,49 ACT2,49 Hyams et al. ,52 UC-SUCCESS,51 Suzuki et al. 46).
All included trials were reported according to the intention-to-treat (ITT) principle. However, for two trials (ACT149 and ACT249), although ITT was reported, > 50% of patients in the PBO group and > 30% of patients in IFX groups did not complete the trial. Similarly, in another IFX trial,52 the numbers of patients withdrawing from the study were unbalanced across groups with > 50% of patients withdrawing from the every 12-week dose group. In the UC-SUCCESS trial,51 there was also a high level of attrition and an imbalance between treatment groups (AZA, 34%; IFX, 18%; IFX/AZA, 21%). In one of the ADA trials (ULTRA245), although ITT analysis was undertaken, there was a high level of attrition and an imbalance between treatment groups (PBO, 44.2%; ADA, 36.4%). In the GOL maintenance trial (PURSUIT-Maintenance48), withdrawal of > 10% was evident across all treatment groups. These trials were all considered to be at high risk of bias for this domain. Of note, the trial of ADA reported by Suzuki et al. 46 was considered at low risk of attrition bias for the induction phase (Figures 3 and 4). A high risk of attrition bias was evident for the maintenance phase (> 10% withdrawing). The maintenance active treatment group comprised participants receiving both licensed and unlicensed doses of ADA during induction (data not used in this report). Details of the numbers of participants withdrawing and reasons for withdrawal by trial are presented in Appendix 3. The extent of reporting of the reasons for withdrawals was variable between studies.
FIGURE 3.
Risk-of-bias summary: review authors’ judgements about each risk-of-bias item for each included study.
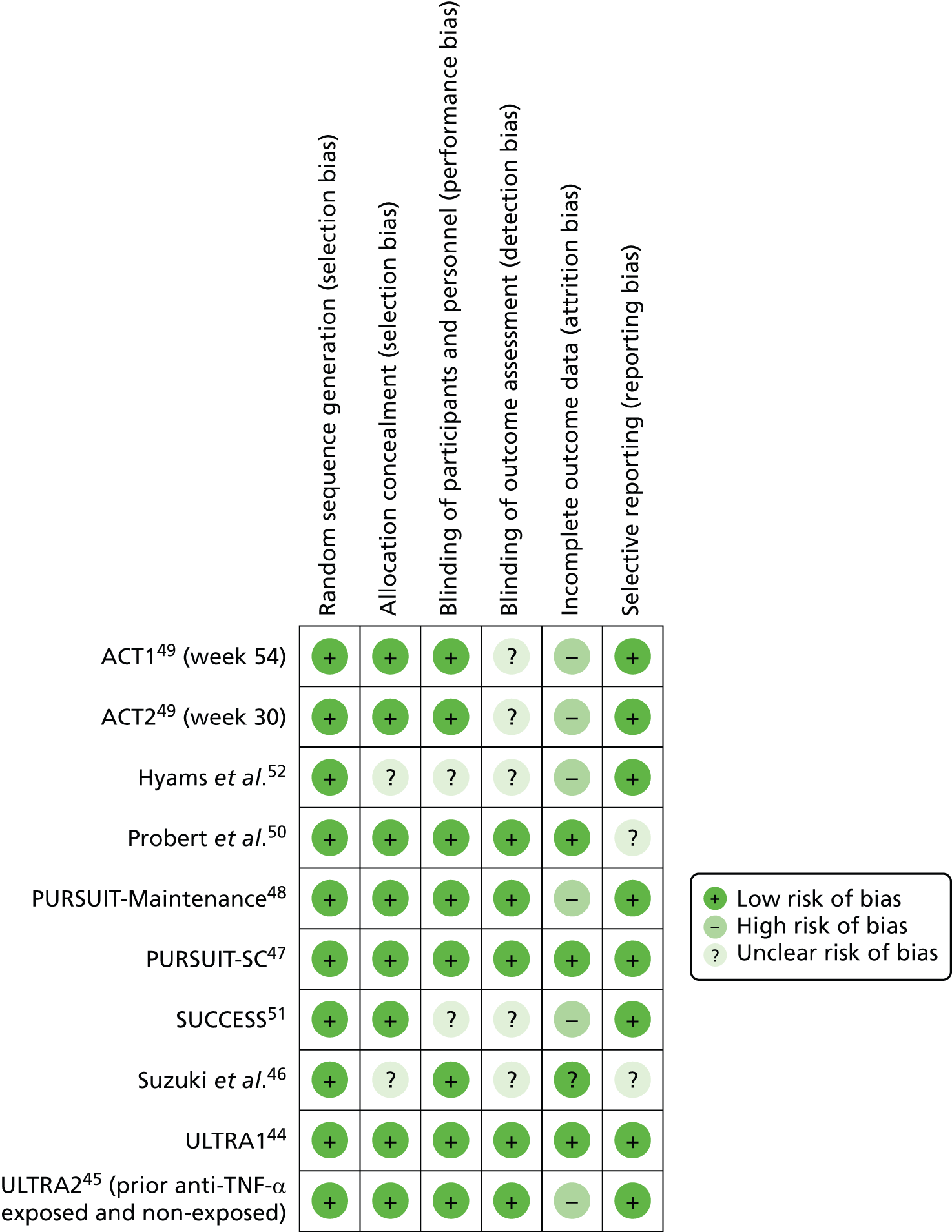
FIGURE 4.
Risk-of-bias graph: review authors’ judgements about each risk-of-bias item presented as percentages across all included studies.
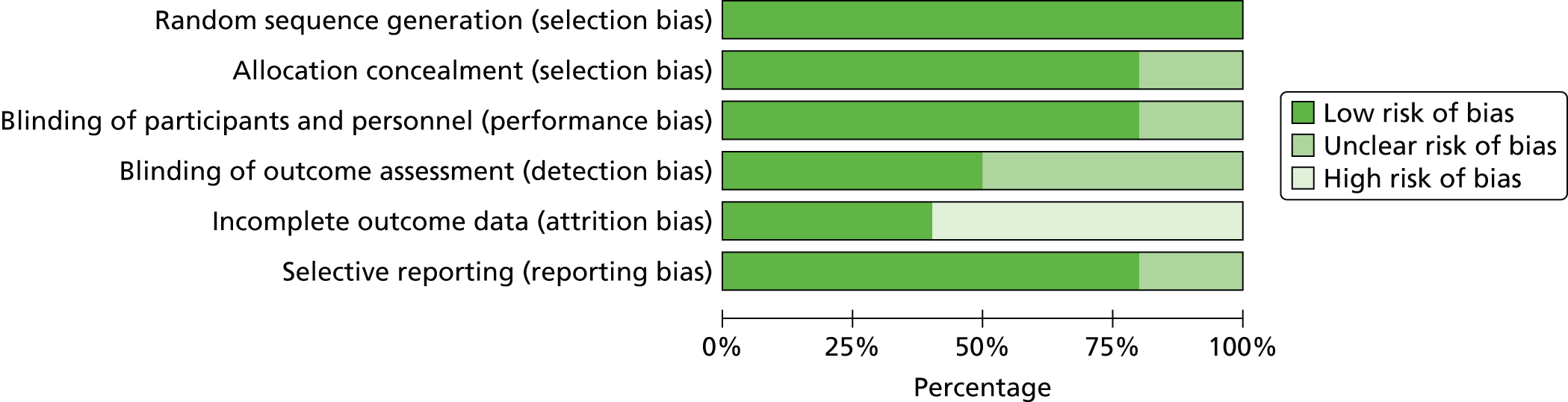
Selective outcome reporting was assessed based on ClinicalTrials.gov records, trial protocols and CSRs when provided by the manufacturers. Adequate data were available across ClinicalTrials.gov records and CSRs (available for some trials only) to compare outcomes with those reported in the associated peer-review publications for all included trials with the exception of Probert et al. 50 Stated primary outcomes were compared between published reports and trial protocols (for those RCTs for which trial protocols were provided by manufacturers). With the exception of Probert et al. 50 and Suzuki et al. ,46 all included RCTs were considered to be of at low risk of bias for this domain. Probert et al. 50 and Suzuki et al. ,46 were judged as being at unclear risk of selective reporting bias.
Population characteristics
The baseline characteristics of participants in the included RCTs are presented in Tables 8 and 9. In addition to comparator arm data, only data relating to licensed doses of interventions are presented.
| Trial identifier (NCT number), primary publication details | Age, mean years (SD) | Male participants (%) | Ethnicity, Caucasian (%) | Disease extent/location and disease duration, mean years (SD) | Disease severity at baseline: Mayo score, mean (SD) | CRP at baseline, mean mg/dl | Medications at baseline | Weight kg, mean (SD) | Smoking status |
|---|---|---|---|---|---|---|---|---|---|
| ADA | |||||||||
| ULTRA144 | Mean NR, median (range) PBO = 37 (18–72), ADA 160 mg/80 mg = 37 (18–75) | PBO = 63.1% ADA 160 mg/80 mg = 63.8% |
NR | PBO = extensive colitis,73/130 (56.2%); left-sided colitis, 42/130 (32.3%); other,15/130 (11.5%), duration median (range) 5.35 (0.3–34.1) ADA 160 mg/80 mg = extensive colitis, 60/130 (46.2%); left-sided colitis, 61/130 (46.9%); other, 9/130 (6.9%), duration median (range) 6.06 (0.2–34.4) |
PBO = 8.7 (1.56) ADA 160 mg/80 mg = 8.8 (1.61) |
Mean NR, median (range) PBO = 0.32, ADA 160 mg/80 mg = 0.33 | PBO = CS, 55/130 (41.5%); IMM, 18/130 (13.8%); CS + IMM, 34/130 (26.1%); no CS or IMM, 23/130 (17.7%); aminosalicylates, 98/130 (75.4%) ADA 160 mg/80 mg = CS, 48/130 (36.9%); IMM, 28/130 (21.5%); CS + IMM, 23/130 (17.7%); no CS or IMM, 31/130 (23.8%); aminosalicylates, 105/130 (80.8%) Dosages of concomitant UC medication(s) stable throughout study |
PBO = 78.7 (17.4) ADA 160 mg/80 mg = 75.5 (14.2) |
NR |
| ULTRA245 | PBO = 41.3 (13.22) ADA 160 mg/80 mg/40 mg = 39.6 (12.47) |
PBO = 152/246 (61.8%) ADA 160 mg/80 mg/40 mg = 142/248 (57.3%) |
NR | PBO = pancolitis, 120/248 (48.8%); descending colon, 96/246 (39.0%); other, 30/246 (12.2%), duration 8.5 (7.37) ADA 160 mg/80 mg/40 mg = pancolitis, 120/248 (48.4%); descending colon, 96/248 (38.7%); other, 32/248 (12.9%), duration 8.1 (7.09) |
PBO = 8.9 (1.75) ADA 160 mg/80 mg/40 mg = 8.9 (1.50) |
PBO = 1.3 ADA 160 mg/80 mg/40 mg = 1.5 |
PBO = CSs 140/246 (56.9%); AZA/6-MP 80/246 (32.5%); aminosalicylates 155/246 (63.0%); AZA/6-MP and/or steroids 175/246 (71.1%); AZA/6-MP and steroids 45/246 (18.3%) (41.1%) ADA 160 mg/80 mg/40 mg = CSs150/248 (60.5%); AZA/6-MP 93/248 (37.5%); aminosalicylates 146/248 (58.9%); AZA/6-MP and/or steroids 193/248 (77.8%); AZA/6-MP and steroids 50/248 (20.2%) Prior anti-TNF-α treatment PBO = 101/246 (41.1) ADA 160 mg/80 mg/40 mg = 98/248 (39.1) Concomitant UC medications held stable except steroids, which could be tapered after week 8 at discretion of investigator for patients with satisfactory clinical response |
PBO = 77.1 (17.31) ADA 160 mg/80 mg/40 mg = 75.3 (17.71) |
NR |
| Suzuki et al., 201446 | PBO = 41.3 (13.6) ADA 160 mg/80 mg/40 mg = 42.5 (14.6) |
PBO = 70/96 (72.9%) ADA 160 mg/80 mg/40 mg = 61/90 (67.8%) |
Exclusively Japanese | PBO = pancolitis, 59/96 (61.5%); descending colon, 35/96 (36.5); other, 2/96 (2.1%); duration 7.8 (6.6) ADA 160 mg/80 mg/40 mg = pancolitis, 63/90 (70.0%); descending colon, 27/90 (30.0); other 0/96 (0%); duration 7.8 (7.1) |
PBO = 8.5 (1.6) ADA 160/80/40 mg = 8.6 (1.4) |
Mean NR Median (range) PBO = 0.34 (0.05–8.72) ADA 160 mg/80 mg/40 mg = 0.22 (0.05–6.28) |
PBO = 5-ASAs, 89/96 (92.7%); IMMs (AZA, 6-MP), 52/96 (54.2%); systemic CSs, 58/97 (60.4%) ADA 160 mg/80 mg/40 mg = 5-ASAs, 83/90 (92.2%); IMMs (AZA, 6-MP), 41/90 (45.6%); systemic CSs, 57/90 (63.3%) Changes in doses of UC concomitant medications not permitted during study (other than CSs). After 8 weeks, patient responders permitted to taper CS dose |
PBO = 60.8 (14.1) ADA 160 mg/80 mg/40 mg = 60.1 (12.3) |
Tobacco non-smoker PBO = 55/96 (57.3%) ADA 160 mg/80 mg/40 mg = 50/90 (55.6%) |
| GOL | |||||||||
| PURSUIT-SC (all randomised patients), Sandborn et al., 201447 | PBO = 39.0 (13.04) GOL 200 mg/100 mg = 40.0 (13.54) |
PBO = 175/331 (52.9%) GOL 200 mg/100 mg = 180/331 (54.4%) |
PBO = 263/331 (79.5%) GOL 200 mg/100 mg = 271/331 (81.9%) |
PBO = n = 330, limited to left side of colon, 188/330 (57.0); extensive = 142/330 (43.0); duration 6.0 (6.65) GOL 200 mg/100 mg = n = 331, limited to left side of colon, 193/331 (58.3); extensive = 138/331 (41.7), duration 6.4 (6.17) |
PBO = 8.3 (1.50) GOL 200 mg/100 mg = 8.6 (1.53) |
PBO = 1.1 GOL 200 mg/100 mg = 1.1 |
PBO = patients receiving any UC medication (%) = 310/331 (93.7), CS (excluding budesonide) = 134/331 (40.5), ≥ 20 mg/d PEq = 78/331 (23.6), < 20 mg/d PEq = 56/331 (16.9), budesonide = 8/331 (2.4), immunomodulatory drugs = 106/331 (32.0), 6-MP/AZA = 102/331 (30.8), MTX = 4/331 (1.2), aminosalicylates = 276/331 (83.4) GOL 200 mg/100 mg = patients receiving any UC medication (%) = 302/331 (91.2), CS (excluding budesonide) = 142/331 (42.9), ≥ 20 mg/d PEq = 85/331 (25.7), < 20 mg/d PEq = 57/331 (17.2), budesonide = 6/331 (1.8), immunomodulatory drugs = 105/331 (31.7), 6-MP/AZA = 100/331 (30.2), MTX = 5/331 (1.5), aminosalicylates = 270/331 (81.6) Patients maintained stable doses of concomitant UC treatments during study |
NR | NR |
| PURSUIT- Maintenance (randomised patients), Sandborn et al., 201448 | PBO = 40.2 (14.05) GOL 50 mg = 41.4 (13.84) GOL 100 mg = 39.1 (13.11) |
PBO = 75/156 (48.1%) GOL 50 mg = 77/154 (50.0%) GOL 100 mg = 89/154 (57.8%) |
PBO = 137/156 (87.8%) GOL 50 mg = 138/154 (89.6%) GOL 100 mg = 130/154 (84.4%) |
Disease extent/location NR PBO = mean 6.9 (6.96), median 4.2 (IQR NR) GOL 50 mg = mean 6.8 (6.93), median 4.5 (IQR NR) GOL 100 mg = mean 7.2 (7.04), median 4.8 (IQR NR) |
PBO = 8.3 (1.37) GOL 50 mg = 8.1 (1.38) GOL 100 mg = 8.5 (1.34) |
PBO = 1.0 GOL 50 mg = 0.9 GOL 100 mg = 0.9 |
PBO = Any UC medication = 148 (94.9), CS = 83 (53.2) (excluding budesonide), ≥ 20 mg/day PEq = 59 (37.8), < 20 mg/day PEq = 24 (15.4), budesonide = 5 (3.2), immunomodulatory drugs = 52 (33.3), 6-MP/AZA = 51 (32.7), MTX = 1 (0.6), 5-ASA = 125 (80.1) GOL 50 mg = any UC medication = 144 (93.5), CS = 77 (50.0), ≥ 20 mg/day PEq = 52 (33.8), < 20 mg/day PEq = 25 (16.2), budesonide 6 (3.9), immunomodulatory drugs = 47 (30.5), 6-MP/AZA = 45 (29.2), MTX = 2 (1.3), 5-ASA = 128 (83.1) GOL 100 mg = Any UC medication = 143 (92.9), CS = 79 (51.3), ≥ 20 mg/day PEq = 55 (35.7), < 20 mg/day PEq = 24 (15.6), budesonide = 4 (2.6), immunomodulatory drugs = 48 (31.2), 6-MP/AZA = 48 (31.2), MTX = 0, 5-ASA = 119 (77.3) Patients receiving 5-ASAs/immunosuppressants at baseline of induction studies required to have held stable doses during induction and maintenance. After induction, patients in clinical response receiving concomitant CSs at baseline of PURSUIT-Maintenance required to taper CSs |
NR | NR |
| IFX | |||||||||
| ACT1 Rutgeerts et al., 200549 |
PBO = 41.4 (13.7) IFX 5 mg/kg = 42.4 (14.3) |
PBO = 72/121 (59.5%) IFX 5 mg/kg = 78/121 (64.5%) |
PBO = 111/121 (91.7%) IFX 5 mg/kg = 116/121 (95.9%) |
PBO = left side, 66/120 (55.0%); extensive, 54/120 (45.0%); duration, 6.2 (5.9) IFX 5 mg/kg = left side, 63/121 (52.9%); extensive, 56/119 (47.1%); duration, 5.9 (5.4) |
PBO = 8.4 (1.8) IFX 5 mg/kg = 8.5 (1.7) |
PBO = 1.7 (2.7) IFX 5 mg/kg = 1.4 (1.9) |
PBO = CS, 79 (65.3%); ≥ 20 mg/day, 54 (44.6%); 5-ASA, 85 (70.2%); IMM, 53 (43.8%); AZA, 36 (29.8%); MP, 17 (14.0%). CS-refractory disease, 38 (31.4%) IFX 5 mg/kg = CS, 70 (57.9%); ≥ 20 mg/day, 45 (37.2%); 5-ASA, 82 (67.8%); IMM, 66 (54.5%); AZA, 45 (37.2%); MP, 21 (17.4%). CS-refractory disease, 36 (29.8%) Doses of concomitant medications kept stable apart from CS, tapered by 5 mg/week after week 8 until dose of 20 mg/day reached, thereafter dose reduced by 2.5 mg/week until discontinuation |
PBO = 76.8 (16.2) IFX 5 mg/kg = 80.0 (17.8) |
Current smoker PBO = 7/121 (5.8%) IFX 5 mg/kg = 2/121 (1.7%) |
| ACT2 Rutgeerts et al., 200549 |
PBO = 39.3 (13.5) IFX 5 mg/kg = 40.5 (13.1) |
PBO = 71/123 (57.7%) IFX 5 mg/kg = 76/121 (62.8%) |
PBO = 117/123 (95.1%) IFX 5 mg/kg = 116/121 (95.9%) |
PBO = left side, 70/120 (58.3%); extensive, 50/120 (41.7%); duration, 6.5 (6.7) IFX 5 mg/kg = left side, 70/118 (59.3%); extensive, 48/118 (40.7%); duration, 6.7 (5.3) |
PBO = 8.5 (1.5) IFX 5 mg/kg = 8.3 (1.5) |
PBO = 1.6 (2.9) IFX 5 mg/kg = 1.3 (2.3) |
PBO = CS, 60 (48.8%); ≥ 20 mg/day, 43 (35.0%); 5-ASA, 89 (72.4%); IMM, 54 (43.9%); AZA, 35 (28.5%); MP, 19 (15.4). CS-refractory disease, 36 (29.3) IFX 5 mg/kg = CS, 60 (49.6%), ≥ 20 mg/day, 40 (33.1%), 5-ASA, 92 (76.0%), IMM, 52 (43.0%), AZA, 41 (33.9%), MP, 11 (9.1). CS-refractory disease, 35 (28.9) Doses of concomitant medications kept stable apart from CS, tapered by 5 mg/week after week 8 until dose of 20 mg/day reached, thereafter dose reduced by 2.5 mg/week until discontinuation |
PBO = 76.1 (17.4) IFX 5 mg/kg = 78.4 (17.8) |
Current smoker PBO = 6/123 (4.9%) IFX 5 mg/kg = 8/121 (6.6%) |
| Probert et al., 200350 | Mean NR Median (IQR) PBO = 40 (29–43.5) IFX 5 mg/kg = 41 (35.5–50.5) |
NR | NR | PBO = extensive UC, 13/20, left-side, 3/20, distal colitis, 4/20; median (IQR) duration 59 (35–96) months IFX 5 mg/kg = extensive UC, 14/23, left-side 5/23, distal colitis 4/23; median (IQR) duration 75 (39–141) months |
Mean (SD) UC severity score PBO = 8.5 (2) IFX 5 mg/kg = 8 (2) Mean (SD) Baron score PBO = 2.4 (0.5) IFX 5 mg/kg = 2 (0.5) |
PBO = 12 (10) IFX 5 mg/kg = 9 (9) |
PBO = AZA use, 7/20 (35%); prednisolone equivalent (mg/day), mean 28 (7 SD), median 30 (IQR 25–30); duration of steroid treatment (days), median 28 (IQR 14–45) 28 (11.5–42) IFX 5 mg/kg = AZA use, 6/23 (26%); prednisolone equivalent (mg/day), mean 32 (11 SD), median 30 (IQR 30–30); duration of steroid treatment (days), median 28 (IQR 11.5–42) Doses of 5-ASA and AZA/6-MP kept stable during study. Glucocorticoids kept stable during screening then permitted to be changed ‘according to clinical demands’, with goal of reducing daily dose by 5 mg prednisolone equivalent per week |
Mean NR Median (IQR) PBO = 72 (60–8 as reported) IFX 5 mg/kg = 66 (61–78) |
NR |
| UC-SUCCESS Panaccione et al., 201451 |
AZA = 40.7 (13.2) IFX 5 mg/kg = 38.5 (12.7) IFX 5 mg/kg + AZA = 38.0 (12.2) |
AZA = 33/79 (41%) IFX 5 mg/kg = 42/78 (54%) IFX 5 mg/kg + AZA = 48/80 (60%) |
NR | Disease extent/duration NR Disease duration AZA = 6.6 (7.8) IFX 5 mg/kg = 6.3 (6.5) IFX 5 mg/kg + AZA = 5.2 (5.1) |
AZA = 8.5 (1.4) IFX 5 mg/kg = 8.1 (1.4) IFX 5 mg/kg + AZA = 8.6 (1.3) |
NR | AZA = CS use, 27/79 (34.2%); prior immunomodulatory therapy, 8/79 (10%) IFX 5 mg/kg = CS use, 31/78 (39.7%); prior immunomodulatory therapy, 8/78 (10.3%) IFX 5 mg/kg + AZA = CS use, 38/80 (47.5%); prior immunomodulatory therapy, 8/80 (10.0%) Baseline concomitant treatments kept stable during study. Patients receiving CSs at baseline tapered to 0 mg by week 14 unless medically contraindicated |
NR | NR |
| Trial identifier (NCT number), primary publication details | Age, mean years (SD) | Male participants (%) | Ethnicity, Caucasian (%) | Disease extent/location and disease duration, mean years (SD) | Disease severity at baseline: Mayo score, mean (SD) | CRP at baseline, mean mg/dl | Medications at baseline | Weight kg, mean (SD) | Smoking status |
|---|---|---|---|---|---|---|---|---|---|
| Hyams et al., 201252 (NCT0033649, C0168T72) | Mean NR Median (IQR) Maintenance IFX 5 mg/kg every 8 weeks = 15.0 (12.0–16.0) Maintenance IFX 5 mg/kg every 12 weeks = 15.0 (12.0–16.0) |
Maintenance IFX 5 mg/kg every 8 weeks = 10/22 (45.5%) Maintenance IFX 5 mg/kg every 12 weeks = 10/23 (43.5%) |
Maintenance IFX 5 mg/kg every 8 weeks = 20/22 (90.9%) Maintenance IFX 5 mg/kg every 12 weeks = 19/23 (82.6%) |
Maintenance IFX 5 mg/kg every 8 weeks = left side of colon, 6/22 (27.3%); extensive, 16/22 (72.7%); duration median (IQR) 1.8 (0.6–2.4) Maintenance IFX 5 mg/kg every 12 weeks = left side of colon, 4/23 (17.4%); extensive, 19/23 (82.6%); duration median (IQR) 1.1 (0.6–1.9) |
Mean NR Median (IQR) Maintenance IFX 5 mg/kg every 8 weeks = 7.5 (7.0–9.0); median PUCAI (IQR) 50.0 (35.0–55.0) Maintenance IFX 5 mg/kg every 12 weeks = 8.0 (7.0–10.0); median PUCAI (IQR) 57.5 (50.0–65.0) |
Mean NR Median (IQR) Maintenance IFX 5 mg/kg every 8 weeks = 0.3 (0.3–1.5) Maintenance IFX 5 mg/kg every 12 weeks = 0.3 (0.3–2.2) |
Maintenance IFX 5 mg/kg every 8 weeks = at least 1 concomitant medication, 22/22 (100%); CSs (parenteral or oral), 14/22 (63.6%); ≤ 1 mg/kg prednisone-equivalent, 10/22 (45.5%); ≤ 1 mg/kg prednisone-equivalent, 4/22 (18.2%); CSs (budesonide), 1/22 (4.5%); CSs (rectal), 2/22 (9.1%); immunomodulatory agents, 11/22 (50.0%); 6-MP/AZA, 10/22 (45.5%); MTX,1/22 (4.5%); aminosalicylates, 10/22 (45.5%); antibiotics, 0/22 (0%) Maintenance IFX 5 mg/kg every 12 weeks = at least 1 concomitant medication, 23/23 (100%); CSs (parenteral or oral), 14/23 (60.9%); ≤ 1 mg/kg prednisone-equivalent, 10/23 (43.5%); > 1 mg/kg prednisone-equivalent, 4/23 (17.4%); CSs (budesonide), 0/23 (0%); CSs (rectal),1/23 (4.3%); immunomodulatory agents, 13/23 (56.5%); 6-MP/AZA, 11/23 (47.8%); MTX, 2/23 (8.7%); aminosalicylates, 12/23 (52.2%); antibiotics, 0/23 (0%) UC therapies to remain stable, CS could be tapered if clinically indicated |
Maintenance IFX 5 mg/kg every 8 weeks = 51.54 (18.294) [median 50.40 (range 26.2 to 91.6, IQR 36.10–61.50)] Maintenance IFX 5 mg/kg every 12 weeks = 52.80 (16.855) [median 52.30 (range 24.5–86.4, IQR 40.30–68.60)] |
NR |
Population: adults aged ≥ 18 years with moderately to severely active ulcerative colitis who have had an inadequate response to conventional therapy including corticosteroids and mercaptopurine or azathioprine, or who are intolerant to, or have medical contraindications against, such therapies
Mean and median reported ages of participants were considered consistent across included adult population trials, ranging from 37 to 42.5 years. Mayo scores at baseline were also consistent across trials and spanned from 8.1 to 8.9. Average proportions of male participants ranged from 41% to 73% and the majority of included patients (when reported) were Caucasian in ethnicity (79.5–95.9%), with the exception of the Suzuki et al. 46 study, which included exclusively Japanese patients. Mean and median disease duration of participants ranged from 59 months (4.9 years) to 8.5 years. Conventional UC medications at baseline were variable between the included trials. In none of the included studies had all participants previously been trialled on corticosteroids and AZA or 6-MP, as required by the wording used in the final NICE scope36 population and the wording of the European Medicines Agency (EMA) licensing for IFX, ADA and GOL. Although it is noted that AZA and 6-MP may be used more typically in clinical practice as maintenance therapies owing to their longer initiation of effect, it is debatable whether or not the included trial populations would represent patients who had failed or were intolerant to previous conventional therapies. All trials related to anti-TNF-α-naive populations, with the exception of ULTRA245 (which permitted the inclusion of anti-TNF-α experienced patients) and PURSUIT-Maintenance48 (in which patients responding to prior GOL induction therapy were randomised to GOL maintenance regimens or PBO). Data at induction were reported according to anti-TNF-α experience. Data relating to patients who were anti-TNF-α-naive for maintenance time points were requested and received from the manufacturer of ADA (AbbVie). Weight and smoking status were both relatively poorly reported across included studies.
Population: children and adolescents aged 6–17 years (inclusive) with severely active ulcerative colitis who have had an inadequate response to conventional therapy including corticosteroids and mercaptopurine or azathioprine, or who are intolerant to, or have medical contraindications against, such therapies
The included trial population52 averaged 15 years of age in both treatment groups, was 43.5–45.5% male and mostly Caucasian in ethnic origin (82.6–90.9%) with average disease duration of 1.1–1.8 years. Patients had a median Mayo score of 7.5 to 8.0 points and a median PUCAI score of 50–57.5 points, for which a PUCAI of score of ≥ 65 would indicate severe disease (and, therefore, were a mixture of patients with moderate and severe disease, while IFX is licensed in paediatric patients with severe disease only). Participants were required to have had prior use of at least one conventional therapy (with 61% to 64% receiving corticosteroids, 50% to 57% immunosuppressants and 46% to 52% aminosalicylates at baseline).
Assessment of effectiveness
Population: adults aged ≥ 18 years with moderately to severely active ulcerative colitis who have had an inadequate response to conventional therapy including corticosteroids and mercaptopurine or azathioprine, or who are intolerant to, or have medical contraindications against, such therapies
Rates of and duration of response, relapse and remission
Clinical response and remission data were well reported across the included trials for the adult population. It was assumed by the Assessment Group that the numbers of patients who were reported in the trial publications as being in clinical response also included those patients who were in clinical remission. Data relating to transitions of patients between no response, response and remission categories at maintenance time points were requested and received from the manufacturers (MSD and AbbVie). The induction trial data (as reported in the trial publications) and maintenance transition data (received from the manufacturers) from eligible trials were analysed using NMA methods (see page 69). Definitions of clinical response and clinical remission used in the included trials are presented in Table 10.
| Trial | Definition of clinical response | Definition of clinical remission | Measurement time points |
|---|---|---|---|
| ACT149 | Decrease from baseline in total Mayo score of ≥ 3 points and ≥ 30%, with accompanying decrease in subscore for rectal bleeding of ≥ 1 point or absolute rectal bleeding subscore of 0 or 1 | Total Mayo score of ≤ 2 points, with no individual subscore of > 1 | Clinical response and remission assessed at weeks 8, 30 and 54 |
| ACT249 | As above | As above | Clinical response and remission assessed at weeks 8 and 30 |
| Probert et al., 200350 | No definition of response, mean UC ‘severity score’ and improvement reported only | UCSS (i.e. Mayo score). Clinical remission = UCSS ≤ 2 | Outcomes reported at week 2 and 6 |
| UC-SUCCESS51 | Decrease in total Mayo score of ≥ 3 points and ≥ 30% decrease from baseline Mayo score | CS-free remission = total Mayo score of ≤ 2 points, with no individual subscore of > 1 point without the use of CSs | Mayo scores assessed at weeks 0, 8 (partial Mayo) and 16 |
| ULTRA144 | Decrease in Mayo score of ≥ 3 points and ≥ 30% from baseline plus decrease in subscore for rectal bleeding of ≥ 1 point or absolute rectal bleeding subscore of 0 or 1 | Mayo score of ≤ 2 points, with no individual subscore of > 1 | Mayo scores recorded at weeks 0 and 8 |
| ULTRA245 | Decrease from baseline in total Mayo score of ≥ 3 points and ≥ 30% plus decrease in subscore for rectal bleeding of ≥ 1 point or absolute rectal bleeding subscore of 0 or 1 | Total Mayo score of ≤ 2 points, with no individual subscore of > 1 | Clinical response and remission measured at weeks 8, 32 and 52/early termination |
| Suzuki et al., 201446 | Decrease from baseline in total Mayo score of ≥ 3 points and ≥ 30%, with accompanying decrease in subscore for rectal bleeding of ≥ 1 point or absolute rectal bleeding subscore of ≤ 1 | Total Mayo score of ≤ 2 points, with no individual subscore of > 1 | Clinical response and remission assessed at weeks 8, 32 and 52 |
| PURSUIT-SC47 | Decrease from baseline in Mayo score of ≥ 3 points and ≥ 30% plus decrease in subscore for rectal bleeding of ≥ 1 point or absolute rectal bleeding subscore of 0 or 1 | Mayo score of ≤ 2 points, with no individual subscore of > 1 | Mayo scores recorded at weeks 0 and 6 |
| PURSUIT-Maintenance48 | Decrease from baseline value (observed in preceding induction study) in Mayo score of ≥ 3 points and ≥ 30% plus decrease in subscore for rectal bleeding of ≥ 1 point or absolute rectal bleeding subscore of 0 or 1 | Mayo score of ≤ 2 points, with no individual subscore of > 1 | Mayo scores calculated at weeks 0, 30 and 54 |
Four ADA trials presented clinical response and remission data (ULTRA1,44 ULTRA2,45 ULTRA354 and Suzuki et al. 46).
At week 8, more patients in the ADA 160 mg/80 mg induction treatment arm of ULTRA144 achieved clinical response (54.6% vs. 44.6%; p-value not reported) and twice as many reached clinical remission (18.5% vs. 9.2%; p = 0.031) than PBO patients. 44 Subgroup analyses demonstrated that patients with a Mayo score of ≥ 10 points at baseline of ULTRA1 were less likely to achieve remission at week 8 than patients with lower baseline Mayo scores. 44 Baseline C-reactive protein (CRP) levels > 10 mg/l and baseline weight of ≥ 82 kg were also linked with lower remission rates in ULTRA1. 44 When baseline prior UC medications were considered, the treatment effect of 160 mg/80 mg of ADA compared with PBO was most pronounced in patients who had received immunomodulator treatment (i.e. AZA/6-MP) at baseline without corticosteroids, and patients who had received no prior aminosalicylates. 44 Clinical response rates at week 8 in the PBO group, when stratified by geographical region, appeared to be higher in Canada and Eastern Europe (than in USA/Puerto Rico and Western Europe) although reasons for this are unclear. 44
In ULTRA2,45 patients in the ADA 160 mg/80 mg induction group were more likely to achieve clinical response (50.4% vs. 34.6%; p < 0.005) and clinical remission (16.5% vs. 9.3%; p < 0.05) at week 8 than in the PBO group. 45 Similarly, among patients who had received no prior anti-TNF-α treatment, greater proportions of patients in the ADA 160 mg/80 mg induction group reached clinical response (59.3% vs. 38.6%; p < 0.005) and clinical remission (21.3% vs. 11.0%; p < 0.05) at week 8 than PBO-treated patients. 45 Patients receiving ADA as maintenance therapy in ULTRA245 were also more likely at week 52 to be in clinical response (30.2% vs. 18.3%; p < 0.05) or clinical remission (17.3% vs. 8.5%; p < 0.005) than subjects in the PBO group. 45 Anti-TNF-α-naive ADA-treated patients were also more likely to achieve clinical response (36.7% vs. 24.1%; p = 0.019) or remission (22.0% vs. 12.4%; p = 0.029) at week 52 than those in the PBO group. 45 Patients in the ADA group were more likely to achieve sustained response (ITT 21.8%, anti-TNF-α-naive 26.7%; both p < 0.05 vs. PBO) and sustained remission (ITT 8.1%, anti-TNF-α-naive 10.7%; both p < 0.05) than PBO group subjects (sustained response: ITT 11.4%, anti-TNF-α-naive 16.6%; sustained remission ITT 2.4%, anti-TNF-α-naive 3.4%). 58 At week 52 of ULTRA2, corticosteroid-free remission was achieved by more ADA group patients versus PBO (both p < 0.05). 59 A post-hoc analysis of ULTRA245 data at week 52, which included patients from the PBO arm who switched to ADA, demonstrated that mean days in clinical response (134.58 vs. 94.55; p < 0.001) and mean days in clinical remission were also greater for ADA-treated patients (85.32 vs. 52.87; p < 0.001). 60 For patients with no prior anti-TNF-α use, stool frequency and rectal bleeding Mayo subscores of ≤ 1 point at week 8 were most likely to be achieved in patients receiving ADA than PBO (both p < 0.05). 45 At week 52, the proportions of patients who had discontinued corticosteroid use and achieved sustained clinical remission at both weeks 32 and 52 (among patients with baseline corticosteroid use) were 10.0% and 1.2% in the ADA (no prior anti-TNF-α use) and PBO groups, respectively (p = 0.014). 45 At week 52, for patients with no prior anti-TNF-α use, 20.3% of the ADA group and 6.2% of the PBO group were in corticosteroid-free clinical remission (p < 0.05). 59
The open-label extension study ULTRA354 presented the 4-year efficacy and safety results of 588 patients from ULTRA144 and ULTRA245 who were followed. Of the 588 patients who entered the ULTRA354 extension study, 52.2% (307/588) were in remission at entry according to partial Mayo scores. Partial Mayo scores were calculated at each study visit and at week 156, 46.4% (273/588) of patients had achieved clinical remission.
Patients who received ADA for induction in the Suzuki et al. 46 trial were more likely to be in clinical response (50% vs. 35%; p < 0.05) by week 8 but not in clinical remission (10% vs. 11%; p-value not reported) than PBO group patients. 46 At week 8, a statistically significant greater proportion of patients in the ADA arm reached a subscore of ≤ 1 for physician’s global assessment domain than in the PBO arm (p ≤ 0.05); differences in the other Mayo subscores were not statistically significant. 46 Within the Suzuki et al. 46 trial, greater proportions of ADA maintenance-treated patients were in clinical response than PBO group patients (31% vs. 18%; p < 0.05) and clinical remission (23% vs. 7%; p < 0.01) through week 52. At week 52, a greater proportion of subjects in the ADA group versus PBO experienced subscores of ≤ 1 point for physician’s global assessment and stool frequency subscore (both p ≤ 0.05). 46 The proportions of patients in steroid-free clinical remission at week 52 were 14.2% and 6.9% in the ADA and PBO arms, respectively (p-value not reported). 46
In the PURSUIT-SC induction trial,47 clinical response and remission data were reported for both Phase II and Phase III. By week 6, in the Phase II analyses (plus additional Phase II randomised patients), more patients receiving GOL were in clinical response (46.5% vs. 37.7%; p-value not reported) and remission (18.3% vs. 10.1%; p-value not reported) than the PBO group. Similarly, more GOL-treated patients achieved clinical response (51.0% vs. 30.3%; p < 0.0001) and remission (17.8% vs. 6.4%; p < 0.0001) than PBO-treated patients by week 6 in the Phase III analyses.
In the PURSUIT-Maintenance study,48 proportions of patients maintaining clinical response (47.0% vs. 31.3%; p = 0.010) and in clinical remission [33.1% (50 mg of GOL; p = 0.068), 33.8% (100 mg of GOL, p = 0.011) vs. 22.1%] through week 54 were larger for the GOL groups than PBO. PURSUIT-Maintenance patients who maintained clinical response and were corticosteroid-free among those who receiving corticosteroids at maintenance baseline were 38.5% in the 50 mg of GOL group (p = 0.026), 30.5% in the 100 mg of GOL group (p = 0.138) and 20.7% in the PBO group.
By week 8 of the ACT1 trial,49 more patients treated with 5 mg/kg of IFX were in clinical response (69.4% vs. 37.2%; p < 0.001) and remission (38.8% vs. 14.9%; p < 0.001) than those who received PBO. At week 54, more IFX group patients were in clinical response (45.5% vs. 19.8%; p < 0.001) and remission (34.7% vs. 16.5%; p = 0.001) than PBO-treated subjects. 49 Patients who sustained clinical response at weeks 8, 30 and 54 were 38.8% in the IFX group and 14.0% in the PBO group (p < 0.001). 49 Proportions of patients who sustained clinical remission at weeks 8, 30 and 54 were 19.8% and 6.6% in the IFX and PBO treatment arms, respectively (p = 0.002). 49 Of the 5 mg/kg of IFX group, 25.7% were in clinical remission and had discontinued corticosteroids at week 54, compared with 8.9% in the PBO group (p = 0.006). 49
In ACT2,49 more patients in the 5 mg/kg of IFX group were in clinical response (64.5% vs. 29.3%; p < 0.001) and remission (33.9% vs. 5.7%; p < 0.001) at week 8 compared with PBO. By week 30, more 5 mg/kg of IFX-treated patients were in clinical response (47.1% vs. 26.0%; p < 0.001) and remission (25.6% vs. 10.6%; p = 0.003) than PBO. 49 The proportions of patients who sustained clinical response (41.3% vs. 15.4%; p < 0.001) and clinical remission (14.9% vs. 2.4%; p < 0.001) at weeks 8 and 30 were also higher in the 5 mg/kg of IFX group than patients receiving PBO. 49
No statistically significant differences were observed between the IFX and PBO treatment groups through week 6 of the Probert et al. 50 trial in terms of clinical remission (as defined by a UCSS of ≤ 2) (39% vs. 30%; p = 0.76). Remission rates among patients receiving AZA were 67% for IFX and 33% for PBO groups (p = 0.89). 50
A greater proportion of patients in the UC-SUCCESS study51 who received combination treatment with IFX plus AZA were in steroid-free clinical remission at week 16 (39.74%) than in the IFX monotherapy (22.08%, p vs. IFX = 0.017) and AZA monotherapy (23.68%, p vs. IFX = 0.813; p vs. IFX/AZA = 0.032) groups. 51
No included trials reported data on rates or duration of relapse.
Data relating to clinical response and remission are summarised in Table 11.
| Study name | Treatment arm | Time point | Rates of and duration of response | Rates of and duration of remission |
|---|---|---|---|---|
| ULTRA144 | PBO | Week 8 | Clinical response: 58/130 (44.6%) (p-value NR) | Clinical remission: ITT-A3 protocol: 12/130 (9.2%) |
| ULTRA144 | 160 mg/80 mg of ADA | Week 8 | Clinical response: 71/130 (54.6%) | Clinical remission: ITT-A3 protocol: 24/130 (18.5%), p-value vs. PBO = 0.031 |
| ULTRA245 | PBO | Week 52 | Patients with response: 45/246 (18.3%) No prior anti-TNF-α: clinical response 35/145 (24.1%) Prior anti-TNF-α clinical response 10/101 (9.9%) |
Patients with remission: 21/246 (8.5%) No prior anti-TNF-α: clinical remission 18/145 (12.4%) Prior anti-TNF-α clinical remission 3/101 (3.0%) |
| ULTRA245 | 160 mg/80 mg of ADA | Week 52 | Patients with response: 75/248 (30.2%) No prior anti-TNF-α: clinical response 55/150 (36.7%) Prior anti-TNF-α clinical response 20/98 (20.4%) |
Patients with remission: 42/248 (17.3%) No prior anti-TNF-α: clinical remission 33/150 (22.0%) prior anti-TNF-α clinical remission 10/98 (10.2%) |
| Suzuki et al.46 | PBO | Week 8 | Full Mayo score response: 34/96 (35%) | Full Mayo score remission: 11/96 (11%) |
| Suzuki et al.46 | 160 mg/80 mg of ADA | Week 8 | Full Mayo score response: 45/90 (50%): p-value vs. PBO ≤ 0.05 | Full Mayo score remission: 9/90 (10%) |
| Suzuki et al.46 | PBO | Week 52 | Full Mayo score response: 17/96 (18%) | Full Mayo score remission: 7/96 (7%) |
| Suzuki et al.46 | ADA 80 mg/40 mg or ADA 160 mg/80 mg to week 8 then ADA 40 mg EOW | Week 52 | Full Mayo score response: 55/177 (31%); p-value vs. PBO, ≤ 0.05 | Full Mayo score remission: 41/177 (23%); p-value vs. PBO, ≤ 0.01 |
| PURSUIT-SC47 | Phase III PBO | Week 6 | Phase III. PBO. Proportion with clinical response: 76/251 (30.3%) | Phase III. Clinical remission: 16/251 (6.4%) |
| PURSUIT-SC47 | Phase III GOL 200 mg/100 mg phase III | Week 6 | Phase III. GOL 200 mg/100 mg. Proportion with clinical response: 129/253 (51.0%) (p < 0.0001) | Phase III. Clinical remission: GOL 200/100, 45/253 (17.8) (p < 0.0001) |
| PURSUIT-Maintenance48 | PBO | Week 54 | Proportion of patients maintaining clinical response: 31.2%, n = 154 | Clinical remission: 34/154 (22.1%) |
| PURSUIT-Maintenance48 | 50 mg of GOL | Week 54 | Proportion of patients maintaining clinical response: 47.0%, n = 151 (p = 0.010) | Clinical remission: 50/151 (33.1%) (p = 0.068) |
| PURSUIT-Maintenance48 | 100 mg of GOL | Week 54 | Proportion of patients maintaining clinical response: 49.7%, n = 151 (p < 0.001) | Clinical remission: 51/151 (33.8%) (p = 0.011) |
| UC-SUCCESS51 | AZA | Week 16 | Data not available | Patients in steroid-free remission: 18/76 (23.68%); p-value between IFX, 0.813; IFX/AZA, 0.032 |
| UC-SUCCESS51 | IFX 5 mg/kg | Week 16 | Data not available | Patients in steroid-free remission at: 17/77 (22.08%); p-value between IFX/AZA, 0.017 |
| UC-SUCCESS51 | IFX/AZA | Week 16 | Data not available | Patients in steroid-free remission: 31/78 (39.74%) |
| Probert et al.50 | PBO | Week 6 | Data not available | Patients with UCSS of < 2: 6/20 (30%). 95% CI for difference with IFX –19% to 34%; p = 0.76 When remission rates of patients with total disease in each of the two groups were compared, no significant difference was found (p = 0.9) |
| Probert et al.50 | 5 mg/kg of IFX | Week 6 | Data not available | Patients with UCSS of < 2: 9/23 (39%) |
| ACT149 | PBO | Week 8 | Proportion of patients with clinical response: 45/121 (37.2%) | Proportion of patients in clinical remission: 14.9% (18/121) |
| ACT149 | 5 mg/kg of IFX | Week 8 | Proportion of patients with clinical response: 84/121 (69.4%) (p < 0.001) | Proportion of patients in clinical remission: 38.8% (47/121) (p < 0.001) |
| ACT149 | PBO | Week 54 | Proportion of patients with clinical response: 24/121 (19.8%) | Proportion of patients in clinical remission: 16.5% (20/121) |
| ACT149 | 5 mg/kg of IFX | Week 54 | Proportion of patients with clinical response: 55/121 (45.5%) (p < 0.001) | Proportion of patients in clinical remission: 34.7% (42/121) (p = 0.001) |
| ACT249 | PBO | Week 8 | Proportion of patients with clinical response: 36/123 (29.3%) | Proportion of patients in clinical remission: 5.7% (7/123) |
| ACT249 | 5 mg/kg of IFX | Week 8 | Proportion of patients with clinical response: 78/121 (64.5%) (p < 0.001) | Proportion of patients in clinical remission: 33.9% (41/121) (p < 0.001) |
| ACT249 | PBO | Week 30 | Proportion of patients with clinical response: 32/123 (26.0%) | Proportion of patients in clinical remission: 10.6% (13/123) |
| ACT249 | 5 mg/kg of IFX | Week 30 | Proportion of patients with clinical response: 57/121 (47.1%) (p < 0.001) | Proportion of patients in clinical remission: 25.6% (31/121) (p = 0.003) |
Consideration was given to whether or not it would be appropriate to conduct meta-analysis using the response and remission outcomes within the trials included in the clinical effectiveness review. It was acknowledged that the ADA trials differed from the IFX and GOL trials in the method of estimation of Mayo scores, in that the IFX and GOL trials were based on the average Mayo scores over a consecutive 3-day diary period and the ADA trials included scores based on the worst entry over a consecutive 3-day diary period. However, clinical advisors to the Assessment Group did not expect that this difference would preclude a synthesis of the evidence. It was further noted by the Assessment Group that there may be potential issues in the consistency of measurement of Mayo scores and levels of PBO response according to physician experience and geographical location. The comparability of the trial data set in terms of prior UC treatment was improved by the requesting and receipt from the manufacturer of ADA of anti-TNF-α-naive maintenance data from ULTRA2. 45 It should also be noted that the PURSUIT-Maintenance trial48 rerandomised patients who had previously responded to GOL induction therapy in two previous trials; the extent of this potential bias on patient outcomes is unclear.
Clinical response and remission at induction and maintenance in eligible adult population trials were analysed using NMAs. The results of these analyses are presented on page 72. For the sake of brevity, all secondary efficacy and safety outcomes data are presented in Appendices 4 and 5.
Measures of disease activity
At week 8 of the ULTRA1 trial,44 median changes in CRP from baseline were greater in the ADA 160 mg/80 mg group than PBO (–0.77 mg/l vs.–0.09 mg/l). Patients receiving ADA 160 mg/80 mg in ULTRA144 were also more likely to achieve scores of ≤ 1 point for the Mayo rectal bleeding (p = 0.038) and physician global assessment (p = 0.035) subscores. 44 Statistically significant changes from baseline in haemoglobin and red blood cells (both p < 0.001), total protein and albumin levels (both p < 0.01) were observed in the ADA group versus PBO in ULTRA1. 61
In ULTRA2,45 greater proportions of patients receiving ADA achieved Mayo subscores of ≤ 1 point at week 8 than PBO, although only stool frequency and rectal bleeding were statistically significant at the 5% level. Significantly more ADA group patients who had not previously received anti-TNF-α treatment reached a rectal bleeding score of ≤ 1 than PBO (p < 0.001). 45
At week 6 in the Phase II and Phase III components of PURSUIT-SC,47 mean changes from baseline in Mayo score were –2.6 [standard deviation (SD) 2.73] points and –1.8 (SD 2.96) points (Phase II; p = 0.219), followed by –3.1 (SD 2.90) points and –1.6 (SD 2.53) points (Phase III; p < 0.0001) in the GOL 200 mg/100 mg and PBO arms. Mean changes in CRP concentration (mg/l) at week 6 (Phase III) were –3.35 (GOL 200 mg/100 mg) and +1.59 (PBO) (p < 0.0001).
In ACT1,49 the proportion of patients at week 8 who were not refractory to corticosteroid therapy was higher in the IFX group than PBO (66.7 vs. 37.9%; p < 0.001). 49 Proportions of patients not refractory to corticosteroids at week 8 of the ACT2 study49 were 64.8% for 5 mg/kg of IFX and 26.4% for PBO (p < 0.001). 49 As of week 152 of the extension studies, 20 patients remained, of whom 18 (90.0%) had no or mild disease.
Mean improvements in UCSS at week 6 of the Probert et al. 50 study were 4 (SD 3) for both PBO and IFX groups. The mean reduction in daily dose of glucocorticoid was equivalent to 19 mg (SD 15 mg) and 14 mg prednisolone (SD 12 mg) in the IFX and PBO groups, respectively (p = 0.037). 50 No statistically significant changes in CRP levels were observed between IFX and PBO arm patients. 50
At week 8 of the UC-SUCCESS trial,51 65.79% and 36.84% of the AZA arm, 88.31% and 49.35% of the IFX arm and 85.90% and 52.56% of the IFX/AZA combination arm achieved partial Mayo score decreases of ≥ 1 point and ≥ 2 points respectively. 51 Week 8 mean changes from baseline in partial Mayo scores were –2.81 (SD 2.46) points, –3.52 (SD 2.25) points and –4.01 (SD 2.04) points for AZA, IFX and combination IFX/AZA. 51 Mean changes in total Mayo score from baseline at week 16 were –3.00 (baseline 8.50) points for AZA (p vs. IFX/AZA = 0.001), –4.27 (baseline 8.08) points for IFX (p vs. IFX/AZA = 0.001), and –5.28 (baseline 8.54) points for combination IFX/AZA. 51
Mortality
Reported deaths for the included trials are presented in Appendix 4.
No deaths occurred in the ULTRA144 or ULTRA245 ADA trials. Deaths were not reported in Suzuki et al. 46
One death occurred in PURSUIT-SC47 in the unlicensed 400 mg/200 mg of GOL induction treatment arm in a patient receiving concomitant 20 mg of prednisolone with a case of peritonitis and sepsis after surgical complications related to an ischiorectal abscess and subsequent bowel perforation after surgery. In PURSUIT-Maintenance,48 no deaths occurred through week 54 in the PBO arm but one death (from pneumonia and heart failure) occurred after week 54 in a patient who had received PBO induction and maintenance. No deaths were observed in the 50 mg of GOL group of PURSUIT-Maintenance; however, one death was reported after week 54 (in a patient who received 100 mg/50 mg of GOL induction and 50 mg of GOL maintenance) owing to heart dysfunction in the presence of pronounced atherosclerosis and stenosis affecting the aorta, large arteries and coronary arteries. Three deaths were reported through week 54 of PURSUIT-Maintenance in the 100 mg of GOL treatment arm due to malnutrition and sepsis (patient receiving 2 mg/kg of i.v. GOL induction); cardiac failure with history of thrombosis (patient receiving 400 mg/200 mg of GOL, subcutaneous induction); and disseminated TB in patient who tested positive for latent TB on induction study entry and was receiving isoniazid at time of event (receiving 200 mg/100 mg of GOL, subcutaneous induction). Four deaths were reported after week 54 for the 100 mg of GOL group in PURSUIT-Maintenance, including one case of myocardial infarction in a patient with history of myocardial infarction (PBO, subcutaneous induction and 100 mg of GOL maintenance), two deaths due to gallbladder adenocarcinoma with liver metastasis and due to sepsis (patients receiving 2 mg/kg of i.v. GOL induction) and 100 mg of GOL maintenance) and one death due to accidental nitrous oxide overdose (in a patient receiving 200 mg/100 mg of GOL, subcutaneous induction and 100 mg of GOL maintenance).
The only reported deaths in any of the included IFX trials occurred in patients recruited into the ACT studies. 49 No deaths occurred through week 54 in ACT1 and ACT2. After week 54, two patients died in the PBO arm of ACT1 (due to suicide and cerebrovascular accident). After 54 weeks, four patients died who received IFX in the ACT studies (no dose information available), histoplasmosis 4 weeks after last infusion, listeria encephalitis 3 years after last infusion, prostate cancer 3.5 years after last infusion, and natural causes 10 months after last infusion.
Rates of hospitalisation
A total of four included trials reported hospitalisation data for the adult population (ULTRA144 and ULTRA245 for ADA, ACT149 and ACT249 for IFX, no trials for GOL).
In ULTRA1,44 all reported hospitalisation outcome measure data were lower in the 160 mg/80 mg of ADA group than PBO at week 8, indicating more favourable outcomes for the intervention group, including physician visits (p = 0.559), emergency room visits (p-value not reported), hospital admissions (p-value not reported) and days in hospital (p = 0.297). None of these differences were statistically significant. 62 Similarly, for the ULTRA2 trial,45 hospitalisation-related outcome data were also slightly lower for the ADA group than PBO at week 52, although this was only statistically significant for physician visits (physician visits, p = 0.035; emergency room visit, p = 0.847; hospital admissions, p = 0.418; and days in hospital, p = 0.467). 62 A range of hospitalisation-related measures were also reported for ULTRA1 and ULTRA2 data combined. The all-cause hospitalisation incidence rate was lower for ADA than PBO (p = 0.047), as was the UC-related hospitalisation incidence rate (p = 0.002), with a relative risk for UC-related hospitalisation of 0.48 for ADA versus PBO (p < 0.001). 63
No included trials reported hospitalisation data for GOL.
Rates of surgical intervention (both elective and emergency)
Six included trials in the adult population included information on rates of surgical intervention (ULTRA144 and ULTRA245 for ADA, PURSUIT-Maintenance48 for GOL, and ACT1,49 ACT249 and Probert et al. 50 for IFX). No trials reported whether surgical outcomes were elective or emergency in nature.
In ULTRA1,44 colectomies to week 8 were lower in the 160 mg/80 mg of ADA group than PBO (1.4% vs. 3.6%; p-value not reported; elective or emergency not reported). Colectomy rates were very slightly lower through week 52 of ULTRA245 in the ADA group (4%) vs. PBO (4.9%) (p-value not reported; elective or emergency not reported). 63,65
Limited data were available for GOL, which indicated that only 2–3% of GOL induction responders rerandomised to 50 mg or 100 mg of GOL in PURSUIT-Maintenance48 received colectomy at the end of maintenance. 66
Colectomy and ostomy rates through week 54 of ACT149 were both slightly lower in the 5 mg/kg of IFX group (5.8% and 2.5%, respectively) than in the PBO group (7.4% and 4.1% respectively) (p-values not reported). 64 One patient in each case from the PBO arm was reported as having the outcomes of colectomy and an ostomy (0.7% and 0.7%) through week 54 of ACT2, while no patients in the 5 mg/kg IFX group underwent colectomy or ostomy. 64 Limited details were available from the Probert et al. 50 trial to the effect that a single patient in the PBO arm received a colectomy during the intervention period.
Meta-analysis
Colectomy rates during induction were reported by one trial (ULTRA144). The between-group difference was not statistically significant [RR = 0.63 (random effects), 95% CI 0.21 to 1.86; p = 0.40; Figure 5).
FIGURE 5.
Forest plot of comparison: colectomy – adults. Outcome: colectomy – induction-licensed dose. M–H, Mantel–Haenszel.

Colectomy rates during maintenance were reported by one trial evaluating the licensed maintenance dose of ADA comprising a mixed sample of anti-TNF-α exposed and naive participants (ULTRA2,45 517 participants). The between-group difference was not significant [RR = 0.83 (random effects), 95% CI 0.36 to 1.88; p = 0.65). Two trials evaluating the licensed maintenance dose of IFX reported maintenance outcomes at 30 weeks (ACT249) and 54 weeks (ACT149). The pooled effect across these trials (486 participants) was not significant [RR = 0.73 (random effects), 95% CI 0.29 to 1.81; p = 0.49]. The forest plot for these analyses (random effects) is presented in Figure 6.
FIGURE 6.
Forest plot of comparison: colectomy – adults. Outcome: colectomy – maintenance-licensed dose. df, degrees of freedom; M–H, Mantel–Haenszel.
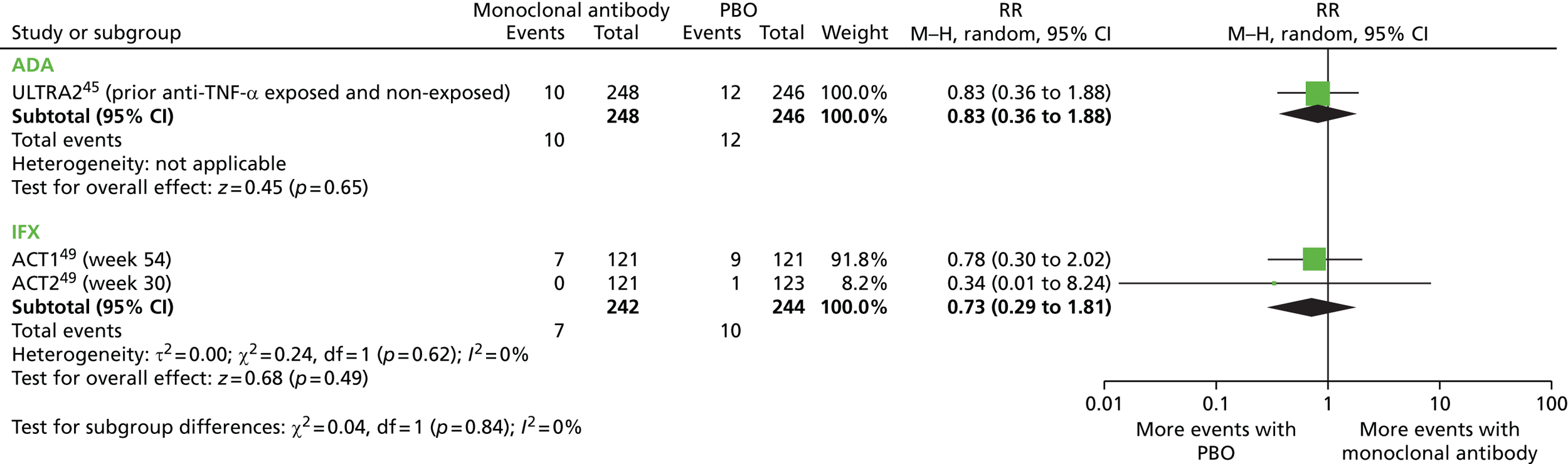
Ostomy rates during maintenance in adults were reported by two trials evaluating the licensed maintenance dose of IFX at 30 weeks (ACT249) and 54 weeks (ACT149). The pooled effect across these trials (486 participants) was not significant [RR = 0.55 (random effects), 95% CI 0.15 to 1.98; p = 0.36]. The forest plot for these analyses is presented in Figure 7.
FIGURE 7.
Forest plot of comparison: ostomy – adults. Outcome: ostomy – maintenance-licensed dose. df, degrees of freedom; M–H, Mantel–Haenszel.

Time to surgical intervention (both elective and emergency)
Very limited data were reported from the included trials in the adult population for the outcome of time to surgical intervention. Sandborn et al. 67 combined data from the ACT1 and ACT2 IFX trials49 and reported that the cumulative incidence of colectomy through 54 weeks was higher for the PBO group (17%) than for the combined IFX group (10%) (p = 0.02) and calculated a hazard ratio of 0.59, indicating a 41% reduction in the risk of colectomy for the combined licensed and unlicensed IFX groups versus PBO.
Health-related quality of life
Health-related quality-of-life data were available from nine included trials in the adult population (ULTRA1,44 ULTRA245 and ULTRA3 for ADA, PURSUIT-SC47 and PURSUIT-Maintenance48 for GOL, and ACT1,49 ACT2,49 UC-SUCCESS51 and Probert et al. 50 for IFX, see Appendix 6). Data related to HRQoL were measured using Inflammatory Bowel Disease Questionnaire (IBDQ), Short Form questionnaire-36 items (SF-36) and the European Quality of Life-5 Dimensions (EQ-5D) [note: total IBDQ scores can range from 32 (very poor) to 224 (perfect HRQoL)].
Adalimumab
In ULTRA1,44 the changes from baseline scores to week 8 in IBDQ were very similar for the 160 mg/80 mg of ADA and PBO groups (153 vs. 152; p-value not reported). Furthermore, the difference between IBDQ mean responses at week 8 in the 160 mg/80 mg of ADA and PBO groups was not statistically significant (70 vs. 75; p = 0.532). Changes from baseline in SF-36 mental and physical component summary scores were also similar at week 8 in the ADA and PBO group (46 vs. 44). ULTRA245 week 52 IBDQ scores were higher in the 160 mg/80 mg of ADA group than PBO, indicating more favourable HRQoL in the ADA group (27 vs. 19; p < 0.05). A greater proportion of patients experienced an increase in IBDQ of ≥ 16 points from baseline by week 52 in the ADA group than PBO (26.2% vs. 16.3%; p < 0.05).
Golimumab
In both Phase II and Phase III of the PURSUIT-SC47 GOL trial, patients in the 200 mg/100 mg of GOL induction arms reported a greater change in IBDQ from baseline to week 6 than the patients of PBO groups [Phase II, mean 24.9 vs. 14.8 (p-value NS); Phase III mean 27.0 vs. 14.8; p < 0.0001]. Greater proportions of patients in each GOL group were also described as achieving ‘any improvement’ to ‘clinically meaningful improvement’ in IBDQ (51.1% vs. 35.2%; p < 0.001), physical component summary (41.0% vs. 31.6%; p = 0.01) and mental component summary scores (42.7% vs. 28.5%; p < 0.001) at week 6.
Infliximab
In the ACT1 trial,49 changes from baseline in SF-36 physical and mental component summary scores to week 8 were larger for the 5 mg/kg of IFX group than the PBO group (both p < 0.05). Statistically significant improvements in IBDQ and SF-36 components were evident in the 5 mg/kg of IFX treatment arm compared with PBO to week 8 for ACT1 and ACT2 trials combined. The greatest changes from baseline to week 16 in both IBDQ and SF-36 physical function were observed in the IFX/AZA combination treatment arm (p < 0.05 vs. AZA, p < 0.05 vs. IFX for both outcomes). Improvements in IBDQ and EQ-5D from baseline to week 6 in Probert et al. 50 were larger in the IFX group than PBO (p-value not reported).
Adverse events of treatment (including leakage and infections following surgery)
The included trials report data relating to AEs associated with the interventions under assessment only (i.e. IFX, ADA and GOL) and do not report safety outcomes (e.g. leakage and infections) post surgery. However, although the clinical effectiveness systematic review does not take these factors into account, these factors are relevant to the economic analysis (see Chapter 4). p-values are provided when available; however, the statistical significance of observed differences in safety outcomes was poorly reported across the included trials.
Discontinuations due to adverse events
Adalimumab
Discontinuations due to AEs at week 8 in ULTRA144 were 5.4% in both 160 mg/80 mg of ADA and PBO groups. 44 Withdrawals due to AEs were slightly lower for ADA than PBO by week 52 of ULTRA2,45 at 23 out of 257 (8.9%) for 160 mg/80 mg of ADA and 34 out of 260 (13.1%) for PBO. 45 More AEs leading to discontinuation occurred in the Suzuki et al. 46 trial in the 40 mg of ADA every other week group versus PBO [n= 22 vs. n= 6; 22.4/100 patient-years (PYs) vs. 13.4/100 PYs].
Golimumab
Numbers of patients who discontinued study agent through week 6 because of at least one AE were relatively low across both 200 mg/100 mg of GOL induction (1/331, 0.3%) and PBO (3/330, 0.9%) groups for PURSUIT-SC. 47 Through week 54 of PURSUIT-Maintenance,48 8/154 (5.2%) of the 50 mg of GOL, 14/154 (9.1%) of the 100 mg of GOL and 10/156 (6.4%) of the PBO groups had discontinued study agent owing to at least one AE. 48
Infliximab
Through week 54 of ACT149 the number of patients with AEs leading to study drug discontinuation was 10 out of 121 (8.3%) and 11 out of 121 (9.1%) for the 5 mg/kg of IFX and PBO groups, respectively. Through week 30 of ACT2,49 discontinuations due to AEs occurred in 2 out of 121 (1.7%) and 12 out of 123 (9.8%) for 5 mg/kg of IFX and PBO arm patients, respectively. Through week 8 of UC-SUCCESS51 AEs leading to discontinuation were highest for AZA (6/79, 8%), compared with 2/78 (3%) for IFX and 3/80 (4%) for combination IFX and AZA. 51
Number of patients experiencing one or more adverse event
Adalimumab
In ULTRA1,44 patients reporting at least one treatment emergent AE were 112 out of 223 (50.2%) and 108 out of 223 (48.4%) for the 160 mg/80 mg of ADA induction and PBO groups, respectively. 44 At week 52 of ULTRA2,44 the proportions of patients reporting any AE were similar between groups; 213 out of 257 (82.9%) for the 160 mg/80 mg of ADA arm and 218 out of 260 (83.8%) of the PBO arm. At week 52 in the Suzuki et al. 46 study, fewer AEs occurred (in terms of events per 100 PYs) in the 40 mg of ADA every other week group than in the PBO group (547.9/100 PYs vs. 609.4/100 PYs).
Golimumab
By week 6 of PURSUIT-SC,47 the proportions of patients with at least one AE were similar for 200 mg/100 mg of GOL induction (124/331, 37.5%) and PBO (126/330, 38.2%). 46 Patients reporting one or more AEs through week 54 of PURSUIT-Maintenance48 were 112 out of 154 (72.7%) in the 50 mg of GOL arm, 113 out of 154 (73.4%) in the 100 mg of GOL arm and 103 out of 156 (66.0%) in the PBO treatment arm.
Infliximab
The proportions of patients through week 54 of ACT149 reporting at least one AE were 106 out of 121 (87.6%) and 103 out of 121 (85.1%) for 5 mg/kg of IFX and PBO respectively. At week 30 of ACT2,49 these values were 99 out of 121 (81.8%) and 90 out of 123 (73.2%) for 5 mg/kg of IFX and PBO, respectively. Through week 8 of UC-SUCCESS,51 patients reporting one or more AE were higher in the AZA group (41/79, 52%) than IFX (26/78, 33%) or combination IFX/AZA (30/80, 38%).
Number of patients experiencing one or more serious adverse event
Definitions of serious adverse events (SAEs) were poorly reported across included RCTs.
Adalimumab
At week 8 in ULTRA1,44 the proportions of patients reporting one or more SAEs were exactly equivalent, at 5.4% (12/223) in the 160 mg/80 mg of ADA group and 5.4% (12/223) in the PBO group. 44 Proportions of ULTRA245 patients reporting any SAEs were also roughly equivalent, with 12.1% (31/257) and 12.3% (32/260) in the 160 mg/80 mg of ADA and PBO groups, respectively. 45 At week 52 of the Suzuki et al. 46 study, a similar number of events per 100 PYs were classed as serious in the 40 mg of ADA every other week group than in the PBO group (33.6/100 PYs vs. 31.3/100 PYs). 46
Golimumab
By week 6 of PURSUIT-SC,47 the proportion of patients reporting at least one SAE was lower in the 200 mg/100 mg of GOL treatment arm (9/331, 2.7%) than the PBO group (20/330, 6.1%). 47 More patients in the 100 mg of GOL group reported one or more SAE (22/154, 14.3%) than patients in the 50 mg of GOL (13/154, 8.4%) or PBO (12/156, 7.7%) groups by week 54 of PURSUIT-Maintenance. 48
Infliximab
Proportions of patients through week 54 of ACT149 who reported SAEs were similar for 5 mg/kg of IFX (26/121, 21.5%) and PBO (31/121, 25.6%) groups. 49 At week 30 of ACT2,49 slightly fewer patients reported SAEs in the 5 mg/kg of IFX group [13/121 (10.7%)] than the PBO group [24/123 (19.5%)]. 49 SAEs were more frequently reported by week 8 of UC-SUCCESS51 among patients receiving AZA (6/79, 8%) than IFX (0/78) or combination IFX and AZA (3/80, 4%).
Infections
Adalimumab
The occurrence of infections at week 8 of ULTRA144 was very similar for the 160 mg/80 mg of ADA group (32/223, 14.3%) and the PBO group (35/223, 15.7%). 44 This was also the case at week 52 of ULTRA2,45 with 45.1% (116/257) and 39.6% (103/260) of patients reporting infections within the 160 mg/80 mg of ADA and PBO groups, respectively. 45 At week 8 of Suzuki et al. ,46 infections occurred in 18.9% (17/90) and 15.6% (15/96) of the 160 mg/80 mg of ADA and PBO groups. 46
Golimumab
At week 6 of PURSUIT-SC,47 12.1% (40/330) of PBO group patients reported at least one infection, of which 7.0% required treatment (23/330); these values were similar to those in the 200 mg/100 mg of GOL induction group (39/331, 11.8%; 15/331, 4.5%). 47 Infections at week 54 of PURSUIT-Maintenance48 were more common in the 50 mg of GOL (60/154, 39.0%; requiring treatment 39/154, 25.3%) and 100 mg of GOL (60/154, 39.0%; requiring treatment 44/154, 28.6%) maintenance groups than PBO (44/156, 28.2%; requiring treatment 24/156, 15.4%). 47
Infliximab
Through week 54 of ACT1,49 infections were slightly more common among patients receiving 5 mg/kg of IFX (53/121, 43.8%; requiring treatment 39/121, 32.2%) than PBO (47/121, 38.8%; requiring treatment 25/121, 20.7%). 49 At week 30 of ACT2,49 infections had occurred in 18 out of 121 (14.9%, requiring treatment 17/121, 14.2%) and 29 out of 123 (23.6%; requiring treatment 15/123, 12.2%) of patients receiving IFX and PBO respectively. 49 Through week 54 of the ACT1 and ACT2 extension studies, infections occurred in 94 out of 242 (39%) of 5 mg/kg IFX and 80 out of 244 (33%) of PBO group patients. 67
Serious infections
Adalimumab
Reported serious infections were low through week 8 of ULTRA144 in both PBO (3/223, 1.3%, one pneumonia, one sepsis, one staphylococcal wound infection) and 160 mg/80 mg of ADA treatment arms (0/223), and remained similarly comparable between treatment arms through week 52 of ULTRA245 (160 mg/80 mg of ADA 4/257, 1.6% vs. PBO 5/260, 1.9%). Serious infections were reported at week 52 of ULTRA3 at a rate of 3.4 events per 100 PYs for patients receiving ADA. 62 No serious infections were reported at week 8 of the Suzuki et al. 46 trial in the PBO arm, while three cases occurred by week 8 in the 160 mg/80 mg of ADA group (3/90, 3.3%).
Golimumab
The proportion of patients reporting one or more serious infections were slightly higher at week 6 of PURSUIT-SC47 in the PBO treatment arm (6/330, 1.8%) than 200 mg/100 mg of GOL induction (1/331, 0.3%, one pneumonia). 47 By week 54 of PURSUIT-Maintenance,48 the occurrence of serious infections was marginally higher in the 50 mg of GOL (5/154, 3.2%) and 100 mg of GOL (5/154, 3.2%) maintenance groups than PBO (3/156, 1.9%).
Infliximab
The proportion of patients with serious infections through week 54 of the ACT149 trial was similar between treatment arms (5 mg/kg of IFX 3/121, 2.5%; PBO 5/121, 4.1%). Numbers of patients with serious infections through week 30 of ACT249 were similar for 5 mg/kg of IFX (2/121, 1.7%) and PBO (1/123, 0.8%). 49 Through week 54 of the ACT1 and ACT2 extension studies, serious infections occurred in 7/242 (2.89%) of 5 mg/kg of IFX and 6/244 (2.46%) of PBO group patients. 67
Serious infections occurred in very low numbers through week 8 of the UC-SUCCESS51 trial (AZA 1/79, 1%; IFX 1/78, 1%; combination IFX/AZA 0/80, 0%).
Meta-analysis
Serious infections associated with the licensed induction dose of ADA in were reported by two trials, one in Western populations (ULTRA1,44 446 participants) and one in Japanese populations (Suzuki et al. ,46 186 participants). The between-group difference in both trials was not significant [RR = 0.14 (random effects) 95% CI 0.01 to 2.75; p = 0.20; RR = 7.46 (random effects) 95% CI 0.39 to 142.47; p = 0.18 respectively]. The forest plot for this analysis (random effects) is presented in Figure 8. Serious infections associated with the licensed induction of GOL in adults were reported by one trial (PURSUIT-SC,47 661 participants). The between-group difference was not significant [RR = 0.17 (random effects), 95% CI 0.02 to 1.37; p = 0.10].
FIGURE 8.
Forest plot of comparison: serious infections – adults. Outcome: serious infections – induction-licensed dose. df, degrees of freedom; M–H, Mantel–Haenszel.

Serious infections associated with the licensed maintenance dose of ADA were reported by one trial comprising a mixed sample of anti-TNF-α exposed and naive participants (ULTRA2,45 517 participants). The between-group difference was not significant [RR = 0.81 (random effects) 95% CI 0.22 to 2.98; p = 0.75]. Serious infections associated with maintenance dose of 50 mg or 100 mg of GOL in adults were reported by one trial (PURSUIT-Maintenance48). The between-group difference for 50 mg of GOL compared with PBO (312 participants) was not significant [RR = 1.67 (random effects) 95% CI 0.41 to 6.85; p = 0.48]. The between-group difference for 100 mg of GOL compared with PBO (310 participants) was also not significant [RR = 1.69 (random effects) 95% CI 0.41 to 6.94; p = 0.47]. Two trials evaluating the licensed maintenance dose of IFX reported maintenance outcomes at 30 weeks (ACT249) and 54 weeks (ACT149). The pooled effect across these trials (486 participants) was not significant [RR = 0.82 (random effects) 95% CI 0.24 to 2.77; p = 0.77]. The forest plot for these analyses is presented in Figure 9.
FIGURE 9.
Forest plot of comparison: serious infections – adults. Outcome: serious infections – maintenance-licensed dose. df, degrees of freedom; M–H, Mantel–Haenszel.

Reactivation of tuberculosis
Adalimumab
No data relating to the reactivation of TB were reported for ULTRA144 or ULTRA2. 45 Reactivation of TB occurred in a single patient (equating to < 0.1 events/100 PYs) by week 52 of ULTRA3. 62 No events occurred in the PBO arm of the Suzuki et al. 46 study through week 8, while for the 40 mg of ADA every other week group, a single event of reactivation of TB was described (1.0 events/100 PYs). 46
Golimumab
No cases of reactivation of TB were reported in the PURSUIT-SC trial. 47 In the PBO maintenance group of PURSUIT-Maintenance,48 one event of reactivation occurred (in a patient who had received unlicensed 4 mg/kg of GOL i.v. induction). 47 No cases were reported for patients receiving 50 mg of GOL maintenance treatment. However, three cases occurred in the 100 mg of GOL maintenance group (one patient each had received induction regimens of 400 mg/200 mg of GOL, subcutaneous; 4 mg/kg i.v.; or 200 mg/100 mg subcutaneous) (including one fatal case: 200 mg/100 mg of GOL, subcutaneous). 47
Reactivation of hepatitis B
Adalimumab
No incidents of reactivation of hepatitis B were reported in any of the included ADA trials.
Golimumab
No cases were described in the included GOL studies.
Infliximab
No events were reported in the included IFX studies.
Administration reactions (injection site reactions/infusion reactions/serious allergic reactions)
Injection site reactions
Adalimumab
Injection site reactions were slightly more frequent at week 8 of ULTRA144 among patients receiving 160 mg/80 mg of ADA (13/223, 5.8%) than PBO (7/223, 3.1%). 44 Injection site reactions were also more frequent in the 160 mg/80 mg of ADA group at week 52 of ULTRA245 (31/257, 12.1%) than for PBO (10/260, 3.8%). 45 Patients receiving ADA through week 52 of ULTRA3 experienced injection site reactions at a rate of 10.5 per 100 PYs. 62 Injection site reactions were more frequent through week 8 of the Suzuki et al. 46 trial in the 160 mg/80 mg of ADA group (7/90, 7.8%) than for PBO (2/96, 2.1%). 46
No serious allergic reactions were described as having occurred in the included ADA trials.
Golimumab
At week 6 of the PURSUIT-SC47 trial, injection site reactions were more common in patients receiving 200/100 mg of GOL induction (11/331, 3.3%) than PBO (5/330, 1.5%). 47 The number of patients reporting one or more injection site reactions through week 54 of PURSUIT-Maintenance48 was higher in the 100 mg of GOL maintenance treatment arm (11/154, 7.1%) than 50 mg of GOL (3/154, 1.9%) and PBO (3/156, 1.9%). 47,48
No serious allergic reactions were reported.
Meta-analysis
Injection site reactions associated with the licensed induction dose of ADA in were reported by two trials, one in Western populations (ULTRA1,44 446 participants) and one in Japanese populations (Suzuki et al. ,46 186 participants). The between-group difference in both trials was not significant [RR = 1.86 (random effects), 95% CI 0.76 to 4.57; p = 0.18; RR = 3.73 (random effects) 95% CI 0.80 to 17.50; p = 0.09 respectively]. The forest plot for this analysis (random effects) is presented in Figure 10. Injection site reactions associated with the licensed induction dose of GOL in adults were reported by one trial (PURSUIT-SC,47 661 participants). The between-group difference was not significant [RR = 2.19 (random effects), 95% CI 0.77 to 6.24; p = 0.14].
FIGURE 10.
Forest plot of comparison: injection site reactions – adults. Outcome: injection site reactions (ADA and GOL only) – induction-licensed dose. df, degrees of freedom; M–H, Mantel–Haenszel.

Injection site reactions associated with maintenance doses of ADA were reported by one trial comprising a mixed sample of anti-TNF-α-exposed and -naive participants (ULTRA2,45 517 participants). The between-group difference was significant in favour of PBO (fewer events) [RR = 3.14 (random effects) 95% CI 1.57 to 6.26; p = 0.001]. The forest plot for this analysis is presented in Figure 11. Injection site reactions associated with maintenance dose of 50 mg or 100 mg of GOL in adults were reported by one trial (PURSUIT-Maintenance48). The between-group difference for 50 mg of GOL compared with PBO (312 participants) was not significant [RR = 1.00 (random effects), 95% CI 0.20 to 4.88; p = 1.00]. The between-group difference for 100 mg of GOL compared with PBO (310 participants) was significant in favour of PBO (fewer events) [RR = 3.71 (random effects), 95% CI 1.06 to 13.06; p = 0.04].
FIGURE 11.
Forest plot of comparison: injection site reactions – adults. Outcome: injection site reactions (ADA and GOL only) – maintenance-licensed dose. df, degrees of freedom; M–H, Mantel–Haenszel.

Infusion reactions
Infliximab
Acute infusion reactions occurred in similar numbers of patients in both treatment arms through week 54 of ACT149 (5 mg/kg of IFX 12/121, 9.9%; PBO 13/121, 10.7%). 49 Infusion reactions were slightly higher in ACT249 patients receiving 5 mg/kg of IFX (14/121, 11.6%) than PBO (10/123, 8.1%). 49
Infusion reactions were rare through week 8 of UC-SUCCESS (AZA 1/79, 1%; IFX 0/78, 0%; combination IFX/AZA 0/80, 0%). 51 Possible delayed hypersensitivity reactions occurred in 2/242 (1%) of the 5 mg/kg of IFX group and 2/242 (1%) of the PBO group through week 54 of the ACT1 and ACT2 extension studies. 67
No serious allergic reactions were reported.
Heart failure
Adalimumab
Heart failure did not occur in any patients in either 160 mg/80 mg of ADA induction or PBO arms by week 8 of ULTRA1. 44 Only one case of heart failure was reported through week 52 of ULTRA2,45 which was in a patient receiving 160 mg/80 mg of ADA for induction (1/257, 0.4%). 45 Heart failure was reported at a rate of 0.2 events per 100 PYs for 40 mg of ADA every other week/every week at week 52 of ULTRA3. 62 Through week 8 of the Suzuki et al. 46 trial, no cases of heart failure were reported.
Malignancies and lymphoproliferative disorders
Adalimumab
Malignancies were reported at low levels through week 8 of ULTRA1,44 with 2 out of 223 events (0.9%, one basal cell carcinoma, one breast cancer) in the PBO group and no cases in the 160 mg/80 mg of ADA group. 44 Two cases of malignancy were reported through week 52 of ULTRA2,45 both of which were in patients receiving 160 mg/80 mg of ADA. 45 Through week 52 of ULTRA3, events (excluding lymphoma) occurred in the 40 mg of ADA maintenance arm at a rate of 1.0 events per 100 PYs and at a rate of 230.1 events per 100 PYs for lymphoma. 62 One case of malignancy (1/90, 1.1%) was described in the 160 mg/80 mg of ADA group at week 8 of the Suzuki et al. 46 trial.
Golimumab
No cases of malignancy were reported for either the 200 mg/100 mg of GOL induction or PBO treatment arms through week 6 of PURSUIT-SC. 47 Although one malignancy (1/156, 0.6%) was described by week 54 of PURSUIT-Maintenance48 in the PBO arm, four cases each were observed in the 50 mg of GOL (4/154, 2.6%) and 100 mg of GOL (4/154, 2.6%) maintenance groups. 47,48
Infliximab
Two cases of malignancy were reported through week 54 of ACT149 in patients receiving 5 mg/kg of IFX. 67 One case of basal cell carcinoma was reported in the PBO arm and one case of rectal adenocarcinoma was described in the 5 mg/kg of IFX arm of ACT249 through week 30. No malignancies were described in the UC-SUCCESS trial. 51
Hepatobiliary events/liver enzyme changes
Adalimumab
No cases were described in ULTRA144 or ULTRA2. 45 Hepatobiliary events were reported at a rate of 0.5 events per 100 PYs in the 40 mg of ADA maintenance arm through week 52 of ULTRA2. 62 By week 8 of the Suzuki et al. 46 trial, events occurred in 1 out of 90 (1.1%) of 160 mg/80 mg of ADA and 1 out of 96 (1.0%) of PBO group patients. 46
Autoimmune processes (e.g. lupus-like syndrome)
Adalimumab
It was stated that no events of lupus-like syndrome occurred in the 160 mg/80 mg of ADA or PBO treatment arms by week 8 of ULTRA1. 44 One case of lupus-like syndrome (1/257, 0.4%) was reported in a patient receiving 160 mg/80 mg of ADA through week 52 of ULTRA2. 45 No cases were reported through week 8 of the Suzuki et al. 46 trial.
Neurological events
Adalimumab
No cases of demyelinating disease occurred in the 160 mg/80 mg of ADA or PBO treatment arms by week 8 of ULTRA144 or by week 52 of ULTRA2. 45 No cases of neurological events were reported through week 8 of the Suzuki et al. 46 trial.
Haematological reactions
Adalimumab
No haematological reactions were described in ULTRA1. 44 One haematological reaction was reported in 5 out of 257 (1.9%) patients receiving 160 mg/80 mg of ADA by week 52 of ULTRA2. 45 Haematological reactions occurred in 1 out of 90 (1.1%) and 1 out of 96 (1.0%) patients receiving 160 mg/80 mg of ADA and PBO, respectively, by week 8 of the Suzuki et al. 46 study.
Golimumab
No haematological reactions were reported for either the 200 mg/100 mg of GOL induction or PBO treatment arms through week 6 of PURSUIT-SC47 or the GOL maintenance or PBO groups of PURSUIT-Maintenance. 47,48
Infliximab
No haematological reactions were described in ACT1,49 ACT2,49 Probert et al. 50 or UC-SUCCESS. 51
Population: children and adolescents aged 6–17 years (inclusive) with severely active ulcerative colitis who have had an inadequate response to conventional therapy including corticosteroids and mercaptopurine or azathioprine, or who are intolerant to, or have medical contraindications against, such therapies
Rates of and duration of response, relapse and remission
Table 12 presents the definitions of clinical response and remission in the included paediatric population RCT.
| Trial | Definition of clinical response | Definition of clinical remission | Measurement time points |
|---|---|---|---|
| Hyams et al., 201252 | Decrease in Mayo score of ≥ 3 points and ≥ 30%, with accompanying decrease in subscore for rectal bleeding of ≥ 1 point or absolute rectal bleeding subscore of 0 or 1 | Mayo score of ≤ 2 points, with no individual subscore of > 1. PUCAI clinical remission = score of < 10 | Mayo scores assessed at weeks 0, 8 and 54. Endoscopy at week 54 optional |
All enrolled patients received induction therapy with 5 mg/kg of IFX. At week 8, clinical response was reached by 44 out of 60 patients (73.3%), while 24 out of 60 (40.0%) of patients achieved clinical remission.
The PUCAI remission rates were evaluated at weeks 30 and 54. A greater proportion of patients in the 5 mg/kg of IFX every 8 weeks treatment group achieved PUCAI remission at week 30 (40.0% vs. 19.0%; p-values not reported) and week 54 (38.1% vs. 18.2%, p-values not reported) than the 5 mg/kg of IFX every 12 weeks group. At week 54, PUCAI remission without the use of corticosteroids was reported for 38.5% of the every 8 weeks group and 0% of the every 12 weeks group. 52
The absence of a PBO or non-IFX control group limits the comparative evaluation of the efficacy of IFX in induction and maintenance of clinical response and remission in paediatric patients. A briefing document68 by Centocor Ltd to the FDA Gastrointestinal Drugs Committee was produced in June 2011 and considered the evidence available from the Hyams et al. 2012/T72 study52 and compared this with the ACT1 and ACT2 trials49 of IFX previously conducted in the adult UC population. The briefing document considered efficacy to be similar between T72 and the ACT1 and ACT2 studies during (1) induction (with clinical response and Mayo remission at week 8 induced in 73.3% and 40.0% of paediatric patients and 66.9% and 36.4% of pooled 5 mg/kg adult patients from ACT1 and ACT2, respectively) and (2) maintenance (with PUCAI remission at week 54 in 38.1% of paediatric subjects in the every 8 weeks group and 34.7% at week 54 of ACT1) (with reported good correlation of 0.75–0.88 between PUCAI and Mayo scores described at baseline and week 8).
Measures of disease activity
At week 8 of the Hyams et al. 52 study, the median reductions in partial Mayo scores were 4 points for both the 5 mg/kg of IFX every 8 weeks group and 5 mg/kg of IFX every 12 weeks group. 52 By week 30, the median reduction in partial Mayo score was approximately 3 points for the every 8 weeks group and 1 point for the every 12 weeks group. 52
Mortality
No deaths were reported in the Hyams et al. 52 trial.
Rates of hospitalisation
No hospitalisation-related outcome data were reported in Hyams et al. 52
Rates of surgical intervention (both elective and emergency)
One of 22 patients (4.5%) in the 5 mg/kg of IFX every 8 weeks group required colectomy through week 54 in the Hyams et al. 52 trial as compared with 2 out of 23 (8.7%) patients in the 5 mg/kg of IFX every 12 weeks treatment arm.
Colectomy rates during maintenance in children were reported by one trial evaluating the licensed dose of IFX every 8 weeks or every 12 weeks (Hyams et al. ,52 45 participants). The between-group at week 54 was not significant [RR = 0.52 (random effects), 95% CI 0.05 to 5.36; p = 0.59; Figure 12].
FIGURE 12.
Forest plot of comparison: colectomy – children. Outcome: IFX maintenance. M–H, Mantel–Haenszel; q8w, every 8 weeks; q12w, every 12 weeks.

Time to surgical intervention (both elective and emergency)
No data were reported in the paediatric population for the outcome of time to surgical intervention.
Health-related quality of life
No HRQoL data were included in the Hyams et al. 52 trial.
Adverse events of treatment (including leakage and infections following surgery)
Through week 54 of the Hyams et al. 52 trial, discontinuations due to at least one AE were higher in the 5 mg/kg of IFX every 12 weeks group than the every 8 weeks frequency group (6/23, 26.1% vs. 3/22, 13.6%). 52
All patients in both treatment arms of the Hyams et al. 52 study reported at least one AE (22/22, 100% vs. 23/23, 100%). 52
The numbers of patients reporting at least one SAE were similar between the 5 mg/kg of IFX every 12 weeks (5/23, 21.7%) and every 8 weeks (4/22, 18.2%) treatment arms. 52
The occurrence of infections was comparable between 5 mg/kg of IFX every 8 weeks (13/22, 59.1%) and every 12 weeks (14/23, 60.9%) treatment groups. 52
No cases of serious infection were reported in the Hyams et al. 52 trial.
No cases were reported.
No cases were reported.
The number of patients experiencing infusion reactions were similar between treatment groups in the Hyams et al. 52 study (4/22, 18.2% vs. 3/23, 13.0%).
Subgroups
As stated in the assessment protocol, the only pre-specified subgroup of interest was duration of disease. However, clinical data reported according to disease duration were very limited. The only studies to evaluate the effect of disease duration on outcomes were ULTRA245 and PURSUIT-Maintenance. 47,48
For ULTRA2,45 the odds ratios (ORs) for the proportion of patients in clinical remission at week 8 for ADA versus PBO were very similar for patients with disease duration of ≤ 2 years (OR 1.91, 95% CI 0.4 to 8.8; p = 0.40) and those with disease duration of > 2 years (OR 1.92, 95% CI 1.1 to 3.4; p = 0.03). However, at week 52, the OR for clinical remission was considerably higher for patients with disease duration > 2 years (OR 3.59, 95% CI 1.9 to 6.9; p < 0.001) than for patients with a shorter disease duration of ≤ 2 years (OR 0.22, 95% CI 0.04 to 1.1; p = 0.05).
PURSUIT-Maintenance48 reported the ORs for comparing the proportion of patients in clinical response in the GOL maintenance group versus the PBO group for GOL-induction responders. The OR for proportion of patients in clinical response through week 54 for 50 mg of GOL versus PBO treatment arms was slightly higher among patients with longer disease duration (> 5 to ≤ 15 years; OR 2.3, 95% CI 1.0 to 5.4; p = 0.056) than those with shorter duration of disease (≤ 5 years; OR 1.4, 95% CI 0.9 to 2.7; p = 0.533). Similarly, OR for 100 mg of GOL versus PBO groups was also reported to be greater among those with a disease duration of > 5 to ≤ 15 years (OR 2.2, 95% CI 1.0 to 4.9; p = 0.068) than for patients with disease duration of ≤ 5 years (OR 1.6, 95% CI 0.8 to 3.1; p = 0.128). However, it was noted that the 95% CIs for these observations overlapped between estimates.
Methods for network meta-analysis
The trials identified in the systematic review formed a connected network such that each trial had at least one treatment in common with at least one other trial. Treatment effects were estimated using NMAs of clinical response and remission as defined by the complete Mayo score.
Selection of evidence contributing to the network meta-analysis
For RCTs to be eligible for inclusion in the NMA they were required to have information about clinical response and/or clinical remission data for either an induction (6–8 weeks) or maintenance (approximately 30 weeks or 52–54 weeks) time point. It should be noted that two adult population RCTs evaluating the use of IFX as an induction treatment (Probert et al. ,50 and UC-SUCCESS51) were excluded from the adult population NMA. These studies were excluded for other reasons, as described in the table of trial characteristics (see Table 6). The base-case analyses utilised data from the anti-TNF-α-naive population rather than the ITT population in ULTRA245 in order to increase comparability of the dataset. The induction base case also incorporated both Phase II (plus additional analysed patients from Phase II) and Phase III data from PURSUIT-SC. 47 The effect of using the ITT (mixed anti-TNF-α experienced) population from ULTRA245 was explored in a sensitivity analysis. As the Suzuki et al. 46 trial was conducted in exclusively Japanese patients, this trial was not included in the base case; however, the addition of this trial to the network was explored in a sensitivity analysis. Therefore, three sensitivity analyses were performed for both induction and maintenance phases to assess the robustness of replacing ULTRA2 anti-TNF-α-naive data with ULTRA2 ITT data (sensitivity analysis 1), including Suzuki et al. 46 (sensitivity analysis 2), and replacing ULTRA2 anti-TNF-α-naive data with ULTRA2 ITT data plus including Suzuki et al. 46 (sensitivity analysis 3).
Clinical response and remission data were defined as outlined in Table 10 and were taken from two different sources. First, data relating to clinical response and remission for the use of interventions as induction treatment were extracted directly from the published RCT reports. Second, data relating to clinical response and remission for the use of interventions as maintenance treatments conditional on outcomes at previous timepoints were requested and received from the manufacturers of the products under assessment (MSD and AbbVie).
Statistical model for the network meta-analysis
Clinical response/remission can be considered as ordered categorical data with three mutually exclusive categories: (1) no response; (2) response; and (3) remission. The model for the data assumed that the treatment effect was the same irrespective of the category. Data available at 6 weeks and 8 weeks were combined, as were data available at 30 weeks and 32 weeks, and 52 weeks and 54 weeks. The likelihood function for the data is described as follows. Let rikj represent the number of patients in arm k of trial i in the mutually exclusive category j = 1, 2, . . . J. The responses rikj will follow a multinomial distribution such that:
The parameters in the model are the probabilities, pikj, that a patient in arm k of trial i has a response equivalent to category j.
We used a probit link function to map the probabilities, pikj, onto the real line such that:
so that:
In this model, the effect of treatment was to change the probit score of the control arm by δi,bk SDs.
The study-specific treatment effects, δi,bkIk ≠ 1, were assumed to arise from a common population distribution with mean treatment effect relative to the reference treatment, which in this analysis was PBO, such that:
We further assumed that there is an underlying continuous latent variable that has been categorised by specifying cut-off points zij, which corresponds to the point at which an individual moves from one category to the next in trial i. The model is rewritten as:
The zij can be treated as fixed, which would assume that these points are the same in each trial and each treatment. Alternatively, they can be treated as random in which they are assumed to vary according to the trial but that within a trial they are the same such that:
We used a model in which the zij were treated as being random because this resulted in a much better fit of the model to the data. Further details of the model are presented in Dias et al. 69
The model was completed by giving the parameters prior distributions. When there are sufficient sample data, we can use conventional reference prior distributions and these will have little influence on the posterior results.
The reference prior distributions used in the analyses were:
-
trial-specific baselines, µi ∼ N(0, 1000)
-
treatment effects relative to reference treatment, d1t ∼ N(0, 1000)
-
between-study SD of treatment effects, τ ∼ U(0,2)
-
population cut-off points, υcj=υcj−1+υc′, υc′∼U(0,5)
-
between-study SD of cut-off points, σz2∼U(0,2).
In both the induction and maintenance phases, there were relatively few studies to allow Bayesian updating of the implausibly vague prior distribution for the between-study SD. Without Bayesian updating, a reference prior distribution that does not represent genuine prior belief will have a significant impact on the results and give posterior distributions that are unlikely to represent genuine posterior beliefs. To allow for this, we used a weakly informative prior distribution (a half-normal distribution) for the between study SD such that τ ∼ HN (0,0.322).
To estimate the absolute probabilities of being in each category for each treatment, we combined the treatment effects with an estimate of the PBO ‘No response’ category (baseline model). We used a Binomial likelihood function for the number of patients, rik1 in each study who were classified as having ‘no response’ when treated with PBO for the baseline model such that:
We used a probit link function such that:
We assumed that the study-specific baselines arose from population of effects such that:
The model was completed by giving the parameters prior distributions such that:
-
population baseline effect, µb ∼ N(0, 1000)
-
between-study SD of the baseline effects, τb ∼ U(0, 2).
Again, in both the induction and maintenance phase there were relatively few studies providing data so a weakly informative prior distribution was used for the between-study SD such that:
All analyses were conducted in the freely available software package OpenBUGS version 3.2.3. 69,70 For the baseline and relative treatment effects models, we used a burn-in of 50,000 iterations of the Markov chain and retained a further 10,000 iterations to estimate parameters. In addition, the NMAs exhibited moderate correlation between successive iterations of the Markov chains so the chains were thinned by retaining every tenth sample.
The total residual deviance was used to formally assess whether or not the statistical model provided a reasonable representation of the sample data. The total residual deviance is the mean of the deviance under the current model minus the deviance for the saturated model, so that each data point should contribute about one to the deviance.
Results of network meta-analyses
A summary of the data used in the NMA is provided in Appendix 7. As described earlier, three sensitivity analyses were undertaken to assess the robustness of replacing ULTRA245 anti-TNF-α-naive data with ULTRA245 ITT data (sensitivity analysis 1), including Suzuki et al. 46 (sensitivity analysis 2), and replacing ULTRA2 anti-TNF-α-naive data with ULTRA245 ITT data and including Suzuki et al. 46 (sensitivity analysis 3). The results presented in Base case: induction phase to Sensitivity analysis 3: maintenance phase 32–52 weeks were derived using weakly informative prior distributions (a half-normal distribution) for the between-study SD such that τ ∼ HN(0, 0.322). Results using vague reference prior distributions [τ ∼ U(0,2)] are presented in Appendix 8.
Base case: induction phase
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the induction phase. Data were available from five studies comparing two treatments. 44,45,47,49 Figure 13 presents the network of evidence for the base-case induction phase.
FIGURE 13.
Base case: network of evidence for the induction phase. Note: solid line indicates a two-arm trial.
Figure 14 presents the effects of each treatment relative to PBO on the probit scale for the base-case induction phase. Figure 15 presents the probabilities of treatment rankings for this analysis. The model fitted the data reasonably well, with the total residual deviance, 18.16, being close to the total number of data points included in the analysis, 20. The between-study SD was estimated to be 0.12 [95% credible interval (CrI) 0.01 to 0.50], which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 14.
Base case: comparative effect of anti-TNF-α treatment on clinical response/remission in the induction phase [SD ∼ HN(0,0.322)].
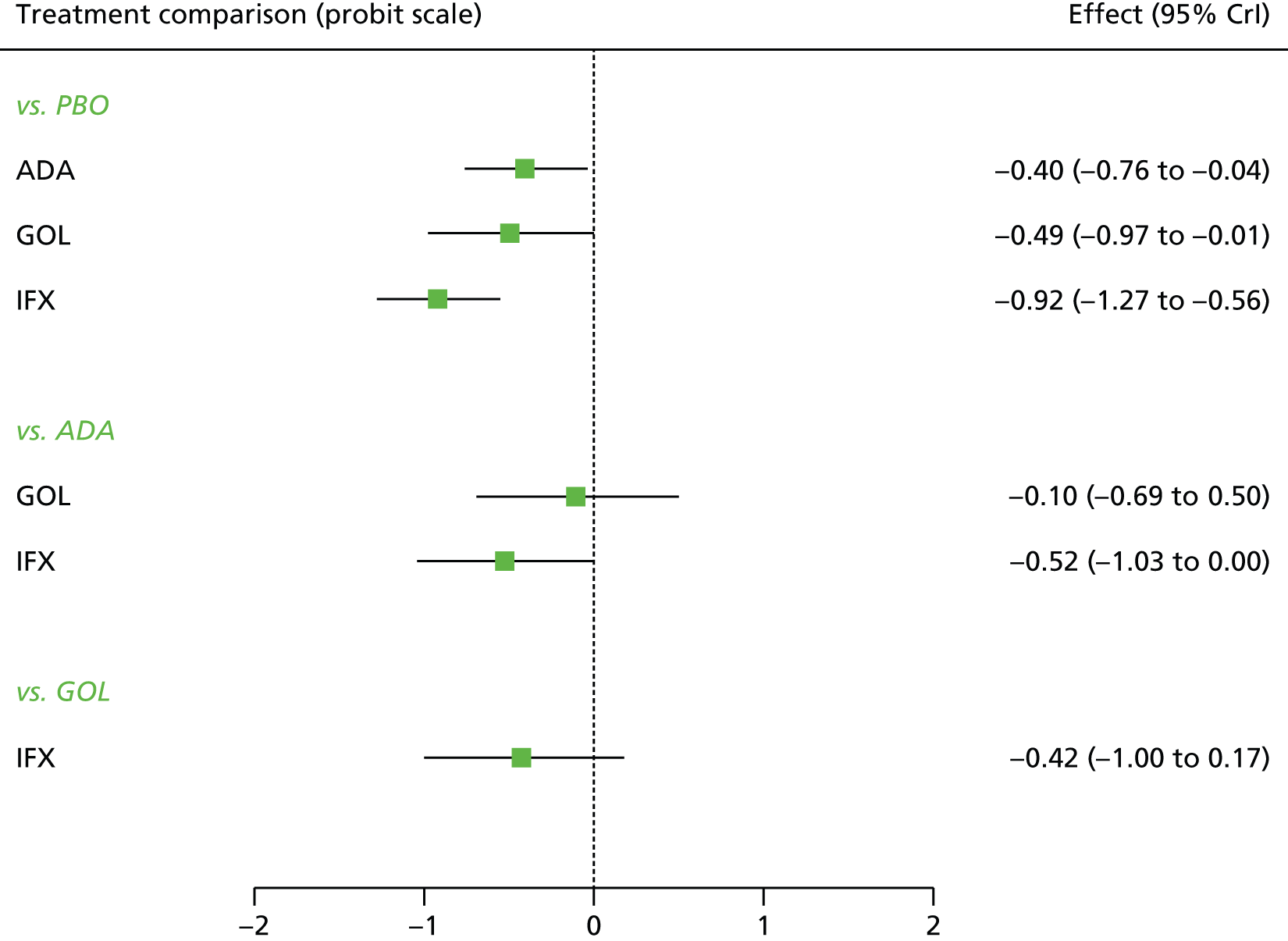
FIGURE 15.
Base case: ranking probability histograms for the induction phase.

All treatments were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with IFX. All treatment effects were statistically significant at a conventional 5% level. IFX was associated with the greatest effect –0.92 (95% CrI –1.27 to –0.56) and was most likely to be the most effective treatment (probability of being the best = 0.93).
Table 13 presents the probabilities of achieving each of the following categories: no response, response and remission for the base-case induction phase. IFX was associated with the highest probability of moving from no response to response and no response to remission respectively. The effects of ADA and GOL on each transition probability were comparable.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.640 | 0.641 | 0.568 to 0.706 | 0.260 | 0.260 | 0.214 to 0.308 | 0.099 | 0.097 | 0.062 to 0.147 |
| ADA | 0.485 | 0.485 | 0.330 to 0.642 | 0.324 | 0.327 | 0.247 to 0.385 | 0.190 | 0.185 | 0.092 to 0.322 |
| GOL | 0.448 | 0.447 | 0.262 to 0.645 | 0.333 | 0.337 | 0.244 to 0.393 | 0.219 | 0.212 | 0.094 to 0.390 |
| IFX | 0.292 | 0.289 | 0.170 to 0.438 | 0.351 | 0.353 | 0.280 to 0.412 | 0.356 | 0.352 | 0.209 to 0.523 |
Base case: maintenance phase 8–32 weeks
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase at 8–32 weeks for patients starting in response. Data were available from four studies comparing two or three treatments. 45,48,49 Figure 16 presents the network of evidence for the base-case maintenance phase at 8–32 weeks for patients starting in response.
FIGURE 16.
Base case: network of evidence for the maintenance phase at 8–32 weeks for patients starting in response. Note: solid line indicates a two-arm trial and dashed line indicates a three-arm study.
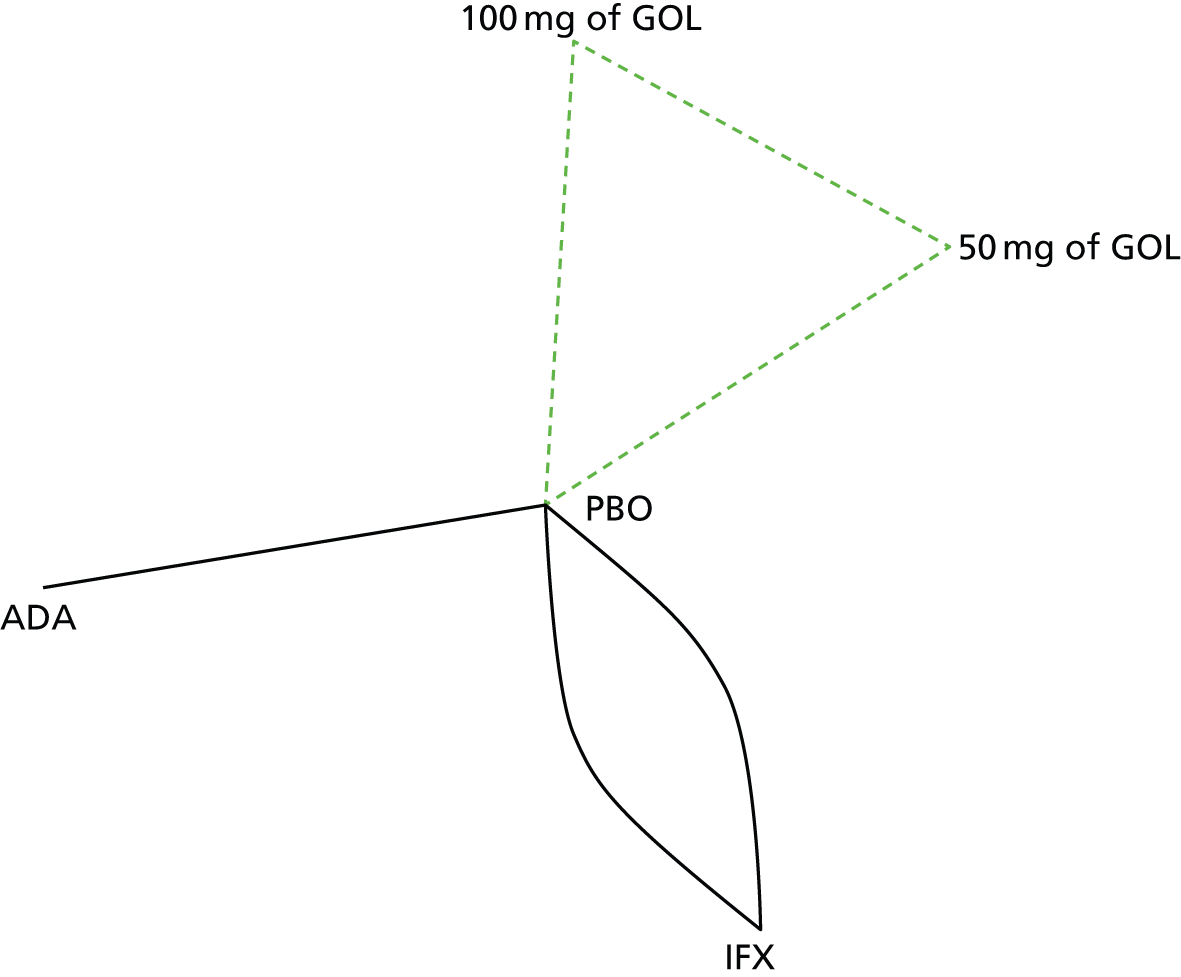
Figure 17 presents the effects of each treatment relative to PBO on the probit scale for the base-case maintenance phase at 8–32 weeks for patients starting in response. Figure 18 presents the probabilities of treatment rankings for this analysis. There was some suggestion that the model did not represent the data well with the total residual deviance, 11.73, being smaller than would be expected given the total number of data points included in the analysis, 18. The probability of observing a value < 11.73 was 0.139, which means that it could be a chance event. All four studies had smaller residual deviances than expected (ULTRA2: deviance 3.0 compared with four data points;45 ACT1: deviance 2.1 compared with four data points;49 ACT2: deviance 2.66 compared with four data points;49 and PURSUIT: deviance 4.0 compared with six data points). 48 The between-study SD was estimated to be 0.17 (95% CrI 0.01 to 0.61), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 17.
Base case: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in response [SD ∼ HN(0,0.322)].

FIGURE 18.
Base case: ranking probability histograms for the maintenance phase at 8–32 weeks for patients starting in response. Note: the horizontal axis represents the rank of each treatment, i.e., from the best rank (left hand side) to the worst rank (right hand side) within each treatment.
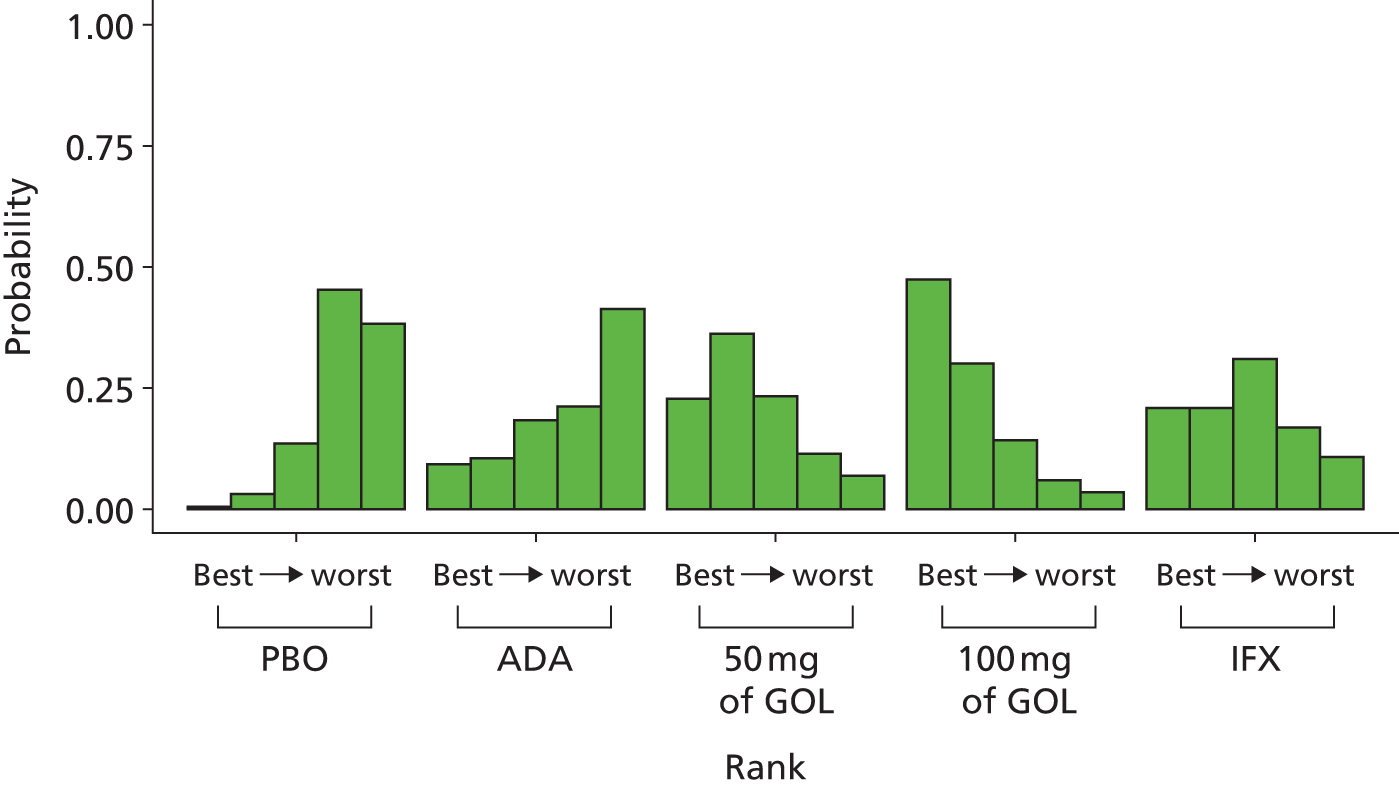
All treatments were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with 100 mg of GOL. However, none of the treatment effects were statistically significant at a conventional 5% level. 100 mg of GOL was associated with the greatest effect –0.42 (95% CrI –0.78 to 0.29) and was most likely to be the most effective treatment (probability of being the best = 0.47).
Table 14 presents the probabilities of achieving each of the following categories: no response, response and remission for the base-case maintenance phase at 8–32 weeks for patients starting in response. 100 mg of GOL was associated with the highest probability of moving from response to remission and staying in the response state at 8–32 weeks. It was also associated with the smallest probability of moving from response to no response. The probabilities of staying in response were comparable among all treatments at 8–32 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.524 | 0.525 | 0.426 to 0.622 | 0.270 | 0.270 | 0.198 to 0.341 | 0.206 | 0.202 | 0.117 to 0.311 |
| ADA | 0.512 | 0.512 | 0.230 to 0.782 | 0.261 | 0.267 | 0.140 to 0.354 | 0.227 | 0.211 | 0.055 to 0.493 |
| 50 mg of GOL | 0.403 | 0.399 | 0.173 to 0.660 | 0.283 | 0.285 | 0.176 to 0.374 | 0.313 | 0.303 | 0.108 to 0.588 |
| 100 mg of GOL | 0.368 | 0.360 | 0.149 to 0.619 | 0.285 | 0.288 | 0.176 to 0.377 | 0.347 | 0.338 | 0.129 to 0.623 |
| IFX | 0.432 | 0.430 | 0.220 to 0.659 | 0.282 | 0.283 | 0.189 to 0.371 | 0.286 | 0.276 | 0.109 to 0.518 |
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase at 8–32 weeks for patients starting in remission. Data were available from four studies comparing two or three treatments. 45,48,49 Figure 19 presents the network of evidence for the base-case maintenance phase at 8–32 weeks for patients starting in remission.
FIGURE 19.
Base case: network of evidence for the maintenance phase at 8–32 weeks for patients starting in remission. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.
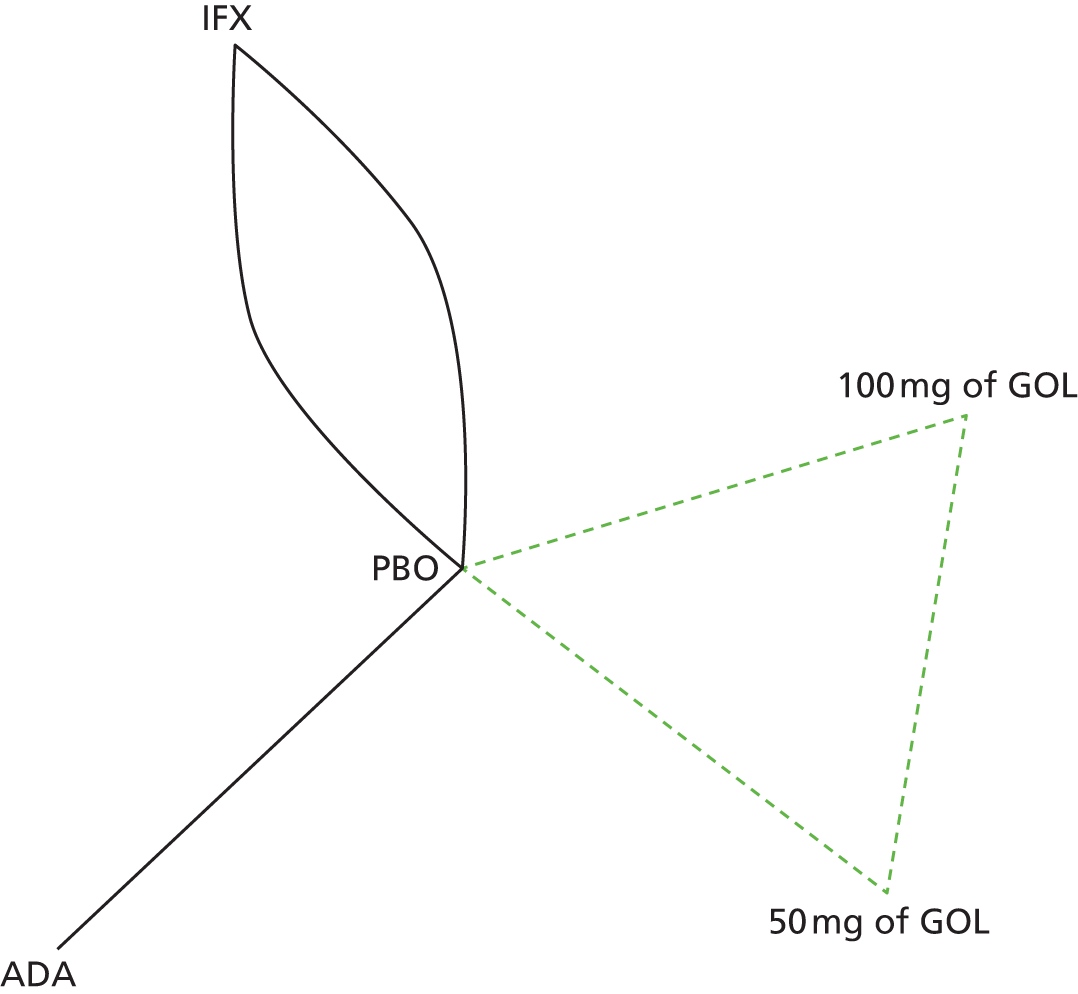
Figure 20 presents the effects of each treatment relative to PBO on the probit scale for the base-case maintenance phase at 8–32 weeks for patients starting in remission. Figure 21 presents the probabilities of treatment rankings for this analysis. The model fitted the data well, with the total residual deviance, 18.20, being close to the total number of data points included in the analysis, 18. The between-study SD was estimated to be 0.18 (95% CrI 0.01 to 0.64), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 20.
Base case: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in remission [SD ∼ HN(0,0.322)].

FIGURE 21.
Base case: ranking probability histograms for the maintenance phase at 8–32 weeks for patients starting in remission.
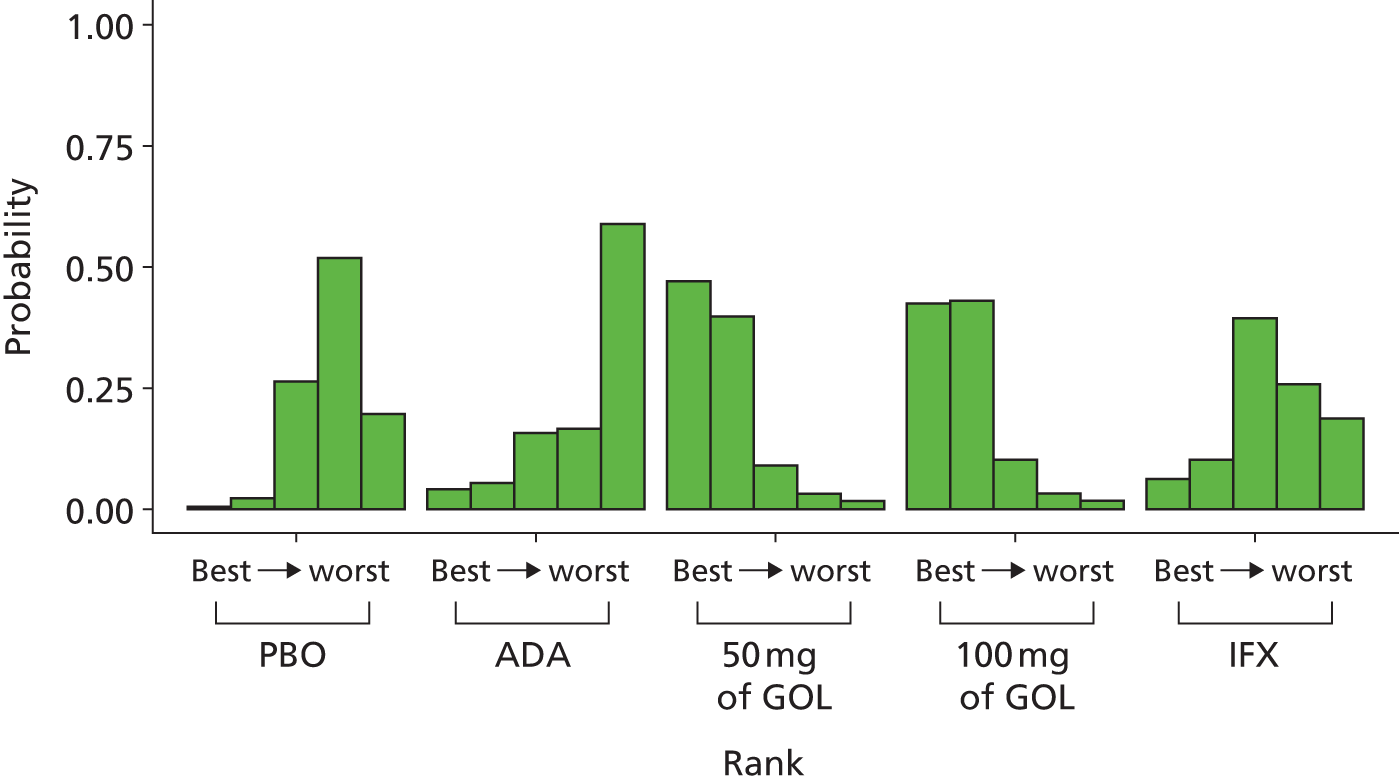
All treatments except ADA were associated with beneficial treatment effects relative to PBO with the greatest effects being associated with 50 mg of GOL (–0.63, 95% CrI –1.36 to 0.11) and 100 mg of GOL (–0.61, 95% CrI –1.32 to 0.11). However, none of the treatment effects was statistically significant at a conventional 5% level. 50 mg and 100 mg of GOL was most likely to be the most effective treatments (probability of being the best = 0.47 and 0.42 respectively).
Table 15 presents the probabilities of achieving each of the following categories: no response, response and remission for the base-case maintenance phase at 8–32 weeks for patients starting in remission. 50 mg and 100 mg of GOL were associated with the highest probability of staying in remission and the smallest probability of moving from remission to response or remission no response at 8–32 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.353 | 0.347 | 0.168 to 0.572 | 0.180 | 0.174 | 0.070 to 0.316 | 0.467 | 0.466 | 0.225 to 0.708 |
| ADA | 0.428 | 0.420 | 0.099 to 0.803 | 0.166 | 0.164 | 0.053 to 0.297 | 0.406 | 0.392 | 0.083 to 0.804 |
| 50 mg of GOL | 0.177 | 0.152 | 0.027 to 0.457 | 0.136 | 0.131 | 0.028 to 0.283 | 0.687 | 0.708 | 0.321 to 0.933 |
| 100 mg of GOL | 0.182 | 0.158 | 0.029 to 0.469 | 0.138 | 0.134 | 0.030 to 0.285 | 0.680 | 0.700 | 0.322 to 0.929 |
| IFX | 0.325 | 0.309 | 0.084 to 0.648 | 0.169 | 0.165 | 0.057 to 0.304 | 0.506 | 0.509 | 0.178 to 0.829 |
Base case: maintenance phase 32–52 weeks
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase for patients starting in response at 32–52 weeks. Data were available from three studies comparing two or three treatments. 45,48,49 Figure 22 presents the network of evidence for the base-case maintenance phase at 32–52 weeks for patients starting in response.
FIGURE 22.
Base case: network of evidence for the maintenance phase at 32–52 weeks for patients starting in response. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.

Figure 23 presents the effects of each treatment relative to PBO on the probit scale for the base-case maintenance phase 32–52 weeks for patients starting in response. Figure 24 presents the probabilities of treatment rankings for this analysis. The model fitted the data reasonably well, with the total residual deviance, 12.88, being close to the total number of data points included in the analysis, 14. The between-study SD was estimated to be 0.21 (95% CrI 0.01 to 0.71), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 23.
Base case: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in response [SD ∼ HN(0,0.322)].

FIGURE 24.
Base case: ranking probability histograms for the maintenance phase at 32–52 weeks for patients starting in response.

All treatments except 100 mg of ADA and GOL were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with 50 mg of GOL; however, none of the treatment effects was statistically significant at a conventional 5% level. IFX was associated with the greatest effect –0.36 (95% CrI –1.33 to 0.62) and was most likely to be the most effective treatment (probability of being the best = 0.56).
Table 16 presents the probabilities of achieving each of the following categories: no response, response and remission for the base-case maintenance phase at 32–52 weeks for patients starting in response. IFX was associated with the highest probability of moving from response to remission and the smallest probability of moving from response to no response at 32–52 weeks. The probabilities of staying in the response state were comparable among treatments at 32–52 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.338 | 0.319 | 0.066 to 0.711 | 0.370 | 0.378 | 0.122 to 0.604 | 0.292 | 0.259 | 0.027 to 0.717 |
| ADA | 0.450 | 0.440 | 0.063 to 0.889 | 0.327 | 0.340 | 0.067 to 0.562 | 0.223 | 0.167 | 0.005 to 0.716 |
| 50 mg of GOL | 0.295 | 0.258 | 0.025 to 0.750 | 0.353 | 0.363 | 0.081 to 0.616 | 0.352 | 0.319 | 0.021 to 0.842 |
| 100 mg of GOL | 0.410 | 0.393 | 0.055 to 0.852 | 0.342 | 0.353 | 0.083 to 0.581 | 0.248 | 0.199 | 0.009 to 0.741 |
| IFX | 0.250 | 0.205 | 0.013 to 0.716 | 0.341 | 0.353 | 0.065 to 0.621 | 0.409 | 0.385 | 0.029 to 0.892 |
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase at 32–52 weeks for patients starting in remission. Data were available from three studies comparing two or three treatments. 45,48,49 Figure 25 presents the network of evidence for the base-case maintenance phase at 32–52 weeks for patients starting in remission.
FIGURE 25.
Base case: network of evidence for the maintenance phase at 32–52 weeks for patients starting in remission. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.

Figure 26 presents the effects of each treatment relative to PBO on the probit scale for the base-case maintenance phase at 32–52 weeks for patients starting in remission. Figure 27 presents the probabilities of treatment rankings for this analysis. The model fitted the data well, with the total residual deviance, 18.46, being close to the total number of data points included in the analysis, 18. The between-study SD was estimated to be 0.21 (95% CrI 0.01 to 0.72), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 26.
Base case: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in remission [SD ∼ HN(0,0.322)].
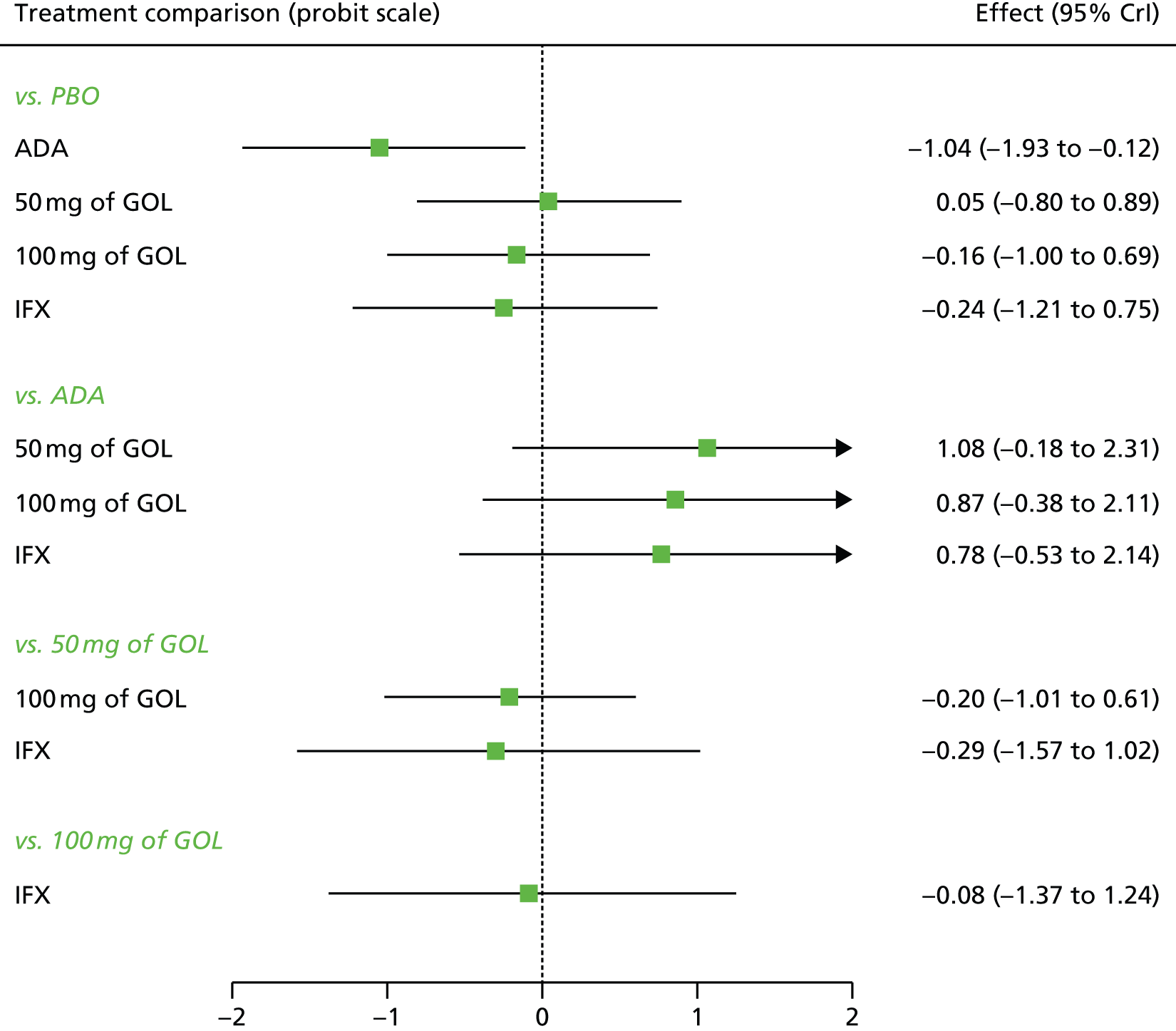
FIGURE 27.
Base case: ranking probability histograms for the maintenance phase at 32–52 weeks for patients starting in remission.

All treatments except 50 mg of GOL were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with ADA. However, only the treatment effects of ADA were statistically significant at a conventional 5% level. ADA was associated with the greatest effect –1.04 (95% CrI –1.93 to –0.12) and was most likely to be the most effective treatment (probability of being the best = 0.84).
Table 17 presents the probabilities of achieving each of the following categories: no response, response and remission for the base-case maintenance phase at 32–52 weeks for patients starting in remission. ADA was associated with the highest probability of staying in remission and the smallest probability of moving from remission to response or from remission to no response at 32–52 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.301 | 0.296 | 0.174 to 0.449 | 0.164 | 0.147 | 0.029 to 0.449 | 0.536 | 0.548 | 0.237 to 0.734 |
| ADA | 0.081 | 0.059 | 0.005 to 0.288 | 0.084 | 0.061 | 0.005 to 0.337 | 0.834 | 0.874 | 0.447 to 0.985 |
| 50 mg of GOL | 0.329 | 0.314 | 0.080 to 0.664 | 0.155 | 0.141 | 0.024 to 0.415 | 0.515 | 0.523 | 0.135 to 0.851 |
| 100 mg of GOL | 0.266 | 0.245 | 0.052 to 0.604 | 0.147 | 0.132 | 0.020 to 0.417 | 0.587 | 0.604 | 0.169 to 0.894 |
| IFX | 0.247 | 0.220 | 0.033 to 0.613 | 0.140 | 0.126 | 0.017 to 0.413 | 0.613 | 0.634 | 0.174 to 0.928 |
Sensitivity analysis 1: induction phase
Sensitivity analysis 1 involved replacing ULTRA2 anti-TNF-α-naive data with ULTRA2 ITT data. A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the induction phase. Data were available from five studies comparing two treatments. 44,45,47,49 Figure 28 presents the network of evidence for the sensitivity analysis 1 induction phase.
FIGURE 28.
Sensitivity analysis 1: network of evidence for the induction phase. Note: solid line indicates a two-arm trial.

Figure 29 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 1 induction phase. Figure 30 presents the probabilities of treatment rankings for this analysis. The model fitted the data reasonably well, with the total residual deviance, 17.08, being close to the total number of data points included in the analysis, 20. The between-study SD was estimated to be 0.11 (95% CrI 0.01 to 0.47), which implies mild heterogeneity between studies in treatment effects.
FIGURE 29.
Sensitivity analysis 1: comparative effect of anti-TNF-α treatment on clinical response/remission in the induction phase [SD ∼ HN(0,0.322)].

FIGURE 30.
Sensitivity analysis 1: ranking probability histograms for the induction phase.
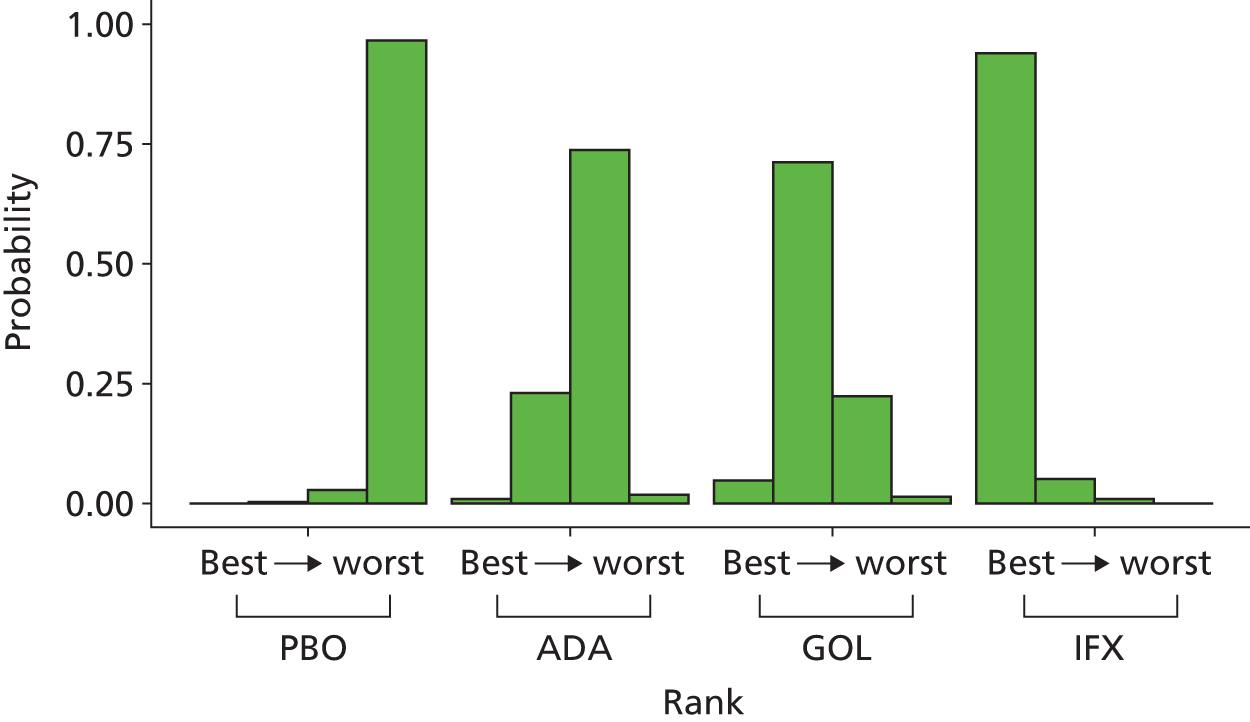
All treatments were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with IFX (–0.91, 95% CrI –1.25 to –0.57). All treatment effects were statistically significant at a conventional 5% level. IFX was most likely to be the most effective treatment (probability of being the best = 0.94).
Table 18 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 1 induction phase. IFX was associated with the highest probability of moving from no response to response and from no response to remission.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.649 | 0.649 | 0.586 to 0.710 | 0.255 | 0.255 | 0.212 to 0.298 | 0.096 | 0.095 | 0.062 to 0.140 |
| ADA | 0.513 | 0.512 | 0.372 to 0.652 | 0.315 | 0.317 | 0.240 to 0.375 | 0.173 | 0.169 | 0.088 to 0.286 |
| GOL | 0.456 | 0.456 | 0.283 to 0.631 | 0.330 | 0.334 | 0.250 to 0.389 | 0.214 | 0.207 | 0.101 to 0.368 |
| IFX | 0.302 | 0.298 | 0.180 to 0.441 | 0.351 | 0.353 | 0.281 to 0.409 | 0.347 | 0.345 | 0.208 to 0.506 |
Sensitivity analysis 1: maintenance phase 8–32 weeks
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase for patients starting in response at 8–32 weeks. Data were available from four studies comparing two or three treatments. 45,48,49 Figure 31 presents the network of evidence for the sensitivity analysis 1 maintenance phase at 8–32 weeks for patients starting in response.
FIGURE 31.
Sensitivity analysis 1: network of evidence for the maintenance phase at 8–32 weeks for patients starting in response. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.
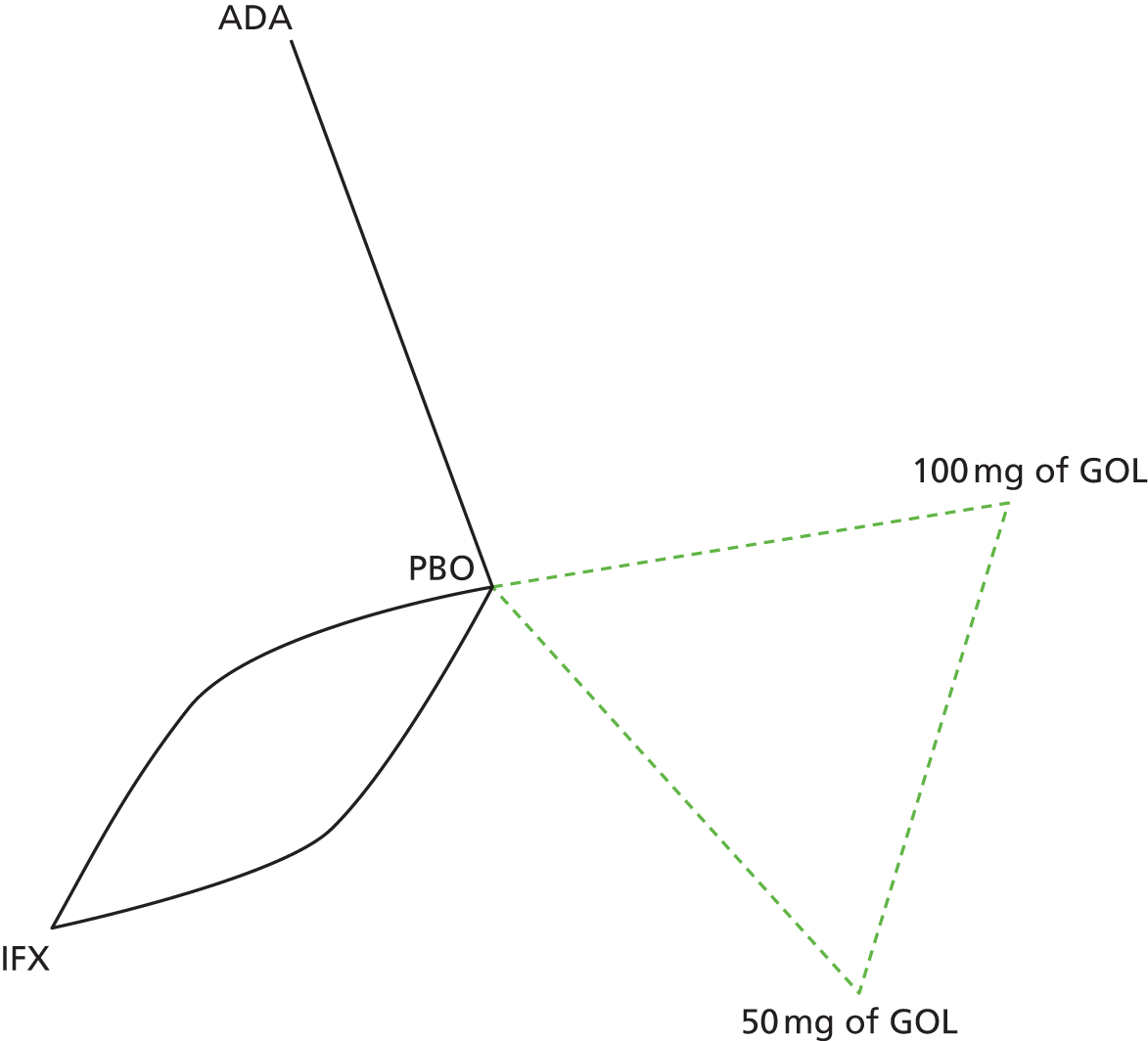
Figure 32 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 1 maintenance phase at 8–32 weeks for patients starting in response. Figure 33 presents the probabilities of treatment rankings for this analysis. There was some suggestion that model did not represent the data well with the total residual deviance, 11.54, being smaller than would be expected given the total number of data points included in the analysis, 18. The probability of observing a value < 11.54 was 0.130, which means that this could be a chance event. Similar to the base-case analysis, all four studies had smaller residual deviances than expected. The between-study SD was estimated to be 0.17 (95% CrI 0.01 to 0.63), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 32.
Sensitivity analysis 1: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in response [SD ∼ HN(0,0.322)].

FIGURE 33.
Sensitivity analysis 1: ranking probability histograms for the maintenance phase at 8–32 weeks for patients starting in response.

All treatments were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with 100 mg of GOL (–0.41, 95% CrI –1.06 to 0.22); however, none of the treatment effects was statistically significant at a conventional 5% level. A treatment of 100 mg of GOL was most likely to be the most effective (probability of being the best = 0.43).
Table 19 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 1 maintenance phase at 8–32 weeks starting in response. A treatment of 100 mg of GOL was associated with the highest probability of moving from response to remission and the smallest probability of moving from response to no response at 8–32 weeks. The probabilities of staying in the response state were comparable among treatments.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.548 | 0.548 | 0.447 to 0.649 | 0.269 | 0.267 | 0.199 to 0.361 | 0.183 | 0.181 | 0.085 to 0.282 |
| ADA | 0.468 | 0.467 | 0.210 to 0.744 | 0.279 | 0.283 | 0.158 to 0.391 | 0.252 | 0.240 | 0.059 to 0.525 |
| 50 mg of GOL | 0.427 | 0.423 | 0.190 to 0.688 | 0.289 | 0.289 | 0.176 to 0.412 | 0.284 | 0.273 | 0.081 to 0.552 |
| 100 mg of GOL | 0.390 | 0.384 | 0.162 to 0.649 | 0.293 | 0.292 | 0.182 to 0.421 | 0.318 | 0.310 | 0.098 to 0.591 |
| IFX | 0.453 | 0.451 | 0.237 to 0.685 | 0.286 | 0.287 | 0.186 to 0.403 | 0.260 | 0.252 | 0.082 to 0.490 |
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase at 8–32 weeks for patients starting in remission. Data were available from four studies comparing two or three treatments. 45,48,49 Figure 34 presents the network of evidence for the sensitivity analysis 1 maintenance phase at 8–32 weeks for patients starting in remission.
FIGURE 34.
Sensitivity analysis 1: network of evidence for the maintenance phase at 8–32 weeks for patients starting in remission. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.
Figure 35 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 1 maintenance phase at 8–32 weeks for patients starting in remission. Figure 36 presents the probabilities of treatment rankings for this analysis. The model fitted the data reasonably well, with the total residual deviance, 15.29, being close to the total number of data points included in the analysis, 18. The between-study SD was estimated to be 0.19 (95% CrI 0.01 to 0.65), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 35.
Sensitivity analysis 1: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in remission [SD ∼ HN(0,0.322)].
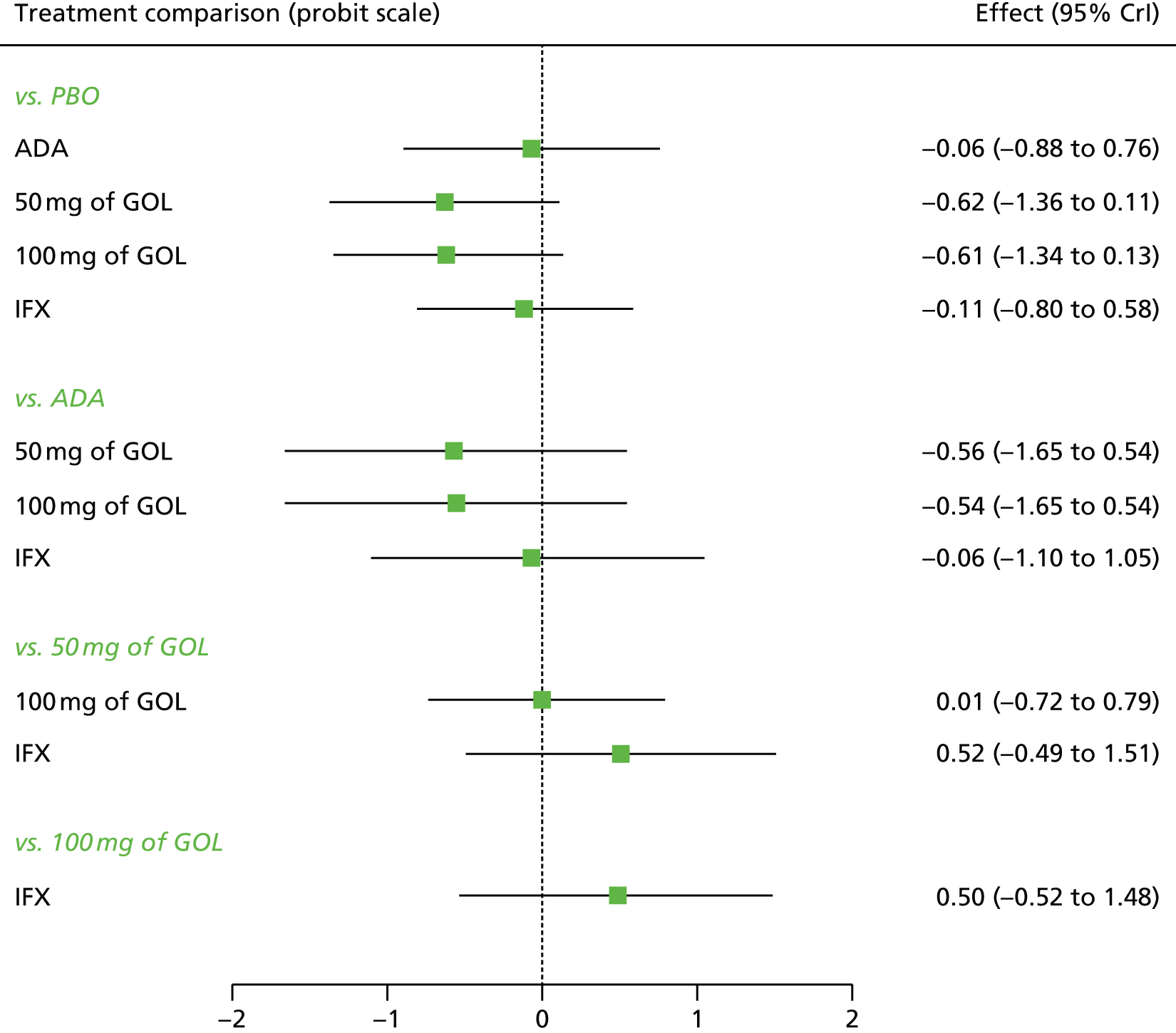
FIGURE 36.
Sensitivity analysis 1: ranking probability histograms for the maintenance phase at 8–32 weeks for patients starting in remission.
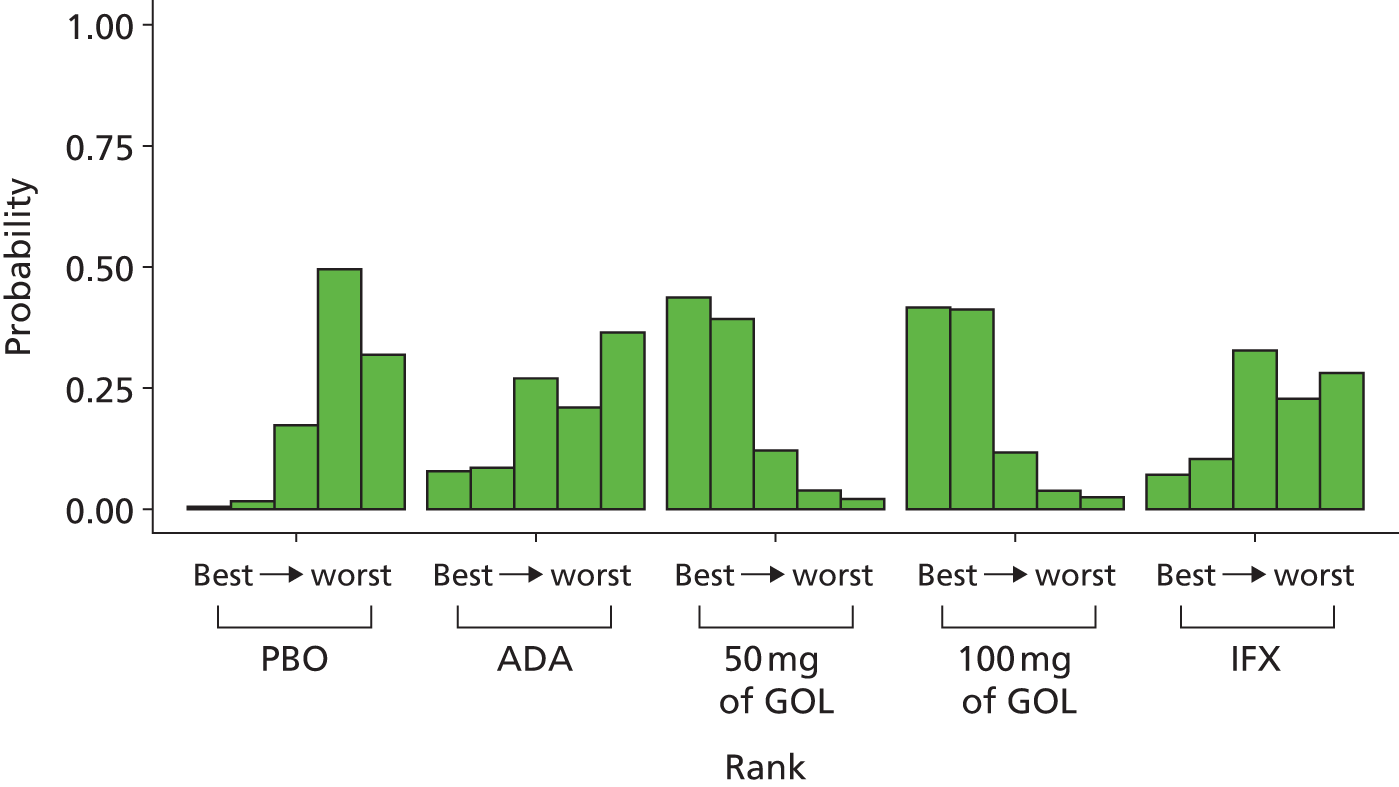
All treatments were associated with beneficial treatment effects relative to PBO with the greatest effects being associated with 50 mg of GOL (–0.62, 95% CrI –1.36 to 0.11) and 100 mg of GOL (–0.61, 95% CrI –1.34 to 0.13). However, none of the treatment effects was statistically significant at a conventional 5% level. A treatment of 50 mg and 100 mg of GOL were most likely to be the most effective (probability of being the best = 0.44 and 0.41 respectively).
Table 20 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 1 maintenance phase at 8–32 weeks starting in remission. A treatment of 50 mg and 100 mg of GOL were associated with the highest probability of staying in remission and the smallest probability of moving from remission to no response and from remission to response at 8–32 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.392 | 0.389 | 0.217 to 0.584 | 0.180 | 0.175 | 0.078 to 0.309 | 0.428 | 0.426 | 0.218 to 0.650 |
| ADA | 0.379 | 0.365 | 0.096 to 0.736 | 0.167 | 0.164 | 0.06 to 0.3 | 0.454 | 0.450 | 0.128 to 0.807 |
| 50 mg of GOL | 0.204 | 0.182 | 0.036 to 0.493 | 0.143 | 0.139 | 0.036 to 0.285 | 0.653 | 0.669 | 0.300 to 0.914 |
| 100 mg of GOL | 0.207 | 0.188 | 0.038 to 0.494 | 0.144 | 0.140 | 0.037 to 0.287 | 0.648 | 0.662 | 0.303 to 0.911 |
| IFX | 0.359 | 0.347 | 0.107 to 0.679 | 0.170 | 0.166 | 0.065 to 0.301 | 0.471 | 0.470 | 0.165 to 0.793 |
Sensitivity analysis 1: maintenance phase 32–52 weeks
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase for patients starting in response at 32–52 weeks. Data were available from three studies comparing two or three treatments. 45,48,49 Figure 37 presents the network of evidence for the sensitivity analysis 1 maintenance phase at 32–52 weeks for patients starting in response.
FIGURE 37.
Sensitivity analysis 1: network of evidence for the maintenance phase at 32–52 weeks for patients starting in response. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.
Figure 38 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 1 maintenance phase 32–52 weeks for patients starting in response. Figure 39 presents the probabilities of treatment rankings for this analysis. The model fitted the data reasonably well, with the total residual deviance, 12.32, being close to the total number of data points included in the analysis, 14. The between-study SD was estimated to be 0.21 (95% CrI 0.01 to 0.72), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 38.
Sensitivity analysis 1: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in response [SD ∼ HN(0,0.322)].
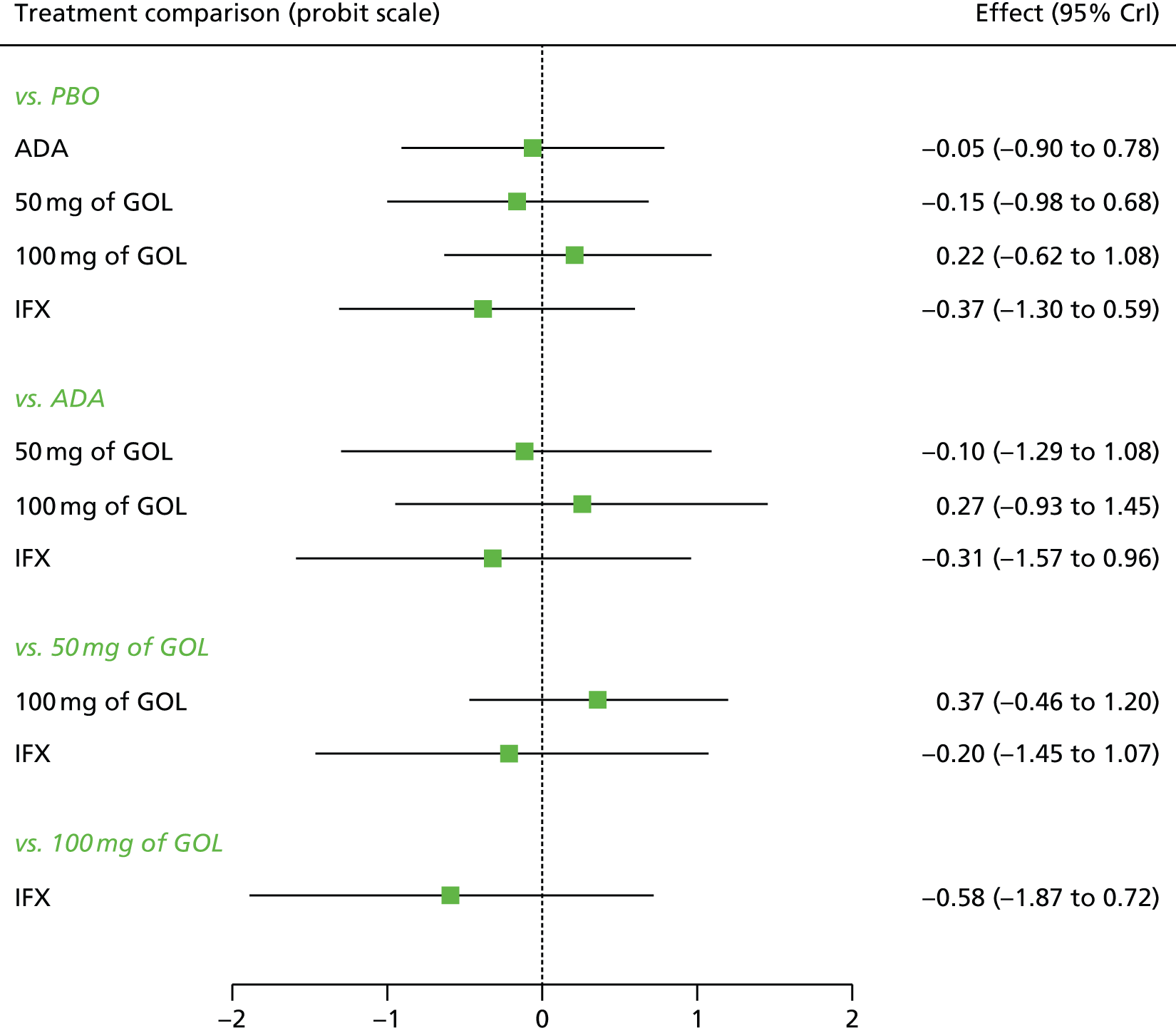
FIGURE 39.
Sensitivity analysis 1: ranking probability histograms for the maintenance phase at 32–52 weeks for patients starting in response.

All treatments except 100 mg of GOL were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with IFX (–0.37; 95% CrI –1.30 to 0.59); however, none of the treatment effects was statistically significant at a conventional 5% level. IFX was most likely to be the most effective treatment (probability of being the best = 0.52).
Table 21 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 1 maintenance phase at 32–52 weeks starting in response. IFX was associated with the highest probability of moving from response to remission and the smallest probability of moving from response to no response at 32–52 weeks. The probabilities of staying in the response state were comparable among treatments at 32–52 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.338 | 0.317 | 0.064 to 0.718 | 0.373 | 0.378 | 0.118 to 0.628 | 0.29 | 0.259 | 0.019 to 0.714 |
| ADA | 0.332 | 0.300 | 0.031 to 0.790 | 0.354 | 0.363 | 0.084 to 0.625 | 0.314 | 0.276 | 0.013 to 0.812 |
| 50 mg of GOL | 0.302 | 0.269 | 0.024 to 0.769 | 0.355 | 0.364 | 0.077 to 0.632 | 0.344 | 0.308 | 0.015 to 0.844 |
| 100 mg of GOL | 0.417 | 0.401 | 0.053 to 0.854 | 0.341 | 0.352 | 0.077 to 0.595 | 0.242 | 0.192 | 0.006 to 0.742 |
| IFX | 0.249 | 0.201 | 0.012 to 0.730 | 0.343 | 0.353 | 0.063 to 0.643 | 0.408 | 0.387 | 0.022 to 0.898 |
Patients starting in remission
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase at 32–52 weeks for patients starting in remission. Data were available from three studies comparing two or three treatments. 45,48,49 Figure 40 presents the network of evidence for the sensitivity analysis 1 maintenance phase at 32–52 weeks for patients starting in remission.
FIGURE 40.
Sensitivity analysis 1: network of evidence for the maintenance phase at 32–52 weeks for patients starting in remission. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.
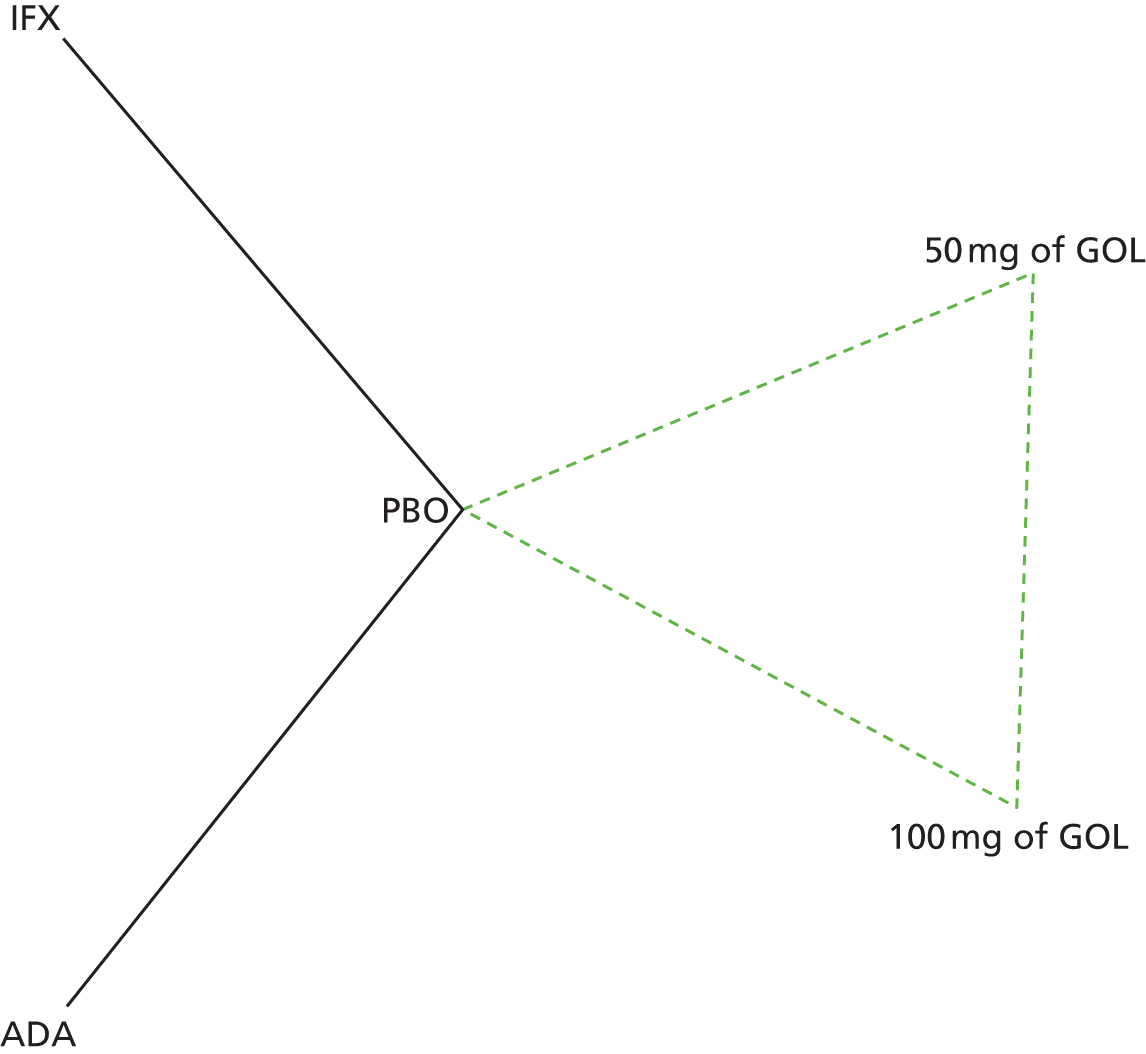
Figure 41 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 1 maintenance phase at 32–52 weeks for patients starting in remission. Figure 42 presents the probabilities of treatment rankings for this analysis. The model fitted the data reasonably well, with the total residual deviance, 17.73, being close to the total number of data points included in the analysis, 14. The between-study SD was estimated to be 0.22 (95% CrI 0.01 to 0.72), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 41.
Sensitivity analysis 1: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in remission [SD ∼ HN(0,0.322)].

FIGURE 42.
Sensitivity analysis 1: ranking probability histograms for the maintenance phase at 32–52 weeks for patients starting in remission.

All treatments except 50 mg of GOL were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with ADA (–0.86; 95% CrI –1.71 to 0.00); however, only the treatment effects of ADA were statistically significant at a conventional 5% level. ADA was most likely to be the most effective treatment (probability of being the best = 0.78).
Table 22 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 1 maintenance phase at 32–52 weeks starting in remission. ADA was associated with the highest probability of staying in remission and the smallest probability of moving from remission to response or from remission to no response at 32–52 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.302 | 0.299 | 0.177 to 0.448 | 0.167 | 0.155 | 0.030 to 0.396 | 0.530 | 0.539 | 0.270 to 0.727 |
| ADA | 0.104 | 0.082 | 0.010 to 0.324 | 0.098 | 0.081 | 0.008 to 0.308 | 0.797 | 0.828 | 0.426 to 0.974 |
| 50 mg of GOL | 0.324 | 0.307 | 0.082 to 0.664 | 0.158 | 0.147 | 0.026 to 0.374 | 0.519 | 0.526 | 0.145 to 0.842 |
| 100 mg of GOL | 0.260 | 0.239 | 0.053 to 0.591 | 0.149 | 0.136 | 0.022 to 0.374 | 0.591 | 0.605 | 0.200 to 0.890 |
| IFX | 0.254 | 0.225 | 0.035 to 0.620 | 0.144 | 0.132 | 0.019 to 0.367 | 0.603 | 0.620 | 0.186 to 0.918 |
Sensitivity analysis 2: induction phase
Sensitivity analysis 2 involved including Suzuki et al. 46 A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the induction phase. Data were available from six studies comparing two treatments. 44–47,49 Figure 43 presents the network of evidence for the sensitivity analysis 2 induction phase.
FIGURE 43.
Sensitivity analysis 2: network of evidence for the induction phase. Note: solid line indicates a two-arm trial.

Figure 44 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 2 induction phase. Figure 45 presents the probabilities of treatment rankings for this analysis. The model fitted the data well, with the total residual deviance, 24.36, being close to the total number of data points included in the analysis, 24. The between-study SD was estimated to be 0.10 (95% CrI 0.01 to 0.41), which implies mild heterogeneity between studies in treatment effects.
FIGURE 44.
Sensitivity analysis 2: comparative effect of anti-TNF-α treatment on clinical response/remission in the induction phase [SD ∼ HN(0,0.322)].
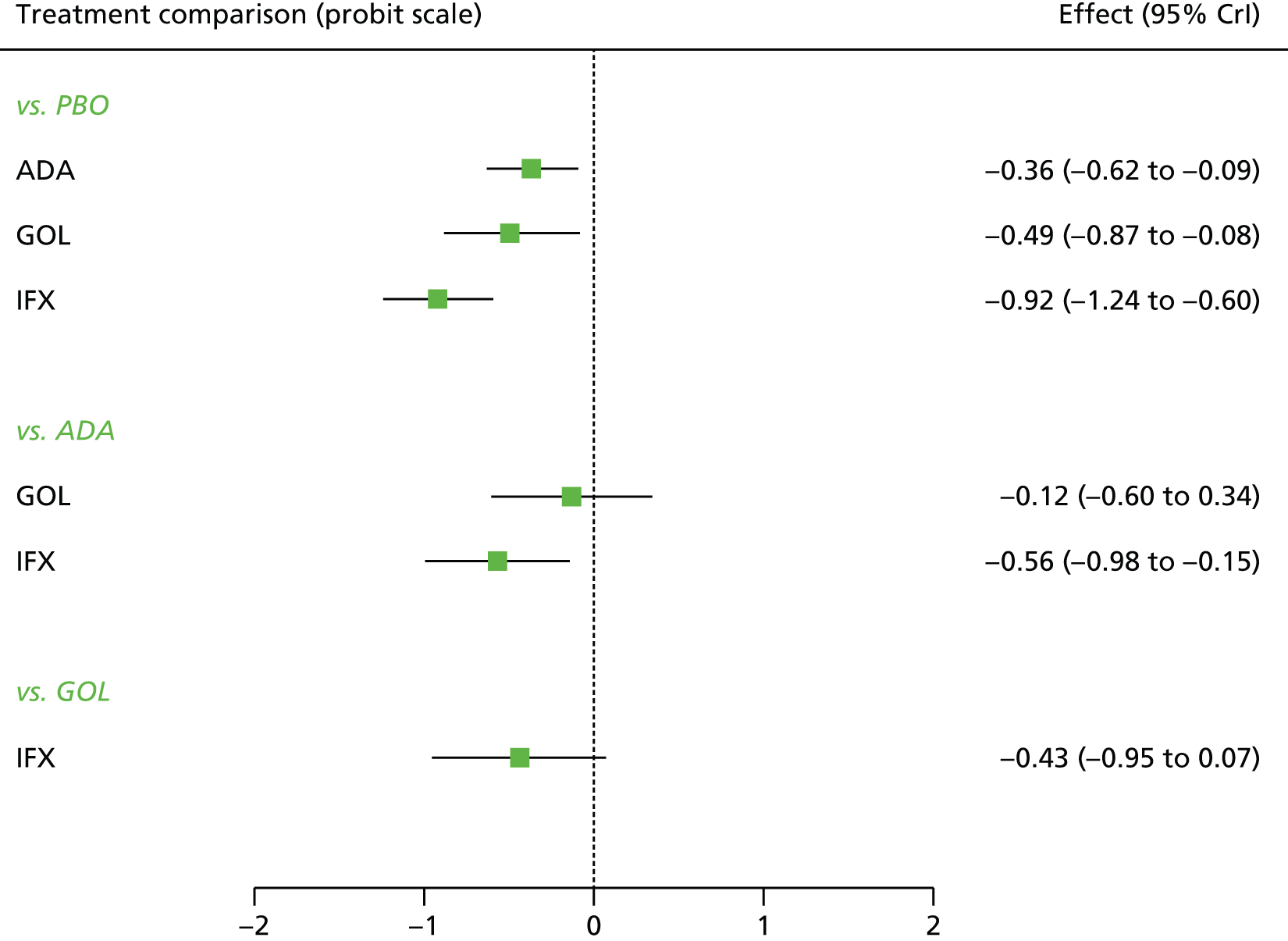
FIGURE 45.
Sensitivity analysis 2: ranking probability histograms for the induction phase.
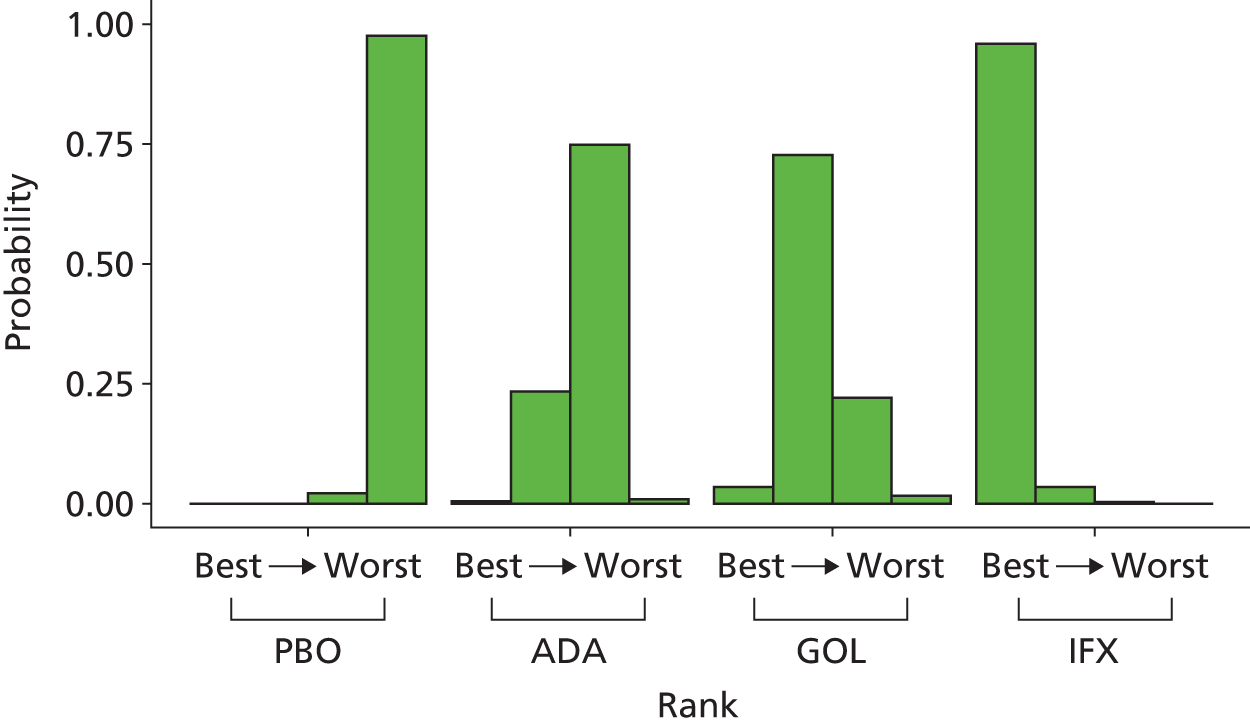
All treatments were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with IFX (–0.92, 95% CrI –1.24 to –0.60). All treatment effects were statistically significant at a conventional 5% level. IFX was most likely to be the most effective treatment (probability of being the best = 0.96).
Table 23 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 2 induction phase. IFX was associated with the highest probability of moving from no response to response and from no response to remission.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.642 | 0.642 | 0.585 to 0.696 | 0.260 | 0.263 | 0.225 to 0.301 | 0.095 | 0.094 | 0.065 to 0.132 |
| ADA | 0.502 | 0.500 | 0.384 to 0.623 | 0.326 | 0.327 | 0.264 to 0.377 | 0.173 | 0.170 | 0.099 to 0.264 |
| GOL | 0.452 | 0.450 | 0.298 to 0.624 | 0.339 | 0.343 | 0.266 to 0.392 | 0.209 | 0.204 | 0.060 to 0.344 |
| IFX | 0.292 | 0.252 | 0.183 to 0.421 | 0.360 | 0.360 | 0.305 to 0.411 | 0.348 | 0.347 | 0.068 to 0.488 |
Sensitivity analysis 2: maintenance phase 8–32 weeks
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase response at 8–32 weeks for patients starting in response. Data were available from five studies comparing two or three treatments. 45,46,48,49 Figure 46 presents the network of evidence for the sensitivity analysis 2 maintenance phase at 8–32 weeks for patients starting in response.
FIGURE 46.
Sensitivity analysis 2: network of evidence for the maintenance phase at 8–32 weeks for patients starting in response. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.
Figure 47 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 2 maintenance phase at 8–32 weeks for patients starting in response. Figure 48 presents the probabilities of treatment rankings for this analysis. There was some suggestion that model did not represent the data well with the total residual deviance, 14.80, being smaller than would be expected given the total number of data points included in the analysis, 22. The probability of observing a value < 14.8 is 0.129, which means that this could be a chance event. Similar to the base-case analysis, all five studies had smaller residual deviance than expected. The between-study SD was estimated to be 0.16 (95% CrI 0.01 to 0.58), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 47.
Sensitivity analysis 2: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in response [SD ∼ HN(0,0.322)].
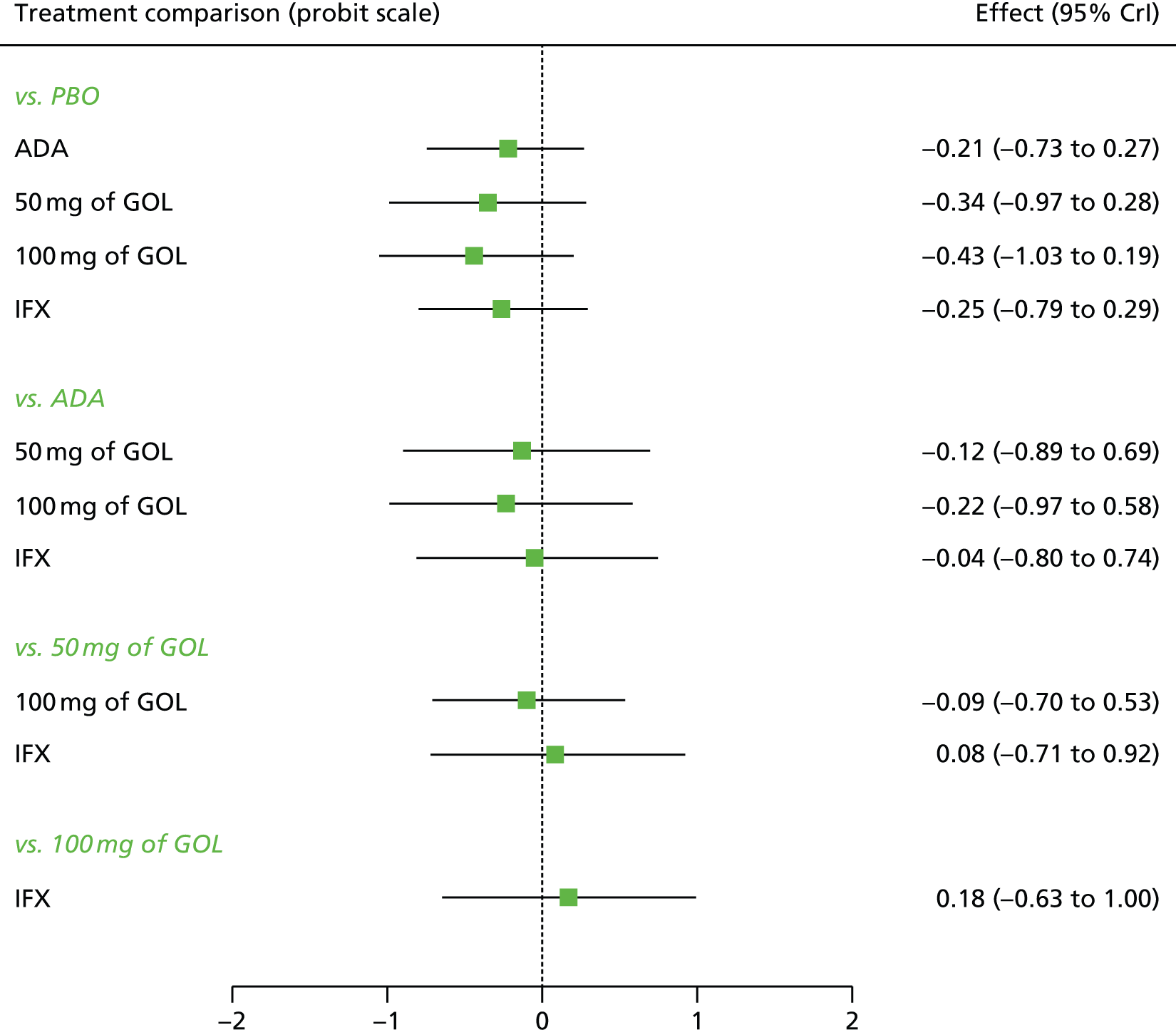
FIGURE 48.
Sensitivity analysis 2: ranking probability histograms for the maintenance phase at 8–32 weeks for patients starting in response.

All treatments were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with 100 mg of GOL (–0.43; 95% CrI–1.03 to 0.19); however, none of the treatment effects was statistically significant at a conventional 5% level. A treatment of 100 mg of GOL was most likely to be the most effective (probability of being the best = 0.44).
Table 24 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 2 maintenance phase at 8–32 weeks for patients starting in response. A treatment of 100 mg of GOL was associated with the highest probability of moving from response to remission and the smallest probability of moving from response to no response at 8–32 weeks. The probabilities of staying in the response state were comparable among treatments.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.525 | 0.525 | 0.437 to 0.609 | 0.274 | 0.274 | 0.220 to 0.329 | 0.201 | 0.199 | 0.130 to 0.286 |
| ADA | 0.441 | 0.441 | 0.238 to 0.647 | 0.286 | 0.288 | 0.209 to 0.354 | 0.273 | 0.263 | 0.116 to 0.485 |
| 50 mg of GOL | 0.398 | 0.393 | 0.174 to 0.649 | 0.289 | 0.292 | 0.199 to 0.357 | 0.313 | 0.305 | 0.116 to 0.569 |
| 100 mg of GOL | 0.363 | 0.356 | 0.158 to 0.617 | 0.291 | 0.293 | 0.200 to 0.360 | 0.346 | 0.338 | 0.132 to 0.598 |
| IFX | 0.429 | 0.425 | 0.222 to 0.657 | 0.287 | 0.289 | 0.204 to 0.352 | 0.284 | 0.276 | 0.110 to 0.504 |
Patients starting in remission
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase at 8–32 weeks for patients starting in remission. Data were available from five studies comparing two or three treatments. 45,46,48,49 Figure 49 presents the network of evidence for the sensitivity analysis 2 maintenance phase at 8–32 weeks for patients starting in remission.
FIGURE 49.
Sensitivity analysis 2: network of evidence for the maintenance phase at 8–32 weeks for patients starting in remission. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.

Figure 50 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 2 maintenance phase at 8–32 weeks for patients starting in remission. Figure 51 presents the probabilities of treatment rankings for this analysis. The model fitted the data well, with the total residual deviance, 21.05, being close to the total number of data points included in the analysis, 22. The between-study SD was estimated to be 0.17 (95% CrI 0.01 to 0.60), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 50.
Sensitivity analysis 2: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in remission [SD ∼ HN(0,0.322)].
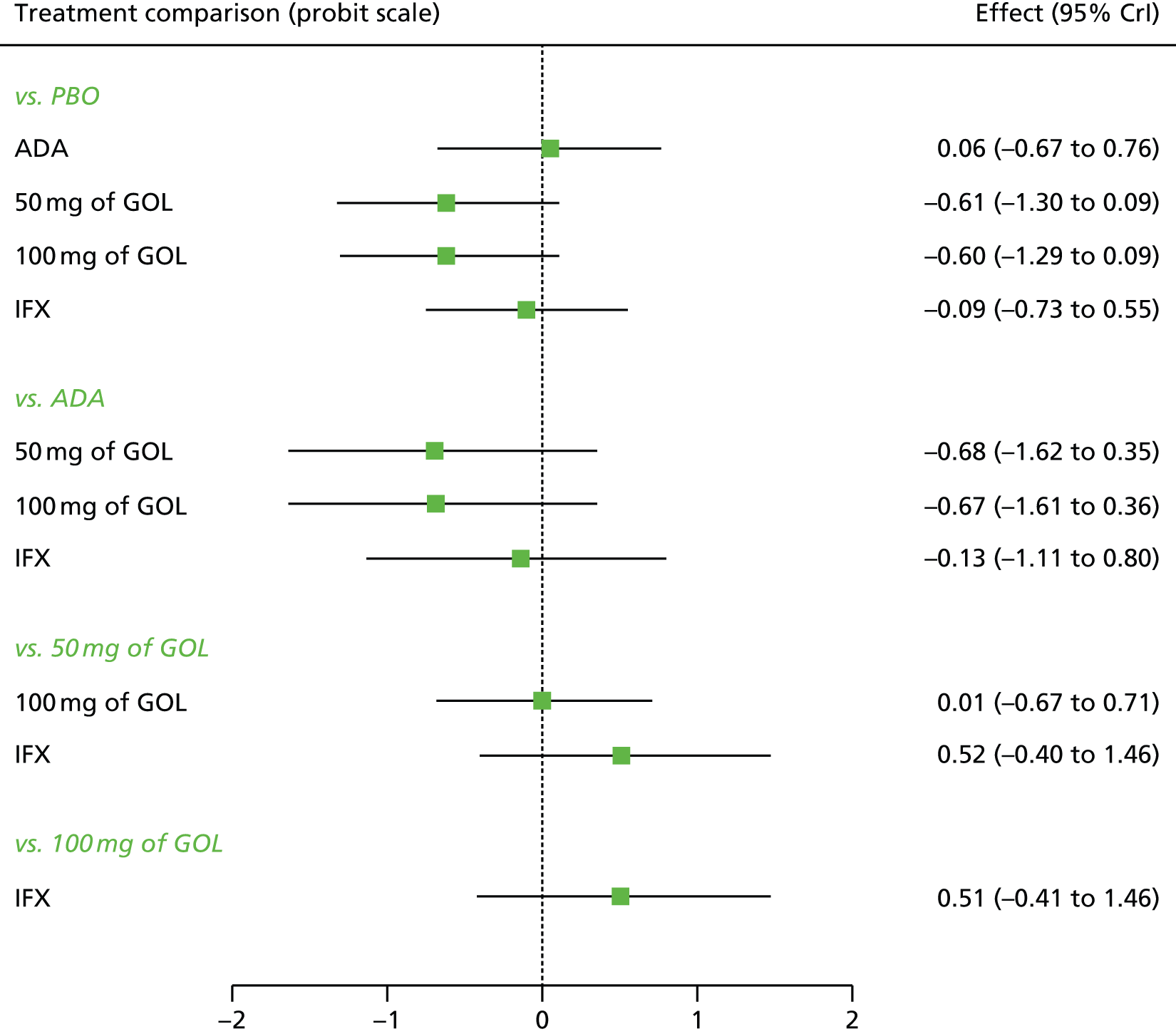
FIGURE 51.
Sensitivity analysis 2: ranking probability histograms for the maintenance phase at 8–32 weeks for patients starting in remission.

All treatments except ADA were associated with beneficial treatment effects relative to PBO with the greatest effects being associated with 50 mg of GOL (–0.61; 95% CrI –1.30 to 0.09) and 100 mg of GOL (–0.60; 95% CrI –1.29 to 0.09); however, none of the treatment effects was statistically significant at a conventional 5% level. Treatments of 50 mg and 100 mg of GOL was most likely to be the most effective (probability of being the best = 0.46 and 0.44 respectively).
Table 25 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 2 maintenance phase at 8–32 weeks starting in remission. Treatments of 50 mg and 100 mg of GOL were associated with the highest probability of staying in remission and the smallest probability of moving from remission to no response and from remission to response at 8–32 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.354 | 0.350 | 0.191 to 0.540 | 0.187 | 0.183 | 0.103 to 0.294 | 0.459 | 0.459 | 0.250 to 0.666 |
| ADA | 0.381 | 0.371 | 0.114 to 0.701 | 0.178 | 0.175 | 0.085 to 0.286 | 0.441 | 0.434 | 0.144 to 0.769 |
| 50 mg of GOL | 0.179 | 0.159 | 0.034 to 0.442 | 0.143 | 0.139 | 0.043 to 0.264 | 0.678 | 0.695 | 0.353 to 0.915 |
| 100 mg of GOL | 0.181 | 0.162 | 0.035 to 0.443 | 0.144 | 0.141 | 0.044 to 0.264 | 0.675 | 0.691 | 0.349 to 0.913 |
| IFX | 0.331 | 0.318 | 0.103 to 0.626 | 0.177 | 0.174 | 0.082 to 0.286 | 0.492 | 0.493 | 0.195 to 0.790 |
Sensitivity analysis 2: maintenance phase 32–52 weeks
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase for patients starting in response at 32–52 weeks. Data were available from four studies comparing two or three treatments. 45,46,48,49 Figure 52 presents the network of evidence for the sensitivity analysis 2 maintenance phase at 32–52 weeks for patients starting in response.
FIGURE 52.
Sensitivity analysis 2: network of evidence for the maintenance phase for patients starting in response at 32–52 weeks. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.

Figure 53 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 2 maintenance phase 32–52 weeks for patients starting in response. Figure 54 presents the probabilities of treatment rankings for this analysis. The model fitted the data reasonably well, with the total residual deviance, 15.92, being close to the total number of data points included in the analysis, 18. The between-study SD was estimated to be 0.20 (95% CrI 0.01 to 0.67), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 53.
Sensitivity analysis 2: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in response [SD ∼ HN(0,0.322)].
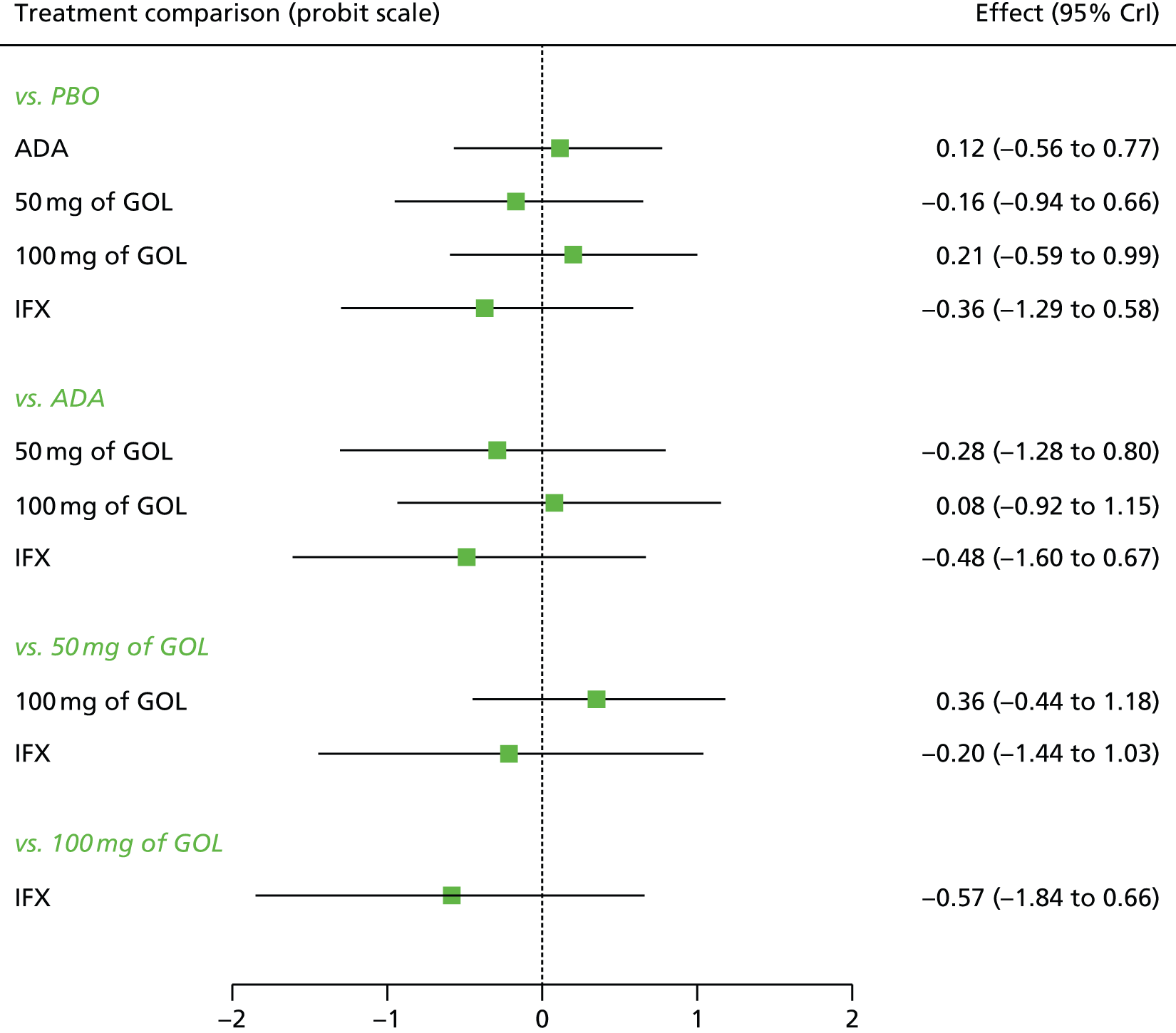
FIGURE 54.
Sensitivity analysis 2: ranking probability histograms for the maintenance phase at 32–52 weeks for patients starting in response.
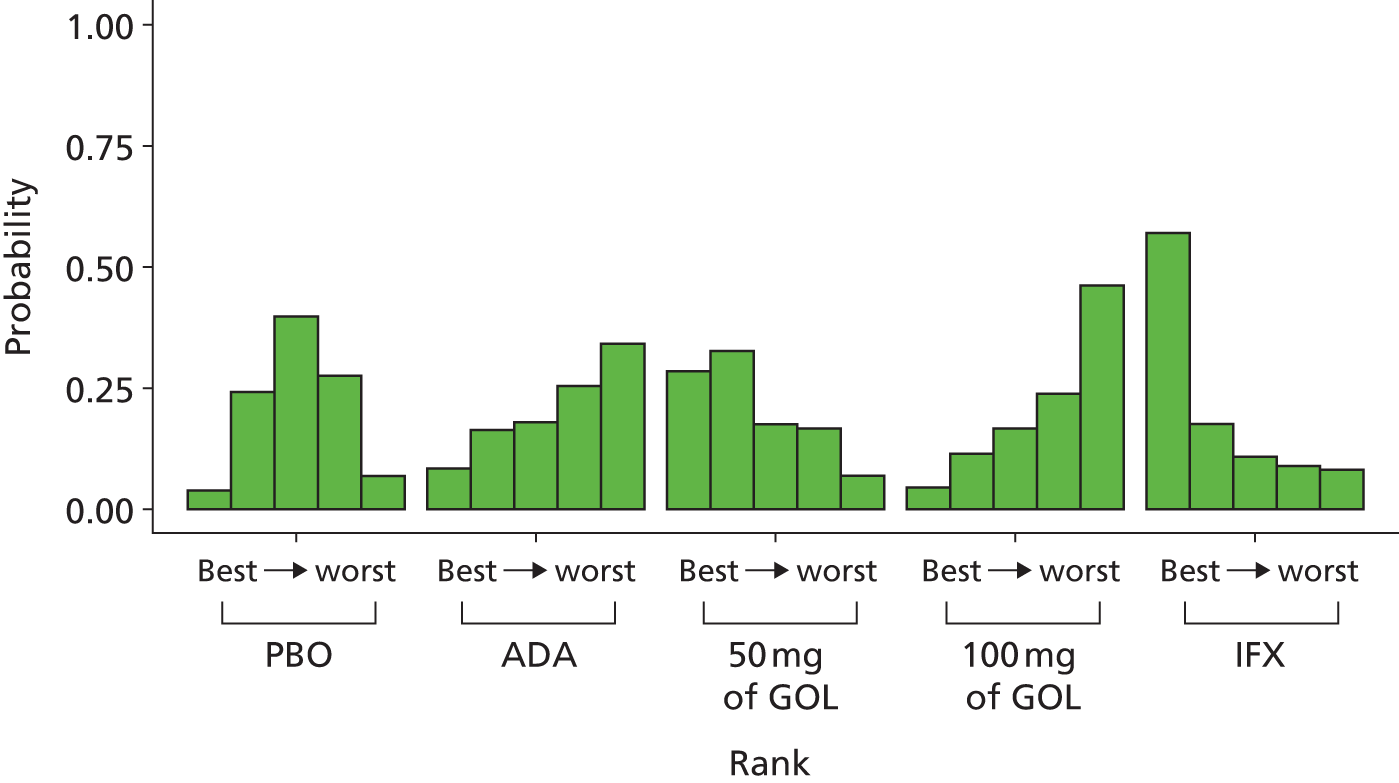
All treatments except ADA and 100 mg of GOL were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with IFX (–0.36; 95% CrI –1.29 to 0.58); however, none of the treatment effects was statistically significant at a conventional 5% level. IFX was most likely to be the most effective treatment (probability of being the best = 0.57).
Table 26 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 2 maintenance phase at 32–52 weeks for patients starting in response. IFX was associated with the highest probability of moving from response to remission and the smallest probability of moving from response to no response at 32–52 weeks. The probabilities of staying in response were comparable among treatments at 32–52 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.322 | 0.318 | 0.189 to 0.472 | 0.393 | 0.398 | 0.200 to 0.544 | 0.286 | 0.278 | 0.107 to 0.520 |
| ADA | 0.371 | 0.363 | 0.130 to 0.661 | 0.371 | 0.378 | 0.176 to 0.524 | 0.259 | 0.238 | 0.05 to 0.576 |
| 50 mg of GOL | 0.283 | 0.265 | 0.066 to 0.606 | 0.370 | 0.378 | 0.153 to 0.545 | 0.347 | 0.332 | 0.069 to 0.713 |
| 100 mg of GOL | 0.404 | 0.395 | 0.128 to 0.730 | 0.359 | 0.368 | 0.153 to 0.521 | 0.237 | 0.211 | 0.035 to 0.579 |
| IFX | 0.230 | 0.202 | 0.030 to 0.575 | 0.352 | 0.363 | 0.115 to 0.542 | 0.418 | 0.410 | 0.081 to 0.820 |
Patients starting in remission
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase at 32–52 weeks for patients starting in remission. Data were available from four studies comparing two or three treatments. 45,48,49 Figure 55 presents the network of evidence for the sensitivity analysis 2 maintenance phase at 32–52 weeks for patients starting in remission.
FIGURE 55.
Sensitivity analysis 2: network of evidence for the maintenance phase at 32–52 weeks for patients starting in remission. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.
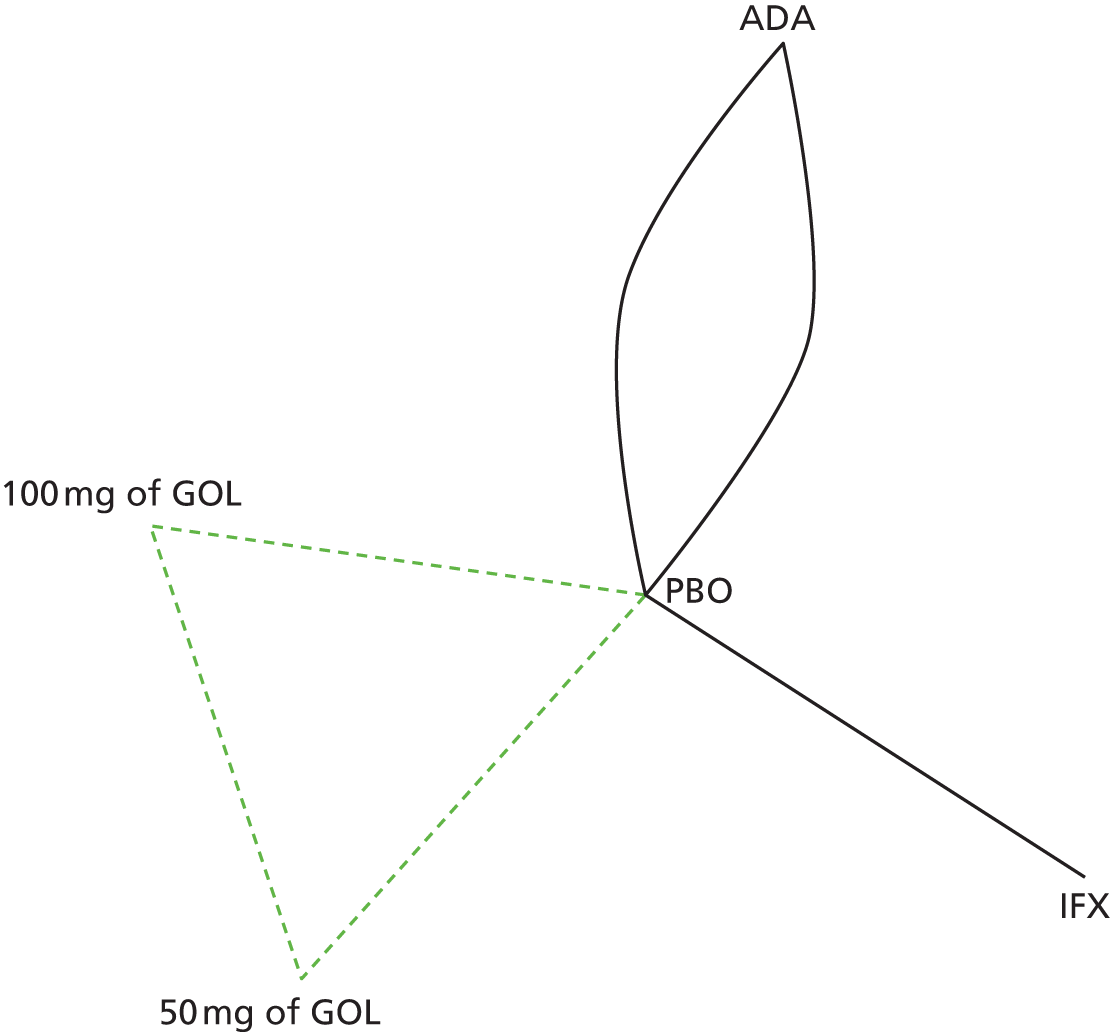
Figure 56 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 2 maintenance phase at 32–52 weeks for patients starting in remission. Figure 57 presents the probabilities of treatment rankings for this analysis. The model fitted the data reasonably well, with the total residual deviance, 21.07, being close to the total number of data points included in the analysis, 18. The between-study SD was estimated to be 0.18 (95% CrI 0.01 to 0.65), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 56.
Sensitivity analysis 2: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in remission [SD ∼ HN(0,0.322)].
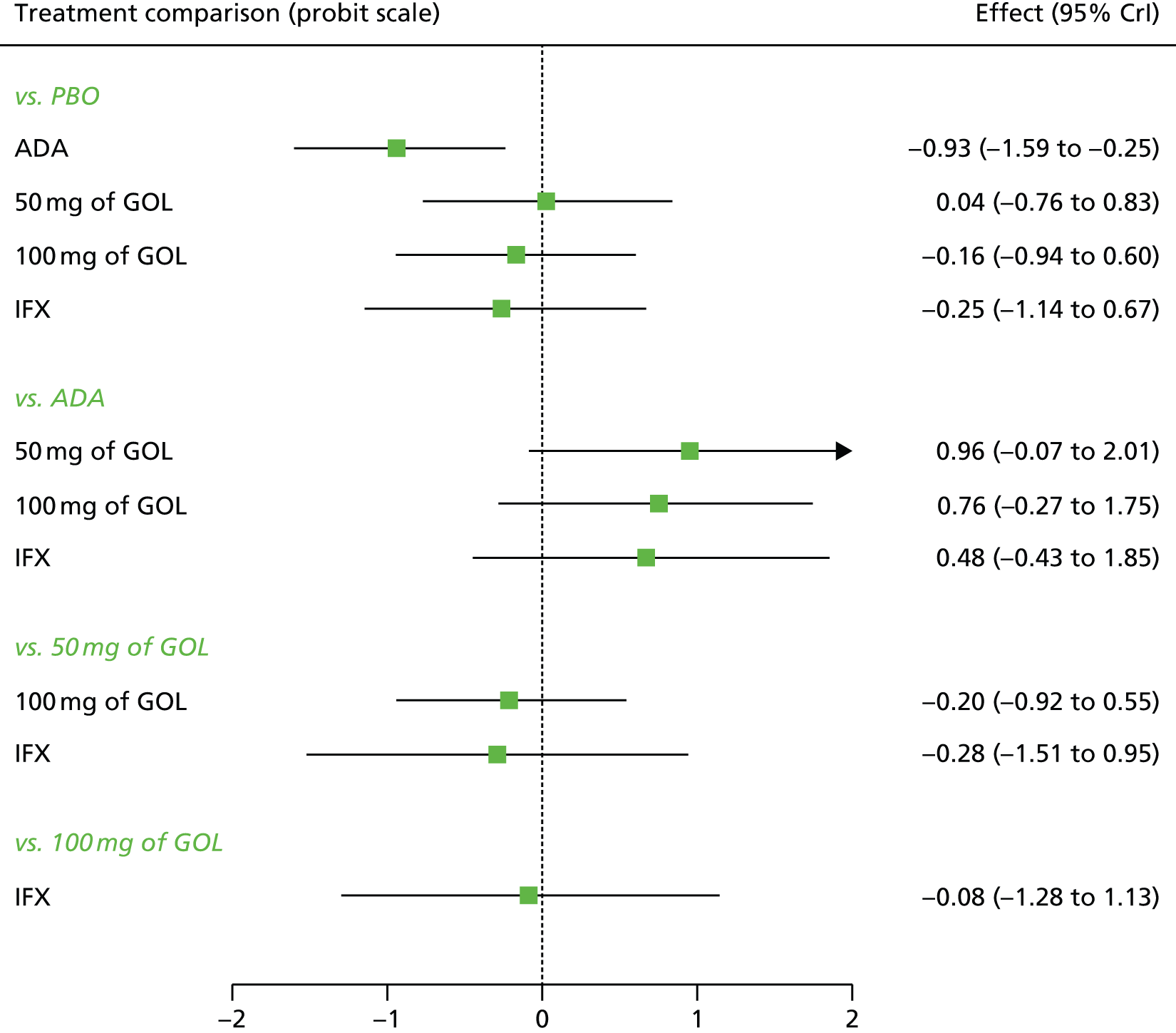
FIGURE 57.
Sensitivity analysis 2: ranking probability histograms for the maintenance phase at 32–52 weeks for patients starting in remission.

All treatments except 50 mg of GOL were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with ADA (–0.93, 95% CrI –1.59 to –0.25); however, only the effect of ADA was statistically significant at a conventional 5% level. ADA was most likely to be the most effective treatment (probability of being the best = 0.84).
Table 27 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 2 maintenance phase at 32–52 weeks starting in remission. ADA was associated with the highest probability of staying in remission and the smallest probability of moving from remission to response or no response at 32–52 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.296 | 0.294 | 0.183 to 0.422 | 0.198 | 0.184 | 0.043 to 0.445 | 0.505 | 0.514 | 0.233 to 0.71 |
| ADA | 0.085 | 0.070 | 0.013 to 0.239 | 0.111 | 0.092 | 0.012 to 0.339 | 0.804 | 0.830 | 0.493 to 0.963 |
| 50 mg of GOL | 0.320 | 0.307 | 0.083 to 0.633 | 0.188 | 0.176 | 0.035 to 0.425 | 0.492 | 0.494 | 0.137 to 0.83 |
| 100 mg of GOL | 0.258 | 0.24 | 0.059 to 0.558 | 0.180 | 0.165 | 0.030 to 0.425 | 0.563 | 0.573 | 0.186 to 0.872 |
| IFX | 0.239 | 0.214 | 0.040 to 0.581 | 0.171 | 0.157 | 0.026 to 0.416 | 0.590 | 0.609 | 0.176 to 0.906 |
Sensitivity analysis 3: induction phase
Sensitivity analysis 3 involved replacing ULTRA2 anti-TNF-α-naive data with ULTRA2 ITT data and including data from Suzuki et al. 46 A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the induction phase. Data were available from six studies comparing two treatments. 44–47,49 Figure 58 presents the network of evidence for the sensitivity analysis 3 induction phase.
FIGURE 58.
Sensitivity analysis 3: network of evidence for the induction phase. Note: solid line indicates a two-arm trial.
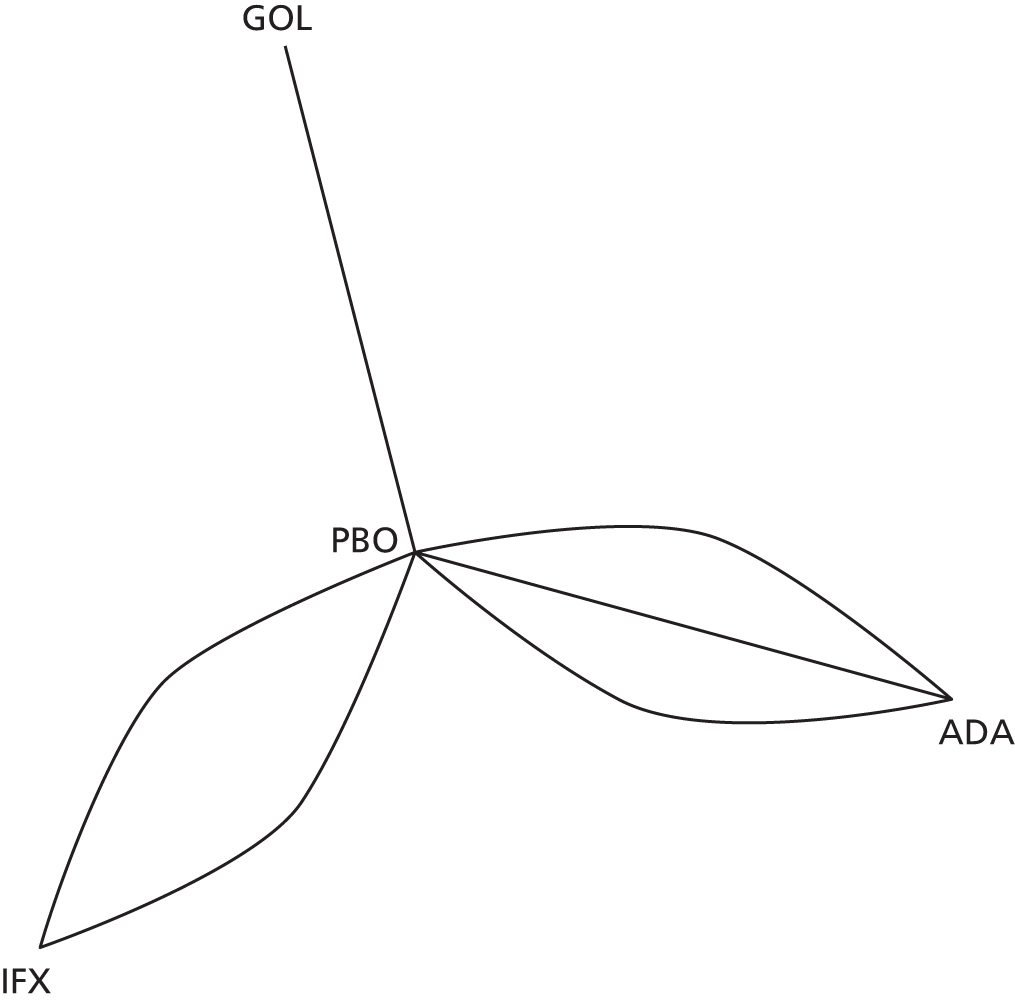
Figure 59 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 3 induction phase. Figure 60 presents the probabilities of treatment rankings for this analysis. The model fitted the data well, with the total residual deviance, 23.63, being close to the total number of data points included in the analysis, 24. The between-study SD was estimated to be 0.09 (95% CrI 0.00 to 0.38), which implies mild heterogeneity between studies in treatment effects.
FIGURE 59.
Sensitivity analysis 3: comparative effect of anti-TNF-α treatment on clinical response/remission in the induction phase [SD ∼ HN(0,0.322)].

FIGURE 60.
Sensitivity analysis 3: ranking probability histograms for the induction phase.
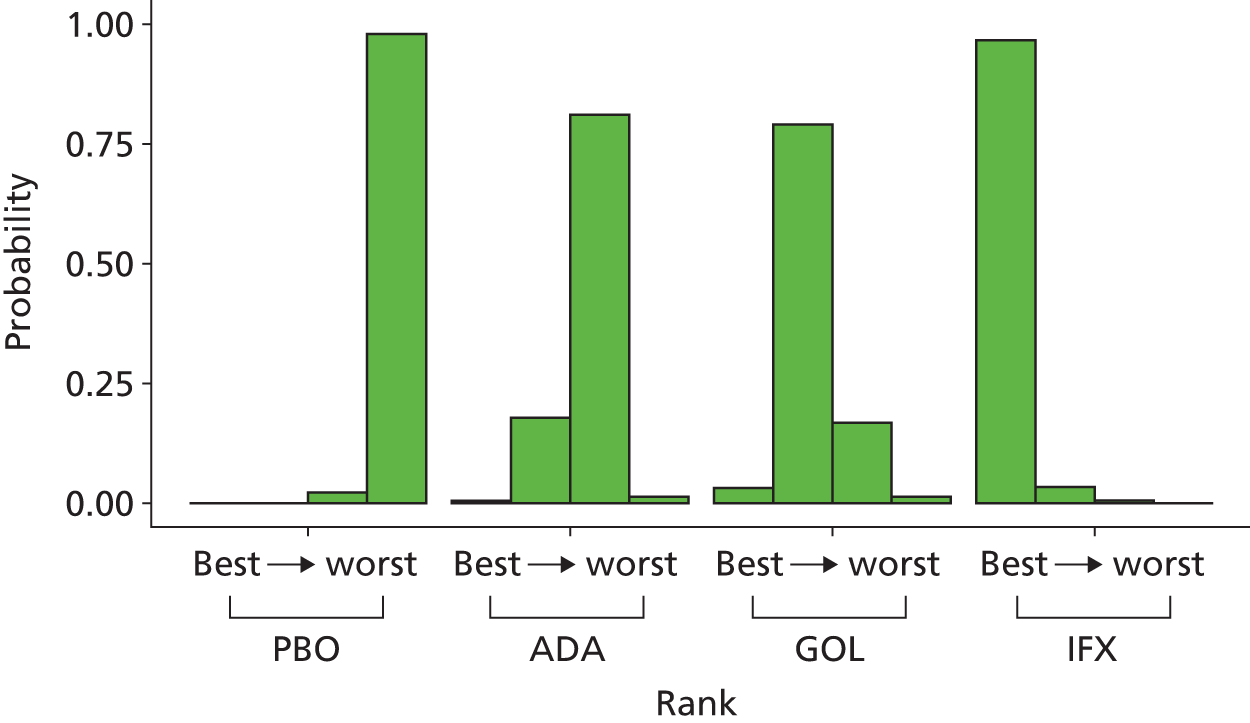
All treatments were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with IFX (–0.91; 95% CrI –1.21 to –0.62). All the treatment effects were statistically significant at a conventional 5% level. IFX was most likely to be the most effective treatment (probability of being the best = 0.97).
Table 28 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 3 induction phase. IFX was associated with the highest probability of moving from no response to response and no response to remission.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.649 | 0.650 | 0.596 to 0.699 | 0.258 | 0.258 | 0.222 to 0.294 | 0.093 | 0.092 | 0.064 to 0.128 |
| ADA | 0.521 | 0.519 | 0.414 to 0.632 | 0.317 | 0.319 | 0.257 to 0.367 | 0.162 | 0.160 | 0.095 to 0.241 |
| GOL | 0.458 | 0.456 | 0.315 to 0.610 | 0.336 | 0.339 | 0.267 to 0.387 | 0.205 | 0.201 | 0.106 to 0.331 |
| IFX | 0.301 | 0.264 | 0.196 to 0.419 | 0.358 | 0.360 | 0.304 to 0.408 | 0.340 | 0.338 | 0.222 to 0.477 |
Sensitivity analysis 3: maintenance phase 8–32 weeks
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase for patients starting in response at 8–32 weeks. Data were available from five studies comparing two or three treatments. 45,46,48,49 Figure 61 presents the network of evidence for the sensitivity analysis 3 maintenance phase at 8–32 weeks for patients starting in response.
FIGURE 61.
Sensitivity analysis 3: network of evidence for the maintenance phase at 8–32 weeks for patients starting in response. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.
Figure 62 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 3 maintenance phase at 8–32 weeks for patients starting in response. Figure 63 presents the probabilities of treatment rankings for this analysis. There was some suggestion that model did not represent the data well with the total residual deviance, 14.05, being smaller than would be expected given the total number of data points included in the analysis, 22. The probability of observing a value < 14.05 is 0.100, which means that this could have occurred by chance. Similar to the base-case analysis, all 5 studies had smaller residual deviance than expected. The between-study SD was estimated to be 0.15 (95% CrI 0.01 to 0.55), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 62.
Sensitivity analysis 3: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in response [SD ∼ HN(0,0.322)].
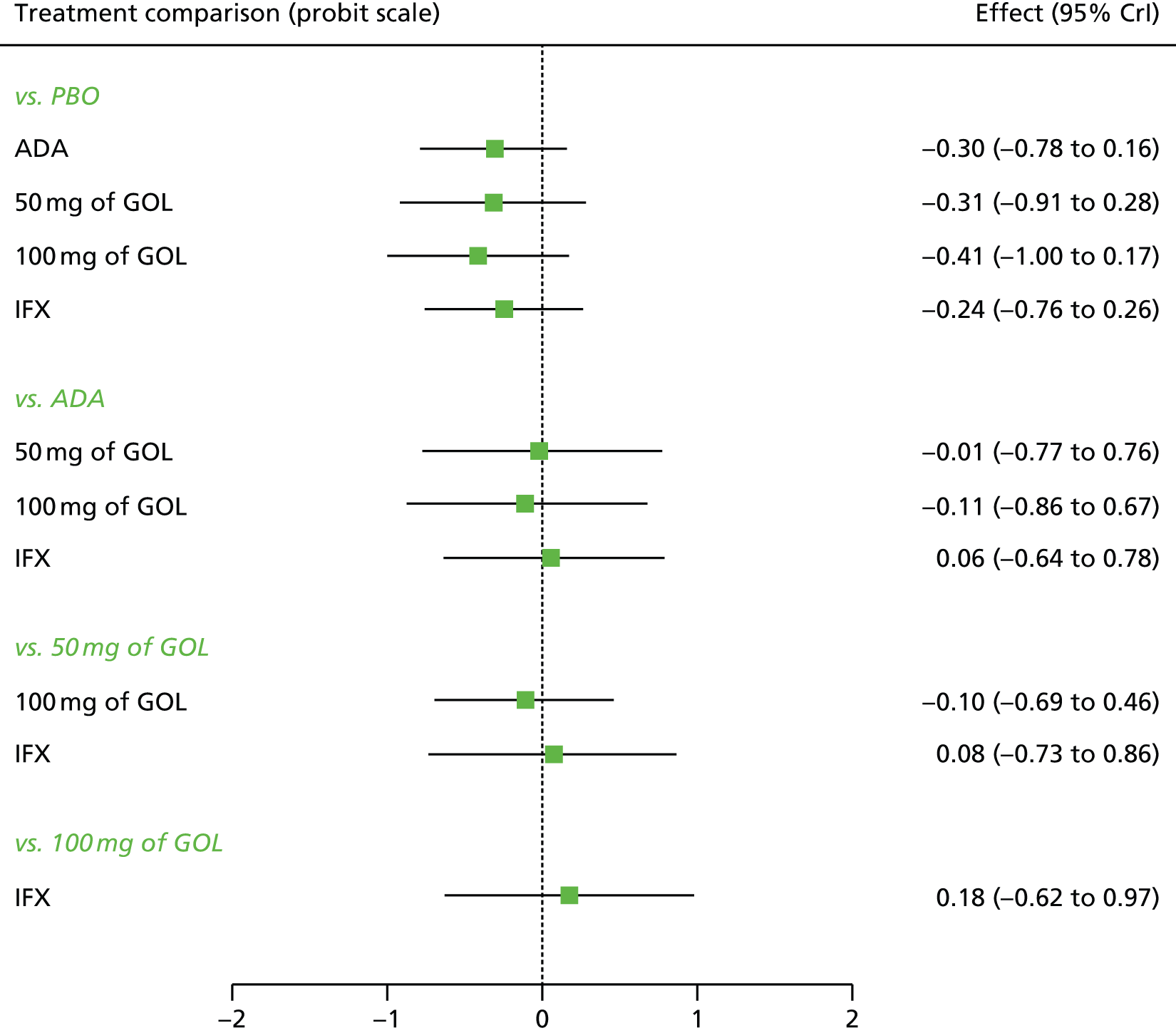
FIGURE 63.
Sensitivity analysis 3: ranking probability histograms for the maintenance phase at 8–32 weeks for patients starting in response.

All treatments were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with 100 mg of GOL; however, none of the treatment effects was statistically significant at a conventional 5% level. A treatment of 100 mg of GOL was most likely to be the most effective (probability of being the best = 0.40).
Table 29 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 3 maintenance phase at 8–32 weeks starting in response. A treatment of 100 mg of GOL was associated with the highest probability of moving from response to remission and the smallest probability of moving from response to no response at 8–32 weeks. The probabilities of staying in response were comparable among treatments.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.545 | 0.545 | 0.459 to 0.630 | 0.270 | 0.270 | 0.223 to 0.32 | 0.185 | 0.183 | 0.120 to 0.260 |
| ADA | 0.427 | 0.425 | 0.243 to 0.626 | 0.292 | 0.293 | 0.223 to 0.353 | 0.280 | 0.274 | 0.129 to 0.470 |
| 50 mg of GOL | 0.425 | 0.422 | 0.202 to 0.669 | 0.289 | 0.290 | 0.204 to 0.354 | 0.287 | 0.276 | 0.107 to 0.523 |
| 100 mg of GOL | 0.386 | 0.382 | 0.176 to 0.628 | 0.293 | 0.294 | 0.213 to 0.356 | 0.321 | 0.313 | 0.129 to 0.560 |
| IFX | 0.451 | 0.449 | 0.246 to 0.664 | 0.287 | 0.289 | 0.211 to 0.349 | 0.262 | 0.255 | 0.109 to 0.465 |
Patients starting in remission
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase at 8–32 weeks for patients starting in remission. Data were available from five studies comparing two or three treatments. 45,46,48,49 Figure 64 presents the network of evidence for the sensitivity analysis 3 maintenance phase at 8–32 weeks for patients starting in remission.
FIGURE 64.
Sensitivity analysis 3: network of evidence for maintenance phase at 8–32 weeks for patients starting in remission. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.
Figure 65 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 3 maintenance phase at 8–32 weeks for patients starting in remission. Figure 66 presents the probabilities of treatment rankings for this analysis. The model fitted the data reasonably well, with the total residual deviance, 17.77, being close to the total number of data points included in the analysis, 22. The between-study SD was estimated to be 0.16 (95% CrI 0.01 to 0.59), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 65.
Sensitivity analysis 3: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in remission [SD ∼ HN(0,0.322)].
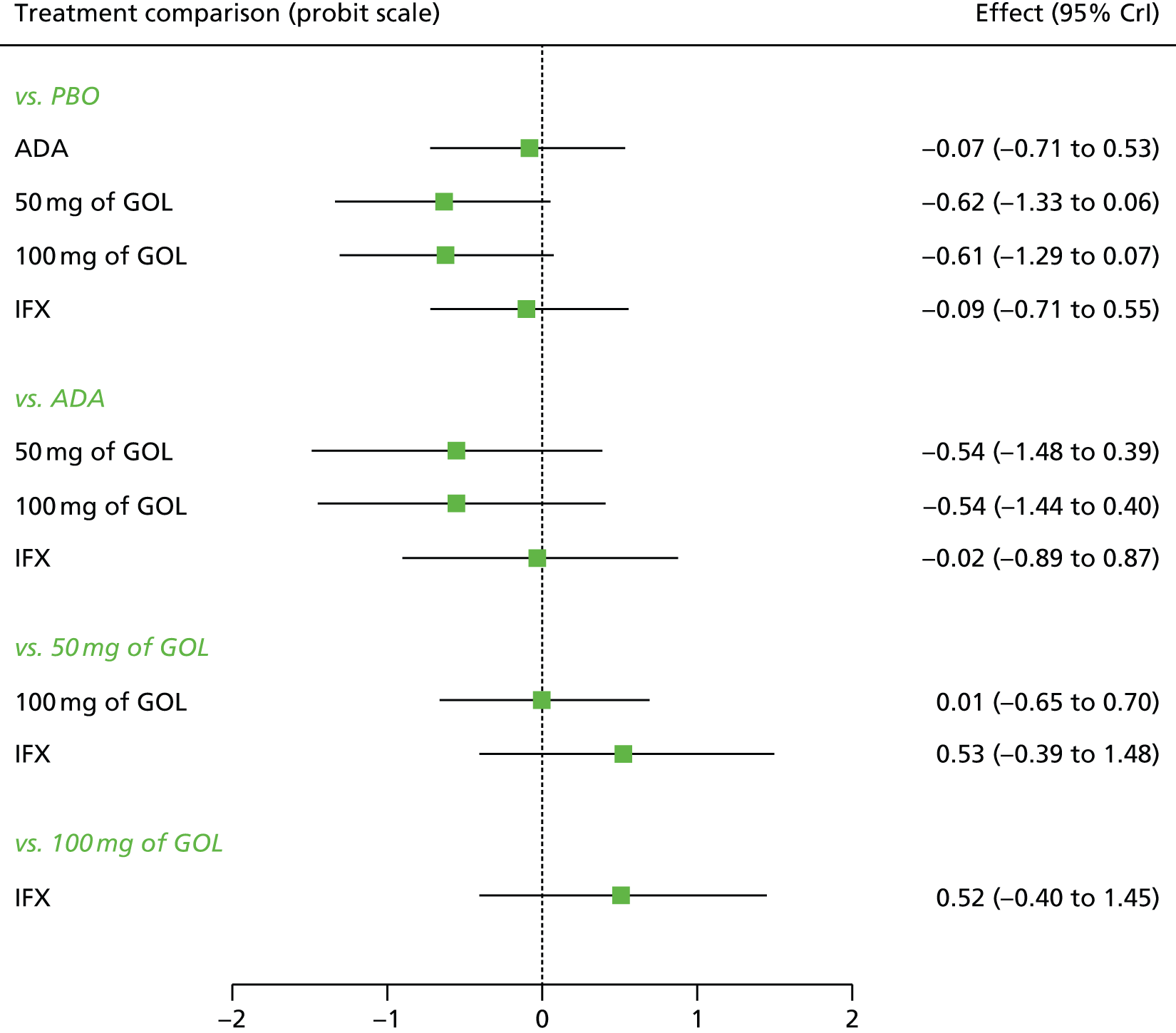
FIGURE 66.
Sensitivity analysis 3: ranking probability histograms for the maintenance phase at 8–32 weeks for patients starting in remission.

All treatments were associated with beneficial treatment effects relative to PBO with the greatest effects being associated with 50 mg of GOL (–0.62, 95% CrI –1.33 to 0.06) and 100 mg of GOL (–0.61, 95% CrI –1.29 to 0.07); however, none of the treatment effects was statistically significant at a conventional 5% level. A treatment of 50 mg and 100 mg of GOL were most likely to be the most effective treatments (probability of being the best = 0.46 and 0.44 respectively).
Table 30 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 3 maintenance phase at 8–32 weeks starting in remission. A treatment of 50 mg and 100 mg of GOL were associated with the highest probability of staying in remission and the smallest probability of moving from remission to no response and from remission to response at 8–32 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.389 | 0.386 | 0.240 to 0.556 | 0.185 | 0.180 | 0.102 to 0.291 | 0.426 | 0.425 | 0.242 to 0.613 |
| ADA | 0.367 | 0.358 | 0.132 to 0.649 | 0.177 | 0.172 | 0.086 to 0.286 | 0.456 | 0.452 | 0.183 to 0.751 |
| 50 mg of GOL | 0.199 | 0.182 | 0.043 to 0.451 | 0.147 | 0.144 | 0.047 to 0.264 | 0.654 | 0.664 | 0.342 to 0.900 |
| 100 mg of GOL | 0.202 | 0.184 | 0.046 to 0.465 | 0.148 | 0.144 | 0.049 to 0.270 | 0.650 | 0.663 | 0.332 to 0.890 |
| IFX | 0.363 | 0.352 | 0.130 to 0.655 | 0.176 | 0.172 | 0.084 to 0.284 | 0.461 | 0.461 | 0.178 to 0.753 |
Sensitivity analysis 3: maintenance phase 32–52 weeks
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase for patients starting in response at 32–52 weeks. Data were available from four studies comparing two or three treatments. 45,46,48,49 Figure 67 presents the network of evidence for the sensitivity analysis 3 maintenance phase at 32–52 weeks for patients starting in response.
FIGURE 67.
Sensitivity analysis 3: network of evidence for the maintenance phase at 32–52 weeks for patients starting in response. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.
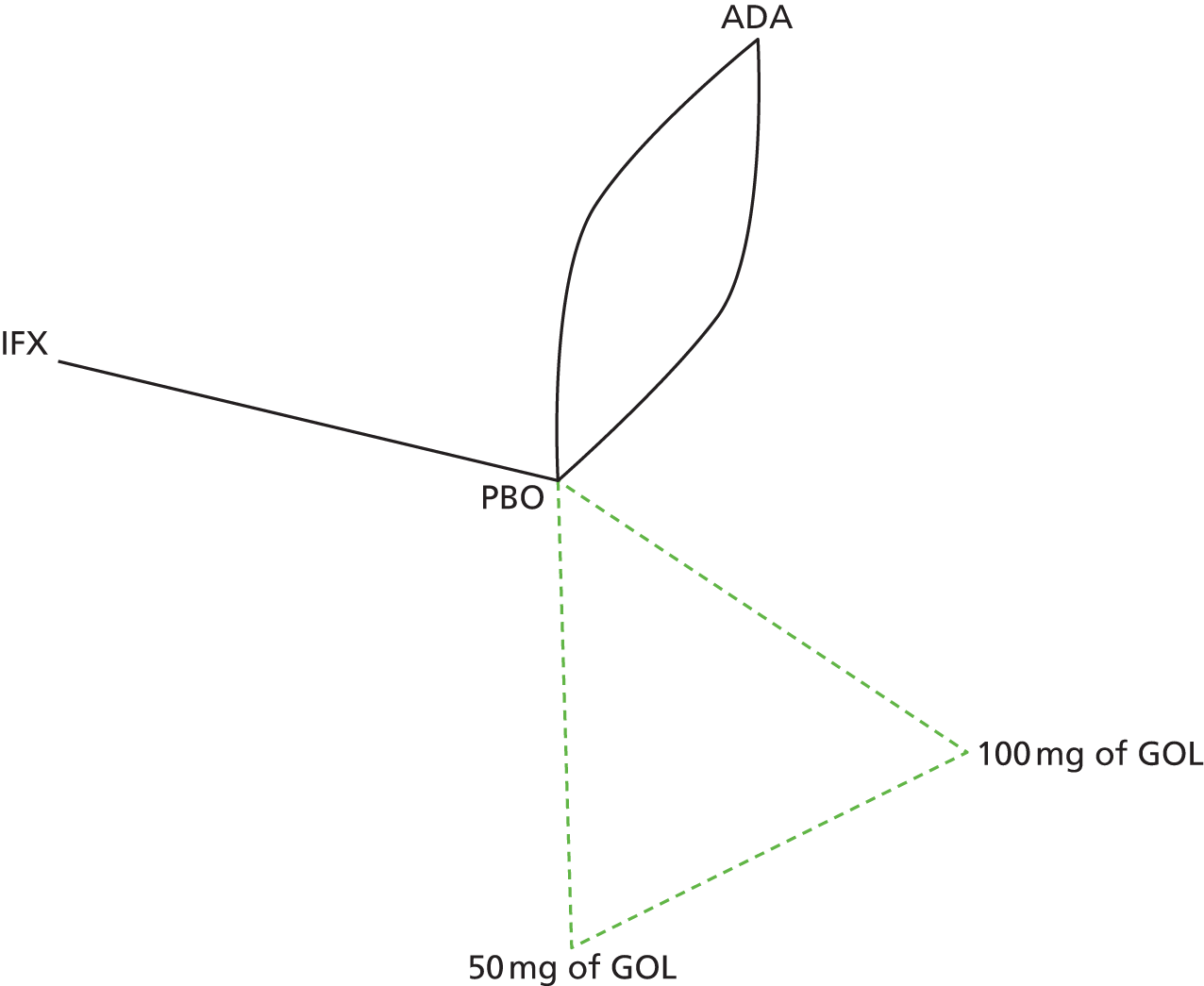
Figure 68 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 3 maintenance phase 32–52 weeks for patients starting in response. Figure 69 presents the probabilities of treatment rankings for this analysis. The model fitted the data reasonably well, with the total residual deviance, 15.21, being close to the total number of data points included in the analysis, 18. The between-study SD was estimated to be 0.18 (95% CrI 0.01 to 0.64), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 68.
Sensitivity analysis 3: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in response [SD ∼ HN(0,0.322)].

FIGURE 69.
Sensitivity analysis 3: ranking probability histograms for the maintenance phase at 32–52 weeks for patients starting in response.
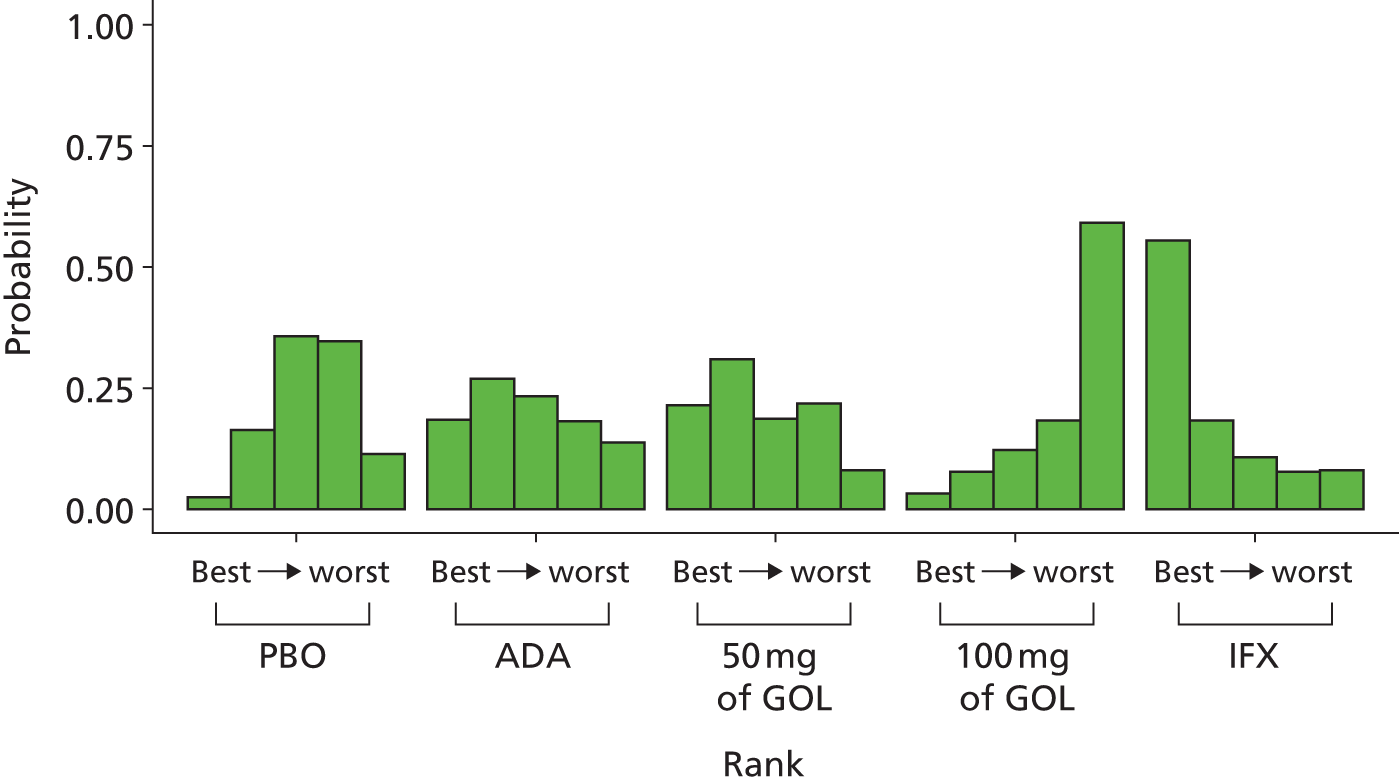
All treatments except 100 mg of GOL were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with IFX (–0.38, 95% CrI –1.27 to 0.55). However, none of the treatment effects was statistically significant at a conventional 5% level. IFX was most likely to be the most effective treatment (probability of being the best = 0.55).
Table 31 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 3 maintenance phase at 32–52 weeks for patients starting in response. IFX was associated with the highest probability of moving from response to remission, and the smallest probability of moving from response to no response at 32–52 weeks. The probabilities of staying in response were comparable among treatments at 32–52 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.344 | 0.340 | 0.218 to 0.484 | 0.393 | 0.395 | 0.252 to 0.514 | 0.263 | 0.257 | 0.114 to 0.449 |
| ADA | 0.314 | 0.304 | 0.109 to 0.580 | 0.382 | 0.385 | 0.219 to 0.520 | 0.305 | 0.290 | 0.086 to 0.605 |
| 50 mg of GOL | 0.309 | 0.293 | 0.081 to 0.625 | 0.374 | 0.380 | 0.191 to 0.514 | 0.317 | 0.302 | 0.067 to 0.661 |
| 100 mg of GOL | 0.436 | 0.431 | 0.149 to 0.759 | 0.354 | 0.363 | 0.172 to 0.490 | 0.210 | 0.187 | 0.031 to 0.520 |
| IFX | 0.240 | 0.213 | 0.041 to 0.575 | 0.358 | 0.369 | 0.151 to 0.513 | 0.402 | 0.392 | 0.088 to 0.771 |
Patients starting in remission
A NMA was used to compare the effects of ADA, GOL and IFX relative to PBO on clinical response in the maintenance phase at 32–52 weeks for patients starting in remission. Data were available from four studies comparing two or three treatments. 45,46,48,49 Figure 70 presents the network of evidence for the sensitivity analysis 3 maintenance phase at 32–52 weeks for patients starting in remission.
FIGURE 70.
Sensitivity analysis 3: network of evidence for the maintenance phase at 32–52 weeks for patients starting in remission. Note: solid line indicates a two-arm trial; dashed line indicates a three-arm study.

Figure 71 presents the effects of each treatment relative to PBO on the probit scale for the sensitivity analysis 3 maintenance phase at 32–52 weeks for patients starting in remission. Figure 72 presents the probabilities of treatment rankings for this analysis. The model fitted the data reasonably well, with the total residual deviance, 20.55, being close to the total number of data points included in the analysis, 18. The between-study SD was estimated to be 0.18 (95% CrI 0.01 to 0.65), which implies mild to moderate heterogeneity between studies in treatment effects.
FIGURE 71.
Sensitivity analysis 3: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in remission [SD ∼ HN(0,0.322)].

FIGURE 72.
Sensitivity analysis 3: ranking probability histograms for the maintenance phase at 32–52 weeks for patients starting in remission.

All treatments except 50 mg of GOL were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with ADA (–0.85, 95% CrI –1.49 to –0.16); however, only the effect of ADA was statistically significant at a conventional 5% level. ADA was most likely to be the most effective treatment (probability of being the best = 0.80).
Table 32 presents the probabilities of achieving each of the following categories: no response, response and remission for the sensitivity analysis 3 maintenance phase at 32–52 weeks for patients starting in remission. ADA was associated with the highest probability of staying in remission and the smallest probability of moving from remission to response or no response at 32–52 weeks.
| Treatment | No response | Response | Remission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| PBO | 0.296 | 0.293 | 0.187 to 0.422 | 0.202 | 0.188 | 0.042 to 0.44 | 0.502 | 0.511 | 0.246 to 0.706 |
| ADA | 0.097 | 0.082 | 0.016 to 0.267 | 0.123 | 0.104 | 0.014 to 0.343 | 0.781 | 0.805 | 0.464 to 0.955 |
| 50 mg of GOL | 0.313 | 0.299 | 0.083 to 0.625 | 0.191 | 0.178 | 0.036 to 0.423 | 0.496 | 0.501 | 0.144 to 0.821 |
| 100 mg of GOL | 0.252 | 0.235 | 0.057 to 0.544 | 0.182 | 0.168 | 0.03 to 0.418 | 0.566 | 0.577 | 0.197 to 0.868 |
| IFX | 0.250 | 0.229 | 0.037 to 0.588 | 0.176 | 0.163 | 0.025 to 0.413 | 0.574 | 0.582 | 0.178 to 0.911 |
Biosimilars to infliximab
As defined by the EMA, a biosimilar is a biological medicine developed with the aim of being similar to an already existing biological medicine (or reference medicine). 71 In this assessment, the reference medicine is IFX. Two biosimilars to IFX were also considered within the scope of this assessment: Remsima and Inflectra. The EMA has stated that a biosimilar and reference medicine may display differences owing to their complex nature and methods of production and that, in the approval process, any differences need to be demonstrated not to affect safety or effectiveness. 71
A submission72 was made to NICE for consideration as part of the current assessment by the manufacturers of Remsima. However, no sponsor submission was presented by Hospira, the manufacturers of Inflectra. EPAR reports were available for both Remsima73 and Inflectra. 74
In June 2013, the EMA CHMP recommended authorisation of Remsima and Inflectra as biosimilars to IFX, reported to be the first authorisation in the European Union for a biosimilar monoclonal antibody. Both Remsima and Inflectra were developed as the product CT-P13.
It was stated in the EPARs for Remsima and Inflectra that an extensive comparability exercise between CT-P13 and Remicade was undertaken, which found the major characteristics and biological activities of Remsima/Inflectra to be comparable with Remicade.
The clinical programme to evaluate CT-P13 was based on two main clinical trials:
-
a pharmacokinetic equivalence study performed in adult patients with AS [Study CT-P13 1.1, Programme evaLuating the Autoimmune disease iNvEstigational drug CT-P13 in ankylosing spondylitis patients (PLANET-AS)]
-
a therapeutic equivalence study of CT-P13 compared with Remicade in adult patients with active rheumatoid arthritis [Study CT-P13 3.1, Programme evaLuating the Autoimmune disease iNvEstigational drug CT-P13 in rheumatoid arthritis patients (PLANET-RA)].
Both studies were planned with a 1-year treatment duration and primary endpoints were evaluated at 30 weeks. Further efficacy and safety data up to 54 weeks were submitted during the EMA assessment.
A third study (CT-P13 1.2) was a small pilot study in RA patients with purpose of facilitating pivotal trial (CT-P13 3.1) conduct.
Study CT-P13 1.1, PLANET-AS
PLANET-AS was a prospective Phase I, randomised double-blind multicentre study, in which 250 patients were randomised (CT-P13 n = 125, Remicade n = 125). Patients received CT-P13 (5 mg/kg) or Remicade (5 mg/kg) at weeks 0, 2 and 6 and then every 8 weeks to week 54. The primary objective of the study was to demonstrate comparable pharmacokinetics of CT-P13 and Remicade at steady state (between weeks 22 and 30). The primary parameters evaluated were the area under the plasma concentration time curve from time zero to time t (AUCT) and maximum serum concentrations (Cmax) after dose 5 (weeks 22–30), with secondary parameters being average concentration at steady state (Cav, ss), minimum concentration immediately before next dose at steady state (Cmin ss), terminal elimination half-life (T1/2), total-body clearance at steady state (CLss) and volume of distribution at steady state (Vss). Additional observed parameters were maximum serum concentrations (Cmax), minimum concentration immediately before next dose (Cmin) and time to reach maximum serum concentrations Cmax (Tmax) after each dose. Efficacy parameters included the proportion of patients achieving clinical response according to the assessment in AS response criteria (ASAS20 and ASAS40). The EPARs reported that PLANET-AS demonstrated that (at 5 mg/kg) pharmacokinetic behaviour between CT-P13 and Remicade was similar, a view that was also supported by pharmacokinetic data from RA patients in study CT-P13 3.1 (PLANET-RA). Furthermore, the EPARs also stated that the proportions of patients experiencing clinical response according to the ASAS20 and ASAS40 criteria at weeks 14 and 30 were similar across the CT-P13 and Remicade groups.
Study CT-P13 3.1, PLANET-RA
PLANET-RA was a prospective Phase III, randomised, double-blind, multicentre study, in which 302 patients were randomised to CT-P13 and 304 to Remicade (randomisation was stratified by geographical region and baseline CRP level). Patients received CT-P13 or Remicade at 3 mg/kg at weeks 0, 2, 6 and then every 8 weeks up to 54 weeks (administered in combination with a stable dose of methotrexate and folic acid). The primary objective of the trial was to demonstrate that CT-P13 was equivalent to Remicade up to week 30 in efficacy as measured by ACR20. Secondary objectives were ACR20, ACR50 and ACR70 responses at weeks 14 and 30, Disease Activity Score 28 at weeks 14 and 30, European League Against Rheumatism response at weeks 14 and 30, Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index at weeks 14 and 30 and SF-36 at weeks 14 and 30.
Fewer patients randomised to the CT-P13 arm (n = 69, 22.8%) discontinued PLANET-RA by week 54 than patients in Remicade arm (n = 82, 27.0%). Patients received CT-P13 and Remicade at the RA dose of 3 mg/kg. It was stated in the EPARs that a similar proportion of patients at week 30 in the CT-P13 (184/302, 60.9%) and Remicade (178/304, 58.6%) arms achieved ACR20 response (Table 33).
| Treatment arm | n/N (%) | Estimate of treatment difference | 95% CI of treatment difference |
|---|---|---|---|
| ACR20: CT-P13 | 184/302 (60.9) | 0.02 | –0.06 to 0.10 |
| ACR20: Remicade | 178/304 (58.6) | ||
| ACR50: CT-P13 | 105/248 (42.3) | 0.02 | –0.07 to 0.10 |
| ACR50: Remicade | 102/251 (40.6) | ||
| ACR70: CT-P13 | 50/248 (20.2) | 0.02 | –0.05 to 0.09 |
| ACR70: Remicade | 45/251 (17.9) |
Furthermore, at week 30, the findings for the secondary end points (including ACR50, ACR70 and decrease in Disease Activity Score 28) were also described as being consistent with the results of the primary end point. Efficacy results were reported to be comparable between treatment arms up to week 54. It was concluded in the EPAR that PLANET-RA provided that robust evidence of therapeutic equivalence between CT-P13 and Remicade. ACR responses between CT-P13 and Remicade remained comparable through the 12-month PLANET-RA extension study. 73
The safety profile of CT-P13 was evaluated in the clinical studies described above. A total of 871 patients were included in the safety population. It was reported in the EPARs that the type and incidence of adverse drug reactions with CT-P13 and Remicade were broadly similar and that no new safety concern was identified. Additionally, it was stated that no marked differences in immunogenicity between CT-P13 and Remicade were observed up to 54 weeks, with comparable effects of antibodies on efficacy and safety. Although there was a numerical imbalance described in SAEs observed in study CT-P13 3.1 (PLANET-RA) (with a higher number of serious infections), reported numbers were stated to be low and, therefore, the CHMP concluded that this observed difference was likely to be due to chance.
In summary, the EMA considered CT-P13 to be biosimilar to the reference product Remicade and judged that the submitted data in the submissions for Remsima and Inflectra allowed for extrapolation to all other indications of Remicade.
An ECCO position statement was presented by Danese and Gomollon75 stating that the use of biosimilars in patients with IBD requires clinical trials in the IBD patient population to allow comparison between the biosimilar and reference products, on the basis of potential differences in manufacturing and structure that could lead to important differences in immunogenicity and efficacy. However, a subsequent statement issued on behalf of the Working Party on Similar Biological (Biosimilar) Medicinal Products of the CHMP argued that no pharmacokinetic and safety issues are known to be particular to IBD, the most responsive known population (RA) was assessed for immunogenicity and that the data submitted allow extrapolation to patients with IBD. 76
Discussion
A total of 10 RCTs were identified in the clinical effectiveness systematic review, of which nine44–51 related to adults and one52 was conducted in a paediatric population. All of the adult RCTs were performed against PBO (with the exception of UC-SUCCESS51) and were a maximum of 1 year in study duration. No head-to-head RCTs were identified in which interventions of interest were assessed against each other.
The risks of bias associated with included RCTs were assessed using the Cochrane risk-of-bias instrument. Only three RCTs could be considered as being at overall low risk of bias as allocation concealment, blinded outcome assessment and completeness of outcome data were all judged as low risk. It should be noted that one of the maintenance trials (PURSUIT-Maintenance48) rerandomised patients who had previously responded to GOL induction therapy in two previous trials; the extent of this potential bias on patient outcomes is unclear.
The outcome measures pre-specified in the final NICE scope were all addressed by the included trial evidence, with the exception of rates of relapse. Clinical response and remission data based on complete Mayo scores were well reported across trials. Evidence was identified to demonstrate that patients receiving IFX, ADA or GOL were more likely than patients receiving PBO to achieve clinical response and remission at induction and maintenance time points. Patients in the UC-SUCCESS51 trial who received combination treatment with IFX and AZA experienced the most favourable rates of steroid-free remission compared with IFX and AZA treatment groups. Seven RCTs performed in adult populations contributed data on clinical response and remission at induction or maintenance time points to NMAs.
Based on the NMA, in the induction phase, all treatments were associated with statistically significant beneficial effects relative to PBO with the greatest effect being associated with IFX. IFX was also associated with the highest probability of moving from no response to response and from no response to remission. The effects of ADA and GOL on these two probabilities were broadly comparable.
For patients classified as responders at the end of the induction phase, treatment effects were not statistically significant, although the greatest effect was associated with 100 mg of GOL at 8–32 weeks. A treatment of 100 mg of GOL was associated with the highest probability of moving from response to remission and staying in response and the smallest probability of moving from response to no response. However, at 32–52 weeks, only IFX and 50 mg of GOL were associated with beneficial effects on clinical response, although the effects were not statistically significant. IFX was associated with the highest probability of moving from response to remission and staying in response and the smallest probability of moving from response to no response at 32–52 weeks. The probabilities of staying in response were comparable among treatments at both 8–32 weeks and 32–52 weeks.
For patients classified as being in remission at the end of the induction phase, all treatments except for ADA were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with 50 mg and 100 mg of GOL, although the effects were not statistically significant at 8–32 weeks. A treatment of 50 mg and 100 mg of GOL were associated with the highest probability of staying in remission and the smallest probability of moving from remission to response and from remission to no response. At 32–52 weeks, all treatments except 50 mg of GOL were associated with beneficial treatment effects relative to PBO, with the greatest effect being associated with ADA (the only treatment with statistically significant effect). ADA was associated with the highest probability of staying in remission and the smallest probability of moving from remission to response and from remission to no response.
Sensitivity analyses were conducted by replacing ULTRA2 anti-TNF-α-naive data with ULTRA2 ITT data (sensitivity analysis 1), including Suzuki et al. 46 (sensitivity analysis 2), and replacing ULTRA2 anti-TNF-α-naive data with ULTRA2 ITT data plus including Suzuki et al. 46 (sensitivity analysis 3). The results suggested that when ULTRA2 ITT data were replaced by ULTRA2 anti-TNF-α-naive data for patients starting in remission at 8–32 weeks and in response at 32–52 weeks, the estimate of the effect of ADA on clinical response changed from being slightly worse than PBO to being slightly better than PBO. However, the estimates were associated with considerable uncertainty.
Available data on hospitalisation outcomes were very limited, but suggested that outcomes may be more favourable for ADA-treated and IFX-treated patients than PBO-treated patients (with no data available from GOL trials). Data on surgical intervention were also very sparse, with a potential inconclusive benefit for intervention-treated patients compared with PBO. No trials reported whether surgical outcomes were elective or emergency in nature; however, more data are required to demonstrate the impact of interventions on hospitalisation and surgical intervention more conclusively. Data were available from a single trial to support the use of IFX in induction and maintenance treatment in a paediatric population.
The main safety issues highlighted in the RCT evidence appeared to be generally consistent with those previously discussed in the respective SmPCs (including serious infections, malignancies and administration site reactions). Deaths occurring during and after the study period were described in some trials evaluating GOL (PURSUIT-Maintenance48) and IFX (ACTs49), of which infection or malignancy were most commonly implicated. This underlines the importance of monitoring and treating serious infections and malignancies in patients receiving immunosuppressive treatment.
The trials included in the clinical effectiveness systematic review typically excluded patients with ulcerative proctitis, patients with fulminant/acute severe disease, those with a history of or at imminent risk of bowel surgery, pregnant or lactating women, and patients with diseases of the central nervous system (e.g. demyelinating disease). Furthermore, patients with history of serious infection and immunodeficiency were also typically excluded, as were individuals with a history of malignancy or signs of dysplasia. Therefore, the effects of ADA, GOL or IFX in these UC populations are unknown.
Two biosimilars (Remsima and Inflectra) to Remicade were considered as part of the evidence base for IFX as part of this assessment. The sponsor submission received from the manufacturers of Remsima and the EPAR reports for Remsima and Inflectra indicated that both biosimilars were approved by the EMA on the basis of reported similar pharmacokinetic and efficacy (demonstrated in AS and RA patients) profiles to Remicade. No further trials of Remsima or Inflectra were identified in the course of this assessment.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
Methods
Identification of studies
A comprehensive search was undertaken to systematically identify literature relating to the cost-effectiveness of IFX, ADA and GOL for treating moderate to severe UC after the failure of conventional therapy. The search strategy comprised the following main elements:
-
searching of electronic databases
-
contact with experts in the field
-
hand-searching of bibliographies of retrieved papers.
The following electronic databases were searched from inception for economic evaluations:
-
MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations: via Ovid – 1946 to January 2014.
-
EMBASE: via Ovid – 1974 to January 2014.
-
Cochrane Library: via Wiley Interscience
-
CDSR – 1996 to January 2014
-
DARE – 1995 to January 2014
-
CCRT – 1995 to January 2014
-
Cochrane Methodology Register – 1904 to January 2014
-
HTA database – 1995 to January 2014
-
NHS EED – 1995 to January 2014.
-
-
CINAHL: via EBSCOhost – 1982 to January 2014.
-
Web of Science Citation Index: via Web of Knowledge – 1900 to January 2014.
-
Conference Proceedings Citation Index: via Web of Knowledge – 1990 to January 2014.
-
BIOSIS Previews: via Web of Knowledge – 1969 to January 2014.
-
EconLit: via Ovid – 1886 to January 2014.
The MEDLINE search strategy is presented in Appendix 9. The search strategy combined free text and MeSH or thesaurus terms relating to ulcerative colitis, with free text and MeSH or thesaurus terms relating to infliximab, adalimumab and golimumab combined with highly sensitive economic filters to retrieve economic evaluations. The search strategy was translated across all databases. No date or language restrictions were applied. Literature searches were conducted during January 2014. References were collected in a bibliographic management database and duplicates were removed.
Inclusion/exclusion criteria
Studies were included in the systematic review if they reported full economic evaluations comparing IFX, ADA and/or GOL, against each other or against any other intervention, within their licensed indications for the treatment of patients with moderate to severe UC. The inclusion and exclusion criteria applied within the systematic review are presented in Box 1. Studies were included only if they were reported as full papers; conference abstracts were excluded from the review as they present insufficient detail to allow for a rigorous assessment of study quality.
-
Full economic evaluations comparing IFX, ADA and/or GOL against each other or any other intervention for the treatment of patients with moderate to severe UC.
-
Studies assessing biologicals in the acute setting (e.g. management of UC exacerbations).
-
Studies in which the same biological is used in all treatment groups within the analysis.
-
Non-comparative studies and partial economic evaluations (e.g. costing studies).
-
Abstracts, letters and commentaries.
-
Studies not reported in English.
-
Studies relating to patients with diseases other than UC.
Review methods
The results of the economic searches were sifted by title and abstract. The full papers of studies that potentially met the inclusion criteria were retrieved for further inspection. Studies included in the systematic review were critically appraised using the Drummond checklist for economic evaluations. 77 In addition, the manufacturers of the products considered within this appraisal submitted economic evidence to NICE; these models were assessed in terms of the extent to which they meet the NICE reference case. 78 The structure and formulae included in the manufacturers’ submission models were scrutinised by two members of the Assessment Group (PT and HB). It should be noted that this appraisal includes an update of Technology Appraisal Guidance 140;79 the economic evaluation reported within the 2007 Schering Plough submission to NICE80 is not included in this review as it has previously been critiqued for NICE;81 however, one of the studies included in the review82 reports an analysis of this model.
Results
The systematic searches identified a total of 907 potentially relevant citations (Table 34 and Figure 73). In addition, 4 manufacturers’ submissions were received by NICE. 62,64,66,72 Two of the four submissions were submitted by the same manufacturer – one relating to GOL and one relating to IFX; as these relate to virtually identical models, they are considered as a single analysis within this assessment. 64,66 Three of the manufacturer’s submissions to NICE62,64,66 included economic analyses; the submission from Celltrion72 did not include any economic analysis. Fourteen studies were excluded as they were available only in abstract form. A total of three published studies and three manufacturers’ submissions reported economic analyses relating to the use of biologicals for the treatment of moderate to severe UC (Table 35).
| Database | Date range | Date searched | Number of results |
|---|---|---|---|
| MEDLINE (via Ovid) | 1946 to January 2014 | 15 January 2014 | 96 |
| EMBASE (via Ovid) | 1974 to January 2014 | 15 January 2014 | 372 |
| CINAHL (via EBSCOhost) | 1982 to January 2014 | 22 January 2014 | 23 |
| Science Citation Index and Social Science Citation Index (via Web of Knowledge) | 1900 to January 2014 | 22 January 2014 | 243 |
| BIOSIS (via Web of Knowledge) | 1969 to January 2014 | 22 January 2014 | 186 |
| Cochrane HTA (via Wiley) | 1991 to January 2014 | 21 January 2014 | 30 |
| Cochrane DARE (via Wiley) | 1991 to January 2014 | 21 January 2014 | 28 |
| Cochrane EED (via Wiley) | 1991 to January 2014 | 21 January 2014 | 24 |
| EconLit (via Ovid) | 1886 to January 2014 | 15 January 2014 | 1 |
FIGURE 73.
Study selection results for review of economic evaluations.

| Study | Year of publication | Perspective | Economic comparisons | Outcome measure | Time horizon | Conflicts of interest |
|---|---|---|---|---|---|---|
| Included published economic evaluations | ||||||
| Park et al.83 | 2012 | USA (payer) | Colectomy + IPAA vs. standard medical care (including IFX) | QALYs | Lifetime | Non-commercial |
| Tsai et al.82 | 2008 | UK NHS | IFX vs. standard care | QALYs | 10 years | Study funded by Schering-Plough |
| Xie et al.84 | 2009 | Canadian (public payer) | IFX vs. usual care | QALYs | 5 years | Three out of six authors disclosed a conflict of interest |
| Included manufacturers’ submissions | ||||||
| AbbVie submission62 | N/A | UK NHS | ADA vs. conventional non-biological treatment | QALYs | 10 years | Manufacturer of ADA (AbbVie) |
| MSD submission64,66 | N/A | UK NHS | Pairwise comparisons of IFX, GOL, ADA and immediate colectomy | QALYs | 10 years | Manufacturer of IFX and GOL (MSD) |
Review of published economic evaluations presents a summary and critical appraisal of the three published economic studies included in this review. 82–84 Cost-effectiveness evaluation of golimumab (with Patient Access Scheme), infliximab and adalimumab relative to colectomy for moderate to severe ulcerative colitis in the UK (MSD submissions64,66) and Adalimumab, golimumab and infliximab, for the treatment of ulcerative colitis (subacute) – AbbVie submission62 present critical reviews of the individual manufacturers’ submissions from AbbVie and MSD, respectively. 62,64,66
Review of published economic evaluations
Park et al.83
Park et al. 83 report the methods and results of an economic analysis of early colectomy plus ileal pouch anal anastomosis (IPAA) versus standard medical therapy in patients with severe UC in the USA. The model population is intended to reflect 21-year old patients with newly diagnosed pancolitis UC confirmed by colonoscopic biopsies. The economic analysis compares two sequences of treatments: (1) immediate colectomy with IPAA; and (2) standard medical therapy, which is assumed to comprise a sequence of (i) 2 g of mesalamine per day; (ii) 125 mg of AZA per day; (iii) 5 mg/kg/dose of IFX every 8 weeks; (iv) 1.5 mg of tacrolimus b.i.d.; and (v) colectomy + IPAA. The analysis does not consider the comparative cost-effectiveness of alternative sequences of medical treatments. The authors purport to have adopted a societal perspective; however, it does not appear that any indirect costs borne outside the health sector (e.g. lost productivity or out-of-pocket expenses) have been included in the economic analysis. It would be more accurate to describe the adopted perspective as that of the health-care payer. Health economic results are presented in terms of the incremental cost per quality-adjusted life-year (QALY) gained over a lifetime horizon. In line with the reference case set out in Gold et al. ,85 costs and health outcomes were discounted at a rate of 3% per year. Costs were valued at 2009 prices.
The economic analysis uses a Markov approach to evaluate relevant events, costs and health outcomes. The duration of each Markov cycle is not entirely clear from the paper;83 the text indicates that the first cycle is 3 months in duration and the cycle length appears to be 8 weekly thereafter. However, the table of parameter values presented within the paper suggests that probability parameters are defined according to various time intervals. In addition, the text does not mention whether or not a half-cycle correction has been applied. The precise health states adopted in the model are also not entirely clear from the text; while a model diagram is presented in the paper, this details the sequences of treatments in each group but does not specify the relevant clinical events that patients may experience. It appears that separate states are assigned for patients who are on treatment, in remission, experiencing UC flare, postcolectomy and experiencing death. With respect to medical treatment, the model appears to separate response and remission based on the Simple Colitis Activity Index (SCAI) score. 86 The model also includes the possibility of patients developing colorectal cancer, which is assumed to result in colectomy. It appears that the model does not include an excess risk of death due to colorectal cancer. Patients enter the model at the point of being hospitalised during their initial flare definitively diagnosing them of pancolitis UC through endoscopic biopsies. Although this may indicate a more severe population than that stated within the scope of this appraisal, the model uses RCT evidence from studies that relate to a moderate to severe UC population49 and assumes that the flare is resolvable without surgery. Following diagnosis, patients are then assumed to receive i.v. methylprednisolone and subsequently mesalamine as maintenance therapy once they are able to tolerate oral medicines. Patients progress along the treatment pathway to IFX, tacrolimus and potentially colectomy + IPAA if remission is not achieved. Patients in the intervention group within the model bypass all medical treatments and immediately undergo surgery. Different cost and HRQoL estimates are applied to each health state.
Treatment benefits are defined differently for surgery and medical treatment. For the standard medical therapy group, treatment benefits are characterised as response, remission and UC flare rates. For the surgery group, treatment benefits are determined according to colectomy success rates, the avoidance of AEs (pouchitis and infertility), the requirement for antibiotics, and remission rates for antibiotics. Effectiveness data were drawn from a number of sources including RCTs and non-randomised studies. 49,87–99 The effectiveness of IFX in inducing and maintaining response and remission was based on the results of the ACT1 and ACT2 studies. 49 The rate of developing colorectal cancer was derived from an observational study. 100 The approach for determining the effectiveness of medical and surgical treatment options is essentially a naive indirect comparison and, as such, the results of the analysis should be interpreted with a degree of caution.
Health utilities were assigned for the following events and states: UC flare (0.48), remission (0.91), post-colectomy (0.87), and infertility following IPAA (0.74). Health utilities were drawn from a variety of sources. 101–104 The elicitation methods from which these estimates were derived were not consistently clear. Although several studies used the time trade-off (TTO) method, the study reportedly used to derive a disutility for female infertility is not a quality-of-life valuation study and actually relates to the medical costs of epididymitis and orchitis in men;104 it is unclear how the information contained within this paper has been used to inform the economic analysis.
The model includes resource costs associated with diagnosis of pancolitis UC, drug therapy, colectomy + IPAA, managing pouchitis and the diagnosis of colorectal cancer. The costs associated with hospitalisations, outpatient visits, procedures and laboratory costs were estimated using national reimbursements from the Centers for Medicare and Medicaid Services and average reimbursement rates from all patient billing records in 2009 at Stanford University Medical Center. The Office of Statewide Health Planning and Development tables were used to validate institutional rates with the intention of reflecting national average cost estimates. Wholesale costs of medical therapies were estimated by prices from online pharmacies and were validated against the drug costs at Lucile Packard Children’s Hospital pharmacy.
The analysis includes deterministic sensitivity analysis and probabilistic sensitivity analysis (PSA). Results are presented as mean costs and QALYs, incremental cost-effectiveness ratios (ICERs), one-way deterministic sensitivity analyses and summary results of the probability of achieving the greatest net benefit at a given willingness-to-pay threshold.
Table 36 presents the headline results of the economic analysis. The analysis indicates that standard medical treatment produces more health (0.06 QALYs) at a considerably greater cost than colectomy + IPAA (US$88,607). The incremental cost-effectiveness of standard medical treatment versus colectomy + IPAA is estimated to be US$1,476,783 per QALY gained.
| Strategy | QALYs (95% CI) | Costs (95% CI) | Incremental QALYs (95% CI) | Incremental costs (95% CI) | ICER (95% CI) |
|---|---|---|---|---|---|
| Colectomy + IPAA | 20.72 (17.53 to 22.76) | US$147,763 (US$137,013 to US$158,904) |
– | – | US$1,476,783 (dominated to US$3,281,923) |
| Standard medical treatment | 20.78 (18.45 to 22.37) | US$236,370 (US$219,057 to US$255,328) | 0.06 (–0.72 to 1.03) | US$88,607 (US$73,726 to US$105,865) |
Assuming a willingness-to-pay threshold of US$50,000 per QALY gained, the probability that colectomy + IPAA produces the greatest net benefit is approximately 1.0. Assuming a willingness-to-pay threshold of US$100,000 per QALY gained, the probability that colectomy + IPAA produces the greatest net benefit is approximately 0.96. The sensitivity analysis indicates that the utility of the cure state after receiving colectomy + IPAA was the only variable which reduced the ICER to below US$100,000 per QALY gained. The authors state that the level of HRQoL for patients with UC would need to be very low in order for exhaustive medical therapy in severe UC to be cost-effective.
The Park et al. 83 study clearly addresses the question of whether or not colectomy + IPAA is cost-effective in comparison with medical management over a lifetime horizon. However, the description of the mathematical model is unclear, hence the assumptions underpinning the analysis are not transparent and their credibility is difficult to judge. The population reflected in the economic analysis is only partially relevant to the scope of this appraisal as the patients considered within the model are by definition hospitalised for UC flare. However, the model also appears to assume that the flare can be resolved; hence, patients may go on to receive biological therapy in a non-acute setting. Given the absence of head-to-head trials comparing medical and surgical management options, the need for an indirect comparison is inevitable and may lead to bias in the model results. It is also noteworthy that the study relates to a US setting and, therefore, its relevance to UK clinical practice may be questionable.
Tsai et al.82
Tsai et al. 82 report the methods and results of an economic analysis that compares two IFX-based strategies with standard care in patients with moderate to severe UC from the perspective of the UK NHS. The model structure and parameter values appear to be very similar to the economic analysis submitted to NICE to inform technology appraisal TA140,28 although it should be noted that the total cost estimates for each group reported by Tsai et al. 82 differ to those reported within the manufacturers’ submission. 80 Patients within the model were assumed to have a mean body weight of 73.1 kg. The base-case scenario evaluated a treatment strategy of 5 mg/kg of IFX every 8 weeks only for patients achieving response while a secondary analysis evaluated 5 mg/kg of IFX every 8 weeks for patients achieving and maintaining remission following induction. Standard care was assumed to include colectomy + IPAA and other medications (5-ASAs, corticosteroids and immunosuppressants). Cost-effectiveness was assessed in terms of the incremental cost per QALY gained over a 10-year time horizon for each comparison of IFX versus standard care. A fully incremental analysis was not undertaken between the two responder/remission stopping rule treatment approaches. The perspective adopted was that of the NHS. Costs borne by Personal Social Services (PSS) were not included in the model. The authors note that although productivity costs are substantial for patients with UC, these were not included in the economic analysis. Costs and health outcomes were discounted at a rate of 3.5% per year. Costs were valued at 2006–7 prices.
The economic evaluation takes the form of a Markov model, as shown in Figure 74. The model appears to include eleven health states: (1) mild (responders); (2) moderate-severe (responders); (3) remission (responders); (4) mild (non-responders); (5) moderate-severe (non-responders); (6) remission (non-responders); (7) temporary discontinuers; (8) surgery (tunnel state); (9) post-surgery remission; (10) post-surgery complications; and (11) death. The cycle length used within the model was specified according to the time intervals of the assessment visits in the ACT1 and ACT2 studies. 49 The first cycle was 8 weeks in duration, followed by 6 weeks in cycle 2, followed by 8 weeks for all subsequent cycles. It should be noted that within the ACT trials, the assessments at these time points were based on partial Mayo scores and may not correspond to full Mayo scores (the latter of which includes endoscopic visualisation). 105 The paper does not mention whether a half-cycle correction was applied to account for the timing of events within the model.
FIGURE 74.
Model diagram presented by Tsai et al. 82 Reproduced with permission from Tsai HH, Punekar YS, Morris J, Fortun P, A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis, Aliment Pharmacol Ther 2008;28:1230–9, © 2008 The Authors. Journal compilation © 2008 Blackwell Publishing Ltd.

All patients enter the model in the moderate to severe health state. At the end of each cycle, patients achieving a Mayo score of 0–2 and 3–5 transit to the remission state and mild health state, respectively, and continue to receive the same treatment. Patients who do not achieve remission or response are classified as non-responders. A ‘temporary discontinuers’ state is included for patients experiencing temporary AEs, which is a tunnel state which is applied for one 8-week cycle. After resolution of AEs, these patients return to their prior health state. Non-responders and patients permanently discontinuing active treatment (e.g. owing to AEs) transit to the corresponding non-responder states and cannot restart IFX treatment. Patients in the moderate to severe states can undergo surgery, which may result in complications. Different costs and utilities are applied to each health state. The model does not include any survival difference between the competing treatments hence the differences in QALYs are driven entirely by differences in sojourn time in each health state.
Transition probabilities in each group were estimated using data from the ACT1 and ACT2 studies. 49 No details are provided within the paper with respect to how these studies were pooled. Transition probabilities for patients in the responder states for IFX and standard care were drawn from the treatment and PBO arms of these trials, respectively. Transition probabilities for non-responders for both groups were drawn from PBO arms of the ACT studies. 49 As ACT1 employed a longer study duration than ACT2, the former trial alone was used to estimate transition probabilities beyond 30 weeks. The ACT1 and ACT2 studies were also used to estimate the probabilities of temporary discontinuation based on the observed AE rates. Transition probabilities for patients undergoing surgery were derived from the literature. 106–108 None of the transition probabilities applied within the model are reported in the paper.
Health-related quality-of-life values are assigned for remission (0.88), mild (0.76), moderate-severe (0.42), temporary discontinuers (0.42), surgery (0.61), post-surgery remission (0.61), post-surgery complications (0.55). Utility values for UC states are stated to have been drawn from an EQ-5D survey of UC patients; however, this appears to be misreferenced as the publication title relates to resource use in patients with CD. 109 Health utility values for patients who were temporary discontinuers and for those with post-surgical complications were drawn from Arseneau et al. 110 The text states that these utilities were indexed to the Woehl utility set to avoid any implausible results using regression analysis. No further details are provided regarding this regression analysis.
The model includes treatment- and state-specific costs associated with drug acquisition and administration, consultant visits, hospitalisations, blood tests and endoscopy. The sources used to value the costs of drug acquisition and administration are unclear. The model includes the costs of concomitant medications based on the baseline characteristics of patients in the ACT1 and ACT2 studies,49 and assumes that the use of immunosuppressants and 5-ASAs remain constant while corticosteroid use declines linearly over time for patients responding and achieving remission. The costs associated with non-SAEs were calculated separately but are not detailed. The costs associated with severe AEs were assumed to be subsumed within the costs of hospitalisation. The costs of colectomy + IPAA were based on NHS Reference Costs. 111 Hospitalisation rates for the IFX and standard care groups were based on the ACT1 and ACT2 trials49 and were valued using NHS Reference Costs. In addition, health-care resource use associated with pre-surgical UC states was estimated from a panel of six UK gastroenterologists; these resource-use estimates were valued using national published cost estimates.
The model results are presented as mean costs and QALYs for each treatment group. The economic analysis includes one-way deterministic sensitivity analysis and PSA. Decision uncertainty is represented using cost-effectiveness planes.
Table 37 presents the results of the economic analysis. Within the base-case analysis, in which medical treatment is assumed to be continued only for those patients in whom response is achieved, IFX is estimated to produce an additional 0.75 QALYs at an additional cost of £20,662, which corresponds to an ICER of £27,424 per QALY gained. Within the secondary analysis, in which patients are assumed to continue treatment only if they achieve and maintain remission, IFX is estimated to produce an additional 0.39 QALYs at an additional cost of £7615, which corresponds to an ICER of £19,696 per QALY gained. It should be noted that the estimates of absolute costs and absolute QALYs for the standard care group differ between the two analyses. Although the alternative treatment stopping rules clearly influence which patients continue to receive IFX and the duration over which patients would receive biological therapy, it is unclear why this would affect outcomes for the standard care group.
| Strategy | QALYs | Costs | Incremental QALYs | Incremental costs | ICER |
|---|---|---|---|---|---|
| Base-case scenario (responders only) | |||||
| IFX | 4.591 | £66,460 | 0.75 | £20,662 | £27,424 |
| Standard care | 3.838 | £45,798 | – | – | |
| Secondary analysis (remission only) | |||||
| IFX | 4.154 | £53,874 | 0.39 | £7615 | £19,696 |
| Standard care | 3.767 | £46,259 | – | – | |
In the responders sensitivity analysis, the ICER ranged from £21,066 per QALY gained (lower patient weight) to £86,320 per QALY gained (1-year time horizon). The results of all other deterministic sensitivity analyses in the responder comparison produced an ICER below £32,000 per QALY gained. In the more stringent remission only analysis, the deterministic sensitivity analyses produced ICERs in the range £14,728 per QALY gained (lower patient weight) to £46,765 per QALY gained (1-year time horizon). The results of all other deterministic sensitivity analyses in the remission only comparison produced ICERs below £23,000 per QALY gained. The results of the probabilistic analysis are presented in the form of cost-effectiveness planes. The authors state that ‘The PSA showed that the results were robust with [the] majority of simulations clustered together. In both responder and remission treatment strategies, IFX SMT resulted in additional QALYs at an additional cost compared to standard care.’82 The probabilistic results for the remission only scenario appear somewhat dubious as the samples appear to be truncated at the y-axis of the cost-effectiveness plane (the estimates of incremental QALYs for IFX vs. standard care cannot drop below zero). The underlying reason for this within the model is unclear.
As noted earlier, the Tsai et al. 82 analysis appears to be based on the same model submitted to NICE as part of TA140 (not reviewed here). 28 While the QALY estimates reported within Tsai et al. 82 are virtually the same as those reported within the manufacturers’ submission to NICE,80 the incremental costs reported by Tsai et al. 82 are lower than those contained within the manufacturers’ submission. Consequently, the ICERs presented by Tsai et al. 82 are lower than those reported within the NICE submission (responders only analysis ICER = £27,424 per QALY gained82 vs. £33,866 per QALY gained;80 remission only analysis £19,696 per QALY gained82 vs. £25,044 per QALY gained80).
Overall, the Tsai et al. 82 model appears to follow a plausible model structure and includes the majority of costs and outcomes relevant to the decision problem. It is also noteworthy that this is the only published UK analysis included in this review. In general, the paper performs well against the Drummond checklist. The two notable issues relate to the absence of other biological therapies (this is reasonable as GOL and ADA did not have a UK marketing authorisation at the time of publication) and immediate colectomy as comparators and the use of a short time horizon.
Xie et al.84
Xie et al. 84 report a cost-effectiveness analysis comparing IFX plus ADA versus usual care in patients with moderate to severe refractory UC in Canada. Patients were assumed to be 40 years of age with a mean body weight of 80 kg. The model adopts a Markov approach and costs and outcomes are evaluated over a 5-year time horizon. Three options were compared within the economic analysis: (1) ‘strategy A’ – ‘usual care’, which includes conventional medical treatment (5-ASAs plus immunosuppressants) without anti-TNF-α drugs; (2) ‘strategy B’ – ‘5 mg/kg of IFX plus ADA initial and maintenance therapy’, which includes 5 mg/kg of IFX followed by a switch to ADA if there is no response to initial therapy or if response is lost during maintenance therapy; and (3) ‘strategy C’ – ‘5 mg/kg and 10 mg/kg of IFX + ADA’, which involves initial therapy using 5 mg/kg of IFX and, if there is no response, the dose is escalated to 10 mg/kg of IFX, then if response is lost during maintenance therapy, switch to ADA (Figure 75). Surgery is included in the pathway for all three treatment groups within the model but is not included as a comparator. Costs and health outcomes were discounted at a rate of 5% per year. All costs were valued at 2008 prices.
FIGURE 75.
Treatment sequences evaluated by Xie et al. 84 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
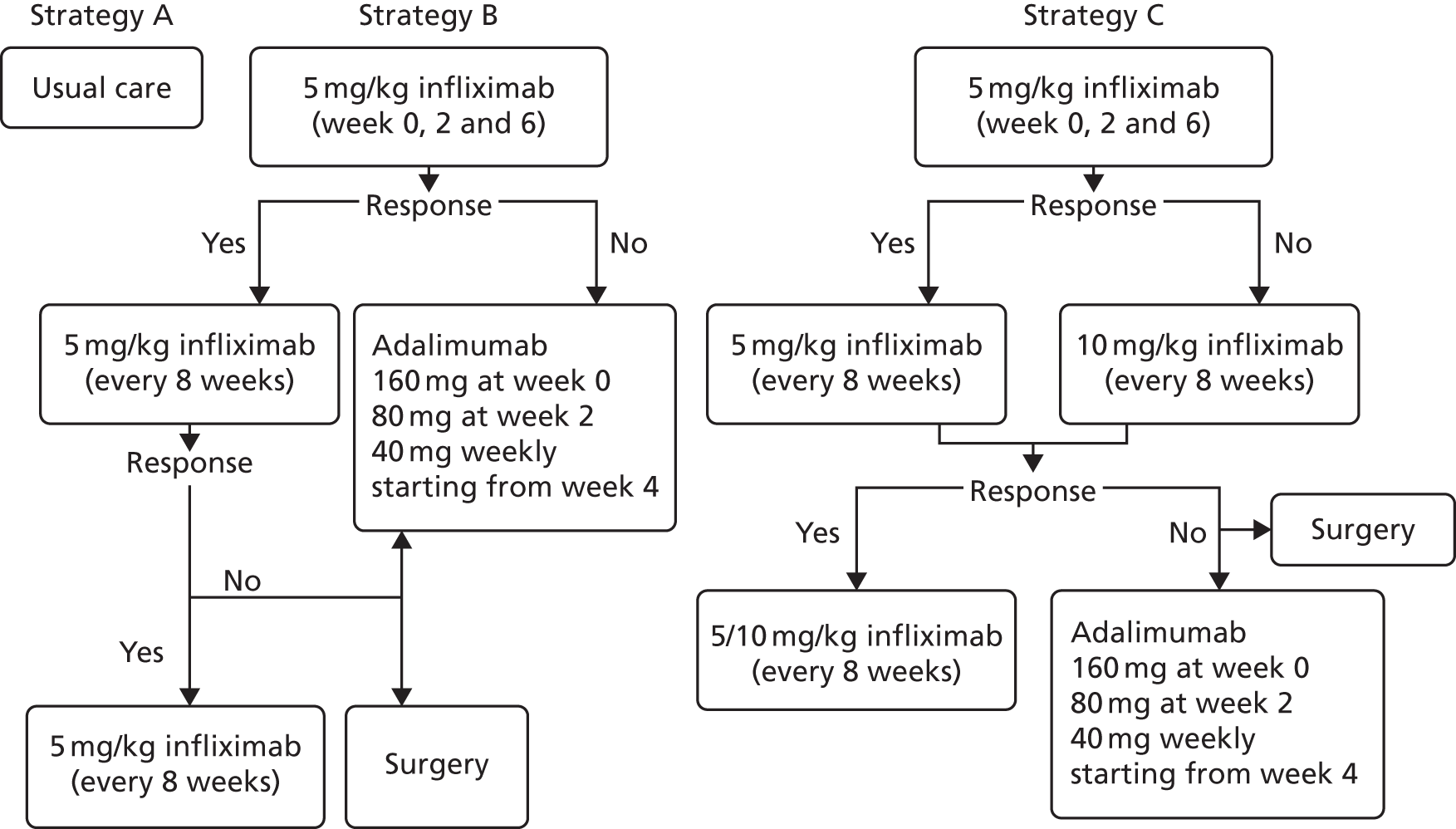
The model includes five mutually exclusive health states: (1) remission, which is defined as a total Mayo score of ≤ 2 points without individual subscores exceeding 1 point; (2) active UC, which includes those patients who do not respond and those who do respond but do not achieve remission; (3) surgery, which is a tunnel state; (4) surgical remission; and (5) surgical complication (Figure 76). The model adopts a variable cycle length according to the timing of full Mayo score assessments adopted in the ACT1 and ACT2 studies (0–8 weeks, 9–30 weeks, 31–54 weeks, then 27 weekly thereafter). 49 Patients enter the model in the active moderate to severe state and following initial therapy either achieve remission or not. Those patients who achieve remission may subsequently lose response and transit to active UC or they may maintain remission. For responders in the active UC state, patients may achieve remission or remain in the active UC state with maintenance. Patients who are non-responders can undergo colectomy + IPAA, switch to ADA (strategy B) or receive an increased dose of IFX (strategy C). Following surgery, patients may experience complications, which may or may not be resolved. These complications may arise immediately after surgery or at a later timepoint. Death is not included as an event in the model owing to the short time horizon. Different costs and utilities are applied to each model health state.
FIGURE 76.
Model diagram presented by Xie et al. 84 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
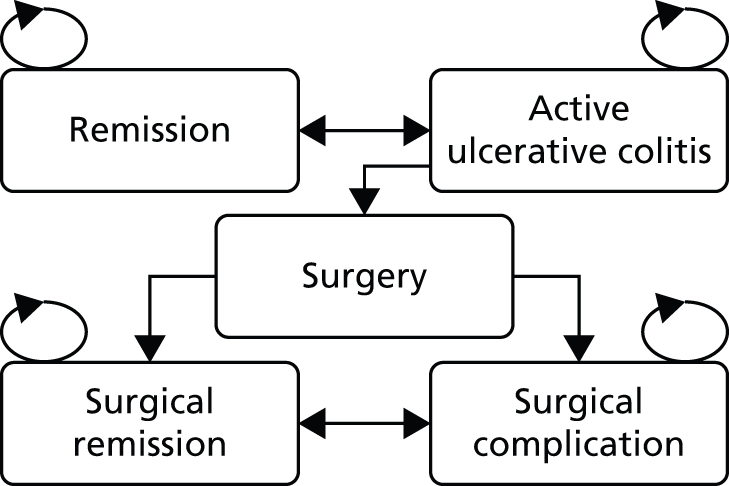
The majority of clinical parameters within the model were drawn from the ACT1 and ACT2 studies,49 derived using a fixed-effects meta-analysis. Remission rates in responders and non-responders were estimated as time-independent parameters based on the ACT1 and ACT2 studies. 49 The authors note that remission rates drawn from the PBO arms of the ACT trials reflect the use of active concomitant medications. Rates of early and late surgery were drawn from a RCT reported by Jarnerot et al. 112 and a non-randomised cohort study reported by Hoie et al. 107 Rates of complications and the probability of their resolution were derived from non-randomised studies. 113,114 Remission rates, the probabilities of maintaining remission over time and the proportion of non-responding patients in those with active UC for each treatment group were modelled as time-dependent parameters based on the ACT1 and ACT2 studies. 49 Owing to the absence of randomised evidence at the time of the analysis, remission rates for ADA were assumed to be equivalent those for 5 mg/kg of IFX. The model did not incorporate the effects of AEs on health outcomes or costs as the ACT1 and ACT2 studies49 reported that the proportions of patients with any AE were similar among the IFX and standard care groups.
The model includes four utility values: remission (0.79), active UC (0.32), surgical remission (0.68) and surgical complications (0.49). The model makes no distinction between the HRQoL outcomes for patients who achieve response but not remission and patients who do not achieve response; this may be considered as a very pessimistic assumption that could bias against IFX and ADA therapy, particularly given the low valuation of HRQoL for patients with active UC. All health utilities were drawn from a previous economic modelling study reported by Arseneau et al. 110 The method of utility elicitation within this study appears to be TTO.
The model includes the costs associated with drug acquisition, drug administration, colectomy and IPAA, medical examination and the management of surgical complications. Drug acquisition costs were drawn from provincial drug benefit lists (including an 8% mark-up). The costs of medical examinations were derived from the Ontario Schedule of Benefits. The costs of surgery were derived from the literature. 115
The results of the economic analysis are presented as mean costs and QALYs and ICERs for each treatment group based on point estimates of parameters. Pairwise comparisons are presented for the IFX and ADA options versus usual care. A fully incremental analysis between all options in the model is reported in the text. PSA was also conducted with decision uncertainty represented using cost-effectiveness acceptability curves (CEACs).
Table 38 presents the headline cost-effectiveness results reported by Xie et al. 84 The model analysis suggests that the strategy B is expected to produce more health gain than strategies A and C. Strategy C is dominated by strategy B. The incremental cost-effectiveness of strategy B versus usual care was estimated to be approximately CA$358,823 per QALY gained.
| Strategy | QALYs | Cost | Incremental QALYs | Incremental cost | ICER |
|---|---|---|---|---|---|
| Strategy B (IFX + ADA) | 2.18 | CA$82,756 | 0.16 | CA$58,488 | CA$358,823 |
| Strategy C [IFX (plus dose escalation) + ADA] | 2.15 | CA$101,272 | – | – | Dominated |
| Strategy A (usual care) | 2.02 | CA$24,268 | – | – | – |
The PSA suggests that assuming a willingness-to-pay threshold of CA$150,000 per QALY gained, the probability that usual care is optimal is approximately 1.0. The deterministic sensitivity analysis suggests that the lowest ICER is achieved by increasing the utility for remission (ICER = CA$273,081 per QALY gained for strategy B vs. strategy A and CA$428,676 per QALY gained for strategy C vs. strategy A), while the highest ICER is achieved by lowering the utility for remission (ICER = CA$527,236 per QALY for strategy B vs. strategy A, CA$889,227 per QALY gained for strategy C vs. strategy A).
Overall, the analysis reported by Xie et al. 84 appears to adequately address the decision problem using a generally appropriate model. However, the analysis is limited by the use of a short time horizon, the absence of surgery as a comparator and questionable assumptions regarding the health gains associated with achieving response without remission.
Discussion of published economic evaluations
Three published economic analyses met the inclusion criteria for the systematic review. One analysis compared early colectomy + IPAA versus standard medical treatment,83 one compared IFX versus usual care,82 and the third compared IFX plus ADA versus usual care. 84 Only one study (Tsai et al. 82) was undertaken from the perspective of the UK NHS. The included studies were broadly consistent in terms of the disease-specific factors included in the analyses; all analyses included remission and response, and surgery as a consequence of ineffective medical treatment. Only one study (Park et al. 83) included the increased risk of colorectal cancer associated with UC within the analysis. One study (Xie et al. 84) did not include mortality for any patient group in the model. Only Park et al. 83 included surgery as a treatment option; the other options focused solely on medical treatment strategies. The study reported by Xie et al. 84 included ADA as part of the pathway; however, owing to a lack of RCT evidence at the time of the analysis, the authors assumed that ADA was equivalent to 5 mg/kg of IFX and this assumption may not be appropriate given more recent evidence. 44,49 None of the included studies evaluated the cost-effectiveness of GOL versus any other treatment. The time horizons considered in the economic analyses differ considerably, ranging from 5 years to the patient’s remaining lifetime. It is also noteworthy that although the study reported by Tsai et al. 82 reported favourable results for IFX (<£30,000 per QALY gained), Xie et al. 84 reported considerably less favourable estimates (> CA$350,000 per QALY gained). This contrasting finding may in part be explained by differences in assumptions regarding the level of HRQoL attributable to patients achieving response but not remission. Overall, none of the included studies present sufficient evidence relating to the cost-effectiveness of IFX, ADA and GOL versus standard medical or surgical treatment options for moderate to severe UC from the perspective of the UK NHS and PSS.
The next sections present a critique of the economic evidence submitted by the manufacturers of IFX, ADA and GOL.
Cost-effectiveness evaluation of golimumab (with Patient Access Scheme), infliximab and adalimumab relative to colectomy for moderate to severe ulcerative colitis in the UK (MSD submissions64,66)
The MSD submissions include details of a systematic review of previous models together with the methods and results of a de novo model developed to assess the cost-effectiveness of ADA, GOL and IFX and standard non-biological treatment for moderate to severe UC. Although MSD submitted two de novo models and two submission reports,64,66 these relate to virtually the same overall model and analysis, hence they are detailed and critiqued together within this section.
Summary of manufacturers’ review of existing economic analyses
The MSD submissions include a systematic review of economic evaluations of treatments for UC. The manufacturer undertook searches in EMBASE, PubMed and the NHS EED to identify published economic evaluations in UC to help inform the model structure and relevant parameters. A total of 12 published health economic analyses were included in the MSD review. 82,84,116–125 Several of these studies do not include biological treatment options and some of the analyses relate to the management of severe UC exacerbations which is beyond the scope of this appraisal. The MSD review highlights the following points with respect to previous health economic analyses:
-
Markov models are commonly used to evaluate treatments for UC
-
all of the published models report outcomes in terms of QALYs/life-years gained
-
none of the studies included all relevant therapies
-
there is variability in parameter sources and values between economic studies
-
resource-use estimates used in published models are typically derived from experts in the field rather than empirical research studies.
The MSD submissions also highlight a distinction between two distinct types of models: (1) Markov models in which the model structure is based on sequences of therapies; and (2) Markov models in which the model structure is based on severity. The MSD submissions do not report the results of these previous economic analyses; hence they are not discussed further here.
MSD model scope
The MSD model compares 160 mg/80 mg/40 mg of ADA, 5 mg/kg of IFX, 200 mg/100 mg/50 mg (100 mg for some patients with higher body weight after the initial induction dosing) of GOL and standard non-biological treatment for patients with moderate to severe UC who have failed previous drug treatment. Standard non-biological treatment is assumed to be immediate colectomy. The perspective of the analysis is that of the UK NHS. GOL is assumed to be given at an initial dose of 200 mg, followed by 100 mg at week 2, then 50 mg every 4 weeks thereafter for patients with body weight < 80 kg. For those patients with body mass ≥ 80 kg, GOL is assumed to be given as an initial dose of 200 mg, followed by 100 mg at week 2, then 100 mg every 4 weeks thereafter. IFX is assumed to be given at a dose of 5 mg/kg followed by additional 5 mg/kg infusion doses at 2 and 6 weeks after the first infusion, then every 8 weeks thereafter. ADA is assumed to be given as 160 mg at week 0 (the dose can be administered as four injections in one day or as two injections per day for two consecutive days) and 80 mg at week 2. After induction treatment, 50% of patients are assumed to receive the recommended dose of 40 mg every other week while the remainder are assumed to receive 40 mg every week. The MSD submission states that 22.9% patients in the ULTRA2 trial require dose escalation but also states that experts advising on the submission suggested that the actual proportion of patients in clinical practice may be as high as 80%. The manufacturer argues that the assumption that 50% patients dose escalate is conservative.
Patients receiving biological treatments who achieve a response or remission at induction are assumed to continue maintenance therapy with the same biological treatment. The model does not include sequences in which alternative biologicals are used. GOL and ADA are assumed to be given as subcutaneous injections while IFX is given as an i.v. infusion. For all treatment options, a proportion of patients are also assumed to receive ongoing ‘background’ non-biological therapies including 5-ASAs, corticosteroids and immunosuppressants. Standard clinical management, defined in the NICE scope as ‘a combination of aminosalicylates (sulfasalazine, mesalazine, balsalazide or olsalazine), corticosteroids (beclomethasone, budesonide, hydrocortisone or prednisolone), and thiopurines (mercaptopurine or AZA), calcineurin inhibitors and surgical intervention’,36 is not included as a treatment option in the MSD economic analysis. Upon model entry, patients are assumed to be 40 years of age and 56% are assumed to be male. The model uses a 2-monthly cycle length. Costs and health outcomes are discounted at a rate of 3.5% each year and are evaluated over a 10-year time horizon.
MSD model structure
The MSD model structure is shown in Figures 77 and 78. The model adopts a hybrid approach whereby an initial decision tree is used to determine the probabilities of induction response or remission for biological drug treatments, together with the probabilities of survival and the incidence of complications for patients undergoing immediate colectomy, while a Markov component is used to estimate long-term outcomes for maintenance drug therapy and surgery. The decision tree structure is identical for all biological drug treatments and includes initial outcomes defined in terms of no response, response and remission. For the standard care (colectomy) option, the decision tree outcomes are different and instead relate to the probabilities of surviving surgery and experiencing early complications resulting from that surgery. The Markov model comprises eight mutually exclusive health states: (1) response (pre-colectomy; maintenance); (2) remission (pre-colectomy; maintenance); (3) response (relapse management); (4) relapse (relapse management); (5) colectomy; (6) remission (post-colectomy); (7) late complications (post-colectomy); and (8) death.
FIGURE 77.
Merck Sharp & Dohme Ltd model structure (redrawn by the Assessment Group).
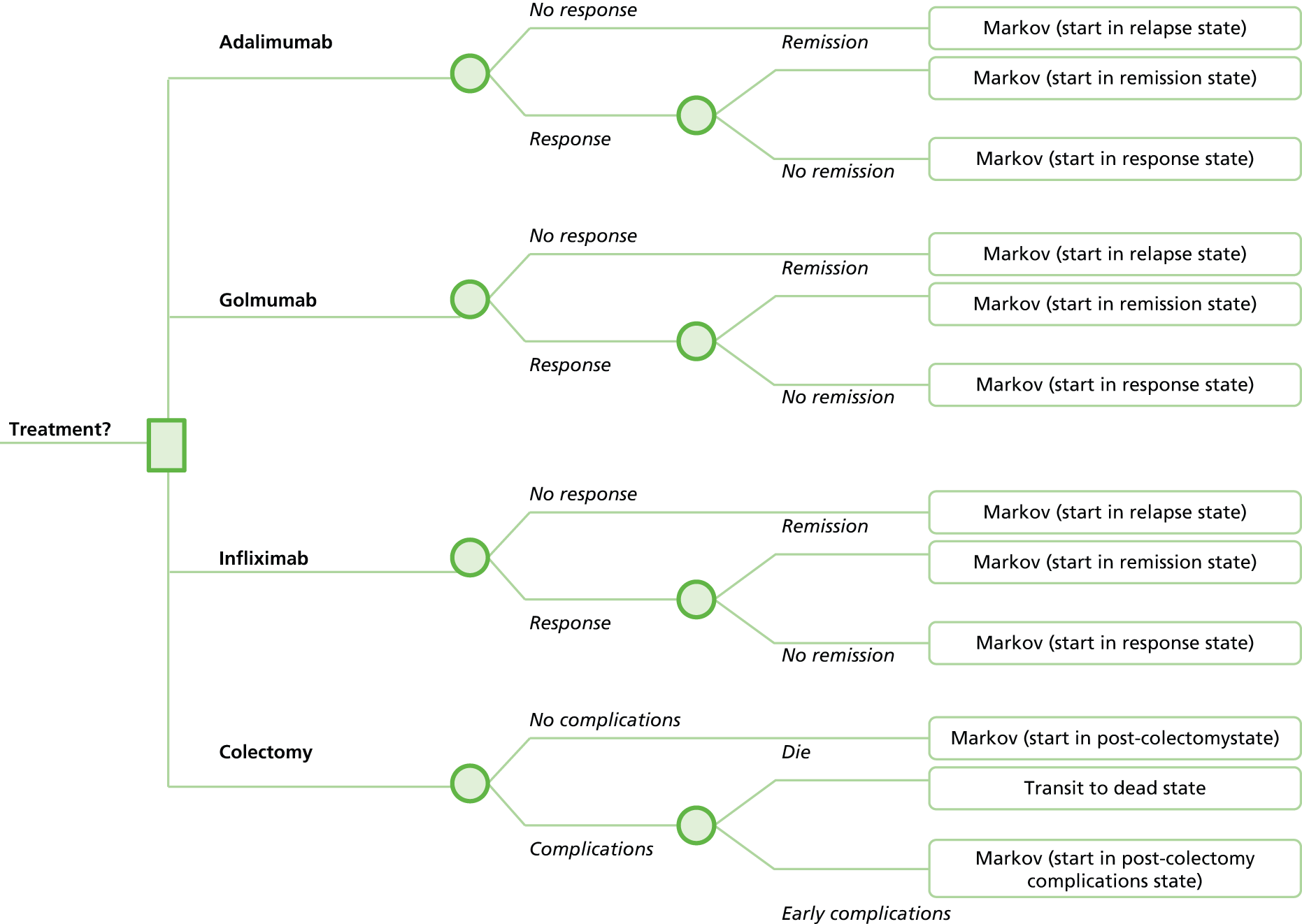
For the biological treatment groups, patients are initially allocated to no response, response or remission based on the results of a de novo NMA of induction therapy trials44,47–49,126 undertaken by the manufacturer. Patients in whom response or remission is achieved at induction are assumed to remain on maintenance treatment using the same biological treatment. Subsequent model transitions are informed by a separate NMA based on the results of the trials of biological maintenance therapies. 44,47,49 Patients who do not respond to induction therapy, and those who lose response during maintenance treatment, are assumed to enter the relapse management state and receive i.v. steroids. Patients who respond to i.v. steroids then transit to the ‘Response (relapse management)’ state at which time they either continue responding or relapse. Patients who do not respond are assumed to undergo immediate colectomy. Colectomy is dealt with as a tunnel state; following surgery a small proportion of patients are assumed to die while the remainder are assumed to be in post-colectomy remission. Patients who survive their surgery are assumed to be at ongoing risk of post-colectomy complications (anal fistula, bowel obstruction and pouchitis). A small proportion of patients receiving drug treatment are assumed to be at risk of serious infection and hospitalisation.
The model uses simple matrix multiplication to determine health state populations during each model cycle based on the state population in the previous Markov cycle and a single time-independent transition matrix over the entire time horizon. Costs and utilities are attached to each health state. Total QALYs are modelled as a function of sojourn time in each health state, mortality associated with colectomy and other-cause (general population risk) mortality.
Evidence sources used to inform the MSD model parameters
A summary of evidence sources used to inform the model’s parameters is presented in Table 39.
| Parameter group | Source |
|---|---|
| Pre-colectomy transition probabilities excluding death: standard non-biological treatment | Manufacturer’s NMA: average study effect of PBO controlled trials in random effects NMA of induction response64,66 |
| ORs for biological treatment effects | ORs derived from manufacturer’s NMA64,66 |
| Effectiveness of i.v. steroids following failure of biological treatment | Model fitted to ensure 27% relapsers require colectomy based on Turner et al.126 |
| Probability of serious infection | Grijalva et al.127 |
| Health utilities for pre-colectomy response/remission | EQ-5D estimates from the PURSUIT trial47,48 in the GOL model, EQ-5D estimates from the ACT1 and ACT249 trials in the IFX model |
| Health utilities for post-colectomy statesa | Woehl et al.109 Tsai et al.82 Health Outcomes Data Analysis Repository, Punekar and Hawkins,120 Chaudhary and Fan,116 Arseneau et al.110 |
| Resource use | PURSUIT trial,47 ACT1 and ACT2 trials49 and interviews with nine gastroenterologists64,66 |
| Unit costsa | Curtis et al.128 NHS Reference Costs129 |
Methods for modelling effectiveness
Estimates of relative effectiveness of biological treatments versus conventional non-biological non-surgical treatment were derived from NMA models of induction and maintenance therapy undertaken by the manufacturer. 64,66
The baseline model employed within the MSD NMA model is not discussed within the submissions. 64,66 The MSD economic model includes a worksheet named ‘Input Efficacy and Trans Prob’ in which the probabilities of response and remission for non-biological therapy are inputted as 0.36 and 0.09 for induction treatment, and 0.83 and 0.86 per 2-month cycle of maintenance therapy respectively. The source is stated in the model as ‘Average study effect of PBO controlled trials in random effects NMA of induction response’. No additional detail on the baseline model is provided within the MSD submissions; thus, it is not possible to determine whether or not these estimates are appropriate.
Relative treatment effects were drawn from de novo NMAs undertaken by the manufacturer, based on the results of a systematic literature review. Separate analyses were undertaken for induction and maintenance therapy. For induction, a NMA was undertaken using data from six RCTs. 44,45,47–49 For maintenance treatment, relative treatment effects were based on a NMA of three RCTs. 45,47,49 The evidence networks employed in the manufacturer’s NMAs are presented in Figures 79 and 80 respectively. It should be noted that the manufacturer’s NMA includes non-licensed indications of IFX, although these are not included in the health economic analysis.
The NMAs use logistic regression models to estimate treatment effects, given an assumption that the data are binomial (separate models are used to estimate the odds of sustained response and the odds of sustained remission respectively). Relative treatment effects were parameterised in terms of ORs and are converted to relative risks in the health economic model. In instances whereby only one RCT informed each treatment (which was predominantly the case for the maintenance outcomes), heterogeneity could not be estimated and, therefore, a fixed-effects model was employed. When multiple studies were available, a random-effects approach was used. 64,66 The NMA model for maintenance therapy includes a complex ‘novel’ imputation of estimates of sustained response and sustained remission for GOL from the PURSUIT trial using data from the non-randomised PBO group (see MSD GOL submission p. 5566). The results of the manufacturer’s NMA are presented in Tables 40 and 41 respectively.
| Intervention | OR | Lower 95% CrI | Upper 95% CrI |
|---|---|---|---|
| Induction response (reference PBO) | |||
| 200 mg/100 mg of GOL | 2.12 | 1.01 | 3.95 |
| 400 mg/200 mg of GOL | 2.47 | 1.19 | 4.65 |
| 5 mg/kg of IFX | 4.12 | 2.08 | 8.14 |
| 10 mg/kg of IFX | 3.81 | 1.95 | 7.59 |
| 160 mg/kg of ADA | 1.87 | 0.96 | 3.65 |
| Induction remission (reference PBO) | |||
| 200 mg/100 mg of GOL | 2.99 | 1.32 | 6.28 |
| 400 mg/200 mg of GOL | 3.32 | 1.56 | 7.23 |
| 5 mg/kg of IFX | 5.27 | 2.60 | 11.64 |
| 10 mg/kg of IFX | 3.90 | 1.88 | 8.56 |
| 160 mg/kg of ADA | 2.25 | 1.08 | 4.72 |
| Intervention | OR | Lower 95% CrI | Upper 95% CrI |
|---|---|---|---|
| Sustained response (reference PBO) | |||
| 40 mg/kg of ADA | 1.31 | 0.67 | 2.59 |
| 5 mg/kg of IFX | 2.12 | 1.02 | 4.54 |
| 10 mg/kg of IFX | 2.51 | 1.17 | 5.51 |
| 50 mg of GOL | 1.51 | 0.94 | 2.47 |
| 100 mg of GOL | 1.75 | 1.08 | 2.84 |
| 50–100 mg of GOL | 1.62 | 1.07 | 2.50 |
| PBO following GOL | 0.78 | 0.47 | 1.28 |
| Sustained remission (reference PBO) | |||
| 40 mg/kg of ADA | 0.76 | 0.22 | 2.56 |
| 5 mg/kg of IFX | 1.30 | 0.44 | 4.05 |
| 10 mg/kg of IFX | 2.26 | 0.73 | 7.49 |
| 50 mg of GOL | 0.83 | 0.29 | 2.40 |
| 100 mg of GOL | 0.98 | 0.36 | 2.78 |
| 50–100 mg of GOL | 0.92 | 0.36 | 2.45 |
| PBO following GOL | 0.45 | 0.15 | 1.34 |
Table 42 illustrates how the ORs are applied within the transition matrix for maintenance therapy within the health economic model, using the ADA treatment group as an example.
| Health state | Response (pre-colectomy; maintenance) | Remission (pre-colectomy; maintenance) | Response (relapse management) | Relapse (relapse management) | Colectomy | Remission (postcolectomy) | Late complications (postcolectomy) | Death related to UC |
|---|---|---|---|---|---|---|---|---|
| Response (pre-colectomy; maintenance) | 0.86a | 0.00 | 0.00 | 0.14b | 0.00 | 0.00 | 0.00 | 0.00 |
| Remission (pre-colectomy; maintenance) | 0.17c | 0.83d | 0.00 | 0.00e | 0.00 | 0.00 | 0.00 | 0.00 |
| Response (relapse management) | 0.00 | 0.00 | 0.83 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 |
| Relapse (relapse management) | 0.00 | 0.00 | 0.73 | 0.00 | 0.27 | 0.00 | 0.00 | 0.00 |
| Colectomy | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.97 | 0.00 | 0.03 |
| Remission (postcolectomy) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.99 | 0.01 | 0.00 |
| Late complications (postcolectomy) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 |
It should be noted that the matrix employed within the MSD models does not match the transitions implied by the diagram within the MSD submissions64,66 (see Figure 78). In the executable model, patients who have previously achieved a response can either maintain or lose that response, but they cannot improve (i.e. they cannot subsequently transit to the remission state). Patients who have previously achieved remission can either maintain or lose that remission. However, upon losing remission, the patient cannot transit directly to relapse – they transit to the response state first. This means that no additional patients can achieve remission after induction and no patients with remission can completely lose response during any given model cycle. It is also noteworthy that patients who discontinue treatment with a biological treatment transit very quickly to colectomy (27% of all non-responding relapsers during each 2-month cycle). This estimate was based on a meta-regression of studies describing the short-term outcomes for adult and paediatric patients treated with i.v. corticosteroids, with or without ciclosporin, for exacerbations of UC. 126
The model also includes a small risk of experiencing serious infection owing to the use of immunosuppressants and biological therapies based on Grijalva et al. 127 The model assumes a hazard ratio of 1.10 for all biological therapies and a baseline risk of 0.16 for non-biological therapy.
Health-related quality of life
The health utility values used in the MSD model are presented in Table 41. Health utilities associated with failure, response, and remission as a result of induction and maintenance treatment, and utility values assigned to the health states ‘Response (pre-colectomy; maintenance)’ and ‘Remission (pre-colectomy; maintenance)’ are assumed to be the same for all biologicals, based on EQ-5D valuations derived from the PURSUIT trial47 within the GOL model and the ACT1 trial49 within the IFX models. The MSD submission suggests that disutilities for AEs associated with biologicals are likely to be captured within these estimates. Different utilities are assumed for the achievement of the same outcome at induction and maintenance (i.e. the utility for response at induction is not the same as the utility for response at maintenance). Utility values for colectomy, postcolectomy and early and late complications of colectomy were based on estimates reported within the literature, although the precise sources are not clear from the MSD submissions64,66 (Table 43).
| Health state | Utility value (GOL model; IFX model) | Valuation method and source (GOL model; IFX model) |
|---|---|---|
| Response (pre-colectomy; induction) | 0.80; 0.79 | EQ-5D PURSUIT trial;47 ACT1 trial49 |
| Remission (pre-colectomy; induction) | 0.86; 0.84 | EQ-5D PURSUIT trial;47 ACT1 trial49 |
| No response (pre-colectomy; induction) | 0.70; 0.70 | EQ-5D PURSUIT trial;47 ACT1 trial49 |
| Response (pre-colectomy; maintenance) | 0.80; 0.82 | EQ-5D PURSUIT trial;47 ACT1 trial49 |
| Remission (pre-colectomy; maintenance) | 0.89; 0.88 | EQ-5D PURSUIT trial;47 ACT1 trial49 |
| Response (relapse management) | 0.76; 0.76 | EQ-5D PURSUIT trial;47 ACT1 trial49 |
| Relapse (relapse management) | 0.42; 0.42 | EQ-5D estimates from Tsai et al.;82 however, the primary source of these estimates (Woehl et al.109) appears to be misreferenced as the cited reference is not a health valuation study and does not report utilities |
| Colectomy | 0.56; 056a | Unclear. Appears to be based on data from Health Outcomes Data Analysis Repository reported within Punekar and Hawkins.120 Disutility for early complications based on TTO study reported by Arseneau et al.110 |
| Remission (postcolectomy) | 0.60; 0.60 | |
| Late complications (postcolectomy) | 0.60; 0.60 |
Resource use and costs
The model includes direct costs of drug acquisition, consultant visits, endoscopy, inpatient hospital admissions, colectomy, management of surgery-related complications, AEs and other UC costs. A Patient Access Scheme (PAS), in which the price of 100 mg of GOL is assumed to be equal to that of 50 mg of GOL, is applied within the model. It should be noted that at the time of writing, this had not been approved by the Department of Health.
Drug acquisition costs
Cost of non-biological ‘background’ therapies
The usage and per-cycle costs of non-biological background therapies assumed within the model are presented in Tables 44 and 45. Patients in the standard non-biological treatment group are assumed to receive 4 g of mesalazine daily (if acute) or 2 g of mesalazine daily (if chronic), 2–2.5 mg/kg of AZA daily, 1–1.5 mg/kg of 6-MP daily, 500 mg of ciprofloxacin twice daily and 25–50 mg of prednisolone per day. The same use of background therapies is assumed for all biological treatment arms. However, it should be noted that in the colectomy group, patients are assumed to undergo immediate colectomy, so the actual drug acquisition cost for the colectomy group is zero within the model. Patients in the biological treatment groups are assumed to receive the same ‘background therapies’ with the exception of ciprofloxacin (although as described above, this is applied as a zero cost in the colectomy group). Resource-use estimates for these therapies appear to be based on the PBO arm of the PURSUIT trial47 within the GOL model and from the PBO arm of the ACT1 and ACT2 trials49 within the IFX model. These are similar, but not the same (see Tables 44 and 45); as with the utility values, the justification for using different assumptions concerning resource use in each model is not clear. The source of the unit costs is not reported within the submission, but estimates appear to be drawn from the British National Formulary (BNF37).
| Treatment group | Background therapies included (proportion of patients) | Cost per cycle (£) |
|---|---|---|
| Induction treatment | ||
| Standard non-biological treatment | Mesalazine (0.83), AZA (0.15), 6-MP (0.15), ciprofloxacin (0.00), prednisolone (1.00) | 251.43a |
| ADA | Mesalazine (0.81), AZA (0.16), 6-MP (0.16), prednisolone (0.44) | 200.03 |
| GOL | Mesalazine (0.81), AZA (0.16), 6-MP (0.16), prednisolone (0.44) | 200.03 |
| IFX | Mesalazine (0.81), AZA (0.16), 6-MP (0.16), prednisolone (0.44) | 200.03 |
| Maintenance treatment | ||
| Standard non-biological treatment | Mesalazine (0.80), AZA (0.16), 6-MP (0.16), ciprofloxacin (0.00), prednisolone (0.49) | 121.15a |
| ADA | Mesalazine (0.80), AZA (0.15), 6-MP (0.15), prednisolone (0.51) | 120.98 |
| GOL | Mesalazine (0.80), AZA (0.15), 6-MP (0.15), prednisolone (0.51) | 120.98 |
| IFX | Mesalazine (0.80), AZA (0.15), 6-MP (0.15), prednisolone (0.51) | 120.98 |
| Relapse management (following prior treatment failure) | ||
| Relapse management | Mesalazine (0.80), AZA (0.16), 6-MP (0.16), ciprofloxacin (0.00), prednisolone (0.49) | 121.15 |
| Relapse management (i.v. steroids) | Mesalazine (0.83), AZA (0.15), 6-MP (0.15), ciprofloxacin (0.00), prednisolone (1.00), i.v. prednisolone (1.00) | 405.43 |
| Treatment group | Background therapies included (proportion of patients) | Cost per cycle (£) |
|---|---|---|
| Induction treatment | ||
| Standard non-biological treatment | Mesalazine (0.71), AZA (0.15), 6-MP (0.15), ciprofloxacin (0.00), prednisolone (1.00) | 233.57a |
| ADA | Mesalazine (0.72), AZA (0.36), 6-MP (0.13), prednisolone (0.54) | 191.11 |
| GOL | Mesalazine (0.72), AZA (0.36), 6-MP (0.13), prednisolone (0.54) | 191.11 |
| IFX | Mesalazine (0.72), AZA (0.36), 6-MP (0.13), prednisolone (0.54) | 191.11 |
| Maintenance treatment | ||
| Standard non-biological treatment | Mesalazine (0.71), AZA (0.15), 6-MP (0.15), ciprofloxacin (0.00), prednisolone (0.57) | 118.10a |
| ADA | Mesalazine (0.72), AZA (0.36), 6-MP (0.13), prednisolone (0.54) | 113.99 |
| GOL | Mesalazine (0.72), AZA (0.36), 6-MP (0.13), prednisolone (0.54) | 113.99 |
| IFX | Mesalazine (0.72), AZA (0.36), 6-MP (0.13), prednisolone (0.54) | 113.99 |
| Relapse management (following prior treatment failure) | ||
| Relapse management | Mesalazine (0.71), AZA (0.29), 6-MP (0.15), ciprofloxacin (0.00), prednisolone (0.57) | 118.10 |
| Relapse management (i.v. steroids) | Mesalazine (0.71), AZA (0.29), 6-MP (0.15), ciprofloxacin (0.00), prednisolone (1.00), i.v. prednisolone (1.00) | 387.57 |
Biological therapies
Table 46 shows the biological acquisition costs per cycle for each treatment group. The table indicates that the estimated costs of induction using IFX is markedly higher than that for ADA and GOL; however, the costs of maintenance therapy per cycle are broadly similar for all biologicals.
| Treatment group | Assumed regimen | Cost per cycle (£) |
|---|---|---|
| Induction treatment (8-week cycle) | ||
| ADA | All patients receive 1 × 160 mg of ADA + 1 × 80 mg of ADA + 3 × 40 mg of ADA | 3169.26 |
| GOL | All patients receive 2 × 100 mg of GOL + 1 × 100 mg of GOL | 3051.88 |
| IFX | All patients receive 12 × 100 mg of IFX over three administrations | 5497.44 |
| Maintenance treatment (2-month cycles) | ||
| ADA | 50% patients receive 40 mg of ADA EW (4.33 doses/cycle); 50% patients receive 40 mg of ADA EW (8.67 doses/cycle) | 2288.91 |
| GOL | 31.6% patients receive 100 mg of GOL every 4 weeks; 68.4% patients receive 50 mg of GOL every 4 weeks | 1653.10 |
| IFX | All patients receive 5 mg/kg of IFX every 8 weeks | 1985.19 |
Health state resource costs
Tables 47 and 48 present the health state costs (excluding drug acquisition) for the biological and colectomy groups respectively. The resource-use estimates underpinning these cost estimates were reported to be based on interviews with nine expert gastroenterologists. Resource use was costed using standard costing sources. 128,130
| State and treatment phase | Consultant visit cost | Endoscopy cost | Inpatient cost | Colectomy cost | Late complications cost | Other UC cost | AE cost | Total cost per cycle |
|---|---|---|---|---|---|---|---|---|
| Response; induction phase | 91.58 | 18.32 | 0.00 | 0.00 | 0.00 | 1.80 | 29.37 | 141.07 |
| Remission; induction phase | 43.70 | 4.97 | 0.00 | 0.00 | 0.00 | 1.80 | 29.37 | 79.84 |
| Failure; induction phase | 162.76 | 40.30 | 0.00 | 0.00 | 0.00 | 1.80 | 29.37 | 234.22 |
| Response (pre-colectomy; maintenance) | 91.58 | 18.32 | 0.00 | 0.00 | 0.00 | 1.80 | 29.37 | 141.07 |
| Remission (pre-colectomy; maintenance) | 43.70 | 4.97 | 0.00 | 0.00 | 0.00 | 1.80 | 29.37 | 79.84 |
| Response (relapse management) | 91.58 | 18.32 | 0.00 | 0.00 | 0.00 | 1.80 | 26.74 | 138.44 |
| Relapse (relapse management) | 162.76 | 40.30 | 350.86 | 0.00 | 0.00 | 3.44 | 26.74 | 584.09 |
| Colectomy | 162.76 | 40.30 | 0.00 | 8967.94 | 0.00 | 3.44 | 0.00 | 9174.42 |
| Remission (postcolectomy) | 53.12 | 45.27 | 0.00 | 0.00 | 0.00 | 0.92 | 0.00 | 99.30 |
| Late complications (postcolectomy) | 67.77 | 26.17 | 0.00 | 0.00 | 2446.85 | 1.85 | 0.00 | 2542.64 |
| State and treatment phase | Consultant visit cost | Endoscopy cost | Colectomy cost without early complications | Cost early complications of colectomy | Colectomy cost | Late complications cost | Other UC cost | Total cost per cycle |
|---|---|---|---|---|---|---|---|---|
| Death due to colectomy. Remission (postcolectomy) due to colectomy | 162.76 | 40.30 | 7619.25 | 4029.61 | 8967.94 | 0.00 | 3.44 | 20,823.28 |
| Remission (postcolectomy) due to colectomy | 162.76 | 40.30 | 7619.25 | 4029.61 | 8967.94 | 0.00 | 3.44 | 20,823.28 |
| Colectomy | 162.76 | 40.30 | 7619.25 | 4029.61 | 8967.94 | 0.00 | 3.44 | 20,823.28 |
| Remission (postcolectomy) | 53.12 | 45.27 | 0.00 | 0.00 | 0.00 | 0.00 | 0.92 | 99.30 |
| Late complications (postcolectomy) | 67.77 | 26.17 | 0.00 | 0.00 | 0.00 | 2446.85 | 1.85 | 2542.64 |
Model evaluation and uncertainty analysis
The results of the economic analysis are presented as pairwise ICERs and are interpreted as net monetary benefits (NMBs) assuming a willingness-to-pay threshold of £30,000 per QALY gained. Incremental CEACs are also presented within the submission (see MSD GOL submission,66 p. 122). Uncertainty surrounding estimates of incremental costs and health outcomes was examined using deterministic sensitivity analyses and PSA. The results of the deterministic analyses are presented as tornado diagrams and the results of the PSA are presented as cost-effectiveness planes and CEACs.
MSD model results
Tables 49 and 50 present the results within the GOL and IFX submissions respectively64,66 (note: the fully incremental analysis presented here has been undertaken by the Assessment Group rather than by the manufacturer).
| Treatment | QALYs | Costs (£) | Incremental QALYs | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| Probabilistic model results | |||||
| IFX | 5.70 | 44,382.28 | 0.16 | 13,003.60 | 80,318 |
| GOL | 5.54 | 31,378.68 | 0.56 | 15,610.91 | 27,994 |
| ADA | 5.49 | 32,096.50 | – | – | Dominated |
| Colectomy | 4.98 | 15,767.78 | – | – | – |
| Results based on point estimates of parameters | |||||
| IFX | 5.65 | 43,091.60 | 0.15 | 12,196.82 | 80,866 |
| GOL | 5.50 | 30,894.78 | 0.55 | 15,100.53 | 27,322 |
| ADA | 5.45 | 31,370.28 | – | – | Dominated |
| Colectomy | 4.95 | 15,794.26 | – | – | – |
| Treatment | QALYs | Costs (£) | Incremental QALYs | Incremental cost(£) | ICER (£) |
|---|---|---|---|---|---|
| Probabilistic model results | |||||
| IFX | 5.71 | 44,189.50 | 0.17 | 12,841.74 | 75,998 |
| GOL | 5.54 | 31,347.76 | 0.57 | 15,522.79 | 27,163 |
| ADA | 5.48 | 32,123.34 | – | – | Dominated |
| Colectomy | 4.97 | 15,824.96 | – | – | – |
| Results based on point estimates of parameters | |||||
| IFX | 5.66 | 42,919.73 | 0.16 | 12,166.45 | 77,599 |
| GOL | 5.51 | 30,753.28 | 0.56 | 14,963.69 | 26,569 |
| ADA | 5.45 | 31,237.38 | – | – | Dominated |
| Colectomy | 4.94 | 15,789.59 | – | – | – |
The model results suggest that IFX is expected to produce the greatest QALY gain, followed by GOL and ADA. ADA is expected to be less effective and more expensive than GOL, hence it is ruled out because of simple dominance. The ICER for GOL versus colectomy is expected to be approximately £27,000–28,000 per QALY gained. The ICER for IFX versus GOL is expected to be approximately £76,000–80,000 per QALY gained. The probabilistic results are slightly different from those derived from point estimates of parameters, but the ICERs appear stable.
Figure 81 presents the results of the PSA in the form of a cost-effectiveness plane for each biological treatment relative to immediate colectomy. It can be seen that the results overlap considerably for ADA and GOL; however, the plane indicates a generally higher overall cost for IFX. The dispersion of sampled incremental QALY gains for IFX versus colectomy is greater than that for ADA and GOL versus colectomy.
Figure 82 presents incremental CEACs for all options in the model. The CEACs suggest that at willingness-to-pay thresholds of £25,000 per QALY gained or lower, immediate colectomy has the highest probability of producing the greatest net benefit. At a willingness-to-pay threshold of £30,000 per QALY gained, GOL has the highest probability of producing the greatest net benefit, although this is only very slightly higher than 0.50.
A number of deterministic sensitivity analyses are also presented; however, these are difficult to interpret as both IFX and GOL are compared in a pairwise manner against colectomy using incremental QALYs, incremental costs and incremental NMB. Deterministic sensitivity analyses are not presented between competing biological therapies. The deterministic sensitivity analyses indicate that the cost-effectiveness results for GOL versus colectomy are sensitive to the utility values for post-colectomy remission,66 while the cost-effectiveness results for IFX versus colectomy are sensitive to the utility value for post-colectomy remission. 64
Critical appraisal of the MSD model
The main issues identified by the Assessment Group are presented in Box 2.
-
Deviations from the NICE reference case and final NICE scope, particularly with respect to omission of conventional non-surgical management as a comparator.
-
Assumption that treatment failure is equivalent to severe exacerbation.
-
Questionable use of evidence concerning improving and worsening of inflammation.
-
Questionable validity of use of novel methods for including non-randomised data from PURSUIT.
-
Lack of clarity regarding the NMA model.
-
Inconsistencies between results of the MSD GOL and IFX models.
-
Lack of clarity regarding the identification, selection and use of certain model parameters.
-
Complex implementation of the model.
-
Failure to undertake an incremental analysis.
-
Inclusion of a PAS for GOL which has not yet been agreed by the Department of Health.
Deviations from NICE reference case and final NICE scope
The extent to which the economic analyses reported in the MSD submissions adhere to the NICE reference case is presented in Table 51.
| Element of HTA | Reference case | Assessment Group comments |
|---|---|---|
| Defining the decision problem | The scope developed by the Institute | The scope of the analysis deviates from the final scope from NICE. Non-biological treatment is assumed to be immediate colectomy. Standard clinical management, defined in the NICE scope as ‘a combination of aminosalicylates (sulfasalazine, mesalazine, balsalazide or olsalazine), corticosteroids (beclomethasone, budesonide, hydrocortisone or prednisolone), and thiopurines (mercaptopurine or AZA), calcineurin inhibitors and surgical intervention’,36 is not included as a treatment option within the model |
| Comparator(s) | As listed in the scope developed by NICE | |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, carers | Health outcomes reflect those of patients with UC |
| Perspective on costs | NHS and PSS | The economic analysis was undertaken from the perspective of the UK NHS. PSS costs are not mentioned in the submission |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | The economic evaluation takes the form of a cost-effectiveness analysis. Analyses are presented as pairwise comparisons rather than a fully incremental economic analysis of all options |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Costs and outcomes are evaluated over a 10-year time horizon. Analyses over a lifetime horizon are not presented in the manufacturers’ submission |
| Synthesis of evidence on health effects | Based on systematic review | Outcomes are synthesised using NMA models using studies identified through a systematic review |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Health outcomes are reported in terms of life-years gained and QALYs gained |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | All utilities except the disutility for surgery-related complications are based on EQ-5D measurements from UC patients and are valued by the general public |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | No equity weighting is applied |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | The economic analysis was undertaken from the perspective of the UK NHS. The sources for prices are not entirely clear |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | The model uses a discount rate of 3.5% for costs and health outcomes |
Overall, the MSD economic analyses are generally in line with the NICE reference case. However, the analysis does make one important deviation from the final NICE scope with respect to the options included in the economic analysis; non-biological treatment is assumed to be immediate colectomy. Standard clinical management, which is defined in the NICE scope as ‘a combination of aminosalicylates (sulfasalazine, mesalazine, balsalazide or olsalazine), corticosteroids (beclomethasone, budesonide, hydrocortisone or prednisolone), and thiopurines (mercaptopurine or AZA), calcineurin inhibitors and surgical intervention’,36 is not included in the MSD model. The omission of non-biological non-surgical treatment options from the MSD model is neither discussed nor justified in the MSD submissions. 64,66
It is also noteworthy that the MSD model adopts a 10-year time horizon; at this point around 96% of patients in the model are still alive in each treatment group. The MSD submissions state that the ‘. . . time horizon of 10 years can be considered sufficiently long to capture differences in the distribution of health states between the compared biologics; after 10 years of follow-up all patients are expected to have discontinued biologic treatment’. The modelled profiles of incremental costs and benefits are slightly different when a longer time horizon is adopted. It is reasonable to suggest that the manufacturer should have examined the impact of using different time horizons within their economic analysis.
Assumption that treatment failure is equivalent to severe exacerbation
Related to the issue regarding the lack of conventional drug therapies as the comparator for the economic analysis (see Deviations from NICE reference case and final NICE scope), the MSD economic models appear to also confuse the severity of the patient populations and the associated treatment pathway included in the model. Although the scope of the appraisal relates to patients with moderate to severe UC who have failed conventional treatment, the modelled pathway after failure of biological therapy, and the choice of non-biological comparators included in the analysis, appear to relate to a population with more severe disease and no further medical treatment options are considered. The pathway represented by the model after failure of biological therapy (or in its absence) appears to assume that failure to achieve an induction response, or that the loss of response during maintenance therapy, is synonymous with an acute UC exacerbation. As noted by the MSD submissions, surgery for UC is typically indicated for (1) patients with life-threatening complications (e.g. toxic megacolon or colonic perforation); (2) dysplasia or proven cancer; or (3) severe disease characterised by treatment refractoriness, frequent flare-ups, extracolonic manifestations, chronic corticosteroid dependence, side effects/intolerance/complications from medications (in particular corticosteroids), or according to clinical judgement. 64,66 After failing biological treatment, the MSD model assumes that all patients who have failed biological therapy will receive i.v. steroids and rapidly progress to colectomy (27% of all relapsing patients during each 2-month cycle). This fails to reflect the possibility that patients may continue to receive, and may still obtain clinical benefit from, non-biological medical treatment options as defined in the NICE scope (5-ASAs, immunotherapies and/or steroids).
After removing mortality, the MSD model suggests that within 1 year (the approximate duration of the maintenance trials45,47,49), 15–20% patients are in the colectomy/post-colectomy health states (Figure 83).
FIGURE 83.
Proportion of patients in post-colectomy states over time (excluding mortality).

This contrasts with the colectomy rates observed within the RCTs included in the systematic review [see Chapter 3, Assessment of effectiveness, Rates of surgical intervention (both elective and emergency) 0.7% to 5.8% in in individual trial arms at approximately 1 year]. The manufacturer’s model also suggests that for patients receiving biological treatments, 59–70% will have undergone surgery within 5 years and 89–93% will have undergone surgery within 10 years (note: the precise values differ by biological treatment group). These rates are very high and fail to reflect both the possibility of benefit from further medical therapies and the element of patient choice in deciding whether or not to undergo colectomy. If surgery really was the only remaining treatment option for these patients, it would not have been possible (or ethical) to undertake any of the trials included in this assessment.
Further to this point, the study reported by Turner et al. ,126 which is used to inform the probability of requiring surgery for active UC, is a systematic review of studies describing the short-term outcome of adult and paediatric patients treated with i.v. corticosteroids, with or without ciclosporin, for exacerbations of UC. Within this published analysis, retrospective and prospective studies evaluating adult or paediatric UC patients admitted for first or subsequent exacerbation, who were severe enough to require i.v. corticosteroid therapy, were included if the short-term outcome and/or analysis of predictors of response were reported. This appears to confuse treatment failure with acute exacerbation of UC. The Assessment Group do not believe that either the narrow choice of remaining viable comparators or the treatment pathways assumed within the MSD models are representative of the clinical management of patients with moderate to severe UC in England and Wales.
Questionable use of evidence concerning improving and worsening of inflammation
The NMA model uses separate models to produce information on the probability of sustained remission and the probability of sustained response. Within the health economic models, the ORs estimated using the NMA models are applied to the probability of remaining in the states of remission and response, respectively. The NMA logistic regression models treat these data as binomial – a patient either stays in their existing state or they do not. However, the data are multinomial – the observed data from the trials indicates that some patients who lost remission transited to response while others transited to no response, and some patients in response subsequently achieved remission, some achieved sustained response and some lost response. The structural assumptions employed within the transition matrix (see Table 42) do not reflect this, with some plausible transitions being assigned probabilities of zero. Although this problem is a likely consequence of the limitations of the published data from the ULTRA2 trial,45 it poorly reflects the characteristics of the actual observed data.
Questionable validity of use of methods for including non-randomised data from PURSUIT
The MSD submissions state that ‘PURSUIT used a non-conventional trial design, and thus, conventional NMA techniques would not have sufficed for producing comparative effect estimates between GOL, IFX, and ADA. This NMA employed novel techniques of optimising the use of all available data.’64,66 This approach was used to ‘downgrade’ the available evidence for PBO within the PURSUIT maintenance trial as patients randomised to PBO were prior GOL induction responders. Based on the information provided in the manufacturers’ submission (see MSD GOL submission,66 table 13 footnotes and text on pp. 55–7), the Assessment Group was unable to logically follow or replicate the calculations used to generate hypothetical values for the PBO group. However, the Assessment Group does believe that the manufacturer’s ‘novel’ method involves omitting the randomised data and instead uses a manipulation of the non-randomised PBO arm data as an input into the NMA. Such manipulation of observed trial data should be viewed with considerable caution. The Assessment Group believe that it would have been more appropriate to use more established methods of bias adjustment (e.g. the methods adopted by Turner and Spiegelhalter131) and/or to use the published ITT data and examine the likely impact of the bias using sensitivity analyses.
Lack of clarity regarding the network meta-analysis model
The NMA model is not reported in detail within either of the MSD submissions and the WinBUGS code was not reported (although this was provided to the Assessment Group during the clarification process). In addition, the baseline model is not described, although the health economic model indicates that baseline probabilities of achieving induction response/remission and maintaining response/remission were derived from ‘Average study effect of PBO controlled trials in random effects NMA of induction response’. The appropriateness of these values is unclear.
Inconsistencies between results of the Merck Sharp & Dohme Ltd golimumab and infliximab models
The IFX model and GOL model are based on the same structure and the same decision problem. However the results are different between the models. In response to a request for clarification on the cause of this discrepancy, the manufacturer stated that the two models use different inputs for health utilities; the IFX model uses utility data from the ACT1 and ACT2 trials while the GOL model uses utility data from the PURSUIT trial. The IFX model also uses different assumptions about the use of conventional non-biological therapies compared with the GOL model. The justification for using different utility and resource-use assumptions in two models that are attempting to reflect exactly the same decision problem is inappropriate. It should also be noted that when the PURSUIT utility vector and resource-use assumptions were inserted into the IFX model, the results still did not coincide.
Lack of clarity regarding the identification, selection and use of certain model parameters
In several instances, the justification for selecting particular parameter sources is unclear. In particular, the justification of the dosing and frequency of background therapies, and the justification for unit costs is not described within the MSD submissions. 64,66
Complex implementation of the model
Conceptually, the submitted MSD models are simple Markov models employing eleven health states and four treatment groups. However, the implementation of these models is complex. The model employs 30 worksheets, many of which were locked as read-only. This limited the ability of the Assessment Group to verify the inputs and formulae used in the model.
Failure to undertake an incremental analysis
The MSD submissions do not include an incremental analysis in which each treatment option is compared against its next best non-dominated alternative. Instead, pairwise comparisons are made using NMB given a willingness-to-pay threshold of £30,000 per QALY gained. The IFX submission states that:
The ICER for infliximab versus standard non-biologic treatment (colectomy) is £37,682. The positive impact of infliximab in terms of reducing the Burden of Illness and mitigating the Wider Societal Impact of the condition represents additional value for consideration by the committee. Taking into account the shortfall in quality of life, and in the ability of people to contribute to society as a result of their experience with moderately to severely active UC, it is likely that infliximab represents a cost-effective treatment in first-line biologic treatment of UC.
MSD. Manufacturer submission of evidence: IFX (Remicade)64
The GOL submission states that ‘At £27,322, the ICER for golimumab falls under a £30,000 threshold, and thus golimumab can be considered a cost-effective treatment option for patients with moderately to severely active UC’. 66
Importantly, both of these economic conclusions are based on a comparison of biological therapy versus immediate colectomy. A fully incremental analysis is presented in Tables 49 and 50. Given the ordering of QALY gains across all treatment options, IFX should be compared against GOL, thus resulting in a considerably higher ICER of approximately £75,000–80,000 per QALY gained (note: the discussion around the discrepancy between model results, see Inconsistencies between results of the Merck Sharp & Dohme Ltd golimumab and infliximab models).
Inclusion of a Patient Access Scheme for golimumab which has not yet been agreed by the Department of Health
Both MSD submissions include a PAS in which 100 mg of GOL will be made available at the same price as 50 mg of GOL (see MSD GOL submission,66 p. 8). However, at the time of this assessment, the proposed PAS had not been agreed with the Department of Health. Although the MSD submissions include a secondary analysis in which the PAS is not included, the absence of fully incremental comparisons by the manufacturer (see Failure to undertake an incremental analysis) clouds the correct interpretation of the economic analysis. The amended results of this fully incremental analysis, which excludes the PAS, are shown in Tables 52 and 53.
| Treatment | QALYs | Costs (£) | Incremental QALYs | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| Probabilistic model results | |||||
| IFX | 5.67 | 44,122.45 | 0.21 | 11,911.17 | 56,268 |
| GOL | 5.50 | 37,306.74 | – | – | Extendedly dominated |
| ADA | 5.45 | 32,211.28 | 0.53 | 16,409.68 | 30,724 |
| Colectomy | 4.92 | 15,801.60 | – | – | – |
| Results based on point estimates of parameters | |||||
| IFX | 5.65 | 43,091.60 | 0.20 | 11,721.32 | 57,980 |
| GOL | 5.50 | 36,805.33 | – | – | Extendedly dominated |
| ADA | 5.45 | 31,370.28 | 0.50 | 15,576.02 | 31,069 |
| Colectomy | 4.95 | 15,794.26 | – | – | – |
| Treatment | QALYs | Costs (£) | Incremental QALYs | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| Probabilistic model results | |||||
| IFX | 5.68 | 44,126.44 | 0.22 | 11,920.92 | 53,258 |
| GOL | 5.51 | 37,198.73 | – | – | Extendedly dominated |
| ADA | 5.46 | 32,205.53 | 0.54 | 16,445.78 | 30,428 |
| Colectomy | 4.92 | 15,759.75 | – | – | – |
| Results based on point estimates of parameters | |||||
| IFX | 5.66 | 42,919.73 | 0.21 | 11,682.36 | 55,5077 |
| GOL | 5.51 | 36,663.51 | – | – | Extendedly dominated |
| ADA | 5.45 | 31,237.38 | 0.51 | 15,447.78 | 30,319 |
| Colectomy | 4.94 | 15,789.59 | – | – | – |
The exclusion of the PAS discount for 100 mg of GOL results in a situation whereby ADA is no longer dominated and GOL is ruled out because of extended dominance. Based on this version of the model, the ICER for ADA versus colectomy is approximately £30,000 per QALY gained. The ICER for IFX versus ADA is at best £53,258 per QALY gained.
Adalimumab, golimumab and infliximab, for the treatment of ulcerative colitis (subacute): AbbVie submission62
The AbbVie submission details the methods and results of a de novo health economic model developed to assess the cost-effectiveness of ADA versus ‘standard of care’ (conventional non-biological therapies) for the treatment of moderate to severe UC.
AbbVie model scope
The AbbVie model includes a comparison of two options: (1) ADA and (2) ‘standard of care’ (standard non-biological therapies) for the treatment of moderate to severe UC from the perspective of the UK NHS. The intervention arm (ADA plus standard non-biological therapies) begins with an induction dose of 160 mg of ADA at week 0, followed by 80 mg of ADA at week 2, and a maintenance dose of 40 mg of ADA every other week starting from week 4. At week 8, those patients who achieve remission or response continue to receive ADA, and those patients who have lost response to the initial treatment can dose-escalate to 40 mg of ADA every week. At week 104, patients in the moderate to severe health state are assumed to discontinue ADA and subsequently receive conventional non-biological treatment only. The comparator group within the model comprises conventional non-biological drug treatments (anti-inflammatory drugs or immunosuppressants). Patients without response or remission in either treatment group can progress to colectomy at any time. Surgery is assumed to be reserved for patients who have failed both biological and non-biological drug treatments but is not evaluated as a treatment comparator in the model. Other biological agents used for the treatment of UC (GOL and IFX) are not included in the AbbVie economic analysis.
The economic evaluation takes the form of a cost–utility analysis whereby the primary health economic outcome is the incremental cost per QALY gained over a 10-year time horizon. The base-case population considered relates to patients with moderate to severe UC who are have not previously been exposed to anti-TNF-α therapy and those who have previously been exposed to anti-TNF-α therapy (excluding ADA). Patients who are naive to anti-TNF-α agents were evaluated as a secondary sensitivity analysis. Patients are assumed to have a mean body mass of 75 kg and the starting age of patients entering the model is unclear in both the submission and the model. Costs and health outcomes are discounted at 3.5%. Costs were valued at 2013 prices.
AbbVie model structure
The model adopts a Markov approach using a 2-week cycle length (Figure 84). The model includes a total of 11 health states: three pre-surgery states for ADA, three pre-surgery states for conventional treatments, one surgery state and four post-surgery states. These states are: (1) mild (ADA); (2) remission (ADA); (3) moderate to severe (ADA); (4) mild (conventional treatment); (5) remission (conventional treatment); (6) moderate to severe (conventional treatment); (7) surgery; (8) post surgery without complications; (9) transient complications; (10) chronic complications; and (11) surgery-related death.
The three pre-surgery health states (remission, mild, and moderate to severe disease states) were defined using the Mayo scoring system (or partial Mayo scores if full Mayo scores were not available).
The model comprises two treatment phases: (1) an induction phase; and (2) a maintenance phase. The induction phase relates to the first 8 weeks of treatment, in line with recommendations from the EMA. 132 For the ADA group, patients who are in the remission or mild disease states at this time point are assumed to continue to receive ADA into the maintenance period (8–52 weeks). At the end of week 8, patients in the moderate to severe disease state are assumed to be non-responders to ADA; these patients discontinue treatment with ADA and subsequently receive conventional non-biological therapy. Between week 8 and week 104, patients who have previously achieved remission or response but subsequently lost that response or remission are assumed to either discontinue ADA treatment or to dose escalate to 40 mg of ADA every week. Within the conventional management group, patients transit between the conventional management health states without entering the biological states.
For both the ADA and the conventional management groups, only patients in the moderate to severe health state are allowed to transit to surgery. Surgery is treated as a tunnel state, whereby patients can remain in that state for one cycle only. Patients can transit between the ‘transient complication’ state and ‘post-surgery without complication’ state during any cycle. Patients experiencing chronic complications are assumed to remain in that state until the time horizon has been exhausted. Patients undergoing surgery are assumed to be at an increased risk of death. Other-cause mortality is not included in the model. All patients enter the model in the pre-surgery moderate to severe state, in line with the inclusion criteria for the ULTRA2 trial. 45 A half-cycle correction was applied to costs and QALYs. The main driver of health benefits within the model relates to HRQoL benefits associated with increased sojourn time in the pre-surgical health states.
Serious and severe AEs were not considered in the AbbVie model; the manufacturers’ submission notes that most AEs experienced by patients in the ULTRA2 trial45 were non-serious and considered to be unrelated to the study drugs. 62 In addition, the manufacturer highlights that the ULTRA2 trial reported slightly higher incidences of serious and severe AEs in the PBO arm than in the ADA arm of the trial; therefore, considering serious and severe AEs in the model would have increased medical costs and reduced health gains within the conventional management group. 62 Therefore, the exclusion of these events represents a conservative assumption.
The model includes the costs associated with drug acquisition, medical costs related to disease states, hospitalisation, surgery, surgery-related complications and costs associated with surgery-related death.
The model uses simple matrix multiplication to determine health state populations during each model cycle based on the state population in the previous Markov cycle and a series of time-dependent transition matrices. Costs and utilities are attached to each health state. Total QALYs are modelled as a function of sojourn time in each health state, together with an indirect survival benefit for ADA as a consequence of reduced rates of surgery (and hence surgical-related mortality) for this group.
Evidence used to inform the model parameters
A summary of evidence sources used to inform the main groups of parameters within the model is presented in Table 54.
| Parameter group | Source |
|---|---|
| Transition probabilities: pre-surgical states | ULTRA2 trial45,126 and ULTRA1/2 extension study62 and other literature121,133 with the cycle length of matrix probabilities adjusted using Eigenvalue decomposition |
| Transition probabilities: rate of surgery | Hillson et al.134 |
| Transition probabilities: post-surgery complications and surgery related-mortality | Transition complications rates estimated from Swenson et al.115 Chronic complication rates estimated using studies by Johnson et al.135 (fertility), Kruasz andDuek136 (male impotence) and Abdelrazeq et al.137 (chronic pouchitis). Perioperative and post-operative mortality risks were estimated using a study reported by Roberts et al.138 |
| Health utilities for pre-colectomy response/remission | EQ-5D study published as a poster by Swinburn et al.139 |
| Health utilities for post-colectomy states | Utility values for postsurgery without complication and transient complication based on estimates reported by Tsai et al.82 Utility values for chronic complications based on Arseneau et al.,110 Hu et al.140 and Smith and Roberts141 |
| Resource use | ADA dosing and dose escalation based on SmPC and experience within the ULTRA2 trial.45,126 Use of conventional non-biological treatments was based on UC-related medication usage rates for all subjects at baseline, as observed in the ULTRA 2trial.45,126 Disease state resource use was based on the estimates reported by Tsai et al.82 Rates of hospitalisation were based on a mixed effects regression analysis of ULTRA1 and ULTRA2 trial data62 |
| Unit costs | Drug acquisition costs (biologicals and conventional treatments) were taken from the MIMS.142 Hospitalisation costs were based on NHS Reference Costs130 Other unit costs derived from literature82,115,143 |
Methods for modelling effectiveness
For the most part, estimates of baseline and relative effectiveness were taken from the ULTRA2 study and the ULTRA1/2 extension study,45,62,126 although other literature was used to inform transitions that were not observed within these studies. 121,133 Efficacy data on response/remission from the ULTRA1 trial were not used in the AbbVie model. Transition probabilities between pre-surgery health states were calculated using trial data from ULTRA245,126 for weeks 8–104 while transitions between states for cycles from week 104 to 520 were based on data from the ULTRA1/2 extension study62 for ADA and ULTRA245,126 for conventional management. Discontinuations owing to other reasons, such as AEs, were also considered based on trial data.
Four matrices of time-dependent transition probabilities are used within the AbbVie model, according to four time intervals; these are described below.
Transition probabilities: adalimumab group
Period 1 (weeks 0–8)
In the induction period, transitions from the moderate to severe state were based on the ADA arm of the ULTRA2 trial. 45,126 As ULTRA2 did not recruit patients with prior response or remission (because it was an induction trial), this study cannot provide information relating to transitions from these states to other states within the first 8-week period. Instead, the probabilities of maintaining remission and response were based on studies reported by Kane et al. 133 (assuming the probability of maintaining remission reflects that of ‘adherent patients’) and Odes et al. 121 A constant hazard was assumed to obtain the 8-week probability in both cases.
Period 2 (weeks 8–52)
Transition probabilities were based on a cross-tabulation of data on the number of patients in each health state from the ADA arm of the ULTRA2 trial. 45,126
Period 3 (weeks 52–104)
Data from the ULTRA1 and ULTRA2 extension study62 were used to derive transition probabilities for the three pre-surgery health states. As patients in the moderate to severe state within the ADA group of the model are assumed to discontinue biological treatment, only those patients who were randomised to the ADA arm and who had remission or mild disease at week 8 in the ULTRA1/2 extension study62 were included in the analysis.
Period 4 (weeks 104–260)
Data from week 48 to week 144 of the ULTRA1/2 extension study62 were used to generate the transition matrix. A multinomial logit regression model was constructed to estimate the transition matrix during each 48-week interval. The dependent variables were the three pre-surgery health states and the independent variables were the health states in the previous visit. The logit model estimates mean predicted probabilities of being in one of the health states given a specific health state at the previous visit.
These four transition matrices were then converted to 2-week probabilities using Eigenvalue matrix decomposition methods reported by Craig and Sendi. 144 The resulting matrices are shown in Table 55.
| From state | To state | |||
|---|---|---|---|---|
| Remission | Mild | Moderate to severe | Surgery | |
| From week 0–8 | ||||
| Remission | 0.9974 | 0.0007 | 0.0019 | – |
| Mild | 0.0003 | 0.9981 | 0.0016 | – |
| Moderate to severe | 0.0551 | 0.0986 | 0.8432 | 0.0031 |
| From week 8–52 | ||||
| Remission | 0.9700 | 0.0164 | 0.0136 | – |
| Mild | 0.0349 | 0.9400 | 0.0251 | – |
| Moderate to severe | 0.0001 | 0.0215 | 0.9753 | 0.0031 |
| From week 52–104 | ||||
| Remission | 0.9889 | 0.0000 | 0.0111 | – |
| Mild | 0.0178 | 0.9436 | 0.0385 | – |
| Moderate to severe | 0.0275 | 0.0217 | 0.9477 | 0.0031 |
| Week 104 onward | ||||
| Remission | 0.9949 | 0.0047 | 0.0004a | – |
| Mild | 0.0113 | 0.9869 | 0.0018a | – |
| Moderate to severe | 0.0037 | 0.0019 | 0.9463a | 0.0031 |
Transition probabilities: conventional management group
Period 1 (weeks 0–8)
In the induction period, transitions from the moderate to severe state were based on the PBO group outcomes within the ULTRA2 trial. 45 As with the ADA matrix for the induction period, estimates of maintaining remission and response were based on studies reported by Kane et al. 133 (assuming the probability of maintaining remission reflects that of ‘non-adherent patients’) and Odes et al. 121 A constant hazard was assumed to obtain the 8-week probability in both cases.
Period 2 (weeks 8–52)
Transition probabilities were based on a cross-tabulation of data on the number of patients in each health state from the PBO arm of the ULTRA2 trial. 45,126
Periods 3 and 4 (weeks 52–260)
Transition probabilities for each cycle were assumed to reflect those estimated for period 2 (weeks 8–52).
As with the ADA group, these four transition matrices were then converted to 2-week probabilities using Eigen matrix decomposition methods reported by Craig and Sendi. 144 The resulting matrices are shown in Table 56.
| From state | To state | |||
|---|---|---|---|---|
| Remission | Mild | Moderate to severe | Surgery | |
| From week 0–8 | ||||
| Remission | 0.9799 | 0.0044 | 0.0157 | – |
| Mild | 0.0013 | 0.9844 | 0.0143 | – |
| Moderate to severe | 0.0291 | 0.0882 | 0.8796 | 0.0031 |
| From week 8–52 (and subsequent cycles) | ||||
| Remission | 0.9696 | 0.0028 | 0.0276 | – |
| Mild | 0.0170 | 0.9217 | 0.0613 | – |
| Moderate to severe | 0.0017 | 0.0074 | 0.9878 | 0.0031 |
Transitions between surgery and post-surgical states
Transitions between surgery and post-surgical health states were based on the literature rather than the clinical studies of ADA. Complication rates were estimated from a study reported by Swenson et al. 115 Chronic complication rates were estimated using studies by Johnson et al. 135 (fertility), Kruasz and Duek136 (male impotence) and Abdelrazeq et al. 137 (chronic pouchitis). Perioperative and post-operative mortality risks were estimated using a study reported by Roberts et al. 138 taking account of background mortality rates. The underlying transition rates are assumed to be time independent. The resulting matrix is shown in Table 57.
| From state | To state | ||||
|---|---|---|---|---|---|
| Surgery | Post-surgery without complication | Transient complication | Chronic complication | Death | |
| Surgery | 0.0000 | 0.7708 | 0.0101 | 0.1919 | 0.0272 |
| Post-surgery without complication | 0.0000 | 0.9893 | 0.0101 | 0.0000 | 0.0006 |
| Transient complication | 0.0000 | 0.9994 | 0.0000 | 0.0000 | 0.0006 |
| Chronic complication | 0.0000 | 0.0000 | 0.0000 | 0.9994 | 0.0006 |
| Death | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 1.0000 |
Health-related quality of life
Table 58 reports the HRQoL values used in the AbbVie model and their sources.
| Disease State | Utility | Source |
|---|---|---|
| Remission | 0.91 | Swinburn et al.139 |
| Mild | 0.80 | Swinburn et al.139 |
| Moderate to severe | 0.55 | Swinburn et al.139 |
| Surgery | 0.55 | Assumed to same as moderate to severe state |
| Post-surgery without complication | 0.61 | Tsai et al.82 |
| Transient complication | 0.55 | Tsai et al.82 |
| Chronic complication | 0.43 | Weighted mean of Arseneau et al.,110 Hu et al.140 and Smith and Roberts141 |
The AbbVie submission argues that although it would have been possible to map SF-6D utility estimates from the ULTRA2 trial onto the EQ-5D, this is likely to overestimate the level of HRQoL of patients with more severe disease. Health utilities for the pre-surgery states were instead sourced from an EQ-5D study of 230 patients with UC reported by Swinburn et al. 139 This study has been published in abstract form only; however, further details are provided in appendix 3 of the AbbVie submission. 62 Utility values for the states of post surgery without complications and post surgery with transient complications were taken from Tsai et al. 82 The utility for the chronic complication state was estimated by using a weighted value of rates and HRQoL impacts of chronic pouchitis (Arseneau et al. 110), infertility (Hu et al. 140 and male sexual dysfunction (Smith and Roberts141).
Resource use and costs
Table 59 summarises the values of the resource use and cost parameters used in the AbbVie model.
| Parametersa | Parameter values | Sources |
|---|---|---|
| ADA dose escalation: increased dose intensity compared with 40 mg EOW | ||
| Week 8–52 maintenance phase | 7.40% | Primary analysis of ULTRA245 and ULTRA1 and ULTRA2 extension study62 |
| Week 52–104 maintenance phase | 24.06% | |
| Beyond week 104 maintenance phase | 21.49% | |
| Use of conventional therapies | ||
| Mesalazine | 47.0% | Based on baseline usage in ULTRA245,126 |
| Sulfasalazine | 7.3% | |
| Balsalazide | 5.9% | |
| Olsalazine | 0.2% | |
| AZA | 28.3% | |
| Mercaptopurine | 6.7% | |
| Drug acquisition costs | ||
| ADA unit price (40 mg) | £352.14 | MIMS (March 2014) |
| Mesalazine | £20.59 | MIMS (March 2014) |
| Sulfasalazine | £2.93 | |
| Balsalazide | £13.10 | |
| Olsalazine | £9.88 | |
| AZA | £2.89 | |
| Mercaptopurine | £105.99 | |
| Total weighted conventional therapy cost per 2-week cycle | £18.60 | |
Drug acquisition costs (adalimumab and conventional management)
Usage of ADA was based on its licensed indication132 together with estimates of relative dose intensity for dose escalating patients based on the primary analysis of data from the ULTRA2 trial45 and the ULTRA1/2 extension study. 62 The use of conventional non-biological therapies was assumed to reflect the baseline usage of these therapies within the ULTRA2 trial. 45,126 The drug acquisition costs for ADA and conventional non-biological therapies were obtained from the Monthly Index of Medical Specialities (MIMS) database. 142
Health state resource costs
Other UC health state costs assumed in the AbbVie model are summarised in Table 60.
| Parameters | Values | Sources |
|---|---|---|
| Hospitalisations per 2-week cycle | ||
| Remission: ADA | 0.0008 | Mixed effects regression analysis of ULTRA1 and ULTRA2 trial data44,126 |
| Mild: ADA | 0.0013 | |
| Moderate to severe: ADA | 0.0042 | |
| Remission: conventional management | 0.0017 | |
| Mild: conventional management | 0.0029 | |
| Moderate to severe: conventional management | 0.0094 | |
| Hospitalisation costs | ||
| Cost per hospitalisation | £3533 | NHS Reference Costs 2012/13130 (Major Gastrointestinal Disorders with CC score 0, elective inpatient, PA25B) |
| Pre-surgery disease state costs (excluding hospitalisation) per 2-week cycle | ||
| Remission | £20.31 | Derived using Tsai et al.82 Includes blood tests, consultation visits, and endoscopies |
| Mild | £67.87 | |
| Moderate to severe | £203.27 | |
| Post-surgery disease state costs per 2-week cycle | ||
| Surgery | £13,071 | Based on Buchanan et al.143 and inflated to 2013 prices |
| Postsurgery without complication | £118.63 | Derived using Tsai et al.82 including blood tests, consultation visits, and endoscopies |
| No hospitalisations were considered for post-surgery without complication | ||
| Transient complication | £8826.05 | Based on Swenson et al.,115 inflated and exchange-rate adjusted to 2013 prices |
| Chronic complication | £118.63 | Assumed to be the same as postsurgery without complication |
| Terminal care | £3533 | NHS Reference Costs 2012/13130 (Major Gastrointestinal Disorders with CC score 0, elective inpatient, PA25B) |
The frequency of hospitalisations per 2-week cycle was estimated according to treatment arm and disease severity using mixed effects regression on pooled data from the ULTRA1 and ULTRA2 trials. 44,126 Other disease state resource use (consultant visits, blood tests and emergency/elective endoscopies) were taken from Tsai et al. 82 and uplifted to current prices. 128 Hospitalisation and post-surgery terminal care costs were obtained from NHS Reference Costs 2012/13. 130 The costs of surgery and managing complications were taken from Buchanan et al. 143 and Swenson et al. 115
Model evaluation and uncertainty analysis
The results of the AbbVie economic analysis are presented as an ICER; this is based on the point estimates of parameters rather than the expectation of the mean. Uncertainty surrounding incremental costs and outcomes was examined using deterministic sensitivity analyses and PSA. The PSA was undertaken over 1000 Monte Carlo samples. The results of the deterministic analyses are presented as tornado diagrams while the results of the PSA are presented as cost-effectiveness planes and CEACs.
AbbVie model results
Tables 61 and 62 present the results of the AbbVie model for the base-case analysis and the secondary analysis of the subgroup of patients who are anti-TNF-α naive. Note that the probabilistic ICERs presented in these tables have been generated by the Assessment Group.
| Treatment | QALYs | Costs | Incremental QALYs | Incremental costs | ICER |
|---|---|---|---|---|---|
| Probabilistic model resultsa | |||||
| ADA | Treatment-specific costs and QALYs not stored in PSA subroutine | 0.73 | £25,335 | £34,590 | |
| Conventional management | – | – | |||
| Results based on point estimates of parameters | |||||
| ADA | 5.73 | £76,392 | 0.74 | £25,446 | £34,417 |
| Conventional management | 4.99 | £50,946 | – | – | |
| Treatment | QALYs | Costs | Incremental QALYs | Incremental costs | ICER |
|---|---|---|---|---|---|
| Probabilistic model resultsa | |||||
| ADA | Subgroup model does not allow for PSA | ||||
| Conventional management | |||||
| Results based on point estimates of parameters | |||||
| ADA | 6.00 | £79,799 | 0.87 | £31,140 | £35,970 |
| Conventional management | 5.140 | £48,659 | – | – | |
The base-case analysis of the model indicates that over a 10-year time horizon, ADA is expected to generate an additional 0.73 QALYs at an incremental cost of £25,335 per patient. This leads to an ICER of £34,590 per QALY gained. The results of the model based on the point estimates of parameters are very similar to those produced using the probabilistic model.
The deterministic subgroup analysis of anti-TNF-α-naive patients indicates that over a 10-year time horizon, ADA is expected to generate an additional 0.87 QALYs at an incremental cost of £31,140 per patient. This leads to an ICER of £35,970 per QALY gained. It was not possible to generate probabilistic estimates for the subgroup analysis as the subgroup model does not include a PSA subroutine.
Figures 85 and 86 present the cost-effectiveness plane and CEACs for the base-case analysis.
FIGURE 85.
Cost-effectiveness plane reported by AbbVie: base-case analysis. 62 Reproduced with permission.

FIGURE 86.
Cost-effectiveness acceptability curve reported by AbbVie: base-case analysis. Adapted from AbbVie submission. 62

The cost-effectiveness plane indicates that ADA is consistently expected to be more effective and more expensive than conventional management. Assuming a willingness-to-pay threshold of £20,000 per QALY gained, the probability that ADA produces more net benefit than conventional management is approximately 0.01. Assuming a willingness-to-pay threshold of £30,000 per QALY gained, the probability that ADA produces more net benefit than conventional management is approximately 0.30.
The deterministic sensitivity analysis undertaken by the manufacturer indicate that the model is most sensitive to assumptions concerning disease state costs and the health state utilities. Given the narrow scope of the AbbVie economic analysis, the cost-effectiveness of ADA compared with other biological therapies or surgery is unknown.
Critical appraisal of the AbbVie model
The main issues identified by the Assessment Group are presented in Box 3 and discussed below.
-
Deviations from the NICE reference case and final NICE scope, particularly with respect to omission of other biologicals and surgery as comparators.
-
Questionable choice of cycle length necessitating the use of other external evidence on transition probabilities, which should not be required.
-
Concerns regarding the selection and use of evidence to inform HRQoL parameters.
-
Questionable source of surgery rate.
Deviations from NICE reference case and final NICE scope
The extent to which the economic analyses reported in the AbbVie submissions adhere to the NICE reference case is presented in Table 63.
| Element of HTA | Reference case | Assessment Group comments |
|---|---|---|
| Defining the decision problem | The scope developed by the institute (NICE) | The scope of the analysis deviates from the final scope from NICE |
| Comparator(s) | As listed in the scope developed by NICE | The comparator is limited to ‘standard of care’ (conventional non-biological therapies) only. The model does not include other biological agents (IFX and GOL) included in the final NICE scope. The model does not include surgery as a comparator |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, carers | Health outcomes reflect those of patients with UC |
| Perspective on costs | NHS and PSS | The economic analysis was undertaken from the perspective of the UK NHS. PSS costs are not mentioned in the submission |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | The economic analysis takes the form of a cost–utility analysis of the two included options |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Costs and outcomes are evaluated over a 10-year time horizon. Analyses over a lifetime horizon are not presented in the manufacturers’ submission nor are they possible within the implemented model structure |
| Synthesis of evidence on health effects | Based on systematic review | Although the submission mentions other relevant trials of IFX and GOL, the manufacturer opted to undertake a ‘within-trial’ analysis of ADA vs. conventional management using efficacy data from the ULTRA2 trial45,126 only |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Health effects are assessed in terms of QALYs. The EQ-5D has been used to assign specific utility values for health states, but weighted averages from other instruments (i.e. TTO) have also been used to value the post-surgery chronic complications health state |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | It would have been possible to map from the SF-6D in the ULTRA2 trial45,126 to the EQ-5D. Instead, the manufacturer used data from Swinburn et al.139 to value pre-surgery states |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | No equity weighting is applied |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | The economic analysis was undertaken from the perspective of the UK NHS |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | The model uses a discount rate of 3.5% for costs and health outcomes |
Overall, the economic analysis undertaken by AbbVie is generally in line with the NICE reference case. However, similar to the MSD submissions,64,66 the two most notable concerns relate to the choice of comparators and the adoption of a short model time horizon (10 years).
The AbbVie economic analysis includes only two treatment options: (1) ADA; and (2) conventional non-biological treatments. The analysis excludes other relevant biological therapies for the treatment of UC (IFX and GOL) and elective surgery. The appendix to the main submission states that:
Other anti-TNF therapies which are being appraised as part of this NICE MTA, namely infliximab and golimumab, were not considered as comparators in the present evaluation as they are not NICE recommended for this patient population and therefore would not form routine standard of care at present.
AbbVie submission to NICE62
However, IFX and GOL were listed in the final NICE scope and hence they should have been included in the economic analysis. As a consequence of their omission, the AbbVie model adopts a very narrow scope and provides no information regarding the comparative cost-effectiveness of the full range of biological treatment options within this appraisal.
The main submission from AbbVie states a number of arguments regarding why it would not be appropriate to undertake a formal NMA (see AbbVie submission,62 pp. 67–8). The main arguments stated are:
-
Differences in Mayo score estimation between the relevant trials.
-
PBO responses have been shown to differ markedly depending on the severity of the trial population, study design and country or region in which the trial was conducted.
-
Other differences in trial design, that is, the use of adaptive design in the PURSUIT trial,47 differences in time points for the assessment of induction response, eligibility criteria relating to prior treatment failures, prior use of biologicals, steroid tapering, open-label escape allowance, timing of efficacy assessments and study durations.
However the Assessment Group do not agree that a NMA is inappropriate and AbbVie’s justifications for not undertaking such an analysis appear to be flawed. Notably, UC is a chronic disease characterised by ongoing inflammation over time; fluctuations in Mayo score evaluations over the course of 3 days are likely to be minor and hence the use of alternative scoring systems between trials is unlikely to produce any substantial bias. Furthermore, no two trials are identical. Although it is useful to highlight potential sources of heterogeneity between studies (and this is done well by AbbVie), the Assessment Group does not believe that the presence of this heterogeneity provides a sufficient basis for ignoring treatment options relevant to the decision problem.
In addition, the AbbVie model explicitly excludes elective colectomy as a comparator from the analysis. The appendix to the main AbbVie submission states that:
Surgery is an important treatment option in UC clinical management and is reserved for patients who have an inadequate response with, are contraindicated to or intolerant of conventional standard of care. Surgery is unlikely to be a first line option for moderately to severely active UC patients. Consistent with this approach, surgery is included in the model as the treatment option for a proportion of patients who failed SOC or ADA+SOC treatment, but not as a comparator to ADA+SOC.
AbbVie submission to NICE62
As this option was specified in the final agreed NICE scope, and because the appraisal does not relate to first-line treatment, it should have been included in the economic analysis.
It should also be noted that the AbbVie model time horizon is constrained to 10 years (260 2-week cycles). This shorter time horizon is used as a justification for excluding other-cause mortality from the model. The model does not include the functionality to consider longer time horizons; it is unclear whether or not the profiles of incremental costs and health outcomes for ADA versus conventional management would be similar over longer time horizons.
Questionable choice of cycle length necessitating the use of other evidence on transition probabilities
The cycle length adopted within the AbbVie model is 2 weeks. This short cycle length was selected ‘to accommodate the ADA dosing schedule.’62 Given that all patients enter the model in the ‘moderate to severe’ health state in line with the ULTRA2 trial, this choice of cycle length leads to a necessity to incorporate data from other literature121,133 to populate the transition probabilities from the ‘mild’ and ‘remission’ health states to other health states. As a consequence, there is some discrepancy between the observed pre-surgery health state distribution following induction in the ULTRA2 trial45,126 and the pre-surgery health state distribution following induction estimated by the model (Table 64). Given a longer cycle length for induction, that is the 6 weeks used in the trial, it would have been unnecessary to include other data on transition probabilities and the predictions of the model would have likely been more accurate.
| Treatment group | No response | Response | Remission |
|---|---|---|---|
| ADA group (observed) | 0.52 | 0.33 | 0.16 |
| ADA group (predicted) | 0.51 | 0.31 | 0.17 |
| Discrepancy in ADA group (observed – predicted) | 0.01 | 0.02 | –0.01 |
| PBO group (observed) | 0.67 | 0.24 | 0.09 |
| PBO group (predicted) | 0.61 | 0.29 | 0.09 |
| Discrepancy in PBO group (observed – predicted) | 0.07 | –0.05 | –0.01 |
Concerns regarding the selection and use of evidence to inform health-related quality-of-life parameters
The ULTRA2 trial45,126 did not collect HRQoL data from patients using the EQ-5D; however, the SF-36 instrument was included and could be used to derive SF-6D utility values. The manufacturer explored mapping the SF-6D values to the EQ-5D but noted that this would likely overestimate the level of HRQoL of patients with more severe disease. Instead, the manufacturer used data from Swinburn et al. 139 to value the pre-surgery health states in the model. Although the Swinburn et al. 139 study has been published only in abstract and poster form, more detail is provided in appendix 3 of the main AbbVie submission. 62 It is noteworthy that the difference in utility for the post-surgery state and the active UC state in the selected utility values within the AbbVie model (0.61–0.55 = 0.06) is smaller than that observed within other EQ-5D UC valuation studies (e.g. Woehl et al. 145 estimated this difference to be approximately 0.71 – 0.41 = 0.30). Therefore, the AbbVie model does not assume that surgery results in a substantial increase in HRQoL in patients with active disease.
It is also noteworthy that the choices made with respect to the HRQoL values for other post-surgery health states are not clear from the submission. In particular, the methods for identifying and selecting studies to value the chronic complications,110,140,141 and the weightings given to each, are unclear from the AbbVie submission. 62 What is clear is that the three valuation studies used to inform the chronic complications utility values used different health instruments; Hu et al. 140 is based on committee valuations using the Health Utility Index, Arseneau et al. 110 reported TTO valuations by UC patients and Smith and Roberts141 report TTO and VAS valuations. Producing a weighted mean utility from studies that use different elicitation methods may produce conceptually inconsistent rankings of identical health states. However, this parameter does not have a material impact on the ICER.
Questionable source of surgery rate
The AbbVie model estimates the 2-week probability of undergoing surgery from a 1-year study reported by Hillson et al. 134 This study was a retrospective analysis of medical claims with and without UC identified from a population of approximately 500,000 employees, retirees and dependants in the USA. This does not specifically relate to a moderate to severe UC population and the use of a 1-year study to estimate long-term risk is concerning, particularly given the availability of other longer studies undertaken in more relevant UC populations (see Evidence used to inform the model’s parameters).
De novo Assessment Group model
Introduction
In light of the limitations of the models submitted by the manufacturers (see Systematic review of existing cost-effectiveness evidence), the Assessment Group developed a de novo health economic model to assess the cost-effectiveness of second-line IFX, ADA and GOL, conventional non-biological therapies and immediate colectomy for the treatment of patients with moderate to severe UC.
Methods
Model scope
The scope of the economic analysis follows the NICE reference case (summarised in Box 4).
Population: patients with moderate to severe UC who have failed at least one prior therapy. a
Interventions and comparators: 160 mg/80 mg/40 mg of ADA; 5 mg/kg of IFX; 200 mg/100 mg/100 mg (50 mg) of GOL; conventional non-biological therapy (comprising a mix of 5-ASAs, immunosuppressants and corticosteroids); and elective surgery.
Economic outcome: incremental cost per QALY gained.
Perspective: NHS and PSS.
Time horizon: lifetime.
Discount rate: 3.5%.
The base-case analysis relates to an adult UC population; a secondary analysis is considered for the paediatric population.
The analysis compares IFX, ADA and GOL against each other and against conventional non-biological therapy (comprising a mix of 5-ASAs, immunosuppressants and corticosteroids) and immediate colectomy. IFX is assumed to be given at a dose of 5 mg/kg on three visits during induction and subsequently at a dose of 5 mg/kg every 8 weeks for patients who go on to receive maintenance therapy. ADA is assumed to be given at one dose of 160 mg, one dose of 80 mg and two doses of 40 mg during the induction phase; a dose of 40 mg every other week is assumed for patients who go on to receive maintenance therapy. A fixed proportion of ADA patients (27%) are assumed to escalate to a 40 mg every week dosing regimen, based on data reported in the AbbVie submission. 62 GOL is assumed to be given as one dose of 200 mg and one dose of 100 mg during induction treatment, with subsequent maintenance therapy given at a dose of 100 mg every 4 weeks for patients with body mass ≥ 80 kg or 50 mg every 4 weeks for patients with body mass < 80 kg. IFX is assumed to be administered in a day-case setting while the administration of GOL and ADA is not assumed to require any additional NHS resources (no costs are included for training patients to self-inject). Patients in the non-surgical treatment groups are assumed to receive conventional background therapies (5-ASAs, immunosuppressants and corticosteroids). Surgery is included in the economic analysis both as a comparator within the analysis and also as a downstream component of the pathway for patients in the biological and non-biological treatment groups.
The population within the economic analysis relates specifically to patients with moderate to severe UC who have failed at least one prior therapy, as reflected in the RCTs included in the systematic review of clinical effectiveness (see Chapter 3). Patient characteristics are based on the trials included in the systematic review. 44,47–49,126 Patients are assumed to enter the model aged 40 years with a mean body mass of 77 kg. Thirty two per cent of patients are assumed to have a body mass > 80 kg. Although the main economic analysis relates to adult patients with UC, a scenario analysis is also presented which compares IFX with conventional drug treatment and immediate colectomy in paediatric patients with UC (note: GOL and ADA do not currently have marketing authorisations in paediatric patients66,132). This secondary analysis should be considered exploratory as the efficacy data are drawn from trials undertaken within an adult UC population. The economic evaluation takes the form of a cost–utility analysis; the primary health economic outcome is the incremental cost per QALY gained. All treatment options are evaluated within a fully incremental analysis within the base case. The perspective of the economic analysis relates to that of the NHS and PSS. All costs and outcomes are discounted at 3.5%. Costs and health outcomes are evaluated over a lifetime horizon in the base case; shorter time horizons are considered as secondary scenario analyses.
Model structure
The Assessment Group model adopts a Markov structure with eight mutually exclusive health states (Figure 87). The model health states are defined according to whether the patient is alive or dead, the non-surgical treatment the patient is currently receiving (biological therapy or non-biological therapy), their prior history of colectomy and their current level of disease control (remission, response and active UC). Remission and response to treatment are classified according to the Mayo score, as defined within the trials included in the systematic review (see Chapter 3). Remission is defined as a Mayo score of ≤ 2 points with no individual subscore of > 1 point. Response is defined as a decrease from baseline in the total Mayo score of at least 3 points and at least 30%, with an accompanying decrease in the subscore for rectal bleeding of at least 1 point or an absolute subscore for rectal bleeding of 0 or 1. As remission is a subset of the broader category of response, these are dealt with as mutually exclusive ordered categorical data (see Chapter 3, Methods for network meta-analysis). Patients without either response or remission are defined as having active (moderate to severe) UC. The model includes the following health states: (1) on biological treatment – active UC; (2) on biological treatment – response; (3) on biological treatment – remission; (4) on conventional treatment – active UC; (5) on conventional treatment – response; (6) on conventional treatment – remission; (7) post-surgery (with or without complications); and (8) dead. Surgery is not included as a state but rather it is incorporated as an event; patients undergoing colectomy are assumed to transit to the post-surgery state if they survive their surgery and the dead state if they do not.
FIGURE 87.
Assessment Group model structure (induction and maintenance phases). a, Patients in the IFX, ADA and GOL groups begin in this portion of the model; b, patients in the conventional non-biological management group begin in this portion of the model; and c, patients in the immediate colectomy group begin in this portion of the model.

The model time horizon is divided into two main phases: (1) induction and (2) maintenance. The model adopts an 8-week cycle length for the induction phase and a 26-week cycle length during the subsequent maintenance phase. During the induction phase, patients receiving biological treatment who achieve response or remission are assumed to continue receiving the same biological treatment as maintenance therapy. Patients who do not achieve response or remission during biological induction therapy are assumed to discontinue that biological treatments and subsequently receive conventional non-biological treatments. Patients in the conventional treatment group are assumed to continue receiving conventional therapy irrespective of their response to induction therapy. Patients in the immediate colectomy group are assumed to undergo surgery during the induction phase of the model and subsequently remain in the post-surgery state. All patients have a probability of dying from other causes during the induction cycle.
During the maintenance phase, patients receiving biological therapy are assumed to continue receiving the same biological treatment for as long as they continue to maintain response/remission. If patients receiving biological therapy lose their response at any point they are assumed to transit to the active UC state and subsequently receive conventional therapy. Patients in the conventional treatment group, and those who have previously achieved but lost response to biological therapy, are assumed to continue receiving conventional therapy irrespective of whether they achieve response or remission to that conventional therapy. A time-independent probability of undergoing surgery is applied to those patients receiving conventional treatment with active UC; the model assumes that this only possible within the active UC state. Patients in the immediate colectomy group, and those who have undergone surgery after receiving biological/conventional treatment, remain in the post-surgery state for the remainder of the model time horizon. All patients have a probability of dying from other causes during each model cycle.
Different levels of HRQoL are assigned to each model health state. Disutilities are assigned to those patients who develop chronic pouchitis – other complications of surgery are assumed to be transient and are assumed not to have a long-term impact on patients’ HRQoL. QALY gains in each arm of the model are driven by sojourn time in each of the model’s health states and differential rates of surgery across the biological groups, the conventional management group and the immediate colectomy group. Resource costs are assigned in terms of drug acquisition, drug administration (IFX only), surgery and related complications and UC health state costs (elective/emergency endoscopy, blood tests, consultant visits and hospitalisations.
The probability of residing in each health state during a given model cycle is estimated using simple matrix multiplication. Transitions between states are handled within a three-stage competing risks framework whereby (1) patients undergo transitions between each of the pre-surgical UC treatment states based on individual transition probabilities estimated using the NMAs (see Chapter 3, Assessment of effectiveness) and the estimated colectomy rate; (2) the populations of the post-surgery and dead states are adjusted to reflect surgical mortality rates; and (3) the remaining surviving population is adjusted to account for other-cause mortality conditional on the patient cohort’s current age. Given the different durations of the induction and maintenance phases, a half-cycle correction is not applied within the model.
Key model assumptions
The Assessment Group model makes the following key assumptions:
-
At the beginning of the maintenance phase, the decision to continue therapy with IFX, ADA and GOL is determined by the achievement of response or remission at the end of induction.
-
During each maintenance cycle, the decision to continue therapy with IFX, ADA and GOL is determined by the achievement/maintenance of response or remission at the end of the previous maintenance cycle.
-
Patients who discontinue biological therapy are assumed to receive conventional treatment.
-
Patients with active UC receiving conventional treatment may undergo colectomy during any cycle; patients receiving biological therapy will receive at least one cycle of conventional treatment before transiting to surgery.
-
Patients’ HRQoL is assumed to be determined by their level of disease control, whether or not they have previously undergone colectomy, and the incidence of post-surgical complications.
-
With the exception of chronic pouchitis, all surgery-related complications are assumed to occur during the first cycle following surgery.
-
With the exception of chronic pouchitis, surgical complications are assumed to be transient and can be resolved either through further surgery or through medical management.
-
The medical management of surgery-related complications is assumed to require a 7-day admission on a gastroenterology ward.
-
The incidence of chronic pouchitis is assumed to be associated with ongoing additional treatment costs and a decrement in patients’ level of HRQoL.
Evidence used to inform the model’s parameters
Table 65 summarises the evidence sources used to inform the groups of parameters within the model. These are described in further detail in the following sections.
| Parameter group | Source(s) used to inform parameter values |
|---|---|
| Patient characteristics (starting age, mean body mass, proportion of patients with body mass > 80 kg, proportion of patients who are female) | Patient age, mean body mass and the probability that a patient is female were derived from the RCTs included in the systematic review of clinical effectiveness (see Chapter 3). The proportion of patients with body mass > 80 kg was taken from the MSD GOL model66 |
| Pre-surgical health state transition rates induction phase | De novo NMA of induction trials |
| Pre-surgical health state transition rates maintenance phase | De novo NMA of maintenance trials |
| Surgery rate during each maintenance cycle | Solberg et al.10 |
| Probability of perioperative mortality | UK IBD Audit 2012146 |
| Probability of other-cause mortality conditional on age and sex | ONS life tables for England and Wales 2009–2011147 |
| Health state utilities for all pre-surgical and post-surgical states | Woehl et al.145 |
| Disutility associated with chronic pouchitis | Arseneau et al.110 |
| Biological drug regimen schedules | Based on the SmPCs and trials for IFX, ADA and GOL132,148,149 |
| Biological drug regimen usage, duration and dosing | Expert opinion (personal communication: Professor Alan Lobo, Consultant Gastroenterologist, Sheffield Teaching Hospitals) |
| Probability of surgery-related complications and proportion of cases requiring surgery/medical treatment | Arai et al.108 |
| Use of other related resources for the management of UC | Tsai et al.82 |
| Relative risk of hospitalisation for biologicals vs. conventional treatment | MSD submissions64,66 |
| Unit costs | BNF37 and NHS Reference Costs 2012/13130 |
Patient characteristics
Patient characteristics were based on data reported within the trials included in the systematic review44,47–49,126 (see Chapter 3). Patients are assumed to enter the model aged 40 years, 43% of patients are assumed to be female and patients are assumed to have a mean body mass of 77 kg. Thirty two per cent of patients are assumed to have a body mass > 80 kg; this estimate was drawn from the MSD GOL model. 66
Transition probabilities for biological and non-biological therapies
The methods for the NMA models are described in Chapter 3, Methods for network meta-analysis. Table 66 presents the means and 95% CrIs for transitions within the model (note: all patients in the colectomy group who survive their surgery are assumed to transit immediately to the post-surgery group).
| Transition | Conventional non-biological treatment (95% CrI) | 5 mg/kg of IFX (95% CrI) | 160 mg/80 mg/40 mg of ADA (95% CrI) | 200 mg/100 mg/50 mg of GOL (95% CrI) | 200 mg/100 mg/100 mg of GOL (95% CrI) |
|---|---|---|---|---|---|
| Induction phase | |||||
| TP no response to no response | 0.64 (0.57 to 0.71) | 0.29 (0.17 to 0.44) | 0.49 (0.33 to 0.64) | 0.45 (0.26 to 0.64) | 0.45 (0.26 to 0.64) |
| TP no response to response | 0.26 (0.21 to 0.31) | 0.35 (0.28 to 0.41) | 0.32 (0.25 to 0.39) | 0.33 (0.24 to 0.39) | 0.33 (0.24 to 0.39) |
| TP no response to remission | 0.10 (0.06 to 0.15) | 0.36 (0.21 to 0.52) | 0.19 (0.09 to 0.32) | 0.22 (0.09 to 0.39) | 0.22 (0.09 to 0.39) |
| Maintenance phase 1 | |||||
| TP no response to no response | 0.85 (0.75 to 0.92) | 1.00a | 1.00a | 1.00a | 1.00a |
| TP no response to response | 0.10 (0.04 to 0.17) | 0.00 | 0.00 | 0.00 | 0.00 |
| TP no response to remission | 0.06 (0.02 to 0.11) | 0.00 | 0.00 | 0.00 | 0.00 |
| TP response to no response | 0.52 (0.43 to 0.62) | 0.43 (0.22 to 0.66) | 0.51 (0.23 to 0.78) | 0.40 (0.17 to 0.66) | 0.37 (0.15 to 0.62) |
| TP response to response | 0.27 (0.20 to 0.34) | 0.28 (0.19 to 0.37) | 0.26 (0.14 to 0.35) | 0.28 (0.18 to 0.37) | 0.29 (0.18 to 0.38) |
| TP response to remission | 0.21 (0.12 to 0.31) | 0.29 (0.11 to 0.52) | 0.23 (0.05 to 0.49) | 0.31 (0.11 to 0.59) | 0.35 (0.13 to 0.62) |
| TP remission to no response | 0.35 (0.17 to 0.57) | 0.32 (0.08 to 0.65) | 0.43 (0.10 to 0.80) | 0.18 (0.03 to 0.46) | 0.18 (0.03 to 0.47) |
| TP remission to response | 0.18 (0.07 to 0.32) | 0.17 (0.06 to 0.30) | 0.17 (0.05 to 0.30) | 0.14 (0.03 to 0.28) | 0.14 (0.03 to 0.28) |
| TP remission to remission | 0.47 (0.23 to 0.71) | 0.51 (0.18 to 0.83) | 0.41 (0.08 to 0.80) | 0.69 (0.32 to 0.93) | 0.68 (0.32 to 0.93) |
| Maintenance phase 2 | |||||
| TP no response to no response | 0.97 (0.93 to 1.00) | 1.00a | 1.00a | 1.00a | 1.00a |
| TP no response to response | 0.02 (0.00 to 0.05) | 0.00 | 0.00 | 0.00 | 0.00 |
| TP no response to remission | 0.01 (0.00 to 0.04) | 0.00 | 0.00 | 0.00 | 0.00 |
| TP response to no response | 0.34 (0.07 to 0.71) | 0.25 (0.01 to 0.72) | 0.45 (0.06 to 0.89) | 0.30 (0.02 to 0.75) | 0.41 (0.05 to 0.85) |
| TP response to response | 0.37 (0.12 to 0.60) | 0.34 (0.06 to 0.62) | 0.33 (0.07 to 0.56) | 0.35 (0.08 to 0.62) | 0.34 (0.08 to 0.58) |
| TP response to remission | 0.29 (0.03 to 0.72) | 0.41 (0.03 to 0.89) | 0.22 (0.01 to 0.72) | 0.35 (0.02 to 0.84) | 0.25 (0.01 to 0.74) |
| TP remission to no response | 0.30 (0.17 to 0.45) | 0.25 (0.03 to 0.61) | 0.08 (0.01 to 0.29) | 0.33 (0.08 to 0.66) | 0.27 (0.05 to 0.60) |
| TP remission to response | 0.16 (0.03 to 0.45) | 0.14 (0.02 to 0.41) | 0.08 (0.00 to 0.34) | 0.16 (0.02 to 0.41) | 0.15 (0.02 to 0.42) |
| TP remission to remission | 0.54 (0.24 to 0.73) | 0.61 (0.17 to 0.93) | 0.83 (0.45 to 0.99) | 0.52 (0.14 to 0.85) | 0.59 (0.17 to 0.89) |
It should be noted that beyond 1 year, the model repeatedly uses the transition probabilities derived within the maintenance phase 2 NMA.
Surgery rate
The rate at which patients with moderate to severe UC progress to colectomy was based on estimates from the literature. A focused MEDLINE search was undertaken to identify studies reporting long-term rates of colectomy in patients with moderate to severe UC. MEDLINE was searched from inception to April 2014 using a simple search comprising two search terms: ‘ulcerative colitis/exp’ and ‘colectomy rate.tw.’ Studies were considered for inclusion in the economic model if they reported on long-term colectomy rates and if they either related to the moderate to severe population as a collective group of patients, or if they reported on colectomy rates in moderate and severe UC populations separately.
The MEDLINE search identified 70 citations. Of these, only six studies were identified that reported on long-term colectomy rates for patients in a selected moderate to severe UC population (Table 67).
| Study | Population | Follow-up duration | Reported colectomy rate |
|---|---|---|---|
| Actis et al.150 | Patients admitted consecutively to study unit with an attack of UC and treated with ciclosporin between January 1991 and December 1999 (responders available for analysis, n = 34) | 7 years | 24/34 (65%) |
| Gower-Rousseau et al.151 | All patients from the EPIMAD registry diagnosed with UC between January 1988 and December 2002 and who were < 17 years old at the time of diagnosis (n = 113) | Median 6.42 years (range 3.83–10.42 years) | Approximately 25% (Kaplan–Meier estimate) |
| Molnár et al.152 | UC patients admitted between 1998 and 2005 to tertiary clinic because of severe exacerbation of UC requiring parenteral CS therapy (n = 183) | Average 4.4 years (range 1.1–10 years) | 16/110 (14.5%) steroid-responders, 29/73 (39.7%) steroid- refractory, overall = 24.6% |
| Mocciaro et al.153 | Two historical cohorts of UC patients with severe relapse refractory to i.v. steroid treatment administered according to the ‘Oxford regimen’ (n = 65) | Mean 6.23 years (± 5.07 years) | IFX group = 60%, ciclosporin group = 30% |
| Gustavsson et al.13 | 158 patients with UC treated in 1975–82 with i.v. CS treatment | Median 14.42 years (range 0.33–22.58 years) | All UC (n = 147): colectomy rate = approximately 50%, mild UC (n = 20): colectomy rate = approximately 40%, moderate UC (n = 45): colectomy rate = approximately 50%, severe UC (n = 61): colectomy rate = approximately 62% |
| Solberg et al.10 | Population-based cohort of 843 patients with IBD was enrolled in south-eastern Norway | Cohort followed up at 1, 5 and 10 years | Cumulative colectomy rate after 10 years = 9.8% (95% CI 7.4% to12.4%) |
Several studies report estimates for patients who have been hospitalised for UC flare; these are likely to overestimate the true colectomy rate in the moderate to severe population. On consideration of the remaining studies, the study reported by Solberg et al. 10 was selected for inclusion in the model as this study was large (423 patients completed 10-year follow-up) and did not specifically relate to patients who had experienced UC flare. A constant 6-month colectomy rate of 0.0051 was applied within the model. Uncertainty surrounding this probability was modelled using a beta distribution.
Mortality
The model includes two types of mortality: perioperative mortality associated with colectomy and other-cause mortality. Additional risks of death, for example owing to the increased risk of colorectal cancer, are excluded from the model as this risk is likely to be small. Perioperative mortality rates were taken from the third round of the UK IBD audit. 146 Within the 2012 publication of the UK IBD audit, there were 28 deaths reported among 807 elective and emergency surgical episodes in adult patients with UC; a probability of death of 0.03 is assumed within the cycle in which the patient undergoes surgery. Other-cause mortality was modelled according to age- and sex-specific life tables from the Office for National Statistics. 147 The annual probability of death during each model cycle was adjusted to reflect the duration of induction and maintenance cycles (8 weeks and 26 weeks respectively) using standard methods. 154 Uncertainty surrounding the perioperative mortality rate was modelled using a beta distribution. No uncertainty was modelled for other-cause mortality.
Probability of experiencing surgery-related complications
The trials used to inform the efficacy parameters do not include details of surgery-related complications. Instead, the model uses data reported in Arai et al. 108 to inform parameters relating to the probability of experiencing transient and chronic surgery-related complications and the probabilities that these complications are treated using medical or surgical approaches. Given the types of complications reported in Arai et al. 108 (Table 68), the model assumes that all are transient with the exception only of pouchitis. Therefore, the model assumes that 47.3% (140/296) patients will develop transient complications, with a further 5% of patients developing chronic pouchitis. Based on the reported timing of complications within the Arai et al. study,108 the model assumes that all transient complications will arise and will be resolved during the first cycle following surgery (in those patients who survive their surgery). Chronic pouchitis is assumed to continue for the remainder of the patient’s lifetime. The model assumes that 19% of complications require further surgery, while the remaining 81% require medical treatment only.
| Complication type | Complication frequency | Treatment approach | ||
|---|---|---|---|---|
| Number of participants with early complication | Number of participants with late complication | Medical | Surgical | |
| Anastomotic stricture | 7 | 56 | 63 | 0 |
| Staple line ulcer | 9 | 31 | 38 | 2 |
| Pouchitis | 0 | 16 | 16 | 0 |
| Bowel obstruction | 6 | 15 | 16 | 5 |
| Proctitis | 1 | 17 | 18 | 0 |
| Pelvic sepsis | 12 | 2 | 1 | 13 |
| Peritoneal abscess | 3 | 0 | 0 | 3 |
| Anal fistula | 0 | 12 | 2 | 8 |
| Incisional hernia | 1 | 11 | 0 | 4 |
| Total | 39 | 160 | 154 | 35 |
Health-related quality of life
Within the model, HRQoL is assigned according to the level of disease control achieved with drug therapy (active UC, response, remission), whether or not the patient has previously undergone colectomy and whether or not the patient is experiencing post-surgical complications. The same utility values are used for all biological and non-biological drug treatments. The Assessment Group undertook a systematic review of studies reporting valuations of states relating to different levels of UC control and postsurgery.
Searches were undertaken to identify utilities literature relating to UC, specifically using the EQ-5D instrument. The following electronic databases were searched from inception for utility published studies:
-
MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations: via Ovid – 1946 to January 2014.
-
EMBASE: via Ovid – 1974 to January 2014.
-
Cochrane Library: via Wiley Interscience
-
CDSR – 1996 to January 2014
-
DARE – 1995 to January 2014
-
CCRT – 1995 to January 2014
-
Cochrane Methodology Register – 1904 to January 2014
-
HTA database – 1995 to January 2014
-
NHS EED – 1995 to January 2014.
-
-
CINAHL: via EBSCOhost – 1982 to January 2014.
-
Web of Science Citation Index: via Web of Knowledge – 1900 to January 2014.
-
Conference Proceedings Citation Index: via Web of Knowledge – 1990 to January 2014.
-
BIOSIS Previews: via Web of Knowledge – 1969 to January 2014.
-
EconLit: via Ovid – 1886 to January 2014.
The MEDLINE search strategies are presented in Appendix 10. The search strategy combined free text and MeSH or thesaurus terms relating to UC combined with terms for specific utility measures or more general utility terms. The search strategy was translated across all databases. No date or language restrictions were applied. Literature searches were conducted during January and February 2014. References were collected in a bibliographic management database, and duplicates were removed. The results of the search are summarised in Table 69.
| Database | Date range | Date searched | Number of results |
|---|---|---|---|
| MEDLINE (via Ovid) | 1946 to January 2014 | 29 January 2014 | 52 |
| EMBASE (via Ovid) | 1974 to January 2014 | 29 January 2014 | 113 |
| CINAHL (via EBSCOhost) | 1982 to January 2014 | 4 February 2014 | 0 |
| Science Citation Index and Social Science Citation Index (via Web of Knowledge) | 1900 to January 2014 | 4 February 2014 | 5 |
| BIOSIS (via Web of Knowledge) | 1969 to January 2014 | 4 February 2014 | 4 |
| CDSR (via Wiley) | 1991 to January 2014 | 29 January 2014 | 0 |
| CENTRAL (via Wiley) | 1991 to January 2014 | 29 January 2014 | 2 |
| Cochrane HTA (via Wiley) | 1991 to January 2014 | 29 January 2014 | 0 |
| Cochrane DARE (via Wiley) | 1991 to January 2014 | 29 January 2014 | 0 |
| Cochrane EED (via Wiley) | 1991 to January 2014 | 29 January 2014 | 0 |
| EconLit (via Ovid) | 1886 to January 2014 | 29 January 2014 | 1 |
Studies were considered potentially includable if they reported EQ-5D utility estimates for multiple UC health states or if they reported valuations of post-surgery states. The study selection process is shown in Figure 88.
FIGURE 88.
Study selection results.

Of the 177 deduplicated, potentially relevant studies, the full papers of 53 citations were retrieved for more detailed examination by the Assessment Group based on their titles and abstracts. Of these 53 citations, 10 studies reported EQ-5D estimates for one or more health states relevant to the model. 47,49,139,145,155 Seven of these studies reported estimates for multiple pre-surgery UC health states64,66,139,145,155–157 and the remaining three studies reported estimates only for post-surgery state only (Table 70). 158–160 Of the 10 potentially relevant EQ-5D studies, those reported by Woehl et al. 145 and Swinburn et al. 139 appear to be the most useful as they are UK based, included a fairly large number of patients (n = 180 and n = 230, respectively) and have the greatest coverage of the health states in the model (see Table 70).
| State/study | ACT1 and ACT264,66 | PURSUIT64,66 | aSwinburn et al.139 | Woehl et al.145 | aCasellas155 | aLeidl156 | Vaizey157 | Van der Valk158 | Richards159 | Kuruvilla160 |
|---|---|---|---|---|---|---|---|---|---|---|
| Study characteristics | ||||||||||
| Sample size | 486b | 464 | 230 | 180 | 528 | 232 | 173 | 982 | 56 | 59 |
| Country | Various | Various | UK | UK | Spain | Germany (UK tariff) | UK | The Netherlands | UK | USA |
| Health state valuations | ||||||||||
| Remission (ranges of utilities reported by the company) | 0.84–0.88 | 0.86–0.89 | 0.91 | 0.87 | 1.00 | 0.91 | 0.86 | NR | NR | NR |
| Response (ranges of utilities reported by the company) | 0.79–0.82 | 0.80 | 0.80 | 0.76 | 0.70 | 0.74 | 0.77 | NR | NR | NR |
| Active UC (utilities) | NR | NR | 0.55 | 0.41 | 0.55 | 0.63 | 0.66 | NR | NR | NR |
| Post surgery (utilities) | NR | NR | 0.59 | 0.71–0.72 | NR | NR | NR | 0.85c | 0.85 | 0.90c |
| Post-surgery complications | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
The study reported by Swinburn et al. 139 examines the impact of colectomy on the HRQoL of patients with UC. A total of 330 participants were recruited into the study, comprising 230 UC patients (30 of whom had previously undergone surgery) together with 100 age- and sex-matched controls. EQ-5D utilities were collected via online survey. For both post-surgery patients versus non-surgery patients and post-surgery patients versus controls, EQ-5D utility scores were compared across IBDQ disease severity. Seventy-eight patients had remission, 47 patients had mild disease, 31 patients had moderate disease and 44 patients had severe disease. The utility for patients post surgery was reported to be 0.59 (95% CI 0.55 to 0.63). For patients who had not undergone surgery, the scores for each disease severity are: remission utility = 0.91 (95% CI 0.87 to 0.95), mild disease utility = 0.80 (95% CI 0.70 to 0.85), moderate disease utility = 0.68 (95% CI 0.58 to 0.78) and severe disease utility = 0.45 (95% CI 0.35 to 0.55). Across the total UC pre-surgery population, the mean EQ-5D utility was reported to be 0.75 (95% CI 0.71 to 0.79). Similarly, for the matched controls, the mean EQ-5D utility was estimated to be 0.79 (95% CI 0.75 to 0.83). Swinburn et al. 139 report that, on average, post-surgery patients reported lower HRQoL scores than non-surgery patients (p = 0.016) and matched controls (p = 0.03).
Woehl et al. 145 collected EQ-5D utility scores from 180 patients with active UC. Within this study population, the mean age was 55.0 years (SD 14.2) and the mean age at diagnosis was 34.1 years (SD 14.6). UC disease severity groups were categorised by SCAI-2 and were compared with patients with IPAA and ileostomy. The mean EQ-5D score was 0.73 (SD 0.29). Mean EQ-5D utilities were reported to be 0.87 (SD 0.15) for remission, 0.76 (SD 0.18) for mild disease, and 0.41 (SD 0.34) for moderate to severe disease. Patients who had undergone IPAA reported an EQ-5D utility of 0.71 (SD 0.29) while patients with an ileostomy reported an EQ-5D score of 0.72 (SD 0.35). Therefore, the health utility scores for these surgery states were slightly below a mild disease severity. The difference between these five groups was statistically significant (p = 0.001).
In the base-case analysis of the Assessment Group model, the Woehl et al. 145 study was selected as the valuation for the surgery state (0.71 to 0.72) is more consistent with the other post-surgery valuations identified158–160 as compared with the Swinburn et al. 139 study.
In order to maintain the ordinal ranking of remission, response and active UC states, remission was modelled as a baseline utility score parameter, with disutilities used to value the reductions in health associated with the loss of remission and the loss of response relative to a baseline of remission. The utility parameter for response is therefore modelled as Utility (remission) minus disutility (loss of remission) while the utility parameter for active UC is modelled as Utility (remission) minus disutility (loss of remission) minus disutility (loss of response). The utility score for post-surgery was based on the mean value reported by Woehl et al. 145 (this parameter was not characterised as a health decrement). Uncertainty surrounding the parameters describing remission utility and post-surgery utility was modelled using beta distributions, assuming that an equal number of patients were in each UC state. The disutility parameters were based on the mean and variance of the differences between the health states; this method ensures that the notionally better health state always has a monotonically better valuation than that for the notionally worse health state.
As the studies identified for inclusion in the review did not identify any studies that employed the EQ-5D to value the health loss associated with surgery-related complications, other sources were required. The Assessment Group model adopts a similar approach to the AbbVie model to value this health decrement based on the difference between the surgery and chronic pouchitis valuations reported by Arseneau et al. 110 (TTO valuation: 0.57 – 0.40 = 0.17). It should be noted that this study used scenario-based TTO elicitation methods and, therefore, deviates from the NICE reference case. 78 Health losses associated with transient complications of surgery are assumed not to last long enough to decrease HRQoL. The utility values in the model were not adjusted by age. The final utility vector for each treatment option is shown in Table 71.
| Treatment | Receiving biological treatment | Receiving standard care | Post surgerya | ||||
|---|---|---|---|---|---|---|---|
| No response | Response | Remission | No response | Response | Remission | ||
| Conventional management | 0.41 | 0.76 | 0.87 | 0.41 | 0.76 | 0.87 | 0.70 |
| IFX | 0.41 | 0.76 | 0.87 | 0.41 | 0.76 | 0.87 | 0.70 |
| ADA | 0.41 | 0.76 | 0.87 | 0.41 | 0.76 | 0.87 | 0.70 |
| GOL | 0.41 | 0.76 | 0.87 | 0.41 | 0.76 | 0.87 | 0.70 |
| Colectomy | 0.41 | 0.76 | 0.87 | 0.41 | 0.76 | 0.87 | 0.70 |
Resource costs
The model includes resource costs related to drug acquisition, drug administration (IFX only), consultant visits, emergency and elective endoscopy, hospitalisations, blood tests, surgery and the management of surgery-related complications.
Biological drug resource use, acquisition and cost
Table 72 shows the dosing regimens and associated costs for each of the biological options within the model. With the exception of GOL induction therapy, the biological regimen assumed reflects the wording of the SmPC for that product. 132,148,149 It should be noted that the SmPC for GOL recommends that continued therapy should be reconsidered in patients who show no evidence of therapeutic benefit within 12–14 weeks (after four doses). However, the PURSUIT-SC trial47 evaluated clinical benefits at 6 weeks (after two doses). The costs and benefits of GOL induction are modelled in line with the design of the PURSUIT-SC trial and, therefore, the costs within the induction phase relate only to the first two doses of GOL. The dose adjustments for ADA were based on the estimate reported within the AbbVie submission. 62
| Treatment group | Mean doses and frequency within cycle | Cycle cost (£) |
|---|---|---|
| Induction phase (8-week duration) | ||
| 5 mg/kg of IFX | 12 × 100 mg of IFX (3 outpatient appointments) | 5035.44 (acquisition) + 893.18 (administration) = 5928.44 |
| 160 mg/80 mg of ADA | 4 × 40 mg ofADA + 2 × 40 mg of ADA (self-administered) | 2817.12 |
| 200 mg/100 mg of GOL | 4 × 50 mg of GOL + 2 × 50 mg of GOL (self-administered) | 4577.82 |
| Maintenance phase (26-week duration) | ||
| 5 mg/kg of IFX | 13.04 × 100 mg of IFX (3.26 outpatient appointments) | 5473.79 (acquisition) + 970.94 (administration) = 6444.73 |
| 40 mg of ADA EOW dosing (72.6% patients) | 13.04 × 40 mg of ADA | 4593.54 |
| 40 mg of ADA EW dosing (27.4% patients) | 26.08 × 40 mg of ADA | 9187.08 |
| 100 mg of GOL (body mass > 80 kg, 31.60% patients) | 13.04 × 50 mg of GOL | 9952.67 |
| 50 mg of GOL (body mass < 80 kg, 68.40% patients) | 6.52 × 50 mg of GOL | 4976.34 |
Non-biological drug resource use
The costs of conventional therapies in each UC state are shown in Table 73. These resource costs were assumed to be the same for all biological options and for the conventional management group. The treatments, doses and their frequencies were based on expert opinion (Professor Alan Lobo, Sheffield Teaching Hospitals, April 2014, personal communication) and BNF dosing recommendations. When multiple products were available, the least expensive was included in the analysis. The model assumes that all patients would receive 5-ASAs using Ipocol® (Sandoz Ltd) at a dose of 2.4 mg/day during induction and 1.2 mg/day during maintenance. Ninety per cent of patients are also assumed to receive topical 5-ASAs (80% enemas, 10% suppositories) during induction; these are assumed to be given for a maximum of 28 days per cycle. Following loss of response, the same therapies may also be used to reinduce response/remission; these same assumptions are applied during each maintenance cycle to the conventional management active UC state only. Eighty per cent of patients are assumed to receive 2.5 mg/kg of AZA daily, with the remaining 20% receiving 6-MP at a dose of 1.5 mg/kg daily (note: it is likely that a lower proportion of patients will actually fulfil the criteria for treatment hence this may be an overestimate). All patients are also assumed to require one course of oral prednisolone during induction with the dose starting at 40 mg being tapered by 5 mg each week until the dose is zero (again, the same assumption is made with respect to reinduction of response/remission in patients in the conventional management active UC state during each maintenance cycle). The model does not include estimates of uncertainty around drug usage.
| Drug, brand | Regimen assumed (% use) | Unit cost | Cost per cycle (for those receiving treatment) |
|---|---|---|---|
| Induction phase (8 weeks) | |||
| 5-ASAs (oral), Ipocol® | 2.4 mg/day for 56 days (100% patients) | 400 mg (120 tablets) = £17.68 | £49.50 |
| 5-ASAs (enema), Asacol®(Warner Chilcott UK Ltd) | 1 metered application/day for 28 days (80% patients) | 1 mg (14 application canister) = £26.72 | £53.44 |
| 5-ASAs (suppository), Asacol | 1.5 g/day for 28 days (10% patients) | 250 mg (20 suppository pack) = £4.82 | £20.24 |
| AZA, non-proprietary | 2.5 mg/kg daily for 56 days (80% patients) | 50 mg (56 tablets) = £3.85 | £14.82 |
| 6-MP, Puri-Nethol® (Teva Pharmaceuticals) | 1.5 mg/kg daily for 56 days (20% patients) | 50 mg (25 tablets) = £50.47 | £261.15 |
| Prednisolone (oral), non-proprietary | 40 mg tapered by 5 mg/week until dose is zero after 56 days (100% patients) | 5 mg (28 tablets) = £1.03 | £9.27 |
| Maintenance phase (26 weeks) | |||
| 5-ASAs (oral), Ipocol® | 1.2 g/day for 182.63 days (100% patients) | 400 mg (120 tablets) = £17.68 | £80.72 |
| 5-ASAs (enema), aAsacol® | 1 metered application/day for 28 days (80% patients) | 1 mg (14 application canister) = £26.72 | £53.44 |
| 5-ASAs (suppository),a Asacol® | 1.5 g/day for 28 days (10% patients) | 250 mg (20 suppository pack) = £4.82 | £20.24 |
| AZA, non-proprietary | 2.5 mg/kg daily for 182.63 days (80% patients) | 50 mg (56 tablets) = £3.85 | £48.34 |
| 6-MP, Puri-Nethol® | 1.5 mg/kg daily for 182.63 days (20% patients) | 50 mg (25 tablets) = £50.47 | £851.66 |
| Prednisolone (oral), non-proprietarya | 40 mg tapered by 5 mg/week until dose is zero after 56 days (100% patients) | 5 mg (28 tablets) = £1.03 | £9.27 |
Other ulcerative colitis health state resource use
Health state costs relating to the use of elective and emergency endoscopy, hospitalisations, consultant visits and blood tests were taken from the previous economic analysis reported by Tsai et al. 82 (Table 74). As Tsai et al. 82 did not report any uncertainty around these resource-use estimates, the standard error was arbitrarily assumed to be equal to 10% of the mean.
| Resource item | Remission | Response | No response | Post-surgery remission | Post-surgery complications |
|---|---|---|---|---|---|
| Consultant visit | 2.00 | 4.50 | 6.50 | 1.50 | 1.75 |
| Hospitalisation episode | 0.30 | 0.30 | 0.30 | 0.00 | 3.25 |
| Blood tests | 3.25 | 3.90 | 6.50 | 1.50 | 3.25 |
| Elective endoscopy | 0.20 | 0.50 | 2.00 | 1.25 | 0.65 |
| Emergency endoscopy | 0.00 | 0.25 | 0.75 | 0.50 | 0.13 |
The MSD submissions to NICE included a meta-analysis in which relative risks were derived for hospitalisations for 160 mg/80 mg/40 mg of ADA and 5 mg/kg of IFX versus conventional treatment. 64,66 The MSD NMA did not include estimates of the relative risk of hospitalisation for GOL versus conventional treatment as this was not measured in the PURSUIT-Maintenance trial. The relative risk for GOL was assumed to be the same as that for ADA (the least favourable option); this assumption favours GOL compared with the other options included in the economic analysis. The relative risks used in the model are shown in Table 75.
Unit costs for ulcerative colitis health state resource use, surgery and complications
The unit costs for UC health state resource use, surgery and complications were taken from NHS Reference Costs130 and are shown in Table 76.
| Item | Unit cost (£) | Standard error (£) | Source |
|---|---|---|---|
| Consultant visit | 123.43 | 3.30 | NHS Reference Costs 2013,130 WF01 A, Gastroenterology, Non-Admitted Face to Face Attendance, Follow-up |
| Hospitalisation | 2722.96 | 101.66 | NHS Reference Costs 2013,130 FZ37 N, Gastroenterology, Inflammatory Bowel Disease, with Single Intervention, with CC score 0–3 |
| Elective endoscopy | 462.36 | 14.96 | NHS Reference Costs 2013,130 FZ51Z, Gastroenterology, Diagnostic Colonoscopy, 19 years and over |
| Emergency endoscopy | 512.62 | 26.20 | NHS Reference Costs 2013,130 FZ51Z, Gastroenterology, Diagnostic Colonoscopy, 19 years and over |
| Blood tests | 1.94 | 0.26 | NHS Reference Costs 2013,130 DAPS03, Integrated Blood Services |
| Colectomy | 13,451.60 | 1345.16 | Buchanan et al.143 |
| Stoma care per maintenance cycle | 214.95 | 21.49 | Buchanan et al.143 |
| Surgical management of complications | 8792.85 | 473.03 | NHS Reference Costs 2013,130 FZ73F, Colorectal Surgery, Very Complex Large Intestine Procedures with CC Score 0–2 |
| Medical management of complicationsa | 4170.95 | 464.59 | NHS Reference Costs 2013,130 WA12D, Colorectal Surgery, Complications of Procedures, with CC score 0 |
Methods for model evaluation
The base-case analysis relates to use of IFX, ADA and GOL within an adult population, based on the expectation of the mean. The cost-effectiveness of competing options are evaluated within a fully incremental analysis according to standard cost-effectiveness decision rules. 77 Results of the probabilistic analyses are presented separately for patients for whom colectomy is a potential option and those for whom it is not. Decision uncertainty is represented using cost-effectiveness planes and CEACs.
A secondary analysis is presented for the paediatric population; this analysis compares IFX with standard non-biological treatments versus colectomy. Given the absence of head-to-head trials of IFX versus any other therapy, this analysis is exactly the same as the base-case analysis except that the patients’ starting age is set to 15 years (the median age within Hyams et al. 52).
In addition to the main analyses, a number of secondary scenario analyses and sensitivity analyses are presented (Box 5). It should be noted that although the base-case economic analysis utilises the results of the NMA models, sensitivity analysis number 4 presents pairwise estimates of cost-effectiveness using direct head-to-head transition probabilities sourced from the individual clinical trials of IFX, ADA and GOL. The pairwise analysis of IFX uses simple pooling of the ACT1 and ACT2 trial data. The pairwise analysis of ADA is based on the anti-TNF-α-naive subgroup from ULTRA2. The GOL analysis is based on the data from the PURSUIT-SC47 trial and the PURSUIT-Maintenance48 trial.
Unless otherwise stated, all results are discounted at a rate of 3.5%.
-
Base-case analysis: NMA using anti-TNF-α-naive subgroup from ULTRA245,126 and excluding Suzuki et al. 46 (probabilistic).
-
Sensitivity analysis 1: NMA using ITT population from ULTRA245 and excluding Suzuki et al. 46 (probabilistic).
-
Sensitivity analysis 2: NMA using anti-TNF-α-naive subgroup from ULTRA245,126 and including Suzuki et al. 46 (probabilistic).
-
Sensitivity analysis 3: NMA using ITT population from ULTRA245,126 and including Suzuki et al. 46 (probabilistic).
-
Sensitivity analysis 4: pairwise head-to-head comparisons of IFX, GOL and ADA vs. conventional management using direct trial evidence from ACT1 and ACT2, ULTRA2 and PURSUIT45,47,49,126 (deterministic).
-
Sensitivity analysis 5: base case using point estimates of parameters (deterministic).
-
Sensitivity analysis 6: time horizon = 20 years (deterministic).
-
Sensitivity analysis 7: time horizon = 10 years (deterministic).
-
Sensitivity analysis 8: time horizon = 5 years (deterministic).
-
Sensitivity analysis 9: all utilities except post-surgical complications drawn from Swinburn et al. 139 (deterministic).
-
Sensitivity analysis 10: utilities of response/remission drawn from ACT1 trial49 (deterministic).
-
Sensitivity analysis 11: utilities of response/remission drawn from PURSUIT-Maintenance trial47 (deterministic).
-
Sensitivity analysis 12: relative risk of hospitalisation for GOL vs. conventional treatment assumed to be 1.0 (deterministic).
-
Sensitivity analysis 13: UC health state costs doubled (deterministic).
-
Sensitivity analysis 14: UC health state costs halved (deterministic).
-
Sensitivity analysis 15: probability of chronic pouchitis doubled (deterministic).
-
Sensitivity analysis 16: probability of chronic pouchitis halved (deterministic).
-
Sensitivity analysis 17: cost of surgery doubled (deterministic).
-
Sensitivity analysis 18: cost of surgery halved (deterministic).
-
Sensitivity analysis 19: probability of surgery in drug groups halved (deterministic).
-
Sensitivity analysis 20: probability of surgery in drug groups based on Gower-Rousseau et al. 151 (deterministic).
-
Sensitivity analysis 21: disutility of pouchitis doubled (deterministic).
-
Sensitivity analysis 22: disutility of pouchitis tripled (deterministic).
Model verification and validation
The Assessment Group undertook a number of measures to ensure the credibility of the model (author/advisor initials are shown in brackets).
-
Peer review of economic analysis by two internal clinical advisors (SH, AL), one external clinical expert (MH) and one external methodological expert (SD).
-
Verification of executable model by a second modeller not involved in its implementation (HB).
-
Double programming of separate Markov models for all five treatment options by the lead modeller (PT).
-
Scrutiny of implemented model coding and formulae by lead modeller (PT).
-
Double-checking of accuracy of all model inputs against sources.
-
Comparison of model results using point estimates of parameters and expectation of the mean.
-
Comparison of mean of all parameter samples against point estimates of parameters.
-
Examination of all identified sources of discrepancy.
-
Model testing using sensitivity analysis and use of extreme parameter values.
-
Comparison of model results against manufacturers’ models (see Systematic review of existing cost-effectiveness evidence).
Assessment Group model results
Central estimates of cost-effectiveness (base-case analysis: adults)
Table 77 presents the base-case results generated using the probabilistic version of the model within an adult population in whom colectomy is an acceptable option. The base-case analysis of the model suggests that colectomy is expected to produce 14.71 QALYs at a cost of approximately £56,268 over the patient’s remaining lifetime. All medical options are expected to produce substantially fewer QALYs at a greater cost than colectomy, hence colectomy is expected to dominate IFX, ADA, GOL and conventional non-biological treatments.
| Option | QALYs | Costs (£) | Incremental QALYs | Incremental cost | ICER |
|---|---|---|---|---|---|
| Colectomy | 14.71 | 56,267.73 | – | – | Dominating |
| ADA | 10.82 | 91,221.71 | – | – | Dominated |
| IFX | 10.81 | 96,594.62 | – | – | Dominated |
| GOL | 10.63 | 90,086.69 | – | – | Dominated |
| Conventional treatment | 10.47 | 73,619.77 | – | – | Dominated |
Figure 89 presents CEACs for IFX, ADA, GOL, conventional treatment and colectomy for the adult population. Assuming a willingness-to-pay threshold of £20,000 per QALY gained, the probability that colectomy produces the greatest amount of expected net benefit is approximately 1.0. The probability that any of the biological treatments produce the greatest amount of expected net benefit at this threshold is approximately zero. Assuming a willingness-to-pay threshold of £30,000 per QALY gained, the probability that colectomy produces the greatest amount of expected net benefit is approximately 1.0. The probability that any of the biological treatments produce the greatest amount of expected net benefit at this threshold is approximately zero.
FIGURE 89.
Cost-effectiveness acceptability curves, base-case analysis, adult patients in whom colectomy is an option (medical and surgical treatments).
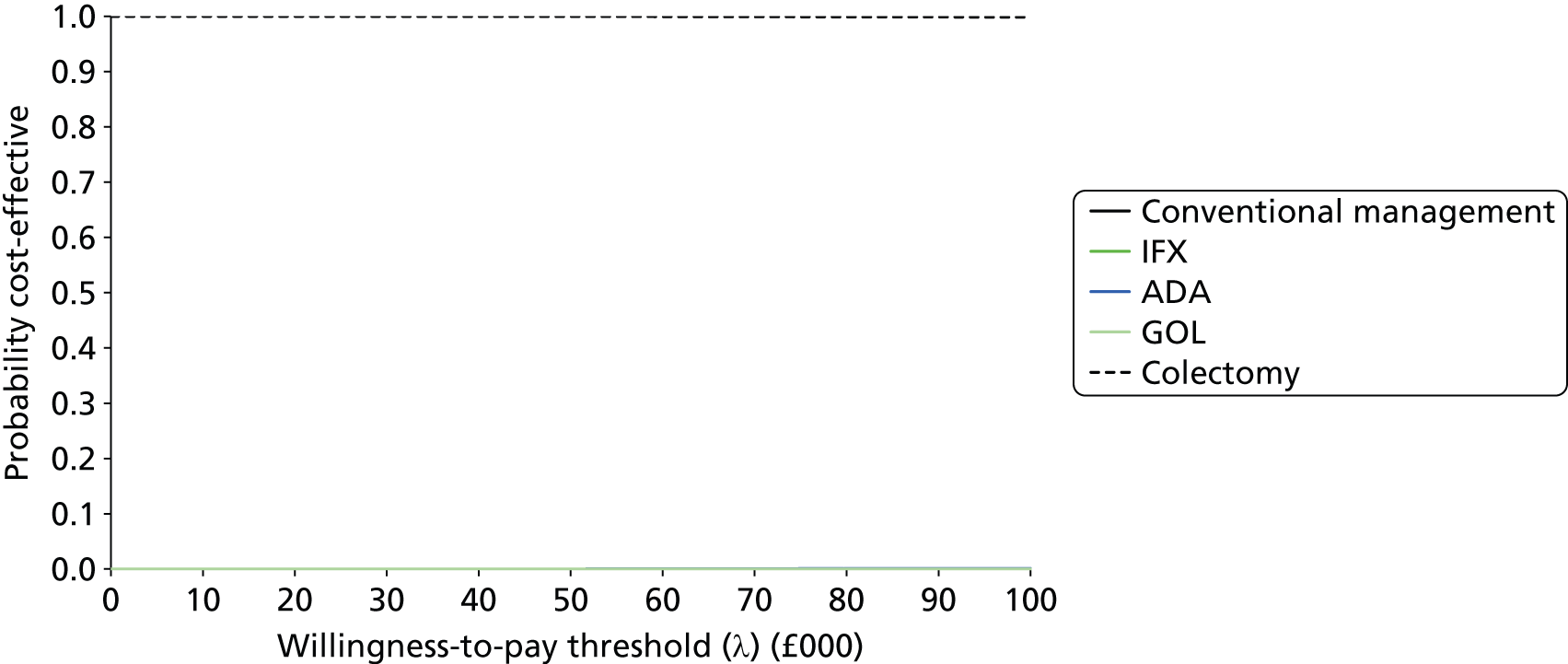
Table 78 presents the probabilistic base-case model results within an adult population in whom colectomy is not an acceptable option, thus relevant treatment options are restricted to medical treatments only (IFX, ADA, GOL and conventional non-biological treatments). The model results suggest that within this population, IFX is expected to be dominated by ADA (note: the difference in QALYs is very small), while GOL is expected to be ruled out because of extended dominance. The incremental cost-effectiveness of ADA versus conventional treatment is expected to be £50,278 per QALY gained.
| Option | QALYs | Costs (£) | Incremental QALYs | Incremental cost (£) | ICER |
|---|---|---|---|---|---|
| ADA | 10.82 | 91,221.71 | 0.35 | 17,601.94 | £50,278 |
| IFX | 10.81 | 96,594.62 | – | – | Dominated |
| GOL | 10.63 | 90,086.69 | – | – | Ext dom |
| Conventional treatment | 10.47 | 73,619.77 | – | – | – |
Figure 90 presents CEACs for IFX, ADA, GOL and conventional treatment within a population in whom colectomy is not an option. Assuming a willingness-to-pay threshold of £20,000 per QALY gained, the probability that conventional non-biological treatment produces the greatest expected net benefit is approximately 1.0. Assuming a WTP threshold of £30,000 per QALY gained, the probability that conventional management produces the greatest expected net benefit is approximately 0.98.
FIGURE 90.
Cost-effectiveness acceptability curves, base-case analysis, adult patients in whom colectomy is not an option (medical treatments only).
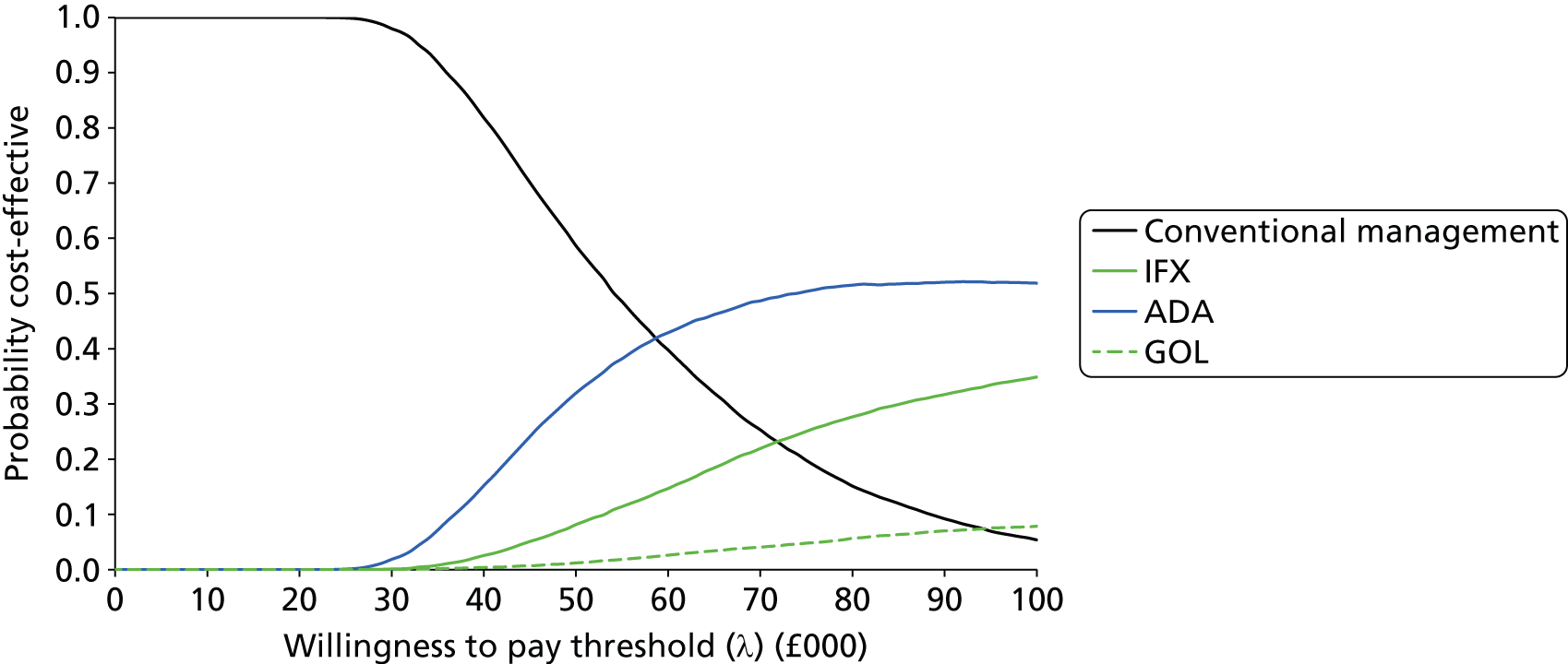
Central estimates of cost-effectiveness (base-case analysis: paediatric population)
Table 79 presents the base-case results generated using the probabilistic version of the model within a paediatric population in whom colectomy is an acceptable option. The base-case analysis of the model suggests that colectomy is expected to produce 17.54 QALYs at a cost of approximately £64,097 over the patient’s remaining lifetime. IFX and conventional management are expected to produce substantially fewer QALYs at a greater cost than colectomy, hence colectomy is expected to dominate these medical options.
| Option | QALYs | Costs (£) | Incremental QALYs | Incremental cost (£) | ICER |
|---|---|---|---|---|---|
| Colectomy | 17.54 | 64,097.18 | – | – | Dominating |
| IFX | 13.00 | 109,438.73 | – | – | Dominated |
| Conventional treatment | 12.65 | 86,280.28 | – | – | Dominated |
Figure 91 presents CEACs for IFX, conventional treatment and colectomy for the paediatric population. Assuming a willingness-to-pay threshold of £20,000 per QALY gained, the probability that colectomy produces the greatest amount of expected net benefit is approximately 1.0. Assuming a willingness-to-pay threshold of £30,000 per QALY gained, the probability that colectomy produces the greatest amount of expected net benefit is approximately 1.0. The probability that IFX produces the greatest amount of expected net benefit at these thresholds is approximately zero.
FIGURE 91.
Cost-effectiveness acceptability curves, base-case analysis, paediatric patients in whom colectomy is an option (medical and surgical treatments).

Table 80 presents the probabilistic base-case model results within a paediatric population in whom colectomy is not an acceptable option, thus relevant treatment options are restricted to IFX and conventional non-biological treatments. The model indicates that within this population, IFX is expected to produce an additional 0.34 QALYs at an additional cost of £23,158 over the patient’s remaining lifetime; the ICER for IFX versus conventional management is expected to be £68,007 per QALY gained.
| Option | QALYs | Costs (£) | Incremental QALYs | Incremental cost (£) | ICER (£) |
|---|---|---|---|---|---|
| IFX | 13.00 | 109,438.73 | 0.34 | £23,158.45 | 68,007 |
| Conventional treatment | 12.65 | 86,280.28 | – | – | – |
Figure 92 presents CEACs for IFX and conventional treatment for the paediatric population. Assuming willingness-to-pay thresholds of £20,000 and £30,000 per QALY gained, the probability that conventional management produces the greatest amount of expected net benefit is approximately 1.0. The probability that IFX produces the greatest amount of expected net benefit at these thresholds is approximately zero.
FIGURE 92.
Cost-effectiveness acceptability curves, base-case analysis, paediatric patients in whom colectomy is not an option (medical options only).

Network meta-analysis sensitivity analyses (sensitivity analyses 1–3, probabilistic)
Table 81 summarises the results of the economic analysis for the adult population based on the three alternative sensitivity analyses.
| Colectomy | IFX | ADA | GOL | Conventional management | |
|---|---|---|---|---|---|
| Adult population in whom colectomy is an option | |||||
| NMA sensitivity analysis number 1: ULTRA2 ITT population, excluding Suzuki et al.46 | |||||
| ICER | Dominating | Dominated | Dominated | Dominated | Dominated |
| P(optimal £20,000/QALY) | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| P(optimal £30,000/QALY) | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| NMA sensitivity analysis number 2: ULTRA2 anti-TNF-α-naive subgroup, including Suzuki et al.46 | |||||
| ICER | Dominated | Dominated | Dominated | Dominated | Dominated |
| P(optimal £20,000/QALY) | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| P(optimal £30,000/QALY) | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| NMA sensitivity analysis number 3: ULTRA2 ITT population, including Suzuki et al.46 | |||||
| ICER | Dominated | Dominated | Dominated | Dominated | Dominated |
| P(optimal £20,000/QALY) | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| P(optimal £30,000/QALY) | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Adult population in whom colectomy is an not option | |||||
| NMA sensitivity analysis number 1: ULTRA2 ITT population, excluding Suzuki et al.46 | |||||
| ICER | N/A | £236,340 (1) | £54,066 (2) | Ext dom(3) | –(4) |
| P(optimal £20,000/QALY) | N/A | 0.00 | 0.00 | 0.00 | 1.00 |
| P(optimal £30,000/QALY) | N/A | 0.00 | 0.01 | 0.00 | 0.99 |
| NMA sensitivity analysis number 2: ULTRA2 anti-TNF-α-naive subgroup, including Suzuki et al.46 | |||||
| ICER | N/A | £546,051 (1) | £56,284 (2) | Ext dom(3) | –(4) |
| P(optimal £20,000/QALY) | N/A | 0.00 | 0.00 | 0.00 | 1.00 |
| P(optimal £30,000/QALY) | N/A | 0.00 | 0.00 | 0.00 | 1.00 |
| NMA sensitivity analysis number 3: ULTRA2 ITT population, including Suzuki et al.46 | |||||
| ICER | N/A | Dominated (2) | £55,637 (1) | Ext dom(3) | –(4) |
| P(optimal £20,000/QALY) | N/A | 0.00 | 0.00 | 0.00 | 1.00 |
| P(optimal £30,000/QALY) | N/A | 0.00 | 0.00 | 0.00 | 1.00 |
In the circumstances for which colectomy is an option, the three NMA sensitivity analyses produce very similar results to the base-case analysis. In all three analyses, colectomy is consistently expected to dominate all medical treatment options. Assuming a WTP threshold of £20,000 per QALY gained, the probability that colectomy produces the greatest amount of net benefit is expected to be approximately 1.0. Assuming a WTP threshold of £30,000 per QALY gained, the probability that colectomy produces the greatest amount of net benefit is expected to be approximately 1.0. When colectomy is not an acceptable option, the results are influenced by which studies are included in the NMA, as the difference in effectiveness between ADA and IFX is very small. GOL is consistently expected to be ruled out of the analysis because of extended dominance. In sensitivity analyses 1 and 2, IFX is expected to produce slightly more QALYs than ADA; however, the ICER for IFX versus ADA is expected to be > £236,000 per QALY gained. In these sensitivity analyses, the ICER for ADA versus conventional non-biological treatment is expected to be > £54,000 per QALY gained. In sensitivity analysis 3, IFX is expected to be ruled out owing to simple dominance; the ICER for ADA versus conventional non-biological treatments is expected to be approximately £55,637 per QALY gained.
Head-to-head analyses (sensitivity analysis 4)
Table 82 presents the results of the economic analysis using the direct head-to-head trial data.
| Option | QALYs | Costs (£) | Incremental QALYs | Incremental cost (£) | ICER |
|---|---|---|---|---|---|
| IFX versus conventional management and colectomy | |||||
| Colectomy | 14.69 | 56,342.42 | – | – | Dominating |
| IFX | 11.62 | 86,609.58 | – | – | Dominated |
| Conventional treatment | 11.44 | 69,583.06 | – | – | Dominated |
| ADA versus conventional management and colectomy | |||||
| Colectomy | 14.69 | 56,342.42 | – | – | Dominating |
| ADA | 10.23 | 90,538.92 | – | – | Dominated |
| Conventional treatment | 10.02 | 75,416.12 | – | – | Dominated |
| GOL versus conventional management and colectomy | |||||
| Colectomy | 14.69 | 56,342.42 | – | – | Dominating |
| GOL | 10.16 | 91,395.09 | – | – | Dominated |
| Conventional treatment | 9.99 | 75,541.49 | – | – | Dominated |
The head-to-head analyses indicate that colectomy is expected to dominate all medical options. Within a population in whom colectomy is not an acceptable option, the incremental cost-effectiveness of IFX versus conventional non-biological treatments is estimated to be £96,403 per QALY gained, the ICER for ADA versus conventional non-biological treatments is estimated to be £69,782 per QALY gained and the ICER for GOL versus conventional non-biological treatments is estimated to be £90,413 per QALY gained.
Sensitivity analyses 5–22: other deterministic sensitivity analyses (medical and surgical options)
Table 83 presents the results of the remaining deterministic sensitivity analysis (analyses 5–22).
| Sensitivity analysis | Incremental cost per QALY gained | ||||
|---|---|---|---|---|---|
| IFX | ADA | GOL | Conventional management | Colectomy | |
| SA5: base case using point estimates of parameters | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA6: time horizon = 20 years | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA7: time horizon = 10 years | Dominated | Dominated | Dominated | – | £886.44 |
| SA8: time horizon = 5 years | Dominated | Dominated | Dominated | – | £8840.14 |
| aSA9: all utilities except post-surgical complications drawn from Swinburn et al.139 | £178,982 (1) | £79,714 (2) | Dominated (3) | Ext dom (4) | – |
| SA10: utilities of response/remission drawn from ACT1 trial49 (0.88, 0.82 for remission and response respectively) | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA11: utilities of response/remission drawn from PURSUIT-Maintenance trial47 (0.89, 0.80 for remission and response respectively) | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA12: relative risk of hospitalisation for GOL vs. conventional treatment assumed to be 1.0 | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA13: UC health state resource use doubled | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA14: UC health state resource use halved | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA15: probability of chronic pouchitis doubled | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA16: probability of chronic pouchitis halved | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA17: cost of surgery doubled | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA18: cost of surgery halved | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA19: probability of undergoing surgery in drug groups halved | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA20: colectomy rate based on Gower-Rousseau et al.151 | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA21: disutility of pouchitis doubled | Dominated | Dominated | Dominated | Dominated | Dominating |
| SA22: disutility of pouchitis tripled | Dominated | Dominated | Dominated | Dominated | Dominating |
The analyses indicate that the model results remain largely unaffected by changes in the model time horizon, assumed patient age, utilities for remission and response, assumptions regarding UC resource use, and the colectomy rate. However, the model is very sensitive to assumptions regarding the relative utilities of remission, response, active UC and postsurgery. Within the sensitivity analysis in which utility values are drawn from Swinburn et al. 139 (analysis number 9), the rank ordering of QALY gains is altered such that colectomy moves from being the most effective option to the least effective option. In this scenario, GOL and conventional non-biological treatments are expected to be ruled out of the analysis, the ICER for ADA versus colectomy is estimated to be approximately £79,714 per QALY gained while the ICER for IFX versus ADA is estimated to be approximately £178,982 per QALY gained.
Budget impact analysis
This section presents an analysis of the expected net budget impact of introducing IFX, ADA and GOL for the treatment of moderate to severe UC in England and Wales. Budget impact estimates are presented annually for a 5-year period. The analysis makes the following assumptions:
-
The prevalence of UC is 240 per 100,000 population.
-
The incidence of UC is 10 per 100,000 population.
-
The population of England and Wales is approximately 56 million.
-
A total of 14.5% of all UC patients would be eligible to receive biological treatments. 80
-
All patients who are eligible for treatment with biologicals will receive these therapies.
-
Discounting is not applied.
These assumptions suggest an eligible prevalent UC cohort of approximately 19,488 patients and an eligible incident cohort of approximately 812 patients per year. Based on the cost distribution over time estimated for each treatment using the Assessment Group model, combined with the estimated eligible prevalent and incident cohorts in each year, this gives rise to the budget impact estimates presented in Table 84. Assuming full uptake of these drugs, the estimated net budget impact of IFX, ADA and GOL for the treatment of moderate to severe UC is estimated to be between £269M and £434M.
| Year | IFX | ADA | GOL | Conventional non-biological treatments |
|---|---|---|---|---|
| Absolute budget impact for each treatment | ||||
| Year 1 | £320,465,072 | £212,829,031 | £274,600,660 | £75,051,491 |
| Year 2 | £145,622,769 | £114,284,053 | £130,417,738 | £69,748,947 |
| Year 3 | £124,230,659 | £108,603,021 | £109,713,744 | £74,396,609 |
| Year 4 | £113,140,687 | £105,994,793 | £101,177,679 | £77,850,322 |
| Year 5 | £107,896,081 | £104,691,559 | £98,410,165 | £80,753,619 |
| Net budget impact for costs of biological less costs of conventional treatments | ||||
| Year 1 | £245,413,582 | £137,777,540 | £199,549,169 | – |
| Year 2 | £75,873,822 | £44,535,106 | £60,668,791 | – |
| Year 3 | £49,834,050 | £34,206,412 | £35,317,135 | – |
| Year 4 | £35,290,365 | £28,144,472 | £23,327,357 | – |
| Year 5 | £27,142,461 | £23,937,940 | £17,656,546 | – |
| Total cost over 5 years | £433,554,281 | £268,601,470 | £336,518,999 | – |
Discussion
The manufacturers of ADA, IFX and GOL submitted economic models to assess the cost-effectiveness of biological therapies versus conventional treatment. The MSD IFX submission model indicates that the estimated ICER for IFX versus standard non-biological treatment (colectomy) is £37,682 per QALY gained. 64 The MSD GOL submission reports an estimated ICER of £27,322 per QALY gained. 66 The AbbVie submission reports a base-case ICER for ADA versus conventional therapy of £34,590 per QALY gained. 62 The Assessment Group scrutinised these models and critiqued the evidence and assumptions which underpin the cost-effectiveness estimates reported by the manufacturers. A number of problems with these models were identified by the Assessment Group, particularly with respect to the exclusion of relevant treatment options specified in the final NICE scope36 and the use of a short time horizon. In addition, the MSD model does not include a fully incremental analysis, confuses evidence from populations with varying degrees of UC severity and inadequately reflects likely UK treatment pathways. As a consequence of these problems, the Assessment Group do not consider that the cost-effectiveness evidence produced by either manufacturer adequately addresses the specified decision problem.
In light of the problems with the manufacturers’ submitted economic analyses, the Assessment Group developed a de novo cost-effectiveness model to assess IFX, ADA, GOL, conventional non-biological treatments and elective surgery within the moderate to severe UC population. The Assessment Group model differs from both the manufacturers’ models in that all relevant medical and surgical options are evaluated over a lifetime horizon, as specified in the final NICE scope. Underpinning the Assessment Group model is a series of complex NMAs, which synthesise all relevant evidence relating to IFX, ADA, GOL and conventional non-biological therapies. A summary of the key differences between the Assessment Group model and the manufacturers’ models is presented in Table 85.
| Element of evaluation | Assessment Group model (base case) | MSD models64,66 | AbbVie model62 |
|---|---|---|---|
| Options evaluated |
|
|
|
| Time horizon | Lifetime | 10 years | 10 years |
| Source of efficacy evidence | NMA using unpublished ordered categorical data | NMA using published binomial data (includes manipulation of PURSUIT-Maintenance trial data) | Unpublished data from ULTRA2 and ULTRA1 and ULTRA2 extension study supplemented using estimates from Kane et al.133 and Odes et al.121 |
| Treatment options following failure of biologic | Conventional non-biological treatments and possible colectomy | Relapse management and imminent colectomy | Conventional non-biological treatments and possible colectomy |
| Possible transitions between active UC states (remission, response, no response) | All transitions in matrix allowed | Patients losing remission transit to response, patients achieving response cannot achieve remission | All transitions in matrix allowed |
| Source of health utilities | Woehl et al.145 (chronic pouchitis valued using Arseneau et al.110) | ACT1/PURSUIT-Maintenance, Woehl et al.,145 complications valued using Arseneau et al.110 | Swinburn et al.,139 Tsai et al.,82 complications valued using weighted average of Arseneau et al.,110 Hu et al.140 and Smith and Roberts141 |
The base-case analysis of the Assessment Group model suggests that within an adult UC population, colectomy is expected to produce 14.71 discounted QALYs at a discounted cost of approximately £56,300 over the patient’s remaining lifetime. All medical options are expected to produce substantially fewer QALYs at a greater cost than colectomy, hence colectomy is expected to dominate IFX, ADA, GOL and conventional non-biological treatments. Importantly however, elective colectomy may not be considered an acceptable or preferable option for some patients. In circumstances whereby only drug options are considered acceptable, the base-case version of the Assessment Group model suggests that IFX and GOL are expected to be ruled out because of dominance, while the incremental cost-effectiveness of ADA versus conventional non-biological treatment is expected to be approximately £50,300 per QALY gained.
The Assessment Group also undertook a separate probabilistic economic analysis of IFX, conventional non-biological treatments and colectomy within a paediatric population (mean age = 15 years). When colectomy is an acceptable treatment option, the economic analysis suggests that this option dominates IFX and conventional non-biological treatments. When colectomy is not an acceptable option, the economic analysis suggests that the incremental cost-effectiveness of IFX versus conventional treatments is approximately £68,000 per QALY gained. However, this analysis is based on adult efficacy evidence, hence it should be interpreted with some degree of caution.
Three separate PSAs were undertaken using data from the Japanese trial reported by Suzuki et al. 46 and using the ITT data rather than anti-TNF-α-naive patients from ULTRA2. 45 Across these three scenarios, the general conclusions of the economic analysis remain unchanged. The Assessment Group also undertook separate comparisons of (1) IFX versus colectomy and conventional treatments; (2) ADA versus colectomy and conventional treatments; and (3) GOL versus colectomy and conventional treatments using the head-to-head trials rather than the NMA models. These analyses indicate that where colectomy is an acceptable option, it is expected to dominate the drug options. When colectomy is not an acceptable option, the ICERs produced from these analyses are all in excess of £69,000 per QALY gained.
A number of simple sensitivity analyses were also undertaken using the point estimates of model parameters. Across these scenarios, the model results appear to be insensitive to changes in these assumptions, with the exception of the HRQoL values assumed. Within the scenario whereby utilities from Swinburn et al. 139 are used in the model (rather than those reported by Woehl et al. 145 as per the base-case analysis), colectomy produces the lowest QALY gain and conventional management and GOL are ruled out because of extended dominance. Within this scenario, the incremental cost-effectiveness of ADA versus elective colectomy is estimated to be £79,714 per QALY gained, while the incremental cost-effectiveness of IFX versus ADA is estimated to be £178,982 per QALY gained. Although these results are very different from the Assessment Group’s base-case analysis, the economic conclusions that should be drawn from this sensitivity analysis are not.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Surgery and patient choice
Surgery may be considered as an option for patients with UC for a number of reasons including complications of disease, perceived risk of or identified dysplasia/neoplasia or lack or loss of efficacy of medical treatments. For a proportion of patients without emergency symptoms, surgery may not represent an acceptable treatment option.
Administration route: intravenous infusions versus subcutaneous injection
The method of drug administration differs between the interventions included in this appraisal. IFX is given via i.v. infusion while ADA and GOL are administered via subcutaneous injection. IFX therefore requires outpatient attendances, additional nursing care and monitoring. These resources are not required for the administration of ADA or GOL. Pre-infusion prophylaxis may be required to minimise the risk of infusion reactions associated with IFX.
Training for subcutaneous injections
When considered appropriate (by the physician), patients, family members and/or carers require training for the administration of subcutaneous injections. This training is associated with additional resource use and costs.
Screening for tuberculosis and other infectious diseases (e.g. hepatitis B)
All of the biological therapies considered in this assessment report may be associated with the reactivation of TB. Patients should be screened for TB (and other infectious disease, e.g. hepatitis B) before initiation of treatment.
Off-license use in children for golimumab and adalimumab
Currently, IFX is the only biological treatment option that is licensed for the treatment of moderate to severe UC in children in the UK.
Chapter 6 Discussion
Statement of principal findings
Principal findings: clinical effectiveness
A total of 10 RCTs were identified in the clinical effectiveness systematic review, of which nine44–51 related to adults and one52 was conducted in a paediatric population. All of the adult RCTs were performed against PBO (with the exception of UC-SUCCESS) and were a maximum of 1 year in duration. No head-to-head RCTs were identified in which interventions of interest were assessed against each other.
The risks of bias associated with included RCTs were assessed using the Cochrane risk of bias instrument. Only three RCTs could be considered as being at overall low risk of bias (as allocation concealment, blinded outcome assessment and completeness of outcome data were all judged as low risk). It should be noted that one of the maintenance trials (PURSUIT- Maintenance) rerandomised patients who had previously responded to GOL induction therapy in two previous trials; the extent of this potential bias on patient outcomes is unclear.
The outcome measures pre-specified in the final NICE scope were all addressed by the included trial evidence, with the exception of rates of relapse. Clinical response and remission data based on complete Mayo scores were well reported across trials. Evidence was identified to demonstrate that patients receiving IFX, ADA or GOL were more likely than patients receiving PBO to achieve clinical response and remission at induction and maintenance time points. Patients in the UC-SUCCESS trial who received combination treatment with IFX and AZA experienced the most favourable rates of steroid-free remission compared with IFX and AZA treatment groups. Seven RCTs performed in adult populations contributed data on clinical response and remission at induction or maintenance time points to NMAs. 44–49
Based on the NMA, in the induction phase, all treatments were associated with statistically significant beneficial effects relative to PBO with the greatest effect being associated with IFX. IFX was also associated with the highest probability of moving from no response to response and from no response to remission. The effects of ADA and GOL on these two probabilities were broadly comparable.
For patients classified as responders at the end of the induction phase, treatment effects were not statistically significant, although the greatest effect was associated with 100 mg of GOL at 8–32 weeks. A treatment of 100 mg of GOL was associated with the highest probability of moving from response to remission and staying in response and the smallest probability of moving from response to no response. However, at 32–52 weeks, only IFX and 50 mg of GOL were associated with beneficial effects on clinical response, although the effects were not statistically significant. IFX was associated with the highest probability of moving from response to remission and staying in response and the smallest probability of moving from response to no response at 32–52 weeks. The probabilities of staying in response were comparable among treatments at both 8–32 weeks and 32–52 weeks.
For patients classified as being in remission at the end of the induction phase, all treatments except for ADA were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with 50 mg and 100 mg of GOL, although the effects were not statistically significant at 8–32 weeks. A treatment of 50 mg and 100 mg of GOL were associated with the highest probability of staying in remission and the smallest probability of moving from remission to response and from remission to no response. At 32–52 weeks, all treatments except 50 mg of GOL were associated with beneficial treatment effects relative to PBO, with the greatest effect being associated with ADA (the only treatment with statistically significant effect). ADA was associated with the highest probability of staying in remission and the smallest probability of moving from remission to response and from remission to no response.
Available data on hospitalisation outcomes were very limited, but suggested that outcomes may be more favourable for ADA-treated and IFX-treated patients than PBO (with no data available from GOL trials). Data on surgical intervention were also very sparse, with a potential inconclusive benefit for intervention-treated patients compared with PBO. No trials reported whether surgical outcomes were elective or emergency in nature; however, more data are required to demonstrate the impact of interventions on hospitalisation and surgical intervention more conclusively. Data were available from a single trial to support the use of IFX in induction and maintenance treatment in a paediatric population.
The main safety issues highlighted in the RCT evidence appeared to be generally consistent with those previously discussed in the respective SmPC (including serious infections, malignancies and administration site reactions). Deaths occurring during and after the study period were described in some trials evaluating GOL (PURSUIT-Maintenance) and IFX (ACT1 and ACT2), of which infection or malignancy was most commonly implicated. This underlines the importance of monitoring and treating serious infections and malignancies in patients receiving immunosuppressive treatment.
The trials included in the clinical effectiveness systematic review typically excluded patients with ulcerative proctitis, patients with fulminant/acute severe disease, those with a history of or at imminent risk of bowel surgery, pregnant or lactating women, and patients with diseases of the central nervous system (e.g. demyelinating disease). Furthermore, patients with a history of serious infection and/or immunodeficiency were also typically excluded, as were individuals with a history of malignancy or signs of dysplasia. Therefore, the effects of ADA, GOL or IFX in these UC populations are unknown.
Two biosimilars (Remsima and Inflectra) to Remicade were considered as part of the evidence base for IFX as part of this assessment. The sponsor submission received from the manufacturers of Remsima and the EPAR reports for Remsima and Inflectra indicated that both biosimilars were approved by the EMA on the basis of reported similar pharmacokinetic and efficacy (demonstrated in AS and RA patients) profiles to Remicade. No further trials of Remsima or Inflectra were identified over the course of this assessment.
Principal findings: cost-effectiveness
The manufacturers of ADA, IFX and GOL submitted economic models to assess the cost-effectiveness of biological therapies versus conventional treatment. The MSD IFX submission model indicates that the estimated ICER for IFX versus standard non-biological treatment (colectomy) is £37,682 per QALY gained. 64 The MSD GOL submission reports an estimated ICER of £27,322 per QALY gained. 66 The AbbVie submission reports a base-case ICER for ADA versus conventional therapy of £34,590 per QALY gained. 62
The Assessment Group identified several issues with the manufacturers’ submitted models, in particular, the exclusion of relevant treatment options specified in the final NICE scope36 and the use of a short time horizon. Given the missing comparators within each of the manufacturers’ submitted economic analyses, it is unclear how these models should be used to inform NICE decision-making.
The Assessment Group developed a de novo cost-effectiveness model to assess IFX, ADA, GOL, conventional non-biological treatments and elective surgery within the moderate to severe UC population. The base-case analysis of the Assessment Group model suggests that within an adult UC population, colectomy is expected to produce 14.71 discounted QALYs at a discounted cost of approximately £56,300 over the patient’s remaining lifetime. All medical options are expected to produce substantially fewer QALYs at a greater cost than colectomy, hence colectomy is expected to dominate IFX, ADA, GOL and conventional non-biological treatments. When colectomy is not considered to be an acceptable option, the base-case analysis of the Assessment Group model suggests that IFX and GOL are expected to be ruled out because of dominance, while the incremental cost-effectiveness of ADA versus conventional non-biological treatment is expected to be approximately £50,300 per QALY gained.
The Assessment Group also undertook a separate probabilistic economic analysis of IFX, conventional non-biological treatments and colectomy within a paediatric population (mean age = 15 years). When colectomy is an acceptable treatment option, the economic analysis suggests that this option dominates IFX and conventional non-biological treatments. When colectomy is not an acceptable option, the economic analysis suggests that the incremental cost-effectiveness of IFX versus conventional treatments is approximately £68,000 per QALY gained.
The results of the Assessment Group model were largely insensitive to changes in model parameter values with the exception of the HRQoL values for each state. The use of utility estimates from Swinburn et al. 139 results in a situation whereby colectomy produces the lowest QALY gain and conventional management and GOL are ruled out because of extended dominance. Within this scenario, the incremental cost-effectiveness of ADA versus elective colectomy is estimated to be £79,714 per QALY gained, while the incremental cost-effectiveness of IFX versus ADA is estimated to be £178,982 per QALY gained.
Strengths and limitations of the assessment
The systematic review was based on rigorous methods, with comprehensive searches for evidence, a good level of consistency between reviewers in study selection and double-checking of data extraction. Clinical response and remission data were widely reported across included trials and study authors were consistent in their use of the complete Mayo score, which aided the comparison of trials. Although no head-to-head RCT evidence was available, NMAs were performed to permit a comparison of the efficacy of interventions in terms of clinical response and remission.
The economic analysis presented by the Assessment Group has several strengths:
-
The treatment pathway represented within the model was based on considerable expert opinion of several leading UC experts.
-
The Assessment Group model is underpinned by a complex NMA across all drug options thereby synthesising relevant efficacy outcomes data within a single network of evidence.
-
The model generally adheres to the NICE reference case and fully addresses the decision problem set out in the final NICE scope.
-
When appropriate and possible, systematic search methods have been used to identify, select and use evidence to inform the model’s parameters (efficacy, HRQoL and colectomy rates).
-
The Assessment Group have undertaken extensive sensitivity analyses to examine the impact of alternative assumptions and sources of evidence on the robustness of the results of the model.
The Assessment Group model is also subject to a number of limitations:
-
There is considerable uncertainty associated with Assessment Group’s extrapolation of short-term trial data (maximum 54 weeks) to a lifetime horizon.
-
The model does not consider an explicit sequential pathway of non-biological treatments; rather, during any cycle, a proportion of patients are assumed to receive 5-ASAs, immunomodulators and prednisolone.
-
Evidence relating to complications of colectomy was identified through consideration of approaches used within previous models rather than through a full systematic review; however, these assumptions were tested within the sensitivity analyses.
Uncertainties
Key uncertainties in this assessment include:
-
the optimal duration of intervention treatment in responding patients
-
the maintenance of efficacy outcomes and safety of interventions beyond the limited study lengths available
-
the maintenance of outcomes in responding patients following cessation of anti-TNF-α treatment
-
the relationship between post-surgical outcomes and HRQoL.
Chapter 7 Conclusions
Based on the NMA for clinical response and remission in the induction phase, all treatments were associated with statistically significant beneficial effects relative to PBO, with the greatest effect being associated with IFX. For patients classified as responders at the end of the induction phase, treatment effects were not statistically significant, although the greatest effect at 8–32 weeks was associated with 100 mg of GOL. At 32–52 weeks, only 50 mg of IFX and GOL were associated with beneficial effects on clinical response. For patients classified as being in remission at the end of the induction phase, all treatments except for ADA were associated with beneficial treatment effects relative to PBO with the greatest effect being associated with 50 mg and 100 mg of GOL, although the effects were not statistically significant at 8–32 weeks. At 32–52 weeks, all treatments except 50 mg of GOL were associated with beneficial treatment effects relative to PBO, with the greatest effect being associated with ADA (the only treatment with statistically significant effect). ADA was associated with the highest probability of staying in remission and the smallest probability of moving from remission to response and from remission to no response.
Available data on hospitalisation outcomes were very limited, but suggested that outcomes may be more favourable for ADA-treated and IFX-treated patients than PBO (with no data available from GOL trials). Data on surgical intervention were also very sparse, with a potential inconclusive benefit for intervention-treated patients compared with PBO. No trials reported whether surgical outcomes were elective or emergency in nature; however, further data are required to demonstrate the impact of interventions on hospitalisation and surgical intervention more conclusively. Data were available from a single trial to support the use of IFX in induction and maintenance treatment in a paediatric population.
The main safety issues highlighted in the RCT evidence appeared to be generally consistent with those previously discussed in the respective SmPCs for each product (including serious infections, malignancies and administration site reactions). Deaths occurring during and after the study period were described in some trials evaluating GOL (PURSUIT-Maintenance) and IFX (ACT1 and ACT2), of which infection or malignancy commonly appeared to be implicated. This underlines the importance of monitoring and treating serious infections and malignancies in patients receiving immunosuppressive treatment.
The base-case analysis of the Assessment Group model suggests that within an adult UC population, colectomy is expected to dominate IFX, ADA, GOL and conventional non-biological treatments. When elective colectomy is not an acceptable option, the Assessment Group model suggests that IFX and GOL are expected to be ruled out because of dominance, while the incremental cost-effectiveness of ADA versus conventional non-biological treatment is expected to be approximately £50,300 per QALY gained. The base-case analysis of the Assessment Group model suggests that within a paediatric UC population, colectomy is expected to dominate IFX and conventional non-biological treatments. When colectomy is not an acceptable option, the incremental cost-effectiveness of IFX versus conventional treatments is approximately £68,000 per QALY gained.
Implications for service provision
The Assessment Group is unaware of any further implications for service provision beyond those addressed in Chapter 5 of this report.
Suggested research priorities
-
Assessment of maintenance of outcomes in responding patients following cessation of anti-TNF-α treatment.
-
Assessment of efficacy, safety and immunogenicity following reintroduction of interventions after interruption in treatment.
-
Assessment of efficacy of interventions under assessment in specific subgroups (e.g. according to disease duration, as specified in the appraisal scope).
-
Head-to-head RCTs of interventions under assessment against each other in the treatment of moderate to severe UC after the failure of conventional therapy.
-
RCTs evaluating use of interventions under assessment in biological switching (i.e. after failure of first anti-TNF-α agent).
-
RCTs of longer duration of follow-up to assess maintenance of outcomes over the longer term.
-
RCTs assessing the clinical effectiveness of biologicals in paediatric patients.
-
Surgical intervention and hospitalisation to be incorporated as outcomes in future RCTs and associated extension studies of interventions in the treatment of moderately to severely active UC after the failure of conventional therapy.
-
Definition of factors that predict an improved patient response to anti-TNF-α treatment.
-
Further exploration of comparative clinical and economic outcomes (including the use of a preference-based utility instrument) associated with medical versus surgical treatments for patients with moderate to severe UC.
-
Consideration of unified universally agreed primary end points in future UC RCTs.
Acknowledgements
We would like to thank Dr Anthony Akobeng (Central Manchester University Hospitals NHS Foundation Trust) and Mr Steven Brown (Sheffield Teaching Hospitals NHS Foundation Trust) for providing clinical advice over the course of this assessment. Dr Stuart Bloom (University College London Hospitals NHS Foundation Trust) also provided clinical advice during the early stages of this assessment but had no subsequent involvement owing to the identification of a potential conflict of interest. We would also like to thank Dr Marcus Harbord (Chelsea and Westminster Hospital NHS Foundation Trust), Mrs Sarah Davis [School of Health and Related Research (ScHARR), University of Sheffield] and Dr Eva Kaltenthaler (ScHARR, University of Sheffield) for commenting on the draft assessment report. Thanks also to Gill Rooney (ScHARR, University of Sheffield) for providing project administration support. We would also like to thank MSD and AbbVie for providing unpublished data to inform the NMAs undertaken as part of this assessment.
About ScHARR: ScHARR is one of the nine departments that comprise the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research, and the application of health economics and decision science to the development of health services and the improvement of public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the NIHR HTA programme on behalf of a range of policy makers, including NICE. ScHARR-TAG is part of a wider collaboration of a number of units from other regions including Health Economics Research Unit and Health Services Research Unit, University of Aberdeen; Southampton Health Technology Assessment Centre, University of Southampton; Liverpool Reviews and Implementation Group, University of Liverpool; Peninsular Technology Assessment Group, University of Exeter; the NHS Centre for Reviews and Dissemination, University of York; Warwick Evidence, University of Warwick; the BMJ Technology Assessment Group, BMJ Evidence Centre and Kleijnen Systematic Reviews Ltd.
Contributions of authors
Rachel Archer acted as principal investigator for this assessment.
Paul Tappenden and Hasan Basarir undertook the health economic review and developed the Assessment Group model.
Shijie Ren, Rebecca Harvey and John Stevens conducted the NMAs.
Rachel Archer, Marrissa Martyn-St James and Christopher Carroll undertook the clinical effectiveness systematic review.
Anna Cantrell carried out the electronic searches.
Alan Lobo (Sheffield Teaching Hospitals NHS Foundation Trust) and Sami Hoque (Barts Health NHS Trust) provided clinical advice and commented on the draft assessment report.
Data sharing statement
Requests for data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Ulcerative Colitis: Management in Adults, Children and Young People. London: NICE; 2013.
- Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365. http://dx.doi.org/10.1056/NEJMra1102942.
- Ford AC, Moayyedi P, Hanauer SB, Kirsner JB. Ulcerative colitis. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f432.
- Bhagat S, Das KM. A shared and unique epitope in the human colon, eye, and joint detected by a monoclonal antibody. Gastroenterology 1994;107.
- Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis 2012;6:965-90. http://dx.doi.org/10.1016/j.crohns.2012.09.003.
- Roth LS, Chande N, Ponich T, Roth ML, Gregor J. Predictors of disease severity in ulcerative colitis patients from Southwestern Ontario. World J Gastroenterol 2010;16:232-6. http://dx.doi.org/10.3748/wjg.v16.i2.232.
- Lee JH, Cheon JH, Moon CM, Park JJ, Hong SP, Kim TI, et al. Do patients with ulcerative colitis diagnosed at a young age have more severe disease activity than patients diagnosed when older?. Digestion 2010;81:237-43. http://dx.doi.org/10.1159/000253850.
- Gibson PR, Vaizey C, Black CM, Nicholls RJ, Weston AR, Bampton P, et al. Relationship between disease severity and quality of life and assessment of health care utilization and cost for ulcerative colitis in Australia: a cross-sectional, observational study. J Crohns Colitis 2014;8:598-606. http://dx.doi.org/10.1016/j.crohns.2013.11.017.
- Ng SC, Kamm MA. Therapeutic strategies for the management of ulcerative colitis. Inflamm Bowel Dis 2009;15:935-50. http://dx.doi.org/10.1002/ibd.20797.
- Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvancarova M, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN study). Scand J Gastroenterol Suppl 2009;44:431-40. http://dx.doi.org/10.1080/00365520802600961.
- Aratari A, Papi C, Clemente V, Moretti A, Luchetti R, Koch M, et al. Colectomy rate in acute severe ulcerative colitis in the infliximab era. Dig Liver Dis 2008;40:821-6. http://dx.doi.org/10.1016/j.dld.2008.03.014.
- Hoie O, Wolters F, Riis L, Aamodt G, Solberg C, Bernklev T, et al. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol 2007;102:1692-701. http://dx.doi.org/10.1111/j.1572-0241.2007.01265.x.
- Gustavsson A, Halfvarson J, Magnuson A, Sandberg-Gertzen H, Järnerot G. Long-term colectomy rate after intensive intravenous corticosteroid therapy for ulcerative colitis prior to the immunosuppressive treatment era. Am J Gastroenterol 2007;102:2513-19. http://dx.doi.org/10.1111/j.1572-0241.2007.01435.x.
- Jess T, Gamborg M, Munkholm P, Sorensen T. Overall and cause-specific mortality in ulcerative colitis: meta-analysis of population-based inception cohort studies. Am J Gastroenterol 2007;102:609-17. http://dx.doi.org/10.1111/j.1572-0241.2006.01000.x.
- Casellas F, Lopez VJ, Casado A, Malagelada JR. Factors affecting health related quality of life of patients with inflammatory bowel disease. Qual Life Res 2002;11:775-81. http://dx.doi.org/10.1023/A:1020841601110.
- Lix LM, Graff LA, Walker JR, Clara I, Rawsthorne P, Rogala L, et al. Longitudinal study of quality of life and psychological functioning for active, fluctuating, and inactive disease patterns in inflammatory bowel disease. Inflamm Bowel Dis 2008;14:1575-84. http://dx.doi.org/10.1002/ibd.20511.
- Waljee AK, Joyce J, Wren PA, Khan TM, Higgins PD. Patient reported symptoms during an ulcerative colitis flare: a Qualitative Focus Group Study. Eur J Gastroenterol Hepatol 2009;21:558-64. http://dx.doi.org/10.1097/MEG.0b013e328326cacb.
- Kane SV. Systematic review: adherence issues in the treatment of ulcerative colitis. Aliment Pharmacol Ther 2006;23:577-85. http://dx.doi.org/10.1111/j.1365-2036.2006.02809.x.
- Higgins PDR, Rubin D, Kaulback K, Schoenfield P, Kane SV. Systematic review: impact of non-adherence to 5-aminosalicylic acid products on the frequency and cost of ulcerative colitis flares. Aliment Pharmacol Ther 2009;29:247-57. http://dx.doi.org/10.1111/j.1365-2036.2008.03865.x.
- Vaizey C, Gibson P, Black CM, Nicholls RJ, Weston AR, Gaya DR, et al. Disease status, patient quality of life, and health care resource use for ulcerative colitis in the United Kingdom: an observational study. Frontline Gastroenterol 2014:1-7.
- Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut 2004;53:1471-8. http://dx.doi.org/10.1136/gut.2004.041616.
- Cooney RM, Warren BF, Altman DG, Abreu MT, Travis SPL. Outcome measurement in clinical trials for ulcerative colitis: towards standardisation. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-17.
- Truelove SC, Witts LJ. Cortisone in ulcerative colitis: final report on a therapeutic trial. Br Med J 1955;2:1041-8. http://dx.doi.org/10.1136/bmj.2.4947.1041.
- Schroeder K, Tremaine W, Ilstrup D. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis-a randomized study. N Engl J Med 1987;317:1625-9. http://dx.doi.org/10.1056/NEJM198712243172603.
- Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133:423-32. http://dx.doi.org/10.1053/j.gastro.2007.05.029.
- Ha C, Bayless TM, Hanauer SB. Advanced Therapy of Inflammatory Bowel Disease Volume 1 IBD and Ulcerative Colitis. Shelton, CT: People’s Medical Publishing House; 2011.
- Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;14:1660-6. http://dx.doi.org/10.1002/ibd.20520.
- Infliximab for Subacute Manifestations of Ulcerative Colitis. London: NICE; 2011.
- Adalimumab for the Treatment of Moderate to Severe Ulcerative Colitis (Terminated Appraisal). London: NICE; 2013.
- Ulcerative Colitis (Acute Exacerbations) – Infliximab: Guidance. London: NICE; 2008.
- Cohen RD, Yu AP, Wu EQ, Xie J, Mulani PM, Chao J. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther 2010;31:693-707. http://dx.doi.org/10.1111/j.1365-2036.2010.04234.x.
- Shih DQ, Nguyen M, Zheng L, Ibanez P, Mei L, Kwan LY, et al. Split-dose administration of thiopurine drugs: a novel and effective strategy for managing preferential 6-MMP metabolism. Aliment Pharmacol Ther 2012;36:449-58. http://dx.doi.org/10.1111/j.1365-2036.2012.05206.x.
- Electronic Medicines Compendium . Summary of Product Characteristics: Remicade 2013. www.medicines.org.uk (accessed 26 May 2014).
- Electronic Medicines Compendium . Summary of Product Characteristics: Humira 2014. www.medicines.org.uk (accessed 26 May 2014).
- Electronic Medicines Compendium . Summary of Product Characteristics: Simponi 2013. www.medicines.org.uk (accessed 26 May 2014).
- Infliximab, Adalimumab and Golimumab for Treating Moderately to Severely Active Ulcerative Colitis After the Failure of Conventional Therapy (Including a Review of TA140 and TA262) – Final Scope. London: NICE; 2013.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- PRISMA . Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement 2009. www.prisma-statement.org/index.htm (accessed 18 December 2015).
- Archer RJ, Martyn-St James M, Cantrell A, Basarir H, Tappenden P, Stevens J. Infliximab, Adalimumab and Golimumab for Treating Moderately to Severely Active Ulcerative Colitis After the Failure of Conventional Therapy: Protocol Record CRD42013006883 2013. www.crd.york.ac.uk/PROSPERO (accessed 20 December 2013).
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011. www.cochrane-handbook.org (accessed 18 December 2015).
- Wright CC, Sim J. Intention-to-treat approach to data from randomized controlled trials: a sensitivity analysis. J Clin Epidemiol 2003;56:833-42. http://dx.doi.org/10.1016/S0895-4356(03)00155-0.
- Xian-Janssen Pharmaceutical Ltd . A Study to Evaluate the Effectiveness and Safety of Infliximab in Chinese Patients With Active Ulcerative Colitis n.d. https://clinicaltrials.gov/ct2/show/NCT01551290 (accessed February 2014).
- Janssen Pharmaceutical KK . A Safety and Effectiveness Study of Golimumab in Japanese Patients With Moderately to Severely Active Ulcerative Colitis n.d. https://clinicaltrials.gov/ct2/show/NCT01863771 (accessed February 2014).
- Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011;60:780-7. http://dx.doi.org/10.1136/gut.2010.221127.
- Sandborn WJ, Van AG, Reinisch W, Colombel JF, D’Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257-65. http://dx.doi.org/10.1053/j.gastro.2011.10.032.
- Suzuki Y, Motoya S, Hanai H, Matsumoto T, Hibi T, Robinson A, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol 2014;49:283-94. http://dx.doi.org/10.1007/s00535-013-0922-y.
- Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85-9. http://dx.doi.org/10.1053/j.gastro.2013.05.048.
- Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:96-109. http://dx.doi.org/10.1053/j.gastro.2013.06.010.
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462-76. http://dx.doi.org/10.1056/NEJMoa050516.
- Probert CS, Hearing SD, Schreiber S, Kuhbacher T, Ghosh S, Arnott ID, et al. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut 2003;52:998-1002. http://dx.doi.org/10.1136/gut.52.7.998.
- Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392-400. http://dx.doi.org/10.1053/j.gastro.2013.10.052.
- Hyams J, Damaraju L, Blank M, Johanns J, Guzzo C, Winter HS, et al. Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol and Hepatol 2012;10:391-9. http://dx.doi.org/10.1016/j.cgh.2011.11.026.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000097.
- Reinisch W, Sandborn WJ, Panaccione R, Huang B, Pollack PF, Lazar A, et al. 52-week efficacy of adalimumab in patients with moderately to severely active ulcerative colitis who failed corticosteroids and/or immunosuppressants. Inflamm Bowel Dis 2013;19:1700-9. http://dx.doi.org/10.1097/mib.0b013e318281f2b7.
- Reinisch W, Sandborn WJ, Rutgeerts P, Feagan BG, Rachmilewitz D, Hanauer SB, et al. Long-term infliximab maintenance therapy for ulcerative colitis: the ACT-1 and -2 extension studies. Inflamm Bowel Dis 2012;18:201-11. http://dx.doi.org/10.1002/ibd.21697.
- COMET Initative . Core Outcome Measures in Effectiveness Trials (COMET) Initative n.d. www.comet-initiative.org (accessed 19 November 2013).
- EMEA Guideline on the Development of New Medicinal Products for the Treatment of Ulcerative Colitis. London: EMA; 2008.
- Ghosh S, Wolf DC, Sandborn W, Colombel JF, Zhou Q, Lazar A, et al. Sustained Efficacy in Patients With Ulcerative Colitis Treated With Adalimumab: Results from ULTRA 2 n.d.
- Van AG, Wolf D, D’Haens G, Sandborn W, Colombel JF, Lazar A, et al. Reduced Steroid Usage in Ulcerative Colitis Patients With Week 8 Response to Adalimumab: Subanalysis of ULTRA 2 n.d.
- Sandborn W, Thakkar R, Lazar A, Skup M, Yang M, Chao J, et al. Time-In-Remission Analysis of Adalimumab Vs. Placebo for the Treatment of Ulcerative Colitis n.d.
- Reinisch W, Sandborn W, Hommes D, Thakkar R, Pollack P, Kumar A, et al. Effect of Adalimumab Induction Therapy on Clinical Laboratory Parameters Suggesting Improved Nutrition and Inflammation Status in Patients With Moderately to Severely Active Ulcerative Colitis n.d.
- AbbVie . Adalimumab, Golimumab and Infliximab, for the Treatment of Ulcerative Colitis (Subacute). 2014.
- Feagan B, Sandborn W, Yang M, Skup M, Thakkar R, Lazar A, et al. Adalimumab Therapy Reduces Hospitalization and Colectomy Rates in Patients With Ulcerative Colitis Among Initial Responders n.d.
- MSD . Manufacturer Submission of Evidence: Infliximab (Remicade). 2014.
- Feagan BG, Sandborn WJ, Lazar A, Thakkar RB, Huang B, Reilly N, et al. Adalimumab therapy is associated with reduced risk of hospitalization in patients with ulcerative colitis. Gastroenterology 2014;146:110-18. http://dx.doi.org/10.1053/j.gastro.2013.09.032.
- MSD . Manufacturer Submission of Evidence: Golimumab (Simponi). 2014.
- Sandborn WJ, Rutgeerts P, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology 2009;137:1250-60. http://dx.doi.org/10.1053/j.gastro.2009.06.061.
- Briefing Document for Food and Drug Administration Gastrointestinal Drugs Advisory Committee. Remicade (infliximab) for Paediatric Ulcerative Colitis. Centocor Research & Development, Inc.; 2011.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials 2011. www.nicedsu.org.uk (accessed March 2014).
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions (with discussion). Stat Med 2009;28:3049-82. http://dx.doi.org/10.1002/sim.3680.
- Questions and Answers on Biosimilar Medicines (Similar Biological Medicinal Products). EMA/837805/2011. London: EMA; 2012.
- Celltrion Healthcare . Infliximab Biosimilar (Remsima®) 2014.
- Assessment Report: Remsima. London: EMA; 2013.
- Assessment Report: Inflectra. London: EMA; 2013.
- Danese S, Gomollon F. ECCO position statement: the use of biosimilar medicines in the treatment of inflammatory bowel disease (IBD). J Crohns Colitis 2013;7:586-9. http://dx.doi.org/10.1016/j.crohns.2013.03.011.
- Kurki P, Bielsky MC. ECCO position challenged by European drug regulators. J Crohns Colitis 2014;8. http://dx.doi.org/10.1016/j.crohns.2014.01.022.
- Drummond ME, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- NICE . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/media/D45/1E/GuideToMethodsTechnologyAppraisal2013.pdf (accessed October 2013).
- NICE . Infliximab for Subacute Manifestations of Ulcerative Colitis 2008. www.nice.org.uk/nicemedia/live/11959/40412/40412.pdf (accessed March 2014).
- Remicade in the Treatment of Ankylosing Spondylitis in England and Wales. A Submission to the National Institute for Health and Clinical Excellence. London: NICE; 2006.
- Hyde C, Bryan S, Juarez-Garcia A, Andronis L, Fry-Smith A. Infliximab for the treatment of ulcerative colitis. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13suppl3/02.
- Tsai HH, Punekar YS, Morris J, Fortun P. A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther 2008;28:1230-9. http://dx.doi.org/10.1111/j.1365-2036.2008.03839.x.
- Park KT, Tsai R, Perez F, Cipriano LE, Bass D, Garber AM. Cost-effectiveness of early colectomy with ileal pouch-anal anastamosis versus standard medical therapy in severe ulcerative colitis. Ann Surg 2012;256:117-24. http://dx.doi.org/10.1097/SLA.0b013e3182445321.
- Xie F, Blackhouse G, Assasi N, Gaebel K, Robertson D, Goeree R. Cost-utility analysis of infliximab and adalimumab for refractory ulcerative colitis. Cost Eff Resour Alloc 2009;7. http://dx.doi.org/10.1186/1478-7547-7-20.
- Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996.
- Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut 1998;43:29-32. http://dx.doi.org/10.1136/gut.43.1.29.
- Barabino A, Tegaldo L, Castellano E, Gandullia P, Torrente F, Marino C, et al. Severe attack of ulcerative colitis in children: retrospective clinical survey. Dig Liver Dis 2002;34:44-9. http://dx.doi.org/10.1016/S1590-8658(02)80058-5.
- Hanauer S, Schwartz J, Robinson M, Roufail W, Arora S, Cello J, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. Am J Gastroenterol 1993;88:1188-97.
- Dignass AU, Bokemeyer B, Adamek H, Mross M, Vinter-Jensen L, Borner N, et al. Mesalamine once daily is more effective than twice daily in patients with quiescent ulcerative colitis. Clin Gastroenterol Hepatol 2009;7:762-9. http://dx.doi.org/10.1016/j.cgh.2009.04.004.
- Kamm MA, Lichtenstein GR, Sandborn WJ, Schreiber S, Lees K, Barrett K, et al. Randomised trial of once- or twice-daily MMX mesalazine for maintenance of remission in ulcerative colitis. Gut 2008;57:893-902. http://dx.doi.org/10.1136/gut.2007.138248.
- Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Porro GB. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut 2006;55:47-53. http://dx.doi.org/10.1136/gut.2005.068809.
- Hawthorne AB, Logan RFA, Hawkey CJ, Foster PN, Axon ATR, Swarbrick ET, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ 1992;305. http://dx.doi.org/10.1136/bmj.305.6844.20.
- Chebli LA, Chaves LD, Pimentel FF, Guerra DM, Barros RM, Gaburri PD, et al. Azathioprine maintains long-term steroid-free remission through 3 years in patients with steroid-dependent ulcerative colitis. Inflamm Bowel Dis 2010;16:613-19. http://dx.doi.org/10.1002/ibd.21083.
- Watson S, Pensabene L, Mitchell P, Bousvaros A. Outcomes and adverse events in children and young adults undergoing tacrolimus therapy for steroid-refractory colitis. Inflamm Bowel Dis 2011;17:22-9. http://dx.doi.org/10.1002/ibd.21418.
- Chew SS, Kerdic RI, Yang JL, Shi EC, Newstead GL, Douglas PR. Functional outcome and quality of life after ileal pouch-anal anastomosis in children and adults. ANZ J Surg 2003;73:983-7. http://dx.doi.org/10.1046/j.1445-2197.2003.t01-5-.x.
- Hahnloser D, Pemberton JH, Wolff BG, Larson DR, Crownhart BS, Dozois RR. Results at up to 20 years after ileal pouch-anal anastomosis for chronic ulcerative colitis. Br J Surg 2007;94:333-40. http://dx.doi.org/10.1002/bjs.5464.
- Waljee A, Waljee J, Morris AM, Higgins PDR. Threefold increased risk of infertility: a meta-analysis of infertility after ileal pouch anal anastomosis in ulcerative colitis. Gut 2006;55:1575-80. http://dx.doi.org/10.1136/gut.2005.090316.
- Stahlberg D, Gullberg K, Liljeqvist L, Hellers G, Löfberg R. Pouchitis following pelvic pouch operation for ulcerative colitis. Incidence, cumulative risk, and risk factors. Dis Colon Rectum 1996;39:1012-18. http://dx.doi.org/10.1007/BF02054692.
- Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, et al. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment Pharmacol Ther 2002;16:909-17. http://dx.doi.org/10.1046/j.1365-2036.2002.01203.x.
- Soderlund S, Brandt L, Lapidus A, Karlen P, Brostrom O, Löfberg R, et al. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology 2009;136:1561-7. http://dx.doi.org/10.1053/j.gastro.2009.01.064.
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583-637. http://dx.doi.org/10.1097/00005650-200006000-00004.
- Muir AJ, Edwards LJ, Sanders LL, Bollinger RR, Koruda MJ, Bachwich DR, et al. A prospective evaluation of health-related quality of life after ileal pouch anal anastomosis for ulcerative colitis. Am J Gastroenterol 2001;96:1480-5. http://dx.doi.org/10.1111/j.1572-0241.2001.03801.x.
- Provenzale D. Cost-effectiveness of screening the average-risk population for colorectal cancer. Gastrointest Endosc Clin N Am 2002;12:93-109. http://dx.doi.org/10.1016/S1052-5157(03)00061-8.
- Gift TL, Owens CJ. The direct medical cost of epididymitis and orchitis: evidence from a study of insurance claims. Sex Transm Dis 2006;33:84-8. http://dx.doi.org/10.1097/01.olq.0000235149.41948.fa.
- Bayless TM, Hanauer SB. Advanced Therapy of Inflammatory Bowel Disease: Ulcerative Colitis. Shelton, CT: PMPH-USA; 2011.
- Penna C, Dozois R, Tremaine W, Sandborn W, LaRusso N, Schleck C, et al. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut 1996;38:234-9. http://dx.doi.org/10.1136/gut.38.2.234.
- Hoie O, Wolters FL, Riis L, Bernklev T, Aamodt G, Clofent J, et al. Low colectomy rates in ulcerative colitis in an unselected European cohort followed for 10 years. Gastroenterology 2007;132:507-15. http://dx.doi.org/10.1053/j.gastro.2006.11.015.
- Arai K, Koganei K, Kimura H, Akatani M, Kitoh F, Sugita A, et al. Incidence and outcome of complications following restorative proctocolectomy. Am J Surg 2005;190:39-42. http://dx.doi.org/10.1016/j.amjsurg.2005.05.001.
- Woehl A, Hawthorne AB, Morgan CL, Punekar Y, McEwan P. The epidemiology and health care resource use in patients with Crohn’s Disease: a population based UK study. Value Health 2007;10. http://dx.doi.org/10.1016/s1098-3015(10)65262-x.
- Arseneau KO, Sultan S, Provenzale DT, Onken J, Bickston SJ, Foley E, et al. Do patient preferences influence decisions on treatment for patients with steroid-refractory ulcerative colitis?. Clin Gastroenterol Hepatol 2006;4:1135-42. http://dx.doi.org/10.1016/j.cgh.2006.05.003.
- NHS Reference Costs 2006/7. London: Department of Health; 2008.
- Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlen P, Granno C, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005;128:1805-11. http://dx.doi.org/10.1053/j.gastro.2005.03.003.
- Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg 1995;222:120-7. http://dx.doi.org/10.1097/00000658-199508000-00003.
- Raval MJ, Schnitzler M, O’Connor BI, Cohen Z, McLeod RS. Improved outcome due to increased experience and individualized management of leaks after ileal pouch-anal anastomosis. Ann Surg 2007;246:763-70. http://dx.doi.org/10.1097/SLA.0b013e31814539b1.
- Swenson BR, Hollenbeak CS, Koltun WA. Factors affecting cost and length of stay associated with the ileal pouch-anal anastomosis. Dis Colon Rectum 2003;46:754-61. http://dx.doi.org/10.1007/s10350-004-6653-7.
- Chaudhary MA, Fan T. Cost-effectiveness of infliximab for the treatment of acute exacerbations of ulcerative colitis in the Netherlands. Biol Ther 2012;3:45-60. http://dx.doi.org/10.1007/s13554-012-0007-0.
- Brereton N, Bodger K, Kamm MA, Hodgkins P, Yan S, Akehurst R. A cost-effectiveness analysis of MMX mesalazine compared with mesalazine in the treatment of mild-to-moderate ulcerative colitis from a UK perspective. J Med Econ 2010;13:148-61. http://dx.doi.org/10.3111/13696990903562861.
- Connolly MP, Nielsen SK, Currie CJ, Poole CD, Travis SPL. An economic evaluation comparing once daily with twice daily mesalazine for maintaining remission based on results from a randomised controlled clinical trial. J Crohns Colitis 2009;3:32-7. http://dx.doi.org/10.1016/j.crohns.2008.10.004.
- Saini SD, Waljee AK, Higgins PDR. Cost utility of inflammation-targeted therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2012;10:1143-51. http://dx.doi.org/10.1016/j.cgh.2012.05.003.
- Punekar YS, Hawkins N. Cost-effectiveness of infliximab for the treatment of acute exacerbations of ulcerative colitis. Eur J Health Econ 2010;11:67-76. http://dx.doi.org/10.1007/s10198-009-0199-5.
- Odes S, Vardi H, Friger M, Esser D, Wolters F, Moum B, et al. Clinical and economic outcomes in a population-based European cohort of 948 ulcerative colitis and Crohn’s disease patients by Markov analysis. Aliment Pharmacol Ther 2010;31:735-44. http://dx.doi.org/10.1111/j.1365-2036.2009.04228.x.
- Buckland A, Bodger K. The cost-utility of high dose oral mesalazine for moderately active ulcerative colitis. Aliment Pharmacol Ther 2008;28:1287-96. http://dx.doi.org/10.1111/j.1365-2036.2008.03856.x.
- Connolly MP, Boersma C, Oldenburg B. The economics of mesalazine in active ulcerative colitis and maintenance in the Netherlands. Neth J Med 2013;70:272-7.
- Prenzler A, Yen L, Mittendorf T, von der Schulenburg JM. Cost effectiveness of ulcerative colitis treatment in Germany: a comparison of two oral formulations of mesalazine. BMC Health Serv Res 2011;11. http://dx.doi.org/10.1186/1472-6963-11-157.
- Yen EF, Kane SV, Ladabaum U. Cost-effectiveness of 5-aminosalicylic acid therapy for maintenance of remission in ulcerative colitis. Am J Gastroenterol 2008;103:3094-105. http://dx.doi.org/10.1111/j.1572-0241.2008.02130.x.
- Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol 2007;5:103-10. http://dx.doi.org/10.1016/j.cgh.2006.09.033.
- Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, et al. Initiation of tumor necrosis factor- antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA 2011;306:2331-9. http://dx.doi.org/10.1001/jama.2011.1692.
- Curtis L. Unit Costs of Health and Social Care 2012. University of Kent, Kent: Personal Social Services Research Unit; 2012.
- NHS Reference Costs 2011–2012. London: Department of Health; 2012.
- Department of Health . NHS Reference Costs 2012–2013 n.d. www.gov.uk/Government/Uploads/System/Uploads/Attachment_Data/File/261154/Nhs_Reference_Costs_2012–13_Acc Pdf 2013 (accessed April 2014).
- Turner RM, Spiegelhalter D. Bias modelling in evidence synthesis. J R Stat Soc Ser A Stat Soc 2009;172:21-47. http://dx.doi.org/10.1111/j.1467-985X.2008.00547.x.
- EMA . Humira: EPAR Product Information 2012. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000481/WC500050870.pdf (accessed 18 December 2015).
- Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med 2003;114:39-43. http://dx.doi.org/10.1016/S0002-9343(02)01383-9.
- Hillson E, Dybicz S, Waters HC, Stuart B, Schaneman J, Dabbous O, et al. Health care expenditures in ulcerative colitis: the perspective of a self-insured employer. J Occup Environ Med 2008;50:969-77. http://dx.doi.org/10.1097/JOM.0b013e31816fd663.
- Johnson P, Richard C, Ravid A, Spencer L, Pinto E, Hanna M, et al. Female infertility after ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum 2004;47:1119-26. http://dx.doi.org/10.1007/s10350-004-0570-7.
- Krausz MM, Duek SD. Restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis and familial adenomatous polyposis: twenty years follow-up in 174 patients. Isr Med Assoc J 2005;7:23-7.
- Abdelrazeq AS, Kandiyil N, Botterill ID, Lund JN, Reynolds JR, Holdsworth PJ, et al. Predictors for acute and chronic pouchitis following restorative proctocolectomy for ulcerative colitis. Colorectal Dis 2008;10:805-13. http://dx.doi.org/10.1111/j.1463-1318.2007.01413.x.
- Roberts SE, Williams JG, Yeates D, Goldacre MJ. Mortality in patients with and without colectomy admitted to hospital for ulcerative colitis and Crohn’s disease: record linkage studies. BMJ 2007;335. http://dx.doi.org/10.1136/bmj.39345.714039.55.
- Swinburn P, Elwick H, Bean K, Curry A, Patel S, Bodger K, et al. The Impact of Surgery on Health Related Quality of Life in Ulcerative Colitis n.d. http://dx.doi.org/10.1136/gutjnl-2012-302514c.127.
- Hu D, Grossman D, Levin C, Blanchard K, Goldie SJ. Cost-effectiveness analysis of alternative first-trimester pregnancy termination strategies in Mexico City. BJOG 2009;116:768-79. http://dx.doi.org/10.1111/j.1471-0528.2009.02142.x.
- Smith KJ, Roberts MS. Quality-of-life utility values for erectile function and sildenafil treatment. Clin Drug Investig 2005;25:99-105. http://dx.doi.org/10.2165/00044011-200525020-00002.
- Monthly Index of Medical Specialities . Monthly Index of Medical Specialities Database n.d. www.mims.co.uk (accessed March 2014).
- Buchanan J, Wordsworth S, Ahmad T, Perrin A, Vermeire S, Sans M, et al. Managing the long term care of inflammatory bowel disease patients: the cost to European health care providers. J Crohns Colitis 2011;5:301-16. http://dx.doi.org/10.1016/j.crohns.2011.02.005.
- Craig BA, Sendi PP. Estimation of the transition matrix of a discrete-time Markov chain. Health Econ 2002;11:33-42. http://dx.doi.org/10.1002/hec.654.
- Woehl A, Hawthorne A, McEwan P. The relation between disease activity, quality of life and health utility in patients with ulcerative colitis. Gut 2008;57.
- Lynch RW, Down C, Roughton M. No increase in surgical complication in patients treated with rescue therapy for acute severe ulcerative colitis: data from the UK IBD audit. Gut 2012;61. http://dx.doi.org/10.1136/gutjnl-2012-302514a.165.
- Office for National Statistics . Interim Life Tables, England and Wales, 2009–11 2011. www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2009–2011/stb-2009–2011.html (accessed April 2014).
- EMA . Remicade. EPAR Product Information 2014. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000240/WC500050888.pdf (accessed March 2014).
- EMA . Assessment Report for Simponi 2014. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000992/WC500052372.pdf (accessed March 2014).
- Actis GC, Fadda M, David E, Sapino A. Colectomy rate in steroid-refractory colitis initially responsive to cyclosporin: a long-term retrospective cohort study. BMC Gastroenterology 2007;7. http://dx.doi.org/10.1186/1471-230X-7-13.
- Gower-Rousseau C, Dauchet L, Vernier-Massouille G, Tilloy E, Brazier F, Merle V, et al. The natural history of pediatric ulcerative colitis: a population-based cohort study. Am J Gastroenterol 2009;104:2080-8. http://dx.doi.org/10.1038/ajg.2009.177.
- Molnár T, Farkas K, Nyari T, Szepes Z, Nagy F, Wittmann T. Response to first intravenous steroid therapy determines the subsequent risk of colectomy in ulcerative colitis patients. J Gastrointestin Liver Dis 2011;20:359-63.
- Mocciaro F, Renna S, Orlando A, Rizzuto G, Sinagra E, Orlando E, et al. Cyclosporine or infliximab as rescue therapy in severe refractory ulcerative colitis: early and long-term data from a retrospective observational study. J Crohns Colitis 2012;6:681-6. http://dx.doi.org/10.1016/j.crohns.2011.11.021.
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press; 2006.
- Casellas F, Lopez VJ, Malagela JR. Previous experience and quiality of life in patients with inflammatory bowel disease during relapse. Rev Esp Enferm Dig 2003;95:471-9.
- Leidl R, Reitmeir P, Konig HH, Stark R. The performance of a value set for the EQ-5D based on experienced health states in patients with inflammatory bowel disease. Value Health 2012;15:151-7. http://dx.doi.org/10.1016/j.jval.2011.08.004.
- Vaizey C, Gibson PR, Black CM, Nicholls RJ, Weston AR, Gaya DR, et al. The Relationship Between Disease Severity, Quality of Life and Health Care Resource Utilization Among United Kingdom Patients With Ulcerative Colitis n.d. http://dx.doi.org/10.1016/s1873-9946(13)60639-x.
- van der Valk ME, Mangen MJ, Dijkstra G, van Bodegraven AA, Fidder H, de Jong DJ, et al. Is There a Difference in Quality of Life and Costs Between Ulcerative Colitis Patients With a Pouch or an Ileostomy? n.d.
- Richards DM, Hughes SA, Irving MH, Scott NA. Patient quality of life after successful restorative proctocolectomy is normal. Colorectal Disease 2001;3:223-6. http://dx.doi.org/10.1046/j.1463-1318.2001.00228.x.
- Kuruvilla K, Osler T, Hyman NH. A comparison of the quality of life of ulcerative colitis patients after IPAA vs ileostomy. Dis Colon Rectum 2012;55:1131-7. http://dx.doi.org/10.1097/DCR.0b013e3182690870.
- Actis GC, Bruno M, Pinna-Pintor M, Rossini FP, Rizzetto M. Infliximab for treatment of steroid-refractory ulcerative colitis. Dig Liver Dis 2002;34:631-4. http://dx.doi.org/10.1016/S1590-8658(02)80205-5.
- Actis GC. Infliximab for ulcerative colitis. Am J Gastroenterol 2003;98. http://dx.doi.org/10.1111/j.1572-0241.2003.07312.x.
- Afif W, Leighton JA, Hanauer SB, Loftus EV, Faubion WA, Pardi DS, et al. Open-label study of adalimumab in patients with ulcerative colitis including those with prior loss of response or intolerance to infliximab. Inflamm Bowel Dis 2009;15:1302-7. http://dx.doi.org/10.1002/ibd.20924.
- Allez M, Karmiris K, Louis E, Van AG, Ben-Horin S, Klein A, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4:355-66. http://dx.doi.org/10.1016/j.crohns.2010.04.004.
- Infliximab (ulcerative colitis): caution is needed due to long-term risks . Prescrire International 2007;16.
- Armuzzi A, De Pascalis B, Lupascu A, Fedeli P, Leo D, Mentella MC, et al. Infliximab in the treatment of steroid-dependent ulcerative colitis. Eur Rev Med Pharmacol Sci 2004;8:231-3.
- Baert F, De Vos M, Louis E, Vermeire S. Immunogenicity of infliximab: how to handle the problem?. Acta Gastroenterol Belg 2007;70:163-70.
- Barbato M, Curione M, Viola F, Versacci P, Parisi F, Amato S, et al. Cardiac involvement in children with IBD during infliximab therapy. Inflamm Bowel Dis 2006;12:828-9. http://dx.doi.org/10.1097/00054725-200608000-00021.
- Barreiro-de AM, Lorenzo A, Mera J, Dominguez-Munoz JE. Mucosal healing and steroid-sparing associated with infliximab for steroid-dependent ulcerative colitis. J Crohns Colitis 2009;3:271-6. http://dx.doi.org/10.1016/j.crohns.2009.06.003.
- Baumgart DC. How many lives does an ulcerative colitis patient have?. Lancet 2010;376. http://dx.doi.org/10.1016/S0140-6736(10)60955-5.
- Ben-Horin S. Randomised placebo-controlled trial of rituximab (anti-CD20) in active ulcerative colitis. Gut 2012;61. http://dx.doi.org/10.1136/gutjnl-2011-300398.
- Bengi G, Akpinar H. What is the importance of infliximab and cyclosporine in the treatment of corticosteroid-refractory severe ulcerative colitis?. Turkish J Gastroenterol 2012;23.
- Biancone L, Petruzziello C, Calabrese E, Zorzi F, Naccarato P, Onali S, et al. Long-term safety of Infliximab for the treatment of inflammatory bowel disease: does blocking TNFalpha reduce colitis-associated colorectal carcinogenesis?. Gut 2009;58. http://dx.doi.org/10.1136/gut.2008.176461.
- Ciclosporin no better than infliximab for acute refractory ulcerative colitis. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e6923.
- Bordeianou L. In flux on infliximab: conflicting studies on surgical outcomes. Inflamm Bowel Dis 2009;15. http://dx.doi.org/10.1002/ibd.20891.
- Borruel N, Navarro E, Robles V, Torrejon A, Casellas F. Factors Influencing Adherence to Treatment With Anti-TNF Agents in Ulcerative and Crohn’s Disease Patients: A Prospective Study n.d.
- Brooklyn TN, Dunnill MG, Shetty A, Bowden JJ, Williams JD, Griffiths CE, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut 2006;55:505-9. http://dx.doi.org/10.1136/gut.2005.074815.
- Bujanover Y, Weiss B. Anti-tumor necrosis factor therapy for pediatric inflammatory bowel diseases. Israel Med Assoc J 2008;10:634-9.
- Busquets D, Aldeguer X. Clinical experience with adalimumab in anti-TNF-naive patients with ulcerative colitis. J Crohns Colitis 2013;7. http://dx.doi.org/10.1016/j.crohns.2012.11.005.
- Carbone J, Perez-Rojas J, Sarmiento E. Infectious pulmonary complications in patients treated with anti-TNF-alpha monoclonal antibodies and soluble TNF receptor. Curr Infect Dis Rep 2009;11:229-36. http://dx.doi.org/10.1007/s11908-009-0034-2.
- Cariñanos I, Acosta MB, Domènech E. Adalimumab for pyoderma gangrenosum associated with inflammatory bowel disease. Inflamm Bowel Dis 2011;17:E153-4. http://dx.doi.org/10.1002/ibd.21723.
- Casteele VN, Compernolle G, Ballet V, Van AG, Gils A, Vermeire S, et al. Individualised Infliximab Treatment Using Therapeutic Drug Monitoring: A Prospective Controlled Trough Level Adapted InfliXImab Treatment (TAXIT) Trial n.d.
- Casteele NV, Compernolle G, Ballet V, Van AG, Gils A, Vermeire S, et al. Results on the Optimisation Phase of the Prospective Controlled Trough Level Adapted Infliximab Treatment (TAXIT) Trial n.d.
- Charles P, Ackermann F, Burdy G, Bletry O, Leport J, Kahn JE. Cytomegalovirus colitis complicating ulcerative colitis treated with adalimumab. Scand J Gastroenterol 2010;45:509-10. http://dx.doi.org/10.3109/00365520903583871.
- Chen M, Black CM, Gurunath S, Jansen JP, Chaudhary MA, Fan T. Network Meta-Analysis of Approved Biologic Interventions for the Maintenance of Response in Ulcerative Colitis n.d.
- Chey WY, Shah A. Infliximab for ulcerative colitis. J Clin Gastroenterol 2005;39. http://dx.doi.org/10.1097/01.mcg.0000180806.51114.eb.
- Chowers Y, Sturm A, Sans M, Papadakis K, Gazouli M, Harbord M, et al. Report of the ECCO workshop on anti-TNF therapy failures in inflammatory bowel diseases: biological roles and effects of TNF and TNF antagonists. J Crohns Colitis 2010;4:367-76. http://dx.doi.org/10.1016/j.crohns.2010.05.011.
- Chuang MH, Singh J, Ashouri N, Katz MH, Arrieta AC. Listeria meningitis after infliximab treatment of ulcerative colitis. J Pediatr Gastroenterol Nutr 2010;50:337-9. http://dx.doi.org/10.1097/MPG.0b013e3181a70f3a.
- Cohen RD. Infliximab in ulcerative colitis: is there a placebo (effect) in the house?. Gastroenterology 2003;124:1990-1. http://dx.doi.org/10.1016/S0016-5085(03)00570-5.
- Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011;141:1194-201. http://dx.doi.org/10.1053/j.gastro.2011.06.054.
- Colombel J-F, Sandborn W, Van AG, D’Haens G, Wolf D, Kron M, et al. Benefit-Risk Assessment of Adalimumab As Maintenance Treatment for Ulcerative Colitis: Near Analysis of Week 8 Responders in Ultra 2 n.d.
- Cottone M, Orlando A, Mocciaro F. Improving patients’ QoL: how the success of treatment can improve workability. Dig Liver Dis Suppl 2008;2:17-8. http://dx.doi.org/10.1016/S1594-5804(08)60019-4.
- Croft A, Walsh A, Doecke J, Cooley R, Howlett M, Radford-Smith G. Outcomes of salvage therapy for steroid-refractory acute severe ulcerative colitis: ciclosporin vs. infliximab. Aliment Pharmacol Ther 2013;38:294-302. http://dx.doi.org/10.1111/apt.12375.
- Cross RK, Lapshin O, Finkelstein J. Patient subjective assessment of drug side effects in inflammatory bowel disease. J Clin Gastroenterol 2008;42:244-51. http://dx.doi.org/10.1097/mcg.0b013e31802f19af.
- Danese S. IBD: golimumab in ulcerative colitis: a ‘menage a trois’ of drugs. Nat Rev Gastroenterol Hepatol 2013;10:511-12. http://dx.doi.org/10.1038/nrgastro.2013.142.
- De Vos M, Dewit O, D’Haens G, Baert F, Fontaine F, Vermeire S, et al. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naive patients with ulcerative colitis. J Crohns Colitis 2012;6:557-62. http://dx.doi.org/10.1016/j.crohns.2011.11.002.
- de Vries HS, van Oijen MG, de Jong DJ. Safety of infliximab in inflammatory bowel disease needs to be debated. Clin Gastroenterol Hepatol 2009;7:603-4. http://dx.doi.org/10.1016/j.cgh.2008.12.021.
- D’Haens G. Infliximab for ulcerative colitis: finally some answers. Gastroenterology 2005;128:2161-4. http://dx.doi.org/10.1053/j.gastro.2005.04.019.
- Dean KE, Hikaka J, Huakau JT, Walmsley RS. Infliximab or cyclosporine for acute severe ulcerative colitis: a retrospective analysis. J Gastroenterol Hepatol 2012;27:487-92. http://dx.doi.org/10.1111/j.1440-1746.2011.06958.x.
- Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel J-F, Allez M, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 2012;6:991-1030. http://dx.doi.org/10.1016/j.crohns.2012.09.002.
- Domènech E, Zabana Y, Manosa M, Garcia-Planella E, Cabre E, Gassull MA. Infliximab reintroduction is not associated to a higher rate of immune-related adverse effects in patients with inflammatory bowel disease initially treated with a three-infusion induction regimen. J Clin Gastroenterol 2010;44:34-7. http://dx.doi.org/10.1097/MCG.0b013e3181962dfa.
- Dranitsaris G, Dorward K, Hatzimichael E, Amir E. Clinical trial design in biosimilar drug development. Invest New Drugs 2012;31:479-87. http://dx.doi.org/10.1007/s10637-012-9899-2.
- Eidelwein AP, Cuffari C, Abadom V, Oliva-Hemker M. Infliximab efficacy in pediatric ulcerative colitis. Inflamm Bowel Dis 2005;11:213-18. http://dx.doi.org/10.1097/01.MIB.0000160803.44449.a5.
- Eriksson U, Janson UA, Strid H, Ohman L, Bajor A. Combination Therapy With Infliximab and Thiopurines Might Reduce Colectomy Rates Compared to Infliximab Monotherapy in Patients With Ulcerative Colitis (UC) n.d.
- Esteve M, Mahadevan U, Sainz E, Rodriguez E, Salas A, Fernandez-Banares F. Efficacy of anti-TNF therapies in refractory severe microscopic colitis. J Crohns Colitis 2011;5:612-18. http://dx.doi.org/10.1016/j.crohns.2011.05.001.
- World Health Organization . International Clinical Trials Registry Platform Record 2014. http://apps.who.int/trialsearch/ (accessed 18 December 2015).
- Fanjiang G, Russell GH, Katz AJ. Short- and long-term response to and weaning from infliximab therapy in pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr 2007;44:312-17. http://dx.doi.org/10.1097/MPG.0b013e31802e98d4.
- Fasanmade AA, Adedokun OJ, Ford J, Hernandez D, Johanns J, Hu C, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol 2009;65:1211-28. http://dx.doi.org/10.1007/s00228-009-0718-4.
- Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther 2010;48:297-308. http://dx.doi.org/10.5414/CPP48297.
- Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JWD, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha(4) beta(7) integrin. N Engl J Med 2005;352:2499-507. http://dx.doi.org/10.1056/NEJMoa042982.
- Feagan B. Infliximab, adalimumab and certolizumab: beneficial and adverse effects (past). Inflamm Bowel Dis 2006;12. http://dx.doi.org/10.1097/00054725-200610003-00013.
- Florholmen J, Overland G, Olsen T, Rismo R, Cui G, Christiansen I, et al. Short-and long-term clinical outcomes of infliximab in fulminant ulcerative colitis. Ulcers 2011;2011. http://dx.doi.org/10.1155/2011/156407.
- Gao J, Jiang XL. Low-dose infliximab for corticosteroid-refractory ulcerative colitis: impact of number of infusions on efficacy and safety. World Chinese J Digestol 2013;21:1453-7. http://dx.doi.org/10.11569/wcjd.v21.i15.1453.
- Gavalas E, Kountouras J, Stergiopoulos C, Zavos C, Gisakis D, Nikolaidis N, et al. Efficacy and safety of infliximab in steroid-dependent ulcerative colitis patients. Hepatogastroenterology 2007;54:1074-9.
- Gearry RB, Falvey JD. Rescue therapy for steroid refractory acute severe ulcerative colitis: more choice, better outcomes?. J Gastroenterol Hepatol 2012;27:417-18. http://dx.doi.org/10.1111/j.1440-1746.2012.07063.x.
- Gies N, Kroeker KI, Wong K, Fedorak RN. Treatment of ulcerative colitis with adalimumab or infliximab: long-term follow-up of a single-centre cohort. Aliment Pharmacol Ther 2010;32:522-8. http://dx.doi.org/10.1111/j.1365-2036.2010.04380.x.
- Ginard D, Khorrami S, Vanrell M. Efficacy and Effectiveness of Biological Therapy in Inflammatory Bowel Disease (IBD): Comparative Study Between Clinical Practice and Pivotal Studies n.d.
- Grosen A, Julsgaard M, Christensen LA. Serum sickness-like reaction due to Infliximab reintroduction during pregnancy. J Crohns Colitis 2013;7. http://dx.doi.org/10.1016/j.crohns.2012.10.006.
- Gustavsson A, Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlen P, et al. Clinical trial: colectomy after rescue therapy in ulcerative colitis – 3-year follow-up of the Swedish-Danish controlled infliximab study. Aliment Pharmacol Ther 2010;32:984-9. http://dx.doi.org/10.1111/j.1365-2036.2010.04435.x.
- Ha CY, Ullman TA, Siegel CA, Kornbluth A. Are Patients Enrolled in Randomized Controlled Trials (RCTs) Representative of the General Inflammatory Bowel Disease (IBD) Patient Population? n.d.
- Halpin SJ, Hamlin PJ, Ford AC. Infliximab rescue therapy in ulcerative colitis, and the effect on subsequent colectomy rates. Aliment Pharmacol Ther 2010;32:1294-5. http://dx.doi.org/10.1111/j.1365-2036.2010.04478.x.
- Halpin S, Hamlin PJ. Commentary: outcomes after escalation of infliximab in moderately active ulcerative colitis. Aliment Pharmacol Ther 2012;35:957-8. http://dx.doi.org/10.1111/j.1365-2036.2012.05034.x.
- Hämäläinen A, Sipponen T, Kolho KL. Infliximab in pediatric inflammatory bowel disease rapidly decreases fecal calprotectin levels. World J Gastroenterol 2011;17:5166-71. http://dx.doi.org/10.3748/wjg.v17.i47.5166.
- Hanauer SB. Infliximab or cyclosporine for severe ulcerative colitis. Gastroenterology 2005;129:1358-9. http://dx.doi.org/10.1053/j.gastro.2005.08.040.
- Hanauer SB, Abreu MT, Kornbluth AA, Present DH, Sandborn WJ. Charging ahead in IBD: challenging issues and recent breakthroughs – a report of a symposium presented on 19 May 2008: San Diego, California. Gastroenterology and Hepatology 2008;4:3-11.
- Hanauer SB, Rubin DT, Sandborn WJ. Treatment guidelines and clinical practice: optimizing foundational therapies for ulcerative colitis. Gastroenterol Hepatol 2008;4:4-13.
- Heraganahally SS, Au V, Kondru S, Edwards S, Bowden JJ, Sajkov D. Pulmonary toxicity associated with infliximab therapy for ulcerative colitis. Intern Med J 2009;39:629-30. http://dx.doi.org/10.1111/j.1445-5994.2009.001999.x.
- Herrlinger KR, Barthel DN, Schmidt KJ, Buning J, Barthel CS, Wehkamp J, et al. Infliximab as rescue medication for patients with severe ulcerative/indeterminate colitis refractory to tacrolimus. Aliment Pharmacol Ther 2010;31:1036-41. http://dx.doi.org/10.1111/j.1365-2036.2010.04267.x.
- Honeywell M, Touchstone K, Caspi A. Infliximab: a chimeric monoclonal antibody against tumor necrosis factor. Pharmacol Ther 2007;32:1052-372.
- Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Stephens M, Evans J, et al. Outcome following infliximab therapy in children with ulcerative colitis. Am J Gastroenterol 2010;105:1430-6. http://dx.doi.org/10.1038/ajg.2009.759.
- Hyams J, Damaraju L, Blank M, Johanns J, Guzzo C, Winter H, et al. A Randomized, Multicenter, Open-Label Phase 3 Study to Evaluate the Safety and Efficacy of Infliximab in Pediatric Patients With Moderate to Severe Ulcerative Colitis n.d.
- Assasi N, Blackhouse G, Xie F, Gaebel K, Marshall J, Irvine EJ, et al. Anti-TNF-alpha drugs for refractory inflammatory bowel disease: clinical- and cost-effectiveness analyses. Database of Abstracts of Reviews of Effects 2009.
- Jackson JM. TNF-alpha inhibitors. Dermatologic Therapy 2007;20:251-64. http://dx.doi.org/10.1111/j.1529-8019.2007.00138.x.
- Järnerot G. Infliximab or cyclosporine for severe ulcerative colitis. Gastroenterology 2006;130. http://dx.doi.org/10.1053/j.gastro.2005.11.037.
- Jimenez JM. Infliximab in the treatment of severe ulcerative colitis. Rev Esp Enferm Dig 2004;96:89-93.
- Joob B, Wiwanikit V. Infliximab for treatment of pyoderma gangrenosum with ulcerative colitis. J Crohns Colitis 2013;7. http://dx.doi.org/10.1016/j.crohns.2012.08.018.
- Kaser A, Tilg H. Inflammatory bowel diseases: highlights from the United European Gastroenterology Week 2008–18–22 October 2008, Vienna, Austria. Expert Opin Ther Targets 2009;13:259-63. http://dx.doi.org/10.1517/14728220802682325.
- Kaur M, Targan SR. Steroid-refractory ulcerative colitis-ciclosporin or infliximab?. Nat Rev Gastroenterol Hepatol 2013;10:8-9. http://dx.doi.org/10.1038/nrgastro.2012.235.
- Kerbleski JF, Gottlieb AB. Dermatological complications and safety of anti-TNF treatments. Gut 2009;58:1033-9. http://dx.doi.org/10.1136/gut.2008.163683.
- Klotz U, Teml A, Schwab M. Clinical pharmacokinetics and use of infliximab. Clinical Pharmacokinet 2007;46. http://dx.doi.org/10.2165/00003088-200746080-00002.
- Kohn A, Prantera C, Pera A, Cosintino R, Sostegni R, Daperno M. Infliximab in the treatment of severe ulcerative colitis: a follow-up study. Eur Rev Med Pharmacol Sci 2004;8:235-7.
- Kohn A, Daperno M, Armuzzi A, Cappello M, Biancone L, Orlando A, et al. Infliximab in severe ulcerative colitis: short-term results of different infusion regimens and long-term follow-up. Aliment Pharmacol Ther 2007;26:747-56. http://dx.doi.org/10.1111/j.1365-2036.2007.03415.x.
- Kohn A. Is there a role for infliximab in severe ulcerative colitis? The European experience. Inflamm Bowel Dis 2008;14:234-5. http://dx.doi.org/10.1002/ibd.20665.
- Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, Filippi J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 2012;380:1909-15. http://dx.doi.org/10.1016/S0140-6736(12)61084-8.
- Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, Filippi J, et al. Cyclosporin Versus Infliximab in Severe Acute Ulcerative Colitis Refractory to Intravenous Steroids: A Randomized Trial n.d.
- Leal RF, de Lourdes Setsuko AM, de Sene Portel OP, Fagundes JJ, Coy CS. Impact of a best clinical therapy management on surgery for ulcerative colitis. Tech Coloproctol 2012;16:321-2. http://dx.doi.org/10.1007/s10151-012-0842-5.
- LeBlanc K, Mosli M, Baker KA, MacDonald JK. The impact of biological interventions for ulcerative colitis on health-related quality of life. Cochrane Database Syst Rev 2013;9. http://dx.doi.org/10.1002/14651858.cd008655.pub2.
- Leblanc S, Allez M, Seksik P, Flourie B, Peeters H, Dupas JL, et al. Successive treatment with cyclosporine and infliximab in steroid-refractory ulcerative colitis. Am J Gastroenterol 2011;106:771-7. http://dx.doi.org/10.1038/ajg.2011.62.
- Levesque BG, Sandborn WJ. Infliximab versus ciclosporin in severe ulcerative colitis. Lancet 2012;380:1887-8. http://dx.doi.org/10.1016/S0140-6736(12)61259-8.
- Levy LC. Advances in inflammatory bowel diseases. Expert Rev Gastroenterol Hepatol 2009;3. http://dx.doi.org/10.1586/egh.09.6.
- Li K, Telesco S, Rutgeerts PJ, Sandborn W, Marano CW, Ma K, et al. Characterization of Molecular Response to Golimumab in Ulcerative Colitis by MRNA Expression Profiling: Results From Pursuit-SC Induction Study n.d.
- Lichtenstein GR. Is infliximab effective for induction of remission in patients with ulcerative colitis?. Inflamm Bowel Dis 2001;7:89-93. http://dx.doi.org/10.1097/00054725-200105000-00002.
- Lichtenstein GR. Applying clinical trial data to biologic treatment of ulcerative colitis. Gastroenterol Hepatol 2009;5:111-15.
- Liu Y, Reichmann B, Yan S, Macaulay D, Skup M, Mulani P, et al. Indirect Comparison of Adalimumab and Infliximab in Ulcerative Colitis: Cost Per Remitter Analysis n.d.
- Löfberg R, Louis EV, Reinisch W, Robinson AM, Kron M, Camez A, et al. Adalimumab produces clinical remission and reduces extraintestinal manifestations in Crohn’s disease: results from CARE. Inflamm Bowel Dis 2012;18:1-9. http://dx.doi.org/10.1002/ibd.21663.
- Lorenzo-Zúñiga V, Boix J, Manosa M, Lezcano C, Cabre E, Moreno de Vega V, et al. Local injection of infliximab in symptomatic isolated mucosal lesions: a novel scenario for endoscopic therapy?. Inflamm Bowel Dis 2013;19:E59-61. http://dx.doi.org/10.1002/ibd.23018.
- Mallow P, Rizzo J, Queener MK, Gathany T, Lofland J. Cost Per Clinical Remission and Clinical Response of Adalimumab, Golimumab, and Infliximab in the Treatment of Patients With Ulcerative Colitis: An Induction Analysis n.d.
- Mallow P, Rizzo J, Queener M, Gathany T, Lofland J. Comparative Efficacy of Adalimumab, Golimumab, and Infliximab in the Treatment of Patients With Ulcerative Colitis: An Induction Analysis n.d.
- Maser EA, Deconda D, Lichtiger S, Ullman T, Present DH, Kornbluth A. Cyclosporine and infliximab as rescue therapy for each other in patients with steroid-refractory ulcerative colitis. Clin Gastroenterol Hepatol 2008;6:1112-16. http://dx.doi.org/10.1016/j.cgh.2008.04.035.
- Matsumoto T. Treatment of inflammatory immunologic disease 2. Anti-cytokine therapies in inflammatory bowel disease. Intern Med 2007;46. http://dx.doi.org/10.2169/internalmedicine.46.1912.
- Mazumdar S, Greenwald D. Golimumab. MAbs 2009;1:422-31. http://dx.doi.org/10.4161/mabs.1.5.9286.
- McCann DA, Smith HL. Infliximab-associated Blastomycosis dermatitidis in treatment of ulcerative colitis. Colorectal Dis 2013;15:e102-3. http://dx.doi.org/10.1111/codi.12050.
- Molnár T, Farkas K, Nagy F, Szepes Z, Wittmann T. Infliximab safety profile and long-term applicability in inflammatory bowel disease: clinical experiences from the eastern side of Europe. Aliment Pharmacol Ther 2010;31:1152-3. http://dx.doi.org/10.1111/j.1365-2036.2010.04260.x.
- Molnár T, Farkas K, Szepes Z, Nagy F, Wittmann T. Long-term outcome of infliximab therapy is highly comparable in a Danish and in a Hungarian tertiary center. Scand J Gastroenterol 2011;46:248-9. http://dx.doi.org/10.3109/00365521.2010.522725.
- Molnár T, Farkas K, Szepes Z, Nagy F, Wittmann T. Infliximab rescue therapy in steroid-refractory ulcerative colitis: is more really more?. Aliment Pharmacol Ther 2011;33:412-13. http://dx.doi.org/10.1111/j.1365-2036.2010.04529.x.
- Moss AC, Farrell RJ. Infliximab for induction and maintenance therapy for ulcerative colitis. Gastroenterology 2006;131:1649-51. http://dx.doi.org/10.1053/j.gastro.2006.09.039.
- Nakase H, Yamamoto S, Matsuura M, Honzawa Y, Chiba T. Cytomegalovirus affects clinical outcome of infliximab in ulcerative colitis refractory to tacrolimus. Aliment Pharmacol Ther 2010;32:510-11. http://dx.doi.org/10.1111/j.1365-2036.2010.04372.x.
- Infliximab (Remicade) for Paediatric Ulcerative Colitis. Birmingham: National Horizon Scanning Centre (NHSC). Birmingham: The NIHR Horizon Scanning Centre; 2011.
- Golimumab (Simponi) for Ulcerative Colitis – Second Line. Birmingham: National Horizon Scanning Centre (NHSC). Birmingham: The NIHR Horizon Scanning Centre; 2011.
- Williams J, Alam F, Alrubaiy L, Clement C, Cohen D, Grey M, et al. COmparison of iNfliximab and ciclosporin in STeroid Resistant Ulcerative Colitis: (CONSTRUCT) pragmatic randomised Trial and economic evaluation. Health Technol Assess 2016.
- Clinical Trials.gov record 2013. https://clinicaltrials.gov/ (accessed February 2014).
- Nguyen GC, Prather CM. Is keeping the colon the ultimate marker of success in ulcerative colitis?. Gastroenterology 2009;137:1204-6. http://dx.doi.org/10.1053/j.gastro.2009.08.024.
- Nielsen OH, Jess T. IBD: can TNF inhibitors be administered during the third trimester?. Nat Rev Gastroenterol Hepatol 2013;10:130-1. http://dx.doi.org/10.1038/nrgastro.2012.248.
- Ochsenkuhn T, Sackmann M, Goke B. Infliximab for acute, not steroid-refractory ulcerative colitis: a randomized pilot study. Eur J Gastroenterol Hepatol 2004;16:1167-71. http://dx.doi.org/10.1097/00042737-200411000-00014.
- Orlando A, Renna S, Mocciaro F, Cappello M, Di MR, Randazzo C, et al. Adalimumab in steroid-dependent Crohn’s disease patients: prognostic factors for clinical benefit. Inflamm Bowel Dis 2012;18:826-31. http://dx.doi.org/10.1002/ibd.21835.
- Oussalah A, Laclotte C, Chevaux JB, Bensenane M, Babouri A, Serre AA, et al. Long-term outcome of adalimumab therapy for ulcerative colitis with intolerance or lost response to infliximab: a single-centre experience. Aliment Pharmacol Ther 2008;28:966-72. http://dx.doi.org/10.1111/j.1365-2036.2008.03811.x.
- Oussalah A, Evesque L, Laharie D, Roblin X, Boschetti G, Nancey S, et al. A multicenter experience with infliximab for ulcerative colitis: outcomes and predictors of response, optimization, colectomy, and hospitalization. Am J Gastroenterol 2010;105:2617-25. http://dx.doi.org/10.1038/ajg.2010.345.
- Panccione R, Ghosh S, Middleton S, Marquez JR, Khalif I, Flint L, et al. Infliximab, Azathioprine, or Infliximab + Azathioprine for Treatment of Moderate to Severe Ulcerative Colitis: The UC Success Trial n.d.
- Panaccione R, Ghosh S, Middleton S, Marquez J, Flint L, Hoogstraten JV, et al. Improvement in Patient Quality of Life During Treatment With Infliximab, Azathioprine, or Combination Infliximab+Azathioprine for Moderate-to-Severe Ulcerative Colitis n.d.
- Pardi DS, Sandborn WJ. Infliximab in ulcerative colitis is associated with an increased risk of postoperative complications after restorative proctocolectomy: commentary. Dis Colon Rectum 2008;51:1207-9.
- Pastore S, Londero M, Gortani G, Abate MV, Marchetti F, Di Leo G, et al. Infliximab-related vasculitis in patients affected by ulcerative colitis. J Pediatr Gastroenterol Nutr 2010;51:226-8. http://dx.doi.org/10.1097/MPG.0b013e3181e5e198.
- Pastorelli L, Pizarro TT, Cominelli F, Vecchi M. Emerging drugs for the treatment of ulcerative colitis. Expert Opin Emerg Drugs 2009;14:505-21. http://dx.doi.org/10.1517/14728210903146882.
- Pearce CB, Lawrance IC. Careful patient selection may improve response rates to infliximab in inflammatory bowel disease. J Gastroenterol Hepatol 2007;22:1671-7. http://dx.doi.org/10.1111/j.1440-1746.2006.04739.x.
- Peyrin-Biroulet L, Laclotte C, Roblin X, Bigard MA. Adalimumab induction therapy for ulcerative colitis with intolerance or lost response to infliximab: an open-label study. World J Gastroenterol 2007;13:2328-32. http://dx.doi.org/10.3748/wjg.v13.i16.2328.
- Pola S, Fahmy M, Evans E, Tipps A, Sandborn WJ. Successful use of infliximab in the treatment of corticosteroid dependent collagenous colitis. Am J Gastroenterol 2013;108:857-8. http://dx.doi.org/10.1038/ajg.2013.43.
- Reinisch WF, Sandborn WJ. Infliximab concentration and clinical outcome in patients with ulcerative colitis. Gastroenterology 2012;142. http://dx.doi.org/10.1016/S0016-5085(12)60431-4.
- Rizzello F, Pratico C, Calabrese C, Gionchetti P. Rescue therapy: ciclosporin or infliximab?. Expert Rev Clin Immunol 2013;9:503-5. http://dx.doi.org/10.1586/eci.13.34.
- Rostholder E, Ahmed A, Cheifetz AS, Moss AC. Outcomes after escalation of infliximab therapy in ambulatory patients with moderately active ulcerative colitis. Aliment Pharmacol Ther 2012;35:562-7. http://dx.doi.org/10.1111/j.1365-2036.2011.04986.x.
- Rubin DT, Mulani P, Chao J, Pollack PF, Bensimon AG, Yu AP, et al. Effect of adalimumab on clinical laboratory parameters in patients with Crohn’s disease: results from the CHARM trial. Inflamm Bowel Dis 2012;18:818-25. http://dx.doi.org/10.1002/ibd.21836.
- Russell GH, Katz AJ. Infliximab is effective in acute but not chronic childhood ulcerative colitis. J Pediatr Gastroenterol Nutr 2004;39:166-70. http://dx.doi.org/10.1097/00005176-200408000-00008.
- Rutgeerts P. Infliximab for ulcerative colitis: the need for adequately powered placebo-controlled trials. Am J Gastroenterol 2002;97:2488-9. http://dx.doi.org/10.1111/j.1572-0241.2002.06049.x.
- Rutgeerts P, Vermeire S, Van AG. Predicting the response to infliximab from trough serum levels. Gut 2010;59:7-8. http://dx.doi.org/10.1136/gut.2009.191411.
- Rutgeerts P, Feagan B, Marano C, Strauss R, Johanns J, Zhang H, et al. Phase 2 3 Randomized, Placebo-Controlled, Double-Blind Study of SC Golimumab Induction in Moderate to Severe UC 2013.
- Rutgeerts P, Feagan BG, Marano C, Strauss R, Johanns J, Zhang H, et al. Phase 3 Study to Evaluate the SC Golimumab Maintenance Therapy in Moderate to Severe UC: Pursuit-Maintenance n.d.
- Salvana EMT, Salata RA. Infectious complications associated with monoclonal antibodies and related small molecules. Clini Microbiol Rev 2009;22:274-90. http://dx.doi.org/10.1128/CMR.00040-08.
- Sandborn WJ, Wagner CL, Fasanmade AA, Diamond RH, Olson A, Marano CW, et al. Effects of immunomodulators on pharmacokinetics and immunogenicity of inffiximab adminstered as 3-dose induction followed by systematic maintenance therapy in IBD. Gastroenterology 2007;132:A504-5.
- Sandborn WJ. Mucosal healing with infliximab: results from the active ulcerative colitis trials. Gastroenterol Hepatol 2012;8:117-19.
- Sandborn WJ, Van Assche GA, Reinisch W, Colombel JF, D’Haens GR, Wolf DC, et al. Induction and maintenance of clinical remission by adalimumab in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2011;140:S123-4. http://dx.doi.org/10.1097/00054725-201112002-00008.
- Sandborn WJ, Wolf DC, Van AG, Yang M, Mulani PM, Reilly N, et al. Rapid Onset of Adalimumab and Long-Term Efficacy Among Week-8 Responders in Adults With Moderate to Severe Active Ulcerative Colitis n.d.
- Sandborn W, Feagan B, Yang M, Skup M, Thakkar R, Lazar A, et al. Adalimumab Therapy Reduces Hospitalization Rates in Patients With Ulcerative Colitis Among Initial Responders n.d.
- Sandborn WJ, Colombel JF, D’Haens G, Van Assche G, Wolf D, Kron M, et al. One-year maintenance outcomes among patients with moderately-to-severely active ulcerative colitis who responded to induction therapy with adalimumab: subgroup analyses from ULTRA 2. Aliment Pharmacol Ther 2013;37:204-13. http://dx.doi.org/10.1111/apt.12145.
- Sandborn WJ, Feagan BG, Marano CW, Strauss R, Johanns J, Zhang H, et al. A Phase 2 3 Randomized, Placebo-Controlled, Double-Blind Study to Evaluate the Safety and Efficacy of Subcutaneous Golimumab Induction Therapy in Patients With Moderately to Severely Active Ulcerative Colitis: Pursuit SC n.d.
- Sandborn W, Feagan B, Lazar A, Thakkar R, Skup M, Yang M, et al. Adalimumab Induction Dose Reduces Hospitalization Risk in Patients With Ulcerative Colitis During the First 8 Weeks of Therapy n.d.
- Sandborn W, Feagan B, Marano C, Strauss R, Johanns J, Zhang H, et al. Clinical Response Is a Meaningful Endpoint in Ulcerative Colitis Clinical Studies n.d.
- Sandborn WJ, Loftus EV. Balancing the risks and benefits of infliximab in the treatment of inflammatory bowel disease. Gut 2004;53:780-2. http://dx.doi.org/10.1136/gut.2003.020552.
- Sands BE, Tremaine WJ, Sandborn WJ, Rutgeerts PJ, Hanauer SB, Mayer L, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis 2001;7:83-8. http://dx.doi.org/10.1097/00054725-200105000-00001.
- Scholmerich J. Balancing the risks and benefits of prolonged use of infliximab. Gut 2009;58:780-2. http://dx.doi.org/10.1136/gut.2008.166702.
- Sciaudone G, Pellino G, Guadagni I, Selvaggi F. Infliximab avoiding colectomy and maintaining remission in pediatric immunosuppressive-naive ulcerative colitis achieving remarkable mucosal healing. Panminerva Med 2010;52:91-2.
- Sciaudone G, Pellino G, Guadagni I, Selvaggi F. Education and imaging: gastrointestinal: herpes simplex virus-associated erythema multiforme (HAEM) during infliximab treatment for ulcerative colitis. J Gastroenterol Hepatol 2011;26. http://dx.doi.org/10.1111/j.1440-1746.2011.06622.x.
- Seirafi M, Treton X, De Vroey B, Cosnes J, Roblin X, Allez M, et al. Anti-TNF therapy and pregnancy in inflammatory bowel disease: a prospective cohort study from the GETAID. Gastroenterology 2011;140.
- Siemanowski B, Regueiro M. Efficacy of infliximab for extraintestinal manifestations of inflammatory bowel disease. Curr Treat Options in Gastroenterol 2007;10:178-84. http://dx.doi.org/10.1007/s11938-007-0011-5.
- Simmons J, Jewell DP. Infliximab for ulcerative colitis. Dig Liver Dis 2002;34:616-18. http://dx.doi.org/10.1016/S1590-8658(02)80201-8.
- Singh S, Loftus J. Management of severe steroid-refractory ulcerative colitis: cyclosporine or infliximab?. Gastroenterology 2013;144:1138-40. http://dx.doi.org/10.1053/j.gastro.2013.03.037.
- Sjöberg M, Walch A, Meshkat M, Gustavsson A, Järnerot G, Vogelsang H, et al. Infliximab or cyclosporine as rescue therapy in hospitalized patients with steroid-refractory ulcerative colitis: a retrospective observational study. Inflamm Bowel Dis 2012;18:212-18. http://dx.doi.org/10.1002/ibd.21680.
- Smith K. Golimumab shows promise in treatment of active ulcerative colitis. Nat Rev Gastroenterol Hepatol 2013;10. http://dx.doi.org/10.1038/nrgastro.2013.108.
- Sokol H, Seksik P, Carrat F, Nion-Larmurier I, Vienne A, Beaugerie L, et al. Usefulness of co-treatment with immunomodulators in patients with inflammatory bowel disease treated with scheduled infliximab maintenance therapy. Gut 2010;59:1363-8. http://dx.doi.org/10.1136/gut.2010.212712.
- Stein OM, Stein P. Prediction of clinical responders to treatment with DIMS0150 a Toll-Like Receptor 9 agonist in therapy refractory ulcerative colitis patients. J Crohns Colitis 2013;7. http://dx.doi.org/10.1016/S1873-9946(13)60429-8.
- Su C, Salzberg BA, Lewis JD, Deren JJ, Kornbluth A, Katzka DA, et al. Efficacy of anti-tumor necrosis factor therapy in patients with ulcerative colitis. Am J Gastroenterol 2002;97:2577-84. http://dx.doi.org/10.1111/j.1572-0241.2002.06026.x.
- Taxonera C, Estelles J, Fernandez-Blanco I, Merino O, Marin-Jimenez I, Barreiro-de AM, et al. Adalimumab induction and maintenance therapy for patients with ulcerative colitis previously treated with infliximab. Aliment Pharmacol Ther 2011;33:340-8. http://dx.doi.org/10.1111/j.1365-2036.2010.04531.x.
- Thorlund K, Druyts E, Mills E, Fedorak R, Marshal J. Adalimumab Versus Infliximab for the Treatment of Moderate-to-Severe Ulcerative Colitis in Adult Patients With No Prior Anti-TNF Experience: An Indirect Comparison Meta-Analysis n.d.
- Toedter G, Ma K, Li K, Marano CW, Macoritto M, Park J, et al. Genes Associated With Reduced Epithelial Permeability and Epithelial-Mesenchymal Transition: Changes in Expression Following Infliximab Therapy in Ulcerative Colitis n.d.
- Toedter G, Li K, Marano C, Ma K, Sague S, Huang CC, et al. Gene expression profiling and response signatures associated with differential responses to infliximab treatment in ulcerative colitis. Am J Gastroenterol 2011;106:1272-80. http://dx.doi.org/10.1038/ajg.2011.83.
- Travis S. Does it all ADA up? Adalimumab for ulcerative colitis. Gut 2011;60:741-2. http://dx.doi.org/10.1136/gut.2010.233403.
- Tursi A, Elisei W, Brandimarte G, Giorgetti G, Penna A, Castrignano V. Safety and effectiveness of infliximab for inflammatory bowel diseases in clinical practice. Eur Rev Med Pharmacol Sci 2010;14:47-55.
- Van Assche G. The optimization of immunosuppressive and biologic cotherapies in inflammatory bowel disease. Gastroenterol Hepatol 2008;4:197-9.
- Van Assche G, Vermeire S, Rutgeerts P. Management of loss of response to anti-TNF drugs: change the dose or change the drug?. J Crohns Colitis 2008;2:348-51. http://dx.doi.org/10.1016/j.crohns.2008.05.011.
- van Casteren-Messidoro C, Prins G, van TA, Zelinkova Z, Schouten J, de Man R. Autoimmune hepatitis following treatment with infliximab for inflammatory bowel disease. J Crohns Colitis 2012;6:630-1. http://dx.doi.org/10.1016/j.crohns.2012.01.017.
- Velayos F, Mahadevan U. Management of steroid-dependent ulcerative colitis: immunomodulatory agents, biologics, or surgery?. Clin Gastroenterol Hepatol 2007;5:668-71. http://dx.doi.org/10.1016/j.cgh.2007.03.028.
- Vermeire S, Ghosh S, Panes J, Dahlerup JF, Luegering A, Sirotiakova J, et al. The mucosal addressin cell adhesion molecule antibody PF-00547,659 in ulcerative colitis: a randomised study. Gut 2011;60:1068-75. http://dx.doi.org/10.1136/gut.2010.226548.
- Warner B, Harris AW. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;143. http://dx.doi.org/10.1053/j.gastro.2012.03.055.
- Waters H, McKenzie RS, Lunacsek O, Lennert BJ. Gastrointestinal-Related Service Utilization and Surgical Procedures Following Infliximab Initiation in Patients With Inflammatory Bowel Disease n.d.
- Waters H, McKenzie R, Lunacsek O, Franklin M, Lennert B, Piech C. Stability of Infliximab Dosing in Ulcerative Colitis: Results from a Chart Review n.d.
- Willert RP, Lawrance IC. Use of infliximab in the prevention and delay of colectomy in severe steroid dependant and refractory ulcerative colitis. World J Gastroenterol 2008;14:2544-9. http://dx.doi.org/10.3748/wjg.14.2544.
- Wolf R, Matz H, Orion E, Ruocco V. Anti-TNF therapies – the hope of tomorrow. Clin Dermatol 2002;20:522-30. http://dx.doi.org/10.1016/S0738-081X(02)00273-0.
- Wolf D, D’Haens G, Sandborn W, Colombel J-F, Van AG, Lazar A, et al. Rate of and Response to Dose Escalation in Patients Treated With Adalimumab for Moderately-to-Severely Active Ulcerative Colitis: Ultra 2 Subanalysis n.d.
- Yamamoto T, Shiraki M. The impact of preoperative infliximab therapy on postoperative complications in patients with ulcerative colitis. J Crohns Colitis 2012;6. http://dx.doi.org/10.1016/j.crohns.2012.06.009.
- Yamamoto-Furusho JK, Uzcanga LF. Infliximab as a rescue therapy for hospitalized patients with severe ulcerative colitis refractory to systemic corticosteroids. Dig Surg 2008;25:383-6. http://dx.doi.org/10.1159/000170882.
- Yapali S, Hamzaoglu HO. Anti-TNF treatment in inflammatory bowel disease. Ann Gastroenterol 2007;20:48-53.
- Sandborn WJ, Van AG, Thakkar RB, Lazar A, Kron M, Yang M, et al. Adalimumab Improves Health-Related Quality of Life for 52 Weeks in Patients With Ulcerative Colitis n.d.
- Sandborn W, Colombel J-F, Feagan B, Marano C, Strauss R, Han C, et al. Early and Sustained Remission After Treatment With Subcutaneously Administered Golimumab Is Associated With Normalized Health-Related Quality of Life in Patients With Moderate to Severe Ulcerative Colitis: Post-HOC Analysis from Pursuit Induction and Maintenance Trials n.d.
- Panaccione R, Ghosh S, Middleton S, Velazquez JRM, Khalif I, Flint L, et al. Infliximab, Azathioprine, or Infliximab + Azathioprine for Treatment of Moderate to Severe Ulcerative Colitis: The UC SUCCESS Trial n.d.
- Lichtenstein GR, Diamond RH, Wagner CL, Fasanmade AA, Olson AD, Marano CW, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210-26. http://dx.doi.org/10.1111/j.1365-2036.2009.04027.x.
- Reinisch W, Sandborn W, Feagan B, Ghosh S, Robinson A, Skup M, et al. Association Between Week Eight Mayo Subscores and Hospitalization Rates in Adalimumab-Treated Patients With Ulcerative Colitis from Ultra 1 and Ultra 2 n.d.
- Reinisch W, Sandborn W, Thakkar R, Lazar A, Huang B, Mulani P, et al. Adalimumab Induction Therapy Improves Health-Related Quality of Life in Patients With Moderately to Severely Active Ulcerative Colitis n.d.
- Feagan B, Gibson P, Marano C, Strauss R, Han C, Johanns J, et al. Impact of Golimumab SC on Disease Specific and Generic Hrqol in Patients With Moderately to Severely Active UC: Pursuit-SC Induction n.d. http://dx.doi.org/10.1097/00054725-201212001-00102.
- Feagan BG, Reinisch W, Rutgeerts P, Sandborn WJ, Yan S, Eisenberg D, et al. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol 2007;102:794-802. http://dx.doi.org/10.1111/j.1572-0241.2007.01094.x.
- Metz DC, Vakil N, Keeffe EB, Lichtenstein GR. Advances in gastrointestinal pharmacotherapy. Clin Gastroenterol Hepatol 2005;3:1167-79. http://dx.doi.org/10.1016/S1542-3565(05)00895-5.
- Reinisch W, Sandborn WJ, Bala M, Yan S, Feagan BG, Rutgeerts P, et al. Response and remission are associated with improved quality of life, employment and disability status, hours worked, and productivity of patients with ulcerative colitis. Inflamm Bowel Dis 2007;13:1135-40. http://dx.doi.org/10.1002/ibd.20165.
Appendix 1 MEDLINE search for clinical effectiveness evidence
Database: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations (via Ovid).
Searched: 1946 to December 2013.
URL: Gateway.ovid.com.
Search strategy
-
Colitis, Ulcerative/
-
ulcerative colitis.tw.
-
colitis ulcerosa.tw.
-
uc.tw.
-
colitis ulcerative.tw.
-
Colitis/
-
colitis.tw.
-
colitides.tw.
-
Inflammatory Bowel Diseases/
-
inflammatory bowel disease$.tw.
-
ibd.tw.
-
(col* and ulcer*).tw.
-
colitis gravis.tw.
-
proctocolitis.tw.
-
or/1-14
-
adalimumab.af.
-
humira.af.
-
d 2e7.af.
-
d2e7.af.
-
331731-18-1.rn.
-
infliximab.af.
-
remicade.af.
-
170277-31-3.rn.
-
ta650.af.
-
ta 650.af.
-
inx.af.
-
remsima.af.
-
inflectra.af.
-
ct p13.af.
-
ctp13.af.
-
golimumab.af.
-
simponi.af.
-
cnto148.af.
-
cnto 148.af.
-
476181-74-5.rn.
-
tnf inhibitor$.tw.
-
anti tnf.tw.
-
antitnf.tw.
-
tnf antagonist$.tw.
-
tnf-alpha blocker$.tw.
-
antitumo?r necrosis factor.tw.
-
Biosimilar Pharmaceuticals/
-
(biosimilar$ or biologic$).tw.
-
or/16-43
-
15 and 44
Terms 1–14 are terms for the condition (ulcerative colitis) which are then combined using OR in term 15. Terms 16–43 are terms for the interventions (infliximab, adalimumab and golimumab) which are then combined using OR in term 44. Terms 15 and 44 are then combined using AND to find studies on the condition and interventions in term 45.
To retrieve RCTs and systematic reviews specially designed highly sensitive search filter were combined with term 45. RCT filter and systematic review filter below.
Randomised controlled trial filter
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
49 placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
or/1-8
-
exp animals/ not humans.sh.
-
9 not 10
Systematic review filter
-
Meta-Analysis/
-
meta analy$.tw.
-
metaanaly$.tw.
-
meta analysis.pt.
-
(systematic adj (review$1 or overview$1)).tw.
-
exp Review Literature/
-
or/1-6
-
cochrane.ab.
-
embase.ab.
-
(psychlit or psyclit).ab.
-
(psychinfo or psycinfo).ab.
-
(cinahl or cinhal).ab.
-
science citation index.ab.
-
bids.ab.
-
cancerlit.ab.
-
or/8-15
-
reference list$.ab.
-
bibliograph$.ab.
-
hand-search$.ab.
-
relevant journals.ab.
-
manual search$.ab.
-
or/17-78
-
selection criteria.ab.
-
data extraction.ab.
-
23 or 24
-
review.pt.
-
25 and 26
-
comment.pt.
-
letter.pt.
-
editorial.pt.
-
animal/
-
human/
-
31 not (31 and 32)
-
or/28-30,33
-
7 or 16 or 22 or 27
-
35 not 34
Appendix 2 Table of excluded studies
| Author and year/NCT number | Reason for exclusion |
|---|---|
| Actis et al., 2002161 | Not a RCT |
| Actis, 2003162 | Not a RCT |
| Afif et al., 2009163 | Not a RCT |
| Allez et al., 2010164 | Not a RCT |
| (No authors listed) 2007165 | Not a RCT |
| Armuzzi et al., 2004166 | Not protocol-eligible population. No prior immunosuppressant use reported |
| Baert et al., 2007167 | Not a RCT |
| Barbato et al., 2006168 | Not a RCT |
| Barreiro-de et al., 2009169 | Not a RCT |
| Baumgart, 2010170 | Not a RCT |
| Ben-Horin, 2012171 | Not treatment of interest (rituximab) |
| Bengi and Akpinar, 2012172 | Not a RCT |
| Biancone et al., 2009173 | Not a RCT |
| (No authors listed) 2012174 | Not a RCT |
| Bordeianou, 2009175 | Not a RCT |
| Borruel et al., 2013176 | Not a RCT |
| Brooklyn et al., 2006177 | Not UC trial population |
| Bujanover and Weiss, 2008178 | Not a RCT |
| Busquets and Aldeguer, 2013179 | Not a RCT |
| Carbone et al., 2009180 | Not a RCT |
| Cariñanos et al., 2011181 | Not a RCT |
| Casteele et al., 2012182,183 | Evaluation of two IFX dosing strategies |
| Charles et al., 2010184 | Not a RCT |
| Chen et al., 2013185 | Not a RCT |
| Chey and Shah, 2005186 | Not a RCT |
| Chowers et al., 2010187 | Not a RCT |
| Chuang et al., 2010188 | Not a RCT |
| Cohen, 2003189 | Not a RCT |
| Colombel et al., 2011190 | No protocol-eligible outcome data |
| Colombel et al., 2011190 | Not a RCT |
| Colombel et al., 2012191 | Parallel publication, duplicate outcome data |
| Cottone, et al., 2008192 | Not a RCT |
| Croft et al., 2013193 | Population outside scope of appraisal (use of biological in acute severe UC following failure of i.v. steroids) |
| Cross et al., 2008194 | Not a RCT |
| Danese, 2013195 | Unable to obtain |
| De Vos et al., 2012196 | Not a RCT |
| de Vries et al., 2009197 | Not a RCT |
| D’Haens, 2005198 | Not a RCT |
| Dean et al., 2012199 | Not a RCT |
| Dignass et al., 2012200 | Not a RCT |
| Domènech et al., 2010201 | Not a RCT |
| Dranitsaris et al., 2012202 | Not a RCT |
| Eidelwein et al., 2005203 | Not a RCT |
| Erikkson et al., 2012204 | Not a RCT |
| Esteve et al., 2011205 | Not a RCT |
| EUCTR2007–006692–37-GB206 | Not UC trial population |
| EUCTR2007–007702–30-IT206 | Not a RCT |
| EUCTR2007–000842–11-AT206 | Not a RCT |
| EUCTR2008–007519–34-SE206 | Not a RCT |
| EUCTR2011–002411–29-SE206 | Not a RCT |
| EUCTR2011–006084–22-GB206 | Not a RCT |
| Fanjiang et al., 2007207 | Not a RCT |
| Fasanmade et al., 2009208 | No protocol-eligible outcome data |
| Fasanmade et al., 2010209 | No protocol-eligible outcome data |
| Feagan et al., 2005210 | Not a RCT |
| Feagan, 2006211 | Not a RCT |
| Florholmen et al., 2011212 | Population outside scope of appraisal (use of biological in acute severe UC following failure of i.v. steroids) |
| Ford et al., 20133 | Not a RCT |
| Gao and Jiang, 2013213 | Not available in English language |
| Gavalas et al., 2007214 | Population outside scope of appraisal (use of biological in acute severe UC) |
| Gearry and Falvey, 2012215 | Not a RCT |
| Gies et al., 2010216 | Not a RCT |
| Ginard et al., 2008217 | Not a RCT |
| Grosen et al., 2013218 | Not a RCT |
| Gustavsson et al., 2010219 | Follow-on study to excluded Järnerot et al., 2005112 |
| Ha et al., 2011220 | Not a RCT |
| Halpin et al., 2010221 | Not a RCT |
| Halpin and Hamlin, 2012222 | Not a RCT |
| Hämäläinen et al., 2011223 | Not a RCT |
| Hanauer, 2005224 | Not a RCT |
| Hanauer et al., 2008225 | Not a RCT |
| Hanauer et al., 2008226 | Not a RCT |
| Heraganahally et al., 2009227 | Not a RCT |
| Herrlinger et al., 2010228 | Not a RCT |
| Honeywell et al., 2007229 | Unable to obtain |
| Hyams et al., 2010230 | Not a RCT |
| Hyams et al., 2011231 | Unable to obtain |
| Assasi, 2009232 | Not a RCT |
| Jackson, 2007233 | Not a RCT |
| Järnerot et al., 2005112 | Population outside scope of appraisal (use of biological in acute UC following failure of i.v. steroids) |
| Järnerot, 2006234 | Not a RCT |
| Jiménez, 2004235 | Not a RCT |
| Joob and Wiwanikit, 2013236 | Not a RCT |
| JPRN-UMIN000006169206 | Not a RCT |
| JPRN-UMIN000007256206 | Not a RCT |
| JPRN-UMIN000007806206 | Not a RCT |
| JPRN-UMIN000010205206 | Not a RCT |
| JPRN-UMIN000013033206 | Not a RCT |
| Kaser and Tilg, 2008237 | Not a RCT |
| Kaur and Targan, 2013238 | Not a RCT |
| Kerbleski and Gottlieb, 2009239 | Not a RCT |
| Klotz, et al., 2007240 | Not a RCT |
| Kohn et al., 2004241 | Not a RCT |
| Kohn et al., 2007242 | Not a RCT |
| Kohn, 2008243 | Not a RCT |
| Laharie et al., 2012244,245 | Population outside scope of appraisal (use of biological in acute severe UC following failure of i.v. steroids) |
| Leal et al., 2012246 | Not a RCT |
| LeBlanc et al., 2013247 | Not a RCT |
| Leblanc et al., 2011248 | Not a RCT |
| Levesque and Sandborn, 2012249 | Not a RCT |
| Levy, 2009250 | Not a RCT |
| Li et al., 2013251 | No protocol-eligible outcome data |
| Lichtenstein, 2001252 | Not a RCT |
| Lichtenstein, 2009253 | Not a RCT |
| Liu et al., 2013254 | Not a RCT |
| Löfberg et al., 2012255 | Not UC trial population |
| Lorenzo-Zúñiga et al., 2013256 | Not a RCT |
| Mallow et al., 2013257 | Not a RCT |
| Mallow et al., 2013258 | Not a RCT |
| Maser et al., 2008259 | Not a RCT |
| Matsumoto, 2007260 | Not a RCT |
| Mazumdar and Greenwald, 2009261 | Not a RCT |
| McCann and Smith, 2013262 | Not a RCT |
| Molnár et al., 2010263 | Not a RCT |
| Molnár et al., 2011264 | Not a RCT |
| Molnár et al., 2011265 | Not a RCT |
| Moss and Farrell, 2006266 | Not a RCT |
| Nakase et al., 2010267 | Not a RCT |
| National Institute for Health Research, 2011268 | Not a RCT |
| National Institute for Health Research, 2011269 | Not a RCT |
| National Institute for Health Research, 2013270 | Not a RCT |
| NCT00207688206 | Not a RCT |
| NCT00421642206 | Not a RCT |
| NCT00488774271 | Unlicensed route of administration for intervention |
| NCT00573794271 | Not a RCT |
| NCT00586599271 | Not a RCT |
| NCT00586807271 | Not a RCT |
| NCT00606346271 | Not a RCT |
| NCT00705484271 | Not a RCT |
| NCT00745329271 | Not a RCT |
| NCT00791557271 | Not a RCT |
| NCT00955123271 | Not a RCT |
| NCT00984568271 | Evaluation of different IFX treatment strategies |
| NCT01346826271 | Evaluation of accelerated IFX infusions |
| NCT01408810271 | Not a RCT |
| NCT01417728271 | Not a RCT |
| NCT01494857271 | Not a RCT |
| NCT01550965271 | Not a RCT |
| NCT01585155271 | Not a RCT |
| NCT01670240271 | Evaluation of biological in treatment of chronic pouchitis (trial currently recruiting) |
| NCT01716039271 | Study evaluating ADA–MTX interaction |
| NCT01787786271 | Not a RCT |
| NCT01846026271 | Not protocol-eligible intervention |
| NCT01848561271 | Not a RCT |
| NCT01851343206 | Not a RCT |
| NCT01900574271 | Not a RCT |
| NCT01947816271 | Not a RCT |
| NCT01960426271 | Evaluation of two dosing methods |
| NCT01971814271 | Not a RCT |
| NCT01988961271 | Not a RCT |
| NCT02057016206 | Not a RCT |
| NCT02073526206 | Not a RCT |
| Nguyen and Prather, 2009272 | Not a RCT |
| Nielsen and Jess, 2013273 | Unable to obtain |
| Ochsenkühn et al., 2004274 | Population outside scope of appraisal: (1) use of biological in acute severe UC, (2) no patients were receiving immunosuppressants/immunomodulators > 10 mg/day prednisolone at baseline and, therefore, not inadequate responders/stated intolerant to conventional therapy options |
| Orlando et al., 2012275 | Not UC trial population |
| Oussalah et al., 2008276 | Not a RCT |
| Oussalah et al., 2010277 | Not a RCT |
| Panncione et al., 2011278 | Parallel publication, duplicate outcome data |
| Panncione et al., 2013279 | Parallel publication, duplicate outcome data |
| Pardi and Sandborn, 2008280 | Not a RCT |
| Pastore et al., 2010281 | Not a RCT |
| Pastorelli et al., 2009282 | Not a RCT |
| Pearce and Lawrance, 2007283 | Not a RCT |
| Peyrin-Biroulet et al., 2007284 | Not a RCT |
| Pola et al., 2013285 | Not a RCT |
| Reinisch and Sandborn 2012286 | No protocol-eligible outcome data |
| Rizzello et al., 2013287 | Not a RCT |
| Rostholder et al., 2012288 | Not a RCT |
| Rubin et al., 2012289 | Not UC trial population |
| Russell and Katz, 2004290 | Not a RCT |
| Rutgeerts, 2002291 | Not a RCT |
| Rutgeerts et al., 2010292 | Not a RCT |
| Rutgeerts et al., 2013293 | Parallel publication, duplicate outcome data |
| Rutgeerts et al., 2013294 | Parallel publication, duplicate outcome data |
| Salvana and Salata, 2009295 | Not a RCT |
| Sandborn et al., 2007296 | Not a RCT |
| Sandborn et al., 200967 | Not a RCT |
| Sandborn, 2012297 | Not a RCT |
| Sandborn et al., 2011298 | Parallel publication, duplicate outcome data |
| Sandborn et al., 2011299 | Parallel publication, duplicate outcome data |
| Sandborn et al., 2012300 | Parallel publication, duplicate outcome data |
| Sandborn et al., 2012301 | Parallel publication, duplicate outcome data |
| Sandborn et al., 2012302 | Parallel publication, duplicate outcome data |
| Sandborn et al., 201260 | Parallel publication, duplicate outcome data |
| Sandborn et al., 2012303 | Unable to obtain |
| Sandborn et al., 2012304 | Unable to obtain |
| Sandborn and Loftus, 2004305 | Not a RCT |
| Sands et al., 2001306 | Population outside scope of appraisal (use of biological in acute severe UC following failure of i.v. steroids) |
| Scholmerich, 2009307 | Not a RCT |
| Sciaudone et al., 2010308 | Not a RCT |
| Sciaudone et al., 2011309 | Not a RCT |
| Seirafi et al., 2011310 | Not a RCT |
| Siemanowski and Regueiro, 2007311 | Not a RCT |
| Simmons and Jewell, 2002312 | Not a RCT |
| Singh and Loftus, 2013313 | Not a RCT |
| Sjöberg et al., 2012314 | Not a RCT |
| Smith, 2013315 | Unable to obtain |
| Sokol et al., 2010316 | Not a RCT |
| Stein and Stein 2013317 | Unable to obtain |
| Su et al., 2002318 | Not a RCT |
| Taxonera et al., 2011319 | Not a RCT |
| Thorlund et al., 2013320 | Not a RCT |
| Toedter et al., 2010321 | No protocol-eligible outcome data |
| Toedter et al., 2011322 | No protocol-eligible outcome data |
| Travis, 2011323 | Not a RCT |
| Tursi et al., 2010324 | Not a RCT |
| Van Assche, 2008325 | Not a RCT |
| Van Assche, et al., 2008326 | Not a RCT |
| van Casteren-Messidoro et al., 2012327 | Not a RCT |
| Velayos and Mahadevan, 2007328 | Not a RCT |
| Vermeire et al., 2011329 | Not treatment of interest (antibody PF-00547,659) |
| Warner and Harris, 2012330 | Not a RCT |
| Waters et al., 2008331 | Unable to obtain |
| Waters et al., 2009332 | Not a RCT |
| Willert and Lawrance, 2008333 | Not a RCT |
| Wolf et al., 2007334 | Not a RCT |
| Wolf et al., 2012335 | Parallel publication, duplicate outcome data |
| Yamamoto and Shiraki, 2012336 | Not a RCT |
| Yamamoto-Furusho and Uzcanga, 2008337 | Not a RCT |
| Yapali and Hamzaoglu, 2007338 | Not a RCT |
Appendix 3 Table of numbers withdrawing and reasons for withdrawal
Participants withdrawing from treatment arms, reasons for withdrawal and risk of attrition bias assessment judgement
| Study number | Treatment arm | Number completing: n/N (%) | Reasons for withdrawal | Attrition bias judgement |
|---|---|---|---|---|
| ULTRA144 | PBO | ITT-A3 (amendment): 121/130 (93%) | ITT-A3 (amendment): AE, 5/130 (4%); lack of efficacy, 4/130 (3%) – overall, 9/130 (7%) | Low risk: ≤ 10% attrition in each group, numbers balanced across groups, ITT analysis presented |
| ULTRA144 | 160 mg/80 mg of ADA | ITT-A3 (amendment): 118/130 (91%) | ITT-A3 (amendment): AE, 6/130 (5%); withdrew consent, 2/130 (1.5%); lost to follow-up, 2/130 (1.5%); lack of efficacy, 2/13 (1.5%) – overall, 12/130 (9%) | |
| ULTRA245 | PBO | 135/260 (52%) switched to open label of which n = 84 dose escalated to 40 mg per week overall, 131/260 (50.4%); completed on 16 mg/80 mg/40 mg dosing, 56/260 (21.5%); switched to open label 40 mg every other week at week 12 (n = 135), 30/260 (11.5%); dose escalated to 40 mg weekly (n = 68), 45/260 (17.3%) | Site non-compliance, 10/258 (4%); lack of efficacy, 63/258 (24%); AE, 12/258 (5%); withdrew consent, 8/258 (3%); lost to follow-up, 1/258 (< 1%); protocol violation, 1/258 (< 1%); other, 9/258 (3%) | High risk: although ITT analysis was undertaken, there was a high level of attrition and an imbalance between treatment groups (PBO, 50%; ADA, 59%) |
| ULTRA245 | 160 mg of ADA at week 0, 80 mg at week 2 and then 40 mg EOW beginning at week 4 | 116/258 (45%) switched to open label of which n = 68 dose escalated to 40 mg week overall, 154/258 (59.7%); completed on 16 mg/80 mg/40 mg dosing, 82/258 (31.7%); switched to open label 40 mg every other week at week 12 (n = 116), 32/258 (12.4%); dose escalated to 40 mg weekly (n = 68), 40/258 (15.5%) | Site non-compliance, 14/260 (5%); lack of efficacy, 70/260 (27%); AE, 25/260 (10%); withdrew consent, 4/260 (1.5%); protocol violation, 5/260 (2%); other, 11/260 (4%) | |
| Suzuki46 | PBO | Week 8: 92/96 (96%), week 52: 73/96 (77%) | Week 8: total discontinued, 4/96 (4%) – lack of efficacy n = 2, AE n = 2. Week 52: total discontinued, 23/96 (23%) – withdrew consent n = 2, lack of efficacy n = 14, AE n = 7; moved to rescue therapy n = 63 | Induction: low risk – < 10% attrition in each group and numbers reasonably balanced across groups. All patients accounted for in the primary outcome analysis |
| Suzuki46 | 80 mg/40 mg of ADA | Week 8: 85/87 (98%) (unlicensed), week 52: 58/87 (67%) | Week 8: total discontinued, 2/87 (2%) – withdrew consent n = 1, lack of efficacy n = 1. Week 52: total discontinued, 29/87 (33%) – withdrew consent n = 3, lack of efficacy n = 17, AE n = 9; moved to rescue therapy n = 50 | Maintenance: high risk – PBO, 23%; ADA, 33% |
| Suzuki46 | 160 mg/80 mg of ADA | Week 8: 86/90 (96%), week 52: 60/90 (67%) | Week 8: total discontinued, 4/90 (4%) – lack of efficacy n = 1, AE n = 3. Week 52: total discontinued, 30/90 (33%) – lack of efficacy n = 16, AE n = 13, other n = 1; moved to rescue therapy n = 46 | |
| ULTRA354 | PBO | 91/121 (75%) | Lack of efficacy, 21/121 (17%); AE, 16/121 (13%); withdrew consent, 3/121 (2%); protocol violation, 1/121 (1%) | Extension study not included in risk of bias assessment |
| ULTRA354 | 80 mg/40 mg of ADA | 86/118 (73%) | Lack of efficacy, 17/118 (14%); AE, 12/118 (10%); withdrew consent, 5/118 (4%); lost to follow-up, 1/118 (1%); protocol violation, 1/118 (1%); other, 4/118 (3%) | |
| ULTRA354 | 160 mg/80 mg of ADA | 95/121 (79%) | Lack of efficacy, 15/121 (%); AE, 10/121 (%); withdrew consent, 4/121 (%); lost to follow-up, 1/121 (%); protocol violation, 1/121 (%) | |
| PURSUIT-SC47 | Phase II PBO | 41/42 plus 26/31 enrolled while Phase II data being analysed | 2/42 ‘other’ reasons | Low risk: ITT reported and withdrawal < 10% across all groups and n balanced |
| PURSUIT-SC47 | Phase II 200 mg/100 mg of GOL all randomised | 41/42 plus 31/31 enrolled while Phase II data being analysed | 1/42 withdrew consent | |
| PURSUIT-Maintenance48 | PBO | PBO 115/156 (73%) randomised completed through week 54 | Discontinued treatment prior to week 52 (n = 43): 17 AE, 19 unsatisfactory therapeutic effect, 1 lost to follow-up, 6 other. Terminated study before week 54 (n = 18): 5 withdrew consent, 3 lost to follow-up, 10 other | High risk: although ITT reported, withdrawal > 10% across all groups |
| PURSUIT- Maintenance48 | 50 mg of GOL | 50 mg of GOL. 120/154 (78%) randomised completed through week 54 | Discontinued treatment prior to week 52 (n = 43): 12 AE, 17 unsatisfactory therapeutic effect, 2 lost to follow-up, 12 other. Terminated study before week 54 (n = 18): 10 withdrew consent, 2 lost to follow-up, 6 other | |
| PURSUIT- Maintenance48 | 100 mg of GOL | 100 mg of GOL. 116/154 (75%) randomised completed through week 54 | Discontinued treatment prior to week 52 (n = 45): 12 AE, 22 unsatisfactory therapeutic effect, 1 lost to follow-up, 10 other. Terminated study before week 54 (n = 21): 11 withdrew consent, 2 lost to follow-up, 8 other | |
| UC-SUCCESS51 | AZA | 53/79 (66%) | AE, 11/80 (14%); withdrew consent, 8/80 (10%); non-compliance with protocol, 5/80 (6%); protocol ineligible, 3/80 (4%) | High risk: although ITT analysis was undertaken, there was a high level of attrition and an imbalance between treatment groups (AZA, 34%; IFX, 18%; IFX/AZA, 21%) |
| UC-SUCCESS51 | IFX | 65/78 (82%) | AE, 7/79 (9%); clinical event, 1/79 (1%); lost to follow-up, 1/79 (1%); withdrew consent, 3/79 (4%); non-compliance with protocol, 1/79 (1%); protocol ineligible, 1/79 (1%) | |
| UC-SUCCESS51 | IFX/AZA | 63/80 (79%) | AE, 8/80 (10%); withdrew consent, 4/80 (5%); non-compliance with protocol, 1/80 (1%); protocol ineligible, 2/80 (3%); administrative reasons, 2/80 (3%) | |
| Probert et al.50 | PBO | 20/20 (100%) | No withdrawals reported | Low risk: all patients accounted for in the primary outcome analysis |
| Probert et al.50 | IFX | 23/23 (100%) | ||
| Hyams et al.52 | 5 mg of IFX every 8 weeks | 18/22 (82%) completed infusions and follow-up | AE, 3/22 (14%); lack of efficacy, 1/22 (5%) | High risk: numbers withdrawing > 10% and unbalanced across groups (every 8 weeks, 21%; every 12 weeks, 51%) |
| Hyams et al.52 | 5 mg of IFX every 12 weeks | 12/23 (52%) complete infusions; 11/23 (49%) completed follow-up | AE, 6/23 (26%); lack of efficacy, 4/23 (17%); other, 1/23 (4%) | |
| ACT149 | PBO | 47 completed study infusions, of whom 46/121 completed follow-up. 74 discontinued study infusions, of whom 18 completed follow-up | In ACT1, similar numbers of patients in each group discontinued treatment because of an AE | High risk: although ITT reported, > 50% in PBO and > 30% in IFX 5 and 10 mg did not complete |
| ACT149 | 5 mg/kg of IFX | 76/121 completed study infusions, of whom 76 completed follow-up. 45 discontinued study infusions, of whom six completed follow-up | ||
| ACT249 | PBO | 67 completed study infusions, of whom 64/123 completed follow-up. 56 discontinued study infusion, nine completed follow-up | In ACT2, more patients in the PBO group than in the two IFX groups discontinued treatment because of an AE | High risk: although ITT reported, > 50% in PBO and > 30% in IFX 5 mg and 10 mg did not complete |
| ACT249 | 5 mg/kg of IFX | 97 completed study infusions, of whom 94/121 completed follow-up. 24 discontinued study infusions, of whom three completed follow-up |
Appendix 4 Additional efficacy outcomes tables
Additional efficacy outcomes (adult population trials)
| Study name | Treatment arm | Time point | Outcome measure |
|---|---|---|---|
| ULTRA144 | PBO | Week 8 | Subgroup analysis results: remission n/N (%): Mayo score < 10: 10/83 (12.0%) Mayo score ≥ 10: 2/47 (4.3%); extensive colitis: 11/73 (15.1%); no extensive colitis: 1/57 (1.8%); CS (without IMM%): 6/55 (10.9%); IMM (AZA and 6-MP without CS%): 0/18 (0%) 2/25 (8.0%) 6/28 (21.4%); IMM + CS: 2/34 (5.9%); no CS + no IMM: 4/23 aminosalicylates: 11/98 (11.2%); no aminosalicylates: 1/32 (3.1%); CRP < 10 mg/l: 7/95 (7.4%); CRP ≥ 10 mg/l: 4/32 (12.5%); weight < 70.0 kg: 5/35 (14.3%); weight ≥ 70.0 kg, < 82.0 kg: 3/43 (7.0%); weight ≥ 82.0 kg: 4/52 (7.7%) Change from baseline in CRP mg/l: median –0.09 (range –274.79 to 88.71) Rectal bleeding subscore of ≤ 1, 86/130 (66.2%) PGA subscore of ≤ 1, 61/130 (46.9%) Stool frequency subscore, 49/130 (37.7%) Reinisch et al.61 Change from baseline: Haemoglobin g/l, 4.4; p-value vs. PBO < 0.001 Haematocrit fraction, 0.014; p-value vs. PBO < 0.001 Red blood cells x1012/l, 0.16; p-value vs. PBO < 0.01 Total protein g/l, 1.5; p-value vs. PBO < 0.05 Albumin g/l, 1.3 CRP mg/l, –0.47 |
| ULTRA1 | 160 mg/80 mg of ADA | Week 8 | Reinisch et al.44 Subgroup analysis results: remission n/N (%): Mayo score < 10: 17/85 (20.0%), difference from PBO (95% CI) 1.5 (–8.7 to 11.8) 8.0 (–3.1 to 19.0) Mayo score ≥ 10: 7/45 (15.6%), difference from PBO (95% CI) 11.3 (–0.8 to 23.4); extensive colitis: 12/60 (20.0%), difference from PBO (95% CI) 4.9 (–8.1 to 18.0); no extensive colitis: 12/70 (17.1%), difference from PBO (95% CI) 15.4 (5.9 to 24.9); CS (without IMM%): 10/48 (20.8%), difference from PBO (95% CI) 11.3 (–3.0 to 25.7) IMM (AZA and 6-MP without CS%): 6/28 (21.4%), difference from PBO (95% CI) 20.7 (5.9 to 35.4); IMM + CS: 2/23 (8.7%), difference from PBO (95% CI) 4.1 (–11.2 to 19.5); no CS + no IMM: 6/31 (19.4%), difference from PBO (95% CI) –1.0 (–20.5 to 18.5); aminosalicylates: 18/105 (17.1%), difference from PBO (95% CI) 5.9 (–3.6 to 15.5); no aminosalicylates: 6/25 (24.0%), difference from PBO (95% CI) 20.9 (3.1 to 38.7); CRP < 10 mg/l: 21/101 (20.8%), difference from PBO (95% CI) 13.4 (3.9 to 22.9); CRP ≥ 10 mg/l: 2/25 (8.0%), difference from PBO (95% CI) –4.5 (–20.1 to 11.1); weight < 70.0 kg: 11/45 (24.4%), difference from PBO (95% CI) 10.2 (–6.9 to 27.2); weight ≥ 70.0 kg, < 82.0 kg: 8/33 (24.2%), difference from PBO (95% CI) 17.3 (0.8 to 33.8); weight ≥ 82.0 kg: 5/52 (9.6%), difference from PBO (95% CI) 1.9 (–8.9 to 12.7) Change from baseline in CRP mg/l: median –0.77 (range –95.09 to 130.41) Rectal bleeding subscore of ≤ 1, 101/130 (77.7%) PGA subscore of ≤ 1, 78/130 (60.0%) Stool frequency subscore, 63/130 (48.5%) Reinisch et al.61 Change from baseline: Haemoglobin g/l, 4.9; p-value vs. PBO < 0.001 Haematocrit fraction, 0.014; p-value vs. PBO < 0.001 Red blood cells × 1012/l, 0.19; p-value vs. PBO < 0.001 Total protein g/l, 1.7; p-value vs. PBO < 0.01 Albumin g/l, 1.7; p-value vs. PBO < 0.01 CRP mg/l, –0.87; p-value vs. PBO < 0.001 |
| ULTRA2 | PBO | Week 8 | Sandborn et al.45 No prior anti-TNF-α treatment: PGA ≤ 1, 63/145 (43.4%); SFS ≤ 1, 43/145 (29.7%); RBS ≤ 1, 86/145 (59.3%); prior anti-TNF-α treatment: PGA ≤ 1, 29/101 (28.7%); SFS ≤ 1, 27/101 (26.7%); RBS ≤ 1, 57/101 (56.4%) |
| ULTRA2 | PBO | Week 32 | EPAR (Humira)34 Number and percentage of subjects taking CSs at baseline who discontinued CS use and achieved clinical remission per Mayo score at week 32 (ITT analysis): clinical remission at week 32 – discontinued CS at any time prior to week 32, 10/140 (7.1%) clinical remission at week 32 – discontinued CS for ≥ 90 days prior to week 32, 9/140 (6.4%) |
| ULTRA2 | PBO | Week 52 | Sandborn et al.45 Discontinued CS use before week 52 and achieved clinical remission at week 52 among patients with baseline CS use, 5/81 (6.2%); discontinued CS use for ≥ 90 days before week 52 and achieved remission at week 52 among patients with baseline CS use, 5/81 (6.2%); discontinued CS use and achieved sustained clinical remission at both weeks 32 and 52 among patients with baseline CS use, 1/81 (1.2%); IBDQ responders at week 52, 31/145 (21.4%); prior anti-TNF-α treatment: discontinued CS use before week 52 and achieved clinical remission at week 52 among patients with baseline CS use, 3/59 (5.1%); discontinued CS use for ≥ 90 days before week 52 and achieved remission at week 52 among patients with baseline CS use, 3/59 (5.1%); discontinued CS use and achieved sustained clinical remission at both weeks 32 and 52 among patients with baseline CS use, 1/59 (1.7%); IBDQ responders at week 52, 9/101 (8.9%) EPAR (Humira)34 Discontinued corticosteroid use for ≥ 90 days before week 52 and achieved remission at week 52, 8/246 (5.7%); discontinued corticosteroid use and sustained remission at both week 32 and week 52, 2/246 (1.4%); IBDQ responders at week 52, 40/246 (16.3%); number and percentage of subjects taking CSs at baseline who discontinued CS use and achieved clinical remission per Mayo score at week 52 (ITT analysis): clinical remission at week 52 – discontinued CS at any time prior to week 52, 8/140 (5.7%); clinical remission at week 52 – discontinued CS for ≥ 90 days prior to week 52, 8/140 (5.7%) Sandborn et al.304 Week 52 CS-free remission: all PBO, 8/140 (5.7%); anti-TNF-α-naive PBO, 5/81 (6.2%); anti-TNF-α-exposed PBO, 3/59 (5.1%) week 52 CS-free: all PBO, 32/140 (22.9%); anti-TNF-α-naive PBO, 20/81 (24.7%); anti-TNF-α-exposed PBO, 12/59 (20.3%) Sandborn et al.60 Post-hoc analysis, week 52 n = 246: mean days in IBDQ remission (IBDQ score of ≥ 170), 79.00; mean SAE adjusted days in clinical remission, 48.23 |
| ULTRA2 | 160 mg of ADA at week 0, 80 mg at week 2 and then 40 mg EOW | Week 8 | Sandborn et al.45 Serum trough concentrations over time by remission status, mean (SD) [min., max.], Nnmiss: 40 mg EOW patients who were remitters (n = 43): 11.4 (5.15) [0.000, 22.8], 41 40 mg EOW patients who were non-remitters (n = 153): 8.49 (4.35) [0.000, 21.8], 110 No prior anti-TNF-α treatment: PGA ≤ 1, 88/150 (58.7%); p-value vs. PBO, 0.009SFS ≤ 1, 69/150 (46.0%); p-value vs. PBO, 0.004RBS ≤ 1, 116/150 (77.3%); p-value vs. PBO, 0.001 IBDQ responders, 102/150 (68.0%); p-value vs. PBO, 0.004 Prior anti-TNF-α treatment: PGA ≤ 1, 26/98 (26.5%); p-value vs. PBO, 0.731SFS ≤ 1, 25/98 (25.5%); p-value vs. PBO, 0.844 RBS ≤ 1, 58/98 (59.2%); p-value vs. PBO, 0.695IBDQ responders, 42/98 (42.9%); p-value vs. PBO, 0.370 |
| ULTRA2 | 160 mg of ADA at week 0, 80 mg at week 2 and then 40 mg EOW | Week 32 | Sandborn et al.45 Serum trough concentrations over time by remission status, mean (SD) [min., max.], Nnmiss: 40 mg EOW patients who were remitters (n = 43), 10.6 (5.64) [0.000, 26.9], 39 40 mg EOW patients who were non-remitters (n = 153), 6.95 (3.98) [0.000, 18.1], 70 |
| ULTRA2 | 160 mg of ADA at week 0, 80 mg at week 2 and then 40 mg EOW | Week 52 | Sandborn et al.45 Serum trough concentrations over time by remission status, mean (SD) [min., max.], Nnmiss: 40 mg EOW patients who were remitters (n = 43), 10.8 (7.45) [0.000, 39.3], 39 40 mg EOW patients who were non-remitters (n = 153), 6.18 (4.22) [0.000, 16.1], 62 No prior anti-TNF-α treatment: discontinued CS use before week 52 and achieved clinical remission at week 52 among patients with baseline CS use, 15/110 (13.6%); p-value vs. PBO, 0.096 discontinued CS use for ≥ 90 days before week 52 and achieved remission at week 52 among patients with baseline CS use, 15/110 (13.6%); p-value vs. PBO, 0.096; discontinued CS use and achieved sustained clinical remission at both weeks 32 and 52 among patients with baseline CS use, 11/110 (10.0%); p-value vs. PBO, 0.014; IBDQ responders at week 52, 48/150 (32.0%); p-value vs. PBO, 0.039; prior anti-TNF-α treatment: discontinued CS use before week 52 and achieved clinical remission at week 52 among patients with baseline CS use, 5/40 (12.5%); p-value vs. PBO, 0.263; discontinued CS use for ≥ 90 days before week 52 and achieved remission at week 52 among patients with baseline CS use, 5/40 (12.5%); p-value vs. PBO, 0.263; discontinued CS use and achieved sustained clinical remission at both weeks 32 and 52 among patients with baseline CS use, 4/40 (10.0%); p-value vs. PBO, 0.155; IBDQ responders at week 52, 17/98 (17.3%); p-value vs. PBO, 0.078 EPAR (Humira)34 Discontinued CS use before week 52 and achieved remission at week 52, 20/248 (13.3%); p-value vs. PBO, 0.035; discontinued corticosteroid use for ≥ 90 days before week 52 and achieved remission at week 52, 20/248 (13.3%); p-value vs. PBO, 0.035; discontinued corticosteroid use and sustained remission at both week 32 and week 52, 15/248 (10.0%); p-value vs. PBO, 0.002; IBDQ responders at week 52, 65/248 (26.2%); p-value vs. PBO, 0.007 Number and percentage of subjects taking CSs at baseline who discontinued CS use and achieved clinical remission per Mayo score (ITT analysis): clinical remission at week 52 – discontinued CS at any time prior to week 52, 20/150 (13.3%); p-value vs. PBO, 0.035;clinical remission at week 52 – discontinued CS for ≥ 90 days prior to week 52, 20/150 (13.3%); p-value vs. PBO, 0.035 Van et al.59 Week 52 CS-free remission – full Mayo: all ADA, 18/90 (20.0%); p-value vs. PBO, < 0.05; anti-TNF-α-naive ADA, 14/69 (20.3%); p-value vs. PBO, < 0.05; anti-TNF-α-exposed ADA, 4/21 (19.0%). Week 52 CS-free remission – partial Mayo: all ADA, 19/90 (21.1%); p-value vs. PBO, < 0.001; anti-TNF-α-naive ADA, 14/68 (20.6%); p-value vs. PBO, < 0.05; anti-TNF-α-exposed ADA, 5/22 (22.7%); p-value vs. PBO, < 0.05 week 52 CS-free – full Mayo: all ADA, 4/90 (45.6%); p-value vs. PBO, < 0.001; anti-TNF-α-naive ADA, 31/69 (44.9%); p-value vs. PBO, < 0.05; anti-TNF-α-exposed ADA, 10/21 (47.6%); p-value vs. PBO, < 0.05 week 52 CS-free – partial Mayo: all ADA, 43/90 (47.8%); p-value vs. PBO, < 0.001; anti-TNF-α-naive ADA, 31/68 (45.6%); p-value vs. PBO, < 0.05; anti-TNF-α-exposed ADA, 12/22 (54.5%); p-value vs. PBO, < 0.05 Sandborn et al.60 Post-hoc analysis, week 52 n = 248: mean days in IBDQ remission (IBDQ score of ≥ 170), 103.93; p-value vs. PBO, 0.025; mean SAE adjusted days in clinical remission, 81.21; p-value vs. PBO, < 0.001 |
| ULTRA3 | 40 mg of ADA EOW or EW | Week 52 | Sandborn et al.339 Clinical remission at week 52 in patients who responded per partial Mayo score at week 8 – ITT-A3 protocol: non-responder imputation, 76/196 (38.8%); modified non-responder imputation, 84/196 (42.9%); as observed 76/131 (58.0%) clinical response at week 52 in patients who responded per partial Mayo score at week 8 – ITT-A3 protocol: non-responder imputation, 113/196 (57.7%); modified non-responder imputation, 131/196 (66.8%); as observed 113/131 (86.3%) proportion of patients in the ITT-A3 population with Mayo subscores indicative of mild disease or remission at week 52: rectal bleeding subscore of ≤ 1: non-responder imputation, 185/390 (47.4%); modified non-responder imputation, 246/390 (63.1%); as observed, 185/279 (67.0%) stool frequency subscore of ≤ 1: non-responder imputation, 145/390 (37.2%); modified non-responder imputation, 175/390 (44.9%); as observed, 145/276 (52.5%); physician’s global assessment ≤ 1: non-responder imputation, 169/390 (43.3%); modified non-responder imputation, 215/390 (55.1%); as observed, 169/276 (61.2%) proportion of patients in the ITT-A3 and ITT-E populations with Mayo subscores indicative of mild disease or remission at week 52: rectal bleeding subscore of ≤ 1: non-responder imputation, 270/575 (47.0%); modified non-responder imputation, 348/575 (60.5%); as observed, 270/290 (93.1%); stool frequency subscore of ≤ 1: non-responder imputation, 210/575 (36.5%); modified non-responder imputation, 251/575 (43.7%); as observed, 210/290 (72.4%); physician’s global assessment ≤ 1: non-responder imputation, 240/575 (41.7%); modified non-responder imputation, 299/575 (52.0%) 240/290 (82.8%); steroid-free remission at week 52; patients using steroids at baseline in the ITT-A3 population (modified non-responder imputation): steroid-free at week 52, 131/234 (56.0%); remission at week 52, 66/234 (28.2%); steroid-free for ≥ 90 days at week 52, 118/234 (50.4%); remission at week 52, patients who were steroid-free for ≥ 90 days, 61/234 (26.1%) |
| ULTRA3 | ADA 40 mg EOW or EW | Weeks 0 to 156 | Sandborn et al.339 Remission per partial Mayo score presented graphically for weeks 0, 8, 12, 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144 and 156. Samples below from: Week 0: All non-responder imputation, 307/588 (52.2%) Entered ULTRA 3 on EOW modified non-responder imputation, 252/447 (56.4%) Week 36: All non-responder imputation, 334/588 (56.8%) Entered ULTRA 3 on EOW modified non-responder imputation, 254/447 (56.8%) Week 60: All non-responder imputation, 325/588 (55.3%) Entered ULTRA 3 on EOW modified non-responder imputation, 229/447 (51.2%) Week 156: All non-responder imputation, 273/588 (46.4%) Entered ULTRA 3 on EOW modified non-responder imputation, 187/447 (41.8%) |
| Suzuki | PBO | Week 8 | Suzuki et al.46 Rectal bleeding subscore of ≤ 1, 65/96 (67.7%); PGA subscore of ≤ 1, 43/96 (44.8%);stool frequency subscore of ≤ 1, 31/96 (32.3%);IBDQ response (increase in IBDQ score of ≥ 16 points from baseline), 38/96 (39.6%); remission by baseline CS use, 10/58 (17.2%); non-use, 1/38 (2.6%); remission by baseline immunomodulator use, 1/52 (1.9%); non-use, 10/44 (22.7%) |
| Suzuki | PBO | Week 32 | Suzuki et al.46 Rectal bleeding subscore of ≤ 1, 27/96 (28.1%) PGA subscore of ≤ 1, 27/96 (28.1%); stool frequency subscore of ≤ 1, 20/96 (20.8%);IBDQ response (increase in IBDQ score of ≥ 16 points from baseline), 2196 (21.9%); steroid-free, 12/58 (n at baseline) (20.7%); steroid-free remission, 5/58 (n at baseline) (8.6%) |
| Suzuki | PBO | Week 52 | Suzuki et al.46 Rectal bleeding subscore of ≤ 1, 22/96 (22.9%) PGA subscore of ≤ 1, 19/96 (19.8%); stool frequency subscore of ≤ 1, 13/96 (13.5%); IBDQ response (increase in IBDQ score of ≥ 16 points from baseline), 12/96 (12.5%); steroid-free, 12/58 (n at baseline) (20.7%); steroid-free remission, 4/58 (n at baseline) (6.9%); remission by baseline CS use, 4/58 (6.9%); non-use, 3/38 (7.9%); remission by baseline immunomodulator use, 1/52 (1.9%); non-use, 6/44 (13.6%) |
| Suzuki | 160 mg/80 mg of ADA | Week 8 | Suzuki et al.46 Rectal bleeding subscore of ≤ 1, 64/90 (71.1%); PGA subscore of ≤ 1, 55/90 (61.1%); p-value vs. PBO ≤ 0.05; stool frequency subscore of ≤ 1, 36/90 (40.0%); IBDQ response (increase in IBDQ score of ≥ 16 points from baseline), 38/90 (42.2%); remission by baseline CS use, 5/57 (8.8%); non-use, 4/33 (12.1%); remission by baseline immunomodulator use, 6/41 (14.6%), p-value vs. PBO ≤ 0.05; non-use, 3/49 (6.1%) |
| Suzuki | 80 mg/40 mg of ADA or 160 mg/80 mg of ADA to week 8 then 40 mg ADA EOW | Week 32 | Suzuki et al.46 Rectal bleeding subscore of ≤ 1, 74/177 (41.8%); PGA subscore of ≤ 1, 66/177 (37.3%); stool frequency subscore of ≤ 1, 57/177 (32.2%); p-value vs. PBO, ≤ 0.05; IBDQ response (increase in IBDQ score of ≥ 16 points from baseline), 55/177 (31.1%); steroid-free, 35/120 (n at baseline) (29.2%); steroid-free remission, 12/120 (n at baseline) (10.0%) |
| Suzuki | 80 mg/40 mg of ADA or 160 mg/80 mg of ADA to week 8 then 40 mg ADA EOW | Week 52 | Suzuki et al.46 Rectal bleeding subscore of ≤ 1, 59/177 (33.3%); PGA subscore of ≤ 1, 57/177 (32.2%); p-value vs. PBO, ≤ 0.05; stool frequency subscore of ≤ 1, 51/177 (28.8%); p-value vs. PBO, ≤ 0.05; IBDQ response (increase in IBDQ score of ≥ 16 points from baseline), 45/177 (25.4%); p-value vs. PBO, ≤ 0.01; steroid-free; p-value vs. PBO, ≤ 0.05; p-value vs. PBO, ≤ 0.05, 39/120 (n at baseline) (32.5%); steroid-free remission; p-value vs. PBO, ≤ 0.05; p-value vs. PBO, ≤ 0.05, 17/120 (n at baseline) (14.2%); remission by baseline CS use, 24/120 (20.0%); non-use, 17/57 (29.8%); p-value vs. PBO use and non-use, ≤ 0.05; remission by baseline immunomodulator use, 24/79 (30.4%), p-value vs. PBO ≤ 0.001; non-use, 17/98 (17.3%) |
| PURSUIT-SC | Phase II PBO | Week 6 | Sandborn et al.47 Mean change (SD) –1.8 (2.96), median change from baseline in Mayo score (IQR) –1.0 (–4.0 to 1.0) |
| PURSUIT-SC | Phase II 200 mg/100 mg of GOL all randomised | Week 6 | Sandborn et al.47 Mean (SD) –2.6 (2.73), median change from baseline in Mayo score (IQR) –2.0 (–4.0 to 0.0); p = 0.219 |
| PURSUIT-SC | Phase III PBO | Week 6 | Sandborn et al.47 Phase III: mean change in CRP concentration at week 6 (mg/l) PBO = 1.59 Phase III: week 6 Mayo score change from baseline PBO, mean (SD) = –1.6 (2.53), median (IQR) = –1.0 (–3.0 to 0.0) Phase III: normal or inactive mucosal disease (endoscopy score = 0) at week 6 PBO = 10/251 (4.0) |
| PURSUIT-SC | Phase III 200 mg/100 mg of GOL phase III | Week 6 | Sandborn et al.47 Phase III: mean change in CRP concentration at week 2 (mg/l) 200 mg/100 mg of GOL = – 6.57 (p < 0.0001) Phase III: mean change in CRP concentrationat week 6 (mg/l) 200 mg/100 mg of GOL = – 3.35 (p < 0.0001) Phase III: week 6 Mayo score change from baseline 200 mg/100 mg of GOL, mean (SD) = –3.1 (2.90), median (IQR) = –3.0 (–6.0 to 0.0) (p < 0.0001) Phase III: normal or inactive mucosal disease (endoscopy score = 0) at week 6 200 mg/100 mg of GOL = 21/253 (8.3) (p = 0.0437) |
| PURSUIT-SC | PBO | Weeks 0 to 6 | Sandborn et al.340 Stool frequency at week 0 [all mean (SD)] PBO = 2.3 (0.8) Stool frequency at week 2 PBO = 2.1 (0.9) Stool frequency at week 4 PBO = 1.9 (0.9) Stool frequency at week 6 PBO = 2 (1) (as reported) Rectal bleeding score at week 0 [all mean (SD)] PBO = 1.50 (0.86) Rectal bleeding score at week 2 PBO = 1.20 (0.91) Rectal bleeding score at week 4 PBO = 1.04 (0.94) Rectal bleeding score at week 6 PBO = 1.04 (0.94) |
| PURSUIT-M | PBO | Week 54 | Sandborn et al.48 PBO: 18.4% (n = 87) were in CS-free clinical remission at week 54 (among those who were receiving CSs at baseline) 31.2% (n = 154) maintained clinical response through week 54. 169 (37.1%) patients in primary analysis population had dose adjustment. PBO = 75 (48.7%) Reduction in median partial Mayo scores observed at baseline of PURSUIT-Maintenance among GOL-induction responders (i.e. decrease of 4 points from induction baseline) maintained in 100 mg and 50 mg groups through weeks 52 and 48, respectively, (but in PBO group increased after week 8 and increased to value approaching that an induction baseline at week 54 Proportion of patients with normal or inactive mucosal disease (i.e. endoscopy score = 0) at week 54 = 13.0% |
| PURSUIT-M | 50 mg of GOL | Week 54 | Sandborn et al.48 28.2% (n = 78, p = 0.279) were in CS-free clinical remission at week 54. 47.0% (n = 151, p < 0.001) maintained clinical response through week 54. 51 (33.8%) had dose adjustment Proportion of patients with normal or inactive mucosal disease (i.e. endoscopy score = 0) at week 54 = 25.8% (p = 0.011) |
| PURSUIT-M | 100 mg of GOL | Week 54 | Sandborn et al.48 23.3% were in CS-free clinical remission at week 54 (n = 82; p = 0.423). 49.7% (n = 151, p < 0.001) maintained clinical response through week 54. 43 (28.5%) had dose adjustment Proportion of patients with normal or inactive mucosal disease (i.e. endoscopy score = 0) at week 54 = 21.9% (p = 0.033) |
| UC-SUCCESS | AZA | Week 8 | Panaccione et al.51 Patients with partial Mayo score decrease of ≥ 1: 50/76 (65.79%); p-value between IFX, 0.002 and IFX/AZA, 0.003; patients with partial Mayo score decrease of ≥ 2: 28/76 (36.84%) Change (SD) in partial Mayo scores from baseline –2.81 (2.46) Faecal calprotectin ≤ 50 µg/g, 12/62 (19.4%); ≤ 250 µg/g, 24/62 (38.7%) ≥ 251 µg/g |
| UC-SUCCESS | AZA | Week 16 | Panaccione et al.51 Patients with Mayo score response: 38/76 (50.00%); p-value between IFX, 0.018 and IFX/AZA, 0.001; total Mayo score change from baseline, mean; n = 71: –3.00 (baseline 8.50); p-value between IFX, 0.013 and IFX/AZA, 0.001 Change (SD) in partial Mayo scores from baseline–2.34 (2.70) A post-hoc analysis was conducted to determine the proportion of patients who achieved a Mayo endoscopy subscore of 0 only at week 16. A greater proportion of patients treated with IFX/AZA combination therapy (29.5%) achieved a Mayo endoscopy subscore of 0 than patients given monotherapy with IFX (11.7%; p = 0.006) and AZA (13.2%; p = 0.014). The difference between the IFX group and the AZA group was not statistically significant (p = 0.783) Faecal calprotectin ≤ 50 µg/g, 12/66 (18.2%); ≤ 250 µg/g, 29/66 (43.9%) Panaccione et al.341 Change from baseline (assume mean): stool frequency –0.97, rectal bleeding –0.77, physician global assessment –0.59, total Mayo score –3.00 |
| UC-SUCCESS | IFX | Week 8 | Panaccione et al.51 Patients with partial Mayo score decrease of ≥ 1: 68/77 (88.31%); p-value IFX/AZA, 0.654; patients with partial Mayo score decrease of ≥ 2: 38/77 (49.35%) Change (SD) in partial Mayo scores from baseline –3.52 (2.25) Faecal calprotectin ≤ 50 µg/g, 15/66 (22.7%); ≤ 250 µg/g, 33/66 (50.7%) ≥ 251 µg/g |
| UC-SUCCESS | IFX | Week 16 | Panaccione et al.51 Patients with Mayo score response: 53/77 (68.83%); p-value IFX/AZA, 0.514; total Mayo score change from baseline, mean; n = 70: –4.27 (baseline 8.08); p-value IFX/AZA, 0.001 Change (SD) in partial Mayo scores from baseline –3.43 (2.26) Faecal calprotectin ≤ 50 µg/g, 11/62 (17.7%); ≤ 250 µg/g, 19/62 (30.6%) Panaccione et al.341 Change from baseline (assume mean): stool frequency –1.23; rectal bleeding –1.14; p-value vs. AZA < 0.05; physician global assessment –1.06; p-value vs. AZA< 0.05; total Mayo –4.27; p-value vs. AZA < 0.05 |
| UC-SUCCESS | IFX/AZA | Week 8 | Panaccione et al.51 Patients with partial Mayo score decrease of ≥ 1: 67/78 (85.90%); patients with partial Mayo score decrease of ≥ 2: 41/78 (52.56%) Change (SD) in partial Mayo scores from baseline –4.01 (SD 2.04); p-value vs. AZA 0.005 Faecal calprotectin ≤ 50 µg/g, 26/63 (41.3%); ≤ 250 µg/g, 42/63 (66.7%) ≥ 251 µg/g |
| UC-SUCCESS | IFX/AZA | Week 16 | Panaccione et al.51 Patients with Mayo score response: 60/78 (76.92%); total Mayo score change from baseline, mean (SD); n = 76: –5.28 (baseline 8.54) Change (SD) in partial Mayo scores from baseline –4.09 (SD 2.18); p-value vs. IFX < 0.001 Faecal calprotectin ≤ 50 µg/g, 22/70 (31.4%); ≤ 250 µg/g, 41/70 (58.6%) Panaccione et al.341 Change from baseline (assume mean): stool frequency –1.54; p-value vs. AZA < 0.05; rectal bleeding –1.25; p-value vs. AZA < 0.05; physician global assessment –1.30; p-value vs. AZA and IFX < 0.05; total Mayo score –5.28; p-value vs. AZA and IFX < 0.05 |
| Probert | PBO | Week 6 | Probert et al.50 UCSS mean (SD), 5 (3); improvement in UCSS, 4 (SD 3); median improvement, 3. Median between-group difference, p = 0.82; Baron score mean (SD), 1 (1); proportion of patients with a Baron score of 0, 6/20 (30%). 95% CI for difference, −30% to 23%; p = 0.96. Mean (SD) improvement in Baron score, 1 (SD 1). Baron score improved by a decrease in score of at least 1 in 3/20 (13%); seven (37%) remained the same and one underwent colectomy; p = 0.67. When remission rates of patients with total disease in each of the two groups were compared, no significant difference was found (p = 0.9). Remission rate in patients receiving AZA, 2/6 (33%). 95% CI for difference, −79% to 45%; p = 0.89 Mean reduction in daily dose of glucocorticoid was equivalent to 14 mg prednisolone (SD 12); p = 0.037 compared with IFX Week 6: CRP median value did not change (data not reported); p-value 0.96 but unclear if change from baseline or between IFX |
| Probert | IFX | Week 6 | Probert et al.50 UCSS mean (SD), 5 (3); improvement in UCSS (n = 18), 4 (SD 3); median improvement, 2.5 for the 18 assessable patients; Baron score mean (SD), 1 (1); proportion of patients with a Baron score of 0, 6/23 (26%). Mean (SD) improvement in Baron score, 1 (1). Baron score improved by a decrease in score of at least 1 in 13/23 (57%); seven (30%) remained the same, and three (13%) deteriorated 5/14 (36%) with total colitis went into remission, 3/5 (60%) with left-sided colitis and 1/4 (25%) with distal colitis (p = 0.5). Remission rate in patients receiving AZA, 4/6 (67%) Mean reduction in daily dose of glucocorticoid was equivalent to 19 mg prednisolone (SD 15) Week 6: CRP median levels rose from 6.5 mg/l to 10 mg/l |
| ACT1 | PBO | Week 2 | Rutgeerts et al.49 Partial Mayo score median (IQR) at baseline, 6.0 (5.0–7.0) Partial Mayo score median (IQR) at week 2, 5.0 (4.0–6.0) |
| ACT1 | PBO | Week 6 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 6, 5.0 (3.0–6.0) |
| ACT1 | PBO | Week 8 | Rutgeerts et al.49 Refractory to CS therapy, 35.3 (12/34) Not refractory to CS therapy, 37.9 (33/87) Partial Mayo score median (IQR) at week 8, 5.0 (3.0–6.0) Daily CS dose in mg (median, IQR) at baseline, 20.0 (10.0–30.0) Daily CS dose in mg (median, IQR) at week 8, 20.0 (10.0–30.0) |
| ACT1 | PBO | Week 30 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 30, 5.0 (3.0–6.0) Daily CS dose in mg, median (IQR), 10.0 (0.8–30.0) |
| ACT1 | PBO | Week 54 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 54, 5.0 (4.0–7.0) Clinical remission and discontinued use of CSs at week 54, 7/79 (8.9) Daily CS dose in mg, median (IQR), 20.0 (0.0–30.0) Lichtenstein et al.342 Clinical response: baseline IMM use: 26% (14/53), no baseline IMM use: 15% (10/68) Clinical remission: baseline IMM use: 21% (11/53), no baseline IMM use: 13% (9/68) |
| ACT1 | 5 mg/kg of IFX | Week 2 | Rutgeerts et al.49 Partial Mayo score median (IQR) at baseline, 6.0 (5.0–7.0) Partial Mayo score median (IQR) at week 2, 3.0 (2.0–5.0) |
| ACT1 | 5 mg/kg of IFX | Week 6 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 6, 3.0 (2.0–5.0) |
| ACT1 | 5 mg/kg of IFX | Week 8 | Rutgeerts et al.49 Refractory to CS therapy, 77.4 (24/31) (p < 0.001) Not refractory to CS therapy, 66.7 (60/90) (p < 0.001) Partial Mayo score median (IQR) at week 8, 2.0 (1.0–4.0) Daily CS dose in mg, median (IQR), at baseline, 20.0 (10.0–25.0) Daily CS dose in mg, median (IQR), at week 8, 20.0 (10.0–25.0) |
| ACT1 | 5 mg/kg of IFX | Week 30 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 30, 3.0 (1.0–6.0) Clinical remission and discontinued use of CSs at week 30, 17/70 (24.3) (p = 0.030) Daily CS dose in mg, median (IQR), at week 30, 5.6 (0.0–20.0) |
| ACT1 | 5 mg/kg of IFX | Week 54 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 54, 3.0 (1.0–6.0) Clinical remission and discontinued use of CSs at week 54, 18/70 (25.7) (p = 0.006) Daily CS dose in mg (median, IQR) at week 54, 5.0 (0.0–20.0) Rutgeerts et al.49 Clinical response: baseline IMM use: 48% (32/66), OR 2.62 (95% CI 1.20 to 5.71); no baseline IMM use: 42% (23/55), OR 4.17 (95% CI 1.77 to 9.84) Clinical remission: baseline IMM use: 35% (23/66), OR 2.04 (95% CI 0.89 to 4.71); no baseline IMM use: 35% (19/55), OR 3.46 (95% CI 1.41 to 8.47) |
| ACT1 | IFX combined | Week 54 | Rutgeerts et al.49 Clinical response: baseline IMM use: 45% (56/125), OR 2.26 (95% CI 1.12 to 4.58); no baseline IMM use: 45% (53/118), OR 4.73 (95% CI 2.21 to 10.1) Clinical remission: baseline IMM use: 34% (42/125), OR 1.93 (95% CI 0.90 to 4.13); no baseline IMM use: 36% (42/118), OR 3.62 (95% CI 1.63 to 8.03) |
| ACT2 | PBO | Week 2 | Rutgeerts et al.49 Partial Mayo score median (IQR) at baseline, 6.0 (5.0–7.0) Partial Mayo score median (IQR) at week 2, 5.0 (4.0–7.0) |
| ACT2 | PBO | Week 6 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 6, 5.0 (4.0–7.0) |
| ACT2 | PBO | Week 8 | Rutgeerts et al.49 Refractory to CS therapy, 37.5 (12/32); not refractory to CS therapy, 37.5 (12/32); partial Mayo score median (IQR) at week 8, 5.0 (3.0–7.0) Daily CS dose in mg, median (IQR), at baseline, 20.0 (15.0–30.0) Daily CS dose in mg, median (IQR), at week 8, 20.0 (15.0–30.0) |
| ACT2 | PBO | Week 30 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 30, 6.0 (3.0–7.0) Daily CS dose in mg, median (IQR), 20.0 (5.6–30.0) Clinical remission and discontinued use of CSs, 2/60 (3.3) Rutgeerts et al.49 Clinical response: baseline IMM use: 26% (14/54); no baseline IMM use: 26% (18/69) Clinical remission: baseline IMM use: 9% (5/54); no baseline IMM use: 12% (8/69) |
| ACT2 | PBO | Week 54 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 54, NR Daily CS dose in mg, median (IQR), at week 54, NR Rutgeerts et al.49 Clinical response at week 54 with baseline immunomodulator use OR = 3.09 (95% CI 1.36 to 6.98) |
| ACT2 | 5 mg/kg of IFX | Week 2 | Rutgeerts et al.49 Partial Mayo score median (IQR) at baseline, 6.0 (5.0–7.0) Partial Mayo score median (IQR) at week 2, 4.0 (2.0–5.0) |
| ACT2 | 5 mg/kg of IFX | Week 6 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 6, 3.0 (1.0–5.0) |
| ACT2 | 5 mg/kg of IFX | Week 8 | Rutgeerts et al.49 Refractory to CS therapy, 63.3 (19/30) (p = 0.053); not refractory to CS therapy, 63.3 (19/30) (p = 0.053) Partial Mayo score median (IQR) at week 8, 2.0 (1.0–4.0) Daily CS dose in mg, median (IQR), at baseline, 5 mg/kg of IFX = 20.0 (10.0–30.0) Daily CS dose in mg, median (IQR), at week 8, 5 mg/kg of IFX = 20.0 (10.0–30.0) |
| ACT2 | 5 mg/kg of IFX | Week 30 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 30, 4.0 (1.0–6.0) Daily CS dose in mg, median (IQR), 7.5 (0.0–20.0) Clinical remission and discontinued use of CSs, 11/60 (18.3) (p = 0.010) Rutgeerts et al.49 Clinical response: baseline IMM use: 52% (27/52), OR 3.09 (95% CI 1.36 to 6.98); no baseline IMM use: 43% (30/69), OR 2.18 (95% CI 1.06 to 4.47) Clinical remission: baseline IMM use: 35% (18/52), OR 5.19 (95% CI 1.76 to 15.3); no baseline IMM use: 19% (13/69), OR 1.77 (95% CI 0.68 to 4.59) |
| ACT2 | 5 mg/kg of IFX | Week 54 | Rutgeerts et al.49 Partial Mayo score median (IQR) at week 54, NR Daily CS dose in mg, median (IQR), NR Rutgeerts et al.49 Clinical response without baseline immunomodulator use, OR = 2.18 (95% CI 1.06 to 4.47) |
| ACT2 | IFX combined | Week 54 | Rutgeerts et al.49 Clinical remission with baseline immunomodulator use, OR = 5.19 (95% CI 1.76 to 15.3) Rutgeerts et al.49 Clinical remission without baseline immunomodulator use, OR = 1.77 (95% CI 0.68 to 4.59) |
| ACT1 and ACT2 extension studies | Randomised patients in the IFX group of the ACT1 or ACT2 trials who entered the extension studies | Weeks 0 to 152 | Reinisch et al.55 Patients with no disease activity (PGA assessment of no disease): Week E0: ACT1, 55.7% (64/115); ACT2, 27.9% (31/111) Week E24: ACT1, 66.4% (73/110); ACT2, 43.3% (42/97) Week E48: ACT1, 70.8% (75/106); ACT2, 52.3% (46/88) Week E72: ACT1, 72.7% (72/99); ACT2, 52.9% (45/85) Week E104: ACT1, 69.5% (57/82); ACT2, 66.2% (51/77) Week E128: ACT1, 79.5% (35/44); ACT2, 66.0% (35/53) Week E152: ACT1, 88.9% (8/9); ACT2, 45.5% (5/11) Patients with no or mild disease activity (PGA assessment of no or mild disease): Week E0: ACT1, 84.3% (97/115); ACT2, 68.5% (76/111) Week E24: ACT1, 90.9% (100/110); ACT2, 91.8% (89/97) Week E48: ACT1, 96.2% (102/106); ACT2, 92.0% (81/88) Week E72: ACT1, 94.9% (94/99); ACT2, 88.2% (75/85) Week E104: ACT1, 96.3% (79/82); ACT2, 92.2% (71/77) Week E128: ACT1, 95.5% (42/44); ACT2, 90.6% (48/53) Week E152: ACT1, 100.0% (9/9); ACT2, 81.8% (9/11) Randomised patients in the IFX group of the extension studies with and without a gap in treatment of > 8 weeks between the last infusion of the main studies and the extension studies week 0 infusions (n = 134) Patients with no disease activity: Week E0: patients without treatment gap (n = 134), 52.3% (69/132); patients with treatment gap (n = 95), 27.7% (26/94) Week E8: patients without treatment gap (n = 134), 59.4% (76/128); patients with treatment gap (n = 95), 44.6% (41/92) Week E24: patients without treatment gap (n = 134), 55.6% (69/124); patients with treatment gap (n = 95), 55.4% (46/83) Week E48: patients without treatment gap (n = 134), 64.8% (79/122); patients with treatment gap (n = 95), 58.3% (42/72) Week E72: patients without treatment gap (n = 134), 64.6% (73/113); patients with treatment gap (n = 95), 62.0% (44/71) Week E104: patients without treatment gap (n = 134), 70.5% (67/95); patients with treatment gap (n = 95), 64.1% (41/64) Week E128: patients without treatment gap (n = 134), 72.5% (37/51); patients with treatment gap (n = 95), 71.7% (33/46) Week E152: patients without treatment gap (n = 134), 62.5% (5/8); patients with treatment gap (n = 95), 66.7% (8/12) Patients with no or mild disease activity: Week E0: patients without treatment gap (n = 134), 84.1% (111/132); patients with treatment gap (n = 95), 66.0% (62/94) Week E8: patients without treatment gap (n = 134), 87.5% (112/128); patients with treatment gap (n = 95), 81.5% (75/92) Week E24: patients without treatment gap (n = 134), 92.7% (115/124); patients with treatment gap (n = 95), 89.2% (74/83) Week E48: patients without treatment gap (n = 134), 94.3% (115/122); patients with treatment gap (n = 95), 94.4% (68/72) Week E72: patients without treatment gap (n = 134), 91.2% (103/113); patients with treatment gap (n = 95), 93.0% (66/71) Week E104: patients without treatment gap (n = 134), 90.5% (86/95); patients with treatment gap (n = 95), 100% (64/64) Week E128: patients without treatment gap (n = 134), 94.1% (48/51); patients with treatment gap (n = 95), 91.3% (42/46) Week E152: patients without treatment gap (n = 134), 87.5% (7/8); patients with treatment gap (n = 95), 91.7% (11/12) All randomised patients in the IFX group who entered the extension studies (n = 229): Week E8: patients with no disease activity (PGA assessment of no disease), 46.4% (102/220); patients with no or mild disease activity (PGA assessment of no or mild disease), 70.9% (156/220) Week E24: patients with no disease activity (PGA assessment of no disease), 50.7% (105/207); patients with no or mild disease activity (PGA assessment of no or mild disease), 80.7% (167/207) Week E48: patients with no disease activity (PGA assessment of no disease), 56.7% (110/194); patients with no or mild disease activity (PGA assessment of no or mild disease), 82.0% (159/194) Week E72: patients with no disease activity (PGA assessment of no disease), 58.7% (108/184); patients with no or mild disease activity (PGA assessment of no or mild disease), 83.2% (153/184) Week E104: patients with no disease activity (PGA assessment of no disease), 66.0% (105/159); patients with no or mild disease activity (PGA assessment of no or mild disease), 88.1% (140/159) Week E128: patients with no disease activity (PGA assessment of no disease), 69.1% (67/97); patients with no or mild disease activity (PGA assessment of no or mild disease), 87.6% (85/97) Week E152: patients with no disease activity (PGA assessment of no disease), 65.0% (13/20); patients with no or mild disease activity (PGA assessment of no or mild disease), 90.0% (18/20) Number of randomised patients in the extension studies (n = 229) who used CSs in the past 8 weeks for UC: Week E8: 0 days, 179/223 (80.3%); 1 to 7 days, 4/223 (1.8%); 8 to 30 days, 8/223 (3.6%); > 30 days, 32/223 (14.3%) Week E24: 0 days, 179/208 (86.1%); 1 to 7 days, 1/208 (0.5%); 8 to 30 days, 2/208 (1.0%); > 30 days, 26/208 (12.5%) Week E48: 0 days, 167/194 (86.1%); 1 to 7 days, 2/194 (1.0%); 8 to 30 days, 4/194 (2.1%); > 30 days, 21/194 (10.8%) Week E72: 0 days, 165/188 (87.8%); 1 to 7 days, 3/188 (1.6%); 8 to 30 days, 4/188 (2.1%); > 30 days, 16/188 (8.5%) Week E104: 0 days, 149/161 (92.5%); 1 to 7 days, 0/161 (0.0%); 8 to 30 days, 1/161 (0.6%); > 30 days, 11/161 (6.8%) Week E128: 0 days, 92/99 (92.9%); 1 to 7 days, 0/99 (0.0%); 8 to 30 days, 0/99 (0.0%); > 30 days, 7/99 (7.1%) Week E152: 0 days, 20/20 (100.0%); 1 to 7 days, 0/20 (0.0%); 8 to 30 days, 0/20 (0.0%); > 30 days, 0/20 (0.0%) |
Additional efficacy outcomes (paediatric population trial)
| Study name | Treatment arm | Time point | Outcome measure |
|---|---|---|---|
| Hyams | 5 mg/kg of IFX q8w | Week 8 | Hyams et al.52 Median reduction in partial Mayo score 4 points median corticosteroid use mg/kg/day: 0 |
| Hyams | 5 mg/kg of IFX q8w | Week 30 | Hyams et al.52 Median reduction in partial Mayo score: approximately 2.5 points (read from source graph). Remission (PUCAI) without CSs: 5/12 (41.7%) EPAR73 Remission (PUCAI) by age (n = 20 evaluable): 6 years, 0/0 (0%); 7 years, 2/2 (100%); 8 years, 0/0 (0%); 9 years, 1/1 (100%); 10 years, 0/1 (0%); 11 years, 1/1 (100%); 12 years, 0/1 (0%); 13 years, 2/4 (50%); 14 years, 0/0 (0%); 15 years, 1/3 (33.3%); 16 years, 0/3 (0%); 17 years, 1/4 (25%) |
| Hyams | 5 mg/kg of IFX q8w | Week 54 | Hyams et al.52 Median reduction in partial Mayo score: approximately 2.5 points (read from source graph). Remission (PUCAI) without CSs: 5/13 (38.5%). Efficacy after step-up decrease of ≥ 2 points in partial Mayo score – patients with data at week 54: 9/10 (90%). Unclear if this value is for IFX 5 mg/q8d group or both groups median corticosteroid use mg/kg/day: 0.04 EPAR73 Clinical response: 3/4 patients who had endoscopy at week 54 (optional) Remission (PUCAI) by age (n = 20 evaluable): 6 years, 0/0 (0%); 7 years, 1/2 (50%); 8 years, 0/0 (0%); 9 years, 1/1 (100%); 10 years, 0/1 (0%); 11 years, 1/1 (100%); 12 years, 0/1 (0%); 13 years, 3/4 (75%); 14 years, 0/0 (0%); 15 years, 1/4 (25.3%); 16 years, 0/3 (0%); 17 years, 1/4 (25%) |
| Hyams | 5 mg/kg of IFX q12w | Week 8 | Hyams et al.52 Median reduction in partial Mayo score: 4 points median corticosteroid use mg/kg/day: 0.15 |
| Hyams | 5 mg/kg of IFX q12w | Week 30 | Hyams et al.52 Median reduction in partial Mayo score: approximately 1 point (read from source graph). Remission (PUCAI) without CSs: 1/13 (7.7%) EPAR73 Remission (PUCAI) by age (n = 21 evaluable): 6 years, 0/1 (0%); 7 years, 0/0 (0%); 8 years, 0/1 (0%); 9 years, 0/0 (0%); 10 years, 0/1 (0%); 11 years, 0/1 (0%); 12 years, 0/0 (0%); 13 years, 0/0 (0%); 14 years, 0/2 (0%); 15 years, 3/4 (75%); 16 years, 1/5 50 (20%); 17 years, 0/5 (0%) |
| Hyams | 5 mg/kg of IFX q12w | Week 54 | Hyams et al.52 Median reduction in partial Mayo score: approximately 1 point (read from source graph). Remission (PUCAI) without CSs: 0/13 (0%) Median CS use mg/kg/day: same as baseline 4 EPAR73 Clinical response: 3/4 patients who had endoscopy at week 54 (optional) Remission (PUCAI) by age (n = 22 evaluable): 6 years, 0/1 (0%); 7 years, 0/0 (0%); 8 years, 0/1 (0%); 9 years, 0/0 (0%); 10 years, 0/1 (0%); 11 years, 0/1 (0%); 12 years, 0/2 (0%); 13 years, 0/0 (0%) 3/4 (75%); 14 years, 0/2 (0%); 15 years, 2/4 (50%); 16 years, 1/5 (20%) 1/8 (12.5%); 17 years, 1/5 (20%) 2/9 (22.2%) |
| Hyams | All patients (n = 60) | Week 8 | Hyams et al.52 Disease activity was more severe at the last visit for patients who discontinued after week 8 [no disease, 1 of 10 (10%); mild, 1 of 10 (10%); moderate, 6 of 10 (60%); severe, 2 of 10 (20%)] than for patients who discontinued before week 8 [mild, 4 of 13 (30.8%); moderate, 4 of 13 (30.8%); and severe disease, 5 of 13 (38.5%)] |
Hospitalisation, surgery and mortality data (adult population trials)
| Study acronym | Treatment arm | Rates of hospitalisation | Rates of surgical intervention | Death |
|---|---|---|---|---|
| ADA | ||||
| ULTRA1 | PBO | From submission62 Week 8 (n = 222, PYs = 19.6) Physician visits number of events (visits) (events/PY), 21 (0.619) Emergency room visits number of events (visits) (events/PY), 8 (0.236) Hospital admissions number of events (admissions) (events/PY), 7 (0.206) Days in hospital number of events (days) (events/PY), 73 (2.153) |
Colectomy, 8/130 (3.6%) during induction, week 8 Elective/emergency NR |
0/223 (0%) |
| ULTRA1 | 160 mg/80 mg of ADA | From submission62 Week 8 (n = 223, PYs = 34.0) Physician visits number of events (visits) (events/PY), 15 (0.441); p-value 0.559 Emergency room visits number of events (visits) (events/PY), 2 (0.059); p-value NA Hospital admissions number of events (admissions) (events/PY), 5 (0.147); p-value NA Days in hospital number of events (days) (events/PY), 26 (0.764); p-value 0.297 |
Colectomy, 5/130 (1.4%) during induction, week 8 Elective/emergency NR |
0/223 (0%) |
| ULTRA2 | PBO | From submission62 (n = 246, PYs = 101.6) Physician visits number of events (visits) (events/PY), 169 (1.663) Emergency room visits number of events (visits) (events/PY), 10 (0.098) Hospital admissions number of events (admissions) (events/PY), 13 (0.128) Days in hospital number of events (days) (events/PY), 105 (0.837) |
Colectomy, 12/246 (4.9%) during follow-up week 52 Elective/emergency NR |
0/260 (0%) |
| ULTRA2 | 160 mg/80 mg of ADA | From submission62 (n = 248, person years = 125.5) Physician visits number of events (visits) (events/PY), 169 (1.347); p-value vs. PBO 0.035 Emergency room visits number of events (visits) (events/PY), 12 (0.096); p-value vs. PBO 0.847 Hospital admissions number of events (admissions) (events/PY), 13 (0.104); p-value vs. PBO 0.418 Days in hospital number of events (days) (events/PY), 120 (1.181); p-value vs. PBO 0.467 |
Colectomy, 10/248 (4%) during follow-up, week 52 Elective/emergency NR |
0/257 (0%) |
| ULTRA1 and ULTRA2 | ADA | Feagan et al.63 ADA Hospitalisation and Colectomy Rates in ULTRA 1 and 2: week 8 ADA responders: All-cause hospitalisation (n/PYs at risk), 46/260.4 All-cause hospitalisation incidence rate (n/PYs at risk), 0.18; All-cause hospitalisation p-value vs. PBO, 0.047 UC-related hospitalisation (n/PYs at risk), 29/266.5 UC-related hospitalisation incidence rate (n/PYs at risk), 0.11 UC-related hospitalisation p-value vs. PBO, 0.002 Colectomy (n/PYs at risk), 6/271.9 Elective/emergency, NR Colectomy incidence rate (n/PYs at risk), 0.02 Colectomy p-value vs. PBO, 0.122 Hospitalisations – all-cause events/PYs, 55/272.7 Hospitalisations – all-cause incidence rate (events/PYs), 0.20 Hospitalisations – all-cause relative risk ADA/PBO, 0.65 p = 0.021 Hospitalisations – UC-related events/PYs, 32/272.7 Hospitalisations – UC-related incidence rate (events/person years), 0.12 Hospitalisations – UC-related relative risk ADA/PBO, 0.48 p < 0.001 |
||
| Feagan et al.65 Non-UC-related hospitalisation categories week 52: General disorder; gastroestinal tract disorder, 3 (0.63%); gynaecological disorder and pregnancy, 1 (0.21%); musculoskeletal and connective tissue disorder; hepatobiliary disorder, 1 (0.21%); neurological disorder, 1 (0.21%); urogenital tract disorder, 3 (0.63%); cardiovascular disorder, 2 (0.42%); endocrine and metabolic disorder; hematologic disorder, 1 (0.21%); infection, 11 (2.28%) 9 (1.88%); malignancy, 1 (0.21%); skin disorder, 2 (0.42%); trauma and surgical/medical procedure, 3 (0.63%) |
||||
| Feagan et al.65 UC-related hospitalisation categories week 52: UC flare, 47 (9.73%) 31 (6.46%); UC leading to colectomy, 19 (3.93%) 15 (3.13%); extraintestinal complication of UC, 6 (1.25%); sequelae of colectomy, 1 (0.21%) |
||||
| Feagan et al.65 Hospitalisation and colectomy analysis: induction period (8 weeks) All-cause hospitalisation, 22 (4.6) UC-related hospitalisation, 17 (3.5%) UC- or drug-related hospitalisation, 19 (4.0%) Colectomy, 5 (1.0%). Elective/emergency, NR |
||||
| Feagan et al.65 Hospitalisation and colectomy analysis: 52-week period, n/PYs at risk (incident rate); RR (relative risk) (95% CI): All-cause hospitalisation, 69/387.5 (0.18); RR, 0.7 (95% CI 0.5 to 1.0); p = 0.03 UC-related hospitalisation, 47/398.1 (0.12); RR, 0.5 (95% CI 0.4 to 0.8); p = 0.002 UC- or drug-related hospitalisation, 55/393.8 (0.14); RR, 0.6 (0.4 to 0.9); p = 0.005 Colectomy, 15/408.1 (0.04) Sensitivity analysis 1: all events that occurred during the open-label period (during ADA therapy) were excluded for the PBO group All-cause hospitalisation, 69/387.5 (0.18); RR, 0.6 (0.4 to 0.9); p = 0.007 UC-related hospitalisation, 47/398.1 (0.12); RR, 0.5 (0.3 to 0.7); p < .0001 UC- or drug-related hospitalisation, 55/393.8 (0.14); RR, 0.5 (0.4 to 0.8); p = 0.001 Colectomy, 15/408.1 (0.04) Elective/emergency, NR Sensitivity analysis 2: all events were attributed to the randomised groups regardless of whether or not patients treated with PBO had switched to open-label ADA therapy All-cause hospitalisation, 69/387.5 (0.18); RR, 0.8 (0.6 to 1.0); p = 0.08 UC-related hospitalisation, 47/398.1 (0.12) 0.7; RR, (0.5 to 1.0); p = 0.03 UC- or drug-related hospitalisation, 55/393.8 (0.14); RR, 0.7 (0.5 to 1.0); p = 0.045 Colectomy, 15/408.1 (0.04) Elective/emergency, NR |
||||
| Reinisch et al.343 Incidence rates for all-cause and UC-related hospitalisations for ADA-treated patients by Mayo subscores at week 8: Mayo subscore 0 (n = 433): stool frequency – all-cause, 0.08 UC-related, 0.05; rectal bleeding – all-cause, 0.13 UC-related, 0.06; PGA – all-cause, 0.11 UC-related, 0.05; endoscopy – all-cause, 0.14 UC-related, 0.06 Mayo subscore 1 (n = 433): stool frequency – all-cause, 0.11 UC-related, 0.05; rectal bleeding – all-cause, 0.13 UC-related, 0.10; PGA – all-cause, 0.11 UC-related, 0.06; endoscopy – all-cause, 0.11 UC-related, 0.06 Mayo subscore 2 (n = 433): stool frequency – all-cause, 0.15 UC-related, 0.11; rectal bleeding – all-cause, 0.22 UC-related, 0.13; PGA – all-cause, 0.20 UC-related, 0.14; endoscopy – all-cause, 0.16 UC-related, 0.10 Mayo subscore 3 (n = 422): stool frequency – all-cause, 0.22 UC-related, 0.16; rectal bleeding – all-cause, 0.29 UC-related, 0.29; PGA – all-cause, 0.23 UC-related, 0.19; endoscopy – all-cause, 0.27 UC-related, 0.21 |
||||
| ULTRA 1 and 2 | PBO | Feagan et al.63 PBO hospitalisation and colectomy rates in ULTRA 1 and 2: week 8 ADA responders: All-cause hospitalisation (n/PYs at risk), 58/222.3 All-cause hospitalisation incidence rate (n/PYs at risk), 0.26 UC-related hospitalisation (n/PYs at risk), 49/223.6 UC-related hospitalisation incidence rate (n/PYs at risk), 0.22 Colectomy (n/PYs at risk), 11/231.7 Elective/emergency, NR Colectomy incidence rate (n/PYs at risk), 0.05 Hospitalisations – all-cause events/PYs, 71/232.8 Hospitalisations – all-cause incidence rate (events/PYs), 0.31 Hospitalisations – UC-related events/PYs, 59/232.8 Hospitalisations – UC-related incidence rate (events/PYs), 0.25 |
||
| Feagan et al.65 Non-UC-related hospitalisation categories week 52: General disorder, 1 (0.21%); gastrointestinal tract disorder, 1 (0.21%); gynaecological disorder and pregnancy, 2 (0.41%); musculoskeletal and connective tissue disorder, 1 (0.21%); hepatobiliary disorder, 0 (0%); neurological disorder, 0 (0%); urogenital tract disorder, 3 (0.62%); cardiovascular disorder, 1 (0.21%); endocrine and metabolic disorder, 1 (0.21%); hematologic disorder, 0 (0%); infection, 11 (2.28%); malignancy, 1 (0.21%); skin disorder, 1 (0.21%); trauma and surgical/medical procedure, 3 (0.62%) |
||||
| Feagan et al.65 UC-related hospitalisation categories week 52: UC flare, 47 (9.73%); UC leading to colectomy, 19 (3.93%); extraintestinal complication of UC, 8 (1.66%); sequelae of colectomy, 1 (0.21%) |
||||
| Feagan et al.65 Hospitalisation and colectomy analysis: induction period (8 weeks) All-cause hospitalisation, 37 (7.7%); p = 0.46 UC-related hospitalisation, 34 (7.0%); p = 0.02 UC- or drug-related hospitalisation, 36 (7.5); p = 0.02 Colectomy, 6 (1.2%); p = 0.77 |
||||
| Feagan et al.65 Hospitalisation and colectomy analysis: 52-week period, n/PYs at risk (incident rate): All-cause hospitalisation, 58/222.3 (0.26) UC-related hospitalisation, 49/223.6 (0.22) UC- or drug-related hospitalisation, 53/223.2 (0.24) Colectomy, 11/231.7 (0.05) Elective/emergency, NR Sensitivity analysis 1: all events that occurred during the open-label period (during ADA therapy) were excluded for the PBO group All-cause hospitalisation, 47/159.2 (0.30) UC-related hospitalisation, 40/159.8 (0.25) UC- or drug-related hospitalisation, 43/159.5 (0.27) Colectomy, 7/166.3 (0.04) Elective/emergency, NR Sensitivity analysis 2: all events were attributed to the randomised groups regardless of whether or not patients treated with PBO had switched to open-label ADA therapy All-cause hospitalisation, 89/377.7 (0.24) UC-related hospitalisation, 68/384.2 (0.18) UC- or drug-related hospitalisation, 76/382.2 (0.20) Colectomy, 19/400.0 (0.05) Elective/emergency NR |
||||
| Suzuki | PBO | NR | NR | NR |
| Suzuki | 80 mg/40 mg of ADA | NR | NR | NR |
| Suzuki | 160 mg/80 mg of ADA | NR | NR | NR |
| Suzuki | 40 mg of ADA EOW | NR | NR | NR |
| Suzuki | Rescue arm | NR | NR | NR |
| PURSUIT-SC | All randomised PBO | NR | NR | NR |
| PURSUIT-SC | All randomised 200 mg/100 mg of GOL | NR | NR | NR |
| PURSUIT-SC | Phase II PBO | NR | NR | NR |
| PURSUIT-SC | Phase II 200 mg/100 mg of GOL all randomised | NR | NR | NR |
| PURSUIT-SC | Phase III PBO | NR | NR | NR |
| PURSUIT-SC | Phase III 200 mg/100 mg of GOL phase III | NR | NR | NR |
| PURSUIT-Maintenance | PBO randomised | NR | NR | Deaths reported through week 54. PBO = 0 deaths reported after week 54. PBO SC induction and maintenance = 1 (pneumonia and heart failure) |
| PURSUIT-Maintenance | 50 mg of GOL randomised | NR | From submission In the PURSUIT trial, only 2–3% of GOL induction responders re-randomised to 50 mg or 100 mg of GOL had a colectomy at the end of maintenance |
Deaths reported through week 54. 50 mg of GOL = 0 deaths reported after week 54. s.c. 100 mg/50 mg of GOL induction, 50 mg maintenance = 1 (heart dysfunction in the presence of pronounced atherosclerosis and stenosis affecting aorta, large arteries and coronary arteries) |
| PURSUIT-Maintenance | 100 mg of GOL randomised | NR | NR | Deaths reported through week 54. 100 mg of GOL = 3 (causes = malnutrition and sepsis (GOL 2 mg/kg i.v. induction), cardiac failure with history of thrombosis (400 mg/200 mg of GOL s.c. induction), disseminated TB in patient who tested positive for latent TB on induction study entry and was receiving isoniazid at time of event (200 mg/100 mg of GOL s.c. induction) Deaths reported after week 54. PBO SC induction, 100 mg of GOL maintenance = 1 (myocardial infarction in patient with history of myocardial infarction). 2 mg/kg of GOL i.v. induction, 100 mg of GOL maintenance = 2 (gallbladder adenocarcinoma with liver metastasis), (sepsis). 200 mg/100 mg of GOL s.c. induction, 100 mg of GOL maintenance = 1 (accidental nitrous oxide overdose) |
| ACT1 | PBO | From submission64 UC-related hospitalisation, mean (SD): 0.22 (0.57) |
From submission64 Colectomy n (%), 9 (7.4) Ostomy n (%), 5 (4.1) |
NR |
| ACT1 | 5 mg/kg of IFX | From submission64 UC-related hospitalisation, mean (SD): 0.11 (0.34); p-value (assume vs. PBO), 0.061 |
From submission64 Colectomy n (%), 7 (5.8) Ostomy n (%), 3 (2.5) |
1 during ACT2 extension |
| ACT2 | PBO | From submission64 UC-related hospitalisation, mean (SD): 0.21 (0.55) |
From submission64 Colectomy n (%), 1 (0.7) Ostomy n (%), 1 (0.7) |
NR |
| ACT2 | 5 mg/kg of IFX | From submission64 UC-related hospitalisation, mean (SD): 0.07 (0.29); p-value (assume vs. PBO), 0.009 |
From submission64 Colectomy n (%), 0 (0.0) Ostomy n (%), 0 (0.0) |
NR |
| UC-SUCCESS | AZA | NR | NR | NR |
| UC-SUCCESS | IFX | NR | NR | NR |
| UC-SUCCESS | IFX/AZA | NR | NR | NR |
| Probert | PBO | One PBO patient underwent colectomy during the intervention period and was recorded as a treatment failure. One patient (unclear which group) refused sigmoidoscopic assessment but by other clinical measures was deemed to be a treatment failure | NR | |
| Probert | IFX | NR | NR | NR |
Hospitalisation, surgery and mortality data (paediatric population trial)
| Study acronym | Treatment arm | Rates of hospitalisation | Rates of surgical intervention | Death |
|---|---|---|---|---|
| IFX | ||||
| Hyams | 5 mg/kg of IFX q8w | NR | Patients requiring colectomy in the 54-week period: 1/22 (4.5%) | NR |
| Hyams | 5 mg/kg of IFX q12w | NR | Patients requiring colectomy in the 54-week period: 2/23 (8.7%) | NR |
| Hyams | All patients to week 8 (n = 60) | NR | Patients requiring colectomy in the 54-week period: 5/60 (8%) (2 out of 15 non-randomised) | NR |
Appendix 5 Safety data tables
Safety: participants experiencing adverse events, serious adverse events and withdrawal due to adverse events (adult population trials)
| Trial name | Treatment arm | Length of safety follow-up/mean number of administrations | Time point | Number of patients experiencing one or more AE, n/N(%) | Number of patients experiencing one or more SAE, n/N (%) (including definition) | Discontinuation owing to AE(s), n/N (%) |
|---|---|---|---|---|---|---|
| ULTRA1 | PBO | NR | Week 8 | Reinisch et al.44 Any severe (not defined) 17/223 (7.6%); any serious [not defined, 17/223 (7.6%)] Submission62 17/223 (7.6%); drug-related SAE, 4/223 (1.8%) |
Reinisch et al.44 108/223 (48.4%) Submission62 108/223 (48.1%); possibly drug related, 48/223 (21.5%) |
Reinisch et al.44 12/223 (5.4%) Submission62 12/223 (5.4%) |
| ULTRA1 | 160 mg/80 mg of ADA | NR | Week 8 | Reinisch et al.44 Any severe (not defined) 19/223 (8.5%); any serious (not defined, 9/223 (4.0%) |
Reinisch et al.44 112/223 (50.2%) |
Reinisch et al.44 12/223 (5.4%) |
| ULTRA1 | 160 mg/80 mg of ADA | NR | Week 8 | Submission62 97/223 (4.0%); drug-related SAE, 1/223 (0.4%) |
Submission62 112/223 (50.2%); possibly drug related, 43/223 (19.3%) |
Submission62 12/223 (5.4%) |
| ULTRA2 | PBO | NR | Week 52 | Sandborn et al.45 Any severe (not defined) 37/260 (14.2%); any serious [not defined, 32/260 (12.3%)] Submission62 32/260 (12.3%) |
Sandborn et al.45 218/260 (83.8%); possibly drug-related, 86/260 (33.1%) Submission62 218/260 (83.8%) |
Sandborn et al.45 34/260 (13.1%) Submission62 34/260 (13.1%) |
| ULTRA2 | 160 mg of ADA at week 0, 80 mg at week 2 and then 40 mg EOW beginning at week 4 | NR | Week 52 | Sandborn et al.45 Any severe (not defined) 41/257 (16.0%); any serious [not defined, 31/257 (12.1%)] Submission62 31/257 (12.1%) |
Sandborn et al.45 213/257 (82.9%); possibly drug-related, 101/257 (39.3%) Submission62 213/257 (82.9%) |
Sandborn et al.45 23/257 (8.9%) Submission62 23/257 (8.9%) |
| ULTRA3 | 40 mg of ADA EOW or EW | NR | Week 52 | Reinisch et al.54 76/577 (13.6%) events, 93 (events per 100 PY, 21.8) |
Reinisch et al.54 421/577 (75.6%) events, 2187 (events per 100 PY, 512.3) |
Reinisch et al.54 78/577 (14.0%) events, 90 (events per 100 PY, 21.1) |
| ULTRA3 | 40 mg of ADA EOW or EW | NR | Week 52 | Submission62 40 mg of ADA EOW/EW n = 1010; PYs, 2338 events (events/100 PYs): 414 (17.7) |
Submission62 40 mg of ADA EOW/EW n = 1010; PYs, 2338 events (events/100 PYs): 8057 (344.6) |
Submission62 40 mg of ADA EOW/EW n = 1010; PYs, 2338 events (events/100 PYs): 249 (10.7) |
| Suzuki | PBO | 52 weeks | Week 8 | Suzuki et al.46 Week 8: 7/96 (7.3%) |
Suzuki et al.46 Week 8: 45/96 (46.9%) |
Suzuki et al.46 Week 8: 4/96 (4.2%) |
| Suzuki | PBO | 52 weeks | Week 52 | Suzuki et al.46 Week 52 (n = 96, PYs = 44.8): events, 273 (events/100 PYs, 609.4) |
Suzuki et al.46 Week 52 (n = 96, PYs = 44.8): events, 14 (events/100 PYs, 31.3) |
Suzuki et al.46 Week 52 (n = 96, PYs = 44.8): events, 6 (events/100 PYs, 13.4) |
| Suzuki | 160 mg/80 mg of ADA | 52 weeks | Week 8 | Suzuki et al.46 Week 8: 4/90 (4.4%) |
Suzuki et al.46 Week 8: 40/90 (44.4%) |
Suzuki et al.46 Week 8: 6/90 (6.7%) |
| Suzuki | 80 mg/40 mg of ADA or 160 mg/80 mg of ADA to week 8 then 40 mg of ADA EOW | 52 weeks | Week 52 | Suzuki et al.46 Week 52 (n = 177, PYs, 98.2): events, 33 (events/100 PYs, 33.6) ADA week 8 responders per full Mayo score (n = 82, PYs, 68.7): 20 (events/100 PYs, 29.1) |
Suzuki et al.46 Week 52 (n = 177, PYs, 98.2): events, 538 (events/100 PYs, 547.9); ADA week 8 responders per full Mayo score (n = 82, PYs, 68.7): 343 (events/100 PYs, 499.3) |
Suzuki et al.46 Week 52 (n = 177, PYs, 98.2): events, 22 (events/100 PYs, 22.4); ADA week 8 responders per full Mayo score (n = 82, PYs, 68.7): 11 (events/100 PYs, 16.0) |
| PURSUIT-SC | PBO | 6.05 weeks Mean 1.98 |
Week 6 | Sandborn et al.47 Patients with ≥ 1 SAE (not defined) 20/330 (6.1) |
Sandborn et al.47 126/330 (38.2) Headache, 17/330 (5.2); nasophayngitis, 11/330 (3.3); pyrexia 7/330, (2.1); nausea 7/330, (2.1); exacerbation of UC, 13/330 (3.9) |
Sandborn et al.47 3/330 (0.9) (viral infection = erythema nodosum, exacerbation of UC) |
| PURSUIT-SC | 200 mg/100 mg of GOL | 6.08 weeks Mean 1.99 |
Week 6 | Sandborn et al.47 9/331 (2.7) |
Sandborn et al.47 124/331 (37.5) Headache, 10/331 (3.0); nasophayngitis, 11/331 (3.3); pyrexia, 6/331 (1.8); nausea, 3/331 (0.9); exacerbation of UC 7/331, (2.1) |
Sandborn et al.47 1/331 (0.3) (worsening of UC/clostridia infection) |
| PURSUIT-M | PBO, n = 156 | 32.7 weeks 8.2 Total number of study agent injections. PBO, 3333 |
Week 54 | Sandborn et al.48 ≥ 1 SAE. PBO,12 (7.7) |
Sandborn et al.48 103 (66.0) (all are treatment-emergent AEs) Exacerbation of UC, 29 (18.6); nasopharyngitis, 11 (7.1); headache, 14 (9.0); arthralgia, 12 (7.7); abdominal pain = 4 (2.6); upper respiratory tract infection, 4 (2.6); rash, 3 (1.9); pharyngitis, 4 (2.6); cough, 5 (3.2) |
Sandborn et al.48 PBO, 10 (6.4) |
| PURSUIT-M | 50 mg of GOL, n = 154 | 44.3 Total number of study agent injections, 11.1. GOL 50 mg, 4392 |
Week 54 | Sandborn et al.48 50 mg of GOL, 13 (8.4) |
Sandborn et al.48 112 (72.7) Exacerbation of UC, 27 (17.5); nasopharyngitis, 14 (9.1); headache, 12 (7.8); arthralgia, 11 (7.1); abdominal pain = 11 (7.1); upper respiratory tract infection = 8 (5.2); rash, 9 (5.8); pharyngitis, 8 (5.2); cough, 5 (3.2) |
Sandborn et al.48 50 mg of GOL, 8 (5.2) |
| PURSUIT-M | 100 mg of GOL, n = 154 | 46.3 Total number of study agent injections, 11.3. GOL 100 mg, 4440 |
Week 54 | Sandborn et al.48 100 mg of GOL, 22 (14.3) |
Sandborn et al.48 113 (73.4) Exacerbation of UC, 24 (15.6); nasopharyngitis, 21 (13.6); headache, 12 (7.8); arthralgia, 8 (5.2); abdominal pain = 11 (7.1); upper respiratory tract infection = 9 (5.8); rash, 7 (4.5); pharyngitis, 5 (3.2); cough, 9 (5.8) |
Sandborn et al.48 100 mg of GOL, 14 (9.1) |
| UC-SUCCESS | AZA | Week 8 and week 8 to 16 |
Week 8 and week 8 to 16 | Panaccione et al.51 Week 8: 6/79 (8%) no definition Week 8 to 16: 0/42 (0%); AFX to IFX/AZA, 1/20 (5%) |
Panaccione et al.51 Week 8: 41/79 (52%) Week 8 to 16: 11/42 (26%); AFX to IFX/AZA, 7/20 (35%) Week 8: abdominal pain, 4/79 (5%); abdominal pain, upper 4/79 (5%); anaemia, 4/79 (5%); fatigue, 4/79 (5%); headache, 8/79 (10%); nausea, 10/79 (13%); pyrexia, 3/79 (4%); vomiting, 6/79 (8%) Week 8 to 16: arthralgia 3/42 (7%); aspergillosis, chest discomfort, conjunctival haemorrhage, drug hypersensitivity, dyspnoea, leucopoenia, nasopharyngitis, painful defecation = pyrexia, UC – all 0/42 (0%); pain in extremity 2/42 (5%) Week 8 to 16: AFX to IFX/AZA: arthralgia, 0/20 (0%); aspergillosis, 1/20 (5); chest discomfort, 1/20 (5); conjunctival haemorrhage, 1/20 (5); drug hypersensitivity, 1/20 (5); dyspnoea, 1/20 (5); leucopoenia, 1/20 (5); nasopharyngitis, 1/20 (5); painful defecation, 1/20 (5); pain in extremity, 0; pyrexia, 1/20 (5); UC, 1/20 (5) |
Panaccione et al.51 Week 8: 6/79 (8%) Week 8 to 16: 1/42 (2%); AFX to IFX/AZA, 3/20 (15%) |
| UC-SUCCESS | IFX | NR | Week 8 and week 8 to 16 | Panaccione et al.51 Week 8: 2/78 (3%) Week 8 to 16: 4/74.5 |
Panaccione et al.51 Week 8: 26/78 (33%) Week 8 to 16: 22/30 (29%) Week 8: abdominal pain, 3/78 (4%); abdominal pain, upper 0/78 (0%); anaemia, 3/78 (4%); fatigue, 0/78 (0%); headache, 4/78 (5%); nausea, 1/78 (1%); pyrexia, 5/78 (6%); vomiting, 0/78 (0%) Week 8 to 16: Arthralgia, 2/74 (3%); aspergillosis, chest discomfort, conjunctival haemorrhage, drug hypersensitivity, dyspnoea, leucopoenia, painful defecation = pain in extremity – all 0/74 (0%); nasopharyngitis 1/74 (1%); pyrexia, 2/74 (3%); UC, 4/74 (5%) |
Panaccione et al.51 Week 8: 2/79 (3%) Week 8 to 16: 3/74 (4%) |
| UC-SUCCESS | IFX/AZA | NR | Week 8 and week 8 to 16 | Panaccione et al.51 Week 8: 3/80 (4%) Week 8 to 16: 1/72 (1%) |
Panaccione et al.51 Week 8: 30/80 (38%) Week 8 to 16: 21/72 (29%) Week 8: abdominal pain, 0/80 (0%); abdominal pain, upper 0/80 (0%); anaemia, 1/80 (1%); fatigue, 1/80 (1%); headache, 4/80 (5%); nausea, 7/80 (9%); pyrexia, 2/80 (3%); vomiting, 1/80 (1%) Week 8 to 16: Arthralgia, 2 (3); aspergillosis, chest discomfort, conjunctival haemorrhage, drug hypersensitivity, dyspnoea, leucopoenia, painful defecation = pain in extremity, pyrexia – all 0/72 (0%); nasopharyngitis, 1/72 (1%); UC, 3/72 (4%) |
Panaccione et al.51 Week 8: 3/79 (4%) Week 8 to 16: 3/72 (4%) |
| Probert | PBO | Week 6 | Week 6 | 2/20 (10%); 1 septic complication, 1 colectomy due to toxic exacerbation and spontaneous perforation | ||
| ACT1 | PBO | 36.2 weeks (all mean = SD NR) 24.2 weeks treatment (mean = SD NR for all) |
Week 54 | Rutgeerts et al.49 31/121 (25.6) states SAEs most commonly related to gastrointestinal system in both studies (no further details) |
Rutgeerts et al.49 103/121 (85.1) AEs occurring in ≥ 10% of any treatment group only reported. Worsening UC, 40/121 (33.1); abdominal pain, 16/121 (13.2 nausea, 14/121 (11.6); upper RTI, 28/121 (23.1); pharyngitis, 10/121 (8.3); sinusitis, 4/121 (3.3); pain = 19/121 (15.7); rash, 16/121 (13.2); arthralgia, 18/121 (14.9); headache, 27/121 (22.3); fever, 10/121 (8.3); anaemia, 12/121 (9.9); fatigue, 11/121 (9.1) |
Rutgeerts et al.49 11/121 (9.1) |
| ACT1 | PBO | Mean duration of treatment, 23 weeks (no SD reported) mean duration of follow-up, 32 weeks (no SD reported) NR |
Week 54 | Sandborn et al.67 57/244 (23) patients with long-term follow-up (mean 30 weeks) patients with ≥ 1 SAE (%), 6/14 (43); UC, 6/14 (43); fever, 1/14 (7) |
Sandborn et al.67 Any AE (%) 196/244 (80) AEs occurring in > 10% of any treatment group: worsening UC 61/244 (25); abdominal pain, 31/244 (13); nausea, 23/244 (9); upper RTI, 43/244 (18); pharyngitis, 16/244 (7); sinusitis, 12/244 (5); pain, 30/244 (12); fatigue, 19/244 (8); arthralgia, 26/244 (11); fever, 22/244 (9); headache, 45/244 (18); anaemia, 25/244 (10) |
Sandborn et al.67 23/244 (9) |
| ACT1 | 5 mg/kg of IFX | 44.9 weeks 34.8 weeks |
Week 54 | Rutgeerts et al.49 26/121 (21.5) |
Rutgeerts et al.49 106/121 (87.6) Worsening UC, 23/121 (19.0); abdominal pain, 11/121 (9.1); nausea, 14/121 (11.6); upper RTI, 20/121 (16.5); pharyngitis, 12/121 (9.9); sinusitis, 8/121 (6.6); pain = 14/121 (11.6); rash, 14/121 (11.6); arthralgia, 21/121 (17.4); headache, 22/121 (18.2); fever, 14/121 (11.6); anaemia, 4/121 (3.3); fatigue, 14/121 (11.6) |
Rutgeerts et al.49 10/121 (8.3) |
| ACT1 | 5 mg/kg of IFX | Mean duration of treatment, 33 weeks (no SD reported); mean duration of follow-up, 41 weeks (no SD reported) NR |
Week 54 | Sandborn et al.67 43/242 (18) patients with long-term follow-up (mean 25 weeks) patients with 1 or more SAEs (%) (AEs included, IFX-related AEs or those requiring hospitalisation for treatment of UC (including colectomy) 5/15 (33); UC 5/15 (33); fever 0 |
Sandborn et al.67 Any AE (%) 208/242 (86) AEs occurring in > 10% of any treatment group: worsening UC 36/242 (15) abdominal pain 22/242 (9) nausea 21 (242 (9) upper RTI 39/16 (16) pharyngitis 23/242 (10) sinusitis 20/242 (8) pain 25/242 (10) fatigue 21/242 (9) arthralgia 40/242 (17) fever 27/242 (11) headache 44/242 (18) anaemia 11/242 (5) |
Sandborn et al.67 14/242 (6) |
| ACT2 | PBO | 21.9 weeks (Mean for all, SD NR) 14.4 weeks Duration of treatment (Mean for all, SD NR) |
Week 54 | Rutgeerts et al.49 | Rutgeerts et al.49 AEs selected as for ACT1: PBO 90/123 (73.2) worsening UC, 20/123 (16.3) abdominal pain = 14/123 (11.4) nausea, 9/123 (7.3) upper RTI, 14/123 (11.4) pharyngitis, 3/123 (2.4) sinusitis, 7/123 (5.7) pain = 11/123 (8.9) rash, 3/123 (2.4) arthralgia, 6/123 (4.9) headache, 18/123 (14.6) fever, 12/123 (9.8) anaemia, 13/123 (10.6) fatigue, 6/123 (4.9) |
Rutgeerts et al.49 12/123 (9.8) |
| ACT2 | 5 mg/kg of IFX | 27.5 weeks 99/121 (81.8) |
Week 54 | Rutgeerts et al.49 | Rutgeerts et al.49 5 mg/kg of IFX 99/121 (81.8); worsening UC, 11/121 (9.1); abdominal pain, mg/kg of IFX 10/121 (8.3); nausea, 6/121 (5.0); upper RTI, 16/121 (13.2); pharyngitis, 7/121 (5.8); sinusitis, 11/121 (9.1); pain, 9/121 (7.4); rash, 2/121 (1.7); arthralgia, 16/121 (13.2); headache, 19/121 (15.7); fever, 13/121 (10.7); anaemia, 6/121 (5.0); fatigue, 6/121 (5.0) |
Rutgeerts et al.49 2/121 (1.7) |
| ACT1, ACT2 extension studies | IFX combined group, n = 230 | Mean (SD) duration of follow-up of 113 (642) weeks (range, 4–184 weeks; median, 128 weeks; 25–75 interquartile range, 96–144 weeks). Mean 2.16 years (no SD) Treatment duration mean (no SD) 1.99 years |
Reinisch et al.55 49/230 (21.3%) experienced SAE. Number SAEs, 21 per 100 PYs. SAE experienced by > 1 patient: UC flare, n = 11; (4.8%), pneumonia; n = 5, (2.2%); gastrointestinal bleeding, n = 4 (1.7%); nausea, n = 3 (1.3%); bone fracture, n = 3 (1.3%); abdominal pain, n = 2 (0.9%); intestinal obstruction, n = 2 (0.9%); fever, n = 2 (0.9%) |
Reinisch et al.55 Number of AEs, 506 per 100 PYs |
Reinisch et al.55 4.63 per 100 PYs |
Safety: participants experiencing adverse events, serious adverse events and withdrawal owing to adverse events (paediatric population trial)
| Trial name | Treatment arm | Length of safety follow-up/mean number of administrations | Time point | Discontinuation due to AE(s), n/N (%) | Number of patients experiencing 1 or more AE, n/N(%) | Number of patients experiencing 1 or more SAE, n/N (%) (including definition) |
|---|---|---|---|---|---|---|
| Hyams | 5 mg of IFX q8w | Mean weeks, 50.4 Mean exposure weeks, 41.0. Total infusions, 165 |
Week 8 | Hyams et al.52 22/22 (100) 22/22 (100) |
Hyams et al.52 4/22 (18.2) (1, serious infection; 1, pancreatitis and UC flare during induction plus viral infection after step-up; 1, UC flare after step-up; 1, anaemia during maintenance) |
Hyams et al.52 3/22 (13.6) |
| Hyams | 5 mg of IFX q12w | Mean weeks, 44.6 Mean exposure weeks, 34.3. Total infusions, 135 |
Week 8 | Hyams et al.52 23/23 (100) |
Hyams et al.52 5/23 (21.7) [1, pharyngitis during induction; 1, urinary tract infection during induction; 1, UC flare during induction (× 1) and UC flare during maintenance (× 1); 1, UC flares during induction (× 2); 1, UC flare after step-up (× 1)] |
Hyams et al.52 6/23 (26.1) |
| Hyams | All patients to week 8 (n = 60) | Mean weeks, 38.0 | Week 8 | Hyams et al.52 57/60 (95) |
Hyams et al.52 14/60 (23.3) |
Hyams et al.52 13/60 (21.7) |
Safety: infections, serious infections, infections requiring treatment, reactivation of TB or hepatitis, injection site reactions, infusion reactions, serious allergic reactions (adult population trials)
| Trial name | Treatment arm | Time point | Infections | Infections requiring treatment | Serious infections | Reactivation of TB | Reactivation of hepatitis B | Injection site reactions (relevant to ADA and GOL) | Infusion reactions (relevant to IFX) | Serious allergic reactions (e.g. anaphylaxis) |
|---|---|---|---|---|---|---|---|---|---|---|
| ULTRA1 | PBO | Week 8 | Reinisch et al.44 35/223 (15.7%) |
NR | Reinisch et al.44 3/223 (1.3%) (pneumonia, 1; sepsis, 1; wound infection staphylococcal, 1) |
NR | NR | Reinisch et al.44 7/223 (3.1%) |
NR | NR |
| ULTRA1 | 80 mg/40 mg of ADA | Week 8 | Reinisch et al.44 26/130 (20.0%) |
NR | Reinisch et al.44 2/130 (1.5%) (abscess rupture, 1; perirectal abscess, 1) |
NR | NR | Reinisch et al.44 7/130 (5.4%) |
NR | NR |
| ULTRA1 | 160 mg/80 mg ADA | Week 8 | Reinisch et al.44 32/223 (14.3%); opportunist infection (oesophageal candidiasis) 1/223 (0.4%) |
NR | Reinisch et al.44 0/223 (0%) |
NR | NR | Reinisch et al.44 13/223 (5.8%) |
NR | NR |
| ULTRA2 | PBO | Week 52 | Sandborn et al.45 103/260 (39.6%); opportunistic infection-related AE (excluding TB) 3/260 (1.2%) |
NR | Sandborn et al.45 5/260 (1.9%) |
NR | NR | Sandborn et al.45 10/260 (3.8%) |
NR | NR |
| ULTRA2 | 160 mg/80 mg ADA | Week 52 | Sandborn et al.45 116/257 (45.1%); opportunistic infection-related AE (excluding TB) 5/257 (1.9%) |
NR | Sandborn et al.45 4/257 (1.6%) |
NR | NR | Sandborn et al.45 31/257 (12.1%) |
NR | NR |
| ULTRA3 | 80 mg/40 mg ADA | Reinisch et al.54 213/577 (38.2%) events, 382 (events per 100 PY, 89.5) opportunistic infection: 5/577 (0.9%) events, 6 (events per 100 PY, 1.4) |
NR | Reinisch et al.54 17/577 (3.1%) events, 17 (events per 100 PY, 4.0) |
NR | NR | Reinisch et al.54 8/577 (1.4%) events, 8 (events per 100 PY, 1.9) |
NR | NR | |
| ULTRA3 | 160 mg/80 mg ADA | Week 52 | NR | NR | Submission62 ADA 40 mg EOW/EW n = 1010; PYs, 2338events (events/100 PYs): serious infection = 79 (3.4); opportunistic infection excluding TB, 6 (0.3) |
ADA 40 mg EOW/EW n = 1010; PYs, 2338 events (events/100 PYs): 1 (< 0.1) | NR | Submission62 ADA 40 mg EOW/EW n = 1010; PYs, 2338 events (events/100 PYs): 246 (10.5) |
NR | NR |
| Suzuki | PBO | Week 8 | Suzuki et al.46 Week 8: 15/96 (15.6%) opportunistic infection (excluding TB); Week 8: 0/96 (0%) |
NR | Suzuki et al.46 Week 8: 0/96 (0%) |
Suzuki et al.46 Week 8: 0/96 (0%) |
NR | Suzuki et al.46 Week 8: 2/96 (2.1%) |
NR | NR |
| Suzuki | PBO | Week 52 | Suzuki et al.46 Week 52 (n = 96, PYs, 44.8): events, 70 (events/100 PYs, 156.3). Opportunistic infection (excluding TB); week 52 (n = 96, PYs, 44.8): events, 0 (events/100 PYs, 0) |
NR | Suzuki et al.46 Week 52 (n = 96, PYs, 44.8): events, 2 (events/100 PYs, 4.5) |
Suzuki et al.46 Week 52 (n = 96, PYs, 44.8): events, 0 (events/100 PYs, 0) |
NR | Suzuki et al.46 Week 52 (n = 96, PYs, 44.8): events, 4 (events/100 PYs, 8.9) |
NR | NR |
| Suzuki | 80 mg/40 mg of ADA | Week 8 | Week 8: 11/87 (12.6%). Opportunistic infection (excluding TB); week 8: 0/87 (0%)46 | NR | Week 8: 0/87 (0%)46 | Week 8: 0/87 (0%)46 | NR | Week 8: 5/87 (5.7%)46 | NR | NR |
| Suzuki | 160 mg/80 mg ADA | Week 8 | Week 8: 17/90 (18.9%). Opportunistic infection (excluding TB); week 8: 1/90 (1.1%)46 | NR | Week 8: 3/90 (3.3%)46 | Week 8: 1/90 (1.1%)46 | NR | Week 8: 7/90 (7.8%)46 | NR | NR |
| Suzuki | 40 mg of ADA EOW | Week 52 | Week 52 (n = 177, PYs, 98.2): events, 134 (events/100 PYs, 136.5). ADA week 8 responders per full Mayo score (n = 82, PYs, 68.7): 90 (events/100 PYs, 131.0). Opportunistic infection (excluding TB); week 52 (n = 177, PYs, 98.2): events, 2 (events/100 PYs, 2.0). ADA week 8 responders per full Mayo score (n = 82, PYs, 68.7): 2 (events/100 PYs, 2.9)46 | Suzuki et al.46 | Week 52 (n = 177, PYs, 98.2): events, 8 (events/100 PYs, 8.1). ADA week 8 responders per full Mayo score (n = 82, PYs, 68.7): 6 (events/100 PYs, 8.7)46 | Week 52 (n = 177, PYs, 98.2): events, 1 (events/100 PYs, 1.0). ADA week 8 responders per full Mayo score (n = 82, PYs, 68.7): 0 (events/100 PYs, 0)46 | Week 52 (n = 177, PYs, 98.2): events, 20 (events/100 PYs, 20.4). ADA week 8 responders per full Mayo score (n = 82, PYs, 68.7): 9 (events/100 PYs, 13.1)46 | NR | ||
| PURSUIT-SC | PBO | Week 6 | Patients with ≥ 1 infection 40/330 (12.1). 1 opportunistic infection (cytomegalovirus infection) (not reported as serious)47 | Patients with ≥ 1 infection requiring treatment 23/330 (7.0)47 | Patients with ≥ 1 serious infection 6/330 (1.8) (1 pneumonia)47 | NR | NR | Patients with ≥ 1 injection site reaction 5/330 (1.5)47 | NR | NR |
| PURSUIT-SC | 100 mg/50 mg of GOL | 8/71 (11.3)47 | 047 | 047 | NR | NR | 4/71 (5.6)47 | NR | NR | |
| PURSUIT-SC | 200 mg/100 mg of GOL | Week 6 | 39/331 (11.8)47 | 15/331 (4.5)47 | 1/331 (0.3) (1 pneumonia)47 | NR | NR | 11/331 (3.3)47 | NR | NR |
| PURSUIT-SC | 400 mg/200 mg of GOL | Week 6 | 41/332 (12.3); 1 opportunistic infection (oesophageal candidiasis) (not reported as serious)47 | 25/332 (7.5)47 | 3/332 (0.9)47 | NR | NR | 10/332 (3.0)47 | NR | NR |
| PURSUIT-M | PBO. n = 156 | Week 54 | ≥ 1 infection (as assessed by investigator), 44 (28.2)48 | 24 (15.4)48 | 3 (1.9) serious opportunistic infection; 200 mg/100 mg of GOL s.c. induction = PBO maintenance, 1 (cytomegalovirus infection approximately 3 months after last GOL dose)48 | TB reported through week 54. GOL 4 mg/kg i.v. induction = PBO maintenance, 148 | NR | Injections with injection site reactions, 18 (0.5) ≥ 1; injection site reactions, 3 (1.9)48 | NR | NR |
| PURSUIT-M | 50 mg of GOL,n = 154 | Week 54 | 60 (39.0)48 | 39 (25.3)48 | 5 (3.2) serious opportunistic infection; 50 mg of GOL maintenance, 048 | 0 | NR | Injections with injection site reactions, 18 (0.4) ≥ 1; injection site reactions, 3 (1.9)48 | NR | NR |
| PURSUIT-M | 100 mg of GOL,n = 154 | Week 54 | 60 (39.0)48 | 44 (28.6)48 | 5 (3.2) serious opportunistic infection; 200 mg/100 mg of GOL induction = 100 mg of GOL maintenance, 1 (Staphylococcus aureus and Nocardia cultured from a brain abscess)48 | 3 (1.9) 100 mg of GOL maintenance (1 each 400 mg/200 mg of GOL s.c., 4 mg/kg i.v. and 200 mg/100 mg s.c. induction) (including fatal case reported previously)48 |
NR | Injections with injection site reactions, 28 (0.6) ≥ 1; injection site reactions, 11 (7.1)48 | NR | NR |
| UC-SUCCESS | AZA | Week 8 | NR | NR | 1/79 (1%)51 | NR | NR | NR | 1/79 (1%)51 | NR |
| UC-SUCCESS | IFX | Week 8 | NR | NR | 1/78 (1%)51 | NR | NR | NR | 0/78 (0%)51 | NR |
| UC-SUCCESS | IFX/AZA | Week 8 | NR | NR | 0/80 (0%)51 | NR | NR | NR | 0/80 (0%)51 | NR |
| ACT1 | PBO | Week 54 | 47/121 (38.8); fungal dermatitis, 8/121 (6.6); pneumonia, 0; varicella-zoster virus infection, 1/121 (0.8); herpes zoster, 049 | 25/121 (20.7)49 | Serious infections, 5/121 (4.1); bacterial infection = 1/121 (0.8); upper RTI, 1/121 (0.8); pneumonia, 0; TB, 0; abscess, 1/121 (0.8); pharyngitis, 1/121 (0.8); gastroenteritis, 0; earache, 0; fever, 0; vaginitis, 0; appendicitis, 0; colitis, 0; surgical wound infection, 1/121 (0.8); pancreatitis, 0; pleurisy, 0; sinusitis, 1/121 (0.8)49 | NR | NR | NR | Acute infusion reaction (any AE occurring ≤ 2 hours after start of infusion), 13/121 (10.7) | NR |
| ACT1 | PBO | ACT1 and ACT2, and ACT2 extension through 54 weeks | 80/244 (33)67 | NR | Serious infections (%), 6/244 (2); bacterial infection, 1/244 (0.4); upper RTI, 1/244 (0.4); pneumonia, 0; TB, 0; abscess, 2/244 (1); pharyngitis, 1/244 (0.4); gastroenteritis, 0; earache, 0; fever, 0; vaginitis, 0; appendicitis, 0; colitis, 0; infection, 1/244 (0.4); (no further details); pancreatitis, 0; pericarditis, 0; pleurisy, 0; pyelonephritis, 0; sinusitis, 1/244 (0.4)67 | NR | NR | NR | Possible delayed hypersensitivity reactions (%), 2/242 (1) | NR |
| ACT1 | 5 mg/kg of IFX | Week 54 | 53/121 (43.8); fungal dermatits, 1/121 (0.8);49 pneumonia, 2/121 (1.7); varicella-zoster virus infection, 1/121 (0.8); herpes zoster, 1/121 (0.8) n = 121 all infections baseline IMM: 32/66 (48.5%); all infections no baseline IMM: 21/55 (38.2%)342 |
39/121 (32.2)49 | Serious infections, 3/121 (2.5); bacterial infection, 0; upper RTI, 0; pneumonia, 0; TB, 0; abscess, 0; pharyngitis, 0; gastroenteritis, 1/121 (0.8); earache, 0; fever, 0; vaginitis, 0; appendicitis, 1/121 (0.8); colitis, 0; surgical wound infection, 0 (0.8); pancreatitis, 1/121 (0.8); pleurisy, 0; sinusitis, 049 n = 121 serious infections baseline IMM: 3/66 (4.5%); serious infections no baseline IMM: 0/55 (0.0%)49 |
NR | NR | NR | 121 (9.9); possible delayed hypersensitivity reactions 2/121 (1.7) 49 n = 121: infusions n with reactions baseline IMM: 7/423 (1.7%); infusions n with reactions no baseline IMM: 8/364 (2.2%); patients n with any infusion reactions baseline IMM: 6/66 (9.1%); patients n with any infusion reactions no baseline IMM: 6/55 (10.9%); n = 121: infusions n with serious reactions baseline IMM: 0/364 (0.0%); infusions n with serious reactions no baseline IMM: 0/423 (0.0%); patients n with serious infusion reactions baseline IMM: 0/66 (0.0%); patients n with serious infusion reactions no baseline IMM: 0/55 (0.0%) |
NR |
| ACT1 | 5 mg/kg of IFX | ACT1 and ACT2, and ACT2 extension through 54 weeks | 94/242 (39)67 | Sandborn et al.67 | Serious infections, (%) 7/242 (3); bacterial infection, 0; upper RTI, 0; pneumonia, 2/242 (1); TB, 0; abscess, 0; pharyngitis, 0; gastroenteritis, 2/242 (1); earache, 1/242 (0.4); fever, 1/242 (0.4); vaginitis, 0; appendicitis, 1/242 (0.4); colitis, 0; infection, 0; pancreatitis, 1/242 (0.4); pericarditis, 0; pleurisy, 0; pyelonephritis, 0; sinusitis 67 | NR | NR | NR | Possible delayed hypersensitivity reactions (%), 2/242 (1)342 | NR |
| ACT1 | 10 mg/kg of IFX | Week 54 | 60/122 (49.2); fungal dermatitis, 3/122 (2.5); pneumonia, 4/122 (3.3); varicella-zoster virus infection, IFX 10 mg 0; herpes zoster, 049 n = 122 all infections baseline IMM: 32/59 (54.2%); all infections no baseline IMM: 28/63 (44.4%)342 |
43/122 (35.2)49 | Serious infections, 8/122 (6.6); bacterial infection, 0; upper RTI, 0; pneumonia, 3/122 (2.5); TB, 1 (0.8); abscess, 2/122 (1.6); pharyngitis, 1/122 (0.8); gastroenteritis, 1/122 (0.8); earache, 0; fever, 1/122 (0.8); vaginitis, 0; appendicitis, 0; colitis, 1/122 (0.8); surgical wound infection, 1/122 (0.8); pancreatitis, 0; pleurisy, 1/122 (0.8); sinusitis, 049 n = 122 serious infections baseline IMM: 6/59 (10.2%); serious infections no baseline IMM: 2/63 (3.2%)342 |
NR | NR | NR | 15/122 (12.3); possible delayed hypersensitivity reactions, 2/121 (1.7)49 n = 122: infusions n with reactions no baseline IMM: 8/403 (2.0%); infusions n with reactions baseline IMM: 8/367 (2.2%); patients n with any infusion reactions baseline IMM: 8/59 (13.6%);342 patients n with any infusion reactions no baseline IMM: 7/63 (11.1%); n = 122: infusions n with serious reactions baseline IMM: 0/367 (0.0%); infusions n with serious reactions no baseline IMM: 0/403 (0.0%); serious infusion reactions baseline IMM: 0/59 (0.0%); serious infusion reactions no baseline IMM: 0/63 (0.0%) |
NR |
| ACT1 | IFX comb | Week 54 | n = 243. All infections baseline IMM: 64/125 (51.2%); all infections no baseline IMM: 49/118 (41.5%); ACT2 week 30 n = 241 all infections baseline IMM: 30/102 (29.4%); all infections no baseline IMM: 37/139 (26.6%)342 | Lichtenstein et al.342 | n = 243 serious infections baseline IMM: 9/125 (7.2%); serious infections no baseline IMM: 2/118 (1.7%); ACT2 week 30 n = 241 serious infusion reactions baseline IMM: 0/102 (0.0%); serious infusion reactions no baseline IMM: 0/139 (0.0%)342 | NR | NR | NR | n = 243: infusions n with reactions baseline IMM: 15/790 (1.9%); infusions n with reactions no baseline IMM: 16/767 (2.1%); patients n any infusion reactions baseline IMM: 14/125 (11.2%); patients n any infusion reactions no baseline IMM: 13/118 (11.0%) n = 243: infusions n with serious reactions baseline IMM: 0/790 (0.0%); infusions n with serious reactions no baseline IMM: 0/767 (0.0%); serious infusion reactions baseline IMM: 0/125 (0.0%); serious infusion reactions no baseline IMM: 0/118 (0.0%)342 |
NR |
| ACT2 | PBO | Week 30 | 29/123 (23.6); fungal dermatitis, 0; IFX pneumonia, 0; IFX varicella-zoster virus infection, 0; herpes zoster, 1/123 (0.8); IFX 549 | 15/123 (12.2)49 | 1/123 (0.8); bacterial infection, 0; upper RTI, 0; pneumonia, 0TB, 0; abscess, 1/123 (0.8); pharyngitis, 0; gastroenteritis, PBO 0; earache, 0; fever, 0; vaginitis, 0; appendicitis, 0; colitis, 0; surgical wound infection, 0; pancreatitis, 0; pleurisy, 0; sinusitis, 049 | NR | NR | NR | Defined as for ACT1: 10/123 (8.1) possible delayed hypersensitivity 049 | NR |
| ACT2 | 5 mg/kg of IFX | Week 30 | 18/121 (14.9); fungal dermatitis, 0; pneumonia, 0; varicella-zoster virus infection, 1/121 (0.8); herpes zoster, 2/121 (0.8)49 n = 121 all infections baseline IMM: 17/52 (32.7%); all infections no baseline IMM: 16/69 (23.2%)342 |
2/121 (1.7); bacterial infection, 0; upper RTI, 0; pneumonia, 0; TB, 0; abscess, 0; pharyngitis, 0; gastroenteritis, 1/121 (0.8); earache, 1/121 (0.8); fever, 1/121(0.8); vaginitis, 0; appendicitis, 0; colitis, 0; surgical wound infection = 0; pancreatitis, 0; pleurisy, 0; sinusitis, 049 n = 121 serious infections342 baseline IMM: 1/52 (1.9%); serious infections no baseline IMM: 1/69 (1.4%) |
14/121 (11.6) possible delayed hypersensitivity 049 n = 121; infusions n with reactions baseline IMM: 4/242 (1.7%); infusions n with reactions no baseline IMM: 12/316 (3.8%); patients n with any infusion reactions baseline IMM: 3/52 (5.8%); patients n with any infusion reactions no baseline IMM: 11/69 (15.9%). n = 121; infusions n with serious reactions baseline IMM: 0/242 (0.0%); infusions n with serious reactions no baseline IMM: 0/316 (0.0%); patients n with serious infusion reactions baseline IMM: 0/52 (0.0%); patients n with serious infusion reactions no baseline IMM: 0/69 (0.0%) |
NR | ||||
| ACT2 | 10 mg/kg of IFX | Week 30 | 17/120 (14.2); fungal dermatitis, 10 mg/kg 1 (0.8); pneumonia, 10 mg/kg 2/120 (1.7); varicella-zoster virus infection, 0; herpes zoster, 1/120 (0.8)49 | Rutgeerts et al.49 | 3/120 (2.5); bacterial infection, 0; upper RTI, 0; pneumonia, 0; TB, 0; abscess, 1/120 (0.8); pharyngitis, 0; gastroenteritis, 0; earache, 0; fever, 0; vaginitis, 1/120 (0.8); appendicitis, 0; colitis, 0; surgical wound infection, 1/120 (0.8); pancreatitis, 0; pleurisy, 0; sinusitis, 049 | NR | NR | Rutgeerts et al.49 | 14/120 (11.7); possible delayed hypersensitivity 1/120 (0.8)342 | NR |
| ACT2 | All treated patients, safety at week 30, n = 121 | Week 30 | 5 mg/kg of IFX WITH immunomodulators, 17/52 (32.7); 5 mg/kg of IFX WITHOUT immunomodulators, 16/69 (23.2)342 n = 120. All infections baseline IMM: 13/50 (26.0%); all infections no baseline IMM: 21/70 (30.0%)342 |
NR | 5 mg/kg of IFX WITH immunomodulators, 1/52 (1.9)342 n = 120 serious infections baseline IMM: 1/50 (2.0%); serious infections no baseline IMM: 2/70 (2.9%)342 |
NR | NR | NR | 5 mg/kg of IFX WITH immunomodulators, 3/52 (5.8); 5 mg/kg of IFX WITHOUT immunomodulators, 11/69 (15.9); serious infusion reactions. 5 mg/kg of IFX WITH immunomodulators, 0/52 (0); serious infusion reactions. 5 mg/kg of IFX WITHOUT immunomodulators, 0/69 (0);342 n = 120:342 infusions n with reactions baseline IMM: 6/224 (2.7%); infusions n with reactions no baseline IMM: 15/318 (4.7%); patients n with any infusion reactions baseline IMM: 5/50 (10.0%); patients n with any infusion reactions no baseline IMM: 9/70 (12.9%);n = 120: infusions n with serious reactions baseline IMM: 0/224 (0.0%); infusions n with serious reactions no baseline IMM: 0/318 (0.0%); serious infusion reactions baseline IMM: 0/50 (0.0%); serious infusion reactions no baseline IMM: 0/70 (0.0%) |
NR |
| ACT2 | IFX combined group | Week 30 | ACT1 week 54 n = 243 all infections baseline IMM: 64/125 (51.2%); all infections no baseline IMM: 49/118 (41.5%); ACT2 week 30 n = 241 all infections baseline IMM: 30/102 (29.4%); all infections no baseline IMM: 37/139 (26.6%)342 | NR | ACT1 week 54 n = 243 serious infections baseline IMM: 9/125 (7.2%); serious infections no baseline IMM: 2/118 (1.7%); ACT2 week 30 n = 241 serious infusion reactions baseline IMM: 0/102 (0.0%); serious infusion reactions no baseline IMM: 0/139 (0.0%)342 | NR | NR | NR | n = 241: infusions n with reactions baseline IMM: 10/466 (2.2%); infusions n with reactions no baseline IMM: 27/634 (4.3%); n patients any infusion reactions baseline IMM: 8/102 (7.82%); n patients any infusion reactions no baseline IMM: 20/139 (14.4%); n = 241: n infusions with reactions no baseline IMM: 27/634 (4.3%); n infusions with serious reactions baseline IMM: 0/466 (0.0%); serious infusion reactions baseline IMM: 0/102 (0.0%); serious infusion reactions no baseline IMM: 0/139 (0.0%)342 | NR |
| ACT1, ACT2 extension studies | IFX combined group n = 230 | NR | Number infections, 99 per 100 PYs55 | Number infections requiring antimicrobial treatment, 41 per 100 PYs55 | Patients (4.3%) had serious infection. Number serious infections 3.4 per 100 PYs. During extension studies, no reports of TB or other opportunistic infections55 | NR | NR | NR | NR | NR |
Safety: infections, serious infections, infections requiring treatment, reactivation of tuberculosis or hepatitis, injection site reactions, infusion reactions, serious allergic reactions (paediatric population trial)
| Trial name | Treatment arm | Time point | Infections | Infections requiring treatment | Serious infections | Reactivation of TB | Reactivation of hepatitis B | Injection site reactions (relevant to ADA and GOL) | Infusion reactions (relevant to IFX) | Serious allergic reactions (e.g. anaphylaxis) |
|---|---|---|---|---|---|---|---|---|---|---|
| Hyams | 5 mg of IFX q8w | Week 8 | 13/22 (59.1%)52 | NR | NR | NR | NR | NR | 4/22 (18.2%)52 | NR |
| Hyams | 5 mg of IFX q12w | Week 8 | 14/23 (60.9%)52 | NR | NR | NR | NR | NR | 3/23 (13.0%)52 | NR |
| Hyams | All patients to week 8 (n = 60) | Week 8 | 31/60 (51.7%)52 | NR | NR | NR | NR | NR | 8/60 (13.3%)52 | NR |
Safety: heart failure, malignancies, hepatobiliary events, autoimmune processes, neurological events, haematological reactions (adult population trials)
| Trial name | Treatment arm | Time point | Heart failure | Malignancies and lymphoproliferative disorders | Hepatobiliary events/liver enzyme changes | Autoimmune processes (e.g. lupus-like syndrome) | Neurological events | Haematological reactions | Other |
|---|---|---|---|---|---|---|---|---|---|
| ULTRA1 | PBO | Week 8 | 0/223 (0%)44 | 2/223 (0.9%) – basal cell carcinoma, 1; breast cancer, 144 | NR | Demyelinating disease, 0/223 (0%)44 | Demyelinating disease, 0/223 (0%) | NR | Submission MedDRA System Organ Class and Preferred Term: any AE, 218/223 (83.8%); colitis ulcerative, 21/223 (9.4%) |
| ULTRA1 | 160 mg/80 mg of ADA | Week 8 | 0/130 (0%) | 0/130 (0%)44 | NR | Lupus-like syndrome, 0/130 (0%)44 | Demyelinating disease, 0/130 (0%)44 | NR | Submission MedDRA System Organ Class and Preferred Term: any AE, 213/223 (82.9%); colitis ulcerative, 13/223 (5.8%) |
| ULTRA2 | PBO | Week 52 | 0/260 (0%)45 | 0/260 (0%)45 | NR | Lupus-like syndrome, 0/260 (0%)45 | Demyelinating disease, 0/260 (0%)45 | 0/260 (0%)45 | NR |
| ULTRA2 | 160 mg of ADA at week 0, 80 mg at week 2 and then 40 mg EOW beginning at week 4 | Week 52 | 1/257 (0.4%)45 | Malignancies: 2/257 (0.8%)45 | NR | Lupus-like syndrome, 1/257 (0.4%)45 | Demyelinating disease, 0/257 (0%)45 | 5/257 (1.9%)45 | Submission MedDRA System Organ Class and Preferred Term: any AE, 213/257 (82.9%); anaemia, 10/257 (3.9%); iron deficiency anaemia, 7/257 (2.7%); colitis ulcerative, 58/257 (22.6%); abdominal pain, 20/257 (7.8%); nausea, 15/257 (5.8%); fatigue, 16/257 (6.2%); pyrexia, 11/257 (4.3%); gastroenteritis, 9/257 (3.5%); nasopharyngitis, 45/257 (17.5%); pharyngitis, 9/257 (3.5%); URTI, 11/257 (4.3%); arthralgia, 20/257 (7.8%); headache, 22/257 (8.6%); oropharyngeal pain, 15/257 (5.8%) |
| ULTRA3 | 40 mg ofADA EOW or EW | Week 52 | 1/577 (0.2%) events, 1 (events per 100 PY, 0.2) | 3/577 (0.5%) events, 3 (events per 100 PY, 0.7) | NR | Demyelinating disease: 1/557 (0.2%); 1 event (events per 100 PY, 0.2) | NR | 11/577 (2.0%) events, 13 (events per 100 PY, 3.0) | NR |
| ULTRA3 | 40 mg of ADA EOW or EW | Week 52 | Submission n = 1010; PYs, 2338; events (events/100 PYs): 4 (0.2) |
Submission PYs, 2338; events (events/100 PYs): excluding lymphoma, 23 (1.0); lymphoma, 3 (0.1) |
Submission n = 1010; PYs, 2338; events (events/100 PYs): 12 (0.5) |
Submission n = 1010; PYs, 2338; events (events/100 PYs): demyelinating disease, 3 (0.1) |
NR | NR | Submission n = 1010; PYs, 2338; events (events/100 PYs): UC worsening, 588 (25.2); flare, 588 (25.2) |
| Suzuki | PBO | Week 8 | NR | Week 8: 0/96 (0%)46 | Week 8: 1/96 (1.0%)46 | NR | NR | Week 8: 1/96 (1.0%)46 | UC worsening/flare: Week 8: 8/96 (8.3%)46 |
| Suzuki | PBO | Week 52 | NR | Week 52 (n = 96, PYs, 44.8): events, 0 (events/100 PYs, 0)46 | Week 52 (n = 96, PYs, 44.8): events, 3 (events/100 PYs, 6.7)46 | NR | NR | Week 52 (n = 96, PYs, 44.8): events, 4 (events/100 PYs, 8.9)46 | Week 52 (n = 96, PYs, 44.8): events, 15 (events/100 PYs, 33.5)46 |
| Suzuki | 160 mg/80 mg of ADA only | Week 8 | NR | 1/90 (1.1%)46 | 1/90 (1.1%)46 | NR | NR | 1/90 (1.1%)46 | UC worsening/flare: week 8: 2/90 (2.2%)46 |
| Suzuki | 80 mg/40 mg of ADA or 160 mg/80 mg of ADA to week 8 then 40 mg of ADA EOW | Week 52 | NR | Week 52 (n = 177, PYs, 98.2): events, 2 (events/100 PYs, 2.0) ADA Week 8 responders per full Mayo score (n = 82, PYs, 68.7): 1 (events/100 PYs, 1.5) |
Week 52 (n = 177, PYs, 98.2): events, 5 (events/100 PYs, 5.1) ADA Week 8 responders per full Mayo score (n = 82, PYs, 68.7): 3 (events/100 PYs, 4.4) |
NR | NR | Week 52 (n = 177, PYs, 98.2): events, 6 (events/100 PYs, 6.1) ADA Week 8 responders per full Mayo score (n = 82, PYs, 68.7): 4 (events/100 PYs, 5.8) |
UC worsening/flare: week 52 (n = 177, PYs, 98.2): events, 18 (events/100 PYs, 18.3) ADA Week 8 responders per full Mayo score (n = 82, PYs, 68.7): 7 (events/100 PYs, 10.2) |
| PURSUIT-SC | Phase III PBO | Week 6 | NR | NR | NR | NR | NR | NR | Proportion of patients reporting AEs through week 6, 38.247 |
| PURSUIT-SC | Phase III 200 ml/100 mg of GOL Phase III | Week 6 | NR | NR | NR | NR | NR | NR | Proportion of patients reporting AEs through week 6, 37.547 |
| PURSUIT-M | PBO | Week 54 | NR | Neoplasm benign = malignant and unspecified. PBO, 1 (0.6). Breast cancer was reported in a patient who had received only PBO during induction and maintenance48 | NR | NR | NR | NR | NR |
| PURSUIT-M | 50 mg of GOL | Week 54 | NR | 50 mg of GOL, 4 (2.6)48 | NR | NR | NR | NR | NR |
| PURSUIT-M | 100 mg of GOL | Week 54 | NR | 100 mg of GOL, 4 (2.6).48 Three malignancies were reported through week 54 in patients receiving 100 mg of GOL maintenance; two of these (rectal cancer and thyroid cancer) presented with symptoms while the patients were receiving SC PBO induction and one (lung adenocarcinoma) occurred in a patient with a 40-year smoking history who received 200 mg/100 mg GOL s.c. induction therapy | NR | NR | NR | 0 | NR |
| UC-SUCCESS | AZA | Week 8 | NR | NR | 13/79 (16%)51 | NR | NR | NR | NR |
| UC-SUCCESS | IFX | Week 8 | NR | NR | 3/78 (4%)51 | NR | NR | NR | NR |
| UC-SUCCESS | IFX/AZA | Week 8 | NR | NR | 5/80 (6%)51 | NR | NR | NR | NR |
| ACT1 | PBO | Week 54 | NR | One (basal cell carcinoma) one colonic dysplasia through week 54 in RESULTS-UC67 | NR | NR | NR | NR | AEs of particular interest (%): fungal dermatitis, 8/244 (3); pneumonia, 0; varicella zoster virus infection, 1/244 (0.4); herpes zoster, 1/244 (0.4)67 |
| ACT1 | 5 mg/kg ofIFX i.v. | Week 54 | NR | n = 2. One patient with prostatic adenocarcinoma with 2-year history of elevated PSA concentration. One patient with colonic dysplasia. Two (prostate adenocarcinoma, rectal adenocarcinoma) through 54 weeks in RESULTS-UC, one new cancer (squamous cell skin carcinoma) developed in 5 mg/kg of IFX group patient, plus one colonic dysplasia67 | NR | 0 | One patient with optic neuritis67 | NR | AEs of particular interest (%): fungal dermatitis, pneumonia, varicella zoster virus infection = herpes zoster67 |
| ACT2 | PBO | Week 30 | NR | n = 1. Basal-cell carcinoma49 | NR | 0 | 0 | NR | At week 30 proportion with positive tests for antibodies to IFX who had infusion reaction 50.0 (6/12)49 |
| ACT2 | IFX | Week 30 | NR | n = 1. Rectal adenocarcinoma49 | NR | n = 1. One patient with lupus-like reaction (considered SAE)49 | n = 1. One patient with optic neuritis49 | NR | NR |
| ACT2 | IFX | Week 30 | NR | 0 | NR | 0 | n = 1. One patient with multifocal motor neuropathy49 | NR | NR |
| ACT1, ACT2 extension studies | IFX all | NR | Malignancy 1.01 per 100 PYs. Five malignancies reported during extension studies for IFX-treated patients. 19-year-old patient with adenocarcinoma of lung diagnosed (receiving 5 mg/kg of IFX) 1 month after E128 infusion. Patient was non-smoker and died approximately 18 months after completing extension study. One patient each developed breast cancer and prostate cancer, both receiving 5 mg/kg of IFX. Breast cancer diagnosed after week E72 infusion in 33-year-old patient with no family history of breast cancer. IFX discontinued and patient treated. Prostate cancer diagnosed approximately 2 weeks after E72 infusion in 64-year-old patient with pre-existing prostatitis (elevated PSA levels) at week E32. IFX discontinued and patient treated. Two patients, each on 10 mg/kg of IFX, developed a skin neoplasm. Neither resulted in discontinuation of treatment. One patient with extensive disease and 10-year UC history at main study baseline received 5 mg/kg of IFX and demonstrated colonic dysplasia during extension studies55 | NR | NR | No cases of optic neuritis or multifocal motir neuropathy were reported during extension studies55 | NR | NR |
Safety: heart failure, malignancies, hepatobiliary events, autoimmune processes, neurological events, haematological reactions (paediatric population trial)
| Trial name | Treatment arm | Time point | Heart failure | Malignancies and lymphoproliferative disorders | Hepatobiliary events/liver enzyme changes | Autoimmune processes (e.g. lupus-like syndrome) | Neurological events | Haematological reactions | Other |
|---|---|---|---|---|---|---|---|---|---|
| Hyams | IFX q8w | NR | NR | NR | NR | NR | NR | NR | NR |
| Hyams | IFX q12w | NR | NR | NR | NR | NR | NR | NR | NR |
Appendix 6 Quality-of-life tables
Quality-of-life outcomes
| Study name | Treatment group | HRQoL instrument and domain, time point, mean (SD) [median] values, n/N (%) reporting improvement |
|---|---|---|
| ULTRA1 | PBO | Reinisch et al.344 Change from baseline at week 4: IBDQ overall, 146 SF-36 mental and physical component summary, 43344 Change from baseline value at week 8: IBDQ overall, 152 SF-36 mental and physical component summary, 44 Reinisch et al.61 IBDQ mean response (SD) at week 8 (n = 130): 75 (57.7) |
| ULTRA1 | ADA 160/80 mg | Reinisch et al.344 Change from baseline at week 4: IBDQ overall, 149 SF-36 mental and physical component summary, 45; p-value vs. PBO, < 0.05344 Change from baseline value at week 8: IBDQ overall, 153 SF-36 mental and physical component summary, 46 Reinisch et al.61 IBDQ mean response (SD) at week 8 (n = 130): 70 (53.8); p-value vs. PBO, 0.532 |
| ULTRA2 | PBO | Sandborn et al.339 IBDQ (domain NR) Value at week 8: 20 (36), value at week 32: 20 (41), value at week 52: 19 (41) Sandborn et al.339 Increase in IBDQ ≥ 16 points from baseline: value at week 8: 112/246 (45.5%), value at week 32: 54/246 (22.0%), value at week 52: 40/246 (16.3%), value at week 8, 32 and 52: 30/246 (12.2%) |
| ULTRA2 | ADA 160/80 mg | Sandborn et al.339 IBDQ (domain NR) value at week 8: 29 (36); p-value vs. PBO < 0.05, value at week 32: 28 (41); p-value vs. PBO < 0.05, value at week 52: 27 (42); p-value vs. PBO < 0.05 Sandborn et al.339 Increase in IBDQ ≥ 16 points from baseline: value at week 8: 144/248 (58.1%); p-value vs. PBO, p < 0.05, value at week 32: 86/248 (34.7%); p-value vs. PBO, p < 0.05, value at week 52: 65/248 (26.2%); p-value vs. PBO, p < 0.05, value at week 8, 32 and 52: 58/248 (23.4%); p-value vs. PBO, p < 0.05 |
| ULTRA3 | Sandborn et al.339 Value at week 12: IBDQ overall, 178.2 (34.60); SF-36 physical, NASF-36 mental, N/A339 Value at week 48: IBDQ overall, 177.2 (34.94); SF-36 physical, 49.6 (8.24); SF-36 mental, 46.1 (10.77)339 Value at week 108: IBDQ overall, 176.3 (37.15); SF-36 physical, 49.4 (8.13); SF-36 mental, 46.0 (11.00) |
|
| PURSUIT-SC | Phase II PBO | Sandborn et al.47 Phase II PBO change from baseline in IBDQ overall, n = 41, mean (SD) 14.8 (37.16), median (IQR) 14.0 (–2.0 to 34.0) Read from source graph: Randomised in Phase II, 13 Randomised while Phase II data analysed, 12.5 Randomised in Phase III, 12.5 |
| PURSUIT-SC | Phase II GOL 100/50 mg (regimen discontinued after Phase II) | Sandborn et al.47 Phase II 100 mg/50 mg of GOL change from baseline in IBDQ overall, n = 40, mean (SD) 26.2 (39.71), median (IQR) 24.5 (–5.5 to 55.0) p = 0.287) |
| PURSUIT-SC | Phase II GOL 200/100 mg all randomised | Sandborn et al.47 Phase II 200 mg/100 mg of GOL change from baseline in IBDQ overall, n = 40, mean (SD) 24.9 (36.89), median (IQR) 16.0 (–2.5 to 49.5) (p = 0.318) Read from source graph: Randomised in Phase II, 14 Randomised while Phase II data analysed, 25 Randomised in Phase III, 27 |
| PURSUIT-SC | Phase II GOL 400/200 mg all randomised | Sandborn et al.47 Phase II 400 mg/200 mg of GOL change from baseline in IBDQ, n = 40, mean (SD) 31.6 (26.21), median (IQR) 33.0 (9.0–54.0) (p = 0.021) Read from source graph: Randomised in Phase II, 32 Randomised while Phase II data analysed,30 Randomised in Phase III, 25 |
| PURSUIT-SC | Phase III PBO | Sandborn et al.47 Phase III change from baseline IBDQ PBO, n = 251, mean (SD) 14.8 (31.25), median (IQR) = 11.0 (–3.0 to 29.0) |
| PURSUIT-SC | Phase III GOL 200/100 mg phase III | Sandborn et al.47 Phase III change from baseline 200 mg/100 mg of GOL, n = 252/253, mean (SD) 27.0 (33.72), median (IQR) 22.5 (0.5–48.5) (p < 0.0001) |
| PURSUIT-SC | Phase III GOL 400/200 mg phase III | Sandborn et al.47 Phase III change from baseline IBDQ 400 mg/200 mg of GOL, n = 255/257, mean (SD) 26.9 (34.28), median (IQR) 21.0 (0.0–50.0) (p < 0.0001) |
| PURSUIT-SC | Feagan et al.345 Compared with PBO, significantly greater improvements experienced in combined GOL-treated group in IBDQ (27.2 vs. 14.6; p < 0.001), SF-36 physical component summary (4.14 vs. 2.46; p < 0.01) and mental component summary (4.89 vs. 1.60; p < 0.001) at week 6. Mean improvements in IBDQ (27.4 and 27.0), physical component summary (4.51 and 3.78) and mental component summary (4.69 and 5.10) comparable for 200 mg/100 mg of GOL and 400 mg/200 mg of GOL groups. Distributions of IBDQ score changed from mean of 129.4 (SD 33.9) at baseline to 156.5 (SD 39.8) at week 6 in GOL-treated patients, with 45.2% patients achieving IBDQ remission vs. PBO group mean of 144.2 (SD 37.1) with 28.1% achieving IBDQ remission (p < 0.001 vs. combined GOL group) Feagan et al.345 In cumulative percentage curve vs. PBO, greater proportions of patients in each GOL group achieved ‘any improvement’ to ‘clinically meaningful improvement’ in IBDQ (51.1% vs. 35.2%; p < 0.001), SF-36 physical component summary (41.0% vs. 31.6; p = 0.01) and mental component summary (42.7% vs. 28.5%; p < 0.001) at week 6 |
|
| PURSUIT-SC and PURSUIT-M | Sandborn et al.340 GOL-treated patients achieving clinical remission at week 6 displayed greater mean improvement in SF-36 physical component summary, mental component summary, EQ-5D and IBDQ than those not achieving remission [physical component summary 8.0 vs. 2.9 (p < 0.001), mental component summary 10.7 vs. 2.6 (p < 0.001), EQ-5D 21.4 vs. 7.2 (p < 0.001) and IBDQ 54.7 vs. 17.7 (p < 0.001)] Sandborn et al.340 Patients in clinical remission more likely to achieve normalised SF-36 physical component summary, normalised mental component summary and IBDQ remission than those not achieving clinical remission [physical component summary 53.6% vs. 25.3% (p < 0.001), mental component summary 63.6% vs. 31.6% (p < 0.001), IBDQ 85.5% vs. 32.2% (p < 0.001)]. Furthermore, GOL-treated patients achieving clinical remission during induction and maintained clinical remission at week 54 in maintenance were also more likely to achieve normalised physical component summary, mental component summary and IBDQ remission than those not [physical component summary 73.5% vs. 22.7% (p < 0.001), mental component summary 63.3% vs. 28.4% (p < 0.001), IBDQ remission 89.8% vs. 22.7 (p < 0.001)] |
|
| UC-SUCCESS | AZA | Panaccione et al.51 Change from baseline at week 8: IBQD overall, n = 50: 37.84; p-value between IFX, 0.539 and IFX/AZA, 0.070. Change from baseline at week 8: SF-36 physical function, n = 58: 3.45; p-value between IFX, 0.422 and IFX/AZA, 0.044 Change from baseline at week 16: IBQD overall, n = 53: 32.51; p-value between IFX, 0.482 and IFX/AZA, < 0.001. Change from baseline at week 16: SF-36 physical function, n = 54: 4.13; p-value between IFX, 0.522 and IFX/AZA, 0.052 |
| UC-SUCCESS | IFX | Panaccione et al.51 Change from baseline at week 8: IBQD, n = 53: 33.42; p-value between IFX/AZA, 0.003. Change from baseline at week 8: SF-36 physical function, n = 63: 3.24; p-value between IFX/AZA, 0.010 Change from baseline at week 16: IBQD, n = 58: 38.55; p-value between IFX/AZA, 0.004. Change from baseline at week 16: SF-36 physical function, n = 59: 4.10; p-value between IFX/AZA, 0.022 |
| UC-SUCCESS | IFX/AZA | Panaccione et al.51 Change from baseline at week 8: IBQD, n = 53: 49.83. Change from baseline at week 8: SF-36 physical function, n = 59: SF-36 vitality, 16.6 (22.0); 6.42 Change from baseline at week 16: IBQD, n = 57: 57.70. Value at week 16: SF-36 physical function, n = 59: SF-36 vitality, 16.6 (22.0); 7.70 |
| Probert | PBO | Probert et al.50 Change from baseline at week 6: IBQD (domain NR), 25 (28) Change from baseline at week 6: European quality of life (domain NR) 4 (16) |
| Probert | IFX | Probert et al.50 Change from baseline at week 6: IBQD (domain NR), 36 (49); p-value vs. PBO, 0.22 Change from baseline at week 6: European quality of life (domain NR) 7 (17); p-value vs. PBO, 0. 3 |
| ACT1 | PBO | Feagan et al.346 Change from baseline at week 8: SF-36 physical component summary, 4.5 (6.8) SF-36 mental component summary, 3.1 (9.7) Feagan et al.346 Change from baseline at week 8: SF-36 physical component summary, 2.9 (6.0) SF-36 mental component summary, 3.1 (9.7) |
| ACT1 | PBO | Metz et al.347 Mean IBDQ scores (read from source graph): Week 8, 20.70 Week 30, 17.83 Week 54, 12.33 |
| ACT1 | 5 mg/kg of IFX | Feagan et al.346 Change from baseline at week 8: SF-36 physical component summary, 6.8 (7.8); p-value vs. PBO < 0.05; SF-36 mental component summary, 5.6 (10.2); p-value vs. PBO < 0.05 Feagan et al.346 Change from baseline at week 8: SF-36 physical component summary, 6.8 (7.4); p-value vs. PBO < 0.05; SF-36 mental component summary, 6.1 (10.8); p-value vs. PBO < 0.05 |
| ACT1 | 5 mg/kg of IFX | Metz et al.347 Mean IBDQ scores (read from source graph): Week 8, 41.72 Week 30, 33.31 Week 54, 32.38 |
| ACT1 | 10 mg/kg of IFX | Metz et al.347 Mean IBDQ scores (read from source graph): Week 8, 34.71 Week 30, 35.01 Week 54, 31.42 |
| ACT1 | 10 mg/kg of IFX | Feagan et al.346 Change from baseline at week 8: SF-36 physical component summary, 5.6 (7.8); SF-36 mental component summary, 6.6 (12.0); p-value vs. PBO < 0.05 Feagan et al.346 Change from baseline at week 8: SF-36 physical component summary, 6.2 (7.9); p-value vs. PBO < 0.05; SF-36 mental component summary, 6.2 (10.7); p-value vs. PBO < 0.05 |
| ACT1 | IFX combined | Feagan et al.346 Change from baseline at week 8: SF-36 physical component summary, 6.2 (7.8); p-value vs. PBO < 0.05; SF-36 mental component summary, 6.1 (11.1); p-value vs. PBO < 0.05 Feagan et al.346 Change from baseline at week 8: SF-36 physical component summary, 6.5 (7.7); p-value vs. PBO < 0.05; SF-36 mental component summary, 6.2 (10.7); p-value vs. PBO < 0.05 |
| ACT1 | IBDQ: responders who were not in remission (n = 150) | Reinisch et al.348 Change from baseline at week 8 IBDQ (domain NR), 47 (p < 0.001 vs. non-responders) |
| ACT1 | IBDQ: responders who were in remission (n = 206) | Reinisch et al.348 Change from baseline at week 8 IBDQ (domain NR), 65 |
| ACT1 | Patients who had not discontinued CSs at week 30 (n = 21) | Reinisch et al.348 Change from baseline at week 30: IBDQ 55.2 (58.0), SF-36 physical component summary 5.9 (7.4), SF-36 mental component summary 11.4 (9.6) |
| ACT1 | Patients who had discontinued CSs at week 30 (n = 70) | Reinisch et al.348 Change from baseline at week 30: IBDQ (domain NR), 64.7 (65.5), SF-36 physical component summary 9.8 (10.4), SF-36 mental component summary 11.0 (9.2) |
| ACT2 | PBO | Metz et al.347 Mean IBDQ scores (read from source graph): Week 8, 19.81 Week 30, 17.87 |
| ACT2 | IFX 5 mg/kg | Metz et al.347 Mean IBDQ scores (read from source graph): Week 8, 38.65 Week 30, 31.64 |
| ACT2 | IFX 10 mg/kg | Metz et al.347 Mean IBDQ scores (read from source graph): Week 8, 35.75 Week 30, 35.99 |
| ACT1 and ACT2 combined | IBDQ: non-responders (n = 137) | Reinisch et al.348 Mean change from baseline IBDQ (no SD for all), 12 Reinisch et al.348 Significantly greater proportions of patients in responder and remission subgroups achieved at least a 16-point increase (87% and 96%, respectively) or a 32-point increase (68% and 87%, respectively) in total IBDQ score vs. patients classed as non-responders (39% and 26% respectively; p < 0.001 for all comparisons) |
| ACT1 and ACT2 combined | PBO | Feagan et al.346 Change from baseline at week 8, n = 244: IBDQ bowel, 7.9 (9.7); IBDQ emotional, 6.2 (10.6); IBDQ systemic, 3.0 (4.8); IBDQ social, 3.8 (6.0); SF-36 physical functioning, 6.0 (17.3); SF-36 role-physical, 22.4 (39.7); SF-36 bodily pain, 13.1 (24.7); SF-36 general health, 5.6 (15.8); SF-36 vitality, 11.5 (20.7); SF-36 social functioning, 15.8 (24.8); SF-36 role-emotional, 12.4 (47.6); SF-36 mental health, 5.0 (18.4); SF-36 physical component summary, 3.7 (6.5); SF-36 mental component summary, 3.0 (9.6) Feagan et al.346 Percentage of patients who achieved clinically meaningful improvement at value at week 8: IBDQ change ≥ 16, 49.6%; IBDQ change ≥ 32, 32.6%; SF-36 physical component summary change ≥ 3, 40.6%; SF-36 mental component summary change ≥ 3, 32.4%; SF-36 physical component summary change ≥ 5, 34.0%; SF-36 mental component summary change ≥ 5, 29.2% |
| ACT1 and ACT2 combined | IFX 5 mg/kg | Feagan et al.346 Change from baseline week to 8 n = 242: IBDQ bowel, 14.5 (11.7); p-value vs. PBO < 0.05; IBDQ emotional, 12.7 (12.6); p-value vs. PBO < 0.05; IBDQ systemic, 5.7 (5.9); p-value vs. PBO < 0.05; IBDQ social, 7.4 (8.0); p-value vs. PBO < 0.05; SF-36 physical functioning, 12.8 (19.3); p-value vs. PBO < 0.05; SF-36 role-physical, 29.6 (41.0); SF-36 bodily pain, 20.2 (22.5); p-value vs. PBO < 0.05; SF-36 general health, 10.0 (16.9); p-value vs. PBO < 0.05; SF-36 vitality, 16.6 (22.0); p-value vs. PBO < 0.05; SF-36 social functioning, 21.2 (24.8); p-value vs. PBO < 0.05; SF-36 role-emotional, 15.5 (46.1); SF-36 mental health, 10.6 (17.5); p-value vs. PBO < 0.05; SF-36 physical component summary, 6.8 (7.6); p-value vs. PBO < 0.05; SF-36 mental component summary, 5.9 (10.5); p-value vs. PBO < 0.05 Feagan et al.346 Percentage of patients who achieved clinically meaningful improvement at value at week 8: IBDQ change ≥ 16, 69.7%; p-value vs. PBO < 0.05; IBDQ change ≥ 32, 56.8%; p-value vs. PBO < 0.05; SF-36 physical component summary change ≥ 3, 62.0%; p-value vs. PBO < 0.05; SF-36 mental component summary change ≥ 3, 52.1%; p-value vs. PBO < 0.05; SF-36 physical component summary change ≥ 5, 48.8%; p-value vs. PBO < 0.05; SF-36 mental component summary change ≥ 5, 40.9%; p-value vs. PBO < 0.05 |
| ACT1 and ACT2 combined | IFX combined | Feagan et al.346 Change from baseline week to 8 n = 484: IBDQ bowel, 13.7 (11.8); p-value vs. PBO < 0.05; IBDQ emotional, 12.0 (12.6); p-value vs. PBO < 0.05; IBDQ systemic, 5.4 (5.9); p-value vs. PBO < 0.05; IBDQ social, 6.8 (7.5); p-value vs. PBO < 0.05; SF-36 physical functioning, 11.0 (18.9); p-value vs. PBO < 0.05; SF-36 role-physical, 31.1 (42.5); p-value vs. PBO < 0.05; SF-36 bodily pain, 20.0 (23.4); p-value vs. PBO < 0.05; SF-36 general health, 10.4 (18.1); p-value vs. PBO < 0.05; SF-36 vitality, 18.3 (22.3); p-value vs. PBO < 0.05; SF-36 social functioning, 21.0 (25.9); p-value vs. PBO < 0.05; SF-36 role-emotional, 18.2 (45.4) SF-36 mental health, 10.5 (18.2); p-value vs. PBO < 0.05; SF-36 physical component summary, 6.4 (7.7); p-value vs. PBO < 0.05; SF-36 mental component summary, 6.1 (10.9); p-value vs. PBO < 0.05 Feagan et al.346 Percentage of patients who achieved clinically meaningful improvement at value at week 8: IBDQ change ≥ 16, 68.7%; p-value vs. PBO < 0.05; IBDQ change ≥ 32, 54.7%; p-value vs. PBO < 0.05; SF-36 physical component summary change ≥ 3, 59.1%; p-value vs. PBO < 0.05; SF-36 mental component summary change ≥ 3, 49.0%; p-value vs. PBO < 0.05; SF-36 physical component summary change ≥ 5, 50.0%; p-value vs. PBO < 0.05; SF-36 mental component summary change ≥ 5, 43.0%; p-value vs. PBO < 0.05 |
| ACT1 and ACT2 combined | IFX combined | Reinisch et al.348 Mean change in PCS and MCS and individual SF-36 scale scores from baseline to week 30 by response status (read from source graph): Physical component summary: non-responders (n = 137), 40.00; responders who were not in remission (n = 150), 44.78; remission (n = 206), 49.77 Physical functioning: non-responders (n = 137), 44.18; responders who were not in remission (n = 150), 48.54; remission (n = 206), 51.66 Role-physical: non-responders (n = 137), 39.24; responders who were not in remission (n = 150), 44.85; remission (n = 206), 50.76 Bodily pain: non-responders (n = 137), 42.07; responders who were not in remission (n = 150), 47.05; remission (n = 206), 52.35 General health: non-responders (n = 137), 36.19; responders who were not in remission (n = 150), 40.24; remission (n = 206), 45.54 Vitality: non-responders (n = 137), 41.20; responders who were not in remission (n = 150), 47.11; remission (n = 206), 51.48 Social functioning: non-responders (n = 137), 40.61; responders who were not in remission (n = 150), 48.39; remission (n = 206), 52.13 Role-emotional: non-responders (n = 137), 44.06; responders who were not in remission (n = 150), 48.42; remission (n = 206), 51.58 Mental health: non-responders (n = 137), 45.02; responders who were not in remission (n = 150), 48.76; remission (n = 206), 50.95 Mental component summary: non-responders (n = 137), 44.12; responders who were not in remission (n = 150), 49.1; remission (n = 206), 51.6 |
| ACT1 and ACT2 combined | PBO/IFX combined | Metz et al.347 Mean baseline and change at week 8 scores per question for each IBDQ dimension (read from source graph): Bowel: baseline, 3.33; week 8, 4.48 Emotional: baseline, 3.62; week 8, 4.44 Systemic: baseline, 2.89; week 8, 3.83 Social: baseline, 3.41; week 8, 4.57 |
| Mean baseline and change at week 8 in norm-based SF-36 scale scores (read from source graph): Physical functioning: baseline, 42.78; week 8, 45.97 Role-physical: baseline, 33.89; week 8, 41.23 Bodily pain: baseline, 39.03; week 8, 45.10 General health: baseline, 34.28; week 8, 37.16 Vitality: baseline, 36.88; week 8, 42.30 Social functioning: baseline, 34.68; week 8, 41.07 Role-emotional: baseline, 41.42; week 8, 45.26 Mental health: baseline, 41.14; week 8, 44.34 |
||
| Mean baseline and change at week 8 scores per question for each IBDQ dimension (read from source graph): Bowel: baseline, 3.34; week 8, 5.12 Emotional: baseline, 3.80; week 8, 5.00 Systemic: baseline, 3.00; week 8, 4.22 Social: baseline, 3.74; week 8, 5.47 |
||
| Mean baseline and change at week 8 in norm-based SF-36 scale scores (read from graph): Physical functioning: baseline, 44.07; week 8, 49.49 Role-physical: baseline, 35.81; week 8, 45.07 Bodily pain: baseline, 39.68; week 8, 48.30 General health: baseline, 35.89; week 8, 40.68 Vitality: baseline, 37.84; week 8, 46.14 Social functioning: baseline, 36.93; week 8, 46.82 Role-emotional: baseline, 42.39; week 8, 48.14 Mental health: baseline, 42.11; week 8, 47.85 |
Appendix 7 Network meta-analysis tables
Induction phase
| Treatments | Treatment 1 | Treatment 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | No response | Response | Remission | Total | No response | Response | Remission | Total | |
| Base-case data | ||||||||||
| ULTRA 1 | PBO | ADA | 72 | 46 | 12 | 130 | 59 | 47 | 24 | 130 |
| ULTRA 2 (anti-TNF-α-naive) | PBO | ADA | 89 | 40 | 16 | 145 | 61 | 57 | 32 | 150 |
| PURSUIT-SC Phases II + III | PBO | GOL | 218 | 79 | 23 | 320 | 162 | 104 | 58 | 324 |
| ACT1 | PBO | IFX | 76 | 27 | 18 | 121 | 37 | 37 | 47 | 121 |
| ACT2 | PBO | IFX | 87 | 29 | 7 | 123 | 43 | 37 | 41 | 121 |
| Sensitivity analysis 1 | ||||||||||
| ULTRA 1 | PBO | ADA | 72 | 46 | 12 | 130 | 59 | 47 | 24 | 130 |
| ULTRA 2 (ITT) | PBO | ADA | 161 | 62 | 23 | 246 | 123 | 84 | 41 | 248 |
| PURSUIT-SC Phases II + III | PBO | GOL | 218 | 79 | 23 | 320 | 162 | 104 | 58 | 324 |
| ACT1 | PBO | IFX | 76 | 27 | 18 | 121 | 37 | 37 | 47 | 121 |
| ACT2 | PBO | IFX | 87 | 29 | 7 | 123 | 43 | 37 | 41 | 121 |
| Sensitivity analysis 2 | ||||||||||
| ULTRA 1 | PBO | ADA | 72 | 46 | 12 | 130 | 59 | 47 | 24 | 130 |
| ULTRA 2 (anti-TNF-α-naive) | PBO | ADA | 89 | 40 | 16 | 145 | 61 | 57 | 32 | 150 |
| PURSUIT-SC Phases II + III | PBO | GOL | 218 | 79 | 23 | 320 | 162 | 104 | 58 | 324 |
| ACT1 | PBO | IFX | 76 | 27 | 18 | 121 | 37 | 37 | 47 | 121 |
| ACT2 | PBO | IFX | 87 | 29 | 7 | 123 | 43 | 37 | 41 | 121 |
| Suzuki et al. | PBO | ADA | 62 | 23 | 11 | 96 | 45 | 36 | 9 | 90 |
| Sensitivity analysis 3 | ||||||||||
| ULTRA 1 | PBO | ADA | 72 | 46 | 12 | 130 | 59 | 47 | 24 | 130 |
| ULTRA 2 (ITT) | PBO | ADA | 161 | 62 | 23 | 246 | 123 | 84 | 41 | 248 |
| PURSUIT-SC Phases II + III | PBO | GOL | 218 | 79 | 23 | 320 | 162 | 104 | 58 | 324 |
| ACT1 | PBO | IFX | 76 | 27 | 18 | 121 | 37 | 37 | 47 | 121 |
| ACT2 | PBO | IFX | 87 | 29 | 7 | 123 | 43 | 37 | 41 | 121 |
| Suzuki et al. | PBO | ADA | 62 | 23 | 11 | 96 | 45 | 36 | 9 | 90 |
Further maintenance NMA data removed owing to commercial-in-confidence status. Commercial-in-confidence information has been removed.
Appendix 8 Network meta-analysis figures
Results when using conventional reference prior for the between study standard deviation
FIGURE 93.
Base case: comparative effect of anti-TNF-α treatment on clinical response/remission in the induction phase [SD ∼ U(0,2)].
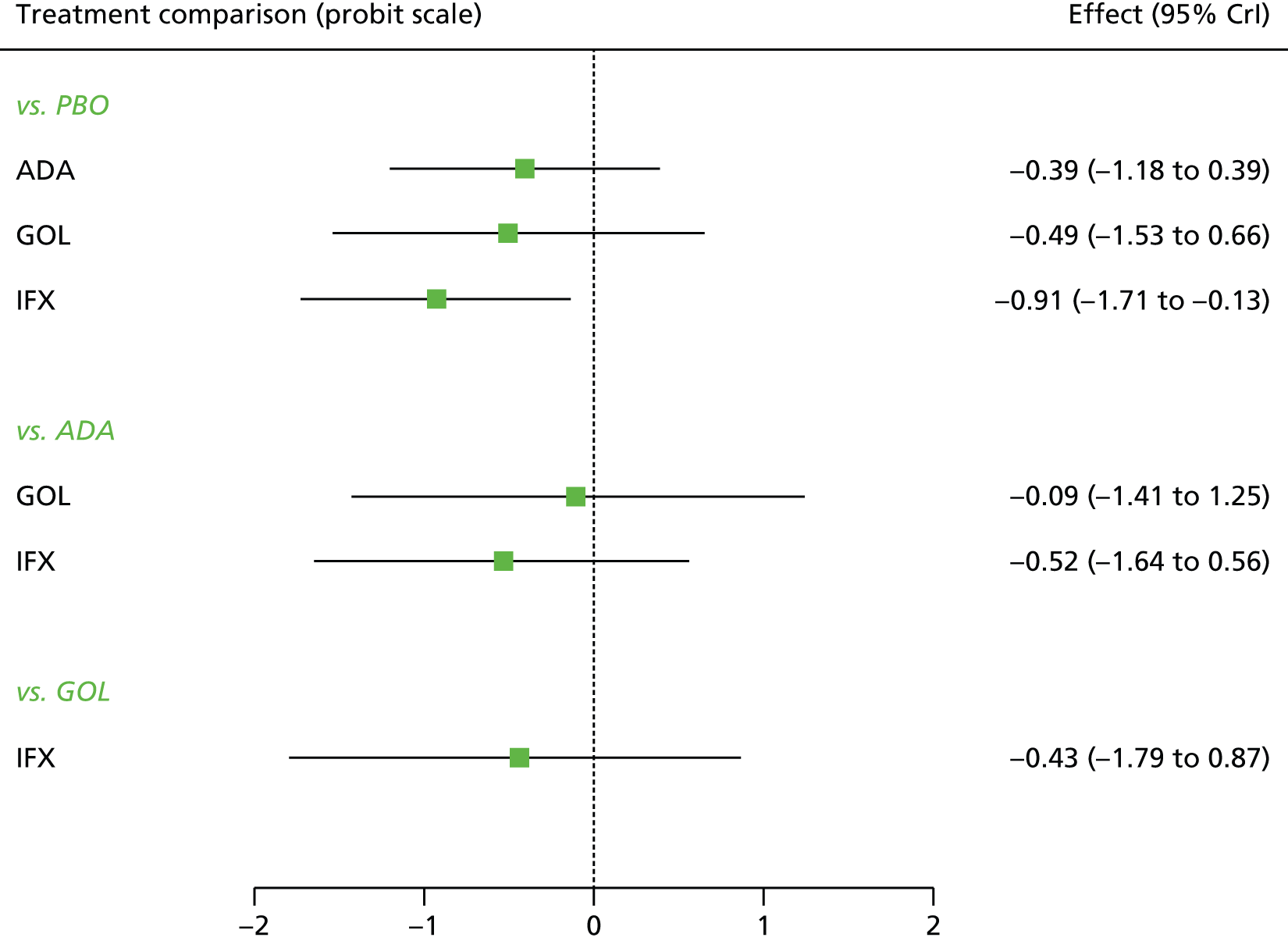
FIGURE 94.
Base case: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in response [SD ∼ U(0,2)].

FIGURE 95.
Base case: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in remission [SD ∼ U(0,2)].
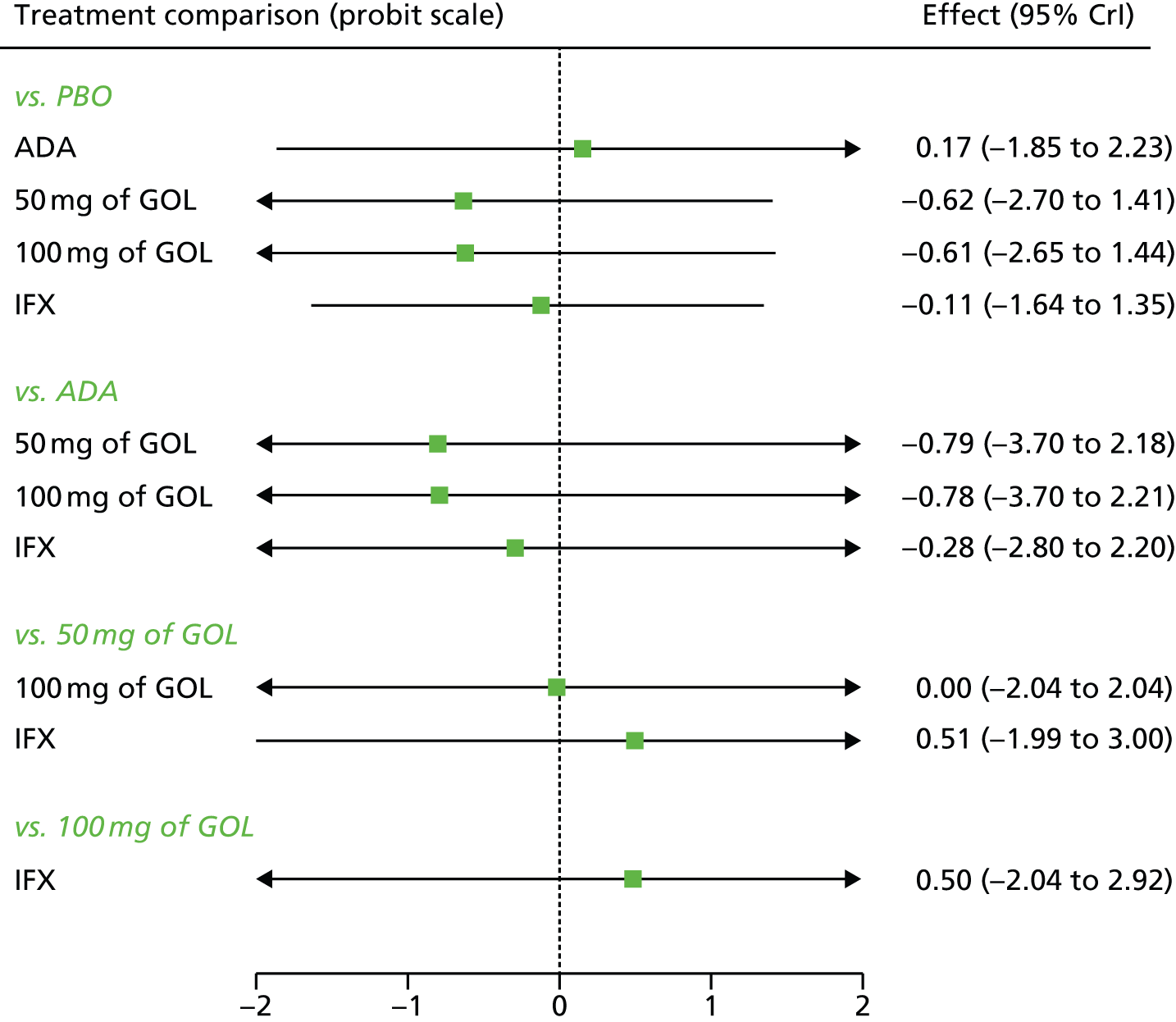
FIGURE 96.
Base case: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in response [SD ∼ U(0,2)].
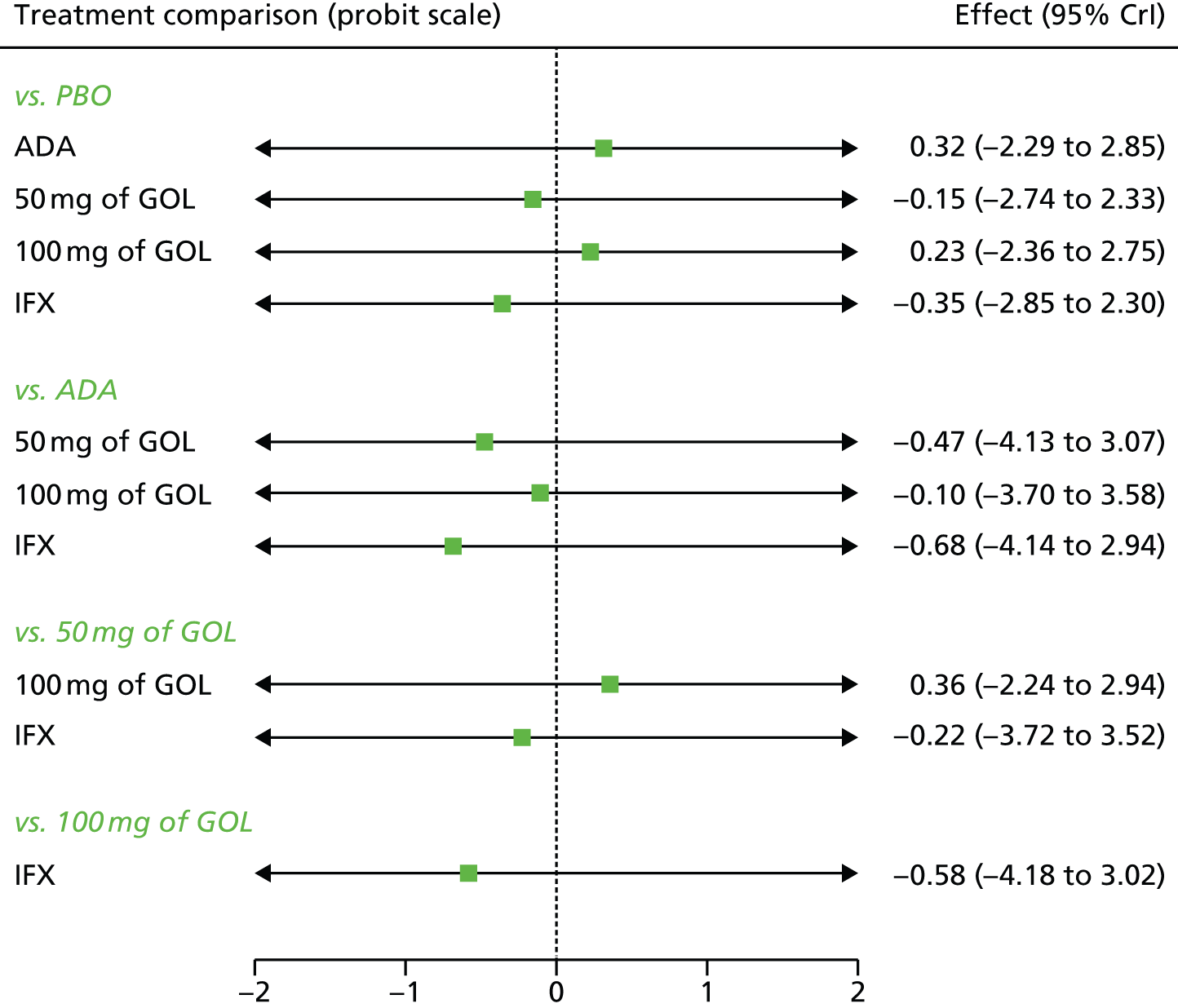
FIGURE 97.
Base case: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in remission [SD ∼ U(0,2)].

FIGURE 98.
Sensitivity analysis 1: comparative effect of anti-TNF-α treatment on clinical response/remission in the induction phase [SD ∼ U(0,2)].
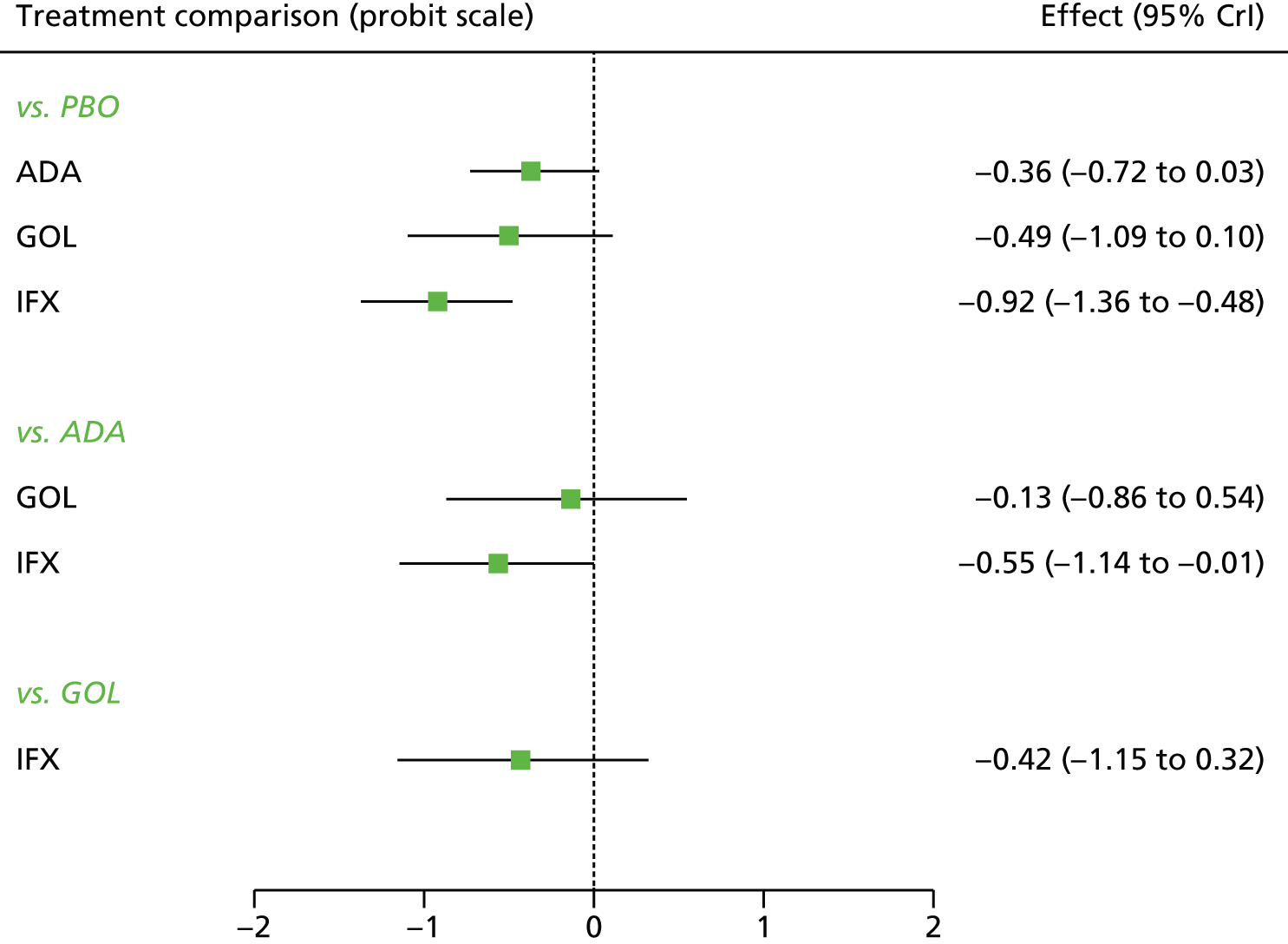
FIGURE 99.
Sensitivity analysis 1: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in response [SD ∼ U(0,2)].
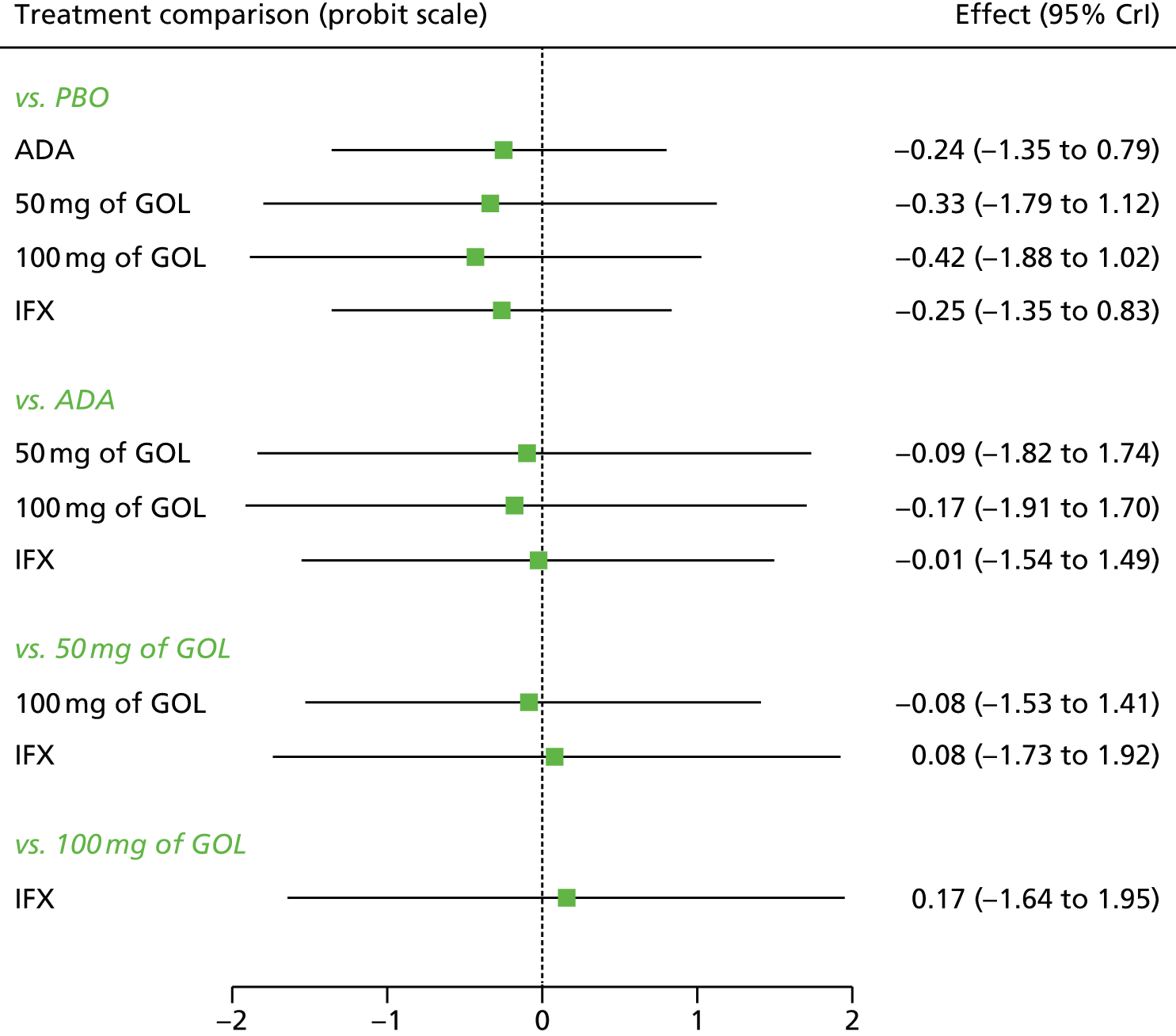
FIGURE 100.
Sensitivity analysis 1: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in remission [SD ∼ U(0,2)].

FIGURE 101.
Sensitivity analysis 1: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in response [SD ∼ U(0,2)].
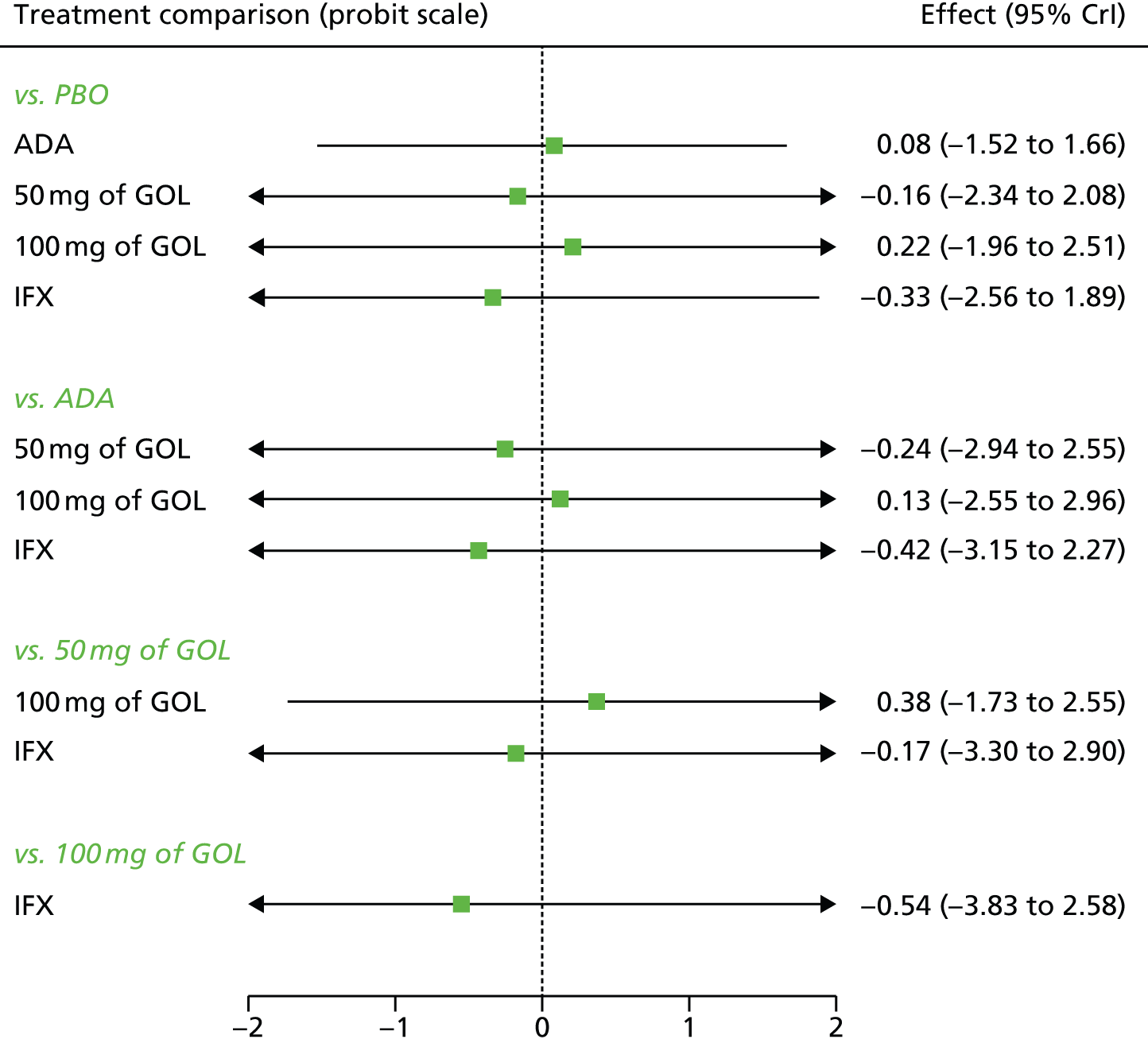
FIGURE 102.
Sensitivity analysis 1: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in remission [SD ∼ U(0,2)].

FIGURE 103.
Sensitivity analysis 2: comparative effect of anti-TNF-α treatment on clinical response/remission in the induction phase [SD ∼ U(0,2)].

FIGURE 104.
Sensitivity analysis 2: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in response [SD ∼ U(0,2)].
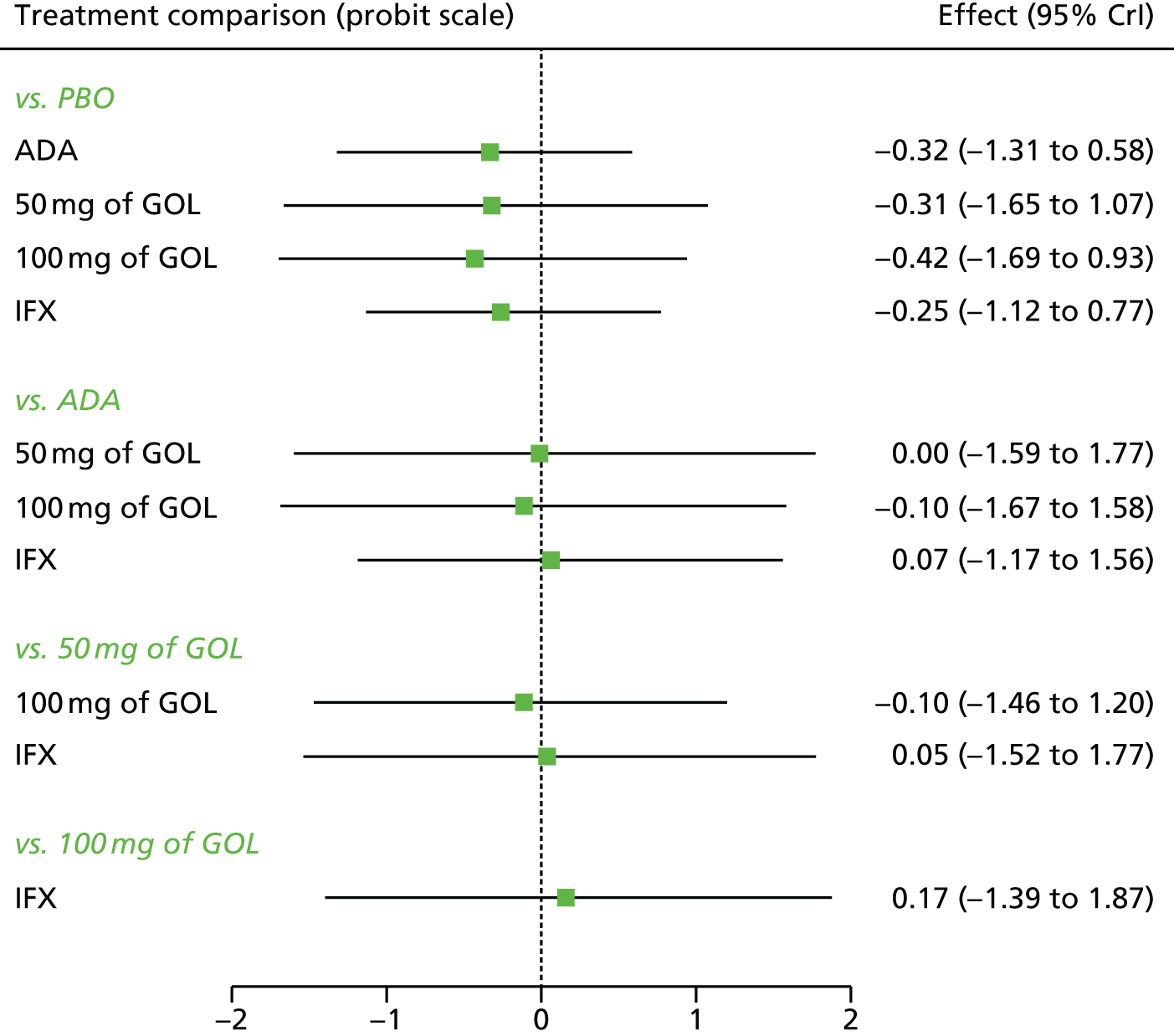
FIGURE 105.
Sensitivity analysis 2: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in remission [SD ∼ U(0,2)].
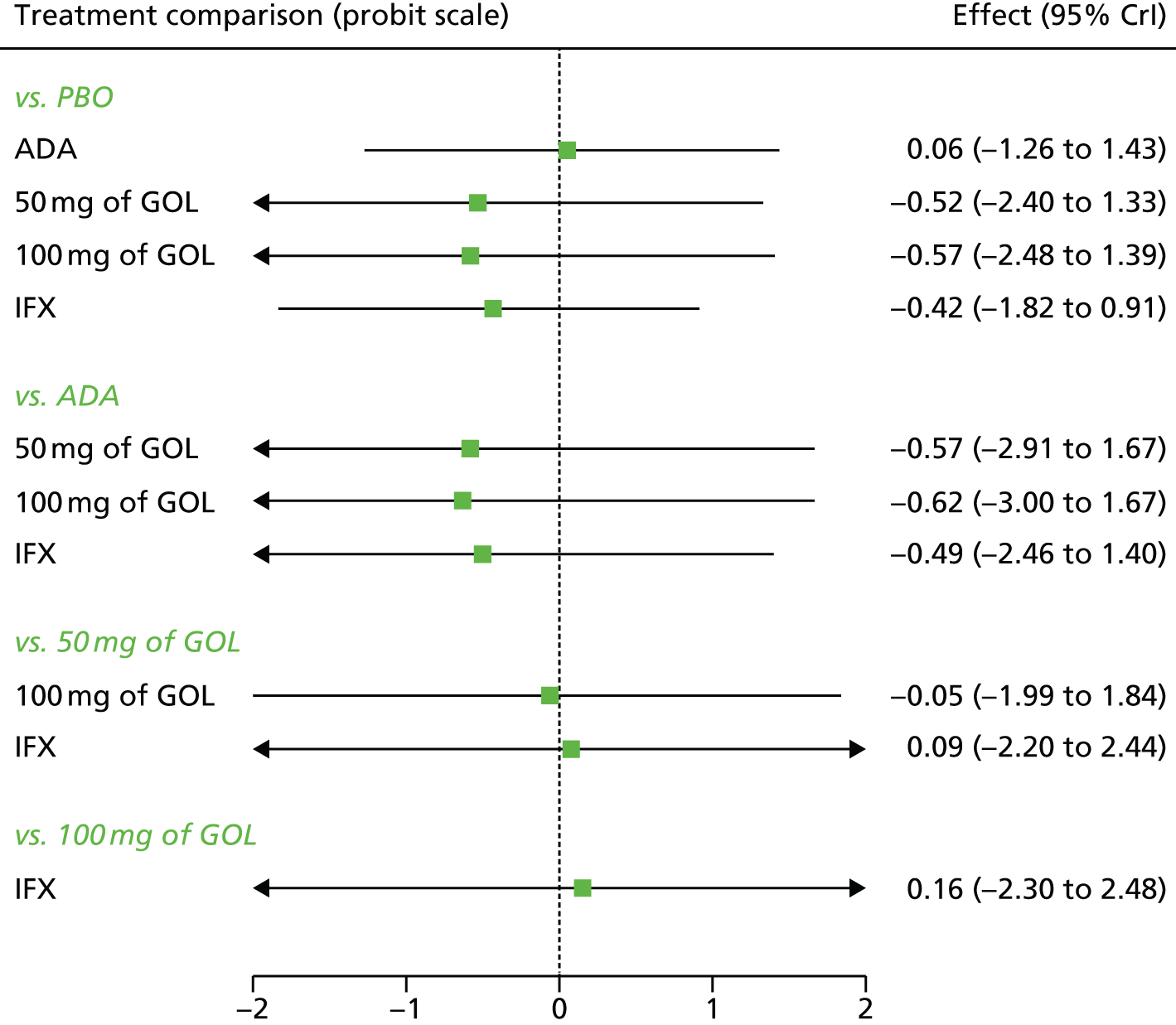
FIGURE 106.
Sensitivity analysis 2: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in response [SD ∼ U(0,2)].
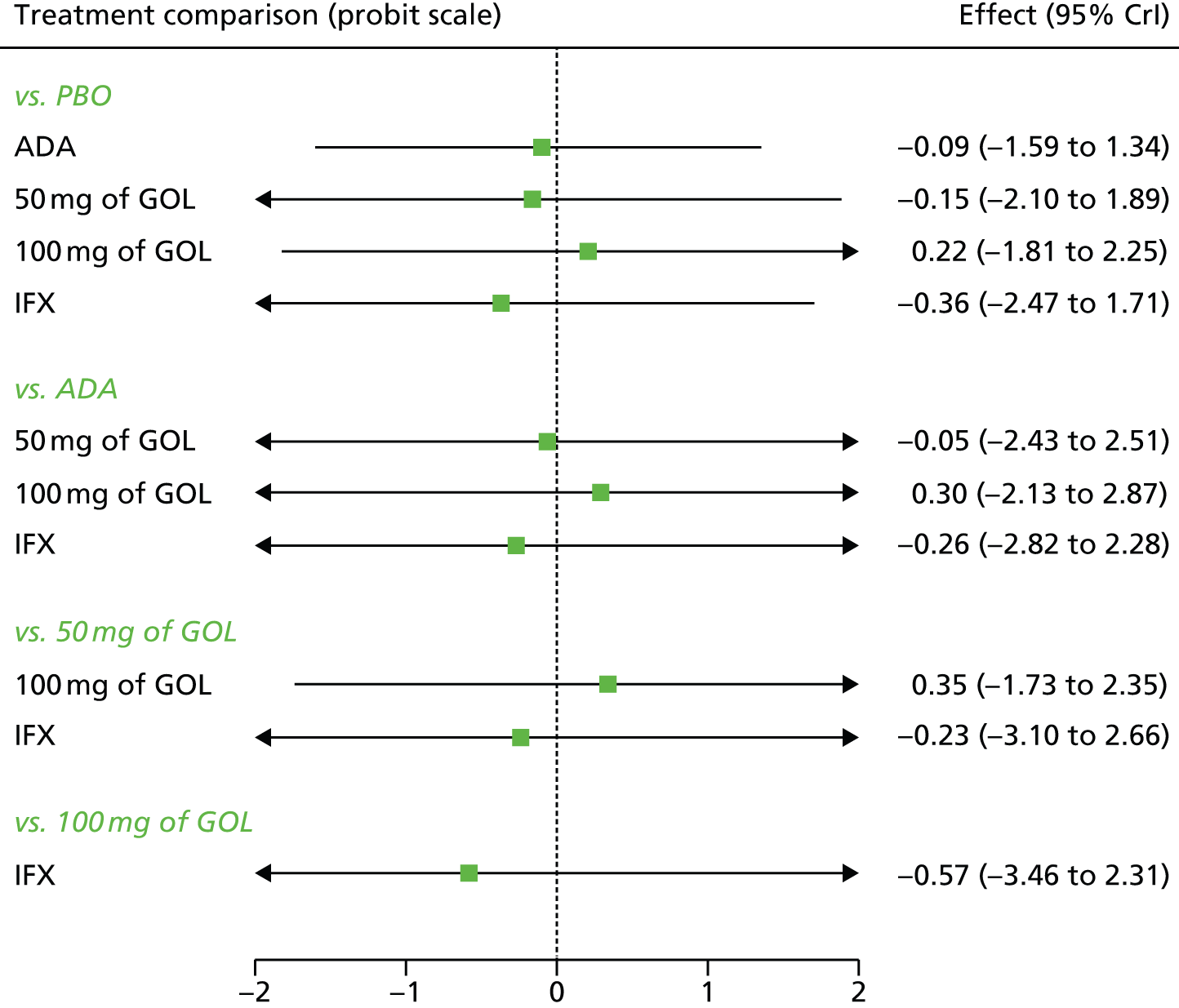
FIGURE 107.
Sensitivity analysis 2: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in remission [SD ∼ U(0,2)].
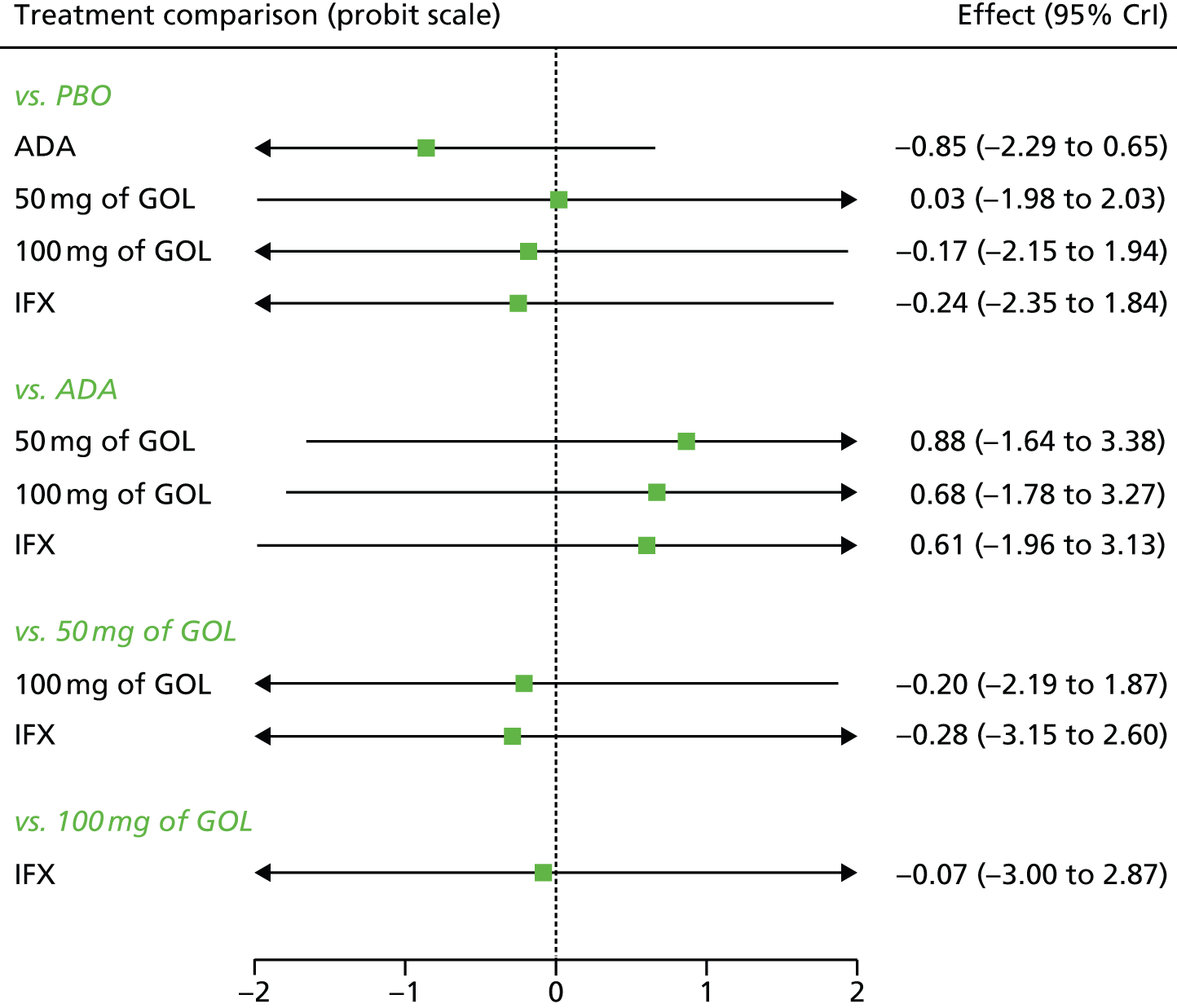
FIGURE 108.
Sensitivity analysis 3: comparative effect of anti-TNF-α treatment on clinical response/remission in the induction phase [SD ∼ U(0,2)].
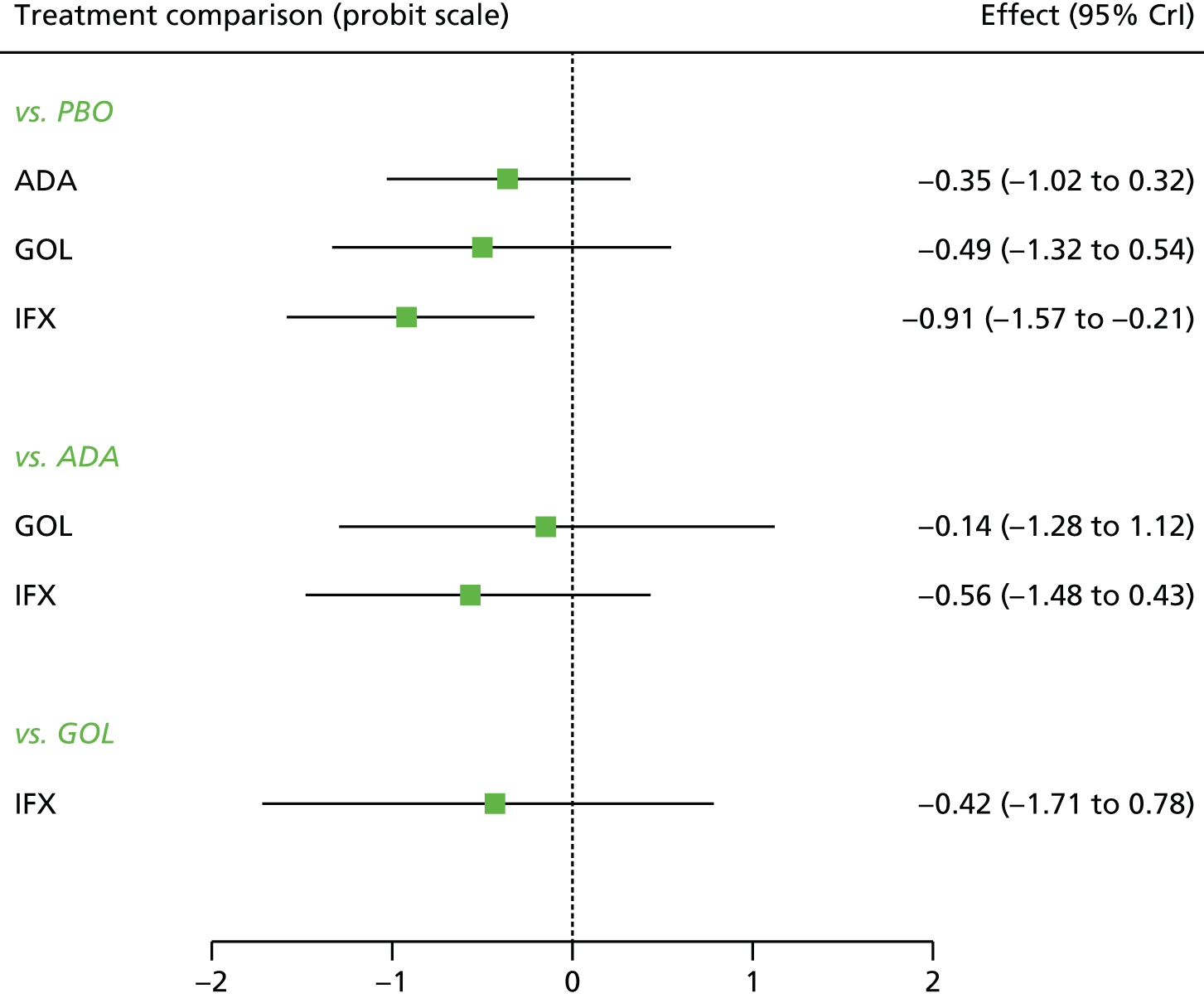
FIGURE 109.
Sensitivity analysis 3: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in response [SD ∼ U(0,2)].

FIGURE 110.
Sensitivity analysis 3: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 8–32 weeks for patients starting in remission [SD ∼ U(0,2)].
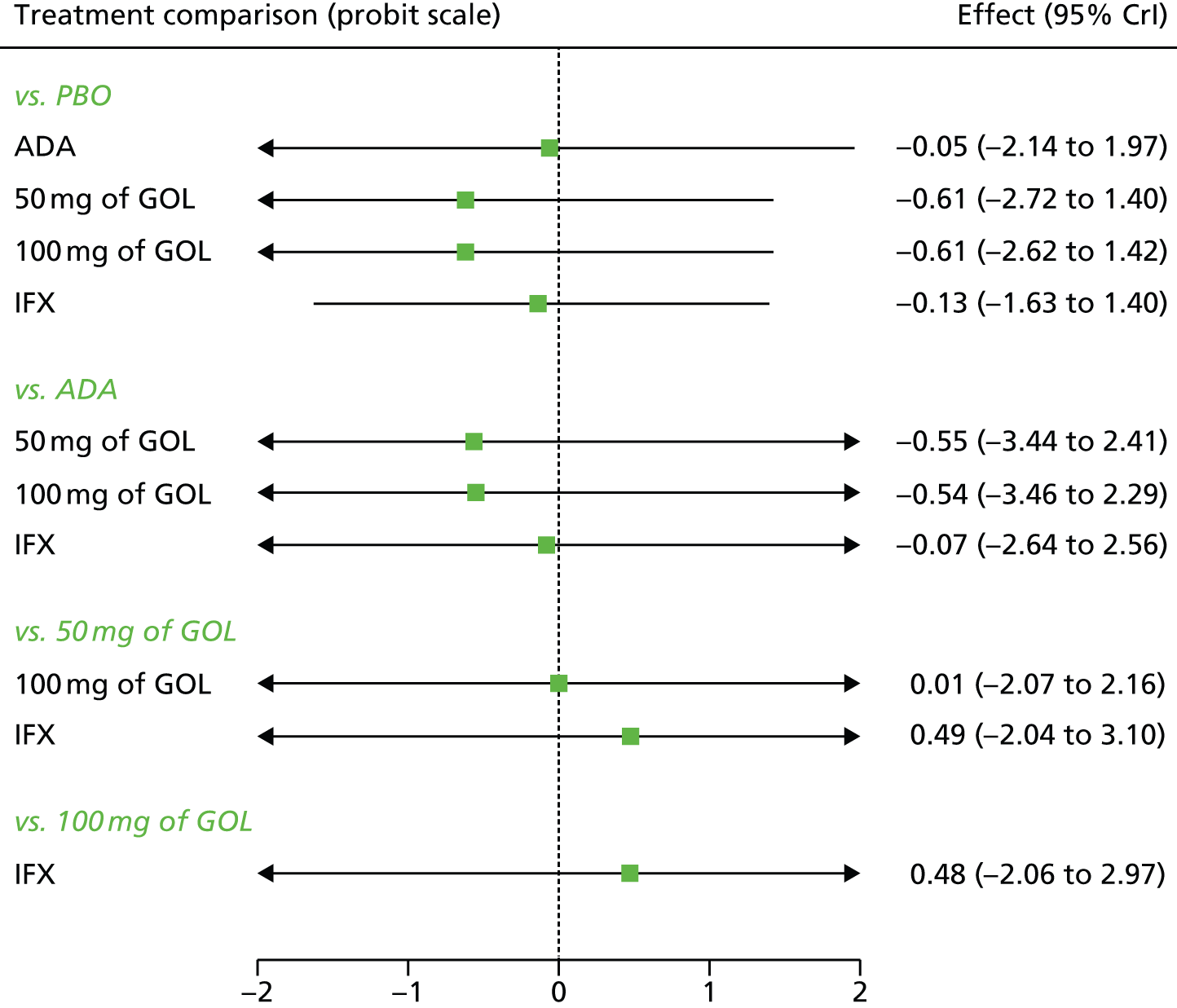
FIGURE 111.
Sensitivity analysis 3: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in response [SD ∼ U(0,2)].
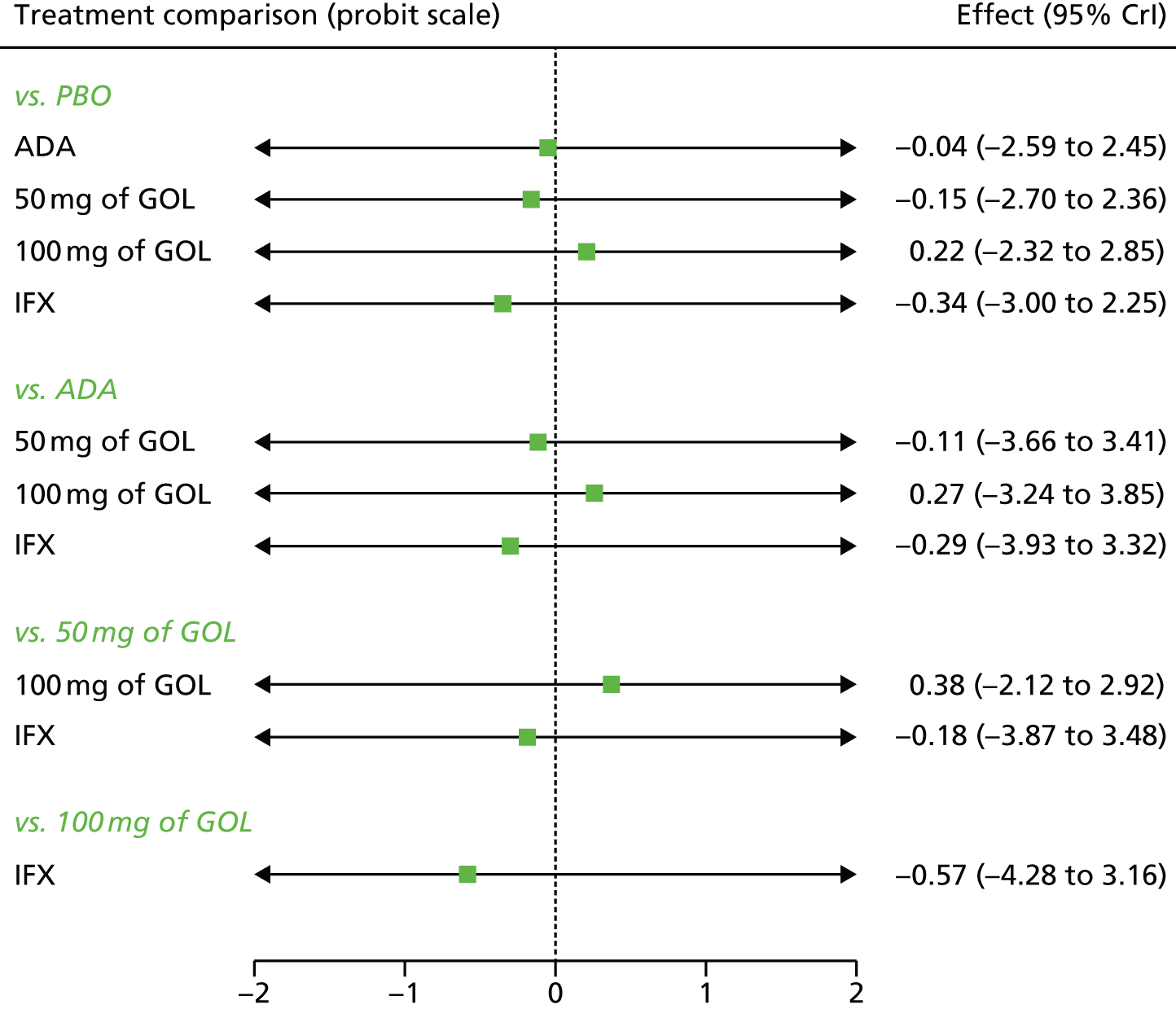
FIGURE 112.
Sensitivity analysis 3: comparative effect of anti-TNF-α treatment on clinical response/remission in the maintenance phase at 32–52 weeks for patients starting in remission [SD ∼ U(0,2)].
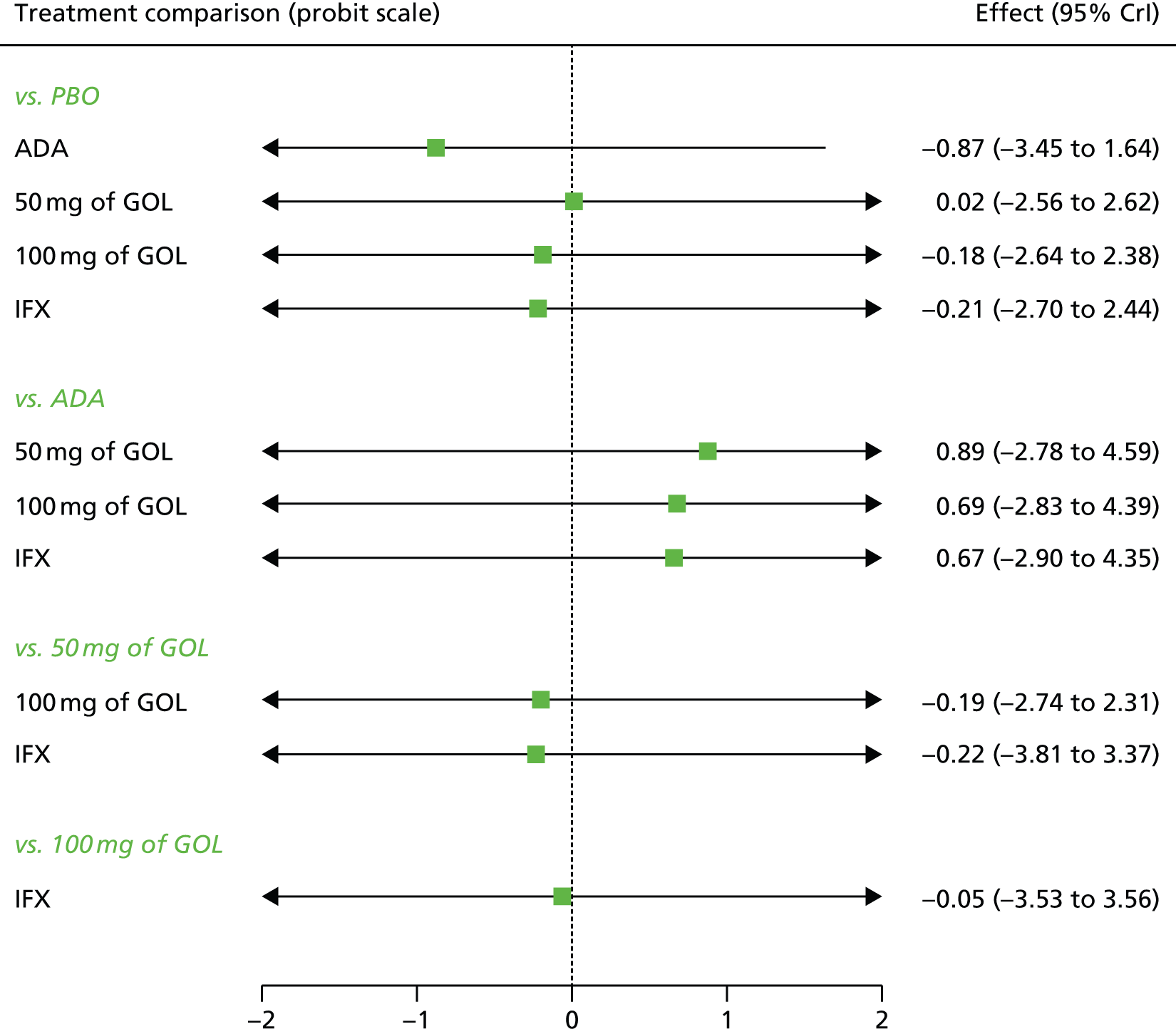
Appendix 9 Searches for cost-effectiveness studies
Database: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations (via Ovid).
Searched: 1946 to January 2014.
Search strategy
-
Colitis, Ulcerative/
-
ulcerative colitis.tw.
-
colitis ulcerosa.tw.
-
uc.tw.
-
colitis ulcerative.tw.
-
Colitis/
-
colitis.tw.
-
colitides.tw.
-
Inflammatory Bowel Diseases/
-
inflammatory bowel disease$.tw.
-
ibd.tw.
-
(col* and ulcer*).tw.
-
colitis gravis.tw.
-
proctocolitis.tw.
-
or/1-14
-
adalimumab.af.
-
humira.af.
-
d 2e7.af.
-
d2e7.af.
-
331731-18-1.rn.
-
infliximab.af.
-
remicade.af.
-
170277-31-3.rn.
-
ta650.af.
-
ta 650.af.
-
inx.af.
-
remsima.af.
-
inflectra.af.
-
ct p13.af.
-
ctp13.af.
-
golimumab.af.
-
simponi.af.
-
cnto148.af.
-
cnto 148.af.
-
476181-74-5.rn.
-
tnf inhibitor$.tw.
-
anti tnf.tw.
-
antitnf.tw.
-
tnf antagonist$.tw.
-
tnf-alpha blocker$.tw.
-
antitumo?r necrosis factor.tw.
-
Biosimilar Pharmaceuticals/
-
(biosimilar$ or biologic$).tw.
-
or/16-43
-
15 and 44
Terms 1–14 are terms for the condition (ulcerative colitis) which are then combined using OR in term 15. Terms 16–43 are terms for the interventions (infliximab, adalimumab and golimumab) which are then combined using OR in term 44. Terms 15 and 44 are then combined using AND to find studies on the condition and interventions in term 45.
To retrieve economic evaluations specially designed highly sensitive search filter were combined with term 45. Economics filter below.
Economic filter
-
exp “costs and cost analysis”/
-
economics/
-
exp economics, hospital/
-
exp economics, medical/
-
economics, nursing/
-
exp models, economic/
-
economics, pharmaceutical/
-
exp “fees and charges”/
-
exp budgets/
-
budget$.tw.
-
ec.fs.
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimi$)).ab.
-
(economic$ or pharmacoeconomic$ or pharmaco-economic$).ti.
-
(price$ or pricing$).tw.
-
(financial or finance or finances or financed).tw.
-
(fee or fees).tw.
-
(value adj2 (money or monetary)).tw.
-
quality-adjusted life years/
-
(qaly or qalys).af.
-
(quality adjusted life year or quality adjusted life years).af.
-
or/1-22
Appendix 10 European Quality of Life-5 Dimensions search
Database: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations (via Ovid).
Searched: 1946 to January 2014.
Search strategy
-
Colitis, Ulcerative/
-
ulcerative colitis.tw.
-
colitis ulcerosa.tw.
-
uc.tw.
-
colitis ulcerative.tw.
-
Colitis/
-
colitis.tw.
-
colitides.tw.
-
Inflammatory Bowel Diseases/
-
inflammatory bowel disease$.tw.
-
ibd.tw.
-
(col* and ulcer*).tw.
-
colitis gravis.tw.
-
proctocolitis.tw.
-
or/1-14
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
15 and 16
List of abbreviations
- 5-ASA
- 5-aminosalicylate
- 6-MP
- 6-mercaptopurine
- ACT
- Active Ulcerative Colitis Trial
- ADA
- adalimumab
- AE
- adverse event
- AS
- ankylosing spondylitis
- AZA
- azathioprine
- BIOSIS
- Bioscience Information Service
- BNF
- British National Formulary
- CCRT
- Cochrane Central Register of Controlled Trials
- CD
- Crohn’s disease
- CDSR
- Cochrane Database of Systematic Reviews
- CEAC
- cost-effectiveness acceptability curve
- CHMP
- Committee for Human Medicinal Products
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CrI
- credible interval
- CRP
- C-reactive protein
- CSR
- clinical study report
- DARE
- Database of Abstracts of Reviews of Effects
- ECCO
- European Crohn’s and Colitis Organisation
- EMA
- European Medicines Agency
- EPAR
- European Public Assessment Report
- EQ-5D
- European Quality of Life-5 Dimensions
- FDA
- Food and Drug Administration
- GOL
- golimumab
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- i.v.
- intravenous
- IBD
- inflammatory bowel disease
- IBDQ
- Inflammatory Bowel Disease Questionnaire
- ICER
- incremental cost-effectiveness ratio
- IFX
- infliximab
- IPAA
- ileal pouch anal anastomosis
- ITT
- intention to treat
- MeSH
- medical subject heading
- MSD
- Merck Sharp & Dohme Ltd
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NMB
- net monetary benefit
- NYHA
- New York Heart Association
- OR
- odds ratio
- PAS
- Patient Access Scheme
- PBO
- placebo
- PLANET-AS
- Programme evaLuating the Autoimmune disease iNvEstigational drug CT-P13 in ankylosing spondylitis patients
- PLANET-RA
- Programme evaLuating the Autoimmune disease iNvEstigational drug CT-P13 in rheumatoid arthritis patients
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PUCAI
- Paediatric Ulcerative Colitis Activity Index
- PURSUIT
- Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment
- PY
- patient-year
- QALY
- quality-adjusted life-year
- RA
- rheumatoid arthritis
- RCT
- randomised controlled trial
- RR
- risk ratio
- SAE
- serious adverse event
- SCAI
- Simple Colitis Activity Index
- ScHARR
- School of Health and Related Research
- ScHARR-TAG
- ScHARR Technology Assessment Group
- SD
- standard deviation
- SDAI
- Simplified Disease Activity Index
- SF-36
- Short Form questionnaire-36 items
- SmPC
- Summary of Product Characteristics
- TB
- tuberculosis
- TNF-α
- tumour necrosis factor alpha
- TTO
- time trade-off
- UC
- ulcerative colitis
- UCSS
- Ulcerative Colitis Symptom Score
- ULTRA
- Ulcerative colitis Long-Term Remission and maintenance with Adalimumab treatment of moderate to severe ulcerative colitis
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed commercial-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence data removed and replaced by the statement ‘commercial-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.