Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/78/03. The contractual start date was in September 2008. The draft report began editorial review in December 2014 and was accepted for publication in August 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
John G Williams was a member of the National Institute for Health Research (NIHR) Efficacy and Mechanism Evaluation Board from 2008 to 2011 and the NIHR Health Services and Delivery Research Researcher-Led Board from 2009 to 2014.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Williams et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and literature review
Background
Ulcerative colitis (UC) is a chronic debilitating disease that affects about 150,000 people in the UK. 1,2 Some 25% of patients with UC present either for the first time or later, with acute severe ulcerative colitis (ASC) requiring hospital admission. 3 In patients with ASC, intravenous steroids are the first-line treatment. 4 However, about 30–40% of these patients are resistant to intensive steroid therapy. 5,6 Previously, colectomy was the only available option for these patients. 5 Although mortality following emergency colectomy has fallen over time, 10% of patients die within 3 months of surgery. 7
The use of intravenous or oral ciclosporin (Sandimmun® or Neoral®, Novartis Pharmaceuticals UK Ltd),8,9 a calcineurin inhibitor that selectively inhibits T-cell function, and infliximab (Remicade®, Merck Sharp & Dohme Ltd),10,11 a monoclonal antibody that targets tumour necrosis factor α, then offered hope for the treatment of steroid-resistant UC.
Several studies support the use of infliximab in patients with moderate or severe UC,12–15 especially steroid-resistant UC patients who do not tolerate ciclosporin. 12 A recent systematic review and meta-analysis of 34 infliximab studies found an average short-term response and remission of 68% and 40%, respectively, and an average long-term response and remission of 53% and 39%, respectively. 15 However, there are concerns about high rates of later relapses. 16,17 Two large randomised controlled trials (RCTs) also found highly significant improvements in total Inflammatory Bowel Disease Questionnaire (IBDQ)18 score and Short Form questionnaire-36 items physical and mental component scores19 for infliximab patients at 8 weeks when compared with placebo. 20 The current UK National Institute for Health and Care Excellence (NICE) guidelines allow the use of infliximab only when ciclosporin is contraindicated or as part of a research study. 21
Several studies support the use of ciclosporin as a safe and effective treatment for steroid-resistant UC,22–24 although it has been associated with side effects including dose-related toxicity23,25,26 and long-term failure. 23–25,27 A systematic review and meta-analysis of 31 ciclosporin studies reported a mean short-term response rate of 71%;28 one study reported that 65% of patients relapsed after 1 year and 90% after 3 years. 27 Another review of 32 studies reported a 51% short-term success rate. 29 However, the relevant Cochrane review concluded that there was limited evidence that ciclosporin was more effective than standard treatment for severe UC and that long-term benefits were unclear. 30 It also advocated research into the long-term effects of ciclosporin on quality of life (QoL) and its cost-effectiveness.
Review of literature
We searched MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials, original studies, systematic reviews and meta-analyses that compare ciclosporin and infliximab in the management of acute UC resistant to steroid therapy up to the 31 July 2014. We used the search terms: ‘ciclosporin’, ‘infliximab’, ‘ulcerative colitis’, ‘acute severe ulcerative colitis’ and ‘steroid resistant ulcerative colitis’. We included synonyms, different spellings and drug brand names in our search to identify all relevant articles. We used the electronic search strategies checklist of the Cochrane Collaboration. 31
We identified nine observational studies and one RCT32–41 that compared the efficacy and safety of ciclosporin with those of infliximab (Table 1). Although both ciclosporin and infliximab were effective in steroid-resistant UC, results did not agree which drug was better. 32–35 One small retrospective study34 of 38 patients with acute UC resistant to steroids showed a higher rate of colectomy in patients who received ciclosporin (63% and 68% at 3 and 12 months, respectively) than in those who received infliximab (21% and 37% at 3 and 12 months, respectively); there was no significant difference between groups in adverse events (AEs) or steroid dependence. A prospective study36 found the colectomy-free rate at discharge, and at 3 and 12 months from admission, was significantly higher in patients who had infliximab as a rescue therapy (n = 45) compared with those who had ciclosporin (n = 38). A minute retrospective study35 of two cohorts of patients (15 on ciclosporin and six on infliximab) showed a higher rate of colectomy and opportunistic infection in patients who received ciclosporin compared with those who received infliximab. A retrospective study of 49 patients on infliximab and 43 patients on ciclosporin showed that colectomy frequencies were significantly lower after rescue with ciclosporin than with infliximab, with no death or opportunistic infection. 32 Mocciaro et al. 33 retrospectively examined the outcomes of 30 patients who received ciclosporin and 30 patients who received infliximab for steroid-resistant acute UC and reported that the rate of colectomy at 12 months was 48% in the ciclosporin group compared with 17% in the infliximab group (p = 0.01); both drugs were equally safe without severe AEs. A recent UK inflammatory bowel disease (IBD) audit reported increased use of infliximab compared with ciclosporin in managing ASC; the clinical response rate was higher in patients who received infliximab. 42
| Study | Number of patients | Main outcomes reported | Follow-up period |
|---|---|---|---|
| Laharie et al., 201241 |
|
Treatment failure: 60% with ciclosporin and 54% with infliximab (p = 0.52) SAEs: 16% with ciclosporin and 25% with infliximab Mucosal healing: 47% with ciclosporin and 45% with infliximab (p = 0.85) UK-IBDQ scores improved by 78 points with ciclosporin and 100 points with infliximab (p = 0.19) Colectomies: 17% in the ciclosporin and 21% in the infliximab group |
98 days |
| Leblanc et al., 201138 |
|
Colectomy rate: 54% in infliximab group and 67% in ciclosporin group Clinical remission: 25% in infliximab group and 14% in ciclosporin group AEs: 23% in infliximab group and 24% in ciclosporin group |
22 months |
| Mañosa et al., 200940 |
|
Six patients (37.5%) required colectomy and 19% of patients had SAEs | 195 days |
| Maser et al., 200837 |
|
Clinical remission: 40% in the infliximab-salvage group and 33% in the ciclosporin-salvage group The median duration of remission: 13.6 months in the infliximab-salvage group and 21.0 months in the ciclosporin-salvage group Colectomy rate: 40% in the infliximab group and 44% in the ciclosporin group SAEs: one in the infliximab group and two in the ciclosporin group |
Average duration of follow-up was 7.8 months for infliximab and 17.7 months for ciclosporin |
| Dean et al., 201234 |
|
Colectomy rate: 63% for ciclosporin and 21% for infliximab (p = 0.0094) at 3 months. By 12 months the rates were 68% and 37% for ciclosporin and infliximab, respectively (p = 0.06) Steroid dependence at 12 months was 50% for ciclosporin and 25% for infliximab (p = 0.36) There was no statistical difference in AEs between the two groups (p = 0.17) |
12 months |
| Chaparro et al., 201239 |
|
Colectomy rate was 30% SAE rate was 23% |
58 weeks |
| Croft et al., 201336 |
|
Colectomy rate: 44% for ciclosporin and 16% for infliximab at discharge (p = 0.006). At 3 months the colectomy rates were 47% vs. 24% (p = 0.04), and at 12 months colectomy rates were 58% vs. 35% (p = 0.04) for ciclosporin and infliximab, respectively | 12 months |
| Sjoberg et al., 201232 |
|
Colectomy rates: 5% vs. 27% at 15 days, 7% vs. 23% at 3 months and 23% vs. 43% at 12 months for ciclosporin and infliximab, respectively (p < 0.05) | 12 months |
| Mocciaro et al., 201233 |
|
Colectomy rates: 28.5% vs.17% (p = 0.25) at 3 months and 48% vs. 17% at 12 months (p = 0.007) for ciclosporin and infliximab, respectively The 1–2–3 year cumulative colectomy rates were 48%, 54%, 57% in the ciclosporin group and 17%, 23%, 27% in the infliximab group (p < 0.05) |
The mean follow-up was 74.7 months for ciclosporin and 33.6 months for infliximab |
| Daperno et al., 200435 |
|
Clinical remission: 53% in the ciclosporin group vs. 67% in the infliximab group Colectomy rates: 47% in the ciclosporin group vs. 33% in the infliximab group |
49 months |
Four studies37–40 explored the use of infliximab and ciclosporin as a second-line rescue therapy by crossing them over after failure of these agents as a first-line therapy. The studies concluded that the use of ciclosporin or infliximab as second-line rescue therapy induced remission in up to two-thirds of patients. 38 However, this remission was of limited duration, the rate of colectomy was around 40%, and the rate of serious adverse events (SAEs) ranged from 16% to 23%. 37–40
A recent meta-analysis of 321 patients in six retrospective cohort studies32–35,37,38 concluded that infliximab and ciclosporin are comparable when used as rescue therapy in acute severe steroid-refractory UC. However, the outcome measures were limited to colectomy rates, adverse drug reactions (ARs) and postoperative complications over 12 months. 43
Against this background of observational studies that compared these two drugs only indirectly, la Groupe d’Etude Thérapeutique des Affections Inflammatoires Digestives (GETAID) recently reported on the trial CycloSporine versus InFliximab (CySIF),41 the first head-to-head comparison of these two drugs. CySIF found no significant differences in ‘treatment failure’ within 98 days, defined as any of the following: (i) no clinical response after 7 days; (ii) no remission without steroids after 98 days; (iii) relapse between 7 and 98 days; (iv) SAE leading to treatment interruption; (v) colectomy; or (vi) death. However, CySIF recruited only 110 patients, followed them for only 98 days, reported no data on QoL and collected no data on costs or from participants.
The qualitative research reported in the literature on infliximab focuses on its role in treating rheumatoid arthritis. 44 There is no qualitative study that explores the use of ciclosporin in the treatment of acute UC. One qualitative study exploring patient and parent experiences of infliximab in paediatric gastroenterology, found favourable views of the drug when used in a hospital environment. 45 To our knowledge, no studies, qualitative or otherwise, have explored health professionals’ views of drug administration for treating steroid-resistant UC. This is disappointing as qualitative methods are well suited to investigate personal experience, individual perception and belief and meaning systems,46,47 enabling triallists to clarify patients’ and clinicians’ understandings of clinical practice and drug regimes. 48,49 Therefore, there is a need for a trial that also seeks the experiences and views of patients with acute UC about treatments and changes in health over time and of health-care professionals about ease of drug handling and drug preference.
Patients with UC incur substantial health-care costs over many years. As well as the direct costs of treatment by drug or surgery, UC patients consume a wide range of health-care resources including spells in hospital, attendances at emergency departments, outpatient visits, endoscopies and other investigations. Nevertheless, no study has assessed the cost-effectiveness of infliximab and ciclosporin in a head-to-head clinical trial. Instead, Markovian economic models have created hypothetical cohorts of patients with acute UC resistant to steroids to assess the cost-effectiveness of infliximab compared with ciclosporin and surgery. 50,51 These models used published evidence6,8,10,52 to extrapolate the costs and effects in quality-adjusted life-years (QALYs) gained by each drug. Although these models conclude that infliximab is a cost-effective treatment in comparison with ciclosporin and surgery, we need to interpret this claim with caution. Theoretical models cannot capture all aspects of disease progression and their costs; and require assumptions to replace unavailable primary data. Furthermore, they excluded patient mortality and side effects while assuming that infliximab had a better side effect profile and mortality rate than both ciclosporin and surgery. Therefore, direct comparison of the clinical effectiveness and cost-effectiveness of infliximab and ciclosporin in patients with acute UC is essential.
In summary, infliximab and ciclosporin are often effective in the short term, but there is little long-term evidence about their relative clinical effectiveness and cost-effectiveness. The Evidence Review Group report commissioned by NICE concluded: ‘The results consistently indicate that the move from standard care to ciclosporin is highly cost-effective’. 53 Thus the policy issue is clear: should the NHS make a further move from ciclosporin to infliximab?
Hence we designed Comparison Of iNfliximab and ciclosporin in STeroid Resistant Ulcerative Colitis: pragmatic randomised Trial and economic evaluation (CONSTRUCT) to achieve a rigorous, comprehensive, long-term comparison of these drugs. In particular, during the trial we enhanced measurement of QoL and costs in four ways:
-
extending data collection for all trial participants, whenever recruited, until 28 February 2014
-
adding questionnaires at 18, 30 and 36 months to those at 3, 6, 12 and 24 months
-
adding four questionnaires following colectomy and any ensuing corrective surgery
-
planning to use the techniques of survival analysis and statistical imputation of missing values to impute costs and QoL for all CONSTRUCT participants who generate data on survival, colectomy or QoL after randomisation.
Aim and objectives
The aim of this trial was to compare the clinical effectiveness and cost-effectiveness of infliximab and ciclosporin for patients with steroid-resistant UC over a period of up to 3 years.
Specific objectives were to:
-
compare health-related quality of life (HRQoL) after these two treatments
-
compare mortality, morbidity and disease activity between treatments
-
compare colectomy rates between treatments
-
compare cost-effectiveness of treatments in cost per QALY
-
investigate the views of patients about their health and treatments
-
investigate the views of health-care professionals about the treatments and their ease of administration.
Chapter 2 Methods
Trial design
We conducted an open-label parallel-group, pragmatic randomised trial using mixed methods: quantitative (including health economics and routinely collected data) and qualitative. 54
Our primary outcome measure was quality-adjusted survival (QAS)55 weighted by participants’ scores on the Crohn’s and Ulcerative Colitis Questionnaire (CUCQ), our extension of the validated UK-IBDQ to include severe colitis and post-colectomy states. Secondary outcomes included two generic measures of QoL [European Quality of Life-5 Dimensions (EQ-5D) and Short Form questionnaire-12 items (SF-12)], emergency and planned colectomy rates, AEs and mortality.
We assessed the relative cost-effectiveness of the trial drugs through cost–utility analysis from the perspective of the NHS and Personal Social Services, as recommended by NICE. 56 These analyses assessed differences between groups in total costs and QALYs.
Our qualitative studies aimed to enrich our quantitative results by exploring participants’ experiences of UC and the trial drugs, and their priorities for health and well-being. We also explored health-care professionals’ preferences between the trial drugs and their administration.
We used the Method for Aggregating The Reporting of Interventions in Complex Studies (MATRICS), which we had previously developed in another complex study, to integrate and compare the findings from the mixed methods used in CONSTRUCT. 57 We classified our outcomes into effects on participants; effects on gastroenterological services and professionals; and effects on the rest of the NHS and society. Using the MATRICS template, we combined all comparable findings into summary statements and highlighted where different methods resulted in inconsistent statements.
As we expected difficulty in recruiting acutely ill patients in hospital and completing their baseline data, we created a comprehensive cohort. We invited patients with known or suspected UC to join this cohort soon after admission. We explained that, if they had UC and did not respond to intravenous steroids, they might need other drug treatment; so, if they were suitable, we would invite them to further treatment as part of a clinical trial. To increase the chance of recruiting them, we collected their baseline data as soon as possible after they had consented.
From May 2010 until the end of February 2013 we recruited from the cohort to the trial those participants with UC who failed to respond to intravenous steroids over about 2–5 days, but did not then need surgery. After full written and oral explanation we invited participants who fulfilled the trial inclusion and exclusion criteria to consent to randomisation between infliximab and ciclosporin. Placebo controls would have been unethical, as these severely ill patients need treatment, as the NICE Evidence Review Group recognised. 53
We shall supplement our designed research data with routinely collected data held by the Health & Social Care Information Centre in England, the Secure Anonymised Information Linkage database in Wales, or the Health Informatics Centre in Scotland. As these data are not yet available for the full period of the trial, we shall analyse and report them in due course. We have consent from participants in both cohort and trial to access their routine data for 10 years from recruitment, and from trial participants to send them questionnaires over that period.
Recruitment
Trial sites
Via the British Society of Gastroenterology we asked consultant gastroenterologists to express interest in taking part in the study and to complete a questionnaire. We considered sites that had treated four or more patients with steroid-resistant UC in the previous 12 months to be eligible and invited them to seek local approval. As a result we initiated the trial in 67 NHS trusts or health boards, covering both teaching and district general hospitals in England, Scotland and Wales. We phased the initiation of these sites, reflecting the time needed to gain local research and development (R&D) approval.
Participants in cohort and trial
The target population for the cohort were inpatients with ASC, known or suspected, who were potentially eligible for the trial. The target population for the trial were cohort members who failed to respond to a course of about 2–5 days of intravenous steroid medication, but did not then need surgery. We invited eligible patients into the cohort as soon as feasible and into the trial once the clinical team had confirmed steroid resistance. The treatment of patients who did not consent to cohort or trial did not change in any way.
Participants in qualitative study
We used purposive quota sampling to identify 12 representative consenting participants from each arm of the trial for interview on two occasions (see Appendix 1). To include participants from sites starting later in the trial pro rata, we maintained a list of eligible participants.
We also interviewed principal investigators (PIs) and nurses responsible for administering and monitoring the drugs across the trial sites. We included both professions to explore drug administration, physical effects of both drugs on patients, personal preferences and their participation in CONSTRUCT. We used purposive sampling to recruit 12 PIs from three strata: sites that recruited well to cohort and trial; sites that recruited well to cohort but less well to trial; and sites that recruited poorly to both cohort and trial. However, we recruited all eight nurses from good recruiting sites as others would have little relevant experience of the trial drugs.
Informed consent
Patients eligible for the cohort received cohort participant information sheets (see Appendix 2) and oral explanation from consultant gastroenterologists or research professionals (usually research nurses); in response, patients gave written consent by signing and dating a cohort consent form (see Appendix 3). Cohort participants who became eligible for the trial received the patient information sheet (RCT) (see Appendix 4), and gave written consent by signing and dating a trial consent form (see Appendix 5). For both cohort and trial, those taking consent countersigned and dated the form to confirm that the participant had fully understood the nature of the study and had an opportunity to ask questions; they also put a copy of that consent in the participant’s medical record and gave another copy to the participant.
Research professionals could take consent to the cohort if authorised to do so on the site delegation log following appropriate training, including in good clinical practice (GCP). Although they could also explain the trial to cohort patients, responsibility for countersigning lay with the site PI or another doctor with delegated authority on the site delegation log. The trial consent form included consent to take part in qualitative interviews.
Withdrawal
The procedure for consenting participants stressed that they could withdraw from the cohort or trial whenever they wished without giving a reason and without affecting their care in any way. However, we documented any reasons given. Participants could stipulate the level of withdrawal: from the allocated treatment, from completion of further participant questionnaires, from consent to the use of any of their routine, or from any combination of these. We also encouraged site staff to trace participants lost to follow-up and document reasons when possible.
Between randomisation and the end of the trial, there were decisions to change allocated treatments, but these did not require withdrawal from this pragmatic trial. These included failure to respond to treatment with infliximab or ciclosporin, usually leading to surgical intervention.
Trial inclusion criteria
Patients admitted as emergency admissions with severe colitis (according to Truelove and Witts,4 a Mayo score of at least 2 on endoscopic finding or clinical judgement) who fail to respond to about 2–5 days of intravenous hydrocortisone therapy, who also had either:
-
histological diagnosis of UC in this episode
-
histological diagnosis of indeterminate colitis in this episode when clinical judgement (based on macroscopic appearance, disease distribution or previous history) suggested a diagnosis of UC rather than Crohn’s disease
-
symptoms typical of UC awaiting histology; or
-
history of UC confirmed histologically.
Trial exclusion criteria
-
Aged < 18 years of age on admission.
-
Histological diagnosis before randomisation inconsistent with UC.
-
Enteric infection confirmed before randomisation by stool microscopy, culture or histology (including Salmonella, Shigella, Clostridium difficile, Campylobacter and cytomegalovirus).
-
Vulnerable patient.
-
Unable to consent.
-
Positive pregnancy test or current lactation.
-
Woman of childbearing potential unwilling to use contraception during and for 6 months after treatment with infliximab in accordance with the Summary of Product Characteristics (SPC).
-
Current malignancy, except basal cell carcinoma.
-
Serious comorbidity, including immunodeficiency, myocardial infarction within last month, moderate or severe heart failure (New York Heart Association class III or IV), acute stroke within last month, respiratory failure, renal failure, hepatic failure and active or suspected tuberculosis.
-
Other severe infections including sepsis, abscesses and opportunistic infections.
-
History of hypersensitivity to infliximab, ciclosporin or polyethoxylated oils (notably Sandimmun Concentrate for Solution for Infusion).
-
Current use of tacrolimus or rosuvastatin.
-
English not good in absence of local translator.
-
Need for emergency colectomy without further medical treatment.
-
Currently taking part in another clinical trial.
-
Treatment with either infliximab or ciclosporin in the 3 months before admission.
-
Any other contraindication to treatment with infliximab or ciclosporin.
Sample size and power
Our original target analysable sample size was 360 participants, based on a primary outcome of a change in HRQoL over 2 years. 54 However, in 2012 slower recruitment than predicted led us to seek agreement from the Trial Steering Committee (TSC), Data Monitoring and Ethics Committee (DMEC) and Health Technology Assessment (HTA) programme to revise the primary outcome and reduce the analysable sample size to 250. We also proposed to analyse the area under the curve (AUC) of scores on the CUCQ collected every 6 months up to 3 years from randomisation; and to include participants who had undergone colectomy by developing and validating a post-colectomy extension to the CUCQ, which we termed the Crohn’s and Ulcerative Colitis Questionnaire with post-colectomy extension (CUCQ+).
The changes required statistical imputation to exploit the resulting data set. As these techniques are difficult to incorporate into power calculations, we used a simpler calculation based on t-tests of mean CUCQ scores at 12 months. To detect an effect size of 0.35 in these scores with 80% power when using a 5% significance level, required that we analyse at least 250 trial participants. We used the techniques of statistical imputation applied successfully by the Cancer of the Oesophagous or Gastricus: New Assessment of the Technology of Endosonography58 and Folate Augmentation of Treatment – Evaluation of Depression (FolATED)59 trials to achieve an effective sample size of 250 for CONSTRUCT.
Randomisation
We allocated at random between infliximab or ciclosporin all participants who completed baseline assessment, met the trial inclusion criteria and gave informed consent. We used a password-protected website that accessed an adaptive algorithm to protect against subversion while ensuring that each trial arm was balanced by centre. 60 To validate each request for randomisation, the website asked:
-
for the participant’s trial number, and month and year of birth
-
for the name of person requesting randomisation (limited to those trained and authorised)
-
if consent had been given
-
if the participant had met the inclusion criteria
-
if the participant had none of the exclusion criteria
-
if the baseline questionnaire been completed.
If the responses to all four questions were ‘yes’, the website gave the name of the drug allocated to the participant and immediately confirmed the trial number and drug by e-mail, and recorded those on the randomisation database.
Hospital pharmacies at trial sites held the trial drugs. After each randomisation, the research staff confirmed study number and drug by fax to the relevant pharmacy who labelled the drug with the European Union Drug Regulating Authorities Clinical Trials number, sponsor, participant’s trial number, name and address of supplier, dose and ‘For Clinical Trial Use Only’.
Blinding
As this was an open trial, there was no need for procedures to inform sites about allocated treatments. However, the chief investigator, trial methodologist, outcomes specialist, health economists and statisticians remained blind to them until the TSC and DMEC had reviewed and approved the analysis of the primary outcome.
Interventions
Participants randomised to infliximab received it as Remicade in 5-mg/kg intravenous infusions over 2 hours – forthwith, and at 2 and 6 weeks after the first infusion – in accordance with local prescribing guidelines.
Participants randomised to ciclosporin received it as Sandimmun by continuous infusion of 2 mg/kg/day. We asked sites to change the infusion every 6 hours, using non-polyvinyl chloride (PVC) bags and administration sets. Intravenous treatment continued for up to 7 days if successful. They switched participants responding to ciclosporin to twice-daily oral doses delivering 5.5 mg/kg/day, and adjusted doses to achieve trough ciclosporin concentration of 100–200 ng/ml. They measured whole-blood ciclosporin levels according to local practice, ideally 48 hours after oral therapy and then every 2 weeks. After 12 weeks, treatment was at the discretion of the participant’s consultant.
We asked centres to consult the SPC for Remicade or Sandimmun and oral ciclosporin (all available online) at the time of first prescription.
For both treatments we gave centres discretion to start azathioprine or 6-mercaptopurine at therapeutic doses in week 4. We asked them to eliminate steroids by week 12 in participants who remained well, but to reinstate them in participants who became symptomatic. We also asked centres to give co-trimoxazole as prophylaxis against Pneumocystis jiroveci (formerly carinii) pneumonia in both groups.
Outcome measures
Primary outcome
The primary outcome was the AUC of CUCQ scores. This is equivalent to QAS weighted by scores on the disease-specific CUCQ. We concurrently validated the CUCQ, which extends the validated UK-IBDQ61 to cover acute illness and colectomy.
Secondary outcomes
-
(a) Disease-specific QoL, measured by the CUCQ.
-
(b) and (c) Generic QoL, measured by the SF-12. 62
-
(d) Mortality.
-
(e) Colectomies, both emergency and planned.
-
(f) AEs.
-
(g) Readmissions, including those for causes other than UC.
-
(h) Malignancies.
-
(i) Serious infections.
-
(j) Renal disorders.
-
(k) Disease activity, using the criteria proposed by Truelove and Witts. 4
Economic outcomes
-
(l) NHS costs.
-
(m) HRQoL, measured by EQ-5D. 63
-
(n) Participants’ time off work.
Qualitative outcomes
-
(o) Participants’ views of their drugs and their consequences.
-
(p) Professional views of both drugs and their consequences.
Adverse events
Definitions
The Medicines for Human Use (Clinical Trials) Regulations 2004: SI 2004/1031 describe thus:64
-
AE: any untoward medical occurrence in a subject to whom a medicinal product has been administered, including occurrences which are not necessarily caused by, or related to, that product.
-
AR: any untoward and unintended response to a medicinal product which is related to any dose administered to the subject.
-
Unexpected adverse reaction: an adverse reaction, the nature and severity of which is not consistent with the information in the SPC for the medicinal product.
-
SAE or serious adverse reaction (SAR) or suspected unexpected serious adverse reaction (SUSAR): any AE, adverse reaction or unexpected adverse reaction, respectively, that:
-
results in death
-
is life-threatening
-
requires hospitalisation or prolongation of existing hospitalisation
-
results in persistent or significant disability or incapacity; or
-
is a congenital anomaly or birth defect.
-
Causality
Causality is the degree to which an untoward medical occurrence can be attributed to the trial intervention rather than the underlying UC. We used the subjective scale: unrelated, unlikely to be related, possibly related, probably related or definitely related. We classed only the last three as ARs or SARs having a causal relationship. However, we did not class events caused by UC, notably worsening colitis or initial steroid resistance, as ARs. We reported SARs to the Medicines and Healthcare products Regulatory Agency (MHRA) annually.
Expectedness
We considered AEs, ARs, SAEs and SARs as ‘unexpected’ if their nature and severity was not consistent with the relevant SPC. Expected events included:
-
progression or exacerbation of the participant’s underlying UC, including clinical sequelae of progression, such as worsening diarrhoea or abdominal pain
-
medical or surgical procedures including surgery and endoscopy; however, we considered events that led to procedures and surgical sequelae, such as pleural effusion or small bowel obstruction, separately
-
conditions or symptoms present or detected before the first dose that did not worsen
-
recognised undesirable effects found in the current SPCs for Remicade, Sandimmun or Neoral; as SPCs updated regularly during the trial, we recommended that PIs consult the versions online.
Monitoring adverse events
In view of the extensive side effects of both trial drugs documented in their SPCs, and the many sequelae of disease progression, the protocol stipulated that we did not require expedited reporting of serious events from sites, unless they were unexpected, in which case we asked for notification within 24 hours.
We designed adverse event screening forms (AESFs) to enable local PIs to assess seriousness, causality and expectedness in a logical sequence (see Appendix 6). Sites sent AESFs, once countersigned by local PI or authorised person, securely via FaxPress to the trial office. The CONSTRUCT data manager was responsible for initial screening of AESFs, paying particular attention to completeness, raising queries with the local team and immediately notifying the chief investigator of events that might be classified as SUSARs. If uncertain, the chief investigator discussed them with the local PI before the final decision. We entered screened AESFs onto our Generic Clinical Information System (GeneCIS; version 10i, Swansea University, Swansea) and regularly checked that they were consistent with data on colectomies reported through case report forms (CRFs). Clinicians within the trial team reviewed the accumulating data on AEs, and commented on GeneCIS, if necessary after discussion with the local team.
At the end of the study, two clinicians in the trial team reviewed all SAEs to ensure consistency of interpretation in the final report, informed by data from sites on the duration of use of the drugs beyond the 3-month intervention period specified in the protocol. Once that period was complete, clinical management varied from participant to participant according to their progress and the clinical judgement of the local team. The reviewers also took the different pharmacokinetic profiles of the trial drugs into account because the bio-availability of ciclosporin is short-lived, whereas bio-availability of infliximab can persist until 6 months after the last infusion. With the exception of malignancy, therefore, we judged relatedness unlikely if the event occurred more than 1 month after ciclosporin, or more than 6 months after infliximab.
Problems caused by UC and the trial drugs may coincide in a single event. When an AESF documented a serious event, usually admission, and included more than one problem (e.g. abnormal liver and renal function tests), we used clinical judgement to decide which related to the drug and which was the prime cause of admission, and classified SAEs and reactions by body system.
Data collection
Baseline and follow-up data
At recruitment to cohort, we collected:
-
sociodemographic details: age, sex, ethnic group and truncated post codes, used to generate measures of social deprivation (Indices of Multiple Deprivation for England, Welsh Index of Multiple Deprivation, Carstairs Deprivation Scores for Scotland and Townsend scores for all three countries)
-
details of admission
-
disease history, including presenting complaint, time since first diagnosis and previous treatment, including any previous surgery, or biologic or steroid therapies
-
comorbidities, in particular cardiorespiratory, liver or renal disease, diabetes mellitus or hypertension
-
UC signs and symptoms, including duration of symptoms in current episode, stool frequency, blood pressure, pulse and temperature
-
current treatment, including type, dose and duration of steroid therapy
-
pathology results, including full blood count, inflammatory markers, liver and renal function tests, and total cholesterol
-
site and extent of disease according to Montreal classification of IBD65
-
histopathology results, including stool culture and histological diagnosis
-
family history of IBD
-
height, weight and smoking status.
At 3 and 6 months after randomisation, and at 6-monthly intervals until 36 months, we collected clinical and resource-use data from participants’ records (see Appendix 7).
Process of data collection
Research staff at trial sites asked all trial participants to complete the Participant Baseline Questionnaire including the CUCQ, SF-12, EQ-5D and questions on primary care resource use (see Appendix 8); and the similar Participant Follow-up Questionnaire (PFQ) which also included questions about intercurrent events at 3, 6 and 12 months, and at 18, 24, 30 and 36 months if they reached these time points before March 2014 (see Appendix 9). Following admission a baseline CRF was completed by research staff to document demographic and clinical details (see Appendix 10).
We asked trial sites to arrange outpatient appointments to coincide with these times whenever compatible with routine clinical management. Local research staff posted PFQs to participants and asked them to bring completed questionnaires to clinics. Participants could either complete and return them by post or seek help at the clinic. We also asked sites to complete CRFs recording intercurrent events, secondary care resource use and drug treatment at these times, preferably at the outpatient appointments, or else from medical records (see Appendix 11).
The trial office sent Post-Colectomy Questionnaires (PCQs) and business reply envelopes to trial participants who underwent colectomy on discharge following surgery and at 4, 8 and 12 weeks thereafter (see Appendix 12). This PCQ included the CUCQ+, the post-colectomy version of the CUCQ and the EQ-5D.
Electronic data capture
We captured designed data on our existing GeneCIS, based on the Oracle(R) object-relational database management system (RDBMS version 10g, Oracle Corporation UK Ltd, Reading). The system is implemented in a three-tier architecture and remotely hosted in a professionally managed, secure environment. With support from local information technology departments, we provided access to hospital sites over the NHS N3 network.
We customised GeneCIS to support the trial following a detailed evaluation of requirements, including process mapping. The resulting electronic data capture structure reflected trial data requirements organised in a clinically logical manner. GeneCIS includes data validity controls, including predefined pick lists, and format and range constraints. We provided contextual guidance as help text and used system alerts to warn users when specific combinations of entered data affected eligibility, the potential for AEs or other important items.
Sites could choose either to enter data collection forms into GeneCIS locally or send completed forms by secure FaxPress to the trial office. Sixteen sites used local data entry; the rest faxed paper forms. The basic system and user interface were identical at sites and the trial office.
Data management and record keeping
All data acquisition, storage, transmission and use complied with the Data Protection Act. 66 The trial office recorded forms received on a bespoke Microsoft Access® 2007 database (Microsoft Corporation, Redmond, WA, USA) for tracking paperwork, and stored all forms in locked cabinets within a secure office with controlled access. We backed up GeneCIS and Access databases every day.
All GeneCIS users had role-based access to the system, including trial staff engaged in system configuration, data entry, helpdesk support or quality assurance. Those with authorised access to identifiable data did not contribute to analysis. Qualitative researchers had access to identifiable data to contact participants, but not to their clinical data. Trial sites had access only to identifiable data for their participants and could not view any other records.
We extracted data for analysis in pseudonymised form identified by participants’ trial numbers.
Data quality and information governance
We asked PIs to maintain site delegation logs authorising staff to perform defined tasks, to sign off any changes and to send them to the trial manager. When we updated trial documents including CRFs, we circulated new versions to sites with appropriate instructions. Sites replaced previous versions but retained them in trial site files.
We asked those making essential changes to data collected on paper to strike the original entry only once, insert the new data, and record the date and initials of the person responsible. GeneCIS maintained an electronic audit trail by annotating all data with the user, date and time of entry. It also checked data at entry, notably for correct format and within specified ranges. We subjected the resulting data to rigorous quality assurance, compared all anomalies with the paper source documents and consulted local staff when necessary.
We transcribed and reviewed interview data as soon as possible. We analysed the final transcripts without identifying patient, professional or hospital.
Routine data
Funded research data collection continued until March 2014. We plan to supplement the designed baseline data collected from cohort and trial participants with routinely collected data, including Hospital Episode Statistics (and the equivalent in Wales and Scotland), and mortality from the Office for National Statistics. We plan to continue follow-up for up to 10 years by linking routine mortality, inpatient and primary care data. Record linkage will use the existing facilities of the Farr Institute at Swansea University Medical School. Using these information sources, we plan to monitor all participants’ long-term outcomes, notably mortality, colectomies both emergency and elective, and major morbidity including hospitalisation and surgery, and thus most of their NHS costs. Hence we aim to achieve long-term follow-up of both trial participants and a large comprehensive cohort of patients with UC.
Qualitative methods
Trial participant interviews
To understand trial participants’ experiences and perceptions of treatment by infliximab, ciclosporin and surgery, we conducted telephone interviews at about 3 and 12 months after recruitment. We aimed to investigate their priorities for their health and well-being, and their perceptions of taking the drugs, side effects and response to treatment.
We first sought consent to interview when recruiting participants to the CONSTRUCT cohort. We sampled interviewees from those subsequently recruited to the trial. Hence they had all been admitted with ASC and received either infliximab or ciclosporin. We contacted those sampled following their first outpatient appointment after discharge, invited them to take part and arranged convenient 1-hour slots to enable them to talk freely without feeling rushed. As more than 50 trial sites across the UK contributed participants, interviews by telephone to participants’ homes enabled us to cover the trial population, minimise cost and ensure confidentiality.
Trained qualitative researchers used a semistructured approach to guide participants through the interview questions and give them the opportunity to develop their responses and raise issues that were important to them. The opening questions of the first interview schedule encouraged participants to think and talk about their health, what was important about it, and what good and bad health meant to them (see Appendix 13). More specific questions followed about their experiences of treatment, drug regimes and health care, and about their outcomes. We soon recognised that some participants had already required surgery for their colitis. We therefore adapted the interview schedule to capture the views of these participants (see Appendix 14).
At the end of each of these initial interviews, we asked participants to take part in a second interview about 12 months later to explore their subsequent experiences. For those, we used similar interview schedules but added questions, notably to explore changes in their health, in their opinions of treatment and in their interactions with health-care professionals (see Appendix 15). Again, we arranged convenient 1-hour slots for telephone interviews.
Health-care professional interviews
These aimed to gain insight into professional preferences between the two drugs and their personal contribution to the trial. Our specific objectives were to explore their views about:
-
administration of the two drugs, including ease of handling
-
effects of the drugs on health-care provision
-
personal preferences between the drugs
-
drug regulation and current policy
-
surgery for UC
-
equipoise in recruiting to the trial.
We therefore planned semistructured interviews lasting 30–40 minutes with flexibility for interviewees to expand on important issues and access their broad knowledge base. Our schedule covered interviewees’ beliefs and ways of working; aspects of drug provision that may affect preferences; their interaction with patients and others; and their contribution to the trial (see Appendices 16 and 17). We offered interviews face to face or over the telephone. We conducted separate interviews with consultants and nurses to provide a richer understanding of differences between professional groups. With participants’ consent we recorded and transcribed interviews for analysis.
Definition and validation of outcome measures
Background
We are entirely committed to the philosophy, expounded by NICE and implemented by the National Institute for Health Research (NIHR), that the ultimate criteria for interventions in health care are clinical effectiveness and cost-effectiveness in improving the survival and QoL of patients over extended periods. Although we admire how the GETAID investigators implemented the CySIF trial with little funding, they recruited only 110 patients, followed them for only 98 days and reported only on ‘treatment failures’. This is not a good basis for NHS investment decisions worth hundreds of millions of pounds. Instead we chose QAS as our primary outcome and recruited enough participants to yield the power to discriminate between two contrasting drugs. That still left us with the task of developing and validating a comprehensive patient-reported outcome measure (PROM) applicable across the broad spectrum of disease severity within UC.
There are several disease-specific QoL measures for patients with IBD. 18,67–71 The most widely used is the McMaster Inflammatory Bowel Disease Questionnaire. 18,67,68,72,73 As it had not been validated for the UK, we previously conducted a development and validation study to anglicise it as the UK-IBDQ. 61 As this was a community study, however, we recognised in designing CONSTRUCT within acute care that, to avoid ceiling effects, we had to modify the UK-IBDQ by extending the range of responses for all items to include more severe replies; the number of items to include more questions addressing severe symptoms; and the frequency of administration in participants undergoing surgery. Furthermore, the UK-IBDQ has no questions about the impact on QoL of colectomy, which reportedly happens to 9–21% of those with UC. 74–76 We therefore derived from the UK-IBDQ a PROM for patients suffering from moderate to severe symptoms of Crohn’s or UC with or without stoma. We called this the CUCQ in its basic form, and the CUCQ plus stoma extension (CUCQ+) in its extended form. Preliminary validation of the CUCQ confirmed that it met essential psychometric criteria,77 with the result that the national IBD registry adopted it. Although we developed the CUCQ as a tool for both conditions, we validated it only on UC patients. As the development and concurrent validation of CUCQ and CUCQ+ underpin the primary analysis of CONSTRUCT, we now describe that validation.
Methods
We used the standard psychometric approach outlined by Streiner and Norman78 to develop and validate the CUCQ and CUCQ+ in three stages:
-
item generation
-
initial development in the CONSTRUCT cohort
-
definitive validation in the CONSTRUCT trial sample.
We used the 32 UK-IBDQ items [see Appendix 8, Section A: Crohn’s and Colitis Questionnaire (CCQ)], as the basis for developing the CUCQ and CUCQ+. We also reviewed the literature on PROMs in gastroenterology to identify additional items. After drafting the CUCQ and CUCQ+, we recruited an expert panel of gastroenterologists, outcome specialists, statisticians and patients to review the resulting questions and response options, and ensure they were appropriate for UC patients.
We piloted the draft questionnaires on a sample of 20 UC patients with or without stoma from Neath Port Talbot Hospital, Port Talbot, who were not participating in CONSTRUCT. We asked them to complete CUCQ or CUCQ+ as appropriate and added four supplementary questions:
-
Did you find any of the questions difficult to understand?
-
Was there any question you did not want to answer?
-
Was there any aspect of your bowel condition not covered by these questions?
-
Did you find any of these questions not applicable to you?
For our initial validation, we used patients recruited to the CONSTRUCT cohort but not the trial, who had therefore completed the CUCQ at baseline. Of the 32 CUCQ questions (see Appendix 18), six were not relevant to post-colectomy participants:
-
Q1: On how many days over the last 2 weeks have you had loose or runny bowel movements?
-
Q2: On how many days in the last 2 weeks have you noticed blood in your stools?
-
Q6: On how many days over the last 2 weeks have you opened your bowels more than three times a day?
-
Q9: On how many days over the last 2 weeks have your bowels opened accidentally?
-
Q24: On how many days over the last 2 weeks have you wanted to go back to the toilet after you thought you had emptied your bowels?
-
Q26: On how many days over the last 2 weeks have you had to rush to the toilet?
We therefore designed the CUCQ+ (see Appendix 9, For patients with stoma), with 10 stoma-specific questions replacing these six questions, making a total of 36 questions.
-
S1: On how many days over the last 2 weeks have you been afraid that other people might hear your stoma?
-
S2: On how many days over the last 2 weeks have you been worried that other people might smell your stools?
-
S3: On how many days over the last 2 weeks have you been worried about possible leakage from your stoma bag?
-
S4: On how many days over the last 2 weeks have you had problems with care for your stoma?
-
S5: On how many days over the last 2 weeks have you found the skin around your stoma irritated?
-
S6: In the last 2 weeks have you felt embarrassed because of your stoma?
-
S7: In the last 2 weeks have you felt less complete because of your stoma?
-
S8: In the last 2 weeks have you felt less attractive as a result of your stoma?
-
S9: In the last 2 weeks have you felt less feminine/masculine as a result of your stoma?
-
S10: In the last 2 weeks have you been dissatisfied with your body as a result of your stoma?
To calculate scores for participants who had not undergone surgery, we used the 32 CUCQ questions. For post-colectomy participants, we used the 10 stoma-specific questions and the 26 stoma-relevant CUCQ questions to calculate CUCQ+ scores. In analysing and validating both CUCQ and CUCQ+, we calculated scores as follows:
-
We scored questions with four responses as 0, 1, 2 or 3 in ascending severity.
-
We scored questions with responses between 0 and 14 days as the actual value.
-
We reversed the scoring of questions with wording in the reverse direction (Q7, Q22 and Q32) to code all questions in the same direction.
-
We rescaled questions between 0 and 1 by dividing actual responses by their maximum score (3 or 14).
-
We calculated CUCQ scores for non-colectomy participants, and CUCQ+ scores for post-colectomy participants, by summing all valid responses and dividing by the number of completed questions.
-
So the lower the CUCQ+ (or CUCQ score), the better the respondent’s health. In analysing the AUC, however, it is necessary to subtract the total CUCQ+ (or CUCQ) score from 1, so that higher scores show better health, consistent with both EQ-5D and SF-12.
However, we calculated CUCQ and CUCQ+ scores only when participants had responded to at least 75% of the questions – 24 out of 32 or 27 out of 36, respectively. If participants had completed fewer than 75% of questions, we treated the total CUCQ or CUCQ+ score as missing. To give equal weight to each question answered, our analysis plan used the original 32 CUCQ questions for non-colectomy participants and the 36 CUCQ+ questions for post-colectomy participants. To test the sensitivity of our findings to this simple approach, we repeated the analysis in two ways. First, we gave equal weight to core scores and stoma-specific scores, thus giving more weight to the latter. Second, we gave the stoma-specific scores 6 out of 26 of the weight given to core scores, as the original UK-IBDQ comprised 26 questions relevant to stomas and 6 questions inapplicable to stomas, thus giving less weight to stoma-specific scores.
Still following Streiner and Norman,78 we conducted initial psychometric development of the CUCQ on CONSTRUCT cohort patients (who had not had a colectomy):
-
We examined the 32 sets of response frequencies for floor or ceiling effects.
-
We calculated the Kaiser–Meyer–Olkin measure of sampling adequacy and Bartlett’s test to judge whether or not principal component analysis was appropriate.
-
We calculated Cronbach’s alpha (which should exceed 0.7 for good internal consistency).
-
We calculated item-total correlations for each question (which should exceed 0.2 for good homogeneity).
-
We undertook principal component analysis to assess the underlying structure; we considered factors important if their eigenvalues were clearly > 1, and individual questions as useful if their factor loadings exceeded 0.4.
-
We assessed the construct validity of the scale by examining the correlation between the CUCQ and two generic QoL questionnaires (EQ-5D and SF-12).
Then we undertook definitive validation of the CUCQ and CUCQ+ on the CONSTRUCT trial sample. First, we compared demographic characteristics of that sample with the cohort to ensure that they were similar. Then we analysed the trial sample in essentially the same psychometric way as the cohort, and tested whether or not the principal components arising from these two analyses were consistent. Finally, we repeated the principal component analysis of the trial sample after 12 months and tested whether or not principal components arising from the CUCQ for non-colectomy patients and those arising from the CUCQ+ for post-colectomy patients were consistent.
We also analysed reliability and responsiveness for the trial sample, initially combining CUCQ and CUCQ+ and then comparing them. We assessed test–retest reliability of the scales on participants who reported no change in their condition at successive assessments; we considered scales reproducible if intraclass correlation exceeded 0.75. We assessed responsiveness of the scales to change on participants who reported a change in their condition; we considered scales responsive if the responsiveness ratio exceeded 0.5.
Analysis
Clinical effectiveness
Primary analysis was by treatment allocated, reflecting the pragmatic nature of the trial design. Figure 1 illustrates the primary outcome measure – the AUC defined by CUCQ scores at baseline and all available time points before 31 March 2014 – also known as ‘QAS’. Within research into PROMs, there is a general convention that for generic PROMs ‘higher is better’ whereas for condition-specific PROMs ‘lower is better’. However, in order to be consistent with both EQ-5D and SF-12 we have subtracted calculated CUCQ scores from 1, so that higher is better.
FIGURE 1.
Primary outcome measure: area under the CUCQ curve.

We calculated this area by summing the areas of the component trapezia, thus assuming linearity between successive CUCQ scores, and setting the score at the end of follow-up equal to the last recorded score (last one carried forward). This method accommodates both missing values and extra values like those arising from PCQs.
The main analysis used a general linear model to estimate differences in QAS between groups, adjusting for covariates that may affect this measure, including trial site; data collected while assessing eligibility for cohort and trial, notably the sociodemographic variables age, gender, ethnic group, QoL, disease severity; current immunosuppressant therapy (using a binary indicator set equal to 1 for participants taking azathioprine, 6-mercaptopurine or methotrexate); and time in follow-up. We combined rare categories in factors like ethnic group, taking account only of observed numbers in each category and the coherence of new groupings.
Secondary analyses adjusted for the same covariates as primary analysis and compared between groups: QAS per day (again using general linear models); QoL scores (using methods for repeated measures); proportion of participants undergoing colectomy (using binary logistic regression); time to colectomy (censored at the end of follow-up, and analysed by Cox regression); proportion of participants suffering one or more AEs (using binary logistic regression); and mortality.
We examined residual diagnostics in analyses that assume normality, with the options of data transformation and bootstrapping when residual distributions were markedly non-normal. We excluded identified outliers and reanalysed the revised data sets. We supplemented analyses by descriptive comparisons between groups in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines, including estimates with 95% confidence intervals (CIs), representing two-tailed tests at the 5% significance level.
Imputation of missing data
We used statistical imputation of censored and missing data to impute costs and QoL for all participants who generate data on survival, colectomy or QoL after randomisation. Thus we excluded participants without follow-up data, as calculation of QAS requires one or more CUCQ scores in follow-up.
None of the questionnaires has an official algorithm for imputing missing answers. So we imputed missing values within participant interviews using the Expectation Maximisation option in the Missing Value Analysis module within Statistical Package for the Social Sciences version 22 (SPSS Inc., Chicago, IL, USA), and calculated scores according to the instructions for the measure in question. When necessary we imputed missing scale and subscale scores by regression using the available values of that score at other times and the allocated treatment group. To avoid introducing outliers, we restricted imputed scores to the range observed for that measure in that group.
Cost-effectiveness
We collected data on NHS resource use from CRFs and PFQs completed at each follow-up time point, supplemented by data from other sources, notably, PCQs completed by patients undergoing colectomy, SAE forms and any relevant information provided by sites. Together CRFs covered resource use over the whole period during which participants were in the trial because each CRF recorded resource use since the previous CRF. To minimise recall bias, however, PFQs reported resource use over the previous 3 months. 79 This left gaps in the data, which we imputed. We estimated all costs in 2012–13 prices inflated when necessary using the NHS Pay and Prices Index. We applied a discount rate of 3.5% per annum to costs occurring beyond 12 months. We applied the same annual discount rate of 3.5% for QALYs beyond 12 months.
Participant Baseline Questionnaires recorded data on resource use in the 3 months before their consent for use as a covariate to account for any existing imbalances in resource use. As this resource use preceded randomisation, we did not include it in the costs analysed.
We based the cost of infusing ciclosporin (Sandimmun) on reported dose and duration in whole vials; and oral ciclosporin on reported dose and duration in whole dispensing packs (Table 2). We also added the cost of monitoring ciclosporin levels. We based the cost of infliximab (Remicade) on the reported dose in whole vials and, for infusions after the initial episode in hospital, we added the cost of admitting participants as day cases (£311).
| Drug | Dose | Unit cost (£) |
|---|---|---|
| Ciclosporin (Sandimmun) | 50 mg/ml, 1-ml ampoules | £1.94 |
| Oral ciclosporin | 100 mg, 30-capsule pack | |
| Neoral | £72.57 | |
| Deximune® (Dexcel Pharma Ltd) | £51.30 | |
| Capimune® (Mylan) | £51.30 | |
| Infliximab (Remicade) | 100-mg vial | £419.62 |
We also estimated the cost of preparing and delivering drug infusions from a questionnaire completed by 42 trial sites (81% of the 52 recruiting sites). For typical infusions they reported who mixes the infusion (nurse or pharmacist); the time taken to prepare the infusion; and the frequency of bag changes for ciclosporin participants. We multiplied by relevant unit costs:81 nurse £41 per hour; pharmacist £47 per hour; and £1.50 per infusion or bag change. For centres that did not respond to the questionnaire we imputed preparation and delivery costs by mean substitution.
Case report forms recorded data on hospital episodes, including the episode leading to recruitment. PFQs reported data on admissions to hospitals other than the trial site. To these we applied unit costs from either the 2012–13 Healthcare Resource Group (HRG) code FZ37 (non-elective episodes of inflamatory bowel disease without interventions, 19 years and over) or the HRG code FZ74 (non-elective episodes complex large intestine procedure, 19 years and over) and ‘complications and comorbidity’ (CC) codes based on participants’ length of stay in hospital (see Table 3). We costed days in hospital beyond the average for that HRG-CC at the published rate. For subsequent surgical procedures we used expert clinical opinion to identify the 2012–13 HRG code most appropriate to the information recorded as free text on the CRF, which often detailed specific procedures undertaken during each operation. Table 3 shows selected secondary procedures and their HRG codes, but not the many CC codes per HRG. Again, we used CC codes based on participants’ lengths of stay and costed excess days at published rates.
| HRG code | National average unit cost (£) | Average length of stay (days) | Cost per day (£) | Excess bed-day rate (£) | |
|---|---|---|---|---|---|
| Without surgerya | |||||
| Non-elective: IBD without interventions, 19 years and over (FZ37) | CC 0 | 1546 | 3.78 | 409 | 243 |
| CC 1–2 | 1692 | 4.39 | 385 | 237 | |
| CC 3–4 | 2218 | 5.62 | 395 | 230 | |
| CC 5+ | 12,630 | 7.82 | 336 | 229 | |
| With surgery (primary procedure)a | |||||
| Non-elective: complex large intestine procedure, 19 years and over (FZ74) | CC 0–2 | 5822 | 8.92 | 653 | 196 |
| CC 3–5 | 7573 | 12.60 | 601 | 162 | |
| CC 6–8 | 8623 | 17.21 | 501 | 390 | |
| CC 9+ | 10,136 | 19.93 | 509 | 229 | |
| With surgery (selected subsequent procedures)b | |||||
| Closure of stoma | FZ13 | Costs dependent on CC code | |||
| Ileoanal pouch | FZ69 | ||||
| Reversal of ileostomy | FZ83 | ||||
| Proctecomy, ileoanal pouch, loop ileostomy | FZ73 | ||||
| Completion proctectomy | FZ77 | ||||
Case report forms also recorded data on non-trial drugs administered to participants as inpatients. When the CRF did not record dose, we assumed that the participant had received the lowest dose recommended in the British National Formulary. 80 When the CRF did not record duration, we assumed that participants took any prescribed gastroenterology drugs throughout their hospital stay; we extended this rule to seven other drugs – alendronic acid, amlodipine, bisoprolol fumarate, clopidogrel, gliclazide, losartan potassium and rampiril. For all other inpatient drugs without specified duration we costed a single day’s dose. As we costed over 1800 drugs and formulations, we do not report them all here.
Participant Follow-up Questionnaires also reported data on non-trial drugs prescribed while participants were in the community. We costed these according to the dose and duration of treatment recommended by the British National Formulary in whole packs. Where duration was not recorded we assumed that the participant received a single pack.
Finally, PFQs reported on participants’ other encounters with the NHS, including general practitioners (GPs), nurses and other professionals at surgery, at home, in the community or by telephone; NHS Direct or NHS24; hospital emergency departments; and outpatient clinics (Table 4). Our main source of unit costs was Curtis,81 who included salaries and expenses, costs of training and qualifications, and capital and overhead costs.
| Resource | Details | Source | Unit cost (£) |
|---|---|---|---|
| NHS contacts | |||
| A&E visits | Treatments leading to admitted (not admitted) £146 (£112) | Curtis 201283 | 112.00 |
| Admitted nights | NIHR Clinical Research Network industry costing template | NIHR 201484 | 386.37 |
| Clinic visit | As outpatient appointment. Weighted average £135 (p. 107) | Curtis 201381 | 135.00 |
| Consultant: medical (p. 245) – per contract hour £139 | |||
| Clinic visit | Length of contact assumed same as GP (11.7 minutes) at £27.11 | Curtis 201381 | 27.11 |
| Telephone call | Length of telephone call assumed same as GP (7.1 minutes) at £16.45 | Curtis 201381 | 16.45 |
| Consultant: psychiatric (p. 246) – per contract hour £140 | |||
| Clinic visit | Length of contact assumed same as GP (11.7 minutes) at £27.29 | Curtis 201381 | 27.29 |
| Telephone call | Length of telephone call assumed same as GP (7.1 minutes) at £16.57 | Curtis 201381 | 16.57 |
| Consultant: surgical (p. 246) – per contract hour £140 | |||
| Clinic visit | Length of contact assumed same as GP (11.7 minutes) at £27.30 | Curtis 201381 | 27.30 |
| Dietitian | (p. 226) Hourly rate £35. Assumed session length same as GP practice nurse – 15.5 minutes | Curtis 201381 | 9.04 |
| GP (including travel, direct staff and qualification costs) (p. 191) | |||
| At practice | Per patient contact lasting 11.7 minutes | Curtis 201381 | 45.00 |
| Home visit | Per out of surgery visit lasting 23.4 minutes | Curtis 201381 | 114.00 |
| Telephone call | Per telephone consultation lasting 7.1 minutes | Curtis 201381 | 27.00 |
| Health visitor | |||
| Home visit | (p. 185) Per hour of home visiting – £71. Assumed length of visit same as GP home visit – 23.4 minutes | Curtis 201381 | 27.69 |
| Telephone call | (p. 185) Per hour of patient-related work – £59. Assumed length of telephone call same as GP practice nurse (6 minutes) | Curtis 201381 | 5.90 |
| Hospital (telephone call) | Assumed contact with clinical nurse specialist (p. 189) per hour of client contact – £90. Rate per 6 minutes’ telephone contact – £9 | Curtis 201381 | 9.00 |
| Midwife | (p. 186) Costed as nurse specialist (community) band 6 – £49 per hour. Length of contact assumed same as GP practice nurse – 15.5 minutes | Curtis 201381 | 12.66 |
| NHS Direct (telephone call) | Digitalhealth.net 201385 | 20.00 | |
| Nurse (GP practice) | GP practice nurse (p. 188) per hour of face-to-face contact rate – £52. Per average contact lasting 15.5 minutes – £13.43 | Curtis 201381 | 13.43 |
| Nurse (home visit) | Community nurse (including district nursing sister, district nurse) (p. 183) per hour of home visiting (including travel) – £70. Assumed length of home visit same as GP (23.4 minutes) – £27.30 | Curtis 201381 | 27.30 |
| Nurse specialist (p. 186) hourly rate £49 | |||
| Clinic visit | Assumed length of visit same as GP practice nurse – 15.5 minutes | Curtis 201381 | 12.66 |
| Home visit | Assumed length of visit same as GP home visit – 23.4 minutes | Curtis 201381 | 19.11 |
| Telephone call | Length of telephone call assumed as calls to hospital (6 minutes) at £4.90 | Curtis 201381 | 4.90 |
| Outpatient visit: gastroenterology | Service code 301 | Department of Health 201582 | 137.00 |
| Outpatient visit: general | Weighted average £135 (p. 107) | Curtis 201381 | 135.00 |
| Paramedic visit | Ambulance services – see, treat and refer (p. 107) | Curtis 201381 | 177.00 |
| Pharmacist | (p. 228) Per hour of direct clinical patient time – £94. Assumed length of session same as GP visit – 11.7 minutes | Curtis 201381 | 18.33 |
| Phlebotomist: | NHS Jobs (www.jobs.nhs.uk) specify that phlebotomists are clinical support workers with a salary between £14,000 and £22,000 p.a. | Curtis 201381 | 4.10 |
| Clinic visit | Clinical support worker (p. 237) hourly rate £21. Assumed length of contact same as GP visit – 11.7 minutes | Curtis 201381 | 4.10 |
| Home visit | Clinical support worker (community) (see p. 187) hourly rate – £30. Assumed length of contact same as GP home visit 23.4 minutes | Curtis 201381 | 11.70 |
| Physiotherapist | (p. 223) Hourly rate – £36. Assumed session length – 30 minutes | Curtis 201381 | 18.00 |
| Psychologist | (p. 179) Clinical psychologist – £134 per hour of client contact. Length of contact assumed same as GP practice nurse – 15.5 minutes | Curtis 201381 | 34.62 |
| Ultrasound | Less than 20 minutes | Department of Health 201582 | 51.00 |
| Walk-in centre | (p. 109) Walk-in services leading to not admitted | Digitalhealth.net 201385 | 41.00 |
| Tests and investigations | |||
| Ciclosporin levels | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 22.00 | |
| Abdominal X-ray | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 65.00 | |
| Barium enema | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 215.00 | |
| Barium follow through | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 350.00 | |
| Barium meal | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 175.00 | |
| Calcium and phosphate | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 4.10 | |
| Chest X-ray | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 50.00 | |
| Clotting profile | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 3.28 | |
| Colonoscopy with biopsy | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 827.00 | |
| Colonoscopy without biopsy | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 767.00 | |
| C-reactive protein | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 3.00 | |
| CT scan | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 475.24 | |
| Erythrocyte sedimentation rate | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 3.28 | |
| Flexible sigmoidoscopy | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 344.00 | |
| Full blood count | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 3.95 | |
| Liver function tests | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 4.45 | |
| MRI scan | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 574.91 | |
| Oesophagogastroduodenoscopy | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 610.00 | |
| Rigid sigmoidoscopy | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 210.11 | |
| Stool culture/testing | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 2.70 | |
| Thiopurine methyltransferase testing | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 31.70 | |
| Urea and electrolytes | Cardiff and Vale and Abertawe Bro Morgannwg University Health Boards | 3.65 | |
Case report forms also recorded data on the number and type of investigations undertaken. We estimated unit costs for these from two local but dissimilar trial sites – in Cardiff and Swansea (see Table 4). When participants died or withdrew further access to medical records, sites completed the next CRF from data available up to that time.
The primary outcome for economic analysis was QALYs estimated from the EQ-5D questionnaire within the PFQ administered at baseline and subsequent assessments. Participants who underwent colectomy also completed the EQ-5D within the PCQ at discharge from surgery and 4, 8 and 12 weeks thereafter. EQ-5D assesses HRQoL on five dimensions – mobility, self-care, usual activities, pain or discomfort, and anxiety or depression – using three levels for each dimension. We converted these to a single utility using the UK tariff. 86
We used the methods described above to impute missing data, which can happen in three ways:
-
CRF or PFQ received missing individual resource items
-
CRF or PFQ received but not covering the entire period since previous CRF; or
-
CRF or PFQ due but not received.
In these three scenarios we imputed thus:
-
We used age, gender, weight, ethnic group, smoking status and hospital data to impute missing items. If more than two were missing, we used mean regression imputation; as regression assumes normally distributed errors, we used log-transformation when the data broke this assumption.
-
We assumed that resource use varied linearly and therefore replaced missing data by the mean of the data available before and after, weighted if necessary.
-
After checking whether or not non-responders differed from responders in sociodemographic characteristics, we using the method described in (2).
-
Censoring data occurred because of the change to protocol which meant that not every participant could be followed up for the same length of time. We studied the mechanism of censoring87 on a range of variables including study arm. As these showed all censoring to be missing completely at random, no adjustments were made to resource data to account for any censoring effects.
We estimated total costs by aggregating participants’ resource use. Although data collection for early participants could extend for 36 months, there were very few of these. Our primary analysis therefore compared costs and assessed cost-effectiveness over 30 months.
The calculation of the cost-effectiveness point estimate included all participants for whom we had EQ-5D data. In principle we aggregated their costs and QALYs over their (variable) periods in the study. To ensure that costs and QALYs covered the same periods, we compared the number of days covered by CRFs and PFQs. We fitted statistical models for NHS costs and QALYs using allocated drug, days since randomisation and the logarithm of those days as independent variables. We used the result coefficients to adjust NHS costs and QALYs to a period of 730 days.
We undertook two contrasting sensitivity analyses: the first restricted analysis to 12 months, and thus analysed all 270 participants but none of their data beyond 12 months; the second restricted analysis to 24 months and those participants who took part for at least that duration.
To account for uncertainty in the costs and QALYs used to estimate incremental cost-effectiveness in these analyses, we used non-parametric bootstrapping to generate 5000 replicates, shown in the scatterplots of the cost-effectiveness planes. The resulting cost-effectiveness acceptability curves (CEAC) show the proportion of replicates considered cost-effective at each threshold of willingness to pay for an additional QALY (including the £20,000 and £30,000 thresholds currently used by NICE). 56
We are developing a Markov model to predict costs and QALYs beyond the follow-up period of the study. As the long-term effectiveness of both trial drugs in UC is unknown, the model will explore uncertainty in outcomes over 20 years. It will estimate the long-term probability of colectomy effects on costs and QALYs. 88
Data to be collected for 5 years after the end of recruitment will monitor costs and effects of both trial drugs on trial patients. At regular intervals we or our successors will update the Markov model, and thus our conclusions, using the most recent data from trial participants. Any discrepancies between costs and effects modelled beyond the trial period and those actually demonstrated by the long-term follow-up will help to validate the use of economic modelling in economic evaluation for this condition.
Qualitative analysis of trial participant interviews
We analysed the 3-month interviews using both thematic and schematic methods. Thematic analysis is a structured approach that uses coding to conceptualise research data and classify them into meaningful and relevant categories. 89 Schema analysis identifies patterns and themes within data in line with study aims and interview topics; it thus combines elements of the linguistic and sociological traditions. It posits that people use cognitive simplifications to help make sense of the complex information they receive. Analysis starts with careful reading of the texts to identify linked themes paying attention to patterns of speech and the repetition of key words and phrases, noting metaphors and commonalities in their reasoning. 90
We began thematic analysis of the initial interviews after completing four interviews. Three qualitative researchers (ACS, SW, FLR) read the transcripts and met to discuss emerging findings. They developed an analysis framework and iterative process for subsequent transcripts to refine and clarify themes and their linked categories.
A subset of the CONSTRUCT trial team then undertook the schema analysis as a group. We chose this sequence for three reasons: to validate the thematic framework by verifying that all elements were cogent and comprehensible; to engage a range of contributing disciplines; and to give those analysing other trial data sets insight into the lives of participants. We asked each subgroup member to read transcripts from three participants; one treated with infliximab, another with ciclosporin and the last with ciclosporin followed by surgery; and to write one-page schematic overviews of the main features of each transcript about participants’ stories relating to QoL and treatment regimes. We circulated these overviews to all group members for discussion and synthesis; and the thematic analysis framework for refinement and validation.
We began the thematic analysis of the 12-month interviews after completing the analysis of the 3-month interviews. The same three qualitative researchers read the transcripts of the first four interviews and again met to discuss their findings. Another iterative process followed to identify themes that had not emerged at 3 months.
Qualitative analysis of health-care professional interviews
These transcripts underwent a thematic analysis technique known as ‘framework analysis’ to develop a template linking data to study aims and objectives (see Appendix 19). Framework analysis is a semistructured approach that, like generic thematic analysis, uses coding to conceptualise and classify research data. 89 Three qualitative researchers (FLR, CC, ACS) individually coded transcripts as they were completed. Then they met to derive a unified coding structure including essential and incidental themes and their linked categories. Continuing group work enabled them to refine the developing framework as interviews progressed, with major themes retaining both nuance and ambiguity.
Using MATRICS to synthesise results
We used the MATRICS from our previous study57 to synthesise CONSTRUCT’s findings in five steps. The MATRICS proforma comprises three ‘layers’ (see Appendix 20):
-
potential effects of the intervention(s) under evaluation (derived from the aims and objectives of the study)
-
methods used in that evaluation
-
research findings.
Step 1: identify, categorise and code effects
We derived the potential effects of CONSTRUCT from its aims and added effects that emerged over the course of the trial; we grouped them in three columns:
-
on participants
-
on gastroenterology services and professionals
-
on the rest of NHS and society.
We populated layer 1 with these effects using one cell per effect under the three defined columns, and numbered each effect.
Step 2: code methods used by CONSTRUCT
We listed each method or instrument used (e.g. CRFs, questionnaires, interviews and routine data) in layer 2 and gave each method a unique letter.
Step 3: create joint alphanumeric codes
We assigned letters to each effect in layer 1 showing how we had investigated them. For example, if disease-specific QoL was effect 2 investigated by the CUCQ questionnaire labelled B, we recorded B alongside effect 2 in layer 1. Similarly, we assigned numbers to each method in layer 2 showing all the effects they had investigated. For example, we recorded effect 2 alongside method B in layer 2. Effects investigated by more than one method have extra letters in layer 1; methods investigating more than one effect have extra numbers in layer 2.
Step 4: identify and code all research findings
We listed the individual findings from each component of CONSTRUCT in layer 3 of the MATRICS proforma. We illustrated them textually or by summary statistics, and labelled them with codes derived from layers 1 and 2 to identify the corresponding effects and methods.
Step 5: synthesise complementary findings and reorder contrasting findings
We merged comparable findings into composite statements, irrespective of which effects were investigated or methods used and listed all codes associated with each. This enabled us to show that more than one effect or method had reported the same outcome. When findings were not comparable, even opposing, we kept them as separate rows in layer 3, but put them together.
Governance and management
Ethics and research governance
The Research Ethics Committee (REC) for Wales gave ethical approval (Ref 08/MRE09/42) and each participating trust or health board gave NHS R&D approval. The trial has European Union Drug Regulating Authorities Clinical Trials number (2008-001968-36) and clinical trial authorisation from the MHRA. We have sought to conform with the Research Governance Frameworks for England,91 Scotland92 and Wales;93 the principles of GCP outlined by the International Conference on Harmonisation (www.ich.org/), the European Union Directive 2001/20/EC94 and The Medicines for Human Use (Clinical Trials) Regulations 2004. 64
Trial management
We established:
-
a TSC which oversaw the trial by meeting twice per year
-
a DMEC which monitored trial data in accordance with an agreed charter (see Appendix 21) and reported to the TSC
-
a Trial Management Group (TMG) comprising academics, health professionals, researchers and a representative of service users which undertook general management of the trial, met every month and reported to the TSC and funders through reports every 6 months.
The available members of the research team also met every week.
Service users
In accordance with the relevant West Wales Organisation for Rigorous Trials in Health standard operating procedure,95 we included service users as active contributors at all stages of this trial. They provided separate members of TSC, DMEC and TMG, contributed to the research process and provided valuable insights into UC. In particular they contributed to the development of the CUCQ+, the primary outcome measure; helped to maximise recruitment rates; attended the series of report writing days; and gave much valuable help and advice outside these meetings. Appendix 22 summarises the personal experience of one service user.
Feasibility and piloting
Before the trial began, we undertook a feasibility study to refine our understanding of the patient pathway, the CUCQ and the economic resource-use questionnaires. We then conducted a pre-pilot study to test the recruitment process up to but not including randomisation. Thereafter we used the resulting cohort of between 20 and 40 patients to test aspects of trial design beyond initial recruitment. Following the pre-pilot study, we piloted GeneCIS (our online trial data management system), participant recruitment and randomisation, and data collection processes. We asked each active centre to recruit and randomise one trial participant. We then met site PIs to learn from the pilot. As we encountered no major problems we incorporated cohort and trial participants recruited during the pilot in the main study.
Safety monitoring and reporting
Clinical trial agreements were in place at each site. Thus we delegated responsibility for adhering to GCP and reporting AEs in accordance with clinical trial regulations to site PIs and their research teams. These PIs, or others authorised by the site delegation log, recorded all AEs on AESFs and assessed the seriousness, causality and expectedness of each. If sites could not make these decisions, they referred them to the chief investigator. In view of the established and extensive side effect profile of both drugs, the protocol required expedited reporting of SUSARs, but not AEs, ARs, SAEs or SARs. We reported the number of notified SAEs and SARs to each meeting of the DMEC; and of SARs in annual safety reports to MHRA and the REC for Wales.
To help identify AEs, we gave trial participants membership cards showing their trial numbers and asked them to show this whenever they saw doctors outwith the team treating them for UC.
Quality assurance
We sought to comply with the principles of quality assurance described by the Medical Research Council (MRC) Guidelines for Good Clinical Practice in Clinical Trials96 the European Union Directive 2001/20/EC,94 The Medicines for Human Use (Clinical Trials) Regulations 2004,64 and the Research Governance Frameworks for England,91 Scotland92 and Wales. 93
We anonymised all research data and stored them securely. All research team members attended GCP training every 2 years and undertook trial-specific training in the protocol, recruiting participants, completing CRFs and reporting AEs. The TMG developed and reviewed a quality plan describing the quality assurance and quality control processes implemented within the trial to ensure a high level of quality. We developed fieldwork, data collection and GeneCIS handbooks to maintain consistency across trial sites.
Dissemination
We seek to comply with the CONSORT guidelines. 97 We seek to publish widely in high-impact peer-reviewed journals and disseminate trial findings at national and international conferences. We are committed to give appropriate recognition to all who have worked on the trial. We have registered CONSTRUCT in the public registry (www.controlled-trials.com/isrctn) with ISRCTN22663589.
Data sharing
In accordance with the MRC–Wellcome Trust data sharing policy, we shall make data from CONSTRUCT available to the scientific community with as few restrictions as feasible, while retaining exclusive use until the publication of major outputs. In particular we shall deposit anonymised data in the University of Essex data archive and thereby encourage wider use.
Summary of protocol changes
Changes to the protocol were approved by the DMEC, TSC, MHRA, REC and the NIHR HTA programme.
Before protocol publication
Number of sites
We had initially planned to work with 40 sites, but it became clear that a smaller number of patients were being recruited per site than we had anticipated so we increased the number of sites.
Power calculation
In June 2012 we obtained approval to increase the target effect size from 0.3 to 0.35, still in the range of small ‘effect’ sizes; this needed at least 250 patients to provide data on survival, colectomy or QoL in each group.
The design of CONSTRUCT required a combination of survival analysis and statistical imputation to get full value from the resulting data set. As these techniques were difficult to incorporate into power calculations, we presented a simpler calculation based on t-tests of mean CUCQ scores at 12 months. For CONSTRUCT to detect an effect size of 0.35 in these scores (i.e. a difference between infliximab and ciclosporin groups of at least 0.35 of the population standard error), with 80% power when using a 5% significance level, required that we analysed at least 250 of the 270 participants recruited to the trial. Although more than 10% of CONSTRUCT participants were likely to drop out over the follow-up period of at least 12 months, all analyses exploited the techniques of statistical imputation used successfully by the Cancer of the Oesophagus or Gastricus: New Assessment of the Technology of Endosonography58 and Folate Augmentation of Treatment – Evaluation of Depression59 trials to maintain the effective sample size at 250. Our initial, more conservative, power calculation had proposed to recruit 480 participants (to yield an analysable sample of 360); not to impute missing data statistically, therefore to allow for 25% loss to follow-up; and thus to yield 80% power to detect a slightly smaller effect size of 0.30 in CUCQ scores. We amended our target to 270 participants once the difficulty of identifying patients with acute steroid-resistant UC became clear early in recruitment.
Trial intervention
Our original protocol stated that ciclosporin should be given for up to 5 days, but it was felt that stopping at 5 days might deprive patients of a response. On 21 January 2010 approval was given to increase this to 7 days and included approval for a recommendation that sites should use non-PVC bags and administration sets for ciclosporin.
Analysis
On 21 January 2010, following a suggestion by our DMEC to change the analysis, we obtained approval to change from ‘quality of life at 24 months’ to ‘analysis using an area under the curve approach to obtain each patient’s quality of life over 24 months’.
On 6 June 2012 approval was given for the primary analysis of CONSTRUCT to use full statistical imputation.
Trial inclusion/exclusion criteria
Without changing the basic content of our inclusion/exclusion criteria, we rewrote them to make them clearer to follow and approval was given on 21 January 2010.
To ensure that cohort patients were not missed, on 22 September 2010 we gained approval to:
-
amend the protocol to clarify that potential patients for the cohort were those admitted with ‘suspected or known colitis’ rather than ‘acute severe colitis’
-
remove ‘with blood apparent’ from the cohort inclusion criteria as it may not always be apparent in patients with colitis.
To ensure that trial participants were not missed approval was given to:
-
change the Mayo score (colitis severity score) in the RCT inclusion criteria from 3 to ≥ 2 as it was felt eligible patients would be excluded because of the higher score.
In June 2012 we gained approval to relax the requirement for sigmoidoscopy to assess eligibility, as feedback from sites indicated that in a patient with a previous history of UC, further sigmoidoscopy might not be done.
We were also permitted to clarify that 2–5 days of intravenous steroid treatment before a decision was made whether or not a patient had responded, was an approximate length of time as this reflected what happens in normal clinical practice.
Recruitment of participants
We had initially planned to complete recruitment over a 1-year period. However, following unforeseen delays in R&D approval and a smaller number of patients recruited per site than we had anticipated, we realised this would not be possible. Approval to lengthen the recruitment period until recruitment to the trial was complete was given on 22 September 2010. Subsequently, the NIHR HTA programme granted us a 24 month-funded extension to complete the trial.
In December 2010 we gained approval to make payments to sites to cover investigator costs at £50 per cohort and £100 per trial participant recruited.
Data collection
We took the opportunity of the 24-month extension to enhance our data collection to capture more data about QoL post colectomy and to collect this more frequently. We did this by:
-
extending data collection for all trial participants, whenever recruited, until early 2014
-
adding questionnaires at 18, 30 and 36 months; participant reconsent was sought where necessary
-
adding four questionnaires following colectomy and any ensuing corrective surgery; participant reconsent was sought where necessary
-
planning to use survival analysis, statistical missing value imputation and economic modelling to impute QoL and costs for all participants who generate data after randomisation.
We also sought approval to:
-
conduct interviews with health-care professionals to elicit their views about the two drugs and their different modes of administration in response to emerging evidence of potential hidden costs in the administration of ciclosporin in particular
-
conduct the second participant interview at 12 months rather than at 6 months.
Outcome measures
With agreement of the TSC and DMEC we changed the primary outcome measure to QAS weighted by scores on CUCQ and approval was given on 6 June 2012.
Since protocol publication
Economic analysis
-
The economic analysis covered a 30-month period.
-
The comparison of NHS resource use between the two groups did not adjust for inpatient stay in intensive care unit and high-dependency unit.
-
Patient-borne costs were limited to time off work.
-
The proposed long-term economic modelling has not yet been completed.
Chapter 3 Results
Recruitment
A total of 68 sites agreed to take part in the study: 62 in England, three in Scotland and three in Wales. The sites ranged from large teaching hospitals to large and small district general hospitals. One site was deactivated because of a protocol deviation.
Sixty-four sites recruited patients to the cohort and 52 of these recruited participants to the trial. The first site was activated in March 2010 with the first cohort patient recruited in May 2010 and the first trial participant randomised in June 2010. We randomised the last trial participant in February 2013 and completed follow-up in February 2014.
Over the course of the study, two sites that had recruited a total of eight cohort patients, withdrew citing the following reasons:
-
one replacement PI with a treatment preference
-
one site not recruiting and felt unlikely to recruit in the near future.
In addition, two sites that had not recruited to the cohort withdrew citing the following reasons:
-
one PI leaving and no replacement
-
one site lack of research support.
Figure 2 shows that 2065 patients were screened for the cohort and 1614 consented to join it. Of these, 276 were eligible for the trial and were randomised. Six participants were randomised in error: five randomised to ciclosporin were found to have raised cholesterol levels between randomisation and treatment and one randomised to infliximab was found to have cytomegalovirus after randomisation and one dose of infliximab. These six were therefore judged to have been randomised in error and were removed from analysis.
FIGURE 2.
Participant recruitment. BCC, basal cell carcinoma; i.v., intravenous; RP, research professional.
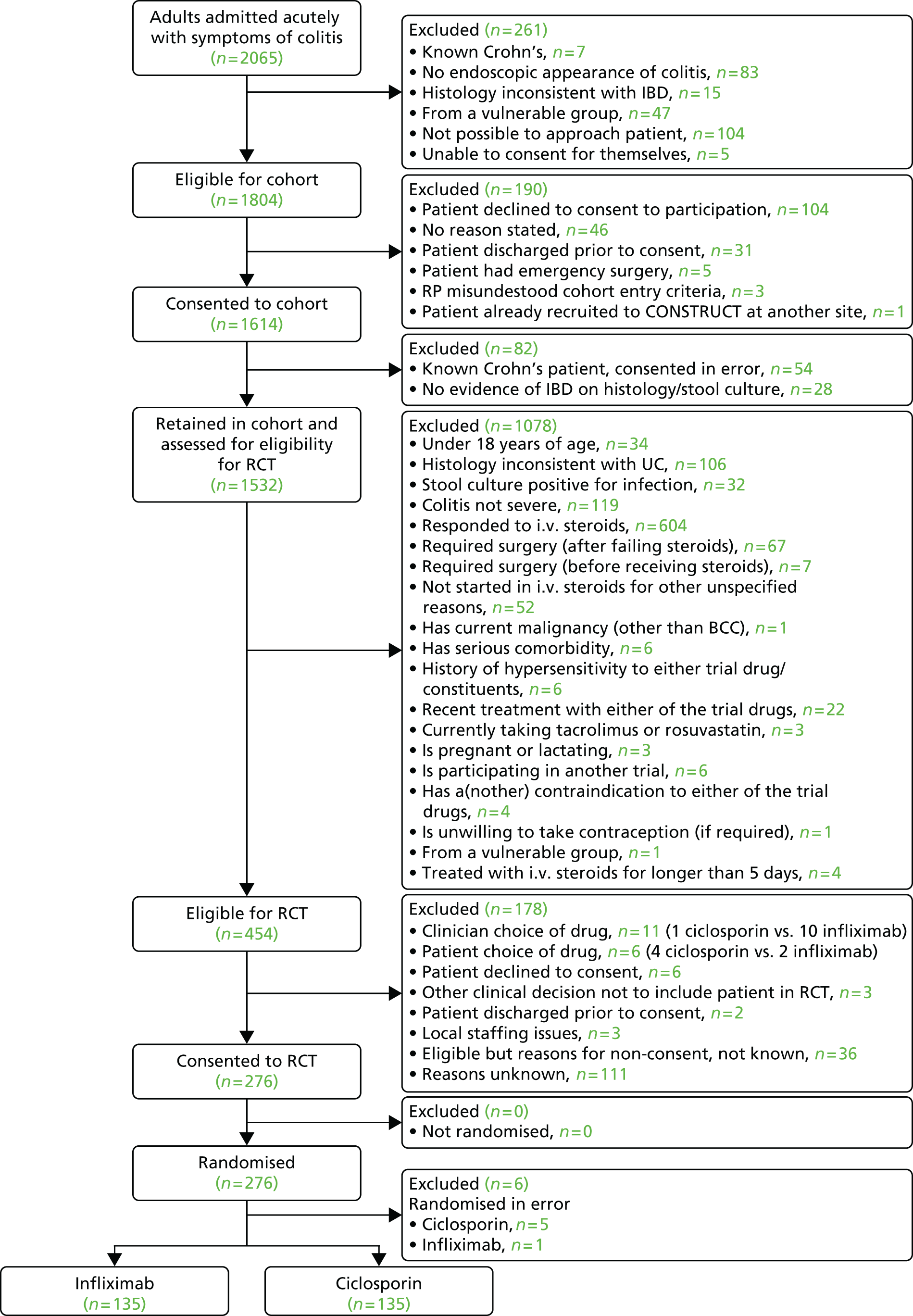
Centre differences in recruitment patterns
Eight sites recruited 11 or more trial participants, 17 sites recruited four to eight participants, 18 recruited two or three participants, nine recruited one participant, with 12 sites recruiting to the cohort only, whereas three sites did not recruit at all (see Appendix 23).
Participant flow
Figure 2 presents the CONSORT flow diagram and summarises patient throughput from recruitment of the first participant in May 2010 to randomisation of the final participant in February 2013.
Figure 3 summarises participant follow-up over the 3-, 6-, 12-, 18-, 24-, 30- and 36-month time points to the end of follow-up on 28 February 2014; 90% of participants contributed to definitive analysis.
FIGURE 3.
Participant follow-up.
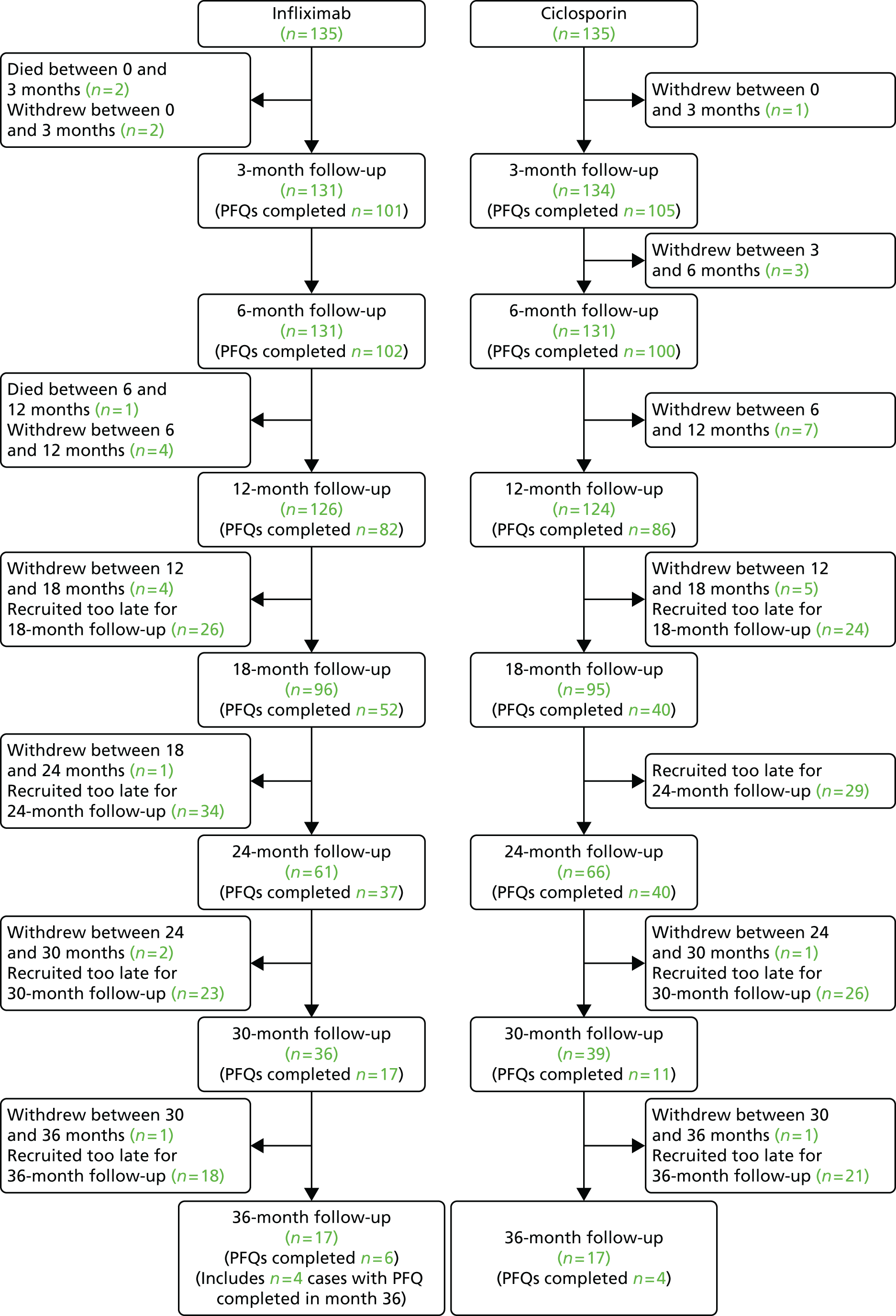
Table 5 shows the data that were obtained as the trial progressed.
| Follow-up period (months) | In trial at period start | Data obtained | Died | Withdrew further access to medical records | Yielded no further data after period end |
|---|---|---|---|---|---|
| Infliximab | |||||
| 0–3 | 135 | 135 | 2 | 2 | 0 |
| 3–6 | 131 | 130 | 0 | 0 | 0 |
| 6–12 | 129 | 124 | 1 | 1 | 21 |
| 12–18 | 105 | 76 | 0 | 0 | 38 |
| 18–24 | 67 | 63 | 0 | 0 | 28 |
| 24–30 | 38 | 32 | 0 | 0 | 23 |
| 30–36 | 15 | 8 | 0 | 0 | – |
| Ciclosporin | |||||
| 0–3 | 135 | 135 | 0 | 2 | 0 |
| 3–6 | 133 | 132 | 0 | 0 | 0 |
| 6–12 | 133 | 129 | 0 | 1 | 28 |
| 12–18 | 104 | 70 | 0 | 0 | 32 |
| 18–24 | 72 | 67 | 0 | 1 | 30 |
| 24–30 | 41 | 28 | 0 | 0 | 23 |
| 30–36 | 18 | 10 | 0 | 0 | – |
Table 6 lists the secondary data available for analysis at each time point. The patients who withdrew from further access to medical records are included in the data available when there were enough data to impute the rest of the secondary care resources. For example, if the patient withdrew soon after the surgery at baseline, the data on hospital costs and trial drugs was used to impute the remainder of resource use to 3 months.
| Follow-up period (months) | In trial at period start | Data obtained | Died | Withdrew further access to medical records | Yielded no further data after period end |
|---|---|---|---|---|---|
| Infliximab | |||||
| 0–3 | 135 | 135 | 2 | 2 | 0 |
| 3–6 | 131 | 131 | 0 | 1 | 0 |
| 6–12 | 131 | 131 | 1 | 3 | 21 |
| 12–18 | 106 | 84 | 0 | 1 | 38 |
| 18–24 | 67 | 65 | 0 | 0 | 28 |
| 24–30 | 39 | 27 | 0 | 1 | 23 |
| 30–36 | 15 | 8 | 0 | 0 | – |
| Ciclosporin | |||||
| 0–3 | 135 | 135 | 0 | 2 | 0 |
| 3–6 | 133 | 133 | 0 | 0 | 0 |
| 6–12 | 133 | 133 | 0 | 2 | 28 |
| 12–18 | 103 | 85 | 0 | 1 | 32 |
| 18–24 | 72 | 71 | 0 | 1 | 30 |
| 24–30 | 49 | 30 | 0 | 0 | 23 |
| 30–36 | 26 | 9 | 0 | 0 | – |
Feasibility and pilot study
Because of the complexity of CONSTRUCT, we conducted a feasibility study at our local health board to test our patient identification and recruitment processes up to randomisation, pilot a modified version of the UK-IBDQ and health resource-use questionnaire, and construct a list of frequently used drugs. The feasibility study ran from March 2009 to June 2009 when 20 patients had been recruited. A report from the local research professional provided helpful insights about points of admission for patients with colitis and how best to identify them; demonstrated that the consent process worked; and showed how to capture a discharge to ensure appropriate study follow-up. It allowed us to refine some of our study documentation and test the drugs list and health resource-use questionnaire. The data from the 20 patients were used to validate the modified UK-IBDQ (see Appendix 8).
We planned a pre-pilot study, including pseudo-randomisation, in five sites and, during the training for this, queries and suggestions from PIs and research professionals led us to make some amendments; where appropriate approval was sought from REC for Wales and the MHRA for these amendments.
The pre-pilot study started in September 2009 in our local health board, but did not commence at the other four sites because of delays caused by R&D approval, staff on leave and staff requiring consent training. By January 2010 several other sites had obtained R&D approval for the full study and to avoid further delay a decision was made at the TMG that our local health board had fulfilled the pre-pilot requirements and we should start the main study. A decision was reached by the TMG that as sites gained R&D approval they would start the study using paper documentation prior to GeneCIS implementation. The documentation would have a ‘pilot’ watermark to distinguish the documents used pre GeneCIS and sites were encouraged to feed back so that amendments could be made before GeneCIS was finalised. When GeneCIS implementation began, sites were sent a revised set of documents with the ‘pilot’ watermark removed.
The first trial participant recruited at each site was considered to be a pilot. After the first trial participant was recruited, we checked the documentation and processes and sought feedback from the PI or research professional about any issues that had occurred.
Validation of outcome measures
Item generation: devising the items for the CUCQ and CUCQ+
Following exploration of the UK-IBDQ and the gastrointestinal literature related to PROMs, we developed the CUCQ and CUCQ+. Appendix 18 shows the major differences between the UK-IBDQ and the CUCQ and CUCQ+. Question 7 from the original UK-IBDQ (related to admission to hospital) was removed from the CUCQ and CUCQ+. A new question was added to the CUCQ/CUCQ+ (Q15, related to getting up to use the toilet after going to bed). In addition, where the original UK-IBDQ had four fixed-response options relating to questions ‘in the past 2 weeks, how often . . .?’, the CUCQ/CUCQ+ used open-response options to take account of any potential floor and ceiling effects.
As the UK-IBDQ did not have any questions for post-colectomy patients, we developed 10 specific stoma-related questions for the CUCQ+ (S1–S10).
Piloting the CUCQ and CUCQ+
We did not modify the CUCQ or CUCQ+ following piloting as none of the patients regarded any of the questions difficult to answer or that there were any specific aspects that were not covered by the questionnaire. The patients with a stoma did not answer the six questions that we had previously identified as not being relevant and instead completed the additional 10 stoma questions.
Baseline demographics of the development and validation samples
We had data from a total of 1240 patients in our cohort development sample and 270 patients in our RCT validation sample (total 1510). We compared the baseline demographic characteristics of the two samples. Table 7 gives details of the characteristics of the two samples.
| Patient characteristic | Cohort sample (maximum n = 1240) | RCT sample (maximum n = 270) | p-value |
|---|---|---|---|
| Gender | |||
| Male | 656 | 170 | 0.003* |
| Female | 584 | 100 | |
| Mean age at recruitment, years (SD) | 41.42 (17.66) | 40.06 (15.31) | 0.240 |
| Ethnicity | |||
| White | 1133 | 250 | 0.377 |
| Asian or Asian British | 65 | 12 | |
| Black or black British | 8 | 3 | |
| Other ethnic groups | 18 | 2 | |
| Mixed | 11 | 0 | |
| Missing | 5 | 3 | |
| Smoking status | |||
| Never smoked | 514 | 112 | 0.829 |
| Non-smoker (history unknown) | 95 | 19 | |
| Current smoker | 102 | 27 | |
| Ex-smoker | 481 | 105 | |
| Mean weight, kg (SD) | 73.05 (17.63) | 74.14 (15.09) | 0.353 |
| Mean height, m (SD) | 1.70 (0.11) | 1.71 (0.09) | 0.160 |
| Truelove and Witt classification4 | |||
| Severe | 1033 | 251 | 0.000* |
| Not severe | 174 | 16 | |
| Mayo score | |||
| 0 | 35 | 4 | 0.000* |
| 1 | 103 | 4 | |
| 2 | 391 | 68 | |
| 3 | 483 | 183 | |
| Family history | |||
| Yes | 147 | 46 | 0.023* |
| No | 1072 | 221 | |
| Comorbidity: IHD | |||
| Yes | 49 | 6 | 0.165 |
| No | 1179 | 263 | |
| Comorbidity: CVD | |||
| Yes | 16 | 2 | 0.446 |
| No | 1213 | 267 | |
| Comorbidity: resp | |||
| Yes | 70 | 15 | 0.934 |
| No | 1157 | 254 | |
| Comorbidity: liver | |||
| Yes | 7 | 3 | 0.320 |
| No | 1221 | 266 | |
| Comorbidity: BP | |||
| Yes | 102 | 18 | 0.376 |
| No | 1112 | 248 | |
| Mean duration of symptoms (SD) | 32.71 (43.64) | 39.60 (51.95) | 0.025* |
| Mean EQ-5D (SD) | 0.57 (0.31) | 0.51 (0.30) | 0.040* |
| Mean CUCQ (SD) | 0.40 (0.16) | 0.36 (0.13) | 0.001* |
| Mean SF-6D (SD) | 0.57 (0.11) | 0.56 (0.11) | 0.090 |
| Mean blood: Hb (SD) | 12.30 (2.10) | 12.82 (2.16) | 0.918 |
| Mean blood: ESR (SD) | 39.63 (28.52) | 41.50 (27.24) | 0.607 |
| Mean blood: CRP (SD) | 61.30 (70.62) | 83.30 (81.13) | 0.000* |
| Mean blood: Alb (SD) | 36.71 (6.88) | 33.18 (6.57) | 0.000* |
| Mean blood: Cr (SD) | 77.02 (26.33) | 77.20 (19.44) | 0.915 |
| Mean blood: Chol (SD) | 4.09 (1.37) | 3.81 (1.03) | 0.147 |
| Mean TW-CCRF-SF (SD) | 11.15 (6.77) | 12.63 (6.76) | 0.021* |
| Mean TW-CCRF-BSF (SD) | 10.48 (6.71) | 11.75 (6.95) | 0.007* |
| Mean TW-CCRF-HR (SD) | 88.79 (17.79) | 89.55 (16.76) | 0.526 |
| Mean TW-CCRF-Temp (SD) | 36.80 (0.69) | 36.91 (0.69) | 0.021* |
Psychometric validation of the CUCQ in the validation sample (CONSTRUCT cohort sample)
We examined the data prior to undertaking principal component analysis. The Kaiser–Meyer–Olkin measure of sampling adequacy was 0.924 and the Barlett’s test of sphericity was 0.000, indicating that the data were suitable principal component analysis.
All 32 CUCQ questions had a response rate of at least 93% in the cohort development sample (Table 8). Based on our set criteria of responding to at least 24 out of the 32 (75%) total questions, there were 1226 (99%) patients for whom we were able to calculate a CUCQ score.
| Question | Number of missing responses, n (%) |
|---|---|
| 1 | 9 (0.73) |
| 2 | 14 (1.13) |
| 3 | 12 (0.97) |
| 4 | 12 (0.97) |
| 5 | 13 (1.05) |
| 6 | 15 (1.21) |
| 7 | 14 (1.13) |
| 8 | 19 (1.54) |
| 9 | 19 (1.54) |
| 10 | 13 (1.05) |
| 11 | 10 (0.81) |
| 12 | 15 (1.21) |
| 13 | 14 (1.13) |
| 14 | 15 (1.21) |
| 15 | 11 (0.89) |
| 16 | 18 (1.46) |
| 17 | 26 (2.11) |
| 18 | 14 (1.13) |
| 19 | 17 (1.38) |
| 20 | 11 (0.89) |
| 21 | 17 (1.38) |
| 22 | 10 (0.81) |
| 23 | 13 (1.05) |
| 24 | 12 (0.97) |
| 25 | 14 (1.13) |
| 26 | 15 (1.21) |
| 27 | 11 (0.89) |
| 28 | 86 (6.96) |
| 29 | 17 (1.38) |
| 30 | 16 (1.30) |
| 31 | 18 (1.46) |
| 32 | 18 (1.46) |
The internal consistency of the CUCQ was excellent with a Cronbach’s alpha of 0.900. Correlations of all 32 questions with the total score exceeded 0.2 (Table 9). None of the questions had a response frequency > 80%. The data therefore did not exhibit any floor or ceiling effects.
| Question | Item-total correlation | Maximum response rate (%) |
|---|---|---|
| On how many days over the last 2 weeks have you had loose or runny bowel movements? | 0.344 | 76.2 |
| On how many days in the last 2 weeks have you noticed blood in your stools? | 0.257 | 49.3 |
| On how many days over the last 2 weeks have you felt tired? | 0.530 | 66.8 |
| In the last 2 weeks have you felt frustrated? | 0.532 | 38.7 |
| In the last 2 weeks has your bowel condition prevented you from carrying out your work or other normal activities? | 0.513 | 33.5 |
| On how many days over the last 2 weeks have you opened your bowels more than three times a day? | 0.425 | 72.2 |
| On how many days over the last 2 weeks have you felt full of energy? | 0.347 | 73.3 |
| In the last 2 weeks did your bowel condition prevent you from going out socially? | 0.539 | 36.4 |
| On how many days over the last 2 weeks have your bowels opened accidentally? | 0.261 | 42.9 |
| On how many days over the last 2 weeks have you felt generally unwell? | 0.554 | 58.6 |
| In the last 2 weeks have you felt the need to keep close to a toilet? | 0.586 | 47.1 |
| In the last 2 weeks has your bowel condition affected your leisure or sports activities? | 0.549 | 50.2 |
| On how many days over the last 2 weeks have you felt pain in your abdomen? | 0.441 | 47.5 |
| On how many nights over the last 2 weeks have you been unable to sleep well (days if you are a shift worker)? | 0.516 | 46.9 |
| On how many nights in the last 2 weeks have you had to get up to use the toilet because of your bowel condition after you have gone to bed? | 0.466 | 50.1 |
| In the last 2 weeks have you felt depressed? | 0.539 | 49.7 |
| In the last 2 weeks have you had to avoid attending events where there was no toilet close at hand? | 0.524 | 35.8 |
| On how many days over the last 2 weeks have you had a problem with large amounts of wind? | 0.207 | 32.5 |
| On how many days over the last 2 weeks have you felt off your food? | 0.423 | 24.5 |
| Many patients with bowel problems have worries about their illness. How often during the last 2 weeks have you felt worried? | 0.540 | 33.7 |
| On how many days over the last 2 weeks has your abdomen felt bloated? | 0.415 | 33.1 |
| In the last 2 weeks have you felt relaxed? | 0.460 | 46.9 |
| In the last 2 weeks have you been embarrassed by your bowel problem? | 0.480 | 37.1 |
| On how many days over the last 2 weeks have you wanted to go back to the toilet immediately after you thought you had emptied your bowels? | 0.501 | 27.9 |
| In the last 2 weeks have you felt upset? | 0.586 | 57.6 |
| On how many days over the last 2 weeks have you had to rush to the toilet? | 0.561 | 48.2 |
| In the last 2 weeks have you felt angry as a result of your bowel problem? | 0.444 | 45.0 |
| In the last 2 weeks, has your sex life been affected by your bowel problem? | 0.361 | 41.0 |
| On how many days over the last 2 weeks have you felt sick? | 0.419 | 27.2 |
| In the last 2 weeks have you felt irritable? | 0.505 | 53.7 |
| In the last 2 weeks have you felt lack of sympathy from others? | 0.314 | 61.5 |
| In the last 2 weeks have you felt happy? | 0.398 | 55.1 |
The principal component analysis indicated that there were seven factors with an eigenvalue of > 1 and which explained approximately 57% of the variance in the data. The scree plot indicated that there was an elbow in the scree plot between the fourth and fifth factors. We rotated the solution based on four factors using direct oblimin principal component analysis (Table 10). The principal component analysis identified that the first factor covered emotional symptoms; the second bowel symptoms; the third social activities; and the fourth general symptoms.
| Factor | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Percentage of factor’s contribution | 26.716 | 8.408 | 6.162 | 5.452 |
| Eigenvalue | 8.549 | 2.691 | 1.972 | 1.745 |
| CUCQ16 | 0.760 | |||
| CUCQ25 | 0.744 | |||
| CUCQ27 | 0.740 | |||
| CUCQ30 | 0.708 | |||
| CUCQ4 | 0.610 | |||
| CUCQ20 | 0.607 | |||
| CUCQ23 | 0.558 | |||
| CUCQ31 | 0.530 | |||
| CUCQ22 | ||||
| CUCQ32 | ||||
| CUCQ1 | 0.754 | |||
| CUCQ6 | 0.751 | |||
| CUCQ26 | 0.642 | |||
| CUCQ2 | 0.615 | |||
| CUCQ24 | 0.563 | |||
| CUCQ15 | 0.535 | |||
| CUCQ14 | ||||
| CUCQ18 | ||||
| CUCQ8 | –0.837 | |||
| CUCQ12 | –0.790 | |||
| CUCQ5 | –0.768 | |||
| CUCQ17 | –0.723 | |||
| CUCQ11 | –0.623 | |||
| CUCQ28 | ||||
| CUCQ10 | 0.629 | |||
| CUCQ3 | 0.611 | |||
| CUCQ13 | 0.578 | |||
| CUCQ7 | 0.554 | |||
| CUCQ19 | 0.497 | |||
| CUCQ29 | 0.433 | |||
| CUCQ9 | –0.403 | |||
| CUCQ21 | ||||
The CUCQ scores achieved significant correlations with the two generic HRQoL scales (SF-12 mental and physical component scores and the EQ-5D) scores demonstrating good construct validity (Table 11).
| Scale | CUCQ |
|---|---|
| SF-12 MCS | 0.588a |
| SF-12 PCS | 0.444a |
| EQ-5D | 0.429a |
Table 12 illustrates the percentage variance that each of the 32 questions contributed to the total CUCQ score in the CONSTRUCT cohort development sample. Ten of the 32 questions contributed over 90% of the variance in the total score. These 10 questions could be future candidates for a shortened version of the CUCQ. We will undertake further work to test the validity of a shortened version of the CUCQ.
| Question | Cumulative % of variance |
|---|---|
| Q25: In the last 2 weeks have you felt upset? | 38.6 |
| Q11: In the last 2 weeks have you felt the need to keep close to a toilet? | 59.5 |
| Q3: On how many days over the last 2 weeks have you felt tired? | 70.6 |
| Q14: On how many nights over the last 2 weeks have you been unable to sleep well (days if you are a shift worker)? | 76.3 |
| Q21: On how many days over the last 2 weeks has your abdomen felt bloated? | 79.9 |
| Q17: In the last 2 weeks have you had to avoid attending events where there was no toilet close at hand? | 82.9 |
| Q4: In the last 2 weeks have you felt frustrated? | 85.4 |
| Q26: On how many days over the last 2 weeks have you had to rush to the toilet? | 87.4 |
| Q19: On how many days over the last 2 weeks have you felt off your food? | 89.2 |
| Q23: In the last 2 weeks have you been embarrassed by your bowel problem? | 90.4 |
| Q12: In the last 2 weeks has your bowel condition affected your leisure or sports activities? | 91.5 |
| Q6: On how many days over the last 2 weeks have you opened your bowels more than three times a day? | 92.5 |
| Q30: In the last 2 weeks have you felt irritable? | 93.3 |
| Q13: On how many days over the last 2 weeks have you felt pain in your abdomen? | 94.2 |
| Q28: In the last 2 weeks has your sex life been affected by your bowel problem? | 94.9 |
| Q16: In the last 2 weeks have you felt depressed? | 95.6 |
| Q18: On how many days over the last 2 weeks have you had a problem with large amounts of wind? | 96.2 |
| Q29: On how many days over the last 2 weeks have you felt sick? | 96.7 |
| Q5: In the last 2 weeks has your bowel condition prevented you from carrying out your work or other normal activities? | 97.1 |
| Q24: On how many days over the last 2 weeks have you wanted to go back to the toilet immediately after you thought you had emptied your bowels? | 97.4 |
| Q20: Many patients with bowel problems have worries about their illness. How often during the last 2 weeks have you felt worried? | 97.8 |
| Q15: On how many nights in the last 2 weeks have you had to get up to use the toilet because of your bowel condition after you have gone to bed? | 98.1 |
| Q2: On how many days in the last 2 weeks have you noticed blood in your stools? | 98.3 |
| Q32: In the last 2 weeks have you felt happy? | 98.7 |
| Q31: In the last 2 weeks have you felt lack of sympathy from others? | 98.9 |
| Q10: On how many days over the last 2 weeks have you felt generally unwell? | 99.1 |
| Q9: On how many days over the last 2 weeks have your bowels opened accidentally? | 99.3 |
| Q27: In the last 2 weeks have you felt angry as a result of your bowel problem? | 99.5 |
| Q8: In the last 2 weeks did your bowel condition prevent you from going out socially? | 99.6 |
| Q22: In the last 2 weeks have you felt relaxed? | 99.8 |
| Q7: On how many days over the last 2 weeks have you felt full of energy? | 99.9 |
| Q1: On how many days over the last 2 weeks have you had loose or runny bowel movements? | 100 |
Psychometric validation of the CUCQ in the validation sample (CONSTRUCT randomised controlled trial sample)
For the purposes of this report, we calculated CUCQ+ scores for stoma extension patients on the basis of equal weighting for each score. Further analysis will be carried out to explore weighting of the stoma-specific questions.
The internal consistency of the CUCQ in the RCT sample was excellent, with a Cronbach’s alpha of 0.845. Table 13 illustrates the percentage variance that each of the 32 questions contributed to the total CUCQ score in the RCT validation sample.
| Questions | Cumulative % of variance |
|---|---|
| Q10: On how many days over the last 2 weeks have you felt generally unwell? | 34.5 |
| Q30: In the last 2 weeks have you felt irritable? | 58.8 |
| Q12: In the last 2 weeks has your bowel condition affected your leisure or sports activities? | 67.4 |
| Q3: On how many days over the last 2 weeks have you felt tired? | 74.0 |
| Q23: In the last 2 weeks have you been embarrassed by your bowel problem? | 79.1 |
| Q24: On how many days over the last 2 weeks have you wanted to go back to the toilet immediately after you thought you had emptied your bowels? | 81.8 |
| Q16: In the last 2 weeks have you felt depressed? | 84.0 |
| Q29: On how many days over the last 2 weeks have you felt sick? | 86.2 |
| Q5: In the last 2 weeks has your bowel condition prevented you from carrying out your work or other normal activities? | 87.8 |
| Q18: On how many days over the last 2 weeks: have you had a problem with large amounts of wind? | 89.5 |
| Q32: In the last 2 weeks have you felt happy? | 90.8 |
| Q28: In the last 2 weeks has your sex life been affected by your bowel problem? | 91.7 |
| Q14: On how many nights over the last 2 weeks have you been unable to sleep well (days if you are a shift worker)? | 92.7 |
| Q20: Many patients with bowel problems have worries about their illness. How often during the last 2 weeks have you felt worried? | 93.7 |
| Q17: In the last 2 weeks have you had to avoid attending events where there was no toilet close at hand? | 94.5 |
| Q2: On how many days in the last 2 weeks have you noticed blood in your stools? | 95.2 |
| Q26: On how many days over the last 2 weeks have you had to rush to the toilet? | 95.8 |
| Q31: In the last 2 weeks have you felt lack of sympathy from others? | 96.3 |
| Q21: On how many days over the last 2 weeks has your abdomen felt bloated? | 96.7 |
| Q27: In the last 2 weeks have you felt angry as a result of your bowel problem? | 97.1 |
| Q22: In the last 2 weeks have you felt relaxed? | 97.5 |
| Q19: On how many days over the last 2 weeks have you felt off your food? | 97.8 |
| Q13: On how many days over the last 2 weeks have you felt pain in your abdomen? | 98.1 |
| Q11: In the last 2 weeks have you felt the need to keep close to a toilet? | 98.4 |
| Q9: On how many days over the last 2 weeks have your bowels opened accidentally? | 98.7 |
| Q4: In the last 2 weeks have you felt frustrated? | 98.9 |
| Q15: On how many nights in the last 2 weeks have you had to get up to use the toilet because of your bowel condition after you have gone to bed? | 99.2 |
| Q7: On how many days over the last 2 weeks have you felt full of energy? | 99.4 |
| Q8: In the last 2 weeks did your bowel condition prevent you from going out socially? | 99.6 |
| Q25: In the last 2 weeks have you felt upset? | 99.7 |
| Q1: On how many days over the last 2 weeks have you had loose or runny bowel movements? | 99.9 |
| Q6: On how many days over the last 2 weeks have you opened your bowels more than three times a day? | 100 |
Table 14 indicates the number of patients for whom we collected a CUCQ/CUCQ+ score at each time period within the RCT validation sample.
| Time point | Scale (total sample, N = 270) |
|---|---|
| CUCQ/CUCQ+, n (%) | |
| Baseline | 267 (98.9) |
| 3 months | 202 (74.8) |
| 6 months | 200 (74.1) |
| 12 months | 168 (62.2) |
| 18 months | 91 (33.7) |
| 24 months | 77 (28.5) |
| 30 months | 27 (10) |
| 36 months | 10 (3.7) |
The CUCQ scores achieved significant correlations at baseline with the two generic HRQoL scales (SF-12 mental and physical component scores and the EQ-5D) in the RCT validation sample scores demonstrating good construct validity (Table 15).
| Scale | CUCQ |
|---|---|
| SF-12 MCS | 0.588a |
| SF-12 PCS | 0.452a |
| EQ-5D | 0.459a |
Testing reliability and responsiveness of the CUCQ
We assessed the reproducibility or test–retest reliability of the CUCQ on those patients who reported no change in their condition between completion of follow-up questionnaires. We included those patients who had indicated no change in their bowel condition on the transition question between the 3- and 6-month periods (How has your QoL changed since the last time you filled in a questionnaire?).
There were 34 patients who reported no change in their condition at 3–6 months. The Cronbach’s alpha was 0.582, indicating moderate agreement between the CUCQ scores. Further exploration of the test–retest scores is needed to determine any differences in the reliability of the CUCQ scale between stoma and non-stoma patients. The judgement of ‘stability’ was based on subjective assessment by the patient. The clinical assessment of stability and how this affects the test–retest reliability will also be explored. In addition, the correlation between the CUCQ test–retest reliability and the generic EQ-5D and SF-12 reliability requires further analysis.
We assessed the responsiveness of the CUCQ on those patients who had reported an improvement or a deterioration in their condition on the transition question between the 3- and 6-month period. There were 146 patients who reported a change in their condition in this period. The responsiveness ratio (mean change in scores for those patients who reported a change divided by the standard deviation (SD) of the scores of the stable patients) was 0.200. This indicated moderate responsiveness. Further analysis is required to explore differences in the responsiveness of the scale between the stoma and non-stoma patients.
Principal component analysis of the CUCQ showed that there were four underlying dimensions that made clinical sense. Internal consistency of the total CUCQ score was demonstrated by an excellent Cronbach’s alpha. The construct validity of the CUCQ was demonstrated by significant correlations with the SF-12 mental and physical component scores and with the EQ-5D. Moderate intraclass correlations between the test and retest scores demonstrated the reproducibility of the instrument. In addition, the scale was found to be responsive to change.
We will undertake further work to explore whether or not there are any differences in the validity of the scale between the stoma and non-stoma patients. In addition, we will carry out additional analysis to explore the CUCQ clinical subscales and the potential for developing a shortened version of the CUCQ.
Clinical effectiveness
Baseline characteristics
We compared the trial participants in the two groups in terms of various characteristics (Table 16); there are no statistically significant differences between the groups.
| Variables | Infliximab (n = 135) | Ciclosporin (n = 135) |
|---|---|---|
| Age at randomisation (years), mean (SD) [n] | 39.3 (15.5) [135] | 39.8 (15.0) [135] |
| Gender: proportion, n/N (%) | ||
| Female | 46/135 (34.1) | 54/135 (40.0) |
| Male | 89/135 (65.9) | 81/135 (60.0) |
| Ethnicity: proportion, n/N (%) | ||
| White | 126/134 (94.0) | 124/133 (93.2) |
| Asian or Asian British | 5/134 (3.7) | 7/133 (5.3) |
| Black or black British | 2/134 (1.5) | 1/133 (0.8) |
| Other ethnic groups | 1/134 (0.7) | 1/133 (0.8) |
| Weight (kg), mean (SD) [n] | 74.3 (15.0) [135] | 73.9 (15.3) [134] |
| Smoking: proportion, n/N (%) | ||
| Never smoked/non-smoker | 58/130 (44.6) | 75/134 (56.0) |
| Current/ex-smoker | 72/130 (55.4) | 59/134 (44.0) |
| Family history: proportion, n/N (%) | ||
| Yes (any one of mother, father, sibling, child) | 28/132 (21.2) | 19/135 (14.1) |
| No | 104/132 (78.8) | 116/135 (85.%) |
| Condition severity (using Truelove and Witt’s criteria4): proportion, n/N (%) | ||
| Severe | 97/133 (72.9) | 95/131 (72.5) |
| Not severe | 36/133 (27.1) | 36/131 (27.5) |
| Montreal Score: proportion, n/N (%) | ||
| E1 | 7/124 (5.6) | 10/126 (7.9) |
| E2 | 64/124 (51.6) | 54/126 (42.8) |
| E3 | 53/124 (42.7) | 62/127 (49.2) |
| Mayo score: proportion, n/N (%) | ||
| 0 | 2/131 (1.5) | 1/128 (0.8) |
| 1 | 2/131 (1.5) | 2/128 (1.6) |
| 2 | 35/131 (26.7) | 35/128 (27.3) |
| 3 | 92/131 (70.2) | 90/128 (70.3) |
| Receiving any of azathioprine, 6-mercaptopurine or methotrexate at baseline, n/N (%) | ||
| At least one | 16/135 (11.9) | 26/135 (19.3) |
| None | 119/135 (88.1) | 109/135 (80.7) |
| Duration of symptoms for current episode (days), mean (SD) [n] | 37.6 (46.0) [135] | 41.4 (57.5) [131] |
| EQ-5D, mean (SD) [n] | 0.5185 (0.2961) [132] | 0.4958 (0.3142) [133] |
| CUCQ score, mean (SD) [n] | 0.3664 (0.1334) [134] | 0.3574 (0.1325) [133] |
Data analysed
Recruitment to the RCT started when participant UCL0001 was randomised on 24 June 2010 and continued until participant ABH0016 was randomised on 26 February 2013; the CONSORT diagram (see Figure 2) summarises recruitment between these dates and the number of PFQs (see Figure 3) actually completed at each stage. Our primary outcome, the QAS,55 can be computed for n = 242 cases with at least one post-randomisation value of the CUCQ or CUCQ+.
Analysis
Outcomes were analysed using an appropriate model. Specifically, we used linear models for measurement outcomes (assuming normality, also assessed via residual diagnostics) in which trial site appeared as a random factor; logistic regression for binary (yes/no) outcomes; Cox survival regression models for times to events; we also analysed the three profiles of QoL scores for each participant using a repeated-measures linear model. Analyses, in which a group effect was always included, considered the following covariates and factors: gender; weight; age at randomisation; ethnicity; smoking status; family history; duration of symptoms; disease severity (as assessed by the criteria proposed by Truelove and Witt4); immunosuppressant therapy (using binary indicator set equal to 1 for participants taking azathioprine, 6-mercaptopurine or methotrexate at baseline); EQ-5D and CUCQ scores at baseline; and time in follow-up. We progressed by eliminating, in turn, and starting with the least significant, all covariates and factors found to be not statistically significant at the 5% level, and concluded when all remaining covariates and factors were statistically significant.
Tables 17–20 provide raw and adjusted comparisons between groups, some indication of the extent of the intracluster correlation in variables between participants at the same site and details of statistically significant factors and covariates. The adjusted comparison reflects the nature of the variable under consideration: we present an odds ratio (OR) for logistic regression models for binary variables; a hazard ratio for survival analyses and an additive group effect (Δ, in same units as dependent variable) for linear models for continuous variables.
| Outcome | Raw data | Adjusted comparison | 95% CI | Intracluster correlation | ||
|---|---|---|---|---|---|---|
| Infliximab | Ciclosporin | Estimate | 95% CI | |||
| QASa | ||||||
| Mean | 564.0 | 587.0 | Δ = 7.90 (p = 0.60) | –21.97 to 37.77 | 0.065 | 0.015 to 0.147 |
| SD | 241.9 | 226.2 | ||||
| n | 121 | 121 | ||||
| QAS per dayb | ||||||
| Mean | 0.7052 | 0.7331 | Δ = 0.0297 (p = 0.129) | –0.0088 to 0.0682 | 0.094 | 0.028 to 0.189 |
| SD | 0.1811 | 0.1580 | ||||
| n | 121 | 121 | ||||
| Participants subsequently undergoing colectomy: proportion, n/N (%) | 55/135 (40.7) | 65/135 (48.1) | OR = 1.350 (p = 0.223) | 0.832 to 2.188 | 0 | n/a |
| Time to colectomy (days)c | ||||||
| Mean | 810.8 | 744.1 | HR = 1.234 (p = 0.251) | 0.862 to 1.768 | 0 | n/a |
| n | 135 | 135 | ||||
| Total number of SARs | 16 | 10 | ER = 0.938 (p = 0.788) | 0.590 to 1.493 | 0 | n/a |
| One SAR per participant | 12 | 8 | ||||
| Two SARs per participant | 2 | 1 | ||||
| Participants with one or more SARs proportion, n/N (%)d | 14/135 (10.4) | 9/135 (6.7) | OR = 0.660 (p = 0.338) | 0.282 to 1.546 | 0.050 | 0.008 to 0.132 |
| Total number of SAEs | 21 | 25 | ER = 1.075 (p = 0.807) | 0.603 to 1.917 | 0 | n/a |
| One SAE per participant | 12 | 13 | ||||
| Two SAEs per participant | 3 | 2 | ||||
| Three SAEs per participant | 1 | 0 | ||||
| Four SAEs per participant | 0 | 2 | ||||
| Participants with one or more SAEs proportion, n/N (%)e | 16/135 (11.9) | 17/135 (12.6) | OR = 0.999 (p = 0.998) | 0.473 to 2.114 | 0 | n/a |
| Post-randomisation LOS (days)f | ||||||
| Mean | 10.32 | 12.21 | Δ = 1.542 (p = 0.28 | –1.297 to 4.38 | 0.025 | 0.002 to 0.089 |
| SD | 13.55 | 10.18 | ||||
| n | 135 | 135 | ||||
| Logarithm of post-randomisation LOSg | ||||||
| Mean | 1.878 | 2.289 | Δ = 0.421 (p < 0.001) | 0.245 to 0.597 | 0.024 | (0.001 to 0.085) |
| SD | 0.887 | 0.626 | ||||
| n | 135 | 135 | ||||
| Mortality: proportion, n/N (%) | 3/135 (2.2) | 0/135 (0) | ||||
| Outcome | Raw data, mean (SD) [n] | Adjusted comparison | 95% CI | |
|---|---|---|---|---|
| Infliximab | Ciclosporin | |||
| CUCQa | ||||
| Month 0 | 0.3664 (0.1334) [134] | 0.3574 (0.1325) [133] | Δ = 0.0195 (p = 0.319) | –0.0191 to 0.0581 |
| Month 3 | 0.7455 (0.1830) [99] | 0.7187 (0.1855) [103] | ||
| Month 6 | 0.7497 (0.1952) [101] | 0.7505 (0.2083) [99] | ||
| Month 12 | 0.7284 (0.2110) [82] | 0.7927 (0.1738) [86] | ||
| Month 18 | 0.7837 (0.1769) [52] | 0.8179 (0.1321) [39] | ||
| Month 24 | 0.8102 (0.1702) [36] | 0.8264 (0.1256) [40] | ||
| Month 30 | 0.8099 (0.1644) [17] | 0.8502 (0.1140) [10] | ||
| Month 36 | 0.7611 (0.0966) [6] | 0.8380 (0.1390) [4] | ||
| SF-6Db | ||||
| Month 0 | 0.5632 (0.1066) [128] | 0.5517 (0.1047) [127] | Δ = 0.0051 (p = 0.737) | –0.0250 to 0.0353 |
| Month 3 | 0.7194 (0.1357) [96] | 0.7066 (0.1383) [100] | ||
| Month 6 | 0.7401 (0.1439) [99] | 0.7384 (0.1513) [95] | ||
| Month 12 | 0.7610 (0.1479) [78] | 0.7624 (0.1551) [85] | ||
| Month 18 | 0.7449 (0.1459) [50] | 0.7954 (0.1323) [39] | ||
| Month 24 | 0.7782 (0.1485) [37] | 0.7867 (0.1197) [37] | ||
| Month 30 | 0.7947 (0.1190) [17] | 0.8095 (0.1065) [11] | ||
| Month 36 | 0.7413 (0.1159) [6] | 0.7603 (0.1334) [4] | ||
| EQ-5Dc | ||||
| Month 0 | 0.5185 (0.2961) [132] | 0.4958 (0.3142) [133] | Δ = 0.0144 (p = 0.527) | –0.0304 to 0.0592 |
| Month 3 | 0.8000 (0.2090) [99] | 0.7791 (0.2409) [103] | ||
| Month 6 | 0.7957 (0.2387) [102] | 0.8107 (0.2111) [100] | ||
| Month 12 | 0.8021 (0.2235) [81] | 0.8327 (0.2336) [86] | ||
| Month 18 | 0.8238 (0.2179) [51] | 0.8821 (0.1290) [40] | ||
| Month 24 | 0.8634 (0.1835) [37] | 0.8678 (0.1871) [38] | ||
| Month 30 | 0.8815 (0.1229) [17] | 0.9225 (0.1193) [11] | ||
| Month 36 | 0.8430 (0.1361) [6] | 0.8203 (0.1300) [4] | ||
| Outcome | Raw data:a outcome, mean (SD) [n] | Adjusted comparisonb,c | 95% CI | |
|---|---|---|---|---|
| Infliximab | Ciclosporin | |||
| Interval 1d | ΔGroup = 0.0130 (p = 0.511) | –0.0259 to 0.0519 | ||
| All | 0.6877 (0.2128) [145] | 0.6926 (0.1951) [157] | ΔColectomy = –0.0476 (p = 0.004) | –0.0800 to –0.0152 |
| BC | 0.7658 (0.1822) [75] | 0.7216 (0.1941) [76] | ||
| PC | 0.6041 (0.2125) [70] | 0.6653 (0.1932) [81] | ||
| Interval 2 | ||||
| All | 0.7425 (0.2076) [112] | 0.7398 (0.2008) [118] | ||
| BC | 0.7750 (0.1856) [72] | 0.7881 (0.1918) [68] | ||
| PC | 0.6841 (0.2338) [40] | 0.6743 (0.1957) [50] | ||
| Interval 3 | ||||
| All | 0.7313 (0.2134) [87] | 0.7700 (0.1783) [100] | ||
| BC | 0.7648 (0.1983) [55] | 0.8168 (0.1530) [50] | ||
| PC | 0.6737 (0.2289) [32] | 0.7231 (0.1906) [50] | ||
| Interval 4 | ||||
| All | 0.7577 (0.1903) [58] | 0.8099 (0.1352) [45] | ||
| BC | 0.7782 (0.1779) [38] | 0.8358 (0.1403) [27] | ||
| PC | 0.7190 (0.2112) [20] | 0.7710 (0.1206) [18] | ||
| Interval 5 | ||||
| All | 0.7999 (0.1742) [38] | 0.8226 (0.1267) [39] | ||
| BC | 0.8054 (0.1812) [27] | 0.8407 (0.1168) [28] | ||
| PC | 0.7861 (0.1633) [11] | 0.7763 (0.1446) [11] | ||
| Interval 6 | ||||
| All | 0.8140 (0.1684) [17] | 0.7190 (0.2588) [16] | ||
| BC | 0.8770 (0.1006) [11] | 0.8446 (0.1284) [8] | ||
| PC | 0.6983 (0.2137) [6] | 0.5934 (0.3016) [8] | ||
| Interval 7 | ||||
| All | 0.7611 (0.0966) [6] | 0.8380 (0.1390) [4] | ||
| BC | 0.7398 (0.1158) [4] | 0.8948 (0.0978) [3] | ||
| PC | 0.8039 (0.0313) [2] | 0.6673 (n/a) [1] | ||
| Outcome | Raw data, mean (SD) [n] | Adjusted comparisona | 95% CI | |
|---|---|---|---|---|
| Infliximab | Ciclosporin | |||
| Interval 1b | ΔGroup = 0.0266 (p = 0.367) | –0.0317 to 0.0848 | ||
| All | 0.6119 (0.214) [84] | 0.6649 (0.1979) [104] | ΔColectomy = 0.0271 (p = 0.244) | –0.0185 to –0.0726 |
| BC | 0.6510 (0.2091) [14] | 0.6631 (0.2184) [23] | ||
| PC | 0.6041 (0.2125) [70] | 0.6653 (0.1932) [81] | ||
| Interval 2 | ||||
| All | 0.6842 (0.2254) [53] | 0.6903 (0.1980) [61] | ||
| BC | 0.6847 (0.2077) [13] | 0.7630 (0.2008) [11] | ||
| PC | 0.6841 (0.2338) [40] | 0.6743 (0.1957) [50] | ||
| Interval 3 | ||||
| All | 0.6701 (0.2248) [39] | 0.7207 (0.1928) [52] | ||
| BC | 0.6537 (0.2209) [7] | 0.6596 (0.3299) [2] | ||
| PC | 0.6737 (0.2289) [32] | 0.7231 (0.1906) [50] | ||
| Interval 4 | ||||
| All | 0.7001 (0.2159) [23] | 0.7752 (0.1186) [19] | ||
| BC | 0.5740 (0.2481) [3] | 0.8512 (n/a) [1] | ||
| PC | 0.7190 (0.2112) [20] | 0.7710 (0.1206) [18] | ||
| Interval 5 | ||||
| All | 0.7673 (0.1689) [123] | 0.7763 (0.1446) [11] | ||
| BC | 0.5595 (n/a) [1] | (n/a) | ||
| PC | 0.7861 (0.1633) [11] | 0.7763 (0.1446) [11] | ||
| Interval 6 | ||||
| All | 0.6983 (0.2137) [6] | 0.5934 (0.3016) [8] | ||
| BC | (n/a) | (n/a) | ||
| PC | 0.6983 (0.2137) [6] | 0.5934 (0.3016) [8] | ||
| Interval 7 | ||||
| All | 0.8039 (0.0313) [6] | 0.6673 (n/a) [1] | ||
| BC | (n/a) | (n/a) | ||
| PC | 0.8039 (0.0313) [2] | 0.6673 (n/a) [1] | ||
Quality-adjusted survival and quality-adjusted survival per day
Figures 4 and 5 show the mean QAS and QAS per day for the two groups, together with 95% CIs for the means. For both variables, the mean for the ciclosporin group is higher than its infliximab counterpart, although the clear overlap in the 95% CIs indicate that observed differences are not statistically significant, as is confirmed in our analysis. There are relatively high values for the intracluster correlations in these variables, albeit with wide CIs (reflecting the relatively small – in statistical terms – sample sizes).
FIGURE 4.
Means and 95% CI for QAS (the QAS or area under the CUCQ curve from randomisation until end of follow-up on 28 February 2014) for the two groups.
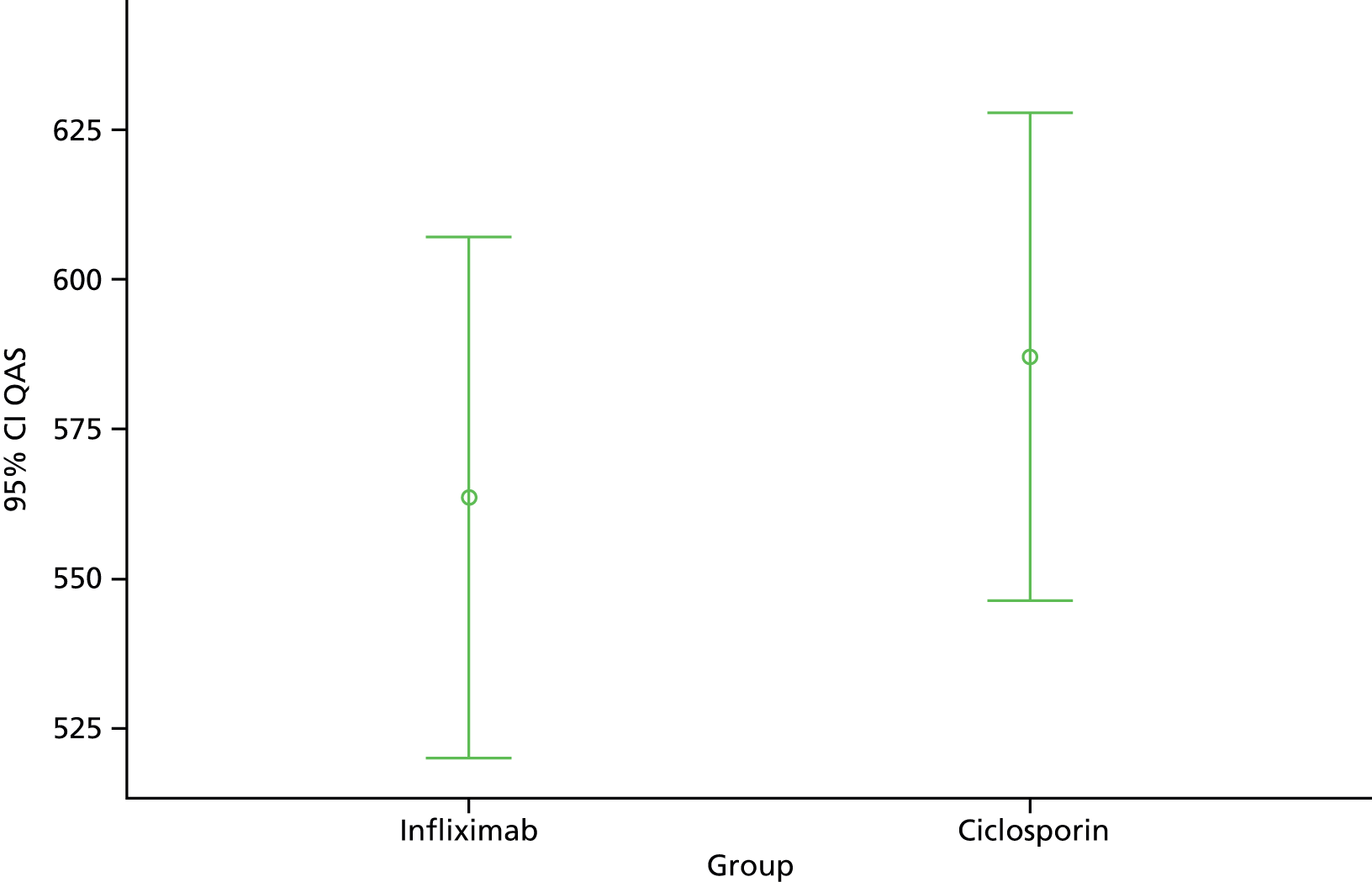
FIGURE 5.
Means and 95% CI for QAS per day [defined as QAS divided by the time (in days) from randomisation until the end of follow-up] for the two groups.
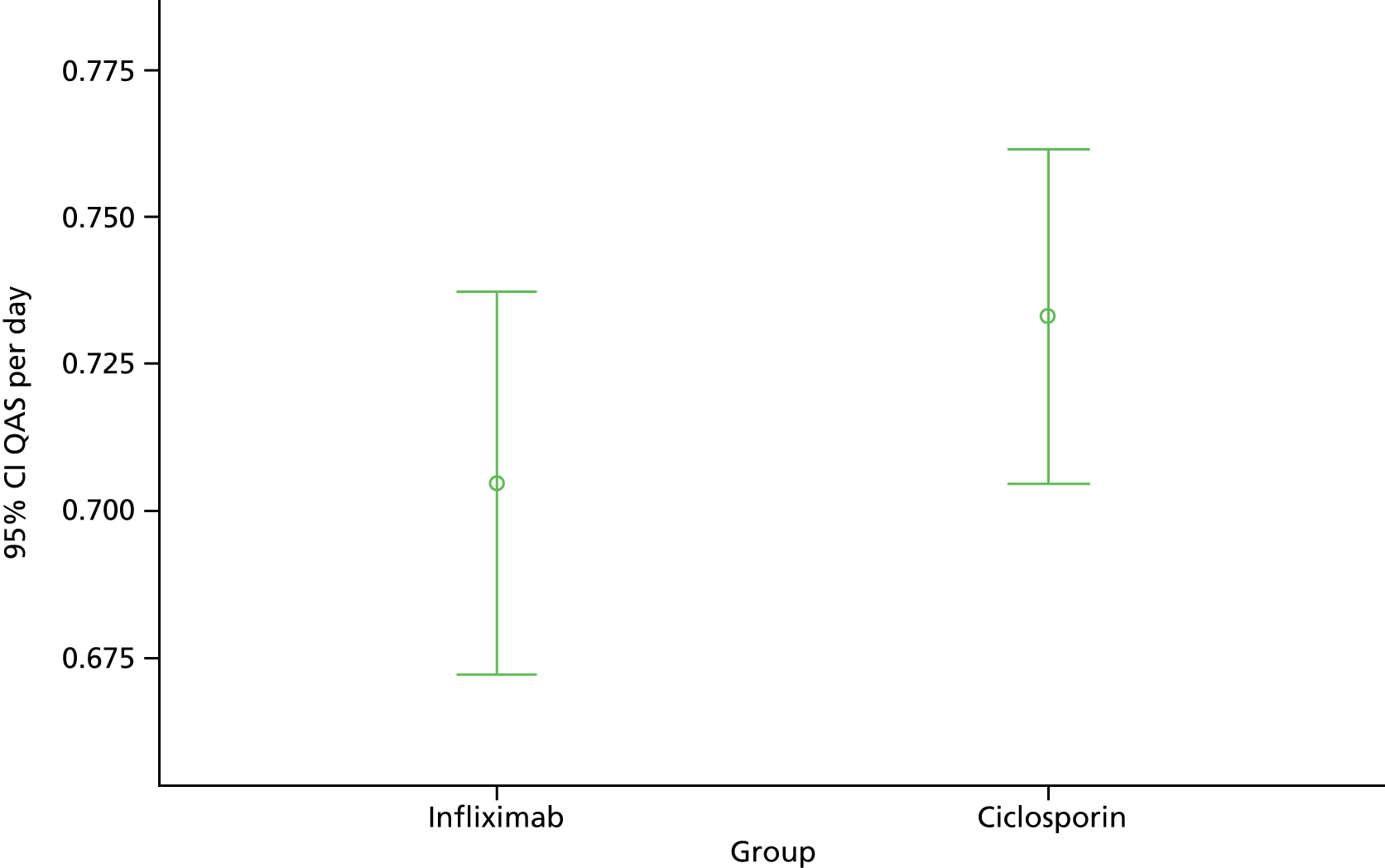
Proportions undergoing colectomy: time to colectomy
Table 17 shows that the observed difference in the proportions of participants subsequently undergoing colectomy in the two groups is not statistically significant; nor is the difference in time to colectomy. Figure 6, the Kaplan–Meier curves of the time to colectomy in the two groups, illustrates the higher proportion of colectomies in the ciclosporin group.
FIGURE 6.
Kaplan–Meier plots showing the proportion remaining colectomy-free in the two groups over time (in days) in follow-up, censored at the end of follow-up on 28 February 2014.
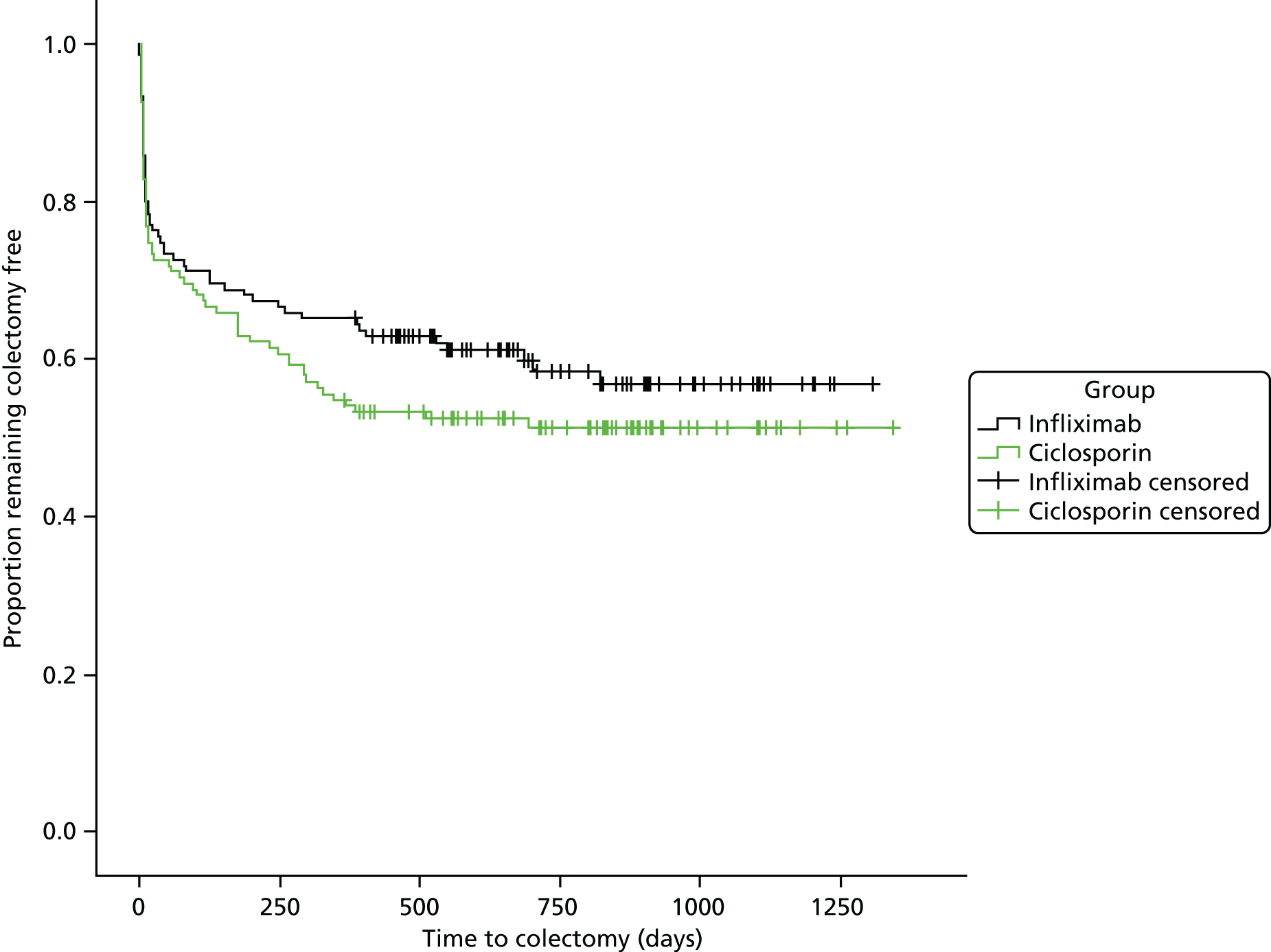
Proportions with serious adverse reactions and serious adverse events
Table 17 shows that the observed difference in the proportions of participants reporting a SAR in the two groups is not statistically significant; the same is also true for the proportions of participants reporting a SAE.
Post-randomisation length of stay
Figure 7a displays box plots of the post-randomisation length of stay data for the two groups, and, together with the numerical summaries in Table 17, illustrates the skewed nature of these values. Transformed values (based on taking natural logarithms) are shown in Figure 7b, which shows that the transformation has considerably reduced the original skewness. Formal analysis shows that, although differences in the raw data are deemed to be not statistically significant, the corresponding analysis of the transformed data leads to the contrary conclusion that differences on the logarithmic scale are statistically different. As assumptions underpinning statistical models are more appropriate to the analysis of the transformed data, we should not readily accept conclusions based on the analysis of the raw data: the evidence on the equality of post-randomisation lengths of stay is thus rather more nuanced than an initial examination indicates.
FIGURE 7.
Box plots of post-randomisation LOS for the two groups. (a) Post-randomisation LOS in days; and (b) natural logarithms (Ln) of post-randomisation LOS. LOS, length of stay.
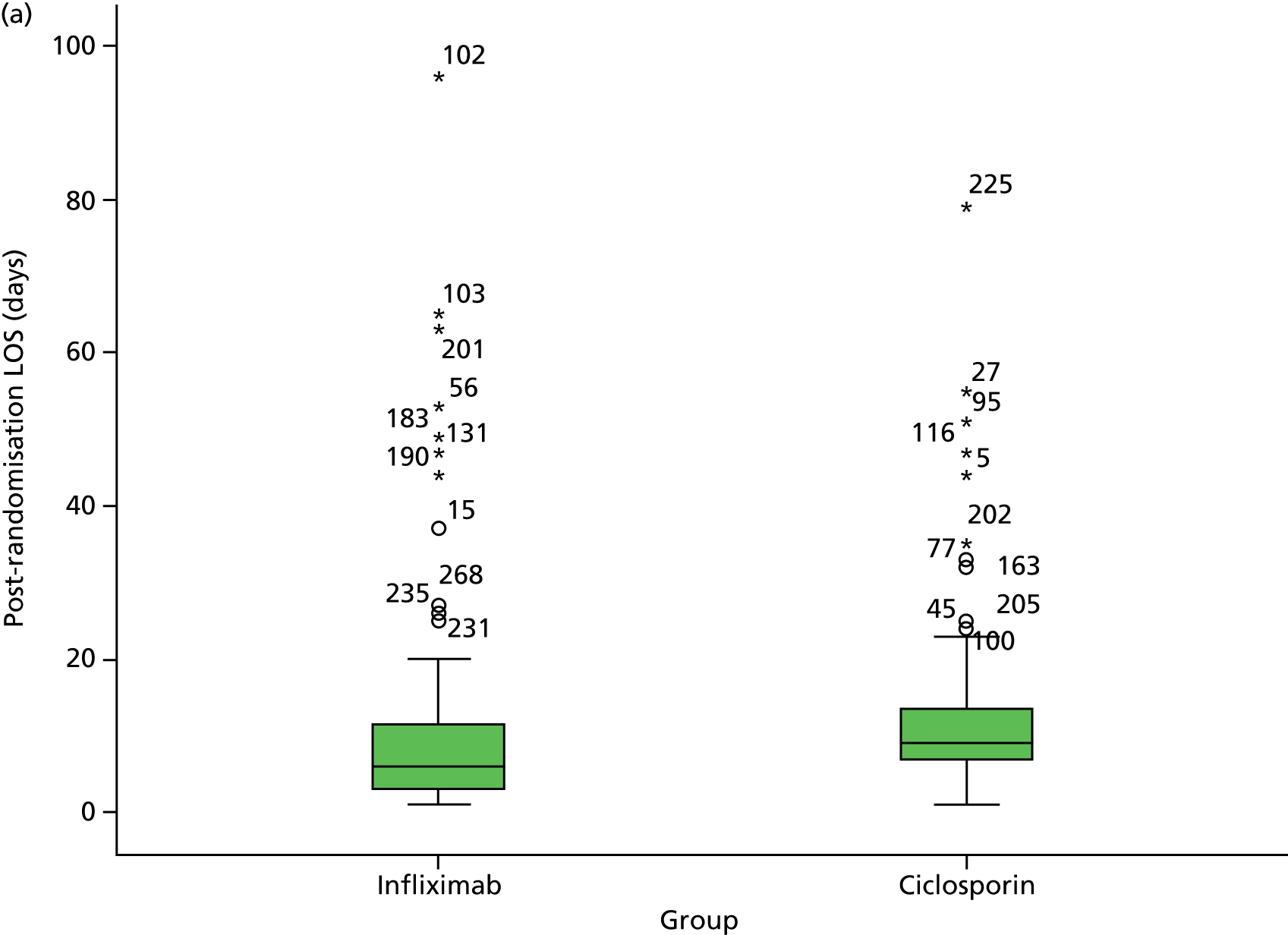
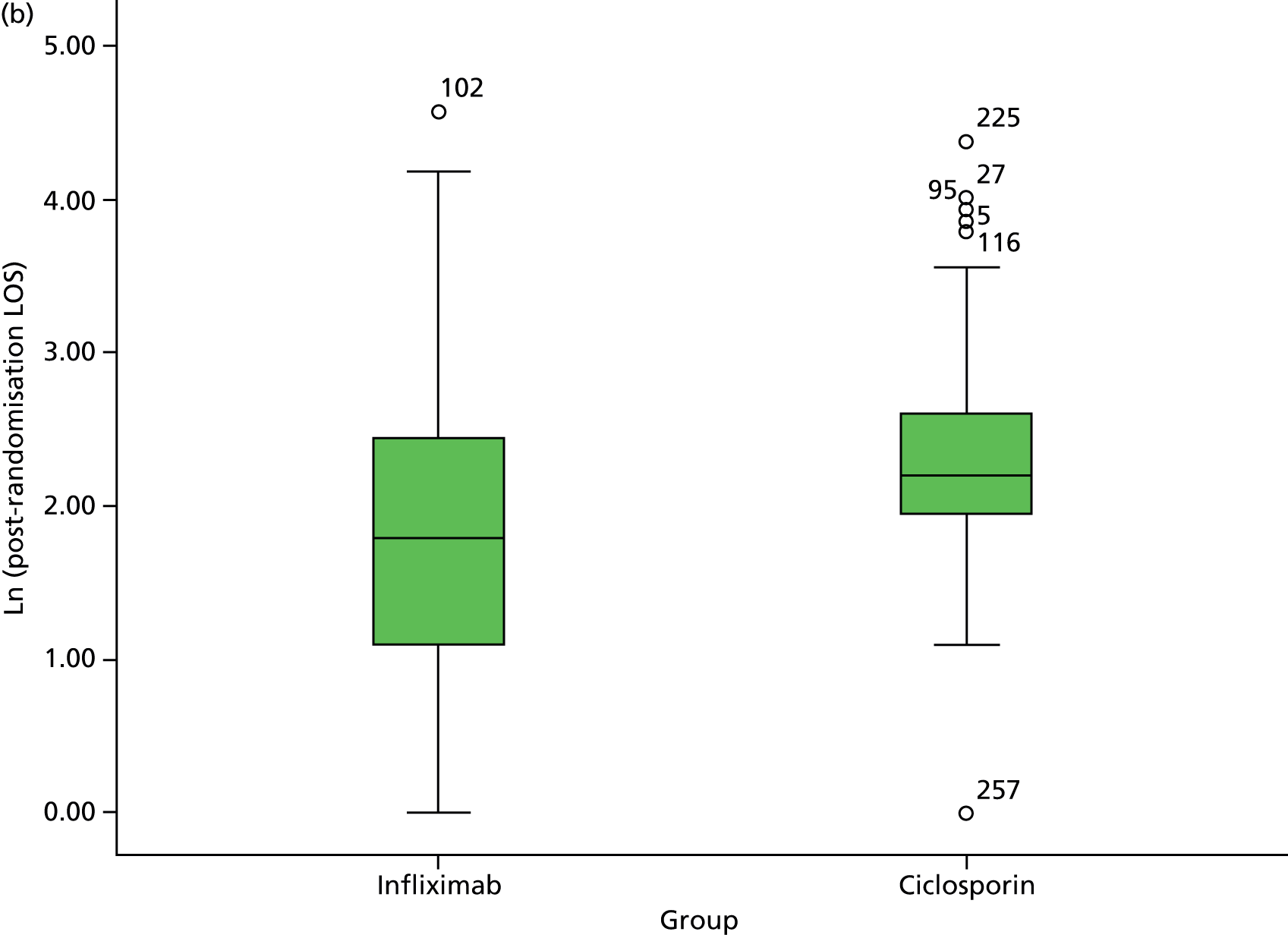
Quality-of-life profiles
Table 18 presents numerical summaries of the three QoL measures [namely CUCQ, Short Form Questinnaire-6 dimensions (SF-6D), EQ-5D] at the various stages in follow-up. As discussed elsewhere, these summaries include, for the CUCQ, values obtained from the CUCQ+ when the extended version of the questionnaire was administered to patients post colectomy, and treats these as pari passu with other CUCQ scores; however, the summaries do not include values generated via specific PCQs, as such questionnaires are not always readily associated with a specific time in follow-up. (For the avoidance of doubt, it may again here be emphasised that CUCQ+ scores obtained from PCQs are included in participants’ CUCQ profiles, and hence contribute to QAS and QAS per day.)
The profiles of the three QoL measures, shown in Figures 8 (CUCQ), 9 (SF-6D) and 10 (EQ-5D) are broadly very similar. They show an initial rise over 1 year from values at baseline followed by a levelling off; the profile for the two groups are also very similar to each other. Formal analysis, summarised in Table 18, confirms that the observed differences are not statistically significant.
FIGURE 8.
Mean CUCQ scores and 95% CI for the two groups at specific points in follow-up.
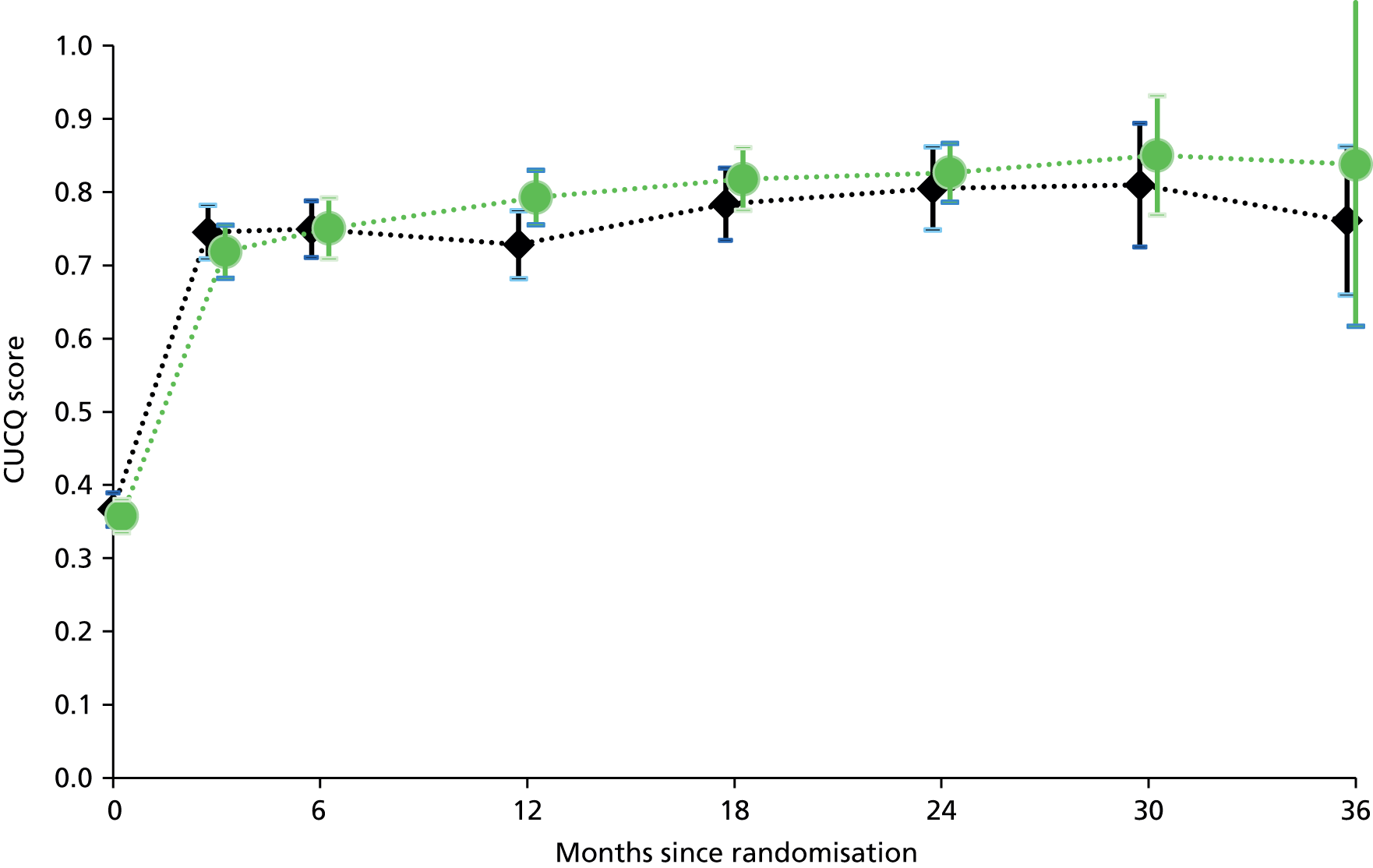
FIGURE 9.
Mean SF-6D values and 95% CI for the two groups at specific time points in follow-up.

FIGURE 10.
Mean EQ-5D values and 95% CI for the two groups at specific time points in follow-up.
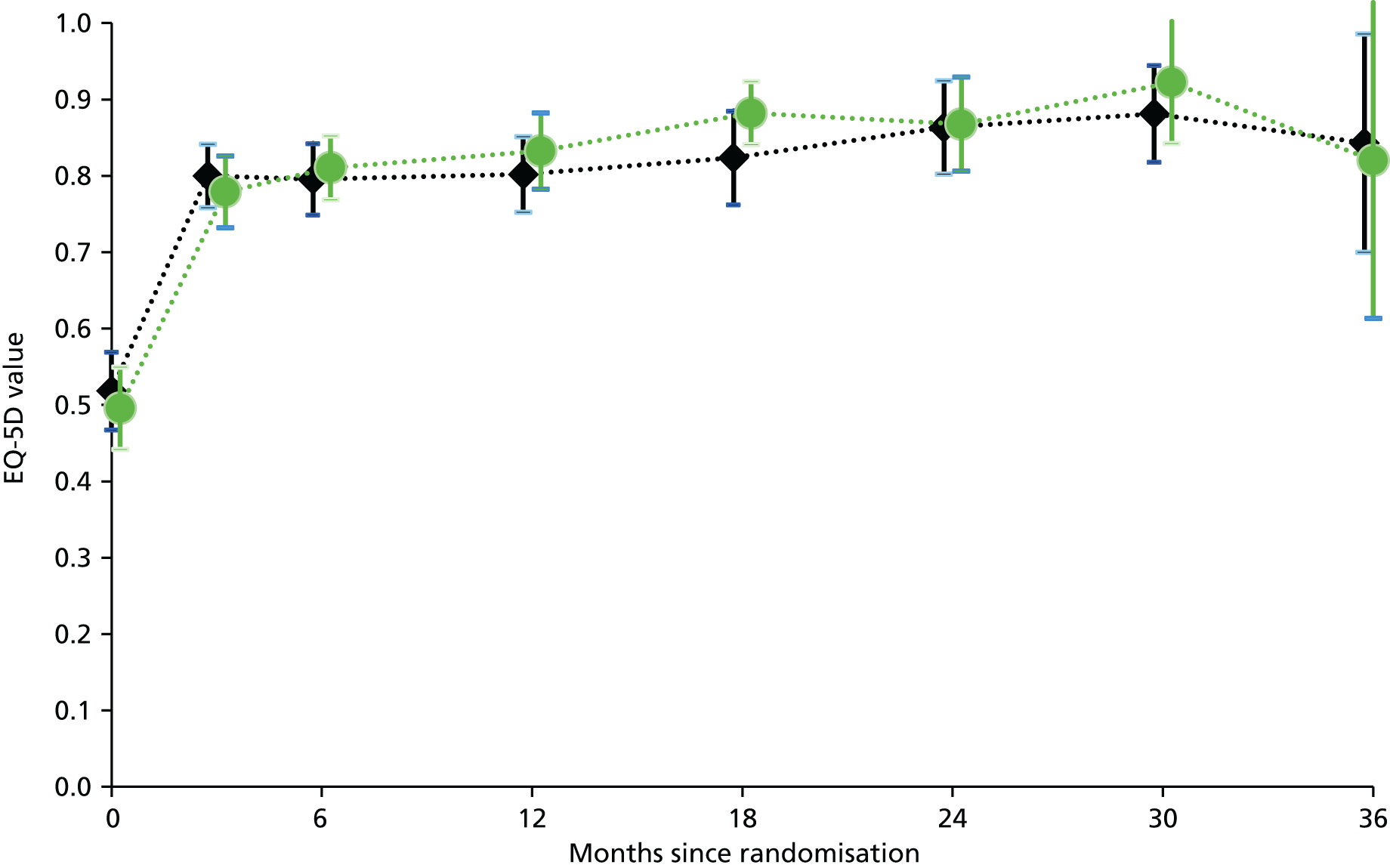
Adverse events
Tables 21–23 summarise the categories of AEs reported on each drug and the clinical system affected.
| Event | Infliximab | Ciclosporin |
|---|---|---|
| SUSAR | 0 | 0 |
| SAR | 16 | 10 |
| SAE total | 145 | 178 |
| SAE IBD related | 36 | 47 |
| SAE surgery related | 88 | 106 |
| SAE other | 21 | 25 |
| AR | 48 | 75 |
| AE | 91 | 91 |
| SAE | Infliximab | Ciclosporin |
|---|---|---|
| Infection | 8 | 16 |
| Gastrointestinal | 1 | 1 |
| Chest infection | 1 | 4 |
| Skin infection | 1 | 2 |
| Post surgical | 4 | 4 |
| UTI | 0 | 2 |
| Non-specific | 1 | 3 |
| Neurological | 1 | 0 |
| Gastrointestinal | 5 | 2 |
| Renal | 0 | 0 |
| Respiratory | 2 | 0 |
| Cardiovascular | 0 | 3 |
| Haematological | 0 | 0 |
| Psychiatric | 1 | 1 |
| Musculoskeletal | 1 | 1 |
| Venous thromboembolism | 1 | 1 |
| Other | 2 | 1 |
| SAR | Infliximab | Ciclosporin |
|---|---|---|
| Infection | 8 | 1 |
| C. diff | 1 | 0 |
| Chest infection | 3 | 0 |
| Skin infection | 0 | 1 |
| Post surgical | 1 | 0 |
| Others | 3 | 0 |
| Neurological | 2 | 3 |
| Gastrointestinal | 1 | 2 |
| Renal | 0 | 2 |
| Malignancya | 1 (colorectal cancer) | 1 (endometrial cancer) |
| Allergy/infusion reaction | 2 | 0 |
| Psychiatric | 1 | 0 |
| Respiratory | 1 | 0 |
| Hepatic | 0 | 1 |
| Others | 0 | 0 |
Overall, 16 patients who had infliximab and 17 patients who had ciclosporin reported 21 and 25 (respectively) SAEs that were not related to disease progression or surgery. Fourteen patients who had infliximab and nine patients who had ciclosporin had 16 and 10 (respectively) SARs. There was no statistical significant difference between the two drugs in relation to the total SAEs (p = 0.807) and SARs (p = 0.788).
Three participants died, all following infliximab. In two cases this was due to sepsis in the presence of multiple comorbidities (including diabetes mellitus), at 20 days (preoperative) and 65 days (postoperative) following randomisation. One participant died from disseminated colorectal cancer at 278 days. This case was unusual in that signet ring cells were identified on histology of the resected specimen, raising the possibility of a lung primary but the multidisciplinary team concluded that primary was colorectal.
Continuation of treatment
As this was a pragmatic trial, local PIs were able to continue treatment with the trial Investigational Medicinal Products (IMPs) at their discretion and to add immunosuppressive drugs. Figure 11 displays the continuation of infliximab and ciclosporin.
FIGURE 11.
Kaplan–Meier plot of the treatment duration (in days) for the two groups; there is no attempt to distinguish further subgroups with different reasons for discontinuation of treatment, nor is there any censoring for cases when the end of treatment is not recorded.
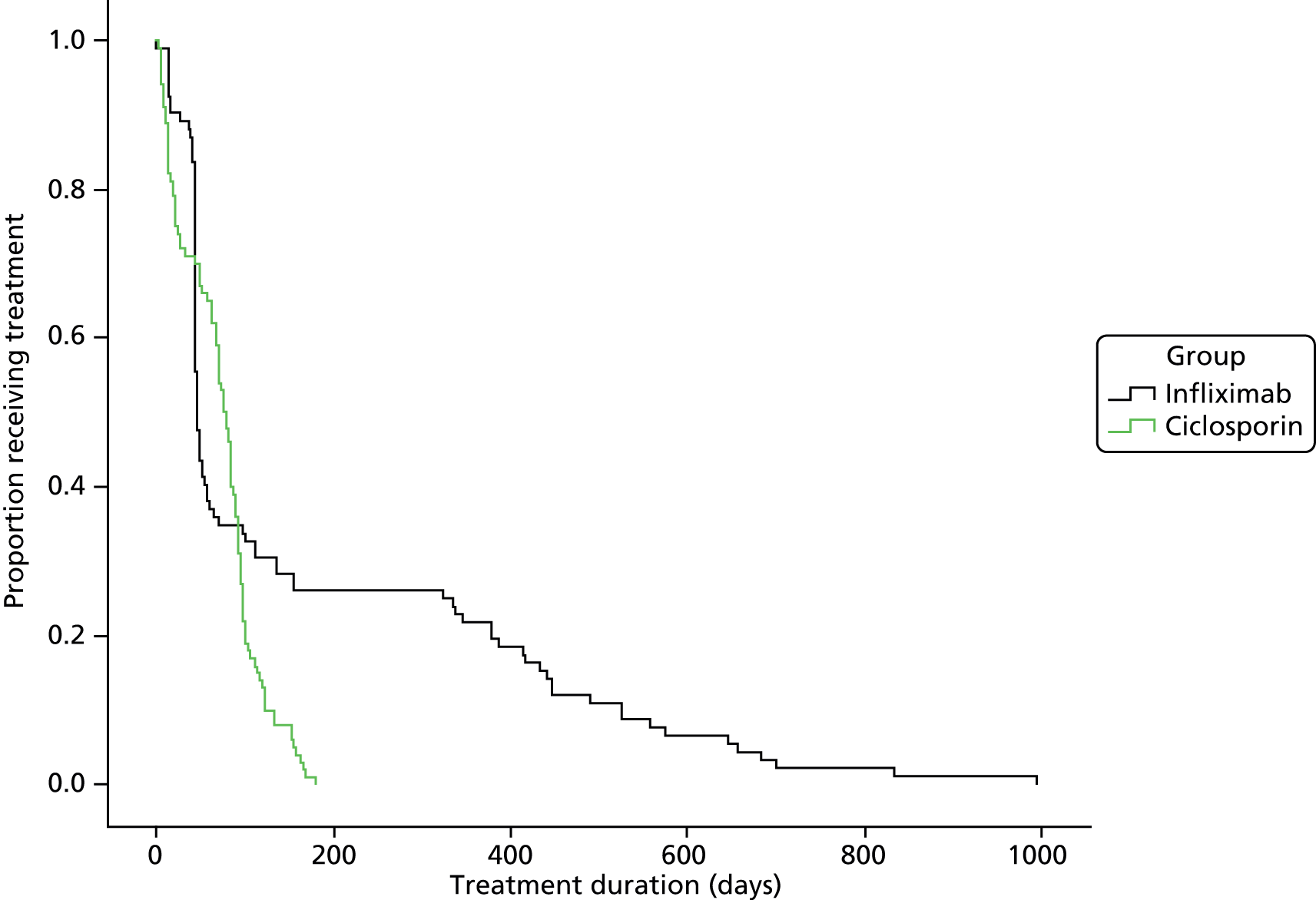
Tables 24–30 show the numbers of individual patients on immunosuppressants at baseline and following randomisation. Table 24 summarises the number of patients receiving immunosuppressants prescribed (azathiporine, 6-mercaptopurine, methotrexate) and Tables 25–30 break the figures down by individual drugs and those given in combination. No patient received more than two immunosuppressants at any one time. No significant differences have been identified. These data are derived from the patient-completed questionnaires at each time point.
| Period | Total | Infliximab, n (% within arm) | Ciclosporin, n (% within arm) |
|---|---|---|---|
| Pre baseline | 42 | 16 (11.9) | 26 (19.3) |
| 3 months | 122 | 56 (42.7) | 66 (49.3) |
| 6 months | 113 | 56 (42.7) | 57 (43.5) |
| 12 months | 84 | 39 (31.0) | 45 (36.3) |
| 18 months | 42 | 23 (24.0) | 19 (20.5) |
| 24 months | 38 | 18 29.5) | 20 (30.3) |
| 30 months | 19 | 10 (27.8) | 9 (23.1) |
| 36 months | 7 | 4 (23.5) | 3 (17.6) |
| Period | Total | Infliximab, n (% within arm) | Ciclosporin, n (% within arm) |
|---|---|---|---|
| Pre baseline | 28 | 14 (10.4) | 14 (10.4) |
| 3 months | 100 | 50 (38.2) | 50 (37.3) |
| 6 months | 80 | 40 (30.5) | 40 (30.5) |
| 12 months | 62 | 28 (22.2) | 34 (27.4) |
| 18 months | 31 | 16 (16.7) | 15 (15.8) |
| 24 months | 25 | 12 (19.7) | 13 (19.7) |
| 30 months | 14 | 8 (22.2) | 6 (15.4) |
| 36 months | 6 | 3 (17.6) | 3 (17.6) |
| Period | Total | Infliximab, n (% within arm) | Ciclosporin, n (% within arm) |
|---|---|---|---|
| Pre baseline | 8 | 2 (1.5) | 6 (4.4) |
| 3 months | 13 | 3 (2.3) | 10 (7.5) |
| 6 months | 24 | 15 (11.5) | 9 (6.9) |
| 12 months | 19 | 11 (8.7) | 8 (6.5) |
| 18 months | 9 | 6 (6.3) | 3 (3.2) |
| 24 months | 13 | 6 (9.8) | 7 (10.6) |
| 30 months | 5 | 2 (5.6) | 3 (7.7) |
| 36 months | 1 | 1 (5.9) | 0 |
| Period | Total | Infliximab, n (% within arm) | Ciclosporin, n (% within arm) |
|---|---|---|---|
| Pre baseline | 2 | 0 | 2 (1.5) |
| 3 months | 3 | 0 | 3 (2.2) |
| 6 months | 5 | 1 (0.8) | 4 (3.1) |
| 12 months | 3 | 0 | 3 (2.4) |
| 18 months | 1 | 0 | 1 (1.1) |
| 24 months | 0 | 0 | 0 |
| 30 months | 0 | 0 | 0 |
| 36 months | 0 | 0 | 0 |
| Period | Total | Infliximab | Ciclosporin |
|---|---|---|---|
| Pre baseline | 0 | 0 | 0 |
| 3 months | 2 | 1 | 1 |
| 6 months | 2 | 0 | 2 |
| 12 months | 0 | 0 | 0 |
| 18 months | 0 | 0 | 0 |
| 24 months | 0 | 0 | 0 |
| 30 months | 0 | 0 | 0 |
| 36 months | 0 | 0 | 0 |
| Period | Total | Infliximab | Ciclosporin |
|---|---|---|---|
| Pre baseline | 4 | 0 | 4 |
| 3 months | 1 | 0 | 1 |
| 6 months | 1 | 0 | 1 |
| 12 months | 0 | 0 | 0 |
| 18 months | 1 | 1 | 0 |
| 24 months | 0 | 0 | 0 |
| 30 months | 0 | 0 | 0 |
| 36 months | 0 | 0 | 0 |
| Period | Total | Infliximab | Ciclosporin |
|---|---|---|---|
| Pre baseline | 0 | 0 | 0 |
| 3 months | 3 | 2 | 1 |
| 6 months | 0 | 0 | 0 |
| 12 months | 0 | 0 | 0 |
| 18 months | 0 | 0 | 0 |
| 24 months | 0 | 0 | 0 |
| 30 months | 0 | 0 | 0 |
| 36 months | 0 | 0 | 0 |
Cost-effectiveness
Analysis of missing data
Table 31 reports the number of observations missing at each data collection time between the two arms of the trial.
| Resource item | Baseline to 3 months | 6 months | 12 months | 18 months | 24 months | 30 months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infliximab | Ciclosporin | Infliximab | Ciclosporin | Infliximab | Ciclosporin | Infliximab | Ciclosporin | Infliximab | Ciclosporin | Infliximab | Ciclosporin | |
| Trial drug administration | 41 (30) | 24 (18) | – | – | – | – | – | – | – | – | – | – |
| Number of subsequent infusions of infliximab | 0 | n/a | – | – | – | – | – | – | – | – | – | – |
| Number of patients on oral ciclosporin | n/a | 2 | – | – | – | – | – | – | – | – | – | – |
| Tests/investigations/drugs in hospital CRF | 2 | 1 | 1 | 6 | 7 | 6 | 16 (12) | 18 (13) | 5 | 2 | 5 | 12 (9) |
| NHS resource-use PFQ | 26 (20) | 27 (20) | 27 (21) | 29 (22) | 43 (34) | 36 (30) | 30 (37) | 30 (43) | 25 (41) | 28 (43) | 8 (30) | 17 (60) |
The amount of missing data was greater for items reported on the PFQ which was completed by the participant as compared with the CRF which was completed by the research nurse. Participants who withdrew from the study were more likely to withdraw only from responding to further questionnaires (PFQ) than from accessing their medical records (CRF) leading to more missing data from the PFQ.
Data on trial drug administration costs were missing for 41 infliximab and 24 ciclosporin participants. Data on oral ciclosporin were missing for two participants who had completed the protocol single infusion.
We used age, gender, study arm, ethnicity, smoking, weight, and baseline SF-6D, EQ-5D and CUCQ scores to assess randomness of missing data.
For CRF data, the pattern of missing data on tests and investigations and drugs delivered to inpatients was studied at 18, 24 and 30 months and no significance was shown.
At 3 months, the pattern of missing PFQ data showed statistical significance for gender, with 41 males (25%) and 12 females (12%) not providing responses (p = 0.017). At 12 months, younger people (mean age 36.35 years, SD 14.59 years) were less likely to provide responses than the rest of the cohort (mean age 40.44 years, SD 15.85 years) (p = 0.045). This was also the case at 24 months, younger people (mean age 36.89 years, SD 14.10 years) versus the rest of the cohort (mean age 43.52 years, SD 14.56 years) (p = 0.012).
At each follow-up point, however, missing data were evenly distributed between study arms, suggesting that the imputation process adopted should not have led to any estimation bias.
Comparison of costs
For cost comparisons, we analysed data on all participants for whom we had cost data for each follow-up period. In these comparisons, we performed tests for statistical significance independently for each period, owing to the different sample sizes at each time point. For the incremental cost-effectiveness analysis we analysed cost data for those participants for whom we had effectiveness data and summed total costs over the whole of the follow-up period.
Trial drug costs
A total of 42 of 52 of sites that recruited participants to the RCT responded to our survey regarding preparation and delivery of trial drugs. Pharmacists prepared infliximab infusions at 11 sites and prepared ciclosporin infusions at seven sites. All other infusions were prepared by nurses. For infliximab, the mean administration costs were £17.66 (SD £25.05) per infusion. For ciclosporin they were £35.41 (SD £28.48) per day during which the infusion was received.
Eighty-eight infliximab participants received the three infusions specified in the protocol; 98 received two infusions and 134 received a single infusion. Thirty-four participants received additional infusions (minimum = 1, maximum = 13) with a mean of 1.20 (SD 2.71) additional infusions.
One hundred and thirty-two ciclosporin participants received the single infusion specified in the protocol with a mean (SD) infusion duration of 5.0 (SD 1.8) days. Of these, 100 were switched to oral following their single infusion with a mean duration on oral ciclosporin of 52.0 (SD 48.8) days.
Table 32 shows the mean costs of treatment with the two trial drugs. As most participants on oral ciclosporin completed their treatment within 3 months, all oral ciclosporin costs have been attributed to the first 3 months. Infliximab is clearly the more costly treatment with a mean cost in the first 3 months of £4823 (SD £2199) compared with £880 (SD £649) for ciclosporin and with costs for additional infliximab infusions continuing beyond this period.
| Follow-up period (months) | Ciclosporin | Infliximab | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) cost (£) | 95% CI (£)a | n | Mean (SD) cost (£) | 95% CI (£)a | |
| 0–3b | 135 | 880 (649) | 569 to 734 | 135 | 4823 (2199) | 4464 to 5181 |
| 4–6 | – | – | – | 131 | 638 (1474) | 399 to 902 |
| 7–12 | – | – | – | 131 | 837 (2179) | 486 to 1232 |
| 13–18 | – | – | – | 84 | 773 (1942) | 385 to 1211 |
| 19–24 | – | – | – | 65 | 337 (1450) | 37 to 693 |
| 25–30 | – | – | – | 27 | 175 (907) | 0 to 510 |
| Total (£) | 118,800 | 935,837 | ||||
Baseline spell in hospital: length of stay
All participants were recruited and randomised while inpatients. Mean length of stay of this baseline spell in hospital (i.e. from randomisation to discharge) was slightly higher for ciclosporin participants but the difference was not statistically significant: ciclosporin 12.21 (SD 10.18) days, infliximab 10.32 (SD 13.55) days (95% CI –1.06 to 4.69 days; p = 0.20) as shown in Table 33.
| Participants | Ciclosporin | Infliximab | Mean difference (ciclosporin – infliximab) (days) | 95% CIa (days) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) (days) | n | Mean (SD) (days) | Lower | Upper | |||
| All | 135 | 12.21 (10.18) | 135 | 10.32 (13.55) | 1.90 | –1.06 | 4.69 | 0.20 |
| No surgery | 101 | 9.13 (5.45) | 107 | 7.02 (11.07) | 2.11 | –0.34 | 4.17 | 0.08 |
| Surgery | 34 | 21.38 (14.65) | 28 | 22.89 (14.90) | –1.55 | –9.03 | 5.91 | 0.68 |
Twenty-eight participants in the infliximab arm (20.7%) had surgery during the baseline spell compared with 34 in the ciclosporin arm (25.2%). The mean length of stay for those who had surgery was similar between groups: infliximab 22.89 (SD 14.90) days versus ciclosporin 21.38 (SD 14.65) days (95% CI –9.03 to 5.91 days; p = 0.68).
A total of 107 participants in the infliximab arm (79.2%) were discharged from their baseline spell in hospital without having surgery compared with 101 in the ciclosporin arm (74.8%). For those who did not have surgery, the mean length of stay was higher for ciclosporin participants at 9.13 (SD 5.45) days versus 7.02 (SD 11.07) days for infliximab, although the difference was not statistically significant (95% CI –9.03 to 5.91 days; p = 0.08).
Cost of hospitalisation without surgery
The cost of the baseline spell in hospital for participants who did not have surgery is shown in Table 34, which also shows costs for all subsequent non-surgical hospital admissions. The mean differences were not statistically significant in any period.
| Follow-up period | Ciclosporin | Infliximab | Mean difference (ciclosporin – infliximab) (£) | 95% CIa | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Admitted, n | Sample, n | Mean (£) | SD (£) | Admitted, n | Sample, n | Mean (£) | SD (£) | Lower (£) | Upper (£) | |||
| Baseline spell, no surgery | 101 | 135 | 2124 | 1691 | 107 | 135 | 1796 | 2590 | 328 | –201 | 793 | 0.220 |
| End of baseline spell to 3 months | 24 | 135 | 890 | 2413 | 30 | 135 | 941 | 2729 | –52 | –662 | 538 | 0.870 |
| 4–6 months | 23 | 133 | 604 | 1677 | 14 | 131 | 365 | 1330 | 238 | –126 | 614 | 0.201 |
| 7–12 months | 32 | 133 | 927 | 2377 | 30 | 131 | 1094 | 2761 | –167 | –778 | 425 | 0.598 |
| 13–18 months | 13 | 85 | 464 | 1326 | 13 | 84 | 589 | 1708 | –125 | –592 | 312 | 0.596 |
| 19–24 months | 10 | 71 | 740 | 2508 | 14 | 65 | 571 | 1299 | 169 | –463 | 910 | 0.618 |
| 25–30 months | 1 | 30 | 129 | 705 | 3 | 27 | 315 | 1061 | 186 | –688b | 266b,c | 0.445 |
| Total (£) | 706,303 | 655,851 | ||||||||||
Cost of hospitalisation with surgery
Table 35 shows the number of participants who had colectomies or other surgical procedures by follow-up period. None of the differences was statistically significant (chi-squared test).
| Follow-up period | Ciclosporin (n = 135) | Infliximab (n = 135) | p-valuea | ||
|---|---|---|---|---|---|
| Colectomy, n | Other procedures, n | Colectomy, n | Other procedure, n | ||
| Baseline spell | 34 | – | 28 | – | 0.235 |
| Discharge to 3 months | 8 | 1 | 10 | 1 | 0.533 |
| 4–6 months | 9 | 3 | 4 | 4 | 0.356 |
| 7–12 months | 11 | 13 | 5 | 13 | 0.239 |
| 13–18 months | 2 | 11 | 5 | 7 | 0.169 |
| 19–24 months | 1 | 67 | 2 | 5 | 0.279 |
| 25–30 months | 0 | 1 | 1 | 3 | 0.460 |
Table 36 shows the costs of surgical admissions at each follow-up point. There were no statistically significant differences between groups at any point.
| Follow-up period | Ciclosporin | Infliximab | Mean difference (ciclosporin – infliximab) (£) | 95% CIa | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (£) | SD (£) | n | Mean (£) | SD (£) | Lower (£) | Upper (£) | |||
| Baseline spell | ||||||||||
| Colectomy | 135 | 2432 | 4640 | 135 | 2074 | 4451 | 358 | –749 | 1466 | 0.519 |
| Discharge to 3 months | ||||||||||
| Colectomy | 135 | 354 | 1535 | 135 | 525 | 2195 | –170 | –642 | 253 | 0.461 |
| Other | 135 | 18 | 205 | 135 | 5 | 53 | 13 | –13b,c | 58c | 0.474 |
| 4–6 months | ||||||||||
| Colectomy | 133 | 348 | 1446 | 131 | 170 | 1005 | 178 | –123 | 494 | 0.247 |
| Other | 133 | 0 | 0 | 131 | 8 | 88 | –8 | –29b,d | 7d | 0.319 |
| 7–12 months | ||||||||||
| Colectomy | 133 | 439 | 1542 | 131 | 214 | 1105 | 226 | –95 | 562 | 0.172 |
| Other | 133 | 589 | 2415 | 131 | 656 | 2665 | –67 | –694 | 546 | 0.830 |
| 13–18 months | ||||||||||
| Colectomy | 85 | 46 | 425 | 84 | 204 | 967 | –158 | –402b,e | 47e | 0.173 |
| Other | 85 | 435 | 1332 | 84 | 526 | 2329 | –91 | –700 | 420 | 0.757 |
| 19–24 months | ||||||||||
| Colectomy | 71 | 204 | 1721 | 65 | 110 | 648 | 94 | –246b,f | 604f | 0.670 |
| Other | 71 | 590 | 2555 | 65 | 281 | 1037 | 309 | –278 | 1059 | 0.350 |
| 25–30 months | ||||||||||
| Colectomy | 30 | 0 | 0 | 27 | 289 | 1501 | –289 | –1076b,g | 217g | 0.327 |
| Other | 30 | 192 | 1051 | 27 | 234 | 677 | –42 | –444b,h | 457h | 0.856 |
| Total (£) | ||||||||||
| Colectomy | 499,175 | 433,258 | ||||||||
| Other | 165,392 | 156,426 | ||||||||
NHS resource use
The costs of NHS resource use in the 3 months prior to baseline are shown in Table 37. There were no statistically significant differences across individual resource items apart from prescription drugs in which the mean cost for ciclosporin participants was significantly higher: £224 (SD £223) versus £166 (SD £185), mean difference £58 (95% CI £9 to £107; p = 0.020). Total costs were not significantly different with a mean cost for ciclosporin participants of £1115 (SD £1291) versus with £1034 (SD £1047), mean difference £80 (95% CI –£194 to £352; p = 0.573).
| NHS service | Ciclosporin | Infliximab | Mean difference (ciclosporin – infliximab) (£) | 95% CIa | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (£) | SD (£) | n | Mean (£) | SD (£) | Lower (£) | Upper (£) | |||
| GP surgery visits | 135 | 119 | 119 | 134 | 124 | 120 | –5 | –34 | 25 | 0.761 |
| Home visits | 135 | 14 | 56 | 134 | 20 | 61 | –6 | –20 | 8 | 0.378 |
| Phone calls | 135 | 46 | 89 | 134 | 34 | 46 | 12 | –5 | 31 | 0.166 |
| A&E attendances | 135 | 41 | 82 | 135 | 50 | 99 | –8 | –30 | 13 | 0.453 |
| Clinic visits | 135 | 210 | 256 | 135 | 168 | 313 | 42 | –24 | 104 | 0.229 |
| Hospitalisation | 135 | 380 | 882 | 135 | 554 | 1128 | –174 | –411 | 55 | 0.159 |
| Prescription drugs | 135 | 224 | 223 | 135 | 166 | 185 | 58 | 9 | 107 | 0.020 |
| Total | 135 | 1115 | 1291 | 135 | 1034 | 1047 | 80 | –194 | 352 | 0.573 |
The total costs of all NHS contacts (as itemised above) are shown in Table 38. Table 38 also shows the costs of prescribed drugs other than the trial drugs given to participants as inpatients and by prescription in the community, and the costs of tests and investigations. There were no statistically significant differences between arms for any items during any period.
| Follow-up period (months) | Ciclosporin | Infliximab | Mean difference (ciclosporin – infliximab) (£) | 95% CIb | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (£) | SD (£) | n | Mean (£) | SD (£) | Lower (£) | Upper (£) | |||
| Cost of all NHS contacts | ||||||||||
| 0–3 | 105 | 204 | 397 | 105 | 165 | 363 | 40 | –62 | 137 | 0.455 |
| 4–6 | 99 | 86 | 98 | 104 | 93 | 116 | –8 | –36 | 21 | 0.609 |
| 7–12 | 85 | 80 | 110 | 82 | 77 | 110 | 3 | –30 | 35 | 0.871 |
| 13–18 | 40 | 69 | 96 | 50 | 72 | 89 | –3 | –41 | 36 | 0.871 |
| 19–24 | 37 | 55 | 75 | 36 | 64 | 81 | –9 | –46 | 28 | 0.627 |
| 25–30 | 11 | 26 | 44 | 18 | 29 | 42 | –4 | –35 | 32 | 0.826 |
| Total (£) | 41,815 | 39,737 | ||||||||
| Cost of prescribed drugs (hospital inpatient) | ||||||||||
| 0–3 | 133 | 66 | 157 | 131 | 61 | 141 | 6 | –30 | 43 | 0.757 |
| 4–6 | 127 | 11 | 43 | 130 | 7 | 32 | 4 | –5 | 14 | 0.392 |
| 7–12 | 127 | 19 | 99 | 124 | 10 | 37 | 9 | –8 | 33 | 0.350 |
| 13–18 | 67 | 9 | 39 | 68 | 18 | 100 | –9 | –34 | 10 | 0.509 |
| 19–24 | 69 | 35 | 193 | 60 | 9 | 29 | 26 | –11 | 84 | 0.278 |
| 25–30 | 18 | < 1 | < 1 | 22 | 9 | 29 | –9 | –23c | < 1c | 0.166 |
| Total (£) | 15,606 | 12,103 | ||||||||
| Cost of prescribed drugs (community) | ||||||||||
| 0–3 | 105 | 215 | 223 | 105 | 168 | 168 | 47 | –6 | 100 | 0.086 |
| 4–6 | 100 | 186 | 221 | 104 | 165 | 187 | 21 | –34 | 78 | 0.459 |
| 7–12 | 85 | 150 | 188 | 82 | 164 | 190 | 14 | –69 | 43 | 0.628 |
| 13–18 | 40 | 138 | 128 | 50 | 194 | 203 | –57 | –125 | 10 | 0.110 |
| 19–24 | 37 | 136 | 188 | 36 | 196 | 184 | –60 | –146 | 26 | 0.170 |
| 25–30 | 11 | 155 | 158 | 18 | 134 | 175 | 20 | –102 | 142 | 0.745 |
| Total (£) | 66,182 | 67,416 | ||||||||
| Costs of clinical tests and investigations | ||||||||||
| 0–3 | 134 | 676 | 705 | 133 | 684 | 744 | –8 | –179 | 164 | 0.927 |
| 4–6 | 127 | 255 | 434 | 130 | 193 | 357 | 63 | –33 | 161 | 0.209 |
| 7–12 | 127 | 388 | 653 | 124 | 346 | 550 | 42 | –106 | 194 | 0.581 |
| 13–18 | 67 | 170 | 296 | 68 | 286 | 485 | –117 | –254 | 12 | 0.093 |
| 19–24 | 69 | 312 | 715 | 60 | 223 | 466 | 89 | –111 | 311 | 0.399 |
| 25–30 | 18 | 140 | 259 | 22 | 236 | 425 | –96 | –323 | 109 | 0.384 |
| Total (£) | 207,683 | 196,986 | ||||||||
| Costs of clinic visits | ||||||||||
| 0–3 | 134 | 408 | 351 | 134 | 416 | 274 | –8 | –82 | 66 | 0.834 |
| 4–6 | 130 | 285 | 279 | 131 | 259 | 238 | 26 | –39 | 92 | 0.422 |
| 7–12 | 125 | 387 | 400 | 123 | 378 | 386 | 10 | –84 | 102 | 0.856 |
| 13–18 | 67 | 371 | 421 | 68 | 334 | 300 | 38 | –86 | 165 | 0.556 |
| 19–24 | 67 | 355 | 417 | 60 | 286 | 363 | 69 | –65 | 204 | 0.322 |
| 25–30 | 18 | 240 | 194 | 22 | 319 | 316 | –79 | –244 | 66 | 0.338 |
| Total (£) | 193,059 | 183,057 | ||||||||
| Costs of A&E attendances | ||||||||||
| 0–3 | 134 | 29 | 82 | 134 | 30 | 77 | 1 | –20 | 19 | 0.932 |
| 4–6 | 130 | 25 | 90 | 131 | 25 | 76 | 0 | –20 | 21 | 0.985 |
| 7–12 | 125 | 38 | 108 | 123 | 36 | 108 | 2 | –24 | 28 | 0.877 |
| 13–18 | 67 | 12 | 48 | 68 | 21 | 76 | –10 | –32 | 9 | 0.375 |
| 19–24 | 67 | 22 | 62 | 60 | 21 | 70 | 1 | –22 | 23 | 0.919 |
| 25–30 | 18 | 6 | 26 | 22 | 15 | 72 | –9 | –48d | 17 | 0.588 |
| Total (£) | 14,272 | 14,741 | ||||||||
Incremental cost-effectiveness
Results relate to a ‘within-trial’ cost-effectiveness analysis using primary data collected within the period of study and without lifetime extrapolation. Base-case costs and QALYs to be reported below cover 30 months adjusted by the number of days over which each participant contributed data. Table 39 shows that the mean number of days that ciclosporin participants contributed CRF data was slightly higher than for infliximab participants: 645.9 (SD 204.8) days versus 623.7 (SD 224.0) days, but the mean difference of 22.2 days was not statistically significant (95% CI –32.2 to 77.4 days; p = 0.433). A similar pattern was shown for PFQ data: 673.0 (SD 226.0) days versus 653.2 (SD 224.8) days, mean difference 19.7 days (95% CI –36.8 to 76.5 days; p = 0.509).
| Data collection method: recruitment to 30 months | Ciclosporin | Infliximab | Mean difference (ciclosporin – infliximab) (days) | 95% CI | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (days) | SD (days) | n | Mean (days) | SD (days) | Lower (days) | Upper (days) | |||
| CRF | 113 | 645.92 | 204.80 | 117 | 623.70 | 223.95 | 22.22 | –32.23 | 77.36 | 0.433 |
| PFQ | 113 | 672.89 | 226.03 | 117 | 653.21 | 224.84 | 19.67 | –36.83 | 76.54 | 0.509 |
Base-case costs and QALYs are shown in Table 40. The mean difference in QALYs was 0.023 in favour of ciclosporin, but this is not statistically significant (95% CI –0.053 to 0.101; p = 0.563). The mean difference in costs was –£5632 in favour of ciclosporin and is statistically significant (95% CI –8348 to –2880; p < 0.001).
| QALYs and costs | Ciclosporin | Infliximab | Mean difference (ciclosporin – infliximab) | 95% CIa | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Lower | Upper | |||
| QALYs | 113 | 1.921 | 0.29 | 117 | 1.898 | 0.31 | 0.023 | –0.053 | 0.101 | 0.563 |
| Costs (£) | 113 | 14,609 | 10,838 | 117 | 20,241 | 10,433 | –5632 | –8348 | –2880 | 0.000 |
The cost-effectiveness plane representing 5000 bootstrap replications is shown in Figure 12. Most observations are in the south-east quadrant suggesting that ciclosporin dominates infliximab.
FIGURE 12.
Base-case (30-month) cost-effectiveness plane adjusted for by participants’ length of time in study.
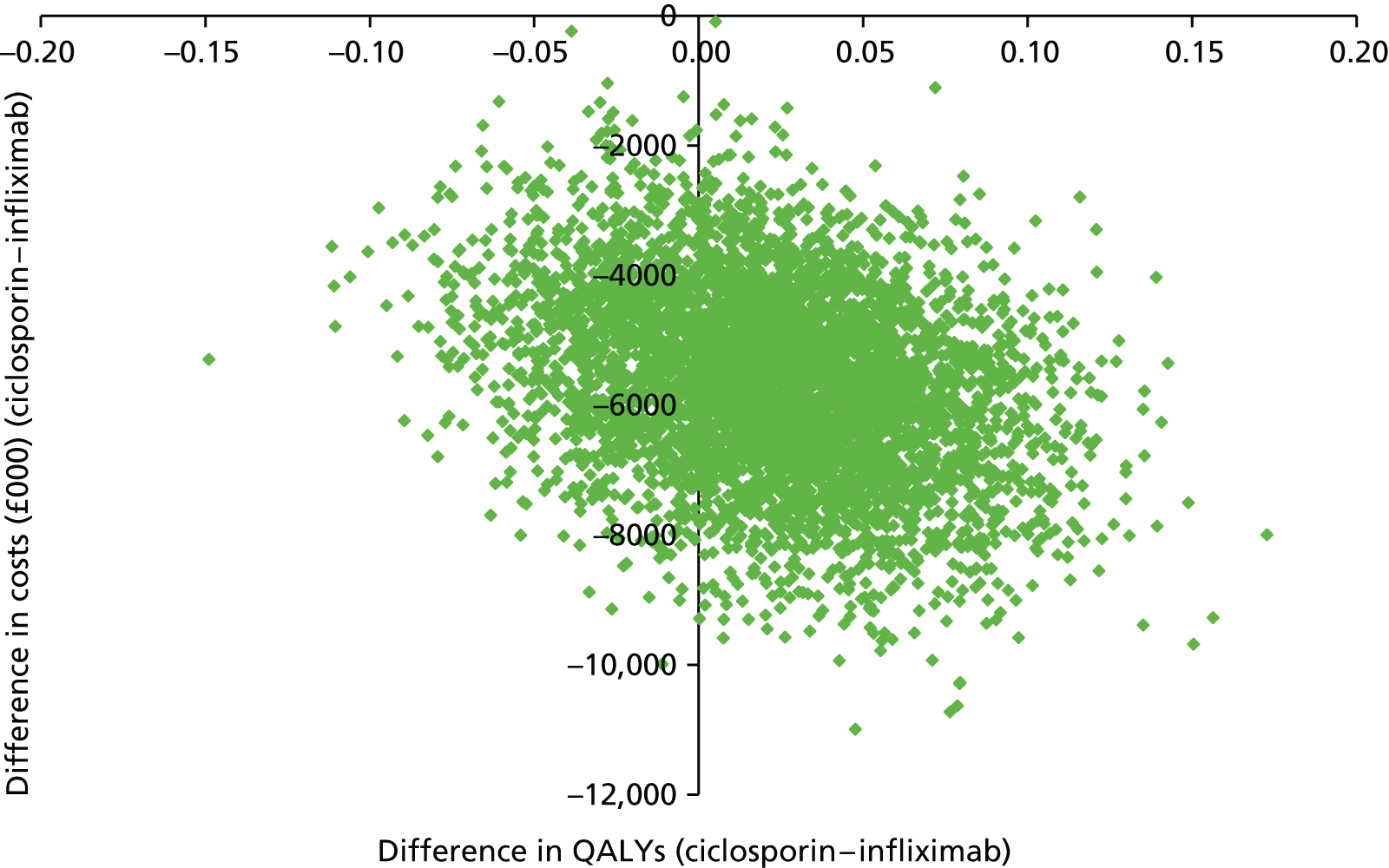
The CEAC shown in Figure 13 shows ciclosporin to have a 73–74% probability of being cost-effective over virtually all willingness-to-pay thresholds. The almost horizontal curve is due to the difference in effects being close to zero. 59,98
FIGURE 13.
Base-case (30-month) CEAC adjusted by participants’ length of time in study.
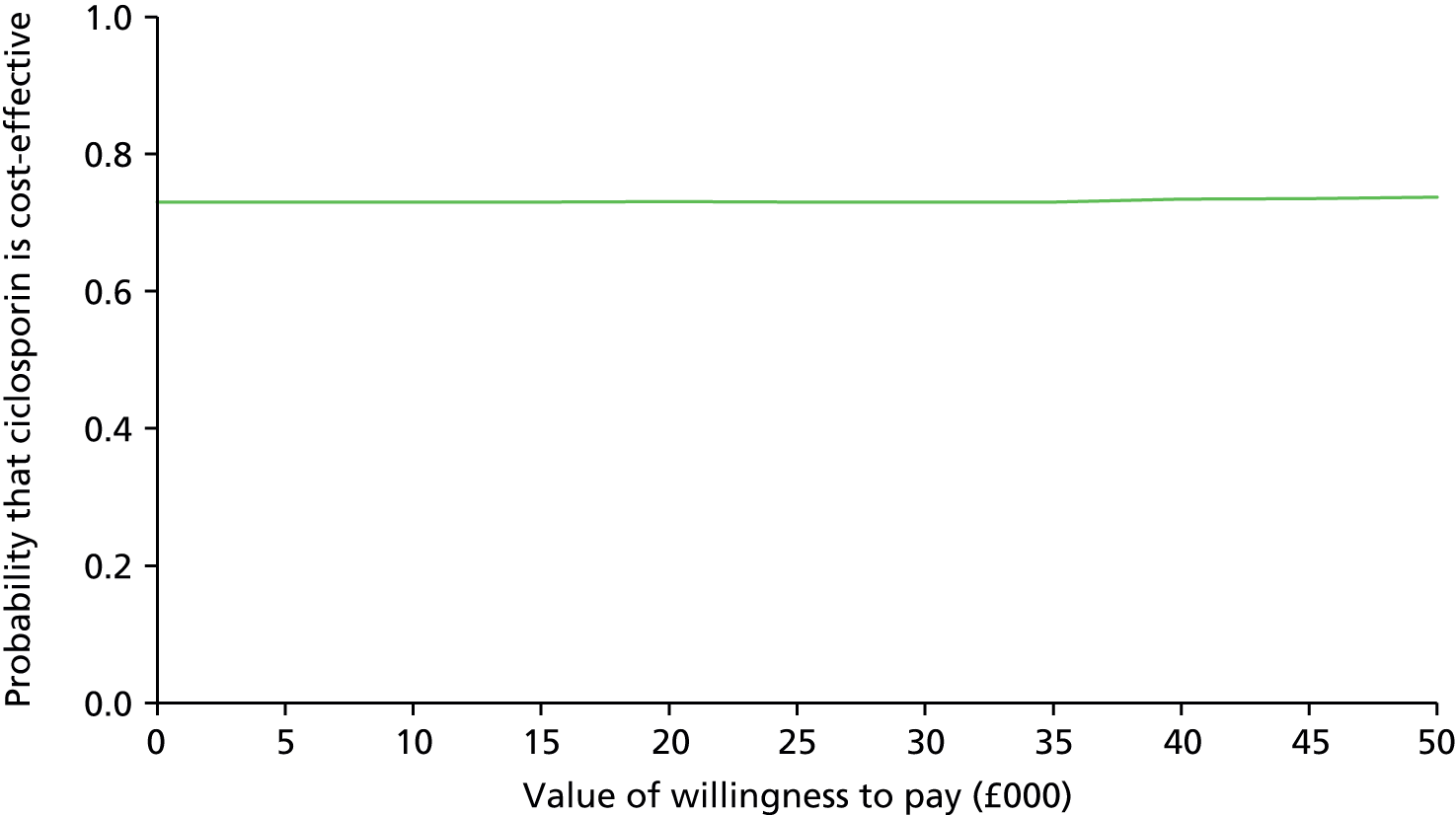
Table 41 shows base-case costs and QALYs over 30 months further adjusted using baseline EQ-5D and CUCQ scores and participants’ weight as covariates for QALYs and baseline cost as a covariate for costs. The mean difference in QALYs remains in favour of ciclosporin but is slightly reduced by 0.002 to 0.021 QALYs, which is not statistically significant (95% CI – 0.032 to 0.096; p = 0.35). The mean difference in costs (–£5632) is unchanged and is statistically significant (95% CI –£8305 to –2773; p < 0.001).
| QALYs and costs | Ciclosporin | Infliximab | Mean difference (ciclosporin – infliximab) | 95% CIa | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Lower | Upper | |||
| QALYs | 113 | 1.921 | 0.18 | 117 | 1.900 | 0.16 | 0.021 | –0.032 | 0.096 | 0.350 |
| Costs (£) | 113 | 14,609 | 593 | 117 | 20,241 | 695 | –5632 | –8305 | –2773 | 0.000 |
The cost-effectiveness plane is shown in Figure 14. The decrease in spread is due to reduced standard errors. Ciclosporin continues to dominate infliximab with the CEAC (Figure 15) showing ciclosporin to have an 85% chance of being cost-effective over all willingness-to-pay thresholds.
FIGURE 14.
Base-case (30-month) cost-effectiveness plane weighted for participants’ time in study and adjusted for baseline covariates.

FIGURE 15.
Base-case (30-month) CEAC weighted for participants’ time in study and adjusted for baseline covariates.
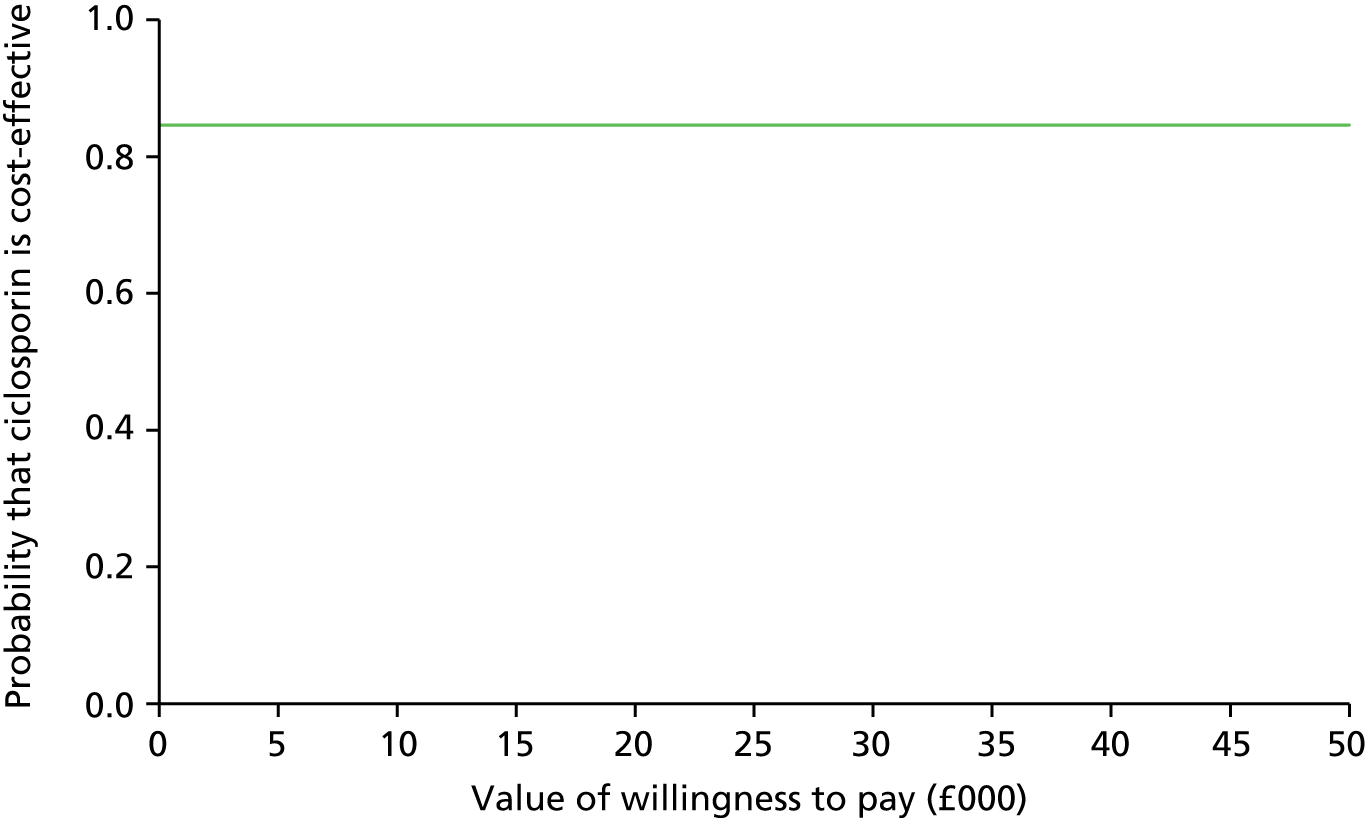
Sensitivity analyses
Table 42 below shows results at 12 and 24 months which are similar to the base case, in that the differences in QALYs at both time points are in favour of ciclosporin and are small (0.027 and 0.022 QALYs at 12 and 24 months, respectively). The mean difference at 12 months is marginally statistically significant (p = 0.049), but not at 24 months (p = 0.479). Mean differences in costs in both cases are again in favour of ciclosporin (–£4568 and –£4498 and are statistically significant; 12 months (p < 0.001), 24 months (p < 0.001). These two sensitivity analyses are consistent with the base case showing ciclosporin to dominate infliximab.
| QALYs and costs | Ciclosporin | Infliximab | Mean difference (ciclosporin – infliximab) | 95% CIa | p-value | ||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||||
| QALYs: 12 months | 109 | 0.802 (0.106) | 115 | 0.775 (0.079) | 0.027 | –0.001 to 0.054 | 0.049 |
| Total NHS costs (£): 12 months | 109 | 10,796 (138) | 115 | 15,364 (169) | –4568 | –4609 to –4528 | 0.000 |
| QALYs: 24 months | 62 | 1.650 (0.166) | 58 | 1.628 (0.169) | 0.022 | –0.039 to 0.082 | 0.479 |
| Total NHS costs (£):24 months | 62 | 14,773 (1607) | 58 | 19,271 (2181) | –4498 | –5231 to –3799 | 0.000 |
Participants’ time off work
Mean participants’ time off work in the 3 months prior to baseline was similar at 7.70 (SD 15.62) days and 8.27 (SD 14.32) days for infliximab and ciclosporin participants, respectively. There were very few differences between groups at other follow-up points suggesting that treatment had no differential impact on participants’ time off work as shown in Table 43.
| Period | Ciclosporin | Infliximab | Mean difference (ciclosporin – infliximab) | p-value (95% CI) | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||
| Baseline | 133 | 8.27 (14.32) | 131 | 7.70 (15.62) | 0.57 | 0.76 (–3.06 to 4.20) |
| 3 months | 103 | 16.19 (29.45) | 101 | 15.64 (27.39) | 0.55 | 0.89 (–7.30 to 8.40) |
| 6 months | 96 | 6.48 (15.97) | 102 | 3.92 (15.75) | 2.56 | 0.26 (–1.89 to 7.01) |
| 12 months | 85 | 4.31 (16.67) | 81 | 4.30 (14.34 | 0.01 | 1.00 (–4.77 to 4.79) |
| 18 months | 40 | 5.68 (18.03) | 52 | 1.71 (3.62) | 3.96 | 0.18 (–1.88 to 9.81) |
| 24 months | 40 | 2.74 (10.52) | 40 | 2.99 (13.56) | –0.25 | 0.93 (–5.65 to 5.15) |
| 30 months | 13 | 1.15 (3.87) | 18 | 1.58 (5.62) | –0.43 | 0.80 (–3.92 to 3.06) |
Participant interviews
Number of interviews completed
The qualitative section in Chapter 2 explained that we planned to conduct a total of 24 interviews at 3- and 12-month intervals with participants after randomisation and treatment. However, after 20 3-month interviews had been analysed, data saturation was reached and the TMG confirmed that no further interviews were needed.
The number of 3-month interviews completed was split evenly between those randomised to infliximab and those to ciclosporin, with 10 participants in each group, of which three in each group had also had a colectomy. There were three females and seven males in each treatment group and their ages ranged from 21 to 75 years, as shown in Table 44.
| Participants | Infliximab | Ciclosporin | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Number interviewed | 7 | 3 | 7 | 3 |
| Age range (years) | 23–64 | 21–44 | 27–75 | 31–59 |
| Mean age (years) | 44 | 32 | 51 | 43 |
To assess the representativeness or otherwise of the 20 participants selected for interview, we compared the primary outcome, QAS per day, in terms of group and interview status. The numerical summaries of means and SDs in Table 45 are consistent with the box plots in Figure 16, and show that, in both groups, those interviewed are reasonably similar to those not interviewed.
| Group | Infliximab, mean (SD) [n] | Ciclosporin, mean (SD) [n] |
|---|---|---|
| Interview status | ||
| Interviewed | 0.6863 (0.2227) [9] | 0.7754 (0.0778) [10] |
| Not interviewed | 0.7062 (0.1786) [112] | 0.7293 (0.1630) [111] |
FIGURE 16.
Graphical summaries of QAS per day.
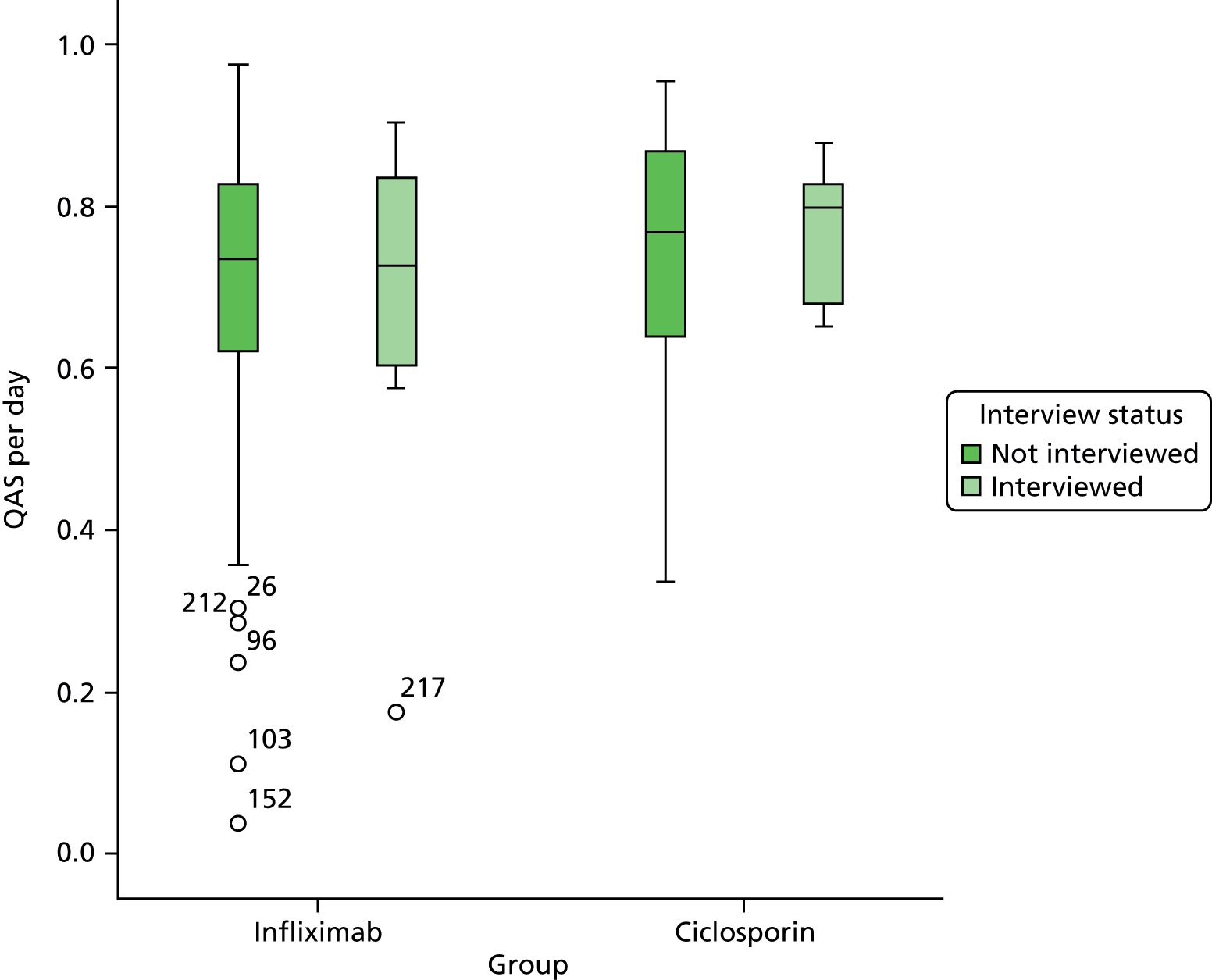
It is worth noting the wide age range of the interviewees, but that their views and experiences were similar and the only differences seemed to relate to views about reversal procedures for those who had had surgery to treat their UC. This is explained below.
The length of time since the participants had been diagnosed with UC varied from just a few weeks to as long as 30 years. However, those who had only recently been diagnosed had generally been experiencing symptoms for several months.
With many participants working, the interviews often took place when people returned home from work. The interviews lasted between 15 minutes (unusually short) and 45 minutes in length (more common), and all participants agreed that the interviews could be recorded.
As soon as possible after an interview had taken place, recordings were transcribed and then reviewed by the interviewer. The interview transcripts were anonymised and any names or geographical details were removed from the transcripts.
At 12 months, 15 of the interviewees agreed to a second interview (eight infliximab, seven ciclosporin); one participant had died, two did not want to take part in the second interview and two could not be contacted. One participant in each treatment group, one male and one female, had undergone colectomy since their first interview and one male participant who had undergone a colectomy before the first interview had since had two further operations, pouch surgery followed by ileostomy closure. In total, 35 interviews were conducted with 20 participants.
Analysis of data
Thematic analysis
When the three qualitative study researchers met (including the interviewer and qualitative study lead), they concurred on the themes that were emerging from the four completed 3-month interviews and developed a first draft of an analysis framework. An iterative process followed as more interviews were completed and the analysis framework continued to develop (see Appendix 24).
When 20 interviews had been completed, the three researchers met to discuss whether or not any new themes were emerging and considered that data saturation had been reached. A proposal was put to the TMG that sufficient interviews had been undertaken and it was agreed that no more 3-month interviews were required.
Once the 3-month interview analysis was complete, analysis of the 12-month interviews commenced. The same iterative process followed as more 12-month interviews were completed and the analysis framework extended.
Group analysis
Seven members of the study team (in addition to the three qualitative researchers) agreed to take part in group analysis of the 3-month interviews and were sent three transcripts each (one from a participant who had taken infliximab, one who had taken ciclosporin and one who had taken ciclosporin followed by a colectomy). Following clear instructions (see Chapter 2), each person wrote three one-page schematic overviews, which were circulated to all those taking part together with the draft analysis framework. Group members looked through the various documents ahead of a full group meeting.
During the meeting, each one-page overview was reviewed and it was clear that each member of the group had created something that covered the main emergent themes derived from the analysis framework.
Findings
Main issues arising
The main issues that emerge from the findings are presented ahead of a more detailed presentation of the qualitative findings for ease of access by the reader and to ensure the main points are not lost:
-
Participants express a liking for infliximab because of its positive outcome, relatively simple method of administration and lack of side effects.
-
The dramatically debilitating symptoms of UC that impact on participants’ QoL and, consequently, on family and friends, is particularly noticeable in this disease type.
-
Participants have to live with the ongoing unpredictability of symptoms and treatment and it is this unpredictability of the disease that makes it particularly difficult for patients and health-care professionals to manage their health.
-
Unlike other chronic diseases, UC is considered an embarrassing disease making it an isolating and awkward experience for patients because of its impact on managing their life and work.
-
The lack of apparency and visibility of either symptoms or outcome also impacts on patients’ sharing knowledge of the disease with others.
-
Surgery is feared, but following it most participants at 3 months experience relief and recognise the health benefits.
-
At 12 months the difficulties for some of living with a stoma were coming to the fore.
-
Participants would like to understand what causes UC and its links with stress and diet and would welcome greater information provision.
-
Ready access to an IBD nurse was suggested as particularly important for members of this patient group.
Introduction
In spite of the variation in the duration of the disease among participants, similar stories were revealed about living and coping with UC; the physical, mental and emotional impact of the condition; the treatments; and people’s concerns and hopes for the future.
The findings that follow are presented as a story so the 13 themes derived from the analysis framework (see Appendix 24), are presented as a narrative rather than under thematic headings. This enables data to be understood in accordance with participants’ presentations of their own illness story, according to a progressive journey from their realisation of the problem, receipt of treatment and management of the condition, to their views of the future. Presenting the findings in this way indicates how UC becomes present and understandable in people’s lives as time passes, whereas presenting the findings thematically would have provided a much more disjointed picture of an illness which is, in effect, often a very long-term chronic condition.
As one of the main aims of CONSTRUCT is the comparison of the two drugs in the treatment of steroid-resistant ASC, after a brief section about interviewees’ views on their general health, we will present their views on the drugs themselves. During their admission all the participants had received one of the drugs and their views illuminate strength of opinion around both.
Following the comparison of participants’ views of the drugs, is a presentation of how the disease impacts on participants’ lives and the effect of symptoms on participants’ health; the impact on wider family members and friends and their understanding of the disease; participants’ experiences of treatment and sharing in the decision-making process with health-care professionals; views on surgery and experiences of undergoing a colectomy; and finally, hopes and expectations for the future.
As there were similarities in participant dialogue at 3 and 12 months, any similarities will not be reiterated. Rather, the report concentrates on new views and experiences in relation to the original 13 themes.
By presenting outcomes in this way, we have aimed to identify both personal experiences of health and illness, and care and treatment options from an individualistic perspective over a period of a year.
All the quotes in this section are from trial participants; they are shown in italics and include an anonymised code and the line number of the quote in the transcript.
Participants’ views on their general health
For many of the participants their UC was the only significant illness they had experienced, and even for those with longer term UC, their recent inpatient stay was often their first admission with the disease. Thus, it soon became apparent that many participants had not given much thought to their health, up to that point, considering themselves fit and healthy. It is against this background that participants reflected on more general questions about their health.
A basic desire to lead a ‘normal’ life, to have sufficient energy and to be fit and healthy to do everyday things was a strong element of the response to general health questions. Participants considered ‘normal life’ and ‘everyday things’ as including the ability to be able to socialise, work and travel, and spoke in terms of wanting to have the freedom and be without constraints, to allow them to pursue these activities. They considered good health as having plenty of energy, not being reliant on medication, not having symptoms or pain and not having to go to the toilet every few minutes. In contrast, participants related bad health to being ‘imprisoned’ by their illness symptoms, by which they meant being housebound, unable to socialise, as well as being physically constrained to having pain and discomfort, lacking energy and being dependent on others.
These issues were raised again when people were asked how their UC had affected their QoL and it was here that the intensity of the suffering caused by UC became strongly apparent. This was particularly the case for those who had experienced symptoms for a only a few weeks or months before becoming so ill that they had to be admitted to hospital, it was a shock that they had reached this state so quickly: ‘a bit of a bolt out of the blue’ (GHI0028.164), it had been a frightening experience and at only 3 months after their treatment they were still trying to adjust to this life-changing event.
At 12 months, while the course of the disease for the participants had differed, their basic desire to live a normal life, to be able to socialise, work and travel, had not changed.
Treatment in the trial: participants’ views of infliximab and ciclosporin
When participants discussed their views of either ciclosporin or infliximab, for some recall about the earliest days of their treatment was hazy as they were so ill that they just could not remember: ‘For the first few days I wasn’t sure what they were giving me’ (PQR0053.216). However, most were able to recall details later on, and their views are presented below.
Ciclosporin
Participants who were randomised to ciclosporin recalled having this while in hospital as an intravenous infusion or drip. There was a sense of relief that treatment was being provided and that this just ‘happened’. However, some found being hooked up to a drip continuously, and for several days, a nuisance. It was also restrictive, because either the drip stand had to be taken everywhere: ‘me and my mate’ (RST0021.271) or the participant had to ask a nurse to disconnect them: ‘loss of freedom, I found that quite irritating, personally found that frustrating’ (BCD0012.218).
Some found the fact that the intravenous ciclosporin bag needed changing frequently was also inconvenient as it meant being woken at night, and for one participant it meant not being able to relax, knowing that the bag needed changing. No one criticised nursing staff and there was praise for the care received. However, a participant at a site with no previous experience of ciclosporin, suggested that more training was required as the participant found himself ‘telling them but they couldn’t do it until someone else came so could be waiting for bag to be changed’ (RHS0021.348).
Some found the intravenous ciclosporin easier than the oral form of ciclosporin because once they started the tablets they had to have tests to monitor the levels in their blood which meant going back to hospital for the test and then returning for the results and further treatment. The oral ciclosporin was noted as having a distinctive smell, a ‘different’, ‘strange’ smell like beer. While none of the participants said it was unpleasant, one said there were others in hospital who disliked it. The tablets were also noted for their large size: ‘they’re like horse pills’ (SHT0001.109), although no one said this made taking them difficult. The advantage of the tablets was that there were ‘no needles’ involved and they could be taken at home.
Participants described the following side effects from ciclosporin: mood swings; tingling in the fingers and toes; a head rush; increased facial and body hair; tiredness; a rash on the arms; hand tremors; and cramping in hands and feet. Women were particularly concerned about an increase in facial hair, but found that it fell out or decreased once they stopped the ciclosporin.
Two participants did not respond to intravenous ciclosporin and required emergency surgery. The others did respond and started oral ciclosporin and made comments such as: ‘It worked so we love ciclo. It saved my bowel’ (TUV0001.310); ‘unbelievably, not unbelievably better but miles better than what I had been’ (RST0021.319); ‘feel myself getting progressively better’ (BCD0012.403); ‘got the condition under control’ (CDE0010.190).
One participant who initially responded to ciclosporin had a relapse and the tablets were restarted, but there was no improvement and she was switched to infliximab. After the switch the participant reported that she realised she had not been feeling that good on ciclosporin: ‘You sort of think yeah I’m sort of finally cured, I’m managing well but now I think, uh, uh, I wasn’t’ (PQR0053.344).
It was difficult for participants to comment on whether or not they felt the ciclosporin was still having an effect and working for them, as most were no longer taking it, or were on other medications so found it difficult to attribute their current health to a specific medication.
Infliximab
Participants who were randomised to infliximab recalled having their first infusion as an inpatient in hospital. There was a sense that being hooked up to a drip for a few hours was not an issue, you were in hospital anyway: ‘I was just lying in a hospital bed so I could doze off, read a book’ (KLM0010.153) and: ‘it was fantastic, machine did it all, just pumped into me’ (FGH0011.87). A couple of participants felt that having treatment given as an intravenous infusion meant that it was more ‘direct’ and reached ‘your system’ more quickly.
After discharge participants returned every 6–8 weeks for a further infusion and this was viewed as a good way of having treatment as they did not have to take tablets: ‘there is not any managing of it because it’s a 2-hour infusion and then you’re not due back in for another 6–8 weeks so in that way it’s fantastic, I’m not having to get up every morning and take about 20 different tablets’ (OPQ0005.395).
The side effects reported from infliximab tend to be fairly immediate with separate participants commenting on a weird sensation in the legs; feeling a bit strange – vague, sort of dopey; becoming ‘roasting hot’ during the first infusion; feeling a little hot and cold after the second infusion; getting hot and sweaty in bed for a week, some 2–3 weeks following infusion.
Three participants did not respond to infliximab and required surgery. The participant mentioned above who switched from ciclosporin to infliximab, noticed the difference with her condition improving within a day. Others who responded used comments such as: ‘straightaway noticed a difference. All benefits and no negatives. Saved me from surgery’ (JKL0033.347); ‘It was my saviour . . . real amazing cure . . . miracle drug for me’ (MNO0034.331); ‘It does work, it definitely does work’ (OPQ0005.213); ‘Really worked on me so they might not have to operate’ (IJK0014.170); ‘Colon saver’ (FGH0011.167).
As with ciclosporin, it was difficult for participants to comment on whether or not they felt infliximab was still having an effect and working for them, as most were taking other medications so it was difficult to attribute their current health to a specific medication: ‘whether it is still the infliximab having an effect, it’s possible kind of thing . . . ‘ (MNO0034.368). A couple of participants felt that the effects were still lasting, but two participants did comment that as they neared the time of their next infusion, they felt that the effects were beginning to wear off.
Reflections on ciclosporin and infliximab at 12 months
By the time of the second interview none of the participants was receiving ciclosporin or infliximab. One infliximab participant had been concerned about stopping after just three doses but was relieved that he had experienced no problems since. Four of those randomised to infliximab and two to ciclosporin had experienced no flare-up of their UC since they completed their treatment with the trial drugs. Flare-ups had occurred for two participants, one from each group, but at the time of the interview both were improving.
The majority of participants spoke more about the treatments they had received since the trial treatment (see Treatment for ulcerative colitis), but thinking back, one ciclosporin participant recalled that she developed ‘terrible facial, arm and leg hair’ which took 3 months to settle, but she reiterated ‘we love ciclosporin, apart from the smell’ (TUV0001.348). Another stated that ciclosporin ‘kept him going’ but that he could not stay on it because ‘it was quite toxic’ (ABC0010.82). One interviewee, randomised to infliximab but who did not respond and had surgery during the same admission, had been treated with ciclosporin several years ago and considered it one of the most successful of all treatments he had received. In contrast, one participant was relieved not to be on ciclosporin and steroids because of side effects and ‘autoimmune issues’ (BCD0012.597)
Three participants commented on infliximab as ‘convenient’ (LMN0009.87), ‘easy in comparison with just having ordinary pills’ (QRS0028.336), and that they ‘would have it again’ (FGH0011.283) and that it meant ‘not taking medication daily’ (PQR0053.509). A participant who initially had infliximab followed quickly by surgery, thought that having treatment with infliximab ‘every so often would have been easy-peasy’ (QRS0028.342) in comparison with the living with a ‘bag’.
One participant had responded well to three doses of infliximab and wanted to continue it as she wanted to start a family. Because of funding restrictions, a case was put to the local primary care trust to allow her to continue but this was refused which she described as ‘quite a blow’ (MNO0034.352). At the time of the second interview she was 3 months pregnant and having a flare-up: ‘colitis and the pregnancy don’t seem to get along’ (MNO0034.170). She felt that infliximab would have kept her well and her ‘quality of life would be much better now if I was able to continue it’ (MNO0034.780).
To reiterate, the presentation of participant views and experiences has begun with their views about the two drugs, ciclosporin and infliximab, at 3 and 12 months, in line with the study’s main aim of comparing clinical effectiveness and the objectives of comparing QoL and investigating participant views of the two treatments. From here on we discuss outcomes of the qualitative interviews in terms of the participants’ journey through their experiences of symptoms, diagnosis, treatment and the impact of UC on their lives.
Onset and diagnosis
It was clear from the interviews that the onset of UC varies from person to person. There were some people who were diagnosed many years ago, some in the last year and some who were diagnosed when they were admitted with severe symptoms that had only been present for a few weeks.
A picture has emerged of a convoluted and sometimes frustrating treatment story from early onset to misdiagnosis. If often begins with mild symptoms such as going to the toilet more frequently and having some bleeding, to an eventual GP visit with a misdiagnosis of haemorrhoids:
I went to the doctor a couple of times but he told me I’d got piles. So I wasted about a month going to see the doctor and he just gave me stuff for piles.
LMN0009.111
Some participants visited their GP several times but it was only when their symptoms did not settle and indeed became worse, particularly if participants reported weight loss, that they were referred for an endoscopy:
I kept going back and saying something is not right. It wasn’t until I went to the doctors and said, I know I’m overweight . . . 100K [kg] and a week, 8, 9, 10 days after I had lost 10K [kg] in that short time and they thought there is something seriously wrong and then they decided to ‘let’s get you in and get a scope done’.
NOP0004.349
At endoscopy some participants were given a clear diagnosis of UC and some were told that they had some ‘inflammation of the bowel’ and the word ‘colitis’ may have been used.
There were some examples of people who knew that they had UC but soldiered on nevertheless with their symptoms: ‘you just put up with it because you just think well I’m just getting old or just, you know, that’s how life is, I can’t afford time off’ (RST0021.90), but this particular participant explained that ‘it shook me to the core’ (RST0021.86) when his consultant would not let him go home and he realised just how ill he was.
An interesting observation from a couple of participants was their attitude to being in hospital as they felt like ‘frauds’ among some of the seriously ill people in beds nearby: ‘I’m looking around going I feel like a fraud really . . . there are a lot of sick people in there that you know, weren’t probably coming out of the place’ (RST0021.289).
Unpredictability of ulcerative colitis
The unpredictability of the course of the disease was a problem for many people who had no idea when their next flare-up might occur. Some commented that between flare-ups, having UC does not impact on one’s QoL, but that when a flare-up occurs, experiences are of pain and discomfort. These are exacerbated by an increase in bowel movements, often with urgency and bloody diarrhoea, which necessitates frequent, very rushed visits to the toilet. Participants described this loss of bodily function as being unable to rely on their body, leading to a sense of giving up control over their bodies. Some people, with longer-term disease, used the term ‘rumbling along’ to describe the way in which their UC was generally controlled by medication, but with occasional flare-ups that required an adjustment to their medication. These participants were used to planning their lives around toilets because of the urgency and frequency their UC caused and would often have a change of clothing with them; some spoke about the worry of what would happen if they were on a train and had an ‘accident’ when they could not reach a toilet in time:
Get used to the fact that you are going to have to change your clothes once maybe twice a day . . . even though you are prepared for it because you know it’s going to happen but actually you are fighting it every minute of the day.
QRS0028.109
I was quite impressed by my planning – it feels like a military procedure at times.
RST0021.223
However, participants jokingly commented on their knowledge of all the toilet stops on a regular car journey, for example to and from work, or the distances between motorway service stations and explained that their first action in a new venue or location was to locate the toilets as soon as they arrived; thus, planning outings around toilets just became a part of their lives.
Participants described how, if a flare-up was not controlled adequately with medication, within a matter of weeks their symptoms began to have a massive impact on their life, and how normal life ceased and many everyday activities were curtailed. On a practical side, there was a need to be near a toilet, as participants had increased bowel movements and for some the increasing symptoms eventually meant not being able to leave the home:
When I’m ill, you’re a bit stuck, especially with UC, when you’re having a flare-up you’re on the edge, you can be a bit worried about going out if you’ve got to think about going to the loo and things like that.
KLM0010.22
Colitis can make you a prisoner that you don’t venture far away from a toilet . . . have to memorise where all the toilets are and things like that. It makes you a prisoner to the disease.
DEF0016.60&67
At 12 months the disease had caused no further problems for four infliximab and two ciclosporin participants; one had been so well that at times she felt as though she did not have UC. One ciclosporin and two infliximab participants had experienced flare-ups; one of the latter associated the flare-up with pregnancy. All were on medication and continued to be followed up and were clear that if symptoms developed they would quickly make contact with their IBD nurse or GP for advice because they ‘would want to catch it earlier’ (BCD0012.214). They understood that a prompt change in medication could prevent them becoming as ill as they had been before.
This section has highlighted some of the practicalities of living with UC, but, as described, when the symptoms are not adequately controlled they can have a massive impact on the life of someone with UC. Impact of symptoms on life is described in more detail below.
Impact of symptoms on life
The impact of symptoms like diarrhoea and disturbed nights resulted in people’s health deteriorating, often within only a few weeks, reducing them from leading an active life – socialising, going to work and exercising – to being housebound. They often suffered from a lack of energy, weight loss and exhaustion, the latter being an issue that many emphasised and referred to several times during their interview:
Basically tiredness was one of the things that was really difficult.
NOP0004.91
Participants spoke frequently about how an important part of their life was being able to go out and socialise with friends and family. However, for many participants life had to be put on hold and they spoke about this as being hard to cope with and a significant change:
Flare-up – it was dictating to me what I was able to do, not me dictating it.
RST0021.25
The condition made attending sporting events, such as football and cricket, particularly difficult because of the travel involved and the busy toilets during break times. One participant explained that before his diagnosis he had followed his football team across the country but had been unable to do so as he could not risk needing the toilet urgently while travelling on coaches for hours:
I used to do a lot of football and travel to football all over the country, that’s what I love doing, but the last year I haven’t been able to do that . . . have to sit on a bus . . . don’t feel comfortable doing that . . . used to travel every weekend but haven’t done it for the last, oh since I got diagnosed. I don’t feel comfortable doing it.
NOP0004.27
A cricket goer explained that he could not eat with everyone else but had to wait until after the lunch break:
Go and watch the cricket . . . take some lunch but I was conscious that you know getting close to lunch, . . . I’m thinking to myself well I can’t have anything to eat at lunch because the moment I have something to eat within minutes I have got to go to the loo and I know there are 20,000 people coming out of the ground going into the toilet and I can’t queue for the toilet.
RST0021.204
Those in employment explained that they had tried to continue working for as long as possible, coping with frequent visits to the toilet, until they reached a point at which it was simply physically impossible for them to carry on. This could depend on the type of employment people were in; for instance, people working as teachers or nurses could not simply drop what they were doing to dash to the toilet: ‘you can’t just walk off at any given moment because you are responsible for patients and you may well be the only nurse in a certain area so it was very difficult’ (MNO0034.85). One participant commented on how difficult it was at work when speaking to somebody and suddenly knowing that there was a need to urgently go to the toilet, meaning that they were no longer able to pay attention. An issue for some younger UC participants was the potential impact on careers which could be affected by ongoing flare-ups requiring time off work. The condition can cause huge changes in lifestyle, impacting on not only the participant but close others as well:
I couldn’t be around my kid when I was feeling pretty low.
QRS0028.101
There appears to be a trajectory for participants of any age in that they go from being someone with an active life, to the disease having an increasingly detrimental physical impact, when they are tied to home and too unwell to do anything more than sit or lie down, which often leads to mental and emotional stress as life narrows:
I was spending all day in a chair or in bed barely able to eat and my life just consisted of going to the toilet and sitting still trying to occupy my mind and not go crazy.
GHI0028.119
It is clear that for some participants, UC had taken them entirely by surprise, changing from someone with good health to someone with a chronic condition that is difficult to control. For many, life had taken a different and unexpected path:
Holidays were a nightmare because like just going to an airport or flying became a major chore.
QRS0028.100
For those wishing to travel there was the added difficulty of obtaining travel insurance following an admission with their UC.
During the second-round interviews, with their symptoms under control, the QoL for participants had improved; the football fan was pleased to report that he had returned to watching games and following surgery and the cricket goer was able to plan his days more easily. Several people commented on the fact that they had been on holiday and could plan events with more confidence.
Participants reflected on the last 12 months of their lives according to changes at work following extreme illness and being diagnosed with a chronic disease. One participant had ‘grabbed’ the opportunity to take a job which involved travelling all over the country, saying: ‘I can’t not do it, you just never know what could happen but I’m not going to say no, just in case’ (NOP0004.36). The period off work made a participant realise that she could get by and as a result she moved house and starting working for herself. Another explained that the experience had made her and her husband rethink and plan their lives a bit more; they got paperwork organised, went on holiday and had long weekends away (TUV0001.414). A surgery participant who had moved from manual to office work reversed the situation as he could wear more comfortable clothes which made managing his stoma easier.
It is clear that the symptoms of UC and being diagnosed with a chronic illness can have a massive impact on participants’ lives but it is the nature of the symptoms of UC that can make it difficult for sufferers to talk about their disease with others, as shown in the next section.
Embarrassment about sharing knowledge with others
In contrast to some other chronic conditions, many UC sufferers consider it an embarrassing illness because of its association with ‘bottoms’, ‘bleeding from your bottom’, diarrhoea and going to the toilet. Because of the embarrassing connotations, participants explained how they select who they share knowledge with, often discussing it only with close family members and friends:
. . . three men wouldn’t stand around a bar and one say to the other two ‘I’ve been for a camera today’, you know, it’s not the sort of thing . . . I sort of relate it a bit to probably to ladies, to breast cancer a few years ago and I think men with the prostate cancer, ‘cos that’s a bit of a taboo subject but it is starting to come out there now.
RST0021.127
It was often in the context of work that participants started to describe the embarrassment that they felt about their condition, as it is an illness that is difficult to talk about with colleagues: ‘it’s hardly a glamorous disease that you wish to chit chat about’ (MNO0034.88). However, participants found that when they did explain their disease to their employers and colleagues, those people were understanding and supportive; several participants emphasised just how supportive they found their place of work to be:
Work have been absolutely brilliant, I couldn’t ask for any more to be perfectly honest with you.
NOP0004.150
Some participants felt that their UC made going out as part of a larger group more difficult. On occasion the group needed to be made aware that a very urgent toilet stop was required but this was difficult to communicate to a group and caused embarrassment for the person needing the toilet.
We go on motorcycle trips and they’ll just think, oh we’ll go from A to B and that will take us two and a half hours without having to go to the loo. Me and my partner we can just stop but it’s difficult if you’re in a group of, might be 10 people on bikes, it’s difficult to . . . it’s that kind of . . . that’s where you think we won’t go on this trip or something, we’ll just go by ourselves.
KLM0010.97
It is a disease that for the most part does not manifest itself openly and, thus, others are unaware of UC patients’ suffering; several participants raised the issue about the lack of a broader awareness of UC as a chronic condition. They felt that it had a low media profile in comparison with some other chronic conditions (such as asthma), and participants wished that more famous people with the condition might come forward to champion their disease and raise awareness. Those coping with the outcomes of bowel surgery for UC raised the same issues. In general, the low profile was attributed to the embarrassing nature of the disease, which was seen to set it apart from other chronic conditions.
At 12 months similar issues arose, but a participant reflected that ‘you need to be able to speak to people and share what’s happened to you’ (TUV0001.584), whereas two surgery participants were keen to share their experiences with other sufferers to help them to understand the impact of surgery and coping with a stoma.
Impact of ulcerative colitis on family and friends
The life-changing nature of UC clearly impacts on families and friends, particularly during periods of a flare-up. Those with young children described how their UC left them unable to look after and play with their children, increasing the need for others to help. Others acknowledged that it affected aspects of their partner’s social life and, for some, the limitations on travel impacted on family travel plans:
I’m retired and one of the things I would like to do is to go around the world with my wife and we can afford to do it now but I simply, at the moment haven’t really got the energy for it.
ABC0010.86
However, the predominant observation made by participants was about the support provided by their families and friends. The different types of support included hospital visits, the understanding shown by relatives to participants’ needs and their support in practical aspects of a participant’s life, such as helping to plan a journey. This support also helped participants emotionally as the condition became more difficult to cope with when a flare-up occurred:
My mum . . . ended up working a bit less and my dad . . . having to go into hospital . . . at the end of the day he was working. And my older brother . . . he was having to visit me, so he had to forego a few things he would usually do. . . so mainly in hospital that it had the biggest impact on my family.
GHI0028.69
At 12 months, the impact of UC on family and friends was spoken about in particular by those who had had surgery, either before the first interview or since (see Surgery). However, one participant felt that friends and family got bored with the disease: ‘so talking to an absolute, vague stranger, there’s something quite soothing about it . . . just go through it from start to finish’ (MNO0034.729).
One participant explained that he had visited his workplace to discuss returning to work and was aware that word had got out that he had a stoma. He said that some individuals did not look at his face but were ‘constantly staring to see if they could see this thing’ (DEF0016.860). He felt that this was rude and said that his wife was so annoyed that she had to walk away.
Acquiring knowledge about ulcerative colitis
Participants appeared to be well informed about their condition. For those diagnosed many years ago their experiences and interactions with doctors and nurses had informed them about the condition and its treatment, while many UC sufferers have used the internet to find out more information. One participant, diagnosed 30 years ago, regretted not using the internet to look for developments in treatment over the years, as he felt that he may have missed out on a treatment that might have helped, instead of putting up with his symptoms all those years.
Those newly diagnosed with UC can be provided with information about ‘Crohn’s and Colitis UK’ a national organisation dedicated to ‘Inform, Support and Research’ or be put in touch with an IBD nurse specialist at their local hospital. Crohn’s and Colitis UK (previously National Association for Crohn’s and Colitis) provides a lot of information about UC, living with and managing the condition and about its treatment. It provides information on a website, or paper information can be provided, and has local support groups in many parts of the country.
Some spoke about their IBD nurse, explaining that they had direct access and could contact the nurse at any time for information and help when their symptoms increased:
I do come up with questions from time to time but I must admit the IBD nurse is brilliant, you just pick the phone up to him and he is more than happy to speak to you about anything so definitely the support I have received is excellent.
NOP0004.423
However, IBD nurses are not necessarily available in all hospitals and a couple of participants expressed strong opinions about the need for their presence and the very valuable role they play in helping to manage UC.
In spite of the information that was available, participants had a number of unanswered questions about the cause of their disease and mentioned the need for more research in this area in order to ensure that other family members could be supported if they did get UC. Participants were also unsure about what triggered a flare-up and wanted more information on this particular aspect of their disease, including information about the links between UC, stress and diet:
Colitis is very difficult, it’s very difficult to manage and certain foods can set it off, etc. and stress is another thing so trying to identify the foods that set it off and stay away from stress is key.
OPQ0005.370
With regard to diet it was acknowledged that there may be some misinformation on the internet, and several people commented on contradictory advice about whether or not there is a link between diet and UC.
Ultimately, participants wanted to see a cure for the condition; although they recognised that it might be too late for them, they wished to prevent others from having to suffer with the condition:
I would love somebody to come up with a cure for it even though the stable door has . . . been bolted for me.
DEF0016.767
Participants appeared to be less questioning about UC at 12 months, as only a few comments arose about a cure for UC and the relationship between stress, diet and disease. The fact that they knew they had easy access to care appeared to be more important for the stage they were in and the course of the disease.
Treatment for ulcerative colitis
The cacophony of drugs
If diagnosed with UC, participants were started on medication; they spoke about this ‘controlling’ their symptoms, some for many years without any problems, and how, when experiencing a flare-up, an adjustment to existing medication or a change in therapeutic pathway brought their symptoms back under control. However, some participants had experienced greater difficulties in keeping their UC under control and spoke about various medications – azathioprine, mesalazine (Pentasa®, Ferring Pharmaceuticals Ltd; Asacol®, Warner Chilcott Ltd), 6-mercaptopurine, methotrexate, steroids and foam enemas – effectively a cacophony of drugs, that they had tried, but against a background of the unpredictability of not knowing what might work and for how long and when a different treatment might be required:
Other things like Salazopyrin [Pfizer Ltd], you’re having 2, 4 times a day, imagine that during the day if you’re based at the office or something, it’s quite hard work.
QRS0028.161
All of the participants had been admitted to hospital approximately 3 months earlier because their UC symptoms were uncontrolled and they needed treatment with intravenous steroids. Those who knew that they had UC before admission appeared to have had a good understanding of their situation and knew that if they failed to respond to intravenous steroids their treatment options were narrowing to a more powerful drug or surgery; they recognised this as a last chance before surgery:
I’m in the last chance saloon I suspect.
ABC0010.281
However, although many were very familiar with the drugs, some were less familiar with infliximab and ciclosporin; they knew of them but perhaps not in as much detail. One participant said that had she been at home when she knew one of these drugs was going to be used, she would have looked it up on the internet, as she would have liked a bit more information, particularly about the long-term effects.
Those who were newly diagnosed had to assimilate the information about their condition and treatment options quickly, at a time when they were extremely ill. It was clear from all participants’ comments that they were so ill at the time that decisions about their treatment almost passed them by:
I was having so many injections and blood tests at the time in hospital that I didn’t even notice it.
QRS0028.134
You could have put a blank cheque in front of me and I would have signed it.
STU008.216
Once discharged, participants had to continue taking medication and more often than not they were on more than one drug. For those who had previously been well, there was a period of adjustment, not only coming to terms with their diagnosis but also getting used to taking medication: ‘just remembering to always have them with me and going on holiday . . . suddenly settle into a routine . . . I mean now it’s so much part of my life but that just seems normal but to begin with seemed quite alien’ (BCD0012.375).
On the whole, people were fairly philosophical about having to take medication and were prepared to take anything that would work: ‘whatever is going to help me to get better or at least help me fight towards getting better’ (OPQ0005.414).
There were mixed experiences for those who had remained on medical therapy over the 12-month period, and these participants spoke about how it had been trial and error to find treatments they could tolerate that would keep their disease under control. Some participants explained that taking medication had become part of their normal life, whereas for one it was a ‘challenge fitting taking medication into routine’ (MNO0034.452).
The unpredictability of treatment was still clear, with participants understanding that some treatments, such as 6-mercaptopurine, could be taken for a limited time only. The uncertainty as to what would happen after that was highlighted by the view that medical treatments were not always successful.
Shared treatment decisions
As mentioned earlier, participants were well informed about their UC; they knew the signs and symptoms that indicated a flare-up and the various treatments available and, armed with this knowledge, they took part in shared decision-making with clinicians about their treatment. This was particularly evident when participants spoke about surgery as a treatment option:
Been hospitalised more in last 5 years than previous 30 and spoke long and hard to the surgeon and medical doctors and we all agreed that my QoL would be improved if the bowel was gone basically.
DEF0016.362
Discussed surgery quite strongly really but again the consultant is quite happy to try and get it treated first, you know, try and get it under control with some medication.
RST0021.677
However, participants clearly understood that if they failed to respond to the various treatments, there may be a time when their ‘body’ made the treatment decision for them:
If you are so bad and it gets to the point where it could put your life at risk then obviously you have to have it done.
JKL0033.521
Several participants commented on their reasons for taking part in the trial. They felt that it was a positive step as they received more attention and had ready access to the research nurse if they had any queries. One participant was relieved to enter the trial because it removed the responsibility of making a decision between ciclosporin and infliximab: ‘there could never be any comeback on what decision I made’. (MNO0034). However, several participants said that they just wanted to help others and to prevent others suffering in the same way.
The participants all continued to attend hospital for follow-up at 12 months and commented on positive relationships with their doctors and IBD nurses. Some also highlighted how helpful their GPs had been. However, one participant had become frustrated by seeing different doctors at each appointment and having to explain his history and treatment on each occasion.
Those on medical treatment understood that they had to take responsibility and seek out help when they experienced symptoms that could indicate a flare-up of their UC and, over the course of 12 months, became familiar with the medical therapies and side effects. Although it was apparent that they discussed their treatment with their doctors and nurses, there was also a sense that shared treatment decisions were limited, as decisions had to be based on whether a treatment worked or caused side effects.
Surgery
The majority of participants spoke about surgery as being absolutely the last treatment they wanted to have. It was certainly a shock to the newly diagnosed that they could be facing surgery. Terms such as ‘saving’ or ‘losing’ colons were used, and participants did not want to have to wear a bag:
It was worthwhile because I walked out of there without a bag [laughs] . . . that’s what meant the most to me. It’s just the fear of the unknown, the effect it can have on your life I suppose, life changes.
TUV0001.242
So realistically if it wasn’t, didn’t try this, it was going to be down the route of surgery which I didn’t want to go down that route at this moment . . . it’s quite a high probability at some point . . . but I’m 28 and I’d rather try and leave it for as long as I possibly could.
NOP0004.266
However, most were philosophical about the possibility of future surgery and realised that they would have very little choice if it became necessary:
If it presents me, if Dr X said to me, I won’t have choice, my body will make it for me.
TUV0001.262
If it’s something I have to have done, I can’t say no I don’t want it, I have to anyway and to be fair they’ve explained the operation and it’s not so bad, come on, they’re trying to fix me you know, there will be no need for any pills, no need for running to the hospital and all the NHS spending money on me if they can just cut it out. I know I would have to have the bag for a few months but then they stitch me back so it’s not permanent, it’s temporary again. That would annoy me if I were to end up with a bag really, just the idea . . . if I know it is for 3 months, fine.
PQR0053.533
. . . rather have it now when I’ve still got some energy left than in my 70s when I’m too old to do anything.
ABC0010.354
Those who had undergone surgery actually appeared to reach a point during their hospital admission at which they became resigned to the fact that it was going to happen; they knew that the infliximab or ciclosporin was not working and that an operation was inevitable, and there was an acceptance of what was to come:
I felt really fed up so I was actually quite happy, to be honest to have the operation by the time it came round. . . it wasn’t really a difficult decision to make in the end.
GHI0028.207
I’ve had it for 30 years, mentally I was prepared for the surgery so to a degree maybe we could have carried on with the drug but I had had enough by then. So it was frustrating really, the drug had improved it but it hadn’t improved enough, they were prepared to maybe stick it out more but I mentally wasn’t prepared for much more.
QRS0028.214
Those who had undergone surgery acknowledged that had the drug treatment worked they would have been happy to continue with that, as they would not have known any different, but after the surgery they were actually relieved that it had been done, as it meant an end to their UC:
Once I’d had the operation there was a sort of a bit of a peace in my body, it was like there was a fight going on forever, even when I think it’s under control, there’s still this sort of underlying . . . that’s what I think of it as a fight, because it’s not settled . . . my body shouting at me and that’s not there anymore.
KLM0010.262
I wish [patient emphasis] I’d had this operation the day I was diagnosed to tell you the truth. It was the moment I woke up after the operation I thought, cor this is fantastic, . . . the fog had cleared and when you are living with it you just deal with it, you just get on with life, you don’t realise how much it did effect me over those 10 years. So yeah, quite honestly, if I could have had the operation then I would have had it done and now I would have been 10 years down the line . . . it’s like hindsight isn’t it.
STU008.188
However, the relief was not felt by all. One man who had fought against surgery when diagnosed 55 years before said ‘I’m so glad I did because I now know the pitfalls of surgery now I’ve had it and I wouldn’t have liked to have thought that I had that as a young man’ (DEF0016.99). He had felt suicidal after his surgery, saying that he ‘felt like jumping off a roof’ but ‘my wife and family have been instrumental in making me shake myself and realise that basically it’s either that or end up in a graveyard and when you look at it in that respect it’s not so bad’ (DEF0016.580).
Although most felt that surgery had been a good thing, it did create issues for some in relation to the practicalities of managing the stoma and the bag, restrictions with regard to clothing and concerns about how others would react to seeing the bag, which for some led to a change in attitude to being able to go swimming:
Go down to the beach . . . I’ve got to keep my T-shirt on which is something I wouldn’t look forward to because I like to jump in the sea . . . I don’t want people staring all the time.
STU008.155
it’s cumbersome and it’s a pain but what price is being healthy really, . . . I’m happy to have it but in a way I’m not happy to have it, because of the inconvenience, but no I’m just happy I’m well.
STU008.256
Participants conveyed that being older made it a little easier to cope mentally with the outcomes of surgery and suggested that they would have been less tolerant when they were younger:
Old enough now that it’s a bit of a laugh and joke. If I was younger I’m sure I wouldn’t have taken to it. It is quite intrusive but I think when you’re a bit older it is a lot easier to deal with, mentally really.
QRS0028.307
In the same way that participants were selective about whom they told about the condition, they were selective about who knew about their surgery, in part because they felt that people would not understand and in part ‘because some people can be cruel’ (DEF0016.613). Participants tended to tell family and close friends, and would inform colleagues where necessary:
. . . with friends and family I haven’t found it difficult talking about the illness and having the stoma and what needs to happen with it. Not had too much of an impact on my relationship with my girlfriend . . . she’s been very understanding about it.
GHI0028.268 & 281
In spite of the support and understanding, some participants were clearly very conscious of the physical changes to their body and referred to how this impacted on personal relationships: ‘my love life is not what it was’ (DEF0016.496). They empathised with their partners having to see the bag but would then comment on how understanding and supportive their partners were.
As with UC, there was a feeling that there was a low media profile about living with a stoma because of the stigma associated with it: ‘there is nobody famous with a colostomy that will admit to having one’ (DEF0016.779).
Several participants spoke about the possibility of having reversal surgery and, again, age was shown to influence attitudes, with younger participants keen to get on with the process of getting life back to normal and older participants appearing more likely to accept life with the stoma:
If I was, don’t know, 20 years younger, I’d probably go for reversal and take my chance because I wouldn’t want to live with the bag but at 50 I’m hardly likely to head down the beach and stuff like that.
QRS0028.289
The surgery participants were aware that the outcome of reversal surgery was more successful if it was completed sooner rather than later and were well informed of the risks involved in further surgery. They were also aware that they would still need to use the toilet more frequently than most people but, having suffered from UC, that was not a concern:
They say I’m always going to be a little bit more regular than a normal human being [laughs], if you see what I mean, but it’s something you live with anyway with colitis, don’t you, because you’re always going to the loo a lot more times than people without it so that’s something I can live with because I’ve already got used to that bit.
STU008.146
One participant felt that the consultant treating him was quite keen that he should have surgery, but as a self-employed person he expressed concern about needing extra time off work for the initial surgery and reversal operations, saying that ‘financially I can’t afford to have this time off work at the moment’ (RST0021.652).
Reflections on surgery after 12 months
The views and experiences of the participants who had had a colectomy were markedly different. Just one participant, a young man diagnosed with UC followed by infliximab and then surgery within the same admission, had gone on to have two further operations to achieve reversal. He said it had been a ‘long haul’ and that surgery was ‘not a magic wand that could make me as healthy and normal as I was before’ (GHI0028.189), but that his QoL improved by not having a stoma. He had no regrets and was happy not to have to medically manage UC.
Two men had found that intimate relationships had suffered, one from the outset because he ‘does not like to look at the bag’, a ‘constant reminder’ (QRS0028.452), and the other when he had experienced a significant deterioration in his sex life:
My personal and sex life has absolutely plummeted because . . . well, it’s not a very nice thing to have flapping about and you wear shirts and things like that but it’s not the same, my wife is very frustrated about it and we try to deal with it but . . .
STU0008.250
In contrast, a young woman reported that her relationship with her partner had not been affected by the stoma and was so pleased with the outcome of laparoscopic colectomy and the control having a stoma gave her that she did not think she would have the procedure reversed. An older man had a similar view; he was not convinced by what he had heard of the success of reversal surgery but did state that had he been younger, with an active sex life, ‘reconnection’ would have been important.
The 12-month interviews highlighted that although some considered surgery as a cure, two participants spoke about still having colitis, as the rectal stump was affected by UC which meant they continued on medical treatment as well as managing their stoma. Although one was coping with this, the other said that he ‘still felt like a prisoner’ as he was waiting for surgery to remove the remaining bowel. In addition, the practicalities of emptying the bags were mentioned, which required an ongoing awareness of the location of public toilets.
A couple of participants commented on the conflict of provision of stoma bags and equipment by stoma nurses funded by manufacturers. This resulted in being offered a limited choice when there may be more suitable styles; these other styles were available to patients, but the onus was on the patient to know what to ask for.
The two participants who had surgery since the first interview felt that there could be better information provision before and after surgery, particularly with regard to the stoma, with one commenting that they would have liked to have spoken to someone who actually had a stoma.
Concerns, hopes and aspirations for the future
The most significant concern for participants who had not had surgery was the unpredictable nature of the course of UC and, as a consequence, the uncertainty surrounding their future treatment. For those concerned with what the future would bring, surgery was often at the back of their mind:
If you can ward off the surgery for a decade it’s worth, I think it’s worth doing. It may come to it, I may have to have it one day and it is always at the back of my mind but I would rather have it later than sooner.
JKL0033.533
Some of those who were only recently diagnosed were finding it hard to adjust to the fact that they had a chronic disease that would require ongoing care. They reflected on others they knew learning to cope with long-term chronic conditions such as asthma, but although they had responded to treatment and were reasonably physically fit, they were mentally vulnerable:
My symptoms have been non-existent since the infliximab pretty much but all of my emotions and everything else is still kind of, almost seems way out of proportion to the bowel symptoms.
MNO0034.286
A lot of the stuff that I like to do that I couldn’t do and even now I probably could do it but I’m wary about doing anything. So it’s still in the back of my mind all the time.
NOP0004.119
Participants were interviewed only a few months after they had been hospitalised and many were going through a transition period with their medication. They spoke about the drugs they were on and what was planned, but did so in the context of uncertainty about what would work and for how long: ‘where we need to go, where do we go from here, stay on the ciclosporin or what really’ (RST0021.474). Some were aware about the funding issues with infliximab and were concerned about whether or not they would be allowed to continue on it in spite of the fact that it was working for them.
Many participants spoke about what had become their ‘normal for me’ health and were happy to accept an increased need to use the toilet only three or four times per day in comparison with 20–30 times per day, understanding that this could be achieved only by using medication. However, for those whose symptoms had completely resolved, taking medication was a constant reminder: ‘I could almost sort of forget that I have been ill . . . it’s a shame that I always have to take them because that’s a constant reminder actually that you know, I’ve been ill and it might come back’ (BCD0012.520&600).
Some younger participants expressed concerns about the impact of the various drugs on their future plans of having a family and were worried about the effects on their fertility: ‘only got married last year and we haven’t had a family yet and with most of these drugs there are issues with getting pregnant’ (MNO0034.513).
Some of those who had surgery said that they hoped to have the reversal procedures and looked forward to a time when it was all over and they could get their life back on track. Others, who tended to be the older participants, were less interested in having a reversal; they accepted the stage they had reached, were relieved that their UC had been dealt with and were prepared to live with a stoma and with the practicalities and restrictions it presented.
The future 12 months later
Life had changed for the participants by the time of the second interview. Seven of the eight who had not required surgery appeared to have come to terms with having a chronic illness that required ongoing treatment and living with the uncertainties of that treatment, but seemed more confident about being able to control the disease. They appeared to be reassured by the ready access to advice from IBD nurses; in particular, those who had a flare-up commented on the speed of the response when it was needed.
The eighth stated that that she had ‘not adjusted to it from the point of view of . . . just feeling angry or sorry for yourself . . . I feel I should feel happier’ (MNO0034.240). However, this participant was coping with a flare-up that she associated with being pregnant, the pregnancy being classed as high risk because of her disease and the fact that she had not been allowed to stay on infliximab. She said that it ‘. . . is not how I ever pictured being pregnant’ (MNO0034.220).
At the time of the second interviews it was some of the surgery participants who were unsure of what the future held as they were thinking about whether or not to have reversal surgery and the ongoing impact of the stoma on their personal relationships, and two expressed concerns about managing the stoma as they got older and the thought of someone else having to deal with it for them.
Conclusion
The findings from these interviews confirm the liking that patients have for infliximab, because of the treatment regime described, fewer side effects and ease of handling the drug. Ciclosporin was less favourably described, with more side effects, greater problems of drug handing and a more onerous treatment regime.
The physical symptoms that UC sufferers described, particularly when experiencing a flare-up, are dramatic and debilitating clearly indicated by these data in terms of the effects of symptoms on patients’ lives, leading to social isolation as their health deteriorates. Efforts should be made to ensure prompt diagnosis, to encourage known UC patients to report changes in their symptoms as soon as possible and to offer appropriate treatment that patients favour, which should be administered as quickly as possible.
A significant issue that emerged was the need for better support and greater information to enable patients to manage the impact of unpredictable symptoms and ongoing treatment regimes.
More research needs to be conducted to explore the views of UC patients post surgery, and to provide more information that could support the decision-making process of those facing surgery. As the views of participants following surgery were generally positive, there should be further research into the surgical treatment of UC as a real alternative to medical treatment.
An awareness raising campaign about UC (and Crohn’s disease) including details about surgery would encourage people to seek help earlier and help to destigmatise UC, thereby reducing the embarrassment felt by sufferers and those living with the outcomes of surgery.
Professional interviews
Main issues arising
The main issues that emerge from the findings above are:
-
Professional interviews indicate a clear preference for infliximab among nurses.
-
Most doctors are more equivocal and prepared to wait for evidence of effectiveness and safety before making up their minds.
-
Some doctors are strongly in favour of infliximab, wishing to see it as the drug of choice in view of its ability to deal with the many complex symptoms this disease group displays; its ease of administration; fewer adverse side effects; greater familiarity; convenience; greater perceived effectiveness; and ease of handling.
-
Ciclosporin is more cumbersome to administer and requires additional monitoring, which puts pressure on an already overstretched workforce.
-
Professionals view nurse colleagues as more familiar with the administration of infliximab and note the fewer demands it puts on nurses’ time.
-
Ciclosporin is more restrictive on patients’ movements, leaving them frustrated and in need of more intensive support from nurses who must be present to manage complications.
-
Professionals question current NICE guidelines and government regulations around drug use and the restrictions that this places on their professional autonomy.
-
Professionals want to gain a clearer understanding of how the drugs affect patients’ lives.
-
Professionals complement the trial for shining a light on this area of study which they see as seriously under-researched.
Introduction
Data saturation was achieved following the completion of 23 interviews. Fifteen consultants and eight nurses formed the interview cohort. The consultant stratification was as follows: eight from high trial and cohort recruiting sites; four from high cohort/no or low trial sites; and three from poor cohort and trial sites. The nurses were all from high trial recruiting sites (the same sites as the eight consultants) and nurses from nil or low trial sites, or poor overall trial and cohort sites were not included (see Chapter 2). The analysis framework (see Appendix 19), led to a rich data set comprising (a) descriptions of the practicalities of administering and monitoring the two drugs and (b) contextual factors influencing professionals’ views of the effects of infliximab and ciclosporin on the alleviation of symptoms or QoL. In addition, analysis led to (c) greater understanding of professional personal preference for one or other drug or other therapies; (d) clarification of professional experience and entry into the trial; and (e) perspectives on current government policy regarding the regulation of drugs and of NICE guidelines. Underpinning views and preferences from both nurses and consultants was an ongoing concern for patient welfare, informed patient choice and joint decision-making.
The main themes and categories derived from interviews are displayed in Box 1 and described in detail below alongside verbatim quotations. All quotations are coded to indicate whether a consultant or nurse was speaking and the transcription line numbers.
Lead-up to treatment.
Drug administration and effectiveness.
Adverse effects.
Drug management.
Longer-term effects and drug maintenance.
Personal preference and involvement in the trialPersonal preference of consultants and nurses.
Views on other health-care professionals’ preferences.
Being part of a trial.
Surgery.
Equipoise.
Negotiated care and shared decision-making.
CostsCosts of the two drugs.
Comparative costs.
Evidence and guidelinesEvidence related to NICE guidelines.
The regulation of drugs and drug policy.
Drug administration and management
Consultants and nurses, when asked about the administration of infliximab and ciclosporin, were keen to point out that consideration must not only be given to the administration process but also to the lead-up procedure (‘work up to treatment’, consultant, HP7.45). Patient work-up was seen as a vitally important step in administering the drugs. However, work-up was said to be something that was neither given enough consideration within the drug guidelines, nor discussed in terms of positive and negative consequences and its effect on the rest of the administration process, such as its effect on drug handling. Interviewees described the work-up necessary for the provision of infliximab and ciclosporin in similar terms; however, following initial work-up administration, infliximab was much easier to handle than the administration activities necessary for the provision of ciclosporin. First, there was less to be concerned about during that period of time, and, second, the process had less impact on workload, especially for nurses, while both drugs demanded due care and attention. Attention to detail was described according to the careful mixing of drugs and other preparations necessary for treatment, prescreening of patients and patient preparedness for the intravenous infusions. In this respect ciclosporin was consistently described as the more complicated of the two, with a longer administration time (continuous as opposed to 2 hours for infliximab), and with the need for frequent changes of intravenous bags (infliximab is a one-off infusion). This had a knock-on effect on health-care professionals’ work schedules:
It [ciclosporin] goes on over a longer period of time obviously, so the need [for nurses] to continually make up bags and things over a longer period of time rather than just the one off infusion.
Consultant, HP13.25
It’s [ciclosporin] time consuming for the nurses and slightly messy. You know it’s complicated and it’s work for the nursing staff.
Consultant, HP15.19
Nurses’ views accorded with consultant opinion on this matter. In addition, nurses were keen to emphasise the complexities resulting from contextual and geographical factors that had to be contended within a busy hospital setting, such as managing patients on different wards requiring an infusion and, as a consequence, the greater number of nurses with advanced prescribing capabilities necessary on the wards at any one time:
Having to make it up [ciclosporin] every 6 hours is time consuming, changing lines, always having two nurses to check it because the way the GI [gastrointestinal] unit is split is there’s a corridor up between the two wards so obviously bed cover etc. but only to have one trained [nurse] each side is a bit difficult . . . geography of the wards . . . we’ve no one else to check the drugs.
Nurse, HPN19.21/28/29
You have to be mindful that the continuous infusion [ciclosporin] has to be prescribed to cover the weekend until Monday.
Nurse, HPN18.27
The practicalities of a lengthier ciclosporin infusion had implications for patients’ well-being too, with health professionals having to spend more extensive periods of time in hospital dealing with patients who were ‘frustrated’ (nurse, HPN21.98) with highly restricted movement:
[Ciclosporin] fairly cumbersome for both the staff and equally importantly for patients because once you are tied to the drip and associated drip stand, it sort of restricts patients moving around.
Consultant, HP12.10
Patients don’t particularly like being hooked up to it for such a long period . . . it’s [ciclosporin] restrictive.
Nurse, HPN25b.52/57
Views on the ease of administering individual drugs were clearly influenced by an individual’s familiarity with the drug in question. Thus, those with more experience of ciclosporin tended to be more positive about that drug, and there were suggestions that nurses should be trained in both drugs as they faced a steep ‘learning curve’ (consultant, HP13.123) particularly in relation to ciclosporin. Nevertheless, support for the administration of infliximab was stronger and it was repeatedly described as the ‘easier’ (consultant HP5.18 and nurse HPN18.169) and more ‘convenient’ (consultant, HP12.78) drug:
Infliximab has an advantage (over ciclosporin) in that it’s just a 2 hour infusion and then it’s done . . . there’s no problem with infliximab, I think it’s a good drug to administer, I think it’s an easy drug to administer . . . I like infliximab because once you’ve done it, you’ve done it for two weeks.
Consultant, HP14.26/30/33
Although both drugs were perceived to be effective, ciclosporin was faster acting and the slower response time with infliximab presented a challenge in terms of whether to continue with infliximab or look towards a different treatment. Ciclosporin was more ‘clear-cut’ (consultant, HP11.81) and consultants felt more confident in their decision-making and timings with regard to this drug. In addition, consultants worried about the ambiguous information available for ciclosporin response rates, as these did not appear to match patients’ actual responsiveness, made all the more complex by the need for an extended administration period:
Tend to use ciclosporin as acting more quickly.
Consultant, HP4.171
This is the one thing I don’t like about infliximab, because there is no data to give us a clear timescale or timeline for decision-making.
Consultant, HP11.94
Sometimes it’s a bit challenging that you have given them infliximab if they are going to take sort of a week to 10 days time would you hold your nerve?
Consultant, HP12.47
In addition to response times, adverse effects were of major importance in treating patients with one or other drug. Ciclosporin in particular was seen to have a range of associated adverse effects, which included drug toxicity, renal failure, neurological impairment, seizures, tremors and hypertension. Consultants were ‘uneasy’ (consultant, HP9.56) about the drug, seeing it as ‘dangerous’ (consultant, HP6.72). Even those advocating its use tended to be apprehensive about the adverse effects in the longer term, thus viewing it as more of a bridging therapy. These views were reinforced by a perceived lack of evidence of ciclosporin’s longer-term benefits.
Don’t want to use it [ciclosporin] in the longer term because of side effects, so switch to azathioprine as soon as possible.
Consultant, HP6.99
I’m always a little bit more nervous with it [ciclosporin] . . . and I think that’s from the side effects of renal impairment . . . I think the side effects profile of ciclosporin, although maybe it has not come out in studies, still concerns us more.
Consultant, HP16.278/281
. . . potentially quite significant side effects that can happen . . . slightly uncomfortable feeling about it [ciclosporin].
Consultant, HP9.60
It’s [ciclosporin] a bit debatable although I use it, I worry about toxicity and the liver, use it half-heartedly, when no choice.
Consultant, HP3.29/32/33
An adverse risk profile for ciclosporin, we were told, results in increased patient monitoring and checking of drug and blood levels, which, in addition to a more resource-intensive process, impacts on nurses’ time. This is particularly noticeable on a busy ward where there are difficulties with nurse-to-patient ratios, and nurses had the sense that their profession was struggling with workload increases, impacting on their ability to give equal time to all patients under their care. They reported occasionally ‘forgetting’ (nurse, HPN24.255) to monitor ciclosporin patients fully. Infliximab, on the other hand, did not warrant any additional monitoring and no issues associated with monitoring were raised:
It’s [ciclosporin] time consuming with regards to observation, particularly on a busy ward when you’ve got one nurse to 10 patients, it can take quite a huge part of your workload.
Nurse, HPN21.16
We have to monitor this patient closely for any side effects . . . it [ciclosporin] takes 3 hours because you’ve got to do obs [observations] for a couple of hours, but then sometimes we tend to forget because we get busy with other patients . . . so ideally it should be one to one.
Nurse, HPN24.249/255
Although infliximab also carried a risk profile that included adverse reactions, causing particular hesitancy in prescribing it for the older patient, it was favoured for its fewer side effects and longer-term efficacy:
Most of the time it [infliximab] goes without incident.
Nurse, HPN19.83
Have only ever seen minor reactions to the infliximab.
Nurse, HPN21.82
However, consultants pointed out that they felt frustrated about policy restrictions and government guidelines that prevented them from using infliximab in the way in which they wished to, including the need to justify its use in the longer term:
We now have to request the funding for patients with UC on long-term infliximab so that’s a bit of a challenge and it means that decisions are made differently to whether a patient is going into maintenance infliximab.
Nurse, HPN19.154/156
It’s already an issue because we can’t really treat as maintenance with infliximab so obviously if a patient responds well we’ve had to get exceptional funding and things like that so there is a case for it I think.
Nurse, HPN22.103
Personal preference and trial involvement
Consultants clearly recognised the effect that their own experience of using one or other drug had on their personal preference. Some consultants, as indicated in Drug administration and management, have a preference for infliximab, based on its ease of preparation; easier administration; lower negative impact on nurse care provision, staffing and workloads; longer-term benefits; and reduced adverse effects. In addition, both consultants and nurses pointed to greater patient tolerance and enhanced patient convenience. Further reasons for stated preferences included familiarity with the drug, drug effectiveness, greater clinical expertise and knowledge and general sense of ‘unease’ (consultant, HP9.58) with the use of ciclosporin:
My personal view is that they probably have equal efficacy, I think infliximab has less side effects so if I was given a free choice, if I was asking it for me or for my loved ones, I would opt for infliximab.
Consultant, HP5.65
When a patient was given infliximab I was rather pleased and when they were given ciclosporin I was less enthusiastic . . . we wondered about the tolerance for the patient and convenience for the patient.
Consultant, HP15.380/394
Nurses’ views mimicked those of their consultant counterparts, expressing a preference for infliximab based on its ease of administration, perceived patient benefits and personal experience and familiarity with the drug. They highlighted the restrictions of patients being hooked up to a ciclosporin infusion for longer periods of time while suffering from bowel disturbances, and noted patients’ preference for infliximab in this respect. Ciclosporin was described as ‘high maintenance’ (nurse, HPN25a, 174):
I prefer it when they have infliximab . . . I think it’s easier for the patients because it’s just one infusion . . . it’s a couple of hours, instead of being hooked up. I mean keep in mind they have profuse diarrhoea . . . I think it’s easier to administer as a nurse and it’s easier for the patient to receive.
Nurse, HPN18.165/169/173/178/189
Personally I feel that the infliximab is better but that’s only because of the experiences we’ve had with the infliximab rather than the ciclosporin . . . seen patients do very well on it, particularly when before perhaps there wouldn’t have been any other outcome than surgery.
Nurse, HPN21.209/217
This personal preference was not, however, across the whole interview cohort, with two consultants describing their preference for ciclosporin, based on personal familiarity with the drug, its quicker-acting properties and its ability to disappear from the system once the drug administration had been halted. However, even these two interviewees stressed the need to retain a level of vigilance and to ‘be more wary’:
Rather more relaxed about it [ciclosporin], paradoxically, simply because I know when the IV [intravenous] infusion is stopped then the drug disappears.
Consultant, HP10.75
I’ve used it [ciclosporin] for a very long time and I’m comfortable with it.
Consultant, HP11.160
In spite of these personal preferences, there remained a genuine sense of uncertainty as to which drug was the more beneficial at the time of the trial, particularly in relation to people’s views of trial entry, trial management and patient recruitment. Most health-care professionals commented that effectiveness was equivocal across the two drugs, genuinely wanting answers from the trial team as to the ‘better’ of the two. This desire was described as patient-driven, based on which of the two was more effective and well received by patients.
If the result was that ciclosporin was a lot better we’d use it and if the results were that infliximab was a lot better we’d use it.
Consultant, HP16.622
The difference (between drugs) is not sufficiently dramatic that I would say it was unethical to put them into a randomised study.
Consultant, HP15.460
Professionals welcomed the CONSTRUCT trial and congratulated the trial team for moving this body of work forward so successfully, in spite of what was described as a somewhat ‘ambiguous’ area of research. They discussed the fact that they had attempted to actively recruit patients into the trial, despite, in the lower recruiting sites, a lack of success, which was put down to busy workloads and lack of joined-up working patterns.
Surgery
Health professionals had mixed views about using alternative therapies, such as surgery, for acute UC. Colectomy, for example, was generally seen as a ‘last resort’ (nurse, HPN21.247) when all other therapies had failed, with some health-care professionals describing feeling ‘disappointed’ (consultant, HP9.210) with colectomy being needed. Consultants argued that although surgery was an option, other medical avenues should be exhausted first before reaching that point:
It’s [colectomy] always a last resort . . . It’s not something I’m comfortable sending them off to have surgery.
Nurse, HPN21.247
Having a colectomy was also seen as a difficult decision to get patients to agree to, and discussions around surgery had to be highly individualised and dealt with on a patient-by-patient basis. Most professionals described colectomy as an option that needed discussion first, to provide both counselling for patients and an assessment of patients’ views: ‘once it comes out you cannot put it back’ (consultant, HP13.338). Patients were seen to be often ‘nervous’ (consultant, HP13.330) of surgery and wanted to put it off for as long as possible, and for this reason alone it was important to have medical options available to prolong the time before surgery. However, at times surgery was seen as being put off for ‘too long’ (consultant, HP20.192), resulting in more difficult decision-making and complications for patients:
I think it comes down to a personal decision, I think it’s very important to have lots of discussions about the options, medical or surgical . . . it’s very difficult if they’re newly diagnosed and they come in with their first presentation . . . some people can come with obvious pre-conceived ideas about things anyway and I think it’s important to explore those feelings.
Nurse, HPN19.254/267
Colectomy was recognised as a ‘lifesaving’ procedure for some patients to which patients responded positively once surgery was over and symptoms were alleviated:
My personal thoughts are that you know that’s their last resort and I think the majority of patients feel the same that they would try any form of medical management.
Nurse, HPN25b.137
Policy development and drug regulation
Health-care professionals were clearly aware of the cost of infliximab and saw the implications of the high cost to be driving policy development and guidelines for practice. Guidelines around the use of the drug ultimately affected clinical decision-making:
There is an issue of cost of course, we’re pushed all the time to stop it for cost reasons.
Consultant, HP15.120
Although health-care professionals understood the need for rigorous guidelines around the use of the drugs, many also linked guidelines to an enforced restriction of the use of infliximab, emphatically arguing that the NICE guidelines were outdated, outmoded and out of step with more recent evidence. This left them feeling frustrated and constrained in their ability to provide the best and most appropriate care for their patients. They described the situation as one in which they had ‘their hands tied’ (consultant, HP6, 127.131), indicating that the more recent clinical evidence and their own personal experience showed infliximab as the drug that needed to be used more widely. As a consequence, they urged that guidelines became more flexible in order to accommodate patients’ needs more appropriately:
It’s very hard to find anyone who supports the NICE guidance in its present format.
Consultant, HP16.516
I sort of treat it with a bit of contempt.
Consultant, HP9.239
The point was repeatedly reinforced that, although the guidelines were there to be adhered to, there was a clear sense of rebellion within the profession, with clinicians ‘ignoring’ (consultant, HP9.236) guidelines when they could find ways around them in order to best meet patient needs. Thus, health-care professionals welcomed a much-needed change to the NICE guidelines that allowed for the greater flexibility of drug use, led primarily from an assessment of patient need:
You can usually find a reason why ciclosporin would be contraindicated . . . I don’t think it’s a particularly sensible NICE guidance based on the lack of evidence that they have to make their decision and therefore in all honesty because we can, we slightly ignore it.
Consultant, HP9.232/236
I think it’s unduly restrictive and I think it will eventually change. I think it’s actually quite hard to adhere to as well. I mean you’ve got a patient who you want to give treatment to, you’re duty bound to give the best treatment and in some cases that’s going to be infliximab.
Consultant, HP14.208
Patient benefit and negotiated care
Throughout the discussions around the administration of the drugs, treatment of patients, personal preference, the value of surgery, and adherence to guidelines and regulations, was a professional focus on patient benefit and need. Nurses and consultants gave many examples of the decisions that were being made that took into account what was best for the patient, especially when it came to a consideration of a patient’s personal circumstances:
I like to tailor the treatment to the patient.
Consultant, HP10.144
The drug we choose ultimately depends on the patient.
Consultant, HP5.75
Nurses were more influenced by the patient experience than the consultants were, perhaps as a result of their extended contact with the patients, and nurses discussed their preference in the context of patient convenience and QoL:
It worked really well on that patient . . . there’s a quicker response time with infliximab . . . patients pass comments and they always tell me I feel much better after this one.
Nurse, HPN24.33/118
It’s whatever is best for the patient . . . I think it’s different for each patient and I think that they need to be given the choice and have all the information because for some patients it can be a very good treatment [colectomy] and give them back their quality of life.
Nurse, HPN22.139/157
As evident in the last quote, the notion of negotiated care was central to the consultation as far as consultants were concerned, and ongoing patient care and decision-making was perceived as being shared across and within multidisciplinary teams. This led to a shared professional responsibility, with the patient–clinician interaction the ultimate example of this:
Our surgeons are very active in decision-making, and multidisciplinary team meetings twice a week.
Consultant, HP5.121
Care is negotiated with the patient, patient input is important, but predefined pathways are according to previous discussions.
Consultant, HP4.113
Patient views are as important as anything else, combined with weighing up risks and benefits, we involve them hugely.
Consultant, HP7.103
The results of this interview study indicate that doctors would make a judgement between the two drugs largely on effectiveness but that there is a clear preference among nurses for infliximab. Ciclosporin is more complex and cumbersome to administer, requiring additional monitoring which tends to be particularly problematic on busy wards with extensive demands on an already overstretched health-care professional workforce. The longer administrative process restricts patient’s movements and leaves them feeling frustrated. It is also excessively demanding on the time of specialist nurses and other health-care professionals, who need to be present to manage any complications, and there is more intensive patient monitoring due to the drug’s adverse risk profile. Consequently, professionals question current NICE guidelines and government regulations around drug use and suggest a sense of rebellion about the restrictions placed on their autonomy and their practice.
Not only are professionals keen to change regulations surrounding current prescribing and drug administration, they also want changes to longer-term patient maintenance and monitoring, preferring infliximab as the longer-term drug of choice in view of its success in dealing with the many complex symptoms that the disease group displays, as well as it being the more familiar, convenient, effective and easy to handle of the two.
Chapter 4 Results synthesis MATRICS
Table 46 illustrates the effects that we investigated in the CONSTRUCT trial, in relation to effects on patients; effects on gastroenterology services and professionals; and effects on the rest of NHS and society. Some effects related to more than one category. We decided that patient views about the drugs and side effects and patient views about their illness including family involvement were effects related to both the patient and the rest of NHS and society. We also decided that professional views about the drugs, preferences, guidelines and equipoise were an effect related to both gastroenterology services, and the NHS and the rest of society.
| Layer 1: effects | ||
|---|---|---|
| Effects on patients | Effects on gastroenterology services and professionals | Effects on the rest of NHS and society |
| 1. Quality-adjusted patient survival [A] | ||
| 2. Patient disease-specific QoL [B] | ||
| 3. Patient generic QoL [C, D] | ||
| 4. Health gain [C] | ||
| 5. Mortality [E] | ||
| 6. Colectomies – planned and emergency [E][H][I] | ||
| 7. Readmissions [E] | ||
| 8. AEs [E] | ||
| 9. Malignancies [E] | ||
| 10. Serious infections [E] | ||
| 11. Renal disorders [E] | ||
| 12. Other new symptoms [E] | ||
| 13. Disease activity [F] | ||
| 14. Patient time off work [G] | ||
| 15. Patient views about the drugs and side effects [H] | 15. Patient views about the drugs and side effects [H] | |
| 16. Patient views about their illness including family involvement [H] | 16. Patient views about their illness including family involvement [H] | |
| 17. Professional views about the drugs, preferences, guidelines and equipoise [I] | 17. Professional views about the drugs, preferences, guidelines and equipoise [I] | |
| 18. NHS costs [J, K] | ||
| 19. Patient time off work [G] | ||
Table 46 illustrates layer 1 and Table 47 illustrates layer 2 of the MATRICS and the specific methods that we used to investigate the effects.
| Layer 2: methods | |
|---|---|
| Code | Method |
| A [1] | Analysis of AUC using the CUCQ |
| B [2] | Analysis of CUCQ scores (patient questionnaire) |
| C [3, 4] | Analysis of EQ-5D scores (patient questionnaire) |
| D [3] | Analysis of SF-12 scores (patient questionnaire) |
| E [5, 6, 7, 8, 9, 10, 11, 12] | Hospital administrative data patient questionnaires |
| F [13] | Calculation of TrueLove and Witts score,4 full blood count, inflammatory markers and albumin |
| G [14, 18] | Patient time off work (patient questionnaire) |
| H [15, 16] | Semistructured patient interviews |
| I [17] | Semistructured interviews with health professionals |
| J [18] | NHS costs (patient questionnaire) |
| K [18] | Hospital activity data |
Table 48 illustrates layer 3 of the MATRICS with the synthesised findings from CONSTRUCT.
| Layer 3: findings | |
|---|---|
| Code | Finding(s) |
| Effects on patients | |
| 1A, 2B, 3C, 3D, 5E, 6E, 7E, 8E, 9E, 10E, 11E, 12E, 13F | There was no significant difference between ciclosporin and infliximab in QAS; disease-specific and generic QoL; mortality and colectomy rates; readmissions; AEs; malignancies; serious infections; renal disorders; other new symptoms |
| 17I | Nursing preference for infliximab because of resource-intensive intravenous administration of ciclosporin. Some doctors perceive fewer side effects, greater patient benefits and easier management of long-term care |
| 4C: health gain EQ-5D | There was no significant difference in generic health gain |
| 14G: patient time off work | There was no significant difference in patients’ time off work |
| 15H: patient views about the drugs and side effects | Patients participants want to normalise their lives, go back to work, feel well again and be less drug dependent From experience, patients who received infliximab reported fewer side effects and more manageable treatment regime when compared with patients who received ciclosporin However, they would favour whichever drug will enable this to happen as easily and quickly as possible |
| 16H: patient views about their illness including family involvement | Patients emphasised extensive impact of the disease on their QoL Patients would welcome early diagnosis and reduction to the effect of lengthy illness and the cacophony of drugs on their lives and the lives of their families Family understanding and support is crucial Patients have to live with unpredictability of symptoms and treatment This is, unlike other chronic conditions, considered an embarrassing disease, making it isolating and awkward Lack of visibility of symptoms makes it hard to share experiences with others Patients and family members seek better and more readily available information and more IBD specialist nurses to support their specific needs Patients fear and avoid surgery for stoma, but many who undergo surgery report on its positive effects on QoL Patients want to understand what causes the disease and possible links to diet and stress |
| Effects on gastroenterology services and professionals | |
| 17I: professional views about the drugs, preference, guidelines and equipoise | Clinician preference for infliximab over ciclosporin as regards treatment and ongoing management: easier administration, fewer side effects, greater patient benefits, easier management of long-term care Views that NICE guidelines need to be brought in line with professional preference suggesting current practice and regulations around drug use should be reconsidered. Maintenance and monitoring easier with infliximab Lack of equipoise among those interviewed Benefits of trial for seeking answers to which drug is more efficacious and supporting regulatory change Consultants’ views that nurses are less familiar with administering ciclosporin and increased nurse workload for ciclosporin patients |
| Effects on the rest of NHS and society | |
| 18J, 18K: NHS costs | Total health-care costs were higher for infliximab patients due solely to the higher drug acquisition cost |
| 19G: patient time off work | Treatment given had no effect on patients’ time off work |
Chapter 5 Discussion
Summary of findings
The CONSTRUCT trial recruited 270 participants from 52 hospitals in England, Scotland and Wales between May 2010 and February 2013 and followed them up until March 2014, that is, for between 1 and 3 years following treatment with either infliximab or ciclosporin, allocated at random. Rigorous analysis of 242 (90%) patients, excluding 28 who provided no analysable data, showed no significant difference between the two drugs in the primary outcome of QAS, equivalent to the AUC of QoL scores from the CUCQ (mean difference in AUC/day 0.0297 favouring ciclosporin, 95% CI –0.0088 to 0.0682; p = 0.129). There was also no significant difference in EQ-5D scores (QALY mean difference 0.021 favouring ciclosporin, 95% CI –0.032 to 0.096; p = 0.350); SF-6D scores (mean difference 0.0051 favouring ciclosporin, 95% CI –0.0250 to 0.0353; p = 0.737); colectomy rates (OR 1.350 favouring infliximab, 95% CI 0.832 to 2.188; p = 0.223); time to colectomy (hazard ratio 1.234 favouring infliximab, 95% CI 0.862 to 1.768; p = 0.251), the incidence of SARs (14 participants on infliximab vs. 9 on ciclosporin, OR 0.660 favouring ciclosporin, 95% CI 0.282 to 1.546; p = 0.338) or the incidence of SAEs (16 participants on infliximab vs. 17 on ciclosporin, OR 0.999 favouring infliximab, 95% CI 0.473 to 2.114; p = 0.998). Three patients died, all of whom had received infliximab (p = 0.25).
Although length of hospital stay after randomisation ostensibly did not differ between drugs (mean adjusted difference 1.542 days more for ciclosporin, 95% CI –1.297 to 4.381 days, assuming normal distribution of residuals in general linear model; p = 0.286), that distribution was so skewed as to invalidate the assumption of normality; hence, we transformed these stays by taking logarithms and estimated that the geometrical mean of adjusted stays after ciclosporin was a factor of 1.523 times longer than that after infliximab (95% CI 1.278 to 1.817; p < 0.001). Although infliximab thus used fewer hospital resources, its much higher acquisition cost resulted in a much lower total NHS cost for ciclosporin (mean difference –£5632, 95% CI –£8305 to –£2773; p < 0.001).
Interviews with patients revealed the substantial impact of UC on their QoL and the potential benefits from these medical treatments and from surgery. Participants treated with infliximab generally spoke more positively about the treatment than did those treated with ciclosporin. Interviews with nurses showed a preference for infliximab, largely because of the resource-intensive infusion protocol for ciclosporin and the resulting restrictions on patients. Although some consultants favoured infliximab, most were indifferent, perceiving both drugs as effective, with the more predictable speed of benefit with ciclosporin balancing its perceived higher rate of side effects.
Trial progress and conduct
This trial took nearly 7 years to complete from the first notification of intention to fund in November 2007. Recruitment started in 2010. Our initial plan was to recruit 480 patients and follow them all for 2 years, measuring change in disease-specific QoL as our primary outcome. Research governance delays99 and slow recruitment led us to seek approval from the DMEC, TSC and HTA for a change in the primary outcome in 2010 and a funded trial extension in 2011. The essential changes were:
-
Change in the primary outcome from change in disease-specific QoL scores at 2 years to QAS. 55 QAS is derived from the AUC described by scores from disease-specific QoL questionnaires completed by participants 6-monthly for 1–3 years after randomisation.
-
Reduction in target analysable sample size from 360 to 250.
-
Follow-up to last for 1–3 years depending on recruitment date.
The implementation of these changes required additional follow-up visits and patient-completed questionnaires.
Sixty-seven hospitals from England, Scotland and Wales collaborated with us on this study, of which 62 recruited patients to the cohort and 52 recruited participants to the trial. This large number of sites was necessary because of the relative infrequency of admission with ASC, which is a life-threatening condition and patients are very sick when admitted to hospital. To facilitate recruitment to the trial the opportunity was explained to patients on admission and baseline data collected at that time. This generated a cohort of 1532 patients with ASC, from which the trial participants were recruited when they failed to respond adequately to treatment with intravenous hydrocortisone. The patients in this cohort have given consent for their progress to be monitored for 10 years using routinely collected data. Fifty per cent of the patients in the cohort (n = 775) settled without steroids or responded to initial treatment with intravenous hydrocortisone and 74 were so ill they went for colectomy before randomisation was possible. We eventually recruited 270 patients to the trial and followed them up for 1–3 years.
We measured disease-specific QoL using the CUCQ and used the scores to compare QAS. The CUCQ is derived from the UK-IBDQ, an anglicised version of the Inflammatory Bowel Disease Questionnaire that we validated 14 years prior to this study. 61 Modification was required because the UK-IBDQ has only been validated for stable or moderately active IBD and we needed an instrument that would be appropriate to use when the disease was severe as well as mild or in remission. We validated the CUCQ concurrently with the trial, using data from participants and other sources. 100
The validated 32-question CUCQ that we used is different from the McMaster Inflammatory Bowel Disease Questionnaire18 in several aspects (see Appendix 18). The wording of the questions in the CUCQ are modified and anglicised to use in the UK. The response options of the CUCQ were simplified using a combination of close-ended and a four-level Likert scale answers instead of the seven-level Likert answers used in the IBDQ. In order to avoid acquiescence bias (yes-set),101,102 three questions about happiness, being relaxed and having energy were phrased in a way that the higher the number, the higher level of QoL for that attribute. Their scores were then reversely coded before adding them to the total score. The items covered by the 32 questions in both the CUCQ and IBDQ are relatively the same. Therefore, we did not use the IBDQ in the construct validity testing of the CUCQ and used the SF-12, EQ-5D and disease severity indices instead. However, the CUCQ includes a question about urgency and rushing to toilet, which does not exist in the IBDQ. The IBDQ asks about the need to get up at night to go to toilet and the lack of good sleep in one question, whereas the CUCQ asks about these two items in two separate questions. The advantages of using the CUCQ are its simplicity, wider coverage of the symptoms of acute IBD, and that it is free to use by health-care professionals.
To be able to continue to measure QoL after surgery we developed and validated an extension to the CUCQ which asked questions more relevant to patients with a stoma (CUCQ+). This was sent to participants post colectomy by the trial office, on discharge and 4, 8 and 12 weeks post discharge.
Deaths and withdrawals meant that QoL questionnaire scores were available from 121 participants in each group up to the end of their participation in the trial, a retention rate for the primary outcome of 90%. Secondary outcomes were measured using data extracted from patient records by research professionals and recorded in CRFs (with completion rates as shown in Table 6). The retention rate to 2 years is excellent but falls off at 30 and 36 months, reflecting mainly non-attendance of participants as their condition has improved. The completion rate at 18 months is low because the 18-month data collection point was added after the protocol was modified and required reconsent, meaning that some participants were missed.
The 121 participants in each group with evaluable primary outcome data were sufficient to demonstrate clearly that there is no significant difference between the two treatments in clinical effectiveness over the first 3 years after treatment. This does, however, also reflect the QoL after surgery, a high colectomy rate in both arms of the study (41% following infliximab; 48% after ciclosporin) and continuation of treatment with infliximab or ciclosporin beyond the prescribed intervention period at the discretion of the attending physician. Ciclosporin was discontinued by all participants by 180 days, whereas infliximab was continued for 1 year in one-quarter of participants and up to 3 years in a few.
In 2009 we applied to the HTA programme and the MRC to use the CONSTRUCT trial to explore the possibility and validity of using data collected at the point of care to support a pragmatic trial that was embedded in clinical care. This Electronic records Underpinning REsearch and CAre (EURECA) proposal was not funded but we nevertheless took the opportunity to explore the feasibility and acceptability of using GeneCIS, a non-commercial clinical management system that has been successfully deployed for many years in a small number of disciplines in six hospitals, to support the trial, while also giving participating sites the opportunity to use this system to support clinical care. The system required customisation to underpin some aspects of the trial and enabled extensive quality control to be applied to the data, especially where it was possible to triangulate data received from different sources (CRFs, AE reports and patient questionnaires). Intensive efforts were made to verify or clarify data that was ambiguous or in conflict (e.g. the dates and nature of an operation). Sixteen sites took up the opportunity to enter data directly into the system, the rest returning their CRFs and patient questionnaires to the trial office for data entry. Although our experience with CONSTRUCT has shown that an operational clinical information system can support a pragmatic RCT and it is likely that the concept of integrated data collection for clinical care and research will take some time to take root. In spite of the failure of the EURECA proposal to secure funding to evaluate this in detail, we believe the principle of using high-quality point-of-care clinical data to support prospective pragmatic trials remains valid, particularly if national standards are applied to the structure and content of electronic patient records. 103
The management and co-ordination of the CONSTRUCT trial was based on PRojects IN Controlled Environments Version 2 principles. We held weekly meetings of the core team in the trial manager’s office, at which we reviewed recruitment and retention, SAEs that needed discussion and any other issues raised, such as progress with the concurrent validation of the CUCQ. All of the research team attended monthly TMG meetings and we used these meetings more formally to review trial progress, including action, risk and issue logs. When specific issues needed to be addressed we set up one-off TMG ‘operational group meetings’ to resolve the issues or make plans. The TSC and DMEC met to review progress and give valuable advice at key milestones in the project. The chief investigator and trial manager liaised with sites mainly through e-mail, or by telephone if the issue was complex or urgent.
We were fortunate in this study to have benefited from enthusiastic, expert and thoughtful input from representatives of the patient community. Mike Hilton was a regular and wise contributor to our monthly TMG meetings, providing particularly useful advice when we were developing a post-colectomy extension to the primary outcome QoL instrument. He was also instrumental in improving recruitment by jointly authoring a letter to sites with the chief investigator. Laura Hawes and Peter Canham regularly attended the TSC and DMEC meetings, respectively, and provided feedback and useful advice over the course of the study. Clare Baggridge also gave us very useful insights from the perspective of a health professional who has undergone colectomy for UC. They have all given us permission to recognise their input in this way. Mike Hilton’s thoughts on his engagement with the study are included at Appendix 22. The input of lay members throughout has been invaluable and we believe it adds weight to the external validity of our findings.
Strengths and weaknesses of the study
The CONSTRUCT trial has been a complex trial to run for many reasons. The severity of the illness, nature of the symptoms and relative rarity of the presentation of ASC meant that case ascertainment, recruitment, retention and data collection have all been challenging. Methodologically we took a pragmatic approach, allowing local investigators flexibility in the clinical situation. For example, we allowed local clinical judgement to over-ride the results of objective tests when assessing the response to intravenous steroids. This was important, as it is well known that high doses of steroids can suppress markers of disease severity, giving a false and dangerous impression of recovery. We also allowed local flexibility in the duration of treatment with steroids and clinicians were allowed to exercise clinical judgement regarding the continuation or change in treatment once the per-protocol intervention was complete. As a result many participants continued on the allocated treatment for some time, more continuing for longer on infliximab than ciclosporin. The use of concomitant immunosuppressive therapy with azathioprine or 6-mercaptopurine after the intervention period was similar in both groups. We believe that this pragmatic acceptance of clinical judgement means that the results of the trial genuinely reflect current clinical practice.
Because of the severe and incapacitating nature of the symptoms suffered by patients with ASC we informed them about the trial at the earliest opportunity after admission and sought their consent for baseline data collection at that time. This meant that disease-specific QoL (the primary outcome measure) was completed by potential participants before treatment with steroids and some days before they were randomised into the trial. In spite of this, the two groups were well matched at baseline in terms of demographic characteristics and baseline CUCQ scores. There was, however, an unexpected preponderance of males in the trial in both groups. There are a number of possible explanations for this, which we are investigating.
The transition from cohort to trial varied from site to site, and the CONSORT diagram identifies 178 paticipants who were not randomised, although they were potentially eligible. For some of these potential participants, we have not been able to ascertain the reasons why they did not take part in the trial, but we suspect that patient or professional preference for a treatment may have inhibited consent and randomisation in some cases. The participants interviewed had experience of only one treatment, but there appeared to be more enthusiasm and satisfaction for infliximab than for ciclosporin. For both participants and professionals this was largely because of the cumbersome and constraining requirements for intravenous infusion of ciclosporin and it is possible that oral treatment may be more popular. We chose intravenous ciclosporin, before a transfer to the oral version, as we feared that tablets might be poorly tolerated and absorbed in very sick patients; however, given the effectiveness of ciclosporin, we feel that first-line oral therapy should be explored further, particularly as colonic release preparations become available. 104
The primary outcome (QAS) was compared using data from QoL questionnaires (PFQs) completed at baseline, 3 months and 6 months and then 6-monthly until February 2014. All participants were, therefore, eligible for follow-up for 1 year, but only 34 (17 in each arm) were recruited early enough to be followed up for 3 years. The number of PFQs completed at each time point, and the reasons for shortfall, are shown in the CONSORT diagram. Some participants failed to return a PFQ at one or more time points but, overall, primary outcome data were available for 121 participants in each arm. There were three deaths and 31 withdrawals (14 infliximab and 17 ciclosporin) over the course of the study, which account for some gaps, but in some cases, and despite reminders, expected questionnaires were not returned. This is understandable: UC is a debilitating and depressing condition for the sufferer and, when well, patients do not like being reminded of their illness.
The incidence of SARs was similar in both arms of the trial. As the known side effect profile of both drugs is very extensive, we specified in the protocol that we did not require expedited reporting of serious events unless they were thought to be unexpected. Assessment of expectedness included featuring in the specification of product characteristics or being attributable to disease progression or surgical intervention. At the start of the trial we stressed in the Fieldwork Handbook that AESFs have dual purpose. The first is to aid the decision process that will define whether or not the event is unexpected and, through this, enable us to detect any trend towards SUSARs to the treatment which might indicate a threat to patient safety. We detected no SUSARs, which is not surprising given the very extensive clinical experience with drugs that have both been in use for the treatment of IBD for over 20 years. The second purpose of the reporting system was to give us the data to be able to compare the incidence of events which are known side effects of the two drugs as a secondary outcome measure. This was a complex process that we addressed as rigorously as possible. Although all events had been monitored during the course of the study, two clinicians in the research team reviewed all SAEs again at the end of the study in order to ensure the consistency of our interpretation of their relatedness to the IMPs. When considering the relatedness of each event to the IMP, decisions were informed by data from sites on the duration of use of treatment with the IMP beyond the 3-month intervention period specified in the protocol. It was important that we did this because once the intervention period was complete, clinical management was no longer per protocol and therefore varied from subject to subject according to their progress and the clinical judgement of the local team. We also took into account the different pharmacokinetic profiles of the two IMPs. It is known that the bio-availability of ciclosporin is short-lived after cessation of treatment, whereas bio-availability of infliximab, although varying from subject to subject, can persist up to 6 months after the last infusion. 105 Therefore, with the exception of malignancy, we considered that relatedness would be unlikely if the event occurred more than 1 month after cessation of treatment with ciclosporin, but could be related up to 6 months after infliximab. These principles were agreed with the Clinical Trials Unit and the DMEC before we undertook the review.
Both UC and the drugs being evaluated are known to cause many clinical problems, some of which may be manifest together in a single event. When an AE report documented a serious event (usually admission) but included more than one problem (e.g. abnormal liver and renal function test results), we used our clinical judgement to decide which (if any) could be related to the IMP and which was the prime cause for admission. In addition, and in order to capture all problems reported, we analysed the events by both the total number of events, and the total number of problems, which we categorised by affected body system. The results of this re-evaluation of serious events led to few changes in category (which were all agreed with local PIs) and overall there was no difference between the two drugs in their toxicity profile in the study.
There were three malignancies: a case of endometrial cancer on ciclosporin, and a basal cell skin cancer and a colorectal cancer on infliximab. The last of these was unusual in that, although it was detected in the colonic specimen removed at colectomy, the histology identified signet ring cells, suggestive of a lung primary. Although dual primaries were considered, this was thought to be unlikely on review by the local multidisciplinary team.
Three participants died, all following treatment with infliximab, a difference from ciclosporin that is not statistically significant. Two deaths were due to sepsis (one pre operative and one post operative). In both cases this was associated with multiple comorbidities, including diabetes mellitus. One was from disemminated malignancy from colorectal cancer.
We took a rigorous approach to data quality. We maintained close contact with sites by e-mail and repeatedly stressed the importance of data completeness. We reviewed all the primary outcome data entered onto GeneCIS before analysis. The overall item omission or transcription error rate was < 1% and those identified were corrected. Many secondary outcome data were derived from a variety of sources, giving us the opportunity to triangulate many items. We undertook monitoring visits to eight sites, including six of the highest recruiting hospitals. These did not identify any data issues except for a few AEs that had not been reported. None of these was a SUSAR but two were SARs.
An evidence review commissioned by NICE in 200853 expressed concerns over the uncertainty in estimates of effectiveness, particularly with regard to colectomy rates, which had been used to model the cost-effectiveness of infliximab versus ciclosporin. This was because of the very small number of RCTs, which themselves were small, and to criticism of the use of evidence from mixed-treatment comparisons. The CONSTRUCT trial provided the opportunity to gain trial-based data from a sufficiently powered head-to-head comparison.
The economic evaluation showed very little difference in effectiveness at 30 months using all available data and adjusting for the number of days each patient had been in the study (0.021 QALYs in favour of ciclosporin). Very similar results were shown using more conventional complete-case analyses at 12 and 24 months where the mean difference in QALYs were 0.027 and 0.022 QALYs, respectively, in favour of ciclosporin. Infliximab, however, is considerably more costly in terms of direct treatment costs, whereas the two treatments did not differ significantly in terms of other NHS resources. Given the similar clinical effectiveness this means that infliximab is dominated by ciclosporin. The probabilistic sensitivity analysis, which takes into account the joint uncertainty in costs and effects, shows ciclosporin to have an 85% chance of being cost-effective when considered against a range of willingness-to-pay thresholds, including the £20,000–30,000 per extra QALY currently used by NICE.
These results differ from a modelling exercise by Punekar and Hawkins,51 which showed infliximab to be more costly but also more effective than ciclosporin. The base-case incremental cost per additional QALY gained was £19,545, which, being below the current NICE threshold, was judged to be cost-effective. A more recent application of the same model to the Netherlands50 produced an incremental cost-effectiveness ratio of €24,277. Modelling, however, requires assumptions which may not be borne out in clinical practice. For example, both studies assumed that infliximab patients receive three infusions as per protocol, whereas in our pragmatic study the number of infliximab infusions was at the discretion of clinicians who delivered more than three infusions to 34 of 135 infliximab patients and up to 13 infusions in one case, and not all participants received the initial three infusions. Similarly, cost-effectiveness results of the models were shown to be sensitive to assumptions about body weight which the UK base case assumed was 80 kg per patient and the Netherlands base case assumed was 70 kg. We were able to use each participant’s treatment using their actual weights: mean 74.0 kg for ciclosporin participants and 74.4 kg for infliximab participants.
The mixed-methods approach we adopted for the study shows the importance of considering a wider range of information. Given the lack of any significant difference between the two drugs in quantitative comparison of their clinical effectiveness, the results of our qualitative research assume greater importance and the validity of our findings requires detailed scrutiny. The views we obtained from participants about infliximab, ciclosporin and surgery are important, unique, detailed, from a broad age range and UK wide. The interviewees were chosen at random, but stratified by age, treatment and surgery, and the demographic and baseline characteristics of the 20 participants who were interviewed were no different from the rest of the participants in the study. Applying the criteria of credibility, transferability, dependability and confirmability set out by Guba,106 we believe we used a rigorous qualitative approach which we have described and reference in detail (see Appendix 19). 107–111
Our approach and conduct of interviews with professionals were similarly rigorous. Many doctors volunteered a treatment preference and those who preferred infliximab tended to hold strong views, although most stressed that clinical effectiveness and safety were their prime concerns, hence their support for the trial and willingness to adopt a position of equipoise. Nurses were more strongly critical of ciclosporin, in particular the cumbersome and constraining intravenous regimen. We were alert to a potential lack of equipoise at some sites and monitored this. We believe that the final distribution of participants from across the UK, and the close matching of baseline data from the two groups, demonstrates that there was no bias relating to this.
The establishment of a cohort of 1532 subjects who were admitted to hospital with ASC, and have given their consent for us to monitor their clinical progress over 10 years, is a considerable strength of the study, particularly as we also have consent to monitor the progress of the trial participants by questionnaires over this time.
As a trial to explore effectiveness rather than efficacy our outcome measures were subjectively patient focused, rather than objective measures of disease activity, but the value of both drugs in terms of efficacy has already been established by the CySIF trial41 and our results are concordant with their findings.
Owing to the small number of participants who contributed cost data at 36 months, we conducted the economic evaluation to 30 months rather than 36 as in the effectiveness evaluation. The number of missing data varied between resource items. However, this was low for the main cost drivers and all missing data were randomly distributed according to patients’ clinical and sociodemographic variables and study arm.
We are aware that our attempt to capture all costs may have led to some double counting. For example, although the CRF would capture all accident and emergency attendances at the centre in which the participants was receiving their treatment (case notes), it would miss any that may have occurred at other hospitals (e.g. while the participant was on holiday). A question about accident and emergency attendances at other hospitals was therefore included in the PFQ but despite the clarity of the instruction it is possible that some participants reported study centre accident and emergency attendances which were already recorded on the CRF.
Our qualitative data from participants were obtained by telephone interview. We confirmed that the demographic characteristics matched those of the overall study participation and saturation was reached with professional interviews as with patient interviews.
External validity
Participants were recruited from a good mix of large and small district general and teaching hospitals, distributed throughout Great Britain, and we believe our study population is representative of severe UC in the UK. The participants were predominantly Caucasian and further analysis is needed to explore the extent to which ethnicity and age reflect the UK distribution of ASC.
Our findings are concordant with the results of the GETAID CySIF study,41 an efficacy trial in 116 subjects that found no difference in patient response rates, colectomy rates or AEs 3 months after treatment (Table 49).
| Outcome | Infliximab (%) | Ciclosporin (%) |
|---|---|---|
| Treatment failure | 54 | 60 |
| Response at day 7 | 86 | 84 |
| Colectomy rate at 3 months | 23 | 18 |
| SAEs | 29 | 18 |
The methodologies and the findings of the two studies are complementary. CySIF used evidence from hospitals in Western Europe to establish that both drugs are equally effective, whereas we used a UK-wide study to show that this also applies to their clinical effectiveness.
The CySIF and CONSTRUCT trials are the only two fully reported randomised trials of these two drugs in this condition, and provide compelling evidence that there is no difference between ciclosporin and infliximab in efficacy or clinical effectiveness. Although, overall, ciclosporin is cheaper than infliximab, the single infusion is more resource intensive to administer and is less well liked by both patients and nurses who administer the infusions. The side effect profile is similar and, given orally, it would be much cheaper to administer. We were concerned that oral administration would not be feasible in very sick patients, but this would undoubtedly be possible in some patients, and we believe that the use of oral ciclosporin to pre-empt admission is worth evaluating. A very small trial comparing oral ciclosporin with intravenous infliximab in 30 participants has been reported in abstract form only and found no difference in efficacy 2–3 years after treatment. 112
The study started to recruit in 2010 and, now that Remicade is off patent, biosimilar biologic treatments have started to appear,113 but this study will provide a benchmark for their effectiveness. The cost of infliximab is likely to reduce with this competition. Newer biologic treatments are also appearing, some of which can be given orally.
The pragmatic approach taken in CONSTRUCT reflects the real world of clinical practice. In particular, we believe that the clinical judgement allowed in determining how ill potential participants were, the flexibility permitted in assessing their response to intravenous steroids and the clinical freedom encouraged in deciding how long to continue treatment after the intervention period was complete all contribute to the external validity of our results.
Similarly, our primary outcome includes the impact of continuing medical treatment and of colectomy undertaken when treatment fails. Although the impact of surgery on the participant tends to be positive, this is seen equally in both arms of the study. Although this may distort the apparent beneficial effect of the primary intervention, both in terms of clinical outcome and cost, it will not impact on the comparative results. As would be expected, we have shown that there is a dip in QoL after surgery, but this recovers.
Our data so far do suggest that colectomy is not a bad outcome. This conclusion reflects our previous analysis of routinely collected data about IBD from the whole of England, which demonstrated a continuing mortality after admission with UC without colectomy. 7
Patients fear colectomy, largely because of the threat of an ileostomy, but surgical techniques and expertise mean that a stoma is not necessarily a long-term outcome. The patients we interviewed who had undergone surgery were pleased with the result, particularly those who had suffered long-term ill health before their final exacerbation. Given the epidemiological evidence that mortality after admission with UC without surgery is as high 3 years later as mortality from emergency colectomy,7 there are reasons to consider surgery earlier rather than later. Whether or not this requires a large-scale randomised trial to compare medical and surgical treatment should be debated, particularly as observational studies have shown that treatment with both drugs is associated with a rising colectomy rate as time goes on – as high as 80% at 7 years after treatment with ciclosporin (Table 50) – although the rate appears to be lower, and declining, after infliximab. 122 Long-term follow-up of our trial and cohort patients will be very important to corroborate or refute these findings. We will analyse routinely collected data about readmission, colectomy and mortality over 10 years to look at this.
| Study | Drug | 2 weeks | Late | Details |
|---|---|---|---|---|
| Teisner et al., Denmark114 | Infliximab | 37% | Median follow-up 22 months; range 4–57 months | |
| Mortensen et al., Denmark115 | Infliximab | 18% | 39% | Median follow-up 17 days; range 2–651 days |
| Jarnerot, Sweden116 | Infliximab | 46% | At 2 years | |
| Jakobovits et al., Oxford117 | Infliximab | 15% | 57% | At 3 months |
| Waters et al., Exeter118 | Infliximab | 16% | 64% | At 4 years |
| Campbell et al., Oxford119 | Ciclosporin | 58% | At 7 years | |
| Molnar et al., Hungary120 | Ciclosporin | 22% | 63% | Mean follow-up 2.9 years |
| Actis et al., Italy9 | Ciclosporin | 65% | At 7 years | |
| Moskovitz et al., Belgium121 | Ciclosporin | 88% | At 7 years |
Implications
The comprehensive design of CONSTRUCT has greatly strengthened the conclusions of CySIF, a small European trial that studied few clinical outcomes. There is no difference between infliximab and ciclosporin in the effectiveness with which they treat steroid-resistant ASC, not only in the incidence of ‘treatment failure’ but also in HRQoL, both generic and specific to UC. Nevertheless, nurses generally disliked the greater resource demands of intravenous ciclosporin, which led to participants on that drug spending a mean of 2 extra days as inpatients. Although 20 representative trial participants generally spoke more favourably about infliximab, gastroenterologists were more equivocal. Given current NHS budgetary constraints the dominant finding of our rigorous evaluation is that, even after subtracting the difference in hospital costs because of longer hospital stays, the net mean cost of ciclosporin to the NHS per participant was still £5632 lower than that of infliximab.
As NICE is currently consulting on the use of infliximab in severe colitis, these findings are timely. The clear conclusion from our quantitative data is that, despite the substantial difference in cost, the two drugs are similar in effectiveness and toxicity. Nevertheless, nurses prefer to administer infliximab and patients who received it seem to be more satisfied than those who were given ciclosporin. In designing CONSTRUCT we chose to start with ciclosporin given intravenously before switching to oral administration, as we believed that most patients would be too sick for immediate oral treatment. However, it is clear from our qualitative findings that intravenous ciclosporin is inconvenient and disliked by many patients and professionals and hence we wonder whether or not oral ciclosporin would have been effective from the start, and could even pre-empt admission in patients who are developing severe symptoms. These are important questions for research, especially as colonic release formulations are already in development.
Future research
We hope to follow both trial and cohort patients for up to 10 years, thus yielding important conclusions on long-term outcomes, notably on further colectomies and hospital admissions. Before then, CONSTRUCT has generated several questions which we plan to address through further analysis including:
-
How representative are our participants of UK patients with ASC?
-
Do females respond to steroids better than males?
-
How well do our baseline data predict subsequent need for colectomy?
-
How quickly does QoL improve after colectomy?
-
Can CONSTRUCT help to address whether or not colectomy is more clinically effective and cost-effective than medical treatment?
-
Do the characteristics of investigators influence their attitude to treatments?
We suggest that there is a strong case for new trials to evaluate:
-
the relative clinical effectiveness and cost-effectiveness of medical and surgical treatment for ASC
-
the relative clinical effectiveness and cost-effectiveness of intravenous versus oral ciclosporin for acute UC especially if colonic release ciclosporin becomes available
-
the relative clinical effectiveness and cost-effectiveness of using oral ciclosporin to pre-empt admission in severe but pre-acute colitis
-
the relative clinical effectiveness and cost-effectiveness of new anti-tumour necrosis factor drugs, currently the subject of a NICE appraisal and intravenous or oral ciclosporin. 123
Conclusions
The total cost to the NHS was much higher for infliximab than ciclosporin. Nevertheless, there was no significant difference between the two drugs in clinical effectiveness, colectomy rates, incidence of SAEs or reactions, or mortality.
Acknowledgements
Contributions of authors
All 13 authors contributed to design and data collection or analysis and interpretation, commented on successive drafts and approved the version to be published. More specifically:
John G Williams (Professor of Health Services Research and Consultant Gastroenterologist) was principal applicant and chief investigator of the trial. He wrote the first draft and co-ordinated the editing of the final report.
M Fasihul Alam (Health Economist and Modeller) contributed to analysing and interpreting the economic data and drafting this report, and led designing the cost-effectiveness modelling.
Laith Alrubaiy (Clinical Academic Trainee in Gastroenterology) contributed to the validation of the CUCQ and CUCQ+, screening and analysing AEs and drafting this report, in which he contributed in particular the first draft of the background and literature review.
Clare Clement (Qualitative Research Officer) collected and analysed qualitative data; she led the writing of the professional views.
David Cohen (Professor of Health Economics) was coapplicant and PI for health economics.
Michelle Grey (Data Manager) co-ordinated data collection.
Mike Hilton (service user) contributed to the design of the trial and drafting this report.
Hayley A Hutchings (Associate Professor) developed and validated the CUCQ and CUCQ+ analysis, led the MATRICS results and contributed to interpreting data and drafting this report.
Mirella Longo (Health Economist) contributed to analysing and interpreting the economic data, and drafting this report.
Jayne M Morgan (Information Scientist) was co-applicant and led the development and operational use of the data management system, GeneCIS.
Frances L Rapport (Professor of Qualitative Health Research) was coapplicant and the PI for the qualitative research.
Anne C Seagrove (Trial Manager/Research Officer) was co-applicant and trial manager; she contributed to developing and implementing the design and management of the trial. She also collected and analysed qualitative data, led the writing of the participant views and contributed to drafting and co-ordinating this report.
Alan Watkins (Senior Trials Statistician) was the trial statistician; he undertook primary statistical analysis and contributed to interpreting data and drafting this report.
Other acknowledgements
We would like to thank the external members of the TSC for their advice and support for the project: Professor Chris Hawkey, chairperson (University of Nottingham), Ms Laura Hawes (service user), Dr Stephen Grainger (Barking, Havering and Redbridge University Hospitals NHS Trust), Dr John Mansfield (Newcastle upon Tyne Hospitals NHS Foundation Trust) Professor Jon Rhodes (University of Liverpool) and Dr Simon Travis (Oxford Radcliffe Hospitals NHS Trust). Our thanks go also to the DMEC comprising Professor Stirling Bryan (University of British Columbia), Mr Peter Canham (service user), Professor Tim Peters (University of Bristol), Dr Chris Probert (University of Liverpool) and Professor Phil Routledge (Cardiff University).
We would like to thank a number of people who helped towards the successful completion of the study:
The NIHR HTA programme for funding CONSTRUCT and their staff for generous and patient support. The study was adopted by the NIHR research portfolio.
The study participants, giving so generously of their time and sharing their experiences with us, especially for completing questionnaires for up to 3.5 years.
Ian Russell gave methodological support until his retirement in March 2015.
Claire Baggridge, Peter Canham and Laura Hawes provided useful advice from a service user perspective. Peter Canham and Laura Hawes also played a helpful role on the TSC and DMEC.
The PIs and the research nurses who played an invaluable role in helping to identify, recruit, randomise participants and collect data; their clinical colleagues for referrals to the study and pharmacy personnel who helped dispense trial treatment. In addition, the research support teams who provided helpful assistance throughout the study.
| PI | Trust/health board |
|---|---|
| Dr Tariq Ahmad | Royal Devon and Exeter Foundation Trust |
| Dr Navneet Alhuwalia | Stockport NHS Foundation Trust |
| Dr Yeng Ang and Dr Neeraj Prasad | Wrightington, Wigan and Leigh NHS Foundation Trust |
| Dr Anurag Argawal | Doncaster and Bassetlaw Hospitals NHS Foundation Trust |
| Dr Ian Arnott | NHS Lothian |
| Dr Bijay Baburajan | Maidstone and Tunbridge Wells NHS Trust |
| Dr Jamie Barbour | Gateshead Health NHS Foundation Trust |
| Dr P Basumani and Dr Yousif Mohamed | Rotherham NHS Foundation Trust |
| Dr Conrad Beckett | Bradford Teaching Hospitals NHS Foundation Trust |
| Dr Stuart Bloom | University College London Hospitals NHS Foundation Trust |
| Dr Jake Burdsall | Wye Valley NHS Trust |
| Dr Faheem Butt | South Tyneside NHS Foundation Trust |
| Dr Simon Campbell | Central Manchester University Hospitals NHS Trust |
| Dr Tim Card and Dr A Shonde | Sherwood Forest Hospitals NHS Foundation Trust |
| Dr Andy Cole | Derby Hospitals NHS Foundation Trust |
| Dr Tom Creed | University Hospitals Bristol NHS Foundation Trust |
| Dr Fraser Cummings | University Hospital Southampton NHS Trust |
| Dr Howard Curtis | South London Healthcare NHS Trust |
| Dr Helen Dallal and Dr Arvind Ramadas | South Tees Hospitals NHS Foundation Trust |
| Dr Jayne Eaden | University Hospital Coventry and Warwickshire NHS Trust |
| Dr Cathryn Edwards | South Devon Healthcare NHS Foundation Trust |
| Dr David Elphick | Chesterfield Royal Hospital NHS Foundation Trust |
| Dr Alex Ford | Leeds Teaching Hospitals NHS Trust |
| Dr Carol Francis | Countess of Chester Hospital NHS Foundation Trust |
| Dr Vivek Goel | Aneurin Bevan University Health Board |
| Dr John Gordon | Hampshire Hospitals NHS Foundation Trust |
| Dr Stephen Grainger | Barking Havering and Redbridge University Hospitals NHS Trust |
| Dr Barney Hawthorne | Cardiff and Vale University Health Board |
| Dr David Hobday | City Hospitals Sunderland NHS Foundation Trust |
| Dr Chris Hovell | Dorset County Hospitals NHS Foundation Trust |
| Dr Tariq Iqbal | University Hospitals Birmingham NHS Foundation Trust |
| Dr Alan Ireland | Brighton and Sussex University Hospitals NHS Trust |
| Dr Peter Isaacs | Blackpool Teaching Hospitals NHS Foundation Trust |
| Dr Sauid Ishaq | The Dudley Group NHS Foundation Trust |
| Dr Aida Jawhari | Nottingham University Hospitals NHS Foundation Trust |
| Dr Sarah Langlands | Frimley Park Hospital NHS Foundation Trust |
| Dr Keith Leipera and Dr S Subramanian | Royal Liverpool and Broadgreen University Hospitals NHS Trust |
| Dr Andy Li | Western Sussex Hospitals NHS Trust |
| Dr Chee Lim | Heart of England NHS Foundation Trust |
| Dr Jimmy Limdi | Pennine Acute Hospitals NHS Trust |
| Dr James Lindsay | Barts Health NHS Trust |
| Dr Alan Lobo | Sheffield Teaching Hospitals NHS Foundation Trust |
| Dr Chris Macdonald | North Cumbria University Hospitals NHS Trust |
| Dr A di Mambro and Dr David Parker | Weston Area Health NHS Trust |
| Dr John Mansfield | The Newcastle upon Tyne Hospitals NHS Foundation Trust |
| Dr Joel Mawdsley | West Middlesex University Hospital NHS Trust |
| Dr Brian McKaig | Royal Wolverhampton NHS Trust |
| Dr John O’Donohue | Lewisham and Greenwich NHS Trust |
| Dr Richard Pollok | St George’s University of London |
| Dr Lindsay Potts | NHS Highland |
| Dr Munuswamy Pushpakaran | Basildon and Thurrock University NHS Foundation Trust |
| Dr S Ramakrishnan | Warrington and Halton Hospitals NHS Foundation Trust |
| Dr Andrew Robinson | Salford Royal NHS Foundation Trust |
| Dr Matt Rutter | North Tees and Hartlepool NHS Foundation Trust |
| Dr Othman Saraj | York Teaching Hospital NHS Foundation Trust |
| Dr Sebastian Shaji | Hull and East Yorkshire NHS Trust |
| Dr Ian Shaw | Gloucestershire Hospitals NHS Foundation Trust |
| Dr Achuth Shenoy | Colchester Hospital University NHS Foundation Trust |
| Dr Mark Smith | Royal Shrewsbury and Telford NHS Trust |
| Dr Alan Steel | Chelsea and Westminster NHS Foundation Trust |
| Dr Linzi Thomas | Abertawe Bro Morgannwg University Health Board |
| Dr Paul Thomas | Taunton and Somerset NHS Foundation Trust |
| Dr Simon Travis | Oxford Radcliffe Hospitals NHS Trust |
| Dr David Watts | NHS Forth Valley |
| Dr Gill Watts | University Hospital South Manchester NHS Foundation Trust |
| Dr Sean Weaver | Royal Bournemouth and Christchurch Hospital NHS Foundation Trust |
| Dr Greg Whatley | Tameside Hospital NHS Foundation Trust |
Dr John Mansfield (Newcastle upon Tyne) for his advice and support in acquiring adoption of CONSTRUCT by the NIHR Research Portfolio.
Mr Hugh Barr (Gloucester), Mr John Beynon (Abertawe Bro Morgannwg University Health Board), Dr Keith Bodger (University of Liverpool), Mr Martyn Evans (Abertawe Bro Morgannwg University Health Board) and Dr Chris Venables (retired surgeon, Newcastle upon Tyne) for their help with the costing of surgery.
Dr Sam Groves (University of South Wales) and Mr Morro Touray (University of South Wales) for their help with economic data analysis.
Gaynor Demery, Jane Draper, Emma Riordan and Judy Williams (Swansea University Medical School) who provided administrative support to the trial.
Ashley Akbari (Information and Data Analyst/Research Officer, Swansea University Medical School) and Dr Kym Thorne (Data Analyst/Research Officer, Swansea University Medical School) who helped develop the data collection tools and the implementation of GeneCIS.
Giles Croft (Clinical Informatics Specialist), Hayley Dickinson (GeneCIS Technical Manager) and Tracy Hughes (Clinical Coding Specialist) supported the use of GeneCIS.
Sarah Wright (Swansea University Medical School) who helped with analysis of the participant interview data.
Dr Stephen Roberts (Swansea University Medical School) for development of the routine data element of the study.
Dr Wai-Yee Cheung (Swansea University Medical School) for her contribution to the design of the study and PROMs.
Dr Daphne Russell (Swansea University Medical School) for her contribution to the design of the study.
Kathy Malinovsky and Leanne Quinn (National Institute for Social Care and Health Research – Clinical Research Centre (NISCHR CRC) who provided helpful guidance in the set up of CONSTRUCT.
Kathie Wareham (Director, Joint Clinical Research Facility, Abertawe Bro Morgannwg University Health Board/Swansea University Medical School) who provided most helpful advice about data quality, study closure and archiving.
Publications and presentations
Williams JG. CONSTRUCT Breaking News. Oral presentation at British Society of Gastroenterology Annual Meeting, 17 June 2014, Manchester, UK.
Seagrove AC, Clement C, Rapport FL, Wright S, Williams JG. Infliximab or Ciclosporin: Patient Views on Treatment and the Impact of Ulcerative Colitis (UC) on Their Lives. Poster presentation at British Society of Gastroenterology Annual Meeting, 17 June 2014, Manchester, UK.
Williams JG. CONSTRUCT. Oral presentation at United European Gastroenterology Week, Vienna, October 2014.
Williams JG, Fasihul Alam M, Alrubaiy L, Arnott I, Clement C, Cohen D, et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial [published online ahead of print 22 June 2016]. Lancet Gastroenterol Hepatol 2016.
Sponsor
Swansea University agreed to act as sponsor for the research.
Data sharing statement
All available data can be obtained from the corresponding author via e-mail to construct@swansea.ac.uk.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut 2004;53:V1-16. http://dx.doi.org/10.1136/gut.2004.043372.
- Rubin GP, Hungin AP, Kelly PJ, Ling J. Inflammatory bowel disease: epidemiology and management in an English general practice population. Aliment Pharmacol Ther 2000;14:1553-9. http://dx.doi.org/10.1046/j.1365-2036.2000.00886.x.
- Dinesen LC, Walsh AJ, Protic MN, Heap G, Cummings F, Warren BF, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis 2010;4:431-7. http://dx.doi.org/10.1016/j.crohns.2010.02.001.
- Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955;2:1041-8. http://dx.doi.org/10.1136/bmj.2.4947.1041.
- Travis SP. Review article: the management of mild to severe acute ulcerative colitis. Aliment Pharmacol Ther 2004;20:88-92. http://dx.doi.org/10.1111/j.1365-2036.2004.02056.x.
- Jarnerot G, Rolny P, Sandberg-Gertzen H. Intensive intravenous treatment of ulcerative colitis. Gastroenterology 1985;89:1005-13.
- Roberts SE, Williams JG, Yeates D, Goldacre MJ. Mortality in patients with and without colectomy admitted to hospital for ulcerative colitis and Crohn’s disease: record linkage studies. BMJ 2007;335. http://dx.doi.org/10.1136/bmj.39345.714039.55.
- Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994;330:1841-5. http://dx.doi.org/10.1056/NEJM199406303302601.
- Actis GC, Aimo G, Priolo G, Moscato D, Rizzetto M, Pagni R. Efficacy and efficiency of oral microemulsion cyclosporin versus intravenous and soft gelatin capsule cyclosporin in the treatment of severe steroid-refractory ulcerative colitis: an open-label retrospective trial. Inflamm Bowel Dis 1998;4:276-9.
- Sands BE, Tremaine WJ, Sandborn WJ, Rutgeerts PJ, Hanauer SB, Mayer L, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis 2001;7:83-8. http://dx.doi.org/10.1097/00054725-200105000-00001.
- Chey WY, Hussain A, Ryan C, Potter GD, Shah A. Infliximab for refractory ulcerative colitis. Am J Gastroenterol 2001;96:2373-81. http://dx.doi.org/10.1111/j.1572-0241.2001.04039.x.
- Aberra FN, Lichtenstein GR. Infliximab in ulcerative colitis. Gastroenterol Clin North Am 2006;35:821-36. http://dx.doi.org/10.1016/j.gtc.2006.09.002.
- Ochsenkuhn T, Sackmann M, Goke B. Infliximab for acute, not steroid-refractory ulcerative colitis: a randomized pilot study. Eur J Gastroenterol Hepatol Eur 2004;16:1167-71. http://dx.doi.org/10.1097/00042737-200411000-00014.
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462-76. http://dx.doi.org/10.1056/NEJMoa050516.
- Gisbert JP, Gonzalez-Lama Y, Mate J. Systematic review: infliximab therapy in ulcerative colitis. Aliment Pharmacol Ther 2007;25:19-37. http://dx.doi.org/10.1111/j.1365-2036.2006.03131.x.
- Yamada S, Yoshino T, Matsuura M, Minami N, Toyonaga T, Honzawa Y, et al. Long-term efficacy of infliximab for refractory ulcerative colitis: results from a single center experience. BMC Gastroenterol 2014;14. http://dx.doi.org/10.1186/1471-230X-14-80.
- Zhou YL, Xie S, Wang P, Zhang T, Lin MY, Tan JS, et al. Efficacy and safety of infliximab in patients with ulcerative colitis: experiences from a single medical center in South China. J Dig Dis 2014;15:483-90. http://dx.doi.org/10.1111/1751-2980.12161.
- Mitchell A, Guyatt G, Singer J, Irvine EJ, Goodacre R, Tompkins C, et al. Quality of life in patients with inflammatory bowel disease. J Clin Gastroenterol 1988;10:306-10. http://dx.doi.org/10.1097/00004836-198806000-00014.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. http://dx.doi.org/10.1097/00005650-199206000-00002.
- Feagan BG, Reinisch W, Rutgeerts P, Sandborn WJ, Yan S, Eisenberg D, et al. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol 2007;102:794-802. http://dx.doi.org/10.1111/j.1572-0241.2007.01094.x.
- Ulcerative Colitis (Acute Exacerbations) – Infliximab (TA163). London: NICE; 2008.
- Naftali T, Novis B, Pomeranz I, Leichtman G, Maor Y, Shapiro R, et al. Cyclosporin for severe ulcerative colitis. Isr Med Assoc J 2000;2:588-91.
- Hawthorne AB. Ciclosporin and refractory colitis. Eur J Gastroenterol Hepatol 2003;15:239-44. http://dx.doi.org/10.1097/00042737-200303000-00005.
- Message L, Bourreille A, Laharie D, Quinton A, Galmiche JP, Lamouliatte H, et al. Efficacy of intravenous cyclosporin in moderately severe ulcerative colitis refractory to steroids. Gastroenterol Clin Biol 2005;29:231-5. http://dx.doi.org/10.1016/S0399-8320(05)80754-7.
- Haslam N, Hearing SD, Probert CS. Audit of cyclosporin use in inflammatory bowel disease: limited benefits, numerous side-effects. Eur J Gastroenterol Hepatol 2000;12:657-60. http://dx.doi.org/10.1097/00042737-200012060-00015.
- Van Assche G, D’Haens G, Noman M, Vermeire S, Hiele M, Asnong K, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology 2003;125:1025-31. http://dx.doi.org/10.1016/S0016-5085(03)01214-9.
- Creed TJ, Probert CS. Review article: steroid resistance in inflammatory bowel disease – mechanisms and therapeutic strategies. Aliment Pharmacol Ther 2007;25:111-22. http://dx.doi.org/10.1111/j.1365-2036.2006.03156.x.
- Garcia-Lopez S, Gomollon-Garcia F, Perez-Gisbert J. Cyclosporine in the treatment of severe attack of ulcerative colitis: a systematic review. Gastroenterol Hepatol 2005;28:607-14. http://dx.doi.org/10.1016/S0210-5705(05)71523-5.
- Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol 2007;5:103-10. http://dx.doi.org/10.1016/j.cgh.2006.09.033.
- Shibolet O, Regushevskaya E, Brezis M, Soares-Weiser K. Cyclosporine A for induction of remission in severe ulcerative colitis. Cochrane Database Sys Rev 2005;1. http://dx.doi.org/10.1002/14651858.cd004277.pub2.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011. www.cochrane-handbook.org (accessed 3 November 2015).
- Sjoberg M, Walch A, Meshkat M, Gustavsson A, Jarnerot G, Vogelsang H, et al. Infliximab or cyclosporine as rescue therapy in hospitalized patients with steroid-refractory ulcerative colitis: a retrospective observational study. Inflamm Bowel Dis 2012;18:212-18. http://dx.doi.org/10.1002/ibd.21680.
- Mocciaro F, Renna S, Orlando A, Rizzuto G, Sinagra E, Orlando E, et al. Cyclosporine or infliximab as rescue therapy in severe refractory ulcerative colitis: early and long-term data from a retrospective observational study. J Crohns Colitis 2012;6:681-6. http://dx.doi.org/10.1016/j.crohns.2011.11.021.
- Dean KE, Hikaka J, Huakau JT, Walmsley RS. Infliximab or cyclosporine for acute severe ulcerative colitis: a retrospective analysis. J Gastroenterol Hepatol 2012;27:487-92. http://dx.doi.org/10.1111/j.1440-1746.2011.06958.x.
- Daperno M, Sostegni R, Scaglione N, Ercole E, Rigazio C, Rocca R, et al. Outcome of a conservative approach in severe ulcerative colitis. Dig Liver Dis 2004;36:21-8. http://dx.doi.org/10.1016/j.dld.2003.04.001.
- Croft A, Walsh A, Doecke J, Cooley R, Howlett M, Radford-Smith G. Outcomes of salvage therapy for steroid-refractory acute severe ulcerative colitis: ciclosporin vs. infliximab. Aliment Pharmacol Ther 2013;38:294-302. http://dx.doi.org/10.1111/apt.12375.
- Maser EA, Deconda D, Lichtiger S, Ullman T, Present DH, Kornbluth A. Cyclosporine and infliximab as rescue therapy for each other in patients with steroid-refractory ulcerative colitis. Clin Gastroenterol Hepatol 2008;6:1112-16. http://dx.doi.org/10.1016/j.cgh.2008.04.035.
- Leblanc S, Allez M, Seksik P, Flourie B, Peeters H, Dupas JL, et al. Successive treatment with cyclosporine and infliximab in steroid-refractory ulcerative colitis. Am J Gastroenterol 2011;106:771-7. http://dx.doi.org/10.1038/ajg.2011.62.
- Chaparro M, Burgueno P, Iglesias E, Panes J, Munoz F, Bastida G, et al. Infliximab salvage therapy after failure of ciclosporin in corticosteroid-refractory ulcerative colitis: a multicentre study. Aliment Pharmacol Ther 2012;35:275-83. http://dx.doi.org/10.1111/j.1365-2036.2011.04934.x.
- Mañosa M, López San Roman A, Garcia-Planella E, Bastida G, Hinojosa J, Gonzalez-Lama Y, et al. Infliximab rescue therapy after cyclosporin failure in steroid-refractory ulcerative colitis. Digestion 2009;80:30-5. http://dx.doi.org/10.1159/000212075.
- Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, Filippi J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 2012;380:1909-15. http://dx.doi.org/10.1016/S0140-6736(12)61084-8.
- Lynch RW, Lowe D, Protheroe A, Driscoll R, Rhodes JM, Arnott ID. Outcomes of rescue therapy in acute severe ulcerative colitis: data from the United Kingdom inflammatory bowel disease audit. Aliment Pharmacol Ther 2013;38:935-45. http://dx.doi.org/10.1111/apt.12473.
- Chang KH, Burke JP, Coffey JC. Infliximab versus cyclosporine as rescue therapy in acute severe steroid-refractory ulcerative colitis: a systematic review and meta-analysis. Int J Colorectal Dis 2013;28:287-93. http://dx.doi.org/10.1007/s00384-012-1602-8.
- Marshall NJ, Wilson G, Lapworth K, Kay LJ. Patients’ perceptions of treatment with anti-TNF therapy for rheumatoid arthritis: a qualitative study. Rheumatology 2004;43:1034-8. http://dx.doi.org/10.1093/rheumatology/keh237.
- Hazen ACM, Smith FJ, Taylor KMG, Rawcliffe C, Medcalf L, Keady S. Paediatric infliximab therapy, patients and parents perspectives on treatment options. Paediatr Perinat Drug Ther 2008;8:177-81. http://dx.doi.org/10.1185/146300908X292097.
- Silverman S. Qualitative Research: Theory, Method and Practice. London: Sage Publications Ltd; 2004.
- Denzin NK, Lincoln YS. The SAGE Handbook of Qualitative Research. London: Sage Publications Ltd; 2005.
- Rapport F, Storey M, Porter A, Snooks H, Jones K, Peconi J, et al. Qualitative research within trials: developing a standard operating procedure for a clinical trials unit. Trials 2013;14:1-8. http://dx.doi.org/10.1186/1745-6215-14-54.
- Popay J, Williams G. Qualitative research and evidence-based healthcare. J R Soc Med 1998;91:32-7.
- Chaudhary MA, Fan T. Cost-effectiveness of infliximab for the treatment of acute exacerbations of ulcerative colitis in the Netherlands. Biol Ther 2013;3:45-60. http://dx.doi.org/10.1007/s13554-012-0007-0.
- Punekar YS, Hawkins N. Cost-effectiveness of infliximab for the treatment of acute exacerbations of ulcerative colitis. Eur J Health Econ 2010;11:67-76. http://dx.doi.org/10.1007/s10198-009-0199-5.
- D’Haens G, Lemmens L, Geboes K, Vandeputte L, Van Acker F, Mortelmans L, et al. Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology 2001;120:1323-9. http://dx.doi.org/10.1053/gast.2001.23983.
- Bryan S, Andronis L, Hyde C, Connock M, Fry-Smith A, Want D. Evidence Review Group Report on Behalf of NICE: Infliximab for the Treatment of Acute Exacerbations of Ulcerative Colitis 2010. www.journalslibrary.nihr.ac.uk/hta/volume-14/suppl-1-article-2 (accessed 4 November 2015).
- Seagrove AC, Alam MF, Alrubaiy L, Cheung WY, Clement C, Cohen D, et al. Randomised controlled trial. Comparison Of iNfliximab and ciclosporin in STeroid Resistant Ulcerative Colitis: trial design and protocol (CONSTRUCT). BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005091.
- Glasziou PP, Simes RJ, Gelber RD. Quality adjusted survival analysis. Stat Med 1990;9:1259-76.
- Process and Methods Guides: The Guidelines Manual. London: NICE; 2012.
- Thorne K, Jezembek GS, Cheung WY, Cohen D, Hutchings HA, Rapport F, et al. MATRICS: a method for aggregating the reporting of interventions in complex studies. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-S1-A147.
- Russell IT, Edwards RT, Gliddon AE, Ingledew DK, Russell D, Whitaker R, et al. Cancer of the oesophagous or gastricus: new assessment of the technology of endosonography (COGNATE). Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17390.
- Bedson E, Bell D, Carr D, Carter B, Hughes D, Jorgensen A, et al. Folate augmentation of treatment-evaluation for depression (FolATED): randomised trial and economic evaluation. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18480.
- Russell D, Hoare ZS, Whitaker R, Whitaker CJ, Russell IT. Generalized method for adaptive randomization in clinical trials. Stat Med 2011;30:922-34. http://dx.doi.org/10.1002/sim.4175.
- Cheung WY, Garratt AM, Russell IT, Williams JG. The UK IBDQ-a British version of the inflammatory bowel disease questionnaire. Development and validation. J Clin Epidemiol 2000;53:297-306. http://dx.doi.org/10.1016/S0895-4356(99)00152-3.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- The Medicines for Human Use (Clinical Trials) Regulations 2004. London: The Stationery Office; 2004.
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749-53. http://dx.doi.org/10.1136/gut.2005.082909.
- Data Protection Act. London: The Stationery Office; 1998.
- Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989;96:804-10.
- Irvine EJ, Feagan B, Rochon J, Archambault A, Fedorak RN, Groll A, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s Relapse Prevention Trial Study Group. Gastroenterology 1994;106:287-96.
- Drossman DA, Leserman J, Li ZM, Mitchell CM, Zagami EA, Patrick DL. The rating form of IBD patient concerns: a new measure of health status. Psychosom Med 1991;53:701-12. http://dx.doi.org/10.1097/00006842-199111000-00010.
- Drossman DA, Li Z, Leserman J, Patrick DL. Ulcerative colitis and Crohn’s disease health status scales for research and clinical practice. J Clin Gastroenterol 1992;15:104-12. http://dx.doi.org/10.1097/00004836-199209000-00005.
- Farmer RG, Easley KA, Farmer JM. Quality of life assessment by patients with inflammatory bowel disease. Cleve Clin J Med 1992;59:35-42. http://dx.doi.org/10.3949/ccjm.59.1.35.
- Irvine EJ, Feagan BG, Wong CJ. Does self-administration of a quality of life index for inflammatory bowel disease change the results?. J Clin Epidemiol 1996;49:1177-85. http://dx.doi.org/10.1016/0895-4356(96)00136-9.
- Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol 1996;91:1571-8.
- Hoie O, Wolters FL, Riis L, Bernklev T, Aamodt G, Clofent J, et al. Low colectomy rates in ulcerative colitis in an unselected European cohort followed for 10 years. Gastroenterology 2007;132:507-15. http://dx.doi.org/10.1053/j.gastro.2006.11.015.
- Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from Olmsted County, Minnesota. Gastroenterology 2006;130:1039-46. http://dx.doi.org/10.1053/j.gastro.2005.12.037.
- Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol 2004;2:1088-95. http://dx.doi.org/10.1016/S1542-3565(04)00543-9.
- Alrubaiy L, Cheung WY, Russell I, Williams J. Developing a short form Crohn’s and Colitis Questionnaire (CCQ) for patients with inflammatory bowel disease. J Crohns Colitis 2012;6:S46-47. http://dx.doi.org/10.1016/S1873-9946(12)60112-3.
- Streiner GL, Norman RD. Health Measurement Scales. A Pratical Guide to Their Development and Use. Oxford: Oxford University Press; 2008.
- Thorn JC, Coast J, Cohen D, Hollingworth W, Knapp M, Noble SM, et al. Resource-use measurement based on patient recall: issues and challenges for economic evaluation. Appl Health Econ Health Policy 2013;11:155-61. http://dx.doi.org/10.1007/s40258-013-0022-4.
- British National Formulary (Online). London: BMJ Group and Pharmaceutical Press; n.d.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2013.
- National Schedule of Reference Costs: 2012–13. London: Department of Health; 2015.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2012.
- National Institute of Health Research . NIHR Clinical Research Network Industry Costing Template 2014. www.crn.nihr.ac.uk/wp-content/uploads/Industry/Secondary%20care%20medtech%20costing%20template-Apr14.xls (accessed 22 September 2014).
- Digitalhealth.net . NHS Direct 0845 Closure Costs £70m 2013. www.digitalhealth.net/news/28663//nhs-direct-0845-closure-costs-£70m (accessed 30 July 2015).
- EuroQol 2014. www.euroqol.org/ (accessed 28 July 2015).
- Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Ryan GW, Bernard HR, Denzin NK, Lincoln US. Handbook of Qualitative Research. Newbury Park, CA: Sage; 2000.
- Rapport F, Iredale R, Jones W, Sivell S, Edwards A, Grey J, et al. Decision aids for familial breast cancer: exploring women’s views using focus groups. Health Expect 2006;9:232-44. http://dx.doi.org/10.1111/j.1369-7625.2006.00392.x.
- Research Governance Framework for Health and Social Care. London: Department of Health; 2005.
- Research Governance Framework for Health and Community Care. Edinburgh: Scottish Executive Health Department; 2006.
- Research Governance Framework. Cardiff: Wales Office for Research and Development for Health and Social Care; 2009.
- European Parliament and Council . Directive 2001 20 EC of the European Parliament and of the Council of 4 April 2001 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to the Implementation of Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use n.d. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2001:121:0034:0044:en:PDF (accessed 18 February 2014).
- Evans BA, Bedson E, Bell P, Hutchings H, Lowes L, Rea D, et al. Involving service users in trials: developing a standard operating procedure. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-219.
- Guidelines for Good Clinical Practice in Clinical Trials. MRC Clinical Trials Series. London: MRC; 1998.
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c869.
- Fenwick E, Marshall DA, Lev AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res 2006;6. http://dx.doi.org/10.1186/1472-6963-6-52.
- Snooks H, Hutchings H, Seagrove A, Stewart-Brown S, Williams J, Russell I. Bureaucracy stifles medical research in Britain: a tale of three trials. BMC Med Res Methodol 2012;12. http://dx.doi.org/10.1186/1471-2288-12-122.
- Alrubaiy L, Cheung WY, Dodds P, Hutchings HA, Watkins A, Russell IT, et al. Development of a short questionnaire to assesss the quality of life in Crohn’s disease and ulcerative colitis. J Crohns Colitis 2015;9:66-7. http://dx.doi.org/10.1093/ecco-jcc/jju005.
- Messick SJ. The psychology of acquiescence: an interpretation of research evidence. ETS Research Bulletin Series 1966;1966:i-44.
- Hinz A, Michalski D, Schwarz R, Herzberg PY. The acquiescence effect in responding to a questionnaire. Psychosoc Med 2007;4.
- Health and Social Care Information Centre (HSCIC), Academy of Medical Royal Colleges . Standards for the Clinical Structure and Content of Patient Records, 2013 n.d. www.aomrc.org.uk/publications/reports-a-guidance# (accessed 3 December 2014).
- Keohane K, Rosa M, Coulter IS, Griffin BT. Enhanced colonic delivery of ciclosporin A self-emulsifying drug delivery system encapsulated in coated minispheres [published online ahead of print June 2015]. Drug Dev Ind Pharm 2015. http://dx.doi.org/10.3109/03639045.2015.1044905.
- Adedokun OJ, Sandborn WJ, Feagan BG, Rutgeerts P, Xu Z, Marano CW, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014;147:1296-307. http://dx.doi.org/10.1053/j.gastro.2014.08.035.
- Guba EG. Criteria for assessing the trustworthiness of naturalistic inquiries. ECTJ 1981;29:75-91.
- Denscombe M. The Good Research Guide for Small-Scale Social Research Projects. Buckingham: Open University Press; 1998.
- Lincoln YS, Guba EG. Naturalistic Inquiry. Berverly Hills, CA: Sage; 1985.
- Shenton AK. Strategies for ensuring trustworthiness in qualitative research projects. Educ Info 2004;22:63-75.
- Stake RE, Denzin NK, Lincoln YS. Handbook of Qualitative Research. Thousand Oaks, CA: Sage; 1994.
- Yin RK. Case Study Research: Design and Methods. Thousand Oaks, CA: Sage Publishing; 1994.
- Scimeca D, Bossa F, Annese V, Biscaglia G, Caruso N, Accadia L, et al. Infliximab Vs Oral Cyclosporin in Patients With Severe Ulcerative Colitis Refractory to Intravenous Steroids: A Controlled, Randomised Study. United European Gastroenterology Week n.d. www.ueg.eu/education/document-detail/?name=infliximab_vs_oral_cyclosporin_in_patients_with_severe_ulcerative_colitis_refractory_to_intravenous_steroids._a_controlled_randomized_study&file=98100 (accessed 4 November 2015).
- Kang YS, Moon HH, Lee SE, Lim YJ, Kang HW. Clinical experience of the use of CT-P13, a biosimilar to infliximab in patients with inflammatory bowel disease: a case series. Dig Dis Sci 2015;60:951-6. http://dx.doi.org/10.1007/s10620-014-3392-z.
- Teisner AS, Ainsworth MA, Brynskov J. Long-term effects and colectomy rates in ulcerative colitis patients treated with infliximab: a Danish single center experience. Scand J Gastroenterol 2010;45:1457-63. http://dx.doi.org/10.3109/00365521.2010.510572.
- Mortensen C, Caspersen S, Christensen NL, Svenningsen L, Thorsgaard N, Christensen LA, et al. Treatment of acute ulcerative colitis with infliximab, a retrospective study from three Danish hospitals. J Crohns Colitis 2011;5:28-33. http://dx.doi.org/10.1016/j.crohns.2010.09.004.
- Jarnerot G. Infliximab or cyclosporine for severe ulcerative colitis. Gastroenterology 2006;130. http://dx.doi.org/10.1053/j.gastro.2005.11.037.
- Jakobovits SL, Jewell DP, Travis SP. Infliximab for the treatment of ulcerative colitis: outcomes in Oxford from 2000 to 2006. Aliment Pharmacol Ther 2007;25:1055-60. http://dx.doi.org/10.1111/j.1365-2036.2007.03300.x.
- Waters O, Saunders M, Clarke M, Daneshmend T, Ahmad T. Infliximab rescue therapy for steroid refractory acute severe ulcerative colitis in the Exeter IBD cohort. Gut 2011;60. http://dx.doi.org/10.1136/gut.2011.239301.454.
- Campbell S, Travis S, Jewell D. Ciclosporin use in acute ulcerative colitis: a long-term experience. Eur J Gastroenterol Hepatol 2005;17:79-84. http://dx.doi.org/10.1097/00042737-200501000-00016.
- Molnar T, Farkas K, Szepes Z, Nagy F, Wittmann T. Long-term outcome of infliximab therapy is highly comparable in a Danish and in a Hungarian tertiary centre. Scand J Gastroenterol 2011;46:248-9. http://dx.doi.org/10.3109/00365521.2010.522725.
- Moskovitz DN, Van Assche G, Maenhout B, Arts J, Ferrante M, Vermeire S, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760-5. http://dx.doi.org/10.1016/j.cgh.2006.04.001.
- Reich KM, Chang HJ, Rezaie A, Wang H, Goodman KJ, Kaplan GG, et al. The incidence rate of colectomy for medically refractory ulcerative colitis has declined in parallel with increasing anti-TNF use: a time-trend study. Aliment Pharmacol Ther 2014;40:629-38. http://dx.doi.org/10.1111/apt.12873.
- Ulcerative Colitis (Moderate, Severe) – Infliximab (Review TA140), Adalimumab (Review TA262) and Golimumab (2nd Line). London: NICE; 2015.
Appendix 1 CONSTRUCT flow chart

Appendix 2 Patient information sheet (cohort)
Appendix 3 Consent form (cohort)
Appendix 4 Patient information sheet (randomised controlled trial)
Appendix 5 Consent form (randomised controlled trial)
Appendix 6 Adverse event screening form
Appendix 7 Summary of data to be collected and source
| Type of data | Initial inpatient stay | 3.5-year designed research data collection and record linkage | Potential annual follow-up (years 3.5–10) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCT and cohort | RCT | Cohort | RCT | Cohort | ||||||||
| Time (months) | 0+ | 3 | 6 | 12 | 18 | 24 | 30 | 36 | 12 | 24 | Yearly | Yearly |
| Demographic | O | |||||||||||
| Administrative | O | |||||||||||
| Clinical | O | O/R | O/R | O/R | O/R | O/R | O/R | O/R | R | R | R | R |
| Pathology results | O | O | O | O | O | O | O | O | R | R | ||
| Outcomes | ||||||||||||
| QoL | P | P | P | P | P | P | P | P | R | R | P | |
| Mortality | O/R | O/R | O/R | O/R | O/R | O/R | O/R | R | R | R | R | |
| Readmissions | O/R | O/R | O/R | O/R | O/R | O/R | O/R | R | R | R | R | |
| Colectomy | O/R | O/R | O/R | O/R | O/R | O/R | O/R | R | R | R | R | |
| Additional data collection for colectomy patients | ||||||||||||
| Hospital costs (excluding drugsa) | O | O | O | O | O | O | O | O | R | |||
| Other NHS costs | P | P | P | P | P | P | P | P | ||||
| Patient-reported AEs | P | P | P | P | P | P | P | |||||
| Patient-borne costs | P | P | P | P | P | P | P | P | P | |||
| Patient views | P | P | ||||||||||
| Professional views | Interviews conducted | |||||||||||
Appendix 8 Participant Baseline Questionnaire
Appendix 9 Participant Follow-Up Questionnaire
Appendix 10 Cohort case report form
Appendix 11 Randomised controlled trial case report form
Appendix 12 Post-Colectomy Questionnaire
Appendix 13 First interview schedule (randomised controlled trial only)
Appendix 14 First interview schedule (colectomy)
Appendix 15 Second interview schedule (randomised controlled trial and surgery)
Appendix 16 Health-care professional interview schedule 1
Appendix 17 Health-care professional interview schedule 2
Appendix 18 McMaster IBDQ, UKIBDQ, CUCQ and CUCQ+ questions and response options
| McMaster IBDQ: question and response | UK-IBDQ: question and response | CUCQ: question and response | CUCQ+: question and response |
|---|---|---|---|
| How frequent have your bowel movements been during the last 2 weeks? McMaster response option A |
On how many days over the last 2 weeks have you had loose or runny bowel movements? UK-IBDQ response option A |
On how many days over the last 2 weeks have you had loose or runny bowel movements? CUCQ response option A |
On how many days over the last 2 weeks have you had loose or runny bowel movements? CUCQ+ response option C |
| How often has the feeling of fatigue or of being tired and worn out been a problem for you during the last 2 weeks? McMaster response option B |
On how many days over the last 2 weeks have you felt tired? UK-IBDQ response option A |
On how many days over the last 2 weeks have you felt tired? CUCQ response option A |
On how many days over the last 2 weeks have you felt tired? CUCQ+ response option A |
| How often during the last 2 weeks have you felt frustrated, impatient, or restless? McMaster response option B |
In the last 2 weeks have you felt frustrated? UK-IBDQ response option B |
In the last 2 weeks have you felt frustrated? CUCQ response option B |
In the last 2 weeks have you felt frustrated? CUCQ+ response option B |
| How often during the last 2 weeks have you been unable to attend school or do your work because of your bowel problem? McMaster response option B |
In the last 2 weeks has your bowel condition prevented you from carrying out your work or other normal activities? UK-IBDQ response option B |
In the last 2 weeks has your bowel condition prevented you from carrying out your work or other normal activities? CUCQ response option B |
In the last 2 weeks has your bowel condition prevented you from carrying out your work or other normal activities? CUCQ+ response option B |
| How much of the time during the last 2 weeks have your bowel movements been loose? McMaster response option B |
On how many days over the last 2 weeks have you opened your bowels more than three times a day? UK-IBDQ response option A |
On how many days over the last 2 weeks have you opened your bowels more than three times a day? CUCQ response option A |
On how many days over the last 2 weeks have you opened your bowels more than three times a day? CUCQ+ response option C |
| How much energy have you had during the last 2 weeks? McMaster response option C |
On how many days over the last 2 weeks have you felt full of energy? UK-IBDQ response option A |
On how many days over the last 2 weeks have you felt full of energy? CUCQ response option A |
On how many days over the last 2 weeks have you felt full of energy? CUCQ+ response option A |
| How often during the last 2 weeks did you feel worried about the possibility of needing to have surgery because of your bowel problem? McMaster response option B |
In the last 2 weeks have you been worried about being admitted to hospital because of your bowel problem? UK-IBDQ response option B |
No comparable question | No comparable question |
| How often during the last 2 weeks have you had to delay or cancel a social engagement because of your bowel problem? McMaster response option B |
In the last 2 weeks did your bowel condition prevent you from going out socially? UK-IBDQ response option B |
In the last 2 weeks did your bowel condition prevent you from going out socially? CUCQ response option B |
In the last 2 weeks did your bowel condition prevent you from going out socially? CUCQ+ response option B |
| No comparable question | On how many days over the last 2 weeks have your bowels opened accidentally? UK-IBDQ response option A |
On how many days over the last 2 weeks have your bowels opened accidentally? CUCQ response option A |
On how many days over the last 2 weeks have your bowels opened accidentally? CUCQ+ response option C |
| How often during the last 2 weeks have you felt generally unwell? McMaster response option B |
On how many days over the last 2 weeks have you felt generally unwell? UK-IBDQ response option A |
On how many days over the last 2 weeks have you felt generally unwell? CUCQ response option A |
On how many days over the last 2 weeks have you felt generally unwell? CUCQ+ response option A |
| How often during the last 2 weeks have you been troubled because of fear of not finding a washroom? McMaster response option B |
In the last 2 weeks have you felt the need to keep close to a toilet? UK-IBDQ response option B |
In the last 2 weeks have you felt the need to keep close to a toilet? CUCQ response option B |
In the last 2 weeks have you felt the need to keep close to a toilet? CUCQ+ response option B |
| How much difficulty have you had, as a result of your bowel problems, doing leisure or sports activities you would have liked to have done during the last 2 weeks? McMaster response option D |
In the last 2 weeks has your bowel condition affected your leisure or sports activities? UK-IBDQ response option B |
In the last 2 weeks has your bowel condition affected your leisure or sports activities? CUCQ response option B |
In the last 2 weeks has your bowel condition affected your leisure or sports activities? CUCQ response option B |
| How often during the last 2 weeks have you been troubled by pain in the abdomen? McMaster response option B |
On how many days over the last 2 weeks have you felt pain in your abdomen? UK-IBDQ response option A |
On how many days over the last 2 weeks have you felt pain in your abdomen? CUCQ response option A |
On how many days over the last 2 weeks have you felt pain in your abdomen? CUCQ+ response option A |
| How often during the last 2 weeks have you had problems getting a good night’s sleep, or been troubled by waking up during the night? McMaster response option B |
On how many nights over the last 2 weeks have you been unable to sleep well (days if you are a shift worker)? UK-IBDQ response option A |
On how many nights over the last 2 weeks have you been unable to sleep well (days if you are a shift worker)? CUCQ response option A |
On how many nights over the last 2 weeks have you been unable to sleep well (days if you are a shift worker)? CUCQ+ response option A |
| How often during the last 2 weeks have you felt depressed or discouraged? McMaster response option B |
In the last 2 weeks have you felt depressed? UK-IBDQ response option B |
In the last 2 weeks have you felt depressed? CUCQ response option B |
In the last 2 weeks have you felt depressed? CUCQ+ response option B |
| How often during the last 2 weeks have you had to avoid attending events where there was no washroom close at hand? McMaster response option B |
In the last 2 weeks have you had to avoid attending events where there was no toilet close at hand? UK-IBDQ response option B |
In the last 2 weeks have you had to avoid attending events where there was no toilet close at hand? CUCQ response option B |
In the last 2 weeks have you had to avoid attending events where there was no toilet close at hand? CUCQ+ response option B |
| Overall, in the last 2 weeks how much of a problem have you had with passing large amounts of gas? McMaster response option E |
On how many days over the last 2 weeks have you had a problem with large amounts of wind? UK-IBDQ response option A |
On how many days over the last 2 weeks have you had a problem with large amounts of wind? CUCQ response option A |
On how many days over the last 2 weeks have you had a problem with large amounts of wind? CUCQ+ response option A |
| No comparable question | On how many days over the last 2 weeks have you felt off your food? UK-IBDQ response option A |
On how many days over the last 2 weeks have you felt off your food? CUCQ response option A |
On how many days over the last 2 weeks have you felt off your food? CUCQ+ response option A |
| Many patients with bowel problems often have worries and anxieties related to their illness. These include worries about getting cancer, worries about never feeling any better and worries about having a relapse. In general, how often during the last 2 weeks have you felt worried or anxious? McMaster response option B |
Many patients with bowel problems have worries about their illness. How often during the last 2 weeks have you felt worried? UK-IBDQ response option B |
Many patients with bowel problems have worries about their illness. How often during the last 2 weeks have you felt worried? CUCQ response option B |
Many patients with bowel problems have worries about their illness. How often during the last 2 weeks have you felt worried? CUCQ+ response option B |
| How much of the time during the last 2 weeks have you been troubled by a feeling of abdominal bloating? McMaster response option B |
On how many days over the last 2 weeks has your abdomen felt bloated? UK-IBDQ response option A |
On how many days over the last 2 weeks has your abdomen felt bloated? CUCQ response option A |
On how many days over the last 2 weeks has your abdomen felt bloated? CUCQ+ response option A |
| How often during the last 2 weeks have you felt relaxed and free of tension? McMaster response option B |
In the last 2 weeks have you felt relaxed? UK-IBDQ response option B |
In the last 2 weeks have you felt relaxed? CUCQ response option B |
In the last 2 weeks have you felt relaxed? CUCQ+ response option B |
| How much of the time during the last 2 weeks have you had a problem with rectal bleeding with your bowel movements? McMaster response option B |
On how many days over the last 2 weeks have you noticed blood with your bowel movements? UK-IBDQ response option A |
On how many days in the last 2 weeks have you noticed blood in your stools? CUCQ response option A |
On how many days in the last 2 weeks have you noticed blood in your stools? CUCQ+ response option C |
| How much of the time during the last 2 weeks have you felt embarrassed as a result of your bowel problem? McMaster response option B |
In the last 2 weeks have you been embarrassed by your bowel problem? UK-IBDQ response option B |
In the last 2 weeks have you been embarrassed by your bowel problem? CUCQ response option B |
In the last 2 weeks have you been embarrassed by your bowel problem? CUCQ+ response option B |
| How much of the time during the last 2 weeks have you been troubled by a feeling of having to go to the bathroom even though your bowels were empty? McMaster response option B |
On how many days over the last 2 weeks have you wanted to go back to the toilet immediately after you thought you had emptied your bowels? UK-IBDQ response option A |
On how many days over the last 2 weeks have you wanted to go back to the toilet immediately after you thought you had emptied your bowels? CUCQ response option A |
On how many days over the last 2 weeks have you wanted to go back to the toilet immediately after you thought you had emptied your bowels? CUCQ+ response option C |
| How much of the time during the last 2 weeks have you felt tearful or upset? McMaster response option B |
In the last 2 weeks have you felt upset? UK-IBDQ response option B |
In the last 2 weeks have you felt upset? CUCQ response option B |
In the last 2 weeks have you felt upset? CUCQ+ response option B |
| How much of the time during the last 2 weeks have you been troubled by accidental soiling of your underpants? McMaster response option B |
On how many days over the last 2 weeks have you had to rush to the toilet? UK-IBDQ response option A |
On how many days over the last 2 weeks have you had to rush to the toilet? CUCQ response option A |
On how many days over the last 2 weeks have you had to rush to the toilet? CUCQ+ response option C |
| How much of the time during the last 2 weeks have you felt angry as a result of your bowel problem? McMaster response option B |
In the last 2 weeks have you felt angry as a result of your bowel problem? UK-IBDQ response option B |
In the last 2 weeks have you felt angry as a result of your bowel problem? CUCQ response option B |
In the last 2 weeks have you felt angry as a result of your bowel problem? CUCQ+ response option B |
| To what extent has your bowel problem limited sexual activity during the last 2 weeks? McMaster response option F |
In the last 2 weeks has your sex life been affected by your bowel problem? UK-IBDQ response option B |
In the last 2 weeks has your sex life been affected by your bowel problem? CUCQ response option B |
In the last 2 weeks has your sex life been affected by your bowel problem? CUCQ+ response option B |
| How much of the time during the last 2 weeks have you been troubled by nausea or feeling sick to your stomach? McMaster response option B |
On how many days over the last 2 weeks have you felt sick? UK-IBDQ response option A |
On how many days over the last 2 weeks have you felt sick? CUCQ response option A |
On how many days over the last 2 weeks have you felt sick? CUCQ+ response option A |
| How much of the time during the last 2 weeks have you felt irritable? McMaster response option B |
In the last 2 weeks have you felt irritable? UK-IBDQ response option B |
In the last 2 weeks have you felt irritable? CUCQ response option B |
In the last 2 weeks have you felt irritable? CUCQ+ response option B |
| How often during the past 2 weeks have you felt a lack of understanding from others? McMaster response option B |
In the last 2 weeks have you felt lack of sympathy from others? UK-IBDQ response option B |
In the last 2 weeks have you felt lack of sympathy from others? CUCQ response option B |
In the last 2 weeks have you felt lack of sympathy from others? CUCQ+ response option B |
| How satisfied, happy, or pleased have you been with your personal life during the past 2 weeks? McMaster response option G |
In the last 2 weeks have you felt happy? UK-IBDQ response option B |
In the last 2 weeks have you felt happy? CUCQ response option B |
In the last 2 weeks have you felt happy? CUCQ+ response option B |
| How often during the last 2 weeks have you been troubled by cramps in your abdomen? McMaster response option B |
No comparable question | No comparable question | No comparable question |
| Overall, in the last 2 weeks, how much of a problem have you had maintaining or getting to, the weight you would like to be at? McMaster response option E |
No comparable question | No comparable question | No comparable question |
| No comparable question | No comparable question | On how many nights in the last 2 weeks have you had to get up to use the toilet because of your bowel condition after you have gone to bed? CUCQ response option A |
On how many nights in the last 2 weeks have you had to get up to use the toilet because of your bowel condition after you have gone to bed? CUCQ+ response option A |
| Stoma-specific questions | |||
| No comparable question | No comparable question | No comparable question | On how many days over the last 2 weeks have you been afraid that other people might hear your stoma? CUCQ+ response option D |
| No comparable question | No comparable question | No comparable question | On how many days over the last 2 weeks have you been worried that other people might smell your stools? CUCQ+ response option D |
| No comparable question | No comparable question | No comparable question | On how many days over the last 2 weeks have you been worried about possible leakage from your stoma bag? CUCQ+ response option D |
| No comparable question | No comparable question | No comparable question | On how many days over the last 2 weeks have you had problems with care for your stoma? CUCQ+ response option D |
| No comparable question | No comparable question | No comparable question | On how many days over the last 2 weeks have you found the skin around your stoma irritated? CUCQ+ response option D |
| No comparable question | No comparable question | No comparable question | In the last 2 weeks have you felt embarrassed because of your stoma? CUCQ+ response option B |
| No comparable question | No comparable question | No comparable question | In the last 2 weeks have you felt less complete because of your stoma? CUCQ+ response option B |
| No comparable question | No comparable question | No comparable question | In the last 2 weeks have you felt less attractive as a result of your stoma? CUCQ+ response option B |
| No comparable question | No comparable question | No comparable question | In the last 2 weeks have you felt less masculine/feminine as a result of your stoma? CUCQ+ response option B |
| No comparable question | No comparable question | No comparable question | In the last 2 weeks have you been dissatisfied with your body as a result of your stoma? CUCQ+ response option B |
| Questionnaire response options | |||
| McMaster IBDQ response options | UK-IBDQ response options | CUCQ response options | CUCQ+ response options |
| A: 1 = Bowel movements as or more frequent that they have ever been 2 = Extremely frequent 3 = Very frequent 4 = Moderate increase in frequency of bowel movements 5 = Some increase in frequency of bowel movements 6 = Slight increase in frequency of bowel movements 7 = Normal, no increase in frequency of bowel movements B: 1 = All of the time 2 = Most of the time 3 = A good bit of the time 4 = Some of the time 5 = A little of the time 6 = Hardly any of the time 7 = None of the time C: 1 = No energy at all 2 = Very little energy 3 = A little energy 4 = Some energy 5 = A moderate amount of energy 6 = A lot of energy 7 = Full of energy D: 1 = A great deal of difficulty; activities made impossible 2 = A lot of difficulty 3 = A fair bit of difficulty 4 = Some difficulty 5 = A little difficulty 6 = Hardly any difficulty 7 = No difficulty; the bowel problems did not limit sports or leisure activities E: 1 = A major problem 2 = A big problem 3 = A significant problem 4 = Some trouble 5 = A little trouble 6 = Hardly any trouble 7 = No trouble F: 1 = No sex as a result of bowel disease 2 = Major limitation as a result of bowel disease 3 = Moderate limitation as a result of bowel disease 4 = Some limitation as a result of bowel disease 5 = A little limitation as a result of bowel disease 6 = Hardly any limitation as a result of bowel disease 7 = No limitation as a result of bowel disease G: 1 = Very dissatisfied, unhappy most of the time 2 = Generally dissatisfied, unhappy 3 = Somewhat dissatisfied, unhappy 4 = Generally satisfied, pleased 5 = Satisfied most of the time, happy 6 = Very satisfied most of the time, happy 7 = Extremely satisfied, could not have been more happy or pleased |
A: None, 1–2; 3–7; 8–14 (i.e. more than every other) days B: No, not at all; yes, some of the time; yes, most of the time; yes, all of the time |
A: Open response option (0–14) B: No, not at all; yes, some of the time; yes, most of the time; yes, all of the time |
A: Open response option (0–14) B: No, not at all; yes, some of the time; yes, most of the time; yes, all of the time C: Open response (0–14) Not applicable option for bowel surgery patients D: None, 1–2; 3–7; 8–14 (i.e. more than every other) days |
Appendix 19 Health professional interview analysis framework
Assessment of quality of interviews
According to Guba106 the following criteria should be applied to qualitative data to assess their trustworthiness, corresponding to constructs employed by positivistic researchers such as internal and external validity, reliability and objectivity:
-
credibility (in preference to internal validity) – ‘congruence of findings with reality’
-
transferability (in preference to external validity/generalisability) – ‘application of findings to other situations’
-
dependability (in preference to reliability) – ‘processes in the study reported in enough detail that others can repeat the work’
-
confirmability (in preference to objectivity) – ‘findings are the result of participants’ experiences and ideas not researcher preference’.
Credibility can be assessed according to whether or not the operational methods that are being applied to the concepts under study are applied correctly. 108 We would argue that the questions asked in this study, and the adoption of both face-to-face interviewing methods and telephone interviewing, with both health-care professionals and participants, over an extended trial period, gave enough detail and sufficient information to clarify people’s views. Interviews were undertaken not at a static time point but over time, as participants (patients) were brought on board, at 3 months and then again at 12 months post drug treatment. This also included patients who had also undergone surgery. The extensive data sets collected were also rich in detail and captured information across the study aims in accordance with a question schedule that related not only to both drugs being administered but also to people’s views of their health, their health care, their relationship with professionals and others, their illness and personal preferences for drug allocation (professionals’ interviews). Indeed, additional information was obtained from health-care professionals, using these qualitative methods, about the trial itself and about professionals’ hope for trial information to guide them in their future practice.
Transferability can be assessed, in qualitative terms, according to Denscombe107 and Stake,110 if a particular study can be seen as an example within a broader group. This can be assured if sufficient contextual information is supplied about the sites involved and the participants within those sites, so any reader can make this kind of transfer. 111 In this trial we are confident that we have supplied sufficient detail of participant characteristics, participant views and trial site allocation across all UK locations for the reader to build a picture of what occurred and with whom. We have also provided information of how participants and sites were involved in this trial, differences between locations and differences between groups of participants. In addition, we have explained how health-care professionals were chosen for interview and how they were recruited, so that this work can be repeated by others in other settings with other drug groups if appropriate.
In qualitative data terms, dependability suggests that the work processes should be reported in enough detail so that any future researcher could repeat the work; thus, the research design can be viewed as a ‘prototype model’. 109 Shenton reminds us that this kind of detail allows the reader to also be reassured that appropriate research practices were followed or, at the very least, that the extent to which they were followed can be assessed. We have demonstrated in this report how participants were approached, the kinds of questions they were asked and the order in which those questions were asked. We have also reported their comments in quotation form and described how we assured participants (both patients and health-care professionals) that ethical standards would be upheld throughout the trial. This included assurances about each individual’s anonymity. We provided participants with information about the removal of aspects of data from the study data sets if they so wished and explained that they could withdraw from the study at any stage if they so wished. We were keen to clarify the research design in this report and to explain the qualitative data capture and analysis methods in the ‘methods’ sections, so that others might follow similar procedures in their own work.
Confirmability is the ‘qualitative investigator’s comparable concern to objectivity’. 109 Confirmability ensures that findings link clearly and precisely to the study informants’ data rather than to the assumptions, preferences or personal opinions of any one of the qualitative researchers involved in a study or to others with whom they work. In this study, we always worked as a team during analytic framework development and data assessment, in terms of both participant and health-care professional data. We developed out trial analytic frameworks from both data sets independently, and conferred about all key findings, which were shared with the wider team. We were aware of the need to achieve consensus of opinion in group discussion and conferred on not only the iterative process of revealing the thematic results but also the emergent key and incidental themes. During each group analysis meeting, we made sure that three qualitative researchers, including the qualitative trial lead, were present to discuss aspects of data capture, data content and analysis. In addition, we worked with the wider team (seven additional members) through a half-day group-working session that employed schema analysis to triangulate patient data, and through the group-working process we were able to add to the veracity of working methods. During schema analysis the core team (three qualitative researchers) examined whether or not their views aligned appropriately and sufficiently with the wider group and whether or not data were being appropriately managed and clarified. We were assured that this was the case, that no new themes were suddenly revealed and that consensus of opinion regarding key emergent themes could be achieved in a manner that was uncontested.
We therefore believe that our qualitative data are robust, although the number of interviewees is inevitably small in comparison with the quantitative data. The data we obtained were extensive, not only about patient and professional views of the interventions but also about participants’ views about the impact of colitis on their health and lifestyle. Participants were willing to speak very openly about their disease, and the views we obtained came from adults of all ages and from across the UK. We thus feel that the data are important and deserve careful consideration alongside the clinical effectiveness and cost-effectiveness results.
Appendix 20 MATRICS proforma
MATRICS
| Effects on patients | Effects on the NHS specialty being investigated | Effects on the rest of NHS and society |
|---|---|---|
| Code | Method |
|---|---|
| Code(s) | Finding(s) |
|---|---|
| Effects on patients | |
| Effects on NHS specialty being investigated | |
| Effects on the rest of NHS and society | |
Appendix 21 Data Monitoring and Ethics Committee charter
Appendix 22 A personal experience
I am a 64-year-old male who prior to retirement was a Senior Officer in the Fire and Rescue Service. In 1999 I was diagnosed with Inflammatory Bowel Disease (IBD) and in 2005 I had a total colectomy following a steroid-resistant flare-up. I have been a member of Crohn’s and Colitis UK for 15 years and it was via them that I was invited to join the Trial Management Group (TMG) of the Construct Trial.
My first encounter with the professional researchers working on the trial was not easy and for the first few meetings I did feel out of my depth. I initially had the fear that I was only there because the National Institute for Health Research (NIHR) make public/patient involvement mandatory in the trials they sponsor. After my first couple of meetings it became apparent that my earlier misgivings were unfounded and could not be further from the truth. The Lead Researcher, Professor Williams, always made a point of involving me in discussions and my views were considered along with those of the other members.
I found that my membership of the TMG was a two-way process and that I was able to feed in information from my personal experience of IBD. This I believe helped the professional researchers to more fully understand how IBD impacts on the life of individuals. I also gained a lot more knowledge about Ulcerative Colitis and its medical effects on the body and the various treatments which are available.
One of the main ways I was able to get involved outside of the normal TMG meetings was in the production of the health questionnaires. In particular the one which dealt with patients who had had surgery when their treatment failed. In these cases the individuals are left with a temporary or permanent Stoma. My personal experience of both the physical and mental trauma of this procedure and the long-term effects were helpful in shaping some of the questions. It is all too easy when you are highly trained in a subject to devise questions which are not explicit or easy for people to understand. I worked on the theory that if I could understand the questions other patients would have a fighting chance.
At one point in the trial we had a recurring problem with patients who where taking part in the trial but not returning questionnaires. It was agreed that a joint letter from Professor Williams and myself would be sent to the individuals who had not returned the questionnaires. This did have some impact, which may have been because the recipients of the letters were influenced by the fact that a fellow sufferer was writing to them in conjunction with the Lead Researcher.
Being a member of a Trial Management Group is a long-term commitment covering years rather than months. My advice would be if you have the time, energy and the will, you may be able to help improve the lives of people you are unlikely to meet and that can be very rewarding.
Appendix 23 Participant recruitment

Appendix 24 Participant analysis framework
-
Longevity of disease.
Living with UC.
Time.
Course of disease: over longer period; short-term unpredictability.
-
Serious suffering.
Description of suffering.
Intensity of suffering.
Negative experience of suffering.
Loss of bodily control/function: gradual/sudden demise.
Unpredictability of symptoms/relapse.
(Toileting.) (Pain.)
-
Acceptance, coping, toleration.
Positive perception of life.
Surprise at positive moments.
Counter-intuitive return to better state.
Normalising illness/wellness/taken for granted.
Trivialising illness (within relationships).
-
Knowledge and information sharing.
Nurse.
Crohn’s and Colitis UK: self-help group low profile/some groups disbanded.
Internet.
Shared decision-making.
Media: UC low profile, not publicised.
Health-care professional/service want issues highlighted.
Containment: people not forthcoming about illness.
Trial removes treatment decision.
-
Life changes.
Being overwhelmed by illness.
Taking different and unexpected paths.
Taken off guard.
Stopping doing things.
Giving up control/taking control.
Restriction/constraint: unpredictability of disease.
-
Body: core of disease.
Within the body but ability to reflect on.
Failure of the body to function: different type of failure to other chronic conditions/physical consequences.
IBD inside/outside.
Loss of body parts.
Defining self (loss of colon).
Body centric.
Visceral notion of UC.
-
Physical, mental and emotional impact.
Trajectory from physical to mental, to social to emotional impact.
Stress (leading to flare-ups).
Directional change (physical becomes mental).
Fear and despair/uncertainty.
Impact of physical, mental, emotional impact on relationships.
(Defining self by symptoms.)
Loss of hope.
Physically fit/mentally vulnerable.
-
Expectations of health and well-being.
‘Normal’ life.
Happy/unhappy life.
Comfort/discomfort.
Inconvenience.
Managing life/practicalities.
Freedom/imprisonment.
Having energy.
Being fit and healthy.
Link between good and bad health.
-
Cacophony of drugs.
Other treatments alongside drugs.
Other treatments instead of drugs.
Number of drugs taken together.
Complex drugs pathway.
Unpredictable drug pathway.
-
Process and outcome of treatment.
Treatment practicalities.
What treatment has achieved/meant to patients.
Surgical intervention.
Side effects.
Unpredictability of treatment.
Proactive: difficult for health-care professional because of unpredictably.
Health-care professional aim is for people to live normal live.
-
Hindsight.
Post-surgery views.
Changes in perception.
Living with colostomy.
Views about reversal.
Stoma.
Submissive regarding choices.
-
Relationships.
Family, friends, professionals.
Support.
Understanding.
Giving.
Sharing/withholding information: work.
Infertility: awareness/lack of impact of disease (male and female).
Pregnancy: awareness/lack of impact of disease (male and female).
Disclosure.
Lack of awareness of others.
Lack of awareness of concerns.
-
Work.
Impact before treatment on work.
Positive effects of treatment on work.
Aftermath: future work arrangements.
Work identity: share with colleagues.
Impact of UC on career/work.
List of abbreviations
- AE
- adverse event
- AESF
- adverse event screening form
- AR
- adverse drug reaction
- ASC
- acute severe ulcerative colitis
- AUC
- area under the curve
- CC
- complications and comorbidity
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CONSTRUCT
- Comparison Of iNfliximab and ciclosporin in STeroid Resistant Ulcerative Colitis: pragmatic randomised Trial and economic evaluation
- CRF
- case report form
- CUCQ
- Crohn’s and Ulcerative Colitis Questionnaire
- CUCQ+
- Crohn’s and Ulcerative Colitis Questionnaire with post-colectomy extension
- CySIF
- CycloSporine versus InFliximab
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- European Quality of Life-5 Dimensions
- EURECA
- Electronic records underpinning REsearch and CAre
- GCP
- good clinical practice
- GeneCIS
- Generic Clinical Information System
- GETAID
- la Groupe d’Etude Thérapeutique des Affections Inflammatoires Digestives
- GP
- general practitioner
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IBD
- inflammatory bowel disease
- IBDQ
- Inflammatory Bowel Disease Questionnaire
- IMP
- Investigational Medicinal Product
- MATRICS
- Method for Aggregating The Reporting of Interventions in Complex Studies
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PCQ
- Post-Colectomy Questionnaire
- PFQ
- Participant Follow-up Questionnaire
- PI
- principal investigator
- PROM
- patient-reported outcome measure
- QALY
- quality-adjusted life-year
- QAS
- quality-adjusted survival
- QoL
- quality of life
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAR
- serious adverse reaction
- SD
- standard deviation
- SF-12
- Short Form-12 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SPC
- Summary of Product Characteristics
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UC
- ulcerative colitis
- UK-IBDQ
- United Kingdom Inflammatory Bowel Disease Questionnaire