Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/34/03. The contractual start date was in July 2011. The draft report began editorial review in November 2013 and was accepted for publication in October 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Debi Bhattacharya reports funding from the University of East Anglia throughout the duration of the study. Clare F Aldus reports grants, personal fees and non-financial support from the University of East Anglia. Garry Barton, Sathon Boonyaprapa, Richard Holland, Christina Jerosch-Herold, Charlotte Salter, Lee Shepstone, Steven Waton and David Wright report grants and personal fees from the University of East Anglia. Christine Bond reports non-financial support from the University of Aberdeen. Christine Walton reports grants and personal fees from NHS Anglia Commissioning Support Unit.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Bhattacharya et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Approximately 50% of patients do not take their prescribed medication as recommended by the prescriber,1 with researchers most commonly describing such behaviours as either unintentional or intentional. Unintentional non-adherence is not the result of any conscious decision by patients not to take their medication, and has been associated with impaired cognitive function and practical problems such as difficulty accessing medication from its packaging or swallowing the dosage form, while intentional non-adherence, a conscious decision to deviate from their prescribed regimen, is associated with patients’ beliefs and their experience of the medicine. As these definitions seem superficially distinct, it is argued that unintentional reasons, such as forgetting to take medication, may actually represent a subconscious decision to not prioritise medication taking and, therefore, is actually largely intentional in its origins. While intentional non-adherence is addressed through effective communication, psychological interventions and selecting therapies which are more patient acceptable, unintentional non-adherence requires interventions which act as memory cues or overcome physical barriers to medicine taking.
Medication organisation devices (MODs) are medical devices intended to address unintentional non-adherence1–3 by enabling patients to identify whether they have or have not taken their medicines and by enhancing the accessibility of the medicine. MODs are known by a wide range of terms, including monitored dosage system, multicompartment compliance aid and domiciliary dosage system (DDS), but all have similar design features. MODs comprise either a rigid pill box or semi-rigid blister pack, divided into days of the week, with several compartments per day to allow for the different timing of doses. Medicines are placed in the appropriate locations in the box or pack, which are clearly empty once the medicine has been removed. Figure 1 provides examples of MODs commonly used within the NHS in the UK. The Nomad Clear™ (Surgichem Ltd, Cheshire, UK) device has the 7 days of the week marked across the top with different dosage times down the left-hand side and therefore provides 1 week’s medication at a time. The Venalink™ (Venalink, Flintshire, UK) system, by contrast, provides the days of the week down the left-hand side and dosage times across the top.
FIGURE 1.
Examples of MODs commonly used in the UK. (a) Nomad Clear and Nomad Clear XL; and (b) Venalink.

It has been estimated that 100,000 people are currently using MODs in the UK3 to reduce unintentional non-adherence and, therefore, MODs potentially play an important role in ensuring that patients receive the full benefit from their medication. Non-adherence to prescribed therapy is one of the factors believed to contribute to decisions to transfer patients from their own homes into care homes. Consequently, MODs may additionally play a pivotal role in maintaining patients in their own home and prolonging their autonomy, which is in accordance with government targets to promote independence. 4
The national pharmacy contract provides for MODs supplied in accordance with the Equality Act. 5 In such cases, provision of medicines in MODs is deemed a reasonable adjustment for those individuals who, as a result of disability, are unable to safely take their medicines without such a device. Great variation in NHS funding of MOD provision exists, and in some localities MOD provision by pharmacists is commissioned using NHS funds, whereas in other localities where there are no such arrangements they are provided at the expense of the patient.
Despite the current disparity, existing evidence is insufficient to underpin the decision-making to either discontinue NHS funding of MODs or provide clear guidance to practitioners regarding their initiation. 6,7 The few randomised controlled trials (RCTs) that have been conducted have been limited by small sample sizes or insufficient data to characterise the sample population or have focused on a specific disease area. It has been estimated that £23M is being spent annually on a non-evidence-based intervention. The National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme, therefore, requested that a study be conducted to identify the most appropriate methodological approach to test the effect of MODs compared with usual packaging in older patients.
The absence of adequate large-scale studies means that there is a need to identify the most appropriate participants, to characterise participants, to select the most appropriate MOD and to define standard care and the intervention itself, while identifying and measuring the right outcomes, optimising recruitment and estimating variation in the primary outcome measure to power a definitive study. These different issues are, therefore, considered in turn.
Participant identification
A key consideration is to target patients who are unintentionally non-adherent. Considerable research has been conducted in order to establish the predictors of non-adherence and, while there is still much uncertainty, a positive association between magnitude of non-adherence and regimen complexity has been frequently reported. 8–12 Research suggests that older patients are prescribed an average of three regular medications; thus, a large proportion of the older population has at least one risk factor for non-adherence. It is, therefore, patients prescribed multiple medications who are at the greatest risk of non-adherence and to whom MODs are most frequently provided (Sharma S, Malik W, Shah A, Desborough J, Bhattacharya D, University of East Anglia, 2007, unpublished data).
Intentional non-adherence is associated with numerous factors such as beliefs about medicines13 and the quality of the patient–prescriber relationship. 14–16 The proportion of non-adherence that is attributable to intentional factors varies, usually ranging from 4% to 17%,15,17 but with figures as high as 37% reported in older people. 18 Owing to the high proportion of patients whose non-adherence is intentional, it is important that this behaviour is excluded from any trial involving MODs which is designed to address unintentional non-adherence. Furthermore, the provision of medicines in single compartments of an MOD makes differentiating between medications challenging for the patient, thereby reducing the ability of patients to choose which medications to take and not take. Such a situation may result in intentionally non-adherent patients omitting to take all medication stored in a MOD compartment. 15
Categorising non-adherence as intentional or unintentional can only be achieved by establishing the motivation for the deviation. A number of self-report tools have been developed to identify intentional non-adherence.
Therefore, this study proposed to identify the prevalence of intentional non-adherence in older people who are receiving polypharmacy regimes using a range of self-report tools, with a view to identifying a suitable tool and cut-off threshold to be used to exclude intentionally non-adherent participants from a definitive study.
Participant characterisation
Once an unintentionally non-adherent patient has been identified, reasons for his or her non-adherence need to be considered to ensure that these are taken into account when choosing a MOD and are also assessed as part of any evaluation to allow for confounding. 19 The most commonly reported factors that impair patients’ ability to adhere to their prescribed regimen are deficits of cognitive function, manual dexterity and visual acuity. 6 Although no clear relationship has been demonstrated between adherence and age, the prevalence of factors known to contribute to unintentional non-adherence increases as individuals age. An Australian survey of older patients (n = 120), with a mean age of 81.8 years, characterised participants in terms of cognitive function and visual acuity and then assessed ability to open a variety of commercially produced medication packaging. It was reported that 78.3% of participants were unable to open one or more of the medication packages in order to access the medication, with inability to access medication significantly associated with lower cognitive function and manual dexterity. 20 For trial purposes, the use of measures that can be replicated is necessary and, therefore, when available, the use of validated measures to test cognitive and functional ability is desirable.
Medication organisation device selection
In the absence of guidance, MODs are currently initiated to a wide range of patients with varying degrees of confusion. A survey of 10 purposively sampled pharmacists reported that eight would select a MOD without involving the patient in the decision-making and all pharmacists had a preferred MOD, thus suggesting that patient needs would not be the primary driver of MOD selection. 3 A larger survey of 105 pharmacists reported that pharmacists perceived that checking patients’ ability to use a MOD was the most important factor when considering whether or not to provide a patient with a MOD; however, it did not suggest that patients were actually being given a choice of MODs to select from.
Commercially available MODs are produced by different manufacturers and, consequently, vary considerably in terms of their size and method of medication access. 6 Systems used in pharmacies are sealed once the medication has been dispensed into them and are designed to be tamper evident. Some commercially available devices which are usually filled by the patient or carers, for example Dosett™ (Swereco, Stockholm, Sweden),21 are not funded by the NHS.
The ideal study, in line with best current practice, should allow participants to select the type of MOD they believe best meets their needs in discussion with the pharmacist.
Defining standard care
Medication organisation devices are heat-moulded (often) lidded plastic trays with wells, each sufficient in size to take multiple solid oral dose forms (SODFs) configured in four (labelled with times of the day) by seven (labelled with days of the week) format. They are intended to target unintentional non-adherence1–3 by providing medication in packaging which acts as a memory aid and which enhances accessibility for the patient. They come in a number of shapes and sizes and it was important to identify MODs acceptable to participants and to fit the NIHR research remit.
A number of factors have been cited as the rationale for MODs supporting adherence, including providing medicine storage which is easily accessible to the patient; reducing the complexity of adhering to a regimen; minimising dose amount and timing errors; and acting as a memory aid. A further benefit may be the weekly dispensing, which results in greater contact with the pharmacy team or a carer by virtue of the medication being supplied on a weekly rather than the more usual monthly basis. Research has demonstrated that reducing monitoring frequency from weekly to fortnightly reduces adherence to therapy and, therefore, it may follow that reducing medication supply frequency may have a similar effect. 22 It is possible that the beneficial effect thought to be associated with MODs could be obtained by dispensing medicine supplies in standard packaging (increasingly blister packs) and supplying at weekly intervals.
The MOD is, therefore, a two-component intervention: weekly supply and an aide-mémoire. Clearly, weekly supply in standard containers is cheaper than weekly supply in a MOD, and it is important to quantify the relative contribution of these two components of the intervention (the container and the dispensing frequency) to any observed improved adherence.
Outcomes
Adherence measurement
Studies of MODs have frequently focused on a specific disease, with a single therapeutic outcome or detection of particular chemicals in body fluids being used as a measure of adherence. Thus, little guidance is available to guide targeting of the wider population of patients for MODs in routine practice. Historically, direct measures of adherence, such as observation and detection of chemicals in bodily fluids, have been considered the gold standard. Observation clearly has significant cost implications for large-scale studies and is subject to the Hawthorne effect. Detection of chemicals in body fluids has the merit of being objective; however, it can be invasive and costly, and there remains the potential for patients to alter their medication-taking behaviour in the days prior to sample provision. Some of the disadvantages associated with observation as an adherence measure are exemplified by a trial which randomised patients to receive potassium supplementation or placebo tablets. 23 Measurement of urine potassium levels identified a reduction over time, which was most likely attributable to reduced patient compliance with 24-hour urine sample collection as the trial progressed; hence measured potassium levels were artificially low. The taking of blood samples would overcome the issues of patient compliance with inconvenient 24-hour urine samples; however, the acceptability to patients of frequent blood samples is even lower and has been demonstrated to adversely affect trial recruitment, with 52% of patients not consenting to trial participation, citing fear of phlebotomy. 24 An additional problem associated with such direct measures is intra- and interpatient variability in drug handling. This can be overcome to a certain extent by estimating individual variation in drug handling via repeated samples over a short period of time; however, this type of invasive assessment has low patient acceptability. Alternatively, Bayesian methodology can be used; however, this is complex and again provides only an estimate of variability.
The dosage unit count (DUC) is generally accepted as the pragmatic approach to adherence assessment. It is based on the assumption that, if the medication is not in the container, it has been taken by the patient. This is problematic when attempting to identify intentional non-adherence because patients may deliberately remove and discard tablets in order to disguise non-adherence. However, the assumption is valid if patients are predominantly unintentionally non-adherent. Previous research has demonstrated that use of the DUC method in the older patient population is feasible and acceptable. 15
More recently, electronic adherence monitoring (EAM) systems have enabled objective measurement of medication adherence that is less susceptible to the Hawthorne effect by virtue of being less intrusive and less conspicuous to the patient than direct adherence measures or DUC. The most widely used EAM system is the Medication Event Monitoring System™ (MWV Healthcare, Richmond, VA, USA). The Medication Event Monitoring System is a bottle thats cap contains a microprocessor that records the date and time of each bottle opening event; it has been widely used in clinical trials to assess medication adherence. 12,23,25,26 Trials have, therefore, generally approached objective adherence monitoring by decanting medication from usual packaging to the monitoring bottles. This has the limitation of not assessing adherence in a naturalistic setting, as usual dispensing is now routinely in manufacturer-issued blister packaging. A number of EAM systems are under development, and a 2-month pilot study (n = 52) of one EAM system to assess feasibility and acceptability for usual-care blister packaging reported promising results. Adherence data were obtained from 94.3% of participants and 67.4% of participants reported that they would consider using the EAM system for a long-term study. 27 Therefore, it was proposed that bespoke versions of an EAM system be evaluated to determine whether or not an EAM system can enable medication-taking events from MODs and ‘usual-care’ blister packs to be objectively and accurately recorded and compared.
Cost-effectiveness
In addition to assessing whether or not MOD provision affects adherence to medication regimes, it will be important to assess whether or not it confers health or economic benefits. Measures to detect changes in the utilisation of health or social services will be put in place and tested to determine utility for a definitive study.
Patient autonomy
In addition to the impact of MODs on adherence, it is important to establish patient acceptability. No studies have reported the impact of MODs on patient autonomy or ability to manage one’s own medication; however, there is anecdotal evidence of reduced autonomy, as patients are unable to differentiate one medication from another when medicines are packed in MODs and, therefore, cannot choose to omit a single type of medication if they so desire (e.g. to delay taking a diuretic when embarking on a long journey), sometimes resulting in the omission of all doses. 1 Conversely, patients may report that they feel enabled by feeling confident about managing their medication. A number of studies have explored patient autonomy with respect to medication taking in the context of describing the extent to which patients feel involved in the decision-making process. 28–30 However, exploration of whether or not patients feel as though they have some control over the medication-taking process is limited. Development of a tool to assess the impact of MODs on patients’ confidence in their ability to manage their own medication, on their satisfaction with their packaging and the service they receive was included. Carers and relatives are closely involved in the daily lives of older people with multiple comorbidities and, therefore, it is also important to assess the impact of providing MODs to older people on their carers or close relatives. An additional tool to determine carer perceptions of the impact of medicine packaging on patients’ confidence and their ability to manage their medicines and effects on the resource provided by carers and relatives was also included.
Patient medication administration errors
Studies evaluating patient medication administration errors have cited access to extra medication as a source of errors and, therefore, reducing the amount of medication to which a patient has access may also be a further source of error reduction. 31 For the duration of the study it was important for participants to have access only to the new stock of drugs supplied to them. Therefore, development of an appropriate process to ensure that errors in compliance could not be attributed to extra medication was included.
General dispensing and administration errors
The most substantial review to date of errors associated with MOD use was conducted in Australia; the Australian Incident Monitoring Study31 reported that 0.43% (52 out of 12,000) of medication-related errors were associated with MODs. In 26 cases, there was a problem with filling the MOD, such as wrong dose, dose omission or wrong medication. In 21 of these cases, nursing staff were responsible for the error, with the remainder being attributable to pharmacy staff or a carer. On 16 occasions problems using the MOD were cited as a reason for an error; however, the nature of these problems was not reported. Factors contributing to the reported problems included patient confusion/distraction and the MOD being inappropriate for the patient. 31 A further Australian audit32 of dispensing errors associated with 6972 dispensed MODs detected an error rate of 4.3%. 32
A 2007 UK evaluation of dispensing error rate associated with the pharmacy usual dispensing process33 reported 1.7% content errors out of 2859 dispensed items. Content errors were errors of omission, incorrect drug, incorrect strength, incorrect dosage form, added or missing dose units and expired medication. A similar US-based study conducted in 2003 reported an identical 1.7% error rate. 33 While general dispensing error rates are 1.7%, there are no UK data for MOD error rates and, therefore, it is necessary to record error rates for dispensing into both MODs and usual packaging.
In summary, comparative data in terms of error rate associated with MODs and usual dispensing are not available. Data regarding the incidence of dispensing errors associated with usual dispensing are available, but may not be generalisable to prescriptions assembled for an older population with more multiple items. A reasonable estimate of error rate requires a large sample size and significant resources. Within a relatively small-scale feasibility study it is, therefore, only appropriate to test tools designed to collect error data and to quantify and describe any identified errors.
Recruitment strategies
While recruitment via medical practice invitation letters is convenient in terms of research administration, response rates have historically been low, as the method requires the patient to be proactive in responding to a letter invitation – consent rates are frequently between 30% and 40%. 16,34 Waiting room recruitment by researcher, while more labour intensive, and thus costly, has yielded substantially higher response rates. 35–38 Identification of the most cost-effective approach to recruitment is, therefore, required within any feasibility study.
Summary
Despite the large amount of both NHS and private funds devoted to MODs, evidence of their effectiveness and cost-effectiveness is limited, as indicated by a Department of Health-commissioned literature review conducted by Bhattacharya. 6 A 2006 Cochrane review7 concluded that MODs may improve adherence ‘with selected conditions examined to date’; however, further research is necessary to improve targeting. In order to achieve this, the impact of MODs on a more heterogeneous population needs to be established.
Preliminary work to determine the feasibility of conducting such a trial is required. An approach to measuring adherence which minimises the Hawthorne effect and which is discrete and compatible with both MODs and usual care needs to be identified from the potentially suitable EAM systems becoming available. The effect of MODs on patient autonomy requires consideration to ensure that the intervention is acceptable to patients. The potential for increasing dispensing errors because of the additional complexity of dispensing into MODs also requires consideration, as does the identification of the most appropriate method of recruitment. Finally, MODs are designed to address unintentional non-adherence and are a method for identifying and differentiating between different types of non-adherence requires development.
This feasibility study to determine the optimal design of a trial testing the effectiveness and cost-effectiveness of MODs was carried out in two main phases. The first was the determination of optimal design features and identification of features that require testing for feasibility and acceptability. The second was the trial of this design and review of the procedures to further refine the trial design.
The initial RCT design was refined as a result of a systematic review and pre-trial focus groups. Post-trial focus groups of patient and health-care professional participants were used to further refine the design of the trial and to determine feasibility and acceptability. Findings and recommendations are presented in the following chapters.
Chapter 2 Systematic review
Rationale
The NIHR funded this feasibility study to design a definitive study to determine the effectiveness and cost-effectiveness of MODs based, in part, on a recent systematic review conducted by Mahtani et al. in 2011. 39 The review concluded that findings from one favourable study are not sufficient to justify the allocation of NHS resources on MODs. In addition to this, the study focused on adherence rather than on the social and economic aspects important to the design of a definitive study. Therefore, under this study, the review was updated with respect not only to adherence but also to informing a definitive study with respect to key social and economic factors.
Objectives
The objectives of this systematic review were to identify and update the current evidence for the impact of MODs on adherence, health and humanistic and economic outcomes in patients self-administering prescribed medicines compared with usual packaging to inform a definitive study.
Methods
The protocol for this systematic review was registered with PROSPERO (registration number CRD42011001718).
Article selection
Inclusion criteria
Studies were eligible for inclusion when they met the following criteria.
Population
Patients, of any age, with any medical condition, in any setting and self-administering medication, where ‘self-administered’ is defined as medication taken by patients (with or without help from a carer) and where medical staff are not directly responsible for administration.
Interventions
Medication supplied in a MOD, packed by a health-care professional, a carer, the patient or a manufacturing company. MODs were defined as any container which permits patient or pharmacy placement of multiple solid oral dose medicines into wells arranged by time and/or date and with room for at least seven days’ medication.
Comparator
Standard care and standard packaging only. No comparator is necessary for descriptive data regarding the number of errors, or the monetary or time costs associated with filling MODs.
Outcomes
Any of the following outcomes reported:
-
adherence to medicines (using only the objective adherence measures of adherence pill counts or electronic monitoring)
-
health outcomes
-
health-related quality of life
-
health or social care utilisation
-
dispensing or administration errors
-
prescribing or medicine supply, financial and staffing costs.
Study design
All study designs involving new data collection and analysis.
Mode of dissemination
No restrictions were applied. For example, conference abstracts and book chapters were eligible.
Exclusion criteria
-
Any MOD incorporating additional reminder systems, such as visual or auditory alarms, telephone or SMS messaging services, or provision of daily medication-taking ‘tick’ charts. Studies were not excluded if training in the use of the MOD was provided.
-
There was direct observation of medicine administration by a health-care professional.
-
A MOD was used as part of a complex intervention where the independent effect of the MOD on outcomes could not be isolated.
-
Language of publication is not English.
-
No novel empirical evidence is presented (i.e. review articles which do not collect new data).
Information sources
The following electronic databases were searched. Relevant reviews were identified in order to access their bibliographies (see the following for augmented searches).
-
The library of the Cochrane collaboration (www.thecochranelibrary.com) including the databases Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Database of Abstracts of Reviews of Effects, HTA Database, NHS Economic Evaluation Database (searched from inception to 2012).
-
MEDLINE (via Ovid; searched from 1966 to 2012).
-
EMBASE (via Ovid; searched from 1980 to 2012).
-
PsycINFO (via Ovid; searched from 1874 to 2012).
-
Allied and Complementary Medicine Database (AMED; via Ovid; searched from 1985 to 2012).
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL; via EBSCOhost; searched from 1982 to 2012).
-
Trials listed as complete in Current Controlled Trials (http://controlled-trials.com/; searched from inception to 2012).
-
York Centre for Review and Dissemination databases (Database of Abstracts of Reviews of Effects, NHS Economic Evaluation Database and the HTA database; www.crd.york.ac.uk/crdweb/SearchPage.asp; searched from inception to 2012).
Search terms of medical subject headings, free text and trade names (see Appendix 1) were applied to title, abstract and whole text of articles within the Ovid search tool. For databases outside the Ovid platform, the same terms were utilised but with appropriate translation of syntax (truncation, wildcards, adjacency and Boolean operators). No restrictions were placed on year of publication and the full back-catalogues of the available databases were searched up to the end of 2012; the first search was run on 8 February 2012 and the search was updated on 11 January 2013.
In addition to formal searching of the above databases, searching was augmented via:
-
keyword searches in the Google Scholar™ search engine (http://scholar.google.com)
-
hand-searching of reference lists of included articles
-
hand-searching of identified review articles
-
hand-searching of reference lists of articles relevant to the project but excluded because of exclusion criteria (e.g. compared MODs to non-standard care, used additional interventions or measured adherence only via self-report)
-
personal communication with packaging companies
-
personal communication with research groups with an expertise in adherence.
Study selection
Titles and abstracts of all identified articles were screened for relevance by two reviewers (CA, LC, EP or TB) with any disagreements resolved by discussion. When agreement could not be reached, a third reviewer moderated the final decision. Full-text articles were similarly screened by two reviewers (CA, EP or TB). In the case of articles identified as potentially relevant after full-text screening, data were independently extracted by two reviewers (EP and SW), with any differences resolved by discussion. Authors conducting the review were not blinded to any element of the identified articles.
Data collection process
Data were extracted independently by two reviewers (EP and SW) using a standardised Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) spreadsheet. Table 1 provides a summary of the data that were extracted to the spreadsheet.
| Data description | Data extracted |
|---|---|
| Generic items | Author names, year and place of publication |
| Design | Population, including sampling |
| Intervention and comparator descriptions | Outcomes and their measurement including cognitive function, manual dexterity or visual acuity |
| Quality | Cochrane risk of bias tool (see Risk of bias) |
Source of funding
Sources of funding were examined to identify any potential conflict of interest.
Risk of bias
Risk of bias was assessed using items from the Cochrane risk of bias tool (randomisation procedure, concealment of allocation, blinding of assessors, blinding of treatment providers, whether or not attrition was variable between groups and how this was accounted for, whether or not all outcomes were reported, and whether or not there were any other clear threats to validity) as appropriate to the study design. When the study design did not permit randomisation or blinding of assessors, it was noted that such designs have greater risk of bias.
Summary measures
Meta-analysis was intended where measures were consistently reported across trials; however, owing to a lack of comparability, none was performed. Results are tabulated and described according to study outcomes.
Results
Study selection
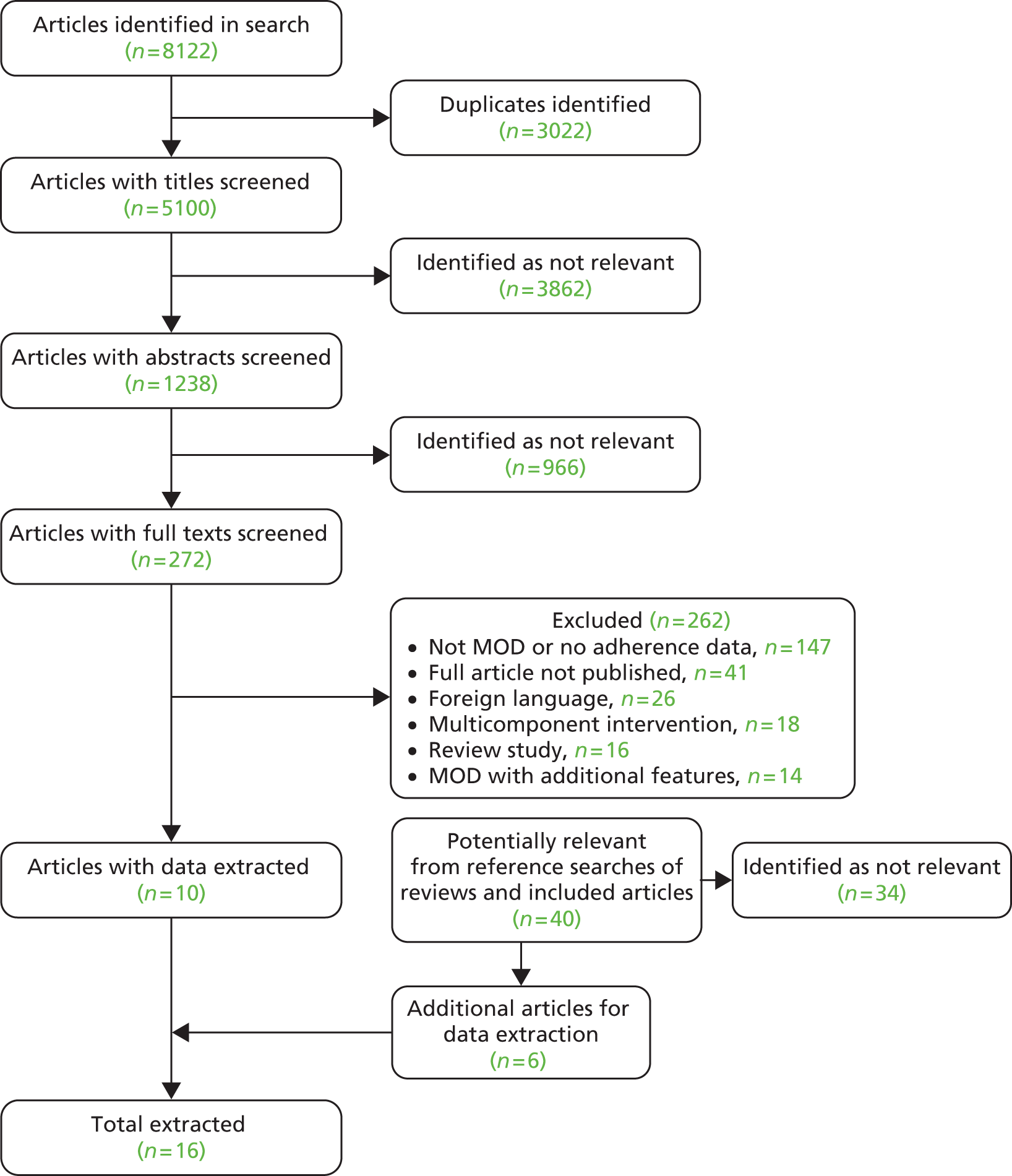
A total of 8122 studies were identified. After automatic removal of duplicates, 5100 articles were screened. Following this, 272 articles were retained for full-text screening. Three authors (CA, EP and TB) assessed these texts for eligibility and 10 articles were included. Screening of identified review articles and included articles, and those retrieved for detailed review, identified a further 40 potentially relevant studies, which yielded an additional six studies. A flow diagram summarising this process is detailed in Figure 2. No studies declared any conflict of interest.
FIGURE 2.
Flow diagram of articles included in review.
Overview of studies
A summary of included studies is shown in Table 2. Of the 16 included studies, seven were undertaken in the USA, five in the UK and four in Australia and New Zealand. Six of the 10 non-US studies were identified via augmented searching, indicating a possible US bias in the availability of articles via academic databases.
| First author and year | Study design | Location | Condition | Setting | Average age (years) | Age range (years) | Type of MOD | Filler of MOD | Total n |
|---|---|---|---|---|---|---|---|---|---|
| Becker 198640 | RCT | USA | Hypertension | Hospital outpatient | – | – | Unsealed reusable MOD | Automatic device | 165 |
| aCarruthers 200832 | Audit | Australia | Mixed | Care home | – | – | Unsealed reusable MOD | Unclear | – |
| Crome 198241 | RCT | UK | Mixed | Hospital inpatient | 80.29 | 68–98 | Unsealed reusable MOD | Unclear | 78 |
| Feetam 198242 | Prospective | UK | Mental illness | Community | 42.40 | 18–68 | Sealed MOD | Unclear | 10 |
| Huang 20002 | RCT | USA | Vitamin supplementation | Community | 58.00 | – | Sealed MOD | Patient | 183 |
| Levings 199931 | Audit | Australia | Mixed | Community | 78.00 | – | Unclear | Unclear | –b |
| MacIntosh 200743 | RCT | Canada | Cancer | Hospital outpatient | 64.00 | 42–81 | Unclear | Researchers | 21 |
| McElnay 199244 | Cross-section | UK | None | Community | – | – | Sealed MOD | Pharmacists/pharmacy technicians | 6 |
| Petersen 200745 | Prospective | USA | HIV infection | Community | 44.00 | 38–49 | Unclear | Unclear | 269 |
| Rehder 198046 | RCT | USA | Hypertension | Hospital outpatient | 51.35 | 31–69 | Sealed MOD | Unclear | 50 |
| Roberts 200447 | Multiple | Australia | Mixed | Community | 76.80 | – | Mixed | Mixed | 353 |
| Ryan-Woolley 200548 | RCT | UK | Mixed | Care home | 78.80 | 67–92 | Sealed MOD | Unclear | 62 |
| Simmons 200049 | RCT | New Zealand | Diabetes mellitus | Community | 54.06 | – | Unsealed reusable MOD | Unclear | 68 |
| Skaer 199350 | RCT | USA | Hypertension | Pharmacy | 56.49 | – | 30-day tray | Unclear | 163 |
| Stewart 200151 | Cross-section | UK | Mixed | Community | – | – | Unclear | Nurses | 96 |
| Wong 198752 | RCT | USA | Mixed | Community | 79.00 | 66–90 | Unsealed reusable MOD | Unclear | 17 |
Eight studies were conducted in 2000 or later, and the majority were in domiciliary settings (n = 12). Seven studies did not target a specific illness and the remaining studies targeted hypertension,3 diabetes mellitus, mental health, cancer and human immunodeficiency virus (HIV) infection. One study44 examined the effect of MODs on dispensing procedures only. Nine studies were RCTs. Two studies were audits and so did not assess any adherence behaviour. 31,32 The combined population from all the studies was 1541.
The type and number of MODs assessed varied across studies. Unsealed reusable MODs were used in five studies40,42,49,52,54 and pre-sealed units were used in five studies;42,44,50 in five studies,31,43,45,50,51 the seal type was not specified. One study52 used multiple types of MODs.
Only one study used patient-filled MODs,2 although in Feetam and Kelly42 it was unclear who filled the MODs. In other studies, the units were filled before being supplied to the patient by researchers in one study,43 were filled by an automatic filling device in one study,40 were filled by community nurses in one study51 and were filled by unspecified professionals in the remaining studies. McElnay and Thompson44 timed the filling of MODs by five pharmacists and a pharmacy technician.
Figure 3 provides the number of studies published over each 5-year period. It can be seen that the frequency of relevant studies has dramatically increased over the past 10 years.
FIGURE 3.
Frequency of studies published per 5-year period.

Risk of bias
There was considerable risk of bias in many studies, which is summarised in Table 3. Only three of the nine RCT studies included information on randomisation,2,43,49 and none mentioned blinding. Other risks of bias identified included baseline differences between groups not accounted for in analysis,40,53 minimal participant information42,52 and minimal information on analysis. 42 Levings et al. 31 performed an audit of errors reported in the Australian Incident Monitoring Study and noted that some types of errors may be more likely to be reported than others (e.g. overdose). It is therefore difficult to determine whether or not the errors identified are representative of all errors. McElnay and Thompson44 determined the time taken to fill MODs, but they used fictional patients with only three medicines which may have led to an underestimation of the actual time required to fill.
| First author and year | Study design | Randomisation procedure | Concealment of allocation | Blinding of assessors | Blinding of treatment providers | Attrition addressed | All outcomes reported | Other risks of bias |
|---|---|---|---|---|---|---|---|---|
| Becker 198640 | RCT | ? | ? | ? | ✗ | ? | ? | ✗ |
| Crome 198241 | RCT | ? | ? | ? | ? | ✓ | ? | ✓ |
| Huang 20002 | RCT | ✓ | ✓ | ? | ? | ? | ? | ✓ |
| MacIntosh 200743 | RCT | ✓ | ? | ? | ? | ✓ | ? | ✓ |
| Rehder 198046 | RCT | ? | ? | ? | ? | ? | ? | ✓ |
| Ryan-Woolley 200548 | RCT | ? | ? | ? | ? | ✓ | ? | ✓ |
| Simmons 200049 | RCT | ✓ | ? | ? | ✗ | ✓ | ? | ✓ |
| Skaer 199350 | RCT | ? | ? | ? | ? | ? | ? | ✓ |
| Wong 198752 | RCT | ? | ? | ? | ? | ✗ | ? | ✗ |
| Carruthers 200832 | Audit | ? | ? | ? | ? | ? | ? | ✓ |
| Feetam 198242 | Prospective | NA | NA | NA | NA | NA | ? | ✗ |
| Levings 199931 | Audit | NA | NA | NA | NA | NA | ? | ✗ |
| McElnay 199244 | Cross-section | NA | NA | NA | NA | NA | ✗ | ✗ |
| Petersen 200745 | Prospective | ✗ | ✗ | ? | ? | ✓ | ? | ✓ |
| Roberts 200453 | Mixed | NA | NA | NA | NA | NA | ? | ✗ |
| Stewart 200151 | Cross-section | NA | NA | NA | NA | NA | ? | ✓ |
Effects of medication organisation devices
Medication adherence
Eight studies from seven papers estimated the effect of MODs on adherence. 40,41,43,45,46,52 Seven of these studies were RCTs. 2,40,41,43,46,52 These studies are presented in Table 4. All studies estimated adherence via pill count, with additional electronic monitoring for patients who were not using a MOD in one study. 45 Of the eight studies, four40,45,46,52 suggested improved adherence in the MOD group. Owing to overall heterogeneity, a meta-analysis was not possible.
| First author and year | Design | Adherence measure | Adherence MOD intervention (%) | MOD intervention, n | Adherence control (%) | Control, n | Adherence effect |
|---|---|---|---|---|---|---|---|
| Becker 198640 | RCT | Per cent of sample taking > 80% of medicines | 84 | 86 | 75.3 | 85 | + ns |
| Crome 198241 | RCT | Per cent of doses missed | 26.1 | 40 | 26.2 | 38 | = |
| aHuang 2000a2 | RCT | Per cent of sample taking > 90% of medicines | 91 | 90 | 94 | 94 | = |
| aHuang 2000b2 | RCT | Per cent of sample taking > 90% of medicines | 87 | 148 | 93 | 149 | – |
| MacIntosh 200743 | RCT | Per cent of sample taking 100% of medicines | 81 | 21 | 86 | 21 | – |
| Petersen 200745 | Prospective | Difference in prescribed doses consumed | 4.1% improvement in adherence | – | – | – | +ss |
| Rehder 198046 | RCT | Per cent of sample taking > 95% of medicines | 89 | 25 | 47 | 25 | +ss |
| Wong 198752 | RCT | Per cent doses missed | 2.04 | 17 | 9.17 | 17 | +ss |
Becker et al. 40 found that 84% of participants took > 80% of their medicines when supplied with a foil-sealed blister pack MOD (time and day to take medicines on the back), compared with only 75.3% of participants without a MOD, but this difference was not statistically significant.
Petersen et al. 45 tracked a cohort of 269 HIV-infected patients between 1998 and 2005, of whom 163 were given a MOD according to clinical need. The effects of MODs on adherence were estimated via bootstrap sampling with double robust estimation to compensate for the lack of random allocation. MODs increased adherence by 4.1% [95% confidence interval (CI) 1.1% to 7.1%].
Rehder et al. ,46 in a study of patients with hypertension, showed that patients supplied with their medication in a Mediset™ MOD (Health Care Logistics, Circleville, OH, USA) were more adherent than those supplied with medication in a safety-capped vial. Of patients who received their medication in a Mediset™, 89% took more than 95% of their pills ,compared with 47% of those who received their medication in vials (the overall proportion of medicines taken was 95% compared with 87%, respectively).
In a crossover trial with older patients receiving either standard care or separate pre-sealed blister packs with doses required for each meal time (analogous to MODs), Wong and Norman52 found that patients in the blister group omitted to take fewer doses than in the usual-care group (2.04% with the MOD vs. 9.17% with standard care; p < 0.01).
Two studies found that the MOD had no effect on adherence. 2,41 In a study conducted by Crome et al. ,41 participants provided with medicines in weekly blister strips with the date and time for taking the dose on the back (C-Pak™, manufacturer information not available; analogous to a MOD) missed 26.1% of doses, compared with 26.2% of doses missed by participants receiving standard care.
Similarly, Huang2 found that participants taking vitamins from patient-filled MODs (one box/day) had a median adherence of 100% of pills taken in the MOD group (91% took > 90% of their medicines) and a median of 99% pills taken in the comparison group (94% took > 90% of their medicines). When they repeated this on a second cohort, median adherence was 99% for both groups but the proportion of patients achieving > 90% adherence was significantly lower in the MOD group than in the comparison group (87% vs. 93% respectively).
One other study also suggested poorer or equivalent adherence in the MOD group. In a study of patients taking capecitabine for breast or gastrointestinal cancer,43 adherence was generally high, with only 3 out of 24 of those in their first cycle and 4 out of 18 in their second cycle not 100% adherent. In a subsample of 21 participants taking part in a crossover design for a MOD (7-day container with exact doses for morning and evening) versus standard pill vials, a difference between groups of 81% in the MOD group versus 86% in the control group was not significant and amounted to only one participant not achieving perfect adherence in the MOD group (p = 1).
Health outcomes
Seven studies from six papers,40,45,46,49,53 including four RCTs,2,40,45,49,53 estimated the impact of MODs on health outcomes and are presented in Table 5. Three studies measured blood pressure changes,40,42,46 one vitamin serum levels,2 one viral load45 and one adverse drug reactions (ADRs) and patient function. 53
| First author and year | Design | Outcome measure | Change in outcome | MOD intervention, n | Control, n | p-value |
|---|---|---|---|---|---|---|
| Becker 198640 | RCT | Diastolic BP | 1.45 | 86 | 85 | 0.259 |
| Huang 2000a2 | RCT | Change in vitamin C serum concentration | –0.9 | 90 | 94 | 0.47 |
| Change in vitamin E serum concentration | –2.4 | – | – | 0.06 | ||
| Huang 2000b2 | RCT | Change in vitamin E serum concentration | 0.9 | 148 | 149 | 0.53 |
| Petersen 200745 | Prospective | Viral load | 0.36 | – | – | < 0.05 |
| Rehder 198046 | RCT | Change in diastolic BP | 1 | 25 | 25 | > 0.05 |
| Change in systolic BP | Not reported | – | – | > 0.05 | ||
| Roberts 200453 | Mixed | Number of ADRs | Non-MOD group reported more ADRs (47.79%) than non-pharmacist supplied MOD group (43.24%) and pharmacist-supplied MOD group (32.56%) | – | – | 0.022 |
| OARS-IADL | Patients supplied with MODs by pharmacists had lower ability scores (10.25/14) than patients supplied by other health-care professionals (12.70/14) or not supplied with a MOD (12.34/14) | – | – | 0.001 | ||
| Simmons 200049 | RCT | Change in diastolic BP | –5.9 | 36 | 32 | 0.0041 |
| Change in systolic BP | –0.7 | – | – | 0.89 | ||
| Change in HbA1c | –1.0 | – | – | 0.026 |
Of the three studies measuring changes in blood pressure, only one suggested a positive impact. 40,46,49 Simmons et al. ,49 in a study of patients with diabetes mellitus, found a significant reduction in diastolic blood pressure and glycosylated haemoglobin (HbA1c) in the MOD group compared with the standard care group. However, the reduction in systolic blood pressure was not significantly different between the groups. Becker et al. 40 did not find that use of a MOD impacted on diastolic blood pressure when change in blood pressure was controlled for baseline and pre-enrolment blood pressure measures, and age. Rehder et al. 46 did not report values for the impact of MODs on systolic blood pressure but noted that the difference was not statistically significant, and estimation of changes in diastolic blood pressure from graphical data showed an increase of 5 mmHg in the intervention group and 6 mmHg in the control group.
Huang et al. 2 found that use of patient-filled MODs did not significantly affect serum vitamin C levels [mean reduction –0.9 mg/dl (95% CI –3.4 mg/dl to 1.6 mg/dl) in the Trial of Antioxidant Vitamins C and E (TRACE) cohort; mean increase 0.9 mg/dl (95% CI –2.0 mg/dl to 3.8 mg/dl) in the Vitamins, Teachers, and Longevity (VITAL) cohort] or serum vitamin E levels [mean reduction –2.4 mg/dl (95% CI –4.8 mg/dl to 0.00 mg/dl) in the TRACE study].
The study by Peterson et al. 45 in patients with HIV suggested the benefit of MODs, as indicated by a mean reduction of 0.36 log10 copies/ml (95% CI 0.09 to 0.63 log10 copies/ml) in the MOD group compared with the standard care and an odds ratio for the increase in the proportion of participants with a viral load below 400 copies/ml of 1.91 (95% CI 1.27 to 2.90).
The study by Roberts53 in older people also suggested that MODs provide benefit, especially when filled by pharmacists. Fewer ADRs were reported when pharmacist-filled MODs were used (32.56%, compared with 43.24% for non-pharmacist-filled MODs or 47.79% for no MODs). Participant ability was assessed using the Older Americans Resource Scale for Instrumental Activities of Daily Living (OARS-IADL). Overall, these ratings were considered to be high in all groups on average, but patients who were supplied with a MOD by a pharmacist had significantly lower abilities scores with a mean [standard error (SE)] score of 10.45 (SE 0.24) out of 14, compared with 12.70 (SE 0.39) for patients who were supplied with a MOD by a non-pharmacist and 12.34 (SE 0.19) for patients who did not have a MOD. However, the direction of effect between MODs impacting on participant ability versus participant ability impacting on the decision to prescribe a MOD cannot be determined in this study.
Health-care utilisation
Table 6 summarises the studies examining the effects of MODs on health-care utilisation. It can be seen that this potential effect was explored in only three studies. 48,50,53
| Author and year | Design | Health-care utilisation measure | Health-care utilisation MOD | MOD intervention, n | Health-care utilisation control | Control, n | p-value |
|---|---|---|---|---|---|---|---|
| Roberts 200453 | Mixed | Number of different doctors visited regularly | 2.02 (pharmacist supplied), 2.91 (non-pharmacist supplied) | 209 | 2.41 | 144 | 0.012 |
| Number of doctors visited in last 2 months | 2.54 (pharmacist supplied), 2.05 (non-pharmacist supplied) | – | 3.05 | – | 0.03 | ||
| Number of hospital admissions (past 12 months) | 1.36 (pharmacist supplied), 0.56 (non-pharmacist supplied) | – | 0.78 | – | 0.001 | ||
| Per cent patients requiring hospitalisation in last 3 months | 59.54% (pharmacist supplied), 35.14% non-pharmacist supplied) | – | 35.14% | – | – | ||
| Ryan-Woolley 200548 | RCT | GP visits | 1.5 | 31 | 1.3 | 31 | 0.07 |
| Number of prescribed medicines | 4.2 | – | 4.8 | – | 0.024 | ||
| Skaer 199350 | RCT | Medicaid archive data of health-care spending per patient | –US$13.66 total spend per patient | 85 | – | 78 | > 0.05 |
Ryan-Woolley and Rees48 found that after 3 months the number of general practitioner (GP) visits was higher in the MOD group than in the standard care group [1.5 (range 1–3) vs. 1.3 (range 0–3) GP visits, respectively; p = 0.070], but that the mean number of medicines prescribed was lower [4.2 (range 2–9) vs. 4.8 (range 2–8 ) medicines per patient, respectively]. These values were more similar at baseline [4.5 (range 1–10) vs. 4.6 (range 1–9) medicines per patient, respectively].
Skaer et al. 50 found non-statistically significant reductions in physicians’ costs (–US$32.85), laboratory costs (–US$3.06) and hospital costs (–US$25.92), which led to provision of MODs resulting in a non-statistically significant reduction in overall costs of approximately US$13.66 per person.
Roberts53 found that the mean (SE) number of different doctors visited was lower in the group supplied with a pharmacist-filled MOD than in the group not supplied with a MOD [2.02 (SE 0.01) vs. 2.41 (SE 0.12) different doctors visited]. However, the number of different doctors visited by patients with non-pharmacist-supplied MODs was 2.91 (SE 0.22). Considering only the last 2 months of the study, the number of doctors visited was lower both for patients with pharmacist-supplied MODs [2.54 (SE 0.16)] and for those with non-pharmacist supplied MODs [2.05 (SE 0.33)] than for those without a MOD [3.05 (SE 0.22)]. Patients receiving MODs from their pharmacist required more hospitalisations [1.34 (SE 0.14)] than those receiving a non-pharmacist MOD [0.56 (SE 0.14)] or those without a MOD [0.78 (SE 0.11) ]. Similarly, the proportion of patients hospitalised was higher among those with a pharmacist MOD than among those not receiving a MOD or those receiving a non-pharmacist MOD (59.54% vs. 42.34% and 35.14%, respectively).
Dispensing errors
Only three studies investigated the frequency of dispensing errors. 31,32,53 There was little consistency of findings because of differences in definition and methods.
Carruthers et al. 32 found errors in 4.3% of Webster-paks™ audited by local health authority staff (297 errors in total from all audited 6972 packs). Of these, the most common reason was omission of a medicine (99 out of 297; 33.3%). Other reasons were supplying a discontinued medicine (37 out of 279), wrong strength (32 out of 297), incorrect instructions (32 out of 297), failure to deliver medicines (13 out of 297), wrong medicine (12 out of 297) and wrong label (7 out of 297). Errors occurred most commonly in pharmacies (125 out of 297).
In a smaller study of only 190 observations, and regardless of packer role (pharmacist, dispensary assistant or pre-registration pharmacy student), Roberts53 found a researcher-reported error rate for 44.7% of packs compared with only 5.7% when reported by staff. It was suggested that, when observed, the packers made more errors and also had a more stringent definition of errors. The most frequent causes were omission of medication (39%), adding extra tablets that had not been prescribed (24%) and placing tablets in the wrong compartment (12%). Higher error rates were associated with larger facilities, longer duration of time spent packing (r = 0.342; p = 0.004) and interruptions (r = –0.337; p = 0.003).
Finally, Levings et al. 31 audited the first 12,000 incidents reported in the Australian Incident Monitoring Study and identified 52 incidents involving MODs (referred to in their study as dosette boxes). Of these, 50% occurred during filling. There was no reported denominator to allow estimation of an error rate.
Supply procedures and costs
Four studies estimated the time taken to fill MODs,42,44,51,53 and two studied costs. 42,53
Feetam and Kelly42 found that a Medidos™ MOD (Allied Health, Perth, UK) took an average of 3 minutes to fill and 24 minutes to label.
In a simulated study involving five pharmacists and a pharmacy technician using medicines for five fictitious patients, McElnay and Thompson44 compared six different MODs for time to fill, user preferences and acceptability (Table 7). Extrapolated results suggest that an average time taken to fill a box would be 7 minutes per month (4 × 105 seconds) per patient, longer than for standard systems. The rank that pharmacists gave ‘ease of filling’ matched the ranking given for fill time.
| MOD device | Time to fill device, minutes : seconds (n = 6) | Perceived ease of filling (0–10) (n = 6) |
|---|---|---|
| Dosette box | 1 : 45 | 8.3 |
| Medidosa | 2 : 52 | 7.8 |
| Medi-Wheela,b | 3 : 20 | 7.5 |
| Pill Millc,d | 2 : 27 | 5 |
| Superceld,e | 9 : 59 | 1.5 |
| MedSystem Week Pouchd,f | 2 : 48 | 7.7 |
Roberts et al. 53 identified that the time required to pack a MOD ranged from 3.2–8.6 minutes for large packing operations to 14–18.5 minutes for small packing operations. Time spent checking MODs ranged from 1.13–2.13 minutes for large operations to 3.01–8.61 minutes at small operations. Those filled using automated packing systems took less time to pack (0.99 minutes), while blister packs (3.34 minutes) and dosette boxes (10 minutes) took longer.
In a survey of 153 Scottish community nurses, 96 (63%) reported having experience of filling MODs. 51 The estimated average time for a nurse to fill one MOD was 34.2 minutes. The survey also identified concern at the impact of this workload on their schedules, lack of any formal training (most had received no training, 25% had received informal training) and lack of knowledge about which medicines could be placed in the devices. Nearly 60% of the nurses felt that pharmacists should fill the compliance devices.
Feetam and Kelly42 also estimated the cost of supplying Medidos MODs for 6 months to be 10 pence per week, compared with 21 pence per week for seven disposable bottles. Similarly, labelling was less expensive (1 pence vs. 4 pence), as was time spent labelling and filling (16 pence vs. 24 pence). Overall, this produced an estimated cost of 27 pence per week for a Medidos containing seven medicines, compared with 49 pence per week for seven pill bottles. Conversely, Roberts et al. 53 found that the annual cost of original packaging (US$942.73) was less than that of a MOD (US$1859.00 per year).
Quality of life and social services utilisation
No studies were identified which measured the impact of MODs on quality of life or social service utilisation.
Summary
Overall, the studies were of poor quality and used a wide range of MODs, populations/conditions and definitions and measures of adherence. No clear trend in any of the outcomes was observed. The reported information suggested that screening of patients to only include those demonstrating unintentional non-adherence was infrequent. For the outcomes of adherence, clinical improvement, health-care utilisation, processing time and costs, there were studies suggesting both that MODs were beneficial compared with standard care, and the converse. The review included studies published at any time, but the first studies identified were in the 1980s, and there was a possible indication of most activity between 2000 and 2008. No studies were identified with a publication date later than 2008.
The search terms were developed in order to identify MODs in the form of a container organising at least 7 days of medicines together. This, therefore, excluded systems which organise medicines into individual dosing times but do not arrange these into the days of the week as a fixed system. Systems such as individual plastic pouches each containing all of the medicines that are prescribed to be taken at the same time, such as the Unit Bag system1, were therefore excluded. This approach was adopted in order to minimise the heterogeneity of the intervention.
As with any review, it is possible that relevant papers were not identified by our search strategy. However, the search terms were inclusive and applied to relevant databases, reference lists of retrieved papers were searched and hand-searching was undertaken. Contacting authors for additional information may have further enhanced the data presented.
With respect to the individual studies, the biggest limitation was study design and inadequate reporting of details to allow risk of bias to be fully assessed. In particular, without access to pre-study protocols it was impossible to determine whether or not studies reported all intended outcomes.
Chapter 3 The design phase
Introduction
Medical Research Council guidance for the development of complex interventions recommends that feasibility and pilot work is undertaken prior to embarking on a definitive study. 47 Feasibility studies are ‘pieces of research done before a main study and are used to estimate important parameters that are needed to design the main study’55 and are used to determine the acceptability of an intervention, measure demand for it, test its implementation and identify the most appropriate outcome measures with respect to sensitivity and practicability. 56 Feasibility studies are also used to determine the variance around the outcome measure to enable power calculations to be performed for the final definitive trial.
A review of the literature is now widely accepted as an essential component of studies to inform trial design. Systematic reviews offer the most rigorous methodological approach to identifying the existing literature through collating all empirical evidence that fits pre-specified eligibility criteria. They use explicit and systematic methods to minimise bias in answering a specific research question. 57,58 Systematic reviews are, however, resource intensive, whereas literature reviews offer a more rapid appraisal of existing evidence and are appropriate for supplementing researcher expertise.
Although commonly associated with full trials, process evaluation is equally, if not more, important in a feasibility study, especially when there are many unknown and to-be-determined variables, contexts and design issues. In a feasibility study it is important to scrutinise and assess both the quantity and the quality of what is proposed. 59 Furthermore, qualitative research can be well utilised in order to assess many aspects of why, how and in what context a specific aspect of a trial, or potential trial, works. 60
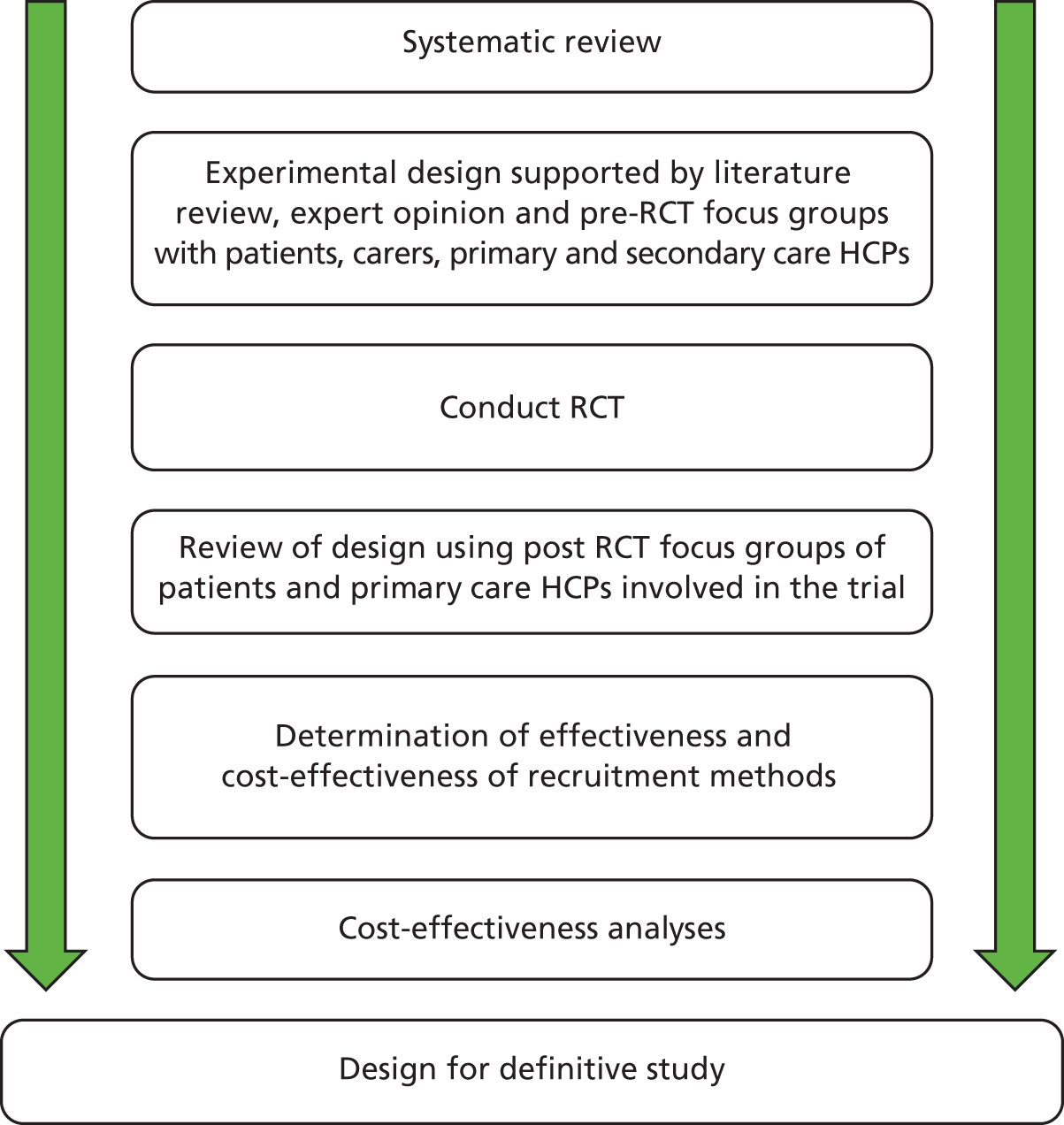
This project aimed to define the optimum design of a study to trial the effectiveness and cost-effectiveness of MODs. Mixed quantitative and qualitative methods were used to develop and evaluate the study design, as illustrated by Figure 4. The project comprised two phases:
-
Phase 1 – identifying the most appropriate design for a definitive study.
-
Phase 2 – investigating the acceptability of the design elements to trial participants and collaborators using focus groups comprising patients, carers and health-care professionals from both primary and secondary care sectors.
FIGURE 4.
Schematic illustrating the mixed-method design of the feasibility study. HCP, health-care professional.
Phase 1: literature review to inform trial design
Method
A review of peer-reviewed and grey literature using appropriate search terms to identify existing knowledge regarding the key design features was supplemented with expert opinion. The design elements investigated were identification of appropriate participants; characterisation of participants’ cognitive, manual and visual ability; selection of appropriate medication organisation devices; definition of standard care and the intervention; objective adherence monitoring; optimum recruitment strategies; and a suitable database.
Results
Identification of appropriate participants
The chief investigator (DB) had previously conducted a literature review regarding the characteristics of participants most likely to receive any benefit from a MOD, that is those patients at greatest risk of unintentional non-adherence. 6 The data from this literature review were used to generate the following initial parameters for participant identification and supplementary searches were used where necessary for further targeting.
Regimen complexity
Evidence suggests that patients who are prescribed multiple medications are at the greatest risk of non-adherence. Norfolk Medicines Support Service is a locally commissioned service which includes a patient home visit in order to establish the medicine-related support needs. The provision of a MOD is a frequently used intervention, and three was reported to be the minimum number of SODFs usually prescribed when a MOD is recommended, except when severe cognitive impairment has been identified.
Age
The funding brief for the study was to target older people. The National Service Framework for older people defines older people as aged ≥ 65 years. 4 However, UK wards specialising in medical care for older people and the British Geriatric Society use 75 years as the lower age boundary. 19
Unintentionally non-adherent
The MOD is intended to support unintentional non-adherence and thus it was essential to exclude patients at risk of intentional non-adherence. The literature review identified a number of self-report tools for identifying non-adherence; however, few provided sufficient information to distinguish between intentional and unintentional non-adherence. The four-item Medication Adherence Questionnaire is the most widely used self-report adherence measure, however, it demonstrates poor sensitivity and offers little information about the cause of non-adherence. 61
Members of the study team (DB and SW) had previously developed and tested a questionnaire designed to identify patients at risk of non-adherence. This questionnaire was informed by a meta-analysis of factors related to adherence conducted by SW. The key factors for intentional non-adherence identified were attitude towards prescribed therapy, patient–doctor relationship, risk-taking behaviour, perceived stress and mental well-being. 62 This questionnaire was a composite of validated tools and questions designed to capture the key factors.
Characterisation of participants’ cognitive, manual and visual ability
An Ovid MEDLINE title search using the terms ‘adherence + cognit*’ with no other limitations yielded 109 articles. Duplicate removal and title screening identified 23 relevant articles. Excluded articles were those describing cognitive therapy or adherence unrelated to medication taking, for example adherence to diet. Abstract and full-text searching resulted in exclusion of a further 10 articles as follows: not English language (n = 1), not cognitive impairment (n = 3), not primary research (n = 4) and children only (n = 2). The remaining 13 articles fell into two groups: multiple measures and single measures of cognitive function. Multiple measures, ranging from 4 to 19 items, to test a diverse range of cognitive abilities were used in eight articles. While these provide a detailed profile of a patient’s cognitive function, they present an excessive burden to the research participant, as this clinical level of detail is not required for the purposes of characterising a research participant. Single measures of cognitive function were used by the remaining five articles, the Dementia Rating Scale63 and the Trail Making Test64 were each used in one study, and three used the Mini Mental State Examination (MMSE). 65 The MMSE is an 11-item assessment scale scored out of 30. Traditionally, cognitive impairment is defined as a score of ≤ 23, at which the sensitivity is 83% and specificity 96%. 66
Discussions held with an expert in upper arm and hand function identified that manual dexterity is a composite of motor, sensory and visual skills. Commonly used tests of hand function with respect to medication adherence are the Purdue tests, grooved tests and nine-hole peg tests (9-HPTs). The Purdue pegboard is commonly used to assess the suitability of candidates for production line work when very high levels of dexterity are required in assembling fine components. This test was considered unsuitable in the context of personal pill taking. 67 The grooved pegboard test comprises metal pegs which are akin to small keys (30 mm long and 5 mm at their widest point) which must be rotated appropriately before insertion into keyhole-like depressions arranged in a 5 × 5 array. 68 The high visual requirement and very small pegs make this test unsuitable for the study population, as has been demonstrated by previous work in this area by Adams et al. 69 The 9-HPT comprises nine plastic dowels (each 6 mm in diameter), which are slotted into nine round holes arranged as a 3 × 3 array, and then removed from the holes. This test of finger dexterity has been described as particularly appropriate for use by the very old and very young and is also simple and quick to administer. 70 Additionally, the pegs for this test have a short axis that is very similar to those of tablets or capsules.
Visual skills required for successfully taking medication are those related to near vision only. Expert advice indicated that the test should assign a text size threshold at habitual visual acuity, that is in usual lighting conditions, at usual distance and using usual visual aids and that reading ability need not be assessed. Bailey–Lovie reading cards fulfil this requirement. 71
Selection of appropriate medication organisation devices
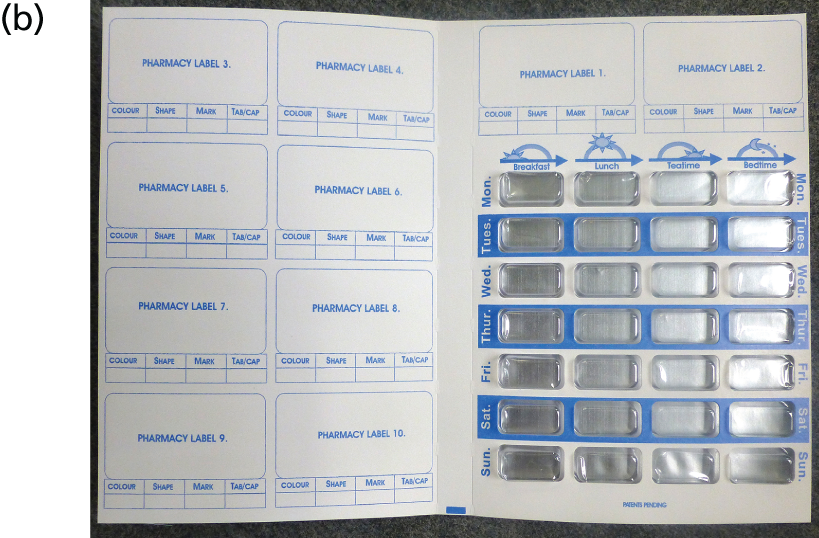
A survey of pharmacies to establish the MODs that are supplied had previously been undertaken by DB. This identified three MODs most frequently used: dosette boxes, Nomad Clear and Venalink. Figure 5 provides the specification for each of these. The dossette box, although frequently recommended by pharmacies, does not conform to recommendations for MOD characteristics as it is not a sealed unit; thus, medicines dispensed into the MOD are not protected from water vapour and atmospheric gases. 72 This means that medicines dispensed in the MOD are at increased risk of degradation. The Nomad Clear and Venalink are both sealed containers and thus afford greater protection to the medication. Furthermore, it is recommended that MODs should have tamper-evident seals, which dosette boxes do not have.
FIGURE 5.
Medication organisation devices.
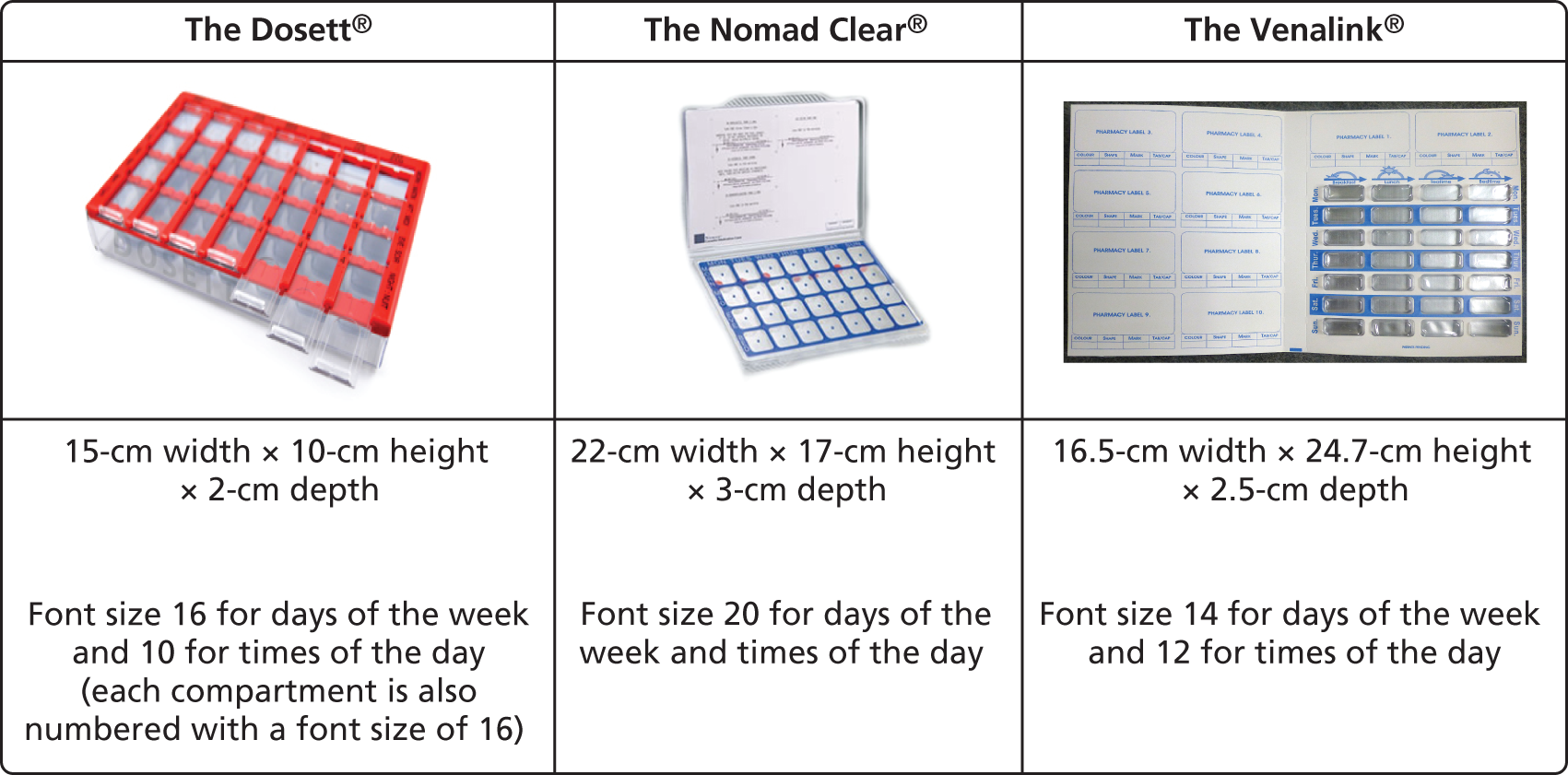
Nomad Clear is a lidded, clear rigid plastic tray of pre-formed compartments. It is cold sealed using a flat plastic adhesive film through which pills are accessed. The film is labelled with days of the week and times of the day and is perforated at the perimeter of each compartment to facilitate ease of access. Nomad Clear XL™ (Surgichem Ltd, Cheshire, UK) is identical to Nomad Clear but, having larger compartments, is better suited to those prescribed more medicines. The Venalin is a cold-sealed device which closely represents blister packaging. It comprises a non-rigid plastic tray into which pills are dispensed. This non-rigid tray is then inserted into a cardboard wallet labelled with days of the week and times of the day and sealed using an opaque adhesive cover.
Defining standard care and the intervention
Expertise within the research team was used to define standard care and the intervention. Prescription medication packaging of solid oral dose medications in the UK is largely 28-dosage units labelled with the days of the week.
Research has demonstrated that reducing monitoring frequency from weekly to fortnightly reduces adherence to therapy and, therefore, it may follow that reducing medication supply frequency may have a similar effect. 22 It is possible that the beneficial effect seen with MODs could be obtained by dispensing medicine supplies in standard packaging (increasingly blister packs) and supplying at weekly intervals.
Primary outcome
The primary outcome measure, as stipulated by the funding agreement, was EAM. EAM overcomes the problem of self-presentation bias which is associated with self-reported adherence. EAM may, however, be susceptible to reactivity bias as the device used for EAM acts as a constant reminder of trial participation and thus may alter usual patient medication-taking behaviour, resulting in artificially inflated adherence. 73,74
Broad-ranging web-based searches were carried out to identify candidate objective adherence monitoring technology and manufacturers were approached to determine whether or not they could supply suitable technology for these purposes.
These searches identified that technological advances mean that it is now possible to objectively measure adherence. This type of monitoring is less susceptible to the Hawthorne effect by virtue of being less intrusive and less conspicuous to the patient than direct adherence measures or DUCs. Electronic medication monitoring systems were initially developed as bottle-based systems containing a switch and data logger in the cap. Such electronic medication monitoring systems have been widely used in clinical trials to assess medication adherence. 11,24–26 The data logger records the date and time of each bottle opening event. However, usual dispensing is now generally in manufacturer issued packaging which is in blister pack form. Trials have, therefore, generally approached this issue by decanting medication from usual packaging into the monitoring bottles. This has the limitation of not assessing adherence in a naturalistic setting.
Similar technological principles have been applied to blister packs; however, there is a further challenge to then translate this to electronic monitoring of manufacturer supplied blister packaging. There is no standardisation to the size and shape of solid oral dosage forms; thus, there is a huge array of different-sized and -shaped packaging.
The main requirements of an electronic monitoring system are that it is discrete, accurate and robust, and consistent for both MODs and usual-care blister packaging; in addition, the comparator must look and feel like usual care and the intervention should provide a good approximation of the intervention as it would in a real setting.
A number of potential candidate technologies were identified for EAM of usual packaging and three organisations were approached for information or to supply devices for the project [Med-ic MMD Solution™, Qolpac b.v. NL and Future Technology (UK)].
Qolpac b.v. (NL) provides electronics-based e-health solutions. Its Objective Therapy Compliance Monitoring (OtCM™, Qolpac, NL, Amsterdam, the Netherlands) system is a powered, single-circuit and resistor-based system printed using silver or copper ink onto a clear plastic sticky-backed film which records each time a blister seal is broken, assuming that this equates to ingestion. Data are downloaded to computer software which records the blister position and time it was broken to within 10 seconds of the actual time. Figure 6 shows a representation of the Qolpac b.v. OtCM system.
FIGURE 6.
Representation of an EAM system for blister packs.

Future Technology (UK) Ltd (Banbury, Oxfordshire, UK) has recently developed SMARTpack™, which is similar to the Qolpac product in design and data produced but uses mobile phone technology to transfer data from a logging device to a remote server.
GP Solutions (UK) Ltd supplied the E-wallet™ (Manchester, UK): a system that enables medication-taking events from blister packs and MODs to be recorded in the same manner as the previous two systems. Further details of the systems are presented in Tables 8 and 9 and in Appendix 2.
| Characteristic | Qolpac b.v. NL, OtCM | Future Technology (UK) Ltd, SMARTpack | GP Solutions UK Ltd, E-wallet |
|---|---|---|---|
| Description | Discrete unit for which the patients’ usual pill pack is overlaid with a flex-circuit (flexible self-adhesive film on which circuit is printed) and a small additional unit measuring approximately 40 mm × 40 mm × 4 mm attached directly to the pill pack comprising power source, data logger and magnetic circuit connection to measure adherence | Unit dose or multidose blister sealed directly with a flex-circuit. The blister is inserted into a lightweight plastic holder that contains a power source, data logger, multipin circuit connection unit and messaging system to measure adherence. The messaging system can be GPRS using mobile phone technology or NFC | Bespoke blister packs overlaid with flex-circuit to which a monitoring unit measuring approximately 40 mm × 40 mm × 4 mm comprising power source, data logger and multipin circuit connection unit are attached. The whole package is housed within a cardboard outer to measure adherence |
| Recyclability | EAM unit designed to be reused | EAM unit designed to be reused | EAM not designed to be reused |
| Software | Appropriate software in place | Appropriate software in place | Appropriate software in place |
| Date | Yes | Yes | Yes |
| Time | Yes | Yes | Yes |
| Pill count | Yes | Yes | Yes |
| Pack temperature | No | Yes | Yes |
| Adherence | Yes | Yes | Yes |
| Data | |||
| Monitored | Resistivity at 30-second intervals | Circuit integrity at 30-second intervals | Circuit integrity at 30-second intervals |
| Stored | Data logger within EAM unit | Data logger within EAM unit | Data logger within EAM unit |
| Captured | NFC/scan | GPRS/NFC/scan | NFC/scan |
| Design principle | Qolpac b.v. NL, OtCM | Future Technology UK Ltd, SMARTpack | GP Solutions UK Ltd, E-wallet |
|---|---|---|---|
| MOD design | Under development | Prototype | 1 × commercial model |
| Blister pack design | Under development | Under development | 4 × commercial models |
| In-house evaluation | Yes | Yes | Yes |
| In-house evaluation blister pack | Yes | Yes | Yes |
| In-house validation MOD | Yes | Yes | Yes |
| In-house validation blister pack | Yes | Yes | Yes |
| Multisite validation MOD | No | No | No |
| Multisite validation blister pack | No | No | No |
| Advantages/limitations |
|
|
|
All systems require precision-designed detection circuits which fit discretely to the surface of the blister pack. Each blister pack requires a slightly different design to ensure accurate recording of pill-taking events, that is if the circuitry does not cross the pill position then the removal of a pill will not be recorded. For a study of one medication, such systems are relatively cost-effective; however, in the case of studies involving multiple different medicines, each with individually shaped packaging, the cost of developing a detection circuit bespoke for each different type of medication would be prohibitive.
A 2-month pilot study (n = 52) of this technology to assess feasibility and acceptability reported promising results. Adherence data were obtained from 94.3% of participants, and 67.4% of participants reported that they would consider using the electronic monitoring system for a long-term study. 27 The original protocol for the pilot RCT set out to include adults aged ≥ 75 years if they were prescribed three or more SODF medications which were expected to be continued for 12 months, were registered with one of six participating medical practices and an associated pharmacy and were capable of providing informed consent. However, owing to technical difficulties with the supply of a suitable EAM system, the eligibility criteria were revised. The EAM systems were not universal and because of cost restrictions could be developed for only a limited range of pack sizes. Therefore, eligibility criteria were relaxed to enable adults aged ≥ 75 years who were taking three or more SODFs to be recruited. At least two of the participants’ medications needed to be from a defined list of 11 medications sourced from one supplier and identified by study GPs as those most commonly taken by patients in the over-75-years age group to enable electronic monitoring of adherence to at least two medicines.
Although all components of MODs and usual-care EAM systems are readily available, no prototype units from the preferred supplier were available for testing and no other EAM technology could be provided for this project within the necessary timescale. In the absence of an electronic monitoring system, DUCs are the most appropriate approach to quantitative adherence measurement.
The DUC is based on the assumption that, if the medication is not in the container, the patient has taken it. This is problematic when attempting to identify intentional non-adherence because patients may deliberately remove and discard medication in order to disguise non-adherence. In essence, however, the electronic monitoring systems are open to the same criticism. Retrospective DUCs are, however, less likely to cause reactivity bias, and previous research has demonstrated that conducting DUCs on the older patient population is feasible and acceptable to patients. 15
Accurate DUCs require accurate knowledge of the quantities of medication to which the patient has access. Hoarding of medication is a regular occurrence; thus, accurate DUCs require that patient access to medicine stores be prohibited. However, the removal of what is, essentially, the property of the patient to a place of safety while also guaranteeing, the integrity of the medications during storage and then their safe and ethical return is problematic.
While pharmacies are secure environments with appropriate medication storage facilities and thus the most intuitive solution to storing patient hoarded medication during a trial, liaison with the pharmacies participating in this feasibility study identified that it would not be an acceptable solution. Limited space was a common reason cited plus ethical considerations; it was considered undesirable to remove a patient’s property and then to be unable to return it because it was out of date or because its provenance could not be guaranteed.
These issues may be circumvented by allowing the patient to retain their non-trial medicines within their home but packaging them into a sealed container so that the contents cannot be accessed without destroying the container. The additional merit of this process is that the patient is able to access their medication in case of an emergency.
Optimum recruitment strategies
Passive recruitment using postal invitation has the merit of being able to simultaneously target a large number of people with minimal cost; however, response rates to this passive method have historically been low, as the method requires the patient to be proactive in responding to a letter of invitation. 75 Consent rates are frequently between 30% and 40% using this method. 16,34 Active recruitment processes such as waiting room recruitment by researcher, while more labour intensive and thus costly, have been reported as yielding substantially higher response rates. 35 While the relative merits of the active and passive approaches to recruitment are known, the most cost-effective approach is yet to be determined.
Database
A dedicated study database was designed, built and maintained by Norwich Clinical Trials Unit. The database was Microsoft SQL based (Microsoft Corporation, Redmond, WA, USA). The data were resident on a secure server at University of East Anglia (UEA), directly accessible only to the database management team at Norwich Clinical Trials Unit and to UEA information technology and computing services personnel. Access for study personnel was via a study website containing forms to enter and review study data. Access to these pages was controlled by personal usernames and passwords under control of the database manager. Web traffic was encrypted using standard secure sockets layer technology.
Secondary outcomes
Patient autonomy
In addition to the impact of MODs on adherence, it is important to establish patient acceptability. No studies have reported the impact of MODs on patient autonomy or ability to manage one’s own medication; however, there is anecdotal evidence of reduced autonomy, as patients are unable to differentiate one medication from another in a MOD and therefore cannot select one type of medication to omit over another. When this is desired (e.g. delaying taking a diuretic when taking a long journey) the result may be omission of all medicines. 1 Conversely, patients may report that they feel enabled by feeling confident about managing their medication. A number of studies have explored patient autonomy with respect to medication taking in the context of describing the extent to which patients feel involved in the decision-making process. 28 However, exploration of whether or not patients feel as though they have some control over the medication-taking process is limited. A questionnaire was developed based on a Client Satisfaction Questionnaire (CSQ-8) to assess the impact of MODs on patient confidence in their ability to manage their own medication and on their satisfaction with their packaging and the service they have received.
Dispensing and administration errors
The most substantial review to date of errors associated with MOD use was conducted in Australia; the Australian Incident Monitoring Study31 reported that 0.43% (52 out of 12,000) of medication-related errors were associated with MODs. In 26 cases, there was a problem with filling the MOD, such as wrong dose, dose omission or wrong medication. In 21 of these cases, nursing staff were responsible for the error, with the remainder being attributable to pharmacy staff or a carer. On 16 occasions problems using the MOD were cited as a reason for an error; however, the nature of these problems was not reported. Factors contributing to the reported problems included patient confusion/distraction and the MOD being inappropriate for the patient. 31
A further Australian audit of dispensing errors associated with 6972 dispensed MODs detected an error rate of 4.3%. 32 A 2007 UK evaluation of dispensing error rate associated with the pharmacy ‘usual’ dispensing process33 reported 1.7% content errors among 2859 dispensed items. Content errors were errors of omission, incorrect drug, incorrect strength, dosage form, added or missing dose units and expired medication. While general dispensing error rates are 1.7%, there are no UK data for MOD error rates, and those from Australia suggest widely different potential error rates. It is necessary, therefore, to record error rates for dispensing into MODs and usual packaging.
In summary, comparative data in terms of error rate associated with MODs and usual dispensing are not available. Data regarding the incidence of dispensing errors associated with usual dispensing are available but may not be generalisable to prescriptions assembled for an older population with multiple items. A reasonable estimate of error rate requires a large sample size and significant resources. Within a relatively small-scale feasibility study it is, therefore, only appropriate to quantify and describe any identified errors.
Phase 2: trial design focus groups
Introduction
Trial design must be feasible and acceptable from the perspective not only of researchers but also of patients and health-care professionals. Focus groups were carried out to identify design elements that would or would not be acceptable and, when necessary, to refine the experimental design to meet both researcher and stakeholder requirements.
Pre-specified objectives for the patient and carer focus groups were to:
-
describe the practical difficulties experienced by patients and carers in adhering to complex medication regimes
-
explore the appropriateness and acceptability of MODs
-
consider the benefits and disadvantages of MODs including any potential adverse outcomes
-
establish the feasibility and appropriateness of trial participant requirements including recruitment documentation, participant information sheets, survey tools and proposed adherence measures.
Pre-specified objectives for the health-care practitioner focus groups were to model and gain consensus expert opinion on:
-
the appropriateness and feasibility of the research design
-
participant inclusion and exclusion criteria
-
recommended MODs and criteria for selection
-
any clinically important difference in patient adherence.
Methods
The feasibility study of a RCT was funded by the NIHR HTA programme and started in August 2011 (ref. HTA 09/34/03). The trial was approved by the Cambridge East Research Ethics Committee (ref. 11/EE/0141) and Norfolk Primary Care Trust (ref. 55252). The trial is registered with UK Clinical Research Network (ref. UKCRN 11256). The protocol describing pre-RCT focus group study design is appended (see Appendix 3).
Six medical practices and the geographically close pharmacies in Norwich, UK, expressed an interest in study involvement. Potential participants were identified by medical practice staff via a computerised search and the resulting list was manually screened by either the prescribing team or GP. Three practices recruited patients receiving, or with a history of having received, a MOD, one practice recruited patients not currently receiving, and with no history of having received, a MOD and two practices recruited carers of patients in receipt of a MOD.
Focus group participant identification and recruitment
Patients and informal carers were purposively sampled to represent a wide variety of medical, social and demographic characteristics.
Inclusion criteria for patients were:
-
age ≥ 75 years
-
exhibiting any medication-taking behaviour (thought to be fully adherent or intentionally non-adherent or unintentionally non-adherent)
-
representing a range of regimen complexity (regularly prescribed three or more medicines and prescribed multiple formulations, e.g inhalers, eye drops and cream or ointments)
-
currently using MODs or who have previously declined the use of a MOD
-
with mild cognitive impairment (sufficient in the clinician’s opinion to allow provision of informed consent and engagement with a focus group)
-
with manual dexterity problems.
Exclusion criteria for patients were:
-
on the mental health register with, for example, a diagnosis of schizophrenia or bipolar disorder, but not depression
-
on the dementia register
-
unable to read or speak English and/or to provide informed consent
-
deemed by the health-care team to be inappropriate for study inclusion.
Informal carers were defined as friends and relatives aged over 18 years who support patients in their medication organisation and/or taking but receive no remuneration, were required to be registered with participating medical practices and were known by practice staff to support a person aged over 75 years in managing medication.
Patients and carers were invited to take part via a recruitment pack posted from their medical practice which comprised a covering letter from the medical practice, participant information leaflet and consent form. Consenting participants returned consent forms with contact details directly to the researchers. After 2 weeks a follow-up letter was sent from practices to non-responders.
Written informed consent was sought from patients and carers to allow contact from researchers with a view to arranging focus groups. Patients and their carers were not invited to participate together in the same focus group, as this may have prevented participants from speaking freely.
Carers in sheltered housing
Wardens were recruited by mail using details obtained from Norfolk County Council Adult Social Services and requested to distribute the carer information leaflets and consent forms to carers supporting people in sheltered housing within Norwich.
Health-care practitioners
Primary care practitioners were recruited from the six medical practices and pharmacies taking part in the study. Secondary care practitioners were recruited from a Department of Older People’s Medicine at the local teaching hospital. Purposive sampling was used to ensure representation from GPs, practice prescription managers, pharmacists, community nurses plus consultants and hospital nurses specialising in the care of older people. Study information leaflets were given to all potential participants and a minimum of 24 hours allowed before a decision regarding participation was requested. Written informed consent was obtained from all participants.
Focus group conduct and data collection
Topic guides were developed from pre-specified study objectives. Meetings were scheduled to last for 90 minutes and were designed to encourage free discussion and to generate a wide range of ideas and opinions. Audio-recordings were made with the consent of participants. A broad introductory session on general areas of organising, giving and taking regular medicines was followed by in-depth discussion surrounding MODs and study procedures. At the relevant points in the discussion, samples of proposed study documentation and a selection of sample MODs were introduced to serve as trigger material to promote discussion of appropriate documentation and MOD types for the proposed study.
Patients and carers
Two mixed focus groups of patients and carers took place at a community centre and at the UEA in Norwich. Refreshments were served, transport offered or reimbursed and a £20 gift card provided on attendance.
The patient and carer focus group topic guide is appended (see Appendix 3). It was designed to obtain experiences, thoughts and opinions on:
-
organising and taking or giving regular medicines
-
using MODs
-
study procedures.
Health-care practitioners
One focus group of primary care practitioners was convened at a medical practice and another focus group of secondary care practitioners was held at the Norfolk and Norwich University Hospital Foundation Trust. Refreshments were provided and expenses including locum cover were reimbursed.
The focus group topic guide was designed to obtain experiences, thoughts and opinions on:
-
patients’ medication management strategies and problems
-
the effects of MODs
-
study design including recruitment procedures and outcome measures.
All focus groups were moderated by the principal investigator (an academic pharmacist), with a research team member in assistance. Two other research team members were present as observers. Audio-recordings were transcribed verbatim and NVivo software (versions 9 and 10; QSR International, Warrington, UK) were used to manage, sort and facilitate analysis of the transcript data and field notes. A thematic analysis was adopted.
Transcripts of each focus group were read and a coding framework was independently developed by two researchers. The coding framework, map and main themes were discussed and selected. Extracts were labelled using participant number and focus group attended. Illustrative anonymised quotations were selected to elucidate study findings. All processes of the analysis were shared with the study management group to enhance transparency and validity of interpretation. A summary report of the major findings was also reviewed by the research team as an additional validity check.
Results
Fifteen patients and carers, comprising broadly equal numbers of males and females, participated in two focus groups with 66% having some experience of either self-filled (46%) or pharmacy-filled MODs (20%). Five health-care professionals took part in the primary care focus group and six attended the secondary care focus group. All focus groups lasted between 85 and 90 minutes. Table 10 provides details of participants attending focus groups.
| Details for participants | Number of participants | Coding assigned to participants |
|---|---|---|
| Patient and carer FG1 | 9 | – |
| Patient | 7 | P1 FG1 to P7 FG1 |
| Carer | 2 | C1 FG1, C2 FG1 |
| Patient and carer FG3 | 6 | – |
| Patient | 1 | P1 FG3 |
| Carer | 5 | C1 FG3 to C5 FG3 |
| Health-care professional FG2 | 5 | – |
| Community pharmacist | 2 | HCP2 FG2, HCP3 FG2 |
| General practitioner | 1 | HCP5 FG2 |
| Prescriptions manager | 1 | HCP4 FG2 |
| Physiotherapist | 1 | HCP1 FG2 |
| Health-care professional FG4 | 6 | – |
| Hospital pharmacist | 2 | HCP1 FG4, HCP2 FG4 |
| Registrar (MFOP) | 1 | HCP4 FG4 |
| Consultant (MFOP) | 1 | HCP5 FG4 |
| Nurse | 2 | HCP3 FG4, HCP6 FG4 |
Domains discussed by all groups were medication management strategies and the difficulties associated with taking and giving regular medicines; the use and effects of MODs; and study design, procedures and outcome measures.
Following analysis of transcripts the findings fell broadly into four themes: barriers to adherence; patient autonomy; using MODs; and the acceptability of the proposed pilot study.
Barriers to adherence
Barriers to adherence have previously been well described, and those identified under this focus group (forgetfulness, distraction, difficulty in opening packs, difficult regimes) were closely aligned with published literature.
Forgetfulness
Both patients and carers reported that forgetting to take medication on time and forgetting the directions given by their doctor were sometimes an issue. Health-care professionals also thought that memory problems were the most common barrier to patients taking medication as prescribed. Box 1 provides quotes illustrating these thoughts.
. . . in the first place, I couldn’t remember, taking each tablet out at different times.
P6 FG1
. . . often that too when you get your medication, the pharmacist has put ‘take as directed by your doctor’ well sometimes you get out the door and you completely forgot what he’s said so . . .
C6 FG3
Also patients who go to the doctor, he or she says ‘oh, you just do this with this one and that one’, finally get home and they have totally forgotten.
P1 FG3
I’ve been aware of a few different types of difficulty with people who have memory problems.
HCP1 FG1
Another group is the ones who do start to lose their memory and struggle that way. And so it seems to go down two paths, most sorts of common ones . . .
HCP2 FG1
The elderly and they have a lot of medication particularly in the hospital setting where they have had a lot of medication changed before they go out of hospital. Also if they have slight issues around memory . . .
HCP2 FG4
Distractions
With medication taking closely linked to daily routines, it was reported by both patients and carers that interruptions may lead to forgotten doses. Furthermore, carers revealed a hierarchy of prioritisation by identifying that they may forget to take their own medication because they were distracted but always ensured that the person they were caring for had received their medication on time. Box 2 provides comments related to these themes.
The only time when it goes wrong is if the routine of the day is interrupted.
C3 FG3
. . . I might neglect myself because I am so concerned about my husband.
P4 FG1
. . . what happens is I’ve forgot to take . . . the neighbour comes round and you make them a cup of tea or the phone goes and you suddenly find it’s dinner time and you find that you haven’t taken the mid-morning tablet.
P2 FG1
I only have two, one before your meal and one after, just for blood pressure, and I forget my own, I am so busy making sure he has them but I do tend to forget my own.
C1 FG3
Access to medicines
Opening medication packaging and dropping or losing doses once accessed can be an issue for older people, particularly those with impaired vision or manual dexterity. There was strong consensus across patient and health-care professionals that patients experience difficulties opening bottle caps and pushing pills out of blister packs, and this seems to be compounded if increasing numbers of pills need to be taken from blister packaging at the same time. Some of these challenges are described in Box 3.
. . . there is the point of pushing them out and the mobility of fingers, um and the only thing would be I’d change from doing it from a bulk lot to changing to doing it each time we needed the tablet, which I might forget.
C1 FG1
. . . someone who has arthritis right? First of all, they can’t even pop the pills out, and if they drop if they can’t even pick it up because it is so small
HCP5 FG2
. . . we’ve had that from elderly patients, that they’ll take if (they have) got seven packets, they might take five today, ‘I’ve popped up five, that’s enough’. . .
HCP4 FG2
. . . Some of the packaging now, we are all familiar with child lock caps, we’ve have now got a lot of these child-resistant foils coming in. I had a lady coming back the other day who had something like lansoprazole and she said ‘I can’t pop them out’, my assistant had tried to help her and she wasn’t able to and so my assistant came to me and said ‘look we can’t pop these out’ and then we realise that it said ‘Peel here’, and we realised we had to peel it back to take it out so it’s not just childproof it’s adult proof as well.
HCP2 FG2
Medication regimes
Complicated regimes were reported to cause confusion and were time-consuming to manage. Confusion is associated with the amount of medication that needs to be taken, the timing of doses and how to take it. It was perceived that with complex regimes, daily life revolves around the routines of medicine taking. Secondary health-care professionals were aware of the high risk of confusion when multiple medicine changes are implemented as a result of hospital visits or stays. Box 4 gives example comments about medication regimes.
. . ., he has to take so many in the morning, and then once a week he has half a tablet, and then he will have to have one for three days of the week and it’s a real problem getting it sorted.
P4 FG1
. . . the fact that the way we are putting the medicines together and the regime, once you get over a certain number of tablets, there is no patient who is going sit there and pop eight packets of tablets out. They’re just going to miss out on them so there is a cut-off point where someone – where we give them an amount of a medication and with the best in the world they are going to miss one of those packets.
HCP4 FG2
. . . they have a lot of medication particularly in the hospital setting where they have had a lot of medication changed before they go out of hospital.
HCP2 FG2
Health-care professionals raised the issue of intentional non-adherence because of experience of medication side effects or lack of tangible benefits. This topic was not raised by either patients or carers. The health-care professionals’ comments related to this topic are provided in Box 5.
. . . sometimes it can be the side effects of the medication which can stop people from remembering to take it.
HCP2 FG2
. . . , we start these routine medications, people start off fine because your blood pressure is coming down your headaches are getting better, you feel fine, then as your problems (are) over and you’re fine there is a time that everyone will go through, they will start missing tablets.
HCP5 FG2
Patient autonomy
An interesting and important finding was that individual patients or carers reported independently designed innovative and varied strategies to promote medication adherence which were actually very similar to those independently designed by other patients and carers. Their strategies were designed to provide the most convenient and practical approach to meet their personal needs. Patients, carers and health-care professionals agreed that maintaining autonomy is important where possible. Health-care professionals also acknowledged that people are individuals and may be reluctant to change their medication management procedures. Some patients indicated that they would trust their own strategy in preference to using a MOD. The thoughts of the patients, carers and health-care professionals are summarised in Box 6. The different systems used to remind patients to take their medication as prescribed are grouped and summarised in Table 11.
She doesn’t want an organiser; she has a list in the bathroom. And again, with the triggers for taking them during the day. So it’s fairly simple.
C3 FG3
I’ve tried putting them in an organiser myself but I find that it doesn’t work out at all. So I have a collection of wine glasses and shot glasses, I do 10 days of these at a time . . . And I just couldn’t make organiser work. It’s . . . me not being very good at it but, um . . . I just find my wine glasses and shot glasses work better for me from my point of view.
C1 FG1
. . . she is on five different medications, she’s got different bowls, and she’s got one in each bowl, and she recognises each by the bowl. Even though she’s half blind and can’t see, she’s got the box and this is this and she keeps that on her kitchen table and then she takes it that way. And no matter whether you try to put her on to something else she’s not going to she’s quite resistant to it.
HCP5 FG2
I think that’s the tricky point as well, that we make a situation for ourselves and then we go into people with their crisis and do we actually make the situation worse because were going in there and interfering, aren’t we?
HCP1 FG2
| System | Example |
|---|---|
| Daily routine or trigger activities | Take the morning tablet after shaving |
| Audible alarm | Have a buzzer setting at certain times |
| Personal reminder | Somebody telephones to remind the patient to take the medicines |
| Placement | Put the night-time tablet with the glass of water by the bedside |
| Medication chart or record card | Generate a chart for the times of the day and medicines to be taken |
Using medication organisation devices
All participants recognised that MODs may be helpful for some patients but also acknowledged some disadvantages. Table 12 summarises the perceived advantages and disadvantages of using MODs.
| Subcategory | Advantages of using MODs | Disadvantages of using MODs |
|---|---|---|
| Convenience |
|
|
| Reminder |
|
|
| Adherence |
|
|
| Accuracy |
|
|
| Economic |
|
|
A number of different MODs are commercially available. A selection of MODs were presented to participants, who handled and discussed the various types. Consistent responses indicated that each appeared to have some limitations, with no one design accommodating all needs. The desirable characteristics of MODs identified from participant comments are summarised in Table 13.
| Factor | Desirable characteristic |
|---|---|
| Compartments |
|
| Material |
|
| Accessibility |
|
| Reminder equipment |
|
The types of patients for whom, in the opinion of participants, MODs are appropriate are:
-
older people
-
people with impaired cognition
-
people with impaired manual dexterity
-
people with impaired visual acuity
-
those prescribed multiple medications
-
those prescribed a complex medication regimen
-
people with no social support
-
those with history of readmission due to poor adherence.
Acceptability of proposed study
A key aim of the focus group was to identify participant opinion regarding the proposed study design. Importantly, no participants voiced any major concerns about the proposed research in relation to study design and materials as written in the study plan. However, it is important to note that the proposed intervention was highly complex and, although the study team took all possible care to promote understanding (providing study flow charts, MOD boxes, study documentation and representations of electronic monitoring devices), there was some necessary simplification because of time constraints. Furthermore, the moderator of both focus groups was a pharmacist and, although all reasonable steps were taken to ensure that participants, particularly carers and patients, felt able to talk freely and to ask questions, we are aware that some participants may have felt unable to query information/misunderstandings or admit to not understanding.
Table 14 provides a summary of the different aspects which were included in the final trial either following recommendation by the research team and with the agreement by the focus groups or as additional recommendations derived from the focus groups. Health-care professionals commented that effect size was, in some cases, entirely dependent on condition. For example, for patients with myasthenia gravis, adherence needs to be 100% at all times. For the general population it was accepted that realistic adherence rates to specific medicines can be estimated at 50%. They agreed that 80% adherence would be acceptable and reasonable for most patients and conditions and, therefore, that an improvement in adherence of around 30% should be the aim.
| Area | Suggestion | Suggested by | Action taken |
|---|---|---|---|
| Inclusion criteria | Patients with moderate physical impairment, e.g. poor sight or poor manual dexterity, may need help to take medicines | Patient, HCP | Do not exclude those with moderate physical impairment |
| Those with cognitive impairment frequently need help to take medicines | HCP | Do not exclude those with moderate cognitive impairment | |
| Older people who are de-skilled with respect to taking their own medicines cannot be expected to relearn to manage medicines by themselves | Carer, HCP | Include only patients who are self-medicating | |
| People who have complex medication regimes often need help to take medicines correctly | HCP | Inclusion criteria to include regime complexity | |
| Prescribed minimum of three SODFsa | HCP | Include patients prescribed three or more medicines | |
| Prescribed minimum of four SODFsa | HCP | Not included | |
| Age can be associated with patients needing more help | HCP | Inclusion criteria to state a lower age limit | |
| Aged ≥ 75 years | Researcher | Include those over 75 years as pre-specified | |
| Age combined with medication complexity and chaotic lives may mean that more help with medication is required. Aged ≥ 50 years is appropriate | HCP | Not included for this study | |
| Those aged ≥ 60 years may need help with medicines | Patient | Not included for this study | |
| Exclusion criteria | People who live independently at home | Patient | Inclusion criteria to exclude those in care homes |
| It is important not to interrupt the medication regime for those with Parkinson’s disease | HCP | Those with Parkinson’s disease to be excluded | |
| Severe mental illness may mean safety issues for researchers | HCP | Patients known to have severe mental illness to be excluded | |
| Deemed inappropriate by the health-care team | HCP | GP screening of lists to ensure that patients considered inappropriate are excluded | |
| Patients already using a MOD cannot be moved to usual care | Patient, HCP | Patients already using a MOD excluded | |
| Recruitment | Utilise pharmacy to identify eligible patients | HCP | Not included |
| Poster in waiting area | Patient | Not included | |
| Maintenance of autonomy | Novel medicine management systems are used by some people, e.g. shot glasses or ice cube trays | Carer, Patient, HCP | Capture information regarding existing strategies and screen out those with strategies incompatible with study requirements |
| Choice of MODs | Patients may need to take many medicines each day so MOD compartments need to be big enough for all the tablets that might go into each compartment | Patient | Inclusion of Nomad XL (relatively large compartments) into the selection of MODs |
| Patients may have difficulty using hands as a result of, for example, arthritic fingers | Patient | Top-opening and blister-type MODs included to accommodate varied manual dexterity | |
| Allow participants to select their preferred MOD | Patient | Participants to select from choice of three MODs | |
| Adjustable compartment size would be beneficial | Patient | No such device is supported under NHS funding and therefore it is not included | |
| Reminder buzzer/alarm at medicine taking times | Patient | ||
| More compartments would be useful | Patient | ||
| Nomad Clear™ acceptable | Patient | To be included | |
| Venalink™ acceptable | Patient | To be included | |
| PIL | Be more explicit about the processes involved in continuing with a MOD | HCP | Processes will be described in PIL and SOP for pharmacy |
| Be very explicit about not sharing data with GPs | HCP | To be included in PIL | |
| Emphasise that the aim is to capture usual medication-taking habits | HCP | Explained to patients and included in PIL | |
| Study design | Crossover design to limit the effect of increased information provision on adherence | HCP | Not incorporated |
| Strategy needed to avoid loss to follow-up as a result of hospitalisation | HCP | Not incorporated as inpatients cannot be self-medicating | |
| Ignore first 2 weeks of adherence data to allow for ‘bedding in’ | HCP | Not possible for this study because of the short duration but a recommendation will be made for inclusion in future study design | |
| Collect clinical outcomes such as blood pressure and biochemistry such as lipid profile | HCP | Not incorporated. No single morbidity for the patient group | |
| Allow extended monitoring period, ideally for at least 6 months to measure normal behaviour | HCP | Not incorporated for this study but to be recommended for definitive study | |
| Invitation given to any patient with multiple scripts | HCP | Not incorporated as unsuitable patients may be approached | |
| Collect time taken to dispense medication and estimate cost | HCP | Incorporated | |
| Important measures and outcomes | Use of an EAM system is acceptable | Patient, HCP | Adherence monitoring system to be included |
| Health and quality of life to be assessed before and after study | HCP | EQ-5D-3L and ICECAP-O incorporation | |
| Collation of GP, hospital and social services use data | HCP | Collection of GP, HES and social care data included | |
| Identify cost issues for MODs vs. usual care | HCP | Health economic evaluation included | |
| Identify whether or not medicine waste is reduced | HCP | Not included in this study but to be recommended for definitive study |
Summary
Separate focus group discussions with secondary and primary health-care professionals and with patients and carers were undertaken in order to refine the trial design. The findings were grouped under four domains: barriers to adherence; patient autonomy; using MODs; and the acceptability of the proposed pilot study design.
In line with published research, barriers to adherence included forgetfulness, distractions, inability to access packaging and complicated regimes. Maintaining patient autonomy was universally agreed by participants to be important, and many patients trusted their own unique strategies for ordering and remembering their medication, for example alarms, lists, charts, wine glasses and bowls.
No one MOD design suited all, but aesthetic and ergonomic factors, such as size and accessibility, were key determinants of acceptability. The main advantages included reduction in packaging and improved visual evidence of adherence. Disadvantages included challenges to access medication from the MOD; inability to accommodate multiple and complex regimes; and potential removal of knowledge and control from patient and carer. Economic arguments balanced better efficiency of medication supply and less wastage, with increased workload demands on pharmacists. The patients identified as most likely to benefit from MODs were older people with impairments of cognition, dexterity and vision who were prescribed complex and multiple medication regimes with little social support and history of readmittance to hospital.
With regards to study design, participants identified no challenges to the proposed methods. The desirable characteristics of study participants were confirmed and largely accommodated by the study inclusion and exclusion criteria with the exception of reducing the minimum recruitment age from 75 years to 50 or 60 years. Alternative recruitment strategies, such as recruitment from pharmacies and by using posters, were proposed but not included as they would not allow targeting of eligible patients.
Chapter 4 Randomised controlled trial
Introduction
Chapter 1 provides a detailed background describing the rationale for this trial. A number of studies quantifying medication adherence for MODs compared with usual care have been identified and the systematic review results are presented in Chapter 2. The review illustrates that most studies in this area are small scale and provide no definitive evidence of either clinical or cost benefit. In addition to this, there is no guidance on the type of patient who would benefit from receiving their medications in a MOD. Chapter 3 provides a description of the development of the proposed design elements for a definitive study. This chapter describes the RCT in order to determine the feasibility of using elements of the proposed design as part of a definitive study to compare MODs with usual care and to define the population most likely to gain benefit from this intervention.
Aim
To test study methods and identify refinements for a definitive trial to investigate the clinical effectiveness and cost-effectiveness of MODs.
Objectives
-
To identify the most effective method of participant recruitment.
-
To estimate the prevalence of intentional non-adherence within an older population.
-
To estimate the prevalence and magnitude of participants’ unintentional non-adherence within a 3-week period.
-
To describe the functional abilities of an older participant population.
-
To compare medication adherence for MODs and usual care.
-
To compare medication adherence for weekly and monthly supply.
-
To compare the effects of MODs and usual care plus weekly and monthly supply.
-
To provide a point estimate of the effect size of MODs relative to usual packaging.
Exploration of each of the objectives is undertaken in this chapter, with the exception of the first objective; the cost-effectiveness of two different recruitment methods is examined in detail in Chapter 6.
Methods
Trial design, funding and approval
The RCT was a single-centre (multisite) individually randomised 2 × 2 factorial design comparing the effect of MODs with medication dispensed in usual packaging and of weekly compared with a monthly medication supply. The trial was conducted across six NHS Norfolk GP practices and 14 pharmacies which served the patients registered at these GP practices. Five of the GP practices and 13 of the pharmacies were situated in the Norwich area, including its suburbs, and one GP practice and pharmacy was based in a small, nearby market town.
The trial was funded by the NIHR HTA programme and started in August 2011 (ref. HTA 09/34/03). The trial was approved by the Cambridge South Research Ethics Committee (ref. 12/EE/0251), Norfolk Primary Care Trust (ref. 99999) and additionally by Norfolk County Council (in respect of social services usage data only). The trial is registered with the International Standard Randomised Controlled Trial Register (ref. ISRCTN 30626972) and the UK Clinical Research Network (ref. UKCRN 12739).
A number of changes to procedure were necessary as a result of the failure to obtain a suitable EAM system. Table 15 provides a summary of deviations from the protocol with reasons for the deviation. All deviations were appropriately notified as amendments to the protocol.
| Protocol section | Protocol instruction | Protocol deviation | Reason for deviation |
|---|---|---|---|
| 3.3.1 | Adherence measured using electronic monitoring | EAM was not carried out | No suitably universal or discrete EAM system was supplied |
| 3.2.2 | Inclusion criterion: prescribed three or more SODFs expected to be continued for 12 months | Inclusion criterion changed to: prescribed three or more SODFs (two from a defined list) expected to be continued for 12 months | Lack of universality of EAM systems meant that a limited range of medication packs were planned for monitoring |
| 3.7.1 | 3 weeks pre-baseline (T–3) participants are provided with 1 month’s medication. At baseline (T0), unintentionally non-adherent participants are randomised. At T0–T12 medicines are supplied to participants according to randomisation | At T0 no EAM system had been provided so patients were maintained on usual care pending arrival of the system. No system was received so DUC was used | No suitably universal or discrete EAM system was supplied |
| 3.7.1 | From T0–T12 (3 months) | In consultation with sponsors the duration of the trial was reduced to 2 months | Delay was a result of lack of suitable electronic monitoring system |
Participant eligibility
The pilot had two phases. Phase 1 established patients’ adherence; only patients identified as unintentionally non-adherent progressed to phase 2, which was randomisation.
As described in Chapter 3, refinements to the eligibility criteria were necessary because of constraints of the proposed EAM system.
Eligibility criteria
The process for ensuring that all randomised participants fulfilled the inclusion and exclusion criteria was multistage and is summarised in Figure 7. Some eligibility criteria are repeated at the different stages where appropriate multiple information sources were used to confirm eligibility.
FIGURE 7.
Process for determining participant eligibility.
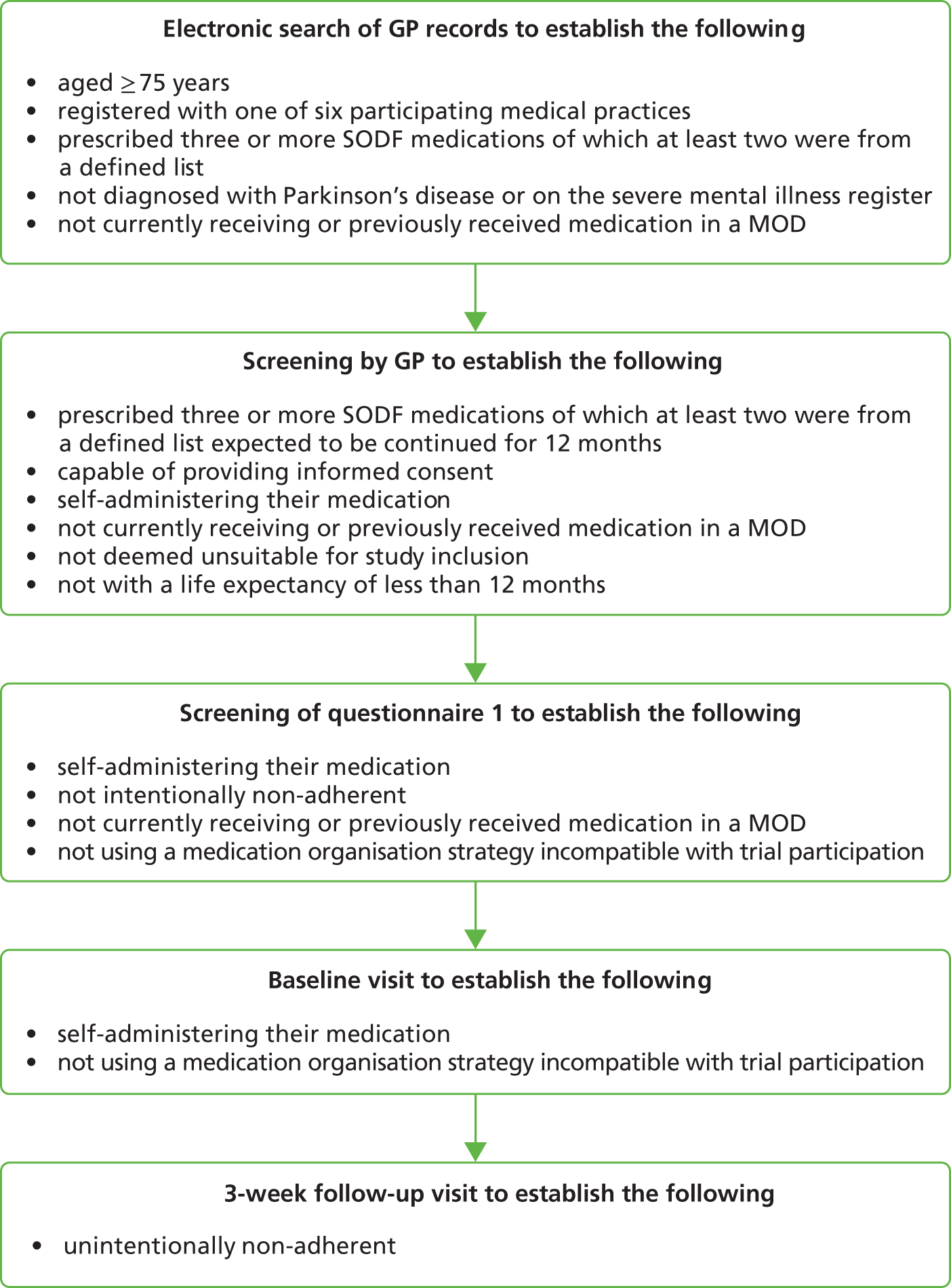
Inclusion
Patients were included if they were:
-
aged ≥ 75 years
-
registered with one of six participating medical practices
-
prescribed three or more SODF medications expected to be continued for 12 months (of which at least two were from a defined list in Table 16)
-
capable of providing informed consent.
| Medication name | Strength |
|---|---|
| Simvastatin tablet | 40 mg |
| Aspirin dispersible tablet | 75 mg |
| Levothyroxine tablet | 25, 50 and 100 mcg |
| Ramipril capsule | 5 and 10 mg |
| Bendroflumethiazide tablet | 2.5 mg |
| Omeprazole capsule | 20 mg |
| Amlodipine tablet | 5 and 10 mg |
| Lansoprazole capsule | 15 and 30 mg |
| Atenolol tablet | 25 and 50 mg |
| Metformin tablet | 500 mg |
| Furosemide tablet | 20 and 40 mg |
Exclusion
Patients were excluded if they:
-
were regularly prescribed medication not currently dispensed by a pharmacy recruited to the study
-
had a life expectancy of less than 12 months
-
were currently involved in a clinical trial
-
were not self-administering their medication
-
were currently receiving or had previously received medication in a MOD
-
were diagnosed with Parkinson’s disease or a severe mental health disorder such as schizophrenia
-
were deemed by the health-care team to be unsuitable for study inclusion for other reasons
-
were using a medication organisation strategy incompatible with trial participation
-
were intentionally non-adherent.
Participant identification and recruitment
Identification
One member of each of the six recruited medical practices undertook a database search to identify potentially eligible patients. A GP subsequently screened the list to exclude any patients deemed unsuitable. The reasons for exclusion were recorded in order to further refine the exclusion criteria of a definitive study.
Recruitment
Two patient recruitment methods were tested, passive and active, to determine their acceptability and effectiveness. For consenting patients, responses to questionnaire 1 (see Appendix 5) were used to identify whether or not the patient should be excluded on the following criteria:
-
were using a medication organisation strategy incompatible with trial participation
-
were intentionally non-adherent.
Passive recruitment
Patients identified by GP database search, and subsequently GP screened for suitability, were sent recruitment packs. Recruitment packs comprised a letter of invitation on GP practice-headed paper (see Appendix 6), patient information leaflet (PIL; see Appendix 7), consent form (see Appendix 8), questionnaire 1 (see Appendix 5) and a pre-paid envelope. Participants were asked to return completed documents directly to researchers. Questionnaire 1 was not attached to the consent form and so patients were able to choose to return both, either or neither. Implicit consent was assumed for any questionnaires returned. One reminder was sent to each non-responding patient 3 weeks after initial posting.
A standard operating procedure (SOP) for passive recruitment was prepared (see Appendix 9). Three practices used only passive recruitment which commenced during August 2012 and was completed in February 2013.
Active recruitment
This process involved one of six researchers recruiting patients when they presented for routine appointments at the participating GP practices. Patients identified by GP database search, and subsequently GP screened for suitability, were highlighted on the GP system, which enabled practice reception staff to identify the potentially eligible patients who would be presenting at the medical practice on a recruitment day. Such patients were provided with a PIL and an explanation that there was a researcher present who would like to talk to them about the study if they were interested. Patients who chose to approach the researcher were informed about the study and given a recruitment pack which they could complete at the medical practice or take away. If patients volunteered information indicating that they were ineligible (e.g. they were already using a MOD), they were informed of their ineligibility and thanked for their time. The SOP for active recruitment is provided in Appendix 10.
Three practices used active recruitment for a 3-week period followed by passive recruitment, as previously described. Active recruitment was carried out in 3-week tranches during the period September to November 2012.
Intervention and comparator
Participants were randomised to one of the following arms for 8 weeks:
-
four MODs dispensed monthly either collected by the patient or delivered to the patient’s home, according to usual patient routine
-
one MOD delivered to the patient’s home weekly
-
usual medication packaging dispensed monthly and either collected by patient or delivered to the patient’s home, according to usual patient routine
-
usual-care medication packaging delivered to the patient’s home weekly.
Other prescribed medication that could not be dispensed in a MOD, for example inhalers, creams, liquids and when-required medication, was supplied in usual packaging with the MOD.
Medication organisation devices
Nomad Clear, Nomad Clear-XL and a Venalink device were used. MODs contained all SODFs prescribed to the patient unless they were unsuited to being dispensed in a MOD [e.g. because they are to be taken ‘when required’, their administration requires special processes, such as dissolving or chewing, or their dose is likely to change frequently or at short notice (e.g. warfarin)]. Monthly MODs were either collected from the pharmacy or delivered from the pharmacy to the patient according to the usual routine of the patient. Weekly MODs were delivered to the patient.
Usual care
Medication supplied in the manufacturer’s usual packaging or in accordance with usual dispensing procedures when this is inappropriate, for example prescription requests for a quantity other than the manufacturer’s pack size. Monthly packaging was either delivered to the participant or collected from the pharmacy according to the participant’s usual routine. Weekly packaging was delivered to the participant’s home.
Outcome measures and outcomes
Primary outcome measure
-
Per cent adherence in terms of dosage units taken.
Secondary outcome measures
-
Self-reported autonomy (adapted enablement) and satisfaction (adapted CSQ-8).
-
Mortality.
-
Economic outcome measures will be considered in Chapter 7.
Other outcomes
-
The proportion of respondents reporting characteristics associated with intentional non-adherence.
-
The proportion of participants identified by DUC to be unintentionally non-adherent.
-
The magnitude of unintentional non-adherence identified within a 3-week period.
-
Participant ability described in terms of cognitive function, manual dexterity and visual acuity.
-
The effects of MODs and usual care plus weekly and monthly medication supply.
Data collection
Questionnaire data were collected from patients by postal return or in their own homes if participants requested help to complete forms. Data on pharmacy activities and the empty packaging for pill counts were obtained from each participating pharmacy. Patient data on comorbidities and use of health-care services for the period 2 months prior to and 2 months after the start of the trial were obtained from participating GP practices. Data on use of social care services were obtained from Norfolk County Council Social Services Department.
Prevalence of intentional non-adherence
All consenting participants were screened for factors including intentional non-adherence, non-self-medication, current or previous use of a MOD and complex medication management strategies using questionnaire 1. Intentional non-adherence was investigated using eight statements in four pairs across three sections of the questionnaire and an arbitrary cut-off of extreme agreement (strongly agree or ‘yes’ response) with three or more of the eight statements on intentional non-adherence was used to indicate likely intentional non-adherence. Statements included widely accepted reasons for intentional non-adherence in a UK setting and addressed the need for treatment breaks, addiction concerns, cost–benefit, perceived toxicity, medication necessity and side effects.
Questionnaire 1 was also used to identify participants already using MODs or using methods of medication organisation which would either be incompatible with EAM or unethical to remove from a participant.
Participants not meeting the inclusion criteria because of intentional non-adherence, use of MOD or other trial-incompatible medication organisation strategy were thanked for their interest by letter and informed we would be making no further contact with them (see Appendix 11).
Participants identified as potentially eligible after completing questionnaire 1 were contacted by telephone to arrange a preliminary baseline visit in their own home, and their usual pharmacy was identified.
Prevalence and magnitude of unintentional non-adherence
The purpose of the baseline visit was multifold: to meet with patients (often for the first time); to confirm that all non-medical inclusion criteria had been identified (e.g. that there were no obvious unreported medication-taking strategies in place); to remove existing prescribed medication so as to ensure that the patient did not accidentally take medicines from a non-study pack; and to deliver a new 28-day medication supply to be taken over the following 4-week period, which would allow the study team to ascertain whether or not patients may be unintentionally non-adherent.
Prior to the baseline visit, participants were asked to collect all medicines together. At the baseline visit, researchers stressed that they would not reveal information to their GPs about how well participants took their medicine, as researchers were interested only in measuring the participants’ usual medication-taking habits. Researchers recorded name, form and quantity of all existing medicines and then removed them from use by placing them in one or more large clear bags secured with a cable tie. Details were recorded on administration form 1 (see Appendix 12) and participants were asked to endorse the record. Medications no longer required or expired were returned to the pharmacy for destruction with participant consent.
Each secure bag was labelled clearly with the participant’s name and instructions not to use the contents unless absolutely necessary. The sealed medicines were returned to the participant for safe keeping and participants were asked to inform researchers if they needed to open the bag during the study. A follow-up appointment was arranged for 3 weeks (± 2 days) after the initial visit.
The assessment of unintentional non-adherence was made by DUC. If a participant had taken fewer than expected SODFs, they were identified as unintentionally non-adherent. Researchers also confirmed the participant’s willingness to continue in the study and carried out randomisation. Participants were informed of their randomisation status. If participants were randomised to receive a MOD, they were shown the three study MODs (Nomad, Nomad XL and Venalink) and asked to select their preferred MOD. Their choice and confirmation that they were able to use it were recorded using administration form 2 (see Appendix 13).
Irrespective of adherence status or willingness to continue in the trial, participants were asked to continue to take the 1-week (approximately) medication supply remaining in the packs delivered at the baseline visit and then to continue to obtain their prescribed medications according to their usual supply routine. Pharmacists were informed of randomisation status for all participants.
Adherence monitoring
In the absence of a suitable EAM system, DUC was used to determine adherence to prescribed SODFs. Patients were provided with a number of large, clearly labelled, sealable plastic wallets in which to store used medication packaging. Wallets were returned to pharmacies by patients or delivery drivers coinciding with usual collection from the pharmacy. Researchers contacted patients for whom medicines had not been returned to the pharmacy. The number of doses returned for each medicine was counted and recorded.
Characterisation of the participant population
Cognitive function was assessed using the 11-item MMSE. It is scored out of 30, with scores of 23 and above indicative of cognitive function within the normal range. 65
Manual dexterity was assessed using the 9-HPT, which was scored by measuring the period of time, in seconds, taken to move pegs, similar in dimension to medication capsules, from a tray into a 3 × 3 array of holes which accommodate the pegs in a vertical orientation, and then to remove them and place them back into the tray. 76
Visual acuity was assessed using the smallest font size that could be read on the Bailey–Lovie Near Chart. 71 Participants used their usual visual aids and held the chart at their normal reading distance (approximately 40 cm). The test took place under the lighting conditions that patients would usually be exposed to when taking their day-time medicines.
General practitioner records were accessed by a member of the research team in order to obtain participant comorbidities.
Participants also completed baseline health and quality-of-life measures as described in Chapter 7.
Randomisation
Randomisation was carried out at the participant’s home by researchers. Participants identified as unintentionally non-adherent were randomised to phase 2. Researchers used mobile internet to connect to the secure study database. The randomisation sequences were generated automatically using an online system developed and managed by Norwich Clinical Trials Unit. Allocation was automatically recorded in the study database. Randomisation was done on a permuted block basis. There were six strata, one per GP medical practice. There were four randomisation arms (usual supply weekly, usual supply monthly, MOD weekly, MOD weekly). The codes for each stratum were blocked randomly into groups of four or eight to try and even out the distribution across randomisation arms.
Blinding
Given the nature of the interventions under study (MOD and frequency of dispensing), blinding was not possible for patients, researchers or pharmacists involved in the study.
Trial logistics
Administration and timings
At commencement of the intervention, researchers visited the participant and explained procedural details emphasising how and when medicines would be supplied and asking that all medication packaging was retained for researchers. It was again stressed that researchers would not feed information back to GPs about how well participants were taking their medicine and that they were only interested in measuring the participants’ usual medication-taking habits. At this visit, researchers delivered the first medicines of the intervention and follow-up questionnaires: EQ-5D-3L combined with the Investigating Choice Experiment CAPability measure for Older people (ICECAP-O, questionnaire 2), a patient satisfaction survey (questionnaire 3, see Appendix 14). A further questionnaire and letter of invitation for any person living with or caring for the participant (questionnaire 4, see Appendix 15) was also left with the participant. Researchers agreed to telephone participants 1 week prior to completion of the intervention to explain the procedure for returning to usual care.
General practice and pharmacy participation
General practice participation at this stage of the trial was limited to routine prescription preparation and required only minimal interaction between researchers and prescription managers.
Pharmacists were informed directly after randomisation of the randomisation status of their participant. They were asked to follow the instructions provided in the SOP for pharmacies (see Appendix 16). Briefly, pharmacists prepared medicines according to randomisation. In the case of medicines that were to be dispensed in MODs, after the first delivery (carried out by researcher), these were delivered to the participant by the pharmacist via their usual delivery service for weekly supply or were collected by participants. Medicines that were dispensed in usual packaging were delivered weekly to patients randomised to usual care and delivered or collected in accordance with usual practice for patients randomised to monthly supply.
Pharmacy data collection
Pharmacists were asked to record participant details, medicines supplied, time taken to dispense medicines and any near-miss dispensing or prescribing errors identified on administration form 3 (see Appendix 17), with further details, if necessary, on pro-forma follow-on sheets. They were also asked to record any other comments about the trial.
Adverse events
This pilot trial was not a clinical trial of an investigational medicinal product, and thus no formal process for recording adverse events (AEs) was implemented. Any potential AEs identified were defined in accordance with European Clinical Trial Directive 2001/20/EC as new or the worsening of pre-existing symptoms.
Serious adverse events
Serious adverse events (SAEs) are defined by European legislation as the development of an undesirable medical condition or the deterioration of an existing medical condition following or during exposure to an investigational medicinal product, whether or not it is considered causally related to that product, which results in hospitalisation or prolongation of hospitalisation; immediately life-threatening illness; persistent or significant disability; and incapacity or death.
Descriptions of all AEs were recorded using UEA SOP 206 with associated SAE form (see Appendices 19 and 20). Reports were made to the UEA research sponsor and local research and governance representative within stipulated time limits. Governance committees assessed the reports. All AEs identified were also reported to management and steering committees. The participant’s usual GP was contacted for an opinion on probable causality, that is whether the GP considered it unlikely, possible or likely that events occurred as a result of trial participation.
Sample size
The sample size was determined as follows. A total of 120 participants were required for the RCT. With four groups of 30 participants, the coefficient of variation of the SE estimates for any continuous outcome measure for each group was expected to be about 13% using a linear approximation and assumption of normality. These SE estimates, would have been used to inform the power calculation of a definitive trial and would have been within about 25% of their true values based on the expected half-width of a 95% CI. It was estimated that, in order to obtain 120 participants who are primarily unintentionally non-adherent, 720 potential participants needed to be screened using a self-report questionnaire. This estimate was based on the assumption that approximately 20% of participants would be intentionally non-adherent17,18,77,78 and thus excluded. The remaining 576 participants would be monitored for non-adherence using DUC. This stage was expected to result in 30% attrition, and thus 403 participants would remain, of whom 160 (40%) were expected to be non-adherent. These participants would then be randomised and monitored for 3 months, during which process a further 25% attrition was expected, leaving 120 participants. (See Figure 8 for the flow diagram summarising participant pathway through trial.) Participants suspected of intentional non-adherence were advised to consult their pharmacist for a medicines use review or their GP.
The primary outcome measure was the mean difference in per cent adherence (measured by pill count) between participants in receipt of a MOD and participants who received their medication in usual-care packaging. This should provide an estimate of the effect of the factor packaging. As there are no reported trials with a similar population, that is pre-screened to remove participants likely to be adherent or demonstrate intentional non-adherence, data were not available to estimate the variance of adherence measured in this population. However, an upper estimate of the precision was determined by considering the dichotomisation of this outcome to the binary measure adherent versus non-adherent. Making the conservative assumption that 50% of participants would be adherent and with 60 participants receiving a MOD and 60 receiving usual-care packaging, the half-width of a 95% CI around the difference between these groups should be less than 18%.
No interim analyses or stopping guidelines were included in the study design.
Statistical methods
A dedicated study database was designed, built and maintained by Norwich Clinical Trials Unit, data were analysed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) and PASW statistics version 18 (SPSS Inc., Chicago, IL, USA). Descriptive analyses were used to report recruitment and consent rates, social and health (comorbidities) status, participant functional abilities in addition to prevalence of intentional and unintentional non-adherence. Unintentional non-adherence during a 3-week screening period was calculated as follows:
The magnitude of unintentional non-adherence was determined from median and interquartile divergence for all unintentionally non-adherent participants.
Unintentional non-adherence during the 8-week trial period was identified and magnitude calculated as described above for the 3-week screening. Percentage medication adherence was reported for participants randomised to MODs or usual care and weekly or monthly delivery for each medication. This approach took into account that there were varied individual daily dose regimes and that not all participants returned all packs. Analyses were undertaken for any medicines returned by participants for whom at least 1 week’s medication packaging was returned. Previous research indicated that the data may be negatively skewed with minimal deviation from 100% adherence. 15 Secondary analysis was, therefore, undertaken using a dichotomisation of 100% versus less than 100% adherence.
Analyses were carried out with respect to incidence of reported AEs. Fisher’s exact (two-tailed) test was used to determine the statistical significance of the events with respect to type of packaging and frequency of supply.
Although the study was not powered to detect differences of a particular size, and accepting the limitations of a feasibility study, the observed differences between the study groups were presented. Exploratory analyses were also undertaken to identify any indications that variables such as cognitive function, number of prescribed medicines and manual dexterity affect the primary outcome (adherence). This was to identify whether or not any subgroups of participants might benefit from MODs.
Results
The inability to obtain a suitable objective EAM solution also delayed a number of trial timelines. The initial intent was to carry out the 3-week assessment of unintentional non-adherence with immediate randomisation of eligible participants. As a result of difficulties identifying a suitable adherence monitoring device, there was a delay of 2–3 months between randomisation and the start of the RCT.
Recruitment
Figure 8 [Consolidated Standards of Reporting Trials (CONSORT) diagram] illustrates the flow of patients through the study. The most significant attrition during recruitment was at the stages of determining eligibility for EAM, followed by ineligibility as a result of medication-taking strategies that were inconsistent with MODs or electronic monitoring. A completed questionnaire 1 was received from 288 participants, of whom 11 (3.8%) were excluded because of current participation in a medication trial.
FIGURE 8.
Flow of participants.
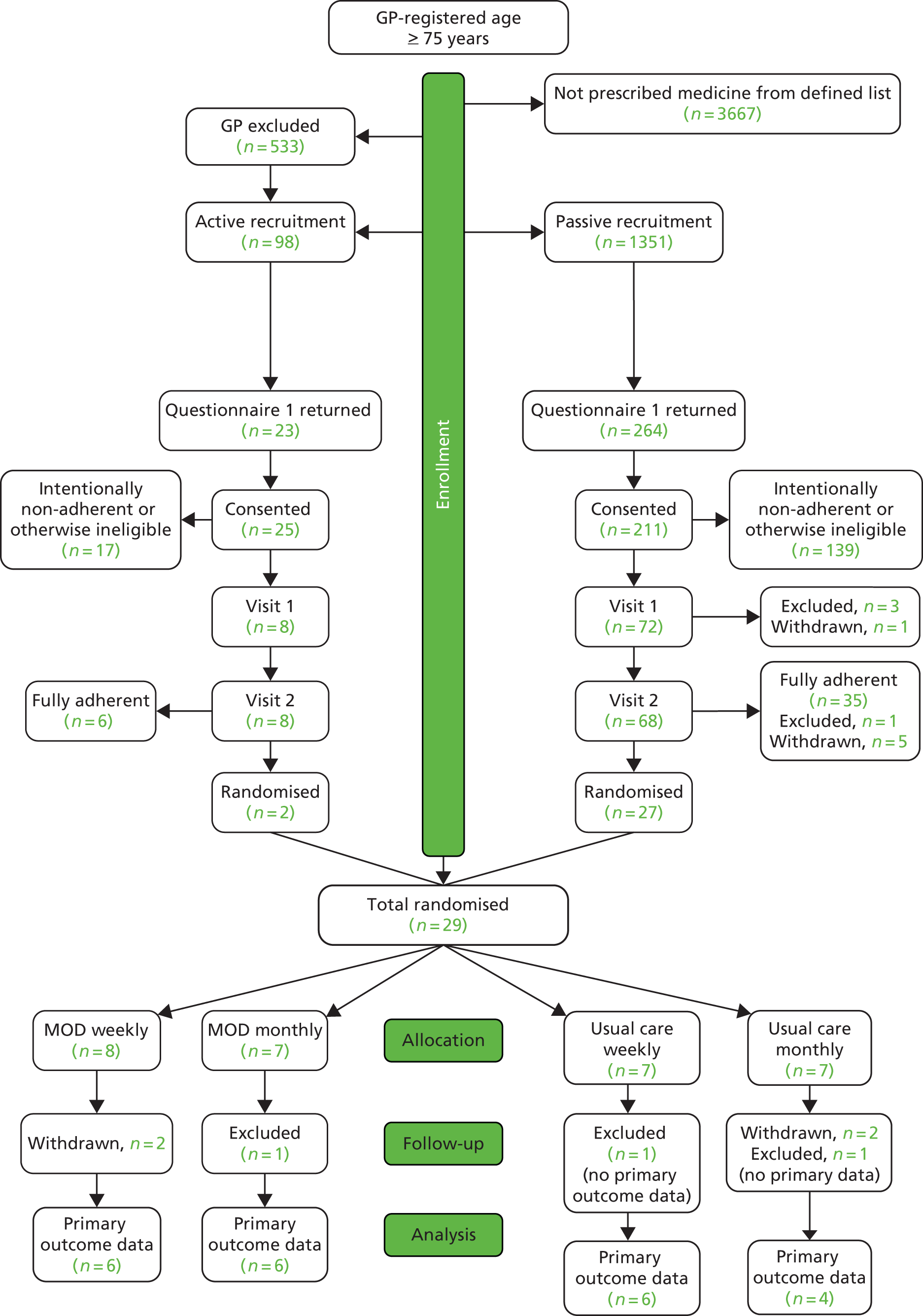
Participants using a medication organisation device or medication organisation strategy incompatible with trial participation
One hundred and two participants (35.4%) reported in questionnaire 1 using a MOD and were thus excluded. A further 27 (9.4%) participants reported use of strategies to aid correct medication taking which were incompatible with the study design. Six reported that they were not self-medicating, one reported using an alarm, three reported popping out their pills in advance into other containers, for example bottles or cups, which would have been incompatible with EAM. The remaining 17 participants reported using other equipment to organise their medicines. For example, some used a series of shot glasses and others ice cube trays.
Prevalence of non-adherence
Intentional non-adherence
Screening of questionnaire 1 responses identified 71 (24.7%) participants indicating extreme agreement with one of eight statements exploring intentional non-adherence. Eighteen (6.3%) indicated extreme agreement with two of eight statements and two (0.7%) participants indicated extreme agreement with three statements and were thus excluded from the study.
Unintentional non-adherence
All 80 eligible participants presented their medicines to the researchers during visit 1. Some medications were neatly presented with the correct blisters in their original packaging in an organised fashion and others appeared to have been randomly tossed into buckets, bags and boxes. One participant, who was subsequently withdrawn from the study, had very poor eyesight and used the colours and sizes of his medicine boxes to distinguish one from another. This participant had incorrect blisters inside boxes.
Many patients had medicines surplus to reasonable requirement in their possession, as summarised in Table 17. As an extreme example, one participant presented 17 unused tubes of Ovestin cream and seven unopened boxes of codeine phosphate. Many participants had excessive numbers of surplus prescribed painkillers. No patients reported any discomfort with or declined the removal from use of their old medicine stocks.
| Type of medication | No. of patients | Mean no. of excess days’ supply |
|---|---|---|
| Antisecretory drugs and mucosal protectants | 35 | 88 |
| Positive inotropic drugs | 2 | 51 |
| Diuretics | 40 | 115 |
| Antiarrhythmic drugs | 2 | 3 |
| Beta-adrenoceptor blocking drugs | 32 | 80 |
| Hypertension and heart failure | 61 | 229 |
| Antianginal therapy | 39 | 94 |
| Anticoagulants and protamine | 10 | 35 |
| Antiplatelets | 48 | 39 |
| Lipid regulating | 58 | 107 |
| Respiratory system | 2 | 58 |
| Central nervous system | 25 | 512 |
| Endocrine system | 50 | 208 |
| Obstetrics, gynaecology and urinary tract disorders | 15 | 182 |
| Malignant disease and immunosuppression | 5 | 90 |
| Drugs for nutrition and blood | 10 | 192 |
| Drugs for musculoskeletal and joint diseases | 9 | 76 |
| Drugs for ear, nose and oropharynx | 1 | 25 |
| Anaesthesia | 1 | 22 |
Dosage unit count data were available for 76 subjects, of whom 41 (53.9%) were ‘fully adherent’ and thus excluded from further study participation. The remaining 35 participants (46.1%, 95% CI 34.8% to 57.3%) were unintentionally non-adherent. Five of these participants withdrew prior to randomisation and one was excluded after visit 2 because of concerns about his mental health on discussion with his health-care team.
Of the 29 randomised participants, three subsequently withdrew prior to receiving the intervention: one participant citing problems with delivery (n = 1 MOD weekly) and two participants stating that they preferred not to take part (n = 1 MOD weekly; n = 1 usual supply monthly). A further patient was withdrawn after randomisation to the usual supply monthly group because his eyesight was extremely poor and there were concerns that he was unable to manage his medicines safely; his health-care team were informed.
Twenty-five participants commenced the intervention, but primary outcome data were not received from three of these participants and thus they were excluded from analysis: one participant was admitted to hospital during the trial (n = 1 MOD monthly), while the other two participants (n = 1, usual care weekly; n = 1, usual care monthly) did not return any medication blister strips to enable the primary outcome measure to be calculated.
Table 18 summarises the baseline characteristics of participants randomised to each of the four groups. It can be seen that the participants were relatively evenly matched across the four groups.
| Randomised population | MOD weekly | MOD monthly | Usual packaging weekly | Usual packaging monthly |
|---|---|---|---|---|
| No. of participants | 8 | 7 | 7 | 7 |
| No. (%) male | 3 (37.5) | 4 (57.1) | 2 (28.6) | 3 (42.9) |
| Median age (years) (IQR) | 77.6 (75.6–86.5) | 80.7 (76.4–83.7) | 80.1 (77.8–82.5) | 83.0 (77.0–85.1) |
| No. living alone (%) | 5 (62.5) | 3 (42.9) | 2(28.6) | 4 (57.1) |
| No. retired (%) | 8 (100) | 7 (100) | 6 (100)a | 7 (100) |
| Median no. of SODFs (IQR) | 5 (4.3–7.5) | 8.0 (5.0–11.0) | 6.0 (4.0–9.0) | 5 (4.0–8.0) |
| Median Charlson score (IQR)b | 5 (4–7) | 5 (5–6) | 5 (0–6) | 4 (0–6) |
Table 19 illustrates the cognitive function, manual dexterity and visual acuity of participants. No randomised participant had significant cognitive impairment. There was substantial variation in manual dexterity and 12 (41.4%) participants had manual dexterity that was poorer than the normative value for the population aged over 71 years. 79
| Function test | n | Mean | SD | Median | Semi-IQR | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| MMSE | 29 | 27.9 | 1.36 | 28.0 | 1.00 | 24.0 | 30.0 |
| Dexterity – lefta | 28 | 31.9 | 14.9 | 26.5 | 5.45 | 18.5 | 92.3 |
| Dexterity – right | 29 | 29.0 | 12.4 | 25.6 | 2.68 | 18.0 | 80.5 |
| Dexterity – dominantb | 26 | 29.1 | 13.1 | 25.5 | 4.16 | 18.0 | 80.5 |
| Visual acuity (font size) | 29 | 9.6 | 14.2 | 6.0 | 1.50 | 4.0 | 80.0 |
Intervention effect on adherence
An estimate of medication adherence could be made for all participants randomised to the MOD arms as at least one MOD was returned from each of these participants. However, some participants in the usual-care group returned packs for some medicines and not for others. Additionally, some patients returned empty packs without the blister strips. One participant reported that he had returned his empty usual-care packs to the pharmacy but in some cases researchers were unable to retrieve packs from pharmacies. Discussion with the pharmacy team suggested that returned usual packaging may, in some cases, have been discarded. Only boxes returned with blister strips were included in the analyses. Twenty-two subjects had primary outcome data (i.e. adherence measure) available for analysis.
Table 20 presents the adherence data from DUC during an 8-week trial period. The distribution of adherence was found to be very negatively skewed (skewness = –2.48), with only 8 of the 22 not being 100% adherent. A non-parametric approach was, therefore, taken to compare treatment groups. Further, a secondary analysis was carried out using a dichotomisation of 100% versus less than 100% adherence. A comparison of the treatment groups is presented in Table 20.
| Item | MOD group | Usual-care group | p-value | Monthly group | Weekly group | p-value |
|---|---|---|---|---|---|---|
| n | 12 | 10 | – | 10 | 12 | – |
| Mean (SD) | 97.3% (6.9%) | 95.1% (9.3%) | – | 96.9% (7.0%) | 95.5% (9.4%) | – |
| Median (semi-IQR) | 100% (0.8%) | 100% (3.1%) | p = 0.619a | 100% (1.7%) | 100% (1.0%) | p = 0.789a |
| Fully adherent | 8 (66.7%) | 6 (60.0%) | – | 6 (60.0%) | 8 (66.7%) | – |
| Not fully adherent | 4 (33.3%) | 4 (40.0%) | p = 1.00b | 4 (40.0%) | 4 (33.3%) | p = 1.00b |
It can be seen that the MOD group and usual-care group had very similar levels of adherence, being very high in each group. There was no statistically significant difference with respect to the median level of adherence (p = 0.619, Mann–Whitney U-test) or with respect to the proportion being fully adherent (p = 1.00, Fisher’s exact test). Similarly, there was no indication of any difference in adherence between the monthly and weekly supply groups, either with respect to the median level of adherence (p = 0.789, Mann–Whitney U-test) or the proportion being fully adherent (p = 1.00, Fisher’s exact test).
Relationship of adherence to cognitive and physical function
The relationships between adherence and MMSE scores, dexterity scores and visual acuity were explored for possible factors predicting adherence among the 22 subjects in whom adherence was measured in the trial. Spearman’s rank correlation (rs) was used to quantify the relationship between adherence and these variables, as shown in Table 21.
| Item | MMSE | Dexterity – left | Dexterity – right | Dexterity – dominant | Visual acuity |
|---|---|---|---|---|---|
| n | 22 | 22 | 22 | 21 | 22 |
| r s a | 0.047 | –0.516 | –0.318 | –0.254 | 0.131 |
| 95% CI | –0.39 to 0.47 | –0.77 to –0.11 | –0.66 to 0.13 | –0.63 to 0.21 | –0.32 to 0.53 |
| p-valueb | 0.837 | 0.014 | 0.150 | 0.267 | 0.560 |
One correlation coefficient was statistically significantly different from zero. This was for the relationship between adherence and dexterity of the left hand (rs = –0.516; p = 0.014). This value was negative, indicating that the longer the time to complete the dexterity test with the left hand, the lower the adherence score was likely to be. No other coefficient was statistically significant.
Adverse events
A total of five AEs (n = 3) or SAEs (n = 2) were identified in the 25 participants receiving the intervention. Events comprised a hypoglycaemic episode, three falls leading to hip fracture, with death in one patient, and one temporary physical incapacitation. All five events were identified in groups randomised to receive MODs. Using Fisher’s exact test (two-tailed), the association between groups (MODs/usual care) and outcomes (harm/no harm) was statistically significant (p = 0.0391). Two events occurred in participants receiving care on a monthly basis and three in those receiving on a weekly basis. Using Fisher’s exact test (two-tailed), the association between groups (weekly care/monthly care) and outcomes (harm/no harm) was not statistically significant (p = 1.000). The SAEs and AEs are detailed in Tables 22–26. No events were identified in participants randomised to usual care.
| Randomisation group | MOD supplied weekly | ||
|---|---|---|---|
| Participant details | Sex: male | Age: 90 years | Living status: independent, alone |
| Study ID: 0040 | Pre-RCT adherence: 79% | RCT adherence: 76% | |
| Prescribed medication | |||
| Sertraline hydrochloride 50 mg once daily, ferrous fumarate 200 mg twice daily, enalapril maleate 10 mg once daily, amlodipine 10 mg once daily and simvastatin 40 mg once daily | |||
| Comorbidities | |||
| Depressed mood (2008), primary repair of inguinal hernia (2007), COPD (2005), skin lesion cystic removed (2000), type 2 diabetes mellitus (1996), labyrinthine vertigo (1992), essential hypertension (1990), raised blood pressure (1989), osteoarthritis (1980), fracture of ankle (1980), and antral and bilateral nasal washout (1967) | |||
| Mechanism of identification | |||
| CFA (Trial Manager) informed by telephone call from participant’s pharmacist. Pharmacist informed by a family member that the patient had been admitted to hospital | |||
| Event | |||
| Fall leading to broken hip and hospital admission | |||
| Outcome | |||
| Death on 20 June 2013 without being discharged from hospital | |||
| Study team assessment | |||
| This participant was 3 weeks into the trial (start date 30 April) when the AE occurred. It is a possibility that study participation improved medication adherence in this patient, leading to hypotension, which in turn may have led to a fall | |||
| NNUH/UEA Joint Research Governance Committee | |||
| Event possibly linked to study intervention | |||
| GP assessment | |||
| . . . From my knowledge of [the patient] prior to his death, I think it is extremely unlikely that the fall/serious adverse event was linked to participation in the MOD study. [The patient] had significant comorbidity including chronic kidney disease stage 4 and COPD and type II diabetes which was making him increasingly frail . . . The discharge summary I have from the hospital suggested his fall was “accidental”. So I feel it is unlikely that it was related to the MOD study | |||
| Randomisation group | MOD supplied monthly | ||
|---|---|---|---|
| Participant details | Sex: female | Age: 84 years | Living status: independent, alone |
| Study ID: 0080 | Pre-RCT adherence: 86% | RCT adherence: no data | |
| Prescribed medication | |||
| Ranitidine 300 mg one twice a day, aspirin 75 mg once daily, bisoprolol 2.5 mg once daily, losartan 25 mg once daily, alendronic acid 70 mg one weekly, furosemide 20 mg one in the morning, warfarin sodium 5 mg once daily, warfarin sodium 1 mg four times daily, primidone 250 mg once daily, solifenacin 5 mg once daily, ezetimibe 10 mg once daily and co-codamol 8/500 mg, one or two tablets four times daily. | |||
| Comorbidities | |||
| Epilepsy (1939), appendectomy (1944), laparotomy (1955), excision of Dupuytren’s contracture of the palm (1979), nasal polyp (1979), cholecystectomy (1983), presbyacusis (1985), angina (1986), fracture of patella (1988), myringotomy (1991), closed Colles’ fracture (1992), fracture NOS (1996), myringotomy (2000), essential hypertension (2003), accidental fall (2006), phacoemulsification of the lens (2007), chronic kidney disease stage 3 (2007), fit hearing aid (2007), ischaemic heart disease (2007), percutaneous balloon angioplasty of coronary artery (2008), acute MI (2009), coronary artery operation (2009), pulmonary embolism (2012), anticoagulant therapy (2013) and fracture of the femur neck (2013) | |||
| Mechanism of identification | |||
| TB (Researcher) informed by telephone call from ward nurse that the patient had been admitted to hospital | |||
| Event | |||
| Fall leading to hip fracture and hospital admission | |||
| Outcome | |||
| Hospital discharge 16 July 2013. Recovery progressing well but experiencing some mobility problems | |||
| Study team assessment | |||
| This patient was 3 days from completion of the trial (start date 25 April). It is a possibility that study participation led to improved medication adherence and thus hypotension, which, in turn, may have led to a fall | |||
| NNUH/UEA Joint Research Governance Committee | |||
| Event possibly linked to study intervention | |||
| GP assessment | |||
| Thank you for your letter . . . regarding this patient’s hip fracture. This occurred when she tripped in the garden and lost her footing. She does have vascular risk factors, namely atrial fibrillation and peripheral vascular disease but in my opinion, the fall was most likely to be related to the trip and not related to her medication | |||
| Randomisation group: | Medication organisation device supplied monthly | ||
|---|---|---|---|
| Participant details | Sex: Female | Age: 81 years | Living status: independent; lives with spouse |
| Study ID: 0230 | Pre RCT adherence: 96% | RCT adherence: 93% | |
| Prescribed medication | |||
| Aspirin 75 mg once daily, lansoprazole 15 mg once daily, spironolactone 25 mg once daily, furosemide 40 mg once daily, isosorbide mononitrate 60 mg, one and a half tablets once daily, clopidogrel 75 mg once daily, ramipril 10 mg once daily, metformin 500 mg once daily, pravastatin 20 mg once daily and bisoprolol 5 mg once daily | |||
| Comorbidities | |||
| Acute coronary syndrome (2012), triple-vessel disease of the heart (2012), eye operation (2012), acute non-ST-segment elevation MI (2012), concentric left ventricular hypertrophy (2012), mild–moderate MR (2012), type 2 diabetes mellitus (2011), blood hyperglycaemia (2010), shoulder joint replacement (2010), arthroscopy NEC L knee (2006), osteoarthritis hips, lumbar (2005), acute MI NOS (2001), asthma (1999), osteoarthritis (1992), Ménière’s disease (1990), hiatus hernia (1990), labyrinthitis (1987), otosclerosis (198?) and total abdominal cyst (1978) | |||
| Mechanism of identification | |||
| Patient reported via free-text section in patient satisfaction questionnaire | |||
| Event | |||
| Fall leading to bruising | |||
| Outcome | |||
| Fully recovered | |||
| Study team assessment | |||
| It is a possibility that study participation improved the medication adherence in this patient, leading to hypotension, which in turn may have led to a fall | |||
| NNUH/UEA Joint Research Governance Committee | |||
| Event possibly linked to study intervention | |||
| General Practitioner assessment | |||
| Not returned | |||
| Randomisation group: | Medication organisation device supplied weekly | ||
|---|---|---|---|
| Participant details Study ID: 0133 |
Sex: Male | Age: 96 years | Living status: independent |
| Pre-RCT adherence: 86% | RCT adherence: 96% | ||
| Prescribed medication | |||
| Lisinopril 20 mg once daily, lisinopril 10 mg once daily, metformin 500 mg, two tablets in the morning and two in the evening, gliclazide 80 mg, one tablet in the morning and two in the evening, amlodipine 5 mg once daily and furosemide 40 mg once daily | |||
| Comorbidities | |||
| High ligation of the long saphenous vein (1993), shoulder injury (2000), hypertensive disease (2001), circumcision (2002), persistent microscopic haematuria (2004), open-angle glaucoma (2007), type 2 diabetes mellitus (2008), excision of basal cell carcinoma (2008), increased frequency of urination (2010), glaucoma suspect (2011), ocular hypertension (2011) and primary open-angle glaucoma (2012) | |||
| Mechanism of identification | |||
| Patient reported directly to researcher at routine visit | |||
| Event | |||
| Hypoglycaemic episode | |||
| Outcome | |||
| Fully recovered | |||
| Study team assessment | |||
| It was known that this patient was non-adherent to his diabetes oral hypoglycaemic medicines prior to randomisation. It was also known that he suffered poor blood glucose control and that he had recently been prescribed an increased dose of oral hypoglycaemic medicine. It is a possibility that study participation improved the medication adherence in this patient, leading to the reported hypoglycaemic episode | |||
| NNUH/UEA Joint Research Governance Committee | |||
| Event possibly linked to study intervention | |||
| GP assessment | |||
| . . . I think it is possible that the increase in use of his prescribed medication was related to symptoms of hypoglycaemia but in general it seems that the effect of the study for [the participant] has been positive . . . | |||
| Randomisation group: | Medication organisation device supplied weekly | ||
|---|---|---|---|
| Participant details Study ID: 0138 |
Sex: female | Age: 76 years | Living status: independent, alone |
| Pre-RCT adherence: 85% | RCT adherence: 98% | ||
| Prescribed medication | |||
| Aspirin dispersible 75 mg once daily, amlodipine 5 mg once daily, simvastatin 20 mg one at night, telmisartan 40 mg once daily, tolterodine 1 mg, one tablet twice a day, levothyroxine 25 mcg, one tablet in the morning, levothyroxine 100 mcg, two tablets in the morning, bendroflumethiazide 2.5 mg once daily and metformin 750 mg, one tablet twice a day | |||
| Comorbidities | |||
| Tubal occlusion (1973), frozen shoulder (1993), breast lump (2001), hypothyroidism (2002), tinnitus (2002), type 2 diabetes mellitus (2002) and osteoarthritis of the knee (2007) | |||
| Mechanism of identification | |||
| Social services data for patient provided post study | |||
| Event | |||
| Patient felt unwell and took a bath. Once in the bath she was unable to get out of the bath and remained there for 12 hours until rescued | |||
| Outcome | |||
| Recovered and under close medical supervision. Although prior to the trial this patient was unknown to social services, she is now receiving regular social care | |||
| Study team assessment | |||
| It is a possibility that study participation improved the medication adherence in this patient, leading to the AE | |||
| NNUH/UEA Joint Research Governance Committee | |||
| No details available | |||
| GP assessment | |||
| Not returned | |||
Pharmacists were asked to record any near misses and the type of near miss for the duration of the RCT. Pharmacy data collection forms were returned for all participants; the two near misses that were reported are presented in Table 27.
| Event date | Care type | Supply frequency | Medicine | Dose | Description of near miss |
|---|---|---|---|---|---|
| 16 November 2012 | Usual | Weekly | Levothyroxine | 25 mcg | Wrong dose requested by surgery |
| 1 May 2013 | MOD | Weekly | Perindopril | 4 mg | Wrong strength stated on label |
Summary
This study aimed to test the methods informed by the preceding chapters and identify refinements for a definitive RCT to investigate clinical effectiveness and cost-effectiveness of MODs. The single-centre, multisite factorial 2 × 2 pragmatic RCT examined the effects of MOD versus usual packaging and weekly versus monthly medication supply. The feasibility study design also tested a passive method of recruitment using mail compared with an active method of a researcher recruiting patients attending their general practice.
Eligible participants were aged over 74 years, prescribed and self-administering three or more solid oral dose medications from a defined list and unintentionally non-adherent. Unintentional non-adherence was determined from a composite of patient self-report and 3-week DUC data. Patients who used techniques to organise their medicines which were incompatible with use of a MOD were excluded.
The intervention and comparator were as follows:
-
four MODs dispensed monthly collected by patient/delivered to patient home
-
one MOD delivered to patient’s home weekly
-
usual medication dispensed monthly collected by patient/delivered to patient’s home
-
usual medication delivered to patient’s home weekly.
The primary outcome measure was percentage adherence, which was intended to be measured using electronic monitoring. A working system suitable for EAM in the usual-care groups could not be sourced within the time frame of the study. Adherence was, therefore, measured by DUC at 8 weeks and a secondary outcome was mortality. Further secondary outcomes were self-reported quality of life (EQ-5D and ICECAP-O), which is reported in Chapter 6, and self-reported autonomy (patient enablement scale) and satisfaction (adapted CSQ-8), which are reported in Chapter 5.
Of the 80 potentially eligible participants identified, 76 completed the pre-baseline 3-week DUC. From the self-reported adherence questionnaire and DUC data, 35 (46.1%) participants were unintentionally non-adherent. Five participants withdrew before randomisation and two were excluded after randomisation (because of mental health and eyesight problems), leaving 28, of whom three withdrew prior to receiving the intervention. Of the remaining 25 participants, no primary outcome data were available for three.
Adherence data suggested almost perfect adherence in all groups, with no significant differences observed. Five AEs were observed among the 13 patients randomised to receive the MOD intervention, compared with none in the 12 participants allocated to usual care (Fisher’s exact test; p = 0.039). Two of the AEs were classified as serious, as both participants were hospitalised and one died.
Chapter 5 Review of the randomised controlled trial design
Introduction
The overarching aim of the study was to design and assess the feasibility of a definitive study to determine the effectiveness and cost-effectiveness of MODs. This chapter describes the assessment of the study methods and management in terms of acceptability both to patients and to health-care professionals.
Aims and objectives
The aim was to review the design of the RCT element (see Chapter 4) of this feasibility study. This chapter investigates the feasibility and acceptability of the study design to identify lessons learnt and aspects of the trial that may need to be changed before progressing to a definitive trial. The objectives were to describe:
-
participant satisfaction with medication packaging, trial management and participation
-
carers’ perceptions of the effect of elements of the RCT on the person they care for
-
participant and health-care professional acceptability of trial participation and design through group discussions.
Methods
Questionnaires and group discussions were used to enable both quantitative and qualitative review of the RCT element of the study.
Questionnaire data collection
Participants were provided with two questionnaires during the visit at which they were randomised: a participant experience questionnaire and a carer experience questionnaire. Participants were asked to complete and return the participant experience questionnaire immediately after completing the RCT. Participants who did not return the questionnaire within 2 weeks of completion and who were thought to have completed the trial were reminded once by telephone.
The carer experience questionnaire left with participants was accompanied by a letter of invitation and participants were encouraged to give the questionnaire and letter to the most appropriate carer (formal carer, spouse or other relative). The letter invited completion and return of the questionnaire when the person they cared for had completed the RCT. Carers were unknown to researchers and, therefore, were not reminded to complete the questionnaire.
Participant questionnaire content
An 11-item questionnaire was developed incorporating validated and non-validated items. Non-validated items were developed from the phase 1 focus group findings; the trial management committee was used to assess content validity and the independent steering committee comprising lay members was used to assess face validity. Three non-validated items related to satisfaction with the medication packaging (MOD or manufacturer’s packaging) and the extent to which it met the participant’s needs. Responses were sought on a 4-point Likert scale, with the most positive responses having the highest score.
Participants’ autonomy, confidence and anxiety associated with medicine taking were assessed using six items developed through appropriate adaptation of the patient enablement instrument and previous work undertaken by the research team. 80 Responses were sought on a 5-point Likert scale, with the most positive responses having the highest score. The questionnaire is appended (see Appendix 14).
Carer questionnaire content
The carer questionnaire had six items related to autonomy, confidence and anxiety, as previously described for the participant questionnaire. These questions were adapted to be relevant for a carer or relative to complete, for example:
My confidence in their ability to take their medicines correctly is . . .
My level of anxiety about them taking their medicines wrongly is . . .
It also included a health economics section to capture the amount and type of care provided to the person involved in the study. A copy of this questionnaire is provided in Appendix 15.
Post-trial discussions
Two group discussions were convened in order to capture the experiences of patients and health-care professionals involved in the trial. The discussions were structured to gain opinions on each stage of the trial process to identify what worked well and less well with a view to optimising definitive study design. Focus group 5 (FG5) comprised RCT participants and focus group 6 (FG6) comprised a range of health-care professionals involved in the delivery of the RCT.
Participant identification and recruitment
The feasibility study information leaflet and consent form invited participants to express an interest in attending a group discussion convened once the trial was complete. These participants were purposively sampled to provide even representation of all four arms of the trial (MOD weekly, MOD monthly, usual care weekly and usual care monthly) and invited by telephone to attend the group discussion. All health-care professionals involved in the study (pharmacists, practice managers and GPs) were invited to take part via telephone and those confirming interest were mailed recruitment packs (invitation letter, PIL and consent form). Consenting health-care professionals were then purposively sampled to ensure even representation of all groups.
Data collection
Discussion guides were based on activities for each stage of the RCT element of this feasibility study, designed to capture both positive and negative experiences and tailored to the perspectives of each group (Appendices 21 and 22). A copy of study information sheets, consent forms and questionnaires was provided to each participant and example MODs were provided for each group. The group discussions took place at the UEA and were designed to last no longer than 90 minutes.
The discussions were moderated by the principal investigator and a researcher independent of the study. Proceedings were audio-recorded and transcribed verbatim. NVivo version 9 software was used to organise the themes of the discussions. Two researchers (SB and CA) read and coded the transcripts independently. An expert in qualitative research (CS) reviewed the final framework comprising themes and subthemes. A selection of anonymised quotes from the transcripts was extracted to illustrate identified themes.
Results
Participant satisfaction with medication packaging, trial management and participation
Twenty-three participants completed the study, of whom 21 (91%) returned patient satisfaction questionnaires. Table 28 provides a comparison of the patient ratings of the different medication packaging and supply formats trialled. It can be seen that patient rating of their medication packaging was generally high, with MOD weekly achieving a median score of 3 on a 4-point scale, where 4 indicates maximal satisfaction. Although MOD weekly was consistently scored highest or equal highest for all criteria, no significant differences were observed in this small sample.
| Criterion | MOD weekly (n = 8) | MOD monthly (n = 7) | Usual packaging weekly (n = 7) | Usual packaging monthly (n = 7) | p-value Kruskal–Wallis |
|---|---|---|---|---|---|
| Number (% respondents) | 5 (62.5) | 6 (85.7) | 7 (100) | 5 (71.4) | – |
| Median (IQR) score for satisfaction criterion | |||||
| Rating of medication packaging | 3 (1–3) | 2.5 (2–3) | 2 (2–3) | 2 (2–2.5) | 0.79 |
| Extent to which packaging meets needs | 3 (2–3) | 3 (2–3) | 2 (2–3) | 3 (2.5–3) | 0.35 |
| Satisfaction with medication packaging | 3 (2–3) | 2.5 (2–3) | 2 (2–3) | 3 (2.5–3) | 0.72 |
Table 29 illustrates the distribution in participant responses regarding perceived change in confidence to self-manage their medication and health plus the extent to which the group to which they were allocated affected any stress associated with medication taking. The median score of 2 on a 5-point scale indicates a response of ‘the same’ for the majority of criteria. This in turn suggests that patient confidence and stress remained largely unchanged before and after randomisation.
| Criterion | MOD weekly (n = 8) | MOD monthly (n = 7) | Usual packaging weekly (n = 7) | Usual packaging monthly (n = 7) | p-value Kruskal–Wallis |
|---|---|---|---|---|---|
| Number (% respondents) | 5 (62.5) | 6 (85.7) | 7 (100) | 5 (71.4) | – |
| Median (IQR) score for confidence, autonomy and anxiety | |||||
| Confidence to correctly take medication | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–2) | 0.219 |
| Confidence in ability to self-manage medication | 2 (2–2) | 3 (1.75–4) | 2 (2–3) | 2 (2–2) | 0.054 |
| Difficulties experienced in taking medication | 2 (2–2) | 3 (2.75–4) | 2 (2–3) | 3 (2–3) | 0.528 |
| Time spent worrying about medication | 2 (2–2) | 3 (3–4) | 2 (2–3) | 3 (3–3) | 0.274 |
| Anxiety about taking medication incorrectly | 2 (2–2) | 2.5 (0–3) | 2 (1–2) | 1 (0–0) | 0.598 |
| Confidence in ability to self-manage health | 2 (2–2) | 2 (1.75–3) | 2 (2–3) | 3 (2–3) | 0.608 |
Table 30 summarises the carers’ responses to the post-study questionnaire assessing their perception of any changes in the participant’s ability to manage their medication or autonomy. From the 23 participants that completed the study, 13 (57%) carer questionnaires were returned. Results indicate that type of device had no significant effect on any of the autonomy items, as rated by the carers, although there was an observed trend to lower scores in the monthly usual packaging group.
| Criterion | MOD weekly | MOD monthly | Usual packaging weekly | Usual packaging monthly | p-value Kruskal–Wallis |
|---|---|---|---|---|---|
| Median (IQR) autonomy | |||||
| Number (% respondents) | 3 (38) | 4 (57) | 4 (57) | 2 (29) | – |
| Confidence to correctly take medication | 3 (2–3) | 3 (0.5–4) | 2 (2–2) | 2.5 (1–2.5) | 0.95 |
| Confidence in ability to self-manage medication | 2.5 (2–2.5) | 3 (2–3) | 2 (2–2) | 2.5 (1–2.5) | 0.44 |
| Difficulties experienced in taking medication | 2.5 (2–2.5) | 2 (1–2) | 2 (0–2) | 0 (0–0) | 0.857 |
| Time spent worrying about medication | 2.5 (2–2.5) | 1.5 (0–1.5) | 2 (0–2) | 0.5 (0–0.5) | 0.208 |
| Anxiety about taking medication incorrectly | 2.5 (2–2.5) | 3.5 (3–3.5) | 2 (0–2) | 2 (0–2) | 0.360 |
| Confidence in ability to self-manage health | 2.5 (2–2.5) | 3 (2–3) | 2 (2–2) | 2 (0–2) | 0.731 |
Of the 29 participants randomised, 17 expressed an interest in participating in a group discussion; purposive sampling led to eight attending the group discussion.
Participant characteristics
Eight participants of the pilot RCT participated in the first focus group and seven health-care professionals in the second. Table 31 provides a summary of participant characteristics.
| Details for participants | Number of participants | Coding assigned to participants |
|---|---|---|
| Focus group 5 | ||
| Total participants | 8 | P1 FG5 to P8 FG5 |
| MOD monthly | 3 | P1 FG5, P4 FG5, P6 FG5 |
| MOD weekly | 2 | P2 FG5, P3 FG5 |
| Usual care monthly | 2 | P5 FG5, P8 FG5 |
| Usual care weekly | 1 | P7 FG5 |
| Focus group 6 | ||
| Total health-care professionals | 7 | HCP1 FG6 to HCP7 FG6 |
| Pharmacist | 3 | HCP2 FG6, HCP3 FG6, HCP6 FG6 |
| Dispenser | 1 | HCP5 FG6 |
| GP | 1 | HCP7 FG6 |
| Practice manager | 1 | HCP1 FG6 |
| Pharmacy store manager | 1 | HCP4 FG6 |
Discussion across both focus groups fell into four broad areas: the general experience of taking part; medication-related issues; comments about specific tools used within the study; and comments regarding a future trial. These are summarised with illustrative quotes, with identifiers (P for patient and HCP for health-care professional), reported in boxes at the end of each section.
General experience of taking part
There were five subthemes under the overarching theme of general experiences: reasons for taking part, communication about the study, patient behavioural changes, impact of study on medical practices and pharmacies, and feedback to pharmacists.
Varied reasons were provided for taking part by patients and health-care professionals. Patients wanted to take part to help others (P7 FG5), to help themselves (P8 FG5), to give payback to the NHS (P2 FG5, P4 FG5), to avoid stockpiling (P2 FG5), P3 FG5) and because it is a good thing to do (P7 FG5). Pharmacy-based health-care professionals were interested from a health-economic/health-benefit perspective (HCP4 FG6, HCP2 FG6, HCP3 FG6, HCP6 FG6) and wanted to know whether or not the resource they put into preparing MODs was worthwhile with respect to patient benefit. Practice-based health-care professionals had chosen to support this study within their Primary Care Research Network (PCRN) research remit and because they were keen to know if MODs (trays) do benefit their patients (HCP1 FG6, HCP7 FG6). Box 7 provides statements of reasons for taking part for patients and Box 8 provides them for health-care professionals.
Well me, when it was put to me by [research associate] I thought there must be a reason for it and if it’s going to help us as we get older, you know, I mean when you get to some people at 86 and 87 who can’t even remember what the day is let alone their pills, if it’s individually and they can read, it would help them. But I’m OK at the moment but I don’t know what I would be like in 4 years’ time. That’s me anyway.
P7 FG5
I feel like this lady too. As you get older you become more confused and I thought it would be easier to have the pill box rather than having to count out your things yourself.
P8 FG5
Well I thought it might do somebody some good, you know, because I just have them once a month, a little pill box, so I thought well, I just hope it does somebody else some good. That’s it.
P5 FG5
I think I’d go along with that because at my age you get a lot of free help from the National Health Service and if I can give back even a small amount then so be it, that’s very good.
P2 FG5
So I found once I could get a month’s supply done for me, I thought this is great and I’m very pleased.
P4 FG5
I thought well if you get them delivered on a basis where each one is prescribed on a daily basis you can’t get a surplus, so I’ll give it a whirl and that really is one of the reasons that I joined in because I had acquired a surplus of metformin, lisinopril and one other one, I forget which one it was. I thought well perhaps, you know, at the moment – or previously I had to sort of when I was reordering at the chemist, I would have to say ‘well I won’t have that one this month and on, I won’t have that one’ . . . Well I thought that by having the tablets supplied on a weekly, daily system, I wouldn’t acquire a surplus.
P2 FG5
Yeah, you wouldn’t stockpile. I found I stockpiled.
P3 FG5
I think deep down we’re all proud that we did it, you know. It’s going to help other people in years to come isn’t it? It’s nice
P7 FG5
And also nice having people round.
P4 FG5
Obviously, you know, within the store we’ve got a lot of experience of different levels of DDS, so from the people that are completely incapable to those that are sort of using it just literally as an aid for administration and just to see from a different perspective when people are put onto it, perhaps not necessarily at the recommendation of a GP but through another means, whether it was still sort of a sustainable thing for them. So although they’d been identified through a specific set, it was sort of interesting to see, having it come from a different angle, whether or not it would still be beneficial to them.
HCP4 FG6
. . . it does create a lot of workload for us and it just seemed really interesting to actually find some research and do some research into, you know, do people really benefit or are we doing it because it feels like it’s the right thing? But can we have some cold hard stats that say yes, this does help people and so it will be interesting to find out what happened from there.
HCP2 FG6
. . . Obviously it’s such a labour-intensive process putting them into the DDS packs so it will be quite interesting to look into the difference it actually makes to people in terms of their compliance because you think oh, I’m putting all this in here, if they didn’t have it would they actually be just taking it pretty much the same anyway, therefore it’s not really worthwhile. Or, is it actually make a real difference to people? I know some people I talk to it does make a real difference but to have a more structured study into it interests me.
HCP3 FG6
It’s a lot of work. As you say, we don’t know the benefit. I mean a lot of it seems to be sort of initiated by relatives, just because they don’t want to do it themselves, they get the pharmacist to do it and the medicine support people say that usually, if a carer’s going in it’s just ordinary boxes is fine. So I just wanted to contribute to see if we can get some consensus.
HCP6 FG6
From our point of view as a general practice surgery we’re extremely active in the PCRN network and looking at the study with our patient demographics and the problems we’ve got with compliance with a number of our patients, it seemed that there was benefit in getting involved in this piece of feasibility work . . . I think really we were supportive of the feasibility itself. Obviously there’s a number of research topics you need to take up during the level 2, level 3 initiative and we’re in a fortunate position I suppose where we can look at these and pick and choose to a certain extent and with the conversations we had with [research associate], we agreed to support her and see how it went.
HCP1 FG6
Yeah, well in a sense of course I’m very much in [name] position in that I’m the principal person in the practice who does decide whether we do take part in a study. I mean there are a number of reasons why we do. A, we’re a level 3 practice so we are committed to taking on recruiting and being involved in a number of research projects a year. But obviously the other aspect is sort of deciding what ones actually suit us as a practice. And yeah, we are regularly putting patients onto weekly trays and to look at a project where it’s actually wondering, you know, is this expensive process actually beneficial at the end of the day, is quite interesting.
HCP7 FG6
Communication about the study
This was a complex study and frequent and effective communication was required. Most communication was required between researchers, pharmacists and patients. Representative statements from focus group participants are presented in Box 9. The GP participant did not experience problems with communication (HCP7 FG6). Problems caused by the inability to source a suitable EAM caused delays to project timelines, and this was reflected in negative comments (HCP5 FG6). All patients were signed up to the automatic prescription order and collect system for preparation of medicines during the trial so that they would seamlessly revert to usual care at the end of the 2-month intervention. The process was not clearly defined in the study SOP and was not clear to all pharmacists as reflected in example comments (HCP4 FG6). However, some found the process very smooth (HCP6 FG6).
I mean I stood back at this stage. As far as I understand the communication was absolutely fine. There was no problem. Scripting essentially were told: they did what they were told!
HCP7 FG6
We kept given a date and then it would get put back and put back and then they came with these scripts and said ‘these need doing for Monday’ . . . I was told they all had to be done for a certain date and that’s why I came in and did late nights and everything to make sure they were done and then it turned out that they didn’t really need to be all done for that day . . . I got given a bunch of things, as I said ‘we need these done realistically for such a date’. It gave me about 3, 4, maybe 5 days to get them all done but nowhere in there did they say to me ‘but, some of these are weekly and they’re going to go out weekly; some of these are monthly, some of these are two monthly’, so I rushed, did them all and then they sat on my shelf for several weeks, some of them . . . My other work just got put on hold so that I could get this out and then they sat on the shelf for several weeks some of them and I was like ‘why?’
HCP5 FG6
And in terms of just communicating, just a final point on communication and specifically with patients, the end of the trial has been very poorly communicated . . . As to what happens now because obviously from our perspective the trial is being run by the UEA so we didn’t particularly expect to have to have any input and we have had quite a few people that have brought their last lot in and gone ‘what happens now?’
HCP4 FG6
I think there’s also a case for like the independence side of things as well for people who are, you know, we’ve touched on the delivery side of things but also for people that order their own medication as well, for a couple of people it was a bit kind of ‘so we’re not having to do anything now’ and it’s just that kind of – and whether or not that would be helped by perhaps a different approach to the initial conversations. There’s a possibility around that but it certainly did feel from – I don’t know if the others would echo – but a little bit more I think understanding almost from a pharmacy perspective, from a community pharmacy perspective, of how DDS works and that then being explained to people would have probably just – things like explaining around the ordering, around the collection and actually a more kind of ‘yes, you’re going to be part of this study’ but a real kind of ‘this is step by step exactly, from the pharmacy perspective, how it’s going to work for you’. And we weren’t there so we can’t say whether or not that was done or not but it certainly didn’t come across that necessarily it had been.
HCP4 FG6
I found it OK.
HCP6 FG6
Did you?
HCP5 FG6
Yeah. One lady wanted to stay with us but didn’t phone to say she’s gone back to Boots, but she really wants me to do it. The other ones were fine, they just – one chap was weekly. I got a message through from UEA saying ‘can you continue to do it but he’d like it monthly?’ so he must have been contacted and it was very smooth.
HCP6 FG6
Patient behavioural changes
There is a body of evidence suggesting that participants do change their behaviour when they know they are being observed. It was therefore a concern that participants may change their usual behaviour as a result of their being observed. Evidence presented in Box 10 suggests that behaviour may have changed as a result of trial participation, with participants applying greater care than usual to take their medication as prescribed.
Make sure I take that, make sure I take that, make sure I – whereas other times I know some I must take and others I’m a little bit thinking oh, I didn’t take so and so and I would take it any old time. But I was really good, knowing that Big Brother was after us sort of thing, you know. I don’t know about anybody else . . . It’s amazing what you do, you’re good when you know someone’s – [watching you].
P7 FG5
Impact of study on medical practices and pharmacies
Health-care professionals were asked about the impact of the study on surgeries or pharmacies to identify any knock-on issues. Representative statements are presented in Box 11. No impact on GP medical practices was identified (HCP7 FG6). Pharmacists identified that it had encouraged them to consider practical aspects of provision of MODs (HCP4 FG6, HCP5 FG6). HCP4 FG6 identified that a benefit of taking part would be patient loyalty to their pharmacy. Pharmacists reported taking a closer look at patients’ needs as a direct result of the trial (HCP5 FG6).
It’s just a matter of making sure we file everything in our research file . . . No, I think as far as I was concerned again that was all fairly clear. Actually, as I say, my main role was going to be on checking recruitment, checking searches and that side of recruitment.
HCP7 FG6
It’s actually very interesting to sit and just think for eight patients over x number of weeks, just those eight, what that actually amounted to in terms of both dispensing, pharmacist and [accredited checking technician] time. So for that side of it, yeah, really really useful. Quite scary! [laughing]
HCP4 FG6
It was amazing how much time it took up with all the paperwork. The initial paperwork even, putting them on our system as, because I wrote that they were all on the UEA trial so that nobody would take their scripts and do it in another part of the dispensary and stuff. There was just so much input put on.
HCP5 FG6
And primarily the benefit for pharmacies with DDS is that it is virtually 100% lock-in. 95% of the time those patients will stay with you . . . From that perspective it is. From time, from effort, from any other perspective it’s a chore.
HCP4 FG6
Because of this trial though, that’s made us look at our patients more closely and we’ve actually discovered now that patients that we’ve had on there for like 2, 3, maybe 4 years, initially they had Dossit boxes but now they’ve got carers and nobody’s ever told us that they’ve got carers so they’ve actually got carers who are just that lazy that they’re not dispensing them themselves, they’re just getting them out the Dossit boxes, but because of this trial it’s made us look at each individual patient a little bit more and we’ve actually discovered that there are quite a few that have now got carers who no longer need a Dossit box because the carer’s responsible for giving it.
HCP5 FG6
Pharmacists were required to provide study participants with their medicines according to randomisation group and to complete a proforma providing error data and resource data for the health economic analysis. These activities were time-consuming, but, once systems were established, both pharmacists and GPs found taking part in the study to be easy and acceptable, as evidenced in the interactions presented in Boxes 12 and 13.
In terms of the actual initial part of it, it was quite time-consuming but once it was done and it was set up, I mean it’s very much the same sort of set-up that we use once a patient gets set up anyway.
HCP4 FG6
It’s fairly straightforward to understand. It was just
HCP2 FG6
It’s just initially setting it up.
HCP5 FG6
Just writing it out the first time.
HCP2 FG6
Provision of medicines to participants
Yes. Yeah, yeah, for most of the trial and it was very easy, yeah, because they [researchers] took all the medicines away from them so it was just literally ‘this is what they’re on, can we have prescriptions for 8 weeks of this’ and yeah, it was really straightforward. And the girls did a really good job with it.
HCP2 FG6
Completion of pharmacy proforma
It’s fairly routine isn’t it?
HCP4 FG6
Yeah, it’s quite simple.
HCP5 FG6
Well I think our prescribing team just weren’t sure initially what they were going to be asking from the point of what prescriptions were going to be required, would it be weekly or monthly, were they going to be chopping and changing, were different things going to be asked at different times? But actually it just didn’t come down to that. They knew the set of patients and actually [name and name] would phone up and he just got in contact and said ‘actually, I need 28 days of this patient’s drugs’ and then you sorted it out. I mean that was – I sort of took a step backwards at that stage and I haven’t really had to have any more involvement. So that’s my impression. I mean [name] you were with our practice for the most part weren’t you?
HCP7 FG6
A vehicle for patients to feedback to pharmacists
A number of comments, presented in Box 14, indicated that taking part in the study had an effect on the pharmacy. Some patients who took part in the trial had asked to stay on MODs (HCP5 FG6). There was consensus that pharmacists rarely get feedback, particularly when patients have medicines delivered to them (HCP4 FG6).
We’ve got three patients that had never even thought about having a Dossit box, never even knew it existed, that came on the trial and all three of them have asked to stay with us to stay and have a Dossit box and two of them have said that their health is so much better because they were forgetting their tablets previously and one man’s absolutely made up and he’s like ‘I feel so much better because I know I was forgetting my medication and stuff’ and he’s just so grateful now that he knows that there is such a service, because there’s a lot of people that don’t even know the service exists.
HCP5 FG6
And I think quite a lot of the time you put somebody onto a DDS tray and perhaps we don’t get – or we put them onto an assisted medication and we don’t actually necessarily get the feedback from the patient.
HCP4 FG6
Especially a lot from the delivery patients.
HCP3 FG6
Exactly. It just becomes kind of we do it on a monthly basis and the compliance side of things or the improvement in health is perhaps something that, you know, is fed back maybe through the GP or perhaps doesn’t get fed back through to pharmacy.
HCP4 FG6
Yeah, because some of ours, they’ve been going for like maybe 3 or 4 years and they started 3 or 4 years ago. You don’t know, you know, you don’t get any feedback whatsoever. I don’t even know what some of my patients look like because they’ve had them for that long and they don’t come in, so it was really nice to actually have customers come in and actually speak to you about it and say how they felt about the box. It’s good.
HCP5 FG6
Medication-related issues
Under this theme, the subthemes emerging related to the MODs, the removal of medicines, the delivery of medicines, the return of packaging and wasted medicines.
Medication Organisation Devices
Patient perspectives on MODs are presented in Box 15. Medicines not included in the MODs were of concern (P6 FG5, P2 FG5), so it may be that better arrangements need to be made to integrate them into the system, such as synchronisation of supply. In addition to this, there were comments on difficulties in getting pills out of the box (P4 FG5, P1 FG6) and the usefulness of the MOD as an aide-mémoire.
I found the pill boxes were a limited help. What I got was five pills in daily doses but I’m also on warfarin and warfarin really is depending on your INR [International Normalised Ratio] tests, so that was excluded from the test. And also like my friend here, I’m on eye drops three times a day and they were excluded. So while it was helpful in a way, it wasn’t totally helpful. I still had to budget how to order eye drops and warfarin.
P6 FG6
Yeah, that’s right. If you’ve got two or three items that are separate from the pills, you’ve still got to go to the pharmacy to pick them up.
P2 FG6
Of course with our ones, sometimes the pill sticks to it doesn’t it when it goes out?
P4 FG6
I find it a bit hard to press that. I don’t know why because my hands are painful and I really try hard to open that.
P1 FG6
Again it’s elderly people like a friend of mine who’s died recently, she was getting confused but her daughter would ring her and say ‘have you taken your tablets?’ and she’d say ‘yes, I think so’ so she’d say ‘well go and have a look. What have you got there?’, ‘I’ve got Wednesday’, ‘well it’s Thursday today’. So those are a help for her. So she says ‘so you didn’t take yesterday’s’, you know. And I think it was worrying her too but that may come to all of us. We don’t know, do we?
P7 FG6
Isolation of non-study medicines
Medicines were isolated from participants’ immediate use to prevent inadvertent use of previous supply instead of the study supply. They were placed into clear plastic bags which were sealed with a cable tie and stored in the usual place that participants stored medicines. The idea was that patients would cut through the bag to release medicines at the end of the study or could do so during the study if for any reason their planned medication supply was disrupted.
Removal of medicines was expected to cause participants the greatest concern. However, no negative sentiments were expressed, participants seemed relaxed about the medication removal process (P2 FG5) and it was readily accepted (Box 16). However, the focus group identified that some patients had difficulty getting into their medicines after the study (P4 FG5) and that inadvertent or even deliberate taking of removed medicines during the study would be very difficult without it being obvious to researchers (P7 FG5).
But to get into them, did you say you managed to get into them? It’s very difficult isn’t it?
P4 FG5
Well it was one of those little fiddly things that you think –well I got a pair of scissors and cut through it . . . So it’s in bits if they want it . . . I thought can I take them out and nobody will know that I’ve been there. You just could not.
P7 FG5
A sense of loss having my tablets taken away from me. [laughing] And tied up in a bag. [laughing] All those surplus ones. [laughing] But no, it was easy.
P2 FG5
No, I thought it was excellent. The fact that it was – well it was professional, it was done properly, you know, and you can’t fault it from that point of view . . . Yeah, it was good.
P4 FG5
Removing old medicines was another important change. Patients on monthly medicines, by virtue of non-adherence and mixed pack sizes, for example 28- or 30-day pack, have a range of medicines in their possession that are at varying stages of use (full packs/half packs/nearly empty packs). Maintaining supply can therefore necessitate a high degree of organisation (P4 FG5, P2 FG5). The timing of visits to see the GP when new medicines are prescribed also impacts on this problem (HCP7 FG6). By removing previous medicines and supplying everything as new that degree of complexity has been removed. It is likely that alignment of supply could have a positive impact on adherence. Comments on alignment of medicines are presented in Box 17.
Well I found that I was getting fed up with trying to get through on the phone to get repeat prescriptions and I take about nine different types of medication all over 10 tablets a day and I could never get them to coincide with timing.
P4 FG5
Yeah, I had thoughts about it because I take quite a lot of pills like most people and because the amount you take per day you don’t necessarily tie up with the amount you get in the boxes, you can end up with a surplus on certain types.
P2 FG5
Because I think one of the problems is when we add in another antihypertensive and it’s out of synch with their other drugs, you know, having somebody who then gets it all into sync again would be really helpful and in a sense I just wonder whether that – I mean it may be that that is as important as sophisticated time release boxes.
HCP7 FG6
Delivery of medicines
Patients who were randomised to a weekly supply had their medicines delivered to them, patients on a monthly supply who usually had medicines delivered continued to have their medicines delivered to them and those who usually collected their medicines continued to collect them. Medicine deliveries were the aspect of the study eliciting most negative feedback and causing most concern. Comments pertaining to delivery and collection are presented in Box 18. Although the principle of delivery was acknowledged as potentially useful (P4 FG5), in practice, delivery in rural areas was problematic (P2 FG5). Erratic delivery or collection occurred for some (P7 FG5, P8 FG5), whereas others had no problem (P6 FG5) and it is intuitive that there would be more problems for weekly than for monthly delivery (eight deliveries compared with two; P7 FG5). Late and missed deliveries caused real inconvenience to patients (P2 FG5).
One thing I would say about that, I mean although I’m 21 up here and 90 down there, you know, I’m still mobile enough to go into the chemist and pick up things. But for someone who’s got a problem, either they live too far away or got mobility problems, it’s a godsend. Perhaps I shouldn’t have been on the programme but I can see the fors and the against.
P4 FG5
But when it came to the actual working of the system, it fell down badly for me. I don’t know about other people. Probably because I live out in the sticks a bit. And out of the eight deliveries, three were missed and in the end I said ‘forget about it, I’ll collect them from the dispensary’. But then the next bloke who came along after I said I didn’t want to have them delivered, he delivered them perfectly and he said ‘dead easy to find. Sat nav brought me straight to your drive’. But three of them, I don’t suppose they could find an ice-cream shop in Sorrento, you know.
P2 FG5
Well I had a funny experience too. [research associate] brought me my quota for the week on the Thursday, right, so my own tablets were put away. Saturday afternoon another one came so I thought oh, but that had number 2 on it so I thought right, now I’ve got 1 and 2. The next week number 3 came and 4 and then I thought oh and then I didn’t get any. So I went into the chemist and said ‘look, I’ve got four which I’ve now started on, but nobody’s delivered number 5’. They looked around and they said ‘oh here it is’, so I said ‘look, next week I’ll collect it myself’ and when I got there ‘no, we haven’t got one’ and they said ‘here it is’ so I took it. When I’d finished it and put all my little boxes in it, it was number 7. So number 6 is somewhere. Nobody’s got it.
P7 FG5
I think the delivery leaves a lot to be desired because they say that they’re going to deliver on such and such a day, you get down to almost 1 or 2 tablets and you panic because you think you won’t get them and you ring and tell them ‘oh but we delivered to you on Saturday’ – this probably is on Wednesday – and eventually obviously they have to come out and deliver so yeah, it is a bit hit and miss, delivery.
P8 FG5
It seemed to work all right for me. I got the first delivery at my home and that was four weekly packs and they were numbered 1, 2, 3, 4 . . . Towards the end of the fourth week I phoned up the pharmacy and said ‘I’m on this trial, have you got the next consignment?’, ‘yes, if you come and collect them’, which I did and it seemed to work.
P6 FG5
Yes, I suppose that was better them doing it a month at a time rather than individual weeks, which mine were you see. And then [research associate] said ‘they’re supposed to deliver it to you’, I said ‘well number 2 was missing. I’d got 1 and 3 but not number 2’, so I said ‘now, I’m collecting it’. So we ended up with me collecting it, apart from the two that were delivered one Saturday afternoon.
P7 FG5
Well the thing that was annoying was waiting in all day Saturday when they were scheduled to deliver and then nothing turned up . . . So we went shopping about 8 o’clock at night.
P2 FG5
And I think we had, you know, probably 50% of the patients that we actually took on weren’t questioned about that and when it came to us doing it, it was ‘yeah, you need to deliver this on a Friday or a Saturday’, so we sent them all out and 50% of the patients came back in and said ‘why am I suddenly getting this delivered to me? I’ve never had it before, I’m quite happy to come in’. So that’s just something that you’ll probably need to add, you know, if you were going to do it on a larger scale to that initial, if that was an initial face-to-face conversation rather than a mailing process, that would certainly need to be included.
HCP4 FG6
We did have a couple that said ‘oh yes, we’ll definitely collect’, the others were just like ‘oh they’re all definitely deliveries’ and we had a couple that were like ‘I’m not going to be in every Friday to receive my box! Once a month would have done me’, you know, there was quite a bit of – one bloke was really harassed because he got a delivery because he’d got a dog and then it wakes his wife up. You know, I think they do need to be asked thoroughly ‘Do you want it to be delivered? Do you want a collection?’
HCP5 FG6
There was definitely some confusion, like some of the people who – well, one lady in particular who was meant to be collecting. I think she was just on the monthly so she was only coming in once but she kept trying to come in early for it and I was saying ‘no, it needs to be regulated so they can see what’s going on’ and then she came in again and in the end I just let her have it a couple of days early because it was just getting a bit ridiculous. But people didn’t seem to understand fully how it was meant to be working when they were used to just ordering their own medication to then be told ‘that’s all right, we’re sorting it out for you, don’t worry, just come in on this date’. They sort of seemed a bit anxious and wanted to come and pick it up earlier and not follow how I thought it was meant to be working.
HCP3 FG6
They feel vulnerable because it’s like ‘what if the driver can’t get here? What if you haven’t done it? What if you’ve forgotten me?’
HCP5 FG6
Health-care professionals identified confusion in patients who did not know why they had been allocated to delivery when they were happy to collect, annoyance in allocation to delivery in others and a misunderstanding on timing of collections (HCP4 FG6, HCP5 FG6, HCP3 FG6). Importantly, apart from the annoyance of waiting in for a delivery that did not materialise, it was identified that removal of autonomy with respect to collection of medicines made some patients feel vulnerable (HCP5 FG6). One participant suggested that patients should be asked whether they wanted to collect their medicines or to have it delivered.
Return of packaging
For successful adherence assessment in a definitive trial, return of medication packaging to the pharmacy would be required. Return is necessary in the case of DUC so that counts can be made without the need for an additional researcher visit and in the case of electronic monitoring so that data can be downloaded and monitoring units can be recycled onto new packs. Returns would be planned to coincide with usual pharmacy or patient delivery and collection, that is when a patient picks up their new supply they deposit their previous packs. Despite one-to-one and group training sessions, provision of itemised information sheets and SOPs for pharmacies and GPs and provision of containers in which to place medicines for return and explanation to patients at visits, it is clear that this aspect of the study design has been challenging. Information regarding pack return was either not retained or misunderstood by pharmacists and patients alike as evidenced by comments presented in Box 19.
Pharmacists indicated that they were unaware that packaging had to be returned to the pharmacy (HCP5 FG5) and that some patients may have been unaware of the requirement (HCP2 FG5).
Can I just say one thing though? When it was first done as well, nobody at any point said to us that when you’re delivering out you’ve got to collect their old boxes back each week, OK. Nobody said nothing and we had a couple that were brought in. We got rid of them because nobody told us that we was to keep them. It was only halfway through that someone said ‘actually, you’re supposed to be keeping those boxes and actually, could you make a delivery and actually do a double delivery so that they bring back the old boxes?’. We weren’t told that till halfway through so half our stuff, half the patients’ stuff is missing because we was never told that – as far as I was concerned they was going to be left at the patient’s house and at the end somebody would collect it all and it was only halfway through that someone actually said ‘oh actually, they’re supposed to be bringing them back every week’. Well we should have been told that at the beginning. We actually got rid of some of it.
HCP5 FG6
In a similar vein, I don’t know if all the patients had that explained as well or whether they understood it. It could be that they’d just forgotten but we had one patient who had been having the packs and when she’d finished one, she would just chuck it out in the rubbish and she said that she hadn’t been told she had to hold onto them, although she had been given one of the large bags so I wondered whether she just hadn’t either understood the message or retained the message. But I think she chucked away two or three of them before I phoned her and spoke to her and she said ‘oh, I’ve been chucking them away’ and I obviously told her ‘no, don’t do that!’. . . But whether that was just a communication thing or whether that was just, you know, that particular patient not retaining the information, I’m not sure.
HCP2 FG6
Wasted medicines
Quarantining of existing drug stocks at the study start highlighted for professional participants the stockpiling and potential waste of medicines in patients’ homes and reminded them of Christmas and Easter ordering patterns. This is illustrated by comments presented in Box 20.
Well I feel that some people, they actually order everything on the list every month and they’ve probably not even used half of it, just because they can. Then you go to the house and they’ve got this store of medication.
HCP5 FG6
I’m sure you would have seen a lot more patients’ houses than us and I’m guessing you’ve found some
HCP2 FG6
Were you in the surgery that day when I dumped those bags?
HCP7 FG6
Oh, the eleven or twelve bin bags?
HCP2 FG6
Those big plastic bin bags and I said, you know, we could
HCP7 FG6
I’ve never seen anything like that.
HCP2 FG6
From that patient’s house.
HCP7 FG6
Yeah. Unbelievable.
HCP2 FG6
They’d been hoarded. I mean of course we hear the other side though because the patients sometimes come in and say ‘oh the pharmacy is ordering my drugs and I just don’t want all these’. So you know we get the other side . . .
HCP7 FG6
People do like medication.
HCP6 FG6
Stockpiling.
HCP3 FG6
It’s a very reassuring thing to have some medication there and there’s enough for the next – [laughing]
HCP6 FG6
Foreseeable future! [laughing]
HCP3 FG6
It’s true, it’s so true. It is so true.
HCP5 FG6
Christmas, you order everything you’ve ever had in your life!
HCP6 FG6
Oh it’s crazy, it’s crazy.
HCP7 FG6
Christmas and Easter.
HCP5 FG6
Study tools
This theme included the subthemes of participants’ comments on the test of participants’ ability, electronic monitoring and the return to usual care.
Tests of participant ability
Participant functional abilities were tested in order to assess the appropriateness of MOD supply. Comments on the tests used are presented in Box 21. No negative comments were received.
I think it’s right. They’ve got to satisfy themselves that you can read these things, which is repeated I notice in this questionnaire.
P4 FG5
I’ve got good eyesight for reading and yes she did, she asked me, I said ‘well how far down?’ and I think I read the one from the bottom, so I don’t think she bothered to test me any more on that.
P7 FG5
Cognitive ability test
I was a little amused about the trial on one’s memory . . . But I thought that was good, just to indicate whether you are really in trouble with your memory, but I think most of us probably couldn’t remember everything. I didn’t! [laughing]
P4 FG5
Well it was quite good really. I must admit, I sat there thinking am I really going through a memory test at my age? But it just proved that I did need it because I did, she came back to it straight afterwards and said ‘can you remember what I said to you just now?’ and I said ‘yes, apple and – oh dear, I’ve forgotten the other one’. It was only seconds but I’ve remembered it ever since.
P7 FG5
Electronic monitoring
Electronic monitoring was part of the original study design but was not implemented during the trial. Exploration of electronic monitoring issues identified mixed feelings. These are represented in Box 22. One interaction correctly identified that monitoring of packs cannot identify that a patient has ingested the medicines and therefore could be considered an expensive waste of money (P7 FG5). One indicated that they thought its use showed a lack of confidence in the patient but it is not clear whether this was a lack of confidence in ability or honesty (P4 FG5). One participant thought it would be helpful for people with dementia (P4 FG5), while another thought it would be worrying for a person with ‘real’ (perhaps meaning severe) dementia (P6 FG5).
I can’t see how it would work though. I mean right, fair enough, you pop it but there’s no proof that you’ve actually taken it.
P7 FG5
No, there isn’t.
I (moderator)
So to me that would be an absolute waste of money.
P7 FG5
I thought the same thing.
P4 FG5
Did you?
P7 FG5
Yes I did. And I thought
P4 FG5
I thought all that money, there’s no proof that you’ve taken it. You could have thrown it down the toilet.
P7 FG5
And furthermore, it did suggest that the whole scheme has got a lack of confidence in the patient.
P4 FG5
Yes.
P7 FG5
Which is not good.
P4 FG5
Yes, that’s true. I hadn’t thought of it that way but I could see this is an easy way that people – because there’s no proof they’ve taken it, is there? Mind you, you should do for your own sake.
P7 FG5
Well if it could be developed it must be of help to those with dementia but as you say, the technology’s not quite there yet. But no doubt the research will be going on.
P6 FG5
But then you see with a dementia patient, real dementia, they would need someone to do it for them to make sure they took it, because I’ve looked after my mother-in-law and there’s no way she would have ever taken a pill if I hadn’t run around after her saying ‘come on, come’ and all that. I had a friend who’s, well, on the borders of it which as I said a little while ago she’s recently died – she was 88 – and there’s no way she would have taken her tablets yet she could go to the shops, come back, ring me, talk and that, but these pills she just could not remember to take them. So I can’t see where that would have helped her. But maybe, you see, it helps some people.
P7 FG5
Well, I think one of the things that intrigued me, I have to say if it comes down to brass tacks, is these super-duper fancy gizmo reporting Dossit boxes that [research associate] told us about at the very beginning and how they’re actually going to work with these time stamped little chippy things in them.
HCP1 FG6
It was a bit disappointing to not have the films because that sounded really good, that you could actually see what time they had actually gone to take the medicine, not ‘yes, I take it in the morning’ and you’d never actually know. So then it obviously hasn’t delivered the amount of detail that perhaps we thought it would do at the start and that seemed something quite novel to find out actually when are they going into the box rather than just send it out and then it comes back empty but you don’t know when they’ve – they might have just done it all at the end of the week and put them all in the bin for all we know.
HCP3 FG6
Yes, I mean the high-tech stuff did look really interesting and it was going to be – It was going to be really, really interesting to see what people are actually doing and so that was a bit disappointing.
HCP2 FG6
And that’s where the technological side of things would – I don’t want to keep dredging it up but that would have been, you know, that would have been a real – and I think for all of us, from all the various, it’s a real shame and I’m sure your lot agree as well!
HCP4 FG6
Yeah, because I think you’re only going to – I’m not sure how many answers one can get through just using the weekly trays. I think it really does need a better way or a sort of clearer way of looking at actual concordance – as medical students are now told to say rather than compliance – so I think yeah, it will take more than that.
HCP7 FG6
The latest version of the Pivotel actually has a mobile phone chip in it and if you are non-concordant, rather than non-compliant, it will actually phone up a pre-designated mobile phone and alert somebody. Also if it falls over on its back or something and it isn’t picked up again, it will phone them. So there’s levels of sophistication in these devices now that are becoming affordable.
HCP1 FG6
Well I think there are a number of issues. As a GP going into these patients’ homes and seeing how the boxes are used, you do see quite haphazard use with the boxes and I think you know, that has got to be somehow looked at because, you know, you can even have something that times when they take it out but what you still can’t be sure is whether it actually ends up in their mouth and there is going to be a limit to what you can do but certainly timing when the various pockets are opened is crucial because I can certainly tell you that, you know, I know some of the ones, the boxes that come back to yourselves, they can look quite haphazard can’t they? Well we can see that haphazard arrangement just in the home. You’ll suddenly see some tablets here and you’ve got this nice box and you realise that they’ve
HCP7 FG6
Health-care professionals were very much in favour of electronic monitoring and identified with its clinical utility rather than research application. They expressed an interest in identifying the details of what people were really doing and the importance of having an objective way to determine that.
Return to usual care
Health-care professionals discussed difficulties surrounding the return to usual care at the end of the study. The possibility that patients may wish for continuation of their medication in a MOD had been carefully considered and ethical approval had been given for participants expressing such a wish to be referred to the Norfolk Medicines Support service by researchers or pharmacists. There was no plan to ask patients in receipt of a MOD for the study to be approached to determine whether or not they would like to continue on one. This position was clearly documented.
Most (22 out of 23) participants completing the RCT continued in the care of their usual pharmacist for the duration of the trial. All had been enrolled on the automatic order and collect scheme on recruitment to the study. They were informed that researchers would deregister them from the scheme on request. No participants requested deregistration. Pharmacist perceptions were that ‘the end of the trial has been very poorly communicated’ (HCP4 FG6) and this would include return to usual care. Comments on return to usual care are presented in Box 23. Although in some cases the return to usual care was smooth (HCP6 FG5) there was some consensus that a final visit or post-study pharmacy consultation with the patient would have been beneficial (HCP5 FG6, HCP4 FG6, HCP5 FG6).
One lady wanted to stay with us but didn’t phone to say she’s gone back to [name], but she really wants me to do it. The other ones were fine, they just – one chap was weekly. I got a message through from UEA saying ‘Can you continue to do it but he’d like it monthly?’ so he must have been contacted and it was very smooth.
HCP6 FG6
Because I felt I had to ring UEA and say ‘what is going on? What is happening to these patients?’ because then they were like ‘so [company name] have let us down now’, ‘no, [company name] haven’t let you down. We’ve done as we were told. We’ve done your 8 weeks, we did it as, you know’ – and there was one man that was like ‘I’ve come to [company name] for years, I’ve done this for you and now you’ve let me down’, but it wasn’t us that let him down, it was the UEA that let him down.
HCP5 FG6
There just needs to be an understanding of the impact, like on the patient, I think and actually for something that is a complete change in terms of medication, there has to be some form of planned-in consultation at the end.
HCP4 FG6
I think they should have had a last visit on the last week. I honestly think they should have had somebody go out to them and say ‘This is your last week. After today, you know, can you sort your prescriptions out because in a few days you’re going to run out of tablets’? I think they should have had a last visit, definitely.
HCP5 FG6
Definitive study design considerations
In this final section, comments which would inform a future study are reported under the subthemes of issues for future participation, the types of patients to include and the way the study should be managed.
Issues for future participation
General experiences were mixed, but the overall response to the question ‘So then, now, having been through it, if you had your time over again, would you still have said yes?’ from all patients that commented was a resounding ‘yes’, indicating that, in general, the experience had been a positive one. Participants indicated willingness, ability and capacity to take part in a definitive study. While, overall, there was probably a net benefit to GP practices in terms of time, this was not the case for pharmacies. Should larger numbers of patients be involved per pharmacy in any future study, it would be challenging from both organisational and financial perspectives (Boxes 24 and 25).
Patient management if you like, you know, with the way we work with our surgery all of our patients don’t actually, very rarely, order their medication at all now once they’re on a DDS tray. The changes are done and liaised direct from the GP, through the GP’s practice to the pharmacy and it’s very much a managed and organised service where the patient has little involvement. So in terms of the bigger scale I don’t think it would have – it would have an impact in terms of the workload of the increased number but it wouldn’t be any different from the current way it’s done in pharmacy. That would be my
HCP4 FG6
Because of the nature of the DDS, because it is so labour intensive, we have to organise it. If not, they’d come in ‘I need my 4 weeks’, ‘Do you want to wait for an hour?’, so that’s why we do take on organising it because if not, if you left it to the patient some of them, you know, that is what would happen, they would just appear and it’s just impossible.
HCP3 FG6
I’m not sure we could take on that much more. . . My workload really did go up because I had so much that I had to do because I literally did it on my own and it was the ordering of the [???], it was doing all the paperwork. There was just so much and so many more man hours that it took to physically do them people because it weren’t as straightforward as just being on the computer like normal, we’ve got our sheets already done. We had to redo all the sheets, we had to put a lot of input into it and I felt that the patients we had was enough because we have got such a big workload anyway of patients. They were umming and arring whether they were actually even going to let us do it because we have got so many.
HCP5 FG6
I think you’re going to find, in terms of a pharmacy perspective, it’s going to be on an individual pharmacy basis as to what their current running capacity is and what, you know, it would have to be negotiated almost on a one-to-one basis that these are the patients that we’ve identified, can you support that? If you came to us tomorrow and said ‘can you support 40 additional patients?’ I’d chase you out the building. [laughing]
HCP4 FG6
And that is something that moving forward would have to be looked at and I’m sure that’s not just, you know, [pharmacy name], that is each individual pharmacy as you go round the area.
M4 FG6
I mean from that point of view, costing how much something like that would take, would be very important.
HCP7 FG6
Absolutely.
HCP4 FG6
Because if they were to come to you saying ‘can you support 40 more of these?’, it would have to be costed appropriately.
HCP7 FG6
Absolutely.
HCP4 FG6
My understanding is at the moment, it barely breaks even with what you’re doing. Is that correct? That’s my understanding.
HCP7 FG6
DDS in pharmacy is patient care, it’s not a profit.
HCP4 FG6
It’s not a profit, no. So it would be important that if you add that much then the cost would have to be done properly and you couldn’t take it on just on that basis.
HCP7 FG6
On that basis, no. And I think without, because of the workload that’s involved and the way it works, it would almost be a prior costing which isn’t always feasible within research or within projects.
HCP4 FG6
And therein lies the problem. It’s not as if we can get to the stage where you can say right, we’re going to give you forty and here is x amount of funding. It’s very much once we get to the end of it we’ll sort of – but without actually knowing that.
HCP4 FG6
You see we had no budget to have an extra person in to help with their boxes, so I was coming in on my day off, I stayed late, I came in early, because we wanted to be part of it and I didn’t mind doing that as a one-off but I wouldn’t want to do that on a regular basis because – because the money wasn’t there to begin with to say ‘right, you can have x amount of staff and you can have so many extra hours so we’ll get somebody in’, there wasn’t that available so I had to just make myself available. So yeah, it was hard work.
HCP5 FG6
I think they had a lot more workload than we ended up with. I think the initial stage was fairly easy but ultimately we only had a couple of people finish on the final stage so we didn’t really, you know, it was very hard to compare. I think if we had had
HCP2 FG6
You see we still had eight at the end.
HCP5 FG6
Yeah, if we’d had a lot more it would have taken a lot more. I mean
HCP2 FG6
We had about eight as well but that was just I got my pre-reg essentially did most of the putting together and we just blitzed it in a couple of days but that was all she was doing for a couple of days.
HCP3 FG6
So yeah, but I think looking forward if you’re talking bigger numbers there you are going to have to, you would have to think of the impact.
HCP4 FG6
Yeah, definitely.
HCP5 FG6
Types of patient
Health-care professionals described the type of patients who should be considered for additional help with adherence, that is who are suited to MODs and therefore to a definitive study. Comments are presented in Box 26 and indicate that those who collect medicines on a haphazard basis (HCP7 FG6, HCP2 FG6) or who run out of medicines (HCP7 FG6) should be targeted.
. . . really the ones that really need to be targeted are those ones who seem to be collecting medication on a haphazard basis and then as [name] says though, whether actually regular face-to-face contact with them in their home, taking away all the rubbish, might actually be– . . . actually the ones you’re wanting to capture are actually in a sense, particularly the ones that you’re wanting to capture, are the ones who aren’t taking the medication very well . . . Actually it’s you spotting or certainly starting with the patients who seem to be asking for things haphazardly.
HCP7 FG6
Yeah. We all know that people that we see twice a week in our pharmacy coming in and they’re the ones where you’re thinking, you know, I’ve seen you so many times recently, what’s going on and it can’t always be they’ve had to come in and see the doctor for something new. A lot of it is just everything’s out of sync and that is going to make it harder for them to manage it. We’ve all had the phone call at 5.30, ‘oh my God, I’ve run out of my medicines, I’ve got none for tonight and it’s those
HCP2 FG6
Yeah, I mean there’s the patients who run out of their medication at a weekend. I mean quite often those aren’t always the elderly . . . I mean with doing out of hours, you know, sometimes those can be young people who haven’t got a Ventolin inhaler and that sort of thing.
HCP7 FG6
Study management
Some comments were made on study management. Exchanges are presented in Box 27 and indicate that the relationship between patients, researchers and health-care professionals, albeit not perfect, was acceptable. Maintaining this good relationship in a larger study would be essential for successful completion.
No, I just thought it was very efficient.
P2 FG5
So did I.
P4 FG5
I agree.
P8 FG5
Yes. They explained everything very well. You knew straight away what you’d got to do didn’t you?
P7 FG5
Yeah.
P2 FG5
Yeah, they were very good.
P7 FG5
From that point of view it was efficient all the way through . . .
P2 FG5
No, I thought it was excellent. The fact that it was – well it was professional, it was done properly, you know, and you can’t fault it from that point of view.
P4 FG5
I think the girls were very good, you know, if you did have a problem you could ring them and they would – and if you couldn’t get them they would always ring you back. They were absolutely brilliant.
HCP5 FG6
In terms of their initial relationship building with us as well, those initial kind of how they approached pharmacies specifically, I mean I don’t know if they had much sort of . . .
HCP4 FG6
No, I mean there wasn’t – they came in and talked with the prescribing team, you know.
HCP7 FG6
We found that . . .
HCP4 FG6
Came back and always were there if there were any worries.
HCP7 FG6
From that perspective I think they were very . . .
HCP4 FG6
Good liaison.
HCP7 FG6
Yeah.
HCP4 FG6
They were very good. They were very professional.
HCP5 FG6
Very quick to build a rapport.
HCP4 FG6
And you felt that no matter what, you could ring them, you weren’t bothering them, you could ring them. They were really good.
HCP5 FG6
Yes.
HCP3 FG6
Yeah.
HCP4 FG6
Summary
The secondary outcome measures of participant satisfaction and autonomy associated with medicine-taking were captured in a final trial evaluation questionnaire, which also captured satisfaction with the trial process and is reported in this chapter. This chapter further evaluated the study design implemented in Chapter 4 using two group discussions: one comprising eight study participants, the other comprising five members of staff from the participating pharmacies and two members of medical practice staff involved in the study.
The trial evaluation questionnaire was completed by 91% of the 23 participants who completed the trial. MOD weekly was consistently scored highest or equal highest for all criteria related to medication packaging, although no significant differences were observed in this small sample. No indication of any difference in participant-perceived autonomy in terms of their confidence and anxiety related to the method of medication packaging was observed between the randomisation groups. Carer evaluation of the effect of medication packaging on participant autonomy resulted in no significant differences between groups. There was, however, a trend towards carers reporting less positive evaluations for participants randomised to monthly usual packaging.
From the focus group discussions, the trial design, including the questionnaires, DUC and assessment of cognitive and physical function, was generally considered acceptable. From the participant and medical practice perspectives, the communication experience with the research team was good. Some pharmacy representatives, however, felt that greater clarity of processes was required. A particular concern is that some pharmacy staff did not retain returned medication packaging for DUC; thus, there were a number of deviations from the SOPs on which they received training. It was clear that trial involvement did present a significant burden to pharmacies and, thus, it is essential that any future study ensures that participating pharmacies have the required resources to fulfil the trial dispensing and management requirements.
Some participants allocated to weekly medication supply, and thus delivery, were dissatisfied with this element, as they found that deliveries were sometimes late and erratic, which presented significant inconvenience. Any future study should thus provide participants with the choice of delivery or collection.
Chapter 6 Cost-effectiveness of different recruitment strategies
Introduction
Successful recruitment in terms of numbers and costs are key to the success of any trial and particularly important for large-scale definitive studies. Two recruitment strategies were devised and used under the RCT. Results were compared to inform the most appropriate recruitment strategy for a definitive study.
While passive recruitment via medical practice invitation letters is convenient in terms of research administration, response rates have historically been low,16,34 as the method requires the patient to be proactive in responding to a letter of invitation; consent rates are frequently between 30% and 40%. 16,34 Active recruitment processes such as waiting room recruitment by researcher, which are potentially more labour intensive and thus costly, have yielded substantially higher response rates. 35–38 Given these potential differences, we sought to compare the cost-effectiveness of these two types of recruitment strategies.
Methods
Recruitment was carried out as described in Chapter 4. The two recruitment strategies adopted were:
-
passive (postal) recruitment (questionnaire sent from the patient’s GP)
-
active (face-to-face) recruitment by GP and researcher.
Briefly, all GP practices (regardless of strategy) undertook a search of their patient lists to identify those who were potentially eligible, that is who were aged ≥ 75 years and taking three or more SODF medications, two of which were from a defined list (see Table 16). GPs checked lists of patients identified by the aforementioned search to determine their suitability for participation. Subsequent actions for each of the two recruitment strategies were as follows.
Recruitment strategies
Passive recruitment:
-
Mailshot recruitment pack sent to eligible patients from the patient’s GP, comprising PIL, consent form, request and collect repeat prescription form, invitation letter (on headed paper), questionnaire 1 and a pre-paid return envelope. A reminder letter, on headed paper, was sent to non-responding potential recruits.
Active recruitment by general practitioner and researcher:
-
Practice staff flagged any attending patients who were identified by the aforementioned search on the patient database.
-
On attendance the GP/nurse briefly explained that there was a trial going on, provided a PIL and asked the patient to see the researcher in the waiting room if interested in taking part in the trial.
-
Patients who chose to speak to the researcher and were interested in taking part, they were provided with a recruitment pack (as for passive recruitment but without GP invitation letter) to fill in at the surgery or to take away with them as they preferred.
Active recruitment took place over a 3-week period for each practice. At the end of that period all potentially eligible participants who had been identified by GP searches were posted recruitment packs. Some participants who were registered in the three active recruitment practices were therefore recruited via the mailshot process. These participants are not included in the analyses, which sought only to compare the efficiency of the two recruitment strategies.
For each recruitment strategy, efficiency was assessed in relation to two measures:
-
the number of people who consented to take part
-
the number of people who were randomised.
The former was chosen to enable simple comparison of consent rates between strategies. The latter was chosen to examine eligibility rates for consenting participants between recruitment strategies. Consenting participants may exhibit one of three adherence types: intentional non-adherence, full adherence or unintentional non-adherence. Only unintentionally non-adherent participants were eligible for randomisation. The recruitment strategy may differentially affect consent and eligibility rates, and therefore randomisation rate, and it may be that different proportions of consenting participants in each recruitment group are later identified as ineligible. For example, it may be that by explaining the study to the patient in person, a lower percentage of non-eligible people (e.g. intentionally non-adherent) will give consent.
Consenting eligible participants were subsequently visited, and provided with 1 month’s supply of medication (visit 1). They were visited again 3 weeks later to determine adherence (visit 2). If they were < 100% adherent, were still otherwise eligible and wished to continue in the study, they were randomised.
Costs associated with the activities listed for each of the recruitment strategies, plus costs associated with both visits 1 and 2 (excluding medication prescribed) were estimated. Medication costs were excluded as, although participants were given 1 month’s supply of medication at visit 1, this was akin to them receiving their next month’s supply earlier than would otherwise have been the case (they continued with their old stock after visit 2 if they were not randomised into the study). Costs were based on the 2012/13 financial year, as this was the time of recruitment (active recruitment took place between September and November 2012, and passive recruitment was undertaken in the three practices between August and September 2012). At the time of analysis, health-care professional costs were available only for the financial year 2011/12 and, thus, these were inflated by 2.6% (the rate of Hospital and Community Health Services pay inflation between 2010/11 and 2011/12) to provide estimated 2012/13 costs. Subsequently, costs associated with each strategy (excluding visit costs) were compared with the number who consented via each strategy (visit costs were excluded as the visits took place after consent was provided), and when visit costs were included the overall costs associated with each strategy were compared with the number who were randomised.
The costing method used varied according to the item costed. For example, when assigning the researcher time in the surgery to the individual participants, the top-down costing method81 was used, whereby the total cost of the researcher time was equally apportioned across the appropriate participants. Conversely, when estimating the costs associated with a visit to the participant’s home, the method was more akin to the bottom-up approach,81 as the resources associated with different cost items were estimated on an individual participant basis (although no particular questionnaire was completed prospectively to monitor these items).
Results
Passive recruitment
Passive (postal) recruitment took place in three practices and a total of 995 patients were identified as potentially eligible, based on a search of the three practice lists. Each of these practices was paid a £175 set-up fee. GPs excluded 185 of the 995 patients (no additional cost to the aforementioned £175 was added for this activity) and subsequently 810 were sent recruitment packs. The cost of each recruitment pack sent was estimated to be £1.28 (including postage, but excluding the cost of return postage), which included the PIL (£0.12), consent form and request and collect repeat prescription form (£0.05), questionnaire 1 (£0.16), a pre-paid return envelope (£0.04) and postage (£0.90). Thus, the total cost of sending all recruitment packs amounted to £1036.80 (Table 32). After a further 2–3 weeks, a total of 703 non-responders were subsequently sent a reminder recruitment pack (unit cost = £1.28). In total, 152 people responded (return postage unit cost = £0.48), of whom 135 returned questionnaire 1 only and 113 returned both questionnaire 1 and the consent form. Table 32 shows the costs associated with each aforementioned component part. When these were assumed and divided by the number who consented, the cost per consent obtained amounted to £22.40.
| Activity | Passive | Active |
|---|---|---|
| Search of practice list | £525.00 | £525.00 |
| Questionnaire 1 distribution | £1004.40 (n = 810) | £19.52 (n = 52) |
| Sending questionnaire 1 reminders | £896.61 (n = 703) | – |
| Responding to questionnaire 1 | £72.96 (n = 152) | £15.36 (n = 32) |
| Travel cost associated with practice visits | – | £405.00 |
| Cost of researcher time associated with practice visits | – | £616.15 |
| Researcher time in surgery | – | £2422.26 |
| Subtotal | £2531.37 | £4003.29 |
| Cost per consent obtained | £22.40 (n = 113) | £190.63 (n = 21) |
| Pre-visit 1 | £2167.58 (n = 39) | £444.63 (n = 8) |
| Visit 1 | £1683.32 (n = 39) | £345.30 (n = 8) |
| Visit 2 | £1218.30 (n = 37) | £263.42 (n = 8) |
| Total | £7600.56 | £5056.64 |
| Cost per randomisation | £506.70 (n = 15) | £2528.32 (n = 2) |
Active recruitment/consent rate
Active recruitment was also undertaken in three practices, where 987 patients were identified as potentially eligible, based on a search of the three practice lists. Again, each of these practices were paid £175 set-up fee and the cost of the search was assumed to equate to this value. When any of the 987 patients who were suitable to participate made a routine appointment to see their GP, the researcher was informed and attended the relevant surgery. The average distance to each surgery was estimated to be 10 miles (mileage was reimbursed at £0.45 per mile in the study) and, thus, the travel cost associated with each GP surgery visit equated to £9.00. Across the three practices a total of 45 visits were made, giving an estimated total travel cost of £405.00.
The average salary of the researchers who undertook the surgery visits in this study was estimated to be £26,000. Assuming salary on-costs (employers’ national insurance and superannuation contribution) equated to 24% of the salary cost and that they worked 37.5 hours per week 42 weeks per year, the cost per hour of researcher time was £20.47. Based on an average speed of 29.9 miles per hour (mph), which is the average free-flow vehicle speed in a 30 mph limit,82 then it would take 2.01 minutes to travel 1 mile and the researcher time associated with the 20-mile round trip would thereby be 40.13 minutes. The cost of the researcher time associated with travel thereby amounted to £616.15 (see Table 32).
The time associated with each visit to the practice was as follows. The researcher was assumed to arrive 15 minutes before the time of the first pre-arranged participant appointment. This was important, as older patients often arrive well in advance of their appointment. The researcher would then be visible at the time the receptionist made the patient aware of the presence of the researcher. It also allowed time for preparation (arranging desk/chairs in position, sorting paperwork and checking for cancellations with the receptionist) so that researchers were available to speak to any potentially eligible participant who approached them after their appointment with the GP/practice nurse. Researcher time at the practice was dependent on how many potentially eligible participants had appointments in that session. Additionally, it was assumed researchers would stay an additional 10 minutes from the start time of the last appointment of any potentially eligible participant (to allow for the consultation with the GP/nurse to finish), 30 minutes to explain the study to any potentially eligible participant that approached them (if applicable), 10 minutes for discussion with practice staff (no additional payment, beyond the £175 practice payment, was made to the practice for this time) and a further 15 minutes to clear up (reinstate the room and clear paper work). The total attendance time (per session) was thereby equal to the time between the appointment times of the first and last potentially eligible participant plus 80 minutes (15 minutes’ preparation, 10 minutes’ appointment time, 30 minutes for discussion, 10 minutes’ discussion with receptionist and 15 minutes to clear up) if the last potentially eligible participant approached them (this applied to 38 of the 45 sessions) and 50 minutes (15 minutes’ preparation, 10 minutes’ appointment time, 10 minutes’ discussion with receptionist and 15 minutes to clear up) if the last potentially eligible participant did not approach them (this applied to 7 of the 45 sessions). The average time between the appointment times of the first and last potentially eligible participant was 82.44 minutes. Over the 45 sessions, the total time the researcher was in the surgery amounted to 7100 minutes (118 hours). At a cost of £20.47 per hour this gave a cost of £2422.26 for the time in the surgery (see Table 32).
Within these 45 sessions, a total of 98 potentially eligible participants approached the researcher, 52 of whom took a recruitment pack containing PIL (£0.12), consent form and request and collect form (£0.05), questionnaire 1 (£0.16) and a pre-paid return envelope (£0.04). Of these, 32 responded, 21 of whom completed both questionnaire 1 and the consent form. Table 32 shows the costs associated with each component part of the active recruitment; the cost per consent obtained amounted to £190.63.
Passive and active recruitment post consent
Those who consented and were deemed not intentionally non-adherent or otherwise ineligible were visited in their own home by the researcher (visit 1). The purpose of this visit was to provide the participant with 1 month’s supply of medication and to remove old medication stocks so that they could be visited 3 weeks later (at visit 2) to assess adherence. Thus, prior to the visit it was necessary for (1) the researcher to call the pharmacist, in order to arrange to collect medicines for the participant to be visited (10-minute telephone call), (2) the pharmacist to prepare the medication and (3) the researcher to collect it. The pre-visit pharmacy time for these three activities was assumed to average 45 minutes [at a cost per hour of employment of £51.30 (£50.0083 inflated by 2.6%)]; thus, the pharmacist cost would be £38.48 per participant. Assuming that the aforementioned travel costs to the GP practice also applied to the pharmacy (20-mile round trip, with an average speed of 29.9 mph), the total researcher time per pharmacy visit would be 40.31 minutes, which gave a total researcher time of 50.13 minutes when the 10-minute telephone call was included. Based on the aforementioned £20.47 per hour of employment, the pre-visit 1 researcher cost was £17.10. Thirty-nine participants from the three passive recruitment practices received visit 1, hence the total pre-visit cost for these participants was £2167.58. Eight participants from the active recruitment practices received visit 1, hence a total pre-visit 1 cost of £444.63 (see Table 32).
At visit 1 participants had their current stock of medication removed and were given 1 month’s supply of new medication. This visit was assumed to last 1 hour (excluding research-related time) and have the same travel time and cost as that for the aforementioned GP visits (although participants often lived further away, it was possible to combine some of these visits). Total researcher time per visit was thereby 1 hour 40.13 minutes at a cost of £34.16, with travel costs of £9.00, giving a total visit 1 cost of £43.16. Total visit 1 costs for the eight active recruitment participants and the 39 passive recruitment participants are shown in Table 32.
Visit 2 took place 3 weeks after visit 1 and was primarily undertaken to monitor adherence by pill count. If participants had taken either more or less than 100% of their medication they were deemed unintentionally non-adherent. After excluding the time associated with research activities (e.g. MMSE, vision test, dexterity test, pill count, randomisation), the time for assessment and to ensure that the participant could use their allocated medication packaging amounted to 30 minutes. Using the same travel costs as visit 1, the total cost per visit 2 amounted to £32.97, which was applied to eight active recruitment participants and the 37 passive recruitment participants.
When the costs associated with each of the aforementioned activities (both pre and post consent) are summed together, the total cost was estimated to be £7600.56 and £5056.64, for passive and active recruitment, respectively (see Table 32). At the end of visit 2 a total of 15 participants were randomised from the three passive practices, compared with two from the active practices, giving a cost per-participant randomisation rate of £506.70 and £2528.32, respectively.
Summary
Within this chapter we have compared the cost-effectiveness of two different recruitment strategies [passive (postal) recruitment and active (face-to-face) recruitment by a researcher]. Both of these strategies were undertaken in three different practices. A total of 113 participants consented as a result of the passive recruitment, 15 of whom went on to be randomised. The numbers actively recruited was 21 and 2, respectively. The costs associated with the passive strategy (up to the point of consent) included a search of the practice list, posting of recruitment packs and return postage and amounted to £22.40 per consent obtained, compared with £190.63 per consent obtained in the active recruitment strategy (which also included the cost of the researcher time in the practice surgery and associated travel costs). After consent, participants were visited to issue medication and later to check adherence; 21 were subsequently randomised from the passive strategy group compared with two from the active group. The cost per randomised participant was £506.70 in the passive group compared with £2528.32 in the active group. Thus, it was concluded that passive recruitment was the more efficient strategy.
Chapter 7 Economic evaluation
Background
For a successful definitive study of clinical effectiveness and cost-effectiveness, it is important to incorporate appropriate measurement methods. This component of the study aimed to evaluate the methodology for, and assess the feasibility of, conducting a definitive economic evaluation of the effectiveness of MODs in this population and to undertake an early-stage cost-effectiveness analysis of the MOD.
Objectives
Key objectives were to:
-
identify the resources and outcomes likely to differ as a result of MOD use
-
test data collection instruments
-
provide an early-stage valuation of the resources and outcomes observed
-
comment on the feasibility of collecting resource use and quality-of-life data in a subsequent more definitive study.
Methods
Measuring costs
Intervention
The intervention comprised the provision of the participants’ medication in a MOD delivered or collected by the patient on either a weekly or monthly basis. Thus, the resource use associated with the MOD itself, packing it (dispensing time) and the associated delivery cost (if the participant chose not to collect their medication from the pharmacy) was identified. Each MOD contains 1 week’s supply of medication, so, for those in the MOD monthly arm, four MODs were prepared at a time, and two delivery trips were undertaken (if applicable) in the 2-month follow-up period. Conversely, for those in the MOD-weekly arm, the pharmacy prepared either one MOD box (1 week’s supply every week) or four MOD boxes at a time (4 weeks’ supply, dispatching one each week and storing the remainder), so eight delivery trips were undertaken (if applicable). In the light of this, pharmacists were asked to record dispensing times (time taken to put the medication into the MOD and time taken for the pharmacist to check the MOD) at least once per month, for each participant in the study. When one MOD was filled at a time (1 week’s supply each week) and the associated times were recorded twice for a participant, the mean of these two values was assumed to apply to the remaining six times a MOD was filled for the same participant but no associated time was recorded. Pharmacists were also asked to record dispensing times for those in the monthly and usual supply weekly arms in the same manner, for which, again, the number of delivery trips would be eight and two, respectively (if applicable). Finally, medication details, for participants in all arms, were also ascertained from the pharmacist.
Other costs
The UK National Institute for Health and Care Excellence recommends that costs should be calculated from the perspective of the NHS and Personal Social Services. 84 Accordingly, primary care medical records for each participant in the study were accessed in order to ascertain the details of health-professional visits (both primary/community care and other outpatient appointments), accident and emergency visits, day-case attendances and hospital admissions. Information on these variables were obtained for 2 months pre RCT and for the period of the RCT.
Additionally, at the 2-month follow-up point the participants’ carer or close relative (if applicable) was provided with a self-report questionnaire (see Appendix 15). Section 2 was designed to evaluate health-economic aspects of their caring role. Briefly, they were asked whether or not they helped the participant with organising or taking their medication. If they reported ‘yes’ to this, they were subsequently asked (1) how many times per week they helped them with organising or taking their medication, (2) the average length of time they spent on each occasion and (3) whether or not they received any payment for helping them. Carers were also asked if they provided any other type of help. If they responded ‘yes’, the same three follow-up questions (how many times, average length of time and whether or not they received payment) were also asked.
Assigning costs to items of resource use
Costs were estimated at 2011/12 financial year levels, as such values were reported in the latest edition of Curtis85 available at the time of analysis.
Intervention
Curtis85 reported the community pharmacist cost per hour of employment to be £50, which was based on an average salary of £38,000. Pharmacy technicians had an estimated salary of £23,000 and, assuming other non-salary costs are proportional to salary costs, as for a pharmacist (as reported by Curtis85), their cost per hour of employment was estimated to be £30.21. Dispensing times were thereby costed at either £50.00 or £30.21 per hour for a pharmacist or pharmacy technician, respectively. The cost of the MOD itself and delivery costs were recorded within the study (although the intervention took place post 2011/12, it was assumed that the difference between the costs incurred in the study period and 2011/12 prices was negligible). Medication costs were taken from the prescription cost analysis for England,86 which were based on Edition 62 of the British National Formulary. However, it should be noted that costs were only applied to prescribed SODF medication that was prescribed as a regular dose, as it was only these types of medication that could be used in the MOD box. All medication costs were estimated on an individual participant basis, based on individual patient-level data.
Other costs
The costs associated with health-care professional visits, accident and emergency visits and day-case attendances were taken from Curtis. 85 To cost hospital attendances, the length of stay was extracted from primary care records, and this was assigned a cost per bed-day from the National Schedule of Reference Costs. 87
With regard to carer time (either helping participants with organising or taking their medication or receiving any other type of help) the rate of average hourly earnings (in 2011)88 was applied to this time, assuming that the times applied to the whole of the 8-week period, consistent with the human capital approach. 89 However, since the costing of such care is sometimes considered controversial,90 these costs are reported separately to the aforementioned NHS costs.
Overall and incremental costs
Overall, mean costs for each of the aforementioned main cost categories [intervention costs (medication costs were reported separately) and other costs] were estimated over the 2-month intervention period. These are reported for each of the four trial groups, as well as overall (undertaken because of the small numbers), in order to provide an indication of the main cost drivers for the population group and, in particular, the proportion of overall costs that the intervention costs constitute.
Measuring outcomes
To estimate the impact on health-related quality of life, participants were asked to complete the EQ-5D89 at baseline and 2 months post randomisation. The EQ-5D has five questions, which ask the respondent to report the level of problems they have (no problems, some/moderate problems or severe/extreme problems) with regard to mobility, self-care, usual activities, pain and anxiety/depression. 89 The three-level version of the EQ-5D (EQ-5D-3L) was used. Responses to the five dimensions are converted into one of 243 different EQ-5D health state descriptions, which range between no problems in all five dimensions (11111) and severe/extreme problems in all five dimensions (33333). A utility score (a scale where death is equal to 0 and full health 1) was assigned to each of these 243 health states using the York A1 tariff91 (producing EQ-5D scores ranging from –0.594 to 1.00). The EQ-5D is accompanied by a visual analogue scale (European Quality of Life-Visual Analogue Scale, EQ-VAS), on which people are asked to indicate how good or bad their health state is (on the day they complete the questionnaire), where zero corresponds to worst imaginable health state and 100 to best imaginable health state. 89
In addition to the EQ-5D, participants were also asked to complete the ICECAP-O92 at baseline and at the 2-month follow-up. The ICECAP-O is designed to capture both quality of life and capability, for which the focus is on an individual’s own perception of their capabilities on the five attributes of attachment, security, role, enjoyment and control. 92 Each of the attributes has four levels: the most preferred state, all of . . . (e.g. the love and friendship) I want (4), a lot of . . . (3), a little of . . . (2) and none of . . . (1). 92 The best–worst scaling technique was used to assign a value to each of the capability descriptions that are ascertained from the ICECAP-O, where these range from 0 (no capability: 11111) to 1 (full capability: 44444).
Cost-effectiveness analysis
The ability to perform an analysis of cost-effectiveness is limited by the small numbers in this study. Thus, we do not seek to estimate the incremental cost and incremental effect associated with the different intervention groups, as we consider that it would be difficult to perform regression-type analyses in such circumstances. The estimated overall unadjusted mean costs and effects are, however, briefly discussed, although such estimates must be treated with caution.
Value of information analysis
When making decisions about the allocation of scarce resources two questions have been argued to be fundamental. 93,94 First, which option is estimated to be cost-effective on the basis of current evidence? Second, should further research be undertaken to reduce the level of uncertainty associated with that decision?
To answer the first question, the option with the highest incremental cost-effectiveness ratio (ICER) below the cost-effectiveness threshold is identified,93 for which dominated options (those that are more costly and less effective than another option) and those that are subject to extended dominance (combinations of other options can provide a higher level of benefit for the same cost) are excluded. 95
In relation to the second question, it has been argued96 that it is useful to plot the cost-effectiveness acceptability curve (CEAC)97 and the cost-effectiveness acceptability frontier (CEAF). 97 The CEAC depicts the probability that an option is cost-effective at different levels of the cost-effectiveness threshold. 96,97 The CEAF is equivalent to plotting the CEAC over the range of thresholds for which each option is estimated to be most cost-effective. 96,97 To answer the second question, the expected value of perfection information (EVPI) can be calculated. 94 The EVPI provides an upper estimate of the value of undertaking further research and is equivalent to the probability of making the wrong decision (shown by the CEAF) multiplied by the consequences of that wrong decision. 94,98
Here, we calculated the EVPI (for an individual patient) using the non-parametric approach outlined by Briggs et al. 94 Bootstrapping was used to produce 5000 iterations of the mean cost and effect, which are equivalent to the mean value of samples of n values drawn from the observed data, with replacement. Here the n is the number of participants in each of the four groups for whom complete cost and quality-adjusted life year (QALY) data were available. The cost variable used was the aforementioned total cost over the 2-month follow-up period from the perspective of the NHS. We used the EQ-5D data to estimate QALYs based on the area under the curve method (with adjustment for baseline differences),99 so the baseline and 2-month follow-up EQ-5D scores were used to estimate the per-patient QALY gain (or loss) which accrued over the 2-month trial period. The CEAF was also estimated, where this was equivalent to the proportion of iterations for which the optimal intervention had the highest level of net benefit at different levels of the cost-effectiveness threshold.
Results
Participants
Participant allocations and characteristics are summarised in the CONSORT diagram (see Figure 7) and baseline characteristics table (see Table 18). It should be noted that 3 of the 29 randomised participants withdrew consent prior to receiving the intervention (two from the MOD weekly arm and one from the usual supply monthly arm). After the point of withdrawal for these three participants, no further data could be collected. Thus, dispensing time data were not available for these three participants and their primary record data had also not been accessed at this point; consequently, data from these three participants are not used in the subsequent costs analyses. Additionally, one participant from the MOD weekly arm died; data from this participant are used up to the point of death. Resource use data were also available for the participant who had poor eyesight (usual supply monthly arm). Cost data were thereby available for up to seven participants in the MOD weekly arm, six in the MOD weekly arm, six in the usual supply monthly arm and seven in the usual supply weekly arm. With regard to outcomes, it was possible to use the baseline data for the 29 randomised participants (the three who withdrew completed the measures prior to withdrawing consent). Follow-up data were, however, only available for up to 26 participants. These numbers differ from those in the CONSORT, as the CONSORT is based on the primary outcome (pill count), for which levels of missing data differed from resource use and quality-of-life data (all available data were used in the economic analysis).
Intervention costs
In this study each MOD covered a 1-week period and had a cost of 40p (this cost was assigned to all types of MODs used). Two participants were admitted to hospital (where they did not use a MOD as they were not self-medicating); the remainder of those in both the MOD monthly and MOD weekly arms were provided with eight MODs in the study period. Thus, the mean number of MODs used in each of these arms was eight (the one participant admitted to hospital in this study arm was in the last week of the 2-month study period, so the cost of eight MODs was still included for that participant) and 7.36 (one of the six participants in this arm was admitted to hospital in their fifth week in the study), respectively, giving mean associated MOD costs of £3.20 and £3.00, respectively.
Table 33 summarises the direct costs associated with the four study arms. Medication delivery costs were £5 per trip, and the mean cost associated with these is also shown in Table 33. Dispensing times were recorded for 25 of the 26 participants. The mean time taken to dispense 1 month’s supply of medication (including filling the MOD, if applicable) varied between 19.50 minutes for usual supply monthly (n = 5) and 43.00 minutes for MOD weekly. Once the appropriate figures for pharmacist/pharmacist technician cost per hour of employment (Table 34) were assigned to these costs, it can be seen that (for both usual supply and MOD) mean dispensing costs were higher for those in the weekly arm than for those in the monthly arm and that mean costs in both MOD arms were higher than in the usual supply arm.
| Item | Levels of resource use (range) | Mean cost, £ (range) | ||||||
|---|---|---|---|---|---|---|---|---|
| Monthly MOD (n = 7) | Weekly MOD (n = 6) | Monthly usual supply (n = 6) | Weekly usual supply (n = 7) | Monthly MOD | Weekly MOD | Monthly usual supply | Weekly usual supply | |
| MODs supplied: intervention period | 8.0 (8–8) | 7.5 (5–8) | – | – | 3.20 (3.20–3.20) | 3.00 (2.00–3.20) | – | – |
| Delivery trips: intervention period | 1.1 (0–2) (d = 4; c = 3) | 7.5 (5–8) (d = 6; c = 0) | 0.8 (0–2) (d = 2; c = 3) | 6.9 (0–8) (d = 6; c = 1) | 5.71 (0–10) | 37.50 (25–40) | 4.00a (0–10) | 34.29 (0–40) |
| Dispensing time: intervention period (for 1 month’s supply of medication) | 38.9 minutes (25–60) | 43.0 minutes (25–90) | 19.5 minutesa (5–50) | 38.5 minutes (20–112) | 48.08 (28.48–70.32) | 54.63 (31.77–130.21) | 22.30 (3.68–63.55) | 41.82 (18.40–124.67) |
| Medication: pre-randomisation (number of prescriptions) | 7.9 (3–12) | 5.5 (4–9) | 5.7 (2–9) | 6.3 (4–9) | 70.48 (£20.61–£149.81) | 31.45 (£11.76–£104.41) | 57.66 (5.18–£226.96) | 62.51 (8.07–140.68) |
| Medication: intervention period (number of prescriptions) | 7.9 (3–13) | 5.5 (4–9) | 5.8 (2–9) | 6.6 (4–9) | 70.44 (£20.61–£148.87) | 31.45 (£11.76–£104.41) | 57.97 (5.18–226.96) | 63.85 (8.07–140.68) |
| Item | Estimated unit cost |
|---|---|
| Pharmacist (cost per hour of employment)a | £50.00 |
| Pharmacy technician (cost per hour of employment)b | £30.21 |
| GP visita (practice) | £43.00 |
| GP visita (home) | £110.00 |
| Practice nurse visita (practice) | £11.63 |
| Practice nurse visita (home) | £27.30 |
| Health-care assistanta (practice) | £6.46 |
| Outpatient visit (weighted average of all procedures)a | £139.00 |
| Accident and emergency visit (non-admitted cost used)a | £112.00 |
| Day case (weighted average of all procedures)a | £680.00 |
| Hospital admission (cost per day)c | £254.00 |
| Medication (average cost per prescription)d | £8.97 |
| Hourly pay (excluding over time) | £14.76 |
Pre-intervention, the mean number of medications per participant varied between 5.50 (MOD weekly) and 7.86 (MOD weekly), where the associated cost also varied between £31.45 and £70.48, respectively (see Table 33). Within the 2-month intervention period, four of the 26 participants had a change in their medication recorded by the pharmacist, where this was the addition of one medication for two participants, the addition of two medications for one participant and the removal of one medication for one participant. Costs/levels of resource use were only estimated for prescribed SODF medication that was prescribed as a regular dose.
Other costs
Medical records were accessed for each of the 26 participants. The mean number of primary/community care visits, outpatient visits, A&E visits, day-case attendances and hospital admissions, within each of the four groups are summarised in Table 35. Unit costs (see Table 35) were subsequently assigned to each of these items of resource use, in order to estimate the mean costs for each of these categories (see Table 35). It can be seen that many of the values are low, and this can be explained by the fact that the most common value for each item was zero. Moreover, it can be seen that one or two individuals have quite an effect on the overall mean, for example there was just one participant who was admitted to hospital in the MOD weekly arm (their length of stay was 32 days).
| Item | Mean levels of resource usea | Mean cost (£) | ||||||
|---|---|---|---|---|---|---|---|---|
| Monthly MOD (n = 7) | Weekly MOD (n = 6) | Monthly usual supply (n = 6) | Weekly usual supply (n = 7) | Monthly MOD | Weekly MOD | Monthly usual supply | Weekly usual supply | |
| Primary/community care visits: pre-randomisation | 2.0 (6, 1) | 1.0 (5, 1–3) | 1.0 (3, 1–4) | 1.4 (4, 1–6) | 42.00 | 24.73 | £50.54 | 30.11 |
| Primary/community care visits: intervention period | 1.3 (5, 1–4) | 1.8 (5, 1–3) | 4.7 (5, 1–16) | 1.3 (4, 1–5) | 23.96 | 48.38 | £84.95 | 34.40 |
| Outpatient visits: pre-randomisation | 1.4 (5, 1–3) | 0.5 (2, 1–2) | 0.8 (3, 1–3) | 0.9 (2, 2–4) | 198.57 | 69.50 | 115.83 | 119.14 |
| Outpatient visits: intervention period | 0.3 (0, –1) | 0.3 (1, 1) | 1.5 (4, 1–6) | 0.7 (4, 1–2) | 39.71 | 46.33 | 208.50 | 99.29 |
| Accident and emergency visits: pre-randomisation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.00 | 0.00 | 0.00 | 0.00 |
| Accident and emergency visits: intervention period | 0.1 (1, 1) | 0.2 (1, 1) | 0 (0–0) | 0 (0–0) | 16.00 | 18.67 | 0.00 | 0.00 |
| Day-case visits: pre-randomisation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.00 | £0.00 | 0.00 | 0.00 |
| Day-case visits: intervention period | 0.1 (1, 1) | 0 (0) | 0 (0) | 0 (0) | 97.14 | 0.00 | 0.00 | 0.00 |
| Hospital admissions (length of stay): pre-randomisation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.00 | 0.00 | 0.00 | 0.00 |
| Hospital admissions (length of stay): intervention period | 0.6 (1, 4) | 5.3 (1, 32) | 0 (0) | 0 (0) | 145.34 | 1356.54 | 0.00 | 0.00 |
Nine carer questionnaires were returned at the 2-month follow-up point, although in one of these questionnaires the person reported that they were the daughter of the participant and not a carer (they met with the participant on a weekly basis); consequently, this participant was assumed to receive no help from a carer. All of the remaining eight carers reported that they were family members, seven of whom lived with the participant. Six of the carers answered the question regarding whether or not they helped the participant with organising or taking their medication, three of whom gave the answer of ‘yes’ (one of these three reported that they received payment for this help). Two of those three were in the MOD weekly arm; of these two, the reported time providing help was once per week for 5 minutes and seven times for 3 minutes on each occasion. Assuming these times applied to the whole of the 8-week period, this would amount to 0.67 hours and 2.80 hours, respectively, and lost productivity costs of £9.84 and £41.33, respectively, when assigned the rate of average hourly earnings in 2011 (£14.76). The other carer who reported that they helped with organising or taking medication was in the MOD weekly arm and reported providing help three times per week (for 2 minutes on each occasion); this equated to a lost productivity cost of £11.81.
In order to calculate average levels of lost productivity, it was necessary to make assumptions with regard to those for whom the carer questionnaire was not returned, specifically whether or not this was because they did not have a carer (and consequently received no such help), and whether or not carer data should be treated as missing. Participants were asked to return the carer questionnaire at the same time as the EQ-5D questionnaire. Thus, we assumed that those who returned the EQ-5D questionnaire, but not the carer questionnaire, did not have a carer.
This applied to 13 participants. For the remaining participants who did not return the EQ-5D questionnaire, or who returned the carer questionnaire but did not complete the question with regard to help regarding medication (n = 2), we assumed the data were missing with regard to whether or not they had a carer and received any associated help. The estimated mean lost productivity costs associated with organising or taking medication across each of the four groups are shown in Table 36, in which the three aforementioned lost productivity values are combined with values of £0.00 for the three participants who reported that they did not provide such help, the one person who returned the carer questionnaire but reported that she was not a carer and the 13 who returned the EQ-5D questionnaire but not the carer questionnaire.
| Costs | Monthly MOD (n = 7) | Weekly MOD (n = 6) | Monthly usual supply (n = 6) | Weekly usual supply (n = 7) | Overall (n = 26) |
|---|---|---|---|---|---|
| Intervention costs (1) (£) | 57.00 (31.68–83.52) | 95.13 (65.55–173.41) | 31.42 (3.68–63.55) | 76.11 (18.40–164.67) | 65.04 (3.68–173.41) |
| Medicationa (SODF) costs (2) (£) | 70.44 (20.61–148.87) | 31.45 (11.76–104.41) | 57.97 (5.18–226.96) | 63.85 (8.07–140.68) | 56.79 (5.18–226.96) |
| Other NHS costs (3) (£) | 322.16 (6.46–1294.23) | 1469.92 (12.92–8251.21) | 293.45 (103.33–939.38) | 133.69 (0.00–317.46) | 529.66 (0.00–8251.21) |
| Total NHS costs (1 + 2 + 3) | 449.60 (94.43–1514.41) | 1596.50 (134.77–8329.24) | 382.84 (180.86–970.23) | 273.65 (97.60–513.60) | 651.49 (94.43–8329.24) |
| Lost productivityb | |||||
| Medication-related care (£) | 8.53 (6, 2) | 5.90 (2, 1) | 0.00 (4, 0) | 0.00 (7, 0) | 3.31 (19, 3) |
| Other care (£) | 93.48 (6, 2) | 275.52 (3, 1) | 0.00 (5, 0) | 68.88 (6, 1) | 90.04 (20, 4) |
Seven of the eight responding carers answered the question regarding other types of help, six of whom reported that they provided such help (two of the six reported that they received a payment for such help). One of those six failed to complete the questions regarding the frequency/length of help, and another (from the usual supply weekly arm) reported that they felt unable to answer this question as they provided help ‘all day’ (providing help 14 hours a day for 7 days a week would equate to a lost productivity cost of £11,571.84 over 2 months). The times reported by the remaining four ranged between 2.5 minutes twice a week (to remind the participant about appointments) and 1 hour 7 days a week (for personal care). The inferred lost productivity costs for these four are combined with the 15 who were assumed to have received no such help (the one carer who reported that they provided no help, the one who returned the questionnaire but reported she was not a carer and the 13 who returned the EQ-5D questionnaire but not the carer questionnaire) in Table 36 (for the purposes of this analysis the responses for the one carer who reported that they provided help ‘all day’, the carer who reported they provided help but did not report the frequency/length and the one carer who returned the questionnaire but did not answer this specific question were treated as missing data).
Overall costs
Missing data
The following assumptions were made in order to enable all 26 participants to be assigned a cost for each component cost. The one participant (from the usual supply monthly arm) for whom the method of delivery/dispensing times were not recorded had been found to have difficulties with regard to eyesight and been withdrawn from usual supply by his GP [he was instead provided with a MOD (by the NHS outwith this study)]. In line with the intention-to-treat principle, we included this participant in the analysis, in the arm to which they were originally allocated. The costs associated with the method of delivery/dispensing times for this participant were assumed to equate to the mean for the seven participants in the MOD weekly arm. Similarly, in line with all participants in the MOD weekly arm, he was assumed to have used eight MOD boxes within the study period.
When the costs associated with the intervention [MOD boxes (if applicable), dispensing and delivery (if applicable), but excluding medication] are summed together, it can be seen that these are relatively small compared with other NHS costs. Over the 26 participants, on average, they can be seen to be approximately 10% of the overall cost. In terms of the MOD intervention itself, it can be seen that the mean intervention cost for those in the MOD monthly arm is just over £20 more than that in the monthly usual-care arm, with a similar, although slightly smaller, mean difference in the intervention cost between the MOD weekly arm and usual supply weekly arm (see Table 35). There is also no evidence to suggest that the higher intervention costs are off-set by lower other NHS costs; indeed, the mean other NHS costs for both usual supply arms are lower than those for both of the MOD arms. It should, however, be noted that these costs are highly influenced by the two participants who were admitted to hospital (both of whom were in one of the MOD arms).
Outcomes
At baseline, the EQ-5D, EQ-VAS and ICECAP-O were completed by 28 of the 29 participants (see Table 37). Follow-up scores were completed by 22 of the 29 participants (84.6% response rate). Looking at the change in scores, it is difficult to draw firm conclusions, but there is little to suggest that the mean scores for those who received a MOD, whether this be on a weekly or monthly basis, were superior to those who received usual supply. Indeed, of the four groups, a usual supply arm had the highest mean change according to the EQ-5D (usual supply weekly), EQ-VAS (usual supply monthly) and ICECAP-O (usual supply weekly).
Cost-effectiveness analysis
Given the small numbers, formal cost-effectiveness analysis is considered inappropriate. There is, however, little to suggest that levels are greater for the MOD intervention, as for each of the measures, based solely on the mean unadjusted results, it can be seen that a usual supply arm dominates (has lower mean costs and higher mean effects) both of the MOD arms (see Table 36 for costs and Table 37 for outcomes).
| Quality-of-life measures | Monthly MOD (n = 7) | Weekly MOD (n = 6) | Monthly usual supply (n = 6) | Weekly usual supply (n = 7) | Overall (n = 26) |
|---|---|---|---|---|---|
| EQ-5D score | |||||
| Baseline | 0.720 (0.516 to 1) | 0.809 (0.587 to 1) | 0.763 (0.620 to 1) | 0.524 (0.055 to 0.796) | 0.698 (0.055 to 1) |
| 2-month follow-up | 0.709 (n = 6) (0.516 to –0.883) | 0.528 (n = 4)a (0.000 to 0.796) | 0.640 (n = 5) (0.159 to 1) | 0.662 (0.195 to 1) | 0.645 (n = 22) (0 to 1) |
| Change | –0.016 (n = 6) (–0.150 to 0.087) | –0.264 (n = 4)a (–0.850 to 0.000) | –0.152 (n = 5) (–0.568 to 0.150) | 0.138 (0.000 to 0.532) | –0.043 (n = 22) (–0.850 to 0.532) |
| EQ-VAS score | |||||
| Baseline | 71.43 (20–90) | 74.33 (40–98) | 73.33 (50–100) | 75.57 (40–97) | 75.57 (n = 22) (40–97) |
| 2-month follow-up | 70.00 (n = 6) (38–82) | 63.75 (n = 4) (40–80) | 80.00 (n = 5) (60–95) | 72.43 (30–97) | 71.91 (n = 22) (30–97) |
| Change | 0.50 (n = 6) (–20 to 18) | –5.75 (n = 4) (–18 to 0) | 2.00 (n = 5) (–10 to 15) | –3.14 (–45 to 10) | –1.45 (n = 22) (–45 to 18) |
| ICECAP-O score | |||||
| Baseline | 0.888 (0.794 to 0.979) | 0.834 (0.556 to 1) | 0.908 (0.745 to 1) | 0.836 (0.556 to 0.975) | 0.8636 (0.556 to 1) |
| 2-month follow-up | 0.859 (n = 6) (0.633 to 0.979) | 0.770 (n = 4) (0.441 to 0.904) | 0.920 (n = 5) (0.883 to 0.961) | 0.832 (0.567 to 0.975) | 0.848 (n = 22) (0.441 to 0.979) |
| Change | –0.017 (n = 6) (–0.271 to 0.099) | –0.008 (n = 4) (–0.115 to 0.064) | –0.020 (n = 5) (–0.085 to 0.093) | –0.004 (–0.072 to 0.047) | –0.012 (n = 22) (–0.271 to 0.099) |
Value of information analysis
We based our value of information calculations on those participants for whom complete cost and effect data were available. The resulting estimates of the within-trial mean cost and QALY gain/loss for each of the four groups are shown in Table 38. The option of usual supply weekly was optimal (had the highest ICER below the cost-effectiveness threshold) for values of λ ≥ £119 per QALY. Consequently, the CEAF (shown in Figure 9) was equal to the probability that usual supply weekly was estimated to be cost-effective for threshold values greater than this value of λ and took the value of the probability for MOD weekly for λ values < £119. The per-participant EVPI was subsequently calculated and is shown in Figure 9. The EVPI never exceeded £50 and is equivalent to an estimated value of £2.91 at λ = £20,000 per QALY (see Table 38). The EVPI is equivalent to the probability of making the wrong decision multiplied by the consequences of that wrong decision, and this low EVPI estimate can be largely explained by the fact that the probability of making the wrong decision (as depicted by the CEAF in Figure 9) was ≤ 15% for values of λ > £10,000 per QALY and ≤ 5% for values of λ > £20,000 per QALY.
| Type of data | Monthly MOD (n = 6) | Weekly MOD (n = 4) | Monthly usual supply (n = 5) | Weekly usual supply (n = 7) |
|---|---|---|---|---|
| Total NHS costs (£) (range) | 272.13 (94.43–764.49) | 2310.54 (207.73–8329.24) | 406.92 (180.86–970.23) | 273.65 (97.60–513.60) |
| QALY change | –0.001 (–0.001 to 0.013) | –0.019 (–0.059 to 0.000) | –0.013 (–0.047 to 0.013) | 0.011 (0.000 to 0.044) |
| ICER | – | Dominated by MOD weekly and usual supply weekly | Dominated by MOD weekly and usual supply weekly | £118.31 compared with MOD weekly |
| CEAC at λ = £20,000 per QALY (%) | 4.2 | 0.2 | 0.7 | 94.9 |
| Net benefit at λ = £20,000 per QALY (£) | –296.83 | 2698.20 | –656.87 | –44.76 |
FIGURE 9.
Estimates of the CEAF and EVPI at different levels of the cost-effectiveness threshold.

If one took the EVPI per-participant value of £2.91 at face value, then one would probably conclude that the potential value of undertaking further research is relatively low. However, there are a number of reasons why we consider that the EVPI estimates produced here should be treated with caution. First, although complete data were available for a large proportion of the sample (22 out of 26), with such small numbers in the sample it can be seen that the mean estimates for those with complete cost and effect data (see Table 38) are somewhat different from those for participants for whom only cost data were complete (see Table 36). Contributory factors include the fact that the participant with the highest NHS cost in the monthly MOD group failed to complete the EQ-5D, as did the participant with the lowest NHS cost in the MOD weekly group. Thus, the mean cost estimates, based on those with complete data, may underestimate for the monthly MOD group and overestimate for the MOD weekly group. Furthermore, a key assumption of bootstrapping (which was used to produce the iterations on which the mean cost and effect data are based) is that the observed data are representative of the population and that estimates of the mean of the population can therefore be made by sampling from the observed data. Given the small numbers in this study, and the fact that those who have missing data may differ from those with complete data, the above assumption is unlikely to hold and the use of bootstrapping may therefore be inappropriate. A further issue is that the mean cost and QALY estimates here were based on the within-trial period of just 2 months. As such, they may underestimate the changes in lifetime costs and effects (we thought it too speculative to produce such estimates based on the data available to us) and the resulting estimates of the consequences of making the wrong decisions which is used in the EVPI calculations. In the light of the above, we consider that the value of information results produced here should be treated with caution. Indeed, similar to other pilot studies that have included an economic component, for example Clarke et al. ,100 we would prefer to concentrate on the completion rates for the different measures (to assess whether or not it would be feasible to use these measures within any subsequent definitive trial).
Summary
Within this chapter we have summarised the data on resource use, quality of life (EQ-5D) and capability (ICECAP-O). Resource use data were available from medical records for all 26 participants for whom it was requested, compared with 22 (84.6%) for quality-of-life and capability data. The feasibility of collecting health economic data to assess the cost-effectiveness of MODs in a subsequent definitive study was thereby demonstrated. A relatively low response rate was, however, obtained regarding carer input, where it was sometimes unclear whether this was because of non-response or because the participant did not have a carer (in a future study participants might therefore be asked to report whether or not they have a carer when recruited and follow-up questionnaires can then be tailored accordingly). The completion rate for both the EQ-5D and ICECAP-O was identical. The two instruments measure different concepts (see Brooks89 and Coast et al. 92); thus, we recommend that both are used in any subsequent definitive study. However, in the light of the development of a five-level version of the EQ-5D (EQ-5D-5L101) and an associated valuation mechanism102 (which was not available at the start of this study), we would recommend that the EQ-5D-5L is used in any subsequent definitive study. Additionally, as MODs could well be used beyond the 2-month follow-up period in this study, it is also recommended that any future definitive study considers the addition of a long-term model to extrapolate the costs and benefits beyond the trial period.
Chapter 8 Discussion
This feasibility study of MODs compared with usual medication packaging has identified refinements in the study design essential for a successful definitive study and has offered an early indication of the effects of MODs. The systematic review has supplemented the findings of Mahtani et al. 39 Their systematic review of MOD RCTs yielded a small number of studies which did not report all potential effects of MODs such as impact on social and psychological well-being. 7 It also included studies which had components additional to the MOD as part of a complex intervention to improve adherence. Their review concluded that MODs may increase adherence and improve clinical outcomes in selected conditions but that more research was needed to inform NHS decision-making.
This systematic review was based on a broader search than previous reviews, including a wide range of databases, inclusive search terms, searching reference lists of retrieved papers and hand-searching. It included all study designs and focused on interventions which only included a MOD (i.e. no other component such as patient education or additional separate reminders) with the intent of capturing those studies excluded by Mahtani et al. 39 and relevant to the aim of determining the independent effects of MODs. This approach identified that MOD studies have a range of objectives, study designs, populations and outcomes and thus preclude meta-analysis.
The studies identified in the present systematic review were largely of poor quality in terms of methodological rigour. For the outcomes of adherence, clinical improvement, health-care utilisation, processing time and costs there were studies suggesting both that MODs were beneficial compared with standard care and the converse. No study included data on all of these outcomes, so triangulation of observed effects was not possible. Inadequate study reporting prevented comprehensive risk of bias assessment, in particular when pre-study protocols were unavailable it was not possible to determine whether or not studies reported all intended outcomes. Contacting authors to supplement the published information may have reduced the gaps in the evidence synthesis. Finally, measurement of adherence was largely dependent on pill counts, and it was unclear whether or not there had been screening of patients to only include those with unintentional non-adherence.
Uncertainty remains regarding the effect of MODs. Of the eight studies reporting adherence, half suggested this was improved with the use of a MOD. However, only one of these studies reported a clinical outcome (a prospective cohort study showing improved viral load), so while it might be assumed improved adherence leads to better clinical outcomes, there is little direct evidence. Similarly, the three studies reporting effects on health-care utilisation reported neither clinical outcomes nor adherence. One study suggested a MOD was associated with a small increase in GP visits; however, another reported decreased visits to doctors but increased hospitalisations. This study also reported decreased AEs. The tentative conclusion is therefore that MODs might increase adherence, however, depending on the clinical condition and therapeutic window this may not be enough to improve outcomes. No study reported any humanistic outcomes such as quality of life. There is, therefore, a need for studies that capture all potential MOD effects while maintaining rigorous trial design. With eight relevant studies published during the previous 12 years, a systematic review update is recommended in 6 years.
The feasibility study was designed from a composite of a priori technique, literature review and refinement through consultation with patients and health-care professionals. Most of the methods proposed by researchers were acceptable to patients and health-care professionals. The refinements suggested emphasise the importance of involving relevant stakeholders in the design stage of a project. For example, it was established at an early stage that storage of excess patient medication at the participating pharmacies was unacceptable because of space, legal and ethical issues and thus it was possible for an alternative strategy to be devised.
There was less clarity regarding the minimum age for recruiting patients to the study, with the suggested minimum age ranging from 50 years by health-care professionals to 75 years which is indicated as ‘old age’ in many national guidelines. Furthermore, pharmacists indicated that from their experience, the frequency with which MODs are initiated increases from the age of 70 years and that this coincides with increasing polypharmacy. Maintaining the minimum recruitment age at 75 years as initially proposed resulted in over one-third of patients being ineligible for study participation because they already used a MOD. It is clear, therefore, that a subsequent study must recruit from a lower age band in order to fully capture the population that is initiated on MODs through usual care. The ethical restriction of being unable to recruit patients already receiving a MOD means that patients who were most likely to demonstrate benefit may have been excluded. It is therefore likely that the results of this feasibility study and any definitive trial would provide a conservative estimate of any MOD benefits.
Recruitment was lower than anticipated, with significant attrition in potentially eligible patients occurring at two stages: patients needing to be prescribed medicines from a limited list for which EAM was intended and patients not already using a MOD. Prior to a definitive RCT, a universal EAM system must therefore be developed and validated in order to prevent attrition of potential participants owing to requirements that certain medicines are prescribed. A lower age band for recruitment is also necessary so that patients identified as unintentionally non-adherent can be recruited prior to a MOD being initiated as a result of usual-care processes.
As expected, the active recruitment approach achieved a much higher consent rate than the passive approach. However, the time taken to recruit these participants and thus increased cost was not off-set by the increased success rate. The data from this feasibility study unequivocally indicate that the passive approach is most cost-effective for recruiting this population to a study of MODs.
The consent rate of just under 20% from the passive recruitment process was lower than the anticipated 30%. 16,34 The primary motivations for trial participation by older people are altruism, social engagement and anticipated personal benefits. 103–105 These mirror precisely the findings of the pre-study focus groups and post-study discussions with participants. Participants cited study involvement as an opportunity to payback the NHS, and with 57% of participants in the MOD RCT group remaining on MODs and 29% of the usual-care group being initiated into MODs post-study completion, there is a clear element of personal benefit. Given that discussion could only be held with patients that consented, determining the reasons for non-consent are challenging. Previous research has cited inconvenience/burden of trial involvement and risk of harm. 103–105 The nature of the intervention is such that potential participants are unlikely to have perceived it as a high risk of harm. It therefore follows that the trial processes were considered a burden that outweighed the potential benefits. The study design focus groups did not give rise to any concerns regarding the proposed burden to participants; however, this attitude may not have translated to the wider population, as the more motivated patients will consent to focus group participation.
The crossover design proposed during the pre-study focus groups with health-care professionals was considered appropriate, as it would allow measurement of both within- and between-patient variation plus equivalent power with fewer than half the number of participants. No significant carryover effect with MOD intervention was anticipated and therefore a washout period was not necessary. However, given that such a large proportion of intervention participants remained on a MOD post study completion, ethical constraints or high dropout rates because patients do not wish to revert to usual care may prevent a crossover design. The motivation of health-care professionals to participate in the research was to help identify whether or not the expensive process of providing MODs is cost-effective.
The proportion of patient participants reporting at least one questionnaire item response associated with intentional non-adherence is comparable to previous reports of the prevalence of intentional non-adherence106 and thus offers some confidence in the screening approach for this study. The reported satisfaction by participants allocated MODs further reinforces that MODs were not provided to patients intentionally not adhering to their prescribed medication.
The prevalence of unintentional non-adherence at 46.1% is in accordance with previous studies examining a similar population using DUC. 15 This affords some confidence in the use of DUC for screening purposes. The counting of tablets at the end of the 30-week period enables the participant’s usual packaging to remain unchanged, which may have minimised the mere-measures effect of changing behaviour simply through its measurement. 107 Furthermore, as patients demonstrating characteristics associated with intentional non-adherence were excluded from trial participation, intentional alteration of the number of remaining dosage units by the participant is less likely.
The participants who were randomised were evenly matched across the randomisation groups and, with multiple comorbidities plus polypharmacy, they reflected the population that is usually in receipt of a MOD. 108 The vision of the majority of participants posed no restriction in terms of reading medication instruction labels either generated by the pharmacy or on the MODs. Although there was no intention to exclude participants based on results from functional ability, researchers did exclude a participant with severely impaired vision which had not been suitably corrected. The study procedures were that researchers would ensure that participants were able to safely use whichever medication supply procedure they had been allocated. The participant in question was randomised to the usual-care group but researchers had identified that he was unable to manage usual care effectively, as, during DUC, incorrect blisters were found inside his pill boxes. It may be more appropriate for a subsequent study to exclude based on visual ability prior to randomisation in order to prevent bias. This approach was not initially adopted in order to reduce the burden to participants of being assessed for multiple medication supply methods of which only one would be implemented. Despite targeting an older population with an average age exceeding 75 years, no participants had impaired cognitive function.
Relative to normative values, the majority of the study participants had poorer manual dexterity than expected for the over-71-years age group. However, studies of this age group have reported large variations in ability and therefore a larger study may produce average results more comparable with normative values. Despite the poorer than average dexterity, no patients reported difficulty in physically manipulating any of the MODs trialled via their satisfaction surveys. However, post-RCT focus groups did provide a comment from one participant indicating that she had experienced some difficulty. This supports recent evidence provided by Adams et al. 69 which demonstrated that the ease with which patients are able to use different MODs varies. Adams et al. 69 investigated patient ability to use MODs using a scale on which participants rated MODs according to various criteria which may have provided greater sensitivity for identifying variations in ease of using the MOD than the methods adopted in the present study. A definitive study may therefore be enhanced by use of the Adams et al. tool.
Given that all participants had been screened and identified within a 3-week DUC period to be non-adherent, there was an unexpectedly high level of adherence post randomisation. This is counterintuitive given that the monitoring occurred over a longer time period and, thus ,the expectation is that there will be a decline in adherence as patients return to their usual medication-taking behaviour, which is likely to be more frequent dose omissions. This increased adherence may be attributable to more pronounced Hawthorne effects post randomisation compared with the screening stage. 107 This hypothesis is upheld by post-trial discussions with study participants identifying that they altered their behaviour during trial participation in order to achieve greater adherence than their usual behaviour. An extended monitoring duration of 1 year with a 2-month bedding in period is recommended for a definitive study to overcome the Hawthorne effect.
Although a number of EAM systems were identified during the developmental stage of this feasibility study, no working EAM system suitable for multiple size and shaped medication packaging was available within the study period. Further work is therefore essential in order to ensure that the outcome measure of adherence is assessed using an objective methodological approach which induces minimal Hawthorne effects. A study to develop and test an EAM system that is effective and acceptable for use in adherence studies is, therefore, required.
The study appeared to be acceptable to recruited patients, as attrition in those agreeing to take part in the RCT was entirely a result of hospital admission. The post-study discussions did, however, identify that some experienced difficulties with the process of medications being delivered. The strategy for all medication supplies to be delivered for those allocated to weekly supplies was adopted to prevent the burden from increased supply frequency negatively impacting on patient participants. However, some participants were anxious about running out of medication before receiving the next delivery and were inconvenienced by waiting at home for medication to be delivered. It would, therefore, be more appropriate in a subsequent study to offer participants the option of delivery or collection and establish this information prior to randomisation so that allocation could be stratified accordingly.
The health-care professional post-study discussion identified no problems with the study design with the exception of one participating pharmacy that felt that communication from trial team members to both patients and health-care professionals could be improved. Despite a well-attended training session, a summary flow chart of procedures, each pharmacy being visited by the trial manager and an invitation to contact a member of the trial team if any problems or questions arose, there were some deviations from the SOPs by one participating pharmacy. The two deviations of note were that the pharmacy dispensed several months of prescriptions at once as it believed this was a study requirement. The result was a high burden to the dispenser and minimal storage space. This may be overcome by including this stage into the data collection form so that the people dispensing are alerted to the trial requirements and complete contemporaneous records indicating that they have adhered to the SOP. The second deviation was that the medication packaging returned by trial participants to the pharmacy were discarded; entering this information was already a requirement of the data collection form. Even with EAM systems it is likely that the return of medication packaging which will carry the data logger will be necessary. As both of the above SOP deviations were made by one of the eight pharmacies to which patients were randomised and not endorsed by the other pharmacies, the implications for a future study are perhaps less relevant. The same pharmacy also reported that the end-of-trial procedures had been poorly communicated to patients and pharmacies. The consequences of patients wishing to continue to receive their medicines in a MOD had been carefully considered by the trial team at the design phase. The strategy implemented for management of such cases was that pharmacies refer patients to a locally funded service where, after assessment of need, MODs are provided to eligible patients at no personal cost. The opinions expressed by this pharmacy were not reflected in the patient focus group and thus no major changes are proposed for a definitive study.
The high response rates to the economic measures requiring patient participant completion suggest that these were acceptable and the post-study discussions with participants do not offer any contradictory information. The magnitude of success with the carer-completed questionnaire is, however, less clear, as baseline data regarding the presence of a carer and the nature of their involvement in supporting the participant in medication taking were not collected. The result is that the extent to which the completed carer questionnaires represent the participants who had carers cannot be determined. A definitive study must therefore collect these baseline data, which will also enable follow-up questionnaires to be sent to any non-responding carers to optimise response rate. Collection of economic outcomes was well executed by pharmacies and the post-study discussions suggest that the process was acceptable.
Economic data collection from GP-held records by the research team was resource intensive but successfully executed. Processes are available to expedite extraction of the necessary information from GP software and it may therefore be more cost-effective in a definitive study to allow sufficient time for the research team to be appropriately trained in the use of the software to enable rapid data extraction for a much larger number of participants. Collection of social service data was novel to this feasibility study of MODs, as demonstrated by the systematic review. A considerable amount of time was taken working with social services to develop the process for data collection with a successful outcome.
As a feasibility study, this study was not powered to detect differences in the primary outcome; therefore, no significant differences were identified between supply frequency of monthly versus weekly and MODs versus usual-care packaging. The findings have provided estimates of adherence magnitude and the variance around this outcome for each study arm. This is consistent with expected feasibility study outcome. A definitive study should therefore consider supply frequency in addition to MOD and usual-care packaging. The early-stage cost-effectiveness analysis of the MOD was similarly heavily compromised by the small sample size and large variation in costs between participants, thus even a tentative estimation of cost-effectiveness is inappropriate. However, this study has established the feasibility of determination of the effectiveness and cost-effectiveness of MODs.
This was not a clinical trial of an investigational medicinal product; only the SODFs usually prescribed to patients were provided in MODs. The study was not therefore designed to capture AEs. However, 38% of participants randomised to MODs experienced AEs compared with none in the usual packaging group. While a definitive causal link between the MOD and the AE cannot be made, the relationship is probable. All of the participants experiencing an AE were prescribed at least one medicine which may contribute to a fall. All study participants had been confirmed as being previously unintentionally non-adherent and the use of the MODs may, therefore, have resulted in an increased dose of the prescribed medicine and thereby increased risk of iatrogenic events. 109
The findings of this study are in agreement with others designed to promote adherence which have reported increased mortality and health-care costs for participants receiving the intervention. 110,111 It is intuitive that increasing adherence to a medication without reflecting on the implications of an increased dosage may result in an AE. Furthermore, as the majority of AEs were associated with falls, a definitive study should capture and stratify participant allocation according to risk factors for falls and the frequency of falls 1 year prior to randomisation. As MODs are already used in practice, a trial to test their safety is urgently required.
Other outcomes explored were risk of dispensing error and effect on patient-perceived autonomy. Information regarding dispensing errors made by pharmacies is extremely sensitive, as under current UK legislation a dispensing error is a criminal offence. No attempt was therefore made to collect dispensing error information from pharmacies. The process of decriminalising dispensing errors is being undertaken in the UK and therefore this obstacle will soon be removed. Many pharmacies routinely collect data regarding dispensing errors that are identified before they reach the patient and are often termed a ‘near miss’. It was therefore planned that this near-miss data would be collected from pharmacies and compared between the different randomisation groups in order to estimate whether or not dispensing into MODs poses an increased risk of dispensing error. Liaison with representatives from the participating pharmacies at a very early stage identified that near-miss data are also considered commercially very sensitive information. The reported frequency of near misses is considerably lower than previous reports which may indicate reporting bias. 112 To avoid compromising the validity of error data, appropriate measures must be implemented to ensure and assure pharmacy participants of confidentiality.
The MOD weekly arm reported slightly greater confidence in ability to self-manage their medication; however, as indicated previously, the small sample size prohibits any firm conclusions from being drawn. There were no other observable differences between the study arms. The pre-study focus groups and post-study discussion with participants did, however, raise the issue of many medicines dispensed in MODs being indistinguishable to patients, and thus patients have to trust that the medication has been correctly dispensed. This anxiety generated from not being able to check that the correct medication has been dispensed was not reflected in participant responses to the self-report question that related to anxiety about taking medication correctly. Refinement of the wording of this questionnaire is therefore necessary in order to elicit a valid response.
The participant completion rate for economic measures of outcome was in agreement with that of other pilot studies. 100 This was also the case for the intervention and other NHS costs; however, there were considerable research costs associated with the collection of data from primary care. The low response to the carer questionnaire needs to be addressed in a definitive study. It was unclear whether this was because the participant had no carer or because the carer elected not to respond. Any future study, when completing baseline measures, should ask the participant whether or not they have a carer and the carer elements should be combined with the participant questionnaire.
Owing to the low patient numbers, interpretations should be treated with caution. The relatively low incremental cost of approximately £20 associated with the MOD intervention weekly and monthly relative to usual care weekly and monthly, respectively, is small in relation to other NHS costs. It constitutes, on average, approximately 10% of the total NHS costs; however, looking at both the mean total NHS costs and the economic outcomes (both unadjusted for differences between groups) there was little evidence to suggest the MOD intervention was cost-effective.
The main limitations of this study were the small sample size and exclusion of patients already using a MOD and not prescribed at least two medicines from a pre-defined list. The results of this feasibility study may not therefore be replicated in a more generalisable sample. One strength in terms of economic data collection was that dispensing times were recorded for 25 of the 26 participants, which would suggest that it would be feasible for pharmacists to collect such data in any future study (even if it is just for a sample of the MOD/usual supply dispensing that is undertaken). However, within this study pharmacies were provided with a set-up fee of £175 and a further £75 for each randomised participant – without such fees it is unclear whether or not such high completion rates would have been achieved. Such fees, which did not differ across arms, were not incorporated into the analyses, as it was considered that pharmacists would not receive such a payment if the MOD intervention were rolled out across the NHS.
Summary of implications for practice
-
Medication organisation devices and other medication management strategies are being used by many older people without this information being recorded in their medical notes. It may be appropriate for GPs to discuss and record a patient’s medication management strategies during routine medication reviews in order to maintain a complete picture.
-
The prevalence of patients potentially choosing not to adhere to their prescribed medication is a reminder to practitioners to regularly discuss with patients their thoughts and attitudes towards their prescribed medication and adopt a concordant approach to prescribing.
-
It is intuitive that increasing adherence to a medication may result in a dose-related AE. It may therefore be appropriate to review the prescribed dose of all medication prior to introduction of an adherence enhancing intervention such as a MOD. Particular attention should be applied to medication associated with dose-related AEs such as antihypertensive and oral hypoglycaemic agents. Patients should also be closely monitored after introduction of the intervention to ensure that treatment is optimised.
Summary of recommendations for further research
-
The risk of AEs associated with MOD initiation must be examined and prioritised over estimating its effect on other outcomes. If a relationship is identified, strategies to mitigate this effect, such as dose reduction prior to initiation with subsequent up-titration, should be explored.
-
An EAM system compatible with MODs and usual-care packaging is required to enable comparison of medication adherence to MODs compared with usual care.
-
A definitive study using EAM systems is required to determine the effect of MODs on adherence compared with usual care to inform policy on whether or not to provide MODs and if so, to whom:
-
Targeting the older population at risk of unintentional medication non-adherence requires a lower age band of 60 years for recruitment to capture patients before a MOD is initiated through routine clinical care.
-
Acknowledgements
We are extremely grateful to the participants who made this study possible. We are also grateful to staff at the GP practices and pharmacies recruited to the study. We are indebted to local clinical commissioning groups, PCRN and Patient and Public Involvement in research (PPIres) groups for their support, particularly Roberta Aldred. We thank the research team, Trish Boyton and Estelle Payerne, for day-to-day research activities. We also thank Laura Cawley, Trish Boyton and Estelle Payerne for search development and review of systematic review articles; Dr Tracey Sach for study design with respect to health economics; and Dr Helen May, Consultant in Medicines for Older people, for invaluable help and advice. Final thanks go to the trial management and steering committee members for their expertise and support.
The trial was registered with the International Standard Randomised Controlled Trial Register (ref. ISRCTN 30626972) and the UK Clinical Research Network (ref. UKCRN 12739).
Contribution of authors
Debi Bhattacharya led on the design of the study in response to the commissioned call and took overall responsibility for the conduct of the study.
Debi Bhattacharya, Clare F Aldus and Sathon Boonyaprapa further refined the study.
Clare F Aldus was responsible for day-to-day trial management.
Debi Bhattacharya and Clare F Aldus were involved in all stages of data analysis; Christine M Bond and Steve Watson led on Chapter 2, Sathon Boonyaprapa and Charlotte Salter led on the qualitative analysis of Chapters 3 and 5, Lee Shepstone led on Chapter 4 and Garry Barton on Chapters 6 and 7. All authors were involved in data interpretation, drafting the report and have approved the final version.
Data sharing statement
Data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Bhattacharya D, Wright DJ, Purvis JR, Corlett AJ. Pharmacist Domiciliary Visiting: Derivation of a Viable Service Model 2003.
- Huang H-Y, Maguire MG, Miller ER, Appel LJ. Impact of pill organizers and blister packs on adherence to pill taking in two vitamin supplementation trials. Am J Epidemiol 2000;152:780-7. http://dx.doi.org/10.1093/aje/152.8.780.
- Nunney JM, Raynor DK. How are multi-compartment compliance aids used in primary care?. Pharm J 2001;267:784-9. http://dx.doi.org/10.2165/11587180-000000000-00000.
- National Service Framework for Older People. London: Department of Health; 2001.
- Equality Act 2010. London: The Stationery Office; 2010.
- Bhattacharya D. Literature Review of Indications for Monitored Dosage System Provision 2005. www.primarycarecontracting.nhs.uk/98.php (accessed 28 August 2007).
- Heneghan CJ, Gasziou P, Perera R. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database Syst Rev 2006;1.
- Kendrick R, Bayne JR. Compliance with prescribed medication by elderly patients. Can Med Assoc J 1982;127:961-2.
- Hulka BS. Medication use and misuse: physician-patient discrepancies. J Chronic Dis 1975;28:7-21. http://dx.doi.org/10.1016/0021-9681(75)90045-4.
- Sung JCY, Nichol MB, Venturini F, Bailey KL, Cody M. Factors affecting patient compliance with antihyperlipidemic medications in an HMO population. Am J Managed Care 1998;4:1421-30.
- Paes AHP, Baker A, Soe-Agnie CJ. Impact of dosage frequency on patient compliance. Diabetes Care 1997;20:1512-17. http://dx.doi.org/10.2337/diacare.20.10.1512.
- Cramer J, Vachon L, Desforges C, Sussman NM. Dose frequency and dose interval compliance with multiple antiepileptic medications during a controlled clinical trial. Epilepsia 1995;36:1111-17. http://dx.doi.org/10.1111/j.1528-1157.1995.tb00469.x.
- Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother 2004;38:303-12. http://dx.doi.org/10.1345/aph.1D252.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999;47:555-67. http://dx.doi.org/10.1016/S0022-3999(99)00057-4.
- Bhattacharya D, Wright DJ, Purvis JR, Corlett AJ. Non-adherence in older people; intentional or unintentional?. Int J Pharm Pract 2004;12.
- Desborough J, Bhattacharya D, Wright D, Holland R. The REPLY trial: what effect do different recipients have on patient reported adherence and satisfaction with the medication aspects of general practice consultations?. Int J Pharm Pract 2006;14.
- Atkins L, Fallowfield L. Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer 2006;42:2271-6. http://dx.doi.org/10.1016/j.ejca.2006.03.004.
- Cooper JK, Love DW, Raffoul PR. Intentional prescription nonadherence (noncompliance) by the elderly. Am Geriatr Soc 1982;30:329-33. http://dx.doi.org/10.1111/j.1532-5415.1982.tb05623.x.
- Conroy S, Cooper N. Definition of Older People in the Context of Medicine for Older People 2010. www.bgs.org.uk/index.php?option = com_content&view = article&id = 44:gpgacutecare&catid = 12:goodpractice&Itemid = 106 (accessed 17 March 2011).
- Atkin PA, Finnegan TP, Ogle SJ, Shenfield GM. Functional ability of patients to manage medication packaging: a survey of geriatric in-patients. Age Ageing 1994;23:113-16. http://dx.doi.org/10.1093/ageing/23.2.113.
- Walker R. Which medication compliance device?. Pharm J 1992;249:605-7. http://dx.doi.org/10.1186/1471-2466-13-72.
- Patel NC, Crismon ML, Miller AL, Johnsrud MT. Drug adherence: effects of decreased visit frequency on adherence to clozapine therapy. Pharmacotherapy 2005;25:1242-7. http://dx.doi.org/10.1592/phco.2005.25.9.1242.
- Dlugos DJ, Scattergood TM, Ferraro TN, Berrettinni WH, Buono RJ. Recruitment rates and fear of phlebotomy in pediatric patients in a genetic study of epilepsy. Epilepsy Behav 2005;6:444-6. http://dx.doi.org/10.1016/j.yebeh.2005.01.014.
- Nuesch R, Schroeder K, Dieterle T, Martina B, Battegay E. Relation between insufficient response to antihypertensive treatment and poor compliance with treatment: a prospective case–control study. BMJ 2001;323:142-6. http://dx.doi.org/10.1136/bmj.323.7305.142.
- Hamilton GA. Measuring adherence in a hypertension clinical trial. Eur J Cardiovasc Nurs 2003;2:219-28. http://dx.doi.org/10.1016/S1474-5151(03)00058-6.
- Diiorio C, McCarty F, Resnicow K, McDonnell Holstad M, Soet J, Yeager K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care 2008;20:273-83. http://dx.doi.org/10.1080/09540120701593489.
- Jekle C, Kramer I. OtCM (Objective therapy Compliance Measurement): smart blister packages for measuring patient compliance. Hosp Pharm Eur 2008;40:47-50.
- Litt IF, Cuskey WR, Rosenberg A. Role of self-esteem and autonomy in determining medication compliance among adolescents with juvenile rheumatoid arthritis. Pediatrics 1982;69:15-7.
- McCrea J, Ranelli P, Boyce E, Erwin W. Preliminary study of autonomy as a factor influencing medication-taking by elderly patients. Am J Health Syst Pharm 1993;50:296-8.
- Rai GS, Gluck T, Wientjes HJ, Rai SGS. The Functional Autonomy Measurement System (SMAF): a measure of functional change with rehabilitation. Arch Gerontol Geriatr 1996;22:81-5. http://dx.doi.org/10.1016/0167-4943(95)00680-X.
- Levings B, Szep S, Helps S. Towards the safer use of dosettes. J Qual Clin Pract 1999;19:69-72. http://dx.doi.org/10.1046/j.1440-1762.1999.00303.x.
- Carruthers A, Naughton K, Mallarkey G. Accuracy of packaging of dose administration aids in regional aged care facilities in the Hunter area of New South Wales. Med J Aust 2008;188:280-2.
- Flynn EA, Barker KN, Carnahan BJ. National observational study of prescription dispensing accuracy and safety in 50 pharmacies. J Am Pharm Assoc 2003;43:191-200. http://dx.doi.org/10.1331/108658003321480731.
- Junghans C, Feder G, Hemingway H, Timmis A, Jones M. Recruiting patients to medical research: double blind randomised trial of ‘opt-in’ versus ‘opt-out’ strategies. BMJ 2005;331. http://dx.doi.org/10.1136/bmj.38583.625613.AE.
- Nazemi H, Abrahamson Larkin A, Sullivan MD, Katon W. Methodological issues in the recruitment of primary care patients with depression. Int J Psychiatry Med 2001;31:277-88. http://dx.doi.org/10.2190/Q8BW-RAA7-F2H3-19BF.
- Csipke E, Serfaty M, Buszewicz M. Optimizing recruitment from primary care: methods of recruiting older people with depression. Prim Health Care Res Dev 2006;7:116-23. http://dx.doi.org/10.1191/1463423606pc283oa.
- Holland R, Lenaghan E, Harvey I, Smith R, Shepstone L, Lipp A, et al. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. Br Med J 2005;330. http://dx.doi.org/10.1136/bmj.38338.674583.AE.
- Holland R, Brooksby I, Lenaghan E, Ashton K, Hay L, Smith R, et al. Effectiveness of visits from community pharmacists for patients with heart failure: HeartMed randomised controlled trial. BMJ 2007;334. http://dx.doi.org/10.1136/bmj.39164.568183.AE.
- Mahtani KR, Heneghan CJ, Glasziou PP, Perera R. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database Syst Rev 2011;9. http://dx.doi.org/10.1002/14651858.cd005025.pub3.
- Becker LA, Glanz K, Sobel E, Mossey J, Zinn SL, Knott KA. A randomized trial of special packaging of antihypertensive medications. J Fam Pract 1986;22:357-61.
- Crome P, Curl B, Boswell M, Corless D, Lewis RR. Assessment of a new calendar pack – the ‘C-Pak’. Age Ageing 1982;11:275-9. http://dx.doi.org/10.1093/ageing/11.4.275.
- Feetam C, Kelly D. An assessment of a new compliance aid: the Medidos. Br J Pharm Pract 1982;4:5-12.
- MacIntosh P, Pond G, Pond B, Leung V, Siu L. A comparison of patient adherence and preference of packaging method for oral anticancer agents using conventional pill bottles versus daily pill boxes. Eur J Cancer Care 2007;16:380-6. http://dx.doi.org/10.1111/j.1365-2354.2006.00758.x.
- McElnay J, Thompson J. Dispensing of medicines in compliance packs. Int Pharm J 1992;6:10-4.
- Petersen ML, Wang Y, van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: a marginal structural model analysis. Clin Infect Dis 2007;45:908-15. http://dx.doi.org/10.1086/521250.
- Rehder TL, McCoy LK, Blackwell B, Whitehead W, Robinson A. Improving medication compliance by counseling and special prescription container. Am J Hosp Pharm 1980;37:379-85.
- Anderson R. New MRC guidance on evaluating complex interventions. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1937.
- Ryan-Woolley BM, Rees JA. Initializing concordance in frail elderly patients via a medicines organizer. Ann Pharmacother 2005;39:834-9. http://dx.doi.org/10.1345/aph.1E148.
- Simmons D, Upjohn M, Gamble GD. Can medication packaging improve glycemic control and blood pressure in 2 diabetes? Results from a randomized controlled trial. Diabetes Care 2000;23:153-6. http://dx.doi.org/10.2337/diacare.23.2.153.
- Skaer TL, Sclar DA, Markowski DJ, Won JK. Effect of value-added utilities on prescription refill compliance and health care expenditures for hypertension. J Hum Hypertens 1993;7:515-18.
- Stewart D, Ogilvie E, Kennedy E, Hansford D, Neil E. Medication compliance devices in primary care: activities of community-based nurses. Int J Pharm Pract 2001;2:91-5. http://dx.doi.org/10.1111/j.2042-7174.2001.tb01036.x.
- Wong BS, Norman DC. Evaluation of a novel medication aid, the calendar blister-pak, and its effect on drug compliance in a geriatric outpatient clinic. J Am Geriatr Soc 1987;35:21-6. http://dx.doi.org/10.1111/j.1532-5415.1987.tb01314.x.
- Roberts M. Effectiveness and Cost Effectiveness of Dose Administration Aids (DAAs). Brisbane: Pharmacy Guild of Australia; 2004.
- Church C, Smith J. How stable are medicines moved from original packs into compliance aids?. Pharm J 2006;276:75-81.
- NIHR Evaluation, Trials and Studies Coordinating Centre . Definition of Pilot and Feasibility Studies n.d. www.nets.nihr.ac.uk/glossary?result_1655_result_page = P (accessed 4 November 2013).
- Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol 2010;10. http://dx.doi.org/10.1186/1471-2288-10-67.
- Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts: treatments for myocardial infarction. JAMA 1992;268:240-8. http://dx.doi.org/10.1001/jama.1992.03490020088036.
- Oxman AD, Guyatt GH. The science of reviewing research. Ann N Y Acad Sci 1993;703:125-33. http://dx.doi.org/10.1111/j.1749-6632.1993.tb26342.x.
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Cooper C, et al. Process evaluation in complex public health intervention studies: the need for guidance. J Epidemiol Community Health 2013;68:101-2. http://dx.doi.org/10.1136/jech-2013-202869.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002889.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67-74. http://dx.doi.org/10.1097/00005650-198601000-00007.
- Watson S. Development of a Novel Validated Tool for Predicting Patient Adherence to Prescribed Medication. Norwich: University of East Anglia; 2013.
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-14. http://dx.doi.org/10.1212/WNL.43.11.2412-a.
- Parkington JE, Leiter RG. Partington’s Pathway Test. Psychol Serv Cent Bull 1949;1:9-20.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Aevarsson O, Skoog I. A longitudinal population study of the mini-mental state examination in the very old: relation to dementia and education. Dement Geriatr Cogn Disord 2000;11:166-75. http://dx.doi.org/10.1159/000017231.
- Desrosiers J, Hebert R, Bravo G, Dutil E. The Purdue Pegboard Test: normative data for people aged 60 and over. Disabil Rehabil 1995;17:217-24. http://dx.doi.org/10.3109/09638289509166638.
- Schmidt SL, Rosinda MO, Rocha FR, Abreu-Villaca Y. Influences of handedness and gender on the Grooved Pegboard Test. Brain Cognition 2000;44:445-54. http://dx.doi.org/10.1006/brcg.1999.1204.
- Adams R, May H, Swift L, Bhattacharya D. Do older patients find multi-compartment medication devices easy to use and which are the easiest?. Age Ageing 2013;42:715-20. http://dx.doi.org/10.1093/ageing/aft113.
- Wang YC, Magasi SR, Bohannon RW, Reuben DB, McCreath HE, Bubela DJ, et al. Assessing dexterity function: a comparison of two alternatives for the NIH Toolbox. J Hand Ther 2011;24:313-20. http://dx.doi.org/10.1016/j.jht.2011.05.001.
- Bailey IL, Lovie JE. New design principles for visual acuity letter charts. Am J Optom Physiol Opt 1976;53:740-5. http://dx.doi.org/10.1097/00006324-197611000-00006.
- Improving Patient Outcomes; The Better Use of Multi-Compartment Compliance Aids. London: Royal Pharmaceutical Society; 2013.
- Liu H, Golin C, Miller L, Hays R, Beck C, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med 2001;134:968-77. http://dx.doi.org/10.7326/0003-4819-134-10-200105150-00011.
- Wetzels G, Nelemans P, Schouten J, van Wijk B, Prins M. All that glisters is not gold: a comparison of electronic monitoring versus filled prescriptions – an observational study. BMC Health Serv Res 2006;6. http://dx.doi.org/10.1186/1472-6963-6-8.
- Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002360.
- Bornstein RA. Normative data on selected neuropsychological measures from a nonclinical sample. J Clin Psychol 1985;41:651-9. http://dx.doi.org/10.1002/1097-4679(198509)41:5%3C651::AID-JCLP2270410511%3E3.0.CO;2-C.
- Heath KV, Singer J, O’Shaughnessy MV, Montaner JS, Hogg RS. Intentional nonadherence due to adverse symptoms associated with antiretroviral therapy. J Acquir Immune Defic Syndr 2002;31:211-17. http://dx.doi.org/10.1097/00126334-200210010-00012.
- Lowry KP, Dudley TK, Oddone EZ, Bosworth HB. Intentional and unintentional nonadherence to antihypertensive medication. Ann Pharmacother 2005;39:1198-203. http://dx.doi.org/10.1345/aph.1E594.
- Oxford Grice K, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available Nine Hole Peg Test for finger dexterity. Am J Occup Ther 2003;57:570-3. http://dx.doi.org/10.5014/ajot.57.5.570.
- Howie JGR, Heaney DJ, Maxwell M, Walker JJ, Freeman GK, Rai H. Quality at general practice consultations: cross sectional survey. BMJ 1999;319:738-43. http://dx.doi.org/10.1136/bmj.319.7212.738.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2005.
- Free Flow Vehicle Speeds on Built-Up Roads in Great Britain, Annual from 2006 to 2011. London: Department for Transport statistics; 2011.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: PSSRU, University of Kent; 2012.
- Guide to the Methods of Technology Appraisal. London: NICE publications; 2008.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU, University of Kent; 2011.
- Prescription Cost Analysis, England – 2012. London: Health and Social Care Information Centre, Prescribing and Primary Care Services; 2013.
- Reference Costs 2011–2012. London: Department of Health; 2012.
- Office of National Statistics . Annual Survey of Hours and Earnings, 2011 Revised Results (SOC 2010) n.d. www.ons.gov.uk/ons/rel/ashe/annual-survey-of-hours-and-earnings/2011-revised-results--soc-2010-/index.html (accessed 4 November 2013).
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Sach TH, Whynes DK. Measuring indirect costs: is there a problem?. Appl Health Econ Health Policy 2003;2:135-9.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JJ, et al. Valuing the ICECAP capability index for older people. Soc Sci Med 2008;67:874-82. http://dx.doi.org/10.1016/j.socscimed.2008.05.015.
- Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute For Clinical Excellence (NICE). Lancet 2002;360:711-15. http://dx.doi.org/10.1016/S0140-6736(02)09832-X.
- Briggs AH, Sculpher MJ, Claxton K. Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press; 2006.
- UK BEAM Trial Team . United Kingdom Back pain Exercise And Manipulation (UK BEAM) randomised trial: cost effectiveness of physical treatments for back pain in primary care. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.38282.607859.AE.
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effective decisions: the role of the cost-effectiveness acceptability curve (CEAC), cost-effectiveness acceptability frontier (CEAF) and expected value of perfect information (EVPI). Value Health 2008;11:886-97. http://dx.doi.org/10.1111/j.1524-4733.2008.00358.x.
- Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res 2006;6. http://dx.doi.org/10.1186/1472-6963-6-52.
- Claxton K. Bayesian approaches to the value of information: implications for the regulation of new pharmaceuticals. Health Econ 1999;8:269-74. http://dx.doi.org/10.1002/(SICI)1099-1050(199905)8:3%3C269::AID-HEC425%3E3.0.CO;2-D.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Clarke CE, Furmston A, Morgan E, Patel S, Sackley C, Walker M, et al. Pilot randomised controlled trial of occupational therapy to optimise independence in Parkinson’s disease: the PD OT trial. J Neurol Neurosurg Psychiatry 2009;80:976-8. http://dx.doi.org/10.1136/jnnp.2007.138586.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. http://dx.doi.org/10.1007/s11136-011-9903-x.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. http://dx.doi.org/10.1016/j.jval.2012.02.008.
- Nappo S, Iafrate G, Sanchez Z. Motives for participating in a clinical research trial: a pilot study in Brazil. BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-19.
- Stunkel L, Grady C. More than the money: a review of the literature examining healthy volunteer motivations. Contemp Clin Trials 2011;32:342-52. http://dx.doi.org/10.1016/j.cct.2010.12.003.
- Marcantonio ER, Aneja J, Jones RN, Alsop DC, Fong TG, Crosby GJ, et al. Maximizing clinical research participation in vulnerable older persons: identification of barriers and motivators. J Am Geriatr Soc 2008;56:1522-7. http://dx.doi.org/10.1111/j.1532-5415.2008.01829.x.
- McHorney CA, Spain CV. Frequency of and reasons for medication non-fulfillment and non-persistence among American adults with chronic disease in 2008. Health Expect 2011;14:307-20. http://dx.doi.org/10.1111/j.1369-7625.2010.00619.x.
- McCambridge J, Butor-Bhavsar K, Witton J, Elbourne D. Can research assessments themselves cause bias in behaviour change trials? A systematic review of evidence from Solomon 4-Group Studies. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0025223.
- Desborough J, Wright D, Bhattacharya D, Holland R. An audit of the impact of a compliance-focused medication support service on emergency hospital admissions in service users. Int J Pharm Pract 2006;14:B104-5.
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356:1255-9. http://dx.doi.org/10.1016/S0140-6736(00)02799-9.
- Lenaghan E, Holland R, Brooks A. Home-based medication review in a high risk elderly population in primary care – the POLYMED randomised controlled trial. Age Ageing 2007;36:292-7. http://dx.doi.org/10.1093/ageing/afm036.
- Pacini M, Smith RD, Wilson EC, Holland R. Home-based medication review in older people: is it cost effective?. Pharmacoeconomics 2007;25:171-80. http://dx.doi.org/10.2165/00019053-200725020-00008.
- Avery T, Barber N, Ghaleb M, Dean Franklin B, Armstrong S, Crowe S, et al. Investigating the Prevalence and Causes of Prescribing Errors in General Practice: The PRACtICe Study. London: General Medical Council; 2012.
Appendix 1
EMBASE
Date range searched: Inception to 31 December 2012.
Date of search: 11 January 2013.
Search strategy
-
reminder system/
-
(complian* or noncomplian* or non-complian*).ti.
-
(adher* or nonadher* or non-adher*).ti.
-
(patient* adj5 (attitude* or accept* or satisf*)).tw.
-
treatment compliance/
-
attitude to health/
-
attitude to illness/
-
persist*.ti.
-
(refusal or refuse*).tw.
-
(improve* adj5 (followup or follow up)).tw.
-
(dropout* or drop out*).tw.
-
(treatment adj5 (stop* or abandon*)).tw.
-
((medicat* or dispen* or pack* or assembl*) adj2 error*).tw.
-
health economics/
-
economic evaluation/
-
health care cost/
-
pharmacoeconomics/
-
(econom* or expenditure* or cost* or pric* or pharmacoeconomic*).tw
-
(value adj2 money).tw
-
Quality of Life/
-
QoL.ti.
-
HRQL/
-
Health status/
-
well being/
-
(cost adj2 illness).tw.
-
sickness impact profile.tw.
-
quality adjusted life years.tw.
-
tertiary prevention/
-
karnofsky.tw.
-
((Hospital or carehome) adj2 (admiss* or admit*)).tw.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30
-
reminder system/
-
blister Pack/
-
drug packaging/
-
(blister adj2 (pack* or pak*)).tw.
-
(calendar adj2 (pack* or pak*)).tw.
-
(c-pak or c-pack or c-cap*).tw.
-
(bubble adj2 (pack* or pak*)).tw.
-
((pill* or medication* or special* or pharma*) adj2 (pack* or organizer* or organiser* or delivery system* or container* or box* or dispenser* or device*)).tw.
-
((multicompartment or multi-compartment) adj2 (pack* or organizer* or organiser* or delivery system* or container* or box* or dispenser* or device*)).tw.
-
pillbox*.tw.
-
(doset* or dosset*).tw.
-
((prescription* or refill* or medication*) adj2 reminder*).tw.
-
((prescription* or medication* or drug* or compliance or adherence) adj2 refill*).tw.
-
((adherence or compliance or persist* or reminder or prompt*) adj (device* or aid or aids)).tw.
-
mediset.tw.
-
medidos.tw.
-
manrex.tw.
-
medi-dispenser*.tw.
-
pre-pack*.tw.
-
nomad*.tw.
-
medi wallet.tw.
-
webster-pak.tw.
-
venalink.tw.
-
medisure.tw.
-
qube.tw.
-
(unit adj3 (dose or pack*)).tw.
-
32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57
-
31 and 58
-
limit 59 to human
Google Scholar™
Date range searched: Inception to 15 February 2012.
Date of search: 15 February 2012.
-
drug packaging
-
c-pak
-
c-pack
-
c-cap
-
pillbox
-
doset
-
dosset
-
mediset
-
medidos
-
manrex
-
medi-dispenser
-
nomad
-
medi wallet
-
webster-pak
-
venalink
-
medisure
-
qube
Appendix 2 Technical specifications for electronic adherence monitoring systems
Appendix 3 Phase 1 (pre-randomised controlled trial) focus group protocol
Appendix 4 Phase 1 (pre-randomised controlled trial) focus group topic guides
Appendix 5 Questionnaire 1
Appendix 6 Letter of invitation to patients for randomised controlled trial participation
Appendix 7 Patient consent form for randomised controlled trial
Appendix 8 Patient information sheet for randomised controlled trial
Appendix 9 Standard operating procedure for general practitioner practices (passive recruitment)
Appendix 10 Standard operating procedure for general practitioner practices (active recruitment)
Appendix 11 Letter of thanks to participants
Appendix 12 Administration form 1
Appendix 13 Administration form 2
Appendix 14 Questionnaire 3
Appendix 15 Questionnaire 4
Appendix 16 Standard operating procedure for pharmacies
Appendix 17 Administration form 3
Appendix 18 Standard operating procedure for managing and reporting adverse events
Appendix 19 Significant adverse event reporting form
Appendix 20 Phase 2 patient participant focus group topic guide (post randomised controlled trial)
Appendix 21 Focus group topic guide post randomised controlled trial (health-care professional)
Glossary
- Active recruitment
- Recruitment directly by researchers.
- C-Pak
- A proprietary medication organisation device.
- Dosett™
- A proprietary medication organisation device.
- Intentional non-adherence
- Planned non-adherence.
- Med-ic MMD Solutions™
- An electronic adherence monitoring system.
- Medidos™
- A proprietary medication organisation device.
- Mediset™
- A proprietary medication organisation device.
- Nomad Clear™
- A proprietary medication organisation device.
- Nomad Clear XL™
- A proprietary medication organisation device with relatively deep pockets to accommodate more or larger pills.
- Norfolk Medicines Support Service
- A local NHS-funded service, unique to Norfolk, that assesses the level and type of support needed for patients having problems with medication.
- Passive recruitment
- Recruitment by letter.
- SMART PACK™
- An electronic adherence monitoring system.
- Unintentional non-adherence
- Unplanned non-adherence.
- Venalink™
- A proprietary medication organisation device.
- Webster-paks™
- A proprietary medication organisation device.
List of abbreviations
- 9-HPT
- nine-hole peg test
- ADR
- adverse drug reaction
- AE
- adverse event
- CEAC
- cost-effectiveness acceptability curve
- CEAF
- cost-effectiveness acceptability frontier
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CSQ
- Client Satisfaction Questionnaire
- DDS
- domiciliary dosage system
- DUC
- dosage unit count
- EAM
- electronic adherence monitoring
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-VAS
- European Quality of Life-Visual Analogue Scale
- EVPI
- expected value of perfection information
- GP
- general practitioner
- HIV
- human immunodeficiency virus
- HTA
- Health Technology Assessment
- ICECAP-O
- Investigating Choice Experiment CAPability measure for Older people
- ICER
- incremental cost-effectiveness ratio
- MMSE
- Mini Mental State Examination
- MOD
- medication organisation device
- NIHR
- National Institute for Health Research
- OtCM™
- Objective Therapy Compliance Monitoring
- PCRN
- Primary Care Research Network
- PIL
- patient information leaflet
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SE
- standard error
- SODF
- solid oral dose form
- SOP
- standard operating procedure
- TRACE
- Trial of Antioxidant Vitamins C and E
- UEA
- University of East Anglia