Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/303/205. The contractual start date was in April 2007. The draft report began editorial review in December 2013 and was accepted for publication in October 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Professor James reports trial support (free trial drug for the Phase II part of the trial and a grant awarded to investigation sites for recruitment of Phase III patients with trial numbers 301–700) and lecturing fees from Sanofi-aventis. He also reports free trial drug for the Phase II part of the trial followed by a trial-linked discount for sites ordering trial drug for Phase III patients and lecturing fees from Novartis Pharmaceuticals UK Ltd. In addition, there was a trial-linked discount for sites ordering trial drug from GE Healthcare. Professor James also reports trial support, consultancy work and lecture fees from Sanofi-aventis and Novartis Pharmaceuticals UK Ltd who were directly related and consultancy work and lecture fees from Bayer HealthCare, Algeta, Amgen, Janssen, Astellas Pharma and Affinity OncoGeneX Pharmaceuticals Inc. who were related to prostate cancer but not the drugs in this study. Dr Pope reports trial support (free trial drug for the Phase II part of the trial and a grant awarded to investigation sites for recruitment of Phase III patients with trial numbers 301–700) from Sanofi-aventis; trial support (free trial drug for the Phase II part of the Phase III patients) from Novartis Pharmaceuticals UK Ltd; and trial support (trial-linked discount for sites ordering trial drug) from GE Healthcare from GP Health Care, during the conduct of the study. Dr Parker reports personal fees from Bayer HealthCare, BN ImmunoTherapeutics Inc. (BNIT), Astellas Pharma, Janssen, Sanofi-aventis and Takeda UK Ltd, outside the submitted work. Dr Stanley reports that Teva Pharmaceutical Industries Ltd supported his attendance at European Society for Medical Oncology, he received personal fees from Calgene, Inc., and Amgen, Inc. supported part of his attendance at British Oncology Pharmacy Association, outside the submitted work. Dr Brown reports personal fees and non-financial support from Novartis Pharmaceuticals UK Ltd for advisory board in different cancer and writing assistance for different cancer outside the submitted work. Dr Billingham reports personal fees from Eli Lilly, and from Pfizer, both for expenses paid for contributing to educational events, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by James et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Prostate cancer
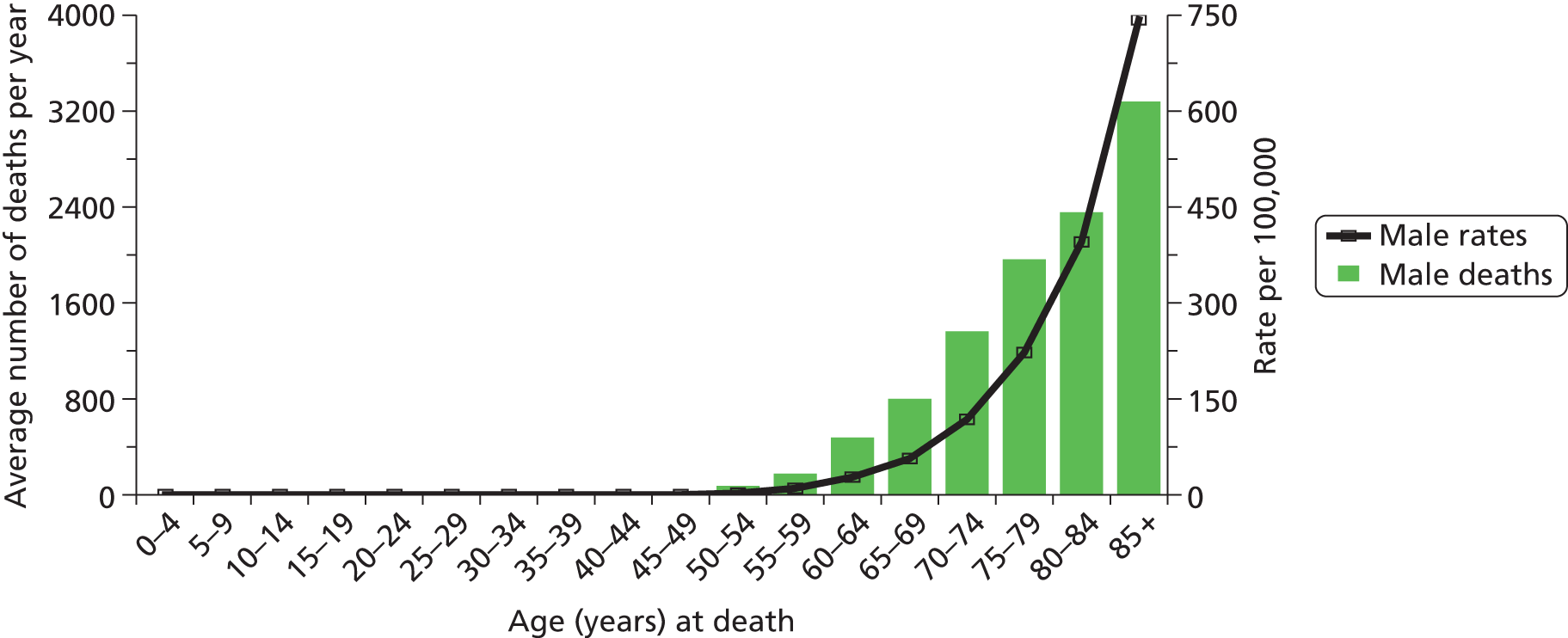
Prostate cancer is a major worldwide health problem which accounts for nearly one-fifth of all newly diagnosed male cancers. In the UK, approximately 35,000 men are diagnosed with prostate cancer each year, and in 2008 almost 10,000 men died from the disease. 1 The disease is mostly one of older age, but significant numbers of men of working age will develop the disease. Figure 1 summarises the age distribution of incident cases and deaths.
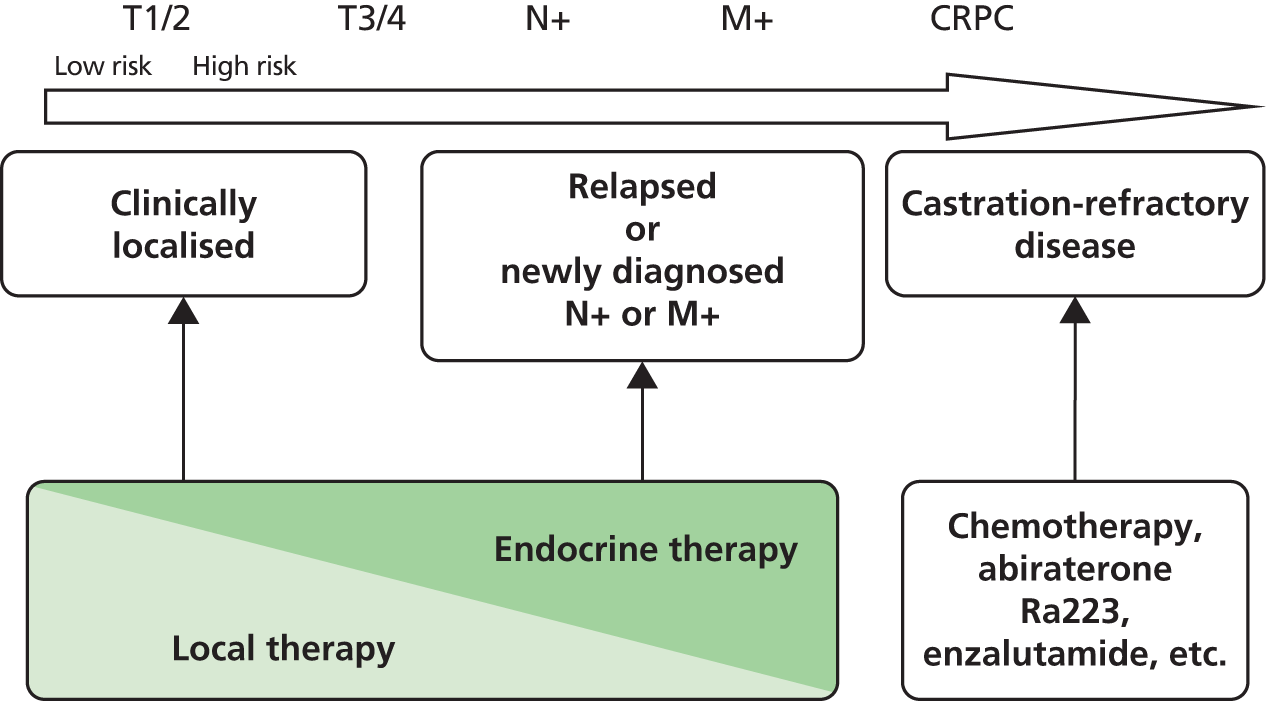
Although adenocarcinoma of the prostate most often presents as local (stage T1 or T2) disease, in which the malignancy is confined to the prostate, a significant proportion of patients progress despite initial treatment with ablative surgery or radiotherapy, often in combination with hormonal therapy. A minority of patients present with de novo metastatic disease. Figure 2 summarises the treatment options across the disease spectrum.
FIGURE 2.
Prostate cancer treatment paradigm. Cancer has spread to the pelvic lymph nodes (N+) or to lymph nodes, organs, or bones distant from the prostate (M+). CRPC, castration-refractory prostate cancer; Ra223, Radium-223.
Hormone therapy
A hormone (from the Greek ὁρμή, meaning ‘impetus’) is a chemical released by a cell in one part of the body to affect cells in other parts of the organism. Cells respond to a hormone when they express a specific receptor for that hormone. The hormone binds to the receptor protein, resulting in the activation of a signal transduction mechanism that ultimately leads to cell type-specific responses. Hormone therapies can thus work on a number of points in this pathway and there are examples of all of these in prostate cancer, which are summarised in Table 1.
| Target | Example in prostate cancer therapy |
|---|---|
| Block synthesis of regulator of hormone | Gonadotropin-releasing hormone analogues and antagonists, e.g. goserelin, leuprorelin, triptorelin |
| Block binding of secreted hormone to receptor | Bicalutamide, enzalutamide, cyproterone acetate |
| Block post-receptor effects | Enzalutamide |
| Block synthesis of hormone | Cyp17 inhibitors, e.g. abiraterone |
| Add alternative hormones to alter environment | Diethylstilboestrol, dexamethasone |
Hormone therapy has been a mainstay of prostate cancer since the seminal studies of Huggins and Hodges,5 published in 1941, demonstrating substantial and prolonged remissions from prostate cancer with the use of either surgical castration or oestrogen therapy. Diethylstilboestrol is the first example of a successful drug treatment for advanced cancer, and, while now supplanted in this role, it remains in use 70 years later. As is now well known, while responses to hormone therapy may be dramatic, with durations running into many years, they are rarely curative and typically last 18–24 months depending on disease stage. This period after failure of initial androgen deprivation therapy has been known by many terms over the years, including androgen-independent prostate cancer and castration-refractory prostate cancer (CRPC). However, with the recognition that relapsing tumours remain dependent on androgen receptor-mediated pathways and the licensing in relapsing disease of abiraterone,6–8 a steroid synthesis inhibitor, and enzalutamide,9 an androgen receptor-targeting agent, the term castration-refractory prostate cancer is increasingly used. This term is, however, unpopular with patient groups and, while accurate, may yet also be supplanted if anyone can think of a term with less pejorative overtones.
Broadly speaking, there are two routes into long-term hormone therapy: via localised disease, radical therapy and relapse, and de novo advanced disease (Figure 3).
FIGURE 3.
Pathways to advanced disease. Natural history for metastatic patients. ADT, androgen deprivation therapy; PSA, prostate-specific antigen.

Figure 3 shows disease burden expressed via the prostate-specific antigen (PSA) level on the vertical axis. For most purposes, however, PSA does equate with disease burden. In particular, in late-stage disease managed with non-hormonal therapies the relationship is not that close and PSA is not recognised as a surrogate end point for clinical trials. In early hormone-sensitive disease, the concordance between PSA changes and clinical ones is close. One consequence of the use of the PSA test is that managment tends to be PSA-driven rather than clinically-driven. In the case of patients relapsing after failed local therapy, clinicians are faced with a rising PSA but often no radiological evidence of disease for many years – termed a biochemical relapse. Patients in this situation will often be started on hormone therapy many years before any clinical consequences of relapse. Randomised trials in this setting have shown that intermittent therapy is as good as continuous therapy and probably should be regarded as the standard of care.
Management of metastatic disease
Initial management of men with locally advanced or metastatic prostate cancer is some form of androgen deprivation therapy. This will generally control disease for 1–3 years, following which progressive clinical failure will ensue – CRPC. In patients with metastatic CRPC (mCRPC), one of the most common sites of spread is bone. The development of bone metastasis, and the associated pain, results in a high level of mobility problems, leading to a loss of functional independence in men, and is a major cause of mortality [bone marrow failure, pathological fractures, spinal cord compression (SCC) and other bone-related complications]. Bone morbidity is often quantified in clinical trials via a composite end point termed the skeletal-related events (SREs). The elements that make up this end point are summarised as:
-
pathological fracture
-
SCC
-
radiotherapy to bone
-
hypercalcaemia
-
change in anticancer treatment to treat bone pain.
The reduction in the frequency or severity of SREs that any particular patient experiences during the individual disease pathway may provide additional health-related quality-of-life (HRQoL) benefits. The true benefit in terms of HRQoL is not yet completely known, although recent data from clinical trials have begun to show the HRQoL benefits of bisphosphonates. 10,11 In addition to the potential quality-of-life (QoL) benefits, patients may also gain actual survival benefit from either mono or combination therapy. Although bisphosphonates therapy and/or chemotherapy may be considered as central to the treatment of patients with bone metastases, other therapies such as radioisotopes are available and are widely used for patients with mCRPC.
Chemotherapy
For many years chemotherapy was considered too toxic to be of value in men with advanced prostate cancer. There were a number of reasons for this, including later diagnosis in the pre-PSA era, difficulty in assessing responses and problems in managing toxicity, such as nausea and vomiting. The advent of PSA-driven diagnosis and management, while remaining controversial in terms of use as a screening test, has undoubtedly resulted in a strong trend to earlier diagnosis now dating back several decades. This in turn has meant that men are diagnosed younger with advanced disease. Secondly, the use of PSA monitoring post-primary treatment has meant that men relapsing after failed radical therapy are picked up early and so, when mCRPC does develop, treatment can be instigated when men remain fit enough to cope with it. Definitive proof of benefit from palliative chemotherapy came from a landmark National Cancer Institute of Canada trial led by Ian Tannock from Toronto. The trial compared prednisone alone with prednisolone plus mitoxantrone given 3-weekly for up to 10 cycles. This relatively small study of 161 patients published in 1996 set out to compare palliative end points rather than survival-based ones. 12
A palliative response was observed in 23 out of 80 patients who received mitoxantrone plus prednisone, compared with 10 out of 81 patients who received prednisone alone. In an additional seven patients in each group, analgesic medication was reduced without an increase in pain. The duration of palliation was longer in patients who received chemotherapy (with a median of 43 weeks to symptom worsening) than in those treated with prednisone alone (median of 18 weeks to symptom worsening). There was significant crossover from the prednisone arm to the chemotherapy arm and no difference in overall survival. Thus, this study clearly established the principle that chemotherapy could provide palliative benefit but did not show a survival benefit. Subsequent mitoxantrone trials produced similar results, although the crossover between the chemotherapy and no-chemotherapy arms means that, essentially, it is not known whether or not chemotherapy with this agent produces a survival benefit.
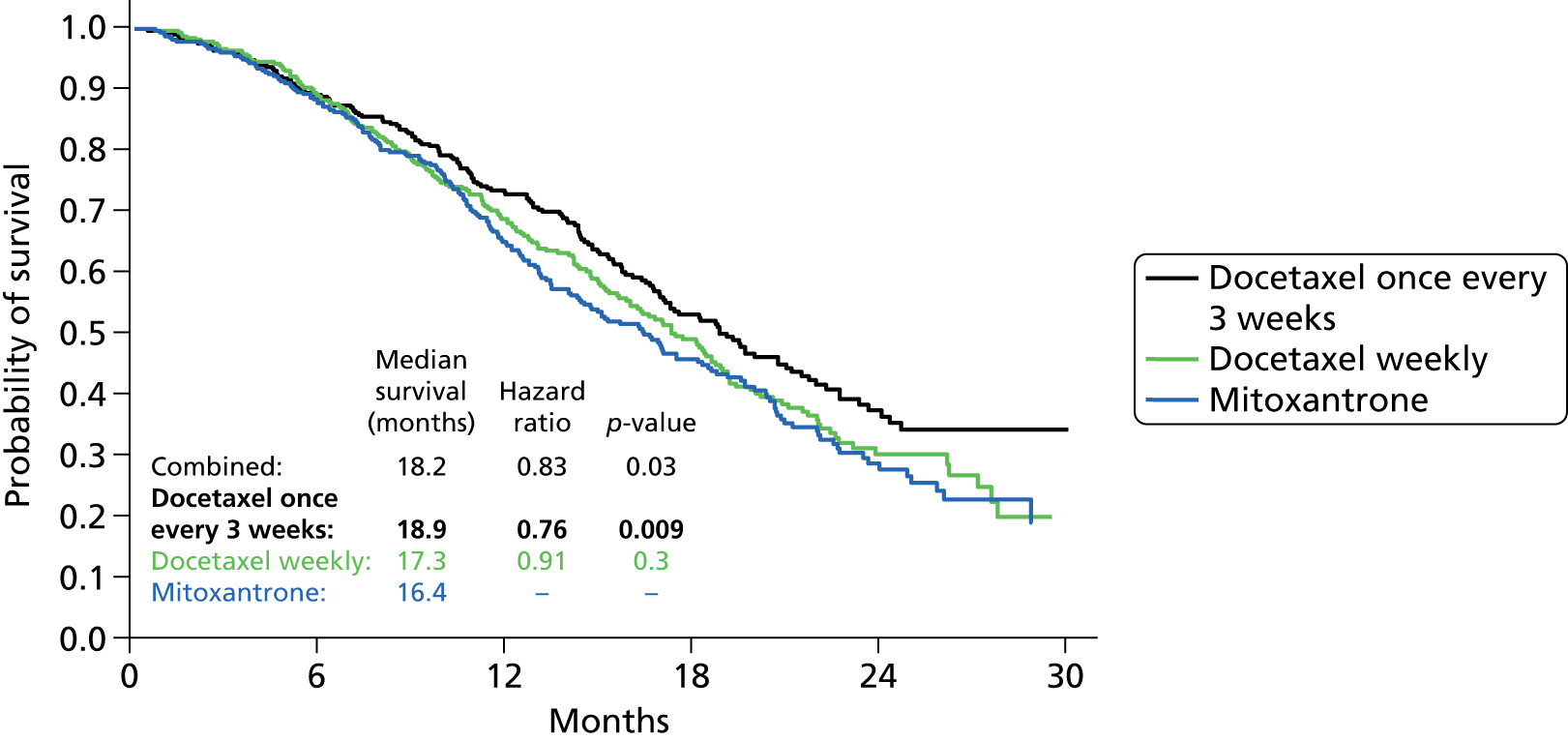
In the late 1990s, a variety of agents started to be evaluated in what was then called hormone-refractory prostate cancer (HRPC). Docetaxel emerged as the lead candidate for evaluation in large phase trials, and two landmark studies were published in the New England Journal of Medicine in 2004. 13,14 One trial, the TAX327 study,13 compared weekly or 3-weekly docetaxel with the Tannock mitoxantrone regimen. The second trial (SWOG 991614) compared a combination of docetaxel and estramustine with the same control arm. Both trials showed improved palliative outcomes compared with mitoxantrone and, very importantly, an overall survival (OS) advantage for 3-weekly docetaxel and the docetaxel–estramustine combination with hazard ratios (HRs) of 0.76 and 0.8, respectively, despite significant crossover to docetaxel in the mitoxantrone arms of both studies. All patients in both trials received prednisone as per the original Tannock paper. These trials confirmed unequivocally that chemotherapy could both prolong survival and give worthwhile palliation without undue toxicity. They also established that docetaxel is a superior agent to mitoxantrone. On the basis of these trials, a 3-weekly schedule of docetaxel plus prednisolone for up to 10 cycles has emerged as the standard of care for mCRPC and was approved by the National Institute for Health and Care Excellence (NICE) for this purpose in 2006 (Figure 4).
FIGURE 4.
Comparison of estimated probability of overall survival of mitoxantrone and docetaxel for CRPC. 15
A number of agents have been studied in the second-line chemotherapy setting. Of these, to date only cabazitaxel has shown a survival advantage and obtained a licence. The key trial, TROPIC, compared cabazitaxel with mitoxantrone given on the standard Tannock trial schedule and showed an improvement in median survival from 12.7 to 15.1 months. 16 Cabazitaxel was licensed in 2010 ahead of abiraterone, which obtained a licence in 2011 in the same post-docetaxel setting. As both drugs improve survival, although they have completely different modes of action, there is clearly an unresolved issue over choice and sequencing (or indeed combination) of agents. The position has now been further complicated by the licensing of enzalutamide post chemotherapy based on the AFFIRM trial,9 plus the extension of the abiraterone licence to chemo-naive patients. 6 The licence for enzalutamide was also expanded to cover pre-chemotherapy patients following the PREVAIL trial. 17
Additionally, the recent publication of the results of the ALSYMPCA trial of radium-22318 demonstrated both improved OS and reduced skeletal complications with six injections, one every 28 days, of radioisotope compared with placebo. 18 How chemotherapy should best be integrated with other therapeutic options for patients with bone metastasis is at present not defined.
Bisphosphonates
Bisphosphonates inhibit bone catabolism by reducing the numbers of functioning osteoclasts and have been used to manage bone metastases. Zoledronic acid (ZA), but not some older bisphosphonates, also arrest cell proliferation, induce apoptosis, and inhibit the growth factor stimulation of cultured prostate cancer cells. 14 In trials in relapsing mCRPC, ZA reduced the time to SRE as well as the frequency of subsequent SREs. 19,20 The ZA licensing trials19,20 have proved very controversial, as the fracture end point was assessed by regular skeletal survey with blinded radiological assessment. Hence, there is significant doubt as to whether many of the small fractures detected were precursors of a subsequent real ‘clinical’ SRE or radiological features of no significance. ZA is not currently recommended for use in the UK by NICE because of doubts as to its cost-effectiveness.
Radioisotopes have been used to palliate bone pain for over 20 years. A variety of radioisotopes are available; the most commonly used during the trial recruitment era were strontium-89 (Sr-89)21,22 and samarium-153. 23 Both accumulate selectively in bone metastases compared with non-involved bone. There is some evidence that Sr-89 may reduce overall health-care costs compared with standard methods of delivering radiotherapy. 24 There are a number of previous studies of combined use of chemotherapy with radioisotopes. Of particular note, Tu et al. 25 combined combination chemotherapy with Sr-89 in a small randomised trial with promising results suggesting a survival advantage in chemotherapy responders allocated to Sr-89.
Since the publication of the MRC PR05 study,26,27 more potent bisphosphonates have been evaluated in mCRPC. The most widely studied has been zoledronate, which has a 40- to 850-fold higher potency than clodronate in pre-clinical models of bone resorption. 28 It has also been shown to be more effective than pamidronate (90 mg) in controlling malignant hypercalcaemia29,30 In addition, zoledronate has demonstrated direct anticancer activity, including inhibition of proliferation of breast cancer and prostate cancer cells in vitro. 31,32
In prostate cancer trials in relapsing mCRPC, ZA reduced the time to SREs as well as the frequency of subsequent SREs. 19,20 However, it is clear from looking at the components that make up the SREs that these vary hugely in clinical significance and, in addition, are to a degree subjective. In particular, the ZA licensing trials19,20 have proved very controversial as the fracture end point was assessed by regular skeletal survey with blinded radiological assessment. As such there is significant doubt about whether many of the small fractures detected were precursors of a subsequent real ‘clinical’ SRE or radiological features of no significance. The subsequent trials comparing ZA with denusomab33 used the same methodology and so can be subject to the same criticism. As a result, neither agent is recommended for use in the UK by NICE. The impact of ZA on SREs is illustrated in Figure 5; the bisphosphonate showing decreases in skeletal complications in both lytic and blastic lesions in a comparison with pamidonate. 20
FIGURE 5.
Proportion of patients with SREs, demonstrating reduction with ZA.

In vitro evidence suggests synergistic killing of breast and prostate cancer cells when combined with chemotherapy. 32 Furthermore, ZA was licensed in the ‘pre-docetaxel’ era; hence, whatever the merits of SRE prevention, the role of zoledronate in the chemotherapy era was effectively undefined. It was therefore logical to evaluate docetaxel with ZA in men with mCRPC affecting bone. In view of the controversy over the SRE as an end point, we did not undertake routine skeletal evaluations as in the zoledronate and denosumab licensing trials but collected data only on ‘clinical’ SREs; that is, those reported by the patient or diagnosed on the basis of symptoms such as those from SCC. We combined this clinically orientated approach to the SRE with a health economic assessment of the impact of the various trial interventions, the intention being that, if the clinical utility of combination therapy were confirmed, we should be able to produce robust estimates of the cost-effectiveness at the same time.
Radioisotopes
A variety of radioisotopes are available, the most commonly used during the trial recruitment era being Sr-89 and samarium-153. Both accumulate selectively in bone metastases compared with uptake rates in non-involved bone. Sr-89, a bone-seeking radionuclide, is a pure β-emitter with a half-life of 50 days, has a high uptake in osteoblastic metastases, and remains in tumour sites for up to 100 days. Sr-89 provides pain relief in up to 80% of patients, and complete freedom from pain in approximately 10%, for periods that can exceed 3 months. 21,34 In a randomised controlled Phase III trial, the combination of Sr-89 injection and external beam radiotherapy improved pain relief, delayed disease progression and enhanced some QoL measures compared with external beam radiotherapy alone. 21 However, another Phase III randomised controlled trial has suggested that, in some patients, systemic Sr-89 may be inferior to local-field radiotherapy in terms of survival (7.2 months vs. 11.0 months; p = 0.0457). 22 The selection of patients has a significant impact on outcome, response and duration of response to radionuclide therapy, as bone pain palliation is reduced in those who have widespread metastatic disease or a short life expectancy. 35–38 Consequently, the use of radionuclides appears to be optimal at an early stage in disease management. However, their efficacy is reduced or lost with repeated use, and overtreatment can also lead to irreversible pancytopenia. As noted above (see Bisphosphonates), there is some evidence that Sr-89 may reduce overall health-care costs compared with standard methods of delivering radiotherapy. 39
There are a number of previous studies of combined use of chemotherapy with radioisotopes. Tu et al. combined combination chemotherapy with Sr-89 in a small randomised trial with promising results suggesting a survival advantage in chemotherapy responders allocated to Sr-89. 25 More recently, Fizazi et al. ,40 Tu et al. 41 and Morris et al. 42 have combined docetaxel with samarium-153 in Phase I/II trials, confirming safety for the combination. No published randomised trials have addressed the safety or efficacy of docetaxel with either Sr-89 or samarium-53.
As new treatments have appeared for CRPC, these treatments have been less frequently used. However, recent data with a new radioisotope radium-223 seem set to change this picture. Like Sr-89, radium-223 is a calcium mimetic. Recently completed placebo-controlled Phase III trials in symptomatic CRPC patients showed a prolongation of survival and also a delay and reduction in symptomatic (as opposed to radiological) SREs. 18 Levels of adverse reactions reported in the trial were low. The agent was licensed in 2013 and is an important new therapeutic option for men with CRPC, especially as the trial included men both pre and post chemotherapy, as well as those deemed unfit to ever receive chemotherapy.
Osteoporosis
Patients eligible for the study are at risk of osteoporosis in view of their previous therapy (androgen deprivation, possible steroid exposure, age) as well as from some on-study therapies (steroids, docetaxel). Osteoporosis was therefore considered in the causality of any SRE. A bone density substudy formed part of this trial.
Chapter 2 Methods
Trial design
This was originally a four-arm randomised controlled Phase II trial, which proceeded seamlessly to a Phase III trial. In order to increase efficiency and reduce the trial duration, the Phase III design was switched from a four-arm comparison to a two-by-two factorial design. The end points changed as the trial progressed from Phase II to Phase III, as summarised in Table 2.
| Phase | Primary | Secondary | Tertiary |
|---|---|---|---|
| II |
|
|
|
| III |
|
|
|
The Phase II objectives were to compare the four trial arms with respect to feasibility, tolerability and safety. The Phase III objectives were to assess treatments with respect to efficacy within a two-by-two factorial design framework; that is, the trial compared ZA versus no ZA (stratified for Sr-89 use) and Sr-89 versus no Sr-89 (stratified for ZA use). The Phase III trial had dual primary end points of effect of each treatment on time to bony disease progression and cost and cost-effectiveness.
During the chemotherapy treatment period, participants were assessed at 3-weekly intervals. Irrespective of treatment arm, all patients were assessed at the end of the sixth cycle of chemotherapy to ensure their fitness to receive Sr-89.
Phase II participants ceased primary trial treatment after cycle 6 of Sr-89 administration, where relevant. Clinicians were encouraged to give further docetaxel off-trial up to a total of 10 cycles in keeping with NICE guidance, where appropriate. In order to streamline data collection, cycles 7 to 10 of docetaxel were designated as trial therapy for Phase III of the study.
Participants
Male patients over the age of 18 years were recruited into the trial. The trial recruited sufficient patients to ensure that at least 618 participants reached the primary end points. The entry criteria primarily included proven mCRPC, with one or more of progressive sclerotic bone metastases, progression of measurable malignant lesions or elevated and rising PSA levels on blood analysis. Consenting participants had to have had an Eastern Cooperative Oncology Group (ECOG) scale score of up to 2, be fit enough to receive trial treatment and have adequate haematological, renal and hepatic function.
Exclusion criteria included prior chemotherapy or radionuclide therapy for CRPC, prior radiotherapy to more than 25% of bone marrow or whole-pelvic irradiation, prior bisphosphonate therapy within 2 months of trial entry, other malignant disease within the previous 5 years (excluding adequately treated basal cell carcinoma), known brain metastases, symptomatic peripheral neuropathy of National Institutes of Health National Cancer Institute’s Common Terminology for the Criteria for Adverse Events grade 2 or more, concurrent participation in any other clinical trial involving an investigational therapeutic compound or treatment with other investigational compound within the 30 days prior to trial entry.
Owing to the nature of the treatments under investigation, this was not a blinded trial for patients or caregivers.
Interventions
Arm A: control – docetaxel plus prednisolone
Docetaxel 75 mg/m2 (up to a maximum dose of 165 mg) was administered intravenously at 3-weekly intervals (21 days). Participants also received oral prednisolone 10 mg daily throughout trial treatment or until disease progression or associated treatment toxicity.
Trial chemotherapy ceased after cycle 6 for Phase II participants but continued for up to 10 cycles for Phase III participants, ceasing for pain or tumour disease progression, or other cause decided by the treating clinician or patient choice. As noted above (see Trial design) patients could receive further chemotherapy off-trial in keeping with NICE guidance.
Arm B: docetaxel, prednisolone plus zoledronic acid
Docetaxel and prednisolone were administered as per the control arm. ZA was administered intravenously after completion of docetaxel administration at a dose of 4 mg, subject to pre-treatment creatinine clearance being greater than 60 ml/minute; creatinine clearance of < 60 ml/minute would incrementally reduce the dose given, as detailed in section 6.1.3 of the protocol (see Appendix 1). Following the completion of chemotherapy, participants received continuing ZA at 4-weekly intervals, as clinically indicated, until pain or tumour disease progression or withdrawal. It was recommended that patients treated with ZA also receive vitamin D and calcium supplements throughout treatment.
Arm C: docetaxel, prednisolone plus strontium-89
Docetaxel and prednisolone were administered as per the control arm, for six cycles. Subject to satisfactory haematological and clinical parameters on clinical assessment 21 days after the sixth docetaxel treatment, participants received a single 150-MBq dose of Sr-89 on the 28th day after the sixth cycle.
Chemotherapy ceased after cycle 6 for Phase II participants, but for Phase III participants continued for up to 10 cycles after a period of between 28 and 56 days of Sr-89 administration, allowing for bone marrow function to be adequately recovered.
Arm D: docetaxel, prednisolone, zoledronic acid plus strontium-89
Patients in this arm received docetaxel, prednisolone and ZA for six cycles, as per arm B participants, plus clinical and haematological assessment and Sr-89 administration, as per arm C participants. Following a recovery period of between 28 and 56 days, chemotherapy, prednisolone and ZA treatment resumed until disease progression, associated treatment toxicity or patient withdrawal. As per the arm B treatment regime, following the end of chemotherapy, patients received continuing ZA administrations at 4-weekly intervals, as clinically indicated, until disease progression or until other discontinuation criteria were met. It was again recommended that patients treated with ZA also receive vitamin D and calcium supplements throughout treatment.
Further off-study treatment
All further off-study treatment, for example chemotherapy, bisphosphonate and radioisotope therapy, as well as newer drugs, such as abiraterone, enzalutamide and radium-223, received after study treatment were captured on the Concomitant Medication Running Form. The choice of further treatment was at the discretion of the participant’s clinician.
Objectives
The primary objective of the Phase II component was to assess the feasibility, tolerability and safety of the four treatment arms.
Phase III assessed treatments within a two-by-two factorial design framework; that is, ZA versus no ZA (stratified for Sr-89 use) and Sr-89 versus no Sr-89 (stratified for ZA use). Each of these treatment comparisons was made in terms of clinical efficacy, with primary outcome clinical progression-free survival (CPFS) interval and health economic outcomes. In addition, the trial assessed the presence of any association between biomarkers and clinical outcomes.
Data collection
Case report forms
Data collected on each subject were recorded by the investigator or his/her designee on case report forms (CRFs). Originals of the CRF were returned to the trial management office, whereas photocopies were retained by the site.
Quality-of-life data
All eligible participants were asked to consider taking part in the QoL part of the study. QoL was assessed using patient-completed questionnaires, i.e. the European Quality of Life 5-Dimensions (EQ-5D) and Functional Assessment of Cancer Therapy – Prostate (FACT-P), while pain and analgesic use diaries were used to facilitate changes in participants’ pain perception and management. An example of both the QoL booklet and pain diary are part of the protocol in Appendix 1. A QoL booklet and pain diary were completed at baseline and subsequently prior to each treatment and follow-up visit. Completion of these documents remained voluntary and continued throughout patient follow-up (pre and post clinical progression), irrespective of any further therapy a patient may have received.
Monitoring
The study was conducted under the auspices of the Cancer Research Clinical Trials Unit (CRCTU) according to current guidelines for good clinical practice. Participating centres were monitored by CRCTU staff to confirm compliance with the protocol and the protection of patients’ rights as detailed in the Declaration of Helsinki.
Participating centres were monitored by checking incoming forms for compliance against the protocol, consistent data, missing data and timing. CRCTU onsite monitoring was carried out as detailed by the trial’s risk assessment, primarily of all sites that had enrolled four or more patients into the trial. Patients’ records to be audited at such visits were selected randomly from the different treatment arms.
Sample size
Sample size calculations were based on the primary outcome measure of CPFS. The calculations were the same for both the comparison of ZA with no ZA and that of Sr-89 with no Sr-89. The trial aimed to detect a HR of 0.76, which would be equivalent to 1-year CPFS rates of 30% versus 40%, assuming that CPFS follows an exponential distribution. The number of events required to detect this difference in each group for either treatment comparison, using a two-sided 5% significance level and 80% power, was 206. It was estimated that approximately 294 participants would be required in each group, that is 588 patients in total, to observe this number of events at 1 year’s follow-up. We aimed to recruit a minimum of 618 evaluable patients, which allowed for 5% dropout.
The analysis of the Phase II component of the trial was entirely descriptive and did not involve any statistical hypothesis testing. The primary outcomes were feasibility, tolerability and safety, and these will be measured as proportions or means, as appropriate. Recruitment of 50 patients into each arm ensured that percentages could be estimated with a precision of at least 15% and provided sufficient data to be able to assess the arms in terms of their suitability for progression into the Phase III component of the trial.
Randomisation
Stratified randomisation
Stratification was used to ensure the balance of participant characteristics as well as numbers within each treatment group. Patients were randomised to treatment arms in a 1 : 1 : 1 : 1 allocation ratio using a computerised minimisation algorithm. If the minimisation is balanced, then allocation is random with equal chance of allocation to all arms. Randomisation was stratified by centre and ECOG performance score (0, 1 or 2) to avoid imbalance.
Implementation
Prior to randomisation, patients gave their informed consent to take part in the trial and the clinician or research nurse completed a pre-randomisation checklist to ascertain that the patient met all the entry criteria.
The process of entering a patient into the trial was conducted by telephone with the CRCTU randomisation office. Using either a computerised randomisation program or a paper equivalent should the computer system be out of commission, the CRCTU randomisation officer re-ascertained the patient’s eligibility, after which the computer program allocated the next available trial number and randomised treatment arm for the participant. When the randomisation was conducted while the computer was out of commission, systems were in place to allocate the next available trial number and random treatment.
The allocated trial number and treatment arm were communicated to the site by telephone and confirmed by fax.
Follow-up
Patients were assessed every 3 weeks during the study treatment period. After treatment completion or withdrawal for any reason except disease progression (pain or tumour growth), participants were followed up monthly for 3 months and subsequently every 3 months until either patient death or withdrawal of the patient’s consent for further follow-up.
Patients progressed to 3-monthly follow-up following clinical progression; that is, increasing pain, tumour growth or SREs.
Trial management
Trial Management Group
The Trial Management Group comprised the chief investigator, a few co-investigators and members of the CRCTU, as detailed in the front sleeve of the protocol (see Appendix 1). The Trial Management Group was responsible for the day-to-day running and management of the trial and met by teleconference or in person, as required.
Data Monitoring Committee
An independent Data Monitoring Committee (DMC; see Appendix 2), comprising an independent statistician, oncologist and urologist, met approximately annually to review the accumulating confidential trial data. Their main objective was to advise the Trial Steering Committee (TSC) whether or not there was any evidence or reason to amend or terminate the trial based on the recruitment rate or safety. Reports to the DMC were produced by the CRCTU.
Trial Steering Committee
An independent TSC (see Appendix 2) provided overall supervision for the trial and advised the trial management group. Members included an independent statistician, oncologist and two urologists. The ultimate decision regarding continuation of the trial lay with the TSC, based on the advice received from the DMC. The TSC met approximately annually, shortly after the DMC met.
Outcomes
Primary end points
Phase II: feasibility, tolerability and safety
The primary end points of the Phase II study were feasibility, tolerability and the safety of each treatment arm. Analysis was purely descriptive, while the control arm data acted as a benchmark against which to assess the experimental treatment arms. Percentages and means were calculated, and 95% confidence intervals (CIs) constructed as appropriate.
Phase III: clinical progression-free survival
The primary Phase III analysis compared ZA versus no ZA (stratified for Sr-89 use) and Sr-89 versus no Sr-89 (stratified for ZA use) in terms of CPFS. CPFS was defined as the number of whole days from the date of randomisation to the first occurrence of SRE, pain progression or death. Patients not experiencing clinical progression were censored at the date last known to be progression free.
Economic analyses
Economic evaluations were carried out to assess the cost-effectiveness of the relevant comparisons – ZA versus no ZA and Sr-89 versus no Sr-89 – for patients with mCRPC. The analyses were carried out from the perspective of the NHS and Personal Social Services and involved calculating estimates of mean per-patient costs and health outcomes for each of the compared treatment options. Costs were calculated on the basis of treatment acquisition and administration costs, cost of concomitant medications and use of NHS primary and secondary care resources. Outcomes were expressed as quality-adjusted life-years (QALYs), calculated on the basis of patients’ responses to the EQ-5D (three-level) instrument. Mean values were reported together with their 95% CIs. To account for the skewed distributions of costs and QALYs, CIs were obtained through bias-corrected and accelerated bootstrap methods. In line with current recommendations, costs and QALYs accruing in the future were discounted at an annual rate of 3.5%.
Incremental analysis was undertaken to obtain a ratio of the difference in costs over the difference in QALYs for each comparison. Results were presented in the form of incremental cost-effectiveness ratios (ICERs), reflecting the extra cost for an additional QALY. 43 To account for the inherent uncertainty as a result of sampling variation, the joint distribution of differences in cost and QALYs was derived by carrying out a large number of non-parametric bootstrap simulations. 44,45 The simulated cost and effect pairs were depicted on a cost-effectiveness plane46 and were plotted as cost-effectiveness acceptability curves (CEACs). 47,48 A series of sensitivity analyses was carried out to assess the impact of key assumptions on the obtained results. Given the short expected survival time of mCRPC patients and the long-term follow-up of patients in the trial, lifetime costs and effects were largely observed and so extrapolation beyond the trial was not necessary.
Secondary end points
Skeletal-related event-free interval
A skeletal-related event-free interval (SREFI) was defined as the time in whole days from the date of randomisation to the date of the first occurrence of a SRE. A SRE was defined as any one of the following:
-
symptomatic pathological bone fracture
-
spinal cord or nerve root compression likely to be related to cancer or treatment
-
cancer related surgery to bone
-
radiation therapy to bone (including use of radioisotopes)
-
change of antineoplastic therapy to treat bone pain due to prostate cancer
-
hypercalcaemia.
Patients who did not experience a SRE were censored at death or the date last known to be alive.
Pain progression-free interval
Pain progression-free interval (PPFI) was defined as the time in whole days from the date of randomisation to the date of clinician-determined pain progression. Patients not experiencing pain progression were censored at the date of death or the date last known to be alive.
Overall survival
Overall survival was defined as the number of whole days from the date of randomisation to the date of death from any cause. Patients alive at the date of analysis were censored at the date last known to be alive.
Quality of life
Quality-of-life questionnaires included the EQ-5D, which consisted of the health-state scale, the descriptive three-level system and the visual analogue scale (VAS); the FACT-P version 4; and a health-problems questionnaire focusing predominantly on resource use. The QoL form was collected 3-weekly during treatment and then monthly for 3 months and, finally, 3-monthly until death.
The EQ-5D is a generic preference-based measure of HRQoL. The instrument was designed to be self-completed and so, where possible, data were provided by the patient. Responses to the descriptive system of the EQ-5D were translated into a single summary utility index ranging from –0.59 to 1 by using a UK-relevant value set. Patients’ rating of their QoL was also collected through a vertical 20-cm VAS with the bottom end point representing the worst imaginable health state and the top end point showing the best imaginable health state. The VAS resembles a thermometer and takes values between 0 (worst imaginable state) and 100 (best imaginable state).
The FACT-P is a 40-item self-reported cancer therapy questionnaire with an additional 12-item prostate cancer subscale. Six measures were generated by this questionnaire: social well-being, personal well-being, emotional well-being, functional well-being, prostate cancer-specific score and an overall FACT-P score ranging from zero to 156.
Toxicity
The analysis of toxicity was purely descriptive. Proportions and means were calculated and 95% CIs constructed as appropriate.
Ancillary end points
Bone mineral density changes and biomarker substudies are detailed in the protocol (see Appendix 1); tertiary end points will not be presented at this time in this report.
Statistical methods
The definitive study analysis was conducted on an intention-to-treat basis. All tests of statistical significance were conducted at the 5% two-sided significance level. All analysis was carried out using Stata version 12.1 (StataCorp LP, College Station, TX, USA).
Descriptive comparisons not involving hypothesis testing will be presented as medians, interquartile ranges (IQRs) and ranges for numerical variables, and percentages will be given for categorical variables. Percentages will not always total exactly 100% due to rounding errors associated with reporting results to one decimal place. Percentage totals have been rounded to the nearest integer. Time-to-event analysis, multiple event analysis and QoL analysis are detailed at the start of the appropriate section. No direct statistical analysis of between randomisation arms has been conducted. The factorial design of the study assumes there is no interaction between the two agents and any treatment effects are assumed to be additive; therefore, the trial was not powered for this analysis.
Summary of changes to the trial protocol
Phase II treatment consisted of six cycles of docetaxel chemotherapy plus an additional four cycles off-study at the discretion of the treating physician. NICE, however, recommended that up to 10 cycles of docetaxel chemotherapy should be administered in one treatment block. This was not stated clearly in the Phase II protocol and the previous trial design had the inadvertent effect of preventing some patients from receiving cycles 7 to 10 at a later stage because of local policy. Adopting the NICE recommendation formally into the clinical trial design ensured that all patients had access to the NICE-recommended schedule of chemotherapy and that the control treatment arm was considered the true ‘standard of care’ (Tables 3 and 4).
| Protocol version no./date | Brief description of previous amendments |
|---|---|
| Versions 1–3 (12 July 2004, 2 August 2004, 16 August 2004) |
|
| TRAPEZE, Phase II: version 4 (1 September 2004) |
|
| Version 5 (23 March 2005) |
|
| Version 6 (7 June 2005) |
|
| Version 7 (4 May 2007) |
|
| TRAPEZE, Phase III: version 8 (24 September 2008) |
|
| Version 9 (12 April 2011) |
|
| Version 10 (25 May 2011) |
|
| Version 11 (17 February 2012) | Substantial amendments:
|
Non-substantial amendments:
|
Chapter 3 Results
Consolidated Standards of Reporting Trials diagram
A Consolidated Standards of Reporting Trials (CONSORT) diagram summarising trial participation figures and analysis is included as Figure 6.
FIGURE 6.
Consolidated Standards of Reporting Trials (CONSORT) diagram. ITT, intention to treat.

Recruitment
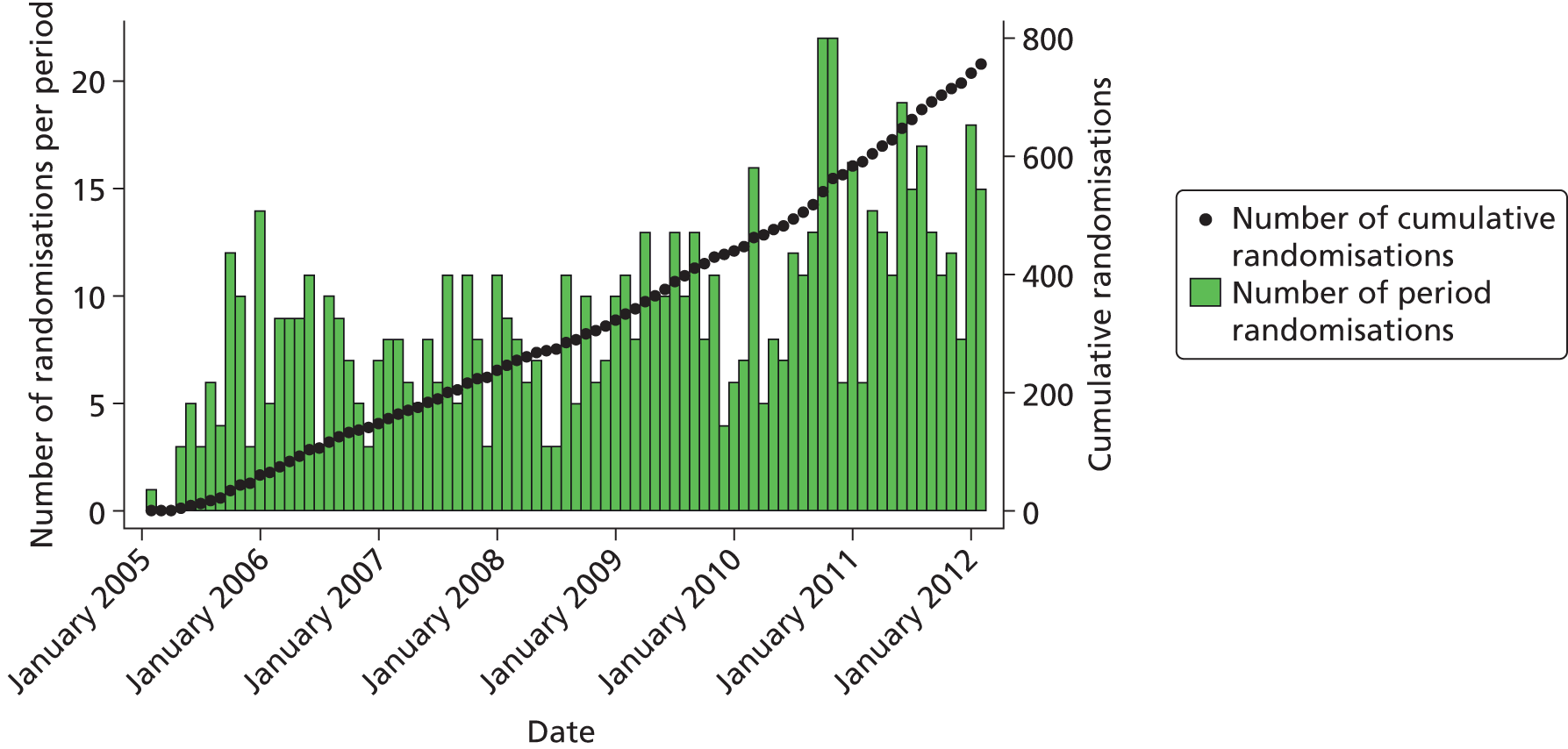
Figure 7 shows trial recruitment both by monthly randomisation periods and cumulatively over the course of the trial. Table 78 (see Appendix 5) shows recruitment by centre.
FIGURE 7.
Recruitment from January 2005 to February 2012.
Losses and exclusions
Ineligible
In total, 27 patients were found to be ineligible following randomisation. Five were randomised to docetaxel alone, 10 to docetaxel + ZA, seven to docetaxel + Sr-89 and five to docetaxel, ZA and Sr-89. All ineligible patients are included in intention-to-treat analysis.
There were three main categories of ineligibilities. These were (1) pre-randomisation blood pressure and blood tests were missed or performed outside of the allowed time frame, (2) progression on trial entry was not appropriately documented and (3) hormone therapies were not stopped at the appropriate time point, for example if bicalutamide had been stopped within 4 weeks of starting trial treatment rather than within 4 weeks of randomisation as stipulated in the eligibility criteria.
Protocol deviations
In total, 71 protocol deviations were reported: 15 in the docetaxel arm, 17 in the docetaxel and ZA arm, 18 in the docetaxel and Sr-89 arm and 21in the docetaxel, ZA and Sr-89 arm.
Table 5 provides a complete summary of all protocol deviations reported during the course of the trial.
| Deviation reason | n (N = 71) |
|---|---|
| Administrative error | 2 |
| Blood pressure consistently not done | 2 |
| Blood pressure not done at baseline | 10 |
| Bloods not done before chemotherapy | 1 |
| Calcium supplements not given with ZA | 1 |
| Calcium supplements stopped at incorrect time for Sr-89 | 1 |
| Chemotherapy capped at wrong BSA | 9 |
| Clinician chose to give lower dose of docetaxel because of patient’s age and comorbidities | 1 |
| Cycle delayed over 14 days | 6 |
| Docetaxel dose capped at BSA of 2 m2 by medical decision to prevent possible excess toxicity | 1 |
| Docetaxel dose escalated | 1 |
| Docetaxel dose reduction not per protocol | 7 |
| Dose not recalculated to BSA at cycle 5: 160 mg given instead of 150 mg | 1 |
| Incorrect dose of strontium | 1 |
| Patient did not receive scheduled ZA | 1 |
| Patient received intended dose of 67.2 mg/m2 because of diarrhoea | 1 |
| Patient received different trial arm | 5 |
| Patient recommenced bicalutamide while on study | 1 |
| Patient sensitive to prednisolone, therefore commenced on 1.5 mg of dexamethasone | 1 |
| Patient stopped taking LHRH agonist | 1 |
| Post-docetaxel assessment not done prior to Sr-89 | 6 |
| Post-docetaxel assessment performed late | 1 |
| Pre-ZA creatinine not done | 2 |
| Premature discontinuation | 2 |
| Sr-89 given at wrong time point | 5 |
| Sr-89 given prior to post-docetaxel assessment | 1 |
| Total | 71 |
Patient withdrawal of consent
Full consent for any further participation in the trial, including follow-up, has been withdrawn by 21 patients. In addition, 28 patients have withdrawn from one or more of the trial substudies. Table 6 contains a breakdown of all non-treatment withdrawals by randomisation arm and Table 7 by comparison group; a complete list of all patients who have withdrawn full consent can be found in Appendix 5.
| Withdrawal | Docetaxel (N = 191) | Docetaxel + ZA (N = 188) | Docetaxel + Sr-89 (N = 190) | Docetaxel + ZA + Sr-89 (N = 188) | Overall (N = 757) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Full withdrawal of consent | 6 | 3.1 | 13 | 6.9 | 6 | 3.2 | 3 | 1.6 | 28 | 3.7 |
| No withdrawal | 178 | 93.2 | 168 | 89.4 | 177 | 93.2 | 174 | 92.6 | 697 | 92.1 |
| Partial withdrawal: blocks | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 1 | 0.1 |
| Partial withdrawal: proteomics | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 1 | 0.5 | 2 | 0.3 |
| Partial withdrawal: Qol | 7 | 3.7 | 5 | 2.7 | 7 | 3.7 | 5 | 2.7 | 24 | 3.2 |
| Partial withdrawal: Qol + blocks | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 |
| Partial withdrawal: Qol + proteomics | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 4 | 2.1 | 4 | 0.5 |
| Total | 191 | 100 | 188 | 100 | 190 | 100 | 188 | 100 | 757 | 100 |
| Withdrawal | No ZA (N = 381) | ZA (N = 376) | No Sr-89 (N = 379) | Sr-89 (N = 378) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Full withdrawal of consent | 12 | 3.1 | 16 | 4.3 | 19 | 5 | 9 | 2.4 |
| No withdrawal | 355 | 93.2 | 342 | 91 | 346 | 91.3 | 351 | 92.9 |
| Partial withdrawal: blocks | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 |
| Partial withdrawal: proteomics | 0 | 0.0 | 2 | 0.5 | 1 | 0.3 | 1 | 0.3 |
| Partial withdrawal: Qol | 14 | 3.7 | 10 | 2.7 | 12 | 3.2 | 12 | 3.2 |
| Partial withdrawal: Qol + blocks | 0 | 0.0 | 1 | 0.3 | 1 | 0.3 | 0 | 0.0 |
| Partial withdrawal: Qol + proteomics | 0 | 0.0 | 4 | 1.1 | 0 | 0.0 | 4 | 1.1 |
| Total | 381 | 100 | 376 | 100 | 379 | 100 | 378 | 100 |
Withdrawal of trial treatment
Docetaxel
In total, 408 (54%) patients received fewer than the protocol-defined number of treatment cycles, which was originally six and then increased to 10. In total, 220 (29%) patients received only six cycles because of the original protocol limitation. Table 8 shows the reasons for withdrawal from docetaxel by randomisation arm and Table 9 shows the reasons by comparison group.
| Withdrawal reason | Docetaxel (N = 107) | Docetaxel + ZA (N = 100) | Docetaxel + Sr-89 (N = 106) | Docetaxel + ZA + Sr-89 (N = 95) | Overall (N = 408) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Administration error | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | 1 | 1.1 | 2 | 0.5 |
| Change in treatment | 4 | 3.7 | 1 | 1.0 | 3 | 2.8 | 4 | 4.2 | 12 | 2.9 |
| Clinician decision | 2 | 1.9 | 3 | 3.0 | 5 | 4.7 | 8 | 8.4 | 18 | 4.4 |
| Death | 5 | 4.7 | 3 | 3.0 | 9 | 8.5 | 7 | 7.4 | 24 | 5.9 |
| Disease progression | 35 | 32.7 | 27 | 27.0 | 33 | 31.1 | 23 | 24.2 | 118 | 28.9 |
| Other condition | 36 | 33.6 | 30 | 30.0 | 31 | 29.2 | 24 | 25.3 | 121 | 29.7 |
| Patient choice | 7 | 6.5 | 7 | 7.0 | 8 | 7.5 | 6 | 6.3 | 28 | 6.9 |
| Toxicity | 11 | 10.3 | 18 | 18.0 | 9 | 8.5 | 12 | 12.6 | 50 | 12.3 |
| Unknown | 7 | 6.5 | 10 | 10.0 | 8 | 7.5 | 10 | 10.5 | 35 | 8.6 |
| Total | 107 | 100.0 | 100 | 100.0 | 106 | 100.0 | 95 | 100.0 | 408 | 100.0 |
| Withdrawal reason | No ZA | ZA | No Sr-89 | Sr-89 | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Administration error | 0 | 0.0 | 2 | 1.0 | 1 | 0.5 | 1 | 0.5 |
| Change in treatment | 7 | 3.3 | 5 | 2.6 | 5 | 2.4 | 7 | 3.5 |
| Clinician decision | 7 | 3.3 | 11 | 5.6 | 5 | 2.4 | 13 | 6.5 |
| Death | 14 | 6.6 | 10 | 5.1 | 8 | 3.9 | 16 | 8.0 |
| Disease progression | 68 | 32.0 | 50 | 25.6 | 62 | 29.9 | 56 | 27.9 |
| Other condition | 67 | 31.4 | 54 | 27.7 | 66 | 31.9 | 55 | 27.4 |
| Patient choice | 15 | 7.0 | 13 | 6.7 | 14 | 6.8 | 14 | 6.9 |
| Toxicity | 20 | 9.4 | 30 | 15.4 | 29 | 14.0 | 21 | 10.4 |
| Unknown | 15 | 7.0 | 20 | 10.3 | 17 | 8.2 | 18 | 8.9 |
| Total | 213 | 100.0 | 195 | 100.0 | 207 | 100.0 | 201 | 100.0 |
Strontium-89
Of the 378 patients randomised to receive Sr-89, 253 (67%) did so. The reasons for not receiving Sr-89 are reported in Table 10.
| Withdrawal reason | Docetaxel + Sr-89 (N = 67) | Docetaxel + ZA + Sr-89 (N = 58) | Overall (N = 125) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Administration error | 0 | 0.0 | 1 | 1.7 | 1 | 0.8 |
| Change in treatment | 2 | 3.0 | 3 | 5.2 | 5 | 4.0 |
| Clinician decision | 0 | 0.0 | 2 | 3.4 | 2 | 1.6 |
| Death | 6 | 9.0 | 7 | 12.1 | 13 | 10.4 |
| Disease progression | 22 | 32.8 | 12 | 20.7 | 34 | 27.2 |
| Other condition | 19 | 28.4 | 14 | 24.1 | 33 | 26.4 |
| Patient choice | 3 | 4.5 | 2 | 3.4 | 5 | 4.0 |
| Toxicity | 5 | 7.5 | 6 | 10.3 | 11 | 8.8 |
| Unknown | 10 | 14.9 | 11 | 19.0 | 21 | 16.8 |
| Total | 67 | 100.0 | 58 | 100.0 | 125 | 100.0 |
Lost to follow-up
Six patients in total have been reported as being lost to follow-up by site: three randomised to docetaxel alone, one randomised to docetaxel and Sr-89 and two randomised to docetaxel, ZA and Sr-89. Two of these reached the primary end point prior to being lost, one subsequently died and, although some follow-up information remains missing, the death information was obtained.
Data maturity
In total, 618 patients have been followed up until death. Of the remaining 139 patients, 78 have reached the primary CPFS end point, leaving 61 patients alive without having reached the primary end point.
The average follow-up of alive patients was 1.84 years (IQR 1.4–2.4 years), and the average follow-up of the 61 patients who have not reached the primary end point was 1.7 years (IQR 1.4–2.1 years). Table 11 shows the average follow-up of the surviving patients split by randomisation arm.
| Duration of follow-up (years) | Docetaxel (N = 37) | Docetaxel + ZA (N = 32) | Docetaxel + Sr-89 (N = 35) | Docetaxel + ZA + Sr-89 (N = 35) | Overall (N = 139) |
|---|---|---|---|---|---|
| n | 37 | 32 | 35 | 35 | 139 |
| Median | 1.7 | 1.8 | 1.9 | 1.9 | 1.8 |
| IQR | 1.4–2.3 | 1.4–2.3 | 1.6–2.6 | 1.5–2.4 | 1.4–2.4 |
Figure 8 shows the time between the date of randomisation and the date when the patient was last seen and the time from that date to the date of the analysis. Each point represents a patient, and the solid black dots are patients who have not reached the primary end point of the trial. The solid black line indicates where the patients would appear on the graph if they were seen on the date of the analysis. The dashed line represents 6 months before the analysis and the dotted line represents 12 months prior to the analysis.
FIGURE 8.
Duration of follow-up. Pain, pain progression.

Stratification variables
Two stratification factors were used during the randomisation process: centre and ECOG performance status. These can be seen in Tables 12 and 13.
| Stratification variable | Docetaxel (N = 191) | Docetaxel + ZA (N = 188) | Docetaxel + Sr-89 (N = 190) | Docetaxel + ZA + Sr-89 (N = 188) | Overall (N = 757) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| ECOG performance status score | ||||||||||
| 0 | 77 | 40.3 | 76 | 40.4 | 76 | 40.0 | 76 | 40.4 | 305 | 40.3 |
| 1 | 98 | 51.3 | 97 | 51.6 | 97 | 51.1 | 97 | 51.6 | 389 | 51.4 |
| 2 | 16 | 8.4 | 15 | 8.0 | 17 | 8.9 | 15 | 8.0 | 63 | 8.3 |
| Randomisation centre | ||||||||||
| Aberdeen Royal Infirmary | 5 | 2.6 | 5 | 2.7 | 5 | 2.6 | 6 | 3.2 | 21 | 2.8 |
| Ayr Hospital | 5 | 2.6 | 6 | 3.2 | 5 | 2.6 | 5 | 2.7 | 21 | 2.8 |
| Beatson West of Scotland Cancer Centre | 15 | 7.9 | 15 | 8.0 | 15 | 7.9 | 16 | 8.5 | 61 | 8.1 |
| Bradford Royal Infirmary | 3 | 1.6 | 4 | 2.1 | 4 | 2.1 | 2 | 1.1 | 13 | 1.7 |
| Cheltenham General Hospital | 4 | 2.1 | 4 | 2.1 | 4 | 2.1 | 4 | 2.1 | 16 | 2.1 |
| Christie Hospital | 30 | 15.7 | 31 | 16.5 | 30 | 15.8 | 31 | 16.5 | 122 | 16.1 |
| Dorset County Hospital | 2 | 1.0 | 1 | 0.5 | 1 | 0.5 | 1 | 0.5 | 5 | 0.7 |
| Forth Valley Royal Hospital | 2 | 1.0 | 1 | 0.5 | 2 | 1.1 | 1 | 0.5 | 6 | 0.8 |
| Gloucester Royal Hospital | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 1 | 0.1 |
| Huddersfield Royal Infirmary | 2 | 1.0 | 2 | 1.1 | 3 | 1.6 | 2 | 1.1 | 9 | 1.2 |
| Ipswich Hospital | 5 | 2.6 | 4 | 2.1 | 4 | 2.1 | 4 | 2.1 | 17 | 2.2 |
| Maidstone Hospital | 7 | 3.7 | 7 | 3.7 | 7 | 3.7 | 8 | 4.3 | 29 | 3.8 |
| Poole Hospital | 0 | 0.0 | 5 | 0.0 | 0 | 0.0 | 1 | 0.5 | 1 | 0.1 |
| Queen Alexandra Hospital | 4 | 2.1 | 6 | 2.7 | 4 | 2.1 | 4 | 2.1 | 17 | 2.2 |
| Queen Elizabeth Hospital | 32 | 16.8 | 15 | 16.5 | 32 | 16.8 | 31 | 16.5 | 126 | 16.6 |
| Royal Albert Edward Infirmary | 2 | 1.0 | 4 | 0.5 | 2 | 1.1 | 2 | 1.1 | 7 | 0.9 |
| Royal Bournemouth Hospital | 2 | 1.0 | 4 | 0.5 | 1 | 0.5 | 1 | 0.5 | 5 | 0.7 |
| Royal Derby Hospital | 6 | 3.1 | 31 | 3.2 | 7 | 3.7 | 7 | 3.7 | 26 | 3.4 |
| Royal Free Hospital | 3 | 1.6 | 1 | 2.1 | 2 | 1.1 | 3 | 1.6 | 12 | 1.6 |
| Royal Marsden Hospital London | 1 | 0.5 | 1 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 |
| Royal Marsden Hospital Sutton | 7 | 3.7 | 0 | 4.3 | 7 | 3.7 | 8 | 4.3 | 30 | 4.0 |
| Royal Preston Hospital | 9 | 4.7 | 2 | 4.8 | 8 | 4.2 | 8 | 4.3 | 34 | 4.5 |
| Southampton General Hospital | 4 | 2.1 | 4 | 1.6 | 4 | 2.1 | 3 | 1.6 | 14 | 1.8 |
| St James’s University Hospital | 7 | 3.7 | 7 | 3.7 | 7 | 3.7 | 6 | 3.2 | 27 | 3.6 |
| Velindre Hospital | 1 | 0.5 | 5 | 1.1 | 2 | 1.1 | 2 | 1.1 | 7 | 0.9 |
| Western General Hospital | 28 | 14.7 | 6 | 14.4 | 28 | 14.7 | 28 | 14.9 | 111 | 14.7 |
| Weston General Hospital | 3 | 1.6 | 15 | 1.6 | 4 | 2.1 | 3 | 1.6 | 13 | 1.7 |
| Wishaw General Hospital | 2 | 1.0 | 4 | 0.5 | 1 | 0.5 | 1 | 0.5 | 5 | 0.7 |
| Stratification variable | No ZA (N = 381) | ZA (N = 376) | No Sr-89 (N = 379) | Sr-89 (N = 378) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| ECOG performance status score | ||||||||
| 0 | 153 | 40.2 | 152 | 40.4 | 153 | 40.4 | 152 | 40.2 |
| 1 | 195 | 51.2 | 194 | 51.6 | 195 | 51.5 | 194 | 51.3 |
| 2 | 33 | 8.7 | 30 | 8.0 | 31 | 8.2 | 32 | 8.5 |
| Randomisation centre | ||||||||
| Aberdeen Royal Infirmary | 10 | 2.6 | 11 | 2.9 | 10 | 2.6 | 11 | 2.9 |
| Ayr Hospital | 10 | 2.6 | 11 | 2.9 | 11 | 2.9 | 10 | 2.6 |
| Beatson West of Scotland Cancer Centre | 30 | 7.9 | 31 | 8.2 | 30 | 7.9 | 31 | 8.2 |
| Bradford Royal Infirmary | 7 | 1.8 | 6 | 1.6 | 7 | 1.8 | 6 | 1.6 |
| Cheltenham General Hospital | 8 | 2.1 | 8 | 2.1 | 8 | 2.1 | 8 | 2.1 |
| Christie Hospital | 60 | 15.7 | 62 | 16.5 | 61 | 16.1 | 61 | 16.1 |
| Dorset County Hospital | 3 | 0.8 | 2 | 0.5 | 3 | 0.8 | 2 | 0.5 |
| Forth Valley Royal Hospital | 4 | 1.0 | 2 | 0.5 | 3 | 0.8 | 3 | 0.8 |
| Gloucester Royal Hospital | 1 | 0.3 | 0 | 0.0 | 0 | 0.0 | 1 | 0.3 |
| Huddersfield Royal Infirmary | 5 | 1.3 | 4 | 1.1 | 4 | 1.1 | 5 | 1.3 |
| Ipswich Hospital | 9 | 2.4 | 8 | 2.1 | 9 | 2.4 | 8 | 2.1 |
| Maidstone Hospital | 14 | 3.7 | 15 | 4.0 | 14 | 3.7 | 15 | 4.0 |
| Poole Hospital | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 |
| Queen Alexandra Hospital | 8 | 2.1 | 9 | 2.4 | 9 | 2.4 | 8 | 2.1 |
| Queen Elizabeth Hospital | 64 | 16.8 | 62 | 16.5 | 63 | 16.6 | 63 | 16.7 |
| Royal Albert Edward Infirmary | 4 | 1.0 | 3 | 0.8 | 3 | 0.8 | 4 | 1.1 |
| Royal Bournemouth Hospital | 3 | 0.8 | 2 | 0.5 | 3 | 0.8 | 2 | 0.5 |
| Royal Derby Hospital | 13 | 3.4 | 13 | 3.5 | 12 | 3.2 | 14 | 3.7 |
| Royal Free Hospital | 5 | 1.3 | 7 | 1.9 | 7 | 1.8 | 5 | 1.3 |
| Royal Marsden Hospital London | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 |
| Royal Marsden Hospital Sutton | 14 | 3.7 | 16 | 4.3 | 15 | 4.0 | 15 | 4.0 |
| Royal Preston Hospital | 17 | 4.5 | 17 | 4.5 | 18 | 4.7 | 16 | 4.2 |
| Southampton General Hospital | 8 | 2.1 | 6 | 1.6 | 7 | 1.8 | 7 | 1.9 |
| St James’s University Hospital | 14 | 3.7 | 13 | 3.5 | 14 | 3.7 | 13 | 3.4 |
| Velindre Hospital | 3 | 0.8 | 4 | 1.1 | 3 | 0.8 | 4 | 1.1 |
| Western General Hospital | 56 | 14.7 | 55 | 14.6 | 55 | 14.5 | 56 | 14.8 |
| Weston General Hospital | 7 | 1.8 | 6 | 1.6 | 6 | 1.6 | 7 | 1.9 |
| Wishaw General Hospital | 3 | 0.8 | 2 | 0.5 | 3 | 0.8 | 2 | 0.5 |
Baseline data
In total, 752 (99%) baseline forms were returned. Table 14 shows the baseline information recorded on the on-study form by randomisation arm.
| Patient characteristic | Randomisation arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Docetaxel (N = 191) | Docetaxel + ZA (N = 187) | Docetaxel + Sr-89 (N = 188) | Docetaxel + ZA + Sr-89 (N = 186) | Overall (N = 752) | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| ECOG performance status score | ||||||||||
| 0 | 76 | 43.4 | 71 | 40.8 | 70 | 39.3 | 64 | 37.0 | 281 | 40.1 |
| 1 | 83 | 47.4 | 88 | 50.6 | 90 | 50.6 | 95 | 54.9 | 356 | 50.9 |
| 2 | 15 | 8.6 | 15 | 8.6 | 18 | 10.1 | 14 | 8.1 | 62 | 8.9 |
| 3 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 |
| Missing | 16 | – | 13 | – | 10 | – | 13 | – | 52 | – |
| Diagnostic indicator | ||||||||||
| Adenocarcinoma | 156 | 81.7 | 146 | 78.9 | 150 | 80.2 | 149 | 81.0 | 601 | 80.5 |
| PSA only | 35 | 18.3 | 39 | 21.1 | 37 | 19.8 | 35 | 19.0 | 146 | 19.5 |
| Missing | 0 | – | 2 | – | 1 | – | 2 | – | 5 | – |
| Staging: T | ||||||||||
| T1 | 2 | 1.4 | 5 | 3.8 | 2 | 1.7 | 1 | 0.7 | 10 | 1.9 |
| T1b | 1 | 0.7 | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 | 2 | 0.4 |
| T1c | 0 | 0.0 | 1 | 0.8 | 2 | 1.7 | 1 | 0.7 | 4 | 0.7 |
| T2 | 19 | 13.3 | 16 | 12.2 | 11 | 9.1 | 17 | 12.1 | 63 | 11.8 |
| T2a | 2 | 1.4 | 2 | 1.5 | 0 | 0.0 | 0 | 0.0 | 4 | 0.7 |
| T2b | 4 | 2.8 | 1 | 0.8 | 1 | 0.8 | 2 | 1.4 | 8 | 1.5 |
| T3 | 54 | 37.8 | 53 | 40.5 | 49 | 40.5 | 63 | 44.7 | 219 | 40.9 |
| T3a | 4 | 2.8 | 3 | 2.3 | 5 | 4.1 | 4 | 2.8 | 16 | 3.0 |
| T3b | 12 | 8.4 | 10 | 7.6 | 10 | 8.3 | 9 | 6.4 | 41 | 7.6 |
| T4 | 28 | 19.6 | 22 | 16.8 | 20 | 16.5 | 25 | 17.7 | 95 | 17.7 |
| TX | 16 | 11.2 | 18 | 13.7 | 20 | 16.5 | 18 | 12.8 | 72 | 13.4 |
| T2c | 1 | 0.7 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 2 | 0.4 |
| Missing | 48 | – | 56 | – | 67 | – | 45 | – | 216 | – |
| Staging: M | ||||||||||
| M0 | 44 | 30.8 | 41 | 31.3 | 33 | 27.3 | 40 | 28.4 | 158 | 29.5 |
| M1a | 20 | 14.0 | 20 | 15.3 | 22 | 18.2 | 21 | 14.9 | 83 | 15.5 |
| M1b | 8 | 5.6 | 7 | 5.3 | 3 | 2.5 | 2 | 1.4 | 20 | 3.7 |
| M1c | 4 | 2.8 | 10 | 7.6 | 6 | 5.0 | 5 | 3.5 | 25 | 4.7 |
| MX | 26 | 18.2 | 14 | 10.7 | 17 | 14.0 | 26 | 18.4 | 83 | 15.5 |
| M1 | 41 | 28.7 | 39 | 29.8 | 40 | 33.1 | 47 | 33.3 | 167 | 31.2 |
| Missing | 48 | – | 56 | – | 67 | – | 45 | – | 216 | – |
| Staging: N | ||||||||||
| N0 | 59 | 41.3 | 57 | 43.5 | 46 | 38.0 | 58 | 41.1 | 220 | 41.0 |
| N1 | 42 | 29.4 | 28 | 21.4 | 32 | 26.4 | 39 | 27.7 | 141 | 26.3 |
| NX | 42 | 29.4 | 46 | 35.1 | 43 | 35.5 | 44 | 31.2 | 175 | 32.6 |
| Missing | 48 | – | 56 | – | 67 | – | 45 | – | 216 | – |
| Gleason score | ||||||||||
| 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 1 | 0.2 |
| 4 | 2 | 1.4 | 0 | 0.0 | 1 | 0.7 | 0 | 0.0 | 3 | 0.5 |
| 5 | 2 | 1.4 | 3 | 2.1 | 2 | 1.4 | 3 | 2.2 | 10 | 1.8 |
| 6 | 9 | 6.3 | 12 | 8.4 | 12 | 8.7 | 6 | 4.5 | 39 | 7.0 |
| 7 | 43 | 29.9 | 48 | 33.6 | 39 | 28.3 | 41 | 30.6 | 171 | 30.6 |
| 8 | 30 | 20.8 | 24 | 16.8 | 35 | 25.4 | 25 | 18.7 | 114 | 20.4 |
| 9 | 57 | 39.6 | 55 | 38.5 | 41 | 29.7 | 54 | 40.3 | 207 | 37.0 |
| 10 | 1 | 0.7 | 1 | 0.7 | 8 | 5.8 | 4 | 3.0 | 14 | 2.5 |
| Missing | 47 | – | 44 | – | 50 | – | 52 | – | 193 | – |
| Prior radiotherapy received? | ||||||||||
| No | 114 | 59.7 | 107 | 57.2 | 95 | 50.8 | 98 | 52.7 | 414 | 55.1 |
| Yes | 77 | 40.3 | 80 | 42.8 | 92 | 49.2 | 88 | 47.3 | 337 | 44.9 |
| Missing | 0 | – | 0 | – | 1 | – | 0 | – | 1 | – |
| Method of castration | ||||||||||
| Surgery | 5 | 2.6 | 4 | 2.1 | 3 | 1.6 | 2 | 1.1 | 14 | 1.9 |
| Ongoing LHRH agonists | 186 | 97.4 | 183 | 97.9 | 185 | 98.4 | 184 | 98.9 | 738 | 98.1 |
| Anti-androgen received? | ||||||||||
| No | 10 | 5.2 | 19 | 10.2 | 17 | 9.1 | 14 | 7.6 | 60 | 8.0 |
| Yes | 181 | 94.8 | 168 | 89.8 | 170 | 90.9 | 171 | 92.4 | 690 | 92.0 |
| Missing | 0 | – | 0 | – | 1 | – | 1 | – | 2 | – |
| Flutamide, nilutamide or cyproterone acetate received? | ||||||||||
| No | 151 | 83.9 | 141 | 83.9 | 142 | 84.0 | 133 | 77.8 | 567 | 82.4 |
| Yes | 29 | 16.1 | 27 | 16.1 | 27 | 16.0 | 38 | 22.2 | 121 | 17.6 |
| Missing | 11 | – | 19 | – | 19 | – | 15 | – | 64 | – |
| Bicalutamide received? | ||||||||||
| No | 11 | 6.1 | 14 | 8.3 | 8 | 4.7 | 15 | 8.8 | 48 | 7.0 |
| Yes | 170 | 93.9 | 154 | 91.7 | 162 | 95.3 | 156 | 91.2 | 642 | 93.0 |
| Missing | 10 | – | 19 | – | 18 | – | 15 | – | 62 | – |
| Method of progression at study entry | ||||||||||
| All | 26 | 13.7 | 27 | 14.6 | 27 | 14.4 | 27 | 14.5 | 107 | 14.3 |
| Elevated PSA | 42 | 22.1 | 48 | 25.9 | 42 | 22.3 | 44 | 23.7 | 176 | 23.5 |
| New lesion | 15 | 7.9 | 16 | 8.6 | 21 | 11.2 | 19 | 10.2 | 71 | 9.5 |
| Objective | 5 | 2.6 | 1 | 0.5 | 1 | 0.5 | 1 | 0.5 | 8 | 1.1 |
| Objective + new lesion | 3 | 1.6 | 4 | 2.2 | 7 | 3.7 | 5 | 2.7 | 19 | 2.5 |
| PSA + new lesion | 94 | 49.5 | 85 | 45.9 | 82 | 43.6 | 88 | 47.3 | 349 | 46.6 |
| PSA + objective | 5 | 2.6 | 4 | 2.2 | 8 | 4.3 | 2 | 1.1 | 19 | 2.5 |
| Missing | 1 | – | 2 | – | 0 | – | 0 | – | 3 | – |
| Baseline pain diary completed? | ||||||||||
| No | 30 | 15.7 | 37 | 19.8 | 42 | 22.3 | 34 | 18.3 | 143 | 19.0 |
| Yes | 161 | 84.3 | 150 | 80.2 | 146 | 77.7 | 152 | 81.7 | 609 | 81.0 |
| Baseline QoL booklet completed? | ||||||||||
| No | 22 | 11.6 | 19 | 10.3 | 28 | 14.9 | 25 | 13.7 | 94 | 12.6 |
| Yes | 168 | 88.4 | 165 | 89.7 | 160 | 85.1 | 158 | 86.3 | 651 | 87.4 |
| Missing | 1 | – | 3 | – | 0 | – | 3 | – | 7 | – |
Table 15 repeats the baseline characteristics reported above but split by comparison groups.
| Patient characteristic | Comparison group | |||||||
|---|---|---|---|---|---|---|---|---|
| No ZA (N = 379) | ZA (N = 373) | No Sr-89 (N = 378) | Sr-89 (N = 374) | |||||
| n | % | n | % | n | % | n | % | |
| ECOG performance status score | ||||||||
| 0 | 146 | 41.4 | 135 | 38.9 | 147 | 42.1 | 134 | 38.2 |
| 1 | 173 | 49.0 | 183 | 52.7 | 171 | 49.0 | 185 | 52.7 |
| 2 | 33 | 9.3 | 29 | 8.4 | 30 | 8.6 | 32 | 9.1 |
| 3 | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 |
| Missing | 26 | – | 26 | – | 29 | – | 23 | – |
| Diagnostic indicator | ||||||||
| Adenocarcinoma | 306 | 81.0 | 295 | 79.9 | 302 | 80.3 | 299 | 80.6 |
| PSA only | 72 | 19.0 | 74 | 20.1 | 74 | 19.7 | 72 | 19.4 |
| Missing | 1 | – | 4 | – | 2 | – | 3 | – |
| Staging: T | ||||||||
| T1 | 4 | 1.5 | 6 | 2.2 | 7 | 2.6 | 3 | 1.1 |
| T1b | 2 | 0.8 | 0 | 0.0 | 1 | 0.4 | 1 | 0.4 |
| T1c | 2 | 0.8 | 2 | 0.7 | 1 | 0.4 | 3 | 1.1 |
| T2 | 30 | 11.4 | 33 | 12.1 | 35 | 12.8 | 28 | 10.7 |
| T2a | 2 | 0.8 | 2 | 0.7 | 4 | 1.5 | 0 | 0.0 |
| T2b | 5 | 1.9 | 3 | 1.1 | 5 | 1.8 | 3 | 1.1 |
| T3 | 103 | 39.0 | 116 | 42.6 | 107 | 39.1 | 112 | 42.7 |
| T3a | 9 | 3.4 | 7 | 2.6 | 7 | 2.6 | 9 | 3.4 |
| T3b | 22 | 8.3 | 19 | 7 | 22 | 8.0 | 19 | 7.3 |
| T4 | 48 | 18.2 | 47 | 17.3 | 50 | 18.2 | 45 | 17.2 |
| TX | 36 | 13.6 | 36 | 13.2 | 34 | 12.4 | 38 | 14.5 |
| T2c | 1 | 0.4 | 1 | 0.4 | 1 | 0.4 | 1 | 0.4 |
| Missing | 115 | – | 101 | – | 104 | – | 112 | – |
| Staging: M | ||||||||
| M0 | 77 | 29.2 | 81 | 29.8 | 85 | 31.0 | 73 | 27.9 |
| M1a | 42 | 15.9 | 41 | 15.1 | 40 | 14.6 | 43 | 16.4 |
| M1b | 11 | 4.2 | 9 | 3.3 | 15 | 5.5 | 5 | 1.9 |
| M1c | 10 | 3.8 | 15 | 5.5 | 14 | 5.1 | 11 | 4.2 |
| MX | 43 | 16.3 | 40 | 14.7 | 40 | 14.6 | 43 | 16.4 |
| M1 | 81 | 30.7 | 86 | 31.6 | 80 | 29.2 | 87 | 33.2 |
| Missing | 115 | – | 101 | – | 104 | – | 112 | – |
| Staging: N | ||||||||
| N0 | 105 | 39.8 | 115 | 42.3 | 116 | 42.3 | 104 | 39.7 |
| N1 | 74 | 28.0 | 67 | 24.6 | 70 | 25.5 | 71 | 27.1 |
| NX | 85 | 32.2 | 90 | 33.1 | 88 | 32.1 | 87 | 33.2 |
| Missing | 115 | – | 101 | – | 104 | – | 112 | – |
| Gleason score | ||||||||
| 3 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 1 | 0.4 |
| 4 | 3 | 1.1 | 0 | 0.0 | 2 | 0.7 | 1 | 0.4 |
| 5 | 4 | 1.4 | 6 | 2.2 | 5 | 1.7 | 5 | 1.8 |
| 6 | 21 | 7.4 | 18 | 6.5 | 21 | 7.3 | 18 | 6.6 |
| 7 | 82 | 29.1 | 89 | 32.1 | 91 | 31.7 | 80 | 29.4 |
| 8 | 65 | 23.0 | 49 | 17.7 | 54 | 18.8 | 60 | 22.1 |
| 9 | 98 | 34.8 | 109 | 39.4 | 112 | 39.0 | 95 | 34.9 |
| 10 | 9 | 3.2 | 5 | 1.8 | 2 | 0.7 | 12 | 4.4 |
| Missing | 97 | – | 96 | – | 91 | – | 102 | – |
| Prior radiotherapy received? | ||||||||
| No | 209 | 55.3 | 205 | 55.0 | 221 | 58.5 | 193 | 51.7 |
| Yes | 169 | 44.7 | 168 | 45.0 | 157 | 41.5 | 180 | 48.3 |
| Missing | 1 | – | – | – | – | – | 1 | – |
| Method of castration | ||||||||
| Surgery | 8 | 2.1 | 6 | 1.6 | 9 | 2.4 | 5 | 1.3 |
| Ongoing LHRH agonists | 371 | 97.9 | 367 | 98.4 | 369 | 97.6 | 369 | 98.7 |
| Anti-androgen received? | ||||||||
| No | 27 | 7.1 | 33 | 8.9 | 29 | 7.7 | 31 | 8.3 |
| Yes | 351 | 92.9 | 339 | 91.1 | 349 | 92.3 | 341 | 91.7 |
| Flutamide, nilutamide or cyproterone acetate received? | ||||||||
| No | 293 | 84.0 | 274 | 80.8 | 292 | 83.9 | 275 | 80.9 |
| Yes | 56 | 16.0 | 65 | 19.2 | 56 | 16.1 | 65 | 19.1 |
| Missing | 30 | – | 34 | – | 30 | – | 34 | – |
| Bicalutamide received? | ||||||||
| No | 19 | 5.4 | 29 | 8.6 | 25 | 7.2 | 23 | 6.7 |
| Yes | 332 | 94.6 | 310 | 91.4 | 324 | 92.8 | 318 | 93.3 |
| Missing | 28 | – | 34 | – | 29 | – | 33 | – |
| Method of progression at study entry | ||||||||
| All | 53 | 14.0 | 54 | 14.6 | 53 | 14.1 | 54 | 14.4 |
| Elevated PSA | 84 | 22.2 | 92 | 24.8 | 90 | 24.0 | 86 | 23.0 |
| New lesion | 36 | 9.5 | 35 | 9.4 | 31 | 8.3 | 40 | 10.7 |
| Objective | 6 | 1.6 | 2 | 0.5 | 6 | 1.6 | 2 | 0.5 |
| Objective + new lesion | 10 | 2.6 | 9 | 2.4 | 7 | 1.9 | 12 | 3.2 |
| PSA + new lesion | 176 | 46.6 | 173 | 46.6 | 179 | 47.7 | 170 | 45.5 |
| PSA + objective | 13 | 3.4 | 6 | 1.6 | 9 | 2.4 | 10 | 2.7 |
| Missing | 1 | – | 2 | – | 3 | – | 0 | – |
| Baseline pain diary completed? | ||||||||
| No | 72 | 19.0 | 71 | 19.0 | 67 | 17.7 | 76 | 20.3 |
| Yes | 307 | 81.0 | 302 | 81.0 | 311 | 82.3 | 298 | 79.7 |
| Baseline QoL booklet completed? | ||||||||
| No | 50 | 13.2 | 44 | 12.0 | 41 | 11.0 | 53 | 14.3 |
| Yes | 328 | 86.8 | 323 | 88.0 | 333 | 89.0 | 318 | 85.7 |
| Missing | 1 | – | 6 | – | 4 | – | 3 | – |
| n | n | n | n | |||||
| Age at randomisation (years) | ||||||||
| N | 379 | 373 | 378 | 374 | ||||
| Median | 68.4 | 69.0 | 68.9 | 68.6 | ||||
| IQR | 63.6–73.6 | 64.1–73.4 | 64.3–73.8 | 63.2–73.1 | ||||
| Range | 45.9–83.8 | 45.0–83.7 | 45.0–83.8 | 49.4–82.0 | ||||
| Days from baseline ECOG to randomisation | ||||||||
| N | 349 | 339 | 341 | 347 | ||||
| Median | 2.0 | 2.0 | 2.0 | 2.0 | ||||
| IQR | 0.0–8.0 | 0.0–8.0 | 0.0–8.0 | 0.0–7.0 | ||||
| Range | 0.0–367.0 | 0.0–73.0 | 0.0–51.0 | 0.0–367.0 | ||||
| Months from diagnosis to randomisation | ||||||||
| N | 379 | 373 | 378 | 374 | ||||
| Median | 30.1 | 37.8 | 34.0 | 33.3 | ||||
| IQR | 18.7–61.6 | 20.2–62.1 | 19.2–57.0 | 19.0–70.1 | ||||
| Range | 1.3–246.2 | 0.3–190.3 | 0.3–246.2 | 0.4–187.2 | ||||
Treatment
In total, 4488 treatment forms were returned. Table 16 shows the treatment information split by cycle for each of the randomisation arms and Table 17 shows the same details split by comparison groups. The data show that only 17% of patients received 10 cycles of docetaxel, with 45% stopping at cycle 6. It is important to take into account that 29% of patients were only ever intended to receive six cycles of treatment, as previously detailed in Withdrawal of trial treatment, docetaxel.
| Treatment details | Cycle | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 (N = 729) | C2 (N = 692) | C3 (N = 665) | C4 (N = 623) | C5 (N = 588) | C6 (N = 527) | C7 (N = 202) | C8 (N = 179) | C9 (N = 154) | C10 (N = 129) | |||||||||||
| Docetaxel: days since randomisation | ||||||||||||||||||||
| n | 186 | 179 | 170 | 158 | 148 | 128 | 45 | 38 | 34 | 26 | ||||||||||
| Median | 6.0 | 28.0 | 49.0 | 70.0 | 91.0 | 112.0 | 133.0 | 154.0 | 175.5 | 196.0 | ||||||||||
| IQR | 2.0–8.0 | 24.0–31.0 | 45.0–54.0 | 67.0–76.0 | 88.0–98.0 | 110.0–119.0 | 130.0–140.0 | 151.0–162.0 | 173.0–183.0 | 194.0–204.0 | ||||||||||
| Docetaxel + ZA: days since randomisation | ||||||||||||||||||||
| n | 180 | 167 | 163 | 156 | 148 | 132 | 57 | 53 | 43 | 34 | ||||||||||
| Median | 6.0 | 27.0 | 49.0 | 70.0 | 91.0 | 113.0 | 135.0 | 156.0 | 178.0 | 199.0 | ||||||||||
| IQR | 3.0–9.0 | 24.0–32.0 | 45.0–53.0 | 67.5–75.0 | 88.0–97.0 | 110.0–119.0 | 131.0–139.0 | 152.0–161.0 | 173.0–183.0 | 194.0–203.0 | ||||||||||
| Docetaxel + Sr-89: days since randomisation | ||||||||||||||||||||
| n | 181 | 175 | 166 | 152 | 144 | 130 | 49 | 44 | 40 | 35 | ||||||||||
| Median | 7.0 | 28.0 | 49.0 | 70.0 | 92.0 | 113.0 | 174.0 | 195.5 | 218.0 | 241.0 | ||||||||||
| IQR | 3.0–9.0 | 25.0–31.0 | 46.0–53.0 | 68.0–74.0 | 89.0–97.0 | 110.0–118.0 | 170.0–183.0 | 190.5–205.0 | 212.5–228.0 | 233.0–254.0 | ||||||||||
| Docetaxel + ZA + Sr-89: days since randomisation | ||||||||||||||||||||
| n | 182 | 171 | 166 | 157 | 148 | 137 | 51 | 44 | 37 | 34 | ||||||||||
| Median | 6.0 | 27.0 | 49.0 | 70.0 | 91.0 | 112.0 | 175.0 | 197.0 | 217.0 | 239.5 | ||||||||||
| IQR | 2.0–8.0 | 24.0–30.0 | 46.0–53.0 | 67.0–75.0 | 88.5–96.0 | 110.0–118.0 | 169.0–185.0 | 189.5–207.0 | 211.0–229.0 | 232.0–250.0 | ||||||||||
| Docetaxel: total dose (mg) | ||||||||||||||||||||
| n | 186 | 179 | 169 | 158 | 148 | 128 | 44 | 38 | 34 | 26 | ||||||||||
| Median | 150.0 | 147.0 | 145.0 | 145.0 | 145.0 | 145.0 | 146.5 | 140.0 | 142.0 | 139.5 | ||||||||||
| IQR | 140.0–155.0 | 140.0–152.0 | 135.0–150.0 | 130.0–150.0 | 130.0–150.0 | 130.0–150.0 | 130.0–150.0 | 120.0–150.0 | 120.0–150.0 | 120.0–150.0 | ||||||||||
| Docetaxel + ZA: total dose (mg) | ||||||||||||||||||||
| n | 180 | 167 | 162 | 155 | 148 | 132 | 57 | 53 | 43 | 34 | ||||||||||
| Median | 150.0 | 150.0 | 150.0 | 150.0 | 148.0 | 148.0 | 143.0 | 142.0 | 140.0 | 140.0 | ||||||||||
| IQR | 140.0–156.0 | 140.0–155.0 | 140.0–155.0 | 135.0–152.0 | 130.0–155.0 | 130.0–155.0 | 130.0–152.0 | 130.0–152.0 | 130.0–150.0 | 130.0–150.0 | ||||||||||
| Docetaxel + Sr-89: total dose (mg) | ||||||||||||||||||||
| n | 181 | 175 | 166 | 152 | 144 | 130 | 49 | 44 | 40 | 35 | ||||||||||
| Median | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 148.0 | 150.0 | 150.0 | 149.0 | 150.0 | ||||||||||
| IQR | 140.0–158.0 | 138.0–156.0 | 135.0–156.0 | 135.0–156.0 | 130.0–156.0 | 130.0–156.0 | 130.0–156.0 | 122.5–156.0 | 124.5–155.5 | 120.0–158.0 | ||||||||||
| Docetaxel + ZA + Sr-89: total dose (mg) | ||||||||||||||||||||
| n | 182 | 171 | 166 | 157 | 148 | 137 | 51 | 44 | 37 | 34 | ||||||||||
| Median | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | ||||||||||
| IQR | 140.0–155.0 | 140.0–155.0 | 135.0–155.0 | 135.0–155.0 | 135.0–155.0 | 135.0–155.0 | 130.0–150.0 | 125.0–150.0 | 135.0–150.0 | 120.0–150.0 | ||||||||||
| Treatment details | C1 (N = 729) | C2 (N = 692) | C3 (N = 665) | C4 (N = 623) | C5 (N = 588) | C6 (N = 527) | C7 (N = 202) | C8 (N = 179) | C9 (N = 154) | C10 (N = 129) | ||||||||||
| Score | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| Docetaxel: ECOG performance status score | ||||||||||||||||||||
| 0 | 70 | 46.4 | 65 | 42.2 | 51 | 36.2 | 53 | 40.2 | 52 | 44.1 | 40 | 37.7 | 14 | 37.8 | 12 | 34.3 | 10 | 32.3 | 8 | 34.8 |
| 1 | 70 | 46.4 | 75 | 48.7 | 80 | 56.7 | 68 | 51.5 | 62 | 52.5 | 63 | 59.4 | 20 | 54.1 | 20 | 57.1 | 19 | 61.3 | 14 | 60.9 |
| 2 | 9 | 6.0 | 14 | 9.1 | 10 | 7.1 | 10 | 7.6 | 3 | 2.5 | 3 | 2.8 | 3 | 8.1 | 3 | 8.6 | 2 | 6.5 | 1 | 4.3 |
| 3 | 2 | 1.3 | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Missing | 578 | – | 538 | – | 524 | – | 491 | – | 470 | – | 421 | – | 165 | – | 144 | – | 123 | – | 106 | – |
| Docetaxel + ZA: ECOG performance status score | ||||||||||||||||||||
| 0 | 67 | 43.8 | 59 | 43.1 | 53 | 39.8 | 49 | 38.0 | 47 | 39.5 | 40 | 37.4 | 23 | 45.1 | 24 | 48.0 | 24 | 64.9 | 14 | 45.2 |
| 1 | 77 | 50.3 | 70 | 51.1 | 71 | 53.4 | 71 | 55.0 | 65 | 54.6 | 65 | 60.7 | 27 | 52.9 | 22 | 44.0 | 11 | 29.7 | 17 | 54.8 |
| 2 | 9 | 5.9 | 8 | 5.8 | 8 | 6.0 | 8 | 6.2 | 7 | 5.9 | 2 | 1.9 | 1 | 2.0 | 4 | 8.0 | 2 | 5.4 | 0 | 0.0 |
| 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Missing | 576 | – | 555 | – | 532 | – | 494 | – | 469 | – | 420 | – | 151 | – | 129 | – | 117 | – | 98 | – |
| Docetaxel + Sr-89: ECOG performance status score | ||||||||||||||||||||
| 0 | 66 | 44.3 | 59 | 40.4 | 59 | 44.4 | 58 | 44.3 | 42 | 33.6 | 37 | 35.2 | 19 | 41.3 | 19 | 50.0 | 17 | 47.2 | 9 | 30 |
| 1 | 68 | 45.6 | 75 | 51.4 | 64 | 48.1 | 65 | 49.6 | 77 | 61.6 | 62 | 59.0 | 26 | 56.5 | 19 | 50.0 | 18 | 50.0 | 20 | 66.7 |
| 2 | 15 | 10.1 | 11 | 7.5 | 9 | 6.8 | 7 | 5.3 | 6 | 4.8 | 6 | 5.7 | 1 | 2.2 | 0 | 0.0 | 1 | 2.8 | 1 | 3.3 |
| 3 | 0 | 0.0 | 1 | 0.7 | 1 | 0.8 | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Missing | 580 | – | 546 | – | 532 | – | 492 | – | 463 | – | 422 | – | 156 | – | 141 | – | 118 | – | 99 | – |
| Docetaxel + ZA + Sr-89: ECOG performance status score | ||||||||||||||||||||
| 0 | 63 | 40.4 | 50 | 35.7 | 44 | 31.0 | 37 | 28.7 | 30 | 22.9 | 34 | 29.6 | 16 | 33.3 | 17 | 40.5 | 18 | 51.4 | 10 | 34.5 |
| 1 | 83 | 53.2 | 83 | 59.3 | 89 | 62.7 | 87 | 67.4 | 89 | 67.9 | 69 | 60.0 | 30 | 62.5 | 25 | 59.5 | 16 | 45.7 | 17 | 58.6 |
| 2 | 9 | 5.8 | 7 | 5.0 | 8 | 5.6 | 4 | 3.1 | 10 | 7.6 | 12 | 10.4 | 2 | 4.2 | 0 | 0.0 | 1 | 2.9 | 2 | 6.9 |
| 3 | 1 | 0.6 | 0 | 0.0 | 1 | 0.7 | 1 | 0.8 | 2 | 1.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Missing | 573 | – | 552 | – | 523 | – | 494 | – | 457 | – | 412 | – | 154 | – | 137 | – | 119 | – | 100 | – |
| Docetaxel: antibiotics given? | ||||||||||||||||||||
| No | 155 | 83.8 | 153 | 85.5 | 142 | 83.5 | 135 | 85.4 | 130 | 88.4 | 117 | 92.1 | 40 | 88.9 | 34 | 89.5 | 30 | 88.2 | 24 | 92.3 |
| Yes | 30 | 16.2 | 26 | 14.5 | 28 | 16.5 | 23 | 14.6 | 17 | 11.6 | 10 | 7.9 | 5 | 11.1 | 4 | 10.5 | 4 | 11.8 | 2 | 7.7 |
| Missing | 544 | – | 513 | – | 495 | – | 465 | – | 441 | – | 400 | – | 157 | – | 141 | – | 120 | – | 103 | – |
| Docetaxel + ZA: antibiotics given? | ||||||||||||||||||||
| No | 154 | 85.6 | 143 | 85.6 | 139 | 85.8 | 137 | 88.4 | 132 | 89.8 | 120 | 90.9 | 50 | 87.7 | 45 | 84.9 | 36 | 83.7 | 31 | 91.2 |
| Yes | 26 | 14.4 | 24 | 14.4 | 23 | 14.2 | 18 | 11.6 | 15 | 10.2 | 12 | 9.1 | 7 | 12.3 | 8 | 15.1 | 7 | 16.3 | 3 | 8.8 |
| Missing | 549 | – | 525 | – | 503 | – | 468 | – | 441 | – | 395 | – | 145 | – | 126 | – | 111 | – | 95 | – |
| Docetaxel + Sr-89: antibiotics given? | ||||||||||||||||||||
| No | 155 | 85.6 | 145 | 83.3 | 137 | 83.5 | 135 | 88.8 | 129 | 90.2 | 117 | 90.0 | 40 | 81.6 | 39 | 88.6 | 34 | 85.0 | 28 | 82.4 |
| Yes | 26 | 14.4 | 29 | 16.7 | 27 | 16.5 | 17 | 11.2 | 14 | 9.8 | 13 | 10.0 | 9 | 18.4 | 5 | 11.4 | 6 | 15.0 | 6 | 17.6 |
| Missing | 548 | – | 518 | – | 501 | – | 471 | – | 445 | – | 397 | – | 153 | – | 135 | – | 114 | – | 95 | – |
| Docetaxel + ZA + Sr-89: antibiotics given? | ||||||||||||||||||||
| No | 160 | 88.9 | 152 | 88.9 | 149 | 89.8 | 140 | 89.2 | 134 | 90.5 | 121 | 88.3 | 46 | 90.2 | 43 | 97.7 | 35 | 94.6 | 33 | 97.1 |
| Yes | 20 | 11.1 | 19 | 11.1 | 17 | 10.2 | 17 | 10.8 | 14 | 9.5 | 16 | 11.7 | 5 | 9.8 | 1 | 2.3 | 2 | 5.4 | 1 | 2.9 |
| Missing | 549 | – | 521 | – | 499 | – | 466 | – | 440 | – | 390 | – | 151 | – | 135 | – | 117 | – | 95 | – |
| Docetaxel: analgesics received? | ||||||||||||||||||||
| No | 101 | 55.2 | 105 | 58.7 | 100 | 58.8 | 93 | 59.2 | 86 | 58.5 | 77 | 60.6 | 25 | 55.6 | 22 | 57.9 | 21 | 61.8 | 13 | 50 |
| Yes | 82 | 44.8 | 74 | 41.3 | 70 | 41.2 | 64 | 40.8 | 61 | 41.5 | 50 | 39.4 | 20 | 44.4 | 16 | 42.1 | 13 | 38.2 | 13 | 50 |
| Missing | 546 | – | 513 | – | 495 | – | 466 | – | 441 | – | 400 | – | 157 | – | 141 | – | 120 | – | 103 | – |
| Docetaxel + ZA: analgesics received? | ||||||||||||||||||||
| No | 97 | 54.5 | 96 | 57.5 | 96 | 58.9 | 93 | 60.4 | 96 | 65.3 | 84 | 64.1 | 44 | 77.2 | 41 | 78.8 | 33 | 76.7 | 27 | 79.4 |
| Yes | 81 | 45.5 | 71 | 42.5 | 67 | 41.1 | 61 | 39.6 | 51 | 34.7 | 47 | 35.9 | 13 | 22.8 | 11 | 21.2 | 10 | 23.3 | 7 | 20.6 |
| Missing | 551 | – | 525 | – | 502 | – | 469 | – | 441 | – | 396 | – | 145 | – | 127 | – | 111 | – | 95 | – |
| Docetaxel + Sr-89: analgesics received? | ||||||||||||||||||||
| No | 106 | 58.6 | 101 | 58.0 | 99 | 60.4 | 88 | 57.9 | 82 | 57.7 | 78 | 60.5 | 36 | 75.0 | 32 | 74.4 | 30 | 75 | 24 | 68.6 |
| Yes | 75 | 41.4 | 73 | 42.0 | 65 | 39.6 | 64 | 42.1 | 60 | 42.3 | 51 | 39.5 | 12 | 25.0 | 11 | 25.6 | 10 | 25.0 | 11 | 31.4 |
| Missing | 548 | – | 518 | – | 501 | – | 471 | – | 446 | – | 398 | – | 154 | – | 136 | – | 114 | – | 94 | – |
| Docetaxel + ZA + Sr-89: analgesics received? | ||||||||||||||||||||
| No | 97 | 53.6 | 102 | 59.6 | 103 | 62.0 | 104 | 66.2 | 92 | 62.6 | 85 | 63.0 | 30 | 58.8 | 24 | 54.5 | 23 | 62.2 | 24 | 72.7 |
| Yes | 84 | 46.4 | 69 | 40.4 | 63 | 38.0 | 53 | 33.8 | 55 | 37.4 | 50 | 37.0 | 21 | 41.2 | 20 | 45.5 | 14 | 37.8 | 9 | 27.3 |
| Missing | 548 | – | 521 | – | 499 | – | 466 | – | 441 | – | 392 | – | 151 | – | 135 | – | 117 | – | 96 | – |
| Docetaxel: GCSF received? | ||||||||||||||||||||
| No | 176 | 95.1 | 172 | 96.1 | 165 | 97.1 | 154 | 97.5 | 143 | 97.3 | 125 | 98.4 | 45 | 100.0 | 38 | 100.0 | 33 | 97.1 | 26 | 100.0 |
| Yes | 9 | 4.9 | 7 | 3.9 | 5 | 2.9 | 4 | 2.5 | 4 | 2.7 | 2 | 1.6 | 0 | 0.0 | 0 | 0.0 | 1 | 2.9 | 0 | 0.0 |
| Missing | 544 | – | 513 | – | 495 | – | 465 | – | 441 | – | 400 | – | 157 | – | 141 | – | 120 | – | 103 | – |
| Docetaxel + ZA: GCSF received? | ||||||||||||||||||||
| No | 173 | 96.1 | 162 | 97.0 | 157 | 96.3 | 148 | 95.5 | 141 | 95.9 | 127 | 96.2 | 56 | 98.2 | 52 | 98.1 | 43 | 100.0 | 32 | 94.1 |
| Yes | 7 | 3.9 | 5 | 3.0 | 6 | 3.7 | 7 | 4.5 | 6 | 4.1 | 5 | 3.8 | 1 | 1.8 | 1 | 1.9 | 0 | 0.0 | 2 | 5.9 |
| Missing | 549 | – | 525 | – | 502 | – | 468 | – | 441 | – | 395 | – | 145 | – | 126 | – | 111 | – | 95 | – |
| Docetaxel + Sr-89: GCSF received? | ||||||||||||||||||||
| No | 175 | 96.7 | 167 | 96.0 | 160 | 97.6 | 146 | 96.1 | 138 | 96.5 | 125 | 96.2 | 48 | 98.0 | 44 | 100.0 | 40 | 100.0 | 35 | 100.0 |
| Yes | 6 | 3.3 | 7 | 4.0 | 4 | 2.4 | 6 | 3.9 | 5 | 3.5 | 5 | 3.8 | 1 | 2.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Missing | 548 | – | 518 | – | 501 | – | 471 | – | 445 | – | 397 | – | 153 | – | 135 | – | 114 | 94 | – | |
| Docetaxel + ZA + Sr-89: GCSF received? | ||||||||||||||||||||
| No | 176 | 97.2 | 164 | 95.9 | 160 | 96.4 | 153 | 97.5 | 143 | 96.6 | 132 | 96.4 | 49 | 96.1 | 42 | 95.5 | 35 | 94.6 | 32 | 94.1 |
| Yes | 5 | 2.8 | 7 | 4.1 | 6 | 3.6 | 4 | 2.5 | 5 | 3.4 | 5 | 3.6 | 2 | 3.9 | 2 | 4.5 | 2 | 5.4 | 2 | 5.9 |
| Missing | 548 | – | 521 | – | 499 | – | 466 | – | 440 | – | 390 | – | 151 | – | 135 | – | 117 | – | 95 | – |
| Docetaxel + ZA: reason ZA discontinued | ||||||||||||||||||||
| GFR increases to > 1.5 ULN | 0 | 0.0 | 1 | 20.0 | 1 | 25.0 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Hypersensitivity to ZA | 0 | 0.0 | 1 | 20.0 | 1 | 25.0 | 1 | 25.0 | 1 | 25.0 | 1 | 12.5 | 1 | 50.0 | 1 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| Other | 4 | 100.0 | 3 | 60.0 | 2 | 50.0 | 2 | 50.0 | 3 | 75.0 | 7 | 87.5 | 1 | 50.0 | 2 | 66.7 | 3 | 100.0 | 2 | 100.0 |
| Missing | 725 | – | 687 | – | 661 | – | 619 | – | 584 | – | 519 | – | 200 | – | 176 | – | 151 | – | 127 | – |
| Docetaxel + ZA + Sr-89: reason ZA discontinued | ||||||||||||||||||||
| Hypersensitivity to ZA | 0 | 0.0 | 1 | 25.0 | 1 | 25.0 | 2 | 100.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Other | 2 | 100.0 | 3 | 75.0 | 3 | 75.0 | 0 | 0.0 | 1 | 50.0 | 4 | 100.0 | 1 | 100.0 | 3 | 100.0 | 0 | 0.0 | 2 | 100.0 |
| Missing | 727 | – | 688 | – | 661 | – | 621 | – | 586 | – | 523 | – | 201 | – | 176 | – | 154 | – | 127 | – |
| Docetaxel + ZA: ZA dose administered (mg) | ||||||||||||||||||||
| 0 | 2 | 1.1 | 5 | 3.0 | 4 | 2.5 | 5 | 3.2 | 3 | 2.1 | 8 | 6.1 | 2 | 3.6 | 3 | 5.7 | 3 | 7.0 | 2 | 6.1 |
| 3 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 1 | 0.6 | 2 | 1.4 | 2 | 1.5 | 2 | 3.6 | 2 | 3.8 | 1 | 2.3 | 0 | 0.0 |
| 3.3 | 7 | 3.9 | 5 | 3.0 | 5 | 3.1 | 6 | 3.8 | 3 | 2.1 | 7 | 5.3 | 1 | 1.8 | 1 | 1.9 | 1 | 2.3 | 2 | 6.1 |
| 3.5 | 7 | 3.9 | 6 | 3.6 | 8 | 4.9 | 5 | 3.2 | 4 | 2.7 | 5 | 3.8 | 2 | 3.6 | 1 | 1.9 | 0 | 0.0 | 0 | 0.0 |
| 4 | 164 | 91.1 | 151 | 90.4 | 145 | 89.0 | 139 | 89.1 | 134 | 91.8 | 110 | 83.3 | 49 | 87.5 | 46 | 86.8 | 38 | 88.4 | 29 | 87.9 |
| Missing | 549 | – | 525 | – | 502 | – | 467 | – | 442 | – | 395 | – | 146 | – | 126 | – | 111 | – | 96 | – |
| Docetaxel + ZA + Sr-89: ZA dose administered (mg) | ||||||||||||||||||||
| 0 | 2 | 1.1 | 5 | 2.9 | 4 | 2.4 | 2 | 1.3 | 2 | 1.4 | 3 | 2.2 | 1 | 2.0 | 3 | 6.8 | 0 | 0.0 | 1 | 2.9 |
| 3 | 4 | 2.2 | 5 | 2.9 | 5 | 3.0 | 3 | 1.9 | 2 | 1.4 | 2 | 1.5 | 1 | 2.0 | 1 | 2.3 | 1 | 2.7 | 1 | 2.9 |
| 3.3 | 7 | 3.8 | 5 | 2.9 | 4 | 2.4 | 4 | 2.5 | 3 | 2.0 | 3 | 2.2 | 1 | 2.0 | 1 | 2.3 | 0 | 0.0 | 0 | 0.0 |
| 3.5 | 8 | 4.4 | 6 | 3.5 | 6 | 3.6 | 5 | 3.2 | 5 | 3.4 | 2 | 1.5 | 2 | 3.9 | 1 | 2.3 | 1 | 2.7 | 2 | 5.9 |
| 4 | 161 | 88.5 | 150 | 87.7 | 147 | 88.6 | 143 | 91.1 | 136 | 91.9 | 127 | 92.7 | 46 | 90.2 | 38 | 86.4 | 35 | 94.6 | 30 | 88.2 |
| Missing | 547 | – | 521 | – | 499 | – | 466 | – | 440 | – | 390 | – | 151 | – | 135 | – | 117 | – | 95 | – |
| Treatment details | Cycle | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | |||||||||||
| No ZA: days since randomisation | ||||||||||||||||||||
| n | 367 | 354 | 336 | 310 | 292 | 258 | 94 | 82 | 74 | 61 | ||||||||||
| Median | 6.0 | 28.0 | 49.0 | 70.0 | 91.0 | 113.0 | 167.5 | 189.0 | 210.0 | 232.0 | ||||||||||
| IQR | 2.0–9.0 | 24.0–31.0 | 46.0–53.0 | 67.0–76.0 | 89.0–97.0 | 110.0–118.0 | 134.0–175.0 | 155.0–196.0 | 176.0–220.0 | 197.0–244.0 | ||||||||||
| ZA: days since randomisation | ||||||||||||||||||||
| n | 362 | 338 | 329 | 313 | 296 | 269 | 108 | 97 | 80 | 68 | ||||||||||
| Median | 6.0 | 27.0 | 49.0 | 70.0 | 91.0 | 112.0 | 145.0 | 164.0 | 189.5 | 215.0 | ||||||||||
| IQR | 2.0–9.0 | 24.0–31.0 | 46.0–53.0 | 67.0–75.0 | 88.0–96.0 | 110.0–118.0 | 134.0–175.0 | 155.0–196.0 | 177.0–217.0 | 198.5–239.5 | ||||||||||
| No Sr-89: days since randomisation | ||||||||||||||||||||
| n | 366 | 346 | 333 | 314 | 296 | 260 | 102 | 91 | 77 | 60 | ||||||||||
| Median | 6.0 | 27.0 | 49.0 | 70.0 | 91.0 | 112.0 | 134.0 | 155.0 | 177.0 | 197.0 | ||||||||||
| IQR | 2.0–9.0 | 24.0–31.0 | 45.0–53.0 | 67.0–76.0 | 88.0–97.0 | 110.0–119.0 | 131.0–140.0 | 151.0–162.0 | 173.0–183.0 | 194.0–203.5 | ||||||||||
| Sr-89: days since randomisation | ||||||||||||||||||||
| n | 363 | 346 | 332 | 309 | 292 | 267 | 100 | 88 | 77 | 69 | ||||||||||
| Median | 6.0 | 28.0 | 49.0 | 70.0 | 91.0 | 113.0 | 175.0 | 196.0 | 217.0 | 240.0 | ||||||||||
| IQR | 3.0–9.0 | 24.0–30.0 | 46.0–53.0 | 67.0–75.0 | 89.0–96.0 | 110.0–118.0 | 169.0–184.0 | 190.0–206.0 | 212.0–229.0 | 233.0–251.0 | ||||||||||
| No ZA: total dose (mg) | ||||||||||||||||||||
| n | 367 | 354 | 335 | 310 | 292 | 258 | 93 | 82 | 74 | 61 | ||||||||||
| Median | 150.0 | 149.0 | 147.0 | 145.0 | 145.0 | 145.0 | 148.0 | 148.0 | 148.0 | 140.0 | ||||||||||
| IQR | 140.0–155.0 | 140.0–155.0 | 135.0–155.0 | 135.0–155.0 | 130.0–155.0 | 130.0–155.0 | 130.0–155.0 | 120.0–155.0 | 124.0–155.0 | 120.0–155.0 | ||||||||||
| ZA: total dose (mg) | ||||||||||||||||||||
| n | 362 | 338 | 328 | 312 | 296 | 269 | 108 | 97 | 80 | 68 | ||||||||||
| Median | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 145.0 | 145.0 | 146.5 | 144.0 | ||||||||||
| IQR | 140.0–155.0 | 140.0–155.0 | 135.5–155.0 | 135.0–153.5 | 135.0–155.0 | 135.0–155.0 | 130.0–150.0 | 130.0–150.0 | 130.0–150.0 | 127.0–150.0 | ||||||||||
| No Sr-89: total dose (mg) | ||||||||||||||||||||
| n | 366 | 346 | 331 | 313 | 296 | 260 | 101 | 91 | 77 | 60 | ||||||||||
| Median | 150.0 | 150.0 | 150.0 | 145.0 | 145.0 | 145.0 | 144.0 | 140.0 | 140.0 | 140.0 | ||||||||||
| IQR | 140.0–155.0 | 140.0–155.0 | 135.0–152.0 | 135.0–152.0 | 130.0–152.0 | 130.0–151.0 | 130.0–150.0 | 130.0–152.0 | 130.0–150.0 | 120.0–150.0 | ||||||||||
| Sr-89: total dose (mg) | ||||||||||||||||||||
| n | 363 | 346 | 332 | 309 | 292 | 267 | 100 | 88 | 77 | 69 | ||||||||||
| Median | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | ||||||||||
| IQR | 140.0– 155.0 | 140.0–155.0 | 135.0–155.0 | 135.0–155.0 | 135.0–155.0 | 130.0–155.0 | 130.0–150.0 | 122.5–152.5 | 130.0–150.0 | 120.0–155.0 | ||||||||||
| Treatment details | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | ||||||||||
| Score | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| No ZA: ECOG performance status score | ||||||||||||||||||||
| 0 | 136 | 45.3 | 124 | 41.3 | 110 | 40.1 | 111 | 42.2 | 94 | 38.7 | 77 | 36.5 | 33 | 39.8 | 31 | 42.5 | 27 | 40.3 | 17 | 32.1 |
| 1 | 138 | 46.0 | 150 | 50.0 | 144 | 52.6 | 133 | 50.6 | 139 | 57.2 | 125 | 59.2 | 46 | 55.4 | 39 | 53.4 | 37 | 55.2 | 34 | 64.2 |
| 2 | 24 | 8.0 | 25 | 8.3 | 19 | 6.9 | 17 | 6.5 | 9 | 3.7 | 9 | 4.3 | 4 | 4.8 | 3 | 4.1 | 3 | 4.5 | 2 | 3.8 |
| 3 | 2 | 0.7 | 1 | 0.3 | 1 | 0.4 | 2 | 0.8 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| ZA: ECOG performance status score | ||||||||||||||||||||
| 0 | 130 | 42.1 | 109 | 39.4 | 97 | 35.3 | 86 | 33.3 | 77 | 30.8 | 74 | 33.3 | 39 | 39.4 | 41 | 44.6 | 42 | 58.3 | 24 | 40.0 |
| 1 | 160 | 51.8 | 153 | 55.2 | 160 | 58.2 | 158 | 61.2 | 154 | 61.6 | 134 | 60.4 | 57 | 57.6 | 47 | 51.1 | 27 | 37.5 | 34 | 56.7 |
| 2 | 18 | 5.8 | 15 | 5.4 | 16 | 5.8 | 12 | 4.7 | 17 | 6.8 | 14 | 6.3 | 3 | 3.0 | 4 | 4.3 | 3 | 4.2 | 2 | 3.3 |
| 3 | 1 | 0.3 | 0 | 0.0 | 1 | 0.4 | 2 | 0.8 | 2 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| No Sr-89: ECOG performance status score | ||||||||||||||||||||
| 0 | 137 | 45.1 | 124 | 42.6 | 104 | 38.0 | 102 | 39.1 | 99 | 41.8 | 80 | 37.6 | 37 | 42.0 | 36 | 42.4 | 34 | 50.0 | 22 | 40.7 |
| 1 | 147 | 48.4 | 145 | 49.8 | 151 | 55.1 | 139 | 53.3 | 127 | 53.6 | 128 | 60.1 | 47 | 53.4 | 42 | 49.4 | 30 | 44.1 | 31 | 57.4 |
| 2 | 18 | 5.9 | 22 | 7.6 | 18 | 6.6 | 18 | 6.9 | 10 | 4.2 | 5 | 2.3 | 4 | 4.5 | 7 | 8.2 | 4 | 5.9 | 1 | 1.9 |
| 3 | 2 | 0.7 | 0 | 0.0 | 0 | 0.0 | 2 | 0.8 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Sr-89: ECOG performance status score | ||||||||||||||||||||
| 0 | 129 | 42.3 | 109 | 38.1 | 103 | 37.5 | 95 | 36.5 | 72 | 28.1 | 71 | 32.3 | 35 | 37.2 | 36 | 45.0 | 35 | 49.3 | 19 | 32.2 |
| 1 | 151 | 49.5 | 158 | 55.2 | 153 | 55.6 | 152 | 58.5 | 166 | 64.8 | 131 | 59.5 | 56 | 59.6 | 44 | 55.0 | 34 | 47.9 | 37 | 62.7 |
| 2 | 24 | 7.9 | 18 | 6.3 | 17 | 6.2 | 11 | 4.2 | 16 | 6.3 | 18 | 8.2 | 3 | 3.2 | 0 | 0.0 | 2 | 2.8 | 3 | 5.1 |
| 3 | 1 | 0.3 | 1 | 0.3 | 2 | 0.7 | 2 | 0.8 | 2 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| No ZA: antibiotics given? | ||||||||||||||||||||
| No | 310 | 84.7 | 298 | 84.4 | 279 | 83.5 | 270 | 87.1 | 259 | 89.3 | 234 | 91.1 | 80 | 85.1 | 73 | 89.0 | 64 | 86.5 | 52 | 86.7 |
| Yes | 56 | 15.3 | 55 | 15.6 | 55 | 16.5 | 40 | 12.9 | 31 | 10.7 | 23 | 8.9 | 14 | 14.9 | 9 | 11.0 | 10 | 13.5 | 8 | 13.3 |
| ZA: antibiotics given? | ||||||||||||||||||||
| No | 314 | 87.2 | 295 | 87.3 | 288 | 87.8 | 277 | 88.8 | 266 | 90.2 | 241 | 89.6 | 96 | 88.9 | 88 | 90.7 | 71 | 88.8 | 64 | 94.1 |
| Yes | 46 | 12.8 | 43 | 12.7 | 40 | 12.2 | 35 | 11.2 | 29 | 9.8 | 28 | 10.4 | 12 | 11.1 | 9 | 9.3 | 9 | 11.3 | 4 | 5.9 |
| No Sr-89: antibiotics given? | ||||||||||||||||||||
| No | 309 | 84.7 | 296 | 85.5 | 281 | 84.6 | 272 | 86.9 | 262 | 89.1 | 237 | 91.5 | 90 | 88.2 | 79 | 86.8 | 66 | 85.7 | 55 | 91.7 |
| Yes | 56 | 15.3 | 50 | 14.5 | 51 | 15.4 | 41 | 13.1 | 32 | 10.9 | 22 | 8.5 | 12 | 11.8 | 12 | 13.2 | 11 | 14.3 | 5 | 8.3 |
| Sr-89: antibiotics given? | ||||||||||||||||||||
| No | 315 | 87.3 | 297 | 86.1 | 286 | 86.7 | 275 | 89.0 | 263 | 90.4 | 238 | 89.1 | 86 | 86.0 | 82 | 93.2 | 69 | 89.6 | 61 | 89.7 |
| Yes | 46 | 12.7 | 48 | 13.9 | 44 | 13.3 | 34 | 11.0 | 28 | 9.6 | 29 | 10.9 | 14 | 14.0 | 6 | 6.8 | 8 | 10.4 | 7 | 10.3 |
| No ZA: analgesics received? | ||||||||||||||||||||
| No | 207 | 56.9 | 206 | 58.4 | 199 | 59.6 | 181 | 58.6 | 168 | 58.1 | 155 | 60.5 | 61 | 65.6 | 54 | 66.7 | 51 | 68.9 | 37 | 60.7 |
| Yes | 157 | 43.1 | 147 | 41.6 | 135 | 40.4 | 128 | 41.4 | 121 | 41.9 | 101 | 39.5 | 32 | 34.4 | 27 | 33.3 | 23 | 31.1 | 24 | 39.3 |
| ZA: analgesics received? | ||||||||||||||||||||
| No | 194 | 54.0 | 198 | 58.6 | 199 | 60.5 | 197 | 63.3 | 188 | 63.9 | 169 | 63.5 | 74 | 68.5 | 65 | 67.7 | 56 | 70.0 | 51 | 76.1 |
| Yes | 165 | 46.0 | 140 | 41.4 | 130 | 39.5 | 114 | 36.7 | 106 | 36.1 | 97 | 36.5 | 34 | 31.5 | 31 | 32.3 | 24 | 30.0 | 16 | 23.9 |
| No Sr-89: analgesics received? | ||||||||||||||||||||
| No | 198 | 54.8 | 201 | 58.1 | 196 | 58.9 | 186 | 59.8 | 182 | 61.9 | 161 | 62.4 | 69 | 67.6 | 63 | 70.0 | 54 | 70.1 | 40 | 66.7 |
| Yes | 163 | 45.2 | 145 | 41.9 | 137 | 41.1 | 125 | 40.2 | 112 | 38.1 | 97 | 37.6 | 33 | 32.4 | 27 | 30.0 | 23 | 29.9 | 20 | 33.3 |
| Sr-89: analgesics received? | ||||||||||||||||||||
| No | 203 | 56.1 | 203 | 58.8 | 202 | 61.2 | 192 | 62.1 | 174 | 60.2 | 163 | 61.7 | 66 | 66.7 | 56 | 64.4 | 53 | 68.8 | 48 | 70.6 |
| Yes | 159 | 43.9 | 142 | 41.2 | 128 | 38.8 | 117 | 37.9 | 115 | 39.8 | 101 | 38.3 | 33 | 33.3 | 31 | 35.6 | 24 | 31.2 | 20 | 29.4 |
| No ZA: GCSF received? | ||||||||||||||||||||
| No | 351 | 95.9 | 339 | 96.0 | 325 | 97.3 | 300 | 96.8 | 281 | 96.9 | 250 | 97.3 | 93 | 98.9 | 82 | 100.0 | 73 | 98.6 | 61 | 100.0 |
| Yes | 15 | 4.1 | 14 | 4.0 | 9 | 2.7 | 10 | 3.2 | 9 | 3.1 | 7 | 2.7 | 1 | 1.1 | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 |
| ZA: GCSF received? | ||||||||||||||||||||
| No | 349 | 96.7 | 326 | 96.4 | 317 | 96.4 | 301 | 96.5 | 284 | 96.3 | 259 | 96.3 | 105 | 97.2 | 94 | 96.9 | 78 | 97.5 | 64 | 94.1 |
| Yes | 12 | 3.3 | 12 | 3.6 | 12 | 3.6 | 11 | 3.5 | 11 | 3.7 | 10 | 3.7 | 3 | 2.8 | 3 | 3.1 | 2 | 2.5 | 4 | 5.9 |
| No Sr-89: GCSF received? | ||||||||||||||||||||
| No | 349 | 95.6 | 334 | 96.5 | 322 | 96.7 | 302 | 96.5 | 284 | 96.6 | 252 | 97.3 | 101 | 99.0 | 90 | 98.9 | 76 | 98.7 | 58 | 96.7 |
| Yes | 16 | 4.4 | 12 | 3.5 | 11 | 3.3 | 11 | 3.5 | 10 | 3.4 | 7 | 2.7 | 1 | 1.0 | 1 | 1.1 | 1 | 1.3 | 2 | 3.3 |
| Sr-89: GCSF received? | ||||||||||||||||||||
| No | 351 | 97.0 | 331 | 95.9 | 320 | 97.0 | 299 | 96.8 | 281 | 96.6 | 257 | 96.3 | 97 | 97.0 | 86 | 97.7 | 75 | 97.4 | 67 | 97.1 |
| Yes | 11 | 3.0 | 14 | 4.1 | 10 | 3.0 | 10 | 3.2 | 10 | 3.4 | 10 | 3.7 | 3 | 3.0 | 2 | 2.3 | 2 | 2.6 | 2 | 2.9 |
| ZA: reason ZA discontinued | ||||||||||||||||||||
| GFR increases to > 1.5 ULN | 0 | 0.0 | 1 | 11.1 | 1 | 12.5 | 1 | 16.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Hypersensitivity to ZA | 0 | 0.0 | 2 | 22.2 | 2 | 25.0 | 3 | 50.0 | 2 | 33.3 | 1 | 8.3 | 1 | 33.3 | 1 | 16.7 | 0 | 0.0 | 0 | 0.0 |
| Other | 6 | 100.0 | 6 | 66.7 | 5 | 62.5 | 2 | 33.3 | 4 | 66.7 | 11 | 91.7 | 2 | 66.7 | 5 | 83.3 | 3 | 100.0 | 4 | 100.0 |
| No Sr-89: reason ZA discontinued | ||||||||||||||||||||
| GFR increases to > 1.5 ULN | 0 | 0.0 | 1 | 20.0 | 1 | 25.0 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Hypersensitivity to ZA | 0 | 0.0 | 1 | 20.0 | 1 | 25.0 | 1 | 25.0 | 1 | 25.0 | 1 | 12.5 | 1 | 50.0 | 1 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| Other | 4 | 100.0 | 3 | 60.0 | 2 | 50.0 | 2 | 50.0 | 3 | 75.0 | 7 | 87.5 | 1 | 50.0 | 2 | 66.7 | 3 | 100.0 | 2 | 100.0 |
| Sr-89: reason ZA discontinued | ||||||||||||||||||||
| Hypersensitivity to ZA | 0 | 0.0 | 1 | 25.0 | 1 | 25.0 | 2 | 100.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Other | 2 | 100.0 | 3 | 75.0 | 3 | 75.0 | 0 | 0.0 | 1 | 50.0 | 4 | 100.0 | 1 | 100.0 | 3 | 100.0 | 0 | 0.0 | 2 | 100.0 |
| ZA: ZA dose administered (mg) | ||||||||||||||||||||
| 0 | 4 | 1.1 | 10 | 3.0 | 8 | 2.4 | 7 | 2.2 | 5 | 1.7 | 11 | 4.1 | 3 | 2.8 | 6 | 6.2 | 3 | 3.8 | 3 | 4.5 |
| 3 | 4 | 1.1 | 5 | 1.5 | 6 | 1.8 | 4 | 1.3 | 4 | 1.4 | 4 | 1.5 | 3 | 2.8 | 3 | 3.1 | 2 | 2.5 | 1 | 1.5 |
| 3.3 | 14 | 3.9 | 10 | 3.0 | 9 | 2.7 | 10 | 3.2 | 6 | 2.0 | 10 | 3.7 | 2 | 1.9 | 2 | 2.1 | 1 | 1.3 | 2 | 3.0 |
| 3.5 | 15 | 4.1 | 12 | 3.6 | 14 | 4.3 | 10 | 3.2 | 9 | 3.1 | 7 | 2.6 | 4 | 3.7 | 2 | 2.1 | 1 | 1.3 | 2 | 3.0 |
| 4 | 325 | 89.8 | 301 | 89.1 | 292 | 88.8 | 282 | 90.1 | 270 | 91.8 | 237 | 88.1 | 95 | 88.8 | 84 | 86.6 | 73 | 91.3 | 59 | 88.1 |
| No Sr-89: ZA dose administered (mg) | ||||||||||||||||||||
| 0 | 2 | 1.1 | 5 | 3.0 | 4 | 2.5 | 5 | 3.2 | 3 | 2.1 | 8 | 6.1 | 2 | 3.6 | 3 | 5.7 | 3 | 7.0 | 2 | 6.1 |
| 3 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 1 | 0.6 | 2 | 1.4 | 2 | 1.5 | 2 | 3.6 | 2 | 3.8 | 1 | 2.3 | 0 | 0.0 |
| 3.3 | 7 | 3.9 | 5 | 3.0 | 5 | 3.1 | 6 | 3.8 | 3 | 2.1 | 7 | 5.3 | 1 | 1.8 | 1 | 1.9 | 1 | 2.3 | 2 | 6.1 |
| 3.5 | 7 | 3.9 | 6 | 3.6 | 8 | 4.9 | 5 | 3.2 | 4 | 2.7 | 5 | 3.8 | 2 | 3.6 | 1 | 1.9 | 0 | 0.0 | 0 | 0.0 |
| 4 | 164 | 91.1 | 151 | 90.4 | 145 | 89.0 | 139 | 89.1 | 134 | 91.8 | 110 | 83.3 | 49 | 87.5 | 46 | 86.8 | 38 | 88.4 | 29 | 87.9 |
| Sr-89: ZA dose administered (mg) | ||||||||||||||||||||
| 0 | 2 | 1.1 | 5 | 2.9 | 4 | 2.4 | 2 | 1.3 | 2 | 1.4 | 3 | 2.2 | 1 | 2.0 | 3 | 6.8 | 0 | 0.0 | 1 | 2.9 |
| 3 | 4 | 2.2 | 5 | 2.9 | 5 | 3.0 | 3 | 1.9 | 2 | 1.4 | 2 | 1.5 | 1 | 2.0 | 1 | 2.3 | 1 | 2.7 | 1 | 2.9 |
| 3.3 | 7 | 3.8 | 5 | 2.9 | 4 | 2.4 | 4 | 2.5 | 3 | 2.0 | 3 | 2.2 | 1 | 2.0 | 1 | 2.3 | 0 | 0.0 | 0 | 0.0 |
| 3.5 | 8 | 4.4 | 6 | 3.5 | 6 | 3.6 | 5 | 3.2 | 5 | 3.4 | 2 | 1.5 | 2 | 3.9 | 1 | 2.3 | 1 | 2.7 | 2 | 5.9 |
| 4 | 161 | 88.5 | 150 | 87.7 | 147 | 88.6 | 143 | 91.1 | 136 | 91.9 | 127 | 92.7 | 46 | 90.2 | 38 | 86.4 | 35 | 94.6 | 30 | 88.2 |
The 47 reasons for discontinuation of ZA which are reported as ‘other’ in Table 16 are summarised in Table 18.
| Other reasons | Number of patients |
|---|---|
| Hypocalcaemia | 6 |
| Breathlessness | 1 |
| Clinical decision | 6 |
| Dental treatment | 10 |
| Raised creatinine | 3 |
| Anaemia | 1 |
| Osteonecrosis | 1 |
| Patient choice | 1 |
| Unspecified side effects | 2 |
| Toxicities | 9 |
| Ulcer on gums | 1 |
| Omitted in error | 4 |
| Hypophosphataemia | 1 |
| Investigation of jaw pain | 1 |
Delivery of strontium-89
Table 19 shows the timings of delivery and dose of Sr-89 administered for the 253 patients who received Sr-89 split firstly by randomisation arm and then by comparison groups. Reasons for not receiving Sr-89 are detailed in withdrawal Sr-89 section.
| Sr-89 details | Randomisation arm | Comparison group | |
|---|---|---|---|
| Docetaxel + Sr-89 (N = 123) | Docetaxel + ZA + Sr-89 (N = 130) | All patients receiving Sr-89 (N = 253) | |
| Days from randomisation to Sr-89 | |||
| n | 123 | 130 | 253 |
| Median | 144.0 | 145.5 | 145.0 |
| IQR | 140.0–149.0 | 140.0–152.0 | 140.0–151.0 |
| Range | 132.0–203.0 | 133.0–182.0 | 132.0–203.0 |
| Sr-89 dose (MBq) | |||
| n | 123 | 130 | 253 |
| Median | 150.0 | 150.0 | 150.0 |
| IQR | 150.0–150.0 | 149.0–150.0 | 150.0–150.0 |
| Range | 89.0–165.0 | 89.0–169.0 | 89.0–169.0 |
Tables 20 and 21 show that most patients who were able to receive Sr-89 received the total dose, independent of whether or not they were randomised to also receive ZA.
| Dose reduction details | C1 (N = 729) | C2 (N = 692) | C3 (N = 665) | C4 (N = 623) | C5 (N = 588) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Did the patient have a dose reduction? | ||||||||||
| No | 723 | 99.2 | 627 | 90.6 | 625 | 94.0 | 579 | 92.9 | 550 | 93.9 |
| Yes | 6 | 0.8 | 65 | 9.4 | 40 | 6.0 | 44 | 7.1 | 36 | 6.1 |
| Missing | 0 | – | 0 | – | 0 | – | 0 | – | 2 | – |
| Split by randomisation arm | ||||||||||
| Docetaxel: was the dose reduced? | ||||||||||
| No | 184 | 98.9 | 164 | 91.6 | 161 | 94.7 | 142 | 89.9 | 136 | 92.5 |
| Yes | 2 | 1.1 | 15 | 8.4 | 9 | 5.3 | 16 | 10.1 | 11 | 7.5 |
| Docetaxel + ZA: was the dose reduced? | ||||||||||
| No | 180 | 100.0 | 148 | 88.6 | 153 | 93.9 | 147 | 94.2 | 139 | 94.6 |
| Yes | 0 | 0.0 | 19 | 11.4 | 10 | 6.1 | 9 | 5.8 | 8 | 5.4 |
| Docetaxel + Sr-89: was the dose reduced? | ||||||||||
| No | 178 | 98.3 | 157 | 89.7 | 156 | 94.0 | 145 | 95.4 | 136 | 94.4 |
| Yes | 3 | 1.7 | 18 | 10.3 | 10 | 6.0 | 7 | 4.6 | 8 | 5.6 |
| Docetaxel + ZA + Sr-89: was the dose reduced? | ||||||||||
| No | 181 | 99.5 | 158 | 92.4 | 155 | 93.4 | 145 | 92.4 | 139 | 93.9 |
| Yes | 1 | 0.5 | 13 | 7.6 | 11 | 6.6 | 12 | 7.6 | 9 | 6.1 |
| Docetaxel: reasons for reduction | ||||||||||
| Non-study drug related | 0 | 0.0 | 2 | 13.3 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 |
| Study drug-related haematological toxicity | 1 | 50.0 | 9 | 60.0 | 6 | 66.7 | 8 | 50.0 | 5 | 45.5 |
| Study drug-related non-haematological toxicity | 0 | 0.0 | 2 | 13.3 | 0 | 0.0 | 2 | 12.5 | 5 | 45.5 |
| Study drug-related both | 0 | 0.0 | 1 | 6.7 | 0 | 0.0 | 0 | 0.0 | 1 | 9.1 |
| Study drug-related other, specify | 1 | 50.0 | 1 | 6.7 | 3 | 33.3 | 5 | 31.3 | 0 | 0.0 |
| Docetaxel + ZA: reasons for reduction | ||||||||||
| Non-study drug related | 0 | 0.0 | 1 | 5.3 | 1 | 10.0 | 1 | 11.1 | 0 | 0.0 |
| Study drug-related haematological toxicity | 0 | 0.0 | 8 | 42.1 | 5 | 50.0 | 5 | 55.6 | 1 | 12.5 |
| Study drug-related non-haematological toxicity | 0 | 0.0 | 2 | 10.5 | 2 | 20.0 | 1 | 11.1 | 5 | 62.5 |
| Study drug-related both | 0 | 0.0 | 4 | 21.1 | 1 | 10.0 | 1 | 11.1 | 1 | 12.5 |
| Study drug-related other, specify | 0 | 0.0 | 4 | 21.1 | 1 | 10.0 | 1 | 11.1 | 1 | 12.5 |
| Docetaxel + Sr-89: reasons for reduction | ||||||||||
| Non-study drug related | 0 | 0.0 | 1 | 5.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related haematological toxicity | 0 | 0.0 | 10 | 55.6 | 5 | 50.0 | 3 | 42.9 | 4 | 50.0 |
| Study drug-related non-haematological toxicity | 0 | 0.0 | 3 | 16.7 | 3 | 30.0 | 3 | 42.9 | 1 | 12.5 |
| Study drug-related both | 1 | 33.3 | 3 | 16.7 | 1 | 10.0 | 1 | 14.3 | 1 | 12.5 |
| Study drug-related other, specify | 2 | 66.7 | 1 | 5.6 | 1 | 10.0 | 0 | 0.0 | 2 | 25.0 |
| Docetaxel + ZA + Sr-89: reasons for reduction | ||||||||||
| Non-study drug related | 0 | 0.0 | 1 | 7.7 | 1 | 9.1 | 2 | 16.7 | 1 | 11.1 |
| Study drug-related haematological toxicity | 1 | 100.0 | 9 | 69.2 | 7 | 63.6 | 5 | 41.7 | 4 | 44.4 |
| Study drug-related non-haematological toxicity | 0 | 0.0 | 2 | 15.4 | 2 | 18.2 | 2 | 16.7 | 2 | 22.2 |
| Study drug-related other, specify | 0 | 0.0 | 1 | 7.7 | 1 | 9.1 | 3 | 25.0 | 2 | 22.2 |
| Dose reduction details | C6 (N = 527) | C7 (N = 202) | C8 (N = 179) | C9 (N = 154) | C10 (N = 129) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Did the patient have a dose reduction? | ||||||||||
| No | 493 | 93.7 | 191 | 94.6 | 162 | 90.5 | 147 | 95.5 | 120 | 93.8 |
| Yes | 33 | 6.3 | 11 | 5.4 | 17 | 9.5 | 7 | 4.5 | 8 | 6.3 |
| Missing | 1 | 0 | 0 | 0 | 1 | |||||
| Split by randomisation arms | ||||||||||
| Docetaxel: was the dose reduced | ||||||||||
| No | 121 | 94.5 | 42 | 93.3 | 32 | 84.2 | 32 | 94.1 | 23 | 88.5 |
| Yes | 7 | 5.5 | 3 | 6.7 | 6 | 15.8 | 2 | 5.9 | 3 | 11.5 |
| Docetaxel + ZA: was the dose reduced? | ||||||||||
| No | 123 | 93.9 | 53 | 93.0 | 51 | 96.2 | 42 | 97.7 | 31 | 91.2 |
| Yes | 8 | 6.1 | 4 | 7.0 | 2 | 3.8 | 1 | 2.3 | 3 | 8.8 |
| Docetaxel + Sr-89: was the dose reduced? | ||||||||||
| No | 123 | 94.6 | 46 | 93.9 | 40 | 90.9 | 38 | 95.0 | 34 | 100.0 |
| Yes | 7 | 5.4 | 3 | 6.1 | 4 | 9.1 | 2 | 5.0 | 0 | 0.0 |
| Docetaxel + ZA + Sr-89: was the dose reduced? | ||||||||||
| No | 126 | 92.0 | 50 | 98.0 | 39 | 88.6 | 35 | 94.6 | 32 | 94.1 |
| Yes | 11 | 8.0 | 1 | 2 | 5 | 11.4 | 2 | 5.4 | 2 | 5.9 |
| Docetaxel: reasons for reduction | ||||||||||
| Study drug-related haematological toxicity | 4 | 57.1 | 2 | 66.7 | 2 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related non-haematological toxicity | 1 | 14.3 | 1 | 33.3 | 4 | 66.7 | 2 | 100.0 | 3 | 100.0 |
| Study drug-related both | 2 | 28.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Docetaxel + ZA: reasons for reduction | ||||||||||
| Non-study drug related | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related haematological toxicity | 3 | 42.9 | 1 | 25.0 | 1 | 50.0 | 0 | 0.0 | 2 | 66.7 |
| Study drug-related non-haematological toxicity | 2 | 28.6 | 2 | 50.0 | 0 | 0.0 | 1 | 100.0 | 1 | 33.3 |
| Study drug-related other, specify | 1 | 14.3 | 1 | 25.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 |
| Docetaxel + Sr-89: reasons for reduction | ||||||||||
| Non-study drug related | 0 | 0.0 | 1 | 33.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related haematological toxicity | 3 | 42.9 | 0 | 0.0 | 1 | 25.0 | 1 | 50.0 | 0 | 0.0 |
| Study drug-related non-haematological toxicity | 2 | 28.6 | 1 | 33.3 | 1 | 25.0 | 1 | 50.0 | 0 | 0.0 |
| Study drug-related both | 1 | 14.3 | 1 | 33.3 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related other, specify | 1 | 14.3 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 |
| Docetaxel + ZA + Sr-89: reasons for reduction | ||||||||||
| Non-study drug related | 2 | 18.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related haematological toxicity | 4 | 36.4 | 1 | 100.0 | 3 | 60.0 | 1 | 50.0 | 1 | 50.0 |
| Study drug-related non-haematological toxicity | 4 | 36.4 | 0 | 0.0 | 2 | 40.0 | 1 | 50.0 | 1 | 50.0 |
| Study drug-related other, specify | 1 | 9.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
Dose intensity
The protocol-defined starting dose of docetaxel was 75 mg/m2 (up to a maximum dose of 165 mg). Figures 9 and 10, split by comparisons, show the actual dose of docetaxel received (lines) and the numbers of patients off-treatment (bar) over the 10 cycles of the trial.
FIGURE 9.
Docetaxel administration by ZA comparison.

FIGURE 10.
Docetaxel administration by Sr-89 comparison.
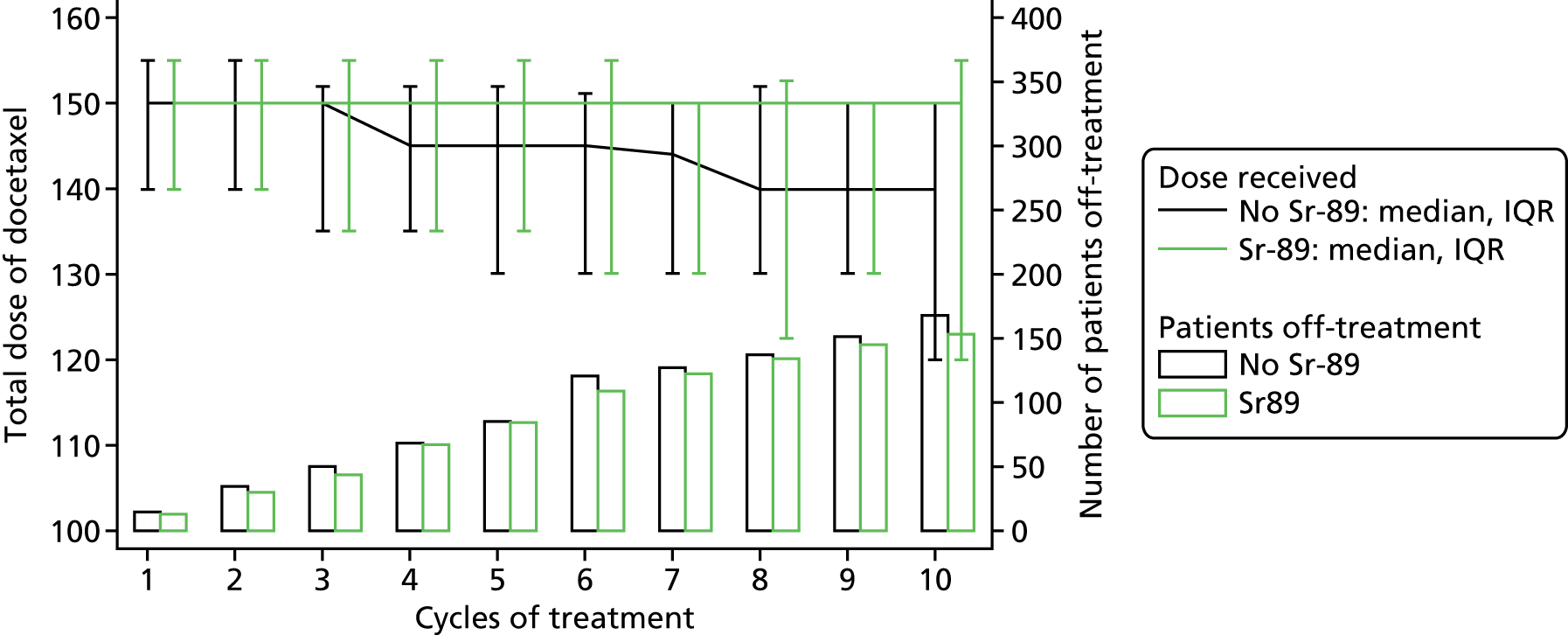
The graphs show that there were only minimal changes in the total doses of docetaxel given during the course of the trial.
Dose reductions and delays
Reductions
Table 20 shows the number of and reasons for dose reductions by randomisation arm over time for cycles 1–5 and Table 21 for cycles 6–10. In total, 267 dose reductions were reported across all 10 cycles, which equated to 6% of all for docetaxel cycles given being reduced.
Delays
Table 22 shows the numbers of docetaxel delays over time and by randomisation arm for cycles 1–5 and Table 23 for cycles 6–10. In total, there were 297 delays reported, equating to delays in 7% of all docetaxel cycles received.
| Dose delay details | C1 (N = 729) | C2 (N = 692) | C3 (N = 665) | C4 (N = 623) | C5 (N = 588) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Did the patient have a dose delay? | ||||||||||
| No | 712 | 97.7 | 637 | 92.1 | 617 | 92.8 | 571 | 91.7 | 545 | 92.8 |
| Yes | 17 | 2.3 | 55 | 7.9 | 48 | 7.2 | 52 | 8.3 | 42 | 7.2 |
| Missing | 0 | 0 | 0 | 0 | 1 | |||||
| Split by randomisation arm | ||||||||||
| Docetaxel: was cycle delayed? | ||||||||||
| No | 183 | 98.4 | 163 | 91.1 | 158 | 92.9 | 144 | 91.1 | 134 | 90.5 |
| Yes | 3 | 1.6 | 16 | 8.9 | 12 | 7.1 | 14 | 8.9 | 14 | 9.5 |
| Docetaxel + ZA: was cycle delayed? | ||||||||||
| No | 177 | 98.3 | 154 | 92.2 | 152 | 93.3 | 140 | 89.7 | 136 | 92.5 |
| Yes | 3 | 1.7 | 13 | 7.8 | 11 | 6.7 | 16 | 10.3 | 11 | 7.5 |
| Docetaxel + Sr-89: was cycle delayed? | ||||||||||
| No | 173 | 95.6 | 162 | 92.6 | 155 | 93.4 | 142 | 93.4 | 133 | 92.4 |
| Yes | 8 | 4.4 | 13 | 7.4 | 11 | 6.6 | 10 | 6.6 | 11 | 7.6 |
| Docetaxel + ZA + Sr-89: was cycle delayed? | ||||||||||
| No | 179 | 98.4 | 158 | 92.4 | 152 | 91.6 | 145 | 92.4 | 142 | 95.9 |
| Yes | 3 | 1.6 | 13 | 7.6 | 14 | 8.4 | 12 | 7.6 | 6 | 4.1 |
| Docetaxel: reasons for delay | ||||||||||
| Non-study drug related | 0 | 0.0 | 4 | 25.0 | 3 | 27.3 | 2 | 14.3 | 7 | 50.0 |
| Study drug-related haematological toxicity | 0 | 0.0 | 4 | 25.0 | 1 | 9.1 | 2 | 14.3 | 0 | 0.0 |
| Study drug-related non-haematological toxicity | 0 | 0.0 | 1 | 6.3 | 1 | 9.1 | 2 | 14.3 | 2 | 14.3 |
| Study drug-related other, specify | 3 | 100.0 | 7 | 43.8 | 6 | 54.5 | 8 | 57.1 | 5 | 35.7 |
| Docetaxel + ZA: reasons for delay | ||||||||||
| Non-study drug related | 2 | 66.7 | 0 | 0.0 | 1 | 9.1 | 6 | 37.5 | 2 | 18.2 |
| Study drug-related haematological toxicity | 0 | 0.0 | 3 | 23.1 | 3 | 27.3 | 3 | 18.8 | 1 | 9.1 |
| Study drug-related non-haematological toxicity | 0 | 0.0 | 3 | 23.1 | 3 | 27.3 | 1 | 6.3 | 3 | 27.3 |
| Study drug-related both | 0 | 0.0 | 2 | 15.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related other, specify | 1 | 33.3 | 5 | 38.5 | 4 | 36.4 | 6 | 37.5 | 5 | 45.5 |
| Docetaxel + Sr-89: reasons for delay | ||||||||||
| Non-study drug related | 3 | 37.5 | 0 | 0.0 | 0 | 0.0 | 2 | 20.0 | 2 | 18.2 |
| Study drug-related haematological toxicity | 0 | 0.0 | 5 | 38.5 | 2 | 18.2 | 0 | 0.0 | 2 | 18.2 |
| Study drug-related non-haematological toxicity | 0 | 0.0 | 0 | 0.0 | 3 | 27.3 | 3 | 30.0 | 1 | 9.1 |
| Study drug-related both | 0 | 0.0 | 2 | 15.4 | 1 | 9.1 | 1 | 10.0 | 0 | 0.0 |
| Study drug-related other, specify | 5 | 62.5 | 6 | 46.2 | 5 | 45.5 | 4 | 40.0 | 6 | 54.5 |
| Docetaxel + ZA + Sr-89: reasons for delay | ||||||||||
| Non-study drug related | 2 | 66.7 | 2 | 15.4 | 1 | 7.1 | 2 | 16.7 | 2 | 33.3 |
| Study drug-related haematological toxicity | 0 | 0.0 | 5 | 38.5 | 1 | 7.1 | 1 | 8.3 | 0 | 0.0 |
| Study drug-related non-haematological toxicity | 0 | 0.0 | 3 | 23.1 | 3 | 21.4 | 4 | 33.3 | 0 | 0.0 |
| Study drug-related both | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 16.7 |
| Study drug-related other, specify | 1 | 33.3 | 3 | 23.1 | 9 | 64.3 | 5 | 41.7 | 3 | 50.0 |
| Dose delay details | C6 (N = 527) | C7 (N = 202) | C8 (N = 179) | C9 (N = 154) | C10 (N = 129) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Did the patient have a dose delay? | ||||||||||
| No | 493 | 93.5 | 182 | 90.1 | 162 | 90.5 | 145 | 94.2 | 125 | 97.7 |
| Yes | 34 | 6.5 | 20 | 9.9 | 17 | 9.5 | 9 | 5.8 | 3 | 2.3 |
| Missing | 0 | 0 | 0 | 0 | 1 | |||||
| Split by randomisation arm | ||||||||||
| Docetaxel: was cycle delayed? | ||||||||||
| No | 125 | 97.7 | 43 | 95.6 | 35 | 92.1 | 34 | 100.0 | 25 | 96.2 |
| Yes | 3 | 2.3 | 2 | 4.4 | 3 | 7.9 | 0 | 0.0 | 1 | 3.8 |
| Docetaxel + ZA: was cycle delayed? | ||||||||||
| No | 121 | 91.7 | 52 | 91.2 | 49 | 92.5 | 40 | 93.0 | 33 | 97.1 |
| Yes | 11 | 8.3 | 5 | 8.8 | 4 | 7.5 | 3 | 7.0 | 1 | 2.9 |
| Docetaxel + Sr-89: was cycle delayed? | ||||||||||
| No | 118 | 90.8 | 43 | 87.8 | 39 | 88.6 | 35 | 87.5 | 33 | 97.1 |
| Yes | 12 | 9.2 | 6 | 12.2 | 5 | 11.4 | 5 | 12.5 | 1 | 2.9 |
| Docetaxel + ZA + Sr-89: was cycle delayed? | ||||||||||
| No | 129 | 94.2 | 44 | 86.3 | 39 | 88.6 | 36 | 97.3 | 34 | 100.0 |
| Yes | 8 | 5.8 | 7 | 13.7 | 5 | 11.4 | 1 | 2.7 | 0 | 0.0 |
| Docetaxel: reasons for delay | ||||||||||
| Non-study drug related | 1 | 33.3 | 1 | 50.0 | 1 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related non-haematological toxicity | 0 | 0.0 | 0 | 0.0 | 1 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related other, specify | 2 | 66.7 | 1 | 50.0 | 1 | 33.3 | 0 | 0.0 | 1 | 100.0 |
| Docetaxel + ZA: reasons for delay | ||||||||||
| Non-study drug related | 3 | 27.3 | 2 | 40.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related haematological toxicity | 2 | 18.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related non-haematological toxicity | 0 | 0.0 | 2 | 40.0 | 2 | 50.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related other, specify | 6 | 54.5 | 1 | 20.0 | 2 | 50.0 | 3 | 100.0 | 1 | 100.0 |
| Docetaxel + Sr-89: reasons for delay | ||||||||||
| Non-study drug related | 2 | 16.7 | 1 | 16.7 | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 |
| Study drug-related haematological toxicity | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related non-haematological toxicity | 4 | 33.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related both | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related other, specify | 6 | 50.0 | 5 | 83.3 | 3 | 60.0 | 4 | 80.0 | 1 | 100.0 |
| Docetaxel + ZA + Sr-89: reasons for delay | ||||||||||
| Non-study drug related | 3 | 37.5 | 1 | 14.3 | 2 | 40.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related haematological toxicity | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related non-haematological toxicity | 1 | 12.5 | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related both | 1 | 12.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Study drug-related other, specify | 3 | 37.5 | 6 | 85.7 | 1 | 20.0 | 1 | 100.0 | 0 | 0.0 |
Figures 11 and 12 show the overall (line) dose reductions and delays, and the reasons (bar) for these, as a percentage of the number of patients who received each cycle. Figure 11 was split by Sr-89 and Figure 12 by ZA.
FIGURE 11.
Reasons for docetaxel reductions and delays by Sr-89. SDR, study-related drug. (a) No Sr-89: delays; (b) no Sr-89: reductions; (c) Sr-89:delays; and (d) Sr-89: reductions.


FIGURE 12.
Reasons for docetaxel reductions and delays by ZA. SDR, study-related drug. (a) No ZA: delays; (b) no ZA: reductions; (c) ZA: delays; and (d) ZA: reductions.




It is clear in both figures that study drug-related haematological toxicities tended to result in dose reductions rather than delays. For patients receiving Sr-89 there appeared to be a peak in delays to cycle 7 following Sr-89 admission, this fits with a drop in the percentage of patients needing dose reductions at that cycle.
Concomitant medications
Concomitant medications were collected at randomisation and during treatment visits. In addition, all concomitant medications given in response to serious adverse events (SAEs) were recorded on the SAE form. At the point of tumour, pain or PSA progression, sites were specifically asked about radiotherapy, radioisotopes and bisphosphonates and given the option to record any other treatments provided at that time.
In total, 9637 concomitant medications were reported, of which 2152 (22%) were related to concomitant medications that were already being taken at randomisation. The majority, 5233 (54%), were taken prior to the patient experiencing CPFS, and 1969 (20%) were taken following CPFS. In addition to this, in the case of 304 (3%) concomitant medications, the date on which they were taken was unavailable.
Details of all concomitant medications reported by more than 20 patients can be found in Appendix 5, Tables 82–87, split by both randomisation arm and comparison groups. The data within these tables are also split by whether the concomitant medications were administered at baseline, post baseline but prior to CPFS or following CPFS.
Analgesic concomitant medications
Patients were asked to complete pain diaries for the 7 days prior to each visit. Patients were asked to list all pain medication taken during these days. This does not give a complete overview of analgesic use; however, as recording was the same in all arms, any difference should be representative of overall use.
In total, 40,029 instances of receiving analgesic medications were reported by 575 patients; 19,494 (79%) were opioid analgesics and 20,535 (51%) non-opioid. These were evenly distributed both between randomisation arms and across comparison groups; details are presented in Tables 24 and 25.
| Analgesic | Docetaxel | Docetaxel + ZA | Docetaxel + Sr-89 | Docetaxel + ZA + Sr-89 | Overall |
|---|---|---|---|---|---|
| Number of patients | |||||
| Non-opioid | 87 | 82 | 88 | 90 | 347 |
| Opioid | 59 | 64 | 5 | 44 | 225 |
| Missing | 1 | 1 | 1 | 0 | 3 |
| Number of instances | |||||
| Non-opioid | 5203 | 5106 | 5188 | 5038 | 20,535 |
| Opioid | 5016 | 4742 | 5081 | 4493 | 19,332 |
| Missing | 53 | 55 | 25 | 29 | 162 |
| Analgesic | No ZA | ZA | No Sr-89 | Sr-89 |
|---|---|---|---|---|
| Number of patients | ||||
| Non-opioid | 175 | 172 | 169 | 178 |
| Opioid | 112 | 113 | 123 | 102 |
| Missing | 2 | 1 | 2 | 1 |
| Number of instances | ||||
| Non-opioid | 10,391 | 10,144 | 10,309 | 10,225 |
| Opioid | 10,097 | 9235 | 9758 | 9574 |
| Missing | 78 | 84 | 108 | 54 |
Details of the types of analgesic medications being taken can be found in Appendix 5, Tables 88–93, split by both randomisation arm and comparison groups. The data within these tables are also split by whether the concomitant medication was administered at baseline, post baseline but prior to CPFS or following CPFS.
Clinical progression-free survival
Statistical methods
Descriptive analysis of CPFS events is presented as percentages. Kaplan–Meier curves were created and used to calculate CPFS percentages at 6, 12, 18 and 24 months. Analysis of CPFS has been carried out using both an unadjusted approach and an adjusted approach. The first analysis of the primary outcome was an unadjusted stratified log-rank test comparing ZA with no ZA, stratified by Sr-89, and comparing Sr-89 with no Sr-89, stratified by ZA. Conclusions were based on a two-sided 5% significance level. No adjustments for multiple testing were made.
The second analysis of the primary end point used an adjusted Cox regression model, including both treatment comparisons and stratification factors (ECOG and randomising centre). The use of stratification factors within the design leads to correlation between the treatment groups. These correlations, when not adjusted for, can lead to upwards biased standard error rates for treatment effects, CIs which are too wide, type 1 error rates which are too low and a subsequent reduction in power. 49,50 Owing to these potential implications, our primary outcome conclusions have been based on the adjusted Cox regression analysis. The use of both log-rank and Cox regression models was pre-specified in the trial protocol.
Further analysis included all prognostic factors recorded at baseline that were either statistically (stratification factors: ECOG, centre) or clinically (age, histology, prior radiotherapy use, anti-androgen use, presence of measurable lesions on bone scan, baseline alkaline phosphatase levels and baseline PSA levels) important. No optimal model-building techniques were employed. All factors listed were adjusted for in the model.
Clinical progression-free survival results
Tables 26 and 27 show a breakdown of the first CPFS event split by randomisation arms and comparison groups.
| Type of first CPFS event | Docetaxel (N = 173) | Docetaxel + ZA (N = 174) | Docetaxel + Sr-89 (N = 171) | Docetaxel + ZA + Sr-89 (N = 178) | Overall (N = 696) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Death | 45 | 26.0 | 61 | 35.1 | 42 | 24.6 | 61 | 34.3 | 209 | 30.0 |
| SRE | 34 | 19.7 | 27 | 15.5 | 39 | 22.8 | 29 | 16.3 | 129 | 18.5 |
| Pain | 73 | 42.2 | 71 | 40.8 | 59 | 34.5 | 61 | 34.3 | 264 | 37.9 |
| Death SRE | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 |
| SRE pain | 21 | 12.1 | 14 | 8.0 | 31 | 18.1 | 27 | 15.2 | 93 | 13.4 |
| Total | 173 | 100.0 | 174 | 100.0 | 171 | 100.0 | 178 | 100.0 | 696 | 100.0 |
| Type of first CPFS event | No ZA (N = 344) | ZA (N = 352) | No Sr-89 (N = 347) | Sr-89 (N = 349) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Death | 87 | 25.3 | 122 | 34.7 | 106 | 30.5 | 103 | 29.5 |
| SRE | 73 | 21.2 | 56 | 15.9 | 61 | 17.6 | 68 | 19.5 |
| Pain | 132 | 38.4 | 132 | 37.5 | 144 | 41.5 | 120 | 34.4 |
| Death SRE | 0 | 0.0 | 1 | 0.3 | 1 | 0.3 | 0 | 0.0 |
| SRE pain | 52 | 15.1 | 41 | 11.6 | 35 | 10.1 | 58 | 16.6 |
| Total | 344 | 100.0 | 352 | 100.0 | 347 | 100.0 | 349 | 100.0 |
Thirty per cent of patients did not experience a SRE or pain progression prior to death. An audit was conducted of patients in this study which checked to see whether or not SREs or pain progression had gone unreported. The audit concluded that this was not the case and confirmed that approximately 30% of patients died without experiencing a preceding event.
In total, 696 of the 757 patients (92%) randomised experienced clinical progression. The median follow-up time of the 61 surviving patients who had not yet progressed is 20.9 months (IQR 16.3–24.8 months).
Zoledronic acid versus no zoledronic acid
In total, there were 696 events; 352 (51%) occurred in the ZA group and 344 (49%) in the no ZA group. A stratified log-rank test was performed comparing ZA and no ZA. No difference in CPFS between the two groups was observed (χ2 = 0.10; p = 0.7553). The median survival time in the ZA group was 9.43 months (95% CI 8.51 to 9.89 months), compared with 8.57 months (95% CI 7.36 to 9.33 months) in the no ZA group. A Cox proportional hazards model was used to estimate the HR (0.98, 95% CI 0.84 to 1.13; p = 0.762).
Table 28 shows the estimated clinical-related progression-free survival percentages at 6-monthly intervals, with 95% CIs.
| Time point | % survival (95% CI), no ZA | % survival (95% CI), ZA |
|---|---|---|
| 6 months | 68 (64 to 73) | 74 (69 to 78) |
| 12 months | 34 (29 to 39) | 34 (30 to 39) |
| 18 months | 20 (16 to 24) | 20 (16 to 24) |
| 24 months | 14 (10 to 18) | 11 (8 to 15) |
It can be clearly seen that the estimated survival percentage is almost exactly the same at 12 and 18 months.
Figure 13 shows the Kaplan–Meier survival estimates split by comparison ZA. The Kaplan–Meier curve shows very clearly that there was virtually no difference between the ZA and no ZA groups.
FIGURE 13.
Kaplan–Meier survival estimates of CPFS by ZA comparison. Reproduced with permission from JAMA Oncol, 2016;2(4):493–9. Copyright © (2016) American Medical Association. All rights reserved. 51

Strontium-89 versus no strontium-89
In total, there were 696 events: 349 (50%) in the Sr-89 group and 347 (50%) in the no Sr-89 group. A stratified log-rank test comparing Sr-89 and no Sr-89 revealed no difference in CPFS between the two groups (χ2 = 2.38; p = 0.1230). The median survival time in the Sr-89 arm was 9.56 months (95% CI 8.74 to 10.25 months), compared with 8.38 months (95% CI 7.36 to 9.07 months) in the no Sr-89 arm. A Cox proportional hazards model was used to estimate the HR (0.88, 95% CI 0.76 to 1.03; p = 0.108).
Table 29 shows the estimated CPFS survival percentages at 6-monthly intervals with 95% CIs.
| Time point | % survival (95% CI), no Sr-89 | % survival (95% CI), Sr-89 |
|---|---|---|
| 6 months | 71 (66 to 75) | 71 (67 to 76) |
| 12 months | 31 (26 to 35) | 38 (33 to 42) |
| 18 months | 18 (14 to 22) | 21 (17 to 25) |
| 24 months | 11 (8 to 15) | 14 (10 to 18) |
Figure 14 shows the Kaplan–Meier survival estimates split by comparison of Sr-89 treatment. A small difference from approximately 6 months to 3 years is visible, although this is not statistically significant according to the log-rank test.
FIGURE 14.
Kaplan–Meier survival estimates of CPFS by Sr-89 comparison. Reproduced with permission from JAMA Oncol, 2016;2(4):493–9. Copyright © (2016) American Medical Association. All rights reserved. 51

Interaction
The two-by-two factorial design assumed no interaction between Sr-89 and ZA. This assumption was investigated both graphically and by inclusion of interaction terms within a Cox model, which revealed no evidence of an interaction between the two treatments (χ2 = 0.70; p = 0.4035).
Secondary analysis adjusting for stratification factors
The secondary analyses of the primary outcome used a Cox multivariable proportional hazards model, including both treatment comparisons, ECOG performance status (fixed effect) and randomising centre (random effect).
Cox model
Table 30 shows the HRs, p-values and the 95% CIs for the HRs from the Cox model.
| Variable | HR | p-value | 95% CI | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Sr-89 (ref.: no Sr-89) | ||||
| Sr-89 | 0.847 | 0.031 | 0.729 | 0.985 |
| ZA (ref.: no ZA) | ||||
| ZA | 0.982 | 0.808 | 0.845 | 1.141 |
| ECOG (ref.: ECOG 0) | ||||
| ECOG 1 | 1.547 | < 0.001 | 1.315 | 1.821 |
| ECOG 2 | 2.136 | < 0.001 | 1.603 | 2.847 |
| Random effect of centre | ||||
| Centre | – | < 0.001 | – | – |
A Cox proportional hazards model looking at treatment effects while controlling for important covariates demonstrated a statistically significant effect of Sr-89, albeit with only a moderate HR (0.85, 95% CI 0.73 to 0.99; p = 0.03); however, there was no statistically significant effect of ZA (HR 0.98, 95% CI 0.84 to 1.14; p = 0.80).
Analysis adjusting for all potential prognostic factors
Table 31 shows the results of Cox proportional hazards model analysis adjusting for all statistically and clinically significant factors.
| Variable | HR | p-value | 95% CI | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Sr-89 (ref.: no Sr-89) | ||||
| Sr-89 | 0.814 | 0.010 | 0.696 | 0.952 |
| ZA (ref.: no ZA) | ||||
| ZA | 0.993 | 0.933 | 0.851 | 1.160 |
| ECOG (ref.: ECOG 0) | ||||
| ECOG 1 | 1.605 | < 0.001 | 1.350 | 1.909 |
| ECOG 2 | 1.895 | < 0.001 | 1.397 | 2.570 |
| Age (years) | ||||
| 1-year increase | 0.977 | < 0.001 | 0.965 | 0.988 |
| Histology (ref.: adenocarcinoma) | ||||
| Elevated PSA | 0.970 | 0.771 | 0.791 | 1.190 |
| Prior radiotherapy (ref.: no) | ||||
| Yes | 1.221 | 0.016 | 1.039 | 1.436 |
| Anti-androgens (ref.: no) | ||||
| Yes | 0.773 | 0.084 | 0.578 | 1.035 |
| Measurable lesions on bone scan (ref.: no) | ||||
| Yes | 1.181 | 0.058 | 0.994 | 1.403 |
| Baseline alkaline phosphatase | ||||
| 100-unit increase | 1.025 | < 0.001 | 1.016 | 1.033 |
| Baseline PSA | ||||
| 100-unit increase | 1.016 | 0.019 | 1.003 | 1.030 |
| Random effect of centre | ||||
| Centre | – | < 0.001 | – | – |
A Cox proportional hazards model looking at treatment effects while controlling for important covariates demonstrated a statistically significant effect of Sr-89 (HR 0.81, 95% CI 0.70 to 0.95; p = 0.01), but no statistically significant effect of ZA (HR 0.99, 95% CI 0.85 to 1.16; p = 0.93). Other factors shown to be associated with reduced risk of CPFS were good performance status, increased age, no previous radiotherapy, low PSA and low alkaline phosphatase levels. The proportionality assumption was assessed and found to be upheld.
Skeletal-related events
Statistical methods
Descriptive analysis of types of SREs is presented as percentages, progression between different types of SREs and the time between them diagrammatically and Kaplan–Meier curves were used to present SREFIs.
The primary analysis was a stratified log-rank comparing ZA with no ZA, stratified by Sr-89 and Sr-89, with no Sr-89 stratified by ZA. Conclusions were based on a two-sided 5% significance level. No adjustments for multiple testing were made.
Sensitivity analysis
Owing to the high proportion of patients who were randomised to Sr-89 but unable to receive it, a per-protocol analysis was conducted on a per-protocol population. The per-protocol population is defined as all eligible patients who received six cycles of docetaxel and did not experience pain progression or a SRE prior to 21 days following the sixth administration of docetaxel and therefore would have been able to receive Sr-89. The definition of SREFIs will remain the same as detailed above, as will the Kaplan–Meier method and stratified log-rank test used.
Multiple failure model
The previously defined log-rank time-to-event analysis took into account only the time-to-first SRE, although patients were at risk of experiencing multiple SREs throughout the course of the trial. It was, therefore, conceivable that a medication may have had little or no effect on the time-to-first event but could prevent patients experiencing multiple events. To take account of this, a multiple failure model was employed.
There were three factors to consider when deciding on the appropriate multiple failure methodology. First, a patient could experience multiple SREs on the same day. Second, SREs were ordered in the sense that a second event could not occur without a first event having been experienced. Finally, for subsequent SREs the time-to-event needed to be measured from the date of the previous SRE and not from randomisation. For example, once a patient had experienced a first SRE, his risk of having a subsequent event changed, as it was considered feasible to assume that having the first SRE would change the underlying hazard in this disease setting. To take account of all of these factors, a multiple failure model was used, specifically the conditional risk set model proposed by Prentice et al. 52 Once the model was set up to account for the multiple events, Kaplan–Meier stratified log-rank methods were employed as previously described.
Descriptive statistics of skeletal-related events
Tables 32 and 33 show the breakdown of the types of SREs that occurred, split by randomisation arm and comparison groups. The occurrence of more serious SREs, that is fracture, SCC and surgery, was consistently higher in the no ZA group than the ZA group, although the overall distribution of SREs within groups was similar.
| Type of SRE | Docetaxel | Docetaxel + ZA | Docetaxel + Sr-89 | Docetaxel + ZA + Sr-89 | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Fractures | 12 | 3.8 | 5 | 2.3 | 18 | 6.3 | 7 | 3.4 | 42 | 4.1 |
| SCC | 31 | 9.8 | 13 | 6 | 30 | 10.4 | 21 | 10.2 | 95 | 9.2 |
| Surgery | 7 | 2.2 | 4 | 1.8 | 14 | 4.9 | 2 | 1 | 27 | 2.6 |
| Radiation | 221 | 69.7 | 158 | 72.5 | 171 | 59.4 | 140 | 68 | 690 | 67.1 |
| Change of therapy | 46 | 14.5 | 38 | 17.4 | 53 | 18.4 | 36 | 17.5 | 173 | 16.8 |
| Hypercalcaemia | 0 | 0 | 0 | 0 | 2 | 0.7 | 0 | 0 | 2 | 0.2 |
| Total | 317 | 100.0 | 218 | 100.0 | 288 | 100.0 | 206 | 100.0 | 1029 | 100.0 |
| Type of SRE | No ZA | ZA | No Sr-89 | Sr-89 | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Fractures | 30 | 5 | 12 | 2.8 | 17 | 3.2 | 25 | 5.1 |
| SCC | 61 | 10.1 | 34 | 8 | 44 | 8.2 | 51 | 10.3 |
| Surgery | 21 | 3.5 | 6 | 1.4 | 11 | 2.1 | 16 | 3.2 |
| Radiation | 392 | 64.8 | 298 | 70.3 | 379 | 70.8 | 311 | 63 |
| Change of therapy | 99 | 16.4 | 74 | 17.5 | 84 | 15.7 | 89 | 18 |
| Hypercalcaemia | 2 | 0.3 | 0 | 0 | 0 | 0 | 2 | 0.4 |
| Total | 605 | 100.0 | 424 | 100.0 | 535 | 100.0 | 494 | 100.0 |
Table 34 shows number of patients experiencing SREs by comparison group. The numbers of patients experiencing multiple SREs is lower in the ZA group.
| Number of SREs | ZA comparison | Sr-89 comparison | ||
|---|---|---|---|---|
| No ZA | ZA | No Sr-89 | Sr-89 | |
| n (%) | n (%) | n (%) | n (%) | |
| None | 147 (39) | 173 (46) | 157 (41) | 163 (43) |
| 1 | 80 (21) | 100 (27) | 90 (24) | 90 (24) |
| 2 | 57 (15) | 45 (12) | 52 (14) | 50 (13) |
| 3 | 40 (10) | 29 (8) | 35 (9) | 34 (9) |
| 4 | 21 (5) | 13 (3) | 15 (4) | 19 (5) |
| 5 | 22 (6) | 10 (2) | 17 (5) | 15 (4) |
| ≥ 6 | 14 (4) | 6 (2) | 13 (3) | 7 (2) |
| Total number of patients | 381 (100) | 376 (100) | 379 (100) | 378 (100) |
| Number reporting at least one SRE | 234 (61) | 203 (54) | 222 (59) | 215 (57) |
Diagrams showing the order in which different types of SREs occurred and the time between these events can be found in Appendix 5, Figures 55–74. These show that the overall time to the first SRE is longer in the no ZA group than in the ZA group. The difference is approximately 1.5 months if radiation, fracture or change of therapy was the first SRE and approximately 5.5 months if the first SRE was SCC. In both ZA and no ZA groups, radiation was by far the most common first SRE, with change in therapy being the second most common, followed by SCC. The proportion of first SREs which were SCCs in the no ZA arm was almost double that in the ZA arm. The ZA group also saw fewer instances of subsequent SREs being for surgery or SCC.
In terms of the Sr-89 comparison, there were fewer differences, with both the no Sr-89 and the Sr-89 groups having similar distributions of the type of events and times to the first events. The only exception was when SCC was the first event, in which case the time to first event was 2.5 months longer for those receiving Sr-89 than for those who were not.
Skeletal-related event-free interval
Zoledronic acid versus no zoledronic acid
In total, there were 437 events: 234 (54%) in the no ZA group and 203 (46%) in the ZA group. A stratified log-rank test was performed comparing ZA with no ZA. A statistically significant difference in SREFIs between the two groups was observed (χ2 = 6.49, p = 0.011). The median survival time in the ZA group was 13.60 months (95% CI 11.76 to 16.62 months), compared with 11.17 months (95% CI 9.76 to 13.01 months) in the no ZA group.
A Cox proportional hazards model was used to estimate the HR (0.78, 95% CI 0.65 to 0.95; p = 0.011). Figure 15 shows the Kaplan–Meier survival estimates split by comparison group: ZA.
FIGURE 15.
Kaplan–Meier survival estimates of SREFIs by comparison group: ZA. Reproduced with permission from JAMA Oncol, 2016;2(4):493–9. Copyright © (2016) American Medical Association. All rights reserved. 51

Strontium-89 versus no strontium-89
In total, there were 437 events: 222 (51%) in the no Sr-89 group and 215 (49%) in the Sr-89 group. A stratified log-rank test was performed comparing Sr-89 with no Sr-89. No difference in SREFI between the two groups was observed (χ2 = 1.89; p = 0.169). The median survival time in the Sr-89 group was 13.04 months (95% CI 11.14 to 14.69 months), compared with 11.70 months (95% CI 10.58 to 13.60 months) in the no Sr-89 group.
A Cox proportional hazards model was used to estimate the hazard ratio (HR 0.88, 95% CI 0.73 to 1.06; p = 0.170). Figure 16 shows the Kaplan–Meier survival estimates split by Sr-89 comparison.
FIGURE 16.
Kaplan–Meier survival estimates of SREFI by comparison group: Sr-89. Reproduced with permission from JAMA Oncol, 2016;2(4):493–9. Copyright © (2016) American Medical Association. All rights reserved. 51
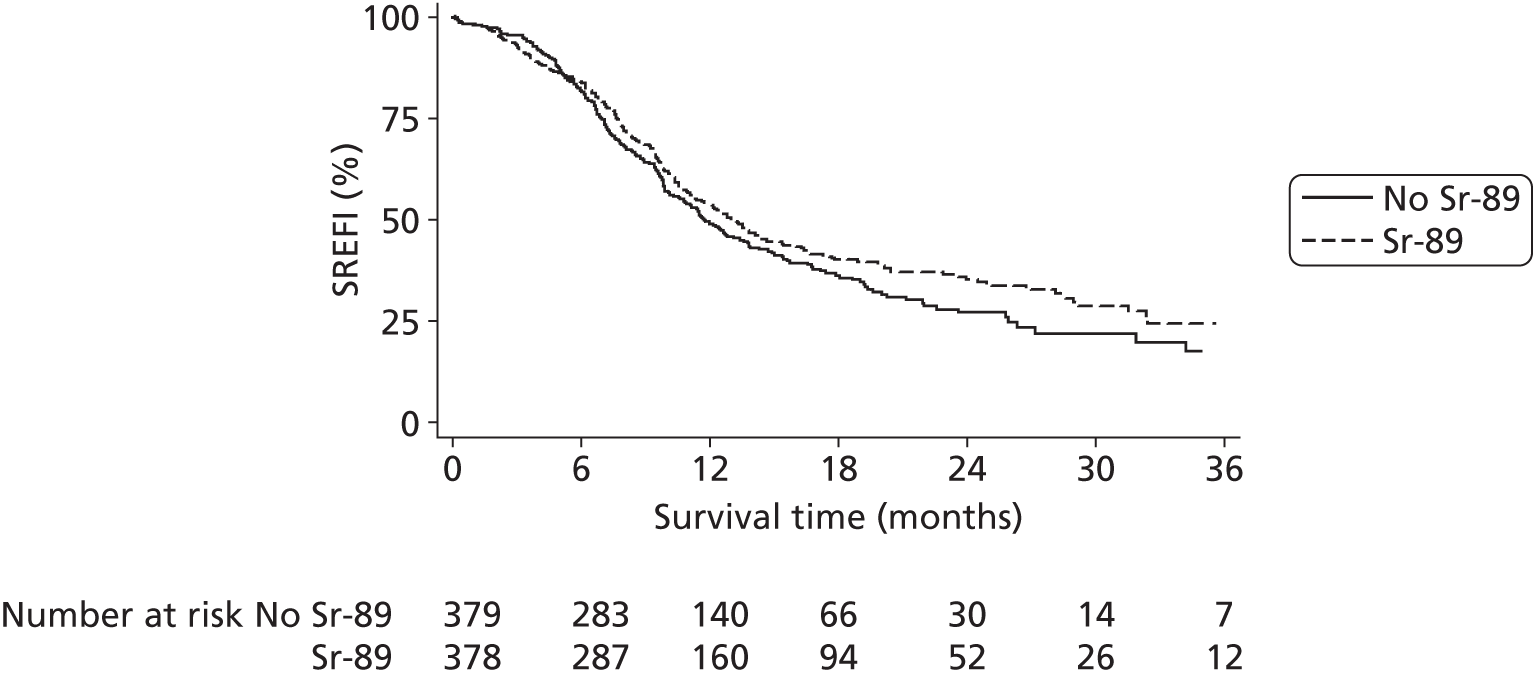
Landmark analysis for strontium-89
In total, 531 (70%) patients received six cycles of docetaxel and were therefore eligible to receive Sr-89.
Of a total of 321 events, 162 (50%) occurred in the no Sr-89 group and 159 (50%) in the Sr-89 group. A stratified log-rank test was performed comparing Sr-89 with no Sr-89. No difference in SREFI between the two groups was observed (χ2 = 2.27; p = 0.132). The median survival time in the Sr-89 arm was 14.16 months (95% CI 12.52 to 17.74 months), compared with 12.75 months (95% CI 11.43 to 15.38 months) in the no Sr-89 arm.
A Cox proportional hazards model was used to estimate the HR (HR 0.85, 95% CI 0.68 to 1.05; p = 0.170). Figure 17 shows the Kaplan–Meier survival estimates split by comparison Sr-89.
FIGURE 17.
Kaplan–Meier survival estimates of SREFIs by Sr-89.

Multiple failure model
As seen in the Descriptive statistics of skeletal-related events, patients experience multiple SREs. The previous time-to-event analysis looks at only time-to-first event, not taking multiple SREs into account. The following section takes the multiple events into account, as detailed in this section’s statistical methods.
There were 33 instances where patients experienced multiple SREs on the same day; for the purposes of this analysis such events were counted as a single event.
Zoledronic acid versus no zoledronic acid
In total, there were 996 events: 584 (59%) in the no ZA group and 412 (41%) in the ZA group. A stratified log-rank test comparing ZA with no ZA demonstrated a statistically significant difference in SREFIs between the two groups (χ2 = 31.39; p< 0.001). The median survival time in the ZA group was 11.5 months (95% CI 10.61 to 12.65 months), compared with 8.87 months (95% CI 7.85 to 9.66 months) in the no ZA group.
A Cox proportional hazards model was used to estimate the HR (HR 0.70, 95% CI 0.62 to 0.79; p < 0.001). Figure 18 shows the Kaplan–Meier survival estimates split by comparison ZA.
FIGURE 18.
Kaplan–Meier survival estimates for SREFI multiple failure model by ZA comparison.
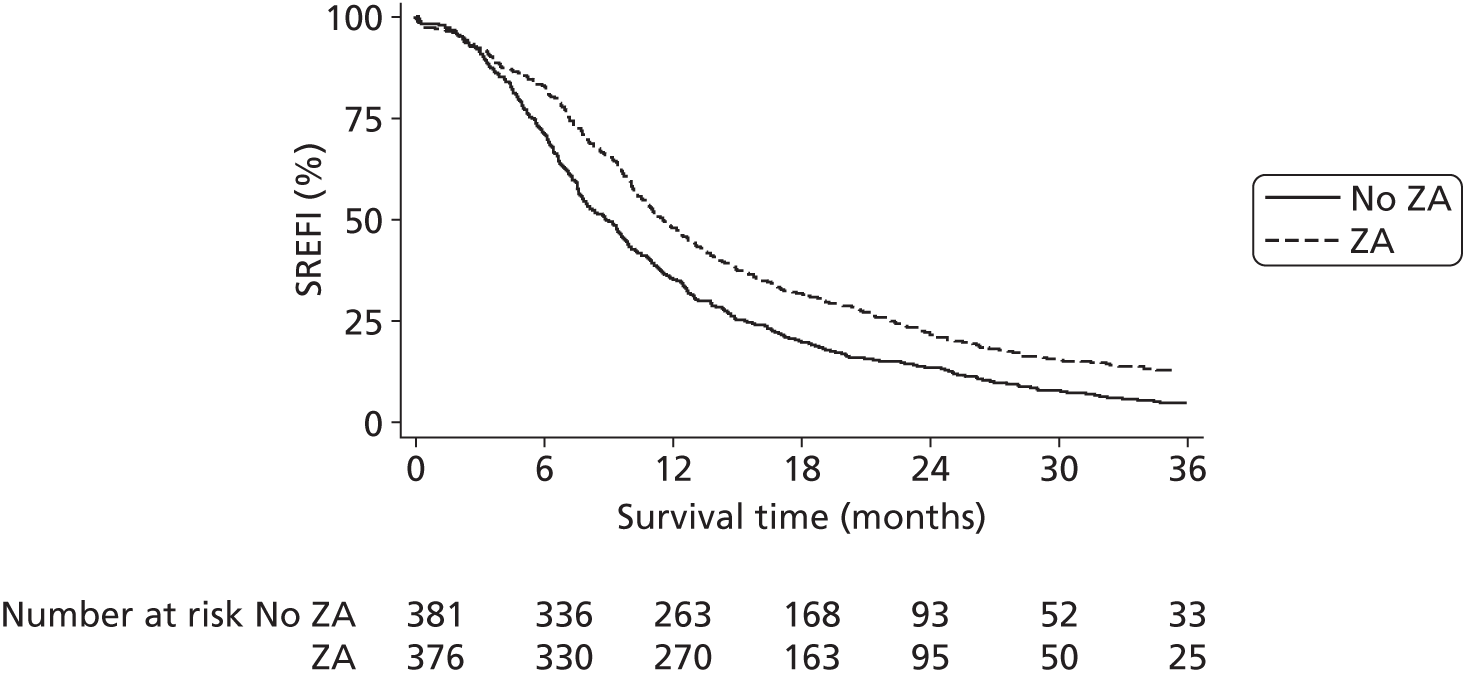
Strontium-89 versus no strontium-89
In total, there were 996 events: 518 (52%) in the no Sr-89 group and 478 (48%) in the Sr-89 group. A stratified log-rank test performed to compare Sr-89 with no Sr-89 observed no difference in SREFI between the two groups (χ2 = 3.11; p = 0.078). The median survival time in the Sr-89 group was 10.41 months (95% CI 9.69 to 11.14 months), compared with 9.86 months (95% CI 9.13 to 10.81 months) in the no Sr-89 group.
A Cox proportional hazards model was used to estimate the HR (HR 0.89, 95% CI 0.79 to 1.01; p = 0.078). Figure 19 shows the Kaplan–Meier survival estimates split by Sr-89 comparison.
FIGURE 19.
Kaplan–Meier survival estimates for SREFI multiple failure model by Sr-89 comparison.
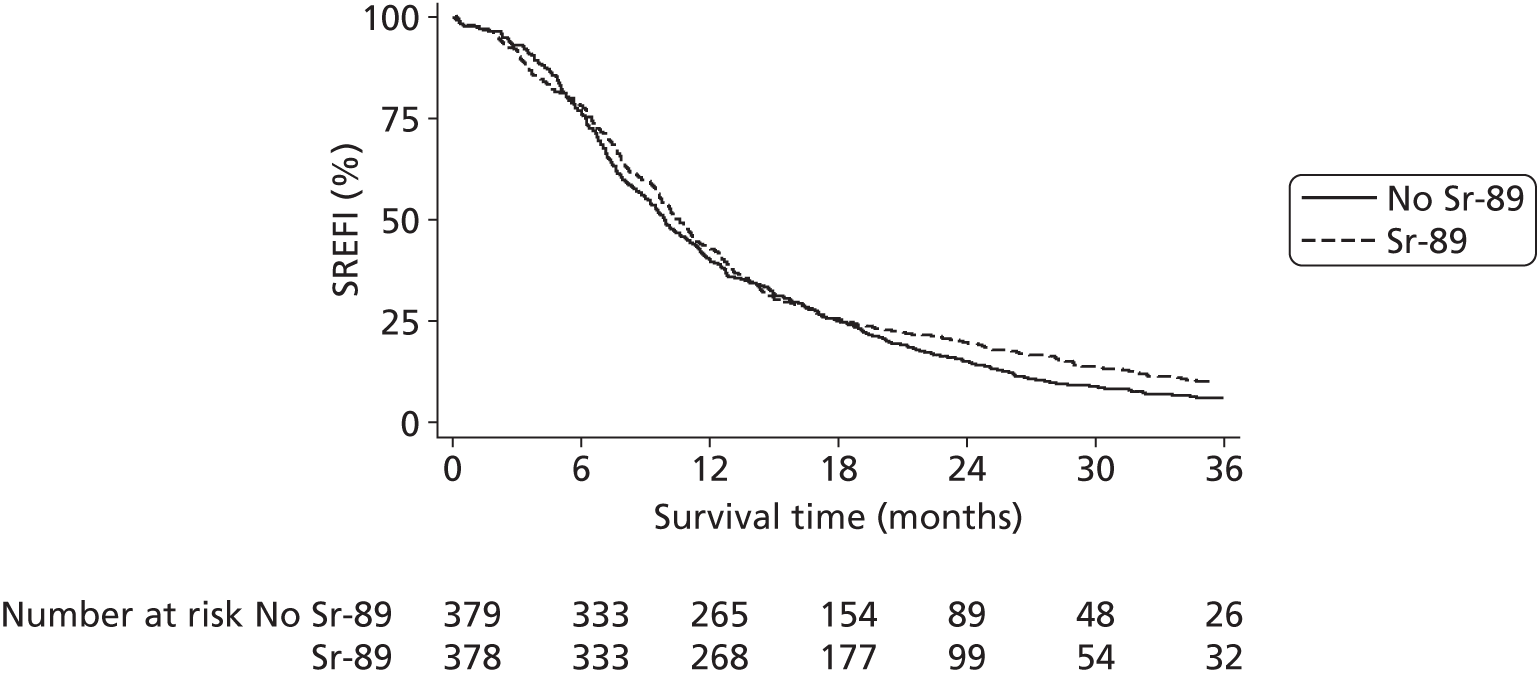
Pain progression-free interval
Statistical methods
The primary analysis was a stratified log-rank test comparing ZA with no ZA, stratified by Sr-89, and Sr-89 with no Sr-89, stratified by ZA. Conclusions were based on a two-sided 5% significance level. No adjustments for multiple testing were made.
Zoledronic acid versus no zoledronic acid
In total, there were 432 events: 225 (52%) in the no ZA group and 207 (48%) in the ZA group. A stratified log-rank test was performed comparing ZA with no ZA. No difference in PPFI between the two groups was observed (χ2 = 1.02; p = 0.3127). The median progression-free survival time in the ZA group was 12.19 months (95% CI 10.78 to 15.38 months), compared with 11.76 months (95% CI 10.55 to 13.37 months) in the no ZA group.
A Cox proportional hazards model was used to estimate the HR (HR 0.91, 95% CI 0.75 to 1.10; p = 0.313). Figure 20 shows the Kaplan–Meier survival estimates split by ZA comparison.
FIGURE 20.
Kaplan–Meier survival estimates for PPFI by ZA.
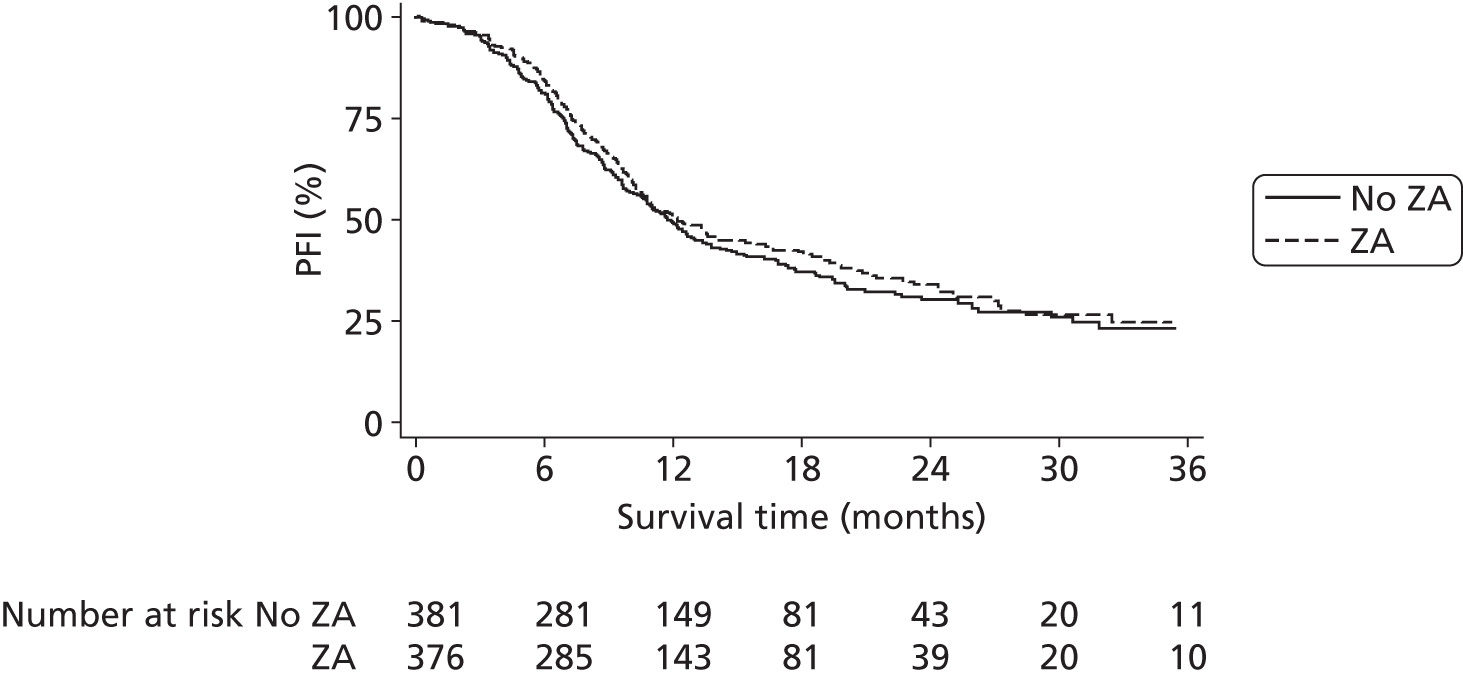
Strontium-89 versus no strontium-89
In total, there were 432 events: 215 (50%) in the no Sr-89 group and 217 (50%) in the Sr-89 group. A stratified log-rank test was performed comparing Sr-89 with no Sr-89. No difference in PPFI between the two groups was observed (χ2 = 0.40; p = 0.3991). The median PPFI time in the Sr-89 group was 12.22 months (95% CI 10.94 to 14.09 months), compared with 11.76 months (95% CI 10.32 to 13.54 months) in the no Sr-89 group.
A Cox proportional hazards model was used to estimate the HR (HR 0.92, 95% CI 0.76 to 1.11; p = 0.387). Figure 21 shows the Kaplan–Meier survival estimates split by Sr-89 comparison.
FIGURE 21.
Kaplan–Meier survival estimates for PPFI by Sr-89.

Overall survival
Statistical methods
The primary analysis was a stratified log-rank comparing ZA with no ZA, stratified by Sr-89, and Sr-89 versus no Sr-89, stratified by ZA. Conclusions were based on a two-sided 5% significance level. No adjustments for multiple testing were made.
Descriptive statistics of deaths
In total, 618 patients died. Tables 35 and 36 list the reported causes of death split by randomisation arm and comparison groups.
| Cause of death | Docetaxel (N = 154) | Docetaxel + ZA (N = 156) | Docetaxel + Sr-89 (N = 155) | Docetaxel + ZA + Sr-89 (N = 153) | Overall (N = 618) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Disease related | 122 | 91.0 | 118 | 84.9 | 128 | 92.1 | 122 | 89.1 | 490 | 89.3 |
| Trial treatment related | 0 | 0.0 | 4 | 2.9 | 0 | 0.0 | 0 | 0.0 | 4 | 0.7 |
| Disease and trial treatment related | 0 | 0.0 | 1 | 0.7 | 0 | 0.0 | 1 | 0.7 | 2 | 0.4 |
| Other cancer | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 1 | 0.2 |
| Other non-cancer | 11 | 8.2 | 15 | 10.8 | 10 | 7.2 | 12 | 8.8 | 48 | 8.7 |
| Non-trial treatment related | 1 | 0.7 | 1 | 0.7 | 1 | 0.7 | 1 | 0.7 | 4 | 0.7 |
| Unknown | 20 | – | 17 | – | 16 | – | 16 | – | 69 | – |
| Total | 134 | 100.0 | 139 | 100.0 | 139 | 100.0 | 137 | 100.0 | 549 | 100.0 |
| Cause of death | No ZA (N = 309) | ZA (N = 309) | No Sr-89 (N = 310) | Sr-89 (N = 308) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Disease related | 250 | 91.6 | 240 | 87 | 240 | 87.9 | 250 | 90.6 |
| Trial treatment related | 0 | 0 | 4 | 1.4 | 4 | 1.5 | 0 | 0 |
| Disease and trial treatment related | 0 | 0 | 2 | 0.7 | 1 | 0.4 | 1 | 0.4 |
| Other cancer | 0 | 0 | 1 | 0.4 | 0 | 0 | 1 | 0.4 |
| Other non-cancer | 21 | 7.7 | 27 | 9.8 | 26 | 9.5 | 22 | 8 |
| Non-trial treatment related | 2 | 0.7 | 2 | 0.7 | 2 | 0.7 | 2 | 0.7 |
| Unknown | 36 | – | 33 | – | 37 | – | 32 | – |
| Total | 273 | 100 | 276 | 100 | 273 | 100 | 276 | 100 |
Overall survival: zoledronic acid versus no zoledronic acid
In total, there were 618 events: 309 (50%) occurred in each group. A stratified log-rank test performed to compare ZA with no ZA demonstrated no difference in OS between the two groups (χ2 = 0.01; p = 0.909). The median survival time in the ZA group was 16.99 months (95% CI 16.07 to 19.23 months), compared with 17.61 months (95% CI 16.10 to 18.96 months) in the no ZA group.
A Cox proportional hazards model was used to estimate the HR (HR 0.99, 95% CI 0.84 to 1.16; p = 0.870). Figure 22 shows the Kaplan–Meier survival estimates split by ZA comparison.
FIGURE 22.
Kaplan–Meier estimates of OS by ZA.

Strontium-89 versus no strontium-89
In total, there were 618 events: 310 (50%) in the no Sr-89 group and 308 in the Sr-89 (50%) group. A stratified log-rank test performed to compare Sr-89 with no Sr-89 demonstrated no difference in OS between the two groups (χ2 = 0.93; p = 0.3359). The median survival time in the Sr-89 group was 18.17 months (95% CI 16.66 to 19.12 months), compared with 16.59 months (95% CI 15.61 to 18.27 months) in the no Sr-89 group.
A Cox proportional hazards model was used to estimate the HR (HR 0.92, 95% CI 0.79 to 1.08; p = 0.323). Figure 23 shows the Kaplan–Meier survival estimates split by Sr-89 comparison.
FIGURE 23.
Kaplan–Meier estimates of OS by Sr-89.
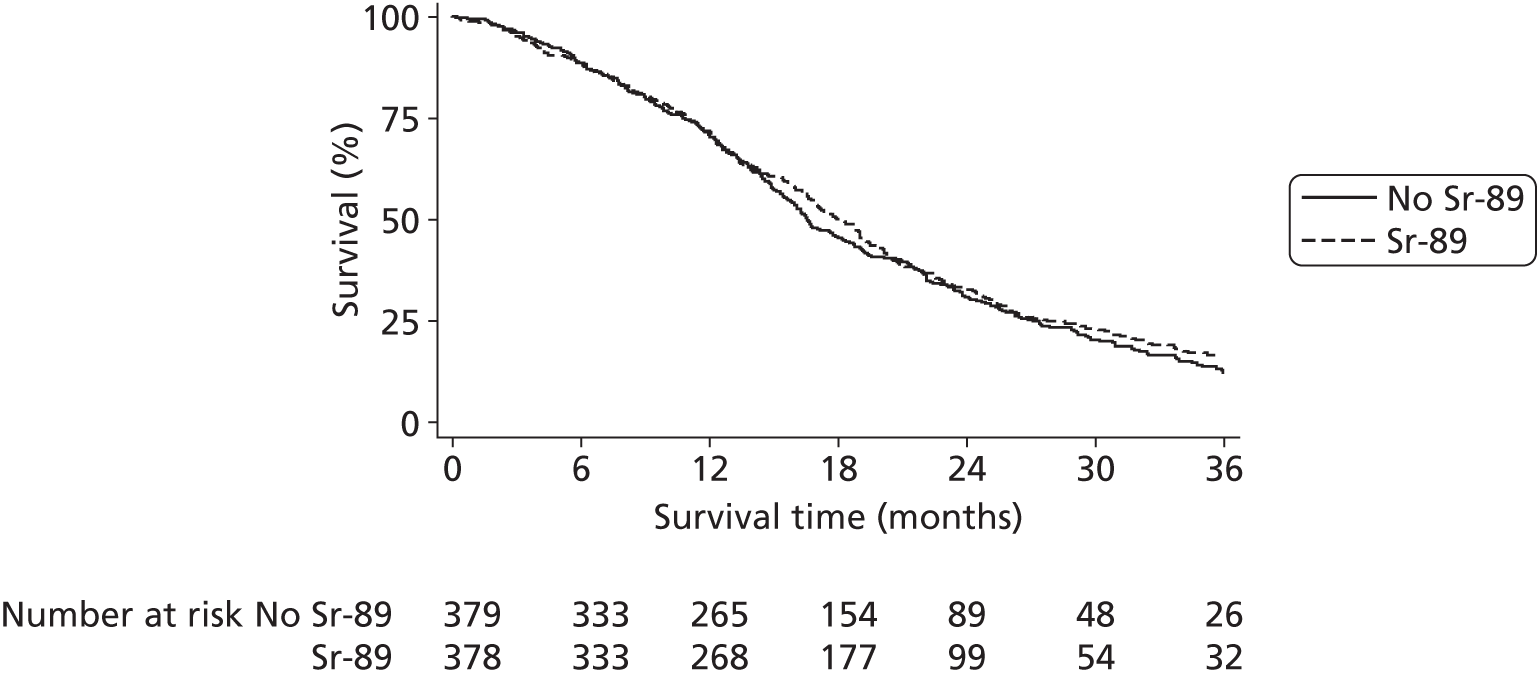
Quality of life
Statistical methods
The EQ-5D, VAS and FACT-P are presented descriptively using means, standard deviations and ranges for the comparison groups. In addition, all are presented graphically to look for patterns emerging over time. It was also considered that SREs may have an effect on patients’ QoL; therefore, patients were categorised as having no SREs, mild SREs or severe SREs, and these were investigated descriptively. In all cases, missing data for single items on questionnaires were dealt with in accordance with the manuals provided.
Quality-adjusted life-year analysis using subject-based approaches
Quality-adjusted life-years were utilised in two ways: subject- and group-based approaches. The first approach to QALY analysis was area-under-the-curve analysis, which was employed to investigate whether or not there were any differences between the comparison groups. As is usual with QoL data, there are more missing data towards the end of life. Therefore, three different methods were used to calculate QALYs to assess the impact of different assumptions, which can be seen in Figure 24. All deceased patients were treated in the same way. As only minimal changes in EQ-5D scores over time had been observed, the last QoL score was carried forward to the date the patient was last known to be alive and then a diagonal line was imputed from that date to date of death (see Figure 24a). For those patients still alive at the time of analysis, three separate approaches were taken. The first (see Figure 24b) made no imputation and the curve was dropped straight down at the date of the last EQ-5D score. This is a conservative approach, as it makes the inference that the patient died on the day his last EQ-5D questionnaire was collected. The other extreme of this was termed the optimistic approach (see Figure 24d); this approach carried the last EQ-5D score forward to the date last seen and then assumed that the patient survived for the same amount of time as the longest-surviving patient in the study and a diagonal line was imputed to that point. The third approach (see Figure 24c) is a middle ground between the conservative and optimistic approaches. The EQ-5D score is carried forward to the date last seen and drops to zero at that point, therefore imputing EQ-5D scores up to the date that the patient was known to be alive but not beyond this point.
FIGURE 24.
Method of calculating QALYs. (a) Deceased patients; (b) alive patients: conservative approach; (c) alive patients: standard approach; and (d) alive patients: optimistic approach.
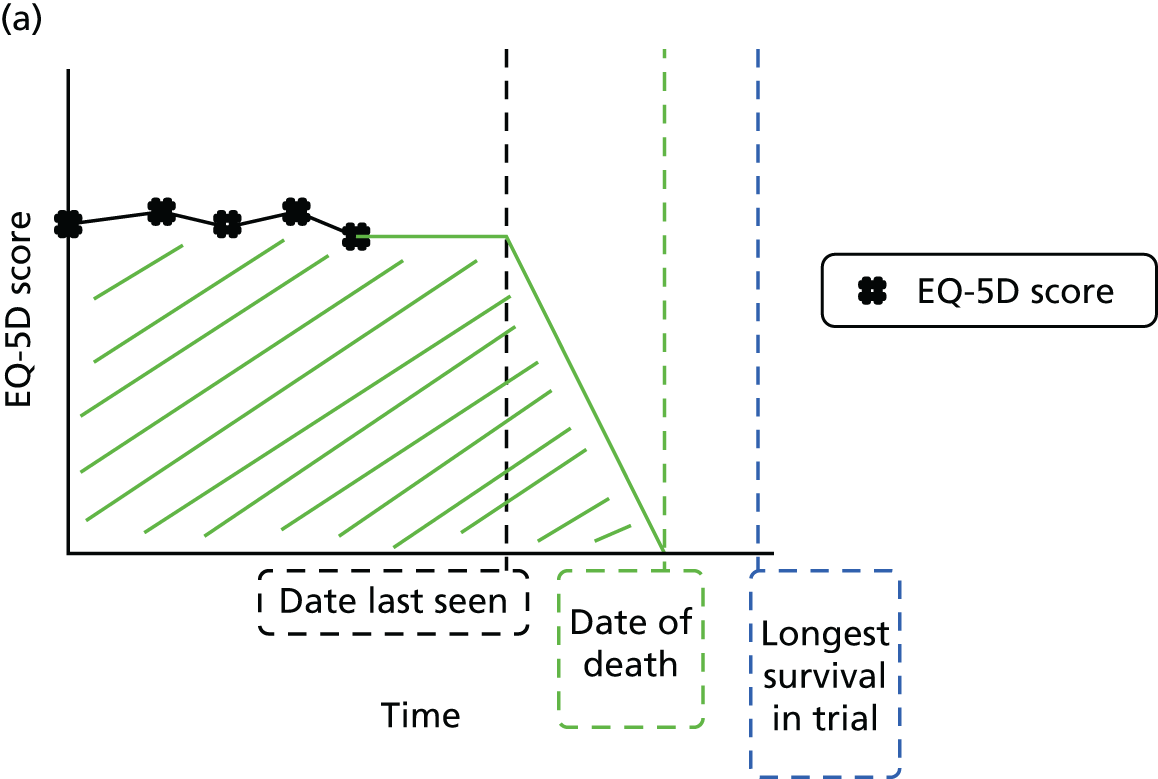



Quality-adjusted life-year analysis using group-based approaches
The second approach to QALY analysis was a pre-specified group-based quality-adjusted survival analysis conducted to assess the balance between QoL and survival. This approach takes account of dropout due to death and censoring. As the median overall survival in the trial was 1.4 years, 2 years was deemed the appropriate cut-off point for the quality-adjusted survival. The integrated quality survival product is the product of the survival53 and EQ-5D QoL measures over the 2-year time period of interest. It is calculated using the following equation:
where S(t) is the proportion of patients who survive to time t and Q(t) is the mean EQ-5D QoL associated with those survivors. This methodology of integrating QoL and survival was carried out at a group level for both the ZA and Sr-89 comparisons. The area under the curve at 2 years gave the mean QALY for each group. Standard errors were calculated and 95% CIs constructed using bootstrapping techniques with 1000 replications.
Results
In total, 6100 QoL booklets were returned: 5802 (95%) had complete EQ-5D data, 5806 (95%) had complete EQ-5D VAS data and 5573 (91%) had complete FACT-P data.
The 6100 forms were completed by 707 patients, leaving 50 patients for whom no QoL data were available. These patients are therefore excluded from this section of the report.
Patients returned, on average, six questionnaires: 40 patients returned only one form and one patient returned 22 forms.
Of the 707 patients, 572 had died at the time of analysis and 135 remained alive and on follow-up. The median time from completing the last QoL form to death was 193 days (IQR 86–381 days), meaning that, on average, two questionnaires are missing at the end of a patient’s life. For those patients alive, the median time from last QoL form to date last seen was 231 days (IQR 59–434 days). Again, there were, on average, two missing questionnaires. Figure 25 shows the points at which each questionnaire was returned.
FIGURE 25.
Quality-of-life questionnaire timings. TNO, trial number.

Six distinct lines can be seen at the start of the graph; these relate to the first six cycles of treatment. Subsequently, among those patients receiving Sr-89, there would be a longer gap between cycles 6 and 7. At this point, a large number of patients also started to progress and, thus, were withdrawn from treatment. As a result, their schedule of completion of QoL questionnaires changed, which explains why there was no discernible pattern after the first six chemotherapy cycles.
Health state thermometer
As mentioned previously, the EQ-5D VAS is a self-completed measure resembling a thermometer ranging from 0 to 100, on which patients mark how well they feel on any specific day. Table 37 shows the average EQ-5D VAS scores for each of the comparison groups throughout the trial.
| Health state (score range: 0–100) | No ZA (N = 2905) | ZA (N = 2996) | No Sr-89 (N = 2920) | Sr-89 (N = 2981) |
|---|---|---|---|---|
| n | 2905 | 2996 | 2920 | 2981 |
| Mean (SD) | 70.4 (19.5) | 72.4 (17.6) | 70.9 (18.4) | 71.9 (18.7) |
| Range | 0.0–100.0 | 0.0–100.0 | 0.0–100.0 | 0.0–100.0 |
There is no discernible difference in health state overall. Figure 26 shows the health state by ZA comparison over time and the number of deaths over time; it also shows this split by SRE severity.
FIGURE 26.
Visual analogue scale scores over time (lines) and number of deaths (boxes) by ZA comparison. (a) VAS score by ZA; and (b) VAS score by worst SRE and ZA.

Once the treatment period is complete, at about 6 months, the QoL of patients not receiving ZA seems to be consistently lower, particularly among those experiencing severe SREs, as would be expected.
Figure 27 below shows the health state by Sr-89 comparison over time and the number of deaths over time; it also shows this split by SRE severity.
FIGURE 27.
Visual analogue scale scores over time (lines) and number of deaths (boxes) by Sr-89 comparison. (a) VAS score by Sr-89; and (b) VAS score by worst SRE and Sr-89.

Again, the QoL of those receiving Sr-89 appears to be consistently lower following the completion of treatment.
Functional Assessment of Cancer Therapy – Prostate
Table 38 shows the average score over the whole trial for each of the FACT-P subscales split by comparison groups.
| FACT-P | No ZA (N = 3005) | ZA (N = 3095) | No Sr-89 (N = 3022) | Sr-89 (N = 3078) |
|---|---|---|---|---|
| Physical well-being (score range: 0–28) | ||||
| n | 2901 | 2993 | 2913 | 2981 |
| Mean (SD) | 21.7 (5.0) | 22.1 (4.7) | 21.7 (5.0) | 22.1 (4.8) |
| Range | 0.0–28.0 | 0.0–28.0 | 0.0–28.0 | 0.0–28.0 |
| Social well-being (score range: 0–28) | ||||
| n | 2914 | 3000 | 2921 | 2993 |
| Mean (SD) | 22.7 (4.5) | 23.2 (4.3) | 23.1 (4.5) | 22.8 (4.2) |
| Range | 0.0–28.0 | 0.0–28.0 | 0.0–28.0 | 0.0–28.0 |
| Emotional well-being (score range: 0–24) | ||||
| n | 2866 | 2970 | 2885 | 2951 |
| Mean (SD) | 18.9 (4.4) | 19.3 (4.0) | 18.9 (4.4) | 19.2 (4.0) |
| Range | 0.0–24.0 | 2.0–24.0 | 0.0–24.0 | 0.0–24.0 |
| Functional well-being (score range: 0–28) | ||||
| n | 2897 | 3004 | 2923 | 2978 |
| Mean (SD) | 18.4 (6.5) | 18.9 (6.2) | 18.5 (6.4) | 18.8 (6.3) |
| Range | 0.0–28.0 | 0.0–28.0 | 0.0–28.0 | 0.0–28.0 |
| Prostate cancer (score range: 0–48) | ||||
| n | 2901 | 3012 | 2916 | 2997 |
| Mean (SD) | 32.9 (7.7) | 33.2 (7.4) | 32.9 (7.5) | 33.2 (7.6) |
| Range | 3.0–48.0 | 6.5–48.0 | 3.0–48.0 | 3.0–48.0 |
| FACT-P (score range: 0–156) | ||||
| n | 2729 | 2844 | 2746 | 2827 |
| Mean (SD) | 114.6 (22.2) | 116.9 (20.8) | 115.2 (21.8) | 116.3 (21.2) |
| Range | 29.0–156.0 | 44.0–156.0 | 44.0–156.0 | 29.0–156.0 |
As with health state scales, there are very few differences across the comparison groups overall. Figure 28 shows a series of six graphs of the FACT-P subscale score over time split by ZA groups. Figure 28f also includes a bar chart of the numbers of deaths over time.
FIGURE 28.
Functional Assessment of Cancer Therapy – Prostate subscales over time by ZA. (a) Physical well-being; (b) social well-being; (c) emotional well-being; (d) functional well-being; (e) prostate cancer; and (f) FACT-P.





Each of the curves appears to show an increase in QoL within the first 2 months. It is most pronounced in the prostate cancer subscale and perhaps shows the positive effect of treatment in this patient population. The scales level off relatively quickly and remain constant and there is no visible decline in QoL; this is almost certainly at least in part due to missing data at the end of patients lives. The ZA line is very slightly higher in all of the graphs, and this is particularly apparent on the FACT-P graph, which is a summation of all of the subscales combined. Figure 29 shows the same series of graphs for Sr-89.
FIGURE 29.
Functional Assessment of Cancer Therapy – Prostate subscale over time by Sr-89. (a) Physical well-being; (b) social well-being; (c) emotional well-being; (d) functional well-being; (e) prostate cancer; and (f) FACT-P.



As seen previously, there is an increased QoL in the first 2 months. However, there appears to be no difference between the Sr-89 comparison groups in terms of social well-being, and this has contributed to the much reduced differences observed in the overall FACT-P scale. Figures 30 and 31 show the same six graphs, but they also split by SRE severity.
FIGURE 30.
Functional Assessment of Cancer Therapy – Prostate subscale over time by SRE severity and ZA. (a) Physical well-being; (b) social well-being; (c) emotional well-being; (d) functional well-being; (e) prostate cancer; and (f) FACT-P.



FIGURE 31.
Functional Assessment of Cancer Therapy – Prostate subscale over time by SRE severity and Sr-89. (a) Physical well-being; (b) social well-being; (c) emotional well-being; (d) functional well-being; (e) prostate cancer; and (f) FACT-P.

European Quality of Life 5-Dimensions
Gains in QALYS measured using the three methods are detailed in Table 39, split by Sr-89 and ZA.
| QALYs | No ZA (N = 357) | ZA (N = 350) | No Sr-89 (N = 357) | Sr-89 (N = 350) |
|---|---|---|---|---|
| Conservative | ||||
| n | 357 | 350 | 357 | 350 |
| Mean QALYs gained (SD) | 0.8 (0.6) | 0.8 (0.6) | 0.8 (0.6) | 0.8 (0.6) |
| Range | –0.2 to 4.2 | –0.1 to 3.6 | –0.1 to 4.2 | –0.2 to 4.1 |
| Standard | ||||
| n | 357 | 350 | 357 | 350 |
| Mean QALYs gained (SD) | 0.9 (0.7) | 0.9 (0.7) | 0.9 (0.7) | 0.9 (0.7) |
| Range | –0.2 to 4.3 | –0.1 to 3.6 | –0.1 to 4.3 | –0.2 to 4.1 |
| Optimistic | ||||
| n | 357 | 350 | 357 | 350 |
| Mean QALYs gained (SD) | 1.2 (1.1) | 1.2 (1.1) | 1.1 (1.1) | 1.2 (1.1) |
| Range | –0.2 to 5.2 | –0.4 to 4.6 | –0.1 to 5.2 | –0.4 to 4.7 |
These findings again support the previous QoL investigations in that no difference between comparison groups is apparent. The three different methods of calculating QALYs do affect the estimates, but the differences between the groups remained consistent. Figure 32 shows the actual returned values of the EQ-5D over time split by SRE severity and ZA.
FIGURE 32.
European Quality of Life 5-Dimensions over time by ZA and SRE Severity. (a) EQ-5D by ZA; and (b) EQ-5D by worst SRE and ZA.

The increase in QoL in the first 2 months is again present. Figure 33 is the same as Figure 32, but split by Sr-89.
FIGURE 33.
European Quality of Life 5-Dimensions over time by Sr-89 and SRE severity. (a) EQ-5D by Sr-89; and (b) EQ-5D by worst SRE and Sr-89.
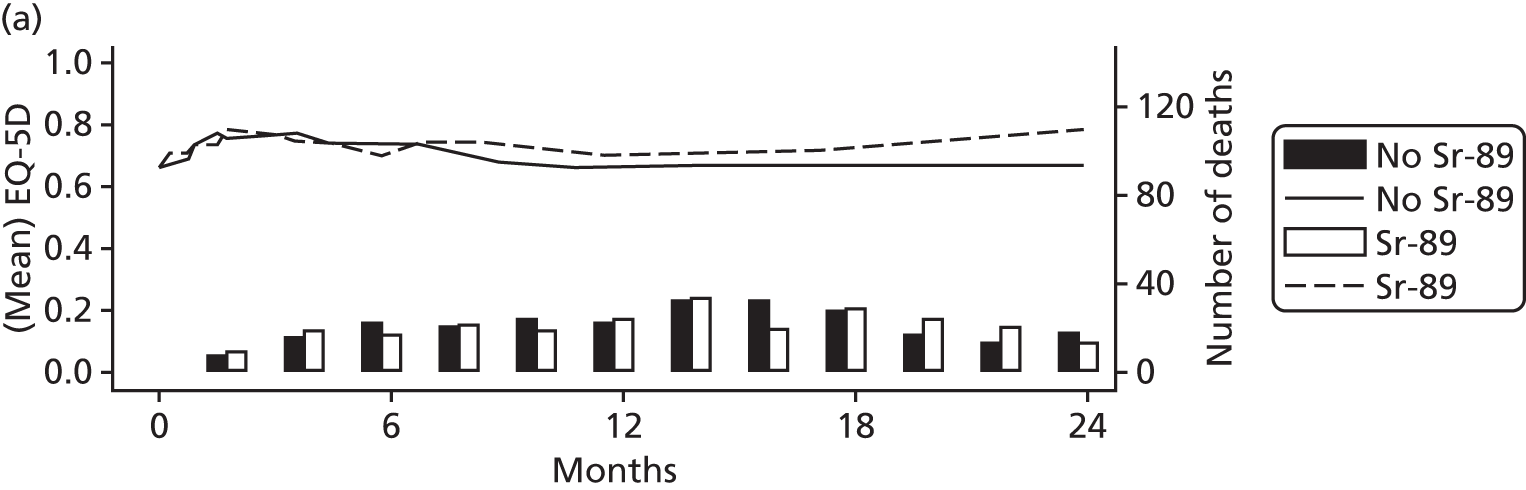

Quality-adjusted survival
Quality-adjusted survival within 2 years split by the comparison groups is detailed in Table 40.
| Comparitor | No ZA | ZA | No Sr-89 | Sr-89 |
|---|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | |
| Mean life-years gained (within 2 years) | 1.47 (1.34 to 1.58) | 1.42 (1.34 to 1.59) | 1.38 (1.30 to 1.52) | 1.51 (1.39 to 1.59) |
| Quality-adjusted survival (within 2 years) | 1.00 (0.93 to 1.10) | 1.04 (0.98 to 1.10) | 0.97 (0.991 to 1.03) | 1.05 (0.99 to 1.12) |
Adjusting for QoL does not change the conclusions of the overall survival analysis alone. There were no statistically or clinically meaningful differences between either comparison group. The 2-year quality-adjusted survival is approximately 1 year in all comparison arms, which is 4 to 6 months shorter than the survival analysis alone. Figures 34 and 35 illustrate the QoL function, the survival function and the integrated quality survival product for the two comparison groups.
FIGURE 34.
Quality-adjusted survival split by ZA group. (a) EQ-5D QoL function, Q(t); (b) survival function, S(t); and (c) integrated quality survival product.

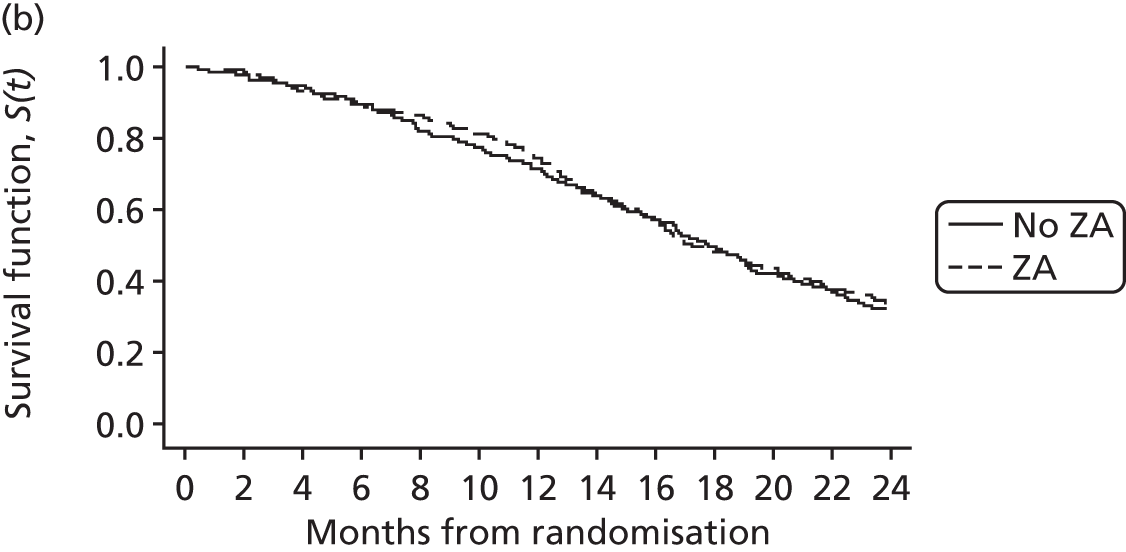
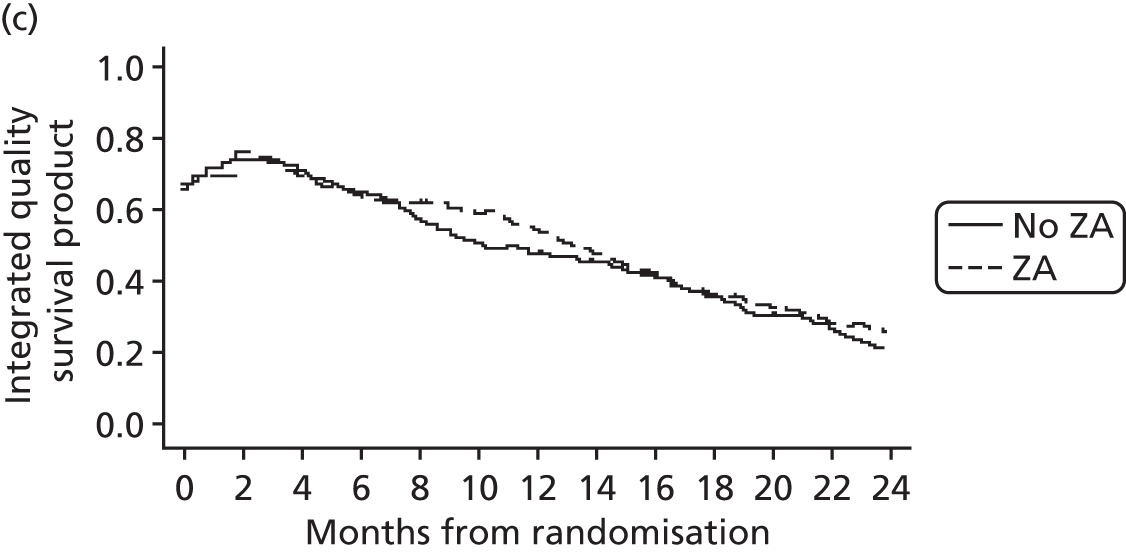
FIGURE 35.
Quality-adjusted survival split by Sr-89 group. (a) EQ-5D QoL function, Q(t); (b) survival function, S(t); and (c) integrated quality survival product.
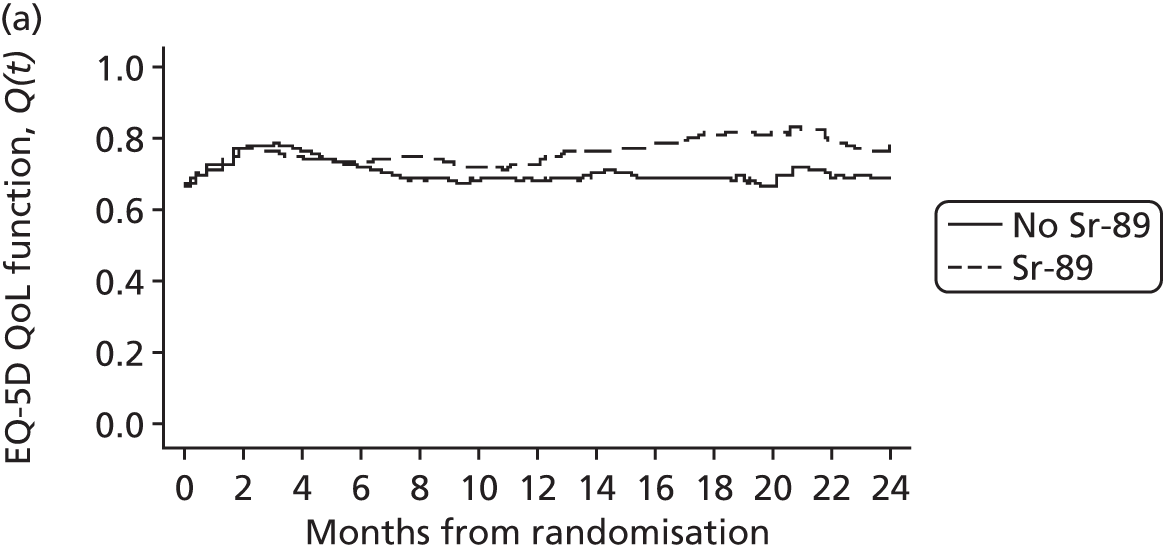

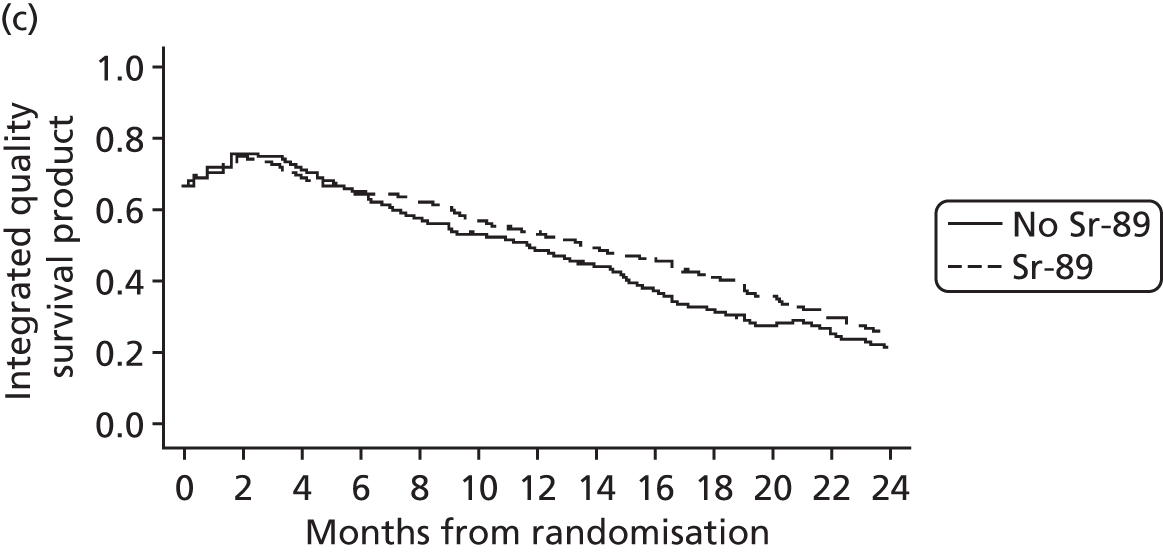
The Sr-89 integrated survival product does show a slight difference, with those receiving Sr-89 gaining approximately one additional quality-adjusted survival month.
Adverse events
Timing of events
In total, 493 grade 3 or 4 adverse events (AEs) were reported. In addition, 313 events without a grade were reported and 161 without a start date were reported, making it impossible to determine whether these events occurred on- or off-treatment.
Additionally, there were 178 (24%) patients who reported no grade 3 or 4 AEs, while 27 patients reported experiencing AEs but did not receive any treatment. Table 41 shows the grading and timing of all AEs.
| Time point | Grade 3 (N = 447) | Grade 4 (N = 46) | Missing (N = 313) | Overall (N = 806) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Pre-treatment | 13 | 2.9 | 4 | 8.7 | 8 | 2.6 | 25 | 3.1 |
| Cycle 1 | 101 | 22.6 | 11 | 23.9 | 38 | 12.1 | 150 | 18.6 |
| Cycle 2 | 55 | 12.3 | 10 | 21.7 | 23 | 7.3 | 88 | 10.9 |
| Cycle 3 | 52 | 11.6 | 5 | 10.9 | 18 | 5.8 | 75 | 9.3 |
| Cycle 4 | 31 | 6.9 | 5 | 10.9 | 16 | 5.1 | 52 | 6.5 |
| Cycle 5 | 38 | 8.5 | 3 | 6.5 | 7 | 2.2 | 48 | 6.0 |
| Cycle 6 | 81 | 18.1 | 4 | 8.7 | 54 | 17.3 | 139 | 17.2 |
| Cycle 7 | 18 | 4.0 | 2 | 4.3 | 2 | 0.6 | 22 | 2.7 |
| Cycle 8 | 10 | 2.2 | 0 | 0.0 | 1 | 0.3 | 11 | 1.4 |
| Cycle 9 | 5 | 1.1 | 0 | 0.0 | 4 | 1.3 | 9 | 1.1 |
| Cycle 10 | 3 | 0.7 | 1 | 2.2 | 0 | 0.0 | 4 | 0.5 |
| Follow-up 1 | 6 | 1.3 | 0 | 0.0 | 5 | 1.6 | 11 | 1.4 |
| Follow-up 2 | 1 | 0.2 | 1 | 2.2 | 0 | 0.0 | 2 | 0.2 |
| Follow-up 3 | 3 | 0.7 | 0 | 0.0 | 1 | 0.3 | 4 | 0.5 |
| Follow-up 4 | 5 | 1.1 | 0 | 0.0 | 0 | 0.0 | 5 | 0.6 |
| Unknown | 25 | 5.6 | 0 | 0.0 | 136 | 43.5 | 161 | 20.0 |
| Total | 447 | 100.0 | 46 | 100.0 | 313 | 100.0 | 806 | 100.0 |
Tables 42 and 43 show the percentages of patients at each time point who reported at least one grade 3 or 4 event, split by randomisation arm and comparison group.
| Time point | Docetaxel (%) | Docetaxel + ZA (%) | Docetaxel + Sr-89 (%) | Docetaxel + ZA + Sr-89 (%) | Overall (%) |
|---|---|---|---|---|---|
| Cycle 1 | 14.0 | 17.2 | 12.7 | 17.6 | 15.4 |
| Cycle 2 | 12.8 | 4.8 | 8.6 | 11.1 | 9.4 |
| Cycle 3 | 7.1 | 8.6 | 11.4 | 7.2 | 8.6 |
| Cycle 4 | 4.4 | 5.1 | 10.5 | 3.2 | 5.8 |
| Cycle 5 | 7.4 | 6.1 | 6.3 | 8.1 | 7.0 |
| Cycle 6 | 10.2 | 13.6 | 17.7 | 22.6 | 16.1 |
| Cycle 7 | 20.0 | 10.5 | 6.1 | 3.9 | 9.9 |
| Cycle 8 | 2.6 | 3.8 | 4.5 | 11.4 | 5.6 |
| Cycle 9 | 2.9 | 9.3 | 0 | 0 | 3.2 |
| Cycle 10 | 3.8 | 5.9 | 2.9 | 0 | 3.1 |
| Follow-up 1 | 0 | 3.2 | 0.7 | 0 | 1.0 |
| Follow-up 2 | 0.7 | 0 | 0.7 | 0 | 0.3 |
| Follow-up 3 | 0 | 0.7 | 1.6 | 0 | 0.6 |
| Follow-up 4 | 0.9 | 3.4 | 0 | 0 | 1.1 |
| Time point | No ZA (%) | ZA (%) | No Sr-89 (%) | Sr-89 (%) |
|---|---|---|---|---|
| Cycle 1 | 13.4 | 17.4 | 15.6 | 15.2 |
| Cycle 2 | 10.7 | 8.0 | 9.0 | 9.8 |
| Cycle 3 | 9.2 | 8.0 | 7.8 | 9.3 |
| Cycle 4 | 7.4 | 4.2 | 4.8 | 6.8 |
| Cycle 5 | 6.8 | 7.1 | 6.8 | 7.2 |
| Cycle 6 | 14.0 | 18.2 | 11.9 | 20.2 |
| Cycle 7 | 12.8 | 7.4 | 14.7 | 5.0 |
| Cycle 8 | 3.7 | 7.2 | 3.3 | 8.0 |
| Cycle 9 | 1.4 | 5.0 | 6.5 | 0 |
| Cycle 10 | 3.3 | 2.9 | 5.0 | 1.4 |
| Follow-up 1 | 0.3 | 1.6 | 1.6 | 0.3 |
| Follow-up 2 | 0.7 | 0 | 0.3 | 0.4 |
| Follow-up 3 | 0.8 | 0.4 | 0.4 | 0.8 |
| Follow-up 4 | 0.5 | 1.7 | 2.2 | 0 |
Tables 41–43 indicate that there was a peak in adverse events at cycle 6. This may have been a function of increased scrutiny at this time point to ensure that patients were well enough to receive additional treatment, which was particularly important for those randomised to receive Sr-89.
Grade and number of adverse events
Figures 36 and 37 show the grade and number of AEs over time, split by comparisons. The graphs show that grade 3 and 4 AEs peak at cycle 1 and cycle 6. There appear to be additional events associated with Sr-89 at cycle 6, which makes sense, as this would include the time during which Sr-89 is administered, but there is no evidence to suggest that these effects are long-lasting. In terms of ZA, increased numbers of events are seen at both peaks. However, it is worth noting that the magnitude of these differences is only 10 individual events.
FIGURE 36.
Grade and number of AEs over time by ZA comparison. (a) No ZA; and (b) ZA.
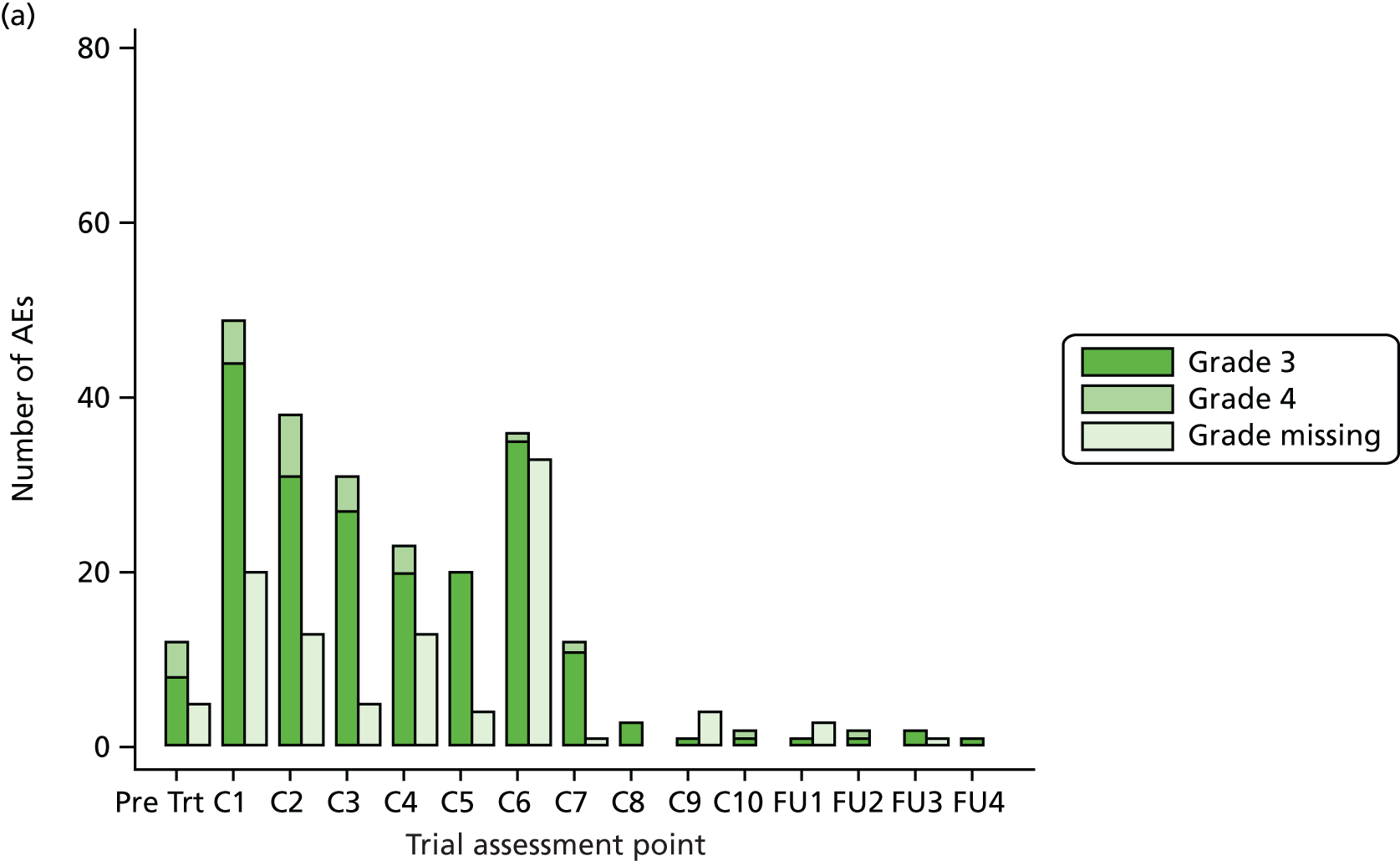

FIGURE 37.
Grade and number of AEs over time by Sr-89 comparison. (a) No Sr-89; and (b) Sr-89.


Figure 38 shows the time and type of any AE where at least five or more instances were observed at one time point. This is split by comparison groups.
FIGURE 38.
Frequency of AEs over time. (a) No ZA; (b) no Sr-89; (c) ZA; and (d) Sr-89.


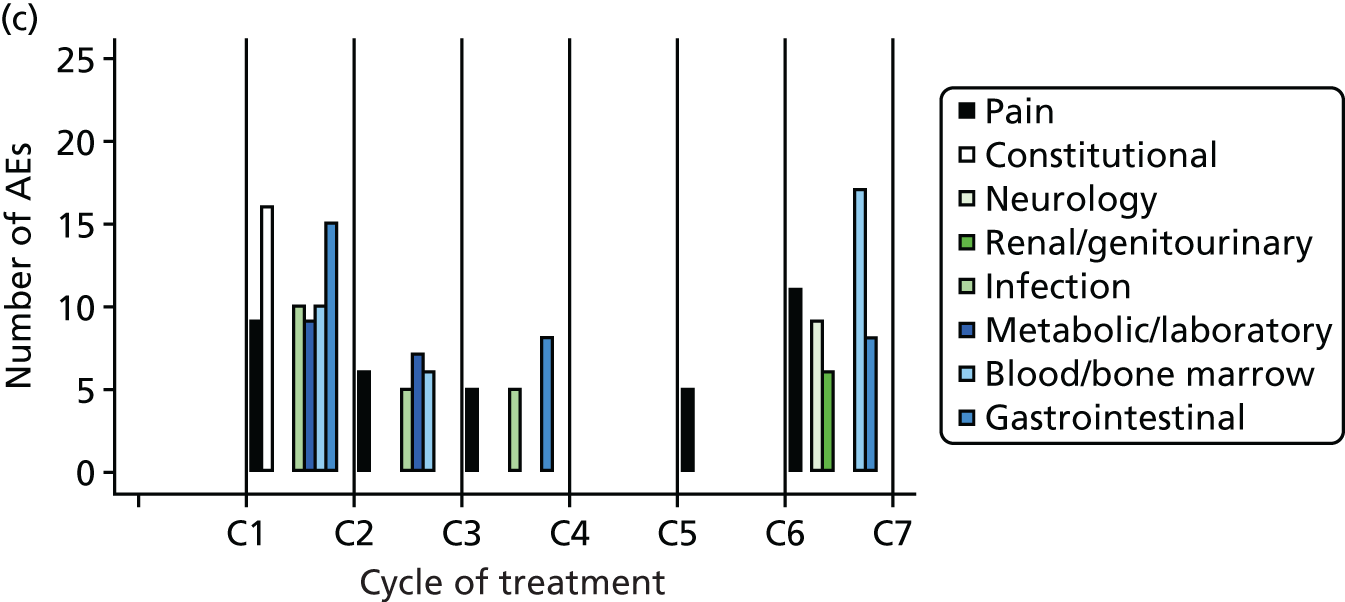

Adverse event symptoms
Tables 44–46 show the adverse event symptoms reported by randomisation arm separately for grades 3 and 4 and missing grades.
The most common grade 4 events were infection- and blood/bone marrow-related. However, it did not appear to be the case that the combined arm containing both ZA and Sr-89 was experiencing more grade 4 AEs.
| Category as defined in Common Terminology Criteria for Adverse Events, version 354 | Docetaxel (N = 106) | Docetaxel + ZA (N = 109) | Docetaxel + Sr-89 (N = 117) | Docetaxel + ZA + Sr-89 (N = 115) | Overall (N = 447) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Pulmonary/upper respiratory | 8 | 7.5 | 3 | 2.8 | 0 | 0.0 | 0 | 0.0 | 11 | 2.5 |
| Pain | 16 | 15.1 | 10 | 9.2 | 16 | 13.7 | 11 | 9.6 | 53 | 11.9 |
| Haemorrhage/bleeding | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 1 | 0.9 | 2 | 0.4 |
| Ocular/visual | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 1 | 0.2 |
| Constitutional symptoms | 13 | 12.3 | 10 | 9.2 | 20 | 17.1 | 12 | 10.4 | 55 | 12.3 |
| Allergy/immunology | 3 | 2.8 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 4 | 0.9 |
| Musculoskeletal/soft tissue | 1 | 0.9 | 4 | 3.7 | 5 | 4.3 | 2 | 1.7 | 12 | 2.7 |
| Neurology | 7 | 6.6 | 10 | 9.2 | 8 | 6.8 | 5 | 4.3 | 30 | 6.7 |
| Lymphatics | 4 | 3.8 | 2 | 1.8 | 2 | 1.7 | 9 | 7.8 | 17 | 3.8 |
| Renal/genitourinary | 0 | 0.0 | 5 | 4.6 | 2 | 1.7 | 7 | 6.1 | 14 | 3.1 |
| Dermatology/skin | 6 | 5.7 | 7 | 6.4 | 6 | 5.1 | 2 | 1.7 | 21 | 4.7 |
| Vascular | 0 | 0.0 | 1 | 0.9 | 1 | 0.9 | 4 | 3.5 | 6 | 1.3 |
| Infection | 16 | 15.1 | 15 | 13.8 | 12 | 10.3 | 12 | 10.4 | 55 | 12.3 |
| Metabolic/laboratory | 9 | 8.5 | 12 | 11.0 | 11 | 9.4 | 15 | 13.0 | 47 | 10.5 |
| Blood/bone marrow | 13 | 12.3 | 20 | 18.3 | 21 | 17.9 | 17 | 14.8 | 71 | 15.9 |
| Cardiac general | 0 | 0.0 | 0 | 0.0 | 2 | 1.7 | 0 | 0.0 | 2 | 0.4 |
| Sexual/reproductive function | 0 | 0.0 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| Cardiac arrhythmia | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 1.7 | 2 | 0.4 |
| Syndromes | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 1 | 0.9 | 2 | 0.4 |
| Endocrine | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 1 | 0.2 |
| Gastrointestinal | 10 | 9.4 | 8 | 7.3 | 9 | 7.7 | 13 | 11.3 | 40 | 8.9 |
| Total | 106 | 100.0 | 109 | 100.0 | 117 | 100.0 | 115 | 100.0 | 447 | 100.0 |
| Category as defined in Common Terminology Criteria for Adverse Events, version 354 | Docetaxel (N = 15) | Docetaxel + ZA (N = 9) | Docetaxel + Sr-89 (N = 12) | Docetaxel + ZA + Sr-89 (N = 10) | Overall (N = 46) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Pulmonary/upper respiratory | 1 | 6.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 2.2 |
| Pain | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 10.0 | 1 | 2.2 |
| Neurology | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 | 1 | 10.0 | 2 | 4.3 |
| Vascular | 1 | 6.7 | 3 | 33.3 | 0 | 0.0 | 0 | 0.0 | 4 | 8.7 |
| Infection | 5 | 33.3 | 1 | 11.1 | 4 | 33.3 | 2 | 20.0 | 12 | 26.1 |
| Metabolic/laboratory | 1 | 6.7 | 2 | 22.2 | 1 | 8.3 | 0 | 0.0 | 4 | 8.7 |
| Blood/bone marrow | 5 | 33.3 | 3 | 33.3 | 6 | 50.0 | 6 | 60.0 | 20 | 43.5 |
| Cardiac general | 2 | 13.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 4.3 |
| Total | 15 | 100.0 | 9 | 100.0 | 12 | 100.0 | 10 | 100.0 | 46 | 100.0 |
| Category as defined in Common Terminology Criteria for Adverse Events, version 354 | Docetaxel (N = 91) | Docetaxel + ZA (N = 73) | Docetaxel + Sr-89 (N = 78) | Docetaxel + ZA + Sr-89 (N = 71) | Overall (N = 313) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Pulmonary/upper respiratory | 2 | 2.5 | 1 | 1.8 | 2 | 3.2 | 3 | 5.5 | 8 | 3.1 |
| Pain | 22 | 27.5 | 14 | 25.0 | 15 | 23.8 | 14 | 25.5 | 65 | 25.6 |
| Haemorrhage/bleeding | 1 | 1.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 |
| Ocular/visual | 0 | 0.0 | 0 | 0.0 | 1 | 1.6 | 0 | 0.0 | 1 | 0.4 |
| Constitutional symptoms | 6 | 7.5 | 9 | 16.1 | 5 | 7.9 | 3 | 5.5 | 23 | 9.1 |
| Allergy/immunology | 1 | 1.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 |
| Musculoskeletal/soft tissue | 4 | 5.0 | 5 | 8.9 | 2 | 3.2 | 3 | 5.5 | 14 | 5.5 |
| Neurology | 2 | 2.5 | 1 | 1.8 | 5 | 7.9 | 3 | 5.5 | 11 | 4.3 |
| Lymphatics | 4 | 5.0 | 4 | 7.1 | 4 | 6.3 | 2 | 3.6 | 14 | 5.5 |
| Renal/genitourinary | 0 | 0.0 | 2 | 3.6 | 2 | 3.2 | 2 | 3.6 | 6 | 2.4 |
| Dermatology/skin | 2 | 2.5 | 5 | 8.9 | 8 | 12.7 | 4 | 7.3 | 19 | 7.5 |
| Infection | 5 | 6.3 | 3 | 5.4 | 2 | 3.2 | 2 | 3.6 | 12 | 4.7 |
| Metabolic/laboratory | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 3.6 | 2 | 0.8 |
| Blood/bone marrow | 4 | 5.0 | 3 | 5.4 | 2 | 3.2 | 4 | 7.3 | 13 | 5.1 |
| Cardiac general | 1 | 1.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 |
| Cardiac arrhythmia | 0 | 0.0 | 0 | 0.0 | 1 | 1.6 | 0 | 0.0 | 1 | 0.4 |
| Syndromes | 3 | 3.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 1.2 |
| Endocrine | 0 | 0.0 | 0 | 0.0 | 2 | 3.2 | 0 | 0.0 | 2 | 0.8 |
| Gastrointestinal | 22 | 27.5 | 9 | 16.1 | 12 | 19.0 | 13 | 23.6 | 56 | 22.0 |
| Secondary malignancy | 1 | 1.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 |
| Missing | 11 | – | 17 | – | 15 | – | 16 | – | 59 | – |
| Total | 80 | 100.0 | 56 | 100.0 | 63 | 100.0 | 55 | 100.0 | 254 | 100.0 |
Serious adverse events
In total, 583 SAEs were reported, with 1064 associated symptoms, relating to 373 (49%) patients. Tables 47 and 48 show the categorisation of the 583 SAEs by both randomisation arm and comparison group.
| SAE categorisation | Docetaxel (N = 133) | Docetaxel + ZA (N = 164) | Docetaxel + Sr-89 (N = 146) | Docetaxel + ZA + Sr-89 (N = 140) | Overall (N = 583) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Unrelated SAE | 56 | 42.1 | 96 | 58.5 | 67 | 45.9 | 67 | 47.9 | 286 | 49.1 |
| SAR | 73 | 54.9 | 65 | 39.6 | 73 | 50.0 | 65 | 46.4 | 276 | 47.3 |
| Non-fatal/life-threatening SUSAR | 1 | 0.8 | 1 | 0.6 | 3 | 2.1 | 1 | 0.7 | 6 | 1.0 |
| Fatal/life-threatening SUSAR | 3 | 2.3 | 2 | 1.2 | 3 | 2.1 | 7 | 5.0 | 15 | 2.6 |
| Total | 133 | 100.0 | 164 | 100.0 | 146 | 100.0 | 140 | 100.0 | 583 | 100.0 |
| SAE categorisation | No ZA (N = 279) | ZA (N = 304) | No Sr-89 (N = 297) | Sr-89 (N = 286) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Unrelated SAE | 123 | 44.1 | 163 | 53.6 | 152 | 51.2 | 134 | 46.9 |
| SAR | 146 | 52.3 | 130 | 42.8 | 138 | 46.5 | 138 | 48.3 |
| Non-fatal/life-threatening SUSAR | 4 | 1.4 | 2 | 0.7 | 2 | 0.7 | 4 | 1.4 |
| Fatal/life-threatening SUSAR | 6 | 2.2 | 9 | 3.0 | 5 | 1.7 | 10 | 3.5 |
| Total | 279 | 100.0 | 304 | 100.0 | 297 | 100.0 | 286 | 100.0 |
Tables 49 and 50 show the number of SAEs experienced per patient, split by randomisation arm and comparison group.
| Number of SAEs per patient | Docetaxel (N = 191) | Docetaxel + ZA (N = 188) | Docetaxel + Sr-89 (N = 190) | Docetaxel + ZA + Sr-89 (N = 188) | Overall (N = 757) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| 1 | 48 | 25 | 72 | 38 | 64 | 34 | 49 | 26 | 233 | 31 |
| 2 | 24 | 13 | 17 | 9 | 23 | 12 | 24 | 13 | 88 | 12 |
| 3 | 11 | 6 | 11 | 6 | 5 | 3 | 11 | 6 | 38 | 5 |
| 4 | 1 | 0 | 5 | 3 | 4 | 2 | 1 | < 1 | 11 | 1 |
| 5 | 0 | 0 | 1 | < 1 | 1 | < 1 | 0 | 0 | 2 | < 1 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | < 1 | 1 | < 1 |
| Patients with ≥ 1 SAE | 84 | 44 | 106 | 56 | 97 | 51 | 86 | 46 | 373 | 49 |
| Number of SAEs per patient | No ZA (N = 381) | ZA (N = 376) | No Sr-89 (N = 379) | Sr-89 (N = 378) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| 1 | 112 | 30 | 121 | 32 | 120 | 32 | 113 | 30 |
| 2 | 47 | 12 | 41 | 11 | 41 | 11 | 47 | 12 |
| 3 | 16 | 4 | 22 | 6 | 22 | 6 | 16 | 4 |
| 4 | 5 | 1 | 6 | 2 | 6 | 1 | 5 | 1 |
| 5 | 1 | < 1 | 1 | < 1 | 1 | < 1 | 1 | < 1 |
| 6 | 0 | 0 | 1 | < 1 | 0 | 0 | 1 | < 1 |
| Patients with ≥ 1 SAE | 181 | 47 | 192 | 51 | 190 | 50 | 183 | 48 |
Approximately 50% of patients in each comparison group experienced at least one SAE, with approximately 20% going on to have multiple SAEs.
Reasons for serious adverse events
Tables 51 and 52 show the reasons for SAEs split by both randomisation arm and comparison groups.
| New reason | Docetaxel (N = 133) | Docetaxel + ZA (N = 164) | Docetaxel + Sr-89 (N = 146) | Docetaxel + ZA + Sr-89 (N = 140) | Overall (N = 583) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Death | 1 | 0.8 | 7 | 4.3 | 8 | 5.5 | 9 | 6.4 | 25 | 4.3 |
| Death and disability | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| Death and hospitalisation | 4 | 3.0 | 5 | 3.0 | 7 | 4.8 | 9 | 6.4 | 25 | 4.3 |
| Death and other | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 1 | 0.2 |
| Death, hospitalisation and disability | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| Death, life-threatening and hospitalisation | 1 | 0.8 | 2 | 1.2 | 0 | 0.0 | 2 | 1.4 | 5 | 0.9 |
| Death, life-threatening, hospitalisation and other | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 1 | 0.2 |
| Disability | 0 | 0.0 | 2 | 1.2 | 1 | 0.7 | 1 | 0.7 | 4 | 0.7 |
| Hospitalisation | 115 | 86.5 | 124 | 75.6 | 117 | 80.1 | 111 | 79.3 | 467 | 80.1 |
| Hospitalisation and disability | 3 | 2.3 | 4 | 2.4 | 1 | 0.7 | 3 | 2.1 | 11 | 1.9 |
| Hospitalisation and other | 2 | 1.5 | 2 | 1.2 | 2 | 1.4 | 0 | 0.0 | 6 | 1.0 |
| Hospitalisation, disability and other | 1 | 0.8 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 2 | 0.3 |
| Life-threatening | 0 | 0.0 | 1 | 0.6 | 1 | 0.7 | 0 | 0.0 | 2 | 0.3 |
| Life-threatening and hospitalisation | 3 | 2.3 | 12 | 7.3 | 4 | 2.7 | 3 | 2.1 | 22 | 3.8 |
| New primary cancer | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| Other | 1 | 0.8 | 3 | 1.8 | 5 | 3.4 | 0 | 0.0 | 9 | 1.5 |
| Total | 133 | 100.0 | 164 | 100.0 | 146 | 100.0 | 140 | 100.0 | 583 | 100.0 |
| Reason | No ZA (N = 279) | ZA (N = 304) | No Sr-89 (N = 297) | Sr-89 (N = 286) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Death | 9 | 3.2 | 16 | 5.3 | 8 | 2.7 | 17 | 5.9 |
| Death and disability | 0 | 0.0 | 1 | 0.3 | 1 | 0.3 | 0 | 0.0 |
| Death and hospitalisation | 11 | 3.9 | 14 | 4.6 | 9 | 3.0 | 16 | 5.6 |
| Death and other | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 |
| Death, hospitalisation and disability | 1 | 0.4 | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 |
| Death, life-threatening and hospitalisation | 1 | 0.4 | 4 | 1.3 | 3 | 1.0 | 2 | 0.7 |
| Death, life-threatening, hospitalisation and other | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 |
| Disability | 1 | 0.4 | 3 | 1.0 | 2 | 0.7 | 2 | 0.7 |
| Hospitalisation | 232 | 83.2 | 235 | 77.3 | 239 | 80.5 | 228 | 79.7 |
| Hospitalisation and disability | 4 | 1.4 | 7 | 2.3 | 7 | 2.4 | 4 | 1.4 |
| Hospitalisation and other | 4 | 1.4 | 2 | 0.7 | 4 | 1.3 | 2 | 0.7 |
| Hospitalisation, disability and other | 1 | 0.4 | 1 | 0.3 | 2 | 0.7 | 0 | 0.0 |
| Life-threatening | 1 | 0.4 | 1 | 0.3 | 1 | 0.3 | 1 | 0.3 |
| Life-threatening and hospitalisation | 7 | 2.5 | 15 | 4.9 | 15 | 5.1 | 7 | 2.4 |
| New primary cancer | 1 | 0.4 | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 |
| Other | 6 | 2.2 | 3 | 1.0 | 4 | 1.3 | 5 | 1.7 |
| Total | 279 | 100.0 | 304 | 100.0 | 297 | 100.0 | 286 | 100.0 |
By far the most common reason for reporting an SAE was hospitalisation, which alone accounted for 80% of all SAEs.
Serious adverse event symptoms by serious adverse event categorisation by randomisation arm
Multiple symptoms were associated with each SAE in a total of 1064 reported symptoms. Tables 53 and 54 split these symptoms by type of SAE and randomisation arm.
| Category as defined in Common Terminology Criteria for Adverse Events, version 354 | Docetaxel (N = 87) | Docetaxel + ZA (N = 154) | Docetaxel + Sr-89 (N = 103) | Docetaxel + ZA + Sr-89 (N = 120) | Overall (N = 464) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Pulmonary/upper respiratory | 8 | 9.2 | 3 | 1.9 | 5 | 4.9 | 10 | 8.3 | 26 | 5.6 |
| Pain | 18 | 20.7 | 20 | 13.0 | 19 | 18.6 | 15 | 12.5 | 72 | 15.6 |
| Haemorrhage/bleeding | 1 | 1.1 | 7 | 4.5 | 3 | 2.9 | 3 | 2.5 | 14 | 3.0 |
| Ocular/visual | 0 | 0.0 | 4 | 2.6 | 1 | 1.0 | 0 | 0.0 | 5 | 1.1 |
| Constitutional symptoms | 10 | 11.5 | 10 | 6.5 | 7 | 6.9 | 6 | 5.0 | 33 | 7.1 |
| Musculoskeletal/soft tissue | 8 | 9.2 | 14 | 9.1 | 14 | 13.7 | 10 | 8.3 | 46 | 9.9 |
| Neurology | 10 | 11.5 | 13 | 8.4 | 8 | 7.8 | 9 | 7.5 | 40 | 8.6 |
| Lymphatics | 1 | 1.1 | 3 | 1.9 | 2 | 2.0 | 3 | 2.5 | 9 | 1.9 |
| Auditory/ear | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| Renal/genitourinary | 3 | 3.4 | 23 | 14.9 | 11 | 10.8 | 8 | 6.7 | 45 | 9.7 |
| Dermatology/skin | 0 | 0 | 1 | 0.6 | 0 | 0.0 | 1 | 0.8 | 2 | 0.4 |
| Vascular | 2 | 2.3 | 2 | 1.3 | 2 | 2.0 | 9 | 7.5 | 15 | 3.2 |
| Surgery/intraoperative injury | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| Infection | 3 | 3.4 | 8 | 5.2 | 2 | 2.0 | 10 | 8.3 | 23 | 5.0 |
| Metabolic/laboratory | 3 | 3.4 | 4 | 2.6 | 4 | 3.9 | 3 | 2.5 | 14 | 3.0 |
| Blood/bone marrow | 5 | 5.7 | 8 | 5.2 | 1 | 1.0 | 7 | 5.8 | 21 | 4.5 |
| Cardiac general | 2 | 2.3 | 4 | 2.6 | 3 | 2.9 | 2 | 1.7 | 11 | 2.4 |
| Death | 2 | 2.3 | 9 | 5.8 | 7 | 6.9 | 5 | 4.2 | 23 | 5.0 |
| Cardiac arrhythmia | 1 | 1.1 | 0 | 0.0 | 2 | 2.0 | 3 | 2.5 | 6 | 1.3 |
| Syndromes | 0 | 0.0 | 1 | 0.6 | 1 | 1.0 | 0 | 0.0 | 2 | 0.4 |
| Gastrointestinal | 9 | 10.3 | 18 | 11.7 | 10 | 9.8 | 16 | 13.3 | 53 | 11.4 |
| Secondary malignancy | 1 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| Missing | 0 | – | 0 | – | 1 | – | 0 | – | 1 | – |
| Total | 87 | 100 | 154 | 100 | 102 | 100 | 120 | 100 | 463 | 100 |
| Category as defined in Common Terminology Criteria for Adverse Events, version 354 | Docetaxel (N = 142) | Docetaxel + ZA (N = 133) | Docetaxel + Sr-89 (N = 142) | Docetaxel + ZA + Sr-89 (N = 131) | Overall (N = 548) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Pulmonary/upper respiratory | 11 | 8.1 | 11 | 8.4 | 4 | 2.8 | 6 | 4.6 | 32 | 5.9 |
| Pain | 4 | 2.9 | 4 | 3.1 | 8 | 5.7 | 7 | 5.4 | 23 | 4.3 |
| Haemorrhage/bleeding | 2 | 1.5 | 2 | 1.5 | 6 | 4.3 | 2 | 1.5 | 12 | 2.2 |
| Ocular/visual | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| Constitutional symptoms | 23 | 16.9 | 12 | 9.2 | 19 | 13.5 | 12 | 9.2 | 66 | 12.3 |
| Allergy/immunology | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 0 | 0.0 | 1 | 0.2 |
| Musculoskeletal/soft tissue | 2 | 1.5 | 0 | 0.0 | 1 | 0.7 | 3 | 2.3 | 6 | 1.1 |
| Neurology | 6 | 4.4 | 10 | 7.6 | 4 | 2.8 | 3 | 2.3 | 23 | 4.3 |
| Lymphatics | 2 | 1.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.4 |
| Renal/genitourinary | 2 | 1.5 | 8 | 6.1 | 2 | 1.4 | 4 | 3.1 | 16 | 3.0 |
| Dermatology/skin | 2 | 1.5 | 0 | 0.0 | 1 | 0.7 | 0 | 0.0 | 3 | 0.6 |
| Vascular | 2 | 1.5 | 4 | 3.1 | 2 | 1.4 | 2 | 1.5 | 10 | 1.9 |
| Infection | 27 | 19.9 | 29 | 22.1 | 35 | 24.8 | 23 | 17.7 | 114 | 21.2 |
| Metabolic/laboratory | 3 | 2.2 | 8 | 6.1 | 1 | 0.7 | 11 | 8.5 | 23 | 4.3 |
| Blood/bone marrow | 18 | 13.2 | 20 | 15.3 | 26 | 18.4 | 23 | 17.7 | 87 | 16.2 |
| Cardiac general | 1 | 0.7 | 1 | 0.8 | 2 | 1.4 | 2 | 1.5 | 6 | 1.1 |
| Death | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 1 | 0.8 | 2 | 0.4 |
| Cardiac arrhythmia | 3 | 2.2 | 2 | 1.5 | 3 | 2.1 | 2 | 1.5 | 10 | 1.9 |
| Gastrointestinal | 28 | 20.6 | 19 | 14.5 | 25 | 17.7 | 29 | 22.3 | 101 | 18.8 |
| Missing | 6 | – | 2 | – | 1 | – | 1 | – | 10 | – |
| Total | 136 | 100 | 131 | 100 | 141 | 100 | 130 | 100 | 538 | 100 |
Tables 55 and 56 split the symptoms associated with SAE by category of SAE and comparison groups.
| Category as defined in Common Terminology Criteria for Adverse Events, version 354 | Docetaxel (N = 2) | Docetaxel + ZA (N = 1) | Docetaxel + Sr-89 (N = 6) | Docetaxel + ZA + Sr-89 (N = 1) | Overall (N = 10) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Ocular/visual | 0 | 0.0 | 0 | 0.0 | 1 | 16.7 | 0 | 0.0 | 1 | 10.0 |
| Coagulation | 0 | 0.0 | 0 | 0.0 | 1 | 16.7 | 0 | 0.0 | 1 | 10.0 |
| Neurology | 0 | 0.0 | 0 | 0.0 | 1 | 16.7 | 0 | 0.0 | 1 | 10.0 |
| Dermatology/skin | 0 | 0.0 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 1 | 10.0 |
| Vascular | 0 | 0.0 | 0 | 0.0 | 1 | 16.7 | 0 | 0.0 | 1 | 10.0 |
| Infection | 1 | 50.0 | 0 | 0.0 | 1 | 16.7 | 0 | 0.0 | 2 | 20.0 |
| Cardiac arrhythmia | 0 | 0.0 | 0 | 0.0 | 1 | 16.7 | 1 | 100.0 | 2 | 20.0 |
| Gastrointestinal | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 10.0 |
| Total | 2 | 100 | 1 | 100 | 6 | 100 | 1 | 100 | 10 | 100 |
| Category as defined in Common Terminology Criteria for Adverse Events, version 354 | Docetaxel (N = 7) | Docetaxel + ZA (N = 8) | Docetaxel + Sr-89 (N = 6) | Docetaxel + ZA + Sr-89 (N = 21) | Overall (N = 42) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Pulmonary/upper respiratory | 3 | 42.9 | 2 | 25.0 | 1 | 16.7 | 2 | 10.0 | 8 | 19.5 |
| Pain | 0 | 0.0 | 1 | 12.5 | 0 | 0.0 | 1 | 5.0 | 2 | 4.9 |
| Haemorrhage/bleeding | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 1 | 5.0 | 2 | 4.9 |
| Constitutional symptoms | 0 | 0.0 | 1 | 12.5 | 0 | 0.0 | 0 | 0.0 | 1 | 2.4 |
| Neurology | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 5.0 | 1 | 2.4 |
| Hepatobiliary/pancreas | 0 | 0.0 | 0 | 0.0 | 1 | 16.7 | 0 | 0.0 | 1 | 2.4 |
| Renal/genitourinary | 0 | 0.0 | 1 | 12.5 | 1 | 16.7 | 2 | 10.0 | 4 | 9.8 |
| Vascular | 1 | 14.3 | 0 | 0.0 | 1 | 16.7 | 1 | 5.0 | 3 | 7.3 |
| Infection | 1 | 14.3 | 3 | 37.5 | 1 | 16.7 | 4 | 20.0 | 9 | 22.0 |
| Blood/bone marrow | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 15.0 | 3 | 7.3 |
| Cardiac general | 1 | 14.3 | 0 | 0.0 | 0 | 0 | 3 | 15.0 | 4 | 9.8 |
| Death | 0 | 0.0 | 0 | 0.0 | 1 | 16.7 | 0 | 0.0 | 1 | 2.4 |
| Gastrointestinal | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 10.0 | 2 | 4.9 |
| Missing | 0 | – | 0 | – | 0 | – | 1 | – | 1 | – |
| Total | 7 | 100 | 8 | 100 | 6 | 100 | 20 | 100 | 41 | 100 |
Chapter 4 Economic evaluation
Parts of this chapter are based on the study by Andronis et al. 55
There is considerable uncertainty whether or not the addition of ZA and/or Sr-89 to standard chemotherapy for advanced CRPC patients would represent a cost-effective use of resources. In particular, it is unclear whether or not the extra cost of treatments seeking to alleviate bone-related problems would be balanced out by reduced use of resources – for example, because of a possible reduction in SREs – and improved outcomes. As such, an economic analysis was undertaken on the basis of patient-level data collected alongside the factorial TRAPEZE trial, to assess the cost-effectiveness of two relevant comparisons:
-
ZA versus no ZA
-
Sr-89 versus no Sr-89.
The results of these analyses are expressed in terms of additional cost per QALY gained. The comparison was conducted on the basis of findings suggesting no significant interactions between ZA and Sr-89 in terms of costs (p-value = 0.12) or QALYs (p-value = 0.2). The economic evaluation was based on 707 patients (93% of the total number of 757 patients) for whom the calculation of QALYs was possible, as detailed previously. Two separate analyses are reported for the comparison between ZA and no ZA; the first analysis considers ZA as a branded pharmaceutical, while the second analysis reflects the availability of ZA as a generic product. The ZA patent in the EU expired in May 2013 after completion of the trial.
As this exploration aims to inform clinical practice and resource allocation decisions within the NHS, the methods followed are in agreement with recent recommendations for conducting health technology appraisals. 46 On this basis, the analyses were undertaken from the perspective of the NHS and Personal Social Services, while costs and outcomes occurring in the future were discounted at a rate of 3.5%. Monetary values throughout the study are expressed in UK pounds sterling in 2011–12 prices, when ZA was available only as a proprietary medication. We have recalculated the impact of generic pricing available since May 2013 as a further analysis.
Methods
This section describes the methods followed in estimating the mean per-patient costs and QALYs and the ICERs for the compared options.
Resource use and cost
NHS resource use was captured through CRFs and patient-completed questionnaires and falls into three overarching categories:
-
trial treatment
-
use of related concomitant treatments
-
use of hospital and primary care services.
Trial treatments comprised combinations of docetaxel and prednisolone with (1) ZA (docetaxel, prednisolone and ZA), (2) Sr-89 (docetaxel, prednisolone and Sr-89) or (3) ZA and Sr-89 (docetaxel, prednisolone, ZA and Sr-89). Data on trial treatments provided were obtained from CRFs completed by research nurses. Information on use of treatments or care provided concomitantly with the trial treatments was also obtained from CRFs. Data on duration of inpatient stay and number of outpatient visits were obtained from CRFs for care that were completed during the treatment period, and from patient questionnaires for services provided after the treatment period.
Trial treatment acquisition and administration
The acquisition cost of trial treatments (docetaxel, prednisolone, ZA and Sr-89) was calculated by multiplying patient-specific doses and numbers of cycles received by published unit cost estimates obtained from the British National Formulary (BNF). 56 An alternative unit cost estimate was used to reflect the lower acquisition price for ZA owing to the drug being available as a generic product (Table 57). With regards to docetaxel, patients received different doses according to their body surface area, which resulted in the use of vials of different volumes. For example, a patient who was administered a dose of docetaxel between 80 mg and 100 mg would require one 4-ml vial and one 1-ml vial at a total cost of £663, whereas a patient administered a dose between 100 mg and 140 mg would require one 7-ml vial at a cost of £720 (see Table 57). Prednisolone at a dose of 10 mg per day was given orally together with docetaxel. The daily cost of prednisolone was calculated at £0.64 (approximately £13.50 per 21-day treatment cycle). ZA was provided in doses of up to 4 mg per cycle at a cost of £174 for the branded product or £58 for the generic alternative in additional analyses. Finally, Sr-89 was given as a single fraction of 150 MBq. As the cost of Sr-89 is not available from the BNF, a value was obtained from the Nuclear Medicine Department of the University Hospital Birmingham, Birmingham, UK. This value was varied in sensitivity analyses.
| Drugs | Dose | Constituent parts | Cost (£) | Source |
|---|---|---|---|---|
| Docetaxel | 1–20 mg | One 1-ml (20-mg) vial | 155 | BNF54 |
| 21–40 mg | Two 1-ml (40-mg) vials | 309 | BNF54 | |
| 41–80 mg | One 4-ml (80-mg) vial | 508 | BNF54 | |
| 81–100 mg | One 4-ml (80-mg) vial and one 1-ml (20-mg) vial | 663 | BNF54 | |
| 101–140 mg | One 7-ml (140-mg) vial | 720 | BNF54 | |
| 141–160 mg | One 16-ml (160-mg) viala | 1070 | BNF54 | |
| 161–180 mg | One 16-ml (160-mg) viala and one 1-ml (20-mg) vial | 1224 | BNF54 | |
| Prednisolone | 10 mg daily | 30-tablet pack of 5 mg | 0.64 a day | BNF54 |
| ZA (branded product) | Range of doses up to 3.5 mg | One 5-ml (4-mg) vial | 174 | BNF54 |
| ZA (generic alternative) | One 4 mg/100 ml infusion bottle | 58 | Department of Health’s electronic market information tool | |
| Sr-89 | 150 MBq | N/A | 1710 | Nuclear Medicine Department, University Hospital Birmingham, Birmingham, UK |
The cost of a chemotherapy administration was taken from the National Schedule of Reference Costs 2011–12,15 with the exception of Sr-89 administrations, the cost of which was obtained from the Nuclear Medicine Department of the University Hospital Birmingham, Birmingham, UK (Table 58). No administration cost was incurred for prednisolone, as the drug is taken orally. When ZA was administered together with docetaxel, no additional cost on top of the administration cost of docetaxel was incurred. However, when ZA was administered as a stand-alone follow-up treatment, it incurred the cost of an outpatient appointment for chemotherapy administration.
Concomitant treatments
Castration-refractory prostate cancer patients received further care or medications provided concomitantly with their trial treatment. Examples include radiotherapy, abiraterone, cabazitaxel, mitoxantrone, blood transfusions, as well as docetaxel, Sr-89 and ZA. The cost of each of these treatments was obtained by multiplying their use, obtained from CRFs, by unit cost estimates available from various sources (Table 59). The cost of docetaxel, ZA and Sr-89 given as concomitant medications is the same as in Table 57. As information on specific doses for concomitant medications was not available from CRFs, the cost of such medications was calculated on the basis of their recommended dosage as detailed in the BNF. 56
| Drugs | Unit cost | Source |
|---|---|---|
| Radiotherapy | £813 cost of radiotherapy preparation plus £118 cost of radiotherapy fraction | National Schedule of Reference Costs 2011–12 15 |
| Abiraterone | £98 per day | BNF54 |
| Cabazitaxel | £3696 per cycle | BNF54 |
| Mitoxantrone | £100 per cycle | BNF54 |
| Blood | £123 per unit plus intravenous cannula (£1) and blood-giving set (£4) | NHS Blood and Transplant57 |
The cost of administration of concomitant medications given intravenously was obtained from the National Schedule of Reference Costs 2011–1215 and is given in Table 60. No administration cost was accounted for abiraterone, which is provided as an oral treatment. The administration cost for docetaxel, Sr-89 and ZA given as second-line treatments is the same as the cost of administration shown in Table 58.
| Administration | Unit cost (£) | Source |
|---|---|---|
| Cabazitaxel | 144 | National Schedule of Reference Costs 2011–12 15 |
| Mitoxantrone | 245 | National Schedule of Reference Costs 2011–12 15 |
| Blood transfusion | 172 | National Schedule of Reference Costs 2011–12 15 |
Patients who experienced SREs, such as SCC and fractures, would typically receive surgery and/or radiotherapy. Each of the undertaken surgical procedures was matched with a Healthcare Resources Group code and was assigned a cost using the NHS reference cost schedules15 (Table 61). It was assumed that these services were provided on a non-elective basis. Typically, a course of radiotherapy would be on consecutive days and would include a single planning session. Where the gap between fractions was more than 2 weeks, it was assumed that this represented treatment to a different site and a further planning session was added to the model for this separate course of treatment. On this basis, the cost of a radiotherapy session consisted of the cost of the planning visit and the cost of the radiotherapy fraction itself.
| Intervention | Unit cost (£) | Source |
|---|---|---|
| Decompression for SCC | 9573 | National Schedule of Reference Costs 2011–12 15 |
| Laminectomy | 6893 | National Schedule of Reference Costs 2011–12 15 |
| Intramedullary nailing | 4995 | National Schedule of Reference Costs 2011–12 15 |
| Hip replacement | 8038 | National Schedule of Reference Costs 2011–12 15 |
| Pathological fractures with complications | 3888 | National Schedule of Reference Costs 2011–12 15 |
| SCC | 7816 | National Schedule of Reference Costs 2011–12 15 |
As NHS reference cost estimates for surgeries include the cost of an average length of inpatient stay, to avoid double-counting of inpatient stay, the average length of stay specific to each surgery was deducted from the total length of inpatient stay recorded for the patient. The cost of inpatient stay was calculated separately, as described in the next section.
Use of hospital and primary care services
Outpatient appointments, inpatient stays and GP visits which took place during the trial treatment period were obtained from CRFs, while post-treatment stays and visits were obtained from patient-completed questionnaires.
The former were filled in by health-care professionals and were complete for 707 patients, 100% of the available sample for the economic evaluation, whereas the latter presented missing data for 126 patients (approximately 18% of the sample). Multiple imputation by chained equations was used to impute the missing observations. 58 The imputation model made use of predictive mean matching to ensure that the imputed values were consistent with the range of observed values in the trial. 59 Predictor variables used in the model included patient characteristics (age, survival after randomisation, treatment arm allocation), resource use (number of cycles of trial treatment received, use of concomitant medications, number of radiotherapy sessions received, surgeries), as well observed (non-missing) data on outpatient appointments, inpatient stay and GP visits. Unit costs for outpatient appointments, inpatient stay and GP visits were obtained from the NHS reference cost schedules and the Personal Social Services Research Unit’s Unit Cost of Health and Social Care 2012 report60 (Table 62).
Health-related quality of life and quality-adjusted life-years
A QALY score was derived for each patient on the basis of his responses to the three-level EQ-5D instrument, a generic preference-based measure commonly used in valuing HRQoL. Responses to the instrument’s health status classification system were translated into a single preference-based (utility) score using a UK-specific value set. 61 QALYs were calculated as the area under the curve connecting utility scores reported at different time points from baseline to death, at which point utility was assigned a value of zero. 62 Details on the analysis of QoL data and the calculation of QALYs are given in Chapter 3, Quality of life. For missing questionnaires, no multiple imputation was conducted. The reason for this was that observed responses at time t could not be used as predictors of missing responses at time t as they corresponded to different time points in patients’ treatment and follow-up trajectory.
Analysis
A total NHS cost and a total number of QALYs were calculated for each of the 707 patients for whom information on costs and preference-based QoL (EQ-5D) was available. The base-case analysis, in which the unit cost of ZA was obtained from the BNF, was supplemented by an additional analysis reflecting the availability of generic ZA at a considerably lower price than the proprietary product. The mean total cost and mean total QALYs across all patients under a given treatment were then calculated. As the distribution of costs and QALYs are typically skewed, 95% CIs around mean values were obtained on the basis of 1000 replications using the bias-corrected and accelerated (BCa) bootstrap method.
Incremental analysis was carried out to determine the difference in mean total costs and QALYs between the compared options. These differences were summarised in the form of an ICER, a measure that reflects the extra cost associated with a gain of one additional QALY. 43 To account for the inherent uncertainty in the results because of sampling variation, non-parametric bootstrapping was used to replicate the joint distribution of the differences in cost and QALYs. 63 This generated 5000 paired estimates of incremental costs and QALYs, which were subsequently represented graphically on a cost-effectiveness plane and plotted on CEACs. 47,48 Cost-effectiveness planes show the bootstrap estimates on a four-quadrant plane. Depending on the quadrant in which cost-effectiveness results are located, a treatment may be more effective and more costly (north-east quadrant), more effective and less costly (south-east quadrant), less effective and less costly (south-west quadrant) or less effective and more costly (north-west quadrant) than an alternative treatment. CEACs show the probability of each option being cost-effective across a range of possible values of willingness to pay for an additional QALY. Data management tasks were undertaken in Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) and statistical analyses, including multiple imputation for missing values, were carried out in Stata version 12.
The impact of different assumptions employed in the cost-effectiveness analysis was assessed in a series of sensitivity analyses. In line with recommendations, and to avoid biased estimates of QALY scores, the analysis controlled for any possible between-group imbalance in baseline EQ-5D QoL scores, using multivariate ordinary least squares regression. 64 Additional sensitivity analyses explored the impact of different plausible values of uncertain parameters in the results, including alternative unit costs for Sr-89, docetaxel and mitoxantrone. As per NICE recommendations, discount rates were varied to no discounting and discounting at 6% for both costs and benefits. 46
Results of comparison between zoledronic acid and no zoledronic acid
The following section reports the results of the comparison between the ZA and no ZA options in terms of resource use and costs, QALYs and ICERs. Two analyses are reported; the first is based on ZA being a branded product, while the second takes into account the availability of ZA as a generic alternative.
Resource use and cost
The number and proportion of patients that received a trial treatment, concomitant treatment and inpatient, outpatient and primary care services are given in Chapter 3 and Appendix 6. The mean cost for main resource use items are given in Table 63.
| ZA (n = 350) | No ZA (n = 357) | Difference (ZA vs. no ZA) | |||||
|---|---|---|---|---|---|---|---|
| Mean (£) | SD (£) | Mean (£) | SD (£) | Cost difference (£) | Lower CI (£) | Upper CI (£) | |
| Trial treatments | |||||||
| Docetaxel + prednisolone | 2502 | 760 | 2441 | 749 | 60 | –49 | 169 |
| ZA | 1044 | 456 | 0 | 0 | 1044 | 1091 | 996 |
| Sr-89 | 769 | 1033 | 724 | 1018 | 45 | –107 | 197 |
| ZA as follow-up treatment | 1157 | 1886 | 4 | 67 | 1153 | 955 | 1351 |
| Concomitant medications and treatments | |||||||
| Radiotherapy | 764 | 1093 | 1021 | 1264 | –257 | –429 | –85 |
| Abiraterone | 1811 | 4198 | 2150 | 4478 | –339 | –993 | 316 |
| ZA as concomitant medication | 326 | 1109 | 141 | 681 | 185 | 45 | 324 |
| Sr-89 as concomitant medication | 98 | 476 | 132 | 539 | –34 | –109 | 41 |
| Blood units | 23 | 150 | 19 | 125 | 4 | –16 | 24 |
| Cabazitaxel | 301 | 1710 | 293 | 2230 | 8 | –288 | 304 |
| Docetaxel as concomitant medication | 372 | 1543 | 433 | 2049 | –61 | –338 | 216 |
| Mitoxantrone | 51 | 245 | 26 | 179 | 25 | –6 | 56 |
| Surgery | 116 | 988 | 377 | 1974 | –261 | –495 | –27 |
| Outpatient appointments and inpatient stay | |||||||
| Hospital outpatient appointment | 672 | 1015 | 591 | 804 | 81 | –51 | 213 |
| Hospital inpatient stay | 3494 | 6216 | 3786 | 6562 | –292 | –1217 | 632 |
| GP appointments | 278 | 319 | 319 | 384 | –42 | –95 | 12 |
As expected, the most substantial difference in costs between the ZA and no ZA groups was as a result of the use of ZA (as protocol and follow-up treatment) in the ZA arm. The mean difference in cost between groups associated with the use of ZA was £2197 (BCa 95% CIs £1971 to £2422). With the exception of ZA, patients in the ZA arm presented lower resource use and costs compared with those in the no ZA arm. In particular, there were significant differences in the use of radiotherapies and surgeries. As such care is provided in response to SREs, the lower use and cost of radiotherapy and surgeries is representative of the fact that people in the ZA arm experienced significantly fewer skeletal-related problems.
Patients in the ZA arm also showed lower, although not significantly different, costs because of abiraterone, Sr-89 and docetaxel as concomitant medication and lower costs as a result of fewer hospital stays and GP appointments. On the other hand, ZA was associated with higher, although non-significantly different, costs for docetaxel and prednisolone – because of patients in ZA receiving on average more cycles of docetaxel, prednisolone – Sr-89 as trial treatment, blood units, cabazitaxel, mitoxantrone and hospital outpatient appointments.
The estimated mean total cost per patient was £13,776 (BCa 95% CIs £12,824 to £14,728) for ZA and £12,457 (BCa 95% CIs £11,465 to £13,449) for no ZA. This resulted in a mean cost difference of £1319 (BCa 95% CIs –£34 to £2671) (Table 64).
| Treatment | Mean (£) | SD (£) | BCa 95% CIs (£) | Difference in cost (ZA vs. no ZA) (£) | BCa 95% CIs of difference (£) | ||
|---|---|---|---|---|---|---|---|
| Lower CI | Upper CI | Lower CI | Upper CI | ||||
| ZA | 13,776 | 9118 | 12,824 | 14,728 | 1319 | –34 | 2671 |
| No ZA | 12,457 | 9453 | 11,465 | 13,449 | |||
The distribution of the total cost can be seen in the box plot in Figure 39 and histogram in Figure 40.
FIGURE 39.
Box plot summarising the distribution of total per-patient cost for ZA and no ZA.

FIGURE 40.
Histogram depicting the distribution of the mean total cost for the ZA and no ZA arms. (a) no ZA; and (b) ZA.
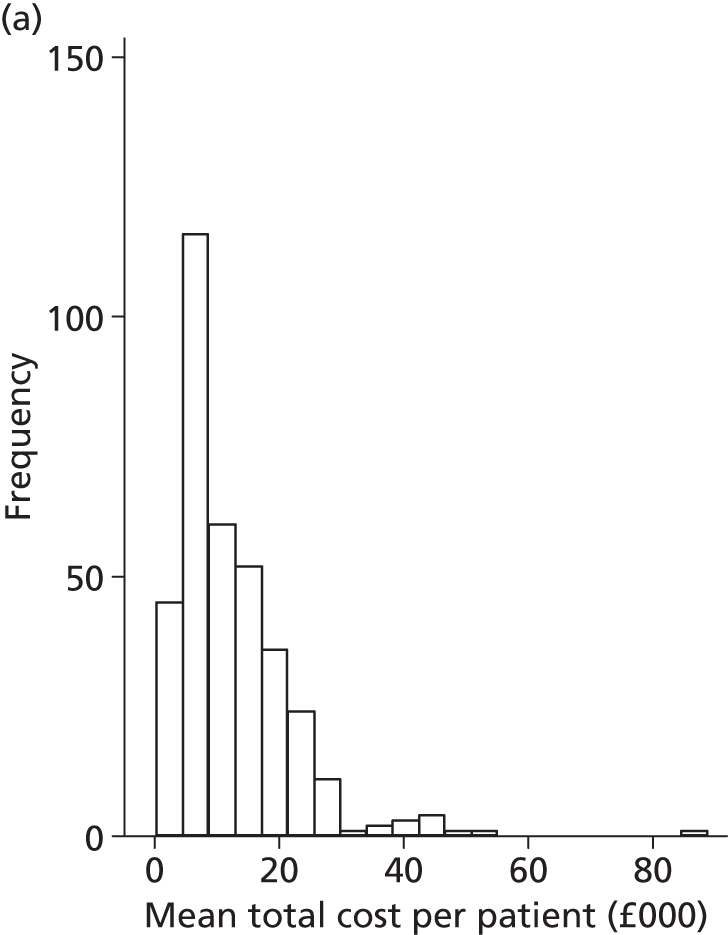
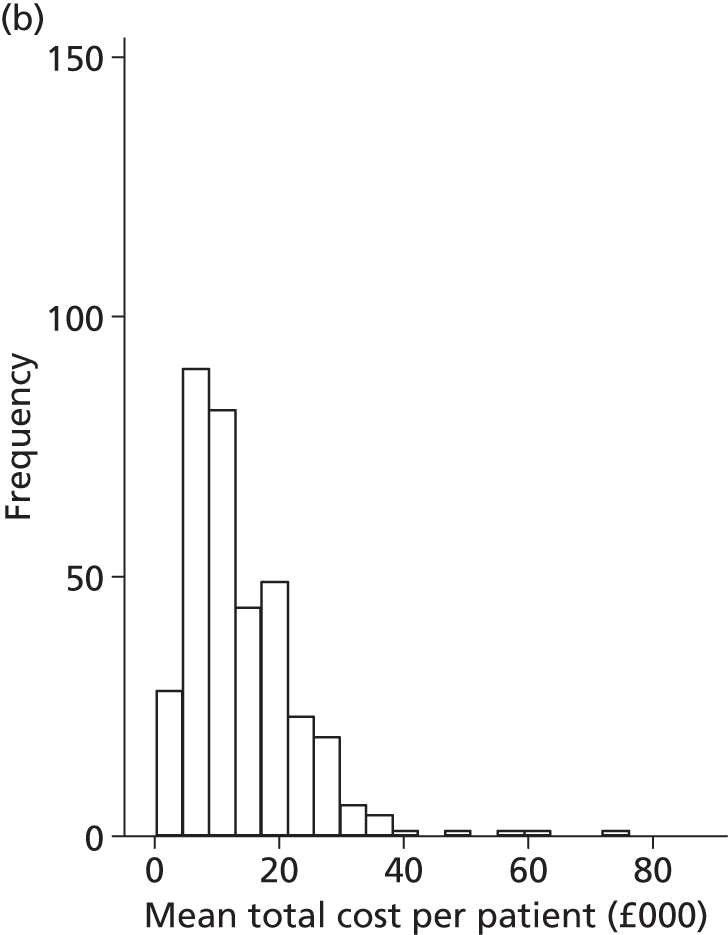
Quality-adjusted life-years
Figure 41 depicts the mean EQ-5D scores across treatment groups at three points in time (date of randomisation, 6 and 12 months after randomisation). Mean EQ-5D scores between randomisation and 6 months after randomisation increased in the no ZA group and decreased in the ZA group. This trend was reversed in the period between 6 and 12 months after randomisation, when the mean QoL score for patients in the ZA group increased, while the equivalent value for the no ZA group decreased. It must be noted that caution is needed in comparing EQ-5D scores across time points, as patients returning a questionnaire at the same point in time after randomisations may be at different stages along their treatment and follow-up trajectories (i.e., at 6 months, some patients were still on treatment while others had completed or discontinued their treatment).
FIGURE 41.
Box plot showing the distribution of EQ-5D scores across different time points, for the ZA and no ZA groups.

Mean numbers of QALYs gained for each treatment are given in Table 65. In total, patients in the ZA group gained an average of 0.91 QALYs, suggesting an improvement of 0.03 QALYs over their counterparts in the no ZA group. The distribution of the discounted QALYs is illustrated in Figure 42.
| ZA (n = 350) | No ZA (n = 357) | Difference | BCa 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Lower CI | Upper CI | ||
| QALYs gained (undiscounted) | 0.915 | 0.697 | 0.884 | 0.712 | 0.031 | –0.073 | 0.134 |
| QALYs gained (discounted) | 0.908 | 0.683 | 0.876 | 0.693 | 0.031 | –0.07 | 0.133 |
FIGURE 42.
Box plot summarising the distribution of discounted QALYs gained for ZA and no ZA.

Cost-effectiveness results
Summary cost and effectiveness results, expressed in terms of a point estimate ICER, are shown in Table 66. ZA appeared to be more costly than no ZA, resulting in an estimated incremental cost of £1319. This difference is mainly driven by the additional cost of ZA in the ZA group. In terms of QALYs gained, ZA appeared to be slightly more effective than no ZA, resulting in a gain of 0.03 QALYs. Given the above, the point estimate ICER for ZA compared with no ZA is £42,047 per additional QALY. This value is considerably greater that the ratio of £20,000 to £30,000 per QALY which is perceived to represent a cost-effective use of resources by NICE. 46
| Treatment | Total cost (£) | Total QALYs | Difference in cost (ZA vs. no ZA) (£) | Difference in QALYs (ZA vs. no ZA) | ICER (£) |
|---|---|---|---|---|---|
| ZA | £13,776 | 0.908 | 1319 | 0.031 | 42,047 |
| No ZA | £12,457 | 0.876 |
Figure 43 depicts the results of 5000 bootstrap replications plotted on the cost-effectiveness plane. Each point represents a pair of incremental cost and incremental effectiveness estimates for the comparison between ZA and no ZA. Approximately 71% of the simulated pairs are located in the north-east quadrant, indicating that ZA is likely to be more costly and more effective than no ZA. About 26% of the points appear in the north-west quadrant, which indicates that ZA is more costly and less effective than no ZA. There are also a small number of points located in the southern half of the plane, indicating that ZA may be less costly and more effective than no ZA (south-east quadrant; 1% of all points) or less costly and less effective than no ZA (south-west quadrant; 2% of all points).
FIGURE 43.
Cost-effectiveness plane showing incremental cost and effect (QALYs) pairs for the comparison between ZA and no ZA.
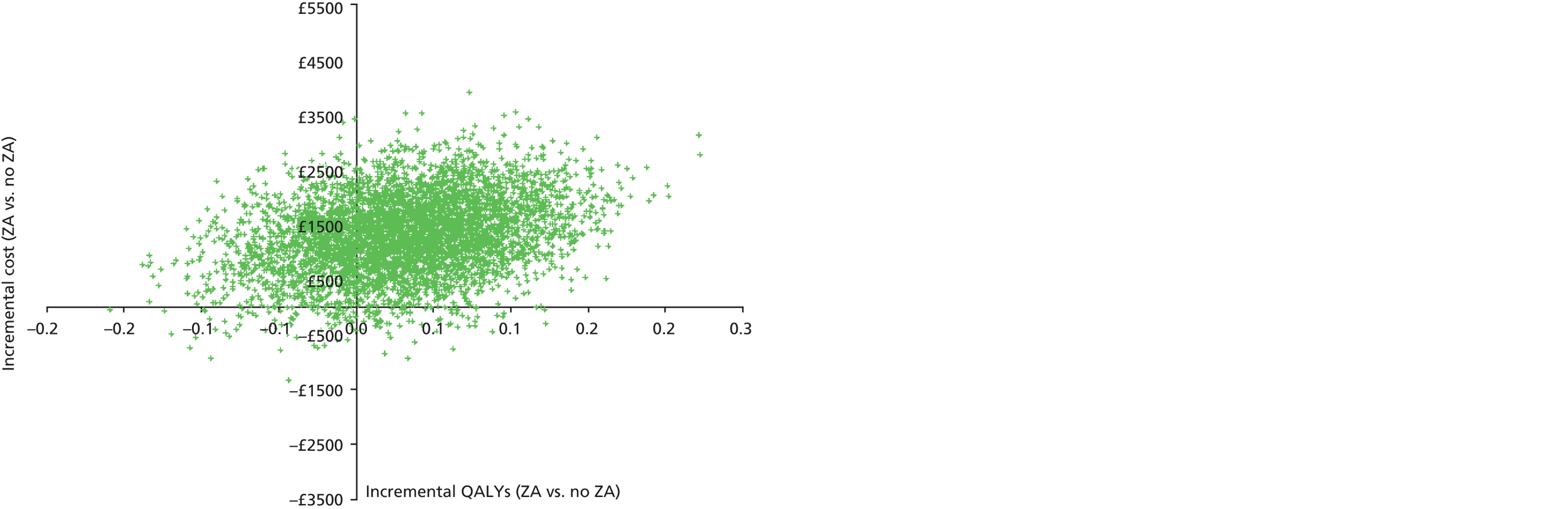
Figure 44 depicts the CEACs for ZA and no ZA, showing the probability of each of the option being cost-effective at different values of a decision-maker’s willingness to pay for a QALY (ceiling ratio). In a situation in which the decision-maker is not prepared to pay any amount for additional health benefits (i.e. the ceiling ratio is zero), the probabilities of ZA and no ZA being cost-effective are 3% and 97%, respectively. At values of the ceiling ratio between £20,000 and £30,000 per QALY, the probability of ZA being cost-effective rises from 26% to 40%; it exceeds 50% (i.e. appears to be the most cost-effective treatment) for ceiling ratios over £42,000 per QALY.
FIGURE 44.
Cost-effectiveness acceptability curves showing the probability of ZA and no ZA being cost-effective at different values of the ceiling ratio.
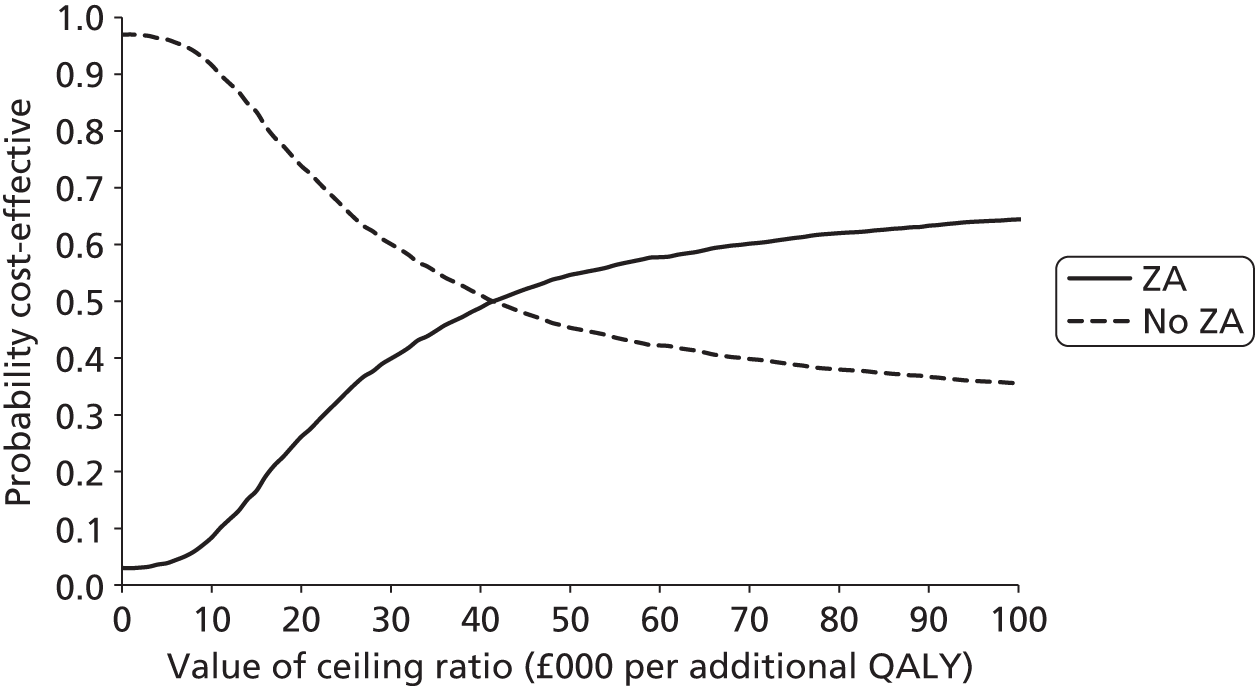
Additional analysis to account for the availability of generic zoledronic acid
Generic alternatives to ZA have now become available and are in use in NHS hospitals, at a significantly lower price than the proprietary alternatives (Zometa®, East Hanover, NJ, USA, or Aclasta®, Surrey, UK). Given this, additional analysis was undertaken to reflect the fact that NHS hospitals are likely to face a lower acquisition cost for ZA.
An estimate of the average price paid by NHS hospitals for ZA in 2013 (£58 per 4 mg/100 ml infusion bottle) was obtained from the electronic market information tool (eMIT) of the Department of Health Commercial Medicines Unit. 65
The use of this unit cost estimate resulted in a marked decrease in the cost of ZA when given as trial treatment, follow-up treatment and concomitant medication (Table 67). As expected, the cost associated with the use of ZA in the ZA group was still higher than the equivalent cost in the no ZA group; nonetheless, the difference between groups is now considerably smaller.
| Treatment | ZA (n = 350) | No ZA (n = 357) | Difference (ZA vs. no ZA) | ||||
|---|---|---|---|---|---|---|---|
| Mean (£) | SD (£) | Mean (£) | SD (£) | Cost difference (£) | Lower 95% CI (£) | Upper 95% CI (£) | |
| ZA | 346 | 151 | 0 | 0 | 346 | 330 | 361 |
| ZA as follow-up treatment | 837 | 1358 | 3 | 48 | 834 | 692 | 977 |
| ZA as concomitant medication | 235 | 801 | 101 | 492 | 134 | 36 | 230 |
As the cost of ZA itself is a main driver of the total cost of ZA, the use of lower prices for ZA led to a decrease in the total per-patient cost for ZA and, consequently, the difference in total costs between ZA and no ZA was reduced to £251 (BCa 95% CI £–1099 to £1602) (Table 68).
| Treatment | Mean (£) | SD (£) | BCa 95% CIs (£) | Difference in cost (ZA vs. no ZA) (£) | BCa 95% CIs of difference (£) | ||
|---|---|---|---|---|---|---|---|
| Lower CI | Upper CI | Lower CI | Upper CI | ||||
| ZA | 12,667 | 8795 | 11,724 | 13,612 | 251 | –1099 | 1602 |
| No ZA | 12,417 | 9433 | 11,436 | 13,397 | |||
The reduced incremental cost combined with the observed change in QALYs gave a point estimate ICER of £8005 per additional QALY (Table 69).
| Treatment | Total cost (£) | Total QALYs | Difference in cost (ZA vs. no ZA) (£) | Difference in QALYs (ZA vs. no ZA) | ICER (£) |
|---|---|---|---|---|---|
| ZA | 12,667 | 0.908 | 251 | 0.031 | 8005 |
| No ZA | 12,417 | 0.876 |
Uncertainty around incremental costs and QALYs, propagated through 5000 bootstrap replications, is plotted on the cost-effectiveness plane in Figure 45. Approximately half (51%) of the simulated pairs are located in the north-east quadrant, indicating that ZA is likely to be more costly and more effective than no ZA. About 14% of the pairs appear in the north-west quadrant (i.e. ZA is more costly and less effective than no ZA), while 21% of the pairs are located in the south-east quadrant (i.e. ZA is less costly and more effective than no ZA). The remaining 14% of the pairs are located in the south-west quadrant (i.e. ZA is less costly and less effective than no ZA).
FIGURE 45.
Cost-effectiveness plane showing incremental cost and effect (QALYs) pairs for the comparison between ZA and no ZA, based on the availability of generic ZA.
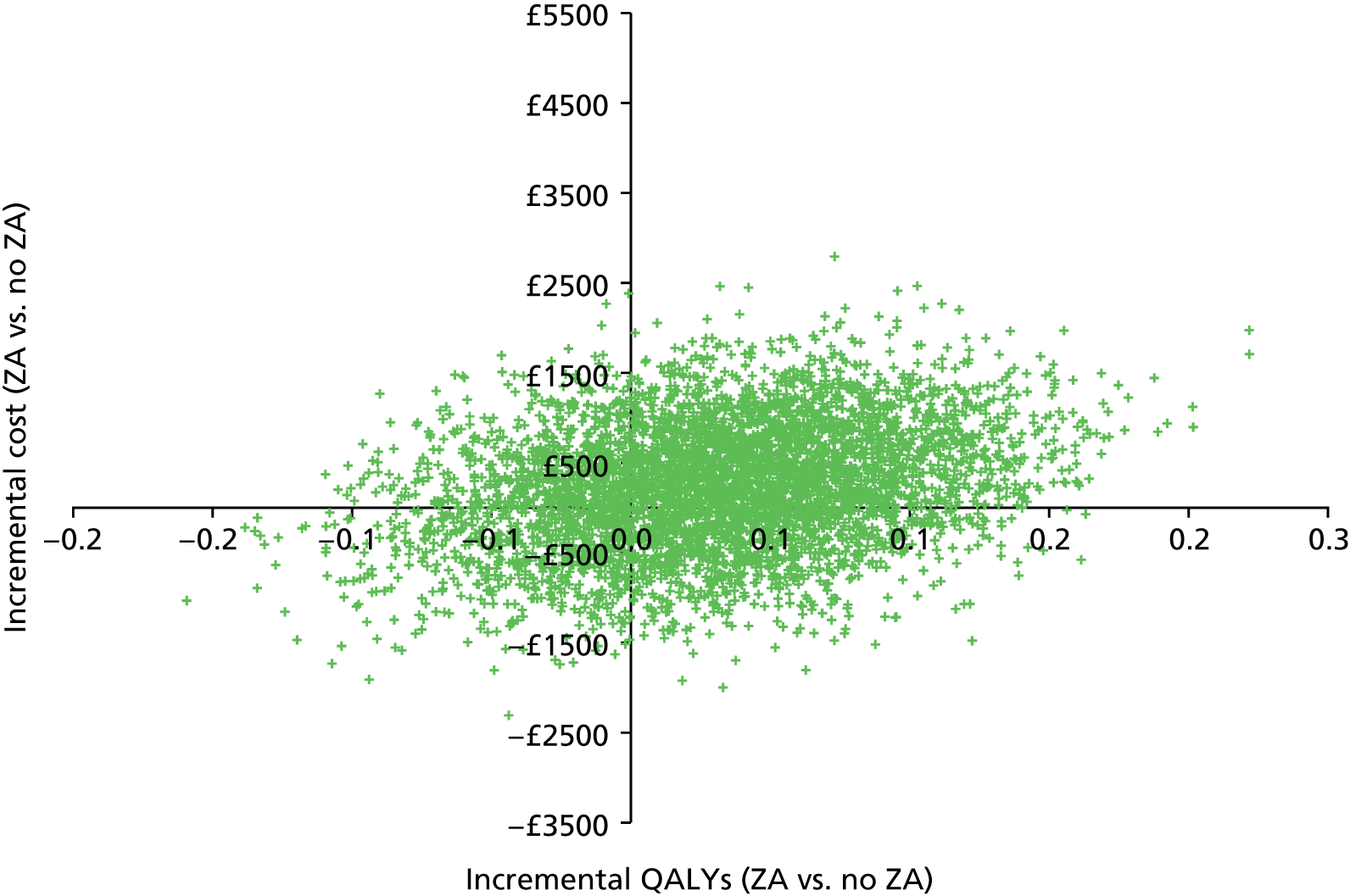
Cost-effectiveness acceptability curves showing the probability of ZA and no ZA being cost-effective at different ceiling ratios are depicted in Figure 46. In a situation in which the decision-maker is not prepared to pay any amount for additional health benefits (i.e. the ceiling ratio is zero), the probabilities of ZA and no ZA being cost-effective are 35% and 65%, respectively. ZA appears to be the most cost-effective option at ceiling ratios over £8000 per QALY, and at £20,000 and £30,000 per QALY the probability of ZA being cost-effective is 64% and 68%, respectively.
FIGURE 46.
Cost-effectiveness acceptability curves showing the probability of generic ZA and no ZA being cost-effective at different values of the ceiling ratio. Reproduced and amended with permission from Andronis et al. 55 In the source figure, only the ZA CEAC was plotted; a CEAC for No ZA has been added here.

Figure 47 depicts ICER values for ZA as a function of possible prices of ZA. For prices of ZA between £0 and £31, the total per-patient cost of ZA is lower than that of no ZA and, given the fact that ZA is associated with additional QALYs, this treatment option dominates its comparator. For prices between £31 and £98, ZA results in ICERs up to £20,000 per QALY, and it is thus cost-effective at this ceiling ratio. For prices of ZA higher than £98, the resulting ICER exceeds £20,000 per QALY.
FIGURE 47.
Incremental cost-effectiveness ratio for ZA vs. no ZA for different prices of ZA.
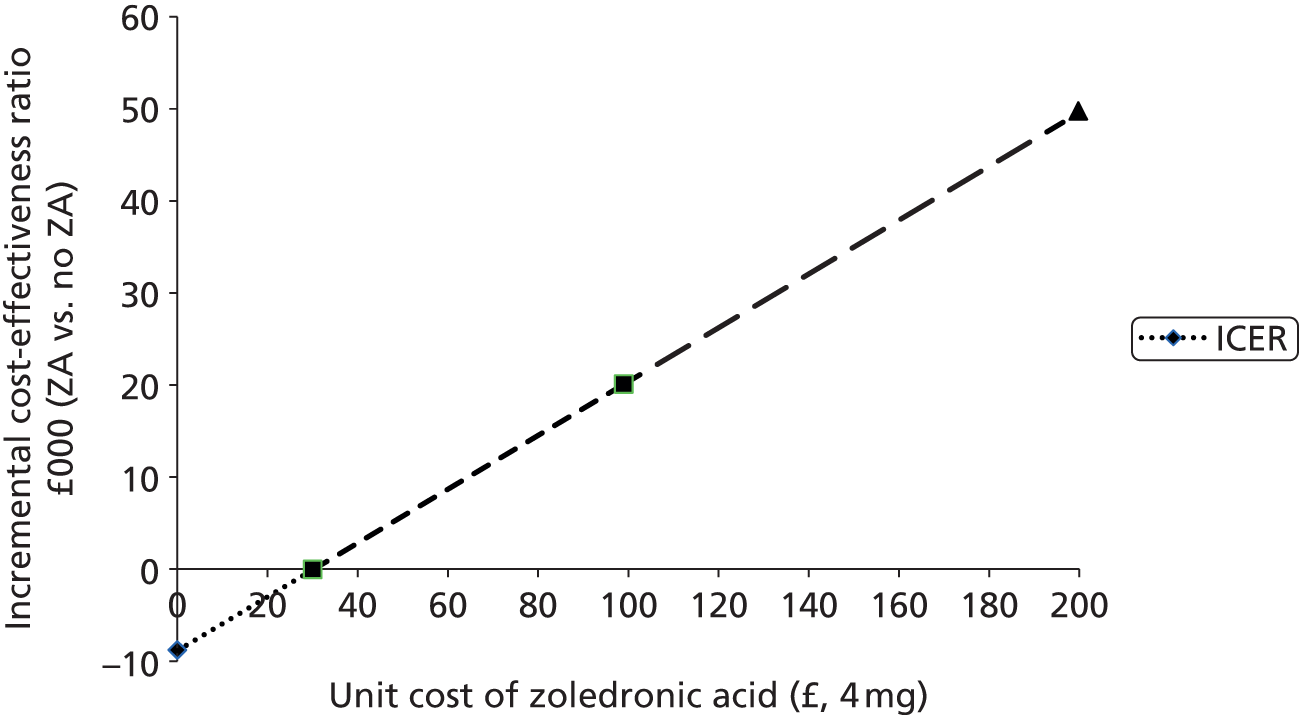
Sensitivity analysis
Most of the alternative assumptions explored in sensitivity analyses appeared to have a small effect on the magnitude of cost and benefits and, consequently, a limited impact on the resulting ICER. First, when future costs and benefits were not discounted, there was a small increase in both costs and QALYs, giving a slightly increased ICER value for ZA of £42,500 per additional QALY. On the other hand, discounting at a higher rate of 6% per annum led to a small decrease in the ICER, which, under this scenario, assumed a value of £41,851. Using alternative unit cost estimates for docetaxel and mitoxantrone from the NHS Commercial Medicines Unit65 had a small effect on the total mean cost per patient, with lower prices of docetaxel and mitoxantrone compared with the base-case values from BNF resulting in ICERs of £43,250 and £41,950, respectively. Sensitivity analyses around the price of Sr-89 had no significant effect on the differences in costs between the ZA and no ZA arms, and gave ICERs close to the £42,000 per QALY mark.
A different pattern was observed when adjusting QALYs for baseline imbalances in EQ-5D scores. Such an adjustment had a significant impact on the difference in QALYs between the compared groups, with no ZA appearing to be slightly more effective than ZA (difference of –0.001 QALYs, 95% CI –0.096 to 0.094). Under this scenario, the ZA group appears to be more expensive and less effective than no ZA, and thus it is extendedly dominated by the latter (Table 70).
| ZA | No ZA | ICER (£) | |||
|---|---|---|---|---|---|
| Mean cost (£) | Mean QALYs | Mean cost (£) | Mean QALYs | ||
| Base-case results | 13,776 | 0.908 | 12,457 | 0.876 | 42,047 |
| No discounting | 13,897 | 0.915 | 12,592 | 0.884 | 42,449 |
| Discounting at 0.06 per annum for both costs and benefits | 13,695 | 0.903 | 12,366 | 0.871 | 41,851 |
| QALYs adjusted for baseline imbalances in EQ-5D | 13,776 | 0.875 | 12,457 | 0.876 | ZA dominated |
| Unit cost of docetaxel from eMIT (£34.29 for 140 mg) | 12,618 | 0.908 | 11,261 | 0.876 | 43,251 |
| Unit cost of mitoxantrone from eMIT (£60.36 for 25 mg) | 13,770 | 0.908 | 12,454 | 0.876 | 41,956 |
| Unit cost of Sr-89 (75% of available estimate) | 13,757 | 0.908 | 12,431 | 0.876 | 42,261 |
| Unit cost of Sr-89 (125% of available estimate) | 13,795 | 0.908 | 12,483 | 0.876 | 41,832 |
Results of comparison between strontium-89 and no strontium-89
The following section reports the results of the comparison between Sr-89 and no Sr-89 in terms of resource use and costs, QALYs and ICERs.
Resource use and cost
The number and proportion of patients who received a trial treatment, concomitant treatment and inpatient, outpatient and primary care are given in Appendix 5. The mean costs for main resource use items are given in Table 71.
| Sr-89 (n = 350) | No Sr-89 (n = 357) | Difference (Sr-89 vs. no Sr-89) | |||||
|---|---|---|---|---|---|---|---|
| Mean (£) | SD (£) | Mean (£) | SD (£) | Cost difference (£) | Lower 95% CI (£) | Upper 95% CI (£) | |
| Trial treatments | |||||||
| Docetaxel + prednisolone | 2497 | 738 | 2445 | 771 | 52 | –61 | 165 |
| ZA | 525 | 613 | 508 | 613 | 18 | –71 | 107 |
| Sr-89 | 1507 | 988 | 0 | 0 | 1507 | 1407 | 1608 |
| ZA as follow-up treatment | 539 | 1337 | 609 | 1549 | –69 | –279 | 141 |
| Concomitant medications | |||||||
| Radiotherapy | 803 | 1033 | 983 | 1318 | –180 | –349 | –11 |
| Abiraterone | 1905 | 4279 | 2058 | 4408 | –153 | –814 | 508 |
| ZA as concomitant medication | 205 | 866 | 259 | 975 | –54 | –192 | 84 |
| Sr-89 as concomitant medication | 110 | 527 | 120 | 492 | –9 | –85 | 66 |
| Blood units | 21 | 150 | 21 | 124 | 0 | –20 | 21 |
| Cabazitaxel | 375 | 2192 | 221 | 1765 | 154 | –134 | 443 |
| Docetaxel as concomitant medication | 415 | 2057 | 390 | 1545 | 25 | –233 | 283 |
| Mitoxantrone | 39 | 218 | 39 | 211 | 0 | –32 | 31 |
| Surgery | 325 | 1954 | 172 | 1064 | 153 | –84 | 390 |
| Outpatient appointments and inpatient stay | |||||||
| Hospital outpatient appointment | 609 | 889 | 653 | 940 | –44 | –178 | 89 |
| Hospital inpatient stay | 3630 | 6294 | 3653 | 6491 | –23 | –950 | 903 |
| GP appointments | 281 | 350 | 316 | 357 | –35 | –86 | 16 |
As expected, the most prominent difference in mean costs between the Sr-89 and no Sr-89 groups is a result of the cost of Sr-89 itself. Apart from higher cost of Sr-89, the Sr-89 group was associated with greater cost for docetaxel and ZA given as protocol treatments, higher cost of cabazitaxel and docetaxel provided as concomitant medications and increased cost as a result of surgeries. On the other hand, the Sr-89 group was associated with lower use of radiotherapies, abiraterone, ZA and Sr-89 as concomitant medications, as well as fewer inpatient days, outpatient appointments and GP visits.
The estimated mean total cost per patient was £13,787 (BCa 95% CI £12,862 to £14,713) for Sr-89 and £12,446 (BCa 95% CI £11,489 to £13,403) for no Sr-89. This resulted in a mean cost difference of £1341 (BCa 95% CI –£66 to £2748) (Table 72).
| Treatment | Mean | SD | Bootstrapped 95% CIs | Difference | Bootstrapped 95% CIs of difference | ||
|---|---|---|---|---|---|---|---|
| Lower CI | Upper CI | Lower CI | Upper CI | ||||
| Sr-89 | £13,787 | £9295 | £12,862 | £14,713 | £1341 | –£66 | £2748 |
| No Sr-89 | £12,446 | £9281 | £11,489 | £13,403 | |||
The distribution of the total cost can be seen in the box plot Figure 48 and histogram Figure 49.
FIGURE 48.
Box plot summarising the distribution of total per-patient cost for Sr-89 and no Sr-89.
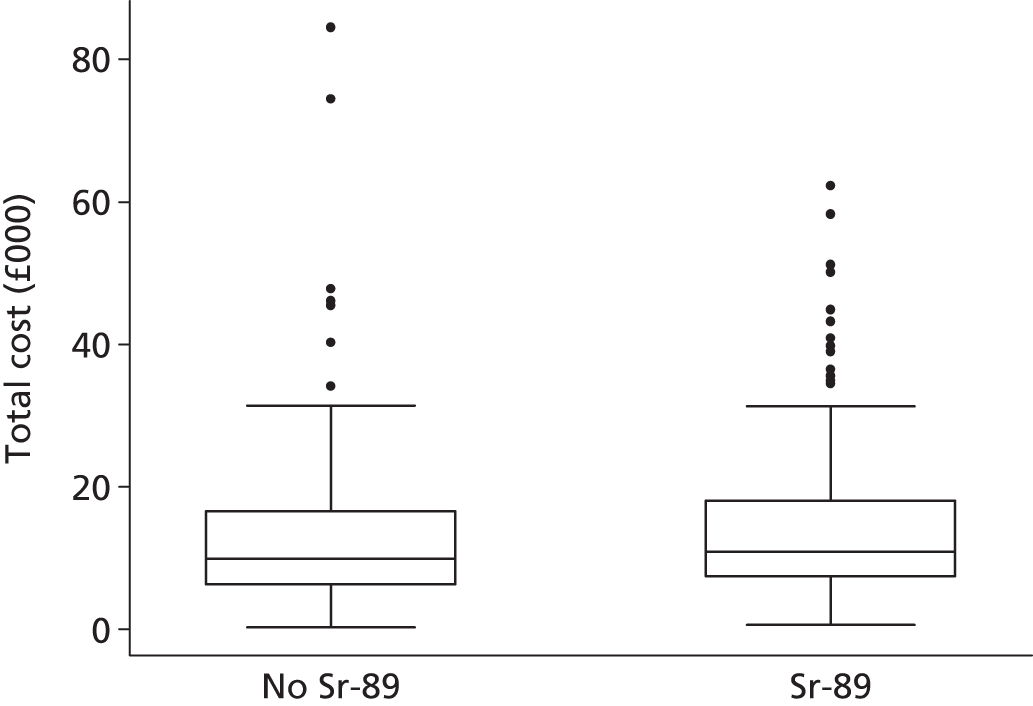
FIGURE 49.
Histogram depicting the distribution of the mean total cost for the Sr-89 and no Sr-89 groups. (a) No Sr-89; and (b) Sr-89.

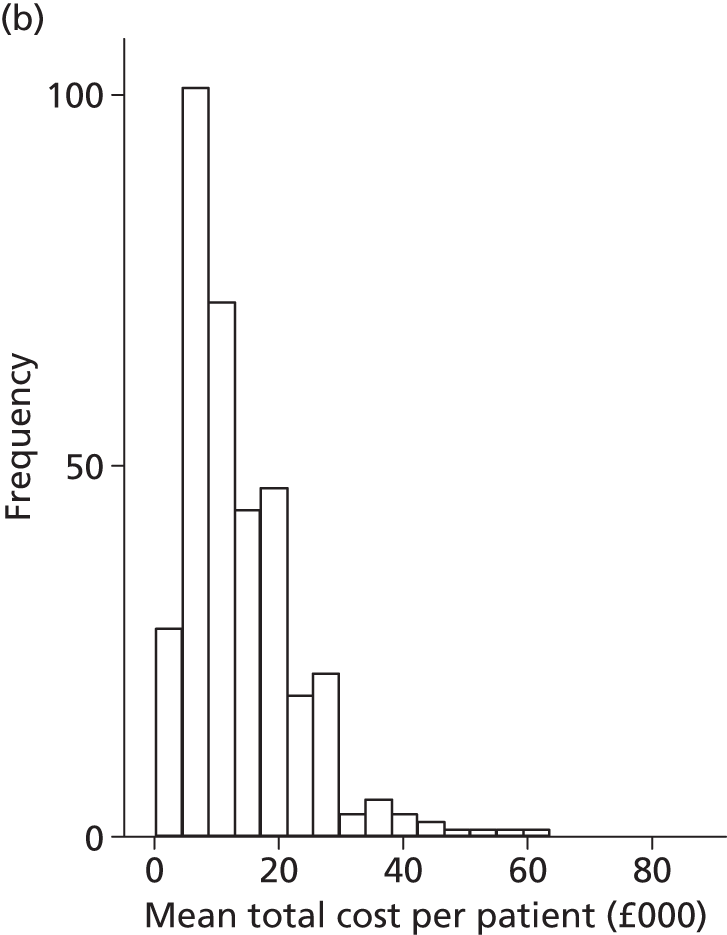
Quality-adjusted life-years
Figure 50 shows the distribution of EQ-5D scores at baseline and at 6 and 12 months after randomisation for the Sr-89 and no Sr-89 groups. Throughout the first year after randomisation, both groups presented approximately the same average increase in QoL. Again, it must be noted that, while responses relate to a specific point in time (e.g. 6 months), patients at this point in time may be at different stages of their treatment and follow-up pathways.
FIGURE 50.
Box plot showing the distribution of EQ-5D scores across different time points, for the Sr-89 and no Sr-89 groups.

The mean and standard deviation of the distribution of discounted and undiscounted QALYs for each treatment are given in Table 73. These distributions are depicted as box plots in Figure 51.
| Sr-89 (n = 350) | No Sr-89 (n = 357) | Difference | BCa 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Lower CI | Upper CI | ||
| QALYs (undiscounted) | 0.941 | 0.741 | 0.859 | 0.665 | 0.082 | –0.021 | 0.184 |
| QALYs (discounted) | 0.933 | 0.725 | 0.852 | 0.648 | 0.081 | –0.019 | 0.181 |
FIGURE 51.
Box plot summarising the distribution of QALYs for Sr-89 and no Sr-89.
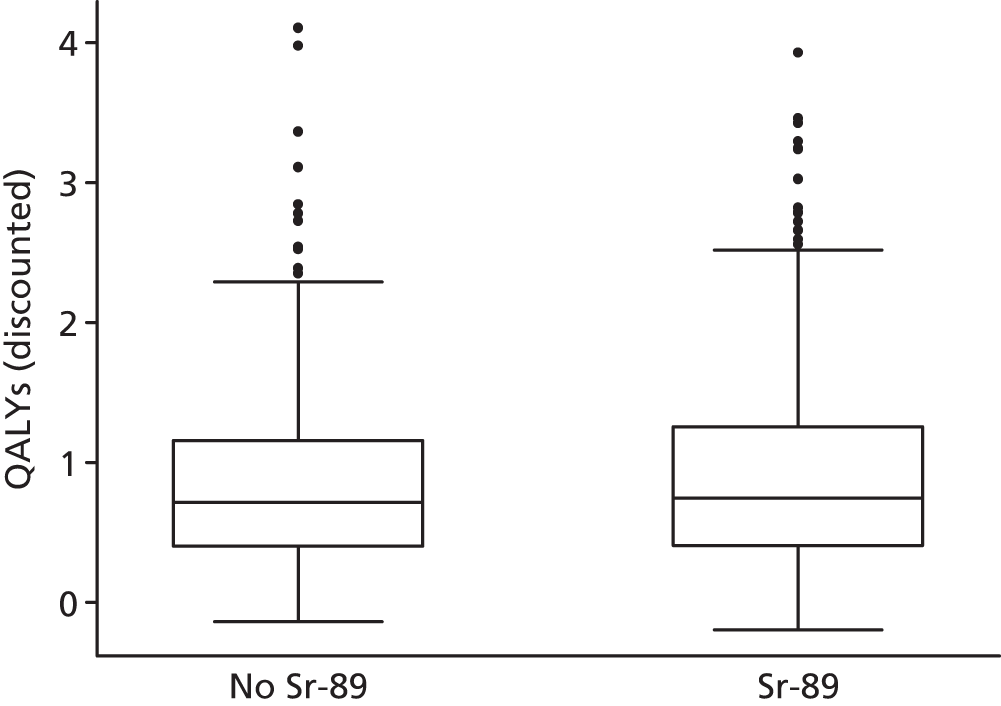
Cost-effectiveness results
Differences in mean total per-patient cost and mean overall QALYs between the Sr-89 and no Sr-89 groups can be seen in Table 74. Sr-89 appears to be more costly than no Sr-89, resulting in a between-group difference of approximately £1350. In terms of QALYs, Sr-89 is slightly more effective, indicating a gain of 0.08 QALYs over no Sr-89. Given these differences in costs and QALYs, the point estimate ICER for Sr-89 compared with no Sr-89 was calculated at £16,590 per additional QALY. This value is well below the £20,000 per QALY ratio which NICE considers to represent effective use of the NHS resources. 46
| Treatment | Total cost (£) | Total QALYs | Difference in costs (£) (Sr-89 vs. no Sr-89) | Difference in QALYs (Sr-89 vs. no Sr-89) | ICER (£) |
|---|---|---|---|---|---|
| Sr-89 | 13,787 | 0.933 | 1341 | 0.081 | 16,590 |
| No Sr-89 | 12,446 | 0.852 |
Pairs of differences in costs and QALYs between the two groups, generated through 5000 bootstrap replications, are depicted on the cost-effectiveness plane (Figure 52). Approximately 91% of the simulated pairs are located in the north-east quadrant, pointing to a substantial likelihood that Sr-89 is more costly and at the same time more effective than no Sr-89. About 6% of the points appear in the north-west quadrant, which indicates that Sr-89 may be more costly and less effective than no Sr-89. The rest of the points – approximately 3% of the 5000 estimates – fall in the southern half of the plane, indicating that Sr-89 may be less costly and more effective than no Sr-89 (south-east quadrant; 2% of all points) or less costly and less effective than no Sr-89 (south-west quadrant; 1% of all points).
FIGURE 52.
Cost-effectiveness plane showing incremental cost and effect (QALYs) pairs for the comparison between Sr-89 and no Sr-89.

The CEAC for Sr-89 and no Sr-89 is given in Figure 53. In the situation that the decision-maker is not prepared to pay any amount for additional QALYs – that is, the ceiling ratio is zero – the likelihoods of Sr-89 and no Sr-89 being cost-effective are 97% and 3%, respectively. As the value of the ceiling ratio rises, the likelihood of Sr-89 being the most cost-effective option increases steadily and reaches 50% at about £16,600 per QALY. For ceiling ratios between £20,000 and £30,000 per QALY gained, the probability that Sr-89 is more cost-effective than no Sr-89 ranges from 61% to 76%. If society is willing to pay more than £20,000 for an additional QALY, the chance that Sr-89 is the most cost-effective option is in excess of 61%.
FIGURE 53.
Cost-effectiveness acceptability curve showing the probability of Sr-89 and no Sr-89 being cost-effective at different values of the ceiling ratio. Reproduced and amended with permission from Andronis et al. 55 In the source figure, only the Sr-89 CEAC was plotted; a CEAC for No Sr-89 has been added here.

Sensitivity analysis
Different scenarios were explored in the sensitivity analysis and are presented in Table 75. Most scenarios appeared to have a limited impact on the resulting ICER. In particular, not discounting costs and QALYs and discounting costs and benefits at 0.06% per year resulted in ICERs for Sr-89 of £16,520 and £16,650 per QALY, respectively. After adjusting for baseline utility, the calculated QALYs for the Sr-89 group remained greater than the QALYs of the no Sr-89 group (difference of 0.073 QALYs, 95% CI –0.019 to 0.166). This resulted in an ICER of £18,325. Using alternative unit cost estimates for docetaxel and mitoxantrone from the NHS Commercial Medicines Unit65 had a minimal effect on the total mean per-patient cost, with prices of docetaxel and mitoxantrone lower than the base-case values from BNF, resulting in ICERs of £16,150 and £16,590 per QALY, respectively. The use of lower acquisition cost for ZA to reflect the availability of generic alternatives had a limited effect on the difference in cost between Sr-89 and no Sr-89 and, thus, it had a minimal impact on the resulting ICER.
| Sr-89 | No Sr-89 | ICER (£) | |||
|---|---|---|---|---|---|
| Mean cost | Mean QALYs | Mean cost (£) | Mean QALYs | ||
| Base-case results | 13,787 | 0.933 | 12,446 | 0.852 | 16,590 |
| No discounting | 13,920 | 0.941 | 12,570 | 0.859 | 16,517 |
| Discounting at 0.6 per annum for both costs and benefits | 13,698 | 0.927 | 12,363 | 0.847 | 16,646 |
| QALYs adjusted for baseline imbalances in EQ-5D | 13,787 | 0.925 | 12,446 | 0.852 | 18,325 |
| Unit cost of docetaxel from eMIT (£34.29 for 140 mg) | 12,592 | 0.933 | 11,287 | 0.852 | 16,151 |
| Unit cost of mitoxantrone from eMIT (£60.36 for 25 mg) | 13,783 | 0.933 | 12,442 | 0.852 | 16,591 |
| Unit cost of ZA (50% lower than BNF price) | 13,371 | 0.933 | 12,012 | 0.852 | 16,777 |
| Unit cost of ZA (90% lower than BNF price) | 13,038 | 0.933 | 11,665 | 0.852 | 16,952 |
| Unit cost of ZA (£57.71 for 4 mg) | 13,230 | 0.933 | 11,865 | 0.852 | 16,851 |
| Unit cost of Sr-89 (75% of available estimate) | 13,488 | 0.933 | 12,446 | 0.852 | 12,889 |
| Unit cost of Sr-89 (125% of available estimate) | 14,086 | 0.933 | 12,446 | 0.852 | 20,292 |
As expected, sensitivity analyses around the price of Sr-89 had a more profound effect on the differences in costs between Sr-89 and no Sr-89; a lower price of Sr-89 showed Sr-89 to be associated with an ICER of £12,900 per QALY, whereas a higher price for this radioisotope resulted in an ICER for Sr-89 slightly over £20,000 per QALY. The relationship between the price of Sr-89 and the resulting ICER for Sr-89, as compared with no Sr-89, is depicted in Figure 54; it can be seen that for prices of Sr-89 up to £2120, the ICER for ZA is below £20,000 per QALY and, thus, Sr-89 is cost-effective at the ceiling ratio of £20,000 per QALY.
FIGURE 54.
Incremental cost-effectiveness ratio for Sr-89 vs. no Sr-89 for different prices of Sr-89.

Chapter 5 Discussion
Interpretation
There are two strands to the discussion: the clinical effectiveness of the two treatments and the health economic aspects. As there was no interaction between the effects of the two therapies, Sr-89 and ZA, the effects can be considered separately.
Strontium-89
The addition of Sr-89 to chemotherapy did favourably affect time to bony disease progression and had a modest effect on QoL, but no effect on OS. Surprisingly, given the previous data for Sr-89, there was no impact on SREs in terms of time to first SRE, total numbers or distribution. Given the lack of impact on events such as SREs, there was little impact on downstream costs. However, the observed QoL gain and modest additional cost (£1341) translated into an ICER of £16,590 per additional QALY.
Despite these positive outcomes, it is less clear whether or not findings will significantly alter the use of Sr-89, for a number of reasons. First, the gain in CPFS is modest – about 1 month – while, at the same time, there was no impact on SRE frequency or time to first event. At the time of study inception, this gain in CPFS, coupled with a QoL gain, would certainly have been practice-changing at an ICER well below £20,000 per QALY. However, we have seen a number of new treatments licensed in the last few years that improve OS after chemotherapy and bring improvements in QoL and SREs. These include abiraterone,8 enzalutamide,9 cabazitaxel16 and, of particular relevance to this study, radium-223. 18 Radium-223 is a radioisotope with a similar uptake mechanism of action to Sr-89, being a calcium-mimetic agent. However, it is more intensely radioactive and the key ALSYMPCA trial18 shows improvements in both OS and symptomatic SREs. In addition, the SRE benefit appears to be potentiated by concurrent use of bisphosphonates.
Zoledronic acid
The addition of ZA to docetaxel did not impact on OS or affect CPFS after chemotherapy. There was, however, a substantial impact on symptomatic SREs. As indicated in Chapter 1, Bisphosphonates, SREs are controversial as they are a composite end point, and in the ZA licensing trials a key component, pathological fracture, was assessed repeatedly by a blinded radiologist. The consequence was that many SREs in the trials were of uncertain clinical significance. In contrast, in TRAPEZE and in some other recent trials in CRPC such as the ALSYMPCA study with radium-223,18 only symptomatic SREs have been collected; hence the clinical and economic consequences are much clearer. The US Food and Drug Administration now refers to symptomatic SREs as ‘symptomatic skeletal events’, which is probably a helpful and relevant distinction. We shall continue to use the term SRE in this report, as this is the terminology used throughout the study. In the trial, ZA produced a substantial increase in time to first SRE (13.6 months vs. 11.17 months; HR 0.78), a substantial decrease in SREs (total SREs 605 vs. 424), as well as a decrease in SREs per patient (see Figure 15). Furthermore, when the distribution of SREs by type is considered, the biggest effect of ZA was on the SREs that may be considered the most severe (fracture, SCC and surgery to bone), with a near 50% reduction, compared with radiotherapy, which reduced these SREs by about one-third (see Table 34).
With this background of clinical effect, the QoL data are of considerable interest. The first feature to note is that QoL is well preserved across the course of the illness. This accords with clinical impression, which is that patients remain well in the majority of cases until the final terminal period. The data collected are undoubtedly incomplete, because the final terminal phase does not seem to be well captured as patients are generally not attending trials clinics in that period. The second feature is that ZA had a positive, albeit minimal, effect on QoL, despite a marked change in distribution of SREs in particular. How may we explain this, as events such as pain leading to radiotherapy, fracture and SCC must certainly impair QoL? There are a number of possibilities. The first relates to the fact that the timing of completion of forms was variable and hence may well have occurred only in the stable outpatient environment of the trials clinic, once problems were resolved. The second is that, after a serious event such as a fracture, patients cease to attend the trial clinic and so the detriment to QoL from a SRE is not well captured. The third is that as the serious SREs are a minority, any effect is drowned by the QoL impact of radiotherapy, which, it could be postulated, is as good a way of maintaining QoL as regular ZA infusions. In truth it is likely that all of these explanations are partially true.
From an economic viewpoint, our cost-effectiveness analysis showed the ZA group to be associated with an additional cost of £1319 compared with no ZA, on the premise that ZA is purchased as a branded product at the price reported in the BNF. Combined with the small increase in QoL, ZA appeared to be more costly and more effective than no ZA, resulting in an ICER near the £42,000 per QALY mark.
However, since the completion of the TRAPEZE trial, ZA has become available as a generic product at a significantly lower price – less than one-third of the price of the branded Zometa. Indeed, according to the NHS Commercial Medicines Unit, the average price that hospitals across England pay for ZA is one-third of the price of proprietary products listed in BNF. As the cost of adding ZA to chemotherapy is a main driver of the difference in total cost between ZA and no ZA, accounting for this showed lower additional costs associated with ZA (£251) and a markedly lower ICER of £8005 per QALY. In addition, prevention of serious events, such as fracture, surgery and cord compression (all associated with frequent and prolonged admissions), is a high priority for NHS trusts with great pressures on beds. Therefore, a predictable outpatient therapy with modest net acquisition costs may well be attractive to trusts if it prevents emergency, unpredictable visits. We did not carry out a patient preference study; however, it may well be the case that patients would prefer a preventative treatment such as ZA to a reactive approach.
Limitations
The main limitations of the data presented are largely discussed above. Specifically, the development of new treatments for CRPC makes Sr-89 in particular less relevant, with the advent of better radioisotope therapy such as radium-223. The limitations on the ZA data are more complex, as the effects on SREs are substantial and result in reduced costs associated with surgery and radiotherapy at a modest additional cost. It is likely that these benefits are complementary to those achieved with other post-docetaxel therapies. In particular, there are data available showing that the benefit of radium-223 on SREs may be increased by ZA. Improved bone-protecting agents that prevent hospital visits may also alter the potential benefits of these agents.
A further problem with the QoL data is the effect of missing data, which we have attempted to model but which could clearly influence outcomes, as it is likely that we are missing data, in particular relating to QoL around SREs and in the terminal phases. In line with recommendations, health benefits accruing from the compared treatments were measured in terms of QALYs on the basis of patient responses to the EQ-5D (three-level) preference-based QoL instrument. However, it must be noted that, in such terminal phases, benefits perceived to be relevant by patients may not be fully captured by instruments such as the EQ-5D and they may be inadequately reflected on QALYs. As Gomes et al. 66 point out, this is largely because, at the end-of-life stage, patients deem improvements in survival and QoL as secondary considerations. Despite this, no instruments are available to measure benefits of end-of-life care for the purposes of economic evaluations. Costs in this paper have been derived from patient-reported resource use data and, thus, may be subject to recall bias.
Generalisablity
Docetaxel remains a mainstay of therapy for CRPC despite the development of new treatments for the disease. Of note is the fact that, although all patients recruited to the study had relapsing bone mCRPC, around 40% of patients died without experiencing a single SRE in the docetaxel arm (fewer with the addition of ZA). Hence, docetaxel itself can be imputed to effectively prevent SREs, as it is highly likely that the majority of patients managed without this agent (in the era prior to the new therapies listed previously) would have had many SREs. The data on QoL are striking for the way that QoL is maintained in a highly disease-burdened population. QoL also rose on commencement of chemotherapy, underlining its value as a palliative treatment.
As noted, Sr-89 has probably been superseded by radium-223, as well as by other treatments of higher effectiveness for CRPC. On the other hand, bone-protecting agents such as ZA may offer complementary benefits via their impact on SREs, particularly serious ones.
Overall evidence
Strontium-89 after six cycles of docetaxel improved CPFS but not OS. ZA did not improve CPFS or OS but did significantly improve median SREFI, mostly after progression, suggesting a role as post-chemotherapy maintenance therapy. QoL was well maintained in all treatment arms but with differing patterns of care resulting from the effects of Sr-89 on time to progression and ZA on SREFI and total SREs.
The addition of Sr-89 to docetaxel chemotherapy resulted in an additional cost of £1341, mainly because of the cost of the Sr-89 acquisition and administration. Combined with a positive, although small, increase in QALYs, this option resulted in an ICER of £16,590 per QALY compared with no use of Sr-89. This value is below the commonly cited willingness-to-pay value of £20,000 per additional QALY.
The addition of ZA to docetaxel resulted in an extra cost of about £1320 (for proprietary ZA) or £251 (given the availability of generic alternatives). These additional costs and the small but positive change in QALYs in favour of ZA resulted in ICERs of £42,047 for the proprietary product or £8005 for the alternative generic-based price. Whether or not the addition of ZA to chemotherapy represents a cost-effective use of resources depends largely on the acquisition cost of a 4-mg dose of ZA. In the likely case that NHS trusts pay less than £98 for 4 mg of ZA, the ICER for ZA is below £20,000 per QALY, and thus ZA represents a cost-effective option at this ceiling ratio.
Chapter 6 Conclusion
Implications for health care
Docetaxel appears to prevent patients with relapsing bone disease from developing skeletal complications. Its use improves QoL and it should remain a component of the treatment options for men with mCRPC. Sr-89 showed modest benefits at a modest cost, resulting in an ICER lower than £20,000 per QALY, though it is likely that this radioisotope has now been superseded, in particular by the recently licensed radium-223. ZA reduced serious skeletal complications at a modest additional cost and showed a small gain in QALYs. Analysis using generic ZA resulted in a low additional cost of £251 and, coupled with the gain in QALYs, showed an ICER below the commonly cited value of £20,000 per QALY.
Recommendations for research
Further modelling of the costs of therapy using Hospital Episode Statistics data is desirable and we plan to complete this. Further research into the use of ZA (and other bone-targeting therapies) with newer prostate cancer therapies is desirable.
Acknowledgements
During Phase II of the trial, Sanofi-aventis and Novartis Pharmaceuticals UK Ltd provided educational grants to Cancer Research Clinical Trial Unit, Cancer Research UK, School of Cancer Sciences (formerly the Institute for Cancer Studies) at the University of Birmingham. Sanofi-aventis and Novartis Pharmaceuticals UK Ltd also provided study drugs Taxotere® (docetaxel) and Zometa (ZA), respectively, free of charge for the first 300 patients recruited into the trial.
Sanofi-aventis continued to support the Phase III trial with a £300 grant to recruiting centres, via Cancer Research Clinical Trials Unit, for each patient recruited into the trial with trial numbers 301 to 700.
Novartis Pharmaceuticals UK Ltd offered a trial discount of 28.2% (on the regular NHS tariff) for the purchase of Zometa for specified TRAPEZE patients.
GE Healthcare Ltd offered a trial discount of 5% for the purchase of a single dose of Metastron® (Sr-89) per patient for patients entered into the TRAPEZE trial.
Contribution of authors
Nicholas James was the chief investigator and lead author.
Sarah Pirrie was the trial statistician and a member of the TMG and contributed to study oversight, review of protocol changes and editorial review of the manuscript.
Ann Pope, Darren Barton, Duncan McLaren, Joe O’Sullivan, Chris Parker, Emilio Porfiri, John Staffurth, Andrew Stanley, James Wylie and Lucinda Billingham and were members of the TMG and contributed to study oversight, review of protocol changes and editorial review of the manuscript.
Lazaros Andronis led on the economic aspect of the study.
Lazaros Andronis and Ilias Goranitis analysed the health economics data and wrote Chapter 4 of this report, in addition to contributing to editorial review of the manuscript.
Stuart Collins, who was trial statistician up until his premature death in 2011, was also a member of the TMG.
Duncan McLaren and James Wylie were primary investigators in centres contributing more than 10% of the trial patients. They also contributed to editorial review of the manuscript.
Sharon Beesley, Alison Birtle, Janet Brown, Prabir Chakraborti and Martin Russell were primary investigators from high-recruiting centres who also contributed to editorial review of the manuscript.
Other contributors
Mr Kaisheng Wen, Senior Research Fellow, University of Birmingham School of Cancer Sciences, for analysis of proteomic plasma samples for the p1NP biomarker.
Publications
James ND, Pirrie S, Barton D, Brown JE, Billingham L, Collins SI, et al. Clinical outcomes in patients with castrate-refractory prostate cancer (CRPC) metastatic to bone randomised in the factorial TRAPEZE trial to docetaxel (D) with Sr-89 (Sr-89), zoledronic acid (ZA), neither or both. Oral abstract presented at the Meeting of the American Society of Clinical Oncology, Chicago, IL, 3 June 2013.
James ND, Pirrie S, Barton D, Brown JE, Billingham L, Collins SI, et al. Clinical outcomes in patients with castrate-refractory prostate cancer (CRPC) metastatic to bone randomised in the factorial TRAPEZE trial to docetaxel (D) with Sr-89 (Sr-89), zoledronic acid (ZA), neither or both. Oral and poster abstract presented at National Cancer Research Institute, Liverpool, 4 November 2013.
James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitis I, et al. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with docetaxel alone or with strontium-89, zoledronic acid, or both: the TRAPEZE randomized clinical trial. JAMA Oncol 2016;2:493–9.
Andronis L, Goranitis I, Pirrie S, Pope A, Barton D, Collins S, et al. Cost-effectiveness of zoledronic acid and strontium-89 as bone protecting treatments in addition to chemotherapy in patients with metastatic castrate-refractory prostate cancer: results from the TRAPEZE trial (ISRCTN 12808747) [published online ahead of print June 3 2016]. Brit J Urol Int 2016.
Data sharing statement
At the time of publication there are no plans by the authors to formally publish the full anonymised study data set on a publically assessable database/study archive system. Any requests for additional information, access to data (TRAPEZE anonymised clinical data set) should be submitted in writing to the TRAPEZE Management Team at the Cancer Research Clinical Trials Unit (CRCTU), University of Birmingham, Birmingham, UK (at crctu-generalenquiries@trials.bham.ac.uk).
The CRCTU are supportive of data sharing and will endeavour to assist external investigators who wish to share clinical trial data. Given the diversity of the CRCTU portfolio, all requests for data sharing are dealt with on a case-by-case basis and as specified below.
The request should clearly document the following:
-
the scientific rational of the proposal
-
aims and objectives
-
outcome measures
-
data variables required
-
how the data will be analysed
-
indicate what acknowledgement the Trial Management Group and the CRCTU will receive on any publications resulting from the work.
In some circumstances investigators may also be asked to complete a CRCTU New Business Committee form.
On receipt of a valid request to share data the CRCTU will ensure that:
-
external parties and trial oversight committees (e.g. Trial Steering Committee) are supportive of the request
-
the necessary legal, ethical and regulatory permissions to allow data sharing are in place
-
completely anonymised data can be supplied
-
there is sufficient resource within the CRCTU to deal with the request.
If the above conditions are met the CRCTU will provide the requested data. In some circumstances this may be subject to a Data Sharing Agreement being put in place.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- CancerStats Key Facts: Prostate Cancer. Cancer Research UK; 2011.
- Office of National Statistics . Mortality Statistics: Deaths Registered in England and Wales n.d. www.ons.gov.uk/ons (accessed January 2014).
- National Records of Scotland . Deaths Time Series Data, Deaths in Scotland n.d. www.gro-scotland.gov.uk/statistics/theme/vital-events/deaths/time-series.html (accessed March 2014).
- Northern Ireland Statistics and Research Agency . Deaths by Cause n.d. www.nisra.gov.uk/demography/default.asp14.htm (accessed 23 October 2015).
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1941;1:293-7.
- Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138-48. http://dx.doi.org/10.1056/NEJMoa1209096.
- Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012;13:983-92. http://dx.doi.org/10.1016/S1470-2045(12)70379-0.
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-200. http://dx.doi.org/10.1056/NEJMoa1014618.
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg C, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-97. http://dx.doi.org/10.1056/NEJMoa1207506.
- Luis Costa XB, Chow E, Lipton A, Wardley A. Impact of skeletal complications on patients’ quality of life, moblity and functional independance. Support Care Cancer 2008;16:879-89. http://dx.doi.org/10.1007/s00520-008-0418-0.
- Marnix GEH, Lam AD, Wil HM, de Klerk, Zonnenberg BA. Combined use of Zoledronic acid and 153Sm-EDTMP in hormone-refractory prostate cancer patients with bone metastases. Eur J Nucl Med Mol Imaging 2008;35:756-65. http://dx.doi.org/10.1007/s00259-007-0659-z.
- Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996;14:1756-64.
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Kim N, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-12. http://dx.doi.org/10.1056/NEJMoa040720.
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513-20. http://dx.doi.org/10.1056/NEJMoa041318.
- National Schedule of Reference Costs 2011–12. London: DH; 2012.
- de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiel JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376:1147-54. http://dx.doi.org/10.1016/S0140-6736(10)61389-X.
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424-33. http://dx.doi.org/10.1056/NEJMoa1405095.
- Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan, Fossa SD, et al. α-emitter radium-223 and increased survival in metastatic prostate cancer. N Engl J Med 2013;369:213-23. http://dx.doi.org/10.1056/NEJMoa1213755.
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002;94:1458-68. http://dx.doi.org/10.1093/jnci/94.19.1458.
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004;96:879-82. http://dx.doi.org/10.1093/jnci/djh141.
- Bolger JJ, Dearnaley DP, Kirk D, Lewington VJ, Mason MD, Quilty PM, et al. Strontium-89 (Metastron) versus external beam radiotherapy in patients with painful bone metastases secondary to prostatic cancer: preliminary report of a multicenter trial. UK Metastron Investigators Group. Semin Oncol 1993;20:32-3.
- Quilty PM, Kirk D, Bolger JJ, Dearnaley DP, Lewington VJ, Mason MD, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol 1994;31:33-40. http://dx.doi.org/10.1016/0167-8140(94)90411-1.
- Serafini AN. Current status of systemic intravenous radiopharmaceuticals for the treatment of painful metastatic bone disease. Int J Radiat Oncol Biol Phys 1994;30:1187-94. http://dx.doi.org/10.1016/0360-3016(94)90327-1.
- McEwan AJ, Amyotte GA, McGowan DG, MacGillivray JA, Porter AT. A retrospective analysis of the cost-effectiveness of treatment with Metastron in patients with prostate cancer metastatic to bone. Eur Urol 1994;26:26-31.
- Tu SM, Millikan RE, Mengistu B, Delpassand ES, Amato RJ, Pagliaro LC, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 2001;357:336-41. http://dx.doi.org/10.1016/S0140-6736(00)03639-4.
- Dearnaley DP, Mason MD, Parmar MKB, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol 2009;10:872-6. http://dx.doi.org/10.1016/S1470-2045(09)70201-3.
- Dearnaley DP, Sydes MR, Mason MD, Scott M, Powell CS, Robinson AC, et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J Natl Cancer Inst 2003;95:1300-11. http://dx.doi.org/10.1093/jnci/djg038.
- Boissier S, Magnetto S, Frappart L, Cuzin B, Ebetino FH, Delmas PD, et al. Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Res 1997;57:3890-4.
- Major P, Lortholary A, Hon J, Abdi E, Mills G, Menssen HD, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001;19:558-67.
- Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 2001;7:377-87.
- Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res 2000;60:2949-54.
- Jagdev SP, Coleman RE, Shipman CM, Rostami HA, Croucher PI. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br J Cancer 2001;84:1126-34. http://dx.doi.org/10.1054/bjoc.2001.1727.
- Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813-22. http://dx.doi.org/10.1016/S0140-6736(10)62344-6.
- Lewington VJ, McEwan AJ, Ackery DM, Bayly R, Keeling D, Macleod P, et al. A prospective, randomised double–blind crossover study to examine the efficacy of strontium-89 in pain palliation in patients with advanced prostate cancer metastatic to bone. Eur J Cancer 1991;27:954-8. http://dx.doi.org/10.1016/0277-5379(91)90257-E.
- Kraeber-Bodere F, Campion L, Rousseau C, Bourdin S, Chatal JF, Resche I. Treatment of bone metastases of prostate cancer with strontium-89 chloride: efficacy in relation to the degree of bone involvement. Eur J Nucl Med 2000;27:1487-93. http://dx.doi.org/10.1007/s002590000315.
- Robinson RG, Preston DF, Spicer JA, Baxter KG. Radionuclide therapy of intractable bone pain: emphasis on strontium-89. Semin Nucl Med 1992;22:28-32. http://dx.doi.org/10.1016/S0001-2998(05)80154-0.
- Robinson RG, Preston DF, Schiefelbein M, Baxter KG. Strontium 89 therapy for the palliation of pain due to osseous metastases. JAMA 1995;274:420-4. http://dx.doi.org/10.1001/jama.1995.03530050068035.
- Jager PL, Kooistra A, Piers DA. Treatment with radioactive (89)strontium for patients with bone metastases from prostate cancer. BJU Int 2000;86:929-34. http://dx.doi.org/10.1046/j.1464-410x.2000.00780.x.
- Porter AT, McEwan AJ. Strontium-89 as an adjuvant to external beam radiation improves pain relief and delays disease progression in advanced prostate cancer: results of a randomized controlled trial. Semin Oncol 1993;20:38-43.
- Fizazi K, Beuzeboc P, Lumbroso J, Haddad V, Massard C, Gross-Goupil M, et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol 2009;27:2429-35. http://dx.doi.org/10.1200/JCO.2008.18.9811.
- Tu SM, Mathew P, Wong FC, Jones D, Johnson MM, Logothetis CJ. Phase I study of concurrent weekly docetaxel and repeated samarium-153 lexidronam in patients with castration-resistant metastatic prostate cancer. J Clin Oncol 2009;27:3319-24. http://dx.doi.org/10.1200/JCO.2008.20.5393.
- Morris MJ, Pandit-Taskar N, Carrasquillo J, Divgi CR, Slovin S, Kelly WK, et al. Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer. J Clin Oncol 2009;27:2436-42. http://dx.doi.org/10.1200/JCO.2008.20.4164.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Anderson J, et al. STAMPEDE: Systemic Therapy for Advancing or Metastatic Prostate Cancer--a multi-arm multi-stage randomised controlled trial. Clin Oncol 2008;20:577-81. http://dx.doi.org/10.1016/j.clon.2008.07.002.
- Parmar MK, Barthel FM, Sydes M, Langley R, Kaplan R, Eisenhauer E, et al. Speeding up the evaluation of new agents in cancer. J Natl Cancer Inst 2008;100:1204-14. http://dx.doi.org/10.1093/jnci/djn267.
- Guide To The Methods Of Technology Appraisal 2013. London: NICE; 2013.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-14. http://dx.doi.org/10.1177/0272989X9001000308.
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. http://dx.doi.org/10.1002/hec.4730030505.
- Kahan BC, Morris TP. Improper analysis of trials ranomised using stratified block or minimisation. Stat Med 2011;31:328-40. http://dx.doi.org/10.1002/sim.4431.
- Yu L, Chan A, Hopewell S, Deeks JJ, Altman DG. Reporting on covariate adjustment in randomised controlled trials before and after revision of the 2001 CONSORT statement: a literature review. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-59.
- James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitis I, et al. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with docetaxel alone or with strontium-89, zoledronic acid, or both: the TRAPEZE randomized clinical trial. JAMA Oncol 2016;2:493-9. http://dx.doi.org/10.1001/jamaoncol.2015.5570.
- Prentice RL, William BJ, Peterson AV. On the regression analysis of multivariate failure time data. Biometrika 1981;68:373-9.
- Billingham LJ, Abrams KR. Simultaneous analysis of quality of life and survival data. Stat Methods Med Res 2002;11:25-48. http://dx.doi.org/10.1191/0962280202sm269ra.
- Cancer Therapy Evaluation Program . Common Terminology Criteria for Adverse Events, Version 3.0 2003. http://ctep.cancer.gov (accessed October 2013).
- Andronis L, Goranitis I, Pirrie S, Pope A, Barton D, Collins S, et al. Cost-effectiveness of zoledronic acid and strontium-89 as bone protecting treatments in addition to chemotherapy in patients with metastatic castrate-refractory prostate cancer: results from the TRAPEZE trial (ISRCTN 12808747) [published online ahead of print June 3 2016]. Brit J Urol Int 2016. http://dx.doi.org/10.1111/bju.13549.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- Strategic Plan 2012–2017. London: NHS Blood and Transplant; 2011.
- Lee KJ, Carlin JB. Multiple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol 2010;171:624-32. http://dx.doi.org/10.1093/aje/kwp425.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. http://dx.doi.org/10.1002/sim.4067.
- Curtis L. Unit Cost of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Billingham LJ, Abrams KR, Jones DR. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol Assess 1999;3.
- Glick HA, Briggs AH, Polsky D. Quantifying stochastic uncertainty and presenting results of cost-effectiveness analyses. Expert Rev Pharmacoecon Outcomes Res 2001;1:25-36. http://dx.doi.org/10.1586/14737167.1.1.25.
- Manca A, Palmer S. Handling missing data in patient-level cost-effectiveness analysis alongside randomised clinical trials. Appl Health Econ Health Policy 2005;4:65-7. http://dx.doi.org/10.2165/00148365-200504020-00001.
- Electronic Market Information Tool. London: DH; 2012.
- Gomes B, Harding R, Foley KM, Higginson IJ. Optimal approaches to the health economics of palliative care: report of an international think tank. J Pain Symptom Manage 2009;38:4-10. http://dx.doi.org/10.1016/j.jpainsymman.2009.04.008.
Appendix 1 Trial protocol
Appendix 2 Composition of the Data Monitoring Committee and Trial Steering Committee
Data Monitoring Committee
Professor Mario Eisenberger (Chairperson), Professor of Oncology & Urology, The Blausteine Cancer Research Building, John Hopkins University, Baltimore, MD, USA.
Professor Fred Saad, Professor of Surgery/Urology, Université de Montréal, Chum/Hôpital Notre-Dame, Montreal, Québec, Canada.
Mr Matthew Sydes, Senior Medical Statistician, Medical Research Council, MRC Clinical Trials Unit Aviation House London, UK.
Trial Steering Committee
Professor Richard Gray, Professor of Medical Statistics, Clinical Trial Service Unit and Epidemiological Studies Unit, Oxford, UK.
Professor Noel Clark, Consultant Oncologist, Christie Hospital NHS Foundation Trust, Manchester UK.
Professor Robert Coleman, Cancer Research Building, School of Medicine & Biomedical Sciences, Weston Park Hospital, Sheffield, UK.
Mr John Anderson (deceased 2013), Department of Urology, Royal Hallamshire Hospital, Sheffield, UK.
Appendix 3 Dates of regulatory and ethical approvals and timelines
| Event | Date |
|---|---|
| MHRA approval of Phase II stage of trial MREC approval of Phase II stage of trial – Protocol V4 & patient and GP Information & consent documents V2 |
11 November 2004 9 November 2004 |
| First site opened | 1 January 2005 |
| Recruitment of first patient | 4 February 2005 |
| Protocol V5 amendment | April 2005 |
| Protocol V6 amendment | June 2005 |
| Protocol V7 amendment | June 2007 |
| MHRA approval of Phase III stage of trial MREC approval of Phase III stage of trial – Protocol V8 & patient and GP information & consent documents V3 |
9 January 2009 29 January 2009 |
| MHRA approval of CTA amendment MREC approval of CTA amendment (i.e. generic docetaxel and ZA use) |
20 January 2011 17 February 2011 |
| Protocol V9 amendment & patient and GP information & consent documents V3 | May 2011 |
| Protocol V10 amendment | 10 June 2011 |
| Patient information addendum to V3 (for patients randomised to ZA treatment arms) | July 2011 |
| Closure to recruitment | 29 February 2012 |
| Protocol V11 amendment | 17 February 2012 |
Appendix 4 Patient information
TRAPEZE
Research Trial Of Treatments For Patients With Bony Metastatic Cancer Of The Prostate
Patient Information Form
Your Oncologist has explained to you that your prostate cancer is no longer responding to hormonal treatment. We would like to invite you to take part in a research trial to treat you with chemotherapy. Before you decide whether to take part it is important for you to understand why the research is being done and what it will involve. Please take time to read the following information carefully and discuss it with friends, relatives and your GP if you wish. Ask us if there is anything that is not clear or if you would like more information. Take time to decide whether or not you wish to take part.
Further information and a summary of the principles of clinical trials can be found on the Cancer Research UK’s patient website (www.cancerhelp.org.uk) together with information about this trial.
Purpose of the trial
It is believed that chemotherapy may be beneficial in treating your prostate cancer. Chemotherapy is currently a standard treatment for prostate cancer that has spread to the bone. The main aim of this trial is test the effects of combining two further known treatments for prostate cancer at different time points, with chemotherapy. The three treatments involved in this trial are described below:
-
Docetaxel is a chemotherapy drug and is approved in the UK for the treatment of advanced breast and lung cancer. Docetaxel has been approved for use within clinical trials for the treatment of prostate cancer. Recently published studies (including an international prostate cancer clinical trial called TAX-327) demonstrate that docetaxel improves symptom control and survival times.
-
Zoledronic acid is a bone-strengthening agent approved in the UK for treating cancer affecting the bone.
-
Strontium-89 is a type of radiotherapy (given by an injection), which is also approved in the UK for treating cancer affecting the bone. Early studies show that it may provide additional pain relief when combined with chemotherapy and may improve your condition.
The aim of the trial is to assess how effective and safe zoledronic acid or strontium-89 is in treating your disease when given in combination with chemotherapy.
We aim to recruit 618 evaluable patients with cancer no longer responding to hormone treatment to take part in this trial. The trial will be open to recruitment for up to 5 years. A patient enrolled onto this trial will be expected to visit the hospital every 3 weeks for chemotherapy treatment for 32 weeks. After this period patients will be expected to attend the hospital on a regular basis for a maximum follow-up period of 2 years. This trial may also be known under the shorter title of ‘TRAPEZE’, named after the treatments involved which include docetaxel, radioisotope and zoledronic acid.
Taking part in the trial
It is up to you to decide whether or not to take part. If you do decide to take part you will be given this information sheet to keep and be asked to sign a consent form. The original consent form will be stored by your hospital and a copy of the consent form will be sent to the coordinating centre.
If you decide to take part you are still free to withdraw at any time and without giving a reason. This will not affect the standard of care you receive.
Description of the trial
When we do not know which way of treating patients is best, we need to make comparisons. Everyone who agrees to take part in this trial will be put into a treatment group.
The treatment you receive will be chosen by a process called randomisation; the treatment is randomly allocated by computer, which is like making a choice by tossing a coin. This means that you have an equal chance of being treated with one of the above treatments. You and your Oncologist will know which treatment you are receiving.
The four treatment groups in this trial are:
-
Docetaxel
-
Docetaxel + zoledronic acid
-
Docetaxel + strontium-89
-
Docetaxel + zoledronic acid + strontium-89.
If you agree to take part, your Oncologist will perform a number of tests and examinations before, during and after the trial. You will also be asked to complete a number of questionnaires. These are summarised below:
-
General medical and physical examinations
-
Blood tests
-
X-rays, CT and bone scans – to measure your cancer response to treatment
-
DXA Scan (bone density scans) – to measure bone density
-
Pain diary
-
Quality-of-life questionnaires
Docetaxel, zoledronic acid and strontium-89 are given by a drip into a vein in your arm. This is called an infusion. You will receive one of the following treatments:
-
Docetaxel 75 mg/m2 as a one hour intravenous infusion every 3 weeks for a maximum of 10 cycles.
-
Docetaxel as a one hour intravenous infusion every 3 weeks for a maximum of 10 cycles with zoledronic acid every 3 weeks. Zoledronic acid will then continue alone every 4 weeks until you or your Oncologist wishes to discontinue it.
-
Docetaxel as a one hour intravenous infusion every 3 weeks for a maximum of 10 cycles and one treatment of strontium-89 given 28 days after the sixth dose of docetaxel as a short intravenous injection. Cycles 7–10 will then follow after a 28 day recovery period.
-
Docetaxel as a 1-hour intravenous infusion every 3 weeks for a maximum of 10 cycles, followed by one treatment of strontium-89 given 28 days after the sixth dose of docetaxel as a short intravenous injection. Cycles 7–10 will then follow after a 28 day recovery period. Zoledronic acid will be given every 3 weeks throughout the treatment, and will then continue alone every 4 weeks until you or your Oncologist wishes to discontinue it.
You may receive less than 10 cycles of docetaxel chemotherapy cycles. The exact number of cycles that you will receive will be determined by your Oncologist after consultation with you.
As part of your main treatment you will also be given steroid tablets (prednisolone) to take during your course of treatment with docetaxel. In addition, you will receive extra steroid tablets (dexamethasone) for a few days around each infusion of chemotherapy to decrease the potential side-effects of docetaxel (allergic reactions and fluid retention).
You will be required to visit the hospital every 3 weeks until the end of therapy. The duration of treatment will be approximately 35 weeks.
After the end of treatment your Oncologist will see you monthly for 3 months and then every 3 months in order to assess the status of your disease.
The flow chart below explains the visits you will make to the hospital and at which time.
Restrictions
It is important that you inform your Oncologist of any changes in your health, whether or not you think it is due to the treatment. You should also tell your Oncologist of any changes to your medicines, either those prescribed by your GP or those you buy at the chemist.
Other treatments available
Your Oncologist will discuss the different treatment options available to treat your disease.
| Tests and procedures | Before start of chemotherapy | Before each administration of chemotherapy | After chemotherapy | Follow-up (every month for 3 months then every 3 months thereafter) |
|---|---|---|---|---|
| Read information | ✓ | – | – | – |
| Sign consent | ||||
| Scanning procedures (CT scan, MRI scan, bone scan and\or ultrasound) | ✓ | – | – | ✓ (as clinically indicated) |
| DXA bone density scan | ✓ | ✓ (1 year after start of treatment) | ||
| Other current medication/side-effect information collected | ✓ | ✓ | ✓ | ✓ (30 days after last infusion) |
| Medical history information collected | ✓ | – | – | – |
| Height measured | ✓ | – | – | – |
| Weight and physical exam and vital signs | ✓ | ✓ | ✓ | ✓ |
| Blood tests including PSA | ✓ | ✓ | ✓ | ✓ |
| Pain and quality-of-life assessments | ✓ | ✓ | ✓ | ✓ |
Potential side effects and risks
As with all medicines of this type there may be some unwanted side-effects. You should discuss these with your Oncologist. The more common side-effects are listed below; there may also be other side-effects that we cannot predict. Other medicines will be given to make side-effects less serious and less uncomfortable.
With docetaxel you may experience nausea and/or vomiting, mouth irritation, diarrhoea, fatigue, a pins and needles sensation in your hands or feet, hair loss, changes in your skin and nails, muscular pain, decrease in blood cell counts, infection, and swelling due to fluid retention. Your blood pressure may also fall while the drug is being given, and this will be checked carefully. The infusion of docetaxel may cause temporary local irritation and bruises if it is given into a small vein. All these side-effects have been experienced by some patients during previous studies and most of them are reversible. (The items underlined may not be reversible).
With docetaxel + zoledronic acid you may experience the same effects as stated for docetaxel above with a rise in temperature, and flu-like symptoms, consisting of fever and bone pain due to the zoledronic acid. Zoledronic acid may also affect your kidney function. Blood samples will be taken prior to zoledronic acid infusion at every trial visit to check that your kidney function has not been affected. Uncommonly, zoledronic acid can cause breakdown (osteonecrosis) of the jaw. This is associated with long-term use of zoledronic acid (usually over 24 months) particularly in patients who have dental disease. Zoledronic acid should be discontinued if you need a tooth extraction.
With docetaxel + zoledronic acid + strontium-89 you may experience the same side-effects as stated above for docetaxel and zoledronic acid. The addition of strontium-89 to docetaxel and zoledronic acid may cause some bone pain lasting 36 to 72 hours following injection. This can usually be controlled by analgesics (pain killers). Strontium-89 can also affect your blood counts following injection; these will be monitored very closely with regular blood tests.
With docetaxel + strontium-89 the addition of strontium-89 to docetaxel may cause some bone pain lasting 36 to 72 hours following injection. This can usually be controlled by analgesics (pain killers). Strontium-89 can also affect your blood counts following injection; these will be monitored very closely with regular blood tests.
As with any chemotherapy it is possible that your treatment could cause problems to an unborn child. You must take full contraceptive precautions if there is any chance of you fathering a child during and for at least 2 months after the treatment. If you have a fever or bruising after receiving either of the drug combinations, it is important that you contact your Oncologist immediately. If you have a fever your Oncologist will perform some blood tests and may prescribe antibiotics.
For more information about risks and side-effects, ask your Oncologist.
You may require one extra bone scan more than you would if you were not taking part in the trial. You may require one additional CT scan and will receive two additional bone density scans (DXA scans) more than you would receive if you were not taking part in the trial. Any potential health risk associated with these or any of the above scans is considered to be low for a patient with your medical condition.
The radioactive strontium is intended to give a very high radiation dose to any parts of your bones that are involved in your cancer. The rest of your body gets a lower radiation dose and your Oncologist will explain possible side-effects with you. Any potential health risk associated with the radiation is considered to be minimal for a patient with your medical condition.
If you have private medical insurance you may wish to consult your medical insurers before agreeing to take part in the trial. This is to ensure that you participation will not affect your medical insurance cover.
What happens when the research trial stops?
At the end of the research trial, or should you withdraw, your Oncologist will assess your symptoms, discuss your options and prescribe appropriate treatment. Rarely companies sponsoring research studies may decide to stop the trial before it has finished. If this happens, your Oncologist will explain the reasons why and arrange appropriate care for you.
Potential benefits
The use of chemotherapy may result in a decrease in pain, improvement in the quality of life and a delay in the progression of your disease and improved survival times. This may be further improved by combining chemotherapy with zoledronic acid and/or strontium-89.
The information we get from this trial may help us to treat other patients with cancer more effectively in future.
Looking at blood serum samples
In addition to your routine blood tests we would also like to take additional blood samples from you during your regular trial visits for additional analyses. We would like to monitor changes in protein levels in your blood during treatment to see if it can help us better predict treatment outcomes.
Your participation in this part of the trial is optional and will not affect the treatment that you receive if you do not consent to providing additional blood samples for these additional tests and research.
Looking at tissue samples
As part of the clinical trial we would like to be allowed to have access to samples of your disease tissue, which were taken as part of your routine care and disease diagnoses. These samples will have been collected by your hospital and stored in paraffin fixed wax blocks. If you agree these samples will be collected and tested for the presence of a number of different chemicals known as biological markers by the School of Cancer Sciences at The University of Birmingham and other collaborative centres.
Your participation in this part of the trial is optional and will not affect the treatment that you receive if you do not consent to the research team having access to your stored tissue samples for additional research.
Your rights regarding tissue/blood samples taken as part of this clinical trial
The results of the analysis of your individual samples will not routinely be given to you unless it is of clinical significance and of importance to your health. You will not benefit financially if this research leads to the development of a new treatment or medical test. Any publications resulting from the collection of these tissue or blood samples will be made available to you, if requested.
Please note that your participation in this part of the trial is optional and will not affect the treatment that you receive, if you do not consent to providing additional blood samples or for the research team to have access to your stored tissue samples detailed above.
New information
Sometimes during a trial new information becomes available about the treatment that is being studied. If this happens your Oncologist will tell you about it and discuss with you whether or not you want to continue. If you decide to withdraw your Oncologist will ensure the continuation of your care off trial. You may be asked, if you decide to continue in the trial, to sign a new consent from.
Voluntary participation and discontinuation
Your participation in this trial is voluntary. If you agree to take part and then change your mind and wish to withdraw, you may do so at any time without this decision affecting your future care. If you decide not to take part your Oncologist will discuss your future care with you. Your legal rights will not be affected by your giving consent to participate.
At the end of the trial your Oncologist will discuss future treatment options. It is not anticipated that patients will be switched routinely to the alternative treatment in the trial.
Confidentiality and patient rights
If you agree to take part in the trial you will need to sign and date the Informed Consent Form attached. Your medical notes will need to be seen by authorised members of our research team so they can collect information needed for this research trial and also to check that it is correct. Your unique registration number will be used to make sure you cannot be identified outside the trial. All information collected about you during the course of the research will be treated as strictly confidential. The confidentiality of your medical records will be respected at all times.
We will continue to contact your hospital in the future to find out how you are getting on. Ideally, we would like to do this for life, but patients often change address or can lose touch with their hospital. If this happens we would still like to be able to collect important basic details (e.g. full name, date of birth, hospital number and NHS number). The Office for National Statistics (ONS) keeps records that can easily provide the information we need, so we would like your permission to ask ONS to pass on this information to us. Any information received in this way remains confidential and is used only for the purposes of that particular trial. Please initial the consent form to indicate you are happy for us to do this. The information that will be collected from ONS will relate only to the status of your disease and current health. The ONS system will not be used to collect information such as your home address.
With your consent your GP will be informed that you wish to take part in a clinical trial. Your GP may be asked to provide information from your records, which are required for the trial.
Anonymised data from the trial may be provided to third parties (e.g. pharmaceutical companies or other academic institutions) for research, safety monitoring or licensing purposes.
Your legal rights will not be affected by agreeing to take part in or withdrawing from the trial. You are free to withdraw from the trial at any time without giving a reason. If you decide to withdraw from the trial, this will not affect the standard of your routine care in any way. Your Oncologist will continue to treat you with the same level of care.
The trial has been reviewed and approved by the South West Multicentre Research Ethics Committee, one of 13 national Research Ethics Committees.
You will be informed of any significant new findings that occur during the trial as this may change your decision to continue.
What if something goes wrong?
You will be closely monitored both during and after therapy and any side effects will be treated as appropriate.
If you are harmed by taking part in this research project, there are no special compensation arrangements. If you are harmed due to someone’s negligence, then you may have grounds for legal action but you may have to pay for legal advice. Regardless of this, if you wish to complain about any aspect of the way you have been approached or treated during the course of this trial, the normal National Health Service complaints mechanisms are available to you.
Results of the trial
At the end of the trial the information collected will be analysed and published in recognised medical journals. Your Oncologist will be informed of any publications and will be able to supply a copy of these publications to you on request. The identity of the patients who took part in the trial will remain confidential. Your Oncologist and trial nurse will also be informed of any results throughout the duration of the trial.
Organisation and funding of the trial
The research trial is being carried out by the Cancer Research UK, School of Cancer Sciences at The University of Birmingham. The research is funded by grants from the Health Technology Assessment (HTA) programme (a governmental funding body which funds clinical research), and pharmaceutical companies Sanofi-aventis and Novartis Pharmaceuticals UK Ltd. During your involvement in this trial no travel costs incurred by you or you family will be paid. Your Oncologist or any other members of staff that are involved in your treatment and care have not been paid for entering you into this clinical trial or receive payment for conducting the trial.
Time to consider
You should take at least 24 hours to decide if you wish to take part.
Who should you contact with questions?
You will be given a copy of this information sheet and the signed consent form to keep. If you have any problems or questions about this trial or your rights as a patient in clinical research you should contact:
Oncologist . . . . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. .. Tel No . . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .
Trial Nurse . . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . . .. Tel No . . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .
24 hour contact number: . . .. . .. . .. . .. . .. . .. . .. . .. . .. . .. . .
The 24 hour contact number can be used out of working hours (9am – 5pm) in the event where you need you contact a hospital doctor immediately.
We would like to thank you for reading the Patient Information Sheet and for considering taking part in this Clinical Trial. If you have any further questions please talk to the trial doctor before considering entry into this clinical trial.
A randomised phase II/III trial of Docetaxel plus Prednisolone vs. Docetaxel plus Prednisolone plus Zoledronic Acid vs. Docetaxel plus Prednisolone plus Strontium-89 vs. Docetaxel plus Prednisolone vs. Docetaxel plus Prednisolone plus Zoledronic Acid plus Strontium-89 in Hormone-refractory Prostate Cancer Metastatic to bone
The TRAPEZE Trial
GP Information Sheet
Your patient has been entered into the TRAPEZE trial for the treatment of their hormone-refractory prostate cancer, which is now metastatic to bone. This is a Phase II/III randomised controlled trial that aims to recruit 618 evaluable patients over 6 years from approximately up to 50 hospitals throughout the UK. The trial will close to recruitment at the end of February 2012. The trial is being coordinated by the CR UK Clinical Trials Unit (CRCTU) at the University of Birmingham. This sheet provides information on the rationale behind the trial and what it will involve for your patient.
Aim of the trial
The treatment of hormone-refractory disease is problematical. There is evidence that cytotoxic chemotherapy can help control symptoms and improve survival. As the disease commonly affects the bones (in around 80% of men) agents targeting bone disease are frequently used. Zoledronic acid has been shown to delay worsening of bone disease and help control pain. There is also laboratory evidence suggesting it may kill prostate cancer cells when combined with chemotherapy.
We therefore wish to examine whether the addition of zoledronic acid to chemotherapy will improve outcomes, including survival, in patients with hormone-refractory disease affecting bone.
Strontium-89 is a radioactive drug which is actively taken up from the blood by bone deposits of the cancer, resulting in targeted radiotherapy. It has also been shown to slow down bone disease and improve pain control. A previous small trial has suggested that there may be benefits from combining Strontium-89 with chemotherapy and we wish to verify or refute this in a larger trial with modern chemotherapy.
Treatment allocation
The details of the trial have been discussed with your patient by their clinician and trial nurse and written informed consent has been obtained. It has been made clear to the patient that they are free to withdraw from the trial at any time without needing to justify their decision and without affecting their future care.
Patients are randomised via computer randomisation at the CRCTU Birmingham, into one of the four possible treatment arms below:
-
Docetaxel 75 mg/m2 as a one hour intravenous infusion every 3 weeks for a maximum of 10 cycles.
-
Docetaxel as above with Zoledronic acid every 3 weeks.
-
Docetaxel as above and Strontium-89 given 28 days after the Cycle 6 docetaxel, as a short intravenous injection. Docetaxel may be continued for up to 10 cycles at his clinician’s discretion, restarting at least one month after the Strontium-89 injection.
-
Docetaxel plus Zoledronic acid and Strontium-89, administered as above. Docetaxel may be continued up to 10 cycles, as above.
Patients are allocated a trial number and been informed as to which of the above treatment arms they have been randomised to.
Possible side effects
Your patient is likely to experience some side-effects associated with the allocated treatment. The more common side-effects are listed below, but there may also be others that we cannot predict.
Docetaxel:
-
Nausea and/or vomiting
-
Myelosuppression – Neutropenia, Thrombocytopenia.
-
Allergy (Anaphylactic and Hypersensitivity reactions)
-
Diarrhoea
-
Stomatitis
-
Peripheral neuropathy
-
Skin toxicity
-
Alopecia
-
Liver toxicity
-
Fluid retention
-
Hyperlacrimation
-
Fatigue
Zoledronic acid treatment arms (b + d):
The patient may experience the same effects as stated for Docetaxel above plus a rise in temperature, and flu-like symptoms, consisting of fever and bone pain due to the zoledronic acid. Long-term use (i.e. > 24 months) of zoledronic acid use has been linked to osteonecrosis of the jaw. This is particularly of concern in patients who have dental disease. Zoledronic acid should be discontinued if a patient requires dental extraction.
Strontium-89 treatment arms (c + d):
The addition of strontium-89 to docetaxel may cause some bone pain lasting 36 to 72 hours following injection. This will be controlled by analgesics. Strontium-89 may also affect marrow reserve. Blood counts will be monitored very closely and strontium-89 will be omitted if there is inadequate marrow reserve.
Your patient has been given a 24 hour contact number to call in the event of suspected neutropenic sepsis, or any other clinical problems arising while on the trial.
The trial will be monitored by an independent data and safety monitoring committee. The committee will advise on whether the trial should be continued in respect to patient safety and results arising from the trial and any other relevant studies.
Quality of life
As part of the TRAPEZE trial, we are also keen to investigate how treatments might affect patients’ quality of life and general well-being. If your patient has agreed to enter this part of the trial they will be asked to complete two questionnaires about their quality of life and general health at each trial visit.
Additional pathology studies
The CRCTU is also interested in biological predictive markers of treatment benefit or toxicity. For this purpose, we have also requested permission from your patient to access a tissue sample from their surgery/biopsy of their prostate, done as part of routine clinical practice. We have also sought consent from your patient for the collection and storage of blood samples taken at intervals during the trial for future proteomic analysis of both known and novel protein markers, using the expertise within the School of Cancer Sciences at the University of Birmingham and other collaborative centres.
Patient confidentiality
All information about your patient will be treated in the strictest of confidence and nothing that may identify them will be revealed to any third parties. Access to your patient’s medical records may be required by authorised members of our research team, to enable them to retrieve or validate information needed for the trial. Unless your patient has given permission for their name to be collected, all research data held centrally at CRCTU about them will be anonymised and identified only by a unique trial number, initials and data of birth.
Contacts
If you have any further questions regarding your patient’s participation in the TRAPEZE trial please do not hesitate to contact your patient’s treating clinician, trial nurse or the TRAPEZE trial office on the numbers below.
| Your patient’s clinician is: | ||
| Contact number: | ||
| 24 hour Hospital switchboard number: | ||
| TRAPEZE Trial Office: | XXXXX | (Mon-Fri 08.00 – 16.00 hrs) |
Appendix 5 Results tables and figures
| Centre | Date of Randomisation | TNO | Randomised treatment |
|---|---|---|---|
| Queen Elizabeth Hospital | 6 January 2006 | 51 | Docetaxel ZA Sr-89 |
| Queen Elizabeth Hospital | 27 January 2006 | 60 | Docetaxel Sr-89 |
| Queen Elizabeth Hospital | 7 March 2006 | 70 | Docetaxel |
| Queen Elizabeth Hospital | 25 August 2006 | 115 | Docetaxel ZA |
| Western Infirmary | 18 December 2006 | 140 | Docetaxel Sr-89 |
| Western General Hospital | 16 August 2007 | 194 | Docetaxel Sr-89 |
| Western General Hospital | 6 February 2008 | 241 | Docetaxel Sr-89 |
| Queen Elizabeth Hospital | 29 September 2008 | 289 | Docetaxel ZA |
| Western General Hospital | 5 August 2009 | 389 | Docetaxel ZA |
| Royal Free Hospital | 9 November 2009 | 422 | Docetaxel ZA |
| Queen Elizabeth Hospital | 11 June 2010 | 477 | Docetaxel ZA |
| Queen Elizabeth Hospital | 7 July 2010 | 485 | Docetaxel ZA |
| Huddersfield Royal Infirmary | 15 July 2010 | 488 | Docetaxel ZA Sr-89 |
| Western General Hospital | 5 October 2010 | 523 | Docetaxel |
| Weston General Hospital | 5 November 2010 | 545 | Docetaxel |
| Royal Derby Hospital | 8 November 2010 | 546 | Docetaxel |
| Royal Derby Hospital | 11 November 2010 | 551 | Docetaxel ZA |
| Aberdeen Royal Infirmary | 19 November 2010 | 557 | Docetaxel ZA Sr-89 |
| Western General Hospital | 30 November 2010 | 561 | Docetaxel ZA |
| Queen Elizabeth Hospital | 21 January 2011 | 579 | Docetaxel ZA |
| Huddersfield Royal Infirmary | 5 April 2011 | 609 | Docetaxel |
| Christie Hospital | 5 April 2011 | 610 | Docetaxel ZA |
| Royal Preston Hospital | 11 May 2011 | 623 | Docetaxel Sr-89 |
| Western General Hospital | 31 August 2011 | 679 | Docetaxel |
| St Mary's Hospital | 4 January 2012 | 725 | Docetaxel ZA |
| Huddersfield Royal Infirmary | 23 January 2012 | 740 | Docetaxel Sr-89 |
| Bradford Royal Infirmary | 2 February 2012 | 743 | Docetaxel ZA |
| Royal Preston Hospital | 13 February 2012 | 748 | Docetaxel ZA |
| Randomisation centre | Docetaxel, n (N = 191) | Docetaxel + ZA, n (N = 188) | Docetaxel + Sr-89, n (N = 190) | Docetaxel + ZA + Sr-89, n (N = 188) | Overall, n (N = 757) |
|---|---|---|---|---|---|
| Aberdeen Royal Infirmary | 5 | 5 | 5 | 6 | 21 |
| Ayr Hospital | 5 | 6 | 5 | 5 | 21 |
| Bradford Royal Infirmary | 3 | 4 | 4 | 2 | 13 |
| Cheltenham General Hospital | 4 | 4 | 4 | 4 | 16 |
| Christie Hospital | 30 | 31 | 30 | 31 | 122 |
| Dorset County Hospital | 2 | 1 | 1 | 1 | 5 |
| Forth Valley Royal Hospital | 2 | 1 | 2 | 1 | 6 |
| Gloucester Royal Hospital | 0 | 0 | 1 | 0 | 1 |
| Huddersfield Royal Infirmary | 2 | 2 | 3 | 2 | 9 |
| Ipswich Hospital | 5 | 4 | 4 | 4 | 17 |
| Maidstone Hospital | 7 | 7 | 7 | 8 | 29 |
| Poole Hospital | 0 | 0 | 0 | 1 | 1 |
| Queen Elizabeth Hospital | 32 | 31 | 32 | 31 | 126 |
| Royal Albert Edward Infirmary | 2 | 1 | 2 | 2 | 7 |
| Royal Bournemouth Hospital | 2 | 1 | 1 | 1 | 5 |
| Royal Derby Hospital | 6 | 6 | 7 | 7 | 26 |
| Royal Free Hospital | 3 | 4 | 2 | 3 | 12 |
| Royal Marsden Hospital London | 1 | 0 | 0 | 0 | 1 |
| Royal Marsden Hospital Sutton | 7 | 8 | 7 | 8 | 30 |
| Royal Preston Hospital | 9 | 9 | 8 | 8 | 34 |
| Southampton General Hospital | 4 | 3 | 4 | 3 | 14 |
| St James’s University Hospital | 7 | 7 | 7 | 6 | 27 |
| Queen Alexandra Hospital | 4 | 5 | 4 | 4 | 17 |
| Velindre Hospital | 1 | 2 | 2 | 2 | 7 |
| Western General Hospital | 28 | 27 | 28 | 28 | 111 |
| Beatson West of Scotland Cancer Centre | 15 | 15 | 15 | 16 | 61 |
| Weston General Hospital | 3 | 3 | 4 | 3 | 13 |
| Wishaw General Hospital | 2 | 1 | 1 | 1 | 5 |
| Centre | Date of randomisation | TNO | Randomised treatment | Reason for ineligibility |
|---|---|---|---|---|
| Queen Elizabeth Hospital | 18 May 2005 | 2 | Docetaxel + ZA + Sr-89 | No proof of progression on study entry. Patient was referred from an external hospital and not all results were here. Please see principal investigator for further information |
| Western General Hospital | 10 November 2005 | 41 | Docetaxel + Sr-89 | Baseline BP not recorded |
| Christie Hospital | 4 January 2006 | 49 | Docetaxel + Sr-89 | 4 January 2006 – PSA progression not demonstrated |
| Western General Hospital | 1 February 2006 | 62 | Docetaxel | Computed tomography and bone scan done after randomisation |
| Western General Hospital | 10 October 2006 | 129 | Docetaxel + ZA | Baseline bloods not within 28 days of randomisation |
| Beatson West of Scotland Cancer Centre | 23 November 2006 | 136 | Docetaxel | Haemoglobin and AST were not within the levels specified for entry – waiver not requested |
| Queen Elizabeth Hospital | 25 January 2007 | 147 | Docetaxel + Sr-89 | Patient GP/urologists stopped Zoladex® (AstraZeneca) prior study entry. We were not informed |
| Western General Hospital | 22 February 2008 | 245 | Docetaxel + ZA | Bicalutamide not stopped before 25 January 2008 and no baseline bloods prior to randomisation |
| Royal Marsden Hospital Sutton | 19 December 2008 | 313 | Docetaxel + ZA + Sr-89 | Inadequate haematological function (Hb < 10 g/dl) |
| Beatson West of Scotland Cancer Centre | 26 February 2009 | 334 | Docetaxel + ZA + Sr-89 | On-study bloods more than 14 days before cycle 1 |
| Wishaw General Hospital | 3 April 2009 | 343 | Docetaxel + ZA | Stop date of bicalutamide not known |
| Royal Marsden Hospital Sutton | 17 April 2009 | 345 | Docetaxel + Sr-89 | Bicalutamide not stopped 4 weeks before treatment start as clinician wanted patient to start chemotherapy earlier as high risk of developing cord compression |
| St James’s University Hospital | 21 April 2009 | 346 | Docetaxel + Sr-89 | On-study bloods not within 28-day time frame |
| Dorset County Hospital | 15 July 2009 | 381 | Docetaxel | Patient did not discontinue bicalutamide until 7 July 2009 – discussed/agreed by Darren Barton, TC |
| Western General Hospital | 5aug2009 | 389 | Docetaxel + ZA | Haematology not within 28 days |
| Christie Hospital | 20 August 2009 | 394 | Docetaxel + ZA | Patients PSA was only 4.4 on randomisation and there is no evidence of new bone mets. His PSA on his first treatment cycle was 6.5 |
| Maidstone Hospital | 12 January 2010 | 438 | Docetaxel + ZA | BP not done at baseline |
| Western General Hospital | 7 July 2010 | 486 | Docetaxel + Sr-89 | Baseline BP not recorded in error |
| Bradford Royal Infirmary | 13 October 2010 | 531 | Docetaxel + ZA | Prior estramustine therapy – randomised 5 days short of 4 weeks |
| St James’s University Hospital | 9 November 2010 | 547 | Docetaxel + ZA | No chest scans at baseline |
| Huddersfield Royal Infirmary | 17 November 2010 | 556 | Docetaxel + Sr-89 | Patient randomised too early in error so bicalutamide date is before 4 weeks; treatment did not commence until after 4 weeks |
| Huddersfield Royal Infirmary | 29 November 2010 | 559 | Docetaxel | BP not done at baseline |
| Queen Alexandra Hospital | 21 December 2010 | 569 | Docetaxel | Not 4 weeks between stopping bicalutamide – was 4 weeks before starting treatment |
| Dorset County Hospital | 16 February 2011 | 589 | Docetaxel + ZA | Baseline bloods taken 17 January 2011, randomised 16 February 2011, outside 28-day time-period |
| Royal Derby Hospital | 4 April 2011 | 607 | Docetaxel + ZA | Bicalutamide stop date not 4 weeks |
| Royal Marsden Hospital Sutton | 23 June 2011 | 643 | Docetaxel + ZA + Sr-89 | Patient had dental extractions before first cycle meaning they were unfit to receive ZA until cycle 4 |
| Christie Hospital | 15 February 2012 | 749 | Docetaxel + ZA + Sr-89 | Still taking a bisphosphonate |
| Sequence | Docetaxel (N = 191) | Docetaxel + ZA (N = 188) | Docetaxel + Sr-89 (N = 190) | Docetaxel + ZA + Sr-89 (N = 188) | Overall (N = 757) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| 0 | 5 | 2.6 | 8 | 4.3 | 9 | 4.7 | 6 | 3.2 | 28 | 3.7 |
| 1 | 7 | 3.7 | 13 | 6.9 | 6 | 3.2 | 11 | 5.9 | 37 | 4.9 |
| 1–2 | 9 | 4.7 | 4 | 2.1 | 9 | 4.7 | 5 | 2.7 | 27 | 3.6 |
| 1–2–3 | 12 | 6.3 | 7 | 3.7 | 14 | 7.4 | 9 | 4.8 | 42 | 5.5 |
| 1–2–3–4 | 10 | 5.2 | 8 | 4.3 | 8 | 4.2 | 9 | 4.8 | 35 | 4.6 |
| 1–2–3–4–5 | 20 | 10.5 | 14 | 7.4 | 14 | 7.4 | 9 | 4.8 | 57 | 7.5 |
| 1–2–3–4–5–6 | 83 | 43.5 | 77 | 41 | 81 | 42.6 | 88 | 46.8 | 329 | 43.5 |
| 1–2–3–4–5–6–7 | 7 | 3.7 | 4 | 2.1 | 5 | 2.6 | 7 | 3.7 | 23 | 3 |
| 1–2–3–4–5–6–7–8 | 4 | 2.1 | 10 | 5.3 | 4 | 2.1 | 5 | 2.7 | 23 | 3 |
| 1–2–3–4–5–6–7–8–9 | 8 | 4.2 | 8 | 4.3 | 5 | 2.6 | 4 | 2.1 | 25 | 3.3 |
| 1–2–3–4–5–6–7–8–9–10 | 26 | 13.6 | 33 | 17.6 | 35 | 18.4 | 33 | 17.6 | 127 | 16.8 |
| 1–2–3–4–5–7–8 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 | 1 | 0.1 |
| 1–2–3–4–5–7–8–10 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 | 1 | 0.1 |
| 1–2–3–4–5–7–8–9 | 0 | 0 | 1 | 0.5 | 0 | 0 | 0 | 0 | 1 | 0.1 |
| 1–2–3–4–5–7–8–9–10 | 0 | 0 | 1 | 0.5 | 0 | 0 | 0 | 0 | 1 | 0.1 |
| Total | 191 | 100.0 | 188 | 100.0 | 190 | 100.0 | 188 | 100.0 | 757 | 100.0 |
| Sequence | No ZA (N = 381) | ZA (N = 376) | No Sr-89 (N = 379) | Sr-89 (N = 378) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| 0 | 14 | 3.7 | 14 | 3.7 | 13 | 3.4 | 15 | 4 |
| 1 | 13 | 3.4 | 24 | 6.4 | 20 | 5.3 | 17 | 4.5 |
| 1–2 | 18 | 4.7 | 9 | 2.4 | 13 | 3.4 | 14 | 3.7 |
| 1–2–3 | 26 | 6.8 | 16 | 4.3 | 19 | 5 | 23 | 6.1 |
| 1–2–3–4 | 18 | 4.7 | 17 | 4.5 | 18 | 4.7 | 17 | 4.5 |
| 1–2–3–4–5 | 34 | 8.9 | 23 | 6.1 | 34 | 9 | 23 | 6.1 |
| 1–2–3–4–5–6 | 164 | 43 | 165 | 43.9 | 160 | 42.2 | 169 | 44.7 |
| 1–2–3–4–5–6–7 | 12 | 3.1 | 11 | 2.9 | 11 | 2.9 | 12 | 3.2 |
| 1–2–3–4–5–6–7–8 | 8 | 2.1 | 15 | 4 | 14 | 3.7 | 9 | 2.4 |
| 1–2–3–4–5–6–7–8–9 | 13 | 3.4 | 12 | 3.2 | 16 | 4.2 | 9 | 2.4 |
| 1–2–3–4–5–6–7–8–9–10 | 61 | 16 | 66 | 17.6 | 59 | 15.6 | 68 | 18 |
| 1–2–3–4–5–7–8 | 0 | 0 | 1 | 0.3 | 0 | 0 | 1 | 0.3 |
| 1–2–3–4–5–7–8–10 | 0 | 0 | 1 | 0.3 | 0 | 0 | 1 | 0.3 |
| 1–2–3–4–5–7–8–9 | 0 | 0 | 1 | 0.3 | 1 | 0.3 | 0 | 0 |
| 1–2–3–4–5–7–8–9–10 | 0 | 0 | 1 | 0.3 | 1 | 0.3 | 0 | 0 |
| Total | 381 | 100 | 376 | 100 | 379 | 100 | 378 | 100 |
| Concomitant medications | Arm | Patients | % | On-study | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|---|
| Antacids and simeticone (GI) | Docetaxel | 7 | 0.92 | 2 | 0.26 | 4 | 0.53 | 2 | 0.29 |
| Docetaxel + ZA | 6 | 0.79 | 2 | 0.26 | 4 | 0.53 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 8 | 1.06 | 1 | 0.13 | 6 | 0.79 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 8 | 1.06 | 1 | 0.13 | 5 | 0.66 | 2 | 0.29 | |
| Antispasmodics | Docetaxel | 6 | 0.79 | 2 | 0.26 | 3 | 0.40 | 1 | 0.14 |
| Docetaxel + ZA | 2 | 0.26 | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 2 | 0.26 | 0 | 0.00 | 2 | 0.26 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 3 | 0.40 | 2 | 0.26 | 1 | 0.13 | 0 | 0.00 | |
| H2 antagonist and ulcer healing (GI) | Docetaxel | 6 | 0.79 | 4 | 0.53 | 0 | 0.00 | 1 | 0.14 |
| Docetaxel + ZA | 4 | 0.53 | 3 | 0.40 | 1 | 0.13 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 2 | 0.26 | 2 | 0.26 | 1 | 0.13 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 10 | 1.32 | 8 | 1.06 | 1 | 0.13 | 1 | 0.14 | |
| Proton pump inhibitors (GI) | Docetaxel | 82 | 10.83 | 57 | 7.53 | 26 | 3.43 | 5 | 0.72 |
| Docetaxel + ZA | 84 | 11.10 | 59 | 7.79 | 23 | 3.04 | 4 | 0.57 | |
| Docetaxel + Sr-89 | 75 | 9.91 | 46 | 6.08 | 26 | 3.43 | 8 | 1.15 | |
| Docetaxel + ZA + Sr-89 | 74 | 9.78 | 53 | 7.00 | 24 | 3.17 | 4 | 0.57 | |
| Antimotility, antidiarrhoea (GI) | Docetaxel | 21 | 2.77 | 1 | 0.13 | 18 | 2.38 | 2 | 0.29 |
| Docetaxel + ZA | 10 | 1.32 | 2 | 0.26 | 7 | 0.92 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 15 | 1.98 | 1 | 0.13 | 13 | 1.72 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 6 | 0.79 | 1 | 0.13 | 4 | 0.53 | 1 | 0.14 | |
| Laxatives (GI) | Docetaxel | 39 | 5.15 | 25 | 3.30 | 13 | 1.72 | 5 | 0.72 |
| Docetaxel + ZA | 58 | 7.66 | 42 | 5.55 | 17 | 2.25 | 3 | 0.43 | |
| Docetaxel + Sr-89 | 49 | 6.47 | 25 | 3.30 | 17 | 2.25 | 9 | 1.29 | |
| Docetaxel + ZA + Sr-89 | 49 | 6.47 | 29 | 3.83 | 17 | 2.25 | 7 | 1.01 | |
| Cardiac glycoside (cardiac) | Docetaxel | 2 | 0.26 | 2 | 0.26 | 1 | 0.13 | 0 | 0.00 |
| Docetaxel + ZA | 13 | 1.72 | 12 | 1.59 | 2 | 0.26 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 2 | 0.26 | 2 | 0.26 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 6 | 0.79 | 5 | 0.66 | 0 | 0.00 | 0 | 0.00 | |
| Diuretics (cardiac) | Docetaxel | 38 | 5.02 | 26 | 3.43 | 12 | 1.59 | 4 | 0.57 |
| Docetaxel + ZA | 43 | 5.68 | 32 | 4.23 | 14 | 1.85 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 35 | 4.62 | 26 | 3.43 | 8 | 1.06 | 5 | 0.72 | |
| Docetaxel + ZA + Sr-89 | 36 | 4.76 | 28 | 3.70 | 10 | 1.32 | 0 | 0.00 | |
| Drugs for arrhythmias (cardiac) | Docetaxel | 4 | 0.53 | 1 | 0.13 | 2 | 0.26 | 0 | 0.00 |
| Docetaxel + ZA | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 4 | 0.53 | 1 | 0.13 | 3 | 0.40 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 1 | 0.13 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 | |
| Beta-blockers (cardiac) | Docetaxel | 19 | 2.51 | 16 | 2.11 | 1 | 0.13 | 1 | 0.14 |
| Docetaxel + ZA | 28 | 3.70 | 26 | 3.43 | 1 | 0.13 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 28 | 3.70 | 27 | 3.57 | 2 | 0.26 | 1 | 0.14 | |
| Docetaxel + ZA + Sr-89 | 34 | 4.49 | 29 | 3.83 | 6 | 0.79 | 0 | 0.00 | |
| Calcium-channel blockers (cardiac) | Docetaxel | 26 | 3.43 | 23 | 3.04 | 4 | 0.53 | 0 | 0.00 |
| Docetaxel + ZA | 25 | 3.30 | 23 | 3.04 | 1 | 0.13 | 1 | 0.14 | |
| Docetaxel + Sr-89 | 27 | 3.57 | 25 | 3.30 | 5 | 0.66 | 1 | 0.14 | |
| Docetaxel + ZA + Sr-89 | 27 | 3.57 | 26 | 3.43 | 2 | 0.26 | 0 | 0.00 | |
| Cardiac – other | Docetaxel | 14 | 1.85 | 11 | 1.45 | 3 | 0.40 | 0 | 0.00 |
| Docetaxel + ZA | 23 | 3.04 | 23 | 3.04 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 18 | 2.38 | 18 2. | 38 | 1 | 0.13 | 1 | 0.14 | |
| Docetaxel + ZA + Sr-89 | 23 | 3.04 | 22 | 2.91 | 1 | 0.13 | 0 | 0.00 | |
| Anticoagulants antiplatelet drugs | Docetaxel | 69 | 9.11 | 45 | 5.94 | 27 | 3.57 | 5 | 0.72 |
| Docetaxel + ZA | 66 | 8.72 | 43 | 5.68 | 26 | 3.43 | 4 | 0.57 | |
| Docetaxel + Sr-89 | 69 | 9.11 | 45 | 5.94 | 21 | 2.77 | 11 | 1.58 | |
| Docetaxel + ZA + Sr-89 | 66 | 8.72 | 45 | 5.94 | 27 | 3.57 | 5 | 0.72 | |
| Blood bone marrow disorders | Docetaxel | 35 | 4.62 | 8 | 1.06 | 24 | 3.17 | 5 | 0.72 |
| Docetaxel + ZA | 27 | 3.57 | 3 | 0.40 | 21 | 2.77 | 6 | 0.86 | |
| Docetaxel + Sr-89 | 31 | 4.10 | 3 | 0.40 | 20 | 2.64 | 6 | 0.86 | |
| Docetaxel + ZA + Sr-89 | 27 | 3.57 | 3 | 0.40 | 19 | 2.51 | 5 | 0.72 | |
| Bronchodilators antihistamines | Docetaxel | 25 | 3.30 | 11 | 1.45 | 13 | 1.72 | 3 | 0.43 |
| Docetaxel + ZA | 28 | 3.70 | 10 | 1.32 | 15 | 1.98 | 2 | 0.29 | |
| Docetaxel + Sr-89 | 28 | 3.70 | 15 | 1.98 | 12 | 1.59 | 6 | 0.86 | |
| Docetaxel + ZA + Sr-89 | 30 | 3.96 | 14 | 1.85 | 15 | 1.98 | 4 | 0.57 | |
| Hypnotics (CNS) | Docetaxel | 11 | 1.45 | 6 | 0.79 | 3 | 0.40 | 2 | 0.29 |
| Docetaxel + ZA | 6 | 0.79 | 2 | 0.26 | 4 | 0.53 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 11 | 1.45 | 8 | 1.06 | 2 | 0.26 | 1 | 0.14 | |
| Docetaxel + ZA + Sr-89 | 9 | 1.19 | 4 | 0.53 | 3 | 0.40 | 2 | 0.29 | |
| Anxiolytics (CNS) | Docetaxel | 4 | 0.53 | 2 | 0.26 | 0 | 0.00 | 2 | 0.29 |
| Docetaxel + ZA | 3 | 0.40 | 1 | 0.13 | 1 | 0.13 | 1 | 0.14 | |
| Docetaxel + Sr-89 | 4 | 0.53 | 1 | 0.13 | 3 | 0.40 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 4 | 0.53 | 1 | 0.13 | 3 | 0.40 | 0 | 0.00 | |
| Antiemetics | Docetaxel | 62 | 8.19 | 13 | 1.72 | 51 | 6.74 | 7 | 1.01 |
| Docetaxel + ZA | 71 | 9.38 | 15 | 1.98 | 54 | 7.13 | 12 | 1.72 | |
| Docetaxel + Sr-89 | 59 | 7.79 | 7 | 0.92 | 44 | 5.81 | 11 | 1.58 | |
| Docetaxel + ZA + Sr-89 | 65 | 8.59 | 13 | 1.72 | 50 | 6.61 | 16 | 2.30 | |
| Central nervous – other | Docetaxel | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 |
| Antibacterials, antifungals and antiviral | Docetaxel | 103 | 13.61 | 14 | 1.85 | 94 | 12.42 | 15 | 2.16 |
| Docetaxel + ZA | 91 | 12.02 | 10 | 1.32 | 82 | 10.83 | 13 | 1.87 | |
| Docetaxel + Sr-89 | 98 | 12.95 | 15 | 1.98 | 84 | 11.10 | 13 | 1.87 | |
| Docetaxel + ZA + Sr-89 | 80 | 10.57 | 10 | 1.32 | 68 | 8.98 | 9 | 1.29 | |
| Diabetes drugs | Docetaxel | 14 | 1.85 | 12 | 1.59 | 2 | 0.26 | 0 | 0.00 |
| Docetaxel + ZA | 15 | 1.98 | 13 | 1.72 | 3 | 0.40 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 15 | 1.98 | 8 | 1.06 | 7 | 0.92 | 1 | 0.14 | |
| Docetaxel + ZA + Sr-89 | 16 | 2.11 | 14 | 1.85 | 1 | 0.13 | 1 | 0.14 | |
| Nitrate | Docetaxel | 13 | 1.72 | 11 | 1.45 | 2 | 0.26 | 1 | 0.14 |
| Docetaxel + ZA | 15 | 1.98 | 14 | 1.85 | 2 | 0.26 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 17 | 2.25 | 13 | 1.72 | 3 | 0.40 | 1 | 0.14 | |
| Docetaxel + ZA + Sr-89 | 11 | 1.45 | 9 | 1.19 | 2 | 0.26 | 0 | 0.00 | |
| ACE inhibitor | Docetaxel | 34 | 4.49 | 29 | 3.83 | 5 | 0.66 | 1 | 0.14 |
| Docetaxel + ZA | 27 | 3.57 | 23 | 3.04 | 4 | 0.53 | 1 | 0.14 | |
| Docetaxel + Sr-89 | 25 | 3.30 | 23 | 3.04 | 2 | 0.26 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 36 | 4.76 | 34 | 4.49 | 6 | 0.79 | 1 | 0.14 | |
| Chemotherapy | Docetaxel | 33 | 4.36 | 0 | 0.00 | 17 | 2.25 | 17 | 2.44 |
| Docetaxel + ZA | 31 | 4.10 | 0 | 0.00 | 17 | 2.25 | 17 | 2.44 | |
| Docetaxel + Sr-89 | 36 | 4.76 | 0 | 0.00 | 17 | 2.25 | 20 | 2.87 | |
| Docetaxel + ZA + Sr-89 | 43 | 5.68 | 0 | 0.00 | 18 | 2.38 | 25 | 3.59 | |
| Statin | Docetaxel | 46 | 6.08 | 40 | 5.28 | 7 | 0.92 | 0 | 0.00 |
| Docetaxel + ZA | 47 | 6.21 | 44 | 5.81 | 3 | 0.40 | 1 | 0.14 | |
| Docetaxel + Sr-89 | 51 | 6.74 | 49 | 6.47 | 6 | 0.79 | 1 | 0.14 | |
| Docetaxel + ZA + Sr-89 | 47 | 6.21 | 45 | 5.94 | 3 | 0.40 | 1 | 0.14 | |
| Antidepressant | Docetaxel | 22 | 2.91 | 18 | 2.38 | 5 | 0.66 | 1 | 0.14 |
| Docetaxel + ZA | 13 | 1.72 | 8 | 1.06 | 4 | 0.53 | 1 | 0.14 | |
| Docetaxel + Sr-89 | 20 | 2.64 | 17 | 2.25 | 4 | 0.53 | 1 | 0.14 | |
| Docetaxel + ZA + Sr-89 | 16 | 2.11 | 11 | 1.45 | 1 | 0.13 | 4 | 0.57 | |
| Alpha blocker | Docetaxel | 24 | 3.17 | 19 | 2.51 | 6 | 0.79 | 2 | 0.29 |
| Docetaxel + ZA | 19 | 2.51 | 15 | 1.98 | 4 | 0.53 | 1 | 0.14 | |
| Docetaxel + Sr-89 | 30 | 3.96 | 26 | 3.43 | 9 | 1.19 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 27 | 3.57 | 22 | 2.91 | 11 | 1.45 | 0 | 0.00 | |
| COX-2 selective inhibitor | Docetaxel | 1 | 0.13 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 |
| Steroid | Docetaxel | 89 | 11.76 | 19 | 2.51 | 59 | 7.79 | 31 | 4.45 |
| Docetaxel + ZA | 88 | 11.62 | 18 | 2.38 | 59 | 7.79 | 21 | 3.02 | |
| Docetaxel + Sr-89 | 75 | 9.91 | 14 | 1.85 | 45 | 5.94 | 29 | 4.17 | |
| Docetaxel + ZA + Sr-89 | 77 | 10.17 | 23 | 3.04 | 53 | 7.00 | 27 | 3.88 | |
| Supplement | Docetaxel | 23 | 3.04 | 15 | 1.98 | 6 | 0.79 | 1 | 0.14 |
| Docetaxel + ZA | 51 | 6.74 | 13 | 1.72 | 36 | 4.76 | 4 | 0.57 | |
| Docetaxel + Sr-89 | 21 | 2.77 | 10 | 1.32 | 10 | 1.32 | 5 | 0.72 | |
| Enzyme inhibitor | Docetaxel + ZA | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| Immunosuppressive | Docetaxel + ZA | 1 | 0.13 | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 |
| Docetaxel + ZA + Sr-89 | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | |
| Radiotherapy | Docetaxel | 87 | 11.49 | 0 | 0.00 | 5 | 0.66 | 82 | 11.78 |
| Docetaxel + ZA | 75 | 9.91 | 0 | 0.00 | 1 | 0.13 | 74 | 10.63 | |
| Docetaxel + Sr-89 | 78 | 10.30 | 0 | 0.00 | 2 | 0.26 | 75 | 10.78 | |
| Docetaxel + ZA + Sr-89 | 75 | 9.91 | 0 | 0.00 | 3 | 0.40 | 73 | 10.49 | |
| Bisphosphonate | Docetaxel | 42 | 5.55 | 2 | 0.26 | 11 | 1.45 | 31 | 4.45 |
| Docetaxel + ZA | 40 | 5.28 | 0 | 0.00 | 26 | 3.43 | 24 | 3.45 | |
| Docetaxel + Sr-89 | 28 | 3.70 | 2 | 0.26 | 8 | 1.06 | 19 | 2.73 | |
| Docetaxel + ZA + Sr-89 | 36 | 4.76 | 1 | 0.13 | 22 | 2.91 | 17 | 2.44 | |
| Hormone therapy | Docetaxel | 66 | 8.72 | 12 | 1.59 | 34 | 4.49 | 32 | 4.60 |
| Docetaxel + ZA | 62 | 8.19 | 15 | 1.98 | 33 | 4.36 | 28 | 4.02 | |
| Docetaxel + Sr-89 | 68 | 8.98 | 14 | 1.85 | 33 | 4.36 | 31 | 4.45 | |
| Docetaxel + ZA + Sr-89 | 67 | 8.85 | 19 | 2.51 | 36 | 4.76 | 23 | 3.30 | |
| NSAID | Docetaxel | 18 | 2.38 | 10 | 1.32 | 7 | 0.92 | 1 | 0.14 |
| Docetaxel + ZA | 12 | 1.59 | 3 | 0.40 | 8 | 1.06 | 1 | 0.14 | |
| Docetaxel + Sr-89 | 14 | 1.85 | 3 | 0.40 | 6 | 0.79 | 1 | 0.14 | |
| Docetaxel + ZA + Sr-89 | 12 | 1.59 | 3 | 0.40 | 6 | 0.79 | 2 | 0.29 | |
| ARB | Docetaxel | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Docetaxel + ZA | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 2 | 0.26 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Radioisotope | Docetaxel | 11 | 1.45 | 0 | 0.00 | 0 | 0.00 | 11 | 1.58 |
| Docetaxel + ZA | 11 | 1.45 | 0 | 0.00 | 1 | 0.13 | 10 | 1.44 | |
| Docetaxel + Sr-89 | 11 | 1.45 | 1 | 0.13 | 3 | 0.40 | 8 | 1.15 | |
| Docetaxel + ZA + Sr-89 | 8 | 1.06 | 0 | 0.00 | 3 | 0.40 | 5 | 0.72 | |
| GCSF | Docetaxel | 9 | 1.19 | 0 | 0.00 | 7 | 0.92 | 1 | 0.14 |
| Docetaxel + ZA | 6 | 0.79 | 0 | 0.00 | 6 | 0.79 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 5 | 0.66 | 0 | 0.00 | 5 | 0.66 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 5 | 0.66 | 0 | 0.00 | 4 | 0.53 | 0 | 0.00 | |
| i.v. fluids for rehydration | Docetaxel + ZA | 1 | 0.13 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 |
| Docetaxel + Sr-89 | 2 | 0.26 | 0 | 0.00 | 2 | 0.26 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 2 | 0.26 | 0 | 0.00 | 2 | 0.26 | 0 | 0.00 | |
| Treatment for glaucoma | Docetaxel | 2 | 0.26 | 0 | 0.00 | 2 | 0.26 | 0 | 0.00 |
| Docetaxel + ZA | 3 | 0.40 | 2 | 0.26 | 1 | 0.13 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 5 | 0.66 | 4 | 0.53 | 2 | 0.26 | 0 | 0.00 | |
| Topical anti-inflammatory | Docetaxel | 2 | 0.26 | 0 | 0.00 | 2 | 0.26 | 0 | 0.00 |
| Docetaxel + ZA | 5 | 0.66 | 2 | 0.26 | 3 | 0.40 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 | |
| Anticonvulsant | Docetaxel | 4 | 0.53 | 3 | 0.40 | 1 | 0.13 | 0 | 0.00 |
| Docetaxel + ZA | 5 | 0.66 | 2 | 0.26 | 2 | 0.26 | 2 | 0.29 | |
| Docetaxel + Sr-89 | 10 | 1.32 | 4 | 0.53 | 3 | 0.40 | 3 | 0.43 | |
| Docetaxel + ZA + Sr-89 | 4 | 0.53 | 2 | 0.26 | 0 | 0.00 | 2 | 0.29 | |
| Other | Docetaxel | 32 | 4.23 | 10 | 1.32 | 20 | 2.64 | 3 | 0.43 |
| Docetaxel + ZA | 47 | 6.21 | 16 | 2.11 | 26 | 3.43 | 7 | 1.01 | |
| Docetaxel + Sr-89 | 46 | 6.08 | 14 | 1.85 | 20 | 2.64 | 9 | 1.29 | |
| Docetaxel + ZA + Sr-89 | 40 | 5.28 | 18 | 2.38 | 24 | 3.17 | 3 | 0.43 |
| Concomitant medications | Arm | Instances | On-study | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|
| Antacids and simeticone (GI) | Docetaxel | 9 | 2 | 22.22 | 5 | 55.56 | 2 | 22.22 |
| Docetaxel + ZA | 8 | 2 | 25.00 | 6 | 75.00 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 8 | 1 | 12.50 | 6 | 75.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 21 | 1 | 4.76 | 13 | 61.90 | 7 | 33.33 | |
| Antispasmodics | Docetaxel | 6 | 2 | 33.33 | 3 | 50.00 | 1 | 16.67 |
| Docetaxel + ZA | 2 | 1 | 50.00 | 1 | 50.00 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 6 | 0 | 0.00 | 6 | 100.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 3 | 2 | 66.67 | 1 | 33.33 | 0 | 0.00 | |
| H2 antagonist and ulcer healing (GI) | Docetaxel | 11 | 4 | 36.36 | 0 | 0.00 | 6 | 54.55 |
| Docetaxel + ZA | 4 | 3 | 75.00 | 1 | 25.00 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 3 | 2 | 66.67 | 1 | 33.33 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 11 | 8 | 72.73 | 1 | 9.09 | 1 | 9.09 | |
| Proton pump inhibitors (GI) | Docetaxel | 96 | 58 | 60.42 | 31 | 32.29 | 5 | 5.21 |
| Docetaxel + ZA | 98 | 60 | 61.22 | 25 | 25.51 | 5 | 5.10 | |
| Docetaxel + Sr-89 | 98 | 48 | 48.98 | 34 | 34.69 | 9 | 9.18 | |
| Docetaxel + ZA + Sr-89 | 90 | 54 | 60.00 | 28 | 31.11 | 4 | 4.44 | |
| Antimotility, antidiarrhoea (GI) | Docetaxel | 27 | 1 | 3.70 | 24 | 88.89 | 2 | 7.41 |
| Docetaxel + ZA | 11 | 2 | 18.18 | 8 | 72.73 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 16 | 1 | 6.25 | 14 | 87.50 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 7 | 1 | 14.29 | 5 | 71.43 | 1 | 14.29 | |
| Laxatives (GI) | Docetaxel | 58 | 32 | 55.17 | 15 | 25.86 | 9 | 15.52 |
| Docetaxel + ZA | 92 | 51 | 55.43 | 30 | 32.61 | 3 | 3.26 | |
| Docetaxel + Sr-89 | 98 | 32 | 32.65 | 42 | 42.86 | 17 | 17.35 | |
| Docetaxel + ZA + Sr-89 | 77 | 37 | 48.05 | 28 | 36.36 | 8 | 10.39 | |
| Cardiac glycoside (cardiac) | Docetaxel | 3 | 2 | 66.67 | 1 | 33.33 | 0 | 0.00 |
| Docetaxel + ZA | 14 | 12 | 85.71 | 2 | 14.29 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 2 | 2 | 100.00 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 6 | 5 | 83.33 | 0 | 0.00 | 0 | 0.00 | |
| Diuretics (cardiac) | Docetaxel | 48 | 27 | 56.25 | 15 | 31.25 | 4 | 8.33 |
| Docetaxel + ZA | 56 | 34 | 60.71 | 19 | 33.93 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 55 | 28 | 50.91 | 19 | 34.55 | 5 | 9.09 | |
| Docetaxel + ZA + Sr-89 | 43 | 29 | 67.44 | 12 | 27.91 | 0 | 0.00 | |
| Drugs for arrhythmias (cardiac) | Docetaxel | 4 | 1 | 25.00 | 2 | 50.00 | 0 | 0.00 |
| Docetaxel + ZA | 1 | 1 | 100.00 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 7 | 2 | 28.57 | 5 | 71.43 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 3 | 0 | 0.00 | 3 | 100.00 | 0 | 0.00 | |
| Beta-blockers (cardiac) | Docetaxel | 20 | 16 | 80.00 | 1 | 5.00 | 1 | 5.00 |
| Docetaxel + ZA | 32 | 27 | 84.38 | 1 | 3.13 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 30 | 27 | 90.00 | 2 | 6.67 | 1 | 3.33 | |
| Docetaxel + ZA + Sr-89 | 38 | 30 | 78.95 | 6 | 15.79 | 0 | 0.00 | |
| Calcium – channel blockers (cardiac) | Docetaxel | 27 | 23 | 85.19 | 4 | 14.81 | 0 | 0.00 |
| Docetaxel + ZA | 28 | 23 | 82.14 | 1 | 3.57 | 1 | 3.57 | |
| Docetaxel + Sr-89 | 34 | 26 | 76.47 | 7 | 20.59 | 1 | 2.94 | |
| Docetaxel + ZA + Sr-89 | 28 | 26 | 92.86 | 2 | 7.14 | 0 | 0.00 | |
| Cardiac – other | Docetaxel | 15 | 11 | 73.33 | 3 | 20.00 | 0 | 0.00 |
| Docetaxel + ZA | 26 | 25 | 96.15 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 20 | 19 | 95.00 | 1 | 5.00 | 1 | 5.00 | |
| Docetaxel + ZA + Sr-89 | 24 | 23 | 95.83 | 1 | 4.17 | 0 | 0.00 | |
| Anticoagulants antiplatelet drugs | Docetaxel | 99 | 46 | 46.46 | 42 | 42.42 | 8 | 8.08 |
| Docetaxel + ZA | 103 | 44 | 42.72 | 48 | 46.60 | 4 | 3.88 | |
| Docetaxel + Sr-89 | 110 | 49 | 44.55 | 37 | 33.64 | 21 | 19.09 | |
| Docetaxel + ZA + Sr-89 | 98 | 49 | 50.00 | 41 | 41.84 | 6 | 6.12 | |
| Blood bone marrow disorders | Docetaxel | 84 | 10 | 11.90 | 58 | 69.05 | 14 | 16.67 |
| Docetaxel + ZA | 53 | 3 | 5.66 | 36 | 67.92 | 13 | 24.53 | |
| Docetaxel + Sr-89 | 56 | 3 | 5.36 | 41 | 73.21 | 10 | 17.86 | |
| Docetaxel + ZA + Sr-89 | 63 | 3 | 4.76 | 38 | 60.32 | 17 | 26.98 | |
| Bronchodilators antihistamines | Docetaxel | 48 | 15 | 31.25 | 23 | 47.92 | 8 | 16.67 |
| Docetaxel + ZA | 42 | 11 | 26.19 | 26 | 61.90 | 3 | 7.14 | |
| Docetaxel + Sr-89 | 53 | 22 | 41.51 | 22 | 41.51 | 6 | 11.32 | |
| Docetaxel + ZA + Sr-89 | 59 | 16 | 27.12 | 29 | 49.15 | 14 | 23.73 | |
| Hypnotics (CNS) | Docetaxel | 13 | 6 | 46.15 | 3 | 23.08 | 2 | 15.38 |
| Docetaxel + ZA | 6 | 2 | 33.33 | 4 | 66.67 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 15 | 8 | 53.33 | 4 | 26.67 | 1 | 6.67 | |
| Docetaxel + ZA + Sr-89 | 11 | 4 | 36.36 | 5 | 45.45 | 2 | 18.18 | |
| Anxiolytics (CNS) | Docetaxel | 5 | 2 | 40.00 | 0 | 0.00 | 3 | 60.00 |
| Docetaxel + ZA | 3 | 1 | 33.33 | 1 | 33.33 | 1 | 33.33 | |
| Docetaxel + Sr-89 | 4 | 1 | 25.00 | 3 | 75.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 4 | 1 | 25.00 | 3 | 75.00 | 0 | 0.00 | |
| Antiemetics | Docetaxel | 351 | 17 | 4.84 | 312 | 88.89 | 20 | 5.70 |
| Docetaxel + ZA | 367 | 15 | 4.09 | 320 | 87.19 | 28 | 7.63 | |
| Docetaxel + Sr-89 | 290 | 7 | 2.41 | 261 | 90.00 | 21 | 7.24 | |
| Docetaxel + ZA + Sr-89 | 335 | 14 | 4.18 | 269 | 80.30 | 52 | 15.52 | |
| Central nervous – other | Docetaxel | 1 | 1 | 100.00 | 0 | 0.00 | 0 | 0.00 |
| Antibacterials, antifungals and antiviral | Docetaxel | 297 | 17 | 5.72 | 242 | 81.48 | 32 | 10.77 |
| Docetaxel + ZA | 292 | 13 | 4.45 | 235 | 80.48 | 37 | 12.67 | |
| Docetaxel + Sr-89 | 349 | 16 | 4.58 | 290 | 83.09 | 40 | 11.46 | |
| Docetaxel + ZA + Sr-89 | 238 | 12 | 5.04 | 203 | 85.29 | 16 | 6.72 | |
| Diabetes drugs | Docetaxel | 20 | 18 | 90.00 | 2 | 10.00 | 0 | 0.00 |
| Docetaxel + ZA | 23 | 19 | 82.61 | 3 | 13.04 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 22 | 10 | 45.45 | 10 | 45.45 | 1 | 4.55 | |
| Docetaxel + ZA + Sr-89 | 22 | 20 | 90.91 | 1 | 4.55 | 1 | 4.55 | |
| Nitrate | Docetaxel | 16 | 13 | 81.25 | 2 | 12.50 | 1 | 6.25 |
| Docetaxel + ZA | 18 | 14 | 77.78 | 3 | 16.67 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 22 | 14 | 63.64 | 3 | 13.64 | 1 | 4.55 | |
| Docetaxel + ZA + Sr-89 | 12 | 9 | 75.00 | 2 | 16.67 | 0 | 0.00 | |
| ACE inhibitor | Docetaxel | 37 | 29 | 78.38 | 6 | 16.22 | 1 | 2.70 |
| Docetaxel + ZA | 31 | 23 | 74.19 | 5 | 16.13 | 1 | 3.23 | |
| Docetaxel + Sr-89 | 33 | 24 | 72.73 | 6 | 18.18 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 45 | 34 | 75.56 | 7 | 15.56 | 1 | 2.22 | |
| Chemotherapy | Docetaxel | 76 | 0 | 0.00 | 30 | 39.47 | 46 | 60.53 |
| Docetaxel + ZA | 102 | 0 | 0.00 | 31 | 30.39 | 71 | 69.61 | |
| Docetaxel + Sr-89 | 110 | 0 | 0.00 | 33 | 30.00 | 77 | 70.00 | |
| Docetaxel + ZA + Sr-89 | 96 | 0 | 0.00 | 42 | 43.75 | 54 | 56.25 | |
| Statin | Docetaxel | 50 | 40 | 80.00 | 7 | 14.00 | 0 | 0.00 |
| Docetaxel + ZA | 54 | 44 | 81.48 | 4 | 7.41 | 1 | 1.85 | |
| Docetaxel + Sr-89 | 57 | 50 | 87.72 | 6 | 10.53 | 1 | 1.75 | |
| Docetaxel + ZA + Sr-89 | 51 | 45 | 88.24 | 3 | 5.88 | 1 | 1.96 | |
| Antidepressant | Docetaxel | 25 | 18 | 72.00 | 6 | 24.00 | 1 | 4.00 |
| Docetaxel + ZA | 14 | 8 | 57.14 | 4 | 28.57 | 1 | 7.14 | |
| Docetaxel + Sr-89 | 25 | 19 | 76.00 | 4 | 16.00 | 1 | 4.00 | |
| Docetaxel + ZA + Sr-89 | 20 | 12 | 60.00 | 2 | 10.00 | 4 | 20.00 | |
| Alpha blocker | Docetaxel | 29 | 19 | 65.52 | 6 | 20.69 | 2 | 6.90 |
| Docetaxel + ZA | 24 | 15 | 62.50 | 5 | 20.83 | 1 | 4.17 | |
| Docetaxel + Sr-89 | 41 | 27 | 65.85 | 12 | 29.27 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 35 | 23 | 65.71 | 11 | 31.43 | 0 | 0.00 | |
| COX-2 selective inhibitor | Docetaxel | 1 | 0 | 0.00 | 1 | 100.00 | 0 | 0.00 |
| Steroid | Docetaxel | 361 | 22 | 6.09 | 274 | 75.90 | 63 | 17.45 |
| Docetaxel + ZA | 329 | 19 | 5.78 | 256 | 77.81 | 52 | 15.81 | |
| Docetaxel + Sr-89 | 341 | 14 | 4.11 | 262 | 76.83 | 63 | 18.48 | |
| Docetaxel + ZA + Sr-89 | 363 | 23 | 6.34 | 260 | 71.63 | 79 | 21.76 | |
| Supplement | Docetaxel | 29 | 21 | 72.41 | 6 | 20.69 | 1 | 3.45 |
| Docetaxel + ZA | 117 | 22 | 18.80 | 68 | 58.12 | 13 | 11.11 | |
| Docetaxel + Sr-89 | 42 | 18 | 42.86 | 18 | 42.86 | 7 | 16.67 | |
| Docetaxel + ZA + Sr-89 | 84 | 20 | 23.81 | 57 | 67.86 | 4 | 4.76 | |
| Enzyme inhibitor | Docetaxel + ZA | 1 | 0 | 0.00 | 0 | 0.00 | 1 | 100.00 |
| Immunosuppressive | Docetaxel + ZA | 3 | 1 | 33.33 | 2 | 66.67 | 0 | 0.00 |
| Docetaxel + ZA + Sr-89 | 1 | 1 | 100.00 | 0 | 0.00 | 0 | 0.00 | |
| Radiotherapy | Docetaxel | 101 | 0 | 0.00 | 5 | 4.95 | 95 | 94.06 |
| Docetaxel + ZA | 75 | 0 | 0.00 | 1 | 1.33 | 74 | 98.67 | |
| Docetaxel + Sr-89 | 81 | 0 | 0.00 | 2 | 2.47 | 78 | 96.30 | |
| Docetaxel + ZA + Sr-89 | 85 | 0 | 0.00 | 3 | 3.53 | 82 | 96.47 | |
| Bisphosphonate | Docetaxel | 70 | 2 | 2.86 | 13 | 18.57 | 55 | 78.57 |
| Docetaxel + ZA | 167 | 0 | 0.00 | 47 | 28.14 | 120 | 71.86 | |
| Docetaxel + Sr-89 | 63 | 3 | 4.76 | 8 | 12.70 | 52 | 82.54 | |
| Docetaxel + ZA + Sr-89 | 115 | 1 | 0.87 | 32 | 27.83 | 82 | 71.30 | |
| Hormone therapy | Docetaxel | 100 | 12 | 12.00 | 46 | 46.00 | 40 | 40.00 |
| Docetaxel + ZA | 96 | 17 | 17.71 | 43 | 44.79 | 33 | 34.38 | |
| Docetaxel + Sr-89 | 109 | 14 | 12.84 | 47 | 43.12 | 46 | 42.20 | |
| Docetaxel + ZA + Sr-89 | 106 | 20 | 18.87 | 52 | 49.06 | 31 | 29.25 | |
| NSAID | Docetaxel | 23 | 10 | 43.48 | 11 | 47.83 | 1 | 4.35 |
| Docetaxel + ZA | 17 | 3 | 17.65 | 12 | 70.59 | 1 | 5.88 | |
| Docetaxel + Sr-89 | 16 | 3 | 18.75 | 6 | 37.50 | 1 | 6.25 | |
| Docetaxel + ZA + Sr-89 | 13 | 3 | 23.08 | 6 | 46.15 | 2 | 15.38 | |
| ARB | Docetaxel | 1 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Docetaxel + ZA | 2 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 2 | 0 | 0.00 | 1 | 50.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 1 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Radioisotope | Docetaxel | 13 | 0 | 0.00 | 0 | 0.00 | 13 | 100.00 |
| Docetaxel + ZA | 11 | 0 | 0.00 | 1 | 9.09 | 10 | 90.91 | |
| Docetaxel + Sr-89 | 14 | 1 | 7.14 | 4 | 28.57 | 10 | 71.43 | |
| Docetaxel + ZA + Sr-89 | 9 | 0 | 0.00 | 3 | 33.33 | 6 | 66.67 | |
| GCSF | Docetaxel | 11 | 0 | 0.00 | 8 | 72.73 | 1 | 9.09 |
| Docetaxel + ZA | 6 | 0 | 0.00 | 6 | 100.00 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 5 | 0 | 0.00 | 5 | 100.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 7 | 0 | 0.00 | 5 | 71.43 | 0 | 0.00 | |
| i.v. fluids for rehydration | Docetaxel + ZA | 1 | 0 | 0.00 | 1 | 100.00 | 0 | 0.00 |
| Docetaxel + Sr-89 | 5 | 0 | 0.00 | 5 | 100.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 3 | 0 | 0.00 | 3 | 100.00 | 0 | 0.00 | |
| Treatment for glaucoma | Docetaxel | 3 | 0 | 0.00 | 3 | 100.00 | 0 | 0.00 |
| Docetaxel + ZA | 4 | 2 | 50.00 | 2 | 50.00 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 3 | 3 | 100.00 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 9 | 4 | 44.44 | 5 | 55.56 | 0 | 0.00 | |
| Topical anti-inflammatory | Docetaxel | 2 | 0 | 0.00 | 2 | 100.00 | 0 | 0.00 |
| Docetaxel + ZA | 6 | 2 | 33.33 | 4 | 66.67 | 0 | 0.00 | |
| Docetaxel + Sr-89 | 1 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Docetaxel + ZA + Sr-89 | 2 | 0 | 0.00 | 0 | 0.00 | 2 | 100.00 | |
| Anticonvulsant | Docetaxel | 4 | 3 | 75.00 | 1 | 25.00 | 0 | 0.00 |
| Docetaxel + ZA | 14 | 2 | 14.29 | 8 | 57.14 | 4 | 28.57 | |
| Docetaxel + Sr-89 | 10 | 4 | 40.00 | 3 | 30.00 | 3 | 30.00 | |
| Docetaxel + ZA + Sr-89 | 4 | 2 | 50.00 | 0 | 0.00 | 2 | 50.00 | |
| Other | Docetaxel | 41 | 12 | 29.27 | 25 | 60.98 | 3 | 7.32 |
| Docetaxel + ZA | 65 | 18 | 27.69 | 34 | 52.31 | 8 | 12.31 | |
| Docetaxel + Sr-89 | 56 | 15 | 26.79 | 24 | 42.86 | 9 | 16.07 | |
| Docetaxel + ZA + Sr-89 | 56 | 21 | 37.50 | 30 | 53.57 | 3 | 5.36 |
| Concomitant medications | ZA | Patients | % | On-study | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|---|
| Antacids and Simeticone | No ZA | 15 | 1.98 | 3 | 0.40 | 10 | 1.32 | 2 | 0.29 |
| ZA | 14 | 1.85 | 3 | 0.40 | 9 | 1.19 | 2 | 0.29 | |
| Antispasmodics | No ZA | 8 | 1.06 | 2 | 0.26 | 5 | 0.66 | 1 | 0.14 |
| ZA | 5 | 0.66 | 3 | 0.40 | 2 | 0.26 | 0 | 0.00 | |
| H2 antagonist and ulcer healing (GI) | No ZA | 8 | 1.06 | 6 | 0.79 | 1 | 0.13 | 1 | 0.14 |
| ZA | 14 | 1.85 | 11 | 1.45 | 2 | 0.26 | 1 | 0.14 | |
| Proton pump inhibitors | No ZA | 157 | 20.74 | 103 | 13.61 | 52 | 6.87 | 13 | 1.87 |
| ZA | 158 | 20.87 | 112 | 14.80 | 47 | 6.21 | 8 | 1.15 | |
| Antimotility, antidiarrhoea | No ZA | 36 | 4.76 | 2 | 0.26 | 31 | 4.10 | 2 | 0.29 |
| ZA | 16 | 2.11 | 3 | 0.40 | 11 | 1.45 | 1 | 0.14 | |
| Laxatives | No ZA | 88 | 11.62 | 50 | 6.61 | 30 | 3.96 | 14 | 2.01 |
| ZA | 107 | 14.13 | 71 | 9.38 | 34 | 4.49 | 10 | 1.44 | |
| Cardiac glycoside | No ZA | 4 | 0.53 | 4 | 0.53 | 1 | 0.13 | 0 | 0.00 |
| ZA | 19 | 2.51 | 17 | 2.25 | 2 | 0.26 | 0 | 0.00 | |
| Diuretics (cardiac) | No ZA | 73 | 9.64 | 52 | 6.87 | 20 | 2.64 | 9 | 1.29 |
| ZA | 79 | 10.44 | 60 | 7.93 | 24 | 3.17 | 0 | 0.00 | |
| Drugs for arrhythmias (cardiac) | No ZA | 8 | 1.06 | 2 | 0.26 | 5 | 0.66 | 0 | 0.00 |
| ZA | 2 | 0.26 | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 | |
| Beta-blockers (cardiac) | No ZA | 47 | 6.21 | 43 | 5.68 | 3 | 0.40 | 2 | 0.29 |
| ZA | 62 | 8.19 | 55 | 7.27 | 7 | 0.92 | 0 | 0.00 | |
| Calcium-channel blockers (cardiac) | No ZA | 53 | 7.00 | 48 | 6.34 | 9 | 1.19 | 1 | 0.14 |
| ZA | 52 | 6.87 | 49 | 6.47 | 3 | 0.40 | 1 | 0.14 | |
| Cardiac – other | No ZA | 32 | 4.23 | 29 | 3.83 | 4 | 0.53 | 1 | 0.14 |
| ZA | 46 | 6.08 | 45 | 5.94 | 1 | 0.13 | 0 | 0.00 | |
| Anticoagulants antiplatelet drugs | No ZA | 138 | 18.23 | 90 | 11.89 | 48 | 6.34 | 16 | 2.30 |
| ZA | 132 | 17.44 | 88 | 11.62 | 53 | 7.00 | 9 | 1.29 | |
| Blood bone marrow disorders | No ZA | 66 | 8.72 | 11 | 1.45 | 44 | 5.81 | 11 | 1.58 |
| ZA | 54 | 7.13 | 6 | 0.79 | 40 | 5.28 | 11 | 1.58 | |
| Bronchodilators antihistamines | No ZA | 53 | 7.00 | 26 | 3.43 | 25 | 3.30 | 9 | 1.29 |
| ZA | 58 | 7.66 | 24 | 3.17 | 30 | 3.96 | 6 | 0.86 | |
| Hypnotics (CNS) | No ZA | 22 | 2.91 | 14 | 1.85 | 5 | 0.66 | 3 | 0.43 |
| ZA | 15 | 1.98 | 6 | 0.79 | 7 | 0.92 | 2 | 0.29 | |
| Anxiolytics (CNS) | No ZA | 8 | 1.06 | 3 | 0.40 | 3 | 0.40 | 2 | 0.29 |
| ZA | 7 | 0.92 | 2 | 0.26 | 4 | 0.53 | 1 | 0.14 | |
| Antiemetics | No ZA | 121 | 15.98 | 20 | 2.64 | 95 | 12.55 | 18 | 2.59 |
| ZA | 136 | 17.97 | 28 | 3.70 | 104 | 13.74 | 28 | 4.02 | |
| Central nervous – other | No ZA | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 |
| Antibacterials, antifungals and antiviral | No ZA | 201 | 26.55 | 29 | 3.83 | 178 | 23.51 | 28 | 4.02 |
| ZA | 171 | 22.59 | 20 | 2.64 | 150 | 19.82 | 22 | 3.16 | |
| Diabetes drugs | No ZA | 29 | 3.83 | 20 | 2.64 | 9 | 1.19 | 1 | 0.14 |
| ZA | 31 | 4.10 | 27 | 3.57 | 4 | 0.53 | 1 | 0.14 | |
| Nitrate | No ZA | 30 | 3.96 | 24 | 3.17 | 5 | 0.66 | 2 | 0.29 |
| ZA | 26 | 3.43 | 23 | 3.04 | 4 | 0.53 | 0 | 0.00 | |
| ACE inhibitor | No ZA | 59 | 7.79 | 52 | 6.87 | 7 | 0.92 | 1 | 0.14 |
| ZA | 63 | 8.32 | 57 | 7.53 | 10 | 1.32 | 2 | 0.29 | |
| Chemotherapy | No ZA | 69 | 9.11 | 0 | 0.00 | 34 | 4.49 | 37 | 5.32 |
| ZA | 74 | 9.78 | 0 | 0.00 | 35 | 4.62 | 42 | 6.03 | |
| Statin | No ZA | 97 | 12.81 | 89 | 11.76 | 13 | 1.72 | 1 | 0.14 |
| ZA | 94 | 12.42 | 89 | 11.76 | 6 | 0.79 | 2 | 0.29 | |
| Antidepressant | No ZA | 42 | 5.55 | 35 | 4.62 | 9 | 1.19 | 2 | 0.29 |
| ZA | 29 | 3.83 | 19 | 2.51 | 5 | 0.66 | 5 | 0.72 | |
| Alpha Blocker | No ZA | 54 | 7.13 | 45 | 5.94 | 15 | 1.98 | 2 | 0.29 |
| ZA | 46 | 6.08 | 37 | 4.89 | 15 | 1.98 | 1 | 0.14 | |
| COX-2 selective inhibitor | No ZA | 1 | 0.13 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 |
| Steroid | No ZA | 164 | 21.66 | 33 | 4.36 | 104 | 13.74 | 60 | 8.62 |
| ZA | 165 | 21.80 | 41 | 5.42 | 112 | 14.80 | 48 | 6.90 | |
| Supplement | No ZA | 44 | 5.81 | 25 | 3.30 | 16 | 2.11 | 6 | 0.86 |
| ZA | 96 | 12.68 | 29 | 3.83 | 66 | 8.72 | 8 | 1.15 | |
| Enzyme inhibitor | ZA | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| Immunosuppressive | ZA | 2 | 0.26 | 2 | 0.26 | 1 | 0.13 | 0 | 0.00 |
| Radiotherapy | No ZA | 165 | 21.80 | 0 | 0.00 | 7 | 0.92 | 157 | 22.56 |
| ZA | 150 | 19.82 | 0 | 0.00 | 4 | 0.53 | 147 | 21.12 | |
| Bisphosphonate | No ZA | 70 | 9.25 | 4 | 0.53 | 19 | 2.51 | 50 | 7.18 |
| ZA | 76 | 10.04 | 1 | 0.13 | 48 | 6.34 | 41 | 5.89 | |
| Hormone Therapy | No ZA | 134 | 17.70 | 26 | 3.43 | 67 | 8.85 | 63 | 9.05 |
| ZA | 129 | 17.04 | 34 | 4.49 | 69 | 9.11 | 51 | 7.33 | |
| NSAID | No ZA | 32 | 4.23 | 13 | 1.72 | 13 | 1.72 | 2 | 0.29 |
| ZA | 24 | 3.17 | 6 | 0.79 | 14 | 1.85 | 3 | 0.43 | |
| ARB | No ZA | 3 | 0.40 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 |
| ZA | 2 | 0.26 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Radioisotope | No ZA | 22 | 2.91 | 1 | 0.13 | 3 | 0.40 | 19 | 2.73 |
| ZA | 19 | 2.51 | 0 | 0.00 | 4 | 0.53 | 15 | 2.16 | |
| GCSF | No ZA | 14 | 1.85 | 0 | 0.00 | 12 | 1.59 | 1 | 0.14 |
| ZA | 11 | 1.45 | 0 | 0.00 | 10 | 1.32 | 0 | 0.00 | |
| i.v. Fluids for Rehydration | No ZA | 2 | 0.26 | 0 | 0.00 | 2 | 0.26 | 0 | 0.00 |
| ZA | 3 | 0.40 | 0 | 0.00 | 3 | 0.40 | 0 | 0.00 | |
| Treatment for glaucoma | No ZA | 3 | 0.40 | 1 | 0.13 | 2 | 0.26 | 0 | 0.00 |
| ZA | 8 | 1.06 | 6 | 0.79 | 3 | 0.40 | 0 | 0.00 | |
| Topical anti-inflammatory | No ZA | 3 | 0.40 | 0 | 0.00 | 2 | 0.26 | 0 | 0.00 |
| ZA | 6 | 0.79 | 2 | 0.26 | 3 | 0.40 | 1 | 0.14 | |
| Anticonvulsant | No ZA | 14 | 1.85 | 7 | 0.92 | 4 | 0.53 | 3 | 0.43 |
| ZA | 9 | 1.19 | 4 | 0.53 | 2 | 0.26 | 4 | 0.57 | |
| Other | No ZA | 78 | 10.30 | 24 | 3.17 | 40 | 5.28 | 12 | 1.72 |
| ZA | 87 | 11.49 | 34 | 4.49 | 50 | 6.61 | 10 | 1.44 |
| Concomitant medications | ZA | Instances | On-study | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|
| Antacids and simeticone | No ZA | 17 | 3 | 17.65 | 11 | 64.71 | 2 | 11.76 |
| ZA | 29 | 3 | 10.34 | 19 | 65.52 | 7 | 24.14 | |
| Antispasmodics | No ZA | 12 | 2 | 16.67 | 9 | 75.00 | 1 | 8.33 |
| ZA | 5 | 3 | 60.00 | 2 | 40.00 | 0 | 0.00 | |
| H2 antagonist and ulcer healing (GI) | No ZA | 14 | 6 | 42.86 | 1 | 7.14 | 6 | 42.86 |
| ZA | 15 | 11 | 73.33 | 2 | 13.33 | 1 | 6.67 | |
| Proton pump inhibitors | No ZA | 194 | 106 | 54.64 | 65 | 33.51 | 14 | 7.22 |
| ZA | 188 | 114 | 60.64 | 53 | 28.19 | 9 | 4.79 | |
| Antimotility, antidiarrhoea | No ZA | 43 | 2 | 4.65 | 38 | 88.37 | 2 | 4.65 |
| ZA | 18 | 3 | 16.67 | 13 | 72.22 | 1 | 5.56 | |
| Laxatives | No ZA | 156 | 64 | 41.03 | 57 | 36.54 | 26 | 16.67 |
| ZA | 169 | 88 | 52.07 | 58 | 34.32 | 11 | 6.51 | |
| Cardiac glycoside | No ZA | 5 | 4 | 80.00 | 1 | 20.00 | 0 | 0.00 |
| ZA | 20 | 17 | 85.00 | 2 | 10.00 | 0 | 0.00 | |
| Diuretics (cardiac) | No ZA | 103 | 55 | 53.40 | 34 | 33.01 | 9 | 8.74 |
| ZA | 99 | 63 | 63.64 | 31 | 31.31 | 0 | 0.00 | |
| Drugs for arrhythmias (cardiac) | No ZA | 11 | 3 | 27.27 | 7 | 63.64 | 0 | 0.00 |
| ZA | 4 | 1 | 25.00 | 3 | 75.00 | 0 | 0.00 | |
| Beta-blockers (cardiac) | No ZA | 50 | 43 | 86.00 | 3 | 6.00 | 2 | 4.00 |
| ZA | 70 | 57 | 81.43 | 7 | 10.00 | 0 | 0.00 | |
| Calcium-channel blockers (cardiac) | No ZA | 61 | 49 | 80.33 | 11 | 18.03 | 1 | 1.64 |
| ZA | 56 | 49 | 87.50 | 3 | 5.36 | 1 | 1.79 | |
| Cardiac – other | No ZA | 35 | 30 | 85.71 | 4 | 11.43 | 1 | 2.86 |
| ZA | 50 | 48 | 96.00 | 1 | 2.00 | 0 | 0.00 | |
| Anticoagulants antiplatelet drugs | No ZA | 209 | 95 | 45.45 | 79 | 37.80 | 29 | 13.88 |
| ZA | 201 | 93 | 46.27 | 89 | 44.28 | 10 | 4.98 | |
| Blood bone marrow disorders | No ZA | 140 | 13 | 9.29 | 99 | 70.71 | 24 | 17.14 |
| ZA | 116 | 6 | 5.17 | 74 | 63.79 | 30 | 25.86 | |
| Bronchodilators antihistamines | No ZA | 101 | 37 | 36.63 | 45 | 44.55 | 14 | 13.86 |
| ZA | 101 | 27 | 26.73 | 55 | 54.46 | 17 | 16.83 | |
| Hypnotics (CNS) | No ZA | 28 | 14 | 50.00 | 7 | 25.00 | 3 | 10.71 |
| ZA | 17 | 6 | 35.29 | 9 | 52.94 | 2 | 11.76 | |
| Anxiolytics (CNS) | No ZA | 9 | 3 | 33.33 | 3 | 33.33 | 3 | 33.33 |
| ZA | 7 | 2 | 28.57 | 4 | 57.14 | 1 | 14.29 | |
| Antiemetics | No ZA | 641 | 24 | 3.74 | 573 | 89.39 | 41 | 6.40 |
| ZA | 702 | 29 | 4.13 | 589 | 83.90 | 80 | 11.40 | |
| Central nervous – other | No ZA | 1 | 1 | 100.00 | 0 | 0.00 | 0 | 0.00 |
| Diabetes drugs | No ZA | 42 | 28 | 66.67 | 12 | 28.57 | 1 | 2.38 |
| ZA | 45 | 39 | 86.67 | 4 | 8.89 | 1 | 2.22 | |
| Nitrate | No ZA | 38 | 27 | 71.05 | 5 | 13.16 | 2 | 5.26 |
| ZA | 30 | 23 | 76.67 | 5 | 16.67 | 0 | 0.00 | |
| ACE inhibitor | No ZA | 70 | 53 | 75.71 | 12 | 17.14 | 1 | 1.43 |
| ZA | 76 | 57 | 75.00 | 12 | 15.79 | 2 | 2.63 | |
| Chemotherapy | No ZA | 186 | 0 | 0.00 | 63 | 33.87 | 123 | 66.13 |
| ZA | 198 | 0 | 0.00 | 73 | 36.87 | 125 | 63.13 | |
| Statin | No ZA | 107 | 90 | 84.11 | 13 | 12.15 | 1 | 0.93 |
| ZA | 105 | 89 | 84.76 | 7 | 6.67 | 2 | 1.90 | |
| Antidepressant | No ZA | 50 | 37 | 74.00 | 10 | 20.00 | 2 | 4.00 |
| ZA | 34 | 20 | 58.82 | 6 | 17.65 | 5 | 14.71 | |
| Alpha blocker | No ZA | 70 | 46 | 65.71 | 18 | 25.71 | 2 | 2.86 |
| ZA | 59 | 38 | 64.41 | 16 | 27.12 | 1 | 1.69 | |
| COX-2 selective inhibitor | No ZA | 1 | 0 | 0.00 | 1 | 100.00 | 0 | 0.00 |
| Steroid | No ZA | 702 | 36 | 5.13 | 536 | 76.35 | 126 | 17.95 |
| ZA | 692 | 42 | 6.07 | 516 | 74.57 | 131 | 18.93 | |
| Supplement | No ZA | 71 | 39 | 54.93 | 24 | 33.80 | 8 | 11.27 |
| ZA | 201 | 42 | 20.90 | 125 | 62.19 | 17 | 8.46 | |
| Enzyme inhibitor | ZA | 1 | 0 | 0.00 | 0 | 0.00 | 1 | 100.00 |
| Immunosuppressive | ZA | 4 | 2 | 50.00 | 2 | 50.00 | 0 | 0.00 |
| Radiotherapy | No ZA | 182 | 0 | 0.00 | 7 | 3.85 | 173 | 95.05 |
| ZA | 160 | 0 | 0.00 | 4 | 2.50 | 156 | 97.50 | |
| Bisphosphonate | No ZA | 133 | 5 | 3.76 | 21 | 15.79 | 107 | 80.45 |
| ZA | 282 | 1 | 0.35 | 79 | 28.01 | 202 | 71.63 | |
| Hormone therapy | No ZA | 209 | 26 | 12.44 | 93 | 44.50 | 86 | 41.15 |
| ZA | 202 | 37 | 18.32 | 95 | 47.03 | 64 | 31.68 | |
| NSAID | No ZA | 39 | 13 | 33.33 | 17 | 43.59 | 2 | 5.13 |
| ZA | 30 | 6 | 20.00 | 18 | 60.00 | 3 | 10.00 | |
| ARB | No ZA | 3 | 0 | 0.00 | 1 | 33.33 | 0 | 0.00 |
| ZA | 3 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Radioisotope | No ZA | 27 | 1 | 3.70 | 4 | 14.81 | 23 | 85.19 |
| ZA | 20 | 0 | 0.00 | 4 | 20.00 | 16 | 80.00 | |
| GCSF | No ZA | 16 | 0 | 0.00 | 13 | 81.25 | 1 | 6.25 |
| ZA | 13 | 0 | 0.00 | 11 | 84.62 | 0 | 0.00 | |
| i.v. fluids for rehydration | No ZA | 5 | 0 | 0.00 | 5 | 100.00 | 0 | 0.00 |
| ZA | 4 | 0 | 0.00 | 4 | 100.00 | 0 | 0.00 | |
| Treatment for glaucoma | No ZA | 6 | 3 | 50.00 | 3 | 50.00 | 0 | 0.00 |
| ZA | 13 | 6 | 46.15 | 7 | 53.85 | 0 | 0.00 | |
| Topical anti-inflammatory | No ZA | 3 | 0 | 0.00 | 2 | 66.67 | 0 | 0.00 |
| ZA | 8 | 2 | 25.00 | 4 | 50.00 | 2 | 25.00 | |
| Anticonvulsant | No ZA | 14 | 7 | 50.00 | 4 | 28.57 | 3 | 21.43 |
| ZA | 18 | 4 | 22.22 | 8 | 44.44 | 6 | 33.33 | |
| Other | No ZA | 97 | 27 | 27.84 | 49 | 50.52 | 12 | 12.37 |
| ZA | 121 | 39 | 32.23 | 64 | 52.89 | 11 | 9.09 |
| Concomitant medications | Sr-89 | Patients | % | On-study | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|---|
| Antacids and simeticone | No Sr-89 | 13 | 1.72 | 4 | 0.53 | 8 | 1.06 | 2 | 0.29 |
| Sr-89 | 16 | 2.11 | 2 | 0.26 | 11 | 1.45 | 2 | 0.29 | |
| Antispasmodics | No Sr-89 | 8 | 1.06 | 3 | 0.40 | 4 | 0.53 | 1 | 0.14 |
| Sr-89 | 5 | 0.66 | 2 | 0.26 | 3 | 0.40 | 0 | 0.00 | |
| H2 antagonist and ulcer healing (GI) | No Sr-89 | 10 | 1.32 | 7 | 0.92 | 1 | 0.13 | 1 | 0.14 |
| Sr-89 | 12 | 1.59 | 10 | 1.32 | 2 | 0.26 | 1 | 0.14 | |
| Proton pump inhibitors | No Sr-89 | 166 | 21.93 | 116 | 15.32 | 49 | 6.47 | 9 | 1.29 |
| Sr-89 | 149 | 19.68 | 99 | 13.08 | 50 | 6.61 | 12 | 1.72 | |
| Antimotility, antidiarrhoea | No Sr-89 | 31 | 4.10 | 3 | 0.40 | 25 | 3.30 | 2 | 0.29 |
| Sr-89 | 21 | 2.77 | 2 | 0.26 | 17 | 2.25 | 1 | 0.14 | |
| Laxatives | No Sr-89 | 97 | 12.81 | 67 | 8.85 | 30 | 3.96 | 8 | 1.15 |
| Sr-89 | 98 | 12.95 | 54 | 7.13 | 34 | 4.49 | 16 | 2.30 | |
| Cardiac glycoside | No Sr-89 | 15 | 1.98 | 14 | 1.85 | 3 | 0.40 | 0 | 0.00 |
| Sr-89 | 8 | 1.06 | 7 | 0.92 | 0 | 0.00 | 0 | 0.00 | |
| Diuretics (cardiac) | No Sr-89 | 81 | 10.70 | 58 | 7.66 | 26 | 3.43 | 4 | 0.57 |
| Sr-89 | 71 | 9.38 | 54 | 7.13 | 18 | 2.38 | 5 | 0.72 | |
| Drugs for arrhythmias (cardiac) | No Sr-89 | 5 | 0.66 | 2 | 0.26 | 2 | 0.26 | 0 | 0.00 |
| Sr-89 | 5 | 0.66 | 1 | 0.13 | 4 | 0.53 | 0 | 0.00 | |
| Beta-blockers (cardiac) | No Sr-89 | 47 | 6.21 | 42 | 5.55 | 2 | 0.26 | 1 | 0.14 |
| Sr-89 | 62 | 8.19 | 56 | 7.40 | 8 | 1.06 | 1 | 0.14 | |
| Calcium-channel blockers (cardiac) | No Sr-89 | 51 | 6.74 | 46 | 6.08 | 5 | 0.66 | 1 | 0.14 |
| Sr-89 | 54 | 7.13 | 51 | 6.74 | 7 | 0.92 | 1 | 0.14 | |
| Cardiac – other | No Sr-89 | 37 | 4.89 | 34 | 4.49 | 3 | 0.40 | 0 | 0.00 |
| Sr-89 | 41 | 5.42 | 40 | 5.28 | 2 | 0.26 | 1 | 0.14 | |
| Anticoagulants antiplatelet drugs | No Sr-89 | 135 | 17.83 | 88 | 11.62 | 53 | 7.00 | 9 | 1.29 |
| Sr-89 | 135 | 17.83 | 90 | 11.89 | 48 | 6.34 | 16 | 2.30 | |
| Blood bone marrow disorders | No Sr-89 | 62 | 8.19 | 11 | 1.45 | 45 | 5.94 | 11 | 1.58 |
| Sr-89 | 58 | 7.66 | 6 | 0.79 | 39 | 5.15 | 11 | 1.58 | |
| Bronchodilators antihistamines | No Sr-89 | 53 | 7.00 | 21 | 2.77 | 28 | 3.70 | 5 | 0.72 |
| Sr-89 | 58 | 7.66 | 29 | 3.83 | 27 | 3.57 | 10 | 1.44 | |
| Hypnotics (CNS) | No Sr-89 | 17 | 2.25 | 8 | 1.06 | 7 | 0.92 | 2 | 0.29 |
| Sr-89 | 20 | 2.64 | 12 | 1.59 | 5 | 0.66 | 3 | 0.43 | |
| Anxiolytics (CNS) | No Sr-89 | 7 | 0.92 | 3 | 0.40 | 1 | 0.13 | 3 | 0.43 |
| Sr-89 | 8 | 1.06 | 2 | 0.26 | 6 | 0.79 | 0 | 0.00 | |
| Antiemetics | No Sr-89 | 133 | 17.57 | 28 | 3.70 | 105 | 13.87 | 19 | 2.73 |
| Sr-89 | 124 | 16.38 | 20 | 2.64 | 94 | 12.42 | 27 | 3.88 | |
| Central nervous – other | No Sr-89 | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 |
| Diabetes drugs | No Sr-89 | 29 | 3.83 | 25 | 3.30 | 5 | 0.66 | 0 | 0.00 |
| Sr-89 | 31 | 4.10 | 22 | 2.91 | 8 | 1.06 | 2 | 0.29 | |
| Nitrate | No Sr-89 | 28 | 3.70 | 25 | 3.30 | 4 | 0.53 | 1 | 0.14 |
| Sr-89 | 28 | 3.70 | 22 | 2.91 | 5 | 0.66 | 1 | 0.14 | |
| ACE inhibitor | No Sr-89 | 61 | 8.06 | 52 | 6.87 | 9 | 1.19 | 2 | 0.29 |
| Sr-89 | 61 | 8.06 | 57 | 7.53 | 8 | 1.06 | 1 | 0.14 | |
| Chemotherapy | No Sr-89 | 64 | 8.45 | 0 | 0.00 | 34 | 4.49 | 34 | 4.89 |
| Sr-89 | 79 | 10.44 | 0 | 0.00 | 35 | 4.62 | 45 | 6.47 | |
| Statin | No Sr-89 | 93 | 12.29 | 84 | 11.10 | 10 | 1.32 | 1 | 0.14 |
| Sr-89 | 98 | 12.95 | 94 | 12.42 | 9 | 1.19 | 2 | 0.29 | |
| Antidepressant | No Sr-89 | 35 | 4.62 | 26 | 3.43 | 9 | 1.19 | 2 | 0.29 |
| Sr-89 | 36 | 4.76 | 28 | 3.70 | 5 | 0.66 | 5 | 0.72 | |
| Alpha-blocker | No Sr-89 | 43 | 5.68 | 34 | 4.49 | 10 | 1.32 | 3 | 0.43 |
| Sr-89 | 57 | 7.53 | 48 | 6.34 | 20 | 2.64 | 0 | 0.00 | |
| COX-2 selective inhibitor | No Sr-89 | 1 | 0.13 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 |
| Steroid | No Sr-89 | 177 | 23.38 | 37 | 4.89 | 118 | 15.59 | 52 | 7.47 |
| Sr-89 | 152 | 20.08 | 37 | 4.89 | 98 | 12.95 | 56 | 8.05 | |
| Supplement | No Sr-89 | 74 | 9.78 | 28 | 3.70 | 42 | 5.55 | 5 | 0.72 |
| Sr-89 | 66 | 8.72 | 26 | 3.43 | 40 | 5.28 | 9 | 1.29 | |
| Enzyme inhibitor | No Sr-89 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| Immunosuppressive | No Sr-89 | 1 | 0.13 | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 |
| Sr-89 | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | |
| Radiotherapy | No Sr-89 | 162 | 21.40 | 0 | 0.00 | 6 | 0.79 | 156 | 22.41 |
| Sr-89 | 153 | 20.21 | 0 | 0.00 | 5 | 0.66 | 148 | 21.26 | |
| Bisphosphonate | No Sr-89 | 82 | 10.83 | 2 | 0.26 | 37 | 4.89 | 55 | 7.90 |
| Sr-89 | 64 | 8.45 | 3 | 0.40 | 30 | 3.96 | 36 | 5.17 | |
| Hormone therapy | No Sr-89 | 128 | 16.91 | 27 | 3.57 | 67 | 8.85 | 60 | 8.62 |
| Sr-89 | 135 | 17.83 | 33 | 4.36 | 69 | 9.11 | 54 | 7.76 | |
| NSAID | No Sr-89 | 30 | 3.96 | 13 | 1.72 | 15 | 1.98 | 2 | 0.29 |
| Sr-89 | 26 | 3.43 | 6 | 0.79 | 12 | 1.59 | 3 | 0.43 | |
| ARB | No Sr-89 | 2 | 0.26 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Sr-89 | 3 | 0.40 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 | |
| Radioisotope | No Sr-89 | 22 | 2.91 | 0 | 0.00 | 1 | 0.13 | 21 | 3.02 |
| Sr-89 | 19 | 2.51 | 1 | 0.13 | 6 | 0.79 | 13 | 1.87 | |
| GCSF | No Sr-89 | 15 | 1.98 | 0 | 0.00 | 13 | 1.72 | 1 | 0.14 |
| Sr-89 | 10 | 1.32 | 0 | 0.00 | 9 | 1.19 | 0 | 0.00 | |
| i.v fluids for rehydration | No Sr-89 | 1 | 0.13 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 |
| Sr-89 | 4 | 0.53 | 0 | 0.00 | 4 | 0.53 | 0 | 0.00 | |
| Treatment for glaucoma | No Sr-89 | 5 | 0.66 | 2 | 0.26 | 3 | 0.40 | 0 | 0.00 |
| Sr-89 | 6 | 0.79 | 5 | 0.66 | 2 | 0.26 | 0 | 0.00 | |
| Topical anti-inflammatory | No Sr-89 | 7 | 0.92 | 2 | 0.26 | 5 | 0.66 | 0 | 0.00 |
| Sr-89 | 2 | 0.26 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 | |
| Anticonvulsant | No Sr-89 | 9 | 1.19 | 5 | 0.66 | 3 | 0.40 | 2 | 0.29 |
| Sr-89 | 14 | 1.85 | 6 | 0.79 | 3 | 0.40 | 5 | 0.72 | |
| Other | No Sr-89 | 79 | 10.44 | 26 | 3.43 | 46 | 6.08 | 10 | 1.44 |
| Sr-89 | 86 | 11.36 | 32 | 4.23 | 44 | 5.81 | 12 | 1.72 |
| Concomitant medications | Sr-89 | Instances | OS | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|
| Antacids and simeticone | No Sr-89 | 17 | 4 | 23.53 | 11 | 64.71 | 2 | 11.76 |
| Sr-89 | 29 | 2 | 6.90 | 19 | 65.52 | 7 | 24.14 | |
| Antispasmodics | No Sr-89 | 8 | 3 | 37.50 | 4 | 50.00 | 1 | 12.50 |
| Sr-89 | 9 | 2 | 22.22 | 7 | 77.78 | 0 | 0.00 | |
| H2 antagonist and ulcer healing (GI) | No Sr-89 | 15 | 7 | 46.67 | 1 | 6.67 | 6 | 40.00 |
| Sr-89 | 14 | 10 | 71.43 | 2 | 14.29 | 1 | 7.14 | |
| Proton pump inhibitors | No Sr-89 | 194 | 118 | 60.82 | 56 | 28.87 | 10 | 5.15 |
| Sr-89 | 188 | 102 | 54.26 | 62 | 32.98 | 13 | 6.91 | |
| Antimotility, antidiarrhoea | No Sr-89 | 38 | 3 | 7.89 | 32 | 84.21 | 2 | 5.26 |
| Sr-89 | 23 | 2 | 8.70 | 19 | 82.61 | 1 | 4.35 | |
| Laxatives | No Sr-89 | 150 | 83 | 55.33 | 45 | 30.00 | 12 | 8.00 |
| Sr-89 | 175 | 69 | 39.43 | 70 | 40.00 | 25 | 14.29 | |
| Cardiac glycoside | No Sr-89 | 17 | 14 | 82.35 | 3 | 17.65 | 0 | 0.00 |
| Sr-89 | 8 | 7 | 87.50 | 0 | 0.00 | 0 | 0.00 | |
| Diuretics (cardiac) | No Sr-89 | 104 | 61 | 58.65 | 34 | 32.69 | 4 | 3.85 |
| Sr-89 | 98 | 57 | 58.16 | 31 | 31.63 | 5 | 5.10 | |
| Drugs for arrhythmias (cardiac) | No Sr-89 | 5 | 2 | 40.00 | 2 | 40.00 | 0 | 0.00 |
| Sr-89 | 10 | 2 | 20.00 | 8 | 80.00 | 0 | 0.00 | |
| Beta-blockers (cardiac) | No Sr-89 | 52 | 43 | 82.69 | 2 | 3.85 | 1 | 1.92 |
| Sr-89 | 68 | 57 | 83.82 | 8 | 11.76 | 1 | 1.47 | |
| Calcium-channel blockers (cardiac) | No Sr-89 | 55 | 46 | 83.64 | 5 | 9.09 | 1 | 1.82 |
| Sr-89 | 62 | 52 | 83.87 | 9 | 14.52 | 1 | 1.61 | |
| Cardiac – other | No Sr-89 | 41 | 36 | 87.80 | 3 | 7.32 | 0 | 0.00 |
| Sr-89 | 44 | 42 | 95.45 | 2 | 4.55 | 1 | 2.27 | |
| Anticoagulants antiplatelet drugs | No Sr-89 | 202 | 90 | 44.55 | 90 | 44.55 | 12 | 5.94 |
| Sr-89 | 208 | 98 | 47.12 | 78 | 37.50 | 27 | 12.98 | |
| Blood bone marrow disorders | No Sr-89 | 137 | 13 | 9.49 | 94 | 68.61 | 27 | 19.71 |
| Sr-89 | 119 | 6 | 5.04 | 79 | 66.39 | 27 | 22.69 | |
| Bronchodilators antihistamines | No Sr-89 | 90 | 26 | 28.89 | 49 | 54.44 | 11 | 12.22 |
| Sr-89 | 112 | 38 | 33.93 | 51 | 45.54 | 20 | 17.86 | |
| Hypnotics (CNS) | No Sr-89 | 19 | 8 | 42.11 | 7 | 36.84 | 2 | 10.53 |
| Sr-89 | 26 | 12 | 46.15 | 9 | 34.62 | 3 | 11.54 | |
| Anxiolytics (CNS) | No Sr-89 | 8 | 3 | 37.50 | 1 | 12.50 | 4 | 50.00 |
| Sr-89 | 8 | 2 | 25.00 | 6 | 75.00 | 0 | 0.00 | |
| Antiemetics | No Sr-89 | 718 | 32 | 4.46 | 632 | 88.02 | 48 | 6.69 |
| Sr-89 | 625 | 21 | 3.36 | 530 | 84.80 | 73 | 11.68 | |
| Central nervous – other | No Sr-89 | 1 | 1 | 100.00 | 0 | 0.00 | 0 | 0.00 |
| Diabetes drugs | No Sr-89 | 43 | 37 | 86.05 | 5 | 11.63 | 0 | 0.00 |
| Sr-89 | 44 | 30 | 68.18 | 11 | 25.00 | 2 | 4.55 | |
| Nitrate | No Sr-89 | 34 | 27 | 79.41 | 5 | 14.71 | 1 | 2.94 |
| Sr-89 | 34 | 23 | 67.65 | 5 | 14.71 | 1 | 2.94 | |
| ACE inhibitor | No Sr-89 | 68 | 52 | 76.47 | 11 | 16.18 | 2 | 2.94 |
| Sr-89 | 78 | 58 | 74.36 | 13 | 16.67 | 1 | 1.28 | |
| Chemotherapy | No Sr-89 | 178 | 0 | 0.00 | 61 | 34.27 | 117 | 65.73 |
| Sr-89 | 206 | 0 | 0.00 | 75 | 36.41 | 131 | 63.59 | |
| Statin | No Sr-89 | 104 | 84 | 80.77 | 11 | 10.58 | 1 | 0.96 |
| Sr-89 | 108 | 95 | 87.96 | 9 | 8.33 | 2 | 1.85 | |
| Antidepressant | No Sr-89 | 39 | 26 | 66.67 | 10 | 25.64 | 2 | 5.13 |
| Sr-89 | 45 | 31 | 68.89 | 6 | 13.33 | 5 | 11.11 | |
| Alpha-blocker | No Sr-89 | 53 | 34 | 64.15 | 11 | 20.75 | 3 | 5.66 |
| Sr-89 | 76 | 50 | 65.79 | 23 | 30.26 | 0 | 0.00 | |
| COX-2 selective inhibitor | No Sr-89 | 1 | 0 | 0.00 | 1 | 100.00 | 0 | 0.00 |
| Steroid | No Sr-89 | 690 | 41 | 5.94 | 530 | 76.81 | 115 | 16.67 |
| Sr-89 | 704 | 37 | 5.26 | 522 | 74.15 | 142 | 20.17 | |
| Supplement | No Sr-89 | 146 | 43 | 29.45 | 74 | 50.68 | 14 | 9.59 |
| Sr-89 | 126 | 38 | 30.16 | 75 | 59.52 | 11 | 8.73 | |
| Enzyme inhibitor | No Sr-89 | 1 | 0 | 0.00 | 0 | 0.00 | 1 | 100.00 |
| Immunosuppressive | No Sr-89 | 3 | 1 | 33.33 | 2 | 66.67 | 0 | 0.00 |
| Sr-89 | 1 | 1 | 100.00 | 0 | 0.00 | 0 | 0.00 | |
| Radiotherapy | No Sr-89 | 176 | 0 | 0.00 | 6 | 3.41 | 169 | 96.02 |
| Sr-89 | 166 | 0 | 0.00 | 5 | 3.01 | 160 | 96.39 | |
| Bisphosphonate | No Sr-89 | 237 | 2 | 0.84 | 60 | 25.32 | 175 | 73.84 |
| Sr-89 | 178 | 4 | 2.25 | 40 | 22.47 | 134 | 75.28 | |
| Hormone therapy | No Sr-89 | 196 | 29 | 14.80 | 89 | 45.41 | 73 | 37.24 |
| Sr-89 | 215 | 34 | 15.81 | 99 | 46.05 | 77 | 35.81 | |
| NSAID | No Sr-89 | 40 | 13 | 32.50 | 23 | 57.50 | 2 | 5.00 |
| Sr-89 | 29 | 6 | 20.69 | 12 | 41.38 | 3 | 10.34 | |
| ARB | No Sr-89 | 3 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Sr-89 | 3 | 0 | 0.00 | 1 | 33.33 | 0 | 0.00 | |
| Radioisotope | No Sr-89 | 24 | 0 | 0.00 | 1 | 4.17 | 23 | 95.83 |
| Sr-89 | 23 | 1 | 4.35 | 7 | 30.43 | 16 | 69.57 | |
| GCSF | No Sr-89 | 17 | 0 | 0.00 | 14 | 82.35 | 1 | 5.88 |
| Sr-89 | 12 | 0 | 0.00 | 10 | 83.33 | 0 | 0.00 | |
| i.v. fluids for rehydration | No Sr-89 | 1 | 0 | 0.00 | 1 | 100.00 | 0 | 0.00 |
| Sr-89 | 8 | 0 | 0.00 | 8 | 100.00 | 0 | 0.00 | |
| Treatment for glaucoma | No Sr-89 | 7 | 2 | 28.57 | 5 | 71.43 | 0 | 0.00 |
| Sr-89 | 12 | 7 | 58.33 | 5 | 41.67 | 0 | 0.00 | |
| Topical anti-inflammatory | No Sr-89 | 8 | 2 | 25.00 | 6 | 75.00 | 0 | 0.00 |
| Sr-89 | 3 | 0 | 0.00 | 0 | 0.00 | 2 | 66.67 | |
| Anticonvulsant | No Sr-89 | 18 | 5 | 27.78 | 9 | 50.00 | 4 | 22.22 |
| Sr-89 | 14 | 6 | 42.86 | 3 | 21.43 | 5 | 35.71 | |
| Other | No Sr-89 | 106 | 30 | 28.30 | 59 | 55.66 | 11 | 10.38 |
| Sr-89 | 112 | 36 | 32.14 | 54 | 48.21 | 12 | 10.71 |
| Analgesic | Arm | Patients | % | On-study | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|---|
| Acetaminophen/paracetamol | Docetaxel | 120 | 15.85 | 52 | 6.87 | 109 | 14.40 | 33 | 4.74 |
| Docetaxel + ZA | 120 | 15.85 | 51 | 6.74 | 107 | 14.13 | 33 | 4.74 | |
| Docetaxel + Sr-89 | 118 | 15.59 | 55 | 7.27 | 105 | 13.87 | 32 | 4.60 | |
| Docetaxel + ZA + Sr-89 | 116 | 15.32 | 50 | 6.61 | 105 | 13.87 | 32 | 4.60 | |
| Aspirin | Docetaxel | 7 | 0.92 | 2 | 0.26 | 6 | 0.79 | 1 | 0.14 |
| Docetaxel + ZA | 4 | 0.53 | 2 | 0.26 | 3 | 0.40 | 1 | 0.14 | |
| Docetaxel + Sr-89 | 7 | 0.92 | 1 | 0.13 | 6 | 0.79 | 2 | 0.29 | |
| Docetaxel + ZA + Sr-89 | 4 | 0.53 | 0 | 0.00 | 4 | 0.53 | 2 | 0.29 | |
| Diclofenac | Docetaxel | 37 | 4.89 | 21 | 2.77 | 32 | 4.23 | 10 | 1.44 |
| Docetaxel + ZA | 48 | 6.34 | 19 | 2.51 | 35 | 4.62 | 12 | 1.72 | |
| Docetaxel + Sr-89 | 43 | 5.68 | 19 | 2.51 | 36 | 4.76 | 13 | 1.87 | |
| Docetaxel + ZA + Sr-89 | 35 | 4.62 | 17 | 2.25 | 31 | 4.10 | 13 | 1.87 | |
| Ibuprofen | Docetaxel | 29 | 3.83 | 6 | 0.79 | 25 | 3.30 | 9 | 1.29 |
| Docetaxel + ZA | 35 | 4.62 | 12 | 1.59 | 30 | 3.96 | 4 | 0.57 | |
| Docetaxel + Sr-89 | 34 | 4.49 | 12 | 1.59 | 29 | 3.83 | 7 | 1.01 | |
| Docetaxel + ZA + Sr-89 | 38 | 5.02 | 18 | 2.38 | 31 | 4.10 | 8 | 1.15 | |
| Naproxen | Docetaxel | 12 | 1.59 | 8 | 1.06 | 9 | 1.19 | 3 | 0.43 |
| Docetaxel + ZA | 10 | 1.32 | 1 | 0.13 | 8 | 1.06 | 2 | 0.29 | |
| Docetaxel + Sr-89 | 8 | 1.06 | 2 | 0.26 | 6 | 0.79 | 2 | 0.29 | |
| Docetaxel + ZA + Sr-89 | 6 | 0.79 | 2 | 0.26 | 5 | 0.66 | 2 | 0.29 | |
| Codeine | Docetaxel | 55 | 7.27 | 20 | 2.64 | 48 | 6.34 | 10 | 1.44 |
| Docetaxel + ZA | 60 | 7.93 | 25 | 3.30 | 49 | 6.47 | 11 | 1.58 | |
| Docetaxel + Sr-89 | 58 | 7.66 | 21 | 2.77 | 48 | 6.34 | 14 | 2.01 | |
| Docetaxel + ZA + Sr-89 | 55 | 7.27 | 22 | 2.91 | 51 | 6.74 | 10 | 1.44 | |
| Dihydrocodeine | Docetaxel | 16 | 2.11 | 7 | 0.92 | 13 | 1.72 | 2 | 0.29 |
| Docetaxel + ZA | 10 | 1.32 | 5 | 0.66 | 9 | 1.19 | 3 | 0.43 | |
| Docetaxel + Sr-89 | 15 | 1.98 | 7 | 0.92 | 13 | 1.72 | 3 | 0.43 | |
| Docetaxel + ZA + Sr-89 | 14 | 1.85 | 2 | 0.26 | 12 | 1.59 | 4 | 0.57 | |
| Morphine | Docetaxel | 54 | 7.13 | 21 | 2.77 | 44 | 5.81 | 22 | 3.16 |
| Docetaxel + ZA | 53 | 7.00 | 21 | 2.77 | 42 | 5.55 | 19 | 2.73 | |
| Docetaxel + Sr-89 | 59 | 7.79 | 21 | 2.77 | 45 | 5.94 | 26 | 3.74 | |
| Docetaxel + ZA + Sr-89 | 46 | 6.08 | 23 | 3.04 | 38 | 5.02 | 18 | 2.59 | |
| Oxycodone | Docetaxel | 16 | 2.11 | 5 | 0.66 | 10 | 1.32 | 7 | 1.01 |
| Docetaxel + ZA | 21 | 2.77 | 4 | 0.53 | 13 | 1.72 | 11 | 1.58 | |
| Docetaxel + Sr-89 | 18 | 2.38 | 6 | 0.79 | 14 | 1.85 | 6 | 0.86 | |
| Docetaxel + ZA + Sr-89 | 12 | 1.59 | 6 | 0.79 | 8 | 1.06 | 3 | 0.43 | |
| Tramadol | Docetaxel | 29 | 3.83 | 11 | 1.45 | 25 | 3.30 | 5 | 0.72 |
| Docetaxel + ZA | 22 | 2.91 | 10 | 1.32 | 18 | 2.38 | 2 | 0.29 | |
| Docetaxel + Sr-89 | 19 | 2.51 | 6 | 0.79 | 14 | 1.85 | 5 | 0.72 | |
| Docetaxel + ZA + Sr-89 | 26 | 3.43 | 13 | 1.72 | 22 | 2.91 | 8 | 1.15 | |
| Fentanyl patch | Docetaxel | 7 | 0.92 | 3 | 0.40 | 6 | 0.79 | 4 | 0.57 |
| Docetaxel + ZA | 10 | 1.32 | 3 | 0.40 | 9 | 1.19 | 7 | 1.01 | |
| Docetaxel + Sr-89 | 7 | 0.92 | 1 | 0.13 | 4 | 0.53 | 4 | 0.57 | |
| Docetaxel + ZA + Sr-89 | 7 | 0.92 | 2 | 0.26 | 6 | 0.79 | 3 | 0.43 |
| Analgesic | Arm | Instances | OS | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|
| Acetaminophen/paracetamol | Docetaxel | 2851 | 192 | 6.73 | 2194 | 76.96 | 465 | 16.31 |
| Docetaxel + ZA | 3090 | 210 | 6.80 | 2309 | 74.72 | 571 | 18.48 | |
| Docetaxel + Sr-89 | 3120 | 214 | 6.86 | 2466 | 79.04 | 440 | 14.10 | |
| Docetaxel + ZA + Sr-89 | 3165 | 214 | 6.76 | 2311 | 73.02 | 640 | 20.22 | |
| Aspirin | Docetaxel | 76 | 4 | 5.26 | 67 | 88.16 | 5 | 6.58 |
| Docetaxel + ZA | 49 | 9 | 18.37 | 26 | 53.06 | 14 | 28.57 | |
| Docetaxel + Sr-89 | 117 | 4 | 3.42 | 91 | 77.78 | 22 | 18.80 | |
| Docetaxel + ZA + Sr-89 | 125 | 0 | 0.00 | 73 | 58.40 | 52 | 41.60 | |
| Diclofenac | Docetaxel | 1209 | 91 | 7.53 | 972 | 80.40 | 146 | 12.08 |
| Docetaxel + ZA | 1097 | 85 | 7.75 | 821 | 74.84 | 191 | 17.41 | |
| Docetaxel + Sr-89 | 1116 | 76 | 6.81 | 867 | 77.69 | 173 | 15.50 | |
| Docetaxel + ZA + Sr-89 | 986 | 68 | 6.90 | 773 | 78.40 | 144 | 14.60 | |
| Ibuprofen | Docetaxel | 665 | 27 | 4.06 | 513 | 77.14 | 125 | 18.80 |
| Docetaxel + ZA | 590 | 49 | 8.31 | 519 | 87.97 | 22 | 3.73 | |
| Docetaxel + Sr-89 | 551 | 57 | 10.34 | 370 | 67.15 | 125 | 22.69 | |
| Docetaxel + ZA + Sr-89 | 616 | 79 | 12.82 | 390 | 63.31 | 147 | 23.86 | |
| Naproxen | Docetaxel | 302 | 36 | 11.92 | 234 | 77.48 | 32 | 10.60 |
| Docetaxel + ZA | 185 | 2 | 1.08 | 163 | 88.11 | 20 | 10.81 | |
| Docetaxel + Sr-89 | 278 | 8 | 2.88 | 258 | 92.81 | 12 | 4.32 | |
| Docetaxel + ZA + Sr-89 | 146 | 14 | 9.59 | 101 | 69.18 | 31 | 21.23 | |
| Codeine | Docetaxel | 1238 | 70 | 5.65 | 999 | 80.69 | 169 | 13.65 |
| Docetaxel + ZA | 1232 | 87 | 7.06 | 944 | 76.62 | 201 | 16.31 | |
| Docetaxel + Sr-89 | 1139 | 84 | 7.37 | 847 | 74.36 | 208 | 18.26 | |
| Docetaxel + ZA + Sr-89 | 1106 | 106 | 9.58 | 879 | 79.48 | 121 | 10.94 | |
| Dihydrocodeine | Docetaxel | 244 | 25 | 10.25 | 183 | 75.00 | 36 | 14.75 |
| Docetaxel + ZA | 184 | 27 | 14.67 | 112 | 60.87 | 45 | 24.46 | |
| Docetaxel + Sr-89 | 285 | 31 | 10.88 | 225 | 78.95 | 29 | 10.18 | |
| Docetaxel + ZA + Sr-89 | 161 | 3 | 1.86 | 137 | 85.09 | 21 | 13.04 | |
| Morphine | Docetaxel | 2262 | 144 | 6.37 | 1566 | 69.23 | 551 | 24.36 |
| Docetaxel + ZA | 2058 | 141 | 6.85 | 1575 | 76.53 | 342 | 16.62 | |
| Docetaxel + Sr-89 | 2259 | 109 | 4.83 | 1656 | 73.31 | 495 | 21.91 | |
| Docetaxel + ZA + Sr-89 | 1740 | 113 | 6.49 | 1211 | 69.60 | 416 | 23.91 | |
| Oxycodone | Docetaxel | 331 | 38 | 11.48 | 169 | 51.06 | 123 | 37.16 |
| Docetaxel + ZA | 547 | 6 | 1.10 | 370 | 67.64 | 169 | 30.90 | |
| Docetaxel + Sr-89 | 711 | 39 | 5.49 | 560 | 78.76 | 112 | 15.75 | |
| Docetaxel + ZA + Sr-89 | 398 | 33 | 8.29 | 282 | 70.85 | 83 | 20.85 | |
| Tramadol | Docetaxel | 462 | 49 | 10.61 | 388 | 83.98 | 25 | 5.41 |
| Docetaxel + ZA | 276 | 35 | 12.68 | 210 | 76.09 | 31 | 11.23 | |
| Docetaxel + Sr-89 | 455 | 30 | 6.59 | 331 | 72.75 | 94 | 20.66 | |
| Docetaxel + ZA + Sr-89 | 765 | 53 | 6.93 | 601 | 78.56 | 111 | 14.51 | |
| Fentanyl patch | Docetaxel | 172 | 21 | 12.21 | 88 | 51.16 | 63 | 36.63 |
| Docetaxel + ZA | 374 | 15 | 4.01 | 261 | 69.79 | 97 | 25.94 | |
| Docetaxel + Sr-89 | 116 | 1 | 0.86 | 84 | 72.41 | 31 | 26.72 | |
| Docetaxel + ZA + Sr-89 | 211 | 12 | 5.69 | 132 | 62.56 | 67 | 31.75 |
| Analgesic | Treatment | Patients | % | On-study | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|---|
| Acetaminophen/paracetamol | No ZA | 238 | 31.44 | 107 | 14.13 | 214 | 28.27 | 65 | 9.34 |
| ZA | 236 | 31.18 | 101 | 13.34 | 212 | 28.01 | 65 | 9.34 | |
| Aspirin | No ZA | 14 | 1.85 | 3 | 0.40 | 12 | 1.59 | 3 | 0.43 |
| ZA | 8 | 1.06 | 2 | 0.26 | 7 | 0.92 | 3 | 0.43 | |
| Diclofenac | No ZA | 80 | 10.57 | 40 | 5.28 | 68 | 8.98 | 23 | 3.30 |
| ZA | 83 | 10.96 | 36 | 4.76 | 66 | 8.72 | 25 | 3.59 | |
| Ibuprofen | No ZA | 63 | 8.32 | 18 | 2.38 | 54 | 7.13 | 16 | 2.30 |
| ZA | 73 | 9.64 | 30 | 3.96 | 61 | 8.06 | 12 | 1.72 | |
| Naproxen | No ZA | 20 | 2.64 | 10 | 1.32 | 15 | 1.98 | 5 | 0.72 |
| ZA | 16 | 2.11 | 3 | 0.40 | 13 | 1.72 | 4 | 0.57 | |
| Codeine | No ZA | 113 | 14.93 | 41 | 5.42 | 96 | 12.68 | 24 | 3.45 |
| ZA | 115 | 15.19 | 47 | 6.21 | 100 | 13.21 | 21 | 3.02 | |
| Dihydrocodeine | No ZA | 31 | 4.10 | 14 | 1.85 | 26 | 3.43 | 5 | 0.72 |
| ZA | 24 | 3.17 | 7 | 0.92 | 21 | 2.77 | 7 | 1.01 | |
| Morphine | No ZA | 113 | 14.93 | 42 | 5.55 | 89 | 11.76 | 48 | 6.90 |
| ZA | 99 | 13.08 | 44 | 5.81 | 80 | 10.57 | 37 | 5.32 | |
| Oxycodone | No ZA | 34 | 4.49 | 11 | 1.45 | 24 | 3.17 | 13 | 1.87 |
| ZA | 33 | 4.36 | 10 | 1.32 | 21 | 2.77 | 14 | 2.01 | |
| Tramadol | No ZA | 48 | 6.34 | 17 | 2.25 | 39 | 5.15 | 10 | 1.44 |
| ZA | 48 | 6.34 | 23 | 3.04 | 40 | 5.28 | 10 | 1.44 | |
| Fentanyl patch | No ZA | 14 | 1.85 | 4 | 0.53 | 10 | 1.32 | 8 | 1.15 |
| ZA | 17 | 2.25 | 5 | 0.66 | 15 | 1.98 | 10 | 1.44 |
| Analgesic | Treatment | Instances | OS | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|
| Acetaminophen/paracetamol | No ZA | 5971 | 406 | 6.80 | 4660 | 78.04 | 905 | 15.16 |
| ZA | 6255 | 424 | 6.78 | 4620 | 73.86 | 1211 | 19.36 | |
| Aspirin | No ZA | 193 | 8 | 4.15 | 158 | 81.87 | 27 | 13.99 |
| ZA | 174 | 9 | 5.17 | 99 | 56.90 | 66 | 37.93 | |
| Diclofenac | No ZA | 2325 | 167 | 7.18 | 1839 | 79.10 | 319 | 13.72 |
| ZA | 2083 | 153 | 7.35 | 1594 | 76.52 | 335 | 16.08 | |
| Ibuprofen | No ZA | 1216 | 84 | 6.91 | 883 | 72.62 | 250 | 20.56 |
| ZA | 1206 | 128 | 10.61 | 909 | 75.37 | 169 | 14.01 | |
| Naproxen | No ZA | 580 | 44 | 7.59 | 492 | 84.83 | 44 | 7.59 |
| ZA | 331 | 16 | 4.83 | 264 | 79.76 | 51 | 15.41 | |
| Codeine | No ZA | 2377 | 154 | 6.48 | 1846 | 77.66 | 377 | 15.86 |
| ZA | 2338 | 193 | 8.25 | 1823 | 77.97 | 322 | 13.77 | |
| Dihydrocodeine | No ZA | 529 | 56 | 10.59 | 408 | 77.13 | 65 | 12.29 |
| ZA | 345 | 30 | 8.70 | 249 | 72.17 | 66 | 19.13 | |
| Morphine | No ZA | 4521 | 253 | 5.60 | 3222 | 71.27 | 1046 | 23.14 |
| ZA | 3798 | 254 | 6.69 | 2786 | 73.35 | 758 | 19.96 | |
| Oxycodone | No ZA | 1042 | 77 | 7.39 | 729 | 69.96 | 235 | 22.55 |
| ZA | 945 | 39 | 4.13 | 652 | 68.99 | 252 | 26.67 | |
| Tramadol | No ZA | 917 | 79 | 8.62 | 719 | 78.41 | 119 | 12.98 |
| ZA | 1041 | 88 | 8.45 | 811 | 77.91 | 142 | 13.64 | |
| Fentanyl patch | No ZA | 288 | 22 | 7.64 | 172 | 59.72 | 94 | 32.64 |
| ZA | 585 | 27 | 4.62 | 393 | 67.18 | 164 | 28.03 |
| Analgesic | Treatment | Patients | % | On-study | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|---|
| Acetaminophen/paracetamol | No Sr-89 | 240 | 31.70 | 103 | 13.61 | 216 | 28.53 | 66 | 9.48 |
| Sr-89 | 234 | 30.91 | 105 | 13.87 | 210 | 27.74 | 64 | 9.20 | |
| Aspirin | No Sr-89 | 11 | 1.45 | 4 | 0.53 | 9 | 1.19 | 2 | 0.29 |
| Sr-89 | 11 | 1.45 | 1 | 0.13 | 10 | 1.32 | 4 | 0.57 | |
| Celecoxib | No Sr-89 | 2 | 0.26 | 1 | 0.13 | 2 | 0.26 | 0 | 0.00 |
| Diclofenac | No Sr-89 | 85 | 11.23 | 40 | 5.28 | 67 | 8.85 | 22 | 3.16 |
| Sr-89 | 78 | 10.30 | 36 | 4.76 | 67 | 8.85 | 26 | 3.74 | |
| Etodolac | No Sr-89 | 3 | 0.40 | 0 | 0.00 | 3 | 0.40 | 0 | 0.00 |
| Flurbiprofen | No Sr-89 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| Ibuprofen | No Sr-89 | 64 | 8.45 | 18 | 2.38 | 55 | 7.27 | 13 | 1.87 |
| Sr-89 | 72 | 9.51 | 30 | 3.96 | 60 | 7.93 | 15 | 2.16 | |
| Indomethacin | No Sr-89 | 1 | 0.13 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 |
| Sr-89 | 1 | 0.13 | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 | |
| Nabumetone | No Sr-89 | 1 | 0.13 | 1 | 0.13 | 1 | 0.13 | 0 | 0.00 |
| Naproxen | No Sr-89 | 22 | 2.91 | 9 | 1.19 | 17 | 2.25 | 5 | 0.72 |
| Sr-89 | 14 | 1.85 | 4 | 0.53 | 11 | 1.45 | 4 | 0.57 | |
| Buprenorphine | No Sr-89 | 4 | 0.53 | 1 | 0.13 | 4 | 0.53 | 2 | 0.29 |
| Sr-89 | 5 | 0.66 | 0 | 0.00 | 3 | 0.40 | 2 | 0.29 | |
| Codeine | No Sr-89 | 115 | 15.19 | 45 | 5.94 | 97 | 12.81 | 21 | 3.02 |
| Sr-89 | 113 | 14.93 | 43 | 5.68 | 99 | 13.08 | 24 | 3.45 | |
| Dextropropoxyphene | No Sr-89 | 2 | 0.26 | 1 | 0.13 | 2 | 0.26 | 0 | 0.00 |
| Sr-89 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 | |
| Dihydrocodeine | No Sr-89 | 26 | 3.43 | 12 | 1.59 | 22 | 2.91 | 5 | 0.72 |
| Sr-89 | 29 | 3.83 | 9 | 1.19 | 25 | 3.30 | 7 | 1.01 | |
| Fentanyl | No Sr-89 | 5 | 0.66 | 0 | 0.00 | 4 | 0.53 | 2 | 0.29 |
| Sr-89 | 4 | 0.53 | 0 | 0.00 | 2 | 0.26 | 2 | 0.29 | |
| Methadone | Sr-89 | 1 | 0.13 | 0 | 0.00 | 0 | 0.00 | 1 | 0.14 |
| Morphine | No Sr-89 | 107 | 14.13 | 42 | 5.55 | 86 | 11.36 | 41 | 5.89 |
| Sr-89 | 105 | 13.87 | 44 | 5.81 | 83 | 10.96 | 44 | 6.32 | |
| Oxycodone | No Sr-89 | 37 | 4.89 | 9 | 1.19 | 23 | 3.04 | 18 | 2.59 |
| Sr-89 | 30 | 3.96 | 12 | 1.59 | 22 | 2.91 | 9 | 1.29 | |
| Tramadol | No Sr-89 | 51 | 6.74 | 21 | 2.77 | 43 | 5.68 | 7 | 1.01 |
| Sr-89 | 45 | 5.94 | 19 | 2.51 | 36 | 4.76 | 13 | 1.87 | |
| Buprenorphine | No Sr-89 | 1 | 0.13 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 |
| Sr-89 | 1 | 0.13 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 | |
| Fentanyl patch | No Sr-89 | 17 | 2.25 | 6 | 0.79 | 15 | 1.98 | 11 | 1.58 |
| Sr-89 | 14 | 1.85 | 3 | 0.40 | 10 | 1.32 | 7 | 1.01 | |
| Codeine paracetamol | No Sr-89 | 10 | 1.32 | 0 | 0.00 | 8 | 1.06 | 2 | 0.29 |
| Sr-89 | 6 | 0.79 | 1 | 0.13 | 3 | 0.40 | 2 | 0.29 | |
| Dihydrocodeine/paracetamol | No Sr-89 | 3 | 0.40 | 0 | 0.00 | 2 | 0.26 | 1 | 0.14 |
| Sr-89 | 1 | 0.13 | 0 | 0.00 | 1 | 0.13 | 0 | 0.00 |
| Analgesic | Treatment | Instances | OS | % | Pre-CPFS | % | Post-CPFS | % |
|---|---|---|---|---|---|---|---|---|
| Acetaminophen/paracetamol | No Sr-89 | 5941 | 402 | 6.77 | 4503 | 75.80 | 1036 | 17.44 |
| Sr-89 | 6285 | 428 | 6.81 | 4777 | 76.01 | 1080 | 17.18 | |
| Aspirin | No Sr-89 | 125 | 13 | 10.40 | 93 | 74.40 | 19 | 15.20 |
| Sr-89 | 242 | 4 | 1.65 | 164 | 67.77 | 74 | 30.58 | |
| Diclofenac | No Sr-89 | 2306 | 176 | 7.63 | 1793 | 77.75 | 337 | 14.61 |
| Sr-89 | 2102 | 144 | 6.85 | 1640 | 78.02 | 317 | 15.08 | |
| Ibuprofen | No Sr-89 | 1255 | 76 | 6.06 | 1032 | 82.23 | 147 | 11.71 |
| Sr-89 | 1167 | 136 | 11.65 | 760 | 65.12 | 272 | 23.31 | |
| Naproxen | No Sr-89 | 487 | 38 | 7.80 | 397 | 81.52 | 52 | 10.68 |
| Sr-89 | 424 | 22 | 5.19 | 359 | 84.67 | 43 | 10.14 | |
| Codeine | No Sr-89 | 2470 | 157 | 6.36 | 1943 | 78.66 | 370 | 14.98 |
| Sr-89 | 2245 | 190 | 8.46 | 1726 | 76.88 | 329 | 14.65 | |
| Dihydrocodeine | No Sr-89 | 428 | 52 | 12.15 | 295 | 68.93 | 81 | 18.93 |
| Sr-89 | 446 | 34 | 7.62 | 362 | 81.17 | 50 | 11.21 | |
| Morphine | No Sr-89 | 4320 | 285 | 6.60 | 3141 | 72.71 | 893 | 20.67 |
| Sr-89 | 3999 | 222 | 5.55 | 2867 | 71.69 | 911 | 22.78 | |
| Oxycodone | No Sr-89 | 878 | 44 | 5.01 | 539 | 61.39 | 292 | 33.26 |
| Sr-89 | 1109 | 72 | 6.49 | 842 | 75.92 | 195 | 17.58 | |
| Tramadol | No Sr-89 | 738 | 84 | 11.38 | 598 | 81.03 | 56 | 7.59 |
| Sr-89 | 1220 | 83 | 6.80 | 932 | 76.39 | 205 | 16.80 | |
| Fentanyl patch | No Sr-89 | 546 | 36 | 6.59 | 349 | 63.92 | 160 | 29.30 |
| Sr-89 | 327 | 13 | 3.98 | 216 | 66.06 | 98 | 29.97 |
FIGURE 55.
Depiction of time between SREs when first event was radiation: no ZA. Dark blue, surgery; mid-blue, change; dark green, radiation; mid-green, fracture; light green, SCC; white, none.
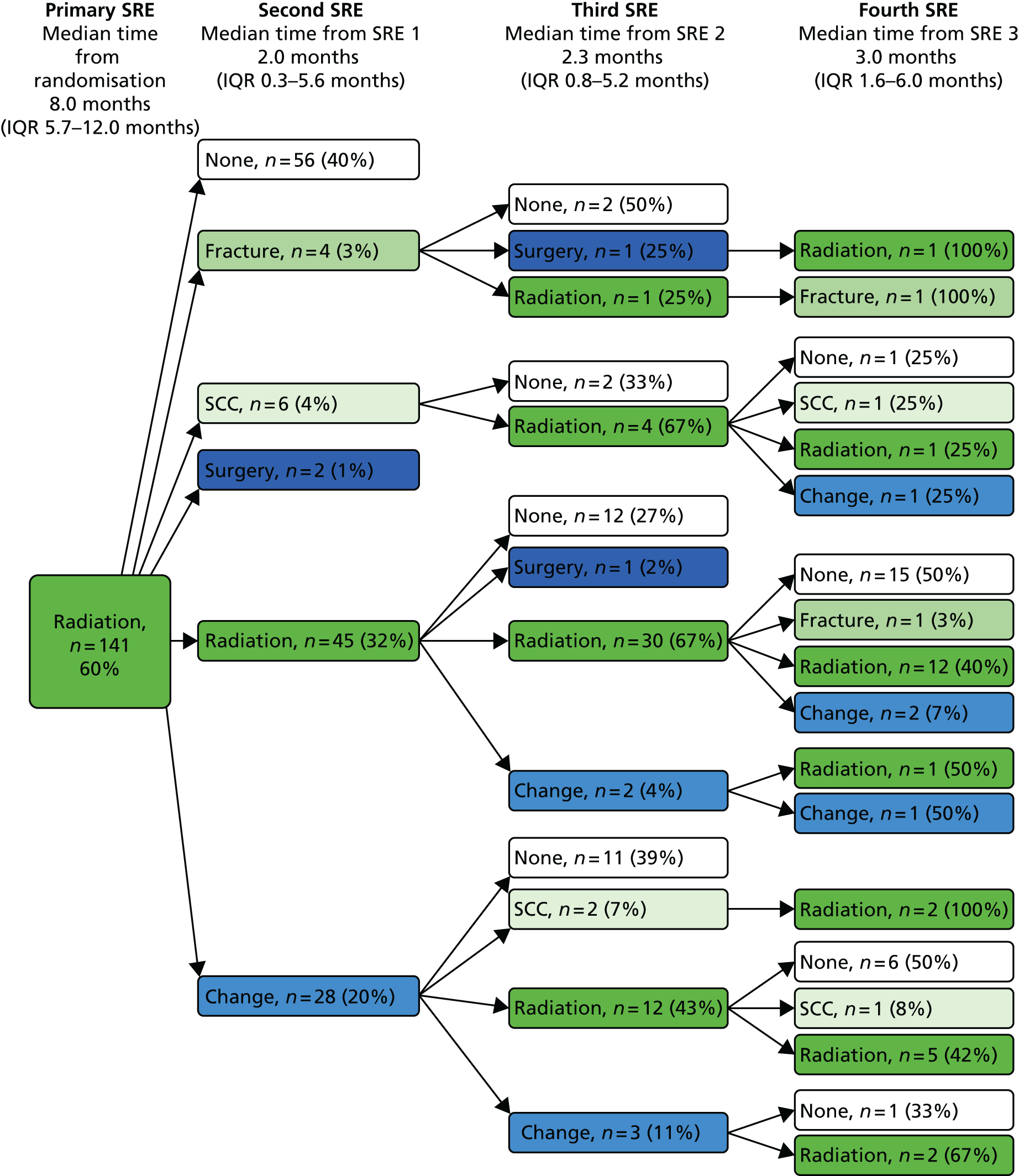
FIGURE 56.
Depiction of time between SREs when first event was radiation: ZA. Mid-blue, change; dark green, radiation; mid-green, fracture; light green, SCC; white, none.

FIGURE 57.
Depiction of time between SREs when first event was fracture: no ZA. Dark blue, surgery; mid-blue, change; dark green, radiation; mid-green, fracture; light green, SCC; white, none.

FIGURE 58.
Depiction of time between SREs when first event was SCC: no ZA. Dark blue, surgery; mid-blue, change; light blue, hypercalcaemia; dark green, radiation; light green, SCC; white, none.
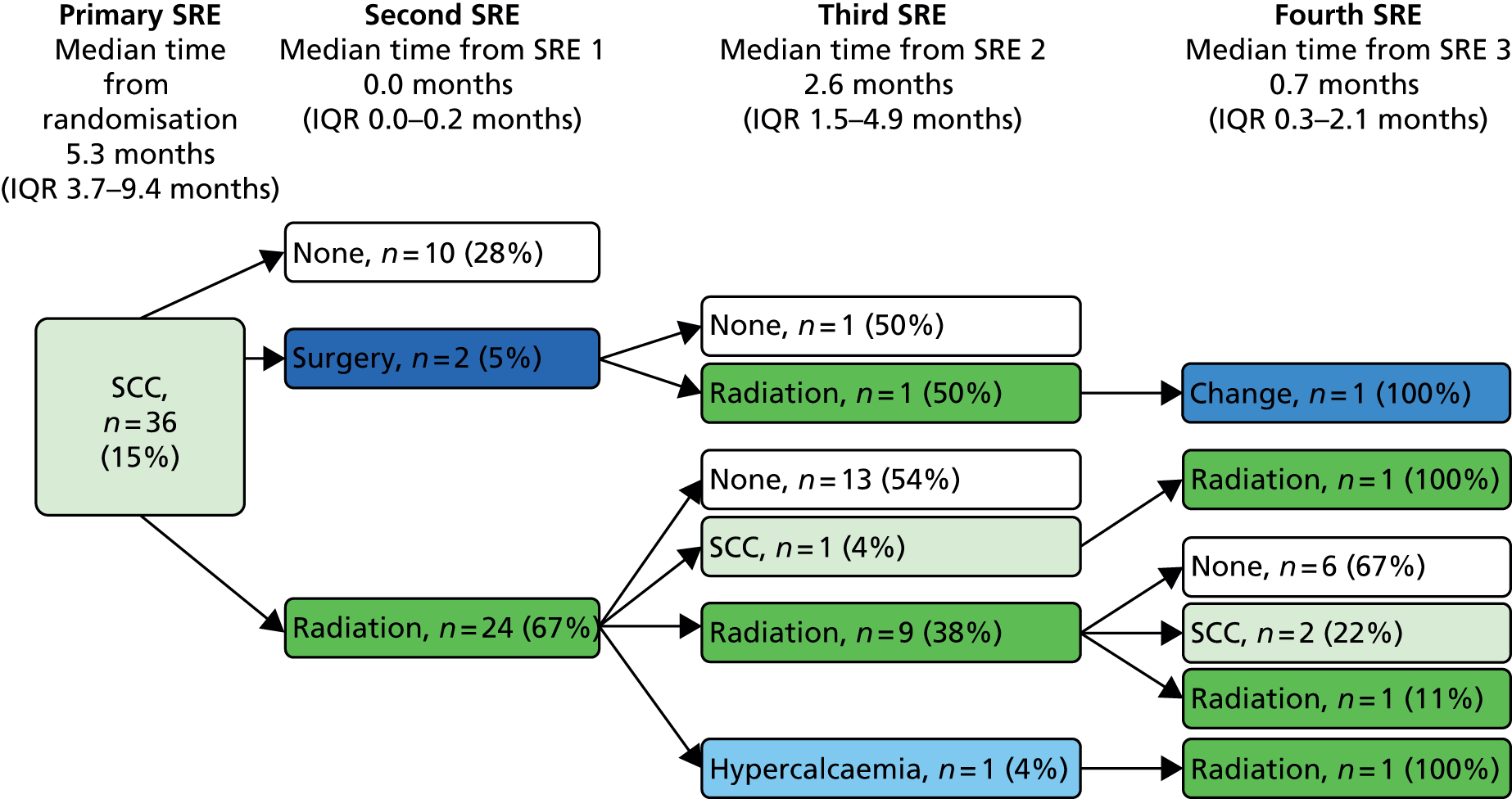
FIGURE 59.
Depiction of time between SREs when first event was fracture: ZA. Dark blue, surgery; mid-blue, change; dark green, radiation; mid-green, fracture; white, none.
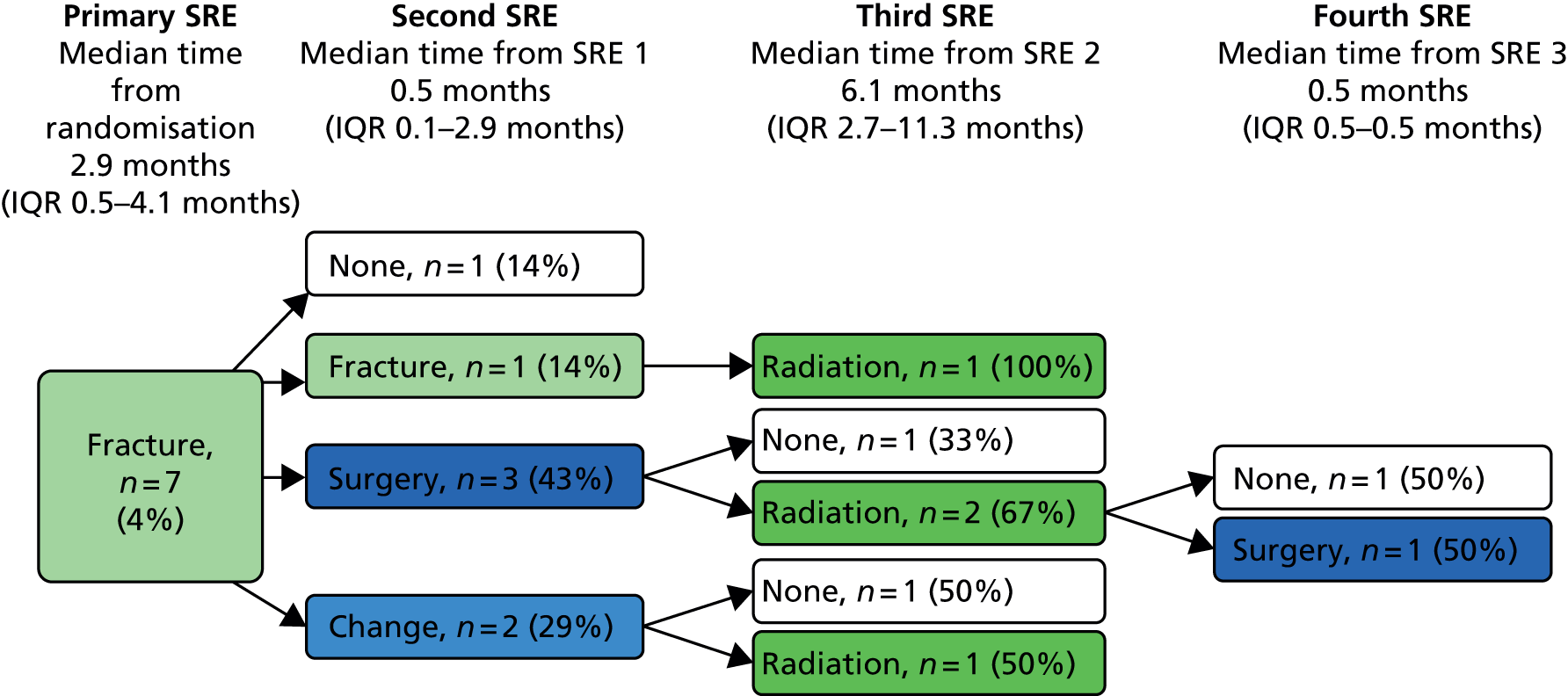
FIGURE 60.
Depiction of time between SREs when first event was SCC: ZA. Dark blue, surgery; mid-blue, change; dark green, radiation; light green, SCC; white, none.
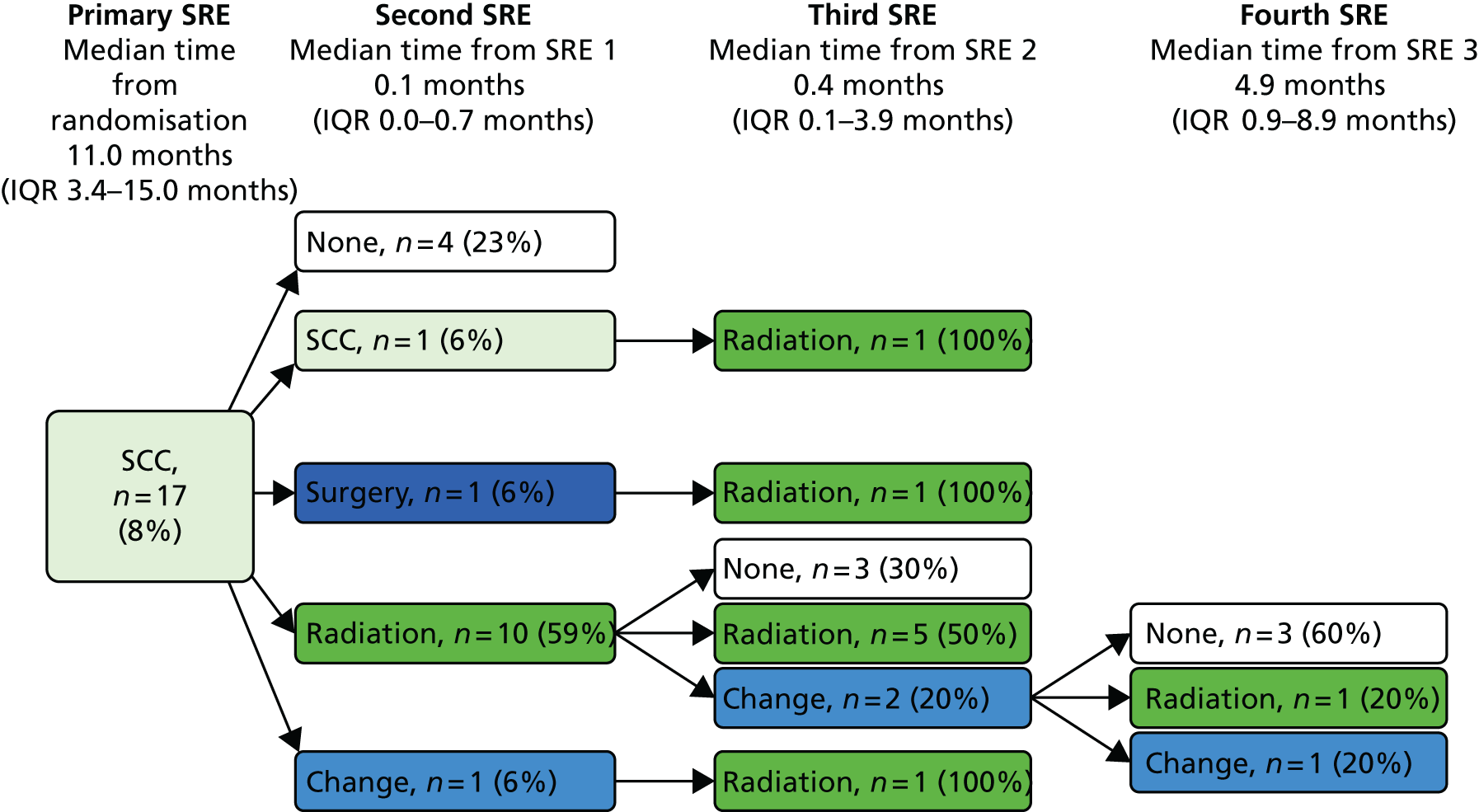
FIGURE 61.
Depiction of time between SREs when first event was change in therapy: no ZA. Dark blue, surgery; mid-blue, change; dark green, radiation; mid-green, fracture; light green, SCC; white, none.

FIGURE 62.
Depiction of time between SREs when first event was surgery: no ZA. Dark blue, surgery; dark green, radiation.
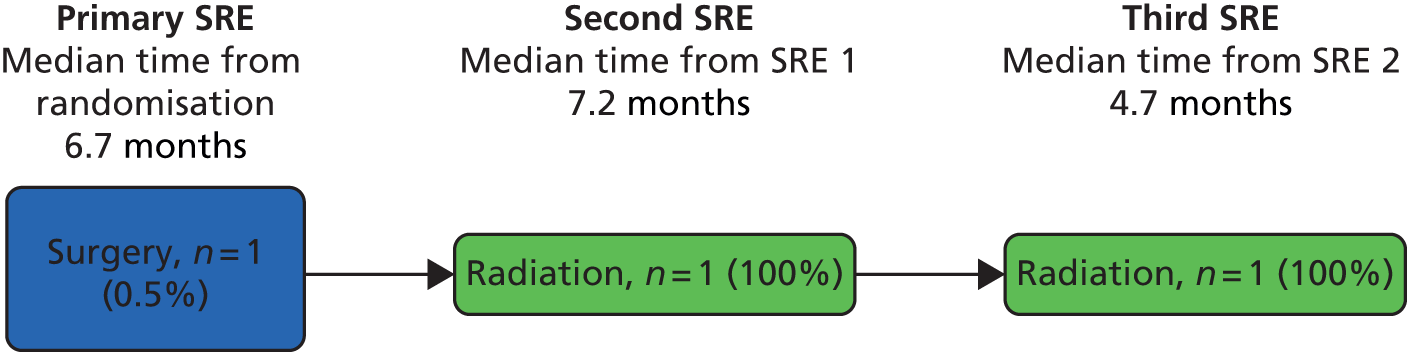
FIGURE 63.
Depiction of time between SREs when first event was hypercalcaemia: no ZA. Light blue, hypercalcaemia.
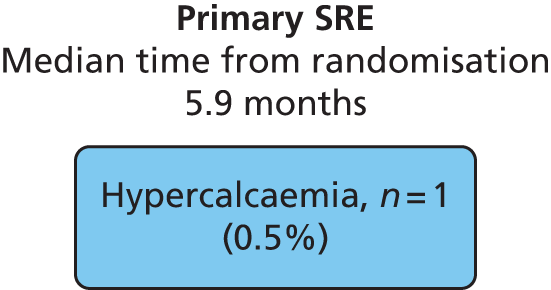
FIGURE 64.
Depiction of time between SREs when first event was change in therapy: ZA. Mid-blue, change; dark green, radiation; light green, SCC; white, none.

FIGURE 65.
Depiction of time between SREs when first event was radiation: no Sr-89. Dark blue, surgery; mid-blue, change; dark green, radiation; mid-green, fracture; light green, SCC; white, none.
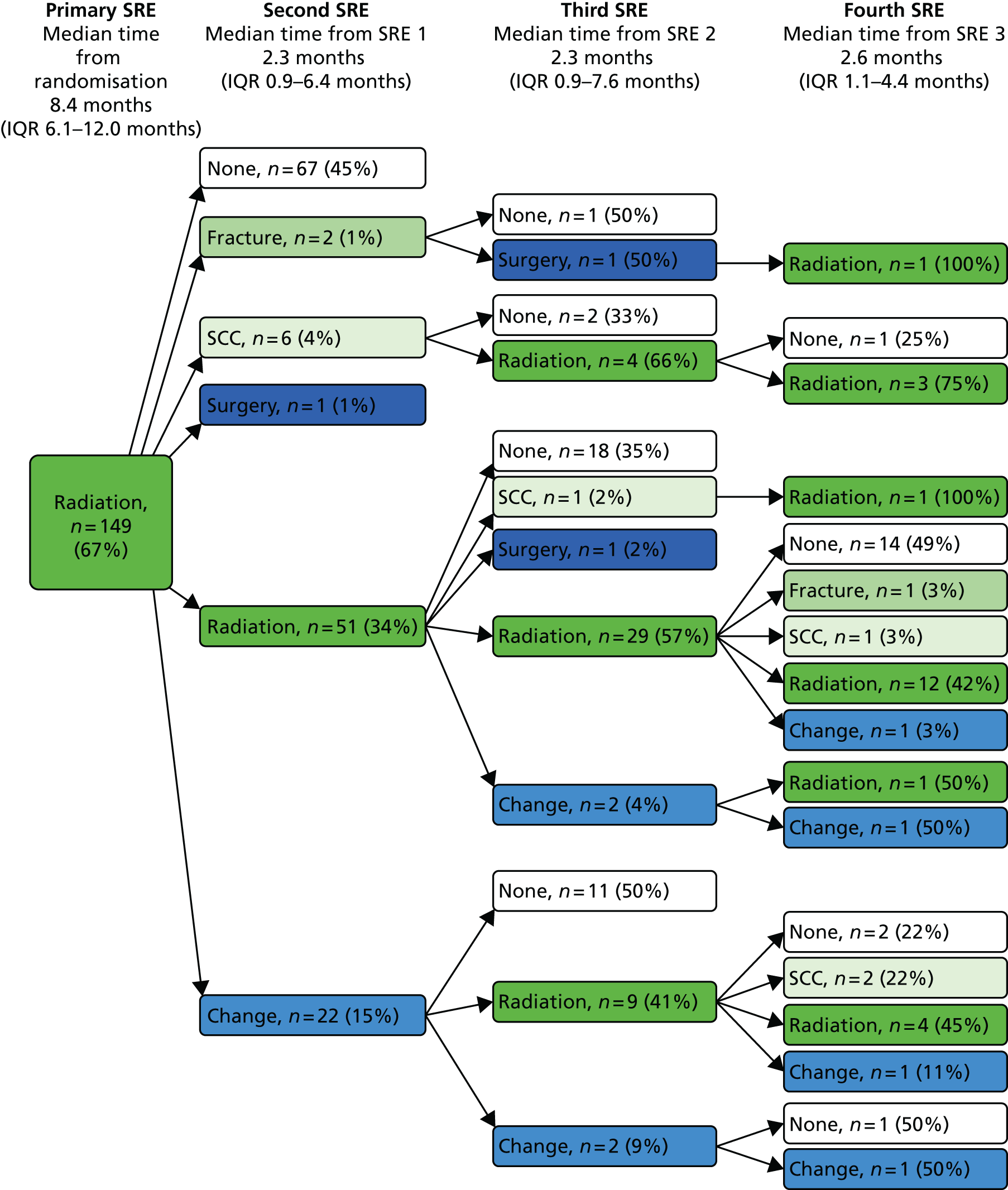
FIGURE 66.
Depiction of time between SREs when first event was radiation: Sr-89. Dark blue, surgery; mid-blue, change; dark green, radiation; mid-green, fracture; light green, SCC; white, none.
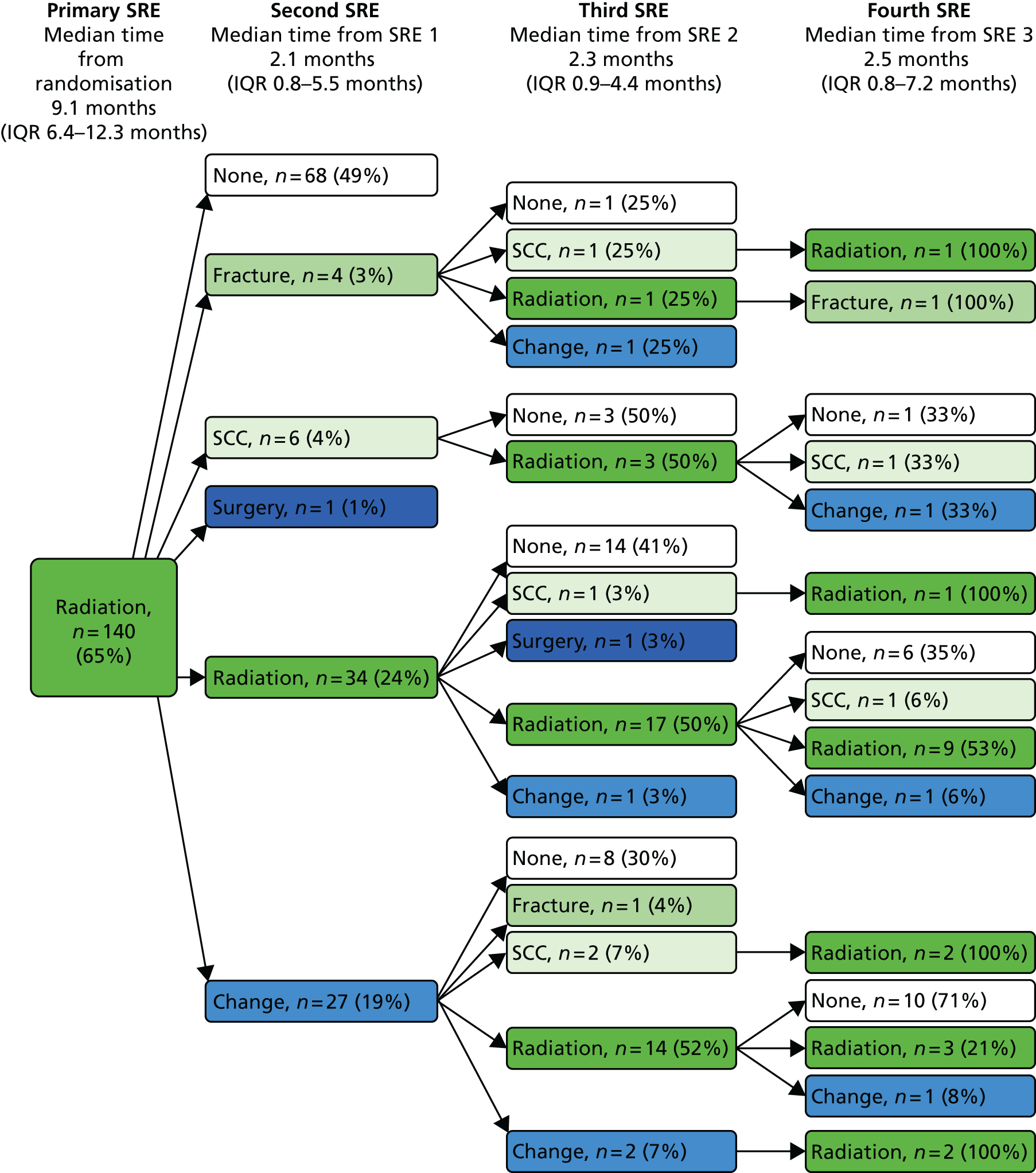
FIGURE 67.
Depiction of time between SREs when first event was fracture: no Sr-89. Dark blue, surgery; mid-blue, change; dark green, radiation; mid-green, fracture; white, none.
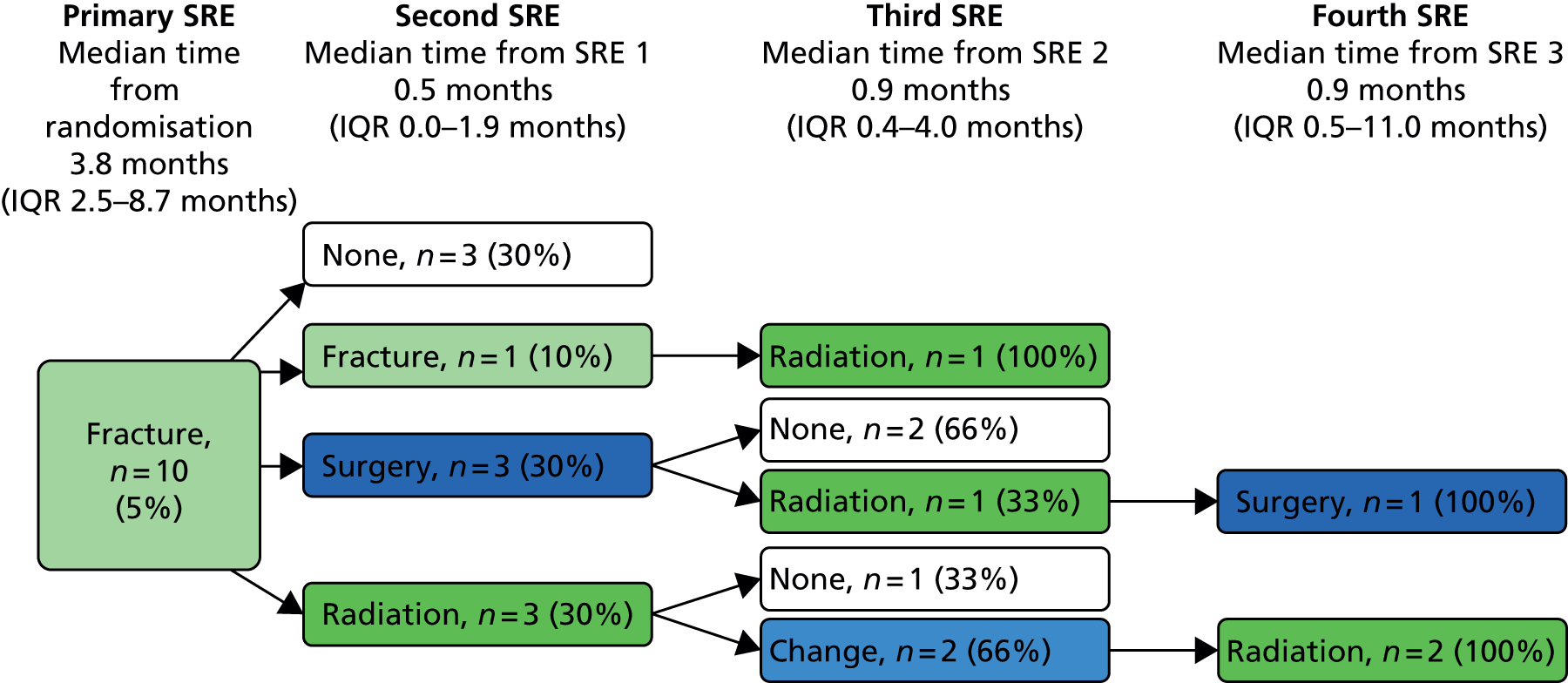
FIGURE 68.
Depiction of time between SREs when first event was SCC: no Sr-89. Dark blue, surgery; mid-blue, change; dark green, radiation; light green, SCC; white, none.
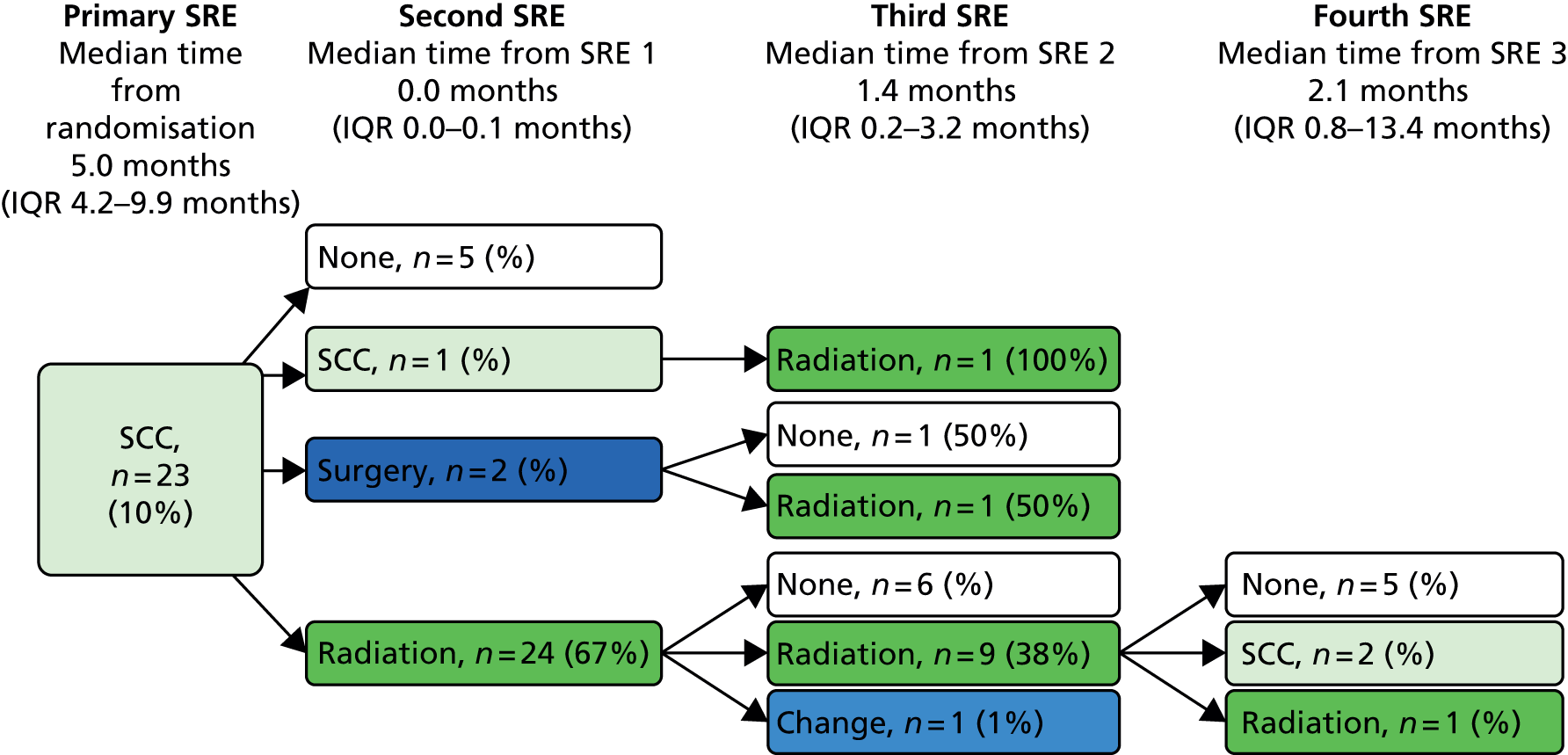
FIGURE 69.
Depiction of time between SREs when first event was fracture: Sr-89. Dark blue, surgery; mid-blue, change; dark green, radiation; mid-green, fracture; light green, SCC; white, none.

FIGURE 70.
Depiction of time between SREs when first event was SCC: Sr-89. Dark blue, surgery; mid-blue, change; light blue, hypercalcaemia; dark green, radiation; light green, SCC; white, none.

FIGURE 71.
Depiction of time between SREs when first event was change in antineoplastic therapy: no Sr-89. Dark blue, surgery; mid-blue, change; dark green, radiation; mid-green, fracture; light green, SCC; white, none.
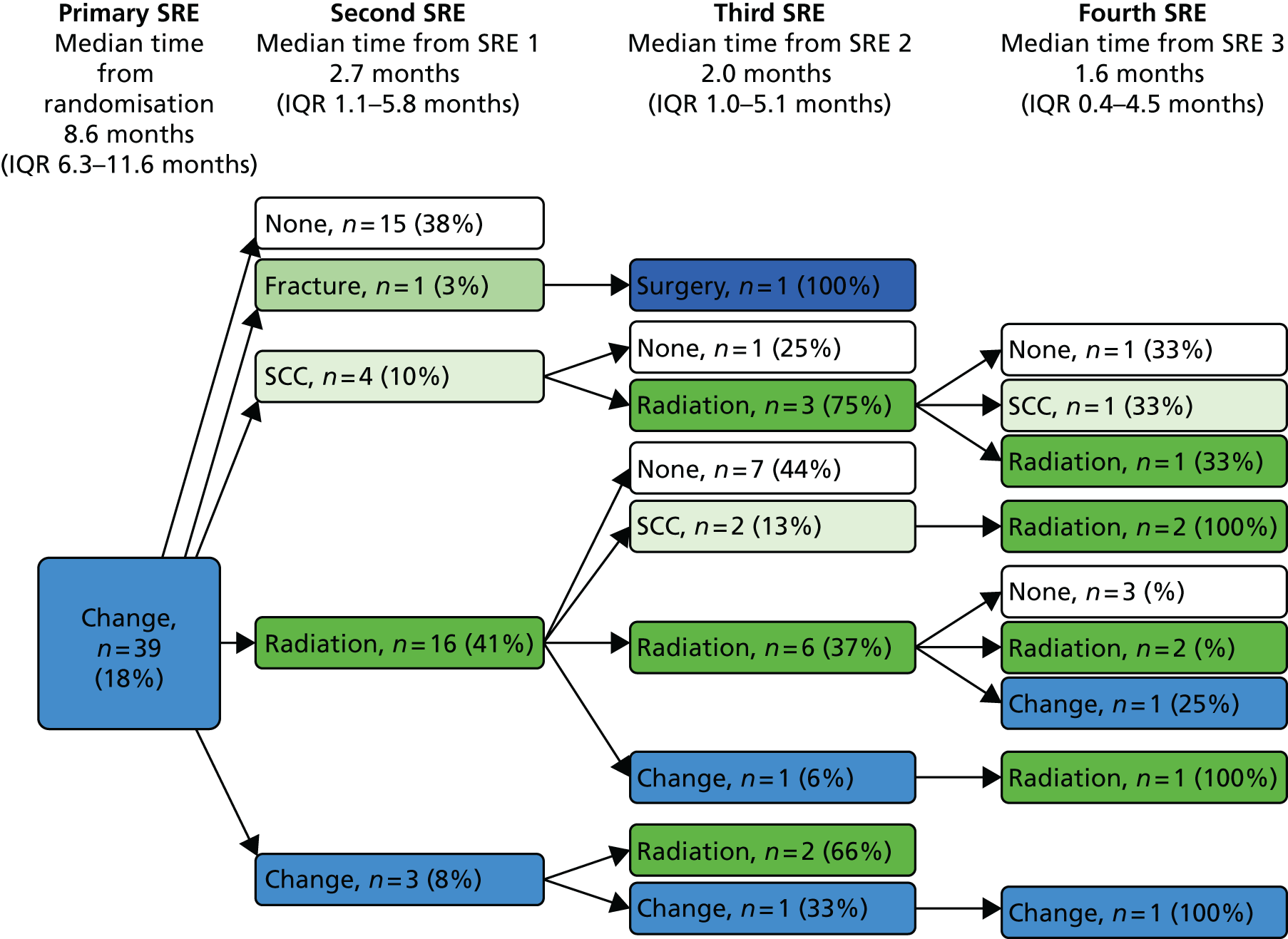
FIGURE 72.
Depiction of time between SREs when first event was surgery: no Sr-89. Dark blue, surgery; dark green, radiation.
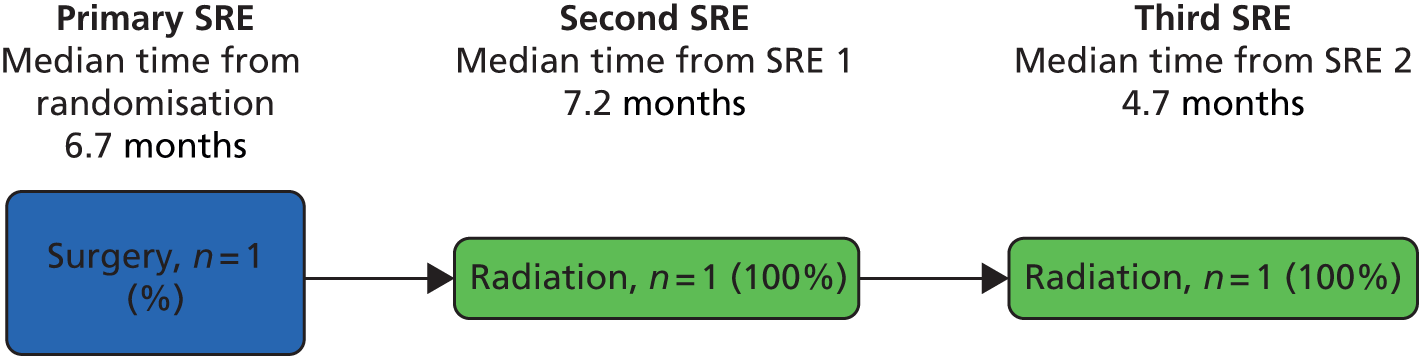
FIGURE 73.
Depiction of time between SREs when first event was hypercalcaemia: Sr-89. Light blue, hypercalcaemia; white, none.
FIGURE 74.
Depiction of time between SREs when first event was change in antineoplastic therapy: Sr-89. Dark blue, surgery; mid-blue, change; dark green, radiation; mid-green, fracture; light green, SCC; white, none.
Appendix 6 Resource use tables for economic evaluation
| Treatment/service | ZA | No ZA | ||||
|---|---|---|---|---|---|---|
| n | % | Mean no. of cycles/units used | n | % | Mean no. of cycles/units used | |
| Trial treatments | ||||||
| Docetaxel + prednisolone | 342 | 98 | 6.31 | 349 | 98 | 6.11 |
| ZA | 342 | 98 | 6.13 | 0 | 0 | 0.00 |
| Sr-89 | 125 | 36 | 1.00 | 120 | 34 | 1.00 |
| ZA as follow-up treatment | 192 | 55 | 5.11 | 1 | 0 | 3.00 |
| Concomitant medications | ||||||
| Radiotherapy | 177 | 51 | 1.65 | 204 | 57 | 1.95 |
| Abiraterone | 56 | 16 | 1.00 | 68 | 19 | 1.00 |
| ZA as concomitant medication | 73 | 21 | 3.77 | 63 | 18 | 1.94 |
| Sr-89 as concomitant medication | 15 | 4 | 1.00 | 21 | 6 | 1.00 |
| Blood transfusion | 31 | 9 | 2.10 | 22 | 6 | 2.50 |
| Cabazitaxel | 18 | 5 | 1.56 | 12 | 3 | 2.33 |
| Docetaxel as concomitant medication | 34 | 10 | 2.94 | 38 | 11 | 3.13 |
| Mitoxantrone | 23 | 7 | 2.30 | 15 | 4 | 1.87 |
| Surgery | 18 | 5 | 1.20 | 5 | 1 | 1.17 |
| Outpatient appointments and inpatient stay | ||||||
| Hospital outpatient appointment | 296 | 85 | 5.79 | 293 | 82 | 5.25 |
| Hospital inpatient stay | 231 | 66 | 8.00 | 250 | 70 | 8.28 |
| GP appointments | 311 | 89 | 5.03 | 328 | 92 | 5.61 |
| Treatment/service | Sr-89 | No Sr-89 | ||||
|---|---|---|---|---|---|---|
| n | % | Mean no. of cycles/units used | n | % | Mean no. of cycles/units used | |
| Trial treatments | ||||||
| Docetaxel + prednisolone | 345 | 99 | 6.23 | 346 | 97 | 6.19 |
| ZA | 172 | 49 | 6.14 | 170 | 48 | 6.12 |
| Sr-89 | 245 | 70 | 1.00 | 0 | 0 | 0.00 |
| ZA as follow-up treatment | 90 | 26 | 5.10 | 103 | 29 | 5.10 |
| Concomitant medications | ||||||
| Radiotherapy | 184 | 53 | 1.67 | 197 | 55 | 1.94 |
| Abiraterone | 59 | 17 | 1.00 | 65 | 18 | 1.00 |
| ZA as concomitant medication | 61 | 17 | 2.85 | 75 | 21 | 2.97 |
| Sr-89 as concomitant medication | 16 | 5 | 1.13 | 20 | 6 | 1.00 |
| Blood transfusion | 26 | 7 | 2.31 | 27 | 8 | 2.22 |
| Cabazitaxel | 19 | 5 | 1.84 | 11 | 3 | 1.91 |
| Docetaxel as concomitant medication | 36 | 10 | 3.11 | 36 | 10 | 2.97 |
| Mitoxantrone | 20 | 6 | 2.00 | 18 | 5 | 2.28 |
| Surgery | 13 | 4 | 1.23 | 10 | 3 | 1.10 |
| Outpatient appointments and inpatient stay | ||||||
| Hospital outpatient appointment | 227 | 65 | 5.27 | 254 | 71 | 5.78 |
| Hospital inpatient stay | 295 | 84 | 8.52 | 294 | 82 | 7.80 |
| GP appointments | 311 | 89 | 5.10 | 328 | 92 | 5.55 |
| Number of instances of radiotherapy | No ZA | ZA | Total |
|---|---|---|---|
| 0 | 153 | 173 | 326 |
| 1 | 108 | 114 | 222 |
| 2 | 42 | 39 | 81 |
| 3 | 29 | 10 | 39 |
| 4 | 12 | 8 | 20 |
| 5 | 9 | 3 | 12 |
| 6 | 3 | 1 | 4 |
| 8 | 1 | 1 | 2 |
| 9 | 0 | 1 | 1 |
| Number of instances of radiotherapy | No ZA | ZA |
|---|---|---|
| Total number of instances of radiotherapy | 398 | 292 |
| Number of instances of radiotherapy | No Sr-89 | Sr-89 | Total |
|---|---|---|---|
| 0 | 160 | 166 | 326 |
| 1 | 111 | 111 | 222 |
| 2 | 36 | 45 | 81 |
| 3 | 25 | 14 | 39 |
| 4 | 12 | 8 | 20 |
| 5 | 8 | 4 | 12 |
| 6 | 2 | 2 | 4 |
| 8 | 2 | 0 | 2 |
| 9 | 1 | 0 | 1 |
| Number of instances of radiotherapy | No Sr-89 | Sr-89 |
|---|---|---|
| Total number of instances of radiotherapy | 383 | 307 |
| Average number of instances of radiotherapy | No ZA | ZA |
|---|---|---|
| For patients who had radiotherapy | 1.95 | 1.65 |
| For all patients in the group | 1.11 | 0.83 |
| Average number of instances of radiotherapy | No Sr-89 | Sr-89 |
|---|---|---|
| For patients who had radiotherapy | 1.94 | 1.67 |
| For all patients in the group | 1.07 | 0.88 |
List of abbreviations
- AE
- adverse event
- BCa
- bias corrected and accelerated
- BNF
- British National Formulary
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CPFS
- clinical progression-free survival
- CRCTU
- Cancer Research Clinical Trials Unit
- CRF
- case report form
- CRPC
- castration-refractory prostate cancer
- CT
- computed tomography
- DMC
- Data Monitoring Committee
- DXA
- dual-energy X-ray absorption scan
- ECOG
- Eastern Cooperative Oncology Group
- eMIT
- electronic market information tool
- EQ-5D
- European Quality of Life 5-Dimensions
- FACT-P
- Functional Assessment of Cancer Therapy – Prostate
- HR
- hazard ratio
- HRPC
- hormone-refractory prostate cancer
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- mCRPC
- metastatic castration-refractory prostate cancer
- NICE
- National Institute for Health and Care Excellence
- OS
- overall survival
- PPFI
- pain progression-free interval
- PSA
- prostate-specific antigen
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- SAE
- serious adverse event
- SCC
- spinal cord compression
- Sr-89
- strontium-89
- SRE
- skeletal-related event
- SREFI
- skeletal-related event-free interval
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- ZA
- zoledronic acid