Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/13/02. The contractual start date was in November 2009. The draft report began editorial review in August 2015 and was accepted for publication in January 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors” report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Graves et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and study objectives
In the UK, the incidence of primary total hip arthroplasty (THA) is 722 per 100,000 operations and the number of all hip procedures performed in 2012 was 86,488. 1,2 Surgical site infections (SSIs) following THAs have been reported to occur in 0.7% of patients,3 but this reflects only 1 year post surgery. Infections are likely to lead to a prolonged hospital stay, increased mortality and reduced quality of life as well as additional expenditures by health services and patients. 4–6 A common consequence of infection is revision surgery, which is very costly and negatively affects the quality of life of patients for a prolonged period. Numerous approaches to preventing these infections exist, but practices vary and the cost-effectiveness of alternative strategies is largely unknown. Systematic reviews of some infection prevention measures have been undertaken, yet they do not address the related questions of which strategies or combination of strategies are most effective in reducing infection risk and what decision-makers should do if they wish to be efficient in the resource-constrained NHS. 7–10 This research is about understanding competing infection prevention methods relevant for THA in the NHS. Using an economic decision model, the cost-effectiveness of each strategy and bundles of strategies are estimated. If a strategy can decrease the incidence of infection following hip arthroplasty, costs could be saved and health outcomes improved. Extra infection prevention does, however, incur additional costs. The change to total costs and health outcomes for each strategy needs to be estimated and presented in a cost-effectiveness framework, and the results then interpreted for decision-making.
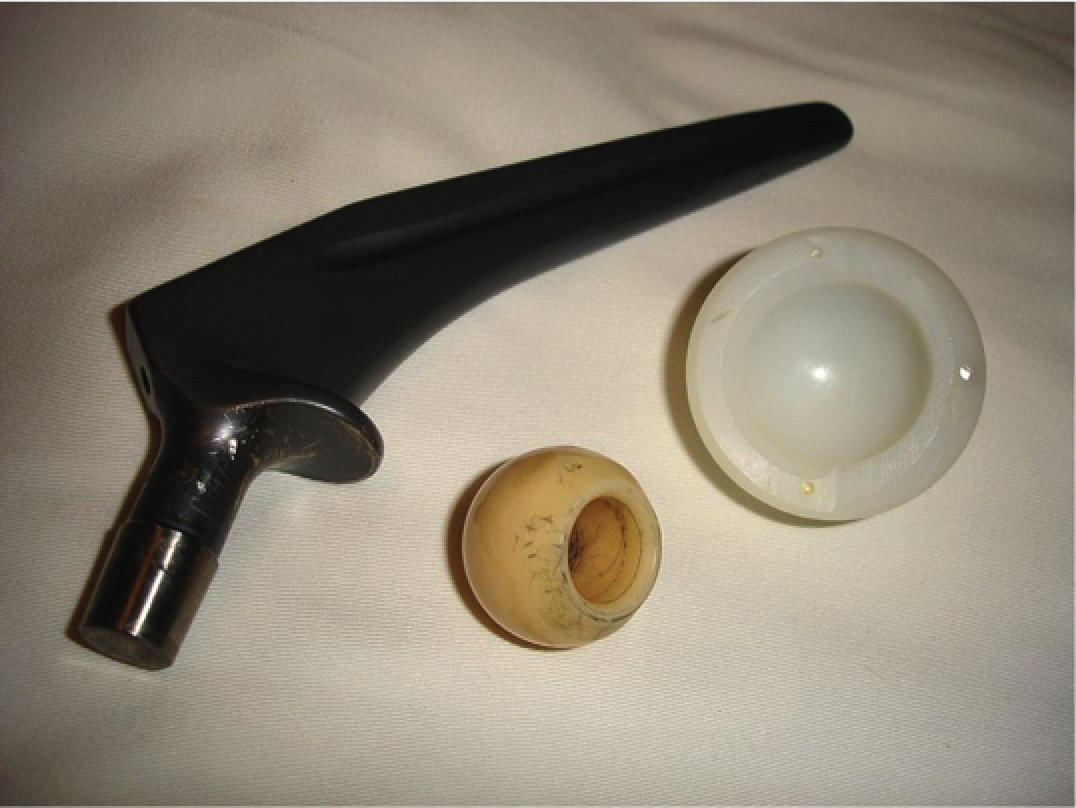
Hip replacements are procedures in which the hip joint is partially or totally replaced by an artificial prosthesis. This can be a primary replacement or a revision of a previous replacement that has failed. The hip joint is a ball-and-socket joint, with the femoral head (top of the femur) sitting in the acetabular cup of the pelvis. The three major components of an implant for THA are the stem, head and cup (Figure 1). Partial replacements involve the replacement of the stem and/or head only, but not the acetabular component. Major revisions involve the removal of the diseased or fractured joint and replacement with a full artificial prosthesis.
FIGURE 1.
Hip arthroplasty components.
Incidence of infections and revisions among hip replacements
Data from 235 NHS and independent sector treatment centres are shown in Table 1. The information was submitted as part of the Public Health England (PHE) national surveillance of SSI service. 3 Data were reported on 549,495 procedures and SSIs organised into 17 surgical categories from April 2008 to May 2013. Fifty-six per cent of hospitals that perform total hip replacement (THR) contributed data for patients who underwent hip prosthesis surgery. They show a low cumulative incidence of SSI of 0.7% within 1 year of hip prosthesis surgery. No clear trend was detected in the incidence over the 5-year period (Figure 2).
| Surgical category | Number of operations | Number of hospitals | Number of inpatients | Inpatient and readmissions, n (%) | Median time to infection (days) | Incidence density/1000 inpatient days |
|---|---|---|---|---|---|---|
| Abdominal hysterectomy | 5073 | 29 | 31 | 77 (1.5, 95% CI 1.2 to 1.9) | 9 | 1.3 (95% CI 0.9 to 1.9) |
| Bile duct, liver and pancreatic surgery | 2124 | 6 | 121 | 137 (6.5, 95% CI 5.4 to 7.6) | 8 | 4.9 (95% CI 4.1 to 5.9) |
| Breasta | 5081 | 20 | 7 | 52 (1.0, 95% CI 0.8 to 1.3) | 13 | 0.7 (95% CI 0.3 to 1.5) |
| Cholecystectomy | 887 | 6 | 31 | 37 (4.2, 95% CI 3.0 to 5.7) | 7 | 4.6 (95% CI 3.1 to 6.6) |
| CABG | 29,144 | 23 | 936 | 1275 (4.4, 95% CI 4.1 to 4.6) | 12 | 3.2 (95% CI 3.0 to 3.4) |
| Cardiac (non-CABG)a | 6497 | 11 | 59 | 83 (1.3, 95% CI 1.0 to 1.6) | 12 | 0.7 (95 % CI 0.6 to 0.9) |
| Craniala | 2832 | 4 | 17 | 45 (1.6, 95% CI 1.2 to 2.1) | 19 | 0.7 (95% CI 0.4 to 1.2) |
| Gastric | 1154 | 8 | 29 | 31 (2.7, 95% CI 1.8 to 3.8) | 8 | 2.6 (95% CI 1.7 to 3.8) |
| Hip prosthesis | 170,158 | 199 | 577 | 1240 (0.7, 95% CI 0.7 to 0.8) | 14 | 0.5 (95% CI 0.5 to 0.6) |
| Knee prosthesis | 182,566 | 193 | 377 | 1096 (0.6, 95% CI 0.6 to 0.6) | 16 | 0.4 (95% CI 0.3 to 0.4) |
| Large bowel | 16,734 | 49 | 1518 | 1772 (10.6, 10.1 to 11.1) | 8 | 8.3 (95% CI 7.9 to 8.7) |
| Limb amputation | 2217 | 19 | 60 | 73 (3.3, 95% CI 2.6 to 4.1) | 10 | 1.8 (95% CI 1.4 to 2.3) |
| Reduction of long bone fractureb | 13,640 | 37 | 91 | 167 (1.2, 95% CI 1.1 to 1.4) | 16 | 0.8 (95% CI 0.6 to 1.0) |
| Repair of neck of femurb | 74,311 | 130 | 905 | 1149 (1.5, 95% CI 1.5 to 1.6) | 14 | 0.7 (95% CI 0.7 to 0.8) |
| Small bowel | 3572 | 20 | 211 | 230 (6.4, 95% CI 5.7 to 7.3) | 8 | 4.9 (95% CI 4.3 to 5.7) |
| Spinalb | 26,249 | 27 | 127 | 283 (1.1, 95% CI 1.0 to 1.2) | 14 | 0.9 (95% CI 0.8 to 1.1) |
| Vascular | 7256 | 30 | 147 | 203 (2.8, 95% CI 2.4 to 3.2) | 11 | 2.2 (95% CI 1.8 to 2.5) |
| Total | 549,495 | 811 | 5244 | 7950 |
FIGURE 2.
Trends in the annual cumulative incidence of SSI (%) in the orthopaedic surveillance categories, with lower and upper 95% CIs, within NHS hospitals in England. (a) Repair of neck of femur; (b) hip prosthesis; (c) reduction of long bone fracture; and (d) knee prothesis. Source: Elgohari et al. 3 Crown copyright and reproduced with the permission of Public Health England under delegated authority from the Controller of HMSO.




The National Joint Registry (NJR)11 collects data that quantify some of the long-term consequences of deep infection, such as revisions. In 2012, 10,040 hip revision procedures were recorded, of which 12% were a result of infection, compared with 11% in 2011. 1 The burden of revision operations lies primarily with NHS hospitals, which carried out 83% of revision procedures, compared with 15% in independent sector hospitals. These statistics contrast with primary hip replacements, 69% of which were carried out in NHS hospitals and 27% in independent hospitals. Patients who had a revision operation were also less fit than patients undergoing primary hip replacement, with one-third of patients for revision surgery being identified as grade 3 under the American Society of Anaesthetists scoring system. 1
Definitions and diagnosis of infection
We excluded superficial infections from this analysis as they are generally easy to treat and do not present serious risks for patients. Reducing deep incisional and organ/space infections is the primary focus. Definitions used have been taken from the Surgical Site Infection Surveillance Service (SSISS) of PHE (formerly the Health Protection Agency). 3
Deep incisional infection
This is defined as infection involving the deep tissues, such as fascia and muscle layers. Infections are recorded if they arise within 30 days of surgery if no implant is in place or within 1 year if an implant is in place and the infection appears to be related to the surgical procedure. At least one of the following criteria must also be met.
-
Criterion 1: purulent drainage from the deep incision, but not from the organ/space component of the surgical site.
-
Criterion 2: the deep incision yields organisms from the culture of aseptically aspirated fluid or tissue, or from a swab and pus cells are present.
-
Criterion 3: a deep incision that spontaneously dehisces or is deliberately opened by a surgeon when the patient has a least one of the following symptoms or signs: fever (> 38 °C), localised pain or tenderness unless the incision is culture negative.
-
Criterion 4: an abscess or other evidence of infection involving the deep incision that is found by direct examination during reoperation, or by histopathological or radiological examination.
-
Criterion 5: diagnosis of a deep incisional SSI by an attending clinician.
Organ/space infection
This is defined as a SSI involving any part of the anatomy, such as an organ or space other than the incision, opened or manipulated during the surgical procedure. Infections are recorded if they occur within 30 days of surgery if no implant is in place or within 1 year if an implant is in place and the infection appears to be related to the surgical procedure. At least one of the following criteria must also be met.
-
Criterion 1: purulent drainage from a drain that is placed through a stab wound into the organ/space.
-
Criterion 2: the organ/space yields organisms from the culture of aseptically aspirated fluid or tissue, or from a swab and pus cells are present.
-
Criterion 3: an abscess or other evidence of infection involving the organ/space that is found by direct examination, during reoperation, or by histopathological or radiological examination.
-
Citerion 4: diagnosis of an organ/space infection by an attending clinician.
These definitions are broadly comparable with both those used by the US Centers for Disease Control and Prevention, which implement the National Nosocomial Infections Surveillance System,12 and those used by the European Centre for Disease Control Health Care Associated Infection – Net Consortium. 13
Aetiology, microbiology and risk factors
Pathogens that cause SSI may be derived from the patient’s own microbial flora on their skin or body, from the skin or mucous membranes of operating personnel, or from the operating room environment (instruments or equipment used during the procedure and airborne particles). Occasionally, micro-organisms from a distant infection in the body can establish a SSI by attaching to a prosthesis or other implant left in the operative site. 14
Practices to prevent SSI are primarily aimed at minimising the number of micro-organisms introduced into the operative site via these routes. Procedures aimed at removing micro-organisms that normally colonise the patient’s skin include preoperative showering and disinfection of the skin at the surgical site; personnel involved in the operation disinfecting their hands and wearing sterile clothing to minimise the risk of introducing their own microbial flora into the wound; and air ventilation systems to minimise the risk of airborne contamination of the incision or instruments. 12 Prophylactic antibiotics are used to prevent micro-organisms introduced to the operative site multiplying, and surgeon technique in minimising tissue damage is important to enhancing the patients’ defences against infection. 15,16 Wound dressings are used to prevent access of micro-organisms into the incision postoperatively. 12
Gram-positive micro-organisms are responsible for over half of SSIs that occur following orthopaedic surgical procedures, with Staphylococcus aureus being the most common pathogen (PHE SSI report 201317 – there are other SSISS reports18 that detail the distribution of pathogens causing SSIs in orthopaedics in the English data).
Diagnosis and treatment
The decision regarding the selection of an adequate treatment strategy for SSI after a hip replacement should be well planned and made by a team of orthopaedic surgeons and infectious disease/microbiology specialists. The goal is not only to eradicate infection but also to preserve function of the hip joint. 19 As a number of different treatment options exist, individual circumstances have to be taken into consideration before treatment can begin. The main factors that influence the treatment and its success are the nature of the organism, site of infection, local factors relating to the bone and tissue condition, the time of infection onset and the patient’s general health status. 19–21
The first step should be diagnosis of the infection and its microbiological properties, especially when antibiotics are considered for treatment. 20 Only after the virulence and antimicrobial susceptibility of the organism and immune status of the host have been assessed can an appropriate antibiotic agent be selected. 22 There is no set approach for diagnosing SSIs or prosthesis-related infections, but a physical examination and a discussion of the patient’s history and symptoms should always be incorporated. 22 The following list of strategies can be regarded as a gold standard, but in practice a combination of only some of these individual strategies is pursued. 21,23,24
-
Imaging [e.g. standard radiography (X-ray), contrast autography, computerised tomography, ultrasonography, magnetic resonance imaging].
-
Laboratory [e.g. repeated measurement of leucocyte count in the synovial fluid (found in the cavities between joints to reduce friction)].
-
Histopathology (e.g. testing for neutrophils in tissue specimen).
-
Microbiology (e.g. swabs from pre- or intraoperative specimen or from removed fragments of the implant).
Once the diagnosis and type of infection has been established, the treatment options are surgical debridement with retention of the prosthesis, revision surgery, lifelong suppressive antibiotics or permanent resection. 19,25,26
Surgical debridement
If an infection manifests early in the post-operative period, debridement in combination with antibiotic therapy and retention of the prosthesis is a potentially successful treatment option. The success rate described in studies varies greatly from 2.8% to 100%, but more recent estimates suggest that this is closer to 80%. 27 The criteria proposed for the use of this technique are a duration of symptoms of < 1 month, infection with staphylococci or streptococci, no loosening of the prosthesis and no evidence of poor soft-tissue integrity as a result of prior surgical procedures. 28 The debridement should be performed as early as possible after the onset of symptoms of infection to avoid treatment failure, ideally within 2–5 days. 27,29,30 As this treatment avoids major revision surgery to replace the joint, it is particularly advantageous for elderly patients. 31
Revision surgery
Revision surgery can be performed as either a one- or a two-stage revision, each combined with antimicrobial therapy. In a one-stage revision, all infected tissue is removed and the prosthetic components are exchanged in the same operation. Even though this method gives good results in > 80% of patients and permits early mobility, the patient faces the risk that any remaining bacteria may reinfect the newly implanted prosthetic device. It is generally accepted that a one-stage revision should be used only if bone grafting is not required, there is no fistula present, the infection is not a result of difficult or resistant bacteria, debridement was extensive enough that others would not be able to repeat the procedure and antibiotic cement is used. 30
A two-stage revision involves the resection of all infected tissue and the removal of the prosthesis, after which the patient undergoes antibiotic treatment for a period usually ranging from 6 weeks to several months, until the infection is under control. 27,28,30 The patient’s movement is limited during that time and it can be very painful. In the second stage a new prosthesis is inserted in a reimplantation arthroplasty. 28,32 For this technique to be used, the patient must have adequate bone stock and minimal comorbidities that might otherwise affect their suitability for multiple surgical procedures. 30 Two-stage revisions are a common treatment for chronic infections in the USA, with studies showing consistently high cure rates of > 90% and a good prognosis of relapse-free survival. 22 However, this method is expensive, can result in significant skeletal defects, demands long hospitalisation periods, and can result in severe functional impairment and sometimes death. 28,30
Lifelong suppressive antibiotics
Lifelong suppression of the infection with antibiotics is infrequently used in practice, but might be the only option for morbid, bedridden or inoperable patients. 33 Antibiotic therapy without implant removal has an estimated failure rate of > 90%,34 as it only eases the symptoms without eliminating the infection. 19
Permanent resection
Permanent resection of the prosthesis, also known as Girdlestone resection, can be seen as a last resort after all other treatment options, including revision surgery, have failed. 35 This salvage technique is seen as acceptable for patients who are not fit for further revision operations because of poor bone stock or a high risk of recurring infection. 36 Other indications include poor quality of soft tissue, infection with multiple or resistant organisms, poor general health or high complexity of reconstruction arthroplasty. 37 The main purpose of the Girdlestone procedure is to reduce pain, as overall quality of life and patient satisfaction may not be improved because of substantially worse functional outcomes. 19,35,36 Patients are often left with a stiff joint and limited mobility, limb shortening as a result of bone loss and scar tissue, and a requirement for a walking aid. 37
Impact on patients and costs to health services
Deep SSIs are associated with substantial costs. Direct costs include a prolonged hospital stay, hospital readmission, outpatient visits, reoperation, additional antibiotic treatment, radiological and laboratory tests, home visits by health services and medication costs, as well as additional mortality and morbidity. Indirect costs include lost productivity of the patient and their family but could also comprise the damage to a clinician’s or hospital’s reputation or loss of staff morale. 38 Other consequences of SSIs are a serious impact on the quality of life and mental state of the generally elderly patients having to face further major surgery. Orthopaedic SSI has been reported to increase the mortality risk by 50% and the hospital stay by, on average, 11.5–14 days and to double the rehospitalisation rates. 4,5,39 A UK study by Vanhegan et al. 40 compared the costs of revision operations for aseptic loosening, dislocation, deep infection and periprosthetic fracture. Clinical, demographic and economic data were collected for 305 consecutive-revision THRs in 286 patients in a single tertiary referral unit. The authors found that the mean inpatient stay for patients with deep infection was significantly longer. Mean total costs were £21,937 in deep infection cases, compared with £11,897, £18,185 and £10,893 for aseptic, dislocation and periprosthetic cases, respectively. A recent cost-analysis study in France found that costs of septic revisions were 3.6 times higher following a THA than following a primary THA, mainly because of longer hospitalisation periods and rehabilitation after hospital discharge. 41 The treatment or revision of an infected joint can be very time-consuming and resource intensive. 42,43 Costs of SSIs usually increase with the depth of infection (i.e. superficial infections incur fewer costs than deep infections). 38 The economic burden of SSI after hip arthroplasty was reported to increase health-care costs by more than 300%, but dollar estimates differ widely, from US$400 to US$60,000 per SSI treated. 5,38,41,44,45 Apart from obvious reasons for the wide range of estimated costs, such as severity of infection or differing hospital fees in different countries, there is no consistent methodology used to calculate costs of infection. 46
The measurement of cost requires identifying the quantity of resource use and the unit costs or prices for using the resources. 47 However, in this context not only costs attributed to the occurrence of infections and related resources are relevant. The implementation of additional infection prevention will also incur costs, details of which are important for an accurate representation of cost outcomes. Often cost savings resulting from infection prevention programmes are overstated, making them appear more desirable than they actually are. Using hospital accounting methods rather than an economic approach biases the results in this context; they include fixed costs, such as wages, which are not affected by the incidence or control of infection. 48 Similarly, costs of infection are overstated when total hospital costs and total length of stay of infected patients are compared with uninfected patients. Patients who are more susceptible to infection often display severe cases of illness and are thus likely to incur higher costs throughout their hospital stay. Only costs incurred after the onset of infection should be attributed to infections. 49
For the economic evaluation in this project, the costs of infection prevention interventions are direct costs of implementation and continuous use of each strategy. Ultraclean air surgery, for example, would require the installation of specialised air handling systems. These capital costs would be valued by calculating the equivalent annual costs that integrate opportunity costs, as well as depreciation of the investment. 47 These costs, along with resource costs, such as additional electricity consumption per procedure or increased staffing requirements, would have to be taken into consideration. Each of the competing infection prevention strategies may be associated with different costs. The cost estimates for the occurrence of infection include direct in-hospital and post-discharge costs. Direct in-hospital costs can be measured as treatment costs, resource consumption and extra mortality and morbidity resulting from infection, whereas direct post-discharge costs are measured as out-of-pocket costs for patients and the use of primary care services. Treatment costs refer to the different treatment alternatives described in the model.
Current general infection prevention measures in the NHS
Guidelines on infection prevention precautions have been developed for NHS professionals to ensure the safety of patients and health-care personnel, as well as those who visit the health-care environment. General infection prevention measures include hand hygiene, respiratory hygiene, personal protective equipment, occupational exposure, management of care equipment, safe care of linen, control of environment and safe waste disposal.
-
Hand hygiene: good hand hygiene is very important for reducing the transmission of infectious agents.
-
Respiratory hygiene: respiratory hygiene has been added to the guidelines to reduce the risk of an influenza pandemic.
-
Personal protective equipment: the use of gloves, gowns, masks or goggles is essential for the health and safety of both patients and those caring for them.
-
Occupational exposure management: health-care staff must report incidents of exposure to infectious agents or needle-stick injuries and deal with incidents promptly.
-
Management of care equipment: care equipment needs to be carefully managed to limit the risk of contamination with micro-organisms.
-
Safe management of linen: appropriate handling of soiled fabric/linen is important to avoid transmission of micro-organisms.
-
Control of environment: the term ‘environment’ refers to any general horizontal or frequently touched surfaces in the environment. Routine cleaning is required to minimise transfer of micro-organisms from the environment to patients/clients.
-
Safe waste management: disposing of waste appropriately can minimise the risk of transmitting micro-organisms.
Specific guidelines on control of SSIs are available in UK, providing advice on the preoperative, intraoperative and postoperative phase (Table 2). 50–52
| Preoperative phase | Intraoperative phase | Postoperative phase |
|---|---|---|
| Preoperative showering | Hand decontamination | Changing dressings |
| Hair removal | Incise drapes | Postoperative cleansing |
| Patient theatre wear | Sterile gowns | Topical antimicrobial agents for wound healing by primary intention |
| Staff theatre wear | Gloves | Dressings for wound healing by secondary intention |
| Staff leaving the operating area | Antiseptic skin preparation | Antibiotic treatment of SSI and treatment failure |
| Nasal decontamination | Diathermy | Debridement |
| Mechanical bowel preparation | Maintaining patient homeostasis | Specialist wound care services |
| Remove hand jewellery, artificial nails and nail polish | Wound irrigation and intracavity lavage | |
| Antibiotic prophylaxis | Antiseptic and antimicrobial agents before wound closure | |
| Wound dressings |
Current cost-effectiveness evidence
The following databases were searched for relevant cost-effectiveness evidence: PubMed, Health Technology Assessment Database, NHS Economic Evaluation Database, MEDLINE, Academic Search Elite, Australia/New Zealand Reference Centre, Cumulative Index to Nursing and Allied Health Literature and EconLit. Searches were initially based on applicable medical subject headings/subject terms for research undertaken in this field (‘Arthroplasty, Replacement, Hip’, ‘Surgical wound infection’, ‘Decision Support Techniques’, ‘Models, economic’, ‘Costs and Cost Analysis’). In order to make the searches more specific, and to find further references, individual search terms were combined with medical subject headings (‘infection’, ‘cost-effectiv*’, ‘intervention’, ‘control, ‘antimicrobial’, ‘prophylaxis’, ‘antibiotic’, ‘cement’, ‘gentamicin’, ‘pre-operative’, ‘showering’, ‘antisep*’, ‘skin preparation’, ‘hair’, ‘nutrition’, ‘sterile’, ‘gown’, ‘surgical attire’, ‘mask’, ‘ultra-clean air’, ‘laminar’, ‘operating room’, ‘oxygen’, ‘suction drain’, ‘patient warming’, ‘UV radiation’, ‘wound dressing’, ‘surveillance’).
Studies were screened for relevance using the eligibility criteria outlined in Table 3.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Published between January 1995 and July 2014 | Partial economic evaluations (cost or effectiveness study) |
| Economic evaluation of infection prevention strategy for THA | No comparator |
| Evaluation based on decision model | Procedure other than hip arthroplasty (e.g. knee, shoulder, general surgery) |
| Assessment of adult population | Economic evaluation of infection diagnosis/treatment |
| Language is English | Prevention of transfusion-associated infection (e.g. human immunodeficiency virus infection, hepatitis) |
| Accessible in full |
Searches were limited to match these criteria as much as possible; for example, searches were restricted to the relevant time frame and English language only.
Studies were selected in a four-step process, illustrated in Figure 3. First, all titles in the selected search results (n = 199) were screened for inclusion. Titles not meeting the eligibility criteria at this stage were excluded (n = 133), for example because the study focus was on a different type of surgical procedure, such as knee or shoulder arthroplasty, or had a different objective, such as prevention of thromboembolism. Second, the abstracts of relevant studies (n = 66) were screened for inclusion.
FIGURE 3.
Review process of articles retrieved in the medical literature.
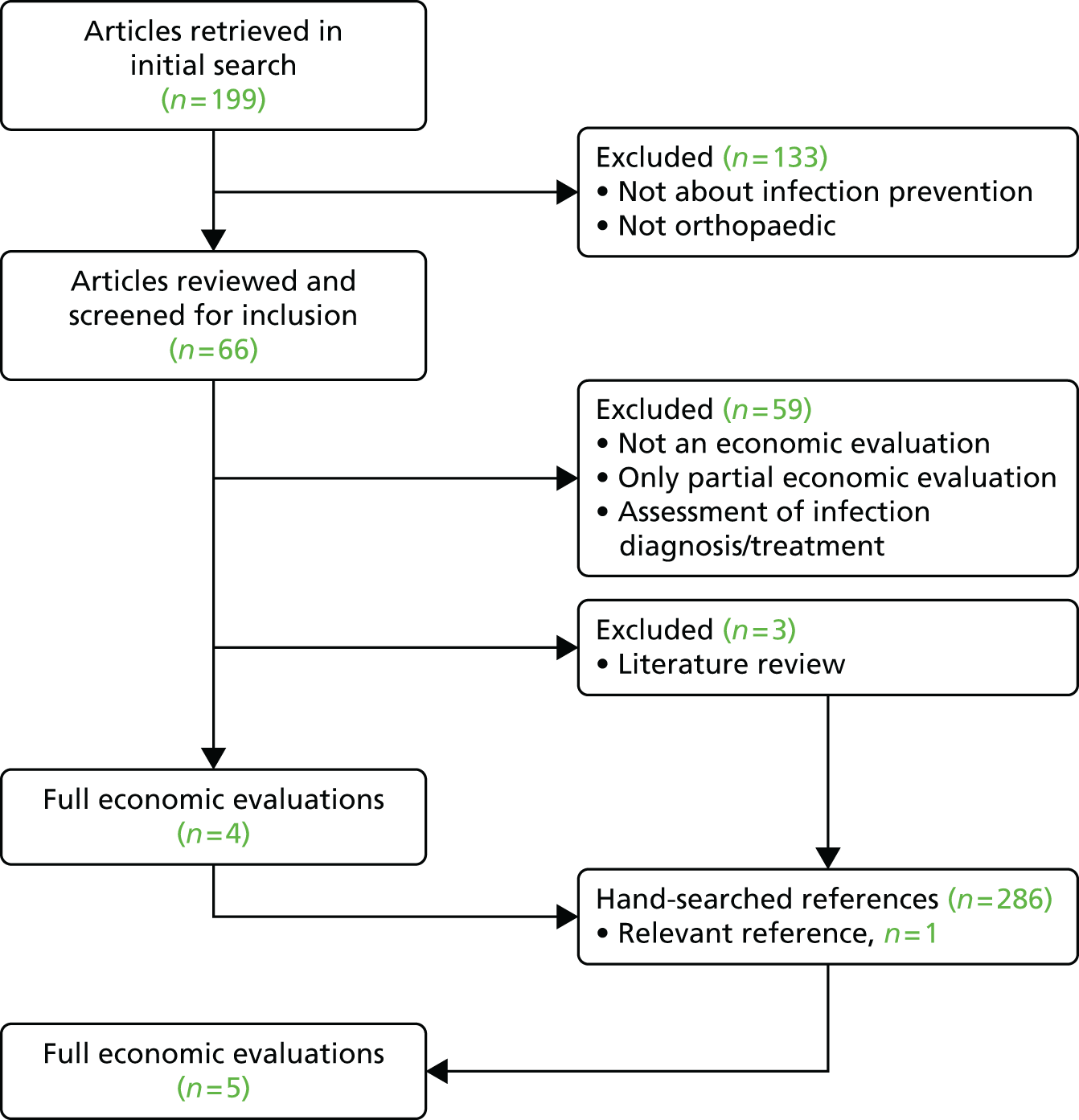
The most common reasons for exclusion were partial economic analysis, especially cost analysis, or studies for infection treatment or diagnosis rather than control. If decisions about inclusion were unclear, full articles were retrieved and reviewed. Studies were included if they met all of the above-described criteria. References of eligible studies (n = 4) and excluded literature reviews (n = 3) were hand-searched for further relevant studies.
Description of cost-effectiveness evidence
The literature searches resulted in five cost-effectiveness evaluations matching the inclusion criteria. 53–57 However, two studies were conducted by the same author group: one was a cost-effectiveness evaluation and the second was a systematic review with a cost-effectiveness evaluation. 53 As the same decision problem and decision model were used in both these publications, only the more recent and updated article was included. 55 The characteristics of the remaining four studies are summarised in Table 4.
| Characteristic | Study | |||
|---|---|---|---|---|
| Cummins et al. (2009)54 | Elliott et al. (2010)55 | Courville et al. (2012)56 | Merollini et al. (2013)57 | |
| Research question | Is the use of antibiotic-impregnated bone cement in primary THA cost-effective when compared with cement without antibiotics? | Is there a threshold of MRSA prevalence at which switching to routine glycopeptide-based antibiotic prophylaxis becomes cost-effective? | Is preoperative nasal mupirocin treatment cost-effective in preventing SSI in total hip and knee arthroplasty? | Are strategies claiming to reduce the risk of SSI in hip arthroplasty cost-effective? |
| Patient cohort | Patients undergoing primary THA for the treatment of osteoarthritis (not fracture); baseline average age: 68 years | Patients undergoing primary THA | Patients with end-stage hip or knee osteoarthritis for whom THA and TKA were recommended because of failed medical management | Patients undergoing THA; baseline age: 65 years |
| Perspective | Unclear (USA) | Cost perspective of the UK NHS and Personal Social Services | Societal perspective, but limited to costs and effects directly affecting the target population | Health services perspective |
| Comparators | Antibiotic-impregnated cement vs. polymethylmethacrylate bone cement without antibiotics | Vancomycin vs. cephalosporin vs. combination of vancomycin and cephalosporin | Preoperative nasal screening and mupirocin treatment vs. preoperative mupirocin treatment vs. no treatment or screening | Antibiotic prophylaxis vs. antibiotic-impregnated cement and antibiotic prophylaxis vs. laminar air operating rooms and antibiotic prophylaxis vs. no antibiotic prophylaxis |
| Time frame | Model cycles until all hypothetical patients are in a death state | Unclear (possibly during hospitalisation) | Within 1 year of the primary operation | 30 years |
| Outcome measure | Rate of revision because of infection and rate of all revisions | Rates of superficial/deep MRSA infection/non-MRSA infection | ICER | QALYs and cost related to infection prevention strategies |
| Findings | ICER for antibiotic-impregnated cement: US$37,355/QALY gained. Antibiotic-impregnated cement dominated standard bone cement | If the MRSA infection rate is ≥ 0.25% and the rate of infections with cephalosporin prophylaxis is ≥ 0.2%, combined antibiotic prophylaxis is optimal | The treat-all and screen-and-treat strategies both had lower costs and greater benefits than the no-treatment strategy | Antibiotic prophylaxis and antibiotic-impregnated cement dominated the other three strategies (no antibiotic prophylaxis, antibiotic prophylaxis, antibiotic prophylaxis and laminar air operating rooms) |
Cummins et al. 54 evaluated the cost-effectiveness of using antibiotic-impregnated bone cement in primary THA compared with standard bone cement. They used the rate of revisions and, in particular, revisions resulting from infection as primary outcome measures in their Markov model. The cohort simulation showed that parameters with the greatest influence on model results were costs of cement and baseline age. If revision resulting from infection was the primary outcome measure, the incremental cost-effectiveness ratio (ICER) for the use of antibiotic cement was US$37,355 per quality-adjusted life-year (QALY) gained. When the outcome measure was the rate of all revisions, antibiotic-impregnated cement was more effective and less costly than standard bone cement. The study demonstrated that antibiotic-impregnated cement was cost-effective for a relatively young patient group (< 71 years) and for a low cost of cement (< US$650). As most THA patients in the USA are older, the authors concluded that the use of antibiotic-impregnated cement as a control measure is of limited use in the US setting unless the price is reduced.
Another economic evaluation, by Elliott et al. ,55 focused on the control of meticillin-resistant Staphylococcus aureus (MRSA) infections using different antibiotic prophylaxis strategies, prior to surgery. Primary hip arthroplasty was used by way of example in the decision-analytic model and it was assumed that all patients received some form of antibiotic prophylaxis: non-glycopeptide, glycopeptide or a combination. The choice of agent has different implications: glycopeptides (e.g. vancomycin) are known to actively fight resistant strains but pose a risk of increasing bacterial resistance; and non-glycopeptides (e.g. cephalosporins) are routinely used but do not represent an effective barrier to MRSA infection. The aim of the study was to investigate whether or not routine glycopeptide-based prophylaxis would be cost-effective beyond a certain threshold prevalence of MRSA. Assuming a threshold of £30,000 per QALY gained, the authors found that, for hip arthroplasty, prophylaxis with cephalosporin alone was optimal at a 0.0% MRSA SSI rate or if the MRSA SSI rate was ≥ 0.2% and the rate of other infections was ≤ 0.1%, and vancomycin alone was to be preferred where the MRSA SSI rate was ≤ 0.15% and the rate of other infections was ≤ 0.1%. Combined administration of cephalosporin and vancomycin was optimal where the MRSA SSI rate was ≥ 0.25% and the non-MRSA SSI rate was ≥ 0.2%. The authors noted high levels of uncertainty and concluded that more work was needed to fully understand the mechanisms of antibiotic resistance and how it affects the effectiveness of glycopeptides.
A study by Courville et al. 56 examined the cost-effectiveness of preoperative nasal mupirocin treatment in patients with total hip or knee arthroplasty. Three strategies were compared in a decision tree model: preoperative screening for all patients and treatment with mupirocin for patients testing positive for S. aureus; preoperative administration of mupirocin to all patients and no screening; and neither preoperative treatment nor screening. The main outcome, costs and health benefits, was assessed within 1 year of the primary operation. Courville et al. 56 found that both treat-all and screen-and-treat strategies had lower costs and greater benefits than the no-treatment strategy. The result is robust, even if the cost of mupirocin was over US$100 and the cost of SSI ranged between US$26,000 and US$250,000. Treating all patients remains the best strategy when the prevalence of S. aureus carriers and SSIs is varied across plausible values as well as when the prevalence of mupirocin-resistant strains is high. Owing to imperfect sensitivity of the screening test, the authors suggested that the treat-all approach is the most likely to decolonise S. aureus-colonised patients to prevent deep SSI.
A cost-effectiveness analysis by Merollini et al. 57 evaluated different strategies claiming to reduce the risk of SSI in hip arthroplasty in Australia. The baseline strategy, antibiotic prophylaxis, was compared with no antibiotic prophylaxis, the combination of antibiotic prophylaxis and antibiotic-impregnated cement, and the combination of antibiotic prophylaxis and laminar air operating rooms, in a Markov model. 58 The model showed that stopping the routine use of antibiotic prophylaxis would increase costs by over AU$1.5M and result in a loss of 163 QALYs. In both baseline and uncertainty analysis, the combination of antibiotic prophylaxis and antibiotic-impregnated cement was both less costly and showed greater health benefits than the other strategies. As a result, the authors recommended the use of antibiotic prophylaxis combined with antibiotic-impregnated cement, but recommend against the use of laminar air operating rooms to reduce both the costs and the risk of SSI.
Quality assessment
The quality of eligible studies was assessed on aspects of structure, data and consistency, using a checklist adapted from Philips et al. 59 (Table 5).
| Dimension of quality | Study | |||
|---|---|---|---|---|
| Cummins et al. (2009)54 | Elliott et al. (2010)55 | Courville et al. (2012)56 | Merollini et al. (2013)57 | |
| Structure | ||||
| Statement of decision problem | Yes | Yes | Yes | Yes |
| Statement of perspective | No | Yes | Yes | Yes |
| Definition of comparators | Yes | Yes | Yes | Yes |
| Statement of model assumptions | Yes | Yes | Yes | Yes |
| Illustration of model structure | Yes | Yes | Yes | Yes |
| Appropriate time horizon | Yes | Unclear | Yes | Yes |
| Data | ||||
| Data identification process transparent | Yes | Yes | Yes | Yes |
| Sources of all data given in detail | No | No | Yes | Yes |
| Quality of model input parameters | see Figure 4 | see Figure 5 | see Figure 6 | see Figure 7 |
| Assessment of uncertainty | Yes | Yes | Yes | Yes |
| Identification of key parameters | Yes | Yes | Yes | Yes |
The structural quality of all four economic evaluations was high, although Cummins et al. 54 failed to clearly state the perspective of the evaluation and Elliott et al. 55 did not clearly define the time horizon. Dimensions of data quality were fulfilled by most studies, but two, by Cummins et al. 54 and Elliott et al. ,55 failed to provide sources of data for at least one parameter. Owing to their importance for the quality of model, input parameters were assessed in more detail.
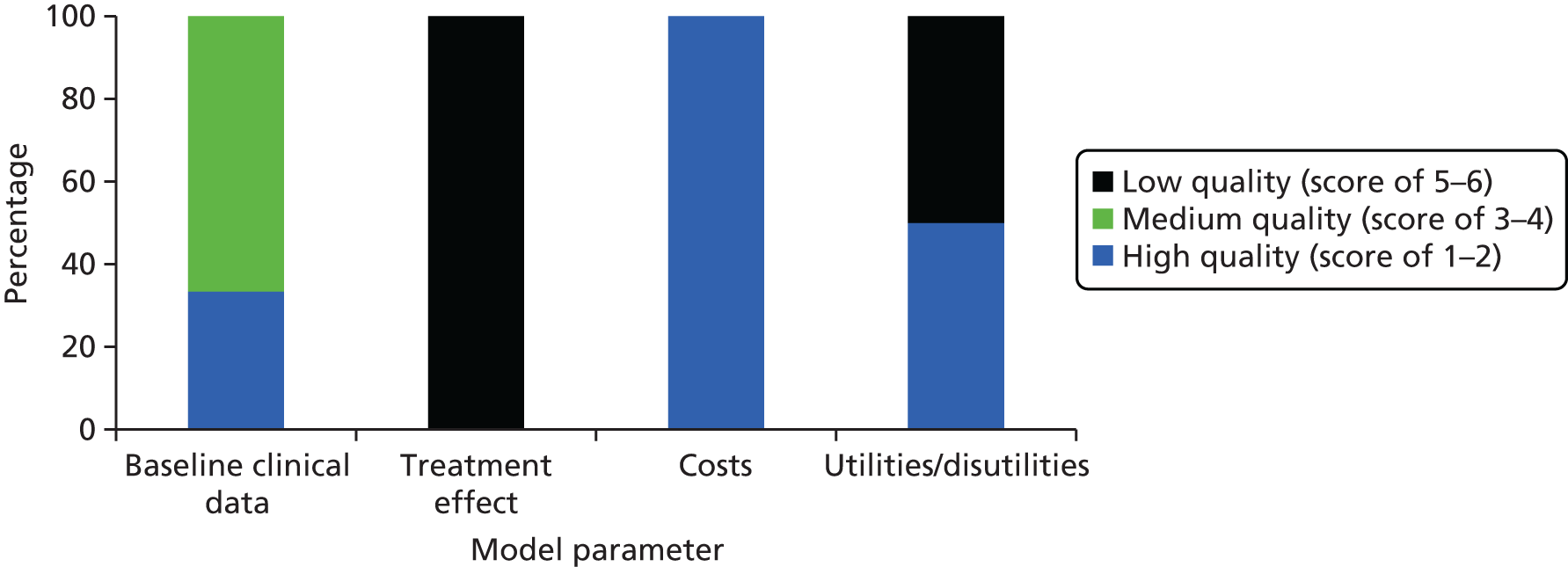
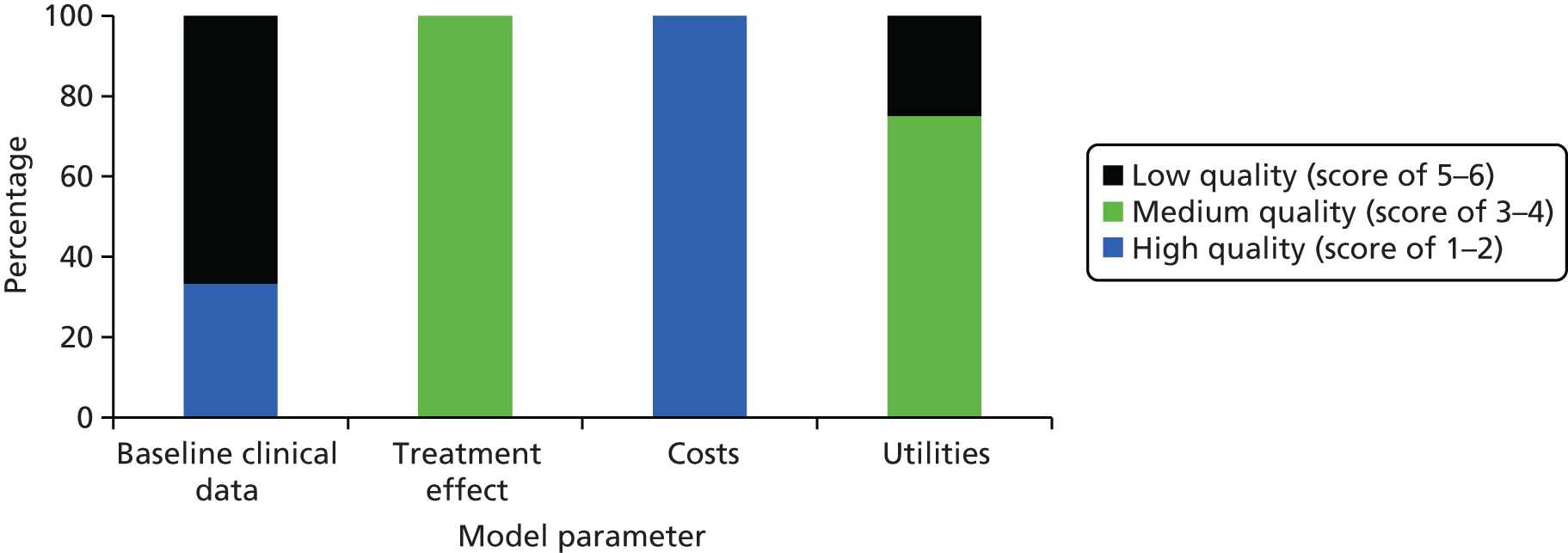
Model parameters were ranked using a hierarchy developed by Cooper et al. 60 for evaluating decision model parameters. Using this hierarchy, evidence levels range from 1 to 6, with higher numbers indicating better quality. The quality of baseline clinical data, treatment effect, costs and utilities is summarised for the economic evaluation by Cummins et al. 54 in Figure 4 and for Elliott et al. 55 in Figure 5. If the evidence used did not match the specified description of evidence, the next most suitable category was chosen. Neither of the studies commented on the assessments of internal consistency of the decision model. Therefore, it is unclear whether or not the mathematical logic of the model has been evaluated in these publications.
Figure 4 shows that the quality of baseline clinical data used by Cummins et al. 54 was medium to high, low for treatment effect and high for cost estimates, whereas the quality of utilities used ranged from low to high.
Cummins et al. 60 derived baseline infection rates from the Norwegian Arthroplasty Registry, which can be classed as a recent ‘reliable administrative database covering patients solely from another jurisdiction’,60 representing a quality score of 3. 60 Baseline surgical mortality was assumed to be the same for primary THA and revision surgery, and was also taken from the Norwegian Arthroplasty Registry. Underlying mortality was informed by recent US life tables, earning a quality score of 2. Owing to a lack of high-quality data, the clinical effectiveness (treatment effect) of antibiotic-impregnated bone cement was derived from a non-analytic study and is, therefore, classified as low quality. Cost parameters assigned to primary THA, increased cost of aseptic revision and revision resulting from infection were adopted from recently published cost calculations of high quality from the same jurisdiction. The quality of data for the extra costs of using antibiotic cement was also high, as these estimates were based on original figures from the authors’ institution. Utility parameters for primary THA and aseptic revision were of high quality, adopted from a study designed for the patient group of interest. The utility for aseptic revision, on the other hand, was estimated by the authors as no published estimate was found, representing the lowest level of data quality according to the classification system used. It was unclear how disutility was measured, as no reference was stated and no measurement method was described. However, the results of the Markov model showed that, in the sensitivity analysis, disutility values had no effect on the model. 54
Figure 5 shows that the quality of baseline clinical data used by Elliott et al. 55 was high. The quality of treatment effect data was rated as medium, whereas cost parameters data were a mix of high and low quality and utility inputs data were of low quality. Elliott et al. 55 used a possible range of infection rates for their baseline estimate, based on two different high-quality sources from the literature. Mortality estimates for superficial and deep SSIs were informed by a recent case series from the same jurisdiction. Underlying mortality rates were taken from UK life tables, which are high-quality data. As no treatment effect describing antibiotic prophylaxis for MRSA prevalence was available in the orthopaedic setting, results from a cardiac clean surgery trial were used instead. Using data from a randomised controlled trial (RCT) conducted in a different setting may compromise the credibility of model outcomes but, in this case, as both procedures are clean surgery, the most appropriate ranking for this input is 4. Different costs for antibiotic prophylaxis and treatment costs of superficial, deep MRSA and non-MRSA infections were derived through personal communication with the British Medical Association and Royal Pharmaceutical Society of Great Britain, all representing high-quality data inputs. Recent UK case series and a previously published economic evaluation informed costs of barrier nursing and inpatient days, and administration costs were set at local NHS contract costs. The reference specified for wound exploration costs did not describe any costs but stated that this strategy can be used to treat infections and hence this parameter was classed as low quality. 61 QALYs for infected joints were derived from a previous study that used expert opinion and was therefore given the lowest quality categorisation. 55,62
Figure 6 shows that the baseline clinical data used by Courville et al. 56 was a mix of low and high quality: the quality of treatment effect data was medium, cost parameters data were of high quality and utility inputs data were a mix of low and medium quality. For the base case, Courville et al. 56 used the S. aureus colonisation rate from their local population of patients with total joint arthroplasty. This is high quality according to the Cooper et al. 60 classification system. However, the specificity and sensitivity of the screening test for S. aureus were estimated based on personal communications and unpublished data in the original source. The treatment effect of mupirocin in reducing SSIs was derived from a meta-analysis that included both RCT and before-and-after trials for patients undergoing different surgical procedures and is therefore ranked at level 3 for quality. The probabilities of SSI among mupirocin-treated carriers and untreated non-carriers were sourced from a single case–control study, representing medium quality. The quality of data for costs of screening tests and treatment was high, being based on fees from the authors’ medical centre. Similarly, the costs of primary total joint arthroplasty and revisions were estimated from published papers where cost calculations were based on reliable data sources in the USA, and were therefore also of high quality. Utility scores for primary total knee replacement, septic knee revision and THA were based on previous studies using the time trade-off method. The quality of utility data for septic hip revision was classified as low, having been taken from the expert opinion estimates used by Cummins et al. 54
Figure 7 shows that the quality of baseline clinical data used by Merollini et al. 57 was mostly high and that treatment effect data were of medium to high quality, whereas cost parameters data were a mix of high and low quality and utility inputs data were a mix of low and medium quality. Merollini et al. 57 sourced the occurrence of deep infection and other transition probabilities from high-quality hospital records in Queensland, Australia. Mortality probabilities for deep infection were taken from a retrospective review of surveillance data in the UK, which is classified as medium quality. Mortality probabilities for revision surgery were calculated based on data from the Australian Orthopaedic Association National Joint Replacement Registry by Merollini et al. ,57 giving a quality score of 1. The quality of underlying mortality probabilities was also high, as Australian life tables were used. The clinical effects of antibiotic prophylaxis and antibiotic-impregnated cement were taken from meta-analyses and are therefore classified as high quality. A large cohort study provided medium-quality data on clinical effect on laminar air systems in operating theatres. Costs of antibiotic prophylaxis and ultraclean air systems were based on expert opinion and conservative estimates by the authors, and are therefore of low quality. On the other hand, the cost assigned to the additional use of antibiotic cement was obtained through personal communication with the Prince Charles Hospital in Brisbane, QLD, Australia, which is classified as high quality. Utility scores for patients with no infection, revision operations and successful treatments were derived from a study that estimated quality of life using the 15D health-related quality-of-life (HRQoL) survey (a 15-dimensional, standardised questionnaire) and an observational study (using the Short Form questionnaire-36 items) that was referred to estimate utility for deep infection. However, the utility of permanent and temporary resection was based on expert opinion and was therefore classified as low-quality evidence.
Related economic evidence
As the literature review of cost-effectiveness analyses of infection prevention in hip arthroplasty resulted in only four eligible studies, related economic evaluations were examined. Findings of these studies could be of relevance even if they did not match the specified inclusion criteria. Most of these studies were excluded because they focused on infection prevention other than hip arthroplasty procedures, did not use a decision model, explored the cost-effectiveness of infection treatment or only focused on cost or effectiveness aspects.
An economic evaluation was published by a Swedish group in 199963 but was excluded as it did not report the use of a decision model and did not investigate health outcomes. Persson et al. 63 assessed the economics of preventing revisions following THA. The risk of revision because of aseptic loosening was calculated for different types of cement, and the risk of revision resulting from deep infection was assessed for no prophylaxis or different combinations of systemic antibiotics, gentamicin-impregnated cement, surgical enclosure and exhaust-ventilated suits. Although the Swedish Arthroplasty Register was used to measure most parameters, weighted average costs for proportions of one-stage and two-stage revision were retrieved from a single Swedish hospital. The authors estimated the expected health-care costs for each infection prevention strategy and then compared the alternatives in terms of their rate of revision and costs. Dominated strategies were excluded (e.g. strategies with higher costs at the same rate of revision). The results showed that every combination with exhaust-ventilated suits resulted in an increased rate of revision. This led the authors to the conclusion that this strategy should never be used as a preventative measure. Effective strategies were systemic antibiotics only, systemic antibiotics in combination with antibiotic cement and, lastly, systemic antibiotics, antibiotic cement and surgical enclosures. 64 The last was the most effective strategy, with revision rates resulting from deep infection of 0.19% and an average cost of prophylaxis of US$331 for each primary THA performed (based on 100 operations performed per year). 64 These additional costs, especially of a surgical enclosure, were not offset by the cost savings because of infection prevention and the cost-effectiveness of different strategies varied with the number of THAs performed per year. For orthopaedic departments already using antibiotic prophylaxis and antibiotic-impregnated cement, the extra use of a surgical enclosure would cost US$314,000 for each deep infection avoided.
Another related full economic evaluation by Fisman et al. 28 used a Markov decision model to compare the clinical effectiveness and cost-effectiveness of two management strategies: surgical debridement with retention of the prosthesis or two-stage exchange arthroplasty for infected THA. The model results were assessed and reported for a 65- and 80-year-old patient cohort. This study fulfilled all criteria for a good economic evaluation,47 but the authors noted the need of RCTs to evaluate management strategies for infected hips in order to increase the quality of model parameters. The results were highly dependent on the annual relapse rate after debridement for each treatment strategy as well as on the age at the initial diagnosis of infection. Debridement and retention resulted in a better cost-effectiveness ratio in all cohorts and increased the life expectancy by 2.2–2.6 quality-adjusted life-months.
A systematic review of effectiveness and cost-effectiveness of antimicrobial prophylaxis in THA was performed by Glenny and Song. 65 The authors assessed the quality of all studies available and performed different meta-analyses in order to combine effectiveness outcomes and evidence of a number of antimicrobial agents and routes of administration. 28 A total of 25 RCTs were included; the overall conclusion was that antimicrobial prophylaxis is effective for the control of surgical wound infections in total joint arthroplasty and the efficacy of most treatment regimens studied was similar.
Numerous partial economic evaluations exist, focusing on cost or effectiveness aspects only rather than cost-effectiveness (a reason for exclusion in the review). Many of these were related to costs of antibiotic prophylaxis as a control measure or treatment costs of infection in orthopaedics. 42,43,66–75
D’Angelo and Ogilvie-Harris71 reviewed nine cases of septic arthritis following arthroscopic procedures on the knee or shoulder in terms of their costs and the possible monetary benefits of antibiotic prophylaxis. Although they mentioned a cost–benefit analysis, this cannot be interpreted in the sense of an economic evaluation because their estimates did not reflect individual preferences (welfarism) nor did they include a cost–benefit ratio or single outcome measure.
The most relevant cost analysis was published by Lidwell72 in 1984. He estimated additional costs of antibiotic prophylaxis, ultraclean air and body exhaust suits used individually in joint replacements, and put these costs in context with cost savings associated with these preventative measures. The conclusion was that overall cost savings were achieved for the hospital if antibiotic prophylaxis or clean-air techniques were used. 72
Arens et al. 42 estimated the substantial economic burden for hospitals treating infections after joint replacements as a result of inappropriate reimbursement. They argued that the high economic burden justified a sound cost evaluation by health insurers and more research in infection prevention. 42 Bozic and Ries43 focused on measuring the impact of deep infection after THA on surgeon and hospital resource utilisation by analysing clinical and economic data of 25 infected patients. The results were increased total medical costs for revisions resulting from infection (2.8 times higher than revisions resulting from aseptic loosening), significantly more hospitalisations, prolonged hospital stay, more operations, more outpatient visits, more outpatient charges and more complications than for THA without complication or revision because of aseptic loosening. 43 A British study by Edwards et al. 73 evaluated data on hip fracture patients regarding infection risk factors and costs for deep or superficial wound infections, and found significantly increased treatment costs and length of stay, with doubled operative costs and quadrupled ward costs for deep infection. Iribarren et al. 74 and Kurtz et al. 75 also reported higher hospital charges and an increased length of stay because of periprosthetic infection or SSI after total hip or knee arthroplasty.
Klouche et al. 41 performed a retrospective cost analysis using hospital data from approximately 500 hip arthroplasties performed in a French hospital. 41 Treatment costs of infected hips after THA included preoperative tests, medicosurgical management during the hospital stay, orthopaedic rehabilitation, antibiotic therapy after revision because of infection and home-based hospitalisation costs. 41 They found that in their institution, the average hospital stay was 7.5 days for primary THA and 30.6 days for revisions because of infection. 41
Vanhegan et al. 40 compared the costs of revision operations for aseptic loosening, dislocation, deep infection and periprosthetic fracture. Clinical, demographic and economic data were collected for 305 consecutive revision THRs in 286 patients in a single tertiary referral unit. They found that the mean inpatient stay for patients with deep infection was significantly longer and mean total costs were £21,937 in deep infection cases, compared with £11,897, £18,185 and £10,893 for aseptic, dislocation and periprosthetic cases, respectively. 40
Research goals and objectives
The economic paradigm is that enhanced infection prevention in NHS hospitals will change cost and health outcomes. Costs will rise with more aggressive infection prevention programmes, but savings will accrue when cases of infection are prevented. Infection prevention strategies will either increase or decrease total costs depending on their cost and effectiveness. Health outcomes will only improve as infection-related morbidity and mortality risk are avoided. The goal of this research is to assess the cost-effectiveness of infection prevention strategies for hip replacements performed in NHS hospitals.
The information generated will address a gap in the scientific knowledge about how the risks of infection following THR should be managed. The major benefit of this work will be that infection prevention arrangements for THR will be improved and this will improve NHS efficiency.
There are five tasks:
-
Synthesise evidence on the effectiveness of strategies to reduce risk of infection after primary hip replacement (see Chapters 2 and 3).
-
Design a decision-analytic model to predict cost and health outcomes from infection prevention strategies (see Chapter 4).
-
Identify, select and synthesise the remaining evidence required to update the model (see Chapters 5 and 6).
-
Evaluate the model and characterise uncertainty among the predictions of cost-effectiveness (see Chapter 6).
-
Interpret the findings with the needs of policy-makers in mind (see Chapters 7 and 8).
A formal modelling framework will be used and updated with existing data. This will inform decision-making for infection prevention and risk reduction. This represents an advance on the existing research in which either the effectiveness or cost-effectiveness of single interventions to manage risks is studied in isolation. Answering the research questions with a prospective clinical trial would be too complex and costly, and unlikely to survive review by an ethics committee.
Chapter 2 Synthesis of effectiveness evidence methods
The purpose of this chapter is to report the methods used to synthesise current effectiveness evidence and quantify the relative effectiveness of infection prevention strategies for reducing the risk of SSI following THR. Sections of this chapter have been published open access in Zheng et al. 9 under the terms of the Creative Commons Attribution Non-Commercial (CC BY-NC 3.0) licence. We focus on key infection prevention strategies with clinical and cost implications for the prevention of THR-related infection. These are antibiotic prophylaxis, antibiotic-impregnated cement and laminar airflow operating systems, and were chosen based on national clinical infection prevention guidelines in the UK,76 the National Institute for Health and Care Excellence (NICE) publication on Surgical Site Infection – Prevention and Treatment of Surgical Site Infection50 and through elicitation of expert opinion.
Mixed-treatment comparison models
We used a mixed-treatment comparison (MTC) model for the evidence synthesis, as it allows coherent judgement to be made on which of multiple treatments is the most effective and produces estimates of the relative effects of each treatment compared with every other treatment in a network. 77,78 A MTC enables simultaneous comparison of multiple treatments from trials that individually do not compare all treatment options under consideration. 78,79
A MTC is achieved by pooling direct and indirect evidence on each relative treatment effect from all trials in the evidence network for comparison. 77 The model assumes that the relative effect of one treatment compared with another is the same across the entire set of trials for the fixed-effect analysis,80 and the odds ratios (ORs) in each trial are different, but form a single common distribution that is the same across all sets of the trials for the random-effects analysis. 77,79 It is under such an assumption that models have been developed to simultaneously synthesise all available evidence using an extended meta-analysis model without breaking randomisation. 79,81
Regression-based methods have been developed to fit MTC models. 82–85 The basic model specification for MTC methods is an extension of the Bayesian specification for standard pairwise meta-analysis of binary data using a logistic regression model:86
where pjk is the probability of the event for treatment k in trial j; µjb is the log-odds of the event for the reference (baseline) treatment b in trial j. The study effects µjb are treated as unrelated nuisance parameters. 77 δjbk is the trial specific log-OR of treatment k relative to the reference treatment b in trial j (k > b signifies that k is numerically after b).
The random-effects model
The trial-specific log-odds δjbk are assumed to be normally distributed with mean dbk and a between-study variance τ2 as specified below:
τ2 accounts for the random effect resulting from between-study variation.
The fixed-effects model
When the between-study variance τ2 = 0, the above random-effects model is reduced to a fixed-effects model:86
If A is treated as the overall MTC reference (baseline) treatment, then the effects of treatment B, C, D, . . . K relative to A, dAB, dAC, dAD . . ., dAK are considered to be basic parameters and dAA = 0. All other parameters that define treatment effect of one treatment relative to another in the model are called functional parameters. 77 These functional parameters are derived from the basic parameters under the assumption that both direct and indirect evidence estimate the same underlying treatment effect on each pairwise comparison:77
The full random-effects model
The full random-effects model takes into account the correlation structure induced by multiarm trials. 81 Multiarm trials on treatments A, X and Y, for example, induce a covariance between δjAX and δjAY. Under the assumption of homogeneous variance in these trials, this covariance is reduced81,84 and is completely accounted for in the model for any multiarm trials.
The choice of baseline treatment has no impact on the comparisons made. 77 This covariance is accounted for by formulating a correlation structure for any number of arms by decomposition of a multivariate normal distribution as a series of conditional univariate distributions:86
with the conditional univariate distributions being:
Evidence search strategy
There is a wide range of infection prevention measures in current clinical practice, yet we focused on key infection prevention strategies with critically important clinical and health-care cost implications. These strategies include antibiotic prophylaxis, antibiotic-impregnated cement and laminar airflow systems. We followed the systematic review guidelines as outlined in the Quality of Reporting of Meta-Analyses statements87 and the guide to the methods of technology appraisal by NICE88 for the evidence synthesis.
The first stage of the literature search used existing systematic reviews on antibiotic prophylaxis, antibiotic-impregnated cement and laminar airflow systems to locate relevant studies for inclusion in the current evidence synthesis. The second-stage literature search was designed to update existing systematic reviews by identifying new primary interventions for inclusion in the evidence synthesis. The major electronic databases searched included MEDLINE (via EBSCOhost), EMBASE, Cumulative Index to Nursing and Allied Health Literature and the Cochrane Central Register of Controlled Trials.
Relevant journals, conference proceedings and bibliographies of retrieved papers were hand-searched. Orthopaedic surgeons and infection prevention experts were also consulted. Owing to language resource constraints, the search was limited to only English-language papers. The selection of evidence was conducted by two independent reviewers and discrepancies were resolved by consensus. The search terms and electronic search strategies used can be found in Appendix 1.
Definition of the outcome measure
The outcome measure used for the current evidence synthesis was deep SSI following primary THR. Failure to use objective criteria to define SSIs has been shown to substantially affect reported SSI rates. 89 For this review process, which included international evidence, we relied on the definition used in the Guideline for Prevention of Surgical Site Infection by the US Centers for Disease Control and Prevention. 12 This definition is described in Chapter 1.
Inclusion criteria
Studies were included if all of the following applied:
-
They were primary interventions with THR-related deep SSIs reported as an outcome.
-
Antibiotic prophylaxis, antibiotic-impregnated cement or laminar airflow system was a trial arm.
-
Antibiotic delivery methods were specified (systemically, via cement or both).
-
The type of ventilation system used in the operating theatre was indicated; otherwise, a conventional ventilation system was assumed.
Some studies defined early deep infection as those requiring a revision procedure within 6 months of the initial operation and used revision rates as early deep infection rates. Given that deep infections that developed within this time frame were most likely caused by bacterial contamination at the time of surgery, studies that only reported revision rates resulting from primary THR-related infection were also included.
Exclusion criteria
Studies were excluded if any of the following applied:
-
Only superficial infection following THR was reported as an outcome measure, or superficial and deep infection were treated as one outcome measure without separating one from the other.
-
Only joint replacement-related infection was reported as an outcome measure without separating THR-related infection from knee replacement-related infection.
-
The outcome measure was revision not caused by SSI.
We treated antibiotic prophylaxis as one intervention arm without differentiating between types, doses and durations of the administration of different antibiotic regimens. This is because there is no convincing evidence to suggest that one type of antibiotic regimen is more effective than another, that extending the duration of an antibiotic regimen beyond 24 hours postoperatively further reduces THR-related SSIs, or that single-dose or short-term administration is not as effective as long-term administration. 65 Therefore, primary interventions that compared different types, doses or durations of antibiotic regimens were treated as one-arm trials and excluded from the network meta-analysis, as MTC relies on there being at least one comparison of two arms that can become part of the connected network. 79 The process used for the literature search is shown in Figure 8.
FIGURE 8.
Two-stage literature search flow chart. CINAHL, Cumulative Index to Nursing and Allied Health Literature. Reproduced from Zheng H, Barnett AG, Merollini K, Sutton A, Cooper N, Berendt T, et al. Control strategies to prevent total hip replacement-related infections: a systematic review and mixed treatment comparison. BMJ Open 2014;4:e003978. 9 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/.

Data extraction
Data were extracted by two independent reviewers and consensus was reached. The key data extracted from the included studies for the evidence synthesis included the total number of THRs performed and the total number of deep SSIs diagnosed following the THR operations in each trial; information regarding the use of antibiotic prophylaxis and its delivery mode, for example systemically, via cement or both; information about the ventilation system used in the operating theatre; and special surgical clothing used, for example exhaust body suits. Where information regarding the use of the type of cement, antibiotic impregnated or plain, was unavailable, plain cement was assumed. If a study reported both early and late deep infection as outcome measures, the former was chosen over the latter in line with the Centers for Disease Control and Prevention definition of SSI. 12
Quality assessment
We conducted a quality assessment of the included studies. The method for quality grading was adapted from NICE’s Methods for the Development of NICE Public Health Guidance (Third Edition)93 and NHS Centre for Reviews and Dissemination guidance. 94 Each study was categorised by study type and graded based on the extent to which the potential sources of bias were minimised. The type of study coupled with the quality evaluation decided the level of evidence. The level of evidence table was based on the checklist of NICE’s Methods for the Development of NICE Public Health Guidance (Third Edition) that was adapted from the Scottish Intercollegiate Guidelines Network. We also assessed the internal validity of the included studies against the critical appraisal criteria based on the checklists of NICE’s Methods for the Development of NICE Public Health Guidance (Third Edition) and NHS Centre for Reviews and Dissemination guidance. Quality scores were allocated to the included studies using quality scoring systems adapted from that used by the Cochrane Musculoskeletal Injuries Group for RCTs and by Tooth et al. 95 for observational studies (see Tables 62–66 in Appendix 3 and see Table 6 for results of the quality assessment).
The choice of reference treatment and testing the statistical approach
We chose treatment (T) 1 to be ‘no antibiotic prophylaxis, plain cement and conventional ventilation without laminar airflow system’. This was the reference treatment as it was trialled against the highest number of other infection prevention strategies. The choice was made to minimise correlations that may otherwise be induced between mean treatment effects for each pair of treatments compared. A number of issues required investigation and the use of some diagnostics: variation in follow-up among the studies, model fit and deviance, and consistency. The models were fitted within a Bayesian framework using WinBUGS (MRC Biostatistics Unit, Cambridge, UK) and relevant code by Dias and colleagues. 77 The absolute and relative treatment effects and the ranking of infection prevention strategies were generated and analysed.
Complementary log–log link to model variation in follow-up durations
The main model used for the evidence synthesis is a binomial likelihood, logit link random-effects model, adjusting for multiarm trials. This model did not account for the variation in follow-up durations. Given that THR-related SSIs tended to occur soon after the operation, the use of the logit link MTC models was justified. However, as there was significant variation in the duration of follow-up in the included studies, we conducted a sensitivity analysis using the following complementary log–log link that models the effect of follow-up duration on the number of events occurring, taken from Dias et al. 77
where φik is the event rate with respect to follow-up duration fi and δi,bk the treatment effects representing log-hazard ratios:
Modelling the baseline effect
In order to estimate absolute effects of infection prevention strategies, we modelled a baseline effect using an absolute natural history model within a Bayesian framework:
where θik is a trial-specific baseline effect (µ) in trial i in arm k. The trial-specific baselines are drawn from a distribution (a normal distribution assumed) of effects with a common mean and variance. Vague priors are put on the mean and variance: m ∼ N(0, 1002), and δm ∼ Uniform(0, 5).
Assessing model fit and deviance
We used the posterior mean of the residual deviance and the deviance information criterion (DIC) to assess the goodness of fit of MTC models. 77 The posterior mean of the residual deviance, D¯res, is defined as the deviance for the fitted model minus the deviance for the saturated model. 77 Each unconstrained data point i has a contribution D¯i to the residual deviance:
It is expected that each data point should contribute approximately 1 to the posterior mean deviance. 77,96 Therefore, under the null hypothesis that the model adequately fits the data, D¯res would have a mean equal to the number of unconstrained data points for a perfectly fitted model. 77,96 The DIC is defined as:
where pD denotes the effective number of parameters, which is the sum of the leverages of each individual observation, defined as the relative influence that each observation has on its own fitted value.
where D¯ is the deviance calculated at the posterior mean of the model parameters or of the fitted values for each data point (the predicted number of events estimated from the model) when non-linearity exists between the likelihood and the model parameters. 97 The DIC provides a measure of model fit that penalises model complexity. Lower DIC values suggest a better-fitted model. 97 When the model fit was poor, we explored how each data point affected the model fit by plotting D¯i (each data point’s contribution to D¯res) against its contribution to pD (leverage). 97 These summaries were displayed in a plot of leverage versus dri for each data point, where dri=±D¯i with sign given by the difference between the posterior mean of the predicted and observed values for observation i. Curves of the form x2 + y = c with c = 1, 2, 3 and 4 were plotted as they represented the lines of each contribution to DIC. Points lying on such parabolas each contributed an amount c to DIC, with points lying outside the line c = 3 identified as contributing to the model’s poor fit.
Checking consistency by node splitting
The underlying assumption of MTC models is that direct and indirect sources of evidence estimate the same underlying treatment effect across the MTC network. However, patient populations may differ in their responsiveness to infection prevention strategies. We therefore examined the consistency of the MTC models by using node splitting. Node splitting is based on splitting sources of information about a node in a directed acyclic graph, which represents the dependency structure of a model. 77 It allows the conflict between the inferences on a node from different sources of information to be examined. 77 We assessed the inconsistency between the direct and indirect evidence for each treatment effect by splitting the information in the model into direct and indirect information. 77 Given that only pairs of treatments that are part of a closed loop have both direct and indirect evidence available,84 and there can be no inconsistency in multiarm trials,77 only five pairwise comparisons that formed two independent three-way loops (see Figure 9, T3, T4 and T1, and T9, T8 and T7) in the evidence network needed to be checked for consistency (see Appendix 4).
Two posterior distributions were obtained from the mean treatment effect dXY: one based on studies comparing treatment X and Y directly, with mean dXYDir, and another indirectly with mean dXYInd from a MTC meta-analysis of all the remaining indirect evidence.
The inconsistency parameter was:
A test of the null hypothesis showed that ωXY = 0 would provide evidence of consistency. 77 We used the posterior mean of the residual deviance D¯res and the DIC to compare the full MTC model with the model where a particular node was split. A reduction in D¯res or DIC for the split model would suggest an inconsistency between the different sources of evidence for a treatment. We also plotted each point’s contribution to the DIC to identify which point in the data contributed to the poor model fit and how their contribution changed when different nodes were split. 77
Addressing heterogeneity
Between-study variability in intervention effects is broadly termed ‘heterogeneity’. It can be induced by clinical diversity in terms of patient population, intervention or setting, or by variability in study design and risk of bias. The former is commonly known as clinical heterogeneity and the latter as methodological heterogeneity. We attempted to address both forms of heterogeneity with metaregression and bias adjustment.
Metaregression on patient subgroup effects
Among the risk factors known to affect SSIs are patient age, sex and previous surgery. As the included trials did not report sex-specific THR-related SSIs (nor significant variation in patient mean age), we focused our attention on previous surgery as a potential covariate interacting with treatment effects and conducted a metaregression using the following subgroup random-effects model:77
where θik is the linear predictor in arm k of trial i, µi the trial-specific baseline effects in trial i, and xi is the trial-level covariate for trial i, which represents a patient subgroup:
δ is the trial-specific log-ORs of the SSIs in the intervention compared with the control for the patient group with no previous surgery reported, and β measures the change in the log-ORs for previous surgery.
The trial-specific log-ORs have a common distribution: δi∼N(d,σ2). 77
d, β and σ are given independent priors in the Bayesian framework: d, β ∼ N(0,1002) and σ ∼ Uniform(0,5). 77
Metaregression on follow-up duration effects
Given the significant variation in follow-up durations of included studies, we conducted a metaregression on follow-up duration as a potential risk of bias for intervention effects using a centred covariate model:
where xi is a follow-up duration covariate in trial i and x¯ is the mean covariate value. The intervention effects were estimated at the mean covariate value, and uncentred and transformed to produce mean intervention effects at a given covariate value z:
Estimating and adjusting for bias in the mixed-treatment comparison network
With the assumption that the mean and variance of study-specific biases are the same for each treatment in the MTC network, it is possible to simultaneously estimate treatment effects and bias effects in a single analysis, and thus to produce treatment effects that are based on the entire body of data, including both low- and high-quality studies, and also adjust for bias.
We used the following model to estimate and adjust for bias of mixed-quality studies (RCTs and observational studies) contained in the MTC network:
where xi = 1 if study i is of low quality (observational) and considered to be at risk of bias and zero for RCTs; βik is the trial-specific bias of the treatment in arm k relative to the treatment in arm 1 of trial i.
Chapter 3 Results of synthesis of effectiveness evidence
Description of the evidence and interventions
From the 12 studies identified by the process shown in Figure 8, six were RCTs98–103 and six were observational studies. 104–109 They included 123,788 THRs and nine infection prevention strategies, as shown in the MTC network (Figure 9). The data from the included papers are shown in Table 6.
FIGURE 9.
Mixed-treatment comparison network consisting of 12 studies, with nine infection prevention strategies. Note that the lines represent direct evidence comparisons, boxes represent infection control strategies involving multiple infection control measures and the numbers on the lines represent the numbers of comparisons. The three-way loops in bold lines represent loops only formed by a multiarm trial. Reproduced from Zheng H, Barnett AG, Merollini K, Sutton A, Cooper N, Berendt T, et al. Control strategies to prevent total hip replacement-related infections: a systematic review and mixed treatment comparison. BMJ Open 2014;4:e003978. 9 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/.

| Comparison of infection prevention strategies | Author (year), study design, country | Study number | Strategy | Number of THR-related SSIs | Number of THRs | Evidence level and quality assessment (scorea) |
|---|---|---|---|---|---|---|
| The reference strategy T1 (no systemic antibiotics, plain cement, conventional ventilation) and T2 (systemic antibiotics, plain cement, conventional ventilation) | Carlsson et al. (1977),99 RCT, Sweden | 1 | T1 | 7 | 58 | Evidence level: 1– C1, 1; C2, 2; C3, 2; C4, 1; C5, 1; C6, 1; C7, 3; C8, 3; total 14 |
| T2 | 0 | 60 | ||||
| Schulitz et al. (1980),100 RCT, Germany | 2 | T1 | 8 | 89 | Evidence level: 1– C1, 1; C2, 1; C3, 1; C4, 1; C5, 2; C6, 2; C7, 3; C8, 2; total 13 |
|
| T2 | 1 | 105 | ||||
| T2 (systemic antibiotics, plain cement, conventional ventilation) and T4 (systemic antibiotics, plain cement, laminar airflow) | Salvati et al. (1982),106 observational study, Italy | 5 | T2 | 11 | 761 | Evidence level: 2– C1, 1; C2, 2; C3, 3; C4, 2; C5, 1; C6, 2; C7, 1; C8, 3; total 15 |
| T4 | 13 | 1518 | ||||
| Fitzgerald (1992),101 RCT, USA | 7 | T2 | 4 | 1739 | Evidence level: 1– C1, 1; C2, 2; C3, 1; C4, 1; C5, 1; C6, 1; C7, 1; C8, 2; total 10 |
|
| T4 | 1 | 1682 | ||||
| Kelly et al. (1996),107 observational study, UK | 8 | T2 | 0 | 236 | Evidence level: 2– C1, 1; C2, 1; C3, 1; C4, 1; C5, 1; C6, 3; C7, 1; C8, 2; total 11 |
|
| T4 | 3 | 207 | ||||
| T2 (systemic antibiotics, plain cement, conventional ventilation) and T5 (no systemic antibiotics, antibiotic-impregnated cement, conventional ventilation) | Josefsson et al. (1981),102 RCT, Sweden | 4 | T2 T5 |
10 2 |
812 821 |
Evidence level: 1– C1, 1; C2, 2; C3, 1; C4, 1; C5, 1; C6, 1; C7, 3; C8, 3; total 13 |
| McQueen et al. (1990),103 RCT, UK | 6 | T2 | 1 | 190 | Evidence level: 1– C1, 1; C2, 2; C3, 1; C4, 1; C5, 1; C6, 2; C7, 3; C8, 3; total 14 |
|
| T5 | 2 | 190 | ||||
| T6 (systemic antibiotics, antibiotic-impregnated cement, conventional ventilation) and T7 (systemic antibiotics, antibiotic-impregnated cement, laminar airflow) | Brandt et al. (2008),105 observational study, Germany | 11 | T6 | 99 | 10,966 | Evidence level: 2+ C1, 2; C2, 3; C3, 1; C4, 3; C5, 2; C6, 2; C7, 2; C8, 3; total 18 |
| T7 | 242 | 17,657 | ||||
| The reference strategy T1 (no systemic antibiotics, plain cement, conventional ventilation), T2 (systemic antibiotics, plain cement, conventional ventilation), T3 (no systemic antibiotics, plain cement, laminar airflow) and T4 (systemic antibiotics, plain cement, laminar airflow) | Hill et al. (1981),98 RCT, France | 3 | T1 | 31 | 596 | Evidence level: 1– C1, 2; C2, 2; C3, 2; C4, 2; C5, 2; C6, 3; C7, 2; C8, 3; total 18 |
| T2 | 4 | 590 | ||||
| T3 | 4 | 471 | ||||
| T4 | 6 | 480 | ||||
| The reference strategy T1 (no systemic antibiotics, plain cement, conventional ventilation), T2 (systemic antibiotics, plain cement, conventional ventilation), T5 (no systemic antibiotics, antibiotic-impregnated cement, conventional ventilation) and T6 (systemic antibiotics, antibiotic-impregnated cement, conventional ventilation) | Espehaug et al. (1997),108 observational study, Norway | 9 | T1 | 3 | 276 | Evidence level: 2+ C1, 2; C2, 3; C3, 2; C4, 3; C5, 2; C6, 3; C7, 3; C8, 3; total 21 |
| T2 | 25 | 4586 | ||||
| T5 | 3 | 239 | ||||
| T6 | 8 | 5804 | ||||
| Engesæter et al. (2003),109 observational study, Norway | 10 | T1 | 3 | 280 | Evidence level: 2+ C1, 2; C2, 3; C3, 2; C4, 3; C5, 2; C6, 3; C7, 3; C8, 3; total 21 |
|
| T2 | 46 | 5960 | ||||
| T5 | 3 | 254 | ||||
| T6 | 50 | 15,676 | ||||
| T6 (systemic antibiotics, antibiotic-impregnated cement, conventional ventilation), T7 (systemic antibiotics, antibiotic-impregnated cement, laminar airflow), T8 (systemic antibiotics, antibiotic-impregnated cement, conventional ventilation, body exhaust suit) and T9 (systemic antibiotics, antibiotic-impregnated cement, laminar ventilation, body exhaust suit) | Hooper et al. (2011),104 observational study, New Zealand | 12 | T6 | 17 | 31,939 | Evidence level: 2+ C1, 2; C2, 2; C3, 2; C4, 3; C5, 1; C6, 3; C7, 2; C8, 3; total 18 |
| T7 | 9 | 8772 | ||||
| T8 | 4 | 2696 | ||||
| T9 | 16 | 8078 |
The quality of evidence summarised in Table 6 was variable, with quality score between 11 and 21 (see Tables 65 and 66 in Appendix 3 for the scoring tools used). Five of the six RCT studies99–103 provided no information on random sequence generation, four100–103 provided no information on blinding assessors and only one98 reported prior calculation of the sample size. The statistical power for most RCTs was generally low. Only one98 RCT reported primary analysis based on all randomised cases, whereas the rest did not report intention to treat. Of the six observational studies, three105,108,109 identified and adjusted for confounding variables, one106 reported that cases and control groups were comparable on diagnostic confounding factors and two108,109 described and included in the analysis the outcomes of the patients who withdrew. Three studies101,108,109 used objective measures to assess the outcomes and were adequately powered with large sample size ranging from 10,905 to 51,485.
Effectiveness outcomes
For every strategy in the connected network a relative effect was estimated against another infection prevention strategy using an OR of SSI. We chose ‘no systemic antibiotics, plain cement and conventional ventilation’ as the reference strategy (T1), as it was compared with the greatest number of other strategies.
Thirty-six relative effects involving nine infection prevention strategies were estimated in the MTC network using models that did and did not adjust for duration of follow-up (Table 7). The results from both models were almost identical, as were estimates of the model fit; therefore, the differences in follow-up duration had little effect on the effectiveness of the infection strategies. Therefore, we report the results of the model without adjustment for follow-up from now on. The 36 ORs for all pairwise comparisons are presented in the forest plot in Figure 10. The probability and median rank of a strategy being the most effective strategy is shown in Table 8. The models were fitted in a Bayesian framework using WinBUGS and code by Dias et al. 110
| Treatment | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | |
| T2 | 0.31 (0.12 to 0.65) | |||||||
| T3 | 0.26 (0.03 to 0.95) | 0.92 (0.11 to 3.39) | ||||||
| T4 | 0.25 (0.06 to 0.66) | 0.84 (0.28 to 1.97) | 1.93 (0.20 to 7.58) | |||||
| T5 | 0.38 (0.09 to 1.12) | 1.28 (0.38 to 3.38) | 3.28 (0.27 to 14.15) | 1.96 (0.37 to 6.54) | ||||
| T6 | 0.13 (0.03 to 0.35) | 0.44 (0.13 to 1.13) | 1.12 (0.09 to 4.62) | 0.67 (0.12 to 2.12) | 0.43 (0.09 to 1.24) | |||
| T7 | 0.27 (0.03 to 0.93) | 0.90 (0.13 to 3.14) | 2.47 (0.11 to 10.22) | 1.41 (0.14 to 5.35) | 0.88 (0.09 to 3.10) | 1.96 (0.52 to 5.37) | ||
| T8 | 0.52 (0.03 to 2.12) | 1.77 (0.11 to 7.20) | 5.78 (0.10 to 21.12) | 2.89 (0.12 to 11.73) | 1.71 (0.08 to 6.93) | 3.72 (0.38 to 13.75) | 2.26 (0.22 to 8.48) | |
| T9 | 0.74 (0.05 to 2.69) | 2.49 (0.20 to 9.11) | 13.15 (0.18 to 27.4) | 4.11 (0.22 to 14.92) | 2.44 (0.15 to 8.62) | 5.00 (0.73 to 16.87) | 3.14 (0.42 to 10.41) | 2.53 (0.23 to 10.41) |
| Model fit statistic (posterior mean residual deviance) 34.3a | Model fit statistic (DIC) 180.6 | Heterogeneity (between-study deviation) 0.62 | ||||||
FIGURE 10.
Forest plot of ORs of SSI for infection prevention strategies (random effects). Reproduced from Zheng H, Barnett AG, Merollini K, Sutton A, Cooper N, Berendt T, et al. Control strategies to prevent total hip replacement-related infections: a systematic review and mixed treatment comparison. BMJ Open 2014;4:e003978. 9 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/.

| Infection prevention strategies | Probability | Median rank (95% CrI) |
|---|---|---|
| T1 | 0.00 | 9 (7 to 9) |
| T2 | 0.00 | 6 (3 to 8) |
| T3 | 0.24 | 3 (1 to 8) |
| T4 | 0.06 | 4 (1 to 8) |
| T5 | 0.02 | 6 (2 to 8) |
| T6 | 0.47 | 2 (1 to 5) |
| T7 | 0.08 | 3 (1 to 8) |
| T8 | 0.10 | 5 (1 to 9) |
| T9 | 0.02 | 7 (2 to 9) |
The five infection prevention strategies associated with a statistically significant reduction in THR-related SSI compared with the reference strategy (T1) were:
-
T6 with an OR of 0.13 [95% credible interval (CrI) 0.03 to 0.35]; systemic antibiotics, antibiotic-impregnated cement and conventional ventilation
-
T4 with an OR of 0.25 (95% CrI 0.06 to 0.66); systemic antibiotics, plain cement and laminar airflow
-
T3 with an OR of 0.26 (95% CrI 0.03 to 0.95); no systemic antibiotics, plain cement and laminar airflow
-
T7 with an OR of 0.27 (95% CrI 0.03 to 0.93); systemic antibiotics, antibiotic-impregnated cement and laminar airflow
-
T2 with an OR of 0.31 (95% CrI 0.12 to 0.65); systemic antibiotics, plain cement and conventional ventilation.
Statistically non-significant reductions in THR-related SSI compared with the reference strategy, T1, were:
-
T5 with an OR of 0.38 (95% CrI 0.09 to 1.12); no systemic antibiotics, antibiotic-impregnated cement and conventional ventilation
-
T8 with an OR of 0.52 (95% CrI 0.03 to 2.12); systemic antibiotics, antibiotic-impregnated cement, conventional ventilation and body exhaust suit
-
T9 with an OR of 0.74 (95% CrI 0.05 to 2.69); systemic antibiotics, antibiotic-impregnated cement, laminar ventilation and body exhaust suit.
When T7 was compared with T6, the OR of SSI was 1.96 (95% CrI 0.52 to 5.37), suggesting that laminar airflow could increase infection risk. Similarly, when T8 was compared with T6, the OR was 3.72 (95% CrI 0.38 to 13.75), suggesting that body exhaust suits may also increase infection risk, at least where there is conventional ventilation. There was no high-quality evidence that antibiotic-impregnated cement without systemic antibiotics was more effective in reducing infection than plain cement and systemic antibiotics (T2 vs. T5; OR 1.28, 95% CrI 0.38 to 3.38). All comparisons and interpretations are summarised in Table 9.
| Treatment | No systemic antibiotics | Plain cement | Conventional ventilation | Systemic antibiotics | Antibiotic-impregnated cement | Laminar airflow | Body exhaust suit | OR compared with T1 | 95% CrI |
|---|---|---|---|---|---|---|---|---|---|
| T1 | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | Reference | |
| T2 | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | 0.31 | 0.12 to 0.65 |
| T3 | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | 0.26 | 0.03 to 0.95 |
| T4 | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | 0.25 | 0.06 to 0.66 |
| T5 | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✗ | 0.38 | 0.09 to 1.12 |
| T6 | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | 0.13 | 0.03 to 0.35 |
| T7 | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | 0.27 | 0.03 to 0.93 |
| T8 | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | 0.52 | 0.03 to 2.12 |
| T9 | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | 0.74 | 0.05 to 2.69 |
Model fit and evidence consistency
The model fit statistics indicate that the fit was less than adequate (see Table 7). This was confirmed by diagnostic plots that showed infection prevention strategies T2 and T5 of study 4102 and the T1 strategy of study 10109 were outliers contributing to the inadequate model fit (Figure 11).
FIGURE 11.
Leverage vs. deviance residual superimposed on curves y = –x2 + c, where c = T1, T2, T3 and T4, representing the amount contributed to DIC. Reproduced from Zheng H, Barnett AG, Merollini K, Sutton A, Cooper N, Berendt T, et al. Control strategies to prevent total hip replacement-related infections: a systematic review and mixed treatment comparison. BMJ Open 2014;4:e003978. 9 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/.
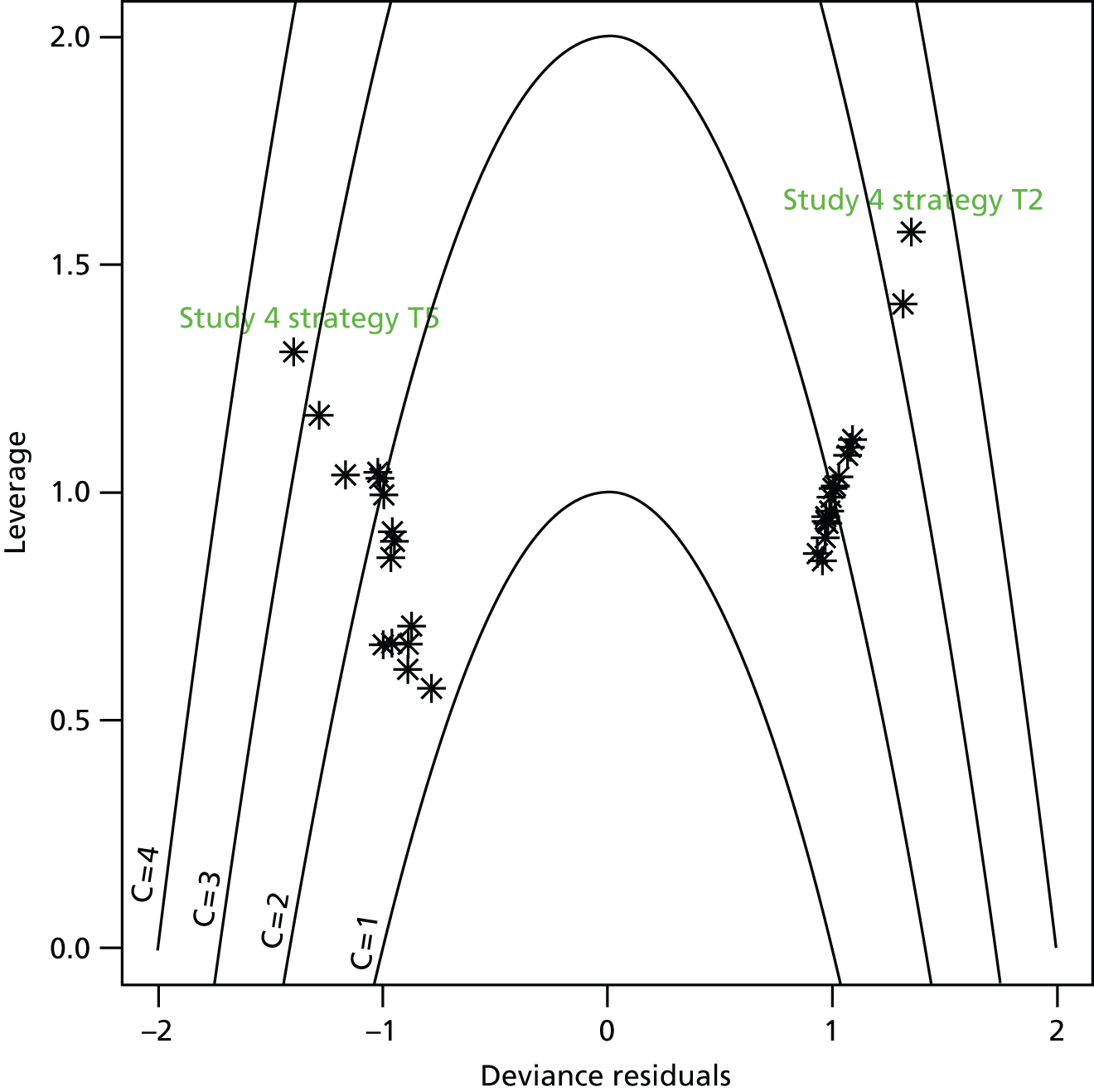
Curves of the quadratic function were plotted as they represented the lines of each contribution to DIC. Points lying outside the line c = 3, were identified as contributing to the inadequate model fit. The plot shows the first and second arms, strategies T2 and T5, of study 4102 are outliers contributing to the inadequate model fit.
After exclusion of both the first and second arms of study 4102 (T4 and T1, and T4 and T2, respectively), the model fitted the data well and the heterogeneity was significantly reduced, but the results were little changed (Figure 12).
FIGURE 12.
Sensitivity analysis excluding the first and second arms of study 4102 (T4 and T1, and T4 and T2, respectively). Leverage vs. deviance residual superimposed on curves: y = –x2 + c, where c = T1, T2, T3 and T4, representing the amount contributed to DIC. Reproduced from Zheng H, Barnett AG, Merollini K, Sutton A, Cooper N, Berendt T, et al. Control strategies to prevent total hip replacement-related infections: a systematic review and mixed treatment comparison. BMJ Open 2014;4:e003978. 9 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/.

A further sensitivity analysis was undertaken that removed the first arm of study 10109 (T1 and T10) (Figure 13). After excluding study 10109 from the network, all the remaining data points lay below the quadratic curve with c = 3, suggesting that the contribution of the remaining data points to the DIC was unimportant, which in turn would improve the model fit.
FIGURE 13.
Sensitivity analysis by further excluding the first arm of study 10109 (T1 and T10). Leverage vs. deviance residual superimposed on curves: y = –x2 + c, where c = T1, T2, T3 and T4, representing the amount contributed to DIC. Reproduced from Zheng H, Barnett AG, Merollini K, Sutton A, Cooper N, Berendt T, et al. Control strategies to prevent total hip replacement-related infections: a systematic review and mixed treatment comparison. BMJ Open 2014;4:e003978. 9 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/.

Following these sensitivity analyses, infection prevention strategy T6 remained dominant, with the highest probability (64%) and highest median rank of being the most effective strategy (Table 10).
| Infection prevention strategies | Probability | Median rank (95% CrI) |
|---|---|---|
| T1 | 0.00 | 9 (7 to 9) |
| T2 | 0.01 | 6 (2 to 8) |
| T3 | 0.14 | 4 (1 to 8) |
| T4 | 0.05 | 4 (1 to 7) |
| T5 | 0.00 | 8 (4 to 9) |
| T6 | 0.64 | 1 (1 to 4) |
| T7 | 0.05 | 3 (1 to 7) |
| T8 | 0.10 | 4 (1 to 8) |
| T9 | 0.01 | 6 (2 to 9) |
The MTC results are shown in Table 11. The sensitivity analysis that excluded studies 4102 and 10109 from the MTC network showed that model fit was improved; the DIC was reduced from 180.6 to 141.8. The posterior mean residual deviance was also reduced from 34.3 to 25.3. Heterogeneity measured in between-study standard deviation across the MTC network also reduced from 0.63 to 0.43.
| Treatment | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | |
| T2 | 0.22 (0.08 to 0.43) | |||||||
| T3 | 0.20 (0.03 to 0.60) | 1.00 (0.15 to 3.21) | ||||||
| T4 | 0.19 (0.06 to 0.43) | 0.87 (0.35 to 1.86) | 1.61 (0.23 to 5.78) | |||||
| T5 | 0.76 (0.11 to 2.23) | 3.39 (0.61 to 10.42) | 7.09 (0.48 to 28) | 4.80 (0.62 to 16.16) | ||||
| T6 | 0.09 (0.02 to 0.25) | 0.43 (0.09 to 1.27) | 0.63 (0.09 to 1.92) | 0.63 (0.09 to 1.92) | 0.19 (0.24 to 0.69) | |||
| T7 | 0.33 (0.02 to 0.56) | 1.05 (0.11 to 2.90) | 3.92 (0.09 to 6.67) | 1.74 (0.12 to 4.22) | 0.46 (0.03 to 1.43) | 1.85 (0.67 to 4.20) | ||
| T8 | 0.83 (0.02 to 1.16) | 10.75 (0.09 to 15.88) | 43.12 (0.08 to 12.92) | 6.84 (0.10 to 8.44) | 1.04 (0.03 to 2.83) | 3.83 (0.44 to 10.33) | 2.05 (0.27 to 6.33) | |
| T9 | 0.66 (0.03 to 1.44) | 3.42 (0.19 to 7.35) | 10.32 (0.16 to 16.24) | 4.60 (0.20 to 10.58) | 1.29 (0.06 to 3.57) | 4.72 (1.00 to 10.27) | 3.23 (0.58 to 7.56) | 2.39 (0.31 to 8.37) |
| Model fit statistic (posterior mean residual deviance) 25.3a | Model fit statistic (DIC) 141.8 | Heterogeneity (between-study deviation) 0.43 | ||||||
The direct evidence from all conventional pairwise meta-analyses is presented in Table 12. There was broad agreement among the direct evidence from conventional pairwise meta-analyses, the direct and indirect evidence from node splitting and the evidence from the MTC model. Tests for inconsistency between direct and indirect evidence from node splitting suggested that there was no statistically significant evidence of inconsistency. The model fit statistics for the node splitting and the MTC models were similar, implying that there was no conflict between the direct and indirect evidence. It is worth noting that the 95% CrIs for some pairwise comparisons widened greatly following node splitting. This is explained by the node splitting reducing the evidence available to inform the variance.
| Inconsistency estimate [node split, mean (95% CrI)] | Treatments | All evidence [MTC, mean (95% CrI)] | Direct | Indirect evidence [node split, mean (95% CrI)] | Test for inconsistency, node split (Bayesian p-value) | Posterior mean residual deviance | DIC | Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Pairwise meta-analysis, mean (95% CI) | Node split, mean (95% CrI) | |||||||
| (MTC) 25.3 | (MTC) 141.8 | (MTC) 0.4 | ||||||||
| –0.7 (–2.6 to 1.1) | T1 | T2 | –1.6 (–2.5 to –0.8) | –1.7 (–2.6 to –0.8); I2 = 24.5% | –1.7 (–2.8 to –0.9) | –1.0 (–2.7 to 0.6) | 0.2 | 25 | 142.4 | 0.5 |
| –2 (–197.5 to 194.2) | T1 | T3 | –1.9 (–3.5 to –0.5) | –1.86 (–2.91 to –0.81); I2 = 0% | –1.9 (–3.5 to –0.5) | 0.0 (–196.2 to 195.5) | 0.5 | 25.4 | 141.9 | 0.4 |
| 0.7 (–1.2 to 2.8) | T1 | T4 | –1.8 (–2.9 to –0.8) | –1.47 (–2.35 to –0.58); I2 = 0% | –1.5 (–3.0 to –0.1) | –2.2 (–3.8 to –0.9) | 0.8 | 25.1 | 142.5 | 0.5 |
| –1.9 (–198.0 to 193.5) | T2 | T3 | –0.3 (–1.9 to –1.2) | 0.23 (–1.16 to 1.62); I2 = 0% | –0.2 (–1.8 to 1.4) | 1.7 (–193.7 to 197.7) | 0.5 | 25.5 | 141.8 | 0.4 |
| –1.9 (–198.5 to 194.3) | T2 | T4 | –0.2 (–1.1 to 0.6) | –0.2 (–0.9 to 0.5); I2 = 19% | –0.2 (–1.0 to 0.7) | 1.7 (–194.6 to 198.3) | 0.5 | 25.4 | 141.8 | 0.4 |
| 0.8 (–1.4 to 3.2) | T3 | T4 | 0.1 (–1.5 to 1.8) | 0.39 (–0.88 to 1.66); I2 = 0% | 0.4 (–1.3 to 2.2) | –0.4 (–2.5 to 1.6) | 0.8 | 25.1 | 142.5 | 0.5 |
| 0.1 (–3.8 to 3.9) | T1 | T5 | –0.7 (–2.2 to 0.8) | 0.1 (–1.0 to 1.3); I2 = 0% | –0.4 (–2.3 to 1.5) | –0.6 (–3.7 to 3.1) | 0.5 | 26.2 | 143.8 | 0.5 |
| –0.7 (–196.9 to 195.6) | T2 | T5 | 0.9 (–0.5 to 2.3) | 0.6 (–0.2 to 1.4); I2 = 0% | 0.8 (–0.7 to 2.1) | 1.5 (–194.8 to 197.8) | 0.5 | 25.3 | 141.8 | 0.4 |
| –0.4 (–97.8 to 104.7) | T1 | T6 | –2.8 (–4.1 to –1.4) | –2.0 (–2.5 to –0.7); I2 = 0% | –2.6 (–3.9 to –1.0) | –3 (–107.3 to 95.1) | 0.5 | 25.3 | 141.8 | 0.4 |
| 23.6 (–100.6 to 124.8) | T2 | T6 | –1.2 (–2.4 to 0.2) | –1.0 (–1.4 to –0.6); I2 = 13.9% | –1.3 (–2.7 to –0.1) | –25 (–126.1 to 99.2) | 0.7 | 25.3 | 141.9 | 0.4 |
| 10.0 (75.8 to 96.8) | T5 | T6 | –2.1 (–3.7 to –0.4) | –1.7 (–2.6 to –0.8); I2 = 0% | –2.1 (–3.7 to –0.4) | –12.1 (–98.7 to 73.8) | 0.6 | 25.3 | 141.8 | 0.4 |
| –2.1 (–198.7 to 194.4) | T6 | T7 | 0.5 (–0.4 to 1.4) | 0.4 (0.2 to 0.7); I2 = 0% | 0.5 (–0.4 to 1.5) | 2.6 (–193.9 to 199.1) | 0.5 | 25.2 | 141.7 | 0.4 |
| –1.7 (–197.4 to 194.3) | T6 | T8 | 0.9 (–0.8 to 2.3) | 1.03 (–0.06 to 2.12); I2 = 0% | 0.9 (–0.7 to 2.3) | 2.6 (–193.5 to 198.2) | 0.5 | 25.4 | 141.9 | 0.4 |
| –1.9 (–197.4 to 193.4) | T7 | T8 | 0.4 (–1.3 to 1.8) | 0.37 (–0.81 to 1.55); I2 = 0% | 0.4 (–1.3 to 1.8) | 2.2 (–193.0 to 197.8) | 0.5 | 25.3 | 141.8 | 0.4 |
| –1.6 (–197.7 to 194.1) | T6 | T9 | 1.2 (0.0 to 2.5) | 1.32 (0.63 to 2.00); I2 = 0% | 1.3 (0.1 to 2.5) | 2.9 (–192.9 to 199.0) | 0.5 | 25.3 | 141.8 | 0.4 |
| –1.3 (–197.3 to 194.4) | T7 | T9 | 0.8 (–0.5 to 2.0) | 0.66 (–0.16 to 1.48); I2 = 0% | 0.7 (–0.6 to 2.0) | 2.0 (–193.8 to 198.0) | 0.5 | 25.3 | 141.8 | 0.4 |
A test of interaction between RCTs and observational studies was not statistically significant, suggesting that combining these study types was not inappropriate (Table 13).
| Models (10 studies included) | The posterior mean residual deviance | DIC | β (subgroup interaction term) | Heterogeneity (between-study standard deviation) |
|---|---|---|---|---|
| The random-effects metaregression model | 24.3 | 141 | 1.4 (95% CrI –0.3 to 3.5) | 0.4 |
| The random-effects MTC model | 25.3 | 141.8 | Not applicable | 0.4 |
The results were little changed by excluding the RCT by Hill et al. 98 (Table 14) or by including the RCT by Lidwell et al. 111 (Table 15).
| Treatment | Probability of each strategy being the best | Median rank (95% CrI) |
|---|---|---|
| T1 | 0.00 | 8 (5 to 8) |
| T2 | 0.01 | 5 (2 to 7) |
| T4 | 0.16 | 3 (1 to 7) |
| T5 | 0.01 | 7 (3 to 8) |
| T6 | 0.63 | 1 (1 to 4) |
| T7 | 0.06 | 3 (1 to 6) |
| T8 | 0.11 | 4 (1 to 8) |
| T9 | 0.02 | 5 (2 to 8) |
| Treatment | Probability of each strategy being the best | Median rank (95% CrI) |
|---|---|---|
| T1 | 0.00 | 9 (7 to 9) |
| T2 | 0.00 | 5 (3 to 7) |
| T3 | 0.01 | 7 (2 to 8) |
| T4 | 0.03 | 4 (1 to 7) |
| T5 | 0.00 | 8 (3 to 9) |
| T6 | 0.83 | 1 (1 to 3) |
| T7 | 0.04 | 2 (1 to 6) |
| T8 | 0.09 | 4 (1 to 8) |
| T9 | 0.01 | 5 (2 to 8) |
Strategy T6 (systemic antibiotics, antibiotic-impregnated cement and conventional ventilation) remained dominant in both these further analyses, with the highest probability of being a cost-effective decision (63% and 83%, respectively) and highest median rank of being the most effective strategy (see Tables 14 and 15).
Chapter 4 Cost-effectiveness methods
Background
The purpose of this cost-effectiveness study is to evaluate change in cost and health outcomes arising from each of the identified infection prevention strategies. The main decision-making group in the NHS is NICE, and it uses cost-effectiveness information to compare the health benefits forgone from competing configurations of NHS services. This approach meets the social objective of making improvements to health when the budget for services is fixed. It requires a systematic assessment of the changes to costs and the changes to health from alternate configurations of services. 112
Costs
The change in costs is shown as ΔC and includes the positive costs from implementing an infection prevention strategy and all the cost savings that arise from the avoided infections, such as reduced length of stay and revisions. Costs are estimated as quantities of resource used, q, and prices, p. The latter are estimated using existing market prices or shadow prices. In processes of production, costs can be fixed or variable. Fixed costs cannot be changed short term and do not vary with fluctuations in productivity or output, whereas variable costs are flexible and change with productivity levels. 47 The sum of both these costs is the total cost. For the interpretation of costs of competing health-care alternatives we will estimate the incremental change to total costs. Incremental costs are more helpful for decision-making as they show the costs of achieving an additional unit of health benefit. 113
The perspective for the analysis determines the scope of the costs included, and for this project we assume only those costs incurred by the NHS are relevant. Private costs are excluded because of the difficulty in assigning a shadow price to certain items, such as volunteer time.
Costs that occur in the present are usually given more value than future costs and hence are discounted. To avoid inconsistencies in the conclusions, both costs and benefits arising in the future need to be discounted, typically at a rate of 3%; this is the rate used for this research. Without discounting, inconsistencies in the overall allocation of resources could arise, as we would be treating the value of health care differently compared with economic benefits of other sectors in the economy. 47
Health outcomes
The change in health outcomes/effects are shown as ΔE. This summarises the gain in health outcomes from a decision to adopt a treatment strategy. In this case we use the QALY, which serves as a generic measure of health outcomes. It is widely used since it allows a combination of both morbidity, measuring quality of life, and mortality, measuring length of life, into a single score. 114
Utility scores describe the quality of life in a certain health state where perfect health is valued 1 and death 0. A QALY is the product of a utility score and the time spent in that health state. In Figure 14 an example of two competing alternatives with different health outcomes is shown. Alternative A generates two additional life-years at a utility of 0.8, whereas alternative B offers four additional life-years at a lower utility of 0.6. The calculation of QALYs below shows that alternative B generates 0.8 extra QALY outcomes than alternative A. Health benefits are not discounted in this example.
FIGURE 14.
Example of a QALY calculation of two alternatives.

A widely used method for valuing the utility of different health states is a multiattribute utility scale. 115 Examples of instruments using a multiattribute utility scale include the European Quality of Life-5 Dimensions (EQ-5D) questionnaire, developed by the EuroQoL Group, and the Assessment of Quality of Life and Short Form questionnaire-6 Dimensions. 47 These classification systems allow the derivation of preferences for health states using prescored measurements called utility weights. The utility weights have been valued previously, by a representative sample of the population for which it is used. The EQ-5D is one of the most widespread instruments and measures five dimensions – mobility, self-care, usual activities, pain/discomfort and anxiety/depression – with three levels for each and so defines a total of 243 health states. 115 People with a certain disease or health outcome are asked to respond to a question for each dimension and their answers are used to determine which health state best fits their condition. The UK value set generated by Dolan116 for the EQ-5D was used for this project.
Incremental cost-effectiveness
The ICER is shown below as the cost incurred to gain 1 QALY:
It arises by comparing the costs and health outcomes of novel treatments or health programmes (CT) with the costs of the baseline comparator (CC). In Table 16 we show hypothetical outcomes of current practice compared with a novel infection prevention strategy, and how the resulting ICER is calculated.
| Costs (£) | QALYs | |
|---|---|---|
| Current practice | 1,800,000 | 210 |
| Novel strategy | 2,000,000 | 250 |
| Difference | 200,000 | 40 |
| ICER | 5000/QALY | |
A decision to adopt a new treatment or service depends on a number of factors, most importantly the ability and willingness to pay for marginal health benefits. This value is reflected by the ceiling or threshold ratio, such as £25,000–£35,000, traditionally used by NICE. 117 An evidence-based approach to finding a threshold was presented recently by Claxton et al. ,118 who found that values of around £18,000 were more appropriate. Combined uncertainty in the estimates showed that the probability that the threshold is < £20,000 per QALY is 0.64 and the probability that it is < £30,000 is 0.92. 118 Other factors influencing health policy decisions are social value judgements, such as equity and efficiency, the level of need in the community or the severity of a given outcome. 117,119
Cost-effectiveness planes are used to summarise results, and Figure 15 shows the ICER from the example in Table 16. Current practice is at the origin, the x-axis represents the effectiveness and the y-axis represents the costs associated with the infection prevention alternative. The ceiling ratio in green indicates the maximum willingness to pay for one extra unit of outcome (i.e. 1 QALY).
FIGURE 15.
Cost-effectiveness plane showing an ICER example of a novel strategy in the context of a ceiling ratio.
Strategies can fall into one of four quadrants describing possible combinations of incremental costs and benefits. Strong dominance exists for strategies that fall in quadrants II and IV. As infection prevention alternatives in quadrant IV cost less and are more effective, they should always be accepted and are described as dominant. On the other hand, strategies falling into quadrant II cost more and give less benefit. These are described as dominated and should always be rejected by decision-makers. For strategies in quadrant I or III, the decision is more complex. Strategies falling into quadrant III are less costly but also have a lower effectiveness, whereas those falling into quadrant I are more costly but return better health outcomes. 120,121 For these cases the ceiling ratio is crucial for making a decision about accepting or rejecting an alternative strategy. Strategies with an ICER below the ceiling ratio are likely to be accepted, and those above are likely to be rejected.
The novel strategy in the hypothetical example shows higher incremental costs and benefits than current practice and lies in quadrant I. At £5000 per QALY gained, it falls below the ceiling ratio and we would therefore expect the decision-maker to adopt the new treatment strategy.
Models versus prospective trials
Cost-effectiveness studies can be undertaken either as decision models or alongside prospective clinical trials. The latter are called ‘piggyback’ economic evaluations, meaning that economic data are collected alongside trial-specific clinical data. The positive aspects of this approach are low marginal costs of collecting economic data and the availability of patient-specific data for both costs and outcomes. But the fact that clinical trials are designed to assess clinical issues, such as treatment effects, can be problematic. Economic models focus on informing a specific decision, whereas trials never include all treatment alternatives relevant to a health-care decision and often compare new treatment with a placebo alternative. Economic models can include all relevant options, represented in a model structure. It is important to always compare current practice with the alternative of ‘doing nothing’, which can be simulated in a hypothetical model without randomising real patients. 122,123
Randomised trials allocate patients into groups, have relatively small sample sizes and are designed to capture short to intermediate outcomes. Follow-up of patients usually ends at a clinically significant time point, often when the efficacy of a treatment or medication is established. But other long-term effects, such as mortality beyond study end points, may nevertheless play an important role for the implementation of an alternative. Economic models have the advantage that they can simulate large patient cohorts over their lifetimes and, therefore, estimate long-term health outcomes without the time and money constraints of clinical studies. 47 Further advantages of models are that they can make indirect comparisons between alternatives for which no RCT is available. They can also be used to observe how outcomes change when certain parameters or assumptions are varied. 122
Randomised controlled trials are a gold standard measurement of outcomes and may differ from real-world scenarios. Economic models, on the other hand, synthesise data from different sources, including RCTs. Therefore, they can integrate data specific to the country of interest, such as particular occurrence and management strategies of a disease. 118 Consequently, RCTs often cannot be generalised, whereas economic models are better at reflecting real-world settings using real-world data inputs. 123
Evidence used in decision models
Decision models employ data from a range of sources to inform the model structure and parameters. Economic analysis based on data from a single RCT could introduce bias because of the method of analysis, confounding or patient selection. 60,123 In decision models, evidence is commonly required for model pathways with related probabilities, clinical effect size, baseline clinical data, resource use, costs and utilities. Possible data sources have been categorised as research based, real world based and reference based. 118,124 Research-based sources of evidence include RCTs, meta-analyses of RCTs, observational studies and economic models. Examples of real-world data sources are administrative databases, hospital or other health-care statistics and disease registers. Reference-based data sources refer to established standards in a decision-making context, such as guidelines, disease classifications or drug formularies. 125
Depending on the model component, different data sources might be more suitable than others. For the assessment of treatment effects, well-conducted clinical trials or systematic reviews of clinical trials including meta-analyses are favoured. 122,126 However, systematic reviews can very rarely be performed for each single model input because of time and resource constraints. In this case, alternative search techniques can be used, known as ‘pearl growing’ and ‘berrypicking’, which start with reviewing relevant studies and studies related to these studies until enough evidence is collected. 127,128 Although it is hard to determine when sufficient evidence has been identified, economic models can be used to establish the value of collecting more information. 129 Furthermore, systematic reviews are not always required or the ideal source of data. 118 Cost and resource use are often informed by local sources, by primary data collection or routinely collected data, as they can vary greatly in different settings.
A good model will include the most important events that impact on costs and health outcomes, rather than all possible events. 125 The model structure is typically developed and validated with the help of experts in the field, and the structure of models can be updated as new evidence becomes available. 130
Potential hierarchies of data sources have been developed to facilitate the selection of appropriate evidence. 60 Rankings of data components included in the hierarchy are clinical effect sizes, adverse events and complications, baseline clinical data, resource use, costs and utilities. For baseline clinical data, for example, the best available evidence would be a case series or a reliable database specifically developed for the study of interest, with patients from the jurisdiction of interest. Evidence of poorer quality would be recent case series not developed for the study or include patients from another jurisdiction. Further down in the hierarchy are old case series, estimates from RCTs, estimates from previously published economic analyses and, finally, expert opinion.
It is essential that the best available evidence is used to inform decision models as the results are only as reliable as the data input with the lowest quality. 60 However, the identification of all relevant evidence is very demanding and most search strategies practised lack uniformity and transparency. 124 There are no clear guidelines on this process and a recent review of modelling guidelines reported inconsistent advice. 59 This methodological issue has been recognised and more guidance on searches for model parameters is anticipated. 59,125
Uncertainty in parameters
Model parameters such as probabilities, relative risk, costs and utilities are estimates and their true value is usually unknown. In order to reduce the risk of bias and improve the usefulness of a model for decision-making, it is crucial to assess the extent of uncertainty around parameter estimates and its overall effect on model outcomes. A semi-Bayesian framework is suitable to capture uncertainty in model parameters. 131 Instead of using only point estimates the whole distribution of a parameter can be used in probabilistic sensitivity analyses (PSAs). The first step is to fit distributions around individual model parameters. Some distributions are more suitable for certain parameters because of their natural features, for example cost parameters are always positive and skewed, hence gamma distributions are a good fit (Figure 16).
FIGURE 16.
Examples of gamma distributions.
Possible shapes of beta distributions are shown in Figure 17. Beta distributions take values between 0 and 1 and are therefore suited to distributions of probabilities in the model.
FIGURE 17.
Examples of beta distributions.
Briggs et al. 132 summarises the following types of distributions, typically used to fit data likelihood functions to model parameters (Table 17).
| Parameter | Form of data/method of estimation | Candidate distribution |
|---|---|---|
| Probability (0 ≤ π ≤ 1) | Binomial/multinomial: estimated proportion(s). Time to event: survival analysis | Beta(α,β),α,β > 0 Log-normal(lm,lv),lm,lv > 0 |
| Relative risk (θ > 0) | Binomial: ratio of estimated proportions | Log-normal(lm,lv),lm,lv > 0 |
| Cost (θ ≥ 0) | Weighted sum of resource counts: mean | Gamma(α,β),α,β > 0 Log-normal(lm,lv),lm,lv > 0 |
| Utility (θ ≥ 0) | Continuous non-zero: mean | Gamma(α,β),α,β > 0 Log-normal(lm,lv),lm,lv > 0 |
| All parameters | Any distribution of data | Normal(µ,σ2),σ2 > 0 |
Once distributions are fitted around model parameters a Monte Carlo simulation is used in which values are drawn randomly for each parameter from the assigned distribution. The model is run multiple times, each time with new values selected at random. The result is a joint posterior distribution of costs and effects. 133 This output distribution can be plotted on the cost-effectiveness plane illustrating incremental costs and incremental effectiveness for each of the simulations.
Figure 18 shows the PSA results for the novel infection prevention strategy example. Instead of giving the results of thousands of simulations, only 10 simulations are illustrated here, for simplification purposes. The initial ICER is surrounded by possible outcomes generated by the Monte Carlo simulation, represented by the blue circles. The area of uncertainty forms a cloud of point estimates.
FIGURE 18.
Example of uncertainty cloud on the cost-effectiveness plane.
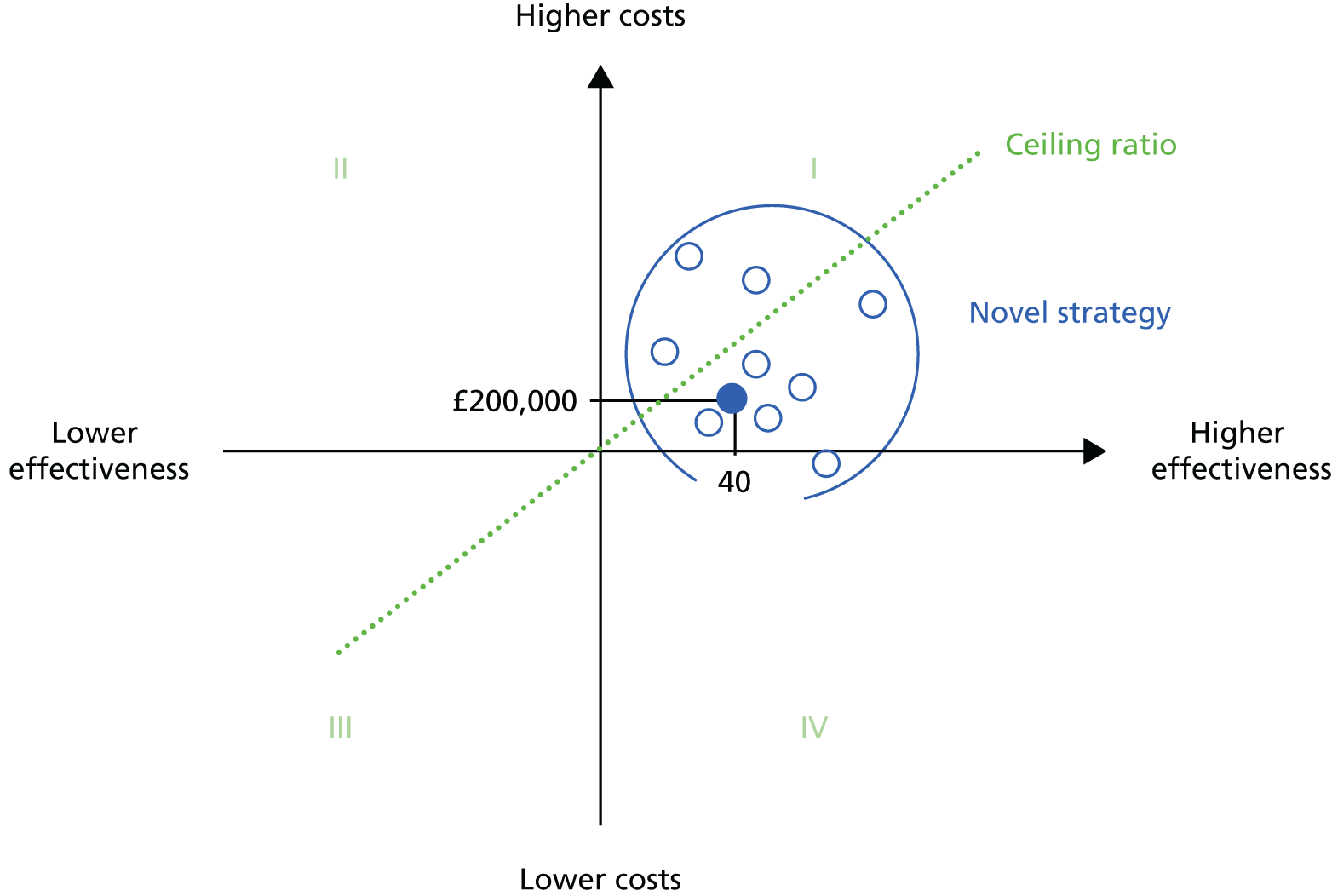
Whether or not a strategy is cost-effective depends on the proportion of the point estimates generated by the Monte Carlo simulation that fall in the cost-effective part of the plane, under the ceiling ratio line. As seven point estimates in the example are below the given ceiling ratio, there is a 70% chance that the novel strategy is cost-effective and, therefore, optimal for decision-makers. However, this approach is only useful when there is one choice compared with a current practice comparator. When multiple options are available to decision-makers, another interpretation of the results is required.
The concept of incremental net monetary benefit (NMB) is appropriate and represents a single-figure summary of the outcome measures of cost and health benefits (QALYs). 134 It is derived by rearranging the formula for calculating ICERs and incorporating the ceiling ratio, λ.
The NMB is calculated by multiplying the ceiling ratio by the average incremental health benefits and, subsequently, deducting average incremental costs:
Employing the NMB simplifies the statistical interpretation of decision model results. The alternative with the highest mean NMB is the best option for decision-makers as it maximises health benefits under conditions of scarce resources. 135 This may not be the outcome with the highest probability of being the best decision, and the cause of the discrepancy is worth discussing. Halton et al. 136 shows exactly why this occurs via an example reproduced in Table 18.
| Baseline | Treatment A | Treatment B | Optimal choice | |
|---|---|---|---|---|
| Simulation 1 | 140 | 150 | 160 | B |
| Simulation 2 | 100 | 110 | 120 | B |
| Simulation 3 | 110 | 100 | 100 | Baseline |
| Simulation 4 | 100 | 150 | 130 | A |
| Simulation 5 | 130 | 120 | 110 | Baseline |
| Average of the incremental NMB | 116 | 126 | 124 | Baseline/A/B = 40%/20%/40% |
These results show that treatment A is optimal only once out of the five simulations from the model parameters, giving a 20% chance it is the best choice and an 80% chance it is the wrong choice. Treatment B or remaining with baseline reveals higher chances of being an optimal decision, yet treatment A has the highest NMB at 126 and so is the best decision if the objective is to maximise health benefits under conditions of scarce resources. The reason for this apparent contradiction is that the distribution of NMBs is skewed. There could be a few large outlier values for treatment A that drag the mean NMB statistic to a higher value; in this case the value of 150 shown twice in Table 18 is fulfilling this. The only way to interpret the information in Table 18 is to say that treatment A is best, but there is great uncertainty in this conclusion with an 80% chance it is incorrect. In this case more research might be required to reduce this uncertainty. 135 If the NMB outcome were normally distributed, then the treatment with the highest probability of being cost-effective would also be the one with the highest NMB. 135 The generalisation of the decision rule to multiple comparators implies we choose the option with the maximum incremental NMB.
Other sources of uncertainty
In addition to parameter uncertainty, other types of uncertainty should also be considered: uncertainty in model structure or assumptions, patient heterogeneity and variability. An approach favoured by NICE to test model assumptions is called scenario analysis and can be performed as part of the PSA. In a scenario analysis, the model assumptions are varied and the PSA run for each change. The different scenarios are compared and the most robust one is selected. 137 Patient heterogeneity is a type of uncertainty that can be explained, for example, by different age or sex of patients. Variability, on the other hand, refers to random differences between patients that cannot be explained, that is, differences occurring by chance. 132 In order to deal with heterogeneity between patient groups, multiple acceptability curves can be employed, for example for different age groups or according to sex. Decision-makers can subsequently identify which infection prevention strategies are cost-effective for certain patient subgroups (e.g. 80-year-old males). A scenario analysis can also be performed for variability, such as different types of hospitals or levels of surgeon experience, but this depends on the information available.
Markov models
The mechanism of Markov models is explained in this section using a very simple example: a model with only two health states and a death state. Model health states are mutually exclusive and death is an absorbing state, because once an individual has entered the state, they must remain there. 138 As Markov models generally start with a cohort of people at risk of a disease, the example starts with a cohort of patients at risk of developing an infection. The model can capture the probability of patients remaining in the initial state (‘no infection’) or moving into one of the other health states (‘infection’ or ‘death’). These transitions occur within a previously defined time period, called a ‘Markov cycle’. 132,138 The length of model cycle depends on the research question but could be hours, days, months or years and can be run for any period of time, up to the full lifetime of a patient or cohort of patients. In each model cycle individuals have a certain probability of moving between health states, shown by the arrows in Figure 19.
FIGURE 19.
Possible transitions between Markov model health states in a given cycle.
In this example it is possible for an individual to remain in their current health state, move to the other one or die in any cycle. The fictional transition probabilities for this example are summarised in a transition matrix, illustrated in Table 19.
| Health states | Transition to | |||
|---|---|---|---|---|
| No infection | Infection | Death | Total | |
| No infection | 0.80 | 0.15 | 0.05 | 1.00 |
| Infection | 0.60 | 0.35 | 0.05 | 1.00 |
| Death | 0.00 | 0.00 | 1.00 | 1.00 |
This table is to be read from left to right; for example, the probability of moving from ‘no infection’ (first column) to ‘infection’ (third column) is 0.15, representing 15% of patients. All transition probabilities sum to 1, as it is not possible to leave the model. As indicated by the ‘0’ in the matrix, transitions from the death state to other health states are not possible. The transition probabilities in this simplified example are constant but they can be time dependent and adjusted or varied for different cycles. Commonly, the probability of dying is adjusted as the mortality rate increases with age.
A Markov trace can be computed using the probabilities in the transition matrix for all model cycles. The Markov trace for the first five cycles of the example model is shown in Table 20. The model simulation starts with a cohort of 1000 patients free of infection. Over the five cycles, patients move between health states in accordance with the transition probabilities specified in Table 19.
| Cycle | No infection | Infection | Death | Total |
|---|---|---|---|---|
| 0 | 1000 | 0 | 0 | 1000 |
| 1 | 800 | 150 | 50 | 1000 |
| 2 | 730 | 173 | 97 | 1000 |
| 3 | 688 | 170 | 142 | 1000 |
| 4 | 652 | 163 | 185 | 1000 |
| 5 | 619 | 155 | 226 | 1000 |
The transitions in cycle 1 are straightforward: 800 patients remain without infection (1000 × 0.8), 150 patients developed an infection (1000 × 0.15) and 50 patients die (1000 × 0.05). Cycle 2 becomes more complex as patients are now moving from both the ‘no infection’ and the ‘infection’ states. The total number of people without infection in this cycle is the sum of patients moving from both health states in the previous cycle: (800 × 0.8) + (150 × 0.6) = 730. Typically, cohort simulations are run until all patients are absorbed by the ‘death’ state.
Model development and validation
Models do not aim to capture all possible health states found in the real world and should be as simple as possible while portraying key elements of a given decision and incorporating the best-existing evidence. 130 In order to meet these requirements, a thorough understanding of events related to the occurrence of infection following primary THA was necessary.
The iterative process of developing the model structure required reviewing the literature and consulting with orthopaedic surgeons and infection prevention experts. Some basic questions helped identify the fundamental elements of the model.
-
What happens to a patient with an infection?
-
Does the clinical management depend only on the type and onset of infection or also on other factors?
-
Which pathways are important to the decision-making process?
-
Can some pathways be left out because of insignificance of cost and quality-of-life outcomes?
The decision model is designed to predict cost and health outcomes of competing infection prevention strategies from a health services perspective. A Markov model is used to show how a hypothetical cohort of patients moves between different health states over time. The model simulation ends when all patients in the cohort are in a ‘dead’ state in order to compare long-term health and cost outcomes. It was not feasible to include all related outcomes from a societal perspective because of financial and time constraints. The model drafts were shown to orthopaedic surgeons and orthopaedic infection experts to validate the model structure. Comments and feedback were taken into account, the literature was reviewed once more regarding some key questions and the model was refined accordingly.
Model description and comparisons
The model takes the health services perspective. The final model structure is outlined in Figure 20 and was based on the following assumptions.
-
No infection is the primary state for the model.
-
Deep SSIs occur within 12 months following a THA.
-
Patients remain in the ‘deep infection’ state until they receive treatment.
-
Treatment options for patients with deep infection are usually debridement, antibiotics and implant retention (DAIR), one-stage revision or two-stage revision.
-
A last resort option is ‘permanent resection’ which patients will remain in until death.
-
Patients either experience successful treatment outcomes or treatment failure.
-
Patients move into the ‘successful treatment’ state after treatment for infection.
-
If treatment was unsuccessful, patients can undergo further treatment (i.e. move directly into treatment health states DAIR, one-stage revision, two-stage revision or permanent resection).
FIGURE 20.
Structure of an economic decision model: a Markov model.

The model begins with a cohort of infection-free patients who underwent THA. In each model cycle, patients can move between health states. Patients remain in the ‘no infection’ state, die or develop an infection. During the following cycles, all patients diagnosed with a deep infection within 12 months move to the health state ‘deep infection’. Patients stay in this health state until they receive treatment or die. The standard treatment options are DAIR, one-stage revision or two-stage revision. A less common treatment option is permanent resection of the prosthesis, used for severe cases. After the initial treatment for deep infection, patients are assumed to be infection free and move to the ‘successful treatment’ state. Patients remain in this health state unless further treatment is required because of recurring or persisting symptoms of infection. In this case, patients can undergo any of the four treatment options multiple times until the treatment is successful or until they are absorbed by death or permanent removal of the prosthesis. The model we have developed will be used to estimate the costs and health outcomes, measured by QALYs, arising from nine competing approaches to managing risk of SSI among THR.
Chapter 5 Data linkage
Five national databases were linked together in order to inform the decision model that will address the aims of this research.
NHS Hospital Episode Statistics
The NHS Hospital Episode Statistics (HES) is a data warehouse containing records of all patients admitted to NHS hospitals in England. It includes private patients treated in NHS hospitals, patients who were resident outside England and care delivered by treatment centres, including those in the independent sector, funded by the NHS. It processes over 125 million admitted patient, outpatient and accident and emergency records each year. The HES data are designed to enable secondary use (i.e. for non-clinical purposes). Each HES record contains a wide range of information about an individual patient admitted to a NHS hospital, including clinical information about diagnoses and operations; information about the patient, such as age group, sex and ethnicity; administrative information, such as waiting times, and dates and methods of admission and discharge; and geographical information, such as where patients were treated and the area in which they live.
A data extract was obtained from the Health and Social Care Information Centre (HSCIC) for a cohort of patients, with specific Office of Population, Censuses and Surveys, Classification of Surgical Operations and Procedures (OPCS) codes relating to THR for the financial years 2008–12. The HES records for the episode of operation were extracted and subsequently all other records of hospital episodes relating to this same cohort of patients were extracted within the given time period, including critical care data (see Appendix 5). These included patient identifiers such as NHS number, date of birth and provider number, as well as HSCIC identifiers ‘encryptedhesid’, ‘epikey’ and ‘susrecid’. The NHS number, date of birth and HSCIC identifiers were used to link the HES admitted patient care (APC) data to other data sets, including:
-
critical care
-
patient-reported outcome measures (PROMs)
-
the Office for National Statistics (ONS) death registrations
-
NJR data from the Healthcare Quality Improvement Partnership
-
PHE SSISS data.
Office for National Statistics
The ONS is the UK’s largest independent producer of official statistics and is the recognised national statistical institute for the UK. It is responsible for collecting and publishing statistics related to the economy, population and society at national, regional and local levels. It also conducts the census in England and Wales every 10 years. The ONS plays a leading role in national and international good practice in the production of official statistics. An extract of ONS mortality data for the cohort of hip replacement patients described above was obtained from the HSCIC. The extract contained the variables listed in Appendix 5 and included the identifier ‘encryptedhesid’ to link these records to HES APC.
Patient-reported outcome measures
Patient-reported outcome measures data sets hold information on the effectiveness of care delivered to NHS patients, as perceived by the patients themselves. Patients rate their health by scoring the severity or difficulty in completing certain tasks or routine activities. The tools used are the EQ-5D index and the EQ-5D visual analogue scale. The Oxford hip score is also collected for those undergoing THR. PROMs data collection is co-ordinated by NHS England, but delivered by organisations such as hospital trusts that perform relevant procedures and the HSCIC. An extract of PROMs data was obtained from the HSCIC for the period 2009–12 for the cohort of hip replacement patients obtained from HES records. The PROMs extract included the variables listed in Appendix 5, in addition to the identifier ‘epikey’ which was used to link these records to HES APC.
Surgical Site Infection Surveillance Service
The SSISS holds data on SSIs reported by NHS hospitals and independent sector NHS treatment centres. All hospitals participating in national SSI surveillance are required to follow the surveillance protocol outlining the follow-up methods and case definitions. Each hospital collects data prospectively on all eligible patients in a surgical category over a 3-month period. The follow-up period is set at 30 days for non-implant procedures and 1 year for implant procedures.
Baseline risks for SSI and rates of revision, by patient characteristics, are available from the surveillance data. Acute care hospitals in England that participated in mandatory SSI surveillance from 1 April 2004 to 31 March 2014 have contributed data for patients who underwent hip replacement. An extract of hip replacement data was downloaded from the PHE SSISS database for the period 2008–12. The SSISS extract contained the variables listed in Appendix 5 and included the identifiers ‘patient name’, ‘NHS number’ and ‘date of birth’ to link these records to HES APC. When NHS numbers were unavailable, these data were obtained from the Demographic Batch Service.
National Joint Registry for England, Wales and Northern Ireland
The purpose of the NJR data collection is to provide an early warning system for patient safety issues. Furthermore, in an effort to continue to improve quality and cost-effectiveness of care, the high-quality data collected about joint replacement surgery in the UK are used to report on, and monitor, patient outcomes and to support research. NJR data from 2008 to 2012 were provided by the Healthcare Quality Improvement Partnership and included the variables listed in Appendix 5. The patient identifiers, NHS number and date of birth were used to link these records to HES APC.
Data linkage and pseudonymisation
The data sets were linked to the HES APC data set using the patient identifiers and HSCIC identifiers described above for each data set. Prior to analysis the data were anonymised to reduce the chance of disclosure by transforming NHS number and HSCIC identifiers to new identification numbers that bore no relationship to the originals. Data, such as date of birth, were ‘blurred’ by calculating age in whole years, rounded down, or time intervals between two incidents in units larger than days, generating a new code for providers/institutions to distinguish between but not identify institutions. All patient identifiers were then removed from the linked data set. Access to the data was restricted to a finite number of named individuals. The data linkages are shown in Figure 21.
FIGURE 21.
Data linkage of national data sets.
There are large numbers of data in HES and ONS data sets that have little value for this project, as they do not relate to THR. The identification of a cohort of patients by the HSCIC, using OPCS codes for THR, has reduced this to a minimum.
In Figure 21, the dark-green section (A) is where we focus our efforts, as these patients can potentially be linked across HES, PROMs, NJR, SSISS and ONS mortality data sets. The HES APC data included 316,208 distinct NHS numbers. The mid-green section (B) includes a subset of 276,858 patients of interest from HES who are not linked to PROMs data, as PROMs data were not available prior to 2009; however, they can potentially be linked to the NJR and SSISS. Whether or not they can be linked to ONS data will depend on their mortality status. The light-green section (C) is a subset of 3117 NJR and 57,017 SSISS patients who were unable to be linked to HES inpatient data. The dark-blue section (D) represents a hypothetical subset of patients whose data are present in PROMs and link to NJR and SSISS databases, but who cannot be linked to HES.
Only about 65% of patients in SSISS had a NHS number. This increased to 80% after tracing by the Demographics Batch Service. The light-blue section (E) represents patients with SSISS data for whom a missing NHS number could not be traced and, therefore, whose record could not be linked to other data sets. The NHS number could not be traced for 36,096 patients (19.23% of 187,673) patients in the SSISS data extract. The final linked data sets may, therefore, be compromised if missing data from patients in sections C, D and E were excluded for non-random reasons (e.g. if these patients were different in some way to the included patients in their demographics, treatment pathways or prognosis).
Chapter 6 Model parameters and results
Transition probabilities
We simulated a cohort of 77,321 patients who had a primary THR in 2012. 11 A rate of deep infection to reflect risks under strategy T1 (no systemic antibiotics, plain cement and conventional ventilation) for a time period of 2.5 years was estimated by Lidwell et al. 111 This study of 8055 operations for replacement of hips (84%) and knees (16%) was undertaken in 19 hospitals in England, Scotland and Sweden between 1974 and 1979. The conditions relevant to T1 were met for 1161 of these procedures and there were 39 cases of deep infection. The rate of infection under T1 conditions for 2.5 years was therefore 3.4%. To adjust this for a 12-month follow-up appropriate to this model, we used information from Ong et al. 139 Ong et al. 139 evaluated the incidence of early-onset (< 2 years) and late-onset (> 2 years) deep joint infection after primary THA for the period 1997–2006. At 1 year post surgery 66.7% of all deep infections had been identified. This information was used to predict risks of infection for 12 months following primary surgery under conditions of T1 for the model.
We accounted for the fact that 32.9% of patients had a cemented hip replacement11 and so the remainder would have an uncemented procedure. The impact was to change the numbers or individuals in the cohort who would have incurred costs and gained health benefits from any strategy that included antibiotic-impregnated cement (T5–9).
The average risks among the cohort of 77,321 patients under conditions of T1 were used to predict 1887 cases of deep infection for 12 months following primary THR (95% uncertainty interval 1253–2621 cases). These patients then progressed through treatment pathways shown in Figure 20, which is a Markov model that simulates patients daily. We used the information described in Chapter 5 to estimate the probabilities of progressing through all model states for 5 years. A major advantage of accessing original data is that treatment probabilities are time dependent. A hypothetical cohort of patients will move through the model proportionally to the real patient data. It is not necessary to assume constant transitions, for example average yearly transitions. Accurate ‘real-world’ transitions between model health states can be used and the model cycle length adjusted to reflect this. Transition probabilities were calculated for each cycle for the occurrence of deep infection, first treatment with DAIR, one-stage revision, the first stage of a two-stage revision followed by the second stage two-stage revision and permanent resection, and further treatment if the initial treatment failed. Each simulation continued until every simulated patient had died. The means of the daily transition probabilities are shown in Table 21.
| State | State | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | 0.9998898 | 0.0000620 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0000482 |
| 2 | 0 | 0.5097793 | 0.2058724 | 0.2058724 | 0.0588207 | 0.0147052 | 0.0049017 | 0 | 0.0000482 |
| 3 | 0 | 0.0000371 | 0.9980240 | 0.0003707 | 0.0000371 | 0 | 0 | 0.0014829 | 0.0000482 |
| 4 | 0 | 0.0003407 | 0.0002271 | 0.9979362 | 0.0001703 | 0.0000852 | 0 | 0.0011923 | 0.0000482 |
| 5 | 0 | 0.0009203 | 0 | 0.0018407 | 0.9902882 | 0.0064424 | 0 | 0.0004602 | 0.0000482 |
| 6 | 0 | 0 | 0.0000872 | 0.0002616 | 0 | 0.9982076 | 0 | 0.0013954 | 0.0000482 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0.9992798 | 0.0006720 | 0.0000482 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.9999518 | 0.0000482 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.0000000 |
All patients started in the ‘primary’ state. Deep infections and their subsequent treatments were identified using the International Classification of Diseases, Tenth Edition (ICD-10) codes listed in Appendix 6, and the presence of a subsequent revision procedure listed in Appendix 7. All deep infections must have occurred within 12 months of the primary THR, in accordance with SSISS follow-up periods. The ICD-10 codes listed in Appendix 6 were used to identify infections leading to any of the treatment strategies. Revision resulting from deep SSI could be performed on one or more components of the prosthesis. ICD-10 organism codes were used to indicate the presence of infection but not the severity of infection, such as deep or superficial (see Appendix 8). The probability of death was taken from UK life table data for the years 2010–12 and was downloaded from the ONS website (see Appendix 9). Each patient’s starting age was randomly selected from the distribution of ages of primary THRs in the hip data. To acknowledge the uncertainty in the transition probabilities we randomly sampled probabilities using a beta distribution for each simulation. Hence our simulations include uncertainty about the parameter estimates as well as stochastic uncertainty. The plot of daily death probability by age is shown in Figure 22.
FIGURE 22.
Plot of daily death probabilities by age.
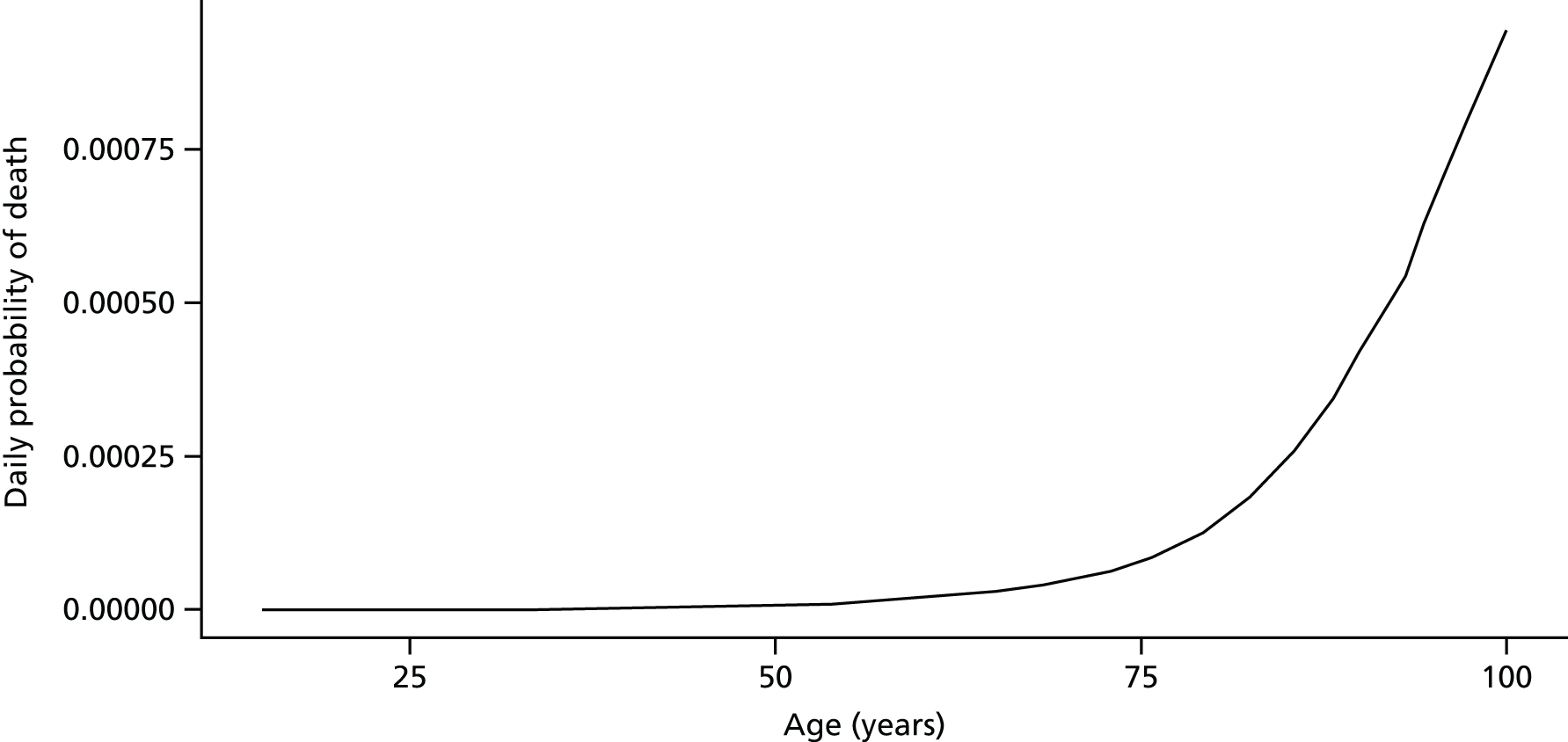
Effectiveness evidence
Estimates of the clinical effectiveness of the competing infection prevention strategies are available from the material included in Chapters 2 and 3. Descriptions of the strategies are provided in Table 22.
| Treatment strategy | Details |
|---|---|
| T1 | No systemic antibiotics, plain cement and conventional ventilation |
| T2 | Systemic antibiotics, plain cement and conventional ventilation |
| T3 | No systemic antibiotics, plain cement and laminar airflow |
| T4 | Systemic antibiotics, plain cement and laminar airflow |
| T5 | No systemic antibiotics, antibiotic-impregnated cement and conventional ventilation |
| T6 | Systemic antibiotics, antibiotic-impregnated cement and conventional ventilation |
| T7 | Systemic antibiotics, antibiotic-impregnated cement and laminar airflow |
| T8 | Systemic antibiotics, antibiotic-impregnated cement, conventional ventilation and body exhaust suit |
| T9 | Systemic antibiotics, antibiotic-impregnated cement, laminar ventilation and body exhaust suit |
Probability ratios are the estimated probabilities of deep infection for strategies T2–9 divided by the probability of deep infection for T1. The simulations were conducted using Queensland University of Technology’s high-performance computer. Probability ratios of deep infection using T1 as the reference strategy are shown in Figure 23. The vertical lines in blue show a probability ratio of 1 (i.e. no change in probability of infection compared with T1). The probability ratio for deep infection for T2 compared with T1 has a mode near to 0.5.
FIGURE 23.
Probability ratios of deep infection using T1 as the reference strategy. (a) T2 vs. T1; (b) T3 vs. T1; (c) T4 vs. T1; (d) T5 vs. T1; (e) T6 vs. T1; (f) T7 vs. T1; (g) T8 vs. T1; and (h) T9 vs. T1.




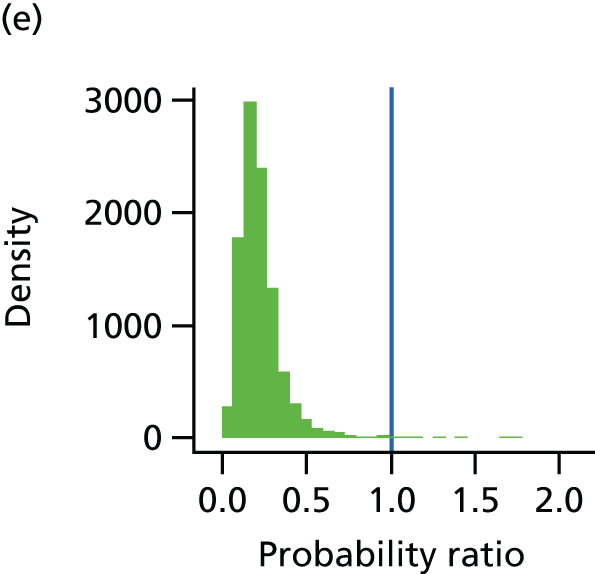
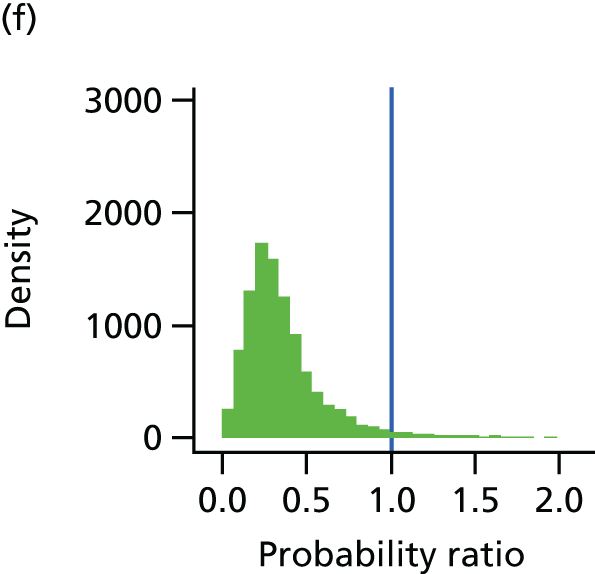

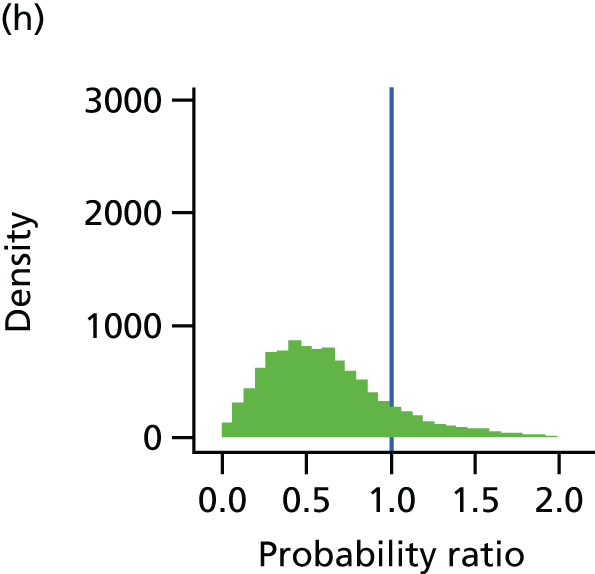
The descriptive statistics for the probability ratios are shown in Table 23.
| Treatment | Probability ratios | |||
|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | |
| T2 | 0.46 | 0.15 | 0.02 | 1.30 |
| T3 | 0.35 | 0.25 | 0.00 | 1.87 |
| T4 | 0.38 | 0.18 | 0.01 | 1.85 |
| T5 | 0.51 | 0.23 | 0.04 | 1.74 |
| T6 | 0.22 | 0.12 | 0.01 | 1.75 |
| T7 | 0.36 | 0.23 | 0.00 | 1.97 |
| T8 | 0.50 | 0.34 | 0.00 | 1.98 |
| T9 | 0.61 | 0.35 | 0.00 | 1.98 |
The probability ratio for deep infection for T2 compared with T1 has a mean of 0.46. Therefore, the average reduction in deep infection for this strategy is around half of the probability of deep infection for T1.
Membership of model states for 5 years after primary procedure
For the starting cohort of 77,321, the number of patients with deep infection for each of the treatments T1–9, and their subsequent occupancy of model states, is shown in Table 24.
| Treatment | Deep infection | One-stage revision | DAIR | Stage 1 of two-stage revision | Stage 2 of two-stage revision | Excision |
|---|---|---|---|---|---|---|
| T1 | 1887 (1253 to 2621) | 925 (587 to 1350) | 1077 (684 to 1548) | 361 (198 to 566) | 342 (18 to 549) | 36 (3 to 105) |
| T2 | 870 (345 to 1655) | 427 (157 to 813) | 497 (194 to 953) | 166 (55 to 337) | 158 (57 to 322) | 17 (1 to 52) |
| T3 | 670 (90 to 1937) | 329 (44 to 1009) | 382 (52 to 1146) | 128 (17 to 386) | 121 (16 to 377) | 13 (1 to 49) |
| T4 | 721 (192 to 1589) | 355 (90 to 769) | 412 (110 to 914) | 138 (33 to 322) | 131 (32 to 308) | 14 (1 to 49) |
| T5 | 950 (286 to 2059) | 466 (142 to 1043) | 544 (161 to 1169) | 182 (51 to 408) | 172 (47 to 388) | 18 (1 to 56) |
| T6 | 406 (90 to 964) | 200 (47 to 473) | 230 (51 to 540) | 77 (16 to 193) | 74 (15 to 177) | 8 (1 to 30) |
| T7 | 666 (101 to 2017) | 327 (48 to 1016) | 380 (56 to 1148) | 127 (16 to 382) | 121 (15 to 371) | 14 (1 to 55) |
| T8 | 905 (77 to 2499) | 446 (35 to 1290) | 516 (42 to 1449) | 173 (13 to 493) | 164 (12 to 494) | 18 (1 to 74) |
| T9 | 1126 (143 to 2827) | 555 (67 to 1405) | 642 (86 to 1668) | 216 (28 to 566) | 204 (25 to 547) | 23 (1 to 80) |
These results are for the first 5 years following primary procedure and show that T6 prevented the most cases and had the fewest treatments.
We used the programing software R version 3.0.3 (The R Foundation for Statistical Computing, Vienna, Austria) for all analyses.
Costing treatments
The cost/price year for all estimates is 2012. The positive incremental costs of implementing each strategy are estimated from unit cost values for each component of the strategy. Unit costs were estimated for antibiotics used for prophylaxis, antibiotic-impregnated cement, laminar airflow systems and the use of exhaust body suits.
Antibiotics used for prophylaxis
Hickson et al. 140 estimated the most common prophylaxis regimes used by NHS trusts for knee and hip replacements. There are three regimes used in the absence of a penicillin allergy: flucloxacillin in combination with gentamicin, cefuroxime alone and teicoplanin in combination with gentamicin. In the presence of penicillin allergy either teicoplanin or teicoplanin in combination with gentamicin is used.
The regime used and the doses given differs in NHS trusts and so for each regime the average dose across trusts was estimated under the assumption that the average patient weighs 75 kg. The expected cost for each regime was based on data from the British National Formulary. 141 As hospitals are able to negotiate discounts with their suppliers, list prices were discounted by 30%. This is in line with other publications142 and variability is accounted for in the PSA. The average costs of regimes in the absence of a penicillin allergy (Tables 25–27) and in the presence of one (Tables 28 and 29) were calculated. We assumed that 10% of patients were treated as allergic to penicillin. 143
| Drug | Dose | Average number of doses | Average dose | List price (£) | Discounted price (£) |
|---|---|---|---|---|---|
| Flucloxacillin | Induction | 1 | 1 g | 4.90 | 3.43 |
| Postoperative | 1.77 | 1 g | 8.68 | 6.08 | |
| Gentamicin | Induction | 0.42 | 160 mg | 1.64 | 1.15 |
| 0.58 | 2 mg/kg | 2.12 | 1.48 | ||
| Postoperative | 0 | 0 | 0 | 0 | |
| Expected cost | 17.34 | 12.14 |
| Drug | Dose | Average number of doses | Average dose | List price (£) | Discounted price (£) |
|---|---|---|---|---|---|
| Cefuroxime | Induction | 1 | 1.5 g | 5.05 | 3.54 |
| Postoperative | 0.20 | 1.5 g | 1.03 | 0.72 | |
| Expected cost | 6.08 | 4.26 |
| Drug | Dose | Average number of doses | Average dose | List price (£) | Discounted price (£) |
|---|---|---|---|---|---|
| Teicoplanin | Induction | 0.8 | 400 mg | 5.86 | 4.10 |
| 0.2 | 10 mg/kg | 2.75 | 1.92 | ||
| Postoperative | 0.32 | 1.15 g | 6.73 | 4.71 | |
| Gentamicin | Induction | 0.32 | 160 mg | 1.25 | 0.87 |
| 0.68 | 225 mg | 3.73 | 2.61 | ||
| Postoperative | 0 | 0 | 0.00 | 0.00 | |
| Expected cost | 20.31 | 14.22 |
| Drug | Dose | Average number of doses | Average dose | List price (£) | Discounted price (£) |
|---|---|---|---|---|---|
| Teicoplanin | Induction | 0.92 | 400 mg | 6.71 | 4.70 |
| 0.08 | 6 mg/kg | 0.69 | 0.48 | ||
| Postoperative | 0.22 | 850 mg | 3.46 | 2.42 | |
| Expected cost | 10.85 | 7.60 |
| Drug | Dose | Average number of doses | Average dose | List price (£) | Discounted price (£) |
|---|---|---|---|---|---|
| Teicoplanin | Induction | 0.93 | 400 mg | 6.84 | 4.79 |
| 0.07 | 10 mg/kg | 0.90 | 0.63 | ||
| Postoperative | 0.26 | 1.15 g | 5.55 | 3.89 | |
| Gentamicin | Induction | 0.36 | 160 mg | 1.41 | 0.99 |
| 1.76 | 2 mg/kg | 6.43 | 4.50 | ||
| Postoperative | 0 | 0 | 0.00 | 0.00 | |
| Expected cost | 21.13 | 14.79 |
This information is used to estimate a weighted average cost for prophylaxis with antibiotics.
Plain and antibiotic-impregnated cement
We assumed that a hip replacement requires 120 g of cement: 40 g for the cup and another 80 g for the femur. The cement used can either be plain or antibiotic impregnated. It comes in 40-g packets and, therefore, we assume that three packets will be used for each THR. There are nine types of plain cement and 12 types of antibiotic-impregnated cement available for use in the NHS. With no data available for the frequency with which each of these is used, it was assumed that each type of cement was used at a similar rate. As with the antibiotic prophylaxis, the list prices for cements were discounted by 30%. The estimated average cost of plain cement and antibiotic-impregnated cement is shown in Tables 30 and 31.
| Cement description | Supplier | Price (£) (three packets) | Discounted price (£) (three packets) |
|---|---|---|---|
| Palacos 1 × 40 g low viscosity (b1) | Heraeus Medical GmBH (Wehrheim, Germany) | 113.10 | 79.17 |
| Palacos R plain 1 × 40 g high viscosity (b1) | Heraeus Medical GmBH | 50.13 | 35.09 |
| Palacos MV medium viscosity plain 1 × 40 g (b1) | Heraeus Medical GmBH | 50.13 | 35.09 |
| HV bone cement, 40 g | DePuy Synthes UK (Leeds, UK) | 70.14 | 49.10 |
| MV bone cement, 40 g | DePuy Synthes UK | 70.14 | 49.10 |
| CMW 1 bone cement, 40 g | DePuy Synthes UK | 122.48 | 85.73 |
| CMW 2 bone cement, 40 g fast set | DePuy Synthes UK | 122.48 | 85.73 |
| HI FATIGUE high viscosity cement, 1 × 40 g | Zimmer Ltd (Warsaw, IN, USA) | 182.97 | 128.08 |
| SIMPLEX P radio opaque, 40-g pack full dose | Stryker Orthopaedics (Kalamazoo, MI, USA) | 95.98 | 67.18 |
| Mean | 97.50 | 68.25 |
| Cement description | Supplier | Price (£) (three packets) | Discounted price (£) (three packets) |
|---|---|---|---|
| Palacos R + gentamicin 1 × 40 g high viscosity (b1) | Heraeus Medical GmBH | 116.7 | 81.69 |
| Palacos + gentamicin 1 × 40 g low viscosity (b1) | Heraeus Medical GmBH | 150.54 | 105.38 |
| Palacos MV + gentamicin 1 × 40 g medium viscosity (b1) | Heraeus Medical GmBH | 116.67 | 81.67 |
| GHV gentamicin bone cement, 40 g | DePuy Synthes UK | 95.20 | 66.64 |
| GMV gentamicin bone cement, 40 g | DePuy Synthes UK | 93.53 | 65.47 |
| CMW 1 gentamicin bone cement, 40 g | DePuy Synthes UK | 194.84 | 136.39 |
| CMW 2 gentamicin bone cement, 40 g fast set | DePuy Synthes UK | 194.84 | 136.39 |
| REFOBACIN bone cement r, 2 × 20 g | Biomet Merck Ltd (Warsaw, IN, USA) | 126.18 | 88.33 |
| HI-FATIGUE bone cement g, 1 × 40 g | Zimmer Limited | 195.6 | 136.92 |
| SIMPLEX AB cement with tobramycin 40-g pack full dose | Stryker Orthopaedics | 177.26 | 124.08 |
| CEMEX high viscosity with gentamicin, 1 × 40 g | Ortho Dynamics (Paterson, NJ, USA) | 85.35 | 59.75 |
| CEMEX medium viscosity green cement gentamicin, 1 × 40 g | Ortho Dynamics | 85.35 | 59.75 |
| Mean | 136.00 | 95.20 |
Conventional ventilation, laminar airflow and body exhaust suit costs
The only information found regarding the costs of different ventilation systems was published by Evans,8 who acknowledged laminar airflow was historically a high-cost technology. US data144 showed that, in 1974, the costs of installation were US$50,000, which is equivalent to US$232,853 in 2012 prices.
The technology has matured, and Evans8 estimates that the costs of construction and installation of an exponential laminar airflow system into a new operating room for 2011/12 range from US$60,000 to US$90,000. A lifetime of 5 years was assumed for the installation. Based on an exchange rate of US$1 to £0.66 (source OANDA;145 New York, NY, USA), this estimates annual capital costs between £7920 and £11,880. Using a typical caseload of five surgeries per day for a 5-day week, 50 weeks per year, the mean cost per case is between £6.33 and £9.50.
The costs of body exhaust suits were based on US prices as no UK data could be found, and prices were converted to Pounds Sterling as above. The Stryker Steri-Shield system (Stryker, Kalamazoo, MI, USA) was used as the case study146 and costs were included for the helmet without lights (US$3515), disposable hoods (US$86 for a pack of 32), power pack and cord (US$750), and charger (US$7000). Based on the same volume assumptions, the cost per surgical patient would be £7.72 (Table 32).
| Costs illustrated | Cost (US$) | Cost (£) |
|---|---|---|
| Helmet | 3515 | 2320 |
| Power pack | 750 | 495 |
| Charger | 7000 | 4620 |
| Total fixed cost | 11,265 | 7435 |
| Divided by 1250 cases, volume assumption | 9.01 | 5.95 |
| Variable cost per hood | 2.69 | 1.78 |
| Cost per patient | 11.70 | 7.72 |
Incremental per patient cost of infection prevention strategies
The components of and total costs for each infection prevention strategy are shown in Tables 33–41. It was assumed that plain cement and conventional ventilation would have been used regardless, so these items attract zero cost.
| Costs illustrated | Expected cost (£) |
|---|---|
| Plain cement | 0 |
| Conventional ventilation | 0 |
| Total | 0 |
| Costs illustrated | Expected cost (£) |
|---|---|
| Systemic antibiotics | 10.09 |
| Plain cement | 0.00 |
| Conventional ventilation | 0.00 |
| Total | 10.09 |
| Costs illustrated | Expected cost (£) |
|---|---|
| Plain cement | 0.00 |
| Laminar airflow | 7.90 |
| Total | 7.90 |
| Costs illustrated | Expected cost (£) |
|---|---|
| Systemic antibiotics | 10.09 |
| Plain cement | 0.00 |
| Laminar airflow | 7.90 |
| Total | 17.99 |
| Costs illustrated | Expected cost (£) |
|---|---|
| Antibiotic-impregnated cement | 95.20 |
| Conventional ventilation | 0.00 |
| Total | 95.20 |
| Costs illustrated | Expected cost (£) |
|---|---|
| Systemic antibiotics | 10.09 |
| Antibiotic-impregnated cement | 95.20 |
| Conventional ventilation | 0.00 |
| Total | 105.29 |
| Costs illustrated | Expected cost (£) |
|---|---|
| Systemic antibiotics | 10.09 |
| Antibiotic-impregnated cement | 95.20 |
| Laminar airflow | 7.90 |
| Total | 113.19 |
| Costs illustrated | Expected cost (£) |
|---|---|
| Systemic antibiotics | 10.09 |
| Antibiotic-impregnated cement | 95.20 |
| Conventional ventilation | 0.00 |
| Body exhaust suit | 7.72 |
| Total | 113.01 |
| Costs illustrated | Expected cost (£) |
|---|---|
| Systemic antibiotics | 10.09 |
| Antibiotic-impregnated cement | 95.20 |
| Laminar airflow | 7.90 |
| Body exhaust suit | 7.72 |
| Total | 120.91 |
Cost outcomes for each model state
The costs of a patient spending one cycle in each model state is estimated from the linked data set assembled for this project. Data for patients who went on to develop an infection following a THR were analysed with information relating to their THR and subsequent treatments for the infection, informing cost estimates.
Each patient in the data set has OPCS – version 4 (OPCS-4) codes assigned to each of the procedures they received. OPCS refers to the former Office of Population, Censuses and Surveys, originally responsible for the classification system, which has since been merged into what is now the ONS. The OPCS-4 classification system is designed to translate operations and surgical procedures into codes for statistical and epidemiological analysis. 147
The NHS mandatory standard for data collection is the OPCS-4 classification system. It is the required classification system for Admitted Patient Care Commissioning Data Sets and other national data sets required for secondary use.
Case-mix classifications are used to categorise patients based on their expected health-care resource use, primarily for the purposes of reimbursing providers. The NHS in England primarily uses Healthcare Resource Groups (HRGs). 148 HRGs are derived from OPCS-4 and ICD-10 codes in patient records and represent clinical groupings of patient activity.
Each patient’s OPCS-4 codes were assigned to a HRG using the HRG4+ Code to Group spreadsheet, which is available online. 147–149 It was possible to assign a HRG to each OPCS-4 code; however, each HRG has different levels based on diagnoses scores or complication and comorbidity information. In the absence of information that would allow us to specify each patient’s HRG level, it was assumed that patients had intermediate diagnoses and complication/comorbidity.
The HRG costs were taken from the NHS Reference Costs 2012 to 2013. 150 HRGs associated with trauma and orthopaedics for elective inpatients were used. Each model state was associated with an expected cost calculated as the weighted average of the cost of the treatments received (Tables 42–53).
| Description of treatment | OPCS-4 code | HRG-4 code | Percentage of patients |
|---|---|---|---|
| Primary total prosthetic replacement of hip joint using cement | W371 | HB12 | 37 |
| Primary total prosthetic replacement of hip joint not using cement | W381 | HB12 | 45 |
| Unspecified total prosthetic replacement of hip joint not using cement | W389 | HB12 | 1 |
| Primary total prosthetic replacement of hip joint NEC | W391 | HB12 | 7 |
| Primary hybrid prosthetic replacement of hip joint using cemented acetabular component | W931 | HB12 | 1 |
| Primary hybrid prosthetic replacement of hip joint using cemented femoral component | W941 | HB11 | 8 |
| Primary hybrid prosthetic replacement of hip joint using cement NEC | W951 | HB11 | 1 |
| Total | 100 |
| HRG-4 code | Description | National average unit cost (£) | Lower quartile unit cost (£) | Upper quartile unit cost (£) |
|---|---|---|---|---|
| HB11A | Major hip procedures for non-trauma, category 2, with major CC | 10,146.59 | 7847.75 | 11,420.82 |
| HB11B | Major hip procedures for non-trauma, category 2, with intermediate CC | 6450.12 | 5674.46 | 7024.51 |
| HB11C | Major hip procedures for non-trauma, category 2, without CC | 6067.55 | 5465.07 | 6457.65 |
| HB12A | Major hip procedures for non-trauma, category 1, with major CC | 8201.72 | 6742.72 | 9259.28 |
| HB12B | Major hip procedures for non-trauma, category 1, with intermediate CC | 6459.47 | 5577.83 | 7108.78 |
| HB12C | Major hip procedures for non-trauma, category 1, without CC | 5880.43 | 5117.28 | 6428.14 |
| Description | OPCS-4 code | HRG-4 code | Percentage of patients |
|---|---|---|---|
| Revision of total prosthetic replacement of hip joint using cement | W373 | HR07 | 11 |
| Revision of one component of total prosthetic replacement of hip joint using cement | W374 | HR07 | 3 |
| Revision of total prosthetic replacement of hip joint not using cement | W383 | HR07 | 10 |
| Revision of one component of total prosthetic replacement of hip joint not using cement | W384 | HR07 | 2 |
| Revision of total prosthetic replacement of hip joint NEC | W393 | HR07 | 2 |
| Revision of one component of total prosthetic replacement of hip joint NEC | W395 | HR07 | 9 |
| Revision of hybrid prosthetic replacement of hip joint using cemented acetabular component | W933 | HR07 | 1 |
| Revision of hybrid prosthetic replacement of hip joint using cemented femoral component | W943 | HR07 | 3 |
| Renewal of prosthesis in organ NOC | Y032 | UZ05a | 6 |
| Removal of prosthesis from organ NOC | Y037 | UZ05a | 1 |
| Attention to total prosthetic replacement of hip joint NEC | W394 | HB12 | 5 |
| Total | 53 |
| HRG-4 code | Description | National average unit cost (£) | Lower quartile unit cost (£) | Upper quartile unit cost (£) |
|---|---|---|---|---|
| HR07A | Orthopaedic reconstruction with intervention score of ≤ 43, with diagnosis score of ≤ 22 | 6212.21 | 4989.24 | 7501.67 |
| HR07B | Orthopaedic reconstruction with intervention score of ≤ 43, with diagnosis score of 23–60 | 7539.30 | 5878.98 | 9149.87 |
| HR07C | Orthopaedic reconstruction with intervention score of ≤ 43, with diagnosis score of 61 or more | 8911.37 | 6933.32 | 11,123.38 |
| HB12A | Major hip procedures for non-trauma, category 1, with major CC | 8201.72 | 6742.72 | 9259.28 |
| HB12B | Major hip procedures for non-trauma, category 1, with intermediate CC | 6459.47 | 5577.83 | 7108.78 |
| HB12C | Major hip procedures for non-trauma, category 1, without CC | 5880.43 | 5117.28 | 6428.14 |
| HB99Z | Other procedures for non-trauma | 3532.34 | 2879.31 | 4100.53 |
| Description | OPCS-4 code | HRG-4 code | Percentage of patients |
|---|---|---|---|
| Attention to total prosthetic replacement of hip joint NEC | W394 | HB12 | 2 |
| Open debridement and irrigation of joint | W801 | HB99 | 49 |
| Open debridement of joint NEC | W802 | HB99 | 2 |
| Other specified debridement and irrigation of joint | W808 | HB99 | 1 |
| Unspecified debridement and irrigation of joint | W809 | HB99 | 2 |
| Debridement of skin NEC | S571 | JC42 | 10 |
| Open irrigation of joint NEC | W803 | HB99 | 9 |
| Aspiration of joint | W901 | HB99 | 6 |
| Total | 81 |
| HRG-4 code | Description | National average unit cost (£) | Lower quartile unit cost (£) | Upper quartile unit cost (£) |
|---|---|---|---|---|
| HB12A | Major hip procedures for non-trauma, category 1, with major CC | 8201.72 | 6742.72 | 9259.28 |
| HB12B | Major hip procedures for non-trauma, category 1, with intermediate CC | 6459.47 | 5577.83 | 7108.78 |
| HB12C | Major hip procedures for non-trauma, category 1, without CC | 5880.43 | 5117.28 | 6428.14 |
| HB99Z | Other procedures for non-trauma | 3532.34 | 2879.31 | 4100.53 |
| JC42A | Intermediate skin procedures, aged ≥ 13 years | 1539.88 | 1077.03 | 1774.94 |
| Description | OPCS-4 code | HRG-4 code | Percentage of patients |
|---|---|---|---|
| Revision of total prosthetic replacement of hip joint using cement | W373 | HR07 | 4 |
| Revision of one component of total prosthetic replacement of hip joint using cement | W374 | HR07 | 3 |
| Revision of total prosthetic replacement of hip joint not using cement | W383 | HR07 | 2 |
| Revision of one component of total prosthetic replacement of hip joint not using cement | W384 | HR07 | 1 |
| Revision of one component of total prosthetic replacement of hip joint NEC | W395 | HR07 | 1 |
| Primary excision arthroplasty of joint NEC | W572 | HB99 | 1 |
| Removal of prosthesis from organ NOC | Y037 | UZ05a | 9 |
| Attention to total prosthetic replacement of hip joint NEC | W394 | HB12 | 1 |
| Total | 22 |
| HRG-4 code | Description | National average unit cost (£) | Lower quartile unit cost (£) | Upper quartile unit cost (£) |
|---|---|---|---|---|
| HR07A | Orthopaedic reconstruction with intervention score of ≤ 43, with diagnosis score of ≤ 22 | 6212.21 | 4989.24 | 7501.67 |
| HR07B | Orthopaedic reconstruction with intervention score of ≤ 43, with diagnosis score of 23–60 | 7539.30 | 5878.98 | 9149.87 |
| HR07C | Orthopaedic reconstruction with intervention score of ≤ 43, with diagnosis score of ≥ 61 | 8911.37 | 6933.32 | 11,123.38 |
| HB12A | Major hip procedures for non-trauma, category 1, with major CC | 8201.72 | 6742.72 | 9259.28 |
| HB12B | Major hip procedures for non-trauma, category 1, with intermediate CC | 6459.47 | 5577.83 | 7108.78 |
| HB12C | Major hip procedures for non-trauma, category 1, without CC | 5880.43 | 5117.28 | 6428.14 |
| HB99Z | Other procedures for non-trauma | 3532.34 | 2879.31 | 4100.53 |
| Description | OPCS-4 code | HRG-4 code | Percentage of patients |
|---|---|---|---|
| Revision of total prosthetic replacement of hip joint using cement | W373 | HR07 | 4 |
| Revision of total prosthetic replacement of hip joint not using cement | W383 | HR07 | 9 |
| Revision of one component of total prosthetic replacement of hip joint not using cement | W384 | HR07 | 2 |
| Revision of total prosthetic replacement of hip joint NEC | W393 | HR07 | 3 |
| Revision of one component of total prosthetic replacement of hip joint NEC | W395 | HR07 | 1 |
| Renewal of prosthesis in organ NOC | Y032 | UZ05a | 1 |
| Removal of prosthesis from organ NOC | Y037 | UZ05a | 1 |
| Total | 21 |
| HRG-4 code | Description | National average unit cost (£) | Lower quartile unit cost (£) | Upper quartile unit cost (£) |
|---|---|---|---|---|
| HR07A | Orthopaedic reconstruction with intervention score of ≤ 43, with diagnosis score of ≤ 22 | 6212.21 | 4989.24 | 7501.67 |
| HR07B | Orthopaedic reconstruction with intervention score of ≤ 43, with diagnosis score of 23–60 | 7539.30 | 5878.98 | 9149.87 |
| HR07C | Orthopaedic reconstruction with intervention score of ≤ 43, with diagnosis score of ≥ 61 | 8911.37 | 6933.32 | 11,123.38 |
| HB12A | Major hip procedures for non-trauma, category 1, with major CC | 8201.72 | 6742.72 | 9259.28 |
| HB12B | Major hip procedures for non-trauma, category 1, with intermediate CC | 6459.47 | 5577.83 | 7108.78 |
| HB12C | Major hip procedures for non-trauma, category 1, without CC | 5880.43 | 5117.28 | 6428.14 |
| HB99Z | Other procedures for non-trauma | 3532.34 | 2879.31 | 4100.53 |
| Description | OPCS-4 code | HRG-4 code | Percentage of patients |
|---|---|---|---|
| Primary excision arthroplasty of joint NEC | W572 | HB99 | 100 |
| HRG-4 code | Description | National average unit cost (£) | Lower quartile unit cost (£) | Upper quartile unit cost (£) |
|---|---|---|---|---|
| HB99Z | Other procedures for non-trauma | 3532.34 | 2879.31 | 4100.53 |
Health benefits: attaching preference-based utilities to each health state
Despite having EQ-5D data from the PROMs data set, none of the scores was collected close enough in time to the health events included in the model. We sought an EQ-5D score within 14 days of the date of infection, or within 3 months of DAIR or one- or two-stage revision. In the absence of these primary data we used estimates from published sources, and these are shown in Table 54.
| Parameter description | Estimate (variance) (SD) | Distribution | Source | Instrument/method |
|---|---|---|---|---|
| No infection ≤ 12 months post THA | 0.86 (0.0117) | Beta | Räsänen et al.151 | 15D HRQoL |
| Deep infection | 0.40 (0.0514) | Beta | Cahill et al.152 | AQoL |
| Revision (DAIR, one-stage revision, two-stage revision) | 0.81 (0.0176) | Beta | Räsänen et al.151 | 15D HRQoL |
| Permanent/temporary resection | 0.6 (95% CI 0.5 to 0.75) | Uniform | Fisman et al.28 | Expert opinion |
| Successful treatment | 0.82 (0.0198) | Beta | Räsänen et al.151 | 15D HRQoL |
These estimates emerged from a reproducible review that was not published, but formed part of a doctor of philosophy thesis (see Merollini et al. 57). The relevant sections have been reproduced in Appendix 10, and the entire thesis is available online.
Cost-effectiveness evaluation
Simulations were made from all prior distributions specified in the model to make 1000 estimates of the change to total cost and QALYs, arising from a decision to adopt each of the treatments among the starting cohort of 77,321 patients. The most effective strategy is T6 and the least effective is T9, shown by the expected change to the number of cases of deep infection (Table 55).
| Treatment | Number of deep infections | Mean reduction in cases |
|---|---|---|
| T1 | 1887 | Not applicable |
| T2 | 869 | 1018 |
| T3 | 670 | 1217 |
| T4 | 722 | 1165 |
| T5 | 949 | 938 |
| T6 | 406 | 1481 |
| T7 | 668 | 1219 |
| T8 | 906 | 981 |
| T9 | 1126 | 761 |
The impacts on cost and QALY outcomes of choosing another treatment compared with T1, with all parameter uncertainties included, are shown in Figures 24 and 25, respectively.
FIGURE 24.
Change to total costs from a decision to adopt T2–9 compared with T1.
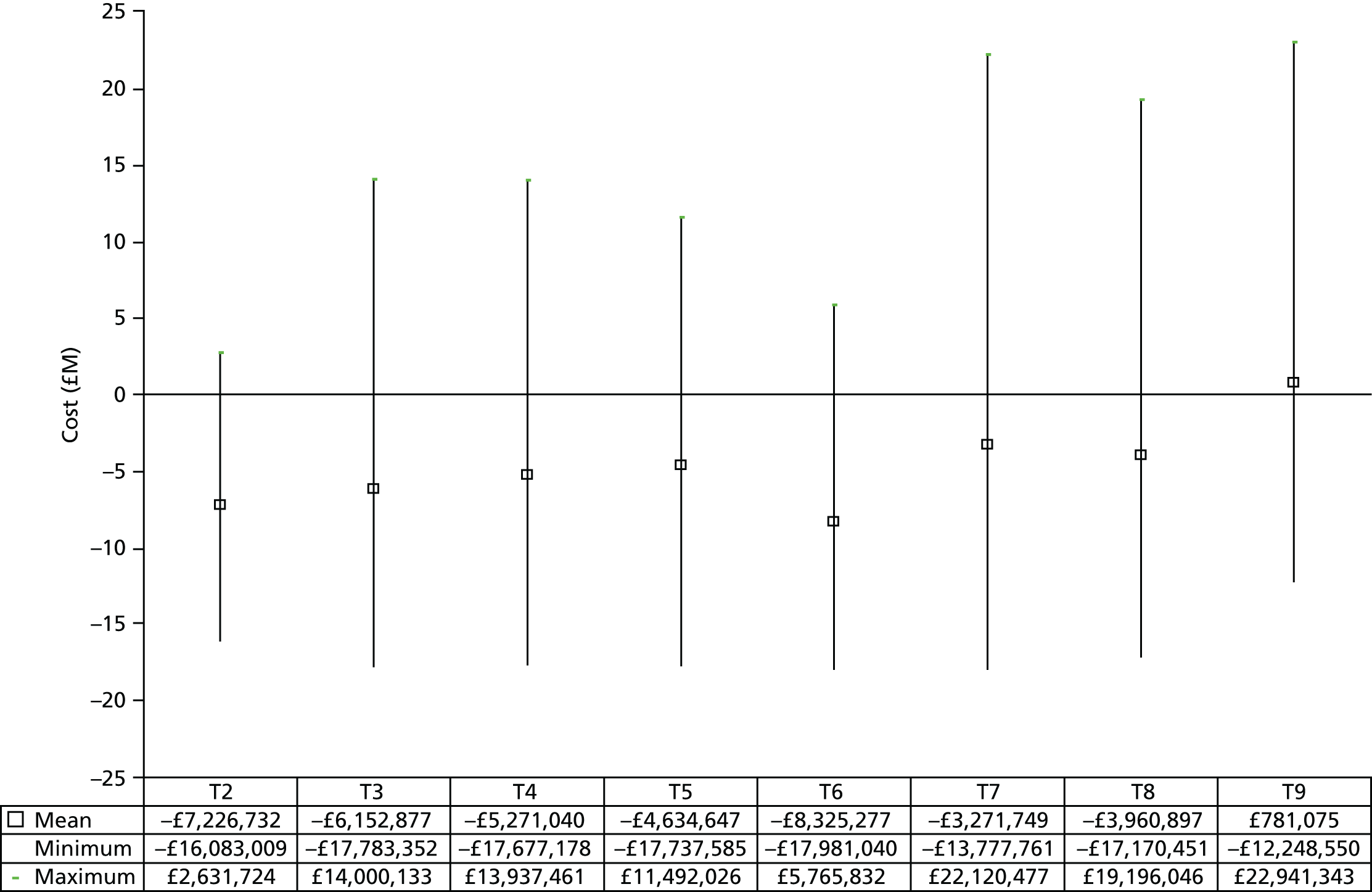
FIGURE 25.
Change to total QALYs from a decision to adopt T2–9 compared with T1.

Costs are lower than T1 for every other treatment except T9. Costs among patients in the cohort are reduced most for T6, with estimated savings of £8,325,749; however, T9 increases actual costs by £781,075. The uncertainty in these estimates is large, shown by the error bars in Figure 24. For all treatments the error bars rise above zero change in cost, indicating a chance that costs will increase as a result of a decision to adopt a treatment strategy different from T1. The probability that any of the treatment strategies will be cost saving is high (Table 56).
| Strategy | Probability (%) |
|---|---|
| T2 | 96 |
| T3 | 94 |
| T4 | 94 |
| T5 | 96 |
| T6 | 92 |
| T7 | 83 |
| T8 | 64 |
| T9 | 47 |
The change to total QALYs when other treatments are compared with T1 is shown in Figure 25.
This shows that QALYs for the cohort are likely to increase, compared with T1, regardless of the treatment strategy chosen. The mean change to QALYs is greatest for T6, at 147 QALYS gained, and least for T9 at 62 QALYs gained. There is large uncertainty in these estimates and the probability that treatment strategies will increase health benefits is moderate (Table 57).
| Strategy | Probability (%) |
|---|---|
| T2 | 65 |
| T3 | 67 |
| T4 | 67 |
| T5 | 62 |
| T6 | 70 |
| T7 | 68 |
| T8 | 66 |
| T9 | 57 |
The mean of the joint distributions of the change to costs and QALYs for each strategy, compared with T1, are shown in Figure 26.
FIGURE 26.
The mean of the joint distributions of the change to costs and QALYs for each strategy compared with T1.
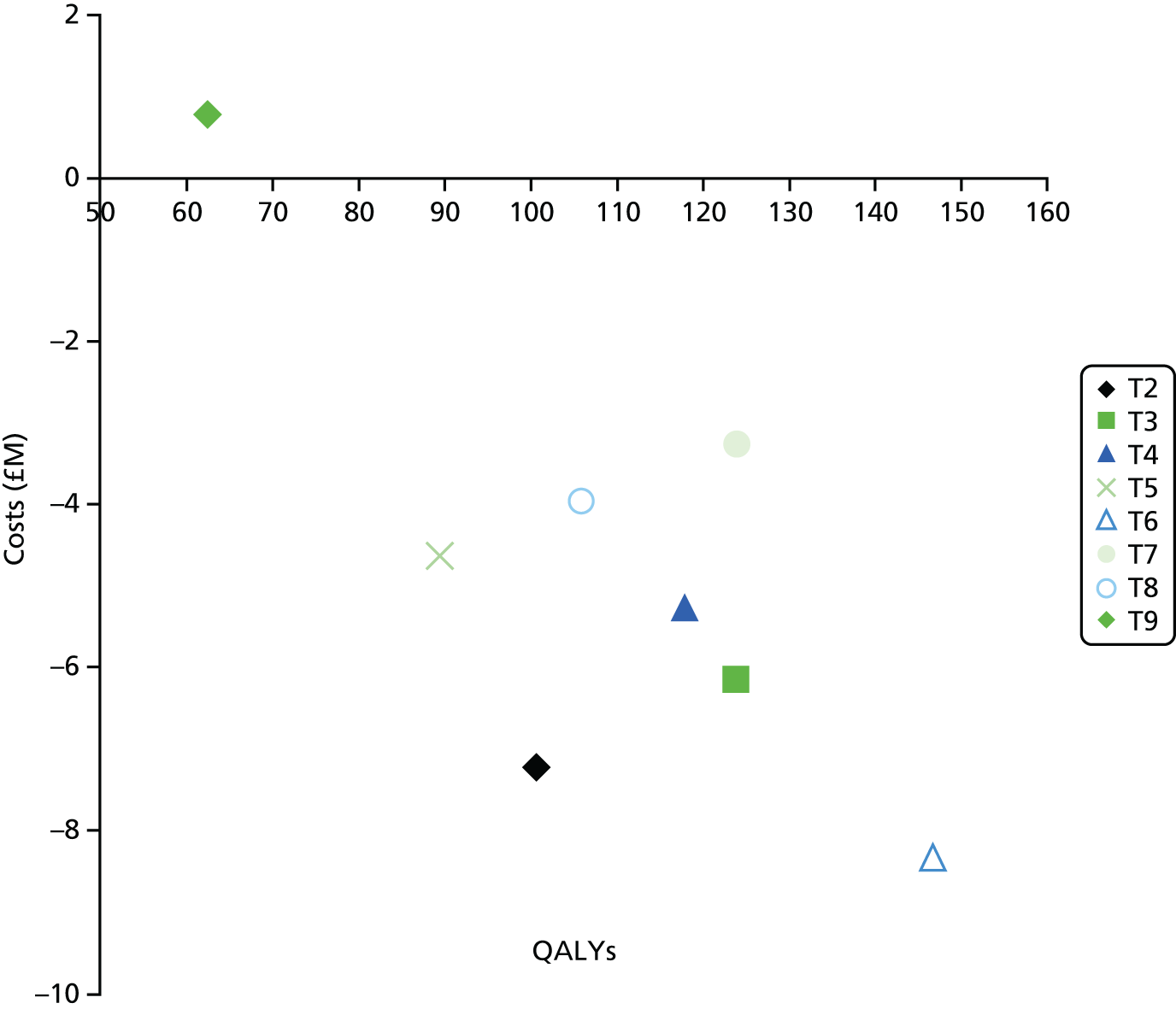
The joint distributions for each comparison are shown in Figures 27–33. For Figures 26–34, T1 is at the origin of both axes.
FIGURE 27.
T2 compared with T1, change to cost and QALY outcomes.
FIGURE 28.
T3 compared with T1, change to cost and QALY outcomes.

FIGURE 29.
T4 compared with T1, change to cost and QALY outcomes.
FIGURE 30.
T5 compared with T1, change to cost and QALY outcomes.
FIGURE 31.
T6 compared with T1, change to cost and QALY outcomes.

FIGURE 32.
T7 compared with T1, change to cost and QALY outcomes.

FIGURE 33.
T8 compared with T1, change to cost and QALY outcomes.

FIGURE 34.
T9 compared with T1, change to cost and QALY outcomes.
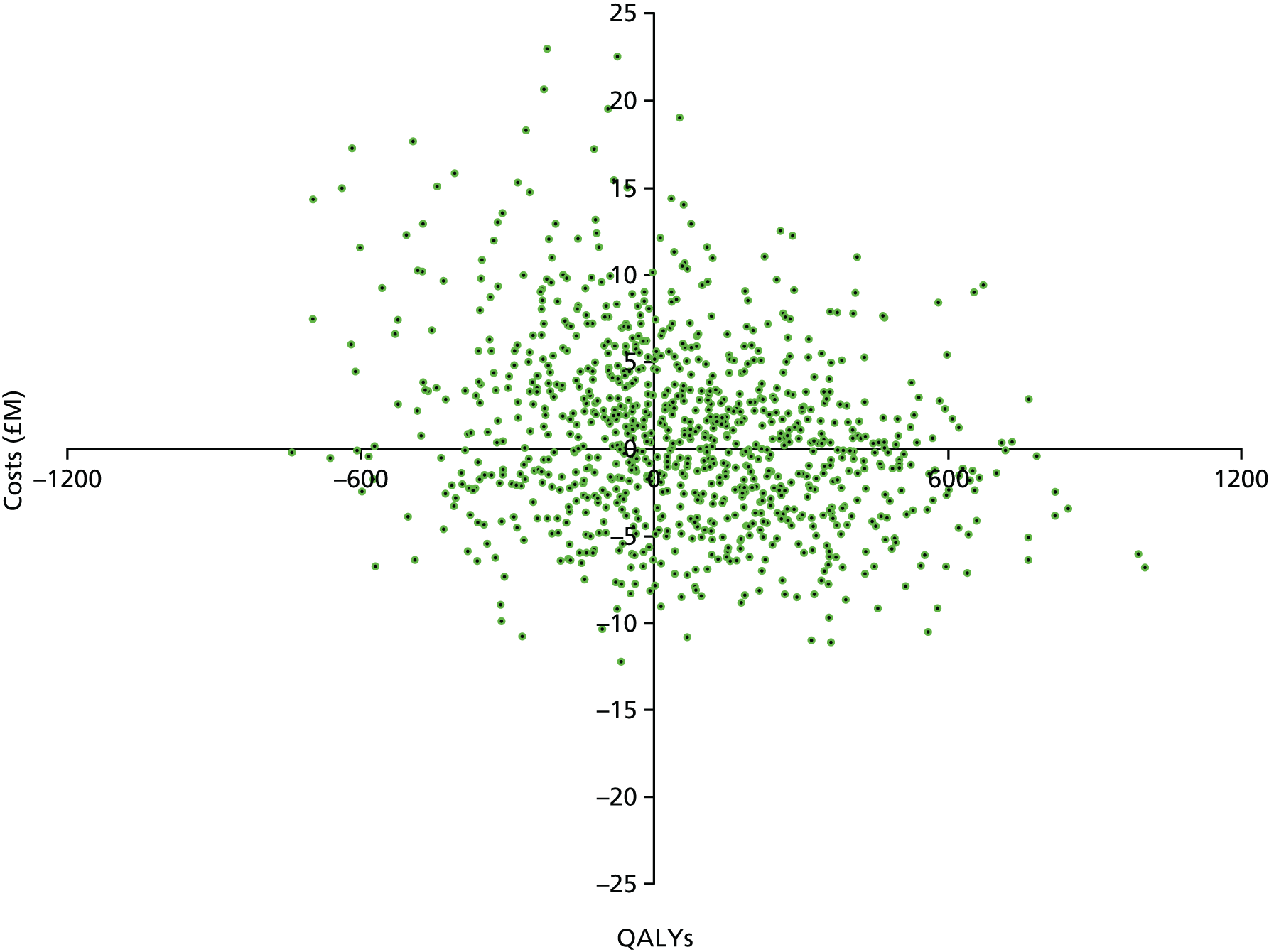
Interpretation of results for decision-making
To help decision-makers understand these results, we calculated the expected incremental NMB from adopting each treatment compared with T1. Deriving this statistic is discussed in Chapter 4. It is a linear measure of the change to QALYs expressed in monetary units less the change to costs, and so it reflects a net profit or loss for society from any adoption decision.
Positive NMB suggests an adoption decision is a good outcome for society and negative values suggest the opposite. QALYs were valued at £18,000 each, in line with recent research. 118 In Figure 35 the mean incremental NMB for each treatment option compared with T1 is shown.
FIGURE 35.
Mean incremental NMB for each treatment option compared with T1.

Treatment 6 shows the highest expected incremental NMB at £10,964,883 for the cohort and reflects the large cost savings and the value of the incremental QALYs gained. T9 shows the lowest expected incremental NMB at £342,619. The large uncertainty in these conclusions remains, with error bars overlapping for every treatment and all the time crossing zero.
The probability that each treatment is cost-effective and has positive incremental NMBs is shown by Table 58.
| Strategy | Probability strategy is cost-effective (%) |
|---|---|
| T2 | 15 |
| T3 | 18 |
| T4 | 10 |
| T5 | 7 |
| T6 | 32 |
| T7 | 6 |
| T8 | 11 |
| T9 | 1 |
The probabilities in Table 58 were calculated by comparing 1000 simulations of the NMB for each treatment strategy and choosing, for each simulation, the treatment that returned the highest value. T9 returned the highest NMB value in only 10 out of 1000 simulations, or 1% of the time. Therefore, we interpret this decision (T9) as having a 99% chance of being incorrect. T6 returned the highest NMB value 32% of the time, making this treatment the most likely to be cost-effective. T6 also prevented the greatest number of cases of deep infection (see Table 55), saved the most costs and generated the greatest QALY gains (see Figures 24 and 25).
Chapter 7 Discussion
Summary and conclusions
We used a MTC analysis to compare the effectiveness of nine competing strategies for reducing the risk of infection following THA and applied the results to a Markov model of the treatment pathways for patients who develop an infection following THA. This enabled an assessment of the costs and health effects of the nine treatment strategies in a single coherent framework. To evaluate uncertainty in the estimates of effectiveness and parameters in the Markov model, a Monte Carlo simulation of prior uncertain parameters was undertaken. The output of the simulation gives an estimate of the probability of cost-effectiveness of each of the treatment strategies and can be used as a decision-making tool in the context of the resource-limited NHS.
The results show that, compared with T1, T6 is the strategy that prevents the greatest number of deep infections following THA. T6 is a treatment of systemic antibiotics, antibiotic-impregnated cement and conventional ventilation. It prevents an extra 1481 cases of deep infection and leads to the largest annual cost savings of –£8,325,277 (95% uncertainty interval –£17,981,040 to £5,765,832). The mean gains to health benefits measured by QALYs are greatest at 147 (95% uncertainty interval –585 to 1157). T6 is the optimal decision with the highest probability of being cost-effective, at 32%. T2 competed closely with T6, delivering cost savings of £7,226,732 and QALY gains of 101. The NMB of a decision to adopt T2 was second highest, at £9,039,481. The only difference between T6 and T2 was the removal of antibiotic-impregnated cement for the cemented procedures. This suggests that adding antibiotic-impregnated cement is worthwhile. Two poor treatments for cost and clinical effectiveness outcomes were T9 and T7, which involved adding in laminar ventilation and a body exhaust suit. The addition of laminar air only (T7) drives costs upwards by £5,032,528 each year and reduces QALYs by 23. The further addition of a body exhaust suit increases costs by £9,106,352 and reduces QALYs by 84. This happens because laminar ventilation and body exhaust suits were found to increase risk of infection.
T6 dominates all other options by both cost and effect. These findings have to be considered against the limitations of the research method, data used and large uncertainties shown.
Limitations and strengths
As a relatively new evidence synthesis tool, MTC provides a powerful analytical tool that enables all available evidence, direct and indirect, to be included in an evidence base, synthesised and ranked according to its relative efficacy with associated probability statements. 78 Like any analytical model, MTC is based on a range of assumptions and the use of non-informative priors within a Bayesian framework. A key assumption of the MTC model used is that intervention effects are exchangeable across all trials in the network. In other words, both direct and indirect sources of evidence in each pairwise comparison are assumed to estimate the same treatment effects across the network of all trials. However, the assumption is appropriate only provided that the baseline characteristics of patient populations and intervention protocols are homogeneous. Variation in these parameters inevitably poses certain threats to both the internal and external validity of MTC models. In order to assess and adjust for the risk of estimation bias, we assessed the model fit and checked the consistency of intervention effect estimates from direct and indirect evidence sources. We conducted a metaregression on previous surgery and follow-up duration as potential sources of heterogeneity. We also estimated and adjusted for bias associated with studies with mixed quality. We were limited to English-language literature, and this may cause a bias in the results, but we were unable to describe the size of it or direction it might take. There were limited data available, and the MTC model was unable to adjust for potential confounders such as case mix, particularly patient comorbidity, in different hospital settings, different types of laminar airflow systems used and temporal changes in clinical practices, infection control technology and patient profiles that may have taken place over the past several decades. Despite the inherent limitations of MTC models and the limited number of studies available, the current evidence using MTC has the potential to change our understanding about the effectiveness of infection prevention strategies or a combination of multiple infection prevention strategies. This in turn may help contribute to the identification and implementation of evidence-based best infection prevention strategies for reducing the risk of, and preventing, THR-related SSI.
The baseline risks of infection for T1 were based on the Lidwell et al. 111 study, conducted in the 1970s and reporting 39 cases of deep infection among 1161 patients who had surgery under conditions that were closely correlated with T1. The 3.4% rate of infection describes 2.5 years, yet the model we ran counted infection outcomes for 12 months only. The rate of 3.4% was reduced based on US data by Ong et al. ,139 who showed that 66.7% of all infections they found over a 9-year period presented in the first 12 months. Whether or not these assumptions lead to an estimate that reliably describes the baseline risk of infection for T1 in NHS hospitals is unknown. If this estimate is inaccurate, however, it will not impact on the conclusions drawn, as the incremental change to outcomes is the key for decision-making. Absolute values are not particularly relevant.
A large effort was made to include parameter uncertainties in the model, and these large uncertainties can be seen in the results tables and figures, especially Figures 24, 25 and 35. We assumed that there was no structural uncertainty arising from the design of the Markov model. We did, however, spend time consulting with orthopaedic surgeons and infection prevention experts. A process was completed to identify the events most likely to impact cost and health outcomes while attempting to keep the model as simple as possible.
A further limitation of the model relates to the data used to inform patient outcomes. PROMs data could not be linked to the relevant time points for primary THA, deep infection or revision procedures. As a result, we made use of utility values from a previous review of health outcomes relating to THA, to attach health benefits to each state in our Markov model. These had been used in a previous model, in an Australian context. There are no strong reasons to believe that these values are not appropriate to the NHS setting. A further limitation is the scope of costs included. The data sets that were available for this project describe only the acute sector, and the costs of primary care, private out-of-pocket expenses and personal social services costs were excluded. We do feel that the costs of revisions of the primary hip and other surgical interventions were appropriately quantified and are likely to be the largest component of total costs.
Perhaps the biggest limitation of our model is the uncertainty regarding the effectiveness of the different treatment options. The best possible data were sourced and appropriate methods used to interpret them. A prospective randomised trial to address the research questions addressed here would be impossible; the cost would be prohibitive and it would not be passed by an ethics committee.
A major strength of this analysis, therefore, is the ability to compare nine different treatment strategies for the prevention of infection, together, using currently available evidence, without the need for a costly and time-consuming clinical trial. Large numbers of patient-level data were available, which are routinely collected in the NHS. This enabled a thorough analysis of THA patient demographics and treatment strategies as a result of deep infection following THA. Similarly, effectiveness data from a number of trials were available and were able to be synthesised using MTC techniques into one comparative analysis of all relevant treatment strategies. In addition, modelling studies have significant advantages over RCTs and other clinical studies in that they enable estimates of long-term cost and effectiveness of treatments. Similarly, findings from modelling studies are generalisable as they are not restricted to controlled trial criteria, and real-world populations are considered.
Chapter 8 Conclusions and recommendations
The conclusion from this research is that T6 is the best decision for NHS hospitals. More studies could be done on the effectiveness of laminar airflow systems and body exhaust suits to reduce the large uncertainties we see in this modelling study. The next steps include education of relevant health-care professionals about these research findings. Further introduction of new laminar airflow systems into NHS hospital operating rooms should be carefully considered, as these are expensive and our research shows that they might harm patients and increase costs. The same applies to the routine use of body exhaust suits.
The literature revealed some information deficiencies. There were only a small number of studies available for evidence synthesis and this reduced the statistical power making the CrIs wide for many of the comparisons. Owing to limited data, the MTC model could not be used to adjust for potential confounders. Examples of potential confounders are case mix, particularly patient comorbidity in different hospital settings; different types of laminar airflow systems used (such as horizontal and vertical systems); changes in clinical practice over time; infection control technology, such as the use of ultra-high airflows in modern conventional operating theatres; forced air blankets; and patient characteristics that may have changed over the past several decades.
Acknowledgements
We would like to thank Nick Hinton (Data Analyst, PHE) for expertise in data management and linkage, as well as Joanna Conneely (Operations Manager, PHE) and Robert Kyffin (Data and Information Policy and Partnerships Lead, PHE) for assistance with contracts and data reuse agreements.
This project was made possible through the co-operation of the data custodians of various data sets and we would like to acknowledge:
-
the Confidentiality Advisory Group at the Health Research Authority (formerly the National Information Governance Board) for reviewing our application for access to confidential patient information without consent under the Health Service (Control of Patient Information) Regulations 2002
-
the Healthcare Quality Improvement Partnership for providing data from the NJR on patients following hip replacement
-
the HSCIC and ONS for co-ordinating the release of HES, PROMs and ONS mortality data for the cohort of patients undergoing hip replacement.
For the information arising from the HSCIC: Copyright © 2014, Re-used with the permission of the Health & Social Care Information Centre. All rights reserved.
For the PROMs the licensee acknowledges that the data has been collected using a series of PROMs which contain copyright material licensed to the Department of Health by third parties. In particular, the licensee acknowledges that all questions reproduced from the:
-
Oxford Hip and Knee Scores were created by Professor Ray Fitzpatrick and Dr Jill Dawson and are copyright of Isis Innovation Limited 1996
-
EQ-5D standardised instrument for use as a measure of health outcomes are copyright of the EuroQol Group 1990 and EQ-5D is a trade mark of the EuroQol Group
-
London School of Hygiene and Tropical Medicine’s comorbidity and complications PROM were created by Professor Nick Bale and others and are copyright of the London School of Hygiene and Tropical Medicine.
For the ONS information, we acknowledge the ONS as the provider of selected materials. The ONS bears no responsibility for their further analysis or interpretation.
From 1 April 2013, the copyright in the material included in the annual reports of PHE has been assigned to PHE, a UK Crown body, by virtue of the transfer of all Health Protection Agency functions to PHE. However, the copyright in this document does not have Crown Copyright status as it was created before 1 April 2013. Table 1 and Figure 2 are Crown copyright and are reproduced with the permission of PHE under delegated authority from the Controller of HMSO.
We also acknowledge the Health Care Associated Infection Service Users Research Forum for their input to the project.
Contributions of authors
Nicholas Graves wrote the grant, designed the methods, managed the research and drafted sections of the report.
Catherine Wloch managed the process of data linkage from the NHS databases used.
Jennie Wilson contributed to study design, data collection, analysis and drafted sections of the report.
Adrian Barnett undertook the modelling and wrote the results sections.
Alex Sutton designed the methods and managed the research.
Nicola Cooper designed the methods and managed the research.
Katharina Merollini designed the methods and managed the research.
Victoria McCreanor undertook data collection and wrote sections of the report.
Qinglu Cheng undertook data collection and wrote sections of the report.
Edward Burn undertook data collection and wrote sections of the report.
Theresa Lamagni managed the process of data linkage from the NHS databases used and wrote sections of the report.
Andre Charlett designed the methods, managed the research and managed the process of data linkage from the NHS databases used.
Publication
Zheng H, Barnett AG, Merollini K, Sutton A, Cooper N, Berendt T, et al. Control strategies to prevent total hip replacement-related infections: a systematic review and mixed treatment comparison. BMJ Open 2014;4:e003978.
Data sharing statement
Data can be obtained from the corresponding author after approvals have been gained from the data custodians (PHE and ONS).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- 10th Annual Report 2013. Hemel Hempstead: NJR; 2013.
- Hospital Episode Statistics: Admitted Patient Care – 2012–13. Leeds: Health and Social Care Information Centre; 2013.
- Elgohari S, Mihalkova M, Harrington P, Wloch C, Lamagni T, Charlett A, et al. Surveillance of Surgical Site Infections in NHS Hospitals in England 2012/13. London: Public Health England; 2013.
- Coello R, Charlett A, Wilson J, Ward V, Pearson A, Borriello P. Adverse impact of surgical site infections in English hospitals. J Hosp Infect 2005;60:93-103. http://dx.doi.org/10.1016/j.jhin.2004.10.019.
- Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol 2002;23:183-9. http://dx.doi.org/10.1086/502033.
- Jenks PJ, Laurent M, McQuarry S, Watkins R. Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. J Hosp Infect 2014;86:24-33. http://dx.doi.org/10.1016/j.jhin.2013.09.012.
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008;90:915-19. http://dx.doi.org/10.1302/0301-620X.90B7.20498.
- Evans RP. Current concepts for clean air and total joint arthroplasty: laminar airflow and ultraviolet radiation: a systematic review. Clin Orthop Relat Res 2011;469:945-53. http://dx.doi.org/10.1007/s11999-010-1688-7.
- Zheng H, Barnett AG, Merollini K, Sutton A, Cooper N, Berendt T, et al. Control strategies to prevent total hip replacement-related infections: a systematic review and mixed treatment comparison. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-003978.
- Wang J, Zhu C, Cheng T, Peng X, Zhang W, Qin H, et al. A systematic review and meta-analysis of antibiotic-impregnated bone cement use in primary total hip or knee arthroplasty. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0082745.
- 12th Annual Report 2015. Hemel Hempstead: NJR; 2015.
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999;20:250-78. http://dx.doi.org/10.1086/501620.
- Suetens C, Hodson K, Kinross P. HELICSwin.Net (HWN) 1.3.8 User Manual. Stockholm: European Centre for Disease Prevention and Control; 2013.
- David TS, Vrahas MS. Perioperative lower urinary tract infections and deep sepsis in patients undergoing total joint arthroplasty. J Am Acad Orthop Surg 2000;8:66-74. http://dx.doi.org/10.5435/00124635-200001000-00007.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg 1973;107:206-10. http://dx.doi.org/10.1001/archsurg.1973.01350200078018.
- Cruse PJ, Foord R. The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds. Surg Clin North Am 1980;60:27-40.
- Public Health England . Surveillance of Surgical Site Infections in NHS Hospitals in England 2013 14 2014. www.gov.uk/government/uploads/system/uploads/attachment_data/file/386927/SSI_report_2013_14_final__3_.pdf (accessed July 2014).
- Public Health England . Surgical Site Infection Surveillance Service 2014. www.gov.uk/guidance/surgical-site-infection-surveillance-service-ssiss (accessed July 2014).
- Hernigou P, Flouzat-Lachianette CH, Jalil R, Uirassu Batista S, Guissou I, Poignard A. Treatment of infected hip arthroplasty. Open Orthop J 2010;4:126-31.
- Zimmerli W. Implant infections. What must an internist know?. Internist 2005;46:652-8. http://dx.doi.org/10.1007/s00108-005-1401-1.
- Lehner B, Witte D, Suda AJ, Weiss S. Revision strategy for periprosthetic infection. Orthopade 2009;38:681-8. http://dx.doi.org/10.1007/s00132-009-1434-6.
- Moyad TF, Thornhill T, Estok D. Evaluation and management of the infected total hip and knee. Orthopedics 2008;31:581-8. http://dx.doi.org/10.3928/01477447-20080601-22.
- Zimmerli W. Infection and musculoskeletal conditions: prosthetic-joint-associated infections. Best Pract Res Clin Rheumatol 2006;20:1045-63. http://dx.doi.org/10.1016/j.berh.2006.08.003.
- Chryssikos T, Parvizi J, Ghanem E, Newberg A, Zhuang H, Alavi A. FDG-PET imaging can diagnose periprosthetic infection of the hip. Clin Orthop Relat Res 2008;466:1338-42. http://dx.doi.org/10.1007/s11999-008-0237-0.
- Theis JC, Gambhir S, White J. Factors affecting implant retention in infected joint replacements. ANZ J Surg 2007;77:877-9. http://dx.doi.org/10.1111/j.1445-2197.2007.04263.x.
- Cramer J, Ekkernkamp A, Ostermann PA. The infected endoprosthesis with the example of the hip joint endoprosthesis. An increasing danger to patient and society. Z Arztl Fortbild Qualitatssich 2001;95:195-201.
- Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, et al. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis 2006;42:471-8. http://dx.doi.org/10.1086/499234.
- Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis 2001;32:419-30. http://dx.doi.org/10.1086/318502.
- Choong PF, Dowsey MM, Carr D, Daffy J, Stanley P. Risk factors associated with acute hip prosthetic joint infections and outcome of treatment with a rifampin based regimen. Acta Orthop 2007;78:755-65. http://dx.doi.org/10.1080/17453670710014527.
- Bernard L, Hoffmeyer P, Assal M, Vaudaux P, Schrenzel J, Lew D. Trends in the treatment of orthopaedic prosthetic infections. J Antimicrob Chemother 2004;53:127-9. http://dx.doi.org/10.1093/jac/dkh033.
- Theis JC. Implant retention in infected joint replacements: a surgeon’s perspective. Int J Artif Organs 2008;31:804-9.
- Magnan B, Regis D, Biscaglia R, Bartolozzi P. Preformed acrylic bone cement spacer loaded with antibiotics: use of two-stage procedure in 10 patients because of infected hips after total replacement. Acta Orthop Scand 2001;72:591-4. http://dx.doi.org/10.1080/000164701317269003.
- Giulieri SG, Graber P, Ochsner PE, Zimmerli W. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection 2004;32:222-8. http://dx.doi.org/10.1007/s15010-004-4020-1.
- Uckay I, Lew DP, Jarvis WR. Bennett & Brachman’s Hospital Infections. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
- Yamamoto PA, Lahoz GL, Takata ET, Masiero D, Chamlian TR. Evaluation of the function and quality of life of patients submitted to Girdlestone’s resection arthroplasty. Acta Ortop Bras 2007;15:214-17.
- Sharma H, Leeuw JD, Rowley DI. Girdlestone resection arthroplasty following failed surgical procedures. Int Orthop 2005;29:92-5. http://dx.doi.org/10.1007/s00264-004-0633-3.
- Sharma H, Dreghorn CR, Gardner ER. Girdlestone resection arthroplasty of the hip: current perspectives. Orthop Trauma 2005;19:385-92. http://dx.doi.org/10.1016/j.cuor.2005.06.005.
- Urban JA. Cost analysis of surgical site infections. Surg Infect 2006;7:19-22. http://dx.doi.org/10.1089/sur.2006.7.s1-19.
- Odom-Forren J. Preventing surgical site infections. Nursing 2006;36:58-63. http://dx.doi.org/10.1097/00152193-200606000-00045.
- Vanhegan IS, Malik AK, Jayakumar P, Ul Islam S, Haddad FS. A financial analysis of revision hip arthroplasty: the economic burden in relation to the national tariff. J Bone Joint Surg Br 2012;94:619-23. http://dx.doi.org/10.1302/0301-620X.94B5.27073.
- Klouche S, Sariali E, Mamoudy P. Total hip arthroplasty revision due to infection: a cost analysis approach. Orthop Traumatol Surg Res 2010;96:124-32. http://dx.doi.org/10.1016/j.otsr.2009.11.004.
- Arens S, Kutscha-Lissberg F, Hebler U, Wingenfeld C, Kälicke T, Muhr G. Pyogenic infection after joint replacement operations: incidence and economic effects. Kongressbd Dtsch Ges Chir Kongr 2002;119:738-42.
- Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am 2005;87:1746-51. http://dx.doi.org/10.2106/JBJS.D.02937.
- Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis 2003;36:1157-61. http://dx.doi.org/10.1086/374554.
- Sia IG, Berbari EF, Karchmer AW. Prosthetic joint infections. Infect Dis Clin North Am 2005;19:885-914. http://dx.doi.org/10.1016/j.idc.2005.07.010.
- Yasunaga H, Ide H, Imamura T, Ohe K. Accuracy of economic studies on surgical site infection. J Hosp Infect 2007;65:102-7. http://dx.doi.org/10.1016/j.jhin.2006.07.008.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2005.
- Graves N, Harbarth S, Beyersmann J, Barnett A, Halton K, Cooper B. Estimating the cost of health care-associated infections: mind your p’s and q’s. Clin Infect Dis 2010;50:1017-21. http://dx.doi.org/10.1086/651110.
- Samore M, Harbarth S, Mayhall CG. Hospital Epidemiology and Infection Control. Philadelphia, PA: Lippincott Williams & Wilkins; 2004.
- Surgical Site Infection: Prevention and Treatment of Surgical Site Infection. London: NICE; 2008.
- Hoffman PN, Williams J, Stacey A, Bennett AM, Ridgway GL, Dobson C, et al. Microbiological commissioning and monitoring of operating theatre suites. J Hosp Infect 2002;52:1-28. http://dx.doi.org/10.1053/jhin.2002.1237.
- Prevention and Control of Healthcare-Associated Infections Overview. NICE; 2014.
- Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al. A systematic review and economic model of switching from non-glycopeptide to glycopeptide antibiotic prophylaxis for surgery. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12010.
- Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesaeter LB, Finlayson SR. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am 2009;91:634-41. http://dx.doi.org/10.2106/JBJS.G.01029.
- Elliott RA, Weatherly HL, Hawkins NS, Cranny G, Chambers D, Myers L, et al. An economic model for the prevention of MRSA infections after surgery: non-glycopeptide or glycopeptide antibiotic prophylaxis?. Eur J Health Econ 2010;11:57-66. http://dx.doi.org/10.1007/s10198-009-0175-0.
- Courville XF, Tomek IM, Kirkland KB, Birhle M, Kantor SR, Finlayson SR. Cost-effectiveness of preoperative nasal mupirocin treatment in preventing surgical site infection in patients undergoing total hip and knee arthroplasty: a cost-effectiveness analysis. Infect Control Hosp Epidemiol 2012;33:152-9. http://dx.doi.org/10.1086/663704.
- Merollini KM, Crawford RW, Whitehouse SL, Graves N. Surgical site infection prevention following total hip arthroplasty in Australia: a cost-effectiveness analysis. Am J Infect Control 2013;41:803-9. http://dx.doi.org/10.1016/j.ajic.2012.11.015.
- Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250-61. http://dx.doi.org/10.1056/NEJMoa074311.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Cooper N, Coyle D, Abrams K, Mugford M, Sutton A. Use of evidence in decision models: an appraisal of health technology assessments in the UK since 1997. J Health Serv Res Policy 2005;10:245-50. http://dx.doi.org/10.1258/135581905774414187.
- Blom AW, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total hip arthroplasty. The Avon experience. J Bone Joint Surg Br 2003;85:956-9. http://dx.doi.org/10.1302/0301-620X.85B7.14095.
- Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making 2002;22:475-81. http://dx.doi.org/10.1177/0272989X02238300.
- Persson U, Persson M, Malchau H. The economics of preventing revisions in total hip replacement. Acta Orthop Scand 1999;70:163-9. http://dx.doi.org/10.3109/17453679909011256.
- Merollini K. Evaluation of the Cost-Effectiveness of Strategies Claiming to Reduce the Risk of Surgical Site Infections Following Primary Total Hip Arthroplasty 2012.
- Glenny A, Song F. Antimicrobial prophylaxis in total hip replacement: a systematic review. Health Technol Assess 1999;3.
- Fry DE, Harbrecht PJ, Polk HC. Systemic prophylactic antibiotics: need the ‘cost’ be so high?. Arch Surg 1981;116:466-9. http://dx.doi.org/10.1001/archsurg.1981.01380160076016.
- Borgquist L, Lidgren L, Lindberg L. Operating box or antibiotic prophylaxis? A comparison of costs. Lakartidningen 1978;75:1705-6.
- Heydemann JS, Nelson CL. Short-term preventive antibiotics. Clin Orthop Relat Res 1986;205:184-7. http://dx.doi.org/10.1097/00003086-198604000-00022.
- Germiniani R. Prevention of infections in surgery. Costs and benefits of infections of surgical wounds and of their prevention with antibiotics. Minerva Chir 1989;44:789-92.
- Henderson PJ, Gillespie WJ. Costs of prophylaxis against infection for joint replacement. N Z Med J 1989;102.
- D’Angelo GL, Ogilvie-Harris DJ. Septic arthritis following arthroscopy, with cost/benefit analysis of antibiotic prophylaxis. Arthroscopy 1988;4:10-4. http://dx.doi.org/10.1016/S0749-8063(88)80004-5.
- Lidwell OM. The cost implications of clean air systems and antibiotic prophylaxis in operations for total joint replacement. Infect Control 1984;5:36-7.
- Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br 2008;90:770-7. http://dx.doi.org/10.1302/0301-620X.90B6.20194.
- Iribarren BO, Alvarez CA, Rodríguez CC, Ferrada MM, Hernández VH, Dorn HL. Cost and outcome of hip’s arthroplasty nosocomial infection. Case and control study. Rev Chilena Infectol 2007;24:125-30. http://dx.doi.org/10.4067/S0716-10182007000200006.
- Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty 2008;23:984-91. http://dx.doi.org/10.1016/j.arth.2007.10.017.
- Primary Total Hip Replacement: A Guide to Good Practice. London: British Orthopaedic Association; 2006.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. http://dx.doi.org/10.1002/sim.3767.
- Sutton A, Ades AE, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmaco Economics 2008;26:753-67. http://dx.doi.org/10.2165/00019053-200826090-00006.
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900. http://dx.doi.org/10.1136/bmj.331.7521.897.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in metaanalysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. http://dx.doi.org/10.1016/S0895-4356(97)00049-8.
- Higgins JP, Whitehead A. Borrowing strength from external trials in a meta-analysis. Stat Med 1996;15:2733-49. http://dx.doi.org/10.1002/(SICI)1097-0258(19961230)15:24%3C2733::AID-SIM562%3E3.0.CO;2-0.
- Ades AE, Claxton K, Sculpher M. Evidence synthesis, parameter correlation and probabilistic sensitivity analysis. Health Econ 2006;15:373-81. http://dx.doi.org/10.1002/hec.1068.
- Ades AE. A chain of evidence with mixed comparisons: models for multi-parameter synthesis and consistency of evidence. Stat Med 2003;22:2995-3016. http://dx.doi.org/10.1002/sim.1566.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. http://dx.doi.org/10.1002/sim.1875.
- Cooper BS, Fang LQ, Zhou JP, Feng D, Lv H, Wei MT, et al. Transmission of SARS in three Chinese hospitals. Trop Med Int Health 2009;14:71-8. http://dx.doi.org/10.1111/j.1365-3156.2009.02346.x.
- Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Stat Med 2009;28:1861-81. http://dx.doi.org/10.1002/sim.3594.
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 1999;354:1896-900. http://dx.doi.org/10.1016/S0140-6736(99)04149-5.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Ehrenkranz NJ, Shultz JM, Richter EL. Recorded criteria as a “gold standard” for sensitivity and specificity estimates of surveillance of nosocomial infection: a novel method to measure job performance. Infect Control Hosp Epidemiol 1995;16:697-702. http://dx.doi.org/10.2307/30141912.
- Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop 2008;79:335-41. http://dx.doi.org/10.1080/17453670710015229.
- Block JE, Stubbs HA. Reducing the risk of deep wound infection in primary joint arthroplasty with antibiotic bone cement. Orthopedics 2005;28:1334-45.
- Whitehead S, Bending M, Trueman P, Saxby R, Duffy S. Cost-Effectiveness of Hospital Design: Options to Improve Patient Safety and Wellbeing: Systematic Literature Review Of Ventilation. York: York Health Economics Consortium; 2008.
- Methods for the Development of NICE Public Health Guidance (Third Edition). London: NICE; 2012.
- Systematic Reviews. York: University of York; 2009.
- Tooth L, Ware R, Bain C, Purdie DM, Dobson A. Quality of reporting of observational longitudinal research. Am J Epidemiol 2005;161:280-8. http://dx.doi.org/10.1093/aje/kwi042.
- Dias S, Welton NJ, Ades AE. Study designs to detect sponsorship and other biases in systematic reviews. J Clin Epidemiol 2010;63:587-8. http://dx.doi.org/10.1016/j.jclinepi.2010.01.005.
- Spiegelhalter DJ, Best NG, Bradley CP, van der Linde A. Bayesian measures of model complexity and fit. J R Statist Soc 2002;64:583-639. http://dx.doi.org/10.1111/1467-9868.00353.
- Hill C, Flamant R, Mazas F, Evrard J. Prophylactic cefazolin versus placebo in total hip replacement. Report of a multicentre double-blind randomised trial. Lancet 1981;1:795-6. http://dx.doi.org/10.1016/S0140-6736(81)92678-7.
- Carlsson AK, Lidgren L, Lindberg L. Prophylactic antibiotics against early and late deep infections after total hip replacements. Acta Orthop Scand 1977;48:405-10. http://dx.doi.org/10.3109/17453677708992017.
- Schulitz KP, Winkelmann W, Schoening B. The prophylactic use of antibiotics in alloarthroplasty of the hip joint for coxarthrosis. Arch Orthop Trauma Surg 1980;96:79-82. http://dx.doi.org/10.1007/BF00433285.
- Fitzgerald RH. Total hip arthroplasty sepsis. Prevention and diagnosis. Orthop Clin North Am 1992;23:259-64.
- Josefsson G, Lindberg L, Wiklander B. Systemic antibiotics and gentamicin-containing bone cement in the prophylaxis of postoperative infections in total hip arthroplasty. Clin Orthop Relat Res 1981;159:194-200. http://dx.doi.org/10.1097/00003086-198109000-00027.
- McQueen MM, Hughes SP, May P, Verity L. Cefuroxime in total joint arthroplasty. Intravenous or in bone cement. J Arthroplasty 1990;5:169-72. http://dx.doi.org/10.1016/S0883-5403(06)80236-6.
- Hooper GJ, Rothwell AG, Frampton C, Wyatt MC. Does the use of laminar flow and space suits reduce early deep infection after total hip and knee replacement?: the ten-year results of the New Zealand Joint Registry. J Bone Joint Surg Br 2011;93:85-90. http://dx.doi.org/10.1302/0301-620X.93B1.24862.
- Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg 2008;248:695-700. http://dx.doi.org/10.1097/SLA.0b013e31818b757d.
- Salvati EA, Robinson RP, Zeno SM, Koslin BL, Brause BD, Wilson PD. Infection rates after 3175 total hip and total knee replacements performed with and without a horizontal unidirectional filtered air-flow system. J Bone Joint Surg Am 1982;64:525-35.
- Kelly AJ, Bailey R, Davies EG, Pearcy R, Winson IG. An audit of early wound infection after elective orthopaedic surgery. J R Coll Surg Edinb 1996;41:129-31.
- Espehaug B, Engesæter LB, Vollset SE, Havelin LI, Langeland N. Antibiotic prophylaxis in total hip arthroplasty. Review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg Br 1997;79:590-5. http://dx.doi.org/10.1302/0301-620X.79B4.7420.
- Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 2003;74:644-51. http://dx.doi.org/10.1080/00016470310018135.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalized Linear Modelling Framework for Pair-Wise and Network Meta-Analysis of Randomised Controlled Trials. Sheffield: University of Sheffield, NICE Decision Support Unit; 2011.
- Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Stanley SJ, Lowe D. Effect of ultraclean air in operating rooms on deep sepsis in the joint after total hip or knee replacement: a randomised study. Br Med J 1982;285:10-4. http://dx.doi.org/10.1136/bmj.285.6334.10.
- Claxton K, Walker S, Palmer S, Schiulpher M. Appropriate Perspectives for Health Care Decisions. York: University of York, Centre for Health Economics; 2010.
- Torgerson DJ, Spencer A. Marginal costs and benefits. BMJ 1996;312:35-6. http://dx.doi.org/10.1136/bmj.312.7022.35.
- Prieto L, Sacristán JA. Problems and solutions in calculating quality-adjusted life years (QALYs). Health Qual Life Outcomes 2003;1. http://dx.doi.org/10.1186/1477-7525-1-80.
- Brazier J, Deverill M, Green C. A review of the use of health status measures in economic evaluation. J Health Serv Res Policy 1999;4:174-84.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224-7. http://dx.doi.org/10.1136/bmj.329.7459.224.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta19140.
- George B, Harris A, Mitchell A. Cost-effectiveness analysis and the consistency of decision making: evidence from pharmaceutical reimbursement in Australia (1991 to 1996). PharmacoEconomics 2001;19:1103-9. http://dx.doi.org/10.2165/00019053-200119110-00004.
- O’Brien B, Heyland D, Richardson W, Levine M, Drummond M. Users’ guides to the medical literature. XIII. How to use an article on economic analysis of clinical practice. B. What are the results and will they help me in caring for my patients? Evidence-Based Medicine Working Group. JAMA 1997;277:1802-6. http://dx.doi.org/10.1001/jama.1997.03540460066034.
- Briggs AH, O’Brien BJ. The death of cost-minimization analysis?. Health Econ 2001;10:179-84. http://dx.doi.org/10.1002/hec.584.
- Buxton MJ, Drummond MF, Van Hout BA, Prince RL, Sheldon TA, Szucs T, et al. Modelling in economic evaluation: an unavoidable fact of life. Health Econ 1997;6:217-27. http://dx.doi.org/10.1002/(SICI)1099-1050(199705)6:3%3C217::AID-HEC267%3E3.0.CO;2-W.
- Sculpher MJ, Claxton K, Drummond M, McCabe C. Whither trial-based economic evaluation for health care decision making?. Health Econ 2006;15:677-87. http://dx.doi.org/10.1002/hec.1093.
- Cooper NJ, Sutton AJ, Ades AE, Paisley S, Jones DR. Use of evidence in economic decision models: practical issues and methodological challenges. Health Econ 2007;16:1277-86. http://dx.doi.org/10.1002/hec.1297.
- Glanville J, Paisley S, Shemilt I, Mugford M, Vale L, Marsh K, et al. Evidence-Based Decisions And Economics: Health Care, Social Welfare, Education And Criminal Justice. Oxford: Wiley-Blackwell; 2010.
- Hanratty B, Craig D, Nixon J, Rice S, Christie J, Drummond M. Are the best available clinical effectiveness data used in economic evaluations of drug therapies?. J Health Serv Res Policy 2007;12:138-41. http://dx.doi.org/10.1258/135581907781543067.
- Schlosser RW, Wendt O, Bhavnani S, Nail-Chiwetalu B. Use of information-seeking strategies for developing systematic reviews and engaging in evidence-based practice: the application of traditional and comprehensive Pearl Growing. A review. Int J Lang Commun Disord 2006;41:567-82. http://dx.doi.org/10.1080/13682820600742190.
- Bates MJ. The design of browsing and berrypicking techniques for the online search interface. Online Review 1989;13:407-24. http://dx.doi.org/10.1108/eb024320.
- Sculpher M, Claxton K. Establishing the cost-effectiveness of new pharmaceuticals under conditions of uncertainty–when is there sufficient evidence?. Value Health 2005;8:433-46. http://dx.doi.org/10.1111/j.1524-4733.2005.00033.x.
- Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices – Modeling Studies. Value Health 2003;6:9-17. http://dx.doi.org/10.1046/j.1524-4733.2003.00234.x.
- O’Hagan A, Luce BR. A Primer on Bayesian Statistics in Health Economics and Outcomes Research. Sheffield: University of Sheffield, The Centre for Bayesian Statistics in Health Economics; 2003.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press; 2006.
- Muenning P, Muenning P. Cost-Effectiveness Analysis In Health: A Practical Approach. San Francisco, CA: Jossey-Bass; 2008.
- Briggs A, Jones AM. The Elgar Companion to Health Economics. Cheltenham: Edward Elgar Publishing; 2006.
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97. http://dx.doi.org/10.1111/j.1524-4733.2008.00358.x.
- Halton KA, Cook DA, Whitby M, Paterson DL, Graves N. Cost effectiveness of antimicrobial catheters in the intensive care unit: addressing uncertainty in the decision. Crit Care 2009;13. http://dx.doi.org/10.1186/cc7744.
- Claxton K, Sculpher M, McCabe C, Briggs A, Akehurst R, Buxton M, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ 2005;14:339-47. http://dx.doi.org/10.1002/hec.985.
- Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. PharmacoEconomics 1998;13:397-409. http://dx.doi.org/10.2165/00019053-199813040-00003.
- Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty 2009;24:105-9. http://dx.doi.org/10.1016/j.arth.2009.04.027.
- Hickson CJ, Metcalfe D, Elgohari S, Oswald T, Masters JP, Rymaszewska M, et al. Prophylactic antibiotics in elective hip and knee arthroplasty: an analysis of organisms reported to cause infections and national survey of clinical practice. Bone Joint Res 2015;4:181-9. http://dx.doi.org/10.1302/2046-3758.411.2000432.
- British National Formulary. NICE; 2014.
- Gray A, Holman R, Clarke P, Leal J. Performance of the UKPDS outcomes model for prediction of myocardial infarction and stroke in the ADDITION-Europe trial cohort: does the ADDITION validation add up?. Value Health 2014;17:895-6. http://dx.doi.org/10.1016/j.jval.2014.07.008.
- Bhattacharya S. The facts about penicillin allergy: a review. J Adv Pharm Technol Res 2010;1:11-7.
- Haslam KR. Laminar air-flow air conditioning in the operating room: a review. Anesth Analg 1974;53:194-9. http://dx.doi.org/10.1213/00000539-197403000-00003.
- OANDA . Currency Converter n.d. www.oanda.com/currency/converter/ (accessed 1 July 2012).
- Flyte Steri Shield: Personal Protection System. Kalamazoo, MI: Stryker Instruments; 2015.
- Health and Social Care Information Centre . Background to OPCS-4 Classification 2014. http://systems.hscic.gov.uk/data/clinicalcoding/codingstandards/opcs4/background (accessed January 2014).
- Health and Social Care Information Centre . Introduction to Healthcare Resource Groups 2014. www.hscic.gov.uk/hrg (accessed January 2014).
- Health and Social Care Information Centre . HRG4+ 2013 14 Reference Costs Grouper 2014. www.hscic.gov.uk/article/4698/HRG4-201213-Reference-Costs-Grouper-and-Documentation (accessed January 2014).
- NHS Reference Costs 2012 to 2013. London: Department of Health; 2013.
- Räsänen P, Paavolainen P, Sintonen H, Koivisto AM, Blom M, Ryynänen OP, et al. Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthop 2007;78:108-15. http://dx.doi.org/10.1080/17453670610013501.
- Cahill JL, Shadbolt B, Scarvell JM, Smith PN. Quality of life after infection in total joint replacement. J Orthop Surg 2008;16:58-65.
- Cochrane . Cochrane Musculoskeletal 2016. http://musculoskeletal.cochrane.org/ (accessed 1 July 2014).
- Joanna Briggs Institute . Joanna Briggs Institute Reviewer’s Manual 2011. http://joannabriggs.org/assets/docs/sumari/ReviewersManual-2011.pdf.
- O’Connor D, Green S, Higgins JPT, Higgins J, Green S. Cochrane Handbook of Systematic Reviews of Intervention 5.0.0. London: The Cochrane Collaboration; 2008.
Appendix 1 Search terms and strategies used for updating existing evidence
MEDLINE search strategy and output
Date of search: August 2011.
Date range searched: January 1966 to June 2011.
Search strategy
-
MH “arthroplasty, replacement, hip’ (12,088)
-
MH”Hip prosthesis” (16,064)
-
or/1-2 (23,923)
-
MH “Surgical wound infection” (24,907)
-
MH “prosthesis-related infections” (5875)
-
MH “sepsis+” (78,337)
-
MH “bacterial infections+” (651,285)
-
or/4-7 (718,898)
-
MH “infection control+’ (44,638)
-
infection prevent*/ (33,276)
-
MH “antibiotic prophylaxis” (6889)
-
MH “anti-infective agents+” (427,375)
-
MH “Anti-Bacterial Agents+” (226,839)
-
intravenous antibiotics/ (3008)
-
systemic antibiotics/ (2001)
-
or/9-15 (489,991)
-
MH “bone cements” (7563)
-
Antibiotic cement/ (448)
-
antibiotic bone cement (smart text searching)/ (7715)
-
Antibiotic-impregnated cement (smart text searching)/ (733)
-
Antibiotic-impregnated bone cement (smart text searching )/ (1001)
-
Antibiotic-loaded cement (smart text searching)/ (1129)
-
Antibiotic-loaded bone cement (smart text s)/ (7969)
-
MH “Environment, Controlled+” (218,646)
-
MH “ventilation” (4202)
-
MH “Air conditioning” (2075)
-
MH “operating rooms” (9370)
-
Operating theatre/ (1937)
-
laminar air flow (smart text searching)/ (3374)
-
laminar airflow (smart text searching )/ (244)
-
laminar air flow system (smart text searching) (4345)
-
ultra-clean air (smart text searching )/ (2527)
-
ultra clean air (smart text searching)/ (2527)
-
ultra-clean air system (smart text searching) (4496)
-
conventional operating room (smart text searching)/ (22,235)
-
conventional operating theatre (smart text searching)/ (2777)
-
Turbulent air flow (smart text searching)/ (2613)
-
or/25-38 (256,457)
-
3 and 8 and 16 (697)
-
3 and 8 and 24 (292)
-
3 and 8 and 39 (87)
-
40 or 41 or 42 (834)
-
Limit 43 to 2004-2011 (01/01/2004-01/06/2011) (343)
-
Limit 44 to English (289)
Cumulative Index to Nursing and Allied Health Literature search strategy and output
Date of search: August 2011.
Date range searched: January 1966 to June 2011.
Search strategy
-
MH “arthroplasty, replacement, hip’ (4105)
-
MH “joint prosthesis” (2008)
-
Hip prosthesis (smart text searching) (1894)
-
or/1-3 (5474)
-
MH “Surgical wound infection” (3410)
-
MH “prosthesis-related infections” (529)
-
MH “sepsis+” (7640)
-
MH “bacterial infections+” (39,831)
-
or/5-8 (48,616)
-
MH “infection control+’ (31,116)
-
infection prevent*/ (10,134)
-
MH “antibiotic prophylaxis” (2227)
-
MH “anti-infective agents+” (45,136)
-
Anti-Bacterial Agents (smart text searching)/ (2578)
-
intravenous antibiotics (smart text searching)/ (1301)
-
systemic antibiotics (smart text searching)/ (665)
-
or/10-16 (79,414)
-
MH “bone cements” (804)
-
Antibiotic cement (smart text searching) (96)
-
antibiotic bone cement (smart text searching)/ (610)
-
Antibiotic-impregnated cement (smart text searching)/ (107)
-
Antibiotic-impregnated bone cement (smart text searching )/ (126)
-
Antibiotic-loaded cement (smart text searching)/ (144)
-
Antibiotic-loaded bone cement (smart text s)/ (638)
-
MH “Environment, Controlled+” (4265)
-
MH “ventilation+” (747)
-
MH “Air conditioning” (118)
-
MH “operating rooms” (4319)
-
Operating theatre (smart text searching) (663)
-
laminar air flow (smart text searching) (126)
-
laminar airflow (smart text searching )/ (38)
-
laminar air flow system (smart text searching) (137)
-
ultra-clean air (smart text searching )/ (40)
-
ultra clean air (smart text searching)/ (40)
-
ultra-clean air system (smart text searching) (126)
-
conventional operating room (smart text searching)/ (359)
-
conventional operating theatre (smart text searching)/ (664)
-
Turbulent air flow (smart text searching)/ (52)
-
or/26-39 (9280)
-
4 and 9 and 17 (216)
-
4 and 9 and 25 (59)
-
4 and 9 and 40 (24)
-
41 or 42 or 43 (233)
-
Limit 44 to 2004-2011 (01/01/2004-01/06/2011) (196)
-
Limit 45 to English (196)
The Cochrane Central Register of Controlled Trials search strategy and search output
Date of search: August 2011.
Date range searched: January 1966 to June 2011.
Search strategy
#1. MH “arthroplasty, replacement, hip +”/exp (1254)
#2. MH”Hip prosthesis”/exp (942)
#3. or/1-2 (1949)
#4. MH “Surgical wound infection”/exp (2470)
#5. MH “prosthesis-related infections”/exp (127)
#6. MH “sepsis ”/exp (2684)
#7. MH “bacterial infections”/exp (13,168)
#8. or/4-7 (17,095)
#9. MH “infection control”/exp (1116)
#10. infection prevent*/ (16,554)
#11. MH “antibiotic prophylaxis”/exp (1040)
#12. MH “anti-infective agents”/exp (44,153)
#13. MH “Anti-Bacterial Agents”/exp (18,759)
#14. intravenous antibiotics/ (2375)
#15. systemic antibiotics/ (1220)
#16. or/9-15 / (55,871)
#17. MH “bone cements”/ exp (579)
#18. Antibiotic cement/ (39)
#19. antibiotic bone cement/ (32)
#20. Antibiotic-impregnated cement/ (7)
#21. Antibiotic-impregnated bone cement/ (7)
#22. Antibiotic-loaded cement/ (3)
#23. Antibiotic-loaded bone cement/ (3)
#24. or/17-23 (601)
25. MH “Environment, Controlled”/exp (1948)
26. MH “ventilation”/exp (52)
27. MH “Air conditioning”/exp (25)
28. MH “operating rooms”/exp (230)
29. Operating theatre/ (402)
30. laminar air flow/ (39)
31. laminar airflow/ (11)
32. laminar air flow system/ (7)
33. ultra-clean air/ (5)
34. ultra clean air/ (6)
35. ultra-clean air system/ (2)
36. conventional operating room/ (184)
37. conventional operating theatre/ (59)
38. Turbulent air flow/ (5)
39. or/25-38 (2697)
40. 3 and 8 and 16/ (58)
41. 3 and 8 and 24/ (9)
42. 3 and 8 and 39/ (11)
43. 40 or 41 or 42/ (59)
44. Limit 43 to 2004-2011 (15)
45. Limit 44 to English (15)
EMBASE search strategy and search output
Date of search: August 2011.
Date range searched: January 1966 to June 2011.
Search strategy
#1. ‘hip arthroplasty’/exp (32,814)
#2. ‘hip prosthesis’/exp (26,568)
#3. #1 OR #2 (32,814)
#4. ‘surgical infection’/exp (18,425)
#5. ‘prosthesis infection’/exp (2624)
#6. ‘sepsis’/exp (129,060)
#7. ‘bacterial infection’/exp (667,479)
#8. #4 OR #5 OR #6 OR #7 (767,273)
#9. ‘infection control’/exp (55,345)
#10. ‘infection prevention’/exp (31,360)
#11. ‘antibiotic prophylaxis’/exp (16,495)
#12. ‘antiinfective agent’/exp (1,827,014)
#13. ‘antibiotic agent’/exp (833,849)
#14. ‘intravenous’/exp AND ‘antibiotics’/exp (57,368)
#15. systemic AND ‘antibiotics’/exp (70,947)
#16. #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 (1,886,128)
#17. ‘bone cement’/exp (9336)
#18. ‘antibiotic’/exp AND ‘cement’/exp (1532)
#19. ‘antibiotic’/exp AND ‘bone’/exp AND ‘cement’/exp (340)
#20. ‘antibiotic’/exp AND impregnated (1038)
#21. ‘gentamicin bone cement’/exp (343)
#22. ‘antibiotic loaded’ AND ‘cement’/exp (204)
#23. ‘antibiotic loaded’ AND ‘bone’/exp AND ‘cement’/exp (31)
#24. #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 (10,339)
#25. ‘microclimate’/exp (30,703)
#26. ‘air conditioning’/exp (10,256)
#27. ‘operating room’/exp (15,854)
#28. ‘laminar airflow’/exp (566)
#29. laminar AND ‘air’/exp AND ‘flow’/exp AND system (29)
#30. ‘ultra clean’ AND ‘air’/exp (15)
#31. ultra AND clean AND ‘air’/exp (16)
#32. ‘ultra clean’ AND ‘air’/exp AND system (2)
#33. conventional AND operating AND room (886)
#34. conventional AND operating AND theatre (135)
#35. ‘turbulent flow’/exp (2813)
#36. #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 (49,434)
#37. #3 AND #8 AND #16 (828)
#38. #3 AND #8 AND #24 (226)
#39. #3 AND #8 AND #36 (37)
#40. #37 OR #38 OR #39 (915)
#41. #37 OR #38 OR #39 AND [english]/lim AND [2004-2011]/py (529)
#42. #37 OR #38 OR #39 AND [english]/lim AND [embase]/lim NOT [medline]/lim AND [2004-2011]/py (140)
Appendix 2 Excluded studies
| Studies excluded from the MTC | Reasons for exclusion |
|---|---|
| Bryan CS, Morgan SL, Caton RJ, Lunceford EM Jr. Cefazolin versus cefamandole for prophylaxis during total joint arthroplasty. Clin Orthop Relat Res 1988;228:117–22 | Without separating THRs from TKRs |
| Chiu FY, Lin CF, Chen CM, Lo WH, Chaung TY. Cefuroxime-impregnated cement at primary total knee arthroplasty in diabetes mellitus. A prospective, randomised study. J Bone Joint Surg Br 2001;83:691–5 | Outcome measure was TKR-related infection |
| Chiu F-Y, Chen C-M, Lin C-FJ, Lo W-H. Cefuroxime-impregnated cement in primary total knee arthroplasty: a prospective, randomized study of three hundred and forty knees. J Bone Joint Surg Am 2002;84:759–62 | Outcome measure was TKR-related infection |
| Davies AJ, Lockley RM, Jones A, el-Safty M, Clothier JC. Comparative pharmacokinetics of cefamandole, cefuroxime and cephradine during total hip replacement. J Antimicrob Chemother 1986;17:637–40 | Only compared different types of antibiotic agents |
| Davis WA, Kane JG. Antimicrobial prophylaxis for arthroplasty: a comparative study of cefonicid and cefazolin. Orthopedics 1987;10:1405–9 | Only compared different types of antibiotic agents |
| DeBenedictis KJ, Rowan NM, Boyer BL. A double-blind study comparing cefonicid with cefazolin as prophylaxis in patients undergoing total hip or knee replacement. Rev Infect Dis 1984;6(Suppl. 4):901–4 | Only compared different types of antibiotic agents |
| Doyon F, Evrard J, Mazas F, Hill C. Long-term results of prophylactic cefazolin versus placebo in total hip replacement. Lancet 1987;1:860 | We included the study by Hill et al.98 instead as it was a long-term follow-up study (both studies have the same patient population) |
| Gunst JP, Deletang S, Rogez JM, Blanloeil Y, Baron D, Dixneuf B. [Prophylactic antibiotic therapy with cefamandole in total hip surgery replacement using Charnley’s tent. A randomized study.] Pathol Biol 1984;32:567–9 | In French |
| Heydemann JS, Nelson CL. Short-term preventive antibiotics. Clin Orthop Relat Res 1986;205:184–7 | Without separating THRs from TKRs |
| Jones RN, Wojeski WV. Single-dose surgical prophylaxis using ticarcillin/clavulanic acid (Timentin): a prospective, randomized comparison with cefotaxime. Diagn Microbiol Infect Dis 1987;7:219–23 | Without separating THRs from other joint replacements. The study covered gastrointestinal, obstetrics and gynaecology, orthopaedic and other procedures with limited data for joint replacements |
| Jones RN, Wojeski W, Bakke J, Porter C, Searles M. Antibiotic prophylaxis of 1036 patients undergoing elective surgical procedures. A prospective, randomized comparative trial of cefazolin, cefoxitin, and cefotaxime in a prepaid medical practice. Am J Surg 1987;153:341–6 | Without separating THRs from other joint replacements. The study covered gastrointestinal, obstetrics and gynaecology, orthopaedic and other procedures with limited data for joint replacements |
| Jones RN, Slepack JM, Wojeski WV. Cefotaxime single-dose surgical prophylaxis in a prepaid group practice. Comparisons with other cephalosporins and ticarcillin/clavulanic acid. Drugs 1988;35(Suppl. 2):116–23 | Without separating THRs from other joint replacements. The study covered gastrointestinal, obstetrics and gynaecology, orthopaedic and other procedures with limited data for joint replacements |
| Mauerhan DR, Nelson CL, Smith DL, Fitzgerald RH Jr, Slama TG, Petty RW, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am 1994;76:39–45 | Only compared two different types of antibiotic agents |
| Mollan RA, Haddock M, Webb CH. Teicoplanin vs cephamandole for antimicrobial prophylaxis in prosthetic joint implant surgery: (preliminary results). Eur J Surg 1992;567:19–21 | Without separating THRs from TKRs |
| Periti P, Stringa G, Donati L, Mazzei T, Mini E, Novelli A. Teicoplanin – its role as systemic therapy of burn infections and as prophylaxis for orthopaedic surgery. Italian Study Groups for Antimicrobial Prophylaxis in Orthopaedic Surgery and Burns. Eur J Surg 1992;567:3–8 | Without separating THRs from TKRs |
| Periti P, Stringa G, Mini E. Comparative multicenter trial of teicoplanin versus cefazolin for antimicrobial prophylaxis in prosthetic joint implant surgery. Italian Study Group for Antimicrobial Prophylaxis in Orthopedic Surgery. Eur J Clin Microbiol Infect Dis 1999;18:113–19 | Without separating THRs from TKRs |
| Ritter MA, Campbell E, Keating EM, Faris PM. Comparison of intraoperative versus 24 hour antibiotic prophylaxis in total joint replacement. A controlled prospective study. Orthop Rev 1989;18:694–6 | Without separating THRs from TKRs |
| Soave R, Hirsch JC, Salvati EA, Brause BD, Roberts RB. Comparison of ceforanide and cephalothin prophylaxis in patients undergoing total joint arthroplasty. Orthopedics 1986;9:1657–60 | Only compared two antibiotic agents |
| Vainionpää S, Wilppula E, Lalla M, Renkonen OV, Rokkanen P. Cefamandole and isoxazolyl penicillins in antibiotic prophylaxis of patients undergoing total hip or knee-joint arthroplasty. Arch Orthopaed Trauma Surg 1988;107:228–30 | Only compared two antibiotic agents |
| Wall R, Klenerman L, McCullough C, Fyfe I. A comparison of teicoplanin and cefuroxime as prophylaxis for orthopaedic implant surgery: a preliminary report. J Antimicrob Chemother 1988;21(Suppl. A):141–6 | Without separating THRs from TKRs |
| Wollinsky KH, Oethinger M, Büchele M, Kluger P, Puhl W, Merhkens HH. Autotransfusion – bacterial contamination during hip arthroplasty and efficacy of cefuroxime prophylaxis. A randomized controlled study of 40 patients. Acta Orthop Scand 1997;68:225–30 | The purpose of the study was to examine bacterial contamination |
| Evrard J, Doyon F, Acar JF, Salord JC, Mazar F, Flamant R. Two-day cefamandole versus five-day cephazolin prophylaxis in 965 total hip replacements. Report of a multicentre double blind randomised trial. Int Orthop 1988;12:69–73 | Only compared two different types of antibiotic agents |
| Wymenga A, van Horn J, Theeuwes A, Muytjens H, Slooff T. Cefuroxime for prevention of postoperative coxitis. One versus three doses tested in a randomized multicenter study of 2651 arthroplasties. Acta Orthop Scand 1992;63:19–24 | Only compared two different doses of an antibiotic agent |
| Suter F, Avai A, Fusco U, Gerundini M, Caprioli S, Maggiolo F. Teicoplanin versus cefamandole in the prevention of infection in total hip replacement. Eur J Clin Microbiol Infect Dis 1994;13:793–6 | Only compared two different types of antibiotic agents |
| Pollard JP, Hughes SP, Scott JE, Evans MJ, Benson MK. Antibiotic prophylaxis in total hip replacement. Br Med J 1979;1:707–9 | Only compared two different types of antibiotic agents |
| Studies excluded from the MTC | Reasons for exclusion |
|---|---|
| Josefsson G, Kolmert L. Prophylaxis with systematic antibiotics versus gentamicin bone cement in total hip arthroplasty. A ten-year survey of 1688 hips. Clin Orthop Relat Res 1993;292:210–14 | This study had the same patient population as Jossefson et al.,102 but had a longer follow-up period |
| McQueen M, Littlejohn A, Hughes SP. A comparison of systemic cefuroxime and cefuroxime loaded bone cement in the prevention of early infection after total joint replacement. Int Orthop 1987;11:241–3 | This study did not report the number of THRs assigned to systemic or cement antibiotic treatment |
| Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two-stage reimplantation protocol. Clin Orthop Relat Res 1994;301:205–12 | This study could not be located in the author’s references |
| Josefsson G, Gudmundsson G, Kolmert L, Wijkström S. Prophylaxis with systemic antibiotics versus gentamicin bone cement in total hip arthroplasty. A five-year survey of 1688 hips. Clin Orthop Relat Res 1990;253:173–8 | This study had the same patient population as Jossefson et al.,102 but had a longer follow-up period |
| Pfarr B, Burri C. [Prospective study on the effect of gentamycin-Palacos in 200 total hip prostheses.] Aktuelle Probl Chir Orthop 1979;12:207–10 | In German |
| Wannske M, Tscherne H. [Results of prophylactic use of Refobacin-Palacos in implantation of endoprostheses of the hip joint in Hannover.] Aktuelle Probl Chir Orthop 1979;12:201–5 | In German |
| Buchholz HW, Engelbrecht H. [Depot effects of various antibiotics mixed with Palacos resins.] Chirurg 1970;41:511–5 | In German |
| Buchholz HW, Gartmann HD. [Infection prevention and surgical management of deep insidious infection in total endoprosthesis.] Chirurg 1972;43:446–53 | In German |
| Buchholz HW, Engelbrecht H, Rottger J, Siegel A. Erkentnisse nach Wechsel von uber 400 infizierten Huftendoprothesen. Orthop Prax 1977;12:1117–20 | In German |
| Thierse L. [Experiences with Refobacin-Palacos with regard to deep late infections following hip-joint endoprosthesis surgery. A 4-years’ study (author’s transl).] Z Orthop Ihre Grenzgeb 1978;116:847–52 | In German |
| Röttger J, Buchholz HW, Engelbrecht E, Siegel A. [Results with Refobacin-Palacos in the changing of infected prostheses. Results of prostheses exchange under cover of Refobacin-Palacos in Hamburg.] Aktuelle Probl Chir Orthop 1979;12:211–13 | In German |
| Chiu FY, Lin CF, Chen CM, Lo WH, Chaung TY. Cefuroxime-impregnated cement at primary total knee arthroplasty in diabetes mellitus. A prospective, randomised study. J Bone Joint Surg Br 2001;83:691–5 | Outcome measure was TKR-related infection |
| Chiu F-Y, Chen C-M, Lin C-FJ, Lo W-H. Cefuroxime-impregnated cement in primary total knee arthroplasty: a prospective, randomized study of three hundred and forty knees. J Bone Joint Surg Am 2002;84:759–62 | Outcome measure was TKR-related infection |
| Persson U, Persson M, Malchau H. The economics of preventing revisions in total hip replacement. Acta Orthop Scand 1999;70:163–9 | An economic evaluation study citing infection data from Lidwell et al.111 |
| Malchau H, Herberts P, Ahnfelt L. Prognosis of total hip replacement in Sweden. Follow-up of 92,675 operations performed 1978-1990. Acta Orthop Scand 1993;64:497–506 | Only investigated risk factors for revision |
| Havelin LI, Espehaug B, Vollset SE, Engesaeter LB. The effect of the type of cement on early revision of Charnley total hip prostheses. A review of eight thousand five hundred and seventy-nine primary arthroplasties from the Norwegian Arthroplasty Register. J Bone Joint Surg Am 1995;77:1543–50 | Revision was the outcome measure |
| Espehaug B, Engesaeter LB, Vollset SE, Havelin LI, Langeland N. Antibiotic prophylaxis in total hip arthroplasty. Review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg Br 1997;79:590–5 | Only investigated patient-related risk factors for early revision |
| Buchholz HW, Elson RA, Heinert K. Antibiotic-loaded acrylic cement: current concepts. Clin Orthop Relat Res 1984;190:96–108 | A semi-review rather than a primary study |
| Murray WR. Use of antibiotic-containing bone cement. Clin Orthop Relat Res 1984;190:89–95 | THRs were not separated from revisions |
| Lynch M, Esser MP, Shelley P, Wroblewski BM. Deep infection in Charnley low-friction arthroplasty. Comparison of plain and gentamicin-loaded cement. J Bone Joint Surg Br 1987;69:355–60 | The study could not be connected to the MTC network |
| Studies excluded from the MTC | Reasons for exclusion |
|---|---|
| Charnley J. Postoperative infection after total hip replacement with special reference to air contamination in the operating room. Clin Orthop Relat Res 1972;87:167–87 | Information about the use of antibiotic was unavailable |
| Berthelot P, Loulergue P, Raberin H, Turco M, Mounier C, Tran Manh Sung R, et al. Efficacy of environmental measures to decrease the risk of hospital-acquired aspergillosis in patients hospitalised in haematology wards. Clin Microbiol Infect 2006;12:738–44 | Outcome was pulmonary aspergillosis infection |
| Clark RE, Amos WC, Higgins V, Bemberg KF, Weldon CS. Infection control in cardiac surgery. Surgery 1976;79:89–96 | Outcome measure was cardiac infection |
| Davidson AI, Smylie HG, Macdonald A, Smith G. Ward design in relation to postoperative wound infection: part II. Br Med J 1971;1:72–5 | Outcome measure was wound infection in general |
| Drake CT, Goldman E, Nichols RL, Piatriszka K, Nyhus LM. Environmental air and airborne infections. Ann Surg 1977;185:219–23 | Outcome measure was wound infection in general |
| Franco JA, Baer H, Enneking WF. Airborne contamination in orthopedic surgery. Evaluation of laminar air flow system and aspiration suit. Clin Orthop Relat Res 1977;122:231–43 | THRs were not separated from TKRs, with culture bacteria being the outcome measure |
| Gruenberg MF, Campaner GL, Sola CA, Ortolan EG. Ultraclean air for prevention of postoperative infection after posterior spinal fusion with instrumentation: a comparison between surgeries performed with and without a vertical exponential filtered air-flow system. Spine 2004;29:2330–4 | Outcome measure was not THR-related infection |
| Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Stanley SJ, Lowe D. Effect of ultraclean air in operating rooms on deep sepsis in the joint after total hip or knee replacement: a randomised study. Br Med J 1982;285:10–14 | THRs were not separated from TKRs |
| Millar KJ. The impact of a new operating theatre suite on surgical wound infections. Aust N Z J Surg 1979;49:437–40 | Outcome measure was not THR-related infection |
| Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001;66:257–62 | Outcome measure was not THR-related infection |
| Sanderson MC, Bentley G. Assessment of wound contamination during surgery: a preliminary report comparing vertical laminar flow and conventional theatre systems. Br J Surg 1976;63:431–2 | THRs were not separated from joint replacements |
| Simsek SY, Bicer Y, Yapici N, Kalaca S, Aydin OO, Camur G, et al. Analysis of risk factors for sternal surgical site infection: emphasizing the appropriate ventilation of the operating theaters. Infect Control Hosp Epidemiol 2006;27:958–63 | Outcome measure was not THR-related infection |
| Wilson L. Large building air conditioning: a case for central systems. Heat Piping Air Cond 1982;54:61 | Irrelevant outcome |
| Nelson JP, Glassburn AR Jr, Talbott RD, McElhinney JP. The effect of previous surgery, operating room environment, and preventive antibiotics on postoperative infection following total hip arthroplasty. Clin Orthop Relat Res 1980;147:167–9 | Previous surgery history was suspected to be a serious confounder, masking true treatment effect, and no pertinent data were available to explain the observed difference in the incidence of infection by the authors |
Appendix 3 Study quality assessment tools
| Study type | Studies included |
|---|---|
| 1 | Meta-analysis, systematic reviews of RCTs or RCTs including cluster RCTs |
| 2 | Systematic reviews of, or individual, non-RCTs, case–control studies, cohort studies, controlled before-and-after studies, interrupted time series studies and correlation studies |
| 3 | Non-analytical studies such as case reports, case-series studies |
| 4 | Expert opinion, formal consensus |
| Study quality | Evaluation |
|---|---|
| ++ | All or most of the quality criteria have been fulfilled. Where the criteria have been fulfilled, the conclusions of the study or the review are thought to be very unlikely to alter |
| + | Some of the criteria have been fulfilled. Where the criteria have been fulfilled, the conclusions of the study or the review are thought to be unlikely to alter |
| – | Few or no criteria have been fulfilled. The conclusions of the study are thought to be likely or very likely to alter |
| Level of evidence | Explanation |
|---|---|
| 1++– | High-quality meta-analysis, systematic reviews of RCTs or RCTs (including cluster RCTs) with a very low risk of bias |
| 1+ | Well-conducted meta-analysis, systematic reviews of RCTs or RCTs (including cluster RCTs) with a low risk of bias |
| 1– | Meta-analyses, systematic reviews of RCTs or RCTs (including cluster) with a high risk of bias |
| 2++ | High-quality systematic reviews of these types of studies, or individual, non-RCTs, case–control studies, cost–benefit analysis studies and correlation studies with a low risk of confounding, bias or chance, and a high probability that the relationship is causal |
| 2+ | Well-conducted non-RCT, case–control studies, cohort studies, cost–benefit analysis studies and correlation studies with a low risk of confounding, bias or chance, and a moderate probability that the relationship is causal |
| 2– | Non-RCTs, case–control studies, cohort studies, cost–benefit analysis studies, interrupted time series and correlation studies with a high risk or chance of confounding bias, and a significant risk that that relationship is not causal |
| 3 | Non-analytical studies (e.g. case reports, case series) |
| 4 | Expert opinion, formal consensus |
| Code | Criterion | Definition |
|---|---|---|
| C1 | Random sequence generalisation Was the assignment to the treatment groups truly random? |
Adequate: sequence generalisation was truly random (computer-generated, random numbers table or coded packages) Inadequate: use of such means as alternation, case record number, birth date, etc. Unknown: no details were provided in the paper of random sequence generalisation |
| C2 | Blinding of subjects Were patients blinded to treatment allocation? |
Adequate: adequate measures were adopted to ensure patients were blinded to treatment allocation Inadequate: there was some possibility of disclosure of treatment allocation Unknown: no details were provided in the paper of treatment allocation |
| C3 | Blinding of assessors Were the assessors of the outcome blinded to treatment status? |
Adequate: actions were taken to blind assessors or outcomes so that bias is unlikely Inadequate: there may be some possibility that assessors or outcomes were not blinded Unknown: no details were provided in the text |
| C4 | Sample size Was a priori calculation of sample size undertaken? |
Yes No/not mentioned |
| C5 | Baseline characteristics and comparability Were the treatment and control groups similar at baseline in terms of prognostic factors? |
Unconfounded: treatment and control groups were comparable at baseline/or confounding were adjusted Some degree of confounding: mentioned, but not adjusted for Significant potential for confounding or confounding not discussed |
| C6 | Intention to treat Were the outcomes of subjects who withdrew described and included in the analysis (intention to treat)? |
Intention to treat: primary analysis based all randomised cases Analysis unmodified: numbers and reasons for withdraw were indicted but not considered in the analysis No mention: intention to treat not mentioned |
| C7 | Outcome assessment Was the assessment of the method of wound infection defined and applied consistently between parent groups? |
Microbiological diagnosis based on a predefined protocol Microbiological diagnosis may be included in definite criteria Clinical decision with no specific criteria or assessment methods unstated |
| C8 | Statistical analysis Was appropriate statistical analysis used? |
Appropriate statistical analysis was used It was unclear whether or not appropriate statistical analysis was used Inappropriate statistical analysis was used |
| Code | Criterion | Definition |
|---|---|---|
| 1 | Were the patients at a similar point in their disease progression? | The patients were at a similar point in their disease progression (3 points) It was unclear whether or not the patients were at a similar point in their disease progression (2 points) The patients were not at a similar point in their disease progression (1 point) |
| 2 | Were confounding variables identified and their effects adequately adjusted for? | Confounding variables were identified and their effects adequately adjusted for (3 points) It was unclear whether or not confounding variables were identified and their effects adequately adjusted for (2 points) Confounding variables were not identified and their effects were not adequately adjusted for (1 point) |
| 3 | Was bias minimised regarding the selection of cases and controls (cases and control groups comparable on all the prognostic confounding factors)? | The bias regarding the selection of cases and controls was minimised (3 points) The bias regarding the selection of cases and controls was inadequately addressed (2 points) The bias regarding the selection of cases and controls was not addressed (1 point) |
| 4 | Were outcomes assessed using objective measures or criteria (self-recall questionnaire is not)? | Outcomes were assessed using objective measures or criteria (3 points) Outcomes were assessed using limited objective measures and criteria (2 points) Outcomes were not assessed using objective measures and criteria (1 point) |
| 5 | Was outcome assessment blind to exposure status? | Outcome assessment was blind to exposure status (3 points) It was unclear whether or not outcome assessment was blind to exposure status (2 points) Outcome assessment was not blind to exposure status (1 point) |
| 6 | Was follow-up carried out over a sufficient period of time (long enough for the outcome to occur)? | Follow-up was carried out over a sufficient period of time (3 points) It was unclear whether or not follow up was carried out over a sufficient period of time (2 points) Follow-up was carried out over an insufficient period of time (1 point) |
| 7 | Were the outcomes of the patients who withdrew described and included in the analysis? | The outcomes of the patients who withdrew were described and included in the analysis (3 points) The outcomes of the patients who withdrew were unclear nor were their inclusion in the analysis (2 points) The outcomes of the patents who withdrew were not included in the analysis (1 point) |
| 8 | Was appropriate statistical analysis used? | Appropriate statistical analysis were used (3 points) It was unclear whether or not appropriate statistical analysis was used (2 points) Statistical analysis used was inappropriate (1 point) |
Appendix 4 Primary total hip replacement OPCS codes
The primary THR OPCS codes are detailed below.
W371 Primary total prosthetic replacement of hip joint using cement.
W378 Other specified total prosthetic replacement of hip joint using cement.
W379 Unspecified total prosthetic replacement of hip joint using cement.
W381 Primary total prosthetic replacement of hip joint not using cement.
W388 Other specified total prosthetic replacement of hip joint not using cement.
W389 Unspecified total prosthetic replacement of hip joint not using cement.
W391 Primary total prosthetic replacement of hip joint not elsewhere classified (NEC).
W398 Other specified other total prosthetic replacement of hip joint.
W399 Unspecified other total prosthetic replacement of hip joint.
W931 Primary hybrid prosthetic replacement of hip joint using cemented acetabular component.
W938 Other specified hybrid prosthetic replacement of hip joint using cemented acetabular component.
W939 Unspecified hybrid prosthetic replacement of hip joint using cemented acetabular component.
W941 Primary hybrid prosthetic replacement of hip joint using cemented femoral component.
W948 Other specified hybrid prosthetic replacement of hip joint using cemented femoral component.
W949 Unspecified hybrid prosthetic replacement of hip joint using cemented femoral component.
W951 Primary hybrid prosthetic replacement of hip joint using cement NEC.
W958 Other specified hybrid prosthetic replacement of hip joint using cement.
W959 Unspecified hybrid prosthetic replacement of hip joint using cement.
Appendix 5 Process information for the data linkage
| HES variable | New variable | Pseudonymised variable |
|---|---|---|
| admincat | Retained | |
| endage | Retained | |
| startage | Retained | |
| dob | Deleted | |
| dob_cfl | Deleted | |
| ethnos | Retained | |
| lopatid | Deleted | |
| newnhsno | Deleted | |
| encrypted_hesid | Deleted | |
| homeadd | Deleted | |
| sex | Retained | |
| admidate | Retained | |
| adm_cfl | Retained | |
| elecdate | Deleted | |
| elec_cfl | Deleted | |
| admimeth | Retained | |
| admisorc | Retained | |
| firstreg | Retained | |
| elecdur | Retained | |
| disdate | Retained | |
| dis_cfl | Retained | |
| disdest | Retained | |
| dismeth | Retained | |
| bedyear | Deleted | |
| spelbgin | Retained | |
| epiend | Retained | |
| epistart | Retained | |
| speldur | Retained | |
| spelend | Retained | |
| epidur | Retained | |
| epiorder | Retained | |
| epie_cfl | Retained | |
| epis_cfl | Retained | |
| epistat | Deleted | |
| epitype | Retained | |
| provspno | Deleted | |
| disreadydate | Deleted | |
| diag_01 | Retained | |
| diag_02 | Retained | |
| diag_03 | Retained | |
| diag_04 | Retained | |
| diag_05 | Retained | |
| diag_06 | Retained | |
| diag_07 | Retained | |
| diag_08 | Retained | |
| diag_09 | Retained | |
| diag_10 | Retained | |
| diag_11 | Retained | |
| diag_12 | Retained | |
| diag_13 | Retained | |
| diag_14 | Retained | |
| diag_15 | Retained | |
| diag_16 | Retained | |
| diag_17 | Retained | |
| diag_18 | Retained | |
| diag_19 | Retained | |
| diag_20 | Retained | |
| cause | Retained | |
| opertn_01 | Retained | |
| opertn_02 | Retained | |
| opertn_03 | Retained | |
| opertn_04 | Retained | |
| opertn_05 | Retained | |
| opertn_06 | Retained | |
| opertn_07 | Retained | |
| opertn_08 | Retained | |
| opertn_09 | Retained | |
| opertn_10 | Retained | |
| opertn_11 | Retained | |
| opertn_12 | Retained | |
| opertn_13 | Retained | |
| opertn_14 | Retained | |
| opertn_15 | Retained | |
| opertn_16 | Retained | |
| opertn_17 | Retained | |
| opertn_18 | Retained | |
| opertn_19 | Retained | |
| opertn_20 | Retained | |
| opertn_21 | Retained | |
| opertn_22 | Retained | |
| opertn_23 | Retained | |
| opertn_24 | Retained | |
| opdate_01 | Retained | |
| opdate_02 | Retained | |
| opdate_03 | Retained | |
| opdate_04 | Retained | |
| opdate_05 | Retained | |
| opdate_06 | Retained | |
| opdate_07 | Retained | |
| opdate_08 | Retained | |
| opdate_09 | Retained | |
| opdate_10 | Retained | |
| opdate_11 | Retained | |
| opdate_12 | Retained | |
| opdate_13 | Retained | |
| opdate_14 | Retained | |
| opdate_15 | Retained | |
| opdate_16 | Retained | |
| opdate_17 | Retained | |
| opdate_18 | Retained | |
| opdate_19 | Retained | |
| opdate_20 | Retained | |
| opdate_21 | Retained | |
| opdate_22 | Retained | |
| opdate_23 | Retained | |
| opdate_24 | Retained | |
| operstat | Deleted | |
| posopdur | Retained | |
| preopdur | Retained | |
| classpat | Deleted | |
| intmanig | Retained | |
| mainspef | Retained | |
| tretspef | Deleted | |
| domproc | Retained | |
| HRGLATE | Deleted | |
| HRGLATE35 | Deleted | |
| hrgnhs | Retained | |
| hrgnhsvn | Retained | |
| suscorehrg | Retained | |
| sushrg | Retained | |
| sushrgvers | Retained | |
| susspellid | Deleted | |
| procode | Deleted | |
| procode3 | Deleted | |
| procodet | Deleted | |
| protype | Retained | |
| susrecid | Deleted | |
| epikey | Deleted | |
| nhsnum_trans | Generated | |
| hesid_trans | Generated | |
| susrec_trans | Generated | |
| epikey_trans | Generated | |
| procode-trans | Generated | |
| procode3_trans | Generated | |
| procodet_trans | Generated | |
| age at operation | Generated (whole years, round down) | |
| HES variable critical care | ||
| ccstartdate | Retained | |
| ccdisdest | Retained | |
| ccdisloc | Retained | |
| ccdisdate | Retained | |
| susrecid | Deleted | |
| susrecid_trans | Generated | |
| PROMs variable | New variable | Pseudonymised variable |
|---|---|---|
| PROMS_SERIAL_NO | Deleted | |
| PROMS_PROC_CODE | Retained | |
| PROMS_PROC_GROUP | Retained | |
| PATIENT_DEATH | Retained | |
| COMPLETE | Retained | |
| Q1_COMPLETE | Retained | |
| Q1_COMPLETED_DATE | Retained | |
| Q1_ASSISTED | Retained | |
| Q1_ASSISTED_BY | Retained | |
| Q1_SYMPTOM_PERIOD | Retained | |
| Q1_PREVIOUS_SURGERY | Retained | |
| GENDER | Retained | |
| Q1_LIVING_ARRANGEMENTS | Retained | |
| Q1_GENERAL_HEALTH | Retained | |
| Q1_DISABILITY | Retained | |
| Q1_PROCODE | Deleted | |
| Q2_COMPLETE | Retained | |
| Q2_COMPLETED_DATE | Retained | |
| Q2_ASSISTED | Retained | |
| Q2_ASSISTED_BY | Retained | |
| Q2_SURGERY_DATE | Retained | |
| Q2_ALLERGY | Retained | |
| Q2_URINE | Retained | |
| Q2_BLEEDING | Retained | |
| Q2_WOUND | Retained | |
| Q2_READMITTED | Retained | |
| Q2_FURTHER_SURGERY | Retained | |
| Q2_SATISFACTION | Retained | |
| Q2_SUCCESS | Retained | |
| Q2_LIVING_ARRANGEMENTS | Retained | |
| Q2_GENERAL_HEALTH | Retained | |
| Q2_DISABILITY | Retained | |
| EQ5D_INDEX_CHANGE | Retained | |
| EQ5D_SCALE_CHANGE | Retained | |
| Q1_EQ5D_PROFILE | Retained | |
| Q1_EQ5D_INDEX | Retained | |
| Q1_EQ5D_HEALTH_SCALE | Retained | |
| Q1_MOBILITY | Retained | |
| Q1_SELF_CARE | Retained | |
| Q1_ACTIVITY | Retained | |
| Q1_DISCOMFORT | Retained | |
| Q1_ANXIETY | Retained | |
| Q2_EQ5D_PROFILE | Retained | |
| Q2_EQ5D_INDEX | Retained | |
| Q2_EQ5D_HEALTH_SCALE | Retained | |
| Q2_MOBILITY | Retained | |
| Q2_SELF_CARE | Retained | |
| Q2_ACTIVITY | Retained | |
| Q2_DISCOMFORT | Retained | |
| Q2_ANXIETY | Retained | |
| Q1_EQ5D_SCALE_COMPLETE | Retained | |
| Q1_EQ5D_PROFILE_COMPLETE | Retained | |
| Q2_EQ5D_SCALE_COMPLETE | Retained | |
| Q2_EQ5D_PROFILE_COMPLETE | Retained | |
| SCORE_CHANGE | Retained | |
| HR_Q1_SCORE | Retained | |
| HR_Q1_SCORE_COMPLETE | Retained | |
| HR_Q2_SCORE | Retained | |
| HR_Q2_PAIN | Retained | |
| HR_Q2_SUDDEN_PAIN | Retained | |
| HR_Q2_NIGHT_PAIN | Retained | |
| HR_Q2_WASHING | Retained | |
| HR_Q2_TRANSPORT | Retained | |
| HR_Q2_DRESSING | Retained | |
| HR_Q2_SHOPPING | Retained | |
| HR_Q2_WALKING | Retained | |
| HR_Q2_LIMPING | Retained | |
| HR_Q2_STAIRS | Retained | |
| HR_Q2_STANDING | Retained | |
| HR_Q2_WORK | Retained | |
| HR_Q2_SCORE_COMPLETE | Retained | |
| HESID_MATCHED | Retained | |
| HESID_RANK | Retained | |
| EPISODE_MATCHED | Retained | |
| EPISODE_MATCH_RANK | Retained | |
| Q2_MATCHED | Retained | |
| Q2_MATCH_RANK | Retained | |
| STATUS_DATE | Retained | |
| MODIFIED_DATE | Retained | |
| EQ5D_VERSION | Retained | |
| Q1_SCORE_COMPLETE | Retained | |
| Q1_LANGUAGE | Retained | |
| Q1_SCAN_DATE | Retained | |
| Q1_EQ5D_VERSION | Retained | |
| Q2_SCORE_COMPLETE | Retained | |
| Q2_FORM_VERSION | Retained | |
| Q2_LANGUAGE | Retained | |
| Q2_SCAN_DATE | Retained | |
| Q2_EQ5D_VERSION | Retained | |
| STATUS | Retained | |
| EPIKEY | Deleted | |
| HES_YEAR | Deleted | |
| HES_SCHEMA | Deleted | |
| procode_trans | Generated | |
| epikey_trans | Generated |
| ONS variable | New variable | Pseudonymised variable |
|---|---|---|
| dod (Date of death) | Deleted | |
| sex | Retained | |
| cause_of_death | Retained | |
| encrypted_hesid | Deleted | |
| hesid_trans | Generated | |
| Age at death | Generated (whole years, round down) | |
| Time primary to death | Generated (whole months, round down) | |
| Time revision to death | Generated (whole months, round down) | |
| Time debridement to death | Generated (whole months, round down) | |
| Flag: default date used | Generated (Y/N) |
| PHE SSI variable | New variable | Pseudonymised variable | Comment |
|---|---|---|---|
| SurveyDataID | Deleted | ||
| PatientName | Deleted | ||
| PatientSurname | Deleted | ||
| NHSNumber | Deleted | ||
| SerialNumber | Deleted | ||
| HospitalCode | Deleted | ||
| HospitalName | Deleted | ||
| HospitalOrgCode | Deleted | ||
| NHSTrustName | Deleted | ||
| NHSTrustCode | Deleted | ||
| HPARegion | Deleted | ||
| ParticipationPeriod | Retained | ||
| PeriodStartDate | Retained | ||
| PeriodEndDate | Retained | ||
| Category | Retained | ||
| Gender | Retained | ||
| DateOfBirth | Deleted | ||
| DateHospitalAdmission | Retained | ||
| DateOperation | Retained | ||
| WeightKG | Retained | ||
| HeightCM | Retained | ||
| PrimaryIndication | Retained | ||
| OPCSCode1 | Retained | ||
| OPCSDescr1 | Retained | ||
| OPCSCode2 | Retained | ||
| OPCSDescr2 | Retained | ||
| OPCSCode3 | Retained | ||
| OPCSDescr3 | Retained | ||
| RevisionOfHipProsthesis | Retained | ||
| surgerytype | Retained | ||
| AntibioticCement | Retained | ||
| AntimicrobialProphylaxis | Retained | ||
| ASAScore | Retained | ||
| Woundclass | Retained | ||
| DurationOperation | Retained | ||
| SurgeonGrade | Retained | ||
| SurgeonCode | Deleted | ||
| SurgeonCode2 | Deleted | ||
| RiskIndex | Retained | ||
| ProstheticImplant | Retained | ||
| MultipleSurgProced | Retained | ||
| ReasonSurvDiscont | Retained | ||
| PDDdate | Retained | Date inpatient surveillance stopped | |
| PDDquestComplete | Retained | Patient questionnaire completed | |
| PatientReviewed | Retained | Patient reviewed as part of systematic post discharge | |
| PDDCompleteDate | Retained | Date patient questionnaire completed | |
| PatReviewedDate | Retained | Date of review as part of systematic post discharge surveillance | |
| PatientPDQGiven | Retained | Patient given questionnaire | |
| Calculated BMI | Retained | ||
| Detection | Retained | ||
| DateOnset | Retained | ||
| SSIType | Retained | ||
| SSIIncisionalType | Retained | ||
| OrganismCode1 | Retained | ||
| OrganismCode2 | Retained | ||
| OrganismCode3 | Retained | ||
| Criteria | Deleted | ||
| nhsnum_trans | Generated | ||
| hospitalcode_trans | Generated | ||
| age at operation | Generated (whole years, round down) | ||
| surv_id_trans | Generated | ||
| ssi_comb recid | Generated |
| NJR variable | New variable | Pseudonymised variable |
|---|---|---|
| PrimaryNJRIndexNo | Deleted | |
| PrimaryProcedureID | Deleted | |
| PrimaryDB | Retained | |
| PrimaryLocalID | Deleted | |
| PatientForenames | Deleted | |
| PatientSurname | Deleted | |
| PatientGender | Retained | |
| PatientDOB | Deleted | |
| AgeAtPrimary | Retain and round down | |
| Postcode | Deleted | |
| NHSNumber | Deleted | |
| PrimaryBMI | Retained | |
| PrimaryASA | Retained | |
| Joint | Retained | |
| Side | Retained | |
| PrimaryProcedureType | Retained | |
| PrimaryPatientProcedure | Retained | |
| Approach | Retained | |
| nhstrustcode | Deleted | |
| Trust | Deleted | |
| Hospital | Deleted | |
| organisationcode | Deleted | |
| Organisation_Type | Retained | |
| LeadSurgeonGrade | Retained | |
| FirstAssisitantGrade | Deleted | |
| PrimaryOpDate | Retained | |
| IndForImp_Osteoarthritis | Deleted | |
| IndForImp_AvascularNecrosis | Deleted | |
| IndForImp_OtherInflammatoryArthropathy | Retained | |
| IndForImp_IndicationOther | Retained | |
| IndForImp_Previousinfection | Retained | |
| CementComponentType | Retained | |
| CementImplantType | Retained | |
| StemCentralizerImplantType | Delete | |
| StemCentralSectionImplantType | Delete | |
| FemoralCanalPlugImplantType | Delete | |
| TaperAdapterImplantType | Delete | |
| OutcomeType | Retained | |
| PrimaryToOutcomeYears | Retained | |
| AgeAtDeath | Retain and round down | |
| RevisionNJRIndexNo | Delete | |
| RevisionProcedureID | Delete | |
| RevisionDate | Retained | |
| RevisionProcedureType | Retained | |
| RevisionPatientProcedure | Retained | |
| IndForRevHip_Infection | Retained | |
| Laminar_Flow_Theatre | Retained | |
| minimally_invasive_surgery_used | Deleted | |
| image_guided_surgery_used | Deleted | |
| patient_position | Retained | |
| incision_approach | Retained | |
| surgical_approach | Retained | |
| IndForRevHip_Other | Retained | |
| IndForRevHip_Other_text | Retained | |
| RevisionDB | Retained | |
| nhsnum_trans | Generated | |
| orgcode_trans | Generated |
Appendix 6 International Classification of Diseases, Tenth Edition T-codes: complications of surgical and medical care
The ICD-10 T-codes are detailed below.
T845 Infection and inflammatory reaction due to internal joint prosthesis.
T846 Infection and inflammatory reaction due to internal fixation device.
T847 Infection and inflammatory reaction due to other internal orthopaedic prosthetic devices, implants and grafts.
T857 Infection and inflammatory reaction due to other internal prosthetic devices, implants and grafts.
T814 Infection following a procedure.
T813 Disruption of wound, not elsewhere classified.
Appendix 7 Treatment path codes/combinations
We are interested only in treatment paths related to deep infection. Revisions that occur for other reasons are not relevant to the model.
Hierarchy rules (where several procedures occur on the same date)
Debridement, antibiotics and implant retention plus any other code from section Hip revision codes or Excision codes. The latter takes precedence ≥ one-stage revision, part of a two-stage or an excision.
One-stage revision and two-stage revision, the latter takes precedence ≥ two-stage revision. However, to qualify as a two-stage procedure, the code in question must be followed by, or subsequent to, another relevant code on a different date.
2 × DAIR codes are collapsed into one ≥ DAIR.
The ICD-10 code in 3.B.3 (Y831) was used only to corroborate revision procedures and does not indicate a separate procedure if there are other relevant codes present on the same date. If there are no other revision codes, Y831 can be used to indicate a revision.
For debridement, antibiotics and implant retention classification
Any code from section Debridement (owing to deep infection) codes. A DAIR may be carried out as part of another revision procedure on the same date. Where this is the case, the procedure should be classified accordingly (i.e. not as a DAIR alone).
For one-stage revision classification
Any code from section Hip revision codes. To classify as a one-stage revision, the procedure in question must not be followed by any other revision procedure. A patient may have several one-stage procedures.
For two-stage revision classification
We used the following codes to identify clear cases of two-stage operations: Y703 – first stage of staged operation; and Y711 – subsequent stage of staged operation. If these codes are not present, there must be two revision codes on two different dates and the two stages must occur within 180 days of each other. If the subsequent procedure does not occur within this time frame (and Y703/Y711 is not present) the procedures are to be classified as two one-stage operations.
For stage 1-only classification
We used W572* – primary excision arthroplasty of joint NEC; Y037* – removal of prosthesis from organ NOC (not otherwise classified); W394^ – attention to total prosthetic replacement of hip joint NEC; and W954^ – attention to hybrid prosthetic replacement of hip joint using cement NEC (^ may indicate DAIR or part of a two-stage revision, these codes are often followed by another revision code).
For stage 1 or 2 classification
We used any code from Hip revision codes. These are the same codes used to define a one-stage revision and so should only be classified as part of a two-stage revision if the rules for the two-stage revision are satisfied.
For permanent excision classification
We used any code from section Excision codes followed by any other revision or DAIR procedure on a subsequent date. An excision procedure may be performed as part of a two-stage revision. Where this is the case, it should be classified as part one of a two-stage revision, not a permanent excision. DAIR may be performed at the same time as an excision. Where this occurs, patients are to be classified into the permanent excision category.
Hip revision codes
W373 Revision of total prosthetic replacement of hip joint using cement.
W374 Revision of one component of total prosthetic replacement of hip joint using cement.
W383 Revision of total prosthetic replacement of hip joint not using cement.
W384 Revision of one component of total prosthetic replacement of hip joint not using cement.
W393 Revision of total prosthetic replacement of hip joint NEC.
W395 Revision of one component of total prosthetic replacement of hip joint NEC.
W933 Revision of hybrid prosthetic replacement of hip joint using cemented acetabular component.
W943 Revision of hybrid prosthetic replacement of hip joint using cemented femoral component.
W953 Revision of hybrid prosthetic replacement of hip joint using cement NEC.
Y032* Renewal of prosthesis in organ NOC.
Excision codes
Y037* Removal of prosthesis from organ NOC.
W572* Primary excision arthroplasty of joint NEC.
Debridement (owing to deep infection) codes
W801* Open debridement and irrigation of joint.
W802* Open debridement of joint NEC.
W808* Other specified debridement and irrigation of joint.
W809* Unspecified debridement and irrigation of joint.
Appendix 8 International Classification of Diseases, Tenth Edition organism codes
The ICD-10 organism codes are detailed below.
B951 Streptococcus, group B, as the cause of diseases classified elsewhere.
B954 Other streptococci as the cause of diseases classified elsewhere.
B956 Staphylococcus aureus as the cause of diseases classified elsewhere.
B957 Other staphylococci as the cause of diseases classified elsewhere.
B958 Unspecified staphylococci as the cause of diseases classified elsewhere.
B961 Klebsiella pneumoniae as the cause of diseases classified elsewhere.
B962 Escherichia coli (E. coli) as the cause of diseases classified elsewhere.
B964 Proteus (mirabilis) (morganii) as the cause of diseases classified elsewhere.
B965 Pseudomonas (aeruginosa) (mallei) (pseudomallei) as the cause of diseases classified elsewhere.
B966 Bacteroides fragilis (B. fragilis) as the cause of diseases classified elsewhere.
B968 Other specified bacterial agents as the cause of diseases classified elsewhere.
Appendix 9 Daily death probabilities from Office for National Statistics
| Age (years) | Probability |
|---|---|
| 13 | 0.0000002 |
| 14 | 0.0000003 |
| 15 | 0.0000004 |
| 16 | 0.0000005 |
| 17 | 0.0000008 |
| 18 | 0.0000009 |
| 19 | 0.0000010 |
| 20 | 0.0000010 |
| 21 | 0.0000010 |
| 22 | 0.0000010 |
| 23 | 0.0000011 |
| 24 | 0.0000011 |
| 25 | 0.0000012 |
| 26 | 0.0000012 |
| 27 | 0.0000013 |
| 28 | 0.0000014 |
| 29 | 0.0000015 |
| 30 | 0.0000016 |
| 31 | 0.0000017 |
| 32 | 0.0000018 |
| 33 | 0.0000019 |
| 34 | 0.0000021 |
| 35 | 0.0000022 |
| 36 | 0.0000024 |
| 37 | 0.0000026 |
| 38 | 0.0000029 |
| 39 | 0.0000031 |
| 40 | 0.0000034 |
| 41 | 0.0000036 |
| 42 | 0.0000039 |
| 43 | 0.0000042 |
| 44 | 0.0000047 |
| 45 | 0.0000050 |
| 46 | 0.0000053 |
| 47 | 0.0000056 |
| 48 | 0.0000062 |
| 49 | 0.0000068 |
| 50 | 0.0000073 |
| 51 | 0.0000082 |
| 52 | 0.0000091 |
| 53 | 0.0000099 |
| 54 | 0.0000108 |
| 55 | 0.0000119 |
| 56 | 0.0000132 |
| 57 | 0.0000142 |
| 58 | 0.0000156 |
| 59 | 0.0000170 |
| 60 | 0.0000187 |
| 61 | 0.0000204 |
| 62 | 0.0000221 |
| 63 | 0.0000236 |
| 64 | 0.0000259 |
| 65 | 0.0000282 |
| 66 | 0.0000320 |
| 67 | 0.0000350 |
| 68 | 0.0000378 |
| 69 | 0.0000428 |
| 70 | 0.0000482 |
| 71 | 0.0000527 |
| 72 | 0.0000583 |
| 73 | 0.0000632 |
| 74 | 0.0000708 |
| 75 | 0.0000778 |
| 76 | 0.0000872 |
| 77 | 0.0000972 |
| 78 | 0.0001091 |
| 79 | 0.0001211 |
| 80 | 0.0001365 |
| 81 | 0.0001543 |
| 82 | 0.0001733 |
| 83 | 0.0001951 |
| 84 | 0.0002190 |
| 85 | 0.0002443 |
| 86 | 0.0002732 |
| 87 | 0.0003043 |
| 88 | 0.0003379 |
| 89 | 0.0003815 |
| 90 | 0.0004225 |
| 91 | 0.0004581 |
| 92 | 0.0004991 |
| 93 | 0.0005393 |
| 94 | 0.0006037 |
| 95 | 0.0006618 |
| 96 | 0.0007234 |
| 97 | 0.0007765 |
| 98 | 0.0008306 |
| 99 | 0.0008870 |
| 100 | 0.0009455 |
Appendix 10 How evidence was found for health utilities
Search strategy
The first step was to define key components in order to focus the review. Commonly, these are identified as the population, interventions and outcomes of interest. 155
Population
People aged > 18 years who underwent primary THA surgery in hospital/other acute care setting and did/did not develop an infection within the 12 months post surgery.
Interventions
No deep SSI ≤ 12 months post primary THA, deep SSI ≤ 12 months post primary THA treated with:
-
permanent resection (Girdlestone arthroplasty)
-
DAIR
-
one-stage revision
-
two-stage revision
-
no infection (successful treatment) after initial treatment for SSI post THA.
Outcomes
Quality of life (instrument and scores).
The following search terms used were combinations of medical subject headings with relevant keywords. A librarian with experience in systematic reviews was consulted for advice in regards to search terms and strategies.
Medical subject headings
‘Arthroplasty, Replacement, Hip’, ‘Arthroplasty, Replacement’, ‘Reoperation’, ‘Infection’, ‘Hip joint – surgery’, ‘Surgical Wound Infection’, ‘Prosthesis-Related Infections’, ‘Quality of Life’, ‘Mortality’.
Keywords
Quality of life, utility, QoL, QALY, SF-36, SF-12, EQ-5D, infection, surgical site infection, SSI, prosthetic joint infection, PJI revision, revision arthroplasty, arthroplasty, hip, total hip arthroplasty, hip replacement, joint replacement, debridement, DAIR, resection, Girdlestone, cost-effectiveness, CEA, CUA, mortality, death, outcomes.
Databases searched
EBSCOhost: Academic Search Elite; Australia/New Zealand Reference Centre; Cumulative Index to Nursing and Allied Health Literature; MEDLINE; EconLit; The Cochrane Library; The Cochrane Database of Systematic Reviews; Database of Abstracts of Reviews of Effectiveness; The Cochrane Central Register of Controlled Trials; The Cochrane Database of Methodology Reviews; Information about the Cochrane Collaboration; Health Technology Assessment database; NHS Economic Evaluation Database; and PubMed.
The following selection criteria were applied for the selection of relevant articles:
Inclusion criteria
Published between January 2000 and July 2012; involving an adult patient group (as defined by study); assessment of health outcomes related to interventions described above; quality of life reported as Short Form questionnaire-12 items, Short Form questionnaire-36 items, 15D HRQoL, Short Form questionnaire-6 Dimensions or EQ-5D; language is English; accessible in full (not only abstract).
Exclusion criteria
Procedures related to hip fracture.
Review process
The literature searches retrieved 108 relevant titles, of which all abstracts were reviewed. As a result, 66 articles were excluded and reasons for exclusion of each study were recorded. The most common reason for exclusion was not reporting quality of life but instead other outcomes after THA like treatment failure or success rates (Figure 36). The full content of the remaining 42 articles was reviewed and another 18 articles were excluded thereafter. The review revealed a total of 24 papers matching the inclusion criteria. Of these, 10 reported mortality outcomes, eight reported quality-of-life outcomes and six reported economic evaluations using both quality-of-life and mortality outcomes. In a next step, the quality of these studies was assessed and put in hierarchical order.
FIGURE 36.
Review process of articles assessing health outcomes. HUI, Health Utilities Index; QoL, quality of life; WOMAC, Western Ontario and McMaster University Osteoarthritis Index.

List of abbreviations
- APC
- admitted patient care
- CrI
- credible interval
- DAIR
- debridement, antibiotics and implant retention
- DIC
- deviance information criterion
- EQ-5D
- European Quality of Life-5 Dimensions
- HES
- Hospital Episode Statistics
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HSCIC
- Health and Social Care Information Centre
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- MRSA
- meticillin-resistant Staphylococcus aureus
- MTC
- mixed-treatment comparison
- NEC
- not elsewhere classified
- NICE
- National Institute for Health and Care Excellence
- NJR
- National Joint Registry
- NMB
- net monetary benefit
- NOC
- not otherwise classified
- ONS
- Office for National Statistics
- OPCS
- Office of Population, Censuses and Surveys, Classification of Surgical Operations and Procedures
- OPCS-4
- Office of Population, Censuses and Surveys, Classification of Surgical Operations and Procedures – version 4
- OR
- odds ratio
- PHE
- Public Health England
- PROM
- patient-reported outcome measure
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SSI
- surgical site infection
- SSISS
- Surgical Site Infection Surveillance Service
- T
- treatment
- THA
- total hip arthroplasty
- THR
- total hip replacement