Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/30/02. The contractual start date was in April 2014. The draft report began editorial review in July 2015 and was accepted for publication in November 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
During this study Simon Lalor was an employee of Opcare, a company that provides orthotic and prosthetic services to the UK NHS. This company does not manufacture orthotic devices, although a sister company ORTHO C FAB does. Cynthia Iglesias is a member of the National Institute for Health and Care Excellence Medical Technologies Assessment Committee and member of the European Clinical Research Infrastructure Network.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by O’Connor et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Introduction
This project was undertaken in response to a commissioning brief from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) to address the question of which devices are in use in the UK NHS for instability of the knee in adults with neuromuscular disease (NMD), which conditions these devices are used for, and what further research is needed. The purpose of the commissioned research was to inform development of a future substantive research question to assess the clinical effectiveness and cost-effectiveness of different types of orthotic management of the knee in people with neuromuscular and central nervous system (CNS) diseases.
Neuromuscular disease
The term ‘NMD’ encompasses a heterogeneous group of conditions, and terminology can vary. Neurology practice in the UK recognises NMDs as conditions that primarily affect the peripheral nerve, muscle and/or neuromuscular junction. Hilton-Jones et al. 1 describe the term as covering any condition caused by dysfunction of the motor unit (the motor nerve and the muscle it controls). They identify four anatomical sites: the anterior horn cell/motor neuron (e.g. poliomyelitis and motor neurone disease); the peripheral nerve [e.g. Charcot–Marie–Tooth (CMT) disease]; the neuromuscular junction (e.g. myasthenia gravis) and the muscle (e.g. muscular dystrophy). 1 There is a wide variety of pathologies, motor impairments and comorbidities across these neuromuscular disorders, for instance peripheral nerve conditions, which may be sensory as well as motor; neuromuscular junction conditions, for which there may be a large element of variability and physiological fatigue; and muscle conditions, which will vary in comorbidities such as cardiomyopathy and respiratory impairment. The exact muscle groups affected will vary both between and within individual conditions. However, there are common factors, particularly muscle weakness and fatigue, which affect mobility and lower limb function.
The term NMD is sometimes used in a more inclusive way, encompassing upper motor neuron conditions that have a common end point of affecting muscle function. This definition would therefore include CNS disorders, such as multiple sclerosis and stroke. Clinical management of people with primarily CNS conditions will often differ from the conditions described above because of the effect of upper motor neuron features on lower limb function. For example, spasticity will influence the prescription of orthoses in conditions such as multiple sclerosis. Likewise, in patients who have experienced a stroke, issues such as spasticity, neglect and spatial awareness will have an impact, plus there is acute onset usually with improvement and plateau. At the request of the commissioners, the term NMD is used inclusively to also encompass knee instability that related to CNS conditions, and both groups of conditions are included in this research.
Knee instability in neuromuscular disease
There are several mechanisms that may lead to knee instability in NMD. The knee is a polycentric hinge joint that also has a rotatory action, with articular surfaces between the femur and the tibia, and the patella and femur. The muscle groups that have a direct effect on the knee are the knee extensors, comprising the quadriceps femoris and tensor fasciae latae, and the knee flexors that include the hamstring group – sartorius, gracilis and gastrocnemius. Weakness in any of these muscles is one mechanism that may lead to knee instability. This would be particularly common in muscle conditions that are predominantly limb girdle in origin; peripheral nerve conditions that affect these muscles, such as diabetic amyotrophy or poliomyelitis; or in upper motor neuron conditions that affect the lower limb. This could be unilateral or bilateral, according to condition. Weakness in these muscle groups, or in more remote muscle groups, might also lead to secondary impairment of the tendons, ligaments and cartilage, such as laxity and contracture, associated with the knee due to altered alignment and redistribution of force across the joints and soft tissue. Muscle weakness, or overactivity remote from the muscles directly affecting the knee, may also cause knee instability due to the secondary effects on posture, such as excessive plantar flexion leading to abnormal anterior progression of the ground reaction force under the foot.
Sensory impairment can affect joint control where proprioceptive loss may reduce awareness of the knee position. Reduced pain or pressure perception may also increase skin vulnerability to excessive pressure or friction, for example from an orthosis. Knee instability itself may be in any of the planes in which the knee can move, that is, anteroposterior, medial–lateral and rotational (transverse plane).
In the case of CNS conditions, spasticity in the muscles acting around the knee can also cause knee instability. For example, spasticity in the gastrocnemius, which causes the foot to plantarflex in stance, shifts the ground reaction force anterior to the knee. Over time, this overstretches the posterior capsule of the knee joint, causing the knee to hyperextend. Similarly, spasticity in the hamstring muscles causes the knee to be flexed in stance phase, inducing knee flexion and associated joint instability.
Orthotic devices for knee instability
Knee instability due to muscle weakness or ligamentous laxity is often treated using orthoses. The commonly stated goals of lower limb orthoses are to improve the ability and quality of walking, protect, stabilise and improve function. 2
A knee–ankle–foot orthosis (KAFO) is usually prescribed when other forms of bracing, such as an ankle–foot orthosis (AFO) or knee orthosis (KO), are insufficient to adequately control knee instability due to weakness or joint laxity. 2 KAFOs span the knee, ankle and foot to stabilise the joints and assist safe ambulation. There are many types of KAFO designs. ‘Conventional’ KAFOs, made of metal and leather, have been used for centuries. Modern KAFOs, made from thermoplastics or carbon fibre composites, are lighter and fit more closely, potentially affording better control of the limb. Modern KAFOs tend to combine plastic and metal components, commonly polypropylene for calf and thigh shells and shoe inserts; aluminium, magnesium, titanium or steel for uprights; and steel for joints. 3 Devices can also vary in the type of knee joint, type of knee locking mechanism, types of knee pads and bands and whether or not there is frontal plane control. 3
Historically, KAFOs were either entirely locked or entirely unlocked at the knee. 4 Most KAFOs incorporate knee joints that lock the knee straight during walking and unlock only when the user sits. Using a KAFO with a locked knee requires the individual to alter their gait to allow their foot to clear the ground in the swing phase of walking. Polycentric knee joints can be locked or unlocked and allow a more natural knee motion, although they have more moving parts, require more maintenance and are therefore more expensive. 3 They can also be heavier and bulkier, require the wearer to have voluntary hip extension and can be problematic when walking on uneven surfaces. Recent years have seen the introduction of stance control knee joint technology, through which mechanical or microprocessor controlled knee joints allow the knee to flex during the swing phase of walking but lock when the knee is extended during stance phase of walking and when weight is borne through the leg to provide stability to the knee in order to allow a more normal walking pattern. These are generally known as stance control KAFOs (SCKAFOs). KAFOs can be worn unilaterally or bilaterally as required.
Hip KAFOs (HKAFOs) are KAFOs that extend across the hip joint connecting to a pelvic band or lumbar/thoracic spinal support. 4 Bilateral KAFOs are linked via hip joints with flexion stops and a release mechanism for sitting. 4 Hip guidance orthoses (HGOs) and reciprocating gait orthoses (RGOs) are examples of HKAFOs with different locking/unlocking mechanisms. Ambulation with these devices by individuals with extensive paresis or paralysis of the lower limbs usually requires additional walking aids, such as crutches or walking frames. 4 These devices were originally designed for patients with higher-level spinal cord dysfunction, who might otherwise not have been able to walk. 5 Modifications of RGOs include the advanced RGO (ARGO) and the isocentric RGO (IRGO). 5
Knee stability can also be improved by the use of knee braces or KOs, or in some cases by a type of AFO known as a ground reaction AFO (GRAFO). An AFO provides direct control of the ankle and foot, and is used to support mobility in people who experience joint instability, muscle weakness or muscle spasticity at the ankle joint. However, it can also provide indirect control of the knee and hip, and may be of benefit for knee instability. Quality Improvement Scotland has issued a Best Practice Statement on the use of AFOs following stroke. 6 The statement recommends that assessment for the orthotic device should be undertaken jointly by a specialist orthotist and specialist physiotherapist, and that the design specification should be based on biomechanical principles and desired functional outcomes of the patient.
The above devices can be prefabricated/off-the-shelf or fully custom-made. Some off-the-shelf devices may be customised to the individual. The clinical effectiveness of the various orthotic options for knee instability related to NMD is unclear. Diagnosis is thought to be a poor predictor of the type of orthosis that will be most effective. Orthosis design should be driven by the specific biomechanical impairments of each individual patient, the rehabilitation goals of the multidisciplinary team (MDT) and the patient’s own goals. In addition to technical and structural considerations to ensure stability and safety, the clinician who is fitting the device needs to understand the goals of the orthosis within a patient’s living and working circumstances. 7 Devices are generally fitted for the individual patient, and the correct supply and fitting of orthotic devices is important to allow a patient to manage his or her condition or prevent future problems.
Factors to be considered when prescribing and fitting a device are the type of deformity or instability present, the biomechanical deficit to be addressed, patient weight and activity level, and lifestyle issues. Patients with significant fixed deformity may benefit from a weight-relieving brim in the orthosis, so that some of the patient’s weight can be offloaded from the affected leg. The biomechanical deficit will influence the way that forces are applied to the limb by the orthosis, the trimlines of the orthosis and the type of materials chosen. Weight and activity level will influence material choice, including the type of side bars and joints used. An important consideration when prescribing and fitting a device is whether off-the-shelf orthoses will meet the needs of the patient or a bespoke device is required. This decision will be influenced by the size and body habitus of the patient, the need to control certain movements as a result of the weakness in the limb, and the experience and skill of the orthotist in fabricating a device.
Most orthotic devices are classified as class 1 (lowest level of risk) with a Conformité Européenne (CE) mark. 8 There is a legal requirement for class 1 devices to meet the requirements of the European Union Medical Device Directive. Following registration with the Medicines and Healthcare products Regulatory Agency (MHRA) and self-declaration, the CE mark (which declares conformity with the Medical Devices Directive) can then be put on the product. A CE mark is not required for custom-made orthoses, although they must meet the requirements of the relevant sections of the Medical Devices Regulations. 9 A custom-made device is defined by MHRA as being ‘manufactured specifically in accordance with a written prescription of a registered medical practitioner, or other authorised person . . . which gives under his responsibility, specific characteristics as to its design; and intended for the sole use of a particular patient’. 9 The prescription can take the form of a letter or a moulded impression of the shape of device required, with a request to ‘make as pattern’. 9
Provision of orthoses
Orthotists in the NHS work closely with several clinical specialties, ranging from orthopaedics to diabetes care to rheumatology, and they provide services to people with a range of other conditions as well as NMD. There are an estimated 1.2 million people using orthotic services in the NHS. 10 An Audit Commission report in 2000 found that in most hospital trusts, orthotics services were small-scale, with an annual expenditure of < £500,000, although there were also a small number of trusts with an expenditure of > £2M. 11 The Audit Commission report11 estimated that in the study sites, 8% of orthotic expenditure was on KAFOs, 9% on HKAFOs and 1% on AFOs. Lower limb orthosis repair accounted for 2% of expenditure. They found a wide variation in the prices quoted by different suppliers for identical products: across four suppliers the cost of a KAFO ranged from £390 to £650 and the cost of an AFO ranged from £40 to £130 (approximate values read from graph). 11
Orthotics services are normally provided in secondary care using a number of different models of provision: in-house service employing a NHS orthotist; employing a NHS orthotist in conjunction with neighbouring trusts; outsourcing the service to a commercial supplier; or a mix of public and private provision. 11 Based on a survey of 150 orthotics managers in acute trusts, the Audit Commission found that 20% employed their own orthotist. 11
Physiotherapists, neurologists and rehabilitation medicine physicians are also involved in the prescription of orthoses for people with NMD. Physiotherapists are often the first professionals who may prescribe orthoses. These tend to be off-the-shelf devices. For more complex situations, the physiotherapist will refer to, and liaise with, an orthotics clinic. Some clinics have a specialist physiotherapist as part of the team but this is not universal. A survey, in 2004–5, of clinicians running specialist clinics for adults with NMD found that the availability of specialist orthotics in the form of an orthotist with experience in NMDs was low in the 32 clinics surveyed. 12
Patient perspective
There is uncertainty about the acceptability of these devices to patients, the extent to which prescribed devices are used, and the factors that determine their usage. Information on acceptability and use has not been collated systematically in relation to people with neuromuscular conditions that lead to knee instability. The lack of a consistent and reliable method of reporting on the daily use of KAFOs after the patient leaves the clinic or laboratory setting has been highlighted. 2
Phillips et al. 13 explored perceptions and experiences of the disadvantages and benefits associated with use of AFO in 15 people with CMT disease, using individual interviews and nominal group technique. Barriers to use that were identified related to the functional use of the AFO (such as lack of mobility in tight spaces); discomfort when wearing the device, including rubbing and digging into the skin; concerns about the appearance of the device and its potential for drawing attention to disability; and problems with finding suitable footwear. The main perceived advantages of wearing the AFO were linked to improvements in walking. Vinci and Gargiulo14 explored acceptability of AFOs to a study sample of 8 male and 17 female participants with CMT disease, who had severe bilateral foot drop, and who had been prescribed AFOs at least 4 months before the start of the study. Results from this qualitative interview study indicated that adherence to the prescribed AFOs was poor, with only five people (20% of the sample) wearing the devices. Reasons for not wearing AFOs were that patients preferred to manage without them because they felt that the AFOs highlighted their disability, the AFOs were uncomfortable, and they had difficulty finding footwear to accommodate the devices. A third study15 explored the differences in presentation and gait function of people with CMT disease who wore AFOs for daily mobility (n = 11) and a group who did not (n = 21). Six of the non-users had been prescribed AFOs but chose not to wear them. Their physical characteristics resembled the non-AFO group more than the AFO group for measures of muscle strength and disease severity. The authors of this study15 concluded that severity of presentation seems to determine whether or not people with CMT disease will use AFOs, and the timing of prescription may accordingly assist with acceptability of the devices.
Garralda et al. 16 explored the views and adjustment of families with a child with Duchenne muscular dystrophy in relation to use of KAFOs. The authors conducted interviews with 17 parents and 9 children (all boys aged 8–18 years) seeking views about the use of KAFOs. Findings from the study revealed the emotional significance for parents of the introduction of KAFOs as an indicator of illness deterioration and re-affirmation of the severity and life-limiting nature of the disorder. Most parents expressed satisfaction with the use of KAFOs, but some wished to have had more discussion about practical aspects beforehand.
In a trial of AFOs,17 only 50% of stroke patients actually wore their orthoses. Long-term rejection rates of KAFOs have been found to be high in people with paraplegia and with spinal cord lesions. 7 One study suggested that a patient selection and training programme could help target the orthoses at those who were more likely to have successful outcomes. 18 The importance of appropriate training with the orthosis and associated assistive devices has been previously emphasised. 19 It has been suggested that rejection might, in some instances, be due solely to lack of appropriate gait training. 2 The need for training for orthosis users in the care of the orthosis and care of the skin to avoid adverse effects has also been recommended. 19
Other problems that have been cited include auditory distraction, memory of old leather and steel orthoses for patients with poliomyelitis, and damage to clothing. 20 If a patient has unrealistic and unfilled expectations then this may lead to abandoning the orthosis. 2 A patient’s prior history with orthoses and any reasons for previous failure may be important. 2
In summary, the existing research literature relating to patients’ perceptions and experiences of using orthoses for knee instability is limited in several respects. Studies that have been conducted to date are mainly small-scale; some do not include in-depth exploratory techniques, such as face-to-face interviews, and existing studies are not fully representative of the diverse populations with NMD and CNS conditions for whom prescription of an orthosis may be an appropriate measure.
Previous research on effectiveness
Scoping searches were undertaken prior to commencing the current review to establish whether or not a systematic review had been previously undertaken. No previous systematic reviews were identified that assessed the effectiveness of orthotic devices in the specific population of interest: knee instability in adults with NMD and CNS disorders. A systematic review conducted in 2013 (searches to November 2011) found that an AFO fitted in patients following stroke had a statistically significant effect on ankle kinematics, knee kinematics in stance phase, kinetics and energy cost, although not on knee kinematics in swing phase, hip kinematics or energy expenditure. However, the focus was on gait biomechanics, and the effects and acceptability of long-term usage have not been evaluated. 21 A Cochrane review22 has investigated any intervention for CMT disease and identified a single controlled study of foot orthoses (FOs); however, the device was not being used for knee instability – although FOs can influence the alignment of the knee on the coronal plane, they do not correct sagittal plane knee instability. A review of non-surgical interventions for people with CMT disease did not identify any studies evaluating an orthotic device. 23 The literature searches for these reviews were undertaken in 2007 and 2006, respectively, and therefore need to be updated.
An overview of the evidence on KAFOs and HKAFOs for all conditions in 20064 concluded that the level of evidence was generally low and consisted mainly of small study sample sizes and inadequate study design, although these aspects were not addressed in any detail. Two Cochrane reviews24,25 were identified which assessed interventions for ankle instability or reduced range of motion in the ankle in people with NMD, but did not investigate knee instability. A review published in 2012 (searching to 8 November 2010) of stance control orthoses (SCOs) for any condition found benefits of SCOs in comparison with locked KAFOs but these studies had methodological limitations. 26
Aims and objectives
The project aimed to address the commissioned research question of which orthoses are in use in the NHS for instability of the knee, for which NMD and CNS disorders, and what further research is needed. There were four objectives.
-
Assess the evidence base for the effectiveness of orthotic devices for management of instability of the knee in adults who have NMD or a CNS disorder To meet this objective, we undertook a systematic review of the best available evidence on the effectiveness of orthotic devices in this population.
-
Identify the types of orthotic devices currently being provided by the NHS for the management of instability of the knee in adults with NMD or a CNS disorder, the frequency of their use and their cost To meet this objective, we conducted a survey of orthotists and physiotherapists, and undertook a costing analysis of orthoses for knee instability.
-
Identify the most important outcomes for patients To meet this objective, we used qualitative research methods to collate the views of people with NMD or a CNS disorder, who have been fitted with an orthotic device for knee instability.
-
Identify any implications for clinical practice, any gaps in the evidence and future research needs To meet this objective, we interrogated and integrated the three sources of evidence: health-care professionals (HCPs), patients and the systematic review.
Chapter 2 Methods
Overview
To address the research objectives we undertook (1) a systematic review of the effectiveness of orthotic devices for management of instability of the knee in adults with NMD or CNS disorders; (2) a survey of orthotists and physiotherapists and a costing analysis; and (3) a qualitative study of the perspective of users of orthotic devices. Details of the methods used for each component are outlined below.
Systematic review
The systematic review was designed to identify and evaluate the best available evidence on orthotic devices for the management of instability of the knee in adults with NMD or CNS disorders. We undertook it following the principles recommended by CRD guidance27 and we have reported the review following PRISMA guidelines. 28 The protocol was registered with PROSPERO, registration number CRD42014010180.
Selection criteria and searching
In order to identify all relevant evidence, we formulated the following selection criteria.
Population Adults (≥ 16 years) with a neuromuscular disorder, who have impaired walking ability due to instability of the knee. Neuromuscular disorders included conditions that primarily affect the peripheral nerve, muscle and neuromuscular junction, for example motor neurone disease, muscular dystrophy, myasthenia gravis, spinal muscular atrophy, CMT disease, poliomyelitis, myopathies and inclusion body myositis. Knee instability related to CNS conditions was also included, for example spinal cord injury and stroke.
Intervention Orthoses with the clinical aim of controlling knee instability, for example KAFOs, AFOs and KOs or mixed designs. Orthoses of any design or material, custom or prefabricated; locked knee joint, eccentric knee joint or stance control design (KAFO), with and without an electronic component, were eligible. Studies evaluating the use of functional electrical stimulation (FES) were excluded.
Comparator Studies using any of the above orthoses as a comparator, including studies comparing different designs of the same orthosis, or no intervention.
Outcomes Studies reporting any of the following outcomes were eligible for the review:
-
condition-specific or generic patient-reported outcomes measures assessing function, disability, independence, activities of daily living, quality of life or psychosocial outcomes
-
pain
-
walking ability
-
other functional ability, for example sit to stand, short turns in confined spaces
-
biomechanical analysis
-
adverse effects, for example tissue damage, falls
-
usage
-
patient satisfaction and the acceptability of a device
-
resource utilisation data, such as number of follow-up appointments, device malfunction or other problems.
Study design Randomised controlled trials (RCTs) and other study designs, with and without a comparator group, were eligible for the review such as non-randomised controlled studies, before-and-after studies and case series. There was no minimum study size. Owing to the risk of bias, non-RCTs have limitations in providing robust evidence about the effectiveness of interventions; however, there are relatively few RCTs undertaken in this field. In addition, a key focus of our research was to inform future research. Therefore, a broad range of study designs was included to provide a comprehensive overview of the research available.
Studies were eligible provided that the orthosis had been used in a real-life setting (i.e. not solely in a laboratory/experimental setting). Outcomes could be assessed in a laboratory or clinic setting; however, participants had to have had the opportunity to have used the device outside that setting. Studies entirely in the laboratory setting focusing on biomechanical outcomes have an important role in identifying effective devices; however, they are at an earlier stage of development. Although the clinical trial phases for pharmaceuticals do not directly translate across to devices, the entirely laboratory-based studies are closer to a Phase II pharmaceutical trial than a Phase III trial investigating efficacy or effectiveness.
Published and unpublished studies from any country and reported in any language were eligible for inclusion in the review.
Search strategy
We developed a comprehensive search strategy to ensure that all of the relevant sources of data were located. For full details of the search strategies used see Appendix 1. Searches were designed in conjunction with an experienced information specialist.
We searched MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, Cumulative Index to Nursing and Allied Health, EMBASE, PASCAL, Scopus, Science Citation Index, BIOSIS Previews, Physiotherapy Evidence Database, Recal Legacy, Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), HTA database and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception.
Information on studies in progress, unpublished research or research reported in the grey literature was identified by searching the Conference Proceedings Citation Index: Science, Health Management Information Consortium, ClinicalTrials.gov, International Clinical Trials Registry Platform and the National Technical Information Service (NTIS). Selected websites were also searched: the International Society for Prosthetics and Orthotics, British Association of Prosthetists & Orthotists (BAPO), American Orthotic & Prosthetic Association and the American Academy of Orthotists and Prosthetists, and the associated Journal of Prosthetics and Orthotics.
Searches were run in May 2014 by the information specialist. No limits on date, language or study design were applied in any of the searches. We carried out an update of the searches in November 2014 in all the above databases, with the exception of BIOSIS, PASCAL, Recal Legacy and NTIS. The same procedures as above were followed.
The reference lists of all included studies, any related systematic reviews and key background papers were checked to identify any further relevant studies. The results of all searches were imported into EndNote bibliographic software (version XVII, Thomson Reuters, CA, USA) and deduplicated.
Two researchers independently screened the bibliographic references in EndNote for relevance, based on the inclusion criteria. The full texts of any potentially relevant papers were ordered. Full papers were loaded into EPPI-Reviewer 4 software (version 4.6.0.1, EPPI-Centre, Social Science Research Unit, Institute of Education, University of London) and read to determine relevance. Reasons for exclusion of studies were documented in EPPI-Reviewer. Disagreements were resolved through discussion and consultation with another member of the project team if necessary. Authors were contacted if eligibility was uncertain from the information provided in the publication.
Data extraction
A data extraction form was developed in EPPI-Reviewer. This was piloted on a small number of studies and adjusted as necessary. Guidelines on its use were produced to enhance consistency among the team. Data from multiple publications of the same study (linked papers) were extracted and reported as a single study. Data extracted included details of the study methods, country, patient characteristics, intervention, comparators, analysis methods and results. Data were extracted as stated by authors and not transformed in any way. Between-group differences were extracted from studies with a comparator. For studies without a comparator, pre- and post-intervention data were extracted.
Data were extracted by one researcher and checked by a second researcher. Studies in languages other than English were extracted by a native speaker who was also a researcher. These were checked by a second researcher for consistency only. We planned to extract data to allow calculation of between-group differences and confidence intervals; however, as a result of the generally poor reporting of data, it was not possible to consistently do this across studies. When data were available, these were extracted; when the appropriate data were not reported, the description of the results provided in the paper was extracted and the lack of summary data was noted.
Assessment of risk of bias
The Cochrane risk-of-bias criteria were used to assess included RCTs. 29 There is a lack of consensus about how risk of bias should be assessed in non-randomised studies, and there is no gold-standard tool for assessing risk of bias of case series or other observational designs. 30,31 However, there is a broad consensus in the methodological literature that selection bias and confounding are key sources of bias in observational study designs. 30,31 We used a similar approach to Siegfried et al. 32 for non-randomised studies with a control group. These studies were assessed for external validity, performance bias, detection bias and selection bias/control of confounding based on eight criteria (gender, age, cause of muscle weakness, presence of sensory disturbance, whether or not the orthosis was used for proximal or distal muscle weakness, previous use of an orthosis, acclimitisation time and type of orthosis used) (see Appendix 2). There are no consensus criteria for assessment of case series. The criteria used were adapted from the assessment of controlled studies and criteria used in a previous systematic review. 33 Assessment of risk of bias was undertaken independently by two researchers (except for non-English-language studies). Discrepancies were resolved by discussion.
Analysis and synthesis
Data extracted from the studies were tabulated and discussed in a narrative synthesis grouped by condition then by type of orthosis and outcome, in conjunction with an assessment of the quality of the studies. Given the diversity of the studies and the insufficient data, neither a meta-analysis nor planned subgroup analyses on the presence of proximal or distal weakness and the presence of sensory disturbance could be undertaken.
Outcomes have been grouped under categories: patient-reported outcomes included measures of satisfaction, measures of functionality and usage of the orthotic device. Objective assessments were those conducted in a laboratory or clinic setting and usually involved gait analysis, including walking ability, energy consumption and muscle activity. Resource utilisation included measures of device function including breakages and cost. Adverse event data were also recorded.
Qualitative study of patient views
This exploratory qualitative study aimed to:
-
explore perceptions relating to acceptability, effectiveness and usage of orthoses
-
identify important outcomes among people who have been fitted with an orthotic device for knee instability across a broad range of diverse NMDs
-
explore the factors influencing the perceived likelihood of achieving the outcomes for those individuals within the context of their condition and care pathway.
Ethics considerations
The study received research ethics (REC reference 14/LO/1132) and governance approvals to recruit patients through NHS sites. Research governance approval was sought and obtained from the University of York’s Department of Health Sciences’ Research Governance Committee in order to recruit study participants via patient support groups. The study was also registered with the ISRCTN registry (ISRCTN 65240228). All study participants were given verbal and written information relating to the study aims and their involvement. Written consent was obtained prior to interviews and participants were given assurances concerning the confidentiality and anonymity of their responses. Participants were also reassured that their care would not be affected in any way whether or not they decided to take part in an interview, and it was made clear that they could withdraw from the study at any time.
Study design
A qualitative in-depth interview study was carried out to elicit peoples’ views and experiences of using an orthotic device, perceptions of the treatment they had received and views of treatment goals and outcomes.
Twenty-four people were recruited for interview from both in and outside the NHS, using a topic guide that was planned to reflect the research aims and objectives. A patient adviser was consulted to assist with design of the interview topic guide. This adviser helped to guide the development of the interview schedule by commenting on the relevance of content, comprehensiveness, the ‘flow’ of questioning and language used. The aim was to develop an instrument sufficiently structured to ensure consistency in information gathering but flexible enough to allow participants to recount their individual experiences. 34
The study protocol included a proposal to undertake a small number of focus groups (2–4) with a sample of participants experiencing a particular neuromuscular condition with instability of the knee (poliomyelitis was identified as a condition of interest). Focus groups were not undertaken for two main reasons. First, half of the study sample (12 participants) had a diagnosis of poliomyelitis and it was therefore not clear that focus groups would have added to the information obtained from the individual interviews. Second, study participants indicated a strong preference to be interviewed in their own home, as attending a focus group could pose problems related to their personal mobility or family or work commitments. Analysis of the data from the 12 in-depth interviews with participants with poliomyelitis suggests that data saturation was achieved in the categories of interest; that is to say, no new findings emerged from the later interviews. 35 We are, therefore, reassured that it is unlikely that focus groups would have yielded new insights.
Conduct of qualitative interviews
Most (n = 21) interviews with participants were conducted by the researchers (DM) and (CJ) in participants’ homes or workplace; three interviews were conducted by telephone when this was more convenient for the participant and/or researcher. The majority of interviews lasted around 1 hour, although some were shorter or longer, and all were audio-recorded. In a number of cases, the participant’s spouse was present during the interview and participated to a greater or lesser extent.
Data sources
Study sites
NHS patients and members of patient support groups were purposively sampled to include people with different types of NMD, including CNS conditions. Participants were recruited from three different regions in England: one to the north, one in the middle of the country, and one to the south, referred to as sites 1, 2 and 3, respectively. Regional variations exist between the nature and organisation of orthotic services offered in the three different sites, ranging from large, specialist units with a high throughput of patients, to services provided through smaller units based in local hospitals or attached to rehabilitation centres.
The orthotic service in research site 1 is a large department operating out of six hospital sites across an urban and suburban area serving a population of 800,000 people. Approximately 10,000 items are dispensed each year from a range of appointments from single-handed orthotist-led clinics through to multidisciplinary clinics run in combination with orthopaedic or rehabilitation medicine consultants. The service also provides in-reach to the inpatient wards as well as domiciliary visits. The service is commissioned by a consortium of Clinical Commissioning Groups (CCGs) in the region and is hosted by the acute hospital Trust, although provided by an independent company. The company has an orthotic manufacturing unit in the city. A parallel orthotic service, located in general practitioner (GP) surgeries, also exists in the city and is commissioned by each CCG independently. This latter service tends to see patients with less complicated needs that can be met by a single-handed orthotist.
Research site 2 offers orthotic services across a range of clinical settings, commissioned by the local CCG. Approximately 7000 items are dispensed each year, which range from KAFOs for patients who are new to the service, and include AFOs, insoles and footwear, including footwear adaptations, repeat orders and repairs. Around 10,000 appointments are offered annually – a mix of new patients and review appointments – as well as those including supply of an orthosis. Appointments vary from 15 to 60 minutes, depending on complexity. The team of orthotists deliver care through hospital sites, as well as outreach clinics. An in-house manufacturing workshop is available at one of the sites, and specialises in plastics (AFO and KAFO) and insoles. Referrals are via GPs and consultants within the local Trust, with some specialist pathways to enable podiatry and physiotherapy referral as part of the MDT.
Research site 3 is located within the boundaries of a major city in the south of England, where specialist orthotic services are provided across a range of locations, including a local hospital and a number of clinics. Patients included in the study accessed orthotic care via a team of orthotists specialising in complex rehabilitation problems, many of which require a multidisciplinary approach. Orthotists work closely with a MDT, including physiotherapists, consultants and podiatrists, to enhance care pathways and treatment programmes and promote the best outcomes and patient experience. Referrals to the service are accepted from GPs, consultants and members of the allied health team. This specialist service benefits from an on-site workshop for the manufacture, maintenance and adaptation of orthotic products.
Sampling and recruitment of participants
Participants were recruited from a range of sources to ensure that a wide range of views was captured, namely through the charities, such as the British Polio Fellowship and Charcot–Marie–Tooth UK, as well as through NHS orthotics clinics. Purposive sampling36 was used to select participants for interview, to reflect a range of conditions: age, sex, length of time fitted with an orthosis, high and low usage, and living in different regions in England.
Inclusion criteria for the study were adults (≥ 16 years for NHS participants; > 18 years of age for non-NHS participants) with a neuromuscular disorder who have impaired walking ability primarily due to instability of the knee. Neuromuscular disorder included conditions that primarily affect the peripheral nerve, muscle and neuromuscular junction, for example motor neurone disease, muscular dystrophy, myasthenia gravis, spinal muscular atrophy, CMT disease, poliomyelitis, myopathies and inclusion body myositis. People with knee instability that was related to CNS conditions were also included, for example spinal cord injury, spina bifida and stroke. Participants were people who were able to give informed consent. Principal exclusion criteria were aged < 16 years, people with neuromuscular disorders other than those described above, and people who were unable to give informed consent because of cognitive impairment or for other reasons.
Two methods of recruitment were used, one in and one outside the NHS.
-
NHS recruitment Clinicians were requested to approach patients with NMD who have been fitted with an orthosis to ask if they might be interested in taking part in the study. If a patient expressed an interest, the clinician partner requested written permission to pass his or her telephone number to the qualitative researcher using a confidential, password-protected ‘drop-off’ system in operation at the University of York. The clinician gave the patient a copy of the participant information sheet containing further details of the study. The patient information sheet explained why the interviews were being conducted and what was involved in taking part in the study. After a period of no shorter than 5 days, patients were contacted by the researcher to provide further details and to find out if they would like to be involved in the study. If so, a mutually convenient date and location were arranged for an interview to take place. Nineteen patients were recruited in this way. Two patients contacted declined to take part: one expressed no further interest in being involved; the second patient’s general condition had suddenly deteriorated so he or she was unable to participate.
The study protocol included a proposal of a second method of recruitment by NHS HCPs, by sending out letters of invitation to patients they considered eligible to take part in the study, along with a participant information sheet. However, we did not resort to this method of recruitment, as we were able to obtain an adequately sized study sample using our (preferred) method whereby the clinician approached the patient in person. Moreover, identifying suitable patients for the study through searching clinical records would have been very difficult, as record systems are based on a record of the patient’s clinical condition rather than on causes underlying difficulties with walking, such as knee instability.
-
Non-NHS recruitment Information about the Orthotics for Knee Instability (OKIS) study was provided to the chairpersons/lead representatives of the British Polio Fellowship, Charcot–Marie–Tooth UK, the FSH (facioscapulohumeral muscular dystrophy) Support Group UK and the Muscular Dystrophy Campaign, with a request to forward this to their members, who were invited to contact the qualitative researcher directly. The response to this method of recruitment was lower than anticipated; of the six people who contacted the researcher seeking further information, five agreed to take part in an interview.
Modes of analysis/interpretation
Audio-recordings of interviews were fully transcribed. A random sample of six transcripts was checked by three researchers (LD, CJ and DM) for accuracy. Data were analysed for thematic content following sequential steps of familiarisation with the data by reading and rereading transcripts; development of a coding scheme and attribution of data to individual codes; collating codes into potential themes; and interpretation through seeking meaning, salience and connections. 37 Data handling and retrieval was assisted by the use of the computer software package NVivo, which also enabled intra (within an individual’s responses) and inter (across the whole data set) case comparison. 38 To promote rigour in analysis, the three qualitative researchers involved in the study (LD, CJ and DM) cross-checked coding procedures in a sample (6) of the early transcripts, followed by discussion and modification of the coding framework where necessary, during the initial phases of data analysis. The coding frame, therefore, evolved and expanded to include relevant categories of interest.
The analytic approach used was both systematic and iterative. 35 An inductive approach was undertaken in order to identify themes in a ‘bottom-up’ way as opposed to a theoretical or ‘top-down’ manner. 37 Deviant cases (those that appear to contradict emerging themes) were actively sought throughout the analysis to ensure full interrogation of the data. 35 The Braun and Clarke37 checklist of criteria for a good thematic analysis provided a guide during the analytic and interpretative processes.
The study protocol suggested that, if appropriate, data from the in-depth interviews would also be examined on a case-by-case basis using phenomenological research methods to examine the perspective and experience of the individual in relation to their own condition and care experience. 39,40 These methods have not been used in this final report, although they may be incorporated into future publications arising from the study, in order to capture the ‘lived experience’ of individuals using orthoses in day-to-day life. The aim of this report is rather to present a more general overview of the data, which broadly represents the perspectives of all study participants, although data from particular individuals will be presented in more depth when this seems warranted.
Survey of health-care professionals
We undertook a web survey of orthotists, physiotherapists and doctors in rehabilitation medicine in order to address the second aim of the project, to identify the types of orthoses currently being used by the NHS for the management of instability of the knee in adults with NMD and CNS disorders, the frequency of their use, the resources required to provide them and the care pathways of these patients. To obtain data for costing orthotic devices, telephone interviews with orthotists were also undertaken.
Survey
The target population was orthotists, physiotherapists and doctors in rehabilitation medicine, within the UK, who provide care to NMD and/or CNS patients with knee instability. The sample frame was membership lists of the following:
-
Association of Chartered Physiotherapists Interested in Neurology (ACPIN)
-
BAPO
-
the British Society of Rehabilitation Medicine (BSRM).
This may be an incomplete sampling frame, as the relevant health professionals may not all be members of these organisations. Conversely, not all members of these organisations will be treating the patient population of interest. Because of the way orthotic services are arranged in the NHS, with orthotists in some hospitals employed by private organisations and others by the NHS, as well as variations in which directorate orthotic services come under in different hospitals, any other approach to identifying these professionals would be very resource intensive. Based on previous work in this area, and the knowledge of the steering group members, regional variations are expected in the types of orthotic devices being prescribed and the care pathways of patients. Therefore, a web survey of membership of the relevant professional organisations was chosen to obtain as wide a geographical coverage as possible.
Response rates in surveys of HCPs vary greatly. A recent systematic review and meta-analysis estimated a mean response rate of 38% by health professional to online surveys. 41 An earlier systematic review reported response rates ranging from 9% to 94%. We have taken a conservative estimate of 30% to estimate what an appropriately powered sample would be. 42 The primary purpose of the survey was to provide descriptive information on current NHS practice and the sample size was been calculated on that basis. 43 Based on a 95% confidence level and a 10% margin of error (which would seem reasonable, given the exploratory nature of the survey) the estimated minimum sample size required was 96 and, assuming a more ideal 5% margin of error, the sample size required was 384.
Ethical approval for the web survey, the telephone interviews and focus group used to inform development of the questionnaire and follow-up interviews for costing devices was sought from and granted by the University of York’s Department of Health Sciences’ Research Governance Committee. As per this approval, the personal information collected from these sessions was stored securely, accessible only to the research team and will be securely destroyed after 5 years.
Questionnaire development
To inform the survey questionnaire, two focus groups were planned, to include orthotists, physiotherapists and doctors in rehabilitation medicine. Two focus groups were planned in two separate geographical locations. Recruitment to the focus groups was facilitated by members of the steering group. An invitation e-mail, provided in Appendix 3, was sent to potential focus group members. Potential participants who expressed an interest were subsequently e-mailed an information sheet, provided in Appendix 4, detailing the length of the session, and data protection and storage information.
The focus group for the first geographical location took place with 11 HCPs in attendance. Unfortunately, a suitable date for all participants could not be reached for the second location, so approval for telephone interviews was obtained from the Research Governance Committee. Four telephone interviews were subsequently undertaken. Informed consent was sought from all participants for the focus group and the telephone interviews (see Appendix 5). Both the focus group and the telephone interviews followed the topic guide presented in Appendix 6. Orthotists, physiotherapists, doctors in rehabilitation medicine and a gait scientist participated in these discussions. Participants were asked to discuss orthoses provision to patients with a NMD or a CNS condition with knee instability, around the following:
-
types of orthoses
-
referral and care pathways
-
patient and treatment
-
factors influencing effectiveness and acceptability.
During these discussions, it became clear that patients with a NMD or CNS condition and knee instability are a diverse group. This introduces variation in the types of orthoses being prescribed, referral mechanisms, care pathways and HCPs involved in the care of these patients. The barriers to referral for orthotics services were also raised as an issue. Discussion highlighted the substantial level of individualised care and the personalised nature of the devices that is required for optimal treatment.
The data collected from this consultation, and feedback from the Advisory Group and research team, were used to develop and enhance the planned survey. The questionnaire is presented in Appendix 7, with the following section headings:
-
demographic characteristics of respondents
-
patient demographic
-
patient referrals
-
initial assessment
-
prescription and fitting of orthotic devices
-
types of devices
-
treatment outcomes and acceptability factors
-
additional requests (which included a request for any audit, service evaluation or other type of data and an invitation to the orthotists to take part in telephone interviews focusing on the cost of orthotic devices).
Qualtric® software (Qualtrics LLC, Provo, UT, USA) was used to distribute the survey. The questions were ordered to follow a typical care pathway for a patient and distributed over 78 screen pages – one question per page. In an attempt to reduce the number of questions, adaptive questioning was used throughout the questionnaire – details are available in Appendix 7. Respondents had to answer each question, in turn, in order to progress; some questions included a non-response option such as ‘not applicable’. No consistency or completion checks were undertaken before the questionnaire was submitted (this was not possible with the software used). Respondents were able to review and change their answers using a ‘previous’ and ‘next’ button, and monitor their progress through the questionnaire on a ‘% completed’ bar.
The survey was piloted on HCPs and the Advisory Group members: usability and technical functionality was tested by project team members and colleagues.
Survey distribution
The survey was distributed to respondents as an open survey link, via an e-mail invitation from their relevant organisation. The information necessary to make informed consent was included in the invitation e-mail (see Appendix 8) and participation was taken as implied consent. Participation was voluntary and no incentives were offered or passwords required for completion of the questionnaire. The first distribution letter was e-mailed in November 2014; reminder e-mails were sent at 2 and 4 weeks after distribution and the survey was closed in January 2015.
The survey was advertised on the NHS Orthotics Network forum and the NHS Orthotics Manager Network forum (see Appendix 9) and blogs posted on the project blog site, encouraging HCPs to complete the survey when they received the link.
The results collated in the Qualtric software were downloaded into Microsoft Excel® (2010 version, Microsoft Corporation, Redmond, WA, USA). All responses that were collected for each question were analysed, with the response rate for each question calculated. The length of time taken by respondents to answer questions was not collected and therefore no cut-off points were used. No adjustments were made to adjust for the non-representativeness of our sample. To help compensate for the incomplete sampling frame, those respondents who stated that they did not treat patients with a NMD or CNS condition and knee instability skipped to the end of the survey, and provided only their demographic profile to the survey results.
We have followed the Checklist for Reporting Results of Internet E-Surveys. 44
Costing analysis
Following on from the survey of HCPs, an analysis to estimate the cost of orthotic devices currently being used by the NHS for the management of instability of the knee in adults with NMD and CNS disorders was undertaken. As per the study protocol, the costing analysis involved estimating two components:
-
the resources (e.g. staff and materials) required to provide orthotic devices to patients with NMD and CNS conditions with knee instability
-
the unit costs associated with these resource-use estimates.
Identification of resource-use estimates
The target population to estimate the resources required to provide orthotic devices to patients with NMD and CNS conditions with knee instability were orthotists. Therefore, the information gathered from the survey of HCPs, which included orthotists, was used, where appropriate. This was supplemented by telephone interviews with orthotists, looking specifically at the resources required to provide orthotic devices. These orthotists were recruited through the survey of HCPs. At the end of the questionnaire, orthotists were asked if they would be willing to take part in a telephone interview at a later date (Q65). Respondents were also asked if they had any audit, service evaluation or other type of data, which could be shared with the project team (Q63, Q64). This question elicited some contact with HCPs but did not result in any data being obtained.
Ethical approval for the telephone interviews to estimate the resource-use requirements for costing devices was sought from, and granted by, the University of York’s Department of Health Sciences’ Research Governance Committee. As per this approval, the personal information collected from these sessions was stored securely, accessible only to the research team and will be destroyed after 5 years. Five orthotists e-mailed the research team, were sent a participant information sheet and consent form, and agreed to take part in these interviews. Four telephone interviews were subsequently undertaken. Informed consent was sought from the four participants. The telephone interviews followed the topic guide presented in Appendix 6. The four telephone interviewees were all male, and all worked in a hospital setting. Two interviewees were based in the south of England, one interviewee was based in the north of England, and one interviewee worked as a locum.
The original project protocol stated that the semistructured interviews would be structured around a series of patient profiles and would cover the key types of orthotics used for knee instability in our patient population. However, the patients’ interviews and the HCPs’ focus groups, telephone interviews and survey, highlighted not only that patients with NMD and/or CNS conditions with knee instability are a diverse group of patients, but that the devices being prescribed to them are also diverse and the prescription process undertaken to provide this patient population with the appropriate device is relatively complex. Custom-made devices could be considered to be a personalised medicine, in that although the components of the various devices (e.g. KAFOs) available may be fairly similar, there is the potential for a KAFO to be unique to each patient for whom it is prescribed. Therefore, in the same way that the survey became more detailed, the telephone interviews for the costing analysis became more focused and the scope of the discussions was reduced.
Given the complexity of the prescription process and the number of variables that need to be taken into account to accurately cost orthotic devices for this patient population, a more detailed costing exercise was undertaken. To begin quantifying the cost of a personalised device, the materials required and the determinants of the quantity of these materials required needed to be estimated. Given these additional information requirements, the interviewees were asked to provide estimates only for a KAFO. The KAFO was chosen as the orthotic device of most interest because of the project’s original commissioning brief; the KAFO is also one of the commonly prescribed orthotic devices for this patient population according to our survey. Respondents were asked to consider the following KAFOs:
-
‘conventional’, which are made from conventional materials, such as metal and leather
-
‘cosmetic’, which are made from materials such as carbon fibre and thermoplastic
-
‘hybrid’, which are a combination of the materials used in conventional and cosmetic KAFOs.
Given the volume of information required from these telephone interviews, it was not possible to discuss the resources required to provide KAFOs to particular groups and so patient profiles were not used.
As per the topic guide, the orthotists were asked to discuss orthotic provision to patients with NMD and CNS conditions with knee instability around the following:
-
materials required to manufacture KAFOs
-
staff required to provide KAFOs
-
overheads and types of orthotic service provision in the UK
-
opportunity cost of not prescribing a KAFO to our patient population of interest.
The orthotists were asked to consider costs and resource-use estimates from a NHS perspective only, as per the topic guide.
Identification of cost estimates
As stated in the project protocol, up until 2010, the NHS Purchasing and Supply Agency (PASA) was the main health service purchasing organisation and would have provided estimates for the device costs under consideration in this report. Enquiries were made to NHS Supply Chain, the organisation into which NHS PASA was subsumed. Enquiries were also made to private manufacturer/suppliers of orthotic devices. However, the replies received indicated that price lists could not be made available because of commercial confidentiality agreements. Expert opinion was also sought, for unit cost estimates. The expert opinion provided to the project, was from a senior orthotist, who worked in a large hospital setting where orthotic devices are manufactured on site.
Patient and public involvement
We developed an advert and role description (available from authors) to recruit two patients with NMD or CNS conditions with experience of using orthoses for knee problems to join the project Advisory Group. We approached several voluntary organisations to disseminate the opportunity among members: Charcot–Marie–Tooth UK, the Stroke Association, the Spinal Injuries Association, Muscular Dystrophy UK, and the British Polio Fellowship. One individual joined our Advisory Group. At the outset our view was that it was important that we had input from individuals with specific experience of using the devices of interest, as there was very little information available to us about patient perspectives. This limited the pool of people available to us locally, and distance, as well as daytime meetings for people who worked, was a barrier. However, the expertise our public member brought about orthotic devices and how services operate was essential; in particular, their views were sought on the qualitative study and the survey questionnaire. At their suggestion they joined the meeting by Skype™ (Microsoft Corporation, Redmond, WA, USA) and we undertook some work by e-mail. In future projects we would be clearer in advertisements about the possibility of communicating in various ways in addition to, or instead of, face to face. We also undertook various public engagement activities (see Chapter 7), which created the opportunity to speak with individuals from the Yorkshire and Humber Muscle Group and the Polio Survivors Network in the early stages of the project.
Chapter 3 Results of systematic review
Study selection
The search strategies and allied searching identified 4516 references (including update searches). Titles and abstracts were screened and full copies of 532 papers were obtained and assessed for inclusion in the review. Figure 1 shows the flow of studies through the review process and the numbers excluded at each stage. Overall, we included 21 studies reported in 25 publications. 45–69 Three ongoing studies were identified and further details of these are provided in Appendix 10.
FIGURE 1.
Flow of studies through the review process.
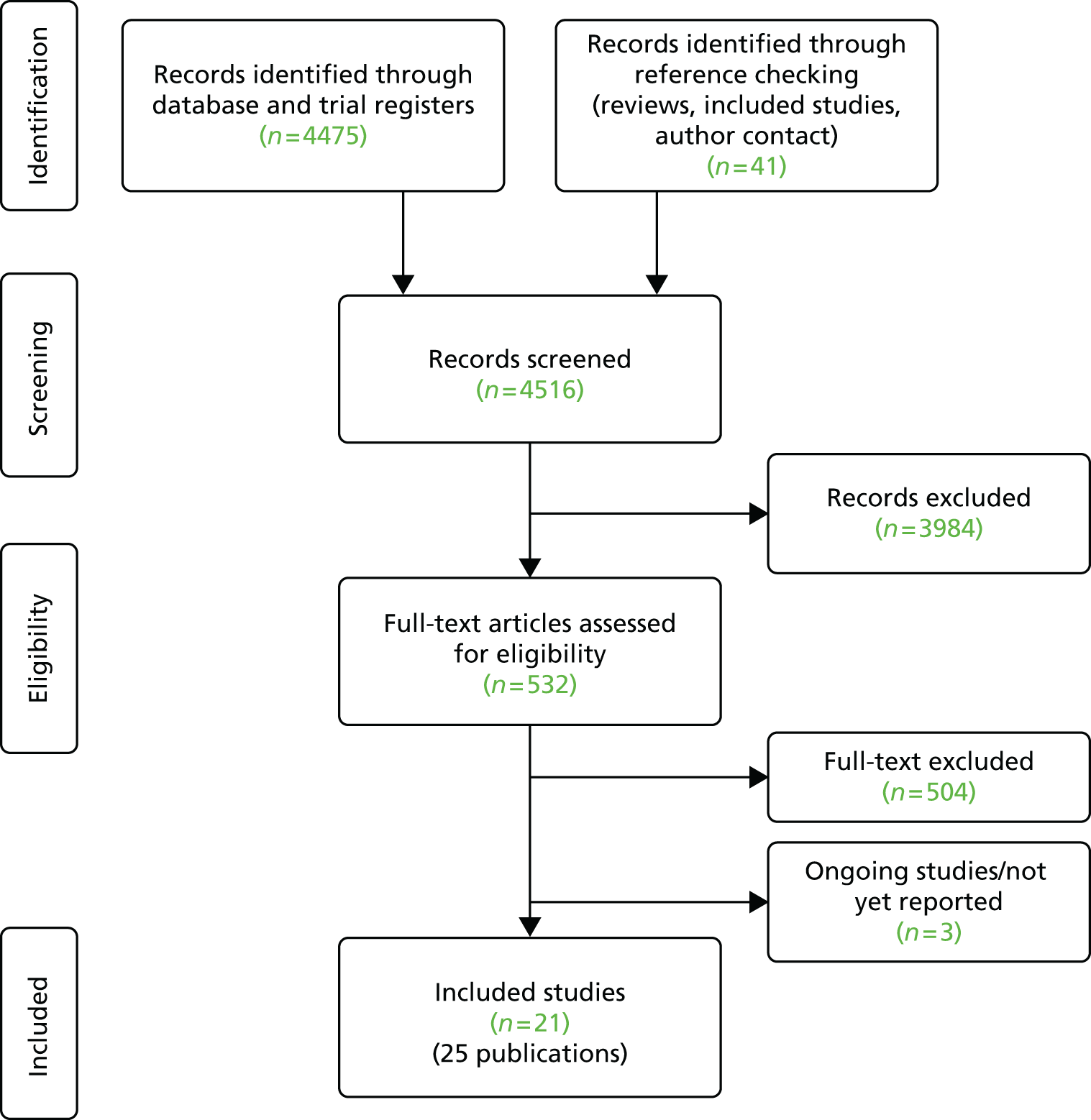
Determining eligibility was quite tricky for some studies, as it was sometimes difficult to definitively determine whether or not the problem being managed was knee instability. This was partly because of poor reporting but also because knee instability was sometimes part of a more complex problem with stability and mobility. Seventy-six studies were identified that took place in laboratory or clinical settings without the patient using the orthosis in the community. These were excluded as participants had not had the opportunity to use the orthosis in everyday life. Other reasons for exclusion were that participants did not have a relevant CNS disorder or NMD (n = 80); they were aged < 16 years or the results for adults and children were not reported separately (n = 41); they did not have knee instability (n = 73); they were not using an orthosis (n = 71); no outcomes were reported (n = 38); an inappropriate comparator (n = 1); not a primary study (n = 109); unavailable (n = 18); and background only (n = 25) (some studies had more than one reason for exclusion). A list of the 504 full papers excluded and reason(s) for exclusion is provided in Appendix 11.
Characteristics of included studies
Twenty-one studies were included in the review. 45,47–50,52,53,56–69 An overview of the included studies is presented in Table 1. Full data extraction tables are provided in Appendix 12.
| Main publication (associated papers) | Study design | Country | Number of participants in study | Number of participants in analysis | Device evaluated |
|---|---|---|---|---|---|
| Post-polio syndrome | |||||
| Bocker 201347 (Bocker 201146) | Case series | Germany | 10 | 6 | I: carbon fibre KAFO C: no comparator |
| Brehm 200749 | Case series | Netherlands | 23 | 20 | I: carbon fibre KAFO (locked knee joint) C: leather/metal or plastic/metal KAFO used previously by same participants |
| Davis 201050 | Case series | Australia | 10 | 10 | I: carbon fibre SCKAFO in stance control mode C: KAFO in locked-knee mode used by same participants |
| Hachisuka 200652 (Hachisuka 200751) | Case series | Japan | 11 | 8 to 11a | I: carbon fibre KAFO C: traditional non-carbon KAFO used by same participants |
| Heim 199756 | Case series | Israel | 30 | 27 | I: carbon fibre KAFO C: no comparator |
| Peethambaran 200061 | Case series | USA | 5 | 5 | I: carbon titanium KAFO (anterior approach design) C: plastic KAFO (posterior approach design) used previously by the same participants |
| Steinfeldt 200363 | Case series | Germany | 55 | 55 | I: carbon fibre KAFO C: no comparator |
| Inclusion body myositis | |||||
| Bernhardt 201145 | Case series | USA | 9 | 6 | I: SCKAFO C: no comparator |
| Post stroke | |||||
| Boudharam 201348 | Case series | France | 11 | Unclear | I: carbon fibre KAFO C: no comparator |
| Kakurai and Akai 199658 | Case series | Japan | 28 | 28 | I: plastic convertible KAFO (to AFO) C: participants who changed to AFO compared with those remaining on KAFO |
| Morinaka 198260 | Cohort study | Japan | 25 | 25 | I: plastic KAFO C: 50 participants fitted with AFOs and a group of 30 healthy adult males |
| Yang 200569 | RCT | China | 67 | 67 | I: KAFO or AFO C: ‘conventional rehabilitation’ |
| Spinal cord injury | |||||
| Harvey 199753 (Harvey 1997;54 199855) | RCT (crossover) | Australia | 10 | 5–10b | I: HKAFO (WO) C: HKAFO (IRGO) |
| Jaspers 199757 | Case series | Belgium | 14 | 14 | I: HKAFO (ARGO) C: no comparator |
| Middleton 199759 | Case series | Australia | 25 | 21 | I: HKAFO (WO) C: no comparator |
| Scivoletto 200062 | Case series | Italy | 24 | 24c | I: HKAFO (RGO) C: no comparator (internal comparison of non-users with users) |
| Summers 198864 | Case series | UK | 20 | 20 | I: HKAFO (HGO ParaWalker; The Orthotic Research & Locomotor Assessment Unit, Robert Jones & Agnes Hunt Orthopaedic Hospital NHS Foundation Trust, Oswestry) C: no comparator |
| Sun 200765 | Case series | China | 20 | 15 | I: HKAFO (RGO) C: no comparator |
| Tang 200966 | Controlled study | China | 58 | Unclear | I: AGO, RGO, KAFO C: rehabilitation training |
| Whittle 199167 | Controlled study (crossover) | UK | 22 | Uncleard | I: HKAFO (HGO ParaWalker) C: HKAFO (RGO) |
| Wu 200368 | Case series | China | 6 | 6 | I: HKAFO (WO) C: no comparator group |
The included studies were published between 198260 and 2013. 48 All were reported as full papers. There were two RCTs (one with a crossover design); two non-randomised studies with a control group (one with a crossover design); one cohort study; and 16 case series (see Table 1). The case series made predominantly before-and-after comparisons either with a previously used device or no device (see Table 1).
The literature is international with studies in China, Japan, Australia, UK, Germany, USA, the Netherlands, France, Belgium, Italy and Israel. Seventeen studies were published in English,45,47–50,52,53,56–62,64,67,68 three in Chinese65,66,69 and one in German. 63 The studies in languages other than English were extracted by a native speaker who was also a researcher with experience of undertaking systematic reviews. These were checked by a second researcher for consistency only.
Overall, 478 patients were included in the review. Sample sizes were small, ranging from 561 to 6769 participants with 11 studies having 20 or fewer participants (see Table 1). Eight of the studies reported knee instability as a result of NMD (153 patients)45,47,49,50,52,56,61,63 (all except one study45 being of patients with post-polio syndrome) and 13 reported knee instability resulting from CNS causes (325 patients),48,53,57–60,62,64–69 either post stroke or spinal cord injury.
Follow-up time was generally short, ranging from 6 weeks61 to 30 months. 56
Study quality
The quality assessments are reported for RCTs (Table 2), non-randomised controlled studies (Table 3) and case series (Table 4). Overall, both RCTs53,69 had a high risk of bias (see Table 2). Owing to poor reporting it was not possible to determine whether or not they were truly randomised studies or to determine whether or not a robust method of allocation concealment had been used. It is not possible to blind participants or clinicians treating them to an orthotic device. Independent outcome assessment would have protected against detection bias; however, there was no evidence of this in either study.
| Study | Selection bias | Performance bias: blinding of participants and personnel | Detection bias: blinding of HCP-assessed outcomes | Attrition bias | Selective outcome reporting | Other | |
|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | ||||||
| Yang69 | Unclear risk | Unclear risk | High risk Not possible due to nature of intervention |
High risk of bias Treating clinician-assessed outcome, which is likely to be influenced by lack of blinding |
Low risk of bias | Unclear | |
| Harvey53 | Unclear risk | Unclear risk | High risk Not possible due to nature of intervention |
High risk of bias Treating clinicians appeared to be involved in gathering data on outcomes that were likely to be influenced by lack of blinding |
High risk of bias for ambulatory outcomes | Unclear | Only a small number of patients wore their second device, suggesting that a crossover design was not appropriate |
| Study | Selection criteria adequately reported? | Representative sample? | Participation rate ≥ 80%? | Performance bias? | Independent outcome assessment? | Follow-up ≥ 80%? | Selection bias? | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||||||
| Morinaka60 | N | U | N | U | N | NAa | Y | Y | U | U | U | U | Nb | U |
| Tang66 | Y | Y | Y | U | N | U | U | U | U | U | U | U | U | U |
| Whittle67 | N | U | U | U | N | Y | U | U | U | U | U | U | U | U |
| Study | Selection criteria adequately reported? | Representative simple? | Participation rate ≥ 80%? | Prospective? | Independent outcome assessment? | Follow-up ≥ 80%? | Prognostic variables reported? | Cointerventions? | Measure of variability? | Other important limitations |
|---|---|---|---|---|---|---|---|---|---|---|
| Bernhardt45 | N | U | U | Y | N | N | Y | N | P | Reporting of results |
| Bocker47 | Y | Y | U | Y | N | N | N | Y | Y | Reporting of results |
| Boudarham48 | Y | U | U | Y | N | U | Y | N | Y | |
| Brehm49 | Y | U | U | Y | N | Y | Y | N | P | Reporting of results |
| Davis50 | Y | U | U | Y | N | Y | Y | Y | Y | Generalisability of assessing two different modes of using orthosis in clinic |
| Hachisuka52 | U | Y | U | Y | N | N | Y | Y | P | Reporting of results |
| Heim56 | N | U | U | Y | N | Y | N | N | N | Reporting of results |
| Jaspers57 | N | U | N | N | Y | N | Y | Y | NA | |
| Kakurai and Akai58 | N | U | U | Y | N | Y | Y | N | Y | Ability to actively control knee a confounder for KAFO and AFO comparisons |
| Middleton59 | Y | U | U | U | N | Ya | Y | Y | Y | Only patients who had successfully completed gait training and continued to used the orthosis were administered a questionnaire |
| Peethambaran61 | Y | U | U | Y | N | Y | Y | N | Y | Generalisability due to small sample |
| Scivoletto62 | U | U | U | Y | N | Ya | Y | N | Y | |
| Steinfeldt63 | N | U | U | N | N | Y | N | N | N | |
| Summers64 | Y | U | U | N | N | NAb | N | Y | NA | Lack of information on interview questionnaire |
| Sun65 | N | U | U | U | N | N | N | U | N | |
| Wu68 | Y | U | U | U | N | Y | Y | Y | Y | Generalisability due to small sample |
Poor reporting was also an issue for assessing risk of bias in the non-randomised controlled studies and case series. Overall, only one of the non-randomised control studies met all of the three criteria that were related to selection of participants into the study; however, it was unclear if the groups were balanced appropriately for key clinical criteria and it was not possible to assess how complete the follow-up was. 66 As with the RCTs, none reported independent outcome assessment (see Table 3).
Ten of the case series were prospective, three were retrospective and for three there was insufficient information to determine the design (see Table 4). There was a risk of selection bias across all of the case series: eight described their inclusion criteria (see Table 4);47–50,59,61,64,68 for two of the studies52,62 it was not possible to determine if the criteria presented were a priori inclusion criteria or a description of who was included in the study; and the remaining studies did not clearly specify the inclusion criteria. Two case series used a method that suggested that they were likely to have a representative sample, such as a consecutive sample of patients from a clinic, although it was unclear whether or not the participation rate was > 80% and, therefore, selection bias may have been introduced here. 47,52 One study57 reported using independent outcome assessment.
Most studies did not have a comparator group of patients. In just one comparative study62 was it possible to determine that performance bias was not present (i.e. treatment groups were not treated differently). One study57 had an independent assessor, with the remainder assessing follow-up through treating health professionals (i.e. the individuals delivering the intervention also assessed the effectiveness of the intervention) thereby introducing the risk of bias in outcome assessment.
Poor reporting was in evidence across the studies in this review; this was in relation to both study methods and results. In terms of methods, 15 studies45,48,49,52,56–60,62–64,67–69 gave no indication of the orthosis ‘dose’ given to patients (i.e. the time per day/week for which they were advised to use their orthosis). Actual use of the orthoses was not provided in 12 studies. 47–50,52,61,63,65–69 Other study aspects that could also impact on results were under-reported. Participants’ previous use of orthoses was not reported in six studies48,62,63,65–67 and co-interventions were not well reported. In general, results were poorly reported. Studies, in the results section, often made statements that were not backed up with numerical data. When no data have been provided to support statements, this has been highlighted in the relevant results sections that follow. Adverse effects were not investigated or not reported in 17 studies. 45,47–50,52,53,59–63,65–69
We planned to extract data to allow calculation of between-group differences and confidence intervals. However, because of the generally poor reporting of data, it was not possible to consistently do this across studies. When data were available, these were extracted; when the appropriate data were not reported, the description of the results provided in the paper was extracted and the lack of summary data was noted.
Results from studies of patients with post-polio syndrome
Seven studies47,49,50,52,56,61,63 (Table 5) assessed the effects of orthoses on patients with knee instability following poliomyelitis (143 patients). None of the studies was conducted in the UK. All were case series, with four studies having ≤ 11 patients. 47,50,52,61
| Author | Population | Intervention | Comparison | Follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Total n, % male, mean age (SD) years | Previous orthotic use | Type of device | Material, manufacture | Orthosis dose | Co-interventions | |||
| Bocker47 | n = 10, 30%, 64.5 | No | KAFO, ‘type eight’, mechanical lockable knee joint plus Glenzack joints to lift foot | Carbon fibre, prefabricated | Used over the whole day | Gait training, physical pain therapy and exercises, (both twice per week for 3 months) | Before-and-after provision of orthosis | 3 months |
| Brehm49 | n = 23, 61%, 55 (9.2) | Yesa | KAFO, locked knee joint | Carbon fibre (weight ranged from 0.9 to 2.1 kg), custom-made | Not reported | Walking aids were used by some participants | Leather and metal or plastic and metal KAFO previously used by same patients (weight ranged from 0.9 to 2.1 kg) | 26 weeks |
| Davis50 | n = 10b, 40%, 61.9 (7.7) | Yesc | SCKAFO | Carbon fibre, prefabricated using Horton Stance Control knee joint | Regular use for > 4 hours/day | Walking aids | Same KAFO used in locked-knee mode | Mean duration of use at time of evaluation 6.2 (SD 5.2) months |
| Hachisuka52 | n = 11, 18%, 53.9 (9.8) | Yesd | KAFO | Carbon fibre (mean weight 0.992 kg, SD 0.168 kg), custom-made | Not reported | Walking aids | Traditional non-carbon fibre KAFO and no orthosis in same participants (mean weight 1.403 kg, SD 0.157 kg) | Not reported |
| Heim56 | n = 30, 33%, 44 | Yes, all | KAFO | Carbon fibre (on average weighed 1.150 kg), prefabricated | Not reported | Not reported | After provision of orthosis only | 30 months |
| Peethambaran61 | n = 5, 40%, 61.4 (12.4) | Yes, all | KAFO, anterior approach, cable control locking mechanism | Carbon titanium, custom-made | Not reported | Not reported | Plastic KAFO used by same patients, posterior approach | 6 weeks |
| Steinfeldt63 | n = 55, 44%, 58 | Not reported | KAFO | Carbon fibre, prefabricated | Not reported | Not reported | Before-and-after provision of orthosis | > 3 months |
Four studies49,50,52,61 compared different types of orthoses used by the same participants, three of these were effectively comparisons before and after provision of a new device; in the fourth study,50 participants were provided with a new stance control device and the comparison was between using the device in stance control mode and using it in locked mode (with the aim of replicating a traditional KAFO design). Comparisons were made before and after use of the orthosis in two studies:47,63 and post-intervention only in one study. 56 All orthoses investigated were types of carbon fibre KAFO.
The outcomes were sparsely reported. Five49,52,56,61,63 of seven studies47,49,50,52,56,61,63 reported measures of patient satisfaction, although not in sufficient detail to assess the robustness of the evaluation. Few of the studies reported assessing device functionality, device usage and quality of life (Table 6). Three studies49,50,52 made a formal assessment of walking ability and four studies47,49,50,52 assessed either energy consumption or particular muscle activity. Resource utilisation data were limited to assessment of device malfunction in four studies49,52,56,61 and cost in one study. 52 Five studies47,49,50,52,63 failed to report adverse effects data or to mention that no adverse effects were identified, therefore, it was unclear if adverse effects had been formally assessed or not reported.
| Study | Patient-reported outcomes | Objective assessments | Resource utilisation | Adverse effects | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Satisfaction with device | Functionality of device | Usage of device | Quality of life | Walking ability | Energy consumption | Muscle activity | Device malfunction | Cost | ||
| Bocker47 | ✓ | ✓ | ||||||||
| Brehm49 | ✓ | ✓ | ✓ | ✓ | ||||||
| Davis50 | ✓ | ✓ | ||||||||
| Hachisuka52 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Heim56 | ✓ | ✓ | ✓ | ✓ | ||||||
| Peethambaran61 | ✓ | ✓ | ✓ | |||||||
| Steinfeldt63 | ✓ | ✓ | ✓ | |||||||
Patient-reported outcomes
Satisfaction
Five studies assessed patient satisfaction. 49,52,56,61,63 Steinfeldt et al. 63 considered patient satisfaction before and after using a carbon fibre KAFO for 3 months. However, this was undertaken retrospectively and it was not clear if participants had previously used an orthosis. Additionally, the views were sought from only 55 of the 78 people who had received a device over the relevant time period; it was unclear why this was the case. Satisfaction was measured in terms of a range of activities measured on a 10-point numerical rating scale, with a higher score indicating better functioning. 63 Satisfaction was greater post device (pre-walking mean 3.8, post-orthosis mean 8.3; sitting pre 4.8, post 8.9; driving a car pre 5.8, post 9.1; comfort pre 4, post 8.8; putting the device on/taking it off pre 4.7, post 8.6). Statistical significance of these data and standard deviations (SDs) were not reported. 63 The authors combined the post-intervention Likert scores and reported that 63% of patients were very satisfied (45–50 points), 32% were satisfied (35–44 points), 3% were neither satisfied nor unsatisfied (20–34 points) and 2% were unsatisfied. 63
Four further studies49,52,56,61 assessed patient satisfaction by comparison with a previously worn device. All four studies49,52,56,61 had improved satisfaction scores for carbon fibre devices compared with older devices. Brehm et al. ,49 in their study of 23 patients, state that mean patient satisfaction scores were 48% higher with the carbon fibre KAFO than with the leather/metal or plastic/metal device previously used by the same participants. However, baseline and follow-up scores were not reported in full, meaning that it was not possible to verify this finding or establish how satisfied participants were with the new device, other than that they were more satisfied. 49 Hachisuka et al. 52 reported that participants were more satisfied with their carbon fibre KAFO than with their previous (unspecified) KAFO especially relating to fatigue during walking, safety during walking and appearance (six patients); however, full details of these results were not provided. Heim et al. 56 reported that 19 out of 27 patients said that they would like to have a permanent carbon fibre orthosis because of it being lighter, better fitting and more aesthetic. Details of the patient structured questionnaire were not provided. In a very small sample of five patients, Peethambaran61 reported that patients were statistically significantly more satisfied with a new carbon fibre anterior approach design KAFO than a conventional posterior approach design plastic KAFO for selected aspects (comfort, gait, appearance, effort, support, putting on/taking off, not catching on clothes and not rubbing on skin), with mean scores ranging from 4.4 to 4.8 on a 5-point scale, but not others (hours of wearing, loss of function, wearing an orthosis, device weight, device soiling clothes and device encouraging perspiration).
Functionality
Two studies considered patient-reported functionality. 61,63 Peethambaran61 assessed patient functionality using a 5-point questionnaire and found that results were statistically significantly in favour of the carbon fibre anterior approach KAFO for ease of putting on and removal, and interference with sitting, but not for strap application, shoe application, maintenance and cleaning, balance, stability in level and uneven ground walking and adequacy for sports compared with a conventional plastic posterior approach KAFO. 61 However, the sample size in this study was very small (n = 5). Steinfeldt et al. 63 reported that two-thirds of patients did not need orthopaedic shoes with the new carbon fibre KAFO (no comparator).
Usage
It was unclear in all studies, except one,56 if patients had actually worn their orthosis as advised. Heim et al. 56 stated that 21 out of 27 patients wore their orthosis ‘throughout the day’, but did not provide further detail on how this was elicited and the precise duration of use. Two further studies47,50 specified an amount of time that the orthosis should be worn, and one study61 specified that patients wore the orthosis for a period of 6 weeks, but it was unclear in either study if participants had used the orthotic device as advised.
Quality of life
At 3-month follow-up of six patients, Bocker et al. 47 noted no significant changes in patient quality of life as measured by the Short Form questionnaire-36 items (SF-36) compared with baseline (no orthosis). SF-36 data were reported in a figure only and it was not possible to extrapolate the data because of the scale. At 26-week follow-up Brehm et al. 49 reported no significant difference in physical function subscale of the SF-36 with a carbon fibre KAFO compared with the leather/metal or plastic/metal device previously worn. Again, the actual data were not reported.
Objective assessments
Four studies47,49,50,52 conducted a gait assessment. Two studies49,52 evaluated walking ability and energy consumption between an older and newer design of orthosis; one study50 assessed these outcomes using a SCKAFO in stance control mode and locked-knee mode. One study47 focused on muscle activity before, and 3 months after, use of the orthosis.
Walking ability
Hachisuka et al. 52 (n = 11 participants) found that walking speed with a custom-made carbon KAFO was significantly faster than walking without an orthosis: 39.5 (SD 9.8) m/minute compared with 31.0 (SD 8.6) m/minute, and walking with an ‘ordinary’ KAFO 42.6 (SD 7.8) m/minute compared with 38.5 (SD 7.0) m/minute; both p < 0.05 (not all patients completed each condition). Based on 10 participants, Davis et al. 50 stated that walking velocity was significantly increased in the stance control condition of the Horton Stance Control orthosis compared with the locked mode [locked mode mean 65.0 cm/second (SD 24.5), SCKAFO mean 72.9 (SD 95.7) cm/second; p = 0.000107). This was reported to be a result of significantly increased cadence and increased step length on the sound limb. 50 Brehm et al. 49 reported that walking speed remained unchanged between the carbon fibre locked KO and previous plastic or leather KAFO (23 participants): 1.8 m/minute (95% CI –4.35 to 0.57 m/minute) (data as reported in original paper, effect lies outside confidence interval).
Energy consumption
Two studies49,52 found that energy costs were lower for the newer device than the older one. Brehm et al. 49 found that the gross and net energy cost of walking were both significantly lower for the new carbon fibre KAFO than the plastic or leather KAFO previously used. Gross energy consumption: mean difference –0.42 (95% CI –0.63 to –0.21) J/kg/minute; 7% reduction. Net energy consumption: mean difference –0.36 (95% CI –0.54 to –0.18) J/kg/minute; 8% reduction. 49 Hachisuka et al. 52 reported that oxygen consumption per body weight (ml/kg/minute), oxygen cost and physiological cost index while walking with a carbon KAFO were significantly lower than while walking without an orthosis (–16%, –35% and –33%, respectively) and with an ordinary KAFO (–9%, –14% and –15%, respectively); all p < 0.05. Davis et al. 50 found no difference in the oxygen cost of walking between the two conditions (nine participants): locked condition 0.213 (SD 0.081) ml/kg/minute, stance control 0.224 (SD 0.069) ml/kg/minute; p = 0.515.
Functionality
One study47 assessed muscle function. Bocker et al. 47 reported an increased stance duration of 24% from baseline to 3 months’ follow-up (p = 0.029) in the leg with the carbon fibre KAFO. They reported that there were no statistically significant differences in the opposite leg during the intervention. Muscle activity was reported in some detail and a summary can be found in the data extraction tables (see Appendix 12).
Resource utilisation
Device malfunction
Four studies49,52,56,63 reported on device problems. Data were provided in the text of the papers, but it was unclear how systematically this information had been gathered. Brehm et al. 49 stated that 7 out of 23 patients reported technical deficits relating to the hinge at the ankle or knee, which could be easily repaired (no details of repair procedures provided). Seven patients reported wear to the cloth upholstery inside the KAFO. One patient needed a replacement of the orthosis as a result of a break of the KAFO. In the study by Hachisuka et al. ,52 follow-up data were available for at least 2 years for this outcome, and the carbon fibre KAFO remained undamaged. Heim et al. 56 noted that within 30 months 19 out of 30 braces had undergone minor repair in the workshop. Steinfeldt et al. 63 commented that ‘patient reports of the need for repairs did not show substantial problems’ (no further details provided). However, none of these studies reported full details of usage of the orthosis, so it is unclear how reliable and generalisable these data are.
Cost
One study undertaken in Japan (published in 2006) by Hachisuka et al. 52 reported that the price of a standard carbon fibre KAFO was 180,000 Japanese yen (US$1700), 50% more expensive than the ordinary KAFO.
Adverse effects
Data on any adverse effects were sparse: the majority of studies did not appear to systematically collect these data. Two studies56,61 (35 participants) reported adverse effects in a limited way. Peethambaran61 stated that the new carbon fibre KAFO was statistically significantly favoured for comfort compared with conventional KAFO in ankle, thigh (back), knee (back) and lower leg (back), but not for thigh (front), knee (front) or lower leg (front). However, this may simply relate to the fit of the device rather than the construction per se. It was an improvement on the previous device in terms of rubbing on skin and catching on clothes, but no difference was noted for the device soiling clothes or causing perspiration (five patients). 61 Heim et al. 56 reported that, at the end of the study, 8 out of 30 patients chose a metal orthosis rather than the carbon fibre brace as a result of skin irritation from the material, excessive sweating due to proximity of orthosis to skin, and inability to alter the shape in accordance with circumferential limb changes. The remaining studies did not report adverse effects or stated that none had occurred.
Results from studies of patients with inclusion body myositis
One case series study45 (Table 7) assessed the effects of orthoses on patients with knee instability as a result of inclusion body myositis (nine patients). This small study of a rare condition was conducted in the USA. Patient selection criteria for the study were not reported in full and it is unclear if the patients are fully representative of those seen in practice.
| Author | Population | Intervention | Comparison | Follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Total n, % male, mean age (SD) years | Previous orthoses use | Type of device | Material, manufacture | Orthoses dose | Co-interventions | |||
| Bernhardt45 | n = 9, 78%, 61 (9) | No | SCKAFO | Not reported Prefabricated, Sensor Walk™ (Otto Bock Health Care, Minneapolis, MN, USA) |
Not reported | Not reported | With and without the orthosis | 6 months |
A comparison was made between patient gait with and without the SCO after 6 months’ use of the device. Patient feedback on the device was also sought. The study45 did not appear to use an independent outcome assessor and follow-up was incomplete (six of nine had outcome data). A gait assessment was conducted to assess walking ability. An author-designed questionnaire was used to elicit patient outcomes but results were not reported in full, just as textual summaries. No data were reported on resource utilisation or adverse effects (Table 8).
| Study | Patient-reported outcomes | Objective assessments | Resource utilisation | Adverse effects | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Satisfaction with device | Functionality of device | Usage of device | Quality of life | Walking ability | Energy consumption | Muscle activity | Device malfunction | Cost | ||
| Bernhardt45 | ✓ | ✓ | ✓ | ✓ | ||||||
Patient-reported outcomes
These were reported by the authors as a narrative summary only; data were not presented. Follow-up was available for six out of nine participants. In terms of functionality of the device, the authors reported that participants felt that the SCKAFO was helpful for protecting against falls and providing stability. The authors further explained that, in relation to patient satisfaction, all participants had complaints regarding size, bulk, cosmesis and noise of the device. They cited difficulty in putting on/taking off the brace. Most participants stated that they would prefer a less-intrusive assistive device. The authors identified that participants with less weakness tended to have positive feedback on the device. This was found to be regardless of the amount of time spent using it. Use of the brace ranged from about 2 hours per day to all day every day. 45
Objective outcomes
Data for most of these outcome measures were available in small-scale graphs only and summary statistics were not presented. At 6-month follow-up participants walked more slowly (p = 0.025) and with a lower cadence (p = 0.0007) with the SCKAFO than when walking without the device. Stride length with the SCKAFO was not significantly different between the two conditions. Participants had a wider step width with the brace (0.035). When wearing the device the weaker participants walked more slowly (p = 0.022) and had a lower cadence (p = 0.019) and a shorter stride length (p = 0.048) than participants with less weakness. Peak knee flexion during swing significantly decreased when using the device [from mean 74.7 (SD 2.9) degrees to mean 62.9 (SD 10.8) degrees; p = 0.021]. There was no significant difference in peak hip flexion during swing when using the device [from mean 41.4 (4.6) degrees to mean 39.7 (6.5) degrees; p = 0.355). 45 No further outcomes were reported.
Results from studies of post-stroke patients
Four studies48,58,60,69 (Table 9) assessed the effects of orthoses on patients with knee instability following stroke (131 patients). None of the studies was conducted in the UK. There was one RCT69 that was limited in its reporting, a cohort study60 and two case series48,58 (one of which had only 11 patients).
| Author | Population | Intervention | Comparison | Follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Total n, % male, mean age (SD) years | Previous orthoses use | Type of device | Material, manufacture | Orthoses dose | Co-interventions | |||
| Boudharam48 | 11, 64%, 51 (15) | Not reported | KAFO | Carbon fibre, custom-made | Required to have worn device daily > 1 month | Not reported | With and without KAFO | Device prescribed within past 6 months |
| Kakurai and Akai58 | 28, 50%, 54.5 | No | KAFO, which was convertible to an AFO | Plastic, custom-made | Not reported | Not reported | Patients changed to AFO vs. those who continued using KAFO | Not reported |
| Morinaka60 | 25, 64%, 56 | Yes, AFO (3) | KAFO | Plastic | Not reported | Not reported | AFO (n = 50), adults who have not had a stroke (n = 30) | Mean 14.6 months (range 1–35) |
| Yang69 | 67, 84%, 58 | No | KAFO/AFO | Not reported | Not reported | Not reported | Conventional rehabilitation | Not reported |
The studies made varying comparisons. Boudharam et al. 48 undertook gait analysis with and without a carbon fibre KAFO. Kakurai and Akai58 compared patients who had changed to a plastic AFO with those who continued to use a plastic KAFO. This study58 was not comparing two types of devices: the comparison was between those who had recovered sufficient control of knee activity to switch to an AFO device and those who had not. Morinaka et al. 60 compared a plastic KAFO to AFO and to normal adult gait. Yang et al. 69 undertook a RCT comparing AFO or KAFO to conventional rehabilitation (not reported in detail). In one study,48 orthoses were made of carbon fibre, two used plastic orthoses,58,60 and the RCT by Yang et al. 69 did not report the material.
None of the studies reported using an independent outcome assessor to assess outcomes. Duration of follow-up was not reported in two studies,58,69 although the RCT by Yang et al. 69 appeared to conduct an assessment of patients two to three months after treatment. In one study, the device had to have been prescribed in the previous 6 months48 and in the fourth study60 the follow-up was 14.6 months, but in the study with the longer follow-up such follow-up was incomplete (< 80%). 60 Outcomes were sparsely reported (Table 10). No studies assessed patient-reported outcomes, with the exception of Morinaka et al. ,60 who reported solely on the usage of the orthotic device. Three studies48,58,60 made a formal assessment of walking ability and two studies58,69 assessed other functional abilities. No studies reported on resource utilisation data. None reported any adverse effects data or mentioned that no adverse effects were identified.
| Study | Patient-reported outcomes | Objective assessments | Resource utilisation | Adverse effects | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Satisfaction with device | Functionality of device | Usage of device | Quality of life | Walking ability | Energy consumption | Functional ability | Device malfunction | Cost | ||
| Boudharam48 | ✓ | |||||||||
| Kakurai and Akai58 | ✓ | ✓ | ||||||||
| Morinaka60 | ✓ | ✓ | ||||||||
| Yang69 | ✓ | |||||||||
Patient-reported outcome
Usage
Morinaka et al. 60 reported that 25 patients continued to wear the orthosis without rejection at the end of the study, but did not provide results for all 36 patients who were fitted with an orthosis during the study period. It is not explained why only a subset of those fitted with a device was evaluated.
Objective assessments
Walking ability
Walking ability was assessed in three studies,48,58,60 two48,60 of which conducted a formal gait assessment. Morinaka et al. 60 reported that 25 patients were able to walk smoothly after fitting of the KAFO (data not reported). The authors60 state that follow-up results were more favourable for the KAFO users than for the AFO users for 8 out of 12 gait characteristics (data available only as small scale graph). The authors60 state that knee flexion was better in AFO users and that KAFO users were about half to one-third faster than AFO users; again, outcomes were not reported in full (only qualitatively and in figures). It is unlikely that the AFO and KAFO groups were clinically similar, as the average time post stroke was 40 months in the AFO group and 20 months in the KAFO group; therefore, aside from inadequate reporting, there is undoubtedly confounding. Boudharam et al. 48 also conducted a formal gait assessment and found that gait velocity was significantly greater with the KAFO than without (21%; p = 0.025). Stride length and cadence were also significantly greater in the KAFO condition (15%, p = 0.030; 11%, p = 0.049). Symmetry between the paretic and non-paretic limbs increased with the KAFO. There was no significant difference between the two conditions for step width (p = 0.384). More details of gait measurements can be found in the data extraction tables in the appendices. 48
In the study by Kakurai and Akai,58 11 out of 28 patients could control their knee actively at between 1.5 and 10 months (average 4 months) after initial prescription of the convertible plastic KAFO. The 11 patients had their KAFOs changed to AFOs (AFO group). The 17 remaining patients were unchanged and continued to use their KAFO (KAFO group). In the AFO group, three were classified as outdoor independent, one as indoor independent and seven as indoor dependent. In the KAFO group, two patients were classified as indoor independent, 11 were classed as indoor dependent and four were classed as non-ambulant. 58
Functional ability
Functional ability was reported in the RCT by Yang et al. 69 and in the case series by Kakurai and Akai. 58 Yang’s reporting69 suggested that, after 2–3 months of treatment, 97% of those in the orthoses group experienced improved motor function recovery, whereas in the conventional rehabilitation group 81% experienced such improvement. The detail of how this was ascertained was unclear. The difference was statistically significant (p < 0.01). Kakurai and Akai58 measured functionality using the Barthel Index. Those wearing an AFO had a higher (better) Barthel Index score (mean 72.8, SD 7.2) than the KAFO group (mean 43.1, SD 4.6); p < 0.01. 58 However, this study58 was not comparing two types of devices: the comparison was between those who had recovered sufficient control of knee activity to switch to an AFO device and those who had not. No further outcomes were reported in relation to resource utilisation and adverse effects.
Results from studies of patients with spinal cord injury
Nine studies53,57,59,62,64–68 (Table 11) assessed the effects of orthoses on patients with knee instability following spinal cord injury (194 patients). Two of the studies64,67 were conducted in the UK. There was one RCT of 10 participants53 and two controlled trials66,67 and the remainder were case series. 57,59,62,64,65,68 Five studies53,57,64,65,68 had 20 or fewer patients. In five studies57,64,65,67,68 it was unclear if the sample was representative of patients seen in practice. In one study53 patients were chosen based on their motivation to walk.
| Author | Population | Intervention | Comparison | Follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| Total n, % male, mean age (SD) years | Previous orthoses use | Type of device | Material, manufacture | Orthoses dose | Co-interventions | |||
| Harvey53 | 10, 90%, 37 (8.4) | Yes, all had KAFO standing experience | HGO (WO) | Not reported | Yesa | Gait training (30–54 hours per orthosis), crutches | IRGO worn by same patients | 28 weeks |
| Jaspers57 | 14, 86%, 33.6 | Yes, long leg brace (4 patients) | ARGO | Not reported | Not reported | Walker (12), crutches (2) | After provision of orthosis only | > 1 year |
| Middleton59 | 25, 76%, 35 (13) | Yes, KAFOs plus backslabs (22) | HGO (WO) | Not reported | Not reported | Parallel bars, forearm crutches or frames | After orthosis only | ≥ 18 months |
| Scivoletto62 | 24, 79%, 33.6 (3.2) | Not reported | RGO | Not reported | Not reported | Not reported | RGO non-users | 1 year |
| Summers64 | 20, 100%, 28 | Yes, long leg callipersb (11) | HGO ParaWalker | Not reported | Not reported | Crutches used as decided by patient | After orthosis only | Mean 20 months |
| Sun65 | 15, 67%, 33.7 | Not reported | RGO | Not reported | 1 hour, twice per day for 2 months | Not reported | After orthosis only | Not reported |
| Tang66 | 58, 83%, 32.4 | Not reported | KAFO, ARGO, AGO | Not reported | 50 minutes, twice per day for 6–8 weeks | Rehabilitation training | Rehabilitation training | 4 monthsc |
| Whittle67 | 22, 82%, 34 | Not reported | HGO (ParaWalker) | Metal foot section | Not reported | Rollator or crutchesd | RGO (plastic foot section) | 4 months for each orthoses |
| Wu68 | 6, 67%, 27.6 | No | HGO (WO) | Plastic | Not reported | Gait training including balance plus walking exercises | Before and after orthosis | Unclear |
All the studies investigated types of HKAFO. Three studies53,59,68 investigated the Walkabout® (Polymedic, QLD, Australia) orthosis (WO), a HGO. Harvey et al. 53 compared the WO with an ISOCENTRIC® RGO (Centre for Orthotics Design, Campbell, CA, USA) used by the same patients. A randomised crossover design was used with a 3-month home trial period and a 2-month washout period of no orthoses use, although the data were analysed as though from a parallel trial. Two studies59,68 had no comparator group.
Two UK studies64,67 investigated the ParaWalker, a HGO. Summers et al. 64 had no comparator group and Whittle et al. 67 compared the HGO to a custom-made RGO worn by the same patients in a crossover study.
The remaining four studies57,62,65,66 investigated types of RGO. Two of these studies57,65 had no comparator group. Scivoletto et al. 62 compared patients using a RGO to those patients not using it, and Tang et al. 66 compared three different types of orthoses (plus rehabilitation training) to rehabilitation training.
All nine studies53,57,59,62,64–68 used patient-reported outcomes, specifically functionality of the device (Table 12). Five53,57,59,64,67 studies reported measures of patient satisfaction, one study66 reported quality of life and five studies53,57,59,62,64 reported usage of the device. There were fewer objective assessments across the studies. Four studies53,65,67,68 made a formal assessment of walking ability. Resource utilisation data were limited to assessment of device malfunction in four studies57,59,64,67 and cost in one study. 67 Six studies53,59,62,66–68 failed to reported adverse effects data or to mention that no adverse effects were identified. This section is grouped by orthotic device.
| Study | Patient-reported outcomes | Objective assessments | Resource utilisation | Adverse effects | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Satisfaction with device | Functionality of device | Usage of device | Quality of life | Walking ability | Energy consumption | Functional ability | Device malfunction | Cost | ||
| Harvey53 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Jaspers57 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Middleton59 | ✓ | ✓ | ✓ | ✓ | ||||||
| Scivoletto 62 | ✓ | ✓ | ||||||||
| Summers64 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Sun65 | ✓ | ✓ | ✓ | |||||||
| Tang66 | ✓ | ✓ | ||||||||
| Whittle67 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Wu68 | ✓ | ✓ | ✓ | |||||||
Hip guidance orthosis: ParaWalker
Two UK studies investigated a HGO (ParaWalker). 64,67 Whittle et al. 67 compared a HGO to a RGO in 22 people. Participants tried one of the devices for 4 months and then switched to the other type. The order in which the device was tried was not randomly allocated. Summers et al. 64 evaluated 20 patients after at least 6 months of home use of the ParaWalker.
Patient-reported outcomes
Satisfaction
Both studies64,67 evaluated patient satisfaction and both found a range of opinions, although details of precisely how satisfaction was assessed were limited. Following patient interviews at a mean follow-up of 20 months, Summers et al. 64 reported that, overall, five patients were highly pleased with the device, 10 were pleased, three were non-committal and two disliked it. Two of 20 patients complained that the HGO was unsightly to wear. 64 Whittle et al. 67 reported that, at the end of the study, 12 of the 22 participants chose to keep the RGO, four chose to keep the HGO and six chose to keep neither orthotic device. The main reasons given for the final choice (no numerical data provided) of the HGO was ease of putting on and taking off, and, for the RGO, cosmesis and ease of standing with hands free. For those who chose neither, fear of developing pressure sores and difficulty using either orthosis were concerns. 67
Functionality
Whittle et al. 67 reported that 17 patients had successfully used the HGO, three did not and two left the study. No details on what constituted ‘successful use’ were provided. Other statements on functionality were not backed up with numerical data. 67 Summers et al. 64 reported that eight patients used the HGO outdoors independently for therapeutic purposes, nine used the HGO independently for therapeutic purposes indoors only, and three abandoned the device. The reasons for abandoning the device were that it was too tiring to use (n = 2) and one patient with an unrelated arm injury (crutches needed to be used with the device) hoped to return to using it. 64 All three patients were dependent therapeutic indoor walkers. In total, 17 of 20 patients achieved independent putting on/taking off of the device, and standing from, and sitting in, a wheelchair. The three individuals who could not manage these functions stopped using the device. Ten patients were able to get into the passenger seat of a car, with some difficulty. Two patients had driven a car with the HGO on but found it difficult and did not often repeat this task. 64
Usage
One study64 reported on usage of the device as determined by patient interview: four patients used the HGO > 3 times a week, 11 patients used it 1–3 times a week, two patients used it < 1 times a week, and three patients abandoned it. 64 Of the 17 users, two used the device for > 3 hours on each occasion, 13 users for 1–3 hours, and two users for < 1 hour per occasion. 64
Objective assessments
Walking ability
One study67 reported making an objective assessment of walking ability; however, no data were provided: the authors stated that there were no significant differences between the HGO and RGO in gait parameters of cadence, stride length and velocity after 4 months (no data provided). The average walking velocity was about 0.24 m/s. 67
Resource utilisation
Device malfunction
Summers et al. 64 stated that minor repairs of the HGO were usually required at 6-monthly follow-up sessions but there were no breakages. Whittle et al. 67 reported that frequent adjustments were needed initially for both the HGO and RGO and this was undertaken by the on-site orthotist. No major failures occurred with the HGO. The RGO was damaged in two cases as a result of overstressing. In 4 months of use for both orthoses, the authors stated that about one-third of participants’ devices needed minor repairs, replacements or adjustments. 67
Cost
One study67 reported on cost. Whittle et al. 67 stated that fabrication of the RGO was £1772, compared with £1116 for the HGO (paper published in 1991). Training and out-of-pocket expenses were reported to be similar between the orthoses. 67 The combined cost of training and 4 months’ maintenance was about £330 for each device. 67 Patients and their carers had an average of 8 days off work and out-of-pocket expenses of £160–200. 67
Adverse effects
One study64 reported adverse effects. Summers et al. 64 reported that there were no pressure sores in the study. The authors stated that the RGO particularly required attention to ensure that patients did not get pressure sores (no data provided), although this relates to fitting of the device. They stated that ‘most’ patients had one or two falls during early use and one patient sustained a significant injury (fractured distal end of radius) (unclear how data were ascertained). 64
Hip guidance orthosis: Walkabout orthosis
Three studies evaluated the WO, a HGO. 53,59,68 Harvey et al. 53 used a crossover design comparing the WO with an IRGO in 10 people. This was described as a RCT; however, details of the method of randomisation or allocation concealment were not reported. Middleton et al. 59 assessed 25 patients after provision of the device, and Wu et al. 68 undertook a before-and-after assessment of six orthoses users.
Patient-reported outcomes
Satisfaction
Two studies53,59 evaluated patient satisfaction. Harvey et al. 53 found that seven participants preferred the IRGO at completion of the study and three participants preferred the WO (p = 0.17). Middleton et al. 59 stated that > 90% of patients reported physical benefits, around 50% reported psychological benefits, around 40% reported exercise benefits and < 10% reported functional benefits (approximate percentages read from small-scale graph) at the follow-up review after 18 months of use.
Functionality
All three studies reported functional outcomes, although in a limited way. Harvey et al. 53 stated that all participants could sit in a wheelchair with the WO on but no participants could do so with the IRGO. Middleton et al. 59 reported that 24 out of 25 patients were able to apply the WO independently and transfer themselves between standing and sitting while wearing it, although three patients could not be successfully trained in using the device and ‘several’ preferred to have assistance for taking the device on and off. The authors59 state that improved standing stability was reported by all patients after using the orthosis. 59 The authors gave several examples of improved functionality in household tasks but stated that overall indoor accessibility was not improved and was often hampered by the accompanying walking aid. Numerical data were not provided. Wu et al. 68 found a functional improvement after use of the orthoses (length of follow-up not specified) as measured by the Barthel Index [pre-treatment mean 26 (SD 8), post-treatment mean 47 (SD 7); p < 0.01].
Usage
Two studies53,59 reported on usage of the orthosis. Harvey et al. 53 stated that there was no significant difference in the number of times that the WO and IRGO orthoses were used over the course of the 28-week study. During the home trial period no participant wore either orthosis for > 2 hours at any one time. The most common reasons for using either orthosis were for exercise, practice or for the long-term benefits. 53 Based on information provided in a graph, it appears as though the device provided at crossover (i.e. the second device) was not worn at all by 6 of the 10 participants. Six participants used the WO at home, with one of these participants requiring assistance. Four of seven participants using the IRGO needed assistance, mainly for sitting and standing. Three participants wore the WO under clothes but no participants wore the IRGO under clothes. 53 Participants could sit in their wheelchair wearing the WO but not the IRGO. When participants were asked why they did not make more use of their orthoses, the most common response was being ‘too busy’. Additionally, participants stated that using the orthoses (type not specified) prevented them from undertaking certain activities in the home, as their hands were unavailable due to holding elbow crutches. 53
Middleton et al. 59 found that 16 of 25 patients still used the WO at follow-up with 15 of these having continued for > 18 months. The mean intensity of WO usage was 150 (SD 24) minutes/week at the first review at 7–12 months and 169 (SD 36) minutes/week at the second review 12 months later. The median at both time points was 120 minutes. 59 Five patients discontinued usage of the WO device between 7 and 20 months, three patients were unsuccessfully trained because of spinal immobility and one patient was lost to follow-up. Four of five who discontinued usage reported a lack of functional enhancement over the orthosis that they had previously used (KAFO, RGO) or a wheelchair. 59 One of these patients had problems with ankle contractures. The fifth patient was satisfied with the device but was forced to withdraw after 11 months because of previous surgery-related back pain. 59
Objective assessments
Walking ability
Two studies53,68 evaluated walking ability after use of the WO. Harvey et al. 53 found that after 8 weeks’ training the participants walked significantly faster with the IRGO than with the WO on the flat surface (eight participants) [IRGO mean = 0.34 (SD 0.18) m/second; WO mean = 0.14 (SD 0.12) m/second; p = 0.002]. Participants also walked significantly faster with the IRGO than with the WO up and down ramps (five participants) [IRGO mean = 0.25 (SD 0.09) m/second; WO mean = 0.1 (SD 0.06) m/second; p = 0.02.]. 53 Wu et al. 68 reported that 1 week after use of the orthosis all six patients could stand or walk better between parallel bars (no data provided). After 2 weeks of exercises with the orthoses, Wu et al. 68 state that patients could continuously walk for 40 m and complete therapeutic walking.
Energy consumption
Harvey et al. 53 found that there were no differences in heart rate or oxygen uptake between the WO and IRGO for any surface. When heart rate and oxygen uptake were expressed relative to walking speed (method of calculation not reported), physical cost index and oxygen cost of gait were significantly greater with WO than IRGO on all surfaces. Participants’ physical cost index (beats/minute) ranged from 8.4–10.3 beats/minute with WO compared with 4.3–7.0 beats/minute with IRGO; p < 0.05. Oxygen cost during WO gait was 3.95–4.91 ml/kg/minute compared with 1.65–1.80 ml/kg/minute for IRGO gait; p < 0.05. 53
Functionality
Harvey et al. 53 reported that there was no significant difference between WO and IRGO in the extent of assistance required to put on/take off their orthoses; get up and down stairs and kerbs; and walk on the flat. Participants required significantly more assistance when using the WO to walk over inclined surfaces (p = 0.03) compared with the IRGO.
Resource utilisation
Adverse effects
None of the studies reported on adverse effects.
Reciprocating gait orthoses
Four studies57,62,65,66 evaluated RGOs. Jasper et al. 57 undertook the assessment of the ARGO (ARGO, developed by Steeper) after patients had been using their device for at least 12 months (range 19–56 months). It was possible to contact only 14 of the 23 patients who had received a device during the study period. 57 Scivoletto et al. 62 primarily focused on the differences between people who used and did not use their RGO, and there was limited information on the outcomes of interest to this review. Tang et al. 66 compared RGO, KAFO and what they described as an alternative gait orthosis (AGO) plus rehabilitation to rehabilitation only. It is unclear how participants were allocated to the different management strategies. Sun et al. 65 evaluated functioning of 20 patients following fitting of a RGO and up to 30 days’ rehabilitation; there was no comparator.
Patient-reported outcomes
Satisfaction
One57 of the studies evaluating ARGO assessed patient satisfaction using a telephone interview. Jaspers et al. 57 reported that all 14 patients stated that they had been well informed about the possibilities of walking with the ARGO prior to fitting, and two patients said that they were disappointed in their expectations. Of the 12 active ARGO users, two found it ‘very good’, six ‘good’, three did not state their opinion and one found it ‘bad’. Of the two non-users, one rated the device ‘good’ and one did not answer. 57
Functionality
All four studies57,62,65,66 reported on functionality. Jaspers et al. 57 stated that all patients used the ARGO mainly to stand and walk, based on responses to the telephone interview. A few (unspecified) tried to use it at work but did not continue, as they considered the ARGO to be too heavy and cumbersome for use in a really functional way. 57 They also found the walking speed to be too slow. 57 One user could make a transfer into a car without much difficulty; two others were able to transfer but found it too difficult to do on a regular basis. The other participants had never tried this. 57
In the study by Sun et al. ,65 12 patients achieved household ambulation, five patients achieved community ambulation and three patients achieved therapeutic ambulation at the end of the study. 65 Tang et al. 66 assessed functionality using the Barthel Index and functional independence measure (FIM); however, because of lack of clarity in the analysis and reporting, it is unclear whether or not there were any between group differences 8 weeks after the devices were fitted.
Usage
Two studies57,62 reported on usage. Jaspers et al. 57 stated that 12 out of 14 users were still using the ARGO on a regular basis at least 1 year following provision. Principal reasons for abandoning ARGO use were mechanical problems with the ARGO and lack of time owing to employment or preoccupation because of various interests. 57 The frequency of using the ARGO ranged from daily to twice a month, with an average of three times a week. On each occasion, the ARGO was used for 1–2 hours. 57 However, Scivoletto et al. 62 reported in their study that 11 out of 24 patients (46%) no longer used the RGO at 1-year follow-up. This included one patient with a fractured femur and several patients (number unspecified) finding the orthosis uncomfortable or too difficult to put on/take off, too slow or too hard to use, or poor fitting. 62
Quality of life
Tang et al. 66 assessed quality of life using the World Health Organization Quality of Life-BREF (WHOQOL-BREF; a subset of 26 questions from the WHOQOL-100, which measures the following broad domains – physical health, psychological health, social relationships, and environment). However, because of lack of clarity in the analysis and reporting it is unclear whether there were any between group differences 8 weeks after the devices were fitted.
Objective assessments
Resource utilisation
Device malfunction
One study by Jaspers et al. 57 found that most complaints, according to the patient telephone interview, were related to the functioning of the knee-locking cables and the knee-locking mechanism when rising and sitting down (numbers unclear). Further complaints about the ARGO were its weight, the fact that it was too big to use in an active wheelchair and the discomfort while sitting due to the back tube.
Adverse effects
Two studies57,65 reported on adverse effects. Jaspers et al. 57 found that there were no complications of a physiological nature according to patient report. Two patients had fallen but without serious injury. 57 Sun et al. 65 reported that there were no adverse effects but it was unclear how this was ascertained.
Chapter 4 Results of qualitative study of orthoses users’ perspectives
Sample characteristics
Twenty-four people were recruited into the study. Nineteen patients were recruited across the three NHS sites; five patients were recruited in site 1 (P1, P2, P4, P13, P17); five in site 2 (P10, P11, P12, P20, P21); and nine patients were recruited in site 3 (P3, P5, P6, P7, P8, P9, P19, P23, P24). The five people recruited outwith the NHS (P14, P15, P16, P18, P22) were located across different areas of England. Of the total sample, 12 people had been diagnosed as having poliomyelitis; five people said they had multiple sclerosis; two people had CMT disease; three people had experienced spinal injury; one person had a diagnosis of spina bifida; and one participant had experienced a stroke. Participants’ ages ranged from 36 years to 80 years, and the median age was 64.5 years. Half of the study participants were engaged in either full- or part-time paid employment, whereas the other half described themselves as retired. Three-quarters of those interviewed said that they lived with their spouse and/or other family members. Details of the study sample are given in Appendix 13.
Findings from the qualitative study are reported under the following subheadings:
-
impact of NMD and CNS conditions on walking and mobility
-
use of orthotic devices
-
wearing orthotic devices
-
use of mobility aids
-
fitting/acquisition of orthotic devices
-
how use of orthotic devices (KAFOs, AFOs, knee braces) may impact on skin
-
positive and negative aspects of orthotic devices
-
appearance connected to orthoses
-
footwear
-
desired treatment goals and outcomes
-
care pathways and factors that impact on experiences of care
-
interactions with HCPs.
Impact of neuromuscular disease and central nervous system conditions on walking and mobility
Study participants reported a range of symptoms and sequelae associated with neuromuscular and CNS conditions that limited their ability to walk and mobilise as they might wish. They described experiencing pain (sometimes severe) in the knee or ankle joints; hyperextension of the knee joint; muscle weakness in the lower limb and/or of the muscles supporting joints; limbs of unequal length; having feet that are ‘frozen’ in an abnormal position; varying degrees of paralysis in lower limbs and feet; toes that are ‘curling’ inwards; and drop foot. Participants described resultant difficulties associated with walking and mobility: frequent falls, often preceded by a feeling that their leg was about to ‘give way’ under them; loss of sense of balance and stability; a ‘lop-sided’ gait; dragging their feet; constantly tripping, especially on uneven surfaces; difficulty standing for any length of time or walking for any but short distances; and fatigue, as well as wear and tear in their ‘good’ or unaffected limb due to transfer of weight and effort in walking.
I went, I am in pain, and then he examined me and goes, of course, it’s not normal, your body is lop-sided.
P1
I am very good at falling over . . . it’s because my muscles are so bad [weak] and you need lots of muscles to hold you up straight.
P18
I fall all the time because my knee can give way . . . sometimes it’s very serious falls and sometimes it’s minor falls.
P1
My knee giving way, like the muscles suddenly weakened . . . as I was picking up heavy things, my legs felt weak . . . I am used to them crumbling underneath me and sometimes I catch myself from falling.
P7
I started to take some heavy falls because my one leg kept giving way underneath me and it was then that they decided, well hang on a minute, you know, you’ve got some form of instability of not walking properly and I was forever going over on my ankles because I’ve got weak ankles and it was then that I started to go see somebody about getting some orthotics fitted.
P22
I wouldn’t be picking my feet up properly and I would trip and go full length.
P2
I can walk about but I have to keep sitting down and have a bit of a rest and I can’t, you know, stand talking to somebody, you think, oh, I’ve got to sit down.
P11
It was just my right knee that got a lot of wear and tear because the right leg is less straight than the left, and that’s the one that’s been bearing the brunt of not walking very well.
P2
Participants also commented on psychosocial impacts arising from their experiences of chronic pain and impaired ability to walk and be fully mobile. They expressed feelings of fearfulness and anxiety about falling which, in turn, led to diminished self-confidence and independence.
The last time I was on the tube I got pushed, literally, and I lack confidence and get scared, so if I haven’t got somebody with me, I won’t use the tube.
P23
Usually when I go out of the house always my partner has walked at the back of me, always, and he needs a medal.
P4
A number of participants expressed concerns about their future ability to carry on driving, or working, with implications for becoming dependent on others. Deterioration of their neuromuscular condition, leading to possible further impairment in the future, was a pre-occupying concern of almost everyone interviewed.
I used to be able to go there quite quickly . . . I used to work for this mobile crèche and I used to get on trains confidently . . . but I can’t do that now.
P7
I was . . . in a very well paid job and of course one of the main things of Charcot–Marie–Tooth does, not only does it affect your walking but it also affects your hands and it affects your dexterity. Well as an electronics engineer that was the one thing that I actually depended on was my hands and my dexterity and of course I had to stop work . . . and so what am I going to do now for the rest of my life because, you know, I’ve still got a long working career in front of me . . . my condition has got worse as I’ve got older.
P22
I am concerned that the knee is going to get worse. I’m 64, not desperately old yet. You know in 10 years’ time, it’s not going to be good. I can feel that it’s changing, my leg is changing shape. My foot now sticks out at an angle and my knee goes it, so it is all sort of collapsing a bit really. The knee definitely needs support.
P14
A few respondents revealed feeling socially isolated from friends while others expressed feelings of regret that they were unable to pursue activities they once enjoyed.
Because obviously I walk slower so I am on my own quite a bit because I have got good friends who will walk with me but a lot of people [won’t] . . . so it does actually make you an alone sort of person because society doesn’t really accommodate, it’s intolerant of people who have a lot of weaknesses . . . people who have never had that problem just don’t understand.
P2
We used to go to [place name] a lot but the last couple of years we haven’t because you have to go up the side steps . . . when it started to get a bit difficult for walking.
Husband of P13
Many of the people interviewed indicated that they were proactive in pursuing preventative measures to postpone and/or abate the impact of future deterioration related to their neuromuscular condition on their ability to mobilise independently. These measures included keeping their weight under control; exercising to maintain or enhance general levels of fitness through, for example, regular walking or swimming; and undertaking exercise regimens recommended by NHS or private physiotherapists.
I am going to have problems later on and I want to keep independent, if I could prolong independence . . .
P15
I saw how much I deteriorated in the last 5 years you know . . . I try to help myself, I am trying, like with my weight for example, I have to work on that and my exercise . . . I am active.
P22
I’ve been referred to a physio . . . I was also sent for some hydrotherapy treatments . . . but I mean I used to go swimming anyway but I don’t any more because I find it’s too difficult to get in and out of the pool and I’ve lost a lot of upper body strength as well over the last 2 years, so swimming I now find very difficult . . . basically it’s down to me to monitor my condition and then if I think I need to do something about it then I’ll go and see my GP and then my GP points me in the area of where I need to go.
P22
None of the 19 study participants recruited via the NHS reported belonging to a patient support group, whereas four out of the five participants recruited outside the NHS were recruited via patient support groups, including British Polio Fellowship and Charcot–Marie–Tooth UK. (The fifth non-NHS recruited participant had read about the study on the dedicated web page and made contact with the study team.) The benefits of support groups cited were related to obtaining information and gaining mutual support from people with similar experiences.
People say, oh, I’ve tried this and I’ve done that and you can get information from people and it’s just being around people with the same condition because when I first got diagnosed with this [CMT] I felt very isolated . . . the reaction from medical staff was ‘what the hell is that?’.
P22
Of the 12 participants who had a diagnosis of poliomyelitis, four (P15, P16, P18 and P23) spoke (unprompted) about their concerns about future deterioration linked to post-polio syndrome, although P15 and P18 commented that they had encountered scepticism among HCPs about this diagnosis. These four interviewees were anxious about muscle wasting in their upper body, arms and hands, and associated potential impacts on their functional ability. P16 revealed his reservations about advice from physiotherapists because of his perception that non-expert advice could result in further damage.
Somebody told me that there’s a neurological physiotherapy department at [name of hospital] you know, sort of cut above the rest as it were and my quack referred me and I went along really quite apprehensive to talk to them about what they were planning on doing because I didn’t really want to be damaged and the problem with multiplex polio is exercise makes it worse rather than better. Exercise will improve your suppleness and what have you but if you start working the muscles too hard, you further damage the validity and quite a few people with polio ended up sort of worse off through having physiotherapy.
P16
Obtaining the ‘right’ orthotic device(s) to help with current and future mobility was, understandably, a central concern of all those interviewed for the study. Participants’ perceptions of the desirable features of a suitable orthotic device (or devices) will be discussed in the body of the report below.
Use of orthotic devices
The orthotic devices that patients reported that they had received varied according to their diagnosis and their individual needs. Generally speaking, patients who had been diagnosed with poliomyelitis had received KAFOs, which they frequently referred to as ‘callipers’; these had mainly been obtained though NHS orthotic services, although two people had sought devices through private suppliers, and others were considering doing so. Most of these people had more than one device. Some were in the process of being fitted for a new KAFO and were also using their ‘old’ device while they were, in their words, ’breaking the new one in’.
Some differentiated between KAFOs that they used for indoor and outdoor wear; most people were very keen to retain at least one ‘spare’ KAFO that they could fall back on in the event of breakage or need for repair of a newer device. Three people with a diagnosis of multiple sclerosis reported having been given an AFO, as did the two respondents with CMT disease. The study participants with spinal injury and the one patient with a diagnosis of stroke reported using knee braces and inserts in their shoes, which had been fitted and provided through NHS orthotics services. We also asked patients about other aids to mobility that they use, and participants reported using these aids in combination with, or sometimes, instead of, the orthotic device with which they had been issued. Table 13 lists the devices and/or aids that they said they had received or were using at time of their interview.
| Participant | Diagnosis | KAFO | AFO | Crutches/stick | Wheelchair/mobility scooter | Brace or splint | Insert in shoe | ‘FlopStop’a |
|---|---|---|---|---|---|---|---|---|
| P1 | Poliomyelitis | ✓ | ✓ | ✓ | ||||
| P2 | Spinal injury | ✓ | ✓ | |||||
| P3 | Poliomyelitis | ✓ | ✓ | |||||
| P4 | Stroke | ✓ | ✓ | |||||
| P5 | Poliomyelitis | ✓ | ✓ | |||||
| P6 | Poliomyelitis | ✓ | ✓ | |||||
| P7 | Multiple sclerosis | ✓ | ✓ | ✓ | ||||
| P8 | Poliomyelitis | ✓ | ✓ | ✓ | ||||
| P9 | Poliomyelitis | ✓ | ||||||
| P10 | Multiple sclerosis | ✓ | ✓ | ✓ | ||||
| P11 | Multiple sclerosis | ✓ | ||||||
| P12 | Multiple sclerosis | ✓ | ✓ | ✓ | ✓ | |||
| P13 | Multiple sclerosis | ✓ | ✓ | ✓ | ||||
| P14 | Poliomyelitis | ✓ | ||||||
| P15 | Poliomyelitis | ✓ | ||||||
| P16 | Poliomyelitis | ✓ | ✓ | ✓ | ✓ | |||
| P17 | Spinal injury | ✓ | ✓ | ✓ | ||||
| P18 | Poliomyelitis | ✓ | ✓ | ✓ | ||||
| P19 | Spina bifida/amputation | ✓ | ✓ | ✓ | ||||
| P20 | Spinal injury | ✓ | ✓ | |||||
| P21 | CMT disease | ✓ | ||||||
| P22 | CMT disease | ✓ | ✓ | |||||
| P23 | Poliomyelitis | ✓ | ✓ | |||||
| P24 | Poliomyelitis | ✓ | ✓ |
Wearing orthotic devices
Participants’ reports of the wearing of their orthotic devices ranged along a continuum from ‘full-time’ (daytime) use to non-use of issued devices. Some people (P3, P5, P6, P7, P19 and P23) reported that their device is essential for them to be able to mobilise independently and, consequently, they said that they would wear it for all daily activities, including at work, using public transport, and participating in social events and gatherings.
Other participants reported partial use of their device for a variety of reasons: some (e.g. P21) said that they might use their device(s) outdoors, but that they could manage to move around inside their own home without it, sometimes by holding on to furniture, as reported by P13. Other participants reported wearing their device only intermittently because they were ‘breaking in’ a new device (P6, P14); because they felt that they did not always need the support of the device, only on ‘bad’ days (P20); or because of discomfort (P18). P18, for example, commented that she benefited from using her KAFO for walking, but found it uncomfortable when she was seated as it ‘stops the circulation’.
Participant 13, with a diagnosis of multiple sclerosis, commented on a (KAFO) device that had been proposed to her in the past; she had given it a week-long trial but had rejected it because she could not operate the locking mechanism because of a lack of muscle power in her hands. Her husband appeared angry and frustrated that she had not been offered a more suitable alternative.
She [orthotist] told us to try it . . . it didn’t work because that’s what you’ve got to do, lock and unlock it . . . we had our hopes for that calliper thing . . . until we found out what we had to do with it.
P13’s husband
Participant 1 was the only person interviewed who responded that she was not wearing the provided orthoses at all; during her interview, she retrieved her KAFO from another room in the house, still in its plastic wrapper, and said that she preferred to rely on a stick for assistance with mobilising.
Most people who relied on a KAFO indicated that they viewed it as essential to hang on to their ‘old’ device during and after fitting of a new one, and some people appeared reluctant to make the transition to a new device, which could pose challenges to them in having to work through a period of teething troubles and possibly have to adjust their gait to some extent. They revealed that they would choose to wear the ‘old’ device for specific activities. For example, P15 had purchased a new, high-tech device from a private supplier and was trying to become accustomed to it at the time of interview; she commented that although she was endeavouring to use her new device for increasing periods of time, she relied on her ‘old’ calliper for her daily walking, which was extensive (up to 8 miles per day) and which she regarded as essential for her physical and mental health.
Interestingly, some participants commented that they did not use their devices all the time because they wanted to try to remain as mobile as possible without it and feared becoming totally reliant on their device.
I feel that if I completely rely on it I won’t be able to do without it.
P18
Use of mobility aids
Most of the people interviewed indicated that in addition to (or sometimes instead of) their orthotic device, they made use of mobility aids, such as sticks and crutches, wheelchairs and mobility scooters.
People commented that using one or two sticks afforded them extra confidence with balance and stability, particularly when walking outdoors, and more so if the ground is icy or slippy. Participants also commented that a stick could help with transfer and distribution of body weight, a strategy that they used to assist with pain management in their knee joint. P23, like many of those interviewed, had purchased a stick himself, without any form of assessment or measurement, and he commented that he was experiencing pain in his arm on the side that he used the stick; he thought that he should really be using two sticks all of the time to achieve a more even transfer of body weight, but was reluctant to have to rely on two sticks for walking, in addition to his KAFO, because he would have to recognise and come to terms with the continuing deterioration in his condition.
I’m at the stage now where I need two sticks to walk. If I want to go for a walk in the park, I take the two sticks with me and it’s so much easier, so much easier because I compensate a lot for my balance and I don’t realise how much effort I am putting, how much pressure I am putting . . .
P23
Some participants (P8, P16 and P17) stated that they needed to use crutches in addition to their orthotic device for walking even short distances. Crutches were also reported as being used for getting up in the night to go to the toilet, or when relaxing at home after work, after taking off the KAFO device, or for ‘popping’ out to local shops, when the sustained support from the KAFO was not needed. However, one participant commented that she had found it difficult to obtain comfortably fitting crutches.
I mean even my elbow crutches, they don’t make these elbow crutches to fit me any more, so I spent another two or three years chasing round the services to get these commissioned because the Health Service en masse provide the adjustable ones but they’re too long for me here. They don’t come small enough here for me because of my physique.
P19
Views about wheelchair use were polarised across the sample. For some people, combining the use of a wheelchair along with wearing of their orthotic device represented freedom and independence, for example to cover long distances, much further than they could walk, to travel abroad for work or leisure and to carry out activities (such as visiting shopping malls or supermarkets, or a Christmas fair) that would otherwise be denied them. P5 described ‘whizzing around’ in his electrically powered wheelchair, which he had purchased through an Access to Work scheme.
Qualities sought in a wheelchair were that it should be light (for easy transfer in and out of a car) and preferably not too large, so that it could easily be accommodated in the home. Some study participants who relied heavily on a wheelchair, such as P16, commented that they had one wheelchair for indoor use and one for outdoors, one of which had been supplied by the NHS and the other purchased privately. P19, who owned a manually operated wheelchair, commented that she has bought a hoist, at her own expense, in order to be able to load it into and out of her car without assistance.
Two study participants (P4 and P10) described their reluctance to use a wheelchair, although both felt that the benefits outweighed the drawbacks. P4 did not like the stares she attracted when out in her wheelchair. P10 said that she had avoided using a wheelchair for as long as possible but, as her condition deteriorated, she had had to accept that she could benefit from using one; when interviewed, she was planning to take her wheelchair with her on an overseas trip to see a close family member, and she also recounted how using her wheelchair enabled her to go out on day trips with friends.
Participants 1 and 2 viewed wheelchairs as anathema: P2 described having to use a wheelchair as her ‘worst fear’, whereas P1 said that she felt pity for people in wheelchairs because ‘they’re like thrown away, lost causes’.
The advantages of disability scooters cited by participants were similar to those of wheelchairs; P18 said that she had a disability scooter for ‘getting around’ and a ‘luggie’, a small disability scooter that was useful for travel as it could be folded up for taking on an aeroplane. P12 said that he had resisted buying a mobility scooter for a long time, but then saw one in action at a festival and realised how useful a scooter could be; for instance, it facilitated independent mobility without the need to have someone else to push the scooter.
Fitting/acquisition of orthotic devices
Fitting/acquisition of knee–ankle–foot orthoses
Study participants reported a range of issues relating to the fitting of their orthotic devices. The fitting of devices appeared to be perceived as potentially particularly fraught with problems by patients who had a diagnosis of poliomyelitis and who had been fitted for KAFOs. Problems cited included the protracted length of time it could take to achieve a well-fitting and comfortable device, and frustration at the number of appointments that this could necessitate, and a perception that when appointments were delayed or cancelled no progress was being made; the need to have a reliable and comfortable spare device while being fitted for a new one, and during the period of ‘breaking in’ the new device; measurements being taken and lost before transfer to manufacturers; and participants feeling removed and remote from the manufacturers of devices. Quotations from individuals are reproduced here to illustrate the above points.
The latest device has been in progress for the last 3 years . . . the last two and a half years of my life I’ve really struggled to mobilise.
P19
So it was nearly 3 years before I got the brace completely right and then I wanted another one as back-up.
P15
If you’re not getting the appointments, you’re not getting the work done.
P19
They took a total of four casts of my leg because they kept losing the casts.
P9
I think there is a factor of distance and anonymity . . . it is exacerbated in our system by the remoteness of the patient from the manufacturers who are actually doing something bespoke.
P8
The thing that is upsetting me is that you get measured for these callipers, they keep sending them away, I never actually see the people who make the calliper or who do the adjustments.
P18
It takes the clerks weeks to send the order out and I managed to get them to agree to let me go over there [to the manufacturers] and do the trial fitting there and then I went back a few days later and they’d finished and I got the callipers in about 8 days.
P16
A minority of people reported that, after a lengthy period of fittings, they were disappointed to receive a device so ill-fitting that it was unwearable.
It was too big, too high, so it was going right into my buttocks, it was too wide, so I wasn’t getting support at the knee and it was like it wasn’t made for me.
P15
It wasn’t a question of being uncomfortable, it didn’t fit. It just did not fit, it didn’t fit and they took it away – I didn’t even leave with it.
P8
Participants expressed mixed views about the length of time that was available for fitting during appointments. Some people mentioned being allocated double appointments, allowing for more time, whereas others commented that the time available was not sufficient for them to identify problems with devices, which are really recognised only once the patient returns home, and then they had to wait for further appointments to follow-up with questions, comments and suggestions.
You know you don’t really sort of pick up that sort of a problem when you’re having a short appointment. You try it on and you think, oh these are a bit odd, I don’t know how this is going to be. But you’re only there for 20 minutes, half an hour and then you take it home with you in a bag. You only think about the questions afterwards.
P14
In clinic time you don’t really have time to try it out properly, you don’t necessarily have enough time to adjust how it’s feeling and what’s pulling and what’s feeling uncomfortable and what’s feeling stiff . . . you can only do that if you’ve tried it on at home . . . then you might have to wait three to six weeks to report back on it.
P19
Three participants, two with a diagnosis of poliomyelitis (P5 and P14) and one with a diagnosis of spina bifida (P19), considered themselves to be ‘complex’ cases, posing particular challenges to those fitting them with an orthosis, because they are not ‘standard’ cases. P5, for example, reported that his foot had been ‘fused’ to assist with walking; he commented that he had undergone a long and fruitless period of fitting and re-fitting for a new device before being seen by an orthotist who adopted a different approach. During his interview, he suggested that perhaps it would be beneficial to have case conferences for complex cases, involving more than one clinician, to focus on specific problems from different perspectives, so that lessons could be learnt.
It was not a standard case, it made it much harder and I think for those first 2 or 3 years where it seemed I was going backwards and forwards to [place] and I felt that no real progress was being made and I think it was almost when someone else came on who looked at it with different eyes and thought of a different type of solution, that’s when it clicked really I would say,
P5
When things are taking that length of time, what I would have assumed is that they would have had some form of case meeting with all the orthotists and say, look this is atypical let’s get our brains altogether around it, rather than just allowing one person to struggle with it . . . because at the moment the system is that you turn up for, let’s say an hour’s appointment and that’s it really. So each clinician has their patients . . . which is fine in the normal run of things but when you’ve got an atypical case like myself where you’d have thought that someone managing the clinician side would say, oh for the last year we’ve had no progress really, can we have a look at this or could we collectively look at this and then also let’s even write it up to help future clinicians with an atypical cases so there is a resource there to say, oh this is what we, at the time, thought what the issues were. This is what we thought the options were. This is what we tried. This is what worked and what didn’t work and then there’d be some form of learning within that profession as well.
P5
The need for people to be fitted for and receive appropriate footwear at the same time as being fitted for a new KAFO, was highlighted by P23, among others:
When you do a new calliper you need new boots okay, because no matter what, no matter how accurate the measurements are, they can’t get it spot on. So every calliper kind of needs a new boot to kind of fit it, so I was wearing the old calliper and had to wear it with these boots which didn’t kind of work with the calliper.
P23
Three participants (P8, P15 and P24) included in the study described seeking devices through private suppliers. P8 commented that he was motivated to do this because of poorly fitting devices that he had received through NHS services. He referred to a KAFO that he had recently received via the NHS as ‘a disaster’ and described how he had made contact with a specialist orthotics unit located outside the UK, saying that he was impressed by the thorough assessment and fitting processes that were being proposed.
Then he [specialist clinician] said, what we would normally now do with you is take you to our gait room where we have cameras linked into a computer programme where we get you to walk and up and down stairs and for and back, side to side and we work out your gait. So I’ve had none of that in UK . . . and then he said, once we’ve done that, we will then draw up a specification, a CadCam model and then we will take a close plaster fit of it and then we will work with that and then we will make a, this wasn’t the word he used but it describes it, we will make a unique prototype for you which will be nowhere near the finished but it will be the fit and then you will come back and then we will go through the whole process again and then when you’re satisfied and we’re satisfied, we will then finish it.
P8
In the case of P15 and P24, their prime motivation for purchasing a device from a private company was the desire to explore new options for technologically advanced devices that might not be available to them through NHS services.
I came to realise that there were new devices out there that could enable me to have the height of my left leg adjusted to the same as my right leg so that I’d have bendy knees so that I could develop a more normal [gait].
P15
[Name of company] are . . . brilliant . . . it’s amazing what they do there . . . it’s unbelievable the technology!
P24
In the study sample, one person (P2) said that she was waiting to take up an appointment at a gait clinic for gait analysis to be carried out; P19 reported having had her gait assessed on two occasions during the past 3–4 years; and P8 commented that he was told that if he chose to pursue the acquisition of a KAFO via a private clinic he would undergo a thorough gait analysis. Other participants did not mention having had gait analysis carried out.
Fitting/acquisition of ankle–foot orthoses
Participants with an AFO commented that either they had been fitted with their device after a plaster cast had been taken, or they had received an ‘off-the-shelf’ device from an orthotics unit; overall, most people were satisfied with the fit of their device.
I had a casting like a cast put on my feet, quite a nice warm feeling.
P7
They had a look and tried one or two, they’d got various ones that fit right, and we went from there, and this one is quite adjustable . . . you can lengthen it or shorten it.
P11
However, being measured for an AFO did not seem to guarantee satisfaction with the finished device. P21, with a diagnosis of CMT disease, described having a plaster cast and mould taken prior to be given new ‘splints’, but she reported that they were uncomfortable and painful to wear, and expressed fears about developing sores on her leg and foot.
I’ve just had a new pair . . . at the moment I’ve been bedding them in but something is not quite right with them . . . I’ve worn them for about a month but my foot has started to hurt again and I think the padding is not quite in the right place . . .
P21
This participant commented on how important it was to keep her ‘old splints’ for wear until the problem with the fit of her new ones was resolved. Like many of the people interviewed, she appeared to have a disinclination to let go of the device that she had grown accustomed to over a long period of time, in exchange for a new one, which she perceived as not being an exact replica of the old one. P21 appreciated having an ‘open appointment’ to attend her local orthotic services, which meant that she could return at any point during the coming year with any problems relating to her device(s) without having to be referred by her GP.
Acquisition of knee braces
During their interviews, two participants (P4 and P13) focused on how they had acquired their knee braces, one of which (belonging to P4) was described as a ‘Swiss knee brace’. The devices issued to these two patients were said to be ‘off-the-shelf’ items; in neither case were measurements taken nor did the devices fit properly, with the result that the recipients were reluctant to wear them.
Participant 4 commented that she had received a device that was too small for her, having been told ‘they only do small and large’ and the one she was issued with apparently ‘dug in’ when she walked, resulting in pain and bruising, and affecting her mood adversely. This patient indicated that she would have liked to have been measured for her brace and felt she was offered a restricted range of options.
I just couldn’t bear it. When I walked it just dug in, you can see the bruising . . . I got, not tearful, but sad . . .
P4
They said there’s nothing else, only these . . . but there are about a dozen on the internet . . . I would have preferred a bespoke one right from the start.
P4
Participant 13’s husband commented that the brace his wife had recently been sent was ‘too big, they’ve sent us the wrong one . . . any time we need a new one we just phone up and they send us one out but they’ve sent the wrong one out, they’ve sent a large one out’.
One-quarter of the sample participants mentioned making adaptations to their devices themselves to improve the fit of their device, to protect their skin or improve the function of the device.
I have to like pad out the knee to keep my knee really straight and they’ve tried making the straps tighter fitting and stronger but I still at the moment have a sock wedged in the strap because if my leg is not completely straight . . .
P15
How use of orthotic devices (knee–ankle–foot orthosis, ankle–foot orthosis, knee brace) may impact on skin
Most of those interviewed said that they were not experiencing ill effects on their skin due to wearing a KAFO or AFO, in part seemingly due to measures that they themselves took to prevent skin damage, such as wearing tights or leggings or pyjamas under the device to protect the skin, adding extra padding at ‘pressure points’ or, in one case, (P9) buying his own ankle straps, made of softer leather than those that were supplied. One participant (P16) stated that he had worn devices made from plastic in the past, which had resulted in skin rashes, and he expressed a preference for callipers that were made from metal and leather because he thought that devices made from carbon fibre would cause problems similar to those made of plastic.
Wearing the device in summer if the weather is warm was associated with discomfort and some participants referred to the pleasure of being able to take the device off at the end of the day, to allow their skin to ‘breathe’.
During the summer I quite often wear pyjama bottoms underneath the straps and leather of the calliper . . . they have to be cotton and easily washable.
P9
One year I was on holiday and it was very hot, very hot and I didn’t have any tights on and, oh, it just rubs your skin, you can’t wear it against the skin. I had blisters . . .
P18
It’s a bit like taking your shoes off . . . when I take the calliper off the skin can breathe a bit more.
P3
Skin damage was mainly associated with newly acquired and/or ill-fitting devices, when some people said that they might expect some ‘marking’ or ‘bruising’ at pressure points, such as where the knee is in close proximity to the joint on the KAFO. P4 commented that she had received an ill-fitting knee brace after her stroke, and suggested that this had caused her to develop bruising. P21 reported padding out her AFO to protect the skin on her shin after receiving a new device, and expressed concern about wearing her newly issued device, specifically because of her anxiety about possible skin damage. In the past, she had suffered an open sore associated with her device, for which she had needed to seek treatment from podiatry services. P16 was the only person interviewed who described experiencing a long-standing leg ulcer during the time that he has been wearing a KAFO. The cause of his leg ulcer had apparently not been established, but P16 described a difficult period of non-healing of the ulcer, with frequent recurrence, despite intensive treatment in a specialist clinic. He suggested that non-healing of the ulcer may have been protracted by friction from his device.
They [ulcers] kept forming and they thought they’d cleared but all they were doing was getting them to heal over but there was still rot inside, the thing seemed to be caused by either pressure or something on the side of the ankle from my boots, so the orthotist built the boots out and then put different types of pads in . . . foam pads on and things like this. None of them stopped the reoccurrence and I guessed that what was happening was we were getting sheer forces from the action of the calliper and the boot because as you stand and put weight on one leg with a calliper, inevitably you get a sort of piston effect of your leg within the calliper . . .
P16
Positive and negative aspects of orthotic devices
Positive aspects of devices
Participants’ perceptions of positive aspects of their orthoses were closely linked to perceived effectiveness of the device to control pain and offer support for the knee or ankle and/or assist with lifting the foot; reliability and durability; and capacity of the device(s) to promote self-confidence and independence through enabling mobility and participation in work and social activities. People who were satisfied with their orthoses talked about the ‘transformative’ effect that they had made on their lives, as illustrated in the quotations, below, from one person who wears a KAFO and one using an AFO on each lower limb.
Oh transformative really . . . before the brace I would say that as soon as I got into the pub, I’d have to sit down because of the pain. I couldn’t necessarily stand up at the bar and talk to people at the same level because of intense knee pain really, so in that way it’s really transformed my life also from here to [place name] may not appear sort of a long distance but for me I don’t think I could have done it beforehand, now I can which is a major sort of step forward . . . it’s made me much more mobile than I ever was before really.
P5
. . . the best thing I’ve ever had. I’ve got to say it, they are because I was walking I was frightened to go out . . . It’s just like, they just give me a new lease, you know, they give me my independence to go out on my own which without them I haven’t got, you know. As I say walking around the house is different. I’ve always got something to hold on. It’s when I get out there, I need the confidence to and I feel with these splints I’ve got that my independence. I can go out without my husband, I can go out with my friends, you know.
P21
Study participants commented in detail on the physical properties of their devices that they liked and appreciated, which were mainly associated with comfort, durability, cosmesis and ‘user-friendliness’. Properties cited included ease of getting the device on and off; light weight; fastenings that are easy to manage and reliable [some people preferred Velcro (Velcro Ltd, Cheshire, UK), whereas others preferred leather fastenings]; ease of use with preferred clothing, for example, ‘normal’ trousers; ‘breathability’ of materials used, and, of central importance, a device that can be relied upon to withstand wear and tear without sudden failure to function. A quotation from P3, who wears a carbon fibre KAFO every day in his job as a teacher, serves to illustrate a number of these points, alongside a series of briefer quotations.
It has carbon fibre, so it’s lighter and it’s meant to be better. So I think it was made in [European country] and personally as someone deeply in favour of getting the latest technology . . . the benefit is to have one joint so it’s not as wide, I can use most trousers. The other one [previous device] is a lot wider underneath, so then if I go to Primark as I don’t have any trousers that fit, so it’s better to be lighter, looks better design which is better and the issue I have with it is the joint because I use the calliper quite a lot, with teaching, you know, go up and down a lot of the stairs. There’s a lot of wear and tear . . .
P3
I like it . . . it feels comfortable, I quite like all the Velcro bits. In the old days, it was a sort of buckles system.
P5
It’s speedy . . . you can put it on quickly.
P6
It’s a full 5 minutes job [to put her KAFO on].
P19
It’s more comfortable than the old one, than the plastic ordinary one . . . it’s fibre, not like the older ones . . .
P7
He said, we want to make you a carbon fibre and he showed me one at the time, which looked pretty cool . . . it just looked really, really good.
P8
Modern day materials are absolutely fabulous . . . there is no weight in them and they are very strong.
P22
Negative aspects of devices
Negative aspects of devices highlighted by study participants included devices that they judged to be ineffective; ill-fitting (causing pain, discomfort or skin damage); unreliable; uncomfortable; heavy, bulky or cumbersome; aesthetically displeasing; damaging to clothing and/or footwear; difficult to put on or take off; susceptible to breakage; and/or requiring high maintenance in the form of frequent repairs and readjustments (see Figure 2). When negative aspects of a device were perceived to outweigh positive ones, participants indicated limited or non-wearing of the device.
Of the total 24 participants interviewed, P1, with a diagnosis of poliomyelitis, expressed the most extreme negative feelings towards the orthotic devices that she had received. P1 reported that she was not wearing the KAFO and AFO that had been supplied for her, preferring to mobilise with the use of a stick. This participant, a woman in her thirties, works to support her family in an occupation requiring her to be physically mobile and able to stand for long periods of time during the working day. Her disappointment with the devices she had received became very apparent as the interview proceeded. The Velcro fastenings on her KAFO did not hold it in place, making it unwearable. She felt the devices supplied for her were a ‘complete waste of NHS money’ and that the orthotist had not listened to her when she was describing her problems and needs. P1 said that she had stopped attending appointments with local orthotics services, although was waiting to be referred to a different orthotist after a visit to a rehabilitation consultant. P1’s disappointment and frustration are reflected in the following quotations.
Ankle–foot orthosis
Perceived as ineffective
It’s supposed to help my ankle.
Causing pain and discomfort
It was cutting me and the shoe . . .
Damage to footwear
The ankle one, I’ve given up on it because to be honest, it’s ruined my shoes.
I put it inside my boot, which it has ripped my boot and damaged them.
Knee–ankle–foot orthosis
Perceived as not secure
This [brace] is for my knee . . . this doesn’t stay on my knee, as I am walking it falls right to the bottom . . . it doesn’t even stay on my leg.
Can you imagine, as I am walking it’s slipping down.
Perceived as ineffective
If it worked, it would be OK . . . there’s no benefit in it.
It just doesn’t do nothing . . . I swear, I feel completely hopeless.
Difficulty in putting devices on
These items are becoming like a toy . . . like a jigsaw puzzle that I’m having to deal with. I have to put my knee on, then I have to put my leg on before I can put my shoe on, and I still have to use my walking stick by the way with these on.
Non-wearing of device
I’m not going to lie or say I wear it, it’s just sat in the bag looking pretty.
Frustration with orthotic service
The last appointment I didn’t even know, because I didn’t go, because I was just like, I’m wasting my time . . . I just cancelled it.
Doctor [name] took a picture of them . . . he put them up on his table and took a picture of them and laughed, he couldn’t believe it . . . the device they gave me.
Male and female participants reported having to restrict their choice of clothing and/or footwear to accommodate their devices, as well as damage to clothing that caused inconvenience and expense. In particular, it was reported that fabric would be damaged when it comes into contact with the joint of a device. Male participants commented that they were restricted in their choice of trousers, which could prevent them from being able to appear smartly dressed on formal business or social occasions.
All callipers are not great in terms of heat in summer and in hot weather you’ve got plastic and metal against your skin, and you have to wear socks and boots in August . . .
P6, female
You get ladders quite a lot [in tights] . . . my tights aren’t 50 pence a pack . . . you’re going through them very rapidly . . . Velcro snags very easily . . .
P19, female
The other thing that callipers do, if you get quite expensive trousers, they tear them up and just rip, you know, even linen or wool trousers, they will just tear . . .
P6, female
Two wears and the trousers are gone . . . it’s really annoying, I can’t buy decent trousers.
P23, male
I don’t wear super tight jeans!
P9, male
It’s difficult to get trousers that are really wide . . . so I buy women’s jeans . . . from second-hand shops, the charity shops . . . callipers damage them, I can’t pay £50–70.
P16, male
It’s really annoying, I can’t buy decent trousers.
P23, male
Issues concerning footwear were commonly reported by participants in relation to their orthotic devices, and will be discussed in more depth below. Some participants experienced difficulties and discomfort when trying to accommodate a new device with their existing footwear; some said the orthosis caused shoes to wear out more quickly, whereas others appeared reluctant to exchange their usual footwear for new footwear that would more easily accommodate a new device.
Getting a shoe on with my [new] calliper is very difficult . . . I mean it’s not very comfortable in the shoes that I’ve got because they’re not purpose built for the device.
P14
You just can’t get in most of the shoes and it’s very bulky because of this strap going round your ankle . . . you’ve got to be wearing the right shoes . . . you couldn’t really wear the shoes I’ve got on now, or my work shoes. You’ve really got to wear outdoor shoes that are very wide . . . in fact we only managed to get it in one very, very big pair of trainers . . . and I just said straightaway, I’m never going to be able to wear this, it was just horrible, very, very impractical.
P12, discussing an AFO supplied to him but which was not used
I buy [brand name] shoes because they are a bit more sturdy but even then they might only last 6 months. Most of them really should last 2–3 years, so that is a cost.
P3
The look and feel of the orthotic device appeared important to both male and female participants, with the ideal suggested being a lightweight, sleek, discreet device. However, some participants reported having devices which they found heavy and cumbersome.
It was a big clunky piece of machinery . . . the whole thing was just too clunky. It was substantially heavier and almost cruder.
P9
I’m not really getting on with it [new KAFO] . . . it is such a cumbersome, difficult thing.
P14
Several participants expressed a wish to obtain devices that are more technologically advanced than the ones they currently had.
I don’t know if they’ve got electronic knee devices now that I can stand up without having to hand lock it . . . I’ve been wearing this for years and years. Technology has been zero on it, absolutely zero . . . I mean there are so many new materials available, I am sure they could use titanium, all kinds of other materials.
P23
A dislike of Velcro fastenings was expressed by number of those interviewed, the main reasons cited being that Velcro did not hold the device securely in place, or that it ‘snags’ on tights, and collects fabric fibres and fluff, making it ineffective; one participant (P16) said he disliked Velcro fastenings because they could not be quietly adjusted, for instance, during a meeting.
I have buckles, I hate Velcro . . . you can’t adjust it quietly so if you’re in a meeting or something and it’s too tight . . .
P16
The aspects of devices that were considered highly negative by participants, and significantly concerning, were the likelihood of a device to fail in some way and a need for frequent repair and/or readjustment. Both had the potential to have a hugely negative impact on patients’ confidence, ability to be mobile and get on with their daily lives. The breakage of a device was viewed as a crisis. Participants suggested that there might be a potential trade-off between the lightness and elegance of a device, and its durability. Some people questioned the quality of various components used in the manufacture of devices, suggesting that, for reasons of cost, the most effective or enduring components might not be routinely used. Comments on the functioning of rivets and hinges in KAFO devices were frequent. Participants provided vivid accounts of specific incidents when a device malfunctioned in some way, especially concerning if it happened when they were travelling abroad. Fear of failure of a device seemed to be the major underlying reason for participants repeatedly commenting on the importance of having a spare device that could be used as a replacement while the new model was being repaired.
if it breaks, you’re kind of thrown really . . . I came out of work, it was about half past six, and I got across the road and the calliper just went, it just snapped, it was one of those carbon fibres . . .
P6
As soon as this thing breaks down, I’m in silly street . . . that’s not a place I want to go.
P19
I would say the source of failure now, as opposed to the wear and tear, is the hinges, you need reliability in the hinges . . . this is one of the problems with this kit [device] . . . one of the hinges just failed without warning. So that’s an immediate demobilisation . . . so in the normal course of circumstances if you didn’t have a spare to hand, which I didn’t when I was away . . . one of the problems is how do you get that emergency repair effected immediately?
P8
Let’s assume that the strongest rivets are stainless steel rivets, well I was saying, well why don’t you use the stronger rivets to begin with. It’s almost as if they’ve got three levels of rivet, a bronze, silver and gold and you start with the bronze and they say, oh they’re not surprised that went and then they take you to silver and you almost say, well let’s assume that the gold variety of rivets was stainless steel, let’s assume, it may cost a few quid extra but it would make a huge difference because it holds the whole thing together really.
P5
When I had this one, I found that the rivets on it broke but these have been replaced but I always worry that these will go as well because they’ve been going and I’ve been told that there are two or three different types of rivets and I think it’s more when you’re twisting on the leg, it creates a torsion whereby the rivets after one or two pop it creates almost like a quick mechanism for the rest of them to go really. So that’s been an issue really.
P5
I get the knee joints failing which is terrifying because you go flying across the room on a good day and hit the floor on a bad day and you’ve completely lost confidence in that calliper then.
P16
Last year we went to [American city] and literally, literally we’d just landed at [ ] airport and I’m walking to get my suitcase and I fell flat on my face . . . what happened this, the thingy here, see there, it just went. Really is this screw holding me, I mean can’t you think of something stronger than that! That’s what I’m saying . . . the whole thing is very basic. All these should have been tightened up. Another time, the same thing here, the thing that they solder in the middle, the solder went, so that slipped out and again . . . the most unhelpful place on earth, [ ] airport, but yeah the holiday was ruined because I could walk but I had to do everything by hand and I sort of held it with thread and things. I couldn’t relax, you know, it was a short trip.
P23
Even a seemingly relatively simple procedure, such as having leather straps replaced when they wear out, could cause difficulties and inconvenience for device users.
The knee strap by virtue of the fact that it’s made of leather will after a while stretch, there’s no getting around that. So last time I had one replaced, I took it in and it got replaced and it was sent back out to me, but actually someone had put it on back to front . . . so I had to take it back.
P19
Participant 15 reported on negative aspects of the device that she had procured from a private supplier (a stance control model) including that it is adapted for walking only on level ground, which left her feeling frightened of walking downhill with it on. She said that she had to concentrate hard on thinking about walking while wearing it, as it seemed to require different walking patterns from her usual ones; she added that she had experienced a bad fall while wearing the device, resulting in damage to ligaments.
Participants’ perceptions of the negative and positive aspects of their orthoses are summarised in Figure 2.
FIGURE 2.
Study participants’ perceptions of positive and negative aspects of their orthoses.
Appearance connected to orthoses
For many people, appearance of their device seemed less important than other aspects, such as reliability and comfort, which they said they valued more.
Given the choice of a bad-looking orthotic appliance compared to one that is snazzy, obviously everybody would like a smart looking one but to me that’s not the be all and end all. I’m looking for reliability that’s the most important thing to me and one that doesn’t need a lot of repair.
P3
I’ve got to an age now accepting that I’ve got to be comfortable.
P2
Most of the women included in the study commented that they tended to choose to wear trousers, in part to conceal their device, as well as for more practical reasons. P18, a woman in her early seventies, commented that she ‘detests’ wearing her device, always wearing long skirts or trousers to hide it.
It’s a little bit of vanity as regards wearing something on top.
P4
It is horrible, really horrible to wear. I hate it. I detest it with a passion. When I wear it I always wear really long skirts to hide it or trousers.
P18
The desire to appear as ‘normal’ as possible when wearing an orthosis was expressed by both male and female participants. Among the men included in the study, P12 displayed strongly felt views on the importance of appearance in relation to wearing a device and he suggested that clinicians ought to pay it more attention. P12 commented that he did not want to have a device that made him look or feel ‘trussed up like a turkey’.
I think the appearance, I would always want to look normal as possible . . . and maybe other people don’t notice that you’ve got it on sort of thing.
P12
Orthotics a long, long time ago said that I ought to look at having something and it was something that went right down to my toes and virtually up to just below my knee and . . . you needed two or three sizes bigger shoe. In fact I think we only managed to get it in one pair of shoes and that was a very big pair of trainers and I said straightaway, well I’m never actually going to be able to ever wear this. It was just horrible, very, very impractical. I’m not even sure why they actually gave it me at the time.
P12
If it was left to them you’d be trussed up with, you know, yes things that make you walk absolutely great but you’d look like a clown in the street sort of thing rather than thinking now what would make you look normal, that’s really what I mean. They work on thinking they’ve got to make device as effective as possible to get over the disability and blow the what it looks like whereas I think you’ve got to have a bit of a compromise at times, that’s what I would say.
P12
A number of participants expressed the wish for uniform colouring of their KAFO, and for the last few inches of a device (towards the ankle) to be in a colour which matched their skin tone. This was underlined by P5 (male) and P19 (female) who pointed out that they regarded their device as an extension of themselves, integral to their identity. P19 stated that ‘the cosmesis is really important to me otherwise we are back in the dark ages’.
Why have we got the blue leather with the flesh colour . . . I don’t get it . . . why have we got flesh colour leather if we’re going to put the blue/grey round the outside? . . . actually this is how I present to the world and it’s very important to me . . . I refuse to be Polly Patchwork . . .
P19
So I was thinking, oh I’ve seen prostheses with various skin colour and they do whatever it is the fibreglass with different colours and I’m thinking why isn’t that given as a possibility in terms of different types of skin colour when you’re sort of wearing it and things, just where you feel, it’s an extension of your own leg but sort of it’s part of you in a way.
P5
Participants reported that they could feel conspicuous when their orthosis was visible to other people because of the stares they could attract; some people mentioned feeling discouraged from going to the gym or swimming pool because they could feel people looking at them, or because they sensed the discomfort of the people around them. Other participants commented that they did not want to evoke feelings of pity in others, nor to feel stigmatised or concerned about their personal safety if they could be identified as ‘disabled’.
If I go to the gym, there is only one piece of equipment I can actually use but people don’t seem comfortable even about advising you . . .
P6
When I go on holiday there’s a pool and I can swim, you know, without an audience.
P23
You walk into a restaurant, and where are the eyes?
P4
I’m not trying to make people feel sorry for me . . . I don’t want nobody to pity me. I don’t want them to feel sorry for me. I want to get on with my life. I know I have got a disability but do I have to let the whole world know that I am disabled?
P1
Sometimes I wear shorts in summer but when people see me they act different to me . . . the fact they can see my calliper.
P3
People can come up to you and you never know when, any kind of disability can trigger you know, nice and not so nice behaviour and so I suppose in a way even though like covering it up, you know, you are identified as disabled, it feels a bit more safer . . .
P6
A few male participants downplayed the importance of the visibility of their orthotic device. P8 suggested that he was quite uninhibited about wearing his device with shorts and a tee shirt when on holiday abroad. By way of contrast, he recounted how the ‘invisibility’ of a device was important to a business man who, he had heard, had sought a discreet, high-tech device that he could conceal from view.
It’s easy for a man, you just stick [the orthosis] in your trousers and nobody notices it when you are walking about.
P11
I’m in my Bermuda shorts . . . so I wear my undercarriage [device], training shoes and a tee shirt, so there was good visibility of the undercarriage and I went for a walk through town, I was comfortable, I wasn’t the slightest bit bothered at all.
P8
This Aston Martin carbon fibre device built for this business man, he wanted its actual existence to be invisible . . . he had a strap that came into his pocket in his bespoke design suits and he just puts his hands in his pockets and click it and sit down as if it was a normal leg . . . he doesn’t want people to know.
P8
Footwear
People interviewed for the study referred to the footwear that they used with their orthoses by various terms, including shoes, boots, orthopaedic shoes and surgical shoes. People who used KAFOs and/or AFOs stated that they might be fitted for new shoes at the same time as being fitted for their orthosis in order to accommodate that particular device, whereas others said that they used their device with ‘normal’ shoes that they purchased themselves. Many respondents mentioned using orthotic devices, such as insoles and inserts, in their shoes; others described how each shoe of a pair might need to be of different width or height, depending on their individual needs.
Participants whose first preference was to wear ‘normal’ shoes seemed to accept that they might, or would, have to move to having their shoes supplied through orthotic services at some point in the future. A number of issues connected to footwear emerged as important to participants, namely the fit of the shoes; damage to skin/and or deeper tissue; repairs; number of pairs of shoes supplied to individuals; choice of colour or material; weight of shoes; level of comfort; absence of ‘grip’; and appearance (Figure 3).
FIGURE 3.
Study participants’ perceptions of positive and negative aspects of footwear.
A major priority identified by everyone spoken to was that shoes should fit well. Participants with a diagnosis of poliomyelitis commented that they can require shoes of different width and height for each of their feet. Some people commented that they needed to receive shoes via orthotic services to accommodate their orthotic devices. P14 had recently received her first KAFO device but was not wearing it because she was facing difficulties using the device with her ‘normal’ shoes, although she reported that the orthotist had advised her to try to manage with her usual footwear, at least initially.
Since I took possession of it [orthotic device] I’ve been back to see the orthotist twice and he said, well do try and get on with it, but he said, if you really can’t we’ll think about getting some shoes made. My foot is stuck in the equinus position so the calliper means I have trouble with shoes . . . my shoes don’t fit . . . I can’t get it in my shoes, it’s uncomfortable and it just seems easier not to use it, which is terrible really because I should be using it.
P14
The importance of having feet assessed and measured frequently, to detect any possible changes, was highlighted. For example, P5 stated that recently his toes had started to curl under his foot, rubbing against the leather on one of shoes, causing pain, and he said his shoe had started to feel too small; he thought he would require new shoes, as well as a possible intervention through podiatry services. Only two of the people interviewed mentioned experiencing difficulties in getting their feet measured. P9 commented that he had been ‘wearing the same type of shoe without a refit since 1970’ and his wife suggested he needed to have a new mould of his feet taken. P15, who said she had not had her feet measured for > 10 years, found that the shoes she was receiving were becoming increasingly ill-fitting, and that recently she had ‘insisted’ on having a review and having her feet measured:
I had to insist and say, look none of your shoes is fitting me properly . . . I haven’t had them reviewed since the 90s and I’m a lot older . . . every time I come, something is wrong.
P15
Almost all participants who wore shoes that were provided through orthotic services suggested that they were ‘entitled’ to a certain number of pairs of shoes a year, and most people thought that they could have up to two pairs of shoes at any one time, which allowed for one pair to be undergoing repairs while the other pair was being worn. Individuals reported different time frames for shoes to be provided and repairs to be effected.
Participants said that shoes required repair frequently, as heels and soles wore out quickly as a result of their particular gait. Long waiting times were said to be frustrating, and people could feel anxious having only one pair of shoes for everyday use. Some people reported that they had been told to expect shoes to be delivered by a particular date, and feeling disappointed when they were not available when promised. P23 said that he had hoped to pick up a pair of boots on the day before a job interview but they did not arrive, and he added that he had been waiting > 3 months to receive a new pair of boots. He suggested that provision of footwear would be improved if the people making the shoes were on the same site as those involved in provision of orthoses, so that their particular expertise is readily available.
I think personally, if the shoemakers were on-site it would make a big difference because [name of orthotist] has to relay to that person and that person has to relay to that person, it’s like too much. Well that thing could have been resolved in a second, you know.
P23
The convenience of delivery of new or repaired shoes to a home address was appreciated. P3, who works full-time, said that a pair of new shoes were waiting for him to collect, but that he would be unable to pick them up until his half-term holiday, whereas extended opening hours of the orthotics department would enable him to pick them up sooner.
Choice of colour, fastening (Velcro or lace-up), material (leather or suede) and style of shoes were deemed more or less important by everyone interviewed. Several people mentioned that they tended to choose black or brown shoes as they ‘go with anything’ and are particularly suitable for work or business, and a few people seemed to think these colours were the only ones on offer. One individual (P5) made a strong case for having a choice of brighter colours, saying that wearing brightly coloured shoes is one of the ways in which he expresses his personality.
Because of my personality, I like bright shoes, for example, I always wore bright green shoes . . . these are quite bright red really, you can only go for one of the five colours that are provided really . . . I would be happy to pay for it, it is part of your identity.
P5
Participant 16 said that, in his view, choices concerning durability, appearance and comfort of shoes would vary according to the purpose or occasion for which the shoes were wanted, and he stated that people should not feel ‘pressurised’ into having black or brown shoes.
If you want some boots for everyday work, durability and comfort are important things, and appearance comes lower down the list. If you are the bride’s mother, appearance is number 1, durability is zero because you are only going to wear them once and comfort doesn’t come into it either, but without that information, they [manufacturers] are not able to match what they are producing to the requests, demands, or expectations of the customer.
P16
Participants 1 and 2 suggested that comfort was more important than appearance, although they said they would prefer footwear that was discreet.
There is no point in weeping over kitten heels because I am never going to wear those. But something reasonably presentable, that is not too intrusive, because you’ve got enough to be getting on in life with without having some people staring at things you might be wearing because obviously I am a bit conscious of the way I do walk . . . and people do stare a bit, so something that can be quite discreet is important.
P2
A normal shoe, but built inside, so it’s normal . . . so you can’t tell no difference, but build inside . . . if you don’t want somebody to notice that you are wearing something or that you’re disabled.
P1
Many people expressed a dislike of ‘heavy, clumpy’ orthotic shoes because heavy shoes compromised the ease with which they could walk. P22 felt that features of orthotics shoes have improved substantially in recent years to great benefit, but he was less happy about having to be referred to the orthotic service each time by his GP when he required a new pair of shoes.
Well compared to the original ones, they’re a lot more comfortable, modern day orthotics now have come a long way especially in the last sort of five years you know. My shoes that they make me are sort of a quarter of the weight that they was when I first started having orthotics . . . it’s all right having these things to help you to walk but it’s the weight aspect. Because obviously the more weight you’ve got on, that causes problems in itself because of the weakness in your legs, you know.
P22
. . . each time I have to go back to my GP surgery for them to refer me back to orthotics . . . it’s quite a long-winded process . . . I can’t go straight to orthotics.
P22
Choice of fastening for shoes (Velcro or laces) was important to some people, whether to accommodate a particular device or for ease of use.
I always wore lace-ups because I always thought Velcro was for old ladies . . . and he’s a lovely orthotist, he actually ordered me a pair of lace-ups and Velcro so I could choose between them and when I actually used the Velcro I could see that if I look like an old lady, tough, that is what I wanted.
P10
I just choose something that’s easy for me to bend down and put on . . . just a Velcro to get on in a morning.
P7
She’d like to wear something different but she can’t.
Husband of P13
I can’t . . . I wear laces all the time . . . with ankle boots.
P13
Several participants mentioned that the grip on the soles of shoes supplied to them was often not very good, making them anxious about walking on muddy, icy or uneven surfaces, and it was suggested that a walking shoe type of sole could improve confidence with walking outdoors. Two female participants (P18 and P21) who were purchasing their own shoes, favoured a particular make of shoes for their width, allowing accommodation of their device, and their suppleness and comfort. P21 mentioned that she was advised by the orthotist to try this particular brand.
Well, I have [brand name] shoes . . . the [brand name] shoe is flexible enough, it’s got room enough and the [brand name] has an insole which can be taken out and then the calliper fits in.
P18
She told me to try [brand name] shoes because they do really wide fittings . . . I like my tie-ups because I can lift the tongue and slide my feet in . . .
P21
Desired treatment goals and outcomes
During their interviews, study participants were asked to identify their desired treatment goals and outcomes and their responses related to a range of physical and psychosocial factors that were deemed important.
Orthotic treatment that achieved effective support for the affected joint (the knee or ankle joint) was identified as the prime desired outcome, along with a reduction in the number of falls and/or trips experienced. Participants highly valued gaining, or maintaining, the ability to mobilise as independently as possible. Reduction of joint pain was identified as an important enabler of increased mobility. Freedom from having to worry that their joint might ‘give way’ was cited as an important treatment goal.
People associated an ability to mobilise confidently and safely with numerous benefits for their physical and mental health: increased self-esteem; possibilities for employment and assurance about financial security for themselves and their family; enjoyment of ‘ordinary’ family and social life; opportunities for travel; maintaining an interest in daily activities (e.g. gardening, days out with friends and family); and prevention of deterioration of their physical health or mental outlook, through being able to undertake regular exercise, such as swimming and walking.
Most of those interviewed seemed to have accepted that they were unlikely to ever be able to walk very fast or for very far, without a degree of fatigue, and they showed little interest in increasing the speed of their walking, or distance covered, as treatment goals per se.
Participants defined their own goals for mobility in terms of what they needed to achieve to enable them to lead their lives in the way that they wanted, as far as realistically possible, based on their own knowledge of themselves as individuals and the circumstances of their lives. P16 vividly described his own treatment goals in terms of being able to take just a few steps from his wheelchair, which allows him to engage in family life, something that was clearly of great importance to him.
My walking is so limited. If I can stand and get something out of a cupboard and walk a few steps and get back to the wheelchair or whatever, that’s what I can do, and that’s what I need to do. For example in [name] mother’s house I can’t get the wheelchair in . . . because of the threshold, so I have to get out, walk a couple of steps over the threshold and then get back in the wheelchair and to be able to just walk those few steps to stand is an enormous advantage. If I was paraplegic and couldn’t get out of the wheelchair, there is no way I could get into their house. Same as my son’s house in [place], they’ve just moved actually to one where hopefully we will be able to get in but that just been able to walk, you know, four or five steps is fantastic . . . There’s a bigger difference between me and a paraplegic than there is between [name] and I, you know, if you are paraplegic and cannot stand at all that is an incredibly limiting thing. The fact that I can’t walk more than a couple of steps isn’t that bad because I can zoom over in a wheelchair, so that is a tremendous advantage to me and I would be very reluctant to lose that ability . . . you can’t imagine how useful that is . . .
P16
Participants associated different activities with the prime treatment goal of enhanced independent mobility and attached varying degrees of importance to them.
I think the key outcome is independence actually . . . for me, it’s just having that freedom not having to worry that my leg is going to give way and I know it is stable and that I can stand for long periods of time . . .
P5
They [her orthoses] just give me a new lease, they give me my independence to go out on my own.
P21
I just do not want to become housebound, so that primarily is the objective of my orthotics.
P22
I need my surgical appliance to get on with my life . . . it needs to be effective and reliable . . . I want to be in the real world. I love it . . . I’m in a working world and I just want to get on with it . . .
P2
It’s just enabling me to keep going as long as possible which is really important to me.
P10
Walking is essential to him . . . to keep active as much as possible.
Wife of P9
Most respondents were prepared to accept and use mobility aids, in conjunction with their orthoses, in pursuit of independence.
In the last 3 or 4 months I’ve been to [name of place], [name of place] and on a family holiday to [name of place], so with the brace and wheelchair combined, it gives me total and utter freedom to do what I want really.
P5
If my legs get a bit funny sometimes, like I’m putting the washing on the line, I just sit in the self-propelling one [wheelchair] because I can move it myself.
P7
I’ve got a [brand name] mobility scooter and I’ve got a luggie which is a small mobility scooter which can be folded up and it can go in an aeroplane and I can go on holidays with that.
P18
Retaining the ability to carry on driving was regarded as vital to the majority of respondents as it allowed patients to pursue opportunities for to work and socialise, both identified as important for their mental well-being.
It’s very important having a car, I couldn’t do my job without a car . . . I try to go by train but by the time I get there, I am exhausted.
P3
I want to drive down and pick my mum up and bring her here.
P10
I’ve got an automatic car . . . I’ve got a mobility car with a hoist in the back which can pick up my scooter.
P18
Participant 2, like others, identified close monitoring of her condition with a view to preventing future deterioration as an important short and long-term treatment goal:
it is sort of critical . . . to know that I am under someone’s care and that I will be looked at and monitored and not just left until it flares up again, I want somebody keeping an eye on it now.
P2
Care pathway and factors that impact on experiences of care
The majority of factors cited by participants as impacting on their care pathway were mentioned by individuals across all three research sites, as well as by people recruited via non-NHS support groups. These generalised perceptions will be discussed with reference to specific research sites where warranted. Details of the research site will be included in attribution of quotation to specific individuals.
Factor 1: referral pathways
The referral pathway into orthotic services was regarded as problematical by around half of those interviewed. Some people described having followed a very circuitous route: they went to their GP (often said to know very little about their particular NMD or condition), who then said that referral to an orthopaedic surgeon was needed, who would then make an assessment before referring to orthotic services. This was perceived as a lengthy process, which negatively impacted on patients through delays in waiting for appointments; waiting periods of up to 1 year to access orthotic services were reported.
I waited to see this GP again, another three to four weeks down the track, to establish that if I want to find out more about spina bifida I can go on-line and find a forum.
P19, site 3
I went to my GP and he said, no the proper way to do it is for him to refer me to an orthopaedic surgeon. But I said, I don’t want an operation on my right knee and he said, no, no, that’s all right, you just need to go . . . so I went . . . and saw Dr [orthopaedic surgeon] and he said, yes, I needed a calliper for my right knee, and he then he recommended me [to orthotic services].
P18, site 3
About once every 12 months I need to go back for a pair of shoes made but each time I want to go back I have to go to my GP to refer me back to the orthotics . . . it’s quite a long-winded process.
P22, non-NHS recruitment
The people who appeared most satisfied with their referral pathway were those who were under the care of a consultant in rehabilitation medicine, whom they saw at regular intervals for review and monitoring. These patients reported that their consultant was alerted to changes and deterioration in their condition during review visits, and could make a direct referral to orthotic and other specialist services, such as specialist neurophysiotherapy or gait assessment clinics. Patients with CMT disease and multiple sclerosis were more likely to report having experienced this more direct referral pathway than those with a diagnosis of poliomyelitis. It was suggested by one participant (P15) that patients with poliomyelitis could gain significantly from this kind of regular review, from a senior clinician, who could initiate appropriate referrals and who might pick up early warning signs of development of post-polio syndrome.
Reported routes for referral to specialist services varied between individuals within the different research sites. Some people suggested that non-specialist physiotherapists could refer directly to specialist physiotherapists, or that orthotists could refer patients to podiatry services, and vice versa, whereas others said that all referrals had to be carried out through their GP.
Factor 2: appointment systems
A majority of respondents made comments relating to their views and experiences of the appointment system in operation within the orthotic departments they attend. There were discernible differences between participants recruited in the different research sites. Participants in research site 2 seemed generally more satisfied with the appointment system than people in sites 1 and 3. Within sites 1 and 3, individual participants often drew a sharp contrast between perceived good clinical care and the administrative system, which, in one case, was described as ‘totally useless’.
My perception is that the clinical side is relatively good. The administrative side is totally useless . . . I have experienced numerous occasions . . . where the administration side have made appointments for me without ever telling me or where they have told me, I’ve turned up and the appointment has been cancelled . . .
P5, site 3
They keep changing the appointments and stretching the time plans, and actually the time plans are important.
P19, site 3
I got delayed for a month because I never got the appointment letter and they said they’d sent it to me but I never got it.
P2, site 1
Problems experienced by participants, which were perceived as affecting them negatively, included delays in waiting for appointments to access services or between appointments; the need to attend multiple appointments over long periods of time, sometimes with little sense of progress (e.g. for fitting or repair of devices); errors in administration of appointments (last-minute cancellations, letters arriving after the appointment date); appointment slots that were considered too short to ask questions or for information or explanation; and the possibility of ‘dropping off’ an appointment list if one or more appointments were missed (which could be due to administrative errors). In general, respondents felt that those who were involved in running the administration and appointment systems had little appreciation or understanding of the detrimental implications and effect of inefficiencies on individuals’ treatment.
The appointment system and the filing system were so wrong and just seemed to be so inefficient . . . you’d get a letter to say you’ve missed your appointment on a date that we never had an appointment or you’ve an appointment on such and such a date when you’d already been.
P9
So actually I got delayed for a month because I never got the appointment letter and they said they’d sent it to me but I never got it.
P2
The thing is they are just so busy . . . they’re just focusing on the leg . . . you feel guilty if you want to ask something . . . you’re just happy to get the calliper.
P23
Participants also mentioned difficulties that they sometimes faced when attending appointments: lack of private transport, yet using public transport was not a viable or preferred alternative; expense of using taxis; need for someone to be free to accompany them to appointments; difficulties of taking time off work (two participants who work full-time suggested extended opening hours would be helpful); orthotic units situated at a distance from their home, sometimes requiring long drives through traffic; and orthotic departments sometimes located a long walk from parking facilities.
One appointment facility (in research site 2) was identified as particularly beneficial by P21. This patient mentioned having been allocated an ‘open appointment’ to her local orthotics service for the duration of 1 year after being fitted with new orthotic devices. This meant she could return at any time during that period without the need for referral from a clinician. Participants in other sites had commented that more frequent and shorter-spaced appointments would be beneficial during the early period of being fitted with a new device.
Factor 3: poor record-keeping and inadequate transfer of information
Record-keeping and sharing and transfer of information across orthotic and other NHS services were viewed as deficient by a minority of respondents. P19 highlighted lack of time as the reason preventing clinicians from completing written records of consultations. Some participants described having to recount their history to different clinicians within a single service, whereas others indicated that information about them was not being shared across services.
He [registrar] had just transferred from another department . . . he had a blank file because he didn’t even have the physiotherapy letters in it as far as I could see and [name of doctor] had written letters back to the GP . . .
P2, site 1
The orthotics person, she always really listens but she never writes anything down . . . I feel as though it is Chinese whispers . . . I don’t think what she has to say gets through to the right people.
P15
I do find I have to remind the guys at times about things we have spoken of . . . if they’re not written effectively in the notes . . . they’re not getting the time to write the notes up either and details are being lost . . . they don’t have time to read the notes even if it’s in the notes.
P19, site 3
Interview accounts also included patients relating many instances of seemingly minor, but frequently occurring, incidents that resulted in treatment delays. For example, the measurements for their devices or shoes were often said to have been ‘lost’ in the system, and had to be taken again, and participants recalled occasions when instructions for repairs were not relayed or correctly followed out, leading to delays in them receiving the finished orthosis.
Factor 4: lack of coordination between services
People in the study described accessing care from a wide range of sources in connection with their knee instability or as a result of other health problems that were related to their neuromuscular condition. Lack of coordination between these services was identified as hampering patients’ progress in achieving the outcomes for which they had hoped. People under the care of a consultant in rehabilitation medicine seemed to have a stronger sense of themselves as at the centre of an integrated health-care service than others. For example, P5 described being shuttled between services while trying to access care for corns on his foot, which associated with his orthotic shoes.
I went to my GP and said, look, I’ve got these corns . . . went to the podiatrist . . . finally they put me in touch with a consultant podiatrist who can operate on things but they can’t deal with corns . . . so they say, you’ve got to go back to podiatry . . . and they say, it’s a recurring thing, you need to go back to [orthotic service] and they say, oh, no, that’s not our thing, it’s more the podiatrist needs to sort that bit out.
P5
My doctor [GP] suggested I go and see [consultant] because that [CMT] is one of her fields . . . and I know if I’ve got any problems she’ll sort it out and try and help . . . I always know that all I’ve got to do is ring her up and either go to see [consultant] or one of her colleagues and they’re pretty good at getting me to see someone.
P21
Factor 5: perceived ‘busyness’ of orthotics services
In general, participants were satisfied with the time available to them for appointments with an orthotist, although some people suggested that they would benefit from having longer or more frequent appointments in the initial period following acquisition of a new device, especially if receiving a KAFO for the first time. Two participants (P9, P16) thought that it would be useful for people receiving a device for the first time to be shown photographs or a video of the kind of device being proposed in order to help prepare them to receive the orthosis.
Individual participants mentioned instances of clinicians booking them double or even triple appointments in one block of time at certain time points, which they appreciated as beneficial, but this practice was not reported as uniform. Participants in site 2 expressed higher overall satisfaction with the number and length of appointments made available to them than respondents in the other two research sites. Reorganisation of services into new configurations was viewed by one participant (P19) as ‘diluting’ service provision in site 3. This long-term service user commented that orthotic services in her area seemed to be increasingly stretched across hospital Trust-wide sites, with the result that ‘her’ orthotist might be required to work across several sites, with increased pressures on the individual clinician. She also suggested that, increasingly, when orthotists were ill or on holiday, there might be no replacement available, resulting in disruption to continuity of care and further delays in treatment.
It’s part of the [name of trust] . . . it means they have kind of stretched the services even further and that means people like [orthotist] have to work from [Trust] on certain days of the week . . . so he’s physically not at [name] as much as he was so that’s even less options for appointments . . . the level of complexity I have doesn’t make it useful for me to see someone who doesn’t know enough about my work . . . they are more pushed and trying to see more people.
P19
Factor 6: complex care
Three participants in the study (P5, site 3; P14, non-NHS; P19, site 3) suggested that they might be viewed as having ‘complex’ care needs, requiring particularly intensive input from orthotic services at various crucial junctures in their care trajectory. P5 would have liked to have been the focus of a ‘case conference’ to help identify and resolve difficulties in his case that were impeding progress with treatment. P5 said he had waited for > 2 years for the ‘breakthrough’ that came when he was seen by a different orthotist. P14 described some of the problems she was experiencing with a new KAFO (she had not had one before) and feeling she would prefer not to have to wait the 3-month period until her next appointment. P19 expressed the view that she ‘selfishly’ felt that there should be a system for prioritising people with complex care needs.
[Orthotist] saying, why don’t we give that a go and see if it works . . . the difficulty that there was initially I think I spent about two or three years trying to explore different options and it was just because of the complexity of having my foot fused which meant the dynamics of the whole walking thing had changed . . . I was mindful that when you are an atypical case there is a sense of oh, we are out of our depth here.
P5
My foot is in the equinus position so the calliper means I am having trouble with shoes . . . I can’t get it in the shoe and it is uncomfortable and it just seems easier not to use it . . .
P14
People such as myself with what I do understand are complex or multiple needs are sat in the same clinic with people who have got fallen arches and bunions being dealt with . . . I’m not suggesting they don’t have a need . . . what I am suggesting is that there are different ends of the scale and you know, actually selfishly I feel I have priority over something like that but the reality is that the health service pushes you together, that’s part of the outcome we’ve got now with people charging into services that are actually specialised for a good reason.
P19
Factor 7: provision of ‘in-house’ facilities for fitting, adjustment and repair of orthotic devices
Availability of an ‘in-house’ workshop, located within the orthotic services department, was viewed by participants as significantly promoting and enhancing the delivery of timely and well-integrated orthotic care. Several participants in site 2 commented favourably on a fast response and turnaround when they had needed ‘emergency’ attention for their orthotic device. Study participants who used an orthotic service provider through which devices had to be sent away to manufacturers (sometimes abroad) expressed the negative impact that this could have on them. They recalled feelings of anxiety about having to relinquish their device, having to attend multiple appointments as the device went back and forth between the orthotic department and the workshop, and experiencing sometimes long delays before they were reunited with their device(s).
I used to go to [name] and they really didn’t have a good or should I say big orthotic department and they used to send my device to [place name] for repair, so even if it was a small thing they had to send it off . . . in [name] they have a special orthotic department where people work on surgical appliances . . . they can do quite a few repairs and sort it out an hour or two later, which makes a big difference.
P3
One of the places I was attached to, I went there and they’d got me a new one [device] and it was slightly out, just needed to be bent, the pins on one side kept popping out of my shoe with every step I took. So I went down there and said, look, can we just, you know, and they said, we haven’t got a workshop here. I said, well if you give me the tool I can do this. He said, no, you’ve got to leave it here and we’ve got to send it to the [place name] and it will be back in two weeks. Now that is just madness. So I went to [name of orthotics department] and they’ve got a fantastic workshop there and by the way it took about two and a half minutes to do . . . the guys there are fantastic.
P24
I just went in and said look I’ve got a bit of a crisis, it’s the only brace I’ve got any chance I’m happy to come first thing in the morning and wait all day to get the rivets sorted so I waited and they did do it there and then.
P5
Factor 8: NHS funding
Issues relating to NHS funding were mentioned by participants, in all three research sites, as seemingly impacting on patient care within orthotic services. Some participants referred to funding decisions relating to orthotic services being made at commissioning level, and sometimes they had appealed against the outcome of these decisions; others referred to ‘cut backs’ in funding, which they linked to restrictions in the number or type of orthotic device (or footwear) available to them; and some people referred to the apparent existence of a postcode lottery, whereby they thought that some people were more likely to receive specialist services (such as neurophysiotherapy) than others.
They would only give me two pairs of shoes . . . for someone who is walking you go out and get one pair wet . . . and one’s being repaired . . . you can be left without any shoes at all and I was saying, I needed three [pairs] and I had to go to [name] and put this complaint in to say I needed three pairs of shoes.
P15
He wouldn’t agree to me having a second calliper, he said no, they couldn’t afford it.
P18
One thing that does concern me is how the budgets are organised and spent, you know, certain things might be available to people in one area that are not available in another and vice versa . . . people in a far worse position than I am that do need these specialist services . . .
P22
Factor 9: perceived lack of business ethos within NHS services, resulting in treatment delays and hold-ups and lack of innovation
One participant (P16, non-NHS) highlighted a lack of ‘business ethos’ within the NHS as a barrier to patients receiving timely treatment through orthotic services. His views of NHS services in general, including provision of orthotic services, were that there is a lack of urgency in meeting contract deadlines, and no repercussions for suppliers or manufacturers if deadlines are not met; a lack of focus on customer service and satisfaction; and inefficiencies that would not be tolerated in a private company, resulting in wastage of clinicians’ and patients’ time. To illustrate his views, he cited the appointment system used by local services, commenting that it would be cheaper and quicker for patients to be texted or telephoned with details of appointments, rather than sending out letters to patients with second-class stamps, which could reach their destination after the date for the appointment had elapsed.
The whole NHS is not like industry or anything else . . . it’s what I call sheltered employment where people always do things the way they’ve done them before, they’ve not thought about the end product . . . the contracts the NHS writes are obviously very slack because the contractors take months in many cases. There’s no penalty clauses in for not returning them within a set period . . . you know, if the customer is waiting you’d say this needs to be returned within 21 days and we’ll deduct 15% for every 7 days you’re over that . . . sometimes months go by without anything happening . . . there is no sense of urgency, no sense of customer service, and I regard myself as a customer, not a patient . . . people are sitting around waiting for things . . . you’ve got people ringing up, chasing things . . . no private company could afford the wastage that the health service has . . .
P16
Participant 24 suggested that constraints within NHS budgets and work pressures resulted in lack of innovation in the technology of orthotic devices. He himself was using a ‘state of the art’ device supplied by specific company with a price label of around £40,000 and he contrasted his feelings about the different approaches of the NHS and the private sector to development of orthotic devices.
I’ve found sometimes through the NHS because of a number of financial constraints and lack of investment sometimes in new materials . . . I think the only change in 30–40 years was in the Velcro . . . no-one used to say to me, look, we’ve got this new idea, what about it . . . it’s almost like, if it works, just leave it . . . I think it’s the pressure they’re under . . . it’s all finances and pressure of work and budgets . . .
P24
[Name of company] are probably one of the best prosthetic leg makers in the world, they’re brilliant . . . they bring new models out . . . unbelievable technology . . . what I love about [name of company] is the way that, you know, like cars, you know the engineering and quality that’s gone into the design and that gives you huge confidence knowing what they’ve done in prosthetics and how good and safe these things are and I think that’s a big positive for going forward.
P24
Factor 10: use of private sector services
Respondents in the study who had contemplated or accessed private orthotic and physiotherapy services cited a number of reasons for doing so: to bypass long waiting times or receive what they considered would be a better-quality or more convenient service, for example by paying privately for a neurophysiotherapist to visit them at home for a longer period than would be available through the NHS; to purchase ‘cutting-edge’ technological devices that they thought would not be funded or available through NHS channels; or because they felt let down by NHS services (not satisfied with the fit and/or performance of KAFOs supplied to them) and were seeking a more personalised service.
She’s a private physio [therapist] . . . she’s does lots of talks and lectures, so I think she is quite well thought of in the area . . . I pay for that privately, my second mortgage!
P11
Maybe I need to speak to people who make non-NHS callipers to find out if there are better devices but I don’t really know where to start because you’ll be going to a commercial organisation.
P14
In 2011 I contacted [name of company] and they checked my legs out and muscle strength and he said, yes, you’ll be an ideal candidate for a new brace that they’ve just started promoting.
P15
They said there’s nothing else they only have these . . . but there are about a dozen on the internet that you can have . . .
P4
The National Health Service probably hasn’t got the money to do something like that.
P13
So he said what we would normally do with you is take you to our gait room where we have cameras linked to a computer programme to work out your gait . . . so I’ve had none of that on the NHS . . . and we will take a close plaster fit of it and then we will make a unique prototype for you . . . and when you’re satisfied and we’re satisfied . . .
P8
Interactions with health-care professionals
Interactions with orthotists
Study participants commented on a range of aspects of their interactions with orthotists that were important to them, such as orthotists’ levels of knowledge and expertise.
The guys there [orthotics department] are excellent you know, I think [name] and [name] are extremely passionate about what they do . . . very professional and they’re always on the cutting edge and improving things.
P24
The two major factors recurring across the data set, which were highlighted consistently and by a majority of participants, related to perception (1) about ease of access to orthotists and (2) concerning orthotists’ communication skills. Specifically, participants focused on orthotists’ willingness and ability to invite and listen to patients describe the issues and problems that they were experiencing, and to engage them in making choices and decisions about available treatment options.
Perceived accessibility of orthotists
Ease of obtaining an appointment with an orthotist when they felt they required one was important to study participants, as was the amount of time available for discussion during appointments. Participants described experiencing varying levels of frustration when they were not able to access input from an orthotist at a particular juncture in their care, when they felt that they needed contact; or because the time available to discuss their needs seemed curtailed; or if the particular clinician they wanted to see was unavailable for whatever reason. Longer-term users of orthotic services (mainly respondents with a diagnosis of poliomyelitis) suggested that seeing the same clinician at consecutive appointments, and building up a relationship of trust with that person over time, were important factors, which affected their views of the quality of care they received. Contrastingly, a few patients commented that it was only when they were seen by an orthotist who was ‘new’ to them that they felt that substantive progress had been made. Study participants in full-time paid employment commented that extending the availability of appointments with orthotists beyond ‘nine-to-five’ hours on weekdays would lead to an improvement in continuity of care for them.
I was wondering whether I need a bit more support rather than an appointment every 3 months in the initial stages just to get me up and running with it [her new orthosis].
P14
When I’m seeing [name of orthotist] on a regular basis things tick over very well. The trouble is we get gaps . . . there were reasons why I couldn’t see him, blah, blah, blah . . . so I’m getting a bit disconnected with him, and that’s not OK . . .
P19
If anything, it was a junior orthotist who came in and provided just ‘fresh eyes’.
P5
I am a teacher, I have to schedule my appointments on the holidays . . . if they opened on a Saturday I could just see somebody on a Saturday.
P3
Perceptions concerning communication skills of individual orthotists and their willingness to engage in patient-centred care
The perceived presence or absence of listening skills in an individual orthotist appeared to have a profound impact on patients’ perceptions of their quality of care. Listening skills seemed to be valued more highly than the clinician’s apparent knowledge and expertise about orthoses. Participants indicated that they wanted orthotists to listen to their specific problems, so that they would gain an understanding of how their condition was affecting them as an individual at a particular point in time. People with poliomyelitis especially commented on the need for orthotists to take time to elicit the wide range of issues that might be affecting them, and how these could be impacting on their lives. Those who felt that their concerns were not listened to perceived clinicians as ‘dismissive’ or ‘arrogant’, provoking feelings of disappointment and/or anger in patients.
Each person is an individual, has an individual problem, polio leaves people with so many different problems . . .
P9
It goes in one ear and out of that one and he [orthotist] gives me what he wants to give me. He gives me stuff, the latest thing, and it’s really good, but it’s no good for me . . . he’s not listening to me . . . it’s like I don’t know what I’m talking about . . .
P1
I’m sure he knows his stuff but . . . he didn’t feel quite so empathetic, that he had a lot of empathy . . . a bit unsympathetic in as much as I was trying to get my head around it . . . almost a bit dismissive.
P2
I’ve not been listened to one iota!
P4
Judgements about clinicians’ listening skills were closely linked to perceptions about the orthotist’s willingness and/or ability to engage with the patient, and include them in the decision-making process about treatment options. Some participants described interactions that they judged as highly satisfactory. During such encounters, the orthotist was said to listen to the patient’s concerns, give information and explanation, discuss treatment options and offer choice where feasible. Study participants frequently drew a contrast between orthotists whom they had encountered whom they thought were ‘good’ or ‘bad’ listeners, revealing the extent of the potential for positive or negative impact on the patient by the orthotist during consultations. P16, with long-term experience as an orthotic services user, suggested that the younger (female) orthotists he has come into contact with more recently tend to be skilled communicators, possibly as a result of improvements in training.
He is very nice, very easy going. He really wants you to be comfortable but he knows that, it’s not that I know better than him, it’s like he knows that I’ve been wearing it [orthosis] for so long, so he sort of listens to what I am telling him . . .
P23
He always listens to what I have to say, he gives me a choice to have a new style or old style calliper. I explained to him my concerns . . . and we had a long discussion and he said to me, the design of this is better . . . so he gave me a choice and he also listened to me if I explained to him as a user what issues are important to me . . . most of the time people have listened to me, but he listens a little bit more.
P3
I go back to before they called themselves orthotics because they just used to be appliance fitters . . . I remember getting an adjustment, you know, spinal brace back after it had been modified and they just sort of strapped it on me and said, oh that’s better isn’t it, and I said, hang on a minute, you have to allow me to tell you whether it’s better or not. I mean that’s the sort of arrogance that you used to get and that was very common. Also they were not receptive to almost listening to what difficulties you were having, you know, they would make the decision with what was wrong with something rather than even if it was completely, you know, you were trying to correct them and say no, that’s not the problem. The problem is hurting here or something like that. Now about eight years ago I noticed a significant change and now again, all the orthotists that I’d seen up to that point have been blokes. Eight years ago I had the first girl, she’s much younger, so I don’t know whether the difference was because she was younger and therefore had a different attitude or she was just forward than the others but she was very good.
P16
So what was good about her? What did you like about her?
She listened. She always asked before she touched me because people can hurt me touching me, you know, they don’t know what hurts and what doesn’t. Very gentle but more to the point listened to the problem, thought about solutions, proposed solutions and worked with me to say we could do this or do that and what have you, so that was a very big difference. It was an enormous difference. I can’t describe what a shock it was. Now she left after a couple of years and they got another orthotist who is the current one and I’ve been with her for about 6 years and she’s very good as well, excellent, and again she’s much younger. So whether the difference is age, whether the difference is training, whether it’s – I mean the whole attitude is different. That’s the biggest difference. So the results tend to be better because they’re listening to the problem and solving the problem rather than not really interested in your input just making their own assessment and what the problem is and going off on their own to do that.
Although communication with orthotists was described as sometimes suboptimal, only one participant (P1) in the sample mentioned the occurrence of a complete breakdown in communication relating to issues concerning supply of a device.
I had [an orthotist] build me a calliper and I couldn’t stand up in it and she told me to take it home . . . and I said to her, I’m not happy. So when I told her, I’d seen her about eight times in between building the calliper and she said ‘well, I’ve had enough, I’m going to send you to somebody else’ . . . they haven’t done me wrong, but if you can’t listen to what I’m telling you about my leg, and what I need for my leg . . .
P1
Interactions with consultants in rehabilitation medicine
Comments on interactions with consultants in rehabilitation medicine came mainly from study participants who were located in research sites 1 and 2. These comments were highly favourable; participants were very pleased with the way that consultants could refer them directly to a variety of services and facilities (e.g. to orthotic services, for specialist neurophysiotherapy, and for specialist assessment at gait clinic). They also valued consultants’ in-depth knowledge of their condition and highly developed interpersonal skills; some people reported that they felt they had been fully understood for the first time when they spoke to the consultants about the issues and problems that they were encountering and trying to deal with. GPs were said to frequently lack knowledge or interest in (relatively) rare conditions, such as CMT disease. Patients appreciated having a consultant to coordinate their care, and felt reassured by regular reviews and monitoring.
I got in a bit quicker [to orthotic services] with going through [consultant] than my GP.
P21
I was very impressed by [consultant]. [Consultant] pulled up a chair and sat down and said, now tell me . . . and [consultant] sat there and listened without putting anything on to me. Just listened to what I was saying . . . somebody you go in and you’ve got confidence.
P2
The registrar had said, oh, we’ll get a brace for your knee, and [consultant] said, no we won’t that’s not what you need. We’ll send you to the walking gait clinic and we’ll get you fixed up with neuro-physiotherapy, and that was that.
P2
[Consultant] actually listens to what you are talking about and [consultant] visualises himself in that situation for [consultant] to understand what you are talking about.
P1
I think it was when I went back to [consultant] and I was saying, this is what I’ve got, and then [consultant] put me in touch with orthotics and they supplied me with the extra bits.
P12
I see [consultant] every 12 months . . . I know if I’ve got any problems [consultant]’ll sort it out and try and help . . .
P21
In contrast, P15, with a diagnosis of poliomyelitis, did not feel that she was being treated as a ‘joined-up person’ and she thought that she would benefit from someone taking a more holistic approach to her overall health and well-being.
I think they’re just not treating me as a joined-up person. They’re not looking at me as a polio person. They are looking at me as someone who has got a weak leg. No-one has been proactive about anything and I think if you had a person overseeing things as you got older, people tend to get osteoporosis where it could be worse for me . . . I think things could be a lot better for people with polio if they did have someone in overall charge of their care and who made sure that at different stages of my life that people came in and said things, like a review of gait education . . .
P15
Interactions with physiotherapists
Participants reported accessing physiotherapist services via GP referrals, referral from consultants in rehabilitation medicine, and through orthotic services. Referral via a consultant was considered the fastest route, although some participants nonetheless mentioned experiencing long waiting times for their initial appointment which caused them anxiety, and/or that the service was pressurised.
I’m still waiting for neuro-physiotherapy . . . if there is only one person who does this specialist physiotherapy you can understand she can only see so many people.
P2
They were having a bit of a crisis because one of their physiotherapists was off ill, so they were really pushed for time and I felt a bit, ‘you’re done’.
P12
I am quite anxious that I am going to get worse and I really want to talk to some more specialist people [neuro-physiotherapists] to know what we could be doing over the next few years.
P2
Many respondents indicated that they were aware of the difference between an ‘ordinary’ physiotherapist and a specialist neurophysiotherapist, and a number of people (mainly, but not entirely, limited to those in research site 2) mentioned paying privately for the services of neurophysiotherapists, sometimes using the money that they received through their disability allowance. There was a widespread perception among participants with poliomyelitis that non-specialist physiotherapy could result in damage to muscles.
The normal one, I did go there but it didn’t do anything.
P21, research site 2, who had been referred for neurophysiotherapy by a consultant
She’s a great physiotherapist but she is not a neurophysiotherapist . . . I know of a very good neurophysio [therapist] in [place name] and I’ve asked to see her.
P10, research site 2
I’ve been having physio [therapy] for 5 or 6 years at least and she has helped me a lot . . . it’s a private one . . . she was with the NHS but she said they don’t give you long enough for your patients, anyway she’s set up on her own . . .
P11, research site 2
I went along really quite apprehensive to talk to them about what they were planning because I didn’t want to be damaged . . . if you start working the muscles too hard, you [can cause] further damage . . . quite a few people with polio end up worse off through having physiotherapy.
P16, non-NHS
Participants who felt assured that they were receiving good advice and treatment from (neuro) physiotherapists were keen to carry out recommended exercises in an attempt to prevent potential future deterioration.
I’ve been giving various exercises by the physiotherapy department . . . I’ve been given a lot of information to manage my condition so really it’s up to me to manage it.
P22
Only 3 of the 24 participants who were interviewed mentioned being given an orthotic device by a physiotherapist; a device to assist with foot drop to P12, and a knee brace to each of P4 and P13.
She [physiotherapist] said, what about splints? . . . so she went and got a splint but a large one, so they put the large one on and although when I stood up it dropped down, still it had that support at the back of my knee . . . nothing digging in anywhere . . . so then I got referred to orthotics, I got referred for a small one.
P4
Some degree of multidisciplinary working between physiotherapists and orthotists was reflected in the comments of P2 and P19. P2 recounted her referral to orthotic services by a physiotherapist, whereas P19 remarked that her orthotist was able to make a direct referral for her to see a physiotherapist with quicker access than she would have achieved if she had needed to go through a GP referral.
I saw the physiotherapist and she put a towel under my foot . . . I was feeling very lopsided . . . and she said, right, I’ll refer you to orthotics because it looks like you might need some help with raising that heel to give you better balance.
P2
It was quite straightforward . . . I guess some kind of consultation between [orthotist] and physio [therapist] . . . physio [therapist] agreed to see me . . . I went and we sorted it out. It wasn’t one of those elongated things where I had to get a consultant involved and a GP and all that nonsense because my GP is hopeless.
P19
Interactions with general practitioners
With few exceptions, study participants viewed GPs’ role in their care as primarily one of referral to specialist services, including orthotic services and physiotherapy. GPs’ involvement in referral decisions was perceived as partly linked to mechanisms for funding, and partly because of GPs lacking the expertise to manage patients with needs for specialist services. Participants reported varying levels of frustration at having to contact their GP when they wanted access to particular services, including orthotic services.
Somebody told me there is a neurological physiotherapy department at [name] and my [GP] referred me.
P16
About probably once every 12 months when I need to go back and have a pair of shoes made . . . I have to go through the GP surgery for them to refer me to the orthotics for them to make me an appointment to go, so it’s quite a long-winded process. I can’t just go straight to the orthotics. You have to go through your GP. It’s all to do with the way funding is allocated.
P22
Less than one-quarter of those interviewed felt that their GP(s) were interested in their condition, or finding out more about it, and a majority reported that their GP lacked both knowledge about and interest in the condition that was their primary diagnosis (poliomyelitis, CMT disease, multiple sclerosis and spina bifida).
I went to my GP who knew absolutely nothing about the condition [CMT] but it was really good and both of us sort if researched it and sort of swapped notes. My first doctor got really involved to try because there wasn’t and there still isn’t a lot of knowledge within the medical community about this condition.
P22
I feel there is almost a black hole with GPs really.
P5
Mostly GPs don’t know very much about it [polio].
P18
Nobody knows anything about me in the surgery now and it hacks me off something chronic . . . I did enquire if I could see someone who was a bit more knowledgeable about the spina bifida side of things. . . I waited to see this GP, another 3 or 4 weeks . . . to establish that if I want to find out more about spina bifida I can go on-line and find a forum.
P19
A few patients reported a lack of response or encountering active resistance from GPs when they had requested particular investigations or services. P2 felt that her GP made referrals for her only when she was ‘at crisis point’ rather than making proactive, preventative referrals on her behalf, whereas P15 had to ‘push’ to be tested for post-polio syndrome when she felt that her condition was deteriorating.
Not referring until I got to crisis point because I had to go to the doctors to get physio [therapy] for my knee. Nobody ever said, it’s starting to give you problems, I think we should . . . if they’d had the knowledge they might have said, I think it would be better if we referred you for some advice.
P2
I went to my doctor about my pins and needles in my left leg and also about weakness in my arms . . . I said I want to be referred to someone . . . so she [GP] said to me, you’re very proactive, she wasn’t terribly happy about it but she did do it and about 3 months later I got an appointment with [name] who did loads of tests.
P15
Interactions with podiatrists
Only three (P5, P7 and P21) of the 24 participants interviewed referred to having any contact with podiatry services. P7 stated that she had been referred for regular review at podiatry services through her contact with a specialist (multiple sclerosis) nurse. P21 described attending a podiatry clinic for treatment of an open sore, over a long period after wearing an ill-fitting AFO. She thought the sore had occurred due to pressure from the device:
I was going to the foot clinic every week and it took them ages and ages to get it right.
P21
Participant 5 had had recent contact with podiatry services because of corns that had developed when wearing his orthotic footwear. He described being passed back and forth between orthotic and podiatry services, with a lack of clarity about who should take responsibility for dealing with his problem:
You need to go back to [orthotic services] to sort this out, and [orthotic services] say, oh, no, it’s not our thing it’s more the podiatrists need to sort that bit out.
P5
Chapter 5 Results of survey of health-care professionals
Response and completion rates
The survey was circulated to members of BAPO (n = 700), ACPIN (n = 2700) and BSRM (n = 300). A total of 238 HCPs agreed to participate in the survey, that is, they clicked into the survey questionnaire via the link in the invitation letter. Based on the sampling frame described above, this equates to a response rate of 6.43%. The response rate by organisation was 12% for BAPO, 3.48% for ACPIN and 16.67% for BSRM. These response rates are an underestimate as a result of the imprecise sampling frame. For example, information supplied by BAPO suggests that approximately three-quarters of their membership are orthotists, not all of whom manage patients with NMD or CNS conditions, leaving a more likely sampling frame of < 400. Similarly, not all participants in the sampling frame for ACPIN and BAPO manage the populations of interest.
The overall completion rate [the number of participants who finished the survey (n = 138)/the number who agreed to participate (n = 238)] was 57.98%; 68% for orthotists, 62.8% for physiotherapists and 54% for doctors in rehabilitation medicine.
The response rate was also calculated for each question in the survey. The denominator used was the number eligible to complete a specific question. As the survey hid certain questions from certain respondents (e.g. all questions that are specifically related to patients with a CNS condition were not asked of respondents who indicated that they did not treat patients with a CNS condition), the denominator varies across questions and across professions. The denominator used ranged from a maximum of 238 for the questions that all participants were eligible to complete to a minimum of 115 when only respondents who fitted devices could respond to a specific question.
The total number of respondents was well above the estimated minimum sample size required, based on a 95% confidence level and a 10% margin of error (n = 96), but insufficient for the more ideal 5% margin of error (n = 384). It was, therefore, not appropriate to undertake subgroup analyses. Descriptive results are presented by HCP as well as the total group; however, any apparent differences should be interpreted with caution.
For each question, the number of responses to each answer option is reported, and the proportion is calculated using the number of respondents who answered that particular question as the denominator. For completeness, the number of non-responders for each question was also reported. This ensured that the proportions of the responses presented were not overestimated. In the tables the total number in the all respondents column is greater than the sum of the three professional groups owing to data not being reported separately for ‘other’ types of health-care professionals and also to missing data on profession. The full questionnaire is provided in Appendix 7.
Demographic characteristics
Although 238 HCPs clicked into the survey via the invitation letter and consented to undertake the survey, only 229 respondents answered the first question. Respondents were asked their occupation (question 1, Q1) and their years of post-qualification experience (Q2). The response rate for Q1 was 96.22% (n = 229) and for Q2 was 95.38% (n = 227).
The respondents included 80 orthotists (34.93% of responders), 94 physiotherapists (41.05% of responders), 50 doctors in rehabilitation medicine (21.83% of responders) and five other types of HCP (one prosthetist/orthotist, two prothestists, one orthotic technician and one podiatrist). Responses for the last group are provided in the overall responses, but not as an individual group, in the tables below.
Most commonly respondents had ≥ 16 years of post-qualification experience (44.49%, n = 101). Similar proportions had up to 5 years’ (19.38%, n = 44), 6–10 years’ (19.38%, n = 44) and 11–15 years’ (16.74%, n = 38) experience. This trend of years of experience held for the physiotherapists and the doctors in rehabilitation medicine, a larger proportion (40%, n = 32) of orthotists who had 0–5 years’ of post-qualification experience, and a small proportion (30%, n = 24) had ≥ 16 years’ experience.
Respondents were asked where their clinical setting was located (Q3). The response rate was 92.44% (n = 220): 96.25% of orthotists, 100% of physiotherapists and 88% of rehabilitation medicine physicians. The vast majority (82.27%, n = 181) of respondents’ clinical setting was in England, 10.45% (n = 23) were based in Scotland, 3.18% (n = 7) in Wales and 3.64% (n = 8) in Northern Ireland.
When asked about the type of clinical setting(s) in which they work (Q4), the question response rate was 92.44% (n = 220); 96.25% of orthotists, 98.94% of physiotherapists and 88% of rehabilitation medicine physicians. The majority of respondents (65.91%, n = 145) were from a NHS setting, 9.09% (n = 20) a private company setting, and both NHS and private settings (18.18%, n = 40). Fifteen respondents (6.82%) chose ‘other’ settings, 11 of whom described their clinical setting, which included private domiciliary (n = 1); a subcontracted private company within the NHS (n = 1); a university setting (n = 3); private community (n = 1); Ministry of Defence (n = 1); self-employed (n = 1) and retired (n = 1).
Respondents were asked how orthotic services in their clinical setting are provided (Q5). No single model predominated: 38.89% (n = 84) reported that orthotic services are provided as an integrated part of a MDT service and 46.76% (n = 101) within a stand-alone prescribing/fitting orthotic service. Thirty-one respondents (14.35%) chose the ‘other’ option (Table 14).
| Service provision | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 216 (90.76%) | n = 77 (96.25%) | n = 92 (97.87%) | n = 43 (86%) |
| As an integrated part of a multidisciplinary service | 84 (38.89) | 31 (40.26) | 27 (29.35) | 24 (55.81) |
| Stand-alone prescribing/fitting orthotic service | 101 (46.76) | 33 (42.86) | 50 (54.35) | 17 (39.53) |
| Other | 31 (14.35) | 13 (16.88) | 15 (16.30) | 2 (4.65) |
| No response | 22 | 3 | 2 | 7 |
Thirteen orthotists chose the ‘other’ option and their responses indicated that the orthotic services in their setting are a combination of both MDTs and stand-alone services. This was also the case for both doctors in rehabilitation medicine who chose ‘other’. There was a variety of responses from the 15 physiotherapists who chose the ‘other’ option: one physiotherapist worked with a podiatrist to access FOs but other orthotic devices prescribed are provided through a stand-alone orthotic service. Five physiotherapists stated that they source orthoses themselves directly from orthotics companies. One physiotherapist stated that their orthotic service was provided as part of a therapy team, which was not a wider MDT. One physiotherapist is part of a privately run rehabilitation service, and patients are referred to the NHS for orthotic devices. Two respondents stated a mix of both stand-alone and MDTs; one respondent stated provision varies depending on whether it is available through the NHS or privately.
The respondents who stated that the orthotic services in their clinical setting are provided by a MDT were asked what HCPs make up their MDT (Q6).
The majority of respondents’ MDTs include a physiotherapist (96.43%), an orthotist (79.76%), occupational therapists (69.05%) and doctors in rehabilitation medicine (60.71%) (Table 15). A smaller proportion reported a gait scientist as part of the MDT (10.71%). Nineteen (22.62%) respondents’ MDTs included HCPs other than those listed.
| HCP | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 84 (78.5%) | n = 32 (91.14%) | n = 28 (93.33%) | n = 24 (77.42%) |
| Physiotherapist | 81 (96.43) | 29 (90.63) | 27 (96.43) | 23 (95.83) |
| Orthopaedic surgeon | 32 (38.10) | 21 (65.63) | 2 (7.14) | 6 (25.00) |
| Doctor in rehabilitation medicine | 51 (60.71) | 16 (50.00) | 12 (42.86) | 22 (91.67) |
| Occupational therapist | 58 (69.05) | 16 (50.00) | 22 (78.57) | 18 (75.00) |
| Gait scientist | 9 (10.71) | 4 (12.50) | 1 (3.57) | 4 (16.67) |
| Neurologist | 14 (16.67) | 6 (18.75) | 6 (21.43) | 2 (8.33) |
| Orthotist | 67 (79.76) | 27 (84.38) | 18 (64.29) | 21 (87.50) |
| Clinical nurse specialist | 30 (35.71) | 9 (28.13) | 11 (39.29) | 9 (37.50) |
| Other HCP | 19 (22.62) | 6 (18.75) | 8 (28.57) | 4 (16.67) |
| No response | 23 | 3 | 2 | 7 |
Six orthotists chose ‘other’ HCPs. Five orthotists stated that their MDTs also included a podiatrist, and one stated they also include community therapists. Eight physiotherapists chose ‘other’ HCPs. Their responses included podiatrists, speech and language therapists, psychologists/neuropsychologists, rehabilitation assistants, inpatient nursing team, geriatricians, social workers, dietitians and one respondent stated ‘patient and family’. Four doctors in rehabilitation medicine chose ‘other’ HCPs. Among the HCPs stated were speech and language therapists, clinical psychologists, dietitians, plastic surgeons, psychologist/neuropsychologist and prothestists.
Patient demographics
A series of questions was asked to ensure that the respondents for the survey are currently treating, or have recently treated, adult patients with NMD and/or CNS with knee instability (Q7–10).
In relation to adult patients with NMD with knee instability, 66.34% (n = 136) of respondents reported that they treat, or have recently treated, this population: 33.66% (n = 69) had not and 33 respondents did not answer this question (response rate to question 86.13%, n = 205).
In relation to adult patients with CNS disorders with knee instability, 83.82% (n = 171) of respondents reported that they treat, or have recently treated, this population, 16.18% (n = 33) had not and 34 respondents did not answer this question (response rate to question 85.71%, n = 204).
The respondents who stated that they currently treat, or have recently treated, these patients were then asked about the types of CNS conditions and/or NMD that they see most frequently. Responses are presented in Table 16.
| Disease/condition | Total respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| NMD | ||||
| Response rate | n = 136 (76.47%) | n = 55 (91.67%) | n = 48 (88.89%) | n = 25 (71.43%) |
| Poliomyelitis | 73 (70.19) | 50 (90.91) | 6 (12.50) | 8 (32.00) |
| Muscular dystrophy | 61 (58.65) | 21 (38.18) | 22 (45.83) | 10 (40.00) |
| Post-polio syndrome | 80 (76.92) | 45 (81.82) | 12 (25.00) | 15 (60.00) |
| Motor neurone disease | 71 (68.27) | 23 (41.82) | 29 (60.42) | 9 (36.00) |
| Inclusion body myositis | 23 (22.12) | 3 (5.45) | 11 (22.92) | 6 (24.00) |
| CMT disease | 71 (68.27) | 40 (72.73) | 9 (18.75) | 14 (56.00) |
| Guillain–Barré syndrome | 68 (65.38) | 14 (25.45) | 31 (64.58) | 18 (72.00) |
| CIDP | 48 (46.15) | 7 (12.73) | 21 (43.75) | 17 (68.00) |
| Other | 10 (9.62) | 4 (7.27) | 1 (2.08) | 4 (16.00) |
| No response | 32 | 5 | 6 | 10 |
| CNS conditions | ||||
| Response rate | n = 171 (91.81%) | n = 64 (92.75%) | n = 72 (90%) | n = 34 (77.27%) |
| Adult cerebral palsy | 79 (50.32) | 43 (67.19) | 15 (20.83) | 21 (61.76) |
| Multiple sclerosis | 130 (82.80) | 54 (84.38) | 51 (70.83) | 25 (73.53) |
| Traumatic brain injury | 110 (70.06) | 39 (60.94) | 43 (59.72) | 28 (82.35) |
| Stroke | 157 (100.00) | 60 (93.75) | 66 (91.67) | 30 (88.24) |
| Acquired brain injury | 102 (64.97) | 37 (57.81) | 37 (51.39) | 28 (82.35) |
| Spinal cord disorders | 98 (62.42) | 32 (50.00) | 37 (51.39) | 29 (85.29) |
| Other | 4 (2.55) | 1 (1.56) | 3 (4.17) | 0 (0.00) |
| No response | 14 | 5 | 8 | 10 |
The most common type of NMD disorder being treated was post-polio syndrome (76.92%), followed by poliomyelitis (70.19%), motor neurone disease (68.27%), CMT disease (68.27%), Guillain–Barré Syndrome (65.38%), muscular dystrophy (58.65%), chronic inflammatory demyelinating polyradiculoneuropathy (CIDP; 46.15%) and inclusion body myositis (22.12%).
The most common type of CNS condition being treated was stroke (100%), followed by multiple sclerosis (82.80%), traumatic brain injury (70.06%), acquired brain injury (64.97%), spinal cord disorders (62.42%) and adult cerebral palsy (50.32%).
The respondents who stated that they do not currently treat, and have not recently treated, patients with a NMD or a CNS condition with knee instability n = 23 (9.66%, n = 238 total) were directed to the end of the survey.
From this section onwards, some questions were asked specifically about NMD/CNS patients with knee instability. Therefore, only the questions relevant to the respondents, based on Q7 and Q9 were displayed to the respondents.
Patient referrals
Those respondents who stated that they treat, or have recently treated, patients with NMD were asked how these patients would routinely be referred to them (Q11). Referrals were from a wide range of sources (Table 17).
| HCP | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 137 (82.04%) | n = 64 (92.75%) | n = 45 (85.19%) | n = 25 (71.43%) |
| GP | 82 (59.85) | 38 (59.38) | 27 (58.70) | 17 (68.00) |
| Physiotherapist | 86 (62.77) | 54 (84.38) | 18 (39.13) | 14 (56.00) |
| Orthopaedic surgeon | 52 (37.96) | 40 (62.50) | 5 (10.87) | 7 (28.00) |
| Doctor in rehabilitation medicine | 56 (40.88) | 30 (46.88) | 20 (43.48) | 6 (24.00) |
| Occupational therapist | 17 (12.41) | 4 (6.25) | 9 (19.57) | 5 (20.00) |
| Gait scientist | 5 (3.65) | 4 (6.25) | 1 (2.17) | 0 (0.00) |
| Neurologist | 87 (63.50) | 40 (62.50) | 33 (71.74) | 14 (56.00) |
| Orthotist | 9 (6.57) | 3 (4.69) | 1 (2.17) | 5 (20.00) |
| Clinical nurse specialist | 18 (13.14) | 6 (9.38) | 7 (15.22) | 5 (20.00) |
| Other | 23 (16.79) | 5 (7.81) | 14 (30.43) | 3 (12.00) |
| No response | 30 | 5 | 8 | 10 |
A substantial proportion of referrals are from GPs (59.85%), physiotherapists (62.77%) and neurologists (63.50%), with orthopaedic surgeons (37.96%) and doctors in rehabilitation medicine (40.88%) also being significant sources of referrals. A total of 23 respondents chose ‘other’ as the HCPs that refers patients with NMD to them; this included 14 (30.43%) of the physiotherapists. The responses provided by these respondents indicated that there can be self-referrals and referrals through inpatient systems. Orthotists can receive referrals from podiatrists, community physiotherapists, the MDT team and self-referrals from patients. Physiotherapists also receive referrals from the inpatient ward, self-referrals from patients, and referrals from oncologists, stroke doctors and case managers. Rehabilitation medicine physicians can receive referrals from community stroke rehabilitation teams.
Those respondents who stated that they treat, or have recently treated, patients with CNS conditions were asked how these patients would routinely be referred to them (Q12).
As for patients with NMD, a substantial proportion of referrals are from GPs (59.64%), physiotherapists (65.66%) and neurologists (55.42%), with doctors in rehabilitation medicine (43.98%) also a significant source of referral (Table 18). A total of 37 respondents chose ‘other’ as the HCPs that refer patients with CNS conditions to them; this included 31 (44.29%) of the physiotherapists. The responses provided by these respondents indicated that referrals can also be self-referrals (by the patients themselves) and referrals through inpatient systems (particularly stroke patients).
| HCP | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 166 (80.98%) | n = 64 (92.75%) | n = 70 (87.5%) | n = 31 (70.45%) |
| GP | 99 (59.64) | 42 (65.63) | 34 (48.57) | 23 (74.19) |
| Physiotherapist | 109 (65.66) | 54 (84.38) | 32 (45.71) | 22 (70.97) |
| Orthopaedic surgeon | 44 (26.51) | 33 (51.56) | 3 (4.29) | 8 (25.81) |
| Rehabilitation medicine physician | 73 (43.98) | 35 (54.69) | 29 (41.43) | 9 (29.03) |
| Occupational therapist | 30 (18.07) | 8 (12.50) | 17 (24.29) | 5 (16.13) |
| Gait scientist | 6 (3.61) | 4 (6.25) | 1 (1.43) | 1 (3.23) |
| Neurologist | 92 (55.42) | 32 (50.00) | 41 (58.57) | 19 (61.29) |
| Orthotist | 11 (6.63) | 4 (6.25) | 2 (2.86) | 5 (16.13) |
| Clinical nurse specialist | 39 (23.49) | 8 (12.50) | 22 (31.43) | 9 (29.03) |
| Other | 37 (22.29) | 2 (3.13) | 31 (44.29) | 4 (12.90) |
| No response | 39 | 5 | 10 | 13 |
Orthotists can also receive referrals from medicolegal sources, community physiotherapists and self-referral from patients. Physiotherapists can also receive referrals from the inpatient ward, stroke units, a MDT member, oncologists, case managers, nursing homes, stroke doctors and self-referral from patients. Doctors in rehabilitation medicine can also receive referrals from the inpatient ward, spinal surgeons and neurologists. One rehabilitation medicine physician stated that he/she would never receive a direct referral for these conditions.
Respondents were asked what information is usually provided on referral of patients with NMD and/or CNS conditions (Q13).
Almost all respondents stated that they are provided with the patient diagnosis (94.64%) and two-thirds are provided with the patient’s medical details (Table 19). Smaller proportions reported being provided with physical assessment details (33.93%), with the aims and goals of the orthotic intervention (32.14%), with the type of orthoses provided (20.24%) and with a gait analysis report (1.79%). Twenty-two respondents chose ‘other’ and some provided further details. The orthotists are also sometimes provided with a suggestion of the orthoses required, what is required from them and medical details from medicolegal reports. Five orthotists also essentially stated that the aims/goals of the intervention would be provided. Physiotherapists are also provided with the reason for referral and care needs reports.
| Information | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 168 (79.25%) | n = 69 (93.24%) | n = 67 (81.71%) | n = 32 (71.11%) |
| Medical details | 112 (66.67) | 37 (53.62) | 50 (74.63) | 24 (75.00) |
| Diagnosis | 159 (94.64) | 65 (94.20) | 62 (92.54) | 32 (100.00) |
| Physical assessment details | 57 (33.93) | 17 (24.64) | 20 (29.85) | 19 (59.38) |
| Gait analysis report | 3 (1.79) | 2 (2.90) | 1 (1.49) | 0 (0.00) |
| The aims/goals of the orthosis (if an orthosis has already been prescribed) | 54 (32.14) | 32 (46.38) | 15 (22.39) | 7 (21.88) |
| Type of orthosis provided (if an orthosis has already been prescribed) | 34 (20.24) | 16 (23.19) | 12 (17.91) | 6 (18.75) |
| Other | 22 (13.10) | 11 (15.94) | 11 (16.42) | 0 (0.00) |
| No response | 44 | 5 | 15 | 13 |
Respondents were asked what symptoms in patients with NMD and/or CNS conditions would trigger a referral to them for assessment (Q14).
The three options available appear to all trigger a substantial number of referrals and this was the case across the types of HCPs (Table 20). A number of respondents (20.12%) chose the ‘other’ option. Symptoms that would trigger a referral to an orthotist also include the patient having gait problems, the patient having poor function, and normal service provision not having worked for the patient. Symptoms that would trigger a referral to a physiotherapist also include the patient having gait problems, the patient having poor balance and a recent change in the patient’s comorbidities. Five physiotherapists stated that they see all patients. A doctor in rehabilitation medicine could also be referred a patient having gait problems.
| Symptoms | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 169 (79.34%) | n = 69 (93.24%) | n = 62 (81.71%) | n = 32 (71.11%) |
| Patient has reported falls | 131 (77.51) | 59 (85.51) | 48 (71.64) | 24 (75.00) |
| Patient-reported pain in knee or lower limb | 110 (65.09) | 51 (73.91) | 39 (58.21) | 19 (59.38) |
| Patient-reported weakness in knee or lower limb | 137 (81.07) | 61 (88.41) | 52 (77.61) | 24 (75.00) |
| Other | 34 (20.12) | 11 (15.94) | 11 (16.42) | 3 (9.38) |
| No response | 44 | 5 | 15 | 13 |
The respondents were asked what other HCPs would assess the patients that are referred to them (Q17). Table 21 shows that physiotherapists would most commonly assess these patients (75.63%), followed by GPs (55%), neurologists (55%), doctors in rehabilitation medicine (50.63%), orthopaedic surgeons (43.75%), orthotists (41.25%), occupational therapists (37.50%), clinical nurse specialists (21.88%) and gait scientists (12.50%).
| HCP | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 160 (75.12%) | n = 65 (87.84%) | n = 64 (78.05%) | n = 30 (66.67%) |
| GP | 88 (55.00) | 42 (64.62) | 32 (50.00) | 14 (46.67) |
| Physiotherapist | 121 (75.63) | 63 (96.92) | 29 (45.31) | 29 (96.67) |
| Orthopaedic surgeon | 70 (43.75) | 44 (67.69) | 13 (20.31) | 13 (43.33) |
| Rehabilitation medicine physician | 81 (50.63) | 36 (55.38) | 35 (54.69) | 10 (33.33) |
| Occupational therapist | 60 (37.50) | 17 (26.15) | 36 (56.25) | 7 (23.33) |
| Gait scientist | 20 (12.50) | 9 (13.85) | 5 (7.81) | 6 (20.00) |
| Neurologist | 88 (55.00) | 39 (60.00) | 35 (54.69) | 13 (43.33) |
| Orthotist | 66 (41.25) | 18 (27.69) | 31 (48.44) | 17 (56.67) |
| Clinical nurse specialist | 35 (21.88) | 8 (12.31) | 21 (32.81) | 6 (20.00) |
| Other | 15 (9.38) | 2 (3.08) | 13 (20.31) | 0 (0.00) |
| No response | 53 | 9 | 18 | 15 |
Fifteen respondents (9.38%) chose the ‘other’ option. One orthotist stated that any of the HCPs listed and any MDT member could potentially also assess these patients. Another orthotist stated that this information would not be available to them on referral. The physiotherapists stated that a consultant, a neurosurgeon and a podiatrist could also assess these patients. Two physiotherapists stated that any of the HCPs listed and any MDT member could potentially also assess these patients, and one physiotherapist stated that his/her patients would not be seen by any other HCP.
Respondents were asked if they thought that there were any barriers to patients being referred to them (Q15). Results are presented in Table 22. The most commonly reported response was that there were ‘sometimes’ barriers to patients being referred (47.85%), followed by ‘rarely’ (30.67%). Although the trend was similar for orthotists and rehabilitation medicine doctors, a higher proportion of physiotherapists felt that there were ‘rarely’ or ‘never’ barriers. This may be explained by the fact that physiotherapist respondents received more referrals from inpatient wards than the other groups; however, the difference is small.
| Perception of barriers | All respondents | Orthotists | Physiotherapists | Doctors in rehabilitation medicine |
|---|---|---|---|---|
| Response rate | n = 163 (76.53%) | n = 66 (89.19%) | n = 65 (79.27%) | n = 31 (69.89%) |
| Never | 20 (12.27) | 5 (7.58) | 12 (18.46) | 3 (9.68) |
| Rarely | 50 (30.67) | 12 (18.18) | 28 (43.08) | 10 (32.26) |
| Sometimes | 78 (47.85) | 42 (63.64) | 22 (33.85) | 13 (41.94) |
| Most of the time | 13 (7.98) | 6 (9.09) | 2 (3.08) | 5 (16.13) |
| Always | 2 (1.23) | 1 (1.52) | 1 (1.54) | 0 (0.00) |
| No response | 50 | 8 | 17 | 14 |
When asked to briefly explain their answers, respondents described both the lack and the presence of barriers to patients being referred to them. Thirty-six of the comments indicated that there were no barriers. It appears that those HCPs with blanket referrals or working in an inpatient setting do not feel that there are barriers to patients being referred to them. However, there were a number of responses for which potential barriers to patient referrals were highlighted.
Lack of awareness and knowledge appear to be the main issues affecting referral of patients to orthotic services. Forty-one comments indicated that a lack of awareness by potential referrers of orthotists’ professional expertise was causing barriers to referrals. Two additional comments noted the lack of knowledge of GPs about patient conditions and four additional comments noted the lack of knowledge of the potential of orthotic devices for this patient population as barriers.
The ability to refer also appears to be affecting referrals for this patient population. Six comments referred to the inability of some HCPs to refer directly to orthotic services. For example, one respondent stated that they could only accept internal referrals; another stated that some areas only allow referrals from consultants and another noted the inability of GPs to provide direct referrals. Five comments indicated that there can be a lack of knowledge by potential referrers in the potential pathway for this patient population. Nine comments indicated that in some cases there are difficulties with the referral pathway, such as criteria required for referrals, making the process less than straightforward.
Seven comments detailed the poor access to service in some areas of the country. One respondent noted a ‘postcode lottery’ and another comment noted the ‘non-existent outpatient facility’ in their setting. Nineteen comments indicated that the cost was a barrier. Cost appears to be a factor both in terms of providing an orthotic service and the cost of referral for GPs. The financial status of the patient themselves was also highlighted, presumably if the cost needs to be borne by the patient. Eighteen comments indicated that long waiting times were causing barriers to referral. Ten comments noted late referrals, from both lack of awareness and long waiting times, as a barrier to treatment. It seems that patients are presenting at orthotic services when their condition has deteriorated to the point at which orthotic provision may no longer be beneficial.
Two comments noted that some settings accept referrals from large geographical areas (one respondent stated that referrals were accepted from all over the country), which provides a barrier as a result of patients having difficulty attending appointments. One comment noted the lack of hospital transport. Two comments also noted that sometimes patients do no seek help, which creates a barrier to referral.
Initial assessment
Respondents were asked the average waiting time for adult patients with NMD-related knee instability between referral of the patient to them and their initial assessment (Q19).
The majority of respondents reported that, on average, patients were seen within 12 weeks of referral (Table 23). Physiotherapists reported the shortest waiting times for referral (86.48% of physiotherapists said that referral occurred within 8 weeks).
| Average waiting time | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 115 (68.45%) | n = 57 (82.61%) | n = 37 (69.81%) | n = 21 (60%) |
| Up to 4 weeks | 41 (35.65) | 11 (19.30) | 23 (62.16) | 7 (33.33) |
| 5–8 weeks | 40 (34.78) | 26 (45.61) | 9 (24.32) | 5 (23.81) |
| 9–12 weeks | 19 (16.52) | 12 (21.05) | 0 (0.00) | 7 (33.33) |
| 13–16 weeks | 9 (7.83) | 5 (8.77) | 3 (8.11) | 1 (4.76) |
| 17–20 weeks | 1 (0.87) | 0 (0.00) | 1 (2.70) | 0 (0.00) |
| 21–24 weeks | 3 (2.61) | 2 (3.51) | 1 (2.70) | 0 (0.00) |
| ≥ 24 weeks | 2 (1.74) | 1 (1.75) | 0 (0.00) | 1 (4.76) |
| No response | 53 | 12 | 16 | 14 |
Respondents were asked about the average waiting time for adult patients with CNS conditions with knee instability between referral of the patient to them and their initial assessment (Q20).
As for patients with NMD, the majority of respondents reported an average time from referral to initial assessment of up to 12 weeks (Table 24). A higher proportion of physiotherapists reported an average waiting time of up to 4 weeks than the other two groups.
| Average waiting time | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 143 (69.76%) | n = 59 (85.51%) | n = 58 (72.5%) | n = 26 (59.1%) |
| Up to 4 weeks | 62 (43.36) | 16 (27.12) | 39 (67.24) | 7 (26.92) |
| 5–8 weeks | 43 (30.07) | 23 (38.98) | 13 (22.41) | 7 (26.92) |
| 9–12 weeks | 22 (15.38) | 11 (18.64) | 2 (3.45) | 9 (34.62) |
| 13–16 weeks | 9 (6.29) | 6 (10.17) | 2 (3.45) | 1 (3.85) |
| 17–20 weeks | 3 (2.10) | 1 (1.69) | 1 (1.72) | 1 (3.85) |
| 21–24 weeks | 3 (2.10) | 2 (3.39) | 1 (1.72) | 0 (0.00) |
| ≥ 24 weeks | 1 (0.70) | 0 (0.00) | 0 (0.00) | 1 (3.85) |
| No response | 62 | 10 | 22 | 18 |
Respondents were asked what assessments they would routinely undertake as part of their initial assessment of patients with NMD and/or CNS with knee instability (Q18). Results are presented in Table 25.
| Assessment | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 153 (71.83%) | n = 63 (85.14%) | n = 60 (73.17%) | n = 28 (62.22%) |
| Ligament laxity | 106 (69.28) | 58 (92.06) | 28 (46.67) | 20 (71.43) |
| Muscle strength | 146 (95.42) | 61 (96.83) | 58 (96.67) | 26 (92.86) |
| Joint ROM and quality of ROM | 146 (95.42) | 62 (98.41) | 58 (96.67) | 25 (89.29) |
| Presence of spasticity (if appropriate) | 142 (92.81) | 59 (93.65) | 57 (95.00) | 25 (89.29) |
| Previous treatments | 128 (83.66) | 55 (87.30) | 48 (80.00) | 25 (89.29) |
| Previous history of pain/falls/walking ability | 134 (87.58) | 58 (92.06) | 52 (86.67) | 24 (85.71) |
| Sensation | 127 (83.01) | 50 (79.37) | 53 (88.33) | 24 (85.71) |
| Observational gait analysis | 136 (88.89) | 59 (93.65) | 55 (91.67) | 22 (78.57) |
| Video recording gait | 16 (10.46) | 5 (7.94) | 10 (16.67) | 1 (3.57) |
| Three-dimensional/video vector gait analysis | 1 (0.65) | 1 (1.59) | 0 (0.00) | 0 (0.00) |
| Balance tests | 88 (57.52) | 18 (28.57) | 55 (91.67) | 15 (53.57) |
| Timed walking tests | 51 (33.33) | 6 (9.52) | 37 (61.67) | 8 (28.57) |
| Patient expectations | 137 (89.54) | 58 (92.06) | 57 (95.00) | 22 (78.57) |
| Activity limitations | 122 (79.74) | 46 (73.02) | 54 (90.00) | 22 (78.57) |
| Aggravating factors | 98 (64.05) | 35 (55.56) | 43 (71.67) | 20 (71.43) |
| Proprioception | 101 (66.01) | 27 (42.86) | 54 (90.00) | 20 (71.43) |
| Imaging (e.g. radiography, MRI or ultrasound) | 29 (18.95) | 6 (9.52) | 6 (10.00) | 17 (60.71) |
| Other | 9 (5.88) | 3 (4.76) | 4 (6.67) | 2 (6.67) |
| No response | 60 | 11 | 22 | 17 |
Respondents reported using a variety of combinations of initial assessments in their clinical practice. The majority of respondents routinely assess a patient’s muscle strength (95.42%), joint range of movement (ROM) and the quality of that movement (95.42%), the presence of spasticity (92.81%), patient expectations (89.54%); observe gait (88.89%); take history of pain/falls/walking ability (87.58%); and assess previous treatments (83.66%), sensation (83.01%), activity limitations (79.74%), ligament laxity (69.82%), proprioception (66.01%), aggravating factors (64.05%) and balance tests (57.52%). Smaller proportions reported using timed walking tests (33.33%) and imaging (18.95%).
It appears that formal gait analysis is rarely used as part of the initial assessment of patients with NMD and/or CNS conditions. Nine respondents chose the ‘other’ option. These respondents stated that they also routinely undertake the following assessments: functional movement tests, such as sit-to-stand or stairs; assessment of psychological limitations and social inclusion; ability/inability to stand; and lifestyle assessments. Respondents stated that they would also undertake the following: review of any imaging or related reports as part of assessment; assessment of pain, balance and confidence in walking with numeric rating scales; assessment of current orthosis; and assessment of patient’s expectations and understanding of the limitations of treatment.
Prescription and fitting of orthotic devices
This section of the survey began by asking our respondents if, in their routine work, they prescribe or fit orthotic devices for adult patients with NMD and/or CNS conditions, with knee instability (Q24).
The majority of respondents (70.21%) prescribe or fit orthotic devices for patients with NMD/CNS conditions, with knee instability (Table 26). All but two orthotists prescribe/fit orthotic devices for this patient group (96.72%). Slightly more of our respondents who were physiotherapists and doctors in rehabilitation medicine do not prescribe/fit orthotic devices for this patient group (50% and 52%, respectively).
| Response | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 141 (66.51%) | n = 61 (82.43%) | n = 55 (68.29%) | n = 25 (55.56%) |
| Yes | 99 (70.21) | 59 (96.72) | 28 (50.00) | 12 (48.00) |
| No | 42 (29.79) | 2 (3.28) | 28 (50.00) | 13 (52.00) |
| No response | 71 | 13 | 26 | 20 |
In order to reduce the burden on respondents, those who answered no to this question subsequently skipped all of the questions that assumed that the respondent prescribed or fitted orthotic devices. Those respondents who stated that they did not prescribe or fit devices to this patient group were asked to which HCP they would refer patients requiring prescription and fitting of orthotic devices (Q25). The results of this question are presented in Tables 27 and 28, and indicated that the most common referrals for prescribing or fitting an orthosis was to an orthotist.
| HCP | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 43 (37.39%) | n = 2 (12.25%) | n = 28 (51.85%) | n = 13 (39.4%) |
| Orthotist | 36 (83.72) | 1 (50.00) | 24 (85.71) | 11 (84.62) |
| Physiotherapist | 2 (4.65) | 0 (0.00) | 0 (0.00) | 2 (15.38) |
| Doctor in rehabilitation medicine | 1 (2.33) | 0 (0.00) | 1 (3.57) | 0 (0.00) |
| Other | 2 (4.65) | 0 (0.00) | 2 (7.14) | 0 (0.00) |
| NA | 2 (4.65) | 1 (50.00) | 1 (3.57) | 0 (0.00) |
| No response | 72 | 14 | 26 | 20 |
| HCP | All respondents | Orthotists | Physiotherapists | Doctors in rehabilitation medicine |
|---|---|---|---|---|
| Response rate | n = 43 (37.39%) | n = 2 (12.25%) | n = 28 (51.85%) | n = 13 (39.4%) |
| Orthotist | 31 (72.09) | 1 (50.00) | 20 (71.43) | 10 (76.92) |
| Physiotherapist | 2 (4.65) | 0 (0.00) | 0 (0.00) | 2 (15.38) |
| Doctor in rehabilitation medicine | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Other | 2 (4.65) | 0 (0.00) | 2 (7.14) | 2 (15.38) |
| NA | 8 (18.60) | 1 (50.00) | 6 (21.43) | 1 (7.69) |
| No response | 72 | 14 | 26 | 20 |
Types of devices
The respondents were asked about what types of orthotic devices they prescribe for patients with NMD and/or CNS conditions with knee instability (Q26). The results for this question are presented in Table 29.
| Orthotic device | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 99 (58.24%) | n = 60 (82.19%) | n = 27 (51.85%) | n = 12 (37.5%) |
| KAFO | 74 (74.75) | 55 (91.67) | 8 (28.57) | 11 (91.67) |
| AFO | 93 (93.94) | 56 (93.33) | 26 (92.86) | 11 (91.67) |
| Knee brace | 88 (88.89) | 58 (96.67) | 19 (67.86) | 11 (91.67) |
| Shoe adaptations | 65 (65.66) | 44 (73.33) | 11 (39.29) | 10 (83.33) |
| Insoles | 69 (69.70) | 46 (76.67) | 13 (46.43) | 10 (83.33) |
| Other | 18 (18.18) | 7 (11.67) | 11 (39.29) | 0 (0.00) |
| None | 0 (0.00) | 1 (1.67) | 0 (0.00) | 0 (0.00) |
| NA | 0 (0.00) | 0 (0.00) | 1 (3.57) | 0 (0.00) |
| No response | 71 | 13 | 26 | 20 |
Ankle–foot orthoses appear to be the device prescribed by most respondents (93.94%), followed closely by knee braces (88.89%) and KAFOs (74.75%). Eighteen respondents (18.18%) chose ‘other’ for the type of orthotic device prescribed. Patients with NMD and CNS conditions with knee instability are also prescribed ‘FOOT-UP’® Orthoses (Össur, Reykjavik, Iceland), Lycra, ‘heel raises’, ankle supports, GRAFOs, taping and anti-hyperextension cages.
Respondents were also asked about what types of orthotic devices they fit for patients with CNS conditions and/or NMD with knee instability (Q27). Only 14 respondents (8.24%) stated that they do not fit any devices, indicating that the majority of respondents both prescribe and fit orthotic devices for patients with CNS conditions and/or NMD with knee instability (Table 30). The responses to this question were very similar to the responses to the previous question on prescribing. The most commonly fitted devices overall were AFOs (89.90%), knee braces (87.88%) and KAFOs (65.66%). Fourteen respondents (14.14%) chose ‘other’ for the type of orthotic device prescribed. Seven orthotists chose ‘other’ and stated that spinal supports, heel raises and Lycra supports could also be fitted. Four physiotherapists chose ‘other’ and stated that FOOT-UPS, taping, GRAFOs and sensory dynamic orthoses could also be fitted. Four doctors in rehabilitation chose ‘other’; responses stated that the orthoses that they prescribe would be fitted by an orthotist.
| Orthotic device | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 99 (58.24%) | n = 59 (81.94%) | n = 28 (52.83%) | n = 12 (37.5%) |
| KAFO | 65 (65.66) | 54(91.53) | 6 (21.43) | 5 (41.67) |
| AFO | 89 (89.90) | 57 (96.61) | 26 (92.86) | 6 (50.00) |
| Knee brace | 87 (87.88) | 58 (98.31) | 23 (82.14) | 6 (50.00) |
| Shoe adaptations | 61 (61.62) | 45 (76.27) | 10 (35.71) | 6 (50.00) |
| Insoles | 67 (67.68) | 46 (77.97) | 16 (57.14) | 5 (41.67) |
| Other | 14 (14.14) | 6 (10.17) | 4 (14.29) | 4 (33.33) |
| None | 13 (13.13) | 7 (11.86) | 4 (14.29) | 2 (16.67) |
| NA | 1 (1.01) | 0 (0.00) | 0 (0.00) | 1 (8.33) |
| No response | 71 | 13 | 25 | 20 |
Respondents were asked what proportion (approximately) of the devices that they prescribe/fit for patients with CNS conditions and/or NMD is custom-made (Q28).
The results indicated that approximately half of all devices prescribed/fitted by our respondents were reported to be custom-made devices (Table 31). This is the case for both orthotists and doctors in rehabilitation medicine; however, physiotherapists indicated that approximately one-third of the devices that they prescribe are custom-made devices. Respondents were then asked what influences their decision to prescribe a custom-made or an off-the-shelf device (Q29). The question response rate was 55.03% (n = 93). The comments indicate that there are patient and device factors that influence the HCPs’ decision.
| HCP | Response rate | Mean (SD) | Median | Minimum, maximum | No response |
|---|---|---|---|---|---|
| All respondents | 57.06% (n = 97) | 50.16 (27.33) | 50 | 0, 100 | 73 |
| Orthotists | 81.94% (n = 59) | 56.75 (21.70) | 60 | 6, 100 | 13 |
| Physiotherapists | 48.15% (n = 25) | 35.62 (33.92) | 33 | 0, 100 | 28 |
| Rehabilitation medicine physicians | 37.5% (n = 12) | 49.33 (26.21) | 44.5 | 3, 94 | 20 |
Patient factors
Forty-one comments stated that the clinical condition of the patients on presentation will influence the decision of what device to prescribe. For example, one comment stated that:
The individual’s functional deficit will dictate the mechanical/biomechanical requirements of the orthosis/orthotic device. Typically custom devices have the potential to exert much greater external moments to the knee and other joints.
Twenty-three comments also discussed the anatomy of the patient and how that influences the decision. For example, one comment stated that ‘ . . . often if thigh is atrophied, a custom brace may be more appropriate’. The ‘other’ factors highlighted as influencing the decision were eight comments indicating that patient preference needs should be taken into account; seven comments noting patients’ past experiences with orthoses; five comments stating the patient’s ability to put the orthosis on and take it off; four comments highlighting patient compliance; and one comment noting the patient’s ability to self-fund.
Device factors
Thirty-one comments highlighted that the decision about the type of device will be influenced by the desired features required by the patients, for example flexibility, control, fit and durability. Eleven comments noted that the availability of devices will influence the decision of which device to prescribe, and 10 comments noted the time to acquire the device. Ten comments stated that the type of device would influence the decision. For example, one respondent wrote: ‘If it is an AFO or KAFO or insole it would be custom-made. Most knee braces are off the shelf unless the knee is a very unusual shape.’ Nine comments stated that an off-the-shelf device may be trialled first on a patient while considering whether or not a custom-made device is needed. Six comments stated that cost influences the decision: ‘If none of these basic items are appropriate, a sound clinical reasoned request is put to manager (budget holder) for the need for alternate item not on the list.’ Two comments stated that off-the-shelf devices are used for assessment. Two comments also noted that someone else would decide whether a custom-made or off-the-shelf device is prescribed.
Respondents were asked where the prescribed custom-made devices are manufactured (Q30). The response rate to this question is particularly low as, although it was set up so that eligible respondents could not skip this question, the function failed.
A higher proportion of respondents reported that custom-made devices (73.58%) are manufactured by a central fabrication manufacturer outside the hospital compared with an on-site workshop (16.98%) (Table 32). Two doctors chose ‘other’ and stated that some are manufactured ‘off-site’ and in an ‘other NHS facility’.
| Site | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 53 (24.88%) | n = 25 (33.78%) | n = 1 (1.06%) | n = 25 (55.56%) |
| On-site workshop in clinical setting | 9 (16.98) | 1 (4.00) | 0 (0.00) | 8 (32.00) |
| Central fabrication manufacturer outside hospital | 39 (73.58) | 24 (96.00) | 1 (100.00) | 14 (56.00) |
| Other | 2 (5.66) | 0 (0.00) | 0 (0.00) | 2 (8.00) |
| NA | 2 (3.77) | 0 (0.00) | 0 (0.00) | 1 (4.00) |
| No response | 160 | 49 | 93 | 20 |
Provision of orthoses
In order to get an indication of how many visits were required to provide orthotic devices, respondents were asked how many visits would be needed depending on whether it was a patient with NMD or CNS condition, and what device they were prescribing (Q34, Q36). Respondents were asked how many visits are normally required to provide orthotic devices for patients with NMD. The results of this question and response rates are presented in Table 33. These underestimate the question response rate, as this is the number of respondents who responded to each element of the answer rather than those who supplied an answer for Q34 and Q36.
| Device | Mean (SD) | Median | Minimum, maximum | Response rate, % (n) | No response |
|---|---|---|---|---|---|
| Custom-made orthoses | |||||
| KAFO | 3 (0.55) | 3 | 2, 4 | 34.18 (n = 54) | 104 |
| AFO | 2.05 (0.58) | 2 | 1, 4 | 37.35 (n = 62) | 104 |
| Knee brace | 2.02 (0.56) | 2 | 1, 4 | 36.97 (n = 61) | 104 |
| Shoe adaptations | 1.78 (0.47) | 2 | 1, 3 | 30.20 (n = 45) | 104 |
| Insoles | 1.84 (0.55) | 2 | 1, 4 | 32.03 (n = 49) | 104 |
| Off-the-shelf orthoses | |||||
| KAFO | 2.64 (0.67) | 3 | 2, 4 | 19.35 (n = 24) | 100 |
| AFO | 1.71 (0.73) | 2 | 1, 4 | 38.27 (n = 62) | 100 |
| Knee brace | 1.80 (0.69) | 2 | 1, 4 | 38.65 (n = 63) | 100 |
| Shoe adaptation | 1.68 (0.47) | 2 | 1, 2 | 25.37 (n = 34) | 100 |
| Insoles | 1.62 (0.61) | 2 | 1, 4 | 31.97 (n = 47) | 100 |
Respondents were also asked how many visits are normally required to provide orthotic devices for patients with CNS conditions. The results of this question are presented in Table 34 (Q35, Q37). As with the previous table, the response rates are an underestimate, as this is the number of respondents who responded to each element of the answer rather than those who supplied an answer for Q35 and Q37.
| Device | Mean (SD) | Median | Minimum, maximum | Response rate, % (n) | No response |
|---|---|---|---|---|---|
| Custom-made orthoses | |||||
| KAFO | 3.03 (0.56) | 3 | 2, 4 | 31.55 (n = 59) | 128 |
| AFO | 2.07 (0.50) | 2 | 1, 4 | 34.36 (n = 67) | 128 |
| Knee brace | 2.09 (0.61) | 2 | 1, 4 | 33.33 (n = 64) | 128 |
| Shoe adaptation | 1.90 (0.47) | 2 | 1, 3 | 33.33 (n = 64) | 128 |
| Insoles | 1.91 (0.53) | 2 | 1, 4 | 29.28 (n = 53) | 128 |
| Off-the-shelf orthoses | |||||
| KAFO | 1.96 (0.71) | 2 | 1, 4 | 18.24 (n = 27) | 121 |
| AFO | 1.66 (0.65) | 2 | 1, 4 | 36.98 (n = 71) | 121 |
| Knee brace | 1.75 (0.63) | 2 | 1, 4 | 36.32 (n = 69) | 121 |
| Shoe adaptation | 1.73 (0.45) | 2 | 1, 2 | 25.31 (n = 41) | 121 |
| Insoles | 1.54 (0.54) | 2 | 0, 2 | 30.86 (n = 54) | 121 |
For all devices and for both patients with NMD and CNS conditions, custom-made devices were found to require additional visits to HCPs compared with off-the-shelf devices in order for the patients to be provided with orthotic devices.
For patients with both NMD and CNS conditions, the custom-made KAFO required the most visits, with an average of three visits to HCPs in order to be fitted with a KAFO; off-the-shelf insoles required the least number of visits (average of 1.62 visits for patient with NMDs and 1.54 for those with CNS conditions).
Typical time frame between appointments
Respondents who stated that they prescribe or fit orthotic devices for patients with NMD and/or CNS conditions were asked a series of questions regarding the typical time frame between particular appointments. The questions were asked for both off-the-shelf devices and for custom-made devices as these times may differ.
Respondents were asked the typical time frame between an initial appointment and the time at which the device is fitted on the patient (Q31). The response rate was 54.12% (n = 92). 76.39% of the orthotists, 54.9% of the physiotherapists and 34.38% of the doctors in rehabilitation medicine responded to the question. The results of this question are presented in Table 35.
| Device | Mean (SD) | Median | Minimum, maximum | No response |
|---|---|---|---|---|
| Custom-made orthoses | ||||
| All respondents | 5.72 (3.51) | 5 | 0, 20 | 78 |
| Orthotists | 5.89 (3.25) | 5 | 2, 20 | 17 |
| Physiotherapists | 3.71 (2.90) | 4 | 0, 10 | 28 |
| Rehabilitation medicine physicians | 8.73 (3.69) | 11 | 3, 12 | 21 |
| Off-the-shelf orthoses | ||||
| All respondents | 3.45 (2.61) | 3 | 0, 12 | 78 |
| Orthotists | 4.09 (2.56) | 4 | 0, 12 | 17 |
| Physiotherapists | 1.91 (2.39) | 1 | 0, 8 | 28 |
| Rehabilitation medicine physicians | 3.45 (2.07) | 4 | 0, 6 | 21 |
The average typical waiting time from initial visit to fitting a custom-made device is longer that the waiting time to fitting an off-the-shelf device (3.45 vs. 5.72 weeks).
Respondents were asked how often they see patients with NMD and/or CNS conditions with knee instability for review (Q22).
The results from this question were quite interesting in that the majority of respondents chose the ‘other’ option (Table 36). It appears from the narrative responses that reviews do not follow a distinctive pattern, do not continue cyclically and are not always available to patients. Eleven physiotherapists stated that the timing of review visit ‘varies’ and eight physiotherapists stated review visits were provided ‘as required’ or ‘as needed’. Twelve respondents (including two orthotists, seven physiotherapists and three doctors in rehabilitation medicine) stated that the frequency of review visits would depend on the patient’s condition. An orthotist also stated that review visits depend on the orthotic device. Six orthotists suggested that review visits depend on the patient’s request.
| Time period | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 144 (67.61%) | n = 61 (82.43%) | n = 57 (71.25%) | n = 26 (59.1%) |
| Weekly | 6 (4.17) | 1 (1.64) | 5 (8.77) | 0 (0.00) |
| Monthly | 7 (4.86) | 2 (3.28) | 4 (7.02) | 1 (3.85) |
| Quarterly | 12 (8.33) | 4 (6.56) | 2 (3.51) | 6 (23.08) |
| Biannually | 21 (14.58) | 10 (16.39) | 1 (1.75) | 10 (38.46) |
| Annually | 5 (3.47) | 5 (8.20) | 0 (0.00) | 0 (0.00) |
| No follow-up | 11 (7.64) | 3 (4.92) | 3 (5.26) | 5 (19.23) |
| Other | 82 (56.94) | 36 (59.02) | 42 (73.68) | 4 (15.38) |
| No response | 69 | 13 | 23 | 19 |
Some alternative timelines were provided. One orthotist stated that review visits happen ‘regularly’, one physiotherapist’s review visits were weekly for 6 weeks, and another physiotherapist’s review visits were fortnightly. Review visits can be daily (according to seven physiotherapists) if the patient is an inpatient. One physiotherapist wrote that review visits are initially every 4 weeks and then every 6 months if the patient is doing well. Another physiotherapist wrote that review visits are quarterly until the patient is discharged. Finally, a doctor in rehabilitation medicine wrote that the initial review visit would be after 1–2 months after which review visits are biannual or annual when the patient is established.
Several respondents (four orthotists and two physiotherapists) found that rather than after a certain time period, review visits take place at specific time points in a patient pathway.
Respondents who stated that they provide review appointments were asked for the typical time frame from fitting of a device to the first review appointment (Q41).
The average time from fitting the device to the first review appointment was 8.28 weeks (Table 37). This was substantially less for physiotherapists, whose average time frame was 1.70 weeks. This may be explained by the larger proportion of physiotherapists who regularly see patients within an inpatient setting.
| HCP | Mean (SD) | Median | Minimum, maximum | No response |
|---|---|---|---|---|
| All respondents Response rate 30.95% (n = 39) |
8.28 (6.74) | 6 | 1, 32 | 87 |
| Orthotists Response rate 55.56% (n = 25) |
8.68 (6.74) | 7 | 2, 32 | 20 |
| Physiotherapists Response rate 17.95% (n = 7) |
3.71 (1.70) | 4 | 1, 6 | 24 |
| Rehabilitation medicine physicians Response rate 23.33% (n = 7) |
11.43 (8.24) | 8 | 4, 27 | 23 |
Typical length of appointments
Respondents who stated that they prescribe or fit orthotic devices for patients with NMD and/or CNS conditions were also asked a series of questions regarding the typical duration of particular appointments discussed. Respondents moved a slider within the range of 0–60 minutes.
The length of appointment required for an initial assessment (Q21) is presented in Table 38.
| HCP | Mean (SD) | Median | Minimum, maximum | No response |
|---|---|---|---|---|
| All respondents Response rate 69.48% (n = 148) |
38 (15.75) | 40 | 0, 60 | 65 |
| Orthotists Response rate 83.78% (n = 62) |
28 (10.59) | 29 | 14, 60 | 12 |
| Physiotherapists Response rate 71.95% (n = 59) |
48 (13.56) | 48 | 0, 60 | 23 |
| Rehabilitation medicine physicians Response rate 60% (n = 27) |
39 (16.09) | 40 | 1, 60 | 18 |
The responses indicate that it takes on average 38 minutes for an initial assessment appointment, with orthotists taking slightly less time than the other HCPs at 28 minutes. These appointments can vary in length, from 0 minutes to 60 minutes.
The length of appointment required for the casting and measuring of orthotic devices (Q32) is presented in Table 39. The response rate was 50.29% (n = 86): 76.39% of the orthotists, 36.54% of the physiotherapists and 28.13% of the doctors in rehabilitation medicine responded to the question.
| HCP | Mean (SD) | Median | Minimum, maximum | No response |
|---|---|---|---|---|
| Custom-made orthoses | ||||
| All respondents | 38.31 (13.27) | 40 | 5, 60 | 85 |
| Orthotists | 36.85 (11.18) | 40 | 15, 60 | 17 |
| Physiotherapists | 42.88 (17.44) | 43.5 | 5, 60 | 33 |
| Rehabilitation medicine physicians | 39.11 (16.37) | 41 | 8, 60 | 23 |
| Off-the-shelf orthoses | ||||
| All respondents | 25.57 (12.83) | 20 | 0, 60 | 85 |
| Orthotists | 24.31 (10.42) | 20 | 10, 60 | 17 |
| Physiotherapists | 30.79 (17.73) | 30 | 0, 60 | 33 |
| Rehabilitation medicine physicians | 22.11 (12.30) | 20 | 3, 41 | 23 |
The respondents required slightly more time to cast and measure custom-made devices than for an off-the-shelf device. On average, for custom-made devices the appointment length was 38.31 minutes compared with 25.57 minutes for off-the-shelf devices. The mean duration for physiotherapists was slightly longer for both custom-made and off-the-shelf devices.
The respondents who stated that they do provide a review appointment were asked how long a review visit lasts, on average (Q23). The mean duration of review appointments was 27 minutes. This ranged from 1 minute to 45 minutes (Table 40). Physiotherapists’ review visits lasted slightly longer on average than those of the other HCPs.
| HCP | Mean (SD) | Median | Minimum, maximum | No response |
|---|---|---|---|---|
| All respondents Response rate 69.48% (n = 148) |
27 (10.62) | 21 | 1, 45 | 65 |
| Orthotists Response rate 81.69% (n = 58) |
22 (7.66) | 20 | 14, 45 | 13 |
| Physiotherapists Response rate 67.09% (n = 53) |
34 (10.99) | 30 | 1, 45 | 26 |
| Rehabilitation medicine physicians Response rate 52.5% (n = 21) |
22 (8.29) | 20 | 22, 45 | 19 |
Patient information at fitting appointments
Respondents were asked what information they provided to patients at fitting appointments (Q38). The majority of the respondents indicated that they relay all of the information in the options provided (Table 41).
| Information provided | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 85 (50%) | n = 52 (72.22%) | n = 24 (44.44%) | n = 9 (28.13%) |
| Instructions on taking the device on and off | 81 (95.29) | 50 (96.15) | 23 (95.83) | 8 (88.89) |
| Instructions on care of the orthosis | 80 (94.12) | 50 (69.15) | 22 (91.67) | 8 (88.89) |
| Instructions on how to monitor the fit of the orthosis | 75 (88.24) | 47 (90.38) | 21 (87.50) | 7 (77.78) |
| Instructions on when to wear the orthosis | 79 (92.94) | 49 (94.23) | 22 (91.67) | 8 (88.89) |
| Instructions on when to seek a review appointment | 76 (89.41) | 51 (98.08) | 18 (75.00) | 7 (77.78) |
| Other | 15 (17.65) | 11 (21.15) | 2 (8.33) | 2 (22.22) |
| None of the above | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| No response | 85 | 20 | 30 | 23 |
Almost all respondents reported providing instructions on taking the device on and off (95.29%); instructions on use of the orthosis (94.12%); when to wear the orthosis (92.94%); when to seek a review appointment (89.41%); and how to monitor the fit of the orthosis (88.24%). Fifteen respondents choose the ‘other’ option.
The response from the orthotists stated that information on the following would also be provided to patients: safety checks, instructions on lifespan and replacement of the orthosis, instructions on adjustment of the orthosis, a review of the goal of the orthosis, instructions on weaning procedure, skin care, and instructions on suitable footwear if an AFO or KAFO is being provided. One doctor in rehabilitation medicine stated that their patient would be provided with the phone number of the rehabilitation consultant’s secretary.
Respondents were asked in what form they provide information to patient at fitting appointments (Q39).
The majority of respondents reported providing information to patients verbally (91.95%), through short leaflets (70.11%) and, to a lesser extent, instruction booklets (44.83%) (Table 42). A small number of respondents (four orthotists and three physiotherapists) direct patients to a website; none provided information on CDs.
| Form of information | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 87 (51.18%) | n = 54 (75%) | n = 24 (44.44%) | n = 9 (28.13%) |
| Verbally | 80 (91.95) | 51 (94.44) | 22 (91.67) | 7 (77.78) |
| Short leaflets | 61 (70.11 | 44 (81.48) | 10 (41.67) | 7 (77.78) |
| Instruction booklets | 39 (44.83) | 20 (37.04) | 14 (58.33) | 5 (55.56) |
| CD | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Direct patient to a website | 7 (8.05) | 4 (7.41) | 3 (12.50) | 0 (0.00) |
| Other | 6 (6.90) | 1 (1.85) | 3 (12.50) | 0 (0.00) |
| None of above | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| No response | 83 | 18 | 24 | 23 |
Six respondents chose the ‘other’ option. The orthotists stated a safety agreement is signed and returned from the patient and retained in the patient’s notes. The physiotherapists stated they used written notes and within discharge reports, and one physiotherapist stated that this would not be their role.
Long-term review appointments
Respondents were asked whether or not they routinely offer long-term review appointments (Q40).
A slightly higher proportion of respondents (54.12%) do not routinely provide long-term review appointments than those who do (45.88%), and this is particularly noticeable in the physiotherapist group (Table 43). Slightly more doctors in rehabilitation medicine do provide long-term review appointments than those who do not.
| Response | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 85 (50%) | n = 52 (72.22%) | n = 24 (44.44%) | n = 9 (28.13%) |
| Yes | 39 (45.88) | 25 (48.08) | 7 (29.17) | 7 (77.78) |
| No | 46 (54.12) | 27 (51.92) | 17 (70.83) | 2 (22.22) |
| None | 85 | 20 | 30 | 23 |
In a separate question, respondents were asked if their practice has a ‘review on request’ option for patients (Q46).
The results show that a substantial majority of respondents operate ‘review on request’ appointments within their clinical setting (88.24%) compared with those who do not (11.76%) (Table 44). The results from both questions on long-term review appointments indicate that instead of prespecified review appointments, review appointments can be instigated by patients.
| Response | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 85 (49.71%) | n = 52 (72.22%) | n = 24 (44.44%) | n = 9 (28.13%) |
| Yes | 75 (88.24) | 51 (98.08) | 16 (66.67) | 8 (88.89) |
| No | 10 (11.76) | 1 (1.92) | 8 (33.33) | 1 (11.11) |
| None | 86 | 20 | 31 | 23 |
Quantifying the success of an orthotic device
Following on from the questions on fitting and reviewing the orthotic devices, respondents were asked how they would normally quantify the success of an orthosis when fitting/reviewing the device (Q42).
Respondents rely on patient feedback (94.87%), family/carer feedback (84.62%), and observational gait analysis (87.18%) a significant proportion of the time to quantify the success of the device (Table 45). Formal gait analysis via video gait analysis or video vector gait analysis appears to be used to a lesser extent (17.95% and 2.56%, respectively). One respondent stated that they used none of the above measures to quantify the success of the device. Two respondents chose the ‘other’ option. Only one respondent provided a text response and stated they were unable to comment as the ‘orthotist would provide this response’.
| Quantifying success | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 39 (30.95%) | n = 25 (55.56%) | n = 7 (17.95%) | n = 7 (23.33%) |
| Patient feedback | 37 (94.87) | 24 (96.00) | 6 (85.71) | 7 (100.00) |
| Family/carer feedback | 33 (84.62) | 24 (96.00) | 4 (57.14) | 5 (71.43) |
| Another clinician or therapist’s feedback | 22 (56.41) | 14 (56.00) | 3 (42.86) | 5 (71.43) |
| Observational gait analysis | 34 (87.18) | 24 (96.00) | 4 (57.14) | 6 (85.71) |
| Video gait analysis | 7 (17.95) | 2 (8.00) | 4 (57.14) | 1 (14.29) |
| Video vector gait analysis | 1 (2.56) | 0 (0.00) | 0 (0.00) | 1 (14.29) |
| Patient-reported outcome measures | 27 (69.23) | 18 (72.00) | 5 (71.43) | 4 (57.14) |
| Clinician-reported outcome measures | 20 (51.28) | 12 (48.00) | 5 (71.43) | 3 (42.86) |
| Other | 2 (5.13) | 0 (0.00) | 1 (14.29) | 1 (14.29) |
| None of the above | 1 (2.56) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| No response | 87 | 20 | 32 | 23 |
Device breakages
The respondents were asked several questions about what happens when orthotic devices break. Respondents were asked separately what procedures are in place in their setting if custom-made or off-the-shelf devices break or are damaged (Q43, Q44).
The most commonly used options in the event of breakage of a custom-made device were provision of a spare orthosis to the patients at the same time as they receive the original device (36.47%); provision of an off-the-shelf device until the prescribed device is repaired (32.94%); and use of on-the-spot repair at an on-site workshop (37.65%) (Table 46). Provision of a wheelchair until the device is repaired was reported by 7.06% of respondents. Twenty-two respondents chose the ‘other’ option (13 orthotists, 8 physiotherapists and 1 doctor in rehabilitation medicine). In the responses provided, the orthotists stated the following: a spare device is provided after first satisfactory review (n = 3); this would depend on the device (n = 3); the patient would have to wait (n = 1); no procedures are in place (n = 1); this is dependent on patient funding (n = 1) or dependent on NHS trust protocol (n = 1); and this is also dependent on whether or not the patient can travel to an off-site workshop (n = 1). The physiotherapists stated that patients are referred to the orthotist for review and/or replacement (n = 6); the patient has to wait (n = 1); and no procedures are in place (n = 1). One doctor in rehabilitation medicine stated that he or she would call the company.
| Procedure | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 85 (50%) | n = 52 (72.22%) | n = 24 (44.44%) | n = 9 (28.13%) |
| A spare orthosis is provided in conjunction with the original device | 31 (36.47) | 29 (55.77) | 1 (4.17) | 1 (11.11) |
| An off-the-shelf orthosis is provided until the prescribed orthosis is fixed | 28 (32.94) | 11 (21.15) | 10 (41.67) | 7 (77.78) |
| Patient is given a wheelchair until their orthosis is fixed | 6 (7.06) | 1 (1.92) | 0 (0.00) | 0 (0.00) |
| Patient comes to an on-site workshop for an on-the-spot repair | 32 (37.65) | 26 (50.00) | 2 (8.33) | 4 (44.44) |
| Other | 22 (25.88) | 13 (25.00) | 8 (33.33) | 1 (11.11) |
| NA | 9 (10.59) | 1 (1.92) | 7 (29.17) | 1 (11.11) |
| No response | 85 | 20 | 30 | 23 |
The most commonly used options in the event of breakage of an off-the-shelf device were use of on-the-spot repair at an on-site workshop (37.65%) and provision of a spare orthosis to the patient at the same time as they receive the original device (30.59%) (Table 47). Provision of a wheelchair until the device is repaired was reported by 3.53% of respondents. Forty respondents chose the ‘other’ option (22 orthotists, 16 physiotherapists and 2 doctors in rehabilitation medicine). Several respondents (four orthotists, four physiotherapists and one doctor in rehabilitation medicine) stated that a replacement is provided on the day if stock is available or soon as possible otherwise. A further six orthotists and two physiotherapists stated that a replacement device is ordered. One orthotist can provide emergency appointments to fit a new device. Four orthotists provide a spare device after the first satisfactory review.
| Procedure | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 85 (50%) | n = 52 (72.22%) | n = 24 (44.44%) | n = 9 (28.13%) |
| A spare orthosis is provided in conjunction with the original device | 26 (30.59) | 22 (42.31) | 2 (8.33) | 2 (22.22) |
| Patient is given a wheelchair until their orthosis is fixed | 3 (3.53) | 1 (1.92) | 0 (0.00) | 2 (22.22) |
| Patient comes to an on-site workshop for an on-the-spot repair | 32 (37.65) | 24 (46.15) | 2 (8.33) | 6 (66.67) |
| Other | 40 (47.06) | 22 (42.31) | 16 (66.67) | 2 (22.22) |
| NA | 8 (9.41) | 1 (1.92) | 6 (25.00) | 1 (11.11) |
| No response | 85 | 20 | 30 | 23 |
Similar to the procedure for custom-made devices, patients sometimes have to wait (two orthotists) and no procedures are currently in place in some settings (two orthotists and one physiotherapist). Four physiotherapists refer the patient to the orthotist for review and/or replacement. Once again, one doctor in rehabilitation medicine stated that he/she would call the company.
Respondents were also asked who repairs the device when it breaks (Q45). The results for this question indicated that off-site technicians most commonly repair broken devices (56.32%) (Table 48). However, given the high response rate of the orthotists in comparison with the other HCPs for this question, this may not be the case for all clinical settings and for all HCPs prescribing and fitting orthotic devices.
| Device repair | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 87 (59.58%) | n = 52 (72.22%) | n = 25 (46.3%) | n = 9 (27.27%) |
| On-site | ||||
| Clinician | 36 (41.38) | 27 (51.92) | 5 (20.00) | 4 (44.44) |
| Technician | 31 (35.63) | 21 (40.38) | 6 (24.00) | 4 (44.44) |
| Off-site | ||||
| Clinician | 6 (6.90) | 1 (1.92) | 5 (20.00) | 0 (0.00) |
| Technician | 49 (56.32) | 40 (76.92) | 6 (24.00) | 3 (33.33) |
| Other | 14 (16.09) | 2 (3.84) | 11 (44.00) | 1 (11.11) |
| No response | 85 | 20 | 29 | 24 |
Fourteen respondents selected the ‘other’ option. This included two orthotists, 11 physiotherapists and one doctor in rehabilitation medicine. The orthotists responses stated that this depends on the site and the required repair. The physiotherapists stated the following: the device is replaced and not repaired (n = 3); the orthotist repairs the device (n = 2); this depends on the device (n = 1); and they do not know who repairs the device when it breaks (n = 4); one did not elaborate.
Treatment outcomes and acceptability factors
Respondents were asked a series of questions exploring treatment outcomes, acceptability factors and patient expectations.
Treatment outcomes
First, respondents were asked to what extent, when trying to manage the expectations of patients, do the following factors influence their decision of what device to prescribe (Q47).
The question response rate was 57.75% (n = 123): 70.27% of the orthotists, 59.26% of the physiotherapists and 48.89% of the doctors in rehabilitation medicine responded to the question. Ninety respondents did not answer this question (which included, where known, 22 orthotists, 33 physiotherapists and 23 doctors in rehabilitation medicine). The responses to this question are presented in Table 49.
| Factor | Responses, n (%) | ||||
|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Most of the time | Always | |
| Cosmetic aspect of the device | 2 (1.63) | 12 (9.76) | 56 (45.53) | 32 (26.02) | 17 (13.82) |
| Weight of the device | 0 (0.00) | 1 (0.81) | 33 (26.83) | 52 (42.28) | 34 (27.64) |
| Material of the device | 1 (0.81) | 12 (9.76) | 39 (31.71) | 34 (27.64) | 34 (27.64) |
| Types of shoes/clothing that can be worn with the device | 0 (0.00) | 9 (7.32) | 38 (30.89) | 45 (36.59) | 28 (22.76) |
| Patients ability to take device on and off | 0 (0.00) | 0 (0.00) | 15 (12.20) | 29 (23.58) | 76 (61.79) |
| Reliability of the device | 0 (0.00) | 3 (2.44) | 12 (9.76) | 40 (32.52) | 65 (52.85) |
| Comfort of the device | 0 (0.00) | 0 (0.00) | 8 (6.50) | 24 (19.51) | 88 (71.54) |
| Other | 34 (27.64) | 1 (0.81) | 11 (8.94) | 5 (4.07) | 18 (14.63) |
The highest proportion of respondents (45.53%) chose ‘sometimes’ when asked about the cosmetic aspects of the device; ‘most of the time’ when asked about the weight of the device (42.28%); ‘sometimes’ when asked about the material of the device (31.71%); and ‘most of the time’ when asked about the types of shoes or clothing that the devices can be worn (36.59%) The majority of respondents (61.79%) chose ‘always’ when asked about patients’ ability to take the device on and off; when asked about the reliability of the device (52.85%); and when asked about the comfort of the device (71.54%). The ‘other’ option was chosen by 69 (56.09%) respondents. The responses are presented in Tables 50–52, grouped by profession.
| Factor | Responses, n (%) | ||||
|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Most of the time | Always | |
| The cosmetic aspects of the device | 0 (0.00) | 3 (5.77) | 24 (46.15) | 12 (23.08) | 13 (25.00) |
| The weight of the device | 0 (0.00) | 0 (0.00) | 11 (21.15) | 21 (40.38) | 20 (38.46) |
| The material of the device | 0 (0.00) | 4 (7.69) | 8 (15.38) | 17 (32.69) | 23 (44.23) |
| Types of shoes or clothing with which the device can be worn | 0 (0.00) | 2 (3.85) | 14 (26.92) | 18 (34.62) | 15 (28.85) |
| Patient’s ability to take the device on and off | 0 (0.00) | 0 (0.00) | 3 (5.77) | 8 (15.38) | 41 (78.85) |
| The reliability of the device | 0 (0.00) | 0 (0.00) | 3 (5.77) | 10 (19.23) | 36 (69.23) |
| The comfort of the device | 0 (0.00) | 0 (0.00) | 4 (7.69) | 6 (11.54) | 42 (80.77) |
| Other | 18 (34.62) | 1 (1.92) | 5 (9.62) | 3 (5.77) | 7 (13.46) |
| Factor | Responses, n (%) | ||||
|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Most of the time | Always | |
| The cosmetic aspects of the device | 2 (4.17) | 6 (12.50) | 21 (43.75) | 14 (29.17) | 2 (4.17) |
| The weight of the device | 0 (0.00) | 1 (2.08) | 13 (27.08) | 20 (41.67) | 11 (22.92) |
| The material of the device | 0 (0.00) | 2 (4.17) | 22 (45.83) | 11 (22.92) | 9 (18.75) |
| Types of shoes or clothing with which the device can be worn | 0 (0.00) | 1 (2.08) | 15 (31.25) | 19 (39.58) | 10 (20.83) |
| Patient’s ability to take the device on and off | 0 (0.00) | 0 (0.00) | 8 (16.67) | 12 (25.00) | 25 (52.08) |
| The reliability of the device | 0 (0.00) | 3 (6.25) | 2 (4.17) | 20 (41.67) | 19 (39.58) |
| The comfort of the device | 0 (0.00) | 0 (0.00) | 2 (4.17) | 9 (18.75) | 34 (70.83) |
| Other | 8 (16.67) | 0 (0.00) | 3 (6.25) | 2 (4.17) | 9 (18.75) |
| Factor | Responses, n (%) | ||||
|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Most of the time | Always | |
| The cosmetic aspects of the device | 0 (0.00) | 3 (13.64) | 11 (50.00) | 6 (27.27) | 2 (9.09) |
| The weight of the device | 0 (0.00) | 0 (0.00) | 8 (36.36) | 11 (50.00) | 3 (13.64) |
| The material of the device | 1 (4.55) | 4 (18.18) | 9 (40.91) | 6 (27.27) | 2 (9.09) |
| Types of shoes or clothing with which the device can be worn | 0 (0.00) | 3 (13.64) | 9 (40.91) | 7 (31.82) | 3 (13.64) |
| Patient’s ability to take the device on and off | 0 (0.00) | 0 (0.00) | 4 (18.18) | 8 (36.36) | 10 (45.45) |
| The reliability of the device | 0 (0.00) | 0 (0.00) | 2 (9.09) | 10 (45.45) | 10 (45.45) |
| The comfort of the device | 0 (0.00) | 0 (0.00) | 2 (9.09) | 8 (36.36) | 12 (54.55) |
| Other | 8 (36.36) | 0 (0.00) | 3 (13.64) | 0 (0.00) | 2 (9.09) |
The highest proportion of orthotists chose ‘sometimes’, when asked about the cosmetic aspects of the device (46.15%); ‘most of the time’ when asked about the weight of the device (40.38%); ‘always’ when asked about the material of the device (44.23%); and ‘most of the time’ when asked about the types of shoes or clothing that the devices can be worn (34.62%). The majority of orthotists chose ‘always’ when asked about patient’s ability to take the device on and off (78.85%); ‘always’ when asked about the reliability of the device (69.23%); and ‘always’ when asked about the comfort of the device (80.77%). The ‘other’ option was chosen by 34 (65.39%), some of whom provided further information.
The orthotists who chose the ‘other’ option stated that the following factors also influence their decision: the cost of the device and the funding available (n = 2); the function of the device (n = 2); the likely benefit to the patient (n = 2); patient acceptability (n = 3); the mental health of the patient (n = 1); and the durability of the device (n = 1).
The highest proportion of physiotherapists chose ‘sometimes’ when asked about the cosmetic aspects of the device (43.75%); ‘most of the time’ when asked about the weight of the device (41.67%); ‘sometimes’ when asked about the material of the device (45.85%); ‘most of the time’ when asked about the types of shoes or clothing with which the devices can be worn (39.58%); ‘always’ when asked about patient’s ability to take the device on and off (52.08%); and ‘most of the time’ when asked about the reliability of the device (41.67%). The majority of physiotherapists (70.83%) chose ‘always’ when asked about the comfort of the device. The ‘other’ option was chosen by 22 physiotherapists (45.84%), some of whom provided further details.
The physiotherapists who chose the ‘other’ option stated that the following factors also influence their decision: the function and effectiveness of the device (n = 3); the availability of the device (n = 1); patient acceptability (n = 1); patient compliance (n = 1); patient prognosis (n = 1); social support available to the patient (n = 1); the fit of the device (n = 1); and advice from the orthotist (n = 1).
The highest proportion of doctors in rehabilitation chose ‘sometimes’ when asked about the cosmetic aspects of the device (50%); ‘most of the time’ when asked about the weight of the device (50%); ‘sometimes’ when asked about the material of the device (40.91%); ‘sometimes’ when asked about the types of shoes or clothing with which the devices can be worn (40.91%); ‘always’ when asked about patient’s ability to take the device on and off (45.45%); ‘always’ or ‘most of the time’ when asked about the reliability of the device (45.45%); and ‘always’ when asked about the comfort of the device (54.55%). The ‘other’ option was chosen by 13 (41.36%) and some provided further details.
The doctors in rehabilitation medicine who chose the ‘other’ option stated that the following factors also influence their decision: the cost to the NHS (n = 1) and the likely benefit and/or discomfort to the patient (n = 1).
Respondents were then asked about what treatment outcomes that they are personally trying to achieve when treating patients with NMD and CNS conditions with knee instability (Q50) (Table 53).
| Aim | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 124 (58.22%) | n = 52 (70.27%) | n = 50 (60.98%) | n = 22 (48.89%) |
| Control joint movement | 108 (87.10) | 49 (94.23) | 45 (90.00) | 14 (63.64) |
| Reducing the number of falls | 110 (88.71) | 48 (92.31) | 42 (84.00) | 20 (90.91) |
| Less pain | 114 (91.94) | 50 (96.15) | 45 (90.00) | 19 (86.36) |
| Increased walking distance | 100 (80.65) | 46 (88.46) | 37 (74.00) | 17 (77.27) |
| Increased walking speed | 69 (55.65) | 31 (59.62) | 26 (52.00) | 12 (54.55) |
| Contracture management | 77 (62.10) | 36 (69.23) | 30 (60.00) | 11 (50.00) |
| Avoid further deterioration | 105 (84.68) | 46 (88.46) | 40 (80.00) | 19 (86.36) |
| Other | 19 (15.32) | 7 (13.46) | 11 (22.00) | 1 (4.55) |
| No response | 89 | 22 | 32 | 23 |
The majority of the outcomes that were presented had a positive response from > 80% of respondents, apart from contracture management and increased walking speed, for which 62.10% and 55.65%, respectively chose these outcomes. Nineteen chose ‘other’. Respondents are also trying to achieve the following treatment outcomes: improved independence (two orthotists and two physiotherapists); improved quality of life (three orthotists); improved mobility (one orthotist and two physiotherapists); improved confidence (two physiotherapists); improved symmetry and reduced deterioration in the other leg (two physiotherapists); support of ligamentous and tendinous structures (one orthotist); improved function (one orthotist); increased social/work integration (one physiotherapist); management of fatigue by reducing effort (one physiotherapist); and prevention of secondary arthritis (one doctor in rehabilitation medicine). One physiotherapist also stated that this would depend on the patient.
Patient preferences
Respondents were asked about the extent to which they agreed that their patients are expressing a preference for particular devices (Q48).
Most commonly respondents stated that they neither agreed nor disagreed that their patients are expressing a preference for particular devices (46.40%) (Table 54). The respondents who chose ‘strongly agree’ or ‘agree’ were asked about what types of devices their patients were expressing a preference for. The responses to this question can be categorised into device factors and patient factors.
| Preference | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 125 (58.69%) | n = 52 (70.27%) | n = 51 (62.2%) | n = 22 (48.89%) |
| Strongly agree | 11 (8.80) | 3 (5.77) | 6 (11.76) | 2 (9.09) |
| Agree | 35 (28.00) | 17 (32.69) | 14 (27.45) | 4 (18.18) |
| Neither agree not disagree | 58 (46.40) | 25 (48.08) | 20 (39.22) | 13 (59.09) |
| Disagree | 18 (14.40) | 7 (13.46) | 10 (19.61) | 1 (4.55) |
| Strongly disagree | 3 (2.40) | 0 (0.00) | 1 (1.96) | 2 (9.09) |
| No response | 88 | 22 | 31 | 23 |
Device factors
Nineteen comments stated that patients are expressing a preference for lightweight devices; nine stated that respondents are expressing a preference for discreet devices; seven stated that patients are expressing a preference for particular devices (e.g. SCKAFOs) and one stated that patients are expressing a preference for FES devices. Patients are also expressing a preference for comfortable devices (four comments), custom-made devices (three comments), cosmetically appealing devices (three comments), functional devices (two comments), durable devices (one comment) and devices that are easy to take on and off (two comments).
Patient factors
Six comments noted that patients may express a preference for devices that they have heard about: ‘Brand they have seen on internet or advised by another MDT member’ or which they have previous experience of using. Two comments also noted that the age of the patient affects their preferences. For example, ‘older patients – callipers/young ones – that fit in nice shoes, and don’t hurt’.
Respondents were also asked the extent to which they thought six individual outcomes were important to patients who have been fitted with an orthotic device (Q51). The question response rate was 58.69% (n = 125): orthotists 70.27%, physiotherapists 60.98% and rehabilitation medicine physicians 48.89%. Eighty-eight respondents did not answer this question (22 orthotists, 31 physiotherapists and 23 doctors in rehabilitation medicine). Table 55 presents the results for all respondents.
| Outcome | Response, n (%) | ||||
|---|---|---|---|---|---|
| Not at all important | Somewhat important | Important | Very important | Extremely important | |
| Comfort | 0 (0.00) | 1 (0.80) | 11 (8.80) | 36 (28.80) | 76 (60.80) |
| Confidence in mobility | 0 (0.00) | 0 (0.00) | 5 (4.00) | 47 (37.60) | 72 (57.60) |
| Increased stability | 0 (0.00) | 2 (1.60) | 19 (15.20) | 59 (47.20) | 45 (36.00) |
| Less energy expenditure | 1 (0.80) | 24 (19.20) | 35 (28.00) | 39 (31.20) | 24 (19.20) |
| Cosmetic aspect of device | 1 (0.80) | 23 (18.40) | 45 (36.00) | 40 (32.00) | 17 (13.60) |
| Other | 30 (24.00) | 4 (3.20) | 6 (4.80) | 3 (2.40) | 5 (4.00) |
Overall, the vast majority of respondents stated that these outcomes are at least somewhat important. Over 80% of respondents chose ‘extremely important’ or ‘very important’ for comfort, confidence in mobility and increased stability. Less energy expenditure was rated as very or extremely important by 50.4% of respondents and the cosmetic aspects of device by 45.6% of respondents. Tables 56–58 present the results for each profession. These followed a similar trend to the overall results.
| Outcome | Responses, n (%) | ||||
|---|---|---|---|---|---|
| Not at all important | Somewhat important | Important | Very important | Extremely important | |
| Comfort | 0 (0.00) | 1 (1.92) | 4 (7.69) | 10 (19.23) | 37 (71.15) |
| Confidence in mobility | 0 (0.00) | 0 (0.00) | 2 (3.85) | 18 (34.62) | 32 (61.54) |
| Increased stability | 0 (0.00) | 0 (0.00) | 6 (11.54) | 18 (34.62) | 28 (53.85) |
| Less energy expenditure | 0 (0.00) | 8 (15.38) | 13 (25.00) | 19 (36.54) | 11 (21.15) |
| Cosmetic aspect of device | 0 (0.00) | 11 (21.15) | 19 (36.54) | 16 (30.77) | 7 (13.46) |
| Other | 14 (26.92) | 1 (1.92) | 3 (5.77) | 1 (1.92) | 2 (3.85) |
| Outcome | Responses, n (%) | ||||
|---|---|---|---|---|---|
| Not at all important | Somewhat important | Important | Very important | Extremely important | |
| Comfort | 0 (0.00) | 0 (0.00) | 5 (9.80) | 16 (31.37) | 29 (56.86) |
| Confidence in mobility | 0 (0.00) | 0 (0.00) | 2 (3.92) | 18 (35.29) | 30 (58.82) |
| Increased stability | 0 (0.00) | 2 (3.92) | 10 (19.61) | 28 (54.90) | 11 (21.57) |
| Less energy expenditure | 1 (1.96) | 11 (21.57) | 13 (25.49) | 16 (31.37) | 9 (17.65) |
| Cosmetic aspect of device | 1 (1.96) | 6 11.76) | 17 (33.33) | 19 (37.25) | 8 (15.69) |
| Other | 10 (19.61) | 0 (0.00) | 1 (1.96) | 2 (3.92) | 3 (5.88) |
| Outcome | Responses, n (%) | ||||
|---|---|---|---|---|---|
| Not at all important | Somewhat important | Important | Very important | Extremely important | |
| Comfort | 0 (0.00) | 0 (0.00) | 2 (9.09) | 10 (45.45) | 10 (45.45) |
| Confidence in mobility | 0 (0.00) | 0 (0.00) | 1 (4.55) | 11 (50.00) | 10 (45.45) |
| Increased stability | 0 (0.00) | 0 (0.00) | 3 (13.64) | 13 (59.09) | 6 (27.27) |
| Less energy expenditure | 0 (0.00) | 5 (22.73) | 9 (40.91) | 4 (18.18) | 4 (18.18) |
| Cosmetic aspect of device | 0 (0.00) | 6 (27.27) | 9 (40.91) | 5 (22.73) | 2 (9.09) |
| Other | 6 (27.27) | 3 (13.64) | 2 (9.09) | 0 (0.00) | 0 (0.00) |
Overall, 18 respondents (14.4%) stated that ‘other’ factors were at least somewhat important. Two orthotists and three physiotherapists also stated that the ease of taking the device on and off was important to patients. The ability to participate in activities of daily living (one orthotist) and improved function (another orthotist) were also important factors. A doctor in rehabilitation medicine stated that cost was also an important treatment outcome for patients. A physiotherapist stated that the weight of the device, lead-in time to fitting, goal achievement and compliance were all important treatment outcomes to patients.
Effectiveness of device
Respondents were asked about the extent to which the following factors affect the effectiveness of the device (Q52). The question response rate (based on the number who completed all the questions) was 38.50% (n = 82) (67.57% of the orthotists, 28.05% of the physiotherapists and 20% of the doctors in rehabilitation medicine). The overall responses to this question are presented in Table 59. A total of 131 respondents did not answer this question (who included 24 orthotists, 59 physiotherapists and 36 doctors in rehabilitation medicine).
| Factor | Responses, n (%) | ||||
|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Most of the time | Always | |
| Acceptability of the device to patient | 0 (0.00) | 0 (0.00) | 7 (8.54) | 26 (31.71) | 49 (59.76) |
| Patient adherence | 0 (0.00) | 0 (0.00) | 4 (4.88) | 29 (35.37) | 49 (59.76) |
| Fit of the device | 0 (0.00) | 1 (1.22) | 1 (1.22) | 27 (32.93) | 53 (64.63) |
| Therapy back-up | 0 (0.00) | 3 (3.66) | 33 (40.24) | 36 (43.90) | 10 (12.20) |
| Medical back-up | 6 (7.32) | 26 (31.71) | 38 (46.34) | 12 (14.63) | 1 (1.22) |
| Surgical back-up | 8 (9.76) | 35 (42.68) | 29 (35.37) | 9 (10.98) | 1 (1.22) |
| Pain due to the device | 0 (0.00) | 0 (0.00) | 14 (17.07) | 11 (13.41) | 58 (70.73) |
| Pressure areas due to the device | 0 (0.00) | 0 (0.00) | 16 (19.51) | 15 (18.29) | 51 (62.20) |
The majority of respondents chose ‘always’ for pain due to the device (70.73%), pressure areas due to the device (62.2%), acceptability of the device to patient (59.76%), patient adherence (59.76%) and for fit of the device (64.63%). The most popular response was ‘most of the time’ for therapy back-up (43.90%), for medical back-up (46.34%) and ‘rarely’ for surgical back-up (42.68%). Tables 60–62 present the results by profession; the results were broadly similar across professions.
| Factor | Responses: n, (%) | ||||
|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Most of the time | Always | |
| Acceptability of the device to patient | 0 (0.00) | 0 (0.00) | 3 (6.00) | 15 (30.00) | 32 (64.00) |
| Patient adherence | 0 (0.00) | 0 (0.00) | 3 (6.00) | 19 (38.00) | 28 (56.00) |
| Fit of the device | 0 (0.00) | 1 (2.00) | 1 (2.00) | 17 (34.00) | 31 (62.00) |
| Therapy back-up | 0 (0.00) | 2 (4.00) | 22 (44.00) | 21 (42.00) | 5 (10.00) |
| Medical back-up | 1 (2.00) | 13 (26.00) | 28 (56.00) | 8 (16.00) | 1 (2.00) |
| Surgical back-up | 2 (4.00) | 18 (36.00) | 23 (46.00) | 6 (12.00) | 1 (2.00) |
| Pain due to the device | 0 (0.00) | 0 (0.00) | 10 (20.00) | 6 (12.00) | 35 (70.00) |
| Pressure areas due to the device | 0 (0.00) | 0 (0.00) | 12 (24.00) | 10 (20.00) | 28 (56.00) |
| Factor | Responses: n, (%) | ||||
|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Most of the time | Always | |
| Acceptability of the device to patient | 0 (0.00) | 0 (0.00) | 3 (13.04) | 9 (39.13) | 11 (47.83) |
| Patient adherence | 0 (0.00) | 0 (0.00) | 1 (4.35) | 6 (26.09) | 16 (69.57) |
| Fit of the device | 0 (0.00) | 0 (0.00) | 0 (0.00) | 7 (30.43) | 16 (69.57) |
| Therapy back-up | 0 (0.00) | 1 (4.35) | 9 (39.13) | 9 (39.13) | 4 (17.39) |
| Medical back-up | 5 (21.74) | 10 (43.48) | 7 (30.43) | 1 (4.35) | 0 (0.00) |
| Surgical back-up | 6 (26.09) | 12 (52.17) | 4 (17.39) | 1 (4.35) | 0 (0.00) |
| Pain due to the device | 0 (0.00) | 0 (0.00) | 4 (17.39) | 2 (8.70) | 17 (73.91) |
| Pressure areas due to the device | 0 (0.00) | 0 (0.00) | 4 (17.39) | 2 (8.70) | 17 (73.91) |
| Factor | Responses: n, (%) | ||||
|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Most of the time | Always | |
| Acceptability of the device to patient | 0 (0.00) | 0 (0.00) | 1 (11.11) | 2 (22.22) | 6 (66.67) |
| Patient adherence | 0 (0.00) | 0 (0.00) | 0 (0.00) | 4 (44.44) | 5 (55.56) |
| Fit of the device | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (33.33) | 6 (66.67) |
| Therapy back-up | 0 (0.00) | 0 (0.00) | 2 (22.22) | 6 (66.67) | 1 (11.11) |
| Medical back-up | 0 (0.00) | 3 (33.33) | 3 (33.33) | 3 (33.33) | 0 (0.00) |
| Surgical back-up | 0 (0.00) | 5 (55.56) | 2 (22.22) | 2 (22.22) | 0 (0.00) |
| Pain due to the device | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (33.33) | 6 (66.67) |
| Pressure areas due to the device | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (33.33) | 6 (66.67) |
Respondents were asked if they thought that there were other factors that affected the effectiveness of the device (Q53). The question response rate was 58.22% (n = 124): 70.27% of the orthotists, 60.98% of the physiotherapists and 48.89% of the doctors in rehabilitation medicine responded to the question. Eighty-six respondents (69.35%) stated that there were no other factors that they thought affected the effectiveness of the device, while 38 respondents (30.65%) stated that there were other factors. Eight-nine respondents did not answer this question. The 38 respondents who thought there were other factors were asked to specify these, and the responses to this question are presented below, categorised into patient factors, device factors and other factors.
Patient factors
Eight comments referred to the patients’ expectations. For example, one respondent stated that ‘they are sometimes referred having been told about a “magical thing” that will solve their ills and so start the process with us already disappointed’. Eight comments also referred to psychological factors affecting the effectiveness of the device. For example, respondents referred to the patients’ motivation, their ability to encompass change, their cognitive ability and how well they feel that they can use the devices. Seven comments noted patient compliance affecting the effectiveness of the device. Six comments described how support from family and friends can have an effect. Five comments described how the patient’s clinical condition can have an effect, in terms of how well the device can work with the patients’ abilities and the progressive nature of some conditions. Finally, four comments noted that the level of involvement of the patient in the decision of what device to prescribe can affect the effectiveness of the device. One respondent stated:
Ensuring the patient is involved in the assessment and understands that there isn’t a magic answer, and the orthosis won’t solve their problem; giving them as much choice as possible; so that they take ownership of how the problem is managed.
Device factors
The device factors that were noted in the comments, which can affect the effectiveness of the device, included ease of use (n = 8), effectiveness (n = 4), cost (n = 3) and comfort (n = 1).
Other factors
Two respondents stated that there needs to be an appropriate time given for assessment, explanation and review. One respondent stated:
I think that available time for orthotic assessment – to achieve an appropriate prescription, and for review – to conduct outcome measures and necessary device fine tuning, will have an impact on orthotic treatment success.
Outcome measures
Respondents were asked what, if any, formal outcome measures they use to assess the effectiveness of orthotic devices (Q55).
There was no single outcome measure that was commonly in use across the sample (Table 63). Thirty-six respondents (29.03%) stated that they do not use a formal outcome measure. The most commonly used measures were a visual analogue scale (50.81%), the 10-m Walk Test (41.13%), the Timed Up and Go Test (35.48%), patient satisfaction questionnaire (33.87%) and the Goal Attainment Scale (27.42%). Twenty (16.13%) respondents stated that they use a formal outcome measure that was not listed in the response options.
| Outcome measure | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physicians |
|---|---|---|---|---|
| Response rate | n = 124 (58.22%) | n = 52 (70.27%) | n = 50 (60.98%) | n = 22 (48.89%) |
| Timed Up and Go Test | 44 (35.48) | 9 (17.31) | 24 (48.00) | 11 (50.00) |
| 10-Walk Test | 51 (41.13) | 6 (11.54) | 34 (68.00) | 11 (50.00) |
| 2-Minute Walk Test | 14 (11.29) | 8 (15.38) | 4 (8.00) | 2 (9.09) |
| 6-Minute Timed Walk Test | 19 (15.32) | 3 (5.77) | 12 (24.00) | 4 (18.18) |
| Visual analogue scale | 63 (50.81) | 21 (40.38) | 32 (64.00) | 10 (45.45) |
| Goal Attainment Scale | 34 (27.42) | 11 (21.15) | 17 (34.00) | 6 (27.27) |
| Patient satisfaction questionnaire | 42 (33.87) | 16 (30.77) | 17 (34.00) | 9 (40.91) |
| Activities Balance Confidence Scale | 6 (4.84) | 0 (0.00) | 4 (8.00) | 2 (9.09) |
| OPUS | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Manchester Oxford Knee Score | 5 (4.03) | 3 (5.77) | 2 (4.00) | 0 (0.00) |
| Do not use a formal outcome measure | 36 (29.03) | 23 (44.23) | 5 (10.00) | 8 (36.36) |
| Other | 20 (16.13) | 3 (5.77) | 15 (30.00) | 2 (9.09) |
| No response | 89 | 22 | 32 | 23 |
Three orthotists chose ‘other’ and stated that patient feedback at review appointment and ’measures against named goals’ were also used. Fifteen physiotherapists chose ‘other’. They reported using the Berg balance scale (n = 5); the ‘timed and videoed functional movement test’ (n = 1); the ‘timed 360 degree test’ (n = 1); the ’Tinetti Test’ (n = 1); the ’Leeds Movement Performance Index’ (n = 1); the ‘Walk 12 Scale’ (n = 1); the ’6-Minute Walk Test’ (n = 1); the ‘Modified Rivermead Mobility Index’ (n = 1); ‘Functional SMART goals’ (n = 1); and ‘TUSS’ (presumably in reference to the Timed Unsupported Steady Stand) (n = 1). One physiotherapist also stated ‘usually being seen by other therapists if having treatment so I will goal set and they will use formal outcome measures’. Two doctors in rehabilitation medicine chose ‘other’. One stated that patient satisfaction measurement by the hospital can also be used.
Acceptability of device to patients
Respondents were asked what factors they think affect the acceptability of devices to patients (Q56). Again, these responses can be divided into patient factors, device factors and ‘other’ factors.
Patient factors
It appears that patients need to perceive a benefit from using the device, and 32 respondents felt that this can affect the acceptability of devices to patients. Previous experience of the device (one comment), seeing others benefit from orthotic devices (two comments) and psychological factors (three comments) can also affect the acceptability of devices to patients. Nine comments noted that pain relief potential of the devices can affect acceptability. One respondent noted that support from family and friends can also affect acceptability.
Device factors
Sixty-one respondents commented that the comfort, or lack of, affected the acceptability of devices to patients. Appearance of device (52 comments), ease of use (45 comments) and effectiveness of device (40 comments) all appear to be common factors affecting the acceptability of devices to patients. The devices weight (28 comments), size/bulk (12 comments) and fit (seven comments) were also noted by respondents as potential factors. Finally, 11 respondents commented that patients’ ability to wear the device under clothing and/or with a range of footwear were also factors that may affect the acceptability of devices to patients.
Other factors
One respondent felt that MDT provision can affect the acceptability of devices to patients.
Respondents were then asked about the cosmetic aspect of the device, and to what extent the cosmetic look of the device affects acceptability to the patient and whether or not he/she wears the device (Q57). The results of this question are presented in Table 64. Additional comments made by respondents are summarised in the text.
| Cosmetic appearance | All respondents | Orthotists | Physiotherapists | Rehabilitation medicine physician |
|---|---|---|---|---|
| Response rate | n = 123 (57.75%) | n = 51 (68.92%) | n = 50 (60.98%) | n = 22 (48.89%) |
| Never | 1 (0.81) | 1 (1.96) | 0 (0.00) | 0 (0.00) |
| Rarely | 8 (6.50) | 1 (1.96) | 5 (10.00) | 2 (9.09) |
| Sometimes | 42 (34.15) | 20 (39.22) | 13 (26.00) | 9 (40.91) |
| Often | 63 (51.22) | 24 (47.06) | 29 (58.00) | 10 (45.45) |
| All of the time | 9 (7.32) | 5 (9.80) | 3 (6.00) | 1 (4.55) |
| No response | 90 | 23 | 32 | 23 |
The majority of respondents stated that the cosmetic look of the device often affects the acceptability of the device to the patient (51.22%). Very few respondents (n = 9) stated that the cosmetic look never or rarely affected the acceptability of the device to the patient and whether or not the patient wears the device.
Respondents were asked to provide details for their answer. Fifty-five respondents expanded on why they responded ‘often’. They stated that patients want discretion, so the device fitting under their clothes and the impact of the device on their choice of footwear available to them is important. Twenty-two respondents stated it depends on the attitude of the patient. For example, one respondent wrote: ‘some patients are more self-conscious than others and struggle to compromise with the device once away from the clinic.’
In terms of the patients most likely to be affected by the cosmetic look of the device, 19 respondents stated that the cosmetic look can sometimes be more important to their female patients. For example, ‘Female patients are more bothered about this as they often wear a skirt. Males can cover it up with trousers’. Seven respondents stated that the cosmetic look is more important for young people.
In contrast, five respondents commented that the cosmetic look of the device did not, or rarely, affect the acceptability of the device. Seventeen respondents felt that function is more important to patients. One respondent wrote: ‘Many patients however see function as more important and would not want to compromise this over cosmesis as sometimes the two just can’t go hand in hand.’ Three respondents stated that the cosmetic look is less important if the patient’s condition is severe and/or painful. Six respondents noted that the degree to which the cosmesis affects the acceptability of the device to patients often depends on the device in question.
Care pathway of patients
Respondents were asked their opinion on the care pathways for these patients. Separate questions were asked for patient with NMDs (Q59, Q60) and patients with CNS conditions (Q61, Q62).
Patients with a neuromuscular disease
Respondents treating patients with NMDs were asked if they felt that there are any aspects of the care pathway for patient with NMDs with knee instability that could be improved. The question response rate was 54.44% (n = 92). A total of 77 respondents did not answer the question. Overall, 78.26% (n = 72) of respondents felt that improvement could be made to the care pathway, compared with 21.74% (n = 20) of respondents who felt that improvement could not be made. The majority of orthotists (41 respondents compared with six respondents), physiotherapists (19 respondents compared with 10 respondents) and doctors in rehabilitation medicine (12 respondents compared with four respondents) felt that improvements could be made. Respondents were asked to provide details for their answer to this question.
Twenty-six respondents felt that improved communication and teamworking across professions would improve the care pathway. Twenty-two respondents felt that reducing the waiting time for appointments with the orthotics service would also improve the pathway. Ten respondents felt that improving awareness of orthotic services in potential referrers would be beneficial. Six respondents believed that making earlier referrals to orthotics services would improve the overall care pathway. Six respondents felt that the removal of budget barriers would improve the care pathway. One respondent suggested that community-based clinics would improve the care pathway. Three respondents felt that the pathway would be improved if differences in the quality of orthotic services were eliminated. Three respondents believed that the pathway would be improved if there was a reduction in the waiting time from orthotic assessment to fitting and delivery of devices. Two respondents felt that improvements could be made through the increase of the time available for orthotic treatment. Four respondents highlighted that improvements in the assessment of patients could lead to improvements in the pathway. For example, one respondent wrote ‘improving the pre/post device with gait assessment would give some form of validated measure of outcome’. Finally, five respondents felt that the care pathway could be improved if improvements were made to patient care in clinic and post device.
In the further information provided, eight respondents reiterated that they did not feel that there any aspects of the care pathway could be improved for patients with NMDs and knee instability. Seven respondents stated that they did not know and one respondent felt that there is no pathway in place for patients with NMDs and knee instability.
Patients with central nervous system conditions
Respondents treating patients with CNS conditions were asked if they felt that any aspects of the care pathway could be improved for patients with CNS conditions and knee instability. The question response rate was 56.59% (n = 116). A total of 89 respondents did not answer the question. Overall, 77.59% (n = 90) of respondents felt that improvement could be made to the care pathway, compared with 22.41% (n = 26) of respondents who felt that improvement could not be made. The majority of orthotists (41 respondents compared with seven respondents), physiotherapists (34 respondents compared with 13 respondents) and doctors in rehabilitation medicine (15 respondents compared with six respondents) felt that improvements could be made. Once again, respondents were asked to provide details for their answer to this question. The responses to this question were very similar to those given for patients with NMDs.
Thirty-five respondents felt that improved communication and team working across professions would improve the care pathway. Twenty-seven respondents felt that reducing the waiting time for appointments with the orthotic service would also improve the pathway. Seven respondents felt that improving awareness of orthotic services in potential referrers would be beneficial. Nine respondents believed that making earlier referrals to orthotics services would improve the overall care pathway. Four respondents felt that the removal of budget barriers would improve the care pathway. Eight respondents felt that the care pathway could be improved if improvements were made to patient care in clinic and post device. Five respondents believed that the pathway would be improved if there was a reduction in the waiting time from orthotic assessment to fitting and delivery of devices. Five respondents highlighted that improvements in the assessment of patients could lead to improvements in the pathway. Three respondents felt that the pathway would be improved if there was an increase in the number of orthotists available. Two respondents felt the pathway would be improved if differences in the quality of orthotic services were eliminated. Finally, one respondent felt that improvements could be made through the increase of the time available for orthotic treatment.
An additional improvement noted by eight respondents, which seems to be specific to patients with CNS conditions in particular, is that an increase in the availability of orthoses would improve the pathway. One respondent noted that ‘stroke patients benefit from strong carbon [fibre] light weight’. Two respondents also stated that changing the referral pathway would improve the overall care pathway. For example, one respondent stated that ‘all of the health professions should be able to refer directly to orthotists without having to send patient to orthopaedic clinic to see a medic first’.
In the further information provided, 11 respondents reiterated that they did not feel that there are any aspects of the care pathway that could be improved for patients with CNS conditions and knee instability. Five respondents stated that they did not know, and three respondents felt that there is no pathway in place for patients with CNS conditions and knee instability.
Chapter 6 Costing analysis
The telephone interviews provided a fairly comprehensive list of the variables that would need to be taken into account to cost custom-made KAFOs. At the beginning of each telephone interview, the orthotists were told to consider only the costs that were associated with providing KAFOs to adult patients with NMD or CNS disorders, and to consider only the costs that would be incurred by the NHS. The results from the telephone interviews are presented below.
Materials
Interviewees were asked about what materials would be used for each component of the KAFO, and they were presented with tables within the topic guide to facilitate the discussion around the materials that would be required to produce a completed KAFO. Tables were provided separately for conventional KAFOs, cosmetic KAFOs and hybrid KAFOs.
Conventional knee–ankle–foot orthoses
All components of a conventional KAFO could potentially be made from metal. Typically, the thigh, calf and foot sections would be the sections that were most likely to be made of leather, although the knee joint and ankle joints could have leather components, which would be used to influence the position of the joint. Foam lining may be used to increase patient comfort.
Cosmetic knee–ankle–foot orthoses
All components of a cosmetic KAFO could potentially be made from thermoplastic. Carbon fibre would typically be used for the thigh, calf and foot sections, that is, not used for the joints. Thermoplastic and carbon fibre would not be used together in the one KAFO. The knee and ankle joints would typically be made from metal. Other materials that could potentially be used within a cosmetic KAFO were leather and, in rare circumstances, a laminate material for the ankle or thigh sections. Lining or padding may also be used to increase patient comfort.
Hybrid knee–ankle–foot orthosis
All of the components of a hybrid KAFO could potentially be made from metal. It appears that titanium is more frequently used than steel or aluminium. The decision regarding which metal to use would depend on the patient’s weight and activity levels. Leather, thermoplastic or carbon fibre could be used for the thigh, calf and foot sections. As with the cosmetic KAFO, either thermoplastic or carbon fibre would be used in the KAFO, but they would not be used together. Additional materials that would be used include fabric, which may be used to cover sections of the device if needed, and foam lining, which may be used to increase patient comfort.
Preliminary discussions indicated that the cosmetic and hybrid KAFOs were more commonly prescribed than the conventional KAFO. One orthotist stated that the vast majority of the KAFOs prescribed in their setting would be carbon fibre KAFOs with titanium joints. Another orthotist stated that they would prescribe a conventional KAFO only if it was a repeat prescription and they would rarely prescribe a conventional KAFO to a new patient.
Discussions also indicated that the main cost driver for KAFOs is the joint elements. Depending on the joint element required, this can significantly increase the cost of manufacturing a KAFO. These joints all appear to require being ordered from a private manufacturer.
One orthotist stated that in his/her clinical setting, the aim was to provide two KAFOs to the patient. This is an important additional cost to take into consideration.
Other materials required
Another associated cost of providing KAFOs is footwear and footwear adaptations. Discussions with one orthotist indicated that the patient population of interest often need adapted footwear or tailor-made footwear in order for the patient to be able to wear the KAFO. It is unclear if this associated cost is necessary for all KAFO prescriptions as another orthotist stated that this may be more common for conventional KAFOs, although the qualitative interviews with orthoses users support the importance of taking footwear into consideration in estimating costs.
Tailor-made shoes or adapted shoes can be an expensive addition that would need to be taken into account in a future cost-effectiveness analysis. The orthotist stated that the policy in their clinical setting was to issue two pairs or tailor-made shoes or up to three adapted shoes. If this policy is widespread then this is an important cost consideration to take into account.
Where the materials are sourced and the knee–ankle–foot orthoses constructed
The consensus from the orthotists interviewed is that the joint sections of the KAFOs are ordered from manufacturing companies. Some orthotists order all of the components individually. One orthotist orders each component apart from leather and plastic, as these are bulk bought for their on-site workshop. The KAFOs can be constructed in an on-site workshop or off-site from the clinical setting.
Deciding factors of which material to use and the quantity of material to use
All of the orthotists stated that patient’s height, weight and activity levels determine the quantity of raw materials that are needed for KAFOs. The patient’s ability, and muscle strength in particular, seems to be the deciding factor on what knee and ankle joint to prescribe. One orthotist stated that their manufacturer provides a matrix to which the patient’s weight and activity levels can be imputed to help with the decision-making process. The patient’s ability to apply the device independently may also affect the prescription.
When deciding what materials to use within the KAFO, the patient’s health condition is taken into consideration. For example, generally speaking, KAFOs being prescribed for patients with diabetes would not include plastic in order to avoid skin contact. Patients with reduced sensation need to be prescribed a KAFO that does not touch the skin as they may not feel the device rubbing on their skin. The weight of the device also appears to be an important consideration that is taken into account by orthotists.
Materials required for shape capture
For the majority of cases, plaster seems to be used to capture the shape of the patient when fitting their KAFO. Tracing the leg and the use of transparent adhesive tape are also options that appear to be used frequently. Computer scanners appear to be used only occasionally and are not always an option available to orthotists. In general, orthotists use one technique to shape capture per patient, that is to say they would not use different techniques for different sections of the patient, although one orthotist stated that the cast of the foot may be taken with plaster and then a trace is taken of the upper leg. Foam shape capture boxes can be used to capture the shape of the underneath of the foot. One orthotist stated that an alignment tool can also be used, which assesses how a patient spreads their weight across both legs, in the prescription of KAFOs.
Staff requirements to prescribe/fit a knee–ankle–foot orthosis
The interviews indicated that, although a multidisciplinary approach would be ideal, in the majority of cases only the orthotist and an orthotic technician are required to prescribe and fit a KAFO. Orthotists require between three and five appointments to prescribe, fit and review a KAFO. The first appointment would be 40–60 minutes long, with the following visits lasting 20–40 minutes. The time required for an orthotic technician to assemble a KAFO varies, depending on the complexity of the device. It was estimated that conventional or more straightforward devices would require 6–8 hours of a technician’s time; more complex devices can require up to 12 hours of a technician’s time.
The following HCPs may also be required:
-
A physiotherapist may be included in the fitting if the patient is a first-time wearer of a KAFO or if it is a complicated prescription. The physiotherapist’s role would be to provide gait training in the new device.
-
An occupational therapist may be required.
-
A gait scientist may walk the patient through a gait laboratory, both wearing and not wearing the KAFO, to get an objective benefit measure of the device and to assess the appropriateness of the device. This would be a 2-hour appointment and for one particular orthotist this would be standard practice for new patients.
-
Rehabilitation medicine physicians may also be involved, given the patient population, but they may not be directly involved in the prescription of the device.
One orthotist stated that they would not be able to refer patients into other services. If the orthotist felt that a patient required, for example, an appointment with a physiotherapist, then the orthotist would need to advise the patient to seek referral through their GP. The neurologist and/or the rehabilitation medicine physicians are ‘often the conductors of the allied health professionals (AHPs)’, who all work separately.
Overheads
During the telephone interviews, it became clear that the overheads, that is, the costs associated with providing the service (not including staff and device costs), vary significantly across clinical settings. The following types of service provision appear to be in place:
-
Some settings have a fully managed service or a block contract. In this scenario, the orthotic services are provided by private companies, subcontracted to the NHS. In this scenario, the company is paid a single fee to cover the entire service, including staff and device costs.
-
Some trusts pay sessional fees: the orthotists are paid sessional fees, such as a daily fee, and then the trust also pays for an item that is prescribed on top of this sessional fee.
-
CCG funding usually is used to cover GP referrals to orthotic services. The CCG would pay a fixed amount per appointment and then pay for items prescribed on top of this. In comparison, a referral from a consultant can come out of the orthotics budget.
-
Finally, some orthotics services are paid per appointment, with the hardware cost included in this fee.
Spending caps
The orthotists were asked whether or not there were any spending restrictions in place in their own clinical settings. It appears that there are no formal restrictions in place apart from high-end items for which approval would need to be requested, but it was not unusual for these requests to be granted if the case for prescribing the device was substantiated. One orthotist stated that a KAFO costing > £2000 would need to be justified, and another stated that approval is needed for some of the more expensive joints such as electronic joints. Approval to prescribe electronic joints often has to go to managerial meetings or be discussed at clinical meetings, at which a consensus would be required that this is necessary for a patient. One specific example of a spending restriction provided was for specialist SCKAFOs. To prescribe these devices would require an application for specialist funding to a board within the Trust.
An orthotic manager stated that although there is no explicit spending cap in place, orthotists need to be mindful of budgets and waiting lists. In their setting, which is a fully managed service, the NHS has its own managers in place whose primary concerns are often budgets and waiting lists and the orthotists are expected to see patients in a timely manner.
It appears that apart from high-cost items, there are very few restrictions in place on what types of KAFOs can be funded, and it is the orthotist’s clinical decision of what orthotic device to prescribe to a patient.
Opportunity cost
The standard care, in terms of either a KAFO or a treatment provided to our patient group, was not clear. Therefore, the orthotists were asked to discuss the cost and effects of not being able to prescribe a KAFO to patients with a NMD or CNS condition with knee instability.
Almost unanimously, the orthotists concluded that if the patient’s condition was severe enough to warrant a KAFO, in these conditions, then their only alternative treatment pathway would be to be given a wheelchair. It seems that an AFO or a knee brace may be of some use for mild knee instability, but if the knee instability is moderate to severe then there is no orthotic alternative to a KAFO. One orthotist stated that, for the conditions in which we are interested for this study, the knee instability would most likely be moderate to severe.
The effects on a patient’s health due to being confined to a wheelchair were discussed with the orthotists, and all agreed that these can have a detrimental effect on a patient’s health compared with using a KAFO regularly.
Unit cost estimates
The orthotists stated during the telephone interviews that conventional KAFOs cost in the region of £1400–1500, and more complex devices can start at £2500; however, these were estimates and may not be generalisable for all clinical settings and for all KAFOs prescribed. Previous literature has used the NHS PASA prices to cost orthotic devices in the UK. Following some investigation, these costs were elicited from NHS Supply Chain Product and Transaction Database; however, these were available for prefabricated (off-the-shelf devices) and for FOs and AFOs only. The average costs for these devices are presented in Table 65.
| Device | Unit cost (£) | ||
|---|---|---|---|
| Minimum | Mean | Maximum | |
| FO | 5.35 | 14.65 | 27.79 |
| AFO | 9.24 | 28.89 | 220.54 |
An expert opinion provided the unit costs for a KAFO and its individual components. These are presented in Table 66. It was estimated the ‘standard’ locked-knee conventional finished product and the ‘standard’ locked-knee cosmetic finished product would require at least 20 hours of labour to be manufactured.
| Component | Cost (£) | Total (£) |
|---|---|---|
| ‘Standard’ locked-knee conventional finished product | ||
| Joints and uprights (steel) | 143.50 | |
| Straps | 6 | |
| Buckles | 4 | |
| Leather | 27.84 | |
| Aluminium square | 8.50 | 189.94 |
| ‘Standard’ locked-knee cosmetic finished product | ||
| Joints (steel) | 120 | |
| Uprights (steel) | 20 | |
| Plastic | 20 | |
| Carbon reinforcements | 25 | |
| Straps | 6 | 191 |
| ‘Standard’ carbon fibre locked-knee KAFO | 2500 | |
| SCKAFO | 2187 | |
| Off-the-shelf KAFO | 900 | |
Staff costs were estimated from NHS reference costs. 70 The unit costs for staff used are presented in Table 67.
| Type of visit | Cost per hour (£) |
|---|---|
| Hospital physiotherapist | 33 |
| First visit with orthotist | 106.56 |
| Subsequent visits with orthotist | 97.27 |
| Occupational therapist | 33 |
| Doctor in rehabilitation medicine | 259 |
Cost estimates for knee–ankle–foot orthoses
Using the information collected in Tables 65–67, combined with the relevant results from the survey of HCPs undertaken, the cost of four different types of KAFOs are estimated. The information taken from the survey to inform this costing analysis is presented in Table 68.
| Information | HCP | Minimum (minutes) | Mean (minutes) | Maximum (minutes) |
|---|---|---|---|---|
| Length of initial assessment appointment | Orthotist | 14 | 28 | 60 |
| Physiotherapist | 0 | 48 | 60 | |
| Doctor in rehabilitation medicine | 1 | 39 | 60 | |
| Length of casting/measuring appointment | ||||
| Custom-made | Orthotist | 15 | 37 | 60 |
| Physiotherapist | 5 | 43 | 60 | |
| Doctor in rehabilitation medicine | 8 | 39 | 60 | |
| Off the shelf | Orthotist | 10 | 24 | 60 |
| Physiotherapist | 0 | 31 | 60 | |
| Doctor in rehabilitation medicine | 3 | 22 | 41 | |
| Length of review appointment | Orthotist | 14 | 22 | 45 |
| Physiotherapist | 1 | 34 | 45 | |
| Doctor in rehabilitation medicine | 9 | 22 | 45 | |
The methodology used below follows that of Simoens et al. 71 The aim of this paper71 was to develop a model that calculates the cost of production and distribution of orthotic braces. The paper began by estimating the cost of production of knee and neck braces and overheads. However, this was beyond the scope of the present exercise and so the cost of production and overheads were assumed to have been included in the unit costs collected.
The device costs are estimated using three scenarios:
-
low-cost scenario, for which the lowest resource-use estimate and minimum values are used
-
average-cost scenario, for which the mean value for all estimates are used
-
high-cost scenario, for which the highest resource-use estimates and maximum values are used.
These scenarios will enable a cost range to be presented.
Cost of custom-made devices
The staff requirements were estimated using the guidelines provided by the orthotists in the telephone interviews and from the survey results. The staff requirements were estimated as follows:
-
Low-cost scenario Patient has appointments with orthotist only. This includes:
-
an initial assessment appointment lasting 14 minutes
-
a casting/measuring appointment lasting 10 minutes
-
a review appointment lasting 14 minutes.
-
-
Average-cost scenario Patient has appointments with orthotist, physiotherapist and rehabilitation medicine physician. This includes:
-
initial assessment appointments (28 minutes with an orthotist; 48 minutes with physiotherapists; and 39 minutes with a doctor in rehabilitation medicine)
-
casting/measuring appointments (24 minutes with an orthotist; 31 minutes with physiotherapists; and 22 minutes with a doctor in rehabilitation medicine)
-
review appointments (22 minutes with an orthotist; 34 minutes with physiotherapists; and 22 minutes with a doctor in rehabilitation medicine).
-
-
High-cost scenario:
-
initial assessment appointments (60 minutes with an orthotist; 60 minutes with physiotherapists; and 60 minutes with a doctor in rehabilitation medicine)
-
casting/measuring appointments (60 minutes with an orthotist; 60 minutes with physiotherapists; and 41 minutes with a doctor in rehabilitation medicine)
-
an additional 40 minute appointment with orthotist
-
review appointments (45 minutes with an orthotist; 45 minutes with physiotherapists; and 45 minutes with a doctor in rehabilitation medicine)
-
40-minute appointment with an occupational therapist
-
1-hour appointment with a gait scientist.
-
The unit costs for KAFOs provided by expert opinion were used to provide the cost estimate for a custom-made KAFO. As stated earlier, it was estimated that the ‘standard’ locked-knee conventional finished product and the ‘standard’ locked-knee cosmetic finished product would require at least 20 hours of labour to be manufactured. In the absence of the appropriate unit cost, orthotic technician time was assumed to be equivalent to the cost of orthotist’s time. This cost is presented in Table 69.
| Estimate | Cost scenario (£) | ||
|---|---|---|---|
| Low cost | Average | High cost | |
| ‘Standard’ locked-knee conventional finished product | |||
| Device | 189.94 | 189.94 | 189.94 |
| Staff | 2009 | 2589 | 2998 |
| Total | 2198.94 | 2778.94 | 3187.94 |
| ‘Standard’ locked-knee cosmetic finished product | |||
| Device | 191 | 191 | 191 |
| Staff | 2009 | 2589 | 2998 |
| Total | 2200 | 2780 | 3189 |
| ‘Standard’ carbon fibre locked-knee KAFO | |||
| Device | 2500 | 2500 | 2500 |
| Staff | 64 | 644 | 1053 |
| Total | 2564 | 3144 | 3553 |
| SCKAFO | |||
| Device | 2187 | 2187 | 2187 |
| Staff | 64 | 644 | 1053 |
| Total | 2251 | 2831 | 3240 |
Our analysis estimates that the cost of a custom-made KAFO could range from £2198 to £3553. However, these estimates are based on some assumptions and may not be reflective of the true cost of providing a KAFO to patients with a NMD or CNS condition with knee instability and, therefore, should be interpreted with caution.
Cost of prefabricated (off-the-shelf) devices
The staff requirements for off-the-shelf devices were estimated from the survey of HCPs and are presented below:
-
Low-cost scenario Patient has appointments with orthotist only. This includes:
-
an initial assessment appointment lasting 14 minutes
-
a casting/measuring appointment lasting 10 minutes
-
a review appointment lasting 14 minutes.
-
-
Average cost scenario Patient has appointments with orthotist, physiotherapist and doctor in rehabilitation medicine. This includes:
-
initial assessment appointments (28 minutes with an orthotist; 48 minutes with physiotherapists; 39 minutes with a doctor in rehabilitation medicine)
-
casting/measuring appointments (24 minutes with an orthotist; 31 minutes with physiotherapists; 22 minutes with a doctor in rehabilitation medicine)
-
review appointments (22 minutes with an orthotist; 34 minutes with physiotherapists; 22 minutes with a doctor in rehabilitation medicine).
-
-
High-cost scenario
-
initial assessment appointments (60 minutes with an orthotist; 60 minutes with physiotherapists; 60 minutes with a doctor in rehabilitation medicine)
-
casting/measuring appointments (60 minutes with an orthotist; 60 minutes with physiotherapists; 41 minutes with a doctor in rehabilitation medicine)
-
review appointments (45 minutes with an orthotist; 45 minutes with physiotherapists; 45 minutes with a doctor in rehabilitation medicine).
-
The unit costs for AFOs provided by the NHS Supply Chain were assumed to be equivalent to the cost of KAFOs and these along with the expert opinion estimate were used to provide the cost estimate for off-the-shelf KAFO. It was assumed that the off-the-shelf devices did not require any labour time for assembly. This cost is presented in Table 70.
| Estimate | Low-cost scenario (£) | Average cost (£) | High-cost scenario (£) |
|---|---|---|---|
| Using NHS Supply Chain unit costs | |||
| Device | 9.24 | 28.89 | 220.54 |
| Staff | 64 | 455 | 998 |
| Total | 73.24 | 483.89 | 1218.54 |
| Using expert opinion cost | |||
| Device | 900 | 900 | 900 |
| Staff | 64 | 455 | 998 |
| Total | 964 | 1355 | 1898 |
Our analysis estimates that the cost of an off-the-shelf KAFO could range from £73.24 to £1898. However, these estimates are based on some assumptions and may not be reflective of the true cost of providing a KAFO to patients with a NMD or CNS condition with knee instability and, therefore, should be interpreted with caution. In addition, the upper range may be an overestimate, as respondents of the survey were considering off-the-shelf and custom-made devices when giving their answers.
Chapter 7 Dissemination and engagement
Strategy development
The project was likely to be of relevance and interest in varying degrees to a wide range of parties. The identified audiences included those involved in the prescribing, supplying and training in the use of orthoses; those using orthoses, carers, and support organisations; and those making policy and commissioning decisions. For some groups (e.g. policy-makers and commissioners), their interests would be served though dissemination of the findings of the project.
However, those using orthotics, carers and support charities were likely to have varying levels of understanding of the research methods to be used in the project. The health professionals on the Advisory Group made us aware that orthotics, in general, is an underdeveloped area of research. This meant that when thinking about the eventual impact of the project results we were unsure of how well developed the channels for dissemination and implementation were to these groups. We decided therefore to undertake some engagement activities from the beginning of the project to help facilitate the planned dissemination of the results on completion of the project. The aim of engagement was to raise awareness within the relevant diverse audiences of the purpose, content and methods of the project, as a whole and within the different elements. Engagement is a two-way process, so we looked at mechanisms that would facilitate a dialogue in order to create a channel for the dissemination of the findings.
Tools for engagement
The key vehicle for engagement was a project-specific blog, which anyone could access and read, and sign up to receive e-mail or Twitter (Twitter, Inc., San Francisco, CA, USA, www.twitter.com) alerts to new content. The link to the blog is http://kneeorthotics.blogspot.co.uk/ (accessed 12 July 2015). The free Google ‘Blogger’ software (Google Inc., Mountain View, CA, USA) was used, and Google Analytics (Google Inc.) was set up to collect statistics on use and users. A project logo (Figure 4) and favicon (Figure 5) (a ‘favourite icon’ that appears next to a webpage title on the tab) were devised in house and the NIHR acknowledgement and disclaimer added to the site.
FIGURE 4.
Project logo.

FIGURE 5.
Project favicon.

The project-specific Twitter account was set up as Orthotics for Knees @OKIS_York, and automated tweets were sent to followers each time a new blog was posted using twitterfeed.com. Users also had the option of following the blog by receiving e-mails via Google FeedBurner (Google Inc.).
A ‘QR’ (Quick Response) code for the blog site was generated for use in articles and on posters (Figure 6). QR codes are a type of bar code that smartphones and tablets can read and decode. When users scanned the OKIS QR code the embedded link took them to a mobile phone-friendly version of the OKIS blog. QR codes are frequently used when space or display time is limited, such as on posters. A ‘digital’ link can be acquired by those who are interested, as an alternative to paper handouts.
FIGURE 6.
Orthotics for Knee Instability QR code.

Fixed pages on the blog site were created for a lay overview of the whole project and biographical details of the research team and Advisory Group members. The site was created and coordinated by the project’s dissemination lead and the blogs were written by various members of the research team. All blogs were edited by the dissemination lead. The posts provided information about the different elements of the project as they progressed and ultimately summaries of the findings. Some additional related topics were included such as a report on AHPs, the NIHR OK to Ask campaign, and the Testing Treatment site. These related topics aimed to raise awareness more generally about the need for high-quality research to support good health-care practice.
A moderated comments facility was made available with each blog post for users to submit their comments and suggestions. A project specific e-mail account was set up with Googlemail (Google Inc.) to facilitate the moderation process, to avoid the risk of a work e-mail account being overloaded.
Engaging with target audiences
The blog was also used to promote involvement in the project where appropriate, for example to advertise for a Patient and Public Involvement representative for the project; and encourage health professionals to complete the survey. Patients considering taking part in the one-to-one interviews or focus groups were referred to the site for further information.
Details of the blog were sent to relevant networks, organisations and charities (Table 71) for general awareness raising. We identified a wide range of diverse groups that were relevant to the broad scope of conditions covered by the project. However, they all had far broader interests than the specific focus of orthotic devices for knee instability. The OKIS Twitter account was set up, with a link to the blog site, and ‘followed’ relevant individuals and charitable and professional organisations to raise visibility and encourage reciprocal links and followers.
| Charities/associations | HCP organisations |
|---|---|
| Muscular Dystrophy Campaign | BAPO |
| FSH-MD Support Group UK | ACPIN |
| Polio Survivors Network | Prosthetics Network (@PROSTHETICSnet) |
| British Polio Fellowship | NHS Orthotics Network |
| Charcot–Marie–Tooth UK | Chartered Society of Physiotherapy |
| The Stroke Association | British Society for Rehabilitation Medicine |
| Spinal Injuries Association | SRR |
| Headway | |
| Neurological Alliance | |
| The Patients Association | |
| Brain and Spine Foundation | |
| Myasthenia Gravis Association | |
| Multiple Sclerosis Society |
The overall project dissemination strategy included a number of activities to make the findings widely available, with many using the blog as a publicity vehicle. The activities include production of a lay summary report on completion of the project. This will give brief background details, information about the quality of evidence, the results and implications to inform all relevant parties. A blog featuring the summary report, and links to this full HTA report for further detail, will be posted and tweeted.
The summary report will also be distributed electronically directly to the organisations listed in Table 71, with links to the blog, and to other relevant parties, such as the Chief Allied Health Professions Officer, NHS England and the British Orthopaedic Association. The professional bodies for GPs and neurologists will also be included, as the survey of health professionals identified these roles as referral sources.
Additional dissemination activities include the submission of papers for peer-reviewed publication and abstracts for oral and poster presentations at conferences; these will also be featured in the blog.
Blog and Twitter activity
Presented here are the figures and other details relating to the project blog and Twitter account as of 14 May 2015. Administrator page views have been excluded from the figures.
A total of 36 blogs were posted in the 12 months of the project. The blogs were all written by research team members. Although support and guidance were offered, pressure of work prohibited the health professional members of the steering group and some of the research team from contributing. The majority of the blog posts were about research methods that were related to the particular stage of the project. The rest were on Advisory Group meetings, general research issues or profession-related topics, and avoided any commercial perspectives. A timeline and summary of blogs posted is provided in Appendix 14.
The top 10 blog posts with their page views are presented in Table 72. The total number of page views was 5777. Page views for About the OKIS Project were 331, The Project Steering Group 319 and The Project Team 315. The page views by country were primarily from the USA (n = 2051) and the UK (n = 1593), followed by Ukraine (n = 409), France (n = 326), Taiwan (n = 260), Russia (n = 186), India (n = 70), Poland (n = 65), Iran (n = 44) and Australia (n = 34).
| Post title | Date published | Page views on last access (12 July 2015) |
|---|---|---|
| AHPs: essential but under valued? Based on an independent report on the value of AHPs |
20 October 2014 | 173 |
| Involving patients and the public in research Focus on OK to Ask campaign |
23 September 2014 | 116 |
| Orthotics provision: Survey of health-care professionals launched Announcing launch of survey through professional organisations |
5 December 2014 | 104 |
| Focus on focus groups About focus group methods and use to inform development of the survey |
15 August 2014 | 102 |
| A complex problem The broad inclusion criteria for the systematic review element |
28 May 2014 | 88 |
| What’s this blog all about? Introductory explanation of the project |
26 Mar 2014 | 87 |
| Orthotics services: who is delivering what? Project aim to map provision of orthotics services |
18 November 2014 | 84 |
| Survey of health professionals: analysis begins Closure of the survey and explanation of next steps |
2 February 2015 | 76 |
| Needles in Haystacks: Part 2. Which haystacks should we look in? Sources to be searched for the systematic review |
5 June 2014 | 76 |
| How can we know what works? Health information on the internet and ‘Testing Treatments’ |
2 September 2014 | 75 |
The OKIS Twitter account gained 59 followers: 16 individuals (specific interest unclear but included some users of orthotic devices); 11 orthotic product manufacturers; 10 HCPs; six charities; five researchers; four professional organisations; three private health-care clinics; two litigation lawyers; one report design company and one research organisation. An additional follower was removed, as it linked inappropriately to material of a sexual nature. The 36 tweets created for the corresponding blogs generated 24 retweets and 12 favourites or mentions.
Although comment boxes were provided with each blog, at moderation the seven comments received were all rejected, as they included advertisements for commercial products or services.
In response to approaches to the charities/patient associations and professional organisations, which included links to the blog, we received requests for articles from the Polio Survivors Network to include in their newsletter; BAPO [article in BAPOmag, issue 2, 2014: Seeking health professional experiences: orthotics for knee instability (OKIS)] and the British Polio Fellowship posted a news item on their website (www.britishpolio.org.uk/latest-news/orthotics-knee-instability/ last accessed 12 July 2015) and later tweeted links to the project blog. The Muscular Dystrophy campaign and Post-Polio news tweeted the link to the project blog site. Others expressed an interest in hearing from us once the results are available. Some sites were not routinely maintained and/or did not feature research, or only the research they funded, and, as anticipated, for others ‘knee instability’ was too small a subgroup for them to be interested in the project.
Recruitment to the qualitative research element of the project was not carried out through the blog. However, we received e-mails from three members of the public who were interested in sharing their experiences and views, having seen articles through the British Polio Fellowship and the Post Polio Network (now known as Polio Survivors Network). They were contacted and offered the opportunity to take part in the one-to-one interviews (according to our procedures for recruiting outside the NHS, approved by the University of York Department of Health Sciences Research Governance Committee) and all three accepted.
Similarly, the survey of health professionals was distributed through professional organisation mailing lists. A blog post raised awareness that the survey had gone out, with a point of contact should anyone have not received the link. This was followed up with a reminder and message about the value and importance of as many different health professionals as possible responding. Response rates increased slightly following each of these posts. However, there were other peaks in response rates, so it is not possible to know what role the blog posts or tweets played in encouraging responses. A blog post about the next steps explained the analysis phase following closure of the survey.
The blog will remain active as it forms part of the dissemination strategy for the final results. A manuscript on our experience using the blog site will be prepared for peer review publication after dissemination of the summary report in a blog.
Chapter 8 Discussion
Principal findings
The project was undertaken in response to a commissioning brief to address the question of what devices are in use in the NHS for instability of the knee in adults with NMD and CNS conditions, for what diseases/conditions, and what further research is needed. We undertook a qualitative study exploring the perspectives of users of orthoses, a systematic review of the effectiveness evidence, a survey of HCPs and a costing analysis. The purpose of the commissioned research was to inform the future research assessing the clinical effectiveness and cost-effectiveness of different types of orthotic management of the knee in people with NMD and CNS conditions. In the sections below we discuss the key findings that are relevant to this purpose and explore areas of agreement and disagreement from the different sources of information.
Perspective of orthoses users
We undertook qualitative interviews with 24 users of orthotic devices for knee instability. Nineteen patients were recruited across three geographically dispersed NHS sites and five people were recruited from outside the NHS across different areas of England. Half of the sample had been diagnosed as having poliomyelitis. Other participants had multiple sclerosis, CMT disease, spinal injury or spina bifida, or had experienced a stroke. Participants’ ages ranged from 36 years to 80 years and the median age was 64.5 years. Half of the study participants were engaged in either full- or part-time paid employment, whereas the other half described themselves as retired.
Study participants reported a range of symptoms and sequelae that were associated with neuromuscular and CNS conditions, which could limit their ability to walk and engage in everyday activities. Participants recounted how fear of falling or of their knee ‘giving way’ could result in diminished self-confidence and circumscribed independence, leading to feelings of social isolation and low mood.
Study participants relied on orthotic devices to enable them to engage, as far as possible, in ‘normal’ daily activities, such as working, driving, using public transport, the pursuit of outdoor activities, and taking part in social events and gatherings. People who had used an orthotic device over a long period of time (e.g. individuals with a diagnosis of poliomyelitis) regarded their orthotic device as an extension of their body, essential for daily functioning and integral to their identity.
Potential for future deterioration of their neuromuscular condition and general health was a preoccupation of many of those interviewed, and was a major consideration for them when seeking advice and assistance from orthotic and other specialist services.
Study participants reported using a range of orthoses (KAFOs, AFOs, knee braces) and mobility aids (sticks, crutches, wheelchairs, mobility scooters), ‘mixing and matching’ these according to differing circumstances and contexts in order to achieve maximum comfort and independence. Views on wheelchair use were polarised: for some, their wheelchair represented possibilities for increased freedom and independence, whereas others said that they dreaded having to use a wheelchair.
Obtaining the ‘right’ orthosis was, understandably, a central concern of all of those interviewed. The features of orthoses that were important to participants related to the fit of the device and how comfortable it was; whether or not it caused any damage to skin; effectiveness, reliability and durability; weight (which affected how far or easily they could walk); appearance; whether or not it caused any damage to clothing or footwear; and ‘user-friendliness’ in terms of ease of putting the device on or getting it off.
Of major importance to patients was whether or not they had a ‘spare’ device in case the currently used device required adjustment or repair, or failed unexpectedly. Participants often appeared reluctant to relinquish an ‘old’ device, to which they had grown accustomed, in exchange for a new one because they envisaged a difficult period of adjustment, during which they would have to ‘break in’ the new device.
Participants spoke at length about the footwear associated with their orthotic device, and expressed a range of views relating to desirable and undesirable characteristics. Central concerns related to careful and frequent measuring of the feet; shoe width and height, which should be sufficient to accommodate the device comfortably; durability; light in weight, preferably with good grip; sufficient number of pairs offered for individual need; in a range of styles and colour; aesthetically pleasing; easy to put on and take off; and prompt delivery of new shoes and return of those sent for repair.
During their interview, participants were asked to identify desired treatment goals and outcomes. The prime desired outcome was to receive treatment that offered effective support for their knee, resulting in a reduction in pain and the number of falls or trips experienced, with improved balance and stability. Effectiveness, reliability, comfort and durability were the features of orthoses that were most valued, and were related to reported use of orthotic devices. Participants defined their own goals for mobility in terms of what they wished to achieve in their daily lives, according to their individual circumstances. Respondents did not discuss treatment outcomes as measured by how far or how fast they could walk. Rather, they focused on different activities that they wished to pursue and judged the success of treatment in terms of how far it enabled them to participate in these activities. The extent to which their orthotic device enabled participants to engage in paid employment, outdoor activities (such as gardening), family visits and social events, was the yardstick used to assess the effectiveness of treatment. Being able to take part in these activities was regarded as important by participants for both their physical and mental well-being.
All participants were concerned about receiving treatment that would slow down, as far as possible, further deterioration in the affected knee joint, and which would not cause collateral, ‘compensatory’ damage to other joints. People with a diagnosis of poliomyelitis revealed concerns about symptoms of post-polio syndrome and were anxious about following advice from physiotherapists who were not specialists in neurophysiotherapy for fear of inadvertent muscle damage.
Respondents in the study commented on a range of factors, related to their care pathway, which they thought impacted on the quality of care they received. The overall picture that emerged was mixed; participants frequently praised individual clinicians (mainly orthotists and consultants in rehabilitation medicine) but were critical of the systems in which they worked.
Participants expressed frustration with referral routes into orthotic services, channelled through GPs and orthopaedic services, which resulted in delays in obtaining effective treatment for their knee instability. People under the care of a consultant in rehabilitation medicine appreciated the consultant’s role in coordinating their care and monitoring their condition, while making proactive and timely decisions to refer them to orthotics and other specialist services, such as to neurophysiotherapy. Three people in the study shared the view that ‘atypical’ or complex cases, who might pose specific challenges related to their orthotic treatment, should be prioritised for a more intensive and in-depth approach; one approach suggested was holding a ‘case conference’ involving more than one orthotist to explore different ways of tackling a problem.
Many of those interviewed expressed a degree of frustration with deficiencies in the appointment systems in operation in orthotic services. They reported delays in receiving treatment, as well as inconvenience and sometimes financial consequences when they had to take time off from work to attend appointments. Other perceived shortfalls in orthotic services that were reported included work pressures from the ‘busyness’ of orthotics departments; poor record-keeping and transfer of information between different parts of the service (e.g. information relating to measurements for devices not reaching manufacturers); lack of coordination between services (such as podiatry and orthotic services); and a perceived lack of ‘business ethos’ in the NHS services, associated with slow production (of orthoses) and toleration of delays, as well as a lack of innovation and investment in new ways of working and technological developments in the field of orthotics.
Several of those sampled said that they had contemplated or had made use of the private sector in relation to acquisition of orthoses. The reasons they gave were mainly related to perceptions about underfunding and investment in NHS orthotic services. These people stated that they were seeking more technologically advanced orthoses than they thought would be available to them through the NHS, and they also hoped to ‘buy in’ to a more personalised care package, with quicker turnaround times than those they had experienced through NHS services.
A particular aspect of orthotic service provision that generated a great deal of commentary among study participants related to provision of ‘in-house’ workshops within orthotics departments for the manufacture, adjustment and repair of orthotic devices. Availability of this facility was associated with delivery of timely, good-quality orthotic care, particularly for minor or emergency repairs to devices. Participants were dissatisfied with long delays and having to attend multiple appointments that they associated with manufacturers being located outside the department – for example, some manufacturers are based abroad.
Aspects of their interactions with individual orthotists that were important to study participants: their level of knowledge and expertise, their accessibility and, most importantly, their listening skills and willingness/ability to engage with patients during consultations in decision-making about treatment options and outcomes. Participants rated most highly those clinicians who took time to listen to them describe the problems that they were experiencing in relation to their knee instability and how these impacted on their daily life; offered information and explanation; and explained the benefits and drawbacks of different devices while involving patients in treatment decisions.
Findings in the context of other studies
Participants in our study described their experiences of lengthy waiting times to access orthotic services, in part because of circuitous referral routes, for example via orthopaedic surgeons. They also reported frequent delays in the provision and repair of custom-made KAFOs and footwear – issues already highlighted elsewhere. 10–12,72
Our study participants emphasised the need for individually tailored care in relation to provision of orthotic devices. Their comments align with Hutton and Hurry’s recommendation10 [based on findings from the Darzi review (Department of Health)73] for a model of service that recognises that ‘orthotic products are not commodities, but individually prescribed solutions suited to each patient’s personal needs’ (p. 10).
Study participants suggested that people receiving an orthotic device for the first time may require intensive support during the initial phases of receiving, adapting to, accepting and using the device, as they adjust to altered self-image. In addition to technical information related to the use of the device, patients may further require psychological support, as found in studies by Vinci and Garguilo14 and Garralda et al. 16 involving people with CMT disease and children with Duchenne muscular dystrophy, and their main carers. In their combined questionnaire and interview study of patient satisfaction with lower limb orthoses, Fisher and McLellan72 found that patients who were dissatisfied with their lower limb orthosis had usually decided not to wear it within the first 2 weeks.
Participants in our study cited functional independence as their prime desired treatment outcome, which they linked closely to their perceived ability to perform a range of work and leisure activities that promoted and enhanced their psychosocial well-being. A study by Schaffalitzky et al. ,74 which included six focus groups with 24 lower limb prosthetic users, highlighted that even small gains in mobility provided by a device may constitute a successful outcome from the patient’s perspective, which mirrors our own findings. Interestingly, in Schaffalitzky et al. ’s study,74 wheelchair use was regarded as anathema by the participants, whereas in our own study, participants were often positive about using a wheelchair and other mobility aids, frequently in combination with their orthotic device, as a means of gaining increased mobility and independence.
Findings from our study concur with previous research with regards to patients’ views of orthotic footwear. Studies by Williams et al. 75 and Fisher and McLellan,72 with 13 and 28 people interviewed, respectively, indicate that the range of choice available to patients in terms of colour, style and material is important, as are cosmesis and weight of the finished shoes, all factors that appear to be associated with likely wearing of prescribed shoes. Williams et al. 75 identify the importance of footwear to people’s self-image, self-expression and identity, something that seemed important equally to both male and female participants in our study.
Three studies13,72,76 have considered the views of patients towards the use of AFOs. These studies,13,72,76 utilising different research techniques and with varying patient populations, found that orthoses that did not fit properly, which were considered uncomfortable, heavy, cumbersome and unsightly, and which drew attention to disability, were less acceptable to patients, results which match findings from our own study. Phillips et al. 13 combined nominal group technique and interviews in her study of people with CMT disease to identify perceived barriers and benefits to the use of AFOs. Results from this study13 revealed a range of factors that were important to patients in determining the likelihood of their wearing a device. Benefits associated with their orthosis were ranked and included enablement of independent mobility, prevention of falls and stumbles, pain reduction, improved balance and prevention of deterioration, similar to factors cited as significant by participants in our own study. However, Phillips et al. ’s study13 revealed marked differences between male and female participants with regard to perceived barriers to using AFOs (e.g. men citing barriers to use of AFOs in the work environment more frequently, and women focusing on difficulties with accessing, and availability of, orthotists and their lack of listening skills). 13 Interview data in our own study did not reveal such marked differentiation of views by gender.
Bernhardt et al. 45 administered a questionnaire to nine people with inclusion body myositis (six of whom were followed up after a 6-month period), who had received a SCO to treat quadriceps weakness. Subjectively, all of the participants felt that the SCO was helpful in safeguarding against falls and providing stability; however, there were complaints about size, bulk, cosmesis and noise of the SCO, as well as difficulty putting on/taking off the brace, and most people noted that they would prefer a less intrusive device. In our own study, comfort, durability, effectiveness and reliability of the device were deemed more important than the size or appearance of the device; participants in our study seemed willing to ‘trade off’ elegance and unobtrusiveness of a device for effectiveness and reliability.
Clinical effectiveness evidence
The systematic review included 21 studies (478 patients) evaluating the effectiveness of orthotic devices for knee instability related to a NMD or CNS condition. The most common CNS conditions were post stroke and spinal cord injury. The NMD studies were of people who had experienced poliomyelitis, with the exception of one study including nine patients with inclusion body myositis. We found that studies were generally small and were not reported in a way to allow confidence that the included patients were representative of patients seen in clinical practice. Only two of the included studies (n = 42 patients) were undertaken in the UK.
Also, the full range of conditions for which devices may be used for knee instability is not reflected in the literature. Although the epidemiology of knee instability in adults, related to NMD or CNS conditions, is unclear, we are aware of other conditions for which a KAFO or AFO may be provided for knee instability. For example, in the qualitative study that we undertook, some of the participants had CMT disease or multiple sclerosis, yet these patients were not represented in the studies included in the review. The survey of HCPs also confirmed that people with a range of other NMD and CNS conditions beyond those found in the studies of effectiveness are users of orthotic devices. This may be related to prevalence. Although some of the NMD and CNS conditions are of relatively high prevalence, the specific problem of knee instability, and being suitable for one of the orthotic devices of relevance, may be relatively small. For example, people with multiple sclerosis may experience walking problems due to balance impairments, fatigue, muscle weakness or spasticity. 77 In addition to the devices under consideration here, other assistive technologies that may be considered for people with multiple sclerosis include walking sticks, crutches, wheeled walkers, and manual/motorised wheelchairs. 77
In the studies in which the participants had experienced poliomyelitis, carbon fibre or plastic KAFOs were investigated. One study investigated a SCKAFO. There was either no comparator or another type of KAFO (one used the same KAFO in locked and stance control mode). The studies of patients who had experienced a stroke compared plastic or carbon fibre KAFOs to no comparator or AFO, except for one study, which compared KAFO or AFO to ‘conventional rehabilitation’. The studies of patients with spinal cord injury assessed HKAFOs, RGOs and HGOs. Predominantly custom-made devices were evaluated across the conditions.
There were three key findings from the review:
-
First, the majority of studies were case series, making before-and-after comparisons and had a substantial risk of bias due to the inherent limitations in this study design to robustly assess the effectiveness of an intervention. In addition, outcomes were not assessed by independent assessors but usually by the treating clinician, and many of the studies were very poorly reported; for example, several made statements about findings without presentation of supporting data.
-
Second, there was a mismatch between the outcomes identified in the qualitative study as important to users of orthotic devices, and also identified in the survey of HCPs as important, and the outcomes that were assessed in the studies of device effectiveness. The literature is dominated by laboratory evaluations of orthoses. During our searching for this review we identified 76 studies that were excluded because the evaluation of the orthosis did not include any use of the orthosis by the patient in a non-clinic setting. Laboratory-based studies can provide useful insights about effectiveness, particularly during development of a device. In this review, we included only studies where patients made use of the orthosis in a ‘real-life’ situation rather than only in a laboratory setting. In this way we hoped to gain the patient perspective of the intervention in addition to objective assessments of gait. However, the most systematically assessed outcomes in the included studies were outcomes such as gait analysis and energy consumption. Although several studies did report patient satisfaction with the device, this was predominantly reported in an anecdotal fashion and it was not possible to assess how robustly the information had been collected. Despite our requirement that participants in studies had used their orthoses outside the clinic there was no assessment of the extent to which the device had been used outside the clinic or use of validated measures of patient function and quality of life. Generally, adverse effects were not systematically reported. It cannot be inferred that there were few adverse events, as authors did not specifically mention that no adverse events were identified. It is apparent from the interviews with orthoses users that concerns (such as falls, avoiding collateral compensatory damage to their other joint, pain, discomfort and skin damage due to an ill-fitting device), and the consequences for them if a device requires frequent repairs and readjustment, were all important. In addition to the mismatch in outcomes that were important to users of devices and those assessed in studies of effectiveness, whereas the majority of studies had follow-up period of < 1 year, users of devices referred to fairly long time frames in terms of adjusting to a new device, with some suggesting > 1 year. Therefore, the studies may not have been long enough in duration to fully capture the effects of using the devices.
The prime desired outcome expressed by participants in the qualitative study was to receive treatment that offered effective support for their knee, resulting in a reduction in pain, the number of falls or trips experienced, improved balance and stability, and participation in work and a range of other family/social activities. Participants defined their own goals for mobility in terms of what they wished to achieve in their daily lives, according to their individual circumstances. Respondents did not discuss treatment outcomes as measured by how far or how fast they could walk. The studies in the systematic review primarily focused on device performance using gait analysis and, in some instances, walking distance and speed, whereas, from a patient perspective, reduction in pain, falls, trips, and improving balance and stability are potential measures of effectiveness. A factor that might contribute to this discrepancy in outcome measurement is the current requirements for device regulation; only evidence of performance and safety is required for medical devices, which may result in a lack of incentives to conduct primary research on efficacy and/or effectiveness. 78
-
Third, the focus of the effectiveness studies tended to be on the device in isolation. Few studies reported the orthosis ‘dose’ given to the patient, i.e. the time per day/week for which they were advised to use the orthosis. In addition, reporting of fitting and training in use of the device and ongoing review was limited. A strong theme emerging from the qualitative study is that users do not see the device itself in isolation from how they were assessed for provision of the device, measured and fitted, how it functions with footwear, cointerventions, ongoing adjustment of the device and review. Given that provision of an orthosis may require a reassessment of self-image, some individuals may require psychological support, as well as technical and other information about the device itself. Provision of an orthosis is essentially a complex intervention and this was not reflected in the effectiveness literature.
Findings in the context of other studies
The broad picture of a very limited evidence base, small single-centre studies and inadequate study design is similar to that identified in other reviews of orthotic devices. 4,17,21 A systematic review of questionnaires that was used to assess patient satisfaction with any limb orthoses found that 63% of the 106 included papers used questionnaires that had been developed for the specific study rather than validated measures, indicating that our findings related to orthoses for knee instability is a broader issue in the field. 79
Survey of health-care professionals
A total of 238 HCPs responded to the survey. Of the 229 who responded to the question on their occupation, there were 80 orthotists, 94 physiotherapists and 50 doctors in rehabilitation medicine (5 other). The majority of respondents had ≥ 16 years’ post-qualification experience. The orthotics services with which they were involved used a number of different models: orthotic provision as part of a MDT, stand-alone services, a combination of both depending on patient group, and other models, such as physiotherapists sourcing orthoses directly. MDTs included physiotherapists, orthopaedic surgeons, specialists in rehabilitation medicine, occupational therapists, neurologists, orthotists, clinical nurse specialists and others. Respondents rarely reported that gait scientists were involved in MDTs. Interestingly, gait analysis is one of the more commonly used outcome measures in the studies that were included in the systematic reviews. There is, therefore, a discrepancy between what is viewed as an important outcome when assessing the effectiveness of a device in a research study and clinical practice. When used in clinical practice, gait analysis may be viewed more as a tool to assess the impact of the orthosis in correcting gait and guiding further intervention rather than a formal measure of outcome. Resources to measure gait may also be a factor here.
The most common NMDs being managed by respondents were poliomyelitis, post-polio syndrome, muscular dystrophy, CMT disease, motor neurone disease and Guillain–Barré syndrome; between 65% and 76% of respondents who managed patients with knee instability that was related to NMDs reported managing these conditions, suggesting that there is no single group that dominates provision. Similarly, no single group dominated provision among CNS conditions, although HCPs most commonly reported managing patients who had experienced a stroke (100%) and multiple sclerosis (83%). Other CNS conditions were adult cerebral palsy, traumatic brain injury, acquired brain injury and spinal cord disorders (50–65%). However, even taking into account the 10% margin of error, there seemed to be a different profile of patient conditions being managed by orthotists and physiotherapists; physiotherapists less commonly reported seeing patients with poliomyelitis, post-polio syndrome and adult cerebral palsy. This is a very tentative suggestion, as the sample size did not permit subgroup analysis; however, it is noted here as it may have implications for how best to recruit specific population groups for future orthotic research.
Falling, experiencing pain or weakness in the lower limb triggered referrals. There were many HCPs involved in assessing the patients in addition to the person referring for an orthosis or providing an orthosis. There is a suggestion in the data that there are some differences between orthotists and physiotherapists in terms of which other HCPs they reported as being involved. This may support that there are differences in the profile of patients they see or may simply suggest differences in how services are organised influencing patterns of referral between HCPs. Again, however, this can be considered as only a very tentative suggestion, but worth investigating further when planning future research.
A range of orthoses are prescribed for knee instability related to NMD or CNS conditions: taking into account the 10% margin of error, broadly similar proportions of respondents reported prescribing KAFOs (75%), AFOs (94%) and knee brace (89%). A substantial proportion also prescribed shoe adaptations (66%) and insoles (70%). Approximately half of the devices prescribed or fitted are custom-made (range 0–100%). There was a range of device and patient factors that influenced the decision to prescribe a custom-made device. Patient factors mainly related to functional deficit and anatomy; device factors mainly related to requirements of patients such as flexibility, control, fit, durability, availability of devices, the type of device (knee braces were rarely custom-made) and, for a small number of respondents, cost. The number of visits that are required to fit a device varied depending on the complexity of the device (least number of visits with insoles and most with a KAFO) and whether or not a device was custom-made. The number of visits was similar across NMD and CNS conditions. For a patient with a NMD the mean number of visits to provide a KAFO was 3 (SD 0.55) and for an insole 1.8 (SD 0.55). The time frame from the initial visit to fitting was shorter for a prefabricated device [mean 3.5 (SD 2.6) weeks; median 3 (range 0–12) weeks] than custom-made [mean 5.7 (SD 3.5) weeks; median 5 (range 0–20) weeks]. The duration of an appointment for casting and fitting a custom-made device was also longer (mean 38 vs. 26 minutes). There was considerable variability in the arrangements for review visits: on average this was 8.3 (SD 6.7) weeks [median 6 (range 1 to 32) weeks]. Respondents were fairly evenly split (taking into account the margin of error) in terms of whether or not they provided long-term review appointments (46% vs. 54%), although 88% reported providing reviews on request.
There was a range of different approaches to dealing with a broken custom device ranging, more commonly, from provision of a spare at the time of receiving the original device (36%), provision of an off-the-shelf device until device is fixed, and on-the-spot repair at an on-site workshop (38%), to rarely that the patient would have to wait for a repair or that there were no procedures in place (single respondents). The picture was broadly similar with off-the-shelf devices. The mechanisms in place to deal with device adjustment, repair or unexpected failure of a device were of major importance to patients. It was beyond the remit of our research to consider whether or not these variations in how repairs are dealt with are associated with variation in the quality of service that patients receive and the extent to which it affects daily functioning.
The majority of respondents were prescribing or fitting an orthotic device in order to achieve the following outcomes: control joint movement, reduce the number of falls, reduce pain, increase walking distance and avoid deterioration. Other treatment goals that were suggested included the broader outcomes of improving quality of life and independence; increasing social and work integration; and management of fatigue, as well as the more focused outcomes of improving symmetry, supporting ligamentous and tendinous structures, and preventing secondary arthritis. The majority of respondents were either neutral or disagreed that patients are expressing a preference for a particular device, although approximately one-third said that patients were expressing preferences for lightweight, discrete and cosmetically appealing orthoses, which are comfortable, functional and durable, easy to take on and off, and specific types of devices, such as SCKAFOs or custom-made devices.
The two treatment outcomes that at least 50% of respondents thought were extremely important were comfort and confidence in mobility. Similarly, a wide range of factors were thought to influence the effectiveness of orthotic devices, reflecting the complexity of the intervention. These ranged from factors related to fit, device-related pain and pressure areas, to acceptability of the device to the patient, each of which the majority of respondents identified as important. Additional factors identified by small proportions of respondents were psychological and cognitive factors, the underlying condition, motivation and expectations, patient involvement in choosing the device and support from family and friends. The majority of respondents (64%) thought that the cosmetic appearance of a device affected the acceptability of the device to patients and whether or not they wore it. They indicated that patients want a discrete device that fits under clothing and that the type of footwear they can use with the device is important to them, although they acknowledged that there was variability in patient views and the factors affecting acceptability depended on the individual patient. The outcomes identified by HCPs and the issues considered to be of concern to patients showed some degree of overlap with those identified by patients themselves in the qualitative interviews. However, what is being evaluated in research is at variance with what is reported as important by both patients and HCPs.
Just over one-quarter of respondents (29%) reported that no formal outcome measure was used to assess the effectiveness of the devices provided. No single outcome measure was used by the remaining respondents. The most commonly used outcome measures were the visual analogue scale (51%), the 10-m Walk Test (41%); and the Timed Up and Go Test (35%). Only 34% reported formally assessing patient satisfaction. Given what patients reported as desired outcomes, it is unlikely that the formal outcome measures used fully capture how useful and effective the devices are for users.
Approximately three-quarters of people who responded to the question on whether or not the care pathway could be improved thought that improvements could be made. There was a wide range of suggestions including improving communication and team working across professions, reducing waiting times, improving awareness of orthotic services, earlier referrals, removal of budget barriers, and improvement of care in clinic, follow-up care and improvements in assessment. Specific to patients with CNS conditions, eight respondents thought that an increase in the availability of orthotic device for stroke patients – such as strong, carbon fibre, lightweight devices – would be beneficial. The concerns of HCPs showed some overlap with the concerns of patients: referral routes, delays and a coordinated approach to care particularly for more complex problems. However, many patients also identified administration-related aspects of orthotics services as an area of concern.
Costing analysis
The costing analysis attempted to give an overview of the complexity of the service provision of KAFOs, as well as the many variables that would need to be taken into account to provide an accurate estimate of the cost of these devices. The telephone interviews highlighted the huge variations in costs that are possible. The following variables, in particular, may alter the price of a KAFO dramatically: the joint elements chosen for the device; the materials chosen for the device; and the HCPs involved in the prescription and/or fitting of the device. Our analysis estimates that the cost of a custom-made KAFO could range from £2198 to £3553 and an off-the-shelf KAFO could range from £73.24 to £1898. However, these estimates are based on some assumptions, may not be reflective of the true cost of providing a KAFO to patients with NMD or CNS conditions with knee instability, and should be interpreted with caution.
Orthotists perceive that the benefits of walking and weight-bearing for the patient as a result of wearing the KAFO far outweigh the cost of the hardware that is required to provide the device; however, this is an area for which future research is needed. Future research could focus on conducting a microcosting of these devices. Such analysis would need to involve device manufacturers and suppliers of KAFO components/raw materials. Raw material and production costs would need to be estimated in more detail; the methodology used by Simeons et al. 71 provides an initial framework to conduct such an analysis.
Findings in the context of other studies
A costing analysis undertaken by Simeons et al. 71 estimated the cost of hard knee and neck braces from a Belgian third-party perspective. This study71 was able to obtain more detailed manufacturing and production costs. The study estimated neck braces to cost between 55 and 150 euros (€) and knee braces to be between €331 and €694. These smaller ranges of cost estimates highlight that there was less uncertainty in Simeons et al. ’s analysis,71 given their increased data on the manufacturing costs of these devices.
Dissemination and engagement
We planned and implemented a combined engagement and dissemination strategy. The engagement process started at the beginning of the project in order to prepare channels of communication. We used a project blog, Twitter feed and articles in newsletters to raise awareness of the project, explain the research methods being used and promote involvement by health professionals in the survey. This prepared the audiences who were less familiar with the research process to understand the background to the project in advance of disseminating the results. The dissemination activities focus on ensuring that all of those who need to know the results receive them and can make sense of them. This includes targeted circulation of this full HTA report, a summary report and blog articles.
The use of social media for awareness-raising was a pragmatic approach involving minimal costs for a potentially wide exposure. Free software options were selected, so the only cost was researcher time in setting up the various elements and maintaining the dialogue. However, not everyone uses the internet and not all of those who do so use social media. We found that many of the charities are not active on Twitter and have out-of-date websites and/or did not cater for news items or articles and/or published only every 6 months. Several of the most active of the organisations we interacted with had developed their own Facebook account and used that rather than a traditional website. Our concerns about using Facebook or its equivalent were primarily about the advertisements that appear alongside a page and also about ensuring control over the content of comments and postings from external sources.
Having the blog site as a focal point when approaching organisations and individuals proved to be a useful resource, and one that we could control. We were aware of the broad range of conditions to be considered in our research and the different types and styles of orthoses for inclusion. The project was very specifically focused on people with a NMD or CNS condition and who had knee instability as a consequence. We did not want to raise expectations in the large number of people with a NMD or CNS condition who need and/or use orthotic devices for reasons other than knee instability. Likewise, the project would not be of direct relevance to people with orthotic devices for knee instability from other causes, such as injuries or arthritis. This was a concern when agreeing the necessarily short titles for the blog (Orthotics for Knee Instability) and Twitter (Orthotics for Knees) accounts. The limitation on the number of characters in the titles precluded specifying the relevant conditions and, for the Twitter account, even that the project was specifically looking at knee instability. In mitigation, the project parameters were explicitly stated on the front pages of both the blog and Twitter sites in order to be as transparent as possible.
At the start of the project we prepared a list of potential blog topics and attempted to allocate members of the team to produce at least first drafts. It was hoped that guest blogs from members of the steering group could be featured, but time pressures for the health professionals prevented this. The research team had similar problems, although some embraced the opportunity to write a short piece in a ‘chatty’ style. Getting the pitch right was a challenge but the focus was on patients, and public and health professionals who did not have a research background.
Our original thoughts were to try and post a blog each week or at least once every 2 weeks on the basis that less-frequent posts and the blog would not attract or retain followers. However, as with all types of social media, blogging has evolved in the way it is used and accessed. Instead of signing up to follow the blog, our audiences signed up to receive tweets. These gave the title of the blog so they could then decide whether to click through to the full post or not. We therefore became more comfortable with tailoring blog post content and timing to our project needs rather than our perceived need of interested audiences. After the research findings have been posted, we plan to do a final blog asking how useful it was to readers, whether or not the content was interesting/boring, pitched at the right level, etc.
It is difficult to assess the success or otherwise of the use of the blog as a medium. Overall it was felt by the team to be most useful as a focal point to refer people to. For example, patients and professionals could easily find out more about the project and who was involved. Anecdotal feedback was positive. For example, prospective health-care professional focus group members and telephone interviewees were directed to information about the project on the blog site. Several participants then mentioned that they had clicked into the blog, seen who was involved and became interested in helping. We also got three valuable contacts from more traditional articles in the magazines of charitable organisations.
Given the increasing simplicity of setting up free websites, this could be a viable alternative to a blog site, as long as there are no advertising issues such as with Facebook. A website may also provide more flexibility in display and content capabilities while achieving the same aims as the blog. However, a cost is usually attached to additional functions, providing little advantage over the simpler blog site.
We will have a full perspective of usefulness of the blog only after the dissemination of the findings, which starts with the publication of this report. It is anticipated that the blog will continue to provide a vehicle for accessing information about the project and all of its outputs, including the summary report, conference presentations and peer-reviewed papers. An assessment after this stage will be included in the planned peer-reviewed journal publication.
If feasible, longer-term news of the impact of this work – for example, any subsequent research commissioned – will also be posted. The blog site has the potential to be linked to, or developed for, use in subsequent related work.
Strengths and limitations of the research
Definition of knee instability
A key challenge across all of the elements of the research was clearly defining the population of interest, specifically the knee instability element. Knee instability is not an explicit and well-defined clinical diagnosis. This created several challenges as the project progressed. When trying to recruit patients to the Advisory Group we attempted to target people using an orthotic device for knee instability. We had limited success. It became apparent from the qualitative study that people do not really define themselves in this way. Therefore, in hindsight this may have limited the number of people approached for involvement in the Advisory Group. It also had implications for recruitment of patients to the qualitative study. In the three recruitment centres, patients were not classified on clinic databases as to whether or not they had knee instability. This also had implications for the screening of studies for inclusion in the systematic review. It was often difficult to determine whether or not the participants in included studies had knee instability. As it is not a diagnosis as such, we sometimes had to rely on the authors’ description of the ambulatory difficulties of the participants and make a judgement. In other instances, knee instability was part of a more complex ambulatory problem and we had to make a judgement as to what the predominant problem was. Therefore, there may be studies included in the systematic review for which it is arguable that knee instability was the main problem and there may be studies that were rejected from the review that do include people with knee instability. However, we would not expect that this would in any way change the overall conclusions of the review about the lack of high-quality evidence or allow conclusions to be made about the effectiveness of specific devices.
Systematic review
For the systematic review we undertook thorough searches for eligible studies, which included systematic searches of 13 electronic bibliographic databases and numerous sources for unpublished and ongoing studies without any language restrictions. Risk of bias in the included studies was assessed and taken into consideration in the synthesis. Standard methods to reduce error and bias in the review process were used at all stages of the review. Several studies provided a descriptive report of some outcomes with no numerical data. Owing to the paucity of evidence we extracted these reports in order to provide as clear a picture as possible of what information is currently available, especially as many of these data related to patient perspectives. Arguably this inflates the amount of evidence that is available. The key limitation of the review was the lack of high-quality generalisable studies; however, it does provide a comprehensive overview of the gaps in the evidence on orthotic devices for knee instability that is related to NMD or CNS conditions.
Qualitative study
The qualitative study was designed to elicit people’s views and experiences of using orthotic devices for knee instability. It is, as far as we are aware, the largest interview study of its kind. Participants were selected to ensure the inclusion of people with different types of neuromuscular conditions and disease or damage of the CNS, and who had experience of orthotic services in one of three different regions of England. We also recruited from NHS and non-NHS settings. The purposive sample of 24 individuals included people of different age, gender and ethnicity; some people lived alone, whereas others lived with family members, and around half of the sample was in paid employment, whereas the remainder were partially or wholly retired. Significant others who were present when participants were interviewed often contributed to the interview, and added a further dimension to the findings, although we did not specifically set out to recruit significant others into the study.
Our study was strengthened by the inclusion of a patient representative at key stages: the patient representative was requested to assist with development of the participant topic guide used in individual interviews, through suggestions on content, language and phrasing of questions. In addition, the individual was included in study team meetings and provided with the opportunity to comment on draft findings from analysis of interview data, during both early and later phases, as well as on the draft final report. Involvement of a patient representative in this way may be viewed as a means of promoting and enhancing ‘trustworthiness’ of the study findings. 36
It might be considered that a weakness of our study was that we did not manage to recruit participants with the full range of pathologies, motor impairments and comorbidity across NMDs. We did not, for example, recruit anyone with a diagnosis of myasthenia gravis or with muscular dystrophy. Nonetheless, we feel reassured that the study included people whose diagnosis (poliomyelitis, stroke, CMT disease, multiple sclerosis, spina bifida and spinal injury) means that they have regular and sustained contact with orthotic services in connection with knee instability.
Owing to the relatively under-researched nature of our research topic, the issue of the generalisability of the qualitative study may be considered less salient here than that of ‘sensitising’ readers to new information captured in the interviews, and new ways of thinking about patients’ perspectives of using orthotic devices and services. These insights can inform our understanding of similar contexts and issues, a form of conceptual generalisability. 35 The small numbers of study participants, with different NMDs, constituting the total study sample urges caution in extrapolating findings in relation to ‘parent’ populations.
Survey
A total of 238 HCPs agreed to participate in the survey equating to a response rate of 6.43%. The response rate by organisation was 12% for BAPO, 3.48% for ACPIN and 16.67% for BSRM. This is a low response rate. However, it is an underestimate, as we did not have a precise sampling frame: membership of the three organisations includes an unknown number of individuals who do not manage people with NMD and CNS conditions with knee instability. The total number of respondents was well above the estimated minimum sample size required based on a 95% confidence level and a 10% margin of error (n = 96) but insufficient for the more ideal 5% margin of error (n = 384). This was the case for most of the questions, although some had fewer than 96 respondents and this is clearly reported throughout the results section. It was not appropriate to undertake subgroup analyses. Given the exploratory nature of the research, descriptive results are presented by professional group as well as the total sample; however, any apparent differences should be interpreted with caution.
Our preliminary focus group and HCP interviews indicated a high level of variability in practice related to individual patient needs, variation in relation to different conditions, variation in how orthotic services are provided and care pathways. This led to a fairly lengthy questionnaire in an attempt to capture the complexity. The overall completion rate was 58%: 68% for orthotists, 63% for physiotherapists and 54% for doctors in rehabilitation medicine, suggesting that the survey was too long. There would be benefit in any follow-up research with HCPs focusing in more detail on specific conditions and specific elements of the care pathway given the complexity.
Costing analysis
There are several device options available depending on the individual patient. In our study we focused on KAFOs. The costing of orthoses used for knee instability is incredibly complex, as many of the devices are custom-made. The telephone interviews with orthotists and the survey of HCPs did not give a clear indication of the useful life of these devices or when a replacement device would be required. One orthotist stated that KAFOs could potentially last for 15–20 years but a standard useful life is not available. This would need to be elicited for a future cost-effectiveness analysis. In addition, because of the large variation across the UK, the cost estimates do not include overheads, replacement device costs and shoes cost. This costing analysis is a stepping stone towards a future cost-effectiveness analysis and has highlighted the areas that would need careful consideration in future analyses. Any future cost-effectiveness analyses would need to consider carefully the devices being compared, the manufacturing costs of these devices and the care setting being assessed. Further research is needed on the service provision of these orthotic services to fully understand the various models of care available and the implications for costs.
Improving the evidence
There is a large gap in the evidence on the effectiveness of KAFOs, AFOs and other orthotic devices for managing knee instability related to NMD and CNS conditions. Robust research addressing this gap is required; RCTs are the most robust way of assessing effectiveness. However, the feasibility of a trial to assess the effectiveness of orthotic devices for knee instability in these populations would need to be carefully considered, as well as to whom the findings would be generalisable and the most appropriate research question.
Population
How the population for a trial should be defined would need consideration, as applying inclusion criteria that are related to knee instability in the context of a trial would be complex. Given the exploratory nature of our research, we were pragmatic and accepted the clinician and/or orthoses user opinion that they had problems with their knee. However, recruitment to the qualitative study was slow and it took us 9 months (September 2014 to May 2015) to recruit 24 people to the study despite strenuous efforts. This was exacerbated by the fact that people were not classified on the basis of knee instability on clinical databases. In addition, patients with knee instability may have had other interventions to manage their condition such as FES or their knee was so unstable that they used a wheelchair and had never used orthoses. Another factor was that some patients had multiple other lower limb problems.
There is also the question of whether or not people with NMD and CNS conditions are a similar enough population and indeed whether or not within these two groupings the clinical problems are similar enough to be included in the same trial on the effectiveness of orthotic management. Clinical management of people with primarily CNS conditions will often differ from the conditions described above because of the effect of upper motor neuron features on lower limb function. For example, in conditions such as multiple sclerosis, spasticity will influence the prescription of orthoses; in patients who have experienced a stroke, issues such as spasticity and spatial awareness will impact prescription of orthoses, additionally there is acute onset usually with improvement and plateau. The importance of this heterogeneity is likely to vary, depending on the specific research question being addressed and needs to be balanced against the fact that including a broader population will make recruitment to a trial more feasible.
The evidence base would also be improved through future studies providing detailed descriptions of the population under investigation so that judgements can be made about the generalisability.
Intervention and comparator
As illustrated by the qualitative research the goals in terms of functioning vary from patient to patient. Given the individualised nature of orthotic provision in these populations with complex needs, use of customised devices is relatively common. The extent of variation that would be permissible if evaluating the effects of a specific type of device would need to be addressed. Similarly, consideration needs to be given to the appropriate comparator. A KAFO is a substantial and intrusive device and there is a view that this tends to be offered when other devices such as an AFO are not likely to help. Therefore, possible comparators are another type of KAFO, no orthosis or use of wheelchair.
As discussed above, being provided with an orthotic device is a complex intervention and consideration would need to be given to where the boundaries of that intervention lie in terms of other assistive devices used, such as walking sticks and the process of measuring, fitting and reviewing the device provided to an individual. Any intervention should be developed with consideration of guidance available on developing complex interventions. 80
As with population characteristics, the evidence base would also be improved through future studies providing detailed descriptions of the intervention and comparators under investigation, so that judgements can be made about the generalisability. In addition to the technical specification of the device and any associated shoes or walking aids, this should include details of any training and/or rehabilitation and advice on wearing.
Outcomes
There is inconsistency between studies in the outcome measures being used and a focus on mechanical outcomes at the expense of other outcomes, such as patient functioning and patient experience. Any future research must consider how best to measure the impact of orthotic devices on patient quality of life, daily functioning outside the clinical setting, use of their device and any adverse effects. Important outcomes for patients in the qualitative study were a reduction in pain, falls or trips, improved balance and stability, and participation in paid employment, outdoor activities (such as gardening), family visits and social events. Brehm et al. 81 have suggested a core set of outcomes for studies of lower limb orthoses, based on the World Health Organization International Classification of Functioning Disability and Health. However, as far as we are aware, a consensus process has not taken place therefore further work is required to achieve consensus between patients, researchers and health-care providers to ensure the acceptability of the measures that have been identified. 81
Patient perspective
The evidence base could also be developed through consideration of the priorities of those using the devices. Findings from our study suggest that provision of an effective and acceptable orthosis for knee instability to people with a range of NMD and CNS conditions has a crucial role to play in maintaining, promoting and enhancing their physical and psychosocial health and well-being.
Our study sample included people in their thirties, forties and fifties, as well as those of older age. Younger participants whose ‘normal’ lives included full-time employment and responsibilities connected to supporting their families, or themselves, in the role of main breadwinner, were reliant on their orthoses to enable them to assume these roles. Older participants frequently described the central importance of their device for their engagement in leisure and family activities, which helped them feel connected to the outside world.
Our findings suggest that acceptability and use of orthoses depend on a range of factors, but that effectiveness, reliability, comfort and durability are the most important considerations for patients in deciding whether or not to use a specific device. Results indicate that people who are receiving their first orthosis (particularly a KAFO), or who are in the initial phases of trialling a new replacement device, may benefit from a period of more frequent appointments than is necessary later on, as they adjust and become accustomed to the device. Participants in the study frequently expressed strong feelings about the appearance of their device and associated footwear, and their individual choices and preferences need to be taken into account as they are likely to be associated with device use.
Results from the study highlight the importance of orthotists (and other HCPs) understanding the ‘lived experience’ of this patient group, so that they can engage with individual patients to identify acceptable management strategies and treatment outcomes. Establishment of a therapeutic relationship in which patients feel listened to and supported would seem to be a necessary prerequisite to promoting patient acceptance and use of orthotic devices. HCPs require the necessary time during consultations and appropriate skills to enable this to be achieved.
Our study findings reveal that many participants experienced a care pathway prone to disruption and delays, primarily due to circuitous referral routes to the specialist services that they require, and administrative inefficiencies within those services. Respondents suggested that improved communication with orthotists, a smoother care pathway and increased continuity of care would enhance their experience of receiving care. Our results indicate that commercial companies producing orthoses offer an attractive option to some people seeking the most technologically advanced products, but information and advice from these sources may be dominated by commercial interests.
Health-care professionals are faced with multiple and complex challenges in caring for patients with a range of neuromuscular and CNS conditions who require orthoses for knee instability. Assessment and measurement of the physical and psychological impact of orthosis use on patients’ quality of life require appropriate, psychometrically valid measurement tools, which may not be currently available or are not in use.
To date there is scant evidence about the views and experiences of people who are given orthoses for knee instability, and other, larger studies are required to investigate further some of the issues raised in our exploratory study. Our study provides preliminary evidence concerning acceptability and use of orthoses for knee instability, alongside patients’ views of desired treatment outcomes, useful information for consideration in the design of any future trial of orthoses.
Summary
There are a number of challenges for any future studies assessing the clinical effectiveness and cost-effectiveness of different types of orthotic management of the knee in NMD and CNS conditions. The challenge in defining the target population, the personalisation of treatment including customisation of devices, the relative rarity of the problem within individual conditions and the potential difficulty in generating generalisable findings suggest that a RCT, although the most robust approach, may not be the most feasible approach to improving knowledge about effectiveness and cost-effectiveness. High-quality observational studies may provide a compromise between robustness and feasibility, and a bridge to designing future trials.
Chapter 9 Conclusions
Implications for service provision
-
Given the paucity of evidence it is not appropriate to make conclusions about the effectiveness of specific orthotic devices for knee instability that is related to NMD or CNS disorders.
-
It is clear from the perspective of orthoses users that these devices can play a crucial role in maintaining, promoting and enhancing physical and psychological health and well-being, as well as enabling participation in paid employment, supporting family, and involvement in social and community activities. Based on the interviews with patients and the survey of HCPs, services are delivered in diverse ways; it is suggested that better understanding of models of delivery that ensure maximum benefit for patients and best value for money would be beneficial, in particular a model of delivery that permits closer integration of orthotics services with other NHS services.
-
Use of a core set of patient-reported outcome measures in the clinical setting would facilitate assessment of the impact of any change in device or management strategy on individual patients, and would also facilitate audit.
Suggested research priorities
-
There is a large gap in the evidence on the effectiveness of KAFOs and AFOs for managing knee instability related to NMD and CNS conditions and using outcome measures relevant to patient’s everyday lives. Robust research addressing this gap is required. RCTs are the most robust way of assessing effectiveness; however, the feasibility of a trial to assess the effectiveness of these devices for knee instability in these populations would need to be carefully considered as well as to whom the findings would be generalisable. It is suggested that any future trial be informed by a feasibility study.
A more feasible strategy for future research may be, in the first instance, to create a national registry for this population to systematically collect data on the ambulatory problem; devices provided; key elements of management of the instability; factors that inform/determine the process of matching patients to orthotic devices; collection of a core set of standardised and validated patient-reported outcome measures; and device and resource use. Although registries do have limitations, this would be a major step from the current evidence base in rigour and generalisability, and would create a population database and an infrastructure from which future RCTs could be undertaken. Any future research regardless of study design should follow reporting standards: www.equator-network.org/ (accessed 12 July 2015).
-
The impact of knee instability on individuals needs to be studied in the context of other aspects of neuromuscular and CNS conditions that affect the structure and function of the lower limb, for example ankle instability or muscle spasticity.
-
Development of a core set of outcome measures would be beneficial [www.comet-initiative.org (accessed 12 July 2015)]. Reduction in pain, falls and trips, and improved balance and stability, as well as participation in paid employment, outdoor activities (such as gardening), family visits and social events were identified as important to patients. Consideration will need to be given to the fact that lower limb orthoses use covers a wide range of different conditions and whether or not some consideration needs to be given to whether or not this is too broad for a shared core outcome set. If undertaken by specific conditions there is a risk that development could be piecemeal. This process could also be informed from progress in identification of suitable instruments to measure effectiveness in other related areas, such as chronic pain; falls (osteoporosis) and self-management.
Following consensus on the core outcomes that are relevant, consensus will also be required as to the appropriate measurement instruments, drawing on methods developed as part of the COSMIN initiative [COnsensus-based Standards for the selection of health Measurement Instruments: www.cosmin.nl/COSMIN.html (accessed 12 July 2015)]. These could also be considered for use in the clinical setting to address the gap identified in the survey in the use of standardised outcome measures in the clinical setting.
-
To date there is scant evidence about the views and experiences of people who are given orthoses for knee instability, and other larger studies are required to investigate further some of the issues that were raised in our exploratory study.
-
Although our research did not set out to explore service provision, the interviews with patients identified that, as well as being concerned with having a device that works for them, aspects of service delivery are an important concern, for example how repairs and breakages are handled, provision of shoes, holistic care and good communication between the different HCPs involved in their care. It is suggested that future research should explore different models of delivery of orthotic service for people with NMD and CNS conditions to identify best practice in terms of greatest benefit to patients and value for money.
-
Once a set of core outcome measures has been put in place, a full economic evaluation would be beneficial to assess the cost-effectiveness of these devices. These future economic evaluations would need to be explicit about the devices being compared and the study settings chosen, as these will have large implications on the quality of care and costs.
Acknowledgements
We thank all of the participants who took part in the study: the patients who took part in qualitative interviews and the clinicians who participated in the survey, focus group and telephone interviews. We also thank everyone who assisted with recruitment for the study and members of the Advisory Group for overseeing its progress.
We would specifically like to thank Ms Samantha Bunting in her role as patient representative. Additionally, thanks are due to Dr Cath Jackson, Valid Research, for undertaking some of the qualitative interviews and the coding of the qualitative data.
We would like to thank Jennifer Brown, Alexis Llewellyn, Nick Meader and Huiqin Yang for help with study selection and data extraction of studies for the systematic review, including non-English language papers. Thanks also to Claire Khouja for compiling the excluded studies table for the systematic review.
Contributions of authors
Joanne O’Connor (Research Associate, health economics) was lead for the HCP survey and costing analysis, and report writing; undertook HCP focus groups/interviews; designed and distributed survey; undertook analysis; contributed to the systematic review (study selection, data extraction, report writing); and contributed to the protocol.
Dorothy McCaughan (Research Fellow, qualitative research) was lead for the qualitative study of patient perspective and report writing; designed topic guide and patient information sheets; wrote ethics application; undertook majority of patient interviews, coding, analysis and report writing; undertook HCP focus group; and commented on drafts of survey questionnaire.
Catriona McDaid (Senior Research Fellow, health services research) was responsible for writing the protocol and had overall responsibility for coordinating and leading the project; provided advice and input to all elements of the project; and contributed to report writing.
Alison Booth (Research Fellow, systematic reviews and dissemination) was lead for dissemination and engagement and report writing; contributed to the systematic review (study selection, quality assessment and report writing); commented on drafts of the survey questionnaire; and contributed to the protocol.
Debra Fayter (Research Fellow, systematic reviews) was lead for the systematic review and report writing; undertook study selection, data extraction, quality assessment, data synthesis; and commented on drafts of survey questionnaire.
Roccio Rodriguez-Lopez (Research Fellow, information specialist) designed and undertook literature searches and the related sections in the report.
Roy Bowers (Senior Teaching Fellow, orthotics) was a member of the Advisory Group, contributed to the protocol, provided clinical and/or methodological advice throughout the project, and commented on drafts of the report.
Lisa Dyson (Research Fellow, qualitative research) was a member of the Advisory Group, contributed to the protocol, provided clinical and/or methodological advice throughout the project, and commented on drafts of the report.
Cynthia P Iglesias (Senior Research Fellow, health economics) was a member of the Advisory Group, contributed to the protocol, provided clinical and/or methodological advice throughout the project, and commented on drafts of the report.
Simon Lalor (Senior Orthotist) was a member of the Advisory Group, contributed to the protocol, provided clinical and/or methodological advice throughout the project, and commented on drafts of the report.
Rory J O’Connor (Consultant in Rehabilitation Medicine) was a member of the Advisory Group, provided clinical advice throughout the project and commented on drafts of the report.
Margaret Phillips (Consultant in Rehabilitation Medicine) was a member of the Advisory Group, contributed to the protocol, provided clinical and/or methodological advice throughout the project, and commented on drafts of the report.
Gita Ramdharry (Associate Professor, physiotherapy) was a member of the Advisory Group, contributed to the protocol, provided clinical and/or methodological advice throughout the project, and commented on drafts of the report.
Data sharing statement
Most of the data are available in the main body of the report and the appendices. Any further data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Hilton-Jones D, Freebody J, Stein J. Neuromuscular Disorders in the Adult. Oxford: Oxford University Press; 2011.
- Hebert JS. Ambulatory KAFOs: a physiatry perspective. J Prosthet Orthot 2006;18:169-74. http://dx.doi.org/10.1097/00008526-200606001-00004.
- Edelstein J, Bruckner J. Orthotics: A Comprehensive Clinical Approach. Thorofare, NJ: Slack; 2002.
- Fatone S. A review of the literature pertaining to KAFOs and HKAFOs for ambulation. J Prosthet Orthot 2006;18:137-68. http://dx.doi.org/10.1097/00008526-200606001-00003.
- Campbell JH. Linked hip-knee-ankle-foot orthoses designed for reciprocal gait. J Prosthet Orthot 2006;18:204-8. http://dx.doi.org/10.1097/00008526-200606001-00011.
- Use of Ankle Foot Orthoses Following Stroke. Best Practice Statement. Edinburgh: Healthcare Improvement Scotland; 2009.
- Michael JW. KAFOs for ambulation: an orthotist’s perspective. J Prosthet Orthot 2006;18:187-91. http://dx.doi.org/10.1097/00008526-200606001-00007.
- Medical Devices: Legal Requirements for Specific Medical Products. London: MHRA; 2014.
- Custom-made Medical Devices. London: MHRA; 2013.
- Hutton J, Hurry M. Orthotic Service in the NHS: Improving Service Provision. York: York Health Economics Consortium; 2009.
- Fully Equipped: The Provision of Equipment to Older or Disabled People by the NHS and Social Services in England and Wales. London: Audit Commission; 2000.
- Hill ME, Phillips MF. Service provision for adults with long-term disability: a review of services for adults with chronic neuromuscular conditions in the United Kingdom. Neuromuscul Disord 2006;16:107-12. http://dx.doi.org/10.1016/j.nmd.2005.11.011.
- Phillips MF, Radford K, Willis A. Ankle foot orthoses for people with Charcot Marie Tooth disease: views of users and orthotists on important aspects of use. Disabil Rehabil Assist Technol 2011;6:491-9. http://dx.doi.org/10.3109/17483107.2010.549899.
- Vinci P, Gargiulo P. Poor compliance with ankle-foot-orthoses in Charcot-Marie-Tooth disease. Eur J Phys Rehabil Med 2008;44:27-31.
- Ramdharry G, Pollard A, Marsden J, Reilly M. Comparing gait performance of people with Charcot-Marie-Tooth disease who do and do not wear ankle foot orthoses. Physiother Res Int 2012;17:191-9. http://dx.doi.org/10.1002/pri.531.
- Garralda ME, Muntoni F, Cunniff A, Caneja AD. Knee-ankle-foot orthosis in children with Duchenne muscular dystrophy: user views and adjustment. Eur J Paediatr Neurol 2006;10:186-91. http://dx.doi.org/10.1016/j.ejpn.2006.07.002.
- Chisholm AE, Perry SD. Ankle-foot orthotic management in neuromuscular disorders: recommendations for future research. Disability 2012;7:437-49. http://dx.doi.org/10.3109/17483107.2012.680940.
- Gatti MA, Sundblad M, Freixes O, Fernández SA, Olmos LE, Rubel IF. Usage follow-up after a knee-ankle-foot orthoses selection and training program in spinal cord injury patients. J Prosthet Orthot 2010;22:146-9. http://dx.doi.org/10.1097/JPO.0b013e3181e98c82.
- Edelstein JE. Ambulatory KAFOs: a physical therapist’s perspective. J Prosthet Orthot 2006;7:183-6. http://dx.doi.org/10.1097/00008526-200606001-00006.
- Genet F, Schnitzler A, Mathieu S, Autret K, Thefenne L, Dizien O, et al. Orthotic devices and gait in polio patients. Ann Phys Rehabil Med 2010;53:51-9. http://dx.doi.org/10.1016/j.rehab.2009.11.005.
- Tyson SF, Sadeghi-Demneh E, Nester CJ. A systematic review and meta-analysis of the effect of an ankle-foot orthosis on gait biomechanics after stroke. Clin Rehabil 2013;27:879-91. http://dx.doi.org/10.1177/0269215513486497.
- Young P, De Jonghe P, Stogbauer F, Butterfass-Bahloul T. Treatment for Charcot-Marie-Tooth disease. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.cd006052.pub2.
- Smith C, Kumar S, Causby R. The effectiveness of non-surgical interventions in the treatment of Charcot foot. Int J Evid Based Healthc 2007;5:437-49.
- Rose KJ, Burns J, Wheeler DM, North KN. Interventions for increasing ankle range of motion in patients with neuromuscular disease. Cochrane Database Syst Rev 2010;2. http://dx.doi.org/10.1002/14651858.cd006973.pub2.
- Sackley C, Disler PB, Turner-Stokes L, Wade DT, Brittle N, Hoppitt T. Rehabilitation interventions for foot drop in neuromuscular disease. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.cd003908.pub3.
- Zacharias B, Kannenberg A. Clinical benefits of stance control orthosis systems: an analysis of the scientific literature. J Prosthet Orthot 2012;24:2-9. http://dx.doi.org/10.1097/JPO.0b013e3182435db3.
- CRD’s Guidance for Undertaking Reviews in Health Care. York: CRD, University of York; 2009.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2535.
- Higgins J, Altman D, Sterne J, Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration; 2011.
- Valentine J, Thompson S. Issues relating to confounding and meta-analysis when including non-randomised studies in systematic reviews of the effects of interventions. Res Synth Methods 2013;4:26-35. http://dx.doi.org/10.1002/jrsm.1064.
- Higgins J, Ramsay C, Reeves B, Deeks J, Shea B, Valentine J. Issues relating to study design and risk of bias when including non-randomised studies in systematic reviews on the effects of interventions. Res Synth Methods 2013;4:12-25. http://dx.doi.org/10.1002/jrsm.1056.
- Siegfried N, Muller M, Deeks J, Volmink J, Egger M, Low N, et al. HIV and male circumcision: a systematic review with assessment of the quality of studies. Lancet Infect Dis 2005;5:165-73. http://dx.doi.org/10.1016/S1473-3099(05)70024-4.
- Llewellyn A, Norman G, Harden M, Coatesworth A, Kimberling D, Schilder A, et al. Interventions for adult Eustachian tube dysfunction: a systematic review. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18460.
- Hennink M, Hutter I, Bailey AM. Qualitative Research Methods. London: Sage; 2011.
- Green J, Thorogood N. Qualitative Methods for Health Research. London: Sage; 2014.
- Flick U. An Introduction to Qualitative Research. London: Sage; 2014.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;33:1314-23. http://dx.doi.org/10.1191/1478088706qp063oa.
- Richards L. Handling Qualitative Data: A Practical Guide. London: Sage; 2005.
- Moustakas C. Phenomenological Research Methods. Thousand Oaks, CA: Sage; 1994.
- Silverman D. Interpreting Qualitative Data: Methods for Analysing Talk, Text and Interaction. London: Sage; 2006.
- Cho I, Johnson T, Vangeest JB. Enhancing surveys of health care professionals: a meta-analysis of techniques to improve response. Eval Health Prof 2013;36:382-407. http://dx.doi.org/10.1177/0163278713496425.
- Braithwaite D, Emery J, de Lusignan S, Sutton S. Using the internet to conduct surveys of health professionals: a valid alternative?. Fam Pract Manag 2003;20:545-51. http://dx.doi.org/10.1093/fampra/cmg509.
- Czaja R, Blair J. Designing Surveys: A Guide to Decisions and Procedures. London: Sage; 2005.
- Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES). J Med Internet Res 2004;6. http://dx.doi.org/10.2196/jmir.6.3.e34.
- Bernhardt K, Oh T, Kaufman K. Stance control orthosis trial in patients with inclusion body myositis. Prosthet Orthot Int 2011;35:39-44. http://dx.doi.org/10.1177/0309364610389352.
- Bocker B, Hölig C, Smolenski UC. Orthosis management in patients after poliomyelitis anterior acuta. J Rehabil Med 2011;49.
- Bocker B, Hoelig C, Smolenski UC. Orthosis management in patients after poliomyelitis anterior acuta. Phy Med Rehab Kuror 2013;23:16-21. http://dx.doi.org/10.1055/s-0032-1331218.
- Boudarham J, Zory R, Genet F, Vigne G, Bensmail D, Roche N, et al. Effects of a knee-ankle-foot orthosis on gait biomechanical characteristics of paretic and non-paretic limbs in hemiplegic patients with genu recurvatum. Clin Biomech 2013;28:73-8. http://dx.doi.org/10.1016/j.clinbiomech.2012.09.007.
- Brehm MA, Beelen A, Doorenbosch CA, Harlaar J, Nollet F. Effect of carbon-composite knee-ankle-foot orthoses on walking efficiency and gait in former polio patients. J Rehabil Med 2007;39:651-7. http://dx.doi.org/10.2340/16501977-0110.
- Davis PC, Bach TM, Pereira DM. The effect of stance control orthoses on gait characteristics and energy expenditure in knee-ankle-foot orthosis users. Prosthet Orthot Int 2010;34:206-15. http://dx.doi.org/10.3109/03093641003773189.
- Hachisuka K, Makino K, Wada F, Saeki S, Yoshimoto N. Oxygen consumption, oxygen cost and physiological cost index in polio survivors: a comparison of walking without orthosis, with an ordinary or a carbon-fibre reinforced plastic knee-ankle-foot orthosis. J Rehabil Med 2007;39:646-50. http://dx.doi.org/10.2340/16501977-0105.
- Hachisuka K, Makino K, Wada F, Saeki S, Yoshimoto N, Arai M. Clinical application of carbon fibre reinforced plastic leg orthosis for polio survivors and its advantages and disadvantages. Prosthet Orthot Int 2006;30:129-35. http://dx.doi.org/10.1080/03093640600574474.
- Harvey LA, Newton-John T, Davis GM, Smith MB, Engel S. A comparison of the attitude of paraplegic individuals to the Walkabout Orthosis and the Isocentric Reciprocal Gait Orthosis. Spinal Cord 1997;35:580-4. http://dx.doi.org/10.1038/sj.sc.3100470.
- Harvey LA, Smith MB, Davis GM, Engel S. Functional outcomes attained by T9-12 paraplegic patients with the walkabout and the isocentric reciprocal gait orthoses. Arch Phys Med Rehabil 1997;78:706-11. http://dx.doi.org/10.1016/S0003-9993(97)90077-0.
- Harvey LA, Davis GM, Smith MB, Engel S. Energy expenditure during gait using the walkabout and isocentric reciprocal gait orthoses in persons with paraplegia. Arch Phys Med Rehabil 1998;79:945-9. http://dx.doi.org/10.1016/S0003-9993(98)90092-2.
- Heim M, Yaacobi E, Azaria M. A pilot study to determine the efficiency of lightweight carbon fibre orthoses in the management of patients suffering from post-poliomyelitis syndrome. Clin Rehabil 1997;11:302-5. http://dx.doi.org/10.1177/026921559701100406.
- Jaspers P, Peeraer L, Van Petegem W, Van der Perre G. The use of an advanced reciprocating gait orthosis by paraplegic individuals: a follow-up study. Spinal Cord 1997;35:585-9. http://dx.doi.org/10.1038/sj.sc.3100462.
- Kakurai S, Akai M. Clinical experiences with a convertible thermoplastic knee-ankle-foot orthosis for post-stroke hemiplegic patients. Prosthet Orthot Int 1996;20:191-4.
- Middleton JW, Yeo JD, Blanch L, Vare V, Peterson K, Brigden K. Clinical evaluation of a new orthosis, the ‘walkabout’, for restoration of functional standing and short distance mobility in spinal paralysed individuals. Spinal Cord 1997;35:574-9. http://dx.doi.org/10.1038/sj.sc.3100459.
- Morinaka Y, Matsuo Y, Nojima M, Morinaka S. Clinical evaluation of a knee-ankle-foot-orthosis for hemiplegic patients. Prosthet Orthot Int 1982;6:111-15.
- Peethambaran A. The relationship between performance, satisfaction, and well being for patients using anterior and posterior design knee-ankle-foot-orthosis. J Prosthet Orthot 2000;12:33-40. http://dx.doi.org/10.1097/00008526-200012010-00008.
- Scivoletto G, Petrelli A, Lucente LD, Giannantoni A, Fuoco U, D’Ambrosio F, et al. One year follow up of spinal cord injury patients using a reciprocating gait orthosis: preliminary report. Spinal Cord 2000;38:555-8. http://dx.doi.org/10.1038/sj.sc.3101047.
- Steinfeldt F, Seifert W, Gunther KP. Modern carbon fibre orthoses in the management of polio patients: a critical evaluation of the functional aspects. Z Orthop Ihre Grenzgeb 2003;141:357-61.
- Summers BN, McClelland MR, el Masri WS. A clinical review of the adult hip guidance orthosis (Para Walker) in traumatic paraplegics. Paraplegia 1988;26:19-26. http://dx.doi.org/10.1038/sc.1988.6.
- Sun JL, Tang D, Ouyang YT, Zhong SZ. Influence of reciprocating gait orthosis on walking function in paraplegic patients after ambulation. J Clin Rehabil Tissue Engineering Res 2007;11:2437-40.
- Tang D, Pei G, Li K. The effects of alternative gait orthosis on activity of daily living and quality of life in patients with spinal cord injury. Chinese J Rehabil Med 2009;24:985-8.
- Whittle MW, Cochrane GM, Chase AP, Copping AV, Jefferson RJ, Staples DJ, et al. A comparative trial of two walking systems for paralysed people. Paraplegia 1991;29:97-102. http://dx.doi.org/10.1038/sc.1991.13.
- Wu JX, Zhou XL, Liu HL, Yin Q, Cong L, Feng L, et al. Effect of the new reciprocating gait orthosis (Walkabout orthosis) in improving paraplegic patients’ independent living ability. Chinese J Clin Rehabil 2003;7:2469-70.
- Yang JL, Xu YL, Wei Y, Sun BJ, Ke YY, Zhan SS. Effects of lower limb orthosis therapy on the recovery of motor function in the post-stroke hemiplegic patients. Chinese J Clin Rehabil 2005;9:6-7.
- Reference Costs 2013–14. London: Department of Health; 2015.
- Simoens S, Debruyne H, Moldenaers I, Guillaume P, De Coster S, Van den Steen D, et al. Do tariffs and prices correspond with costs? A case study of orthotic braces. J Med Econ 2008;11:245-54.
- Fisher LR, McLellan DL. Questionnaire assessment of patient satisfaction with lower limb orthoses from a district hospital. J Prosthet Orthot 1989;13:29-35.
- High Quality Care for All: NHS Next Stage Review Final Report. London: DH; 2008.
- Schaffalitzky E, Gallagher P, Maclachlan M, Ryall N. Understanding the benefits of prosthetic prescription: exploring the experiences of practitioners and lower limb prosthetic users. Disabil Rehabil 2011;33:1314-23. http://dx.doi.org/10.3109/09638288.2010.529234.
- Williams A, Nester C, Ravey M. Rheumatoid arthritis patients’ experiences of wearing therapeutic footwear: a qualitative investigation. BMC Musculoskelet Disord 2007;8. http://dx.doi.org/10.1186/1471-2474-8-104.
- Bulley C, Shiels J, Wilkie K, Salisbury L. User experiences, preferences and choices relating to functional electric stimulation and ankle foot orthoses for foot drop after stroke. Physiotherapy 2011;97:226-33. http://dx.doi.org/10.1016/j.physio.2010.11.001.
- Souza A, Kelleher A, Cooper R, Cooper R, Iezzoni L, Collins D. Multiple sclerosis and mobility-related assistive technology: systematic review of the literature. J Rehabil Res Dev 2010;47:213-24. http://dx.doi.org/10.1682/JRRD.2009.07.0096.
- Iglesias C. Does assessing the value for money of therapeutic medical devices require a flexible approach. Pharmacoecon Outcomes Res 2015;15:21-32. http://dx.doi.org/10.1586/14737167.2015.982098.
- Bettoni E, Ferriero G, Bakhsh H, Bravini E, Massazza G, Franchignoni F. A systematic review of questionnaires to assess patient satisfaction with limb orthoses. Prosthet Orthot Int 2016;40:158-69. http://dx.doi.org/10.1177/0309364614556836.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1655.
- Brehm M, Bus SA, Harlaar J, Nollet F. A candidate core set of outcome measures based on the International Classification of Functioning, Disability and Health for clinical studies on lower limb orthoses. J Prosthet Orthot 2011;35:269-77. http://dx.doi.org/10.1177/0309364611413496.
- ClinicalTrials.gov . The Use of Brace to Retrain Hemiparetic Gait n.d. https://clinicaltrials.gov/ct2/show/study/NCT02082938 (accessed 23 May 2016).
- Frechtel A, Portnoy S, Raveh E, Schwartz I. Prevention of knee hyperextension in stroke patients using a knee orthosis: 3D computational gait analysis and dynamic EMG. Gait Posture 2013;38. http://dx.doi.org/10.1016/j.gaitpost.2013.07.178.
- Kannenberg A, Probsting E. An orthotronic mobility system improves perceived walking capabilities in traditional leg orthosis users. PM R 2014;1:S229-30. http://dx.doi.org/10.1016/j.pmrj.2014.08.525.
Appendix 1 Search strategies for the systematic review
Ovid MEDLINE® In-Process & Other Non-Indexed Citations and Ovid MEDLINE®
Date range searched: 1946 to 21 May 2014.
Date of search: 22 May 2014.
Search strategy
-
Orthotic Devices/ or Braces/ or Splints/ (16,320)
-
Gait/ (17,744)
-
Lower Extremity/ or Leg/ (61,929)
-
Hip/ or Hip Joint/ (28,943)
-
Knee/ or exp Knee Joint/ (51,355)
-
Ankle/ or Ankle Joint/ (16,707)
-
Foot/ or Foot Joints/ (20,388)
-
1 and (2 or 3 or 4 or 5 or 6 or 7) (2732)
-
Foot Orthoses/ (145)
-
8 or 9 (2870)
-
((gait or “lower extremity” or “lower extremities” or “lower limb” or “lower limbs” or leg? or hip? or knee? or ankle? or foot or feet) adj3 (orthos* or orthot* or brace? or bracing or support)).ti,ab. (3590)
-
(heel adj2 (pad? or raise?)).ti,ab. (365)
-
((shoe? and (modification? or insert? or “negative heel” or “negative heels”)) or (rocker? or insole?)).ti,ab. (1507)
-
((HKAFO? or KAFO? or SCKAFO? or AFO? or GRAFO? or RGO? or SWASH? or DAFO? or SAFO?) and (orthos* or orthot* or brace? or bracing)).ti,ab. (387)
-
(SMART? and walker).ti,ab. (10)
-
11 or 12 or 13 or 14 or 15 (5269)
-
10 or 16 (6735)
-
exp Knee Joint/ or Knee/ (51,355)
-
knee?.af. (11,4312)
-
18 or 19 (115,529)
-
17 and 20 (2085)
EMBASE
Date range searched: 1974 to 21 May 2014.
Date of search: 22 May 2014.
Search strategy
-
orthotics/ or brace/ or exp splint/ (16,472)
-
gait/ (27,502)
-
leg/ or leg muscle/ or lower leg/ or thigh/ (76,123)
-
hip/ (32,702)
-
knee/ (41,879)
-
ankle/ (19,516)
-
Foot/ (17,901)
-
1 and (2 or 3 or 4 or 5 or 6 or 7) (1987)
-
exp foot orthosis/ or knee brace/ or leg brace/ (1097)
-
8 or 9 (3017)
-
((gait or “lower extremity” or “lower extremities” or “lower limb” or “lower limbs” or leg? or hip? or knee? or ankle? or foot or feet) adj3 (orthos* or orthot* or brace? or bracing or support)).ti,ab. (4462)
-
(heel adj2 (pad? or raise?)).ti,ab. (410)
-
((shoe? and (modification? or insert? or “negative heel” or “negative heels”)) or (rocker? or insole?)).ti,ab. (1888)
-
((HKAFO? or KAFO? or SCKAFO? or AFO? or GRAFO? or RGO? or SWASH? or DAFO? or SAFO?) and (orthos* or orthot* or brace? or bracing)).ti,ab. (506)
-
(SMART? and walker).ti,ab. (15)
-
11 or 12 or 13 or 14 or 15 (6531)
-
10 or 16 (8134)
-
knee instability/ or knee/ (44,382)
-
knee?.af. (145,743)
-
18 or 19 (145,743)
-
17 and 20 (2436)
Cumulative Index to Nursing and Allied Health Literature Plus
Date of search: 22 May 2014.
Search strategy
S1 (MH “Orthoses”) OR (MH “Orthoses Design”) OR (MH “Orthoses Fitting”) OR (MH “Slings”) OR (MH “Splints”) (6577)
S2 (MH “Gait+”) (5519)
S3 (MH “Lower Extremity”) OR (MH “Leg”) OR (MH “Thigh”) (12,585)
S4 (MH “Hip”) OR (MH “Hip Joint”) (8336)
S5 (MH “Knee”) OR (MH “Knee Joint”) (14,318)
S6 (MH “Ankle”) OR (MH “Ankle Joint”) (6148)
S7 (MH “Foot”) OR (MH “Heel”) OR (MH “Toes”) (8605)
S8 S1 AND (S2 or S3 or S4 or S5 or S6 or S7) (1315)
S9 (MH “Foot Orthoses”) OR (MH “Reciprocating Gait Orthoses”) (1615)
S10 S8 OR S9 (2834)
S11 TX (gait OR “lower extremity” OR “lower extremities” OR “lower limb” OR “lower limbs” OR leg? OR hip? OR knee? OR ankle? OR foot OR feet) N3 (orthos* OR orthot* OR brace? OR bracing (2751)
S12 TX (heel N2 (pad? OR raise?)) (51)
S13 TX ((shoe? AND (modification? OR insert? OR “negative heel” OR “negative heels”)) OR (rocker? OR insole?)) (448)
S14 TX ((HKAFO? OR KAFO? OR SCKAFO? OR AFO? OR GRAFO? OR RGO? OR SWASH? OR DAFO? OR SAFO?) AND (orthos* OR orthot* OR brace? OR bracing)) (127)
S15 TX SMART? AND walker (0)
S16 S11 or S12 or S13 or S14 or S15 (3084)
S17 S10 or S16 (3892)
S18 (MH “Knee”) OR (MH “Knee Joint”) (14,318)
S19 TX knee? OR MW knee? (3121)
S20 S18 or S19 (16,111)
S21 S17 AND S20 (534)
The Cochrane Library (includes Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, Health Technology Assessment and NHS Economic Evaluation Database)
Date of search: 22 May 2014.
Search strategy
#1 MeSH descriptor: [Orthotic Devices] this term only (521)
#2 MeSH descriptor: [Braces] this term only (326)
#3 MeSH descriptor: [Splints] this term only (377)
#4 (#1 or #2 or #3) (1192)
#5 MeSH descriptor: [Lower Extremity] this term only (629)
#6 MeSH descriptor: [Leg] this term only (2593)
#7 MeSH descriptor: [Hip] this term only (296)
#8 MeSH descriptor: [Hip Joint] this term only (814)
#9 MeSH descriptor: [Knee] this term only (573)
#10 MeSH descriptor: [Knee Joint] explode all trees (2304)
#11 MeSH descriptor: [Ankle] this term only (355)
#12 MeSH descriptor: [Ankle Joint] this term only (463)
#13 MeSH descriptor: [Foot] explode all trees (1173)
#14 MeSH descriptor: [Foot Joints] this term only (31)
#15 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 (8061)
#16 #4 and #15 (288)
#17 MeSH descriptor: [Foot Orthoses] this term only (25)
#18 #16 or #17 (311)
#19 ((gait or “lower extremity” or “lower extremities” or “lower limb” or “lower limbs” or leg? or hip? or knee? or ankle? or foot or feet) near/3 (orthos* or orthot* or brace? or bracing or support)):ti,ab (379)
#20 (heel near/2 (pad? or raise?)):ti,ab (16)
#21 ((shoe? and (modification? or insert? or “negative heel” or “negative heels”)) or (rocker? or insole?)):ti,ab (156)
#22 ((HKAFO? or KAFO? or SCKAFO? or AFO? or GRAFO? or RGO? or SWASH? or DAFO? or SAFO?) and (orthos* or orthot* or brace? or bracing)):ti,ab (22)
#23 (SMART? and walker):ti,ab (0)
#24 #19 or #20 or #21 or #22 or #23 (522)
#25 #18 or #24 (700)
#26 MeSH descriptor: [Knee Joint] explode all trees (2304)
#27 MeSH descriptor: [Knee] this term only (573)
#28 knee?:ti,ab,kw (973)
#29 #26 or #27 or #28 (3445)
#30 #25 and #29 (104)
Of 104 total results in The Cochrane Library, 2 were from CDSR, 7 from DARE, 94 from CENTRAL, 1 from HTA and 0 from NHS EED.
PASCAL
Date of search: 22 May 2014.
Search strategy
S1 ti(((gait or “lower extremity” or “lower extremities” or “lower limb” or “lower limbs” or leg? or hip? or knee? or ankle? or foot or feet) NEAR/3 (orthos* or orthot* or brace? or bracing or support))) OR ab(((gait or “lower extremity” or “lower extremities” or “lower limb” or “lower limbs” or leg? or hip? or knee? or ankle? or foot or feet) NEAR/3 (orthos* or orthot* or brace? or bracing or support))) (696)
S2 ti((heel NEAR/2 (pad? or raise?))) OR ab((heel NEAR/2 (pad? Or raise?))) (30)
S3 ti(((shoe? and (modification? or insert? or “negative heel” or “negative heels”)) or (rocker? or insole?))) OR ab(((shoe? And (modification? or insert? or “negative heel” or “negative heels”)) or (rocker? or insole?))) (175)
S4 ti(((HKAFO? or KAFO? or SCKAFO? or AFO? or GRAFO? or RGO? Or SWASH? or DAFO? or SAFO?) and (orthos* or orthot* or brace? Or bracing))) OR ab(((HKAFO? or KAFO? or SCKAFO? or AFO? or GRAFO? or RGO? or SWASH? or DAFO? or SAFO?) and (orthos* or orthot* or brace? or bracing))) (36)
S5 ti((SMART? and walker)) OR ab((SMART? and walker)) (0)
S6 S1 OR S2 OR S3 OR S4 OR S5 (883)
S7 *knee* (36,128)
S8 S6 AND S7 (178)
Scopus
Date of search: 23 May 2014.
Search strategy
((TITLE-ABS-KEY(((gait OR “lower extremity” OR “lower extremities” OR “lower limb” OR “lower limbs” OR leg? OR hip? OR knee? OR ankle? OR foot OR feet) W/3 (orthos* OR orthot* OR brace? OR bracing OR support)))) OR (TITLEABS- KEY(heel W/2 (pad? OR raise?))) OR (TITLE-ABS-KEY((shoe? AND (modification? OR insert? OR “negative heel” OR “negative heels”)) OR (rocker? OR insole?))) OR (TITLE-ABS-KEY(((hkafo? OR kafo? OR sckafo? OR afo? OR grafo? OR rgo? OR swash? OR dafo? OR safo?) AND (orthos* OR orthot* OR brace? OR bracing)))) OR (TITLE-ABS-KEY(smart? AND walker))) AND (TITLE-ABS-KEY(*knee*)) (1190)
Science Citation Index
Date of search 22 May 2014.
Search strategy
# 8 #7 AND #6 (914)
# 7 TOPIC: (*knee*) (96,659)
# 6 #5 OR #4 OR #3 OR #2 OR #1 (3851)
# 5 TOPIC: (((SMART? and walker))) (0)
# 4 TOPIC: ((((HKAFO? or KAFO? or SCKAFO? or AFO? or GRAFO? or RGO? or SWASH? or DAFO? or SAFO?) and (orthos* or orthot* or brace? or bracing)))) (127)
# 3 TOPIC: ((((shoe? and (modification? or insert? or “negative heel” or “negative heels”)) or (rocker? or insole?)))) (743)
# 2 TOPIC: (((heel NEAR/2 (pad? or raise?)))) (109)
# 1 TOPIC: ((((gait or “lower extremity” or “lower extremities” or “lower limb” or “lower limbs” or leg? or hip? or knee? or ankle? or foot or feet) NEAR/3 (orthos* or orthot* or brace? Or bracing or support)))) (3151)
Bioscience Information Service Previews
Date of search: 22 May 2014.
Search strategy
# 8 #7 AND #6 (306)
# 7 TOPIC: (*knee*) (39,826)
# 6 #5 OR #4 OR #3 OR #2 OR #1 (1579)
# 5 TOPIC: (((SMART? and walker))) (0)
# 4 TOPIC: ((((HKAFO? or KAFO? or SCKAFO? or AFO? or GRAFO? or RGO? or SWASH? or DAFO? or SAFO?) and (orthos* or orthot* or brace? or bracing)))) (28)
# 3 TOPIC: ((((shoe? and (modification? or insert? or “negative heel” or “negative heels”)) or (rocker? Or insole?)))) (183)
# 2 TOPIC: (((heel NEAR/2 (pad? or raise?)))) (53)
# 1 TOPIC: ((((gait or “lower extremity” or “lower extremities” or “lower limb” or “lower limbs” or leg? Or hip? or knee? or ankle? or foot or feet) NEAR/3 (orthos* or orthot* or brace? or bracing or support)))) (1366)
Health Management Information Consortium
Date range searched: 1979 to March 2014.
Date of search: 23 May 2014.
Search strategy
-
orthotic devices/ or braces/ or splints/ (57)
-
lower limbs/ or legs/ (113)
-
hip joints/ (141)
-
knees/ or knee joints/ (59)
-
ankles/ or ankle joints/ (9)
-
feet/ or heels/ or toes/ (37)
-
1 and (2 or 3 or 4 or 5 or 6) (5)
-
((gait or “lower extremity” or “lower extremities” or “lower limb” or “lower limbs” or leg? or hip? or knee? or ankle? or foot or feet) adj3 (orthos* or orthot* or brace? or bracing or support)).ti,ab. (12)
-
(heel adj2 (pad? or raise?)).ti,ab. (1)
-
((shoe? and (modification? or insert? or “negative heel” or “negative heels”)) or (rocker? or insole?)).ti,ab. (1)
-
((HKAFO? or KAFO? or SCKAFO? or AFO? or GRAFO? or RGO? or SWASH? or DAFO? or SAFO?) and (orthos* or orthot* or brace? or bracing)).ti,ab. (1)
-
(SMART? and walker).ti,ab. (0)
-
8 or 9 or 10 or 11 or 12 (14)
-
7 or 13 (15)
-
knees/ or knee joints/ (59)
-
knee?.af. (331)
-
15 or 16 (331)
-
14 and 17 (4)
Physiotherapy Evidence Database
Date of search: 22 May 2014.
Search strategy
Knee* ortho* (32)
Knee* brace* (33)
RECAL Legacy
Date of search: 22 May 2014.
Knee Instability Orthoses in Descriptors (41)
Conference Proceedings Citation Index – Science
Date of search: 23 May 2014.
Search strategy
#8 #7 AND #6 (164)
#7 TOPIC: ((*knee*)) (11,585)
#6 #5 OR #4 OR #3 OR #2 OR #1 (936)
#5 TOPIC: (((((shoe? and (modification? or insert? or “negative heel” or “negative heels”)) or (rocker? or insole?))))) (122)
#4 TOPIC: ((((SMART? and walker)))) (0)
#3 TOPIC: (((((HKAFO? or KAFO? or SCKAFO? or AFO? or GRAFO? or RGO? or SWASH? or DAFO? or SAFO?) and (orthos* or orthot* or brace? or bracing))))) (28)
#2 TOPIC: ((((heel NEAR/2 (pad? or raise?))))) (6)
# 1 TOPIC: ((((gait or “lower extremity” or “lower extremities” or “lower limb” or “lower limbs” or leg? Or hip? or knee? or ankle? or foot or feet) NEAR/3 (orthos* or orthot* or brace? or bracing or support)))) (918)
ClinicalTrials.gov
Date of search: 23 May 2014.
Search strategy
(HKAFO OR KAFO OR SCKAFO OR AFO OR GRAFO OR RGO OR SWASH OR DAFO OR SAFO) AND (orthoses OR orthosis OR orthotic OR brace OR bracing) (18)
(gait OR “lower extremity” OR “lower extremities” OR “lower limb” OR “lower limbs” OR leg OR hip OR knee OR ankle OR foot OR feet) AND (orthoses OR orthosis OR orthotic OR brace OR bracing) (158)
International Clinical Trials Registry Platform Portal – World Health Organization
Date of search: 23 May 2014.
Search strategy
knee and ((stability or inestability) AND (orthosis OR orthoses OR orthotic)) (192)
National Technical Information Service
Date of search: 23 May 2014.
Search strategy
Knee AND (orthosis orthoses orthotic) (2)
leg AND (orthosis orthoses orthotic) (10)
ankle AND (orthosis orthoses orthotic) (1)
Appendix 2 Quality assessment criteria
Randomised controlled trials
Possible answers for each criterion were low risk of bias, high risk of bias or unclear risk of bias. Cochrane criteria for judgement of low, high and unclear risk of bias were used. 29
-
Selection bias:
-
owing to inadequate generation of a randomised sequence
-
owing to inadequate concealment of allocations prior to assignment.
-
-
Performance bias:
-
owing to participants’ knowledge of the allocated intervention during the study
-
owing to personnels’ knowledge of the allocated intervention during the study.
-
-
Detection bias:
-
owing to knowledge of the allocated interventions by outcome assessors (for HCP assessed outcomes)
-
-
Attrition bias:
-
owing to number, nature or handling of incomplete outcome data.
-
-
Selective outcome reporting:
-
reporting bias due to selective outcome reporting.
-
-
Other bias:
-
bias due to problems not covered elsewhere.
-
Non-randomised controlled studies
-
External validity:
-
Were the selection criteria adequately reported? (yes/no)
-
Is the sample likely to be representative? (‘yes’ if the sample included all eligible patients with a NMD or CNS condition requiring on orthotic over a defined period of time, such as a consecutive sample or a random or systematic sample of that population/‘no’ if none of above/‘unclear’ if not reported)
-
Was the participation rate adequate? (‘yes’ if percentage participation was ≥ 80%/‘no’ if < 80%; ‘unclear’ if not reported)
-
-
Performance bias:
-
Were there differences in the care received by the two groups? (yes/no/unclear)
-
-
Detection bias:
-
Was there independent outcome assessment (for HCP-assessed outcomes)? (yes/‘no’ if not explicitly stated)
-
-
Attrition bias:
-
Completeness of outcome assessment (‘yes’ if ≥ 80% of participants were included in the final analysis; no if < 80%; unclear if not reported)
-
-
Selection bias/control of confounding (‘yes’ if the group variable was balanced between groups (≤ 10% difference) or adjusted for in analysis):
-
gender
-
age
-
cause of muscle weakness
-
presence of sensory disturbance
-
purpose of orthotic (proximal/distal muscle weakness)
-
previous use of orthotic device
-
acclimatisation time
-
type of orthotic device used.
-
Uncontrolled studies
-
Were the selection criteria adequately reported? (yes/no)
-
Is the sample likely to be representative? (‘yes’ if the sample included all eligible patients with a NMD or CNS condition requiring on orthosis over a defined period of time, such as a consecutive sample or a random or systematic sample of that population/‘no’ if none of the above/‘unclear’ if not reported)
-
Was the participation rate adequate? (‘yes’ if percentage participation was ≥ 80%/’no’ if < 80%/’unclear’ if not reported)
-
Was recruitment prospective? (yes/no/unclear)
-
Was there independent outcome assessment (for HCP-assessed outcomes)? (yes/’no’ if not explicitly stated)
-
Completeness of outcome assessment (‘yes’ if ≥ 80% of participants were included in the final analysis/’no’ if < 80%/’unclear’ if not reported)
-
Were relevant prognostic factors reported? (‘yes’ for all or some of cause of muscle weakness, presence of sensory disturbance, whether or not the orthosis was used for proximal or distal muscle weakness, previous use of an orthosis, acclimatisation time, other relevant)
-
Were other relevant confounding factors reported, such as cointerventions? (yes/no)
-
Was an appropriate measure of variability reported? (yes/no/‘partial’ if reported for some outcomes)
-
Were there any other important limitations?
Appendix 3 Invitation e-mail for health-care professional focus groups/telephone interviews
Do you fit orthotic devices to patients with instability of the knee?
If so, you could help us find out more about what types of devices are being used and what is involved in this process. You are invited to take part in a focus group where you will be asked about your experience, practice and preferences in orthotic devices for the treatment of patients with knee instability due to NMDs or CNS disorders.
There will be between six and eight people in the focus group and two researchers will guide the discussion. We are interested in finding out about the types of orthotic devices being fitted in these patients, and the resources required to provide this service within the NHS.
The focus group will be held on [day date time] in the [location]. The session will last approximately 90 minutes and refreshments will be provided.
This focus group is one element of a larger project with involves researchers and health professionals from the University of York, Nottingham University, University of Strathclyde, Kingston University and Queen Mary’s Hospital Roehampton. The research team is based at the University of York. The study has research governance approval from the University of York Health Sciences Research Governance Committee.
If you are interested in taking part or would like to know more please contact [researcher contact details].
Appendix 4 Participant information sheet for health-care professional focus groups/telephone interviews
Appendix 5 Consent form for health-care professional focus groups/telephone interviews
Appendix 6 Patient interview and orthotist telephone interview topic guides
-
Researcher to ask brief details about age, occupation, ethnicity, family member, etc. to frame and contextualise the interview.
-
Can you tell me a little bit about yourself and how you came to have your orthotic device(s) (such as brace or callipers)? [explore important aspects further, such as diagnosis, referral, care pathway, length of time having an orthosis]
-
How has the orthotic device impacted on your daily life?
-
[from answer to above question] – What factors have affected you most and why? [explore important aspects further]
-
What do you like, or not like, about your orthotic device? Which factors influence your decisions to use your orthotic device and why are they important?
-
How effective do you think your device is in the management of knee instability?
-
How do you feel about the treatment you have received in connection with your knee instability? How could this have been improved?
-
What are the goals of treatment that matter most to you?
-
How do you feel about the interactions you have had with HCPs? How could these have been improved?
-
Any other aspects that have not already been discussed or you would like to expand upon?


Orthotics for knee instability
Topic guide for telephone interviews with orthotists
For the purposes of our analysis, we are interested only in:
-
the costs associated with providing KAFOs to adult patients with NMD or CNS disorders
-
the costs incurred by the NHS only.
Below is a list of all the information that we would like to collate. We understand that this is a very large quantity of information and some aspects of the list are quite detailed. If you cannot answer a particular question – please let us know the reason. This would be just as helpful as filling in an answer.
1 MATERIALS
In this section, the materials required to manufacture/shape and capture conventional, cosmetic and hybrid KAFOs will be discussed.
Components of a conventional KAFO
| Thigh section | Knee joint (including up-rights) | Calf section | Ankle joint | Foot section | Other elements | |
|---|---|---|---|---|---|---|
| Metal | ||||||
| Leather | ||||||
| Other |
Components of a cosmetic KAFO
| Thigh section | Knee joint (including up-rights) | Calf section | Ankle joint | Foot section | Other elements | |
|---|---|---|---|---|---|---|
| Thermoplastic | ||||||
| Carbon fibre | ||||||
| Other |
Components of a hybrid KAFO
| Thigh section | Knee joint (including up-rights) | Calf section | Ankle joint | Foot section | Other elements | |
|---|---|---|---|---|---|---|
| Metal | ||||||
| Leather | ||||||
| Thermoplastic | ||||||
| Carbon fibre | ||||||
| Aluminium | ||||||
| Titanium | ||||||
| Steel | ||||||
| Fabric | ||||||
| Other |
Components used for shape capture
| Thigh section | Knee joint (including up-rights) | Calf section | Ankle joint | Foot section | Other elements | |
|---|---|---|---|---|---|---|
| Computer scanner | ||||||
| Plaster | ||||||
| Synthetic casting tape |
-
Are there other materials required in the manufacturer/fitting/prescription of KAFOs?
-
What decides the quantity of these materials that are used?
| Thigh section | Knee joint (including up-rights) | Calf section | Ankle joint | Foot section | Other elements | |
|---|---|---|---|---|---|---|
| Health condition (i.e. disease) | ||||||
| Primary indication (e.g. support, align, prevent, improve function, etc.) |
-
Who decides what materials can be purchased or are available?
| Health-Care provider | |
| Clinical Commissioning Groups (CCGs) |
2 STAFF
Our survey asked you what HCPs routinely referred patients to you. We would now like to know what HCPs are routinely required to provide a KAFO.
| Number of visits required | Length of visit | |
|---|---|---|
| Physiotherapist | ||
| Doctor in rehabilitation medicine | ||
| Occupational therapist | ||
| Gait scientist | ||
| Neurologist | ||
| Orthotist | ||
| Clinical nurse specialist | ||
| Orthotic technician | ||
| Other |
3 OVERHEADS
We understand that orthotics service provision varies across the UK. Provision can vary from a fully managed service by a private company to a solely NHS provided service.
-
What type of orthotic provision is in place in your clinical setting?
-
How is your orthotic service funded? (e.g. block contract, pay per appointment, pay per item).
-
Are spending caps in place?
4 OPPORTUNITY COST
This section looks at the costs that would be accumulated if our patient population of interest were not prescribed an orthotic device (a KAFO in particular).
| Disease type | Other devices required (e.g. wheelchairs) | Medical staff requirements | More/less visits with particular staff | Inpatient stays required | Other |
|---|---|---|---|---|---|
| NMD patients | |||||
| Poliomyelitis | |||||
| Muscular dystrophy | |||||
| Post-polio syndrome | |||||
| Motor neurone disease | |||||
| Inclusion body myositis | |||||
| Charcot–Marie–Tooth disease | |||||
| Guillain–Barré syndrome | |||||
| CIDP | |||||
| CNS patients | |||||
| Adult cerebral palsy | |||||
| Multiple sclerosis | |||||
| Traumatic brain injury | |||||
| Stroke | |||||
| Acquired brain injury | |||||
| Spinal cord disorders | |||||
Thank you very much for taking part in this telephone interview – we do appreciate it.
As a participant, you will receive a summary of the study findings.
If you wish to follow the progress of the study, you can access the study blog on http://kneeorthotics.blogspot.co.uk
Appendix 7 Survey questionnaire
Appendix 8 Invitation e-mail for survey of health-care professionals
Appendix 9 Advertisement of survey for health-care professionals
Appendix 10 Ongoing studies
| Study details | Participants | Intervention | Outcomes |
|---|---|---|---|
| Cardoso (2014)82 Country: Brazil Before-and-after study Study completion date: January 2014 |
12 post-stroke patients with hemiparesis with genu recurvatum (no participant details provided) | Brace (unspecified) for retraining and control of genu recurvatum | Primary outcome: distribution of plantar pressures during walking at 4 weeks |
| Frechtel (2013)83 Country: Israel Randomised crossover trial Study completion date: April 2014 |
60 post-stroke patients 20 evaluated in interim results [16 male, 4 female; mean age 61 (13.5) years] | Genu Neurexa orthosis (Otto Bock, Germany) | Gait characteristics: spatiotemporal parameters, symmetry index, paretic knee angle in the sagittal plane Dynamic muscle activity patterns Functional measures: 6-Minute Walk Test, 10-Metre Walk Test, Berg Balance Scale, Timed Up and Go Test Satisfaction questionnaire |
| Kannenberg (2014)84 Country: USA Case series Study completion date: Unclear |
13 participants with various neurological conditions causing paresis/paralysis of lower limb muscles | OBS with microprocessor hydraulic stance and swing-phase control | mPEQ, on perceived difficulty to perform 45 activities of daily living Activities of Daily Living rating scale |
Appendix 11 Excluded studies
| Reference | Reason for exclusion |
|---|---|
| Aalam M. [The Kettwig lower-limb orthosis.] Orthopadische Praxis 1984;20:404–8 | No outcomes reported |
| Abe H, Michimata A, Sugawara K, Sugaya N, Izumi S. Improving gait stability in stroke hemiplegic patients with a plastic ankle-foot orthosis. Tohoku J Exp Med 2009;218:193–9 | Not instability of the knee |
| Abe K. Comparison of static balance, walking velocity, and energy consumption with knee-ankle-foot orthosis, walkabout orthosis, and reciprocating gait orthosis in thoracic-level paraplegic patients. J Prosthet Orthot 2006;18:87–91 | Not instability of the knee |
| Abiko T, Shimamura R, Abiko Y, Soma M, Ogawa D, Kamiya A, et al. A case study of a patient with polio and stroke who improved in gait ability by exercise and plastic ankle foot orthosis. Rigakuryoho Kagaku 2011;26:163–7 | Not orthotic intervention |
| Ackermann M, Cozman FG. Automatic knee flexion in lower limb orthoses. J Braz Soc Mech Sci 2009;31:305–11 | Laboratory setting only |
| ACTRN12609000034235. A randomised clinical trial to determine the relative benefits of botulinum toxin injections, ankle foot orthosis or a combination of both on knee hyperextension during walking in people with chronic stroke. 2009 | Unavailable |
| ACTRN12612000218897. Investigating fatigue, balance, falls and mobility in people with multiple sclerosis. 2012 | Not instability of the knee |
| ACTRN12614000260628. The Foot Orthosis versus Hip eXercises (FOHX) trial: predicting success in patellofemoral pain patients. 2014 | No patients with NMD or CNS condition |
| Aguirre-Ollinger G, Colgate JE, Peshkin MA, Goswami A. A one-degree-of-freedom assistive exoskeleton with inertia compensation: the effects on the agility of leg swing motion. Proc Inst Mech Eng H 2011;225:228–45 | No patients with NMD or CNS condition |
| Alemdarotlu E, Mandirotlu S, Ucan H, Celik C. [Evaluation of regular orthosis use in paraplegics after inpatient rehabilitation.] Turk Fiz Tip Rehab D 2013;59:245 | Not orthotic intervention |
| American Academy of Orthotists and Prosthetics Proceedings. Knee-ankle-foot orthoses for ambulation. J Prosthet Orthot 2006;7:131–208 | Unavailable |
| Anderson EG, Frank PL, Henshaw JT, Rae HG. Clinical assessment of a new weight-relieving brace. J Bone Joint Surg Br 1977;59-B:439–45 | No patients with NMD or CNS condition |
| Andrysek J, Klejman S, Kooy J. Examination of knee joint moments on the function of knee-ankle-foot orthoses during walking. J Appl Biomech 2013;29:474–80 | Laboratory setting only |
| Andrysek J, Redekop S, Matsui NC, Kooy J, Hubbard S. A method to measure the accuracy of loads in knee-ankle-foot orthoses using conventional gait analysis, applied to persons with poliomyelitis. Arch Phys Med Rehabil 2008;89:1372–9 | Laboratory setting only |
| Aoki M, Moriizumi S, Toki M, Murakami T, Ishiai S. Rehabilitation and long-term course of nontraumatic myelopathy associated with surfing. Am J Phys Med Rehabil 2013;92:828–32 | Not instability of the knee |
| Appasamy M, Oreste A, Patel NM. Genu recurvatum in adult patients with spastic hemiparesis. Treatment strategies: a case series. PM R 2012;1:S261 | No patients with NMD or CNS condition |
| Aprile I, Bordieri C, Gilardi A, Lainieri Milazzo M, Russo G, De Santis F, et al. Balance and walking involvement in facioscapulohumeral dystrophy: a pilot study on the effects of custom lower limb orthoses. Eur J Phys Rehabil Med 2013;49:169–78 | Not instability of the knee |
| Aprile I, Padua LL, Iosa M, Gilardi A, Bordieri C, Frusciante R, et al. Balance and walking in facioscapulohumeral muscular dystrophy: multiperspective assessment. Eur J Phys Rehabil Med 2012;48:393–402 | Not orthotic intervention |
| Arazpour M, Mehrpour SR, Bani MA, Hutchins SW, Bahramizadeh M, Rahgozar M. Comparison of gait between healthy participants and persons with spinal cord injury when using a powered gait orthosis: a pilot study. Spinal Cord 2014;52:44–8 | Laboratory setting only |
| Arazpour M, Bani MA, Hutchins SW, Sayyadfar M. The Araz medial linkage orthosis: a new orthosis for walking in patients with spinal cord injury: a single patient study. Prosthet Orthot Int 2014;38:155–9 | Laboratory setting only |
| Arazpour M, Ahmadi Bani M, Kashani RV, Tabatabai Ghomshe F, Mousavi ME, Hutchins SW. Effect of powered gait orthosis on walking in individuals with paraplegia. Prosthet Orthot Int 2013;37:261–7 | Laboratory setting only |
| Arazpour M, Chitsazan A, Bani MA, Rouhi G, Ghomshe FT, Hutchins SW. The effect of a knee ankle foot orthosis incorporating an active knee mechanism on gait of a person with poliomyelitis. Prosthet Orthot Int 2013;37:411–14 | Laboratory setting only |
| Arazpour M, Bani MA, Chitsazan A, Ghomshe FT, Kashani RV, Hutchins SW. The effect of an isocentric reciprocating gait orthosis incorporating an active knee mechanism on the gait of a spinal cord injury patient: a single case study. Disabil Rehabil Assist Technol 2013;8:261–6 | Not primary study |
| Arazpour M, Bani MA, Hutchins SW, Jones RK. The physiological cost index of walking with mechanical and powered gait orthosis in patients with spinal cord injury. Spinal Cord 2013;51:356–9 | Laboratory setting only |
| Arazpour M, Chitsazan A, Hutchins SW, Ghomshe FT, Mousavi ME, Takamjani EE, et al. Design and simulation of a new powered gait orthosis for paraplegic patients. Prosthet Orthot Int 2012;36:125–30 | No patients with NMD or CNS condition |
| Arazpour M, Chitsazan A, Hutchins SW, Mousavi ME, Takamjani EE, Ghomshe FT, et al. Evaluation of a novel powered gait orthosis for walking by a spinal cord injury patient. Prosthet Orthot Int 2012;36:239–46 | Laboratory setting only |
| Arfaoui FZ, Karkouri S, Bennis N, Eloumri AA, Hajjaj-Hassouni N. Patient satisfaction survey on knee-ankle-foot orthoses carried out during an apparatus workshop at the El-Ayachi hospital. Ann Phys Rehabil Med 2011;54:e10 | No patients with NMD or CNS condition |
| Atrice MB. Lower extremity orthotic management for the spinal-cord-injured client. Top Spinal Cord Inj Rehabil 2000;5:1–10 | Not primary study |
| Baardman G, IJzerman MJ, Hermens HJ, Veltink PH, Boom HB, Zilvold G. The influence of the reciprocal hip joint link in the Advanced Reciprocating Gait Orthosis on standing performance in paraplegia. Prosthet Orthot Int 1997;21:210–21 | Not instability of the knee |
| Baba M, Saitoh E, Sarai S, Teranishi T, Okada M, Mizuno A. Clinical Experience with a HKAFO System Using a Medial Single Hip Joint for Patients with Locomotive Problems. 8th World Congress of International Rehabilitation Medicine Association, Monduzzi Editore, 1997, Bologna, Italy, pp.1307–10 | No patients with NMD or CNS condition |
| Bakker JP, de Groot IJ, Beckerman H, de Jong BA, Lankhorst GJ. The effects of knee-ankle-foot orthoses in the treatment of Duchenne muscular dystrophy: review of the literature. Clin Rehabil 2000;14:343–59 | Not primary study |
| Bakker JP, de Groot IJ, de Jong BA, van Tol-De Jager MA, Lankhorst GJ. Prescription pattern for orthoses in The Netherlands: use and experience in the ambulatory phase of Duchenne muscular dystrophy. Disabil Rehabil 1997;19:318–25 | All participants aged < 16 years |
| Banala SK, Kim SH, Agrawal SK, Scholz JP. Robot assisted gait training with active leg exoskeleton (ALEX). IEEE Trans Neural Syst Rehabil Eng 2009;17:2–8 | Not orthotic intervention |
| Banala SK, Agrawal SK, Fattah A, Krishnamoorthy V, Hsu WL, Scholz J, Rudolph K. Gravity-balancing leg orthosis and its performance evaluation. IEEE Trans Robot 2006;22:1228–39 | Laboratory setting only |
| Bani MA, Arazpour M, Ghomshe FT, Mousavi ME, Hutchins SW. Gait evaluation of the advanced reciprocating gait orthosis with solid versus dorsi flexion assist ankle foot orthoses in paraplegic patients. Prosthet Orthot Int 2013;37:161–7 | Not instability of the knee |
| Baptiste P. [Biomechanical Effects of Knee Orthoses (experimental characterization and modelling).] PhD thesis. Saint-Étienne: École Nationale Supérieure des Mines de Saint-Étienne; 2013 | No patients with NMD or CNS condition |
| Barbu DM, Barbu I. Dynamical Model for an Original Mechatronical Rehabilitation System. Proceedings of the 14th WSEAS International Conference on Applied Mathematics, 14–16 December 2000, pp. 23–6 | No patients with NMD or CNS condition |
| Barela JA, Whitall J, Black P, Clark JE. An examination of constraints affecting the intralimb coordination of hemiparetic gait. Hum Mov Sci 2000;19:251–73 | Laboratory setting only |
| Barnicle K, Andrews BJ, Phillips GF, Chizeck HJ. Hybrid Orthosis for Paraplegic Standing with Percutaneous Electrodes. Proceedings of the IEEE/Engineering in Medicine and Biology Society Annual Conference, 4–7 November 1988, New Orleans, LA, pp. 164–5 | No outcomes reported |
| Barton CJ, Munteanu SE, Menz HB, Crossley KM. The efficacy of foot orthoses in the treatment of individuals with patellofemoral pain syndrome: a systematic review. Sports Med 2010;40:377–95 | Not primary study |
| Bartonek A, Saraste H, Samuelsson L, Skoog M. Ambulation in patients with myelomeningocele: a 12-year follow-up. J Pediatr Orthop 1999;19:202–6 | Not orthotic intervention |
| Bassett GS, Fleming BW. The Lenox Hill brace in anterolateral rotatory instability. Am J Sports Med 1983;11:345–8 | No patients with NMD or CNS condition |
| Beck C, Drez D, Young J, Cannon WD, Stone ML. Instrumented testing of functional knee braces. Am J Sports Med 1986;14:253–6 | No patients with NMD or CNS condition |
| Beekman C, Perry J, Boyd LA, Newsam CJ, Mulroy SJ. The effects of a dorsiflexion-stopped ankle-foot orthosis on walking in individuals with incomplete spinal cord injury. Top Spinal Cord Inj Rehabil 2000;5:54–62 | Not instability of the knee |
| Beer S, Aschbacher B, Manoglou D, Gamper E, Kool J, Kesselring J. Robot-assisted gait training in multiple sclerosis: a pilot randomised trial. Multiple Sclerosis 2008;14:231–6 | Not instability of the knee |
| Belforte G, Gastaldi L, Pastorelli S, Sorli M. A Novel Actuation for the Pneumatic Active Gait Orthosis. International Conference on Climbing and Walking Robots, Paris, France, 25–27 September 2002 | No patients with NMD or CNS condition |
| Bellantoni L, Heffernan C, Oyedijo O, Reding M. The effect of knee-ankle-foot orthosis on ambulation outcome after stroke. Clin Res 1992;40:A119 | Not instability of the knee |
| Belmahfoud R, Sautreuil P. [The orthotic knee in poliomyelitis patients.] Lett Méd Phys Réadapt 2010;26:150–4 | No outcomes reported |
| Beltramo F. Lower limb orthoses. Ann Readapt Med Phys 2001;44:170–5 | Not primary study |
| Benvenuti E, Galantini P, Del Lungo I, Cecchi F, Giardini S, Federighi G, et al. [Ankle foot orthosis: which one to choose for a correct prescription.] G Geronto 2003;51:107–18 | Unavailable |
| Bergquist RJ. Brace conversion: long leg to short leg. Phys Ther 1973;53:1071 | No patients with NMD or CNS condition |
| Bernardi M, Canale I, Castellano V, Di Filippo L, Felici F, Marchetti M. The efficiency of walking of paraplegic patients using a reciprocating gait orthosis. Paraplegia 1995;33:409–15 | Not instability of the knee |
| Bernhardt KA, Kaufman KR. Loads on the uprights of a knee-ankle-foot orthosis. Prosthet Orthot Int 2011;35:106–12 | Laboratory setting only |
| Bernhardt KA, Irby SE, Kaufman KR. Consumer opinions of a stance control knee orthosis. Prosthet Orthot Int 2006;30:246–56 | All participants aged < 16 years |
| Bertomeu JM, Lois JM, Guillem RB, Pozo AP, Lacuesta J, Molla CG, et al. Development of a hinge compatible with the kinematics of the knee joint. Prosthet Orthot Int 2007;31:371–83 | No patients with NMD or CNS condition |
| Beyl P, Naudet J, Van Ham R, Lefeber D. Mechanical Design of an Active Knee Orthosis for Gait Rehabilitation. Proceedings of the IEEE 10th International Conference on Rehabilitation Robotics, Noordwidjk, The Netherlands, 13–15 June 2007, pp. 100–5 | No patients with NMD or CNS condition |
| Biden EN. Free knee brace system. J Rehabil Res Dev 1991;28:311 | No outcomes reported |
| Biedermann L. [Orthopadie-technische versorgung von knieband- instabilitaten mit einer leichtbau-orthese.] Orthop Tech 1989:119–124 | No outcomes reported |
| Bijlenga G, Cook JK, Gelb J, de Wit JJ. Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: a review. Avian Pathol 2004;33:550–7 | No patients with NMD or CNS condition |
| Bizzini M, Childs JD, Piva SR, Delitto A. Systematic review of the quality of randomized controlled trials for patellofemoral pain syndrome. J Orthop Sports Phys Ther 2003;33:4–20 | Not primary study |
| Blanton S, Grissom SP, Riolo L. Use of a static adjustable ankle-foot orthosis following tibial nerve block to reduce plantar-flexion contracture in an individual with brain injury. Phys Ther 2002;82:1087–97 | Not instability of the knee |
| Blauth W, Ulrich HW, Hahne HJ. [Evaluation of knee orthoses.] Unfallchirurg 1990;93:221–7 | No patients with NMD or CNS condition |
| Bleyenheuft C, Bleyenheuft Y, Hanson P, Deltombe T. Treatment of genu recurvatum in hemiparetic adult patients: a systematic literature review. Ann Phys Rehabil Med 2010;53:189–99 | Not primary study |
| Bocker B, Hoelig C, Smolenski UC. [Orthosis management in patients after poliomyelitis anterior acuta.] Phys Med Rehab Kuror 2013;23:16–21 | Needs translation |
| Bolden I. Full Leg Joint Pad Appliance. Abstract for a Google patent. 2006. URL: www.google.com/patents/US7096507 (accessed 23 May 2016) | No outcomes reported |
| Boldingh EJK, Van Pijkeren T, Wijkmans DW. A study on the value of the modified KBM prosthesis compared with other types of prosthesis. Prosthet Orthot Int 1985;9:79–82 | Not orthotic intervention |
| Bonaroti D, Akers JM, Smith BT, Mulcahey MJ, Betz RR. Comparison of functional electrical stimulation to long leg braces for upright mobility for children with complete thoracic level spinal injuries. Arch Phys Med Rehabil 1999;80:1047–53 | No patients with NMD or CNS condition |
| Bos RPM, Grady JH, Vierhout PAM, de Vries J. A comparison of two custom-made and two off-the-shelf rigid knee orthoses in the treatment of ACL-deficient knees. J Prosthet Orthot 1997;9:25–32 | No patients with NMD or CNS condition |
| Boudarham J, Marchiori C, Zory R, Genet F, Bensmail D, Roche N, et al. Effects of dynamic ankle-foot orthosis on biomechanic gait parameters in hemiparetic patients. Ann Phys Rehabil Med 2011;54:e9–10 | Not instability of the knee |
| Bowers R. Biomechanical basis for use of ankle-foot orthoses after stroke. BJNN 2013;4:20 | Not primary study |
| Branch T, Hunter R, Reynolds P. Controlling anterior tibial displacement under static load: a comparison of two braces. Orthopedics 1988;11:1249–52 | No patients with NMD or CNS condition |
| Brandt JM. To lock or unlock? That is the question. Rehab Manag 2010;23:16 | Not primary study |
| Brehm M, Bus SA, Harlaar J, Nollet F. A candidate core set of outcome measures based on the International Classification of Functioning, Disability and Health for clinical studies on lower limb orthoses. Prosthet Orthot Int 2011;35:269–77 | Not primary study |
| Brill J. Assistive device for locking brace knee joint. J Am Phys Ther Assoc 1963;43:807 | No outcomes reported |
| Bromwich W, James M, Stewart C, Emery N, Quinlivan R. Outcomes using ambulatory ankle foot orthoses in Duchenne muscular dystrophy. Gait Posture 2012;36:S94–5 | All participants aged < 16 years |
| Bromwich W, Emery N, Stewart C, James M, Quinlivan R. A novel ankle foot orthoses/footwear combination to aid walking in Duchenne muscular dystrophy. Neuromuscular Disord 2010;20:S7 | All participants aged < 16 years |
| Brown J, Tindall G, Nitschke R, Haake P, Jackman KV. Hip, knee, ankle foot orthosis: lateral bar design with spring extension assist hip joints. Orthotics Prosthet 1982;36:44–9 | No outcomes reported |
| Brunel P. Concepts of knee-ankle-foot and knee-ankle-heel orthesis used for neurological diseases. Ann Phys Rehabil Med 2011;54:e7 | Not primary study |
| Brutsch K, Schuler T, Koenig A, Zimmerli L, Koeneke SM, Lunenburger L, et al. Influence of virtual reality soccer game on walking performance in robotic assisted gait training for children. J Neuroeng Rehabil 2010;7:15 | All participants aged < 16 years |
| Buckon CE, Thomas SS, Jakobson-Huston S, Sussman M, Aiona M. Comparison of three ankle-foot orthosis configurations for children with spastic hemiplegia. Dev Med Child Neurol 2001;43:371–8 | All participants aged < 16 years |
| Bulea TC, Kobetic R, Audu ML, Triolo RJ. Stance controlled knee flexion improves stimulation driven walking after spinal cord injury. J Neuroeng Rehabil 2013;10:68 | Laboratory setting only |
| Bulea TC, Kobetic R, Triolo RJ. Restoration of stance phase knee flexion during walking after spinal cord injury using a variable impedance orthosis. Conf Proc IEEE Eng Med Biol Soc 2011:608–11 | No patients with NMD or CNS condition |
| Bumbea AM, Popescu R, Traistaru R, Bighea A, Patru S. Rehabilitation program in secondary knee osteoarthritis with GENU recurvatum in hemi-paretic patients. Ann Rheum Dis 2013;71 | Not orthotic intervention |
| Burdett RG, Borello-France D, Blatchly C, Potter C. Gait comparison of subjects with hemiplegia walking unbraced, with ankle-foot orthosis, and with Air-Stirrup brace. Phys Ther 1988;68:1197–203 | Not instability of the knee |
| Butler PB, Farmer SE, Stewart C, Jones PW, Forward M. The effect of fixed ankle foot orthoses in children with cerebral palsy. Disabil Rehabil Assist Technol 2007;b:51–8 | All participants aged < 16 years |
| Butler PB, Farmer SE, Major RE. Improvement in gait parameters following late intervention in traumatic brain injury: a long-term follow-up report of a single case. Clin Rehabil 1997;11:220–6 | Not primary study |
| Byl NN. Mobility training using a bionic knee orthosis in patients in a post-stroke chronic state: a case series. J Med Case Rep 2012;6:216 | Not orthotic intervention |
| Campbell JH. Linked hip-knee-ankle-foot orthoses designed for reciprocal gait. J Prosthet Orthot 2006;18:P204–8 | Not primary study |
| Carlow WA, Almeida MJ. Plastics in lower-limb orthotics. Orthotics Prosthet 1978;32:25–31 | No outcomes reported |
| Carlson JM, French J. Knee orthoses for valgus protection. Experiments on 11 designs with related analyses of orthosis length and rigidity. Clin Orthop Relat Res 1989:175–92 | No patients with NMD or CNS condition |
| Carlson WE, Vaughan CL, Damiano DL, Abel MF. Orthotic management of gait in spastic diplegia. Am J Phys Med Rehabil 1997;76:219–25 | All participants aged < 16 years |
| Carse B, Bowers RJ, Meadows BC, Rowe PJ. Visualisation to enhance biomechanical tuning of ankle-foot orthoses (AFOs) in stroke: study protocol for a randomised controlled trial. Trials 2011;12:254 | Not orthotic intervention |
| Cassvan A, Wunder KE, Fultonberg DM. Orthotic management of the unstable knee. Arch Phys Med Rehabil 1977;58:487–91 | No patients with NMD or CNS condition |
| Cerny K, Walker J, Perry J. The effect of a knee-ankle-foot orthosis on the stance phase of gait. Phys Ther 1988;68:801 | No patients with NMD or CNS condition |
| Cerny D, Waters R, Hislop H, Perry J. Walking and wheelchair energetics in persons with paraplegia. Phys Ther 1980;60:1133–9 | Not instability of the knee |
| Chandrapal M, Chen XQ. Intelligent Active Assistive and Resistive Orthotic Device for Knee Rehabilitation. 7th IEEE International Conference on Control and Automation, Christchurch, New Zealand, 9–11 December 2009. pp. 1880–5 | No patients with NMD or CNS condition |
| Charrette M. When to consider orthotics: research-based recommendations. Dynamic Chiropr 2010;34(9) | Not primary study |
| Charrette M. Lateral knee pain and orthotic support. Dynamic Chiropr 2008;26(26) | Not primary study |
| Charrette MN. Foot care: thirteen ways orthotics can help your patients. Dynamic Chiropr 2008;26(14) | Not primary study |
| Charrette MN. Foot care: musculoskeletal problems and orthotic support. Dynamic Chiropr 2007;25(34) | No outcomes reported |
| Chen CH, Lin KH, Lu TW, Chai HM, Chen HL, Tang PF, Hu MH. Immediate effect of lateral-wedged insole on stance and ambulation after stroke. Am J Phys Med Rehabil 2010;89:48–55 | Not instability of the knee |
| Chisholm AE, Perry SD. Ankle-foot orthotic management in neuromuscular disorders: recommendations for future research. Disabil Rehabil Assist Technol 2012;7:437–49 | Not primary study |
| Chiu YY. The effectiveness of Knee Extension Splint (KES) on weight shifting control of stroke patient: pilot study. Int J Stroke 2012;7:51 | Not instability of the knee |
| Collins NJ, Bisset LM, Crossley KM, Vicenzino B. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta-analysis of randomized trials. Sports Med 2012;42:31–49 | Not primary study |
| Colombo G, Dietz V. Powered Gait Orthosis Improves Locomotor Training in Paraplegic Patients. In Buhler C, H Knops H, editors. Assistive Technology on the Threshold of the New Millenium 1999;6:580–4. Proceedings of the 5th Biennial European Conference for the Advancement of Assistive Technology in Europe, 1999 | Not orthotic intervention |
| Colombo G, Jorg M, Dietz V. Driven Gait Orthosis to do Locomotor Training of Paraplegic Patients. Proceedings of the IEEE Engineering in Medicine and Biology Society Conference, 23–28 July 2000, Chicago, IL, pp. 3159–63 | No patients with NMD or CNS condition |
| Colombo G, Joerg M, Schreier R, Dietz V. Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil R D 2000;37:693–700 | Not orthotic intervention |
| Conil JL, Fico G, Bardot A, Delarque A, Viton JM, Kraenzler R, et al. Knee-ankle-foot orthosis for poliomyelitis sequelae: structural and technical evolution. Eura Medicophys 2001;37:171–9 | No outcomes reported |
| Crossley K, Bennell K, Green S, McConnell J. A systematic review of physical interventions for patellofemoral pain syndrome. Clin J Sport Med 2001;11:103–10 | No patients with NMD or CNS condition |
| Cruz TH, Dhaher YY. Impact of ankle-foot-orthosis on frontal plane behaviors post-stroke. Gait Posture 2009;30:312–16 | Not instability of the knee |
| Cullell A, Moreno JC, Rocon E, Forner-Cordero A, Pons JL. Biologically based design of an actuator system for a knee-ankle-foot orthosis. Mech Mach Theory 2009;44:860–72 | Laboratory setting only |
| Cybulski GR, Jaeger RJ. Standing performance of persons with paraplegia. Arch Phys Med Rehabil 1986;67:103–8 | Not primary study |
| Cybulski G, Jaeger R, Troyk P. Quantitative analysis of standing balance in paraplegic individuals. Conference of the Rehabilitation Engineering Society of North America 1984 | Not instability of the knee |
| Dall P. The function of the reciprocal link in paraplegic orthotic gait. J Prosthet Orthot 2001;13:10–13 | Not primary study |
| Daly JJ, Ruff RL. Construction of efficacious gait and upper limb functional interventions based on brain plasticity evidence and model-based measures for stroke patients. Scientific World J 2007;7:2031–45 | No outcomes reported |
| Daly JJ, Roenigk K, Holcomb J, Rogers JM, Butler K, Gansen J, et al. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke 2006;37:172–8 | Not orthotic intervention |
| Daly JJ, Roenigk KL, Gansen JL, Fredrickson E, Marsolais EB, Rogers J, Ruff RL. Response of sagittal plane gait kinematics to weight-supported treadmill training and functional neuromuscular stimulation following stroke. J Rehabil Res Dev 2004;41:807–20 | Not orthotic intervention |
| Dandurand E, Gaurier E, Lauvin E, Soster D, Valat C. [Helping gait with ankle foot orthoses for hemiplegic patients.] Kinesitherapie 2011;11:55–60 | Not primary study |
| Danielsson A, Sunnerhagen KS. Energy expenditure in stroke subjects walking with a carbon composite ankle foot orthosis. J Rehabil Med 2004;36:165–8 | Laboratory setting only |
| Dao TT, Pouletaut P, Marin F, Aufaure P, Charleux F, Ho Ba Tho MC. Simulation of the Gait of a Patient Specific Model of Post Polio Residual Paralysis (PPRP): Effect of the Orthosis. Third International Conference on the Development of Biomedical Engineering in Vietnam, 2010 | No patients with NMD or CNS condition |
| De Azevedo ERFBM, Cacho EWA, Alonso KC, Martin FTB, Cliquet A. Kinetic and Kinematic Gait Assessment of Paraplegic Patients With and Without Ankle Foot Orthoses. BIODEVICES 2010, Institute for Systems and Technologies of Information Control and Communication 3rd International Conference on Biomedical Electronics and Devices, Valencia, Spain, 20–23 January 2010, Proceedings 2010 | Laboratory setting only |
| De Lecluse J. [Which knee-brace should be prescribed?] Rhumatologie 1997;49:191–5 | Not primary study |
| De Moraes Barros Fucs PM, Svartman C, de Assumpcao RM. Knee flexion deformity from poliomyelitis treated by supracondylar femoral extension osteotomy. Int Orthop 2005;29:380–4 | Not orthotic intervention |
| De Pisi F. Aids and orthoses in patients with stroke consequences. Clin Exp Hypertens 2006;28:383–5 | Not primary study |
| De Seze MP, Bonhomme C, Daviet JC, Burguete E, Machat H, Rousseaux M, et al. Effect of early compensation of distal motor deficiency by the Chignon ankle-foot orthosis on gait in hemiplegic patients: a randomised pilot study. Clin Rehabil 2011;25:989–98 | Not instability of the knee |
| De Vries J. Evaluation of lower leg orthosis use following cerebro-vascular accident. Int J Rehabil Res 1991;14:239–43 | Not instability of the knee |
| De Vries J. [Knee flexion extension orthosis.] Orthop Tech 1987:707–9 | Not primary study |
| De Wit DC, Buurke JH, Nijlant JM, Ijzerman MJ, Hermens HJ. The effect of an ankle-foot orthosis on walking ability in chronic stroke patients: a randomised controlled trial. Clin Rehabil 2004;18:550–7 | Not instability of the knee |
| Del Bianco JD, Fatone S. Comparison of silicone and posterior leaf spring ankle-foot orthoses in a subject with Charcot–Marie–Tooth disorder. J Prosthet Orthot 2008;20:155–62 | Not instability of the knee |
| Dias LS, Tappit-Emas E, Boot EJ, Kelly L. The use of the reciprocating reciprocating hip-knee-ankle-foot orthosis in spina bifida the Childrens Memorial Hospital Chicago Illinois USA experience. Dev Med Child Neurol 1985;27:93–4 | All participants aged < 16 years |
| Dlamini N, Messina S, Padua DL, Main M, Knight R, Manzur A, et al. Quality of life outcomes following rehabilitation in knee-ankle-foot orthoses (KAFOs) in Duchenne muscular dystrophy. Neuromuscular Disord 2005;15:701–2 | All participants aged < 16 years |
| Dogan A, Mengulluoglu M, Ozgirgin N. Evaluation of the effect of ankle-foot orthosis use on balance and mobility in hemiparetic stroke patients. Disabil Rehabil 2011;33:1433–9 | Not instability of the knee |
| Doria L, Minge M, Riedel L, Kraft M. User-centred evaluation of lower-limb orthoses: a new approach. Biomed Tech (Berl) 2013;58 | Laboratory setting only |
| Dos Anjos Fernandes N, Troise DC, Favero FM, Fontes SV, Oliveira ASB. [The importance of orthosis for the lower limbs in Duchenne muscular dystrophy.] Revista Neurociências 2012;20:584–7 | Not primary study |
| Dubowitz V. Clinical myology at the crossroads; the gospel truth. Neuromuscul Disord 2010;20:95–6 | Not primary study |
| Dufek JS, Neumann ES, Hawkins MC, O’Toole B. Functional and dynamic response characteristics of a custom composite ankle foot orthosis for Charcot–Marie–Tooth patients. Gait Posture 2014;39:308–13 | Not instability of the knee |
| Dunlap HV, MacNeil LG, Tarnopolsky MA. Functional impairment in patients with sporadic inclusion body myositis. Can J Neurol Sci 2014;41:253–9 | Not orthotic intervention |
| Dunsky A, Dickstein R, Marcovitz E, Levy S, Deutsch JE. Home-based motor imagery training for gait rehabilitation of people with chronic poststroke hemiparesis. Arch Phys Med Rehabil 2008;89:1580–8 | Not orthotic intervention |
| Dwivedi M, Shetty KD, Nath LN. Design and development of anthropometric device for the standardization of sizes of knee-ankle-foot orthoses. J Med Eng Technol 2009;33:87–94 | Not orthotic intervention |
| Edberg E. Paralytic dysfunction. IV. Bracing for patients with traumatic paraplegia. Phys Ther 1967;47:818–23 | Not primary study |
| Edelstein JE. Ambulatory KAFOs: a physical therapist’s perspective. J Prosthet Orthot 2006;7:183–6 | Not primary study |
| Edelstein JE. Orthotic options for standing and walking. Top Spinal Cord Inj Rehabil 2000;5:11–23 | Not primary study |
| Eng JJ, Pierrynowski MR. Effect of foot orthotics on the kinematics of the knee joint. J Biomech 1989;22:1007 | All participants aged < 16 years |
| Erel S, Uygur F, Engin Simsek I, Yakut Y. The effects of dynamic ankle-foot orthoses in chronic stroke patients at three-month follow-up: a randomized controlled trial. Clin Rehabil 2011;25:515–23 | Not instability of the knee |
| Esquenazi A, Coulter T, Packel A, Saulino M, Talaty M. Safety and performance evaluation of rewalk reciprocating gait orthosis. PM R 2010;1:S158–9 | Not orthotic intervention |
| Farncombe PM. The Swedish knee cage. Management of the hyperextended hemiplegic knee. Physiotherapy 1980;66:33–4 | Not orthotic intervention |
| Farris RJ, Quintero HA, Murray SA, Ha KH, Hartigan C, Goldfarb M. A preliminary assessment of legged mobility provided by a lower limb exoskeleton for persons with paraplegia. IEEE Trans Rehabil Eng 2014;22:482–90 | Laboratory setting only |
| Farris RJ, Quintero HA, Goldfarb M. Performance evaluation of a lower limb exoskeleton for stair ascent and descent with paraplegia. Conf Proc IEEE Eng Med Biol Soc 2012:1908–11 | Laboratory setting only |
| Farris RJ, Quintero HA, Goldfarb M. Preliminary evaluation of a powered lower limb orthosis to aid walking in paraplegic individuals. IEEE Trans Neural Syst Rehabil Eng 2011;19:652–9 | Laboratory setting only |
| Fatone S. A review of the literature pertaining to KAFOs and HKAFOs for ambulation. J Prosthet Orthot 2006;18:P137–68 | Not primary study |
| Fatone S, Gard SA, Malas BS. Effect of ankle-foot orthosis alignment and foot-plate length on the gait of adults with poststroke hemiplegia. Arch Phys Med Rehabil 2009;90:810–18 | Not instability of the knee |
| Felmet G, Piro M. [Report of orthopedic technical experience: 2.5 years with the ULTRATECH knee orthosis.] Orthop Tech 1995:407–15 | No patients with NMD or CNS condition |
| Fengler Reinoud KB, Holtslag Herman R. Compensating weakness of the quadriceps muscles due to poliomyelitis using a custom-made orthopedic shoe. J Prosthet Orthot 2011;23:199–203 | Not primary study |
| Ferguson KA, Polando G, Kobetic R, Triolo RJ, Marsolais EB. Walking with a hybrid orthosis system. Spinal Cord 1999;37:800–4 | Not orthotic intervention |
| Finger S, Paulos LE. Clinical and biomechanical evaluation of the unloading brace. J Knee Surg 2002;15:155–8 | No patients with NMD or CNS condition |
| Fisher LR, McLellan DL. Questionnaire assessment of patient satisfaction with lower limb orthoses from a district hospital. Prosthet Orthot Int 1989;13:29–35 | No patients with NMD or CNS condition |
| Fishman A, Kudelka AP, Tresukosol D, Edwards CL, Freedman RS, Kaplan AL, et al. Leuprolide acetate for treating refractory or persistent ovarian granulosa cell tumor. J Reprod Med 1996;41:393–6 | Not orthotic intervention |
| Flandry F, Burke S, Roberts JM, Hall S, Drouilhet A, Davis G, et al. Functional ambulation in myelodysplasia: the effect of orthotic selection on physical and physiologic performance. J Pediatr Orthop 1986;6:661–5 | All participants aged < 16 years |
| Forin V. Lower limb orthesis and standing position in neuromuscular disease: indications, limits, contraindications, modalities, expected benefits. Ann Readapt Med Phys 2001;44:225–8 | Not primary study |
| Franceschini M, Baratta S, Zampolini M, Loria D, Lotta S. Reciprocating gait orthoses: a multicenter study of their use by spinal cord injured patients. Arch Phys Med Rehabil 1997;78:582–6 | All participants aged < 16 years |
| Freeman D, Orendurff M, Moor M. Case study: improving knee extension with floor-reaction ankle-foot orthoses in a patient with myelomeningocele and 20 degree knee flexion contractures. J Prosthet Orthot 1999;11:63–8 | All participants aged < 16 years |
| Freivogel S, Mehrholz J, Husak-Sotomayor T, Schmalohr D. Gait training with the newly developed ‘LokoHelp’-system is feasible for non-ambulatory patients after stroke, spinal cord and brain injury. A feasibility study. Brain Inj 2008;22:625–32 | Not orthotic intervention |
| Fuchs A, Doderlein L. Orthotics and cerebral palsy. Established treatments and trends in orthopaedic devices for patients with cerebral palsy. Orthopade 2004;33:1173–82 | All participants aged < 16 years |
| Furia JP, Willis FB, Shanmugam R, Curran SA. Systematic review of contracture reduction in the lower extremity with dynamic splinting. Adv Ther 2013;30:763–70 | Not primary study |
| Galvez JA, Budovitch A, Harkema SJ, Reinkensmeyer DJ. Trainer variability during step training after spinal cord injury: Implications for robotic gait-training device design. J Rehabil R D 2011;48:147–60 | Not orthotic intervention |
| Galvez JA, Budovitch A, Harkema SJ, Reinkensmeyer DJ. Quantification of therapists’ manual assistance on the leg during treadmill gait training with partial body-weight support after spinal cord injury. Conf Proc IEEE Eng Med Biol Soc 2007:4028–32 | Not orthotic intervention |
| Garralda ME, Muntoni F, Cunniff A, Caneja AD. Knee-ankle-foot orthosis in children with Duchenne muscular dystrophy: user views and adjustment. Eur J Paediatr Neurol 2006;10:186–91 | All participants aged < 16 years |
| Gatti MA, Freixes O, Fernandez SA, Rivas ME, Crespo M, Waldman SV, et al. Effects of ankle foot orthosis in stiff knee gait in adults with hemiplegia. J Biomech 2012;45:2658–61 | Not instability of the knee |
| Gatti MA, Sundblad M, Freixes O, Fernández SA, Olmos LE, Rubel IF. Usage follow-up after a knee-ankle-foot orthoses selection and training program in spinal cord injury patients. J Prosthet Orthot 2010;22:146–9 | Background |
| Genda E, Oota K, Suzuki Y, Koyama K, Kasahara T. A new walking orthosis for paraplegics: hip and ankle linkage system. Prosthet Orthot Int 2004;28:69–74 | Not primary study |
| Genet F, Schnitzler A, Mathieu S, Autret K, Thefenne L, Dizien O, et al. Orthotic devices and gait in polio patients. Ann Phys Rehabil Med 2010;53:51–9 | Not primary study |
| Geurts MA, Boog GJ, van der Graaff L, Meyer JW, Janssen M. Neuro-arthropathy, a painless but very destructive disorder of frequently one joint. Ned Tijdschr Geneeskd 1996;140:1869–72 | No patients with NMD or CNS condition |
| Gharooni S, Heller B, Tokhi MO. A new hybrid spring brake orthosis for controlling hip and knee flexion in the swing phase. IEEE Trans Rehabil Eng 2001;9:106–7 | No patients with NMD or CNS condition |
| Glinn JE, Huckert G. Building bridges. Rehab Manag 2005;18:34, 36 | Unavailable |
| Gombert C, Percebois-Macadre L, Tambosco L, Rapin A, Belassian G, Coulon JM, et al. Osteoclasis following femoral condylar fracture in a patient suffering from poliomyelitis sequelae. Ann Phys Rehabil Med 2011;54:e26–7 | Not orthotic intervention |
| Goodman MJ, Menown JL, West JM, Barr KM, Vander Linden DW, McMulkin ML. Secondary gait compensations in individuals without neuromuscular involvement following a unilateral imposed equinus constraint. Gait Posture 2004;20:238–44 | No patients with NMD or CNS condition |
| Goodwin V, Jones-Hughes T, Thompson-Coon J, Boddy K, Stein K. Implementing the evidence for preventing falls among community-dwelling older people: a systematic review. J Safety Res 2011;42:443–51 | Not primary study |
| Grant AD, Atar D, Lehman WB. Postpoliomyelitis syndrome problems of knee function: a review. Bull Hosp Jt Dis 1995;53:27–9 | Not primary study |
| Greene PJ, Granat MH. A knee and ankle flexing hybrid orthosis for paraplegic ambulation. Med Eng Phys 2003;25:539–45 | Laboratory setting only |
| Greene WB. Treatment of hip and knee problems in myelomeningocele. J Bone Joint Surg Am 1998;80:1068–82 | Not primary study |
| Grimby G, Li J, Vandenakker C, Sandel ME. Post-polio syndrome: a perspective from three countries. PM R 2009;1:1035–40 | Not primary study |
| Grissom SP, Blanton S. Treatment of upper motoneuron plantarflexion contractures by using an adjustable ankle-foot orthosis. Arch Phys Med Rehabil 2001;82:270–3 | Not instability of the knee |
| Groah SL, Cifu DX. The rehabilitative management of the traumatic brain injury patient with associated femoral neuropathy. Arch Phys Med Rehabil 1995;76:480–3 | Not orthotic intervention |
| Guenther N. Below-knee Orthotic Device. United States Patent No. US 7,112,180 B2. 2006. | Not primary study |
| Guse ST, Alvine FG. Treatment of diabetic foot ulcers and Charcot neuroarthropathy using the patellar tendon-bearing brace. Foot Ankle Int 1997;18:675–7 | Not instability of the knee |
| Hachisuka K, Arai K, Arai M. Carbon fibre reinforced plastic knee-ankle-foot orthosis with a partially flexible thigh cuff: a modification for comfort while sitting on a toilet seat. Prosthet Orthot Int 2007;31:133–7 | Not primary study |
| Hahn HR. Lower extremity bracing in paraplegics with usage follow-up. Paraplegia 1970;8:147–53 | All participants aged < 16 years |
| Handford F. Handford knee brace. Physiotherapy 1987;73:254 | No outcomes reported |
| Hebela N, Keenan MA. Neuro-orthopedic management of the dysfunctional extremity in upper motor neuron syndromes. Eura Medicophys 2004;40:145–56 | Not orthotic intervention |
| Hebert JS. Ambulatory KAFOs: a physiatry perspective. J Prosthet Orthot 2006;74 | Not primary study |
| Hebert JS, Liggins AB. Gait evaluation of an automatic stance-control knee orthosis in a patient with postpoliomyelitis. Arch Phys Med Rehabil 2005;86:1676–80 | Laboratory setting only |
| Hebert JS, Liggins AB. Post-polio gait with an automatic stance control KAFO a case study. Gait Posture 2004;20(Suppl. 1):42–3 | Laboratory setting only |
| Heinemann AW, Magiera-Planey R, Schiro-Geist C, Gimines G. Mobility for persons with spinal cord injury: an evaluation of two systems. Arch Phys Med Rehabil 1987;68:90–3 | Not primary study |
| Heizer D. Structural insufficiency. 3. Bracing design for knee joint instability. Phys Ther 1967;47:859–63 | Not primary study |
| Herr H, Kornbluh R. New Horizons for Orthotic and Prosthetic Technology: Artificial Muscle for Ambulation. In Bar-Cohen Y, editor. Proc SPIE 5385, Smart Structures and Materials 2004: Electroactive Polymer Actuators and Devices (EAPAD), 27 July 2004 | Not instability of the knee |
| Heydarian K, Akbarnia BA, Jabalameli M, Tabador K. Posterior capsulotomy for the treatment of severe flexion contractures of the knee. J Pediatr Orthop 1984;4:700–4 | Not orthotic intervention |
| Hijmans JM, Geertzen JHB. Development of clinical guidelines for the prescription of orthoses in patients with neurological disorders in the Netherlands. Prosthet Orthot Int 2006;30:35–43 | Not primary study |
| Hockin J, Banister G. The extended role of a physiotherapist in an out-patient orthopaedic clinic. Physiotherapy 1994;80:281–4 | Not orthotic intervention |
| Hong C, San Luis EB, Chung S. Follow-up study on the use of leg braces issued to spinal cord injury patients. Paraplegia 1990;28:172–7 | No outcomes reported |
| Horwood A. Lateral wedges. Podiatry Now 2012;15:48 | Unavailable |
| Hruby V. Simple supporting device for genu valgum, genu varum and the unstable knee joint. Zdrav Prac 1974;24:685 | not primary study |
| Hsu CC, Chen JS. On the Design and Implementation of a Wearable Hybrid Assisted Lower Limb Orthosis. Proceedings of the 2011 2nd International Congress on Computer Applications and Computational Science, Tuban, Indonesia, 15–17 November 2011. pp. 43–51 | No patients with NMD or CNS condition |
| Hung JW, Chen PC, Yu MY, Hsieh YW. Long-term effect of an anterior ankle-foot orthosis on functional walking ability of chronic stroke patients. Am J Phys Med Rehabil 2011;90:8–16 | Not instability of the knee |
| Hurley EA. Use of KAFOs for patients with cerebral vascular accident, traumatic brain injury, and spinal cord injury. J Prosthet Orthot 2006;201 | Not primary study |
| Hussey RW, Stauffer ES. Spinal cord injury: requirements for ambulation. Arch Phys Med Rehabil 1973;54:544–7 | No outcomes reported |
| Hwang S, Kang S, Cho K, Kim Y. Biomechanical effect of electromechanical knee-ankle-foot-orthosis on knee joint control in patients with poliomyelitis. Med Biol Eng Comput 2008;46:541–9 | Laboratory setting only |
| Ijzerman MJ, Baardman G, Hermens HJ, Veltink PH, Boom HB, Zilvold G. Comparative trials on hybrid walking systems for people with paraplegia: an analysis of study methodology. Prosthet Orthot Int 1999;23:260–73 | Not primary study |
| Inaba M. Control dysfunction. 3. Bracing the unstable knee and ankle in hemiplegia. Phys Ther 1967;47:838–43 | No patients with NMD or CNS condition |
| Irby SE, Bernhardt KA, Kaufman KR. Gait of stance control orthosis users: the dynamic knee brace system. Prosthet Orthot Int 2005;29:269–82 | All participants aged < 16 years |
| IRCT201101015520N1. Comparison of Pressure Distribution Among Three Kind of AFOs. 2011 | No patients with NMD or CNS condition |
| IRCT201206295520N7. A Comparison between the Effects of Hip Flexion Assist Orthosis and Conventional Ankle Foot Orthosis on Walking in Multiple Sclerosis Patients. 2012 | Laboratory setting only |
| ISRCTN21404893. Ankle-foot Orthoses by Laser-Scanning or Existing Methods. 2010 | Not instability of the knee |
| ISRCTN52126764. Biomechanics Visualisation in Ankle-foot Orthoses (AFO) Tuning for Stroke. 2011 | Not orthotic intervention |
| ISRCTN61814249. A Pilot Study of a Cross Trial with Randomised use of Ankle Foot Orthoses and Ligaflex for People with CMT Disease. 2006 | Not instability of the knee |
| ISRCTN72463123. Clinical Trial to Compare Electrical Stimulation and the Conventional Ankle-foot Orthosis (AFO) in the Treatment of Dropped Foot Following Stroke. 2001 | Not instability of the knee |
| ISRCTN94187624. The Effect of Two Different Ankle-foot-orthoses on Walking and Quality of Life in Children with Hereditary Motor and Sensory Neuropathy (HMSN) – Also Known as Charcot-Marie Tooth (CMT) Disease. 2005 | All participants aged < 16 years |
| Itokazu M, Yamamoto S. Examination of gait improvement in spina bifida elicited by an ankle-foot orthosis with double-braking function. Rigakuryoho Kagaku 2011;26:69–74 | All participants aged < 16 years |
| Iwata Y, Sakamoto T, Maruyama A, Tateishi T, Yorimoto K, Yajima H, et al. Utility of subjective pain scales for assessments of standing with knee-ankle-foot-orthoses in Duchenne muscular dystrophy. Neuromuscul Disord 2012;22:880 | All participants aged < 16 years |
| Jaeger RJ. Electromyographic activity in paraplegic individuals during standing in knee-ankle-foot orthoses. Abstr Soc Neurosci 1984;10:904 | Not orthotic intervention |
| Jagadamma KC, Owen E, Coutts FJ, Herman J, Yirrell J, Mercer TH, et al. The effects of tuning an ankle-foot orthosis footwear combination on kinematics and kinetics of the knee joint of an adult with hemiplegia. Prosthet Orthot Int 2010;34:270–6 | Not primary study |
| Jariod-Gaudes R, Rodríguez-Pérez A, Hidalgo-Mendía B, Bouzas-Pérez D, Ruiz-Alejos Garrido S, Muro-Martínez de Quel J. [KAFO orthosis in glass fibre in patient with hemiparesis and hemiagnosia in post-cerebral stroke.] Rehabilitacion 2008;42:153–7 | Not orthotic intervention |
| Johnson WB, Fatone S, Gard SA. Walking mechanics of persons who use reciprocating gait orthoses. J Rehabil Res Dev 2009;46:435–46 | No patients with NMD or CNS condition |
| Jones Oen, K, Myhr KM, Rundhovde Skar AB, Moen Brandal K, Smedal T. WalkAide: a multidisciplinary model for adjustment and evaluation (#46). Multiple Sclerosis 2012;18:S30 | Not instability of the knee |
| JPRN-UMIN000007422. The Effect of an Ankle-foot Orthosis with Dorsiflexion Resistance on Stance Phase in Hemiplegic Gait. 2012 | No outcomes reported |
| JPRN-UMIN000010308. The Real-Time Effect of Double Upright Ankle Foot Orthosis for Walking Ability with Acute Hemiplegic Patients. 2013 | Not instability of the knee |
| JPRN-UMIN000010917. Development of Ankle Foot Orthosis by applying Magnetic Rheological Fluid Brake. 2013 | Not orthotic intervention |
| Junn C, Groah SL. Rehabilitation of severe guillain-barre syndrome resembling clinical brain death: a case report. PM R 2012;1:S280 | Not orthotic intervention |
| Kameyama S, Osawa A, Maeshima S, Takeda K, Hirano Y, Nishio D, et al. Refinement of the knee ankle foot orthosis for stroke patients with severe hemiplegia. Int J Stroke 2010;5:292 | Not orthotic intervention |
| Kaneko K, Koshi A, Matsuda Y, Kondoh K. Development of knee ankle foot orthosis made of magnesium alloy with high strength and toughness. Keikinzoku 2008;58:617–21 | No patients with NMD or CNS condition |
| Kaoru A. Comparison of static balance, walking velocity, and energy consumption with knee-ankle-foot orthosis, walkabout orthosis, and reciprocating gait orthosis in thoracic-level paraplegic patients. J Prosthet Orthot 2006;18:6 | Laboratory setting only |
| Kaplan LI, Grynbaum BB, Rusk HA, Anastasia T, Gassler S. A reappraisal of braces and other mechanical aids in patients with spinal cord dysfunction: results of a follow-up study. Arch Phys Med Rehabil 1966;47:393–405 | No patients with NMD or CNS condition |
| Karimi MT. The physiological benefits and problems associated with using standing and walking orthoses in individuals with spinal cord injury-a meta-analytic review. JOTR 2012;16:37–40 | Not primary study |
| Karimi MT. What are the next steps in designing an orthosis for paraplegic subjects? Int J Prev Med 2012;3:145–59 | Not primary study |
| Karimi MT, Mostamand J, Fatoye F. The use of motion analysis system and orthosis in patients with neuro-musculoskeletal disorders. J Mech Med Biol 2014;14 | No patients with NMD or CNS condition |
| Karimi MT, Amiri P, Esrafilian A, Sedigh J, Fatoye F. Performance of spinal cord injury individuals while standing with the Mohammad Taghi Karimi reciprocal gait orthosis (MTK-RGO). Australas Phys Eng Sci Med 2013;36:35–42 | Laboratory setting only |
| Kaufman KR, Irby SE. Ambulatory KAFOs: a biomechanical engineering perspective. J Prosthet Orthot 2006;82 | Not primary study |
| Kaufman KR, Irby SE, Mathewson JW, Wirta BW, Sutherland DH. Energy-efficient knee-ankle-foot orthosis: a case study. J Prosthet Orthot 1996;8:79–85 | Laboratory setting only |
| Kawamoto H, Taal S, Niniss H, Hayashi T, Kamibayashi K, Eguchi K, Sankai Y. Voluntary motion support control of Robot Suit HAL triggered by bioelectrical signal for hemiplegia. Conf Proc IEEE Eng Med Biol Soc 2010:462–6 | Not orthotic intervention |
| Kawamoto H, Hayashi T, Sakurai T, Eguchi K, Sankai Y. Development of single leg version of HAL for hemiplegia. Conf Proc IEEE Eng Med Biol Soc 2009:5038–43 | Not orthotic intervention |
| Kelleher KJ, Spence WD, Solomonidis S, Apatsidis D. The effect of textured insoles on gait patterns of people with multiple sclerosis. Gait Posture 2010;32:67–71 | Not instability of the knee |
| Kent HO. Vannini-Rizzoli stabilising orthosis (boot): preliminary report on a new ambulatory aid for spinal cord injury. Arch Phys Med Rehabil 1992;73:302–7 | Not instability of the knee |
| Kesikburun S, Koroglu Omac O, Yasar E, Yilmaz B, Kenan Tan A. Ultrasound guided block of the saphenous neuroma following use of an AFO in a patient with paraplegia. A case report. Eur J Phys Rehabil Med 2014;50:197–86 | Not instability of the knee |
| Khanna I, Roy A, Rodgers MM, Krebs HI, Macko RM, Forrester LW. Effects of unilateral robotic limb loading on gait characteristics in subjects with chronic stroke. J Neuroeng Rehabil 2010;7:23 | Laboratory setting only |
| Kim CM, Eng JJ, Whittaker MW. Effects of a simple functional electric system and/or a hinged ankle-foot orthosis on walking in persons with incomplete spinal cord injury. Arch Phys Med Rehabil 2004;85:1718–23 | Not instability of the knee |
| Kim DH, Joa KL, Shin YK, Lee JJ. Immediate effect of Walkbot robotic gait training on neuromechanical knee stiffness in spastic hemiplegia: a case report. NeuroRehabilitation 2013;32:833–8 | Not orthotic intervention |
| Kim G, Kang S, Kang S, Ryu J, Mun M, Kim K. Unlockable knee joint mechanism for powered gait orthosis. Int J Precis Eng Man 2009;10:83–9 | Not orthotic intervention |
| Kimishima K, Hachisuka K, Ogata H, Tanaka S, Tajima F. Supracondylar knee-ankle-foot orthosis for post-polio syndrome. J UOEH 1991;13:251–5 | Not orthotic intervention |
| Kinali M, Main M, Eliahoo J, Messina S, Knight RK, Lehovsky J, et al. Predictive factors for the development of scoliosis in Duchenne muscular dystrophy. Eur J Paediatr Neurol 2007;11:160–6 | All participants aged < 16 years |
| Kirtley C. Principles and practice of paraplegic locomotion: experience with the walkabout walking system. Aust Orthotics Prosthet Mag 1992;7:4–8 | Unavailable |
| Kobayashi T, Leung AK, Akazawa Y, Hutchins SW. The effect of varying the plantarflexion resistance of an ankle-foot orthosis on knee joint kinematics in patients with stroke. Gait Posture 2013;37:457–9 | Laboratory setting only |
| Kobayashi H, Hashimoto T, Nakayama S, Irie K. Development of an active walker and its effect. JMR 2012;24:275–83 | Laboratory setting only |
| Kobetic R, To CS, Schnellenberger JR, Audu ML, Bulea TC, Gaudio R, et al. Development of hybrid orthosis for standing, walking, and stair climbing after spinal cord injury. J Rehabil R D 2009;46:447–62 | Not orthotic intervention |
| Kobetic R, Marsolais EB, Triolo RJ, Davy DT, Gaudio R, Tashman S. Development of a hybrid gait orthosis: a case report. J Spinal Cord Med 2003;26:254–8 | Not orthotic intervention |
| Kobetic R, Marsolais EB, Triolo RJ, Davy DT, Gaudio R, Tashman S. Technical perspective development of a hybrid gait orthosis: a case report. J Spinal Cord Med 2003;26:254–8 | Not orthotic intervention (duplicate publication) |
| Kofman J, Allard P, Sibille J, Duhaime M, Vanasse M. A Spring-loaded Knee-ankle Orthosis in the Management of Duchenne Muscular Dystrophy Patients. Second International Conference on Rehabilitation Engineering, Ottawa, ON, Canada, 1984. pp. 255–6 | Unavailable |
| Kottink AI, Tenniglo MJ, de Vries WH, Hermens HJ, Buurke JH. Effects of an implantable two-channel peroneal nerve stimulator versus conventional walking device on spatiotemporal parameters and kinematics of hemiparetic gait. J Rehabil Med 2012;44:51–7 | Not orthotic intervention |
| Kottink AI, Hermens HJ, Nene AV, Tenniglo MJ, Groothuis-Oudshoorn CG, IJzerman MJ. Therapeutic effect of an implantable peroneal nerve stimulator in subjects with chronic stroke and footdrop: a randomized controlled trial. Phys Ther 2008;88:437–48 | Not orthotic intervention |
| Koutsou AD, Rocon E, Pons JL, Moreno JC. Output-error model system identification for FES-driven control of knee extensor muscles. Artif Organs 2010;34:A42 | No outcomes reported |
| Krishnamoorthy V, Hsu WL, Kesar TM, Benoit DL, Banala SK, Perumal R, et al. Gait training after stroke: a pilot study combining a gravity-balanced orthosis, functional electrical stimulation, and visual feedback. J Neurol Phys Ther 2008;32:192–202 | Not orthotic intervention |
| Krishnan C, Ranganathan R, Dhaher YY, Rymer WZ. A pilot study on the feasibility of robot-aided leg motor training to facilitate active participation. PLOS ONE 2013;8 | Not orthotic intervention |
| Kuhn DR, Yochum TR, Cherry AR, Rodgers SS. Immediate changes in the quadriceps femoris angle after insertion of an orthotic device. J Manipulative Physiol Ther 2002;25:465–70 | Not instability of the knee |
| La Torre RR, Richards M, Ramcharran S. Ischial thigh knee ankle orthosis. Orthotics Prosthet 1973;27:3–8 | No outcomes reported |
| Laffont I, Julia M, Tiffreau V, Yelnik A, Herisson C, Pelissier J. Aging and sequelae of poliomyelitis. Ann Phys Rehabil Med 2010;53:24–33 | Not primary study |
| Lam WK, Leong JC, Li YH, Hu Y, Lu WW. Biomechanical and electromyographic evaluation of ankle foot orthosis and dynamic ankle foot orthosis in spastic cerebral palsy. Gait Posture 2005;22:189–97 | All participants aged < 16 years |
| Lampe R, Mitternacht J, Schrodl S, Gerdesmeyer L, Natrath M, Gradinger R. Influence of orthopaedic-technical aid on the kinematics and kinetics of the knee joint of patients with neuro-orthopaedic diseases. Brain Dev 2004;26:219–26 | All participants aged < 16 years |
| Lan Y, Xu GQ, Huang DF, Mao YR, Chen SZ, Pei Z, et al. Association between improved trunk stability and walking capacity using ankle-foot orthosis in hemiparetic patients with stroke: evidence from three-dimensional gait analysis. Chin Med J 2013;126:3869–73 | Laboratory setting only |
| Landsman A, Defronzo D, Anderson J, Roukis T. Scientific assessment of over-the-counter foot orthoses to determine their effects on pain, balance, and foot deformities. J Am Podiat Med Assoc 2009;99:206–15 | Not instability of the knee |
| LeBlanc MA. Clinical evaluation of a comprehensive approach to below knee orthotics. Orthotics Prosthet 1973;27:20–35 | No outcomes reported |
| Lee DY, Choi IH, Chung CY, Shim JS. A modified Wagner technique for femoral lengthening in skeletally mature patients with poliomyelitis. Int Orthop 1993;17:154–7 | Not orthotic intervention |
| Lee KH, Johnston R. Effect of below-knee bracing on knee movement: biomechanical analysis. Arch Phys Med Rehabil 1974;55:179–82 | No patients with NMD or CNS condition |
| Lee KH, Johnston R. Bracing below the knee for hemiplegia: biomechanical analysis. Arch Phys Med Rehabil 1973;54:466–70 | No outcomes reported |
| Lefebvre R, Boucher JP. Effects of foot orthotics upon the ankle and knee mechanical alignment. J Biomech 1989;22:1046 | No patients with NMD or CNS condition |
| Lehmann JF. Push-off and propulsion of the body in normal and abnormal gait. Correction by ankle-foot orthoses. Clin Orthop Relat Res 1993;288:97–108 | Not primary study |
| Lehmann JF. Biomechanics of ankle-foot orthoses: prescription and design. Arch Phys Med Rehabil 1979;60:200–7 | No outcomes reported |
| Lehmann JF, Stonebridge JB. Knee lock device for knee ankle orthoses for spinal cord injured patients: an evaluation. Arch Phys Med Rehabil 1978;59:207–11 | Not primary study |
| Lehmann JF, Stonebridge JB, deLatuer BJ. Pneumatic and standard double upright orthoses: comparison of their biomechanical function in three patients with spinal cord injuries. Arch Phys Med Rehabil 1977;58:72–80 | Laboratory setting only |
| Lehmann JF, Warren CG. Restraining forces in various designs of knee ankle orthoses: their placement and effect on the anatomical knee joint. Arch Phys Med Rehabil 1976;57:430–7 | Laboratory setting only |
| Lehmann JF, Warren CG. Ischial and patellar tendon weight bearing braces: function, design, adjustment, and training. Bull Prosthet Res 1973;19:6–19 | No patients with NMD or CNS condition |
| Lehmann JF, deLateur BJ, Price R. Knee-ankle-foot orthoses for paresis and paralysis. Phys Med Rehabil Clin N Am 1992;3:161–83 | Not primary study |
| Lehmann JF, Condon SM, Price R, deLateur BJ. Gait abnormalities in hemiplegia: their correction by ankle-foot orthoses. Arch Phys Med Rehabil 1987;68:763–71 | Not primary study |
| Lehmann JF, Condon SM, deLateur BJ, Price R. Gait abnormalities in peroneal nerve paralysis and their corrections by orthoses: a biomechanical study. Arch Phys Med Rehabil 1986;67:380–6 | No patients with NMD or CNS condition |
| Lehmann JF, Condon SM, deLateur BJ, Smith JC. Gait abnormalities in tibial nerve paralysis: a biomechanical study. Arch Phys Med Rehabil 1985;66:80–5 | No patients with NMD or CNS condition |
| Lehmann JF, Ko MJ, deLateur BJ. Origin of knee moments during ambulation and their modification by ankle foot orthoses. J Biomech 1983;16:283–4 | No patients with NMD or CNS condition |
| Lehmann JF, Esselman PC, Ko MJ, Smith JC, deLateur BJ, Dralle AJ. Plastic ankle-foot orthoses: evaluation of function. Arch Phys Med Rehabil 1983;64:402–7 | Not instability of the knee |
| Lehmann JF, Ko MJ, deLateur BJ. Knee moments: origin in normal ambulation and their modification by double-stopped ankle-foot orthoses. Arch Phys Med Rehabil 1982;63:345–51 | No patients with NMD or CNS condition |
| Lehmann JF, Ko MJ, deLateur BJ. Origin of knee moments during ambulation and their modification by ankle-foot orthoses. Arch Phys Med Rehabil 1982;63:523 | No patients with NMD or CNS condition |
| Lehmann JF, Warren CG, Hertling D, McGee M, Simons BC, Dralle A. Craig-Scott orthosis: a biochemical and functional evaluation. Arch Phys Med Rehabil 1976;57:438–42 | Laboratory setting only |
| Lehmann JF, Warren CG, Kirkpatr G. Distribution of forces on leg in various designs of foot-ankle-knee orthoses. Arch Phys Med Rehabil 1973;54:586 | Laboratory setting only |
| Lehmann JF, Warren CG, deLateur BJ. A biomechanical evaluation of knee stability in below knee braces. Arch Phys Med Rehabil 1970;51:688–95 | No patients with NMD or CNS condition |
| Lehneis HR. The Swedish knee cage. Artificial Limbs 1968;12:54–7 | No outcomes reported |
| Lemaire ED. Mechanical and biomechanical analysis of a linear piston design for angular-velocity-based orthotic control. J Rehabil R D 2013;50:43–52 | No outcomes reported |
| Leung AK, Wong AF, Wong EC, Hutchins SW. The Physiological Cost Index of walking with an isocentric reciprocating gait orthosis among patients with T(12)–L(1) spinal cord injury. Prosthet Orthot Int 2009;33:61–8 | Laboratory setting only |
| Li YFD, Hsiao-Wecksler ET. Gait mode recognition using an inertial measurement unit to control an ankle-foot orthosis during stair ascent and descent. IEEE Int Conf Rehabil Robot 2013, June | No patients with NMD or CNS condition |
| Limbird TJ, Stills M, Elliott D, Wharton G. Lower extremity telescopic orthosis for immediate fitting in paraplegia. Orthopedics 1989;12:851–4 | Laboratory setting only |
| Lister MJ. Paralytic dysfunction. II. Bracing the unstable knee in flacid paralysis. Phys Ther 1967;47:807–15 | Not primary study |
| Liu XH. [Application of lower limb orthosis in paralysis rehabilitation.] CRTER 2007;11:6252–5 | Not primary study |
| Lobley S, Rogerson J, Cullen J, Freed M. Orthotic design from the New England Regional Spinal Cord Injury Center. Phys Ther 1985;65:492–3 | No outcomes reported |
| Loke M. New concepts in lower limb orthotics. Phys Med Rehabil Clin N Am 2000;11:477–96 | Not primary study |
| Long JT, Sirota N, Klein JP, Wertsch JJ, Janisse D, Harris GF. Biomechanics of the double rocker sole shoe: gait kinematics and kinetics. Conf Proc IEEE Eng Med Biol Soc 2004;7:5107–10 | No patients with NMD or CNS condition |
| Lotta S, Fiocchi A, Giovannini R, Silvestrin R, Tesio L, Raschi A, et al. Restoration of gait with orthoses in thoracic paraplegia: a multicentric investigation. Paraplegia 1994;32:608–15 | Not instability of the knee |
| Lugris U, Carlin J, Luaces A, Cuadrado J. Gait analysis system for spinal cord-injured subjects assisted by active orthoses and crutches. Proc Inst Mech Eng K 2013;227:363–74 | Not orthotic intervention |
| MacLean IC, Granger CV. Use of a short leg brace to support the knee in hemiplegia. Arch Phys Med Rehabil 1967;48:250–2 | No outcomes reported |
| Maeshima S, Osawa A, Nishio D, Hirano Y, Takeda K, Kigawa H, et al. Efficacy of a hybrid assistive limb in post-stroke hemiplegic patients: a preliminary report. BMC Neurol 2011;11:116 | Not orthotic intervention |
| Maeshima S, Osawa A, Nishio D, Hirano Y, Kigawa H, Sankai Y. Ambulation support for stroke patient using the hybrid assistive limb (HAL). Int J Stroke 2010;5:298 | Not primary study |
| Magnusson L, Ramstrand N, Fransson EI, Ahlstrom G. Mobility and satisfaction with lower-limb prostheses and orthoses among users in Sierra Leone: a cross-sectional study. J Rehabil Med 2014;46:438–46 | No patients with NMD or CNS condition |
| Magora A, Robin GC, Rozin R, Gonen B, Saltiel J. Investigation of gait. 5. Effect of a below-knee brace on the contralateral, unbraced leg. Electromyogr Clin Neurophysiol 1973;13:355–61 | No patients with NMD or CNS condition |
| Magora A, Robin GC, Adler E, Libai A, Eitan A. Dynamic strain analysis of below-knee orthopedic braces. A preliminary report. Am J Phys Med 1968;47:31–40 | No patients with NMD or CNS condition |
| Major RE, Stallard J, Farmer SE. A review of 42 patients of 16 years and over using the ORLAU Parawalker. Prosthet Orthot Int 1997;21:147–52 | Not instability of the knee |
| Maki BE, Rosen MJ, Simon SR. Modification of spastic gait through mechanical damping. J Biomech 1985;18:431–43 | All participants aged < 16 years |
| Malas BS. What variables influence the ability of an AFO to improve function and when are they indicated? Myelomeningocele. Clin Orthop Relat Res 2011;469:1308–14 | All participants aged < 16 years |
| Martin D, Dixon J, Hatton A, Rome K, Hodgson D, Warnett R, et al. Moderate/severe pain affects function in people with multiple sclerosis. J Pain 2011;1:P26 | No outcomes reported |
| Martin D, Lamoreux L, Skinner H. An ankle-foot orthosis for dynamic alteration of varus valgus knee moments. J Biomech 1985;18:536 | No outcomes reported |
| Martinet N, Maaref K, Paysant J. Quantitative gait analysis of polio patients walking with orthotic devices. Ann Phys Rehabil Med 2010;53:e122 | Not orthotic intervention |
| Massucci M, Brunetti G, Piperno R, Betti L, Franceschini M. Walking with the advanced reciprocating gait orthosis (ARGO) in thoracic paraplegic patients: energy expenditure and cardiorespiratory performance. Spinal Cord 1998;36:223–7 | Not instability of the knee |
| Mat Dzahir MA, Nobutomo T, Yamamoto SI. Development of body weight support gait training system using pneumatic Mckibben actuators: control of lower extremity orthosis. Conf Proc IEEE Eng Med Biol Soc 2013:6417–20 | No patients with NMD or CNS condition |
| Matsumoto S, Kawahira K, Makita M, Nitta H, Hirao K, Fuse Y, et al. Effect of knee brace combined with pneumatic artificial muscles on muscle strength. J Rehabil Med 2012;44:25–6 | No patients with NMD or CNS condition |
| Mazur JM, Sienko-Thomas S, Wright N, Cummings RJ. Swing-through vs. reciprocating gait patterns in patients with thoracic-level spina bifida. Z Kinderchir 1990;45(Suppl. 1):23–5 | All participants aged < 16 years |
| McCall RE, Schmidt WT. Clinical experience with the reciprocal gait orthosis in myelodysplasia. J Pediatr Orthop 1986;6:157–61 | All participants aged < 16 years |
| McCollough NC. Orthotic management in adult hemiplegia. Clin Orthop Relat Res 1978;131:38–46 | Not primary study |
| McCollough NC. Rationale for orthotic prescription in the lower extremity. Clin Orthop Relat Res 1974;102:32–45 | Not primary study |
| McGhee RB, Tomovic R, Yang PY, MacLean IC. An experimental study of a sensor-controlled external knee locking system. IEEE Trans Biomed Eng 1978;25:195–9 | No patients with NMD or CNS condition |
| McHugh B, Campbell J. Below-knee orthoses. Physiotherapy 1987;73:380–5 | Not primary study |
| McMillan AG, Kendrick K, Michael JW, Aronson J, Horton GW. Preliminary evidence for effectiveness of a stance control orthosis. J Prosthet Orthot 2004;16:6–15 | Laboratory setting only |
| McMillan AG, Kendrick K, Zabel R, Aronson J, Horton G. Stance control orthosis to improve functional gait: a pilot study. Platform & poster presentations for CSM 2003. Neurol Report 2002;26:209 | Laboratory setting only |
| Mefoued S. Robust and Intention-based Control of an Active Orthosis for Assistance of Knee Movements. Thesis. Paris: Université Paris-Est; 2012 | Not orthotic intervention |
| Mefoued S, Mohammed S, Amirat Y. Knee Joint Movement Assistance through Robust Control of an Actuated Orthosis. Proceedings of the IEEE International Conference on Intelligent Robots and Systems, 2011 | No patients with NMD or CNS condition |
| Merkel KD, Miller NE, Merritt JL. Energy expenditure in patients with low-, mid-, or high-thoracic paraplegia using Scott-Craig knee-ankle-foot orthoses. Mayo Clin Proc 1985;60:165–8 | Laboratory setting only |
| Merkel KD, Miller NE, Westbrook PR, Merritt JL. Energy expenditure of paraplegic patients standing and walking with two knee-ankle-foot orthoses. Arch Phys Med Rehabil 1984;65:121–4 | Laboratory setting only |
| Merkel KD, Merritt JL, Miller NE. Energy expenditure comparison in paraplegic patients during stance and ambulation with 2 types of knee ankle foot orthoses. Arch Phys Med Rehabil 1981;62:513 | Laboratory setting only |
| Merritt JL, Yoshida MK. Knee-ankle-foot orthoses: indications and practical applications of long leg braces. Phys Med Rehabil 2000;14:395–422 | Not primary study |
| Meyer PR. Lower limb orthotics. Clin Orthop Relat Res 1974;102:58–71 | Not primary study |
| Meyers AH, Lewis MM. Orthotic management of patients following limb sparing procedures. Arch Phys Med Rehabil 1991;72:783 | No patients with NMD or CNS condition |
| Michael JW. KAFOs for ambulation: an orthotist’s perspective. J Prosthet Orthot 2006;18:187–91 | Not primary study |
| Michael JW. Summary from the Academy’s seventh State-of-the-Science conference on knee-ankle-foot orthoses for ambulation. J Prosthet Orthot 2006;18:132–6 | Not primary study |
| Michael JW, McMillan AG, Kendrick K. Stance control orthoses: history, overview and case example of improved KAFO function. Alignment 2003;60–70 | Background |
| Middleton JW, Sinclair PJ, Smith RM, Davis GM. Postural control during stance in paraplegia: effects of medially linked versus unlinked knee-ankle-foot orthoses. Arch Phys Med Rehabil 1999;80:1558–65 | Not primary study |
| Middleton JW, Fisher W, Davis GM, Smith RM. A medial linkage orthosis to assist ambulation after spinal cord injury. Prosthet Orthot Int 1998;22:258–64 | Laboratory setting only |
| Millard JB. Automatic release knee stabilizing calliper. Ann Phys Med 1970;10:243 | No outcomes reported |
| Miller MK, Hamill J, Ricard MD. Effect of ankle orthoses on lower extremity function. Med Sci Sports Exerc 1988;20:S55 | No patients with NMD or CNS condition |
| Millet A, Paquin JM, Ostermann P. [Nicholas’ orthesis.] Revue de Readaptation Fonctionnelle Professionnalle et Sociale 1982;9:35–43 | Unavailable |
| Mishra DK, Daniel DM, Stone ML. The use of functional knee braces in the control of pathologic anterior knee laxity. Clin Orthop Relat Res 1989;213–20 | No patients with NMD or CNS condition |
| Modisane MR, Stewart A, Riley M. The effect of a knee brace on gait parameters of hypertonic hemiplegic patients. SAJPA 2008;64:43–7 | Laboratory setting only |
| Moreno J, Pons JL, Koutsou A. The Rehabot-Knee Project approach for recovery of neuromuscular control of the knee with controllable braces. Proceedings of the 10th Congress of the European Federation for Research in Rehabilitation, Riga, Latvia, 09–12 September 2009. Int J Rehabil Res 2009;32(Suppl. 1):112 | No patients with NMD or CNS condition |
| Moreno JC, Brunetti F, Rocon E, Pons JL. Immediate effects of a controllable knee ankle foot orthosis for functional compensation of gait in patients with proximal leg weakness. Med Biol Eng Comput 2008;46:43–53 | Laboratory setting only |
| Morinaka Y, Matsuo Y, Nojima M, Inami Y, Nojima K. Biomechanical study of a knee-ankle-foot-orthosis for hemiplegic patients. Prosthet Orthot Int 1984;8:97–9 | No outcomes reported |
| Morinaka Y, Matsuo Y, Nojima M, Morinaka S. Clinical evaluation of a knee-ankle-foot-orthosis for hemiplegic patients. Prosthet Orthot Int 1982;6:111–15 | Not primary study |
| Mulder AJ, Veltink PH, Boom HB, Zilvold G. Low-level finite state control of knee joint in paraplegic standing. J Biomed Eng 1992;14:3–8 | Not orthotic intervention |
| Mulroy SJ, Eberly VJ, Gronely JK, Weiss W, Newsam CJ. Effect of AFO design on walking after stroke: impact of ankle plantar flexion contracture. Prosthet Orthot Int 2010;34:277–92 | Not instability of the knee |
| Murray S, Goldfarb M. Towards the use of a lower limb exoskeleton for locomotion assistance in individuals with neuromuscular locomotor deficits. Conf Proc IEEE Eng Med Biol Soc 2012:1912–15 | No patients with NMD or CNS condition |
| Nagarkatti DG, Banta JV, Thomson JD. Charcot arthropathy in spina bifida. J Pediatr Orthop 2000;20:82–7 | All participants aged < 16 years |
| Nakamura T, Saito K, Wang ZD, Kosuge K; IEEE. Realizing a Posture-based Wearable Antigravity Muscles Support System for Lower Extremities. Proceedings of the IEEE 9th International Conference on Rehabilitation Robotics 2005, Chicago, IL, 28 June–1 July 2005. pp. 273–6 | No patients with NMD or CNS condition |
| NCT00199589. Treatment of Spastic Equinovarus Foot After Stroke. 2005 | Not instability of the knee |
| NCT00216320. Efficacy of the WalkAide and AFOs for CVA. 2005 | Not instability of the knee |
| NCT00961103. Motor Development and Orthoses in Spinal Muscular Atrophy (SMA). 2009 | All participants aged < 16 years |
| NCT01006772. Rehabilitation of Early Stroke Patients Using an AFO: An RCT. 2009 | Unavailable |
| NCT01087957. WalkAide Compared to Ankle-Foot Orthosis (AFO) in Stroke Patients. 2010 | Not instability of the knee |
| NCT01251549. Safety and Performance Evaluation of ReWalk Reciprocating Gait Orthosis (RGO). 2010 | Laboratory setting only |
| NCT01320839. Effects of an Ankle-Foot Orthosis on Gait While Performing an Attention Demanding Task. 2011 | Not instability of the knee |
| NCT01415700. Comparison Between Implanted Functional Electrical Stimulation and Foot Orthosis. 2011 | Not instability of the knee |
| NCT01499862. Mobility Training Using a Bionic Knee Orthosis in Patients Chronic Post-Stroke: A Case Series. 2011 | Unavailable |
| NCT01558232. A Prospective Study to Evaluate Use of the Tibion Bionic Leg in Sub-Acute Post-stroke Patients. 2012 | Not orthotic intervention |
| NCT01626417. Evaluation of Transfer Kinematics and Kinetics in Patients Chronic Post-stroke Using the Tibion Bionic Leg. 2012 | Unavailable |
| NCT01704807. Duration of Spinal Manipulation Effects as Influenced by Orthotics. 2012 | No patients with NMD or CNS condition |
| NCT01723046. Therapeutic Effects of a New Upper Limb Robot Assisted Therapy Device for Persons After Stroke. 2012 | Not instability of the knee |
| NCT01796860. Effectiveness of Ankle Foot Orthoses on Gait in Multiple Sclerosis. 2013 | Unavailable |
| NCT01798927. Effect of Lower Extremity Ankle Foot Orthoses in Parkinson’s Disease. 2013 | Unavailable |
| NCT01943669. Exoskeletons for Spinal Cord Injury: A Feasibility Study. 2013 | Not instability of the knee |
| NCT01947582. The Effects of Ankle Foot Orthoses (AFOs) on Mobility in Persons With Multiple Sclerosis (MS). 2013 | Not instability of the knee |
| NCT01961557. Evaluating a New Knee-Ankle-Foot Brace to Improve Crouch Gait in People With Cerebral Palsy. 2013 | All participants aged < 16 years |
| NCT02089880. Comparing Functional Outcomes in Individuals Using Micro-processor Controlled Orthosis Versus Stance Control Orthosis. 2014 | Unavailable |
| NCT02109393. Use of Lokomat in Patients With Progressive Supranuclear Palsy. 2014 | No outcomes reported |
| NCT02113189. Impact of Walking Practice on Persons With PD. 2014 | Not instability of the knee |
| NCT02122783. Comparison of the Functional Walking Outcomes of Two Settings of a Commercially Available Ankle Foot Orthosis in Adult Stroke Patients. 2014 | Unavailable |
| Nolan KJ, Savalia KK, Yarossi M, Elovic EP. Evaluation of a dynamic ankle foot orthosis in hemiplegic gait: a case report. Neurorehabilitation 2010;27:343–50 | Not primary study |
| NTR1930. Timing of providing ankle-foot orthoses in stroke rehabilitation. 2009 | Unavailable |
| Obinata G, Fukada S, Matsunaga T, Iwami T, Shimada Y, Miyawaki K, et al. Hybrid control of powered orthosis and functional neuromuscular stimulation for restoring gait. Conf Proc IEEE Eng Med Biol Soc 2007;4879–82 | Laboratory setting only |
| O’Daniel WE, Hahn HR. Follow-up usage of the Scott-Craig Orthosis in paraplegia. Paraplegia 1981;19:373–8 | Not appropriate comparator |
| Ofir R, Sell H. Orthoses and ambulation in hemiplegia: a ten year retrospective study. Arch Phys Med Rehabil 1980;61:216–20 | Not orthotic intervention |
| Ogilvie C, Messenger N, Bowker P, Rowley DI. Orthotic compensation for non-functioning hip extensors. Z Kinderchir 1988;43(Suppl. 2):33–5 | Not instability of the knee |
| Ohsawa S, Ikeda S, Tanaka S, Takahashi T, Takeuchi T, Utsunomiya M, et al. A new model of plastic ankle foot orthosis (FAFO (II)) against spastic foot and genu recurvatum. Prosthet Orthot Int 1992;16:104–8 | Not instability of the knee |
| Ohta Y, Yano H, Suzuki R, Yoshida M, Kawashima N, Nakazawa K. A two-degree-of-freedom motor-powered gait orthosis for spinal cord injury patients. Proc Inst Mech Eng H 2007;221:629–39 | Laboratory setting only |
| Pardo AC, Do T, Ryder T, Meyer A, Miles L, Wong BL. Combination of steroids and ischial weight-bearing knee ankle foot orthoses in Duchenne’s muscular dystrophy prolongs ambulation past 20 years of age: a case report. Neuromuscul Disord 2011;21:800–2 | Not primary study |
| Parker HG. Chronic anteromedial instability of the knee. Clin Orthop Relat Res 1979;142:123–30 | No patients with NMD or CNS condition |
| Pavlik AJ. The effect of long-term ankle-foot orthosis use on gait in the poststroke population. J Prosthet Orthot 2008;20:49–52 | Not primary study |
| Peaco A, Halsne E, Hafner BJ. Assessing satisfaction with orthotic devices and services: a systematic literature review. J Prosthet Orthot 2011;23:95–105 | Not primary study |
| Peizer E, Lorenze EJ, Dixon M. The genucentric joint orthosis. Med Instrum 1982;16:207–8 | No outcomes reported |
| Perrin C, Condemine A, Giraux P. [Efficacy and satisfaction of a lowered dynamical ankle-foot orthosis in chronic walking hemiparetic subjects.]. Ann Phys Rehabil Med 2012;55:e106–8 | Laboratory setting only |
| Perry J. Lower-extremity bracing in hemiplegia. Clin Orthop Relat Res 1969;63:32–8 | Not primary study |
| Perry J, Mulroy SJ, Renwick SE. The relationship of lower extremity strength and gait parameters in patients with post-polio syndrome. Arch Phys Med Rehabil 1993;74:165–9 | Not orthotic intervention |
| Perry J, Gronley JK, Lunsford T. Rocker shoe as walking aid in multiple sclerosis. Arch Phys Med Rehabil 1981;62:59–65 | Not instability of the knee |
| Phillips GF, Andrews BJ, Chizeck H, Barnicle K. Finite State Control of Paraplegic Gait Using a Hybrid FNS Orthosis. IEEE/Engineering in Medicine and Biology Society Annual Conference, 4–7 November 1988 | No outcomes reported |
| Pierrat B, Calmels P, Molimard J, Navarro L, Avril S. [Mechanical assessment of knee orthoses: finite element modeling. Preliminary data.] Ann Phys Rehabil Med 2012;55:e106–8 | No patients with NMD or CNS condition |
| Plassat R, Perrouin-Verbe B, Stephan A, Rome J, Brunel P, Richard I, et al. Gait orthosis in patients with complete thoracic paraplegia. Review of 43 patients. Ann Readapt Med Phys 2005;48:240–7 | Exclude but flag for qualitative |
| Ploeger HE, Bus SA, Brehm MA, Nollet F. Ankle-foot orthoses that restrict dorsiflexion improve walking in polio survivors with calf muscle weakness. Gait Posture 2014;40:391–8 | Laboratory setting only |
| Portnoy S, Schwartz I. Gait characteristics of post-poliomyelitis patients: standardization of quantitative data reporting. Ann Phys Rehabil Med 2013;56:527–41 | Not orthotic intervention |
| Pratt DJ. Three-dimensional electrogoniometric study of selected knee orthoses. Clin Biomech 1991;6:67–72 | No patients with NMD or CNS condition |
| Quentin B, Wissel J. Recommendations for remobilisation therapies following stroke. Nervenheilkunde 2010;29:537 | No outcomes reported |
| Quintero H, Farris R, Hartigan C, Clesson I, Goldfarb M. A powered lower limb orthosis for providing legged mobility in paraplegic individuals. Top Spinal Cord Inj Rehabil 2011;17:25–33 | Laboratory setting only |
| Quintero HA, Farris RJ, Goldfarb M. Control and Implementation of a Powered Lower Limb Orthosis to Aid Walking in Paraplegic Individuals. Proceedings of the IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–2 July 2011 | Not primary study |
| Quintero HA, Farris RJ, Hartigan C, Clesson I, Goldfarb M. A Powered Lower Limb Orthosis for Providing Legged Mobility in Paraplegic Individuals. The North American Neurorehabilitation Symposium: Advances in Robotics and Neurorehabilitation in SCI and Other Neurotrauma, August 2010, Atlanta, Georgia. Top Spinal Cord Inj Rehabil 2011;17:25–33 | Laboratory setting only |
| Ramdharry GM, Pollard A, Marsden JF, Reilly MM. Comparing gait performance of people with Charcot-Marie-Tooth disease who do and do not wear ankle foot orthoses. J Peripher Nerv Syst 2010;15:32 | Not instability of the knee |
| Ransom CL. Orthoses and adaptive equipment. Continuum (Minneap Minn) 2011;17:494–509 | Not primary study |
| Rasmussen AA, Smith KM, Damiano DL. Biomechanical evaluation of the combination of bilateral stance-control knee-ankle-foot orthoses and a reciprocating gait orthosis in an adult with a spinal cord injury. J Prosthet Orthot 2007;19:42–7 | Laboratory setting only |
| Raveh E, Schwartz I, Portnoy S. Comparison of dynamic muscle activity and 3D gait kinematics acquired while wearing an electronic stance control orthosis versus a locked knee-ankle-foot orthosis: a case study. Gait Posture 2013;38:S23–4 | Laboratory setting only |
| Rocha CT, Hoffman EP. Limb-girdle and congenital muscular dystrophies: current diagnostics, management, and emerging technologies. Curr Neurol Neurosci Rep 2010;10:267–76 | Not orthotic intervention |
| Roehrig S, Yates DA. Case report: effects of a new orthosis and physical therapy on gait in a subject with longstanding hemiplegia. J GeriatrPhys Ther 2008;31:38–46 | Not primary study |
| Roggow D, Strasser DC, Fletcher GF, Frankowiak R. Bilateral knee extensor assist ankle foot orthoses for an 80-year-old man with inclusion body myositis and heart disease. Arch Phys Med Rehabil 1991;72:813 | Not primary study |
| Romkes J, Brunner R. Comparison of a dynamic and a hinged ankle-foot orthosis by gait analysis in patients with hemiplegic cerebral palsy. Gait Posture 2002;15:18–24 | All participants aged < 16 years |
| Rosa MC, Marques AS, Rodrigues J, Metcalf CD, Demain S. Relationship between the time spent in double support phase of gait and the knee strength in subjects with stroke. Cerebrovasc Dis 2013;35:788 | Not orthotic intervention |
| Rosenbaum D, Rodl R, Entrup M, Klein D. Shoe modification or inserts can effect relief of the knee joint. Z Orthop Ihre Grenzgeb 2002;140:579–80 | No patients with NMD or CNS condition |
| Rosenthal RK, Deutsch SD, Miller W. A fixed ankle, below the knee orthosis for the management of genu recurvatum in spastic cerebral palsy. J Bone Joint Surg Am 1975;57:545–7 | All participants aged < 16 years |
| Rosman N, Spira E. Paraplegic use of walking braces: a survey. Arch Phys Med Rehabil 1974;55:310–14 | No patients with NMD or CNS condition |
| Rubin G, Danisi M. A knee stabilising ankle foot orthosis. Orthotics Prosthet 1975;29:11–14 | Not orthotic intervention |
| Saitoh E, Suzuki T, Sonoda S, Fujitani J, Tomita Y, Chino N. Clinical experience with a new hip-knee-ankle-foot orthotic system using a medial single hip joint for paraplegic standing and walking. Am J Phys Med Rehabil 1996;75:198–203 | Laboratory setting only |
| Sarikaya S, Basaran A, Ortancil O, Balbaloglu O. A new modification of KAFO for assistance in knee extension. Disabil Rehabil Assist Technol 2007;2:67–70 | Not primary study |
| Sarno JE, Lehneis HR. Prescription considerations for plastic below-knee orthoses. Arch Phys Med Rehabil 1971;52:503–10 | Not primary study |
| Sautreuil P, Belmahfoud R, Vandermeersch T, Thoumie P, Bendaya S. [Lower limb’s device for poliomyelitic adult patient.] Lett Med Phys Readapt 2009;25:127–35 | Not primary study |
| Scivoletto G, Mancini M, Fiorelli E, Morganti B, Molinari M. A prototype of an adjustable advanced reciprocating gait orthosis (ARGO) for spinal cord injury (SCI). Spinal Cord 2003;41:187–91 | Laboratory setting only |
| Scott CM. Functional Long Leg Brace Research 1971. URL: www.ntis.gov/search/product.aspx?ABBR = PB2165141 | Unavailable |
| Seymour RJ, Knapp CF, Anderson TR, Kearney JT. Paraplegic use of the Orlau swivel walker: case report. Arch Phys Med Rehabil 1982;63:490–4 | Laboratory setting only |
| Sheffler LR, Bailey SN, Chae J. Spatiotemporal and kinematic effect of peroneal nerve stimulation versus an ankle-foot orthosis in patients with multiple sclerosis: a case series. PM R 2009;1:604–11 | Not instability of the knee |
| Shurr D, Miller H, Albright J, Feldick H. The Iowa Knee Orthosis. Orthotics Prosthet 1978;32:20–4 | Not primary study |
| Silver-Thorn B, Herrmann A, Current T, McGuire J. Effect of ankle orientation on heel loading and knee stability for post-stroke individuals wearing ankle-foot orthoses. Prosthet Orthot Int 2011;35:150–62 | Not orthotic intervention |
| Simoens S, Guillaume P, Moldenaers I, Depoorter A, De Coster S, Van den Steen D, et al. International comparison of orthotic brace prices. Eur J Health Econ 2009;10:149–55 | Not orthotic intervention |
| Simoens S, Debruyne H, Moldenaers I, Guillaume P, De Coster S, Van den Steen D, et al. Do tariffs and prices correspond with costs? A case study of orthotic braces. J Med Econ 2008;11:245–54 | Not orthotic intervention |
| Singer M, Kobayahi T, Lincoln L, Orendurff M, Foreman KB. The effect of AFO stiffness on the knee joint during the heel strike phase of gait in stroke patients. FASEB J 2014;1 | Not instability of the knee |
| Sliwa J. Postpolio syndrome and rehabilitation. Am J Phys Med Rehabil 2004;83:909 | Not primary study |
| Smith GC, Moutvic MA, Oh TH. Use of stance-control knee-ankle-foot orthoses in a patient with inclusion body myositis and falls. Poster presented at the Association of Academic Physiatrists Annual Meeting, Nashville, TN, 25 February–1 March 2014 | Unavailable |
| Smits J, Zepf A. [Orthotic management of the unstable knee joint.] Orthop Tech 1993;268–73 | Not primary study |
| Solomonow M, Baratta R, D’Ambrosia R. Standing and walking after spinal cord injury: experience with the Reciprocating Gait Orthosis powered by electrical muscle stimulation. Top Spinal Cord Inj Rehabil 2000;5:29–53 | Not orthotic intervention |
| Solomonow M, Reisin E, Aguilar E, Baratta RV, Best R, D’Ambrosia R. Reciprocating gait orthosis powered with electrical muscle stimulation (RGO II). Part II: medical evaluation of 70 paraplegic patients. Orthopedics 1997;20:411–18 | Not instability of the knee |
| Solomonow M, Baratta R, Hirokawa S, Rightor N, Walker W, Beaudette P, et al. The RGO Generation II: muscle stimulation powered orthosis as a practical walking system for thoracic paraplegics. Orthopedics 1989;12:1309–15 | Laboratory setting only |
| Sonoda S, Imahori R, Saitoh E, Tomita Y, Domen K, Chino N. Clinical application of the modified medially-mounted motor-driven hip gear joint for paraplegics. Disabil Rehabil 2000;22:294–7 | Not primary study |
| Spector P, Davis J. Hip-abduction, knee-extension orthosis. Am J Occup Ther 1982;36:461–2 | Not instability of the knee |
| Stallard J, Henshaw JH, Lomas B, Poiner R. The ORLAU VCG (variable centre of gravity) swivel walker for muscular dystrophy patients. Prosthet Orthot Int 1992;16:46–8 | No outcomes reported |
| Stein RB, Hayday F, Chong S, Thompson AK, Rolf R, James KB, Bell G. Speed and efficiency in walking and wheeling with novel stimulation and bracing systems after spinal cord injury: a case study. Neuromodulation 2005;8:264–71 | Not orthotic intervention |
| Steinfeldt F, Seifert W, Gunther KP. [Modern carbon fibre orthoses in the management of polio patients: a critical evaluation of the functional aspects.] Z Orthop Ihre Grenzgeb 2003;141:357–61 | Not primary study |
| Stevens PM. Lower limb orthotic management of Duchenne muscular dystrophy: a literature review. J Prosthet Orthot 2006;18:111–19 | Not primary study |
| Suga T, Kameyama O, Ogawa R, Matsuura M, Oka H. Newly designed computer controlled knee-ankle-foot orthosis (Intelligent Orthosis). Prosthet Orthot Int 1998;22:230–9 | No patients with NMD or CNS condition |
| Sulzer JS, Gordon KE, Dhaher YY, Peshkin MA, Patton JL. Preswing knee flexion assistance is coupled with hip abduction in people with stiff-knee gait after stroke. Stroke 2010;41:1709–14 | Laboratory setting only |
| Summers P, Singleton C. Sizable functional improvement in a recently diagnosed case of adult onset muscular dystrophy and multiple sclerosis after outpatient therapy failure: a case report. PM R 2013;1:S156 | Not orthotic intervention |
| Suzuki N, Shinohara T, Kimizuka M, Yamaguchi K, Mita K. Energy expenditure of diplegic ambulation using flexible plastic ankle foot orthoses. Bull Hosp Jt Dis 2000;59:76–80 | All participants aged < 16 years |
| Suzuki T, Sonoda S, Saitoh E, Onogi K, Fujino H, Teranishi T, et al. Prediction of gait outcome with the knee-ankle-foot orthosis with medial hip joint in patients with spinal cord injuries: a study using recursive partitioning analysis. Spinal Cord 2007;45:57–63 | Not instability of the knee |
| Suzuki T, Sonoda S, Saitoh E, Murata M, Uno A, Shimizu Y, et al. Development of a novel type of shoe to improve the efficiency of knee-ankle-foot orthoses with a medial single hip joint (Primewalk orthoses): a novel type of shoe for Primewalk orthosis. Prosthet Orthot Int 2005;29:303–11 | Not primary study |
| Swift TA, Strausser KA, Zoss AB, Kazerooni H. Control and Experimental Results for Post Stroke Gait Rehabilitation with a Prototype Mobile Medical Exoskeleton. ASME 2010 Dynamic Systems and Control Conference, 12–15 September 2010, Cambridge, MA | Not instability of the knee |
| Swinnen E, Beckwee D, Meeusen R, Baeyens JP, Kerckhofs E. Does robot-assisted gait rehabilitation improve balance in stroke patients? A systematic review. Top Stroke Rehabil 2014;21:87–100 | Not primary study |
| Taiar R, Boyer FC, Roche N, Pradon D. Technical and smart fabrics fibres orthosis for recurvatum knee in hemiplegic patients. Ann Phys Rehabil Med 2013;56:e25–6 | Laboratory setting only |
| Tanabe S, Hirano S, Saitoh E. Wearable Power-Assist Locomotor (WPAL) for supporting upright walking in persons with paraplegia. Neurorehabilitation 2013;33:99–106 | Not primary study |
| Tatar Y. [Lower extremity orthoses in spinal cord injury.] Turk Fiz Tip Rehab 2006;52:B12–17 | Not primary study |
| Taylor MK. KAFOs for patients with neuromuscular deficiencies. J Prosthet Orthot 2006;3 | Not primary study |
| Thoumie P, Sautreuil P, Mevellec E. [Knee orthosis. Evaluation of clinical efficiency with a review of the literature.] Ann Readapt Med Phys 2002;45:1–11 | Not primary study |
| Thoumie P, Sautreuil P, Mevellec E. [Knee orthosis. First part: evaluation of physiological properties based on a review of the literature.] Ann Readapt Med Phys 2001;44:567–80 | Not primary study |
| Thoumie P, Le Claire G, Beillot J, Dassonville J, Chevalier T, Perrouin-Verbe B, et al. Restoration of functional gait in paraplegic patients with the RGO–II hybrid orthosis. A multicenter controlled study. II: Physiological evaluation. Paraplegia 1995;33:654–9 | Not instability of the knee |
| Tian F, Elahinia M, Hefzy MS. A Dynamic Knee-Ankle-Foot Orthosis with Superelastic Actuators. ASME 2013 Conference on Smart Materials, Adaptive Structures and Intelligent Systems (SMASIS), 16–18 September 2013, Snowbird, UT | No patients with NMD or CNS condition |
| To CS, Kobetic R, Bulea TC, Audu ML, Schnellenberger JR, Pinault G, et al. Sensor-based stance control with orthosis and functional neuromuscular stimulation for walking after spinal cord injury. J Prosthet Orthot 2012;24:124–32 | Not orthotic intervention |
| To CS, Kobetic R, Bulea TC, Audu ML, Schnellenberger JR, Pinault G, et al. Stance control knee mechanism for lower-limb support in hybrid neuroprosthesis. J Rehabil R D 2011;48:839–50 | Laboratory setting only |
| Trotter LC, Pierrynowski MR. Changes in gait economy between full-contact custom-made foot orthoses and prefabricated inserts in patients with musculoskeletal pain: a randomized clinical trial. J Am Podiatr Med Assoc 2008;98:429–35 | No patients with NMD or CNS condition |
| Trotter LC, Pierrynowski MR. The short-term effectiveness of full-contact custom-made foot orthoses and prefabricated shoe inserts on lower-extremity musculoskeletal pain: a randomized clinical trial. J Am Podiatr Med Assoc 2008;98:357–63 | No patients with NMD or CNS condition |
| Tuck WH. The Stanmore cosmetic calliper. J Bone Joint Surg Br 1974;56:115–20 | No outcomes reported |
| Tyson SF, Thornton HA. The effect of a hinged ankle foot orthosis on hemiplegic gait: objective measures and users’ opinions. Clin Rehabil 2001;15:53–8 | Not instability of the knee |
| Tyson SF, Sadeghi-Demneh E, Nester CJ. A systematic review and meta-analysis of the effect of an ankle-foot orthosis on gait biomechanics after stroke. Clin Rehabil 2013;27:879–91 | Not primary study |
| Vicenzino B. Foot orthotics in the treatment of lower limb conditions: a musculoskeletal physiotherapy perspective. Man Ther 2004;9:185–96 | Not primary study |
| Vinci P, Gargiulo P. Poor compliance with ankle-foot-orthoses in Charcot-Marie-Tooth disease. Eur J Phys RehabilMed 2008;44:27–31 | Not instability of the knee |
| Vinci P, Paoloni M, Ioppolo F, Gargiulo P, Santilli V. Gait analysis in a patient with severe Charcot–Marie–Tooth disease: a case study with a new orthotic device for footdrop. Eur J Phys Rehabil Med. 2010;46:355–61 | Not instability of the knee |
| Ward J, Sugar T, Standeven J, Engsberg JR. Stroke Survivor Gait Adaptation and Performance After training on a Powered Ankle Foot Orthosis. Proceedings of the IEEE International Conference on Robotics and Automation, Anchorage, AK, 3–8 May 2010 | Laboratory setting only |
| Waring WP, Maynard F, Grady W, Grady R, Boyles C. Influence of appropriate lower extremity orthotic management on ambulation, pain, and fatigue in a postpolio population. Arch Phys Med Rehabil 1989;70:371–5 | Not instability of the knee |
| Warren CG, Lehmann JF, deLateur BJ. Pelvic band use in orthotics for adult paraplegic patients. Arch Phys Med Rehabil 1975;56:221–3 | Not instability of the knee |
| Watanabe H, Yonemitsu H. Short leg brace for knee extensor weakness (K. U. short leg brace). Kumamoto Med J 1973;26:90–5 | Not primary study |
| Watanabe H, Asami T, Itoh Y, Arizono O. Lower Limb Orthotic Management for Hemiplegia. 8th World Congress of the International Rehabilitation Medicine Association, Kyoto, Japan, 31 August–4 September 1997. pp. 645–8 | No outcomes reported |
| Waters R, Montgomery J. Lower extremity management of hemiparesis. Clin Orthop Relat Res 1974;133–43 | Not primary study |
| Waters RL, Yakura JS, Adkins R, Barnes G. Determinants of gait performance following spinal cord injury. Arch Phys Med Rehabil 1989;70:811–18 | Not primary study |
| Webster JB, Miknevich MA, Stevens P, Hansen C. Lower extremity orthotic management in neurological rehabilitation. Crit Rev Phys Rehabil Med 2009;21:1–23 | Not primary study |
| Wiener-Ogilvie S, Jones RB. A randomised trial of exercise therapy and foot orthoses as treatment for knee pain in primary care. Br J Podiatr 2004;7:43–9 | No patients with NMD or CNS condition |
| Wiest B. A pilot study of Orthodream foot orthose. Preliminary results of a new orthopaedic treatment in torsional low limb disorders. Journal de l’orthopedie 2003;18:793–5 | All participants aged < 16 years |
| Wijesinha C. Hemiplegia and bracing. Med J Aust 1979;2:76–8 | Not primary study |
| Winchester PK, Carollo JJ, Parekh RN, Lutz LM, Aston JW. A comparison of paraplegic gait performance using two types of reciprocating gait orthoses. Prosthet Orthot Int 1993;17:101–6 | Laboratory setting only |
| Wong CK, Bishop L, Stein J. A wearable robotic knee orthosis for gait training: a case-series of hemiparetic stroke survivors. Prosthet Orthot Int 2012;36:113–20 | Not orthotic intervention |
| Wu Q, Ma ZH, He CQ. Comparison of different orthosis for improving gait in patients with spinal cord injury. CJTER 2013;17:4152–60 | Laboratory setting only |
| Wu SK, Jordan M, Shen X. A pneumatically-actuated lower-limb orthosis. Conf Proc IEEE Eng Med Biol Soc 2011;2011:8126–9 | No patients with NMD or CNS condition |
| Xu GX, Gu SQ, Li JA. Poliomyelitis sequela in Pizhou city. Chin J Clin Rehabil 2005;9:238–40 | All participants aged < 16 years |
| Yadav SL, Saha PK, Panwar L. Floor Reaction Orthosis an Alternative Device for Quadriceps Paresis. IEEE/Engineering in Medicine and Biology Society Annual Conference, 1995 | Not primary study |
| Yakimovich T, Lemaire ED, Kofman J. Engineering design review of stance-control knee-ankle-foot orthoses. J Rehabil R D 2009;46:257–67 | Not primary study |
| Yakimovich T, Kofman J, Lemaire ED. Design and evaluation of a stance-control knee-ankle-foot orthosis knee joint. IEEE Trans Rehabil Eng 2006;14:361–9 | Laboratory setting only |
| Yakimovich T, Lemaire ED, Kofman J. Gait evaluation of a new electromechanical stance-control knee-ankle-foot orthosis. Conf Proc IEEE Eng Med Biol Soc 2006;1:5924–7 | Laboratory setting only |
| Yakimovich T, Lemaire ED, Kofman J. Preliminary kinematic evaluation of a new stance-control knee-ankle-foot orthosis. Clin Biomech 2006;21:1081–9 | Laboratory setting only |
| Yakimovich T, Kofman J, Lemaire E. Design, construction and evaluation of an electromechanical stance-control knee-ankle-foot orthosis. Conf Proc IEEE Eng Med Biol Soc 2005;7:6934–41 | Laboratory setting only |
| Yamamoto S, Hagiwara A, Mizobe T, Yokoyama O, Yasui T. Development of an ankle-foot orthosis with an oil damper. Prosthet Orthot Int 2005;29:209–19 | Not orthotic intervention |
| Yang A, Pena S, Spungen AM, Harel NY. Dynamic knee bracing to improve weight bearing during SCI balance training. J Spinal Cord Med 2014;37:454 | Not orthotic intervention |
| Yang W, Burgess HR, Lamb GA, Lanciault P, Perez M, Ramos R, Wong LA. Application of microprocessor stance control knee ankle foot orthoses: a case series report. PM R 2010;1:S160 | No patients with NMD or CNS condition |
| Yano H, Kaneko S, Nakazawa K, Yamamoto SI, Bettoh A. A new concept of dynamic orthosis for paraplegia: the weight bearing control (WBC) orthosis. Prosthet Orthot Int 1997;21:222–8 | No patients with NMD or CNS condition |
| Yokoyama O, Sashika H, Hagiwara A, Yamamoto S, Yasui T. Kinematic effects on gait of a newly designed ankle-foot orthosis with oil damper resistance: a case series of 2 patients with hemiplegia. Arch Phys Med Rehabil 2005;86:162–6 | Laboratory setting only |
| Zacharias B, Kannenberg A. Clinical benefits of stance control orthosis systems: an analysis of the scientific literature. J Prosthet Orthot 2012;24:2–9 | Not primary study |
| Zancan A, Beretta MV, Schmid M, Schieppati M. A new hip-knee-ankle-foot sling: kinematic comparison with a traditional ankle-foot orthosis. J Rehabil R D 2004;41:707–12 | Laboratory setting only |
| Ziter FA, Allsop KG. The value of orthoses for patients with Duchenne muscular dystrophy. Phys Ther 1979;59:1361–5 | All participants aged < 16 years |
Appendix 12 Data extraction tables
Bernhardt (2011) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Bernhardt (2011) Country: USA Language: English Funding source: Myositis Association Conflict of interest: yes, one of the authors is an inventor of the technology used in the study and holds two US patents on the device Study type: case series |
Number of participants: 9 % male: 78 Mean or median age: mean 61 (SD 9) yrs Ethnicity: not reported Weight: not reported BMI: mean 27.2 kg/m2 (SD 4.0 kg/m2) Type of disorder: inclusion body myositis Affected knee: both, braced side was chosen based on participants’ subjective evaluation of the weaker leg Nature of instability: patients had functional deficits due to quadriceps weakness. All participants had knee buckling and falls Previous use of orthotics: no |
Type of orthotic: SCKAFO Manufacture: custom-made SensorWalk (Otto Bock Health Care, Minneapolis), MN,USA Material: not reported Specified orthotic dose: not reported Fitting procedure: fitted by certified orthotist Cointerventions: not reported Comparator: before and 6 months after fitting of SCO |
Duration of follow-up: 6 months How were patient outcomes elicited?: questionnaire covering donning and doffing, brace cosmesis and weight, stability during standing, walking and postural transitions How was gait assessed?: using a 10-camera motion analysis system and four force platforms. Three left and right force plate strikes were included in analysis. Trials were averaged and strides normalised to 100% gait cycle, with 0% being foot-strike and 100% indicating ipsilateral foot-strike Statistical analysis: if normally distributed comparisons were made between the braced and unbraced conditions using a paired t-test and between the two strength groups using a two-sample two-sided t-test. When not normally distributed comparisons were made with the Wilcoxon Signed-Rank test for paired data |
Bernhardt (2011) results
| Patient-reported outcomes |
| Functionality of device: data not reported. The authors state that participants felt that the SCO was helpful for protecting against falls and providing stability Satisfaction with device: data not reported. The authors state that all participants had complaints regarding size, bulk, cosmesis and noise of the SCO as well as difficulty donning and doffing the brace. Most participants stated that they would prefer a less intrusive assistive device. Participants with less weakness tended to have positive feedback on the SCO. This was found to be regardless of the amount of time spent using it. The weakest participants had mixed feelings on the orthotic and had less use of the brace Usage of device: use of the brace ranged from about 2 hrs per day to all day every day |
| Objective assessments |
| Walking ability: data mainly presented in small scale graphs. Participants walked slower (p = 0.025) and with a lower cadence (p = 0.0007) with the SCO. Stride length with the SCO was not significantly different between conditions. Participants had a wider step width with the brace (0.035). When wearing the SCO the weaker participants walked slower (p = 0.022), had a lower cadence (p = 0.019) and a shorter stride length (p = 0.048) when compared to participants with less weakness. Peak knee flexion during swing significantly decreased when using the SCO [from mean 74.7 (2.9) degrees to mean 62.9 (10.8) degrees (p = 0.021)]. There was no significant difference in peak hip flexion during swing when using the SCO [from mean 41.4 (4.6) degrees to mean 39.7 (6.5) degrees (p = 0.355)] |
| Resource utilisation and adverse effects |
| Not reported |
Bocker (2013) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Bocker (2013) Country: Germany Language: English Funding source: not reported Conflict of interest: no Study type: case series |
Number of participants: 10 % male: 30 Mean or median age: 64.5 years Ethnicity: not reported Weight: not reported BMI: not reported Type of disorder: paralysis of the lower limb caused by poliomyelitis Affected knee: not reported Nature of instability: clinical paresis of the lower limb with partial paralysis of the quadriceps, instability of the knee joint: decentralisation of the patella, hyperextension of the knee joint while standing, varus or valgus misalignment Previous use of orthotics: no |
Type of orthotic: KAFO Type-eight orthosis Manufacture: custom-made. Mechanical lockable knee joint as well as Glenzack joints to lift the foot. There is a brace area on the tibia, soft areas of contact proximal and dorsal to the calf and the thigh Material: carbon fibre Specified orthotic dose: orthosis used over the whole day Fitting procedure: not reported Cointerventions: gait training with the orthosis twice a week for 3 months. Physical pain therapy and exercises to prevent cardiopulmonary and muscular reduction twice a week for 3 months Comparator: no comparator group |
Duration of follow-up: 3 months How were patient outcomes elicited?: SF-36 How was gait assessed?: duration of the stance phase and the knee joint angle at the stance-to-swing transition was analysed. Patients walked 4.5 m three times forwards and backwards on flat ground. The Zebris WinGait-HS v3.1.35 (zebris Medical GmbH, Isny, Germany) was used Statistical analysis: Friedman-Test. Median and quartiles were calculated |
Bocker (2013) results
| Patient-reported outcomes |
| Impact on daily living, quality of life: SF-36 including physical and psychological domains. The authors stated that no significant changes were observed in SF-36 at all time points. They stated that in the summary of the physical and mental score no improvement was observed. SF-36 data were reported in a figure only and it was not possible to extrapolate the data due to the scale |
| Objective assessments |
| Muscle activity was described using surface electromyography (S-EMG) at 3 months before the orthosis, at the time of fitting the orthosis and 3 months after getting the orthosis. The s-EMG was analysed while walking and the average value of the amplitude was used as a parameter of the current muscle activity during the process of standing and walking. On the side without the orthosis, statistically significantly increased s-EMG values of m obliquus abdominus internus and m multifideus during standing and of the m gluteus medius during walking were observed. On the side with the orthosis support changes of both abdominal muscles as well as m multifideus, mm.rectus and biceps femoris showed statistically significantly higher s-EMG whereas both vasti and m gluteus medius had decreased values. Full results were provided in the paper. Kinematic gait analysis. There were no significant changes in the knee joint angles. The knee angle of both legs seemed to be stabilised. The angle of the leg with the orthosis showed a difference of 13 degrees from baseline to follow-up. In the leg without the orthosis the knee angle decreased by about 8 degrees. An increased stance duration of 24% was seen in the leg with orthosis whereas there were no differences in the opposite leg during the intervention (not possible to extract actual duration) |
| Resource utilisation and adverse effects |
| Not reported |
Boudarham (2013) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Boudarham (2013) Country: France Language: English Funding source: not reported Conflict of interest: unclear Study type: case series |
Number of participants: 11 % male: 64 Mean or median age: mean 51 (SD 15) years Ethnicity: not reported Weight: mass 70 (SD 15) kg BMI: Not reported Type of disorder: CNS. Hemiplegia after stroke occurring six months prior to study (chronic phase) Affected knee: left 5, right 6 Nature of instability: genu recurvatum with aetiology: spasticity of quadriceps (6), weakness of quadriceps (2), spasticity of triceps surae (3) Previous use of orthotics: not reported |
Type of orthotic: KAFO Manufacture: custom-made (details provided in the text) Material: carbon fibre with polypropylene foot part Specified orthotic dose: reported. Had worn daily for at least one month Fitting procedure: not reported Cointerventions: not reported Comparator: no comparator group Compared gait with and without KAFO |
Duration of follow-up: participants were required to have been prescribed the device within the previous 6 months How were patient outcomes elicited?: no patient outcomes How was gait assessed?: each patient performed two sessions of gait analysis at preferred walking velocity without and with KAFO in a 10 m gait corridor. Six trials were carried out. Each patient performed the two gait analyses successively with a 10-minute rest in between. Gait was analysed using a motion capture system with eight optoelectronic cameras. The trajectories of 30 reflective markers placed on anatomical landmarks were collected and filtered. In KAFO condition reflective markers were placed directly on the KAFO joint in the axis of the centres of rotation of the knee and the ankle of the paretic limb. Ground reaction forces were measured synchronously with the kinematic data using two force plates staggered along the walkway Statistical analysis: a Wilcoxon text was used (control vs. KAFO condition) to assess the effects of KAFO on the primary outcome measure (angle of knee extension during stance) and for secondary outcomes |
Boudarham (2013) results
| Objective assessments |
| Walking ability: spatio-temporal parameters: gait velocity was significantly greater in the KAFO condition than in the control condition (+ 21%, p = 0.025). Stride length and cadence were also significantly greater in the KAFO condition (15%, p = 0.030 and 11%, p = 0.049). There was no significant difference between the two conditions for step width (p = 0.384). Step length of the non-paretic limb was greater in the KAFO condition (14%, p = 0.005) and swing phase duration of the paretic limb was significantly shorter in the KAFO condition (-29%, p = 0.003). Gait symmetry: symmetry between the paretic and non-paretic limbs increased with the KAFO. Asymmetry ratio was significantly lower in the KAFO condition [from 1.93 (0.77) to 1.27 (0.10)]. There was no significant difference between the two conditions for the stance phase duration asymmetry ratio [from 0.82 (0.11) to 0.86 (0.08), p = 0.132]. The knee flexor moment was significantly decreased during initial double stance phase with the KAFO: without KAFO, paretic side median –0.23, mean –0.30 (SD 0.22), with KAFO, paretic side median –0.10, mean –0.12 (SD 0.15), p = 0.047. There were no significant differences between conditions for the knee moment in simple support and final double contact phases. There were no significant differences between conditions for peak knee flexion for the non-paretic limb. Full details of hip, knee and ankle moments are provided in the paper |
| Resource utilisation |
| Number and nature of follow-up appointments One at 6 months Device malfunction Not reported |
| Patient-reported outcomes and adverse events |
| Not reported |
Brehm (2007) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Brehm (2007) Country: the Netherlands Language: English Funding source: grants from Anna Fonds and ZonMw Conflict of interest: unclear Study type: case series |
Number of participants: 23 % male: 61 Mean or median age: mean age 55 years (SD 9.2 years) Ethnicity: not reported Weight: mean body mass 72 kg(SD 11.8 kg) BMI: mean 25.9 kg/m2 (SD 4.1 kg/m2) Type of disorder: neuromuscular. Patients with former polio Affected knee: left, right, both Nature of instability: polio residual disability Previous use of orthotics: yes. All patients had previously used a conventional locked knee-joint KAFO made of leather/metal or plastic/metal with no technical deficits for at least 2 years |
Type of orthotic: KAFO with a locked knee-joint fitted according to total-contact principle. Weight ranged from 0.9 to 2.1 kg Manufacture: custom-made Noppe Orthopaedie BV (The Netherlands) manufactured the new KAFOs Material: carbon fibre Specified orthotic dose: not reported Fitting procedure: reported Cointerventions: some participants used cane(s) as a walking aid Comparator: yes. Leather/metal KAFO or plastic metal KAFO used by the same patients. Weight ranged from 1.0 to 4.1 kg |
Duration of follow-up: 26 weeks How were patient outcomes elicited?: an individualised satisfaction evaluation was made to quantify patient-specific improvements on various aspects of KAFO use. Patients chose five areas for improvement and, at baseline, rated these for satisfaction with regard to the old KAFO on a 10-point Likert scale (1 = extremely unsatisfied, 10 = extremely satisfied). At follow-up the patients rated the items again for the new KAFO SF-36 Physical functioning How was gait assessed?: patient wore KAFO and shoes and used usual walking aids and walked at a comfortable speed along a 10 m walkway. A 3D-movement analysis system was used (OPTOTRAK, Northern Digital Inc., Canada). Patients performed three trials. Energy cost of walking was measured by a portable gas-analysis system (Vmax ST, Sensormedics, The Netherlands). The distance covered during the last 2 minutes of the walking test was registered to calculate the patient’s walking speed Statistical analysis: generalised estimated equations used to test for changes in gross energy cost, net energy cost, walking speed and physical function and to investigate whether changes in gross and net energy cost were associated with changes in 15 biomechanical gait parameters and KAFO weight. Paired t-tests used to assess differences between old and new KAFO for biomechanical gait parameters and patient satisfaction |
Brehm (2007) results
| Patient-reported outcomes |
| Satisfaction with device: mean patient satisfaction scores were 48% higher for the new KAFO than for the old KAFO (p < 0.01) (data not provided) A total of 26 items for improvement were mentioned. Items that were most frequently mentioned for improvement were weight, fitting and cosmesis of the KAFO, stability and walking performance (no data provided) |
| Objective assessments |
| Walking ability: the gross and net energy cost of walking were both significantly lower for the new KAFO than for the old KAFO. Gross energy cost J/kg/m: mean difference (%) –0.42 (–7%, 95% CI –0.63 to –0.21). Net energy cost J/kg/m: mean difference (%) –0.36 (–8%) (95% CI –0.54 to –0.18). Increments in gross and net energy cost above norm values with the old KAFO were both reduced with the new KAFO: Gross energy cost above norm: mean difference (%) –0.47 (–18%) (95% CI –0.27 to –0.67). Net energy cost above norm: mean difference (%) –0.37 (–18%) (95% CI –0.19 to –0.55). Walking speed remained unchanged: speed (m/minute): mean change (%) 1.8 (3%) (95% CI –4.35 to 0.57). An improvement in knee flexion, forward excursion of the centre of pressure, peak ankle moment and timing of peak ankle power were significantly associated with the decrease in energy cost. Reduction in KAFO weight was not significantly associated with decrease in energy cost. Full details are provided in the paper Other functional ability. physical functioning was assessed using the SF-36 physical functioning scale. The authors report there was no significant difference in physical function between the old and new KAFO (no further details reported) |
| Resource utilisation |
| Device malfunction: seven patients reported technical deficits relating to the hinge at the ankle or knee which could be easily repaired. Seven patients reported wear to the cloth upholstery inside the KAFO. One patient needed a replacement of the orthosis due to a break of the KAFO |
| Adverse effects |
| Not reported |
Davis (2010) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Davis (2010) Country: Australia Language: English Funding source: International Society for Prosthetics and Orthotics (Australian National Member Society) Conflict of interest: no Study type: case series |
Number of participants: 10 % male: 40 Mean or median age: mean 61.9 (SD 7.7) years Weight: ranged from 49 kg to 111 kg BMI: not reported Type of disorder: neuromuscular. MND 1, post-polio 9 Affected knee: left, right Nature of instability: significant lower limb weakness or paralysis Previous use of orthotics: yes. Previous use of orthotics included Solid GRAFO (4), LKAFO (1) posterior offset KAFO (1), knee brace (1), none (3) |
Type of orthotic: SCKAFO Manufacture: custom-made. All participants had a SCO that incorporated a Horton Stance Control knee joint Material: Carbon fibre. Laminate with carbon and fibreglass reinforcing Specified orthotic dose: reported. Participants had to regularly use the SCO for at least 4 hours per day Fitting procedure: reported Cointerventions: walking aids – none (4), 2 walking sticks (2), 1 walking stick (2), 2 forearm crutches (1), 1 forearm crutch (1) Comparator: yes. KAFO in locked mode worn by same participants |
Duration of follow-up: the average use was 6.2 (5.2) months How were patient outcomes elicited?: NA How was gait assessed?: a GAITRite walkway (CIR systems, USA) was used to measure temporospatial characteristics. A Cosmed K4b2 metabolic system (Cosmed, Italy) was used to measure oxygen consumption. For temporospatial measurements participants were asked to walk at their comfortable walking velocity over a 9 m GAITRite walkway four times for each condition. A 30-minute break was allowed between the two conditions Statistical analysis: GAITRite data for four trials were averaged to determine average walking velocity, left and right step cadence, stance time and swing time for both conditions for each participant. Net oxygen consumption for walking was calculated for each condition by measuring total oxygen consumption over the 15-minute test and subtracting baseline oxygen consumption averaged over the last 2 minutes of the initial resting period. Oxygen cost was calculated by dividing net oxygen consumed by the distance walked. Values were normalised for body weight. The Physiological Cost Index was calculated as the ratio of heart rate difference (exercise – rest) to walking velocity in metres per minute. Heart rates from the Cosmed system were averaged over the last 2 minutes of the initial rest period and the last 2 minutes of exercise. Where distributions were normal, t-tests for matched pairs were used to compare means between the two orthosis conditions. Otherwise a Wilcoxon signed–rank test was used to compare conditions. Due to testing for both walking velocity and energy expenditure 0.025 was chosen for tests of statistical significance |
Davis (2010) results
| Objective assessments |
| Walking ability: walking velocity was significantly increased in the stance control condition based on the results of 10 participants: locked condition 65.0 cm/s (SD 24.5), stance control 72.9 (95.7) cm/s, p = 0.000107. This was a result of significantly increased cadence and increased step length on the sound limb (p < 0.001). Affected limb step length and unaffected limb swing time were not significantly different (full details in paper). There was no difference in the oxygen cost of walking between the two conditions: (9 participants) locked condition 0.213 (0.081) ml/kg/minute, stance control 0.224 (0.069) ml/kg/minute p = 0.515. There was no difference in the physiological cost index between the two conditions: (8 participants) locked condition 0.65 (0.32) b/m, stance control 0.70 (0.34) b/m p = 0.093. During energy expenditure testing there was no significant difference in walking speed between conditions: (9 participants) locked condition 36.34 (11.3) cm/s, stance control 38.42 (10.2) cm/s p = 0.235. Walking velocity during energy expenditure testing was significantly slower than during temporospatial testing in both the locked and stance control conditions (p = 0.00006 and p = 0.00004 respectively) |
| Patient-related outcomes, resource utilisation and adverse events |
| Not reported |
Hachisuka (2007) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Hachisuka (2007) Country: Japan Language: English Funding source: unclear Conflict of interest: unclear Study type: case series |
Number of participants: 11 % male: 18 Mean or median age: mean 53.9 (SD 9.8) years Ethnicity: not reported Weight: not reported BMI: not reported Type of disorder: neuromuscular. Post-polio Affected knee: both. 10 participants had a carbon KAFO for unilateral lower extremity and one had two carbon KAFOs for bilateral lower extremities Nature of instability: post-polio Previous use of orthotics: yes. Eight of 11 participants had used an ordinary KAFO with double-metal uprights for more than 10 years. Three had no previous use |
Type of orthotic: KAFO Manufacture: custom-made. Carbon fibre frame and sole (Otto Bock Japan and Carbon Fiber Fabrics, Toray Industries Inc., Japan), pair of Swiss knee joints and free ankle joints. Two participants had a solid ankle, one a ring lock knee joint and one off-set knee joint. Mean weight 992 g (SD 168) Material: carbon fibre Specified orthotic dose: not reported Fitting procedure: reported. Fitting included use of a temporary orthosis for 2–4 weeks, modification as required then 2 further weeks trial followed by fabrication of carbon fibre orthosis in the same alignment and shape as the modified temporary orthosis based on discussion with the patient Cointerventions: walking aids. Canes and crutches used as necessary Comparator: yes. ‘Ordinary’ KAFO. Mean weight 1403g (SD 157) and no orthosis |
Duration of follow-up: not reported How were patient outcomes elicited?: eight participants who had experienced both ordinary and carbon KAFOs evaluated walking with a carbon KAFO. The questionnaire had ten categories and used a visual analogue scale to cover: awareness of weight, speed, walking distance, fatigue during walking, safety during walking, back pain, hip pain, knee pain, foot pain and appearance. These were rated on a visual analogue scale with 0% (completely unsatisfied with carbon orthosis and 100% completely satisfied with carbon orthosis when compared to previous orthosis How was gait assessed?: oxygen consumption was measured using a telemetric breath-by-breath gas analyser. Participants had to walk along a 50 m rectangular line in the training room with the condition randomly assigned (without orthosis, with ordinary KAFO, with carbon fibre KAFO). Participants rested before each trial condition until both oxygen consumption and heart rate reached a steady state. They also stood for two minutes before each trial condition. Oxygen consumption and heart rate were averaged for the last 30 seconds of each walking phase. Physiological cost index was obtained from the formula [heart rate (beats/minute) at 3-minute walk – heart rate (beats/minute) at rest] divided by speed (m/minute) Statistical analysis: differences between walking conditions analysed using Wilcoxon matched pairs signed-ranks test or the paired t-test |
Hachisuka (2007) results
| Patient-reported outcomes |
| Satisfaction with device: participants were more satisfied with their carbon KAFO than with their previous ordinary KAFO especially relating to fatigue during walking, safety during walking and appearance (6 patients). |
| Objective assessments |
| Walking ability: all participants walking with both KAFOs showed a significant increase in scores of the functional ambulation category compared with walking without an orthosis, (p <0.05). There was no significant difference between walking with an ordinary KAFO and with a carbon KAFO (data not reported) Other: there were no significant differences in the number of steps per minute between walking without an orthosis and with a carbon KAFO: (steps per minute) 77.0 (12.5) vs. 84.3 (13.2) p > 0.05 nor in walking with an ordinary KAFO and a carbon KAFO (steps per minute) 92.9 (6.3) vs. 92.0 (11.1) p > 0.05. Step length while walking with a carbon KAFO longer when compared with no KAFO: (step length cm) 45.6 (7.0) vs. 39.7 (7.5) and when compared with an ordinary KAFO 45.7 (8.2) vs. 41.3 (7.0), p < 0.1. Speed while walking with a carbon KAFO was significantly faster than walking without an orthosis: (m/minute) 39.5 (9.8) vs. 31.0 (8.6) and walking with an ordinary KAFO 42.6 (7.8) vs. 38.5 (7.0), both p < 0.05 Oxygen consumption per body weight (ml/minute/kg), oxygen cost and physiological cost index while walking with a carbon KAFO were significantly lower than those without an orthosis (–16%, –35% and –33% respectively) and with an ordinary KAFO (–9%, –14% and –15% respectively), all p < 0.05 |
| Resource utilisation |
| Number and nature of follow-up appointments: Not reported Device malfunction: participants were followed up for at least 2 years and the carbon KAFO remained undamaged, but a plastic cable and two steel springs of the Swiss lock knee joints needed to be changed Cost: price of standard carbon KAFO was 180,000 yen (US $1700), 50% more expensive than the ordinary KAFO |
| Adverse effects |
| Not reported |
Harvey (1997) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Harvey (1997) Country: Australia Language: English Funding source: Motor Accident Authority of New South Wales and Center for Orthotic Designs Conflict of interest: unclear Study type: randomised crossover trial. There was a 2-month washout period of nonorthotic use before participants began the trial with the second orthosis |
Number of participants: 10 % male: 90 Mean or median age: Mean: 37 years (SD 8.4 years) Ethnicity: not reported Weight: mean: 70 kg (SD 11.1 kg) BMI: not reported Type of disorder: CNS. Complete spinal injury between 4 and 19 years previously. 4 patients had no spasticity, two had moderate to severe spasticity and four had mild spasticity Nature of instability: T9-12 paraplegia Previous use of orthotics: yes. None had prior experience with HKAFO but all had some standing experience in KAFOs. One patient mainly used KAFOs and walking sticks for ambulation whilst the remainder were dependent on wheelchairs for functional mobility but had undertaken standing or gait training with KAFOs during the initial period of inpatient rehabilitation |
Type of orthotic: HKAFO, Walkabout Orthosis Manufacture: custom-made. KAFO component was manufactured by an experienced on-site orthotist working within Spinal Injuries Unit and other components were made by two companies: Center for Orthotic Designs Inc., The RGO Center, Redwood City, California and Poly Medic Australia, Queensland, Australia Material: not reported Specified orthotic dose: reported. Participants could use each orthosis as they wished but had to complete a brief summary sheet each time the orthosis was worn Fitting procedure: not reported Cointerventions: walking aids, elbow crutches Other: gait training. Patients attended individualised gait training sessions 2–3 times a week over a 6- to 8-week period. Each session lasted between 2 and 3 hours. At the end of each 8-week training period, levels of skill performing 19 specific tasks associated with functional ambulation were assessed Comparator: yes, IRGO |
Duration of follow-up: mean 14 weeks with each orthosis How were patient outcomes elicited?: 18-point questionnaire at end of each training and home trial period. 10-point Likert scale. Summary sheet on context of use (when, where, what for) 5-point scale assessing usefulness. Questionnaire on device preference (beginning and end of study) How was gait assessed?: performed with elbow crutches. Level of independence and time to perform task assessed. Average speed of walking assessed for flat surface and up and down two ramps of different gradients Statistical analysis: only those who could walk with minimal assistance or less in both orthoses included in the analysis. The 19 specific skills associated with functional ambulation were grouped into five categories and a mean score for each patient derived for each cluster of skills. Wilcoxon’s signed-rank test used to determine differences between orthotics. The number of times participants used each orthosis was compared using t-tests. Patient preference for orthotic was assessed with binomial probability distributions. A Fischer Exact test used to determine whether or not participants’ preferences changed over the course of the study |
Harvey (1997) results
| Patient-reported outcomes |
| Functionality of device: all participants could sit in the wheelchair with the WO on but no participants could do so with the IRGO Satisfaction with device: at the end of the study seven people preferred the IRGO and three preferred the WO (p = 0.17). Based on finding to significant difference between groups on any of the items the authors pooled data for both orthoses and across both testing periods and reported satisfaction overall rather than by device (not extracted) Usage of device: there was no significant difference in the number of times that the two orthoses were used. During the home trial period no participant wore either orthosis for more than 2 hours at any one time. The most common reasons for using either orthosis were for exercise, practice or for the long term benefits. Most participants scored both orthoses between 3 (moderately useful) and 5 (extremely useful), (taken from text, numerical data not available). Six participants used the WO at home with one requiring assistance. Four of seven participants using the IRGO needed assistance, mainly for sitting and standing. Three participants wore the WO under clothes but no participants wore the IRGO under clothes. Both orthoses were worn indoors and outdoors by most participants (no further details). When participants were asked why they did not make more use of their orthoses, the most common response was being ‘too busy’. Additionally, participants stated that using the orthoses prevented them from undertaking certain activities in the home as their hands were unavailable due to holding elbow crutches |
| Objective assessments |
| Walking ability: speed of walking on the flat (8 participants): Participants walked significantly faster with the IRGO than with the WO on the flat surface, IRGO mean = 0.34 m/sec (SD 0.18), WO mean = 0.14 m/sec (0.12), p = 0.002. Speed of walking up and down ramps (5 participants). Participants walked significantly faster with the IRGO than with the WO up and down ramps, (IRGO mean = 0.25 m/sec (SD 0.09), WO mean = 0.14 m/sec (SD 0.06), p = 0.02 Other functional ability: there was no significant difference between orthoses in the extent of assistance required to don and doff their orthoses; in the ability of participants to get up and down stairs and curbs; and in the ability of participants to walk on the flat. Participants required significantly more assistance when using the WO to walk over inclined surfaces (medians IRGO = ‘independent’, WO = ‘minimal’, p = 0.03) and significantly less assistance when using the WO to get from sitting to standing and from standing to sitting (medians IRGO = ‘moderate’, WO = ‘minimal’, p = 0.03) Other: no participants required more than minimal assistance to walk on the flat surface using the IRGO but one participant required moderate assistance and one maximal assistance to walk on the flat surface using the WO. No participants required more than minimal assistance to walk up and down ramps using the IRGO but five participants required either moderate or maximal assistance using the WO. Heart rate (HR, beats/minute), Oxygen uptake (VO2, l/minute), expired ventilation (l/minute), O2 cost (ml/kg/m) and PCI measured at rest before and after three 6-minute walking trials (1 over flat-tiled surface, 1 over flat carpeted surface, 1 up a 4 degree concrete ramp). Data were collected using a tight-fitting mask and chest electrodes. There were no differences in HR or VO2 between orthoses for any surface or among surfaces. When HR and VO2 were expressed relative to walking speed, PCI and O2 cost of gait were significantly greater with WO compared with IRGO on all three surfaces. Participants’ PCI (beats/minute) ranged from 8.4 to 10.3 beats/minute with WO compared to 4.3 to 7.0 beats/minute with IRGO (p < 0.05). O2 cost during WO gait was between 3.95 and 4.91 ml/kg/m versus 1.65 to 1.80 ml/kg/m for IRGO gait (p < 0.05) |
| Resource utilisation and adverse effects |
| Not reported |
Heim (1997) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Heim (1997) Country: Israel Language: English Funding source: Lewis National Rehabilitation Institute and orthotic manufacturers Gapim and Eshed Conflict of interest: yes. Manufacturers helped fund the study Study type: case series |
Number of participants: 30 % male: 33 Mean or median age: mean age 44 years Ethnicity: not reported Weight: not reported BMI: not reported Type of disorder:neuromuscular, post-polio Affected knee: left, right, both Nature of instability: post-polio Previous use of orthotics: yes. All patients had used orthoses and were eligible to have new orthotics (replaced every 3 years at the time of study) |
Type of orthotic: KAFO Manufacture: custom-made. Gapim Ltd, Israel (33 braces) Esched Advanced Orthopedics (three braces) Material: carbon fibre braces weighted on average 1150 g (metal braces previously used weighed on average 1720 g) Specified orthotic dose: not reported Fitting procedure: not reported Cointerventions: not reported Comparator: no comparator group |
Duration of follow-up: mean 30 months (range 15 to 39 months) How were patient outcomes elicited?: structured questionnaire (no further details provided) How was gait assessed?: gait not assessed Statistical analysis: not reported |
Heim (1997) results
| Patient-reported outcomes |
| Satisfaction with device: 19 of 27 patients reported that they would like to have a permanent carbon fibre orthosis due to it being lighter, better fitting and more aesthetic Usage of device: 21 of 27 patients wore their orthosis throughout the day |
| Objective assessments |
| Not reported |
| Resource utilisation |
| Device malfunction: within 30 months, 19 braces had undergone minor repair in the workshop |
| Adverse effects |
| Eight patients chose a metal orthosis rather than the carbon fibre brace due to skin irritation from the material, excessive sweating due to proximity of orthosis to skin and inability to alter the shape in accordance with circumferential limb changes |
Jaspers (1997) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Jaspers (1997) Country: Belgium Language: English Funding source: the government agency for Innovation by Science and Technology, Belgium Conflict of interest: unclear Study type: case series |
Number of participants: 14 (out of 23 people fitted with a device in the study period) % male: 86 Mean or median age: mean 33.6 years Ethnicity: not reported Weight: not reported BMI: not reported Type of disorder: CNS. Paraplegia with levels of lesion mid-thoracic to L1 except two patients with high level C7 lesions Affected knee: not reported Nature of instability: result of paraplegia Previous use of orthotics: yes. Four patients had used long leg braces previously |
Type of orthotic: RGO, ARGO Manufacture: custom-made. STEEPER ARGO, Hugh Steeper Limited, London Specified orthotic dose: not reported Fitting procedure: reported Cointerventions: walking aids. 12 patients used a walker, 2 used crutches Other. patients were trained 4 weeks prior to fitting of the orthotic followed by a 4- to 6-week training period with the appliance. which was usually continued at home under the supervision of a private physiotherapist Comparator: no comparator group |
Duration of follow-up: at least 1 year (from 1 year 7 months to 4 years 8 months) How were patient outcomes elicited?: telephone interview (approximately 30 minutes) with an independent researcher using a standardised questionnaire (full details in paper) How was gait assessed?: not applicable Statistical analysis: none |
Jaspers (1997) results
| Patient-reported outcomes |
| Functionality of device: all patients used the ARGO mainly to stand and walk without a real functional goal. A few (unspecified) tried to use it at work but did not continue as they considered the ARGO to be too heavy and cumbersome for use in a really functional way. They also found the walking speed to be too slow. One user could make a transfer into a car without much difficulty, two others were able to transfer but found it too difficult to do on a regular basis. The other participants had never tried this Impact on daily living, quality of life: the ARGO was used outdoors by Four participants (for walking in the garden). No participants used it for walking in the street. Seven out of 14 of the regular users could use the ARGO fully independently. Three needed help with donning, five needed assistance with rising Satisfaction with device: all patients stated that they had been well informed about the possibilities of walking with the ARGO prior to fitting and two said that they were disappointed in their expectations. Of the 12 active ARGO users, two found it ‘very good’, six ‘good’, three did not state their opinion and one found it ‘bad’. Of the two non-users one rated the device ‘good’ and one did not answer Usage of device: 12 of 14 were still using the ARGO on a regular basis. One patient who had stopped using the ARGO was planning to resume use. Principal reasons for abandoning ARGO use were mechanical problems with the ARGO and lack of time due to employment or preoccupation because of various interests. All four patients who had used long leg braces had discontinued use of these braces. The frequency of using the ARGO ranged from daily to twice a month with an average of three times a week. On each occasion, the ARGO was used for 1–2 hours Other: of the 12 regular users, one reported problems with the cosmesis of the ARGO or with the appearance of gait. Although the ARGO could be worn under clothing, none of the users did so |
| Resource utilisation |
| Device malfunction. Most complaints were related to the functioning of the knee-locking cables and the knee-locking mechanism when rising and sitting down (numbers unclear). Further complaints about the ARGO were its weight, the fact that it was too big to use in an active wheelchair and the discomfort whilst sitting due to the back tube |
| Adverse effects |
| There were no complications of a physiological nature. Two patients had fallen but without serious injury |
Kakurai and Akai (1996) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Kakurai and Akai (1996) Country: Japan Language: English Funding source: not reported Conflict of interest: unclear Study type: case series |
Number of participants: 28 % male: 50 Mean or median age: mean 54.5 years Ethnicity: not reported Weight: not reported BMI: not reported Type of disorder: CNS. Hemiplegic patients whose ‘functional recovery was less than expected’. 60.7% had moderate to severe sensory disturbances, 42.9% had shoulder hand syndrome, 34% had aphasia, 25% unilateral spatial neglect, 17.9% apraxia and 17.9% dementia Affected knee: left, right Nature of instability: hemiplegia Previous use of orthotics: no |
Type of orthotic: KAFO (Convertible KAFO to AFO) Manufacture: custom-made Material: plastic solid. Convertible KAFO - plastic shoe-horn type AFO connected with a knee orthosis using metal plunger lock. KAFO can then be replaced by AFO after functional recovery of active knee control Specified orthotic dose: not reported Fitting procedure: reported. Orthosis was fitted when patients began gait training after onset of stroke. In 12 cases this was within 3 months, in nine cases between 3 and 6 months and in seven cases > 6 months after onset of stroke Cointerventions: not reported Comparator: yes. Patients who had changed to AFO during the study were compared with those remaining on KAFO |
Duration of follow-up: not reported How were patient outcomes elicited?: not applicable How was gait assessed?: not reported Statistical analysis: those changing to AFO were compared with those remaining on KAFO using Student’s t-test and chi-squared test to consider differences in ambulation capability using the modified Barthel index |
Kakurai and Akai (1996) results
| Objective assessments |
| Walking ability: during the period of observation (unclear) 11 patients could control their knee actively between 1.5 and 10 months (average 4 months) after initial prescription of the orthosis. Their KAFOs were changed to AFOs (AFO group). The 17 remaining patients were unchanged and continued to use their KAFO (KAFO group). In the AFO group three were outdoor independent, one indoor independent and seven were indoor dependent. In the KAFO group two cases were indoor independent, 11 indoor dependent and four were non-ambulant Other functional ability: mean AFO group Barthel index score was 72.8 (7.2), mean KAFO group Barthel index score was 43.1 (4.6), p < 0.01 |
| Patient-reported outcomes, resource utilisation and adverse effects |
| Not reported |
Middleton (1997) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Middleton (1997) Country: Australia Language: English Funding source: AMP Society, Orthotic Department of the Royal North Shore Hospital and Polymedic Pty Ltd Conflict of interest: unclear Study type: case series |
Number of participants: 25 % male: 76 Mean or median age: mean 35 (SD 13) years Ethnicity: not reported Weight: not reported BMI: not reported Type of disorder: CNS. Spinal cord injury resulting in paralysis (traumatic 76% and non-traumatic 24%) Affected knee: both Nature of instability: paralysis due to spinal cord injury Previous use of orthotics: yes. 22 had previous experience of orthotics including KAFOs and Backslabs |
Type of orthotic: Walkabout orthosis Manufacture: custom-made. All patients required adaptations to the Walkabout orthosis and could not stand or balance well without individual customisation of the device. Adaptations included shoe raises, heel wedges, carbon fibre inserts, medial knee extensions and pads, bridging plates and lateral extension bars fitted with a pelvic strap and an abdominal pad Material: not reported Specified orthotic dose: not reported Fitting procedure: reported Cointerventions: walking aids, parallel bars, forearm crutches or frames Comparator: no comparator group |
Duration of follow-up: at least 18 months for those who continued to use the device after successful training (five discontinued, three were unsuccessfully trained and one was lost to follow-up) How were patient outcomes elicited?: questionnaire at outpatient review or by telephone interview of all patients who completed gait training and continued to use Walkabout. All patients who used the Walkabout for 18 months or more were interviewed at least twice, between 7 and 12 months and approximately 12 months later. Five patients who discontinued use of Walkabout were interviewed after withdrawing from programme How was gait assessed?: not assessed Statistical analysis: analysis of variance of differences in pattern of orthotic usage between complete and incomplete spinal cord injured individuals was conducted |
Middleton (1997) results
| Patient-reported outcomes |
| Functionality of device: 24 of 25 patients were able to apply the Walkabout orthosis independently and transfer themselves between standing and sitting while wearing it. Improved standing stability was reported by all patients. Several examples of improved functionality were given but overall indoor accessibility was not improved and was often hampered by the accompanying walking aid Satisfaction with device: no significant differences were found between patients with complete and incomplete lesions in terms of perceived benefits of the Walkabout device. Physical and psychological benefits were reported more frequently than exercise or functional benefits. Read from graph physical benefits were reported by > 90% of patients, psychological by approximately 50%, exercise approximately 40% and functional benefits by < 10% Usage of device: 16 of 25 patients still use the Walkabout with 15 of these having continued for > 18 months. No statistically significant differences in usage between the two groups of patients were found at either review time point. No significant differences in variables within groups were found over time (data not given). The mean intensity of Walkabout usage for both spinal cord injured groups combined was 150 (24) minutes/week at the first review and 169 (36) minutes/week at the second review. The median at both time points was 120 minutes. Five patients discontinued usage of the Walkabout device between 7 and 20 months, three patients were unsuccessfully trained due to spinal immobility and one patient was lost to follow-up. Four of five who discontinued usage reported a lack of functional enhancement over either pre-programme orthoses (KAFO, RGO) or the wheelchair. One of these patients had problems with ankle contractures. The fifth patient was satisfied with the Walkabout but was forced to withdraw after 11 months due to previous surgery-related back pain |
| Resource utilisation |
| Device malfunction: The authors reported that all devices required adaptation. Once set up, few devices required any adjustment, maintenance or repairs (no data provided) |
| Objective assessments and adverse effects |
| Not reported |
Morinaka (1982) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Morinaka (1982) Country: Japan Language: English Funding source: not reported Conflict of interest: not reported Study type: cohort study |
Number of participants: 25 (of 36 patients fitted with the device) % male: 64 Mean or median age: mean 56 years Ethnicity: not reported Weight: not reported BMI: not reported Type of disorder: CNS. Hemiplegia as a result of stroke. Graded as 3-3-5 in Brunnstrom’s functional classification of hemiplegia Affected knee: not reported Nature of instability: hemiplegia Previous use of orthotics: yes. Three patients had previously received an AFO |
Type of orthotic: KAFO Manufacture: custom-made Material: plastic solid. Long leg shelled, flexible plastic laminate ‘Subortholen’. Weight approx 500 g Specified orthotic dose: not reported Fitting procedure: reported Cointerventions: not reported Comparator: yes. Comparison with 50 patients fitted with AFOs for evaluation of gait. A group of 30 adult males who had not had a stroke was also used for comparison of gait |
Duration of follow-up: mean 14.6 months (range 1–35 months) How were patient outcomes elicited?: not applicable How was gait assessed?: twelve characteristics of gait (10 unfavourable signs) were compared between the 25 cases fitted with KAFOs and 50 cases fitted with AFOs. The time to walk 5–10 m using a straight line drawn on the floor was measured and compared for each group and for a group of 30 adult males who had not had a stroke. The same groups were compared on walking and returning a distance of 10 m in an L-shaped line (a total of 20 m) and walking and returning for 5 m in an S-shaped line (total of 10 m) Statistical analysis: not reported |
Morinaka (1982) results
| Objective assessments |
| Walking ability: the authors state that all 25 patients were able to walk smoothly after fitting of the KAFO. Two could walk independently after 12–15 months, two could ambulate independently but preferred to wear the orthosis. Results of gait characteristics were more favourable for KAFO users than for AFO users for 8 of 12 characteristics (from figure and author summary, statistical significance was not reported). Knee flexion was better in AFO users. The KAFO group was about ‘a half to a third’ faster than AFO group but slightly slower than non-stroke group. The authors state that almost all of KAFO seemed able to turn easily and ambulate on the S-shaped line smoothly. Outcomes were not reported in full (only qualitatively and in figures) Other: all 25 patients continued to wear the orthosis without rejection |
| Patient-reported outcomes, resource utilisation and adverse effects |
| Not reported |
Peethambaran (2000) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Peethambaran (2000) Country: USA Language: English Funding source: not reported Conflict of interest: not reported Study type: case series |
Number of participants: 5 % male: 40 Mean or median age: mean 61.40 (SD 12.44) years Ethnicity: not reported Weight: mean 153 lb (SD 34 lb) BMI: mean BMI 24.14 kg/m2 (SD 2.62 kg/m2) Type of disorder: neuromuscular. Patients with post-polio paralysis Affected knee: not reported Nature of instability: post-polio paralysis Previous use of orthotics: yes. Previously using the conventional KAFO |
Type of orthotic: anterior approach KAFO Manufacture: custom-made Material: carbon titanium with cable control locking mechanism Specified orthotic dose: reported. New anterior approach KAFO was to be worn for 6 weeks Fitting procedure: reported. At the initial visit, subjects were evaluated and casting procedures completed for the new KAFO. The KAFO system was ready for application in 3 weeks. Regular follow-up was carried out to ensure the integrity of the system Cointerventions: not reported Comparator: yes. Within group comparison with posterior approach KAFO. Plastic solid conventional design – standard polypropylene thigh foot and ankle section |
Duration of follow-up: 6 weeks How were patient outcomes elicited?: three questionnaires on 1. satisfaction (5-point scale) 2. performance (5-point scale) and 3. body part discomfort map (6-point scale) How was gait assessed?: patients completed a performance evaluation questionnaire, which included a question on gait Statistical analysis: the non-parametric Wilcoxon signed-rank test to compare performance, satisfaction and wellbeing. A one-tailed test was used with alpha = 0.05 to determine statistical significance |
Peethambaran (2000) results
| Patient-reported outcomes |
| Functionality of device: results were statistically significantly in favour of the new KAFO for ease of putting on [mean 4.4 (SD 0.548) vs. 2.60 (SD 0.894)] and ease of removal [(4.6 (0.548) vs. 3.0 (0.000)] and interference with sitting [4.80 (0.447) vs. 1.20 (0.447)] . It was not statistically significant for the other domains of strap application, shoe application, maintenance and cleaning, balance, stability in level and uneven ground walking and adequacy for sports Pain and disability: the new KAFO was statistically significantly favoured for discomfort in ankle, thigh (back) [4.4 (0.548) vs. 1.6 (0.894)], knee (back) [4.8 (0.447) vs. 2.8 (1.483)], lower leg (back) [4.8 (0.447) vs. 2.0 (1.414)] and foot [4.6 (0.548) vs. 36 (0.894)]. It was not statistically significantly favoured for thigh (front), knee (front) or lower leg (front) Satisfaction with device: patients were statistically significantly more satisfied with the new KAFO in terms of its comfort [4.2 (0.447) vs. 3.4 (0.548)], gait [5.00 (0.000) vs. 3.8 (0.447)], appearance [500 (0.00 vs. 3.00 (0.707)], effort [5.00 (0.00) vs. 4.40 (0.548)], support [4.8 (0.447) vs. 4.00 (0.00)], donning/doffing) 4.80 [0.447) vs. 3.20 (0.837)], not catching on clothes [2.00 (0.00) vs. 1.00 (0.00)] and not rubbing on skin [2.00 (0.00) vs. 1.40 (0.548)]. Other criteria were not statistically significantly different (hours of wearing, loss of function, wearing an orthosis, device weight, device soiling clothes and device encouraging perspiration |
| Adverse effects |
| Pain/discomfort results reported under patient satisfaction |
| Objective assessments and resource utilisation |
| Not reported |
Scivoletto (2000) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Scivoletto (2000) Country: Italy Language: English Funding source: not reported Conflict of interest: not reported Study type: case series |
Number of participants: 24 % male: 79 Mean or median age: mean 33.6 (SD 3.2) years Ethnicity: not reported Weight: not reported BMI: not reported Type of disorder: CNS. Complete spinal cord injury of traumatic aetiology all of thoracic level Affected knee: not reported Nature of instability: result of spinal cord injury Previous use of orthotics: not reported |
Type of orthotic: RGO Manufacture: custom-made Material: not reported Specified orthotic dose: not reported Fitting procedure: not reported Cointerventions: not reported Comparator: yes. RGO non-users |
Duration of follow-up: 1 year How were patient outcomes elicited?: neurological and physical examination and social history at the beginning of the study. At follow-up patients took the Eysenck Personality questionnaire and the Cognitive Behavioural Assessment Schedule 3 for self-rating anxiety and Schedule 8 for self-rating depression. Patients were defined as ‘anxious’ and ‘depressed’ when their scores on the two scales were one standard deviation above the mean of the norms How was gait assessed?: not applicable Statistical analysis: chi-squared test and Student’s t-test used to consider differences between RGO-users (Group A) and RGO-non-users (Group B) |
Scivoletto (2000) results
| Patient-reported outcomes |
| Functionality of device: users and non-users did not differ on donning and doffing time, the need for help with donning and doffing, walking speed, walking aids and ability to go up and down stairs at the end of training (p = 0.003) and at 1 year follow-up (details provided). There was a significant difference between users and non-users both at the end of training (p = 0.005) and at follow-up (p = 0.003) with functional capacity as measured by the Garrett score. RGO users had a functional level between home ambulation with limitations and home ambulation while RGO non-users had a functional level between hospital ambulation and home ambulation with limitations Usage of device: 11 patients (46%) no longer used the RGO. This included one patient with a fractured femur and several (unspecified) finding the orthosis uncomfortable or too difficult to don or doff, or too slow or too hard to use, or poor fitting |
| Objective assessments, resource utilisation and adverse effects |
| Not reported |
Steinfeldt (2003) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Steinfeldt (2003) Country: Germany Language: German Funding source: not reported Conflict of interest: yes. One of the authors was involved in the company manufacturing the orthotic Study type: case series (retrospective) |
Number of participants: 55 (of 78 patients treated in the time period) % male: 44 Mean or median age: mean 58 years Ethnicity: not reported Weight: not reported BMI: not reported Type of disorder: neuromuscular. Polio Affected knee: both Nature of instability: resulting from polio Previous use of orthotics: not reported |
Type of orthotic: KAFO Manufacture: prefabricated Material: carbon fibre Specified orthotic dose: not reported Fitting procedure: reported Cointerventions: not reported Comparator: no comparator group |
Duration of follow-up: at least 3 months How were patient outcomes elicited?: postal questionnaire How was gait assessed?: not assessed Statistical analysis: score counting and calculation of percentages |
Steinfeldt (2003) results
| Patient-reported outcomes |
| Satisfaction with device: patient’s perception of the following activities was retrospectively assessed pre- and post-device (10-point Likert scale): walking: pre 3.8, post 8.3, sitting: pre 4.8, post 8.9, driving a car: pre 5.8, post 9.1, comfort: pre 4, post 8.8, putting the device on/taking it off: pre 4.7, post 8.6 Other: two-thirds of patients did not need orthopaedic shoes with the KAFO |
| Objective assessments |
| Walking ability: maximal walking distance assessed by patient report, increased 1.2–11 times once patients were used to their KAFO compared to pre-intervention but use of other walking aids was only reduced for four patients (7%) |
| Resource utilisation |
| Device malfunction: ‘patient reports of the need for repairs did not show substantial problems’ |
| Adverse effects |
| Not reported |
Summers (1988) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Summers (1988) Country: UK Language: English Funding source: not reported Conflict of interest: unclear Study type: case series |
Number of participants: 20 % male: 100 Mean or median age: mean: 28 years (range 20–39) Ethnicity: not reported Weight: mean 69 kg BMI: not reported Type of disorder: CNS. Spinal cord injury Affected knee: both Nature of instability: paraplegic patients with neurologically complete lesions between C8 and T12 Previous use of orthotics: yes. 11 patients had previously used long leg callipers. At the time of fitting of the ParaWalker 10 of 11 had stopped using these |
Type of orthotic: HGO (Parawalker) Manufacture: custom-made Material: not reported Specified orthotic dose: not reported Fitting procedure: not reported Cointerventions: walking aids: crutches used as decided by patient Comparator: no comparator group |
Duration of follow-up: mean: 1 year 8 month; range 6 months to 3 years 8 months How were patient outcomes elicited?: nine patients were interviewed in person and 11 by phone by one of the study authors. Level of walking ability was classified according to the ability to don, doff and transfer independently or dependently, the value of the orthosis to the patient (functional or therapeutic) and how far and where the patient was able to walk (indoor or outdoor > 50 metres over varying terrain) How was gait assessed?: not assessed Statistical analysis: summary data only, no analysis |
Summers (1988) results
| Patient-reported outcomes |
| Functionality of device: eight patients used the HGO outdoors independently for therapeutic purposes, nine used the device independently for therapeutic purposes indoor only and three abandoned the device (two found it too tiring and one with an arm injury hoped to return to using it). All three were dependent therapeutic indoor walkers. In total 17 of 20 patients achieved independent donning and doffing and standing from and sitting in a wheelchair. The three who could not achieve this abandoned their HGO Ten patients were able to get into the passenger seat of a car, with some difficulty. Two patients had driven a car with the device on but found it difficult and did not often repeat this task Satisfaction with device: two patients complained that the device was unsightly to wear. Overall five patients were highly pleased with the device, 10 were pleased, three were non-committal and two disliked it Usage of device: four patients used the device > 3 times a week, 11 used it 1–3 times a week, two < week and three abandoned it. Of the 17 users, two used the device for > 3 hours on each occasion, 13 for 1–3 hours and two for < 1 hour per occasion |
| Resource utilisation |
| Number and nature of follow-up appointments: Patients were followed up at the gait laboratory every 6 months Device malfunction: the authors stated that minor repairs were usually required at 6-monthly follow-up sessions but there were no breakages |
| Adverse effects |
| Reported. The authors reported that there were no pressure sores. They stated that ‘most’ patients had one or two falls during early use and one patient sustained a significant injury (fractured distal end of radius) |
| Objective assessments |
| Not reported |
Sun (2007) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Sun (2007) Country: China Funding source: not reported Conflict of interest: unclear Study type: case series |
Number of participants: 15 % male: 66.7 Mean or median age: mean 33.7 years Ethnicity: Chinese Weight: not reported BMI: not reported Type of disorder: CNS. Spinal cord injury Nature of instability: unclear Previous use of orthotics: not reported |
Type of orthotic: RGO Manufacture: not reported Material: not reported Specified orthotic dose: reported. Twice per day; 1 hour for each time; for 2 months Fitting procedure: not reported Cointerventions: not reported Comparator: no comparator group |
Duration of follow-up: unclear How were patient outcomes elicited: not applicable How was gait assessed?: not applicable The outcome assessed walking distance within 6 minutes and mean walking time for 10 m |
Sun (2007) results
| Objective assessments |
Walking ability:
|
| Patient-reported outcomes, resource utilisation and adverse effects |
| Not reported |
Tang (2009) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Tang (2009) Country: China Funding source: not reported Conflict of interest: unclear Study type: controlled trial |
Number of participants: 58 % male: 82.8% Mean or median age: mean 32.35 years Ethnicity: Chinese Weight: not reported BMI: not reported Type of disorder: CNS. spinal cord injury Nature of instability: unclear Previous use of orthotics: not reported |
Type of orthotic: KAFO (n = 6), RGO (n = 15), AGO (n = 27) Manufacture: not reported Material: not reported Specified orthotic dose: reported. Twice per day; 50 minutes per time; for 6–8 weeks Fitting procedure: not reported. After fitting there was 6–8 weeks’ standing and walking training Cointerventions: rehabilitation training for 8 weeks (including training in muscle strength, balance, transferring, wheelchair using and activities of daily living) Comparator: yes. Conventional rehabilitation training (n = 10) |
Duration of follow-up: 4 months after commencement of rehabilitation (8 weeks following fitting of device) How were patient outcomes elicited?: Barthel index, Functional Independence Measure and WHO-QoL were administered. How was gait assessed?: not applicable |
Tang (2009) results
| Patient-reported outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Barthel index (BI), functional independence measure (FIM) (The paper also reports p-values for between and within group differences; however, due to the quality of the reproduction we could not confidently distinguish the between and within group comparisons and it was not possible to determine from the accompanying text) GroupBIFIMBaselineBefore fitting the deviceAfter fitting the device at 8 weeksBaselineBefore fitting the deviceAfter fitting the device at 8 weeksAGO42.11 (11.63)71.48 (6.62)80.04 (4.44)81.19 (9.40)98.30 (4.21)105.07 (5.31)RGO43.0 (14.37)73.47 (7.72)82.67 (6.23)83.0 (14.15)97.2 (3.69)105.6 (7.02)KAFO43.33 (11.25)72.50 (8.22)77.5 (6.90)81.5 (7.12)98.5 (3.08)102.5 (6.09)Control44.0 (9.94)72.0 (6.75)74.5 (5.50)83.5 (5.48)97.9 (4.04)98.6 (6.83) WHO-QoL measure (The paper also reports p-values for between and within group differences; however, due to the quality of the reproduction we could not confidently distinguish the between and within group comparisons and it was not possible to determine from the accompanying text) GroupAGORGOKAFOControlPhysicalBaseline37.25 (13.62)36.13 (14.45)36.66 (17.2)38.7 (9.79)Before fitting device53.25 (17.38)56.13 (9.65)54.33 (12.27)53.9 (9.42)After fitting device at 8 weeks68.18 (13.24)70 (10.12)58.51 (4.03)55.8 (9.54)PsychologyBaseline46.70 (13.13)49.06 (15.35)48.33 (11.63)49.9 (10.32)Before fitting device53.85 (18.35)57.06 (13.71)54.5 (7.03)56.5 (12.98)After fitting device at 8 weeks65.81 (12.31)67.46 (12.18)57.83 (11.16)56.91 (1.78)Interpersonal relationshipBaseline52.77 (11.90)52.73 (17.72)54.66 (17.39)55.2 (12.18)Before fitting device54.88 (10.6)53.93 (11.37)55.66 (11.77)53.7 (5.25)After fitting device at 8 weeks54.85 (9.57)55.6 (8.23)55.5 (10.85)55 (8.19)Environment surrounding patientsBaseline49.7 (14.37)48.41 (3.38)48.1 (4.04)49 (8.27)Before fitting device54.74 (15.37)55.73 (14.56)54.16 (12.71)54.9 (15.72)After fitting device at 8 weeks55.4 (12.92)53.66 (8.94)54.16 (10.88)57.5 (11.97) |
Group | BI | FIM | Baseline | Before fitting the device | After fitting the device at 8 weeks | Baseline | Before fitting the device | After fitting the device at 8 weeks | AGO | 42.11 (11.63) | 71.48 (6.62) | 80.04 (4.44) | 81.19 (9.40) | 98.30 (4.21) | 105.07 (5.31) | RGO | 43.0 (14.37) | 73.47 (7.72) | 82.67 (6.23) | 83.0 (14.15) | 97.2 (3.69) | 105.6 (7.02) | KAFO | 43.33 (11.25) | 72.50 (8.22) | 77.5 (6.90) | 81.5 (7.12) | 98.5 (3.08) | 102.5 (6.09) | Control | 44.0 (9.94) | 72.0 (6.75) | 74.5 (5.50) | 83.5 (5.48) | 97.9 (4.04) | 98.6 (6.83) | Group | AGO | RGO | KAFO | Control | Physical | Baseline | 37.25 (13.62) | 36.13 (14.45) | 36.66 (17.2) | 38.7 (9.79) | Before fitting device | 53.25 (17.38) | 56.13 (9.65) | 54.33 (12.27) | 53.9 (9.42) | After fitting device at 8 weeks | 68.18 (13.24) | 70 (10.12) | 58.51 (4.03) | 55.8 (9.54) | Psychology | Baseline | 46.70 (13.13) | 49.06 (15.35) | 48.33 (11.63) | 49.9 (10.32) | Before fitting device | 53.85 (18.35) | 57.06 (13.71) | 54.5 (7.03) | 56.5 (12.98) | After fitting device at 8 weeks | 65.81 (12.31) | 67.46 (12.18) | 57.83 (11.16) | 56.91 (1.78) | Interpersonal relationship | Baseline | 52.77 (11.90) | 52.73 (17.72) | 54.66 (17.39) | 55.2 (12.18) | Before fitting device | 54.88 (10.6) | 53.93 (11.37) | 55.66 (11.77) | 53.7 (5.25) | After fitting device at 8 weeks | 54.85 (9.57) | 55.6 (8.23) | 55.5 (10.85) | 55 (8.19) | Environment surrounding patients | Baseline | 49.7 (14.37) | 48.41 (3.38) | 48.1 (4.04) | 49 (8.27) | Before fitting device | 54.74 (15.37) | 55.73 (14.56) | 54.16 (12.71) | 54.9 (15.72) | After fitting device at 8 weeks | 55.4 (12.92) | 53.66 (8.94) | 54.16 (10.88) | 57.5 (11.97) | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | BI | FIM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | Before fitting the device | After fitting the device at 8 weeks | Baseline | Before fitting the device | After fitting the device at 8 weeks | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AGO | 42.11 (11.63) | 71.48 (6.62) | 80.04 (4.44) | 81.19 (9.40) | 98.30 (4.21) | 105.07 (5.31) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RGO | 43.0 (14.37) | 73.47 (7.72) | 82.67 (6.23) | 83.0 (14.15) | 97.2 (3.69) | 105.6 (7.02) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KAFO | 43.33 (11.25) | 72.50 (8.22) | 77.5 (6.90) | 81.5 (7.12) | 98.5 (3.08) | 102.5 (6.09) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Control | 44.0 (9.94) | 72.0 (6.75) | 74.5 (5.50) | 83.5 (5.48) | 97.9 (4.04) | 98.6 (6.83) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | AGO | RGO | KAFO | Control | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 37.25 (13.62) | 36.13 (14.45) | 36.66 (17.2) | 38.7 (9.79) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Before fitting device | 53.25 (17.38) | 56.13 (9.65) | 54.33 (12.27) | 53.9 (9.42) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| After fitting device at 8 weeks | 68.18 (13.24) | 70 (10.12) | 58.51 (4.03) | 55.8 (9.54) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychology | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 46.70 (13.13) | 49.06 (15.35) | 48.33 (11.63) | 49.9 (10.32) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Before fitting device | 53.85 (18.35) | 57.06 (13.71) | 54.5 (7.03) | 56.5 (12.98) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| After fitting device at 8 weeks | 65.81 (12.31) | 67.46 (12.18) | 57.83 (11.16) | 56.91 (1.78) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Interpersonal relationship | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 52.77 (11.90) | 52.73 (17.72) | 54.66 (17.39) | 55.2 (12.18) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Before fitting device | 54.88 (10.6) | 53.93 (11.37) | 55.66 (11.77) | 53.7 (5.25) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| After fitting device at 8 weeks | 54.85 (9.57) | 55.6 (8.23) | 55.5 (10.85) | 55 (8.19) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Environment surrounding patients | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | 49.7 (14.37) | 48.41 (3.38) | 48.1 (4.04) | 49 (8.27) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Before fitting device | 54.74 (15.37) | 55.73 (14.56) | 54.16 (12.71) | 54.9 (15.72) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| After fitting device at 8 weeks | 55.4 (12.92) | 53.66 (8.94) | 54.16 (10.88) | 57.5 (11.97) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Objective assessments, resource utilisation and adverse effects | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Whittle (1991) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Whittle (1991) Country: UK Language: English Funding source: not reported Conflict of interest: no Study type: case series |
Number of participants: 22 % male: 82 Mean age: 34 years Ethnicity: not reported Weight: not reported BMI: not reported Type of disorder: CNS. Paraplegia due to spinal cord injury Affected knee: both Nature of instability: spinal cord injury Previous use of orthotics: not reported |
Type of orthotic: ParaWalker (adult HGO) Manufacture: custom-made Material: metal solid Specified orthotic dose: not reported Fitting procedure: reported. Participants were placed in pairs matched for age and level of spinal injury. One member used the HGO first, the other the RGO first. Participants performed upper limb and trunk exercise for 4 weeks, the first orthosis was fitted and training given so that the patient could put on and take off the orthosis, stand up and sit down safely and walk at least 30 m. Participants wore the orthosis at home for 4 months then the pattern was repeated for the other orthosis. Training in the use of either orthosis generally took about 3 hours per day for 4–5 days Cointerventions: walking aids: Nineteen patients using the RGO used a rollator, one used crutches. Fourteen patients using the HGO used crutches, five used a rollator Comparator: yes. Custom-made RGO |
Duration of follow-up: 8 months (4 months for each orthotic) How were patient outcomes elicited?: unclear How was gait assessed?: not assessed Statistical analysis: unclear |
Whittle (1991) results
| Patient-reported outcomes |
| Functionality of device: 17 patients successfully used the HGO, three did not and two left the study. Fifteen were successful with the RGO, five were not and two left the trial Impact on daily living, quality of life: the authors made the following statements but did not provide numerical data to support them. The HGO was much quicker to put on or to take off. The RGO was quicker on most other tests but this was statistically significant only for standing up and climbing up a kerb. When sitting in a wheelchair, wearing an orthosis did not cause any serious problems. It was difficult to use a car when wearing one of the orthoses as either driver or passenger although patients reported it was a little easier with the RGO Satisfaction with device: at the end of the study 12 patients chose to keep the RGO, four the HGO and six neither orthotic device. The main reasons given for the final choice (no numerical data provided): HGO – ease of putting on and taking off, RGO – cosmesis and ease of standing with hands free, neither – fear of developing pressure sores and difficulty using either orthosis. Final choice of orthosis did not appear to be influenced by intelligence or by any previous knowledge of one or other orthosis. The RGO was preferred by those who tended to be anxious and by those who did not regard themselves as particularly persistent in the face of difficulty (no data provided) Other: there was little difference in comfort between the two orthoses (no data provided) |
| Objective assessments |
| Walking ability: there were no significant differences between orthoses in gait parameters of cadence, stride length and velocity after 4 months’ use (no data provided). The average walking velocity was about 0.24 m/s (about one-fifth normal speed). The effort involved in walking estimated by changes in pulse rate and oxygen consumption was similar for the two orthoses (no data provided) |
| Resource utilisation |
| Device malfunction: the authors stated that frequent adjustments were needed initially for both orthoses and this was done by the on-site orthotist. The RGO particularly required attention to ensure patients did not get pressure sores (no data provided). No major failures occurred with the HGO. The RGO was damaged in two cases due to overstressing. In 4 months of use for both orthoses about one third of participants' devices needed minor repairs, replacements or adjustments Other: fabrication of the RGO was statistically significantly more expensive (£1772 vs. £1116 for the HGO). Training and out-of-pocket expenses were similar between the orthoses. Combined cost of training and 4 months’ maintenance was about £330 for each device. Patients and their carers had an average of 8 days off work and out-of-pocket expenses of £160 to £200 |
| Adverse effects |
| Not reported |
Wu (2003) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Wu (2003) Country: China Language: English Funding source: not reported Conflict of interest: not reported Study type: case series |
Number of participants: 6 % male: 67 Mean or median age: mean 27.6 years Ethnicity: not reported Weight: not reported BMI: not reported Type of disorder: CNS. Spinal cord injury Affected knee: not reported Nature of instability: paraplegia due to spinal cord injury Previous use of orthotics: no |
Type of orthotic: HKAFO Manufacture: custom-made Material: plastic solid, propene polymer Specified orthotic dose: not reported Fitting procedure: reported Cointerventions: other. Gait training including balance and walking exercises Comparator: no comparator group |
Duration of follow-up: unclear How were patient outcomes elicited?: activities of daily living determined by Barthel index. However results presented were above the 20-point maximum on this scale How was gait assessed?: unclear Statistical analysis: pre–post data for Barthel index, motor plan score and sensory plan score were provided and statistical significance was assessed between the two time points (t-test) |
Wu (2003) results
| Patient-reported outcomes |
| Functionality of device Barthel index: pre treatment 26 (SD 8), post treatment 47 (SD 7), p < 0.01 |
| Objective assessments |
| Walking ability: 1 week after use of the orthotic all six patients could stand or walk better between parallel bars (no data provided). After 2 weeks of exercises with the orthoses patients could 'continuously walk for 40 m and complete therapeutic walking Other functional ability: motor plan score: pre treatment 32 (SD 10), post treatment 37 (SD 6) (p > 0.1); Sensory plan score: pre treatment 54 (SD 12), post treatment 61 (SD 12) (p > 0.1) |
| Resource utilisation and adverse effects |
| Not reported |
Yang (2005) methods
| Study details | Study participants | Interventions | Analysis |
|---|---|---|---|
| Yang (2005) Country: China Funding source: not reported Conflict of interest: unclear Study type: RCT |
Number of participants: 67 % male: 83.6 Mean or median age: intervention group mean 58.2 years control group mean 57.6 years Ethnicity: Chinese Weight: not reported BMI: not reported Type of disorder: CNS. post-stroke hemiplegic patients Nature of instability: knee over-stretching Previous use of orthotics: no |
Type of orthotic: KAFO, AFO Manufacture: not reported Material: not reported Specified orthotic dose: not reported Fitting procedure: not reported Cointerventions: not reported Comparator: yes, conventional rehabilitation, kinaesthetics |
Duration of follow-up: not reported (duration of intervention 2 months) How were patient outcomes elicited?: not reported How was gait assessed?: not reported |
Yang (2005) results
| Objective assessments | ||||||||||||||||||
| EffectivenessnHighly effectiveModerately effectiveNo effect% effectiveIntervention35268197Control32179681 | Effectiveness | n | Highly effective | Moderately effective | No effect | % effective | Intervention | 35 | 26 | 8 | 1 | 97 | Control | 32 | 17 | 9 | 6 | 81 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effectiveness | n | Highly effective | Moderately effective | No effect | % effective | |||||||||||||
| Intervention | 35 | 26 | 8 | 1 | 97 | |||||||||||||
| Control | 32 | 17 | 9 | 6 | 81 | |||||||||||||
| Patient-reported outcomes, resource utilisation and adverse effects | ||||||||||||||||||
| Not reported |
Appendix 13 Demographic characteristics of participants in qualitative study
| Participant | Age (years) | Gender | Ethnicity | Condition |
|---|---|---|---|---|
| P1 (NHS) | 36 | Female | Black African | Poliomyelitis |
| P2 (NHS) | 52 | Female | White British | Spinal injury at birth (?spina bifida/cerebral palsy) |
| P3 (NHS) | 50 | Male | White and black African | Poliomyelitis |
| P4 (NHS) | 70 | Female | White British | Stroke |
| P5 (NHS) | 53 | Male | Asian Indian | Poliomyelitis |
| P6 (NHS) | 59 | Female | White British | Poliomyelitis |
| P7 (NHS) | 55 | Female | Black British | Multiple sclerosis |
| P8 (NHS) | 63 | Male | White British | Poliomyelitis |
| P9 (NHS) | 67 | Male | White British | Poliomyelitis |
| P10 (NHS) | 62 | Female | White British | Multiple sclerosis |
| P11 (NHS) | 73 | Male | White British | Multiple sclerosis |
| P12 (NHS) | 53 | Male | White British | Multiple sclerosis |
| P13 (NHS) | 54 | Female | White British | Multiple sclerosis |
| P14 (non-NHS) | 64 | Female | White British | Poliomyelitis |
| P15 (non-NHS) | 72 | Female | White British | Poliomyelitis |
| P16 (non-NHS) | 64 | Male | White British | Poliomyelitis |
| P17 (NHS) | 72 | Male | White British | Spinal injury/drop foot |
| P18 (non-NHS) | 73 | Female | White European | Poliomyelitis |
| P19 (NHS) | 48 | Female | White British | Spina bifida; amputation |
| P20 (NHS) | 80 | Male | White British | Spinal injury |
| P21 (NHS) | 58 | Female | White British | CMT disease |
| P22 (non-NHS) | 63 | Male | White British | CMT disease |
| P23 (NHS) | 57 | Male | Arab | Poliomyelitis |
| P24 (NHS) | 63 | Male | White British | Poliomyelitis |
Appendix 14 Orthotics for Knee Instability blog post timeline
PICO, population, intervention, comparator, outcomes.


List of abbreviations
- ACPIN
- Association of Chartered Physiotherapists Interested in Neurology
- AFO
- ankle–foot orthosis
- AGO
- alternative gait orthosis
- AHP
- allied health professional
- ARGO
- advanced reciprocating gait orthosis
- BAPO
- British Association of Prosthetists and Orthotists
- BSRM
- British Society of Rehabilitation Medicine
- CCG
- Clinical Commissioning Group
- CDSR
- Cochrane Database of Systematic Reviews
- CE
- Conformité Européenne
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CIDP
- chronic inflammatory demyelinating polyradiculoneuropathy
- CMT
- Charcot–Marie–Tooth
- CNS
- central nervous system
- DARE
- Database of Abstracts of Reviews of Effects
- FES
- functional electrical stimulation
- FIM
- functional independence measure
- FO
- foot orthosis
- FSH
- facioscapulohumeral muscular dystrophy
- GP
- general practitioner
- GRAFO
- ground reaction ankle–foot orthosis
- HCP
- health-care professional
- HGO
- hip guidance orthosis
- HKAFO
- hip–knee–ankle–foot orthosis
- HTA
- Health Technology Assessment
- IRGO
- isocentric reciprocating gait orthosis
- KAFO
- knee–ankle–foot orthosis
- KO
- knee orthosis
- MDT
- multidisciplinary team
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NIHR
- National Institute for Health Research
- NMD
- neuromuscular disease
- NTIS
- National Technical Information Service
- OKIS
- Orthotics for Knee Instability
- PASA
- Purchasing and Supply Agency
- QR
- Quick Response
- RCT
- randomised controlled trial
- RGO
- reciprocating gait orthosis
- ROM
- range of movement
- SCKAFO
- stance control knee–ankle–foot orthosis
- SCO
- stance control orthosis
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- WHOQOL-BREF
- The World Health Organization Quality of Life-BREF
- WO
- Walkabout orthosis