Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 14/69/02. The protocol was agreed in October 2014. The assessment report began editorial review in April 2015 and was accepted for publication in November 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Edwards et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Conditions and aetiologies
Skin cancer is one of the most common cancers in the UK. In 2011, 13,300 cases of malignant melanoma were diagnosed, and around 2200 people died from the disease. 1 In 2010, around 100,000 people were diagnosed with non-melanoma skin cancer, and in 2012 there were 638 deaths from non-melanoma skin cancer. 2
Skin cancer is commonly classified into melanoma skin cancer (also known as malignant melanoma), which develops from pigmented cells (melanocytes) in the epidermis, and non-melanoma skin cancer, which develops from cells that produce keratin (keratinocytes). 1
Non-melanoma skin cancer can be further divided into squamous cell carcinoma (SCC) and basal cell carcinoma (BCC). Malignant melanoma, SCC and BCC make up > 95% of all skin cancers. In addition, there are other rare types of non-melanoma skin cancer including Merkel cell carcinoma, Kaposi sarcoma and T-cell lymphoma of the skin. 3
The main risk factor for developing most types of skin cancer is exposure to ultraviolet radiation in the form of sunlight or from the use of sunbeds. Other factors that may influence the risk of developing skin cancer include age and sex, ethnicity, occupation, personal and family history of skin cancer, socioeconomic status and certain physical characteristics (light eyes or hair; fair skin that sunburns easily; and having a lot of moles, unusually shaped or large moles or a lot of freckles). 1,2,4–6
Melanoma
Malignant melanoma is the fifth most common cancer in the UK, accounting for 4% of all new cases. 2 Like most cancers, skin cancer is more common with increasing age, but malignant melanoma rates are disproportionately high in younger people. 2 Malignant melanoma is almost twice as common in young women (up to age 34 years) as in young men, but more men die from it. 2 Malignant melanoma incidence rates have increased more than fivefold since the mid-1970s. People from more affluent areas are more likely than those from more deprived areas to be diagnosed with malignant melanoma at an early stage. The most common sites of melanoma in men are the trunk, head and neck, and arms, whereas in women they are trunk, legs and arms. 4 Survival rates among patients with malignant melanoma have been improving for the last 25 years and is now among the highest for any cancer. Five-year survival rate ranges from 100% among patients diagnosed at the earliest stage to 8% (men) and 25% (women) among patients diagnosed once the disease has spread. Around two-thirds of malignant melanoma cases are diagnosed at the earliest stage. 2
There are several different types of melanoma:
-
Superficial spreading melanoma makes up approximately 70% of malignant melanomas. Initially this type usually grows outwards with low risk of metastasis, but when it eventually starts to grow down into the dermis it can acquire the capacity for invasion. 4
-
Nodular melanoma is the most aggressive form of malignant melanoma. Fourteen per cent of all melanomas are nodular, and these make up 37% of ultimately fatal lesions. They grow quickly downwards into the skin, and are usually very dark with a raised area of skin, but may not necessarily develop from an existing mole. 7
-
Lentigo maligna melanoma (LMM) arises from lentigo maligna (LM) or Hutchinson’s freckle, which present as macular-pigmented lesions. It most commonly appears on the face or other areas of the skin that has high sun exposure. LM grows outwards very slowly, and it becomes malignant when it starts to grow down into the deeper layers of the skin. Around 10% of malignant melanomas are LMM. 4
-
Acral lentiginous melanoma is a rare form of melanoma most commonly found on the palms of the hand, the soles of the feet or under or around the nails. It is the most common type of melanoma in people with dark skin. 4
-
Amelanotic melanomas lack the dark colour of usual melanomas. They are usually non-pigmented and may appear pink or red with light-brown or grey edges. They make up approximately 5% of melanomas and are difficult to diagnose, as they can easily be mistaken for other skin conditions. 4
Non-melanoma skin cancers
There is known under-recording of non-melanoma skin cancer incidence with an estimated 30–50% of BCC and around 30% of SCC going unrecorded. This is partly because many cases are treated in primary care or privately and are not notified to the cancer registries, and partly because most cancer registries record only the first diagnosis of BCC or SCC. 2 As non-melanoma skin cancer registrations are known to be incomplete, they are usually excluded from incidence totals for all cancers combined. Although non-melanoma skin cancer is extremely common, in the vast majority of cases it is detected early and is not usually life-threatening. However, around 590 people died from non-melanoma skin cancer in 2011 in the UK. 2
Basal cell carcinoma
Basal cell carcinoma is the most common type of non-melanoma skin cancer, making up about 75% of non-melanoma cases. 6 It develops on areas of the skin with a high sun exposure, such as the nose, forehead and cheeks. BCC is slow-growing and rarely spreads or becomes fatal; however, it can invade other types of tissue such as cartilage and bone in the nose or ears. BCCs can be divided into several subtypes based on morphology and development including nodular, superficial, morphoeic and pigmented BCCs.
Basel cell carcinomas are more common in older people; people aged > 75 years are about five times more likely to have a BCC than those people aged between 50 and 55 years. 6 BCCs are also more common in males than females. In the UK, the recorded incidence between 2000 and 2010 was around 36% in males and 32% in females. 8
Squamous cell carcinoma
Squamous cell carcinoma is a more serious, but less common, type of non-melanoma skin cancer than BCC, which has the potential to metastasise to other organs of the body. 9 Around 20% of diagnosed non-melanoma skin cancers are SCCs. 6 The increase in incidence of SCCs from 2000–2 to 2008–10 was 34% in males and 39% in females. 8
Squamous cell carcinoma lesions often develop on sun-exposed skin such as the head and neck, but they can also develop in areas of the skin that have been ulcerated for a long time, in scars, burns or in pre-existing lesions such as Bowen’s disease. SCCs are usually crusty or scaly, but can also present as an ulcer without keratinisation.
Description of technologies under assessment
The aim of skin cancer diagnosis is to identify truly positive lesions while curtailing the number of unnecessary biopsies. Reflectance confocal microscopy (RCM) is a non-invasive technique that allows examination of the epidermis and papillary dermis at cellular resolution. 10
The VivaScope® imaging systems are non-invasive technologies designed to diagnose potentially malignant skin lesions. They capture highly magnified images of the upper layer of the skin. They are designed for use in conjunction with dermoscopy to investigate potentially malignant skin lesions, thus providing a more accurate diagnosis, leading to fewer biopsies of benign lesions and earlier detection of skin cancers. They may also be used as a guide to surgery to provide more accurate presurgical margins, preventing unnecessarily large scars for skin cancers in anatomical areas where tissue preservation is of importance (e.g. face, hands, feet and genitals), and reducing the risk of recurrence.
A near-infrared light source is used to visualise skin structures at different horizontal levels within the upper layer of the skin. 11 The images produced are based on the reflection and scattering of light from the examined tissue section. Different cell structures lead to different reflection patterns, which are seen as shades of grey in the captured image. Melanin, haemoglobin, cellular microstructures and collagen serve as ‘endogenous’ contrast agents. Melanocytic lesions could therefore be potentially well imaged using VivaScope.
VivaScope® 1500
The stationary device of the VivaScope® 1500 (Caliber Imaging and Diagnostics, Rochester, NY, USA) is designed for use on extremities such as the back of the hand or the back, chest, leg, arm, cheek or forehead. The horizontal resolution is reported to be 1.25 µm and the vertical resolution (layer thickness) is 3–5 µm, which corresponds to the layer thickness of normal histological examinations. With the VivaScope 1500 individual images are 500 × 500 µm in size; however, in total, images of an area of between 1 × 1 mm and 8 × 8 mm may be captured. The imaging depth includes the upper layers of the reticular dermis.
VivaScope 1500 is a console-based unit. Examination using the VivaScope 1500 involves applying an adhesive window on the stainless steel ring of the device, which is fixed on the skin over the lesion. The VivaScope 1500 is positioned on the tissue ring and images can be recorded. The VivaScope 1500 also includes an integrated dermoscope.
VivaScope® 3000
The hand-held VivaScope® 3000 (Caliber Imaging and Diagnostics, Rochester, NY, USA) is designed to access difficult-to-reach skin regions such as around the nose, ears and eyes, or between fingers. From the technical specification, VivaScope 3000 can be used for diagnosis, as well as a guide to surgery to provide presurgical margins of tumours. The resolution of the VivaScope 3000 is the same as that of the VivaScope 1500, but the individual images are 1000 × 1000 µm for VivaScope 3000 and the image depth is reported as up to 200 µm depending on the tissue type. The VivaScope 1500 and 3000 can be used as stand-alone units or together.
Earlier versions of VivaScope include VivaScope® 1000 (Lucid Inc., Rochester, NY, USA, or Lucid Inc., MAVIG GmbH, Munich, Germany) and VivaScope® 2500 (Caliber Imaging and Diagnostics, Rochester, NY, USA). VivaScope 1000 is a stationary laser microscope device capable of imaging living tissue at the cellular level. The VivaScope 2500 surgical cellular confocal imager allows the capture cellular resolution images of the skin and supporting stroma. These images are captured from bulk, excised tissue without the need for lengthy staining and sectioning protocols.
Costs of the VivaScope 1500/3000 and training needs
The costs associated with examination of skin lesions with VivaScope comprise the purchase (capital) cost of the VivaScope imaging system, maintenance costs, costs of equipment parts and other consumables required for the examination, and costs of training staff in operating the system and in the assessment and interpretation of the images obtained. They also include costs of staff time required for the examination with VivaScope and subsequent assessment of skin lesions.
According to the company, the purchase price and annual maintenance costs of VivaScope 3000, as an add-on device to VivaScope 1500, is lower than the respective costs of VivaScope 3000 as a stand-alone device (Table 1).
| Item | Cost |
|---|---|
| Indicative price of technology | £90,224.00 for VivaScope System (dermoscopy + RCM integrated)a |
| Consumables | £1.50/adhesive window per patient lesion |
| Service/maintenance cost and frequency | £4380.00 per annum |
| Anticipated life span of technology | 10 years |
| Average length of use per treatment | 10–15 minutes per treatment |
| Average frequency of use | 15–20 per day |
| Average cost per treatmentb | £120.00 |
| Additional costs | |
|
100 per box = £147.00 (for VivaScope onlya) |
|
£55.00 (very durable steel ring, usually no replacement required unless loss)a |
|
£7.80 |
|
£3.30 (usually already available in the hospital, or other disinfectant)a |
|
£3.20 (usually already available in the hospital)a |
|
£192 (two caps are provided with the device, only in case of loss)a |
Training on the use of VivaScope consists of the following (information provided by the company, supplemented by one of the clinical experts providing the training):
-
Introductory training: this is provided on-site for free with the purchase of VivaScope, lasts approximately 1–2 days and involves mainly technical training but some basic clinical information is also offered. The purpose of training is to give technicians and clinicians (i.e. consultant dermatologist, consultant dermatological surgeon, technical assistant, pathologist and researcher) the ability to properly use the machine and the software, provide them with an understanding of the anatomical location of the image on the monitor and detect the most common and evident structures. Participants are given information image acquisition, data management, operational precautions, etc. The training course consists of presentations, the revision of manuals, discussion of imaging guidelines and consideration of appropriate studies of interest.
-
Independent study with textbooks: this is complementary to the introductory training; VivaScope users are expected to revise two sophisticated imaging textbooks.
-
Intensive expert training: this is also provided for free with the purchase of VivaScope and follows the introductory training and independent study. It is a 3-day course currently offered four times a year at the University of Modena and Reggio Emilia in Italy, but there are plans to expand it to referral centres in Europe, including the UK. Four confocal experts who have been working with the VivaScope for > 10 years provide the training in Italy. They guide the participants through the diagnosis of melanocytic lesions, non-melanocytic lesions, inflammatory skin diseases, cosmetic applications and others. It is considered an essential part of the training.
-
Online training course: provided for free with the purchase of VivaScope, this course consists of 100 cases with expert evaluation made available after student evaluation. It is considered part of the intensive expert training and is available with the purchase of VivaScope. The aim of this course is to establish the learning and test the trainee’s skills.
Diagnosis using VivaScope
VivaScope can be used for diagnosis of different kinds of skin cancer by providing detailed images that show the morphology of potentially cancerous cells.
According to the company, the main criteria for a diagnosis of malignant melanoma with VivaScope include the absence of the normal epidermis architecture, lack of delineation of the papillae (non-edged papillae), irregular nests of atypical melanocytes, and the presence of large and highly refractile cells with prominent nuclei in higher epidermal layers. 11
VivaScope can also be used to diagnose BCCs. Five main criteria have been described by the company as characteristic BCC changes that can be identified using the VivaScope: elongated and monomorphic nuclei; polarisation of these cells along an axis; pronounced inflammatory infiltrate; increased as well as dilated blood vessels; and loss of epidermal honeycomb structure. 11 In addition, tumour cell islands with peripheral palisading, distinguishable from the dermis by a dark gap, are often identified in the dermis. This optical gap formation corresponds histologically to the accumulation of mucin.
Squamous cell carcinomas can be difficult to view using imaging techniques because their upper surface is often scaly, which can make it difficult to obtain sufficient resolution detail. 11
Relevant comparators
In clinical practice, lesions suspected of malignancy are assessed by visual examination of the lesion followed by dermoscopy by an experienced diagnostic clinician [dermatologist, plastic surgeon, nurse specialist, general practitioners (GPs)]. Decisions on tumour margin delineation prior to surgery are based on guidelines by the British Association of Dermatology (BAD). 13 For example, all suspected melanomas are excised with a 2.0-mm margin and then re-excision is based on the Breslow thickness. BCCs are generally excised with a 3.0- to 4.0-mm margin unless they are being excised by Mohs surgery, and if they are recurrent a 6.0-mm margin is sometimes used. 13
Care pathways/current practice
According to clinical experts, patients with suspicious skin lesions are referred to secondary care by their GP. After a dermoscopic examination, patients with benign lesions are discharged and those with suspicious clinical and dermoscopic features go straight diagnostic excision biopsy.
Melanoma
Melanoma remains relatively uncommon in primary care settings and, therefore, the opportunities to develop specific diagnostic skills are limited and all suspected melanoma lesions should therefore be referred within 2 weeks to an appropriate core member of the local specialist multidisciplinary skin cancer team, the Local Hospital Skin Cancer Multidisciplinary Team (LSMDT). 13
The National Institute for Health and Care Excellence (NICE)14 has produced the following draft guideline on the assessment and management of melanoma (Box 1).
-
Dermoscopy should be used to assess all pigmented skin lesions referred for further assessment, by health-care professionals trained in this technique.
-
Confocal microscopy or computer-assisted diagnostic tools should not routinely be used to assess pigmented lesions.
-
For a clinically atypical melanocytic lesion that does not need excision at first presentation, baseline photographic images (preferably dermoscopic) should be used to review the clinical appearance of the lesion, 3 months after first presentation to identify early signs of melanoma.
-
All suspected atypical spitzoid lesions should be discussed at the specialist skin cancer multidisciplinary team meeting.
-
Diagnosis of a spitzoid tumour of unknown malignant potential should be made on the basis of the histology, clinical features and behaviour.
-
Spitzoid tumours of unknown malignant potential should be managed as melanoma.
-
Excision
-
Excision with a clinical margin of ≥ 0.5 cm for people with in situ (stage 0) melanoma should be considered.
-
Further management should be discussed with the multidisciplinary team if an adequate histological margin is not achieved after excision for in situ melanoma.
-
Excision should be offered with a clinical margin of at least 1.0 cm to people with AJCC stage I (Breslow thickness < 2.0 mm) melanoma.
-
Excision should be offered with a clinical margin of at least 2.0 cm to people with AJCC stage II (Breslow thickness 2.0 mm or more) melanoma.
-
AJCC, American Joint Committee on Cancer.
Source: adapted from NICE. Melanoma: Assessment and Management of Melanoma. NICE Guideline. London: NICE; 2015. 14
In secondary care, assessment of suspected malignant lesions can be improved using dermoscopy. According to the revised UK melanoma guidelines,13 if malignancy cannot be excluded the lesion should be photographed and then completely excised. The excision biopsy should include the whole tumour with a clinical peripheral margin of 2.0 mm, with a cuff of underlying subdermal fat. Definitive diagnosis is then made by histopathological review of the biopsy. If malignancy is confirmed, subsequent treatment options are then based on the Breslow thickness of the tumour.
In cases where it is not possible to diagnose a lesion as a melanoma or a benign melanocytic naevi (the so-called ‘melanocytic lesion of uncertain malignant potential’,15 the patient should be referred to a specialist skin cancer multidisciplinary team (SSMDT) for clinical and pathological review. 13 A decision to treat as a melanoma should be made by the SSMDT in discussion with the patient.
Incision or punch biopsy may be used for diagnosis of LM or acral melanoma. However, with LM there is a risk of subclinical microinvasion, that is progression into an LMM, which may be missed because of sampling errors when using incisional biopsies.
Surgery is the only curative treatment for melanoma. Following excision biopsy for diagnosis, a wider and deeper margin, based on Breslow thickness, may be needed to ensure complete removal of the primary lesion and any micrometastases. 13 Recommended surgical excision margins are summarised in Table 2. However, the final decision about the size of the margin should be made after discussion with the patient and taking into consideration functional and cosmetic implications of the margin chosen.
| Breslow thickness (mm) | Excision margins |
|---|---|
| In situ | 5.0 mm |
| < 1.00 | 1.0 cm |
| 1.01–2.00 | 1–2 cm |
| 2.10–4.00 | 2.0 cm |
| > 4.00 | 2–3 cm16,17 |
For LM the aim is to excise the lesion completely with a clear histological margin, after which no further treatment is then required. For large in situ LMMs, surgical margins of > 0.5 cm may be necessary to achieve histologically negative margins. 18 There may also be clinical situations where treatment by other methods, such as radiotherapy or observation only, may be appropriate.
Basal cell carcinoma
Lower-risk nodular BCC may be removed in primary care by suitably qualified GPs (only in low-risk sites, below the head and neck, and < 2 cm in diameter). However, if there is uncertainty around the diagnosis or if the BCC is of any other high-risk subtype, it should be referred to a LSMDT. 19 In most cases dermatologists can make a confident diagnosis of BCC by visual examination of the lesion, which may be helped by dermoscopy. If there is uncertainty around the BCC diagnosis or around the subtype of BCC, which may influence prognosis or treatment selection, diagnosis should be confirmed by biopsy and histology. The aim of treatment of BCC is to remove the tumour while resulting in a cosmetic outcome that is acceptable to the patient. 19
The treatment options for BCC depend on if the lesion is classified as having a low or high risk of recurrence following treatment, which depends on a range of prognostic factors including:
-
tumour size (increasing size indicates a higher risk of recurrence)
-
tumour site (lesions on the central face, especially around the eyes, nose, lips and ears, are at higher risk of recurrence)
-
definition of clinical margins (poorly defined lesions are at higher risk of recurrence)
-
histological subtype (certain subtypes leads to a higher risk of recurrence)
-
failure of previous treatment (recurrent lesions are at higher risk of further recurrence).
Techniques that do not allow histological confirmation of tumour clearance are generally used for only low-risk BCC lesions. These include cryosurgery, curettage, radiotherapy, topical treatments such as imiquimod (Aldara®, Meda Pharmaceuticals Ltd, Essex, UK) and photodynamic therapy. The exception is radiotherapy and Mohs surgery, which are also used for high-risk BCCs. Surgical excision is widely used to treat both low- and high-risk BCCs. 19
Squamous cell carcinoma
In common with all suspected melanoma, every SCC presenting in primary care should be referred, under the 2-week rule, to the LSMDT, which will establish the diagnosis histologically. 13
The majority of SCC tumours are at low risk of metastases, but it is essential to identify the estimated 5% of SCC tumours that are high risk. 9 SCC tumours are deemed low or high risk based on several prognostic factors that may influence their metastatic potential, including tumour site, size, thickness and level of invasion; rate of growth; aetiology; presence of perineural or lymphovascular invasion; degree of histological differentiation (subtype); and host immunosuppression. 9 However, the malignant behaviour of SCC tumours varies greatly.
The aim of treatment is complete removal of the primary tumour and any metastases. The success of the treatment is highly dependent on the definition of tumour margin. The gold standard for tumour margin identification is histological assessment. However, determining tumour extent may be challenging, particularly when the margins of the tumour are ill-defined or any metastases are discontinuous from the primary tumour. Locally recurrent tumours may arise either because of the failure to treat the primary tumour or from local metastases. 9
Surgical excision (including Mohs micrographic surgery), a highly specialised surgical method for removing high-risk skin tumours, is the primary treatment option for the majority of SCCs. The advantage of surgical excision is that it provides tissue for histological examination, which allows assessment of the adequacy of treatment and for further surgery if necessary. Other treatment options include curettage and cautery, and cryosurgery for small, well-defined, low-risk tumours, and radiotherapy for non-resectable tumours with ill-defined margins. 9
Place of intervention in diagnosis and treatment pathway
VivaScope 1500 is intended as an add-on test to dermoscopy used in hospital settings to avoid biopsy for potential malignant melanoma, LM, BCC or SCC skin lesions. It may also be used to diagnose skin cancer in patients with equivocal melanocytic skin lesions who would otherwise have been biopsied. VivaScope 3000 can be used for both lesion diagnosis and to define the margins of melanoma, BCC, SCC and LM skin lesions to guide surgical excision.
However, in the latest NICE14 guideline on assessment and management of melanoma, clinicians are advised not to routinely use confocal microscopy (such as VivaScope) or computer-assisted diagnostic tools to assess pigmented lesions in the diagnosis of melanoma.
Therefore, in reviewing the evidence on the use of VivaScope in the diagnosis of malignant melanoma or defining margins of melanoma, this systematic review looks at the evidence beyond the scope of the NICE14 and National Comprehensive Cancer Network draft guideline. 18
Chapter 2 Definition of the decision problem
Decision problem
Population
The VivaScope 1500 and 3000 imaging system was assessed in the diagnosis of skin cancer in the following populations:
-
people with suspected melanomas, who have equivocal lesions following dermoscopy
-
people with suspected BCCs, whose lesions have a positive result on dermoscopy, to confirm diagnosis as an alternative to diagnostic biopsy.
The above populations were considered to be the most relevant to undergo diagnostic assessment with VivaScope, according to clinical experts to the Evidence Assessment Group (EAG). The NICE scope defines the study population as ‘people with equivocal lesions following dermoscopy’; however, clinical experts advised the EAG that suspected BCC lesions are rarely equivocal on dermoscopy and that the use of VivaScope in suspected BCC would be mainly to confirm diagnosis in lesions that were found positive on dermoscopy, as an alternative to diagnostic biopsy.
Equivocal lesions include any lesions that are suspected of being melanoma based on a number of characteristics on dermoscopy, with the exception of clear-positive (cancerous) lesions that have all the dermoscopic characteristics of melanoma and clear-negative (benign) lesions that show no features for melanoma (no changes) on dermoscopy.
The risk of equivocal lesions being malignant is overall low. There are different degrees of ‘equivocalness’, depending on the dermoscopic characteristics of the lesion and subjective experience and interpretation.
Clinical expert advice indicated that highly suspicious equivocal lesions are:
-
lesions with at least two positive dermoscopic features, including one major criterion, or three minor positive features suggestive of melanoma, and/or
-
lesions clearly changed after digital follow-up, and/or
-
new or growing lesions in an adult with at least one dermoscopic positive criterion, or papular/nodular or pink or spitzoid lesions.
In all those cases, excision is prompted and examination with VivaScope does not represent a real advantage as the risk to miss a melanoma remains too high.
Low or moderately suspicious equivocal lesions are:
-
lesions with only one major dermoscopic positive feature or two minor features, and/or
-
no clear history of minor changes.
In such cases, excision is possible but other options could be taken into account, such as digital follow-up, especially in the case of flat lesions in patients with multiple moles; however, digital follow-up can delay a melanoma diagnosis. The majority of low or moderately suspicious equivocal lesions that are excised are benign and examination with VivaScope can play a major role in reducing this burden of unnecessary excisions.
Clinical experts advised that VivaScope is less suitable for the detection and assessment of skin lesions suspected of being SCC, as this type of skin cancer is usually scaly because of severe hyperkeratosis. This often limits the evaluation of SCC lesions, as it is more difficult to capture images of structures deeper in the tissue. Moreover, no evidence on the diagnostic accuracy of VivaScope in this type of skin cancer was identified in the systematic review of clinical evidence. Therefore, it was decided not to include people with skin lesions suspected of being SCC in the diagnostic economic model.
Regarding margin delineation, VivaScope 3000 was assessed in the following population:
-
patients with LM prior to surgical management.
According to clinical expert advice, margin delineation of melanomas with VivaScope is not useful in clinical practice, as the margins of melanomas are clearly defined and can be completely excised following BAD guidance;5 consequently, VivaScope mapping of melanomas does not offer any clinical utility and, therefore, was not considered further for economic modelling.
Clinical experts advised that margin delineation of BCCs using VivaScope may be difficult, as BCCs may be too deep so their margins may not be accurately mapped with VivaScope.
VivaScope is not appropriate for the assessment of SCC lesion margins; in addition to the scaly nature of the lesion, it may be too deep and/or the margin may be poorly defined.
Setting
Secondary care.
Intervention and comparator
Interventions
-
Diagnosis Assessment of the lesion by dermoscopy plus VivaScope or VivaScope alone by an experienced skin cancer specialist.
-
Delineation of lesion margins Assessment of the lesion by dermoscopy plus VivaScope or VivaScope alone by an experienced skin cancer specialist.
Although this report is mainly aimed at the current versions of VivaScope (1500 and 3000), earlier versions such as VivaScope 1000 and 2500 were also considered, as they may provide additional potential information on the current versions.
Comparators
-
The comparator eligible for inclusion for the assessment of both diagnostic accuracy and delineation of lesion margins was visual assessment of the lesion followed by dermoscopy and clinical judgement by an experienced skin cancer specialist.
Reference standard
-
The eligible reference standard for the assessment of diagnostic accuracy and margin delineation was histopathology or biopsy of the excised skin lesion.
Outcomes
The following outcomes were considered subject to available evidence from included studies:
-
Diagnosis
-
diagnostic accuracy
-
time to test result
-
test failure rate, for example imaging failure
-
number of biopsies performed and repeat biopsies
-
morbidity associated with biopsy such as pain and swelling
-
extent of scarring and associated psychological impact
-
adverse events from biopsy including infections
-
adverse events from false test results including patient distress and sequelae
-
health-related quality of life (HRQoL)
-
cost-effectiveness
-
-
Delineation of lesion margins
-
diagnostic accuracy
-
time to result
-
imaging failure rate
-
number of surgical procedures/surgical stages
-
morbidity associated with excision surgery such as pain and swelling
-
recurrence rates
-
extent of scarring and associated psychological impact
-
adverse events from false test results including patient distress and sequelae
-
adverse events from surgery including infections
-
HRQoL
-
cost-effectiveness.
-
Overall aims and objectives of assessment
To evaluate the clinical effectiveness and cost-effectiveness of the non-invasive RCM VivaScope 1500 and 3000 imaging systems, to avoid unnecessary biopsy of equivocal skin lesions suspected to be malignant melanoma, LM, BCC or SCC, relative to current practice.
To evaluate the clinical effectiveness and cost-effectiveness of the non-invasive RCM VivaScope 3000 imaging system in defining the margins of melanoma, BCC, SCC and LM skin lesions, relative to current practice.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing clinical effectiveness
A systematic review was conducted to summarise the evidence on the clinical effectiveness of VivaScope 1500 for lesion diagnosis and VivaScope 3000 for margin delineation. However, the scope was broadened to include previous or earlier versions, such as VivaScope 1000 and 2500, in order to capture data that may be missing by including only the current versions.
The systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination guidance for undertaking reviews in health care20 and in the NICE Diagnostic Assessment Programme manual. 21
Inclusion and exclusion criteria
Inclusion and exclusion criteria in terms of population, interventions and comparators, reference standard test and outcome measures have been described in Chapter 2.
Study design
The following types of studies were eligible for inclusion:
-
randomised controlled trials (RCTs) or observational studies, in which participants are assigned to dermoscopy plus VivaScope or VivaScope alone for diagnosis or skin lesion delineation, and where outcomes are compared at follow-up
-
test accuracy studies assessing the test accuracy of dermoscopy plus VivaScope or VivaScope alone with histology of biopsy as the reference standard.
The following study/publication types were excluded:
-
preclinical and animal studies
-
reviews, editorials and opinion pieces
-
case reports.
Search strategy
The searches combined terms for the condition and terms for the technology being assessed. For the technology we used both generic terms (e.g. RCM) and terms for the specific product (e.g. VivaScope). The search strategy was refined by scanning key papers identified during the review, through discussion with the review team, clinical experts and information specialists.
Electronic sources including MEDLINE, EMBASE and The Cochrane Library were searched. In addition, systematic reviews from the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, the Health Technology Assessment Database and Cochrane Central Register of Controlled Trials were searched as sources of other relevant references or studies
Electronic databases were searched from database inception on 14 October 2014 and results uploaded into EndNote (version 7.2; Thomson Reuters, CA, USA) and deduplicated. Full details of the terms used in the searches are presented in Appendix 1. The searches were updated on 11 February 2015.
Two reviewers independently screened all titles and abstracts according to the inclusion criteria. Full-text manuscripts of any titles/abstracts of potential relevance were obtained and assessed independently by two reviewers. Authors of papers for which insufficient details were available to allow data extraction and/or critical appraisal of study quality were contacted. Discrepancies between the two reviewers were resolved by consensus, with involvement of a third reviewer when necessary.
Potentially important ongoing and unpublished UK-based studies were also searched using: clinicaltrials.gov, controlled-trials.com, clinicaltrialsregister.eu. Reference lists of included papers were assessed for additional relevant studies, and clinical experts were also contacted for additional information on published and unpublished studies.
Relevant reviews and guidelines were identified through searching additional resources, including Clinical Evidence, the NICE website, National Institute for Health Research Health Technology Assessment programme, NHS Evidence, National Library of Guidelines, Scottish Intercollegiate Guidelines Network Guidelines and the Guidelines International Network website.
In addition, abstracts from the following key conference proceedings were searched for relevant studies from 2012:
-
annual meeting of the BAD
-
annual meeting of the British Society of Dermapathology
-
congress of European Association of Dermato-Oncology
-
annual meeting of the American Academy of Dermatology
-
annual meeting of the American Society of Dermapathology.
No limits relating to language of publication were applied to the searches.
Inclusion screening and data extraction
Data were extracted using a standardised data extraction form by one reviewer, and checked by a second reviewer after the pilot of six studies, which was done in duplicate. Information extracted included details of the study’s design and methodology, intervention and comparator tests, reference standard, baseline characteristics of participants and outcome measures, including clinical outcome efficacy and any adverse events. Discrepancies between the two data extractors were resolved by discussion, with involvement of a third reviewer if necessary or contact with study authors for clarification.
Quality assessment
Two reviewers assessed the quality of included studies and the two extractions were compared. Any disagreements were resolved by consensus and, if necessary, a third reviewer was consulted. The quality of diagnostic studies was assessed using the quality assessment of diagnostic accuracy studies (QUADAS-2) tool,22 according to recommendations by the Cochrane Handbook for diagnostic test accuracy reviews. 23 If clinical effectiveness studies that met the eligibility criteria were identified, we assessed their quality according to the study design. The quality of RCTs was assessed in accordance with the recommendations of the Centre for Reviews and Dissemination and the Cochrane Handbook for Systematic Reviews of Interventions24,25 and recorded using the Cochrane risk-of-bias tool. 26 When suitable for inclusion, the quality of cohort studies was assessed using the Newcastle–Ottawa Scale. 27
Methods of analysis/synthesis
Details of test accuracy, clinical effectiveness and quality assessment for each included study are presented in structured tables and as a narrative summary.
For test accuracy data, results of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) are presented in this report. Where these are not reported, absolute numbers of true-positive (TP), false-negative (FN), false-positive (FP) and true-negative (TN) test results were used to calculate sensitivity and specificity values.
Where results could be combined, we intended to use absolute numbers of effect or aggregate data (means) with standard deviations (SDs) in standard frequentist meta-analyses to produce forest plots of pooled data. Heterogeneity was to be assessed by doing a sensitivity analysis regardless of the I2-statistic.
We also planned to analyse accuracy data using patient-level data and not lesion-level data because of the difficulty in estimating within-study variance. 28 Estimates of sensitivity and specificity and their confidence intervals (CIs) were to be plotted in forest plots to explore heterogeneity in the first instance. A random-effects meta-analysis was planned to fit the bivariate summary receiver operating characteristics curve model with the within-study variance fitted as binomial. 29
Results of the assessment of clinical effectiveness
Quantity and quality of research available
Included studies
A total of 7446 records were identified from clinical effectiveness searches in electronic databases. After deduplication, 5122 records were screened for eligibility based on title and abstract (Figure 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for studies included and excluded from the clinical effectiveness review.

Full publications of 347 references were ordered and, after screening for eligibility, 11 studies30–40 met the inclusion criteria. The database searches were updated from October 2014 to February 2015, and a further two studies41,42 that met the inclusion criteria were identified. Three additional studies43–45 were obtained by hand-searching and contacting clinical experts in the field. Thus, in total, 1630–41,43–46 studies were identified that met the inclusion criteria for the review. No study was identified from conference proceedings that met the inclusion criteria.
Figure 1 shows the flow diagram for included and excluded studies of clinical effectiveness. A list of excluded references (with reason for exclusion) is presented in Appendix 2, and Appendix 3 shows a list of ongoing trials identified from searching trial registers.
Study characteristics
Study indication
Out of the 16 included studies, 1330–34,36,38–40,43–46 indicated the use of VivaScope or RCM in diagnosing suspected or equivocal lesions and three35,37,41 were indicated in lesion margin delineation.
Population
There were different inclusion criteria for all the included studies. Patients in the 13 studies on lesion diagnosis had suspicious lesions36,39,40,46 or dermoscopically equivocal lesions (melanoma, BCC). 30–34,38,43,45 The three studies indicated for lesion margin diagnosis enrolled patients with LM lesions > 5 cm (that would require complex reconstructive surgery) or recurrent LM,35 or patients with clinically suggestive BCC37 or surgically removed BCCs. 41
Only three studies specified exclusion criteria. 34,36,42 Reasons for exclusion included LM and lesions of the soles and palms,34 lesions not amenable to RCM (i.e. physically inaccessible site), and if patients had a previous diagnostic biopsy done on the lesion36 or clinical and/or dermoscopic clear-cut epithelial tumours. 42
For the 13 studies on lesion diagnosis, the number of participants enrolled ranged from 4231 to 423,42 while the number of participants in studies for lesion margin delineation ranged from 1037 to 74. 41 However, the unit of analysis in the included studies was patient-level data,30,34,36,45 lesion-level data30–33,38,39,42,43 or the number of positive or negative sites. 35,37,41 The reported median age ranged from 4734 to 62 years,40 and mean age ranged from 44.236 to 71 years. 35
Study design
In 10 out of the 13 studies on lesion diagnosis, consecutive patients were enrolled prospectively from settings including melanoma or dermatology clinics in tertiary or university hospitals,30–32,34,36,38–40,42,43 whereas other studies retrospectively selected images of previously imaged sets of lesions33 or excised lesions. 40,45 Of the three studies on lesion margin diagnosis, one retrospectively assessed and interpreted lesion images in patients previously enrolled in two university-based clinics/hospitals35 and two prospectively recruited patients/lesions randomly from a dermatology department37 or Mohs surgery unit. 41
Intervention and comparator
Of the 13 studies on lesion diagnosis, two used VivaScope 1500 with dermoscopy as a comparator,30,42 four used VivaScope 1500 without dermoscopy as a comparator31,39,40,45 and one study used VivaScope 1500 or VivaScope 3000 with dermoscopy as a comparator. 43 Owing to the lack of data, we included additional studies without dermoscopy as a comparator.
For earlier versions of VivaScope, one study used VivaScope 1000 with dermoscopy as a comparator,36 two used VivaScope 1000 without a comparator32,33 and two studies used both VivaScope 1000 and VivaScope 1500, with one study34 using dermoscopy as a comparator and the other having no comparator. 38 Only one study41 used VivaScope 2500.
Two of the studies on lesion margin diagnosis used VivaScope 1500 with35 or without dermoscopy as a comparator37 and one used VivaScope 2500. 41
The VivaScope used in the included studies were from two companies: VivaScope 1500, 2500 and 3000 (Caliber Imaging and Diagnostics, Rochester, NY, USA) and VivaScope 1000 and VivaScope 1500 (Lucid Inc., Rochester, NY, USA, or Lucid Inc., MAVIG GmbH, Munich, Germany). The source of light in the VivaScope was an 830-nm near-infrared laser beam with a power of either ≥ 35 mW or < 35 mW.
Assessors who reviewed and interpreted images obtained from VivaScope were trained in the RCM technology. All the studies except four37,39,42,45 reported qualitative and/or quantitative diagnostic thresholds using morphological features or algorithms validated in previous published studies.
Dermoscopy, used as a comparator test in some studies, utilised either a dermoscope (DermLite Photo; 3Gen LLC, Dana Point, CA, USA) or a dermoscopic camera attached to a VivaScope. 30,34,36,39,42
Histopathological assessment of excised lesions (biopsy) was used as reference standard in all of the included studies before32–34,41 or after the use of VivaScope. 30,31,35–39,42,43,45 Where histopathology was done before the use of VivaScope, assessors of the results of the histopathology were blinded to the results of the VivaScope. Details regarding histopathological analysis were described in only one study. 37
Characteristics of the studies included in the review are given in Table 3.
| Study, year and location | Study design | Participant and lesion characteristics | Prevalence of skin cancer/lesions in the study population | Index test characteristics | Comparator characteristics | Reference standard |
|---|---|---|---|---|---|---|
| Lesion diagnosis | ||||||
| Alarcon et al., 201430 Spain |
Prospective observational | Patients (n = 343) with equivocal pigmented lesions 343 lesions (92 melanomas, 12 BCCs, and 239 benign naevi and others) Age: median 54.7 years (range 8–89 years) |
Melanoma = 26.8% BCC = 3.5% Benign lesions = 69.7% |
VivaScope 1500. Light source: 830-nm near-infrared laser at maximum power of 35 mW | Dermoscope (DermLite Foto: 3GEN LLC, San Juan Capistrano, CA, USA) | Histopathology |
| Castro et al., 201543 Brazil and the USA |
Prospective observational | Patients (n = 73) with skin lesions suspicious for BCC based on clinical and dermoscopic examination 92 lesions |
BCC = 83% | VivaScope 3000 VivaScope 1500 |
NC | Histopathology |
| Curchin et al., 201131 Australia |
Prospective observational | Patients (n = 42) with equivocal lesions 50 lesions (13 melanomas, 22 benign naevi, nine BCC and six SCC) Age: NR |
Melanoma = 26% BCC = 18% SCC = 12% |
VivaScope 1500 with a dermoscopic camera attached | NC | Histopathology |
| Ferrari et al., 201544 Italy |
Retrospective observational | 322 melanocytic lesions (70 melanomas and 252 naevi) excised on the basis of equivocal clinical and/or dermoscopic features | 70 melanomas and 252 naevi | VivaScope 1500 | Dermoscope | Histopathology |
| Gerger et al., 200632 Austria |
Prospective observational | Patients (n = 119) with skin tumours 27 melanomas, 15 BCC, 90 benign naevi and 30 SK Age: NR |
Melanoma = 16.7% BCC = 9.3% |
VivaScope 1000 Light source: 830-nm near-infrared diode laser Power: < 35 mW |
NC | Histopathology |
| aGerger et al., 200833 Austria |
Retrospective observational | Patients (n = 60) with melanocytic skin tumours 20 melanomas and 50 benign naevi Age: NR |
Melanoma = 28.6% | VivaScope 1000 Light source: 830-nm diode laser at a power of < 35 mW |
NC | Histopathology |
| Guitera et al., 200934 Australia and Italy |
Prospective observational | Patients (n = 326) with equivocal lesions selected for excision after clinical examination 326 lesions (123 melanomas and 203 naevi) Age: median 47 years (range 6–90 years) |
Melanoma = 37.7% | VivaScope 1000 or VivaScope 1500. Light source: 830-nm laser | Dermoscope | Histopathology |
| Guitera et al., 201040 Australia and Italy |
Retrospective observational | Patients (n = 219) with clinically equivocal, macules of the face 284 lesions (81 LM and 203 benign macules) Age: mean 62 years (range 51–72 years) |
LM = 28.5% Benign macules = 71.5% |
VivaScope 1500 Light source: 830-nm laser beam with a maximum power of 35 mW |
NC | Histopathology |
| Langley et al., 200736 Canada |
Prospective observational | Patients (n = 125) scheduled for biopsy of suspected lesions 125 lesions (37 melanomas and 88 melanocytic naevi) Age: mean 44.2 years (range 16–84 years) |
Melanoma = 29.6% | VivaScope 1000 | Dermoscope, specifications NR | Histopathology |
| Pellacani et al., 200738 Italy |
Prospective observational | Patients (n = 332) with melanoma and equivocal lesions 136 melanomas and 215 naevi Age: median 47.7 years (IQR 36–60 years) |
Melanoma = 38.7% | VivaScope 1000/1500 Light source: 830-nm near-infrared laser beam at maximum power of 35 mW |
NC | Histopathology |
| Pellacani et al., 201442 Italy |
Prospective observational | Patients (n = 423) with suspicious lesion requiring a mole check and/or with a suspect of melanoma 493 lesions (29 melanomas, 39 BCC and 425 benign lesions) Age: mean 40.7 years (range 28.5–52.5 years) |
Melanoma = 5.9% BCC = 7.9% Benign lesions = 86.2% |
VivaScope 1500 Light source: 830-nm near-infrared laser beam at a power of 20 mW |
Dermoscopy | Histopathology |
| Rao et al., 201339 USA |
Prospective observational | Patients (n = 334) with lesions selected for removal Nine melanomas, 27 BCC, 43 SCC, 255 naevi, 26 AK and 24 other benign lesions Age: NR |
Melanoma = 2.3% BCC = 7% SCC = 11.2% |
VivaScope 1500 | NC | Histopathology |
| Stanganelli et al., 201545 Italy |
Retrospective observational | Patients (n = 70) with equivocal lesions that lacked clear dermoscopy criteria for melanoma 70 lesions (12 melanomas and 58 benign lesions) Age: NR |
Melanoma = 17.14% Benign lesions = 82.9% |
VivaScope 1500 Light source: 830-nm laser at a maximum power of 20 mW |
NC | Histopathology |
| Lesion margin delineation | ||||||
| Bennassar et al., 201441 Spain |
Prospective observational | Patients (n = 74) with surgically removed BCCs from Mohs surgery 80 BCC, with 480 images |
BCC = 100% | VivaScope 2500 | NC | Histopathology |
| Guitera et al., 201335 Australia and Italy |
Retrospective observational | Patients (n = 37) with large facial lesions requiring surgery 32 LM and five LMM Age: mean 71 years (range 47–88 years) |
LM/LMM = 100% | VivaScope 1500 Light source: 830-nm laser beam at a maximum power of 35 mW |
Dermoscope | Histopathology |
| Pan et al., 201237 China |
Prospective observational | Patients (n = 10) with lesions clinically suggestive of BCC 10 lesions Age: NR |
BCC = 70% | VivaScope 1500 Light source: 830-nm laser, with a power of < 15 mW |
NC | Histopathology |
Outcomes
The outcomes of interest to this review that were reported in the included studies are listed in Table 3. The most commonly reported outcome specified in the methods section is diagnostic accuracy, which was reported as sensitivity, specificity, PPV and NPV. Other diagnostic accuracy data such as FP, FN and TN were rarely reported and had to be estimated/calculated using other reported diagnostic data where possible.
Therefore, because of the absence of more clinical data as specified in the protocol, additional clinical outcomes not specified in the methods section but deemed clinically relevant are reported in Table 4. These included misdiagnosis or misclassification of lesions, and change in management of lesions after confirmation or final diagnosis with histopathology.
| Study and year | Diagnostic accuracy | Time to test failure (e.g. imaging failure rate) | Number of biopsies performed and repeat biopsies | Morbidity associated with excision such as pain and swelling | Extent of scarring and associated psychological impact | Lesion recurrence rates | Adverse events from biopsy or false test results | HRQoL | Misdiagnosis/misclassification of lesions | Change in management of lesions |
|---|---|---|---|---|---|---|---|---|---|---|
| Alarcon et al., 201430 | ✓ | NA | ||||||||
| Bennassar et al., 201441 | ✓ | NA | NA | |||||||
| Castro et al., 201543 | ✓ | NA | ||||||||
| Curchin et al., 201131 | ✓ | NA | ||||||||
| Ferrari et al., 201544 | ✓ | NA | ||||||||
| Gerger et al., 200632 | ✓ | NA | ||||||||
| Gerger et al., 200833 | ✓ | NA | ||||||||
| Guitera et al., 200934 | ✓ | NA | ✓ | |||||||
| Guitera et al. 201040 | ✓ | NA | ||||||||
| Guitera et al., 201335 | ✓ | NA | NA | ✓ | ✓ | |||||
| Langley et al., 200736 | ✓ | NA | ✓ | |||||||
| Pan et al., 201237 | ✓ | NA | NA | |||||||
| Pellacani et al., 200738 | ✓ | NA | ||||||||
| Pellacani et al., 201442 | ✓ | NA | ✓ | |||||||
| Rao et al., 201339 | ✓ | NA | ||||||||
| Stanganelli et al., 201545 | ✓ | NA |
Table 4 shows outcomes of interest reported in included studies.
Quality assessment of studies included in clinical effectiveness review
The QUADAS-2, which separates the evaluation of study quality into two main areas – (1) risk of bias and (2) concerns regarding applicability of patient selection, index test, reference standard and flow of timing – was used to assess quality of included studies.
A summary of the results of the quality assessment of the included studies is shown in Appendix 4. The majority of the included studies had a low risk of bias and low applicability concerns in patient selection (e.g. less concern that included patients did not match the review question),30,32–34,36,38,40–45 conduct of the index test (e.g. the index test, its conduct or interpretation did not differ from the review question)30–34,36–38,40,41,43–45 and reference standard. 31–36,38,40–45 However, concerning flow and timing, the risk of bias in the majority of the studies was unclear (i.e. it was unclear if patient flow did not introduce any bias or also if there was an appropriate interval between the index test and reference standard)32,33,35–45 as a result of poor reporting and/or insufficient data.
Figure 2 shows a summary of risk of bias and applicability concerns of included studies.
FIGURE 2.
Summary of risk of bias and applicability concerns of included studies.

Clinical effectiveness results
Diagnostic accuracy
Lesion diagnosis
Dermoscopy plus VivaScope 1500 versus dermoscopy
Three studies30,42,44 compared dermoscopy with VivaScope 1500 following dermoscopy.
Alarcon et al. 30 assessed the impact of RCM analysis on dermoscopically equivocal pigmented lesions. Of the 343 lesions that underwent RCM examination, only 264 were excised (79 lesions were followed up for 1 year without any melanoma diagnosed). Of the 92 melanomas diagnosed using dermoscopy alone, histopathology proved that there were six FNs and in those diagnosed with dermoscopy plus VivaScope 1500 there were two FNs.
Based on the 264 excised lesions, combined use of dermoscopy and VivaScope was more likely than dermoscopy alone to diagnose melanoma (sensitivity 97.8% vs. 94.6%; p = 0.043), and more likely to diagnose those without melanoma (non-melanoma) (specificity 92.4% vs. 26.74%; p < 0.000001). Similar results were obtained when the analysis was based on all 343 patients who underwent RCM, assuming that all the 79 patients/lesions who were followed up were TNs (Table 5).
| Intervention/comparator | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) |
|---|---|---|---|---|
| Based on excised lesions (n = 264) | ||||
| VivaScope 1500 following dermoscopy | 97.8a (91.6 to 99.6) | 92.4a (87.2 to 95.7) | 87.4 (79.0 to 92.8) | 98.8 (95.1 to 99.8) |
| Dermoscopy alone | 94.6 (87.2 to 98.0) | 26.74b (87.2 to 98.0) | 40.8 (34.2 to 47.8) | 90.2b (77.8 to 96.3) |
| Based on all lesions that underwent RCM (n = 343) | ||||
| VivaScope 1500 following dermoscopy | 97.83 (92.4 to 99.7) | 94.8 (91.3 to 97.2) | 87.0 (79.0 to 93.0) | 99.0 (97.0 to 100.0) |
| Dermoscopy alone | 93.5 (86.3 to 97.6) | 49.0 (42.7 to 55.4) | 40 (34.0 to 47.0) | 95.0 (90.0 to 98.0) |
Pellacani et al. 42 prospectively assessed the potential impact of RCM when implemented in a routine melanoma workflow. At dermoscopy, patients were referred to one of the following pathways:
-
no further examination
-
referral to RCM
-
RCM documentation (lesions with consistent suspicious clinical/dermoscopic criteria, already qualified and scheduled for surgical excision)
-
RCM consultation (equivocal, or moderately suspicious, lesions in which RCM diagnosis would determine the lesion-definite outcome, i.e. either excision or digital follow-up).
-
Of a total of 493 lesions referred for RCM examination, two patients refused RCM imaging so lesions were excised and histopathology reported, one patient had BCC and the other patient had benign lesion. Of the remaining 491 lesions, 183 were referred for RCM documentation and 308 for RCM consultation. In the RCM documentation group, histopathology confirmed 110 RCM positives (23 melanomas, 19 BCCs and 68 benign lesions) and 73 RCM negatives (73 benign lesions). In all melanomas and BCCs identified at histology, RCM had recommended excision.
In the RCM consultation group, RCM identified 81 positives and 227 negatives. Of the 81 RCM positives, excision confirmed six melanomas, 19 BCCs and 56 benign lesions. Of the 227 RCM negatives followed up for 3–12 months, 28 showed significant changes but excision confirmed no malignancy, 178 showed no changes and 21 were lost to follow-up but checks at the local tumour registry identified no excision.
Table 6 shows the sensitivity and specificity (based on a 2 × 2 contingency table) based on two alternative assumptions: (1) all 21 RCM negatives lost to follow-up were TNs; or (2) all 21 RCM negatives lost to follow-up were excluded from the sensitivity and specificity analysis.
| Type of RCM examination | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) |
|---|---|---|---|---|
| RCM documentation | 100.0 (91.5 to 100.0) | 51.8 (43.2 to 60.3) | 38.0 (29.0 to 48.0) | 100.0 (95.0 to 100.0) |
| RCM consultation (based on 227 TNs) | 100.0 (86.2 to 100.0) | 80.2 (75.1 to 84.7) | 31.0 (21.0 to 42.0) | 100.0 (98.0 to 100.0) |
| RCM consultation (based on 206 TNs, i.e. excluding the 21 lesions lost to follow-up) | 100.0 (86.2 to 100.0) | 78.6 (73.2 to 83.4) | 31.0 (21.0 to 42.0) | 100.0 (98.0 to 100.0) |
Ferrari et al. 44 evaluated the most relevant RCM features for the detection of difficult melanomas by dermoscopy: a score of 0–2 represents featureless lesions, a score of 3–4 indicates borderline positive lesions and a score of 5–10 indicates clear-cut positive lesions. For RCM, previously published confocal parameters for melanoma detection were used. In the population with a dermoscopic score of 0–2, the presence of at least one of the two independent parameters accounted for the detection of all six melanomas (100% sensitivity and 82.3% specificity). Similarly, in the population with a dermoscopic score of 3–4, the presence of at least one of the two independent parameters accounted for the detection of 16 out of 17 melanomas (94.1% sensitivity and 62.4% specificity).
Dermoscopy plus VivaScope 1500
Four studies31,39,40,45 reported the diagnostic accuracy of VivaScope 1500 following dermoscopy without a comparator.
Curchin et al. 31 reported sensitivity and specificity data on 50 equivocal lesions in 42 patients. Following dermoscopy, VivaScope 1500 correctly diagnosed 12 out of 13 melanomas (92.3% sensitivity, 75% specificity), 19 out of 22 benign naevi (86% sensitivity, 95% specificity), six out of nine BCCs (66.7% sensitivity, 100% specificity) and six out of six SCCs and its precursors (100% sensitivity, 75% specificity) (Table 7).
| Lesion type | Histopathology-proven cases (n/N) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Melanoma | 12/13 | 92.3 | 75 |
| Benign naevi | 19/22 | 86 | 95 |
| BCC | 6/9 | 66.7 | 100 |
| SCC and its precursors | 6/6 | 100 | 75 |
Guitera et al. 40 assessed which RCM features could distinguish LM from benign macules of the face, such as solar lentigo, actinic keratosis and seborrhoeic keratosis, and to test different algorithms for diagnosing LM.
In addition to describing RCM diagnostic features for LM, an algorithm was developed (LM score) to distinguish LM from benign macules [two major features, each scoring +2 points (non-edged papillae and round, large pagetoid cells > 20 µm), and four minor features, three scoring +1 point each (three or more atypical cells at the dermo-epidermal junction, follicular localisation of atypical cells and nucleated cells within the dermal papillae) and one (negative) feature scoring –1 point (a broadened honeycomb pattern)]. A LM score of ≥ 2 resulted in a sensitivity of 85% and specificity of 76% for the diagnosis of LM (odds ratio for LM 18.6, 95% CI 9.3 to 37.1).
Rao et al. 39 assessed the diagnostic accuracy of VivaScope 1500 compared with histopathology in the diagnosis of cutaneous lesions by two readers with varying degrees of experience: a bedside-trained physician and a distant expert. Reader 1 diagnosed as malignant 66.7% of the histologically diagnosed melanomas, 74.1% of BCCs and 37.2% of SCCs [i.e. 317/334 cases (94.9%) were evaluated with 93.1% sensitivity]. Reader 2, diagnosed as malignant 88.9% of melanomas, 51.9% of BCCs and 72.1% of SCCs [i.e. 323/334 cases (96.7%) were evaluated with 97.4% sensitivity] (Table 8).
| Reader/reviewer | Agreement between VivaScope 1500 and histopathology (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Reader 1 (bedside-trained physician): evaluated 317 of 334 cases (94.9%) | Melanoma = 66.7; BCC = 74.1; SCC = 37.2 | 93.1 | 64.1 |
| Reader 2 (distant expert): evaluated 323 of 334 cases (96.7%) | Melanoma = 88.9; BCC = 51.9; SCC = 72.1 | 97.4 | 80.5 |
| Overall (readers 1 and 2) | NR | 98.6 | 44 |
Stanganelli et al. 45 assessed if dermoscopy followed by imaging with VivaScope 1500 could improve melanoma detection and reduce the number of unnecessary excisions. Thirty out of 70 lesions were classified as melanoma by dermoscopy plus VivaScope 1500; of these, 11 were histologically confirmed as melanoma (TPs) and 19 were FPs. The remaining 40 out of 70 lesions (57%) were classified as benign based on RCM; of these, one was subsequently shown to be melanoma (i.e. a EN). A 2 × 2 contingency table estimated a sensitivity of 91.67% (95% CI 61.52% to 99.79%) and a specificity of 67.24% (95% CI 53.66% to 78.99%) for diagnosing melanoma (Table 9).
| VivaScope 1500 | Reference standard | |
|---|---|---|
| Disease | No disease | |
| Disease | TP = 11 | FP = 19 |
| No disease | FN = 0 | TN = 40 |
Dermoscopy plus VivaScope 1000 versus dermoscopy
Langley et al. 36 evaluated the diagnostic accuracy of VivaScope 1000 compared with dermoscopy in patients with benign and malignant melanocytic lesions. The sensitivity of VivaScope 1000 following dermoscopy compared with dermoscopy alone was 97.3% vs. 89.2% and specificity was 83.0% vs. 84.1%, respectively.
Using a 2 × 2 contingency table to estimate histologically proven positive and negative diagnostic tests, the numbers of patients/lesions correctly (TP + TN) and incorrectly (FP + FN) diagnosed were similar using VivaScope 1000 following dermoscopy and using dermoscopy alone (Table 10).
| Intervention/comparator | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | TP (n) | TN (n) | FP (n) | FN (n) |
|---|---|---|---|---|---|---|---|---|
| VivaScope 1000 | 97.3 | 83.0 | 70.6 | 98.6 | 37 | 72 | 15 | 1 |
| Dermoscope | 89.2 | 84.1 | 70.2 | 94.9 | 33 | 74 | 14 | 4 |
VivaScope 1000
Two publications32,33 from the same trial reported the diagnostic accuracy of VivaScope 1000 without a comparator.
In the trial by Gerger et al. ,32 117 melanocytic skin lesions and 45 non-melanocytic skin lesions were consecutively sampled and examined by four independent observers using VivaScope 1000. The overall (total of the four observers) diagnostic differentiation of benign from malignant lesions (melanoma and BCC) reached a sensitivity of 94.65%, specificity of 96.67%, PPV of 97.50% and NPV of 92.99% based on histopathology (Table 11).
| Diagnostic differentiation of benign from malignant lesions based on biopsy documented lesions | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Observer 1 | 90.48 | 96.6 | NR | NR |
| Observer 2 | 95.24 | 100 | NR | NR |
| Observer 3 | 95.24 | 96.6 | NR | NR |
| Observer 4 | 97.62 | 100 | NR | NR |
| Overall (observers 1–4) | 94.65 | 96.67 | 97.50 | 92.99 |
In a supplementary publication of Gerger et al. ,32 Gerger et al. 33 retrospectively evaluated 3709 selected images of 70 lesions (20 malignant melanomas and 50 benign naevi) obtained by VivaScope 1000. Overall, performance of the four observers who reviewed the images showed a sensitivity of 97.5%, specificity of 99.0%, a PPV of 97.5% and a NPV of 99.0% (Table 12).
| Reader/observer | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Observers 1–3 | 100 | 100 | NR | NR |
| Observer 4 | 90 | 96 | NR | NR |
| Overall (observers 1–4) | 97.5 | 99 | 97.5 | 99 |
VivaScope 1000 or 1500 versus dermoscopy
In a trial by Guitera et al. ,34 the possible additive value of VivaScope 1000 and 1500 in the management of melanocytic lesions was evaluated at two centres. In terms of the diagnosis of melanoma, there was no significant difference in sensitivity between VivaScope 1000/1500 (91%, 95% CI 84.6% to 95.5%) and dermoscopy (88%, 95% CI 80.7% to 92.6%), but specificity differed significantly (VivaScope 1000/1500: 68%, 95% CI 61.1% to 74.3%; dermoscopy: 32%, 95% CI 25.9% to 38.7%).
When VivaScope 1000/1500 is used in addition to dermoscopy, the number of patients correctly diagnosed (histologically proven) with melanoma [TP, n = 100 (81.3%)] or without melanoma [TN, n = 3 (2.4%)] was higher than the number incorrectly diagnosed without melanoma [FP + FN, n = 20 (16.3%)] (Table 13).
| Lesion | Diagnostic test | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Double positive (TP) (correctly diagnosed with melanoma), n (%) | Double negative (TN) (correctly diagnosed without melanoma), n (%) | Single positive (FP + FN) (incorrectly diagnosed without melanoma), n (%) |
|---|---|---|---|---|---|---|
| Melanoma (n = 123) | VivaScope 1000/1500 | 91.0 (84.6 to 95.5) | 68.0 (61.1 to 74.3) | 100 (81.3) | 3 (2.4) | 20 (16.3) |
| Dermoscopy | 88.0 (80.7 to 92.6) | 32.0 (25.9 to 38.7) | ||||
| Benign naevi (n = 203) | VivaScope 1000/1500 | 68.0 | 15.0 | 46 (22.7) | 46 (22.7) | 111 (54.7) |
| Dermoscopy | 32.0 | 11.0 |
VivaScope 1000 or 1500
Pellacani et al. 38 evaluated the sensitivity and specificity of confocal features for the diagnosis of melanoma and benign naevi using RCM score thresholds compared with models obtained from statistical analysis. The VivaScope 1000/1500 demonstrated optimal sensitivity for a score of ≥ 2 (96.3%), with 52.1% specificity.
Dermoscopy plus VivaScope 1500 versus dermoscopy plus VivaScope 3000
Castro et al. 43 compared the accuracy of VivaScope 3000 with VivaScope 1500 in the identification of BCC. Among 54 lesions imaged with both RCM devices, 45 were biopsy-proven BCCs. Comparison between VivaScope 1500 following dermoscopy and VivaScope 3000 following dermoscopy was as follows: sensitivity, 100% vs. 93%; specificity, 78% for both RCMs; PPV, 96% vs. 95%; and NPV, 100% vs. 70% (Table 14).
| Test classification | VivaScope 1500 following dermoscopy | VivaScope 3000 following dermoscopy |
|---|---|---|
| Sensitivity (%) | 100 | 93 |
| Specificity (%) | 78 | 78 |
| PPV (%) | 96 | 95 |
| NPV (%) | 100 | 70 |
Lesion margin delineation
Dermoscopy plus VivaScope 1500 versus dermoscopy
Guitera et al. 35 analysed LM and LMM cases to determine if VivaScope 1500 mapping might alter patient care and management. Out of 60 histopathology-positive LM lesions, 55 had previously been identified as LM by VivaScope 1500 (i.e. FN = 5) and 21 had been so identified by dermoscopy (i.e. FN = 39). Out of 125 suspected LM lesions found to be negative by histopathology, 121 had been previously identified as negative by VivaScope 1500 (FP = 4) and 122 were considered negative by dermoscopy (FP = 3). Histopathology also showed that 17 out of 29 patients with visible lesions had evidence of subclinical disease > 5 mm beyond the edge of the dermoscopically identified margin. In addition, both the length and width of the dermoscopically visible area of the lesion were, on average, 60% smaller than the final corresponding dimensions determined by VivaScope 1500. Thus, the visible area was, on average, < 40% of the area that was treated based on VivaScope 1500 mapping findings (Table 15).
| Finding | Methods of diagnosis | ||
|---|---|---|---|
| Histopathology | Dermoscopy | VivaScope 1500 | |
| Number of sites positive for LM | 60 | 21 (39 FN) | 55 (5 FN) |
| Number of sites negative for LM | 125 | 122 (3 FP) | 121 (4 FP) |
VivaScope 1500
Pan et al. 37 investigated the feasibility of VivaScope 1500 in defining the margins of lesions clinically suggestive of BCC before surgery. The margins of 10 lesions were evaluated using VivaScope 1500, and biopsies of the margins were used to confirm the results. In 7 out of 10 (70%) cases, the margins of the cancer were identified using VivaScope 1500 and confirmed by histopathological analysis. In 3 out of 10 (30%) cases, the margin of the lesions could not be detected because of the unevenness of the surface (Table 16).
| Imaging system | Cases/margins confirmed by histology, n (%) |
|---|---|
| VivaScope 1500 | 7 (70) |
VivaScope 2500
Bennassar et al. 41 evaluated the sensitivity and specificity of ex vivo imaging with fluorescence confocal microscopy for the detection of residual BCC in Mohs tissue excisions, and to calculate the time invested up to the diagnosis for both fluorescence confocal microscopy and frozen sections. The overall sensitivity and specificity of detecting residual BCC in surgical margins were 88% and 99%, respectively. The number of images/mosaic correctly diagnosed as TP was 79 (89%) and TN was 390 (99.7%). There was only one (0.3%) FP. In addition, VivaScope 2500 reduced the evaluation time by 18 minutes (p < 0.001) when compared with the processing of a frozen section.
Lesion recurrence
Lesion diagnosis
None of the included studies on lesion diagnosis reported lesion recurrence data.
Lesion margin delineation
In the trial conducted by Guitera et al. ,35 none of the patients treated surgically after histopathology confirmed LM (n = 17) or LMM (n = 37) developed recurrence during a median follow-up of 37 months. Recurrence was suspected in one imiquimod-treated patient after 1 year’s follow-up, and in three patients treated with radiotherapy (one after 12, one after 24 and one after 36 months’ follow-up) (Table 17).
| Method of treatment of confirmed LM/LMM | Follow-up period | Number of patients with recurrence |
|---|---|---|
| Surgical (n = 17) | 12 months | 0 |
| Non-surgical (n = 20) | ||
| Imiquimod | 12 months | 1 |
| Radiotherapy | 12 months | 1 |
| 24 months | 1 | |
| 36 months | 1 |
Misdiagnosis/misclassification of lesions
Lesion diagnosis
VivaScope 1000/1500 versus dermoscopy
In the trial by Guitera et al. ,34 15 melanomas (12%) were misclassified by dermoscopy, 11 melanomas (9%) were misclassified by the VivaScope 1000/1500 and only 2.4% were misclassified by both techniques.
Dermoscopy plus VivaScope 1000 versus dermoscopy
In the trial by Langley et al. ,36 there were 5 out of 37 melanomas for which VivaScope 1000 following dermoscopy and dermoscopy alone produced differing diagnoses. VivaScope 1000 following dermoscopy correctly classified four out of five melanomas, whereas dermoscopy alone correctly classified one of out five melanomas. Additionally, both methods correctly identified seven benign naevi. Two of the melanomas were misdiagnosed by the investigator using dermoscopy alone, but correctly diagnosed by dermoscopy plus VivaScope 1000 as amelanotic or hypomelanotic melanomas.
Dermoscopy plus VivaScope 1500
In the trial conducted by Pellacani et al. ,42 overall VivaScope 1500-proposed diagnosis was concordant with histopathological diagnosis in 216 out of 283 (76.3%) evaluated cases. BCC was the most accurate diagnosis [37/38 (97.4%)], followed by melanoma [24/28 (85.7%)]. Spitz naevus was the most frequently misclassified diagnosis [accurate diagnosis: 4/13 (30.8%)]: six were misclassified as Clark’s naevi and three as melanoma (Table 18).
| Study | Comparison group | Lesions misdiagnosed/misclassified, n (%) |
|---|---|---|
| Guitera et al.34 | Dermoscopy | Melanoma: 15 (12) |
| VivaScope 1000/1500 | Melanoma: 11 (9) | |
| Dermoscopy plus VivaScope 1000/15000 | Melanoma: 3 (2.4) | |
| Langley et al.36 | Dermoscopy | Melanoma: 4 |
| VivaScope 1000 | Melanoma: 1 | |
| Dermoscopy plus VivaScope 1000 | NR | |
| Pellacani et al.42 | Overall VivaScope 1500 | Overall lesions: 67 (naevi, 42; BCC, 1; melanoma, 4; Spitz naevi, 9) |
Lesion margin delineation
The only included study on lesion margin delineation2 did not report on misdiagnosis or misclassification of lesions.
Change in management of lesions
Lesion diagnosis
No included study on lesion diagnosis reported change in management of lesions after diagnosis.
Lesion margin delineation
In the trial conducted by Guitera et al. ,35 VivaScope 1500 mapping changed the management of lesions in 27 patients (73%): 11 patients had a major change in their surgical procedure and 16 were offered radiotherapy or imiquimod treatment. Treatment was surgical in 17 out of 37 patients.
Adverse events
None of the included studies on lesion diagnosis or lesion margin delineation reported data on adverse events and side effects of excision, including pain, swelling, infections, distress and scarring.
Summary of clinical effectiveness results
The systematic review of clinical effectiveness identified 16 studies, 13 of which are on lesion diagnosis and three on lesion margin delineation. For the index test, included studies used VivaScope 1500 or 1000, or 2500 or 3000, with or without dermoscopy as adjunctive technology or as comparator.
Two studies (Alarcon et al. 30 from Spain and Pellacani et al. 42 from Italy) investigated lesion diagnosis and were deemed to be the most representative of clinical practice in the UK setting (in terms of study population and treatment pathway) from the studies identified.
Alarcon et al. 30 assessed the impact of RCM analysis on dermoscopically equivocal pigmented lesions. Based on the 264 excised lesions, dermoscopy plus VivaScope 1500 was significantly more sensitive than dermoscopy alone in the diagnosis of melanoma (97.8% vs. 94.6%; p = 0.043) and significantly more specific than dermoscopy alone in the diagnosis of non-melanoma (92.4% vs. 26.74%; p < 0.000001).
Pellacani et al. 42 prospectively assessed the potential impact of RCM when implemented in a routine melanoma workflow. Following dermoscopy, patients who were referred to RCM underwent either:
-
RCM documentation (lesions with consistent suspicious clinical/dermoscopic criteria, already qualified and scheduled for surgical excision) or
-
RCM consultation (equivocal, or moderately suspicious, lesions in which RCM diagnosis would determine the lesion-definite outcome, i.e. either excision or digital follow-up).
Of a total of 491 lesions, 183 underwent RCM documentation and 308 underwent RCM consultation. Using a 2 × 2 contingency table, sensitivity and specificity were calculated. Based on the assumption that all of the 21 RCM-negative lesions lost to follow-up in the RCM consultation group were TNs, the sensitivity (RCM documentation 100% vs. RCM consultation 100%) and specificity (RCM documentation 51.77% vs. RCM consultation 78.6%) were calculated. However, when the 21 RCM-negative lesions lost to follow-up were excluded, the sensitivity of RCM consultation was 100% and the specificity was 80.2%.
One study35 investigated lesion margin delineation and was also deemed to be the most representative of clinical practice in the UK setting. Guitera et al. 35 analysed LM and LMM cases to determine if VivaScope 1500 mapping might alter patient care and management. Histopathology showed 17 out of 29 patients with visible lesions had evidence of subclinical disease > 5 mm beyond the edge of the dermoscopically identified margin. In addition, both the length and width of the dermoscopically visible area of the lesion were, on average, 60% smaller than the final corresponding dimensions determined by VivaScope 1500. Thus, the visible area was, on average, < 40% of the area that was treated based on VivaScope 1500 mapping findings.
Generalisability of results
Although none of the included studies in the review of clinical effectiveness was conducted in the UK, two studies30,42 on diagnosis and one study on margin delineation35 were deemed to be the most representative of clinical practice in the UK setting. Our clinical experts validated this and these trials were taken forward for the health economic analysis. It is worth noting that, although PPVs and NPVs were reported, no analyses were performed on them as only studies that were relevant to the UK population were taken forward to the health economic analysis.
Chapter 4 Assessment of cost-effectiveness
Systematic literature review of existing economic evidence
Methods
A systematic review of the literature was undertaken in October 2014 in order to identify published economic evaluations that assessed the cost-effectiveness of VivaScope 1500 and 3000 compared with standard treatment (dermoscopy alone or surgical excision) in the diagnosis of skin lesions suspected of being malignant (i.e. suspected melanoma including LM, suspected BCC and suspected SCC) following an equivocal finding on dermoscopy and in the margin delineation of malignant skin lesions, including LM, prior to surgical treatment.
In addition, two further systematic reviews were conducted, in October and December 2014, aiming to identify:
-
studies reporting resource-use and cost data associated with the care pathways of skin cancer, including the initial assessment and diagnosis of skin lesions suspicious of malignancy, which could be utilised in primary economic modelling
-
studies providing utility (preference-based) data on the HRQoL of people with suspected or confirmed skin cancer, which could be used for the estimation of quality-adjusted life-years (QALYs) in the economic models developed as part of this report.
The following databases were searched:
-
MEDLINE (via Ovid)
-
EMBASE (via Ovid)
-
Health Technology Assessment database
-
NHS Economic Evaluations Database.
Further to the database searches, experts in the field were contacted with a request for details of relevant published and unpublished studies of which they may have knowledge; reference lists of key identified studies were also reviewed for any potentially relevant studies. Finally, the NICE website was searched for any recently published guidance relating to skin cancer that had not been already identified via the database searches.
The search strategy for existing economic evaluations combined terms capturing the interventions (RCM, i.e. VivaScope) and comparators of interest (dermoscopy, surgical excision and biopsy), the target condition (types of skin cancer) and, for searches undertaken in MEDLINE and EMBASE, terms to capture economic evaluations. The search strategies for resource-use and cost data, as well as for utility data, were not restricted by intervention and used terms capturing the target condition; in searches undertaken in MEDLINE and EMBASE, these terms were combined with cost of illness terms (resource-use and cost data searches) and HRQoL terms (searches for utility data).
No restrictions on language or setting were applied to any of the searches. The search for resource-use and cost data was limited to the UK/NHS setting, as the aim of this search was to identify data directly relevant to the NHS context that could inform the economic model; however, no country restrictions were applied to searches for existing economic evaluations or studies reporting utility data relating to skin cancer. Searches for HRQoL data were restricted by date, starting from 1997, because of the high number of search hits if this restriction was not imposed; the year 1997 was selected as this was the year the utility index for the European Quality of Life-5 Dimensions (EQ-5D) was published. Limits were applied to remove animal studies and case studies. Conference abstracts were considered for inclusion from 1 January 2013, as it was expected that high-quality studies reported in abstract form before 2013 were published in a peer-reviewed journal. Full details of the search strategies are presented in Appendix 5.
Two health economists, using predefined eligibility criteria, independently assessed the titles and abstracts of papers identified through the searches for inclusion. Owing to the high number of studies retrieved by the HRQoL search, one health economist reviewed all identified citations and a second health economist reviewed a random sample of 1000 citations, to confirm that the same studies were included for second pass.
The inclusion and exclusion criteria for each of the three systematic reviews described above are outlined in Box 2.
-
Intervention or comparators according to the scope of the assessment, that is, VivaScope 1500 or VivaScope 3000 compared with standard treatment, either dermoscopy alone or surgical excision.
-
Study population according to the scope of the assessment, that is, people with suspected melanoma, including LM, suspected BCC or suspected SCC, who have equivocal lesions following dermoscopy; and people with LM prior to surgical treatment.
-
Full economic evaluations (cost–utility, cost-effectiveness, cost–benefit or cost-consequence analyses) that assess both costs and outcomes associated with the interventions of interest.
-
Economic evaluations that utilise clinical effectiveness data from randomised or non-randomised clinical trials, prospective cohort studies or systematic reviews and meta-analyses of clinical studies; economic analyses that utilise clinical data from studies with a mirror-image or other retrospective design will not be considered.
-
Study population according to the scope of the assessment.
-
UK resource-use or costing studies.
-
Any setting (to be as inclusive as possible).
-
Studies reporting utility data elicited using a generic or a condition-specific preference-based measure, vignettes or self-report and a validated, choice-based technique for valuation (i.e. time trade-off or standard gamble).
-
Utility data referring to specific health states associated with skin cancer through the care pathway.
-
Abstracts with insufficient methodological details.
-
Conference papers pre January 2013.
Results
Economic evaluations
The systematic literature search identified a total of 125 papers. Of those, 91 were excluded on the basis of title and abstract and 29 were duplicates. Therefore, a total of five papers were identified as potentially relevant and were ordered for full review based on the criteria listed in Box 2. Of the five papers ordered, none was considered to meet the predefined inclusion criteria listed in Box 2. Reasons for exclusion of the ordered papers are provided in Appendix 5.
During the development of this report, the company made available to the EAG an unpublished study of the cost-effectiveness of RCM in the diagnosis of skin lesions suspicious for skin cancer (note that this report has now been published). 46 The study had a retrospective design and, therefore, did not meet the inclusion criteria for economic evaluations described in Box 2. Nevertheless, because of the paucity of any relevant economic evidence on the cost-effectiveness of VivaScope, it was decided to relax the inclusion criteria and thus include this study in the systematic literature review. None of the five potentially relevant papers that had been excluded according to the predefined inclusion criteria met the relaxed inclusion criteria. Figure 3 provides the flow chart of the process of the systematic search for economic evaluations.
FIGURE 3.
Flow chart of the process of the systematic search for economic evaluations. HTA, Health Technology Assessment; NHS EED, NHS Economic Evaluation Database.
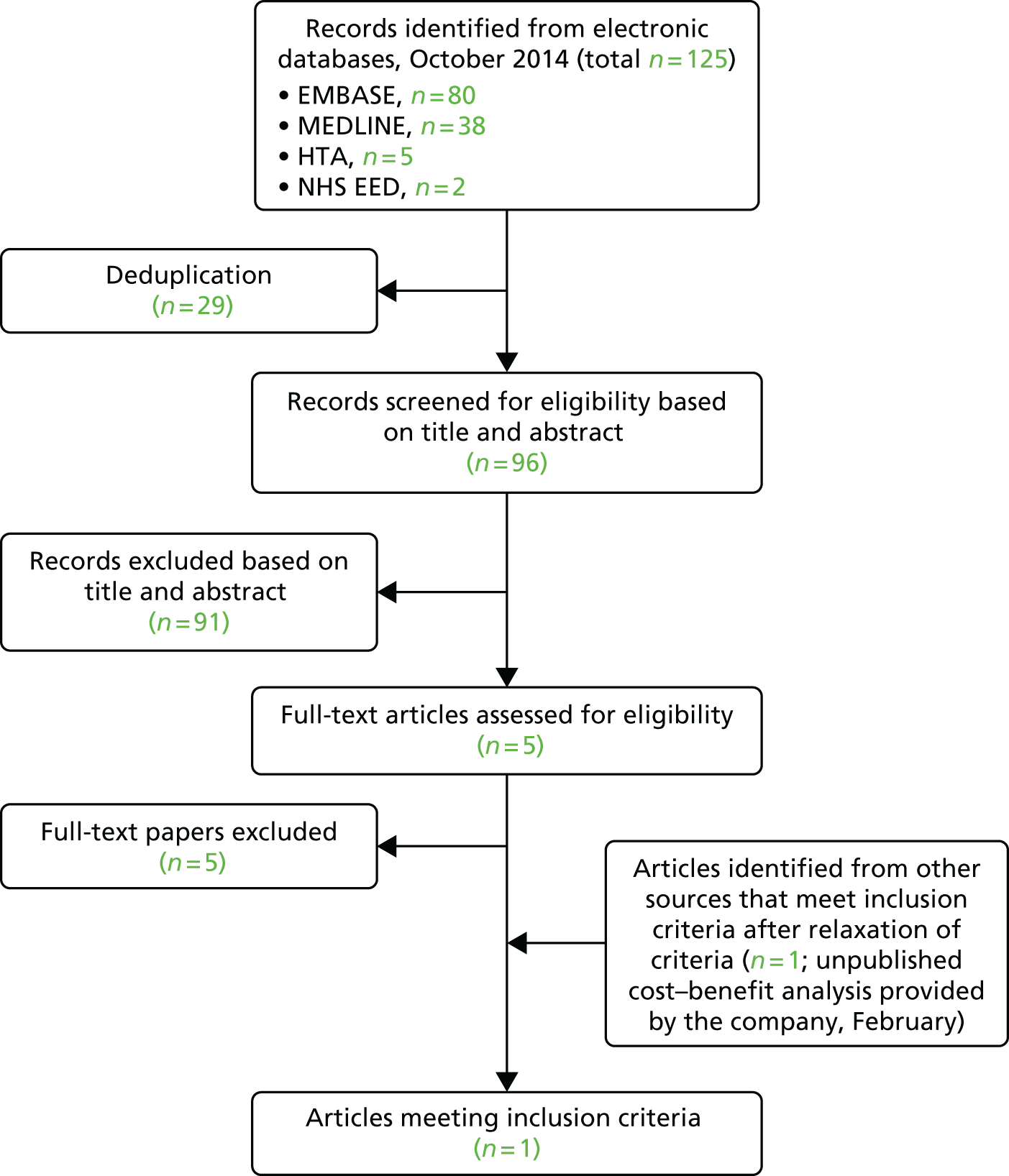
The study by Pellacani et al. 46 assessed the cost-effectiveness of RCM for the diagnosis of melanomas in Italy, under a hospital perspective, by estimating the impact of RCM use on the number of benign lesions needed to excise a malignant melanoma, in terms of clinical outcomes and costs per patient. The study, which had a retrospective design, utilised data from the whole skin cancer activity performed in the Province of Modena in a semester, between 1 January and 30 June 2013, and compared costs and outcomes (in terms of the number of skin neoplasms that were excised) between a territorial service that used exclusively dermoscopy for the diagnosis of malignant skin lesions and a university hospital that used a combination of dermoscopy and RCM for the evaluation of suspicious skin lesions.
Clinical data on number of cases, diagnoses and excision procedures were retrieved from two sources: the digital archive of the province-centralised pathology department, which contained data on all the skin tumours that were excised during the study time frame at the university hospital and the territorial dermatology service; and the database of the melanoma and pigmented lesion clinic of the university hospital. The data of the two databases were matched for consistency.
The cost analysis was performed following a microcosting approach. All procedure costs were estimated by taking into account required staff time and other fixed and variable costs (including device, disposables, etc.). The cost of RCM examination included depreciation and other fixed and variable costs, assuming an operational lifetime of 4 years and the use of the RCM on at least 1800 patients per year. Overhead costs were evaluated for all procedures at 15% of the unit total direct costs. Unit costs (staff, consumables) were taken from the University Hospital of Modena. Training costs for clinicians assessing RCM images were not included. For lesions not excised after RCM examination, the cost of a dermoscopy follow-up examination was added. A surgical excision because of dermoscopy changes was estimated for 14% of lesions referred to digital follow-up. Other direct and indirect costs not directly relevant to the diagnostic process, such as time off work for accessing the hospital services, transportation, morbidity, etc., were not considered in the cost analysis.
The study estimated a large reduction in the number of benign lesions excised at the university hospital (which used RCM in addition to dermoscopy) compared with the territorial dermatology service (which used exclusively dermoscopy), with almost the same number of melanomas excised in both services. The analysis of data showed a number needed to excise (NNE) of 6.25 for the university hospital, compared with 19.41 for the territorial dermatology service.
In terms of costs, the study estimated a mean total cost per patient following the standard diagnostic procedure without RCM of €144, including examination with dermoscopy (70%) or digital dermoscopy (30%), excision, medication, pathology report and conclusive visit. Applied to all cases undergoing excision in the scenario without RCM (corresponding to a NNE of 19.41), the cost corresponded to €2932 per melanoma removed. Introduction of RCM into the standard procedure resulted in a mean total cost per patient of €105, or a cost of €2133 per melanoma removed, using a NNE of 6.25 after RCM and an estimate of 14% of lesions referred to follow-up excised later because of changes on digital dermoscopy monitoring.
Overall, the use of RCM in addition to dermoscopy for the diagnosis of skin lesions suspicious of skin cancer led to a large reduction in the number of benign lesions excised and a reduction in the mean total cost per patient from the point of lesion assessment to the point of excision or discharge.
One limitation of the study was its retrospective design. Another limitation was the omission of training costs associated with the use of RCM. Clinicians assessing skin lesions with RCM should achieve adequate expertise in image reading. According to the authors, a minimum of 6 months’ full-time training, including the evaluation of more than 4000 cases, is required in order to obtain adequate levels of diagnostic accuracy and confidence. However, the authors noted that the development of a teledermatology confocal-dedicated platform might reduce the time needed to effectively implement and exploit the technique into the clinical practice, through distant teaching and diagnostic support to new centres and users.
The study suggests that the use of RCM in addition to dermoscopy for the diagnosis of skin cancer may represent a cost-effective strategy as it leads to better outcomes and cost-savings to the health-care system. It should be noted, however, that, as the study was conducted in Italy, its findings may not be generalisable to the UK setting, as there may be differences between the two health-care settings in terms of prevalence of the various skin cancer types, the population phenotype distribution, the clinical pathways for the diagnosis, assessment and management of skin cancer, the level of experience of clinicians in the use of RCM and relevant unit costs.
The methodological quality of the study by Pellacani et al. 46 assessed against the NICE reference checklist for economic evaluations, is presented in Table 19. The evidence table with the summary of methods and results of the study is provided in Table 20.
| Attribute | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Decision problem | The scope developed by NICE | Broadly yes; RCM assessed as a diagnostic test in addition to dermoscopy in patients with skin lesions suspicious of skin cancer |
| Comparator(s) | Alternative therapies routinely used in the NHS | Yes; comparator is dermoscopy alone, which reflects current practice in the UK with equivocal lesions after dermoscopy |
| Perspective costs | NHS and Personal Social Services | No (Italy, costs estimated in euros). Training costs not considered |
| Perspective benefits | All health effects on individuals | Mostly yes; benefits measured as NNE a melanoma (reflecting number of unnecessary excisions). However, future events associated with progression of non-identified malignant lesions were not considered |
| Form of economic evaluation | Cost–utility analysis | No; cost-effectiveness analysis |
| Time horizon | Sufficient to capture differences in costs and outcomes | Partly; time horizon not defined, but allowed for consideration of future monitoring of non-excised lesions. However, future progression of non-identified malignant lesions was not considered under the time horizon used |
| Synthesis of evidence on outcomes | Systematic review | No; two predetermined databases were retrieved to obtain data for all excised tumours in the study time frame. The percentage of lesions referred for follow-up that undergo surgical excision as a result of dermoscopy changes was taken from the literature, but it is not reported how this source was identified and selected |
| Outcome measure | QALYs | No; number of benign lesions NNE a melanoma |
| Health states for QALY | Described using a standardised and validated instrument | NA |
| Benefit valuation | TTO or standard gamble | NA |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the public | NA |
| Discount rate | An annual rate of 3.5% on both costs and health effects | Not necessary; time horizon of analysis likely to be < 1 year |
| Equity | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | NA |
| Sensitivity analysis | PSA | No |
| Study, country, study type | Intervention and comparator | Study population, study design, data sources | Costs (perspective, description and values) and outcomes (description and values) | Results: cost-effectiveness | Comments |
|---|---|---|---|---|---|
| Pellacani et al.,46 Italy Cost-effectiveness analysis |
RCM added to dermoscopy vs. dermoscopy alone as a diagnostic tool | People with skin lesions suspected of being malignant, examined by a Territorial Dermatology Service or a university hospital dermatology service in Modena, Italy Retrospective design and further modelling Source of clinical data: the digital archive of the province-centralised pathology department and the database of the melanoma and pigmented lesion clinic of the university hospital in Modena, Italy, for the period between 1 January and 30 June 2013 Source of resource-use data: the above sources, published literature and expert opinion Unit costs: local data |
Hospital perspective Costs included: intervention costs (RCM or dermoscopy) including device, consumables, staff time, overheads, depreciation, excision, medication, pathology report, conclusive visit; a dermoscopy follow-up examination was assumed for lesions not excised after RCM examination, and 14% of these lesions were assumed to be subsequently excised because of dermoscopy changes; training costs and patient direct and indirect costs not considered Cost of RCM estimated assuming an operational lifetime of 4 years and the use of the RCM on at least 1800 patients per year Mean total cost per patient:
|
RCM added on dermoscopy dominated dermoscopy alone (both more effective and less costly) | Currency: euro Cost year: likely 2013 Time horizon: not reported, likely < 1 year Discounting: not applied |
Resource-use and costing studies
A total of 277 papers were identified from the systematic search of the literature. Of those, 205 were excluded on the basis of title and abstract and 63 were duplicates. Therefore, a total of nine papers were identified as potentially relevant and were ordered for full review based on the criteria listed in Box 2. On the basis of the full text, six studies were excluded. Reasons for exclusion of the ordered papers are provided in Appendix 5. The remaining three studies identified from the search included relevant UK cost data on skin cancer.
Figure 4 provides the flow chart of the process of the systematic search for resource-use and costing studies. 48–50
FIGURE 4.
Flow chart of the process of the systematic search for resource-use and costing studies.

Of the three studies included in this review, one48 was an economic evaluation of a diagnostic aid (the MoleMate system; MedX Health Corp., Mississauga, ON, Canada) versus best practice in people with pigmented skin lesions in primary care. The other two studies49,50 estimated the cost of skin cancer in England.
Wilson et al. 48 conducted a model-based economic evaluation that assessed the lifetime costs and QALYs associated with the diagnostic assessment of people with at least one suspicious pigmented skin lesion presenting to UK primary care. The economic model consisted of a decision tree and a Markov model that followed TP, TN, FP and FN cases (based on diagnostic assessment) over a lifetime. The analysis, which adopted the NHS perspective, considered explicitly only the costs and outcomes of melanoma, as it did not differentiate between melanoma and non-melanoma skin cancer. Costs included diagnostic assessment costs and costs of TP, TN, FP and FN cases over a lifetime. Costs were calculated using a bottom-up approach. Treatment costs were estimated according to stage of melanoma, including initial treatment (biopsy excision and definitive surgery), investigations, follow-up surgery for positive lymph nodes, treatment of metastatic disease, follow-up and terminal care. Resource use and costs associated with the management of each melanoma stage were reported separately. Resource-use estimates for the treatment of distinct melanoma stages were based on the 2010 UK guidelines for the management of cutaneous melanoma,13 supplemented by expert opinion. Unit costs were based on the NHS Reference Costs 2008 to 2009. 51 The cost year was 2009.
The study appears to report cost data that are potentially useful for economic modelling. However, clinical experts advised that costs associated with the treatment of more advanced melanoma stages (stages III and IV) are likely to have changed recently, with the introduction of new chemotherapeutic agents, such as ipilimumab (YERVOY®, Bristol-Myers Squibb) and vemurafenib (Zelboraf®, Roche Products Ltd), in the treatment of advanced melanoma in the NHS.
Morris et al. 49 reported the costs associated with malignant melanoma and other malignant neoplasms of the skin in England from a societal perspective. Health-care costs included GP assessment, inpatient stays, outpatient attendances and day cases; in addition, travel costs, incapacity benefits and productivity losses were estimated. The cost year was 2002. Costs were estimated using a top-down approach; total costs were divided by the number of registrations to estimate the mean cost per registration. Resource-use data and unit costs were taken from national sources. The study reported the mean NHS and societal cost per registration of melanoma to be £2179 and £20,020, respectively. The mean NHS and societal cost per registration of other malignant skin neoplasms was £1149 and £1413, respectively.
The resource-use data utilised by this study in order to estimate costs are out of date, as some estimates are > 20 years old. Moreover, the top-down approach allows only a rough estimation of relevant costs. Finally, it is noted that the study provides an overall cost per case with skin cancer (either melanoma or non-melanoma) but, in the case of melanoma, does not report costs by stage of skin cancer.
Vallejo-Torres et al. 50 also reported the costs associated with melanoma and non-melanoma skin cancer in England, but from a NHS perspective. The study used both a top-down and a bottom-up approach in order to produce cost estimates. The cost year was 2008. The top-down approach was adapted from Morris et al. 49 using more up-to-date costs, and was not used to estimate a cost per case. The bottom-up approach used a simplified model of skin cancer care in the NHS, which utilised probabilities of people with suspected skin cancer using different treatment pathways; costs for each pathway were estimated separately. Data to populate the model were taken from UK guidelines for the management of skin cancer,13,14 other published reports and clinical expert opinion. Treatment pathways included initial examination, treatment in primary care or referral to a specialist, diagnostic biopsy of suspicious lesions and treatment according to the biopsy results.
Even though the study by Vallejo-Torres et al. 50 uses more up-to-date resource-use figures and unit costs, the probabilities of treatment received by patients may no longer represent clinical practice, as they were based on an outdated study. 52 Moreover, the study provides separately costs per treatment pathway, but, in the case of melanoma, does not report costs by stage of skin cancer.
The overall methods of the resource-use and costing studies, the resource-use elements that are potentially relevant for the economic model developed for this report and the estimated costs associated with management of skin cancer are presented in Table 21.
| Patient population perspective, costs considered, cost year | Methods, sources of resource-use estimates and unit costs | Available resource-use estimates that are potentially relevant to the economic models constructed for this report |
|---|---|---|
| Wilson et al., 201348 | ||
| Adults with at least one suspicious pigmented lesion undergoing diagnostic assessment Following assessment, TP, TN, FP and FN cases are followed over a lifetime. The analysis considered explicitly only the costs melanoma, as it did not differentiate between melanoma and non-melanoma skin cancer NHS perspective Costs included diagnostic assessment costs and costs of TP, TN, FP and FN cases over a lifetime. Treatment costs according to stage of melanoma were estimated Cost year 2009 |
Combination of resource use with respective unit costs using a bottom-up approach Resource-use estimates for treatment of melanoma based on UK guidelines for the management of cutaneous melanoma supplemented by expert opinion Unit costs taken from published national sources |
Diagnostic assessment costs not relevant (MoleMate system, GP examination) Initial treatment All melanomas have a biopsy excision (£132), staging and definitive surgery (£150) Further treatment Stages 0, Ia and Ib undergo no further treatment Stages IIa and above undergo sentinel lymph node biopsy at the same time with definitive surgery (£34) Stages IIb and above undergo chest radiography (£27), CT scan (£151), liver function test (£3) and full blood cell count (£3) Patients with a positive sentinel lymph node biopsy (stages IIIa, IIIb and IIIc) undergo follow-up surgery comprising preoperative CT scan (£143) and radical lymph node dissection (£891) Stage IV melanomas undergo surgery for removal of localised metastases (£738), a course of 10 fractions of radiotherapy (£1962) and six cycles of dacarbazine-based chemotherapy (Medac GmbH) (£1605) Follow-up Stage 0 disease have only one follow-up appointment in dermatology (£82) Stage I disease are followed up every 3 months for 3 years before discharge (12 visits, £919) Stage II and above followed up 3-monthly for 3 years, then twice yearly for 2 years (16 visits, £1200) Terminal care costs Costs in the final year of life are assumed to be the same as for the treatment of metastatic disease (surgical removal of localised metastases, radiotherapy and chemotherapy) totalling £4305 FNs Patients with undiagnosed melanoma are assumed not to incur any costs unless their disease is opportunistically detected (in which case treatment costs are dependent on stage at diagnosis) or they die of their disease, in which case terminal care costs are incurred Total treatment and terminal care costs Stage 0: £361; stages Ia and IIb: £1198; stage IIa: £1505; stages IIb and IIc: £1680; and stages IIIa–IIIc: £2714 Stage IV, £5985; terminal year, £4305 |
| Morris et al., 200949 | ||
| Patients with skin cancer in England Costs estimated separately for malignant melanoma and other malignant skin neoplasms Societal perspective Costs included GP assessment, inpatient stays, outpatient attendances and day cases, travel costs, incapacity benefits and productivity losses Cost year 2002 |
Combination of resource use with respective unit costs using a top-down approach; mean cost per registration estimating by dividing total health-care and societal costs by the number of registrations Health-care resource-use data and taken from national sources |
Mean NHS cost per registration of malignant melanoma: £2179 (mean total societal cost £20,020) Mean NHS cost per registration of other malignant skin neoplasms: £1149 (mean total societal cost £1413) Travel costs, incapacity benefits and productivity losses not relevant |
| Vallejo-Torres et al., 201450 | ||
| Patients with skin cancer in England Costs estimated separately for malignant melanoma and non-melanoma skin cancer NHS perspective Costs included GP assessment and treatment of non-melanoma skin cancers, diagnostic biopsy, treatment of non-melanoma skin cancer (surgical excision, Mohs surgery, cryotherapy, radiotherapy, curettage and cautery, topical treatment with imiquimod, phototherapy), treatment of melanoma (surgical excision, radiotherapy, radical lymph node dissection) and follow-up Cost year 2008 |
Combination of resource use with respective unit costs using a bottom-up and a top-down approach Top-down approach not used in estimation of cost per case Bottom-up approach based on a model simulating skin cancer care in the NHS; resource use based on UK guidelines, other health guides and clinical expert input Data on probabilities of patients following each treatment pathway and unit costs taken from published papers and reports, administrative data and national sources |
Probability and cost of therapy – non-melanoma skin cancer:
|
Studies reporting utility data
A total of 11,497 citations were identified from the systematic literature search. Of those, 3547 were duplicates and 7909 studies were excluded on the basis of title and abstract. A total of 43 full texts were assessed against the inclusion criteria listed in Box 2; these included 41 studies identified from the database search and the two studies identified from the reference list search.
Of the 41 ordered studies identified from the database search, 17 were cost-effectiveness studies that obtained utility values from the literature to estimate QALYs. Consequently, the sources used to inform the utility values in these studies were identified and reviewed for inclusion. Two further studies were identified from the references lists of those 17 cost-effectiveness studies retrieved from the database search. A full list of the sources used to inform the cost-effectiveness studies is provided in Appendix 5. After full-text review, 38 studies were excluded. Reasons for exclusion of the ordered papers are also presented in Appendix 5. Out of the 43 full texts assessed for inclusion, a total of five studies met the inclusion criteria defined in Box 2. 53–57
After the systematic search was completed, the EAG was informed by experts in the field of an additional recently published paper that provided relevant utility data. 58 Subsequently, the EAG included this paper in the systematic review, after assessing the full text against the set inclusion criteria. It should be noted that one of the inclusion criteria specifies a requirement for a choice-based technique for valuation [i.e. time trade-off (TTO) or standard gamble (SG)]; Tromme et al. 58 does not meet this criterion, as valuation of health states was based on the visual analogue scale (VAS). 58 However, as this study reported utility values that were generated using the EQ-5D, which is the preferred measure by NICE, and the EQ-5D has been valued by the Flemish population in Belgium using the VAS, it was decided to relax the inclusion criteria in order to include this study in this review. None of the 38 potentially relevant studies that had been excluded according to the predefined inclusion criteria met the relaxed inclusion criteria.
In total, six studies were included in the review of studies reporting preference-based HRQoL data (utility data) for skin cancer. 53–58
Figure 5 provides the flow chart of the process of the systematic search for studies reporting utility data.
FIGURE 5.
Flow chart of the process of the systematic search for studies reporting utility data. HTA, Health Technology Assessment; NHS EED, NHS Economic Evaluation Database.

Four of the six studies included in the review reported utility values for melanoma-related health states. 53–55,58 One study reported utility values for health states of advanced BCC56 and the other study reported utility values associated with scarring following facial and auricular non-melanoma skin cancer surgery and reconstruction. 57
Askew et al. 53 reported EQ-5D utility values for different melanoma stages derived from 273 patients with melanoma, 75 of whom were undergoing treatment and 198 were undergoing follow-up surveillance at the time of the study; all attended a tertiary cancer care centre in the USA. The median age of the study sample was 52 years, 98% were white and 58% were male. The number of patients at each melanoma stage was 102 at stage I/II, 100 at stage III and 71 at stage IV. The utility values were generated using patient responses on the EQ-5D. The US EQ-5D tariff was used, which has been developed following a valuation survey of 4048 representative members of the US population using TTO. 59
Beusterien et al. 54 reported utility values for various hypothetical advanced melanoma-related health states elicited from 140 members of the general population (77 from Australia and 63 from the UK), using SG. The hypothetical health states (vignettes) included four advanced melanoma treatment-related response states, one symptomatic melanoma state and nine toxicity-related health states. The hypothetical health states were constructed based on published literature and refined following an iterative review by five clinical experts, two oncology nurses, three quality-of-life researchers and a pilot test with individuals from the general public. The four treatment-related response states were defined as follows: partial response was defined by a > 50% decrease in lesion mass; stable disease was defined by a > 25% decrease or increase in lesion mass; progressive disease was defined by the appearance of new lesions or a > 25% increase in lesion mass; and for best supportive care there was no indicated or desired cancer treatment. A symptomatic melanoma health state represented symptoms experienced in advanced melanoma. The health states were described as being treated for cancer (melanoma was not specified), whether or not treatment is working, and changes in tumour size, pain levels, appetite, effort required to perform daily activities and fatigue. Each of the toxicity descriptions was described in association with partial response so that the utility decrements for toxicities could be calculated by subtracting the utility for partial response from the utility of the toxicity state.
King et al. 55 developed vignettes describing health states associated with each of the melanoma stages (I–IV) based on published literature and relevant websites; the hypothetical health states were valued by 163 adult patients with melanoma attending a cancer clinic in the USA using TTO. Patients were divided into new cases (if they were ≤ 1 year from diagnosis) and established cases (if they were > 1 year after diagnosis or > 6 months if stage IV). Patients were asked to value stages other than their own: patients with stage I disease imagined having a new diagnosis of stage II, III or IV disease, while patients with higher-stage disease imagined the impact of a new stage I diagnosis. Utilities derived from new cases, established cases and all patients participating in the study were reported separately.
Tromme et al. 58 reported EQ-5D utility values for different melanoma stages derived from 356 patients with melanoma. Patients completed the five-level version of the EQ-5D (EQ-5D-5L); 39 patients completed the EQ-5D-5L questionnaire twice, as they were seen in two different phases (treatment and follow-up) and/or stages during the study. Patients were classified into eight groups using four melanoma stages (I, II, III and IV), with each stage subdivided into treatment and remission phases.
Patients with stage 0 and Ia melanoma were pooled with the justification that they had marginal differences regarding their surgical treatment and follow-up. Patients with stage Ib and II melanoma were also pooled because, according to the authors, these patients had undergone sentinel lymph node biopsy (SLNB) that had not been followed by elective node dissection and also because of evidence that surgical resection margins did not appear to influence HRQoL.
Based on expert opinion, treatment duration was estimated to be 1, 2 and 3 months for stages 0–Ia, Ib–II and III, respectively, and > 10 months for stage IV. The remission period for stages 0/Ia and Ib/II was estimated at 2 years of follow-up, as it has been shown that after 2 years the HRQoL of these patients is similar to that of the general population. 60 Patients with stage IV melanoma in remission but still under treatment were classified as patients under treatment in order for the impact of side effects on HRQoL to be captured. The mean age of the patients was 52.6 years and 74% were male. EQ-5D-5L profiles were first mapped onto EQ-5D three levels (EQ-5D-3L) profiles, which were subsequently converted into utility values using the Belgian EQ-5D tariff, which has been developed following a valuation survey of 2754 Flemish adults from the general public in Belgium using the VAS. 61
Shingler et al. 56 reported utility values for a number of hypothetical advanced BCC-related health states elicited from a representative sample of 100 members of the UK general public using TTO. The health state vignettes associated with advanced BCC were constructed based on a literature review, consultation with two clinical experts and validation/piloting with three members of the general public. At the end of this process, nine health state vignettes were developed, reflecting level of treatment response. The nine vignettes describing advanced BCC health states were as follows: complete response; post-surgical state; partial response with small (2 cm) or large (6 cm) growth; stable disease with small (2 cm) or large (6 cm) growth or multiple growths (2 cm); and progressed disease with small (2 cm) or large (6 cm) growth.
Seidler et al. 57 developed simple health state vignettes describing the type of repair and subsequent scar after facial and auricular non-melanoma skin cancer surgery and reconstruction. 57 One state comprised surgery for facial non-melanoma skin cancer, a second state described simple repairs or scars (granulation and primary closure) as a result of surgery and a third state described complex repairs or scars (local flap and graft) because of surgery of non-melanoma skin cancer. Five healthy adults from the general public in the USA valued the three health states using TTO.
Table 22 summarises the methods used to derive and value health states associated with skin cancer and the resulting utility scores, as reported in the six studies included in this systematic review.
| Study and year | Definition of health states | Valuation method | Population providing valuations | Health states and corresponding utility scores |
|---|---|---|---|---|
| Melanoma skin cancer | ||||
| Askew et al., 201153 | EQ-5D data from 273 patients with melanoma: 75 undergoing treatment and 198 in follow-up surveillance at a tertiary cancer care centre in the USA Median age 52 years (range 20–79 years); white 98%, male 58% Melanoma stage I/II, n = 102; stage III, n = 100; and stage IV, n = 71 |
TTO | 4048 representative members of the US population | Stage I/II: 0.91 (SD 0.14) Stage III: 0.85 (SD 0.13) Stage IV: 0.86 (SD 0.11) |
| Beusterien et al., 200954 | Vignettes constructed for four advanced melanoma treatment-related response states, one symptomatic melanoma state and nine toxicity-related health states based on published literature and refined following an iterative review by five clinical experts, two oncology nurses, three quality-of-life researchers and a pilot test with individuals from the general public. The health states were described as being treated for cancer (melanoma not mentioned), whether or not treatment is working and changes in tumour size, pain levels, appetite, effort required to perform daily activities and fatigue Toxicity scenarios added on description of partial response The four response states were defined as follows:
|
SG | 140 members of the general population (77 from Australia and 63 from the UK) | UK sample values Clinical response states:
|
| King et al., 201155 | Vignettes describing different stages of melanomas (I, II, III and IV) constructed based on published literature and relevant websites | TTO | 163 adult patients with melanoma in the USA; mean age 51 years; 99% white; 45% male New cases (≤ 1 year from diagnosis); established cases (> 1 year after diagnosis, or > 6 months if stage IV) Stage I: n = 15; 80 established melanoma diagnoses Stage II: n = 4; 11 established melanoma diagnoses Stage III: n = 8; 10 established melanoma diagnoses Stage IV: n = 11; 24 established melanoma diagnoses Patients asked to value stages other that their own |
Stage I
|
| Tromme et al., 201458 | 395 EQ-5D-5L questionnaires from 356 patients with melanoma (mean age 52.6 years, male 74%); 39 patients completed questionnaires twice, as they were seen in two different phases (treatment and follow-up) and/or stages Patients grouped according to melanoma stage (I, II, III and IV) and phase of stage (treatment or remission) Based on expert advice, treatment duration was assumed to be 1, 2 and 3 months for stages 0-Ia, Ib–II and III, respectively; and > 10 months for stage IV Remission period for stages 0/Ia and Ib/II was 2 years Patients with stage IV melanoma in remission but still under treatment were classified as patients under treatment Patient sample size (mean age) by health state:
|
VAS | 2754 Flemish adults from the general public in Belgium | Stage 0/Ia
|
| Non-melanoma skin cancer | ||||
| Shingler et al., 201356 | Health state vignettes associated with advanced BCC constructed based on a literature review, consultation with two clinical experts and validation/piloting with three members of the general public Final health state vignettes:
|
TTO | Representative sample of 100 members of the UK General public (mean age 39.1 years; 96% white; 57% female) |
Complete response: 0.94 (SD 0.08) Post-surgical state: 0.72 (SD 0.24) Partial response
|
| Surgical excision | ||||
| Seidler et al., 200957 | Health state vignettes describing the type of repair and subsequent scar after facial and auricular non-melanoma skin cancer surgery and reconstruction The health states were:
|
TTO | Five healthy people from the general public in the USA (mean age 40 years) | Excision procedure: 0.996 (range 0.984–1) Simple repairs/scars 0.984 (range 0.974–1) Complex repairs/scars 0.974 (range 0.953–1) |
According to NICE guidance47 on the selection of utility values for use in cost–utility analysis, the measurement of changes in HRQoL should be reported directly by people with the condition examined, and the valuation of health states should be based on public preferences elicited using a choice-based method, such as the TTO or SG, in a representative sample of the UK population. When changes in HRQoL cannot be obtained directly from the people with the condition examined, then data should be obtained from their carers. NICE recommends EQ-5D for use in cost–utility analyses of interventions for adults. When EQ-5D scores are not available or are inappropriate for the condition or effects of treatment, NICE recommends that the valuation methods be fully described and comparable to those used for the EQ-5D. 47
None of the studies included in the review meet the above criteria set by NICE. Two of the studies53,58 used the EQ-5D for the description of HRQoL experienced by patients with melanoma. However, none of them used the UK EQ-5D tariff62 for the valuation of health states; Askew et al. 53 used the USA EQ-5D tariff, which was developed using TTO, whereas Tromme et al. 58 used the Belgian EQ-5D tariff, which was developed using the VAS – a valuation method that is not choice based and thus is not among NICE-preferred valuation methods.
All the remaining studies generated utility values for health states described in vignettes. Of those, Beusterien et al. 54 elicited utility values for melanoma-related health states from members of the general population in Australia but also in the UK, so, in this aspect, the study meets the NICE criterion for valuation of states by the UK general population. The same applies to Shingler et al. ,56 who reported utility values for advanced BCC-related health states obtained from members of the UK general public. In contrast, King et al. 55 reported melanoma-related utility values elicited from patients with melanoma in the USA, while Seidler et al. 57 reported utility values associated with facial non-melanoma skin cancer surgery and reconstruction that were elicited from only five healthy adults in the USA.
A comparison of the utility values available for melanoma-related health states according to stage revealed that the utility values reported by Askew et al. 53 for melanoma stages III and IV are considerably higher than those reported by King et al. 55 and Tromme et al. 58 Moreover, the utility values reported by Tromme et al. 58 for melanoma early stages I and II are substantially lower than the utility values reported for the same stages in Askew et al. 53 and King et al. 55 These discrepancies are potentially attributable to differences in measurement and valuation across the three studies. Measurement of HRQoL in two studies53,58 was taken from patients with melanoma using the EQ-5D, whereas King et al. 55 used vignettes to describe the HRQoL associated with melanoma stages. In the two studies reporting EQ-5D-based utility values,53,58 values had been elicited from members of the general population in two different countries (USA and Belgium) using two different valuation techniques (TTO and VAS). King et al. 55 elicited values from patients with melanoma. 55 These differences in valuation may also be responsible for the differences in resulting utility values.
In addition, it is noted that utility values for stage III appear to be lower than utility values for stage IV in Tromme et al. ;58 however, this may be attributable to the variation in values because of the small number of patients providing EQ-5D ratings for stage III in treatment (n = 15) and stage IV in remission (n = 14).
The available utility values for skin cancer, the methods used in their development and underlying limitations as well as their eligibility for use in economic modelling according to NICE criteria are discussed in the appropriate subsections of Economic modelling.
Economic modelling
Introduction: overview of methods
This section gives an overview of the economic modelling approach, the overall objectives and methods employed. The economic analysis consists of three ‘part’ models that were eventually combined into one analysis. The specific methods employed for each ‘part’ model are described separately for each model in the sections below.
Overall objective
The overall objective of the economic analysis, as defined by the scope of this diagnostic assessment, was to assess the cost-effectiveness of the VivaScope 1500 and 3000 imaging systems in the diagnosis of potentially malignant skin lesions, including LM, and the margin delineation of diagnosed malignant lesions, including LM, prior to surgical treatment. Not all potentially malignant or diagnosed skin lesions are suitable for diagnosis or presurgical margin delineation with the use of the VivaScope imaging system. The selection of population groups with suspected (or diagnosed) skin cancer for consideration in economic modelling was determined by the availability of relevant clinical data and clinical expert opinion.
Study population
The VivaScope 1500 and 3000 imaging system was assessed in the diagnosis of skin cancer in the following populations:
-
people with suspected melanoma who have equivocal lesions following dermoscopy
-
people with suspected BCC whose lesions have an equivocal or positive result on dermoscopy, to make the diagnosis or to confirm diagnosis, respectively, as an alternative to diagnostic biopsy.
The above populations were considered to be the most relevant to undergo diagnostic assessment with VivaScope, according to clinical experts to the EAG. Clinical experts also advised that they form an opinion about the type of skin cancer they will find prior to examination with VivaScope, and hence the type of suspected skin cancer was prespecified at early stages of designing the economic model. The NICE scope63 defines the study population as ‘people with equivocal lesions following dermoscopy’; however, clinical experts advised the EAG that the use of VivaScope in suspected BCC lesions has two purposes: (1) to make a diagnosis when results of dermoscopy are not certain; and (2) to confirm diagnosis in lesions that are found positive on dermoscopy. In both cases the VivaScope is used as an alternative to diagnostic biopsy. Thus, the economic model considered all people with suspected BCC lesions eligible for dermoscopy, and not only those with equivocal lesions suspected to be BCC, as the latter are rather a minority of the cases eligible for examination with VivaScope.
Equivocal lesions among those suspected of being melanoma include any suspected melanoma lesions based on a number of characteristics on dermoscopy, with the exception of clearly positive (malignant) lesions that have all the dermoscopic characteristics of melanoma and clearly negative (benign) lesions that show no features of melanoma (no changes) on dermoscopy. The risk of equivocal lesions being malignant is overall low. There are different degrees of ‘equivocalness’, depending on the dermoscopic characteristics of the lesion and subjective experience and interpretation. Clinical expert advice indicated that highly suspicious equivocal lesions are lesions with at least two positive dermoscopic features including one major criterion or three minor positive features suggestive of melanoma, and/or lesions clearly changed after digital follow-up, and/or new or growing lesions in an adult with at least one dermoscopic positive criterion, or papular/nodular or pink or spitzoid lesions. In all of those cases, excision is prompted and examination with VivaScope does not represent a real advantage, as the risk of missing a melanoma remains too high. Moderately or low suspicious equivocal lesions are lesions with only one major dermoscopic positive feature or two minor features, and/or no clear history or minor changes. In such cases, excision is possible but other options could be taken into account, such as digital follow-up, especially in the case of flat lesions in patients with multiple moles; however, digital follow-up can delay a melanoma diagnosis. The majority of moderately or low suspicious equivocal lesions that are excised are benign, and examination with VivaScope can play a major role in reducing this burden of unnecessary excisions.
Clinical experts advised that VivaScope is less suitable for the detection and assessment of skin lesions suspicious of SCC, as this type of skin cancer is usually scaly because of severe hyperkeratosis. This often limits the evaluation of SCC lesions, as it is more difficult to capture images of structures deeper in the tissue. Moreover, no evidence on the diagnostic accuracy of VivaScope in this type of skin cancer was identified in the systematic review of clinical evidence. Therefore, it was decided not to include people with skin lesions suspicious of SCC in the diagnostic economic model.
Regarding margin delineation, VivaScope 3000 was assessed in the following population:
-
patients with LM prior to surgical management.
According to clinical expert advice, margin delineation of melanomas with VivaScope is not useful in clinical practice, as the margins of melanomas are clearly defined and can be completely excised following BAD guidance;13 consequently, VivaScope mapping of melanomas does not offer any clinical utility and, therefore, was not considered further for economic modelling.
Clinical experts advised that margin delineation of BCCs using VivaScope may be difficult, as BCCs may be too deep for their margins to be accurately mapped with VivaScope. Therefore, it was decided not to consider margin delineation of BCC lesions with the use of VivaScope in the economic model, also considering the lack of evidence in this area. Nevertheless, it is acknowledged that, although margin delineation of BCCs using VivaScope prior to surgical excision was not considered in the economic analysis, this may be used as an alternative to Mohs surgery, as advised by clinical experts.
VivaScope is not appropriate for the assessment of SCC lesion margins due to the scaly nature of SCC making it difficult to view using imaging techniques.
Economic models developed: decision problems addressed
According to the study populations that were identified as relevant for the economic evaluation of VivaScope, three separate ‘part’ economic models were developed:
-
Use of VivaScope in the diagnosis of equivocal lesions suspected of being melanoma. This model assessed the cost-effectiveness of VivaScope 1500 and 3000, as one integrated system, assuming that both devices will be available for the diagnosis of equivocal lesions but each will be used as appropriate according to the location of the equivocal lesion to be examined.
-
Use of VivaScope in the diagnosis of suspected BCC lesions following a positive or equivocal finding on dermoscopy. As with the previous model, this model assessed the cost-effectiveness of VivaScope 1500 and 3000, as one integrated system, assuming that both devices will be available for the diagnosis of suspected BCC lesions but each will be used as appropriate according to the location of the skin lesion to be examined.
-
Use of VivaScope for the margin delineation of LM prior to surgical therapy. This model assessed the cost-effectiveness of VivaScope 3000 as a stand-alone device, as only this device is appropriate for margin delineation.
Development of two separate models, one for the diagnosis of equivocal lesions suspected of being melanoma and one for suspected BCC lesions with a positive or equivocal dermoscopy finding, was necessary because both the diagnostic accuracy of VivaScope and the treatment pathways and associated costs and outcomes following diagnosis vary greatly between these two types of skin cancer.
Using the results of the above three ‘part’ models, five economic analyses were undertaken, examining the cost-effectiveness of VivaScope in:
-
the diagnostic assessment of equivocal lesions suspicious of melanoma (integrated use of VivaScope 1500 and 3000)
-
the diagnostic assessment of lesions suspicious of BCC following a positive or equivocal result on dermoscopy (integrated use of VivaScope 1500 and 3000)
-
the diagnostic assessment of skin lesions suspicious of skin cancer, either melanoma (following an equivocal finding on dermoscopy) or BCC (following a positive or equivocal finding on dermoscopy) – this analysis combined the results of the two ‘part’ models.
-
the margin delineation of LM prior to surgical treatment (use of VivaScope 3000 as a stand-alone device)
-
the diagnostic assessment of skin lesions suspected of being either melanoma or BCC, and the margin delineation of LM (integrated use of VivaScope 1500 and 3000) – this analysis combined the results of all three ‘part’ models.
The final economic analysis synthesised all cost and effectiveness data from each of the ‘part’ economic models to obtain an estimate of the overall cost-effectiveness of the VivaScope imaging system used for all indicated purposes assessed in economic modelling in a skin cancer multidisciplinary team (MDT) service.
The analyses that combined results of ‘part’ models used weighted total costs and benefits according to the expected relative number of each type of lesion diagnosed and/or mapped with VivaScope in one dermatology MDT service.
Costs and outcomes considered in the analysis
The perspective of the analysis was that of the NHS and Personal Social Services (PSS). Costs consisted of the costs of the intervention, that is VivaScope (including equipment and maintenance costs, costs of consumables, staff training and staff time required for the examination), the costs associated with the comparators used in the the analysis (such as the costs of biopsy, histological examination and monitoring including any required consultations with clinicians), the costs of management of skin lesions following correct (i.e. TN and TP cases) or incorrect (FN and FP cases) diagnosis, as well as the costs incurred following the presurgical mapping of malignant skin lesions. The costs of management of future events such as progression and recurrence of skin cancer, where relevant, were also considered. All costs were expressed in 2014 prices.
The outcome measure of the economic analysis was the QALY. Discounting of costs and outcomes was applied at an annual rate of 3.5%, in accordance with NICE methodology guidance. 47
Sources of model input parameters
The clinical effectiveness parameters required for the economic models (diagnostic accuracy of VivaScope 1500 and 3000 and clinical outcomes on margin delineation) were informed, where possible, by the systematic review of the clinical evidence reported in Chapter 3. A non-systematic review of model-based economic studies assessing strategies and interventions for the prevention, assessment or management of skin cancer was also undertaken, aiming to provide an insight into the modelling methods in the area of skin cancer and also identify relevant input parameters that could be potentially utilised in the economic models assessing VivaScope. These studies were predominantly identified by re-running the search for existing economic evaluations of VivaScope (described in Systematic literature review of existing economic evidence, Methods) after omitting the terms capturing the interventions and comparators of interest from the search strategy. The search resulted in a very high number of hits (approximately 9000) that did not permit a review of the findings in a systematic way because of time and resource constraints. Nevertheless, this review helped identify a range of useful clinical (as well as resource-use) data and model structural components that contributed to the construction of the model structures for the economic assessment of VivaScope. In addition, relevant NICE guidance (including clinical and public health guidelines, technology appraisals and interventional procedure guidance) was reviewed for clinical and cost data that could be potentially useful in economic modelling.
Preference-based data on the HRQoL of people experiencing health states or events associated with suspected or confirmed skin cancer were derived from the relevant published literature identified in the systematic review, the results of which are provided in Systematic literature review of existing economic evidence, Results.
Following clinical expert advice, the EAG undertook a review of conference abstracts presented at the British Association of Dermatologists’ annual meetings since 2010, which are available from the British Journal of Dermatology. This review aimed to identify audits reporting data on health service use from patients with skin cancer in the UK, as well as recent trends in epidemiological data directly relevant to the UK population that could inform the economic models. Clinical experts also provided references to studies reporting data that were potentially useful in populating the economic models.
Finally, at all steps of designing the economic models, clinical expert opinion was sought to confirm that diagnostic and assessment pathways were consistent with current clinical practice in the UK, as well as with anticipated changes in practice following a potential introduction of VivaScope within the NHS context. Clinical expert opinion was also employed to supplement the economic models with parameter estimates, in areas where relevant published evidence was lacking.
The costs associated with the intervention (VivaScope 1500 and 3000 imaging systems), including the purchase price of the equipment and parts and maintenance costs, were provided by the company. Other health-care unit costs were obtained from national sources such as the NHS drug tariff for February 2015,64 the national Unit Costs of Health and Social Care 201465 and the NHS Reference Costs 2013 to 2014 for 2014. 12 The NHS reference costs were preferred over the Payment by Results tariffs because they represent actual national average costs incurred as a result of health-care services provided by the NHS, and hence they reflect opportunity costs, whereas the Payment by Results tariffs represent payments rather than the actual cost of services to the NHS.
Annual number of cases eligible for examination with VivaScope in a dermatology multidisciplinary team clinic in the UK
The annual number of cases eligible for examination with VivaScope in a dermatology MDT clinic was needed in order to determine the total cost per case associated with a VivaScope examination, as the overall cost of VivaScope (including purchase and maintenance cost, training costs and any other ancillary costs) is spread across the number of lesions examined. Given the high cost of purchasing VivaScope and the considerable training required for obtaining and interpreting VivaScope images, an adequate number of VivaScope examinations needs to be performed every year, so that the benefit from VivaScope use offsets the intervention cost.
In order to estimate the total number of people that are assessed with VivaScope in 1 year, three approaches were followed.
The first approach was to ask clinical experts working in the dermatology department of Guy’s and St Thomas’ Hospital, London, UK, where VivaScope is currently in use, about the annual number of cases examined with VivaScope in their practice.
This approach yielded the following information:
-
Approximately one suspected melanoma is assessed with VivaScope per week; however, it was suggested that this number is probably lower than the typical number of lesions suspicious of melanoma that would normally be examined by a tertiary service and that would be eligible for examination with VivaScope.
-
Approximately 15 suspected BCC lesions are assessed with VivaScope per week; however, it was suggested that this number might be higher than the typical number of suspected BCC lesions that would normally be examined by a tertiary dermatology service.
-
Approximately one or two LMs are mapped with VivaScope per week, but this includes LMs planned for surgical therapy as well as radiotherapy and topical immunotherapy.
Based on the above information, the annual number of lesions examined with VivaScope was estimated to comprise 75–100 equivocal lesions suspicious of melanoma (estimated as 1.5–2 expected to be seen per week × 50 weeks); 500 suspected BCC lesions (estimated as 10 expected to be seen per week × 50 weeks); and 75 LMs prior to treatment (estimated as 1.5 expected to be seen per week × 50 weeks, and considering that the vast majority of LMs are treated surgically, as advised by clinical experts).
The second approach was to seek information from clinical experts working in other dermatology services, who were approached for expert opinion and advice on the preparation of this report, and on the annual number of suspected melanomas, suspected BCCs and LMs eligible for examination with VivaScope that were assessed in their practice.
This approach yielded the following information:
-
The dermatology clinic at the Norfolk and Norwich University Hospital examines approximately 600–800 lesions suspicious of melanoma per year. No information was available on the number of lesions suspicious of BCC or LMs examined per year.
-
The dermatology service at the Lincoln hospitals serves a population of about 0.75 million. Using population incidence data, it was estimated that every year the service diagnoses about 160 melanomas, 1000 BCCs and roughly 60–80 LMs. The vast majority of BCCs are easy to diagnose clinically or with dermoscopy; sometimes they are so typical that no dermoscopy is needed.
-
The Department of Dermatology at the Chelsea and Westminster Hospital examines around 300 suspected BCCs and manages at most 20 LMs annually. No information was available on the number of suspected melanomas examined per year.
Clinical experts advised that, of every five or six lesions that are excised because of suspicion of melanoma, one melanoma is confirmed. Using the estimate of 160 diagnosed melanomas, the number of suspected melanomas examined by the service in Lincoln (i.e. lesions giving a positive or equivocal result on dermoscopy) should be approximately 800–960 per year.
Of the suspected melanomas examined in each service, only those giving an equivocal finding on dermoscopy would be eligible for examination with VivaScope. Therefore, to estimate the number of suspected melanomas eligible for examination with VivaScope in each service, the proportion of equivocal lesions among the number of suspected melanomas examined in each service is needed. For this reason, a review of the studies included in the systematic review of clinical evidence reported in Chapter 3 was undertaken, attempting to identify the proportion of suspected melanomas examined by a dermatology MDT clinic that give an equivocal finding on dermoscopy. The review considered studies reporting prospective or retrospective recruitment of consecutive people attending a dermatology clinic for skin lesions suspicious of melanoma, which were assessed with a dermoscope, as they were likely to be more representative of the population of people with suspected melanomas likely to be seen at a dermatology clinic. Studies that had selectively recruited people with suspicious skin lesions and those that assessed retrospectively lesions that had already been excised on the basis of their dermoscopic features were not considered, as their study samples were not necessarily representative of the study population. In addition, studies that had excluded ‘clear-cut positive lesions on dermoscopy’ from recruitment were not considered useful, as they provided an overestimate of the proportion of equivocal lesions among the total number of lesions examined with dermoscopy.
The only suitable study included in the systematic review of clinical evidence reported in Chapter 3 was that by Alarcon et al. ,30 who assessed the impact of a VivaScope examination of equivocal lesions suspicious of melanoma following diagnostic assessment with dermoscopy. From 5520 patients attending a hospital dermatology unit in Barcelona, the study identified 1534 people with lesions suspicious of melanoma who underwent dermoscopy. In 1191 of these lesions, the finding, according to the authors, was clear and the lesions were scheduled either for immediate excision or for digital follow-up. In the remaining 343 lesions, the findings were equivocal and thus these lesions were suitable for examination with VivaScope 1500. Thus, the percentage of equivocal lesions among all lesions suspicious of melanoma and assessed with dermoscopy was 22.4% (343/1534).
A Belgian observational study assessed the extent of cost reduction resulting from the use of sequential digital dermoscopy in people presenting to dermatologists because of concern about melanoma and having 1–3 equivocal melanocytic lesions. 66 The study reported that, of the 9360 consecutive people with 1–3 lesions suspicious of cancer that were assessed with a dermoscope over 1 year (2009–10), 822 had equivocal lesions, according to dermatologists, making the percentage of equivocal lesions among lesions suspicious of melanoma 8.78%. However, the study population was people presenting to dermatology services rather than being referred, and, therefore, the prevalence of melanoma, and subsequently the prevalence of equivocal lesions, was most likely lower than the prevalence of melanoma and prevalence of equivocal lesions in populations referred to dermatology MDTs from primary care in the UK.
It should be noted that the proportion of equivocal lesions among lesions suspicious of melanoma that are examined with a dermoscope is affected by a number of other factors, such as the experience of the dermatologist performing the examination and the underlying prevalence of melanomas.
Expert opinion indicated that the proportion of equivocal lesions out of lesions suspicious of melanoma undergoing dermoscopic evaluation in England must be between the figures observed in the two studies described above. 30,66 Therefore, estimations of the number of lesions suspicious of melanoma that are suitable for a VivaScope examination (i.e. they have an equivocal finding on dermoscopy) were based on the assumption that approximately 15% of lesions examined by skin cancer MDT services are equivocal.
Using the estimates of suspected melanomas seen annually in each of the two services (600–800 cases at the dermatology clinic at the Norfolk and Norwich University Hospital and 800–960 cases at the dermatology clinic in Lincoln) and a proportion of equivocal lesions following dermoscopy of 15% among all cases examined, the estimated number of equivocal lesions suspicious of melanoma that would be eligible for examination with VivaScope seen by each service per year was 90–120 in Norfolk and Norwich University Hospital and 120–144 in Lincoln.
Regarding BCCs diagnosed at the dermatology clinic at Lincoln Hospitals, the experts considered that the vast majority are easy to diagnose, sometimes without the use of a dermoscope. Expert opinion suggested that, out of 1000 confirmed BCCs, in 600–700 clinical examination would reveal a clear-cut picture (and a positive finding on dermoscopy). The remaining 300–400 confirmed BCC lesions would be identified after roughly 500–600 lesions suspicious of BCC had examined with VivaScope (following an equivocal finding on dermoscopy). In total, at least 500–600 lesions per year would be eligible for examination with VivaScope to make a diagnosis, and 600–700 clear-cut positive BCC lesions would be eligible for examination with VivaScope for confirmation of diagnosis, leading to a total estimate of 1200–1400 lesions suspicious of BCC that would be eligible for examination with VivaScope in the dermatology service of Lincoln hospitals annually.
The number of suspected BCCs examined at the Department of Dermatology of the Chelsea and Westminster Hospital annually is approximately 300. Similar to the above estimations, about 180–200 would be expected to be positive on dermoscopy and 100–120 equivocal; the latter would be identified after roughly 150–200 lesions suspicious of BCC have been examined with VivaScope following an equivocal finding on dermoscopy, resulting in a total estimate of around 330–400 lesions suspicious of BCC that would be eligible for examination with VivaScope in the Department of Dermatology of the Chelsea and Westminster Hospital.
The number of LMs examined annually at the Department of Dermatology of the Chelsea and Westminster Hospital was 20 at maximum, whereas at Lincoln hospitals roughly 60–80 LMs are diagnosed every year. It was assumed that practically all of them are treated surgically.
The third approach was to estimate the numbers of lesions/cases eligible for VivaScope examination indirectly, by estimating the numbers of people being referred to dermatology services each year in the UK and, from that, estimate the number of people in a large dermatology MDT service.
This approach considered dermatology MDT clinics serving a large population of people, which are likely to see a high number of skin lesions suspicious of skin cancer. SSMDT clinics were selected for this purpose, as it is expected that more suspected skin cancers are referred here than in LSMDTs. These serve a catchment population for referral (their own local catchment plus the catchment of referring LSMDTs) of at least 750,000 and serve as the LSMDT for their local (secondary) catchment population. 67
In 2009–10, 882,000 patients were referred to dermatologists in England (approximately 16 per 1000 population). 68 Up to 50% of referrals relate to skin cancer (including both diagnosis and management). Dermatologists screen > 90% of skin cancer referrals and treat approximately 75%. 68 In the period between 2000 and 2007, there was an increase of about 5.6% in new patients visiting dermatology specialists. 69 This is an increase of approximately 0.8% per year. Applying this annual rate of increase to the data from 2010, in 2015 the expected number of people referred to dermatologists in England is 16.63 per 1000 population. With 50% of referrals relating to skin cancer and 90% of them being screened, this results in an estimate of approximately 7.49 examinations for skin cancer per 1000 population per year.
Assuming a catchment area of 750,000, one SSMDT would examine around 5614 people for skin cancer per year. Assuming that the ratio of referred lesions suspicious of BCC, SCC and melanoma is approximately the same as the ratio of confirmed skin cancers, and taking into account the fact that in 2011 there were 102,628 cases of non-melanoma skin cancer in the UK (of which BCCs make up 74%) and 13,348 cases of melanoma in the UK,2 then, of the 5614 people examined for skin cancer annually, 11.5% (646) would be examined for suspected melanoma and 65.5% (3676) would be examined for suspected BCC.
Using an estimated proportion of equivocal lesions among lesions suspicious of melanoma of 15%, a SSMDT serving a population of 750,000 would see 97 equivocal lesions suspicious of melanoma per year.
Clinical expert advice was that the number of 3676 lesions suspicious of BCC appears to be unrealistically high. Therefore, it was assumed that only 50% of them were actually suspected to be BCCs. Using estimates described earlier, it was calculated that roughly 2000–2300 lesions suspicious of BCC would be potentially eligible for a VivaScope examination.
Epidemiological data specific to LM are rather sparse and not routinely available in UK cancer statistics. Incidence data on LM were reported in a US study that identified all adult residents with a first lifetime diagnosis of LM between 1970 and 2007 in Olmsted County, MN, USA. 70 The study reported that the overall age- and sex-adjusted incidence of LM among adults was 6.3 per 100,000 person-years, increasing from 2.2 between 1970 and 1989 to 13.7 between 2004 and 2007. 70 Although the incidence of LM in the UK population, as well as the mixture of the UK population, may be different from that in Minnesota, using the incidence of 13.7 per 100,000 person-years in a population of 750,000 people would result in 103 cases identified and treated in a dermatology service annually.
The estimates derived from the three approaches are summarised in Table 23.
| Approach | Estimates on annual number of skin lesions eligible for examination with VivaScope (including equivocal lesions suspicious of melanoma, suspected BCC lesions and LM prior to treatment) |
|---|---|
| Clinical advice from experts working in UK services where VivaScope is available |
|
| Clinical advice from experts working in UK services and further assumptions on the proportion of lesions eligible for examination with VivaScope |
|
| Synthesis of epidemiological data and national statistics |
|
As can be seen, with the exception of BCC lesions, for which the range of estimates is wide, the estimated number of equivocal lesions suspicious of melanoma and the estimated number of LMs that are eligible for examination with VivaScope using each of the three approaches are very close. In order to estimate the cost of VivaScope per skin lesion assessed, the following estimates in the number of lesions examined annually with VivaScope in a dermatology service were utilised:
-
100 equivocal lesions suspicious of melanoma
-
500 lesions suspicious of BCC (use of a low, relatively conservative estimate, which was based on information derived from the only setting in the UK that currently uses VivaScope for the diagnostic or presurgical assessment of skin lesions)
-
75 LMs prior to treatment.
Lesions suitable for examination with VivaScope 3000
Lesions suspicious of melanoma or BCC that are suitable for examination with VivaScope 3000 are predominantly those on the head or neck. Owing to a lack of relevant data on lesions suspicious of skin cancer, it was assumed that the proportion of equivocal lesions suspicious of melanoma on the head or neck was equal to the proportion of diagnosed melanomas on the head or neck; similarly, the proportion of lesions suspicious of BCC with a positive finding on dermoscopy that present on the head or neck was assumed to equal the proportion of diagnosed BCCs on the head or neck.
The proportion of confirmed melanomas on the head or neck is approximately 14% in females and 22% in males. 71 In 2011, there were 13,348 new cases of melanoma in the UK, 6495 of which were in males,2 so that the proportion of men in the population of people with newly diagnosed melanoma was 48.7%. Using these data, the proportion of melanomas on the head or neck out of all melanomas was estimated to reach 17.9%. This figure was used as a proxy to represent the proportion of lesions suspicious of melanoma with an equivocal finding presenting on the head or neck, because of the lack of more specific data.
Regarding BCC, a review on facial BCC has reported that up to 85% of BCCs are on the head or neck. 72 A study that analysed data on all cases of BCC diagnosed at a single centre of dermatopathology during 1967–96 in Strasbourg, France, reported that BCCs of the head and neck were more frequent in women (85.2%) than in men (81%), independent of their histological subtype. 73 Another study analysing trends in the demographic, clinical and socioeconomic profile of more than 50,000 cases of non-melanoma skin cancer registered between 1994 and 2011 by the Irish National Cancer Registry reported that 69% of diagnosed BCCs over that period were on the face. 74 In the UK, an audit of all BCC excisions performed in a single centre in 2008 showed that 68.1% of those (631/926) were removed from the face. 75 Similarly, a regional audit of BCC histopathology reports (using the Cancer Registry Cancer – Base Enquiry System to extract data on the first 100 BCC de novo cases per trust for the year 2007) showed that, of the 1318 BCC-excised lesions for which the anatomical site was known, 915 (69.4%) were on the head or neck. 76 The figure of 69.4% was used as a proxy to represent the proportion of suspected BCC lesions with a positive dermoscopic finding presenting on the head or neck.
Estimation of the proportion of LMs presenting on the head or neck was not relevant in the context of examination with VivaScope 1500 or 3000, as all LMs are mapped with VivaScope 3000 prior to surgical excision.
Cost of VivaScope 1500 and 3000
This section reports the costs associated with examination of skin lesions with the VivaScope imaging system, either for the diagnostic assessment of lesions suspicious of melanoma or BCC or for the margin delineation of LM prior to surgical treatment.
The costs associated with examination of skin lesions with VivaScope comprise the purchase (capital) cost of the VivaScope imaging system, maintenance costs, the costs of equipment parts and other consumables required for the examination, and the costs of training staff in operating the system and in the assessment and interpretation of the images obtained. They also include costs of staff time required for the examination with VivaScope and subsequent assessment of skin lesions.
The company provided the purchase price of VivaScope 1500 and 3000, as well as the annual maintenance costs. The purchase price and annual maintenance costs of VivaScope 3000 as an add-on device to VivaScope 1500 were stated to be lower than the corresponding costs of VivaScope 3000 as a stand-alone device. For the use of VivaScope in the diagnostic assessment of lesions suspicious of skin cancer, VivaScope 1500 and 3000 have been considered to be used according to indications and suitability (i.e. the economic models assumed that VivaScope 1500 is used for the diagnostic assessment of body skin lesions, whereas VivaScope 3000 is used for the diagnosis of skin lesions on the head or neck). Thus, the lower purchase price and annual maintenance costs for VivaScope 3000 as an add-on device to VivaScope 1500 were utilised in the estimation of costs in all economic analyses that included any of the diagnostic ‘part’ economic models. In contrast, mapping of LMs prior to surgical treatment can be achieved only with the use of VivaScope 3000. Therefore, in the analysis that assessed the cost-effectiveness of VivaScope used exclusively for presurgical margin delineation of LMs, the purchase and annual maintenance costs of VivaScope 3000 as a stand-alone device were used.
The purchase price of VivaScope 1500 and 3000 was annuitised over the expected lifetime of the technology, which was reported by the company to be 10 years. The equivalent annual cost was calculated from the purchase price of the technology and the useful life of the equipment, as advised by the company, using an inflation rate of costs of 3.5%.
The costs of parts refer to the cost of the tissue ring and the cost of a cap for VivaScope 3000. The purchase price of VivaScope includes two tissue rings and two caps. Extra parts need to be purchased only in the event of loss or damage and, therefore, were not considered in the estimation of the total cost of VivaScope.
The consumables required for an examination of a skin lesion with VivaScope include, according to the manufacturer, an adhesive window that is attached to the lesion only for examination with VivaScope 1500; crodamol oil (Croda International Plc, Goole, UK) used as a lubricant; Alcotip sachets (Universal Hospital Supplies, Enfield, UK), which are used for the preparation (disinfection) of the skin; and ultrasound gel. MAVIG GmbH (Munich, Germany), the company that manufactures VivaScope, provided the cost of adhesive windows. For the other consumables, a small cost per lesion examined was assumed, estimated after considering the market prices of the consumables and the fact that only a small portion of each is required per lesion examination.
Training on the use of VivaScope consists of the following (information provided by the company, supplemented by one of the experts providing the training):
-
Introductory training This is provided on-site for free with the purchase of VivaScope, lasts approximately 1–2 days and involves mainly technical training, although some basic clinical information is also offered. The purpose of training is to teach technicians and clinicians (consultant dermatologist, consultant dermatological surgeon, technical assistant, pathologist and researcher) how to use the machine and the software correctly, how to identify the anatomical location of the image on the monitor and how to detect the most common and evident structures. Participants are given information on image acquisition, data management, operational precautions, etc. The training course consists of presentations, the review of manuals, the discussion of imaging guidelines and the consideration of appropriate studies of interest.
-
Independent study with textbooks This is complementary to the introductory training; VivaScope users are expected to revise two sophisticated imaging textbooks.
-
Intensive expert training This is also provided free with the purchase of VivaScope and follows the introductory training and independent study. It is a 3-day course currently offered four times a year at the University of Modena and Reggio Emilia in Italy, but there are plans to expand it to referral centres in Europe, including the UK. Four confocal experts who have been working with the VivaScope for > 10 years in Italy provide the training. They guide participants through the diagnosis of melanocytic lesions, non-melanocytic lesions, inflammatory skin diseases, cosmetic applications and others. It is considered an essential part of the training.
-
Online training course Provided free with the purchase of VivaScope, this course consists of 100 cases, with expert evaluation made available after student evaluation. It is considered part of the intensive expert training. The aim of this course is to establish the learning and test the trainee’s skills.
According to clinical expert opinion, after this ‘first-degree’ level of training, which usually lasts 3–5 weeks, trainees are able to recognise features, describe cases and identify diagnoses following algorithms, but they cannot be considered fully trained for routine activity (i.e. they cannot fully achieve the clinical advantages offered by optimal use of VivaScope). Clinicians trained in the use of VivaScope will need to develop their skills further and gain experience and a good level of confidence in interpreting VivaScope images before they achieve the outcomes described in the literature following examination of skin lesions with VivaScope.
At the University of Modena and Reggio Emilia in Italy, this ‘first degree’ of training is followed by a ‘second degree’ of training, consisting of an intense teaching programme, with a duration ranging from 3 to 5 months. This includes a total of approximately 30 hours of teaching (including a short basic course on histopathology), 50 hours of ‘tutored cases’ (case review with an expert and group discussion of the cases) and 100 hours of activities in the skin cancer unit (systematically using confocal microscopy). After this programme, the trainees should achieve a consistent increase in confidence (translated into clinical benefits from VivaScope use that is comparable to literature data), and also a reduction in some initial mistakes in the management of difficult situations (such as pink lesions, undefined papule/nodules, etc.).
According to clinical experts with experience in the use of VivaScope, the overall training required for a clinician to reach a good level of confidence and expertise, is between 4 and 6 months’ time, and approximately 1000–2000 cases evaluated with confocal microscopy in a setting including a sufficient number of melanomas (> 200). This is broadly consistent with the view expressed by Pellacani et al. ,46 according to which ‘a minimum 6 months full-time training, including the evaluation of more than 4000 cases, is required to obtain adequate levels of diagnostic accuracy and confidence’.
Further to the above training, MAVIG GmbH indicated the availability of the following services:
-
Online expert tutorial Clinicians may send very difficult confocal cases arising uring the daily clinical practice to a confocal expert for a ‘second opinion’. In this way, clinicians may expand their knowledge and increase their ability to diagnose difficult-to-assess lesions with a high degree of reliability and accuracy. This service is intended as an educational tool and requires a revised VivaNet telemedicine service (Lucid, Inc., Rochester, NY, USA).
-
Independent international circle of experts This is a group of expert VivaScope users that offers interdisciplinary discussions in order to establish confocal laser scanning microscopy as the standard in the dermatological diagnosis.
Estimation of training costs and staff time required for examination of skin lesions with VivaScope has been based on the information described above regarding the training courses available and expert advice, according to which a VivaScope facility run by a skin cancer MDT service requires staffing with a band 7 radiographer, who is sufficiently qualified to interpret images, and a well-trained consultant dermatologist or specialist registrar.
The estimation of training costs for the purpose of this evaluation has been based on the information provided by the company regarding the ‘first-level’ training (introductory training and intensive expert training course). No course fees have been considered, as both courses are provided free with the purchase of VivaScope. In terms of staff time, 1.5 days of two radiographers and two dermatologists (for the introductory training) and a further 4 days of two dermatologists (for the 3-day intensive expert training plus travel time to/from Italy) were included in the cost. Moreover, £2000 of travel, hotel and subsistence costs for each dermatologist attending the intensive expert training was included in the estimation of training costs.
It should be noted, however, that the estimate of training costs above does not take into account the substantial further time of ongoing training during routine clinical practice (about 3–5 months) that is required before dermatologists acquire enough confidence and expertise to achieve the full clinical benefits resulting from the use of VivaScope. This means that the conclusions of the economic analysis undertaken to support this report, which has utilised optimal diagnostic accuracy data for VivaScope (as reported in relevant applications), are applicable after dermatologists using VivaScope obtain a good level of expertise (i.e. at about 3–5 months of routine clinical practice following training) in order to achieve the outcomes reported for VivaScope in the literature.
No further training costs for new radiographers and dermatologists using VivaScope in the future were considered, as it has been assumed that the radiographers and dermatologists who were originally trained can subsequently train and pass their experience on to new colleagues expected to use the device on-site and during routine clinical practice.
The total estimated training costs were annuitised over 10 years (equal to the expected lifetime of the device). The equivalent annual cost was calculated using an inflation rate of 3.5%.
In terms of staff time, clinical experts with experience in using VivaScope indicated that examination of skin lesions suspicious of cancer with VivaScope 1500 requires 10 minutes of a radiographer’s time (from the time the patient enters the consultation room until the end of visit, including a radiographer’s time for attaching the adhesive window and obtaining the image) plus 5 minutes of a dermatologist’s time for evaluation of images. Examination of skin lesions suspicious of cancer with VivaScope 3000 requires 10 minutes of a dermatologist’s time (from the time the patient enters the consultation room until the end of the visit, including a dermatologist’s time for obtaining and interpreting the image). In the case of patients with more than one suspected lesion, it was assumed that 50% of a radiographer’s and dermatologist’s time (from the time the patient enters the consultation room until the end of the visit) was fixed, and the remaining 50% was attributed to each lesion examined. Mapping of LMs with VivaScope 3000 prior to surgical treatment requires 30 minutes of a dermatologist’s time.
The unit costs of radiographers and dermatologists were taken from national sources. 65 The unit cost of a hospital band 5 radiographer was £38 per hour in 2014, based on the mean full-time equivalent basic salary for Agenda for Change band 5 for qualified allied health professionals of the July 2013–June 2014 NHS staff earnings estimates. This unit cost included salary (considering also overtime, shift work and geographic allowances) and salary oncosts, capital and other overheads, and qualification costs. The mean annual basic pay of Agenda for Change band 7 qualified allied health professionals is approximately 63% higher than the corresponding pay at Agenda for Change band 565 and, therefore, the unit cost for a band 7 radiographer was estimated to equal £62 per hour (this was estimated by the EAG, as no relevant figures were available in the literature).
The unit cost of a medical consultant was £140 per contract hour in 2014, including, as above, salary and salary oncosts, capital and other overheads, and qualification costs. 65 The unit cost of a specialist registrar was not available (a mean unit cost was available for the registrar group, which comprise a heterogeneous group of registrars, senior registrars, specialist and specialty registrars). The unit cost of an associate specialist was reported to be £124 per hour (under a 40-hour week). For costing purposes, the economic analysis assumed that a consultant dermatologist performs the clinical examination with VivaScope.
The equivalent annual purchase and training and annual maintenance costs of VivaScope were each divided by the annual number of cases (skin lesions) expected to be examined with VivaScope 1500 or VivaScope 3000 for either diagnosis or margin delineation in a skin cancer MDT service in order to distribute these costs across the lesions examined and estimate an annual fixed and training cost per examined lesion. The cost of adhesive windows was omitted from the cost of suspicious skin lesions on the head or neck, as these are examined with VivaScope 3000. As reported in Introduction: overview of methods, it was estimated that approximately 100 suspected melanomas with equivocal dermoscopy findings, 500 lesions suspicious of BCC and 75 diagnosed LMs prior to surgical treatment are eligible for examination with VivaScope by a skin cancer MDT service over 1 year. The percentage of equivocal lesions suspicious of melanoma and of suspected BCC lesions that are on the head or neck was estimated to be 17.9% and 69.4%, respectively.
The economic analysis that assessed the integrated use of VivaScope 1500 and 3000 for the diagnostic assessment of equivocal lesions suspicious of melanoma considered exclusively a number of 100 lesions per year. The analysis that assessed use of VivaScope for diagnostic assessment of lesions suspicious of BCC used a number of 500 lesions per year. The economic analysis considering diagnostic assessment of both types of lesions suspicious of skin cancer with VivaScope assumed an annual number of 600 lesions. The analysis that evaluated the use of VivaScope 3000 in the margin delineation of LMs prior to surgical treatment assumed an annual number of 75 lesions. Finally, the overall analysis of all three uses of VivaScope imaging system that were considered in the economic modelling undertaken for this report utilised an annual number of 675 lesions eligible for examination with VivaScope.
The cost of VivaScope examination per skin lesion examined, by type of skin lesion and analysis considered, is shown in Table 24.
| Characteristics and cost elements of VivaScope 1500 and 3000 | Value |
|---|---|
| Purchase price of VivaScope (no VAT)a | VivaScope 1500 (dermoscope and RCM integrated): £90,224 VivaScope 3000 as an add-on to VivaScope 1500: £41,600 VivaScope 3000 as stand-alone device: £62,300 Combined purchase of VivaScope 1500 and VivaScope 3000: £131,824 |
| Equivalent annual capital cost (assuming 3.5% interest rate and using a 10-year lifetime of equipment, as advised by company) | VivaScope 1500 and VivaScope 3000 as an add-on: £15,315 VivaScope 3000 as stand-alone device: £7238 |
| Annual maintenance cost | £4100 for VivaScope 1500 or VivaScope 3000 as stand-alone device £1400 for VivaScope 3000 as an add-on to VivaScope 1500 |
| Costs of parts (incurred only in case of loss of parts): Tissue ring: £55 (two tissue rings provided with the device) Cap for VivaScope 3000: £192 (two caps provided with the device) |
Not included in the estimation of cost per lesion |
| Costs of consumables: Adhesive windows £147/box (containing 100; needed only for VivaScope 1500) Crodamol oil (lubricant): £7.80 per bottle Alcotip (disinfectant): £1.85 per 100 sachets Ultrasound gel: £3.20 per tube |
£2.97 per lesion examined with VivaScope 1500 £1.50 per lesion examined with VivaScope 3000 (rough estimates) |
| Training cost [includes 1.5 days of introductory training (× 8 hours) for two radiographers band 7, 1.5 days of introductory training + 3 days of intensive expert training (× 8 hours) for two consultant dermatologists, 1 day of travel time for two consultant dermatologists to attend the expert training course and £2000 for travel, hotel and subsistence per consultant dermatologist attending the expert training in Italy] Costs associated with further ongoing training during routine clinical practice (about 3–5 months), which is required so that dermatologists obtain a good level of confidence and expertise in the use of VivaScope, have not been included in the estimated training cost |
£17,816 |
| Equivalent annual training cost (assuming 10 years of training ‘lifetime’ and 3.5% interest rate) | £2070 |
| Mean staff cost per lesion examined Diagnostic assessment VivaScope 1500: 10 minutes of radiographer band 7 + 5 minutes of consultant dermatologist VivaScope 300: 10 minutes of consultant dermatologist Margin delineation of LM: 30 minutes of consultant dermatologist Unit cost of radiographer band 7: £62 per hour, using the unit cost of radiographer band 5 and the ratio of salary of band 7 to band 5 AfC for qualified allied health professionals Unit cost of consultant dermatologist: £140 per hour of contract |
Diagnostic assessment with VivaScope 1500: £22 Diagnostic assessment with VivaScope 3000: £22 for BCC; £23 for melanoma Mapping with VivaScope 3000: £70 |
| Number of lesions examined per year | Suspected melanomas with an equivocal finding on dermoscopy: 100 Suspected BCC with a positive dermoscopic result: 500 LMs assessed for margin delineation: 75 |
| Proportion of lesions suspicious of cancer on the head or neck, that would be suitable for examination with VivaScope 3000 | Suspected melanomas with an equivocal finding on dermoscopy: 17.9% Suspected BCC with a positive dermoscopic result: 69.4% |
| Total cost of VivaScope examination per lesion examined | Per suspected melanoma:
|
Diagnostic economic model on suspected melanoma lesions following an equivocal finding on dermoscopy: methods
Study population
The study population for this model comprised people with lesions suspicious of melanoma and an equivocal finding on dermoscopy.
The BAD guidelines on management of cutaneous melanoma define populations at greatly increased risk of melanoma (> 10 times that of the general population). 13 These include people with a giant congenital pigmented hairy naevus (such as ≥ 20 cm in diameter or 5% of body surface area), people with a strong family history of melanoma or pancreatic cancer (three or more family members), people with two family members affected with melanoma who also have the atypical mole syndrome, or a history of multiple primary melanomas in an individual or pancreatic cancer. This very high-risk subgroup of patients requires regular monitoring (approximately every 6 months), often over a lifetime, as the risk of some of their skin lesions being malignant or their risk of developing a new melanoma over time is high. In very high-risk patient subgroups with multiple lesions, current practice is selection and excision of a number of lesions based on dermoscopy and clinical judgement, and monitoring of the remaining lesions, as it is not possible to excise all suspicious lesions. If a melanoma is not identified, it will probably be picked up during routine monitoring within 6 months to 1 year. Examination with VivaScope would be beneficial in this subgroup of patients, as it would help identify melanomas among the suspicious lesions so that they are excised earlier rather than later and also would help avoid unnecessary diagnostic biopsies of non-malignant lesions. These very high-risk subpopulations were not considered in the economic model as their management (routine monitoring) differs from that of the ‘average’ population with suspected melanoma; populations at greatly increased risk of melanoma make up a very small proportion of people at risk of melanoma, whose management, nevertheless, can be very resource intensive.
Other categories of moderately increased risk patients (approximately 8–10 times that of the general population) include organ transplant recipients, those with either a previous primary melanoma or a large numbers of moles, some of which may be clinically atypical changing naevi, as well as people with other risk factors for melanoma (e.g. aged ≥ 50 years, prior history of cancer), for whom long-term follow-up is not routinely recommended, were included in the study population of the analysis.
The mean age of the study population, that is, people with suspected melanoma with an equivocal finding on dermoscopy, was assumed to be the same as that of the population receiving a diagnosis of melanoma. Clinical expert advice was that the mean age of people with equivocal lesions suspicious of melanoma does not differ from the mean age of people with lesions suspicious of melanoma in general. Malignant melanoma incidence is related to age, but it has an unusual pattern compared with other types of cancer. In the UK, between 2008 and 2010, an average of 27% of cases were diagnosed in those aged < 50 years, and an average of 45% of cases were diagnosed in those aged ≥ 65 years. 2 Age-specific incidence rates increase steadily from around age 20–24 years, reaching a peak at age ≥ 85 years for both sexes (with the increase being sharper for males from age 55–59 years onwards). 2 The mean age of patients at presentation of melanoma has been reported to be 55 years, although different types of melanoma typically present at different ages. 77 A retrospective study of 1769 people with melanoma who had been referred to a tertiary centre in London from 1999 to 2012 showed that the mean age of patients was 58 years. 78 Using the available information, the age of the study population in the economic model was assumed to be 55 years.
In 2011 there were 13,348 new cases of melanoma in the UK, 6495 of which were in males,2 so that the proportion of men in the population of people with newly diagnosed melanoma was 48.7%. This figure was used in the economic model to reflect the percentage of men in the population with suspected melanoma following an equivocal finding on dermoscopy as well as the population of men with (identified or non-identified) melanoma.
The proportion of confirmed melanomas on the head or neck is approximately 14% in females and 22% in males. 71 These figures were also used to reflect the proportion of suspected and confirmed melanomas with an equivocal finding that are on the head or neck in women and men, respectively.
Each person with suspected melanoma may present with more than one equivocal lesion on dermoscopy, although clinical experts advised that the majority of people present with only one lesion suspicious of melanoma. In studies included in the systematic review of clinical evidence that assessed the diagnostic accuracy of VivaScope in identification of melanomas among equivocal lesions and reported both number of study participants and number of equivocal lesions, the number of suspected melanomas with an equivocal finding per person ranged from 1.0030 to 1.17. 42 However, the number of confirmed melanomas per person reported in the studies was 1, and this was in agreement with clinical expert opinion. For simplicity purposes, the economic model assumed that every person presents with one suspected melanoma with equivocal finding on dermoscopy.
The annual number of equivocal lesions suspicious of melanoma examined at a dermatology MDT service in the UK was estimated to be approximately 100, as reported in Annual number of cases eligible for examination with VivaScope in a dermatology multidisciplinary team clinic in the UK.
Intervention and comparator
The intervention assessed in this model was VivaScope 1500 (for body lesions) and VivaScope 3000 (for lesions on the head or neck) for the diagnostic assessment of skin lesions suspicious of melanoma, following an equivocal finding on dermoscopy. The comparator was routine management of equivocal lesions suspicious of melanoma, comprising excision and biopsy for the majority of the equivocal lesions (highly suspicious lesions) and monitoring for the rest of them (moderately/low suspicious lesions). Monitoring consisted of one outpatient dermatology visit at 3 months, followed by discharge if there was no indication of melanoma.
Model structure
A decision tree followed by a Markov model was constructed to assess the cost-effectiveness of VivaScope in the diagnosis of people with lesions suspicious of melanoma following an equivocal finding on dermoscopy. According to the model structure, which was determined by clinical expert advice and the availability of relevant data, people aged 55 years with dermoscopically equivocal lesions suspicious of melanoma were either examined with VivaScope 1500 or VivaScope 3000 as appropriate (according to the location of the lesion) or received routine management, comprising excision and biopsy of the majority of the suspicious lesions and monitoring of a smaller proportion of less suspicious ones.
The model assumed that confirmed cases of skin cancer are of the same type of cancer as initially suspected (in the case of this model, melanoma), although occasionally skin cancers identified may be of a different type from that initially estimated by the clinician via dermoscopy.
People whose lesions were examined with VivaScope received the results of the examination immediately. Lesions found to be positive on VivaScope examination underwent excision and biopsy. The results of the biopsy were received 2 weeks after excision. Lesions found to be TP (i.e. confirmed on biopsy to be melanoma) were further treated as melanoma according to stage, as recommended by national guidelines. Those that were FP (i.e. biopsy showed it was not a melanoma) were assumed to be a benign tumour that did not require treatment and patients were discharged after the (unnecessary) excision and biopsy. People whose lesions were found to be negative on VivaScope examination were discharged and advised to visit their GP if they noticed changes in their skin lesion. If the lesions were TN (i.e. not melanoma), then they were assumed to be a benign tumour that did not require treatment. If the lesions were FN (i.e. an unidentified melanoma), it was assumed that the patient would return to the service at a later time once the melanoma had potentially progressed to a more advanced stage.
People who received routine management, whose lesions were excised and biopsied, received the results of biopsy 2 weeks after the excision. Those who had a positive result (i.e. their lesion was confirmed be a melanoma) were treated for their melanoma according to its stage, as recommended by national guidelines. Those who had a negative result (i.e. biopsy showed that their lesion was not a melanoma) were assumed to have a benign tumour that did not require treatment and were discharged after the (unnecessary) excision and biopsy. People under routine management who were selected for monitoring attended an outpatient dermatology follow-up appointment at 3 months for re-evaluation of their lesion. If the lesion was found to be suspicious of melanoma, it underwent excision and biopsy, which was followed by either further appropriate treatment, if biopsy confirmed the presence of malignancy, or discharge, if the result of biopsy was negative. If at the follow-up appointment the lesion was found not to be suspicious, patients were discharged and advised to visit their GP if they noticed changes in their skin lesion. If the lesion was not malignant, patients were assumed not to require further treatment. If the lesion was malignant but was not identified at the follow-up meeting, it was assumed that the patient would return to the service at a later time once the melanoma had potentially progressed to a more advanced stage. However, if a malignant lesion was identified at the 3-month follow-up meeting, it was assumed not to have progressed to a more advanced stage.
All people undergoing excision and biopsy of their lesion experienced distress because of the procedure; they also experienced anxiety while waiting for the results of biopsy, whether or not they had been examined with VivaScope prior to excision. People with a FP result after VivaScope examination experienced anxiety thinking that they have melanoma, until the results of biopsy were available.
Following the outcome of the diagnostic assessment, people entered a Markov model and followed one of the following pathways:
-
Patients with a confirmed melanoma (i.e. those with a TP result after VivaScope examination and subsequent excision and biopsy, as well as those who, under routine management, had a positive result after excision and biopsy, either immediately or following monitoring) entered a Markov chain of ‘identified melanomas’. All melanomas were assumed to be in situ or stage I (Ia or Ib) at identification, as clinical experts advised that an equivocal finding on dermoscopy suggests early stages of melanoma. All identified melanomas were treated in accordance with national guidelines and were assumed not to progress to a more advanced stage. Patients with an identified melanoma had a reduction in their HRQoL. A proportion of those who had a melanoma on their head or neck experienced an additional permanent reduction in their HRQoL because of the scarring following excision and biopsy. Patients with an identified melanoma stage Ib were at increased risk of mortality, because of their melanoma, for the first 10 years following identification of their melanoma. After the period of 10 years, the risk of mortality of people with identified melanomas returned to that of the general population of the same age. People dying because of their melanoma were assumed to become terminally ill in the year in which they died.
-
Patients with a missed melanoma (i.e. those with a FN result after VivaScope examination, as well as those who, under routine management, were selected for monitoring and were not identified) entered a Markov chain of ‘non-identified melanomas’. All melanomas were assumed to be in situ or stage I (Ia or Ib) at the point of examination; however, they could progress to more advanced stages over time. Every year patients could remain in their undiagnosed status with their melanoma remaining at the same stage or progressing to the next stage (without incurring any costs for its management), or could return to the dermatology service because of changes in their lesion and be diagnosed and treated, or die because of their cancer. Clinical experts advised that any unidentified melanomas would be recognised by the time they reached stage II (IIa, IIb or IIc), and within 5 years at maximum after the initial examination that resulted in the equivocal dermoscopic finding. People with an unidentified melanoma had a HRQoL equal to that of the general population of the same age, until their melanoma was identified, in which case they experienced a reduction in their HRQoL. A proportion of those who had an identified melanoma on their head or neck experienced an additional permanent reduction in their HRQoL because of the scarring following excision and biopsy. Unidentified melanomas did not incur any costs; identified melanomas were treated in accordance with national guidelines and were assumed not to progress to a more advanced stage. People with an unidentified or identified melanoma at stage Ib or II were at increased risk of mortality, because of their melanoma, from the start of the model and for the first 10 years after identification of their melanoma. After that period, their risk of mortality became equal to that of the general population of the same age. People dying because of their melanoma were assumed to become terminally ill in the year in which they died. People with newly identified melanomas entered tunnel states over a period of 10 years, so that the time-dependent risk of mortality over that period could be applied. In this aspect, the economic model was not a Markovian one under a strict definition, given that tunnel states allowed the model to keep memory of the time period people spent with melanoma, once this was identified.
-
People without a melanoma (i.e. those with a FP or TN result after VivaScope examination, as well as those who, under routine management, had a negative result after excision and biopsy, either immediately or following monitoring) entered a Markov chain of ‘no melanomas’. A proportion of people with a benign lesion on their head or neck, who had undergone unnecessary excision and biopsy, experienced a permanent reduction in their HRQoL because of the resulting scarring. Otherwise, the HRQoL in this Markov chain and the mortality risk were equal to that of the general population of the same age.
The care pathways described above were adapted from Wilson et al. ,48 who developed an economic model to assess the cost-effectiveness of a device aiming at the diagnostic assessment of pigmented skin lesions in primary care in the UK. The pathways designed for the model developed for this report were finalised following clinical expert advice.
Management of identified melanomas comprised surgical excision with a wider and deeper margin for all melanomas, SLNB for 50% of melanomas of stage Ib and all stage II melanomas, and follow-up visits. Patients dying because of their cancer incurred terminal disease costs in the year in which they died. These included costs of radiological examination, costs of metastatic disease (costs of chemotherapy including costs of adverse events), inpatient care and outpatient attendances, as well as costs of terminal and palliative care.
The time horizon of the economic model was over a lifetime (up to 100 years of age). The cycle length of the Markov model was 1 year and half-cycle correction was applied.
Figure 6 shows a schematic diagram of the VivaScope diagnostics model on suspected melanoma following an equivocal finding on dermoscopy.
FIGURE 6.
Schematic diagram of the VivaScope diagnostics model on suspected melanoma following an equivocal finding on dermoscopy. (a) Decision tree component; (b) progression of people with melanomas following identification (VivaScope TP or identified at biopsy or during monitoring); (c) progression of people with melanomas following initial non-identification (VivaScope FN or not identified during monitoring); and (d) progression of people without melanoma (VivaScope FP or TN, or identified as negative following biopsy or monitoring).
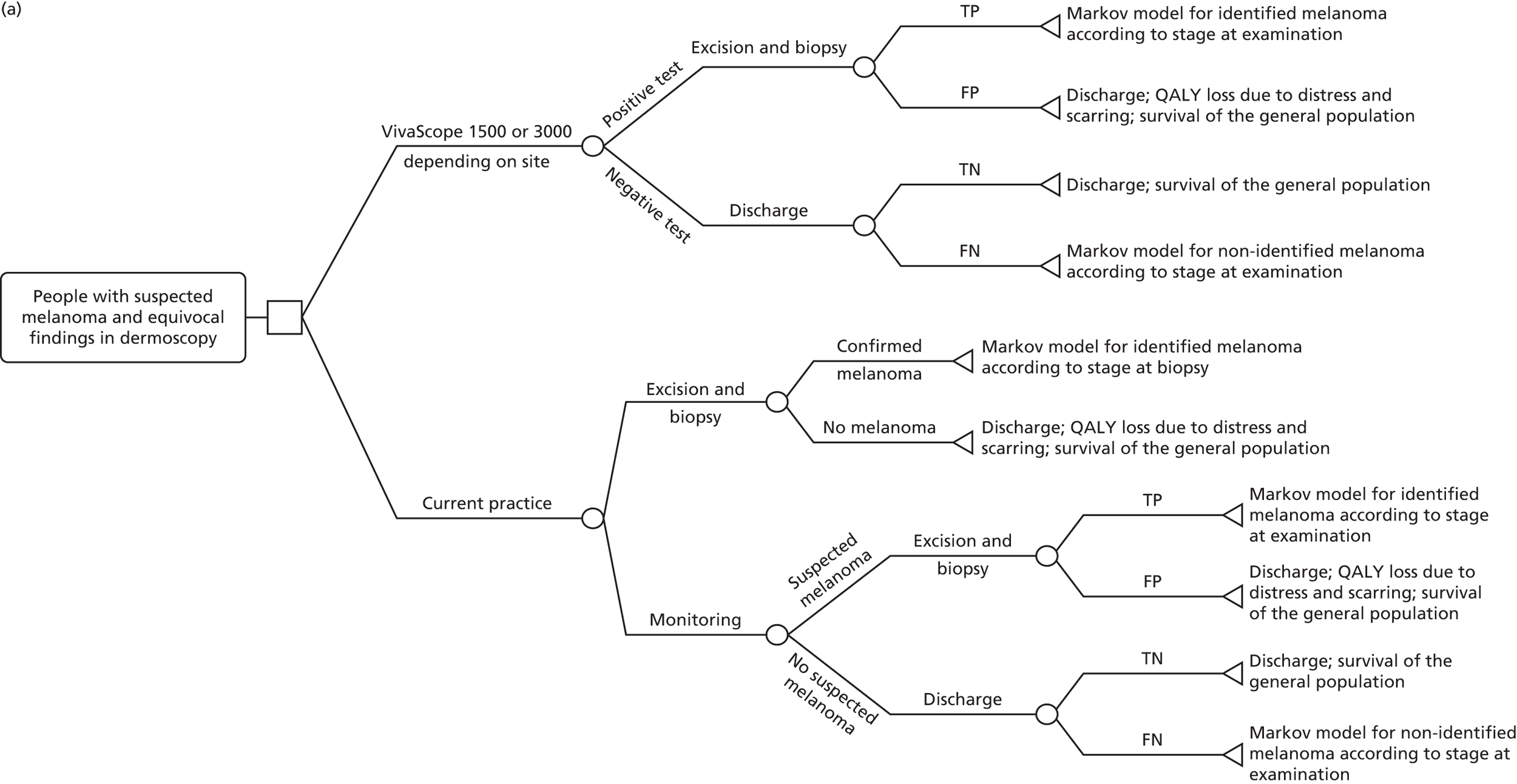


Clinical input parameters
Diagnostic accuracy data
Diagnostic accuracy data for VivaScope were based on the findings of the systematic review of clinical evidence reported in Chapter 3, Results of the assessment of clinical effectiveness. As diagnostic accuracy data were not synthesised, the base-case economic analysis utilised data on the diagnostic accuracy of VivaScope 1500 in people with equivocal lesions suspicious of melanoma from Alarcon et al. 30 and Pellacani et al. 42 in two separate analyses, as these two studies were considered to be the most representative of the UK setting (see Chapter 3, Clinical effectiveness results). The diagnostic accuracy of VivaScope 3000 in equivocal lesions suspicious of melanoma was assumed to be equal to that of VivaScope 1500 in the economic model because of the lack of relevant data specific to VivaScope 3000. However, it is acknowledged that this assumption, which was applied to 17.9% of the study population who had equivocal lesions on their head or neck, may have overestimated the diagnostic accuracy of VivaScope 3000.
In Alarcon et al. ,30 the sensitivity and specificity of VivaScope 1500 in identifying malignant lesions in people with equivocal lesions suspicious of melanoma was 97.8% and 94.8%, respectively. These figures were used for people with equivocal lesions that would have been excised under routine care, as well as for those with equivocal lesions that would have been selected for monitoring under routine care. However, it is acknowledged that diagnostic accuracy may differ across different subpopulations, as it may be affected by the prevalence of the disease. 79
In Pellacani et al. ,42 the sensitivity and specificity of VivaScope 1500 in identifying malignant lesions in people with highly suspicious equivocal lesions (i.e. lesions with consistent suspicious clinical/dermoscopic criteria, already qualified and scheduled for surgical excision) was 100% and 51.8%, respectively, whereas in people with moderately/low suspicious equivocal lesions (i.e. lesions where VivaScope examination would determine whether to excise or monitor digitally) the figures were 100.0% and 80.2%, respectively. 42 These two sets of diagnostic accuracy values were applied to patients with suspected melanomas that would be routinely excised and monitored, respectively. The overall sensitivity and specificity of VivaScope 1500 in people with equivocal lesions suspicious of melanoma was 100.0% and 70.8%, respectively.
Excision and biopsy was considered in the economic model to be the gold standard for the diagnosis of melanoma (i.e. it was assumed to have 100% sensitivity and specificity).
The outcomes of monitoring, in terms of identified and missed melanomas at 3 months, were taken from Altamura et al. ,80 who conducted a study to assess the optimal interval for, and sensitivity of, short-term sequential digital dermoscopy monitoring for the diagnosis of melanoma. The study included 1850 consecutive people with 2602 atypical skin lesions examined at a tertiary referral centre for melanomas whose lesions were monitored using short-term sequential digital dermoscopy imaging. Half of the patients were followed up 6 weeks after dermoscopy, followed by 3-month monitoring if changes were not seen. The remainder underwent 3-month monitoring only. Any change during this time led to excision. Lesions unchanged at 3 months were followed up over a period of time that ranged from 6 months to > 12 months from baseline. According to the study findings, over 3 months, 487 lesions showed changes in digital dermoscopy and were subsequently excised, of which 81 were melanomas (TPs) and 406 were benign lesions (FPs). Of the 2115 lesions that were negative at 3 months, nine proved to be melanomas at follow-up (FNs), 1118 showed no changes or showed changes but proved to be benign following excision (TNs) and 988 were lost to follow-up. Based on these data, the sensitivity and specificity of monitoring were estimated to be 90.0% and 73.4%, respectively.
Proportion of lesions excised versus monitored under routine management
Clinical experts advised that in UK routine clinical practice, about two-thirds of equivocal lesions suspicious of melanoma are excised and the remaining one-third are monitored, as they are less suspicious of malignancy.
Prevalence of melanoma in lesions with an equivocal dermoscopic finding
A review of the prevalence of melanoma in equivocal lesions suspicious of melanoma in relevant studies considered in the systematic review of clinical evidence reported in Chapter 3 gave the following results.
In Alarcon et al. ,30 the prevalence of melanoma in 343 equivocal lesions that were planned for excision was 26.8%. Curchin et al. 31 reported a very similar prevalence of melanoma in 50 equivocal lesions that were excised (26.0%). In Guitera et al. ,34 the prevalence of melanoma in 326 skin lesions that were excised on the basis of clinical suspicion was 37.7%. In Stanganelli et al. ,45 the prevalence of melanoma in equivocal pigmented lesions that lacked clear dermoscopy criteria for melanoma at baseline (all scoring 0–2 points at the 7-point checklist score) but were excised subsequently because of changes during digital monitoring was 17.1%.
In Pellacani et al. ,42 the prevalence of melanoma in 183 equivocal lesions with consistent suspicious clinical/dermoscopic criteria already qualified and scheduled for surgical excision was 12.6%; the prevalence of melanoma in 287 equivocal lesions (308 minus 21 that were lost to follow-up), where VivaScope examination would determine whether to excise or to monitor digitally, was 2.1%. The ratio of the prevalence of melanoma in highly versus moderately suspicious lesions was 6 : 1.
In Ferrari et al. ,44 the prevalence of melanoma in 130 featureless lesions with a 0–2 score on the 7-point checklist score on dermoscopy was 4.6%; in 102 positive borderline lesions with a score of 3 or 4, the prevalence of melanoma was 16.7%. The ratio of the prevalence of melanoma in positive borderline versus featureless lesions was 4 : 1.
Regarding the remaining studies included in the review, Gerger et al. 32 reported a 16.7% prevalence of melanoma in 117 melanocytic skin lesions and 45 non-melanocytic skin tumours examined with VivaScope, whereas the prevalence of melanoma among 70 melanocytic skin tumours included in the study by Gerger et al. 33 was 28.5%. In Langley et al. ,36 the prevalence of melanoma in 125 patients with 125 suspicious pigmented lesions was 29.6%. Rao et al. 39 reported a prevalence of 2.3% for melanoma in 334 lesions selected for removal for either cosmetic or medical reasons.
In Altamura et al. ,80 the prevalence of melanomas in 2602 atypical lesions selected for digital monitoring was 5.58%.
In Tromme et al. ,66 an observational study of people presenting to dermatologists because of their own concern for melanoma and having 1–3 equivocal melanocytic lesions, the prevalence of melanoma in 892 equivocal lesions observed in 822 people was 12.41%.
It should be noted that none of the above studies was conducted in the UK and, therefore, the overall prevalence of melanoma in the study populations may differ from that in the UK population, thus potentially affecting the prevalence of melanoma in equivocal lesions. Moreover, the categorisation of a skin lesion as ‘equivocal’ depends to a significant degree on the experience of the dermatologist undertaking the dermoscopic examination, and the definition of ‘equivocal’ across the studies. Clinical experts advised that, in the UK, out of five to six equivocal lesions that get excised because of dermoscopically equivocal findings, one is histopathologically confirmed to be a melanoma, translating into a prevalence of 16.7–20%.
The economic model utilised an overall prevalence of melanoma in equivocal lesions of 15.0%, and assumed that the prevalence of melanoma in suspicious lesions excised is five times the prevalence of melanoma in suspicious lesions selected for monitoring. In a sample of lesions of which two-thirds are excised and one-third is monitored, as advised by clinical experts for routine UK practice and utilised in the model, these figures and assumptions translate into a prevalence of melanoma of 20.6% in suspicious lesions excised and 4.1% in suspicious lesions selected for monitoring.
Stages of identified and missed melanomas
According to clinical expert opinion, melanomas that give an equivocal finding on dermoscopy are at early stages of development, most likely in situ or stage I, and this was also suggested by the available information in the studies included in the systematic literature review of clinical evidence. Following clinical expert advice, melanomas undergoing diagnostic assessment in the economic model were assumed to be 60% in situ and 40% at stage I. This estimate was applied to both men and women with melanomas that give an equivocal finding on dermoscopy. Melanomas that were not identified by VivaScope examination or after monitoring (i.e. FNs) were expected to be even less advanced; however, the exact staging of FN melanomas would be determined by the diagnostic characteristics of VivaScope and monitoring, and would require further assumptions for its estimation. For this reason, the staging of all melanomas giving an equivocal finding on dermoscopy was assumed to be the same for all melanomas (i.e. 60% in situ and 40% in stage I), regardless of the result (TP or FN) of VivaScope examination or monitoring.
Melanomas in stage I were further classified into stage Ia and stage Ib. Unidentified melanomas that progressed to stage II were further classified into substages IIa, IIb and IIc. Classification of melanomas into substages was essential, as management costs and mortality may differ between substages within the same stage. Initial proportions of melanomas in each substage (i.e. at the stage of identification or progression to the next stage) were estimated using data from Balch et al. ,81 who conducted a multivariate analysis of 30,946 patients with stage I, II or III melanoma and 7972 patients with stage IV melanoma to revise and clarify TNM (tumour, lymph nodes, metastasis) classifications and stage grouping criteria. The number and proportion of people in each melanoma substage are presented in Table 25. After that point in time, the proportion of people in each substage changed because of the different mortality characterising each substage.
| Stage | n (% within stage)a | Survival ratea | Annual probability of death in the model | ||
|---|---|---|---|---|---|
| 5 years | 10 years | Unidentified and first 5 years from identification | Next 5 years from identification | ||
| Ia | 9452 (51.5) | 0.97 | 0.930 | Sex- and age-adjusted mortality of general population | |
| Ib | 8918 (48.5) | 0.92 | 0.860 | 0.017 | 0.012 |
| IIa | 4644 (50.1) | 0.81 | 0.670 | 0.041 | 0.030 |
| IIb | 3228 (34.8) | 0.70 | 0.570 | 0.069 | 0.027 |
| IIc | 1397 (15.1) | 0.53 | 0.390 | 0.119 | 0.030 |
Progression
In the economic model, unidentified melanomas could progress by only one stage and never regressed. The annual rate of progression of unidentified melanomas is unknown, as no naturalistic data that would suggest the rate of progression in identified melanomas are available, as a lack of provision of therapy would be unethical. However, according to a report on the impact of earlier diagnosis of cancer to the NHS published by the Department of Health,82 the mean duration of stage I melanoma is 50 months. Assuming that, at 50 months, 50% of melanomas of stage I progress to the next stage and that progression to the next stage is characterised by exponential function, the annual probability of progression of melanomas stage I to stage II was estimated to be 15.3%. This annual probability was also applied to unidentified in situ melanomas progressing to stage I.
Clinical experts expressed the opinion that all unidentified melanomas should be identified when they reach stage II at the latest, and should have been detected by 5 years after the initial diagnostic assessment. These two hypotheses were broadly satisfied by using an annual probability of identification of 35% in the economic model, which appeared to be a reasonable estimate according to clinical experts. Any unidentified melanomas by year 5 were imposed to be identified at this point.
Mortality
The risk of mortality of people in the model depended on the status of their skin lesions following diagnostic assessment.
People with TN or FP lesions (i.e. people without melanoma) were assumed to have a normal lifespan and, therefore, their mortality rates were assumed to equal those of the UK general population in both arms of the model. Mortality in this group of patients was considered in order to allow estimation of the lifetime permanent disutility experienced because of scarring. Sex- and age-specific mortality rates were taken from recent UK national mortality statistics83 and were applied separately to men and women in every arm of the model. It is acknowledged that the mortality of people in the general population incorporates mortality because of melanoma, but given that the incidence of and mortality from melanoma are rather low in the general population in the UK (17.4 and 2.5 per 100,000 population, respectively),2 general population mortality rates were not adjusted to exclude deaths because of melanoma. Moreover, it is possible that people who had not developed melanoma at the start of the model could develop melanoma later in life and, therefore, applying the overall mortality of the UK general population, which incorporated the future risk of dying from a melanoma, appeared to be valid.
People with TP or FN lesions (i.e. people with identified or unidentified melanoma after the initial diagnostic assessment) were assumed to be at increased risk of mortality because of their melanoma. Balch et al. 81 reported the 5- and 10-year overall survival of patients with melanoma in each stage. These data were used to determine a mean annual mortality rate for years 1–5 and for years 6–10 for each substage, assuming an exponential survivor function (see Table 25). The overall annual mortality risk for stage Ia that was reported in Balch et al. 81 was very similar to the mean mortality of the UK general population of age 55–60 years (i.e. of the model study population over the first 5 years of the Markov model). Clinical experts confirmed that the mortality risk of people with stage Ia melanoma, as well as of people with in situ melanoma, is very close to that of the general population. Therefore, the economic model assumed that people with identified or unidentified melanoma in situ or stage Ia had the same mortality risk with the UK general population of the same sex and age, taken from UK national mortality statistics. 83
Patients with unidentified melanoma were assumed to be at increased mortality risk corresponding to the stage of their melanoma for the whole period over which their melanoma remained unidentified (i.e. maximum 5 years). Patients with identified melanoma were assumed to be at increased mortality risk because of their melanoma over 10 years (5 years at a higher mortality risk and another 5 years at a lower mortality risk, which was, nevertheless, higher than the mortality risk of the general population of same sex and age). The excess risk of mortality estimated by subtracting the sex- and age-specific UK general population mortality83 from the annual mortality risk derived from analysis of data in Balch et al. 81 was attributed to melanoma metastatic disease and was assumed to be associated with metastatic disease and terminal illness costs.
Beyond the 10 years from identification of melanoma, patients with melanoma were assumed to have survived their cancer and to have return to the mortality risk of the general population, according to their sex and age, although there is evidence that a small proportion of patients may present with metastatic melanoma > 10 years after they are diagnosed with melanoma. 84 However, the proportion of patients presenting with late recurrence of melanoma (beyond 10 years) was deemed to be small and, therefore, the assumption of complete cure from melanoma 10 years after identification was considered to be reasonable.
Utility values
People in the model experienced utility (or disutility) associated with one or more of the following:
-
disutility because of the excision and biopsy of a lesion suspected of melanoma that caused distress as well as anxiety while waiting for the results
-
disutility because of the permanent scarring following surgical excision of a lesion on the head or neck
-
health state-related utility, which was associated with the stage of melanoma (in people with melanoma) or with the average utility of the general population (in people without a melanoma).
As reported in Studies reporting utility data, the systematic literature review identified four studies reporting utility data relating to melanoma health states. 53–55,58 Beusterien et al. 54 reported utility data associated with partial response to treatment, stable or progressive disease, best supportive care and toxicity from chemotherapy. The health state descriptions were vignette based. Utility values were elicited from members of the general population in Australia and the UK using SG. The utility values reported by Beusterien et al. 54 referred to health states that did not directly correspond to melanoma stages and, therefore, were unsuitable for use in the economic model.
The remaining three studies53,55,58 reported utility values associated with melanoma stages. None of the studies was conducted in the UK. Two of the studies53,58 used EQ-5D for the description of HRQoL experienced by patients with melanoma. However, none of them used the UK EQ-5D tariff62 for the valuation of health states, as recommended by NICE. Askew et al. 53 used the US EQ-5D tariff, which was developed using TTO, whereas Tromme et al. 58 used the Belgian EQ-5D tariff, which was developed using the VAS – a valuation method that is not choice based and thus is not among NICE-preferred valuation methods. King et al. 55 reported melanoma-related utility values elicited from patients with melanoma in the USA; health state descriptions were based on vignettes. A comparison of the utility values reported in these three studies revealed inconsistencies in the available data. For example, the utility values reported by Askew et al. 53 for melanoma stages III and IV were considerably higher than those reported by King et al. 55 and Tromme et al. ;58 the utility values reported by Tromme et al. 58 for melanoma early stages I and II were substantially lower than the utility values reported for corresponding stages in Askew et al. 53 and King et al. 55 These discrepancies are potentially attributable to differences in measurement and valuation across the three studies. Quite importantly, the utility values reported for all melanoma stages (I–IV) in Askew et al. 53 and for stages I and II in King et al. 55 were considerably higher than reported mean utility values for the UK general population aged 55 years (which was the age of the study population at the start of the model): in Askew et al. ,53 the utility values of melanoma stages I–IV ranged from 0.91 in stages I and II to 0.86 in stage IV. King et al. 55 reported utility values of 0.93 and 0.92 for melanoma stages I and II, respectively. In contrast, Kind et al. ,85 who analysed EQ-5D data obtained from 3395 participants in the measurement and valuation of health survey conducted in the UK in 1993, reported a mean EQ-5D utility value for people in the UK aged 55–64 years of 0.78 for men and 0.81 for women. More recently, Sullivan et al. 86 produced a catalogue of EQ-5D utilities for the UK population by applying the UK EQ-5D tariff62 to EQ-5D descriptive questionnaire responses obtained from participants in the US-based Medical Expenditure Panel Survey. The mean utility value for people aged 50–59 years was 0.798. Consequently, the utility data reported in Askew et al. 53 and King et al. 55 appeared to lack face validity compared with UK population norms and could not be used in the economic model in their ‘raw’ form, as this would result in patients with melanoma having a higher utility than people without melanoma (who are expected to have the utility of the general population of same sex and age).
The other utility study under consideration was the one conducted by Tromme et al. 58 The study reported utility values associated with melanoma stages 0 (in situ)/Ia, Ib/II, III and IV, subdivided into treatment and remission phases. The reported utility values appeared to be sound when compared with mean utility values of the UK general population, as the utility values of treatment phase were always lower than the utility of the UK general population aged 55 years (mean age of patients at presentation of melanoma) and utility values of remission phase were lower than (stages III and IV), or comparable with (stages 0–II), the utility of the UK general population aged 55 years. Utility values reported in Tromme et al. 58 were estimated from EQ-5D responses using the Belgian EQ-5D tariff, which has been developed following a valuation survey of 2754 Flemish adults from the general public in Belgium using the VAS. 61 The Flemish EQ-5D tariff has shown good correlation with the UK EQ-5D tariff that was derived using the VAS,87 with a correlation coefficient of 0.979. 61 Owing to the lack of melanoma utility data more directly relevant to the UK population, melanoma-related utility values from Tromme et al. 58 were selected for use in the economic model.
The utility values obtained from Tromme et al. 58 were adjusted for age in the economic analysis, using a coefficient of –0.00029 per year that was reported by Sullivan et al. ;86 this study involved multiple regression analyses using ordinary least squares, the Tobit model and censored least absolute deviations regression methods and reported regression coefficients for a number of clinical conditions and demographic characteristics of the study population, including age.
Table 26 shows the patient characteristics and mean utility values by melanoma stage reported in Tromme et al. ,58 as well as the resulting utility values for patients with melanoma aged 55 years, after adjusting for age, that were used in the economic model.
| Stage | Time period | Number of respondents | Mean age (years) | Mean utility value (SD) | Mean utility values adjusted for age (55 years) |
|---|---|---|---|---|---|
| 0/Ia – treatment | Month 1 | 68 | 51.7 | 0.687 (0.192) | 0.6860 |
| 0/Ia – remission | Months 2–24 | 98 | 46.5 | 0.809 (0.179) | 0.8065 |
| Ib/II – treatment | Months 1–2 | 33 | 54.5 | 0.579 (0.272) | 0.5789 |
| Ib/II – remission | Months 3–24 | 76 | 53.2 | 0.802 (0.166) | 0.8015 |
| III – treatment | Months 1–3 | 15 | 55.9 | 0.535 (0.278) | 0.5849 |
| III – remission | From month 4 | 50 | 53.3 | 0.703 (0.156) | 0.6860 |
| IV – treatment | From start of treatment | 41 | 61.4 | 0.583 (0.192) | 0.8065 |
| IV – remission | From start of treatment | 14 | 64.8 | 0.796 (0.167) | 0.5789 |
The utility values of stages 0/Ia and Ib/II in remission reported by Tromme et al. 58 were very close to (only slightly higher than) the utility values of the UK general population aged 55 years reported by Kind et al. 85 and Sullivan et al. 86 Tromme et al. 58 reported that treatment duration in stages 0/Ia and Ib/II was 1 and 2 months, respectively, according to expert opinion. Using the treatment and remission utility data and the treatment duration for stages 0/Ia and Ib/II, it was estimated that the reduction in utility over the year within the melanoma was treated was –0.0100 for stage 0/Ia and –0.0371 for stage Ib/II. Tromme et al. 58 stated that patients with stage 0 and Ia melanoma were pooled together because they had very similar management in terms of surgical treatment and follow-up, whereas patients with stage Ib and II melanoma were pooled because they had all undergone SLNB that had not been followed by elective node dissection and because of evidence that surgical resection margins did not appear to influence HRQoL. Therefore, the values of –0.0100 for stage 0/Ia and –0.0371 for stage Ib/II were considered to express the disutility associated with surgical management of early stage melanoma which involved (–0.0371) or did not involve (–0.0100) SLNB in patients with melanoma aged 55 years. These values were applied as one-off disutilities in the economic model (i.e. they were applied once, at the time of treatment of melanomas, without time adjustment), after adjusting for age by applying the age coefficient of –0.00029 reported by Sullivan et al. ,86 for every year above 55 years of age. However, as only 50% of patients with stage Ib melanoma were assumed to undergo SLNB in the economic model, the value of –0.0100 (adjusted for age) was applied to all patients with identified melanoma of stage 0/Ia and 50% of patients with melanoma of stage Ib, and the value of –0.0371 (adjusted for age) was applied to 50% of patients with stage Ib melanoma and all patients with stage II melanoma. Apart from that disutility, which was attributed to surgical management and was applied as a one-off disutility, patients with identified melanoma in stages 0–II were assumed to have the average utility of the UK general population of the same age, which was derived from Sullivan et al. 86 as the utility of stages 0–II in remission reported in Tromme et al. 58 was very close to (in fact it was higher than) the utility of the UK general population reported in Sullivan et al. 86 This assumption is broadly consistent with the results of a German study, according to which the HRQoL in patients with melanoma, without recurrence within 2 years after initial therapy, was comparable to the HRQoL of the general population. 60
Patients with unidentified melanoma and people without melanoma were also assumed to have the average utility of the general UK population of the same age, as reported in Sullivan et al. 86
Table 27 presents the mean utility of the UK general population aged ≥ 55 years, as reported in Sullivan et al. 86 and applied in the economic model, as well as the characteristics of the US population providing responses to EQ-5D that were analysed by Sullivan et al. 86 in order to produce the catalogue of UK utility values. The mean EQ-5D utility for people aged 80–89 years was applied to all people aged ≥ 80 years in the economic model.
| Age (years) | Number of respondents | Mean number of clinical conditions | Median EQ-5D | Mean EQ-5D | SE |
|---|---|---|---|---|---|
| 50–59 | 14,333 | 2.4 | 0.796 | 0.798 | 0.0035 |
| 60–69 | 9028 | 3.1 | 0.796 | 0.774 | 0.0039 |
| 70–79 | 6789 | 4.0 | 0.727 | 0.723 | 0.0049 |
| 80–89 | 3593 | 4.4 | 0.691 | 0.657 | 0.0075 |
People with metastatic melanoma disease/terminal illness (i.e. people dying because of their melanoma) were assumed to have the utility of melanoma stage IV in treatment reported in Tromme et al. ,58 which was adjusted for age using the age coefficient of –0.00029 reported by Sullivan et al. ,86 for every year above 55 years of age.
People undergoing surgical excision and biopsy of their lesion were assumed to experience disutility because of distress as well as anxiety while waiting for the results of the biopsy. The distress because of the excision and biopsy experienced by people whose suspected lesion was melanoma was assumed to have been incorporated in the disutility associated with the surgical management of melanoma. The distress experienced by people whose suspected lesion was not melanoma (FN), who therefore did not proceed to surgical excision with wider margins, was expressed by a one-off disutility of –0.002, which was also used to express the disutility of a diagnostic biopsy for suspected BCC in the relevant economic model. As described later in Utility values, the disutility experienced because of surgical treatment of BCC was derived from Seidler et al. ,57 who reported a disutility of –0.004 associated with an excision procedure because of facial non-melanoma skin cancer. The economic model on lesions suspicious of BCC assumed that a diagnostic biopsy created a disutility of –0.002 to the person, as it was expected to be a less invasive procedure than surgical treatment of BCC (excision or Mohs surgery).
In addition to the distress directly associated with excision and biopsy, people undergoing excision and biopsy for their suspected melanoma lesion were considered to experience a reduction in their HRQoL because of anxiety while waiting for the results of biopsy. The methodology used to estimate the disutility associated with anxiety while waiting for results of biopsy was adopted from a model-based economic evaluation of intraoperative tests for detecting sentinel lymph node metastases in breast cancer88 that was undertaken to inform relevant NICE diagnostics guidance. In that economic model, patients who underwent histopathology experienced some level of disutility because of the associated anxiety of waiting for test results; this disutility was imputed by using the EQ-5D health state valuation equation for the UK reported by Dolan,62 which allows an estimation of a person’s utility based on their responses to the EQ-5D classification system. The system has five dimensions (mobility, self-care, ability to perform usual activities, pain/discomfort and anxiety/depression) and in the version used by Dolan each dimension had three levels of response (no problems, moderate problems and severe problems). Huxley et al. 88 used only the utility decrement because of anxiety/depression, which was expressed by the following equation:
where α = 0.081 is the constant applied to any level of disutility in any of the five EQ-5D dimensions, AD = 0.071 (for each level of disutility associated with anxiety or depression), A2 = 0.094 (for severe anxiety/depression) and N3 = 0.269 (when any of the five dimensions of EQ-5D is severe).
Huxley et al. ,88 as well as the economic model on the diagnostic assessment of equivocal lesions suspicious of melanoma with VivaScope, assumed that people waiting for histopathology results already had a utility of < 1 (so the α value was not applied at the estimation of the utility decrement because of anxiety/depression), that they moved from a state of no anxiety/depression to severe anxiety/depression, and that this anxiety/depression was the only dimension of the EQ-5D they had that was severe. These assumptions resulted in a disutility of –0.505 (i.e. –0.236 – 0.269 = –0.505).
This disutility of –0.505 was applied for only 2 weeks in the model, as clinical experts advised that biopsy results for suspected melanoma are available 2 weeks after excision and biopsy. This gave a 2-week disutility of –0.019 attributed to anxiety while waiting for the results of biopsy. This disutility was applied in every person waiting for results of biopsy, including people who had already undergone examination with VivaScope, people undergoing routine management of equivocal lesions suspicious of melanoma who had their lesions excised immediately or after 3-month monitoring, and people with missed melanomas (FN) who had them excised at a later stage following late identification.
A number of people in the model experienced permanent disutility because of scars on their head or neck from the excision of suspected melanomas. In the economic model it was assumed that 15% of people undergoing excision of their suspected melanoma lesion on their head or neck would experience permanent disutility because of their scar over their lifetime. Seidler et al. 57 reported a disutility of –0.016 for simple repairs/scars (granulation and primary closure) and a disutility of –0.026 for complex repairs/scars (local flap and graft) experienced by people with facial non-melanoma skin cancer. Owing to a lack of more relevant data, data from this study were used to express permanent disutility experienced by people with suspected melanomas on their head or neck because of scars from excision. Clinical expert advice was that all initial excisions of suspected melanomas are undertaken with simple repairs/scars; wider surgical excisions of confirmed melanomas make up 90% of simple and 10% of complex repairs/scars. Based on these estimates and the disutility data reported in Seidler et al. ,57 the permanent disutility from scarring following initial excision and biopsy (people with lesions that were not melanomas) and wider surgical excision (people with melanomas) was estimated to be –0.016 and –0.017, respectively. These disutilities were applied only to people with permanent reduction in their HRQoL because of scarring on their head or neck over their lifetime.
Table 28 provides all utility data applied in the diagnostic economic model on equivocal lesions suspicious of melanoma.
| Type of utility | Utility value | Relevant population in the model | Source of utility data and assumptions |
|---|---|---|---|
| General utility for: | Patients with stage 0–II melanoma (TPs and FNs that were identified at a later stage); patients with unidentified melanoma (FNs) and people without melanoma (TNs and FPs) | Sullivan et al.;86 applied over a lifetime, according to age | |
| 50–59 years | 0.798 | ||
| 60–69 years | 0.774 | ||
| 70–79 years | 0.723 | ||
| ≥ 80 years | 0.657 | ||
| Disutility because of the management of melanoma | –0.010 | All patients treated for in situ or stage Ia melanoma; 50% of patients treated for stage Ib melanoma | Tromme et al.;58 reported disutilities correspond to 55-year-old patients and were age adjusted in the model using an age coefficient of –0.00029;86 applied as one-off disutilities at the time of treatment |
| –0.037 | 50% of patients treated for stage Ib melanoma; all patients treated for stage II melanoma | ||
| Metastatic melanoma/terminal disease (stage IV) | 0.585 | All patients with identified or unidentified melanoma stage Ib or II dying because of their melanoma | Tromme et al.;58 reported value corresponds to 55-year-old patients and was age adjusted in the model using an age coefficient of –0.00029;86 applied in the year within which patients died because of their melanoma |
| Disutility because of the excision and biopsy | –0.002 | People without melanoma who underwent excision and biopsy (FP in VivaScope or monitoring and those undergoing excision under routine management) | Assumption used in the diagnostic model on suspected BCC lesions; value used to express distress because of the diagnostic biopsy; reported disutility experienced as a result of surgical treatment of facial BCC was –0.004 in Seidler et al.;57 applied as a one-off disutility |
| Disutility because of anxiety while waiting for results of biopsy | –0.019 | Any person waiting for results of biopsy, including people who had positive results in examination with VivaScope, people undergoing routine management who had their lesions excised immediately or after 3-month monitoring, and people with missed melanomas (FN) that were excised at a later stage, following identification | 2-week disutility because of anxiety/depression estimated using the EQ-5D UK health state valuation equation,62 assuming that people waiting for biopsy results had already utility of < 1, moved from no to severe anxiety/depression, and this was their only severe EQ-5D dimension |
| Permanent disutility because of scarring on head or neck | –0.016 | 15% of people with lesions on the head or neck who underwent initial excision and biopsy (people with lesions that were not melanomas) | Seidler et al.;57 initial excisions of suspected melanomas assumed to entail simple repairs/scars; wider surgical excisions of confirmed melanomas assumed to comprise 90% simple and 10% complex repairs/scars; applied over lifetime |
| –0.017 | 15% of people with lesions on the head or neck who underwent wider surgical excision (people with melanomas) | ||
Costs
Costs considered in this economic model included the cost of diagnostic assessment of a suspected melanoma with VivaScope following an equivocal finding on dermoscopy, the cost of routine management (cost of excision or monitoring of suspected melanomas), the management cost of confirmed melanomas (TPs) following diagnostic assessment, the cost of missed melanomas (FNs) that were identified at a later time and costs associated with metastatic melanoma and terminal illness.
As reported in Table 24, the cost per suspected melanoma with an equivocal finding on dermoscopy examined with VivaScope was estimated to be £254 if VivaScope is exclusively used for the diagnostic assessment of suspected melanomas with an equivocal finding on dermoscopy; £63 if VivaScope is used only for diagnostic assessment of suspected melanomas giving an equivocal finding on dermoscopy and suspected BCC lesions with a positive dermoscopic finding; and £59 if the device is used not only for the diagnostic assessment of suspected melanomas and BCCs, but also for the mapping of LMs prior to surgical treatment.
The costs of all other procedures and treatments included in the model, with the exception of the cost associated with terminal illness, were taken from either the NHS Reference Costs 2013 to 201412 for 2014 or the Unit Costs of Health and Social Care 2014. 65 Clinical experts advised on the appropriate NHS service, and procedure codes and unit costs corresponding to relevant health-care resource use considered in the model.
The unit cost of excision and biopsy of a lesion suspicious of melanoma was estimated to be £151, corresponding to the national unit cost of outpatient intermediate skin procedures conducted in a dermatology service for people aged ≥ 13 years (service code 330, currency code JC42A). 12
The unit cost of monitoring was £93 and corresponded to an outpatient, face-to-face, consultant-led dermatology follow-up attendance (service code 330, currency code WF01A). 12
The cost of management of melanomas after identification and confirmation with excision and biopsy (i.e. both melanomas identified at initial diagnostic assessment and melanomas missed and identified at a later time) comprised:
-
The cost of surgical excision with a wider and deeper margin for all melanomas (in situ, stage I and stage II), which was £943, corresponding to the national unit cost of an intermediate skin procedure treated as a day case for people aged ≥ 13 years (currency code JC42A). 12 Clinical experts advised that if skin grafts or flaps are required for the excision, the procedure becomes more complex and costly; however, the associated additional cost was not considered because of lack of relevant data.
-
The cost of SLNB for 50% of melanomas of stage Ib and all stage II melanomas. The unit cost of such a procedure was estimated to be £1033, corresponding to a day case procedure on the lymphatic system. 12 Clinical experts advised that this procedure is routinely carried out together with the wider excision and, therefore, it might be reasonable not to apply its unit cost as a separate cost component in the model; nevertheless, other experts advised that it can be a complex procedure, especially when performed in complicated nodal sites, for example in the groin or head and neck. Consequently, it was decided to apply the unit cost of £1033 as an extra cost in patients undergoing SLNB alongside the wide surgical excision of their melanoma.
-
The cost of follow-up visits: these comprised, according to the BAD guidelines:13
-
a single follow-up visit for patients with in situ melanomas, after complete excision, to explain the diagnosis, check the whole skin for further primary melanomas and to teach self-examination for a new primary melanoma
-
four 3-monthly visits in the first year after the excision of the melanoma for patients with stage Ia melanoma
-
3-monthly visits for 3 years and then 6-monthly visits to 5 years after the excision of the melanoma for patients with stage Ib or II melanoma.
-
The unit cost of a follow-up visit was £93 and corresponded to an outpatient, face-to-face, consultant-led dermatology attendance (service code 330, currency code WF01A). 12 It should be noted, however, that clinical experts advised that, in some hospitals, the follow-up of patients with melanoma is nurse led rather than consultant led.
The health-care resource use and associated cost of management of melanomas following excision and biopsy that was utilised in the economic model is presented in Table 29.
| Resource use/cost component | Stage | |||
|---|---|---|---|---|
| In situ | Stage Ia | Stage Ib | Stage 2 | |
| Surgical excision with a wider and deeper margin | £943 | £943 | £943 | £943 |
| Cost of SLNB | NA | NA | 50% of lesions: £1033 | £1033 |
| Follow-up visits | One-off: £93 | 3-monthly × 1 year: £372 | 3-monthly × 3 years and then 6-monthly × 2 years: £1488 | 3-monthly × 3 years and then 6-monthly × 2 years: £1488 |
| Total management cost | £1036 | £1315 | £2948 | £3464 |
The cost of people with unidentified melanomas was assumed to be zero, unless patients died because of their melanoma (in which case they experienced terminal disease before they died and incurred the corresponding costs) or until their melanoma was identified. Costs of identification included a GP visit at a cost of £67,65 an outpatient, face-to-face, consultant-led first attendance at a dermatology clinic for the reassessment of the skin lesion costing £109 (service code 330, currency code WF01B),12 and excision and biopsy for confirmation of the malignancy at a cost of £151.
The cost of terminal illness in the year in which patients died because of their melanoma (cost of management of metastatic disease and terminal care) was based on data reported in the NICE single technology appraisal of ipilimumab for previously untreated advanced (unresectable or metastatic) melanoma (NICE TA319). 89 Based on clinical expert advice, patients with metastatic melanoma and terminal disease in the model were assumed to be treated with either ipilimumab (50%), dacarbazine (15%) or vemurafenib (35%), with the proportions of patients on each drug being based on an economic analysis assessing the cost-effectiveness of adding routine imaging of asymptomatic patients to current standard follow-up in patients with stage III melanoma that was undertaken for the NICE guideline update on melanoma. 14 The drug acquisition costs of ipilimumab and vemurafenib to the NHS are subject to a Patient Access Scheme discount and, therefore, are not known; consequently, it was not possible to estimate the actual costs of chemotherapy to the NHS. The company submission for the NICE TA31989 reported the estimated total metastatic disease and terminal care costs associated with each of the three drugs over a lifetime, as well as the average number of life-years per person for each drug, so it was possible to estimate an average annual cost associated with each drug, although it is acknowledged that costs of chemotherapy and terminal illness are unlikely to be evenly spread across life-years. However, as total lifetime was not long (it did not exceed 3.5 years with any of the three drugs), the estimated mean annual cost was considered a reasonable approximation of metastatic disease/terminal illness cost over the last year of life of patients dying because of their melanoma in the economic model. The single technology appraisal cost figures were derived from a scenario included in the EAG report that considered drugs only as first-line treatments followed by best supportive care and palliative care. 90 These costs included drug acquisition costs, costs of adverse events, costs of radiological examination, inpatient care and outpatient attendances, as well as costs of terminal and palliative care. The metastatic melanoma/terminal disease cost estimated using these data was £16,139, as shown in Table 30.
| Patients on each drug (%)14 | Drug | Total cost (£)90 | Total QALYs90 | Total life-years gained | Total annual cost (£) | Weighted annual cost (£) |
|---|---|---|---|---|---|---|
| 50 | Ipilimumab | 57,760 | 2.353 | 3.35 | 17,230 | 8615 |
| 15 | Dacarbazine | 19,914 | 1.461 | 2.02 | 9876 | 1481 |
| 35 | Vemurafenib | 52,346 | 2.166 | 3.03 | 17,264 | 6042 |
| Total weighted metastatic melanoma and terminal disease cost (last year of life) | 16,139 | |||||
All other health-care and PSS costs incurred by the study population in the model, including the costs incurred by people with a benign lesion (i.e. people with TN or FP results in diagnostic assessment), were estimated to be equal between the two arms of the model and were thus omitted from the analysis.
Diagnostic economic model on lesions suspicious of basal cell carcinoma following a positive or equivocal dermoscopic finding: methods
Study population
The study population for this model comprised people with suspected BCC lesions with a positive or equivocal result on dermoscopy. The aim of examination of the suspected BCC lesions with VivaScope was to make or confirm diagnosis, as an alternative to diagnostic biopsy.
According to NICE guidance,91 patients with low-risk BCC lesions may be identified and managed by GPs in community care settings. However, clinical experts expressed the opinion that this is not routine practice, and, in reality, GPs manage < 10% of low-risk BCCs; therefore, following clinical expert advice, the economic model assumed that all patients with suspected BCC lesions are referred to (and managed by) specialist dermatologist centres.
The mean age of the study population, that is, people referred to a dermatology department with suspected BCC, was assumed to be the same as the mean age of people at diagnosis of BCC. BCC is more common in older people; people aged > 75 years are about five times more likely to have a BCC than those aged between 50 and 55 years. 2 According to a study that analysed trends in the demographic, clinical and socioeconomic profile of > 50,000 patients with non-melanoma skin cancer registered between 1994 and 2011 by the Irish National Cancer Registry, the median age at diagnosis of BCC was 68 years for both men and women. 74 Another study that analysed data on all cases of BCC diagnosed at a single centre of dermatopathology during 1967–96 in Strasbourg, France, reported that the mean age of people at diagnosis of BCC was 65 years. 73 Data on the mean age of patients with suspected or diagnosed BCC in the UK were not possible to identify, so the mean age of the study population (people with suspected BCC) was estimated to be 63 years, based on the available data and after considering the fact that the age-specific incidence rate for BCCs has been increasing in both sexes for all age groups over the years, with the largest overall increase in BCC incidence rates being observed in the youngest age groups. 74
Non-melanoma skin cancers are more common in males than females in the UK, with a ratio of 13 : 10 (which translates to a proportion of 56.5% males in the total population), although the sex difference is wider for SCC than for BCC. 2 Data from the Irish National Cancer Registry indicated that the proportion of men among patients with BCC between 1994 and 2011 was 52.8%. 74 This figure of 52.8%, which is overall consistent with relevant UK information on non-melanoma skin cancer, was used in the economic model because of a lack of relevant UK data on the male-to-female ratio specific to BCC. This figure was used to represent the percentage of men in the population with suspected (rather than confirmed) BCC following a positive or equivocal finding on dermoscopy.
The proportion of suspected and also confirmed BCC lesions on the head or neck in the model was 69.4%,76 based on information reported in Annual number of cases eligible for examination with VivaScope in a dermatology multidisciplinary team clinic in the UK.
Each person with suspected BCC may present in one visit with more than one lesion that has been found positive or equivocal for BCC on dermoscopy. Clinical experts advised that for non-melanoma skin cancer there is a 50% chance of a second non-melanoma skin cancer in a 5-year period, whereas incidental second tumours may be potentially present in about 10% of patients with BCC. The economic model assumed, for simplicity, that the number of suspected BCC lesions with a positive or equivocal dermoscopic finding per person is equal to the number of confirmed BCC lesions per person; the latter was estimated to be 1.09, using audit data from Teoh et al. ,75 who reported 926 confirmed BCC lesions in 849 patients in a retrospective single-centre audit of all BCC excisions performed in 2008. The figure of 1.09 lesions per person is consistent with clinical expert advice. The study that provided the data on the diagnostic accuracy of VivaScope on suspected BCC lesions for the economic analysis reported a mean number of 1.29 lesions suspicious of BCC per study participant (92 lesions in 72 patients), and a mean number of 1.40 confirmed BCC lesions per person with BCC (45 BCCs in 32 patients). 43 The higher figure of 1.40 BCC lesions per person (suspected or confirmed) was tested in sensitivity analysis. It should be noted that, for purposes of simplicity in the model design, it was assumed that all lesions in one person are either malignant (BCC) or not and follow the same pathway [i.e. received the same result on examination with VivaScope, the same (necessary or unnecessary) treatment], and have the same potential impact on HRQoL.
The annual number of lesions suspicious of BCC with a positive or equivocal dermoscopic finding examined at a dermatology MDT service in the UK was estimated to be approximately 500, as reported in Annual number of cases eligible for examination with VivaScope in a dermatology multidisciplinary team clinic in the UK.
Intervention and comparator
The intervention assessed in this model was VivaScope 1500 (for body lesions suspicious of BCC) and VivaScope 3000 (for suspected BCC lesions on the head or neck) for the diagnostic assessment of skin lesions suspicious of BCC. The comparator was diagnostic biopsy, which was considered to reflect routine practice following a positive or equivocal dermoscopic finding for BCC.
Model structure
A decision tree followed by a Markov model was constructed to assess the cost-effectiveness of VivaScope in the diagnosis of people with lesions suspicious of BCC that had a positive or equivocal finding on dermoscopy. According to the model structure, which was determined by clinical expert advice and availability of relevant data, people aged 63 years with lesions suspicious of BCC following a positive or equivocal finding on dermoscopy were either examined with VivaScope 1500 or VivaScope 3000 as appropriate (according to the location of the lesion) or had a diagnostic biopsy for confirmation of BCC. The model assumed that confirmed cases of skin cancer are of the same type of cancer as initially suspected (in the case of this model, BCC), although occasionally skin cancers identified may be of a different type of that initially estimated by the clinician at dermoscopy.
People whose lesions were examined with VivaScope received the results of the examination immediately. Lesions found positive by VivaScope examination were treated for BCC in accordance with national guidelines; treatment was applied to both TP and FP lesions. Lesions found negative by VivaScope examination underwent diagnostic biopsy (because of the dermoscopic outcome that was suggestive of malignancy), and subsequently received treatment if BCC was confirmed (diagnostic biopsy was considered to be the gold standard for the diagnosis of BCC). The results of diagnostic biopsy were available 6 weeks after the biopsy, in accordance with routine clinical practice in the UK. If the results of biopsy were negative, patients were discharged. It is noted that, under this pathway, no BCC lesions remained undiagnosed, as any FN lesions following VivaScope examination would move on to receive a diagnostic biopsy and would eventually be identified. On the other hand, FP lesions following VivaScope examination received unnecessary treatment.
Lesions assessed with diagnostic biopsy following a positive or equivocal finding on dermoscopy received treatment if BCC was confirmed; otherwise, patients were discharged. The results of diagnostic biopsy were received 6 weeks after the biopsy. All people in this arm of the model received treatment in accordance with their true BCC status and, therefore, none of them received unnecessary treatment.
Treatment of BCC lesions in the model comprised a mixture of surgical and non-surgical therapies, in accordance with published guidelines. 92,93 Surgical therapies included surgical excision and Mohs surgery. Non-surgical treatments included photodynamic therapy, radiotherapy and topical treatment with imiquimod or fluorouracil. Other, overall less common treatments for BCC, such as curettage and cautery, cryotherapy and chemotherapy, were not considered in the economic model. However, it is acknowledged that curettage and cautery, as well as cryotherapy, are commonly used treatments for low-risk BCCs, especially superficial ones. In any case, It should be noted that, according to clinical expert advice, there seems to be variation in clinical practice, with some of the therapies being offered more or less routinely at different dermatology centres across the country.
All people undergoing diagnostic biopsy experienced distress because of the biopsy and anxiety while waiting for the results. All people receiving surgical treatment and those treated unnecessarily with any kind of treatment (surgical or non-surgical) experienced distress because of the treatment. Moreover, a proportion of people undergoing diagnostic biopsy or surgical treatment for skin lesions on the head or neck were assumed to experience a permanent reduction in their HRQoL because of the resulting scarring.
People experiencing a permanent reduction in their HRQoL because of scarring entered a very simple Markov model, consisting of only the states of being alive (with permanent disutility attributable to scarring) and dead, in order to estimate the total disutility because of scarring experienced over a lifetime. Apart from this permanent disutility experienced by a proportion of people in each arm of the model, the choice of diagnostic strategy (i.e. either examination with VivaScope followed by diagnostic biopsy for lesions found negative for BCC or diagnostic biopsy of all suspected BCC lesions) did not have any other impact on costs or outcomes beyond the end of treatment. This is because, in both arms of the model, no BCC remained undiagnosed and, therefore, untreated. Consequently, there was no difference in tumour expansion, recurrence or mortality between the two arms of the model. For this reason, tumour expansion or future recurrence (and associated costs and impact on HRQoL) were not considered in the Markov part of the model. Thus, all future costs and outcomes, with the exception of permanent disutility because of scarring, experienced by a proportion of people, were estimated to be the same in both arms of the model and were therefore omitted from the model. The cycle length of the Markov model was 1 year and half-cycle correction was applied.
The time horizon of the economic model was over a lifetime (up to 100 years of age).
A schematic diagram of the VivaScope diagnostics model on suspected BCC following a positive or equivocal finding on dermoscopy is shown in Figure 7.
FIGURE 7.
Schematic diagram of the VivaScope diagnostics model on suspected BCC following a positive or equivocal finding on dermoscopy. (a) Decision tree component; and (b) the Markov model component.
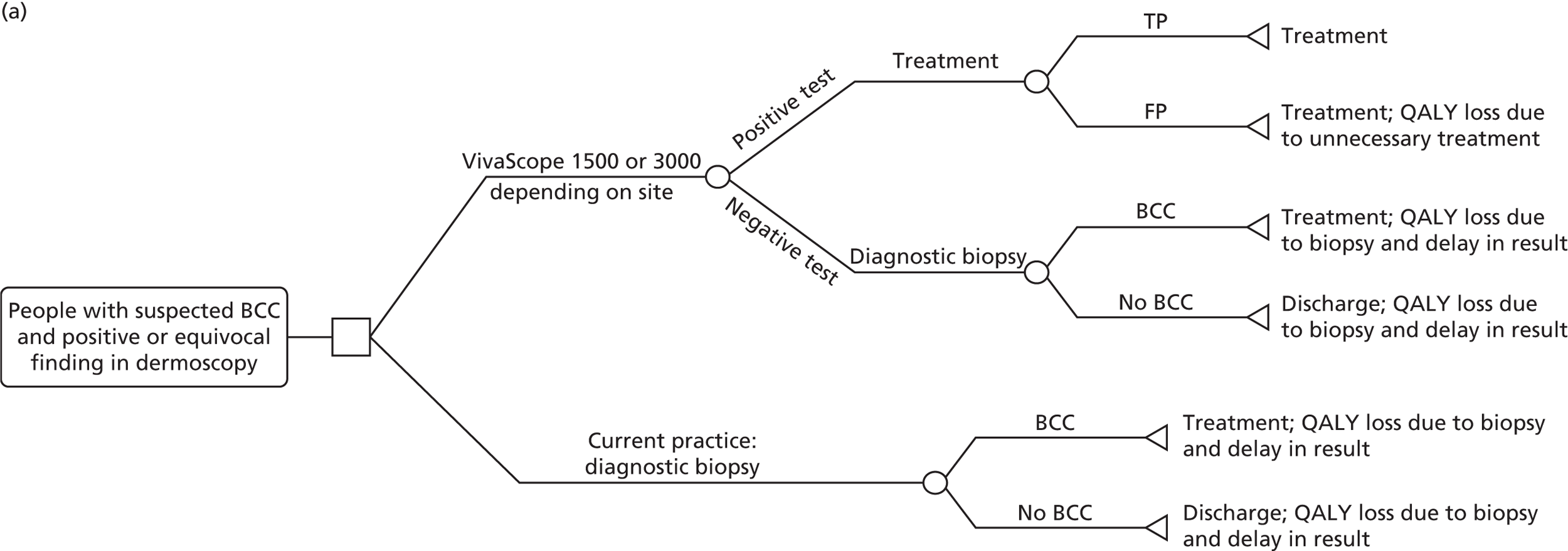

Clinical input parameters
Diagnostic accuracy data
Diagnostic accuracy data for VivaScope were taken from the results of the systematic review of clinical evidence reported in Chapter 3, Clinical effectiveness results. One study43 was found that reported the sensitivity and specificity of both VivaScope 1500 and VivaScope 3000 in the diagnosis of suspected BCC in patients presenting with at least one lesion clinically and dermoscopically suspicious for BCC that were recruited from two dermatology skin cancer clinics. According to this study, the sensitivity of VivaScope 1500 and VivaScope 3000 was 100% and 93.3%, respectively. The specificity of both devices was 77.8%. Diagnostic biopsy was considered in the model to be the gold standard for the diagnosis of BCC (i.e. it was assumed to have 100% sensitivity and specificity).
Prevalence of basal cell carcinoma in lesions with a positive dermoscopic finding
The prevalence of BCC in suspected lesions was shown to be 83.3% in Castro et al. 43 Clinical expert opinion indicated that the prevalence of BCC in lesions suspicious of BCC with a positive dermoscopic finding ranges from 95% to virtually 100%; when suspected BCC lesions with an equivocal finding on dermoscopy are considered, the prevalence of BCC is closer to 95%. The economic model utilised a prevalence value of BCC in lesions suspicious of BCC with a positive or equivocal finding on dermoscopy of 95%.
Mortality
As BCC very rarely metastasises, it has practically no impact on patients’ mortality; therefore, mortality rates in both arms of the model were assumed to equal that of the UK general population. Mortality was considered in the model only to allow estimation of the lifetime permanent disutility experienced because of scarring. Sex- and age-specific mortality rates were taken from recent UK national statistics83 and were applied separately to men and women in every arm of the model.
Utility values
Patients in this model experienced a reduction in their HRQoL for one of the following reasons:
-
diagnostic biopsy that caused distress as well as anxiety while waiting for the results
-
surgical treatment (all people undergoing surgical excision or Mohs surgery in the model) and unnecessary non-surgical treatment (people with FP lesions)
-
permanent scarring following surgical treatment of a lesion on the head or neck.
As reported in Systematic literature review of existing economic evidence, Results, Seidler et al. 57 estimated a disutility of –0.004 associated with an excision procedure because of facial non-melanoma skin cancer using traditional surgical excision or Mohs surgery. They also reported a disutility of –0.016 for simple repairs/scars (granulation and primary closure) and a disutility of –0.026 for complex repairs/scars (local flap and graft).
The study had many limitations and did not meet NICE criteria for use of utility data. Utility values were elicited from five healthy individuals in the USA, who used TTO to value two scenarios relating to surgical excision or Mohs surgery of facial non-melanoma skin cancer. Owing to a lack of better-quality data, the utility values reported in this study were utilised in the economic model. The value of –0.004 was used to reflect the decrement in HRQoL (utility) experienced because of surgical treatment (either surgical excision or Mohs surgery). Owing to the lack of any relevant data on the disutility caused by unnecessary treatment received by people with lesions with a FP result for BCC following examination with VivaScope, it was assumed that the one-off disutility of –0.004 reported in Seidler et al. 57 for surgical treatment applied to any (surgical or non-surgical) unnecessary treatment as well. It was assumed that a diagnostic biopsy created a disutility of –0.002 to the person, as it is expected to be a less invasive procedure than surgical excision or Mohs surgery. The disutility attributable to diagnostic biopsy and the disutility attributable to surgical/unnecessary treatment were applied as one-off disutilities (i.e. they were applied once, at the time of the respective procedure, without time adjustment). These disutilities were assumed to be additive, that is a lesion receiving a diagnostic biopsy followed by surgical treatment created a disutility for the patient of –0.002 + (–0.004) = –0.006 in the year in which it was biopsied and excised. Decrements in utility as a result of diagnostic biopsy or surgical/unnecessary treatment were applied separately to each lesion, so that a person with more than one lesion was assumed to experience a ‘cumulative’ disutility because of procedures experienced on each of their lesions.
In addition to the distress directly associated with diagnostic biopsy, people undergoing a diagnostic biopsy were considered to experience a reduction in their HRQoL because of anxiety while waiting for the results of biopsy. Owing to the lack of any relevant utility data, it was assumed that people experienced moderate anxiety while waiting for a potential positive result for BCC. Moreover, it was assumed that people already had a utility of < 1, and that they moved from a state of no anxiety/depression to moderate anxiety/depression. In the health state valuation equation provided by Dolan62 for EQ-5D (shown in Diagnostic economic model on suspected melanoma lesions following an equivocal finding on dermoscopy: methods), the disutility (coefficient) for moderate depression/anxiety was –0.071. According to clinical expert advice, the results of diagnostic biopsy for suspected BCC are available 6 weeks after the procedure. Therefore, the total reduction in QALYs associated with the anxiety while waiting for the results of diagnostic biopsy for suspected BCC was estimated to be –0.008. This disutility was applied in every person waiting for results, regardless of the person’s number of lesions awaiting diagnosis.
A number of people may experience permanent disutility because of scars on their head or neck caused by diagnostic biopsy of surgical treatment of skin lesions. In the economic model it was assumed that 5% of people undergoing a diagnostic biopsy for a skin lesion on their head or neck and 15% of people undergoing surgery for BCC on their head or neck would experience permanent disutility because of their scar over their lifetime. Clinical expert advice was that 100% of diagnostic biopsies for suspected BCC are undertaken with simple repairs/scars; surgical excisions make up 75% of simple and 25% of complex repairs/scars, whereas in Mohs surgery simple and complex repairs/scars make up 50% each. Based on these estimates and the disutility data reported in Seidler et al. ,57 the permanent disutility from scarring following diagnostic biopsy, surgical excision and Mohs surgery was estimated to be –0.016, –0.019 and –0.021, respectively. These disutilities were applied only to people who experienced a permanent reduction in their HRQoL because of scarring on their head or neck.
It should be noted that, as the general utility of people was not expected to differ between the two arms of the economic model (apart from the disutilities described above associated with certain procedures and resulting scars), the total number of QALYs in each arm in the model, reflecting the overall utility of each model arm from start of the model and over lifetime, was not estimated. The mean number of QALYs reported for each arm of this model is therefore negative, and reflects only the total disutility experienced by each arm of the model because of the biopsy, surgery and/or scarring resulting in permanent disutility over the time horizon of the analysis.
Table 31 provides all utility data applied in the diagnostic economic model on lesions suspicious of BCC.
| Type of utility | Utility value | Relevant population in the model | Source of utility data and assumptions |
|---|---|---|---|
| Disutility because of the diagnostic biopsy | –0.002 | People without BCC who underwent diagnostic biopsy (TN or FN in VivaScope examination and all people undergoing diagnostic biopsy under routine management) | Assumption; applied separately to every lesion undergoing diagnostic biopsy in each person, as one-off disutility |
| Disutility because of surgical treatment of a TP BCC or any unnecessary treatment of a FP BCC | –0.004 | People with BCC undergoing surgical treatment (TP in VivaScope examination or identified from diagnostic biopsy) and people without BCC undergoing unnecessary treatment (FP in VivaScope examination) | Seidler et al.;57 disutility associated with excision procedure because of facial non-melanoma skin cancer using traditional surgical excision or Mohs surgery; applied separately to every lesion undergoing surgical treatment (or unnecessary treatment) in each person, as a one-off disutility |
| Disutility because of anxiety while waiting for results of biopsy | –0.008 | Any person waiting for results of diagnostic biopsy, including people who had negative results in examination with VivaScope and people under routine management | 6-week disutility because of anxiety/depression, estimated using the EQ-5D UK health state valuation equation62 and assuming that people waiting for biopsy results had already a utility value of < 1 and moved from no to moderate anxiety/depression; applied to person (rather than lesion), as a one-off disutility |
| Permanent disutility because of scarring on the head or neck | –0.016 | Five per cent of people with lesions on their head or neck who underwent diagnostic biopsy without surgical treatment (people with TN lesions in VivaScope examination who underwent diagnostic biopsy, people with FN lesions in VivaScope examination who underwent diagnostic biopsy followed by non-surgical treatment, and people with negative lesions undergoing routine management with diagnostic biopsy) | Seidler et al.;57 diagnostic biopsy of suspected BCCs assumed to entail simple repairs/scars |
| –0.019 | 15% of people with BCC on head or neck who underwent surgical excision | Surgical excision of BCCs assumed to make up 75% simple and 25% complex repairs/scars | |
| –0.021 | 15% of people with BCC on head or neck who underwent Mohs surgery | Mohs surgery of BCCs assumed to make up 50% simple and 50% complex repairs/scars; applied over a lifetime |
Costs
Costs considered in this economic model included the cost of diagnostic assessment with VivaScope following a positive result on dermoscopy, the cost of diagnostic biopsy and the cost of treatment (including the cost of unnecessary treatment for skin lesions with a FP result in VivaScope examination).
As reported in Table 24, the cost of VivaScope per suspected BCC lesion examined was estimated to be £71 if VivaScope is exclusively used for the diagnostic assessment of suspected BCC lesions found positive on dermoscopy; £62 if VivaScope is used only for the diagnostic assessment of suspected melanomas giving an equivocal finding on dermoscopy and suspected BCC lesions with a positive dermoscopic finding; and £58 if the device is used not only for the diagnostic assessment of suspected melanomas and BCCs, but also for the mapping of LMs prior to surgical treatment.
The costs of all other procedures and treatments included in the model were taken from either the NHS Reference Costs 2013 to 201412 for 2014 or the NHS national drug tariff for 2015. 64 Clinical experts advised on the appropriate NHS service and procedure codes corresponding to procedures and treatments considered in the model.
The unit cost of diagnostic biopsy was estimated to be £134, corresponding to the national unit cost of outpatient minor skin procedures conducted in a dermatology service for people aged ≥ 13 years (service code 330, currency code JC43A). 12
Treatment comprised a mixture of surgical and non-surgical therapies. Clinical experts indicated that the proportion of BCC lesions treated surgically ranges between 66% and 90% of BCC lesions. The economic model assumed that 75% of BCC lesions are treated surgically. Of those, 85% were assumed to undergo surgical excision and 15% to be treated with Mohs surgery (the proportion of BCC lesions undergoing Mohs surgery among those receiving surgical treatment appears to range between 10% and 20% across services in the UK, as indicated by clinical experts, although a wider variation may potentially exist).
Among lesions managed with non-surgical treatment, the percentage of lesions receiving each treatment was derived from a multicentre audit (seven centres in the Mersey region of north-west England), comprising a retrospective case note review of 50 randomly selected patients per trust who had BCCs managed non-surgically within a 12-month time period (1 January 2012–1 January 2013). 94 In total, 246 patients were selected as being suitable for the audit. The most commonly used agent for treatment was imiquimod (used by ≥ 50% of patients with BCC), followed by photodynamic therapy in 21%, radiotherapy in 19% and fluorouracil in 8%. Based on these data, and after consulting with clinical experts, it was assumed that non-surgical treatment of BCCs in the economic model comprised 60% topical treatment with imiquimod or fluorouracil (30% each), 21% photodynamic therapy and 19% radiotherapy. It should be emphasised that this is not necessarily a typical picture of non-surgical treatments across the country, as the EAG was advised that some of these treatments are not routinely used in some dermatology services, whereas others (such as cryotherapy, and curettage and cautery), which were not included in the economic model, may be more frequently offered in some services for the treatment of low-risk BCC lesions, especially superficial ones. However, regarding non-surgical therapies, as these made up only 25% of the treatment of BCC lesions, the impact of variations in relevant practice across settings on the total cost of BCC treatment was rather insubstantial.
By combining the above resource-use estimates with appropriate unit costs12,64 as recommended by clinical experts, the mean total cost of treatment per BCC lesion was estimated at £475.
All other health-care and PSS costs incurred by people in the model were estimated to be equal between the two arms of the model and were, thus, omitted from the analysis.
Table 32 provides the data and assumptions used at the estimation of the mean weighted treatment cost of BCC.
| Type of treatment | % treated with method | Treatment | % within type | Cost (£) | Data sources and assumptions based on clinical expert estimates |
|---|---|---|---|---|---|
| Surgical | 75 | Surgical excision | 85 | 388 | Assuming 50% is made up of minor skin procedures undertaken as day cases (currency code JC43A, unit cost for people aged ≥ 13 years, £624),12 and 50% is made up of dermatology outpatient, intermediate skin procedures (service code 330, currency code JC42 A, unit cost for people aged ≥ 13 years, £151)12 |
| Mohs surgery | 15 | 943 | Intermediate skin procedure undertaken as day case (currency code JC42A, unit cost for people aged ≥ 13 years £943)12 | ||
| Non-surgical | 25 | Imiquimod | 30 | 142 | Imiquimod 5% cream one pack of 12 sachets is £48.6064 plus one consultant-led, dermatology outpatient follow-up visit (service code 330, currency code WF01A, unit cost £93)12 |
| Fluorouracil | 30 | 126 | Fluorouracil 5% cream one tube £32.9064 plus one consultant-led, dermatology outpatient follow-up visit (service code 330, currency code WF01A, unit cost £93)12 | ||
| Radiotherapy | 19 | 1303 | Involves approximately 10 fractions; cost per fraction £87 plus one-off cost for the mask £433, according to clinical expert opinion | ||
| Photodynamic therapy | 21 | 753 | Two sessions of photodynamic therapy offered as day cases (currency code JC46Z, unit cost £330 each)12 plus one consultant-led, dermatology outpatient follow-up visit (service code 330, currency code WF01A, unit cost of £93)12 | ||
| Mean weighted treatment cost | 475 | ||||
All input parameters utilised in the diagnostic economic model on lesions suspicious of BCC following a positive dermoscopic finding are shown later (see Table 35).
Presurgical margin delineation economic model: methods
Study population
The study population for this model comprised patients with LM, aged 70 years, undergoing margin delineation prior to receiving surgical treatment. The aim of examination of LM with VivaScope prior to surgical removal was accurate definition of tumour margins. Surgical removal of LM needs to balance between sufficiently wide margins to prevent recurrence, and minimal margins to preserve functional and aesthetic areas of the face and neck. Therefore, accurate definition of the surgical margins of LM potentially leads to a low rate of multiple excisions, sparing tissue in functional and aesthetic areas. 95
Epidemiological data specific to LM are rather sparse in the literature, possibly because this is a precancerous condition and it may not always be recorded in cancer registries. Cases of LM are not routinely included in UK cancer statistics. LM is more common in older people. A review of LM and LMM, published in 1995, indicated that patients with LM are generally > 40 years of age, with a mean age of 65 years. 96 LM most commonly affects the sun-exposed skin of the head and neck, with a predilection for the cheek. 96 A recent US study identified all adult residents with a first lifetime diagnosis of LM between 1970 and 2007 in Olmsted County, MN, USA. 70 The study analysed medical records in order to determine demographic, clinical and surgical data, as well as incidence and survival rates associated with LM. 70 According to this study, the mean age of patients at LM diagnosis was 70 years (range 33–97 years), with 64.1% being male. The proportion of LMs on the head or neck was approximately 62%. However, clinical expert advice to the EAG indicated that this percentage might be much higher, and even reach 90%. Based on this information, the study population in the economic model had a mean age of 70 years, with 64% being male and 70% of them having a LM on the head or neck. Each person had only one diagnosed LM that required surgical treatment at the time of the analysis, according to clinical expert opinion.
The annual number of LMs examined for margin delineation at a dermatology MDT service in the UK was estimated to approximate 75, as reported in Annual number of cases eligible for examination with VivaScope in a dermatology multidisciplinary team clinic in the UK.
Intervention and comparator
The intervention assessed in this model was VivaScope 3000 for the margin delineation of LM prior to surgical treatment. The comparator was routine practice, which comprised presurgical assessment of LM margins with a dermoscope and/or clinical judgement.
Model structure
A decision tree followed by a Markov model was constructed to assess the cost-effectiveness of VivaScope in the margin delineation of LMs prior to surgical treatment. According to the model structure, which was determined by clinical expert advice and availability of relevant data, patients of 70 years of age with a LM planned for surgical treatment either had their tumour examined with VivaScope 3000 for margin delineation prior to surgery or underwent routine management, comprising presurgical assessment of LM margins with a dermoscope and/or clinical judgement.
Following margin assessment, LMs in both arms of the model were removed either by surgical excision or by Mohs surgery. A proportion of surgical excisions were incomplete, as determined by histopathology, meaning that some premalignant cells were still present after treatment, despite margin delineation. Incompletely excised tumours required a second surgical excision 4–6 weeks later, after which excision was assumed to be complete and confirmed by histopathology. The proportion of LMs that were incompletely excised was determined by the type of presurgical assessment of the margins (i.e. by VivaScope 3000 or dermoscopy/clinical judgement). Mohs surgery is performed in surgical stages until the surgical margins are clear. The type of presurgical assessment of the margins (i.e. by VivaScope 3000 or dermoscopy/clinical judgement) affected the number of stages of Mohs surgery.
All patients experienced distress because of surgery. Moreover, a proportion of patients with a LM surgically removed from their head or neck experienced a permanent reduction in their HRQoL because of the resulting scarring.
After complete surgical excision or Mohs surgery, all patients in both arms of the decision tree entered the Markov model, which was run in yearly cycles; half-cycle correction was applied. All patients entering the Markov model were at risk of recurrence of their tumour for the first 10 years (i.e. 10 years after the primary surgical removal of their LM). The risk of recurrence depended on the type of initial presurgical margin delineation (i.e. with either VivaScope 3000 or dermoscopy/clinical judgement) and/or the type of initial surgical treatment they had received (i.e. surgical excision or Mohs therapy). Patients experiencing a recurrence either underwent surgical excision or Mohs surgery, as according to clinical expert advice the vast majority of LMs are treated surgically; alternative therapies (such as radiotherapy and topical therapy with imiquimod) are used only if the patient is unfit for surgery or if there is a medical reason preventing surgery, for example if the patient is very frail and elderly. All patients experienced distress because of surgical treatment. A proportion of the patients with LMs on the face could experience permanent disutility because of scarring, if they were not already experiencing permanent disutility as a result of scarring after the initial surgical treatment.
The Markov model consisted of the states of ‘no recurrence, no permanent disutility because of scarring’, ‘no recurrence, permanent disutility because of scarring’, ‘recurrence, no permanent disutility because of scarring’, ‘recurrence, permanent disutility because of scarring’ and ‘death’, which was an absorbing state. Patients moving from the decision tree could enter any of the Markov model states (except death), depending on whether or not they had already experienced permanent disutility because of scarring. Patients in the ‘no recurrence, no permanent disutility because of scarring’ state could remain in this state, experience a recurrence and move to ‘recurrence, no permanent disutility because of scarring’ state, experience a recurrence and a scar that created permanent disutility thus moving to ‘recurrence, permanent disutility because of scarring’ state (this was possible for only patients with LM on the head or neck) or die. Patients in the ‘no recurrence, permanent disutility because of scarring’ state (who were patients with a LM on their head or neck) could remain on this state, experience a recurrence and move to ‘recurrence, permanent disutility because of scarring’ state or die. The two recurrence states with or without permanent disutility because of scarring were only temporary states; patients in these states could only transition to the two non-recurrence states with or without permanent disutility because of scarring, respectively, from which they could transition to a new recurrence or death in the next cycle. After the first 10 years, patients could not experience a recurrence of their tumour and therefore they could either remain in their ‘no recurrence’ state (with or without scarring) or die.
Lentigo maligna in the economic model was assumed not to progress to LMMs, as the relevant risk was low, given that all LMs in the model were treated.
The time horizon of the economic model was over a lifetime (up to 100 years of age). A schematic diagram of the VivaScope margin delineation model is shown in Figure 8.
FIGURE 8.
Structure of the margin delineation model. (a) Decision tree component; and (b) the Markov model component. a, As confirmed by histopathology. Note that dotted lines in the Markov model illustration show points of entrance from the decision tree.
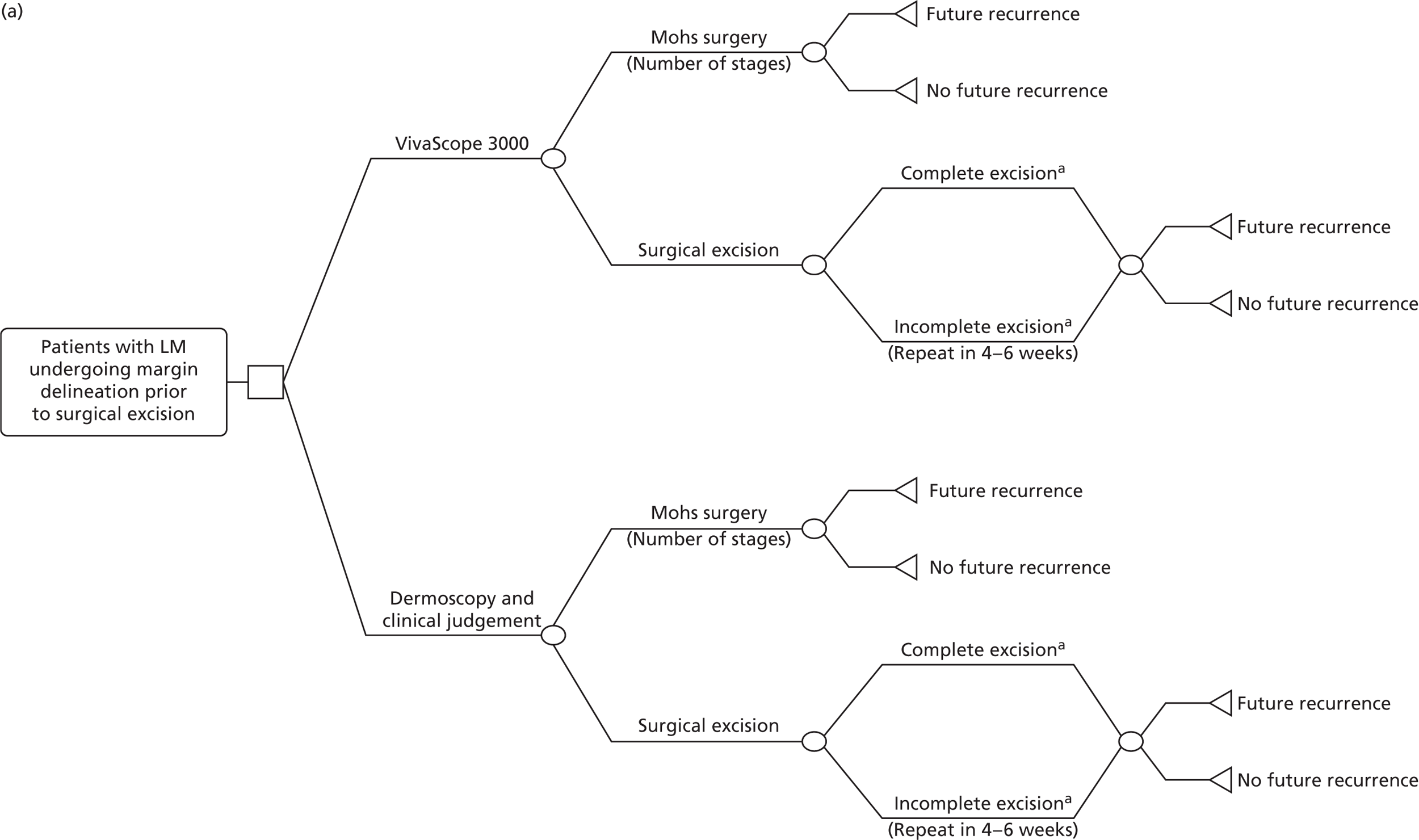
Clinical input parameters
Impact of method of margin delineation on surgical outcomes
The impact of VivaScope on surgical outcomes following presurgical margin delineation of LMs was taken from the results of systematic review reported in Chapter 3, Clinical effectiveness results. The risk of incomplete surgical excisions following margin delineation with VivaScope 3000 was taken from Guitera et al. ,35 who reported that, out of 17 patients who had LM surgically excised, two had VivaScope delineated margins involved after excision (12%). Regarding future recurrence, the study reported that no recurrence of LMs treated surgically was observed in any of the patients by last follow-up (median follow-up 37 months, range 7–66 months). However, this observation was based on a small number of LMs excised. In order to populate the economic model, it was assumed that the risk of recurrence of LMs after margin delineation with the use of VivaScope 3000 was equal to the risk of recurrence of LMs following Mohs surgery, regardless of the type of surgical treatment (i.e. surgical excision or Mohs surgery) following mapping with VivaScope 3000. This was considered by clinical experts to be a conservative assumption.
The risk of incomplete surgical excision and future recurrence following routine margin delineation with dermoscopy and/or clinical judgement was based on a review of published studies and audits reporting relevant data.
A large study evaluating the outcomes of surgical excision in all LM cases treated in Leicestershire between 1987 and 1996 reported that, out of 89 evaluable patients with LM treated with primary excision, eight (i.e. 9%, with 95% CI 4% to 17%) had a histologically incomplete excision. 97 The margins used by surgeons in Leicestershire were 2 mm, in accordance with standard practice in the UK at the time of the study. In completely excised lesions (n = 81), the observed recurrence rate was 20% (95% CI 12% to 30%) at a mean follow-up of 42 months, which was claimed to be similar to previous reports. However, Kaplan–Meier analysis undertaken by the authors estimated a probability of recurrence of 31% (95% CI 19% to 50%), with time to relapse being up to 66 months.
A retrospective review of all melanomas in situ referred to one hospital in Hull between 2001 and 2009 revealed that, of the 75 excisions of LMs, 22 (29.3%) were incomplete. 98 The risk of recurrence in complete excisions was 2.9% at 3 years.
A review of the clinical features, histopathology and treatment options for LM reported that standard excision of LMs with 5-mm margins was insufficient in 50% of cases. 99 The recurrence rate with standard excision was reported to range from 8% to 20%. On the other hand, it was argued that Mohs surgery and staged excision might offer better margin control and lower recurrence rates, around 4–5%. According to the BAD guidelines,13 local recurrence of LM occurs in about 5% of patients by 2 years.
Finally, a US retrospective study of 5-year treatment outcomes of all primary LM cases treated with either wide local excision with 5-mm margins or Mohs surgery in one dermatology setting in Minnesota between 1995 and 2005 reported that, out of 269 lesions treated with wide excision, there were 16 recurrences over 5 years (5.9%), but, out of 154 lesions treated with Mohs surgery, there were three recurrences over 5 years (1.9%). 100
The economic model used a 12% risk of incomplete excision for surgical excisions of LM following mapping with VivaScope and a 30% risk of incomplete excision for surgical excisions after routine margin delineation with a dermoscopic and/or clinical judgement. The 5-year risk of recurrence of LMs mapped with VivaScope (regardless of type of subsequent surgical treatment), as well as the 5-year risk of recurrence of LMs following Mohs surgery (regardless of method of presurgical mapping), was 5% in the model. The 5-year risk of recurrence of LMs after surgical excision was 15% in the model. These figures, which were based on values reported in the literature and were validated by clinical expert opinion, were converted to 1-year probabilities using exponential function and were applied over the first 10 years of the Markov model. After 10 years, it was assumed that the risk of recurrence fell to zero.
Regarding the number of stages in Mohs surgery after margin delineation of LMs, a small UK study of Mohs surgery on 16 LM cases, of which seven had been mapped with VivaScope 3000, reported that cases that were mapped with VivaScope took an average of 1.4 stages to clear (SD 0.53 stages), whereas those that did not undergo mapping took an average of 2.2 stages to clear (SD 1.2 stages). 101 These values were utilised in the economic model because of a lack of any more robust data.
Mortality
As progression of LM to LMM is very low, particularly if treated, and in the very elderly may be unlikely within their lifespan,13 mortality rates in both arms of the model were assumed to equal that of the UK general population. Sex- and age-specific mortality rates were taken from recent UK national statistics83 and were applied separately to men and women in every model arm.
Utility values
Patients in this model experienced a reduction in their HRQoL for one of the following reasons:
-
surgical treatment (either surgical excision or Mohs surgery)
-
permanent scarring following surgical treatment of a LM on the head or neck.
As reported in Systematic literature review of existing economic evidence, Results, Seidler et al. 57 estimated a disutility of –0.004 associated with an excision procedure because of facial non-melanoma skin cancer using traditional surgical excision or Mohs surgery. They also reported a disutility of –0.016 for simple repairs/scars (granulation and primary closure) and a disutility of –0.026 for complex repairs/scars (local flap and graft).
The presence and surgical management of LM was considered to have a similar impact on patients’ HRQoL as the presence and surgical management of BCC. Owing to a lack of more relevant and better-quality data, the value of –0.004 was used to reflect the decrement in HRQoL (utility) experienced because of surgical treatment (either surgical excision or Mohs surgery). This disutility as a result of surgical treatment was applied as a one-off every time a person underwent surgical treatment (i.e. at first surgery, repeat surgery because of incomplete excision or future recurrence).
Disutility because of a patient waiting for a second surgery following incomplete excision was not considered; however, it is acknowledged that waiting time for a second surgery may create additional distress to the patient.
The number of stages in Mohs surgery is expected to affect the patients’ HRQoL, in terms of time and distress. However, the differential utility resulting from differences to the number of stages in Mohs surgery was not factored into the model as it was not possible to estimate a disutility per stage.
A number of people may experience permanent disutility because of scars on their head or neck as a result of the surgical removal of LMs. In the economic model it was assumed that 15% of patients undergoing surgical treatment for their LM on their head or neck (either for the first time or at a future recurrence of the tumour) would experience permanent disutility because of their scar. Clinical expert advice was that surgical removal of LMs, either by surgical excision or by Mohs surgery, is made up of 50% simple and 50% complex repairs/scars. Based on these estimates and the disutility data reported in Seidler et al. ,57 the disutility associated with scarring from surgical treatment of LMs was estimated to be –0.021. This disutility was applied only to people with permanent reduction in their HRQoL because of scarring on the head or neck. An individual who underwent surgical treatment for a LM on the head or neck and did not experience disutility because of scarring was at a 15% risk of experiencing permanent disutility caused by scarring at each potential future recurrence of LM.
As the general utility of people in the model was not expected to differ between the two arms of the economic model (apart from the disutilities described above associated with surgical treatment and resulting scars), the total number of QALYs in each arm in the model, reflecting the overall utility of each model arm from start of the model and over a lifetime, was not estimated. The mean number of QALYs reported for each arm of this model is, therefore, negative and reflects only the total disutility experienced by each arm of the model caused by surgery and/or scarring resulting in permanent disutility over the time horizon of the analysis.
Table 33 provides all utility data applied in the economic model on margin delineation of LMs.
| Type of utility | Utility value | Relevant population in the model | Source of utility data and assumptions |
|---|---|---|---|
| Disutility because of surgical treatment of LM | –0.004 | People with LM undergoing surgical treatment (surgical excision or Mohs surgery) | Seidler et al.57 disutility associated with excision procedure because of facial non-melanoma skin cancer using traditional surgical excision or Mohs surgery; applied every time a person underwent surgical treatment (i.e. at first surgery, repeat surgery because of incomplete excision or future recurrence), as a one-off disutility |
| Permanent disutility because of scarring on the head or neck | –0.021 | 15% of people with BCC on their head or neck who underwent surgical excision or Mohs surgery at the start of the model or because of future recurrence of LM | Seidler et al.57 surgical excision and Mohs surgery of LM assumed to be made up of 50% simple and 50% complex repairs/scars; applied over lifetime |
Costs
Costs included the cost of presurgical mapping of LMs with either VivaScope 3000 or dermoscopy/clinical judgement, the cost of treatment with either surgical excision or Mohs surgery and the cost of potential future treatment because of recurrence.
As reported in Table 24, the cost of margin delineation with VivaScope 3000 per LM mapped was estimated to be £250 if VivaScope is exclusively used for presurgical margin delineation of LMs and £105 if the device is used for the diagnostic assessment of suspected melanomas and BCCs as well as for the mapping of LMs prior to surgical treatment.
Routine presurgical margin delineation of LMs with dermoscopy/clinical judgement was estimated to comprise 5 minutes of a consultant dermatologist’s time. Using the unit cost of a consultant dermatologist of £140 per hour of contract,65 the mean cost of routine presurgical margin delineation was estimated to be £12. The acquisition cost of dermoscopy was not included in the estimation of the cost of routine presurgical margin delineation LMs, as dermoscopes appear to be already in place in dermatology departments and can be used for the assessment of skin lesions.
The proportion of LMs that were treated with surgical excision in the first surgery following margin delineation and in future recurrences was estimated based on clinical expert opinion. It was assumed that 85% of the first surgical treatment of LMs made up surgical excision and 15% Mohs surgery. After tumour recurrence, it was assumed that 80% of LMs were treated with surgical excision and 20% with Mohs surgery.
The unit costs of surgical excision and Mohs surgery were taken from the NHS reference costs for 2014. 12 Clinical experts advised on the appropriate NHS service and procedure codes corresponding to these two types of surgical treatment.
Mohs surgery is undertaken in stages. The number of stages required for Mohs surgery is directly related to an opportunity cost in terms of staff time and consumables; however, it was not possible to identify a unit cost per stage of Mohs surgery. For this reason, it was assumed that the unit cost reflecting Mohs surgery corresponded to the mean number of required stages, across all skin operations requiring Mohs surgery. As it was not possible to identify relevant UK data on the mean number of stages required in Mohs surgery, this figure was derived from a US multicentre prospective cohort study that aimed to evaluate the rate of complications and postoperative pain associated with the treatment of skin cancer using Mohs surgery. 102 The study included 1550 patients with 1792 tumours, the majority of which were BCC (61%) or SCC (31%). The authors reported that the mean number of stages was 1.6, ranging from 1 to 8. Therefore, for the purposes of costing, it was assumed that the national unit cost reflecting the cost of Mohs surgery corresponded to 1.6 Mohs stages, and that 70% of this unit cost was fixed (and independent of the number of stages involved in Mohs surgery), whereas the remaining 30% of the unit cost was variable and in a linear relationship with the number of stages required for the completion of Mohs surgery. This assumption was utilised only in the first surgery following margin delineation of LMs. For Mohs surgery undertaken in future recurrences, the mean cost of Mohs surgery, without adjusting for the number of stages, was used.
All other health-care and PSS costs incurred by people in the model were estimated to be equal between the two arms of the model and were thus omitted from the analysis.
Table 34 provides the percentages of each type of surgical treatment for LM at first surgery following margin delineation and after recurrence, the costs of each type of surgical treatment, as well as the data sources and assumptions used for their estimation.
| Treatment | % at first surgery | % after recurrence | Cost (£) | Data sources and assumptions based on clinical expert estimates |
|---|---|---|---|---|
| Surgical excision | 85 | 80 | 388 | Assuming 50% is made up of minor skin procedures undertaken as day cases (currency code JC43A, unit cost for people aged ≥ 13 years £624),12 and 50% is made up of dermatology outpatient, intermediate skin procedures (service code 330, currency code JC42A, unit cost for people aged ≥ 13 years £151)12 |
| Mohs surgery | 15 | 20 | 943 | Intermediate skin procedure undertaken as day case (currency code JC42A, unit cost for people aged ≥ 13 years £943);12 70% assumed to be fixed and 30% assumed to be linearly determined by number of stages of Mohs surgery (this was applied only to first surgery). Reported cost assumed to correspond to 1.6 stages of Mohs surgery |
All input parameters utilised in the diagnostic economic model on lesions suspicious of BCC following a positive dermoscopic finding are shown later (see Table 35).
Methods of analysis and presentation of the results
Overview of methods of analysis
A deterministic analysis that utilised point estimates of each model input parameter was first undertaken. This was followed by a probabilistic analysis, which was conducted to take account of the uncertainty characterising the input parameter estimates. For this analysis, all relevant input parameters were entered as probability distributions to reflect their imprecision. Probability distributions were determined by the available data or, where data were lacking, by plausible assumptions. Monte Carlo simulation was then employed to reflect this uncertainty in the models results: 10,000 iterations were performed, each drawing random values out of the distributions fitted onto the model input parameters. Results of the probabilistic analysis were averaged across the 10,000 iterations to provide a mean estimate of costs and QALYs for each intervention. In addition, uncertainty in the model input parameters and structural assumptions were explored through deterministic one-way and two-way sensitivity analyses.
The results have been presented in the form of incremental cost-effectiveness ratios (ICERs), except in cases of dominance, which occurs when an intervention results in lower costs and a higher number of QALYs than its comparator. The results of both types of analyses (deterministic and probabilistic) have been depicted in the form of cost-effectiveness planes. The results of the probabilistic analysis have been summarised in the form of cost-effectiveness acceptability curves (CEACs), which show the probability of VivaScope being cost-effective at different cost-effectiveness thresholds, in each of the analyses considered. All input parameters were tested in one-way sensitivity analysis; tornado diagrams were produced for different analyses to show the impact of the most influential parameters on the results. The results of tornado diagrams have been reported using incremental net monetary benefits (INMBs), estimated at a willingness-to-pay (WTP) threshold of £20,000/QALY, rather than ICERs, because use of the whole range of some parameters tested in the tornado diagrams resulted in negative ICERs, because of dominance, which are not meaningful. Additional one-way sensitivity analyses were undertaken to estimate the impact of alternative scenarios and model assumptions on the results. Finally, two-way sensitivity analyses were carried out to test the impact of concurrently varying sensitivity and specificity of VivaScope in the diagnostic assessment of eligible skin lesions suspicious of melanoma or BCC on the cost-effectiveness results.
Summary of all model input parameters, probability distributions and range of values tested in sensitivity analysis
In order to run the probabilistic analysis, all relevant input parameters were entered as probability distributions to reflect their imprecision. Probability distributions were determined by the available data or, where data were lacking, by plausible assumptions.
The annual number of the three types of lesions examined with VivaScope (i.e. suspected melanomas with an equivocal finding on dermoscopy, suspected BCCs with a positive or equivocal dermoscopic finding and LMs undergoing presurgical margin delineation) was given a uniform distribution, with a range of ± 30% of the originally estimated volume.
The diagnostic accuracy characteristics of VivaScope and monitoring (which was part of routine management of equivocal lesions suspicious of melanoma) (i.e. that is sensitivity and specificity) were given a beta distribution. It is acknowledged that sensitivity and specificity are usually correlated, and, as such, a joint distribution should ideally be used. However, as no meta-analysis of diagnostic accuracy data was performed and no summary receiver operating characteristic curves that could indicate the relationship between sensitivity and specificity were possible to produce, as described in Chapter 3, it was considered reasonable to use a beta distribution for sensitivity and specificity, assuming that these are independent from each other, although this assumption is acknowledged as a limitation of the analysis.
All proportions and dichotomous probabilities (e.g. the proportion of men in the study population, the proportion of lesions on the head or neck, the probability of death associated with melanoma, the prevalence of cancer in lesions suspicious of skin cancer, the probability of future recurrence of LM) were given a beta distribution. Utilities were also given a beta distribution, using the method of moments; disutilities were given a distribution of 1 minus beta. Polychotomous transitions and variables were given a Dirichlet distribution.
Staff unit costs (radiographer, consultant dermatologist) and the required staff time to operate the VivaScope system were given a normal distribution. All other costs were assigned a gamma distribution.
Table 35 provides an overview of all input parameters, reporting deterministic values and details on the types and range of probability distributions assigned to each parameter with relevant data sources and justifications.
| Input parameter | Mean (deterministic) value | Probability distribution | Source of data: comments |
|---|---|---|---|
| Parameters determining the cost of VivaScope | |||
| Annual number of lesions examined with VivaScope | Clinical expert advice supplemented by estimates based on national statistics and further assumptions2,67–70 | ||
| Equivocal lesions suspicious of melanoma | 100 | Uniform; range 70–130 | |
| Suspected BCCs positive/equivocal on dermoscopy | 500 | Uniform; range 350–650 | |
| LMs prior to surgical treatment | 75 | Uniform; range 52.9–97.5 | |
| Purchase price of VivaScope | |||
| VivaScope 1500 | £90,224 | No distribution assigned | Information provided by the company |
| VivaScope 3000 stand-alone device | £62,300 | No distribution assigned | Information provided by the company |
| VivaScope 1500 and VivaScope 3000 combined | £131,824 | No distribution assigned | Information provided by the company |
| Annual maintenance cost of VivaScope | |||
| VivaScope 1500 | £4100 | No distribution assigned | Information provided by the company |
| VivaScope 3000 stand-alone device | £4100 | No distribution assigned | Information provided by the company |
| VivaScope 1500 and VivaScope 3000 combined | £5500 | No distribution assigned | Information provided by the company |
| Useful life of VivaScope/training | 10 years | No distribution assigned | Information provided by the company/assumption |
| Interest rate used for annuitisation of costs | 3.5% | No distribution assigned | Assumption |
| Costs of consumables | Including adhesive windows needed for VivaScope 1500, crodamol oil, alcotip and ultrasound gel; based on retail prices and further assumptions | ||
| Per lesion examined with VivaScope 1500 | £2.97 | No distribution assigned | |
| Per lesion examined with VivaScope 3000 | £1.50 | No distribution assigned | |
| Cost of training (cost of staff time) | £17,816 | Distribution determined by staff unit costs | Includes 1.5 days of two radiographers and two consultant dermatologists (introductory training) and 4 days of two consultant dermatologists (intensive expert training plus travel time) plus £2000 travel, hotel and subsistence costs for each dermatologist attending the intensive expert training |
| Staff time per examination | Clinical expert opinion; all distributions imposed to a minimum value of 5 minutes of a radiographer and 3 minutes of a dermatologist for diagnosis with VivaScope 1500; 5 minutes of dermatologist for diagnosis with VivaScope 3000; 20 minutes of a radiographer for mapping; distribution based on assumption | ||
| Diagnosis, VivaScope 1500 – radiographer | 10 minutes | Normal; SE: 0.1 × mean | |
| Diagnosis, VivaScope 1500 – dermatologist | 5 minutes | Normal; SE: 0.1 × mean | |
| Diagnosis, VivaScope 3000 – dermatologist | 10 minutes | Normal; SE: 0.1 × mean | |
| Margin mapping, VivaScope 3000 – dermatologist | 30 minutes | Normal; SE: 0.1 × mean | |
| Staff unit costs | Curtis;65 unit cost of a radiographer band 7 estimated from the unit cost of a radiographer band 5 and the ratio of salary of band 7 to band 5 AfC for qualified allied health professionals; distribution based on assumption Details on the estimation of these costs provided in Table 24 |
||
| Radiographer band 7 – per hour | £62 | Normal; SE: 0.1 × mean | |
| Clinical dermatologist – per hour of contract | £140 | Normal; SE: 0.1 × mean | |
| Cost of VivaScope examination | |||
| Per suspected melanoma | |||
| Exclusive use on suspected melanomas | £254 | ||
| Use on suspected melanomas and BCCs | £63 | ||
| Use across all three types of lesions | £59 | ||
| Per suspected BCC | |||
| Exclusive use on suspected BCCs | £70 | ||
| Use on suspected melanomas and BCCs | £62 | ||
| Use across all three types of lesions | £58 | ||
| Per mapped LM | |||
| Exclusive use on mapping of LM | £250 | ||
| Use across all three types of lesions | £105 | ||
| Diagnostic assessment model on suspected melanomas | |||
| Mean age of the study population | 55 years | NA | Schofield et al.77 |
| Proportion of men in the study population | 0.49 | Beta; α = 6495, β = 6853 | Cancer Research UK2 |
| Proportion of melanomas on head or neck | Statistical Information Team at Cancer Reseach UK71 | ||
| Men | 0.22 | Beta; α = 1429, β = 5066 | |
| Women | 0.14 | Beta; α = 959, β = 5894 | |
| Number of suspected/diagnosed melanomas per person | 1 | No distribution assigned | Clinical expert advice |
| Prevalence of melanoma in equivocal lesions | 0.15 | Beta; α = 15, β = 85 | Review of studies and clinical expert opinion; distribution based on assumption |
| Proportion of equivocal lesions excised under routine management | 0.67 | Beta; α = 67, β = 33 | Clinical expert opinion; distribution based on assumption |
| Ratio of prevalence of melanoma in equivocal lesions excised: monitored under routine management | 1 : 5 | Normal; SD = 0.1 × mean, n = 500 | Average between Pellacani et al.42 and Ferrari et al.;44 distribution based on assumption |
| Prevalence of melanoma in equivocal lesions | Determined by overall prevalence of melanoma in equivocal lesions, proportion of those excised under routine management, and ratio of prevalence of melanoma in lesions excised: monitored under routine management | ||
| Excised | 0.21 | Determined by relevant parameter distributions | |
| Monitored | 0.04 | ||
| Waiting time for biopsy results | 2 weeks | No distribution assigned | Clinical expert advice |
| Diagnostic accuracy of VivaScope | |||
| Alarcon et al.30 | Alarcon et al.30 data for VivaScope 1500; diagnostic accuracy of VivaScope 3000 assumed to be the same | ||
| Sensitivity | 0.978 | Beta; α = 90, β = 2 | |
| Specificity | 0.948 | Beta; α = 238, β = 13 | |
| Pellacani et al.42 | |||
| Sensitivity in highly suspicious lesions | 1.000 | Beta; α = 43, β = 1 | Pellacani et al.;42 data for VivaScope 1500; diagnostic accuracy of VivaScope 3000 assumed to be the same; uninformative prior distribution applied in sensitivity (both types of lesions) to deal with zero observations in β |
| Specificity in highly suspicious lesions | 0.518 | Beta; α = 73, β = 68 | |
| Sensitivity in moderately/low suspicious lesions | 1.000 | Beta; α = 26, β = 1 | |
| Specificity in moderately/low suspicious lesions | 0.802 | Beta; α = 227, β = 56 | |
| Diagnostic accuracy of biopsy | Considered to be the gold standard for diagnosis | ||
| Sensitivity | 1.000 | No distributions assigned | |
| Specificity | 1.000 | ||
| Diagnostic accuracy of monitoring | Altamura et al.80 | ||
| Sensitivity | 0.900 | Beta; α = 81, β = 9 | |
| Specificity | 0.734 | Beta; α = 1118, β = 406 | |
| Proportion of melanomas in situ among melanomas prevalent in equivocal lesions (remaining are stage I) | 0.60 | Beta; α = 60, β = 40 | Review of studies and clinical expert opinion; distribution based on assumption |
| Substages within melanoma stages | Balch et al.81 | ||
| Proportion of stage Ia melanomas among stage I | 0.515 | Beta; α = 9452, β = 8918 | |
| Proportion of stage IIa melanomas among stage II | 0.501 | Dirichlet (4644, 3228, 1397) | |
| Proportion of stage IIb melanomas among stage II | 0.348 | Dirichlet (4644, 3228, 1397) | |
| Proportion of stage IIc melanomas among stage II | 0.151 | Dirichlet (4644, 3228, 1397) | |
| Transitions of people with unidentified melanomas | Based on data reported in a Department of Health report82 and further assumptions; distribution based on assumption | ||
| Progression to next stage | 0.153 | Dirichlet (15.3, 35.0, 49.7) | |
| Identification | 0.350 | Dirichlet (15.3, 35.0, 49.7) | |
| Remaining unidentified | 0.497 | Dirichlet (15.3, 35.0, 49.7) | |
| Mortality | |||
| 5-year mortality – melanoma stage Ia | General population | No distribution assigned | For melanoma stage Ia: UK general population mortality was assumed, based on Office for National Statistics data;83 age- and sex-specific data utilised For all other melanoma stages: Balch et al.;81 annual probability of death estimated assuming exponential survivor function |
| 5-year mortality – melanoma stage Ib | 0.920 | Beta; α = 8,205, β = 713 | |
| 5-year mortality – melanoma stage IIa | 0.810 | Beta; α = 3,762, β = 882 | |
| 5-year mortality – melanoma stage IIb | 0.700 | Beta; α = 2,260, β = 968 | |
| 5-year mortality – melanoma stage IIc | 0.530 | Beta; α = 740, β = 657 | |
| 10-year mortality – melanoma stage Ia | General population | No distribution assigned | For melanoma stage Ia: UK general population mortality was assumed, based on Office for National Statistics data;83 age- and sex-specific data utilised For all other melanoma stages: Balch et al.;81 annual probability of death estimated assuming exponential survivor function and taking into account 5-year mortality |
| 10-year mortality – melanoma stage Ib | 0.860 | Beta; α = 7669, β = 1249 | |
| 10-year mortality – melanoma stage IIa | 0.670 | Beta; α = 3111, β = 1533 | |
| 10-year mortality – melanoma stage IIb | 0.570 | Beta; α = 1840, β = 1388 | |
| 10-year mortality – melanoma stage IIc | 0.390 | Beta; α = 545, β = 852 | |
| Utility values and related variables | |||
| Melanoma related | Tromme et al.;58 distributions determined by method of moments using data reported in the publication; all values adjusted for age; data for stages 0/Ia and Ib/II used to estimate a disutility for stages 0–II, assuming 1-month treatment for stages 0/Ia and 2-months treatment for stages Ib/II. More details in Utility values | ||
| Stage 0/Ia – treatment | 0.687 | Beta; α = 271.8, β = 123.8 | |
| Stage 0/Ia – remission | 0.809 | Beta; α = 381.5, β = 90.1 | |
| Stage Ib/II – treatment | 0.579 | Beta; α = 62.4, β = 45.4 | |
| Stage Ib/II – remission | 0.802 | Beta; α = 350.4, β = 86.5 | |
| Stage IV – treatment | 0.583 | Beta; α = 157.1, β = 112.3 | |
| General population | Sullivan et al.;86 distributions determined by method of moments using data reported in the publication | ||
| Aged 50–59 years | 0.798 | Beta; α = 10,500.0, β = 2657.9 | |
| Aged 60–69 years | 0.774 | Beta; α = 8900.7, β = 2598.9 | |
| Aged 70–79 years | 0.723 | Beta; α = 6029.9, β = 2310.2 | |
| Aged ≥ 80 years | 0.657 | Beta; α = 2631.4, β = 1373.8 | |
| Age coefficient | –0.00029 | Normal; 95% CI –0.0005917 to 0.0000129 | Sullivan et al.86 |
| Disutility because of the first excision of non-melanomas | –0.002 | 1 – beta; α = 827.7, β = 1.66 | Based on assumption and data reported in Seidler et al.57 |
| Disutility because of anxiety while waiting for biopsy results | –0.505 | 1 – beta; α = 3787.0, β = 3863.5 | Based on the UK EQ-5D valuation equation;62 distribution based on assumption; applied for 2 weeks |
| % with permanent disutility from scarring (head or neck) | 0.15 | Beta; α = 15, β = 85 | Clinical expert opinion; distribution based on assumption |
| Probability of simple closure/scar in first excision | 1 | No distribution assigned | Clinical expert opinion; distribution based on assumption |
| Probability of simple closure/scar in wider excision | 0.90 | Beta; α = 90, β = 10 | |
| Disutility because of simple closure | –0.016 | 1 – beta; α = 609.2, β = 9.9 | |
| Disutility because of complex closure | –0.026 | 1 – beta; α = 296.3, β = 7.9 | |
| Disutility because of scar first excision – permanent | –0.016 | Determined by distributions of linked variables | Seidler et al.;57 distributions determined by method of moments using data reported in the publication |
| Disutility because of scar second excision – permanent | –0.017 | ||
| Costs | NHS reference costs;12 for relevant NHS reference cost codes see text; distributions based on assumptions | ||
| Excision and biopsy | £151 | Gamma; SE = 0.1 × mean | |
| Monitoring or follow-up visit | £93 | Gamma; SE = 0.1 × mean | |
| Wide excision | £943 | Gamma; SE = 0.1 × mean | |
| Sentinel lymph node biopsy | £1033 | Gamma; SE = 0.1 × mean | |
| Terminal disease | £16,139 | Gamma; SE = 0.1 × mean | Estimated using data reported in the NICE STA of ipilimumab;89 distribution based on assumption |
| Newly identified melanomas | Curtis65 and NHS reference costs;12 for relevant NHS reference cost codes see text; distributions based on assumptions | ||
| GP visit | £67 | Gamma; SE = 0.1 × mean | |
| Dermatology first visit | £109 | Gamma; SE = 0.1 × mean | |
| First excision and biopsy cost | £151 | Gamma; SE = 0.1 × mean | |
| Diagnostic assessment model on suspected BCC | |||
| Mean age of the study population | 63 years | NA | Based on a review of studies and clinical expert advice |
| Proportion of men in the study population | 0.53 | Beta; α = 2508, β = 2240 | Deady et al.74 |
| Proportion of BCCs on head or neck | 0.69 | Beta; α = 915, β = 403 | Pignatelli et al.76 |
| Number of suspected/diagnosed BCCs per person | 1.09 | Gamma; SE = 0.1 × mean | Teoh et al.;75 distribution based on assumption, value imposed to be ≥ 1 |
| Prevalence of BCC in lesions found positive or equivocal on dermoscopy | 0.95 | Beta; α = 95, β = 5 | Clinical expert opinion; distribution based on assumption |
| Waiting time for biopsy results | 6 weeks | No distribution assigned | Clinical expert advice |
| Diagnostic accuracy of VivaScope 1500 | |||
| Sensitivity | 1.000 | Beta; α = 46, β = 1 | Castro et al.;43 uninformative prior distribution applied in sensitivity to deal with zero observations in β |
| Specificity | 0.778 | Beta; α = 7, β = 2 | |
| Diagnostic accuracy of VivaScope 3000 | |||
| Sensitivity | 0.933 | Beta; α = 42, β = 3 | Castro et al.43 |
| Specificity | 0.778 | Beta; α = 7, β = 2 | |
| Diagnostic accuracy of biopsy | |||
| Sensitivity | 1.000 | No distributions assigned | Considered to be the gold standard for diagnosis |
| Specificity | 1.000 | ||
| Utility values and related variables | |||
| Disutility because of the diagnostic biopsy | –0.002 | 1 – beta; α = 827.7, β = 1.7 | Based on assumption and data reported in Seidler et al.;57 estimated using method of moments Based on the UK EQ-5D valuation equation;62 distribution based on assumption; applied for 6 weeks |
| Disutility because of surgical or unnecessary treatment | –0.004 | 1 – beta; α = 411.7, β = 1.7 | |
| Disutility because of anxiety while waiting for biopsy results | –0.071 | 1 – beta; α = 531.6, β = 40.6 | |
| % with permanent disutility from scarring | |||
| Because of biopsy (head or neck) | 0.05 | Beta; α = 5, β = 95 | Clinical expert opinion; distributions based on assumption |
| Because of surgical treatment (head or neck) | 0.15 | Beta; α = 15, β = 85 | |
| Probability of simple closure/scar | |||
| Suspected BCC biopsy | 1 | No distribution assigned | Clinical expert opinion; distributions based on assumption |
| BCC surgical excision | 0.75 | Beta; α = 75, β = 25 | |
| BCC Mohs surgery | 0.50 | Beta; α = 50, β = 50 | |
| Disutility because of simple closure | –0.016 | 1 – beta; α = 609.2, β = 9.9 | Seidler et al.;57 distributions determined by method of moments using data reported in the publication |
| Disutility because of complex closure | –0.026 | 1 – beta; α = 296.3, β = 7.9 | |
| Disutility because of the scar from the biopsy – permanent | –0.016 | Determined by distributions of linked variables | |
| Disutility because of the scar from surgical treatment – permanent | –0.019 | ||
| Resource use | |||
| Surgical treatment of BCC | |||
| % of BCC treatment that is surgical | 0.75 | Beta; α = 75, β = 25 | Clinical expert advice; distributions based on assumptions |
| % of surgical excision in BCC surgical treatment | 0.85 | Beta; α = 85, β = 15 | |
| No surgical treatment of BCC | |||
| % imiquimod | 0.30 | Dirichlet; (30, 21, 19, 30) | Published audit data,94 modified following clinical expert advice |
| % photodynamic therapy | 0.21 | Dirichlet; (30, 21, 19, 30) | |
| % radiotherapy | 0.19 | Dirichlet; (30, 21, 19, 30) | |
| % 5-fluorouracil | 0.30 | Dirichlet; (30, 21, 19, 30) | |
| Costs | |||
| Diagnostic biopsy | £134 | Gamma; SE = 0.1 × mean | Cost of procedures based on NHS reference costs,12 except cost of radiotherapy, which was based on clinical expert opinion; cost of drugs from NHS Business Services;64 for details see Table 32 |
| Surgical excision | £388 | Gamma; SE = 0.1 × mean | |
| Mohs surgery | £943 | Gamma; SE = 0.1 × mean | |
| Imiquimod | £142 (£49 + £93) | For £93: Gamma; SE = 0.1 × mean | |
| 5-Fluorouracil | £126 (£33 + £93) | For £93: Gamma; SE = 0.1 × mean | |
| Radiotherapy | £753 | Gamma; SE = 0.1 × mean | |
| Photodynamic therapy | £1303 | Gamma; SE = 0.1 × mean | |
| BCC treatment cost | £475 | Determined by distributions of linked variables | |
| Margin delineation model on LMs prior to surgical treatment | |||
| Mean age of the study population | 70 years | NA | Based on a review of studies and clinical expert advice |
| Proportion of men in the study population | 0.64 | Beta; α = 93, β = 52 | Mirzoyev et al.70 |
| Proportion of LMs on head or neck | 0.70 | Beta; α = 70, β = 30 | Clinical expert opinion |
| Number of LMs per person | 1 | No distribution assigned | Clinical expert advice |
| Incomplete surgical excision | |||
| Mapping with VivaScope 3000 | 0.12 | Beta; α = 2, β = 15 | Guitera et al.35 |
| Routine management | 0.30 | Beta; α = 30, β = 70 | Based on a review of studies and clinical expert opinion; distribution based on assumptions |
| Number of stages in Mohs surgery | |||
| Mapping with VivaScope 3000 | 1.40 | Normal; n = 7; SD = 0.53 | Daly et al.101 |
| Routine management | 2.22 | Normal; n = 9; SD = 1.2 | |
| Annual recurrence of LM | |||
| Surgical excision | 0.032 | Beta; α = 3.2, β = 96.8 | Based on a review of studies, clinical expert opinion and further assumptions |
| Mohs surgery (applied also to recurrence after mapping with VivaScope 3000, regardless of type of surgical treatment) | 0.010 | Beta; α = 1, β = 99 | |
| Utility values and related variables | |||
| Disutility because of surgical treatment | –0.004 | 1 – beta; α = 411.7, β = 1.7 | Seidler et al.;57 estimated using method of moments |
| % with permanent disutility from scarring because of surgical treatment (head or neck) | 0.15 | Beta; α = 15, β = 85 | Clinical expert opinion; distribution based on assumption |
| Probability of simple closure/scar in surgical treatment LM | 0.50 | Beta; α = 50, β = 50 | Clinical expert opinion; distribution based on assumption |
| Disutility because of simple closure | –0.016 | 1 – beta; α = 609.2, β = 9.9 | Seidler et al.;57 distributions determined by method of moments using data reported in the publication |
| Disutility because of complex closure | –0.026 | 1 – beta; α = 296.3, β = 7.9 | |
| Disutility because of scarring from surgical treatment – permanent | –0.021 | Determined by distributions of linked variables | |
| Resource use | |||
| Dermatologist’s time for routine mapping | 5 minutes | Normal; SE: 0.1 × mean | Clinical expert opinion; distribution based on assumption, a minimum value of 3 minutes imposed |
| % of surgical excision in surgical treatment | |||
| First surgical treatment | 0.85 | Beta; α = 85, β = 15 | Clinical expert advice; distributions based on assumptions |
| Surgical treatment following recurrence | 0.80 | Beta; α = 80, β = 20 | |
| Mean number of stages in Mohs surgery | 1.6 | No distribution assigned | Merritt et al.102 |
| Costs | |||
| Routine mapping with dermoscopy | £12 | Determined by distribution of dermatologist’s time for routine mapping | |
| Surgical excision | £388 | Gamma; SE = 0.1 × mean | |
| Mohs surgery (70% of cost assumed to be fixed, 30% of cost attributed to 1.6 stages) | £943 | Gamma; SE = 0.1 × mean | NHS reference costs;12 more details on relevant NHS codes provided in the text (see Costs); distributions based on assumptions |
| UK general population mortality risk (applied across all models as appropriate) | Available in National Life Tables for Years 2011–2013, United Kingdom83 | No distribution assigned | Based on UK national mortality statistics UK;83 age- and sex-specific data utilised |
| Annual discount rate | 0.035 | No distribution assigned | As recommended by NICE47 |
Table 36 provides the mean and the range of values of the most influential model input parameters depicted in tornado diagrams, together with a justification of the extreme values used for each parameter.
| Input parameter | Mean value | Low value | High value | Justification of range |
|---|---|---|---|---|
| Annual number of lesions eligible for examination with VivaScope | ||||
| Suspected melanomas | 100 | 70 | 130 | ± 30% of the mean value (assumption) |
| Suspected BCCs | 500 | 250 | 750 | |
| LMs prior to surgery | 75 | 52.5 | 97.5 | |
| Diagnostic assessment of suspected melanomas | ||||
| Prevalence of melanoma in equivocal lesions | 0.15 | 0.075 | 0.225 | ± 50% of the mean value (assumption) |
| Proportion of equivocal lesions excised under routine management | 0.67 | 0 | 1 | Whole plausible range tested |
| VivaScope sensitivity, Alarcon et al.30 | 0.978 | 0.924 | 0.997 | Alarcon et al.30; 95% CIs |
| VivaScope specificity, Alarcon et al.30 | 0.948 | 0.913 | 0.972 | |
| VivaScope sensitivity, highly suspicious lesions, Pellacani et al.42 | 1.000 | 0.915 | 1.000 | Pellacani et al.;42 95% CIs |
| VivaScope specificity, highly suspicious lesions, Pellacani et al.42 | 0.518 | 0.432 | 0.6026 | |
| VivaScope sensitivity, moderately/low suspicious lesions, Pellacani et al.42 | 1.000 | 0.862 | 1.000 | |
| VivaScope specificity, moderately/low suspicious lesions, Pellacani et al.42 | 0.802 | 0.751 | 0.847 | |
| Disutility because of the first excision of non-melanomas | –0.002 | –0.004 | –0.001 | Lower value assumed to be equal to disutility from wide excision; upper value based on assumption |
| Disutility because of anxiety while waiting for biopsy results | –0.505 | –0.556 | –0.051 | Lower value assumed to be 10% lower than the mean; upper value assumed to be 10% of the mean |
| % with permanent disutility from scarring (head or neck) | 0.15 | 0 | 1 | Whole plausible range tested |
| Disutility caused by scar first excision – permanent | –0.016 | –0.032 | –0.001 | Lower value assumed to be 100% lower than the mean; upper value based on assumption |
| Cost of excision and biopsy | £151 | £106 | £196 | ± 30% of the mean value (assumption) |
| Diagnostic assessment of suspected BCCs | ||||
| Number of suspected/diagnosed BCCs per person | 1.09 | 1 | 1.60 | Lower value lowest possible value; upper value based on Castro et al.43 |
| Prevalence of BCC in lesions found positive or equivocal on dermoscopy | 0.95 | 0.83 | 0.99 | Lower value taken from Castro et al.;43 upper value based on assumption |
| Sensitivity of VivaScope 3000 | 0.933 | 0.821 | 0.977 | Castro et al.;43 95% CIs |
| Disutility because of the diagnostic biopsy | –0.002 | –0.004 | –0.001 | Lower value assumed to be equal to disutility from surgical treatment; upper value based on assumption |
| Disutility because of anxiety while waiting for biopsy results | –0.071 | –0.142 | –0.007 | Lower value assumed to be 100% lower than the mean; upper value assumed to be 10% of the mean |
| % with permanent disutility from scarring because of the biopsy (head or neck) | 0.05 | 0 | 0.80 | Assumption |
| % with permanent disutility from scarring because of surgical treatment (head or neck) | 0.15 | 0 | 0.80 | Assumption |
| Permanent disutility caused by scar from biopsy | –0.016 | –0.032 | –0.001 | Lower value assumed to be 100% lower than the mean; upper value based on assumption |
| % of BCC treatment that is surgical | 0.75 | 0.60 | 0.95 | Assumptions based on discussions with clinical experts |
| Cost of diagnostic biopsy | £134 | £94 | £174 | ± 30% of the mean value (assumption) |
| Margin delineation of LMs | ||||
| VivaScope mapping – incomplete surgical excision | 0.12 | 0.033 | 0.343 | Guitera et al.;35 95% CIs |
| Routine management – incomplete surgical excision | 0.30 | 0.15 | 0.45 | ± 50% of the mean value (assumption) |
| Routine management – number of Mohs stages | 2.22 | 1.44 | 3.00 | Daly et al.;101 95% CIs |
| Routine management – annual recurrence after surgical excision | 0.032 | 0.012 | 0.048 | Lower value based on Hou et al.;100 upper value assumed to be 50% higher than the mean |
| VivaScope mapping – annual recurrence after surgical excision | 0.010 | 0.002 | 0.015 | Lower value based on Hou et al.;100 upper value assumed to be 50% higher than the mean |
| Disutility caused by surgical treatment | –0.004 | –0.008 | –0.001 | Lower value assumed to be 100% lower than the mean; upper value based on assumption |
| % with permanent disutility from scar caused by surgical treatment (head or neck) | 0.15 | 0 | 0.80 | Assumption |
| Permanent disutility because of a scar from surgical treatment | –0.021 | –0.042 | –0.001 | Lower value assumed to be 100% lower than the mean; upper value based on assumption |
Additional scenarios tested in one-way sensitivity analysis
Further to tornado diagrams, which depicted the impact of the most influential input parameters on the results of the economic analysis, additional sensitivity analyses were carried out to explore the robustness of the results under alternative scenarios and model assumptions. The following alternative scenarios were explored.
Relating to the cost of VivaScope examination:
-
The estimated staff time cost for the diagnosis of skin lesions suspicious of cancer was replaced by the NHS reference cost of £47 for a direct-access ultrasound scan of < 20 minutes, as a proxy;12 the estimated staff time cost for mapping of skin lesions prior to surgical treatment was replaced by the NHS reference cost of £109 for a consultant-led, outpatient, dermatology first visit. 12
-
The cost associated with training was doubled, to account for the extra training required over the first few months in order for dermatologists to gain experience in the clinical interpretation of the results obtained from the examination of lesions with VivaScope. In addition, the useful time of training was reduced to 5 years.
Relating to the diagnostic model on suspected melanomas:
-
People waiting for the results of biopsy were assumed to experience moderate rather than severe anxiety; therefore, a much lower disutility of anxiety of –0.071 was used in this scenario, as estimated from the health state valuation equation provided by Dolan62 for EQ-5D, rather the value of –0.505 that was used in the base-case analysis.
Relating to the diagnostic model on suspected BCCs:
-
Clinical experts advised that, in reality, not all suspected BCCs receive diagnostic biopsy following dermoscopy, but some move on directly to treatment. Therefore, a scenario was tested where only 70% of suspected BCCs received a diagnostic biopsy under routine care; that is, only the 70% of the diagnostic biopsy cost was applied and only 70% of people were assumed to experience disutility associated with biopsy and permanent scarring following biopsy on the head or neck (unless surgical treatment was received). For simplicity, it was assumed that the percentage of 70% of suspected BCCs that received diagnostic biopsy did not distinguish between true BCCs and no BCCs; in other words, both suspected BCC lesions that proved to be BCCs and suspected BCCs that were not actually BCCs were subject to a 0.7 probability of biopsy under this scenario.
In addition to the above scenarios, the ICERs obtained in each model were plotted against different values of the annual number of each type of lesion examined with VivaScope (i.e. equivocal lesions suspicious of melanoma, suspected BCCs that give a positive or equivocal finding on dermoscopy and LMs prior to surgical treatment) to identify the minimum number of each type of lesion that is required to be examined with VivaScope per year, so that examination with VivaScope is a cost-effective strategy.
Finally, for the diagnostic model on suspected melanomas that utilised diagnostic accuracy data from Pellacani et al. ,42 the impact of the percentage of the suspected melanomas with an equivocal finding on dermoscopy that were excised (i.e. because they were highly suspicious) on the results was assessed by plotting the ICER obtained in the respective analysis against the whole range of the probability of suspected melanomas being excised (i.e. 0–100%). This was decided because the percentage of the suspected melanomas that were excised on the results of the analysis had a twofold impact:
-
An increase in the percentage of suspected melanomas that were excised led to a lower diagnostic accuracy of VivaScope examination, as Pellacani et al. 42 reported a lower specificity for VivaScope in lesions that were chosen for excision as highly suspicious following dermoscopy than the specificity of VivaScope in less suspicious lesions that were selected for monitoring based on the results of dermoscopy. Consequently, an increase in the percentage of suspected melanomas being excised reduced the benefit of VivaScope in the model.
-
At the same time, an increase in the percentage of suspected melanomas that were excised led to an increase in the cost of routine management (as the cost of excision is higher than the cost of monitoring) and an increase in the disutility because of excision, permanent disutility because of scarring (relevant to lesions on the head or neck) and disutility because of anxiety while waiting for the results. Consequently, an increase in the percentage of suspected melanomas that were being excised increased the cost of routine management and reduced its benefit.
Results of economic modelling
Base-case deterministic and probabilistic results
The base-case deterministic and probabilistic results for each of the economic models and analyses considered for this report are provided in Tables 37–46. For each type of lesion, different cost and cost-effectiveness results are presented, depending on the types of lesions expected to be examined with VivaScope; the latter determined the cost of VivaScope, as the total cost associated with acquisition and use of the device was spread across the annual number of lesions examined with VivaScope in order to determine a cost per lesion.
| Intervention | Total cost (£) | Total QALYs |
|---|---|---|
| VivaScope examination | ||
| VivaScope use only for melanoma diagnosis | 517.23 | 13.222 |
| VivaScope use for diagnosis | 326.52 | |
| VivaScope use for all indications | 322.28 | |
| Routine management | 379.24 | 13.206 |
| Incremental | ||
| VivaScope use only for melanoma diagnosis | 137.99 | 0.016 |
| VivaScope use for diagnosis | –52.71 | |
| VivaScope use for all indications | –56.95 | |
| Cost-effectiveness | ||
| VivaScope use only for melanoma diagnosis | 8877/QALY | |
| VivaScope use for diagnosis | VivaScope dominant | |
| VivaScope use for all indications | VivaScope dominant | |
| Intervention | Total cost (£) | Total QALYs |
|---|---|---|
| VivaScope examination | ||
| VivaScope use only for melanoma diagnosis | 524.82 | 13.222 |
| VivaScope use for diagnosis | 327.83 | |
| VivaScope use for all indications | 323.35 | |
| Routine management | 379.52 | 13.206 |
| Incremental | ||
| VivaScope use only for melanoma diagnosis | 145.31 | 0.016 |
| VivaScope use for diagnosis | –51.69 | |
| VivaScope use for all indications | –56.16 | |
| Cost-effectiveness | ||
| VivaScope use only for melanoma diagnosis | 9362/QALY | |
| VivaScope use for diagnosis | VivaScope dominant | |
| VivaScope use for all indications | VivaScope dominant | |
| Intervention | Total cost (£) | Total QALYs |
|---|---|---|
| VivaScope examination | ||
| VivaScope use only for melanoma diagnosis | 556.27 | 13.215 |
| VivaScope use for diagnosis | 365.56 | |
| VivaScope use for all indications | 361.32 | |
| Routine management | 379.24 | 13.206 |
| Incremental | ||
| VivaScope use only for melanoma diagnosis | 177.03 | 0.009 |
| VivaScope use for diagnosis | –13.67 | |
| VivaScope use for all indications | –17.91 | |
| Cost-effectiveness | ||
| VivaScope use only for melanoma diagnosis | 19,095/QALY | |
| VivaScope use for diagnosis | VivaScope dominant | |
| VivaScope use for all indications | VivaScope dominant | |
| Intervention | Total cost (£) | Total QALYs |
|---|---|---|
| VivaScope examination | ||
| VivaScope use only for melanoma diagnosis | 566.91 | 13.214 |
| VivaScope use for diagnosis | 369.63 | |
| VivaScope use for all indications | 365.12 | |
| Routine management | 379.40 | 13.207 |
| Incremental | ||
| VivaScope use only for melanoma diagnosis | 187.51 | 0.007 |
| VivaScope use for diagnosis | –9.78 | |
| VivaScope use for all indications | –14.29 | |
| Cost-effectiveness | ||
| VivaScope use only for melanoma diagnosis | 25,453/QALY | |
| VivaScope use for diagnosis | VivaScope dominant | |
| VivaScope use for all indications | VivaScope dominant | |
| Intervention | Total cost (£) | Total QALYs |
|---|---|---|
| VivaScope examination | ||
| VivaScope use only for BCC diagnosis | 585.82 | –0.025 |
| VivaScope use for diagnosis | 577.50 | |
| VivaScope use for all indications | 572.88 | |
| Routine management | 637.92 | –0.036 |
| Incremental | ||
| VivaScope use only for BCC diagnosis | –52.10 | 0.011 |
| VivaScope use for diagnosis | –60.42 | |
| VivaScope use for all indications | –65.04 | |
| Cost-effectiveness | ||
| VivaScope use only for BCC diagnosis | VivaScope dominant | |
| VivaScope use for diagnosis | VivaScope dominant | |
| VivaScope use for all indications | VivaScope dominant | |
| Intervention | Total cost (£) | Total QALYs |
|---|---|---|
| VivaScope examination | ||
| VivaScope use only for BCC diagnosis | 594.93 | –0.025 |
| VivaScope use for diagnosis | 585.85 | |
| VivaScope use for all indications | 580.91 | |
| Routine management | 644.87 | –0.036 |
| Incremental | ||
| VivaScope use only for BCC diagnosis | –49.93 | 0.011 |
| VivaScope use for diagnosis | –59.02 | |
| VivaScope use for all indications | –63.96 | |
| Cost-effectiveness | ||
| VivaScope use only for BCC diagnosis | VivaScope dominant | |
| VivaScope use for diagnosis | VivaScope dominant | |
| VivaScope use for all indications | VivaScope dominant | |
| Intervention | Total cost (£) | Total QALYs |
|---|---|---|
| VivaScope examination | ||
| VivaScope use only for LM mapping | 801.98 | –0.034 |
| VivaScope use for all indications | 657.12 | |
| Routine management | 731.24 | –0.041 |
| Incremental | ||
| VivaScope use only for LM mapping | 70.75 | 0.007 |
| VivaScope use for all indications | –74.12 | |
| Cost-effectiveness | ||
| VivaScope use only for LM mapping | 10,241/QALY | |
| VivaScope use for all indications | VivaScope dominant | |
| Intervention | Total cost (£) | Total QALYs |
|---|---|---|
| VivaScope examination | ||
| VivaScope use only for LM mapping | 809.69 | –0.034 |
| VivaScope use for all indications | 659.55 | |
| Routine management | 731.16 | –0.040 |
| Incremental | ||
| VivaScope use only for LM mapping | 78.53 | 0.007 |
| VivaScope use for all indications | –71.60 | |
| Cost-effectiveness | ||
| VivaScope use only for LM mapping | 11,651/QALY | |
| VivaScope use for all indications | VivaScope dominant | |
| Intervention | Total cost (£) | Total QALYs |
|---|---|---|
| VivaScope examination | 532.56 | 2.347 |
| Routine management | 591.60 | 2.335 |
| Incremental | –59.04 | 0.012 |
| Cost-effectiveness | VivaScope dominant | |
| Intervention | Total cost (£) | Total QALYs |
|---|---|---|
| VivaScope examination | 543.29 | 2.065 |
| Routine management | 608.13 | 2.054 |
| Incremental | –64.84 | 0.011 |
| Cost-effectiveness | VivaScope dominant | |
Results of the diagnostic model of suspected melanomas are presented in Tables 37 and 38 (results derived when diagnostic data from Alarcon et al. 30 were utilised) and in Tables 39 and 40 (results derived when diagnostic data from Pellacani et al. 42 were utilised). It can be seen that under use of the more optimistic diagnostic data from Alarcon et al. ,30 VivaScope appears to be cost-effective in the diagnostic assessment of suspected melanomas with an equivocal finding on dermoscopy, even when VivaScope is exclusively used for this purpose (with an ICER of £8877 per QALY in deterministic analysis and £9362 per QALY in probabilistic analysis). On the other hand, use of the diagnostic data from Pellacani et al. 42 resulted in an ICER of £19,095 per QALY in deterministic analysis and £25,453 per QALY in probabilistic analysis when VivaScope was considered for only the diagnostic assessment of equivocal lesions suspicious of melanoma. Nevertheless, if VivaScope is expected to be used in the diagnostic assessment of both suspected melanomas and suspected BCCs, or also in the mapping of LMs prior to surgical treatment, then VivaScope becomes dominant in the diagnostic assessment of melanomas, as the cost associated with its use is spread across a larger number of sessions, leading to the total cost associated with VivaScope examination in the economic model being lower than the total cost associated with routine management of equivocal lesions suspicious of melanoma.
VivaScope was shown to be the dominant strategy when used for the assessment of suspected BCCs, regardless of its estimated use exclusively for this purpose or for the assessment of suspected melanomas and LMs as well (see Tables 41 and 42). Consideration of the use of VivaScope for other indications, further to its use on the diagnostic assessment of suspected BCCs, had little impact on the results as the annual number of suspected BCCs is much higher than the annual number of other lesions expected to be examined with VivaScope and, therefore, this number of suspected BCCs drives the cost per lesion examined with BCC and, subsequently, the cost-effectiveness results.
Regarding margin delineation of LMs, mapping with VivaScope was shown to be cost-effective, even if it used exclusively for this purpose, as indicated by an ICER of £10,241 per QALY obtained in deterministic analysis (see Table 43) and £11,651 per QALY in probabilistic analysis (see Table 44). When use of VivaScope was expanded to other indications covered in this economic analysis, then VivaScope became the dominant option.
Overall, in the analyses that combined the different ‘part’ models designed for this report, VivaScope was shown to be the dominant strategy over routine management in the diagnostic assessment of suspected melanomas and BCCs (see Table 45) and in the diagnostic assessment of suspected melanomas and BCCs combined with margin delineation of LMs prior to surgical treatment (see Table 46). The tables show the deterministic results, but probabilistic results were very similar.
The cost-effectiveness planes of all the probabilistic analyses undertaken for this assessment are provided in Appendix 6.
The CEACs for each part model considered in the analysis are provided in Figures 9–12. Figure 9 indicates that, using the diagnostic accuracy data from Alarcon et al. ,30 the probability of VivaScope being cost-effective in the diagnostic assessment of suspected melanomas is zero at a zero WTP per QALY gained, but reaches 0.99 at the lower NICE cost-effectiveness threshold of £20,000 per QALY when VivaScope is used only for this purpose (i.e. diagnostic assessment of suspected melanomas). When VivaScope is used for the diagnostic assessment of suspected melanomas and BCCs or a combination of diagnosis of suspected melanomas and BCCs and presurgical margin delineation of LMs, then the probability of being it cost-effective in the diagnosis of suspected melanomas is 1 and is independent of the level of WTP considered.
FIGURE 9.
Cost-effectiveness acceptability curve: diagnostic model on suspected melanomas – diagnostic data based on Alarcon et al. 30
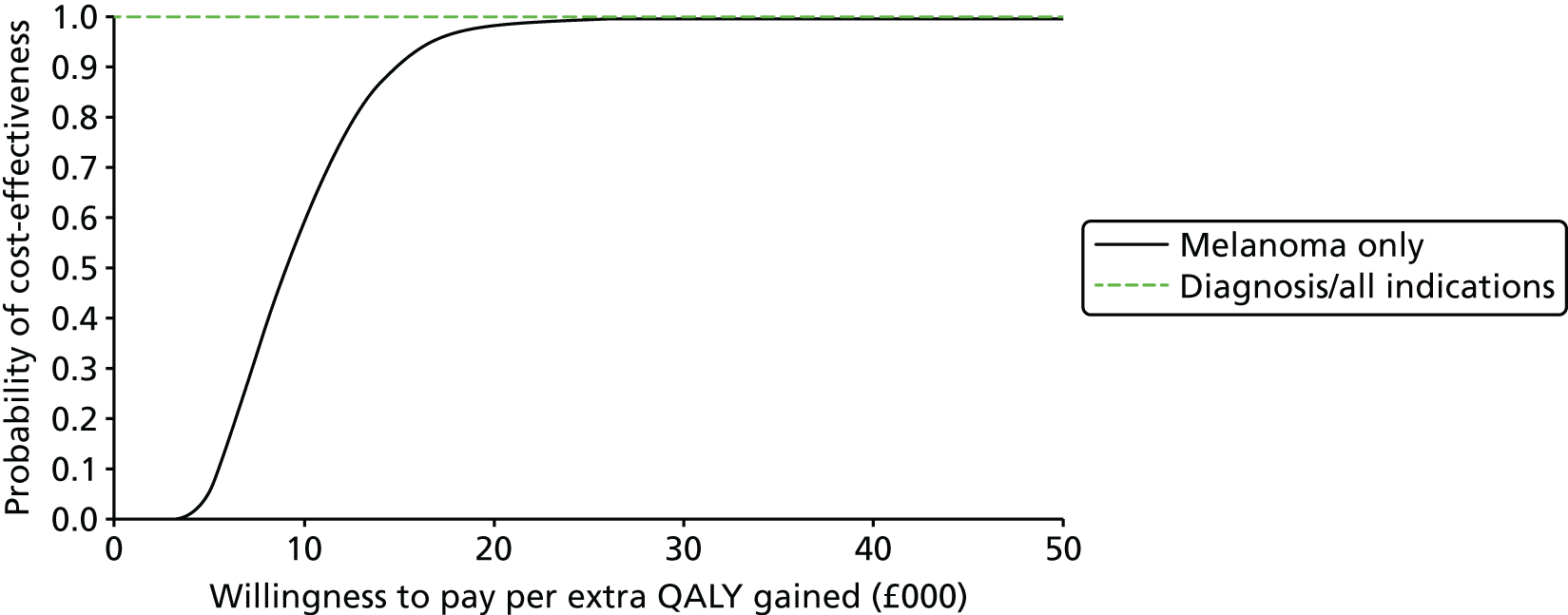
FIGURE 10.
Cost-effectiveness acceptability curve: diagnostic model on suspected melanomas – diagnostic data based on Pellacani et al. 42
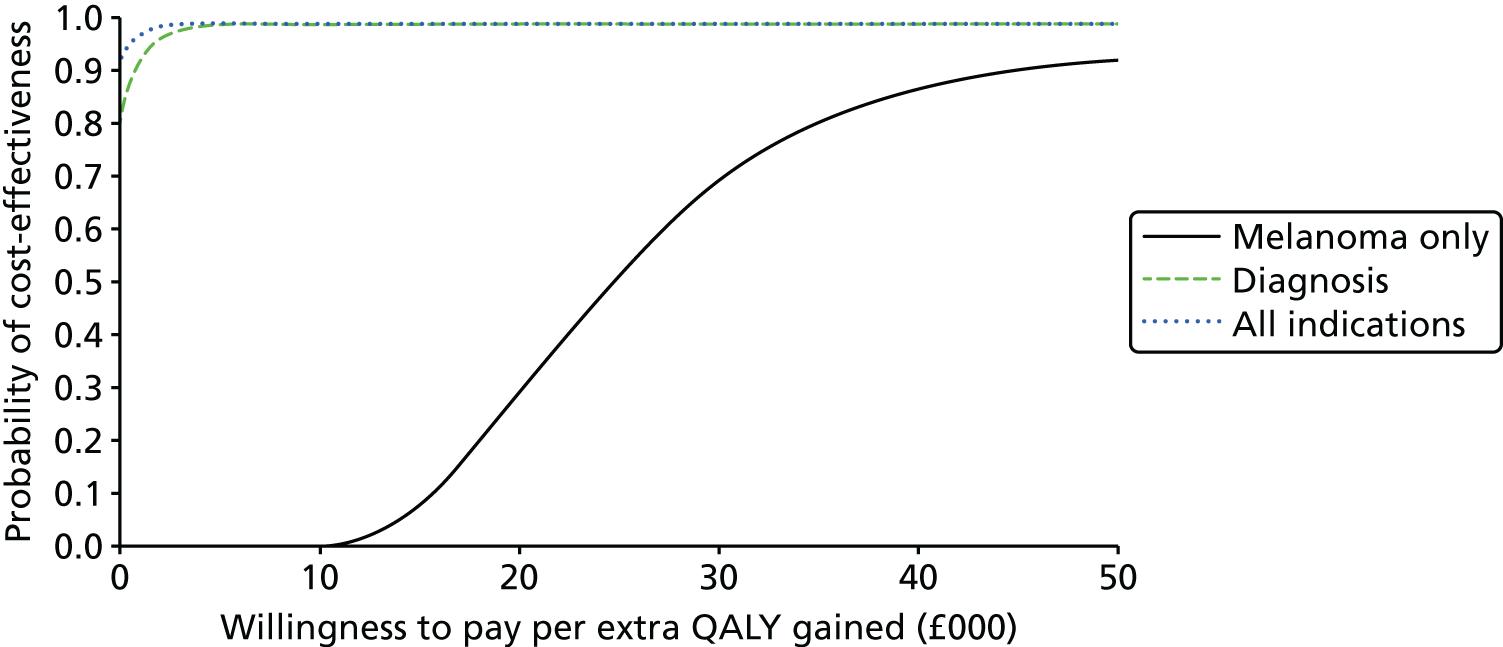
FIGURE 11.
Cost-effectiveness acceptability curve: diagnostic model on suspected BCCs.

FIGURE 12.
Cost-effectiveness acceptability curve: presurgical margin delineation model on LMs.

Figure 10 shows the CEAC derived when using the diagnostic accuracy data for suspected melanomas from Pellacani et al. 42 In this case, the probability of VivaScope being cost-effective when used exclusively in the diagnostic assessment of suspected melanomas is 0.29 and 0.69 at the lower and upper NICE cost-effectiveness threshold, respectively. When the use of VivaScope is expanded to the diagnostic assessment of suspected BCCs or all indications examined in this analysis, its probability of cost-effectiveness in the diagnostic assessment of suspected melanomas reaches 0.99 at the NICE lower cost-effectiveness threshold of £20,000 per QALY.
Regarding the probability of cost-effectiveness of VivaScope in the diagnostic assessment of suspected BCCs, Figure 11 shows that this is 1, regardless of whether VivaScope is used exclusively for this purpose or its use is expanded to other indications examined in this economic evaluation, and is independent of the cost-effectiveness threshold used.
Finally, Figure 12 provides the CEAC for the model assessing the cost-effectiveness of VivaScope in the presurgical margin delineation of LMs. It shows that when VivaScope is used exclusively for this purpose, its probability of being cost-effective is 0.62 and 0.74 at the lower and upper NICE cost-effectiveness threshold, respectively. However, when VivaScope is used for all indications considered in economic modelling, its cost-effectiveness in the presurgical margin delineation of LMs improves, and its probability of being cost-effective rises up to 0.92 and 0.94 at the lower and upper NICE cost-effectiveness threshold, respectively.
Results of one-way and two-way sensitivity analyses
One-way sensitivity analyses were performed on all input parameters that were given a probability distribution in the economic model. The tornado diagrams that present the impact of the most influential input parameters on the results are shown in Appendix 6. It is evident that among the most influential parameters across all models are those relating to permanent disutility because of scarring (such as the percentage of people experiencing permanent disutility as well as the value of disutility itself) and the disutility because of anxiety while waiting for the results of biopsy. Overall, the most influential parameters included the following points.
In the diagnostic assessment of suspected melanomas:
-
the percentage of people experiencing permanent disutility because of scarring
-
the disutility because of anxiety while waiting for the biopsy results
-
the percentage of equivocal lesions excised under routine management (this parameter was not considered in the tornado diagrams when Pellacani et al. 42 data were used, because of its twofold impact on the results, which would lead to a misleading picture in the tornado diagram)
-
the permanent disutility because of scarring from first excision
-
the annual number of suspected melanomas eligible for examination for VivaScope (if VivaScope was used exclusively for examination of suspected melanomas)
-
the VivaScope sensitivity and specificity
-
the prevalence of melanomas in equivocal lesions
-
the cost of first excision
-
the disutility because of the first excision.
It should be noted that when VivaScope was assumed to be used exclusively for the diagnosis of suspected melanomas and diagnostic data from Alarcon et al. 30 were utilised, the only parameter that potentially resulted in negative INMBs in the tornado diagram was the disutility because of anxiety. When VivaScope was assumed to be used exclusively for the diagnosis of suspected melanomas and diagnostic data from Pellacani et al. 42 were utilised, then several parameters resulted in negative INMBs. However, when use of VivaScope was assumed to expand to diagnosis of suspected BCCs as well, none of the influential parameters could result in a negative INMB. Tornado diagrams were not produced for the scenario of VivaScope being used for all indications suggested in this economic analysis, as results were expected to be similar to those produced when diagnosis of both suspected melanomas and suspected BCCs was informed by VivaScope.
In the diagnostic assessment of suspected BCCs:
-
the percentage of people experiencing permanent disutility because of scarring from biopsy
-
the disutility because of anxiety while waiting for the results
-
the diagnostic biopsy cost
-
the prevalence of BCC in examined lesions
-
the permanent disutility because of scarring from biopsy
-
the annual number of suspected BCCs that would be examined with VivaScope
-
the disutility because of the biopsy
-
the percentage of patients treated with surgical therapy
-
the sensitivity of VivaScope 3000
-
the number of lesions per person
-
the percentage of people experiencing permanent disutility because of scarring from surgery.
However, none of the parameters had such an impact so as to turn the INMB to negative values, even when VivaScope was used exclusively in the diagnostic assessment of suspected BCCs. For this reason, tornado diagrams relating to expansion of use of VivaScope for the assessment of other types of lesions were not produced, as expansion of use of VivaScope would only reduce the impact of influential parameters on the results even further.
In the presurgical mapping of LMs:
-
the probability of incomplete surgical excision following routine mapping
-
the probability of annual recurrence after surgical excision
-
the probability of incomplete surgical excision following mapping with VivaScope
-
the permanent disutility because of scarring from surgical treatment
-
the percentage of people with permanent disutility from scarring
-
the probability annual recurrence following VivaScope mapping and surgical excision
-
the VivaScope mapping (staff) time
-
the cost of surgical excision
-
the number of Mohs stages under routine mapping
-
the disutility caused by surgery.
As with the results for suspected melanomas, a number of influential parameters could turn the INMB into a negative value if VivaScope was used only for the mapping of LMs prior to surgical treatment. However, when a wider use of VivaScope was assumed, the INMB remained positive under any values of the influential parameters examined.
The results of the additional sensitivity analyses are shown in Table 47. It can be seen that results for suspected melanoma are negatively affected after application of relevant scenarios, when diagnostic accuracy data from Pellacani et al. 42 are used and VivaScope is assumed to be exclusively used for the diagnostic assessment of suspected melanomas. However, when wider use of VivaScope is assumed, the results are practically unaffected by the scenarios tested.
| Scenario | Intended use of VivaScope | ICER | ||
|---|---|---|---|---|
| Suspected melanomas | Suspected BCCs | LM | ||
| Staff time cost for diagnosis replaced by ultrasound scan unit cost of £47; staff time cost for mapping replaced by outpatient dermatology visit of £109 | Only for this purpose | £10,467/QALY (A)a £21,761/QALY (P)b |
VivaScope dominant | £15,887/QALY |
| Diagnosis | VivaScope dominant (A) £1191/QALY (P) |
VivaScope dominant | NA | |
| All indications | VivaScope dominant (A) £734/QALY (P) |
VivaScope dominant | VivaScope dominant | |
| VivaScope training cost doubled and its useful life reduced to 5 years | Only for this purpose | £12,451/QALY (A) £25,086/QALY (P) |
VivaScope dominant | £20,964/QALY |
| Diagnosis | VivaScope dominant (A and P) | VivaScope dominant | NA | |
| All indications | VivaScope dominant (A and P) | VivaScope dominant | VivaScope dominant | |
| Melanoma – moderate disutility because of anxiety while waiting for the results | Only for this purpose | £22,983/QALY (A) £40,943/QALY (P) |
NA | NA |
| Diagnosis | VivaScope dominant (A and P) | NA | NA | |
| All indications | VivaScope dominant (A and P) | NA | NA | |
| Diagnostic biopsy assumed to be performed in only 70% of suspected BCCs | Only for this purpose | NA | VivaScope dominant | NA |
| Diagnosis | NA | VivaScope dominant | NA | |
| All indications | NA | VivaScope dominant | NA | |
Two-way sensitivity analyses were performed to test the impact of different combinations of sensitivity and specificity of VivaScope on its cost-effectiveness in the diagnostic assessment of equivocal lesions suspicious of melanoma. The results on the diagnosis of suspected melanomas are shown in Tables 48 and 49. The results indicate that VivaScope needs to have a relatively high diagnostic accuracy in order to be cost-effective, in particular when it is used exclusively for the diagnostic assessment of suspected melanomas. A two-way sensitivity analysis for the diagnosis of suspected BCCs showed that any combination of sensitivity and specificity from values as low as 0.40 resulted in VivaScope being a cost-effective strategy (the maximum ICER, when sensitivity and specificity were 0.40, was £7083/QALY).
| Sensitivity of VivaScope | Specificity of VivaScope | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.50 | 0.55 | 0.60 | 0.65 | 0.70 | 0.75 | 0.80 | 0.85 | 0.90 | 0.95 | 1.00 | |
| 0.50 | –£897 | –£866 | –£835 | –£804 | –£773 | –£742 | –£711 | –£680 | –£649 | –£618 | –£587 |
| 0.55 | –£814 | –£783 | –£752 | –£721 | –£690 | –£659 | –£628 | –£597 | –£566 | –£535 | –£504 |
| 0.60 | –£731 | –£700 | –£669 | –£638 | –£607 | –£576 | –£545 | –£514 | –£483 | –£452 | –£421 |
| 0.65 | –£649 | –£618 | –£587 | –£556 | –£525 | –£494 | –£463 | –£432 | –£401 | –£370 | –£339 |
| 0.70 | –£566 | –£535 | –£504 | –£473 | –£442 | –£411 | –£380 | –£349 | –£318 | –£287 | –£256 |
| 0.75 | –£483 | –£452 | –£421 | –£390 | –£359 | –£328 | –£297 | –£266 | –£235 | –£204 | –£173 |
| 0.80 | –£400 | –£369 | –£338 | –£307 | –£276 | –£245 | –£214 | –£183 | –£152 | –£121 | –£90 |
| 0.85 | –£317 | –£286 | –£255 | –£224 | –£193 | –£162 | –£131 | –£100 | –£69 | –£38 | –£7 |
| 0.90 | –£235 | –£204 | –£173 | –£142 | –£111 | –£80 | –£49 | –£18 | £13 | £44 | £75 |
| 0.95 | –£152 | –£121 | –£90 | –£59 | –£28 | £3 | £34 | £65 | £96 | £127 | £158 |
| 1.00 | –£69 | –£38 | –£7 | £24 | £55 | £86 | £117 | £148 | £179 | £210 | £241 |
| Sensitivity of VivaScope | Specificity of VivaScope | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.50 | 0.55 | 0.60 | 0.65 | 0.70 | 0.75 | 0.80 | 0.85 | 0.90 | 0.95 | 1.00 | |
| 0.50 | –£706 | –£675 | –£644 | –£613 | –£582 | –£551 | –£520 | –£489 | –£458 | –£427 | –£396 |
| 0.55 | –£623 | –£592 | –£562 | –£531 | –£500 | –£469 | –£438 | –£407 | –£376 | –£345 | –£314 |
| 0.60 | –£541 | –£510 | –£479 | –£448 | –£417 | –£386 | –£355 | –£324 | –£293 | –£262 | –£231 |
| 0.65 | –£458 | –£427 | –£396 | –£365 | –£334 | –£303 | –£272 | –£241 | –£210 | –£179 | –£148 |
| 0.70 | –£375 | –£344 | –£313 | –£282 | –£251 | –£220 | –£189 | –£158 | –£127 | –£96 | –£65 |
| 0.75 | –£292 | –£261 | –£230 | –£199 | –£168 | –£137 | –£106 | –£75 | –£44 | –£13 | £18 |
| 0.80 | –£209 | –£178 | –£147 | –£116 | –£85 | –£54 | –£23 | £8 | £38 | £69 | £100 |
| 0.85 | –£127 | –£96 | –£65 | –£34 | –£3 | £28 | £59 | £90 | £121 | £152 | £183 |
| 0.90 | –£44 | –£13 | £18 | £49 | £80 | £111 | £142 | £173 | £204 | £235 | £266 |
| 0.95 | £39 | £70 | £101 | £132 | £163 | £194 | £225 | £256 | £287 | £318 | £349 |
| 1.00 | £122 | £153 | £184 | £215 | £246 | £277 | £308 | £339 | £370 | £401 | £432 |
Figures 13–15 show the ICERs obtained in each model plotted against different values of the annual number of each type of lesion examined with VivaScope and help identify the minimum number of each type of lesion required to be examined with VivaScope per year, so that examination with VivaScope is a cost-effective strategy. For suspected melanomas and LMs only, exclusive use of VivaScope for their examination is shown in the graphs, because consideration of wider use of VivaScope resulted in VivaScope being dominant in the diagnosis of suspected melanomas and mapping of LMs, even when a negligible number of lesions examined (close to zero) was assumed.
FIGURE 14.
Incremental cost-effectiveness ratio plotted against annual number of suspected BCCs examined with VivaScope: different uses of VivaScope considered.
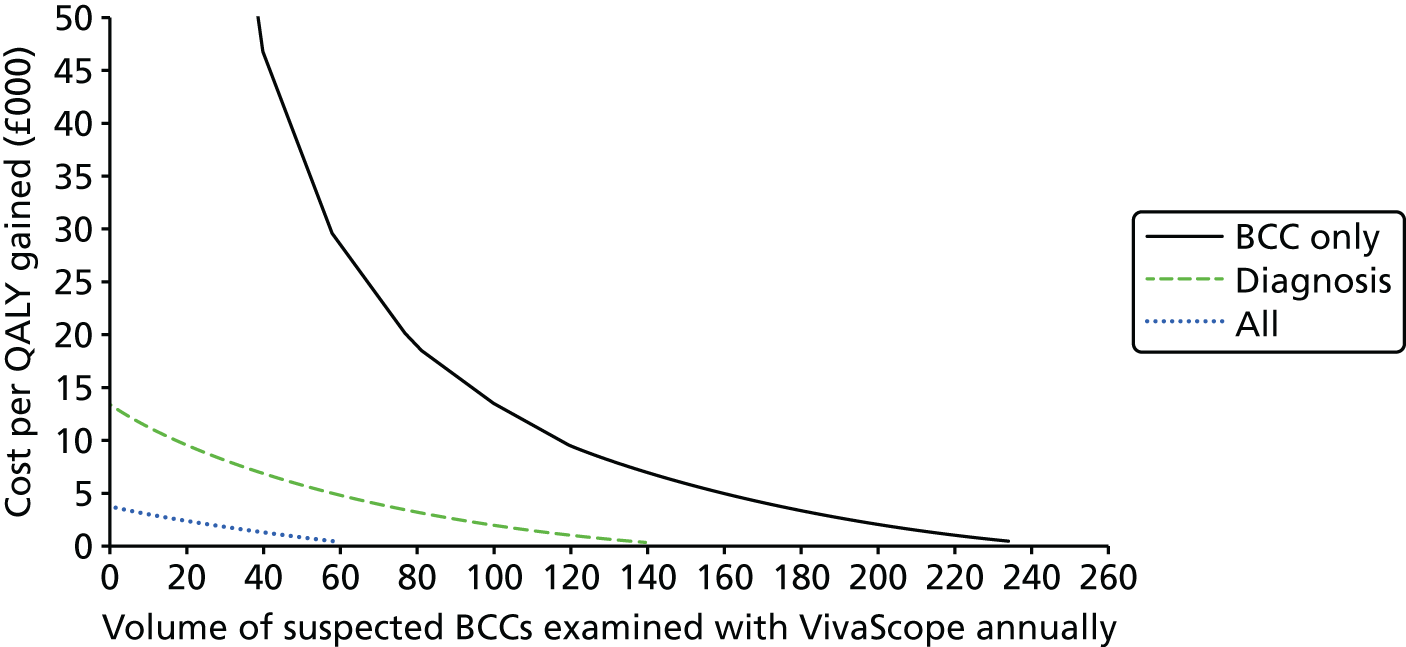
FIGURE 15.
Incremental cost-effectiveness ratio plotted against annual number of LMs mapped with VivaScope prior to surgical treatment: exclusive use of VivaScope for this purpose.

Finally, Figure 16 shows the impact of a change in the percentage of equivocal lesions suspicious of melanoma that are excised under routine management. The shape of the line is determined by the fact that the percentage of equivocal lesions sent for excision affects both the cost and disutility of routine management, but also the diagnostic accuracy of VivaScope, which differs between highly suspicious and low/moderately suspicious lesions in Pellacani et al. 42 The ICER is below the lower NICE cost-effectiveness threshold of £20,000/QALY when the percentage of equivocal lesions excised is approximately ≤ 10% or ≥ 60%.
FIGURE 16.
Incremental cost-effectiveness ratio plotted against the percentage of equivocal lesions suspicious of melanoma that are excised under routine management (highly suspicious lesions): diagnostic data from Pellacani et al. 42

Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
The systematic review of clinical effectiveness identified 16 studies, 13 of which are on investigated lesion diagnosis30–34,36,38–40,43–46 and three lesion margin delineation. 35,37,41 For the index test, included studies used VivaScope 1500 or VivaScope 1000 or VivaScope 2500 or VivaScope 3000 with or without dermoscopy as adjunctive technology or as comparator.
The majority of the included studies had a low risk of bias and low applicability concerns in patient selection, conduct of the index test and reference standard. However, concerning flow and timing, the risk of bias in majority of the studies was unclear because of poor reporting and/or insufficient data.
None of the included studies was conducted in the UK. The majority of the 15 included studies are from countries whose skin cancer rates and treatment pathways may be different from the UK setting (six studies from Australia and Italy,35,38,40,42,44,45 two from Brazil and the USA,39,43 two each from Spain30,41 and Austria,32,33 and one each from China27 and Canada36).
Two studies (Alarcon et al. ,30 conducted in Spain, and Pellacani et al. ,42 conducted in Italy) investigated lesion diagnosis and were deemed to be the most representative of clinical practice in the UK setting (in terms of study population and treatment pathway) from the studies identified. However, Alarcon et al. 30 was the preferred choice as it is the most representative of patients diagnosed with melanoma in the UK. This was validated by our clinical experts and, therefore, formed the basis of the health economic analysis for diagnosis of malignant melanoma.
One study35 that investigated lesion margin delineation was also deemed to be the most representative of clinical practice in the UK setting. Our clinical experts validated this and this trial formed the basis for the health economic analysis of VivaScope assisted margin delineation.
The most commonly reported outcome specified in the protocol was diagnostic accuracy, reported as sensitivity, specificity, PPV, NPV and, in some cases, number of positive or negative test results. Included studies were considered too heterogeneous to have their results combined by meta-analysis. This was because of study design (e.g. not post dermoscopy), patient population (e.g. different prior history of melanoma) or regarding reporting of results (e.g. patient based or lesion based).
Analysis of test accuracy can be performed only when the studies are similar, particularly with regard to the prevalence of the disease in the people studied.
Summary of key results of clinical effectiveness
Diagnostic accuracy of current versions of VivaScope in lesion diagnosis
-
In the trial by Alarcon et al. ,30 the addition of VivaScope 1500 to dermoscopy reduced unnecessary excisions with a high diagnostic accuracy. Based on the 264 excised lesions, combined use of dermoscopy and VivaScope was more likely to diagnose melanoma than dermoscopy alone (sensitivity, 97.8% vs. 94.6%, respectively; p = 0.043), and more likely to diagnose those without melanoma (non-melanoma) (specificity, 92.4% vs. 26.74%, respectively; p < 0.000001). Similar results were obtained when the analysis was based on all 343 patients who underwent RCM, assuming that all the 79 patients/lesions who were followed up were TNs.
-
In the study by Castro et al. ,43 among 54 lesions imaged with both VivaScope 1500 or VivaScope 3000 following dermoscopy, 45 were biopsy-proven BCCs. Comparison between VivaScope 1500 and VivaScope 3000 was as follows: sensitivity, 100% vs. 93%; specificity, 78% for both RCMs; PPV, 96% vs. 95%; and NPV, 100% vs. 70%.
-
Pellacani et al. 42 prospectively assessed the potential impact of RCM when implemented in a routine melanoma workflow. Of 491 lesions, 183 underwent RCM documentation and 308 RCM consultations. In the RCM documentation group, histopathology confirmed 110 RCM positives (23 melanomas, 19 BCCs and 68 benign lesions) and 73 RCM negatives (73 benign lesions). In all melanomas and BCCs identified at histology, RCM had recommended excision. In the RCM consultation group, RCM identified 81 positives and 227 negatives. Of the 81 RCM positives, excision confirmed six melanomas, 19 BCCs and 56 benign lesions. Of the 227 RCM negatives followed up for 3–12 months, 28 showed significant changes but excision confirmed no malignancy, 178 showed no changes and 21 were lost to follow-up but checks at the local tumour registry identified no excision.
-
In the trial by Curchin et al. ,31 on addition of VivaScope 1500 to dermoscopy, 12 out of 13 melanomas (92.3% sensitivity, 75% specificity), six out of nine BCCs (66.7% sensitivity, 100% specificity) and six out of six SCC and its precursors (100% sensitivity, 75% specificity) were diagnosed correctly when compared with final histopathology.
-
In the trial by Rao et al. ,39 VivaScope 1500 provided a high diagnostic accuracy in teleconsultation use. Lesions diagnosed by reader 1 (bedside-trained physician, less experience) as malignant with VivaScope 1500 represented 66.7% of histologically diagnosed melanoma, 74.1% of BCC and 37.2% of SCC. For reader 2 (distant expert, more experience), lesions diagnosed as malignant represented 88.9% of melanoma, 51.9% of BCC and 72.1% of SCC. Out of 284 lesions evaluated by both readers, 212 were benign and 72 malignant based on histopathology.
-
In the trial by Stanganelli et al. ,45 VivaScope 1500 as additional diagnostic tool to dermoscopy can improve melanoma detection and reduce unnecessary excisions. Of 30 out of 70 lesions (43%) classified as melanoma by VivaScope 1500, 11 out of 12 were histologically confirmed (11 TP and one FN) and 19 as FPs.
Diagnostic accuracy of the older version of VivaScope in lesion diagnosis
-
In the trial by Langley et al. ,36 VivaScope 1000 had a relatively higher sensitivity than dermoscopy, but the specificity was similar. The sensitivity of VivaScope 1000 compared with dermoscopy was 97.3% vs. 89.2% and specificity was 83.0% vs. 84.1%.
-
In the trials by Gerger et al. ,32,33 VivaScope 1000 examination was a promising method for non-invasive assessment of melanoma and non-melanoma skin tumours. The overall (total of the four observers/readers) diagnostic differentiation of benign from malignant lesions (melanoma and BCC) reached a sensitivity of 94.65%, specificity of 96.67%, PPV of 97.50% and NPV of 92.99% based on histopathology.
Diagnostic accuracy of the current version of VivaScope in lesion margin delineation
-
In the trial by Guitera et al. 35 in vivo VivaScope 1500 as an addition to dermoscopy provided valuable information facilitating accurate diagnosis. Out of 60 positive sites for LM confirmed by histopathology, 55 (FN = 5) had been confirmed by VivaScope 1500 and 21 (FN = 39) by dermoscopy, and out of 125 LM sites confirmed as negative by histopathology, 121 (FP = 4) had been confirmed by VivaScope 1500 and 122 (FP = 3) by dermoscopy. Both the length and width of the dermoscopically visible area of the lesion were, on average, 60% smaller than the final corresponding dimensions determined by VivaScope 1500.
-
In the trial by Pan et al. ,37 VivaScope 1500 imaging of lesion margins demonstrated the possibility of preoperative mapping of cancer margins. In 7 out of 10 (70%) cases, the margins of the cancer were identified using VivaScope 1500 and confirmed by histopathological analysis. In 3 of 10 (30%) cases, the margin of the lesions could not be detected because of the unevenness of the surface.
-
-
VivaScope 2500 in lesion margin delineation In the trial by Bennassar et al. ,41 the overall sensitivity and specificity of detecting residual BCC in surgical margins was 88% and 99%, respectively. The number of images/mosaic correctly diagnosed as TP was 79 (89%) and as TN was 390 (99.7%). There was only one (0.3%) FP. In addition, average VivaScope 2500 reduced the evaluation time by 18 minutes (p < 0.001) when compared with the processing of a frozen section. Table 50 summarises the consistency and inconsistency of the results of diagnostic accuracy of the included studies in the clinical systematic review.
| Study | Sensitivity and specificity results | Consistency/inconsistency of results |
|---|---|---|
| Current versions of VivaScope in lesion diagnosis | ||
| Alarcon et al., 201430 | The addition of VivaScope 1500 to dermoscopy reduced unnecessary excisions with a high diagnostic accuracy. Based on the 264 excised lesions, combined use of dermoscopy and VivaScope was more likely to diagnose melanoma than dermoscopy alone (sensitivity 97.8% vs. 94.6%, respectively; p = 0.043), and more likely to diagnose those without melanoma (non-melanoma) (specificity 92.4% vs. 26.74%; p < 0.000001). Similar results were obtained when the analysis was based on all 343 patients who underwent RCM, assuming all of the 79 patients/lesions who were followed up were TNs | 264 excisions out of 343 lesions that underwent RCM and hence the reported specificity and sensitivity analysis does not reflect the total number of lesions analysed |
| Castro et al., 201543 | Among 54 lesions imaged with both VivaScope 1500 or 3000, 45 were biopsy-proven BCCs. Comparison between VivaScope 1500 and VivaScope 3000 was as follows: sensitivity (100% vs. 93%), specificity (78% for both RCMs), PPV (96% vs. 95%) and NPV (100% vs. 70%), respectively | Study participants recruited from a tertiary hospital in Brazil and a private skin cancer specialist hospital in USA, hence may not be representative |
| Pellacani et al., 201442 | Of 491 lesions, 183 underwent RCM documentation and 308 RCM consultations. In the RCM documentation group, histopathology confirmed 110 RCM positives (23 melanomas, 19 BCCs and 68 benign lesions) and 73 RCM negatives (73 benign lesions). In all melanomas and BCCs identified at histology, RCM had recommended excision. In the RCM consultation group, RCM identified 81 positives and 227 negatives. Of the 81 RCM positives, excision confirmed six melanomas, 19 BCCs and 56 benign lesions. Of the 227 RCM negatives followed up for 3–12 months, 28 showed significant changes but excision confirmed no malignancy, 178 showed no changes and 21 were lost to follow-up but checks at the local tumour registry identified no excision | The comparison was between RCM documentation (documentation of lesions already qualified and scheduled for surgical excision following consistent clinical and/or dermoscopic criteria for melanoma diagnosis) and RCM consultation (an outcome decision requested from the confocal reader. In this case RCM examination determined the lesion-definite outcome) |
| Curchin et al., 201131 | On the addition of VivaScope 1500 to dermoscopy, 12 out of 13 melanomas (92.3% sensitivity, 75% specificity), six out of nine BCCs (66.7% sensitivity, 100% specificity) and six out of six SCC and its precursors (100% sensitivity, 75% specificity) were diagnosed correctly when compared with final histopathology | No comparator |
| Rao et al., 201339 | Lesions diagnosed by reader 1 (bedside-trained physician, less experience) as malignant with VivaScope 1500 represented 66.7% of histologically diagnosed melanoma, 74.1% of BCC and 37.2% of SCC. For reader 2 (distant expert, more experience), lesions diagnosed as malignant represented 88.9% of melanoma, 51.9% of BCC and 72.1% of SCC. Out of 284 lesions evaluated by both readers, 212 were benign and 72 malignant based on histopathology | Study had no comparator and the only comparison was between reader 1 (bedside-trained physician, less experience) and reader 2 (distant expert, more experience) |
| Stanganelli et al., 201545 | VivaScope 1500 as an additional diagnostic tool to dermoscopy can improve melanoma detection and reduce unnecessary excisions. Of 30 out of 70 lesions (43%) classified as melanoma by VivaScope 1500, 11 out of 12 were histologically confirmed (11 TP and one FN) and 19 as FPs | No comparator, and was based on retrospective study of excised lesions |
| Older versions of VivaScope in lesion diagnosis | ||
| Langley et al., 200736 | VivaScope 1000 had a relatively higher sensitivity than dermoscopy, but the specificity was similar. The sensitivity of VivaScope 1000 compared with dermoscopy was 97.3% vs. 89.2% and specificity was 83.0% vs. 84.1% | Earlier version of VivaScope |
| Gerger et al., 200632 and Gerger et al., 200833 | The overall (total of the four observers/readers) diagnostic differentiation of benign from malignant lesions (melanoma and BCC) reached a sensitivity of 94.65%, specificity of 96.67%, PPV of 97.50%, and NPV of 92.99% based on histopathology | Earlier version of VivaScope and no comparator |
| Current versions of VivaScope in margin delineation | ||
| Guitera et al., 201335 | Out of 60 positive sites for LM confirmed by histopathology, 55 (FN = 5) had been confirmed by VivaScope 1500 and 21 (FN = 39) by dermoscopy, and out of 125 LM sites confirmed as negative by histopathology, 121 (FP = 4) had been confirmed by VivaScope 1500 and 122 (FP = 3) by dermoscopy. Both the length and width of the dermoscopically visible area of the lesion were, on average, 60% smaller than the final corresponding dimensions determined by VivaScope 1500. Thus, the visible area was on average < 40% of the area that was treated based on VivaScope 1500 mapping findings | High-risk population, 15/37 had recurrent LM, including nine with multiple prior recurrence |
| Pan et al., 201237 | VivaScope 1500 imaging of lesion margins demonstrated the possibility of preoperative mapping of cancer margins. In 7 of 10 (70%) cases, the margins of the cancer were identified using VivaScope 1500 and confirmed by histopathological analysis | No comparator |
| Bennassar et al., 201441 | The overall sensitivity and specificity of detecting residual BCC in surgical margins were 88% and 99%, respectively. The number of images/mosaic correctly diagnosed as TP was 79 (89%) and TN was 390 (99.7%). There was only one (0.3%) FP. In addition, average VivaScope 2500 reduced the evaluation time by 18 minutes (p < 0.001) when compared with the processing of a frozen section | Earlier version of VivaScope and no comparator |
Generalisability of results
Although none of the included studies in the review of clinical effectiveness was conducted in the UK, two studies30,42 on diagnosis and one study on margin delineation35 were deemed to be the most representative of clinical practice in the UK setting. Our clinical experts validated this and these trials were taken forward for the health economic analysis.
Cost-effectiveness
Existing evidence on the cost-effectiveness of VivaScope is particularly limited. One economic evaluation that assessed the cost-effectiveness of VivaScope in the diagnostic assessment of suspected melanomas from a hospital perspective in Italy was the only evidence identified. 46 The study estimated the impact of VivaScope use on the number of benign lesions NNE a malignant melanoma, in terms of clinical outcomes and costs per patient, and indicated that VivaScope reduces the NNE of skin lesions suspicious of melanoma and results in cost savings to the hospital. As the study was conducted in Italy, its findings may not be generalisable to the UK setting, as there may be differences between the two health-care settings in terms of prevalence of the various skin cancer types, the population phenotype distribution, the clinical pathways for the diagnosis, assessment and management of skin cancer, the level of experience of clinicians in the use of RCM and relevant unit costs.
The results of primary economic modelling indicate that VivaScope is probably a cost-effective strategy in the diagnostic assessment of skin lesions suspicious of cancer (suspected melanomas with an equivocal finding on dermoscopy and suspected BCCs with an equivocal or positive finding on dermoscopy) and in the margin delineation of LM prior to surgical treatment, even when VivaScope is used exclusively for one of the three indications assessed in the economic analysis. Results were affected by the intended use of VivaScope (i.e. exclusive use on diagnostic assessment of suspected melanomas, or diagnostic assessment of suspected BCCs, or presurgical mapping of LM, or combined use for the diagnosis of suspected melanomas and BCCs, or use in all of the above indications). This is because the capital, maintenance and training costs of VivaScope are spread across a different number of lesions eligible for examination, which affects the intervention cost per lesion examined, and, ultimately, the total cost associated with the use of VivaScope.
The cost-effectiveness of VivaScope in the diagnostic assessment of suspected melanomas with an equivocal finding on dermoscopy was affected by the diagnostic accuracy data utilised in the model, when it was assumed that VivaScope was exclusively used for this purpose. Using the more ‘optimistic’ diagnostic data from Alarcon et al. 30 resulted in a deterministic ICER of £8877 per QALY (£9362 per QALY in probabilistic analysis), while the ‘less favourable’ diagnostic data from Pellacani et al. 42 resulted in a deterministic ICER of £19,095 per QALY (£25,453 per QALY in probabilistic analysis). When the use of VivaScope was expanded to include other indications assessed in the economic analysis, VivaScope became the dominant strategy over routine management of equivocal lesions suspicious of melanoma.
VivaScope was shown to be a dominant strategy when used for the diagnostic assessment of suspected BCCs with a positive or equivocal finding on dermoscopy, and this was independent of the intended use of the device (i.e. it was a dominant strategy when it was exclusively used for this purpose or when it was used for other indications covered by the economic analysis as well).
Regarding margin delineation of LM, mapping with VivaScope was shown to be cost-effective, even if it was used exclusively for this purpose, as indicated by a deterministic ICER of £10,241 per QALY (£11,651 per QALY in probabilistic analysis). When VivaScope was used for diagnosis as well as mapping of LM, then the intervention cost was reduced and it became a dominant strategy.
Overall, in the analyses that combined the different ‘part’ models designed for this report, VivaScope was shown to be a dominant strategy over routine management in the diagnostic assessment of suspected melanomas and BCCs alone or combined with margin delineation of LM prior to surgical treatment.
One-way sensitivity analysis showed that the most influential parameters across all models were those relating to permanent disutility caused by scarring following surgical intervention of skin lesions on the head or neck (such as the percentage of people experiencing permanent disutility as well as the value of disutility itself) and the disutility because of anxiety while waiting for the results of biopsy.
A series of scenario analyses were undertaken to test the impact on the results when using alternative sources for parameter estimates or challenge assumptions in the model. All scenario analyses that were performed exclusively for the diagnostic assessment of suspected melanomas raised the ICER above the base case. However, when wider use of VivaScope was assumed, the results (VivaScope dominance) remained unaffected by the scenarios tested. Overall, the dominance of VivaScope was robust and unaffected by use of alternative data and assumptions when the system was assumed to be used for a combination of indications assessed in the economic analysis.
Strengths and limitations of the assessment
Clinical effectiveness
Strengths
-
This systematic review provides the most up-to-date evidence of the clinical effectiveness of VivaScope 1500 and 3000 for detecting and monitoring skin cancer, and with a low likelihood of missing any key or pivotal trial.
Limitations
-
There is lack of UK data among the included studies and, therefore, the generalisability of the results is limited. This has implications for the NHS.
-
Apart from diagnostic accuracy and lesion recurrence rate (only reported by one study), none of the outcomes specified in the protocol was reported in the included studies.
-
None of the included studies reported diagnostic accuracy results of SCC with VivaScope. This confirms evidence in the literature which suggests that SCCs can be difficult to view using imaging techniques because their upper surface is often scaly, which can make it difficult to obtain detail at sufficient resolution. 11 SCC will, therefore, not be carried through into the economic evaluation.
-
In some of the studies, there was paucity and/or quality of reported data on number of patients with positive and negative test results, making it impossible to construct a 2 × 2 contingency table to calculate sensitivity and specificity.
Cost-effectiveness
Strengths
-
The economic analysis was based on the development of three ‘part’ models, each designed to simulate the care pathways of people with skin lesions eligible for examination with VivaScope who undergo assessment of their skin lesions in a dermatology MDT service. The care pathways were designed based on national guidelines and following advice from clinical experts, and were specific to each type of lesion considered in the economic analysis. The use of national guidance and consultation with clinical experts ensured that the care pathways considered in this model reflect, as close as possible, clinical practice in the NHS, although there appears to be wide variation in the management of suspected and/or confirmed skin cancer across services.
-
The model input parameters were based on national guidelines and other published evidence, clinical expert opinion and national unit costs.
Limitations
-
The diagnostic and mapping accuracy data that were utilised in the model were taken from studies included in the systematic literature review of clinical evidence conducted for this guideline. However, data were limited and it was not possible to synthesise the results in a meta-analysis because of the heterogeneous nature of the studies identified. Moreover, none of the studies was conducted in the UK, which may have implications for the generalisability of not only the clinical, but also the economic, findings as the prevalence of the skin cancer and the population phenotype distribution may affect the diagnostic accuracy of VivaScope.
-
Sensitivity analysis showed that the most influential parameters across all models were those relating to permanent disutility because of scarring following surgical intervention of skin lesions on the head or neck (such as the percentage of people experiencing permanent disutility as well as the value of disutility itself) and the disutility because of anxiety while waiting for the results of biopsy. However, utility data relating to these events were very limited and of poor quality or non-existent, and a number of assumptions were needed in order to inform the model.
-
Other complications of excision and biopsy, which were the main comparators of VivaScope in the diagnostic assessment of suspected cancerous lesions, such as bleeding, bruising, infection or allergic reaction to the topical antibiotic were not considered in the model. Clinical experts acknowledged that these are not common complications, but their omission may have potentially underestimated, to some extent, the cost-effectiveness of VivaScope.
Uncertainties
The annual number of lesions eligible for examination with VivaScope is important in determining the cost of VivaScope per lesion examined and, ultimately, in determining its cost-effectiveness. There appears to be wide variation across dermatology in the UK in terms of the number and type of lesions examined annually. Although this parameter has been tested in sensitivity analysis in the economic model, the cost-effectiveness of VivaScope may potentially vary across different dermatology centres in the UK, depending on the volume and type of lesions assessed and managed at each service.
Other relevant factors
-
Training in the use of VivaScope and the clinical interpretation of the findings is an important factor that is likely to drive the diagnostic accuracy of VivaScope in the diagnostic assessment of suspected skin cancers and the mapping of skin lesions prior to surgical treatment. The economic analysis did consider formal training costs when estimating the cost associated with the use of VivaScope; however, clinical expert advice indicated that, as expected, there is a learning curve following formal training, and the overall training required for a clinician to reach a good level of expertise is between 4 and 6 months’ time, and approximately 1000–2000 cases evaluated with confocal microscopy in a setting including a sufficient number of melanomas (> 200). This means that the benefits and cost savings associated with VivaScope use that were suggested by the results of the economic analysis are likely to take some time to realise, as the diagnostic accuracy of VivaScope utilised in the economic analyses was taken from studies conducted in dermatology centres with expertise in the use of VivaScope, so optimal diagnostic outcomes were obtained.
-
The primary economic analysis considered the costs and benefits associated with use of VivaScope in the diagnostic assessment of skin lesions suspicious of melanoma or BCC and in the margin delineation of LM prior to surgical treatment. However, evidence and clinical expert advice suggest that there may be additional benefits resulting from the use of VivaScope that were not factored in the economic analysis, including:
-
monitoring and selection of suspicious lesions for biopsy in greatly high-risk patients
-
monitoring of less suspicious lesions by digital dermoscopy, given that a high-definition digital dermoscopy has been integrated into all VivaScope in vivo devices
-
post-therapy monitoring of skin lesions
-
margin delineation of LM planned for non-surgical treatment
-
contribution to the monitoring and management of benign skin tumours.
-
Chapter 6 Conclusions
Clinical effectiveness
There is no RCT evidence for both diagnostic accuracy and margin delineation with VivaScope 1500 and 3000. However, the systematic review provides up-to-date non-RCT evidence that indicates that the use of VivaScope subsequent to dermoscopy may improve diagnostic accuracy of equivocal skin lesions compared with dermoscopy alone, particularly for malignant melanomas. In terms of margin delineation, clinical data are very scarce but those that exist suggest that VivaScope 1500 mapping for LM and LMM may improve the accuracy in terms of complete excision of lesions than dermoscopically determined margins.
Cost-effectiveness
The use of VivaScope appears to be a cost-effective strategy in the diagnostic assessment of suspected skin cancer (more specifically, of suspected melanomas with an equivocal finding on dermoscopy and suspected BCCs with a positive or equivocal finding on dermoscopy) and the margin delineation of LM prior to surgical treatment, in particular when VivaScope is used for all three indications considered in the economic analysis.
Implications for service provision
Although the use of VivaScope following dermoscopy may improve patient care and management, there is an absence of UK data in the included studies and, therefore, the generalisability of the results to the UK population is unclear. However, VivaScope could potentially help to reduce the number of unnecessary excisions of benign lesions, minimise the number of patients referred for ongoing digital dermoscopy monitoring and minimise the risk of losing patients at risk of cancer to follow-up. In addition, VivaScope may help to reduce the number of patients with incomplete excision of malignant skin lesions and thus potentially reduce the burden on both patients and the NHS in terms of further surgical procedures and ongoing surveillance.
The results of the economic analysis undertaken for this assessment indicate that use of VivaScope in dermatology MDT services is likely to reduce the patient distress and anxiety associated with diagnostic biopsy and excision of lesions suspicious of skin cancer, reduce future recurrence of LM and the distress to the patients associated with surgical treatment and lead to cost-savings to the NHS. However, the cost-effectiveness of VivaScope may potentially vary across different dermatology centres in the UK, depending on the volume and type of lesions assessed and managed at each service.
Suggested research priorities
High-quality RCTs are required in a UK population to assess diagnostic accuracy of dermoscopy plus VivaScope compared with dermoscopy alone in people with equivocal skin lesions and margin delineation accuracy of VivaScope compared with dermoscopy alone. In addition, RCTs focusing on clinical outcomes such as time to test result; test failure rate, for example imaging failure; number of biopsies performed and repeat biopsies; recurrence rate and morbidity associated with surgery are required. However, this research may not be feasible because of the current lack of expertise and availability of VivaScope in the UK. In addition, research on patient-specific outcomes such as patients’ quality of life, adverse effects and mortality may be of interest to patients and the wider clinical community.
Further research is also needed on the impact of tools and procedures associated with the diagnostic assessment and management of potentially cancerous skin lesions on people’s HRQoL; in particular, the impact of the distress and anxiety associated with excision and biopsy of suspicious lesions and the disutility associated with permanent disfiguring after excision of a facial malignant lesion, in order to determine the cost-effectiveness of alternative diagnostic strategies in this area with higher certainty.
Acknowledgements
The assessment group thanks Dr Andrew Coleman (Head of Non-Ionising Radiation Physics), Dr Emma Craythorne (Consultant Dermatologist and Dermatological Surgeon), Dr Louise Fearfield (Consultant Dermatologist), Dr Jennifer Garioch (Consultant Dermatologist), James Hawkins (Health Economist), Dr Rakesh Patalay (Consultant Dermatologist and Dermatological Surgeon), Professor Giovanni Pellacani (Department of Dermatology, University of Modena and Reggio Emilia), Matthew Prettyjohns (Senior Health Economist) and Dr Julia Schoffield (Consultant Dermatologist) for their input throughout the project. Thanks also to Mr Marc Moncrieff (Consultant Plastic and Reconstructive Surgeon) for reviewing the report. The assessment group would also like to thank Dr Fay Crawford for her contributions to the background section and to the appraisal and data abstraction of the literature retrieved by the clinical searches and Mariana Bacelar for her contribution to the design of the economic model and the acquisition of input data.
Contributions of authors
Steven J Edwards contributed to the editing of the report. He was the overall director of the project and guarantor of the report.
Ifigeneia Mavranezouli provided project management, carried out the appraisal of abstracts retrieved from the cost-effectiveness and HRQoL literature searches, designed and developed the economic model, wrote the report relating to health economics and cost-effectiveness analysis, and contributed to the editing of the report.
George Osei-Assibey carried out appraisal of abstracts retrieved from the literature search, assessed full publications for inclusion, contributed to data extraction and validation, wrote the sections of the report relating to clinical effectiveness and contributed to the editing of the report.
Gemma Marceniuk devised and carried out the literature searches for the review of cost-effectiveness and HRQoL, carried out the appraisal of abstracts retrieved from the cost-effectiveness and HRQoL literature searches, carried out study selection and data extraction, wrote the sections of the report relating to health economics and cost-effectiveness analysis, and contributed to the editing of the report.
Victoria Wakefield provided project management, assessed full publications for inclusion, contributed to validation of extracted data and contributed to the editing of the report.
Charlotta Karner provided project management, devised and carried out the literature searches for the review of clinical effectiveness and carried out appraisal of abstracts retrieved from the clinical effectiveness.
All authors read and commented on draft versions of the report.
Data sharing statement
Requests for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Skin Cancer Prevention: Information, Resources and Environmental Changes. Public Health Guidance PH32. London: NICE; 2011.
- Cancer Research UK . Cancer Statistics, Key Stats 2014. https://publications.cancerresearchuk.org/downloads/Product/CS_KE_SKIN (accessed 6 October 2014).
- Cancer Research UK . Types of Skin Cancer 2014. www.cancerresearchuk.org/about-cancer/type/skin-cancer/about/types-of-skin-cancer (accessed 6 October 2014).
- Cancer Research UK . About Melanoma 2014. www.cancerresearchuk.org/about-cancer/type/melanoma/about/ (accessed 6 October 2014).
- Cancer Research UK . About Skin Cancer 2014. www.cancerresearchuk.org/about-cancer/type/skin-cancer/about/ (accessed 6 October 2014).
- Tidy C. Skin Cancer – An Overview. 2012.
- Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Arch Dermatol 2012;148:30-6. http://dx.doi.org/10.1001/archdermatol.2011.264.
- Non-Melanoma Skin Cancer in England, Scotland, Northern Ireland, and Ireland. London: NCIN; 2013.
- Motley RJ, Preston PW, Lawrence CM. Multi-Professional Guidelines for the Management of the Patient with Primary Cutaneous Squamous Cell Carcinoma. London: British Association of Dermatologists; 2009.
- Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol 1995;104:946-52. http://dx.doi.org/10.1111/1523-1747.ep12606215.
- Welzel J, Ulrich M, Lange-Asschenfeldt S, Hohenleutner U. Confocal Laser Microscopy in Dermatology 2011. www.vivascope.de/fileadmin/user_upload/Downloads/Guideline_09_2011ENG.pdf.
- NHS Reference Costs 2013 to 2014. London: Department of Health; 2014.
- Marsden JR, Newton-Bishop JA, Burrows L, Cook M, Corrie PG, Cox NH, et al. Revised U.K. guidelines for the management of cutaneous melanoma 2010. Br J Dermatol 2010;163:238-56. http://dx.doi.org/10.1111/j.1365-2133.2010.09883.x.
- Melanoma: Assessment and Management of Melanoma, Draft for Consultation. NICE Guideline in Development [GID-CGWAVE0674]. London: NICE; 2015.
- Primary Care Dermatology Society . Benign Melanocytic Naevus (Acquired and Dermal Naevi i.E. Common Moles) 2015. www.pcds.org.uk/clinical-guidance/moles (accessed 3 February 2015).
- Gillgren P, Drzewiecki KT, Niin M, Gullestad HP, Hellborg H, Månsson-Brahme E, et al. 2-cm versus 4-cm surgical excision margins for primary cutaneous melanoma thicker than 2 mm: a randomised, multicentre trial. Lancet 2011;378:1635-42. http://dx.doi.org/10.1016/S0140-6736(11)61546-8.
- Thompson JF, Ollila DW. Optimum excision margins for melanoma. Lancet 2011;378:1608-10. http://dx.doi.org/10.1016/S0140-6736(11)61615-2.
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines on Oncology: Melanoma 2015. www.mmmp.org/mmmpFile/image/conv%20ther/NCCN%20guidelines_Melanoma.pdf (accessed 13 January 2015).
- Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. Br J Dermatol 2008;159:35-48. http://dx.doi.org/10.1111/j.1365-2133.2008.08666.x.
- Centre for Reviews and Dissemination . CRD’s Guidance for Undertaking Reviews in Healthcare 2011. www.york.ac.uk/inst/crd/S.ysRev/!SSL!/WebHelp/SysRev3.htm (accessed 13 January 2015).
- Diagnostics Assessment Programme Manual. London: NICE; 2011.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Diagnostic Test Accuracy Working Group . Handbook for DTA Reviews 2013. www.srdta.cochrane.org/handbook-dta-reviews (accessed 13 January 2015).
- Higgins J, Green SG. Cochrane Handbook for Systematic Reviews of Interventions 2009. www.handbook.cochrane.org/ (accessed 13 January 2015).
- The Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] n.d. http://handbook.cochrane.org/ (accessed 13 January 2015).
- Higgins J, Green SG, Higgins J, Green SG. Cochrane Handbook for Systematic Reviews of Interventions. 2011.
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality in Non-Randomised Studies in Meta-Analyses; 2003. www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (accessed 6 October 2014).
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JAC. A unification of models of meta-analysis for diagnostic accuracy studies. Biostatistics 2007;1:239-51. http://dx.doi.org/10.1093/biostatistics/kxl004.
- Moskowitz CS, Zabor EC, Jochelson M. Breast imaging: understanding how accuracy is measured when lesions are the unit of analysis. Breast J 2012;18:225-6. http://dx.doi.org/10.1111/tbj.12009.
- Alarcon I, Carrera C, Palou J, Alos L, Malvehy J, Puig S, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol 2014;170:802-8. http://dx.doi.org/10.1111/bjd.12678.
- Curchin CE, Wurm EM, Lambie DL, Longo C, Pellacani G, Soyer HP, et al. First experiences using reflectance confocal microscopy on equivocal skin lesions in Queensland. Australas J Dermatol 2011;52:89-97. http://dx.doi.org/10.1111/j.1440-0960.2011.00756.x.
- Gerger A, Koller S, Weger W, Richtig E, Kerl H, Samonigg H, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer 2006;107:193-200. http://dx.doi.org/10.1002/cncr.21910.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, Richtig E, Koller S, Weger W, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol 2008;158:329-33. http://dx.doi.org/10.1111/j.1365-2133.2007.08389.x.
- Guitera P, Pellacani G, Longo C, Seidenari S, Avramidis M, Menzies SW, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol 2009;129:131-8. http://dx.doi.org/10.1038/jid.2008.193.
- Guitera P, Moloney FJ, Menzies SW, Stretch JR, Quinn MJ, Hong A, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol 2013;149:692-8. http://dx.doi.org/10.1001/jamadermatol.2013.2301.
- Langley RG, Walsh N, Sutherland AE, Propperova I, Delaney L, Morris SF, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology 2007;215:365-72. http://dx.doi.org/10.1159/000109087.
- Pan ZY, Lin JR, Cheng TT, Wu JQ, Wu WY, Pan ZY, et al. In vivo reflectance confocal microscopy of Basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg 2012;38:1945-50. http://dx.doi.org/10.1111/j.1524-4725.2012.02587.x.
- Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol 2007;127:2759-65. http://dx.doi.org/10.1038/sj.jid.5700993.
- Rao BK, Mateus R, Wassef C, Pellacani G, Rao BK, Mateus R, et al. In vivo confocal microscopy in clinical practice: comparison of bedside diagnostic accuracy of a trained physician and distant diagnosis of an expert reader. J Am Acad Dermatol 2013;69:295-300. http://dx.doi.org/10.1016/j.jaad.2013.07.022.
- Guitera P, Pellacani G, Crotty KA, Scolyer RA, Li LX, Bassoli S, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol 2010;130:2080-91. http://dx.doi.org/10.1038/jid.2010.84.
- Bennassar A, Vilata A, Puig S, Malvehy J. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol 2014;170:360-5. http://dx.doi.org/10.1111/bjd.12671.
- Pellacani G, Pepe P, Casari A, Longo C. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol 2014;171:1044-51. http://dx.doi.org/10.1111/bjd.13148.
- Castro RP, Stephens A, Fraga-Braghiroli NA, Oliviero MC, Rezze GG, Rabinovitz H, et al. Accuracy of in vivo confocal microscopy for diagnosis of basal cell carcinoma: a comparative study between handheld and wide-probe confocal imaging. J Eur Acad Dermatol Venereol 2015;29:1164-9.
- Ferrari B, Pupelli G, Farnetani F, De Carvalho NT, Longo C, Reggiani C, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol 2015;29:1135-40.
- Stanganelli I, Longo C, Mazzoni L, Magi S, Medri M, Lanzanova G, et al. Integration of reflectance confocal microscopy in sequential dermoscopy follow-up improves melanoma detection accuracy. Br J Dermatol 2015;172:365-71.
- Pellacani G, Witkowski A, Cesinaro AM, Losi A, Colombo GL, Campegna A, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol 2015;30:413-19.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Wilson EC, Emery JD, Kinmonth AL, Prevost AT, Morris HC, Humphrys E, et al. The cost-effectiveness of a novel SIAscopic diagnostic aid for the management of pigmented skin lesions in primary care: a decision-analytic model. Value Health 2013;16:356-66. http://dx.doi.org/10.1016/j.jval.2012.12.008.
- Morris S, Cox B, Bosanquet N. Cost of skin cancer in England. Eur J Health Econ 2009;10:267-73. http://dx.doi.org/10.1007/s10198-008-0127-0.
- Vallejo-Torres L, Morris S, Kinge JM, Poirier V, Verne J. Measuring current and future cost of skin cancer in England. J Public Health 2014;36:140-8. http://dx.doi.org/10.1093/pubmed/fdt032.
- NHS Reference Costs 2008 to 2009. London: Department of Health; 2009.
- Orr DJ, Hughes LE, Horgan K. Management of malignant melanoma of the head and neck. Br J Surg 1993;80:998-1000. http://dx.doi.org/10.1002/bjs.1800800821.
- Askew RL, Swartz RJ, Xing Y, Cantor SB, Ross MI, Gershenwald JE, et al. Mapping FACT-melanoma quality-of-life scores to EQ-5D health utility weights. Value Health 2011;14:900-6. http://dx.doi.org/10.1016/j.jval.2011.04.003.
- Beusterien KM, Szabo SM, Kotapati S, Mukherjee J, Hoos A, Hersey P, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer 2009;101:387-9. http://dx.doi.org/10.1038/sj.bjc.6605187.
- King SM, Bonaccorsi P, Bendeck S, Hadley J, Puttgen K, Kolm PG, et al. Melanoma quality of life: pilot study using utility measurements. Arch Dermatol 2011;147:353-4. http://dx.doi.org/10.1001/archdermatol.2010.340.
- Shingler SL, Garside J, Samanta K, Lear JT, Keohane S, Lloyd AJ, et al. Utilities for advanced basal cell carcinoma. J Med Econ 2013;16:777-83. http://dx.doi.org/10.3111/13696998.2013.800822.
- Seidler AM, Bramlette TB, Washington CV, Szeto H, Chen SC, Seidler AM, et al. Mohs versus traditional surgical excision for facial and auricular nonmelanoma skin cancer: an analysis of cost-effectiveness. Dermatol Surg 2009;35:1776-87. http://dx.doi.org/10.1111/j.1524-4725.2009.01291.x.
- Tromme I, Devleesschauwer B, Beutels P, Richez P, Leroy A, Baurain JF, et al. Health-related quality of life in patients with melanoma expressed as utilities and disability weights. Br J Dermatol 2014;171:1443-50. http://dx.doi.org/10.1111/bjd.13262.
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203-20. http://dx.doi.org/10.1097/00005650-200503000-00003.
- Schlesinger-Raab A, Schubert-Fritschle G, Hein R, Stolz W, Volkenandt M, Holzel D, et al. Quality of life in localised malignant melanoma. Ann Oncol 2010;21:2428-35. http://dx.doi.org/10.1093/annonc/mdq255.
- Cleemput I. A social preference valuations set for EQ-5D health states in Flanders, Belgium. Eur J Health Econ 2010;11:205-13. http://dx.doi.org/10.1007/s10198-009-0167-0.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Skin Cancer: The VivaScope 1500 and 3000 Systems for Detecting and Monitoring Skin Lesions. Final Scope. London: NICE; 2014.
- Prescription Pricing Division. Electronic Drug Tariff for England and Wales. London: Department of Health; 2015.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Tromme I, Devleesschauwer B, Beutels P, Richez P, Praet N, Sacre L, et al. Selective use of sequential digital dermoscopy imaging allows a cost reduction in the melanoma detection process: a Belgian study of patients with a single or a small number of atypical nevi. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0109339.
- Manual for Skin Cancer Measures, Version 1.2. NHS England; 2014.
- Levell N, Jones S, Bunker C. Dermatology 2013. www.rcplondon.ac.uk/guidelines-policy/pain-complex-regional-pain-syndrome (accessed 15 April 2015).
- Centre for Workforce Intelligence . Dermatology CfWI Medical Fact Sheet and Summary Sheet – August 2010 2010. www.cfwi.org.uk/publications/dermatology-cfwi-medical-fact-sheet-and-summary-sheet-august-2010 (accessed 15 April 2015).
- Mirzoyev SA, Knudson RM, Reed KB, Hou JL, Lohse CM, Frohm ML, et al. Incidence of lentigo maligna in Olmsted County, Minnesota, 1970 to 2007. J Am Acad Dermatol 2014;70:443-8. http://dx.doi.org/10.1016/j.jaad.2013.11.008.
- Statistical Information Team at Cancer Research UK . Malignant Melanoma (C43), Percentage Distribution of Cases Diagnosed on Parts of the Body, by Sex, Great Britain, 2008–2010 2011. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/skin-cancer/incidence#heading-Six (accessed 15 April 2015).
- Baxter JM, Patel AN, Varma S. Facial basal cell carcinoma. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5342.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol 2002;147:41-7. http://dx.doi.org/10.1046/j.1365-2133.2002.04804.x.
- Deady S, Sharp L, Comber H. Increasing skin cancer incidence in young, affluent, urban populations: a challenge for prevention. Br J Dermatol 2014;171:324-31. http://dx.doi.org/10.1111/bjd.12988.
- Teoh YL, Halpem SM, Shall L. Factors Associated With Incomplete Excision of Basal Cell Carcinomas n.d.:20-72.
- Pignatelli I, Poirer V, de Berker DAR, Verne J. Audit of Completeness of Cancer Registration for Basal Cell Carcinoma and Its Impact on Use for Quality Assurance n.d.
- Schofield J, Grindlay D, Williams H. Skin Conditions in the UK: A Health Care Needs Assessment. Nottingham: Centre of Evidence-Based Dermatology, University of Nottingham; 2009.
- Alwan W, Karagiannia P, Poulos G, Lacy K. Epidemiological trends in malignant melanoma in a large urban population in England from 1999 to 2012. Abstract from the World Congress on Skin Cancer. Br J Dermatol 2014;171:1-76.
- Fletcher RH. Carcinoembryonic antigen. Ann Intern Med 1986;104:66-73. http://dx.doi.org/10.7326/0003-4819-104-1-66.
- Altamura D, Avramidis M, Menzies SW. Assessment of the optimal interval for and sensitivity of short-term sequential digital dermoscopy monitoring for the diagnosis of melanoma. Arch Dermatol 2008;144:502-6. http://dx.doi.org/10.1001/archderm.144.4.502.
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199-206. http://dx.doi.org/10.1200/JCO.2009.23.4799.
- The Likely Impact of Earlier Diagnosis of Cancer Costs and Benefits to the NHS. National Awareness and Early Diagnosis Inititive (NAEDI). London: Department of Health; 2011.
- National Life Tables for Years 2011-2013, United Kingdom. London: ONS; 2014.
- Leman JA, MacKie RM. Late (> 10 years) recurrence of melanoma: the Scottish experience. Br J Dermatol 2003;148:372-3. http://dx.doi.org/10.1046/j.1365-2133.2003.05097_8.x.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. Discussion Paper 172. York: Centre for Health Economics, University of York; 1999.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. http://dx.doi.org/10.1177/0272989X11401031.
- Gudex C, Dolan P, Kind P, Williams A. Health state valuations from the general public using the visual analogue scale. Qual Life Res 1996;5:521-31. http://dx.doi.org/10.1007/BF00439226.
- Huxley N, Jones-Hughes T, Coelho H, Snowsill T, Cooper C, Meng Y, et al. A systematic review and economic evaluation of intraoperative tests [RD-100i one-step nucleic acid amplification (OSNA) system and Metasin test] for detecting sentinel lymph node metastases in breast cancer. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta19020.
- Bristol-Myers Squibb Pharmaceuticals Ltd . Single Technology Appraisal: Ipilimumab for Previously Untreated Advanced (Unresectable or Metastatic) Melanoma (TA319). Manufacturer Sponsor Submission of the Evidence 2013. www.nice.org.uk/guidance/ta319 (accessed 15 April 2015).
- CRD and CHE Technology Assessment Group University of York . Evidence Review Group’s Final Report (TA319): Ipilimumab for Previously Untreated Unresectable Malignant Melanoma 2013. www.nets.nihr.ac.uk/_data/assets/pdf_file/0017/112490/ERGReport-08-210-01-pdf (accessed 21 July 2016).
- Improving Outcomes for People with Skin Tumours Including Melanoma (Update): The Management of Low-risk Basal Cell Carcinomas in the Community. London: NICE; 2010.
- Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. Br J Dermatol 2008;159:35-48. http://dx.doi.org/10.1111/j.1365-2133.2008.08666.x.
- Electrochemotherapy for Primary Basal Cell Carcinoma and Primary Squamous Cell Carcinoma (IPG 478). London: NICE; 2014.
- Llewellyn RS, Alkali A. Regional Audit of the Nonsurgical Management of Basal Cell Carcinomas n.d.
- Champin J, Perrot JL, Cinotti E, Labeille B, Douchet C, Parrau G, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg 2014;40:247-56. http://dx.doi.org/10.1111/dsu.12432.
- Cohen LM. Lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol 1995;33:923-36. http://dx.doi.org/10.1016/0190-9622(95)90282-1.
- Osborne JE, Hutchinson PE. A follow-up study to investigate the efficacy of initial treatment of lentigo maligna with surgical excision. Br J Plast Surg 2002;55:611-15. http://dx.doi.org/10.1054/bjps.2002.3967.
- Akhtar S, Bhat W, Magdum A, Stanley PR. Surgical excision margins for melanoma in situ. J Plast Reconstr Aesthet Surg 2014;67:320-3. http://dx.doi.org/10.1016/j.bjps.2013.11.014.
- McKenna JK, Florell SR, Goldman GD, Bowen GM. Lentigo maligna/lentigo maligna melanoma: current state of diagnosis and treatment. Dermatol Surg 2006;32:493-504. http://dx.doi.org/10.1097/00042728-200604000-00003.
- Hou JL, Reed KB, Knudson RM, Mirzoyev SA, Lohse CM, Frohm ML, et al. Five-year outcomes of wide excision and mohs micrographic surgery for primary lentigo maligna in an academic practice cohort. Dermatol Surg 2015;41:211-18. http://dx.doi.org/10.1097/DSS.0000000000000248.
- Daly ML, Anjum N, Patel M, Barlow R, Sheth N, Mallepedi R, et al. In Vivo Reflectance Confocal Microscopy Prior to Paraffin-Fixed ‘Slow’ Mohs Micrographic Surgery Can Reduce the Number of Stages Required to Clear Lentigo Maligna n.d.
- Merritt BG, Lee NY, Brodland DG, Zitelli JA, Cook J. The safety of Mohs surgery: a prospective multicenter cohort study. J Am Acad Dermatol 2012;67:1302-9. http://dx.doi.org/10.1016/j.jaad.2012.05.041.
Appendix 1 Clinical effectiveness search strategies
OVID EMBASE (searched on 14 October 2014)
| # | Searches |
|---|---|
| 1 | ((skin* or melano* or cutaneous* or sarcoma* or “non melanoma”) adj3 (secondar* or neoplasm* or cancer* or carcinoma* or adenocarcinom* or tumo?r* or malignan* or metastas* or lesion*)).mp. |
| 2 | ((superficial* adj2 melanoma*) or SSM or nodular* melanoma* or lentigo* maligna* or lentiginous* melanoma* or (Hutchinson* adj2 freckle*) or melanoma* in situ or acral* lentiginous* melanoma* or amelanotic* melanoma*).mp. |
| 3 | exp skin tumour/ |
| 4 | exp amelanotic melanoma/ or exp cutaneous melanoma/ or exp melanoma/ or exp non melanoma skin cancer/ or exp melanoma skin cancer/ |
| 5 | (non melanoma* or BCC or gorlin* syndrome* or rodent ulcer* or basalioma* or NMSC*).mp. |
| 6 | ((basal or basocellular* or basosquamous*) adj2 (carcinoma* or cancer* or neoplasm* or tumo?r* or epithelioma* or malignan*)).mp. |
| 7 | ((squamous adj2 (carcinoma* or tumo?r* or cancer* or neoplasm* or epithelioma* or malignan*)) or Bowen* disease* or squamous* cell* carcinoma* in situ or SCC).mp. |
| 8 | exp basal cell carcinoma/ |
| 9 | exp squamous cell carcinoma/ |
| 10 | exp basal cell nevus syndrome/ |
| 11 | exp eyelid tumour/ |
| 12 | Kaposi* sarcoma*.mp. |
| 13 | Merkel* cell* carcinoma*.mp. |
| 14 | (T*cell lymphoma* or cutaneous* T*cell lymphoma* or CTCL or primary* cutaneous* lymphoma*).mp. |
| 15 | or/1-14 |
| 16 | (((CSLM or laser microscop* or confocal microscop* or confocal scanning microscop* or reflec*) adj confocal adj microscop*) or RCM or confocal laser scanning microscop* or reflectan*-mode confocal microscop*).mp. |
| 17 | exp confocal microscopy/ |
| 18 | VivaScope*.mp. |
| 19 | exp epiluminescence microscopy/ |
| 20 | (Dermatoscop* or dermascop* or dermoscop* or (epiluminescen* adj microscop*) or skin* surface* microscop*).mp. |
| 21 | or/16-20 |
| 22 | 15 and 21 |
OVID MEDLINE (searched on 14 October 2014)
| # | Searches |
|---|---|
| 1 | ((skin* or melano* or cutaneous* or sarcoma* or “non melanoma”) adj3 (secondar* or neoplasm* or cancer* or carcinoma* or adenocarcinom* or tumo?r* or malignan* or metastas* or lesion*)).mp. |
| 2 | ((superficial* adj2 melanoma*) or SSM or nodular* melanoma* or lentigo* maligna* or lentiginous* melanoma* or (Hutchinson* adj2 freckle*) or melanoma* in situ or acral* lentiginous* melanoma* or amelanotic* melanoma*).mp. |
| 3 | exp skin neoplasms/ |
| 4 | exp melanoma/ |
| 5 | (non melanoma* or BCC or gorlin* syndrome* or rodent ulcer* or basalioma* or NMSC*).mp. |
| 6 | ((basal or basocellular* or basosquamous*) adj2 (carcinoma* or cancer* or neoplasm* or tumo?r* or epithelioma* or malignan*)).mp. |
| 7 | ((squamous adj2 (carcinoma* or tumo?r* or cancer* or neoplasm* or epithelioma* or malignan*)) or Bowen* disease* or squamous* cell* carcinoma* in situ or SCC).mp. |
| 8 | exp carcinoma, basal cell/ |
| 9 | exp carcinoma, squamous cell/ |
| 10 | exp Neoplasms, Basal Cell/ |
| 11 | exp Basal Cell Nevus Syndrome/ |
| 12 | exp eyelid neoplasms/ |
| 13 | Kaposi* sarcoma*.mp. |
| 14 | Merkel* cell* carcinoma*.mp. |
| 15 | (T*cell lymphoma* or cutaneous* T*cell lymphoma* or CTCL or primary* cutaneous* lymphoma*).mp. |
| 16 | or/1-15 |
| 17 | (((CSLM or laser microscop* or confocal microscop* or confocal scanning microscop* or reflec*) adj confocal adj microscop*) or RCM or confocal laser scanning microscop* or reflectan*-mode confocal microscop*).mp. |
| 18 | exp Microscopy, confocal/ |
| 19 | VivaScope*.mp. |
| 20 | exp Dermoscopy/ |
| 21 | (Dermatoscop* or dermascop* or dermoscop* or (epiluminescen* adj microscop*) or skin* surface* microscop*).mp. |
| 22 | or/17-21 |
| 23 | 16 and 22 |
The Cochrane Library (searched on 14 October 2014)
| ID | Search |
|---|---|
| #1 | (skin or melano* or cutaneous or sarcoma or non next melanoma) near/3 (secondar* or neoplasm or cancer or carcinoma or adenocarcinom* or tumor or tumour or malignan* or metastas or lesion) |
| #2 | superficial near/2 melanoma or SSM or nodular next melanoma or lentigo next maligna or lentiginous next melanoma or Hutchinson* near/2 freckle or “melanoma in situ” or “acral lentiginous melanoma” or “amelanotic melanoma” |
| #3 | MeSH descriptor: [Skin Neoplasms] explode all trees |
| #4 | MeSH descriptor: [Melanoma] explode all trees |
| #5 | non next melanoma or BCC or gorlin* next syndrome or rodent next ulcer or basalioma or NMSC |
| #6 | (basal or basocellular or basosquamous) near/2 (carcinoma or cancer or neoplasm or tumor or tumour or epithelioma or malignan) |
| #7 | (squamous near/2 (carcinoma or tumor or tumour or cancer or neoplasm or epithelioma or malignan*)) or “Bowen’s disease” or “squamous cell carcinoma in situ” or SCC |
| #8 | MeSH descriptor: [Carcinoma, Basal Cell] explode all trees |
| #9 | MeSH descriptor: [Carcinoma, Squamous Cell] explode all trees |
| #10 | MeSH descriptor: [Neoplasms, Basal Cell] explode all trees |
| #11 | MeSH descriptor: [Basal Cell Nevus Syndrome] explode all trees |
| #12 | MeSH descriptor: [Eyelid Neoplasms] explode all trees |
| #13 | “Kaposi’s sarcoma” |
| #14 | “Merkel cell carcinoma” |
| #15 | “T-cell lymphoma” or “cutaneous T-cell lymphoma” or CTCL or “primary cutaneous lymphoma” |
| #16 | {or #1-#15} |
| #17 | CSLM or laser next microscop* or confocal next microscop* or confocal next scanning next microscop* or reflec* next confocal next microscop* or RCM or confocal next laser next scanning next microscop* or reflectan*-mode next confocal next microscop* |
| #18 | MeSH descriptor: [Microscopy, Confocal] explode all trees |
| #19 | vivascope |
| #20 | MeSH descriptor: [Dermoscopy] explode all trees |
| #21 | Dermatoscop* or dermascop* or dermoscop* or “epiluminescence microscopy” or “skin surface microscope” |
| #22 | {or #17-#21} |
| #23 | #16 and #22 |
Appendix 2 Table of excluded studies with rationale on clinical effectiveness
| Full reference details | Reason for exclusion |
|---|---|
| Gurgen J, Gatti M. Epiluminescence microscopy (dermoscopy) versus visual inspection during Mohs’ microscopic surgery of infiltrative basal cell carcinoma. Dermatol Surg 2012;38:1066–9 | Dermoscopy vs. visual inspection |
| Guardiano RA, Grande DJ. A direct comparison of visual inspection, curettage, and epiluminescence microscopy in determining tumour extent before the initial margins are determined for Mohs’ micrographic surgery. Dermatol Surg 2010;36:1240–4 | Visual inspection vs. curettage vs. dermoscopy |
| Binder M, Schwarz M, Winkler A, Steiner A, Kaider A, Wolff K, et al. Epiluminescence microscopy. A useful tool for the diagnosis of pigmented skin lesions for formally trained dermatologists. Arch Dermatol 1995;131:286–91 | Trained vs. non-trained experts on dermoscopy |
| Argenziano G, Puig S, Zalaudek I, Sera F, Corona R, Alsina M, et al. Dermoscopy improves accuracy of primary care physicians to triage lesions suggestive of skin cancer. J Clin Oncol 2006;24:1877–82 | Accuracy of referrals using dermoscopy |
| Blum A, Hofmann-Wellenhof R, Luedtke H, Ellwanger U, Steins A, Roehm S, et al. Value of the clinical history for different users of dermoscopy compared with results of digital image analysis. J Eur Acad Dermatol Venereol 2004;18:665–9 | Trained vs. untrained clinicians on dermoscopy |
| Blum A, Rassner G, Garbe C, Blum A, Rassner G, Garbe C. Modified ABC-point list of dermoscopy: a simplified and highly accurate dermoscopic algorithm for the diagnosis of cutaneous melanocytic lesions. J Am Acad Dermatol 2003;48:672–8 | Accuracy of ABCD point list on dermoscopy |
| Carli P, De Giorgi V, Crocetti E, Mannone F, Massi D, Chiarugi A, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997–2001. Br J Dermatol 2004;150:687–92 | Dermoscope users vs. non-users |
| Chiacchio N, Hirata SH, Enokihara MY, Michalany NS, Fabbrocini G, Tosti A. Dermatologists’ accuracy in early diagnosis of melanoma of the nail matrix. Arch Dermatol 2010;146:382–7 | Clinicians agreement of nail melanomas with dermoscopy |
| Dolianitis C, Kelly J, Wolfe R, Simpson P. Comparative performance of 4 dermoscopic algorithms by nonexperts for the diagnosis of melanocytic lesions. Arch Dermatol 2005;141:1008–14 | Comparison of four dermoscopic algorithms |
| Dreiseitl S, Binder M, Hable K, Kittler H, Dreiseitl S, Binder M, et al. Computer versus human diagnosis of melanoma: evaluation of the feasibility of an automated diagnostic system in a prospective clinical trial. Melanoma Res 2009;19:180–4 | Experts vs. non-experts in the use of computer-based diagnostic systems |
| Elbaum M, Kopf AW, Rabinovitz HS, Langley RG, Kamino H, Mihm MC Jr, et al. Automatic differentiation of melanoma from melanocytic naevi with multispectral digital dermoscopy: a feasibility study. J Am Acad Dermatol 2001;44:207–18 | Differentiation between melanoma and melanocytic naevi |
| Fruhauf J, Leinweber B, Fink-Puches R, Ahlgrimm-Siess V, Richtig E, Wolf IH, et al. Patient acceptance and diagnostic utility of automated digital image analysis of pigmented skin lesions. J Eur Acad Dermatol Venereol 2012;26:368–72 | Patients acceptance of dermoscopy |
| Garcia Arroyo JL, Garcia ZB, Garcia Arroyo JL, Garcia Zapirain B. Detection of pigment network on dermoscopy images using supervised machine learning and structural analysis. Comput Biol Med 2014;44:144–57 | Only dermoscopy, no RCM |
| Garnavi R, Aldeen M, Bailey J, Garnavi R, Aldeen M, Bailey J. Computer-aided diagnosis of melanoma using border and wavelet-based texture analysis. IEEE Trans Info Technol Biomed 2012;16:1239–52 | Only dermoscopy, no RCM |
| Garnavi R, Aldeen M, Celebi ME, Varigos G, Finch S, Garnavi R, et al. Border detection on dermoscopy images using hybrid thresholding on optimized color channels. Comput Med Imaging Graph 2011;35:105–15 | Only dermoscopy, no RCM |
| Gilmore S, Hofmann-Wellenhof R, Soyer HP, Gilmore S, Hofmann-Wellenhof R, Soyer HP. A support vector machine for decision support in melanoma recognition. Exp Dermatol 2010;19:830–5 | Only dermoscopy, no RCM |
| Haenssle HA, Krueger U, Vente C, Thoms KM, Bertsch HP, Zutt M, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical naevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol 2006;126:980–5 | Only dermoscopy, no RCM |
| Henning JS, Dusza SW, Wang SQ, Marghoob AA, Rabinovitz HS, Polsky D, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol 2007;56:45–52 | Accuracy of dermoscopy algorithm |
| Hoffmann K, Gambichler T, Rick A, Kreutz M, Anschuetz M, Grunendick T, et al. Diagnostic and neural analysis of skin cancer (DANAOS). A multicentre study for collection and computer-aided analysis of data from pigmented skin lesions using digital dermoscopy. Br J Dermatol 2003;149:801–9 | Only dermoscopy, no RCM |
| Iyatomi H, Oka H, Celebi ME, Ogawa K, Argenziano G, Soyer HP, et al. Computer-based classification of dermoscopy images of melanocytic lesions on acral volar skin. J Invest Dermatol 2008;128:2049–54 | Classification of dermoscopy images |
| Iyatomi H, Oka H, Saito M, Miyake A, Kimoto M, Yamagami J, et al. Quantitative assessment of tumour extraction from dermoscopy images and evaluation of computer-based extraction methods for an automatic melanoma diagnostic system. Melanoma Res 2006;16:183–90 | Only dermoscopy, no RCM |
| Kockara S, Mete M, Yip V, Lee B, Aydin K, Kockara S, et al. A soft kinetic data structure for lesion border detection. Bioinformatics 2010;26:i21–8 | Assessment of dermoscopic images |
| Lorentzen H, Weismann K, Petersen CS, Larsen FG, Secher L, Skodt V, et al. Clinical and dermatoscopic diagnosis of malignant melanoma. Assessed by expert and non-expert groups. Acta Derm Venereol 1999;79:301–4 | Only dermoscopy, no RCM |
| Lorentzen H, Weismann K, Secher L, Petersen CS, Larsen FG, Lorentzen H, et al. The dermatoscopic ABCD rule does not improve diagnostic accuracy of malignant melanoma. Acta Derm Venereol 1999;79:469–72 | Accuracy of ABCD rule on dermoscopy |
| Lorentzen HF, Eefsen RL, Weismann K, Lorentzen HF, Eefsen RL, Weismann K. Comparison of classical dermatoscopy and acrylic globe magnifier dermatoscopy. Acta Derm Venereo 2008;88:139–42 | Classical dermoscopy vs. acrylic globe magnifier dermoscopy |
| MacKie RM, Fleming C, McMahon AD, Jarrett P, MacKie RM, Fleming C, et al. The use of the dermatoscope to identify early melanoma using the three-colour test. Br J Dermatol 2002;146:481–4 | Only dermoscopy, no RCM |
| Nachbar F, Stolz W, Merkle T, Cognetta AB, Vogt T, Landthaler M, et al. The ABCD rule of dermatoscopy. High prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol 1994;30:551–9 | Accuracy of ABCD rule on dermoscopy |
| Piccolo D, Ferrari A, Peris K, Diadone R, Ruggeri B, Chimenti S. Dermoscopic diagnosis by a trained clinician vs. a clinician with minimal dermoscopy training vs. computer-aided diagnosis of 341 pigmented skin lesions: a comparative study. Br J Dermatol 2002;147:481–6 | Trained clinician vs. clinician with minimal dermoscopy training vs. computer-aided diagnosis |
| Soyer HP, Argenziano G, Zalaudek I, Corona R, Sera F, Talamini R, et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology 2004;208:27–31 | Experts vs. non-experts on dermoscopy |
| Zalaudek I, Argenziano G, Soyer HP, Corona R, Sera F, Blum A, et al. Three-point checklist of dermoscopy: an open internet study. Br J Dermatol 2006;154:431–7 | Only dermoscopy, no RCM |
| Cosgarea RU. Our 9 years digital dermoscopy experience in the diagnosis of early melanoma. J German Soc Dermatol 2013;11:2–3 | Only dermoscopy, no RCM |
| Gereli MCO. Comparison of two dermoscopic techniques in the melanoma diagnosis: 3-point checklist and 7-point checklist. TÜRKDERM Deri Hastaliklari ve Frengi Arsivi 2008;42:45–50 | Comparison of two dermoscopic techniques |
Appendix 3 List of ongoing trials on clinical effectiveness
| Title, study identifier and link | Type of RCM | Study design | Indication | Status (ongoing or completed |
|---|---|---|---|---|
| Sensitivity/Specificity Study of Non-invasive Imaging for Melanoma Diagnosis (NCT 01556503) https://clinicaltrials.gov/ct2/show/NCT01556503?term=vivascope&rank=1 (accessed 18 November 2014) | VivaScope 1500 and 2500 | Prospective observational | Lesion diagnosis | Ongoing (April 2011–August 2015) |
| Treatment of Basal Cell Carcinoma Using a One-stop-shop With Reflectance Confocal Microscopy: A Randomized Controlled Multicenter Trial (NCT02285790) https://clinicaltrials.gov/ct2/show/NCT02285790?term=vivascope&rank=2 (accessed 18 November 2014) | VivaScope 1500 | RCT | Lesion diagnosis | Ongoing (January 2015–February 2016) |
| Reflectance Confocal Microscopy of Wounds During Mohs’ Surgery: Feasibility Testing of a Mosaicing Algorithm for Intraoperative Imaging of Cancer Margins (NCT01872130) https://clinicaltrials.gov/ct2/show/NCT01872130?term=reflectance+confocal+ microscopy&rank=4 (accessed 18 November 2014) | Not reported | Prospective observational | Margin delineation | Ongoing (May 2013–May 2015) |
| VivaNet Study. A Multicenter Study of Confocal Reflectance Microscopy in Telemedicine (NCT01385943) https://clinicaltrials.gov/ct2/show/NCT01385943?term=reflectance+confocal+microscopy&rank=8 (accessed 18 November 2014) | Not reported | Prospective observational | Lesion diagnosis | Ongoing (April 2011–December 2015) |
| Evaluation of Optical Imaging for Margin Delineation of Non-Melanoma Skin Cancer (NCT00432471) https://clinicaltrials.gov/ct2/show/NCT00432471?term%20=%20reflectance+confocal+microscopy&rank%20=%2014 (accessed 18 November 2014) | Not reported | Prospective observational | Margin delineation | Ongoing (January 2007–January 2016) |
Appendix 4 Quality assessment
| Study and year | Risk of bias: summary of risk-of-bias assessments of parallel RCTs included in review | Applicability concerns | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Comparator | Reference standard | Flow and timing | Patient selection | Index test | Comparator | Reference standard | |
| Lesion diagnosis | |||||||||
| Alarcon et al., 201430 | Low | Low | Low | Unclear | Low | Low | Low | Low | Low |
| Castro et al., 201443 | Low | Low | NC | Low | Unclear | Low | Low | NC | Low |
| Curchin et al., 201131 | Unclear | Low | NC | Low | High | Unclear | Low | NC | Low |
| Ferrari et al., 201444 | Low | Low | Low | Low | Unclear | Low | Low | Low | Low |
| Gerger et al., 200632 | Low | Low | NC | Low | Unclear | Low | Low | NC | Low |
| Gerger et al., 200833 | Low | Low | NC | Low | Unclear | Unclear | Unclear | NC | Low |
| Guitera et al., 200934 | Low | Low | Low | Low | Low | Unclear | Unclear | Unclear | Low |
| Guitera et al., 201040 | Low | Low | NC | Low | Unclear | Low | Low | NC | Low |
| Langley et al., 200736 | Low | Low | Low | Low | Unclear | Low | Low | Low | Low |
| Pellacani et al., 200738 | Low | Low | NC | Low | Unclear | Low | Low | NC | Low |
| Pellacani et al., 201442 | Low | Unclear | Unclear | Low | Unclear | Low | Low | Unclear | Low |
| Rao et al. 201339 | High | High | NC | Unclear | Unclear | High | High | NC | Low |
| Stanganelli et al., 201445 | Low | Low | NC | Low | Unclear | Low | Low | NC | Low |
| Lesion margin delineation | |||||||||
| Bennassar et al., 201441 | Low | Low | NC | Low | Unclear | Low | Low | NC | Low |
| Guitera et al., 201335 | Unclear | Unclear | NC | Low | Unclear | Unclear | Unclear | NC | Low |
| Pan et al., 201237 | Unclear | Low | NC | Low | Unclear | Unclear | Low | NC | Low |
Appendix 5 Health economics search strategy
Search 1: economic evaluations
MEDLINE
Full database title: Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE.
Search range: 1946 to present.
Date of search: 21 October 2014.
| # | Terms | Number of hits |
|---|---|---|
| 1 | ((skin* or melano* or cutaneous* or sarcoma* or “non melanoma”) adj3 (secondar* or neoplasm* or cancer* or carcinoma* or adenocarcinom* or tumo?r* or malignan* or metastas* or lesion*)).mp. | 175,861 |
| 2 | ((superficial* adj2 melanoma*) or SSM or nodular* melanoma* or lentigo* maligna* or lentiginous* melanoma* or (Hutchinson* adj2 freckle*) or melanoma* in situ or acral* lentiginous* melanoma* or amelanotic* melanoma*).mp. | 4241 |
| 3 | exp skin neoplasms/ | 99,460 |
| 4 | exp melanoma/ | 76,818 |
| 5 | (non melanoma* or BCC or gorlin* syndrome* or rodent ulcer* or basalioma* or NMSC*).mp. | 7559 |
| 6 | ((basal or basocellular* or basosquamous*) adj2 (carcinoma* or cancer* or neoplasm* or tumo?r* or epithelioma* or malignan*)).mp. | 19,588 |
| 7 | ((squamous adj2 (carcinoma* or tumo?r* or cancer* or neoplasm* or epithelioma* or malignan*)) or Bowen* disease* or squamous* cell* carcinoma* in situ or SCC).mp. | 134,054 |
| 8 | exp carcinoma, basal cell/ | 14,918 |
| 9 | exp carcinoma, squamous cell/ | 107,922 |
| 10 | exp Neoplasms, Basal Cell/ | 16,143 |
| 11 | exp Basal Cell Nevus Syndrome/ | 1083 |
| 12 | exp eyelid neoplasms/ | 3914 |
| 13 | Kaposi* sarcoma*.mp. | 11,949 |
| 14 | Merkel* cell* carcinoma*.mp. | 1974 |
| 15 | (T*cell lymphoma* or cutaneous* T*cell lymphoma* or CTCL or primary* cutaneous* lymphoma*).mp. | 1645 |
| 16 | or/1- 15 | 344,314 |
| 17 | Health economics.mp. | 2317 |
| 18 | Economic evaluation.mp. | 5602 |
| 19 | exp “Costs and Cost Analysis”/ | 188,506 |
| 20 | exp Cost-Benefit Analysis/ | 62,754 |
| 21 | exp Models, economic/ | 10,609 |
| 22 | exp “Fees and Charges”/ | 27,778 |
| 23 | exp Budgets/ | 12,298 |
| 24 | Cost Effectiveness Analysis.mp. | 6038 |
| 25 | (unit cost or unit-cost or unit-costs or unit costs or drug cost or drug costs or hospital costs or health-care costs or health care cost or medical cost or medical costs).tw. | 23,117 |
| 26 | Cost Minimi?ation Analysis.mp. | 489 |
| 27 | Cost Utility Analysis.mp. | 1327 |
| 28 | (cost adj2 (util$ or effective$ or efficac$ or benefit$ or consequence$ or analys$ or minimi$ or allocation$ or control$ or illness$ or affordable$ or fee$ or charge$)).tw. | 104,630 |
| 29 | (decision adj1 (tree* or analys* or model*)).tw. | 9424 |
| 30 | (econom* or price* or pricing or financ* or fee* or pharmacoeconomic* or pharmaeconomic* or pharmaco-economic*).tw. | 638,050 |
| 31 | ((value or values or valuation) adj2 (money or monetary or life or lives or costs or cost)).tw. | 4538 |
| 32 | Markov*.tw. | 14,886 |
| 33 | or/17-31 | 864,990 |
| 34 | 16 and 33 | 5004 |
| 35 | (letter or editorial or comment or case report or review).pt. | 3,334,906 |
| 36 | (animals not humans).sh. | 3,983,385 |
| 37 | 34 (not 35 or 36) | 3682 |
| 38 | (((CSLM or laser microscop* or confocal microscop* or confocal scanning microscop* or reflec*) adj confocal adj microscop*) or RCM or confocal laser scanning microscop* or reflectan*-mode confocal microscop*).mp. | 10,172 |
| 39 | exp Microscopy, confocal/ | 44,436 |
| 40 | vivascope*.mp. | 22 |
| 41 | exp Dermoscopy/ | 2067 |
| 42 | (Dermatoscop* or dermascop* or dermoscop* or (epiluminescen* adj microscop*) or skin* surface* microscop*).mp. | 3210 |
| 43 | or/38-42 | 53,228 |
| 44 | 43 not (35 or 36) | 30,492 |
| 45 | 37 and 44 | 38 |
EMBASE
Search range: 1974 to 20 October 2014.
Date of search: 21 October 2014.
| # | Terms | Number of hits |
|---|---|---|
| 1 | ((skin* or melano* or cutaneous* or sarcoma* or “non melanoma”) adj3 (secondar* or neoplasm* or cancer* or carcinoma* or adenocarcinom* or tumo?r* or malignan* or metastas* or lesion*)).mp. | 285,165 |
| 2 | ((superficial* adj2 melanoma*) or SSM or nodular* melanoma* or lentigo* maligna* or lentiginous* melanoma* or (Hutchinson* adj2 freckle*) or melanoma* in situ or acral* lentiginous* melanoma* or amelanotic* melanoma*).mp. | 5818 |
| 3 | skin tumor/ or exp skin cancer/ | 214,794 |
| 4 | exp melanoma/ or exp non melanoma skin cancer/ or exp melanoma skin cancer/ or exp amelanotic melanoma/ or exp cutaneous melanoma/ | 268,360 |
| 5 | (non melanoma* or BCC or gorlin* syndrome* or rodent ulcer* or basalioma* or NMSC*).mp. | 10,211 |
| 6 | ((basal or basocellular* or basosquamous*) adj2 (carcinoma* or cancer* or neoplasm* or tumo?r* or epithelioma* or malignan*)).mp. | 25,252 |
| 7 | ((squamous adj2 (carcinoma* or tumo?r* or cancer* or neoplasm* or epithelioma* or malignan*)) or Bowen* disease* or squamous* cell* carcinoma* in situ or SCC).mp. | 143,567 |
| 8 | exp basal cell carcinoma/ | 21,007 |
| 9 | exp squamous cell carcinoma/ | 101,150 |
| 10 | exp basal cell nevus syndrome/ | 1954 |
| 11 | exp eyelid tumor/ | 3553 |
| 12 | Kaposi* sarcoma*.mp. | 18,521 |
| 13 | Merkel* cell* carcinoma*.mp. | 2589 |
| 14 | (T*cell lymphoma* or cutaneous* T*cell lymphoma* or CTCL or primary* cutaneous* lymphoma*).mp. | 18,117 |
| 15 | or/1-14 | 485,211 |
| 16 | exp “cost utility analysis”/ | 5618 |
| 17 | exp “cost benefit analysis”/ | 65,480 |
| 18 | exp “cost effectiveness analysis”/ | 100,676 |
| 19 | exp “cost minimization analysis”/ | 2538 |
| 20 | health economics.mp. | 35,999 |
| 21 | economic evaluation.mp. | 13,996 |
| 22 | statistical model/ | 103,962 |
| 23 | exp fee/ | 34,213 |
| 24 | exp budget/ | 19,880 |
| 25 | (unit cost or unit-cost or unit-costs or unit costs or drug cost or drug costs or hospital costs or health-care costs or health care cost or medical cost or medical costs).tw. | 31,850 |
| 26 | (cost adj2 (util$ or effective$ or efficac$ or benefit$ or consequence$ or analys$ or minimi$ or allocation$ or control$ or illness$ or affordable$ or fee$ or charge$)).tw. | 134,581 |
| 27 | (decision adj1 (tree* or analys* or model*)).tw. | 11,939 |
| 28 | (econom* or price* or pricing or financ* or fee* or pharmacoeconomic* or pharmaeconomic* or pharmaco-economic*).tw. | 768,319 |
| 29 | ((value or values or valuation) adj2 (money or monetary or life or lives or costs or cost)).tw. | 5823 |
| 30 | Markov*.tw. | 16,453 |
| 31 | or/16-30 | 1,124,796 |
| 32 | 15 and 31 | 9954 |
| 33 | (letter or editorial or comment or case report or review).pt. | 3,302,908 |
| 34 | (animal$ not human$).sh,hw. | 3,787,830 |
| 35 | 32 not (33 or 34) | 7260 |
| 36 | (((CSLM or laser microscop* or confocal microscop* or confocal scanning microscop* or reflec*) adj confocal adj microscop*) or RCM or confocal laser scanning microscop* or reflectan*-mode confocal microscop*).mp. | 11,941 |
| 37 | exp confocal microscopy/ | 40,535 |
| 38 | vivascope*.mp. | 155 |
| 39 | exp epiluminescence microscopy/ | 3889 |
| 40 | (Dermatoscop* or dermascop* or dermoscop* or (epiluminescen* adj microscop*) or skin* surface* microscop*).mp. | 4476 |
| 41 | or/36-40 | 54,904 |
| 42 | 41 not (33 or 34) | 36,364 |
| 43 | 35 and 42 | 80 |
Health Technology Assessment database
Search range: inception to 14 October 2014.
Date of search: 14 October 2014.
| Search terms (and fields searched) | #1 (skin or melano* or cutaneous or sarcoma or non next melanoma) near/3 (secondar* or neoplasm or cancer or carcinoma or adenocarcinom* or tumor or tumour or malignan* or metastas or lesion) #2 superficial near/2 melanoma or SSM or nodular next melanoma or lentigo next maligna or lentiginous next melanoma or Hutchinson* near/2 freckle or “melanoma in situ” or “acral lentiginous melanoma” or “amelanotic melanoma” #3 MeSH descriptor: [Skin Neoplasms] explode all trees #4 MeSH descriptor: [Melanoma] explode all trees #5 non next melanoma or BCC or gorlin* next syndrome or rodent next ulcer or basalioma or NMSC #6 (basal or basocellular or basosquamous) near/2 (carcinoma or cancer or neoplasm or tumor or tumour or epithelioma or malignan) #7 (squamous near/2 (carcinoma or tumor or tumour or cancer or neoplasm or epithelioma or malignan*)) or “Bowen’s disease” or “squamous cell carcinoma in situ” or SCC #8 MeSH descriptor: [Carcinoma, Basal Cell] explode all trees #9 MeSH descriptor: [Carcinoma, Squamous Cell] explode all trees #10 MeSH descriptor: [Neoplasms, Basal Cell] explode all trees #11 MeSH descriptor: [Basal Cell Nevus Syndrome] explode all trees #12 MeSH descriptor: [Eyelid Neoplasms] explode all trees #13 “Kaposi’s sarcoma” #14 “Merkel cell carcinoma” #15 “T-cell lymphoma” or “cutaneous T-cell lymphoma” or CTCL or “primary cutaneous lymphoma” #16 {or #1-#15} #17 CSLM or laser next microscop* or confocal next microscop* or confocal next scanning next microscop* or reflec* next confocal next microscop* or RCM or confocal next laser next scanning next microscop* or reflectan*-mode next confocal next microscop* #18 MeSH descriptor: [Microscopy, Confocal] explode all trees #19 vivascope #20 MeSH descriptor: [Dermoscopy] explode all trees #21 Dermatoscop* or dermascop* or dermoscop* or “epiluminescence microscopy” or “skin surface microscope” #22 {or #17-#21} #23 #16 and #22 |
| Number of hits | 5 |
NHS Economic Evaluation Database
Search range: inception to 14 October 2014.
Date of search: 14 October 2014.
| Search terms (and fields searched) | #1 (skin or melano* or cutaneous or sarcoma or non next melanoma) near/3 (secondar* or neoplasm or cancer or carcinoma or adenocarcinom* or tumor or tumour or malignan* or metastas or lesion) #2 superficial near/2 melanoma or SSM or nodular next melanoma or lentigo next maligna or lentiginous next melanoma or Hutchinson* near/2 freckle or “melanoma in situ” or “acral lentiginous melanoma” or “amelanotic melanoma” #3 MeSH descriptor: [Skin Neoplasms] explode all trees #4 MeSH descriptor: [Melanoma] explode all trees #5 non next melanoma or BCC or gorlin* next syndrome or rodent next ulcer or basalioma or NMSC #6 (basal or basocellular or basosquamous) near/2 (carcinoma or cancer or neoplasm or tumor or tumour or epithelioma or malignan) #7 (squamous near/2 (carcinoma or tumor or tumour or cancer or neoplasm or epithelioma or malignan*)) or “Bowen’s disease” or “squamous cell carcinoma in situ” or SCC #8 MeSH descriptor: [Carcinoma, Basal Cell] explode all trees #9 MeSH descriptor: [Carcinoma, Squamous Cell] explode all trees #10 MeSH descriptor: [Neoplasms, Basal Cell] explode all trees #11 MeSH descriptor: [Basal Cell Nevus Syndrome] explode all trees #12 MeSH descriptor: [Eyelid Neoplasms] explode all trees #13 “Kaposi’s sarcoma” #14 “Merkel cell carcinoma” #15 “T-cell lymphoma” or “cutaneous T-cell lymphoma” or CTCL or “primary cutaneous lymphoma” #16 {or #1-#15} #17 CSLM or laser next microscop* or confocal next microscop* or confocal next scanning next microscop* or reflec* next confocal next microscop* or RCM or confocal next laser next scanning next microscop* or reflectan*-mode next confocal next microscop* #18 MeSH descriptor: [Microscopy, Confocal] explode all trees #19 vivascope #20 MeSH descriptor: [Dermoscopy] explode all trees #21 Dermatoscop* or dermascop* or dermoscop* or “epiluminescence microscopy” or “skin surface microscope” #22 {or #17-#21} #23 #16 and #22 |
| Number of hits | 2 |
First pass
Potential economic evaluations reviewed at second pass (n = 5)
| # | Study |
|---|---|
| 1 | Morton, CA, Downie F, Auld S, Smith B, van der Pol M, Baughan P, et al. Community photo-triage for skin cancer referrals: an aid to service delivery. Clin Exp Dermatol 2011:36:248–54 |
| 2 | Stratigos AJ, Katsambas AD. The value of screening in melanoma. Clin Dermatol 2009;27:10–25 |
| 3 | Tromme, I Devleesschauwer B, Beutels P, Richez P, Praet N, Sacré L. Selective use of sequential digital dermoscopy imaging allows a cost reduction in the melanoma detection process: a Belgian study of patients with a single or a small number of atypical nevi. PLOS ONE 2014:9:e109339 |
| 4 | Watts C, Cust A, Meuzies S, Coates E, Mann G, Morton R. Using Multiple Data Sources to Determine the Cost of Managing Individuals in a Clinic for Individuals at High Risk of Primary Melanoma. JDDG – Journal of the German Society of Dermatology Conference: Eighth World Congress of Melanoma, Ninth Congress of the European Association of Dermatology, EADO, Seventh Interdisciplinary Melanoma/Skin Cancer Meeting, Third European Post-Chicago Melanoma Meeting 2013, Hamburg, Germany, 17–20 July 2013. pp. 20130711–12 |
| 5 | Wilson ECF, Emery JD, Kinmonth AL, Prevost AT, Morris MC, Humphrys E, et al. The cost-effectiveness of a novel SIAscopic diagnostic aid for the management of pigmented skin lesions in primary care: a decision-analytic model. Value Health 2013;16:356–66 |
Second pass
Summary of reasons for exclusion, economic evaluations
| Study and year | Reasons for exclusion |
|---|---|
| Wilson et al., 201348 | Clinical experts advised the TAG that the MoleMate system (SiaScopy) is not a relevant intervention; SiaScopy produces images at surface features, whereas the VivaScope can image cells of a histological quality |
| Tromme et al., 201458 | Digital dermoscopy is not a diagnostic test of interest |
| Watts et al., 2013 | Interventions not relevant |
| Stratigos and Katsambas, 2009 | Not an economic evaluation or costing study |
| Morton et al., 2011 | Interventions not relevant |
Search 2: resource-use and cost-of-illness studies
MEDLINE
Full database title: Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE.
Search range: 1946 to present.
Date of search: 17 December 2014.
| # | Terms | Number of hits |
|---|---|---|
| 1 | ((skin* or melano* or cutaneous* or sarcoma* or “non melanoma”) adj3 (secondar* or neoplasm* or cancer* or carcinoma* or adenocarcinom* or tumo?r* or malignan* or metastas* or lesion*)).mp. | 177,999 |
| 2 | ((superficial* adj2 melanoma*) or SSM or nodular* melanoma* or lentigo* maligna* or lentiginous* melanoma* or (Hutchinson* adj2 freckle*) or melanoma* in situ or acral* lentiginous* melanoma* or amelanotic* melanoma*).mp. | 4293 |
| 3 | exp skin neoplasms/ | 100,649 |
| 4 | exp melanoma/ | 77,456 |
| 5 | (non melanoma* or BCC or gorlin* syndrome* or rodent ulcer* or basalioma* or NMSC*).mp. | 7658 |
| 6 | ((basal or basocellular* or basosquamous*) adj2 (carcinoma* or cancer* or neoplasm* or tumo?r* or epithelioma* or malignan*)).mp. | 20,007 |
| 7 | ((squamous adj2 (carcinoma* or tumo?r* or cancer* or neoplasm* or epithelioma* or malignan*)) or Bowen* disease* or squamous* cell* carcinoma* in situ or SCC).mp. | 135,631 |
| 8 | exp carcinoma, basal cell/ | 15,250 |
| 9 | exp carcinoma, squamous cell/ | 108,973 |
| 10 | exp Neoplasms, Basal Cell/ | 16,501 |
| 11 | exp Basal Cell Nevus Syndrome/ | 1089 |
| 12 | exp eyelid neoplasms/ | 3991 |
| 13 | Kaposi* sarcoma*.mp. | 12,016 |
| 14 | Merkel* cell* carcinoma*.mp. | 2022 |
| 15 | (T*cell lymphoma* or cutaneous* T*cell lymphoma* or CTCL or primary* cutaneous* lymphoma*).mp. | 1659 |
| 16 | or/1- 15 | 348,258 |
| 17 | (UK or United Kingdom or England or Wales or Scotland or GB or Great Britain).tw. | 160,549 |
| 18 | exp Great Britain/ | 312,045 |
| 19 | (NHS or National Health Service or DOH or Department of Health or PSSRU or Personal Social Services Research Unit).tw. | 35,310 |
| 20 | or/17-19 | 412,613 |
| 21 | (unit cost or unit-cost or unit-costs or unit costs or drug cost or drug costs or hospital costs or health-care costs or health care cost or medical cost or medical costs).tw. | 23,706 |
| 22 | (econom* or price* or pricing or financ* or fee* or pharmacoeconomic* or pharmaeconomic* or pharmaco-economic*).tw. | 647,359 |
| 23 | exp “cost of illness”/ | 19,141 |
| 24 | or/21-23 | 675,466 |
| 25 | 16 and 20 and 24 | 110 |
| 26 | (letter or editorial or comment or case report or review).pt. | 3,373,280 |
| 27 | (animals not humans).sh. | 4,004,891 |
| 28 | 25 not (26 or 27) | 83 |
EMBASE
Search range: 1974 to 16 December 2014.
Date of search: 17 December 2014.
| # | Terms | Number of hits |
|---|---|---|
| 1 | ((skin* or melano* or cutaneous* or sarcoma* or “non melanoma”) adj3 (secondar* or neoplasm* or cancer* or carcinoma* or adenocarcinom* or tumo?r* or malignan* or metastas* or lesion*)).mp. | 288,557 |
| 2 | ((superficial* adj2 melanoma*) or SSM or nodular* melanoma* or lentigo* maligna* or lentiginous* melanoma* or (Hutchinson* adj2 freckle*) or melanoma* in situ or acral* lentiginous* melanoma* or amelanotic* melanoma*).mp. | 5905 |
| 3 | skin tumor/ or exp skin cancer/ | 216,673 |
| 4 | exp melanoma/ or exp non melanoma skin cancer/ or exp melanoma skin cancer/ or exp amelanotic melanoma/ or exp cutaneous melanoma/ | 262,555 |
| 5 | (non melanoma* or BCC or gorlin* syndrome* or rodent ulcer* or basalioma* or NMSC*).mp. | 10,400 |
| 6 | ((basal or basocellular* or basosquamous*) adj2 (carcinoma* or cancer* or neoplasm* or tumo?r* or epithelioma* or malignan*)).mp. | 25,557 |
| 7 | ((squamous adj2 (carcinoma* or tumo?r* or cancer* or neoplasm* or epithelioma* or malignan*)) or Bowen* disease* or squamous* cell* carcinoma* in situ or SCC).mp. | 145,113 |
| 8 | exp basal cell carcinoma/ | 21,264 |
| 9 | exp squamous cell carcinoma/ | 102,063 |
| 10 | exp basal cell nevus syndrome/ | 1973 |
| 11 | exp eyelid tumor/ | 3589 |
| 12 | Kaposi* sarcoma*.mp. | 18,669 |
| 13 | Merkel* cell* carcinoma*.mp. | 2642 |
| 14 | (T*cell lymphoma* or cutaneous* T*cell lymphoma* or CTCL or primary* cutaneous* lymphoma*).mp. | 18,384 |
| 15 | or/ 1-14 | 487,558 |
| 16 | (unit cost or unit-cost or unit-costs or unit costs or drug cost or drug costs or hospital costs or health-care costs or health care cost or medical cost or medical costs).tw. | 32,693 |
| 17 | (econom* or price* or pricing or financ* or fee* or pharmacoeconomic* or pharmaeconomic* or pharmaco-economic*).tw. | 777,455 |
| 18 | exp “cost of illness”/ | 14,591 |
| 19 | or/16-18 | 806,927 |
| 20 | (UK or United Kingdom or England or Wales or Scotland or GB or Great Britain).tw. | 279,911 |
| 21 | exp United Kingdom/ | 338,464 |
| 22 | (NHS or National Health Service or DOH or Department of Health or PSSRU or Personal Social Services Research Unit).tw. | 45,706 |
| 23 | or/20-22 | 523,277 |
| 24 | 15 and 19 and 23 | 291 |
| 25 | (letter or editorial or comment or case report or review).pt. | 3,322,723 |
| 26 | (animal$ not human$).sh,hw. | 3,800,224 |
| 27 | 24 not (25 or 26) | 194 |
First pass
Resource-use and cost-of-illness studies reviewed at second pass (n = 9)
| # | Studya |
|---|---|
| 1 | Brown B, Diamantopoulos A, Bernier J, Schöffski P, Hieke K, Mantovani L, et al. An economic evaluation of cetuximab combined with radiotherapy for patients with locally advanced head and neck cancer in Belgium, France, Italy, Switzerland, and the United Kingdom. Value Health 2008;11:791–9 |
| 2 | Dixon S, Walters SJ, Turner L, Hancock BW. Quality of life and cost-effectiveness of interferon-alpha in malignant melanoma: results from randomised trial. Br J Cancer 2006;94:492–8 |
| 3 | Johnston K, Levy AR, Lorigan P, Maio M, Lebbe C, Middleton M, et al. Economic impact of healthcare resource utilisation patterns among patients diagnosed with advanced melanoma in the United Kingdom, Italy, and France: results from a retrospective, longitudinal survey (MELODY study). Eur J Cancer 2012;48:2175–82 |
| 4 | Kim K, Amonkar MM, Högberg D, Kasteng F. Economic burden of resected squamous cell carcinoma of the head and neck in an incident cohort of patients in the UK. Head Neck Oncol 2011;3:47 |
| 5 | Morris S et al.49 |
| 6 | Parthan A, Posner MR, Brammer C, Beltran P, Jansen JP. Cost utility of docetaxel as induction chemotherapy followed by chemoradiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck 2009;31:1255–62 |
| 7 | Ramrakha-Jones VS, Herd RM. Treating Bowen's disease: a cost-minimization study. Br J Dermatol 2003;148:1167–72 |
| 8 | Vallejo-Torres et al.50 |
| 9 | Wilson et al.48 |
Second pass
Summary of reasons for exclusion, resource-use and cost-of-illness studies
| Study and year | Reasons for exclusion |
|---|---|
| Brown et al., 2008 | Irrelevant population: SCC of the head and necka |
| Dixon et al., 2006 | Only total incremental costs are reported |
| Johnston et al., 2012 | Irrelevant population: unresectable melanoma treatment pattern used to estimate the cost per user or per patient |
| Kim et al., 2011 | Irrelevant population: SCC of the head and necka |
| Parthan et al., 2009 | Irrelevant population: SCC of the head and necka |
| Ramrakha-Jones and Herd, 2003 | Irrelevant population: Bowen’s disease (provisionally included as a proxy for skin cancer, but later excluded as sources of melanoma and non-melanoma skin cancer were identified) |
Search 3: health-related quality-of-life studies
MEDLINE
Full database title: Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE.
Search range: 1946 to present.
Date of search: 10 October 2014.
| # | Terms | Number of hits |
|---|---|---|
| 1 | ((skin* or melano* or cutaneous* or sarcoma* or “non melanoma”) adj3 (secondar* or neoplasm* or cancer* or carcinoma* or adenocarcinom* or tumo?r* or malignan* or metastas* or lesion*)).mp. | 175,702 |
| 2 | ((superficial* adj2 melanoma*) or SSM or nodular* melanoma* or lentigo* maligna* or lentiginous* melanoma* or (Hutchinson* adj2 freckle*) or melanoma* in situ or acral* lentiginous* melanoma* or amelanotic* melanoma*).mp. | 4237 |
| 3 | exp skin neoplasms/ | 99,426 |
| 4 | exp melanoma/ | 76,777 |
| 5 | (non melanoma* or BCC or gorlin* syndrome* or rodent ulcer* or basalioma* or NMSC*).mp. | 7537 |
| 6 | ((basal or basocellular* or basosquamous*) adj2 (carcinoma* or cancer* or neoplasm* or tumo?r* or epithelioma* or malignan*)).mp. | 19,570 |
| 7 | ((squamous adj2 (carcinoma* or tumo?r* or cancer* or neoplasm* or epithelioma* or malignan*)) or Bowen* disease* or squamous* cell* carcinoma* in situ or SCC).mp. | 133,902 |
| 8 | exp carcinoma, basal cell/ | 14,912 |
| 9 | exp carcinoma, squamous cell/ | 107,853 |
| 10 | exp Neoplasms, Basal Cell/ | 16,136 |
| 11 | exp Basal Cell Nevus Syndrome/ | 1080 |
| 12 | exp eyelid neoplasms/ | 3914 |
| 13 | Kaposi* sarcoma*.mp. | 11,933 |
| 14 | Merkel* cell* carcinoma*.mp. | 1976 |
| 15 | (T*cell lymphoma* or cutaneous* T*cell lymphoma* or CTCL or primary* cutaneous* lymphoma*).mp. | 1643 |
| 16 | or/ 1-15 | 343,965 |
| 17 | ((quality adj3 life) or life quality or QOL).ti,ab. | 165,058 |
| 18 | (HRQL or HRQOL or HRQol).ti,ab. | 10,741 |
| 19 | (value adj2 life).ti,ab. or exp “Value of Life”/ | 6494 |
| 20 | (quality-adjusted life year$1 or QALY or QALYs or quality adjusted life year$1).ti,ab. or exp Quality-Adjusted Life Years/ | 11,098 |
| 21 | (disabilit$3 adj2 life).ti,ab. | 2298 |
| 22 | (sf36 or sf-36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. | 17,444 |
| 23 | (sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. | 1413 |
| 24 | (sf6d or sf 6d or sf-6d or short form 6d or shortform 6d or sf six dimension$1 or short form six dimension$1).ti,ab. | 488 |
| 25 | (sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve).ti,ab. | 3126 |
| 26 | (sf16 or sf 16 or sf-16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab. | 24 |
| 27 | (sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty).ti,ab. | 349 |
| 28 | (euroqol or euro qol or eq5d or eq 5d or eq-5d).tw. | 4590 |
| 29 | (hye or hyes or health$ year$ equivalent$).ti,ab. | 65 |
| 30 | (willing$ adj2 (pay or accept)).tw. | 4195 |
| 31 | standard gamble$.tw. | 708 |
| 32 | (health adj3 (utilit$3 or value$2 or preference$2)).tw. | 7607 |
| 33 | (visual analog$3 scale or VAS).tw. | 41,882 |
| 34 | (person$ trade-off or person$ trade off or PTO).ti,ab. | 626 |
| 35 | (contingent value or contingent valuation).ti,ab. | 440 |
| 36 | discrete choice.ti,ab. | 736 |
| 37 | ((quality adj3 wellbeing index) or QWB).ti,ab. | 181 |
| 38 | (time trade off or time tradeoff or TTO or time trade-off).ti,ab. | 1263 |
| 39 | (utility or utilities).ti,ab. | 129,244 |
| 40 | disutil$.ti,ab. | 255 |
| 41 | ((quality of adj (wellbeing or well-being or well being)) or qwb).ti,ab. | 191 |
| 42 | (health utilities index or HUI or hui$1).ti,ab. | 1388 |
| 43 | or/17-42 | 353,292 |
| 44 | 16 and 43 | 5517 |
| 45 | (letter or editorial or comment or case report or review).pt. | 3,331,203 |
| 46 | (animals not humans).sh. | 3,981,381 |
| 47 | 44 not (45 or 46) | 4394 |
| 48 | limit 47 to yr=“1997 -Current” | 3812 |
EMBASE
Search date: 1974 to 16 October 2014.
Date of search: 17 October 2014.
| # | Terms | Number of hits |
|---|---|---|
| 1 | ((skin* or melano* or cutaneous* or sarcoma* or “non melanoma”) adj3 (secondar* or neoplasm* or cancer* or carcinoma* or adenocarcinom* or tumo?r* or malignan* or metastas* or lesion*)).mp. | 285,106 |
| 2 | ((superficial* adj2 melanoma*) or SSM or nodular* melanoma* or lentigo* maligna* or lentiginous* melanoma* or (Hutchinson* adj2 freckle*) or melanoma* in situ or acral* lentiginous* melanoma* or amelanotic* melanoma*).mp. | 5815 |
| 3 | skin tumor/ or exp skin cancer/ | 214,761 |
| 4 | exp melanoma/ or exp non melanoma skin cancer/ or exp melanoma skin cancer/ or exp amelanotic melanoma/ or exp cutaneous melanoma/ | 268,303 |
| 5 | (non melanoma* or BCC or gorlin* syndrome* or rodent ulcer* or basalioma* or NMSC*).mp. | 10,210 |
| 6 | ((basal or basocellular* or basosquamous*) adj2 (carcinoma* or cancer* or neoplasm* or tumo?r* or epithelioma* or malignan*)).mp. | 25,248 |
| 7 | ((squamous adj2 (carcinoma* or tumo?r* or cancer* or neoplasm* or epithelioma* or malignan*)) or Bowen* disease* or squamous* cell* carcinoma* in situ or SCC).mp. | 143,535 |
| 8 | exp basal cell carcinoma/ | 21,004 |
| 9 | exp squamous cell carcinoma/ | 101,134 |
| 10 | exp basal cell nevus syndrome/ | 1954 |
| 11 | exp eyelid tumor/ | 3551 |
| 12 | Kaposi* sarcoma*.mp. | 18,518 |
| 13 | Merkel* cell* carcinoma*.mp. | 2588 |
| 14 | (T*cell lymphoma* or cutaneous* T*cell lymphoma* or CTCL or primary* cutaneous* lymphoma*).mp. | 18,116 |
| 15 | or/ 1-14 | 485,109 |
| 16 | ((quality adj3 life) or life quality or QOL).ti,ab. | 234,577 |
| 17 | (HRQL or HRQOL or HRQol).ti,ab. | 14,935 |
| 18 | (value adj2 life).ti,ab. | 698 |
| 19 | (quality-adjusted life year$1 or QALY or QALYs or quality adjusted life year$1).ti,ab. or exp quality adjusted life year/ | 16,541 |
| 20 | (disabilit$3 adj2 life).ti,ab. | 2667 |
| 21 | (sf36 or sf-36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. | 24,039 |
| 22 | (sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. | 1537 |
| 23 | (sf6d or sf 6d or sf-6d or short form 6d or shortform 6d or sf six dimension$1 or short form six dimension$1).ti,ab. | 743 |
| 24 | (sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve).ti,ab. | 4495 |
| 25 | (sf16 or sf 16 or sf-16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab. | 35 |
| 26 | (sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty).ti,ab. | 334 |
| 27 | (euroqol or euro qol or eq5d or eq 5d or eq-5d).tw. | 7421 |
| 28 | (hye or hyes or health$ year$ equivalent$).tw. | 110 |
| 29 | (willing$ adj2 (pay or accept)).tw. | 5611 |
| 30 | standard gamble$.tw. | 800 |
| 31 | (health adj3 (utilit$3 or value$2 or preference$2)).tw. | 9274 |
| 32 | (visual analog$3 scale or VAS).tw. | 59,137 |
| 33 | (person$ trade-off or person$ trade off or PTO).ti,ab. | 641 |
| 34 | (contingent value or contingent valuation).ti,ab. | 565 |
| 35 | discrete choice.ti,ab. | 935 |
| 36 | (time trade off or time tradeoff or TTO or time trade-off).ti,ab. | 1598 |
| 37 | (utility or utilities).ti,ab. | 158,757 |
| 38 | disutil$.ti,ab. | 390 |
| 39 | ((quality of adj (wellbeing or well-being or well being)) or qwb).ti,ab. | 207 |
| 40 | (health utilities index or HUI or hui$1).ti,ab. | 1858 |
| 41 | or/16-40 | 464,862 |
| 42 | 15 and 41 | 10,334 |
| 43 | (letter or editorial or comment or case report or review).pt. | 3,302,060 |
| 44 | (animal$ not human$).sh,hw. | 3,786,854 |
| 45 | 42 not (43 or 44) | 8001 |
| 46 | limit 45 to yr=“1997 -Current” | 7400 |
Health Technology Assessment database
Search range: inception to 14 October 2014.
Date of search: 14 October 2014.
| Search terms (and fields searched) | #1 (skin or melano* or cutaneous or sarcoma or non next melanoma) near/3 (secondar* or neoplasm or cancer or carcinoma or adenocarcinom* or tumor or tumour or malignan* or metastas or lesion) #2 superficial near/2 melanoma or SSM or nodular next melanoma or lentigo next maligna or lentiginous next melanoma or Hutchinson* near/2 freckle or “melanoma in situ” or “acral lentiginous melanoma” or “amelanotic melanoma” #3 MeSH descriptor: [Skin Neoplasms] explode all trees #4 MeSH descriptor: [Melanoma] explode all trees #5 non next melanoma or BCC or gorlin* next syndrome or rodent next ulcer or basalioma or NMSC #6 (basal or basocellular or basosquamous) near/2 (carcinoma or cancer or neoplasm or tumor or tumour or epithelioma or malignan) #7 (squamous near/2 (carcinoma or tumor or tumour or cancer or neoplasm or epithelioma or malignan*)) or “Bowen’s disease” or “squamous cell carcinoma in situ” or SCC #8 MeSH descriptor: [Carcinoma, Basal Cell] explode all trees #9 MeSH descriptor: [Carcinoma, Squamous Cell] explode all trees #10 MeSH descriptor: [Neoplasms, Basal Cell] explode all trees #11 MeSH descriptor: [Basal Cell Nevus Syndrome] explode all trees #12 MeSH descriptor: [Eyelid Neoplasms] explode all trees #13 “Kaposi’s sarcoma” #14 “Merkel cell carcinoma” #15 “T-cell lymphoma” or “cutaneous T-cell lymphoma” or CTCL or “primary cutaneous lymphoma” #16 {or #1-#15} |
| Number of hits | 151 |
NHS Economic Evaluation Database
Search range: inception to 14 October 2014.
Date of search: 14 October 2014.
| Search terms (and fields searched) | #1 (skin or melano* or cutaneous or sarcoma or non next melanoma) near/3 (secondar* or neoplasm or cancer or carcinoma or adenocarcinom* or tumor or tumour or malignan* or metastas or lesion) #2 superficial near/2 melanoma or SSM or nodular next melanoma or lentigo next maligna or lentiginous next melanoma or Hutchinson* near/2 freckle or “melanoma in situ” or “acral lentiginous melanoma” or “amelanotic melanoma” #3 MeSH descriptor: [Skin Neoplasms] explode all trees #4 MeSH descriptor: [Melanoma] explode all trees #5 non next melanoma or BCC or gorlin* next syndrome or rodent next ulcer or basalioma or NMSC #6 (basal or basocellular or basosquamous) near/2 (carcinoma or cancer or neoplasm or tumor or tumour or epithelioma or malignan) #7 (squamous near/2 (carcinoma or tumor or tumour or cancer or neoplasm or epithelioma or malignan*)) or “Bowen’s disease” or “squamous cell carcinoma in situ” or SCC #8 MeSH descriptor: [Carcinoma, Basal Cell] explode all trees #9 MeSH descriptor: [Carcinoma, Squamous Cell] explode all trees #10 MeSH descriptor: [Neoplasms, Basal Cell] explode all trees #11 MeSH descriptor: [Basal Cell Nevus Syndrome] explode all trees #12 MeSH descriptor: [Eyelid Neoplasms] explode all trees #13 “Kaposi’s sarcoma” #14 “Merkel cell carcinoma” #15 “T-cell lymphoma” or “cutaneous T-cell lymphoma” or CTCL or “primary cutaneous lymphoma” #16 {or #1-#15} |
| Number of hits | 134 |
First pass
Potential studies reporting utility data reviewed at second pass (n = 41)
| # | Studya |
|---|---|
| Published studies | |
| 1 | Askew et al.53 |
| 2 | Barzey V, Atkins MB, Garrison LP, Asukai Y, Kotapati S, Penrod JR. Ipilimumab in 2nd line treatment of patients with advanced melanoma: a cost-effectiveness analysis. J Med Econ 2013;16:202–12 |
| 3 | Beusterien KM, et al.54 |
| 4 | Brown B, Diamantopoulos A, Bernier J, Schöffski P, Hieke K, Mantovani L, et al. An economic evaluation of cetuximab combined with radiotherapy for patients with locally advanced head and neck cancer in Belgium, France, Italy, Switzerland, and the United Kingdom. Value Health 2008;11:791–9 |
| 5 | Chan AL, Leung HW, Huang SF. Cost effectiveness of cetuximab concurrent with radiotherapy for patients with locally advanced head and neck cancer in Taiwan: a decision-tree analysis. Clin Drug Investig 2011;31:717–26 |
| 6 | Chen T, Bertenthal D, Sahay A, Sen S, Chren MM. Predictors of skin-related quality of life after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. Arch Dermatol 2007;143:1386–92. [Erratum published in J Arch Dermatol 2008;144:230] |
| 7 | Cormier JN, Xing Y, Ding M, Cantor SB, Salter KJ, Lee JE, et al. Cost effectiveness of adjuvant interferon in node-positive melanoma. J Clin Oncol 2007;25:2442–8 |
| 8 | Crott R, Ali F, Burdette-Radoux S. Cost-utility of adjuvant high-dose interferon alpha therapy in stage III cutaneous melanoma in Quebec. Value Health 2004;7:423–32 |
| 9 | Dixon S, Walters SJ, Turner L, Hancock BW. Quality of life and cost-effectiveness of interferon-alpha in malignant melanoma: results from randomised trial. Br J Cancer 2006;94:492–8 |
| 10 | Essers BA, Dirksen CD, Nieman FH, Smeets NW, Krekels GA, Prins MH. Cost-effectiveness of Mohs micrographic surgery vs surgical excision for basal cell carcinoma of the face. Arch Dermatol 2006;142:187–94 |
| 11 | Freedberg KA, Geller AC, Miller DR, Lew RA, Koh HK. Screening for malignant melanoma: a cost-effectiveness analysis. J Am Acad Dermatol 1999;414:738–45 |
| 12 | Hannouf MB, Sehgal C, Cao JQ, Mocanu JD, Winquist E, Zaric GS. Cost-effectiveness of adding cetuximab to platinum-based chemotherapy for first-line treatment of recurrent or metastatic head and neck cancer. PLOS ONE 2012;7:e38557 |
| 13 | Hengge UR, Wallerand A, Stutzki A, Kockel N. Cost-effectiveness of reduced follow-up in malignant melanoma. J Dtsch Dermatol Ges 2007;5:898–907 |
| 14 | Hillner BE. Cost-effectiveness assessment of interferon alfa-2b as adjuvant therapy of high-risk resected cutaneous melanoma. Eur J Cancer 1998;34(Suppl. 3):18–21 |
| 15 | Hillner BE, Kirkwood JM, Atkins MB, Johnson ER, Smith TJ. Economic analysis of adjuvant interferon alfa-2b in high-risk melanoma based on projections from Eastern Cooperative Oncology Group 1684. J Clin Oncol 1997;15:2351–8 |
| 16 | Hirst NG, Gordon LG, Scuffham PA, Green AC. Lifetime cost-effectiveness of skin cancer prevention through promotion of daily sunscreen use. Value Health 2012;15:261–8 |
| 17 | Hollenbeak CS, Lowe VJ, Stack BC. The cost-effectiveness of fluorodeoxyglucose 18-F positron emission tomography in the N0 neck. Cancer 2001;92:2341–8 |
| 18 | Kansal AR, Shaul AJ, Stern S, Busam K, Doucet CA, Chalfin DB. Cost-effectiveness of a FISH assay for the diagnosis of melanoma in the USA. Expert Rev Pharmacoecon Outcomes Res 2013;13:371–80 |
| 19 | King SM et al.55 |
| 20 | Ko CY, Maggard M, Livingston EH. Evaluating health utility in patients with melanoma, breast cancer, colon cancer, and lung cancer: a nationwide, population-based assessment. J Surg Res 2003;114:1–5 |
| 21 | Lear W, Akeroyd JE, Mittmann N, Murray C. Measurement of utility in nonmelanoma skin cancer. J Cutan Med Surg 2008;12:102–6 |
| 22 | Losina E, Walensky RP, Geller A, Beddingfield FC, Wolf LL, Gilchrest BA, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol 2007;143:21–8 |
| 23 | Morton RL, Howard K, Thompson JF. The cost-effectiveness of sentinel node biopsy in patients with intermediate thickness primary cutaneous melanoma. Ann Surg Oncol 2009;16:929–40 |
| 24 | Parthan A, Posner MR, Brammer C, Beltran P, Jansen JP. Cost utility of docetaxel as induction chemotherapy followed by chemoradiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck 2009;31:1255–62 |
| 25 | Seidler et al.57 |
| 26 | Shingler SL et al.56 |
| 27 | Wilson EC et al.48 |
| 28 | Wilson LS, Reyes CM, Lu C, Lu M, Yen C. Modelling the cost-effectiveness of sentinel lymph node mapping and adjuvant interferon treatment for stage II melanoma. Melanoma Res 2002;12:607–17 |
| 29 | National Institute for Health and Care Excellence. Skin Cancer Prevention: Information, Resources and Environmental Changes (PH32). London; NICE; 2011 |
| Conference papers | |
| 30 | Amdahl J, Wang A, Thabane M, Amonkar M, Delea TE. Cost Effectiveness of Trametinib as First-line (1l) Treatment for BRAF v600 Positive Advanced or Metastatic Melanoma – A Canadian Societal Perspective. Value in Health Conference: ISPOR 19th Annual International Meeting, Montreal, QC, Canada, 31 May 2014–4 July 2014. pp. 20140517, 20140533, A20140583 |
| 31 | Dalgard F, Kupfer J, Gieler U. The Psychological Burden of Common Skin Diseases in 13 European Countries. British Journal of Dermatology Conference: 94th Annual Meeting of the British Association of Dermatologists, Glasgow, UK, 1–3 July 2014. pp. 20140171, 20140703 |
| 32 | Delea TE, Amdahl J, Wang A, Amonkar M, Smith HW, Balaratnam S, et al. Cost-Utility Analysis of Dabrafenib/Trametinib Combination (D+T) for BRAFV600 Mutation-Positive Metastatic Melanoma (MM) from the United Kingdom (UK) National Health Service (NHS) Perspective. Value in Health Conference: ISPOR 19th Annual International Meeting, Montreal, QC, Canada, 31 May 2014–4 June 2014. pp. 20140517, 20140533, A20140588 |
| 33 | Klein J, Livergant J, Ringash J. Health-Related Quality of Life in Head-And-Neck Cancer Treated with Radiation Therapy with or Without Chemotherapy: A Systematic Review. International Journal of Radiation Oncology Biology Physics Conference: 55th Annual Meeting of the American Society for Radiation Oncology, ASTRO 2013, Atlanta, GA, USA, 22–25 September 2013. pp. 20130987, 20130922, Suppl. 20130921, S20130605–6 |
| 34 | Radford M, Cortes P, Carrasco J, Gueron B, Gonçalves F. Cost-Effectiveness of Ipilimumab in Previously Treated Patients for Advanced Melanoma in Portugal. Value in Health Conference: ISPOR 18th Annual International Meeting, New Orleans, LA, USA, 18–22 May 2013. pp. 20130516, 20130513, A20130139 |
| 35 | Sebaratnam D, Fernández Peñas P, Morton R, Paver R. Cost Effectiveness Analysis of Mohs Micrographic Surgery Versus Traditional Surgical Excision for Head and Neck Basal Cell Carcinoma. Journal of the American Academy of Dermatology Conference: 71st Annual Meeting of the American Academy of Dermatology, Miami Beach, FL, USA, 1–5 March 2013 pp. 20130368, 20130304, Suppl. 20130301, AB20130159 |
| 36 | Seubring I, van Rijsingen MCJ, Maessen-Visch MB, Alkemade JAC, van Doom R, van de Kerkhof PCM, et al. Cost-effectiveness and Quality of Life on MAL-PDT versus Imiquimod and Simple Surgical Excision in Basal Cell Carcinoma; A decision tree model. Nederlands Tijdschrift voor Dermatologie en Venereologie Conference: 14th Annual Scientific Meeting of the Nederlandse Vereniging voor Experimentele Dermatologie, NVED 2013, Lunteren, the Netherlands, 31 January 2013–1 February 2013. pp. 20130123, 20130131, 20130150-1 |
| 37 | Shih V, ten Ham RMT, Bui CT, Tran DN, Wilson LS. BRAF Targeted Therapies for the Treatment of Metastatic Melanoma: A Cost-Effectiveness Analysis. Value in Health Conference: ISPOR 19th Annual International Meeting Montreal, QC, Canada, 31 May 2014–4 June 2014. pp. 20140517, 20140533, pp. A20140584 |
| Technology appraisals | |
| 38 | National Institute for Health and Care Excellence. Dabrafenib for Treating Unresectable or Metastatic BRAF V600 Mutation-Positive Melanoma (TA321). London; NICE; 2014 |
| 39 | National Institute for Health and Care Excellence. Ipilimumab for Previously Untreated Advanced (Unresectable or Metastatic) Melanoma (TA319). London; NICE; 2014 |
| 40 | National Institute for Health and Care Excellence. Ipilimumab for Previously Treated Advanced (Unresectable or Metastatic) Melanoma (TA268). London; NICE; 2012 |
| 41 | National Institute for Health and Care Excellence. Vemurafenib for Treating Locally Advanced or Metastatic BRAF V600 Mutation-Positive Malignant Melanoma (TA269). London; NICE; 2012 |
Second pass
From the references lists of those 17 cost-effectiveness studies identified from the database search, 17 sources of utility values were identified. Of those, three studies were previously identified from the database search and met the criteria for full-text review at second pass, 12 studies were not considered to meet the inclusion criteria based on a review of the title and abstract, or publication date, and the remaining two studies were ordered for a full-text review.
Source of utility values applied in cost-effectiveness studies identified from the health-related quality of life search, October 2014
| Reference identified from the search | Source of utility values |
|---|---|
| Cormier JN, Xing Y, Ding M, Cantor SB, Salter KJ, Lee JE, et al. Cost effectiveness of adjuvant interferon in node-positive melanoma. J Clin Oncol 2007;25:2442–8 | Kilbridge KL, Weeks JC, Sober AJ, Haluska FG, Slingluff CL, Atkins MB, et al. Patient preferences for adjuvant interferon alfa-2b treatment. J Clin Oncol 2001;19:812–23a Mooney MM, Mettlin C, Michalek AM, Petrelli NJ, Kraybill WG, Mooney MM, et al. Life-long screening of patients with intermediate-thickness cutaneous melanoma for asymptomatic pulmonary recurrences: a cost-effectiveness analysis. Cancer 1997;80:1052–64b Hillner BE, Kirkwood JM, Atkins MB, Johnson ER, Smith TJ. Economic analysis of adjuvant interferon alfa-2b in high-risk melanoma based on projections from Eastern Cooperative Oncology Group 1684. J Clin Oncol 1997;15:2351–8 |
| Crott R, Ali F, Burdette-Radoux S. Cost-utility of adjuvant high-dose interferon alpha therapy in stage III cutaneous melanoma in Quebec. Value Health 2004;7:423–32 | Kilbridge KL, Cole BF, Kirkwood JM, Haluska FG, Atkins MA, Ruckdeschel JC, et al. Quality-of-life-adjusted survival analysis of high-dose adjuvant interferon alpha-2b for high-risk melanoma patients using intergroup clinical trial data. J Clin Oncol 2002;20:1311–18a |
| Barzey V, Atkins MB, Garrison LP, Asukai Y, Kotapati S, Penrod JR, et al. Ipilimumab in 2nd line treatment of patients with advanced melanoma: a cost-effectiveness analysis. J Med Econ 2013;16:202–12. [Erratum published in J Med Econ 2013;16:212] | Beusterien KM, Szabo SM, Kotapati S, Mukherjee J, Hoos A, Hersey P, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer 2009;101:387–9 |
| Hannouf MB, Sehgal C, Cao JQ, Mocanu JD, Winquist E, Zaric GS. Cost-effectiveness of adding cetuximab to platinum-based chemotherapy for first-line treatment of recurrent or metastatic head and neck cancer. PLOS ONE 2012;7:e38557 | NICE. Cetuximab for the Treatment of Recurrent or Metastatic Head and Neck Cancer. London: NICE; 2009c |
| Hillner BE. Cost-effectiveness assessment of interferon alfa-2b as adjuvant therapy of high-risk resected cutaneous melanoma. Eur J Cancer 1998;34(Suppl. 3):18–21 | Goodwin PJ, Feld R, Evans WK, Pater J. Cost-effectiveness of cancer chemotherapy. An economic evaluation of a randomized trial in small-cell lung cancer. J Clin Oncol 1988;6:1537–47d Weeks J, O’Leary J, Fairclough D, Paltiel D, Weinstein M. The ‘Q-tility index’: a new tool for assessing health related quality of life and utilities in clinical trials and clinical practice. Proc Am Soc Clin Oncol 1994;13:1498d |
| Hirst NG, Gordon LG, Scuffham PA, Green AC. Lifetime cost-effectiveness of skin cancer prevention through promotion of daily sunscreen use. Value Health 2012;15:261–8 | Bendeck S, Hadley J, Bonaccorsi P, Brown KM, Lawson DH, Murray DR. Quality of Life Impact by Melanoma as Measured by Utilities. 26th Annual Meeting of the Society for Medical Decision Making, Atlanta, GA, 17–20 October 2004 Beusterien KM, Ackerman SJ, Plante K, Glaspy J, Naredi P, Wood D, et al. The health-related quality-of-life impact of histamine dihydrochloride plus interleukin-2 compared with interleukin-2 alone in patients with metastatic melanoma. Support Care Cancer 2003;11:304–12e Killbridge et al. 2001a Hillner et al. 1997 |
| Kansal AR, Shaul AJ, Stern S, Busam K, Doucet CA, Chalfin DB, et al. Cost-effectiveness of a FISH assay for the diagnosis of melanoma in the USA. Expert Rev Pharmacoecon Outcomes Res 2013;13:371–80 | Beusterien et al. 2009 |
| Losina E, Walensky RP, Geller A, Beddingfield FC, III, Wolf LL, Gilchrest BA, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol 2007;143:21–8 | Bendeck et al. 2004 |
| Morton RL, Howard K, Thompson JF. The cost-effectiveness of sentinel node biopsy in patients with intermediate thickness primary cutaneous melanoma. Ann Surg Oncol 2009;16:929–40 | Killbridge et al. 2001a Bendeck et al. 2004 Torrance GW, Feeny D. Utilities and QALYs. Int J Technol Assess Health Care 1989;5:559–65d Jani AB, Basu A, Heimann R, Hellman S. Sentinel lymph node versus axillary lymph node dissection for early-stage breast carcinoma a comparison using a utility-adjusted number needed to treat analysis. Cancer 2003;97:359–66b Lafuma A, Dreno B, Delaunay M, Emery C, Fagnani F, Hieke K, et al. Economic analysis of adjuvant therapy with interferon alpha-2a in stage II malignant melanoma. Eur J Cancer 2001;37:369–75 Hutton J, Brown R, Borowitz M. A new decision model for cost-utility comparisons of chemotherapy in recurrent metastatic breast cancer. PharmacoEconomics 1996;9:8–22d Van den Hout WB, Van der Linden YM, Steenland E, Wiggenraad RG, Kievit J, De Haes H, Leer JW. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost–utility analysis based on a randomized trial. JNCI 2003;95:222–9b Hillner et al. 1997 Mooney et al. 1997b |
| Wilson EC, Emery JD, Kinmonth AL, Prevost AT, Morris HC, Humphrys E, et al. The cost-effectiveness of a novel SIAscopic diagnostic aid for the management of pigmented skin lesions in primary care: a decision-analytic model. Value Health 2013;16:356–66 | Bendeck S, Hadley JC, Bonaccorsi P, Brown KM, Lawson DH, Murray DR. Can Melanoma Patients Predict the Quality of Life Impact of an Alternate Melanoma Stage? Methods and Applications: Health Services Research Society for Medical Decision Making, Atlanta, GA, 17–20 October 2004 |
| Wilson LS, Reyes CM, Lu C, Lu M, Yen C. Modelling the cost-effectiveness of sentinel lymph node mapping and adjuvant interferon treatment for stage II melanoma. Melanoma Res 2002;12:607–17 | Killbridge et al. 2001a |
| Sebaratnam D, Penas PF, Morton R, Paver R. Cost effectiveness analysis of Mohs micrographic surgery versus traditional surgical excision for head and neck basal cell carcinoma. J Am Acad Dermatol 2013;68(Suppl. 1):AB159 | Not reported |
| NICE. Skin Cancer Prevention: Information, Resources and Environmental Changes. Health Technology Assessment Database. 2011. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32011000297/frame.html (accessed 6 October 2014) | Freedberg KA, Geller AC, Miller DR, Lew RA, Koh HK. Screening for malignant melanoma: a cost-effectiveness analysis. J Am Acad Dermatol 1999;41:738–45 |
| NICE. Dabrafenib for Treating Unresectable or Metastatic BRAF V600 Mutation-Positive Melanoma (TA321). London; NICE; 2014 | Beusterien et al. 2009 |
| NICE. Ipilimumab for Previously Untreated Advanced (Unresectable or Metastatic) Melanoma (TA319). London; NICE; 2014 | Beusterien et al. 2009 |
| NICE. Ipilimumab for Previously Treated Advanced (Unresectable or Metastatic) Melanoma (TA268). London; NICE; 2012 | Beusterien et al. 2009 |
| NICE. Vemurafenib for Treating Locally Advanced or Metastatic BRAF V600 Mutation-Positive Malignant Melanoma (TA269). London; NICE; 2012 | Beusterien et al. 2009 |
Summary of reasons for exclusion: health-related quality of life
| Study | Reasons for exclusion |
|---|---|
| Identified through database search | |
| Dixon S, Walters SJ, Turner L, Hancock BW. Quality of life and cost-effectiveness of interferon-alpha in malignant melanoma: results from randomised trial. Br J Cancer 2006;94:492–8 | Utilities are reported separately for the placebo arm (which are not influenced by patients receiving interferon therapy), but these change over time and cannot be connected to stages in the model. In addition malignant melanoma is not defined |
| Ko CY, Maggard M, Livingston EH. Evaluating health utility in patients with melanoma, breast cancer, colon cancer, and lung cancer: a nationwide, population-based assessment. J Surg Res 2003;114:1–5 | Utilities reported for melanoma, but melanoma is not defined |
| Chan AL, Leung HW, Huang SF, Chan ALF, Leung HWC, Huang SF. Cost effectiveness of cetuximab concurrent with radiotherapy for patients with locally advanced head and neck cancer in Taiwan: a decision-tree analysis. Clin Drug Invest 2011;31:717–26 | Modified utility values from Brown et al. (2008) (see below for full reference) with the incidence rate observed in a recent Chinese clinical trial |
| Chen T, Bertenthal D, Sahay A, Sen S, Chren MM. Predictors of skin-related quality of life after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. Arch Dermatol 2007;143:1386–92. [Erratum published in Arch Dermatol 2008;144:230] | Utility values not reported |
| Essers BA, Dirksen CD, Nieman FH, Smeets NW, Krekels GA, Prins MH, et al. Cost-effectiveness of Mohs micrographic surgery vs surgical excision for basal cell carcinoma of the face. Arch Dermatol 2006;142:187–94 | Utility values not reported |
| Hengge UR, Wallerand A, Stutzki A, Kockel N, Hengge UR, Wallerand A, et al. Cost-effectiveness of reduced follow-up in malignant melanoma. J Deutschen Dermatologischen Gesellschaft 2007;5:898–907 | Utility values not reported |
| Hollenbeak CS, Lowe VJ, Stack BC Jr, Hollenbeak CS, Lowe VJ, Stack BCJ. The cost-effectiveness of fluorodeoxyglucose 18-F positron emission tomography in the N0 neck. Cancer 2001;92:2341–8 | Health states not applicable to the model (report values for modified neck dissection and/or radiation) |
| Hillner BE, Kirkwood JM, Atkins MB, Johnson ER, Smith TJ. Economic analysis of adjuvant interferon alfa-2b in high-risk melanoma based on projections from Eastern Cooperative Oncology Group 1684. J Clin Oncol 1997;15:2351–8 | Method used to estimate utility values not reported, appear to be subjective estimates |
| Klein J, Livergant J, Ringash J. Health-Related Quality of Life in Head-and-Neck Cancer Treated with Radiation Therapy with or without Chemotherapy: A Systematic Review. 55th Annual Meeting of the American Society for Radiation Oncology, Atlanta, GA, 22 September 2013 | Irrelevant population;a utility values not reported; patients within the studies are treated with radiation therapy |
| Parthan A, Posner MR, Brammer C, Beltran P, Jansen JP, Parthan A, et al. Cost utility of docetaxel as induction chemotherapy followed by chemoradiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck 2009;31:1255–62 | Irrelevant population;a completed the QLQ-C30 questionnaire at different time points (crossing walking algorithm to EQ-5D utility scores) |
| Brown B, Diamantopoulos A, Bernier J, Schoffski P, Hieke K, Mantovani L, et al. An economic evaluation of cetuximab combined with radiotherapy for patients with locally advanced head and neck cancer in Belgium, France, Italy, Switzerland, and the United Kingdom. Value Health 2008;11:791–9 | Irrelevant population;a health states considered are not applicable to model (UK oncology nurse completed the EQ-5D) |
| Lear W, Akeroyd JE, Mittmann N, Murray C. Measurement of utility in nonmelanoma skin cancer. J Cutaneous Med Surg 2008;12:102–6 | Methods to estimate utility values were not robust which resulted in unrealistic utility values for BCC (i.e. 0.999) |
| Dalgard F, Kupfer J, Gieler U. The Psychological Burden of Common Skin Diseases in 13 European Countries. 94th Annual Meeting of the British Association of Dermatologists, Glasgow, UK, 1 July 2014 | Utility values not reported; insufficient methodological detail |
| Radford M, Cortes P, Carrasco J, Gueron B, Gonçalves F. Cost-effectiveness of ipilimumab in previously treated patients for advanced melanoma in Portugal. Value Health 2013;16:A139 | Utility values not reported; insufficient methodological detail |
| Shih V, Ten Ham RMT, Bui CT, Tran DN, Wilson LS. Braf Targeted Therapies for the Treatment of Metastatic Melanoma: A Cost-Effectiveness Analysis. ISPOR 19th Annual International Meeting, Montreal, QC, 31 May 2014 | Unable to access full text |
| Seubring I, Van Rijsingen MCJ, Maessen-Visch MB, Alkemade JAC, Van Doorn R, Van De Kerkhof PCM, et al. Cost-Effectiveness and Quality of Life on Mal-PDT versus Imiquimod and Simple Surgical Excision in Basal Cell Carcinoma; A Decision Tree Model. 14th Annual Scientific Meeting of the Nederlandse Vereniging voor Experimentele Dermatologie, NVED, Lunteren, the Netherlands, 31 January 2013 | Unable to access full text |
| Amdahl J, Wang A, Thabane M, Amonkar M, Delea TE. Cost Effectiveness of Trametinib as First-Line (1l) Treatment for braf v600 Positive Advanced or Metastatic Melanoma – A Canadian Societal Perspective. ISPOR 19th Annual International Meeting, Montreal, QC, 31 May 2014 | Utility values based on patients receiving trametinib (Mekinist®, GlaxoSmithKline), dacarbazine (DTIC-Dome®, Bayer) or vemurafenib (Zelboraf®, Roche) |
| Delea TE, Amdahl J, Wang A, Amonkar M, Smith HW, Balaratnam S, et al. Cost-Utility Analysis of Dabrafenib/Trametinib Combination (d+t) for BRAFV600 Mutation-Positive Metastatic Melanoma (MM) from the United Kingdom (UK) National Health Service (NHS) Perspective. ISPOR 19th Annual International Meeting, Montreal, QC, 31 May 2014 | Utility values based on patients receiving vemurafenib or dacarbazine |
| Freedberg KA, Geller AC, Miller DR, Lew RA, Koh HK. Screening for malignant melanoma: a cost-effectiveness analysis. J Am Acad Dermatol 1999;41:738–45 | Report quality adjustment values obtained from dermatologists using the VAS technique which is not the preferred method specified in the protocol but was considered following relaxation of inclusion criteria regarding valuation method; however, study reports decrements (in days) from the projected total quality-adjusted life expectancy, which does not allow straightforward estimation of utility values |
| Identified through reference list search | |
| Bendeck S, Hadley J, Bonaccorsi P, Brown KM, Lawson DH, Murray DR. Quality of Life Impact by Melanoma as Measured by Utilities. 26th Annual Meeting of the Society for Medical Decision Making, Atlanta, GA, 17–20 October 2004 | Conference papers published pre January 2014 – the TAG reviewed the full texts of these papers because of the large number of citations from the cost-effectiveness studies |
| Bendeck S, Hadley JC, Bonaccorsi P, Brown KM, Lawson DH, Murray DR. Can Melanoma Patients Predict the Quality of Life Impact of an Alternate Melanoma Stage? 26th Annual Meeting of the Society for Medical Decision Making, Atlanta, GA, 17–20 October 2004 | |
Appendix 6 Detailed results of economic modelling
Cost-effectiveness planes: all probabilistic analyses
FIGURE 17.
Diagnosis of suspected melanomas using the Alarcon et al. 30 diagnostic accuracy data. (a) VivaScope use only for melanoma diagnosis; (b) VivaScope use for the diagnosis of suspected melanomas and BCCs; and (c) VivaScope use for all indications assessed in the economic modelling.


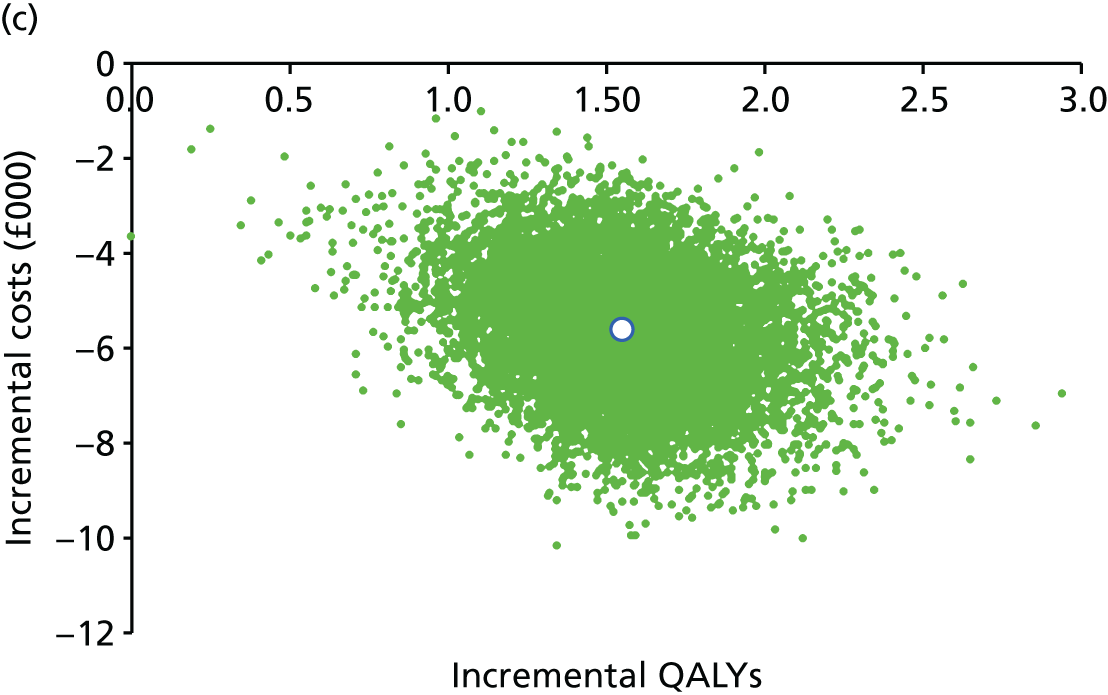
FIGURE 18.
Diagnosis of suspected melanomas using the Pellacani et al. 42 diagnostic accuracy data. (a) VivaScope use only for diagnosis of suspected melanomas; (b) VivaScope use for diagnosis of suspected melanomas and BCCs; and (c) VivaScope use for all indications assessed in the economic modelling.



FIGURE 19.
Diagnosis of suspected BCCs. (a) VivaScope use only for the diagnosis of suspected BCCs; (b) VivaScope use for the diagnosis of suspected melanomas and BCCs; and (c) VivaScope use for all indications assessed in the economic modelling.

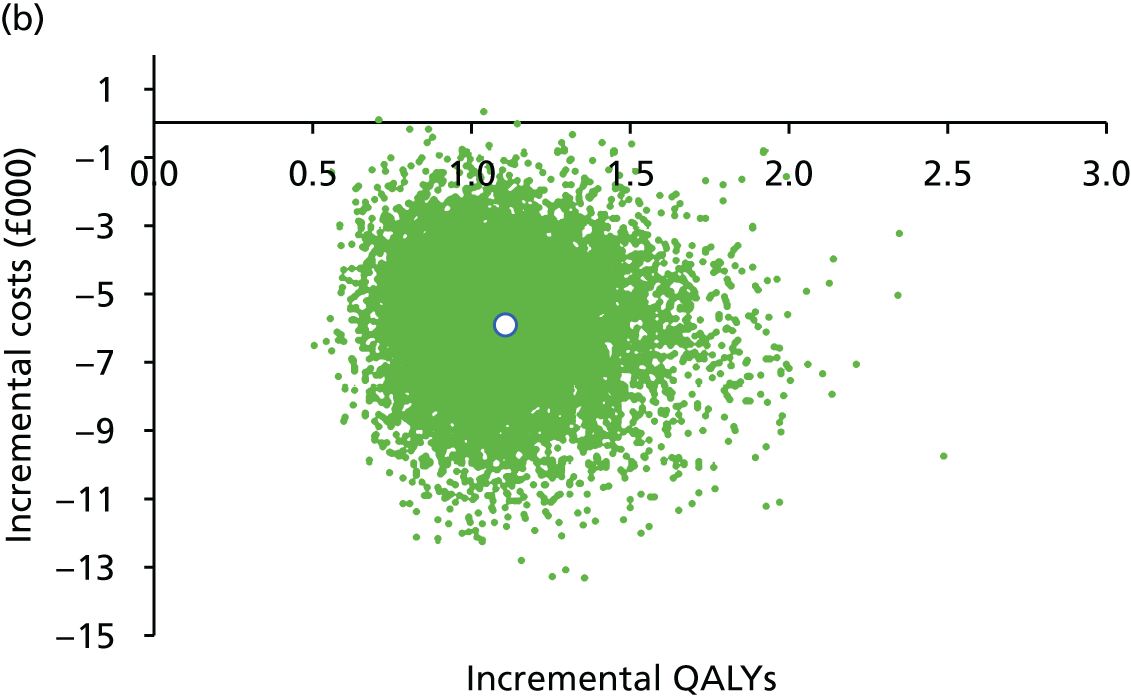
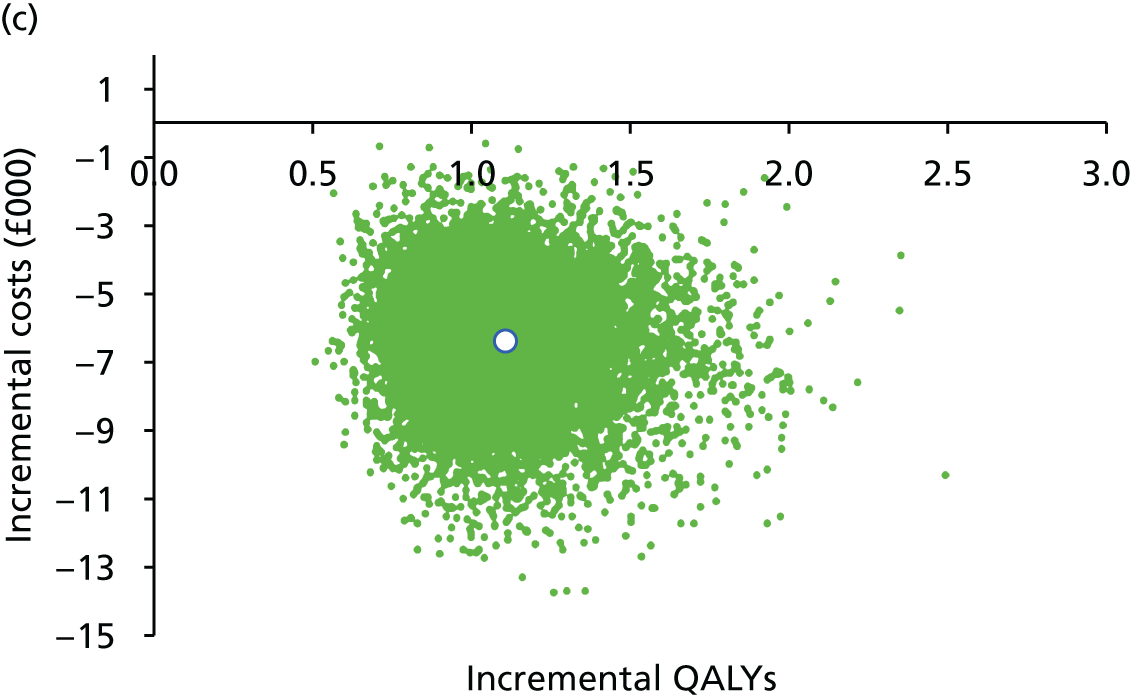
FIGURE 20.
Presurgical margin delineation of LMs. (a) VivaScope use only for the presurgical margin delineation of LMs; and (b) VivaScope use for all indications assessed in the economic modeling.
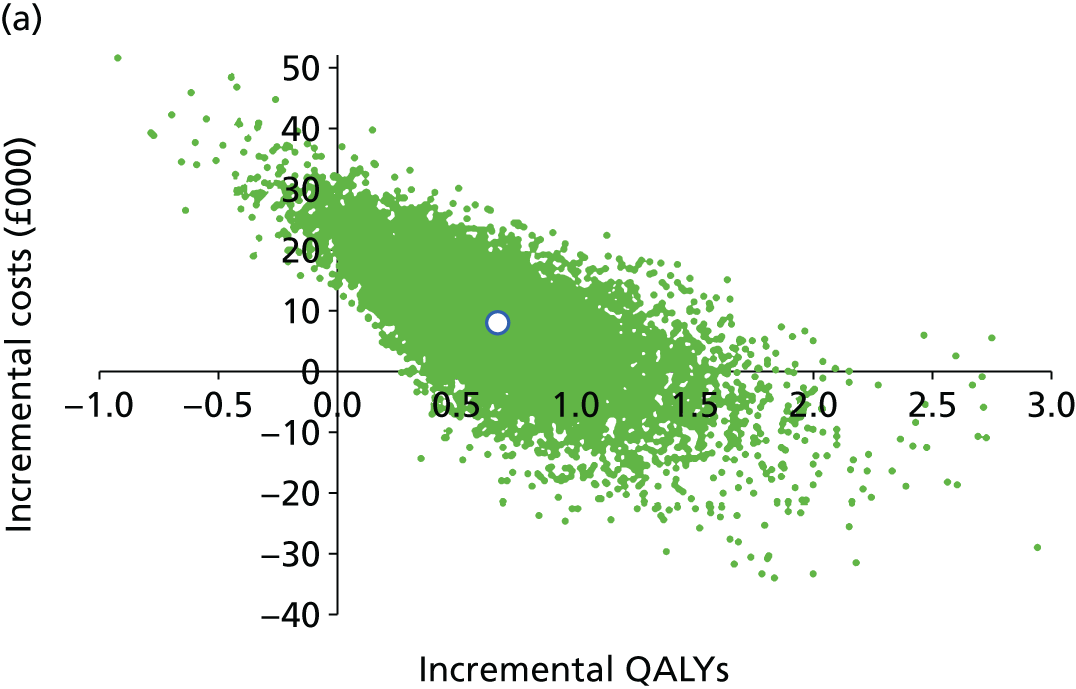

Tornado diagrams: all analyses
FIGURE 21.
Diagnosis of suspected melanomas using the Alarcon et al. 30 diagnostic accuracy data. (a) VivaScope use only for melanoma diagnosis; and (b) VivaScope use for the diagnosis of suspected melanomas and BCCs.

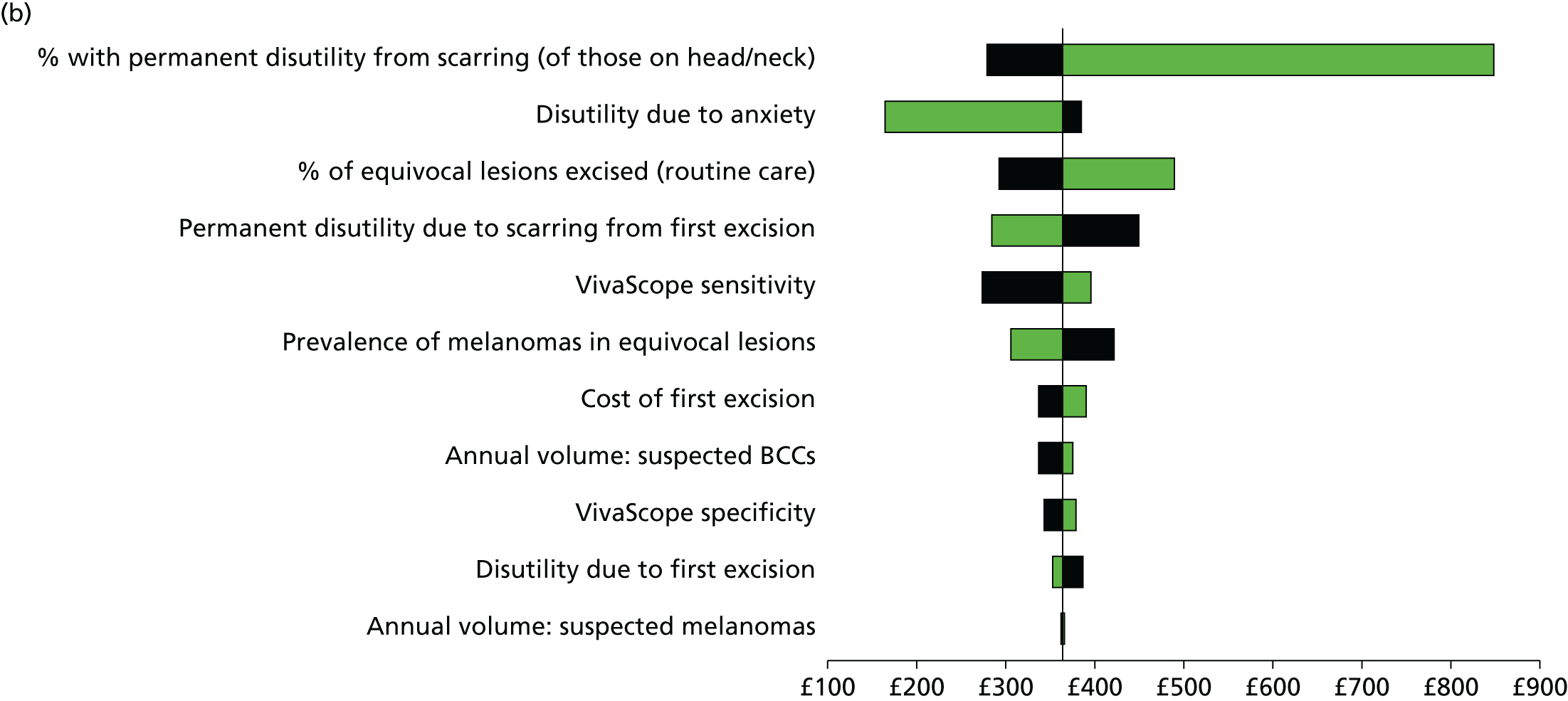
FIGURE 22.
Diagnosis of suspected melanomas using the Pellacani et al. 42 diagnostic accuracy data. (a) VivaScope use only for diagnosis of suspected melanomas; and (b) VivaScope use for the diagnosis of suspected melanomas and BCCs.
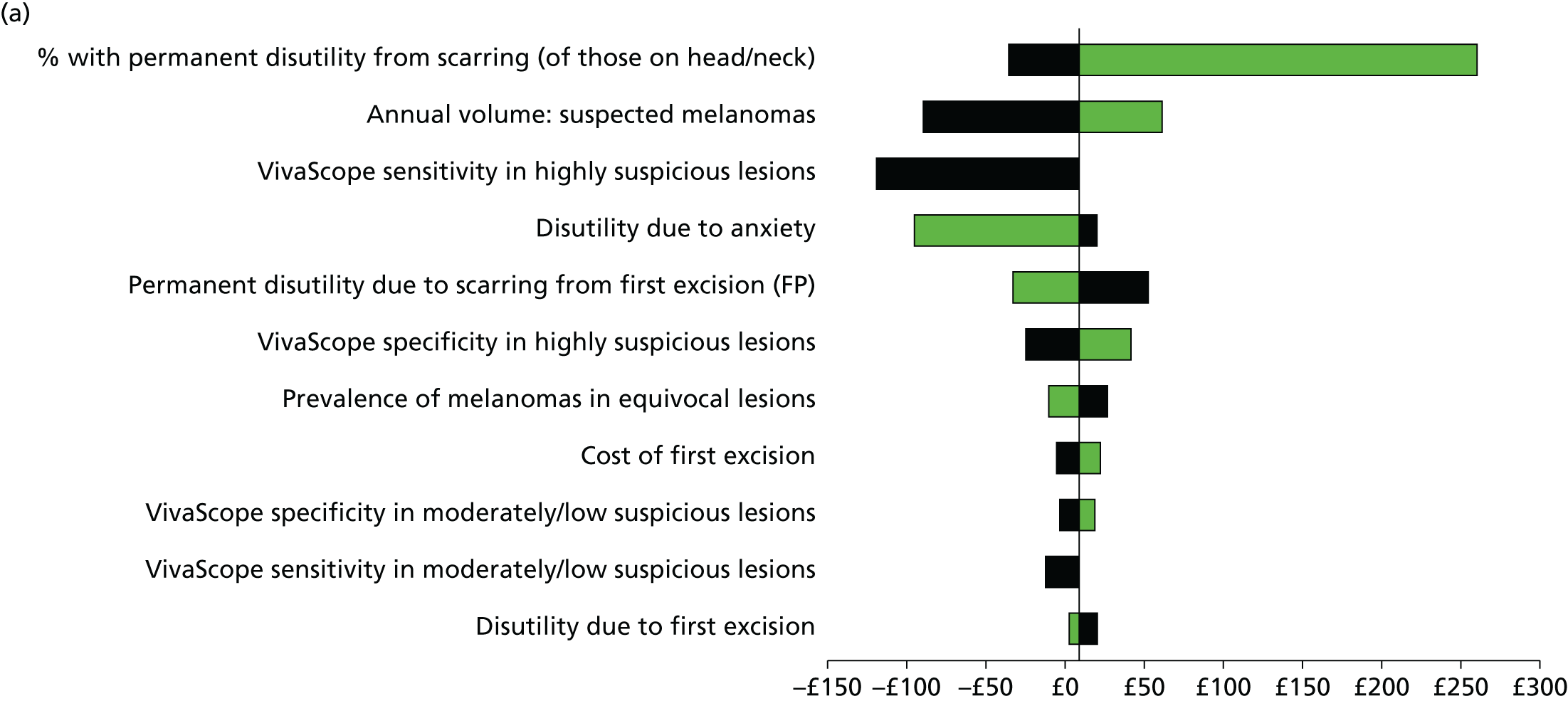

FIGURE 23.
Diagnosis of suspected BCCs: VivaScope use only for the diagnosis of suspected BCCs.
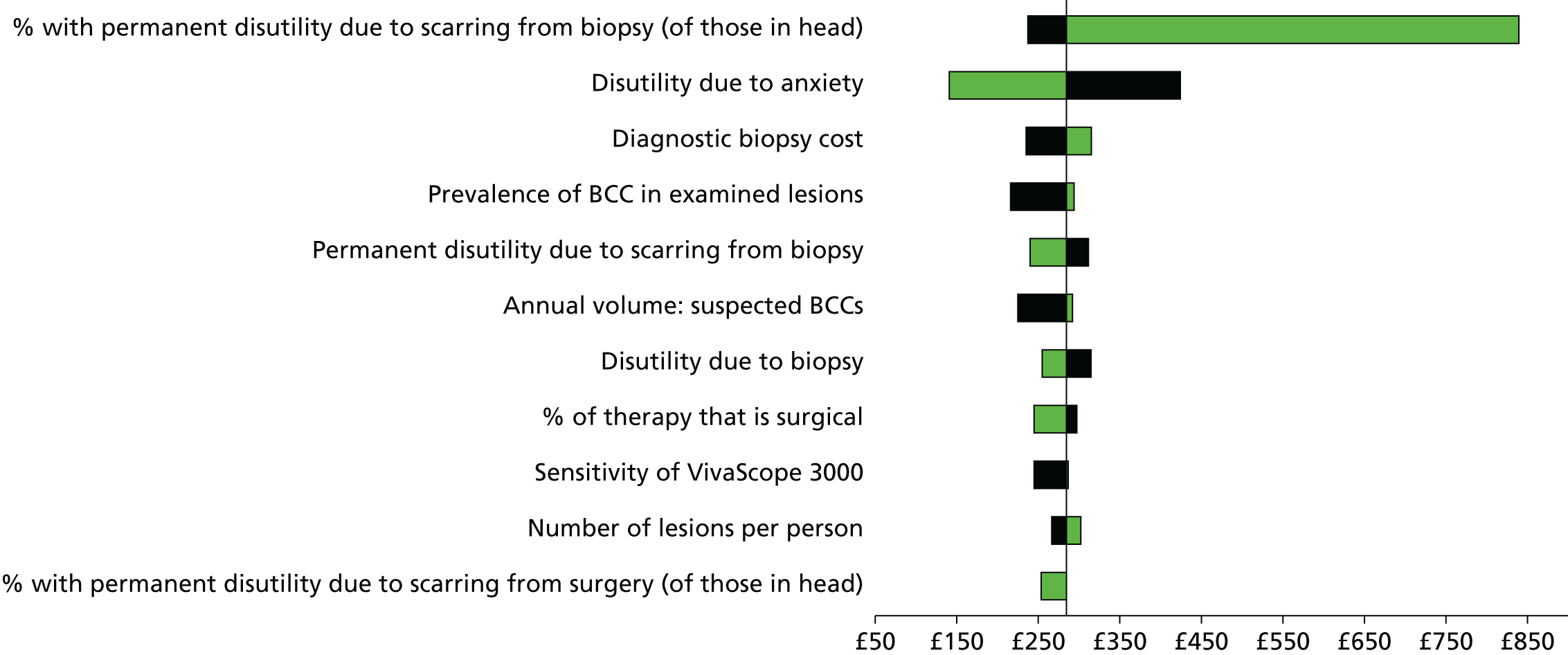
FIGURE 24.
Presurgical margin delineation of LMs. (a) VivaScope use only for the presurgical margin delineation of LMs; and (b) VivaScope use for all indications assessed in the economic modeling.
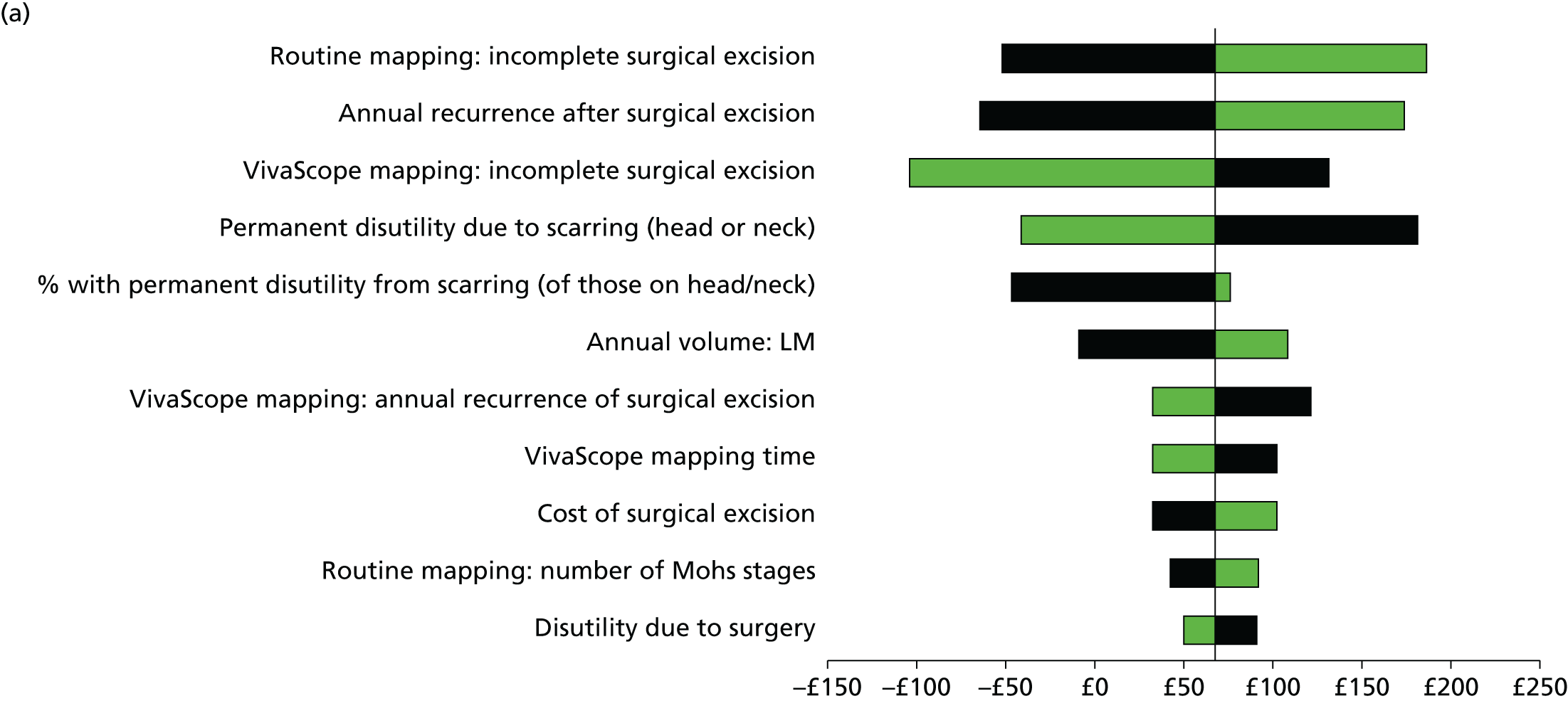

Appendix 7 Data abstraction tables
Alarcon et al. 201430
| Reviewer: George Osei-Assibey | Study ID: #110 | ||
|---|---|---|---|
| Reference details for all references relating to the trial: | Alarcon I, Carrera C, Palou J, Alos L, Malvehy J, Puig S, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol 2014;170:802–8 | ||
| General | |||
| RCT ( ) | Prospective (✓) before-and-after data for dermoscopy and VivaScope | Retrospective ( ) | |
| Indication for test (diagnosis or margin delineation or both): Diagnosis | |||
| Intervention(s): Dermoscopy | |||
| Comparator(s): Dermoscopy + VivaScope 1500 | |||
| Year(s) study was done: 2011–12 | |||
| Setting (e.g. district general, university hospital): Melanoma Unit of the Hospital Clinic of Barcelona, Spain | |||
| Source of funding: Fondo de Investigaciones Sanitarias, Spain; Centro de Investigación Biomédica en Red (CIBER) de Enfermedades Raras of the Instituto de Salud Carlos III, Spain; Catalan Government, Spain; European Commission (GenoMEL); the National Cancer Institute of the US National Institutes of Health | |||
| Conflict of interest: None declared | |||
| Participants’ characteristics | |||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) |
| Inclusion criteria: Patients with dermoscopically equivocal pigmented lesions, assumed to be melanocytic Exclusion criteria: NR |
|||
| Total | Men | Women | |
| Number enrolled | 1534 lesions | NR | NR |
| Number excluded | 1191 | NR | NR |
| Number withdrawn | NR | NR | NR |
| Number lost to follow-up | 79 | NR | NR |
| Number completed | 264 excisions | 136 | 128 |
| Age, mean and range (or data as reported): median 54.7 years (range 8–89 years) | |||
| Lesion- or patient-level data | Lesion ( ) | Patient (✓) | Both ( ) |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: l Lesion site: anatomical location Head and neck = 73; trunk = 135; limbs = 49; acral = 7 |
|||
| Types and number of lesion excised | 92 melanomas | 172 | |
| BCC | 12 | NR | |
| SCC | NR | NR | |
| LM | NR | NR | |
| LMM | NR | NR | |
| Melanocytic naevi | NR | NR | |
| Others | 53 | NR | |
Previous tests or assessments:
|
|||
| Treatment (details of any treatments given): NR | |||
| Mortality (number of study patients reported dead): NR | |||
| Index test | |||
| Equipment (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 1500; Caliber Imaging and Diagnostics, Rochester, NY, USA. Incorporates a near-infrared laser at a wavelength of 830 nm, with a maximum power of 35 mW | |||
| Image interpretation | |||
| Assessors (number of assessors): Three | |||
| Experience in using VivaScope or RCM: Images were independently reviewed by one of the three dermatologists with expertise in RCM | |||
| Qualitative (note how positive and negative findings were defined): Independent and blinded to the pathological outcome but not the clinical information |
|||
Quantitative diagnostic thresholds (e.g. ABCD rule): Four diagnostic features were followed to assess all of the images. The presence of:
|
|||
| Final confirmation method (e.g. histology): Optical sections were obtained of the stratum corneum, granulosum and spinosum dermo-epidermal junction and papillary dermis | |||
| Technical failures (number and reasons): NR | |||
| Comparator test | |||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): DermLite Photo; 3Gen LLC, Dana Point, CA, USA | |||
| Image interpretation | |||
| Assessors (number, expertise, experience in using comparator test): Three | |||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): | |||
Four diagnostic features were followed to assess all of the images. The presence of:
|
|||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Technical failures (number and reasons e.g. lesion site inaccessible with equipment): NR | |||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-a) | NR | ||
| Diameter of excisions (e.g. 2 mm) | NR | ||
| Number of excisions | 264 | ||
| Number of re-excisions | NR | ||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | Breslow thickness:
|
||
| Lymph node involvement or micrometastases | NR | ||
| Test interpretation: NR | |||
| Technical failures: NR | |||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |
| ✓ | |||
| RESULTS | |||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||
| Note threshold(s) where appropriate: | Reference standard | ||
| Disease | No disease | ||
| VivaScope 1500 | Disease | TP = 91 | FP = 14 |
| No disease | FN = 2 | TN = 157 | |
| Dermoscopy | Disease | TP = 87 | FP = 126 |
| No disease | FN = 5 | TN = 46 | |
| B. Sensitivity, specificity, PPV, NPV of dermoscopy and VivaScope 1500 | |||
| Dermoscopy (number of excised lesions = 264) | VivaScope 1500 (number of excised lesions ≠ 264) | ||
| Sensitivity, % (95% CI) | 94.6 (87.2 to 98.0) | 97.8 (91.6 to 99.6) | |
| Specificity, % (95% CI) | 26.74 (87.2 to 98.0) | 92.4 (87.2 to 95.7) | |
| PPV, % (95% CI) | 40.8 (34.2 to 47.8) | 87.4 (79.0 to 92.8) | |
| NPV, % (95% CI) | 90.2 (77.8 to 96.3) | 98.8 (95.1 to 99.8) | |
| C. NNT: defined as the proportion of dermoscopically and RCM equivocal pigmented lesions, assumed to be melanocytic, excised for every melanoma | |||
| Lesions intended for excision | NNT | ||
| Dermoscopy | 343 | 3.73 | |
| Dermoscopy + VivaScope 1500 | 264 | 2.87 | |
| VivaScope 1500 | 103 | 1.12 | |
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Patients with equivocal lesions attending a dedicated melanoma clinic in Barcelona | ||||
| Index test(s) | VivaScope 1500 and dermoscopy | ||||
| Reference standard and target condition | Biopsy | ||||
| Draw a flow for the primary study | |||||
| xxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Consecutive patients presenting at the Melanoma Unit of the Hospital Clinic of Barcelona, Spain, with dermoscopically equivocal pigmented lesions, assumed to be melanocytic, were considered for enrolment. Dermoscopic criteria for diagnosing melanoma and the criteria were used to establish the eligibility of lesions | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| Patients with equivocal lesions were tested with a dermoscope then VivaScope 1500, The aim of the study was to assess the therapeutic impact of VivaScope 1500 on the number of excisions of lesions deemed equivocal using dermoscopy | |||||
| Low risk | High risk | Unclear risk | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
In vivo confocal microscopy was performed with a VivaScope 1500. Four diagnostic features were followed to assess all images. The presence of:
|
|||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear risk | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| Performed by certified dermatopathologists | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear risk | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| All lesion data are included in the 2 × 2 tables | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| Immediately, one after the other | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
Bennassar et al. 201441
| Reviewer: George Osei-Assibey | Study ID: #3635 | ||
|---|---|---|---|
| Reference details for all references relating to the trial: | Bennassar A, Vilata A, Puig S, Malvehy J. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol 2014;170:360–5 | ||
| General | |||
| RCT ( ) | Prospective (✓) | Retrospective ( ) | |
| Indication for test (diagnosis or margin delineation or both): Margin delineation | |||
| Intervention(s): VivaScope 2500 Comparator(s): NR |
|||
| Year(s) study was done: October 2010 and November 2011 | |||
| Setting (e.g. district general, university hospital): Mohs Surgery Unit at the Hospital Clinic, Barcelona, Spain | |||
| Source of funding: Personal grants to AB from Hospital Clinic de Barcelona ‘Emili Letang’ and is partially supported by Fondo de Investigaciones Sanitarias (FIS) grant 09/1393; CIBERER U-726, ISCIII | |||
| Conflict of interest: The VivaScope 2500 was borrowed from Lucid Inc. for 8 months (now Caliber Imaging and Diagnostics) | |||
| Participants’ characteristics | |||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) |
| Inclusion criteria: 80 BCCs (≥ 5 mm in diameter) which have undergone classical Mohs surgery Exclusion criteria: NR |
|||
| Total | Men | Women | |
| Number enrolled | 74 (80 lesions) | 44 | 30 |
| Number excluded | NR | NR | NR |
| Number withdrawn | NR | NR | NR |
| Number lost to follow-up | NR | NR | NR |
| Number completed | 74 (80 lesions) | 44 | 30 |
| Age, mean and range (or data as reported): NR | |||
| Lesion- or patient-level data | Lesion (✓) | Patient ( ) | Both ( ) |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: Lesion location: head and neck, 73 (91%); trunk, 7 (9%) Status of lesions: primary, 63 (79%); recurrent, 17 (21%) |
|||
| Types and number of lesion excised | 80 | ||
| BCC | 80 | NR | |
| SCC | NR | NR | |
| LM | NR | NR | |
| Melanocytic naevi | NR | NR | |
| Others | |||
| Previous tests or assessments: NR | |||
| Treatment (details of any treatments given): | |||
| Mortality (number of study patients reported dead): NR | |||
| Index test | |||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 2500; Caliber Imaging and Diagnostics, Rochester, NY, USA); this FCM version is specially designed for ex vivo imaging of freshly excised tissue samples | |||
| Image interpretation | |||
| Assessors (number of assessors): One | |||
| Experience in using VivaScope or RCM: NR | |||
| Qualitative (note how positive and negative findings were defined): Eight criteria, namely presence of fluorescence, tumour demarcation, nuclear crowding, peripheral palisading, clefting, nuclear pleomorphism, increased nuclear-to-cytoplasm ratio and the presence of stroma, were described, evaluated and validated. These criteria have been demonstrated to be useful in distinguishing BCC nests and strands from adnexal structures. In RCM mosaics, a well-circumscribed mass or lobule of pleomorphic hyperfluorescent bright dots, with a striking tendency to arrange with peripheral palisading next to the clefting, is very likely to be a BCC | |||
| Quantitative diagnostic thresholds (e.g. ABCD rule): NR | |||
| Final confirmation method (e.g. histology): Histopathology | |||
| Technical failures (number and reasons): NR | |||
| Comparator test | |||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): NR | |||
| Image interpretation | |||
| Assessors (number, expertise, experience in using comparator test): NR | |||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Technical failures (number and reasons e.g. lesion site inaccessible with equipment): NR | |||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-a) | NR | ||
| Diameter of excisions (e.g. 2 mm) | NR | ||
| Number of excisions | 80 | ||
| Number of re-excisions | NR | ||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | NR | ||
| Lymph node involvement or micrometastases | NR | ||
| Test interpretation: NR | |||
| Technical failures: NR | |||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |
| NR | NR | ||
| RESULTS | |||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||
| Note threshold(s) where appropriate: | Reference standard | ||
| Disease | No disease | ||
| VivaScope 2500 | Disease | TP = 79 | FP = 1 |
| No disease | FN = 10 | TN = 390 | |
| B. Sensitivity, specificity, PPV, NPV of detecting BCC margins | |||
| Sensitivity (%) | 88 | ||
| Specificity (%) | 99 | ||
| PPV | 98 | ||
| NPV | 97 | ||
| C. Change in evaluation time | |||
| The mean time to obtain VivaScope mosaics in the first Mohs stage (two samples per stage) was 10.1 ± 1.22 minutes, while it took a mean of 28.2 ± 2.2 minutes to process the samples with frozen haematoxylin and eosin-stained slides. Therefore, on average, VivaScope 2500 reduced the evaluation time by 18 minutes (p < 0.001) | |||
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Eighty consecutive BCCs from 74 patients were prospectively collected and the margins scanned with VivaScope 2500 | ||||
| Index test(s) | VivaScope 2500 | ||||
| Reference standard and target condition | Histopathology | ||||
| Draw a flow for the primary study | |||||
| xxxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Eighty consecutive BCCs from 74 patients were prospectively collected and the margins scanned with VivaScope 2500 | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| NR | |||||
| Low risk | High risk | Unclear | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| Confocal mosaics were acquired using a modified version of a commercially available ex vivo laser scanning RCM (VivaScope 2500; Caliber Imaging and Diagnostics, Rochester, NY, USA); this RCM version is specially designed for ex vivo imaging of freshly excised tissue samples. All samples were directly immersed in a 1 mmol/l solution of acridine orange to provide a strong nuclear–dermis contrast, as it specifically stains nuclear DNA | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| NR | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
Castro et al. 201543
| Reviewer: George Osei-Assibey | Study ID: Handsearch | ||
|---|---|---|---|
| Reference details for all references relating to the trial: | Castro RP, Stephens A, Fraga-Braghiroli NA, Oliviero MC, Rezze GG, Rabinovitz H, et al. Accuracy of in vivo confocal microscopy for diagnosis of basal cell carcinoma: a comparative study between hand-held and wide-probe confocal imaging. J Eur Acad Dermatol Venereol 2015;29:1164–9 | ||
| General | |||
| RCT ( ) | Prospective (✓) | Retrospective ( ) | |
| Indication for test (diagnosis or margin delineation or both): Lesion diagnosis | |||
| Intervention(s): VivaScope 1500 and 3000 Comparator(s): NR |
|||
| Year(s) study was done: NR | |||
| Setting (e.g. district general, university hospital): Outpatient dermatology clinic at a tertiary cancer centre in São Paulo, Brazil, and at a private practice that specialises in skin cancer treatment in South Florida, FL, USA | |||
| Source of funding: NR | |||
| Conflict of interest: Dr Rabinovitz is an investigator in a study coordinated by Lucid Inc., manufacturer of a commercial confocal microscope. He has received funding for a fellowship programme and equipment from Lucid Inc. He is also a consultant and has received equipment from 3-Gen, manufacturer of a polarised dermoscope. MC Oliviero is a consultant, speaker for Caliber ID, 3Gen LLC, Canfield and MelaSciences. The other authors have no conflicts of interest to declare | |||
| Participants’ characteristics | |||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) |
| Inclusion criteria: Patients with one or more skin lesions that were deemed suspicious for BCC based on clinical and dermoscopic examination Exclusion criteria: NR |
|||
| Total | Men | Women | |
| Number enrolled | 73 | 44 | 30 |
| Number excluded | NR | NR | NR |
| Number withdrawn | NR | NR | NR |
| Number lost to follow-up | NR | NR | NR |
| Number completed | 73 | NR | NR |
| Age, mean and range (or data as reported): Mean 65 years (range 30–89 years) | |||
| Lesion- or patient-level data | Lesion (✓) | Patient ( ) | Both ( ) |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: 38 (41%) of the lesions were mostly facial; 24 (75%) of the patients had skin phototype II and 8 (25%) skin phototype III. The anatomic distribution of these 45 BCCs was head and neck nine (20%), torso 26 (58%), upper extremities four (9%) and lower extremities six (13%) | |||
| Types and number of lesion excised | |||
| BCC | 92 | NR | |
| SCC | NR | NR | |
| LM | NR | NR | |
| Melanocytic naevi | NR | NR | |
| Others | NR | NR | |
| Previous tests or assessments: Dermoscopy | |||
| Treatment (details of any treatments given): NR | |||
| Mortality (number of study patients reported dead): NR | |||
| Index test | |||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 1500 and 3000 | |||
| Image interpretation | |||
| Assessors (number of assessors): two | |||
| Experience in using VivaScope or RCM: All examinations, including clinical, dermoscopic and RCM imaging, were made by a dermatologist experienced with RCM examination, with supervision by a skin cancer expert | |||
| Qualitative (note how positive and negative findings were defined): Images were evaluated for the presence of previously published RCM criteria for identification of BCC including: the presence of neoplastic aggregates, seen as ‘dark silhouettes’ or as ‘bright tumour islands’ at the level of the dermo-epidermal junction or upper dermis; ‘streaming’ polarisation of nuclei in neoplastic aggregates along the same axis of orientation; ‘peripheral palisading’ of nuclei at the tumour islands’ periphery; dark ‘peritumoral clefts’ around the tumour islands; fibrotic stroma with ‘thickened collagen bundles’; dilated and tortuous ‘linear blood vessels’ and ‘coiled blood vessels’; ‘bright dendritic structures’ within tumour islands; and ‘bright round cells’ in the stroma | |||
| Quantitative diagnostic thresholds (e.g. ABCD rule): A threshold of ≥ 3 RCM criteria to identify BCC, whereby at least one of the criteria had to be the presence of ‘dark silhouettes’ or ‘bright tumour islands’; these latter criteria denote the presence of neoplastic aggregates of BCC and hence need to be observed in all cases identified as BCC by RCM | |||
| Final confirmation method (e.g. histology): Histopathology | |||
| Technical failures (number and reasons): NR | |||
| Comparator test | |||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): NR | |||
| Image interpretation | |||
| Assessors (number, expertise, experience in using comparator test): NR | |||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | |||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-A) | NR | ||
| Diameter of excisions (e.g. 2 mm) | NR | ||
| Number of excisions | 92 | ||
| Number of re-excisions | NR | ||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | NR | ||
| Lymph node involvement or micrometastases | NR | ||
| Test interpretation: NR | |||
| Technical failures: NR | |||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |
| NR | NR | ||
| Results | |||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||
| Note threshold(s) where appropriate: | Reference standard | ||
| Disease | No disease | ||
| VivaScope 1500 | Disease | TP = 45/47 | FP = 2 |
| No disease | FN = | TN = | |
| VivaScope 3000 | Disease | TP = 42/44 | FP = 2 |
| No disease | FN = | TN = | |
| B. Sensitivity, specificity, PPV, NPV of detecting BCC margins | |||
| VivaScope 1500 | VivaScope 3000 | ||
| Sensitivity (%) | 100 | 93 | |
| Specificity (%) | 78 | 78 | |
| PPV (%) | 96 | 95 | |
| NPV (%) | 100 | 70 | |
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Patients with one or more skin lesions that were deemed suspicious for BCC based on clinical and dermoscopic examination, recruited from outpatient dermatology clinic at a tertiary cancer centre in São Paulo, Brazil and at a private practice that specialises in skin cancer treatment in South Florida, FL, USA | ||||
| Index test(s) | VivaScope 1500 and 3000 | ||||
| Reference standard and target condition | Histopathology | ||||
| Draw a flow for the primary study | |||||
| xxxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Patients included in the study were recruited from the population of women and men who underwent skin cancer screening at the outpatient dermatology clinic at a tertiary cancer centre in São Paulo, Brazil and at a private practice that specialises in skin cancer treatment in South Florida, FL, USA. Patients recruited were those presenting with one or more skin lesions that were deemed suspicious for BCC based on clinical and dermoscopic examination. Informed consent was obtained from each study participant | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| Included patients had been clinically and dermoscopically tested | |||||
| Low risk | High risk | Unclear | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| Hand-held RCM imaging was performed with commercially available in vivo RCM system (VivaScope3000). TWP-RCM imaging was performed with a commercially available in vivo RCM system (VivaScope1500) | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| NR | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear | |||
| Could the patient flow have introduced bias? | ✓ | ✓ | |||
| Notes/comments: | |||||
Curchin et al. 201131
| Reviewer: George Osei-Assibey | Study ID: #643 | ||
|---|---|---|---|
| Reference details for all references relating to the trial: | Curchin CE, Wurm EM, Lambie DL, Longo C, Pellacani G, Soyer HP, et al. First experiences using reflectance confocal microscopy on equivocal skin lesions in Queensland. Australas J Dermatol 2011;52:89–97 | ||
| General | |||
| RCT ( ) | Prospective (✓) | Retrospective ( ) | |
| Indication for test (diagnosis or margin delineation or both): Diagnosis | |||
| Intervention(s): VivaScope 1500 with a dermoscopic camera Comparator(s): NR |
|||
| Year(s) study was done: January 2010 to May 2010 | |||
| Setting (e.g. district general, university hospital): Princess Alexandra Hospital Dermatology Department, Woolloongabba QLD, Australia | |||
| Source of funding: NR | |||
| Conflict of interest: NR | |||
| Participants’ characteristics | |||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) |
| Inclusion criteria: Consecutive patients with equivocal lesions recruited from the dermatology departments booking list Exclusion criteria: NR |
|||
| Total | Men | Women | |
| Number enrolled | 42 (50 lesions) | NR | NR |
| Number excluded | 0 | NR | NR |
| Number withdrawn | 0 | NR | NR |
| Number lost to follow-up | 0 | NR | NR |
| Number completed | 42 (50 lesions) | NR | NR |
| Age, mean and range (or data as reported): NR | |||
| Lesion- or patient-level data | Lesion (✓) | Patient ( ) | Both ( ) |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: NR | |||
| Types and number of lesion excised | |||
| BCC | 9 | NR | |
| SCC | 6 | NR | |
| LM | NR | NR | |
| Melanoma | 13 | NR | |
| Benign naevus | 22 | NR | |
| Others | |||
| Previous tests or assessments: NR | |||
| Treatment (details of any treatments given): NR | |||
| Mortality (number of study patients reported dead): NR | |||
| Index test | |||
| Equipment (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 1500 (Lucid Inc., Rochester, NY, USA) | |||
| Image interpretation | |||
| Assessors (number of assessors): One | |||
| Experience in using VivaScope or RCM: Assessor was a novice who had completed a course in RCM | |||
| Qualitative (note how positive and negative findings were defined): Confidence level in diagnosis (low confidence, 1; medium confidence, 2; and high confidence, 5) each image also evaluated for the presence and degree of artefact | |||
Quantitative diagnostic thresholds (e.g. ABCD rule): Superficial layer scrutinised for three possible patterns:
|
|||
| Final confirmation method (e.g. histology): Images blind to the histopathology result | |||
| Technical failures (number and reasons): NR | |||
| Comparator test | |||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): NR | |||
| Image interpretation | |||
| Assessors (number, expertise, experience in using comparator test): NR | |||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | |||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-a) | NR | ||
| Diameter of excisions (e.g. 2 mm) | NR | ||
| Number of excisions | 50 | ||
| Number of re-excisions | NR | ||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | NR | ||
| Lymph node involvement or micrometastases | NR | ||
| Test interpretation: NR | |||
| Technical failures: NR | |||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |
| NR | ✓Pts @ excision clinic | ||
| Results | |||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||
| Note threshold(s) where appropriate: | Reference standard | ||
| Disease | No disease | ||
| Test | Disease | TP | FP |
| No disease | FN | TN | |
| B. Diagnostic accuracy of VivaScope 1500 | |||
| Number correctly diagnosed after histopathology | Sensitivity | Specificity | |
| Melanomas | 12/13 | 92.3% | 75% |
| BCC | 6/9 | 66.7% | 100% |
| SCC | 6/6 | 100% | 75% |
| Benign naevi | 19/22 | 86% | 95% |
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Consecutive patients attending a dermatology department minor excision clinic | ||||
| Index test(s) | VivaScope 1500 | ||||
| Reference standard and target condition | Histopathology | ||||
| Draw a flow for the primary study | |||||
| xxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Consecutive patients already on the dermatology excision clinic list | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| Previous tests not reported, indication = equivocal lesions | |||||
| Low risk | High risk | Unclear risk | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| The dermoscopic and RCM images were aligned over the top of each other so that correlation between the two could be made. RCM images were taken as blocks (a series of individual RCM images digitally stitched together to form a larger mosaic) in the horizontal plane at depths of 30, 60 and 90 mm, approximate levels of the epidermis, dermal–epidermal junction and dermis, respectively. Individual features of interest were identified from the blocks and were imaged further with vertical stacks (a series of individual RCM images taken at the same position but at increasing depths in the vertical plane). Vertical stacks were taken from depths of 0 to 120 mm, 10 mm apart | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear risk | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| Histopathological analysis, details of method not reported | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear risk | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| The patients were already on the excision clinic list and received RCM prior to the excision | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
Ferrari et al. 201544
| Reviewer: George Osei-Assibey | Study ID: Obtained from updated search | ||
|---|---|---|---|
| Reference details for all references relating to the trial: | Ferrari B, Pupelli G, Farnetani F, De Carvalho NT, Longo C, Reggiani C, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol 2015;29:1135–40 | ||
| General | |||
| RCT ( ) | Prospective ( ) | Retrospective (✓) | |
| Indication for test (diagnosis or margin delineation or both): Diagnosis | |||
| Intervention(s): VivaScope 1500 Comparator(s): Dermoscopy |
|||
| Year(s) study was done: 2010 | |||
| Setting (e.g. district general, university hospital): Department of Dermatology, University of Modena and Reggio Emilia, Italy | |||
| Source of funding: None declared | |||
| Conflict of interest: None declared | |||
| Participants’ characteristics | |||
| Consecutive sample | Yes ( ) | No (✓) | Unclear ( ) |
| Inclusion criteria: Only lesions with high-quality dermoscopic images, a complete set of confocal images and histopathology report available were included Exclusion criteria: NR |
|||
| Total | Men | Women | |
| Number enrolled | 322 lesions | NR | NR |
| Number excluded | NR | NR | NR |
| Number withdrawn | NR | NR | NR |
| Number lost to follow-up | NR | NR | NR |
| Number completed | 322 lesions | NR | NR |
| Age, mean and range (or data as reported): NR | |||
| Lesion- or patient-level data | Lesion (✓) | Patient ( ) | Both ( ) |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: among 322 lesions, 70 were melanomas and 252 were naevi | |||
| Types and numbers of lesions excised | 322 | ||
| BCC | NR | NR | |
| SCC | NR | NR | |
| LM | NR | NR | |
| LMM | NR | NR | |
| Naevi | 252 | NR | |
| Melanoma | 70 | NR | |
| Previous tests or assessments: Histopathology | |||
| Treatment (details of any treatments given): NR | |||
| Mortality (number of study patients reported dead): NR | |||
| Index test | |||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): Confocal imaging was performed with near-infrared reflectance-mode confocal laser scanning microscope (VivaScope1500; MAVIG GmbH, Munich, Germany) | |||
| Image interpretation | |||
| Assessors (number of assessors): One | |||
| Experience in using VivaScope or RCM: Dermatologist trained in RCM | |||
| Qualitative (note how positive and negative findings were defined): In the superficial layer it was evaluated the presence of pagetoid cells, the cell shape (roundish or dendritic) and their number (< 5 or ≥ 5 cells per mm2). At the dermo-epidermal junction lesion’s architecture was evaluated for the presence of the following patterns: ringed, meshwork, clods and non-specific pattern, according with previous definition; 16 architectural disorder, corresponding to irregular alternation of different RCM patterns, non-edged papillae extended over the 10% of lesion, and/or tangled filaments/dendrites crossing the papillae; presence of cytological atypia (≥ 5 cells per mm2). In the superficial dermis, the presence of atypical nucleated cells arranged in nests was analysed. Presence of five or more roundish pagetoid cells, architectural disorder at the junction, atypical cells at the junction, and atypical nucleated cells arranged in nests were considered melanoma clues upon RCM examination | |||
| Quantitative diagnostic thresholds (e.g. ABCD rule): NR | |||
| Final confirmation method (e.g. histology): Histopathological analysis | |||
| Technical failures (number and reasons): NR | |||
| Comparator test | |||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): dermoscope | |||
| Image interpretation | |||
| Assessors (number, expertise, experience in using comparator test): Dermatologist trained on dermoscopy | |||
Qualitative (note how positive and negative findings were qualitatively defined): The 7-point checklist score was calculated for each case as well as the frequencies of each distinct dermoscopic feature accounting for the score. Afterwards, lesions were classified according the 7-point checklist score into three categories:
|
|||
Quantitative diagnostic thresholds (e.g. ABCD system): The 7-point checklist score was calculated for each case as well as the frequencies of each distinct dermoscopic feature accounting for the score. Afterwards, lesions were classified according the 7-point checklist score into three categories:
|
|||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | |||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-a) | NR | ||
| Diameter of excisions (e.g. 2 mm) | NR | ||
| Number of excisions | 322 | ||
| Number of re-excisions | NR | ||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | Mean ± SD: 1.05 ± 2.16 mm; range 0–10 mm | ||
| Lymph node involvement or micrometastases | NR | ||
| Test interpretation: NR | |||
| Technical failures: NR | |||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |
| NR | NR | ||
| Results | |||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||
| Note threshold(s) where appropriate: | Reference standard | ||
| Disease | No disease | ||
| Test | Disease | TP | FP |
| No disease | FN | TN | |
| B. Sensitivity and specificity with VivaScope 1500 | |||
| VivaScope 1500 | |||
|
|||
| Dermoscopy | |||
|
|||
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | 322 melanocytic lesions obtained from the Department of Dermatology, University of Modena and Reggio Emilia, Italy | ||||
| Index test(s) | VivaScope 1500 | ||||
| Reference standard and target condition | Histopathological analysis | ||||
| Draw a flow for the primary study | |||||
| xxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Only lesions with high quality dermoscopic images, a complete set of confocal images and histopathology report available were included in the study | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| Study samples included all melanocytic lesions excised on the basis of equivocal clinical and/or dermoscopic features. Before excision, all lesions were recorded by means of digital dermoscopy and RCM. Only lesions with high quality dermoscopic images, a complete set of confocal images and histopathology report available were included in the study | |||||
| Low risk | High risk | Unclear | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| Confocal imaging was performed with VivaScope 1500. A minimum of three mosaics, with a maximum area of 8 × 8 mm, were obtained per lesion, one in the superficial epidermis (stratum granulosum/spinosum), one at the dermo-epidermal junction and one in papillary dermis, to analyse the overall architectural and cytological aspects | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | |||||
| Yes | |||||
| Low risk | High risk | Unclear | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| The histopathological analysis was performed by a Board Certified Pathologist | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
Gerger et al. 200632
| Reviewer: George Osei-Assibey | Study ID: #962 | |||
|---|---|---|---|---|
| Reference details for all references relating to the trial: | Gerger A, Koller S, Weger W, Richtig E, Kerl H, Samonigg H, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer 2006;107:193–200 | |||
| General | ||||
| RCT ( ) | Prospective (✓) | Retrospective ( ) | ||
| Indication for test (diagnosis or margin delineation or both): Diagnosis | ||||
| Intervention(s): VivaScope 1000 Comparator(s): NR |
||||
| Year(s) study was done: NR | ||||
| Setting (e.g. district general, university hospital): Dermato-oncology Clinic at the Department of Dermatology, Medical University of Graz, Graz, Austria | ||||
| Source of funding: Fond zur Forderung der wissenschaftlichen Forschung (project number 16206-B05) | ||||
| Conflict of interest: NR | ||||
| Participants’ characteristics | ||||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) | |
| Inclusion criteria: Patients with melanocytic and non-melanocytic skin tumours were selected Exclusion criteria: NR |
||||
| Total | Men | Women | ||
| Number enrolled | 119 | 62 | 57 | |
| Number excluded | NR | NR | NR | |
| Number withdrawn | NR | NR | NR | |
| Number lost to follow-up | NR | NR | NR | |
| Number completed | NR | NR | NR | |
| Age, mean and range (or data as reported): | ||||
| Lesion- or patient-level data | Lesion (✓) | Patient ( ) | Both ( ) | |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: One hundred seventeen melanocytic skin lesions and 45 non-melanocytic skin lesions (90 benign naevi, 27 malignant melanomas, 15 BCC and 30 seborrhoeic keratoses) | ||||
| Types and number of lesion excised | ||||
| BCC | NR | NR | ||
| SCC | NR | NR | ||
| LM | NR | NR | ||
| LMM | NR | NR | ||
| Melanocytic naevi | NR | NR | ||
| Previous tests or assessments: NR | ||||
| Treatment (details of any treatments given): NR | ||||
| Mortality (number of study patients reported dead): NR | ||||
| Index test | ||||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 1000; Lucid Inc., Rochester, NY, USA | ||||
| Image interpretation | ||||
| Assessors (number of assessors): Four | ||||
| Experience in using VivaScope or RCM: Four independent dermato-oncologists without previous experience in confocal laser scanning microscopy received a standardised instruction about diagnostic RCM features of malignant melanoma, benign naevi, BCC, and seborrhoeic keratosis for 1 hour as a PowerPoint® (Microsoft Corporation, Redmond, WA, USA) presentation. Diagnostic criteria were explained and 26 image examples were demonstrated for training purposes. | ||||
| Qualitative (note how positive and negative findings were defined): Morphological features of melanocytic skin tumours were assessed according to identification of melanocytic cytomorphology and architecture, keratinocyte cell borders, and complex branching dendrites as highly diagnostic criteria. For BCC, vascular architecture, tumour cells in a streaming pattern, and collagen fibre bundles were taken into account for diagnostic decisions. In contrast, SK features were assessed solely based on well-known, standard criteria used in conventional histopathology | ||||
| Quantitative diagnostic thresholds (e.g. ABCD rule): NR | ||||
| Final confirmation method (e.g. histology): NR | ||||
| Technical failures (number and reasons): NR | ||||
| Comparator test | ||||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): NR | ||||
| Image interpretation | ||||
| Assessors (number, expertise, experience in using comparator test): NR | ||||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | ||||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | ||||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | ||||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | ||||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | ||||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-a) | NR | |||
| Diameter of excisions (e.g. 2 mm) | NR | |||
| Number of excisions | 72 | |||
| Number of re-excisions | NR | |||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | NR | |||
| Lymph node involvement or micrometastases | NR | |||
| Test interpretation: NR | ||||
| Technical failures: NR | ||||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | ||
| NR | NR | |||
| Results | ||||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | ||||
| Note threshold(s) where appropriate: | Reference standard | |||
| Disease | No disease | |||
| Test | Disease | TP | FP | |
| No disease | FN | TN | ||
| B. Diagnostic differentiation between lesions | ||||
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| Melanoma and all other lesions based solely on VivaScope 1000 examination | 90.74 | 98.8 | 94.22 | 98.17 |
| Benign vs. malignant skin tumours lesions based solely on VivaScope 1000 examination | 94.05 | 98.75 | 96.3 | 97.94 |
| Benign vs. malignant lesions classification based on only the biopsy documented lesions | 94.65 | 96.67 | 97.50 | 92.99 |
| Overall | 97.5 | 99 | 97.5 | 99 |
| C. Correlation between VivaScope 1000 diagnosis and the assessed pathological or clinical diagnosis | ||||
| VivaScope 100 diagnosis | Pathological diagnosis | |||
| Malignant melanoma | BCC | |||
| Malignant melanoma | 98 | 0 | ||
| BCC | 2 | 58 | ||
| Benign naevus | 3 | 0 | ||
| SK | 5 | 2 | ||
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | One hundred and nineteen patients (62 males and 57 females) recruited prospectively from the Dermato-oncology Clinic at the Department of Dermatology, Medical University of Graz, Austria, over 2 years | ||||
| Index test(s) | VivaScope 1000 | ||||
| Reference standard and target condition | Histopathology | ||||
| Draw a flow for the primary study | |||||
| xxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| One hundred and nineteen patients (62 males and 57 females) recruited prospectively from the Dermato-oncology Clinic at the Department of Dermatology, Medical University of Graz, Austria | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| One hundred and nineteen patients (62 males and 57 females) with 117 melanocytic skin lesions and 45 non-melanocytic skin tumours, including malignant melanoma, benign naevi, BCC and SK, were imaged consecutively by using a confocal microscope | |||||
| Low risk | High risk | Unclear risk | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| Morphological features of melanocytic skin tumours were assessed according to the results of published investigations. The identification of melanocytic cytomorphology and architecture, keratinocyte cell borders, and complex branching dendrites were rated as highly diagnostic criteria. The set of confocal BCC features was selected based on qualitatively described criteria from previously published studies. Vascular architecture, tumour cells in a streaming pattern, and collagen fibre bundles were taken into account for diagnostic decisions. In contrast, SK features were assessed solely based on well known, standard criteria used in conventional histopathology | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear risk | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| NR | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear risk | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | |||||
| Low risk | High risk | Unclear risk | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
Gerger et al. 200833
| Reviewer: George Osei-Assibey | Study ID: #961 | |||
|---|---|---|---|---|
| Reference details for all references relating to the trial: | Gerger A, Hofmann-Wellenhof R, Langsenlehner U, Richtig E, Koller S, Weger W, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol 2008;158:329–33 | |||
| General | ||||
| RCT ( ) | Prospective ( ) | Retrospective (✓) | ||
| Indication for test (diagnosis or margin delineation or both): Diagnosis | ||||
| Intervention(s): VivaScope 1000 Comparator(s): NR |
||||
| Year(s) study was done: study conducted over 10 months | ||||
| Setting (e.g. district general, university hospital): Dermato-oncology clinic, Medical University of Graz, Graz, Austria | ||||
| Source of funding: Fond zur Forderung der wissenschaftlichen Forschung | ||||
| Conflict of interest: NR | ||||
| Participants’ characteristics | ||||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) | |
| Inclusion criteria: Patients with melanocytic skin tumours Exclusion criteria: NR |
||||
| Total | Men | Women | ||
| Number enrolled | 60 | 32 | 28 | |
| Number excluded | 0 | 0 | 0 | |
| Number withdrawn | 0 | 0 | 0 | |
| Number lost to follow-up | 0 | 0 | 0 | |
| Number completed | 0 | 32 | 28 | |
| Age, mean and range (or data as reported): NR | ||||
| Lesion- or patient-level data | Lesion (✓) | Patient ( ) | Both ( ) | |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: NR | ||||
| Types and number of lesion excised | 70 | NR | ||
| Malignant melanoma | 20 | NR | ||
| BCC | NR | NR | ||
| SCC | NR | NR | ||
| LM | NR | NR | ||
| Benign naevi | 50 | NR | ||
| Previous tests or assessments: Dermoscopy | ||||
| Treatment (details of any treatments given): NR | ||||
| Mortality (number of study patients reported dead): NR | ||||
| Index test | ||||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 1000; Lucid Inc., Rochester, NY). The VivaScope 1000 had a diode laser at 830-nm wavelength and a power of < 35 kW at the tissue level. (Reported in reference 14: Gerger, Br J Dermatol 2006;107:193–200) | ||||
| Image interpretation | ||||
| Assessors (number of assessors): Four independent clinical dermato-oncologists | ||||
| Experience in using VivaScope or RCM: Four independent clinical dermato-oncologists with moderate experience in confocal laser scanning microscopy (CLSM) who have received a standardised instruction about diagnostic CLSM features of melanocytic skin tumours assessed the images | ||||
| Qualitative (note how positive and negative findings were defined): Blind to the dermoscopy and biopsy results. | ||||
| Quantitative diagnostic thresholds (e.g. ABCD rule): Morphological features of melanocytic skin tumours were selected and assessed according to recently published studies. Melanocytic cytomorphology and architecture and keratinocyte cell borders were taken into account for diagnostic decisions. All morphological features were defined a priori without reference to the image set of the present study | ||||
| Final confirmation method (e.g. histology): biopsy | ||||
| Technical failures (number and reasons): NR | ||||
| Comparator test | ||||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): NR | ||||
| Image interpretation | ||||
| Assessors (number, expertise, experience in using comparator test): NR | ||||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | ||||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | ||||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | ||||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | ||||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | ||||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-a) | NR | |||
| Diameter of excisions (e.g. 2 mm) | NR | |||
| Number of excisions | 34 | |||
| Number of re-excisions | NR | |||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | Mean (± SD): 1.48 ± 1.60 mm | |||
| Lymph node involvement or micrometastases | NR | |||
| Test interpretation: NR (All 20 malignant melanomas received biopsy but only 14 out of 50 naevi received a biopsy) | ||||
| Technical failures: NR | ||||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | ||
| NR | NR | |||
| Results | ||||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | ||||
| Note threshold(s) where appropriate: | Reference standard | |||
| Disease | No disease | |||
| VivaScope 1000 | Disease | TP = 15 | FP = 0 V | |
| No disease | FN = 0 | TN = 45 | ||
| B. Diagnostic differentiation of benign naevi and malignant melanoma using RCM | ||||
| Sensitivity | Specificity | PPV | NPV | |
| VivaScope 1000 | 97.5% | 99% | 97.5% | 99% |
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Patients were recruited from the dermato-oncology clinic, Medical University of Graz, Graz, Austria, over a period of 10 months. The intended use of the index test was to validate diagnostic confocal examination of melanocytic skin tumours using unselected tumour images | ||||
| Index test(s) | VivaScope 1000 | ||||
| Reference standard and target condition | Histopathological analysis | ||||
| Draw a flow for the primary study | |||||
| xxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Patients were recruited from the dermato-oncology clinic over a period of 10 months. The tumour set in the present study was randomly selected from a consecutively imaged and previously published study set. Overall, 70 melanocytic skin tumours including 50 benign naevi and 20 malignant melanomas (60 patients: 32 male and 28 female) were selected | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| The test set comprised 70 melanocytic skin tumours, including 20 melanomas (all histologically verified) and 50 benign naevi (14 histologically verified) obtained from 60 patients | |||||
| Low risk | High risk | Unclear risk | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| Index test was carried out using confocal laser scanning microscopy. All images obtained in the horizontal plane. From individual tumours, a minimum of 17 and a maximum of 170 images per tumour were obtained. Morphological features of melanocytic skin tumours were selected and assessed according to published studies. Melanocytic cytomorphology and architecture and keratinocyte cell borders were taken into account for diagnostic decisions. All morphological features were defined a priori without reference to the image set of the present study | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear risk | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| Histopathology was performed by well-trained dermato-pathologists, without diagnostic difficulties | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear risk | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
Guitera et al. 200934
| Reviewer: George Osei-Assibey | Study ID: #1057 | ||||
|---|---|---|---|---|---|
| Reference details for all references relating to the trial: | Guitera P, Pellacani G, Longo C, Seidenari S, Avramidis M, Menzies SW, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol 2009;129:131–8 | ||||
| General | |||||
| RCT ( ) | Prospective (✓) | Retrospective ( ) | |||
| Indication for test (diagnosis or margin delineation or both): Diagnosis | |||||
| Intervention(s): VivaScope 1000 and VivaScope 1500 Comparator(s): Dermoscopy |
|||||
| Year(s) study was done: September 2004 to August 2007 | |||||
| Setting (e.g. district general, university hospital): Two referral centres, Sydney Melanoma Diagnostic Centre, University of Sydney, Sydney, NSW, Australia, and the University of Modena, Modena, Italy | |||||
| Source of funding: Study partially supported by a grant from, the Fondazione Cassa di Risparmio di Moderna and Cancer Institute New South Wales, Australia | |||||
| Conflict of interest: NR | |||||
| Participants’ characteristics | |||||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) | ||
| Inclusion criteria: Melanocytic lesions that required excision following standard clinical practices Exclusion criteria: LM and lesions of the soles and palms |
|||||
| Total | Men | Women | |||
| Number enrolled | 326 | 177 | 149 | ||
| Number excluded | Unclear | ||||
| Number withdrawn | Unclear | ||||
| Number lost to follow-up | Unclear | ||||
| Number completed | Unclear | ||||
| Age, mean and range (or data as reported): median, 47 years (range 6–90 years) | |||||
| Lesion- or patient-level data | Lesion ( ) | Patient ( ) | Both (✓) | ||
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: Naevi: compound = 127; dermal = 9; junctional = 42; Spitz n = 25; light coloured = 13; pigmented lesions = 172 Malignant melanoma: median Breslow thickness 0.54 mm (IQR 0 – 0.98 mm); 34 in situ; 86 superficial spreading; three nodular; light coloured, n = 13; pigmented lesions, n = 110 12.2% did not display dermoscopic features of malignant melanomas and 68% of naevi displaying dermoscopic features of malignancy |
|||||
| Types and number of lesion excised | |||||
| Malignant melanoma | 123 | NR | |||
| BCC | NR | NR | |||
| SCC | NR | NR | |||
| LM | Excluded | NR | |||
| Melanocytic naevi | 203 | NR | |||
| Previous tests or assessments: NR | |||||
| Treatment (details of any treatments given): NR | |||||
| Mortality (number of study patients reported dead): NR | |||||
| Index test | |||||
| Equipment (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM):VivaScope 1000 and 1500 Lucid Inc., Henrietta, NY, USA. 830-nm laser source Images correspond to field of view: 500 × 500 µm; lateral resolution, 1.0 µm; axial resolution, 3–5 µm |
|||||
| Image interpretation | |||||
| Assessors (number of assessors): Two image assessors working blind to the dermoscopy and histology results but not the age or site of the lesion. Images from Sydney were judged in Modena and vice versa | |||||
| Experience in using VivaScope or RCM: NR | |||||
| Qualitative (note how positive and negative findings were defined): Six diagnostic features were scored: non-edged papillae and cytological atypia at the dermal–epidermal junction were given a score of 2 each, whereas the presence of round pagetoid cells intraepidermally, widespread pagetoid infiltration in the epidermis, nucleated cells found within the dermal papillae, and cerebriform nests in the dermis all scored 1 each. A score of > 3 corresponded to the threshold for the diagnosis of melanoma | |||||
| Quantitative diagnostic thresholds (e.g. ABCD rule): NR | |||||
| Final confirmation method (e.g. histology): Biopsy | |||||
| Technical failures (number and reasons): NR | |||||
| Comparator test | |||||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): in Sydney, high-resolution, digital, oil-immersion dermoscopic camera (Sentry polytechnics Ltd, Sydney, NSW, Australia); in Modena, hand-held dermascope (Delta 10 Heine, Herrsching, Germany) | |||||
| Image interpretation | |||||
| Assessors (number, expertise, experience in using comparator test): NR | |||||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | |||||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | |||||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-A) | NR | ||||
| Diameter of excisions (e.g. 2 mm) | NR | ||||
| Number of excisions | NR | ||||
| Number of re-excisions | NR | ||||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | Median Breslow thickness of 0.54mm | ||||
| Lymph node involvement or micrometastases | NR | ||||
| Test interpretation: NR | |||||
| Technical failures: NR | |||||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |||
| NR | NR | ||||
| Results | |||||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||||
| Note threshold(s) where appropriate: | Reference standard | ||||
| Disease | No disease | ||||
| Dermoscopy (naevus) | Disease | TP = 138 | FP | ||
| No disease | FN | TN = 65 | |||
| VivaScope 1500 (naevus) | Disease | TP = 65 | FP | ||
| No disease | FN | TN = 138 | |||
| Dermoscopy (malignant melanoma) | Disease | TP = 108 | FP | ||
| No disease | FN | TN = 15 | |||
| VivaScope 1500 (malignant melanoma) | Disease | TP = 112 | FP | ||
| No disease | FN = 11 | TN = 11 | |||
| B. Diagnostic accuracy of dermoscopy and RCM in the biopsied set | |||||
| Diagnosed as benign by dermoscopy | Diagnosed as malignant melanoma by dermoscopy | Diagnosed as benign by RCM | Diagnosed as malignant melanoma by RCM | ||
| Naevus (n = 203) | n (%) | 65 (32%)a | 138 (68%) | 138 (68%)a | 65 (32%) |
| Malignant melanoma (n = 123) | n (%) | 15 (12.2%) | 108 (88%) | 11 (8.9%) | 112 (91%) |
| Odds ratio | 3.4b | NR | 27.5b | NR | |
| 95% CI | 1.8 to 6.3b | NR | 14.5 to 52.3b | NR | |
| C. Misdiagnosis of lesions | |||||
|
|||||
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Lesions (203 naevi and 123 melanomas with a median Breslow thickness of 0.54 mm) recruited from two referral centres in Sydney (Australia) and Modena (Italy) to assess whether or not in vivo RCM enhances secondary evaluation of melanocytic lesions | ||||
| Index test(s) | RCM (VivaScope 1000 and VivaScope 1500, Lucid Inc., Henrietta, NY, USA) | ||||
| Reference standard and target condition | Biopsy of suspected malignant melanoma | ||||
| Draw a flow for the primary study | |||||
| xxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Melanocytic lesions (203 naevi and 123 melanomas with a median Breslow thickness of 0.54 mm) were recruited from two referral centres in Sydney (Australia) and Modena (Italy) | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| No prior testing is reported. Index test used to detect MM, no information about the presentation given | |||||
| Low risk | High risk | Unclear risk | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| RCM images were acquired by means of reflectance confocal laser scanning microscopes (VivaScope 100 and VivaScope 1500, Lucid Inc., Henrietta, NY, USA). A sequence of montage images was acquired for each lesion. Confocal images were scored by experts, retrospectively and blinded to dermoscopy and pathological diagnosis. Six diagnostic features were scored: non-edged papillae and cytological atypia at the dermal–epidermal junction were given a score of 2 each, whereas the presence of round pagetoid cells intraepidermally, widespread pagetoid infiltration in the epidermis, nucleated cells found within the dermal papillae, and cerebriform nests in the dermis all scored 1 each. A score greater than 3 corresponded to the threshold for the diagnosis of melanoma | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear risk | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| NR | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear risk | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
Guitera et al. 201040
| Reviewer: George Osei-Assibey | Study ID: #1465 | ||
|---|---|---|---|
| Reference details for all references relating to the trial: | Guitera P, Pellacani G, Crotty KA, Scolyer RA, Li LX, Bassoli S, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol 2010;130:2080–91 | ||
| General | |||
| RCT ( ) | Prospective ( ) | Retrospective (✓) | |
| Indication for test (diagnosis or margin delineation or both): Diagnosis | |||
| Intervention(s): Dermoscopy + VivaScope 1500 Comparator(s): Dermoscopy |
|||
| Year(s) study was done: 2013 | |||
| Setting (e.g. district general, university hospital): Two tertiary referral melanoma centres (Sydney Melanoma Diagnostic Centre and The Melanoma Institute Australia | |||
| Source of funding: Melanoma Institute Australia, the Melanoma Foundation of the University of Sydney, Cancer Institute New South Wales, and the Australian and New Zealand Melanoma Trials Group | |||
| Conflict of interest: NR | |||
| Participants’ characteristics | |||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) |
Inclusion criteria: Patients with one or more of the following:
|
|||
| Total | Men | Women | |
| Number enrolled | 37 | 11 | 26 |
| Number excluded | NR | NR | NR |
| Number withdrawn | NR | NR | NR |
| Number lost to follow-up | NR | NR | NR |
| Number completed | NR | NR | NR |
| Age, mean and range (or data as reported): mean 71 years (range 47–88 years) | |||
| Lesion- or patient-level data | Lesion (✓) | Patient ( ) | Both ( ) |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: 10 LM lesions were amelanotic, including nine lesions invisible to the naked eye or dermoscopic assessment and one pink lesion. Nine were partially lightly pigmented, 27 were in the cheek, five on the nose, two on the temple, one on the eyebrow, one on the shoulder and one on the lower leg | |||
| Types and number of lesion excised | 37 | ||
| BCC | 0 | ||
| SCC | 0 | ||
| LM | 32 | ||
| LMM | 5 | ||
| Melanocytic naevi | 0 | ||
| Previous tests or assessments: NR | |||
| Treatment (details of any treatments given): NR | |||
| Mortality (number of study patients reported dead): NR | |||
| Index test | |||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 1500; Lucid Inc 830-nm laser beam with a maximum power of 35 mW | |||
| Image interpretation | |||
| Assessors (number of assessors): A team of at least one dermatologist, one plastic surgeon and one radiation oncologist | |||
| Experience in using VivaScope or RCM: All patients were assessed by a MDT (usually at a specialised multidisciplinary LM clinic) including at least one dermatologist | |||
| Qualitative (note how positive and negative findings were defined): When the lesion was clinically visible, the RCM field of view was centred in the middle of the lesion. Confocal images were obtained in four radial directions allowing for anatomical barriers for margin determination until no evidence of LM was seen | |||
| Quantitative diagnostic thresholds (e.g. ABCD rule): The length and width of the visible area were measured retrospectively from the clinical photograph and compared with the length and width of the lesion determined by RCM on the same photograph. The ratio of the RCM and clinical lengths and widths were then calculated. Images were evaluated and the differences were assessed as being greater or less than 5 mm | |||
| Final confirmation method (e.g. histology): Each of the 37 patients had at least one positive site and one negative site biopsied to obtain histopathological correlation. Targeted 2- to 3-mm punch biopsies were performed at the margins of the lesion, in particular when they were considered equivocal by RCM. Pathological assessment of all biopsy specimens included examination of multiple tissue sections (typically 12 sections per 2-mm punch biopsy) | |||
| Technical failures (number and reasons): NR | |||
| Comparator test | |||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): Dermoscope (Nikon D1X digital camera, and with a Nikon F401s camera with a 60-mm lens with dermatophot attachment) | |||
| Image interpretation | |||
| Assessors (number, expertise, experience in using comparator test): NR | |||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | |||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-A) | NR | ||
| Diameter of excisions (e.g. 2 mm) | NR | ||
| Number of excisions | 2 or 3 | ||
| Number of re-excisions | Total number of biopsies per patient ranged from 2 to 12; median, 5; mean, 5 | ||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | Median Breslow thickness for the invasive melanomas was 0.62 mm (range 0.20–7.92 mm) | ||
| Lymph node involvement or micrometastases | NR | ||
| Test interpretation: NR | |||
| Technical failures: NR | |||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |
| ✓ | |||
| Results | |||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||
| Note threshold(s) where appropriate: | Reference standard | ||
| Disease | No disease | ||
| VivaScope 1500 | Disease | TP = 55 | FP = 4 |
| No disease | FN = 5 | TN = 121 | |
| Dermoscopy | Disease | TP = 21 | FP = 3 |
| No disease | FN = 39 | TN = 122 | |
| B. Pathological, RCM and dermoscopic correlations | |||
| Pathological analysis | Dermoscopic evaluation | VivaScope 1500 | |
| Number of sites positive for LM | 60 | 21 (39 FN) | 55 (5 FN) |
| Number of sites negative for LM | 125 | 122 (3 FP) | 121 (4 FP) |
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Patients with suspicious pigmented lesions prospectively recruited from the Division of Dermatology Pigmented Lesion Clinic and the Plastic Surgery Clinics at the Queen Elizabeth II Health Sciences Centre (Canada) to undergo a clinical, dermoscopic and CSLM examination | ||||
| Index test(s) | VivaScope 1000 | ||||
| Reference standard and target condition | Histopathological analysis | ||||
| Draw a flow for the primary study | |||||
| xxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Patients with suspicious pigmented lesions prospectively recruited from the Division of Dermatology Pigmented Lesion Clinic and the Plastic Surgery Clinics at the Queen Elizabeth II Health Sciences Centre (Canada) | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| Male and female patients aged ≥ 16 years and scheduled for biopsy of their lesions because of clinical suspicion of malignancy determined by clinical appearance or a history of change in the lesion after clinical, dermoscopic and vivo CSLM diagnosis | |||||
| Low risk | High risk | Unclear risk | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
The lesion as well as adjacent, uninvolved, clinically normal, and control skin were imaged with VivaScope 1000. A drop of oil was applied to the control/lesional skin, followed by a metal adaptor ring with a tape adhesive. The confocal scanning laser microscope was scanned with a field of view of 450 × 400 µm which was scanned repeatedly over a total area of 13 mm. A single observer with experience in confocal scanning laser microscopy performed the imaging and examined all images in real time. For the diagnosis of melanoma, the architectural and cytological features included:
|
|||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear risk | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| NR | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear risk | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: NR | |||||
Langley et al. 200736
| Reviewer: George Osei-Assibey | Study ID: #1465 | |||||
|---|---|---|---|---|---|---|
| Reference details for all references relating to the trial: | Langley RG, Walsh N, Sutherland AE, Propperova I, Delaney L, Morris SF, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology 2007;215:365–72 | |||||
| General | ||||||
| RCT ( ) | Prospective (✓) | Retrospective ( ) | ||||
| Indication for test (diagnosis or margin delineation or both): Diagnosis | ||||||
| Intervention(s): VivaScope 1000 Comparator(s): Dermoscopy |
||||||
| Year(s) study was done: 2002–5 | ||||||
| Setting (e.g. district general, university hospital): Division of Dermatology Pigmented Lesion Clinic and the Plastic Surgery Clinics at the Queen Elizabeth II Health Sciences Centre, Dalhousie University, Halifax, NS, Canada | ||||||
| Source of funding: Canadian Dermatology Foundation, Nova Scotia Health Research Foundation and the University Internal Medicine Research Foundation | ||||||
| Conflict of interest: NR | ||||||
| Participants’ characteristics | ||||||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) | |||
| Inclusion criteria: Male and female patients aged ≥ 16 years and scheduled for biopsy of their lesions because of clinical suspicion of malignancy determined by clinical appearance or a history of change in the lesion Exclusion criteria: Patients were excluded from the study if their lesions were not amenable to CSLM (i.e. physically inaccessible site), or if they had a previous diagnostic biopsy done on the lesion |
||||||
| Total | Men | Women | ||||
| Number enrolled | 125 | NR | NR | |||
| Number excluded | NR | NR | NR | |||
| Number withdrawn | NR | NR | NR | |||
| Number lost to follow-up | NR | NR | NR | |||
| Number completed | 125 | NR | NR | |||
| Age, mean and range (or data as reported): Mean 44.2 years (range 16–84 years) | ||||||
| Lesion- or patient-level data | Lesion ( ) | Patient ( ) | Both (✓) | |||
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: The study included 125 patients with 125 lesions (88 melanocytic naevi and 37 melanomas) | ||||||
| Types and numbers of lesions excised | 125 | NR | ||||
| BCC | NR | NR | ||||
| SCC | NR | NR | ||||
| LM | NR | NR | ||||
| Melanoma | 37 | NR | ||||
| Melanocytic naevi | 88 | NR | ||||
| Previous tests or assessments: Clinical diagnosis | ||||||
| Treatment (details of any treatments given): NR | ||||||
| Mortality (number of study patients reported dead): NR | ||||||
| Index test | ||||||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 1000, Lucid Inc., Henrietta, NY, USA | ||||||
| Image interpretation | ||||||
| Assessors (number of assessors): Single reviewer | ||||||
| Experience in using VivaScope or RCM: A single observer with experience in confocal scanning laser microscopy performed the imaging and examined all images in real time | ||||||
Qualitative (note how positive and negative findings were defined): For the diagnosis of melanoma, the architectural and cytological features included:
|
||||||
| Quantitative diagnostic thresholds (e.g. ABCD rule): NR | ||||||
| Final confirmation method (e.g. histology): Biopsy | ||||||
| Technical failures (number and reasons): NR | ||||||
| Comparator test | ||||||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): Dermoscope (Nikon D1X digital camera, and with a Nikon F401s camera with a 60-mm lens with dermatophot attachment) | ||||||
| Image interpretation | ||||||
| Assessors (number, expertise, experience in using comparator test): Single reviewer | ||||||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | ||||||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | ||||||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | ||||||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | ||||||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | ||||||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-a) | NR | |||||
| Diameter of excisions (e.g. 2 mm) | NR | |||||
| Number of excisions | 125 | |||||
| Number of re-excisions | NR | |||||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | Median Breslow thickness for the invasive melanomas was 0.62 mm (0.20–7.92 mm) | |||||
| Lymph node involvement or micrometastases | NR | |||||
| Test interpretation: NR | ||||||
| Technical failures: NR | ||||||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | ||||
| NR | NR | |||||
| Results | ||||||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | ||||||
| Note threshold(s) where appropriate: | Reference standard | |||||
| Disease | No disease | |||||
| VivaScope 1000 | Disease | TP = 36.96 | FP = 14.79 | |||
| No disease | FN = 1.03 | TN = 72,23 | ||||
| B. Specificity, sensitivity, PPV, NPV of dermoscopic and RCM | ||||||
| Diagnostic test | Number of benign lesions correctly diagnosed (total 88) | Number of malignant melanomas correctly diagnosed (total 37) | Specificity (%) | Sensitivity (%) | PPV (%) | NPV (%) |
| Dermoscopy | 74 | 33 | 84.1 | 89.2 | 70.2 | 94.9 |
| RCM | 73 | 36 | 83.0 | 97.3 | 70.6 | 98.6 |
| No significant difference (p = 0.3932) was found between the sensitivities or specificities between the two methods RCM had a higher sensitivity than dermoscopy. The difference was 8.11% (95% CI –3.15% to 19.35%; p = 0.1797) Dermoscopy had a higher specificity with a difference of 1.14% (95% CI –7.39% to 9.67%; p = 0.7963) |
||||||
| C. Misdiagnosis of lesions | ||||||
| Diagnosis made using dermoscopy and RCM together agreed on 73 out of 88 total benign naevi, and on 32 out of 37 malignant melanomas There were five melanomas for which RCM and dermoscopy produced differing diagnoses. In these cases, RCM correctly classified four of the melanomas, whereas dermoscopy correctly classified the other melanoma There were no cases where melanoma was misdiagnosed when RCM and dermoscopy were used together There were 15 benign naevi for which the diagnoses made by dermoscopy and RCM differed. Of these, dermoscopy provided the correct diagnosis nine times, and RCM made the correct diagnosis six times There were seven benign naevi for which both diagnoses were incorrect. Two of the melanomas were misdiagnosed by the investigator using dermoscopy, but correctly diagnosed by RCM were amelanotic/hypomelanotic melanomas |
||||||
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Patients with suspicious pigmented lesions prospectively recruited from the Division of Dermatology Pigmented Lesion Clinic and the Plastic Surgery Clinics at the Queen Elizabeth II Health Sciences Centre (Canada) to undergo a clinical, dermoscopic and confocal scanning laser microscopic examination | ||||
| Index test(s) | VivaScope 1000 | ||||
| Reference standard and target condition | Histopathological analysis | ||||
| Draw a flow for the primary study | |||||
| xxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Patients with suspicious pigmented lesions prospectively recruited from the Division of Dermatology Pigmented Lesion Clinic and the Plastic Surgery Clinics at the Queen Elizabeth II Health Sciences Centre (Canada) | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| Male and female patients aged ≥ 16 years and scheduled for biopsy of their lesions because of clinical suspicion of malignancy determined by clinical appearance or a history of change in the lesion after clinical, dermoscopic and in vivo confocal scanning laser microscopic diagnosis | |||||
| Low risk | High risk | Unclear risk | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
The lesion as well as adjacent, uninvolved, clinically normal, and control skin were imaged with VivaScope 1000. A drop of oil was applied to the control/lesional skin, followed by a metal adaptor ring with a tape adhesive. The confocal scanning laser microscope was scanned with a field of view of 450 × 400 µm which was scanned repeatedly over a total area of 13 mm. A single observer with experience in confocal scanning laser microscopy performed the imaging and examined all images in real time. For the diagnosis of melanoma, the architectural and cytological features included:
For the diagnosis of naevi, the architectural and cytological features included:
|
|||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear risk | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| NR | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear risk | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: NR | |||||
Pan et al. 201237
| Reviewer: George Osei-Assibey | Study ID: #1903 | ||
|---|---|---|---|
| Reference details for all references relating to the trial: | Pan ZY, Lin JR, Cheng TT, Wu JQ, Wu WY, Pan ZY, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg 2012;38:1945–50 | ||
| GENERAL | |||
| RCT ( ) | Prospective (✓) | Retrospective ( ) | |
| Indication for test (diagnosis or margin delineation or both): margin delineation | |||
| Intervention(s): VivaScope 1500 Comparator(s): NR |
|||
| Year(s) study was done: NR | |||
| Setting (e.g. district general, university hospital): dermatology department | |||
| Source of funding: NR | |||
| Conflict of interest: None | |||
| Participants’ characteristics | |||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) |
| Inclusion criteria: patients with lesions clinically suggestive of BCC Exclusion criteria: NR |
|||
| Total | Men | Women | |
| Number enrolled | 10 | NR | NR |
| Number excluded | 0 | NR | NR |
| Number withdrawn | 0 | NR | NR |
| Number lost to follow-up | 0 | NR | NR |
| Number completed | 10 | NR | NR |
| Age, mean and range (or data as reported): NR | |||
| Lesion- or patient-level data | Lesion (✓) | Patient ( ) | Both ( ) |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: | |||
| Types and number of lesion excised | 13 | ||
| BCC | 13 | ||
| SCC | 0 | ||
| LM | 0 | ||
| Others | 0 | ||
| Previous tests or assessments: NR | |||
| Treatment (details of any treatments given): NR | |||
| Mortality (number of study patients reported dead): NR | |||
| Index test | |||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 1500 (Lucid Technologies, Henrietta, NY, USA), which uses a diode laser with a wavelength of 830 nm and a power < 15 mW | |||
| Image interpretation | |||
| Assessors (number of assessors): NR | |||
| Experience in using VivaScope or RCM: NR | |||
| Qualitative (note how positive and negative findings were defined): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD rule): NR | |||
| Final confirmation method (e.g. histology): Histopathology (surgical excision) | |||
| Technical failures (number and reasons): NR | |||
| Comparator test | |||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): NR | |||
| Image interpretation | |||
| Assessors (number, expertise, experience in using comparator test): NR | |||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | |||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-a) | NR | ||
| Diameter of excisions (e.g. 2 mm) | NR | ||
| Number of excisions | 13 | ||
| Number of re-excisions | NR | ||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | NR | ||
| Lymph node involvement or micrometastases | NR | ||
| Test interpretation: NR | |||
| Technical failures: NR | |||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |
| NR | NR | ||
| Results | |||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||
| Note threshold(s) where appropriate: | Reference standard | ||
| Disease | No disease | ||
| VivaScope 1500 | Disease | NR | NR |
| No disease | NR | NR | |
| B. Histological confirmation of margins correctly delineated | |||
| n (%) of cases/margins correctly delineated | |||
| VivaScope 1500 | 7 (70%) | ||
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Ten patients with lesions clinically suggestive of BCC and then biopsy proven were recruited randomly from the dermatology department for the margin study | ||||
| Index test(s) | VivaScope 1500 | ||||
| Reference standard and target condition | Histopathological analysis | ||||
| Draw a flow for the primary study | |||||
| xxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Patients with lesions clinically suggestive of BCC and then biopsy proven were recruited randomly from the dermatology department for the margin study. Thirteen patients with biopsy-proven BCC were recruited for surgical excision | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| Ten patients with lesions clinically suggestive of BCC and then biopsy proven were recruited randomly from the dermatology department to investigate the feasibility of RCM in defining the margins of BCC before surgery | |||||
| Low risk | High risk | Unclear risk | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| Confocal imaging was performed using VivaScope 1500 (Lucid Technologies, Henrietta, NY, USA), which uses a diode laser with a wavelength of 830 nm and power of < 15 mW. This system provides high-resolution images (horizontal resolution, 1.0 µm; vertical optical section thickness, 3.0 µm) from a depth of 0 to 250 µm in vivo (from the epidermis to the papillary dermis). Blocks of 2- by 2-mm mosaic image mode were used to detect the margins | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of results of reference? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear risk | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| Biopsy specimens were routinely processed with formalin fixation and paraffin embedding followed by vertical sectioning and haematoxylin and eosin staining. Slides were also examined for findings that appeared to correlate best with RCM structures under analysis | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear risk | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | |||||
| Low risk | High risk | Unclear risk | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
Pellacani et al. 200738
| Reviewer: George Osei-Assibey | Study ID: #1952 | ||
|---|---|---|---|
| Reference details for all references relating to the trial: | Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol 2007;127:2759–65 | ||
| General | |||
| RCT ( ) | Prospective (✓) | Retrospective ( ) | |
| Indication for test (diagnosis or margin delineation or both): diagnosis | |||
| Intervention(s): Dermoscopy + VivaScope 1000 or VivaScope 1500 Comparator(s): NR |
|||
| Year(s) study was done: NR | |||
| Setting (e.g. district general, university hospital): Sydney Melanoma Diagnostic Centre of the Royal Prince Alfred Hospital, University of Sydney, Sydney, NSW, Australia; and the Department of Dermatology of the University of Modena and Reggio Emilia, Italy | |||
| Source of funding: Partially supported by grants from the Fondazione Cassa di Risparmio di Modena, Modena, Italy, the CNR (Centro Nazionale per la Ricerca), Italy, and the Cancer Institute New South Wales, Sydney, NSW, Australia | |||
| Conflict of interest: Authors have no conflict of interest | |||
| Participants’ characteristics | |||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) |
| Inclusion criteria: Patients with melanoma and equivocal melanocytic lesions Exclusion criteria: NR |
|||
| Total | Men | Women | |
| Number enrolled | 332 | 174 | 158 |
| Number excluded | NR | NR | NR |
| Number withdrawn | NR | NR | NR |
| Number lost to follow-up | NR | NR | NR |
| Number completed | NR | NR | NR |
| Age, mean and range (or data as reported): Median 47.7 years (interquartile range: 35.9–60.4) | |||
| Lesion- or patient-level data | Lesion (✓) | Patient ( ) | Both ( ) |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: NR | |||
| Types and number of lesion excised | 351 | ||
| Malignant melanoma | 351 | NR | |
| Melanoma | 136 | NR | |
| Melanocytic naevi | 215 | NR | |
| LMM | NR | NR | |
| Previous tests or assessments: clinical and dermoscopic assessments | |||
| Treatment (details of any treatments given): NR | |||
| Mortality (number of study patients reported dead): NR | |||
| Index test | |||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 1000 and VivaScope 1500, Lucid Inc., Henrietta, New York, NY, USA | |||
| Image interpretation | |||
| Assessors (number of assessors): 2 | |||
| Experience in using VivaScope or RCM: Two expert observers, blinded from anamnestic information, dermoscopy and clinical aspects, but not for the location and the patient’s age | |||
| Qualitative (note how positive and negative findings were defined): Morphological features of RCM images were evaluated for the presence/absence (binary non-parametric data), with the exception of the number and size of pagetoid cells that were dichotomised for statistics considering the presence of more than three pagetoid cells in five 0.5- × 0.5-mm images and pagetoid cells larger than 20 µm, respectively | |||
| Quantitative diagnostic thresholds (e.g. ABCD rule): The total RCM score was also calculated for each lesion evaluating the presence of two major features (non-edged papillae and cellular atypia at dermal–epidermal junction), each scored 2 points, and four minor ones (roundish pagetoid cells, widespread pagetoid infiltration, cerebriform nests and nucleated cells within the papilla), each scored 1 point, and compared with new models obtained by statistical analysis | |||
| Final confirmation method (e.g. histology): Biopsy | |||
| Technical failures (number and reasons): NR | |||
| Comparator test | |||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): NR | |||
| Image interpretation | |||
| Assessors (number, expertise, experience in using comparator test): NR | |||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | |||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-a) | NR | ||
| Diameter of excisions (e.g. 2 mm) | NR | ||
| Number of excisions | NR | ||
| Number of re-excisions | NR | ||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | NR | ||
| Lymph node involvement or micrometastases | NR | ||
| Test interpretation: NR | |||
| Technical failures: NR | |||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |
| NR | NR | ||
| Results | |||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||
| Note threshold(s) where appropriate: | Reference standard | ||
| Disease | No disease | ||
| Test | Disease | TP | FP |
| No disease | FN | TN | |
| B. Sensitivity and specificity for RCM score with different thresholds | |||
| RCM score threshold | Sensitivity | Specificity | |
| ≥ 1 | 96.3% | 49.3% | |
| ≥ 2 | 96.3% | 52.1% | |
| ≥ 3 | 91.9% | 69.3% | |
| ≥ 4 | 79.4% | 77.2% | |
| ≥ 5 | 66.9% | 82.3% | |
| ≥ 6 | 49.3% | 91.6% | |
| ≥ 7 | 23.5% | 98.1% | |
| ≥ 8 | 2.2% | 100% | |
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Patients with malignant melanomas recruited from the Sydney Melanoma Diagnostic Centre of the Royal Prince Alfred Hospital, University of Sydney (Sydney, NSW, Australia) and the Department of Dermatology of the University of Modena and Reggio Emilia (Italy) were evaluated for 37 confocal features | ||||
| Index test(s) | VivaScope 1000 and VivaScope 1500 | ||||
| Reference standard and target condition | Histopathology | ||||
| Draw a flow for the primary study | |||||
| xxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| A total of 351 melanocytic lesions from 332 patients with 351 melanomas, recorded by means of RCM at the Sydney Melanoma Diagnostic Centre (156 lesions) and at the Department of Dermatology of the University of Modena and Reggio Emilia (195 lesions) were included | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| 351 melanocytic lesions from 332 patients (158 female and 174 males; median age of 47.7 years, interquartile range 35.9–60.4 years), of which 136 were melanomas, 215 were melanocytic naevi (49 junctional, 132 compound, nine intradermal and 25 Spitz naevi), recorded by means of RCM. The lesions were located on the head/neck region in 15 cases, on the abdomen and chest in 68, on the back in 135, on the upper limbs in 50, and on the lower limbs in 83, without significant differences between the site distribution of melanomas and naevi | |||||
| Low risk | High risk | Unclear risk | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| RCM images were acquired by means of near-infrared reflectance confocal laser scanning microscopes (VivaScope 1000 and VivaScope 1500. A sequence of montage images (‘block’ images) were acquired for each lesion at the level of the dermo-epidermal junction to explore a 4- × 4-mm field of view per lesion. For large lesions, not completely comprised within the field of view, the device was centred on the lesion or on the portion with the most suspicious dermoscopic features, according to pattern analysis and standard second-step melanoma diagnostic methods. Confocal sections, beginning at the stratum corneum and ending inside the papillary dermis, were recorded at areas of interest. More than 100 capture images per lesion were recorded | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear risk | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear risk | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
Pellacani et al. 201442
| Reviewer: George Osei-Assibey | Study ID: Obtained from updated search | ||||
|---|---|---|---|---|---|
| Reference details for all references relating to the trial: | Pellacani G, Pepe P, Casari A, Longo C. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol 2014;171:1044–51 | ||||
| General | |||||
| RCT ( ) | Prospective (✓) | Retrospective ( ) | |||
| Indication for test (diagnosis or margin delineation or both): Diagnosis | |||||
| Intervention(s): Dermoscopy + VivaScope 1500 (RCM consultation) Comparator(s): Dermoscopy (RCM documentation) |
|||||
| Year(s) study was done: January 2010–December 2010 | |||||
| Setting (e.g. district general, university hospital): Melanoma-Pigmented Lesion Outpatient Clinic of the Dermatology Department, University of Modena and Reggio Emilia, Italy | |||||
| Source of funding: NR | |||||
| Conflict of interest: NR | |||||
| Participants’ characteristics | |||||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) | ||
| Inclusion criteria: Patients with the request of a mole check and/or with a suspect of melanoma Exclusion criteria: Clinical and/or dermatoscopic clear-cut epithelial tumours were not enrolled |
|||||
| Total | Men | Women | |||
| Number enrolled | 1005 | 443 | 562 | ||
| Number excluded | NR | NR | NR | ||
| Number withdrawn | NR | NR | NR | ||
| Number lost to follow-up | NR | NR | NR | ||
| Number completed | NR | NR | NR | ||
| Age, mean and range (or data as reported): NR | |||||
| Lesion- or patient-level data | Lesion ( ) | Patient ( ) | Both (✓) | ||
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: Patients referred for RCM consultation had a higher number of naevi (> 100 naevi; 19%) and of atypical naevi (> 5; 15%) than patients referred for RCM documentation and patients without RCM referral (p < 0.0001). Personal and/or familial history of melanoma was recorded in approximately 8% of patients | |||||
| Types and number of lesion excised | 292 | NR | |||
| BCC | 38 | NR | |||
| Melanoma | 29 | NR | |||
| LM | NR | NR | |||
| Spitz naevi | 13 | NR | |||
| Clark’s naevi | 192 | NR | |||
| Other benign lesions | 9 | ||||
| Previous tests or assessments: Clinical dermoscopic examinations | |||||
| Treatment (details of any treatments given): NR | |||||
| Mortality (number of study patients reported dead): NR | |||||
| Index test | |||||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 1500, MAVIG GmbH, Munich, Germany), which uses an 830-nm laser beam with a maximum power of 20 mW | |||||
| Image interpretation | |||||
| Assessors (number of assessors): One | |||||
| Experience in using VivaScope or RCM: confocal reader | |||||
| Qualitative (note how positive and negative findings were defined): NR | |||||
| Quantitative diagnostic thresholds (e.g. ABCD rule): NR | |||||
| Final confirmation method (e.g. histology): biopsy | |||||
| Technical failures (number and reasons): NR | |||||
| Comparator test | |||||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): Dermoscopic examinations were conducted using the Dermlite HR (3Gen® LLC, San Juan Capistrano, CA, USA) | |||||
| Image interpretation | |||||
| Assessors (number, expertise, experience in using comparator test): NR | |||||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | |||||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | |||||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-A) | NR | ||||
| Diameter of excisions (e.g. 2 mm) | NR | ||||
| Number of excisions | 292 | ||||
| Number of re-excisions | NR | ||||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | NR | ||||
| Lymph node involvement or micrometastases | NR | ||||
| Test interpretation: NR | |||||
| Technical failures: NR | |||||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |||
| NR | NR | ||||
| Results | |||||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||||
| Note threshold(s) where appropriate: | Reference standard | ||||
| Disease | No disease | ||||
| Test | Disease | TP | FP | ||
| No disease | FN | TN | |||
| B. Number (%) of lesions histologically proven | |||||
| Diagnosis | RCM referral | ||||
| Dermoscopy (RCM documentation) | RCM proposed outcome | Dermoscopy + VivaScope 1500 (RCM consultation) | RCM proposed outcome | Total | |
| Histopathologically proven cases | |||||
| Melanoma | 23 (79.3%) | Excised: 23; Follow-up: 0 | 6 (20.7%) | Excised: 6; Follow-up: 0 | 29 |
| BCC | 19 (50%) | Excised: 19; Follow-up: 0 | 19 (50%) | Excised: 19; Follow-up: 0 | 38 |
| Clark’s naevi | 121 (63%) | Excised: 57; Follow-up: 64 | 71 (37%) | Excised: 46; Follow-up: 25 | 192 |
| Spitz naevi | 8 (61.5%) | Excised: 6; Follow-up: 2 | 5 (38.5%) | Excised: 3; Follow-up: 2 | 13 |
| Other benign lesions | 12 (60%) | Excised: 5; Follow-up: 7 | 8 (40%) | Excised: 7; Follow-up: 1 | 19 |
| RCM Documentation lesions: Histopathology results identified 23 melanomas; 19 BCC; 121 Clark’s naevi, 11 Spitz naevi; and 12 other benign lesions In all melanoma and BCCs identified at histology, RCM had recommended excision. In 82.6% of the melanoma (19/23) and in 94.7% of the BCC (18/19), RCM had proposed the same diagnosis as those confirmed at histopathology RCM consultation lesions: Excision was recommended at RCM in all six cases of melanoma, in all 19 cases of BCC, and in 56 benign lesions (46 Clark’s naevi, three Spitz naevi, and seven benign non-melanocytic lesions) |
|||||
| C. Confocal–histopathology concordance | |||||
|
|||||
| D. NNE | |||||
| NNE | Benign : melanoma | ||||
| NNE after RCM examination | 6.8 | 197 : 29 | |||
| NNE after follow-up (end of the study) | 7.7 | 225 : 29 | |||
| Estimated NNE values: | |||||
| (a) Without RCM (overall) – documentation group – consultation group | 14.6 (p < 0.05 vs. actual value) 6.1 47.2 (p < 0.05 vs. actual value) | 424 : 29 – 141 : 23 – 283 : 6 | |||
| (b) Using RCM in all cases (before follow-up) – documentation group – consultation group | 4.3 (p < 0.05 vs. actual value) – 2.9 (p < 0.05 vs. actual value) – 47.2 (significant vs. estimated NNE without RCM) | 124 : 29 – 68 : 23 – 56 : 6 | |||
| Immediate NNE was 6.8, and NNE after the follow-up period was 7.7. In the first hypothesis where RCM evaluations were not considered, the estimated NNE was 14.6 (6.1 for the RCM documentation subgroup, and 47.2 for RCM consultation subgroup; p < 0.05). In the second hypothesis, considering RCM evaluations (in both RCM documentation subgroup and lesions changed after follow-up) the NNE was 4.3 (2.9 for RCM documentation subgroup, and 9.3 for RCM consultation subgroup; p < 0.05) | |||||
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Patients referred to a single Melanoma Clinic were consecutively enrolled | ||||
| Index test(s) | VivaScope 1500 | ||||
| Reference standard and target condition | Histopathology | ||||
| Draw a flow for the primary study | |||||
| xxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Patients with the request of a mole check and/or with a suspect of melanoma were included but patients with clinical and/or dermoscopic clear-cut epithelial tumours were excluded | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| Patients had a request of a mole check and/or with a suspect of melanoma. The purpose of the index test was to prospectively determine its potential impact when implemented in a routine melanoma diagnosis workflow | |||||
| Low risk | High risk | Unclear risk | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| Confocal images were acquired using a near-infrared RCM (VivaScope 1500, MAVIG GmbH, Munich, Germany), which uses an 830-nm laser beam, with a maximum power of 20 mW | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear risk | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| NR | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear risk | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
Rao et al. 201339
| Reviewer: George Osei-Assibey | Study ID: #2108 | ||
|---|---|---|---|
| Reference details for all references relating to the trial: | Rao BK, Mateus R, Wassef C, Pellacani G, Rao BK, Mateus R, et al. In vivo confocal microscopy in clinical practice: comparison of bedside diagnostic accuracy of a trained physician and distant diagnosis of an expert reader. J Am Acad Dermatol 2013;69:295–300 | ||
| General | |||
| RCT ( ) | Prospective (✓) | Retrospective ( ) | |
| Indication for test (diagnosis or margin delineation or both): Diagnosis | |||
| Intervention(s): Dermoscopy + VivaScope 1500 Comparator(s): NR |
|||
| Year(s) study was done: June 2010–September 2011 | |||
| Setting (e.g. district general, university hospital): Teleconsultation | |||
| Source of funding: NR | |||
| Conflict of interest: Drs Pellacani and Rao are both consultants for CaliberID. Dr Mateus and Ms Wassef have no conflicts of interest to declare | |||
| Participants’ characteristics | |||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) |
| Inclusion criteria: patients with lesions that had been selected for removal for either cosmetic or medical reasons Exclusion criteria: NR |
|||
| Total | Men | Women | |
| Number enrolled | 340 | NR | NR |
| Number excluded | 6 | NR | NR |
| Number withdrawn | NR | NR | NR |
| Number lost to follow-up | 17 | NR | NR |
| Number completed | 334 | NR | NR |
| Age, mean and range (or data as reported): NR | |||
| Lesion- or patient-level data | Lesion (✓) | Patient ( ) | Both ( ) |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: Images captured at the superficial spinous/granular layer, dermo–epidemermal junction, papillary dermis and more reticular dermis The lesions were on the trunk (n = 135), face (n = 90), upper limbs (n = 70) and lower limbs (n = 39) |
|||
| Types and number of lesion excised | 334 | ||
| Melanoma | 9 | NR | |
| BCC | 27 | NR | |
| SCC | 43 | NR | |
| LM | NR | NR | |
| LMM | NR | NR | |
| Melanocytic naevi | 182 | ||
| Actinic keratosis | 26 | ||
| Seborrhoeic keratosis (and solar lentigo) | 24 | ||
| Others | 23 | ||
| Previous tests or assessments: NR | |||
| Treatment (details of any treatments given): NR | |||
| Mortality (number of study patients reported dead): NR | |||
| Index test | |||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): VivaScope 1500, CaliberID, Rochester, NY, USA | |||
| Image interpretation | |||
| Assessors (number of assessors): Two | |||
| Experience in using VivaScope or RCM: Images were reviewed by two confocal readers, one in New York, NY, USA (reader 1), and the other in Modena, Italy (reader 2). Reader 1 at the start of the study had less experience reading RCM images than reader 2, who had over 9 years of experience with RCM | |||
| Qualitative (note how positive and negative findings were defined): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD rule): Two viva stacks from the stratum corneum to the stratum corneum to the dermis were taken | |||
| Final confirmation method (e.g. histology): Histopathological analysis, method not reported | |||
| Technical failures (number and reasons): NR | |||
| Comparator test | |||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): NR | |||
| Image interpretation | |||
| Assessors (number, expertise, experience in using comparator test): NR | |||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | |||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-A) | NR | ||
| Diameter of excisions (e.g. 2 mm) | NR | ||
| Number of excisions | 334 | ||
| Number of re-excisions | NR | ||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | NR | ||
| Lymph node involvement or micrometastases | NR | ||
| Test interpretation: NR | |||
| Technical failures: NR | |||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |
| NR | NR | ||
| Results | |||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||
| Note threshold(s) where appropriate: | Reference standard | ||
| Disease | No disease | ||
| VivaScope 1500 | Disease | TP = 79 | FP = 60 |
| No disease | FN = 20 | TN = 175 | |
| B. Comparison of RCM diagnosis by a trained physician vs. distant diagnosis by an expert | |||
| Reader 1 (bedside-trained physician) evaluated 317 of 334 cases (94.9%) | Reader 2 (distant expert) evaluated 323 of 334 cases (96.7%) | Combined sensitivity and specificity | |
| Sensitivity (%) | 93.1 | 97.4 | 98.6 |
| Specificity (%) | 64.1 | 80.5 | 44 |
| Diagnostic performance of the readers (per cent of lesions correctly diagnosed) For reader 1, RCM diagnosis was in agreement with histopathological diagnosis in 83.2% of naevi, 58.3% of seborrhoeic keratosis, and 17.3%% of other benign lesion, 66.7% of melanomas, 74.1% of BCC and 37.2% of SCC RCM diagnosis of reader 2 was the same as the histopathological diagnosis in 83% of naevi, 66.7% of seborrhoeic keratosis, 21.7% of other benign lesions, 88.9% of melanomas, 51.9% of BCC and 72.1% of SCC |
|||
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | The study sought to assess RCM diagnostic accuracy in a support teleconsultation setting in lesions had been selected for removal for either cosmetic or medical reasons | ||||
| Index test(s) | VivaScope 1500 | ||||
| Reference standard and target condition | Histopathological analysis | ||||
| Draw a flow for the primary study | |||||
| xxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
| Patients selected were from the USA and had lesions that had been selected for removal for either cosmetic or medical reasons. A total of 340 lesions were imaged between June 2010 and September 2011. Six cases were excluded from the study because of insufficient information | |||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| The intended test was to assess its diagnostic accuracy in a support teleconsultation | |||||
| Low risk | High risk | Unclear risk | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| Lesions were imaged using VivaScope 1500. An imaging protocol allowed for the capture of one dermoscopic image and four RCM images for each lesion. Series of consecutive high-resolution images starting from the stratum corneum to the dermis were taken. The images were reviewed by two confocal readers. Diagnosis was based on the dermoscopic image and confocal microscopy evaluation before excision | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear risk | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| NR | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear risk | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear risk | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
Stanganelli et al. 201545
| Reviewer: George Osei-Assibey | Study ID: Handsearched | ||
|---|---|---|---|
| Reference details for all references relating to the trial: | Stanganelli I, Longo C, Mazzoni L, Magi S, Medri M, Lanzanova G, et al. Integration of reflectance confocal microscopy in sequential dermoscopy follow-up improves melanoma detection accuracy. Br J Dermatol 2015;172:365–71 | ||
| General | |||
| RCT ( ) | Prospective ( ) | Retrospective (✓) | |
| Indication for test (diagnosis or margin delineation or both): Diagnosis | |||
| Intervention(s): Dermoscopy + VivaScope 1500 Comparator(s): |
|||
| Year(s) study was done: July 2010–12 | |||
| Setting (e.g. district general, university hospital): Skin Cancer Unit at the Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST IRCCS), in Ravenna/Forli and Meldola, Italy | |||
| Source of funding: No external funding | |||
| Conflict of interest: None declared | |||
| Participants’ characteristics | |||
| Consecutive sample | Yes (✓) | No ( ) | Unclear ( ) |
| Inclusion criteria: (i) lesion excised after change at the follow-up visit; (ii) availability of baseline and follow-up dermoscopic images; (iii) availability of a complete standard set of RCM images;21,22 and (iv) availability of histopathology report and slides Exclusion criteria: NR |
|||
| Total | Men | Women | |
| Number enrolled | 70 | 38 | 32 |
| Number excluded | NR | NR | NR |
| Number withdrawn | NR | NR | NR |
| Number lost to follow-up | NR | NR | NR |
| Number completed | 70 | NR | NR |
| Age, mean and range (or data as reported): NR | |||
| Lesion- or patient-level data | Lesion ( ) | Patient ( ) | Both (✓) |
| Lesion characteristics if known at the time VivaScope or RCM was performed and duration of symptoms: The most common skin phototype was type III (n = 50), followed by II (n = 18), I (n = 1) and IV (n = 1). Twenty-six patients (37%) had a history of melanoma. Regarding total naevus counts, 27 patients (39%) had more than 50 melanocytic naevi, 33 patients (47%) had 10–50 naevi and 10 patients (14%) had fewer than 10 naevi | |||
| Types and number of lesion excised | 70 | ||
| Melanoma | 12 | ||
| Benign | 58 | ||
| LM | NR | ||
| LMM | NR | ||
| Melanocytic naevi | NR | ||
| Previous tests or assessments: NR | |||
| Treatment (details of any treatments given): NR | |||
| Mortality (number of study patients reported dead): NR | |||
| Index test | |||
| Equipment: (note machine name and manufacturer of VivaScope 1500 or VivaScope 3000 or RCM): RCM with VivaScope 1500 (Lucid Inc., MAVIG GmbH, Munich, Germany) using an 830-nm laser at a maximum power of 20 mW Dermoscopy with Leica Wild M-650 stereo microscope with a Sony 3CCD DXC-930P colour video camera connected to a workstation with DERMOX application software (Tesi Imaging, Milan, Italy) |
|||
| Image interpretation | |||
| Assessors (number of assessors): Three | |||
| Experience in using VivaScope or RCM: RCM images were evaluated jointly by three expert dermatologists who had no knowledge of the clinical, dermoscopic or histopathology information, and reached a consensus or majority opinion for feature evaluation and diagnostic classification | |||
Qualitative (note how positive and negative findings were defined):
|
|||
Quantitative diagnostic thresholds (e.g. ABCD rule):
|
|||
| Final confirmation method (e.g. histology): Histopathology | |||
| Technical failures (number and reasons): NR | |||
| Comparator test | |||
| Equipment: comparator (e.g. dermoscopy; note machine name and manufacturer and the specification): NR | |||
| Image interpretation | |||
| Assessors (number, expertise, experience in using comparator test): NR | |||
| Qualitative (note how positive and negative findings were qualitatively defined): NR | |||
| Quantitative diagnostic thresholds (e.g. ABCD system): NR | |||
| Technical failures (number and reasons, e.g. lesion site inaccessible with equipment): NR | |||
| Reference standard [test: biopsy (used for confirmation and staging) note any details] | |||
| Method of preparation of the specimen (immunohistochemistry – antibodies; S100, HMB 45 and melan-A) | NR | ||
| Diameter of excisions (e.g. 2 mm) | NR | ||
| Number of excisions | 70 | ||
| Number of re-excisions | NR | ||
| Tumour staging: thickness of the melanoma (Breslow thickness, Clark level, TNM system) | Median 0.4 mm (range 0.2–1.0 mm) | ||
| Lymph node involvement or micrometastases | NR | ||
| Test interpretation: NR | |||
| Technical failures: NR | |||
| Interval between index test and reference standard (excision of the histological specimen): | < 6 weeks | > 6 weeks | |
| NR | NR | ||
| Results | |||
| A. Test accuracy: label all tables as appropriate (add more tables as necessary) | |||
| Note threshold(s) where appropriate: | Reference standard | ||
| Disease | No disease | ||
| VivaScope 1500 | Disease | TP = 11 | FP = 19 |
| No disease | FN = 1 | TN = 39 | |
Quality assessment (QUADAS-2)
| Patients (setting, intended use of index test, presentation, prior testing) | Data on 70 patients with 70 lesions obtained from a database at the Skin Cancer Unit at the Instituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST IRCCS), in Ravenna/Forli and Meldola, Italy | ||||
| Index test(s) | VivaScope 1500 | ||||
| Reference standard and target condition | Histopathology | ||||
| Draw a flow for the primary study | |||||
| xxxxxxxx | |||||
| Domain 1: patient selection | A. Risk of bias | Describe methods of patient selection | |||
Inclusion criteria included:
|
|||||
| Yes | No | Unclear | |||
| Was a consecutive or random sample of patients enrolled? | ✓ | ||||
| Was a case–control design avoided? | ✓ | ||||
| Did the study avoid inappropriate exclusions? | ✓ | ||||
| Low risk | High risk | Unclear | |||
| Could the selection of patients have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Describe included patients (prior testing, presentation, intended use of index test and setting) | ||||
| The population included 32 women (46%), mean age 39 years, and 38 men (54%), mean age 40 years. The index test was conducted to determine whether or not combining it with sequential dermoscopy imaging can improve melanoma detection and reduce the burden of unnecessary excisions | |||||
| Low risk | High risk | Unclear | |||
| Is there concern that the included patients do not match the review question? | ✓ | ||||
| Domain 2: index test(s) | A. Risk of bias | Describe the index test and how it was conducted and interpreted | |||
| RCM images of 0.5 × 0.5 mm were acquired with a lateral resolution of 1 µm and an axial resolution of 3–5 µm and assembled into composite images that covered 4–8 mm2 mosaics. Images were evaluated jointly by three expert dermatologists who had no knowledge of the clinical, dermoscopic or histopathology information, and reached a consensus or majority opinion for feature evaluation and diagnostic classification. Each lesion was classified considering the main melanoma features | |||||
| Yes | No | Unclear | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | ✓ | ||||
| If a threshold was used, was it prespecified? | |||||
| NR | |||||
| Low risk | High risk | Unclear | |||
| Could the conduct or interpretation of the index test have introduced bias? | ✓ | ||||
| B. Concerns regarding applicability | Low risk | High risk | Unclear | ||
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | ✓ | ||||
| Domain 3: reference standard | A. Risk of bias | Describe the reference standard and how it was conducted and interpreted | |||
| NR | |||||
| Yes | No | Unclear | |||
| Is the reference standard likely to correctly classify the target condition? | ✓ | ||||
| B. Concerns regarding applicability | Were the reference standard results interpreted without knowledge of the results of the index test? | ✓ | |||
| Low risk | High risk | Unclear | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | ✓ | ||||
| Is there concern that the target condition as defined by the reference standard does not match the review question? | ✓ | ||||
| Domain 4: flow and timing | A. Risk of bias | Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram) | |||
| NR | |||||
| Describe the time interval and any interventions between index test(s) and reference standard | |||||
| NR | |||||
| Yes | No | Unclear | |||
| Was there an appropriate interval between index test(s) and reference standard? | ✓ | ||||
| Did all patients receive a reference standard? | ✓ | ||||
| Did patients receive the same reference standard? | ✓ | ||||
| Were all patients included in the analysis? | ✓ | ||||
| Low risk | High risk | Unclear | |||
| Could the patient flow have introduced bias? | ✓ | ||||
| Notes/comments: | |||||
List of abbreviations
- BAD
- British Association of Dermatology
- BCC
- basal cell carcinoma
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- EAG
- Evidence Assessment Group
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions-3 levels
- EQ-5D-5L
- European Quality of Life-5 Dimensions-5 levels
- FN
- false negative
- FP
- false positive
- GP
- general practitioner
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- INMB
- incremental net monetary benefit
- LM
- lentigo maligna
- LMM
- lentigo maligna melanoma
- LSMDT
- Local Hospital Skin Cancer Multidisciplinary Team
- MDT
- multidisciplinary team
- NICE
- National Institute for Health and Care Excellence
- NNE
- number needed to excise
- NPV
- negative predictive value
- PPV
- positive predictive value
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QUADAS
- quality assessment of diagnostic accuracy studies
- RCM
- reflectance confocal microscopy
- RCT
- randomised controlled trial
- SCC
- squamous cell carcinoma
- SD
- standard deviation
- SG
- standard gamble
- SLNB
- sentinel lymph node biopsy
- SSMDT
- specialist skin cancer multidisciplinary team
- TN
- true negative
- TP
- true positive
- TTO
- time trade-off
- VAS
- visual analogue scale
- WTP
- willingness to pay