Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/109/02. The contractual start date was in April 2011. The draft report began editorial review in September 2015 and was accepted for publication in April 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Neil Poulter receives grants and personal fees from Servier, outside the submitted work. Janet Powell receives grants from the National Institute for Health Research and the Camelia Botnar Research Foundation and personal fees from the American Heart Association, outside the submitted work. Colin Bicknell receives personal fees from Hansen Medical, Medtronic and Bolton Medical, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Kiru et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and rationale
Introduction
An abdominal aortic aneurysm (AAA) is a ballooning of the infrarenal aorta to either 1.5 times its normal anteroposterior (AP) diameter or to an absolute value of ≥ 3 cm. 1 Small AAAs can be defined as those between 3.0 cm and 5.4 cm in diameter. These small AAAs have a low risk of rupture, whereas the operation to repair them is fatal in approximately 2–3% of patients. 2 Small AAAs are generally managed by optimising cardiovascular health and placing the patient on a surveillance programme to measure the AAA diameter at regular intervals. Once AAAs reach 5.5 cm (or if initially detected at a larger size), they are often repaired as the risk of rupture rises exponentially above this size. If rupture does occur, the results of emergency aneurysm repair are not good, with only about 40% of patients surviving. Without repair few survive, so that overall the survival of AAA rupture is probably < 20%. Although recent reports have suggested that the incidence of aneurysms appears to be in decline,3,4 AAA remains a significant health risk in the older population, with around 4000 deaths each year in England and Wales attributed to AAA rupture. 5
Except when they rupture, most AAAs are usually asymptomatic and so, until recently, they were detected as an incidental finding on clinical examination or ultrasonography, abdominal computerised tomography (CT) or magnetic resonance imaging performed for other purposes. However, in the UK, the NHS Abdominal Aortic Aneurysm Screening Programme (NAAASP) was introduced in 2009 and so many more small AAAs are now being detected early. The programme has been very successful and screened its millionth man in autumn 2015. There is an opportunity to reduce the number of patients needing AAA repair if AAA growth can be slowed or prevented in this growing cohort of patients.
Although data on the effects of angiotensin-converting enzyme inhibitors (ACE-Is) in this context are not consistent, ACE-Is have been associated with a reduced incidence of AAA rupture in analysis of administrative databases. 6 Previous trials of some other drugs to slow AAA growth have been hindered by poor patient compliance. 7 Therefore, this pilot trial was undertaken to assess whether or not ACE-Is could potentially slow AAA growth and are well tolerated in doing so. We are unaware of any other completed randomised controlled trials (RCTs) designed to examine the efficacy of ACE-Is or angiotensin receptor blockers (ARBs) in limiting or inhibiting AAA progression, although two trials of the impact of ARBs on the growth of AAAs are in progress.
Risk factors
A wide variety of risk factors have been attributed to the formation and progression of AAAs. The single most important risk factor for AAAs has consistently been found to be smoking,8–10 although other risk factors including male sex, age, high blood pressure (BP) [particularly raised diastolic BP (DBP)] and family history of AAA are frequently linked with the aetiology of AAA. 11 Low prevalence rates have been observed among African12 and Asian13 men compared with Caucasian men. Several studies have also found a strong coexistence of localised and generalised atherosclerosis and AAA,14,15 an underlying disturbed connective tissue metabolism16 and an increased risk for AAA with increasing alcohol consumption. 17
There are many genetic syndromes that are associated with aortic aneurysmal disease affecting patients often at a very early age, including Marfan syndrome, Ehlers–Danlos syndrome, Loeys–Dietz syndrome and familial thoracic aortic aneurysms and dissections. 18
The NHS Abdominal Aortic Aneurysm Screening Programme
Phased implementation of the NAAASP began in July 2009. It was introduced after data from a number of studies and existing local screening programmes in England showed a reduction in aneurysm-related mortality when men aged ≥ 65 years were offered ultrasound screening.
The Multicentre Aneurysm Screening Study (MASS) was designed to assess whether or not AAA screening would be beneficial. 19 This study enrolled men aged 65–74 years who were randomised to receive screening or not. Extended follow-up of patients confirmed that screening resulted in a reduction in all-cause mortality. Over 13 years there were 224 AAA-related deaths out of the 33,883 participants in the invited group and 381 AAA-related deaths out of the 33,887 participants in the control group, a 42% relative reduction. 20
In 2005 this evidence was assessed by the UK National Screening Committee, which concluded that ultrasound screening should be offered to men in their 65th year, with men aged ≥ 65 years being able to self-refer within the NHS. 5
The initial outcomes from the NAAASP in England identified a lower prevalence of AAA than reported in the MASS (1.4% vs. 4.7%). However, in the MASS, men aged 65–74 years were included, whereas the NAAASP invites men in their 65th year only for screening.
Between 2009 and 31 March 2014 the NAAASP had scanned > 700,000 men and referred > 1000 men with a large AAA for surgery. In the period 2013–14, 491 of the screened men had an elective AAA repair and four of these men died (an elective repair mortality rate of 0.8%). In addition, seven of the 10 screened men who suffered aneurysmal rupture died (a rupture mortality rate of 70%). 21
Because of the NAAASP a greater number of patients with a small AAA are being detected and, if there were effective treatments to slow AAA growth, this could provide an opportunity to intervene before the AAAs expand significantly and rupture. Also, the NAAASP potentially provides a useful pool of patients for research purposes, not only for logistical reasons but also because this group of patients (who were previously unaware of their AAA) may be receiving less clinical/medical intervention than patients who are already receiving monitoring for their AAA. They are therefore of particular interest for interventional studies.
Current guidelines for the management of small abdominal aortic aneurysms
Given the variability in aneurysm expansion rates,22 the optimal interval between surveillance scans remains uncertain. Meta-analysis of small AAA growth rates has demonstrated that the screening interval should be dependent on diameter and that long intervals between screening may be safe for the majority of patients. 23 However, guidelines must balance the need to reduce the cost of surveillance programmes and the need to ensure safety, as well as increasing the face-to-face time for direct cardiovascular risk factor education.
The UK guidelines recommend that rescreening intervals should shorten as the aneurysm enlarges and these guidelines are expected to be updated in 2017. 24 Usual clinical practice in the UK, and for those patients in the NAAASP, involves follow-up surveillance imaging at 12-monthly intervals for patients with an AAA of 3.0–4.4 cm in diameter and at 3-monthly intervals for those patients with an AAA diameter between 4.5 cm and 5.4 cm.
Abdominal aortic aneurysm treatment
In both the USA and the UK, elective surgery by either open or endovascular repair is undertaken to prevent AAA rupture and this is generally recommended for patients with an AAA of ≥ 5.5 cm in diameter, for symptomatic aneurysms or for aneurysms that have increased by > 0.5 cm in the past 6 months. The UK Small Aneurysm Trial (UKSAT) demonstrated that the overall mortality of patients with an aneurysm of < 5.5 cm in diameter who received surveillance was similar to that in patients who received early open surgery. 22 Furthermore, surveillance was the more cost-effective option. Subsequent studies that have randomised patients to surveillance or endovascular treatment of AAAs have corroborated this finding. 25,26 There has been an increasing trend in the proportion of repairs performed as endovascular aneurysm repair (EVAR) procedures, increasing from 54% in 2009 to 66% in 2013. 27
Studies indicate that without surgery the 5-year survival rate for patients with an aneurysm of diameter > 5 cm is about 20%. 28 Surgery to replace the aneurysmal segment or endovascular placement of a covered stent graft excluding the aneurysm is recommended if the risk of aneurysm rupture is high enough to justify the risk of surgery. The rate of rupture of an aneurysm rises exponentially after it reaches 5.5 cm in size, justifying the need for repair in most patients as aneurysm rupture is associated with a high mortality rate. Approximately half of the patients with a ruptured AAA fail to reach hospital and, of those patients who undergo emergency surgery, there is a 35–40% mortality rate at 30 days. 29
Open surgical repair carries a significant risk of perioperative morbidity and mortality. The 2014 National Vascular Registry report stated that, over the 5 years between 2009 and 2013, in-hospital mortality for open repairs was 3.6%. 27 The less-invasive EVAR technique has significantly lowered perioperative morbidity and mortality rates27 but not all patients are anatomically suitable for EVAR and there is still debate regarding the long-term benefits of EVAR. Several trials, including the EVAR,30 DREAM (Dutch Randomized Endovascular Aneurysm Repair)31 and OVER (Open Versus Endovascular Repair)32 trials, have found that the advantage of EVAR over open repair is lost during mid-term follow-up, with survival rates beyond 2 years being similar in both groups.
Given the risks involved with AAA repair, strategies to reduce the need for surgery are needed. Currently, there are no clear recommendations on pharmacological treatment approaches to prevent aneurysm progression or reduce the risk of rupture. 33
Growth rates of small abdominal aortic aneurysms
The growth rate of AAAs is highly variable both between patients and in the same patient over time. Average growth rates increase as the aneurysm enlarges. The average growth rate for a 3.5-cm aneurysm is estimated at 1.90 mm per year, whereas that for a 4.5-cm aneurysm is 3.52 mm/year. Therefore, given an exponentially increasing aneurysm diameter, it would take an average of 6.2 years for a 3.5-cm aneurysm to grow to 5.5 cm, whereas a 4.5-cm aneurysm would grow to 5.5 cm in 2.3 years. 34 These growth rates highlight the need for very accurate measurements of AAA to be obtained in a trial setting.
In the UKSAT, AAA growth was most strongly associated with diameter at baseline22 and smoking was associated with an incrementally increased growth rate of 0.4 mm per year. Multivariate analysis of other potential risk factors demonstrated that the presence of peripheral arterial disease (adversely) or diabetes (beneficially) influenced AAA growth.
The risk factor profile for aneurysm growth has been reproduced in other studies, with AAA growth being increased in smokers8,35 and decreased in patients with diabetes. 36
Average baseline diameters and growth rates reported in the Western Australia screening study,37 MASS,19 Propranolol Aneurysm Trial,7 UKSAT22 and Second Manifestation of ARTerial disease study38 are shown in Table 1.
Rupture rates of small abdominal aortic aneurysms
Aneurysm size is one of the strongest predictors of the risk of rupture, with risk increasing considerably for aneurysm diameters of > 5.5 cm. The UKSAT reported the risk of rupture for AAAs up to 5.5 cm in diameter to be < 1 per 100 person-years in men and 3 per 100 person-years in women. 39
Similarly, the 5-year overall cumulative rupture rate of incidentally diagnosed AAAs in population-based samples is 25–40% for aneurysms of > 5.0 cm diameter and 1–7% for aneurysms of 4.0–5.0 cm in diameter. 40,41
Rupture rates have been found to be doubled in current smokers compared with ex-smokers or non-smokers (p = 0.001) and to increase with mean arterial pressure (per 10 mmHg) (p = 0.001). 42
Blood pressure and abdominal aortic aneurysms
Raised BP was the leading risk factor contributing to the overall global burden of disease in 2010. 43 The recent decrease in case fatality rates associated with acute cardiovascular events in high-income countries has been associated with a rise in the numbers of patients living with cardiovascular disease and the wider use of preventative drugs in the context of primary and secondary prevention.
An association between hypertension and the incidence of AAA is frequently cited. 12,44 The CALIBER (CArdiovascular research using LInked Bespoke studies and Electronic health Records) study used linked electronic health records to assemble a cohort of > 1 million patients aged ≥ 30 years and initially free from cardiovascular disease, one-fifth of whom received BP-lowering treatments. 36
Of all cardiovascular diseases, AAA had the strongest association with DBP [hazard ratio (HR) per 10 mmHg 1.45, 95% confidence interval (CI) 1.34 to 1.56] and mean arterial pressure (HR 1.61, 95% CI 1.48 to 1.75) and the weakest association with systolic BP (SBP) (HR 1.08, 95% CI 1.00 to 1.17). Furthermore, it was the only cardiovascular outcome for which the association with higher pulse pressure was reversed (HR 0.91, 95% CI 0.86 to 0.98). 36
However, mean baseline BP levels reported in several large AAA surveillance studies7,19,22,37 (Table 2) are all above what is currently considered as controlled (< 140/90 mmHg45) and the AAA growth rates observed in these studies (see Table 1) may at least in part be related to these higher BPs.
| Study | Mean baseline BP (mmHg) |
|---|---|
| Western Australia screening study37 | 157/91 |
| MASS19 | 155/83 |
| Propranolol Aneurysm Trial7 | 143/81 |
| UKSAT22 | 157/86 |
Despite the relatively strong association between hypertension and the prevalence of AAA, the association between increased BP and the rate of AAA growth or incidence of rupture is not clear and the evidence supporting increased growth as a result of hypertension is lacking.
Non-pharmacological treatments to reduce the growth and rupture rate of abdominal aortic aneurysms
There is clear observational evidence that smoking increases the likelihood of developing an AAA. 11,12,46 For example, in the large (n = 114,567) Aneurysm Detection and Management (ADAM) screening study, a history of ever smoking was associated with an odds ratio of 2.97 (95% CI 2.65 to 3.32) for 3.0- to 3.9-cm AAAs and 5.07 (95% CI 4.13 to 6.21) for ≥ 4-cm AAAs. 46 In addition, a recent prospective population-based study of 92,728 men in Oxfordshire found that men aged 65–74 years who were current smokers had a 3% 10-year risk of acute AAA, highlighting the need for screening campaigns to reach this high-risk group. 47
Furthermore, several studies8,15,17 and meta-analyses48 have found higher growth rates in current smokers than in past smokers.
Consequently, the standard non-pharmacological treatment for AAA is smoking cessation. However, it has been suggested that smoking cessation may lose some of its importance once significant aortic dilatation has occurred. 35
Pharmacological treatments to reduce the growth and rupture rate of abdominal aortic aneurysms
There remains a significant need to find medical therapies that could reduce the growth and rupture rates of small and medium-sized AAAs.
As well as interest in the development of new AAA-specific treatments, there has also been interest in assessing the impact of treatments already in use for other indications. Early evidence often arises from animal studies but a small number of RCTs in humans have been carried out to assess the efficacy of some of the currently available drugs.
Beta-blockers
Evidence that the use of beta-blockers might reduce the growth of AAAs first arose from animal studies. 49,50 However, a placebo-controlled RCT including 548 patients failed to find an association between beta-blocker use and a significant reduction in AAA expansion. 7 Compliance with the medication was a problem, with 117 of 276 (42%) randomised to propranolol and 73 of 272 (27%) allocated to placebo stopping the drugs because of side effects. Furthermore, the increase in AAA diameter was similar in both the propranolol group and the placebo group (2.2 and 2.6 mm per year, respectively; p = 0.11) based on an intention-to-treat (ITT) analysis. Patients receiving propranolol also reported a significantly worse quality of life, leading the authors to conclude that the drug did not affect the growth rate of small AAAs and patients with AAAs do not tolerate propranolol well. Similarly, no protective association was observed for beta-blockers in a large observational study of patients with an AAA. 6
Statins
The evidence supporting the use of statins for the reduction of growth and rupture rates in AAA is inconsistent. The UK Heart Protection Study (UKHPS) compared simvastatin with placebo for the reduction of cardiovascular events over a mean of 5 years in 20,536 patients with vascular disease or at high risk of vascular disease at baseline. 51 This included 6748 patients with peripheral artery disease. The study reported that the requirement for AAA repair was unaffected (1.2% in both groups). The Tromsø study related statin prescription to the development of AAAs in 4345 subjects who were scanned over 7 years. 44 The use of statins was associated with an increased risk of developing an AAA.
Contrary to the UKHPS51 and Tromsø study,44 a systematic review in 2008 found that statin use was associated with reduced growth rates of AAAs. 52 This included two cohort studies that both showed reduced growth rates in patients taking statins. 53,54 Evidence suggesting that statins may be of benefit was also presented in a population-based combined case–control and follow-up study, which found that statin use was associated with a reduced risk of ruptured AAA and lower case fatality following ruptured AAA. 55
Despite inconsistent evidence, current guidelines recommend statin therapy in patients diagnosed with an AAA because of their high cardiovascular risk. 1
Doxycycline
Doxycycline was investigated as a treatment for AAA as a result of the theory that chlamydia or related infections might be involved in AAA formation and growth. 56 However, clear evidence for the role of infection in the progression of AAAs is limited with small antibiotic trials showing no difference in expansion rate. 57
More recent studies have investigated the effects of doxycycline as an inhibitor of matrix metalloproteinases. Matrix metalloproteinases are thought to play a role in the destruction of elastin and collagen in the aortic wall, leading to degeneration, and several matrix metalloproteinases have been detected in AAAs, importantly in greater proportions at the site of rupture. 58–60 Doxycycline has been found to inhibit aneurysm development and progression in numerous animal models. 61–63 A small randomised pilot trial (n = 32) in patients with small AAAs found that the AAA expansion rate in the doxycycline group was significantly lower than that in the placebo group during both the 6- to 12-month period and the 12- to 18-month period. 64 However, a recent larger randomised trial (n = 286) found that 18 months of doxycycline therapy did not reduce aneurysm growth or influence the need for AAA repair or time to repair. 55 Nevertheless, the results of this trial are being challenged in a new American trial, the Non-Invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (NTA3CT), using transverse aorta CT measurements of AAA growth (NCT01756833). Trials incorporating other protease inhibitors are also expected in the near future.
The role of the renin–angiotensin system
The renin–angiotensin system (RAS) is a peptidergic hormone system that has been recognised to be highly involved in disturbances of the cardiovascular system. RAS blockade by ACE-Is and ARBs has been found to not only decrease arterial pressure but also prevent or reverse endothelial dysfunction and aspects of the atherosclerotic process, which results in a reduction in cardiovascular mortality and morbidity. 65,66
In experimental studies both angiotensinogen and angiotensin type 1 receptors have been found to be upregulated by approximately twofold in the walls of AAAs compared with the walls of atherosclerotic aortas, although the expression of angiotensin type 2 receptors was similar. 67 In hypercholesterolaemic mice, angiotensin II infusion induces medial dissection of the aorta proximal to aortic branch points, with subsequent formation of suprarenal aortic aneurysms, which can be prevented with the use of ACE-Is. 68
Furthermore, in a recent study, perindopril (Coversyl arginine, Servier) inhibited aortic degeneration and AAA formation in the experimental AAA model induced by elastase and calcium chloride. 69
Angiotensin-converting enzyme inhibition
In line with the animal studies that have suggested a potential role of the RAS system in AAA formation and growth, an observational case–control study on a group of > 15,000 patients with AAAs found that patients who received an ACE-I before admission were 20% less likely to present with a ruptured aneurysm. 6 These results remained after adjustments were made for demographic characteristics, comorbidities, contraindications to ACE-Is, measures of health-care use and aneurysm screening. The group noted that the reduction in risk of aortic rupture was distinct from antihypertensive and other medications, suggesting that the mechanism may not be related to a BP-lowering effect. Calcium channel blockers (CCBs) and beta-blockers, for example, were not associated with any reduction in risk. 6 This large-scale study demonstrated an impressive reduction in AAA rupture but there were several potential confounders in this study, not least the compliance with ACE-Is in smokers.
In addition, a recent cohort study of 21,791 patients with AAA identified from Danish registries suggested that treatment with ACE-Is or ARBs was associated with a decreased risk of all-cause death and death from AAA in patients who had not yet undergone surgery for AAA. 70 However, there was no reduction in the need for surgery for AAA.
When considering growth rate modulation by agents acting on the RAS, the evidence is certainly conflicting. The Chichester small AAA surveillance study suggested an association between ARB prescription and reduced AAA progression. 71 However, in contrast, a report from the UK Small Aneurysm Study group reported a small but significant association between ACE-I prescription and increased AAA expansion. 72 This significant difference remained after adjustment for known confounders such as smoking, diabetes, BP and peripheral atherosclerosis.
In summary, currently there is no clear or consistent evidence that medication designed to inhibit the RAS limits AAA progression or leads to a decrease in the risk of rupture.
Designing the trial
To date we are unaware (based on a recent literature review) of any other completed RCTs designed to examine the efficacy of ACE-I or ARBs in limiting or inhibiting AAA progression. This report describes the AARDVARK (Aortic Aneurysmal Regression of Dilation: Value of ACE-Inhibition on RisK) trial, which was commissioned by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme to address this need.
Objectives
Primary
-
To investigate AAAs in a three-arm randomised placebo-controlled pilot trial the hypothesis that the ACE-I perindopril reduces the growth rate of small AAAs.
Secondary
-
To evaluate any BP-independent effects of perindopril on the growth rate of small AAAs.
-
To determine differences in AAA rupture rate and/or time taken to reach an AAA diameter of 5.5 cm and/or referral for surgical intervention among the three randomised groups.
-
To evaluate how well perindopril is tolerated as measured by compliance, adverse events (AEs) and quality of life.
-
To compare the repeatability of ultrasound measurements of internal and external small AAA diameters.
Later, pending the results of this pilot trial, our objective was to work with local and national aneurysm screening programmes to conduct a larger, more definitive RCT to investigate the hypothesis that the use of an ACE-I reduces the rate of AAA-related mortality, rupture or elective surgery.
Chapter 2 Methods
Final study design
This randomised, single-blind, placebo-controlled study took place at 14 investigational sites in England. The trial consisted of three parallel randomised arms, with patients receiving 10 mg of perindopril daily (arginine salt), placebo daily (primary comparison) or 5 mg of amlodipine daily (secondary comparison). The perindopril and amlodipine doses used were estimated to have similar effects on BP reduction73–75 and hence the secondary comparison was included to assess whether or not any benefits of perindopril were independent of BP reduction.
Trial participants
The following factors were taken into consideration when deciding on the inclusion and exclusion criteria for the trial:
-
Abdominal aortic aneurysm size. Patients with an AAA of ≥ 5.5 cm diameter would be considered for surgical intervention as per the current clinical guidelines. Therefore, patients with an AAA of ≤ 5.4 cm diameter were included to minimise the rate of patient withdrawal from the study.
-
Sex. Although a lower prevalence of AAAs has been found in women, there is no evidence to suggest that the trial medications would have a different mechanism of action between the sexes. Therefore, both men and women were included.
-
Age. Assuming potentially differential benefits of ACE-Is for patients with AAAs related to genetic syndromes, a lower age limit (initially 60 years and then amended to 55 years) was set to more effectively screen out this group.
-
Ethnicity. Although a higher prevalence of AAAs has been found in Caucasian men than in black and Asian men, there is no evidence to suggest that the trial medications would have a different mechanism of action between races, specifically on AAA growth. Therefore, patients from all ethnicities were included.
-
Blood pressure. Regarding the treatment of hypertension, the 2011 National Institute for Health and Care Excellence (NICE) guidelines45 state:
-
aim for a target clinic BP of < 140/90 mmHg in people aged < 80 years with treated hypertension
-
aim for a target clinic BP of < 150/90 mmHg in people aged ≥ 80 years with treated hypertension.
-
However, the Quality and Outcomes Framework target for general practitioners (GPs) is a SBP of < 150 mmHg in people aged < 80 years. It seemed reasonable, therefore, to set an inclusion criterion of a SBP of < 150 mmHg in line with this Quality and Outcomes Framework target. Otherwise, eligible patients who had a SBP of ≥ 150 mmHg could be subsequently recruited into the trial but only after suitable BP medication had been supplied and the SBP was controlled to < 150 mmHg (see Planned drug interventions).
-
Medical history. Patients with any medical conditions that would interfere with their participation in the trial or who would be at an increased risk of adverse effects by taking the study medications were excluded from the trial.
-
Concomitant medications. Patients already receiving an ACE-I, a CCB or an ARB could not participate in the trial. The only exception was patients receiving 5 mg of amlodipine because the maximum dosage of amlodipine is 10 mg, thereby allowing the in-trial allocation to a further 5 mg.
The final inclusion and exclusion criteria are listed in the following sections. Only patients who met these criteria were considered for inclusion in the trial.
Inclusion criteria
-
Willing and able to give written informed consent.
-
Men or women aged at least 55 years.
-
With an AAA of 3.0–5.4 cm in diameter by internal or external measurement according to ultrasonography.
-
Systolic BP of < 150 mmHg.
Exclusion criteria
-
Patients who are already required to take an ACE-I, an ARB or a CCB (with the exception of 5 mg of amlodipine).
-
Those with known renal artery stenosis (> 50%) or with a serum creatinine level of > 180 µmol/l.
-
Those unable to give informed consent.
-
Those too frail to travel for 3-monthly surveillance visits.
-
Any clinically significant medical condition that, in the opinion of the investigator, would interfere with the study results and/or reduce life expectancy to < 2 years.
-
Participation in another trial of an investigational product or device within the previous 30 days.
-
Known allergy or sensitivity to perindopril or amlodipine.
-
Unable or unwilling to comply with the requirements of the study, in the opinion of the investigator.
Recruiting centres
Participants were recruited from 14 centres across England (Figure 1). Initially, patients were recruited from five centres:
-
Imperial College Healthcare NHS Trust (St Mary’s Hospital)
-
Imperial College Healthcare NHS Trust (Charing Cross Hospital)
-
Guy’s and St Thomas’ NHS Foundation Trust (St Thomas’ Hospital)
-
Royal Free London NHS Foundation Trust (Royal Free Hospital)
-
University Hospitals Coventry and Warwickshire NHS Trust (University Hospital Coventry).
FIGURE 1.
Organisation of recruiting sites. DAR, Dartford Hospital; ICCH, International Centre for Circulatory Health; KCH, King’s College Hospital; LEW, Lewisham Hospital; MTW, Maidstone and Tunbridge Wells; QE, Queen Elizabeth Hospital, Woolwich; WMX, West Middlesex University Hospital.
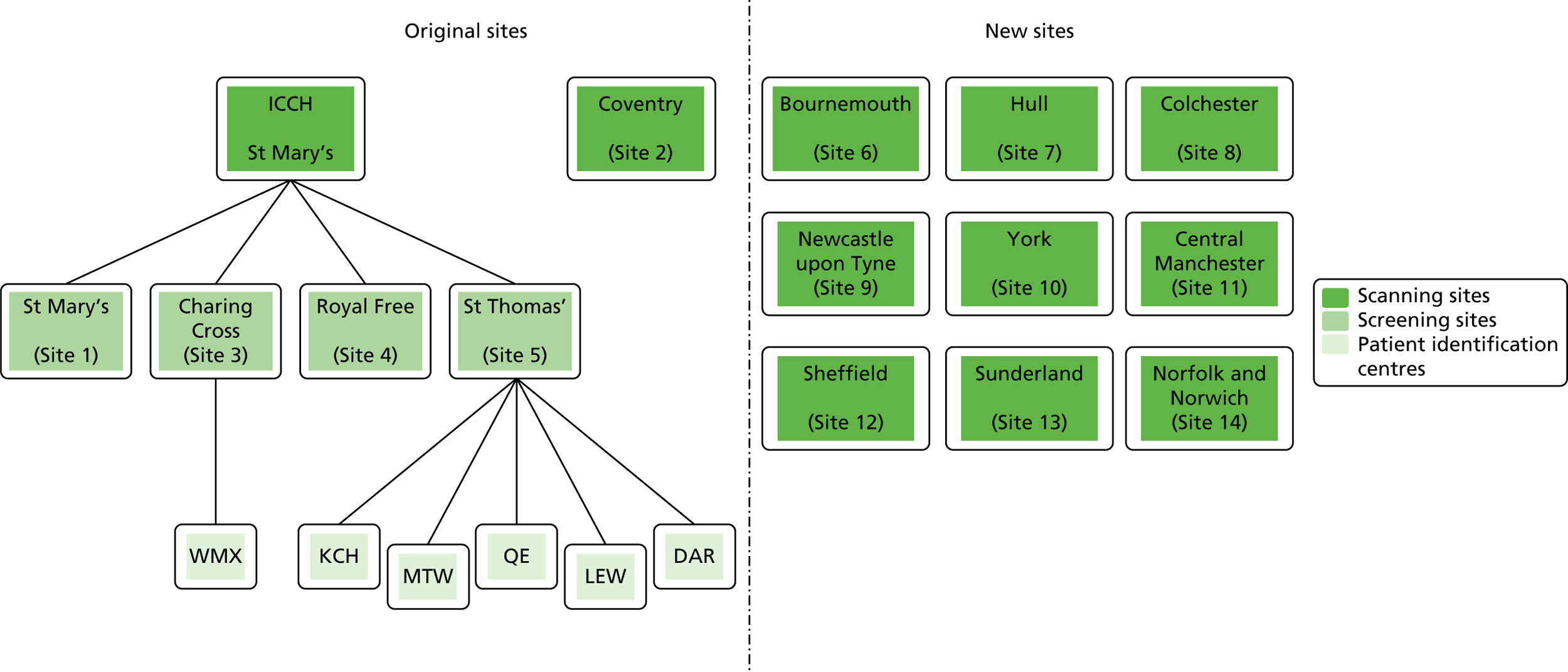
The original arrangement was for there to be only two sonographers in the study, one to perform all of the scans on patients at the London sites with the same mobile ultrasound scanner and one to perform all of the scans on patients from University Hospital Coventry on the same model of ultrasound scanner located in Coventry. This was principally to reduce intersonographer variability.
However, before the first patient was recruited into the study it was decided that patients in London should be screened and recruited at their local research site but that all visits and measurements from baseline onwards would take place at a central hub, the International Centre for Circulatory Health (ICCH), Imperial College London. The advantages of this were that:
-
Patients would have complete flexibility in the days/times of their study visits.
-
In the case of cancellations or missed appointments, patients could be rebooked without restriction, making it less likely for them to fall out of their protocol-defined visit window.
-
Experts in hypertension and antihypertensive medications and their side effects are based on site at ICCH and were available to see at short notice patients who had experienced any AEs.
-
The issue of being able to identify available clinic space to conduct the patient visits at each of the research sites was overcome.
-
In the case that the sonographer was unable to conduct patient visits (because of sickness, annual leave, etc.), the back-up sonographer (who was based at ICCH) was available to conduct visits at short notice with minimal disruption to her other duties.
Nine further sites were later added to enhance recruitment:
-
Hull and East Yorkshire Hospitals NHS Trust (Hull Royal Infirmary)
-
Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust (Royal Bournemouth Hospital)
-
Colchester Hospital University NHS Foundation Trust (Colchester General Hospital)
-
Newcastle upon Tyne Hospitals NHS Foundation Trust (Freeman Hospital)
-
City Hospitals Sunderland NHS Foundation Trust (Sunderland Royal Hospital)
-
York Teaching Hospitals NHS Foundation Trust (York Hospital)
-
Norfolk and Norwich University Hospitals NHS Foundation Trust (Norfolk and Norwich University Hospital)
-
Central Manchester University Hospitals NHS Foundation Trust (Manchester Royal Infirmary)
-
Sheffield Teaching Hospitals NHS Foundation Trust (Northern General Hospital).
Patient identification centres
Several patient identification centres (PICs) were also added to the study to further enhance recruitment (see Figure 1). The PICs identified potential participants for the trial at their sites and referred them to the associated research site for recruitment into the trial. The following PICs were approved for the study:
-
West Middlesex University Hospital NHS Trust (West Middlesex University Hospital)
-
King’s College Hospital NHS Foundation Trust (King’s College Hospital)
-
Maidstone and Tunbridge Wells NHS Trust (Tunbridge Wells Hospital)
-
South London Healthcare NHS Trust (Queen Elizabeth Hospital, Woolwich)
-
Lewisham and Greenwich NHS Trust (Lewisham Hospital)
-
Dartford and Gravesham NHS Trust (Dartford Hospital).
Recruitment
The clinical registries and the NAAASP databases (when relevant) at the study sites were used to identify patients with an AAA. Permission to recruit NAAASP patients was obtained from the NAAASP research committee. All patients were then prescreened against the inclusion and exclusion criteria. Sites were asked to capture the patient initials for each identified patient and to record the reasons for ineligibility or for non-participation to help inform study progress. These patients were entered onto the Consolidated Standards of Reporting Trials (CONSORT) flow chart. Eligible patients were then subsequently invited to consider entry into the trial.
It was the investigators’ responsibility to obtain written informed consent from patients after adequate explanation of the aims, methods, anticipated benefits and potential hazards of the study, and before any study procedures commenced. Potential participants were given a copy of the patient information sheet (PIS) and informed consent form (ICF) (see Appendix 1). The original copy of the signed and dated ICF was retained by the site.
Patients were given at least 24 hours to read the PIS and consider their participation.
Study correspondence for health professionals
Throughout the study, steps were taken to ensure that the relevant clinical personnel were kept updated on patients’ involvement and progress in the trial. The following documents were created, approved by the ethics committee and utilised in the study:
-
General practitioner letter A. The purpose of this letter was to inform the patient’s GP about his or her involvement in the study, including a brief description of the trial and its requirements, with contact information for the trial administration and clinical staff.
-
General practitioner letter B. The purpose of this letter was to inform the patient’s GP of the following clinical issues that might require their attention (the relevant statement could be ticked):
-
the patient’s SBP in clinic was found to be > 150 mmHg and the patient has been commenced/or may require commencement on 1.5 mg of slow-release indapamide to potentially be suitable for the study (see Planned drug interventions)
-
the patient was not found to be taking statin medication and he or she may wish to perform a lipid profile if not already carried out and consider initiating statin therapy
-
the creatinine level was found to be > 180 µmol/l.
-
-
General practitioner letter C. The purpose of this letter was to inform the patient’s GP that, pending BP assessment, the patient might need an alternative antihypertensive medication to maintain normotension. The letter referred to the 2011 NICE guidelines45 and invited the GP to contact the chief investigator for advice if required.
-
End-of-study letter. This was sent to the patient (with a copy sent to the GP and the relevant referring consultant, if applicable) to say thank you for participating in the study and to inform the patient of the medication that he or she was receiving during the trial.
In addition, for patients who had their follow-up visits at ICCH (St Mary’s Hospital), a study results letter was sent to their referring consultant after each visit.
Study documents for patients
All patients were provided with an appointment diary at the start of the study as a method of reducing the number of those missing or forgetting their appointments.
The appointment diary recorded the times and dates of the visits, and space was also available for patients to record any side effects experienced between visits and to record any changes to their concomitant medication or study medication.
Patients were provided with an emergency contact card at the start of the study and were asked to keep this on them at all times. The contact card gave some brief information about the study, including the study name and a list of the potential medications that patients might be receiving as part of the trial. It also provided a 24-hour telephone number for the local site pharmacy who held the unblinding information.
Planned drug interventions
The primary comparison was the effect on AAA growth of the ACE-I compared with placebo; one-third of randomised patients received 10 mg of perindopril arginine daily and one-third received placebo daily. To evaluate the BP-independent effects of the ACE-I, one-third of patients were randomised to a CCB (5 mg of amlodipine daily). It was estimated that, at these doses and in this population, the two drugs would produce similar average BP-lowering effects of approximately 6/4 mmHg. This protocol also allowed both drugs to be compared with placebo to evaluate any BP-lowering impact on AAA growth.
If the SBP of potential trial recruits was > 150 mmHg at screening, sites were asked to arrange for these patients to receive 1.5 mg of slow-release indapamide daily (either prescribed locally or through their GP) or, if this was not appropriate, 5 mg of amlodipine for a 6-week period. Such patients then had their BP measurements repeated after 6 weeks and if their SBP had fallen to < 150 mmHg they were eligible to proceed to randomisation.
The most common side effect of ACE-Is is cough, which affects about 15% of those treated. 76 However, exclusion of those with a pretrial history of ACE-I intolerance was enforced to reduce the incidence of this problem. During the trial, this side effect was monitored, particularly as we anticipated (based on past research) that the majority of patients in the trial would be smokers or ex-smokers who tend to tolerate ACE-Is less well. When in-trial cough was persistent and intolerable, patients stopped medication for 2 weeks and if the cough resolved they were changed to the ARB losartan (100 mg per day). If the cough continued (and hence was deemed to be unrelated to the trial medication), perindopril was restarted.
For all patients recruited into the trial who were not currently receiving a statin, sites were advised to request that the their GP prescribe a drug in this class as per current guidelines.
Visit schedule
The study visit schedule for the AARDVARK trial is shown in Table 3.
| Study procedures | Visita | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Screening and consent | Randomisation | Treatment | ||||||||
| Months | –3 to 0 | 0 | 3b | 6c | 9c | 12b | 15d | 18b | 21d | 24b |
| Inclusion and exclusion criteria | ✗ | Check | ||||||||
| Informed consent | ✗ | Check | ||||||||
| Patient demographics | ✗ | Review | ||||||||
| Past medical historye | ✗ | Review | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Current medical therapies | ✗ | Review | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Ultrasound of AAA | Review | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| BPf | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| AEs | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Dispensing of study medication | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Pill count | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Blood for measurement of creatinine and electrolytesg | ✗ | Review | ✗ | ✗ | ✗ | |||||
| Blood and urine for biomarker studiesh | ✗ | ✗ | ||||||||
| EQ-5D and health resource use questionnaires | ✗ | ✗ | ||||||||
Each patient had a maximum of 10 planned study visits. The visit schedule and interventions were as follows:
-
screening – informed consent, collection of demographic information, past medical history and current medical therapies, review of most recent AAA ultrasound measurement, BP, blood for measurement of creatinine and electrolytes
-
baseline – review of informed consent, review of demographic information, review and checking of past medical history, review and checking of current medical therapies, ultrasound of AAA as per study protocol, BP readings in triplicate, review of screening blood test results, collection of urine and blood for the biomarker study (in a subset of patients), confirmation of patient eligibility, randomisation and dispensing of study medication
-
3 months – review and check medical history, review and check current medical therapies, collect details of any AEs, ultrasound of AAA as per study protocol, BP readings in triplicate, collection of blood for measurement of creatinine and electrolytes, dispensing of study medication and pill count
-
6 months – review and check medical history, review and check current medical therapies, collect details of any AEs, ultrasound of AAA as per study protocol, BP readings in triplicate, dispensing of study medication and pill count
-
9 months – review and check medical history, review and check current medical therapies, collect details of any AEs, ultrasound of AAA as per study protocol, BP readings in triplicate, dispensing of study medication and pill count
-
12 months – review and check medical history, review and check current medical therapies, collect details of any AEs, ultrasound of AAA as per study protocol, BP readings in triplicate, collection of blood for measurement of creatinine and electrolytes, dispensing of study medication and pill count
-
15 months – review and check medical history, review and check current medical therapies, collect details of any AEs, ultrasound of AAA as per study protocol, BP readings in triplicate, dispensing of study medication and pill count
-
18 months – review and check medical history, review and check current medical therapies, collect details of any AEs, ultrasound of AAA as per study protocol, BP readings in triplicate, dispensing of study medication and pill count
-
21 months – review and check medical history, review and check current medical therapies, collect details of any AEs, ultrasound of AAA as per study protocol, BP readings in triplicate, dispensing of study medication and pill count
-
24 months – review and check medical history, review and check current medical therapies, ultrasound of AAA as per study protocol, BP readings in triplicate, collection of urine and blood for biomarker study (in a subset of patients), collection of European Quality of Life-5 Dimensions (EQ-5D) questionnaire, health resource use questionnaire and pill count.
Blood pressure protocol
At each visit three BP recordings were taken in the sitting position using a validated semiautomated device after at least 10 minutes rest. The mean of the second and third readings was used in analyses. Smoking was not permitted in the 30 minutes before BP measurement. Omron 705CP-II machines (OMRON Healthcare, Milton Keynes, UK) were distributed for collection of BP measurements at all except five sites, where BP Plus devices (USCOM, Sydney, NSW, Australia) were used. The purchase of six BP Plus devices was funded by the Foundation for Circulatory Health, Imperial College London. These machines were distributed to the five sites (one machine was kept as a backup) where we expected the highest recruitment levels (St Mary’s Hospital, Royal Bournemouth Hospital, Hull Royal Infirmary, Norfolk and Norwich University Hospital and York Hospital). At these sites both peripheral and central BP recordings were collected from the baseline visit onwards for all patients. At ICCH, 20 patients were already participating in the trial prior to the BP Plus machine arriving; these patients had all of their BP measurements taken using an OMRON machine.
Clinical laboratory samples
Angiotensin-converting enzyme inhibitors commonly cause mild increases in serum creatinine as part of the desired result of reducing intraglomerular pressure. This slight rise in serum creatinine is to be expected and is acceptable after starting ACE-Is. 77 Blood tests for measurement of creatinine and electrolytes were therefore carried out at screening and 3, 12 and 24 months (in keeping with best recommended practice for the management of hypertension with ACE-Is) and results were reviewed regularly by the study team and the Data Safety Monitoring Committee (DSMC). If the serum creatinine level rose > 30% above baseline or progressively increased over time, investigators were advised to discontinue the study medication. Lesser increases in serum creatinine were monitored as required more frequent blood tests.
Quality of life
The EQ-5D is a standardised measure of health status developed by the EuroQol group78 to provide a simple, generic measure of health for clinical and economic appraisal. It is applicable to a wide range of health conditions and treatments, and provides a simple descriptive profile and a single index value for health status that can be used in the clinical and economic evaluation of health care.
The EQ-5D consists of two pages: a descriptive system and a visual analogue scale. The descriptive system has five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each has three levels of severity (no problems, some problems, extreme problems) in the three-level version and five levels of severity (no problems, slight problems, moderate problems, severe problems and extreme problems) in the five-level version. The three-level version was utilised for the AARDVARK trial (see Appendix 2).
The visual analogue scale used to value EQ-5D health states is presented as a 20-cm vertical line calibrated from zero (‘worst imaginable health state’) to 100 (‘best imaginable health state’). It asks respondents to ‘mark an ✗ on the scale to indicate how your health is today’.
This questionnaire was administered after 12 and 24 months of follow-up to allow quality of life to be compared among the three randomised groups during treatment as part of the safety analyses.
Health resource use questionnaire
The health resource use questionnaire (see Appendix 3) was created specifically for this study to evaluate the associated costs and burden of AAA patients to the NHS if there was a significant effect of ACE-Is on aneurysm growth.
The questionnaire collects information relating to patients’ health service use (e.g. visits to their doctor, nurse or GP) for reasons related to their aneurysm, use of social services, the amount of help they receive from their family and carers and service use for reasons not related to their aneurysm.
This questionnaire was administered after 12 and 24 months of follow-up.
Data collection
Data were collected by the study team onto paper case report forms (CRFs) (see Appendix 4) and were then entered onto corresponding electronic forms on the InForm™ ITM (Integrated Trial Management) system (Oracle Corporation UK Ltd, Reading, UK). This is a web-based data entry system that builds an Oracle database for each individual clinical trial. Bespoke web-based electronic CRFs with built-in validation rules were designed to identify data entry errors in real time and provide a full audit trail of data entry and changes. All persons entering data were trained prior to start-up and given personal login details with access to forms restricted according to site and role. The electronic CRFs were designed in accordance with the requirements of the trial protocol and complied with regulatory requirements. An automated audit trail was recorded when (and by which user) records were created, updated or deleted. Error reports were generated when data clarification was required and data queries were resolved by research nurses at trial sites.
Trial reporting
The trial team was required to submit annual reports on trial progress, data completion rates and safety and protocol compliance to the Medicines and Healthcare products Regulatory Agency (MHRA) and the Research Ethics Committee (REC). Reports were also prepared for all trial-oversight committees.
In addition, monthly key figures and 6-monthly reports were prepared for the funding body (the NIHR HTA programme). Monitoring meetings to discuss trial progress were also held with the NIHR at its request.
Trial monitoring
The data collected for the study were monitored on a regular basis to ensure that the integrity of the data and the rights and well-being of participants were protected. Monitoring was completed at a central level and a site level to check for any data errors, deviations or protocol non-compliance.
Validation checks were built into the InForm system, which enabled validation reports to be generated. Any missing values, values out of range or spurious values within the data set were flagged. Queries were sent to sites and followed up for resolution prior to data lock.
All sites were visited prior to opening to recruitment, within 2 weeks of randomising their first patient and then as required to achieve the following:
-
Source data verification of 100% of ICFs signed since previous visit to ensure correct completion.
-
Drug accountability for 100% of patients.
-
Verification that all serious adverse events (SAEs) had been reported correctly.
-
Source data verification of at least 50% of subjects randomised (a list of random patient numbers was provided by the statistician in advance) should have been performed by the end of the study for the following:
-
Eligibility – the data on the screening worksheets should have been checked against the inclusion/exclusion checklist and the subjects’ medical notes; for scanning sites, this eligibility should also have been entered correctly on InForm.
-
Existence – verification that subjects’ name and date of birth on the study worksheets and ICFs match the details in the medical notes.
-
Aneurysm measurements – by comparison of data on InForm against the study worksheets to ensure that the measurements had been entered correctly.
-
EQ-5D – by comparison of data on InForm against the hard copy of the questionnaire completed by the patient.
-
Health resource use questionnaire – by comparison of data on InForm against the hard copy of the questionnaire completed by the patient.
-
AEs and SAEs – by comparison of data on InForm against worksheets and medical notes.
-
Blood pressure – by comparison of data on InForm against the data recorded on the study worksheets. For BP measurements recorded using an OMRON machine with a printer, a printout of the results should have been attached to the worksheets. If a BP Plus machine was used, the measurements entered on InForm should have been checked against the study worksheets and, if available, against the electronic BP Plus database.
-
Randomisation
Randomisation was performed using a 1 : 1 : 1 ratio stratified by centre and by baseline size of aneurysm stratified into two size ranges: 3.0–4.5 cm and 4.51–5.40 cm. The randomisation code was generated by an independent statistician using randomly permutated blocks of varying sizes using SAS computer software (SAS Institute, Cary, NC, USA).
Any subjects successfully screened for the study and found to be eligible to proceed in the study were then randomised via the InForm system by a trained member of the research team after appropriate consent. Patients were allocated a unique study number for use in all future data collection.
Investigational medicinal products
Perindopril, amlodipine and placebo were produced in accordance with good manufacturing practice79 and packaged and labelled by the Royal Free Hospital Pharmacy Manufacturing Unit. Overencapsulation of the tablets for this pilot study proved too costly; therefore, the amlodipine, perindopril and placebo tablets were not identical. The three investigational medicinal products (IMPs) were randomly allocated to either bottle A, bottle B or bottle C by a statistician and this information was provided to the Royal Free Manufacturing Unit to allow the IMPs to be deblistered and packed into the corresponding bottles.
Perindopril arginine (10 mg) was provided by Servier at no cost. All products were checked by a qualified person at the Royal Free Manufacturing Unit prior to release.
The Royal Free Manufacturing Unit held a manufacturer’s authorisation for IMPs (called the Manufacturing and Importation Authorisation at the time of this study). Blinded treatment kits were labelled as per European Commission Good Manufacturing Practice Guidelines, Annex 1379 to enable the treatment to be identified and the batch source of the materials traced.
The IMPs were supplied to the participating sites on a demand basis with minimal wastage of materials. Bottle accountability logs were maintained by all parties to allow full reconciliation of IMPs, including assignment to patients.
Although the study was classed as single blind because the tablets were not identical, the following measures were taken to avoid site staff becoming aware of treatment allocation:
-
At no point during the study were the site staff informed of the contents of bottles A, B and C.
-
Bottles A, B and C were the same colour and opaque so that the tablets could not be seen by participating staff.
-
Sites were advised that returned tablets should be counted only by the pharmacy staff. This was to avoid any members of the research team identifying any of the tablets by their appearance.
Consequently, for all practical purposes the trial might reasonably be considered double blind.
Slow-release indapamide (1.5 mg) or amlodipine (5 mg) for use in the treatment of BP in those with a SBP > 150 mmHg following the initial screening visit was supplied by the site pharmacy or patients’ GPs in blister packs (not blinded). Losartan (100 mg) (an ARB) for use in patients who developed a cough during the trial was supplied by the site pharmacy or patients’ GP in blister packs (not blinded).
Study drug administration and compliance
Either a 3- or a 6-month supply of study drug was dispensed at each visit (depending on the timing of the next visit). For the initial 2 weeks following randomisation, patients were asked to take half doses of the IMP dispensed (i.e. 5 mg of perindopril, 2.5 mg of amlodipine and half of the placebo tablet). This is in line with standard clinical practice for the initiation of perindopril and hence was applied to all three randomised groups. All patients were provided with pill cutters at their randomisation visit for this purpose. After 2 weeks they were instructed to take the full dose. Patients were instructed to take their tablets at the same time each morning.
Patient compliance with the trial IMPs and potential side effects of drug treatment were monitored closely. When an in-trial cough was persistent and intolerable, patients stopped medication for 2 weeks and if the cough resolved they were changed to losartan (100 mg per day). If the cough continued (and hence was deemed to be unrelated to the trial medication), the study treatment was restarted. All patients who were switched to an ARB continued in the trial and were followed up on an ITT basis.
To encourage continued involvement in the trial, retention techniques (follow-up telephone calls, study keyrings and Christmas cards) were used.
Compliance with study medication was evaluated using pill counts. Compliance (expressed as a percentage) was calculated as the ratio of tablets taken (based on pill counts of tablets returned) divided by the number of tablets that should have been consumed based on the dates that they were dispensed and returned.
Unblinding procedures
In the event of a medical emergency it may have been considered important for a clinician to be aware of which treatment a patient had been using. It may have therefore been necessary for the trial code to be broken and the treatment allocation to be revealed.
All sites were advised to provide a number on the patients’ emergency contact card to call for the pharmacist or pharmacist-on-call (out of hours).
It was planned that all unblinding requests be discussed with the chief investigator of the trial, principal investigator (PI) of the site or delegate during working hours.
The pharmacy department held an out-of-hours contact number for the PI (or delegate) so that authorisation to unblind could be given. If the PI or delegate could not be contacted out of hours, the pharmacists were permitted to break the code.
The pharmacists were advised to record the name, post, address and contact number of the person requesting the unblinding, as well as the patient’s identification number, name and reason for unblinding.
Adverse event management
The following AEs were collected as part of the study:
-
SAEs
-
a single diagnosis or symptom that led to discontinuation of the trial drug
-
AEs thought to be secondary to trial medication.
For each AE the following was recorded:
-
start and end date and severity
-
the likely causal relationship between the IMP and the AE in the opinion of the PI or delegate was indicated as possible, probable or definite.
In addition, sites were requested to report any other AEs that impacted on patients’ participation in the trial or that they felt should be reviewed by the medical monitors. AEs were followed up according to local practice until they had stabilised or resolved.
Serious adverse events were defined as any untoward medical occurrence or effect that:
-
resulted in death
-
was life-threatening, that is, an event in which the subject was at risk of death at the time of the event; it did not refer to an event that hypothetically might have caused death if it were more severe
-
required hospitalisation or prolongation of an existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
resulted in a congenital abnormality or birth defect.
Medical judgement was exercised in deciding whether or not an AE/adverse reaction was serious in other situations. Important AEs/adverse reactions that were not immediately life-threatening or that did not result in death or hospitalisation but which may have jeopardised the health of a subject or may have required intervention to prevent one of the other outcomes listed in the definition above were also considered serious.
Any planned/elective hospitalisations that were scheduled prior to signing the informed consent but which took place during participation in the study, as well as elective AAA repair, did not require reporting as SAEs.
Reporting of protocol violations and deviations
Sites were requested to report all deviations from the study protocol. The site research staff were responsible for ensuring that procedures were undertaken and treatment given in accordance with the protocol. Any suspected protocol violation or deviation was reported on a protocol deviation form and also recorded on the InForm system. The AARDVARK management team was responsible for reviewing all protocol deviations and informing the sponsor as appropriate. Participants continued to participate in the trial except if they had been randomised in error or if they requested to be totally withdrawn from the study. Fully consented patients enrolled on the trial were followed up and analysed as per ITT analysis.
Subject confidentiality
All study staff were responsible for ensuring that participant confidentiality was maintained at all times. On the study worksheets or any other documents submitted to the sponsor, subjects were identified by a subject ID number and initials only.
The chief investigator and PIs were permitted direct access to subjects’ records and source documents only for the purposes of monitoring, auditing or inspection by the sponsor, authorised representatives of the sponsor, regulatory authorities and REC.
Retention of trial documents
This trial was coordinated by the Imperial Clinical Trials Unit (ICTU), which has well-established protocols for the protection of data and facilities for the retention of documents in place. Data will be stored for a minimum of 10 years (or according to changes in regulatory requirements) following completion of this trial. Data generated by this work will be processed in accordance with the Data Protection Act 1998. 80 The ICTU adheres to the Imperial College Code of Practice, drawn up in association with the College’s data protection policy, relating to the collection, holding and disclosure of data relating to individuals. The principal applicant and co-applicants act as custodians of the data and are responsible for its security. The chief investigator or delegate will ensure the continued storage of the documents, even if they leave the clinic/practice or retire before the end of the required storage period. Delegation will be documented in writing. The PI at each site is responsible for the archiving of all of the essential trial documents, including the Investigator Site File, in accordance with regulatory requirements.
The chief investigator and PIs were expected to retain a comprehensive and centralised filing system of all study-related documentation that was suitable for inspection by the sponsor and representatives of regulatory authorities.
Primary efficacy variables
The primary outcome measure was the growth of AAA external diameter (measured in the longitudinal plane) as per the results of the intra-/inter-variability studies (see Chapter 3).
In the absence of any convincing evidence regarding the best method of measuring AAAs, it was decided to inspect the within-trial assessments of measurement repeatability and base the primary outcome on the modality (internal or external) with the greatest within-trial repeatability for the AP diameter in the longitudinal plane.
Secondary efficacy variables
Secondary outcome measures included:
-
time taken for the aneurysm to reach the threshold for intervention (diameter of 5.5 cm)
-
aneurysm rupture
-
aneurysm repair/referral for repair
-
repeatability of internal and external aneurysm diameters
-
quality of life (EQ-5D) and a health resource use questionnaire (12 and 24 months)
-
intolerance of ACE-Is
-
drug compliance
-
reduction in BP.
Safety variables
Safety was assessed during the trial by:
-
assessment of AEs and SAEs
-
monitoring changes in serum creatinine levels.
Statistical methods
Sample size
Based on the inclusion of 225 patients with a baseline AAA diameter of ≤ 5.4 cm and an estimated growth rate (based on UKSAT) of 2.6 mm per year,22 the trial was powered to 90% at the 5% level to detect a 38% reduction in growth rate associated with the ACE-I compared with placebo. The detectable reduction in growth rates with 80% and 70% power were 31% and 28%, respectively. On the assumption that the effects on aneurysm progression are specific to ACE-Is rather than other antihypertensive drugs, the trial was powered to detect a smaller difference in growth rate (< 20%) by comparing the ACE-I group with the other two groups. These calculations allowed for 10% attrition (see Attrition).
The placebo-corrected AAA growth rate in the amlodipine group could be used for evaluation of the extent to which any ACE-I effect on AAA growth was attributable to BP reduction. The events of aneurysm repair, aneurysm rupture and death were to be documented and patients censored at these time points or in the absence of these events at the end of the study.
Attrition
Over a 2-year follow-up period, a total attrition rate of 10% was included in the power calculations for the trial. Participants were included in the attrition rate if data from fewer than two study visits were available for analysis and hence they could not contribute data to the measurement of AAA growth rate.
Outcome measures
The primary outcome measure was growth rate of AAA diameter measured using outer-to-outer (OTO) measurements in the longitudinal plane, estimated using multilevel modelling. Secondary outcome measures included AAA rupture, AAA repair/or referral for repair, time taken for the AAA diameter to reach the threshold for intervention (5.5 cm), tolerance of study medication (measured by compliance, AEs and quality of life) and a comparison of the repeatability of measures of internal and external AAA diameter.
Baseline demographics
Baseline demographic variables (including age, sex, ethnicity, smoking, alcohol, height and weight) and other relevant clinical baseline characteristics (including other coexisting medical conditions, use of statins, pulse pressure, BP, AAA diameter and serum creatinine level) are summarised for each treatment group.
Summaries of continuous variables are presented as means and standard deviations (SDs) if normally distributed and as medians and interquartile ranges for skewed data, whereas categorical variables are presented as frequencies and percentages as planned a priori in the statistical analysis plan.
Primary efficacy analysis
The AAA diameter growth from baseline to month 24 was analysed using linear mixed models (multilevel modelling) in which repeated measurements were nested within subjects. The growth rate was estimated from the individual specific trajectories of AAA diameter over time and the multilevel model has the advantage of using all of the available measurements and of taking into account both between- and within-individual variability over time. The model, described in detail below, gives an estimate of the average growth rate and the difference in growth rate between treatment groups. To check for non-linearity in AAA diameter growth with time, quadratic and cubic models were also fitted. We fitted the following random-intercept model (under the standard assumptions):
where yij is the diameter for each subject j at occasion i, timeij is the corresponding time point, the parameters β1 and β2 are the fixed effect, with β1 representing the mean diameter at baseline and β2 the mean difference in diameter for a unit increase in time, ς1j is the random intercept, that is, the deviation of the individual-specific intercept from the population intercept and ϵij is the residual error. Although 3- or 6-monthly measurements were used to fit the model, the results are presented with 1 year as the unit of time (as annual growth rates are most commonly reported in the literature).
We then specified a random-coefficient model adding a random slope ς2j of time to allow patients to differ in their rate of diameter growth:
where ς2j represents the deviation of the individual-specific slope from the population slope.
The model assumption is that the random intercept and slope components had a joint normal distribution, with a zero mean, a constant SD across individual-specific intercepts, a constant SD across individual-specific slopes and a correlation between the random intercepts and random slopes. The residual error was assumed to be normally distributed with a zero mean and constant SD.
To investigate the difference in growth rate between treatment groups we added two dummy variables for the groups and their interaction with time to the fixed part. The primary comparison was perindopril compared with placebo (B vs. A):
where β1 and β2 are the mean intercept and slope for group A, β3 is the parameter representing the difference in estimated mean diameter at baseline in group B compared with group A and β5 is the parameter representing the difference in the estimated change in diameter over time for group B compared with group A (same interpretation for β4 and β6 for group C compared with group A). A further assumption of this model was that the variation between individual intercepts and slopes and the variation of the residual errors did not differ between groups.
We also checked for a potential site effect adding the site in the model as a random effect. In the sensitivity analysis we examined the site effect as a fixed term.
Secondary efficacy analysis
All secondary end points were summarised in the form of frequency distributions and descriptive statistics at each visit. Differences within groups were tested using paired t-tests and differences at different time points between groups were analysed using linear regression adjusted for baseline. Log-transformation was used for non-normally distributed variables.
The survival secondary end point (time taken to reach 5.5 cm or referred to surgery) was analysed using Kaplan–Meier plots for descriptive analysis and the log-rank test was used to assess differences between treatments.
The repeatability of measurement of internal and external aneurysm diameters was analysed using Bland–Altman methodology. Using repeated measurements taken on the same patient on the same day by different sonographers the repeatability coefficient for each sonographer was reported for intrasonographer variability and the 95% limits of agreement for each sonographer compared with the most experienced vascular scientist were reported for intersonographer variability.
General methodology
Histograms and box plots were used to assess the distributional assumptions and check for possible outliers.
All treatment evaluations were performed under the ITT principle unless otherwise specified. All statistical tests were two-tailed at the 5% significance level.
All of the available AAA diameter measurements were included in the analysis (also from patients who underwent AAA repair, who were withdrawn or who were lost to follow-up). An advantage of using multilevel modelling for the analysis of repeated measurements is that all available information can be used and it gives robust estimation with incomplete data under the assumption of ‘missing at random’. Therefore, no extra missing data imputation was performed.
Nevertheless, before starting the data analysis, the level, pattern and likely causes of the missing data in the baseline variables and outcomes were investigated. Some intermittent missing data were anticipated as patients and sites were given the option of undertaking 6-monthly visits (and we can assume that this intermittent missingness is missing at random).
Interim analysis
A preliminary analysis was undertaken in February 2015 and approval for this analysis was given by the DSMC, Trial Steering Committee (TSC), NIHR HTA programme and Trial Management Group (TMG). The main purpose of this analysis was to undertake a practice run of the final analysis to identify any early potential problems and to give thought to a large-scale trial if warranted.
The results of this preliminary analysis were presented to the study writing committee. The writing committee was also unblinded at this stage to help direct and support the writing of the final report. Subsequent to being unblinded, members of the writing committee had no further involvement in data collection for the trial.
Research governance and management
This IMP trial was conducted in accordance with Medical Research Council (MRC) Guidelines for Good Clinical Practice81 and the Medicines for Human Use (Clinical Trials) Regulations 2004. 82 Imperial College London acted as the trial sponsor. A clinical trial authorisation was applied for and received from the MHRA. A site agreement between Imperial College London and the participating sites outlined the responsibilities of all parties and was signed prior to commencement of recruitment at the participating sites.
Three committees were established to govern the conduct of the trial: the TSC, the independent DSMC and the TMG. These committees functioned in accordance with ICTU standard operating procedures.
Trial Steering Committee
The TSC consisted of three independent members [two vascular surgeons (one as chairperson) and a patient representative] and two members of the trial team (chief investigator and trial manager).
The responsibilities of the TSC were to approve the main study protocol and any amendments, monitor and supervise the trial with regard to its interim and overall objectives, review relevant information from other sources, consider the recommendations of the DSMC and resolve problems brought by the trial co-ordinating centres. The TSC, therefore, provided overall supervision for the trial on behalf of the HTA programme and Imperial College London (sponsor) to ensure that the trial was conducted to the rigorous standards set out in the MRC Guidelines for Good Clinical Practice. 81 Meetings were held by teleconference at regular intervals determined by need and not less than once a year.
Data Safety Monitoring Committee
The DSMC consisted of an independent statistician (chairperson), a vascular surgeon, an expert in hypertension and the trial statistician. In addition, two members of the trial team (the chief investigator and trial manager) attended the open sessions of the DSMC meetings.
The DSMC was responsible for reviewing the progress of the trial and accruing data and providing advice on the conduct of the trial to the TMG and TSC. The DSMC was required to inform the chairperson of the TMG if, in its view:
-
there were concerns about the safety of one or more of the treatment arms
-
the results showed a benefit of one treatment arm over another that was so large, and precise, that it was likely to convince a broad range of clinicians to change practice
-
it was evident that if the trial continued it would fail to show a clear benefit for any treatment arm
-
accrual of patients was so low that it was unlikely that a sufficient number of patients would be recruited to provide meaningful results.
It also had a specific role in reviewing the trial’s progress with the aim of:
-
monitoring evidence for treatment harm (e.g. toxicity data, SAEs, deaths)
-
suggesting additional data analyses, for example of main outcome measures, but only when this was relevant to the trial continuing or stopping early
-
deciding whether or not to recommend that the trial continued to recruit participants or if recruitment should be terminated either for everyone or for some treatment groups and/or some participant subgroups
-
monitoring planned sample size assumptions and recommend amendments if appropriate
-
monitoring recruitment figures and losses to follow-up
-
advising on major protocol modifications suggested by investigators or sponsors such as changing the main end points
-
assessing data quality, including completeness
-
monitoring compliance with the protocol by participants and investigators
-
monitoring the continuing appropriateness of patient information
-
assessing the impact and relevance of external evidence.
Trial Management Group
The TMG consisted of the chief investigator, co-applicants, PIs, trial manager, monitors, project administrators, statisticians, research nurses and patient representative. This group was responsible for the day-to-day management of the trial. The group met on a monthly basis during the recruitment period of the trial and on an ad hoc basis during the follow-up and close-out period.
Patient and public involvement
Several patients with small AAAs were consulted to seek their opinions about the design and running of the trial. All of these patients reported that they would feel reassured by the increased surveillance of their AAA. In addition, a close relative of one patient approached emphasised the painful and stressful nature of the surgery that his relative had undergone and how he wished that there had been an alternative to that surgery. Therefore, anything that could be done to slow the growth of the AAA, to prevent or delay the need for distressing major surgery, was seen as positive.
A patient representative was present on the TMG and was involved in the design of the trial as well as review of protocol amendments and changes to the patient literature.
Protocol changes
All changes to the trial protocol and study conduct were reviewed by the sponsor and submitted to the REC and MHRA for approval as appropriate.
A summary of the amendments made prior to participant recruitment, during recruitment and after recruitment are provided in Tables 4–6, respectively.
| Amendment number | Documents amendment relates to | Date of approval |
|---|---|---|
| 1 |
|
11 May 2011 |
| 2 |
|
5 August 2011 |
| Amendment number | Documents amendment relates to | Date of approval |
|---|---|---|
| 3 |
|
30 January 2012 |
| 4 |
|
4 May 2012 |
| 5 |
|
17 July 2012 |
| 6 |
|
4 September 2013 |
| Amendment number | Documents amendment relates to | Date of approval |
|---|---|---|
| 7 |
|
14 November 2014 |
Chapter 3 Ultrasound measurements and quality assurance
Obtaining abdominal aortic aneurysm measurements
Accurate initial and repeat measurements of AAAs are essential to correctly ascertain growth rates in a trial setting and also to direct clinical management. Ultrasonography is a widely used, non-invasive and effective method for screening and obtaining measurements of AAA diameter. Static images are produced by freezing the image obtained using ultrasound and then accurate measurements can be taken using callipers.
Ultrasonography has been found to be accurate when used to assess AAA diameters by trained observers when compared with CT, and 95% of the differences between the two measurements can be expected to be < 3.5 mm. 83 Similar accuracy was obtained for trained screeners in the UKSAT22 and other aneurysm screening19 and observation84 studies.
In a clinical setting, ultrasound studies of AAAs are most often undertaken by vascular scientists and sonographers who have been trained extensively to undertake such studies. However, staff with no or limited previous experience of ultrasonography can be trained rapidly to identify the presence of AAAs reliably. 85–87 This model of training is used extensively in the NAAASP.
The visualisation of the abdominal aorta may be dependent on the sonographer’s experience and the extent of the patient’s bowel gas and body mass index. However, in a study by Hoffman et al. ,88 despite the level of difficulty reported, the accuracy of aortic measurements by the novices was independent of obesity and central adiposity.
Other common difficulties when scanning include ‘eccentric’ aneurysms and tortuous aortas, as well as the incorrect identification of other structures that appear tubular in cross-section (e.g. the inferior vena cava, the superior mesenteric artery and the gall bladder). The accurate selection of the boundaries of the aorta is also clearly important. 89 It is important that these factors are addressed and that quality improvement strategies are appropriately implemented in trials using ultrasound measurements.
When using ultrasound it is generally accepted that AP abdominal aortic diameters are recorded as standard because of their superior repeatability compared with transverse diameters. 90 However, the most reliable method of measuring AAA diameter remains debatable. The two main methods are measuring the internal diameters [i.e. inner anterior wall to the inner posterior wall (inner to inner or ITI) or intima to intima] or measuring the external diameters [i.e. from the outer anterior to the outer posterior wall (OTO) or adventitia to adventitia] (Figure 2). Some studies also use leading edge to leading edge measurements.
FIGURE 2.
An image of an AAA in the transverse plane showing internal and external AP measurements. The crosses (×) indicate the position of the inner anterior and inner posterior wall and the pluses (+) indicate the position of the outer anterior and outer posterior wall. The asterisk indicates the inner border of an area of thrombus on the posterior wall and it is important that the posterior inner wall calliper is not placed on the inner border of the thrombus.
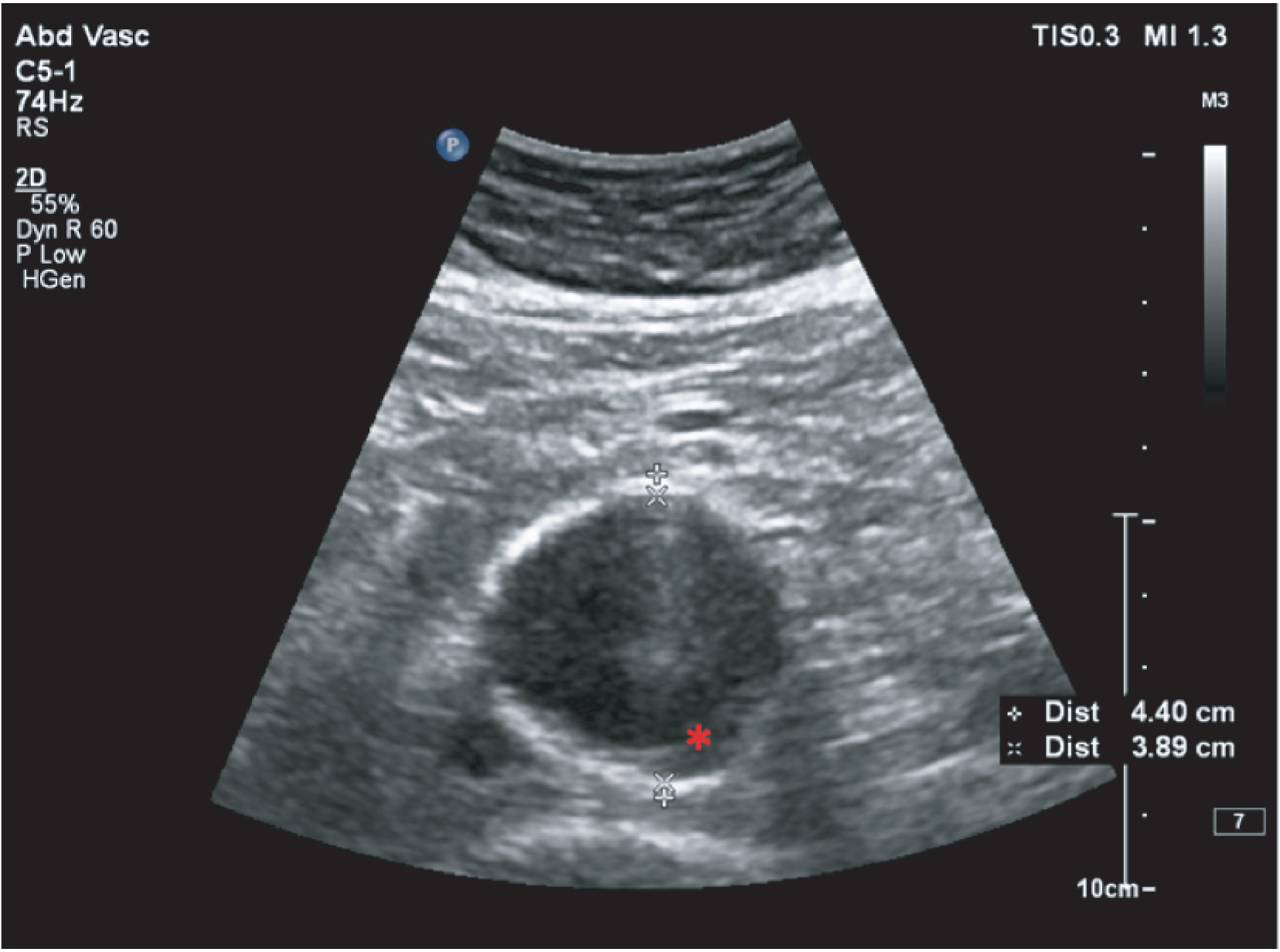
Screening technicians employed by NAAASP are trained to correctly identify and take ITI measurements of AAAs by undertaking a 3-month accredited training programme. For trained screening technicians, the ITI method has been found to have better reliability and reproducibility than OTO measurement. 91 Conversely, when comparing the reliability and reproducibility of measurements taken by vascular scientists, it has been suggested that the OTO method is superior, with the ITI method underestimating the aortic size92 and having greater variability because of difficulty identifying the internal wall because of thrombus93 (see Figure 2), that is, aneurysm growth rates measured using internal diameters have greater noise or scatter than growth rates measured using external diameters. 42
The purpose of the trial quality assurance (QA) process was to:
-
ensure that there was adequate completeness of data collection by all sites
-
ensure the use of a standard protocol in the ultrasound scans carried out across 11 scanning sites by different sonographers on different ultrasound scanning systems
-
ensure the reliability of the results in terms of inter- and intra-observer variability
-
evaluate the reliability of AP AAA measurements taken using internal and external methods in both transverse and longitudinal planes to determine which provided the most repeatable measurements.
Abdominal aortic aneurysm measurement terminology
To ensure accurate comparison with both past and present research it is important to define the terminology used in this report.
Planes
In this study, AP measurements of the AAAs were taken on a transverse plane (referred to as transverse measurements within this report) and on a longitudinal plane (referred to as longitudinal measurements within this report). Figure 3 shows the transverse and longitudinal body planes.
FIGURE 3.
Transverse and longitudinal body planes.
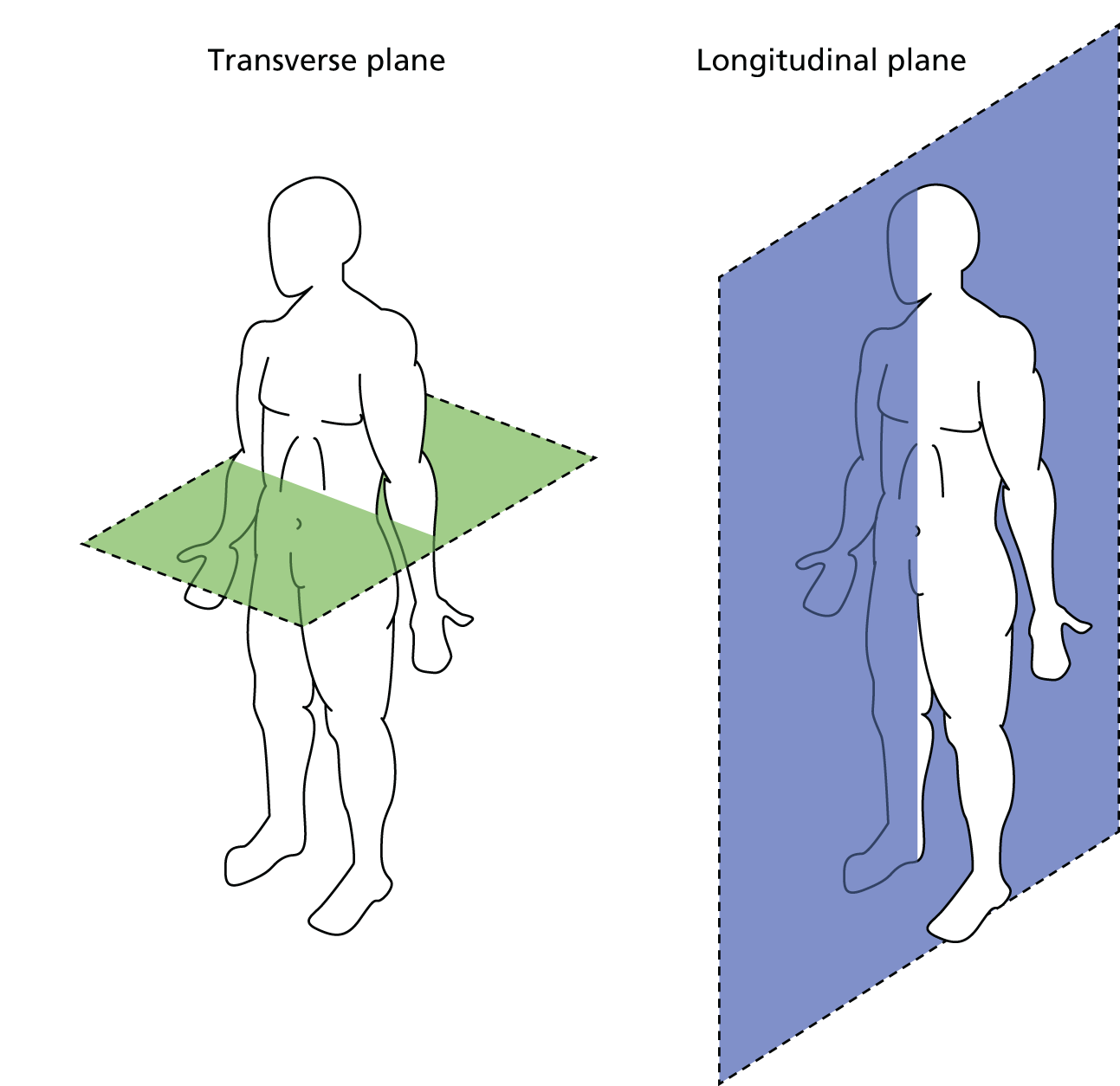
Direction of the ultrasound probe
Figure 4 shows the direction of the probe required to obtain an AP diameter in a longitudinal plane. An example of an image that would be obtained in this view in shown in Figure 5.
FIGURE 4.
Direction of probe required for an image in the longitudinal plane. Reproduced with permission from Fpnotebook.com.
FIGURE 5.
Example of an AP diameter taken in the longitudinal plane.

Figure 6 shows the direction of the probe required to obtain an AP diameter in a transverse plane. An example of an image that would be obtained in this view is shown in Figure 7.
FIGURE 6.
Direction of probe required for an image in the transverse plane. Reproduced with permission from Fpnotebook.com.
FIGURE 7.
Example of an AP diameter taken in the transverse plane.

An example of a lateral wall to lateral wall diameter taken in a transverse plane is shown in Figure 8. Lateral wall to lateral wall diameters were not collected in this trial. This is because tortuosity of the aorta is common in aneurysmal disease as the aorta grows in length as well as width and these diameters can be considerably inaccurate.
FIGURE 8.
Example of an AAA image in the transverse plane with the measurements taken from lateral wall to lateral wall.
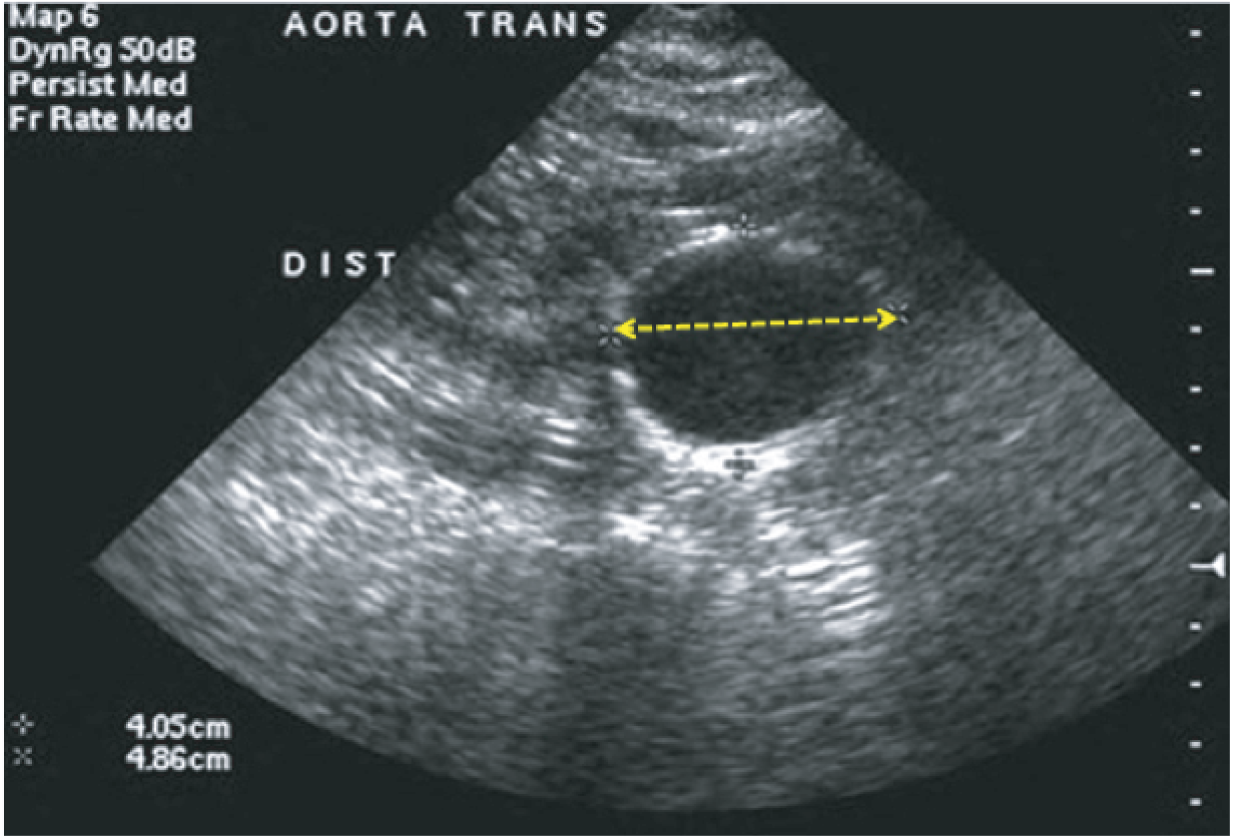
Ultrasound scanning protocol
At the time of this study there still remained debate over the best method of obtaining AAA measurements, as highlighted earlier. Therefore, four measurements in total – both maximum AP internal and external measurements in the transverse and longitudinal planes – were recorded on all patients, to enable a reliable comparison and to inform future practice.
The study was initially designed to have just two dedicated scanning staff between five sites (four in London and one in Coventry) to enhance the accuracy and repeatability of measurements. After 6 months it became clear that fewer patients than anticipated were eligible for the trial at these sites and therefore we were required to expand the study to recruit at 14 sites to meet the recruitment target, with scanning taking place at 11 sites (patients from all four London recruiting sites were scanned at ICCH, St Mary’s Hospital; see Figure 1).
This required more rigorous scanning and quality control protocols to be implemented to ensure maximum accuracy in the measurement of AAAs at all sites. A scanning protocol was provided to all participating sites to ensure the highest possible standard and accuracy in the ultrasound scans carried out across the 11 scanning sites. The final version of the scanning protocol is provided in Box 1.
-
At each site it is requested that a single fully trained sonographer, vascular scientist or ultrasound screening technician performs all scans when possible.
-
When alternative arrangements for the sonographers are made at some sites (e.g. vacation, illness), sonographers should follow identical protocols and should ensure that there is internal consistency in recorded measurements from the site.
-
The same ultrasound system and probes should be used for each subject on each occasion.
-
The patient should be brought into the room and allowed to rest on the couch in the semi-recumbent position for a period of 5 minutes for acclimatisation (while details are entered onto the duplex system).
-
Once scanning is to be performed, the patient should be laid flat on the examination couch with abdomen exposed from xiphisternum to pubic symphysis.
-
The room should be appropriately lit to facilitate accurate scanning in a standard fashion (i.e. similar on all occasions).
-
Review previous image to determine the point from which to begin measurement.
-
Perform an initial scan, checking for compressibility, pulsation and anterior branches, to enable the aorta to be correctly identified.
-
The aorta should then be scanned in both longitudinal and transverse planes from the renal arteries to the bifurcation of the iliac arteries.
-
The point of maximum dilatation in both transverse and longitudinal planes should be identified.
-
Image optimisation should be achieved using gain/depth etc. control to produce an image with the clearest wall appearance.
-
All images must be taken with the highest quality and accuracy.
-
Following maximum point identification, the longitudinal image should be frozen on screen.
-
Four images in total must be saved.
-
Measurements are now taken using the calipers from the anterior side of the aorta to the posterior side. The measurements taken should be at 90° to the wall.
-
An ITI measurement should be made by placing the calipers on the inside of the aortic wall, taking care not to misidentify plaque or thrombus as in the inner wall.
-
This image should be saved after labelling the scan image with LS (for longitudinal view), ITI and the time of the scan within the study (baseline, 3 months, 6 months, etc.) and adding the sonographer’s initials.
-
An OTO measurement should be made by placing the calipers on the outer walls, taking care not to misidentify the surface of the spine or other structures as the posterior wall.
-
This image should be saved after labelling the scan image with LS, OTO and the time of the scan within the study (baseline, 3 months, 6 months, etc.) and adding the sonographer’s initials.
-
The ultrasound probe should be pivoted 90° at the same point (of maximum size) on the aorta. This image in the transverse plane should be frozen.
-
An ITI measurement should be made by placing the calipers on the inside of the aortic wall, taking care not to misidentify plaque or thrombus as in the inner wall.
-
This image should be saved after labelling the scan image with TS (for transverse view), ITI and the time of the scan within the study (baseline, 3 months, 6 months, etc.) and adding the sonographer’s initials.
-
Check the results that have been obtained. If the ITI and OTO measurements in TS and LS are not similar then the patient should be rescanned and checked – if there is a reason for disagreement such as tortuosity of the vessel, make a comment on the record.
-
Problematic measurements: if you are experiencing difficulty defining and measuring the aorta and its aneurysm, please consult a more senior sonographer for assistance and make a note on the ‘Relevant comments’ section of the scanning log.
Images, with measurements and labelled as described in the scanning protocol, will be stored on the hard drive of the scanner and subsequently downloaded onto the Picture Archiving and Communication System or other storage devices at the co-ordinating centre. All sites will be required to download all images onto a CD to be sent to the co-ordinating centre for QA purposes.
Longitudinal anteroposterior inner-to-inner measurements

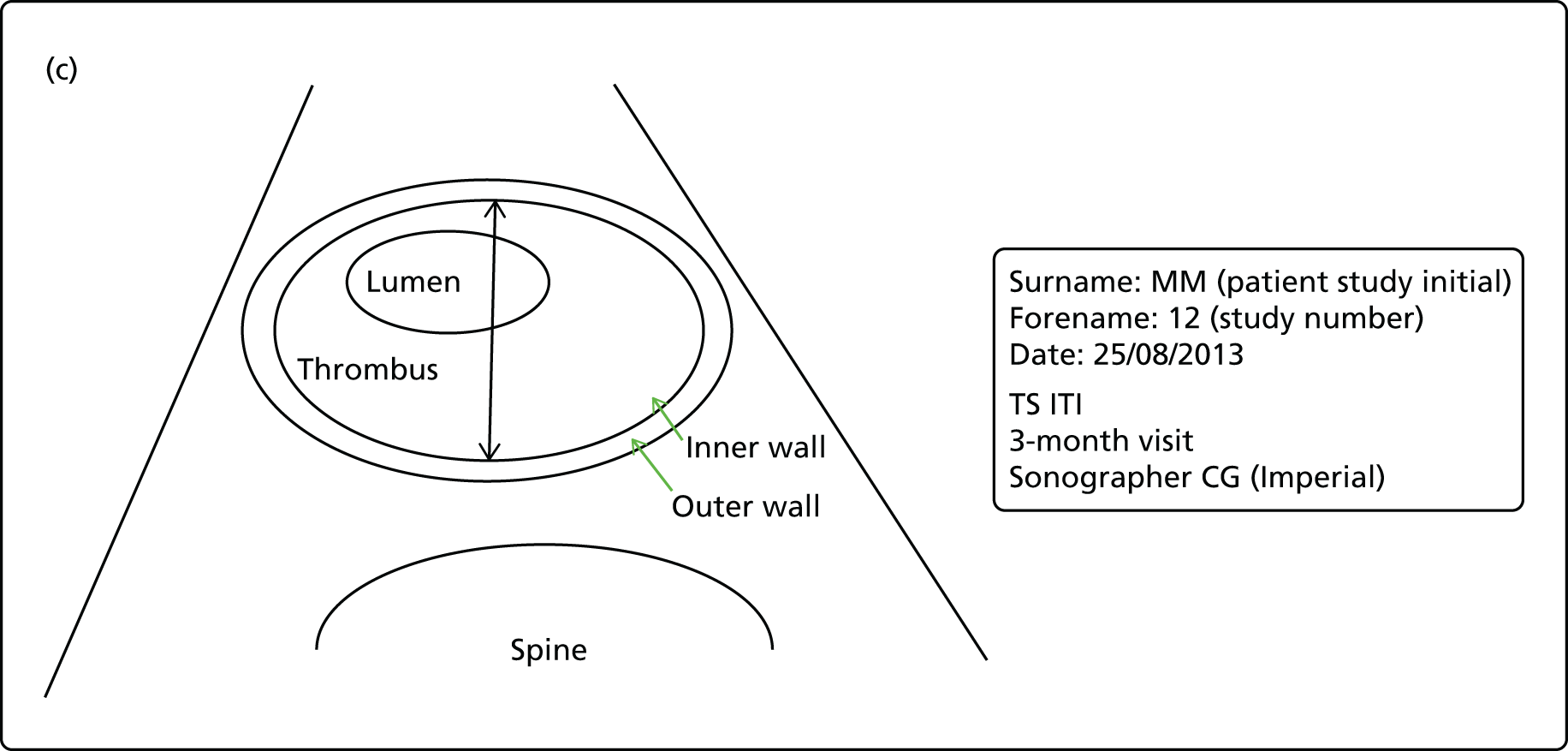

Performance in data collection
Recording of the four AAA measurements was required for each patient at each visit. A key performance indicator for sites was that of completeness of data recording for all four required measurements. The completeness of data collection at each visit was between 94% and 100%. Table 7 describes the percentages of patients who attended who had all four required measurements recorded at the end of the study visit.
| Measurement | Placebo | Perindopril | Amlodipine | Total |
|---|---|---|---|---|
| Randomisation (n) | 79 | 73 | 72 | 224 |
| External diameter longitudinal | 79 (100) | 71 (97) | 71 (99) | 221 (99) |
| Internal diameter longitudinal | 78 (99) | 71 (97) | 68 (94) | 217 (97) |
| External diameter transverse | 79 (100) | 73 (100) | 72 (100) | 224 (100) |
| Internal diameter transverse | 78 (99) | 71 (97) | 69 (96) | 218 (97) |
| 3-month visit (n) | 76 | 68 | 67 | 211 |
| External diameter longitudinal | 74 (97) | 65 (96) | 65 (97) | 204 (97) |
| Internal diameter longitudinal | 74 (97) | 65 (96) | 65 (97) | 204 (97) |
| External diameter transverse | 74 (97) | 65 (96) | 65 (97) | 204 (97) |
| Internal diameter transverse | 74 (97) | 65 (96) | 65 (97) | 204 (97) |
| 6-month visit (n) | 69 | 62 | 62 | 193 |
| External diameter longitudinal | 66 (96) | 61 (98) | 60 (97) | 187 (97) |
| Internal diameter longitudinal | 66 (96) | 61 (98) | 60 (97) | 187 (97) |
| External diameter transverse | 67 (97) | 61 (98) | 60 (97) | 188 (97) |
| Internal diameter transverse | 68 (99) | 61 (98) | 60 (97) | 189 (98) |
| 9-month visit (n) | 64 | 57 | 57 | 178 |
| External diameter longitudinal | 63 (98) | 56 (98) | 54 (95) | 173 (97) |
| Internal diameter longitudinal | 63 (98) | 56 (98) | 54 (95) | 173 (97) |
| External diameter transverse | 62 (97) | 56 (98) | 54 (95) | 172 (97) |
| Internal diameter transverse | 62 (97) | 56 (98) | 54 (95) | 172 (97) |
| 12-month visit (n) | 71 | 61 | 54 | 186 |
| External diameter longitudinal | 68 (96) | 60 (98) | 51 (94) | 179 (96) |
| Internal diameter longitudinal | 69 (97) | 60 (98) | 51 (94) | 180 (97) |
| External diameter transverse | 68 (96) | 60 (98) | 52 (96) | 180 (97) |
| Internal diameter transverse | 69 (97) | 60 (98) | 52 (96) | 181 (97) |
| 15-month visit (n) | 60 | 49 | 47 | 156 |
| External diameter longitudinal | 57 (95) | 47 (96) | 44 (94) | 148 (95) |
| Internal diameter longitudinal | 57 (95) | 47 (96) | 44 (94) | 148 (95) |
| External diameter transverse | 57 (95) | 47 (96) | 44 (94) | 148 (95) |
| Internal diameter transverse | 57 (95) | 47 (96) | 44 (94) | 148 (95) |
| 18-month visit (n) | 61 | 52 | 49 | 162 |
| External diameter longitudinal | 60 (98) | 49 (94) | 47 (96) | 156 (96) |
| Internal diameter longitudinal | 60 (98) | 49 (94) | 47 (96) | 156 (96) |
| External diameter transverse | 60 (98) | 49 (94) | 47 (96) | 156 (96) |
| Internal diameter transverse | 60 (98) | 49 (94) | 47 (96) | 156 (96) |
| 21-month visit (n) | 54 | 44 | 47 | 145 |
| External diameter longitudinal | 52 (96) | 43 (98) | 47 (100) | 142 (98) |
| Internal diameter longitudinal | 52 (96) | 44 (100) | 47 (100) | 143 (99) |
| External diameter transverse | 52 (96) | 43 (98) | 47 (100) | 142 (98) |
| Internal diameter transverse | 52 (96) | 44 (100) | 47 (100) | 143 (99) |
| 24-month visit (n) | 59 | 53 | 49 | 161 |
| External diameter longitudinal | 56 (95) | 52 (98) | 47 (96) | 155 (96) |
| Internal diameter longitudinal | 56 (95) | 52 (98) | 48 (98) | 156 (97) |
| External diameter transverse | 56 (95) | 52 (98) | 47 (96) | 155 (96) |
| Internal diameter transverse | 56 (95) | 52 (98) | 48 (98) | 156 (97) |
Quality assurance events
Inter- and intraobserver QA scanning events were organised to ensure consistency between sonographers, screening technicians and others responsible for aortic measurements and between measurements by the same observers. Specific aims of the QA events were:
-
to ensure the reliability of the results in terms of inter- and intra-observer variability.
-
to evaluate which was the most accurate and repeatable of AP AAA measurements taken using ITI and OTO methods in both transverse and longitudinal planes.
Three QA days were arranged, one each in York, Hull and London. A total of 19 observers scanned patients over the course of the trial (Table 8). Of these, 12 (63%) attended the QA days. At least one observer from each of the 11 scanning sites attended one of the QA events. The numbers of observers attending and scanning centre locations are documented in Table 8. Six volunteers were scanned on 8 July 2013, five volunteers were scanned on 13 September 2013 and five volunteers were scanned on 8 November 2013.
| Site | QA event date | Number of sonographers from the site | Number of sonographers attending | ||
|---|---|---|---|---|---|
| 8 July 2013 | 13 September 2013 | 8 November 2013 | |||
| Royal Bournemouth Hospital | Yes | 1 | 1 | ||
| Norfolk and Norwich University Hospital | Yes | 4 | 1 | ||
| Colchester General Hospital | Yes | 1 | 1 | ||
| Northern General Hospital, Sheffield | Yes | 2 | 2 | ||
| Freeman Hospital, Newcastle | Yes | 2 | 1 | ||
| University Hospital Coventry | Yes | 1 | 1 | ||
| Sunderland Royal Hospital | Yes | 1 | 1 | ||
| York Hospital | Yes | 2 | 1 | ||
| London sites | Yes | 3 | 1 | ||
| Manchester Royal Infirmary | Yes | Yes | 1 | 1 | |
| Hull Royal Infirmary | Yes | 1 | 1 | ||
| AARDVARK trial vascular scientist | Yes | Yes | Yes | NA | NA |
| Total | 3 | 6 | 3 | 19 | 12 |
At each event the following took place:
-
review of the scanning requirements as documented in the protocol
-
discussion of example problem cases as a teaching prompt
-
highlighting of known improvement strategies to reduce variability as needed
-
scanning of volunteer patients for inter- and intraobserver variability studies.
Patients with an AAA who were part of the trial were invited to attend the QA days. Each was informed that they were to have their AAA measured on at least two occasions by each sonographer who was able to attend that QA session.
The scanning protocol was reviewed by all of the clinicians. Teaching on problem cases and strategies for best practice were presented and the structure for the day outlined in full.
All of the sonographers who had scanned trial patients were observed scanning at least one subject following the scanning protocol until the trial sonographer was convinced that appropriate protocols were being followed and adequate images were being obtained consistently. Each of the sonographers from the sites then scanned each of the AAA patients using the scanning protocol. All sonographers were blinded to the clinical details, size and anatomical configurations of the aneurysms of patients as far as possible. Volunteers were not named but were allocated an identifier for anonymity in an attempt to minimise bias. All four required measurements were recorded and handed to the trial manager. All patients were measured on at least two occasions by each sonographer. The trial clinical vascular scientist also performed a set of measurements for each patient.
The mean intraobserver variability is documented in Table 9. The mean intraobserver variability repeatability coefficient is documented for each of the four measurements obtained by observers in the study along with the SD and range. The repeatability coefficient is the value below which the absolute difference between repeated test results is expected to lie with a probability of 95%.
| Measure of intraobserver variability | Longitudinal ITI (cm) | Longitudinal OTO (cm) | Transverse ITI (cm) | Transverse OTO (cm) |
|---|---|---|---|---|
| Mean | 0.32 | 0.33 | 0.48 | 0.50 |
| SD | 0.05 | 0.09 | 0.27 | 0.34 |
| Minimum | 0.24 | 0.21 | 0.23 | 0.20 |
| Maximum | 0.39 | 0.45 | 1.07 | 1.24 |
Clearly, measurements in the longitudinal plane were more repeatable than measurements in the transverse plane. The differences in the mean (range) ITI and OTO intrasonographer repeatability coefficients for longitudinal measurements were similar at 0.32 (0.24–0.39) cm for ITI measurements and 0.33 (0.21–0.45) cm for OTO measurements. This was compared with 0.48 (0.23–1.07) cm and 0.50 (0.20–1.24) cm for transverse ITI and OTO measurements, respectively.
As most sites used more than one observer to follow up the enrolled trial patients, interobserver variability was of great importance.
The differences between the AAA measurements taken by the sonographers and those taken by the senior clinical vascular scientist in the same patients were used to calculate the intersonographer variability by using Bland–Altman 95% limits of agreement (Table 10). The limits of agreement define the range within which 95% of the differences between two measurements made by two observers are likely to fall. Figure 9 shows the variability in the measurements of external longitudinal diameter compared with the measurements of the senior clinical vascular scientist (expert). Thirteen pairs disagreed by > ±4 mm for ITI measurements, whereas seven pairs disagreed by > ±4 mm for OTO measurements. These data suggest that the interobserver repeatability is better for longitudinal OTO measurements than for longitudinal ITI measurements.
| Observera | Intraobserver variability repeatability coefficient (cm) | Interobserver variabilityb Bland–Altman 95% limits of agreement (cm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Long in | Long out | Tran in | Tran out | Long in | Long out | Tran in | Tran out | |
| A1 July | 0.39 | 0.26 | 0.42 | 0.34 | –0.87 to 0.32 | –0.42 to 0.31 | –0.54 to 0.19 | –0.42 to 0.47 |
| A2 July | 0.35 | 0.44 | 0.44 | 0.31 | –0.59 to 0.42 | –0.59 to 0.48 | –0.29 to 0.36 | –0.28 to 0.34 |
| A3 July | 0.34 | 0.42 | 0.33 | 0.36 | –0.32 to 0.25 | –0.30 to 0.60 | –0.32 to 0.33 | –0.25 to 0.54 |
| A1 September | 0.36 | 0.26 | 1.07 | 1.24 | –0.67 to 0.19 | –0.5 to 0.35 | –0.57 to 0.04 | –0.61 to 0.41 |
| A3 September | 0.26 | 0.24 | 0.29 | 0.25 | –0.004 to 0.40 | –0.34 to 0.26 | –0.15 to 0.50 | –0.33 to 0.13 |
| A4 September | 0.37 | 0.45 | 0.41 | 0.49 | –0.06 to 0.71 | –0.26 to 0.64 | –0.05 to 0.61 | –0.34 to 0.68 |
| A5 September | 0.34 | 0.43 | 0.45 | 0.48 | –0.15 to 0.56 | –0.42 to 0.59 | –0.13 to 0.38 | –0.34 to 0.40 |
| A6 September | 0.30 | 0.34 | 0.94 | 1.04 | –0.21 to 0.31 | –0.65 to 0.62 | –0.62 to 1.26 | –0.91 to 1.59 |
| A7 September | 0.24 | 0.21 | 0.68 | 0.75 | –0.44 to 0.22 | –0.4 to 0.65 | –0.53 to 0.16 | –0.4 to 0.63 |
| A1 November | 0.27 | 0.24 | 0.23 | 0.25 | –0.87 to 0.69 | –0.48 to 0.69 | –0.87 to 0.66 | –0.65 to 0.65 |
| A2 November | 0.26 | 0.28 | 0.24 | 0.20 | –1.37 to 0.71 | –0.87 to 0.74 | –1.33 to 0.76 | –0.80 to 0.72 |
| A3 November | 0.39 | 0.42 | 0.28 | 0.24 | –1.10 to 0.69 | –0.77 to 0.48 | –1.25 to 0.94 | –1.01 to 0.76 |
FIGURE 9.
Interobserver repeatability graphs (longitudinal diameters). (a) Longitudinal internal diameter; and (b) longitudinal external diameter. Each symbol on the graphs represents a different AARDVARK scanner (three from the July event, three from the November event and six from the September event). Five or more patients are shown for each event, so 10 vertical scatters have only three points and five vertical scatters have six points. Each vertical scatter is a separate patient.


Quality control of ultrasound scans
The quality of the ultrasound images obtained in trial was assessed centrally to ensure that a reliable standard of ultrasound scans was obtained across the 11 scanning sites by different sonographers on different ultrasound scanning systems. The following protocol was used:
-
The baseline images for all of the patients in the trial were reviewed as soon as possible after the first monitoring visit.
-
All patients at a site or a minimum of 10 patients at a site (for sites with > 10 patients) had each of their 3-monthly images reviewed. Review was undertaken by the senior clinical vascular scientist. Up to 10 random patients were selected by the trial statistician for quality control analysis. If one of the selected patients reached an end point or withdrew from the study or did not attend the visit, the number of images checked might be less than baseline in certain cases. In addition, we asked for additional images to be checked if poor image quality was found at sites. Hence, > 10 images were checked at certain visits. The numbers of recorded images reviewed from each site by the senior clinical vascular scientist are provided in Table 11.
-
All sites were informed of the scans that needed to be forwarded to the trial centre.
-
Each site was asked to save images from longitudinal and transverse planes at the point of maximum dilation to include ITI and OTO calliper placement records.
-
The senior clinical vascular scientist reviewed all images sent to the trial centre. All of the images had patient-identifiable information removed and the senior clinical vascular scientist was blinded to treatment allocations.
-
Each image was assessed on clarity (looking at depth, gain, focus and good wall definition) and calliper placement (ITI and OTO). The following criteria were used to judge an image as acceptable:
-
the image was of the highest image quality possible
-
the callipers were placed correctly for both ITI and OTO measurements in relation to the wall
-
measurements were taken at right angles to the aneurysm sac wall
-
the image was labelled correctly
-
the image was judged to be taken in a true transverse and true longitudinal plane.
-
| Site | Visit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 3 | Month 6 | Month 9 | Month 12 | Month 15 | Month 18 | Month 21 | Month 24 | |
| Colchester General Hospital | 17 | 8 | 11 | 11 | 10 | 11 | 11 | 10 | 10 |
| Freeman Hospital, Newcastle | 5 | 3 | 2 | 2 | 2 | 2 | 2 | 0 | 2 |
| Hull Royal Infirmary | 40 | 22 | 33 | 18 | 8 | 9 | 9 | 10 | 10 |
| Manchester Royal Infirmary | 3 | 3 | 3 | 3 | 1 | 0 | 0 | 0 | 3 |
| Norfolk and Norwich University Hospital | 14 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Northern General Hospital, Sheffield | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 2 |
| Royal Bournemouth Hospital | 50 | 24 | 17 | 11 | 10 | 10 | 10 | 11 | 11 |
| Royal Free Hospital, London | 9 | 9 | 7 | 8 | 8 | 8 | 8 | 7 | 9 |
| St Thomas’ Hospital, London | 25 | 12 | 18 | 11 | 8 | 7 | 7 | 7 | 11 |
| St Mary’s Hospital/Charing Cross Hospital, London | 17 | 10 | 16 | 10 | 9 | 11 | 11 | 11 | 11 |
| Sunderland Royal Hospital | 4 | 4 | 4 | 4 | 3 | 3 | 1 | 0 | 3 |
| University Hospital Coventry | 28 | 14 | 21 | 12 | 9 | 9 | 9 | 9 | 10 |
| York Hospital | 6 | 4 | 4 | 4 | 3 | 0 | 3 | 3 | 3 |
Each image that was reviewed was classified as acceptable or unacceptable.
This protocol was based on the standard operating procedures for the NAAASP [see www.gov.uk/government/collections/aaa-screening-supporting-documents (accessed 3 June 2016)], with the addition of the following assessment criteria: measurements to be taken at right angles to the aneurysmal sac and judged to be in the transverse and longitudinal planes.
If an image was deemed unacceptable, the site was contacted and asked to review the image to ensure that the correct image had been sent, the callipers had been placed correctly and the AARDVARK scanning protocol had been followed. If required, the senior clinical vascular scientist responsible for quality control and the relevant sonographer liaised directly until a resolution was reached.
Sites/observers with unacceptable images were contacted. When a single image taken by an observer was unacceptable, the observer was contacted to review the image. If more than two images were unacceptable then the site was visited and all observers underwent further dedicated training sessions. In these training sessions the site was visited by the lead sonographer for the study and (1) the scanning protocol was reviewed and relevant teaching given and (2) each of the images that was deemed unacceptable was reviewed. This was required at only one site.
Quality control of the final ultrasound data
In addition to quality control of the ultrasound images and the QA events, the ultrasound measurement database as a whole was monitored for any anomalies or spurious results. Sites were requested to measure the maximum AP diameter of the AAA at each visit. Therefore, the ITI transverse and longitudinal measurements and the OTO transverse and longitudinal measurements at each visit were expected to be within ±3 mm of each other.
If the longitudinal and transverse measurements varied by > ±3 mm, sites were contacted and asked to recheck their scans and measurements to ensure that the maximum AP diameter had been accurately measured. Cases were accepted or rejected accordingly and remeasured when an obvious error had been made.
In total, 107 ITI measurements and 35 OTO measurements had differences > ±3 mm. This, together with the results of the interobserver variability study and the use of multiple observers for the trial, suggested that the longitudinal OTO measurement was the most reliable measurement on which to base aneurysm growth rate. Therefore, longitudinal OTO measurements were used for the evaluation of the trial end points.
The 35 OTO measurements were reconsidered as this measurement was the primary measurement to be used in the main trial analyses. Four measurements were amended after obvious measurement errors were made. Adjusted measurements were used for the analyses in the trial.
Justification for the choice of diameter used for primary end point
The mean (range) intrasonographer repeatability coefficient for longitudinal measurements was 0.33 (0.21–0.45) cm for OTO measurements and 0.32 (0.24–0.39) cm for ITI measurements compared with 0.48 (0.23–1.07) cm and 0.50 (0.20–1.24) cm for transverse ITI and OTO measurements, respectively. Longitudinal plane measurements were therefore used for the primary analyses.
However, possibly reflecting the small number of patients included (n = 11), there was no meaningful difference between the variability of OTO and ITI measurements reported in intraobserver variability studies that could determine the superiority of OTO or ITI measurements for use as the primary end point in the study. Considering ITI interobserver variability measurements, 13 pairs of measurements (by an observer and the senior clinical vascular scientist) differed by > ±4 mm, whereas for OTO measurements seven pairs of measurements differed by > ±4 mm. These data suggest that interobserver repeatability was better for longitudinal OTO measurements than for longitudinal ITI measurements
When comparing transverse and longitudinal maximum diameters in the same patient at each time point there was less variation between OTO measurements than between ITI measurements (35 vs. 107 measurements were different by > 0.3 mm, respectively). In addition, there was less variation in the SDs of AAA measurements for most time points in the study of OTO measurements (see Table 19).
Therefore, OTO diameters measured from longitudinal images were used for evaluation of the primary outcome measure.
Chapter 4 Results
Site recruitment
Recruitment was originally to take place at five sites: Imperial College Healthcare NHS Trust (St Mary’s and Charing Cross Hospitals), Royal Free London NHS Foundation Trust, Guy’s and St Thomas’ NHS Foundation Trust and University Hospitals Coventry and Warwickshire NHS Trust. We identified a pool of approximately 860 patients potentially eligible for the trial from existing databases from these four hospital trusts (200, 90, 380 and 189, respectively). We also predicted that expansion of the NAAASP was likely to increase this patient pool before trial recruitment started, with approximately 4% of men screened expected to have an AAA. Against this, we estimated that 30% of patients would be ineligible for the trial because they were already receiving ACE-I therapy or because they had an unsuitable BP, that is, SBP could not be controlled to < 150 mmHg before randomisation. Assuming a 15% refusal rate in the remaining 262 patients, the target of 225 patients seemed achievable given a steady influx of newly screened and eligible patients over the recruitment period.
The first trial site was opened to recruitment in September 2011, with recruitment due to end after 12 months. However, to meet the recruitment target a further nine sites were added during the trial. The four sites in London became recruitment and screening sites, with patients having all other study visits at the ICCH, Imperial College London. The remaining 10 scanning sites were located across the country and performed all study visits locally.
Participant recruitment
A recruitment extension of 7 months was required before the recruitment target was successfully met. The reasons for this were as follows:
-
The number of patients taking ACE-Is was higher than anticipated and this was the main barrier to recruitment. At the time of the HTA programme commissioned call in 2009, approximately 32.3% of the population aged > 60 years received ACE-Is; by 2012 this had increased to 52.9% (Health Survey for England). 94
-
The proportion of patients with an AAA picked up through the NAAASP was less than half the proportion expected: 4% of patients screened were expected to have an AAA, whereas in reality this proportion was 1.2%.
-
Progress was slow in securing NHS research governance approval at some of the participating sites. The quickest research and development approval was granted in 33 working days (approximately 1.5 months), whereas the longest approval took 147 working days (6 months). After waiting 168 working days (7 months) with approval still not granted in one further site, attempts to gain approval were abandoned.
To try and overcome some of the barriers to recruitment, the following strategies and actions were implemented approximately 6 months into the study:
-
Review of methods of approaching patients – sites were requested to review their methods of contacting patients and try different strategies (i.e. speaking to patients at their next visit instead of cold-calling) in an attempt to increase uptake into the trial.
-
Breakdowns of site recruitment were requested monthly and were reviewed at the monthly management meetings to try and identify and address any specific issues.
-
Addition of PICs – some centres had PIC sites set up to widen the pool of potential participants.
-
The age range was expanded to include those aged ≥ 55 years (originally set at ≥ 60 years).
-
Patient invitation letter – this was created because some site investigators felt that the PIS was too lengthy and daunting as a first introduction to the trial.
-
Trial poster – this was created and put on the noticeboards in relevant clinical areas (e.g. in the vascular ultrasound laboratories) at some sites to create awareness of the trial.
-
AARDVARK trial web page – this was set up via the ICTU website and was available as a resource for both patients and site staff. The site contained general information about the trial and was updated regularly with current recruitment numbers.
-
Update/simplify the PIS – some sites felt that the original PIS was written in such a way that the side effects and risks of the study were unduly worrying to potential participants. Therefore, the PIS was reworded and a list of side effects tabulated as an appendix.
-
Additional research sites – by far the most successful strategy for increasing recruitment was increasing the number of research sites, specifically targeting those with large populations and time/resources to conduct the study.
-
Chief investigator presence at site initiation visits – the chief investigator visited all sites as part of the site initiation visits to stress the importance of the trial and answer any clinical queries.
In total, 227 patients were initially randomised to the trial. However, the randomisation of three patients was subsequently discovered to be a protocol violation because these patients did not meet all of the entry criteria for the trial. Table 12 shows the final recruitment numbers by site. The Royal Bournemouth Hospital and Hull Royal Infirmary were the top recruiting sites, with 50 and 40 patients randomised, respectively. The first patient was randomised to the study on 16 December 2011 and the last patient was randomised to the study on 19 April 2013 (Figure 10).
| Site | Randomised, n (%) |
|---|---|
| Charing Cross Hospital, London | 4 (1.8) |
| Colchester General Hospital | 17 (7.6) |
| Freeman Hospital, Newcastle | 5 (2.2) |
| Hull Royal Infirmary | 40 (17.9) |
| Manchester Royal Infirmary | 3 (1.3) |
| Norfolk and Norwich University Hospital | 14 (6.3) |
| Northern General Hospital, Sheffield | 2 (0.9) |
| Royal Bournemouth Hospital | 50 (22.3) |
| Royal Free Hospital, London | 9 (4.0) |
| St Thomas’ Hospital, London | 25 (11.2) |
| St Mary’s Hospital, London | 17 (7.6) |
| Sunderland Royal Hospital | 4 (1.8) |
| University Hospital Coventry | 28 (12.5) |
| York Hospital | 6 (2.7) |
| Total | 224 |
FIGURE 10.
Participant recruitment by month.
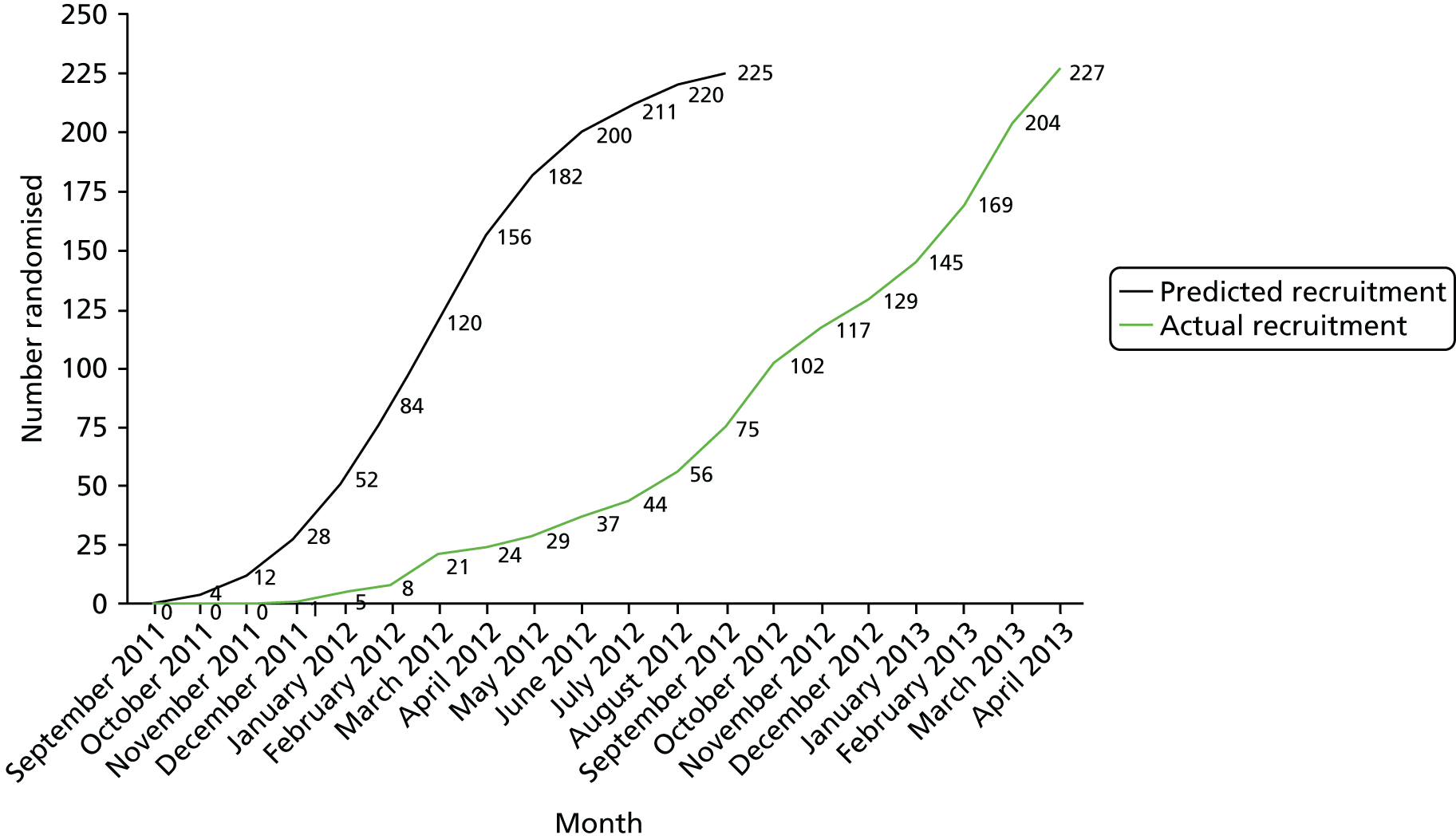
Table 13 shows that the number of patients randomised to each treatment arm was well balanced, with 79 patients randomised to placebo, 73 randomised to perindopril and 72 randomised to amlodipine.
| Site | Placebo | Perindopril | Amlodipine | Total |
|---|---|---|---|---|
| Charing Cross Hospital, London | 1 | 1 | 2 | 4 |
| Colchester General Hospital | 6 | 6 | 5 | 17 |
| Freeman Hospital, Newcastle | 2 | 2 | 1 | 5 |
| Hull Royal Infirmary | 14 | 13 | 13 | 40 |
| Manchester Royal Infirmary | 2 | 1 | 0 | 3 |
| Norfolk and Norwich University Hospital | 5 | 5 | 4 | 14 |
| Northern General Hospital, Sheffield | 1 | 1 | 0 | 2 |
| Royal Bournemouth Hospital | 17 | 16 | 17 | 50 |
| Royal Free Hospital, London | 3 | 3 | 3 | 9 |
| St Thomas’ Hospital, London | 8 | 8 | 9 | 25 |
| St Mary’s Hospital, London | 7 | 5 | 5 | 17 |
| Sunderland Royal Hospital | 1 | 1 | 2 | 4 |
| University Hospital Coventry | 10 | 9 | 9 | 28 |
| York Hospital | 2 | 2 | 2 | 6 |
| Total | 79 | 73 | 72 | 224 |
Non-recruited patients
During the trial recruitment period we requested that the sites capture details of reasons why patients with an AAA were not recruited into the trial. These data were collected by prospective pre-screening by recruiting staff, mainly by case note and clinical database review. Unfortunately, limited research nurse availability and clinical service pressures prevented reliable collection of these data at some centres. However, data were collected from a total of 1912 non-recruited patients. The patients were excluded on the basis of ineligibility (Figure 11) or because they declined to participate (Figure 12). The main reason for ineligibility was already taking ACE-Is; this excluded 643 patients from participating in the trial. Unfortunately, a specific reason for declining to participate was not recorded for 107 patients; however, 56 patients declined because they did not want to participate in a clinical trial.
FIGURE 11.
Reasons for trial ineligibility, n (%) (N = 1595).
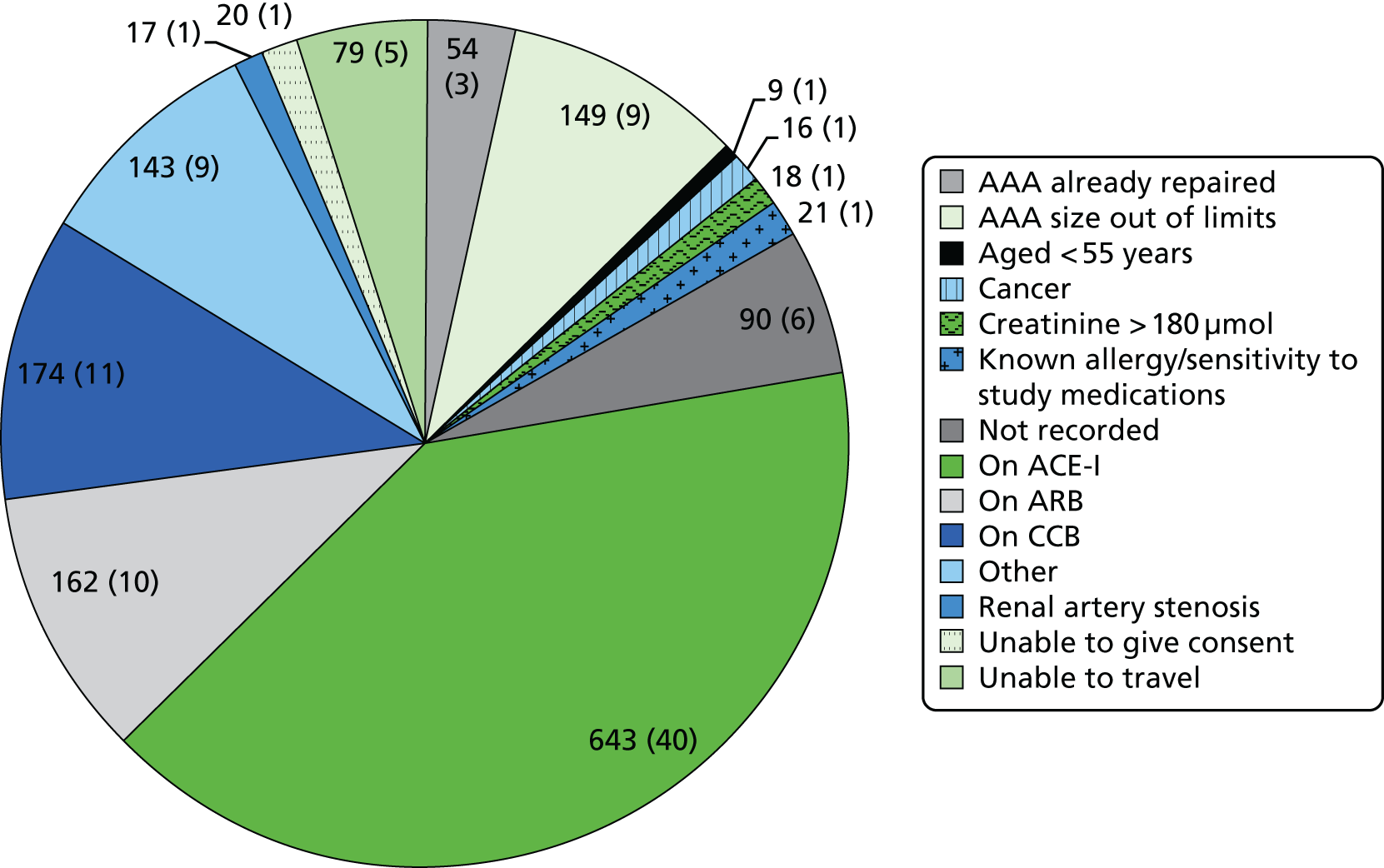
FIGURE 12.
Reasons for declining the trial, n (%) (N = 317).
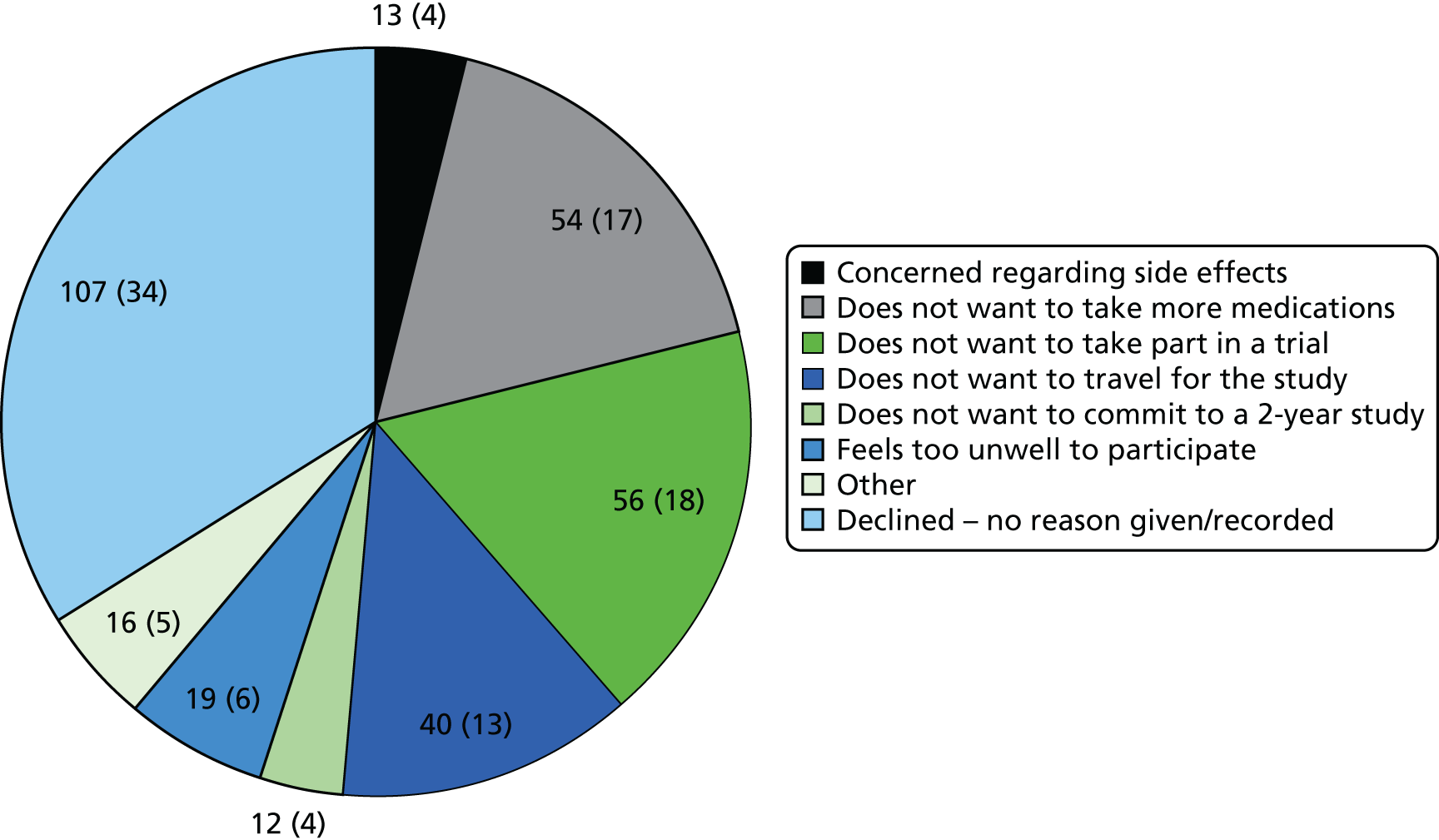
Status of patients in the study
Table 14 shows the numbers of patients who completed each study visit across the three groups. In total, 161 patients (71%) successfully completed all study visits.
| Visit | Placebo | Perindopril | Amlodipine | Total |
|---|---|---|---|---|
| Baseline | 79 | 73 | 72 | 224 |
| Month 3 | 76 | 68 | 67 | 211 |
| Month 6 | 69 | 62 | 62 | 193 |
| Month 9 | 64 | 57 | 57 | 178 |
| Month 12 | 71 | 61 | 54 | 186 |
| Month 15 | 60 | 49 | 47 | 156 |
| Month 18 | 61 | 52 | 49 | 162 |
| Month 21 | 54 | 44 | 47 | 145 |
| Month 24 | 59 | 53 | 49 | 161 |
| Unscheduled | 10 | 2 | 2 | 14 |
As previously described, a 10% attrition rate was included in the power calculations for the trial. Participants were included in the attrition rate if data from fewer than two study visits were available for analysis. A total of 13 patients counted towards the attrition rate: three patients who were randomised in error and 10 patients who withdrew from the study before completing at least two study visits. The patients randomised in error were not included in the final analyses; however, the remaining 10 patients were included on an ITT basis. The final attrition rate for the study was therefore 6%. The CONSORT diagram (Figure 13) shows the status of patients at the end of the trial. Six patients withdrew from the trial because of AEs attributed to the study medications (n = 2 perindopril, n = 4 amlodipine). Four patients (n = 3 perindopril, n = 1 amlodipine) switched to losartan because of cough. No patients suffered AAA rupture and 26 underwent elective surgery (n = 9 placebo, n = 10 perindopril, n = 7 amlodipine) during the trial period.
FIGURE 13.
The AARDVARK trial CONSORT diagram. Reproduced from Bicknell et al. 2016. 95 © Bicknell et al. 2016. Published by Oxford University Press on behalf of the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.

Baseline comparability of randomised groups
The baseline characteristics for all patients in the study are shown in Table 15. Although groups were randomised and generally well matched, there were some small imbalances between the groups that may influence aneurysm growth, for example presence of diabetes [n = 8 (10.1%) placebo, n = 2 (2.7%) perindopril, n = 6 (8.3%) amlodipine] and use of statins [n = 48 (61%) placebo, n = 53 (73%) perindopril, n = 45 (63%) amlodipine]. All patients but one were Caucasian.
| Characteristic | Placebo | Perindopril | Amlodipine |
|---|---|---|---|
| n b | 79 | 73 | 72 |
| Age (years) | 70.7 (7.5) | 71.6 (6.9) | 71.5 (6.7) |
| Male, n (%) | 74 (94) | 71 (97) | 66 (92) |
| Caucasian, n (%) | 79 (100) | 73 (100) | 71 (99) |
| SBP (mmHg) | 131.7 (12.2) | 130.9 (11.5) | 131.9 (13) |
| DBP (mmHg) | 77.9 (7.6) | 76.7 (8) | 78 (7) |
| Use of statins, n (%) | 48 (61) | 53 (73) | 45 (63) |
| AAA external diameter longitudinal (cm) | 4.06 (0.67) | 4.05 (0.65) | 4.03 (0.69) |
| AAA internal diameter longitudinal (cm) | 3.67 (0.67) | 3.66 (0.68) | 3.61 (0.71) |
| AAA external diameter transverse (cm) | 4.05 (0.68) | 4.09 (0.65) | 4.04 (0.67) |
| AAA internal diameter transverse (cm) | 3.65 (0.69) | 3.68 (0.68) | 3.61 (0.7) |
| Current smokers, n (%) | 17 (22) | 21 (29) | 18 (25) |
| Pack-years current smokers | 32.9 (28) | 33.1 (24) | 29.3 (17.3) |
| Past smokers, n (%) | 56 (72) | 41 (57) | 44 (63) |
| Pack-years past smokers | 42.2 (45.5) | 42 (33.8) | 40.5 (36.8) |
| Height (cm) | 174.4 (8.5) | 175.9 (8.3) | 173.7 (8.7) |
| Weight (kg) | 84.3 (16.1) | 84.3 (16.6) | 81.2 (13.8) |
| Diabetes, n (%) | 8 (10.1) | 2 (2.7) | 6 (8.3) |
| Antiplatelet therapy, n (%) | 28 (35.4) | 37 (50.6) | 33 (45.8) |
Abdominal aortic aneurysm growth
The changes in AAA diameter over the duration of the trial are shown in Table 16 (summary longitudinal AAA measurements) and Table 17 (differences in longitudinal AAA measurements). See Appendix 5 for a summary of the transverse AAA measurement data.
| Visit | Longitudinal internal diameter (cm) | Longitudinal external diameter (cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Minimum | Maximum | n | Mean | SD | Minimum | Maximum | |
| Placebo | ||||||||||
| Baseline | 78 | 3.67 | 0.67 | 2.33 | 5.26 | 79 | 4.06 | 0.67 | 3.00 | 5.44 |
| Month 3 | 74 | 3.72 | 0.72 | 2.46 | 5.48 | 74 | 4.10 | 0.72 | 3.01 | 6.08 |
| Month 6 | 66 | 3.74 | 0.65 | 2.61 | 5.55 | 66 | 4.13 | 0.62 | 3.02 | 5.83 |
| Month 9 | 63 | 3.74 | 0.70 | 2.30 | 5.37 | 63 | 4.11 | 0.68 | 3.05 | 5.67 |
| Month 12 | 69 | 3.77 | 0.64 | 2.48 | 5.22 | 68 | 4.13 | 0.64 | 3.00 | 5.51 |
| Month 15 | 57 | 3.78 | 0.68 | 2.52 | 5.41 | 57 | 4.15 | 0.70 | 3.01 | 6.01 |
| Month 18 | 60 | 3.83 | 0.65 | 2.68 | 5.27 | 60 | 4.20 | 0.68 | 3.06 | 5.85 |
| Month 21 | 52 | 3.84 | 0.63 | 2.71 | 5.30 | 52 | 4.18 | 0.67 | 3.04 | 5.86 |
| Month 24 | 56 | 3.79 | 0.63 | 2.39 | 5.37 | 56 | 4.12 | 0.63 | 3.08 | 5.58 |
| Perindopril | ||||||||||
| Baseline | 71 | 3.66 | 0.68 | 2.38 | 5.05 | 71 | 4.05 | 0.65 | 3.04 | 5.40 |
| Month 3 | 65 | 3.65 | 0.68 | 2.27 | 5.02 | 65 | 4.05 | 0.65 | 3.04 | 5.42 |
| Month 6 | 61 | 3.72 | 0.71 | 2.17 | 5.18 | 61 | 4.11 | 0.72 | 3.01 | 5.93 |
| Month 9 | 56 | 3.72 | 0.72 | 2.32 | 5.38 | 56 | 4.10 | 0.69 | 3.04 | 5.78 |
| Month 12 | 60 | 3.76 | 0.73 | 2.51 | 5.50 | 60 | 4.12 | 0.72 | 3.03 | 5.79 |
| Month 15 | 47 | 3.75 | 0.72 | 2.54 | 5.49 | 47 | 4.09 | 0.71 | 3.03 | 5.74 |
| Month 18 | 49 | 3.75 | 0.62 | 2.45 | 5.50 | 49 | 4.09 | 0.64 | 3.08 | 5.83 |
| Month 21 | 44 | 3.74 | 0.65 | 2.57 | 5.74 | 43 | 4.08 | 0.69 | 3.08 | 5.99 |
| Month 24 | 52 | 3.71 | 0.60 | 2.54 | 5.28 | 52 | 4.03 | 0.57 | 3.05 | 5.42 |
| Amlodipine | ||||||||||
| Baseline | 68 | 3.61 | 0.71 | 2.20 | 4.99 | 71 | 4.03 | 0.69 | 3.00 | 5.50 |
| Month 3 | 65 | 3.70 | 0.70 | 2.02 | 5.10 | 65 | 4.10 | 0.67 | 3.05 | 5.60 |
| Month 6 | 60 | 3.78 | 0.74 | 2.27 | 5.24 | 60 | 4.14 | 0.69 | 3.04 | 5.47 |
| Month 9 | 54 | 3.87 | 0.70 | 2.64 | 5.52 | 54 | 4.22 | 0.68 | 3.02 | 5.75 |
| Month 12 | 51 | 3.82 | 0.70 | 2.26 | 5.25 | 51 | 4.18 | 0.69 | 2.97 | 5.80 |
| Month 15 | 44 | 3.82 | 0.71 | 2.15 | 5.21 | 44 | 4.19 | 0.67 | 3.00 | 5.59 |
| Month 18 | 47 | 3.87 | 0.72 | 2.46 | 5.51 | 47 | 4.17 | 0.70 | 3.01 | 5.78 |
| Month 21 | 47 | 3.90 | 0.74 | 2.62 | 5.54 | 47 | 4.21 | 0.72 | 3.00 | 5.88 |
| Month 24 | 48 | 3.83 | 0.73 | 2.44 | 5.50 | 47 | 4.18 | 0.67 | 3.02 | 5.67 |
| Total | ||||||||||
| Baseline | 217 | 3.65 | 0.68 | 2.20 | 5.26 | 221 | 4.05 | 0.66 | 3.00 | 5.50 |
| Month 3 | 204 | 3.69 | 0.70 | 2.02 | 5.48 | 204 | 4.09 | 0.68 | 3.01 | 6.08 |
| Month 6 | 187 | 3.75 | 0.69 | 2.17 | 5.55 | 187 | 4.13 | 0.67 | 3.01 | 5.93 |
| Month 9 | 173 | 3.77 | 0.70 | 2.30 | 5.52 | 173 | 4.14 | 0.68 | 3.02 | 5.78 |
| Month 12 | 180 | 3.78 | 0.69 | 2.26 | 5.50 | 179 | 4.14 | 0.68 | 2.97 | 5.80 |
| Month 15 | 148 | 3.78 | 0.70 | 2.15 | 5.49 | 148 | 4.14 | 0.69 | 3.00 | 6.01 |
| Month 18 | 156 | 3.82 | 0.66 | 2.45 | 5.51 | 156 | 4.16 | 0.67 | 3.01 | 5.85 |
| Month 21 | 143 | 3.83 | 0.67 | 2.57 | 5.74 | 142 | 4.16 | 0.69 | 3.00 | 5.99 |
| Month 24 | 156 | 3.78 | 0.65 | 2.39 | 5.50 | 155 | 4.11 | 0.62 | 3.02 | 5.67 |
| Study period | AAA longitudinal internal diameter (cm) | AAA longitudinal external diameter (cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Minimum | Maximum | n | Mean | SD | Minimum | Maximum | |
| Placebo | ||||||||||
| Month 3 – baseline | 73 | 0.08 | 0.23 | –0.3 | 0.87 | 74 | 0.06 | 0.24 | –0.73 | 1.04 |
| Month 6 – baseline | 65 | 0.11 | 0.27 | –0.54 | 0.84 | 66 | 0.08 | 0.24 | –0.61 | 0.70 |
| Month 12 – baseline | 68 | 0.18 | 0.31 | –0.68 | 0.91 | 68 | 0.14 | 0.28 | –0.57 | 0.92 |
| Month 18 – baseline | 59 | 0.28 | 0.29 | –0.20 | 0.94 | 60 | 0.26 | 0.29 | –0.19 | 1.27 |
| Month 24 – baseline | 55 | 0.31 | 0.35 | –0.27 | 1.20 | 56 | 0.27 | 0.30 | –0.22 | 1.11 |
| Perindopril | ||||||||||
| Month 3 – baseline | 64 | 0.02 | 0.26 | –0.72 | 0.86 | 64 | 0.05 | 0.21 | –0.65 | 0.69 |
| Month 6 – baseline | 60 | 0.11 | 0.24 | –0.33 | 1.06 | 60 | 0.12 | 0.22 | –0.42 | 0.97 |
| Month 12 – baseline | 59 | 0.22 | 0.30 | –0.27 | 1.35 | 59 | 0.19 | 0.27 | –0.40 | 1.18 |
| Month 18 – baseline | 48 | 0.26 | 0.26 | –0.18 | 1.04 | 48 | 0.23 | 0.26 | –0.35 | 1.26 |
| Month 24 – baseline | 51 | 0.27 | 0.21 | –0.16 | 1.00 | 51 | 0.23 | 0.22 | –0.35 | 0.82 |
| Amlodipine | ||||||||||
| Month 3 – baseline | 61 | 0.08 | 0.29 | –0.7 | 1.21 | 64 | 0.05 | 0.24 | –0.69 | 0.90 |
| Month 6 – baseline | 56 | 0.18 | 0.42 | –0.84 | 1.74 | 59 | 0.12 | 0.30 | –0.77 | 1.11 |
| Month 12 – baseline | 47 | 0.29 | 0.49 | –0.69 | 2.61 | 50 | 0.22 | 0.31 | –0.52 | 1.18 |
| Month 18 – baseline | 43 | 0.35 | 0.33 | –0.3 | 1.47 | 46 | 0.27 | 0.32 | –0.32 | 1.16 |
| Month 24 – baseline | 44 | 0.37 | 0.41 | –0.73 | 1.43 | 46 | 0.33 | 0.36 | –0.68 | 1.17 |
| Total | ||||||||||
| Month 3 – baseline | 198 | 0.06 | 0.26 | –0.72 | 1.21 | 202 | 0.05 | 0.23 | –0.73 | 1.04 |
| Month 6 – baseline | 181 | 0.13 | 0.32 | –0.84 | 1.74 | 185 | 0.10 | 0.26 | –0.77 | 1.11 |
| Month 12 – baseline | 174 | 0.22 | 0.36 | –0.69 | 2.61 | 177 | 0.18 | 0.29 | –0.57 | 1.18 |
| Month 18 – baseline | 150 | 0.29 | 0.29 | –0.3 | 1.47 | 154 | 0.25 | 0.29 | –0.35 | 1.27 |
| Month 24 – baseline | 150 | 0.31 | 0.33 | –0.73 | 1.43 | 153 | 0.27 | 0.29 | –0.68 | 1.17 |
There was a small increase in the mean internal and external AAA diameter across all three treatment groups. The mean difference between month 24 and baseline for the longitudinal external diameter was 0.27 cm for the three groups combined. Similar results were observed for the mean external transverse measurements. Comparisons of 2-year changes across the three randomised groups are invalid because of the variable reduction in numbers in each group over time. The SDs of the diameters recorded in the longitudinal plane for the ITI measures (see Table 16) were generally systematically higher than those for the OTO measures at each time point, as were the SDs of the differences between baseline and each time point (see Table 17).
The AAA growth trajectories in each patient by group are shown in Figure 14 (placebo), Figure 15 (perindopril) and Figure 16 (amlodipine). Most of the patients had slow growth rates but there were a small number of patients with faster growth rates in each randomised group.
FIGURE 14.
Abdominal aortic aneurysm growth trajectories by patients in the placebo group.

FIGURE 15.
Abdominal aortic aneurysm growth trajectories by patients in the perindopril group.
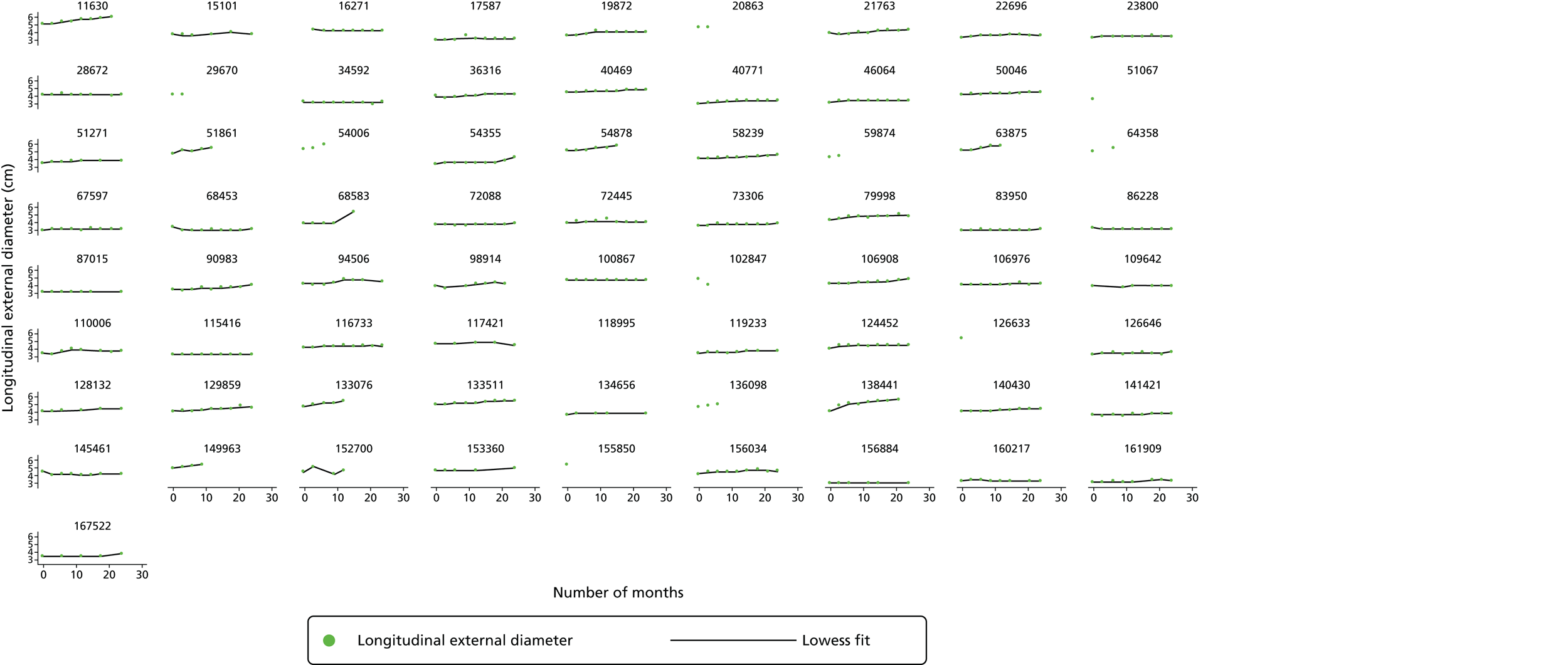
FIGURE 16.
Abdominal aortic aneurysm growth trajectories by patients in the amlodipine group.

Figure 17 shows the observed mean AAA diameter for each treatment group at each visit time point. The numbers of patients were different across each visit, with lower numbers of patients in the later visits. There were no significant differences between the groups over time and any apparent differences between the groups must be considered in the context of the changing numbers of participants. This is especially relevant when patients with an AAA of ≥ 5.5 cm who are taken for surgery and do not complete any further measurements are considered.
FIGURE 17.
Mean longitudinal external diameter over time by randomised group.
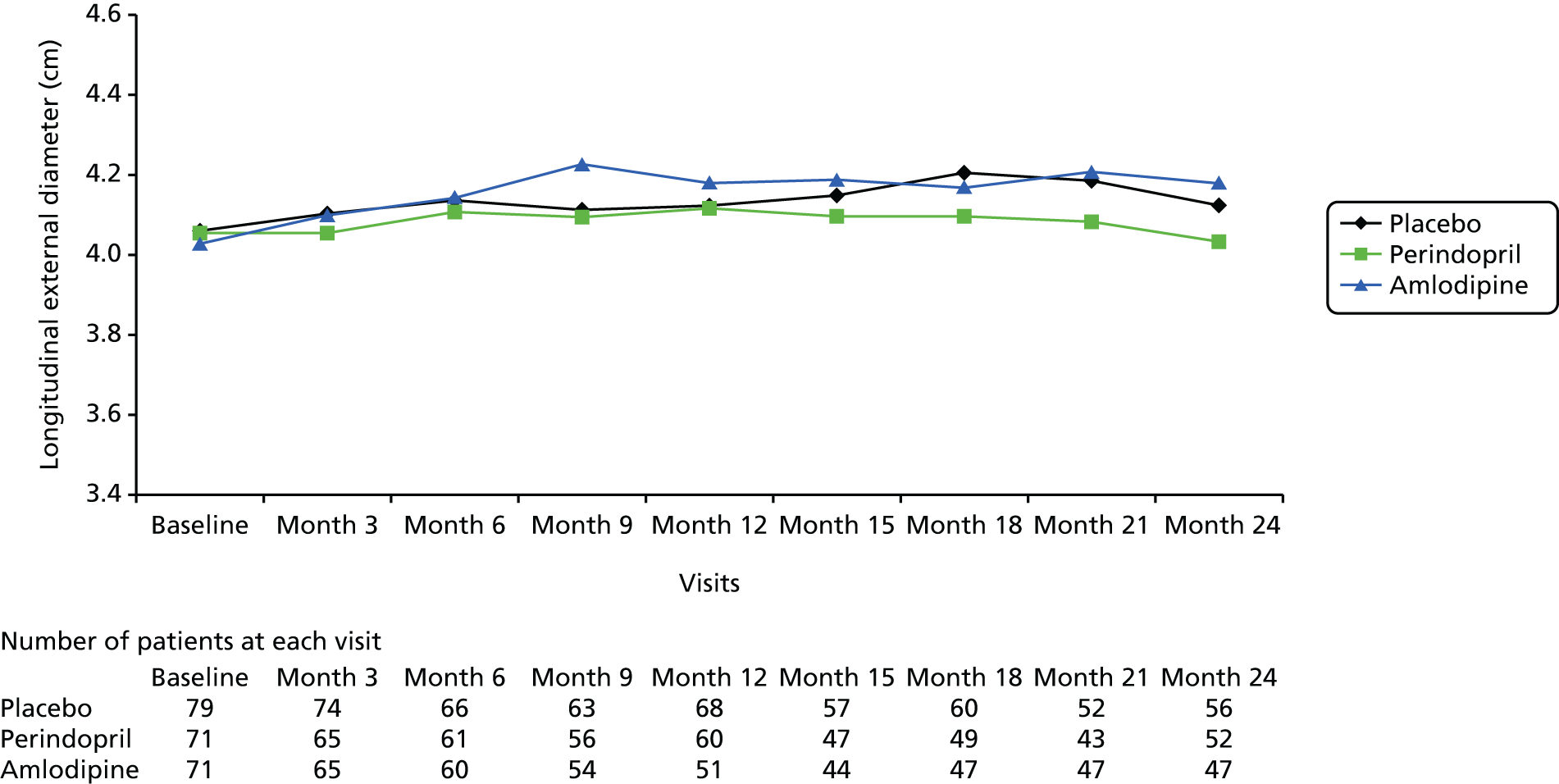
Histograms of change in longitudinal external AAA diameter from baseline to 3, 12 and 24 months are shown in Figures 18–20, respectively (for the histograms at 6 and 18 months see Appendix 6). These histograms show the distribution of the difference in the data between the two specified time points. Overall, no obvious differences were apparent across groups at any of the three time points, whereas in all three histograms growth was apparent overall at 12 and 24 months reflected by the shift of bars to the right of the graphs.
FIGURE 18.
Histograms of change in AAA longitudinal external diameter from baseline to month 3. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
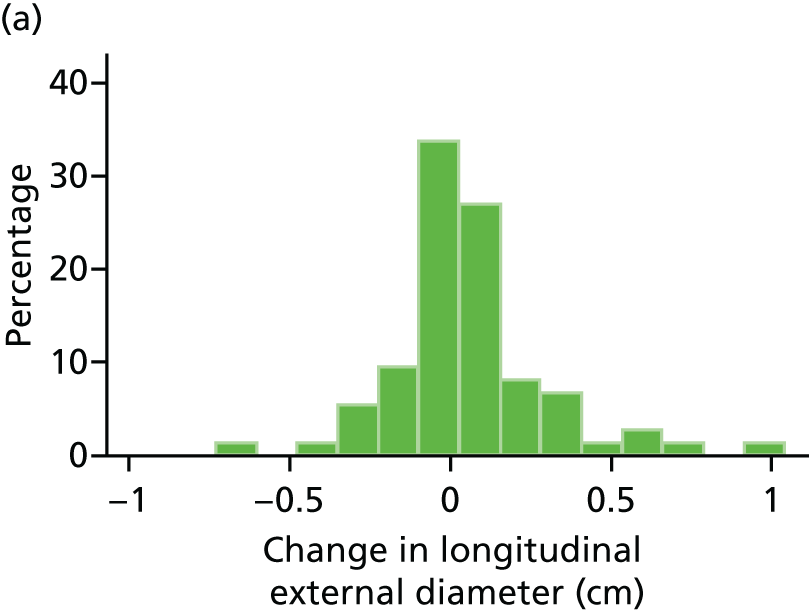
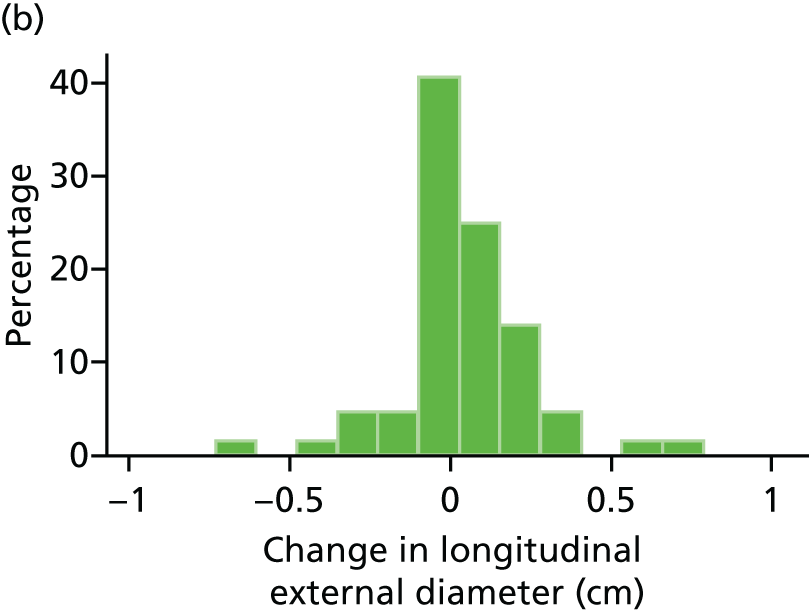


FIGURE 19.
Histograms of change in AAA longitudinal external diameter from baseline to month 12. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
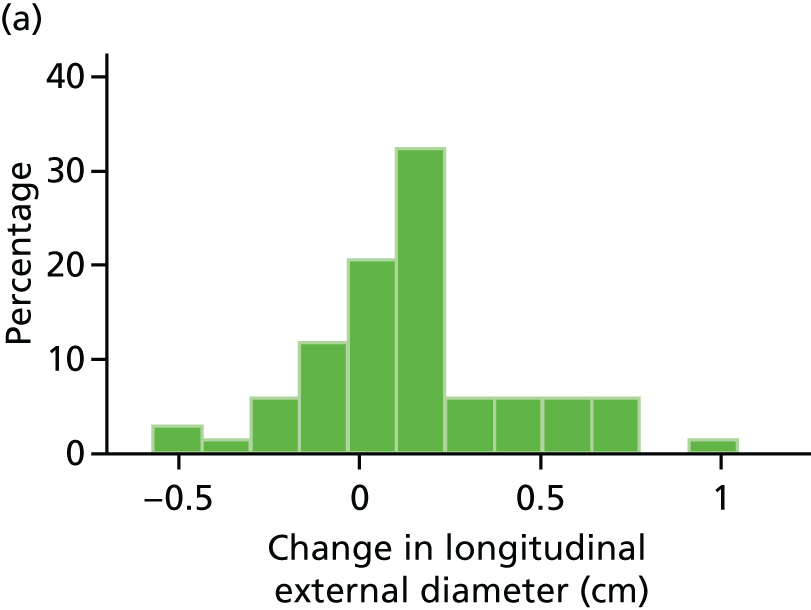
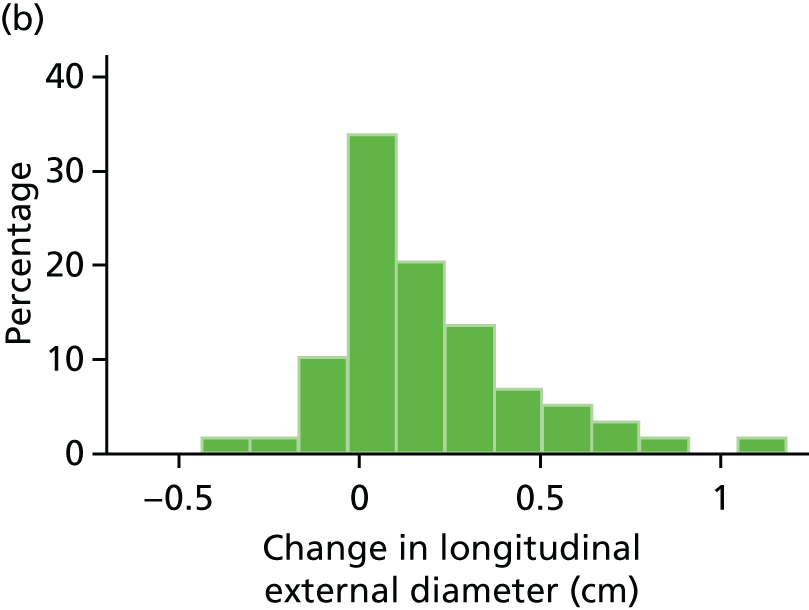


FIGURE 20.
Histograms of change in AAA longitudinal external diameter from baseline to month 24. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
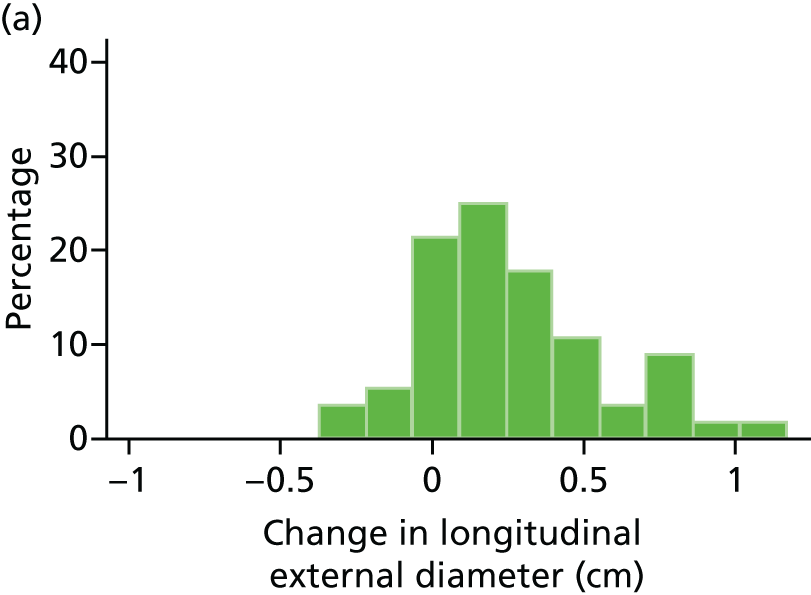



Counting those AAAs that exhibit fast growth rates (as defined by growth of > 0.5 cm per year) in these histograms (n = 14 placebo, n = 8 perindopril, n = 14 amlodipine) suggested possible differential effects among the three treatment groups. Formal analyses of the number of patients who exhibit fast growth rates in each of the three groups (taking into account aneurysm size and adjusting for confounders) was not undertaken as part of the main analyses of this trial but will be considered as part of future research plans.
Box plots of change in longitudinal external AAA diameter from baseline to 3, 12 and 24 months are shown in Figures 21–23, respectively (the box plots for change from baseline to 6 and 18 months can be found in Appendix 7). These figures confirm the findings shown in Figures 18–20 in that there are no apparent differences in change in diameter at 3, 12 or 24 months across the three groups and growth of a similar magnitude is apparent in all three groups at 12 and 24 months.
FIGURE 21.
Box plot of change in AAA longitudinal external diameter from baseline to month 3. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
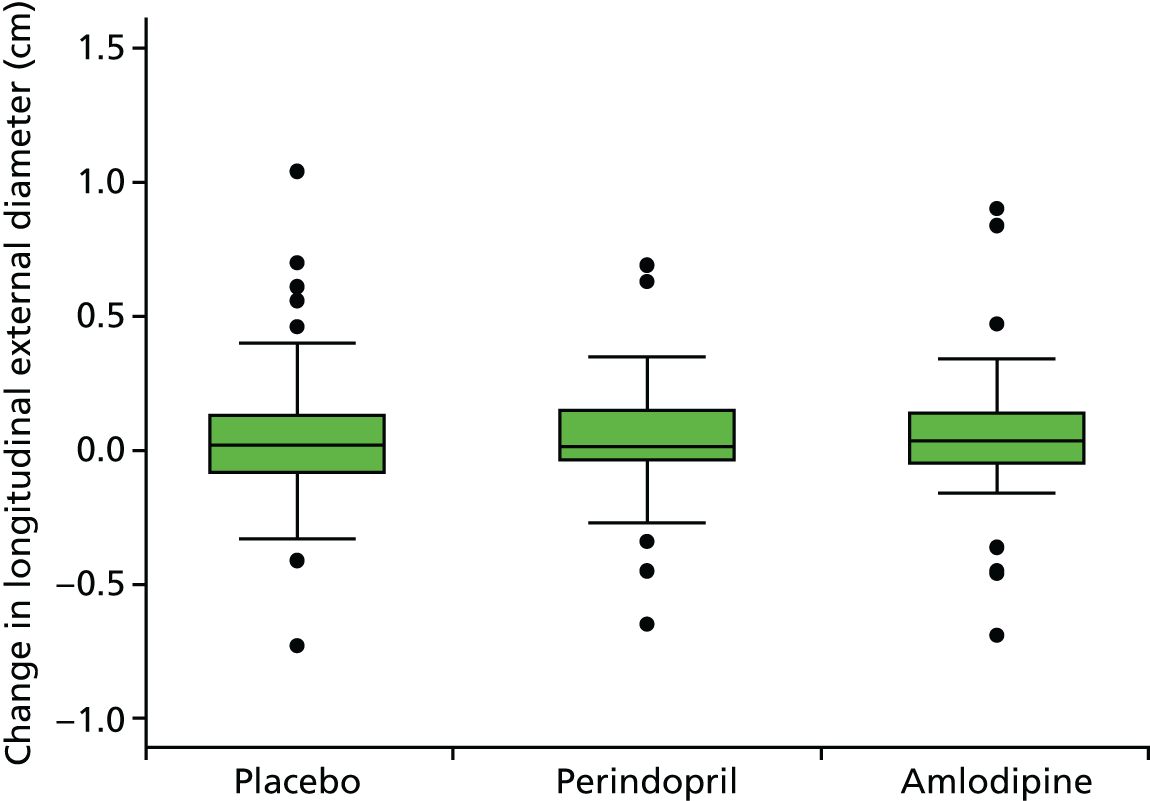
FIGURE 22.
Box plot of change in AAA longitudinal external diameter from baseline to month 12. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

FIGURE 23.
Box plot of change in AAA longitudinal external diameter from baseline to month 24. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
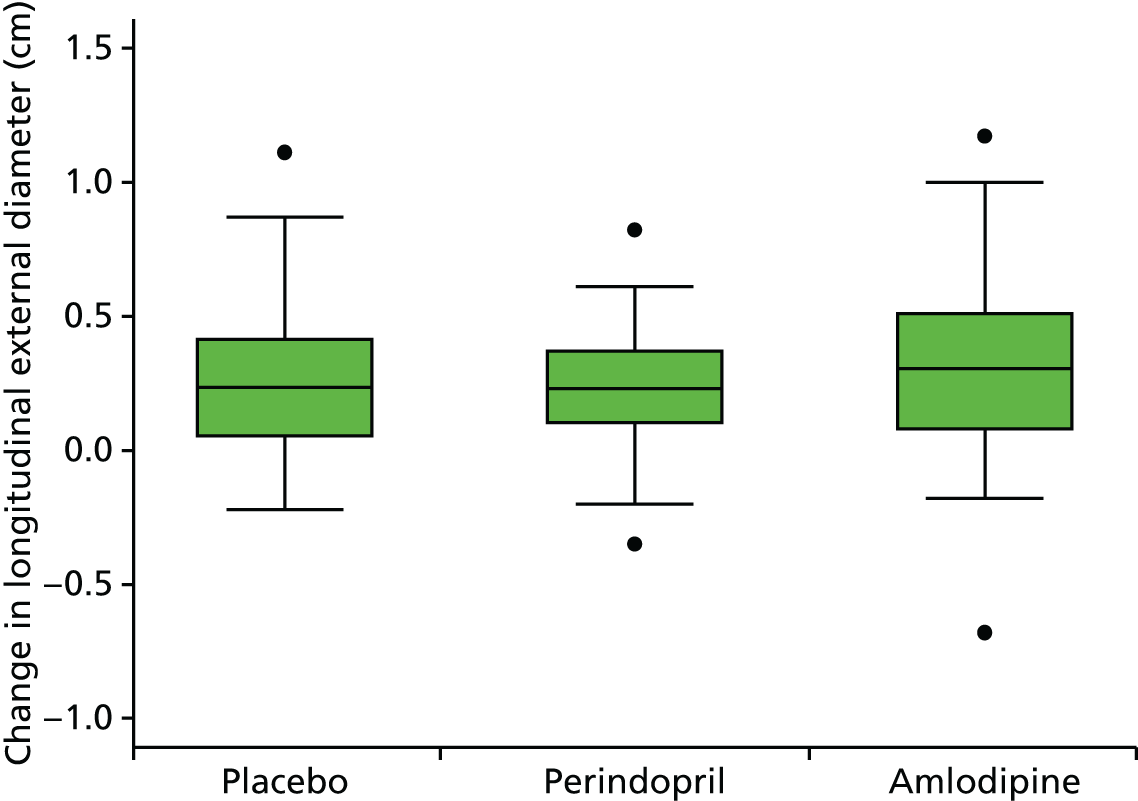
Histograms and box plots of change in diameter for longitudinal internal diameters and transverse external and internal diameters are provided in Appendices 8–13.
Primary outcome
Multilevel modelling was used to determine the maximum likelihood estimates for AAA diameter growth. Table 18 provides these results for the longitudinal external AAA diameter. The estimates for annual diameter growth were as follows:
-
1.68 mm [standard error (SE) 0.02 mm] in the placebo group
-
1.77 mm (SE 0.02 mm) in the perindopril group
-
1.81 mm (SE 0.02 mm) in the amlodipine group.
| Treatment and visit | Linear model with interaction | |
|---|---|---|
| Estimate | 95% CI | |
| Fixed part | ||
| Baseline diameter (cm) for the control group (β1) | 4.074 | 3.931 to 4.218 |
| Change in diameter growth (cm) for a yearly increase in time (β2) | 0.168 | 0.129 to 0.208 |
| Difference in baseline diameter (cm) | ||
| β3(perindopril vs. placebo) | –0.008 | –0.217 to 0.200 |
| β4(amlodipine vs. placebo) | –0.021 | –0.229 to 0.188 |
| Difference in diameter growth (cm) for a yearly increase in time | ||
| β5(perindopril vs. placebo) | 0.008 | –0.050 to 0.065 |
| β6(amlodipine vs. placebo) | 0.012 | –0.046 to 0.070 |
| Random part (cm) | ||
| SD of individual intercepts (ς1j) | 0.646 | 0.587 to 0.710 |
| SD of individual slopes (ς2j) | 0.149 | 0.128 to 0.173 |
| Correlation between random effects | 0.455 | 0.296 to 0.589 |
| SD of residual errors | 0.136 | 0.130 to 0.141 |
The differences in the slopes of modelled growth over time were not significant between perindopril and placebo (p = 0.78) or between perindopril and amlodipine (p = 0.89). The difference in slope between perindopril and placebo plus amlodipine combined was also not significant (p = 0.92).
Sensitivity analyses
Exclusion of patients with diabetes gave very similar results (Table 19). The estimated difference in annual growth rate between perindopril and placebo was –0.01 mm with a 95% CI of –0.6 to 0.6 mm. Adjustment for baseline age, sex, current smoking status and statin use also gave similar results (Table 20).
| Treatment and visit | Linear model with interaction | |
|---|---|---|
| Estimate | 95% CI | |
| Fixed part | ||
| Baseline diameter (cm) for the control group (β1) | 4.046 | 3.895 to 4.198 |
| Change in diameter growth (cm) for a yearly increase in time (β2) | 0.176 | 0.135 to 0.217 |
| Difference in baseline diameter (cm) | ||
| β3(perindopril vs. placebo) | 0.018 | –0.198 to 0.232 |
| β4(amlodipine vs. placebo) | –0.030 | –0.249 to 0.188 |
| Difference in diameter growth (cm) for a yearly increase in time | ||
| β5(perindopril vs. placebo) | –0.001 | –0.059 to 0.057 |
| β6(amlodipine vs. placebo) | 0.009 | –0.051 to 0.069 |
| Random part (cm) | ||
| SD of individual intercepts (ς1j) | 0.646 | 0.585 to 0.712 |
| SD of individual slopes (ς2j) | 0.146 | 0.125 to 0.171 |
| Correlation between random effects | 0.495 | 0.333 to 0.629 |
| SD of residual errors | 0.136 | 0.130 to 0.142 |
| Treatment and visit | Adjusted for baseline age and sex (n = 223) | Adjusted for baseline statin use (n = 223) | Adjusted for baseline current smoking status (n = 223) | |||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Fixed part (cm) | ||||||
| Intercept (β1) | 3.681 | 2.754 to 4.607 | 4.029 | 3.851 to 4.207 | 4.084 | 3.934 to 4.233 |
| Change in diameter for a yearly increase in time (β2) | 0.168 | 0.129 to 0.207 | 0.169 | 0.129 to 0.208 | 0.169 | 0.129 to 0.208 |
| Difference in baseline diameter | ||||||
| β3(perindopril vs. placebo) | –0.014 | –0.222 to 0.195 | –0.018 | –0.227 to 0.191 | –0.005 | –0.214 to 0.204 |
| β4(amlodipine vs. placebo) | –0.025 | –0.233 to 0.183 | –0.022 | –0.230 to 0.186 | –0.019 | –0.228 to 0.189 |
| Difference in diameter growth for a yearly increase in time | ||||||
| β5(perindopril vs. placebo) | 0.008 | –0.049 to 0.066 | 0.008 | –0.049 to 0.066 | 0.008 | –0.049 to 0.066 |
| β6(amlodipine vs. placebo) | 0.012 | –0.046 to 0.070 | 0.012 | –0.046 to 0.070 | 0.012 | –0.046 to 0.070 |
| Random part (cm) | ||||||
| SD of individual intercepts (ς1j) | 0.644 | 0.586 to 0.709 | 0.645 | 0.587 to 0.710 | 0.646 | 0.588 to 0.711 |
| SD of individual slopes (ς2j) | 0.149 | 0.128 to 0.173 | 0.149 | 0.128 to 0.173 | 0.149 | 0.128 to 0.173 |
| Correlation between random effects | 0.455 | 0.296 to 0.589 | 0.459 | 0.301 to 0.592 | 0.460 | 0.300 to 0.595 |
| SD of residual errors | 0.136 | 0.130 to 0.141 | 0.136 | 0.130 to 0.141 | 0.136 | 0.130 to 0.141 |
Blood pressure reduction
Summary BP statistics for the duration of the trial are shown in Table 21 and BP differences from baseline are shown in Table 22. There was an increase in mean SBP in the placebo group from baseline to month 24 (+2.5 mmHg) and a decrease in in mean SBP in both the perindopril (–5.0 mmHg) and amlodipine (–2.8 mmHg) groups from baseline to month 24. There was little change in mean DBP between baseline and month 24 in the placebo group (–0.7 mmHg) but reductions were seen in the perindopril and amlodipine groups (–5.2 mmHg and –3.3 mmHg, respectively). Mean SBP and DBP over the duration of the trial are shown in Figures 24 and 25, respectively.
| Visit | n | SBP (mmHg) | DBP (mmHg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | Mean | SD | Minimum | Maximum | ||
| Placebo | |||||||||
| Baseline | 79 | 131.7 | 12.2 | 101 | 152 | 77.9 | 7.6 | 63 | 95 |
| Month 3 | 76 | 132.5 | 18.0 | 89 | 167 | 77.4 | 10.0 | 54 | 97 |
| Month 6 | 69 | 129.8 | 14.1 | 104 | 164 | 76.5 | 8.2 | 55 | 96 |
| Month 9 | 64 | 131.8 | 13.8 | 102 | 161 | 77.5 | 8.1 | 62 | 96 |
| Month 12 | 70 | 132.8 | 14.7 | 102 | 166 | 77.9 | 9.4 | 61 | 99 |
| Month 15 | 59 | 129.6 | 14.5 | 96 | 167 | 76.7 | 9.5 | 58 | 98 |
| Month 18 | 59 | 129.7 | 15.3 | 89 | 169 | 75.7 | 9.0 | 54 | 100 |
| Month 21 | 53 | 131.3 | 18.3 | 98 | 198 | 76.0 | 9.9 | 52 | 97 |
| Month 24 | 59 | 134.7 | 18.7 | 95 | 197 | 77.3 | 10.3 | 58 | 102 |
| Perindopril | |||||||||
| Baseline | 73 | 130.9 | 11.5 | 105 | 148 | 76.7 | 8.0 | 60 | 98 |
| Month 3 | 68 | 120.4 | 16.3 | 85 | 162 | 71.0 | 9.5 | 47 | 92 |
| Month 6 | 62 | 126.3 | 17.4 | 93 | 182 | 72.8 | 9.3 | 46 | 93 |
| Month 9 | 56 | 122.1 | 14.8 | 94 | 166 | 72.1 | 8.7 | 54 | 92 |
| Month 12 | 60 | 121.4 | 14.1 | 85 | 160 | 71.0 | 8.9 | 44 | 96 |
| Month 15 | 49 | 125.8 | 18.2 | 87 | 172 | 71.9 | 8.4 | 52 | 88 |
| Month 18 | 52 | 120.2 | 15.2 | 89 | 173 | 69.4 | 8.2 | 51 | 89 |
| Month 21 | 44 | 124.0 | 17.2 | 93 | 170 | 71.8 | 9.2 | 51 | 95 |
| Month 24 | 53 | 125.6 | 17.3 | 95 | 169 | 71.8 | 10.9 | 50 | 102 |
| Amlodipine | |||||||||
| Baseline | 72 | 131.9 | 13.0 | 104 | 155 | 78.0 | 7.0 | 63 | 93 |
| Month 3 | 67 | 127.8 | 13.5 | 102 | 176 | 74.5 | 8.0 | 56 | 98 |
| Month 6 | 61 | 127.4 | 14.2 | 98 | 178 | 74.7 | 7.8 | 59 | 93 |
| Month 9 | 56 | 124.7 | 16.2 | 98 | 166 | 73.7 | 8.4 | 54 | 92 |
| Month 12 | 54 | 124.7 | 13.9 | 99 | 159 | 73.3 | 8.2 | 54 | 89 |
| Month 15 | 46 | 127.0 | 17.1 | 97 | 173 | 74.0 | 8.3 | 59 | 96 |
| Month 18 | 49 | 126.3 | 13.3 | 101 | 156 | 73.8 | 7.4 | 59 | 94 |
| Month 21 | 47 | 125.8 | 14.4 | 98 | 165 | 72.8 | 6.5 | 60 | 89 |
| Month 24 | 49 | 127.9 | 15.2 | 107 | 165 | 74.2 | 7.3 | 60 | 91 |
| Total | |||||||||
| Baseline | 224 | 131.5 | 12.2 | 101 | 155 | 77.5 | 7.5 | 60 | 98 |
| Month 3 | 211 | 127.1 | 16.8 | 85 | 176 | 74.4 | 9.6 | 47 | 98 |
| Month 6 | 192 | 127.9 | 15.2 | 93 | 182 | 74.8 | 8.5 | 46 | 96 |
| Month 9 | 176 | 126.5 | 15.4 | 94 | 166 | 74.6 | 8.6 | 54 | 96 |
| Month 12 | 184 | 126.7 | 15.0 | 85 | 166 | 74.3 | 9.3 | 44 | 99 |
| Month 15 | 154 | 127.6 | 16.5 | 87 | 173 | 74.4 | 9.0 | 52 | 98 |
| Month 18 | 160 | 125.6 | 15.1 | 89 | 173 | 73.1 | 8.6 | 51 | 100 |
| Month 21 | 144 | 127.3 | 16.9 | 93 | 198 | 73.7 | 8.8 | 51 | 97 |
| Month 24 | 161 | 129.6 | 17.6 | 95 | 197 | 74.5 | 10.0 | 50 | 102 |
| Visit | n | SBP (mmHg) | DBP (mmHg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | Mean | SD | Minimum | Maximum | ||
| Placebo | |||||||||
| Month 3 – baseline | 76 | 0.8 | 15.5 | –26.5 | 45.0 | –0.6 | 7.1 | –17.5 | 12.5 |
| Month 6 – baseline | 69 | –1.9 | 13.6 | –31.5 | 30.0 | –1.7 | 7.0 | –18.5 | 14.0 |
| Month 12 – baseline | 70 | 0.5 | 14.3 | –33.5 | 44.5 | –0.2 | 7.3 | –22.0 | 12.5 |
| Month 18 – baseline | 59 | –1.8 | 14.2 | –34.5 | 26.0 | –2.1 | 8.4 | –21.5 | 16.5 |
| Month 24 – baseline | 59 | 2.5 | 16.5 | –31.0 | 69.0 | –0.7 | 7.9 | –20.0 | 19.5 |
| Perindopril | |||||||||
| Month 3 – baseline | 68 | –11.1 | 14.2 | –420 | 20.0 | –5.9 | 8.9 | –26.5 | 22.5 |
| Month 6 – baseline | 62 | –5.7 | 17.4 | –34.0 | 43.5 | –4.5 | 8.0 | –20.5 | 17.0 |
| Month 12 – baseline | 60 | –9.5 | 13.1 | –35.0 | 21.5 | –5.8 | 8.1 | –300 | 22.0 |
| Month 18 – baseline | 52 | –9.6 | 14.6 | –42.5 | 38.0 | –7.9 | 7.8 | –24.5 | 15.5 |
| Month 24 – baseline | 53 | –5.0 | 16.3 | –38.5 | 38.5 | –5.2 | 10.0 | –25.0 | 23.5 |
| Amlodipine | |||||||||
| Month 3 – baseline | 67 | –3.7 | 13.0 | –360 | 23.0 | –3.6 | 8.3 | –26.5 | 13.5 |
| Month 6 – baseline | 61 | –4.4 | 14.3 | –51.5 | 290 | –3.5 | 8.1 | –25.5 | 12.0 |
| Month 12 – baseline | 54 | –6.7 | 12.0 | –37.0 | 31.0 | –4.7 | 7.5 | –24.5 | 16.0 |
| Month 18 – baseline | 49 | –5.4 | 10.6 | –28.5 | 22.5 | –4.5 | 6.7 | –17.5 | 11.0 |
| Month 24 – baseline | 49 | –2.8 | 11.7 | –24.5 | 25.5 | –3.3 | 6.3 | –18.5 | 8.5 |
| Total | |||||||||
| Month 3 – baseline | 211 | –4.5 | 15.1 | –42 | 45.0 | –3.3 | 8.4 | –26.5 | 22.5 |
| Month 6 – baseline | 192 | –3.9 | 15.1 | –51.5 | 43.5 | –3.2 | 7.8 | –25.5 | 170 |
| Month 12 – baseline | 184 | –4.9 | 13.9 | –37 | 44.5 | –3.4 | 8.0 | –30.0 | 22.0 |
| Month 18 – baseline | 160 | –5.4 | 13.7 | –42.5 | 38.0 | –4.7 | 8.1 | –24.5 | 16.5 |
| Month 24 – baseline | 161 | –1.6 | 15.4 | –38.5 | 69.0 | –3.0 | 8.4 | –25.0 | 23.5 |
FIGURE 24.
Mean SBP over the duration of the trial by randomised group. (a) Placebo; (b) perindopril; and (c) amlodipine. Vertical bars represent the 95% CIs for the means. Reproduced from Bicknell et al. 2016. 95 © Bicknell et al. 2016. Published by Oxford University Press on behalf of the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
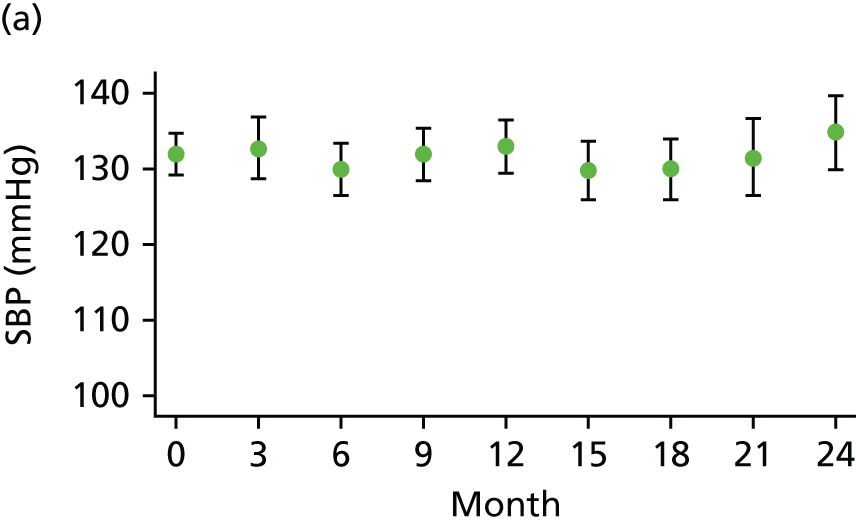
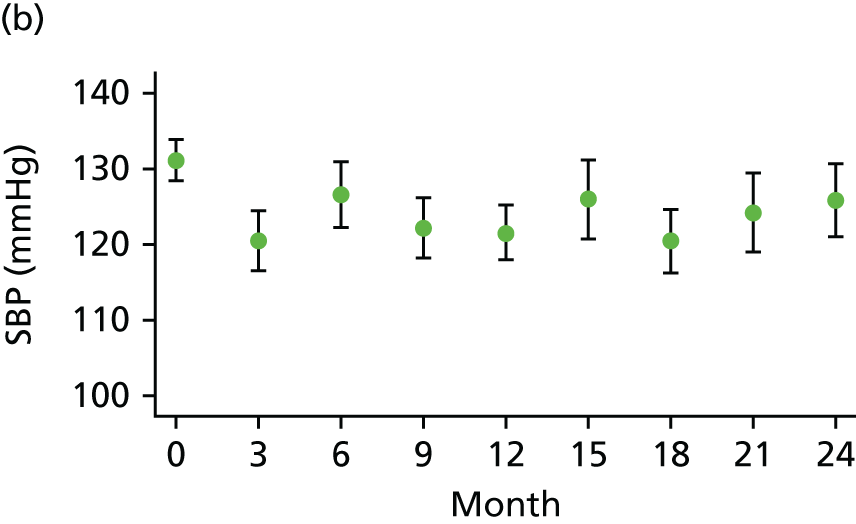

FIGURE 25.
Mean DBP over the duration of the trial by randomised group. (a) Placebo; (b) perindopril; and (c) amlodipine. Vertical bars represent the 95% CIs for the means. Reproduced from Bicknell et al. 2016. 95 © Bicknell et al. 2016. Published by Oxford University Press on behalf of the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.

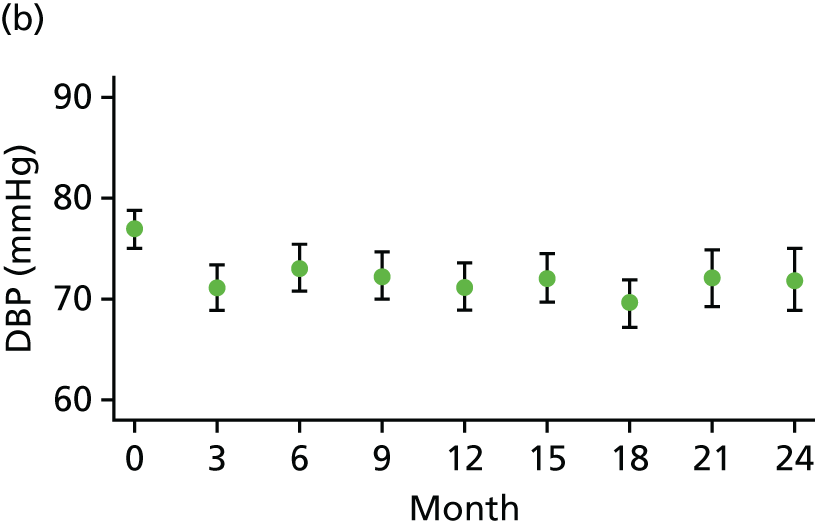
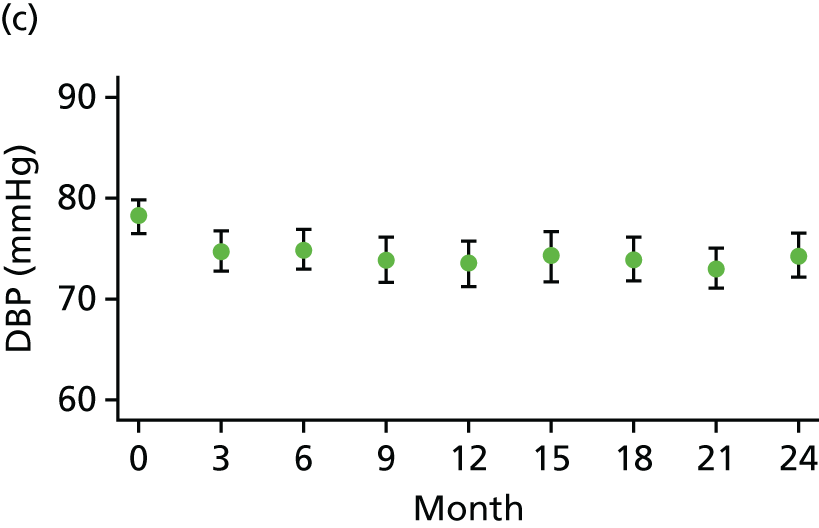
Figures 26–28 show the histograms of change in SBP from baseline to 3, 12 and 24 months, respectively, by randomised group and combined. These histograms show the distribution of the difference in the data between the two specified time points. Negative values indicate a decrease in BP and positive values indicate an increase in BP. The histograms for the placebo group generally follow a normal distribution around zero, whereas reductions in SBP in the perindopril and amlodipine groups are shown by the increased density of bars on the left-hand side of each histogram, particularly at 12 and 24 months. For histograms of the change in SBP from baseline to 6 and 18 months see Appendix 14.
FIGURE 26.
Histograms of change in SBP from baseline to month 3. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.


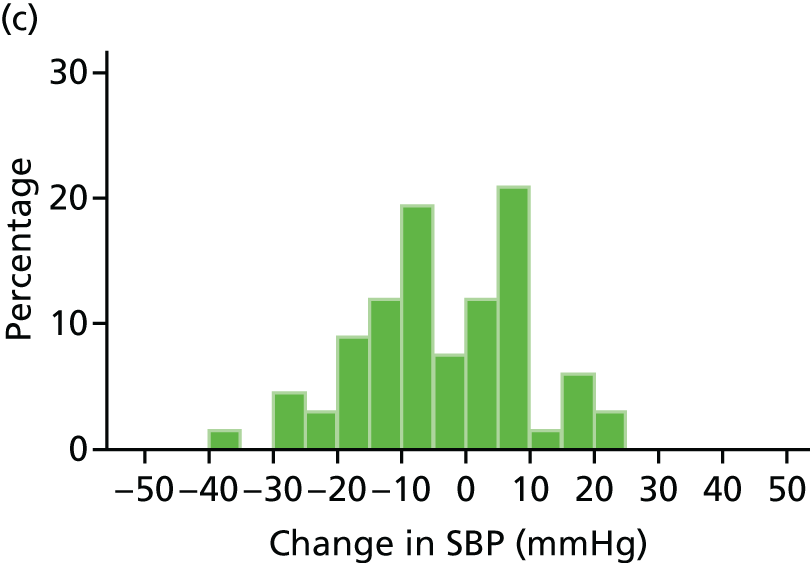
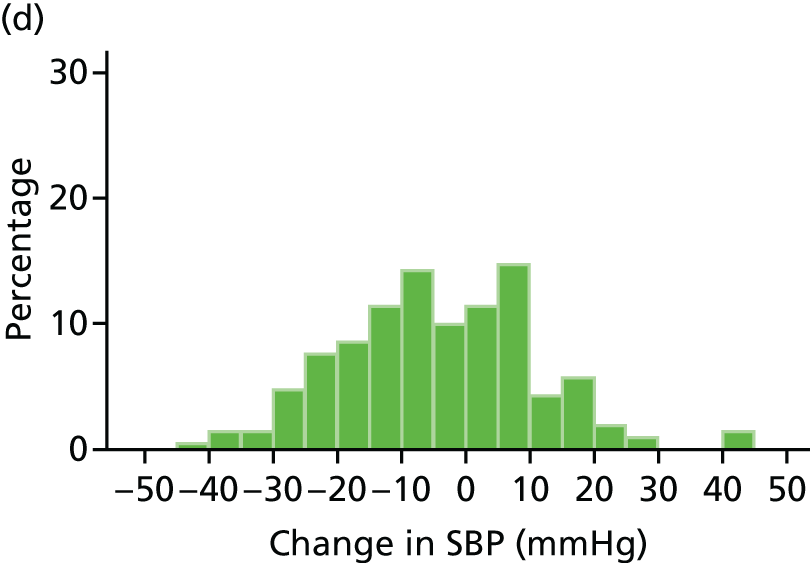
FIGURE 27.
Histograms of change in SBP from baseline to month 12. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.

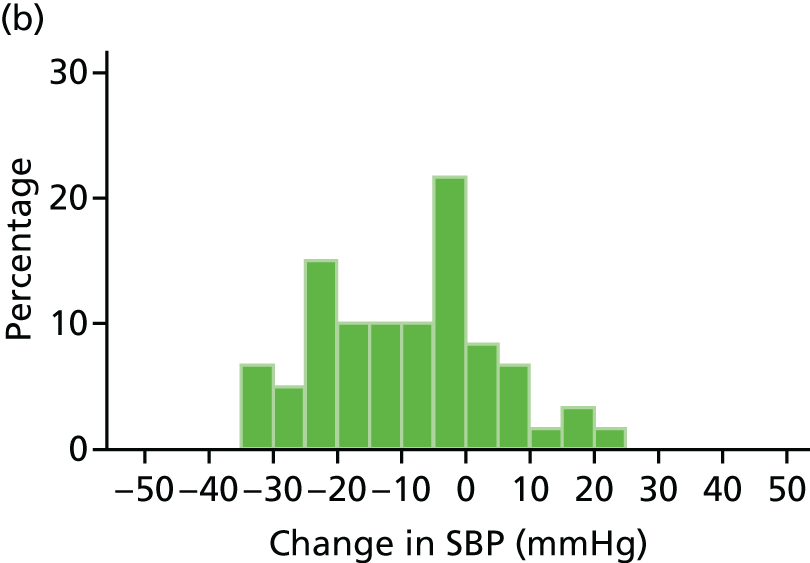
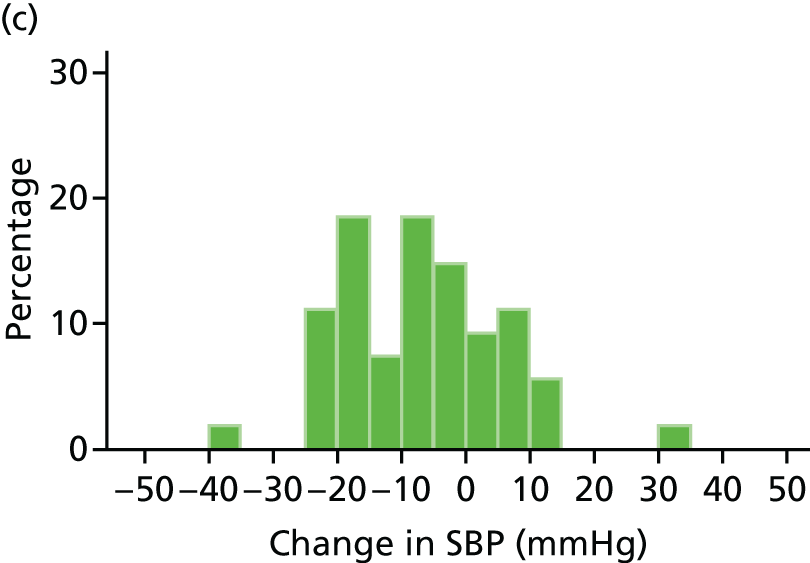
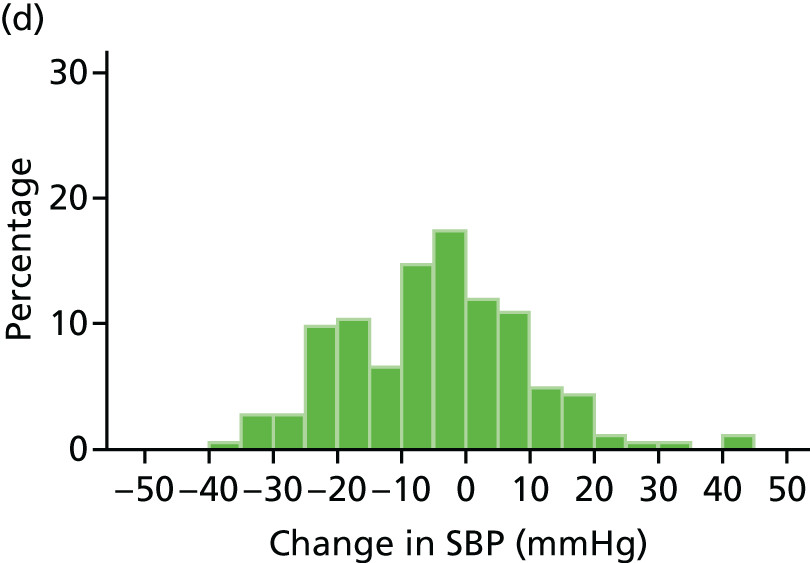
FIGURE 28.
Histograms of change in SBP from baseline to month 24. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
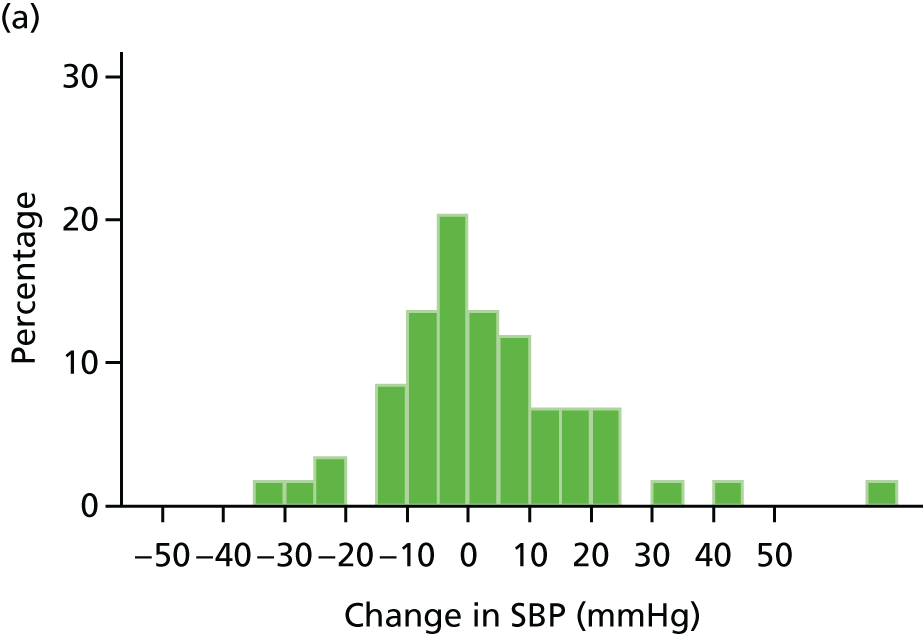


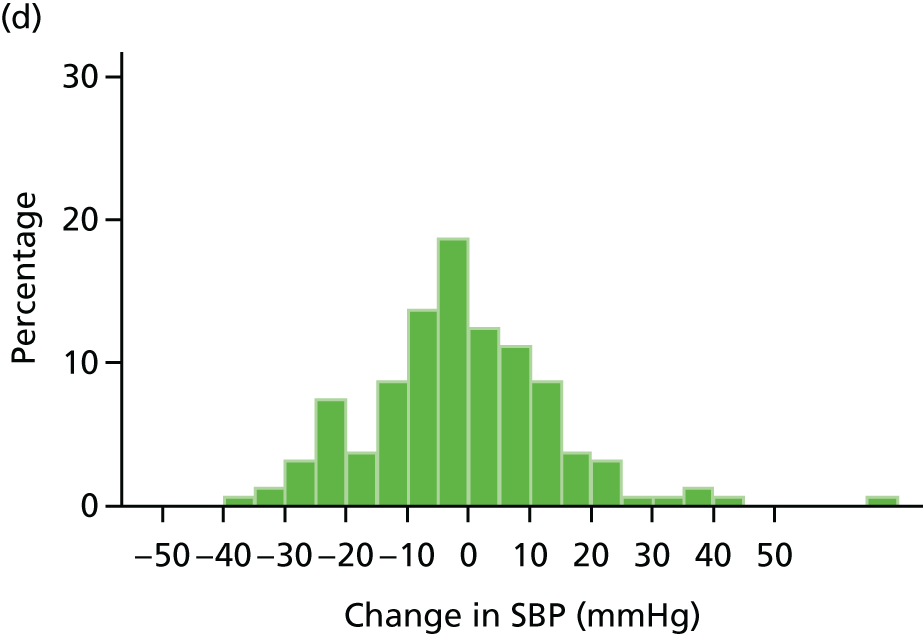
Box plots of change in SBP from baseline to 3, 12 and 24 months are shown in Figures 29–31, respectively. These show that there was little change in SBP in the placebo group but a reduction in SBP in the perindopril and amlodipine groups. For box plots of change in SBP from baseline to 6 and 18 months see Appendix 15.
FIGURE 29.
Box plot of change in SBP from baseline to month 3. p-value from regression of SBP at 3 months on treatment adjusted for SBP at baseline. a, perindopril vs. placebo; b, amlodipine vs. placebo; c, amlodipine vs. perindopril. Circle is outside value. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

FIGURE 30.
Box plot of change in SBP from baseline to month 12. p-value from regression of SBP at 3 months on treatment adjusted for SBP at baseline. a, perindopril vs. placebo; b, amlodipine vs. placebo; c, amlodipine vs. perindopril. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
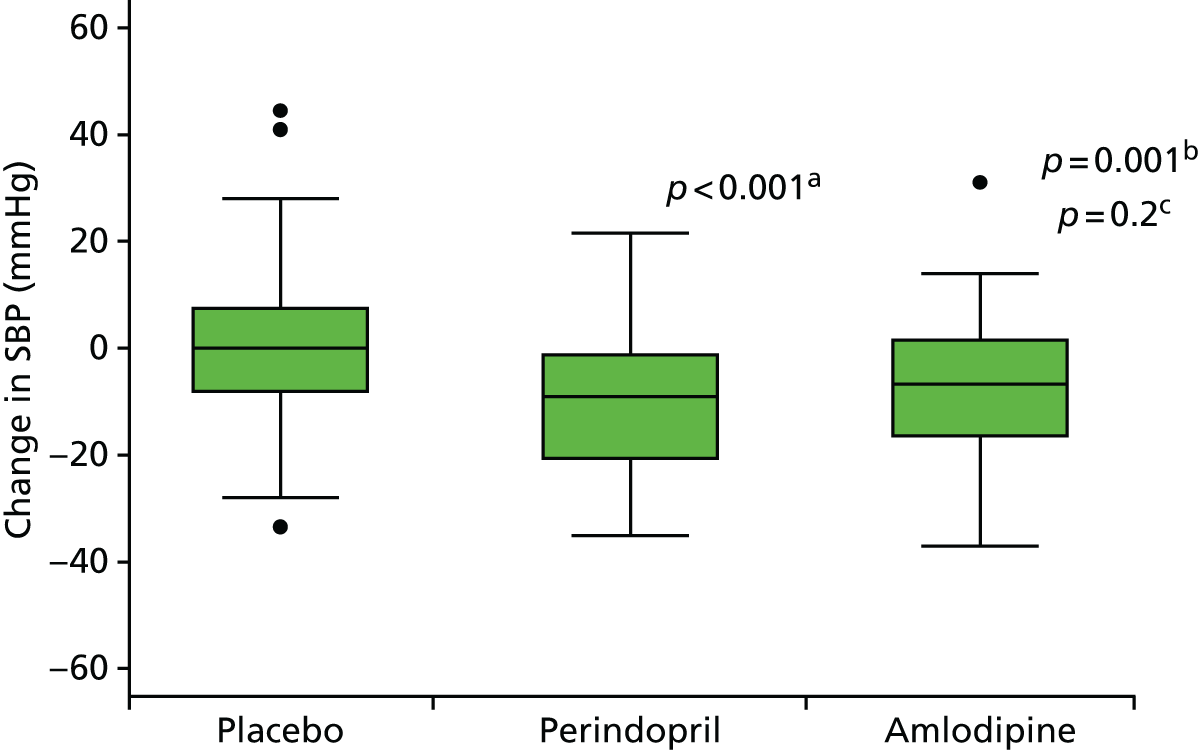
FIGURE 31.
Box plot of change in SBP from baseline to month 24. p-value from regression of SBP at 3 months on treatment adjusted for SBP at baseline. a, perindopril vs. placebo; b, amlodipine vs. placebo; c, amlodipine vs. perindopril. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

Figures 32–34 show the histograms of change in DBP from baseline to 3, 12 and 24 months, respectively. In line with the changes seen in SBP, the histograms for the placebo group generally follow a normal distribution, whereas the reductions in DBP in the perindopril and amlodipine group are shown by the increased density of bars to the left-hand side of each histogram. For histograms of change in DBP from baseline to 6 and 18 months see Appendix 16.
FIGURE 32.
Histograms of change in DBP from baseline to month 3. (a) Placebo; (b) perindopril; (c) amlodipine; and and (d) total.
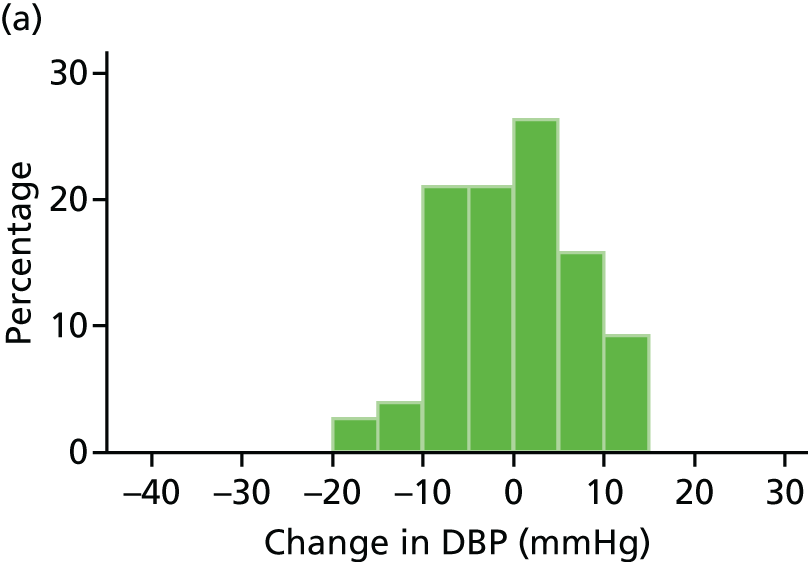



FIGURE 33.
Histograms of change in DBP from baseline to month 12. (a) Placebo; (b) perindopril; (c) amlodipine; (d) total.

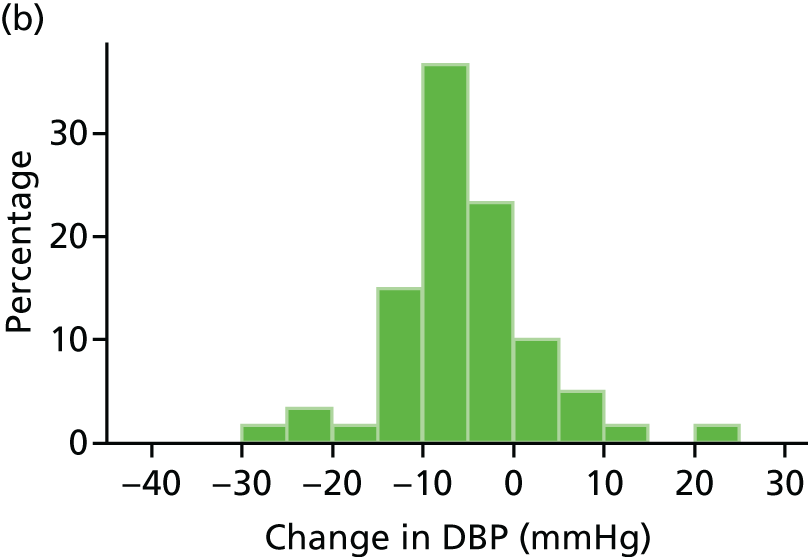
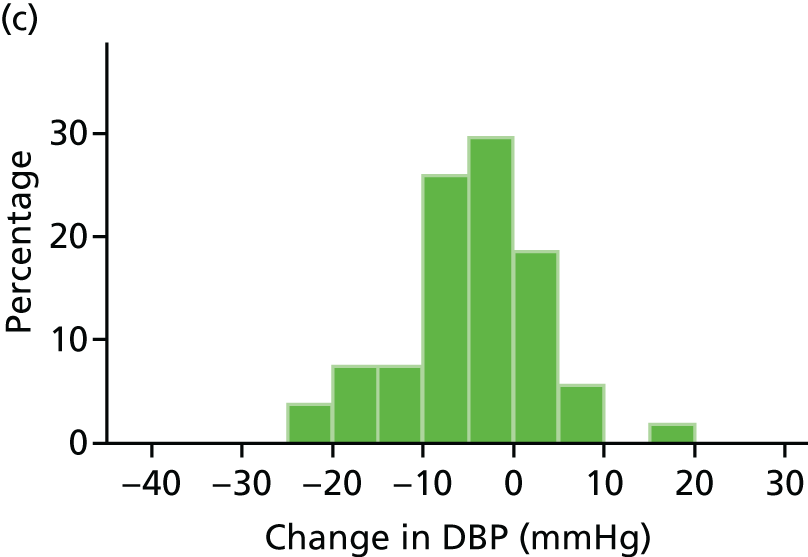
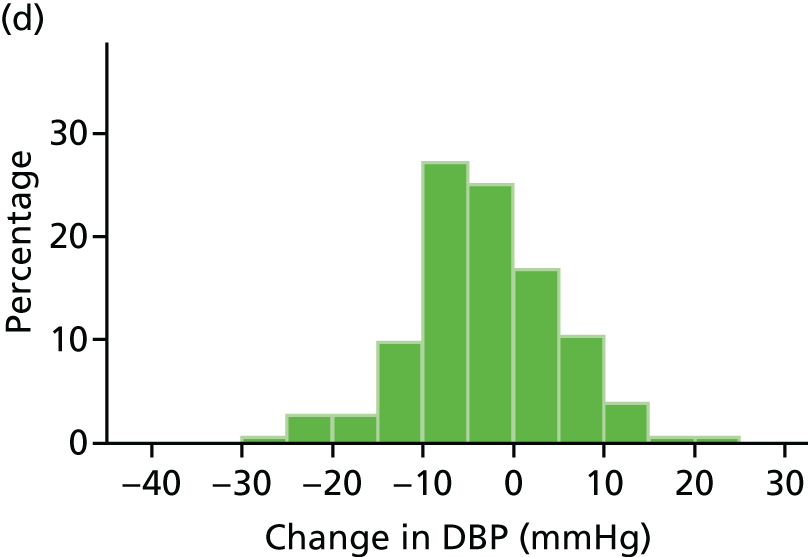
FIGURE 34.
Histograms of change in DBP from baseline to month 24. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
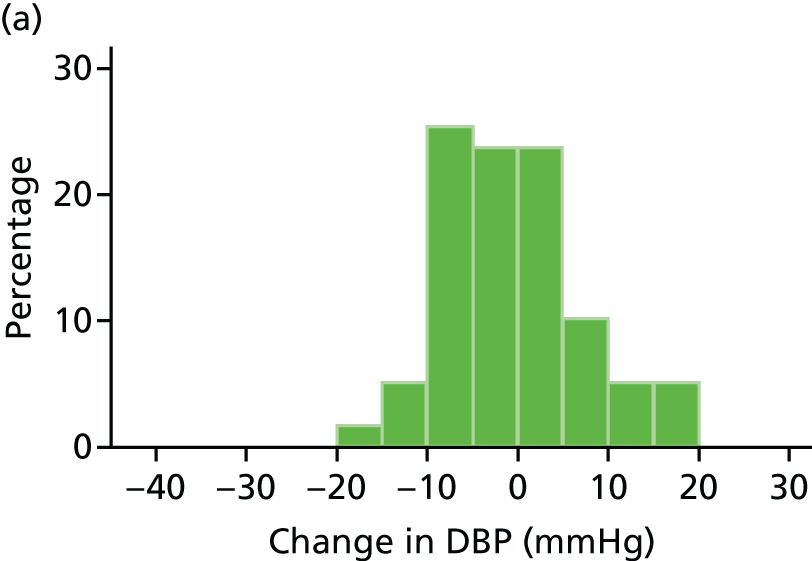

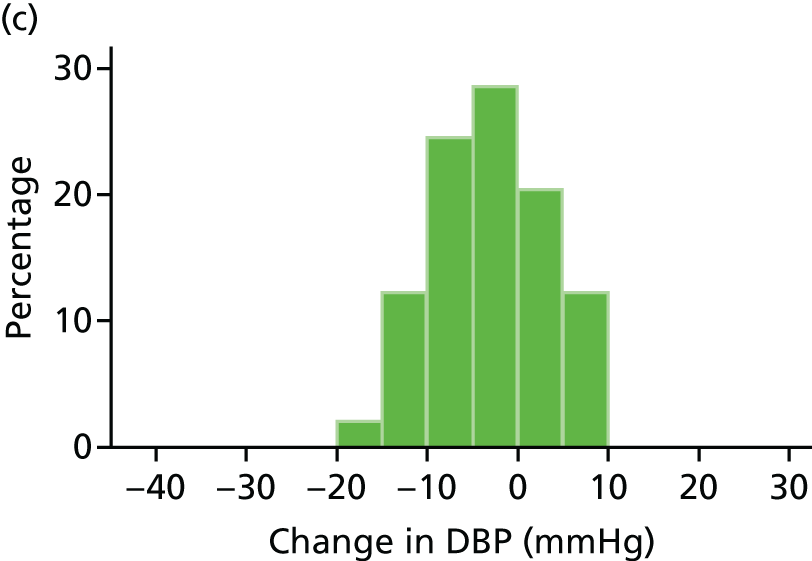

Box plots of change in DBP from baseline to 3, 12 and 24 months are shown in Figures 35–37, respectively. These show that there was little change in DBP in the placebo group but a reduction in DBP in both the perindopril group and the amlodipine group. For the box plots of change in DBP from baseline to 6 and 18 months see Appendix 17.
FIGURE 35.
Box plot of change in DBP from baseline to month 3. p-value from regression of DBP at 3 months on treatment adjusted for DBP at baseline. a, perindopril vs. placebo; b, amlodipine vs. placebo; c, amlodipine vs. perindopril. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

FIGURE 36.
Box plot of change in DBP from baseline to month 12. p-value from regression of DBP at 3 months on treatment adjusted for DBP at baseline. a, perindopril vs. placebo; b, amlodipine vs. placebo; c, amlodipine vs. perindopril. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
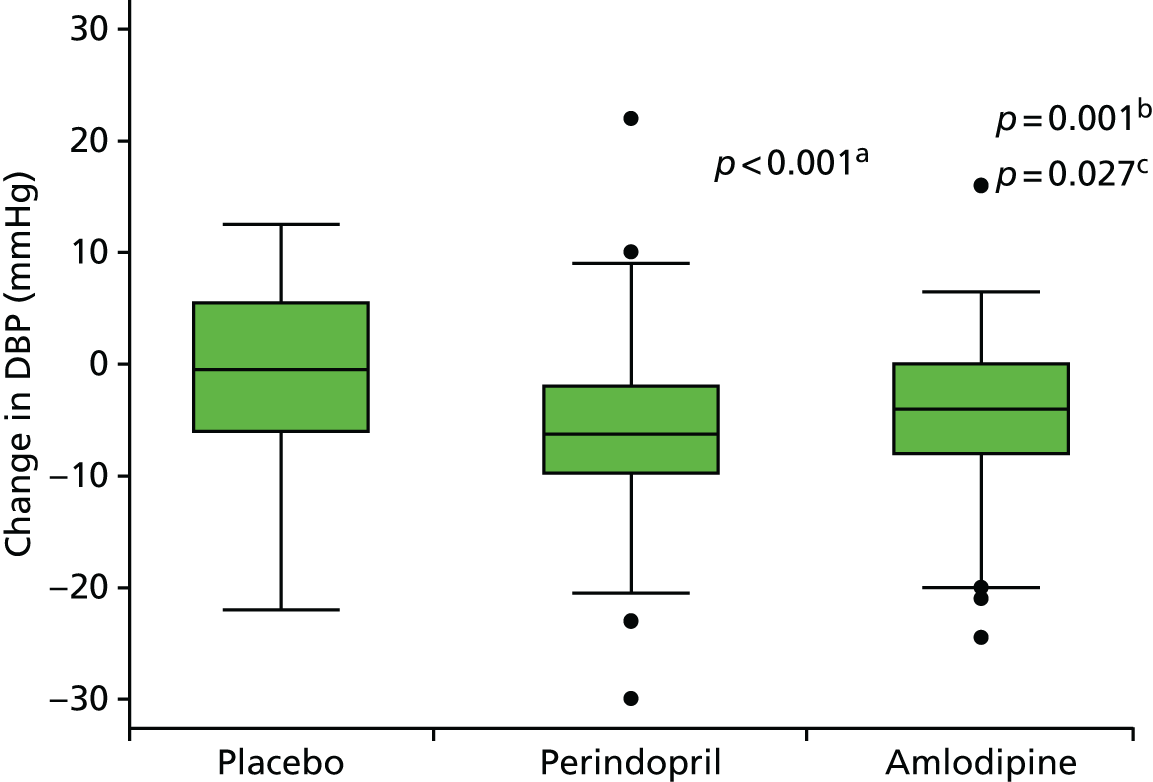
FIGURE 37.
Box plot of change in DBP from baseline to month 24. p-value from regression of DBP at 3 months on treatment adjusted for DBP at baseline. a, perindopril vs. placebo; b, amlodipine vs. placebo; c, amlodipine vs. perindopril. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
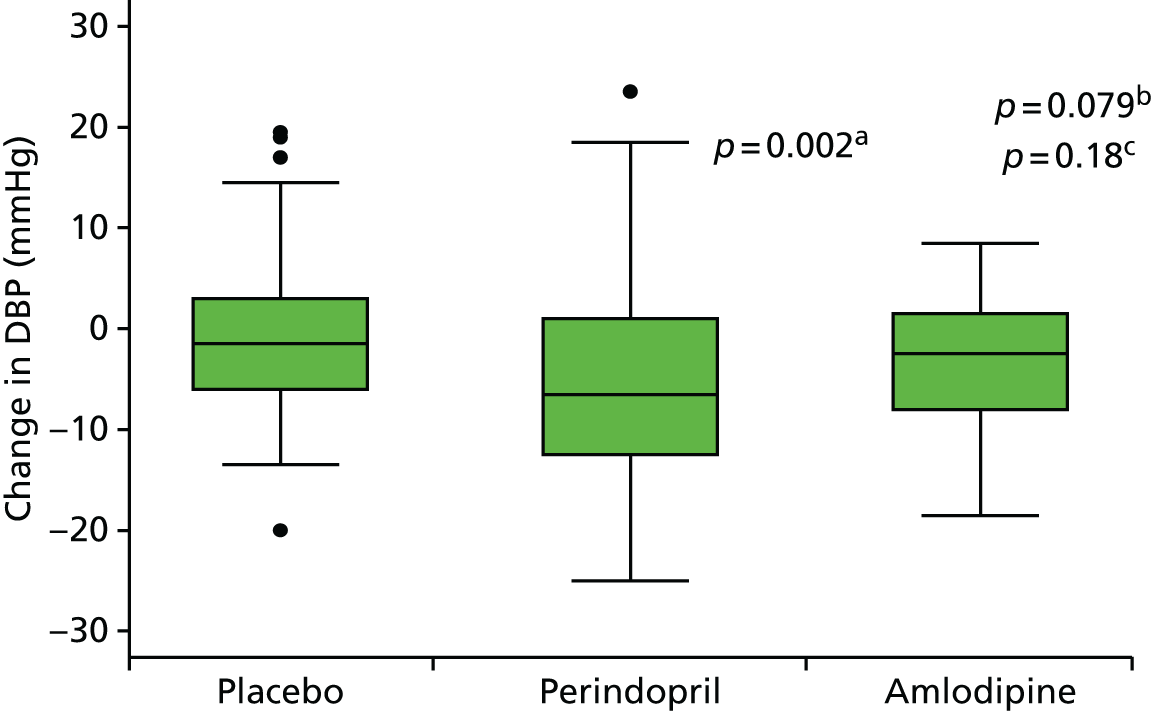
Secondary outcomes
Time to reach an AAA diameter of 5.5 cm, being referred to or having AAA surgery, or AAA rupture
The end points for this analysis were reaching a diameter of 5.5 cm in any of the four measurements (i.e. the first visit when this happened even if the patient continued in follow-up) or having/being referred to surgery. A total of 26 patients reached an AAA diameter of 5.5 cm during the course of the trial, with the same number being referred to/having AAA surgery (Tables 23 and 24). There were no AAA ruptures reported. For patients who reached both end points, the date of reaching an AAA diameter of 5.5 cm was used for analysis. Two cases proceeded to surgery before an AAA diameter of 5.5 cm was recorded in-trial and for these two patients the date of surgery was used. No significant differences were found between the three treatment groups for this combined secondary end point (Figure 38).
| Study end point | Placebo | Perindopril | Amlodipine | Total |
|---|---|---|---|---|
| Reaching 5.5 cm | 11 | 6 | 9 | 26 |
| Being referred to or having AAA surgery | 9 | 10 | 7 | 26 |
| Combined end point | 11 | 10 | 11 | 32 |
| Surgery only | 0 | 4 | 2 | 6 |
| Visit | Placebo | Perindopril | Amlodipine | Total |
|---|---|---|---|---|
| Baseline | 1 | 0 | 1 | 2 |
| Month 3 | 4 | 0 | 1 | 5 |
| Month 6 | 0 | 1 | 0 | 1 |
| Month 9 | 1 | 1 | 2 | 4 |
| Month 12 | 4 | 3 | 2 | 9 |
| Month 15 | 0 | 1 | 0 | 1 |
| Month 18 | 0 | 0 | 0 | 0 |
| Month 21 | 0 | 0 | 1 | 1 |
| Month 24 | 1 | 0 | 2 | 3 |
| Total | 11 | 6 | 9 | 26 |
FIGURE 38.
Kaplan–Meier estimates of the proportions of patients reaching secondary end points. a, 11 patients randomised are not included as they were seen only at baseline; b, apparent disparity in numbers attending their 24-month visit largely because of this visit occurring before 720 days. Reproduced from Bicknell et al. 2016. 95 © Bicknell et al. 2016. Published by Oxford University Press on behalf of the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
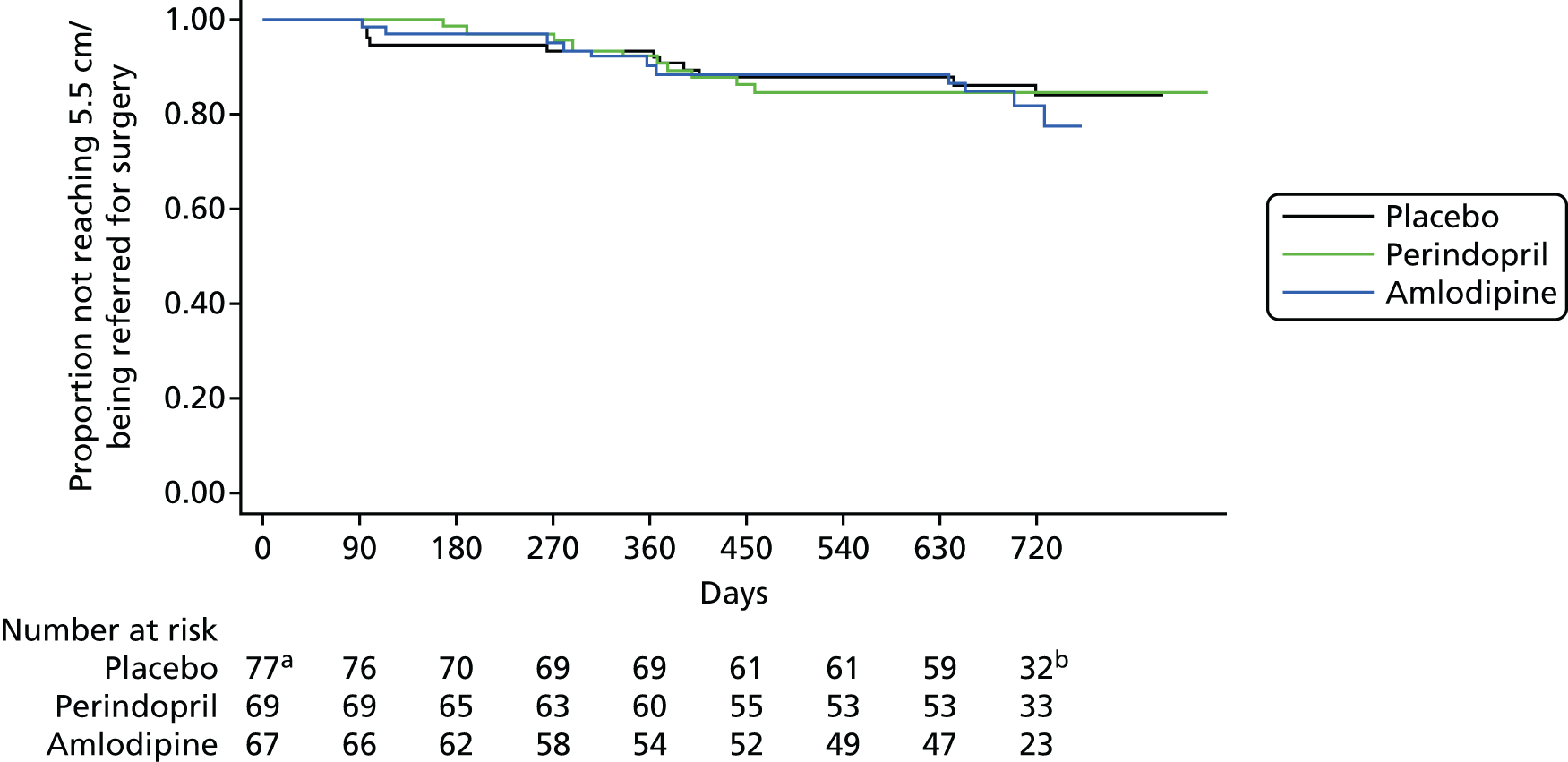
Quality-of-life outcomes
The overall EQ-5D scores at 12 and 24 months are shown in Table 25 (see Appendix 18 for the scores for each of the five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Overall, mean scores were similar across all three treatment groups at 12 months but a reduction in the mean score from 12 to 24 months was observed in the placebo group (from 71.4 to 64.0), representing a reduction in quality of life. A similar reduction in score was not observed in the perindopril or amlodipine group.
| Measure | Placebo | Perindopril | Amlodipine | Total |
|---|---|---|---|---|
| Month 12 | ||||
| n | 69 | 59 | 51 | 179 |
| Mean | 71.4 | 69.6 | 72.7 | 71.2 |
| SD | 24.1 | 27.2 | 24.4 | 25.1 |
| Median | 80 | 80 | 80 | 80 |
| IQR | 25 | 25 | 20 | 30 |
| Month 24 | ||||
| n | 56 | 50 | 45 | 151 |
| Mean | 64.0 | 70.2 | 71.4 | 68.2 |
| SD | 26.9 | 23.5 | 26.4 | 25.7 |
| Median | 70.5 | 75 | 80 | 75 |
| IQR | 33.5 | 20 | 30 | 25 |
Drug compliance
Compliance was evaluated using pill count data (number dispensed minus number returned) for each time period (Table 26). Mean compliance combining all three groups was > 80% for each 3-month period evaluated.
| Study period | n | Mean | SD | Median | P25 | P75 | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|
| Placebo | ||||||||
| Baseline to month 3 | 60 | 80 | 15 | 82 | 76 | 88 | 14 | 100 |
| Month 3 to month 6 | 65 | 75 | 25 | 85 | 71 | 90 | 0 | 100 |
| Month 6 to month 9 | 61 | 80 | 19 | 85 | 73 | 96 | 26 | 100 |
| Month 9 to month 12 | 60 | 77 | 20 | 84 | 69 | 90 | 10 | 100 |
| Month 12 to month 15 | 58 | 81 | 21 | 87 | 78 | 92 | 0 | 100 |
| Month 15 to month 18 | 47 | 82 | 21 | 88 | 80 | 95 | 1 | 100 |
| Month 18 to month 21 | 45 | 80 | 26 | 87 | 77 | 96 | 0 | 100 |
| Month 21 to month 24 | 40 | 82 | 20 | 89 | 76 | 94 | 6 | 100 |
| Perindopril | ||||||||
| Baseline to month 3 | 56 | 76 | 20 | 80 | 74 | 85 | 0 | 100 |
| Month 3 to month 6 | 59 | 84 | 16 | 86 | 81 | 96 | 4 | 100 |
| Month 6 to month 9 | 51 | 82 | 18 | 88 | 73 | 96 | 4 | 100 |
| Month 9 to month 12 | 45 | 78 | 22 | 85 | 75 | 91 | 20 | 100 |
| Month 12 to month 15 | 44 | 79 | 25 | 87 | 78 | 94 | 12 | 100 |
| Month 15 to month 18 | 38 | 76 | 27 | 87 | 75 | 91 | 0 | 99 |
| Month 18 to month 21 | 37 | 85 | 14 | 89 | 78 | 94 | 26 | 100 |
| Month 21 to month 24 | 32 | 84 | 20 | 90 | 81 | 94 | 12 | 100 |
| Amlodipine | ||||||||
| Baseline to month 3 | 60 | 75 | 19 | 77 | 71 | 84 | 1 | 100 |
| Month 3 to month 6 | 59 | 80 | 18 | 81 | 74 | 91 | 0 | 100 |
| Month 6 to month 9 | 50 | 75 | 22 | 81 | 71 | 89 | 12 | 100 |
| Month 9 to month 12 | 48 | 72 | 25 | 82 | 70 | 89 | 0 | 96 |
| Month 12 to month 15 | 43 | 73 | 30 | 84 | 75 | 90 | 3 | 100 |
| Month 15 to month 18 | 40 | 84 | 11 | 85 | 77 | 93 | 58 | 100 |
| Month 18 to month 21 | 37 | 82 | 20 | 89 | 84 | 93 | 3 | 100 |
| Month 21 to month 24 | 31 | 81 | 23 | 89 | 75 | 94 | 0 | 100 |
| Total | ||||||||
| Baseline to month 3 | 176 | 77 | 18 | 80 | 74 | 86 | 0 | 100 |
| Month 3 to month 6 | 183 | 80 | 20 | 85 | 74 | 92 | 0 | 100 |
| Month 6 to month 9 | 162 | 79 | 20 | 84 | 73 | 93 | 4 | 100 |
| Month 9 to month 12 | 153 | 75 | 22 | 83 | 71 | 90 | 0 | 100 |
| Month 12 to month 15 | 145 | 78 | 25 | 86 | 77 | 92 | 0 | 100 |
| Month 15 to month 18 | 125 | 81 | 21 | 87 | 77 | 92 | 0 | 100 |
| Month 18 to month 21 | 119 | 82 | 21 | 88 | 78 | 94 | 0 | 100 |
| Month 21 to month 24 | 103 | 82 | 21 | 89 | 78 | 94 | 0 | 100 |
Safety
Adverse events
Both active treatments were generally well tolerated, with similar numbers of patients as in the placebo group discontinuing therapy for AEs (n = 8, 13 and 14 for placebo, perindopril and amlodipine, respectively). Table 27 provides a summary of AEs and SAEs occurring in the trial. Although there were more SAEs in the perindopril group, none of these SAEs was deemed to be related to the study IMP by the PI of the relevant site.
| AEs and SAEs | Events | Patientsa | Patientsb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Perindopril | Amlodipine | Total | Placebo | Perindopril | Amlodipine | Total | Placebo | Perindopril | Amlodipine | Total | |
| Number of AEs | 175 | 137 | 103 | 415 | 54 | 55 | 36 | 145 | 54 | 55 | 36 | 145 |
| Number permanently discontinued | 8 | 13 | 14 | 35 | 8 | 9 | 9 | 26 | 8 | 9 | 9 | 26 |
| Number of SAEs | 23 | 33 | 17 | 73 | 16 | 19 | 12 | 47 | 16 | 19 | 12 | 47 |
| SAEs related to study (definite or probable) | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 2 |
| Reason defined as a SAE | ||||||||||||
| Death | 3 | 2 | 2 | 7 | 3 | 2 | 2 | 7 | 3 | 2 | 2 | 7 |
| Life-threatening | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 2 |
| Hospitalisation required | 17 | 28 | 11 | 56 | 11 | 17 | 8 | 36 | 10 | 16 | 7 | 33 |
| Other important medical events | 2 | 3 | 3 | 8 | 2 | 3 | 3 | 8 | 2 | 1 | 2 | 5 |
Serum creatinine
Table 28 and Figure 39 show the distribution of serum creatinine levels at 3, 12 and 24 months for the three treatment arms and combined. Table 29 shows the serum creatinine differences between each of the study visits and screening. As expected, there was little difference in mean serum creatinine levels in the placebo and amlodipine groups across the duration of the trial but a small increase was observed in the perindopril group (6% at 3 months). However, this increase was not statistically significant and no patients were withdrawn from the trial because of concerns regarding renal function.
| Visit | n | Mean | SD | Median | P25 | P75 | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|
| Placebo | ||||||||
| Screening | 73 | 86.6 | 20.5 | 79 | 73 | 96 | 56 | 154 |
| Month 3 | 72 | 87.5 | 19.7 | 84 | 72 | 100 | 56 | 153 |
| Month 12 | 63 | 88.2 | 19.8 | 85 | 73 | 99 | 55 | 154 |
| Month 24 | 52 | 85.5 | 16.5 | 83 | 74 | 97 | 58 | 126 |
| Perindopril | ||||||||
| Screening | 69 | 87.0 | 18.7 | 86 | 75 | 95 | 54 | 156 |
| Month 3 | 66 | 92.1 | 19.1 | 88 | 78 | 101 | 66 | 169 |
| Month 12 | 54 | 93.0 | 21.9 | 89 | 75 | 105 | 64 | 161 |
| Month 24 | 49 | 90.3 | 20.7 | 86 | 75 | 101 | 53 | 137 |
| Amlodipine | ||||||||
| Screening | 67 | 90.0 | 20.0 | 87 | 78 | 96 | 55 | 178 |
| Month 3 | 64 | 90.0 | 20.3 | 88 | 74 | 100 | 61 | 161 |
| Month 12 | 50 | 90.3 | 18.8 | 87 | 78 | 96 | 54 | 136 |
| Month 24 | 43 | 89.8 | 18.7 | 89 | 75 | 95 | 65 | 143 |
| Total | ||||||||
| Screening | 209 | 87.8 | 19.7 | 86 | 75 | 96 | 54 | 178 |
| Month 3 | 202 | 89.8 | 19.7 | 87 | 76 | 100 | 56 | 169 |
| Month 12 | 167 | 90.4 | 20.2 | 87 | 77 | 100 | 54 | 161 |
| Month 24 | 144 | 88.4 | 18.7 | 85 | 75 | 98 | 53 | 143 |
FIGURE 39.
Median serum creatinine levels at screening and 3, 12 and 24 months by randomised group. (a) Placebo; (b) perindopril; and (c) amlopidine. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
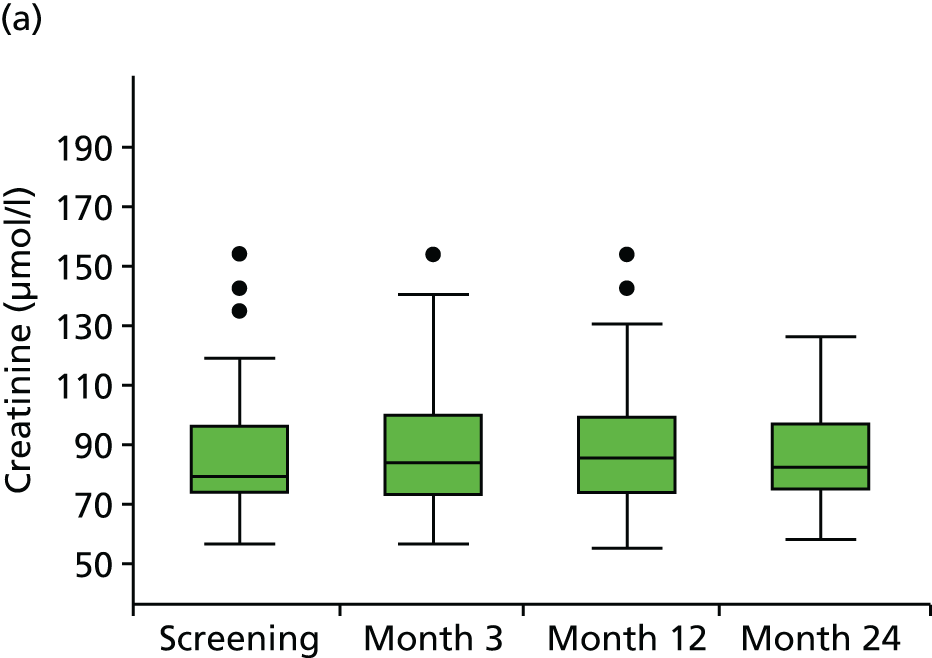
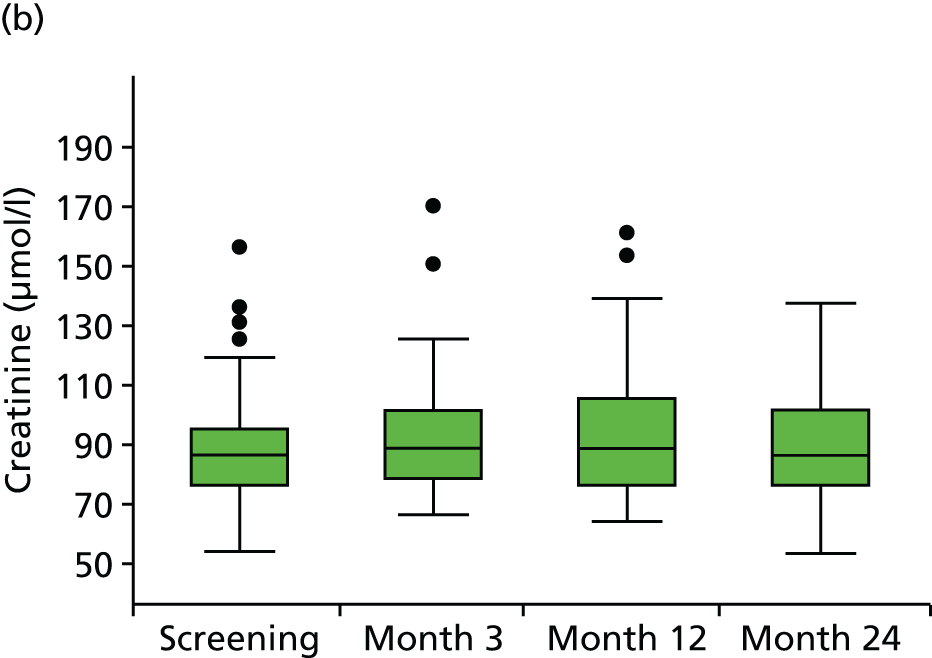

| Study period | n | Mean | SD | Median | P25 | P75 | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|
| Placebo | ||||||||
| Month 3 – screening | 67 | 0.6 | 12.9 | 0 | –4 | 5 | –64 | 49 |
| Month 12 – screening | 61 | 0.5 | 14.6 | 1 | –5 | 6 | –61 | 30 |
| Month 24 – screening | 50 | –0.4 | 13.4 | –1 | –7 | 8 | –51 | 31 |
| Perindopril | ||||||||
| Month 3 – screening | 62 | 5.6 | 10.4 | 4 | –1 | 11 | –9 | 54 |
| Month 12 – screening | 51 | 4.8 | 10.6 | 4 | –2 | 10 | –19 | 37 |
| Month 24 – screening | 47 | 3.8 | 11.2 | 1 | –4 | 11 | –17 | 44 |
| Amlodipine | ||||||||
| Month 3 – screening | 60 | –0.3 | 10.3 | –1 | –7 | 6 | –23 | 35 |
| Month 12 – screening | 46 | 1.7 | 11.5 | 1 | –4 | 6 | –21 | 49 |
| Month 24 – screening | 41 | –0.1 | 8.2 | –2 | –5 | 6 | –18 | 18 |
Chapter 5 Discussion
The AARDVARK trial was designed to evaluate whether or not ACE inhibition induces a beneficial effect on the growth of small AAAs. This trial has shown that 2 years of daily ingestion of the ACE-I perindopril has no impact on the growth rate of small AAAs (the primary end point of the trial) compared with that observed among those randomised to the CCB amlodipine or placebo.
In keeping with this lack of any differential effect on AAA growth among the three randomised treatment arms of the trial, there was also no difference between the groups in the numbers of trial participants whose AAA reached a diameter of 5.5 cm and/or who were referred for/received surgical intervention for their AAA (26 patients), which was a secondary end point of the trial. No ruptures were reported in the study, confirming the safety of a policy of ultrasound surveillance for small AAAs.
Taking into account various sources of data collated during the trial, including three QA events at which trial sonographers and AAA patient volunteers attended, it was clear that, as expected, AAA measurements collected in the longitudinal plane were more repeatable than those collected in the transverse plane and that, overall, OTO measurements were superior to ITI measurements in terms of interobserver repeatability. Hence, for the primary analyses of this trial, OTO measurements in the longitudinal plane were used.
Serum creatinine levels as well as other potential AEs were monitored closely in the study. Although a small and expected increase in creatinine levels was seen in the perindopril group, trial withdrawals because of study drug-related AEs were similar among the three groups. As expected, no suspected unexpected serious adverse reactions were reported during the trial, consistent with the established safety profiles of ACE-Is and CCBs. Compliance with therapy was excellent in all three treatment arms.
The trial was designed to generate equivalent BP reduction in the two actively treated groups, based on previously published data73–75 and the knowledge that ACE-Is tend to be less effective than CCBs in terms of BP lowering among the older age group included in the trial. 45
Both actively treated groups had lower in-trial BPs than those on placebo; however, somewhat surprisingly, BP lowering was greater among those randomised to perindopril than among those receiving amlodipine. Nevertheless, had there been a BP-related benefit in terms of AAA growth rather than a drug class-related effect, such a BP-related effect could have been detected in this trial. However, no differential effects on AAA growth were apparent across the three groups, BP related or otherwise.
Although data are inconsistent, ACE-Is have been associated with a reduced incidence of AAA rupture in analyses of administrative databases. 6,70 Evidence for the benefit of other antihypertensive agents has been lacking,6 suggesting that ACE-Is may act through a BP-independent mechanism; however, it is important to note that analyses of administrative databases are subject to the caveats and shortcomings of observational data.
Meanwhile, evidence implicating the RAS in aortic aneurysm formation and growth in animal models has been reported. 67,68,96,97 For example, infusion of angiotensin II induces suprarenal post-dissection aneurysm formation in animal models and continued infusion has been shown to cause pathological changes in the aneurysmal tissue including infiltration of macrophages and disruption of elastin in the medial layer. 98 The discrepancies between animal and human studies may reflect the challenges of conducting RCTs in AAA patients, including accurately measuring AAA growth and bias in patient recruitment, but may more likely reflect differential associations and physiology across species or differences in the pathology of these aneurysms between humans and artificial animal models.
Strengths
To our knowledge this study is unique in being the only placebo-controlled RCT to have completed an evaluation of the impact of ACE-Is on the growth rate of small AAAs. However, two other RCTs of the ARBs valsartan (NCT01904981) and telmisartan99 are currently in progress. Interestingly, the large Canadian case–control study6 that reported the protective effects of ACE-Is on the rupture of AAAs did not show similar benefits for ARBs.
Although a significant proportion of trial participants did not complete 2 years of follow-up in the AARDVARK trial because of reaching a censoring trial end point (e.g. referral for surgical intervention or death), the great majority continued regular follow-up and the overall attrition rate was only 6% (which was less than the target attrition rate of 10% used in power calculations). Hence, 94% of patients did contribute at least two sequential AAA measurements to facilitate an evaluation of growth.
Drug compliance among attendees was excellent as evaluated by pill counts and data collection was efficient with only a small number of key measurements or investigations (e.g. BP levels) missing in the trial overall.
The trial included a system for quality control. The standardised procedures used for AAA measurements as outlined in the scanning protocol were designed to reduce measurement variability associated with using 19 sonographers from 11 sites. This protocol was taught at specifically designed QA days. Image quality was also robustly assessed and sites with poor-quality scans were reassessed. Lastly, the measurements taken for all time points were audited by the trial senior clinical vascular scientist and, when obvious differences between measurements in the same subject at the same time point existed, a review took place.
Limitations
The profile of the trial participants – largely white men aged ≥ 55 years with a heavy smoking history – is typical of that for UK patients with an AAA but this should be set in the context of those ineligible for the trial or who did not take part (see Figures 11 and 12). Indeed, the limitations of the AARDVARK trial include the potentially restricted generalisability of the trial recruits. Trial inclusion and exclusion criteria predetermined that, although the overall profile of the recruits might at first sight be considered typical of patients from the UK with an AAA, the age range was limited to ≥ 55 years and SBP had to be < 150 mmHg.
Furthermore, it is clear from Figure 11 that most patients considered for the trial were already taking an antihypertensive agent that made them ineligible. Allied with the other reasons why patients declined to join the trial (see Figure 12), it is reasonable to assume that the trial population may not be representative of the true AAA population in the UK as a whole. This conclusion is supported by the mean SBP and DBP levels recorded in other AAA studies (see Table 2), with SBP levels ranging from 143 to 157 mmHg and DBP levels ranging from 81 to 91 mmHg. These levels appear to be significantly higher than the mean BPs found at baseline in all three treatment arms in the AARDVARK trial (131.7/77.9 mmHg, 130.9/76.7 mmHg and 131.9/78.0 mmHg for placebo, perindopril and amlodipine, respectively).
At a more global level, the findings of a study predominantly including UK-based white men may clearly not be generalisable to non-UK populations, women or those of different ethnicities.
The doses of ACE-I and CCB chosen for the trial were expected to cause similar reductions in BP;73–75 however, this was not found to be the case. Although both treatments were effective in lowering BP, the mean differences in SBP from baseline to 24 months in the perindopril and amlodipine groups were –5.0 (SD 16.3) mmHg and –2.8 (SD 11.7) mmHg, respectively, compared with 2.5 (SD 16.5) mmHg in the placebo group. With similar withdrawal rates observed among the three treatment groups and no differences in relation to compliance, the reason for the difference in BP reduction between the perindopril group and the amlodipine group remains unclear. Although ACE-Is are known to be particularly effective in treating hypertension in patients with renal artery stenosis, patients with known renal artery stenosis of > 50% were excluded from the trial. However, no further investigations were undertaken to explore or exclude lesser degrees of renal artery stenosis in the trial population, making it difficult to draw any further conclusions about the observed BP differences in relation to the role of renal artery stenosis.
A potentially crucial limitation of the AARDVARK trial relates to the statistical power. To calculate the sample size of the trial we estimated that the trial would have 90% power at the 5% level to detect a 38% (1-mm) difference in growth rate between those receiving perindopril and those on placebo. This was based on an estimated annual growth in AAA diameter of 2.6 (SD 1.8) mm, as reported in the UKSAT trial. 22 However, given the actual average annual growth rate observed of 1.7 (SD 3.0) mm, 190 patients per group would have been required to detect a 1-mm difference in growth with a power of 90%. Based on the actual sample size (75 per group), we had 51% power to detect a 1-mm difference in growth (between two groups) and 85% power to detect a difference of 1.5 mm (close to the annual growth rate). However, our estimated difference in the slopes of regression between perindopril and placebo was 0.08 mm with a 95% CI of –0.50 mm to 0.65 mm, which statistically excludes a likely reduction of 1 mm. This implies that an important clinical difference in growth rate of small AAAs is unlikely to occur in this population in association with the use of perindopril compared with placebo.
Given the very small changes in AAA size that were expected over the 2-year follow-up period, numerous quality control measures were put in place to obtain accurate AAA measurements. Furthermore, sites were requested to have the same observer scanning the same patient on the same machine for the duration of the study. All sites reported that they adhered to this request to the greatest extent possible, bearing in mind clinical service pressures and staffing changes. Despite this, the mean (range) intrasonographer repeatability coefficient was 0.33 (0.21–0.45) cm for OTO measurements. Although ultrasonography is a cost-effective tool for the identification of AAAs and sufficient to direct clinical management of patients, the accuracy of measurements made in a RCT becomes critical because of its high operator dependence. During the site initiation visits for the trial it became clear that the routine methods for taking ultrasound measurements varied among sites. Despite the protocol and training that were provided for the trial, unfamiliarity with taking some of the AAA measurements required by the trial may explain the greater than expected range in measurement variability.
The greater than expected intra- and inter-observer variability and measurement error add uncertainty to the interpretation of the results. For future studies involving observers at multiple sites it would be pertinent to undertake detailed and practical training sessions prior to the recruitment of patients. Furthermore, measurement variability might be reduced by using more accurate methods of obtaining images, such as performing an electrocardiogram simultaneously with recording ultrasound videos, allowing for electrocardiogram gating in the reading process. 100,101
Chapter 6 Conclusions
We were unable to demonstrate any impact of an ACE-I compared with placebo or a CCB on the growth rate of small AAAs over a 2-year period. Furthermore, a decrease in BP achieved among those allocated to active BP-lowering therapy was not associated with a reduction in AAA growth rate compared with those allocated to placebo. However, because of the small AAA growth rates observed, as well as the greater than expected AAA measurement variability (including measurement error), the trial had only 51% power to detect a 1-mm difference in growth (between two groups) and 85% power to detect a difference of 1.5 mm (close to the annual growth rate). Nevertheless, our estimated difference in slope between those receiving perindopril and those receiving placebo was 0.08 mm, with a 95% CI of –0.50 mm to 0.65 mm, which statistically excludes a likely reduction of 1 mm. Hence, at least in this type of population with relatively well-controlled BP, modest BP reduction with a CCB or an ACE-I offers no obvious benefits in terms of reducing the growth of small AAAs and this trial was unable to show any indication of any BP-independent beneficial effect on the growth of small AAAs of an ACE-I over placebo or a CCB or a combination of placebo and a CCB.
Implications for health care
Despite some earlier evidence suggesting that the rupture rates of AAAs may be lower in patients taking ACE-Is, this trial found no evidence that the growing cohort of patients with a small AAA under surveillance should be prescribed an ACE-I to slow AAA growth. The QA studies undertaken as well as the comparison of various aspects of variability of internal and external measurements provide support for the use of external rather than internal AAA diameter measurements taken in the longitudinal plane as the routine measurement of choice for the screening and follow-up of AAAs.
Recommendations for future research
The results of this pilot trial do not provide strong support for a larger trial to evaluate the effects of ACE-Is on small AAA growth rate.
Other than the apparently negative findings of the trial (albeit with some caveats around the issue of power), the large and increasing numbers of elderly patients with an AAA already receiving some form of RAS blockade (the most common cause of ineligibility for this trial) would make randomisation to an ACE-I or an ARB significantly more difficult, with associated concerns about the generalisability of the findings of any such trial. Nevertheless, patients recruited through the NAAASP were less likely to be already receiving a RAS blocker than those already in a vascular database and could therefore offset some of those concerns.
Patients considered unfit for surgery but with an AAA of larger diameter than those included in the AARDVARK trial may, by virtue of their larger AAA growth rates, provide a further source of eligible patients for a more powerful evaluation of medical interventions with regard to AAA size and/or rupture.
Importantly, whichever groups of patients are included in future trials of AAA, strict protocols around ultrasound measurements and training of trial staff are critical if AAA measurements are key outcomes.
The following research recommendations are made as a consequence of the conduct and findings of the AARDVARK trial:
-
Further work related to the data already collected in the AARDVARK trial:
-
A multivariate analysis of determinants of AAA growth in the AARDVARK trial.
-
Potential differences were observed between the three treatment groups in relation to the number of patients whose AAA grew at a fast rate during the trial (as defined by a growth rate of > 5 mm per year). However formal analyses are still required.
-
An evaluation of the incremental predictive power of baseline and changing central BPs and BP variability with regard to AAA growth rates.
-
-
Further work potentially arising from the AARDVARK trial:
-
An evaluation of currently available data regarding AAA growth rates in those with SBP of < 150 mmHg and ≥ 150 mmHg to investigate whether growth rates could be critically affected by this systolic threshold or other systolic and diastolic thresholds.
-
An evaluation of whether the BP-lowering effect of perindopril and amlodipine are affected by the presence or absence of an AAA.
-
The strong protective effect of type 2 diabetes on the development of AAAs observed in large observational databases merits further investigation.
-
A large measurement variability study to optimise training and standardisation.
-
A trial to evaluate the impact of ACE-Is on the rupture of larger AAAs.
-
Acknowledgements
We would like to thank the NIHR HTA programme for funding the AARDVARK trial and the NIHR Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London for supporting the trial.
Thanks also go to the Foundation for Circulatory Health for funding the central BP substudy and to Servier for providing perindopril at no charge for use in the trial.
We would like to thank all of the staff at the recruiting centres (see AARDVARK trial collaborators) for their work in setting up and running the trial. We also thank all of the staff from the ICTU who dedicated their time to supporting and running the study, including Miriam Adesokan, Dr Sasi Thiagarajah, Dr Andrew Whitehouse, Annette Wilson, Yogeshwari Bhadresa, Sharon Spence and Dr Hilda Tsang. Thank you also to Sandra Griffiths and the InForm team who provided expert database support.
Thanks go to Professor Deborah Ashby, Dr Louise Brown and Nan Wang for their statistical advice and to Corinna Gomm for her assistance with the QA part of the study.
We also thank the independent members of the TSC [Mr Jonothan Earnshaw (chairperson), Professor Cliff Shearman and Mr Barrie Newton] and the DSMC members [Professor Simon Thompson (chairperson), Professor Gareth Beevers and Mr Teun Wilmilk] for their essential advice, support and encouragement during the trial.
Finally, a very special thank you to all of the AARDVARK trial participants.
Grant holders
Professor Neil Poulter, Professor Deborah Ashby, Professor Janet Powell, Mr Colin Bicknell, Professor Christopher Imray, Mr Matthew Waltham and Ms Meryl Davies.
Trial Steering Committee
Mr Jonothan Earnshaw (chairperson), Professor Cliff Shearman and Mr Barrie Newton.
Data Safety Monitoring Committee
Professor Simon Thompson (chairperson), Professor Gareth Beevers and Mr Teun Wilmilk.
AARDVARK trial collaborators
-
Imperial College Healthcare NHS Trust (St Mary’s Hospital): Mr Colin Bicknell (PI), Ms Louise Brown, Ms Jennifer Scriven, Mr Alex Rolls, Ms Candice Coghlan, Ms Rebecca James and Mr Andrew Whitehouse.
-
Imperial College Healthcare NHS Trust (Charing Cross Hospital): Professor Janet Powell (PI) and Ms Angela Williams.
-
Guy’s and St Thomas’ NHS Foundation Trust (St Thomas’ Hospital): Mr Bijan Iodarai (PI), Mr Matthew Waltham (PI) and Ms Susan Clark.
-
Royal Free London NHS Foundation Trust (Royal Free Hospital): Ms Meryl Davis (PI).
-
University Hospitals Coventry and Warwickshire NHS Trust (University Hospital Coventry): Professor Christopher Imray (PI) and Ms Alison Kite.
-
Hull and East Yorkshire Hospitals NHS Trust (Hull Royal Infirmary): Professor Ian Chetter (PI), Ms Emma Clarke and Ms Anna Firth.
-
Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust (Royal Bournemouth Hospital): Mr Dynesh Rittoo (PI) and Ms Sara Baker.
-
Colchester Hospital University NHS Foundation Trust (Colchester General Hospital): Mr Sohail Choksy (PI), Ms Marianne Morgan and Ms Nyasha Nygo.
-
Newcastle Hospitals NHS Foundation Trust (Freeman Hospital): Mr Tim Lees (PI), Ms Lesley Wilson, Ms Vera Wealleans and Ms Noala Parr.
-
City Hospitals Sunderland NHS Foundation Trust (Sunderland Royal Hospital): Mr Andrew Brown (PI), Ms Florrie Mowatt and Ms Ruth Chipp.
-
York Teaching Hospitals NHS Foundation Trust (York Hospital): Mr Andrew Thompson (PI), Mr Andy Gibson, Ms Zoe Coleman and Mr Thomas Smith.
-
Norfolk and Norwich University Hospitals NHS Foundation Trust (Norfolk and Norwich University Hospital): Ms Felicity Meyer (PI) and Ms Mandy Burrows.
-
Central Manchester University Hospitals NHS Foundation Trust (Manchester Royal Infirmary): Mr Vince Smyth (PI), Mr Andrew Schiro and Ms Helna Edlin.
-
Sheffield Teaching Hospitals NHS Foundation Trust (Northern General Hospital): Mr Shah Nawaz (PI), Rebecca Warren, Diane Swift, Craig King, Cheryl Bailey and Faith Okhuoya.
Thank you also to all of the pharmacy staff and scanning staff/sonographers at all of the above sites.
Contributions of authors
Ms Gaia Kiru (Trial Manager) contributed to the design of the study, the day-to-day management and delivery of the trial and interpretation of the results, and was the principal author responsible for the writing and delivery of the final report.
Mr Colin Bicknell (Vascular Surgeon and coinvestigator) contributed his expertise to the design of the study, recruitment, interpretation of the trial findings, and writing and delivery of the final report.
Ms Emanuela Falaschetti (Statistician) was responsible for the interim and final statistical analyses for the study, and contributed to the writing of the statistical methods and results sections.
Professor Janet Powell (Professor of Vascular Medicine and coinvestigator) contributed her expertise to the design of the study, recruitment, interpretation of the trial findings, and writing and delivery of the final report.
Professor Neil Poulter (Professor of Preventative Cardiovascular Medicine and chief investigator) contributed his expertise to the design of the study, recruitment, interpretation of the trial findings and writing and delivery of the final report, and held overall oversight and responsibility for the study.
Publication
Bicknell CD, Kiru G, Falaschetti E, Powell JT, Poulter NR, on behalf of the AARDVARK collaborators. An evaluation of the effect of an angiotensin converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomized placebo-controlled trial (AARDVARK) [published online ahead of print July 1 2016]. Eur J Heart 2016.
Data sharing statement
Requests for available data can be made by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg 2011;41:1-58. http://dx.doi.org/10.1016/j.ejvs.2010.09.011.
- Mitchell DC, Hindley H, Naylor R, Wyatt M, Loftus I. Outcomes after Elective Repair of Infra-Renal Abdominal Aortic Aneurysm. London: Vascular Society of Great Britain and Ireland; 2012.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012;43:161-6. http://dx.doi.org/10.1016/j.ejvs.2011.11.014.
- Svensjö S, Björck M, Gürtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 2011;124:1118-23. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.030379.
- Davis M, Harris M, Earnshaw JJ. Implementation of the National Health Service Abdominal Aortic Aneurysm Screening Program in England. J Vasc Surg 2013;57:1440-5. http://dx.doi.org/10.1016/j.jvs.2012.10.114.
- Hackam DG, Thiruchelvam D, Redelmeier DA. Angiotensin-converting enzyme inhibitors and aortic rupture: a population-based case-control study. Lancet 2006;368:659-65. http://dx.doi.org/10.1016/S0140-6736(06)69250-7.
- Propanolol Aneurysm Trial investigators . Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002;35:72-9. http://dx.doi.org/10.1067/mva.2002.121308.
- MacSweeney ST, Ellis M, Worrell PC, Greenhalgh RM, Powell JT. Smoking and growth rate of small abdominal aortic aneurysms. Lancet 1994;344:651-2. http://dx.doi.org/10.1016/S0140-6736(94)92087-7.
- Chang JB, Stein TA, Liu JP, Dunn ME. Risk factors associated with rapid growth of small abdominal aortic aneurysms. Surgery 1997;121:117-22. http://dx.doi.org/10.1016/S0039-6060(97)90279-8.
- Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA, Scott RA. Quantifying the risks of hypertension, age, sex and smoking in patients with abdominal aortic aneurysm. Br J Surg 2000;87:195-200. http://dx.doi.org/10.1046/j.1365-2168.2000.01353.x.
- Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010;52:539-48. http://dx.doi.org/10.1016/j.jvs.2010.05.090.
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997;126:441-9. http://dx.doi.org/10.7326/0003-4819-126-6-199703150-00004.
- Salem MK, Rayt HS, Hussey G, Rafelt S, Nelson CP, Sayers RD, et al. Should Asian men be included in abdominal aortic aneurysm screening programmes?. Eur J Vasc Endovasc Surg 2009;38:748-9. http://dx.doi.org/10.1016/j.ejvs.2009.07.012.
- Reed D, Reed C, Stemmermann G, Hayashi T. Are aortic aneurysms caused by atherosclerosis?. Circulation 1992;85:205-11. http://dx.doi.org/10.1161/01.CIR.85.1.205.
- Lee AJ, Fowkes FG, Carson MN, Leng GC, Allan PL. Smoking, atherosclerosis and risk of abdominal aortic aneurysm. Eur Heart J 1997;18:671-6. http://dx.doi.org/10.1093/oxfordjournals.eurheartj.a015314.
- Wilmink AB, Quick CR, Hubbard CS, Day NE. The association between connective tissue laxity and the risk of an abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2000;20:290-5. http://dx.doi.org/10.1053/ejvs.2000.1180.
- Wong DR, Willett WC, Rimm EB. Smoking, hypertension, alcohol consumption, and risk of abdominal aortic aneurysm in men. Am J Epidemiol 2007;165:838-45. http://dx.doi.org/10.1093/aje/kwk063.
- Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med 2013;2013. http://dx.doi.org/10.1155/2013/267215.
- Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002;360:1531-9. http://dx.doi.org/10.1016/S0140-6736(02)11522-4.
- Thompson SG, Ashton HA, Gao L, Buxton MJ, Scott RA. MASSM Group . Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg 2012;99:1649-56. http://dx.doi.org/10.1002/bjs.8897.
- NHS Abdominal Aortic Aneurysm Screening Programme . 2013/14/AAA/Screening/Data n.d. http://aaa.screening.nhs.uk/2013-14datareports (accessed 22 March 2015).
- Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 2004;110:16-21. http://dx.doi.org/10.1161/01.CIR.0000133279.07468.9F.
- Sweeting MJ, Thompson SG, Brown LC, Powell JT. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg 2012;99:655-65. http://dx.doi.org/10.1002/bjs.8707.
- Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009;50:2-49. http://dx.doi.org/10.1016/j.jvs.2009.07.002.
- Ouriel K, Clair DG, Kent KC, Zarins CK. Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) investigators . Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010;51:1081-7. http://dx.doi.org/10.1016/j.jvs.2009.10.113.
- Cao P, De Rango P, Verzini F, Parlani G, Romano L, Cieri E, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg 2011;41:13-25. http://dx.doi.org/10.1016/j.ejvs.2010.08.026.
- Waton S, Johal A, Heikkila K, Cromwell D, Loftus I. National Vascular Registry: 2014 Progress Report. London: Royal College of Surgeons of England; 2015.
- Wilmink AB, Quick CR. Epidemiology and potential for prevention of abdominal aortic aneurysm. Br J Surg 1998;85:155-62. http://dx.doi.org/10.1046/j.1365-2168.1998.00714.x.
- Powell JT, Sweeting MJ, Thompson MM, Ashleigh R, Bell R, Gomes M, et al. Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.f7661.
- EVAR trial participants . Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005;365:2179-86. http://dx.doi.org/10.1016/S0140-6736(05)66627-5.
- Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004;351:1607-18. http://dx.doi.org/10.1056/NEJMoa042002.
- Lederle FA, Freischlag JA, Kyriakides TC, Matsumura JS, Padberg FT, Kohler TR, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med 2012;367:1988-97. http://dx.doi.org/10.1056/NEJMoa1207481.
- Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation 2008;117:1883-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.735274.
- Powell JT, Sweeting MJ, Brown LC, Gotensparre SM, Fowkes FG, Thompson SG. Systematic review and meta-analysis of growth rates of small abdominal aortic aneurysms. Br J Surg 2011;98:609-18. http://dx.doi.org/10.1002/bjs.7465.
- Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg 1999;30:1099-105. http://dx.doi.org/10.1016/S0741-5214(99)70049-2.
- Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014;383:1899-911. http://dx.doi.org/10.1016/S0140-6736(14)60685-1.
- Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.38272.478438.55.
- Schlösser FJ, Tangelder MJ, Verhagen HJ, van der Heijden GJ, Muhs BE, van der Graaf Y, et al. Growth predictors and prognosis of small abdominal aortic aneurysms. J Vasc Surg 2008;47:1127-33. http://dx.doi.org/10.1016/j.jvs.2008.01.041.
- Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann Surg 1999;230:289-96. http://dx.doi.org/10.1097/00000658-199909000-00002.
- Lederle FA, Johnson GR, Wilson SE, Ballard DJ, Jordan WD, Blebea J, et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA 2002;287:2968-72. http://dx.doi.org/10.1001/jama.287.22.2968.
- The UK Small Aneurysm Trial Participants . Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998;352:1649-55. http://dx.doi.org/10.1016/S0140-6736(98)10137-X.
- Thompson SG, Brown LC, Sweeting MJ, Bown MJ, Kim LG, Glover MJ, et al. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: implications for surveillance intervals and their cost-effectiveness. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17410.
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224-60. http://dx.doi.org/10.1016/S0140-6736(12)61766-8.
- Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromsø study, 1994–2001. Circulation 2009;119:2202-8. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.817619.
- Hypertension in Adults: Diagnosis and Management. London: NICE; 2011.
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med 2000;160:1425-30. http://dx.doi.org/10.1001/archinte.160.10.1425.
- Howard DP, Banerjee A, Fairhead JF, Handa A, Silver LE, Rothwell PM, et al. Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. Br J Surg 2015;102:907-15. http://dx.doi.org/10.1002/bjs.9838.
- Bown MJ, Sweeting MJ, Brown LC, Powell JT, Thompson SG. Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis. JAMA 2013;309:806-13. http://dx.doi.org/10.1001/jama.2013.950.
- Slaiby JM, Ricci MA, Gadowski GR, Hendley ED, Pilcher DB. Expansion of aortic aneurysms is reduced by propranolol in a hypertensive rat model. J Vasc Surg 1994;20:178-83. http://dx.doi.org/10.1016/0741-5214(94)90004-3.
- Moursi MM, Beebe HG, Messina LM, Welling TH, Stanley JC. Inhibition of aortic aneurysm development in blotchy mice by beta adrenergic blockade independent of altered lysyl oxidase activity. J Vasc Surg 1995;21:792-9. http://dx.doi.org/10.1016/S0741-5214(05)80010-2.
- Heart Protection Study Collaborative Group . Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg 2007;45:645-54. http://dx.doi.org/10.1016/j.jvs.2006.12.054.
- Guessous I, Periard D, Lorenzetti D, Cornuz J, Ghali WA. The efficacy of pharmacotherapy for decreasing the expansion rate of abdominal aortic aneurysms: a systematic review and meta-analysis. PLOS ONE 2008;3.
- Schouten O, van Laanen JH, Boersma E, Vidakovic R, Feringa HH, Dunkelgrün M, et al. Statins are associated with a reduced infrarenal abdominal aortic aneurysm growth. Eur J Vasc Endovasc Surg 2006;32:21-6. http://dx.doi.org/10.1016/j.ejvs.2005.12.024.
- Sukhija R, Aronow WS, Sandhu R, Kakar P, Babu S. Mortality and size of abdominal aortic aneurysm at long-term follow-up of patients not treated surgically and treated with and without statins. Am J Cardiol 2006;97:279-80. http://dx.doi.org/10.1016/j.amjcard.2005.08.033.
- Meijer CA, Stijnen T, Wasser MN, Hamming JF, van Bockel JH, Lindeman JH. Doxycycline for stabilization of abdominal aortic aneurysms: a randomized trial. Ann Intern Med 2013;159:815-23.
- Juvonen J, Juvonen T, Laurila A, Alakärppä H, Lounatmaa K, Surcel HM, et al. Demonstration of Chlamydia pneumoniae in the walls of abdominal aortic aneurysms. J Vasc Surg 1997;25:499-505. http://dx.doi.org/10.1016/S0741-5214(97)70260-X.
- Lindholt JS, Shi GP. Chronic inflammation, immune response, and infection in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2006;31:453-63. http://dx.doi.org/10.1016/j.ejvs.2005.10.030.
- Crowther M, Goodall S, Jones JL, Bell PR, Thompson MM. Localization of matrix metalloproteinase 2 within the aneurysmal and normal aortic wall. Br J Surg 2000;87:1391-400. http://dx.doi.org/10.1046/j.1365-2168.2000.01554.x.
- Mao D, Lee JK, VanVickle SJ, Thompson RW. Expression of collagenase-3 (MMP-13) in human abdominal aortic aneurysms and vascular smooth muscle cells in culture. Biochem Biophys Res Commun 1999;261:904-10. http://dx.doi.org/10.1006/bbrc.1999.1142.
- Nollendorfs A, Greiner TC, Nagase H, Baxter BT. The expression and localization of membrane type-1 matrix metalloproteinase in human abdominal aortic aneurysms. J Vasc Surg 2001;34:316-22. http://dx.doi.org/10.1067/mva.2001.115962.
- Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg 2008;47:166-72. http://dx.doi.org/10.1016/j.jvs.2007.09.016.
- Bartoli MA, Parodi FE, Chu J, Pagano MB, Mao D, Baxter BT, et al. Localized administration of doxycycline suppresses aortic dilatation in an experimental mouse model of abdominal aortic aneurysm. Ann Vasc Surg 2006;20:228-36. http://dx.doi.org/10.1007/s10016-006-9017-z.
- Kaito K, Urayama H, Watanabe G. Doxycycline treatment in a model of early abdominal aortic aneurysm. Surg Today 2003;33:426-33. http://dx.doi.org/10.1007/s10595-002-2513-0.
- Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, et al. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg 2001;34:606-10. http://dx.doi.org/10.1067/mva.2001.117891.
- Unger T. The role of the renin–angiotensin system in the development of cardiovascular disease. Am J Cardiol 2002;89:3-9A. http://dx.doi.org/10.1016/S0002-9149(01)02321-9.
- Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527-35. http://dx.doi.org/10.1016/S0140-6736(03)14739-3.
- Liao S, Miralles M, Kelley BJ, Curci JA, Borhani M, Thompson RW. Suppression of experimental abdominal aortic aneurysms in the rat by treatment with angiotensin-converting enzyme inhibitors. J Vasc Surg 2001;33:1057-64. http://dx.doi.org/10.1067/mva.2001.112810.
- Inoue N, Muramatsu M, Jin D, Takai S, Hayashi T, Katayama H, et al. Involvement of vascular angiotensin II-forming enzymes in the progression of aortic abdominal aneurysms in angiotensin II-infused ApoE-deficient mice. J Atheroscler Thromb 2009;16:164-71. http://dx.doi.org/10.5551/jat.E611.
- Xiong F, Zhao J, Zeng G, Huang B, Yuan D, Yang Y. Inhibition of AAA in a rat model by treatment with ACEI perindopril. J Surg Res 2014;189:166-73. http://dx.doi.org/10.1016/j.jss.2014.01.057.
- Kristensen KE, Torp-Pedersen C, Gislason GH, Egfjord M, Rasmussen HB, Hansen PR. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with abdominal aortic aneurysms: nation-wide cohort study. Arterioscler Thromb Vasc Biol 2015;35:733-40. http://dx.doi.org/10.1161/ATVBAHA.114.304428.
- Thompson A, Cooper JA, Fabricius M, Humphries SE, Ashton HA, Hafez H. An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. J Vasc Surg 2010;52:55-61. http://dx.doi.org/10.1016/j.jvs.2010.02.012.
- Sweeting MJ, Thompson SG, Brown LC, Greenhalgh RM, Powell JT. Use of angiotensin converting enzyme inhibitors is associated with increased growth rate of abdominal aortic aneurysms. J Vasc Surg 2010;52:1-4. http://dx.doi.org/10.1016/j.jvs.2010.02.264.
- Mehta JL, Lopez LM, Vlachakis ND, Gradman AH, Nash DT, O’Connell MT, et al. Double-blind evaluation of the dose–response relationship of amlodipine in essential hypertension. Am Heart J 1993;125:1704-10. http://dx.doi.org/10.1016/0002-8703(93)90762-X.
- Myers MG. A dose–response study of perindopril in hypertension: effects on blood pressure 6 and 24 h after dosing. Perindopril Multicentre Dose–Response Study Group. Can J Cardiol 1996;12:1191-6.
- Chrysant SG, McDonald RH, Wright JT, Barden PL, Weiss RJ. Perindopril as monotherapy in hypertension: a multicenter comparison of two dosing regimens. The Perindopril Study Group. Clin Pharmacol Ther 1993;53:479-84. http://dx.doi.org/10.1038/clpt.1993.54.
- Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129:169S-73S. http://dx.doi.org/10.1378/chest.129.1_suppl.169S.
- Palmer BF. Renal dysfunction complicating the treatment of hypertension. N Engl J Med 2002;347:1256-61. http://dx.doi.org/10.1056/NEJMra020676.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- European Commission . EudraLex – Volume 4 Good Manufacturing Practice (GMP) Guidelines 2010. http://ec.europa.eu/health/documents/eudralex/vol-4/index_en.htm (accessed 3 June 2016).
- Data Protection Act 1998. London: The Stationery Office; 1998.
- MRC Guidelines for Good Clinical Practice in Clinical Trials. London: Medical Research Council; 1998.
- The Medicines for Human Use (Clinical Trials) Regulations 2004 n.d. www.legislation.gov.uk/uksi/2004/1031/contents/made (accessed 3 June 2016).
- Grimshaw GM, Docker MF. Accurate screening for abdominal aortic aneurysm. Clin Phys Physiol Meas 1992;13:135-8. http://dx.doi.org/10.1088/0143-0815/13/2/005.
- Pleumeekers HJ, Hoes AW, Mulder PG, van der Does E, Hofman A, Laméris JS, et al. Differences in observer variability of ultrasound measurements of the proximal and distal abdominal aorta. J Med Screen 1998;5:104-8. http://dx.doi.org/10.1136/jms.5.2.104.
- Riegert-Johnson DL, Bruce CJ, Montori VM, Cook RJ, Spittell PC. Residents can be trained to detect abdominal aortic aneurysms using personal ultrasound imagers: a pilot study. J Am Soc Echocardiogr 2005;18:394-7. http://dx.doi.org/10.1016/j.echo.2004.12.019.
- Nguyen AT, Hill GB, Versteeg MP, Thomson IA, van Rij AM. Novices may be trained to screen for abdominal aortic aneurysms using ultrasound. Cardiovasc Ultrasound 2013;11. http://dx.doi.org/10.1186/1476-7120-11-42.
- Kuhn M, Bonnin RL, Davey MJ, Rowland JL, Langlois SL. Emergency department ultrasound scanning for abdominal aortic aneurysm: accessible, accurate, and advantageous. Ann Emerg Med 2000;36:219-23. http://dx.doi.org/10.1067/mem.2000.108616.
- Hoffmann B, Bessman ES, Um P, Ding R, McCarthy ML. Successful sonographic visualisation of the abdominal aorta differs significantly among a diverse group of credentialed emergency department providers. Emerg Med J 2011;28:472-6. http://dx.doi.org/10.1136/emj.2009.086462.
- Jaakkola P, Hippeläinen M, Farin P, Rytkönen H, Kainulainen S, Partanen K. Interobserver variability in measuring the dimensions of the abdominal aorta: comparison of ultrasound and computed tomography. Eur J Vasc Endovasc Surg 1996;12:230-7. http://dx.doi.org/10.1016/S1078-5884(96)80112-2.
- Ellis M, Powell JT, Greenhalgh RM. Limitations of ultrasonography in surveillance of small abdominal aortic aneurysms. Br J Surg 1991;78:614-16. http://dx.doi.org/10.1002/bjs.1800780529.
- Hartshorne TC, McCollum CN, Earnshaw JJ, Morris J, Nasim A. Ultrasound measurement of aortic diameter in a national screening programme. Eur J Vasc Endovasc Surg 2011;42:195-9. http://dx.doi.org/10.1016/j.ejvs.2011.02.030.
- Meecham L, Rajagopalan S, Fairhead J, Pherwani AD. Re. ‘Ultrasound measurement for abdominal aortic aneurysm screening: a direct comparison of the three leading methods’. Eur J Vasc Endovasc Surg 2014;48:231-2. http://dx.doi.org/10.1016/j.ejvs.2014.04.034.
- Thapar A, Cheal D, Hopkins T, Ward S, Shalhoub J, Shaloub J, et al. Internal or external wall diameter for abdominal aortic aneurysm screening?. Ann R Coll Surg Engl 2010;92:503-5. http://dx.doi.org/10.1308/003588410X12699663903430.
- Health and Social Care Information Centre . Health Survey for England 2013. https://data.gov.uk/dataset/health_survey_for_england (accessed 1 May 2016).
- Bicknell CD, Kiru G, Falaschetti E, Powell JT, Poulter NR. on behalf of the AARDVARK collaborators . An evaluation of the effect of an angiotensin converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomized placebo-controlled trial (AARDVARK) [published online ahead of print 1 July 2016]. Eur J Heart 2016. http://eurheartj.oxfordjournals.org/content/early/2016/07/01/eurheartj.ehw257 (accessed 7 July 2016).
- Lu H, Rateri DL, Cassis LA, Daugherty A. The role of the renin–angiotensin system in aortic aneurysmal diseases. Curr Hypertens Rep 2008;10:99-106. http://dx.doi.org/10.1007/s11906-008-0020-3.
- Alsac JM, Journe C, Louedec L, Dai J, Julia P, Fabiani JN, et al. Downregulation of remodelling enzymatic activity induced by an angiotensin-converting enzyme inhibitor (perindopril) reduces the degeneration of experimental abdominal aortic aneurysms in a rat model. Eur J Vasc Endovasc Surg 2011;41:474-80. http://dx.doi.org/10.1016/j.ejvs.2010.12.007.
- Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2003;23:1621-6. http://dx.doi.org/10.1161/01.ATV.0000085631.76095.64.
- Morris DR, Cunningham MA, Ahimastos AA, Kingwell BA, Pappas E, Bourke M, et al. TElmisartan in the management of abDominal aortic aneurYsm (TEDY): the study protocol for a randomized controlled trial. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-0793-z.
- Sillesen H, Eldrup N, Hultgren R, Lindeman J, Bredahl K, Thompson M, et al. Randomized clinical trial of mast cell inhibition in patients with a medium-sized abdominal aortic aneurysm. Br J Surg 2015;102:894-901. http://dx.doi.org/10.1002/bjs.9824.
- Bredahl K, Eldrup N, Meyer C, Eiberg JE, Sillesen H. Reproducibility of ECG-gated ultrasound diameter assessment of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2013;45:235-40. http://dx.doi.org/10.1016/j.ejvs.2012.12.010.
Appendix 1 Patient information sheet and informed consent form
Appendix 2 The European Quality of Life-5 Dimensions questionnaire
Appendix 3 Health resource use questionnaire
Appendix 4 Data collection forms
Appendix 5 Transverse abdominal aortic aneurysm diameter tables
| Visit | Transverse internal diameter (cm) | Transverse external diameter (cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Minimum | Maximum | n | Mean | SD | Minimum | Maximum | |
| Placebo | ||||||||||
| Baseline | 78 | 3.65 | 0.69 | 2.29 | 5.28 | 79 | 4.05 | 0.68 | 3.00 | 5.64 |
| Month 3 | 74 | 3.74 | 0.72 | 2.52 | 5.40 | 74 | 4.12 | 0.72 | 3.03 | 6.07 |
| Month 6 | 68 | 3.80 | 0.66 | 2.63 | 5.50 | 67 | 4.17 | 0.64 | 3.07 | 5.80 |
| Month 9 | 62 | 3.77 | 0.68 | 2.59 | 5.30 | 62 | 4.12 | 0.67 | 3.08 | 5.60 |
| Month 12 | 69 | 3.78 | 0.64 | 2.66 | 5.46 | 68 | 4.15 | 0.65 | 3.00 | 5.55 |
| Month 15 | 57 | 3.80 | 0.68 | 2.47 | 5.41 | 57 | 4.14 | 0.68 | 3.06 | 5.90 |
| Month 18 | 60 | 3.84 | 0.64 | 2.56 | 5.28 | 60 | 4.19 | 0.66 | 3.07 | 5.72 |
| Month 21 | 52 | 3.85 | 0.63 | 2.75 | 5.28 | 52 | 4.18 | 0.67 | 3.03 | 5.95 |
| Month 24 | 56 | 3.80 | 0.63 | 2.52 | 5.38 | 56 | 4.13 | 0.62 | 3.07 | 5.57 |
| Perindopril | ||||||||||
| Baseline | 71 | 3.68 | 0.68 | 2.39 | 5.17 | 73 | 4.09 | 0.65 | 3.08 | 5.39 |
| Month 3 | 65 | 3.67 | 0.66 | 2.20 | 4.99 | 65 | 4.09 | 0.66 | 3.05 | 5.41 |
| Month 6 | 61 | 3.70 | 0.70 | 2.43 | 5.23 | 61 | 4.12 | 0.73 | 3.05 | 5.90 |
| Month 9 | 56 | 3.72 | 0.69 | 2.29 | 5.29 | 56 | 4.10 | 0.67 | 3.07 | 5.64 |
| Month 12 | 60 | 3.80 | 0.70 | 2.50 | 5.47 | 60 | 4.14 | 0.72 | 3.03 | 6.07 |
| Month 15 | 47 | 3.73 | 0.69 | 2.46 | 5.42 | 47 | 4.09 | 0.67 | 3.06 | 5.74 |
| Month 18 | 49 | 3.77 | 0.61 | 2.59 | 5.50 | 49 | 4.10 | 0.64 | 3.03 | 5.83 |
| Month 21 | 44 | 3.78 | 0.65 | 2.42 | 5.73 | 43 | 4.09 | 0.70 | 3.09 | 6.03 |
| Month 24 | 52 | 3.73 | 0.58 | 2.42 | 5.13 | 52 | 4.04 | 0.58 | 3.03 | 5.42 |
| Amlodipine | ||||||||||
| Baseline | 69 | 3.61 | 0.70 | 2.20 | 4.92 | 72 | 4.04 | 0.67 | 3.00 | 5.35 |
| Month 3 | 65 | 3.71 | 0.68 | 2.18 | 5.12 | 65 | 4.08 | 0.66 | 3.02 | 5.52 |
| Month 6 | 60 | 3.79 | 0.75 | 2.14 | 5.30 | 60 | 4.13 | 0.72 | 3.00 | 5.60 |
| Month 9 | 54 | 3.87 | 0.72 | 2.08 | 5.26 | 54 | 4.22 | 0.66 | 3.05 | 5.66 |
| Month 12 | 52 | 3.85 | 0.70 | 2.39 | 5.21 | 52 | 4.20 | 0.69 | 3.01 | 5.76 |
| Month 15 | 44 | 3.85 | 0.69 | 2.33 | 5.22 | 44 | 4.20 | 0.66 | 3.04 | 5.63 |
| Month 18 | 47 | 3.90 | 0.71 | 2.78 | 5.50 | 47 | 4.18 | 0.70 | 3.03 | 5.88 |
| Month 21 | 47 | 3.89 | 0.76 | 2.58 | 5.46 | 47 | 4.22 | 0.70 | 3.02 | 5.80 |
| Month 24 | 48 | 3.85 | 0.72 | 2.59 | 5.45 | 47 | 4.18 | 0.67 | 3.03 | 5.67 |
| Total | ||||||||||
| Baseline | 218 | 3.65 | 0.69 | 2.20 | 5.28 | 224 | 4.06 | 0.67 | 3.00 | 5.64 |
| Month 3 | 204 | 3.71 | 0.69 | 2.18 | 5.40 | 204 | 4.10 | 0.68 | 3.02 | 6.07 |
| Month 6 | 189 | 3.77 | 0.70 | 2.14 | 5.50 | 188 | 4.14 | 0.69 | 3.00 | 5.90 |
| Month 9 | 172 | 3.78 | 0.69 | 2.08 | 5.30 | 172 | 4.14 | 0.67 | 3.05 | 5.66 |
| Month 12 | 181 | 3.81 | 0.67 | 2.39 | 5.47 | 180 | 4.16 | 0.68 | 3.00 | 6.07 |
| Month 15 | 148 | 3.80 | 0.68 | 2.33 | 5.42 | 148 | 4.14 | 0.67 | 3.04 | 5.90 |
| Month 18 | 156 | 3.84 | 0.65 | 2.56 | 5.50 | 156 | 4.16 | 0.66 | 3.03 | 5.88 |
| Month 21 | 143 | 3.84 | 0.68 | 2.42 | 5.73 | 142 | 4.16 | 0.69 | 3.02 | 6.03 |
| Month 24 | 156 | 3.79 | 0.64 | 2.42 | 5.45 | 155 | 4.12 | 0.62 | 3.03 | 5.67 |
| Study period | n | Transverse internal diameter (cm) | n | Transverse external diameter (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | Mean | SD | Minimum | Maximum | |||
| Placebo | ||||||||||
| Month 3 – baseline | 73 | 0.12 | 0.31 | –0.5 | 1.48 | 74 | 0.09 | 0.24 | –0.64 | 0.83 |
| Month 6 – baseline | 67 | 0.19 | 0.29 | –0.32 | 1.44 | 67 | 0.12 | 0.27 | –0.47 | 0.89 |
| Month 12 – baseline | 68 | 0.24 | 0.34 | –0.67 | 1.49 | 68 | 0.18 | 0.29 | –0.51 | 0.91 |
| Month 18 – baseline | 59 | 0.33 | 0.38 | –0.41 | 1.67 | 60 | 0.26 | 0.27 | –0.28 | 1.36 |
| Month 24 – baseline | 55 | 0.37 | 0.37 | –0.28 | 1.62 | 56 | 0.30 | 0.31 | –0.34 | 1.12 |
| Perindopril | ||||||||||
| Month 3 – baseline | 64 | 0.02 | 0.28 | –0.68 | 0.70 | 65 | 0.06 | 0.22 | –0.69 | 0.78 |
| Month 6 – baseline | 60 | 0.07 | 0.21 | –0.37 | 0.77 | 61 | 0.10 | 0.22 | –0.47 | 0.73 |
| Month 12 – baseline | 59 | 0.24 | 0.28 | –0.29 | 1.27 | 60 | 0.19 | 0.27 | –0.52 | 1.14 |
| Month 18 – baseline | 48 | 0.26 | 0.22 | –0.13 | 0.83 | 49 | 0.21 | 0.27 | –0.53 | 1.15 |
| Month 24 – baseline | 51 | 0.28 | 0.22 | –0.15 | 0.89 | 52 | 0.21 | 0.26 | –0.53 | 0.76 |
| Amlodipine | ||||||||||
| Month 3 – baseline | 62 | 0.08 | 0.29 | –0.68 | 1.19 | 65 | 0.04 | 0.21 | –0.57 | 0.74 |
| Month 6 – baseline | 57 | 0.19 | 0.43 | –0.65 | 2.38 | 60 | 0.10 | 0.26 | –0.75 | 0.76 |
| Month 12 – baseline | 49 | 0.31 | 0.49 | –0.65 | 2.68 | 52 | 0.23 | 0.27 | –0.44 | 0.86 |
| Month 18 – baseline | 44 | 0.37 | 0.37 | –0.3 | 1.57 | 47 | 0.27 | 0.28 | –0.25 | 0.85 |
| Month 24 – baseline | 45 | 0.37 | 0.42 | –0.66 | 1.42 | 47 | 0.32 | 0.33 | –0.63 | 0.95 |
| Total | ||||||||||
| Month 3 – baseline | 199 | 0.07 | 0.30 | –0.68 | 1.48 | 204 | 0.06 | 0.23 | –0.69 | 0.83 |
| Month 6 – baseline | 184 | 0.15 | 0.32 | –0.65 | 2.38 | 188 | 0.11 | 0.25 | –0.75 | 0.89 |
| Month 12 – baseline | 176 | 0.26 | 0.37 | –0.67 | 2.68 | 180 | 0.20 | 0.28 | –0.52 | 1.14 |
| Month 18 – baseline | 151 | 0.32 | 0.34 | –0.41 | 1.67 | 156 | 0.25 | 0.27 | –0.53 | 1.36 |
| Month 24 – baseline | 151 | 0.34 | 0.34 | –0.66 | 1.62 | 155 | 0.27 | 0.30 | –0.63 | 1.12 |
Appendix 6 Histograms of change in abdominal aortic aneurysm longitudinal external measurements (6 and 18 months)
FIGURE 40.
Histograms of change in AAA longitudinal external diameter from baseline to month 6. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
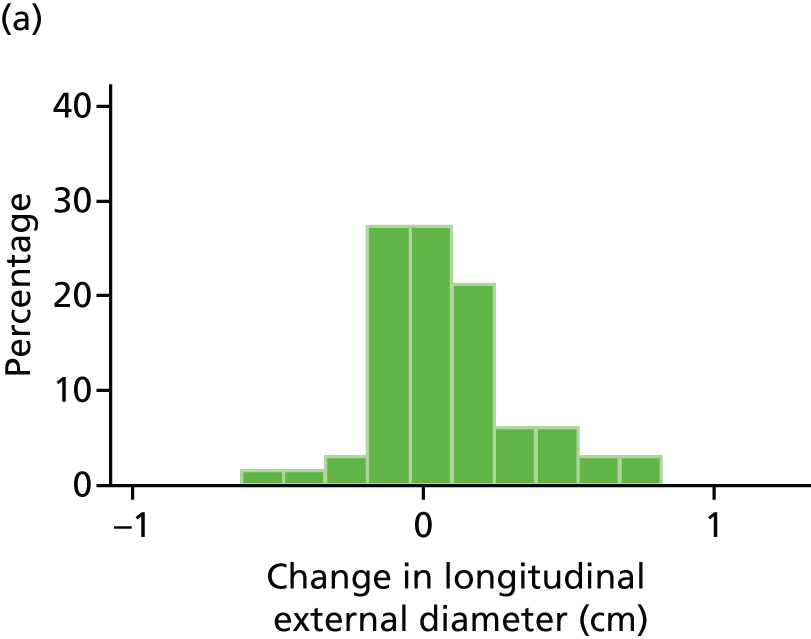
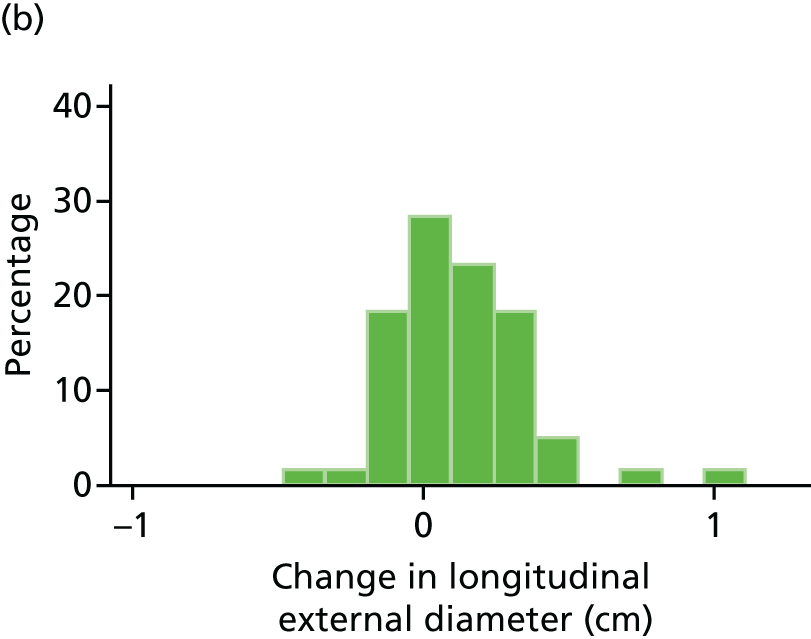
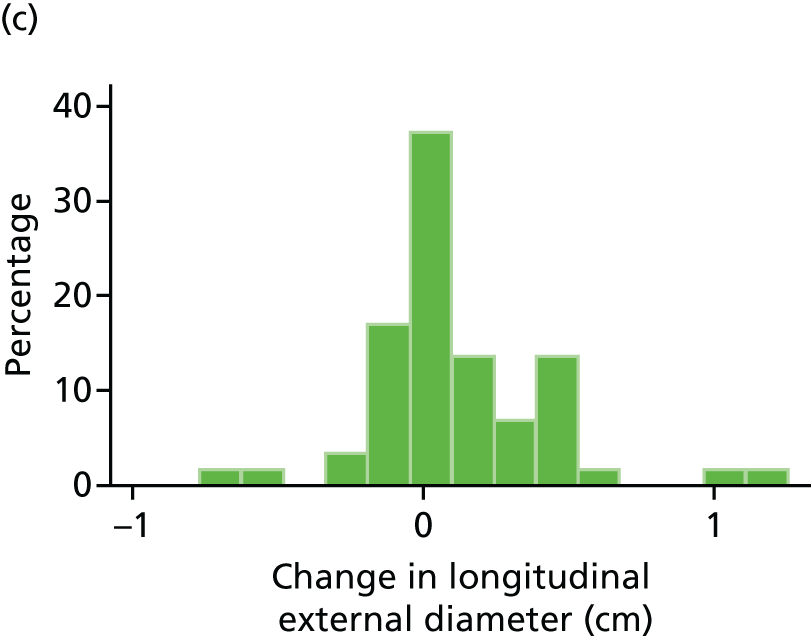

FIGURE 41.
Histograms of change in AAA longitudinal external diameter from baseline to month 18. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.

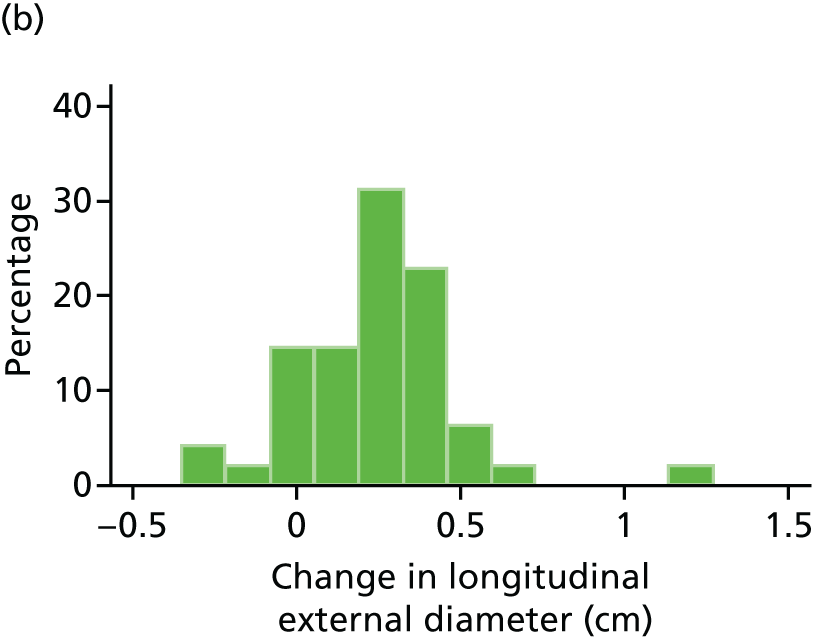


Appendix 7 Box plots of change in abdominal aortic aneurysm longitudinal external measurements (6 and 18 months)
FIGURE 42.
Box plot of change in AAA longitudinal external diameter from baseline to month 6. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
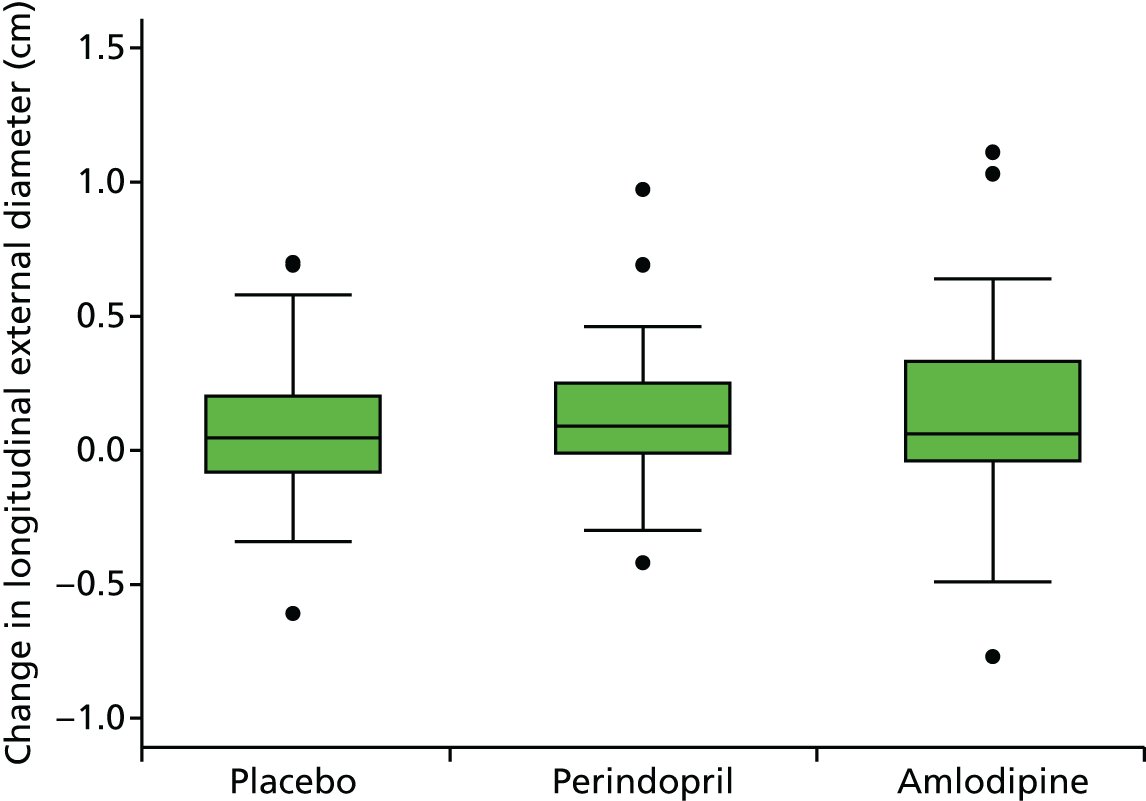
FIGURE 43.
Box plot of change in AAA longitudinal external diameter from baseline to month 18. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

Appendix 8 Histograms of change in abdominal aortic aneurysm longitudinal internal measurements
FIGURE 44.
Histograms of change in AAA longitudinal internal diameter from baseline to month 3. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
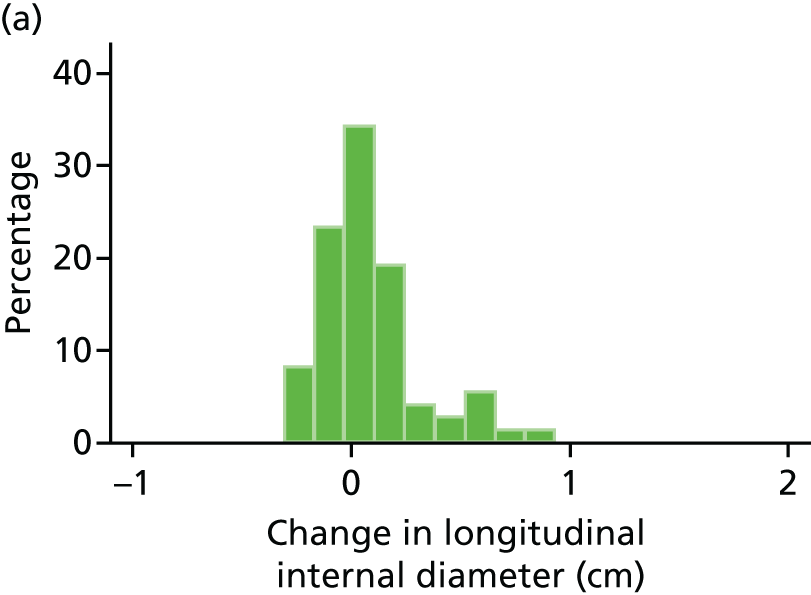



FIGURE 45.
Histograms of change in AAA longitudinal internal diameter from baseline to month 6. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
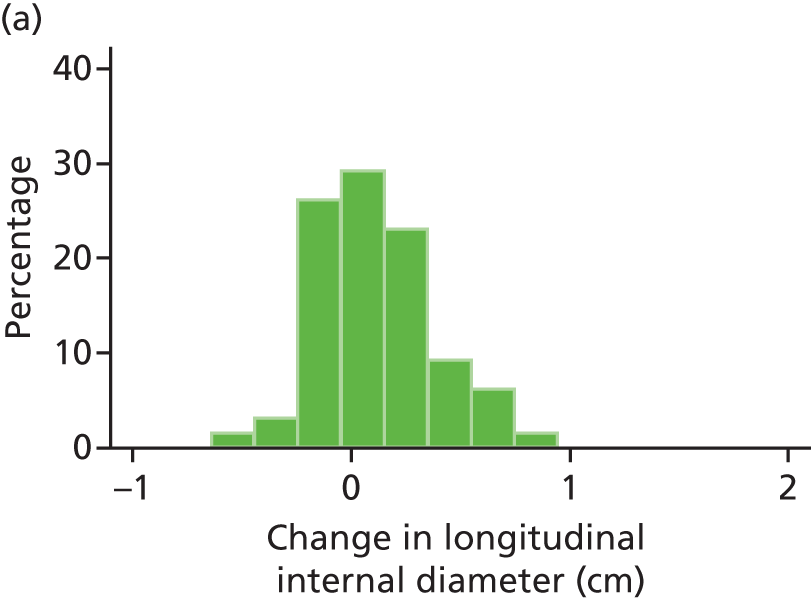
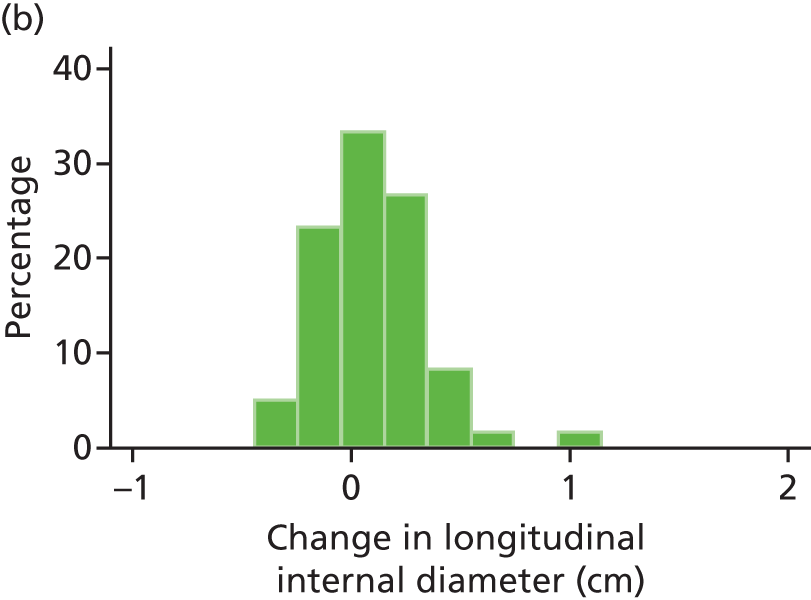
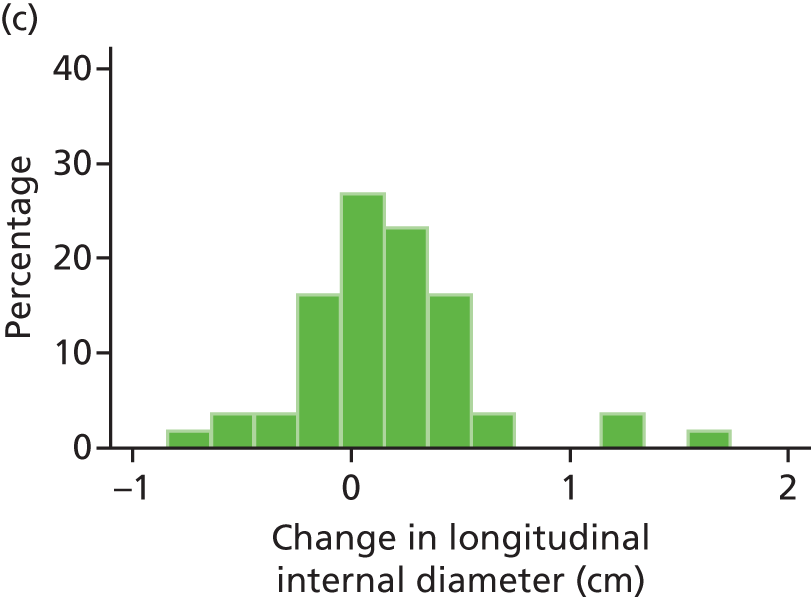
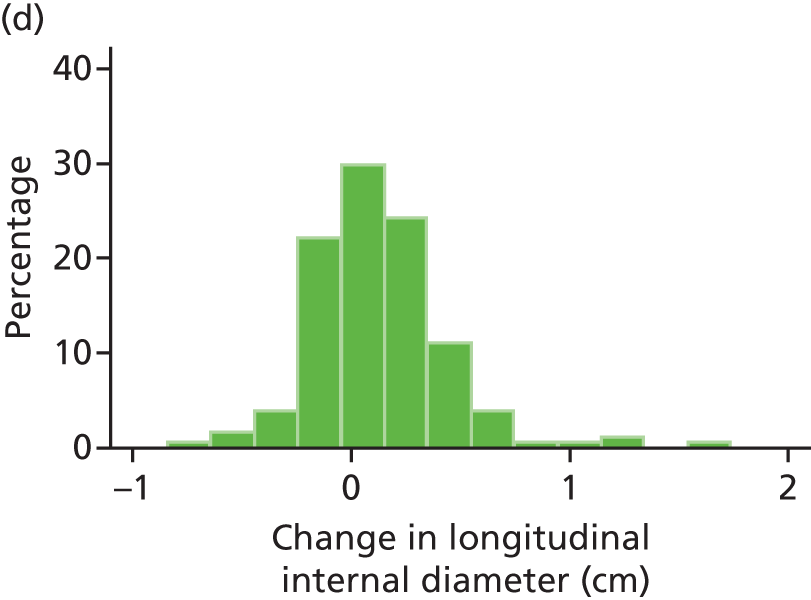
FIGURE 46.
Histograms of change in AAA longitudinal internal diameter from baseline to month 12. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.

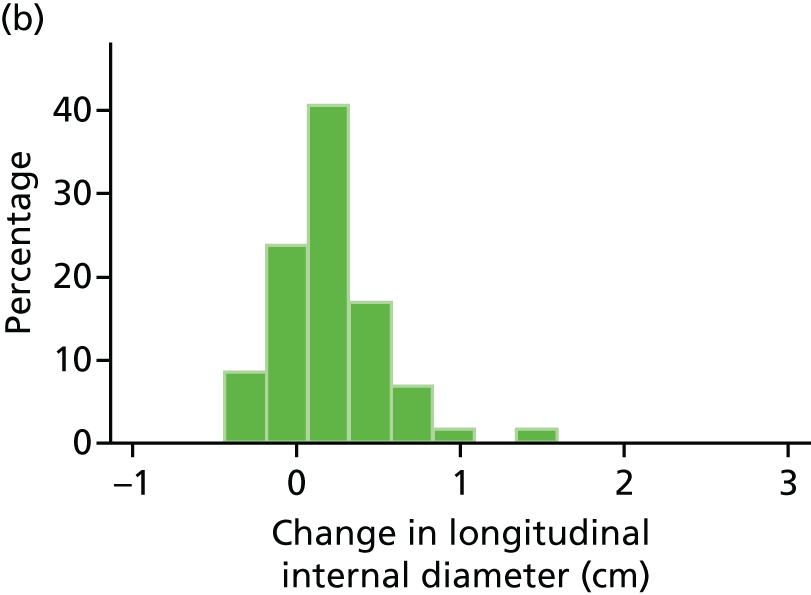

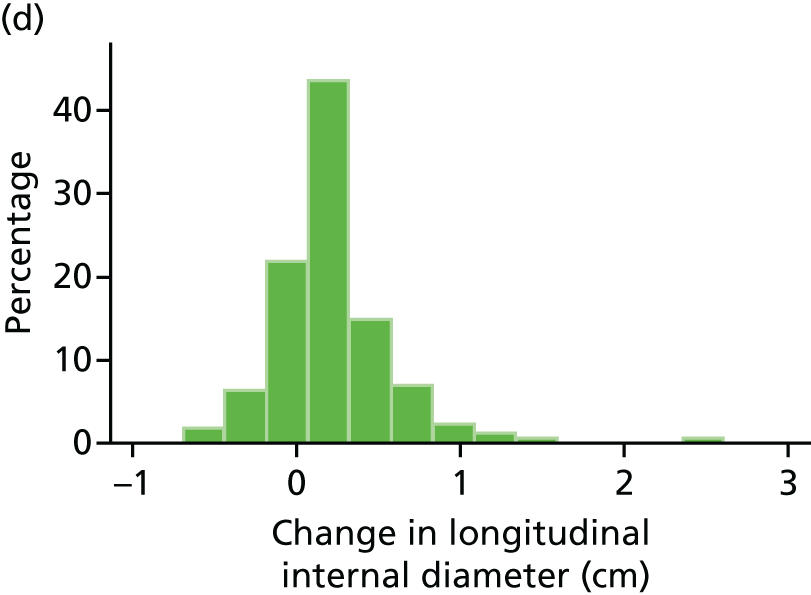
FIGURE 47.
Histograms of change in AAA longitudinal internal diameter from baseline to month 18. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.

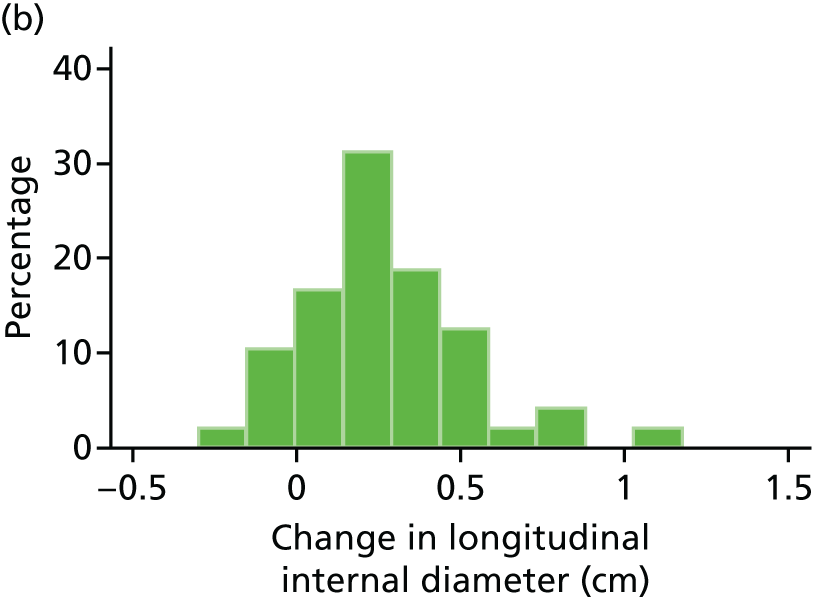
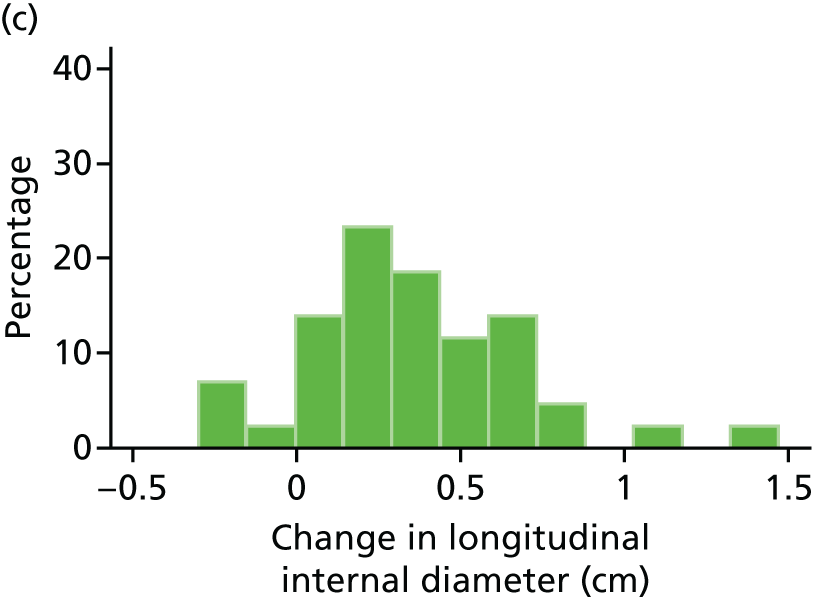
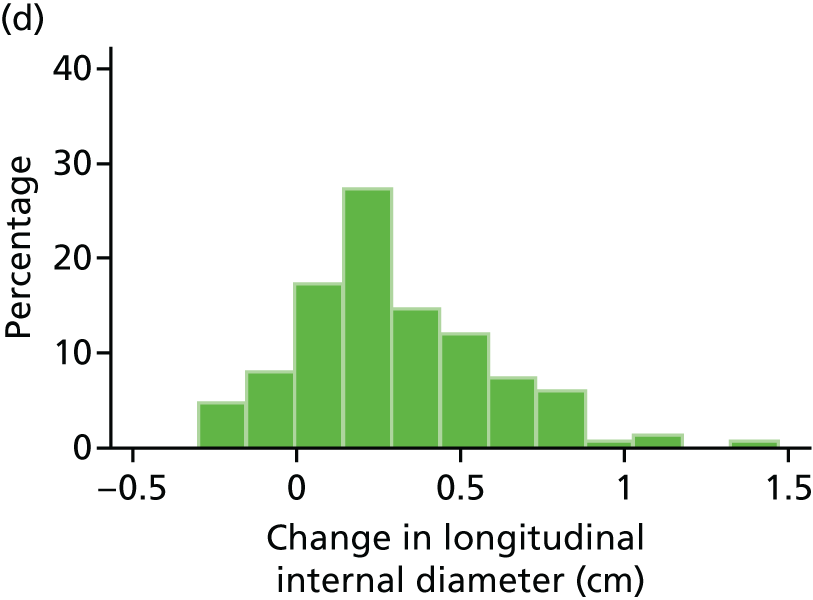
FIGURE 48.
Histograms of change in AAA longitudinal internal diameter from baseline to month 24. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
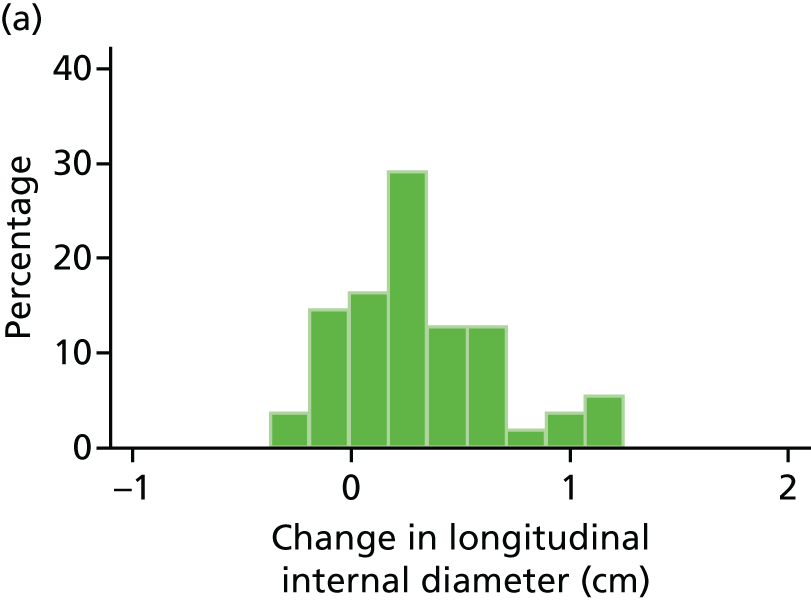
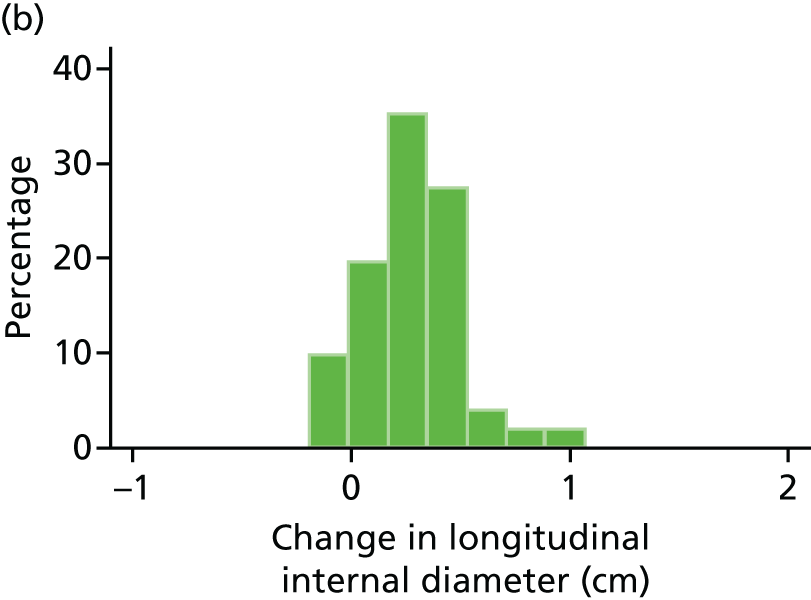


Appendix 9 Box plots of change in abdominal aortic aneurysm longitudinal internal measurements
FIGURE 49.
Box plot of change in AAA longitudinal internal diameter from baseline to month 3. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
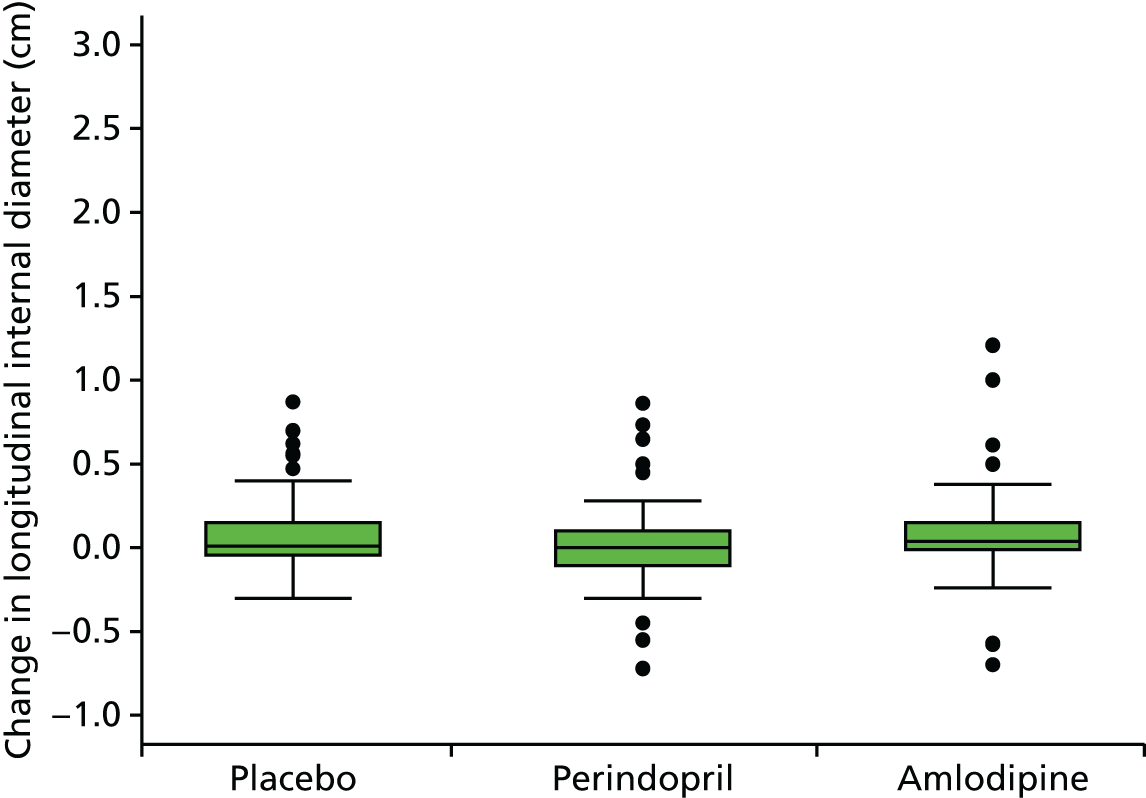
FIGURE 50.
Box plot of change in AAA longitudinal internal diameter from baseline to month 6. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
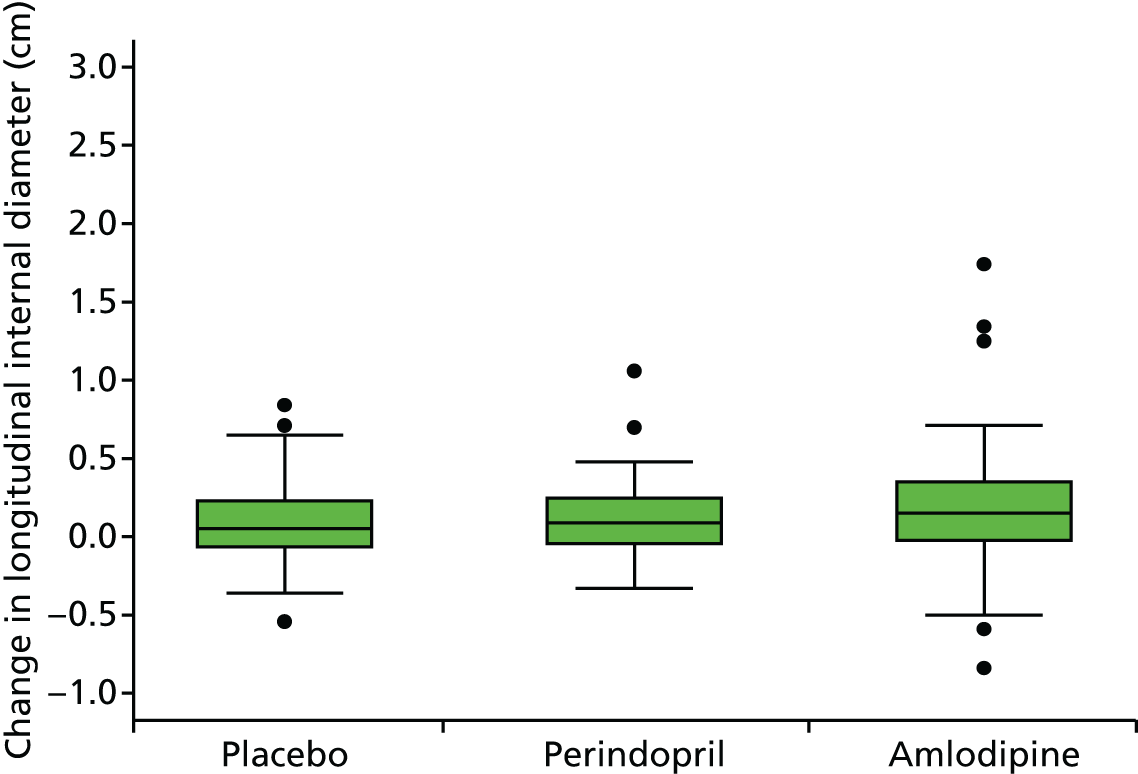
FIGURE 51.
Box plot of change in AAA longitudinal internal diameter from baseline to month 12. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

FIGURE 52.
Box plot of change in AAA longitudinal internal diameter from baseline to month 18. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
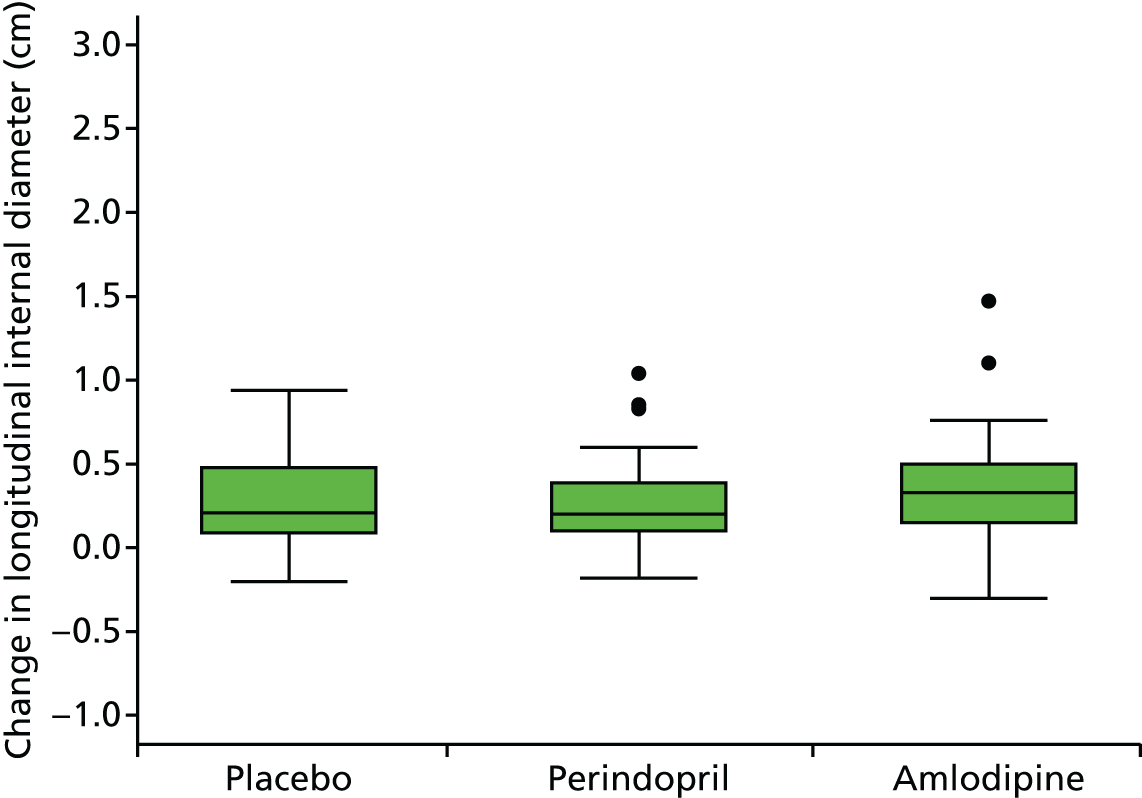
FIGURE 53.
Box plot of change in AAA longitudinal internal diameter from baseline to month 24. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

Appendix 10 Histograms of change in abdominal aortic aneurysm transverse external measurements
FIGURE 54.
Histograms of change in AAA transverse external diameter from baseline to month 3. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
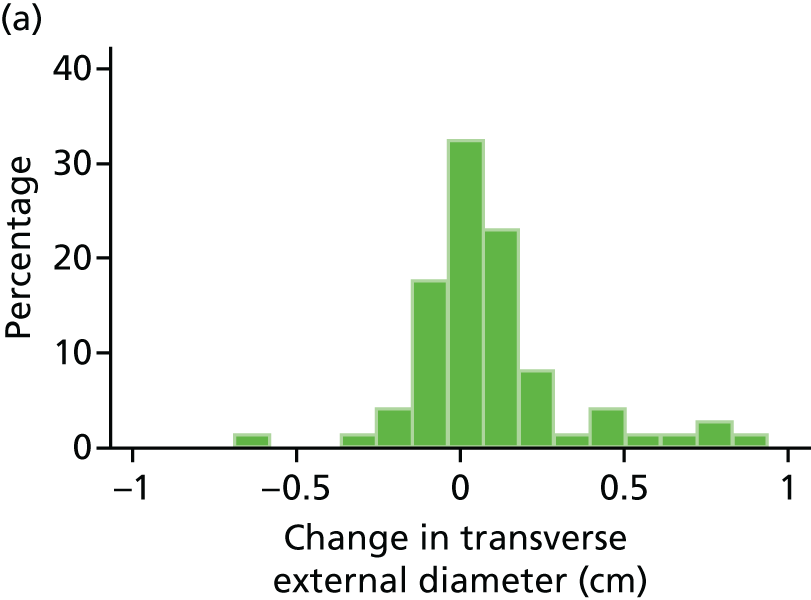
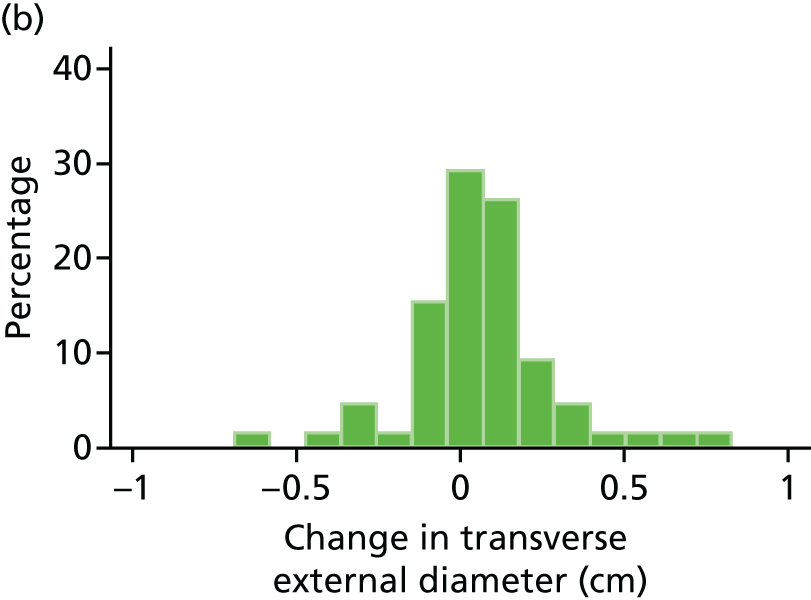

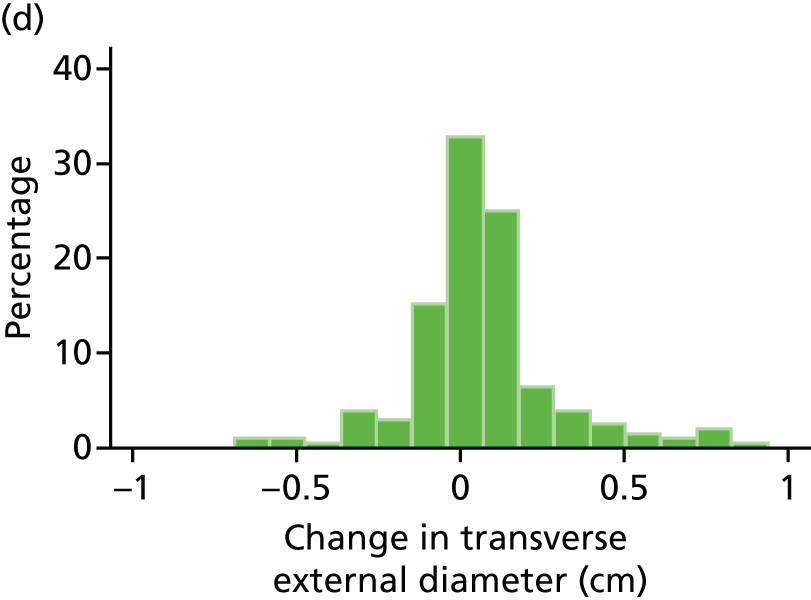
FIGURE 55.
Histograms of change in AAA transverse external diameter from baseline to month 6. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
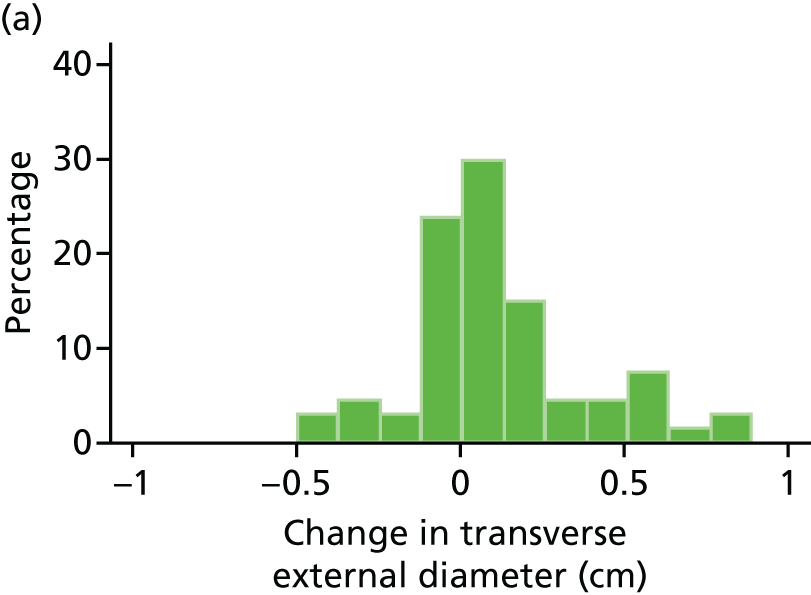


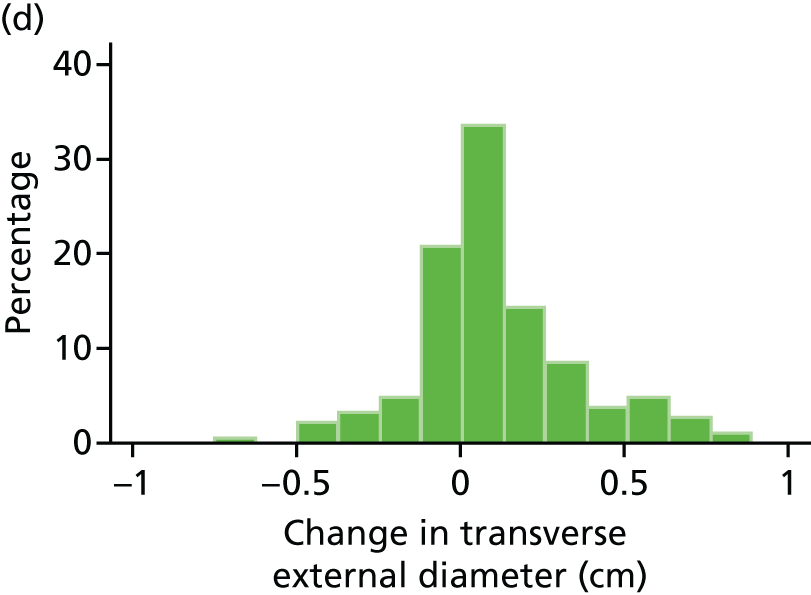
FIGURE 56.
Histograms of change in AAA transverse external diameter from baseline to month 12. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.

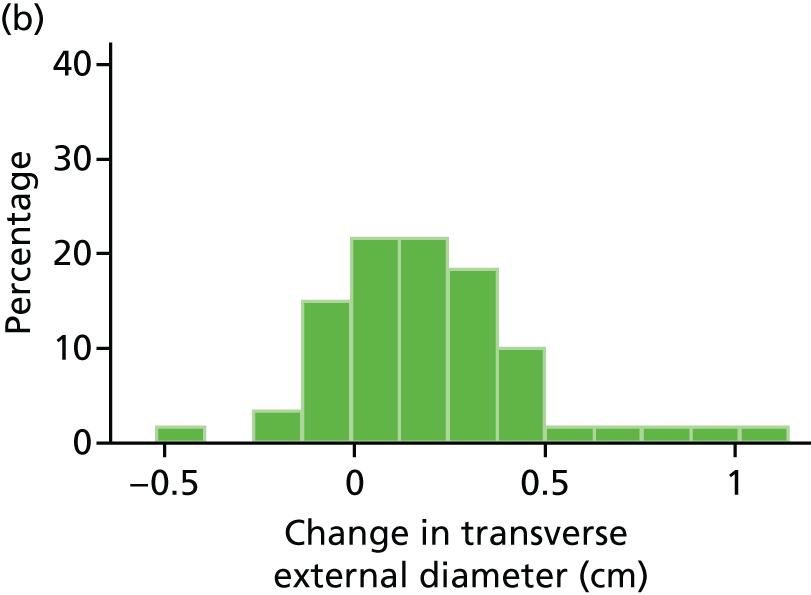

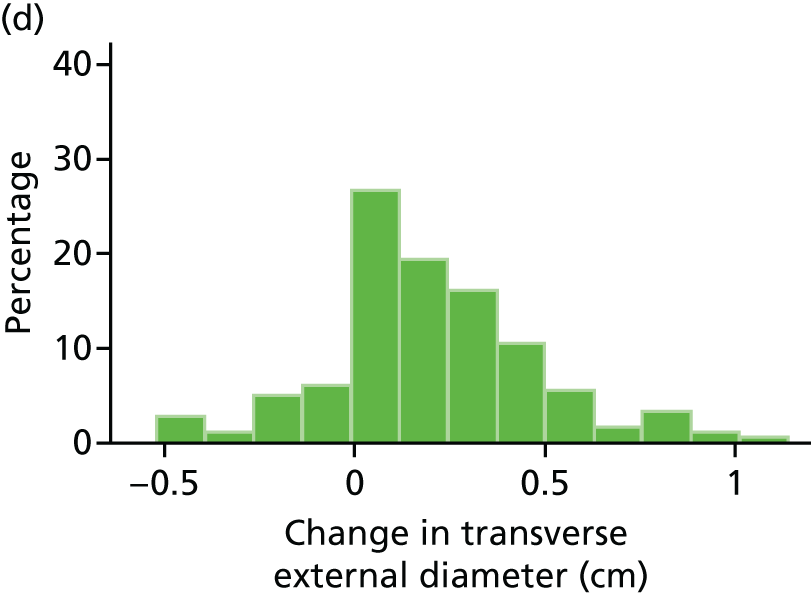
FIGURE 57.
Histograms of change in AAA transverse external diameter from baseline to month 18. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.




FIGURE 58.
Histograms of change in AAA transverse external diameter from baseline to month 24. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.



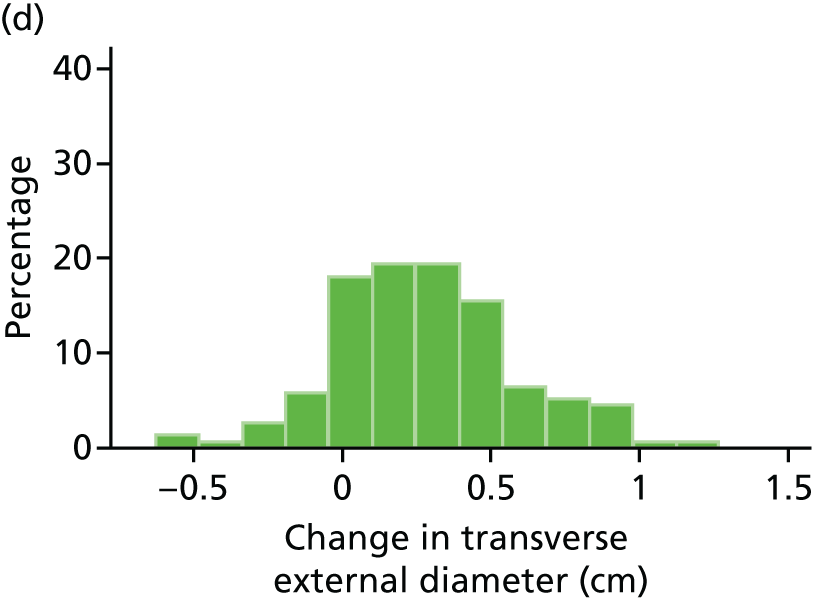
Appendix 11 Box plots of change in abdominal aortic aneurysm transverse external measurements
FIGURE 59.
Box plot of change in AAA transverse external diameter from baseline to month 3. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
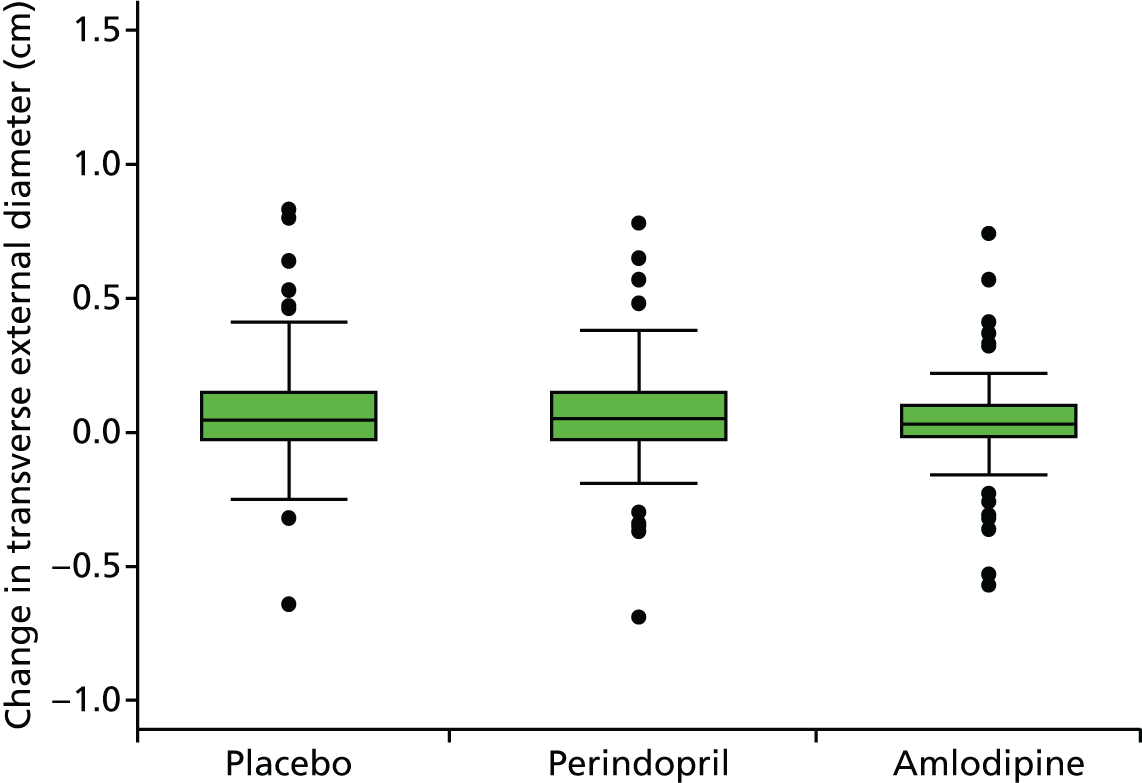
FIGURE 60.
Box plot of change in AAA transverse external diameter from baseline to month 6. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
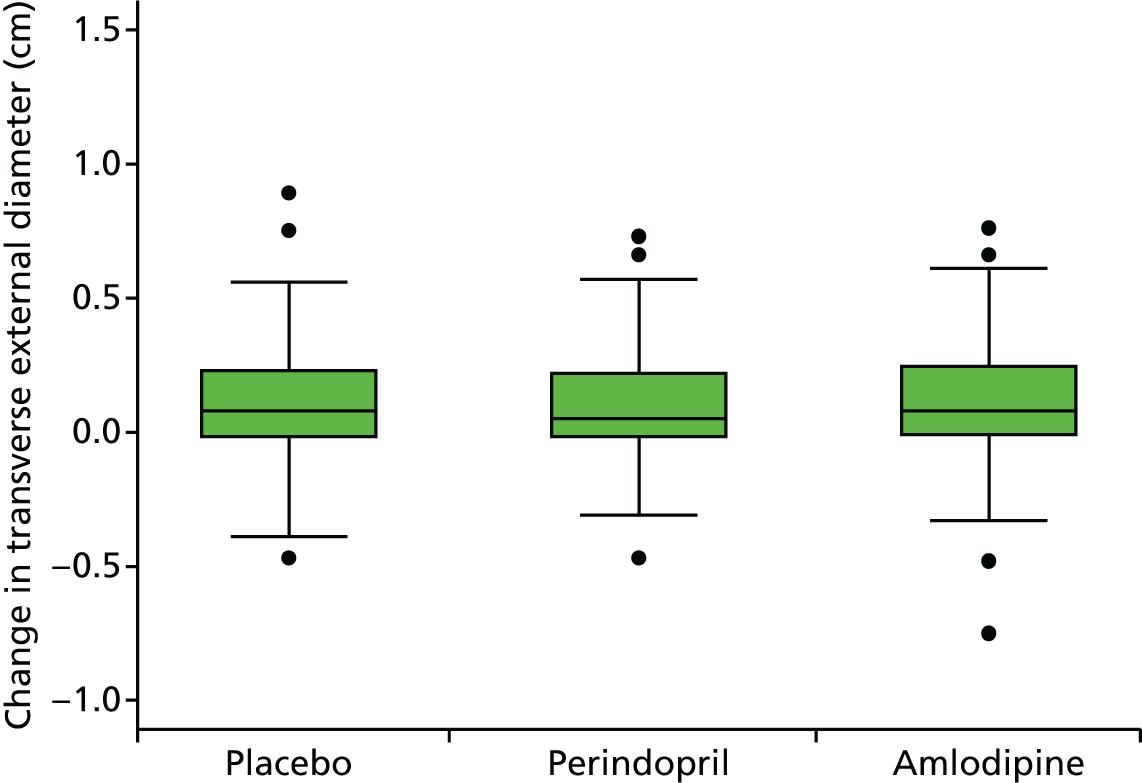
FIGURE 61.
Box plot of change in AAA transverse external diameter from baseline to month 12. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
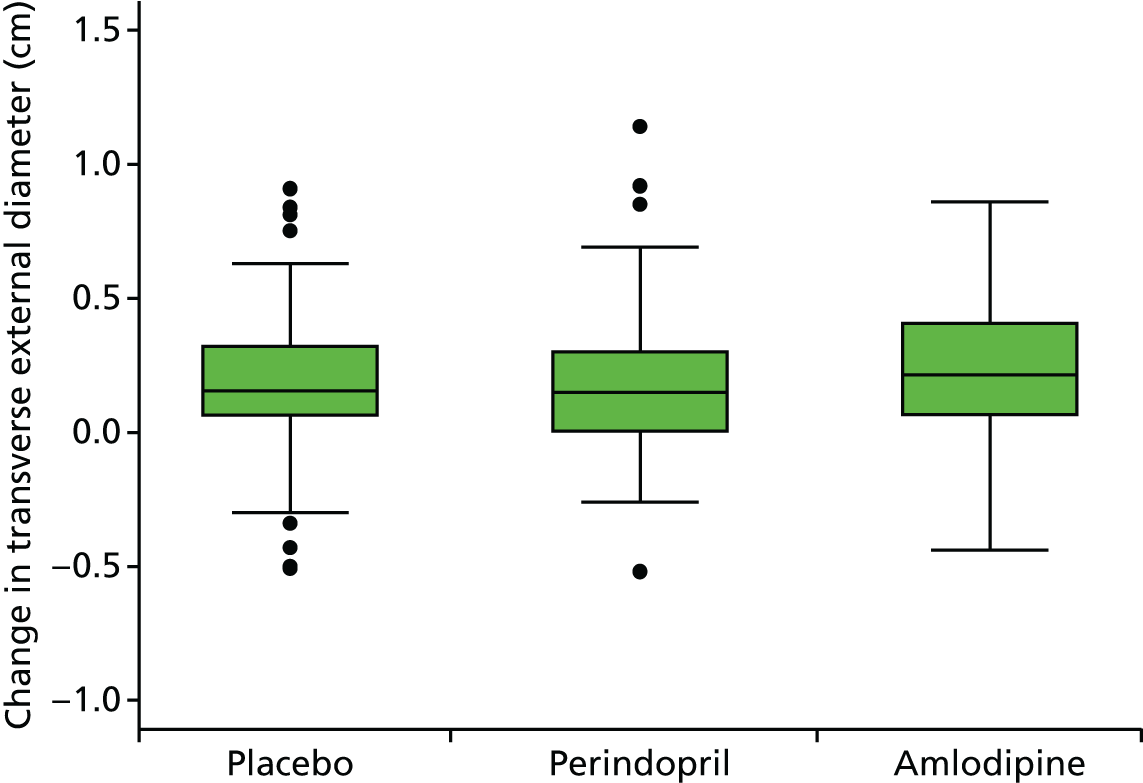
FIGURE 62.
Box plot of change in AAA transverse external diameter from baseline to month 18. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
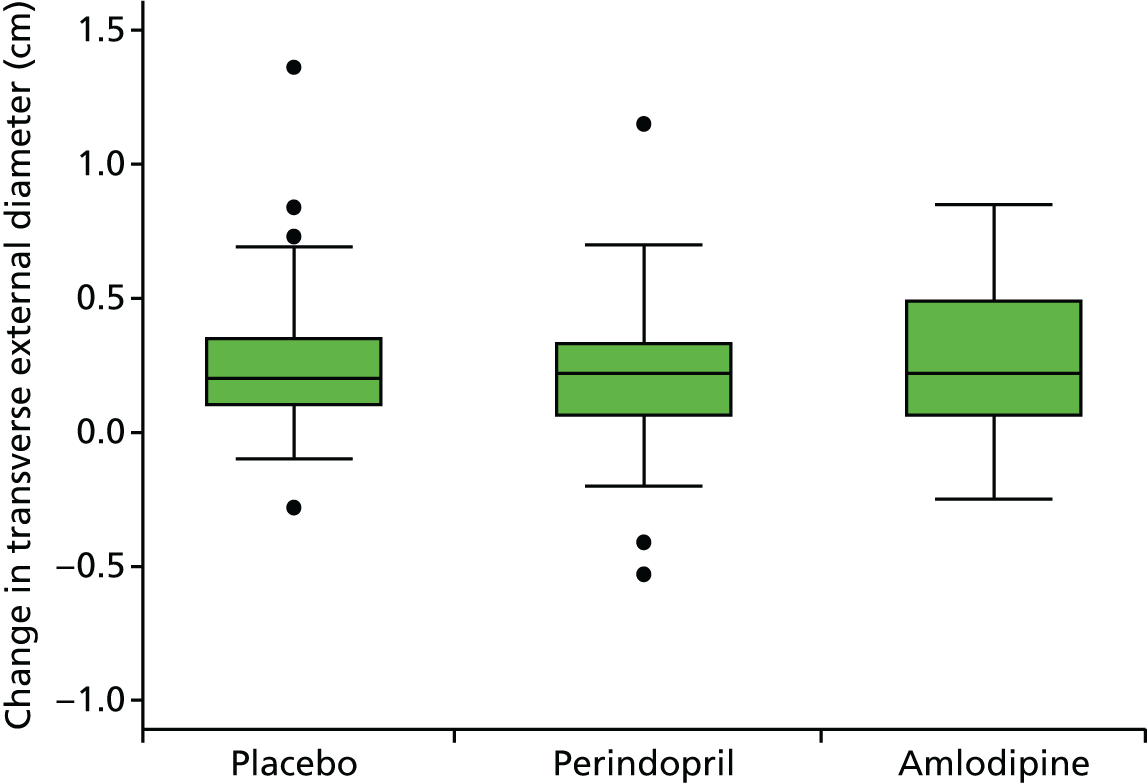
FIGURE 63.
Box plot of change in AAA transverse external diameter from baseline to month 24. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
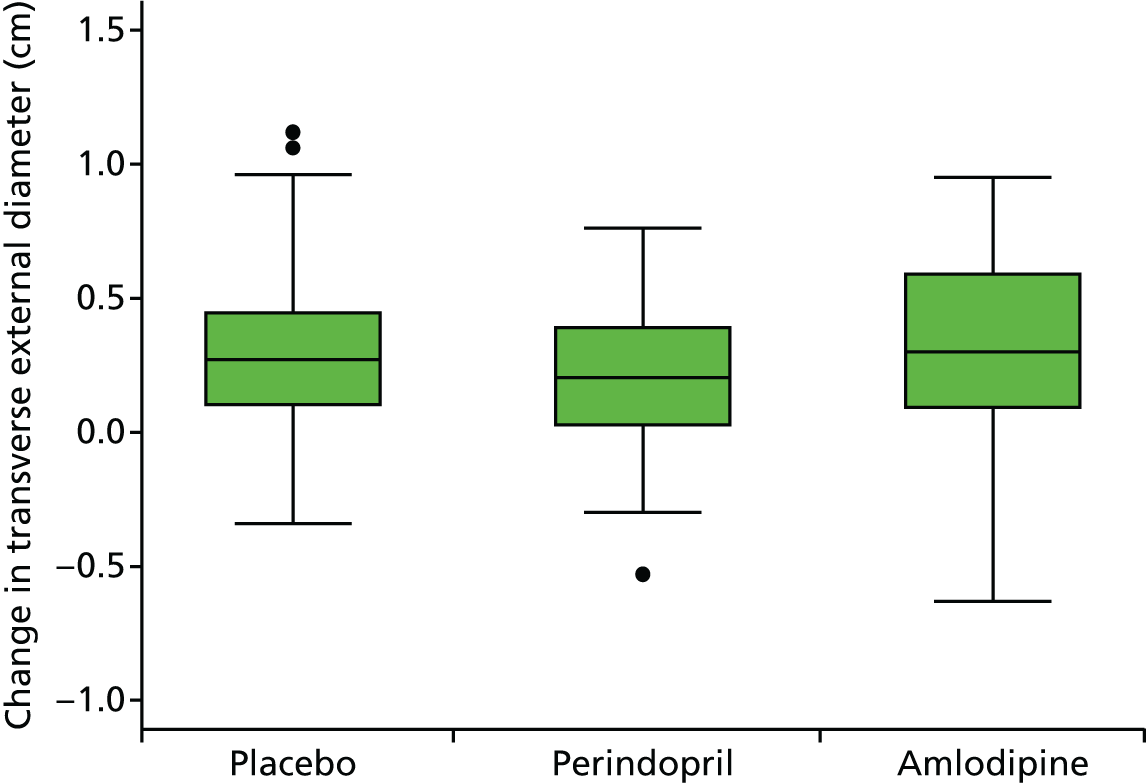
Appendix 12 Histograms of change in abdominal aortic aneurysm transverse internal measurements
FIGURE 64.
Histograms of change in AAA transverse internal diameter from baseline to month 3. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
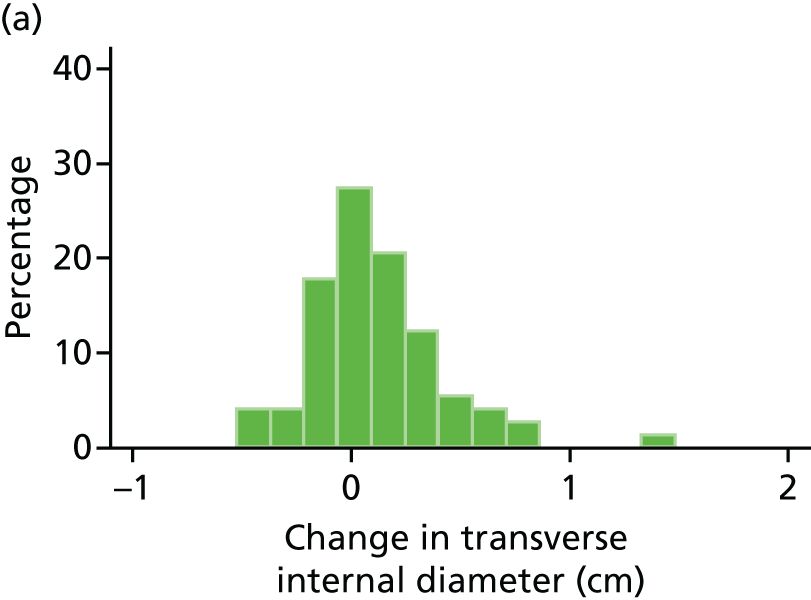
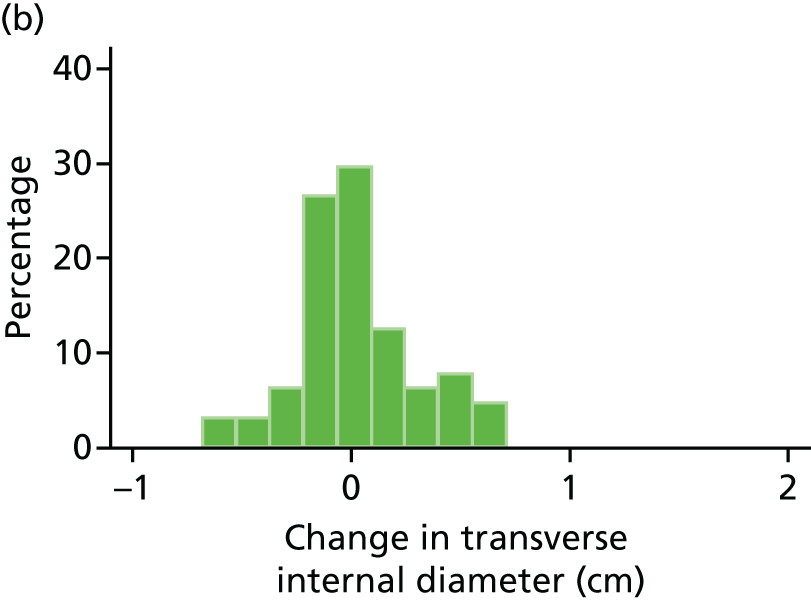
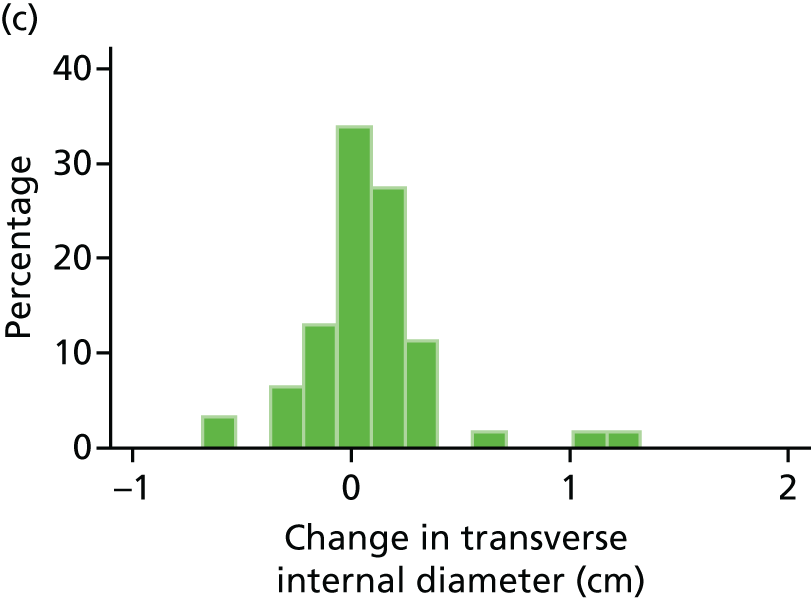

FIGURE 65.
Histograms of change in AAA transverse internal diameter from baseline to month 6. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.


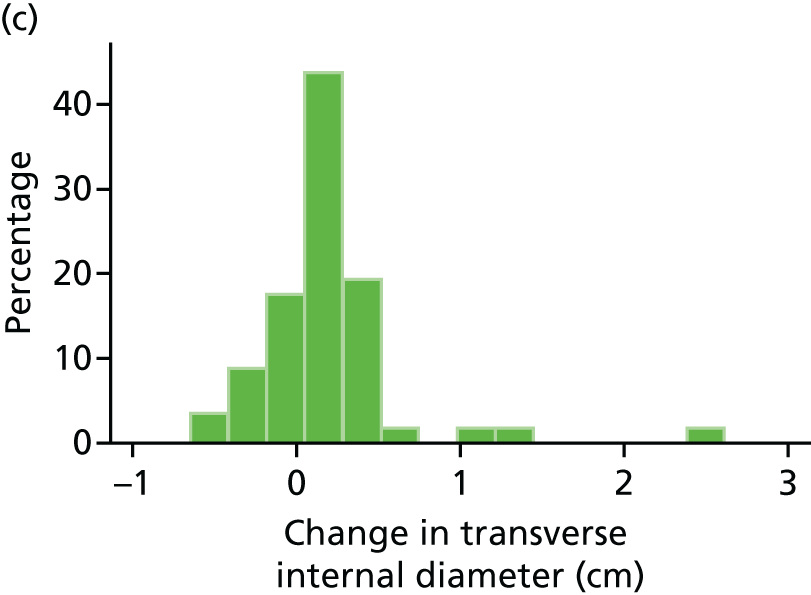
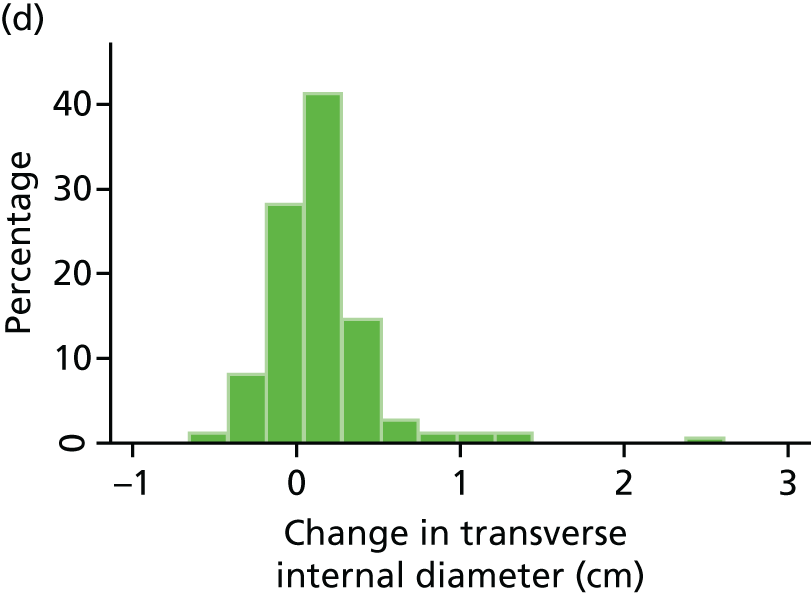
FIGURE 66.
Histograms of change in AAA transverse internal diameter from baseline to month 12. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.


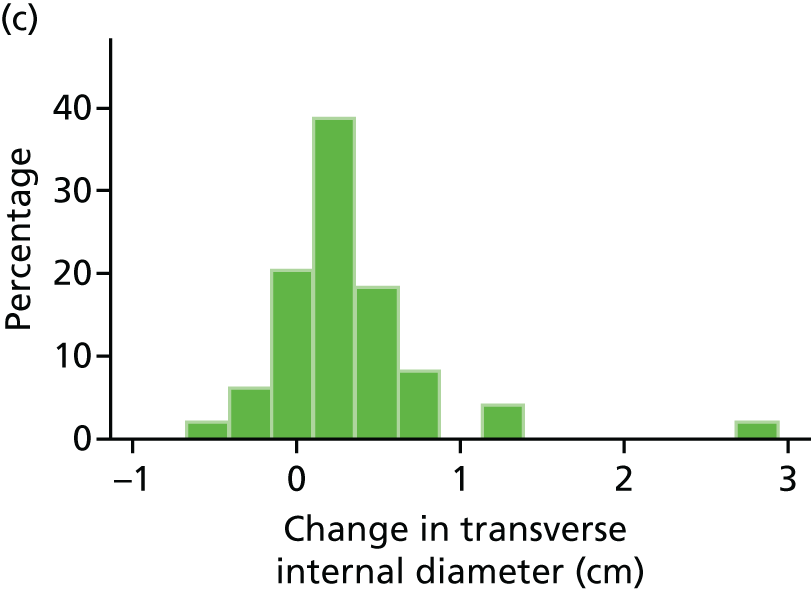

FIGURE 67.
Histograms of change in AAA transverse internal diameter from baseline to month 18. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
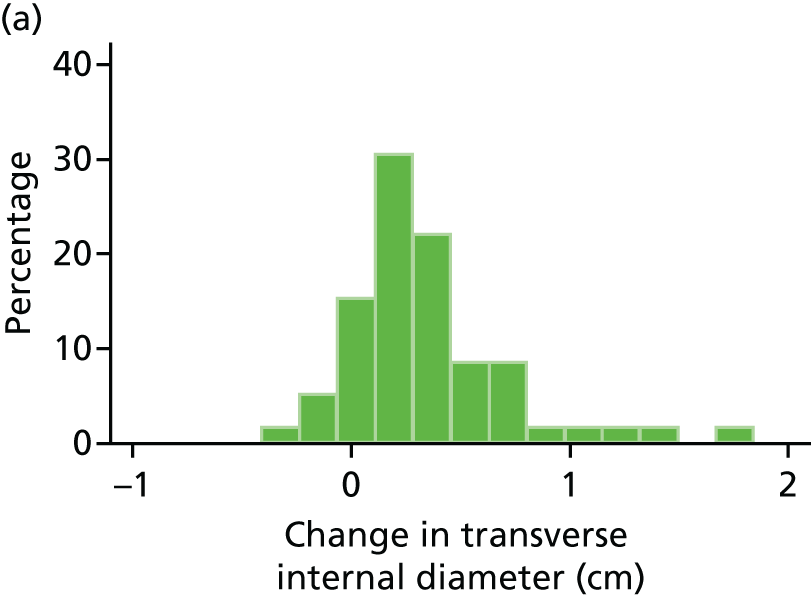


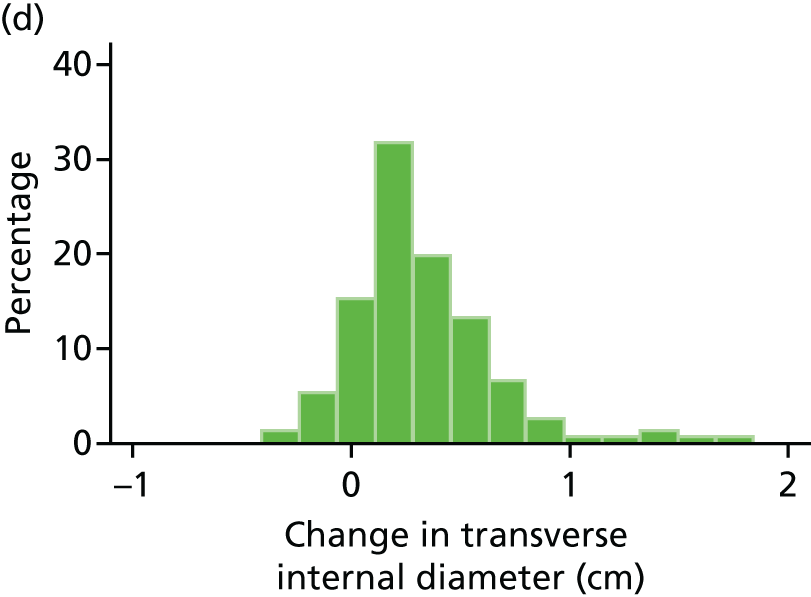
FIGURE 68.
Histograms of change in AAA transverse internal diameter from baseline to month 24. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.

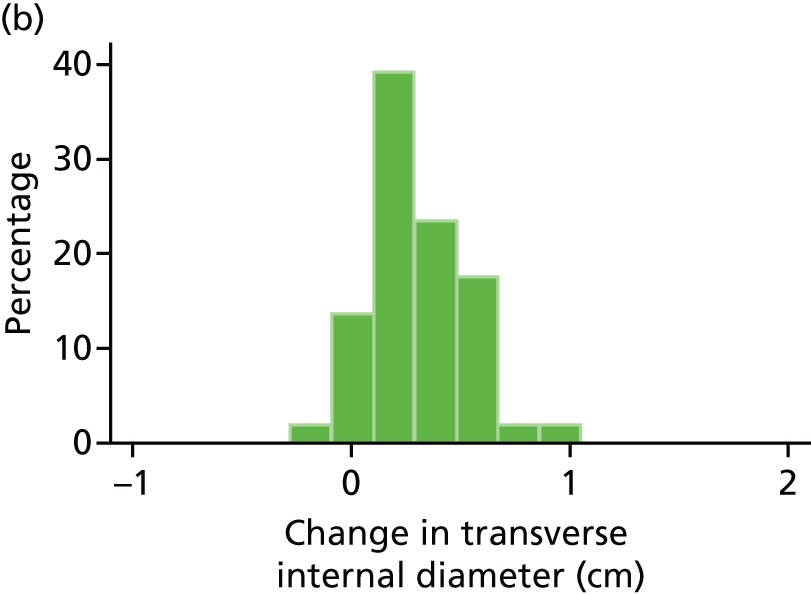
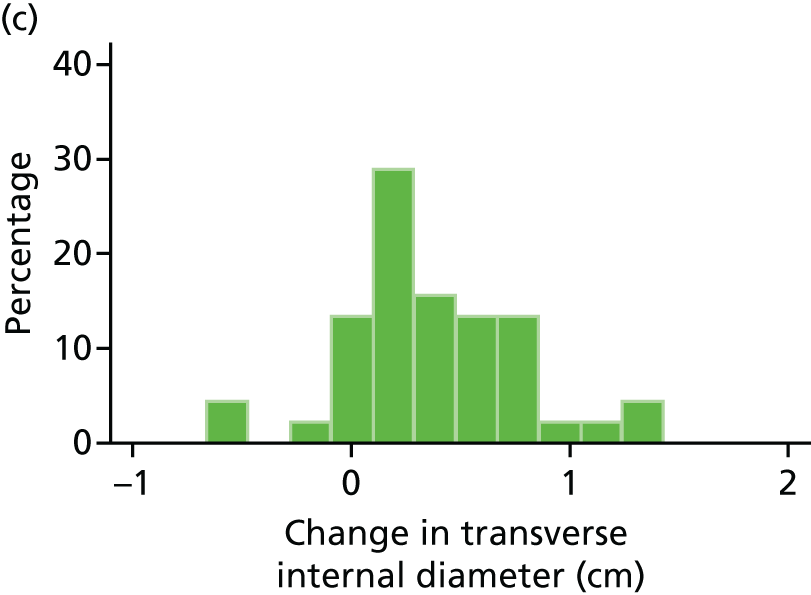

Appendix 13 Box plots of change in abdominal aortic aneurysm transverse internal measurements
FIGURE 69.
Box plot of change in AAA transverse internal diameter from baseline to month 3. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

FIGURE 70.
Box plot of change in AAA transverse internal diameter from baseline to month 6. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
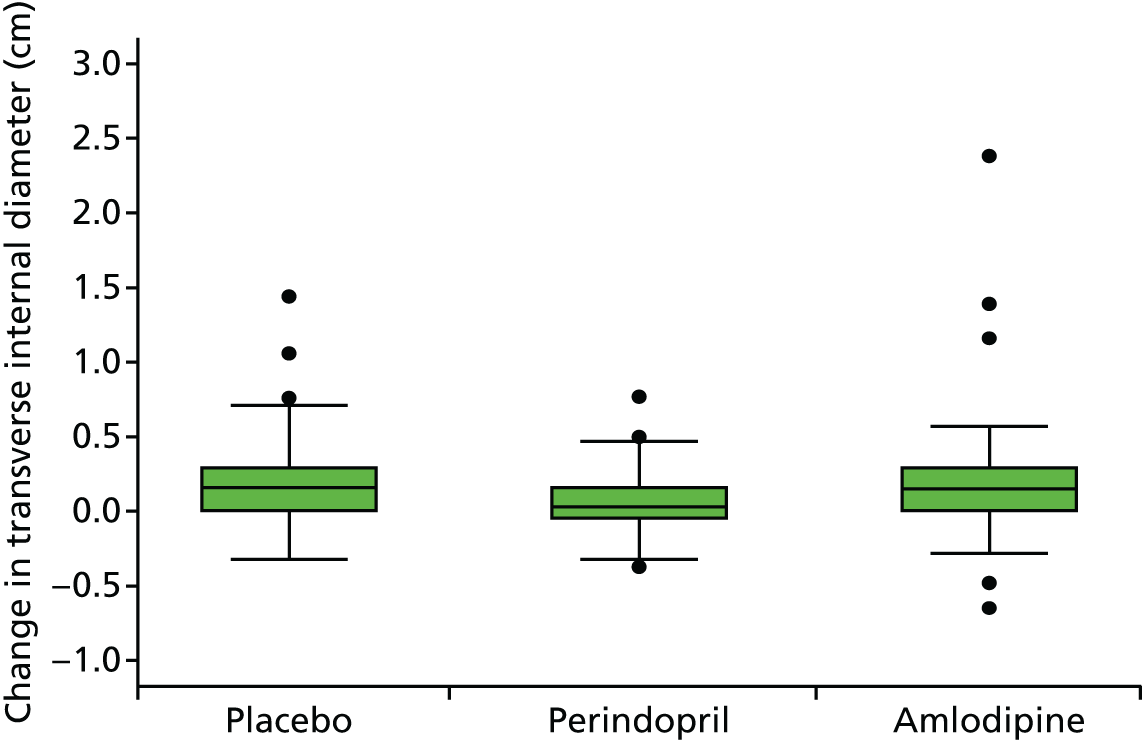
FIGURE 71.
Box plot of change in AAA transverse internal diameter from baseline to month 12. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
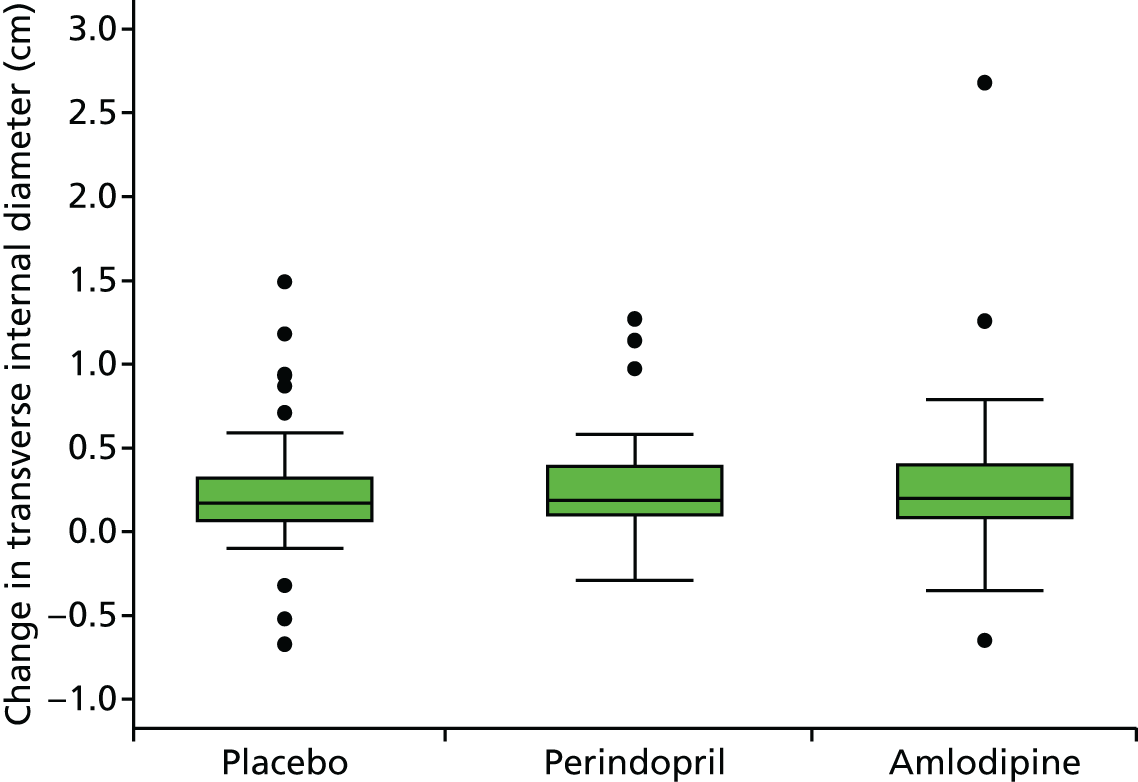
FIGURE 72.
Box plot of change in AAA transverse internal diameter from baseline to month 18. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
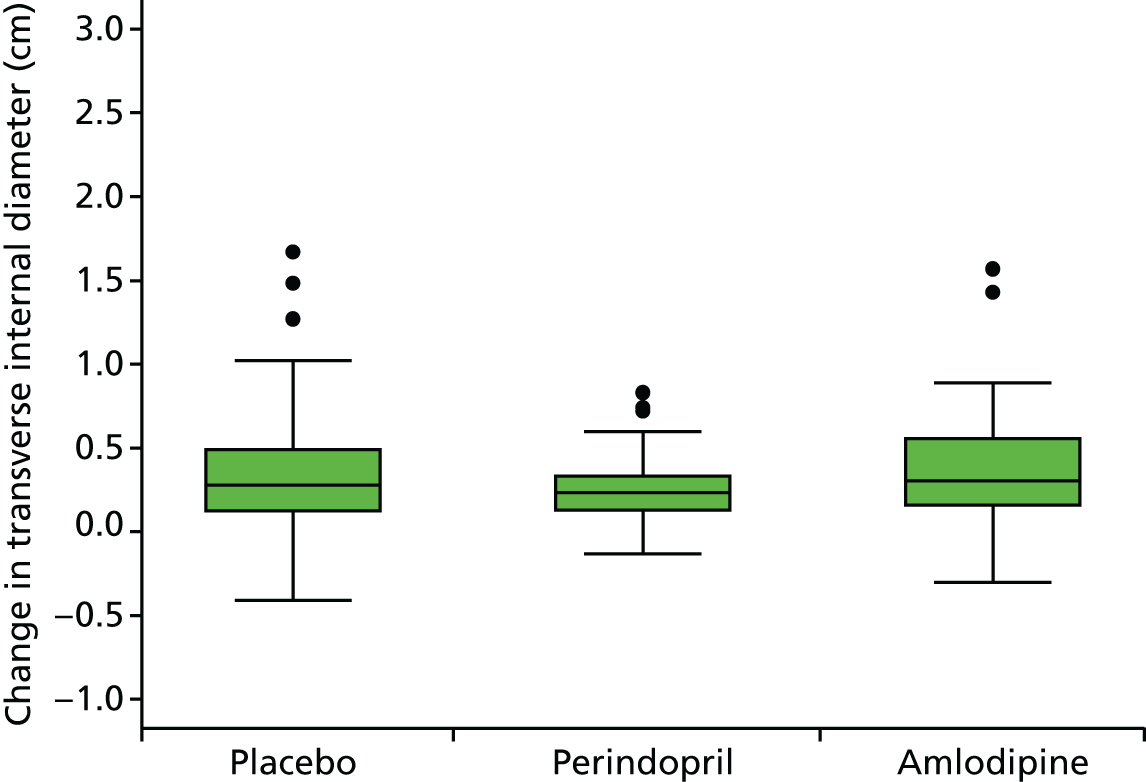
FIGURE 73.
Box plot of change in AAA transverse internal diameter from baseline to month 24. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

Appendix 14 Histograms of change in systolic blood pressure (6 and 18 months)
FIGURE 74.
Histograms of change in SBP from baseline to month 6. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.




FIGURE 75.
Histograms of change in SBP from baseline to month 18. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.


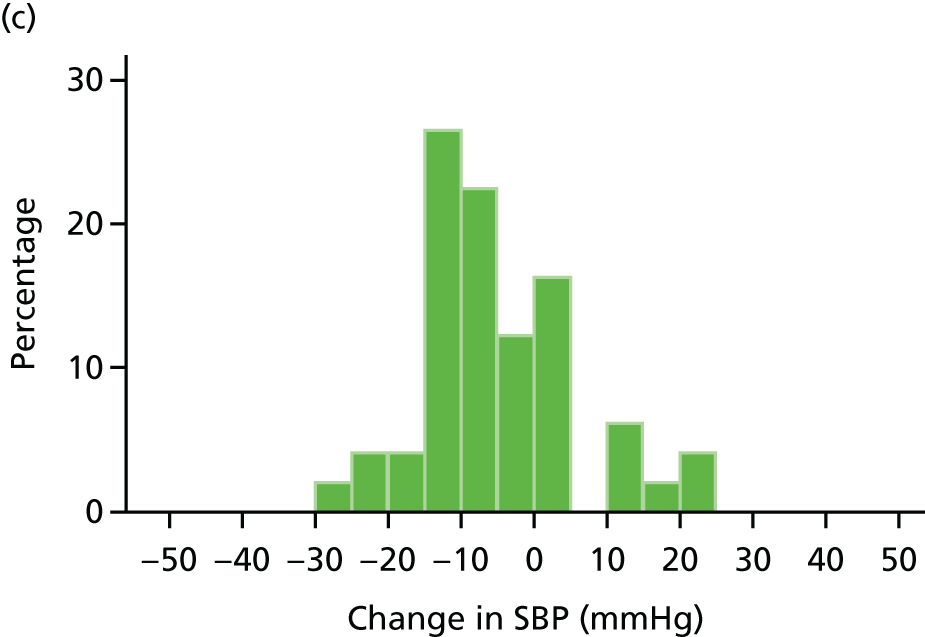
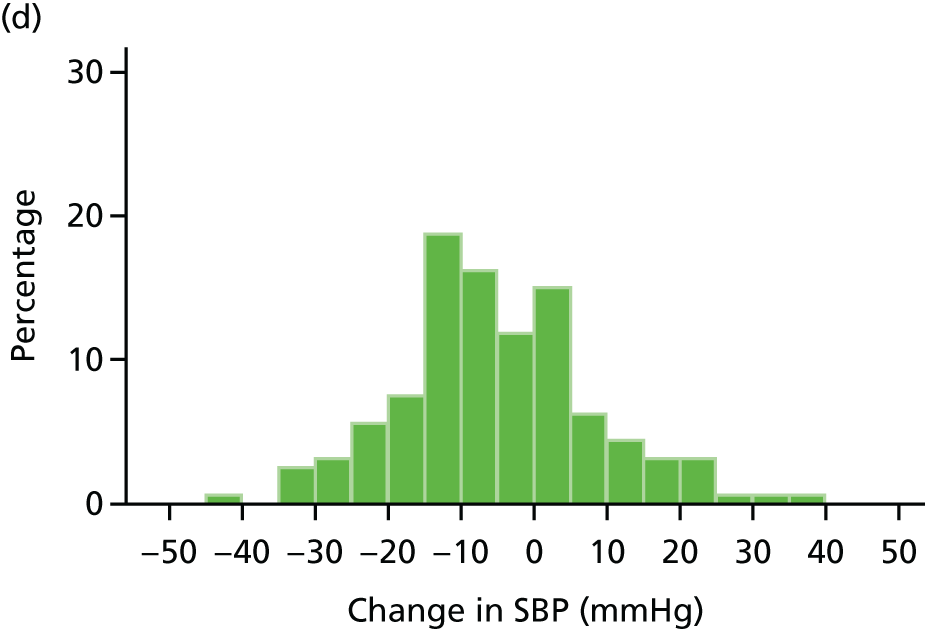
Appendix 15 Box plots of change in systolic blood pressure (6 and 18 months)
FIGURE 76.
Box plot of change in SBP from baseline to month 6. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

FIGURE 77.
Box plot of change in SBP from baseline to month 18. Circles are outside values. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.
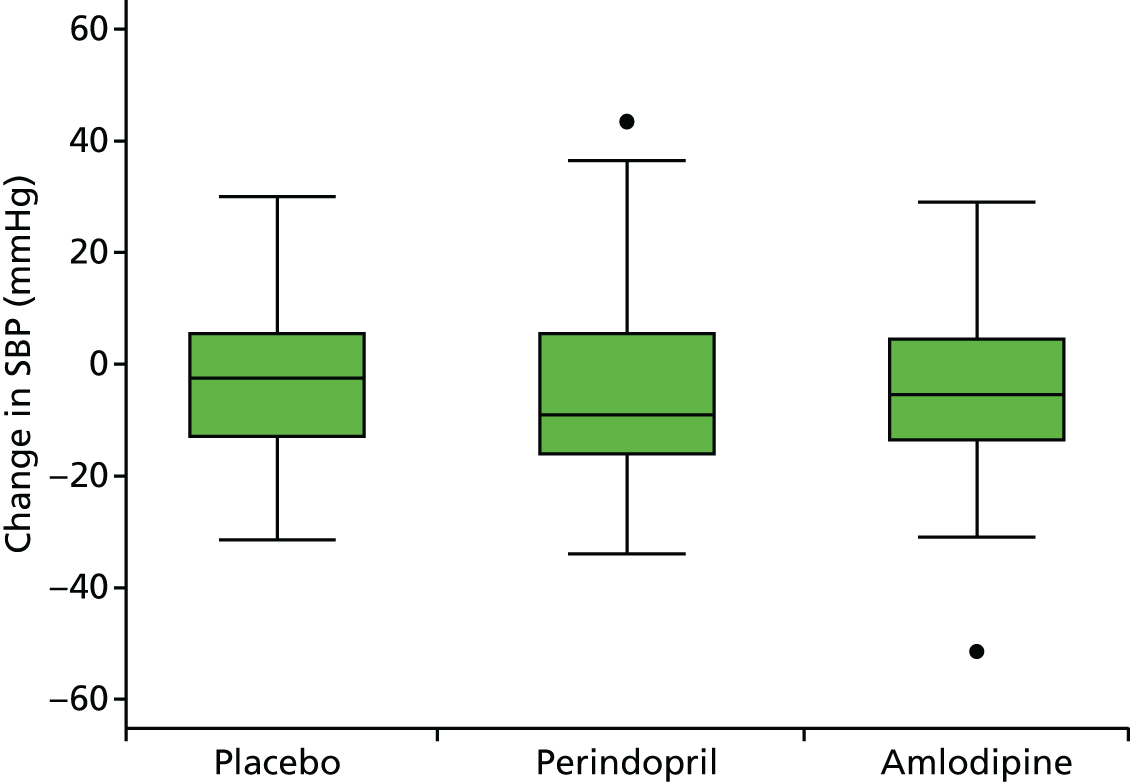
Appendix 16 Histograms of change in diastolic blood pressure (6 and 18 months)
FIGURE 78.
Histograms of change in DBP from baseline to month 6. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
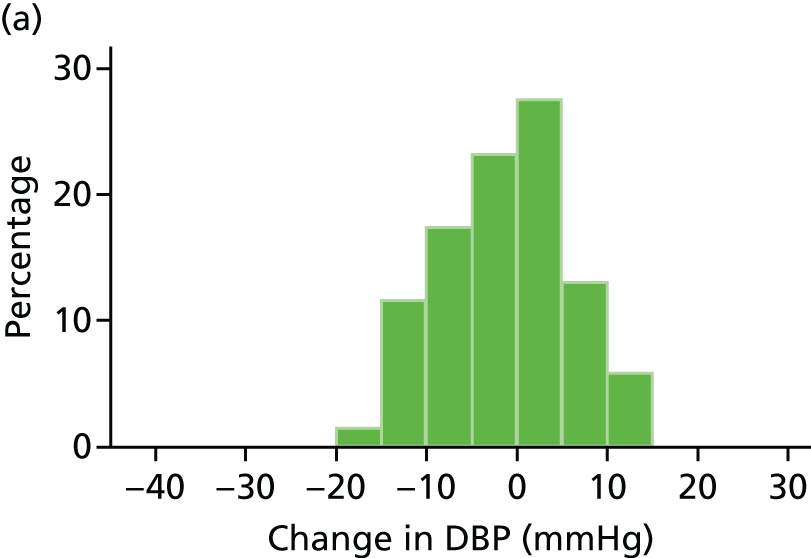
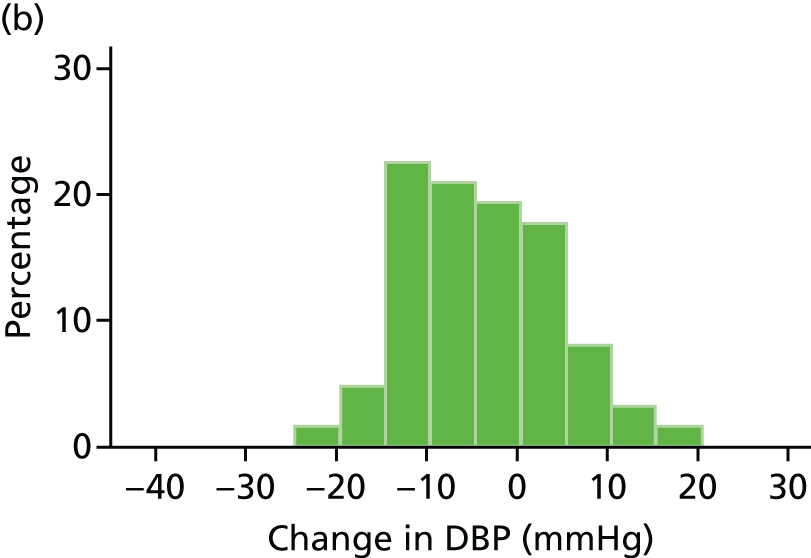
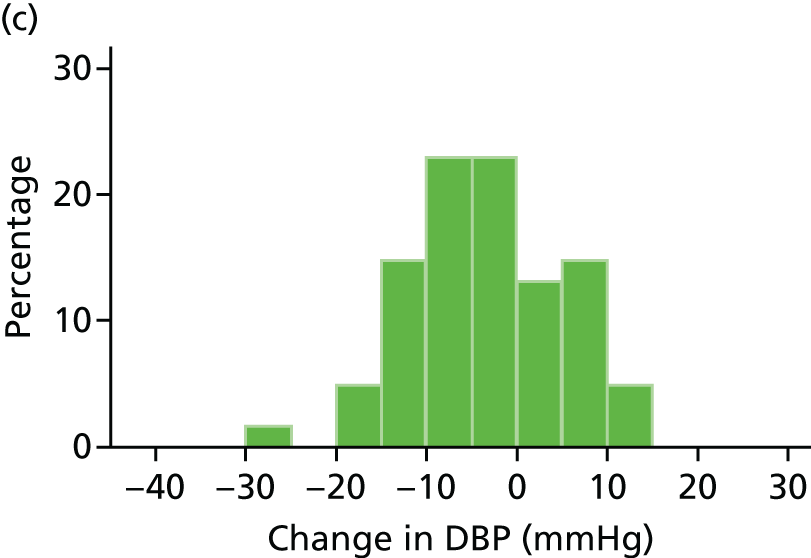

FIGURE 79.
Histograms of change in DBP from baseline to month 18. (a) Placebo; (b) perindopril; (c) amlodipine; and (d) total.
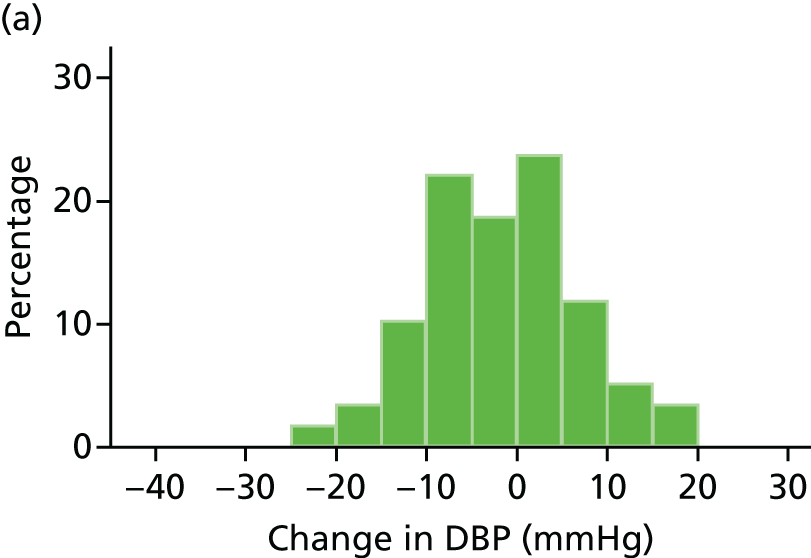



Appendix 17 Box plots of change in diastolic blood pressure (6 and 18 months)
FIGURE 80.
Box plot of change in DBP from baseline to month 6. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

FIGURE 81.
Box plot of change in DBP from baseline to month 18. Circle is an outside value. The box represents the 25th and 75th percentiles and the whiskers are the upper and lower adjacent values.

Appendix 18 Breakdown of European Quality of Life-5 Dimensions scores by domain
| Domain | Placebo | Perindopril | Amlodipine | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Mobility | ||||||||
| Month 12 | ||||||||
| I have no problems in walking about | 34 | 48.6 | 37 | 61.7 | 33 | 63.5 | 104 | 57.1 |
| I have some problems in walking about | 35 | 50 | 23 | 38.3 | 19 | 36.5 | 77 | 42.3 |
| Missing | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| Month 24 | ||||||||
| I have no problems in walking about | 31 | 55.4 | 32 | 61.5 | 32 | 69.6 | 95 | 61.7 |
| I have some problems in walking about | 25 | 44.6 | 20 | 38.5 | 14 | 30.4 | 59 | 38.3 |
| I am confined to bed | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Self-care | ||||||||
| Month 12 | ||||||||
| I am unable to wash or dress myself | 0 | 0 | 1 | 1.7 | 0 | 0 | 1 | 0.5 |
| I have no problems with self-care | 63 | 90.0 | 53 | 88.3 | 48 | 92.3 | 164 | 90.1 |
| I have some problems washing or dressing myself | 6 | 8.6 | 6 | 10 | 4 | 7.7 | 16 | 8.8 |
| Missing | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| Month 24 | ||||||||
| I am unable to wash or dress myself | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| I have no problems with self-care | 50 | 89.3 | 46 | 88.5 | 42 | 91.3 | 138 | 89.6 |
| I have some problems washing or dressing myself | 6 | 10.7 | 6 | 11.5 | 4 | 8.7 | 16 | 10.4 |
| Usual activities | ||||||||
| Month 12 | ||||||||
| I am unable to perform my usual activities | 2 | 2.9 | 0 | 0 | 2 | 3.8 | 4 | 2.2 |
| I have no problems performing my usual activities | 44 | 62.9 | 44 | 73.3 | 43 | 82.7 | 131 | 72.0 |
| I have some problems with performing my usual activities | 23 | 32.9 | 16 | 26.7 | 7 | 13.5 | 46 | 25.3 |
| Missing | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| Month 24 | ||||||||
| I am unable to perform my usual activities | 1 | 1.8 | 0 | 0 | 1 | 2.2 | 2 | 1.3 |
| I have no problems performing my usual activities | 38 | 67.9 | 37 | 71.2 | 34 | 73.9 | 109 | 70.8 |
| I have some problems with performing my usual activities | 17 | 30.4 | 15 | 28.8 | 11 | 23.9 | 43 | 27.9 |
| Pain/discomfort | ||||||||
| Month 12 | ||||||||
| I have extreme pain or discomfort | 5 | 7.1 | 4 | 6.7 | 2 | 3.8 | 11 | 6.0 |
| I have moderate pain or discomfort | 36 | 51.4 | 27 | 45.0 | 18 | 34.6 | 81 | 44.5 |
| I have no pain or discomfort | 28 | 40.0 | 29 | 48.3 | 32 | 61.5 | 89 | 48.9 |
| Missing | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| Month 24 | ||||||||
| I have extreme pain or discomfort | 4 | 7.1 | 4 | 7.7 | 1 | 2.2 | 9 | 5.8 |
| I have moderate pain or discomfort | 25 | 44.6 | 29 | 55.8 | 16 | 34.8 | 70 | 45.5 |
| I have no pain or discomfort | 27 | 48.2 | 19 | 36.5 | 29 | 63.0 | 75 | 48.7 |
| Anxiety/depression | ||||||||
| Month 12 | ||||||||
| I am extremely anxious or depressed | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| I am moderately anxious or depressed | 20 | 28.6 | 9 | 15.0 | 7 | 13.5 | 36 | 19.8 |
| I am not anxious or depressed | 48 | 68.6 | 51 | 85.0 | 45 | 86.5 | 144 | 79.1 |
| Missing | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| Month 24 | ||||||||
| I am extremely anxious or depressed | 2 | 3.6 | 1 | 1.9 | 0 | 0 | 3 | 1.9 |
| I am moderately anxious or depressed | 13 | 23.2 | 10 | 19.2 | 4 | 8.7 | 27 | 17.5 |
| I am not anxious or depressed | 41 | 73.2 | 41 | 78.8 | 42 | 91.3 | 124 | 80.5 |
List of abbreviations
- AAA
- abdominal aortic aneurysm
- AARDVARK
- Aortic Aneurysmal Regression of Dilation: Value of ACE-inhibition on RisK
- ACE-I
- angiotensin-converting enzyme inhibitor
- AE
- adverse event
- AP
- anteroposterior
- ARB
- angiotensin receptor blocker
- BP
- blood pressure
- CCB
- calcium channel blocker
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CT
- computerised tomography
- DBP
- diastolic blood pressure
- DSMC
- Data Safety Monitoring Committee
- EQ-5D
- European Quality of Life-5 Dimensions
- EVAR
- endovascular aneurysm repair
- GP
- general practitioner
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICCH
- International Centre for Circulatory Health
- ICF
- informed consent form
- ICTU
- Imperial Clinical Trials Unit
- IMP
- investigational medicinal product
- ITI
- inner to inner
- ITT
- intention to treat
- MASS
- Multicentre Aneurysm Screening Study
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MRC
- Medical Research Council
- NAAASP
- NHS Abdominal Aortic Aneurysm Screening Programme
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OTO
- outer to outer
- PI
- principal investigator
- PIC
- patient identification centre
- PIS
- patient information sheet
- QA
- quality assurance
- RAS
- renin–angiotensin system
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SBP
- systolic blood pressure
- SD
- standard deviation
- SE
- standard error
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UKHPS
- UK Heart Protection Study
- UKSAT
- UK Small Aneurysm Trial