Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/402/94. The contractual start date was in December 2008. The draft report began editorial review in September 2014 and was accepted for publication in January 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Rachel CM Brierley, Alan Cohen, Alice Miles, Andrew D Mumford, Gavin J Murphy (up to 31 August 2012), Rachel L Nash, Katie Pike, Sarah Wordsworth, Elizabeth A Stokes and Barnaby C Reeves had varying percentages of their salaries paid for by the grant awarded for the trial. Some or all of the time contributed by Gianni D Angelini, Gavin J Murphy (from 1 September 2012) and Chris A Rogers was paid for by the British Heart Foundation. Barnaby C Reeves is a member of the National Institute for Health Research Health Technology Assessment Commissioning Board, Systematic Reviews Programme Advisory Board and the Efficient Studies Design Board.
Disclaimer
The views and opinions expressed are those of the authors and do not necessarily reflect those of the Health Technology Assessment programme, the National Institute for Health Research, the British Heart Foundation, the UK NHS or the Department of Health.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Reeves et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Perioperative anaemia is strongly associated with adverse outcomes in cardiac surgery patients. 1–3 Transfusion of allogenic red blood cells is the preferred treatment to reverse acute anaemia and, on average, > 50% of adult cardiac surgery patients receive a perioperative transfusion. 4,5
Cardiac surgery consumes a substantial proportion of blood supplies – > 6% of all red blood cell use in the UK occurs in cardiac surgery. 6 Red blood cell transfusion is essential in some cardiac surgical patients for the management of life-threatening haemorrhage. In most cases, however, decisions to give a red blood cell transfusion are made because the haemoglobin concentration has fallen to a level or threshold at which the physician is uncomfortable. 2,7,8 The transfusion threshold varies across different cardiac surgery units and between different surgeons, which contributes to the wide variation in blood usage observed in cardiac surgical units (25% to 95%). 4,5,9 The threshold variation stems from a lack of evidence regarding what constitutes a safe level of anaemia following cardiac surgery.
Background and rationale
The clinical benefits of red blood cell transfusion beyond increasing circulating haemoglobin concentrations are unclear. Observational analyses suggest that transfusion after cardiac surgery may not, in fact, improve outcome where, in an apparent paradox, reversal of anaemia with transfusion has been shown to be consistently associated with increased infection, low cardiac output state, acute kidney injury (AKI) and death. 2,10,11 In contrast, randomised controlled trials (RCTs) comparing a restrictive red blood cell transfusion threshold (allowing a participant’s haemoglobin level to drop to a lower level before transfusing) with a more liberal strategy (transfusing a participant at a higher haemoglobin level) have not demonstrated adverse effects directly attributable to transfusion in patients undergoing major surgery or in the critically ill. 12–14
The absence of harm from restrictive practice in RCTs combined with the evidence from observational studies has been interpreted as being supportive of restrictive transfusion practice. 15 Alongside the well-documented risks of more liberal transfusion (including haemolytic and non-haemolytic transfusion reactions and transfusion-associated lung injury,16 increasing demands on blood services17 as well as additional and important cost implications associated with the storage, handling and administration of red blood cell units18), this evidence has led to an emphasis on restrictive transfusion in contemporary blood management guidelines19–21 and increasingly in health policy. 22,23
The Transfusion Indication Threshold Reduction (TITRe2) trial was designed in 2006 and was prompted by the widely varying transfusion thresholds that were being applied at the time and by a detailed observational analysis of data from the hospital in which the triallists worked. 2 Existing RCTs at the time that had compared liberal with restrictive transfusion in cardiac surgery, including our own pilot trial, had lacked sufficient statistical power to demonstrate clinical benefits attributable to restrictive transfusion. 24–26 A contemporary systematic review of RCTs of liberal compared with restrictive transfusion, most of which were not conducted in cardiac surgery, also concluded that there is uncertainty as to the benefits of more restrictive transfusion in patients with unstable cardiac disease. 12
Aims and objectives
To address this uncertainty, we undertook the TITRe2 RCT. The trial was designed to test the hypothesis that a restrictive threshold for red blood cell transfusion (haemoglobin 7.5 g/dl and/or haematocrit 22%) would reduce post-operative morbidity and health service costs compared with a liberal threshold (haemoglobin 9 g/dl and/or haematocrit 27%).
Specific objectives of the TITRe2 trial were:
-
to estimate the difference in the risk of a post-operative infection or ischaemic event between restrictive and liberal transfusion thresholds
-
to compare the effects of restrictive and liberal transfusion thresholds with respect to a range of secondary outcomes
-
to estimate the cost-effectiveness of a restrictive compared with a liberal haemoglobin transfusion threshold.
Chapter 2 Methods
Study design
The study was a multicentre RCT. The objectives were addressed by randomising participants to either a restrictive (transfuse if post-operative haemoglobin dropped below 7.5 g/dl, or haematocrit below 22%) or a liberal (transfuse if post-operative haemoglobin dropped below 9 g/dl, or haematocrit below 27%) strategy for red blood cell transfusion. The trial is registered, number ISRCTN70923932.
Participants provided written, informed consent pre-operatively but only became eligible for randomisation if their haemoglobin fell below 9 g/dl, or haematocrit below 27%, at some point postoperatively. Therefore, postoperatively, haemoglobin/haematocrit levels were monitored according to usual care and if the relevant threshold was breached at any time on the cardiac unit the participant was randomised as soon as possible, at the latest within 24 hours. (Note: thresholds were expressed as haemoglobin or haematocrit, and randomisation or transfusion was indicated if either value fell below the allocated threshold. Hereinafter, haemoglobin should be interpreted as haemoglobin or haematocrit.) A UK NHS Research Ethics Committee approved the study (08/H0606/125). Full details of all methods are reported elsewhere. 7
Changes to study design after commencement of the study
There were no major changes to the study design after commencement. Some changes were made to eligibility criteria and outcomes, which are discussed in Changes to study eligibility criteria after commencement of the study and Changes to study outcomes after commencement of the study, respectively.
Participants
Eligibility criteria
The study inclusion criteria were:
-
adults of either sex, aged ≥ 16 years, undergoing cardiac surgery [defined as coronary artery bypass grafting (CABG), heart valve replacement or repair, aortic surgery or surgical correction of congenital cardiac disease]
-
post-operative haemoglobin level < 9 g/dl at any stage during the patient’s post-operative hospital stay [i.e. on cardiac intensive care unit (CICU) or cardiac surgical ward]
-
written informed consent.
The exclusion criteria were:
-
patients undergoing emergency cardiac surgery
-
patients prevented from having blood and blood products according to a system of beliefs (e.g. Jehovah’s Witnesses)
-
patients with congenital or acquired platelet, red blood cell or clotting disorders
-
patients with ongoing or recurrent sepsis
-
patients with critical limb ischaemia
-
patients unable to give full informed consent for the study (e.g. learning or language difficulties)
-
patients already participating in another interventional research study.
Changes to study eligibility criteria after commencement of the study
In April 2009 (before starting recruitment to the study), two exclusion criteria were removed:
-
patients with a critical carotid artery stenosis (> 75%)
-
patients with flow limiting (> 70% luminal stenosis) coronary artery disease not undergoing complete revascularisation.
These exclusion criteria were included originally on the basis of the exclusion criteria used in the pilot study for this trial. 26 The pilot study used different thresholds, notably a lower haemoglobin threshold of 7 g/dl for the restrictive group. At the time of designing the pilot study it was felt that, because patients entering the pilot study could potentially experience haemoglobin levels as low as 7 g/dl, these exclusion criteria were needed to avoid non-adherence by intensivists, who might consider such patients to be more at risk of experiencing ischaemic adverse effects. TITRe2 used the higher haemoglobin level of 7.5 g/dl for the restrictive threshold and this threshold was already used routinely at some centres for all patients. Therefore, after discussing these exclusion criteria again, the study team believed they were not necessary for TITRe2.
In August 2010, the previously stated upper age limit of 80 years for the inclusion of participants was removed. This decision was a result of feedback from sites that they did not consider older age to be a contraindication for randomisation to the study and that the exclusion would substantially limit the pool of eligible patients for the study. After removal of this criterion surgeons were still able to refuse to include patients aged > 80 years on a case-by-case basis.
Settings
Patients were recruited to the trial in 17 specialist cardiac surgery centres in UK NHS hospitals.
Interventions
The trial compared two thresholds for blood transfusion, liberal and restrictive. The thresholds were defined as follows:
-
Liberal group: participants randomised to this group were eligible for transfusion if their post-operative haemoglobin level fell < 9 g/dl at any time during their post-operative hospital stay on the CICU or cardiac surgical ward. Therefore, all participants in this group should have received one red blood cell unit soon after randomisation. The objective was to maintain the haemoglobin level at or above 9 g/dl.
-
Restrictive group: participants randomised to this group were eligible for transfusion if their post-operative haemoglobin level fell < 7.5 g/dl at any time during their post-operative hospital stay on the CICU or cardiac surgery ward. The objective was to maintain the haemoglobin level ≥ 7.5 g/dl.
The protocol specified that, in both groups, one red blood cell unit should be transfused, the haemoglobin rechecked and a second unit transfused only if the haemoglobin remained below the relevant threshold. Clinicians were allowed to transfuse, or refuse to transfuse, in contravention of the allocated threshold but were required to document their reason for doing this and the haemoglobin level at the time. Furthermore, a clinician could decide it was in the best interests of a participant to permanently discontinue treatment according to the allocated group, which did not constitute a withdrawal and the participant was followed up as normal. Other aspects of post-operative care were provided in accordance with the institution’s usual care.
The duration of intervention in the trial was the duration of the participant’s care under the consultant cardiac surgeon or a maximum of 3 months after the date of randomisation, whichever was shorter. Almost always, the duration of care under the cardiac surgeon was the period of hospitalisation after surgery. However, a few participants who developed serious complications were transferred to the care of another consultant in the same hospital, at which time the interventional period for the study ended.
Outcomes
Primary outcome
The primary outcome was a binary composite outcome of any serious infectious (sepsis or wound infection) or ischaemic event [permanent stroke, myocardial infarction (MI), gut infarction or AKI] in the first 3 months after randomisation. The qualifying events listed in Table 1 were included; the table also describes the manner in which each qualifying event was verified.
| Infectious events | Definition/method of verification |
|---|---|
| Sepsis | During index admission: |
|
|
| In follow-up period: | |
|
|
| Wound infection | ASEPSIS score of > 20.27 Two scores were calculated and summed: |
|
|
| A follow-up score derived from the in-hospital score and a questionnaire either posted for self-completion or administered by telephone, at 3 months post randomisation28,29 | |
| Ischaemic events | Definition/method of verification |
| Permanent stroke | Clinical report of brain imaging (CT or MRI), in association with new onset focal or generalised neurological deficit (defined as a deficit in motor, sensory or co-ordination function) |
| MI | Elevated post-operative peak serum troponin I or T, verified by an adjudication committee. Further details are given in Primary outcome |
| Gut infarction | Documented reason for laparotomy or post-mortem report |
| AKI | AKI network criteria for AKI, stage one, two or three:30 |
| Stage one: | |
|
|
| Stage two: | |
|
|
| Stage three: | |
|
Events occurring after discharge only contributed to the primary outcome if the potentially qualifying event resulted in admission to hospital or death. Wound infection identified as a result of adding post-discharge information was the only exception to this rule. For example, information ascertained using the additional treatment, serous discharge, erythema, purulent exudate, separation of deep tissues, isolation of bacteria, and stay duration as inpatient (ASEPSIS) post-discharge surveillance assessment questionnaire (see Table 1), when added to the ASEPSIS score derived for the index admission, sometimes resulted in a total ASEPSIS score for the index admission that was > 20. Other suspected infectious events treated in the community that did not cause readmission to hospital were not recorded as they could not be validated and are less serious than perioperative infections.
Events suspected to qualify for the primary outcome but that were not supported by objective evidence were referred to an independent adjudication committee. In practice, the adjudication committee only considered suspected MIs because documentary objective evidence for sepsis, stroke, AKI and gut infarction was verified by research nurses at the co-ordinating centre who were blinded to the random allocation. The adjudication committee consisted of a cardiac surgeon, cardiologist and anaesthetist who were blinded to allocation and each other’s assessments. They were required to classify a suspected MI as definite or not based on participant’s medical history, echocardiograms (ECGs) (both pre-operatively and at the time of the suspected MI) and troponin levels at the time of the suspected MI. Agreement between at least two of the three specialists was required to reach a final adjudicated classification.
Death was not included as a component of the primary composite outcome because, if death occurred following a qualifying event, the event would precede death itself. Deaths that occurred for other reasons were not hypothesised to increase because of red blood cell transfusion.
Secondary outcomes
All secondary outcomes were collected in the time between randomisation and 3-month follow-up, unless otherwise stated.
-
Units of red blood cells and other blood components [fresh frozen plasma (FFP), platelets, cryoprecipitate, activated factor VI (NovoSeven, Novo Nordisk) and Beriplex® (CSL Behring UK Ltd)] transfused during a participant’s hospital stay. Red blood cells transfused pre-randomisation (either intraoperatively or postoperatively but prior to randomisation) were collected and described separately. However, it was only possible to collect information about other blood components transfused over the pre-randomisation and post-randomisation periods combined.
-
Occurrence of an infectious qualifying event, defined as sepsis or wound infection.
-
Occurrence of an ischaemic qualifying event, defined as permanent stroke, MI, AKI or gut infarction.
-
Quality of life measured using European Quality of Life-5 Dimensions-3 Level (EQ-5D-3L),31 assessed pre-operatively and at 6 weeks and 3 months post randomisation.
-
Duration of intensive care unit (ICU) or high-dependency unit (HDU) stay; calculated as the total time between randomisation and discharge from the cardiac unit that the participant was in either the CICU, HDU or general ICU wards, including any periods of readmission to that area.
-
Duration of hospital stay, calculated as the time between randomisation and discharge from the cardiac unit.
-
Significant pulmonary morbidity, comprising initiation of non-invasive ventilation [e.g. continuous positive airway pressure (CPAP) ventilation], reintubation/ventilation or tracheostomy.
-
All-cause mortality.
-
Health and Personal Social Services resource use and their costs.
(Durations of ICU, HDU and hospital stay were originally specified as ‘postoperative’. We specified randomisation as the time origin for these durations in the analysis plan, for consistency with the primary and other secondary outcomes.)
Changes to study outcomes after commencement of the study
The following changes were made to study outcomes after the trial had commenced.
-
In April 2009, before starting recruitment, there were some amendments made to the definitions of infectious and ischaemic events that qualified for the primary outcome.
-
In March 2010, an amendment was made to include troponin T in addition to troponin I in defining MI. This amendment was required after discovering that some participating centres habitually used troponin T rather than I. In addition, as part of this change, the troponin threshold for MI that was previously stated was removed; the decision was made, instead, to collect the highest troponin reading for all participants with suspected MI and to adjudicate suspected MIs (see Primary outcome).
-
In March 2011, the secondary outcome ‘significant pulmonary morbidity’ was added. This was initially named transfusion-associated circulatory overload and then subsequently renamed. The outcome was added because information from the haematology community had highlighted pulmonary morbidity as a potentially important outcome for patients receiving blood transfusions. The outcome was defined with respect to data already being collected on the study case report forms (CRFs) before the amendment so that the outcome could be identified consistently across the entire duration of the trial.
-
Furthermore, in March 2011, ‘A&E [accident and emergency] admission’ was removed from the primary outcome as qualifying event. This change arose from discussion with clinicians on the Trial Steering Committee (TSC) who agreed that a participant experiencing any element of the primary outcome would be admitted to hospital if they attended the emergency department (ED) within the follow-up period.
Adverse events
Expected adverse events (AEs) were specified in the study protocol and captured via the study CRFs, both for the post-operative in-hospital period (serious and non-serious), and at the 3-month follow-up (serious only).
A serious adverse events (SAE) is any untoward medical occurrence that either results in death, is life-threatening, requires in-patient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, or results in a congenital anomaly/birth defect.
All other AEs were considered unexpected, any such events satisfying one or more criteria for classification as serious were recorded in detail on purpose-designed SAE forms. Unexpected SAEs were coded using the Medical Dictionary for Regulatory Activities version 14.1 (MedDRA; McLean, VA, USA) independently by two research nurses blinded to randomised allocation. Any discrepancies were resolved by a consultant cardiac surgeon also blinded to allocation.
Sample size
The trial was designed to answer superiority questions. The following steps were taken to calculate the sample size.
-
From observational data, we assumed that approximately 65% of patients would breach the threshold of 9 g/dl and 20% would breach the 7.5 g/dl threshold. 2 Therefore, with complete adherence to the transfusion protocol, we assumed that transfusion rates should be 100% in the liberal group and ≈30% (0.20/0.65) in the restrictive group.
-
In the observational analysis,2 63% of patients with a nadir haematocrit between 22.5% and 27%, and 93% of patients with a nadir haematocrit below 22.5%, were transfused. Therefore, in combination with the proportions of patients expected to breach the liberal and restrictive thresholds, these figures were used to estimate conservative transfusion rates of 74% for the liberal group and ≤ 35% for the restrictive group. These percentages reflected the rates of transfusion documented in the observational study (Figure 1) and assumed non-adherence with the transfusion protocol of approximately 26% in the liberal group and 5% in the restrictive group.
-
The observational frequencies of infectious and ischaemic events for transfused and non-transfused patients were adjusted to reflect the estimated transfusion rates in the two groups (i.e. 74% and ≤ 35%), giving event rates for the proposed composite outcome of 17% in the liberal threshold group and 11% in the restrictive threshold group. A sample size of 1468 was required to detect this risk difference of 6% with 90% power and 5% significance (two-sided test), using a sample size estimate for a chi-squared test comparing two independent proportions (applying a normal approximation correction for continuity) in Stata version 9 (StataCorp LP, College Station, TX, USA).
-
The target sample size was inflated to 2000 participants (i.e. 1000 in each group) to allow for uncertainty about non-adherence and the estimated proportions of participants experiencing the primary outcome. We regarded these parameter estimates as uncertain because (1) they were estimated from observational data, (2) they were based on the red blood cell transfusion rate only in Bristol, (3) they were based on routinely collected data, using definitions for elements of the composite primary outcome which are not identical to those proposed for the trial, and (4) they were based on any compared with no red blood cell transfusion, rather than on the number of units of red blood cells likely to be transfused in participants who breach the liberal threshold. No adjustment was made for withdrawals or loss to follow-up, as both rates were expected to be very low.
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram summarising TITRe2 trial design. Percentages are based on data from the cardiac surgery registry in Bristol for the period January to September 2007. An unknown percentage of patients are excluded by the exclusion criteria because the registry does not contain sufficient detail to apply the definitions proposed for the trial. However, patients meeting one or more of these criteria are extremely rare and we expected all of the exclusion criteria to account for a maximum of 5% of cardiac surgery patients. Hb, haemoglobin.

We expected approximately two-thirds of participants to breach the haemoglobin threshold for eligibility. 2 Therefore, we predicted that we needed to register approximately 3000 participants into the study as a whole to allow 2000 participants to be randomised into the main study.
The main outcome measure for the economic evaluation was quality-adjusted life-years (QALYs), which are derived from EQ-5D-3L utilities measured on a continuous scale and time under observation. The analysis of QALYs requires baseline utility to be modelled as a covariate; the correlation between baseline and 3-month EQ-5D-3L utilities was assumed to be ≥ 0.3. With a total sample size of 2000, the trial had more than 95% power to detect a standardised difference in continuous outcomes between groups of 0.2 with 1% significance (two-sided test). This magnitude of difference is conventionally considered to be ‘small’. 32
Interim analyses
One formal, pre-specified interim analysis was carried out in June 2012 after 50% of the participants had been recruited and followed up for 3 months. Extreme criteria for stopping the trial (p ≤ 0.001) were set and, therefore, no adjustment was made to the sample size and statistical significance levels for this interim analysis.
Randomisation
Participants were randomly allocated to either the liberal or restrictive transfusion strategies using cohort minimisation to achieve balance across the two arms of the trial; minimisation factors were centre and operation type (CABG, valve, CABG and valve combined, or other cardiac surgery). Participants were randomly assigned in a 1 : 1 ratio. Allocations were generated by computer and concealed using an internet-based system provided by Sealed Envelope Ltd (London, UK). Staff in participating centres were able to gain secure limited access to the system using a password and PIN (personal identification number). Information to identify a participant uniquely and to confirm eligibility had to be entered before the system assigned the randomised treatment allocation, ensuring concealment of allocations. Randomisation occurred postoperatively and as soon as possible after the participant’s haemoglobin level fell below 9 g/dl (at the latest within 24 hours). If randomisation did not occur within 24 hours, the patient was considered to have become ineligible and should not have been randomised unless the haemoglobin fell below 9 g/dl again (when the clock for the ‘24 hour rule’ was restarted; see Non-adherence with randomisation protocol).
Blinding
It was not possible to blind clinicians, research staff and other NHS staff caring for participants to the randomised allocation. However, outcomes were defined on the basis of objective criteria as far as possible, in order to minimise susceptibility to bias. Furthermore, both the research nurses reviewing the documentary evidence relating to primary outcome events and the adjudication committee assessing MIs were blinded to treatment allocation.
Every effort was made to blind participants to their allocation. The success of blinding was checked by asking participants if they knew what their allocation was at the time of discharge from hospital and their 3-month post-randomisation follow-up.
Data collection
In-hospital data collection (see Appendix 4 for the CRFs) included the following elements.
-
Screening log of all non-emergency patients having cardiac surgery
-
distinguishing patients but without recording identifiable data electronically
-
whether or not a patient information leaflet (PIL) was sent
-
whether or not a patient was approached for the trial
-
assessment of eligibility; if ineligible, reasons for ineligibility
-
whether or not a patient was asked to give written informed consent for the trial.
-
-
For all registered participants (randomised and non-randomised)
-
pre-operative characteristics, including operation category
-
a summary of blood products received
-
daily haemoglobin levels to check compliance with protocol.
-
-
For all randomised participants
-
date and time when the haemoglobin fell below 9 g/dl
-
operative details, including duration of surgery and use of any blood products
-
observations required for the primary and secondary outcomes, including dates and times of relevant events
-
other key resource use
-
any AEs
-
information about whether or not a participant was blinded to allocation at discharge.
-
Research staff in participating centres collected data on the trial screening log and pre-printed CRFs. These data were transferred promptly to a secure computerised database maintained on a NHS computer, allowing data to be checked centrally. Queries about specific data items were listed on the database and were immediately apparent to centre staff when they logged on.
Post-operative haemoglobin levels in consented participants were measured at regular intervals and the lowest level observed on each post-operative day was recorded on the CRF. After randomisation, the threshold to which a participant had been randomised was communicated to attending medical and nursing staff and recorded on the CRF. Centres used varying methods to highlight to staff that a participant had been randomised. Details of red blood cell transfusions were recorded and haemoglobin levels continued to be monitored. If a non-adherent transfusion decision was made for a randomised participant (i.e. a decision which did not adhere to the allocated protocol, see Non-adherence with transfusion protocol), the attending doctor was required to give a reason for the decision. This reason was documented on the CRF.
Data collection after hospital discharge consisted of the following elements.
-
The EQ-5D-3L was posted to randomised participants at 6 weeks and 3 months after randomisation. Participants who consented to the study but were not randomised also received a postal EQ-5D-3L 3 months after their operation.
-
Three months after the operation, a questionnaire was posted for self-completion, or administered by telephone (if a participant elected to be telephoned or failed to return the postal questionnaire), by staff at the co-ordinating centre. The questionnaire was composed of items eliciting information about:
-
AEs occurring after discharge, with further details of any event suspected to contribute to the primary outcome or meet the definition of a SAE sought from either the admitting hospital or the participant’s general practitioner (GP).
-
surgical wound infections occurring after discharge (ASEPSIS post-discharge surveillance questionnaire). 28,29
-
resource use after discharge from hospital.
-
a participant’s awareness of his/her random allocation.
-
-
Occasionally data collection was delayed beyond the planned follow-up times; when this occurred the following rules were used to determine whether or not data should be included in analyses:
-
EQ-5D-3L – the time between questionnaire completion and operation date was examined by group, blinded to allocation, separately for each time point. The distributions did not differ; therefore, data corresponding to times that were extreme outliers (identified by eye) were excluded but all other data included.
-
Three-month telephone/postal questionnaire – questionnaire items were phrased specifically in relation to the 3-month post-operative period and staff completing the telephone questionnaires were trained only to record information regarding this period. Therefore, data from all questionnaires were used.
-
Data collection is summarised in Table 2.
| Data collected | Pre-surgery | Day of surgery | At randomisation | In CICU/ward | At discharge | 6 weeks after randomisation | 3 months after randomisation |
|---|---|---|---|---|---|---|---|
| Eligibility | ✓a | ||||||
| Written consent | ✓a | ||||||
| Demographics and medical history | ✓a | ||||||
| EQ-5D-3L questionnaire | ✓a | ✓b | ✓a,b | ||||
| Operative details | ✓ | ||||||
| Haemoglobin/haematocrit level | ✓a | ✓a | ✓ | ✓a | |||
| Summary of blood components transfused | ✓a | ||||||
| Details of red blood cell transfusion | ✓ | ✓ | |||||
| Randomised allocation | ✓ | ||||||
| Surgical complications and AEs | ✓ | ✓c | |||||
| ASEPSIS assessment of wound infection | ✓ | ||||||
| Resource use data | ✓ | ✓ | ✓c | ||||
| ASEPSIS post-discharge surveillance | ✓c | ||||||
| Check participant blinded to allocation | ✓ | ✓c |
Adherence
Non-adherence with randomisation protocol
Non-adherence with the randomisation protocol was defined as any of the following:
-
Participant did not meet one or more of the pre-consent study eligibility criteria but was consented into the study. Any randomised participant to whom this applies was classified as ‘randomised in error’ and excluded from the analysis population.
-
Participant consented and met the post-consent inclusion criteria (i.e. haemoglobin dropped below 9 g/dl) but was not randomised. Any randomised participant to whom this applies was not randomised and, therefore, was excluded from the analysis population.
-
Participant did not meet the post-consent eligibility criteria (i.e. haemoglobin did not drop below 9 g/dl) but was randomised. Any randomised participant to whom this applies was classified as ‘randomised in error’ and excluded from the analysis population.
-
Participant was randomised more than 24 hours after meeting the post-consent inclusion criteria (i.e. randomised more than 24 hours after haemoglobin dropping below 9 g/dl). Any participant to whom this applies was classified as non-adherent with the randomisation protocol, but was included in the analysis population.
Non-adherence with transfusion protocol
Measuring and assessing adherence with the transfusion protocol was identified as a critical element of the study owing to the assumptions about adherence made in the sample size calculation. 33 The Data Monitoring and Ethics Committee (DMEC) also highlighted the importance of non-adherence, as they had concern that doctors might otherwise make transfusion decisions in different ways in the two groups. For example, a decision to transfuse in the liberal group could be delayed up to 24 hours without contravening the protocol and such behaviour would have been missed if data about haemoglobin levels measured during this period, their times and consequent actions had not been recorded.
Two types of non-adherence were defined: (1) a participant received a red blood cell transfusion outside of protocol (‘extra’ transfusion) and (2) a participant was not given a red blood cell transfusion that, according to the protocol, should have been given (‘withheld’ transfusion). Adherence was assessed for the period from randomisation to hospital discharge so multiple instances of non-adherence could be documented for a participant. If a participant withdrew or had their treatment according to their allocation discontinued, adherence after the time of withdrawal/discontinuation was not assessed. For both of the above types of non-adherence, instances were classified into mild, moderate or severe (Table 3). Non-adherence was classified as severe only if the non-adherent instant changed the participant’s overall classification as transfused or not.
| Non-adherence type | ‘Extra’ transfusion outside of protocol | ‘Withheld’ transfusion according to protocol |
|---|---|---|
| Mild | N/A | A transfusion took place, but more than 24 hours after the relevant breach of the transfusion threshold |
| Moderate | Participant transfused outside of protocol, but participant breached the threshold for transfusion at least once postoperatively | Participant was not transfused following a breach, but the participant had previously had at least one post-randomisation transfusion |
| Severe | Participant transfused outside of protocol and participant did not breach the threshold for transfusion at any point postoperatively | Participant was not transfused following a breach and participant had no post-randomisation transfusions |
In addition to describing the amount of non-adherence, work has been done to further describe and characterise non-adherence, including:
-
characteristics of each instance of non-adherence (including reasons, haemoglobin levels and timing) have been described
-
logistic regression models were fitted to identify predictors of non-adherence
-
non-adherence trends both by centre and over the course of the trial have been described.
Statistical methods
The analysis and safety populations consisted of all participants randomised into the study, excluding participants who withdrew and who were unwilling for the data already collected to be used. All analyses were performed on an intention-to-treat (ITT) basis and were directed by a pre-specified analysis plan. 34 Continuous variables were summarised via the mean and standard deviation (SD), or median and interquartile range (IQR) if distributions were skewed. Categorical data were summarised as a number and percentage. Pre-randomisation characteristics were described by allocated treatment. Similarly, pre-operative and intraoperative characteristics, transfusions, EQ-5D-3L scores and mortality of randomised and non-randomised (but consented) participants were described but no formal comparisons made.
Comparisons of outcomes
All outcomes were analysed using mixed-effects regression models, adjusting for all factors included in the cohort minimisation: operation type as a fixed effect and centre as a random effect (or a shared frailty term in time-to-event models). The primary outcome and other binary outcomes (numbers of participants experiencing infectious or ischaemic events, any transfusions of red blood cells and non-red blood cell products and significant pulmonary morbidity) were analysed using logistic regression, with treatment estimates presented as odds ratios (OR) and 95% confidence intervals (CI). For the analysis of the transfusion of any red blood cells, treatment estimates were analysed using unadjusted logistic regression, with results presented as a risk ratio (RR) and 95% CI, as the OR proved difficult to interpret and an adjusted model did not converge. Time-to-event outcomes were analysed using Cox proportional hazards models and treatment estimates presented as hazard ratios (HR) and 95% CI. Durations of ICU/HDU stay and hospital stay were censored at the time of death if the participant died before discharge from hospital. All-cause mortality was censored at the time of last follow-up for survivors. A secondary analysis of the primary outcome, analysing the time to first occurrence of the primary outcome, was also undertaken using a Cox proportional hazards model, censoring at the time of last follow-up or death.
Longitudinal data (EQ-5D-3L scores) were analysed using mixed-effects mixed-distribution models;35 this method was used because the distribution of the data was non-monotonic, with many participants scoring perfect health. Both types of score (utility and visual analogue scores) were dichotomised into less than perfect health compared with perfect health. There were two-parts to each fitted model: (1) an occurrence model, a logistic regression model for the occurrence of less than perfect health compared with perfect health, and (2) an intensity model, a log-linear model for the score, conditional on a non-perfect health score. Correlated participant-term random effects (for occurrence and intensity) were included in each model to allow for the repeated measures. Separate parameter estimates were incorporated into models for the mean baseline response across both treatment groups and for each treatment post intervention, avoiding the necessity to either exclude cases with missing baseline measures or to impute missing baseline values. A time by allocation interaction (post intervention) was added to each of the models; an overall treatment effect is reported unless the interaction was statistically significant at the 10% level, in which case separate treatment effects at each post-intervention time are given.
Safety data
The AEs and SAEs were described by allocated treatment but no formal comparisons made.
Subgroup analyses
Pre-planned subgroup analyses were specified because of clinical opinion that transfusion decisions should be influenced by patients’ characteristics, notably that ‘at-risk’ patients should be transfused at a different threshold. The subgroups defined in the protocol were: operation type (isolated CABG vs. other operation types), age (< 75 years vs. ≥ 75 years), pre-operative diagnosis of diabetes (none vs. diet, oral medication or insulin controlled), pre-operative diagnosis of lung disease (none vs. chronic pulmonary disease or asthma), pre-operative renal impairment [estimated glomerular filtration rate (eGFR) > 60 ml/minute vs. eGFR ≤ 60 ml/minute], sex (males vs. females) and ventricular function (good vs. moderate or poor). Such analyses were implemented by adding a relevant treatment allocation by subgroup interaction term into the primary outcome model; the hypothesis for all subgroup analyses was that there would be no interaction. The pre-operative renal impairment subgroup analysis was defined in the study protocol as pre-operative creatinine ≤ 177 µmol/l versus creatinine > 177 µmol/l. However, during the course of the trial, use of pre-operative creatinine for risk stratification was superseded by estimated eGFR and, therefore, the subgroup analysis for renal impairment was based on eGFR as described above (this change was not covered by a protocol amendment).
Sensitivity analyses
A number of sensitivity analyses were pre-specified for the primary outcome in the analysis plan, although such analyses were not specified in the study protocol.
-
Examining treatment effect estimates for the primary outcome by site, ordering sites by rates of severe non-adherence with the transfusion protocol. This was implemented by a forest plot displaying site-specific treatment estimates. It provided a way of assessing the effect of non-adherence on the overall treatment estimate for the primary outcome, without excluding non-adherent participants. (We considered that an analysis excluding non-adherent participants would be inappropriate because it would be very likely to be biased as non-adherent participants were hypothesised to be the sicker participants in the restrictive group and the healthier participants in the liberal group.) As non-adherence represents a dilution of the allocated intervention, we hypothesised that the treatment effect would tend towards the null with increasing non-adherence.
-
Excluding all events that occurred in the first 24 hours after randomisation. The rationale was that such events could have an onset that actually preceded randomisation and, hence, be unrelated to the intervention. Therefore, we hypothesised that the treatment effect would tend away from the null with exclusion of these events.
-
Excluding participants who were transfused before randomisation. The rationale was that transfusions before randomisation, expected to occur with similar frequency in both groups, would dilute any effect of a difference between groups in the number of transfusions after randomisation. Therefore, we hypothesised that the treatment effect would tend away from the null with exclusion of pre-randomisation transfusions.
-
In collecting AKI data it became apparent that prospective data collection by research nurses in centres failed to identify AKI events that were apparent from routinely collected serial creatinine data. We attribute this discrepancy to differences between centres in the ‘baseline’ creatinine value used to define AKI, which can be confusing to implement as the specified creatinine rise should occur in a 48-hour period. 30,36 However, highest daily creatinine levels were recorded separately, so the following sensitivity analyses were planned:
-
excluding AKI events when the clinical diagnosis was not verified by routinely recorded creatinine levels. This analysis would exclude potentially ‘false’ AKI events, although AKI events in these participants may have been ‘true’ events classified on the basis of urine output (which was not documented routinely).
-
including additional AKI events when the participant was reported not to have had AKI according to clinical judgement but when highest daily creatinine levels supported a diagnosis of AKI. This analysis would include AKI events that were missed, assuming that the creatinine levels recorded for usual hospital care were accurately transcribed on to the CRF.
Assuming that false AKI events would arise in proportion to the incidence of true AKI events, they would not bias the treatment effect. Therefore, we hypothesised that the effect in the first analysis would reduce precision but not shift the estimate predictably either towards or away from the null. Similarly, assuming that missed AKI events would arise in proportion to the incidence of true AKI events, they would also not bias the treatment effect. Therefore, we hypothesised that the effect in the second analysis would increase precision but not shift the estimate predictably.
-
-
Including only ‘serious’ primary outcome events, defined as either stroke, MI, gut infarction, AKI stage three events, pre-discharge sepsis plus organ failure [MI, stroke, laparotomy for gut infarction and one or more of reintubation, acute respiratory distress syndrome (ARDS), low cardiac output and/or tracheostomy] and/or post-discharge sepsis that required hospital readmission. This analysis arose from the pre-planned interim analysis that showed a higher primary outcome event frequency than was anticipated when the study was designed, with a large majority of qualifying events arising from sepsis and AKI, which were considered to be clinically less serious. We considered that this sensitivity analysis would better reflect our original intention in formulating the composite outcome and the outcome events that were included in the observational analysis, which led to the superiority hypothesis for the trial. This analysis would necessarily have less precision but we did not have a strong hypothesis about the way in which the treatment effect might be affected. If transfusion were to have the same effect on more and less serious events, the treatment effect should be unaltered; if transfusion were to have a differential effect on more and less serious events, the treatment effect should be moved towards or away from the null in a manner consistent with the differential effect.
Post-hoc analyses
In addition, a secondary post-hoc analysis of severe in hospital events was performed, which involved refitting the primary outcome model with an outcome of death, severe sepsis [as defined in sensitivity analysis (e) above], ARDS, tracheostomy, low cardiac output, MI, AKI stage three, gut infarction and/or stroke. This analysis was performed because it was judged to be of key interest to hospital-based clinicians caring for patients. As for analysis (e) above, it would necessarily have less precision but we did not hypothesise that the treatment effect would be moved towards or away from the null.
Two further post-hoc sensitivity analyses were carried out for the secondary outcome of mortality; these comprised analyses (b) and (c) above (i.e. excluding deaths within 24 hours of randomisation and participants transfused before randomisation). These were suggested during the peer review process, on account of the seriousness of the outcome, and we agreed that they were worthwhile. Our (post hoc) hypotheses were the same as that for the corresponding sensitivity analyses of the primary outcome. These were the only additional analyses requested in this way, the decision to perform them was made without knowing the results and the results are fully reported.
Observational analyses
Three observational analyses were pre-specified in the study protocol.
-
Estimating the relationship between the number of red blood cell units transfused and the risk of mortality and morbidity, stratified by trial arm.
-
Investigating the relationship between percentage decline in haemoglobin from the pre-operative level and the risk of primary and secondary outcomes, taking into account the number of red blood cell units transfused.
-
Investigating whether or not red blood cell age (i.e. time since donation and processing) is associated with the risk of primary and secondary outcomes, achieved by linking batch numbers of all red blood cells transfused to a blood bank database and determining the age of each unit donation and transfusion dates.
For the purposes of these observational analyses, a composite outcome of the trial primary outcome or death was defined in the statistical analysis plan (SAP). In addition, all red blood cell units transfused or haemoglobin levels recorded after the time of the first occurrence of the primary outcome (or censoring) were excluded to ensure the relevant exposure occurred before the outcome. (More complex methods to deal with this issue were outlined in the SAP but these have not been attempted owing to the complexity of the analyses.) For all three analyses, pre-operative and intraoperative characteristics and trial outcomes were described by exposure [i.e. any red blood cells vs. no red blood cells, minimum haemoglobin < 7.5 g/dl vs. ≥ 7.5 g/dl, and transfusion of any red blood cells aged over 21 days old (median age) vs. only younger blood (< 21 days) vs. no red blood cell transfusions].
For analyses (b) and (c), the exposure definitions differ slightly from those used in the protocol/SAP. With respect to analysis (b), the protocol stated that haemoglobin would be defined in terms of percentage decline; however, exploratory analyses suggested this was not sensible (e.g. a participant with pre-operative haemoglobin 12 g/dl and post-randomisation haemoglobin 6 g/dl would be treated in the same way as a participant with pre-operative haemoglobin 18 g/dl and post-randomisation haemoglobin 9 g/dl, as the percentage decline is 50% in both cases) and that it would be more informative to include both pre-operative and post-randomisation haemoglobin levels in any analysis model. For analysis (c), various methods of defining age of blood were described in the SAP (using the age of the ‘oldest’ red blood cell unit given, the mean age of all red blood cells, the use of any red blood cells more than 14 days old, the number or percentage of red blood cells given over 14 days old, the use of red blood cells older than the median age of all red blood cells transfused) and it was stated that the age of the oldest unit would be used as the primary analysis. Owing to the large volume of missing data for age of blood, it was decided instead only to provide descriptive analyses by the receipt of any red blood cells older than the median age.
For parts (a) and (b), further analyses have been undertaken. Univariate analyses exploring the relationship between exposures and the outcome were performed. Two separate adjusted models [one for analysis (a) and one for analysis (b)] were then fitted adjusting for the following, if found to be potential confounders: operation type, centre (as a random effect), European System for Cardiac Operative Risk Evaluation (EuroSCORE), age, sex and pre-randomisation red blood cell transfusions [for analysis (a) only]. A model building strategy was used whereby variables were sequentially added to the model, at each step including the variable that improved the model fit the most (as determined by a likelihood ratio test). Variables were included in the model if they were (1) associated with both the exposure and the outcome but did not lie on the causal pathway between the exposure and outcome, and (2) significantly contributed to the relevant multivariate model (defined by a likelihood ratio p < 0.05 or modifying the effect estimate by greater than 10%). If pairs of variables were considered to be collinear or strongly related (e.g. EuroSCORE and age), only one of the pair was included. In addition, interaction terms were included in models if significant at the 5% level. The parameterisation of the exposure variable (e.g. continuous linear, continuous including additional power terms, ordinal categorical or binary) was explored using fractional polynomial models and likelihood ratio tests to compare nested models. Marginal plots were used to describe interactions between continuous and categorical covariates graphically. Models were refitted separately within each randomised group. Finally, instrumental variable (IV) methods were used to estimate the associations of interest free from confounding, separately for analyses (a) and (b); models used the multiplicative generalised method of moments estimation (the ivpoisson command in Stata).
Meta-analysis
A meta-analysis was performed analysing mortality from TITRe2 and all other RCTs that have compared liberal and restrictive red blood cell transfusion strategies in patients undergoing cardiac surgery. This analysis was undertaken to place the findings of TITRe2 in the context of the evidence base. Eligible RCTs24–26,37,38 were identified from a previous review of RCTs comparing restrictive versus liberal transfusion thresholds12 and an on-going review comparing RCT and observational evidence about the effects of red blood cell transfusion in cardiac surgery patients. 39 The previous Cochrane review searched multiple databases including the Cochrane Injuries Group’s Specialised Register, the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE and ISI Web of Science (both the Science and Conference Proceedings Citation Indices). The review included RCTs with a concurrent control group in which participants were assigned to groups with different transfusion triggers or thresholds, for which the thresholds were defined by a haemoglobin or haematocrit level that a participant had to reach before a red blood cell transfusion could be administered. For the purposes of the current meta-analysis, we included RCTs identified from either review that were deemed to have taken place in the context of cardiac surgery. Therefore, we included RCTs with different group-specific transfusion thresholds to those used in TITRe2 and which included all participants, irrespective of whether or not the liberal threshold was breached (i.e. without the post-operative eligibility criterion adopted in the TITRe2 trial). When writing the SAP, we also intended to perform a meta-analysis for the primary outcome; however, outcomes were too dissimilar between included RCTs. The meta-analysis was performed using standard meta-analysis methods for binary outcomes with a random effects model.
Missing data
Missing data are indicated in all of the tables. Rules for imputing missing data were outlined in the analysis plan, dependent on the level of missing data. However, the majority of outcomes had levels of missing data below the defined thresholds in the plan (5% for outcomes measured at one time point and 20% for longitudinal data) and imputation methods were not generally used. The first exception was for the infectious events secondary outcome (5.6% missing), whereby separate estimates were made prior to hospital discharge and overall. A second exception was the in-hospital component of the ASEPSIS score from which wound infection events were identified; this was one of the rarer components of the primary outcome but the in-hospital component of the score was the outcome data item that was most likely to be missing. If the in-hospital ASEPSIS score was missing, the participant was assumed not to have had a serious wound infection if the following criteria were met: participant did not have antibiotics for suspected wound infection prescribed in hospital and follow-up was completed, and the participant reported no problems with the healing of chest, leg and/or arm wound up to 3 months after the operation.
Significance levels
For hypothesis tests, two-tailed p-values of < 0.05 were considered statistically significant, with the exception of tests for interactions between group and time in longitudinal models when a 10% significance level was used. Likelihood ratio tests were used in preference to Wald tests. No formal adjustment was made for multiple testing. When interpreting the results, consideration has been given to the number of tests performed and the consistency, magnitude and direction of estimates for different outcomes. 40 All data management and analyses were performed in SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) or Stata version 12.1 or 13.1 (StataCorp LP, College Station, TX, USA).
Health economics
Aims and objectives
The economic evaluation aimed to estimate the cost-effectiveness of the restrictive compared with the liberal haemoglobin transfusion threshold as compared in TITRe2. Our main objective was to estimate the incremental cost and the incremental cost-effectiveness of the restrictive compared with the liberal haemoglobin transfusion threshold after cardiac surgery.
Economic evaluation methods overview
A cost–utility analysis was conducted, with outcomes measured using the EQ-5D-3L. The restrictive haemoglobin threshold was considered as cost-effective if the incremental cost-effectiveness ratio (ICER) fell below £20,000, which is generally considered as the threshold at which the National Institute for Health and Care Excellence (NICE) considers an intervention to be cost-effective. 41 Good practice guidelines on the conduct of economic evaluations were followed for the economic evaluation. 42–44 Table 4 summarises the methods for the economic evaluation, with further details provided in the text following the table.
| Aspect of methodology | Strategy used in base-case analysis |
|---|---|
| Form of economic evaluation | Cost–utility analysis for comparison between restrictive and liberal transfusion thresholds |
| Perspective | NHS and Personal Social Services |
| Time horizon | A within-trial analysis, taking a 3-month time horizon (up to the primary clinical time point) |
| Population | All randomised participants were included, except those randomised in error |
| Costs included in analysis | Index admission |
|
|
| Post discharge | |
|
|
| Utility measurement (primary economic outcome) | EQ-5D-3L (administered pre-operatively and at 6 weeks and 3 months postoperatively) |
| QALY calculations | Assume that participants’ utility changes linearly between utility measurements |
| Adjustment for baseline utility | Regression used to adjust QALY calculations for differences in baseline utility |
| Missing data | Mean imputation and multiple imputation |
Form of analysis and primary outcome measure
The primary outcome measure for the economic evaluation was QALYs, as advocated by NICE. 44 This outcome combines quantity and quality of life into a single measure. Our evaluation took the form of a cost–utility analysis in which the difference in mean costs between the two transfusion threshold groups is divided by the difference in mean QALYs between the two groups to calculate an ICER and, specifically, the incremental cost per QALY gained by switching from using a liberal threshold to using a restrictive threshold.
Perspective
The primary perspective of the evaluation was that of the UK NHS and Personal Social Services, as recommended by NICE. 44 However, data were collected on some types of non-NHS costs including expenditure incurred by a participant when travelling to hospital. We planned to include these costs in a wider perspective in a sensitivity analysis, if resource use for these non-NHS costs differed between the trial groups. The perspective for outcomes was that of the participants undergoing treatment.
Time horizon
A within-trial analysis, taking a 3-month time horizon, was conducted. It was anticipated that all major resource use would occur within this timeframe and, therefore, be captured. The start of our analysis was from the point of surgery. Surgery was chosen as the time origin, rather than the point of randomisation as was the case with the analysis of effectiveness, in order to capture the resources that would be required for the intervention from a decision-maker’s perspective, that is, to include all relevant costs (and effects) involved in delivering an intervention. Our time horizon continued until 3 months postoperatively. Ideally, the time point for baseline costs and outcomes should be the same; however, the EQ-5D-3L was collected pre-operatively whereas detailed resource use collection began on the day of surgery.
Population
Our base-case analysis included all participants randomised into the trial except those randomised in error, which is consistent with the main effectiveness analyses. Analyses were performed on an ITT basis.
Collection of resource use and cost data
Resource use data were collected on all significant health service resource inputs for the trial participants up to the point of the 3-month follow-up. The main resource use categories that were costed are listed in the first column of Table 5, along with the sources of information for both the resource use and unit costs. Costing decisions (such as resource use assumed for complications) were made without knowledge of the allocation of participants to trial groups.
| Resource category | Sources for resource use informationa | Sources for unit cost information |
|---|---|---|
| Initial cardiac surgery | CRF C1 | National Schedule of Reference Costs (2012–13);45 NHSBT National Comparative Audit of Blood Transfusion;5 NHSBT price list46 |
| Blood products | CRFs B1, B2 | NHSBT price list;46 primary data collection for the costs of administering blood products (further details in Appendix 3, Table 60) |
| Initial stay in hospital post surgery | CRFs D1, H5 | National Schedule of Reference Costs (2012–13)45 |
| Medications | CRF D2 | eMIT;47 BNF48 |
| Complications, including re-operations; SAEs | CRFs C1, C2–C4, C5, C6, C7, F1–F3, H5, X1 | National Schedule of Reference Costs (2012–13);45 eMIT;47 BNF48 |
| Hospital readmissions | CRF X1, 3-month follow-up questionnaire – section 2a | National Schedule of Reference Costs (2012–13)45 |
| Outpatient attendances and visits to ED | 3-month follow-up questionnaire – sections 2b, 2c | National Schedule of Reference Costs (2012–13)45 |
| Community health and social care contacts | 3-month follow-up questionnaire – section 3 | National Schedule of Reference Costs (2012–13);45 Unit Costs of Health and Social Care49 |
Initial cardiac surgery and blood products
As the type of surgery itself was not the main factor being assessed within the trial, we used published cardiac surgery costs rather than performing a detailed microcosting. We used cost figures from the cardiac surgery Healthcare Resource Group (HRG) codes from the elective inpatient spreadsheet in the National Reference Costs database45 and subtracted costs relating to length of stay (LOS) and blood products to calculate the cost of the surgery itself.
The LOS in hospital was removed by using the average LOS associated with each HRG and each specialty (cardiac surgery or cardiothoracic surgery) at a cost of £392 per day; this cost is a weighted average of elective inpatient excess bed-days for relevant cardiac procedures (see Appendix 3, Table 60 for further details). For valve surgery, there were HRG codes for single-valve procedures and for procedures on more than one valve. The costs are higher for procedures involving more than one valve; 25% of the activity reported in Reference Costs was for procedures involving multiple valves45 but in TITRe2 this proportion is only 10%. To reflect this fact, we created a weighted average of the costs of single- and multiple-valve procedures, with the weighting being according to the proportion of these types of participants recruited to TITRe2.
The costs of blood products (red blood cells, FFP and platelets) were removed from HRG costs by using the average numbers of products reported to be used by CABG, valve, and CABG and valve patients in the NHS Blood and Transplant (NHSBT) national audit in 2011,5 and valued using published NHSBT prices. For our surgery category of ‘other’, the costs of average blood products were removed by applying the information used for CABG and valve participants because the average operation time for ‘other’ was lengthy and most similar to the CABG and valve group.
The total number of red blood cells transfused each day was recorded on the trial CRFs. The costs of administering red blood cell transfusions were added to the costs of the units of red blood cells. The costs of administering transfusions were based on primary data collection of the nursing time and consumables associated with administering transfusions collected by the authors as part of another study (see Appendix 3, Table 60 for more information).
Initial post-surgery hospital stay
In terms of hospital stay following the actual surgery, LOS was collected for CICU/HDU, general ICU and ward during the trial. As time spent on CICU was not reported separately from time spent on HDU, and recognising that these activities probably require a different level of resources, the time of extubation was used to distinguish between time on CICU and time on HDU. Participants had an initial extubation date and time recorded in the trial, along with the dates and times of any further intubations and extubations. If data were missing on extubation date/time, we assumed that for those who died before discharge, that they were intubated until death. For participants without a tracheostomy, and no indication that they were not extubated, we assumed an average intubation duration, based on information from participants with available data. For participants who went on to have a tracheostomy, we calculated their time to tracheostomy and time to discharge and assumed the average intubation time for participants with intubation durations between these two times. Similar assumptions were made for any reintubations. CICU and HDU costs were taken from NHS Reference Costs. 45 To cost time on a cardiac ward, an average bed-day cost was created by weighting the cost of relevant cardiac procedures excess bed-day costs according to activity from the elective inpatient spreadsheet in Reference Costs45 (see Appendix 3, Table 60 for further details).
Medications and fluids
Medications and fluids given during surgery or intensive care, such as inotropes, were costed for each participant. Information on whether or not participants received these medications were collected on pre-specified yes/no tick boxes on the trial CRFs (Form D2). In order to cost these interventions, a member of the trial research team provided an estimate of the likely quantity of fluids a participant would receive. The costs of antibiotics administered after surgery for an infection were summed during the period of initial hospital stay post surgery and during any hospital stay after discharge if participants were readmitted (up to 3 months). The names of specific antibiotics were reported on the trial CRF (Form C5) as free text with the route and duration of the course. We established the most likely dose with the TITRe2 research team. If information was missing on the route of administration (oral or intravenous) or frequency of drugs, we clarified this information with the trial research team and conducted sensitivity analyses around alternative scenarios and drug costs for antibiotic treatment. The costs of antibiotics were included in the costs of complications.
In addition, the regular medications that participants were taking, such as beta-blockers, statins and warfarin, were recorded as on the medication or not by pre-specified tick boxes (yes/no) on CRF Form D2. This was recorded for two time points: at baseline – on admission to the cardiac surgery unit – and at discharge from the cardiac surgery unit. A member of the trial research team estimated the name, dose and mode of delivery (oral or intravenous) for the regular medications that participants were taking at baseline and discharge. We assumed that participants took any medications recorded at discharge for the 3-month follow-up and costed these medications for 3 months. In a separate analysis, we also costed the regular medications participants were taking at baseline and at discharge for a period of one week. Comparisons were then made between the costs of these medications taken at baseline and at discharge from hospital, to determine whether or not there were significant changes in this resource before and after surgery.
Treatment complications and serious adverse events
Primary outcome complications that were costed included serious infection, permanent stroke, MI, gut infarction and AKI. Details of these complications were recorded on CRF Forms C5 and C6. We also included the costs of any procedures or tests required to verify the complications, such as computerised tomography (CT) or magnetic resonance imaging (MRI) scans for permanent stroke, laparotomy for gut infarction and ECG for suspected MIs. For all participants suspected to have had a MI, the costs of diagnostic investigations were included. The costs of all other post-operative complications recorded on CRF C7 were calculated; examples include pacing (both temporary and permanent pacing), CPAP ventilation, tracheostomy and transient ischaemic attack. Cardiac surgery reoperations were also included in complication costs. Care was taken to avoid double counting of complication costs. For example, resource use associated with both ARDS and reintubation was assumed to be a transoesophageal echo and three chest X-rays. If a participant had both complications on the same day, only one echo and three chest X-rays were costed to avoid probable double counting. The trial CRFs were used to gather the types and amounts of complications the participants had experienced and also to capture resource use around SAEs. SAEs were individually reviewed and additional resources were costed if not already captured in complication costs, again to avoid double counting. Tables 64, 65, 67 and 68 in Appendix 3 show all the complications, the corresponding diagnostic tests and treatments assumed, and their unit costs.
Hospital readmissions
The costs of hospital readmissions include all expected and unexpected cardiac surgery and transfusion complications, in terms of AEs and SAEs, but excluded all unexpected unrelated complications. For example, our analysis included the cost of readmissions for hypertension and angina, but excluded the cost of readmissions for cancer treatment. Clinical opinion was sought to clarify whether unexpected complications were possibly related or were unrelated to the index surgery. A bed-day cost for readmissions was created by weighting the non-elective inpatient excess bed-days across all specialties according to activity. The cost of an ED attendance was included if a participant was admitted via ED or referred by their GP (and assumed to be admitted via ED). If participants travelled to hospital via ambulance, this was also costed.
Outpatient attendances, emergency department visits and community health and social care contacts
The type of outpatient appointment was recorded by pre-specified tick boxes on the trial follow-up questionnaire [section 2(c)] which include cardiac surgery, cardiology (non-surgical), renal/dialysis unit, stroke clinic or ‘other’. If participants specified ‘other’, we discussed with the trial research team whether or not these were likely to be linked to the surgery, in order to avoid costing any outpatient visits that were totally unlinked to the trial. Information on the number of ED visits related to the surgery and the reasons for the visits was captured on the trial CRF [section 2(b) of the follow-up questionnaire]. The reasons for visits recorded by participants were reviewed and any unrelated activity excluded. Information was also collected on how the participant travelled to ED to ensure any ambulance costs were captured. Primary care contacts with GPs and practice nurses, whether at the GP surgery or participant’s home, were costed. Other NHS or social services visits at home or elsewhere, including any visits to cardiac rehabilitation clinics or warfarin clinics, were also costed using information collected on the trial follow-up questionnaires (see section 3 of the questionnaire).
Attaching unit costs to resource use
Unit costs for hospital and community health-care resource use were largely obtained from national sources, for example, NHSBT price lists for blood products, the National Schedule of Reference Costs for ICU, HDU and cardiac ward costs, MRI and CT scans and many complications, and Unit Costs of Health and Social Care for community costs. 45,46,49 Resources were valued in 2012/13 pounds sterling (£); if any unit costs were in pre-2012/13 prices, they have been inflated to 2012/13 using the Hospital and Community Health Services (HCHS) inflation index. 49 Costs of drugs given in hospital were taken from the electronic marketing information tool (eMIT)47 when possible, which provides the reduced prices paid for generic drugs in hospital; other drug costs were taken from the British National Formulary (BNF). 48 Tables 63 and 64 in Appendix 3 lists all the medications and their costs used for the trial; further details on all unit costs and their source can be found in Appendix 3, Unit costs and resource use assumed for complications.
Measurement of health-related quality of life and quality-adjusted life-years
Measurement of health-related quality of life
The EQ-5D-3L questionnaire, advocated for use in economic evaluations by NICE,44 was used to measure health-related quality of life. 31 The EQ-5D-3L is a generic measure of health outcome covering five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Responses recorded on the instrument are converted into a single-index value using the UK valuation set, valuations from approximately 3000 members of the UK general population elicited using the time trade-off method;50 scores are then used to facilitate the calculation of QALYs in health economic evaluations. The EQ-5D-3L was used for TITRe2 as the 5-level version was not available at the start of the trial. Our trial participants completed the EQ-5D-3L questionnaire at three time points: in hospital pre-operatively and by post/telephone at 6 weeks and 3 months postoperatively.
Calculation of quality-adjusted life-years
The QALY profile for each participant up to 3 months postoperatively was estimated and the area under the curve of utility measurements used to calculate the number of QALYs accrued by each participant. QALYs were calculated assuming that each participant’s utility changed linearly between each of the time points (pre-operatively, 6 weeks and 3 months postoperatively). For participants who died during the trial, their utility was assumed to change linearly between the preceding time point and the time of death, and a value of zero was given to participants from death onwards.
Total QALYs gained were calculated for each participant by adding together QALYs gained from baseline to 6 weeks and from 6 weeks to 3 months. QALYs gained from baseline to 6 weeks were calculated by averaging a participant’s EQ-5D-3L scores at baseline and 6 weeks and multiplying by 42 days (or number of days until death if this was within 42 days). QALYs gained from 6 weeks to 3 months were calculated in a similar way. Once total QALYs gained were calculated for all participants, the average QALY gain for participants in each group was calculated. Alternative assumptions regarding the analysis of QALYs were investigated in sensitivity analyses, such as using the date of completion of the 6-week EQ-5D-3L rather than assuming this was at 42 days.
Missing data
We first summarised descriptively the volume of missing data for both resource use and EQ-5D-3L scores, which showed that 2.5% of resource use data were completely missing: 2.4% in the restrictive group and 2.5% in the liberal group. Overall, 10.7% of EQ-5D-3L scores were missing across the three time points (pre-surgery, 6 weeks and 3 months); 10.9% in the restrictive group and 10.5% in the liberal group. Although the level of missing data on resource use sounds small, because there are a large number of resource use variables for the trial, any simple methods to deal with the missing data would work poorly. For instance, using complete case analysis would leave only 61% of participants remaining for analysis. Multiple imputation was used to handle this missing data.
When data were partially missing, for example for linked questions for which only the first part was answered, mean imputation was used to handle such missing data. This occurred when a resource use question was in two parts: if participants were asked to respond yes/no to whether or not they used a particular resource (e.g. if they were readmitted to hospital) and then if yes, to record further details on the volume of resource use (e.g. the number of days they were readmitted, or the number of visits). Further details of mean and multiple imputation are described next.
Mean imputation for partially missing data
When data were partially missing, mean imputation was used. For example, if participants reported a readmission to hospital, but information on the LOS was missing, a mean LOS across all readmissions was calculated and this mean was then imputed if data were missing. Similarly if a participant reported GP visits, but did not record the number of visits, the mean number of visits from other participants was calculated and then imputed for those participants whose data were missing. This approach was also used to complete some of the intubation durations.
There were a number of dates and times recorded on the CRFs for events such as extubation, reintubation and re-extubation and movements between wards. If the exact time of an event was unknown, there was provision on the CRFs to record an approximate time of morning, afternoon or overnight. If only an approximate time was available, we used the same assumptions as were made in the effectiveness analyses, which were based on discussions with the research nurses. For calculations involving the date and time of hospital discharge, a discharge time of 18:00 was assumed (consistent with the effectiveness analyses).
Multiple imputation for missing data
Multiple imputation using a series of chained regression equations was used to impute missing resource use and EQ-5D-3L data. Following recent guidelines,51 multiple imputation using chained equations was conducted using the mi command in Stata. Multiple imputation uses regression to predict m-values for each missing data cell (m is often 5 and was here), and enables all variables used in the economic evaluation and demographic data (both complete and incomplete) to be used to predict the values of missing data cells.
Missing resource use data were imputed in two stages. First, the missing data within inpatient resource use were imputed based on the available inpatient resource use data together with independent variables: centre, sex, treatment group, age at operation and cardiac procedure (as four categories) in the regression equations; second, all the post-discharge resource use with missing data were imputed on the same independent variables with the addition of total LOS. All readmission variables, including ICU days, complications and SAEs, were imputed conditional on readmission LOS being greater than zero; that is, only participants who had a readmission could then have complications in the follow-up period. Indicator variables for SAEs included in the imputation only counted participants for whom we attached a cost to their SAE. Missing EQ-5D-3L data were imputed based on the available EQ-5D-3L scores at each of the three time points together with independent variables: centre, sex, treatment group, age at operation, cardiac procedure and total costs. Finally, Rubin’s Rule was used to summarise data across the m datasets. 52 This approach accounts for the variability both within and between imputed datasets and takes uncertainty in the estimated mean into account.
Adjustment for baseline utility
Given that baseline utility directly contributes to QALY calculations, it is important to control for any potential imbalances in baseline utility in the estimation of the mean difference in QALYs between treatment groups, to avoid introducing bias. 53 Regression adjustment also allows for regression to the mean and increases precision. Therefore, we adjusted our QALYs for baseline EQ-5D-3L. For each of the five imputed datasets, we regressed total QALYs on treatment group and baseline EQ-5D-3L and used the Stata command, mi estimate, to combine the five imputed datasets, and used Rubin’s Rule to combine the standard errors (SEs) across the five imputations. This provided an estimate of the QALY difference and its SE between the trial groups, adjusted for baseline EQ-5D-3L. The Stata command nlcom was then used to combine regression coefficients to obtain the mean QALYs in each trial group, adjusted for baseline EQ-5D-3L.
Within-trial statistical analysis of cost-effectiveness results
Most of the cost-effectiveness analyses were conducted in Stata version 12; some of the graphs and unit cost calculations were conducted in Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA).
Initially, resource use, costs and health-related quality of life were summarised using means, SDs and SEs of means, using both the central limit theorem and bootstrapping. ICERs were derived from the average costs and QALYs gained in each trial group, producing an incremental cost per QALY gained by implementing a restrictive threshold in place of a liberal threshold. Non-parametric bootstrapping of costs and QALYs was then used to quantify the degree of uncertainty around the ICER. Bootstrapping was used to avoid making parametric assumptions.
A thousand bootstrap samples were drawn for each of the five imputed datasets. For each bootstrap sample for each imputation, total costs were regressed on treatment group and total QALYs were regressed on treatment group and baseline EQ-5D-3L. The mean cost difference between the groups (restrictive minus liberal) was calculated, as well as the mean QALY difference between the groups adjusted for baseline EQ-5D-3L.
These 5000 bootstrap replicates of the mean difference in costs and QALYs between the groups were used to represent graphically the uncertainty around the ICER on the cost-effectiveness plane. In order that the points could be seen, only 1000 replicates were plotted (200 replicates for each of the five imputations).
For each of the five imputations, the mean and SD of the 1000 cost and QALY differences were estimated. These SDs are SDs of a column of means, so are actually SEs. Rubin’s Rule was then used to combine the SEs across the five imputed datasets. CIs around the cost and QALY differences were then generated based on these SEs rather than SEs based on parametric methods.
Results are expressed in terms of a cost-effectiveness acceptability curve (CEAC), which indicates the likelihood that the restrictive threshold is cost-effective for different levels that health-care decision-makers are willing to pay for health gain. All 5000 bootstrap replicates were used to generate the CEAC. The restrictive threshold would be considered as cost-effective if the ICER falls below £20,000; however, the ICERs and CEACs presented would allow decision-makers to assess cost-effectiveness at a willingness-to-pay threshold of their choice.
Discounting
Costs and effects were not discounted as our time horizon was < 12 months.
Sensitivity analysis
One-way sensitivity analysis was used to investigate the impact on the results of the cost and cost-effectiveness analyses when varying key parameters or major cost drivers and also to investigate the impact of uncertainty on the cost-effectiveness results. Factors that were examined in the sensitivity analysis for costing were:
-
varying the unit costs of treatment of complications, ward stays, reoperations, expensive drugs, oral versus intravenous drug administration and the source of medication unit costs (BNF vs. eMIT) (see Appendix 3, Sensitivity analyses around unit costs)
-
conducting the costing from the point of randomisation rather than the point of surgery as undertaken in the baseline analysis (further details in Appendix 3, Costs from randomisation)
-
exploring the impact of any high-cost participants (outliers) if the cost data are skewed.
Non-NHS costs were also considered and analyses were conducted to determine whether or not these differed between the trial groups and hence whether or not there was a need to conduct a sensitivity analysis from a wider societal perspective (instead of a NHS and Personal Social Services perceptive).
For the sensitivity analysis on outcomes, we varied the assumptions for calculating QALYs; the alternative strategies examined were:
-
not adjusting for baseline utility
-
exploring the use of the last observation carried forward until death rather than assuming utility changes linearly until death
-
using the date of completion of the 6-week EQ-5D-3L rather than assuming it is completed at exactly 6 weeks
-
calculating QALYs from the point of randomisation rather than surgery.
Finally, we carried out a sensitivity analysis examining life-years gained as a secondary outcome measure in the economic evaluation. Previous economic evaluations conducted by the authors have often found that EQ-5D-3L scores are similar across trial groups. 54 Such a finding could reflect reality but could also be a function of the 3-level version of the EQ-5D-3L not being sufficiently sensitivity to changes in quality of life, or that quality-of-life improvements arise before EQ-5D-3L measurements (failing to capture periods of lower quality of life), especially following SAEs.
Subgroup analysis
Subgroup analyses were conducted to investigate whether or not cost-effectiveness results varied between participant subgroups. The pre-specified subgroups used for the effectiveness analyses were used for the cost-effectiveness subgroup analyses:
-
operation type (isolated CABG vs. other operation types)
-
age at operation (< 75 years vs. ≥ 75 years)
-
pre-operative diagnosis of diabetes (none vs. diet, oral medication or insulin controlled)
-
pre-operative diagnosis of lung disease (none vs. chronic pulmonary disease or asthma)
-
pre-operative renal impairment (eGFR ≤ 60 ml/minute vs. eGFR > 60 ml/minute)
-
sex (males vs. females)
-
pre-operative ventricular function (good vs. moderate or poor).
The impact of subgroups was evaluated using ordinary least squares regression predicting total costs and QALYs, conditional on treatment group, subgroup and an interaction between treatment group and subgroup (and baseline EQ-5D-3L for QALYs only). A Bonferroni adjustment was made to allow for the multiple tests conducted across the seven subgroups and two variables; statistical significance was therefore evaluated at the 0.0036 level.
Patient and public involvement
At the time of formulating the research question for TITRe2 and deciding to apply for funding, information about progress on the pilot study26 and the proposed trial was presented to the Research Advisory Group of the Bristol Heart Institute. This group comprised members of the public who are stakeholders in the use of, or delivery of, health care and health-care research, including patients and potential patients, those who commission or deliver health-care services and a representative of the British Heart Foundation. The group agreed that it was important for patients and the NHS to answer the research question and supported our proposal for the trial.
The trial recruited patients who had moderate to high levels of anxiety before surgery because of the life-threatening nature of their condition and the operation, and took place in a particularly acute care setting. Although patients had full capacity when they were approached about the trial, about 90% were randomised and first received the intervention when they were on the ICU or HDU, when they were likely to be artificially ventilated or sedated. In terms of the conduct of the trial, most patient and public involvement (PPI) occurred through the representative on the TSC, Karin Smyth. At the time of her appointment to the TSC, she had recently been a non-executive director of Bristol North Primary Care Trust and a lay/patient representative on the Research Advisory Group.
Information about the trial used when approaching patients to take part was developed with input from patients who had had cardiac surgery previously, both initially and when the information was revised, in order to try to better communicate the possible benefits and risks of withholding or giving extra transfusions. We also consulted a group of past patients when we were considering the option of obtaining follow-up information by postal questionnaire, as well as by telephone. This option was raised when staff in the trials unit were having to spend large amounts of time carrying out telephone follow-ups. We had not budgeted for such a large amount of time and there was a risk that either a backlog of follow-up information would build up or other trial-related tasks would be delayed. We particularly valued the involvement of this group of patients in endorsing the principle that postal follow-up would be an acceptable alternative and in optimising the format of the questionnaire for self-completion by participants, which we believe contributed to the completeness of follow-up information. We are currently involving patients and lay representatives in disseminating information about the results of the trial to participants.
The lay representative of the TSC played an important role towards the end of the trial when there was some concern about emerging findings from the trial based on the data available at the time. Having reassurance from both lay and professional members of the TSC was vital at this time in ensuring successful completion of the trial as planned.
We also would like to draw attention to the reciprocal benefits that PPI can contribute. With her permission, we are reproducing comments that Karin Smyth made spontaneously about her membership of the TSC.
I wanted to put on record my appreciation of being involved in this trial. As I have found in my own work the role of a ‘lay person’ is a peculiar and ill-defined one. When Gavin [Professor Murphy] asked me to be involved it was as someone who had commissioning and health care management experience but who was not, at that point, working in the NHS and could be a lay person. The science has often been beyond my own understanding but I am grateful for your patience and explanation when that was the case. I was made to feel a full part of the team.
Karin Smyth, reproduced with permission
Contractual and financial arrangements
When applying for funding, we chose to adopt a fee-per-participant payment model in order to reimburse the research costs incurred by participating centres in taking part. These research costs arose primarily from the need to collect data during participants’ index admissions but also from the time spent by local research teams helping with collection of follow-up data, for example if a participant was readmitted to a participating centre or a nearby referring hospital after discharge. We preferred this model to one in which each participating centre is given a set amount of funding, for example to employ a part-time research nurse, because it created an incentive for centres to recruit and randomise participants, and contained local research costs.
We developed a spreadsheet to estimate the total locally incurred costs (i.e. for the total target sample size), estimating the amount of a consultant’s, research nurse’s and clerical person’s time per participant needed to identify, approach and consent patients and collect the data required. The spreadsheet took into account the different numbers of patients/participants at each stage of the recruitment process; we projected that 6000 patients would need to be identified, 5000 approached, 3000 consented and registered, and 2000 randomised. Items in the spreadsheet were then classified as research or service support activities. Most but not all of the activities prior to randomisation were considered to represent service support (i.e. approaching and consenting patients, including discussion with a clinician). Therefore, for simplicity, we estimated the fee-per-participant for randomised participants only (including the costs of pre-randomisation tasks within this amount, averaged per randomised participant). The total came to £260 per randomised participant, divided into £100 for service support costs and £160 for local research costs. The latter total included 0.25 hours of consultant time (reviewing and signing SAE forms), 7.75 hours of research nurse time (collecting data, communicating with the participants and usual-care staff and responding to data queries from the coordinating centre) and 2.75 hours of clerical time (primarily entering data into the database).
On the basis of our original assumption that a centre would randomise eight participants, on average, per month, we expected the corresponding income [8 × 12 × (£160 + £100) = £24,960] to generate sufficient research income to pay for approximately 0.6 full time equivalents of a research nurse and the appropriate amounts of consultant and clerical time.
This payment structure was implemented through the contracts (using the model non-commercial agreement) between the Sponsor (University Hospitals of Bristol NHS Foundation Trust) and sites. (A separate contract was in place between the Sponsor and the University of Bristol, which held the grant.) The contract specified that payments would be made in two parts: £120 ‘Upon receipt of complete and accurate data following participant discharge, including documentary evidence of qualifying or suspected qualifying events for the primary outcome as specified in the protocol and case report form’ and an additional £40 ‘Upon receipt of additional data required as a result of 3-month-follow-up (e.g. response to queries on follow-up or SAEs)’. The trial database kept track of data submitted, payments due and payments already invoiced. A query was run quarterly to generate an itemised activity report for each site detailing the payment due. This report formed the basis for an invoice to the University of Bristol, which held the grant.
Chapter 3 Trial cohort
Screened patients
Screening data were provided for 11,483 patients at 17 UK centres (Figure 2). A total of 7918 screened patients were excluded from the study: 3055 were not sent a PIL; 1863 were not approached; 281 were ineligible; and 2719 did not consent. In addition, there were 696 participants who were not sent a PIL and 118 participants who were not approached, as they were already deemed ineligible when they were screened. Similarly, for 10 participants, the reason for non-consent was recorded as ineligibility and reasons are unknown for these participants. The most common reasons for ineligibility were: congenital or acquired platelet, red blood cell or clotting disorder (83 patients); and inability to give full informed consent (62 patients). Similarly, the most prevalent reasons for not consenting were: wanting the standard procedure (1053 patients) and personal reasons (678 patients). Therefore, 3565 participants (31.0% of those screened) consented to take part in the study, of whom 94 were not considered for randomisation (for reasons see Figure 2). Of the remaining 3471 participants, 2007 (57.8%) were randomised, 1004 to the restrictive group and 1003 to the liberal group.
FIGURE 2.
Flow of participants. a, These figures should be interpreted with caution owing to variable data quality between centres. The proportion of screened patients consented into the study varied between centres (range 11–90%). b, Only withdrawals in which participants were unwilling for data collected to be used or for follow-up to continue were treated as exclusions. Some participants withdrew from treatment but were willing for data collection to continue.
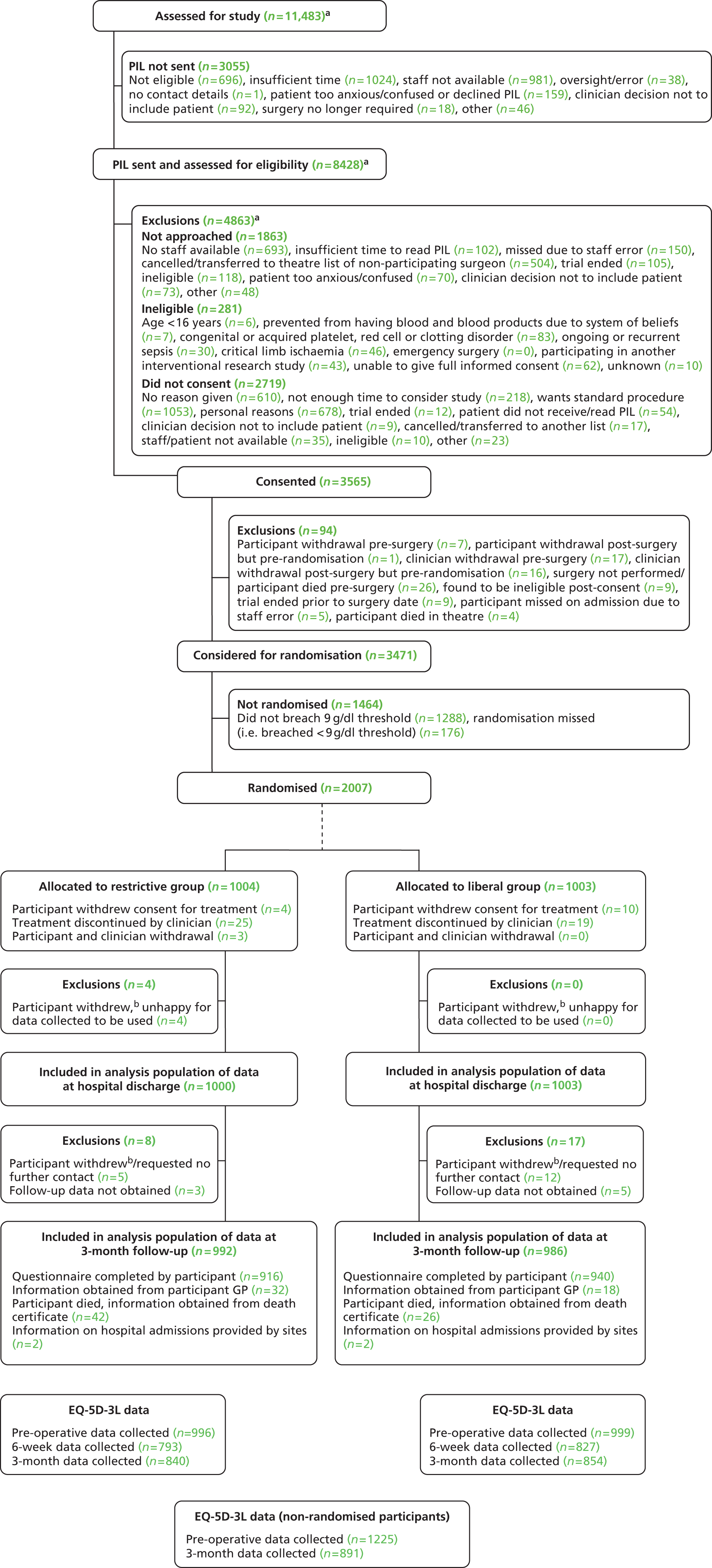
The numbers of patients screened, excluded from the study, consented and randomised are given in Table 6, demonstrating a large variation in screening rates. The percentage of screened patients consented into the study ranges from 11.1% to 90.0%, suggesting that quality in screening (or the completeness of recording screened patients in the log) was very variable between centres. Two centres (site A, consent rate 34.3%, and site H, consent rate 11.1%) were identified as using the screening log as intended, suggesting centres with higher consent rates were perhaps not screening all non-trial patients and, therefore, some of the variation is likely to have arisen from varying data completeness across centres up to the point of consent.
| Centre | Number of months recruiting into study | Screened | Excluded from study | Consented (% of screened patients) | Randomised (% of consented patients) | |||
|---|---|---|---|---|---|---|---|---|
| PIL not sent | Not approached | Ineligible | Did not consent | |||||
| Site A | 44 | 1690 | 50 | 250 | 128 | 683 | 579 (34.3) | 393 (67.9) |
| Site B | 35 | 819 | 64 | 240 | 6 | 135 | 374 (45.7) | 135 (36.1) |
| Site C | 40 | 1077 | 213 | 274 | 1 | 209 | 380 (35.3) | 142 (37.4) |
| Site D | 9 | 20 | 0 | 1 | 0 | 1 | 18 (90.0) | 8 (44.4) |
| Site E | 40 | 282 | 34 | 12 | 7 | 91 | 138 (48.9) | 76 (55.1) |
| Site F | 41 | 902 | 7 | 259 | 40 | 256 | 340 (37.7) | 224 (65.9) |
| Site G | 27 | 271 | 37 | 54 | 6 | 94 | 80 (29.5) | 47 (58.8) |
| Site H | 30 | 3067 | 2232 | 150 | 24 | 320 | 341 (11.1) | 179 (52.5) |
| Site I | 35 | 531 | 0 | 130 | 4 | 78 | 319 (60.1) | 147 (46.1) |
| Site J | 32 | 844 | 243 | 121 | 8 | 222 | 250 (29.6) | 157 (62.8) |
| Site K | 28 | 284 | 38 | 24 | 4 | 64 | 154 (54.2) | 134 (87.0) |
| Site L | 21 | 228 | 2 | 2 | 2 | 72 | 150 (65.8) | 54 (36.0) |
| Site M | 22 | 283 | 1 | 54 | 8 | 123 | 97 (34.3) | 56 (57.7) |
| Site N | 25 | 774 | 70 | 112 | 35 | 312 | 245 (31.7) | 183 (74.7) |
| Site O | 14 | 328 | 32 | 163 | 7 | 55 | 71 (21.6) | 51 (71.8) |
| Site P | 2 | 30 | 11 | 2 | 1 | 3 | 13 (43.3) | 12 (92.3) |
| Site Q | 3 | 53 | 21 | 15 | 0 | 1 | 16 (30.2) | 9 (56.3) |
| Total | 44 | 11483 | 3055 | 1863 | 281 | 2719 | 3565 (31.0) | 2007 (56.3) |
Furthermore, the percentages of consented patients that were randomised ranged from 36.0% to 92.3% (accepting that the latter percentage is based on a relatively small denominator). These differences are likely to have arisen from differences in clinical practice and case mix of patients between centres, as well as from the different strategies used by sites to target certain kinds of patient who were more likely to be randomised (such targeting was encouraged by the trial management team to maximise the yield of randomised participants among those who consented).
Recruitment
Participants were consented to the study between 13 July 2009 and 14 February 2013, and randomised between 15 July 2009 and 18 February 2013. Follow-up data for the last participant were collected 21 August 2013. The 2007 randomised participants were recruited from 17 centres (see Table 6).
Actual cumulative recruitment compared with the original and revised targets are shown in Figure 3. At the start of the study, recruitment was predicted as follows: (1) we expected two centres to start recruiting in month one, with a further two centres opening per month thereafter until eight centres were open; (2) we predicted five consented participants per month at centres in their first 2 months of recruitment, 10 consented participants per month at centres in months three and four of recruitment and 19 consented participants per centre per month thereafter; and (3) we also predicted that two-thirds of consented participants would be randomised.
FIGURE 3.
Recruitment over time. ‘Original recruitment predictions’ are as described in the original protocol. ‘Revised recruitment predictions (1)’ are using the assumptions in the original protocol (five consented participants per month for centres in months one and two of recruitment, 10 consented participants per month for centres in months three and four of recruitment, and 19 consented participants per month thereafter; two-thirds of consented participants would be randomised) applied to the actual numbers of centres recruiting. ‘Revised recruitment predictions (2)’ are as per the trial extension request and are based on the actual recruitment as of the end of October 2010 plus 60 randomised participants per month thereafter.

As the trial progressed, it became clear that these estimates were optimistic: (1) it took 1 year to set up the first eight sites to the point of starting recruitment instead of the 4 months we predicted, mainly due to delays with contracts but also due to other site-specific reasons; (2) the predicted numbers of consented participants per centre per month were not achieved at the majority of centres; and (3) the percentage of consented participants who were randomised was substantially lower than the predicted 66% (at the end of trial it was 56%). Extra centres (over the eight predicted) were opened to try to increase recruitment, but the targets were still not achieved.
Therefore, in November 2010 an extension request was made to the National Institute for Health Research (NIHR). This extension was based on actual cumulative recruitment to the end of October 2010 (399 randomised participants) and predicted that thereafter 60 randomised participants would be recruited each month across all centres. This request was granted and the trial was extended with a revised end of recruitment date of 31 January 2013. For the following 10 months, the new target was not met (a median of 50 participants per month were randomised); however, in September 2011 recruitment took an upward turn and the 60 randomised participants per month target was exceeded for the first time. Subsequently, recruitment remained above target at a median of 63 participants per month for the remainder of the trial. The highest monthly recruitment was achieved in August 2012 when 91 participants were randomised. The last participant was recruited in mid February 2013, just 2 weeks behind target.
Recruited patients
Very few data were collected about patients who did not take part in the study (Table 7). On average, patients who did not take part (for whatever reason) were older than participants who consented [median 71 years (IQR 62–77 years) vs. 68 years (IQR 61–75 years)] and were less likely to be male (66.7% vs. 75.4%).
| Characteristic | Excluded from study | Included in study (N = 3565) | |||
|---|---|---|---|---|---|
| PIL not sent (N = 3055) | Not approached (N = 1863) | Ineligible (N = 281) | Did not consent (N = 2719) | ||
| Age (years), median (IQR) | 71.0 (62.0–78.0) | 70.0 (61.0–76.0) | 71.0 (61.0–77.0) | 71.0 (63.0–77.0) | 68.0 (61.0–75.0) |
| Males, n (%) | 2077 (68.0) | 1303 (69.9) | 170 (60.5) | 1730 (63.6) | 2687 (75.4) |
Characteristics of (1) participants who consented but were not randomised and (2) randomised participants are described in Table 8. As anticipated (because they were by definition not anaemic), non-randomised participants were generally younger [median 66.5 years (IQR 59.5–73.1 years) vs. 70.3 years (IQR 63.5–76.4 years)], more likely to be male (84.8% vs. 68.5%), with a lower risk of perioperative mortality [median EuroSCORE of 4 (IQR 2–5) vs. 5 (IQR 3–7)] and a higher pre-operative haemoglobin [mean 14.4 g/dl (SD 1.3 g/dl) vs. 13.3 g/dl (SD 1.5 g/dl)]. Non-randomised participants were more likely to be having CABG surgery (54.6% vs. 40.6%) and were less likely to be transfused red blood cells (8.1% vs. 79.4%) or other blood products (FFP 6.6% vs. 29.0%, platelets 10.1% vs. 36.8%, cryoprecipitate 1.6% vs. 10.0%) intra-operatively and/or postoperatively. EQ-5D-3L scores were similar. A higher proportion of non-randomised participants were alive at hospital discharge (raw percentages, not taking differences in the composition of the subpopulations, were 99.3% vs. 97.9%). Haemoglobin levels were considerably higher for non-randomised participants than randomised participants (Figure 4), with differences being highest in the first 24 hours postoperatively.
| Characteristic | Consented not randomised (N = 1464) | Randomised (N = 2003) |
|---|---|---|
| Cardiac history | ||
| EuroSCORE,a median (IQR) | 4.0 (2.0–5.0) | 5.0 (3.0–7.0) |
| NYHA class, n/N (%) | ||
| I | 420/1426 (29.5) | 493/1951 (25.3) |
| II | 683/1426 (47.9) | 885/1951 (45.4) |
| III | 301/1426 (21.1) | 525/1951 (26.9) |
| IV | 22/1426 (1.5) | 48/1951 (2.5) |
| CCS class, n/N (%) | ||
| No angina | 437/1430 (30.6) | 718/1962 (36.6) |
| I | 260/1430 (18.2) | 362/1962 (18.5) |
| II | 451/1430 (31.5) | 526/1962 (26.8) |
| III | 236/1430 (16.5) | 281/1962 (14.3) |
| IV | 46/1430 (3.2) | 75/1962 (3.8) |
| Pacemaker, n/N (%) | ||
| No | 1420/1464 (97.0) | 1940/2002 (96.9) |
| Temporary | 10/1464 (0.7) | 10/2002 (0.5) |
| Permanent | 34/1464 (2.3) | 52/2002 (2.6) |
| Heart rhythm, n/N (%) | ||
| AF/flutter | 165/1463 (11.3) | 250/1998 (12.5) |
| Heart block | 14/1463 (1.0) | 43/1998 (2.2) |
| Sinus | 1284/1463 (87.8) | 1705/1998 (85.3) |
| Coronary disease, n/N (%) | ||
| None | 365/1462 (25.0) | 620/1991 (31.1) |
| Single vessel | 162/1462 (11.1) | 225/1991 (11.3) |
| Double vessel | 213/1462 (14.6) | 282/1991 (14.2) |
| Triple vessel | 660/1462 (45.1) | 805/1991 (40.4) |
| Not investigated | 62/1462 (4.2) | 59/1991 (3.0) |
| Disease in left main stem (> 50% stenosis), n/N (%) | 224/1450 (15.4) | 304/1977 (15.4) |
| Non-cardiac history | ||
| Age (years), median (IQR) | 66.5 (59.5–73.1) | 70.3 (63.5–76.4) |
| Males, n/N (%) | 1241/1464 (84.8) | 1373/2003 (68.5) |
| BMI (kg/m2),b mean (SD) | 29.5 (4.8) | 28.2 (4.9) |
| Urgent operative priority, n/N (%) | 132/1464 (9.0) | 245/2003 (12.2) |
| Diabetic, n/N (%) | ||
| No diabetes | 1191/1464 (81.4) | 1604/2003 (80.1) |
| Diet controlled | 54/1464 (3.7) | 69/2003 (3.4) |
| Insulin | 61/1464 (4.2) | 98/2003 (4.9) |
| Oral medication | 158/1464 (10.8) | 232/2003 (11.6) |
| Smoker, n/N (%) | ||
| Non-smoker | 668/1464 (45.6) | 928/2002 (46.4) |
| Ex-smoker (> 1 month) | 652/1464 (44.5) | 922/2002 (46.1) |
| Current smoker | 144/1464 (9.8) | 152/2002 (7.6) |
| Haemofiltration/dialysis, n/N (%) | 4/1464 (0.3) | 19/2001 (0.9) |
| CVA/TIA, n/N (%) | 108/1464 (7.4) | 163/2003 (8.1) |
| Pre-operative tests | ||
| Haemoglobin (g/dl), mean (SD) | 14.4 (1.3) | 13.3 (1.5) |
| eGFRb (ml/minute/1.73m2) median (IQR) | 85.7 (69.2–108) | 73.9 (56.8–93.2) |
| Medications | ||
| Intravenous nitrates until theatre, n/N (%) | 12/1463 (0.8) | 5/2002 (0.2) |
| Unfractionated intravenous heparin within 6 hours of surgery, n/N (%) | 17/1463 (1.2) | 19/2002 (0.9) |
| Low-molecular-weight heparin within 12 hours of surgery, n/N (%) | 14/1463 (1.0) | 23/2002 (1.1) |
| Inotropes until theatre, n/N (%) | 7/1463 (0.5) | 3/2002 (0.1) |
| Aspirin within 5 days of surgery, n/N (%) | 302/1462 (20.7) | 561/1999 (28.1) |
| Clopidogrel within 5 days of surgery, n/N (%) | 32/1463 (2.2) | 78/2000 (3.9) |
| Operative details | ||
| Cardiac procedure, n/N (%) | ||
| CABG only | 800/1464 (54.6) | 814/2003 (40.6) |
| Valve only | 382/1464 (26.1) | 597/2003 (29.8) |
| CABG and valve | 189/1464 (12.9) | 393/2003 (19.6) |
| Other | 93/1464 (6.4) | 199/2003 (9.9) |
| Alive at end of surgery, n/N (%) | 1464/1464 (100) | 2003/2003 (100) |
| Transfusions (intra-operative and postoperative) | ||
| Red blood cells, n/N (%) | 119/1464 (8.1) | 1591/2003 (79.4) |
| FFP, n/N (%) | 97/1464 (6.6) | 581/2003 (29.0) |
| Platelets, n/N (%) | 148/1464 (10.1) | 738/2003 (36.8) |
| Cryoprecipitate, n/N (%) | 23/1464 (1.6) | 201/2003 (10.0) |
| Activated factor VII used, n/N (%) | 3/1464 (0.2) | 12/2003 (0.6) |
| Beriplex used, n/N (%) | 52/1464 (3.6) | 100/2003 (5.0) |
| EQ-5D-3L scores | ||
| Pre-operative utility,c median (IQR) | 0.8 (0.7–1.0) | 0.8 (0.7–1.0) |
| 3-month post-operative utility,d median (IQR) | 0.8 (0.7–1.0) | 0.8 (0.7–1.0) |
| Pre-operative visual analogue score,e median (IQR) | 75.0 (60.0–85.0) | 70.0 (53.0–80.0) |
| 3-month post-operative visual analogue score,f median (IQR) | 80.0 (70.0–90.0) | 80.0 (70.0–90.0) |
| Mortality | ||
| Alive at hospital discharge, n/N (%) | 1454/1464 (99.3) | 1961/2003 (97.9) |
FIGURE 4.
Daily mean nadir haemoglobin levels for randomised/non-randomised participants. The numbers alongside the points are the numbers of participants contributing readings for that data point. The x-axis has an origin of the day of surgery for both groups.

Withdrawals
Participant withdrawals and clinician discontinuations of treatment are summarised in Table 9. Prior to randomisation there were eight participants who withdrew consent, seven of whom did so pre-operatively. There were also 33 pre-randomisation decisions by clinicians to discontinue treatment according to the allocation, 17 of which occurred pre-operatively. The most common reason for discontinuation was the participant’s condition (13 participants).
| Pre-randomisation | |||
|---|---|---|---|
| Withdrawal/discontinuation of treatment type | Total consented participants (n = 3565) | ||
| Participant withdrawals | 8 (0.2%) | ||
| Timing | |||
| Pre-surgery | 7 | ||
| Post surgery but pre-randomisation | 1 | ||
| Reason | |||
| After discussion with family decided to withdraw | 2 | ||
| Surgery rearranged and participant no longer happy to take part | 2 | ||
| Participant decided to take part in another study | 1 | ||
| No reason given | 3 | ||
| Clinician treatment discontinuations | 33 (0.9%) | ||
| Timing | |||
| Pre-surgery | 17 | ||
| Post surgery but pre-randomisation | 16 | ||
| Reason | |||
| Condition of participant | 13 | ||
| Complex procedure | 4 | ||
| Clinician wants haemoglobin at a specific level | 5 | ||
| Change of planned operation | 5 | ||
| Change of surgeon | 6 | ||
| Post randomisation but pre-hospital discharge | |||
| Withdrawl/discontinuation of treatment type | Randomised to restrictive threshold (n = 1004) | Randomised to liberal threshold (n = 1003) | Total randomised participants (N = 2007) |
| Participant withdrawals | 7 (0.7%) | 10 (1.0%) | 17 (0.8%) |
| Participant happy for data already collected to be used | 3 | 10 | 13 |
| Participant happy to participate in follow-up | 2 | 8 | 10 |
| Reason | |||
| Wants normal care | 2a | 0 | 2 |
| Post-operative problems, does not want further intervention | 2 | 2 | 4 |
| Unhappy with allocation | 0 | 1 | 1 |
| Does not want any more transfusions | 0 | 6 | 6 |
| Blood products given before and after surgery | 1b | 0 | 1 |
| Ineligibility (discovered post randomisation) | 1c | 0 | 1 |
| No reason given | 1 | 1 | 2 |
| Clinician treatment discontinuations | 28 (2.8%) | 19 (1.9%) | 47 (2.3%) |
| Reason | |||
| Participant too unstable/unwell | 16 | 6 | 22 |
| Clinician does not want participant to have any more blood | 1b | 7 | 8 |
| Clinician wants participant to have more blood | 1 | 0 | 1 |
| Clinician wants to transfuse at higher haemoglobin | 6a | 0 | 6 |
| Clinician wants to transfuse at lower haemoglobin | 1 | 1 | 2 |
| Participant already had many breaches of threshold | 0 | 2 | 2 |
| Reaction to blood | 0 | 1 | 1 |
| Continued participation would prolong hospital stay | 0 | 1 | 1 |
| Ineligibility (discovered post randomisation) | 1c | 0 | 1 |
| Clinical need (no further details given) | 1 | 0 | 1 |
| No reason given | 1 | 1 | 2 |
| Post-hospital discharge | |||
| Participant withdrawals | 4 (0.4%) | 10 (1.0%) | 14 (0.7%) |
| Reason | |||
| Participant too ill/had complications | 0 | 5 | 5 |
| Participant not contactable as lives abroad | 1 | 1 | 2 |
| Requests no further questionnaires/contact | 1 | 3 | 4 |
| No reason given | 2 | 1 | 3 |
A further 17 participants (seven in the restrictive group and 10 in the liberal group) withdrew consent after randomisation but before hospital discharge, of whom four were unhappy for data collected to be used (all in the restrictive group) and a further three were unhappy for follow-up to continue. The most common reason for withdrawal was that the participant did not want any more transfusions (six participants, all in the liberal group). Clinicians decided to discontinue treatment according to the allocation after randomisation for 47 participants (28 in the restrictive group and 19 in the liberal group), the most common reason being that the participant was too unstable/unwell (22 participants, 16 in the restrictive group and six in the liberal group). Making such a decision did not necessarily mean that the clinician had a definitive opinion about the transfusion needs of the participant but, often, simply that the clinician considered the additional uncertainty or constraint created by the randomised treatment allocation to be undesirable when managing some critically ill participants. Three participants both withdrew and had their treatment discontinued (all in the restrictive group). A further 14 participants withdrew after hospital discharge (four in the restrictive group and 10 in the liberal group).
Participant follow-up
Follow-up data at 3 months post randomisation were obtained for 1978 participants (992 in the restrictive group and 986 in the liberal group), 98.7% of the 2003 eligible participants (see Figure 2). The questionnaire was completed by 1856 participants and by the research team from information supplied by the participant’s GP for 50 participants. Relevant information was extracted from the death certificate for a further 68 participants who died. For the remaining four participants, information on hospital admissions only (which provided the required data to ascertain the primary outcome) was provided by sites. Of the 25 participants with no follow-up data, 17 had withdrawn consent or requested no further contact; the remaining eight were lost to follow-up.
For randomised participants, EQ-5D-3L data were collected for almost all participants (1995/2003) pre-operatively, for 1620/2003 (80.9%) at 6 weeks and 1694/2003 (84.6%) at 3 months post randomisation (see Figure 2). Of the 1464 participants considered for randomisation who were not randomised, EQ-5D-3L data were collected for 1225 participants (83.7%) pre-operatively and 891 participants (60.9%) at 3 months post randomisation.
Numbers analysed
The analysis population consisted of 2003 participants, that is the 2007 randomised participants excluding four withdrawn participants who were unhappy for their data to be used. Primary outcome data were available for 1906 participants (95.2%). For most of the secondary outcomes, very few data were missing with the exception of the infectious event component of the primary outcome and the EQ-5D-3L (see Participant follow-up). Post-operative complication data up to 3 months after randomisation were complete for 1982 participants (99.0%).
Baseline data and operative characteristics
Baseline characteristics are summarised in Table 10 and intraoperative characteristics in Table 11. The median additive EuroSCORE was 5 (IQR 3–7) and logistic EuroSCORE was 4.0 (IQR 2.2–7.2). The median age was 70.3 years (IQR 63.5–76.4 years) and 68.5% of participants were male. Just fewer than 20% of participants were diabetic and 12.2% required urgent operations (i.e. urgent operative priority). Pre-operative haemoglobin concentrations had a mean value of 13.3 g/dl (SD 1.5 g/dl) and the median eGFR was 73.8 ml/minute/1.73 m2 (IQR 56.8–93.2 ml/minute/1.73 m2). In terms of intraoperative characteristics, the median duration of operation was 4.0 hours (IQR 3.3–5.0 hours) and 95.1% of operations were performed using cardiopulmonary bypass (CPB). Most operations were either isolated CABG (40.7%) or valve (30.5%) procedures. Tranexamic acid was used in 80.7% of procedures. All pre-operative and intraoperative characteristics were generally well balanced between the two groups, although the logistic EuroSCORE was slightly higher in the liberal group than the restrictive group [median 4.3 (IQR 2.4–7.5) vs. 3.8 (IQR 2.1–7.0)]. Pre-randomisation red blood cell transfusions are described in Chapter 4 and EQ-5D-3L scores in Chapter 5.
| Characteristic | Randomised to restrictive threshold (N = 1000) | Randomised to liberal threshold (N = 1003) | Overall (N = 2003) |
|---|---|---|---|
| Cardiac history | |||
| Additive EuroSCORE,a median (IQR) | 5.0 (3.0–7.0) | 5.0 (3.0–7.0) | 5.0 (3.0–7.0) |
| Logistic EuroSCORE,a median (IQR) | 3.8 (2.1–7.0) | 4.3 (2.4–7.5) | 4.0 (2.2–7.2) |
| NYHA class, n/N (%) | |||
| I | 235/997 (24.1) | 258/974 (26.5) | 493/1951 (25.3) |
| II | 445/997 (45.5) | 440/974 (45.2) | 885/1951 (45.4) |
| III | 268/997 (27.4) | 257/974 (26.4) | 525/1951 (26.9) |
| IV | 29/997 (3.0) | 19/974 (2.0) | 48/1951 (2.5) |
| CCS class, n/N (%) | |||
| No angina | 365/982 (37.2) | 353/980 (36.0) | 718/1962 (36.6) |
| I | 169/982 (17.2) | 193/980 (19.7) | 362/1962 (18.5) |
| II | 273/982 (27.8) | 253/980 (25.8) | 526/1962 (26.8) |
| III | 139/982 (14.2) | 142/980 (14.5) | 281/1962 (14.3) |
| IV | 36/982 (3.7) | 39/980 (4.0) | 75/1962 (3.8) |
| Pacemaker, n/N (%) | |||
| No | 972/1000 (97.2) | 968/1002 (96.6) | 1940/2002 (96.9) |
| Temporary | 7/1000 (0.7) | 3/1002 (0.3) | 10/2002 (0.5) |
| Permanent | 21/1000 (2.1) | 31/1002 (3.1) | 52/2002 (2.6) |
| Heart rhythm, n/N (%) | |||
| AF/flutter | 119/997 (11.9) | 131/1001 (13.1) | 250/1998 (12.5) |
| Heart block | 18/997 (1.8) | 25/1001 (2.5) | 43/1998 (2.2) |
| Sinus | 860/997 (86.3) | 845/1001 (84.4) | 1705/1998 (85.3) |
| Coronary disease, n/N (%) | |||
| None | 310/993 (31.2) | 310/998 (31.1) | 620/1991 (31.1) |
| Single vessel | 112/993 (11.3) | 113/998 (11.3) | 225/1991 (11.3) |
| Double vessel | 132/993 (13.3) | 150/998 (15.0) | 282/1991 (14.2) |
| Triple vessel | 403/993 (40.6) | 402/998 (40.3) | 805/1991 (40.4) |
| Not investigated | 36/993 (3.6) | 23/998 (2.3) | 59/1991 (3.0) |
| Disease in left main stem (> 50% stenosis) | 159/987 (16.1) | 145/990 (14.6) | 304/1977 (15.4) |
| Non-cardiac history | |||
| Age (years), median (IQR) | 69.9 (63.1–76.0) | 70.8 (64.1–76.7) | 70.3 (63.5–76.4) |
| Males, n/N (%) | 693/1000 (69.3) | 680/1003 (67.8) | 1373/2003 (68.5) |
| BMI (kg/m2),b mean (SD) | 28.2 (5.0) | 28.2 (4.9) | 28.2 (4.9) |
| Urgent operative priority, n/N (%) | 126/1000 (12.6) | 119/1003 (11.9) | 245/2003 (12.2) |
| Diabetic, n/N (%) | |||
| No diabetes | 802/1000 (80.2) | 802/1003 (80.0) | 1604/2003 (80.1) |
| Diet controlled | 33/1000 (3.3) | 36/1003 (3.6) | 69/2003 (3.4) |
| Insulin | 49/1000 (4.9) | 49/1003 (4.9) | 98/2003 (4.9) |
| Oral medication | 116/1000 (11.6) | 116/1003 (11.6) | 232/2003 (11.6) |
| Smoker, n/N (%) | |||
| Non-smoker | 461/1000 (46.1) | 467/1002 (46.6) | 928/2002 (46.4) |
| Ex-smoker (> 1 month) | 472/1000 (47.2) | 450/1002 (44.9) | 922/2002 (46.1) |
| Current smoker | 67/1000 (6.7) | 85/1002 (8.5) | 152/2002 (7.6) |
| Haemofiltration/dialysis, n/N (%) | 7/999 (0.7) | 12/1002 (1.2) | 19/2001 (0.9) |
| CVA/TIA, n/N (%) | 76/1000 (7.6) | 87/1003 (8.7) | 163/2003 (8.1) |
| Pre-operative tests | |||
| Haemoglobin (g/dl) mean (SD) | 13.3 (1.5) | 13.3 (1.5) | 13.3 (1.5) |
| eGFRc (ml/minute/1.73m2) median (IQR) | 74.5 (57.2–92.9) | 72.8 (56.4–93.2) | 73.8 (56.8–93.2) |
| Medications | |||
| Intravenous nitrates until theatre, n/N (%) | 1/1000 (0.1) | 4/1002 (0.4) | 5/2002 (0.2) |
| Unfractionated intravenous heparin within 6 hours of surgery, n/N (%) | 10/1000 (1.0) | 9/1002 (0.9) | 19/2002 (0.9) |
| Low-molecular-weight heparin within 12 hours of surgery, n/N (%) | 13/1000 (1.3) | 10/1002 (1.0) | 23/2002 (1.1) |
| Inotropes until theatre, n/N (%) | 2/1000 (0.2) | 1/1002 (0.1) | 3/2002 (0.1) |
| Aspirin within 5 days of surgery, n/N (%) | 277/999 (27.7) | 284/1000 (28.4) | 561/1999 (28.1) |
| Clopidogrel within 5 days of surgery, n/N (%) | 41/1000 (4.1) | 37/1000 (3.7) | 78/2000 (3.9) |
| Characteristic | Randomised to restrictive threshold (N = 1000) | Randomised to liberal threshold (N = 1003) | Overall (N = 2003) |
|---|---|---|---|
| Duration of operationa (hours) median (IQR) | 4.0 (3.3–5.0) | 4.0 (3.2–5.0) | 4.0 (3.3–5.0) |
| Lowest haematocrit,b mean (SD) | 25.5 (4.2) | 25.6 (4.4) | 25.6 (4.3) |
| CPB used, n/N (%) | 950/999 (95.1) | 953/1003 (5.0) | 1903/2002 (5.1) |
| If yes: total bypass time (minutes), median (IQR) | 97.0 (77.0–132) | 95.0 (75.0–127) | 96.0 (76.0–129) |
| If yes: cumulative cross-clamp timec (minutes), median (IQR) | 66.0 (48.0–92.0) | 63.0 (46.0–86.0) | 65.0 (47.0–90.0) |
| If yes: myocardial protection, n/N (%) | |||
| Blood | 796/948 (84.0) | 809/952 (85.0) | 1605/1900 (84.5) |
| Crystalloid | 107/948 (11.3) | 110/952 (11.6) | 217/1900 (11.4) |
| Other | 30/948 (3.2) | 22/952 (2.3) | 52/1900 (2.7) |
| NA | 15/948 (1.6) | 11/952 (1.2) | 26/1900 (1.4) |
| Operation type | |||
| Cardiac procedure, n/N (%) | |||
| CABG only | 408/1000 (40.8) | 408/1003 (40.7) | 816/2003 (40.7) |
| Valve only | 307/1000 (30.7) | 304/1003 (30.3) | 611/2003 (30.5) |
| CABG + valve | 195/1000 (19.5) | 203/1003 (20.2) | 398/2003 (19.9) |
| Major aortic procedure | 54/1000 (5.4) | 62/1003 (6.2) | 116/2003 (5.8) |
| Other procedure | 36/1000 (3.6) | 26/1003 (2.6) | 62/2003 (3.0) |
| Number of distal coronary anastomoses, n/N (%) | |||
| 0 | 374/1000 (37.4) | 369/1002 (36.8) | 743/2002 (37.1) |
| 1 | 114/1000 (11.4) | 124/1002 (12.4) | 238/2002 (11.9) |
| 2 | 165/1000 (16.5) | 137/1002 (13.7) | 302/2002 (15.1) |
| 3 | 234/1000 (23.4) | 267/1002 (26.6) | 501/2002 (25.0) |
| 4 | 99/1000 (9.9) | 92/1002 (9.2) | 191/2002 (9.5) |
| 5 | 14/1000 (1.4) | 12/1002 (1.2) | 26/2002 (1.3) |
| 6 | 0/1000 (0.0) | 1/1002 (0.1) | 1/2002 (0.0) |
| Aortic valve replaced/repaired, n/N (%) | 431/999 (43.1) | 456/1003 (45.5) | 887/2002 (44.3) |
| MV replaced/repaired, n/N (%) | 154/999 (15.4) | 143/1003 (14.3) | 297/2002 (14.8) |
| TV replaced/repaired, n/N (%) | 32/999 (3.2) | 33/1003 (3.3) | 65/2002 (3.2) |
| Pulmonary valve replaced/repaired, n/N (%) | 7/999 (0.7) | 3/1003 (0.3) | 10/2002 (0.5) |
| Details of other cardiac procedures, n | |||
| Ablation for AF | 1 | 0 | 1 |
| Atrial septal defect closure | 1 | 0 | 1 |
| Atrial septal defect closure + radiofrequency ablation for AF | 1 | 0 | 1 |
| AVR + biopsy of lesion of wall of heart | 1 | 0 | 1 |
| AVR, left atrial appendage occlusion + pulmonary vein isolation | 0 | 1 | 1 |
| AVR, Cox-Maze procedure + left atrial appendage occlusion | 0 | 1 | 1 |
| AVR, MVR, ablation for AF + left atrial Cox-Maze procedure | 1 | 0 | 1 |
| AVR, MVR, TV repair, left atrial appendage removal + radiofrequency ablation for AF | 1 | 0 | 1 |
| AVR, TV repair +/– MVR, ablation for AF + left atrial appendage occlusion | 0 | 1 | 1 |
| AVR +/– TV repair + Morrow procedure | 0 | 1 | 1 |
| CABG + ablation for AF | 1 | 0 | 1 |
| CABG + aneurysmectomy | 3 | 0 | 3 |
| CABG, AVR + left atrial appendage occlusion | 0 | 1 | 1 |
| CABG, AVR, Cox-Maze procedure + left atrial appendage occlusion | 1 | 0 | 1 |
| CABG + left atrial appendage occlusion | 0 | 1 | 1 |
| CABG + left ventricular pacing lead | 0 | 1 | 1 |
| CABG + Cox-Maze procedure | 3 | 0 | 3 |
| CABG, MV repair + ablation for AF | 0 | 1 | 1 |
| CABG, MV repair + left ventricular lead placement | 1 | 0 | 1 |
| CABG, MV repair + Cox-Maze procedure | 0 | 1 | 1 |
| CABG, MV repair + myomectomy | 1 | 0 | 1 |
| CABG, MV repair, TV repair + Cox-Maze procedure | 0 | 1 | 1 |
| CABG, MVR + left ventricular aneurysm | 0 | 1 | 1 |
| CABG, TV repair + atrial septal defect closure | 1 | 0 | 1 |
| CABG, valve + Cox-Maze procedure | 1 | 0 | 1 |
| CABG, valve + replacement of aneurysmal segment | 0 | 1 | 1 |
| CABG, valve replacement + thymectomy | 1 | 0 | 1 |
| Excision of atrial myxoma | 0 | 1 | 1 |
| Left apical aneurysmectomy | 0 | 1 | 1 |
| MV repair + ablation for AF | 2 | 2 | 4 |
| MV repair + atrial septal defect closure + tricuspid | 0 | 1 | 1 |
| MV repair, left atrial appendage occlusion + patent foramen ovale closure | 1 | 0 | 1 |
| MV repair + Cox-Maze procedure | 0 | 1 | 1 |
| MV repair, MV ring + pulmonary vein isolation ablation | 0 | 1 | 1 |
| MV repair + TV repair | 2 | 1 | 3 |
| MVR + ablation for AF | 1 | 0 | 1 |
| MVR + artificial chordae | 1 | 0 | 1 |
| MVR + patent foramen ovale closure | 1 | 0 | 1 |
| MVR + TV repair | 0 | 1 | 1 |
| MVR, TV repair + ablation for AF | 2 | 0 | 2 |
| MVR, TV repair + Cox-Maze procedure | 1 | 0 | 1 |
| MVR, TV repair + left atrial appendage occlusion | 0 | 1 | 1 |
| MVR, TV repair + patent foramen ovale closure | 0 | 1 | 1 |
| MVR, TV repair, patent foramen ovale closure + Cox-Maze procedure | 1 | 1 | 2 |
| MVR +/– TV repair, patent foramen ovale closure, left atrial appendage ligation +/– radiofrequency ablation for AF | 1 | 0 | 1 |
| Reimplantation of anomalous right coronary artery | 1 | 0 | 1 |
| TV repair + atrial septal defect closure | 0 | 1 | 1 |
| TV repair, Cox-Maze procedure, ablation for AF + excision of LATR | 1 | 0 | 1 |
| TV repair, MV repair, left atrial appendage occlusion + left sided Cox-Maze procedure | 1 | 0 | 1 |
| Valve + ablation for AF | 0 | 1 | 1 |
| Valve + radiofrequency ablation for AF | 1 | 0 | 1 |
| Graft conduit harvest sites | |||
| Right arm, n/N (%) | 3/1000 (0.3) | 3/1003 (0.3) | 6/2003 (0.3) |
| Left arm, n/N (%) | 40/1000 (4.0) | 38/1003 (3.8) | 78/2003 (3.9) |
| Right leg, n/N (%) | 212/999 (21.2) | 200/1003 (19.9) | 412/2002 (20.6) |
| Left leg, n/N (%) | 416/999 (41.6) | 416/1003 (41.5) | 832/2002 (41.6) |
| Left internal mammary artery, n/N (%) | 488/1000 (48.8) | 495/1003 (49.4) | 983/2003 (49.1) |
| Right internal mammary artery, n/N (%) | 19/1000 (1.9) | 32/1003 (3.2) | 51/2003 (2.5) |
| Other, n/N (%) | 7/1000 (0.7) | 10/1003 (1.0) | 17/2003 (0.8) |
| Blood saving techniques | |||
| Tranexamic acid, n/N (%) | 806/999 (80.7) | 809/1002 (80.7) | 1615/2001 (80.7) |
| Trasylol, n/N (%) | 39/942 (4.1) | 32/952 (3.4) | 71/1894 (3.7) |
| Cell saver, n/N (%) | 481/999 (48.1) | 503/1003 (50.1) | 984/2002 (49.2) |
Post-operative characteristics that were not specified explicitly as primary or secondary outcomes are summarised in Table 12 and Figure 5. The median haemoglobin at randomisation was 8.5 g/dl (IQR 8.1–8.8 g/dl), the median time between end of surgery and randomisation was 4.9 hours (IQR 1.7–17.7 hours) and the median post-randomisation ventilation time was 3.6 hours (IQR 0.0–10.2 hours). The majority of participants (88.2%) were discharged after cardiac surgery to their homes. There did not appear to be any important difference between the two groups, although the total chest tube drainage at 12 hours was slightly higher in the restrictive group than the liberal group [median 500 ml (IQR 325–790 ml) vs. 475 ml (IQR 300–750 ml)].
| Characteristic | Randomised to restrictive threshold (N = 1000) | Randomised to liberal threshold (N = 1003) | Overall (N = 2003) |
|---|---|---|---|
| Medications used in theatre/postoperatively | |||
| Hydroxyethyl starch, n/N (%) | 230/996 (23.1) | 233/1002 (23.3) | 463/1998 (23.2) |
| Human albumin solution (Zenalb, Bio Products Laboratory Ltd), n/N (%) | 79/996 (7.9) | 90/1002 (9.0) | 169/1998 (8.5) |
| Gelofusine, n/N (%) | 839/996 (84.2) | 834/1001 (83.3) | 1673/1997 (83.8) |
| Inotropes, n/N (%) | 620/995 (62.3) | 612/1000 (61.2) | 1232/1995 (61.8) |
| Randomisation | |||
| Haemoglobin at randomisation (g/dl), median (IQR) | 8.5 (8.1–8.8) | 8.5 (8.1–8.8) | 8.5 (8.1–8.8) |
| Time from surgery to randomisationa (hours), median (IQR) | 5.0 (1.7–17.8) | 4.8 (1.7–17.4) | 4.9 (1.7–17.7) |
| Post-operative details | |||
| Total chest tube drainage at 4 hours (ml),b median (IQR) | 250 (150–425) | 240 (150–400) | 250 (150–425) |
| Total chest tube drainage at 12 hours (ml),b median (IQR) | 500 (325–790) | 475 (300–750) | 480 (320–760) |
| Post-operation cell salvage used, n/N (%) | 55/989 (5.6) | 45/989 (4.6) | 100/1978 (5.1) |
| Post-randomisation ventilation time (hours),c median (IQR) | 3.6 (0.0–11.0) | 3.6 (0.0–9.5) | 3.6 (0.0–10.2) |
| Duration of post-randomisation ward stay (hours),d median (IQR) | 102 (74.1–164) | 104 (75.1–152) | 103 (75.0–152) |
| Discharged from cardiac surgery unit to, n/N (%) | |||
| Another unit in hospitale | 9/1000 (0.9) | 18/1003 (1.8) | 27/2003 (1.3) |
| Home | 882/1000 (88.2) | 885/1003 (88.2) | 1767/2003 (88.2) |
| Other hospital | 74/1000 (7.4) | 74/1003 (7.4) | 148/2003 (7.4) |
| Other | 35/1000 (3.5) | 26/1003 (2.6) | 61/2003 (3.0) |
FIGURE 5.
Daily highest creatinine levels for randomised/non-randomised participants. The numbers alongside the points are the numbers of participants contributing readings for that data point. The x-axis has an origin of the day of randomisation for both groups.

Success of blinding
At discharge, 152 participants of the 1007 questioned thought they knew which group they were allocated to (15.1%), of whom 115 (75.7%) were correct (Table 13). At the 3-month follow-up more participants thought they knew which group they were allocated to (459/1669; 27.5%) but proportionately fewer participants (260/459; 56.6%) were correct. More participants thought that being in the liberal group would be better than the restrictive group, although the proportion was higher at discharge (864/1003; 86.1%) than at follow-up (172/278; 61.9%).
| Described by treatment group | |||||
|---|---|---|---|---|---|
| Aspect of blinding | Randomised to restrictive threshold (n = 1000) | Randomised to liberal threshold (n = 1003) | Overall (n = 2003) | ||
| At hospital discharge | |||||
| Did the participant think being in one group would be better, if so whicha | |||||
| Restrictive | 74 | 65 | 139 | ||
| Liberal | 422 | 442 | 864 | ||
| Did not know | 1 | 3 | 4 | ||
| Did the participant think they knew which group they were in, if so whicha | |||||
| Restrictive | 51 | 21 | 72 | ||
| Liberal | 16 | 64 | 80 | ||
| Did not know | 430 | 425 | 855 | ||
| At 3-month follow-up | |||||
| Did the participant think being in one group would be better, if so whichb | |||||
| Restrictive | 65 | 41 | 106 | ||
| Liberal | 86 | 86 | 172 | ||
| Thought one group was better, did not specify which | 12 | 8 | 20 | ||
| Did not think one group was any better | 553 | 612 | 1165 | ||
| Did the participant think they knew which group they were in, if so whichc | |||||
| Restrictive | 158 | 99 | 257 | ||
| Liberal | 54 | 102 | 156 | ||
| Thought knew which group, did not specify which | 21 | 25 | 46 | ||
| Did not know which group they were in | 581 | 629 | 1210 | ||
| Description of data at hospital discharge vs. data at 3-month follow-up | |||||
| Hospital discharge | At 3-month follow-up | ||||
| Restrictive | Liberal | Thought one group was better, did not specify which | Did not think one group was any better | Did not answer question | |
| Did the participant think being in one group would be better, if so which | |||||
| Restrictive | 16 | 21 | 3 | 77 | 22 |
| Liberal | 38 | 69 | 6 | 547 | 204 |
| Did not know | 0 | 1 | 0 | 1 | 2 |
| Unavailable to answer questions | 52 | 81 | 11 | 540 | 312 |
| Restrictive | Liberal | Thought knew which group, did not specify which | Did not think one group was any better | Did not answer question | |
| Did the participant think they knew which group they were in, if so which | |||||
| Restrictive | 31 | 5 | 4 | 25 | 7 |
| Liberal | 10 | 23 | 4 | 41 | 2 |
| Did not know | 102 | 50 | 14 | 576 | 113 |
| Unavailable to answer questions | 114 | 78 | 24 | 568 | 212 |
Summary
There is some uncertainty about screening data and there were recruitment challenges throughout the trial. However, a significant upturn in recruitment in late 2011 led to the trial completing recruitment just 2 weeks behind the revised target date. Data completeness is excellent and withdrawals and drop-out rates were few; over 98% of participants were followed up 3 months after randomisation. Baseline characteristics were well balanced between the groups.
Chapter 4 Process outcomes
Haemoglobin levels
Haemoglobin levels at the time of randomisation were similar [median 8.5 g/dl (IQR 8.1–8.8 g/dl)] in both the restrictive and liberal groups (see Table 12). After randomisation the groups diverged, daily nadir haemoglobin levels were lower in the restrictive group than the liberal group by approximately 1 g/dl (Figure 6). Day three was pre-specified in the SAP to be used as an overall summary measure; at this time the mean haemoglobin was 8.66 g/dl (SD 1.03 g/dl) in the restrictive group and 9.55 g/dl (SD 1.01 g/dl) in the liberal group.
FIGURE 6.
Mean daily nadir haemoglobin. The error bars are SDs (calculated independently at each time point).

Red blood cell transfusions
Red blood cell transfusions both before and after randomisation are given in Figure 7 and Table 14. Before randomisation, 25.7% of participants were transfused one or more units, with approximately equal numbers of transfused participants in each group. Most of the pre-randomisation red blood cell transfusions were administered intraoperatively and the remaining were given either postoperatively but pre-randomisation or during a reoperation. After randomisation, 53.4% of participants in the restrictive group and 92.2% in the liberal group were transfused one or more units (RR 0.58, 95% CI 0.54 to 0.62; p < 0.0001). The median numbers of red blood cell units transfused after randomisation in the restrictive and liberal groups were 1 unit (IQR 0–2 units) and 2 units (IQR 1–3 units), respectively, and 1494 units were transfused in the restrictive group and 2494 units in the liberal group in total. Most red blood cell units were transfused according to the trial protocol; a small number were given either during a reoperation (when the trial protocol was suspended), after treatment according to allocation was discontinued, or in breach of the protocol. During the entire index admission (i.e. pre-randomisation and/or post randomisation), 63.7% of participants in the restrictive group and 94.9% in the liberal group were transfused. The median numbers of units transfused in the index admission was 1 (IQR 0–3) in the restrictive group and 2 (IQR 1–4) in the liberal group.
FIGURE 7.
Secondary outcome: pre-randomisation and post-randomisation red blood cell transfusions. (a) Pre-randomisation red blood cell transfusions. (b) Post-randomisation red blood cell transfusions.

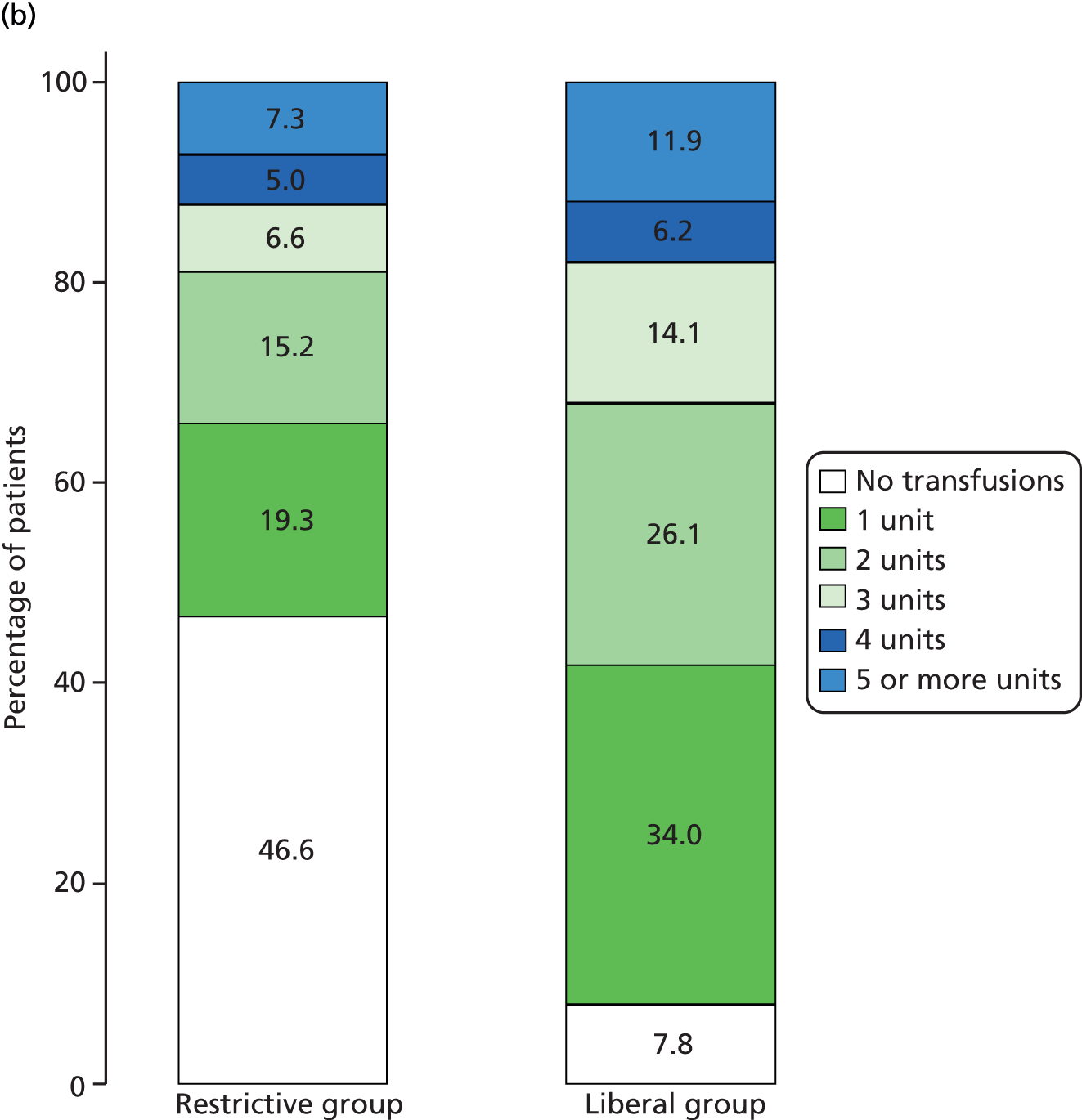
| Outcome | Randomised to restrictive threshold (N = 1000) | Randomised to liberal threshold (N = 1003) | Overall (N = 2003) | RRa (95% CI) | p-value |
|---|---|---|---|---|---|
| Pre-randomisation transfusions | |||||
| Total units transfused pre-randomisation, n/N (%) | |||||
| Median (IQR) units | 0.0 (0.0–0.5) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | ||
| Not transfused | 750/1000 (75.0) | 739/1003 (73.7) | 1489/2003 (74.3) | ||
| 1 unit | 90/1000 (9.0) | 109/1003 (10.9) | 199/2003 (9.9) | ||
| 2 units | 89/1000 (8.9) | 79/1003 (7.9) | 168/2003 (8.4) | ||
| 3 units | 32/1000 (3.2) | 33/1003 (3.3) | 65/2003 (3.2) | ||
| 4 units | 16/1000 (1.6) | 21/1003 (2.1) | 37/2003 (1.8) | ||
| ≥ 5 units | 23/1000 (2.3) | 22/1003 (2.2) | 45/2003 (2.2) | ||
| Total units transfused | 587 | 589 | 1176 | ||
| Intraoperative transfusions, n/N (%) | |||||
| Not transfused | 816/1000 (81.6) | 823/1003 (82.1) | 1639/2003 (81.8) | ||
| 1 unit | 69/1000 (6.9) | 69/1003 (6.9) | 138/2003 (6.9) | ||
| 2 units | 71/1000 (7.1) | 67/1003 (6.7) | 138/2003 (6.9) | ||
| 3 units | 18/1000 (1.8) | 18/1003 (1.8) | 36/2003 (1.8) | ||
| 4 units | 14/1000 (1.4) | 18/1003 (1.8) | 32/2003 (1.6) | ||
| ≥ 5 units | 12/1000 (1.2) | 8/1003 (0.8) | 20/2003 (1.0) | ||
| Post-operative pre-randomisation transfusions, n/N (%) | |||||
| Not transfused | 911/1000 (91.1) | 894/1003 (89.1) | 1805/2003 (90.1) | ||
| 1 unit | 45/1000 (4.5) | 68/1003 (6.8) | 113/2003 (5.6) | ||
| 2 units | 26/1000 (2.6) | 22/1003 (2.2) | 48/2003 (2.4) | ||
| 3 units | 10/1000 (1.0) | 10/1003 (1.0) | 20/2003 (1.0) | ||
| 4 units | 7/1000 (0.7) | 4/1003 (0.4) | 11/2003 (0.5) | ||
| ≥ 5 units | 1/1000 (0.1) | 5/1003 (0.5) | 6/2003 (0.3) | ||
| Transfusions during a pre-randomisation reoperation, n/N (%) | |||||
| Not transfused | 986/1000 (98.6) | 992/1003 (98.9) | 1978/2003 (98.8) | ||
| 1 unit | 6/1000 (0.6) | 2/1003 (0.2) | 8/2003 (0.4) | ||
| 2 units | 3/1000 (0.3) | 5/1003 (0.5) | 8/2003 (0.4) | ||
| 3 units | 3/1000 (0.3) | 3/1003 (0.3) | 6/2003 (0.3) | ||
| 4 units | 2/1000 (0.2) | 1/1003 (0.1) | 3/2003 (0.1) | ||
| Post-randomisation transfusions | |||||
| Total units transfused post randomisation, n/N (%) | |||||
| Median (IQR) units | 1.0 (0.0–2.0) | 2.0 (1.0–3.0) | 1.0 (0.0–3.0) | ||
| Not transfused | 466/1000 (46.6) | 78/1003 (7.8) | 544/2003 (27.2) | 0.58 (0.54 to 0.62) | < 0.0001 |
| 1 unit | 193/1000 (19.3) | 341/1003 (34.0) | 534/2003 (26.7) | ||
| 2 units | 152/1000 (15.2) | 262/1003 (26.1) | 414/2003 (20.7) | ||
| 3 units | 66/1000 (6.6) | 141/1003 (14.1) | 207/2003 (10.3) | ||
| 4 units | 50/1000 (5.0) | 62/1003 (6.2) | 112/2003 (5.6) | ||
| ≥ 5 units | 73/1000 (7.3) | 119/1003 (11.9) | 192/2003 (9.6) | ||
| Total units transfused | 1494 | 2494 | 3988 | ||
| Transfusions during a post-randomisation reoperation, n/N (%) | |||||
| Not transfused | 963/1000 (96.3) | 969/1003 (96.6) | 1932/2003 (96.5) | ||
| 1 unit | 15/1000 (1.5) | 9/1003 (0.9) | 24/2003 (1.2) | ||
| 2 units | 11/1000 (1.1) | 13/1003 (1.3) | 24/2003 (1.2) | ||
| 3 units | 6/1000 (0.6) | 4/1003 (0.4) | 10/2003 (0.5) | ||
| 4 units | 1/1000 (0.1) | 3/1003 (0.3) | 4/2003 (0.2) | ||
| ≥ 5 units | 4/1000 (0.4) | 5/1003 (0.5) | 9/2003 (0.4) | ||
| Transfusions after treatment according to protocol discontinued, n/N (%) | |||||
| Not transfused | 980/1000 (98.0) | 993/1003 (99.0) | 1973/2003 (98.5) | ||
| 1 unit | 4/1000 (0.4) | 2/1003 (0.2) | 6/2003 (0.3) | ||
| 2 units | 4/1000 (0.4) | 2/1003 (0.2) | 6/2003 (0.3) | ||
| 3 units | 2/1000 (0.2) | 0/1003 (0.0) | 2/2003 (0.1) | ||
| 4 units | 2/1000 (0.2) | 0/1003 (0.0) | 2/2003 (0.1) | ||
| ≥ 5 units | 8/1000 (0.8) | 6/1003 (0.6) | 14/2003 (0.7) | ||
| Transfusions in breach of protocol, n/N (%) | |||||
| Not transfused | 727/1000 (72.7) | 896/1003 (89.3) | 1623/2003 (81.0) | ||
| 1 unit | 135/1000 (13.5) | 85/1003 (8.5) | 220/2003 (11.0) | ||
| 2 units | 72/1000 (7.2) | 10/1003 (1.0) | 82/2003 (4.1) | ||
| 3 units | 34/1000 (3.4) | 5/1003 (0.5) | 39/2003 (1.9) | ||
| 4 units | 17/1000 (1.7) | 3/1003 (0.3) | 20/2003 (1.0) | ||
| ≥ 5 units | 15/1000 (1.5) | 4/1003 (0.4) | 19/2003 (0.9) | ||
| Transfusions per protocol, n/N (%) | |||||
| Median (IQR) units | 0.0 (0.0–1.0) | 2.0 (1.0–3.0) | 1.0 (0.0–2.0) | ||
| Not transfused | 577/1000 (57.7) | 87/1003 (8.7) | 664/2003 (33.2) | ||
| 1 unit | 256/1000 (25.6) | 362/1003 (36.1) | 618/2003 (30.9) | ||
| 2 units | 93/1000 (9.3) | 265/1003 (26.4) | 358/2003 (17.9) | ||
| 3 units | 46/1000 (4.6) | 147/1003 (14.7) | 193/2003 (9.6) | ||
| 4 units | 13/1000 (1.3) | 56/1003 (5.6) | 69/2003 (3.4) | ||
| ≥ 5 units | 15/1000 (1.5) | 86/1003 (8.6) | 101/2003 (5.0) | ||
Transfusion of blood products other than red blood cells
Platelets were the most common other blood product transfused (36.8% of participants). FFP was transfused in 29.0% of participants, cryoprecipitate in 10.0% and Beriplex and activated factor VII in only 5.0% and 0.6% of participants, respectively (Figure 8 and Table 15). Use of all other products was similar between the two groups over the duration of the index admission.
FIGURE 8.
Use of (a) FFP, (b) platelets and (c) cryoprecipitate; pre-randomisation and post-randomisation use have been combined.



| Outcome | Randomised to restrictive threshold (N = 1000) | Randomised to liberal threshold (N = 1003) | Overall (N = 2003) | ||
|---|---|---|---|---|---|
| Estimatea (95% CI) | p-value | ||||
| FFP transfusions, n/N (%) | |||||
| Not transfused | 703/1000 (70.3) | 719/1003 (71.7) | 1422/2003 (71.0) | ||
| 1 unit | 12/1000 (1.2) | 11/1003 (1.1) | 23/2003 (1.1) | OR 1.08 (0.88 to 1.33) | 0.45 |
| 2 units | 129/1000 (12.9) | 113/1003 (11.3) | 242/2003 (12.1) | ||
| 3 units | 32/1000 (3.2) | 30/1003 (3.0) | 62/2003 (3.1) | ||
| 4 units | 82/1000 (8.2) | 92/1003 (9.2) | 174/2003 (8.7) | ||
| ≥ 5 units | 42/1000 (4.2) | 38/1003 (3.8) | 80/2003 (4.0) | ||
| Platelet transfusions, n/N (%) | |||||
| Not transfused | 624/1000 (62.4) | 641/1003 (63.9) | 1265/2003 (63.2) | ||
| 1 unit | 196/1000 (19.6) | 177/1003 (17.6) | 373/2003 (18.6) | OR 1.08 (0.89 to 1.31) | 0.42 |
| 2 units | 130/1000 (13.0) | 133/1003 (13.3) | 263/2003 (13.1) | ||
| 3 units | 29/1000 (2.9) | 25/1003 (2.5) | 54/2003 (2.7) | ||
| 4 units | 11/1000 (1.1) | 20/1003 (2.0) | 31/2003 (1.5) | ||
| ≥ 5 units | 10/1000 (1.0) | 7/1003 (0.7) | 17/2003 (0.8) | ||
| Cryoprecipitate transfusions, n/N (%) | |||||
| Not transfused | 901/1000 (90.1) | 901/1003 (89.8) | 1802/2003 (90.0) | ||
| 1 unit | 23/1000 (2.3) | 22/1003 (2.2) | 45/2003 (2.2) | OR 0.99 (0.72 to 1.35) | 0.95 |
| 2 units | 58/1000 (5.8) | 69/1003 (6.9) | 127/2003 (6.3) | ||
| 3 units | 6/1000 (0.6) | 4/1003 (0.4) | 10/2003 (0.5) | ||
| 4 units | 7/1000 (0.7) | 5/1003 (0.5) | 12/2003 (0.6) | ||
| ≥ 5 units | 5/1000 (0.5) | 2/1003 (0.2) | 7/2003 (0.3) | ||
| Activated factor VII used, yes (%) | 7/1000 (0.7) | 5/1003 (0.5) | 12/2003 (0.6) | RR 1.41 (0.45 to 4.45) | 0.56 |
| Beriplex used, yes (%) | 52/1000 (5.2) | 48/1003 (4.8) | 100/2003 (5.0) | OR 1.21 (0.73 to 2.03) | 0.46 |
Adherence
Non-adherence with the randomisation protocol
There were nine participants consented to the trial who were later found to be ineligible, although none of these participants were randomised (Table 16). Of the 1464 non-randomised participants, 176 (12.0%) met the post-consent eligibility criterion (haemoglobin < 9 g/dl) but were not randomised. All of the 2003 randomised participants breached the 9 g/dl threshold. However, randomisation was delayed (i.e. occurred later than 24 hours after the threshold breach occurred) for 65 participants (3.2%) and these instances were classified as non-adherent with respect to the randomisation protocol.
| Non-adherence type | Consented (N = 3565) |
|---|---|
| Participant was randomised more than 24 hours after meeting post-consent eligibility criteria (haemoglobin < 9 g/dl),a n/N (%) | 9/3565 (0.2) |
| Considered for randomisation, but not randomised (n = 1464) | |
| Participant consented and met post-consent eligibility criterion (haemoglobin < 9 g/dl) but was not randomised, n/N (%) | 176/1464 (12.0) |
| Randomised (n = 2003) | |
| Participant did not meet the post-consent eligibility criteria (haemoglobin < 9 g/dl) but was randomised, n/N (%) | 0/2003 (0.0) |
| Participant was randomised more than 24 hours after meeting post-consent eligibility criteria (haemoglobin < 9 g/dl) but was not randomised,b n/N (%) | 65/2003 (3.2) |
Non-adherence with the allocated transfusion threshold
Non-adherence with the allocated transfusion threshold is described in Table 17. There were 1813 deviations from the protocol occurring in 37.6% of participants; 635 deviations in 30.0% of participants in the restrictive group and 1178 deviations in 45.2% of participants in the liberal group. As anticipated, extra transfusions (i.e. given outside of protocol) were more common in the restrictive group (573 in 27.3% of participants vs. 161 in 10.7% of participants) and withheld transfusions were more common in the liberal group (1017 in 38.9% of participants vs. 62 in 5.5% of participants). Approximately one-sixth of all deviations were classified as severe; 24.8% of extra transfusions and 11.1% of withheld transfusions. Therefore, severe protocol deviations were more common in the restrictive group than the liberal group (186 in 9.7% of participants vs. 116 in 6.2% of participants).
| Non-adherence type | Randomised to restrictive threshold | Randomised to liberal threshold | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Events (n) | Participants (N = 1000) (n/N) | % | Events (n) | Participants (N = 1003) (n/N) | % | Events (n) | Participants (N = 2003) (n/N) | % | |
| Any protocol deviation | 635 | 300/1000 | 30.0 | 1178 | 453/1003 | 45.2 | 1813 | 753/2003 | 37.6 |
| Any severe protocol deviation | 186 | 97/1000 | 9.7 | 116 | 62/1003 | 6.2 | 302 | 159/2003 | 7.9 |
| Extra transfusion | 573 | 273/1000 | 27.3 | 161 | 107/1003 | 10.7 | 734 | 380/2003 | 19.0 |
| Moderate | 391 | 180/1000 | 18.0 | 161 | 107/1003 | 10.7 | 552 | 287/2003 | 14.3 |
| Severe | 182 | 93/1000 | 9.3 | 0 | 0/1003 | 0.0 | 182 | 93/2003 | 4.6 |
| Withheld transfusion | 62 | 55/1000 | 5.5 | 1017 | 390/1003 | 38.9 | 1079 | 445/2003 | 22.2 |
| Mild | 34 | 30/1000 | 3.0 | 546 | 204/1003 | 20.3 | 580 | 234/2003 | 11.7 |
| Moderate | 24 | 22/1000 | 2.2 | 355 | 167/1003 | 16.7 | 379 | 189/2003 | 9.4 |
| Severe | 4 | 4/1000 | 0.4 | 116 | 62/1003 | 6.2 | 120 | 66/2003 | 3.3 |
Characteristics of each instance of non-adherence are reported in Table 18. Extra transfusions tended to be given either for the clinical reasons listed on the CRF (36.5%) or for ‘other’ reasons (42.7%), which were generally clinical reasons not listed as specific options on the CRF. The most common reason for a withheld transfusion was ‘oversight/error’ (67.2%). Extra transfusions tended to occur earlier than withheld transfusions and were more likely to occur overnight. There were no clear trends in time of year for either type of non-adherence.
| Characteristic | Extra transfusions | Withheld transfusions | |||||
|---|---|---|---|---|---|---|---|
| Restrictive group (N = 573) | Liberal group (N = 161) | Overall (N = 734) | Restrictive group (N = 62) | Liberal group (N = 1017) | Overall (N = 1079) | ||
| Section Aa | |||||||
| Reason for non-adherence, n/N (%) | |||||||
| Excessive blood loss | 128/558 (22.9) | 55/149 (36.9) | 183/707 (25.9) | N/A | N/A | N/A | |
| Sepsis | 18/558 (3.2) | 2/149 (1.3) | 20/707 (2.8) | N/A | N/A | N/A | |
| Physiological indicators of oxygen debt | 54/558 (9.7) | 1/149 (0.7) | 55/707 (7.8) | N/A | N/A | N/A | |
| Clinical preference | N/A | N/A | N/A | 8/48 (16.7) | 167/815 (20.5) | 175/863 (20.3) | |
| Oversight/error | 98/558 (17.6) | 49/149 (32.9) | 147/707 (20.8) | 26/48 (54.2) | 554/815 (68.0) | 580/863 (67.2) | |
| Other | 260/558 (46.6) | 42/149 (28.2) | 302/707 (42.7) | 14/48 (29.2) | 94/815 (11.5) | 108/863 (12.5) | |
| If, clinical reason/other level of clinician making decision, n/N (%) | |||||||
| Consultant | 203/340 (59.7) | 34/71 (47.9) | 237/411 (57.7) | 5/10 (50.0) | 79/174 (45.4) | 84/184 (45.7) | |
| Registrar | 125/340 (36.8) | 32/71 (45.1) | 157/411 (38.2) | 5/10 (50.0) | 70/174 (40.2) | 75/184 (40.8) | |
| Junior doctor | 9/340 (2.6) | 5/71 (7.0) | 14/411 (3.4) | 0/10 (0.0) | 14/174 (8.0) | 14/184 (7.6) | |
| Nurse practitioner | 3/340 (0.9) | 0/71 (0.0) | 3/411 (0.7) | 0/10 (0.0) | 11/174 (6.3) | 11/184 (6.0) | |
| Haemoglobin levels at time of non-adherence (g/dl), median (IQR) | |||||||
| Any non-adherenceb | 7.8 (7.6–8.4) | 9.0 (8.2–9.5)c | 8.0 (7.6–8.8) | 7.2 (7.0–7.4) | 8.6 (8.3–8.8) | 8.6 (8.3–8.8) | |
| Mild | N/A | N/A | N/A | 7.1 (7.0–7.4) | 8.6 (8.3–8.8) | 8.6 (8.1–8.8) | |
| Moderated | 7.8 (7.5–8.3) | 9.0 (8.2–9.5)c | 8.1 (7.6–8.9) | 7.2 (7.1–7.4) | 8.6 (8.4–8.8) | 8.6 (8.3–8.8) | |
| Severee | 8.0 (7.6–8.5) | N/A | 8.0 (7.6–8.5) | 7.3 (7.2–7.4) | 8.7 (8.5–8.8) | 8.7 (8.4–8.8) | |
| Time between operation end and non-adherence (days), median (IQR) | |||||||
| Any non-adherence | 2.0 (0.4–7.0) | 0.7 (0.3–4.2) | 1.8 (0.3–6.1) | 3.0 (1.8–5.4) | 3.7 (1.8–8.0) | 3.7 (1.8–7.9) | |
| Mild | N/A | N/A | N/A | 2.8 (1.5–7.2) | 3.0 (1.4–8.6) | 3.0 (1.4–8.5) | |
| Moderate | 2.7 (0.5–11.2) | 0.7 (0.3–4.2) | 2.0 (0.4–8.2) | 3.7 (2.7–4.6) | 4.8 (3.4–9.8) | 4.8 (3.2–9.6) | |
| Severe | 0.9 (0.3–3.7) | N/A | 0.9 (0.3–3.7) | 1.9 (1.1–2.8) | 3.0 (1.0–4.6) | 2.9 (1.0–4.4) | |
| Time of day, n/N (%) | |||||||
| Weekday 09:00 to 17:00 | 153/573 (26.7) | 34/161 (21.1) | 187/734 (25.5) | 13/57 (22.8) | 163/923 (17.7) | 176/980 (18.0) | |
| Weekday evenings/overnight | 262/573 (45.7) | 93/161 (57.8) | 355/734 (48.4) | 18/57 (31.6) | 348/923 (37.7) | 366/980 (37.3) | |
| Weekend 09:00 to 17:00 | 65/573 (11.3) | 6/161 (3.7) | 71/734 (9.7) | 8/57 (14.0) | 92/923 (10.0) | 100/980 (10.2) | |
| Weekend evenings/overnight | 93/573 (16.2) | 28/161 (17.4) | 121/734 (16.5) | 18/57 (31.6) | 320/923 (34.7) | 338/980 (34.5) | |
| Section Bf | |||||||
| Day of week, n/N (%) | |||||||
| Sunday | 56/1334 (4.2) | 10/1333 (0.8) | 66/2667 (2.5) | 8/1334 (0.6) | 166/1333 (12.5) | 174/2667 (6.5) | |
| Monday | 63/1428 (4.4) | 20/1431 (1.4) | 83/2859 (2.9) | 8/1428 (0.6) | 124/1431 (8.7) | 132/2859 (4.6) | |
| Tuesday | 76/1489 (5.1) | 40/1491 (2.7) | 116/2980 (3.9) | 4/1489 (0.3) | 97/1491 (6.5) | 101/2980 (3.4) | |
| Wednesday | 108/1552 (7.0) | 36/1524 (2.4) | 144/3076 (4.7) | 3/1552 (0.2) | 105/1524 (6.9) | 108/3076 (3.5) | |
| Thursday | 103/1609 (6.4) | 19/1586 (1.2) | 122/3195 (3.8) | 7/1609 (0.4) | 138/1586 (8.7) | 145/3195 (4.5) | |
| Friday | 106/1558 (6.8) | 22/1569 (1.4) | 128/3127 (4.1) | 12/1558 (0.8) | 132/1569 (8.4) | 144/3127 (4.6) | |
| Saturday | 61/1437 (4.2) | 14/1440 (1.0) | 75/2877 (2.6) | 15/1437 (1.0) | 161/1440 (11.2) | 176/2877 (6.1) | |
| Time of year, n/N (%) | |||||||
| February to April | 88/2291 (3.8) | 42/2368 (1.8) | 130/4659 (2.8) | 13/2291 (0.6) | 204/2368 (8.6) | 217/4659 (4.7) | |
| May to July | 129/2210 (5.8) | 41/2311 (1.8) | 170/4521 (3.8) | 7/2210 (0.3) | 221/2311 (9.6) | 228/4521 (5.0) | |
| August to October | 227/3031 (7.5) | 27/2847 (0.9) | 254/5878 (4.3) | 18/3031 (0.6) | 264/2847 (9.3) | 282/5878 (4.8) | |
| November to January | 129/2875 (4.5) | 51/2848 (1.8) | 180/5723 (3.1) | 19/2875 (0.7) | 234/2848 (8.2) | 253/5723 (4.4) | |
| Level of care, n/N (%) | |||||||
| ICU | 460/4318 (10.7) | 137/4184 (3.3) | 597/8502 (7.0) | 40/4318 (0.9) | 708/4184 (16.9) | 748/8502 (8.8) | |
| Ward | 113/6089 (1.9) | 24/6190 (0.4) | 137/12279 (1.1) | 22/6089 (0.4) | 309/6190 (5.0) | 331/12,279 (2.7) | |
Separate logistic regression models were fitted for (1) extra transfusions and (2) withheld transfusions to identify any characteristics (both at an adherence level and participant level) that predicted non-adherence (Table 19). The odds of an extra transfusion reduced by 3% with each post-operative day and reduced by 22% at weekends. However, the odds of a withheld transfusion increased by 3% with each post-operative day and increased by 79% at weekends. Both types of non-adherence were much more likely in the ICU than on the ward. Centre recruitment rate was important for predicting both types of non-adherence, which was most common in relatively slow recruiting centres (3–4 participants per month). Participants having more complex operation types (CABG and valve and valve-alone surgery) were more likely to have an extra transfusion, as were participants transfused pre-randomisation. Participants with a longer period of time between operation end and randomisation were more likely to have a withheld transfusion, and the odds of a withheld transfusion decreased by 1% with each year of age.
| Characteristic | Extra transfusions | Withheld transfusions | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Adherence characteristics | ||||
| Time from operation end (days) | 0.97 (0.95 to 0.98) | p < 0.001 | 1.03 (1.01 to 1.04) | p < 0.001 |
| Weekend vs. weekday | 0.78 (0.63 to 0.95) | p = 0.013 | 1.79 (1.54 to 2.09) | p < 0.001 |
| ICU vs. ward | 4.68 (3.76 to 5.83) | p < 0.001 | 3.07 (2.55 to 3.69) | p < 0.001 |
| Participant characteristics | ||||
| Centre recruitment rate | ||||
| ≥ 6 participants per month | Reference group | p < 0.001 | Reference group | p = 0.022 |
| 4–6 participants per month | 1.04 (0.77 to 1.39) | 0.84 (0.61 to 1.15) | ||
| 3–4 participants per month | 2.23 (1.62 to 3.05) | 1.39 (0.99 to 1.96) | ||
| < 3 participants per month | 1.54 (0.97 to 2.44) | 0.71 (0.41 to 1.22) | ||
| Time between operation end and randomisation | 1.15 (1.07 to 1.25) | p < 0.001 | ||
| Age (years) | 0.99 (0.97 to 1.00) | p = 0.029 | ||
| Cardiac procedure | p < 0.001 | |||
| CABG only | Reference group | |||
| CABG + valve | 1.36 (1.01 to 1.83) | |||
| Valve only | 1.75 (1.27 to 2.40) | |||
| Other | 1.04 (0.66 to 1.65) | |||
| Transfused pre-randomisation | 1.49 (1.15 to 1.93) | p = 0.003 | ||
Adherence by centre is given in Figure 9, which demonstrates wide variation between centres with no obvious relationship to total recruitment or average recruitment rates.
FIGURE 9.
Adherence by centre. (a) Percentage of participants with any severe/non-severe non-adherence by centre. (b) Percentage of participants having extra and withheld transfusions. Sites are ordered by the average number of participants recruited per month.


Non-adherence was monitored carefully over the course of the trial and various measures were put in place to try and improve non-adherence rates. 33 Some of these measures are described in Table 20.
| Methods implemented by trial management team across all sites | ||
|---|---|---|
| For site research teams | For clinical staff | For clinical and site research staff |
| Regular newsletters were sent to sites to try to motivate staff to improve adherence and maintain interest in study | Regular teaching slots about the trial for new and existing staff, the timing of which was frequently aimed to coincide with the start of residents’ rotations | Colour-coded labels provided for research and clinical staff to add to participants’ notes and charts (to clearly identify TITRe2 participants and allocated group) |
| Mid-study site visits included analysis and discussion of non-adherence with local research teams to try to identify site-specific barriers to adherence and potential solutions | Nurses’ manuals at nursing stations containing trial-specific information and summaries of the protocol for the restrictive and liberal groups | Daily haemoglobin transfusion checks by research nurses to monitor adherence with the protocol for randomisation and treatment according to allocated group and to record instances of non-adherence. These checks were usually done Monday to Friday (owing to research nurse working patterns). These checks provided a useful additional avenue of communication if the clinical team had any trial-related queries and provided a physical presence of the trial on the cardiac units |
| Reports were fed back to sites, both at mid-study visits and thereafter on a quarterly basis, describing site-specific non-adherence over time and non-adherence in relation to other sites | Adherence competitions were trialled but found to be difficult to implement logistically. However, informal prizes were handed out at meetings of study investigators to commend sites that achieved high adherence rates | Trial branded stationery produced to remind staff to check and react to haemoglobin concentrations |
| Methods for avoiding non-adherence adopted by sites with better adherence were shared at meetings of study investigators. Research nurses were primary contributors at these meetings | Study posters in staff rooms | |
| Methods implemented by sites themselves | ||
| Careful ‘handover’ between nursing shifts, highlighting the need to monitor the haemoglobin of a participant carefully and to randomise/transfuse in the event of breaching the allocated threshold (site A) | ||
| Additional plastic wrist band/tag identifying that the patient was taking part in the trial; this band was alongside another band with the participant’s identification details, which doctors and nurses had to check when prescribing/administering a red blood cell transfusion (site E) | ||
| Adding coloured covers to the participant’s paper medical records highlighting that the patient was taking part in research (site C) | ||
| Out of hours/weekend reminder calls to ICU/ward (for participants known to be at risk of breaching their allocated threshold) to ask whether or not a participant’s haemoglobin had been checked | ||
There was no improvement in non-adherence rates over the course of the study despite these measures (Figure 10). In the early stages, rates of non-adherence fluctuated somewhat but by the time that half of the participants had been recruited, rates remained fairly constant.
FIGURE 10.
Change over time in proportions of non-adherent participants. Lines represent cumulative non-adherence rates; triangles represent the number of centres in the study.
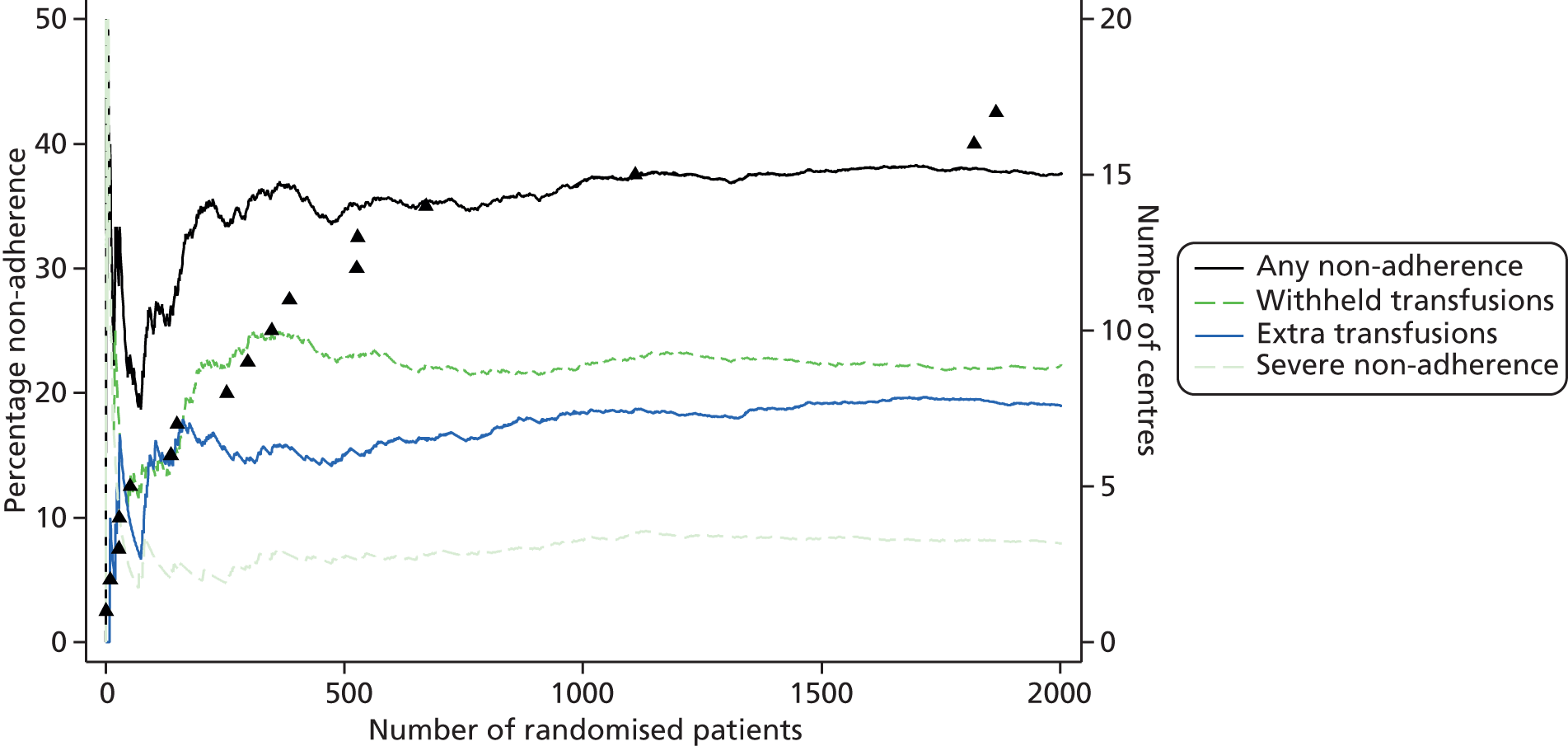
Summary
The proportion of participants with any non-adherence was relatively high (37.6%). However, only 7.9% of participants had non-adherence that was classified as severe and which, by definition, affected overall transfusion rates. This percentage was consistent with the assumptions made when designing the trial. Therefore, we managed to achieve good separation between the groups in terms of both haemoglobin levels (approximately 1 g/dl difference) and transfusion rates (53.4% in the restrictive group vs. 92.2% in the liberal group). Finally, the proportions of participants transfused prior to randomisation, and the proportions given other blood products, were similar in the two groups.
Chapter 5 Primary and secondary outcomes
Primary outcome
Primary analysis
The primary outcome occurred in 35.1% of participants in the restrictive group and 33.0% in the liberal group (Table 21). This difference was not statistically significant, OR 1.11 (95% CI 0.91 to 1.34; p = 0.30). The most common element of the primary outcome was sepsis (21.6%), followed by AKI (13.2%) and wound infection (5.4%); all other components were relatively rare (< 2%). There were 27.1% participants who experienced the primary outcome before hospital discharge and 10.8% after hospital discharge (some participants experienced qualifying events both in hospital and after discharge). The small excess of primary outcome events in the restrictive group appeared to be mainly driven by AKI events. Most of the AKI events occurred before hospital discharge (96.6%) and AKI events had similar frequencies in each of the three AKI stages (34.4% stage one, 28.6% stage two and 37.1% stage three).
| Outcome | Randomised to restrictive threshold (N = 1000) | Randomised to liberal threshold (N = 1003) | Estimate (95% CI) | p-value |
|---|---|---|---|---|
| At any time,a n/N (%) | ||||
| Overall | 331/944 (35.1) | 317/962 (33.0) | OR 1.11 (0.91 to 1.34) | 0.30 |
| Infectious event | 238/936 (25.4) | 240/954 (25.2) | OR 1.02 (0.83 to 1.26) | 0.83 |
| Sepsisb | 210/982 (21.4) | 214/983 (21.8) | ||
| Wound infection | 55/921 (6.0) | 46/936 (4.9) | ||
| Ischaemic event | 156/991 (15.7) | 139/991 (14.0) | OR 1.16 (0.90 to 1.49) | 0.26 |
| Permanent strokec | 15/989 (1.5) | 17/985 (1.7) | ||
| MI | 3/987 (0.3) | 4/981 (0.4) | ||
| Gut infarctiond | 6/987 (0.6) | 1/982 (0.1) | ||
| AKIe | 140/989 (14.2) | 122/989 (12.3) | ||
| Stage one | 49/989 (5.0) | 40/989 (4.0) | ||
| Stage two | 39/989 (3.9) | 35/989 (3.5) | ||
| Stage three | 50/989 (5.1) | 46/989 (4.7) | ||
| Pre-discharge, n/N (%) | ||||
| Overall | 282/988 (28.5) | 253/984 (25.7) | ||
| Infectious event | 184/983 (18.7) | 175/983 (17.8) | ||
| Sepsis | 178/990 (18.0) | 167/993 (16.8) | ||
| Wound infection | 17/990 (1.7) | 15/990 (1.5) | ||
| Ischaemic event | 146/1000 (14.6) | 134/1003 (13.4) | ||
| Permanent stroke | 11/1000 (1.1) | 14/1003 (1.4) | ||
| MI | 1/1000 (0.1) | 3/1003 (0.3) | ||
| Gut infarction | 5/1000 (0.5) | 1/1003 (0.1) | ||
| AKI | 134/1000 (13.4) | 121/1003 (12.1) | ||
| Post discharge, n/N (%) | ||||
| Overall | 104/924 (11.3) | 98/938 (10.4) | ||
| Infectious event | 94/924 (10.2) | 92/937 (9.8) | ||
| Sepsis | 49/987 (5.0) | 55/981 (5.6) | ||
| Wound infection | 55/921 (6.0) | 46/936 (4.9) | ||
| Ischaemic event | 15/987 (1.5) | 6/981 (0.6) | ||
| Permanent stroke | 5/987 (0.5) | 3/981 (0.3) | ||
| MI | 2/987 (0.2) | 1/981 (0.1) | ||
| Gut infarction | 1/987 (0.1) | 0/981 (0.0) | ||
| AKI | 7/987 (0.7) | 2/981 (0.2) | ||
Most participants experiencing the primary outcome encountered their first component event in the first 10 days after randomisation (Figure 11). A planned secondary analysis using a Cox proportional hazards model gave HR 1.09 (95% CI 0.93 to 1.27; p = 0.29) which is very similar to the OR obtained from logistic regression in Table 21.
FIGURE 11.
Kaplan–Meier estimates of time from randomisation to the primary outcome. The HR is not adjusted for cardiac procedure (as was planned) because the proportional hazards assumption was violated if this term was included, and the first events occurring were sepsis (169 restrictive group and 171 liberal group), wound infection (21 restrictive group and 26 liberal group), stroke (12 restrictive group and 12 liberal group), MI (one restrictive group and four liberal group), gut infarction (four restrictive group) and AKI (126 in restrictive group and 105 in liberal group).

The combinations of primary outcome components occurring are described in Table 22. Four hundred and eighty participants experienced one component, of which sepsis alone was the most common (240/480, 50%); 151 participants experienced two components (of which sepsis and AKI was the most common combination; 72/151, 47.7%) and 17 participants experienced three components (of which sepsis, wound infection and AKI was the most common combination; 11/17, 64.7%). More participants in the restrictive group had AKI alone than the liberal group (7.2% vs. 5.9%), but fewer had AKI and sepsis (3.1% vs. 4.1%).
| Number of elements | Sepsis | Wound infection | Stroke | MI | Gut infarction | AKI | Randomised to restrictive threshold (N = 1000) | Randomised to liberal threshold (N = 1003) |
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||||
| None | 613 (61.3) | 645 (64.3) | ||||||
| No | No | No | No | No | No | 613 (61.3) | 645 (64.3) | |
| One | 244 (24.4) | 236 (23.5) | ||||||
| Yes | No | No | No | No | No | 120 (12.0) | 120 (12.0) | |
| No | No | No | No | No | Yes | 72 (7.2) | 59 (5.9) | |
| No | Yes | No | No | No | No | 20 (2.0) | 24 (2.4) | |
| Yes | Missing | No | No | No | No | 14 (1.4) | 14 (1.4) | |
| No | No | Yes | No | No | No | 6 (0.6) | 6 (0.6) | |
| No | Missing | No | No | No | Yes | 2 (0.2) | 3 (0.3) | |
| No | No | No | Yes | No | No | 1 (0.1) | 3 (0.3) | |
| Missing | Missing | Yes | Missing | Missing | Missing | 1 (0.1) | 2 (0.2) | |
| Missing | No | No | No | No | Yes | 3 (0.3) | 0 (0.0) | |
| Yes | Missing | Missing | Missing | Missing | Missing | 1 (0.1) | 2 (0.2) | |
| Yes | No | Missing | Missing | Missing | Missing | 1 (0.1) | 1 (0.1) | |
| Missing | Missing | Missing | Missing | Missing | Yes | 0 (0.0) | 1 (0.1) | |
| Missing | Missing | Yes | No | No | No | 0 (0.0) | 1 (0.1) | |
| Missing | No | Yes | Missing | Missing | Missing | 1 (0.1) | 0 (0.0) | |
| Missing | No | Yes | No | No | No | 1 (0.1) | 0 (0.0) | |
| No | No | No | No | Yes | No | 1 (0.1) | 0 (0.0) | |
| Two | 76 (7.6) | 75 (7.5) | ||||||
| Yes | No | No | No | No | Yes | 31 (3.1) | 41 (4.1) | |
| Yes | Yes | No | No | No | No | 19 (1.9) | 17 (1.7) | |
| Yes | Missing | No | No | No | Yes | 6 (0.6) | 4 (0.4) | |
| No | Yes | No | No | No | Yes | 7 (0.7) | 2 (0.2) | |
| Yes | No | Yes | No | No | No | 2 (0.2) | 4 (0.4) | |
| Yes | Missing | Missing | Missing | Missing | Yes | 2 (0.2) | 3 (0.3) | |
| No | No | Yes | No | No | Yes | 3 (0.3) | 1 (0.1) | |
| Yes | No | No | No | Yes | No | 2 (0.2) | 0 (0.0) | |
| Missing | Missing | Yes | Missing | Missing | Yes | 0 (0.0) | 1 (0.1) | |
| Missing | Yes | No | No | No | Yes | 1 (0.1) | 0 (0.0) | |
| No | No | No | No | Yes | Yes | 1 (0.1) | 0 (0.0) | |
| No | No | No | Yes | No | Yes | 1 (0.1) | 0 (0.0) | |
| Yes | Missing | No | No | Yes | No | 1 (0.1) | 0 (0.0) | |
| Yes | Missing | No | Yes | No | No | 0 (0.0) | 1 (0.1) | |
| Yes | No | Missing | Missing | Missing | Yes | 0 (0.0) | 1 (0.1) | |
| Three | 11 (1.1) | 6 (0.6) | ||||||
| Yes | Yes | No | No | No | Yes | 8 (0.8) | 3 (0.3) | |
| Yes | No | Yes | No | No | Yes | 1 (0.1) | 1 (0.1) | |
| Yes | Missing | Missing | Missing | Yes | Yes | 0 (0.0) | 1 (0.1) | |
| Yes | No | No | No | Yes | Yes | 1 (0.1) | 0 (0.0) | |
| Yes | No | No | Yes | No | Yes | 1 (0.1) | 0 (0.0) | |
| Yes | No | Yes | Missing | Missing | Yes | 0 (0.0) | 1 (0.1) | |
| Missing | 56 (5.6) | 41 (4.1) | ||||||
| No | Missing | No | No | No | No | 45 (4.5) | 26 (2.6) | |
| Missing | Missing | Missing | Missing | Missing | Missing | 5 (0.5) | 8 (0.8) | |
| Missing | No | No | No | No | No | 2 (0.2) | 6 (0.6) | |
| Missing | No | Missing | Missing | Missing | Missing | 2 (0.2) | 1 (0.1) | |
| Missing | Missing | No | No | No | No | 2 (0.2) | 0 (0.0) | |
Sensitivity analyses
Sensitivity analyses were pre-specified in the SAP, but not the study protocol; several of these were planned during data collection in response to knowledge about limitations of the study (e.g. non-adherence) or accruing data (e.g. inconsistency in data characterising renal function) but without any knowledge about how the sensitivity analyses would impact on the findings. The rationale and hypotheses for the sensitivity analyses have been explained previously (see Chapter 2, Sensitivity analyses).
The effect of non-adherence was assessed by estimating centre-specific treatment effects and ordering sites by rates of severe non-adherence (Figure 12). The hypothesis that estimates would tend towards the null with increasing non-adherence was not supported either visually, from the forest plot, or statistically in that a test for heterogeneity suggested no significant differences between sites (p = 0.65).
FIGURE 12.
Sensitivity analysis: primary outcome OR estimates by site, ranked by severe non-adherence rates. The grey vertical lines represent the overall treatment estimate (solid line) and 95% CI (dashed lines) of the primary outcome for the entire analysis cohort. The sizes of the point estimates reflect the centre sizes. Test for heterogeneity between sites; p = 0.65. Estimates have not been calculated for three sites with fewer than 20 participants with complete primary outcome data.

Excluding primary outcome events occurring in the first 24 hours after randomisation did not substantially alter the estimated treatment effect (Table 23), which did not support the hypothesis that the effect would tend away from the null. Excluding participants who received transfused red blood cells prior to randomisation caused the treatment effect estimate to increase (OR 1.23, 95% CI 0.97 to 1.54; p = 0.084), which was consistent with the hypothesis. Excluding AKI events without the relevant creatinine rise did not alter the treatment effect estimate; however, including additional AKI events not picked up via clinical assessment (anticipated to be milder events) caused the treatment effect estimate to increase (OR 1.20, 95% CI 1.00 to 1.44; p = 0.045) and the distribution of AKI events across AKI stages also became more pyramidal, consistent with adding in extra mild events. We had not hypothesised an increase in the treatment effect for this analysis but it was consistent with the observation that the small difference in the primary outcome frequency arose mainly from AKI events (see Table 23) and with the last planned sensitivity analysis, including only serious primary outcome events, which unexpectedly reduced the treatment effect estimate to unity (OR 0.99, 95% CI 0.77 to 1.27; p = 0.94).
| Sensitivity analysis | Randomised to restrictive threshold (N = 1000) | Randomised to liberal threshold (N = 1003) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Excluding primary outcome events occurring in the first 24 hours after randomisation, n/N (%) | 293/943 (31.1) | 284/956 (29.7) | 1.08 (0.88 to 1.31) | 0.47 |
| Excluding participants who were transfused red blood cells pre-randomisation, n/N (%) | 229/707 (32.4) | 202/712 (28.4) | 1.23 (0.97 to 1.54) | 0.084 |
| Excluding AKI events without relevant creatinine rise,a n/N (%) | 328/944 (34.8) | 315/962 (32.7) | 1.10 (0.91 to 1.34) | 0.33 |
| Including additional AKI events identified from routinely collected creatinine data,b n/N (%) | 477/959 (49.7) | 440/970 (45.4) | 1.20 (1.00 to 1.44) | 0.045 |
| Including only ‘serious’ primary outcome events, n/N (%) | ||||
| Any serious event | 145/985 (14.7) | 147/987 (14.9) | 0.99 (0.77 to 1.27) | 0.94 |
| Sepsis | 102/982 (10.4) | 110/983 (11.2) | ||
| Stroke | 15/989 (1.5) | 17/985 (1.7) | ||
| MI | 3/987 (0.3) | 4/981 (0.4) | ||
| Gut infarction | 6/987 (0.6) | 1/982 (0.1) | ||
| AKI | 50/989 (5.1) | 46/989 (4.7) |
Finally, the post-hoc analysis of severe in-hospital events (death, severe sepsis, ARDS, tracheostomy, low cardiac output, MI, AKI stage three, gut infarction and/or stroke) showed that this composite outcome occurred in 94/995 (9.5%) participants in the restrictive group and 90/993 (9.1%) in the liberal group (OR 1.05, 95% CI 0.77 to 1.43; p = 0.75).
Subgroup analyses
Subgroup analyses pre-specified in the study protocol are summarised in Figure 13. The subgroup analysis showing the largest difference between strata contrasted the treatment effect for participants with and without chronic obstructive pulmonary disease or asthma. The analysis suggested that the liberal transfusion strategy might be beneficial for participants with pulmonary comorbidity, although few participants had this comorbidity and the effect is not statistically significant. There was no other evidence of any subgroup effects.
FIGURE 13.
Subgroup analyses. The grey vertical lines represent the overall treatment estimate (solid line) and 95% CI (dashed lines) of the primary outcome for the entire analysis cohort. The sizes of the point estimates reflect the sizes of the subgroups. COPD, chronic obstructive pulmonary disease; LV, left ventricular; mod, moderate.
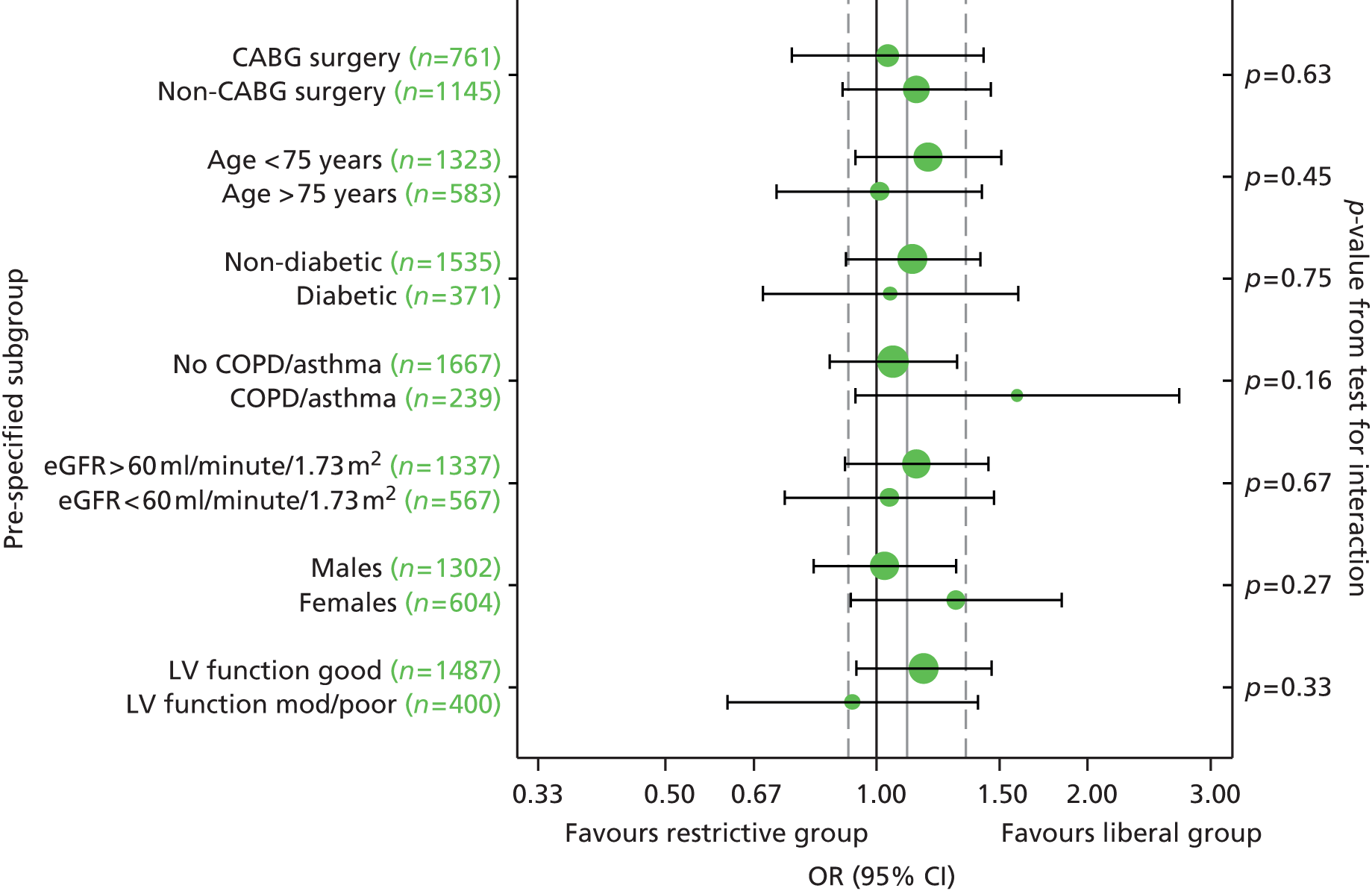
Secondary outcomes
Primary analyses
Infectious and ischaemic events
Infectious and ischaemic events are summarised in Table 21. Infectious events occurred equally often in the two groups, 25.4% in the restrictive group and 25.2% in the liberal group (OR 1.02, 95% CI 0.83 to 1.26; p = 0.83). However, as the number of missing data was over 5% (mainly due to missing post-hospital discharge data), a treatment effect was estimated separately for pre-hospital discharge infections only, as specified in the SAP (OR 1.07, 95% CI 0.85 to 1.36; p = 0.55). Ischaemic events were slightly more common in the restrictive group (15.7%) than the liberal group (14.0%; OR 1.16, 95% CI 0.90 to 1.40; p = 0.26). This small difference appears to arise mainly owing to AKI events – 14.2% in the restrictive group and 12.3% in the liberal group.
Other clinical outcomes
There were significantly more deaths from any cause in the restrictive group (4.2%) than the liberal group (2.6%; HR 1.64, 95% CI 1.00 to 2.67; p = 0.045) (Table 24 and Figure 14). Causes of death and other SAEs that preceded death are given in Table 25. There are no clear causes of death contributing to the excess in the restrictive group; the common causes were cardiac disorders (21 participants), infections/infestations (13 participants) and general disorders and administration site conditions (10 participants). The primary outcome was experienced before death by 65% of participants. With respect to SAEs preceding death, 55% of the deaths in the restrictive group were preceded by an ischaemic SAE, whereas 58% of the deaths in the liberal group were preceded by an infectious SAE.
| Outcome | Randomised to restrictive threshold (N = 1000) | Randomised to liberal threshold (N = 1003) | Effect (95% CI) | p-value |
|---|---|---|---|---|
| All-cause mortality, n/N (%) | 42/1000 (4.2) | 26/1003 (2.6) | HR 1.64 (1.00 to 2.67) | 0.045 |
| Significant pulmonary morbidity, n/N (%) | 127/979 (13.0) | 116/982 (11.8) | OR 1.11 (0.85 to 1.45) | 0.45 |
| Initiation of non-invasive ventilation | 88/989 (8.9) | 77/984 (7.8) | ||
| Re-intubation/ventilation | 50/975 (5.1) | 53/973 (5.4) | ||
| Tracheostomy | 30/988 (3.0) | 32/988 (3.2) | ||
| Duration of ICU/HDU stay (hours),a median (IQR) | 49.5 (21.9–99.7) | 45.9 (20.1–94.8) | HR 0.97 (0.89 to 1.06) | 0.53 |
| Duration of hospital stay (days),b median (IQR) | 7.0 (5.0–10.0) | 7.0 (5.0–10.0) | HR 1.00 (0.92 to 1.10) | 0.94 |
FIGURE 14.
Kaplan–Meier estimates of time from randomisation to all-cause mortality.
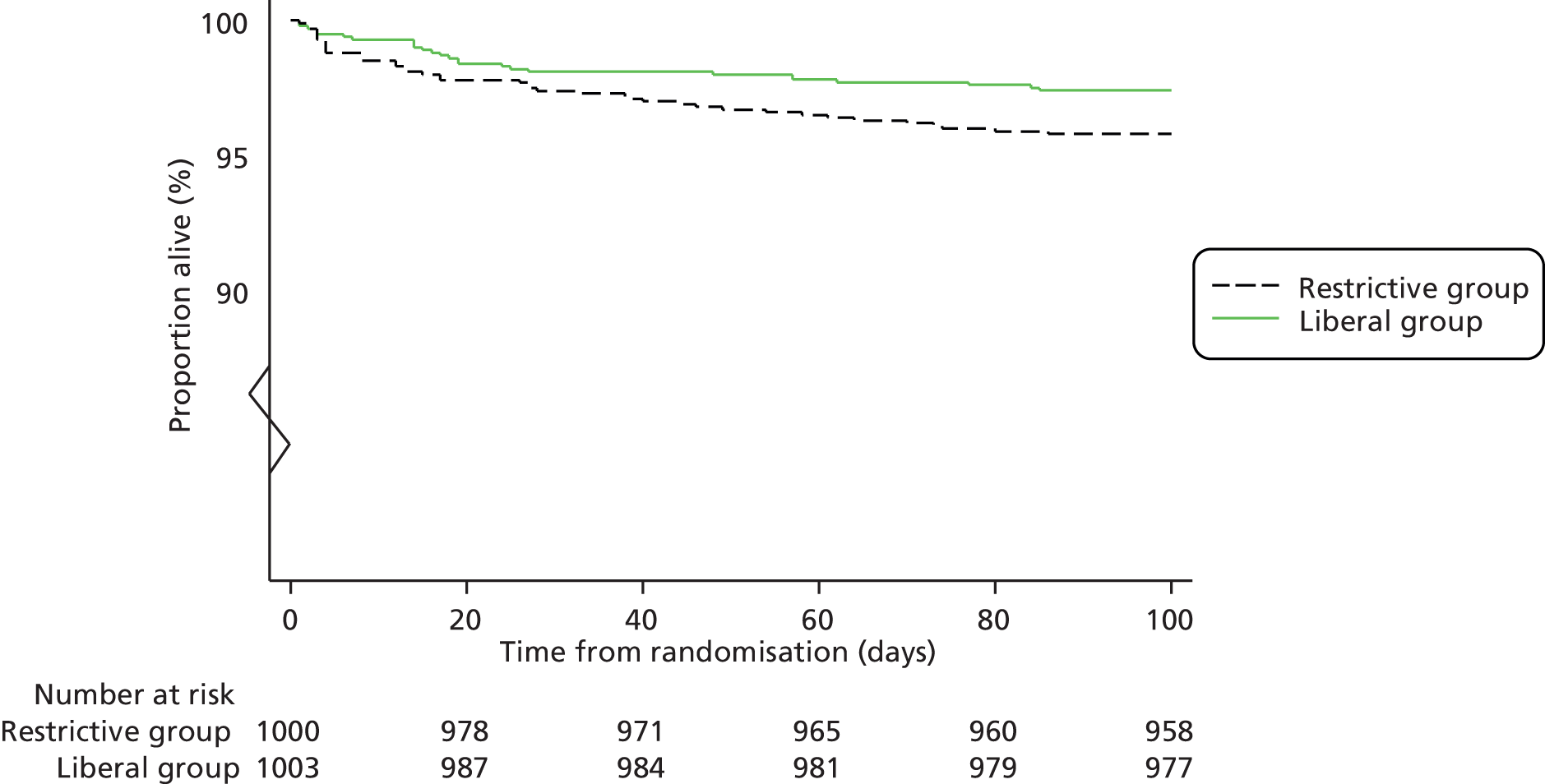
| Causes of deatha | Randomised to restrictive threshold | Randomised to liberal threshold | ||
|---|---|---|---|---|
| Events | Participants (n = 42) | Events | Participants (n = 26) | |
| Blood and lymphatic system disorders | 0 | 0 | 1 | 1 |
| Coagulopathy | 0 | 0 | 1 | 1 |
| Cardiac disorders | 10 | 10 | 11 | 11 |
| Arrhythmia | 4 | 4 | 1 | 1 |
| Cardiac arrest | 1 | 1 | 0 | 0 |
| Cardiac failure | 3 | 3 | 1 | 1 |
| Cardiac failure acute | 0 | 0 | 2 | 2 |
| Cardiac failure congestive | 1 | 1 | 4 | 4 |
| Left ventricular failure | 1 | 1 | 1 | 1 |
| Left ventricular hypertrophy | 0 | 0 | 1 | 1 |
| Pericardial haemorrhage | 0 | 0 | 1 | 1 |
| GI disorders | 6 | 6 | 1 | 1 |
| Duodenal ulcer perforation | 1 | 1 | 0 | 0 |
| GI haemorrhage | 0 | 0 | 1 | 1 |
| Intestinal infarction | 1 | 1 | 0 | 0 |
| Intestinal ischaemia | 3 | 3 | 0 | 0 |
| Peritonitis | 1 | 1 | 0 | 0 |
| General disorders and administration site conditions | 7 | 7 | 3 | 3 |
| Multiorgan failure | 7 | 7 | 3 | 3 |
| Hepatobiliary disorders | 1 | 1 | 0 | 0 |
| Hepatic necrosis | 1 | 1 | 0 | 0 |
| Infections and infestations | 8 | 8 | 5 | 5 |
| Bronchopneumonia | 1 | 1 | 2 | 2 |
| Empyema | 0 | 0 | 1 | 1 |
| Endocarditis | 1 | 1 | 1 | 1 |
| Lower respiratory tract infection | 1 | 1 | 0 | 0 |
| Pneumonia | 3 | 3 | 1 | 1 |
| Sepsis | 2 | 2 | 0 | 0 |
| Neoplasms benign, malignant and unspecified | 1 | 1 | 0 | 0 |
| Brain cancer metastatic | 1 | 1 | 0 | 0 |
| Nervous system disorders | 3 | 3 | 2 | 2 |
| Cerebral haemorrhage | 1 | 1 | 0 | 0 |
| CVA | 1 | 1 | 2 | 2 |
| Haemorrhage intracranial | 1 | 1 | 0 | 0 |
| Renal and urinary disorders | 1 | 1 | 0 | 0 |
| Renal failure | 1 | 1 | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | 4 | 4 | 0 | 0 |
| ARDS | 1 | 1 | 0 | 0 |
| Hypoxia | 1 | 1 | 0 | 0 |
| Pulmonary oedema | 2 | 2 | 0 | 0 |
| Surgical and medical procedures | 3 | 3 | 3 | 3 |
| Cardiac operation | 3 | 3 | 2 | 2 |
| Ventriculocardiac shunt | 0 | 0 | 1 | 1 |
| Vascular disorders | 1 | 1 | 3 | 3 |
| Aortic aneurysm rupture | 1 | 1 | 1 | 1 |
| Haemorrhage | 0 | 0 | 2 | 2 |
| SAEs preceding deatha | Randomised to restrictive threshold | Randomised to liberal threshold | |||
|---|---|---|---|---|---|
| Events, n | Participants (N = 42), n/N (%) | Events | Participants (N = 26), n/N (%) | ||
| Primary outcome | 26/40 (65) | 17/26 (65) | |||
| Sepsis | 11/40 (28) | 15/26 (58) | |||
| Wound infection | 2/42 (5) | 0/26 (0) | |||
| Permanent stroke | 3/42 (7) | 0/26 (0) | |||
| MI | 0/42 (0) | 1/26 (4) | |||
| Gut infarction | 4/42 (10) | 0/26 (0) | |||
| AKI | 16/42 (38) | 12/26 (46) | |||
| Transient ischaemic attack | 0 | 0/42 (0) | 1 | 1/26 (4) | |
| GI complications | 2 | 2/42 (5) | 8 | 7/26 (27) | |
| Post-operative haemorrhage | 1 | 1/42 (2) | 1 | 1/26 (4) | |
| Cardiac tamponade | 0 | 0/42 (0) | 0 | 0/26 (0) | |
| Pulmonary complications | 23 | 14/41 (34) | 29 | 10/26 (38) | |
| Arrhythmias | 15 | 12/42 (29) | 9 | 7/26 (27) | |
| Re-operation | 12 | 11/42 (26) | 4 | 4/26 (15) | |
| Thromboembolic complications | 1 | 1/42 (2) | 0 | 0/26 (0) | |
| Low cardiac output | 6 | 6/42 (14) | 10 | 6/26 (23) | |
| Wound dehiscence | 0 | 0/42 (0) | 6 | 4/26 (15) | |
| Other (unexpected event) | 5 | 5/42 (12) | 1 | 1/26 (4) | |
| Cardiac arrest | 1 | 1/42 | 0 | 0/26 | |
| Cardiac failure | 3 | 3/42 | 0 | 0/26 | |
| Cardiac failure congestive | 0 | 0/42 | 1 | 1/26 | |
| Compartment syndrome | 1 | 1/42 | 0 | 0/26 | |
In the restrictive group, 13.0% of participants experienced significant pulmonary morbidity compared with 11.8% participants in the liberal group (OR 1.11, 95% CI 0.85 to 1.45; p = 0.45). The median duration of post-randomisation ICU/HDU stay was 49.5 hours (IQR 21.9–99.7 hours) in the restrictive group and 45.9 hours (IQR 20.1–94.8 hours) in the liberal group (see Table 24). This difference was not statistically significant (HR 0.97, 95% CI 0.89 to 1.06; p = 0.53). Durations of total post-randomisation hospital stay were very similar in both groups [medians and IQRs for both groups were 7 days and IQR 5–10 days (HR 1.00, 95% CI 0.92 to 1.10; p = 0.94)].
Quality of life
Crude responses to the five EQ-5D-3L component questions show no clear trends between the treatment groups (Table 26), although there was some suggestion of slightly improved scores on the mobility and usual activities domains in the restrictive compared with the liberal group post randomisation. No formal statistical comparisons were performed and the usual activities domain was also slightly imbalanced at baseline. The median utility and visual analogue scale (VAS) scores were similar in the two groups at all three time points (Table 27). Modelling the utility score demonstrated that participants in the restrictive group were slightly less likely to experience a score representing imperfect health than the liberal group, although this difference was not statistically significant (OR 0.89, 95% CI 0.71 to 1.12; p = 0.33). Scores for those participants with imperfect health were similar in the group groups [geometric mean ratio (GMR) 0.99, 95% CI 0.95 to 1.12; p = 0.68). For the VAS, the occurrence model suggested very little difference between the groups in the proportions of participants experiencing imperfect health (OR 1.11, 95% CI 0.57 to 2.15; p = 0.76). Of those with imperfect health on the VAS score, the average score was slightly higher (representing better health) for the restrictive group (GMR 0.97, 95% CI 0.92 to 1.02; p = 0.21). There was no evidence of a treatment by time interaction for either measure, implying that any difference between groups did not change between 6 weeks and 3 months after randomisation.
| EQ-5D-3L component | Randomised to restrictive threshold (N = 1000) | Randomised to liberal threshold (N = 1003) |
|---|---|---|
| Mobility, n/N (%) | ||
| Pre-operative | ||
| I have no problems walking about | 586/995 (58.9) | 583/996 (58.5) |
| I have some problems walking about | 400/995 (40.2) | 410/996 (41.2) |
| I am confined to bed | 9/995 (0.9) | 3/996 (0.3) |
| 6 weeks post randomisation | ||
| I have no problems walking about | 524/790 (66.3) | 521/823 (63.3) |
| I have some problems walking about | 261/790 (33.0) | 299/823 (36.3) |
| I am confined to bed | 5/790 (0.6) | 3/823 (0.4) |
| 3 months post randomisation | ||
| I have no problems walking about | 572/833 (68.7) | 549/850 (64.6) |
| I have some problems walking about | 259/833 (31.1) | 300/850 (35.3) |
| I am confined to bed | 2/833 (0.2) | 1/850 (0.1) |
| Self-care, n/N (%) | ||
| Pre-operative | ||
| I have no problems with self-care | 901/996 (90.5) | 923/997 (92.6) |
| I have some problems with self-care | 90/996 (9.0) | 71/997 (7.1) |
| I am unable to wash and dress myself | 5/996 (0.5) | 3/997 (0.3) |
| 6 weeks post randomisation | ||
| I have no problems with self-care | 648/792 (81.8) | 664/824 (80.6) |
| I have some problems with self-care | 136/792 (17.2) | 152/824 (18.4) |
| I am unable to wash and dress myself | 8/792 (1.0) | 8/824 (1.0) |
| 3 months post randomisation | ||
| I have no problems with self-care | 722/833 (86.7) | 728/849 (85.7) |
| I have some problems with self-care | 108/833 (13.0) | 117/849 (13.8) |
| I am unable to wash and dress myself | 3/833 (0.4) | 4/849 (0.5) |
| Usual activities, n/N (%) | ||
| Pre-operative | ||
| I have no problems with doing my usual activities | 549/995 (55.2) | 513/996 (51.5) |
| I have some problems with doing my usual activities | 372/995 (37.4) | 418/996 (42.0) |
| I am unable to perform my usual activities | 74/995 (7.4) | 65/996 (6.5) |
| 6 weeks post randomisation | ||
| I have no problems with doing my usual activities | 263/787 (33.4) | 257/822 (31.3) |
| I have some problems with doing my usual activities | 455/787 (57.8) | 485/822 (59.0) |
| I am unable to perform my usual activities | 69/787 (8.8) | 80/822 (9.7) |
| 3 months post randomisation | ||
| I have no problems with doing my usual activities | 455/835 (54.5) | 423/850 (49.8) |
| I have some problems with doing my usual activities | 349/835 (41.8) | 394/850 (46.4) |
| I am unable to perform my usual activities | 31/835 (3.7) | 33/850 (3.9) |
| Pain/discomfort, n/N (%) | ||
| Pre-operative | ||
| I have no pain or discomfort | 609/995 (61.2) | 573/995 (57.6) |
| I have moderate pain or discomfort | 356/995 (35.8) | 393/995 (39.5) |
| I have extreme pain or discomfort | 30/995 (3.0) | 29/995 (2.9) |
| 6 weeks post randomisation | ||
| I have no pain or discomfort | 265/791 (33.5) | 279/823 (33.9) |
| I have moderate pain or discomfort | 516/791 (65.2) | 528/823 (64.2) |
| I have extreme pain or discomfort | 10/791 (1.3) | 16/823 (1.9) |
| 3 months post randomisation | ||
| I have no pain or discomfort | 418/836 (50.0) | 432/850 (50.8) |
| I have moderate pain or discomfort | 400/836 (47.8) | 392/850 (46.1) |
| I have extreme pain or discomfort | 18/836 (2.2) | 26/850 (3.1) |
| Anxiety/depression, n/N (%) | ||
| Pre-operative | ||
| I am not anxious or depressed | 683/994 (68.7) | 687/996 (69.0) |
| I am moderately anxious or depressed | 278/994 (28.0) | 284/996 (28.5) |
| I am extremely anxious or depressed | 33/994 (3.3) | 25/996 (2.5) |
| 6 weeks post randomisation | ||
| I am not anxious or depressed | 602/791 (76.1) | 592/823 (71.9) |
| I am moderately anxious or depressed | 181/791 (22.9) | 215/823 (26.1) |
| I am extremely anxious or depressed | 8/791 (1.0) | 16/823 (1.9) |
| 3 months post randomisation | ||
| I am not anxious or depressed | 635/836 (76.0) | 640/850 (75.3) |
| I am moderately anxious or depressed | 186/836 (22.2) | 196/850 (23.1) |
| I am extremely anxious or depressed | 15/836 (1.8) | 14/850 (1.6) |
| EQ-5D-3L score | Randomised to restrictive threshold (n = 1000) | Randomised to liberal threshold (n = 1003) | Occurrence model | Intensity model | Test for treatment by time interactionc | ||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | ORa (95% CI) | p-value | GMRb (95% CI) | p-value | ||
| Utility score | |||||||
| Pre-operatived | 0.81 (0.69–1.00) | 0.81 (0.69–1.00) | |||||
| 6 weeks post randomisatione | 0.76 (0.69–0.81) | 0.76 (0.64–0.81) | |||||
| 3 months post randomisationf | 0.80 (0.69–1.00) | 0.80 (0.69–1.00) | |||||
| Overall estimate of treatment effect | 0.89 (0.71 to 1.12) | 0.33 | 0.99 (0.95 to 1.03) | 0.68 | 0.20 | ||
| VAS | |||||||
| Pre-operativeg | 70.0 (55.0–80.0) | 70.0 (51.0–80.0) | |||||
| 6 weeks post randomisationh | 75.0 (61.0–85.0) | 75.0 (60.0–80.0) | |||||
| 3 months post randomisationi | 80.0 (70.0–90.0) | 80.0 (69.0–90.0) | |||||
| Overall estimate of treatment effect | 1.11 (0.57 to 2.15) | 0.76 | 0.97 (0.92 to 1.02) | 0.21 | 0.60 | ||
Sensitivity analyses
The two sensitivity analyses outlined for the primary outcome that could be applied to the secondary outcome of mortality were performed on a post-hoc basis. The results are shown in Table 28. The treatment effects for both analyses, excluding deaths occurring in the first 24 hours after randomisation and excluding participants who had red blood cells transfused before randomisation, were shifted further away from unity, as was hypothesised.
| Outcome | Randomised to restrictive threshold (N = 1000), n/N (%) | Randomised to liberal threshold (N = 1003), n/N (%) | HR (95% CI) | p-value |
|---|---|---|---|---|
| Excluding deaths occurring in the first 24 hours after randomisation | 41/999 (4.1) | 24/1001 (2.4) | HR 1.73 (1.05 to 2.87) | 0.029 |
| Excluding participants transfused red blood cells pre-randomisation | 23/750 (3.1) | 11/739 (1.5) | HR 2.15 (1.04 to 4.40) | 0.032 |
Adverse events
All expected and unexpected SAEs (excluding the primary outcome and mortality) occurring after randomisation are summarised in Table 29. There were 664 events occurring in 35.7% of participants in the restrictive group and 648 events in 34.2% participants in the liberal group. There were slightly more participants with pulmonary complications (12.9% vs. 10.6%) and arrhythmias (15.3% vs. 12.8%) in the restrictive group than the liberal group, and slightly fewer participants with gastrointestinal (GI) complications (3.8% vs. 5.1%). The most common events were arrhythmias (14.0%), pulmonary complications (11.8%) and other (i.e. unexpected) events (9.0%).
| Event type | Randomised to restrictive threshold | Randomised to liberal threshold | ||||
|---|---|---|---|---|---|---|
| Events (n) | Participants (N = 1000), (n/N) | % | Events (n) | Participants (N = 1003), (n/N) | % | |
| Any event | 664 | 354/991 | 35.7 | 648 | 339/991 | 34.2 |
| Transient ischaemic attack | 6 | 6/987 | 0.6 | 3 | 3/981 | 0.3 |
| GI complications | 40 | 37/986 | 3.8 | 55 | 50/983 | 5.1 |
| Post-operation haemorrhage | 12 | 12/987 | 1.2 | 18 | 17/982 | 1.7 |
| Cardiac tamponade | 2 | 2/987 | 0.2 | 2 | 2/981 | 0.2 |
| Pulmonary complications | 200 | 127/986 | 12.9 | 170 | 105/988 | 10.6 |
| Arrhythmias | 186 | 151/989 | 15.3 | 152 | 126/984 | 12.8 |
| Reoperation | 70 | 63/988 | 6.4 | 80 | 73/983 | 7.4 |
| Thromboembolic complications | 9 | 9/985 | 0.9 | 15 | 12/981 | 1.2 |
| Low cardiac output | 17 | 16/988 | 1.6 | 20 | 16/983 | 1.6 |
| Wound dehiscence | 19 | 16/987 | 1.6 | 20 | 18/981 | 1.8 |
| Other (unexpected event)a | 103 | 88/1000 | 8.8 | 113 | 93/1003 | 9.3 |
A summary measure was created (as a post-hoc analysis) combining the events in Table 29 with the primary outcome and mortality. This measure could be relevant if the mechanisms hypothesised to justify the superiority hypothesis and to classify SAEs as expected or unexpected were subsequently considered to be unsound. In the restrictive group 523 out of 961 (54.4%) participants experienced this composite, compared with 492 out of 971 (50.7%) in the liberal group.
Detailed examination of the events is undertaken in the following sections.
Expected adverse events
Prior to hospital discharge (Table 30) there were 988 expected AEs in the restrictive group occurring in 49.6% of participants, and 938 in 48.4% of participants in the liberal group. Of these, 418 events in the restrictive group (23.2% of participants) and 405 events in the liberal group (21.5% of participants) were classified as serious. The most frequent events were arrhythmias. The numbers of pulmonary complications and arrhythmias were slightly larger in the restrictive group than the liberal group, which is consistent with Table 29.
| Event type | Randomised to restrictive threshold | Randomised to liberal threshold | ||||||
|---|---|---|---|---|---|---|---|---|
| Events | Participants (N = 1000) | Events | Participants (N = 1003) | |||||
| AE | SAE | AE, n/N (%) | SAE, n/N (%) | AE | SAE | AE, n/N (%) | SAE, n/N (%) | |
| Any event | 988 | 418 | 496/1000 (49.6) | 232/998 (23.2) | 938 | 405 | 485/1003 (48.4) | 216/1003 (21.5) |
| Transient ischaemic attack | 4 | 2 | 4/1000 (0.4) | 2/1000 (0.2) | 2 | 1 | 2/1003 (0.2) | 1/1003 (0.1) |
| GI complications | 49 | 20 | 44/1000 (4.4) | 17/999 (1.7) | 46 | 30 | 40/1003 (4.0) | 26/1003 (2.6) |
| Pancreatitis | 2 | 0 | 2/1000 (0.2) | 0/1000 (0.0) | 0 | 0 | 0/1003 (0.0) | 0/1003 (0.0) |
| Intestinal obstruction/perforation | 1 | 0 | 1/1000 (0.1) | 0/1000 (0.0) | 5 | 4 | 5/1003 (0.5) | 4/1003 (0.4) |
| Other GI complications | 46 | 20 | 41/999 (4.1) | 17/999 (1.7) | 41 | 26 | 38/1003 (3.8) | 24/1003 (2.4) |
| Post-operation haemorrhage | 31 | 11 | 31/1000 (3.1) | 11/1000 (1.1) | 37 | 16 | 34/1003 (3.4) | 15/1003 (1.5) |
| Cardiac tamponade | 1 | 1 | 1/1000 (0.1) | 1/1000 (0.1) | 1 | 1 | 1/1003 (0.1) | 1/1003 (0.1) |
| Pulmonary complications | 288 | 137 | 177/998 (17.7) | 81/998 (8.1) | 286 | 118 | 169/1003 (16.8) | 66/1003 (6.6) |
| ARDS | 5 | 4 | 5/999 (0.5) | 4/999 (0.4) | 3 | 0 | 3/1003 (0.3) | 0/1003 (0.0) |
| Reintubation/ventilation | 54 | 35 | 50/1000 (5.0) | 31/1000 (3.1) | 61 | 31 | 53/1003 (5.3) | 25/1003 (2.5) |
| Tracheostomy | 31 | 20 | 30/999 (3.0) | 19/999 (1.9) | 33 | 18 | 32/1003 (3.2) | 18/1003 (1.8) |
| Initiation of mask CPAP | 95 | 29 | 87/1000 (8.7) | 28/1000 (2.8) | 88 | 26 | 77/1003 (7.7) | 23/1003 (2.3) |
| Pneumothorax requiring drainage | 10 | 6 | 10/999 (1.0) | 6/999 (0.6) | 9 | 3 | 9/1003 (0.9) | 3/1003 (0.3) |
| Pleural effusion requiring drainage | 56 | 25 | 54/999 (5.4) | 24/999 (2.4) | 56 | 24 | 49/1003 (4.9) | 22/1003 (2.2) |
| Other pulmonary complications | 37 | 18 | 36/999 (3.6) | 17/999 (1.7) | 36 | 16 | 33/1003 (3.3) | 14/1003 (1.4) |
| Arrhythmias | 489 | 148 | 374/1000 (37.4) | 121/999 (12.1) | 436 | 127 | 354/1003 (35.3) | 108/1003 (10.8) |
| Pacing | 87 | 25 | 84/1000 (8.4) | 24/1000 (2.4) | 62 | 14 | 58/1003 (5.8) | 14/1003 (1.4) |
| SVT/AF requiring treatment | 346 | 96 | 312/1000 (31.2) | 90/1000 (9.0) | 329 | 94 | 292/1003 (29.1) | 83/1003 (8.3) |
| VF/VT requiring treatment | 17 | 8 | 13/1000 (1.3) | 6/1000 (0.6) | 6 | 2 | 6/1003 (0.6) | 2/1003 (0.2) |
| Other arrhythmias | 39 | 19 | 34/999 (3.4) | 17/999 (1.7) | 39 | 17 | 39/1003 (3.9) | 17/1003 (1.7) |
| Reoperation | 68 | 68 | 62/1000 (6.2) | 62/1000 (6.2) | 78 | 78 | 71/1003 (7.1) | 71/1003 (7.1) |
| Thromboembolic complications | 6 | 5 | 6/998 (0.6) | 5/998 (0.5) | 2 | 1 | 2/1003 (0.2) | 1/1003 (0.1) |
| Deep-vein thrombosis | 0 | 0 | 0/1000 (0.0) | 0/1000 (0.0) | 1 | 1 | 1/1003 (0.1) | 1/1003 (0.1) |
| Pulmonary embolus | 0 | 0 | 0/1000 (0.0) | 0/1000 (0.0) | 0 | 0 | 0/1003 (0.0) | 0/1003 (0.0) |
| Other thromboembolic complications | 6 | 5 | 6/998 (0.6) | 5/998 (0.5) | 1 | 0 | 1/1003 (0.1) | 0/1003 (0.0) |
| Low cardiac output | 32 | 14 | 31/1000 (3.1) | 13/1000 (1.3) | 26 | 18 | 22/1003 (2.2) | 14/1003 (1.4) |
| Wound dehiscence | 20 | 12 | 17/1000 (1.7) | 9/1000 (0.9) | 24 | 15 | 22/1003 (2.2) | 13/1003 (1.3) |
Expected SAEs occurred less frequently after hospital discharge (Table 31), when there were 143 SAEs in 11.4% of participants in the restrictive group and 130 SAEs in 10.5% of participants in the liberal group. The most common events were pulmonary complications (5.0%). There were no clear differences between the groups although, again, rates of pulmonary complications and arrhythmias were slightly higher in the restrictive group than the liberal group (5.5% vs. 4.6% and 3.4% vs. 2.4%, respectively).
| Event type | Randomised to restrictive threshold | Randomised to liberal threshold | ||
|---|---|---|---|---|
| SAEs, n | Participants (N = 1000), n/N (%) | SAEs, n | Participants (N = 1003), n/N (%) | |
| Any event | 143 | 113/987 (11.4) | 130 | 103/981 (10.5) |
| Transient ischaemic attack | 4 | 4/987 (0.4) | 2 | 2/981 (0.2) |
| GI complications | 20 | 20/987 (2.0) | 25 | 24/981 (2.4) |
| Pancreatitis | 0 | 0/987 (0.0) | 2 | 2/981 (0.2) |
| Intestinal obstruction/perforation | 0 | 0/987 (0.0) | 0 | 0/981 (0.0) |
| Other GI complications | 20 | 20/987 (2.0) | 23 | 23/981 (2.3) |
| Post-operation haemorrhage | 1 | 1/987 (0.1) | 2 | 2/981 (0.2) |
| Cardiac tamponade | 1 | 1/987 (0.1) | 1 | 1/981 (0.1) |
| Pulmonary complications | 63 | 54/987 (5.5) | 52 | 45/981 (4.6) |
| ARDS | 0 | 0/987 (0.0) | 0 | 0/981 (0.0) |
| Reintubation/ventilation | 0 | 0/987 (0.0) | 0 | 0/981 (0.0) |
| Tracheostomy | 0 | 0/987 (0.0) | 0 | 0/981 (0.0) |
| Initiation of mask CPAP | 1 | 1/987 (0.1) | 0 | 0/981 (0.0) |
| Pneumothorax requiring drainage | 0 | 0/987 (0.0) | 1 | 1/981 (0.1) |
| Pleural effusion requiring drainage | 34 | 31/987 (3.1) | 32 | 30/981 (3.1) |
| Other pulmonary complication | 28 | 26/987 (2.6) | 19 | 17/981 (1.7) |
| Arrhythmias | 38 | 34/987 (3.4) | 25 | 24/981 (2.4) |
| Pacing | 0 | 0/987 (0.0) | 0 | 0/981 (0.0) |
| SVT/AF requiring treatment | 27 | 24/987 (2.4) | 22 | 21/981 (2.1) |
| VF/VT requiring treatment | 0 | 0/987 (0.0) | 0 | 0/981 (0.0) |
| Other arrhythmias | 11 | 10/987 (1.0) | 3 | 3/981 (0.3) |
| Re-operation | 2 | 2/987 (0.2) | 2 | 2/981 (0.2) |
| Thromboembolic complications | 4 | 4/987 (0.4) | 14 | 11/981 (1.1) |
| Deep-vein thrombosis | 2 | 2/987 (0.2) | 5 | 4/981 (0.4) |
| Pulmonary embolus | 1 | 1/987 (0.1) | 7 | 6/981 (0.6) |
| Other thromboembolic complications | 1 | 1/987 (0.1) | 2 | 2/981 (0.2) |
| Low cardiac output | 3 | 3/987 (0.3) | 2 | 2/981 (0.2) |
| Wound dehiscence | 7 | 7/987 (0.7) | 5 | 5/981 (0.5) |
The classification of SAEs occurring at any time (either before or after discharge) suggests that the small differences in the frequencies of GI complications, pulmonary complications and arrhythmias identified between the groups were not due to any particular SAEs within each category (Table 32). Instead, all subcategories of SAEs appear to demonstrate differences between the groups in the same direction, which aggregate to the overall slight differences observed in Table 29.
| Event type | Randomised to restrictive threshold | Randomised to liberal threshold | ||
|---|---|---|---|---|
| SAEs, n | Participants (N = 1000), n/N (%) | SAEs, n | Participants (N = 1003), n/N (%) | |
| Any event | 561 | 311/990 (31.4) | 535 | 293/991 (29.6) |
| Transient ischaemic attack | 6 | 6/987 (0.6) | 3 | 3/981 (0.3) |
| GI complications | 40 | 37/986 (3.8) | 55 | 50/983 (5.1) |
| Pancreatitis | 0 | 0/987 (0.0) | 2 | 2/981 (0.2) |
| Intestinal obstruction/perforation | 0 | 0/987 (0.0) | 4 | 4/981 (0.4) |
| Other GI complications | 40 | 37/986 (3.8) | 49 | 47/983 (4.8) |
| Post-operation haemorrhage | 12 | 12/987 (1.2) | 18 | 17/982 (1.7) |
| Cardiac tamponade | 2 | 2/987 (0.2) | 2 | 2/981 (0.2) |
| Pulmonary complications | 200 | 127/986 (12.9) | 170 | 105/988 (10.6) |
| ARDS | 4 | 4/986 (0.4) | 0 | 0/981 (0.0) |
| Reintubation/ventilation | 35 | 31/988 (3.1) | 31 | 25/983 (2.5) |
| Tracheostomy | 20 | 19/987 (1.9) | 18 | 18/985 (1.8) |
| Initiation of mask CPAP | 30 | 29/987 (2.9) | 26 | 23/982 (2.3) |
| Pneumothorax requiring drainage | 6 | 6/986 (0.6) | 4 | 4/981 (0.4) |
| Pleural effusion requiring drainage | 59 | 52/987 (5.3) | 56 | 52/981 (5.3) |
| Other pulmonary complication | 46 | 42/986 (4.3) | 35 | 31/983 (3.2) |
| Arrhythmias | 186 | 151/989 (15.3) | 152 | 126/984 (12.8) |
| Pacing | 25 | 24/987 (2.4) | 14 | 14/981 (1.4) |
| SVT/AF requiring treatment | 123 | 110/990 (11.1) | 116 | 102/982 (10.4) |
| VF/VT requiring treatment | 8 | 6/987 (0.6) | 2 | 2/981 (0.2) |
| Other arrhythmias | 30 | 27/987 (2.7) | 20 | 20/983 (2.0) |
| Re-operation | 70 | 63/988 (6.4) | 80 | 73/983 (7.4) |
| Thromboembolic complications | 9 | 9/985 (0.9) | 15 | 12/981 (1.2) |
| Deep-vein thrombosis | 2 | 2/987 (0.2) | 6 | 5/981 (0.5) |
| Pulmonary embolus | 1 | 1/987 (0.1) | 7 | 6/981 (0.6) |
| Other thromboembolic complications | 6 | 6/985 (0.6) | 2 | 2/981 (0.2) |
| Low cardiac output | 17 | 16/988 (1.6) | 20 | 16/983 (1.6) |
| Wound dehiscence | 19 | 16/987 (1.6) | 20 | 18/981 (1.8) |
Unexpected serious adverse events
There were 103 unexpected SAEs occurring in 8.8% of participants in the restrictive group and 113 events in 9.3% participants in the liberal group (Table 33). Classifying events according to the MedDRA dictionary suggests that cardiac disorders were the most frequent unexpected SAEs (3.0% of participants), in particular cardiac failure (30 events) and pericardial effusion (20 events). Other types of event were rare. There were more cases of anaemia in the restrictive group (six cases vs. one case) and more cases of cardiac failure in the liberal group (19 cases vs. 11 cases), although the numbers of both events were low. There were no other trends between the groups.
| Event type | Randomised to restrictive threshold (N = 1000) | Randomised to liberal threshold (N = 1003) | ||||
|---|---|---|---|---|---|---|
| Events (n) | Participants, n (%) | Events (n) | Participants, n (%) | |||
| Any event | 103 | 88 (8.8) | 113 | 93 (9.3) | ||
| Description of events (MedDRA terms) | ||||||
| Blood and lymphatic system disorders | 6 | 5 (0.5) | 2 | 2 (0.2) | ||
| Anaemia | 4 | 4 | 1 | 1 | ||
| Haemolytic anaemia | 2 | 1 | 0 | 0 | ||
| Coagulopathy | 0 | 0 | 1 | 1 | ||
| Cardiac disorders | 29 | 26 (2.6) | 41 | 35 (3.5) | ||
| Cardiac arrest | 7 | 7 | 1 | 1 | ||
| Cardiac failure | 11 | 11 | 19 | 18 | ||
| Cardiorespiratory arrest | 0 | 0 | 2 | 1 | ||
| Heart valve incompetence | 0 | 0 | 1 | 1 | ||
| Left ventricular failure | 2 | 2 | 1 | 1 | ||
| Left ventricular hypertrophy | 0 | 0 | 1 | 1 | ||
| MV incompetence | 1 | 1 | 0 | 0 | ||
| Palpitations | 0 | 0 | 1 | 1 | ||
| Pericardial effusion | 8 | 6 | 12 | 11 | ||
| Pericarditis | 0 | 0 | 1 | 1 | ||
| Pericarditis constrictive | 0 | 0 | 1 | 1 | ||
| TV incompetence | 0 | 0 | 1 | 1 | ||
| Eye disorders | 2 | 2 (0.2) | 0 | 0 (0.0) | ||
| Diplopia | 1 | 1 | 0 | 0 | ||
| Visual impairment | 1 | 1 | 0 | 0 | ||
| General disorders and administration site conditions | 12 | 12 (1.2) | 10 | 10 (1.0) | ||
| Adverse drug reaction | 0 | 0 | 1 | 1 | ||
| Chest pain | 2 | 2 | 2 | 2 | ||
| Local swelling | 0 | 0 | 1 | 1 | ||
| Malaise | 0 | 0 | 1 | 1 | ||
| Multiorgan failure | 6 | 6 | 3 | 3 | ||
| Non-cardiac chest pain | 1 | 1 | 2 | 2 | ||
| Oedema peripheral | 1 | 1 | 0 | 0 | ||
| Pain | 1 | 1 | 0 | 0 | ||
| Swelling | 1 | 1 | 0 | 0 | ||
| Hepatobiliary disorders | 1 | 1 (0.1) | 2 | 2 (0.2) | ||
| Alcoholic liver disease | 0 | 0 | 1 | 1 | ||
| Hepatic cyst | 0 | 0 | 1 | 1 | ||
| Hepatic necrosis | 1 | 1 | 0 | 0 | ||
| Immune system disorders | 1 | 1 (0.1) | 0 | 0 (0.0) | ||
| Anaphylactic reaction | 1 | 1 | 0 | 0 | ||
| Infections and infestations | 4 | 4 (0.4) | 7 | 7 (0.7) | ||
| Cellulitis | 0 | 0 | 1 | 1 | ||
| Diverticulitis | 1 | 1 | 1 | 1 | ||
| Empyema | 0 | 0 | 1 | 1 | ||
| Endocarditis | 2 | 2 | 2 | 2 | ||
| Gangrene | 0 | 0 | 1 | 1 | ||
| H1N1 influenza | 1 | 1 | 0 | 0 | ||
| Oral candidiasis | 0 | 0 | 1 | 1 | ||
| Injury, poisoning and procedural complications | 6 | 5 (0.5) | 10 | 9 (0.9) | ||
| Arteriovenous fistula site haemorrhage | 0 | 0 | 1 | 1 | ||
| Fall | 1 | 1 | 3 | 3 | ||
| Femoral neck fracture | 0 | 0 | 2 | 2 | ||
| Nerve injury | 0 | 0 | 1 | 1 | ||
| Overdose | 0 | 0 | 1 | 1 | ||
| Rib fracture | 1 | 1 | 0 | 0 | ||
| Seroma | 1 | 1 | 0 | 0 | ||
| Toxicity to various agents | 2 | 2 | 0 | 0 | ||
| Transfusion reaction | 0 | 0 | 1 | 1 | ||
| Upper limb fracture | 1 | 1 | 0 | 0 | ||
| Wound | 0 | 0 | 1 | 1 | ||
| Investigations | 2 | 2 (0.2) | 3 | 3 (0.3) | ||
| Blood pressure decreased | 0 | 0 | 1 | 1 | ||
| International normalised ratio | 2 | 2 | 1 | 1 | ||
| International normalised ratio increased | 0 | 0 | 1 | 1 | ||
| Metabolism and nutrition disorders | 4 | 4 (0.4) | 1 | 1 (0.1) | ||
| Hypernatraemia | 1 | 1 | 0 | 0 | ||
| Hypoglycaemia | 1 | 1 | 1 | 1 | ||
| Hyponatraemia | 2 | 2 | 0 | 0 | ||
| Musculoskeletal and connective tissue disorders | 7 | 7 (0.7) | 4 | 4 (0.4) | ||
| Back pain | 1 | 1 | 1 | 1 | ||
| Compartment syndrome | 2 | 2 | 0 | 0 | ||
| Muscular weakness | 1 | 1 | 0 | 0 | ||
| Musculoskeletal chest pain | 2 | 2 | 3 | 3 | ||
| Pain in extremity | 1 | 1 | 0 | 0 | ||
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 2 | 2 (0.2) | 1 | 1 (0.1) | ||
| Bladder cancer | 0 | 0 | 1 | 1 | ||
| Brain cancer metastatic | 1 | 1 | 0 | 0 | ||
| Breast cancer | 1 | 1 | 0 | 0 | ||
| Nervous system disorders | 9 | 9 (0.9) | 11 | 10 (1.0) | ||
| Amnesia | 1 | 1 | 0 | 0 | ||
| Brain injury | 0 | 0 | 2 | 1 | ||
| Convulsion | 3 | 3 | 1 | 1 | ||
| Dizziness | 0 | 0 | 1 | 1 | ||
| Grand mal convulsion | 2 | 2 | 1 | 1 | ||
| Headache | 1 | 1 | 1 | 1 | ||
| Loss of consciousness | 0 | 0 | 1 | 1 | ||
| Neuralgia | 0 | 0 | 1 | 1 | ||
| Partial seizures | 0 | 0 | 1 | 1 | ||
| Syncope | 2 | 2 | 2 | 2 | ||
| Psychiatric disorders | 2 | 2 (0.2) | 2 | 2 (0.2) | ||
| Alcohol withdrawal syndrome | 1 | 1 | 1 | 1 | ||
| Confusional state | 0 | 0 | 1 | 1 | ||
| Depression | 1 | 1 | 0 | 0 | ||
| Renal and urinary disorders | 4 | 4 (0.4) | 7 | 7 (0.7) | ||
| Haematuria | 1 | 1 | 1 | 1 | ||
| Renal colic | 1 | 1 | 0 | 0 | ||
| Renal vasculitis | 0 | 0 | 1 | 1 | ||
| Urinary retention | 2 | 2 | 5 | 5 | ||
| Respiratory, thoracic and mediastinal disorders | 1 | 1 (0.1) | 3 | 3 (0.3) | ||
| Dysphonia | 0 | 0 | 1 | 1 | ||
| Epistaxis | 0 | 0 | 1 | 1 | ||
| Hypoxia | 1 | 1 | 1 | 1 | ||
| Surgical and medical procedures | 3 | 3 (0.3) | 6 | 6 (0.6) | ||
| Aortic aneurysm repair | 1 | 1 | 0 | 0 | ||
| Cardiac pacemaker revision | 0 | 0 | 1 | 1 | ||
| Coronary arterial stent insertion | 0 | 0 | 1 | 1 | ||
| Debridement | 0 | 0 | 1 | 1 | ||
| Eye excision | 1 | 1 | 0 | 0 | ||
| Haematoma evacuation | 0 | 0 | 1 | 1 | ||
| Leg amputation | 1 | 1 | 1 | 1 | ||
| Ventriculocardiac shunt | 0 | 0 | 1 | 1 | ||
| Vascular disorders | 8 | 8 (0.8) | 3 | 3 (0.3) | ||
| Aortic aneurysm rupture | 1 | 1 | 1 | 1 | ||
| Haematoma | 1 | 1 | 0 | 0 | ||
| Hypotension | 2 | 2 | 0 | 0 | ||
| Orthostatic hypotension | 2 | 2 | 1 | 1 | ||
| Peripheral ischaemia | 1 | 1 | 0 | 0 | ||
| Peripheral vascular disorder | 1 | 1 | 0 | 0 | ||
| Vasculitis | 0 | 0 | 1 | 1 | ||
The characteristics of the SAEs suggest slightly higher proportions in the restrictive group resulted in death or were life-threatening (17% vs. 13% and 36% vs. 29%, respectively, Table 34). Similarly, slightly higher proportions were classified as of severe intensity in the restrictive group (55% vs. 50%). In total, 41 SAEs were classified as possibly, probably or definitely related to the intervention: 20 (19%) in the restrictive group and 21 (19%) in the liberal group. Of these, 34 were attributed to a red blood cell transfusion being given (14 in the restrictive group and 20 in the liberal group), and seven to a transfusion being withheld (six in the restrictive group and one in the liberal group). Finally, more participants experiencing a SAE had their treatment according to protocol discontinued (14 participants vs. four participants).
| Event type | Restrictive group SAEs (N = 103) | Liberal group SAEs (N = 113) | ||
|---|---|---|---|---|
| Events, % | Participants, % | |||
| Timing of event | ||||
| Pre-discharge | 35 | 34 | 46 | 41 |
| Post discharge | 68 | 66 | 67 | 59 |
| Reason event classified as SAEa | ||||
| Resulted in death | 18 | 17 | 15 | 13 |
| Was life-threatening | 37 | 36 | 33 | 29 |
| Resulted in persistent or significant disability/incapacity | 23 | 22 | 31 | 27 |
| Required hospitalisation | 62 | 60 | 72 | 64 |
| Prolonged ongoing hospitalisation | 26 | 25 | 36 | 32 |
| Other | 5 | 5 | 4 | 4 |
| Maximum intensity | ||||
| Mild | 12 | 12 | 18 | 16 |
| Moderate | 34 | 33 | 39 | 35 |
| Severe | 57 | 55 | 56 | 50 |
| Final outcome | ||||
| Resolved no sequelae | 52 | 50 | 61 | 54 |
| Resolved with sequelae | 33 | 32 | 34 | 30 |
| Died | 18 | 17 | 18 | 16 |
| Relatedness | ||||
| Not related | 61 | 59 | 70 | 62 |
| Unlikely to be related | 22 | 21 | 22 | 19 |
| Possibly related | 14 | 14 | 18 | 16 |
| Probably related | 3 | 3 | 2 | 2 |
| Definitely related | 3 | 3 | 1 | 1 |
| Related to | ||||
| Red blood cell transfusion being given | 14 | 14 | 20 | 18 |
| Red blood cell transfusion being withheld | 6 | 6 | 1 | 1 |
| Treatment according to protocol permanently discontinued | 4 | 4 | 14 | 12 |
Meta-analysis
A meta-analysis of mortality for TITRe2 and the five earlier RCTs24–26,37,38 is shown in Figure 15. The combined estimate suggests an increased risk of death in the restrictive group, of borderline statistical significance, RR 1.41 (95% CI 0.98 to 2.04). It should be noted that the restrictive and liberal thresholds varied across these trials (Table 35). The trials also varied with respect to whether or not the intervention was applied during the operation. In addition, with the exception of TITRe2, all of the studies randomised all participants prior to their operation; hence, they included in their analyses participants who did not breach the liberal threshold and who were almost certainly not transfused. By virtue of randomisation, there should have been similar numbers of participants in each group who did not breach the liberal threshold. Including these participants would be expected to dilute any treatment effect.
FIGURE 15.
Meta-analysis for mortality.

| Study | Restrictive group threshold | Liberal group threshold | Intervention period |
|---|---|---|---|
| Johnson 199224 | 8.3 g/dl | 10.7 g/dl | Postoperative only |
| Bracey 199925 | 8.0 g/dl | 9.0 g/dl | Postoperative only |
| Murphy 200726 | 7.0 g/dl | 8.0 g/dl | Postoperative only |
| Hajjar 201037 | 8.0 g/dl | 10.0 g/dl | Intraoperative and postoperative |
| Shehata 201238 | 7.0 g/dl intraoperatively during CPB; 7.5 g/dl postoperatively | 9.5 g/dl intraoperatively during CPB; 10.0 g/dl postoperatively | Intraoperative and postoperative |
| Murphy 201555 | 7.5 g/dl | 9.0 g/dl | Postoperative only |
Summary
The frequency of the primary outcome was slightly higher in the restrictive group than the liberal group (35.1% vs. 33.0%), mainly owing to ischaemic events and, in particular, AKI. Sensitivity analyses either restricted to participants who were not transfused pre-randomisation or including additional less-severe AKI events augmented this difference. However, restricting the analysis to only the most severe primary outcome events reduced the event frequencies to 14.7% and 14.9% in the restrictive and liberal groups, respectively, and the treatment effect (OR) was reduced to unity. This event frequency was very similar to that assumed at the outset for the sample size justification. There was no evidence of any subgroup effects.
There were significantly more deaths in the restrictive group (4.2%) than the liberal group (2.6%); HR 1.64 (95% CI 1.00 to 2.67). This difference persisted in two post-hoc sensitivity analyses of the mortality outcome. A meta-analysis combining mortality data from five previous RCTs gave a pooled RR 1.41 (95% CI 0.98 to 2.04). There were no statistically significant differences between the groups for any of the other secondary outcomes; however, all estimated treatment differences either favoured the liberal group or were null apart from the EQ-5D-3L utility score. In terms of SAEs, the overall frequency was slightly higher in the restrictive group than the liberal group (35.7% vs. 34.2%).
Chapter 6 Results of the economic evaluation
Quality-adjusted life-years
A summary of the mean EQ-5D-3L scores at each of the time points and the QALYs gained in each group are shown in Figure 16 and in Table 36 (compare with medians reported in Table 27). These figures differ slightly to those presented earlier because we use means rather than medians, in contrast with the description of effectiveness, and include participants who have died with scores of zero from the date of death. The means are supported by SEs. On average, participants’ EQ-5D-3L scores did not fully return to their pre-operative level by 3 months in either treatment group.
FIGURE 16.
Mean EQ-5D-3L utility scores (and 95% CI) at each time point for each treatment group.
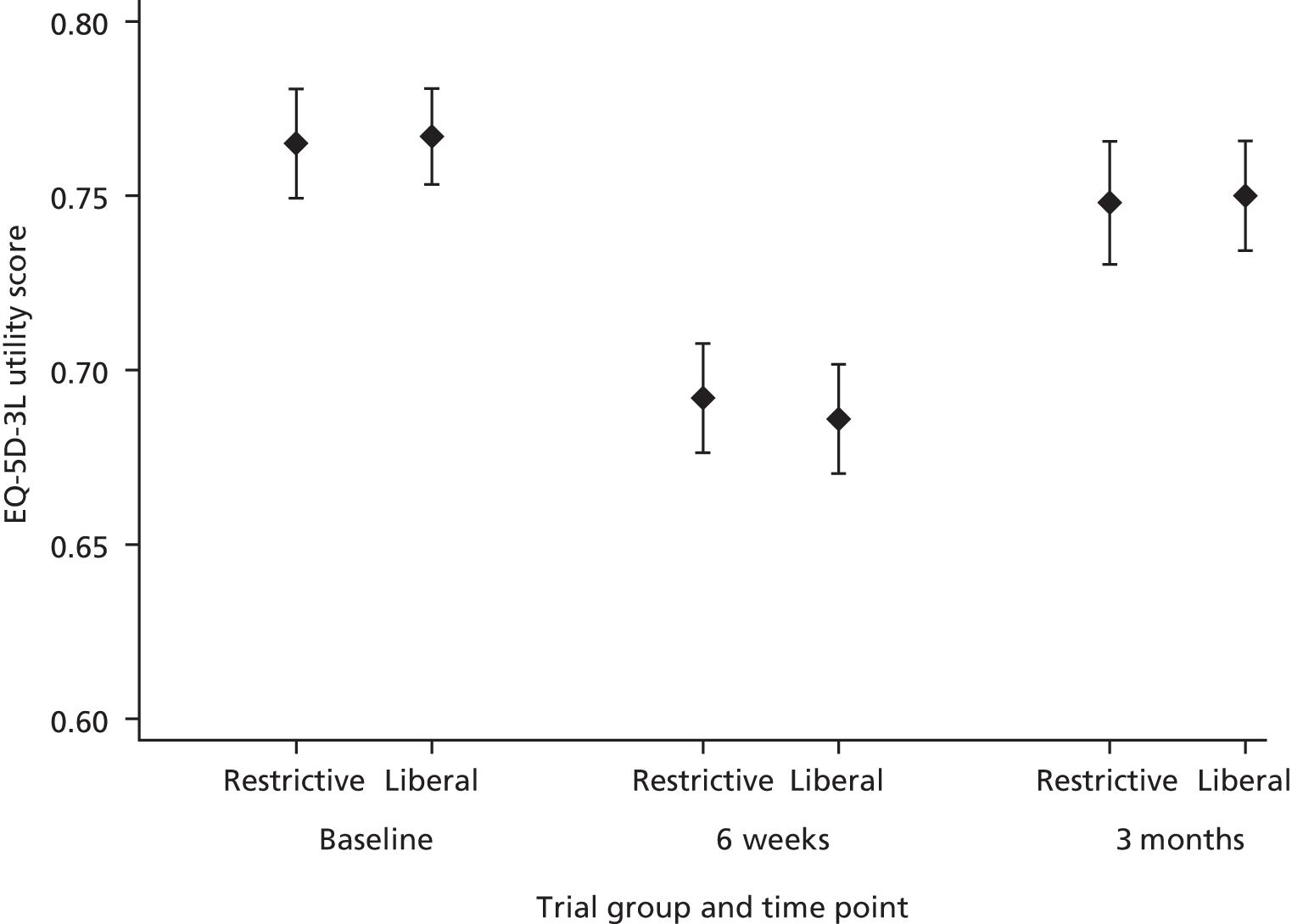
| Time point | Randomised to restrictive threshold (n = 1000), mean (SE) | Randomised to liberal threshold (n = 1003), mean (SE) | Restrictive vs. liberal threshold, mean difference (SE) |
|---|---|---|---|
| EQ-5D-3L time pointa | |||
| Baseline | 0.765 (0.008) | 0.767 (0.007) | –0.001 (0.011) |
| 6 weeks | 0.692 (0.008) | 0.686 (0.008) | 0.006 (0.011) |
| 3 months | 0.748 (0.009) | 0.750 (0.008) | –0.002 (0.012) |
| QALYs to 3 months (adjusted for baseline EQ-5D-3L) | 0.1802 (0.0015) | 0.1798 (0.0016) | 0.0004 (0.0021) |
As Table 36 shows, there is very little difference between the groups for EQ-5D-3L scores at any of the three time points and a tiny (non-significant) difference in QALYs between the groups. Indeed, the QALYs to 3 months are 0.18 for both the restrictive and liberal groups, with a mean difference of only 0.0004 (SE 0.0021). This difference of 0.0004 QALYs is approximately 3.5 quality-adjusted hours. Given the significant difference in deaths between the groups (more deaths in the restrictive group), we explored potential reasons why the difference in deaths did not appear to translate into a difference in QALYs. We would have assumed that the participants who died were more ill, which theoretically could have resulted in them reporting lower EQ-5D-3L scores prior to death. We therefore plotted the QALY data for each group: for all participants, for participants excluding deaths and only for deaths. This investigation revealed that it was not merely the participants who died who had low QALYs, but also many other participants. These low EQ-5D-3L scores for surviving participants had the effect of ‘diluting’ the impact on the means of imputed zero EQ-5D-3L scores for participants who died. Most of the participants who died had total QALYs of 0–0.05. When these participants were excluded, there were a few more participants in the liberal group with QALYs < 0.05 than in the restrictive group and, overall, these low scores in the liberal group appear to have partly balanced out the greater number of deaths in the restrictive group. In addition, there were more participants in the restrictive group in the highest band for QALYs, which could also be balancing out the greater number of deaths in the restrictive group. Therefore, we came to the conclusion that the figures in Table 36 could be showing that there was genuinely no real difference in QALYs between the restrictive and liberal groups, despite the difference in deaths.
Resource use and costs
Table 37 reports information on the main resource use items for the trial groups to 3 months. The table includes information on surgery, blood products, LOS, complications and health-care contacts post discharge. Frequencies are given for binary responses (yes/no) and means and SEs are presented for the number of events or LOS per participant. Red blood cells are the only resource item for which there is a clear difference between the groups, an expected finding given that the liberal group had more red blood cells transfused. The average units of red blood cells transfused (compared with medians in Table 14) in the restrictive group was 2.08 units (SE 0.09 units) per participant, compared with 3.07 units (SE 0.11 units) units per participant in the liberal group, leading to an average difference of 1.00 unit (SE 0.14 units) per participant. For most of the other categories of resource use, the differences between the groups are very small. The differences are slightly larger for inpatient ward LOS, time in other hospitals and readmissions than for some other resource use items, but these are nevertheless small differences.
| Resource use component | Randomised to restrictive threshold (n = 1000), frequency (%) or mean (SE) | Randomised to liberal threshold (n = 1003), frequency (%) or mean (SE) | Restrictive versus liberal threshold, % or mean (SE) difference |
|---|---|---|---|
| Red blood cells, number of units/participant | 2.08 (0.09) | 3.07 (0.11) | –1.00 (0.14) |
| Cardiac procedure, n (%) | |||
| CABG | 408 (41) | 408 (41) | 0 |
| Valve | 307 (31) | 304 (30) | 1 |
| CABG and valve | 195 (20) | 203 (20) | 0 |
| Other | 90 (9) | 88 (9) | 0 |
| Blood products, number of units/participant | |||
| FFP | 1.00 (0.06) | 0.95 (0.06) | 0.05 (0.08) |
| Platelets | 0.65 (0.03) | 0.64 (0.03) | 0.01 (0.05) |
| Cryoprecipitate | 0.23 (0.03) | 0.21 (0.02) | 0.02 (0.04) |
| Inpatient complications, n (%) | |||
| Primary outcome | |||
| Antibiotics for infectious complication | 341 (34) | 344 (34) | 0 |
| Stroke | 14 (1) | 16 (2) | –1 |
| Suspected MI | 3 (0) | 7 (1) | –1 |
| Gut infarction | 5 (1) | 1 (0) | 1 |
| AKI: stage 3 | 60 (6) | 51 (5) | 1 |
| Other complications, n events/participant (%) | |||
| Reoperation | 0.09 (0.01) | 0.10 (0.01) | –0.01 (0.02) |
| Reintubation | 0.07 (0.01) | 0.07 (0.01) | 0.00 (0.01) |
| Tracheostomy | 0.03 (0.01) | 0.03 (0.01) | 0.00 (0.01) |
| Mask CPAP | 0.13 (0.01) | 0.12 (0.01) | 0.01 (0.02) |
| Pneumothorax requiring chest drainage | 0.01 (0.00) | 0.01 (0.00) | 0.00 (0.01) |
| Pleural effusion requiring drainage | 0.06 (0.01) | 0.06 (0.01) | 0.00 (0.01) |
| Pacing | 0.31 (0.02) | 0.31 (0.02) | 0.00 (0.02) |
| SVT/AF requiring treatment | 0.41 (0.02) | 0.39 (0.02) | 0.02 (0.03) |
| VF/VT requiring intervention | 0.02 (0.01) | 0.01 (0.00) | 0.01 (0.01) |
| Low cardiac output | 0.11 (0.01) | 0.11 (0.01) | 0.00 (0.01) |
| Inpatient LOS, days/participant | |||
| CICU | 1.14 (0.12) | 1.12 (0.13) | 0.02 (0.18) |
| HDU | 3.09 (0.12) | 3.05 (0.12) | 0.04 (0.17) |
| Ward | 5.67 (0.15) | 5.83 (0.17) | –0.17 (0.23) |
| Another unit/hospital | 1.27 (0.20) | 1.36 (0.19) | –0.09 (0.27) |
| Blood saving techniques, n (%) | |||
| Tranexamic acid | 807 (81) | 810 (81) | 0 |
| Trasylol | 41 (4) | 35 (3) | 1 |
| Intraoperative cell salvage | 482 (48) | 503 (50) | –2 |
| Post-operative cell salvage | 56 (6) | 46 (5) | 1 |
| Fluids in theatre/CICU/HDU, n (%) | |||
| Inotropes | 624 (62) | 614 (61) | 1 |
| Gelofusine® (B. Braun, Melsungen, Germany) | 843 (84) | 836 (83) | 1 |
| HES | 231 (23) | 233 (23) | 0 |
| Readmissions to hospital | |||
| LOS, days/participant | 1.38 (0.15) | 1.46 (0.16) | –0.08 (0.22) |
| ED attendances | |||
| Total ED visits, number/participant | 0.09 (0.01) | 0.08 (0.01) | 0.01 (0.01) |
| Outpatient appointments, n/participant (%) | |||
| Cardiac surgery outpatient visits | 0.44 (0.02) | 0.51 (0.02) | –0.07 (0.03) |
| Cardiology outpatient visits | 0.28 (0.02) | 0.26 (0.02) | 0.03 (0.03) |
| Other outpatient visits | 0.17 (0.02) | 0.17 (0.02) | 0.00 (0.03) |
| Other health-care contacts, n/participant (%) | |||
| GP at surgery | 1.99 (0.06) | 2.07 (0.07) | –0.09 (0.10) |
| GP at home | 0.43 (0.04) | 0.37 (0.03) | 0.06 (0.06) |
| Practice nurse | 1.56 (0.13) | 1.57 (0.14) | –0.01 (0.19) |
| District nurse | 2.47 (0.20) | 2.21 (0.23) | 0.26 (0.30) |
In terms of unit costs which were attached to the main resource use items, Table 38 provides some information on the main resource unit costs used and the source for the information, presented in 2012–13 prices. More detailed information on unit costs can be found in Appendix 3, Unit costs and resource use assumed for complications.
| Resource use | Unit cost (£) | Source |
|---|---|---|
| Red blood cells (per unit of blood) | 123.31 | NHSBT Price List 2012/1345 |
| Cardiac procedure | ||
| CABG | 6714 | See Appendix 3, Table 61 |
| Valve | 7336 | See Appendix 3, Table 61 |
| CABG and valve | 8054 | See Appendix 3, Table 61 |
| Other | 8298 | See Appendix 3, Table 61 |
| Blood products (per unit) | ||
| FFP | 27.46 | NHSBT Price List 2012/1345 |
| Platelets | 209.30 | NHSBT Price List 2012/1345 |
| Cryoprecipitate | 189.19 | NHSBT Price List 2012/1345 |
| Inpatient complications | ||
| Primary outcome | ||
| Antibiotics for infectious complication | See Appendix 3, Table 63 | eMIT, 2014;46 BNF 66, 201347 |
| Stroke | 139 | NHS Reference Costs 2012/1344 |
| Confirmed by CT scan | 62 | NHS Reference Costs 2012/1344 |
| Confirmed by MRI scan | 248 | NHS Reference Costs 2012/1344 |
| Suspected MI | 1868 | NHS Reference Costs 2012/1344 |
| Gut infarction | 62 | NHS Reference Costs 2012/1344 |
| Confirmed by laparotomy | 2693 | NHS Reference Costs 2012/1344 |
| AKI – stage 3 | 1438 | NHS Reference Costs 2012/1344 |
| Other complications | ||
| Reoperation (duration < 3 hours) | 6608 | NHS Reference Costs 2012/1344 |
| Reoperation (duration ≥ 3 hours) | 8298 | NHS Reference Costs 2012/1344 |
| Reintubation | 395 | NHS Reference Costs 2012/1344 |
| Tracheostomy | 5354 | NHS Reference Costs 2012/1344 |
| Mask CPAP | 539 | NHS Reference Costs 2012/1344 |
| Pneumothorax requiring drainage | 4218 | NHS Reference Costs 2012/1344 |
| Pleural effusion requiring drainage | 4218 | NHS Reference Costs 2012/1344 |
| Pacing | 3073 | NHS Reference Costs 2012/1344 |
| SVT/AF requiring treatment | 4.79 | eMIT, 201446 |
| VF/VT requiring treatment | 2007 | NHS Reference Costs 2012/1344 |
| Low cardiac output | 313 | NHS Reference Costs 2012/1344 |
| Inpatient LOS | ||
| CICU day | 1190 | NHS Reference Costs 2012/1345 |
| HDU day | 619 | NHS Reference Costs 2012/1345 |
| Ward day | 392 | NHS Reference Costs 2012/1345 |
| Another unit/hospital ICU day | 1168 | NHS Reference Costs 2012/1345 |
| Another unit/hospital ward day | 265 | NHS Reference Costs 2012/1345 |
| Blood saving techniques | ||
| Tranexamic acid | 15.50 | BNF 66, 201348 |
| Trasylol | 316.83 | Davies et al.56 |
| Intra- and post-operative cell salvage | 176 | Davies et al.56 |
| Fluids in theatre/CICU/HDU | ||
| Inotropes | 57.30 | eMIT, 201447 |
| Gelofusine | 7.92 | Finance Department South Central, 2013, personal communication |
| HES | 40.60 | BNF 58, 200957 |
| Readmissions to hospital | ||
| Ward day | 265 | NHS Reference Costs 2012/1345 |
| ICU day | 1168 | NHS Reference Costs 2012/1345 |
| ED attendances | ||
| ED visit (not leading to admission) | 101 | NHS Reference Costs 2012/1345 |
| Outpatient appointments | ||
| Cardiac surgery outpatient visit | 299 | NHS Reference Costs 2012/1345 |
| Cardiology outpatient visit | 131 | NHS Reference Costs 2012/1345 |
| Other outpatient visits | See Appendix 3, Tables 70 and 71 | NHS Reference Costs 2012/1345 |
| Other health-care contacts | ||
| GP at surgery | 34 | Unit Costs of Health and Social Care 201349 |
| GP at home | 85 | Unit Costs of Health and Social Care 201349 |
| Practice nurse | 11.37 | Unit Costs of Health and Social Care 201349 |
| District nurse | 39 | Unit Costs of Health and Social Care 201349 |
The combined resource use and unit cost information are presented in Figure 17 in terms of a breakdown of total costs for each treatment group. This clearly shows that there is very little difference in costs between the groups apart from a difference in the average cost of red blood cells, which is to be expected. Key drivers of total costs were surgery, complications and LOS. A breakdown of total costs is given in Table 39. This table is broken down into three cost categories: red blood cells, inpatient episode and post-hospital discharge. A more detailed breakdown of total costs is given in Table 74 in Appendix 3. A separate analysis, which shows that the costs of regular medications were reduced after surgery, is described in Appendix 3, Changes in the use of regular medications between admission to and discharge from the cardiac surgery unit.
FIGURE 17.
Breakdown of total costs for each treatment group. Error bars show SEs around total costs.
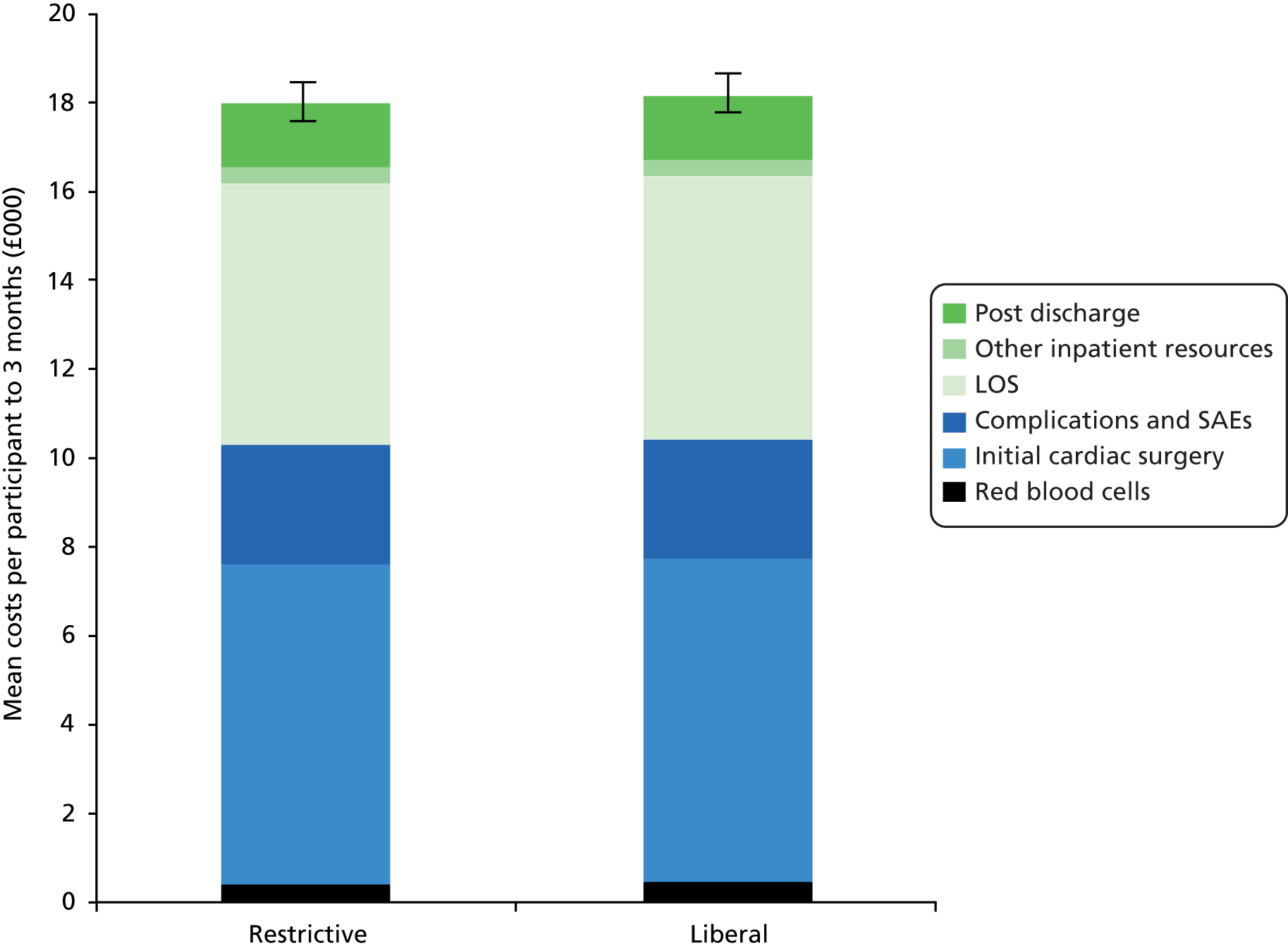
| Cost component | Randomised to restrictive threshold (n = 1000), mean cost (£) (SE) | Randomised to liberal threshold (n = 1003), mean cost (£) (SE) | Restrictive vs. liberal threshold, mean cost (£) difference (SE) |
|---|---|---|---|
| Red blood cells | 287 (13) | 427 (15) | –140 (19) |
| Inpatient episode | |||
| Initial cardiac surgery | 7309 (18) | 7313 (18) | –4 (26) |
| Other blood products | 206 (12) | 199 (11) | 7 (16) |
| Complications and SAEs | 2684 (137) | 2714 (146) | –30 (200) |
| LOSa | 5854 (201) | 5892 (221) | –38 (299) |
| Blood saving techniques | 159 (9) | 152 (8) | 7 (12) |
| Regular medications | 26 (2) | 29 (2) | –3 (3) |
| Fluids | 55 (1) | 55 (1) | 0 (2) |
| Total | 16,293 (309) | 16,353 (339) | –60 (459) |
| Post-hospital discharge | |||
| Readmissions | 770 (85) | 753 (78) | 17 (116) |
| ED visits | 16 (2) | 12 (2) | 4 (3) |
| Outpatient appointments | 202 (6) | 216 (7) | –14 (9) |
| Other medical/social care | 378 (14) | 366 (16) | 12 (21) |
| Total | 1365 (90) | 1347 (82) | 18 (122) |
| Total costs | 17,945 (332) | 18,127 (357) | –182 (488) |
The total cost of care from surgery up to 3 months is £17,945 in the restrictive group and £18,127 in the liberal group, creating a mean difference between the groups of £182 (SE £488). Most of this difference in cost is associated with the higher cost of red blood cells in the liberal group (cost difference of £140). The next main cost difference is in LOS costs, the complications and SAEs, with the liberal group being more expensive than the restrictive group, but only slightly more. In terms of post-discharge costs, the restrictive group costs slightly more than the liberal group for hospital readmissions, with a cost difference of £17 (SE £116) and ‘other’ medical/social care costs, with a difference of £12 (SE £21). The liberal group costs more than the restrictive group for outpatient appointments with a cost difference of £14 (SE £9).
The differences in costs between the groups are small, although there is substantial uncertainty around the differences in costs as shown in the SEs in the final column in Table 39. In terms of the distribution of costs across the trial groups, the histograms presented in Figures 18 and 19 show that the cost data were skewed for both groups, which is a common finding in health economic evaluations. This skewness was enhanced by the existence of a few very high-cost outliers, especially in the liberal group. There were four participants with costs over £100,000, who were all in the liberal group.
FIGURE 18.
Mean total costs (£) per participant in the restrictive group. The inset graph shows that there were outliers in the liberal group but not in the restrictive group.
FIGURE 19.
Mean total costs (£) per participant in the liberal group. The inset graph shows that there were outliers in the liberal group but not in the restrictive group.

Base-case cost-effectiveness results
The ICER for the restrictive threshold compared with the liberal threshold is shown in Table 40. The differences in costs and QALYs between the groups are small and neither difference is statistically significant. The difference between the groups for QALYs is particularly small, that is the denominator for the ICER (the difference in QALYs) is therefore very small. Dividing the difference in costs by a tiny number close to zero, results in a very large ICER (–£428,064). Based on the point estimate, the restrictive threshold is considered cost-effective and the restrictive threshold is dominant over the liberal threshold as it is both more effective and less costly. However, there is great uncertainty around this result, as shown on the cost-effectiveness plane in Figure 20. The black dot is the point estimate of the cost and QALY difference and is close to the origin. The bootstrap replicates of the cost and QALY differences cover all four quadrants of the cost-effectiveness plane, which illustrates that there is actually very little difference between the two groups and much uncertainty. There is a 43% probability that the restrictive threshold dominates the liberal threshold, but also a 20% probability of the reverse scenario, that the liberal threshold dominates the restrictive threshold.
FIGURE 20.
Cost-effectiveness plane.

| Outcome | Total costs (95% CI) | QALYs (95% CI) | ICER (cost/QALY) | Probability that restrictive is | Probability restrictive is cost-effective at a ceiling ratio of | Probability that restrictive is | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Restrictive (n = 1000) | Liberal (n = 1003) | Difference | Restrictive (n = 1000) | Liberal (n = 1003) | Difference | Dominant | Dominated | £20,000 | £50,000 | £100,000 | More effective | Less costly | ||
| QALYs adjusted for baseline EQ-5D-3L | £17,945 (£17,273 to £18,618) | £18,127 (£17,450 to £18,804) | –£182 (–£1108 to £744) | 0.1802 (0.1772 to 0.1832) | 0.1798 (0.1766 to 0.1829) | 0.0004 (–0.0037 to 0.0045) | Restrictive dominant (–£428,064) | 43% | 20% | 65% | 66% | 66% | 58% | 65% |
The CEAC in Figure 21 shows the probability that the restrictive threshold is cost-effective for a range of willingness-to-pay thresholds. If a decision-maker is willing to pay £20,000 for an additional QALY, then the probability of restrictive being cost-effective is 65%. The probability that a restrictive threshold is cost-effective changes little across a broad range of willingness-to-pay thresholds, indicating that this probability is invariant to the willingness-to-pay threshold. Across ceiling ratios from £0 to £100,000, a restrictive threshold has a probability of being cost-effective of 0.65–0.66 and the liberal threshold has a probability of being cost-effective of 0.34–0.35. Clearly the restrictive threshold is more likely to be cost-effective, but there is much uncertainty around this. The dashed lines at 0.1 and 0.9 indicate the 80% confidence limits for the probability that a restrictive threshold is cost-effective. As these horizontal lines do not cut the curve at any point, the 80% confidence limits on cost-effectiveness do not exist. Indeed it is not possible to define even 50% confidence limits on cost-effectiveness across willingness-to-pay thresholds from £0 to £100,000.
FIGURE 21.
Cost-effectiveness acceptability curve.

Sensitivity analyses
Sensitivity analyses for costing were conducted to investigate varying a number of unit costs, moving the time origin from surgery to the time of randomisation and the impact of any high-cost participants. We planned to consider a wider, societal perspective (instead of the NHS and Personal Social Services perceptive taken in the base-case analysis) if non-NHS costs were found to differ between the trial groups. This was not found to be the case (see Appendix 3, Non-NHS costs: did these differ between trial groups?) and, therefore, this sensitivity analysis was not conducted. In terms of outcomes, alternative assumptions for calculating QALYs were implemented in sensitivity analyses. Finally, we examined life-years gained as a secondary outcome measure. Each of these sensitivity analyses is considered in turn.
Sensitivity analyses around unit costs
The results of the sensitivity analyses around the costs of bed-days, antibiotics, complications and outpatient visits are shown in Table 79 in Appendix 3. Varying the costs of bed-days during the index admission by ± 50% had the greatest impact on total costs in each group (increasing and decreasing total costs to approximately £21,000 and £15,000 respectively). However, none of the sensitivity analyses had a great impact on the cost difference between the groups. The cost differences across the sensitivity analyses ranged from –£208 to –£161, bracketing and all very similar to the base-case cost difference of –£182. These findings reinforce how similar resource use is between the groups.
Costing from the point of randomisation
Events that occurred before randomisation were excluded and costs from randomisation to 3 months calculated. Participants were on average randomised 0.8 days after surgery. There is little difference in total costs of care from randomisation to 3 months between the two treatment groups. The total costs from randomisation are £8825 (SE £310) in the restrictive group and £8959 (SE £340) in the liberal group, with a mean difference between the groups of –£134 (SE £460). The costs associated with red blood cells are lower in the restrictive group than the liberal group, as expected. The costs of other inpatient and post-discharge resource use are very similar. Further details on the methods for this analysis and a breakdown of resource use and total costs are provided in Appendix 3, Costs from randomisation.
Total costs from randomisation are considerably less than total costs from surgery. Costs are lower because the costs of surgery and complications occurring before randomisation have been excluded and LOS costs are reduced. The LOS occurring pre-randomisation is at least, in part, time spent in CICU/HDU, as all participants go to CICU/HDU after surgery. Red blood cell costs are also reduced because red blood cells are sometimes transfused during surgery.
Sensitivity analyses around cost outliers
The distribution of total costs per participant is positively skewed in both transfusion groups, as seen in Figures 18 and 19. It is possible that a few high-cost outliers are exerting influence over the mean costs in each group and the overall findings; therefore, we investigated the existence of outliers and their effects.
There were 12 participants with costs over £80,000, of whom seven were in the liberal group and five were in the restrictive group. Participants with the five highest costs were all in the liberal group. Four participants had costs over £100,000 (£101,173; £107,163; £108,865; and one extreme outlier of £144,985). These participants did not have unexpected events; rather, they had large numbers of expected complications and stayed in hospital with a high level of care for some time. Therefore, there were no grounds for excluding these participants from the analyses. Nevertheless, it is instructive to investigate the impact they are having on cost and cost-effectiveness results, as the imbalance across groups of these outliers could easily have arisen by chance.
Table 41 shows the effects on costs and cost-effectiveness results of excluding the highest cost outlier and of excluding the four highest cost outliers with total costs over £100,000. All of these participants were in the liberal group and, therefore, results for the restrictive group are unchanged. If the participant with the highest cost is excluded from the analyses, the difference in costs between the groups reduces from –£182 to –£55. If participants with the four highest costs are excluded, the liberal group becomes less expensive than the restrictive group and the difference in costs between the groups changes from –£182 to +£208. The liberal group also becomes marginally more effective than the restrictive group and the conclusions are reversed, that is, the liberal group dominates the restrictive group as it is both less costly and more effective. While there is much uncertainty around these findings, these four participants are clearly exerting a significant impact on the cost and cost-effectiveness results.
| Sensitivity analysis | Randomised to restrictive threshold (n = 1000) | Randomised to liberal threshold (n = 1003) | Restrictive vs. liberal threshold | ||||
|---|---|---|---|---|---|---|---|
| Mean costs (SE) | Mean QALYs (SE) | Mean costs (SE) | Mean QALYs (SE) | Mean cost difference (SE) | Mean QALY difference (SE) | ICER | |
| Base case, all participants | £17,945 (£332) | 0.1802 (0.0015) | £18,127 (£357) | 0.1798 (0.0016) | –£182 (£488) | 0.0004 (0.0021) | Restrictive dominant (–£428,064) |
| Exclude highest cost participant | £17,945 (£332) | 0.1802 (0.0015) | £18,001 (£335) | 0.1799 (0.0016) | –£55 (£471) | 0.0003 (0.0021) | Restrictive dominant (–£210,078) |
| Exclude four highest cost participants | £17,945 (£332) | 0.1802 (0.0015) | £17,737 (£299) | 0.1803 (0.0016) | £208 (£447) | –0.0001 (0.0021) | Liberal dominant (–£1,835,715) |
Sensitivity analyses around quality-adjusted life-year calculations
We explored various assumptions for calculating QALYs, including the use of last observation carried forward until death (rather than assuming that utility changes linearly until death) and using the date of the 6-week EQ-5D-3L completion (rather than assuming it was completed exactly at 6 weeks). Details of the alternative strategies explored are given in Table 42 and the results are provided in Table 43. In all of these sensitivity analyses the difference in QALYs between the groups remained very small. When last observation carried forward until death was used, QALYs increased slightly in both groups, more so in the restrictive group as there were more deaths in this group and a greater number of participants whose QALYs were increased by this sensitivity analysis (unless their EQ-5D-3L score was less than zero at the previous observation). When the exact timing of the 6-week EQ-5D-3L questionnaire was used in the QALY calculations, the mean QALYs gained up to 3 months were slightly higher in the liberal group than in the restrictive group, a reversal of the base-case findings. Participants on average completed the 6-week questionnaire later than planned, at 51 days rather than 42 days.
| Sensitivity analysis | Aspect of methodology | Strategy used in base-case analysis | Alternative strategy for sensitivity analysis |
|---|---|---|---|
| 1 | QALY calculations: adjusting for baseline utility | Regression used to adjust for differences in baseline utility | No adjustment for baseline utility |
| 2 | QALY calculations for participants who die | Utility was assumed to change linearly between the preceding time point and the time of death | Use last observation carried forward until death |
| 3 | QALY calculations: timing of 6-week EQ-5D-3L | EQ-5D-3L at 6 weeks assumed to be completed at exactly 6 weeks | Use the date of completion of the 6-week EQ-5D-3L |
| 4 | QALY calculations: timing | Calculate QALYs gained from time of surgery to 3 months | Calculate QALYs gained from randomisation to 3 months |
| Sensitivity analysis | Randomised to restrictive threshold (n = 1000), QALYs to 3 months, mean (SE) | Randomised to liberal threshold (n = 1003), QALYs to 3 months, mean (SE) | Restrictive vs. liberal threshold, QALYs to 3 months mean, difference (SE) | |
|---|---|---|---|---|
| Base case | 0.1802 (0.0015) | 0.1798 (0.0016) | 0.0004 (0.0021) | |
| 1 | No adjustment for baseline utility | 0.1801 (0.0018) | 0.1798 (0.0017) | 0.0003 (0.0025) |
| 2 | Last observation carried forward until death | 0.1807 (0.0015) | 0.1800 (0.0016) | 0.0007 (0.0021) |
| 3 | Exact time between operation and 6-week EQ-5D-3L completion | 0.1801 (0.0014) | 0.1802 (0.0014) | –0.0002 (0.0020) |
| 4 | From randomisation | 0.1801 (0.0015) | 0.1795 (0.0015) | 0.0006 (0.0021) |
Life-years
Life-years gained from surgery up to 3 months are shown in Table 44. Given the greater number of deaths in the restrictive group, slightly fewer life-years were gained in the restrictive group than in the liberal group. The cost-effectiveness results using life-years as the outcome measure are also shown in Table 44. This analysis generated the typical trade-off between the treatment effect and the difference in cost. This trade-off is usually the result of a better effect at a higher cost; here, the reverse was the case with the restrictive threshold gaining fewer life-years but at lower cost than the liberal threshold. The ICER of £66,800 is the incremental saving associated with the loss of 1 life-year by adopting a restrictive rather than a liberal threshold. If a decision-maker’s willingness to accept compensation for the loss of 1 life-year was £20,000, then a restrictive threshold would be considered cost-effective.
| Outcome | Total costs (95% CI) | Life-years gained (95% CI) | ICER (cost/life-year) | Probability that restrictive is | Probability restrictive is cost-effective at a ceiling ratio of | Probability that restrictive is | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Restrictive (n = 1000) | Liberal (n = 1003) | Difference | Restrictive (n = 1000) | Liberal (n = 1003) | Difference | Dominant | Dominated | £20,000 | £50,000 | £100,000 | More effective | Less costly | ||
| Life-years | £17,945 (£17,273 to £18,618) | £18,127 (£17,450 to £18,804) | –£182 (–£1108 to £744) | 0.2428 (0.2404 to 0.2452) | 0.2455 (0.2437 to 0.2474) | –0.0027 (–0.0057 to 0.0002) | £66,800 | 2% | 34% | 61% | 55% | 45% | 3% | 65% |
The cost-effectiveness plane for life-years (Figure 22) and the CEAC for life-years (Figure 23) show quite interesting differences compared with those for the QALY analysis. In particular, Figure 22 shows that with life-years, many of the points are located in the bottom left quadrant of the plane (south-west quadrant), indicating that a restrictive threshold is most likely to be less effective and less costly than a liberal threshold. With the QALYs analysis, the plane had most of its points scattered around the origin showing very little difference. Compared with the cost-effectiveness plane for QALYs, the points on the cost-effectiveness plane for life-years have been pulled across to the left as there is a clearer difference in effects, namely a reduction in the number of life-years gained in the restrictive group. The uncertainty around the difference in costs remains. In this analysis, the probability that restrictive is more effective than liberal is just 3% (not statistically significant). There is only a 2% probability that the restrictive threshold dominates the liberal threshold (i.e. is more effective and less costly), but a 34% probability of the reverse scenario – that the liberal threshold dominates the restrictive threshold.
FIGURE 22.
Cost-effectiveness plane for life-years.

FIGURE 23.
Cost-effectiveness acceptability curve for life-years.

The CEAC in Figure 23 shows that if a decision-maker is willing to accept compensation of £20,000 for a life-year, then the probability that restrictive is cost-effective is 61%. As was the case for the CEAC for QALYs, there is much uncertainty around this finding. It is not possible to define 80%, or even 50%, confidence limits on cost-effectiveness across ceiling ratios from £0 to £100,000.
Subgroup analyses
The results of the seven subgroup analyses conducted to investigate whether or not cost-effectiveness results varied between participant subgroups are presented in Appendix 3, Table 82. Comparing costs between subgroups as a whole, the findings are generally as expected. Participants in each low-risk stratum before surgery cost less than those in each high-risk stratum, with the exception of sex for which the high-risk stratum (females) cost less than the low-risk stratum (males), and the possible exception of pulmonary disease/asthma, but the numbers at high risk for pulmonary comorbidity were small.
The cost and QALY differences between the treatment groups within the subgroups are all small relative to their SEs. When the impact of subgroups was evaluated using ordinary least squares regression separately for total costs and for QALYs, and considering interaction terms between treatment group and subgroup, only the interaction term for the subgroup for lung disease for QALYs was found to be significant (p = 0.003). Participants in the restrictive group with chronic pulmonary disease or asthma gained a reduced number of QALYs compared with other participants. This effect is consistent with the corresponding subgroup analysis of the primary outcome (see Chapter 5, Subgroup analyses).
For subgroup analyses 1–4 and 6 (further details in Appendix 3, Table 82), the direction of differences between treatment groups does differ between the subgroups. In the ‘low-risk’ stratum of each subgroup analysis (participants believed to be at lower risk of the primary outcome), the restrictive threshold is both less costly and more effective than the liberal threshold and, therefore, a restrictive threshold is favoured. In the ‘high-risk’ stratum of each subgroup (participants believed to be at higher risk of the primary outcome), the restrictive threshold is both more costly and less effective than the liberal threshold, so the liberal threshold is favoured. Note that negative ICERs need to be interpreted with caution (an ICER is a ratio of two numbers and, if either of the two is negative, the ICER will be negative). Either the new intervention is less costly and more effective, a desirable finding, or the new intervention is more costly and less effective, an undesirable finding. Both scenarios result in negative ICERs, but have very different meanings. These subgroup analyses should be considered as exploratory and further work would be required to confirm these findings.
Summary
There was very little difference between the groups in either costs or effects, and great uncertainty around the cost-effectiveness results. Mean QALYs to 3 months were 0.18 in both groups and there was a tiny difference between the restrictive and liberal groups (mean difference 0.0004, 95% CI –0.0037 to 0.0045). The total cost of care from surgery up to 3 months was £17,945 in the restrictive group and £18,127 in the liberal group, creating a small mean difference of –£182, 95% CI –£1108 to £744. There were several outliers in the liberal group that exerted a substantial influence on the average costs of participants in that group, altering the results when they were excluded.
The point estimate of cost-effectiveness suggested that the restrictive group was more effective and less costly than the liberal group (i.e. dominant) and, therefore, cost-effective. However given the small differences in costs and effects, there was much uncertainty around this result.
There was evidence of one subgroup effect: participants in the restrictive group with chronic pulmonary disease or asthma gained a reduced number of QALYs compared with other participants (p = 0.003).
Chapter 7 Observational analyses
As described in Chapter 2, Observational analyses, all red blood cells administered and haemoglobin levels recorded after the time of the first event that qualified for the primary outcome or after censoring have been excluded. The classification of all red blood cell transfusions as happening before randomisation, after randomisation but before the primary outcome or censoring and after the primary outcome or censoring is given in Table 45. Therefore, for analyses described in this chapter, 1381 participants are classified as having had at least one unit of red blood cells transfused after randomisation and before experiencing the primary outcome or being censored, 484 in the restrictive transfusion threshold stratum (total of 1074 units) and 897 in the liberal transfusion threshold stratum (total of 2030 units).
| Transfusion | Restrictive group | Liberal group | ||
|---|---|---|---|---|
| Number of transfusions of red blood cells (units) | Number of participants receiving any transfusions (%) (n = 1000) | Number of transfusions of red blood cells (units) | Number of participants receiving any transfusions (%) (n = 1003) | |
| Pre-randomisation red blood cell transfusions | 587 | 250 (25.0) | 589 | 264 (26.3) |
| Post-randomisation red blood cell transfusions | 1494 | 534 (53.4) | 2494 | 925 (92.2) |
| Before the primary outcome or censoringa,b | 1074 | 484 (48.4) | 2030 | 897 (89.4) |
| After the primary outcome or censoringa | 420 | 121 (12.1) | 464 | 147 (14.7) |
For the same period (after randomisation and before the time of the primary outcome or censoring), all participants were also classified as having experienced a minimum haemoglobin < 7.5 g/dl versus ≥ 7.5 g/dl. No haemoglobin levels were recorded for two participants between randomisation and the first incidence of the primary outcome or censoring. A total of 595 participants had a minimum haemoglobin level below 7.5 g/dl and 1406 participants had a haemoglobin level ≥ 7.5 g/dl throughout the period.
The analysis population is, therefore, identical to that for the analyses by transfusion threshold stratum reported in Chapter 5. However, the two participants with no haemoglobin measurements after randomisation and before experiencing the primary outcome or censoring are excluded from all models that fitted haemoglobin level.
Red blood cells and haemoglobin levels
Trial characteristics and outcomes
Pre-operative and intraoperative characteristics and trial outcomes are described by red blood cell transfusion status (after randomisation and before experiencing the primary outcome or censoring) in Tables 46 and 47, and by minimum haemoglobin in Tables 48 and 49.
| Characteristic | Transfused (N = 1381) | Not transfused (N = 622) |
|---|---|---|
| Cardiac history | ||
| Additive EuroSCORE,a median (IQR) | 5.0 (3.0–7.0) | 5.0 (3.0–7.0) |
| Logistic EuroSCORE,a median (IQR) | 4.2 (2.4–7.5) | 3.7 (2.0–6.6) |
| NYHA class, n/N (%) | ||
| I | 342/1348 (25.4) | 151/603 (25.0) |
| II | 594/1348 (44.1) | 291/603 (48.3) |
| III | 382/1348 (28.3) | 143/603 (23.7) |
| IV | 30/1348 (2.2) | 18/603 (3.0) |
| CCS class, n/N (%) | ||
| No angina | 477/1354 (35.2) | 241/608 (39.6) |
| I | 259/1354 (19.1) | 103/608 (16.9) |
| II | 350/1354 (25.8) | 176/608 (28.9) |
| III | 208/1354 (15.4) | 73/608 (12.0) |
| IV | 60/1354 (4.4) | 15/608 (2.5) |
| Coronary disease, n/N (%) | ||
| None | 426/1375 (31.0) | 194/616 (31.5) |
| Single vessel | 168/1375 (12.2) | 57/616 (9.3) |
| Double vessel | 184/1375 (13.4) | 98/616 (15.9) |
| Triple vessel | 562/1375 (40.9) | 243/616 (39.4) |
| Not investigated | 35/1375 (2.5) | 24/616 (3.9) |
| Disease in left main stem (> 50% stenosis) | 204/1364 (15.0) | 100/613 (16.3) |
| Non-cardiac history | ||
| Age (years), median (IQR) | 70.8 (64.2–76.8) | 69.5 (62.6–75.5) |
| Males, n/N (%) | 933/1381 (67.6) | 440/622 (70.7) |
| BMI (kg/m2),b mean (SD) | 27.9 (4.8) | 28.7 (5.1) |
| Urgent operative priority, n/N (%) | 181/1381 (13.1) | 64/622 (10.3) |
| Diabetic, n/N (%) | 279/1381 (20.2) | 120/622 (19.3) |
| Haemofiltration/dialysis, n/N (%) | 16/1379 (1.2) | 3/622 (0.5) |
| CVA/TIA, n/N (%) | 112/1381 (8.1) | 51/622 (8.2) |
| Pre-operative tests | ||
| Haemoglobin (g/dl), mean (SD) | 13.2 (1.5) | 13.5 (1.4) |
| eGFR (ml/minute/1.73m2),c median (IQR) | 71.7 (54.7–91.5) | 77.4 (61.3–96.9) |
| Intraoperative characteristics | ||
| Duration of operation (hours),d median (IQR) | 4.0 (3.3–5.0) | 3.9 (3.3–4.9) |
| CPB used, n/N (%) | 1323/1381 (95.8) | 580/621 (93.4) |
| Cardiac procedure, n/N (%) | ||
| CABG only | 542/1381 (39.2) | 274/622 (44.1) |
| Valve only | 428/1381 (31.0) | 183/622 (29.4) |
| CABG + valve | 301/1381 (21.8) | 97/622 (15.6) |
| Other | 110/1381 (8.0) | 68/622 (10.9) |
| Tranexamic acid, n/N (%) | 1112/1380 (80.6) | 503/621 (81.0) |
| Trasylol, n/N (%) | 48/1315 (3.7) | 23/579 (4.0) |
| Cell saver, n/N (%) | 689/1381 (49.9) | 295/621 (47.5) |
| Blood loss at 4 hours (ml),e median (IQR) | 275 (170–460) | 210 (125–328) |
| Blood loss at 12 hours (ml),e median (IQR) | 525 (340–840) | 400 (290–600) |
| Outcome | Transfused (N = 1381) | Not transfused (N = 622) |
|---|---|---|
| Intra- and post-operative use of other blood products | ||
| Pre-randomisation red blood cell transfusion, n/N (%) | 354/1381 (25.6) | 160/622 (25.7) |
| FFP transfusions, n/N (%) | 449/1381 (32.5) | 132/622 (21.2) |
| Platelet transfusions, n/N (%) | 562/1381 (40.7) | 176/622 (28.3) |
| Cryoprecipitate transfusions, n/N (%) | 158/1381 (11.4) | 43/622 (6.9) |
| Activated factor VII used, n/N (%) | 7/1381 (0.5) | 5/622 (0.8) |
| Beriplex used, n/N (%) | 65/1381 (4.7) | 35/622 (5.6) |
| Minimum haemoglobin (g/dl),a median (IQR) | 7.8 (7.2–8.4) | 8.3 (7.8–8.6) |
| Percentage decline in haemoglobin,a median (IQR) | 41.3 (35.1–46.7) | 39.3 (34.7–43.3) |
| Primary outcome, n/N (%) | ||
| Overall primary outcome | 474/1342 (35.3) | 174/564 (30.9) |
| Infectious event | 351/1327 (26.5) | 127/563 (22.6) |
| Sepsis | 311/1360 (22.9) | 113/605 (18.7) |
| Wound infection | 72/1297 (5.6) | 29/560 (5.2) |
| Ischaemic event | 213/1371 (15.5) | 82/611 (13.4) |
| Permanent stroke | 25/1363 (1.8) | 7/611 (1.1) |
| Suspected MI | 6/1357 (0.4) | 1/611 (0.2) |
| Gut infarction | 4/1358 (0.3) | 3/611 (0.5) |
| AKI | 188/1367 (13.8) | 74/611 (12.1) |
| Other trial outcomes | ||
| All-cause mortality, n/N (%) | 51/1381 (3.7) | 17/622 (2.7) |
| Significant pulmonary morbidity, n/N (%) | 192/1367 (14.0) | 51/614 (8.3) |
| Duration of ICU/HDU stay (hours), median (IQR) | 59.6 (24.4–109) | 32.6 (11.3–76.1) |
| Duration of post-randomisation hospital stay (days), median (IQR) | 7.0 (5.0–11.0) | 6.0 (4.0–9.0) |
| Characteristic | Minimum haemoglobin < 7.5 g/dl (N = 595) | Minimum haemoglobin ≥ 7.5 g/dl (N = 1406) |
|---|---|---|
| Cardiac history | ||
| Additive EuroSCORE,a median (IQR) | 5.0 (4.0–7.0) | 5.0 (3.0–7.0) |
| Logistic EuroSCORE,a median (IQR) | 4.2 (2.4–7.2) | 4.0 (2.2–7.2) |
| NYHA class, n/N (%) | ||
| I | 133/580 (22.9) | 360/1370 (26.3) |
| II | 259/580 (44.7) | 625/1370 (45.6) |
| III | 170/580 (29.3) | 355/1370 (25.9) |
| IV | 18/580 (3.1) | 30/1370 (2.2) |
| CCS class, n/N (%) | ||
| No angina | 185/583 (31.7) | 533/1378 (38.7) |
| I | 102/583 (17.5) | 260/1378 (18.9) |
| II | 168/583 (28.8) | 358/1378 (26.0) |
| III | 98/583 (16.8) | 182/1378 (13.2) |
| IV | 30/583 (5.1) | 45/1378 (3.3) |
| Coronary disease, n/N (%) | ||
| None | 178/593 (30.0) | 442/1396 (31.7) |
| Single vessel | 64/593 (10.8) | 161/1396 (11.5) |
| Double vessel | 86/593 (14.5) | 196/1396 (14.0) |
| Triple vessel | 256/593 (43.2) | 547/1396 (39.2) |
| Not investigated | 9/593 (1.5) | 50/1396 (3.6) |
| Disease in left main stem (> 50% stenosis), n/N (%) | 97/590 (16.4) | 206/1385 (14.9) |
| Non-cardiac history | ||
| Age (years), median (IQR) | 70.8 (64.2–76.7) | 70.0 (63.3–76.2) |
| Males, n/N (%) | 391/595 (65.7) | 980/1406 (69.7) |
| BMI (kg/m2),b mean (SD) | 27.6 (4.9) | 28.4 (4.9) |
| Urgent operative priority, n/N (%) | 86/595 (14.5) | 158/1406 (11.2) |
| Diabetic, n/N (%) | 126/595 (21.2) | 273/1406 (19.4) |
| Haemofiltration/dialysis, n/N (%) | 7/595 (1.2) | 12/1404 (0.9) |
| CVA/TIA, n/N (%) | 44/595 (7.4) | 119/1406 (8.5) |
| Pre-operative tests | ||
| Haemoglobin (g/dl), mean (SD) | 13.0 (1.5) | 13.4 (1.4) |
| eGFR (ml/minute/1.73m2),c median (IQR) | 69.3 (52.5–86.7) | 75.8 (58.5–95.4) |
| Intraoperative characteristics | ||
| Duration of operation (hours),b median (IQR) | 4.2 (3.4–5.2) | 4.0 (3.3–5.0) |
| CPB used, n/N (%) | 567/595 (95.3) | 1334/1405 (94.9) |
| Cardiac procedure, n/N (%) | ||
| CABG only | 239/595 (40.2) | 575/1406 (40.9) |
| Valve only | 171/595 (28.7) | 440/1406 (31.3) |
| CABG + valve | 132/595 (22.2) | 266/1406 (18.9) |
| Other | 53/595 (8.9) | 125/1406 (8.9) |
| Tranexamic acid, n/N (%) | 474/595 (79.7) | 1139/1404 (81.1) |
| Aprotinin (Trasylol, The Nordic group), n/N (%) | 24/561 (4.3) | 47/1331 (3.5) |
| Cell saver, n/N (%) | 292/595 (49.1) | 691/1405 (49.2) |
| Blood loss at 4 hours (ml),d median (IQR) | 328 (200–525) | 225 (140–350) |
| Blood loss at 12 hours (ml),d median (IQR) | 630 (380–1000) | 450 (300–700) |
| Outcome | Minimum haemoglobin < 7.5 g/dl (N = 595) | Minimum haemoglobin ≥ 7.5 g/dl (N = 1406) |
|---|---|---|
| Intra- and post-operative use of blood products | ||
| Pre-randomisation red blood cell transfusion, n/N (%) | 181/595 (30.4) | 333/1406 (23.7) |
| Post-randomisation (pre-primary outcome) red blood cell transfusions, n/N (%) | 555/595 (93.3) | 826/1406 (58.7) |
| FFP transfusions, n/N (%) | 243/595 (40.8) | 337/1406 (24.0) |
| Platelet transfusions, n/N (%) | 288/595 (48.4) | 449/1406 (31.9) |
| Cryoprecipitate transfusions, n/N (%) | 82/595 (13.8) | 119/1406 (8.5) |
| Activated factor VII used, n/N (%) | 1/595 (0.2) | 11/1406 (0.8) |
| Beriplex used, n/N (%) | 20/595 (3.4) | 80/1406 (5.7) |
| Percentage decline in haemoglobin, median (IQR) | 46.7 (42.4–50.7) | 38.4 (33.3–42.8) |
| Primary outcome, n/N (%) | ||
| Overall primary outcome | 237/574 (41.3) | 411/1330 (30.9) |
| Infectious event | 161/566 (28.4) | 317/1322 (24.0) |
| Sepsis | 141/583 (24.2) | 283/1380 (20.5) |
| Wound infection | 39/560 (7.0) | 62/1295 (4.8) |
| Ischaemic event | 128/593 (21.6) | 167/1387 (12.0) |
| Permanent stroke | 14/591 (2.4) | 18/1381 (1.3) |
| Suspected MI | 1/588 (0.2) | 6/1378 (0.4) |
| Gut infarction | 3/588 (0.5) | 4/1379 (0.3) |
| AKI | 114/590 (19.3) | 148/1386 (10.7) |
| Other trial outcomes | ||
| All-cause mortality, n/N (%) | 38/595 (6.4) | 30/1406 (2.1) |
| Significant pulmonary morbidity, n/N (%) | 111/590 (18.8) | 132/1389 (9.5) |
| Duration of ICU/HDU stay (hours), median (IQR) | 71.7 (42.3–131) | 42.1 (17.4–87.6) |
| Duration of post-randomisation hospital stay (days), median (IQR) | 8.0 (6.0–13.0) | 6.0 (5.0–9.0) |
Compared with non-transfused participants, transfused participants were on average older [median 70.8 years (IQR 64.2–76.8 years) vs. 69.5 years (IQR 62.6–75.5 years)], had similar additive EuroSCOREs [medians 5 (IQR 3–7)], higher logistic EuroSCOREs [median 4.2 (IQR 2.4–7.5) vs. 3.7 (IQR 2.0–6.6)] and were less likely to be male (67.6% vs. 70.7%). Transfused participants had, on average, lower pre-operative haemoglobin levels [mean 13.2 g/dl (SD 1.5 g/dl) vs. 13.5 g/dl (SD 1.4 g/dl)] and eGFR levels [median 71.7 ml/minute/1.73m2 (IQR 54.7–91.5 ml/minute/1.73m2) vs. 77.4 ml/minute/1.73m2 (IQR 61.3–96.9 ml/minute/1.73m2)]. Their surgery time was slightly longer [median 4.0 hours (IQR 3.3–5.0 hours) vs. 3.9 hours (IQR 3.3–4.9 hours)] and they were less likely to have had CABG surgery (39.2% vs. 44.1%).
Pre-randomisation red blood cell transfusion frequencies were similar in the two groups (25.6% for transfused and 25.7% for non-transfused). However, the transfusion of other blood products was higher in the participants who also had red blood cells transfused; FFP was transfused in 32.5% of participants who had a red blood cell transfusion vs. 21.2% of participants who did not, platelets in 40.7% and 28.3% of participants and cryoprecipitate in 11.4% and 6.9% of participants, respectively. The minimum haemoglobin reached was lower in the transfused participants [median 7.8 g/dl (IQR 7.2–8.4 g/dl) vs. 8.3 g/dl (IQR 7.8–8.6 g/dl)], but the percentage decline in haemoglobin from the pre-operative level was only very slightly more in the transfused participants [median 41.3% (IQR 35.1–46.7%) vs. 39.3% (IQR 34.7–43.3%)]. In terms of trial outcomes, the primary outcome occurred in 35.3% of transfused participants and 30.9% of non-transfused participants, both mortality (3.7% vs. 2.7%) and significant pulmonary morbidity (14.0% vs. 8.3%) rates were higher for transfused participants, and the duration of ICU/HDU stay was longer [median 59.6 hours (IQR 24.4–109 hours) vs. 32.6 hours (IQR 11.3–76.1 hours)].
Compared with participants with a haemoglobin ≥ 7.5 g/dl, participants with a haemoglobin < 7.5 g/dl were of a similar age and had a similar median additive EuroSCOREs but slightly higher median logistic EuroSCOREs [median 4.2 (IQR 2.4–7.2) vs. 4.0 (IQR 2.2–7.2)] and were less likely to be male (65.7% vs. 69.7%). Participants with a post-randomisation haemoglobin < 7.5 g/dl had slightly lower pre-operative haemoglobin levels [mean 13.0 g/dl (SD 1.5 g/dl) vs. 13.4 g/dl (SD 1.4 g/dl)] and eGFR levels [median 69.3 ml/minute/1.73 m2 (IQR 52.5–86.7 ml/minute/1.73 m2) vs. 75.8 ml/minute/1.73 m2 (IQR 58.5–95.4 ml/minute/1.73 m2)].
Participants with a haemoglobin < 7.5 g/dl were more likely to have had a pre-randomisation red blood cell transfusion (30.4% vs. 23.7%), post-randomisation red blood cell transfusion (93.3% vs. 58.7%), FFP transfusion (40.8% vs. 24.0%), platelet transfusion (48.4% vs. 31.9%) and cryoprecipitate transfusion (13.8% vs. 8.5%). In terms of trial outcomes, the primary outcome occurred in 41.3% of participants with a haemoglobin < 7.5 g/dl and 30.9% of participants whose post-randomisation haemoglobin remained ≥ 7.5 g/dl. Both mortality (6.4% vs. 2.1%) and significant pulmonary morbidity (18.8% vs. 9.5%) were more frequent in participants with a haemoglobin < 7.5 g/dl and the duration of ICU/HDU stay was longer [median 71.7 hours (IQR 42.3–131 hours) vs. 42.1 hours (IQR 17.4–87.6 hours)].
Unadjusted relationship between red blood cells, haemoglobin and outcome
The outcome used for these analyses was the primary outcome and/or death, which occurred in 671 out of 1908 (35.2%) participants. In the population as a whole, the risk of this outcome increased with increasing number of transfused units (Table 50). For haemoglobin, the risk of outcome decreases as haemoglobin increases.
| Transfusions/haemoglobin levels | Number of participants | Primary outcome/death, n (%) | Unadjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Post-randomisation (pre-primary outcome) red blood cell transfusions | ||||
| None | 565 | 176 (31.2) | Reference group | 0.0200 |
| One | 577 | 198 (34.3) | 1.15 (0.90 to 1.48) | |
| Two | 396 | 150 (37.9) | 1.35 (1.03 to 1.77) | |
| Three to four | 260 | 96 (36.9) | 1.29 (0.95 to 1.76) | |
| Five or more | 110 | 51 (46.4) | 1.91 (1.26 to 2.89) | |
| Post-randomisation (pre-primary outcome) minimum haemoglobin | ||||
| Hb < 7 g/dl | 216 | 99 (45.8) | 1.91 (1.38 to 2.65) | < 0.0001 |
| 7 g/dl < Hb ≤ 7.5 g/dl | 358 | 154 (43.0) | 1.71 (1.29 to 2.26) | |
| 7.5 g/dl < Hb ≤ 8 g/dl | 363 | 119 (32.8) | 1.10 (0.83 to 1.47) | |
| 8 g/dl < Hb ≤ 8.5 g/dl | 447 | 139 (31.1) | 1.02 (0.78 to 1.34) | |
| Hb ≥ 8.5 g/dl | 522 | 160 (30.7) | Reference group | |
Haemoglobin levels, transfusion status and outcome are described in Table 51 and Figure 24, both for all participants and stratified by transfusion threshold stratum.
| Haemoglobin levels | Transfused participants | Non-transfused participants | All participants | |||
|---|---|---|---|---|---|---|
| Primary outcome/death | Primary outcome/death | Primary outcome/death | ||||
| N | n (%) | N | n (%) | N | n (%) | |
| All participants | ||||||
| Hb < 7 g/dl | 205 | 89 (43.4) | 11 | 10 (90.9) | 216 | 99 (45.8) |
| 7 g/dl < Hb ≤ 7.5 g/dl | 335 | 140 (41.8) | 23 | 14 (60.9) | 358 | 154 (43.0) |
| 7.5 g/dl < Hb ≤ 8 g/dl | 213 | 76 (35.7) | 150 | 43 (28.7) | 363 | 119 (32.8) |
| 8 g/dl < Hb ≤ 8.5 g/dl | 273 | 82 (30.0) | 174 | 57 (32.8) | 447 | 139 (31.1) |
| Hb ≥ 8.5 g/dl | 317 | 108 (34.1) | 205 | 52 (25.4) | 522 | 160 (30.7) |
| Total | 1343 | 495 (36.9) | 563 | 176 (31.3) | 1906 | 671 (35.2) |
| Liberal group | ||||||
| Hb < 7 g/dl | 62 | 29 (46.8) | 6 | 5 (83.3) | 68 | 34 (50.0) |
| 7 g/dl < Hb ≤ 7.5 g/dl | 100 | 43 (43.0) | 2 | 0 (0.0) | 102 | 43 (42.2) |
| 7.5 g/dl < Hb ≤ 8 g/dl | 149 | 48 (32.2) | 2 | 1 (50.0) | 151 | 49 (32.5) |
| 8 g/dl < Hb ≤ 8.5 g/dl | 252 | 74 (29.4) | 24 | 7 (29.2) | 276 | 81 (29.3) |
| Hb ≥ 8.5 g/dl | 314 | 106 (33.8) | 51 | 11 (21.6) | 365 | 117 (32.1) |
| Total | 877 | 300 (34.2) | 85 | 24 (28.2) | 962 | 324 (33.7) |
| Restrictive group | ||||||
| Hb < 7 g/dl | 143 | 60 (42.0) | 5 | 5 (100.0) | 148 | 65 (43.9) |
| 7 g/dl < Hb ≤ 7.5 g/dl | 235 | 97 (41.3) | 21 | 14 (66.7) | 256 | 111 (43.4) |
| 7.5 g/dl < Hb ≤ 8 g/dl | 64 | 28 (43.8) | 148 | 42 (28.4) | 212 | 70 (33.0) |
| 8 g/dl < Hb ≤ 8.5 g/dl | 21 | 8 (38.1) | 150 | 50 (33.3) | 171 | 58 (33.9) |
| Hb ≥ 8.5 g/dl | 3 | 2 (66.7) | 154 | 41 (26.6) | 157 | 43 (27.4) |
| Total | 466 | 195 (41.9) | 478 | 152 (31.8) | 944 | 347 (36.8) |
FIGURE 24.
Relationship between red blood cell transfusion status, minimum haemoglobin concentration and primary outcome/death. Hb, haemoglobin.

A number of observations can be made from Table 51 and Figure 24.
-
At haemoglobin levels below 7.5 g/dl it is difficult to assess the role of transfusion because almost all such participants (540/574; 94.1%) were transfused; however, there is some evidence of a reduced risk of outcome with transfusion. The overall risk at haemoglobin ≤ 7.5 g/dl was 229/540, 42.4% (95% CI 38.2% to 46.7%) for transfused participants and 24/34, 70.6% (95% CI 52.5% to 84.9%) for non-transfused participants. At haemoglobin levels > 7.5 g/dl there was generally a slightly increased risk of outcome associated with transfusion (with the exception of the 8.0 –8.5 g/dl group), although CIs overlap (see Figure 24).
-
The reduced risk of outcome with increasing haemoglobin for the entire population is observed separately within transfused and non-transfused participants.
-
Examining the difference in risk of the outcome in transfused and non-transfused groups in the liberal threshold stratum only is not very informative because most participants in this stratum were transfused. Within the group of transfused participants there is a general trend for the risk of outcome to decrease with increasing haemoglobin. The overall proportion of non-transfused participants experiencing the outcome in the liberal threshold stratum (28.2%) is slightly lower than the overall proportion across both transfusion threshold strata (31.3%), although this is likely to be at least partially attributable to the fact that non-transfused participants in the liberal stratum (arising mainly owing to non-adherence with the study protocol) are likely to have been healthier participants.
-
For participants in the restrictive threshold stratum, there is again a general trend within both transfused and non-transfused participants for the risk of the outcome to decrease with increasing haemoglobin. Similarly, the risk of the outcome appears to reduce among participants transfused at haemoglobin ≤ 7.5 g/dl (although numbers of non-transfused participants are small) and to increase among participants transfused at haemoglobin > 7.5 g/dl (again, arising mainly owing to non-adherence with the study protocol). Outcome event rates for non-transfused participants in the restrictive threshold stratum are similar to the rates for both strata combined, but somewhat higher for transfused participants (except at low haemoglobin levels).
Conventionally adjusted statistical models
As described in Chapter 2, Observational analyses, separate models have been fitted with (1) red blood cell transfusion after randomisation and before the primary outcome or censoring and (2) haemoglobin < 8 g/dl after randomisation and before the pre-primary outcome or censoring as explanatory variables, adjusting for confounders. Models for post-randomisation red blood cells are described in Table 52. Three models are fitted with red blood cells as a categorical variable, an ordinal variable (i.e. fitting the five-level variable as a continuous variable) and a binary variable (i.e. any vs. no red blood cell transfusions).
| Explanatory variables | Model 1: categorical red blood cells | Model 2: ordinal red blood cells | Model 3: binary red blood cells | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Post-randomisation red blood cell units (before primary outcome) | ||||||
| 0 | Reference group | 0.0900 | 1.12 (1.03 to 1.21) | 0.0089 | Reference group 1.28 (1.03 to 1.60) |
0.0280 |
| 1 | 1.17 (0.90 to 1.51) | |||||
| 2 | 1.36 (1.02 to 1.81) | |||||
| 3–4 | 1.27 (0.92 to 1.76) | |||||
| ≥ 5 | 1.70 (1.09 to 2.63) | |||||
| Pre-randomisation red blood cells | 1.64 (1.31 to 2.05) | < 0.0001 | 1.64 (1.31 to 2.05) | < 0.0001 | 1.67 (1.33 to 2.08) | < 0.0001 |
| Logistic EuroSCORE | 1.27 (1.10 to 1.46) | 0.0008 | 1.27 (1.10 to 1.46) | 0.0008 | 1.28 (1.11 to 1.47) | 0.0005 |
| Cardiac procedure | ||||||
| CABG | Reference group | 0.0230 | Reference group | 0.0200 | Reference group | 0.0170 |
| Valve | 0.88 (0.67 to 1.14) | 0.88 (0.67 to 1.14) | 0.88 (0.67 to 1.14) | |||
| CABG + valve | 1.35 (1.01 to 1.79) | 1.35 (1.02 to 1.80) | 1.36 (1.02 to 1.82) | |||
| Other | 1.00 (0.68 to 1.46) | 1.00 (0.68 to 1.46) | 1.01 (0.69 to 1.49) | |||
From Table 52, the best fitting model in terms of deviance is the ordinal model and all models fit well in terms of residual and leverage plots and goodness-of-fit tests. All three models suggest a clear dose–response relationship of increased odds of outcome associated with increasing numbers of red blood cell transfusions; for example, the ordinal model suggests increased odds of outcome of 12% (95% CI 3% to 21%) associated with an increase of one level in the post-randomisation red blood cells variable. The odds of outcome were also significantly increased by transfusion of pre-randomisation red blood cells (OR 1.64, 95% CI 1.31 to 2.05).
The ordinal model from Table 52 was refitted separately within each transfusion threshold stratum (Table 53). Associations are similar to those identified in Table 52, although there is some evidence of a slightly stronger relationship between pre-randomisation red blood cells and outcome in the liberal threshold stratum and, conversely, between cardiac procedure and outcome in the restrictive threshold stratum.
| Explanatory variables | Restrictive threshold stratum | Liberal threshold stratum | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Post-randomisation (pre-primary outcome) red blood cells – ordinal variable | 1.19 (1.06 to 1.35) | 0.0042 | 1.13 (0.99 to 1.28) | 0.072 |
| Any pre-randomisation red blood cells | 1.39 (1.01 to 1.93) | 0.046 | 1.89 (1.38 to 2.59) | 0.0001 |
| Logistic EuroSCORE | 1.24 (1.02 to 1.52) | 0.035 | 1.32 (1.09 to 1.60) | 0.0047 |
| Cardiac procedure | ||||
| CABG | Reference group | 0.047 | Reference group | 0.380 |
| Valve | 0.92 (0.64 to 1.33) | 0.84 (0.57 to 1.22) | ||
| CABG + valve | 1.59 (1.06 to 2.41) | 1.18 (0.79 to 1.77) | ||
| Other | 1.05 (0.62 to 1.79) | 0.98 (0.56 to 1.70) | ||
Models for post-randomisation haemoglobin levels are described in Table 54. Two models are fitted with haemoglobin level as a continuous or binary variable (< 7.5 g/dl vs. ≥ 7.5 g/dl).
| Explanatory variables | OR (95% CI) | p-value |
|---|---|---|
| Post-randomisation (pre-primary outcome) haemoglobina | ||
| CABG participants | 0.92 (0.75 to 1.14) | < 0.0001b |
| Valve participants | 0.62 (0.48 to 0.79) | |
| CABG + valve participants | 0.51 (0.38 to 0.68) | |
| Other participants | 0.68 (0.44 to 1.04) | |
| Pre-operative haemoglobin | 0.91 (0.85 to 0.98) | 0.0094 |
| Logistic EuroSCORE | 1.34 (1.16 to 1.54) | < 0.0001 |
| Females | 0.77 (0.61 to 0.97) | 0.0250 |
| CABG, at haemoglobin = 8 g/dl | Reference group | 0.0600c |
| Valve, at haemoglobin = 8 g/dl | 0.84 (0.64 to 1.10) | |
| CABG + valve, at haemoglobin = 8 g/dl | 1.26 (0.93 to 1.69) | |
| Other, at haemoglobin = 8 g/dl | 1.04 (0.70 to 1.55) | |
| Explanatory variables | OR (95% CI) | p-value |
|---|---|---|
| Post-randomisation haemoglobin ≥ 7.5g/dl (vs. < 7.5g/dl) | ||
| CABG participants | 0.89 (0.63 to 1.26) | < 0.0001a |
| Valve participants | 0.51 (0.35 to 0.75) | |
| CABG + valve participants | 0.37 (0.23 to 0.58) | |
| Other participants | 0.47 (0.23 to 0.93) | |
| Pre-operative haemoglobin (continuous) | 0.90 (0.84 to 0.97) | 0.0044 |
| Logistic EuroSCORE | 1.33 (1.16 to 1.54) | 0.0001 |
| Females | 0.78 (0.62 to 0.99) | 0.0380 |
| CABG, at haemoglobin ≥ 7.5 g/dl | Reference group | 0.2200b |
| Valve, at haemoglobin ≥ 7.5 g/dl | 0.76 (0.55 to 1.04) | |
| CABG + valve, at haemoglobin ≥ 7.5 g/dl | 1.04 (0.73 to 1.47) | |
| Other, at haemoglobin ≥ 7.5 g/dl | 0.89 (0.56 to 1.42) | |
| CABG, at haemoglobin ≥ 7.5 g/dl | Reference group | 0.0018c |
| Valve, at haemoglobin ≥ 7.5 g/dl | 1.33 (0.86 to 2.05) | |
| CABG + valve, at haemoglobin ≥ 7.5 g/dl | 2.52 (1.57 to 4.06) | |
| Other, at haemoglobin ≥ 7.5 g/dl | 1.70 (0.89 to 3.25) | |
All models fit well in terms of residual and leverage plots and goodness-of-fit tests. For both models, an interaction between post-randomisation haemoglobin and cardiac procedure was statistically significant and, therefore, interpretation of the relationship between haemoglobin and outcome is complex. The effect of haemoglobin for each cardiac procedure is given in Table 54, along with the effect of the different cardiac procedures (compared with CABG surgery) at an approximately median haemoglobin value of 8 g/dl (see Table 54a). To visualise this relationship, marginal plots of the haemoglobin effect for different cardiac procedures (from the continuous model) averaged across the other covariates are given in Figure 25.
FIGURE 25.
Marginal plots of the effect of minimum haemoglobin on primary outcome/death for each cardiac procedure type. (a) Without CIs, p-values represent the operation type effect at three different haemoglobin levels. (b) With CIs. Hb, haemoglobin.
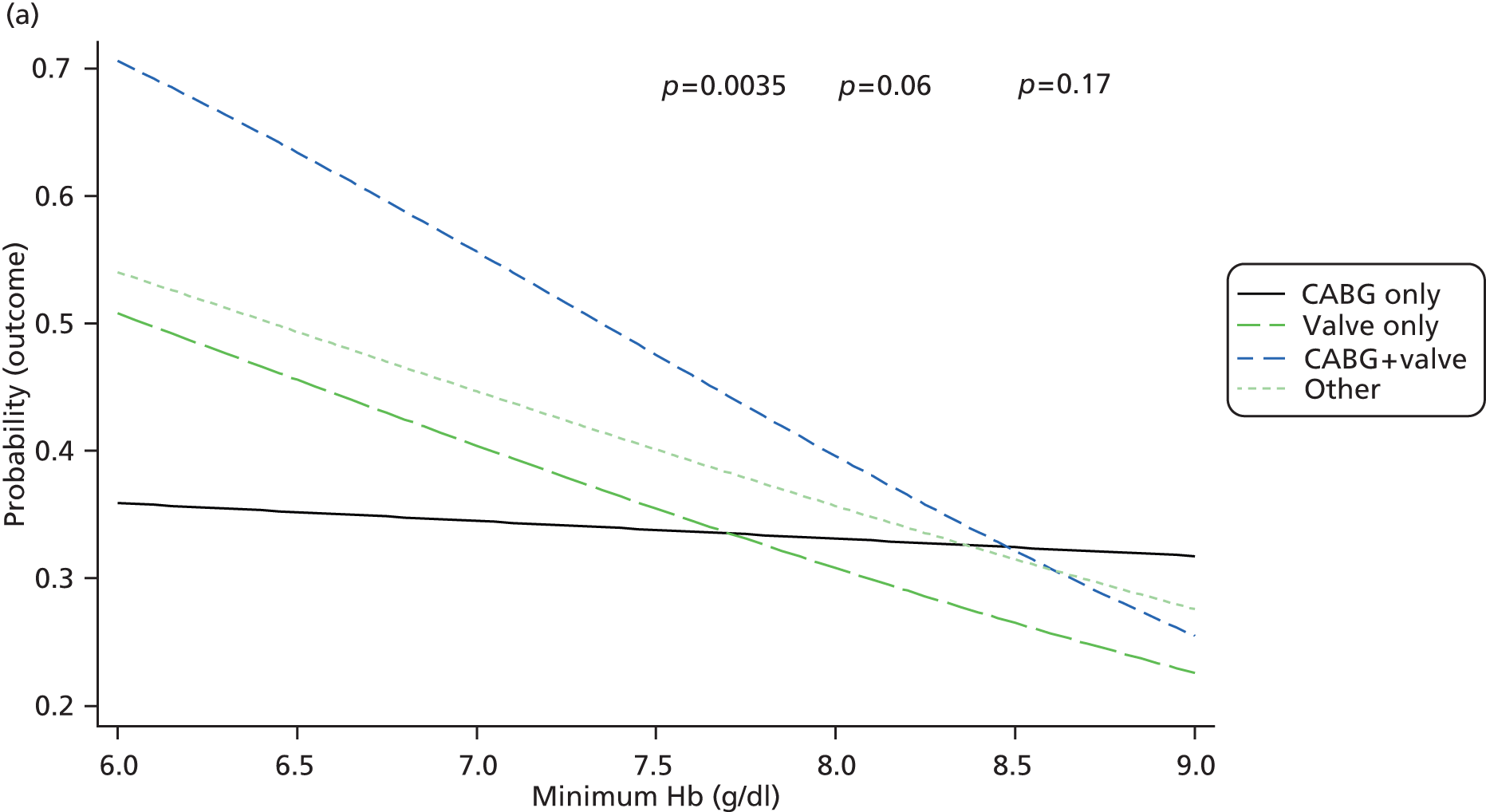
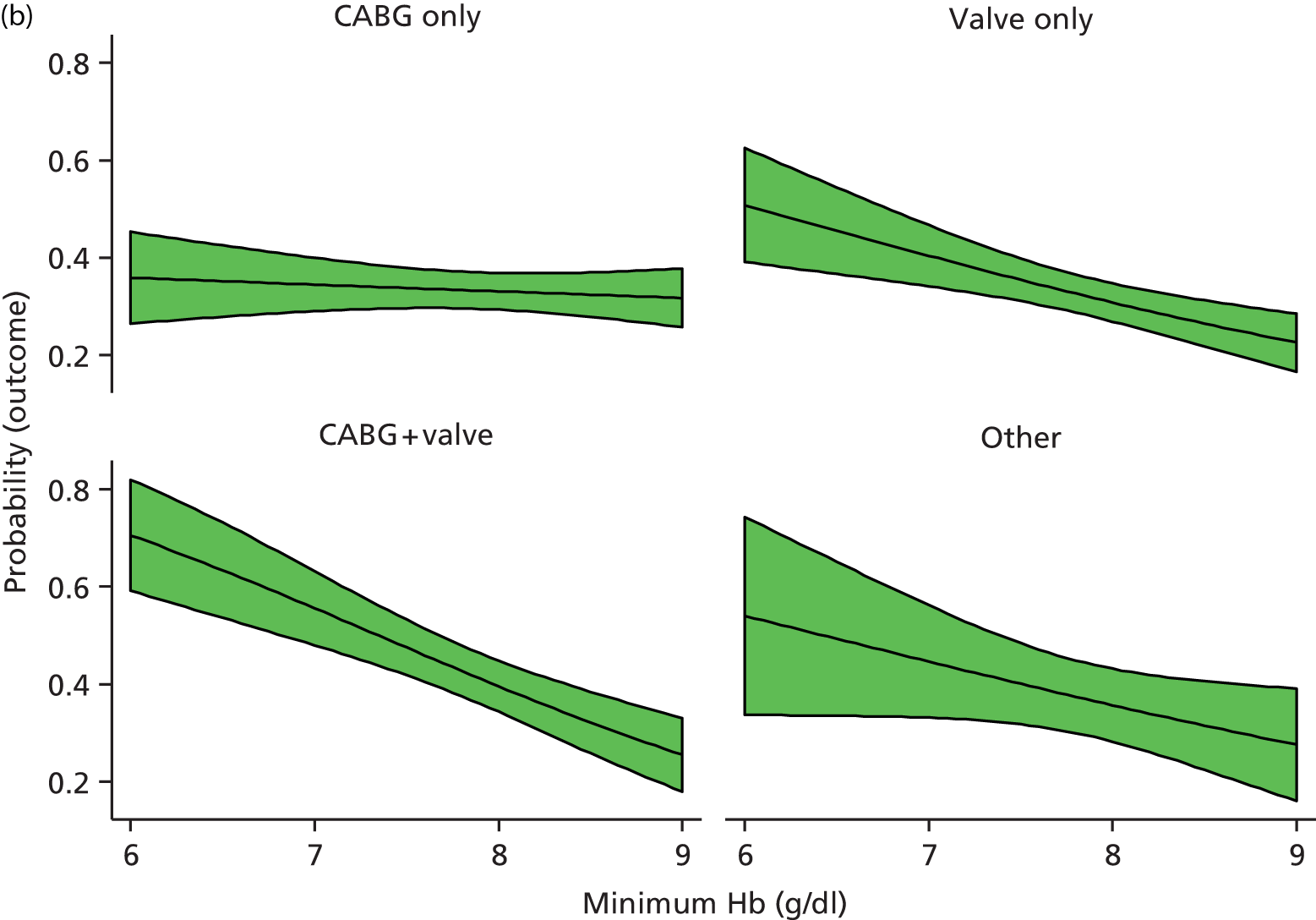
There is evidence of a haemoglobin effect for the non-CABG surgery participants – an increase in haemoglobin of 1 g/dl reduces the odds of outcome (valve surgery: OR 0.62, 95% CI 0.48 to 0.79; CABG and valve surgery: OR 0.51, 95% CI 0.38 to 0.68; other procedures: OR 0.68, 95% CI 0.44 to 1.04), see Table 54 and Figure 25. For CABG participants, there is little evidence of any haemoglobin effect (OR 0.92, 95% CI 0.75 to 1.14). Similarly, the binary model suggests reduced odds of outcome associated with haemoglobin ≥ 7.5 g/dl for non-CABG participants (valve surgery: OR 0.51, 95% CI 0.35 to 0.75; CABG and valve surgery: OR 0.37, 95% CI 0.23 to 0.58; other procedures: OR 0.47, 95% CI 0.23 to 0.93) but little evidence of any effect for CABG participants (OR 0.89, 95% CI 0.63 to 1.26).
The effect of cardiac procedure is estimated at various haemoglobin levels in both models. In the continuous model, cardiac procedure was found to have greater effect at lower haemoglobin levels (see Figure 25). Similarly, in the binary model the effect of cardiac procedure is greater for haemoglobin < 7.5 g/dl participants than haemoglobin ≥ 7.5 g/dl participants. In both the continuous and binary models, a reduced odds of outcome was found with increased pre-operative haemoglobin (continuous model OR 0.91, 95% CI 0.85 to 0.98), and increased odds of outcome associated with both increased logistic EuroSCORE (continuous model OR 1.34, 95% CI 1.16 to 1.54) and sex (continuous model OR 0.77, 95% CI 0.61 to 0.97).
The continuous model from Table 54 was refitted separately within each transfusion threshold stratum (Table 55).
| Explanatory variables | Restrictive group | Liberal group | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Post-randomisation haemoglobin | ||||
| CABG participants | 0.91 (0.67 to 1.23) | 0.0029 | 0.94 (0.68 to 1.29) | 0.0024 |
| Valve participants | 0.63 (0.43 to 0.93) | 0.59 (0.41 to 0.85) | ||
| CABG + valve participants | 0.52 (0.34 to 0.79) | 0.54 (0.35 to 0.84) | ||
| Other participants | 0.67 (0.37 to 1.22) | 0.69 (0.36 to 1.32) | ||
| Pre-operative haemoglobin | 0.84 (0.76 to 0.94) | 0.0013 | 0.98 (0.89 to 1.09) | 0.72 |
| Logistic EuroSCORE | 1.23 (1.00 to 1.51) | 0.049 | 1.47 (1.21 to 1.80) | 0.0001 |
| Females | 0.82 (0.59 to 1.13) | 0.22 | 0.72 (0.52 to 1.01) | 0.054 |
| CABG, at haemoglobin = 8 g/dl | Reference group | 0.16 | Reference group | 0.59 |
| Valve, at haemoglobin = 8 g/dl | 0.83 (0.55 to 1.26) | 0.87 (0.59 to 1.28) | ||
| CABG + valve, at haemoglobin = 8 g/dl | 1.44 (0.90 to 2.30) | 1.16 (0.77 to 1.74) | ||
| Other, at haemoglobin = 8 g/dl | 1.09 (0.61 to 1.97) | 1.02 (0.58 to 1.79) | ||
The effect of post-randomisation haemoglobin was remarkably similar in the two transfusion threshold strata; the equivalent marginal plots are given in Figure 26. Pre-operative haemoglobin had a greater effect on the odds of outcome in the restrictive threshold stratum and EuroSCORE had a greater effect in the liberal threshold stratum.
FIGURE 26.
Marginal plots of the effect of minimum haemoglobin on primary outcome/death for each cardiac procedure type, by transfusion threshold stratum. p-values represent tests of the operation type effect at a haemoglobin level of 8.0 g/dl in each transfusion threshold stratum. (a) Restrictive group. (b) Liberal group. Hb, haemoglobin.

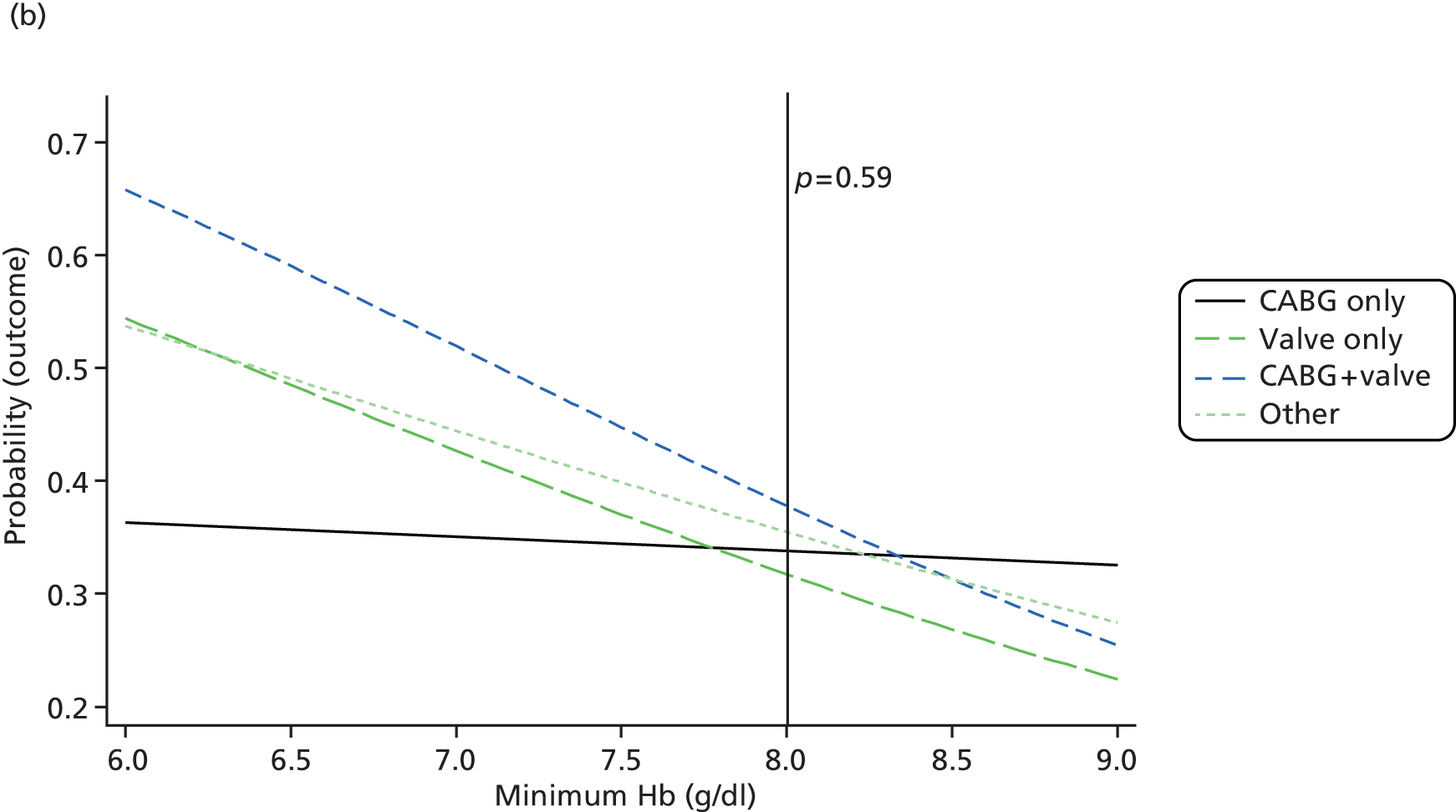
Instrumental variable analysis
The assumptions of IV analysis59 and why we believe that the assumptions are met in our analyses are described below.
-
The instrument (randomised allocation) is associated with the exposure (post-randomisation red blood cell units or post-randomisation haemoglobin); if a regression of the instrument on the exposure is performed, a F-statistic of > 10 is typically used as a cut-off criterion, with values ≤ 10 indicating a weak instrument. There is evidence of a strong relationship between each of the exposure variables and randomised allocation (Table 56). Furthermore, F-statistics from the relevant univariate regression models are as follows: (1) a log-linear model regressing randomised allocation on the ordinal transfusion variable gave a RR of 0.53, 95% CI 0.49 to 0.58; F-statistic 238.8; and (2) a linear regression of randomised allocation on post-randomisation haemoglobin gave a mean difference between groups of –0.44, 95% CI –0.50 to –0.38; F-statistic 181.5.
-
The instrument is independent of confounders between exposure and outcome. We consider this assumption to be met by virtue of the instrument being randomised allocation; there is no evidence of an association between randomised allocation and any variables (other than haemoglobin and red blood cell transfusions).
-
The instrument is independent of the outcome, given the exposure and confounders between the exposure and the outcome. Again we consider this assumption to be met as the instrument is randomised allocation.
| Transfusions/haemoglobin levels | Restrictive group (n = 1000) | Liberal group (n = 1003) |
|---|---|---|
| Post-randomisation (pre-primary outcome) red blood cell transfusions, n (%) | ||
| None | 516 (51.6) | 106 (10.6) |
| One | 211 (21.1) | 379 (37.8) |
| Two | 142 (14.2) | 268 (26.7) |
| Three to four | 94 (9.4) | 176 (17.6) |
| Five or more | 37 (3.7) | 74 (7.4) |
| Post-randomisation (pre-primary outcome) minimum haemoglobin, n (%) | ||
| Hb < 7 g/dl | 153 (15.3) | 71 (7.1) |
| 7 < Hb ≤ 7.5 g/dl | 267 (26.8) | 104 (10.4) |
| 7.5 < Hb ≤ 8 g/dl | 226 (22.7) | 157 (15.7) |
| 8 < Hb ≤ 8.5 g/dl | 186 (18.6) | 289 (28.8) |
| Hb ≥ 8.5 g/dl | 166 (16.6) | 382 (38.1) |
Results from IV models are given in Table 57. In terms of post-randomisation red blood cells, both models show no statistically significant effect of transfusion. If anything, effect estimates indicate reductions in risk of outcome with transfusion (RR 0.89, 95% CI 0.75 to 1.06, for the ordinal red blood cell transfusion model and RR 0.78, 95% CI 0.53 to 1.14, for the binary predictor, i.e. any vs. no red blood cell transfusion). It is interesting to compare these estimates with the main trial ITT estimate between the randomised groups (inverted, i.e. comparing liberal ‘transfused’ to restrictive ‘not transfused’ participants), OR 0.90 (95% CI 0.74 to 1.10; p = 0.30). The estimates are remarkably similar despite the fact that they are estimating different effects (ITT estimate of effect of transfusion threshold on primary outcome only in the context of non-adherence in the trial compared with the effect of red blood cell transfusion on primary outcome or death on participants who were actually transfused, adjusted for confounding, in the IV analysis).
| Instrument used | RR (95% CI) | p-value |
|---|---|---|
| Model 1: post-randomisation red blood cells as an ordinal variable | 0.89 (0.75 to 1.06) | 0.19 |
| Model 2: post-randomisation red blood cells as a binary variable (any transfusion vs. none) | 0.78 (0.53 to 1.14) | 0.20 |
| Model 3: post-randomisation haemoglobin as a continuous variable | 0.83 (0.64 to 1.08) | 0.17 |
| Model 4: post-randomisation haemoglobin as a binary variable (haemoglobin ≥ 7.5 g/dl vs. < 7.5 g/dl) | 0.71 (0.43 to 1.19) | 0.19 |
For post-randomisation haemoglobin, both models show no statistically significant effect with effect estimates indicating reductions in risk of outcome with increasing haemoglobin (RR 0.83, 95% CI 0.64 to 1.08, for the model with haemoglobin fitted as a continuous variable, and RR 0.71, 95% CI 0.43 to 1.19, for the model with haemoglobin fitted as a binary variable comparing haemoglobin levels ≥ 7.5g/dl with < 7.5g/dl). As anticipated, the CIs around effect estimates are relatively wide in all of the IV models.
Next steps
The main limitation of the analyses covered in this section is their restriction to investigating either the effect of red blood cell transfusion or the effect of haemoglobin level. They do not address the combined effects of these factors, preventing us from answering questions such as ‘at what haemoglobin threshold does the receipt of transfusion become beneficial?’. This question is not straightforward to answer because red blood cell transfusion is inevitably associated with haemoglobin level and the lowest haemoglobin level experienced by a patient does not necessarily precede transfusion.
An extension to these analyses that may address this issue is to perform analyses restricted to groups of participants that breach a certain haemoglobin threshold (e.g. 7.5 g/dl or 8 g/dl) and then compare outcomes for participants transfused and not transfused at a haemoglobin below that level. Such an analysis could be performed at a small number of different haemoglobin thresholds to estimate the effect of transfusion at different thresholds of haemoglobin.
Age of blood
Descriptive analyses
The age of each red blood cell unit transfused was unable to be retrieved for a relatively large proportion of units. Of the 3104 units transfused post-randomisation but prior to the time of the primary outcome occurring or censoring, the age was unobtainable for 945 units (30.4%). In terms of participants, of the 1381 participants transfused one or more unit, 581 (42.1%) had one or more unit with unknown age. The volume of blood transfused was a strong predictor of missing age of blood for one or more of the units transfused (Table 58).
| Transfusions | Number of participants | One or more units with missing age, n (%) |
|---|---|---|
| Post-randomisation (pre-primary outcome) red blood cell transfusions | ||
| None | 621 | 0 (0.0) |
| One | 591 | 174 (29.4) |
| Two | 409 | 170 (41.6) |
| Three to four | 271 | 151 (55.7) |
| Five or more | 111 | 86 (77.5) |
For the purposes of initial descriptive analyses, participants have been grouped into four categories:
-
Transfused one or more unit older than 21 days (n = 527).
-
Transfused, but received no units older than 21 days (n = 402).
-
Transfused, but unknown if any units were received older than 21 days (n = 452). [Note: this number is lower than the number of participants quoted above as having one or more unit with unknown age (n = 581) because any participants transfused multiple units with one or more unit older than 21 days will be classified in group (a) above, regardless of any other units with unknown age.]
-
Not transfused any red blood cells (n = 622).
Characteristics and outcomes of participants according to these categories are given in Tables 59 and 60.
| Characteristic | Transfused one or more unit older than 21 days (N = 527) | Transfused, but received no units older than 21 days (N = 402) | Transfused, but unknown if any units were received older than 21 days (N = 452) | Not transfused any red blood cells (N = 622) |
|---|---|---|---|---|
| Cardiac history | ||||
| Additive EuroSCORE,a median (IQR) | 5.0 (4.0–7.0) | 5.0 (3.0–7.0) | 6.0 (4.0–7.0) | 5.0 (3.0–7.0) |
| Logistic EuroSCORE,a median (IQR) | 4.3 (2.4–8.0) | 3.6 (2.2–6.6) | 4.5 (2.6–7.5) | 3.7 (2.0–6.6) |
| NYHA class, n/N (%) | ||||
| I | 138/505 (27.3) | 104/393 (26.5) | 100/450 (22.2) | 151/603 (25.0) |
| II | 208/505 (41.2) | 187/393 (47.6) | 199/450 (44.2) | 291/603 (48.3) |
| III | 143/505 (28.3) | 97/393 (24.7) | 142/450 (31.6) | 143/603 (23.7) |
| IV | 16/505 (3.2) | 5/393 (1.3) | 9/450 (2.0) | 18/603 (3.0) |
| CCS class, n/N (%) | 191/510 (37.5) | 139/395 (35.2) | 147/449 (32.7) | 241/608 (39.6) |
| No angina, n/N (%) | ||||
| I | 103/510 (20.2) | 72/395 (18.2) | 84/449 (18.7) | 103/608 (16.9) |
| II | 121/510 (23.7) | 108/395 (27.3) | 121/449 (26.9) | 176/608 (28.9) |
| III | 73/510 (14.3) | 52/395 (13.2) | 83/449 (18.5) | 73/608 (12.0) |
| IV | 22/510 (4.3) | 24/395 (6.1) | 14/449 (3.1) | 15/608 (2.5) |
| Coronary disease, n/N (%) | ||||
| None | 172/525 (32.8) | 110/400 (27.5) | 144/450 (32.0) | 194/616 (31.5) |
| Single vessel | 66/525 (12.6) | 46/400 (11.5) | 56/450 (12.4) | 57/616 (9.3) |
| Double vessel | 66/525 (12.6) | 55/400 (13.8) | 63/450 (14.0) | 98/616 (15.9) |
| Triple vessel | 211/525 (40.2) | 179/400 (44.8) | 172/450 (38.2) | 243/616 (39.4) |
| Not investigated | 10/525 (1.9) | 10/400 (2.5) | 15/450 (3.3) | 24/616 (3.9) |
| Disease in left main stem (> 50% stenosis), n/N (%) | 78/520 (15.0) | 66/398 (16.6) | 60/446 (13.5) | 100/613 (16.3) |
| Non-cardiac history | ||||
| Age (years), median (IQR) | 70.7 (64.3–76.6) | 70.5 (64.0–77.0) | 70.9 (63.8–76.7) | 69.5 (62.6–75.5) |
| Males, n/N (%) | 350/527 (66.4) | 288/402 (71.6) | 295/452 (65.3) | 440/622 (70.7) |
| BMI (kg/m2),b mean (SD) | 28.0 (5.0) | 27.9 (4.8) | 27.8 (4.7) | 28.7 (5.1) |
| Urgent operative priority, n/N (%) | 81/527 (15.4) | 53/402 (13.2) | 47/452 (10.4) | 64/622 (10.3) |
| Diabetic, n/N (%) | 104/527 (19.7) | 86/402 (21.4) | 89/452 (19.7) | 120/622 (19.3) |
| Haemofiltration/dialysis, n/N (%) | 8/526 (1.5) | 4/402 (1.0) | 4/451 (0.9) | 3/622 (0.5) |
| CVA/TIA, n/N (%) | 47/527 (8.9) | 30/402 (7.5) | 35/452 (7.7) | 51/622 (8.2) |
| Pre-operative tests | ||||
| Haemoglobin (g/dl), mean (SD) | 13.1 (1.5) | 13.3 (1.5) | 13.2 (1.5) | 13.5 (1.4) |
| eGFR (ml/minute/1.73m2),c median (IQR) | 71.2 (54.4–91.9) | 72.1 (55.7–90.4) | 72.5 (54.7–91.6) | 77.4 (61.3–96.9) |
| Intra-operative characteristics | ||||
| Duration of operation (hours),d median (IQR) | 4.0 (3.2–5.2) | 4.1 (3.4–5.0) | 4.0 (3.5–5.0) | 3.9 (3.3–4.9) |
| CPB used, n/N (%) | 511/527 (97.0) | 385/402 (95.8) | 427/452 94.5) | 580/621 (93.4) |
| Cardiac procedure, n/N (%) | ||||
| CABG only | 197/527 (37.4) | 179/402 (44.5) | 166/452 (36.7) | 274/622 (44.1) |
| Valve only | 170/527 (32.3) | 116/402 (28.9) | 142/452 (31.4) | 183/622 (29.4) |
| CABG + valve | 111/527 (21.1) | 82/402 (20.4) | 108/452 (23.9) | 97/622 (15.6) |
| Other | 49/527 (9.3) | 25/402 (6.2) | 36/452 (8.0) | 68/622 (10.9) |
| Tranexamic acid, n/N (%) | 448/527 (85.0) | 334/401 (83.3) | 330/452 (73.0) | 503/621 (81.0) |
| Trasylol, n/N (%) | 17/515 (3.3) | 15/398 (3.8) | 16/402 (4.0) | 23/579 (4.0) |
| Cell saver, n/N (%) | 272/527 (51.6) | 210/402 (52.2) | 207/452 (45.8) | 295/621 (47.5) |
| Blood loss at 4 hours (ml),e median (IQR) | 274 (160–450) | 260 (150–425) | 290 (180–500) | 210 (125–328) |
| Blood loss at 12 hours (ml),f median (IQR) | 525 (350–850) | 500 (325–800) | 550 (350–865) | 400 (290–600) |
| Outcome | One + red blood cells > 21 days (N = 527) | No red blood cells > 21 days (N = 402) | Unknown red blood cells > 21 days (N = 452) | No red blood cell transfusions (N = 622) |
|---|---|---|---|---|
| Intra- and post-operative use of blood products | ||||
| Pre-randomisation red blood cell transfusion, n/N (%) | 143/527 (27.1) | 108/402 (26.9) | 103/452 (22.8) | 160/622 (25.7) |
| Post-randomisation (pre-primary outcome) red blood cell transfusions, n/N (%) | ||||
| No units | 0/527 (0.0) | 0/402 (0.0) | 0/452 (0.0) | 622/622 (100.0) |
| 1 unit | 178/527 (33.8) | 239/402 (59.5) | 173/452 (38.3) | 0/622 (0.0) |
| 2 units | 172/527 (32.6) | 107/402 (26.6) | 130/452 (28.8) | 0/622 (0.0) |
| 3–4 units | 124/527 (23.5) | 48/402 (11.9) | 99/452 (21.9) | 0/622 (0.0) |
| > 4 units | 53/527 (10.1) | 8/402 (2.0) | 50/452 (11.1) | 0/622 (0.0) |
| FFP transfusions, n/N (%) | 185/527 (35.1) | 126/402 (31.3) | 138/452 (30.5) | 132/622 (21.2) |
| Platelet transfusions, n/N (%) | 225/527 (42.7) | 165/402 (41.0) | 172/452 (38.1) | 176/622 (28.3) |
| Cryoprecipitate transfusions, n/N (%) | 68/527 (12.9) | 34/402 (8.5) | 56/452 (12.4) | 43/622 (6.9) |
| Activated factor VII used, n/N (%) | 5/527 (0.9) | 2/402 (0.5) | 0/452 (0.0) | 5/622 (0.8) |
| Beriplex used, n/N (%) | 24/527 (4.6) | 20/402 (5.0) | 21/452 (4.6) | 35/622 5.6) |
| Minimum haemoglobin (g/dl),a median (IQR) | 7.8 (7.1–8.4) | 7.9 (7.3–8.5) | 7.7 (7.1–8.3) | 8.3 (7.8–8.6) |
| Primary outcome, n/N (%) | ||||
| Overall primary outcome | 188/511 (36.8) | 139/390 (35.6) | 147/441 (33.3) | 174/564 (30.9) |
| Infectious event | 138/501 (27.5) | 106/387 (27.4) | 107/439 (24.4) | 127/563 (22.6) |
| Sepsis | 125/515 (24.3) | 92/396 (23.2) | 94/449 (20.9) | 113/605 (18.7) |
| Wound infection | 21/486 (4.3) | 27/375 (7.2) | 24/436 (5.5) | 29/560 (5.2) |
| Ischaemic event | 89/522 (17.0) | 62/399 (15.5) | 62/450 (13.8) | 82/611 (13.4) |
| Permanent stroke | 11/517 (2.1) | 7/398 (1.8) | 7/448 (1.6) | 7/611 (1.1) |
| Suspected MI | 5/514 (1.0) | 1/396 (0.3) | 0/447 (0.0) | 1/611 (0.2) |
| Gut infarction | 1/514 (0.2) | 0/396 (0.0) | 3/448 (0.7) | 3/611 (0.5) |
| AKI | 75/519 (14.5) | 55/398 (13.8) | 58/450 (12.9) | 74/611 (12.1) |
| Other trial outcomes | ||||
| All-cause mortality, n/N (%) | 24/527 (4.6) | 10/402 (2.5) | 17/452 (3.8) | 17/622 (2.7) |
| Significant pulmonary morbidity, n/N (%) | 68/518 (13.1) | 52/400 (13.0) | 72/449 (16.0) | 51/614 (8.3) |
| Duration of ICU/HDU stay (hours), median (IQR) | 66.5 (24.5–117) | 48.1 (23.5–97.0) | 60.3 (26.4–99.3) | 32.6 (11.3–76.1) |
| Duration of post-randomisation hospital stay (hours), median (IQR) | 7.0 (6.0–11.0) | 7.0 (5.0–10.0) | 7.0 (6.0–11.0) | 6.0 (4.0–9.0) |
| Composite outcome | ||||
| Primary outcome or death, n/N (%) | 196/511 (38.4) | 142/390 (36.4) | 157/441 (35.6) | 176/564 (31.2) |
The three groups of transfused participants (older blood, younger blood and unknown) were of similar ages, but compared with those only transfused younger blood, participants transfused older blood were less likely to be male (71.6% vs. 66.4%), had higher average logistic EuroSCORE [median 4.3 (IQR 2.4–8.0) vs. 3.6 (IQR 2.2–6.6)] and were less likely to have had CABG surgery (37.4% vs. 44.5%). It should be noted that some of these apparent associations are likely to have arisen from confounding; for example, female participants may have been more likely to be given older blood because they were more likely to have multiple red blood cell units transfused, increasing the risk of having an older unit transfused.
Rates of pre-randomisation red blood cell transfusion were lower in the group with unknown age of blood (22.8%) than the other three groups (older blood 27.1%, younger blood 26.9% and no blood 25.7%). The number of post-randomisation red blood cells transfused was, on average, higher in those transfused older blood (66.2% transfused two or more units) and unknown age (61.7% transfused two or more units) than those transfused only younger blood (40.5% transfused two or more units). In addition, participants transfused older blood were more likely to have been given non-red blood cell blood products than the other groups but there were no clear trends in average post-randomisation haemoglobin levels between the three groups of transfused participants.
In terms of trial outcomes, the primary outcome occurred in 36.8% of participants transfused older blood, 35.6% of participants transfused only younger blood, 33.3% of those with unknown age of blood and 30.9% of those not transfused. More participants died in the groups transfused older blood (4.6%) and with unknown age of blood (3.8%) than those transfused only younger blood (2.5%) and those not transfused (2.7%). Finally, the composite outcome of primary outcome or death occurred in 38.4% of participants transfused older blood, 36.4% of participants transfused younger blood only, 35.6% of those with unknown age of blood and 31.2% of those not transfused.
Next steps
The results from Descriptive analyses suggest some evidence of a weak association between older blood and poorer outcome, particularly in terms of mortality; however, a major limitation is that no adjustment for confounding has been performed. Therefore, it is unclear from these descriptive analyses whether or not any differences observed are actually due to other factors, for example, the fact that participants transfused older blood received, on average, more red blood cells than those transfused only younger blood.
A further limitation of this work is that blood group was unfortunately not collected in the study and is not considered retrievable. It has been suggested that this could be an important confounding factor, especially if age of blood is defined in terms of giving older vs. younger blood. The rationale for this view is that the turnover of blood stores varies according to the blood group of the donated blood; for example, participants with rare blood groups may be more likely to have older blood. 60
Therefore, an obvious next step would be to fit multivariate models addressing the confounding. This has not been done owing to the number of missing data on age of blood, which is of a sufficiently high level that any analyses ignoring missing data (‘complete-case analyses’) will be inefficient and possibly biased. Addressing this missing data problem is not straightforward. Multiple imputation techniques are most commonly used as they are considered most appropriate and flexible. 61 Imputation (and, therefore, subsequent modelling) could be implemented at the red blood cell unit level (i.e. by imputing the age of each unit of red blood cells) or at level of a participant (e.g. imputing whether a participant received any old blood or not). Further work is required to address the issue of whether imputation should be implemented at the red blood cell unit level or at the participant level.
Another consideration is alternative ways of defining age of blood at the participant level (as described in the methods), including the age of the oldest red blood cell transfused, the mean age of all red blood cells, the use of any blood older than 14 days, and the number or percentage of red blood cells given that are older than 14 or 21 days. These approaches will be affected to different extents by missing data; therefore, we do not intend to proceed with this until we have addressed the missing data problem described above.
Chapter 8 Discussion
Main findings: study conduct
Recruitment
Recruitment was slower than expected and the duration of recruitment had to be extended in order to reach the target sample size. Issues affecting recruitment have been described in Chapter 3, Recruitment. Throughout the trial the rate of recruitment remained frustratingly resistant to any actions the co-ordinating team took to increase it. It did not appear to increase as additional centres were recruited, nor when mid-term site visits were carried out to encourage centre staff to share good practice tips gleaned from the best recruiting centres and to discuss local circumstances that were perceived to be limiting recruitment. However, the UK Comprehensive Research Network made TITRe2 a special focus for their efforts and this is likely to explain the increase in recruitment in the second half of 2011.
Non-adherence to the allocated threshold
We identified non-adherence as a key issue at the outset and were able to put in place appropriate data collection (although time-consuming) to differentiate non-adherence into mild, moderate and severe. The importance of non-adherence was reinforced by the DMEC, both in terms of the threat to the overall power of the trial and also the possibility of differential non-adherence by group. The central trial team reported adherence to the DMEC regularly.
Even with our extreme awareness of the problem and a large investment in data collection, non-adherence was still prevalent in the trial. Fortunately, severe non-adherence occurred for only a small number of participants (9.7% in the restrictive group and 6.2% in the liberal group) and, therefore, good separation was maintained between groups with respect to red blood cells transfused and haemoglobin levels. In calculating the initial target sample size we assumed that no more than 35% of participants in the restrictive group and no less than 74% of participants in the liberal group would be transfused. Although the absolute rates differed, the transfusion rates (53% and 92%, respectively) achieved this separation in the rate of transfusion.
In a similar way to the rate of recruitment, non-adherence was resistant to our efforts to reduce its incidence through a continuous education and awareness campaign and, latterly, detailed feedback to centres about specific non-adherent instances. This resistance perhaps reflects the limited ability of these initiatives to overcome staff shortages on the ground, which was the factor that we believed to be mainly responsible. This leads to the question of whether or not we would advocate monitoring non-adherence in this level of detail, to which our answer is unequivocally yes. Despite non-adherence not being straightforward to predict, knowledge of non-adherence is vital. Even if non-adherence has been considered when justifying the target sample size, there is still a need to monitor its incidence carefully and ensure it is in line with the assumptions made.
The main drawback of trying to measure non-adherence is the demanding data collection required and, if we were to design the study again, we would investigate more streamlined methods of capturing these data, for example downloads of routine data from ICU software now commonly being used to manage patient care. Many reasons for non-adherence were missing and, although these were queried, relevant information was often difficult to retrieve. In addition, we believe the category ‘oversight’ was used as a default reason when a clinician was not asked to justify non-adherence at the time it occurred.
It is difficult to compare non-adherence rates from TITRe2 to rates observed in previous trials that randomised patients to different transfusion strategies. Two of the earliest studies defined non-adherence either only in terms of withheld transfusions62 or extra transfusions. 37 The former study (838 participants) reported non-adherence for six participants (1.4%) in the restrictive group and 18 participants (4.3%) in the liberal group, and the latter (the largest study in cardiac surgery patients to date37) reported four cases (all in the restrictive group) of non-adherence from 502 participants. Both studies reported markedly lower non-adherence rates than TITRe2, although comparisons are not sensible owing to the different definitions used, differences in the populations randomised (both trials included participants who did not breach the liberal threshold) and the limited information about non-adherence that was reported in both studies.
Two more recent studies considered non-adherence both in terms of extra and withheld transfusions. The FOCUS trial13 investigated transfusion strategies following hip surgery; severe protocol deviations that were comparable with our definitions were reported in 9.0% of participants in the liberal strategy group and 5.6% in the restrictive strategy group. These rates are similar to our rates of severe non-adherence, although the differences between the randomised groups are in the opposite direction to TITRe2. This could be due to the different hypotheses being addressed as FOCUS hypothesised that giving transfusion would benefit patients. The second study was a pilot RCT of adherence to transfusion thresholds in cardiac surgery;38 it used non-adherence definitions most comparable to TITRe2 and comprised a similar population. Among the 50 participants recruited, post-operative non-adherence was reported as 18% in the ICU and 0% on the ward in the restrictive group, and 31% in ICU and 86% on the ward in the liberal group. As in TITRe2 for any non-adherence, rates were higher in the liberal group than the restrictive group. The rate in the liberal group was substantially higher than TITRe2.
We believe that we are the first researchers to identify and classify, in detail, non-adherence to a transfusion strategy in a complicated trial setting, in which both the timing of randomisation and intervention were not at a fixed point in time. Adherence remains a key issue in trials comparing restrictive and liberal transfusion strategies and this study has led to better understanding of potential motivations and mechanisms for different types of non-adherence. Some of this understanding can be generalised to other trials with complex interventions. In particular, vigilance and reminders appeared to be the most successful ways to avoid non-adherence. We noted that non-adherence was less common in centres with successful research infrastructures (e.g. a well organised NHS research department, recruiting to multiple NIHR portfolio studies, well-integrated team of research nurses with expertise in managing cardiac surgery patients) and a high throughput of patients. It is clear that having fewer, high-recruiting, sites as opposed to lots of low-recruiting sites is preferable, as the constant presence of trial participants on the ICU or the ward is one of the best reminders to staff. Even if increased vigilance results from higher staffing levels, it is unclear whether or not this is cost-effective. Trials can suffer a large amount of non-adherence and still deliver meaningful results, as we believe has been the case in TITRe2.
Higher-than-expected frequency of the primary outcome
The higher-than-expected frequency of the composite primary outcome and the dominance of less serious events such as sepsis and milder AKI were the most substantive issues experienced. On the one hand, the higher overall frequency meant that the trial had more statistical power than anticipated; however, on the other hand, the dominance by less serious events had the potential to undermine the interpretation of the primary outcome. The question of whether or not to revise the primary outcome was debated by the DMEC before an application was made to extend the scheduled period of recruitment. The DMEC recommended that neither the primary outcome nor the target sample size should be changed (see Strengths and limitations).
Main findings: study results
Summary of findings of the trial
The TITRe2 trial tested the hypothesis that a restrictive red blood cell transfusion threshold is superior to a liberal threshold after adult cardiac surgery, in terms of post-operative morbidity and health service costs. We refuted this hypothesis because we observed no difference in the primary composite outcome between the liberal and restrictive groups. Pre-planned subgroup analyses showed no differences, contrary to beliefs that ‘at-risk’ groups should be transfused at different haemoglobin thresholds.
We carried out a number of planned sensitivity analyses of the primary outcome in order to test the robustness of the primary analysis. When we designed the trial we decided to include participants transfused before randomisation, for example in the operating theatre, as the question the trial sought to answer applied as much to these patients as others. Nevertheless, we recognised that by doing so we might dilute any effect of randomisation as transfusion was the ‘exposure’ of interest and transfusion before randomisation would be distributed similarly. As hypothesised, when we excluded this subgroup in a sensitivity analysis, the effect estimate for the primary outcome moved away from the null, favouring the liberal threshold.
A second sensitivity analysis was planned because of the observation (in the pre-specified interim analysis) that the majority of the primary outcome events were either sepsis or AKI. We considered that a treatment effect for more ‘serious’ events would better reflect our original intention in formulating the composite outcome but did not hypothesise that the effect estimate would move. Therefore, we were surprised to find that effect estimate moved to towards the null.
Two other sensitivity analyses were designed to address uncertainty about the ascertainment of AKI events (see Chapter 2, Sensitivity analyses). We did not hypothesise a change in the magnitude of the treatment effect for either analysis. Excluding AKI events that may have been reported erroneously did not move the effect estimate; however, including additional AKI events that we suspected had been missed did move the effect estimate away from the null, again favouring the liberal threshold. Although we had not hypothesised such a shift, this finding was consistent with the imbalance of qualifying AKI events (both among AKI events that had been reported and AKI events that we suspected had been missed) across the two groups. The small difference in the overall primary outcome event rate (2%) arose because participants in the restrictive group had a 2% higher frequency of AKI events (14% vs. 12%). Including the additional AKI events approximately doubled their frequency in both groups (approximately 28% vs. 24%), increasing the overall difference in the primary outcome from 2% to 4%.
All-cause mortality was the only secondary outcome for which the treatment effect suggested a difference between groups, with more participants dying in the restrictive group. There were no differences in other secondary outcomes, including unexpected SAEs and SAEs that did not qualify for the primary outcome. In the course of peer review of a manuscript reporting the main outcome results, it was suggested that we should carry out (the same) sensitivity analyses for all-cause-mortality. This suggestion was made owing to the potential importance of a difference in mortality and the difficulty in quantifying uncertainty around the observed difference, given that it was one among several secondary outcomes. However, only two of the sensitivity analyses were applicable: first, excluding deaths occurring in the first 24 hours after randomisation and, second, excluding participants who had red blood cells transfused before randomisation. In both analyses, the magnitude of the treatment effect favouring a liberal threshold increased as hypothesised (and its nominal statistical significance was maintained despite both analyses having less power).
Interpreting secondary analyses is challenging when several statistical tests are carried out. 40 Nevertheless, the higher frequency of deaths in the restrictive group is a cause for serious concern. It is not clear how anaemia attributable to the restrictive threshold may have resulted in an increased number of deaths. The difference in haemoglobin between groups was modest (1 g/dl) and assessment of causes of death or SAEs that preceded death did not demonstrate cause and effect, although expecting to deduce a causal mechanism in this way may be unrealistic based on a small number of deaths in a setting in which death typically occurs after a series of AEs.
The TITRe2 trial compared two active interventions, both of which probably reflected usual care although in different centres. Safety is considered here in terms of a restrictive threshold compared with a liberal one. There was a similarly small non-significant difference, again favouring the liberal threshold, in the frequency of all SAEs not included in the primary outcome (35.7% and 34.2% of participants in the restrictive and liberal groups, respectively). The trial did not have adequate power to distinguish any difference in the types of SAEs between groups. The IV analysis estimating the effect of red blood cell transfusion (after randomisation and before the occurrence of the primary outcome or censoring) also provided the most direct test of the safety of transfusion among participants who were transfused, finding no evidence at all that transfusion increased the risk of the primary outcome or death.
Three observational analyses were planned investigating the effects of exposure to red blood cell transfusion, post-operative anaemia and ‘old’ red blood cells (that had been stored for longer than average). The main reasons for doing these analyses were:
-
to try to replicate our observational finding2 and the findings of other observational studies,63 about the risks of transfusion in the trial cohort
-
to try to estimate the effect of transfusion at different levels of anaemia
-
to try to replicate previous reports (e.g. Dzik60 and Koch et al. 64) regarding the risk of poor clinical outcome being attributable to red blood cells stored for longer than average
-
to contrast effect estimates from analyses using conventional and IV methods to adjust for confounding (added when drafting the SAP when recognised the opportunity that the trial afforded to do IV analyses).
The rationale (a) above for analysis may appear odd given that the main finding of the trial did not support our original trial hypothesis about the superiority of a restrictive threshold. Nevertheless, it is interesting to contrast the findings from this conventionally adjusted analysis with our previous observational estimate and the main trial finding (remembering that Murphy et al. 2 compared any versus no transfusion rather than different transfusion thresholds). The conventionally adjusted analysis showed a dose–response relationship between the odds of the composite poor outcome and increasing red blood cell transfusion, as did the previous observational analysis for ischaemic and infectious events, although the gradient of the relationship was shallower in TITRe2. However, it is very difficult to reconcile the dose–response relationship with the main trial finding, which found no difference in the frequency of the composite primary outcome between groups which received substantially different average volumes of red blood cells, forcing us to conclude that this conventionally adjusted result is subject to residual confounding.
We successfully estimated the effect of anaemia in conventionally adjusted models in analysis (b) and the effect estimates were consistent with our expectation – that is, as the nadir haemoglobin increased, the odds of a poor outcome decreased. However, we were unable to investigate how nadir haemoglobin modified the effect of red blood cell transfusion because of the intrinsic link between red blood cell transfusion and haemoglobin level, and the unpredictability of the temporal relationship between transfusion and the lowest haemoglobin level experienced by a patient.
We were unable to investigate the effect of duration of storage of red blood cells [analysis (c)] because (1) the duration of storage was often missing and (2) we did not have access to information about the blood groups of red blood cell donors and recipients, a likely important confounder. Missing duration of storage of red blood cell units transfused was especially critical because the occurrence of such missing data for a participant was associated with the number of red blood cell units transfused. Any simple attempt to deal with these missing data, for example excluding participants who received any red blood cell unit with missing duration of storage, would almost certainly introduce bias. The implications of imputing duration of storage based on participants’ characteristics are uncertain and subject to ongoing analyses.
The IV analysis of the effect of red blood cell transfusion (d) also generated a striking three-way contrast with the results of the conventionally adjusted analyses (a) and the main trial finding. The estimate from the IV analysis was consistent with the main trial finding but different from the conventionally adjusted estimate, implying again that the conventionally adjusted estimate was subject to residual confounding. The conventionally adjusted estimates of the effect of lowest post-operative haemoglobin experienced were complex to interpret, suggesting that the effect varied according to the operation that a participant was undergoing and that the anticipated increased risk from anaemia was mainly apparent for non-CABG operations. The IV analysis could not consider this interaction; it did not find a statistically significant increase in the odds of a poor outcome from anaemia but the point estimates increased with increasing anaemia.
Instrumental variable analyses of trials in which the random allocation is not congruent with exposure to a particular intervention (typically owing to non-adherence) are often reported as estimating ‘the effect of treatment among the treated’. To this extent, the IV analysis provides the most direct estimate possible of the effect of red blood cell transfusion. Although the CIs are wide (and are unadjusted for nadir haemoglobin), the estimates of the IV analyses strongly suggest that transfusion after cardiac surgery setting is safe.
Balance of benefits against harms
Safety has been discussed above (see Summary of findings of the trial) and there was a small non-significant difference, favouring the liberal threshold, in the frequency of all SAEs not included in the primary outcome. The primary outcome was also a composite of SAEs; therefore, consideration of the balance between benefits and harms requires the primary outcome to be combined with deaths and all other SAEs. Combining all of these events increased the risk difference between groups (54.4% and 50.7% of participants in the restrictive and liberal groups, respectively); thus, although none of these differences was statistically significant, the liberal threshold appears to offer the better balance of benefits and harms.
Economic evaluation
The main findings from the economic evaluation are that there is very little difference between the groups in either costs or effects and great uncertainty around the cost-effectiveness results. There was very little difference in total costs per participant between the two groups. Participants in the restrictive group cost were, on average, £182 less than those in the liberal group. When a breakdown of total costs was considered, there was a clear difference in the costs associated with red blood cells between the two groups, as expected, but otherwise, cost components were very similar. (The differences in cost between groups were about the same when considering only the red blood cell costs; however, the difference in costs attributable to red blood cells was estimated more precisely than the overall difference in costs.) Total costs were lower when the time origin was moved from surgery to the point of randomisation, but the mean cost difference between groups did not change substantively. Varying unit costs in a sensitivity analysis made very little difference to the mean cost difference, reinforcing how similar resource use was between the groups. There were several outliers in the liberal group, which exerted a substantial influence on the average costs of participants in that treatment group and reversing the direction of the results described above when they were excluded.
A difference of approximately £200 between the groups is a modest cost difference (approximately 1% of total costs). However, as 34,174 cardiac surgery procedures were undertaken in the UK in 2012/13,65 a difference of £200 in each procedure would have resulted in savings or additional costs of £6.8M. The effect of this cost difference, and whether it is a cost saving or additional cost, is clearly important for the NHS.
The difference between the groups for QALYs is particularly small, creating a very small denominator for the ICER. Dividing the difference in costs by a tiny number close to zero resulted in a very large ICER (–£428,064). The point estimate in our base-case analysis suggests that the restrictive threshold is dominant over the liberal threshold as it is both more effective (very slightly greater QALY gain) and less costly and, therefore, it is cost-effective. However, there is a great deal of uncertainty around this result. This point estimate is close to the origin and the bootstrap replicates of the cost and QALY differences covered all four quadrants of the cost-effectiveness plane. Given the higher mortality rate in the restrictive group, there was a clearer difference between the groups favouring the liberal threshold when life-years were considered as an alternative outcome measure. However, the liberal group was no longer favoured when costs were considered alongside life-years and the cost-effectiveness point estimate suggested that the restrictive threshold was still cost-effective compared with the liberal threshold when life-years were used as the outcome measure in a sensitivity analysis.
There was a single subgroup interaction for QALYs gained, which suggested that patients with chronic obstructive pulmonary disease or asthma may be a particularly vulnerable group with respect to transfusion at a restrictive threshold. This finding was consistent with the subgroup analyses of the primary outcome, in so much as the largest difference between thresholds arose for this subgroup (see Figure 13). It is also intuitive from a clinical perspective, in that patients with chronic respiratory diseases commonly develop a reactive polycythaemia to chronic hypoxia and that these patients would experience a smaller QALY gain when exposed to a more extreme degree of anaemia.
Comparison with results from similar studies
Here, we consider the findings of TITRe2 in the context of the results of other trials both in cardiac surgery populations and other populations. (We do not consider observational studies given that their findings are very likely to be affected by confounding; see Chapter 7.) A Cochrane systematic review including all RCTs comparing restrictive and liberal transfusion thresholds in surgical patients and the critically ill was published in 2012. 12 The authors concluded:
In patients who do not have acute coronary artery disease, blood transfusion can probably be withheld in the presence of haemoglobin levels as low as 7.0 g/dl to 8.0 g/dl as long as there is no notable bleeding. The benefits of minimising allogeneic red cell transfusion are likely to be greatest where there is doubt about the safety of the blood supply.
Reproduced with permission from Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2012;4:CD002042,12 John Wiley & Sons. Copyright © 2010 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
but expressed caution about applying this conclusion to patients with coronary disease:
For the present we recommend the use of a restrictive transfusion trigger, but suggest using caution in patients from high-risk groups such as acute coronary syndrome as there is currently no evidence from randomised controlled trials to guide treatment.
Our result is consistent with the findings of the review and also resonates with the caution expressed by the authors. Four additional trials have been published since the last literature search for the Cochrane review. 13,14,38,66 Given this caution, three of these trials are relevant to this discussion. 13,14,38 (Patients with acute coronary syndrome were excluded from the Transfusion Strategies for Acute Upper Gastrointestinal Bleeding trial,66 the only contemporary trial to have demonstrated a benefit for restrictive transfusion.) The first of these RCTs was a single-centre pilot trial in high-risk cardiac surgery patients to assess adherence to the proposed transfusion thresholds (discussed in the context of adherence above). 38 The trial was very small, recruiting just 50 participants, but reported more AEs in the restrictive group. In the second of these RCTs, which recruited patients with hip fracture,13 63% had cardiovascular disease and the trial found no benefit of restrictive transfusion. The third of these RCTs was a feasibility trial of transfusion thresholds in patients with unstable coronary disease (MI). It also only recruited a small sample (n = 110) but reported a reduced risk of major cardiac morbidity or death of borderline statistical significance with more liberal transfusion. 14
As part of our effort to put our trial results in context, we formally combined the results of five RCTs comparing restrictive versus liberal transfusion thresholds in cardiac surgery patients (see Chapter 5, Meta-analysis). 24–26,37,38 Three of these RCTs were included in the Carson review,24,25,37 the other two were the pilot trial for TITRe226 and another pilot trial. 38 Mortality was the only outcome for which we could synthesise data across all trials, with the pooled estimate indicating an increase in mortality of borderline statistical significance for a restrictive threshold. It should be noted that TITRe2 randomised approximately 50% more participants than the total number randomised in all previous trials and contributed more than 50% of the weight of information in the meta-analysis. We have already described other limitations of this meta-analysis arising from differences in the design of the included trials (see Chapter 5, Meta-analysis), including the possibility that previous trials underestimated their treatment effects by randomising participants who did not breach the liberal threshold. A further limitation is that mortality does not capture the full consequences of different transfusion thresholds.
In summary, although the result of our primary analysis implies non-inferiority of a restrictive threshold compared with a liberal one, we consider that the totality of evidence available at present (including the results of secondary analyses in TITRe2 and the evidence from other trials discussed above) supports the caution expressed in the Cochrane review. Therefore, we are very uncertain about recommending restrictive transfusion after cardiac surgery. The evidence does not lead us to recommend using a liberal threshold after cardiac surgery; however, we believe that it should lead to a new hypothesis that more liberal transfusion may be beneficial.
This new hypothesis is clinically plausible. TITRe2 differed from previous large trials of transfusion thresholds in that all participants had symptomatic cardiovascular disease;67,68 moreover, a significant proportion will have developed oxygen supply dependency in the immediate post-operative period. 69,70 Cardiac surgery patients are, therefore, often at the limits of their cardiovascular reserve and may benefit from higher haemoglobin levels and enhanced oxygen delivery. Patients with symptomatic disease may represent a specific high-risk group when more liberal transfusion thresholds are to be recommended.
Patient and public involvement
The main impacts of PPI in the trial were with respect to:
-
Developing information for participants.
-
Making significant changes in the way in which trial follow-up as conducted and hence promoting the completeness of outcome data; specifically, PPI led to endorsement of the acceptability of postal follow-up and optimisation of the wording of items included in the questionnaire and their format.
-
Discussion of the emerging findings from the trial based on the incomplete information available at the time.
-
Disseminating information about the findings of the trial to participants (ongoing).
We found it difficult to involve patients in operational details of the trial. As previously described (see Chapter 2, Patient and public involvement), the trial needed to recruit anxious patients in an acute care setting. When patients were approached about the trial, they were, unsurprisingly, primarily concerned about the possible benefits and risks of withholding or giving extra transfusions.
We are aware that dissemination of the findings of the trial is also challenging. There is a risk that, despite the weight of the information contributed to the research question, the trial will be seen as inconclusive. The statistical issues are complex and it is challenging to find a lay form of words that reflects the findings while at the same time avoids tipping the reader to favour one or other transfusion threshold.
The challenges of carrying out meaningful PPI in the acute setting of cardiac surgery are ongoing because the trials unit that co-ordinated TITRe2 also manages a portfolio of early phase trials and other studies for the NIHR Bristol Cardiovascular Biomedical Research Unit. In particular, through the Biomedical Research Unit we are investing in PPI to improve the ways in which we approach potential participants to make the experience for research participants better, for example by minimising anxiety, to promote a fuller understanding about our trials and with the aspiration that this will enhance recruitment to these difficult-to-do studies.
Strengths and limitations
The TITRe2 trial has many strengths, most obviously its size compared with previous trials of transfusion thresholds in cardiac surgery patients; it randomised four times more participants than the next largest trial comparing liberal and restrictive transfusion thresholds after cardiac surgery. 24–26,37,38 The sample size was designed to take into account non-adherence, which was observed at a similar level to that expected. The higher-than-expected frequency of the primary outcome meant that the primary finding had more power than it was designed to have. By only randomising participants who breached the liberal threshold, we avoided diluting the treatment effect with similar numbers of participants in each group who would probably not have been transfused. This design contrasts with previous RCTs, which included such patients in their analysis populations. 24–26,37,38
The TITRe2 was also highly pragmatic. Approximately half of all of the specialist cardiac surgery centres in the UK took part and the trial was conducted in a usual-care setting. We are confident that the findings of the trial can be applied to all cardiac surgery centres in the UK. Importantly, and to the great credit of participating units, the trial succeeded (with the help of very many staff in the NHS) in monitoring haemoglobin levels and treating participants according to their allocated thresholds. The separation in both the volumes of red blood cell transfusion and average haemoglobin levels between groups demonstrates the success of the trial in implementing the transfusion thresholds. Local research teams and the co-ordinating centre together achieved excellent completeness of follow-up, with just one of the planned outcomes, infectious events, having more than 5% of missing data. Assessors who were blind to the allocation verified or adjudicated all reported events that qualified for the primary outcome, although we suspect that AKI may have been under-ascertained (see below).
With hindsight, designing TITRe2 to test the superiority of a restrictive threshold may have been a mistake. Answering the question of whether or not a restrictive threshold is non-inferior to a liberal threshold might be considered more pressing. This limitation was assuaged to some extent by the additional power (better precision) of the primary analysis but we have not formally been able to address this question because we did not pre-specify and justify a non-inferiority margin at the outset. The totality of the findings from the trial, and other evidence, make a simple conclusion very difficult. Through no fault of the trial, it is likely that different readers will view the findings as supportive of either restrictive or liberal transfusion practice. The possible 40–60% increase in mortality with a restrictive threshold remains a major concern but a very much larger trial would be needed to provide a definitive answer about this effect.
At the outset, the trial was also presented as a comparison of a new intervention, that is a restrictive threshold, against a usual-care comparator of a liberal threshold. In fact, practice was shifting towards a more restrictive threshold during the course of applying for funding and during the trial, and usual care varied across participating centres (as previously and more recently documented). 5,71 Some readers may want to reverse this perspective to consider liberal compared with restrictive.
Although the trial had greater power than planned, the downside was dominance of the composite primary outcome by less serious events, sepsis and AKI, which was the consequence of implementing objectively verifiable criteria for these events. This limitation was addressed to some extent by a sensitivity analysis of more serious events, which occurred with a frequency of 14.8%, similar to that assumed when estimating the required sample size. Unexpectedly, in this sensitivity analysis the treatment effect for the primary outcome shifted to the null. This result is difficult to reconcile with a difference in mortality and we have no explanation for these divergent findings. Verification of data led us to suspect that AKI events had been underascertained by prospective data collection, leading to the sensitivity analysis including extra AKI events identified from routinely collected serial creatinine data. We believe that this limitation arose because of differences between centres in the baseline creatinine value they used to define AKI. 36 It was interesting that including the extra events generated the expected pyramidal distribution by AKI severity (most mild, least severe), which was not apparent in the distribution of AKI events in the primary analysis.
The main limitation in the conduct of the trial was our inability to blind health-care staff. However, the use of objective end points or adjudication by blinded personnel protected against detection bias. Non-adherence was a challenge throughout the trial but did not prevent us achieving substantial separation in red blood cell transfusion between the groups. Non-adherence that was classified as mild or moderate, despite occurring with greater frequency, was considered unlikely to alter transfusion frequency (as opposed to the average number of units transfused). The nature of protocol non-adherence differed by group but only affected the overall transfusion rate in a small percentage of participants and was similar by group. The question of how much impact the effect of non-adherence (severe or non-severe) has had on the outcome of the trial is difficult to quantify, although we note the consistency between the IV analysis and the main trial finding. A sensitivity analysis excluding non-adherent participants would have been biased because non-adherence arose for different reasons in the two groups, that is participants with non-adherent instances had different characteristics. The consequences of non-adherence are to dilute the treatment effect and, therefore, to provide a more conservative estimate.
Lessons for the future
Choosing specific restrictive and liberal thresholds in RCTs such as TITRe2 is particularly challenging. From the point of view of the feasibility of the trial, the thresholds need to be sufficiently different in order to investigate a clinically important target difference in outcome (whether specified in terms of a superiority or non-inferiority hypothesis) with a sample size that can be achieved in a reasonable duration of recruitment. However, the greater the separation of the thresholds, the more challenging it is to maintain adherence. Moreover, a comparison between any two thresholds (likely to be set in a way that encompasses most of the range of thresholds implemented in usual care) cannot answer the question ‘What threshold is best?’. A modified version of the usual design would be to allocate participants to multiple groups with different thresholds, powered to detect a non-zero gradient in effect across thresholds. Although this design might be logistically more challenging to conduct, it might paradoxically promote recruitment and adherence as fewer participants would be exposed to the highest and lowest thresholds.
When applying for funding, we underestimated the number of data that would be necessary to collect. We make no apology for collecting these data as they supported our assessments of fidelity of implementing the intervention (haemoglobin levels and red blood cell transfusions) as well as non-adherence, which were aspects of the conduct of the trial that had been substantially neglected in the pilot. We undertook a careful appraisal of data collection early in the course of the trial and removed a few items that were considered to be unnecessary. The success of this process, both the initial specification of the data items and removal of some at a later stage, is demonstrated by our use of all of the data collected in analysing the trial findings and writing this report. The important lesson from the trial is that it was possible to collect the data required to monitor non-adherence.
We underestimated the number of data needed because the extent of data collection, particularly with respect to monitoring non-adherence, only became apparent when we were setting up the trial. Collecting information about all blood products transfused, nursing observations of temperature, heart rate and oxygen saturation (used to define sepsis), the lowest haemoglobin recorded each day (used to monitor non-adherence) and creatinine biochemistry each day (used to validate instances of AKI) was all time-consuming. However, the additional time needed was not simply to do with extracting more data, for example, research nurses often had to make repeated visits to participants to check information or liaise with doctors or nurses, given that randomisation and the intervention were not fixed in time.
When seeking extra time to recruit participants for the trial, we also sought extra funding for centres to cover the higher than expected research costs they were incurring. Once we had succeeded in persuading the funder of the need for this funding, implementation of the uplift of £80 per randomised participant (about 4 hours of research nurse time) was relatively straightforward through a variation to the site contract. (The additional funding was paid for participants already randomised as well as participants randomised after the variation to the contract was implemented.)
Had we adopted a payment model in which each participating centre was given a set amount of funding, we would have faced a much more serious challenge in distributing the local research costs. Centres may have resisted an attempt to reduce the amount owing to lower than expected recruitment, on the grounds that we had underestimated the volume of work involved. Moreover, we originally intended to recruit four to eight centres but had recruited 17 by the end of the trial; we think that increasing the number of centres in this way (each participating for different lengths of time) would have been problematic. The total funding award in the extension for the uplift in research costs (£120,000) would also have represented substantially less per centre than we estimated a centre would (on average) generate per year (about £13,000 for the remainder of the trial for an extra centre compared with approximately £15,000 per year for the first group of centres).
We found it difficult to estimate the local research costs that a participating centre was likely to incur. Researchers have to do this task when writing the full application for a project and preparing a budget and may be tempted to reduce this cost, which is inevitably uncertain, rather than the cost of resources that they will use centrally to manage the trial (which they may believe they can estimate more confidently). The importance of the local research income to a centre for a study is likely to become more important in the future as NHS organisations increasingly compete for NIHR portfolio income in a region. If it is clear that ‘boots on the ground’ at participating centres are a rate-limiting step in delivering a trial, increasing the local research costs may be a relatively easy way to enhance recruitment. However, when multiple trials are competing for the same group of patients, there is also a risk that NHS organisations may cherry-pick the trials that generate the most income.
In TITRe2, although we believe that the primary factor determining the recruitment rate and quality of data for a centre was the commitment and research awareness of the local research team (evidenced by the impact of the absence of specific research nurses), the amount of local researcher time available to be spent on the project was also very important. Having a team of research nurses was helpful in maintaining recruitment and data collection over usual periods of annual leave. When this was not the case, such periods doubled the period over which recruitment slowed or stopped, as there was no point in recruiting participants in advance if no one was going to be available to collect the data. Similarly, there was no one available to recruit participants during the period of absence for the usual research nurse, so no recruited participants, for whom data collection was required, having surgery when he/she returned from leave (e.g. 1 week’s annual leave typically affected recruitment for 3 weeks).
There is still debate about the best way to remunerate participating centres for local research costs and whether one or other method enhances recruitment. We believe that a ‘fee’ per participant randomised, as adopted in TITRe2, provides an incentive to recruit and most fairly rewards differential recruitment by centres. In most circumstances, we consider it preferable to providing, for example, funding to each centre for a fixed amount of time of a research nurse. As described in Chapter 2, Contractual and financial arrangements, we successfully implemented a system of activity reports using data submitted from centres, which were used by centres as the basis for invoices to the University of Bristol (the ‘contractor’). However, with payment being made in arrears, this system can cause difficulties and delays in setting up centres that may be sceptical about recruitment rates and unable to deploy staff to work on a trial without advance funding. In current trials, we are using a mixed-economy method, awarding centres a fixed amount to put in place staff to start recruiting participants, then applying the fee-per-participant system. The mixed system will inevitably be somewhat less efficient if some centres are not successful in recruiting participants but this inefficiency may be worthwhile to reduce the average delay in centres starting to recruit. Another way to implement this principle would be initially to offer each centre a fixed amount of research nurse time and subsequently to adjust the funding depending on actual recruitment.
We believe that TITRe2 benefited substantially from efforts made by the cardiovascular specialty group of the UK Comprehensive Research Network. We are unable to describe what measures the Network instituted as they were applied discretely (which may explain their success) and were not disclosed to the trial team.
Other trials competing for the same target population was a final factor affecting recruitment and it was frustrating that the NIHR (across its varied programmes) funded new trials that competed for cardiac surgery patients during the course of TITRe2. Not surprisingly, centres employing chief investigators for these trials tended to prioritise recruitment to their home-grown trials over TITRe2, with a dramatic effect on recruitment at one or two sites. At any one time, specialty networks in the Comprehensive Research Network have an overview of NIHR-funded trials currently recruiting patients with particular conditions but this is after the trials have been funded. We are not aware that these networks feed information back to the NIHR about target populations that are currently ‘over-researched’ and when a new trial may struggle to recruit.
Future research
The most pressing question to answer is whether or not a liberal threshold is superior to a restrictive threshold in cardiac surgery patients who are likely to be at the limits of their cardiovascular reserve. A RCT has recently started to recruit in the Canada and the USA which will help to answer this question. 72 As with TITRe2, the TRICS-III trial is again comparing restrictive and liberal thresholds in patients having cardiac surgery with CPB and the researchers aim to randomise 3592 participants. Key differences between the trials are as follows:
-
TRICS-III hypothesises a restrictive threshold to be non-inferior to a liberal threshold.
-
TRICS-III is recruiting high-risk patients only (EuroSCORE of > 6).
-
TRICS-III thresholds are slightly different and the thresholds are applied both during the operation and subsequently (restrictive < 7.5 g/dl intraoperatively or postoperatively; liberal < 9.5 g/dl intraoperatively or in the ICU and < 8.5 g/dl postoperatively on the ward).
-
TRICS-III has a primary composite outcome consisting only of events that we considered to be serious in our sensitivity analysis (i.e. death, MI, kidney failure requiring dialysis or stroke; expected frequency and non-inferiority margin are not stated in the registration details).
Although not explicitly testing the hypothesis that a liberal transfusion threshold is superior, TRICS-III is recruiting patients who are most likely to be at the limit of their cardiovascular reserve. The weight of information contributed by this trial to a future meta-analysis will be substantial, although it is unclear whether the researchers are randomising preoperatively or only when a participant breaches the liberal threshold. Until this trial concludes, we do not see any particular merit in pursuing an individual participant data meta-analysis of the existing trials. In our opinion, the only benefit of such an analysis would be investigation of relevant subgroups but, even if achievable, these analyses would have low power. A more fruitful approach would be to persuade previous triallists to reanalyse their trial datasets after excluding from both restrictive and liberal groups all participants who did not breach the liberal threshold. These analyses would make these trials more similar to TITRe2 and test the hypothesis that the results of these trials currently underestimate the treatment effects. The revised treatment effects should be combined in a further (aggregate) meta-analysis of mortality. An initiative of this kind might also allow meta-analyses of other outcomes with higher frequencies, which would provide estimates with greater precision.
With respect to the investment already made in TITRe2, we believe that further analyses of the data may be able to estimate the effects of transfusion at different haemoglobin levels and to estimate the effect of longer versus shorter duration of storage of red blood cells (although we are aware that a RCT is currently testing this73,74). Chapter 7, Red blood cells and haemoglobin levels, Next steps and Chapter 7, Age of blood, Next steps have already described the analyses that we propose to pursue.
There are two key areas of further health economic research that would be worthwhile. In this report, we have described a within-trial analysis up to 3 months, consistent with the main analysis of clinical outcomes. It would be useful to extrapolate the information about costs and effects obtained for the trial and to explore different time horizons including a life-time time horizon. With respect to quality of life, the EQ-5D-3L questionnaire was used in the trial because the 5-level version had not been validated at the time. The 5-level version has been designed to be able to discriminate changes in health-related quality of life better than the 3-level questionnaire. Given such small differences between the restrictive and liberal groups, it would be interesting to investigate whether or not the use of the 5-level questionnaire in current cardiac and blood transfusion studies could detect larger differences between trial groups.
Chapter 9 Conclusion
We conclude that both TITRe2 and totality of evidence available at present indicate that a restrictive transfusion threshold is definitely not superior, and probably not inferior, to a liberal threshold. In terms of the economic component of the trial, we also conclude that there is no difference between the two thresholds. However, the same evidence makes it difficult to recommend restrictive transfusion after cardiac surgery, despite the reduction in costs that this might achieve by lowering the consumption of allogeneic red blood cells. A new hypothesis, that more liberal transfusion may be beneficial after cardiac surgery, needs to be investigated.
Acknowledgements
The trial was funded by the UK NIHR Health Technology Assessment programme (reference 06/402/94). Reeves is part funded by the NIHR Bristol Biomedical Research Unit in Cardiovascular Disease and this award also supported the research nurse team in Bristol. Angelini and Murphy are British Heart Foundation (BHF) Professors of Cardiac Surgery and Rogers is a BHF Reader in Medical Statistics.
Contributions of authors
Barnaby C Reeves (chief investigator, Professor of Health Services Research and Co-Director of the Clinical Evaluation and Trials Unit) and Gavin J Murphy (BHF Professor of Cardiac Surgery) conceived the trial.
Katie Pike (research associate in medical statistics) and Rachel L Nash (NIHR Predoctoral Research Methods Fellow) prepared reports during the trial and Katie Pike carried out the statistical analyses, under the supervision of Chris A Rogers (BHF Reader in Medical Statistics and Co-Director of the Clinical Evaluation and Trials Unit).
Rachel CM Brierley (trial co-ordinator), Alice Miles (trial coordinator), Barnaby C Reeves, Chris A Rogers and Gavin J Murphy managed the conduct of the trial with expert clinical input as required from Gianni D Angelini (BHF Professor of Cardiac Surgery), Andrew D Mumford (reader in haematology) and Alan Cohen (Consultant Anaesthetist).
Elizabeth A Stokes (researcher in health economics) and Sarah Wordsworth (Associate Professor in Health Economics) carried out the health economic analyses.
Barnaby C Reeves was the chief investigator and Gavin J Murphy the lead clinical investigator.
Barnaby C Reeves, Gavin J Murphy, Chris A Rogers and Sarah Wordsworth designed the trial.
Barnaby C Reeves, Gavin J Murphy, Chris A Rogers, Gianni D Angelini and Sarah Wordsworth wrote the application for funding to do the trial.
Katie Pike, Barnaby C Reeves, Sarah Wordsworth, Elizabeth A Stokes and Gavin J Murphy drafted the report.
All authors reviewed the report for important intellectual content and approved the final version.
Publications
Brierley RC, Pike K, Miles A, Wordsworth S, Stokes EA, Mumford AD, et al. A multi-centre randomised controlled trial of transfusion indication threshold reduction on transfusion rates, morbidity and healthcare resource use following cardiac surgery: study protocol. Transfus Apher Sci 2014;50:451–61.
Pike K, Nash RL, Murphy GJ, Reeves BC, Rogers CA. Transfusion Indication Threshold Reduction (TITRe2) randomised controlled trial in cardiac surgery: statistical analysis plan. Trials 2015;16:54.
Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery: TITRe2 randomized trial. New Engl J Med 2015;372:997–1008.
Reeves BC, Rogers CA, Murphy GJ. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015;373:193.
Pike K, Maishman RL, Brierley RCM, Rogers CA, Murphy GJ, Reeves BC. Adherence to transfusion strategies in a randomized controlled trial: experiences from the TITRe2 trial. Br J Haematol 2016; in press. http://dx.doi.org/10.1111/bjh.14220
Stokes EA, Wordsworth S, Bargo D, Pike K, Rogers CA, Brierley RCM, et al. Are lower levels of red blood cell transfusion more cost-effective than liberal levels after cardiac surgery? Findings from the TITRe2 randomised controlled trial. BMJ Open 2016:6:e011311.
Reeves BC, Pike K, Murphy GJ, Sterne JAC, Rogers CA. Effects of red cell transfusion after cardiac surgery: estimates from multivariable and instrumental variable analyses of data from the TITRe2 trial. Submitted to Lancet Haematol.
Data sharing statement
Data will not be made available for sharing until after publication of the main results of the study. Thereafter, anonymised individual patient data will be made available for secondary research, conditional on assurance from the secondary researcher that the proposed use of the data is compliant with the Medical Research Council Policy on Data Preservation and Sharing regarding scientific quality, ethical requirements and value for money. We propose that a minimum requirement with respect to scientific quality should be a publicly available pre-specified protocol describing the purpose, methods and analysis of the secondary research, e.g. a protocol for a Cochrane systematic review. A second file containing patient identifiers would be made available for record linkage or a similar purpose, subject to confirmation that the secondary research protocol has been approved by a UK Research Ethics Committee or other similar, approved ethics review body.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Habib RH, Zacharias A, Schwann TA, Riordan CJ, Engoren M, Durham SJ, et al. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcome. Crit Care Med 2005;33:1749-56. http://dx.doi.org/10.1097/01.CCM.0000171531.06133.B0.
- Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544-52. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.698977.
- Karkouti K, Wijeysundera DN, Beattie WS. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation 2008;117:478-84. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.718353.
- Bennett-Guerrero E, Zhao Y, O’Brien SM, Ferguson TB, Peterson ED, Gammie JS, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA 2010;304:1568-75. http://dx.doi.org/10.1001/jama.2010.1406.
- Murphy MF, Murphy GJ, Gill R, Herbertson M, Allard S, Grant-Casey J. National Comparative Audit of Blood Transfusion: 2011 Audit of Blood Transfusion in Adult Cardiac Surgery. Birmingham: NHS; 2013.
- Wells AW, Llewelyn CA, Casbard A, Johnson AJ, Amin M, Ballard S, et al. The EASTR Study: indications for transfusion and estimates of transfusion recipient numbers in hospitals supplied by the National Blood Service. Transfus Med 2009;19:315-28. http://dx.doi.org/10.1111/j.1365-3148.2009.00933.x.
- Brierley RCM, Pike K, Miles A, Wordsworth S, Stokes EA, Mumford AD, et al. A multi-centre randomised controlled trial of Transfusion Indication Threshold Reduction on transfusion rates, morbidity and healthcare resource use following cardiac surgery: study protocol. Transfus Apher Sci 2014;50:451-61. http://dx.doi.org/10.1016/j.transci.2014.02.020.
- Corwin HL, Parsonnet KC, Gettinger A. RBC transfusion in the ICU. Is there a reason?. Chest 1995;108:767-71. http://dx.doi.org/10.1378/chest.108.3.767.
- Stover EP, Siegel LC, Parks R, Levin J, Body SC, Maddi R, et al. Variability in transfusion practice for coronary artery bypass surgery persists despite national consensus guidelines: a 24-institution study. Institutions of the Multicenter Study of Perioperative Ischemia Research Group. Anesthesiology 1998;88:327-33. http://dx.doi.org/10.1097/00000542-199802000-00009.
- Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med 2006;34:1608-16. http://dx.doi.org/10.1097/01.CCM.0000217920.48559.D8.
- Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, Chan CT, Wong PY, et al. Influence of erythrocyte transfusion on the risk of acute kidney injury after cardiac surgery differs in anemic and nonanemic patients. Anesthesiology 2011;115:523-30. http://dx.doi.org/10.1097/ALN.0b013e318229a7e8.
- Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2012;4. http://dx.doi.org/10.1002/14651858.cd002042.pub3.
- Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011;365:2453-62. http://dx.doi.org/10.1056/NEJMoa1012452.
- Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013;165:964-71. http://dx.doi.org/10.1016/j.ahj.2013.03.001.
- Carson JL, Carless PA, Hebert PC. Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA 2013;309:83-4. http://dx.doi.org/10.1001/jama.2012.50429.
- Bolton-Maggs PHB, Poles D, Watt A, Thomas D, Cohen H. Serious Hazards of Transfusion (SHOT) Steering Group . The 2012 Annual SHOT Report 2013. www.shotuk.org/wp-content/uploads/2013/08/SHOT-Annual-Report-2012.pdf (accessed 30 July 2015).
- Greinacher A, Fendrich K, Brzenska R, Kiefel V, Hoffmann W. Implications of demographics on future blood supply: a population-based cross-sectional study. Transfusion 2011;51:702-9. http://dx.doi.org/10.1111/j.1537-2995.2010.02882.x.
- Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010;50:753-65. http://dx.doi.org/10.1111/j.1537-2995.2009.02518.x.
- Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthetists blood conservation clinical practice guidelines. Ann Thorac Surg 2011;91:944-82. http://dx.doi.org/10.1016/j.athoracsur.2010.11.078.
- Napolitano LM, Kurek S, Luchette FA, Anderson GL, Bard MR, Bromberg W, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med 2009;37:3124-57. http://dx.doi.org/10.1097/CCM.0b013e3181b39f1b.
- Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med 2012;157:49-58. http://dx.doi.org/10.7326/0003-4819-157-1-201206190-00429.
- Global Forum for Blood Safety: Patient Blood Management. Priorities for Action. Dubai: WHO; 2011.
- Farmer SL, Towler SC, Leahy MF, Hofmann A. Drivers for change: Western Australia Patient Blood Management Program (WA PBMP), World Health Assembly (WHA) and Advisory Committee on Blood Safety and Availability (ACBSA). Best Pract Res Clin Anaesthesiol 2013;27:43-58. http://dx.doi.org/10.1016/j.bpa.2012.12.007.
- Johnson RG, Thurer RL, Kruskall MS, Sirois C, Gervino EV, Critchlow J, et al. Comparison of two transfusion strategies after elective operations for myocardial revascularization. J Thorac Cardiovasc Surg 1992;104:307-14.
- Bracey AW, Radovancevic R, Riggs SA, Houston S, Cozart H, Vaughn WK, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion 1999;39:1070-7. http://dx.doi.org/10.1046/j.1537-2995.1999.39101070.x.
- Murphy GJ, Rizvi SIA, Battaglia F, Culliford L, Rogers CA, Cohen A, et al. A pilot randomized controlled trial of the effect of transfusion- threshold reduction on transfusion rates and morbidity after cardiac surgery. Transfus Altern Transfus Med 2007;9:41-2.
- Wilson AP, Treasure T, Sturridge MF, Gruneberg RN. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet 1986;1:311-13. http://dx.doi.org/10.1016/S0140-6736(86)90838-X.
- Wilson AP, Weavill C, Burridge J, Kelsey MC. The use of the wound scoring method ‘ASEPSIS’ in postoperative wound surveillance. J Hosp Infect 1990;16:297-309. http://dx.doi.org/10.1016/0195-6701(90)90002-6.
- Gibbons C, Bruce J, Carpenter J, Wilson AP, Wilson J, Pearson A, et al. Identification of risk factors by systematic review and development of risk-adjusted models for surgical site infection. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15300.
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11. http://dx.doi.org/10.1186/cc5713.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New Jersey, NJ: Lawrence Erlbaum Associates; 1988.
- Pike K, Brierley R, Rogers CA, Murphy GJ, Reeves BC. Adherence in a randomised controlled trial comparing liberal and restrictive red blood cell (RBC) transfusion protocols after cardiac surgery (TITRe2). Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-S1-A131.
- Pike K, Nash RL, Murphy GJ, Reeves BC, Rogers CA. Transfusion Indication Threshold Reduction (TITRe2) randomised controlled trial in cardiac surgery: statistical analysis plan. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-0564-x.
- Tooze JA, Grunwald GK, Jones RH. Analysis of repeated measures data with clumping at zero. Stat Methods Med Res 2002;11:341-55. http://dx.doi.org/10.1191/0962280202sm291ra.
- Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, et al. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care 2011;15. http://dx.doi.org/10.1186/cc9960.
- Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559-67. http://dx.doi.org/10.1001/jama.2010.1446.
- Shehata N, Burns LA, Nathan H, Hebert P, Hare GM, Fergusson D, et al. A randomized controlled pilot study of adherence to transfusion strategies in cardiac surgery. Transfusion 2012;52:91-9. http://dx.doi.org/10.1111/j.1537-2995.2011.03236.x.
- Patel NN, Avlonitis VS, Jones HE, Reeves BC, Sterne JAC, Murphy GJ. Indications for red cell transfusion in cardiac surgery: a systematic review and meta-analysis of randomized controlled trials and observational studies. Lancet Haematol 2015. http://dx.doi.org/10.1016/S2352-3026(15)00198-2.
- Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet 2005;365:1591-5. http://dx.doi.org/10.1016/S0140-6736(05)66461-6.
- Social Value Judgements: Principles for the Development of NICE Guidance. London: NICE; 2008.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2005.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- National Schedule of Reference Costs 2012–13. London: Department of Health; 2013.
- NHS Blood and Transplant . NHS Blood and Transplant Price List 2012–2013 2012. http://hospital.blood.co.uk/products/ (accessed 28 September 2012).
- Department of Health Commercial Medicines Unit . Electronic Marketing Information Tool (eMIT). Drugs and Pharmaceutical Electronic Market Information (eMit) n.d. http://cmu.dh.gov.uk/electronic-market-information-tool-emit/ (accessed 10 February 2014).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury, University of Kent: Personal Social Services Research Unit; 2013.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for Euroqol: Results from a UK General Population Survey. York: University of York; 1995.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. http://dx.doi.org/10.1002/sim.4067.
- Rubin DB. Multiple Imputation For Non-Response In Surveys. New York, NY: John Wiley & Sons; 1987.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Dakin HA, Wordsworth S, Rogers CA, Abangma G, Raftery J, Harding SP, et al. Cost-effectiveness of ranibizumab and bevacizumab for age-related macular degeneration: 2-year findings from the IVAN randomised trial. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005094.
- Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015;372:997-1008. http://dx.doi.org/10.1056/NEJMoa1403612.
- Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C. Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10440.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2009.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing . . . presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. http://dx.doi.org/10.1002/hec.766.
- Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol 2000;29. http://dx.doi.org/10.1093/oxfordjournals.ije.a019909.
- Dzik W. Fresh blood for everyone? Balancing availability and quality of stored RBCs. Transfus Med 2008;18:260-5. http://dx.doi.org/10.1111/j.1365-3148.2008.00870.x.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. http://dx.doi.org/10.1016/j.jclinepi.2007.11.008.
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Eng J Med 1999;340:409-17. http://dx.doi.org/10.1056/NEJM199902113400601.
- Reeves BC, Murphy GJ. Increased mortality, morbidity, and cost associated with red blood cell transfusion after cardiac surgery. Curr Opin Cardiol 2008;23:607-12. http://dx.doi.org/10.1097/HCO.0b013e328310fc95.
- Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008;358:1229-39. http://dx.doi.org/10.1056/NEJMoa070403.
- Blue Book Online . How Many [Cardiac Surgery] Operations Are Carried Out Each Year? 2013. http://bluebook.scts.org (accessed 30 July 2015).
- Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11-2. http://dx.doi.org/10.1056/NEJMoa1211801.
- Wijns W, Kolh P, Danchin N, Di Mario C, . European Association for Percutaneous Cardiovascular Interventions (EAPCI) . Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501-55. http://dx.doi.org/10.1093/eurheartj/ehq277.
- Vahanian A, Alfieri O, Andreotti F, . European Association for Cardio-Thoracic Surgery (EACTS) . Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. http://dx.doi.org/10.1093/eurheartj/ehs109.
- Casutt M, Seifert B, Pasch T, Schmid ER, Turina MI, Spahn DR. Factors influencing the individual effects of blood transfusions on oxygen delivery and oxygen consumption. Crit Care Med 1999;27:2194-200. http://dx.doi.org/10.1097/00003246-199910000-00021.
- Utoh J, Moriyama S, Okamoto K, Kunitomo R, Hara M, Kitamura N. The effects of cardiopulmonary bypass on postoperative oxygen metabolism. Surg Today 1999;29:28-33. http://dx.doi.org/10.1007/BF02482966.
- Wells AW, Mounter PJ, Chapman CE, Stainsby D, Wallis JP. Where does blood go? Prospective observational study of red cell transfusion in north England. BMJ 2002;325. http://dx.doi.org/10.1136/bmj.325.7368.803.
- Clinical Trials Registry . Transfusion Requirements in Cardiac Surgery III (TRICS-III) n.d. http://clinicaltrials.gov/show/NCT02042898 (accessed 30 July 2015).
- Lacroix J, Hébert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, et al. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev 2011;25:197-205. http://dx.doi.org/10.1016/j.tmrv.2011.03.001.
- Lacroix J, Hébert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015;372:1410-18. http://dx.doi.org/10.1056/NEJMoa1500704.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2004.
- Lord J, Willis S, Eatock J, Tappenden P, Trapero-Bertran M, Miners A, et al. Economic modelling of diagnostic and treatment pathways in National Institute for Health and Care Excellence clinical guidelines: the Modelling Algorithm Pathways in Guidelines (MAPGuide) project. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17580.
- Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. 3CPO study investigators . A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13330.
- NICE . NICE Medical Technology Guidance 8: The VeriQ System for Assessing Graft Flow During Coronary Artery Bypass Graft Surgery 2011. http://guidance.nice.org.uk/mtg8 (accessed 30 July 2015).
- NICE . Nutrition Support in Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition 2006. www.nice.org.uk/guidance/cg32/resources/costing-report-194884669 (accessed 10 November 2015).
- NHS Business Services Authority, NHS Prescription Services . Drug Tariff. The Electronic Drug Tariff 2014. www.ppa.org.uk/ppa/edt_intro.htm (accessed 21 February 2014).
- NHS Direct National Health Services Trust Annual Report and Accounts 2012/13. London: The Stationery Office; 2013.
Appendix 1 Transfusion Indication Threshold Reduction study investigators
Trial sites
Blackpool Victoria Hospital and Lancaster University
Investigators: Mr Augustine Tang and Dr Palaniappan Saravanan. Research team: Charlotte Waterhouse.
Royal Sussex County Hospital, Brighton
Investigator: Dr Robert Kong. Research team: Nicola Skipper.
University Hospitals NHS Foundation Trust, Bristol
Investigator: Professor Gavin Murphy (until August 2012)/Professor Gianni Angelini (from August 2012). Research team: Emma Hopkins and Penny Lambert.
University Hospital Coventry and Warwickshire NHS Trust, Coventry
Investigator: Mr Sunil K Bhudia. Research team: Denise Gocher.
Castle Hill Hospital, Hull
Investigator: Dr Sean Bennett. Research team: Neil Smith and Adam Walker.
Derriford Hospital, Plymouth
Investigators: Dr Mark Bennett and Mr Malcolm Dalrymple-Hay. Research team: Maxine Pearse.
Essex Cardiothoracic Centre, Basildon
Investigator: Professor Andrew J Ritchie. Research team: Emily Redman and Amanda Solesbury.
Royal Infirmary of Edinburgh, Edinburgh
Investigator: Mr Vipin Zamvar.
Hammersmith Hospital, London
Investigator: Dr Geoffrey Lockwood. Research team: Dr Francesca Fiorentino, Alima Rahman.
King’s College Hospital NHS Foundation Trust
Investigator: Dr Gudrun Kunst. Research team: Georgina Parsons and Fiona Wade-Smith.
The Leeds Teaching Hospitals NHS Trust
Investigator: Dr Michael H Cross. Research team: Stuart Elliot and Zoe Beardow.
Glenfield Hospital, Leicester
Investigator: Professor Tom Sypt. Research team: Martina Williams.
Liverpool Heart and Chest Hospital Foundation Trust
Investigator: Mr Brian Fabri (until December 2012)/Mr Mark Field (from January 2013). Research team: Ian Kemp and Andrea Young.
The James Cook University Hospital, Middlesbrough
Investigator: Dr Nick Stratford. Research team: Heather Robinson.
Freeman Hospital, Newcastle
Investigator: Mr Stephen Clark. Research team: Sarah Rowling and Hazel Forsyth.
University Hospital Southampton Foundation Trust
Investigator: Dr Ravi Gill. Research team: Beverley Wadhams and Kim de Courcy-Golder.
New Cross Hospital, Wolverhampton
Investigators: Dr Ian Morgan. Research team: Emma Greatbach and Alex Ng.
Resource centres
Trial management centre, Clinical Trials and Evaluation Unit, University of Bristol
Professor Barnaby C Reeves, Dr Chris A Rogers, Dr Rachel CM Brierley, Dr Alice Miles, Wendy Underwood, Dr Lucy A Culliford, Jonathan Evans, Katie Pike, Rachel Nash, David Hutton, Emma Hopkins, Penny Lambert, Kate Rajakaruna, Kim Wright, Jenny Wilcox and Rachel Wyatt.
Health Economics Research Centre, University of Oxford
Dr Sarah Wordsworth, Elizabeth A Stokes and Danielle Bargo.
Adjudication Committee
Dr Tom W Johnson, Dr Sally Tomkins and Mr Jon Anderson.
NHS Blood and Transplant
Dr Edwin Massey and Ian Millar.
Appendix 2 Transfusion Indication Threshold Reduction committees
Data Monitoring and Safety Committee
Professor Gordon Murray (chairperson), Professor Tim Walsh and Professor Domenico Pagano.
Trial Steering Committee
Mr Patrick Magee (chairperson, until his death in May 2011), Professor John Pepper (chairperson, from June 2011), Dr Duncan Young, Dr Edwin Massey, Dr Gordon Taylor and Karin Smyth.
Appendix 3 Additional health economic evaluation information
Unit costs and resource use assumed for complications
Note that unit costs not in 2012/13 prices have been inflated to 2012/13 prices using the HCHS inflation index. 48
| Resource | Unit cost (£) | Reference |
|---|---|---|
| Cardiac surgery and reoperations | ||
| CABG | 6714 | NHS Reference Costs 2012/13.45 Elective inpatients. HRG code EA14 for service codes 170 (cardiothoracic surgery) and 172 (cardiac surgery). For each code, the costs associated with the average LOS reported were subtracted at a cost of £392 per day (see Inpatient stay row, Cardiac ward day, in this table), and £227 was subtracted for blood products based on data from an audit of blood transfusion in cardiac surgery (NHSBT, 20115). An average cost for the codes was then generated, weighted by activity |
| Valve | 7336 | NHS Reference Costs 2012/13.45 Elective inpatients. HRG codes EA17 (single valve) and EA52 (more than one valve) for service codes 170 and 172. For each code, the costs associated with the average LOS reported were subtracted at a cost of £392 per day, and £659 was subtracted for blood products (NHSBT, 20115). An average cost for the codes was then generated for single and more than one valve procedures, weighted by activity. Finally these two figures were weighted to produce an average that reflects the proportion of single-valve procedures in TITRe2 participants (90% single, 10% more than 1 valve) |
| CABG and valve | 8054 | NHS Reference Costs 2012/13.45 Elective inpatients. HRG code EA51 for service codes 170 and 172. For each code, the costs associated with the average LOS reported were subtracted at a cost of £392 per day, and £1421 was subtracted for blood products (NHSBT, 20115). An average cost for the codes was then generated, weighted by activity |
| Other | 8298 | NHS Reference Costs 2012/13.45 Elective inpatients. HRG code EA20 for service codes 170 and 172. For each code, the costs associated with the average LOS reported were subtracted at a cost of £392 per day, and £1421 was subtracted for blood products (NHSBT, 20115). An average cost for the codes was then generated, weighted by activity |
| Reoperations < 3 hours, excluding blood and LOS | 6608 | As ‘other’ cardiac procedure above, but the lower quartile unit cost was used rather than the mean cost |
| Reoperations < 3 hours, including blood, excluding LOS | 8029 | As ‘reoperations < 3 hours, excluding blood and LOS’, with £1421 for blood products added back in |
| Reoperations ≥ 3 hours, excluding blood and LOS | 8298 | As ‘other’ cardiac procedure above |
| Reoperations ≥ 3 hours, including blood, excluding LOS | 9719 | As ‘other’ cardiac procedure above, with £1421 for blood products added back in |
| Inpatient stay | ||
| Cardiac ward day | 392 | NHS Reference Costs 2012/13.45 Weighted average of elective inpatient excess bed-day costs for relevant HRGs (EA14, EA16, EA17, EA19, EA20, EA22, EA51, EA52, excluding any service codes for paediatrics) |
| HDU day | 619 | NHS Reference Costs 2012/13.45 Critical Care Services – Adult: Critical Care Unit (XC07Z, 0 organs supported) |
| CICU day | 1190 | NHS Reference Costs 2012/13.45 Critical Care Services – Adult: Critical Care Unit (weighted average of XC01Z – XC06Z, 1–6 organs supported) |
| General ICU day | 1608 | NHS Reference Costs 2012/13.45 Critical Care Services – Adult: Critical Care Unit (weighted average of XC01Z – XC03Z, 4–6 organs supported) |
| Ward day for another unit in the hospital, or at another hospital | 265 | NHS Reference Costs 2012/13.45 Non-elective inpatient excess bed-day cost across all activities |
| Blood products | ||
| Red blood cells | 123.31 | NHSBT Price List 2012/1346 |
| Red blood cell administration cost, first unit | 22 | Primary data collection of the nursing time and consumables associated with requesting blood and administering transfusions undertaken with collaborators on the TOPPS trial, funded by NHSBT. Preliminary analyses show it takes 49 minutes of nursing time and £6 of consumables to request and administer the first unit of red blood cells |
| Red blood cell administration cost, subsequent units | 5 | As above (see Resource row, Red blood cell administration cost, first unit, in this table); analyses found it took 15 minutes of nursing time to administer subsequent units (no additional consumables) |
| FFP | 27.46 | NHSBT Price List 2012/1346 |
| Platelets | 209.30 | NHSBT Price List 2012/1346 |
| Cryoprecipitate | 189.19 | NHSBT Price List 2012/1346 |
| Resource | Assumed quantity | Unit cost (£) | Reference |
|---|---|---|---|
| Tranexamic acid | 5 g intravenously | 15.50 | BNF 66, 201348 |
| Trasylol | 6 million Kallikrein Inhibitor Units intravenously | 316.83 | Davies et al.56 using data from BNF 47, 2004.75 Costs have been inflated using the HCHS inflation index |
| Intraoperative/post-operative cell salvage | 176 | Davies et al.56 Costs have been inflated using the HCHS inflation index | |
| Activated factor VII | 5 mg intravenously | 2486.60 | Transfusion Laboratory of the John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford, 2013, personal communication |
| Human prothrombin complex (Beriplex, CSL Behring UK Ltd) | 1500 IU intravenously | 420 | Transfusion Laboratory at a district general hospital, South Central, 2014, personal communication |
| HES | 1500 ml intravenously | 40.60 | BNF 58, 2009.57 Costs have been inflated using the HCHS inflation index |
| Human albumin solution (Zenalb, Bio Products Laboratory Ltd) | 500 ml intravenously | 36 | Transfusion Laboratory at a district general hospital, South Central, 2014, personal communication |
| Gelofusine | 1500 ml intravenously | 7.92 | Finance Department, teaching hospital, South Central, 2013, personal communication |
| Inotropes | Noradrenaline 1 mg/hour for 5 days | 57.30 | eMIT, 201447 |
| Hartmann’s solution (compound sodium lactate) | 1500 ml intravenously | 2.67 | Finance Departmen, teaching hospital, South Central, 2013, personal communication |
| Gelatin (Isoplex, Beacon Pharmaceuticals) | 1500 ml intravenously | 22.07 | BNF 66, 201348 |
| Drug name | Route | Assumed dose/frequency per day | Daily cost (£) | Source | Daily cost (£) from BNF47 for sensitivity analyses |
|---|---|---|---|---|---|
| Amikacin | i.v. | 500 mg 3 × day | 18.42 | eMIT47 | |
| Amoxicillin | Oral | 500 mg 4 × day | 0.10 | eMIT47 | 0.31 |
| Amoxicillin | i.v. | 500 mg 4 × day | 1.46 | eMIT47 | 2.20 |
| Aztreonam | i.v. | 1 g 3 × day | 28.20 | BNF48 | |
| Benzylpenicillin | i.v. | 1.2 g 4 × day | 7.56 | BNF48 | |
| Caspofungin | i.v. | 70 mg first day, 50 mg 1 × day subsequent days | 416.78 day 1, 327.67 thereafter | BNF48 | |
| Cefalexin | Oral | 250 mg 4 × day | 0.11 | eMIT47 | |
| Cefotaxime | i.v. | 1 g 2 × day | 1.22 | eMIT47 | |
| Ceftazidime | i.v. | 1 g 3 × day | 2.56 | eMIT47 | |
| Ceftriaxone | i.v. | 1 g 1 × day | 0.53 | eMIT47 | |
| Cefuroxime | i.v. | 750 mg 3 × day | 1.37 | eMIT47 | |
| Cefuroxime 1.5 g | i.v. | 1.5 g 3 × day | 2.13 | eMIT47 | |
| Cefuroxime 750 mg | i.v. | 750 mg 3 × day | 1.37 | eMIT47 | |
| Chloramphenicol | i.v. | 1 g 4 × day | 5.56 | BNF48 | |
| Ciprofloxacin | Oral | 500 mg 2 × day | 0.05 | eMIT47 | 0.15 |
| Ciprofloxacin | i.v. | 400 mg 2 × day | 2.02 | eMIT47 | 39.58 |
| Clarithromycin | Oral | 250 mg 2 × day | 0.13 | eMIT47 | |
| Clarithromycin | i.v. | 500 mg 2 × day | 5.24 | eMIT47 | |
| Clindamycin | Oral | 150 mg 4 × day | 0.24 | eMIT47 | |
| Clindamycin | i.v. | 600 mg 3 × day | 7.27 | eMIT47 | |
| Co-amoxiclav | Oral | 375 mg 3 × day | 0.21 | eMIT47 | 0.32 |
| Co-amoxiclav 625 mg | Oral | 625 mg 3 × day | 0.22 | eMIT47 | |
| Co-amoxiclav | i.v. | 600 mg 3 × day | 1.64 | eMIT47 | 3.63 |
| Co-amoxiclav 1.2 g | i.v. | 1.2 g 3 × day | 1.91 | eMIT47 | |
| Colomycin nebs (Colistimethate sodium) | Oral | 1 million units 2 × day | 3.36 | BNF48 | |
| Colistimethate sodium | i.v. | 1 million units 2 × day | 3.36 | BNF48 | |
| Co-trimoxazole | Oral | 960 mg 2 × day | 0.49 | eMIT47 | |
| Co-trimoxazole | i.v. | 960 mg 2 × day | 7.12 | BNF48 | |
| Daptomycin | i.v. | 350 mg 1 × day | 62.00 | BNF48 | |
| Demeclocycline | Oral | 150 mg 4 × day | 11.64 | BNF48 | |
| Doxycycline | Oral | 200 mg first day, 100 mg 1 × day subsequent days | 0.07 day 1, 0.03 thereafter | eMIT47 | 0.28 day 1, 0.14 thereafter |
| Ertapenem | i.v. | 1 g 1 × day | 31.65 | BNF48 | |
| Erythromycin | Oral | 250 mg 4 × day | 0.11 | eMIT47 | 0.24 |
| Erythromycin | i.v. | Erythromycin lactobionate 1 g 4 × day | 43.92 | BNF48 | |
| Flucloxacillin | Oral | 250 mg 4 × day | 0.11 | eMIT47 | |
| Flucloxacillin | i.v. | 0.25 g 4 × day | 1.68 | eMIT47 | 4.92 |
| Fluconazole | Oral | 400 mg first day, 200 mg 1 × day subsequently | 0.19 day 1, 0.09 thereafter | eMIT47 | |
| Fluconazole | i.v. | 400 mg first day, 200 mg 1 × day subsequently | 1.80 day 1, 0.94 thereafter | eMIT47 | |
| Fusidic acid | Oral | 500 mg 3 × day | 1.81 | BNF48 | |
| Gentamicin | i.v. | 80 mg 3 × day | 1.57 | eMIT47 | 5.85 |
| Imipenem | i.v. | 500 mg 4 × day | 17.67 | eMIT47 | |
| Levofloxacin | Oral | 500 mg 1 × day | 0.23 | eMIT47 | |
| Levofloxacin | i.v. | 500 mg 1 × day | 1.87 | eMIT47 | |
| Linezolid | Oral | 600 mg 2 × day | 89.00 | BNF48 | |
| Linezolid | i.v. | 600 mg 2 × day | 89.00 | BNF48 | |
| Meropenem | i.v. | 0.5 g 3 × day | 7.86 | eMIT47 | 24.00 |
| Metronidazole | Oral | 400 mg 3 × day | 0.05 | eMIT47 | 0.21 |
| Metronidazole | i.v. | 500 mg 3 × day | 1.20 | eMIT47 | 9.30 |
| Nitrofurantin | Oral | 50 mg 4 × day | 5.23 | BNF48 | |
| Piperacillin/tazobactam | i.v. | 4.5 g 3 × day | 5.68 | eMIT47 | |
| Rifampicin | Oral | 300 mg 3 × day | 0.42 | eMIT47 | |
| Rifampicin | i.v. | 600 mg 3 × day | 7.66 | eMIT47 | |
| Oseltamivir (Tamiflu®, Roche pharmaceuticals) | Oral | 75 mg 2 × day | 3.08 | BNF48 | |
| Teicoplanin | i.v. | 400 mg 2 × day for three doses, subsequently 400 mg 1 × day | 12.24 day 1, 6.12 thereafter | eMIT47 | 14.64 day 1, 7.32 thereafter |
| Temocillin | i.v. | 1 g 2 × day | 50.90 | BNF48 | |
| Timentin | i.v. | 3.2 g 3 × day | 15.99 | BNF48 | |
| Trimethoprim | Oral | 200 mg 2 × day | 0.03 | eMIT47 | 0.14 |
| Vancomycin | i.v. | 0.5 g 2 × day | 2.32 | eMIT47 | 12.50 |
| Recorded on CRF | Assumed drug | Assumed route | Assumed dose/frequency per day | Daily cost (£) from BNF47 |
|---|---|---|---|---|
| Digoxin | Digoxin | Oral | 125 µg daily | 0.04 |
| Diuretics | Furosemide | Oral | 40 mg daily | 0.03 |
| Beta-blockers | Atenolol | Oral | 25 mg daily | 0.03 |
| Calcium antagonists | Amlodipine | Oral | 5 mg daily | 0.03 |
| Aspirin | Aspirin | Oral | 75 mg daily | 0.03 |
| Oral nitrates | Isosorbide dinitrate | Oral | 80 mg daily | 0.98 |
| Angiotensin 2 blockers | Losartan | Oral | 25 mg daily | 0.04 |
| ACE inhibitors | Ramipril | Oral | 5 mg daily | 0.04 |
| Warfarin | Warfarin sodium | Oral | 3 mg daily | 0.03 |
| Clopidogrel | Clopidogrel | Oral | 75 mg daily | 0.06 |
| Statins | Simvastatin | Oral | 40 mg daily | 0.04 |
| Antiarrhythmic | Amiodarone | Oral | 200 mg daily | 0.06 |
| Heparin/clexane | Enoxaparin sodium | S/C | 20 mg daily | 2.27 |
| Intravenous glyceryl trinitrate/nitrates | Glyceryl trinitrate | i.v. | 25 mg daily | 6.49 |
| FeSO4 | Ferrous Sulphate | Oral | 200 mg (65 mg iron) 3 × day | 0.11 |
| Complication | Treatment/action | Cost (£) | Assumptions |
|---|---|---|---|
| Sepsis | No additional treatment | 0 | Antibiotics recorded separately and costed |
| Wound infection | No additional treatment | 0 | Antibiotics recorded separately and costed |
| Permanent stroke | Rehabilitation (plus scan) | 139 | |
| CT scan | 62 | ||
| MRI scan | 248 | ||
| Suspected MI | Emergency angiography, transthoracic echocardiography, ECG | 1868 | |
| Gut infarction | CT scan | 62 | |
| If confirmed by laparotomy | 2693 | ||
| AKI – stage 3 only | Haemofiltration | 1438 | Assume treatment for 2 days |
| TIA | CT scan | 62 | |
| Pancreatitis | CT scan, parenteral nutrition, intravenous fluids | 275.49 | Reoperations already captured |
| Intestinal obstruction/perforation | Laparotomy, parental nutrition | 2893 | Reoperations already captured |
| Post-operative haemorrhage | Chest radiograph | 41 | Reoperations already captured. Assume no additional costs for participants who have a reoperation on the same day/following day as post-operative haemorrhage |
| ARDS | Transoesophageal echocardiography, three chest radiographs | 395 | Reintubation and intensive care already captured |
| Reintubation/ventilation | Transoesophageal echocardiography, three chest radiographs | 395 | |
| Initiation of mask CPAP | CPAP, chest radiograph | 539 | |
| Tracheostomy | Tracheostomy, chest radiograph | 5354 | |
| Pneumothorax requiring chest drainage | Chest radiograph, chest drain | 4218 | |
| Pleural effusion requiring drainage | Chest radiograph, chest drain | 4218 | |
| Pacing | Temporary pacemaker | 3073 | |
| SVT/AF requiring treatment | Amiodarone | 4.79 | |
| Deep-vein thrombosis | Duplex scan of leg veins, intravenous heparin | 202.43 | Warfarin already captured |
| VF/VT requiring intervention | Transoesophageal echocardiography, emergency coronary angiography, chest radiograph | 2007 | Emergency reoperations and reintubation captured elsewhere |
| Low cardiac output requiring management (including IABP) | Transoesophageal echocardiography, chest radiograph | 313 | |
| Wound dehiscence requiring rewiring/treatment | Minor treatment (£161), or VAC therapy if stated (£3501) | 161 or 3501 | Assume reoperation covers this complication if reoperation the same day or next day |
| Cardiac tamponade | Transoesophageal echocardiography, chest radiograph | 313 | Reoperations and red blood cells already captured |
| Other GI complications | |||
| Abdominal distention/small bowel dilatation/abdominal pain | CT scan | 62 | |
| Coffee ground vomitus | Omeprazole | 12.68 | |
| Colonic pseudo-obstruction | CT scan | 62 | |
| Constipation | Laxatives, enemas | 2.25 | |
| Diabetes inference/exacerbation | Insulin | 138.60 | |
| Diagnostic laparotomy | Laparotomy | 2693 | |
| Diarrhoea/diarrhoea and vomiting | Isolation room, stool culture and i.v. for dehydration | 3592 | |
| Duodenal ulcer | Endoscopy | 676 | |
| Dysphagia/poor swallow/difficult chewing with hoarse voice | Speech and language therapy review, nasendoscopy, oropharyngeal fluoroscopy and maybe CT head | 370 | |
| Gastric bubble | Nasogastric tube insertion | 252 | |
| Gastritis, gastro-oesophageal reflux | Omeprazole | 12.68 | |
| GI bleed | Endoscopy | 676 | |
| GI bleed – duodenal ulcer | Endoscopy and in severe cases laparotomy | 2022 | |
| Haematemesis | Omeprazole | 12.68 | |
| Hepatic impairment | CT scan | 62 | |
| Ileus/gallstone ileus/paralytic ileus | CT scan | 62 | |
| Intestinal ischaemia | Laparotomy, CT scan | 2755 | |
| Ischaemic bowel and GI bleed | Laparotomy, CT scan | 2755 | |
| Laparoscopy | Laparoscopy | 2693 | |
| Melaena | Endoscopy, omeprazole | 688.68 | |
| Melaena and bleeding duodenal ulcers | Endoscopy, omeprazole | 688.68 | |
| Nausea and/or vomiting | Anti-nausea medication | 0.63 | |
| Nasogastric tube inserted | Nasogastric tube inserted | 252 | |
| Not absorbing owing to abdominal aortic aneurysm repair | CT scan | 62 | |
| Rectal bleed | Endoscopy | 676 | |
| Upper GI bleed owing to transoesophageal echocardiography | Endoscopy | 676 | |
| Other pulmonary complications – all assumed to have two chest radiographs, add £82 to all | |||
| Suspected chest infection | No additional treatment | 0 | Antibiotics and reintubation already captured |
| Aspiration pneumonia/pneumonia | No additional treatment | 0 | Antibiotics and reintubation already captured |
| BIPAP commenced | BIPAP | 498 | |
| Basal atelectasis | Chest radiographs, physiotherapy | 180 | CPAP already captured |
| Basal respiratory wheeze | Nebulised 0.9% saline (10 ml) or salbutamol (2.5 mg) 4 times daily | 1035 | |
| Bronchopneumonia | No additional treatment | 0 | Antibiotics and reintubation already captured |
| Fluid overload | No additional treatment | 0 | Antibiotics and reintubation already captured |
| Left and right haemothorax/haemothorax requiring chest drainage | Chest drain | 4177 | |
| Heart failure | Diuretics | 0.15 | Reintubation already captured |
| Increasing oxygen requirements, chest examination | Physiotherapy | 139 | CPAP and reintubation already captured |
| Infection | No additional treatment | 0 | Antibiotics and reintubation already captured |
| Left pneumothorax | Chest radiograph | 41 | |
| Lower respiratory tract infection | No additional treatment | 0 | Antibiotics and reintubation already captured |
| Overloaded | Diuretics | 0.15 | Reintubation already captured |
| Pericardial effusion/pericardial effusions with atelectasis | No treatment in mild cases | 0 | Reoperation and reintubation already captured |
| Pleuritic pain | Analgesia | 6 | |
| Pneumonia and respiratory failure | No additional treatment | 0 | Antibiotics and reintubation already captured |
| Pulmonary oedema | Diuretics | 0.15 | Reintubation already captured |
| Right lower lobe collapse and atelectasis changes on chest radiograph | Chest radiograph, physiotherapy | 180 | CPAP and reintubation already captured |
| Reduced air entry to bases/respiratory distress/respiratory failure | Physiotherapy | 139 | CPAP and reintubation already captured |
| Respiratory arrest | No additional treatment | 0 | Reintubation already captured |
| Slight decrease in entry in both bases | Physiotherapy | 139 | CPAP and reintubation already captured |
| (Small) pleural effusion left side/right side/bilateral | No treatment | 0 | |
| Surgical emphysema | Chest drain, chest radiograph | 4218 | |
| Aspirated on nasogastric tube insertion | Nasogastric tube insertion (if not already included) | 252 | |
| Disconnected chest drain | Chest drain | 4177 | |
| Increased pulmonary artery pressure | No treatment | 0 | |
| Left basal effusion and right basal collapse | Physiotherapy | 139 | CPAP and reintubation already captured |
| Other arrhythmia complications – all assumed to have two ECGs, add £106 to all | |||
| AV block/first degree heart block/third degree AV block/complete heart block | No additional treatment | 0 | Pacing already captured |
| First degree heart block with atrial ectopics | No treatment | 0 | |
| Complete heart block, permanent pacemaker | Permanent pacemaker | 14,564 | |
| Heart block-paced | No additional treatment | 0 | Pacing already captured |
| Arrhythmia | Amiodarone | 4.79 | |
| Asystole/P wave asystole/cardiac arrest/pulseless electrical activity arrest | CPR | 1491 | Reintubation already captured |
| Asystole with permanent pacemaker insertion | Permanent pacemaker | 14,564 | |
| Atrial flutter | Amiodarone | 4.79 | |
| Bradycardia/intermittent bradycardia/nodal bradycardia/sinus bradycardia | No additional treatment | 0 | Pacing already captured |
| Bradycardic episode with left bundle branch block, 24-hour tape performed | 24-hour Holter monitor | 204 | |
| Cardioversion | Cardioversion | 808 | |
| Heart rate irregular, commenced on amiodarone | Amiodarone | 4.79 | |
| Ectopics/multiple ectopics/ventricular ectopics | No treatment | 0 | |
| Fast AF requiring amiodarone and pacing switched off | Amiodarone | 4.79 | |
| Junctional rhythm | No additional treatment | 0 | Pacing already captured |
| Left bundle branch block/new onset of left branch block/right bundle branch block | No treatment | 0 | |
| Loss of cardiac output | CPR | 1491 | Reintubation already captured |
| New AF | No additional treatment | 0 | Pacing already captured |
| Permanent pacemaker implanted | Permanent pacemaker | 14,564 | |
| SVT/flutter | Amiodarone | 4.79 | |
| Sinus tachycardia | No treatment | 0 | |
| Type B Wolff–Parkinson–White pattern | No treatment | 0 | |
| Sinus pauses | No additional treatment | 0 | Pacing already captured |
| Vasovagal episode | No treatment | 0 | |
| Other thromboembolic complications | |||
| CT head confirmed occipital infarction | CT scan | 62 | |
| Cerebral infarct | CT scan | 62 | |
| Saphenous vein graft thrombosed to right coronary artery | Coronary angiography | 1694 | |
| Thrombophlebitis | Analgesia | 6 | |
| Treatment/action | Unit cost (£) | Source for cost information |
|---|---|---|
| 24-hour Holter monitor | 204 | NHS Reference Costs 2012/13.45 Day cases. EA47Z electrocardiogram monitoring and stress testing. 320 cardiology. Lower quartile cost |
| Antibiotics (piperacillin/tazobactam, 4.5 g i.v. 3 × day for 5 days) | 28.40 | eMIT47 |
| Amiodarone (1.2 g i.v., then oral 200 mg 3 × day for 1 week, 2 × day for 1 week) | 4.79 | eMIT47 |
| Analgesia (morphine sulphate, 10 mg i.v. every 4 hours for 5 days) | 6 | eMIT47 |
| Antinausea medication (ondanestron, 4 mg i.v. for 5 days) | 0.63 | eMIT47 |
| BIPAP | 498 | As CPAP |
| Cardioversion | 808 | Lord et al.76 Costs have been inflated using the HCHS inflation index |
| Chest drain | 4177 | NHS Reference Costs 2012/1345 |
| Chest radiograph | 41 | Finance Department, teaching hospital, South Central, 2012, personal communication. Costs have been inflated using the HCHS inflation index |
| Coronary angiography | 1694 | NHS Reference Costs 2012/1345 |
| CPAP | 498 | Grey et al.77 Costs have been inflated using the HCHS inflation index |
| CPR | 1491 | NHS Reference Costs 2012/1345 |
| CT scan | 62 | NHS Reference Costs 2012/13.45 Diagnostic Imaging – Direct Access. RA08 A Computerised Tomography Scan, one area, no contrast, 19 years and over. 100 general surgery |
| Diuretics (furosemide 40 mg orally for 5 days) | 0.15 | BNF48 |
| Drainage of pus under local anaesthesia | 426 | NHS Reference Costs 2012/13.45 Elective inpatients. JC43 A Minor Skin Procedures, 13 years and over. 320 Cardiology |
| Drainage of pus under general anaesthesia | 1919 | NHS Reference Costs 2012/13.45 Elective inpatients. JC43 A Minor Skin Procedures, 13 years and over. 172 Cardiac Surgery |
| Duplex scan of leg veins | 155 | NHS Reference Costs 2012/13.45 Diagnostic Imaging – Outpatients. RA10Z Computerised Tomography Scan, one area, pre and post contrast. 172 Cardiac Surgery |
| ECG | 53 | NHS Reference Costs 2012/13.45 Directly Accessed Diagnostic Services. EA47Z Electrocardiogram Monitoring and stress testing |
| Echocardiography – transthoracic | 121 | NHS Reference Costs 2012/13.45 Diagnostic Imaging – Outpatients. RA60 A Simple Echocardiogram, 19 years and over. 172 Cardiac Surgery |
| Echocardiography – transoesophageal | 272 | NHS Reference Costs 2012/13.45 Day Cases. EA45Z Complex Echocardiogram, including Transoesophageal and Fetal Echocardiography. 320 Cardiology. Lower quartile cost |
| Endoscopy | 676 | NHS Reference Costs 2012/1345 |
| Fluoroscopy | 115 | NHS Reference Costs 2012/13.45 Diagnostic Imaging – Outpatients. RA16Z Contrast Fluoroscopy Procedures, less than 20 minutes. 172 Cardiac Surgery |
| Haemofiltration (assume for 2 days) | 1438 | NHS Reference Costs 2012/13.45 Renal Dialysis at Base. LE01 A. Haemodialysis for Acute Kidney Injury, 19 years and over |
| IABP – used in sensitivity analysis | 2776 | NICE Medical Technology Guidance 8, 2011.78 Costs have been inflated using the HCHS inflation index |
| Intravenous fluids (gelofusine, 1500 ml) | 13.49 | BNF48 |
| Insulin (1000 units for 5 days) | 138.60 | BNF48 |
| i.v. heparin (initial 5000 units, then 15,000 units every 12 hours for 5 days) | 47.43 | BNF48 |
| Isolation room, stool culture and i.v. for dehydration | 3592 | NHS Reference Costs 2012/1345 |
| Omeprazole (i.v. omeprazole 40 mg for 3 days, then 40 mg oral daily for 5 days) | 12.68 | BNF,48 eMIT47 |
| Laparoscopy | 2693 | As laparotomy |
| Laparotomy | 2693 | NHS Reference Costs 2012/1345 |
| Laxatives, enemas (bisacodyl, 5 mg; sodium citrate, assume for 5 days) | 2.25 | BNF,48 eMIT47 |
| Minor treatment for wound dehiscence | 161 | NHS Reference Costs 2012/13.45 Elective inpatients. JC43 A Minor Skin Procedures, 13 years and over. 320 Cardiology, with the costs associated with the average LOS reported subtracted at a cost of £265 per day |
| MRI scan | 248 | NHS Reference Costs 2012/13.45 Diagnostic Imaging – Direct Access. RA07Z Magnetic Resonance Imaging Scan, requiring extensive patient repositioning and/or more than one contrast agent. 320 Cardiology |
| Nasendoscopy | 115 | As fluoroscopy |
| Nasogastric tube insertion | 252 | NHS Reference Costs 2012/1345 |
| Nebulised 0.9% saline (10 ml) or salbutamol (2.5 mg) 4 times daily | 1035 | NHS Reference Costs 2012/1345 |
| Parenteral nutrition (assume 5 days) | 200 | NICE, Nutrition Support in Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition Costing Report, 2006.79 Costs have been inflated using the HCHS inflation index |
| Permanent pacemaker | 14,564 | NHS Reference Costs 2012/1345 |
| Physiotherapy/rehabilitation | 139 | NHS Reference Costs 2012/13.45 Elective inpatients. DZ30Z Chest Physiotherapy. 340 Respiratory Medicine |
| Speech and language therapy review | 109 | NHS Reference Costs 2012/13.45 Non Consultant Led Outpatient Attendances. WF01B Non-Admitted Face to Face Attendance, First. 652 Speech and Language Therapy |
| Stroke (alternative cost used in sensitivity analysis) | 705 | NHS Reference Costs 2012/13.45 Non elective inpatients. AA35E Stroke with CC Score 4–6, 300 General Medicine, with the costs associated with the average LOS reported subtracted at a cost of £265 per day |
| Temporary pacemaker | 3073 | NHS Reference Costs 2012/13.45 Elective inpatients. EA39B Pacemaker Procedure without Generator Implant, including Re-siting and Removal of Cardiac Pacemaker System, with CC Score 2–4, 320 Cardiology, with the costs associated with the average LOS reported subtracted at a cost of £265 per day |
| Tracheostomy | 5313 | NHS Reference Costs 2012/1345 |
| Ultrasound | 67 | NHS Reference Costs 2012/13.45 Diagnostic Imaging – Outpatients. Weighted average of RA25Z Ultrasound Mobile Scan or Intraoperative Procedures, less than 20 minutes; RA26Z Ultrasound Mobile Scan or Intraoperative Procedures, 20 to 40 minutes, RA27Z Ultrasound Mobile Scan or Intraoperative Procedures, more than 40 minutes. 100 General Surgery |
| Negative Pressure Wound Therapy | 3501 | NHS Reference Costs 2012/13.45 Elective inpatients. JC42 A Intermediate Skin Procedures, 13 years and over. 172 Cardiac Surgery, with the costs associated with the average LOS reported subtracted at a cost of £265 per day |
| Resource | Unit cost (£) | Reference: sheet from which costs were taken, specific HRG code and/or specialty code from which costs were taken |
|---|---|---|
| Ward day for readmissions | 265 | NHS Reference Costs 2012/13.45 Non-elective inpatient excess bed-day cost across all activities |
| ICU day for readmissions | 1168 | NHS Reference Costs 2012/13.45 Critical Care Services – Adult: Critical Care Unit (weighted average of XC01Z–XC07Z, 0–6 organs supported) |
| ED attendance, leading to admission | 154 | NHS Reference Costs 2012/13.45 ED services, excluding dental care. Weighted average of all admitted codes |
| ED attendance, not leading to admission | 101 | NHS Reference Costs 2012/13.45 ED services, excluding dental care. Weighted average of all non-admitted codes |
| Ambulance to hospital | 230 | NHS Reference Costs 2012/13.45 Ambulance services, ASS02, see and treat and convey |
| Complication | Treatment/action | Cost (£) | Assumption |
|---|---|---|---|
| Antibiotics | |||
| Site = respiratory | Antibiotics, chest radiograph | 69.40 | |
| Site = surgical wound | Antibiotics, chest radiograph, CT scan | 131.40 | |
| Site = blood | Antibiotics | 28.40 | |
| Site = other – endocarditis | Antibiotics, chest radiograph, CT scan | 131.40 | |
| Site = other – infective endocarditis | Antibiotics, chest radiograph, CT scan, transthoracic echocardiography | 252.40 | |
| Site = other – respiratory tract infection | Antibiotics, chest radiograph | 69.40 | |
| Site = other – wound | Antibiotics, chest radiograph, CT scan | 131.40 | |
| Site = other – all others including UTI | Antibiotics | 28.40 | |
| Deep-vein thrombosis | Duplex scan of leg veins, i.v. heparin, warfarin | 203.78 | |
| Cardiac tamponade | Transoesophageal echocardiography, chest radiograph, two red blood cells | 559.62 | Reoperations captured elsewhere |
| Other GI complications | |||
| Barrett’s oesophagus | Endoscopy | 676 | |
| Dehydration, hypovolaemia secondary to 3 days of diarrhoea | i.v. fluids | 13.49 | |
| Gastroenteritis | i.v. fluids | 13.49 | |
| Oesophageal ulcer | Endoscopy | 676 | |
| Peritonitis | Laparotomy, CT scan | 2755 | |
| Vomiting related to amiodarone. Medication changed | i.v. fluids | 13.49 | |
| Other pulmonary complications: all assumed to have two chest radiographs and an ECG – add £135 to all | |||
| Suspected/possible pulmonary embolism – diagnosed with pleuritic chest pain | CT scan, transthoracic echocardiography | 183 | |
| Acute shortness of breath – treated with diuretics | Diuretics, transthoracic echocardiography | 121.15 | |
| Breathing difficulties at routine outpatients. Kept in overnight for breathing assessment | Transthoracic echocardiography | 121 | |
| Breathlessness and cough | Transthoracic echocardiography | 121 | |
| Chest pain and shortness of breath | Transthoracic echocardiography | 121 | |
| Chest pain on inspiration/coughing | CT scan | 62 | |
| Cough | No treatment | 0 | |
| Dyspnoea | Transthoracic echocardiography | 121 | |
| End stage heart failure | Diuretics, transthoracic echocardiography | 121.15 | |
| Failed extubation | No additional treatment | 0 | Covered in ICU cost |
| Left hydropneumothorax | Chest ultrasonography, chest drain | 4244 | |
| Musculoskeletal chest pain | Analgesia | 6 | |
| Pleural effusion and bilateral pedal oedema | Chest ultrasonography, diuretic therapy | 67.15 | |
| Pleural effusion not requiring drainage | Chest ultrasonography | 67 | |
| Pleural effusion treated with increased dose of furosemide | Furosemide (80 mg) | 0.30 | |
| Pleuritic left lung (not requiring drainage) | Analgesia | 6 | |
| Pulmonary fibrosis | No treatment | 0 | |
| Right sided pleural effusion and empyema, ultrasonography-guided drainage, 2 units blood transfused, treated with i.v. antibiotics | Chest drain, 2 units red blood cells, i.v. antibiotics | 4452.02 | |
| Severe chest pain, possible pulmonary embolism but ruled out following investigations | Transthoracic echocardiography, CT pulmonary angiogram (CT chest) | 183 | |
| Shortness of breath | Transthoracic echocardiography | 121 | |
| Shortness of breath owing to fluid overload, diuretics increased | Transthoracic echocardiography, furosemide (80 mg) | 121.30 | |
| Sudden onset of shortness of breath, small right pleural effusion | Transthoracic echocardiography | 121 | |
| Other arrhythmia complications: all assumed to have two ECGs – add £106 to all | |||
| Accelerated junctional rhythm | No additional treatment | 0 | Pacing already captured |
| Atrial fibrillation | Amiodarone | 4.79 | |
| Chest discomfort, palpitations | No treatment | 0 | |
| Fast atrial flutter | Amiodarone | 4.79 | |
| Paroxysmal AF | Amiodarone | 4.79 | |
| Re-entry tachycardia | No additional treatment | 0 | Only cost permanent pacemaker if clear indication participant had this treatment |
| Other thromboembolic complications | |||
| Pulmonary embolus | Transthoracic echocardiography, CT pulmonary angiogram (CT chest), i.v. heparin for 5 days, warfarin | 231.78 | |
| Apical thrombus | Transthoracic echocardiography, i.v. heparin for 5 days, warfarin | 169.78 | |
| Possible bilateral renal infarcts | CT scan, i.v. heparin, warfarin | 110.78 | |
| Small leg thrombus | Ultrasonography of leg, i.v. heparin, warfarin | 115.78 | |
| Treatment/action | Unit cost (£) | Reference |
|---|---|---|
| Diuretics (furosemide 80 mg orally for 5 days) | 0.30 | BNF48 |
| Warfarin (3 mg daily, assumed given for half of follow up time, 45 days) | 1.35 | BNF48 |
| Specialty | Unit cost (£) | Service code | Reference: sheet from which costs were taken, specific HRG code and/or specialty code from which costs were taken |
|---|---|---|---|
| Anticoagulation service | 25 | 324 | These are all sourced from NHS Reference Costs 2012–13.45 They are all average costs for each specialty (costs taken from the Total – Outpatient Attendances page of the NHS Reference Costs 2012–13, Total activity section) |
| Cardiac rehabilitation | 42 | 327 | |
| Cardiac surgery | 299 | 172 | |
| Cardiology | 131 | 320 | |
| Cardiothoracic surgery | 275 | 170 | |
| Clinical haematology | 151 | 303 | |
| Colorectal surgery | 113 | 104 | |
| Dermatology | 98 | 330 | |
| Diabetic medicine | 136 | 307 | |
| Endocrinology | 152 | 302 | |
| Gastroenterology | 137 | 301 | |
| General medicine | 153 | 300 | |
| General surgery | 128 | 100 | |
| Geriatric medicine | 204 | 430 | |
| Hepatology | 213 | 306 | |
| Infectious diseases | 142 | 350 | |
| Medical oncology | 138 | 370 | |
| Nephrology | 158 | 361 | |
| Neurology | 176 | 400 | |
| Occupational therapy | 63 | 651 | |
| Ophthalmology | 86 | 130 | |
| Physiotherapy | 42 | 650 | |
| Plastic surgery | 88 | 160 | |
| Rehabilitation | 90 | 314 | |
| Respiratory medicine | 150 | 340 | |
| Stroke clinic | 200 | 328 | |
| Thoracic surgery | 253 | 173 | |
| Upper GI surgery | 120 | 106 | |
| Urology | 101 | 101 | |
| Vascular surgery | 142 | 107 |
| Resource | Unit cost (£) | Reference: sheet from which costs were taken, specific HRG code and/or specialty code from which costs were taken |
|---|---|---|
| Renal/dialysis | 157 | NHS Reference Costs 2012/13.45 Renal Dialysis at Base. LD02 A Hospital Haemodialysis or Filtration, with Access via Arteriovenous Fistula or Graft, 19 years and over |
| Outpatient endoscopy | 676 | As endoscopy, previously given (see Table 66) |
| ECG | 53 | As previously given (see Table 66) |
| Electrocardiogram monitoring and stress testing | 204 | As 24-hour Holter monitor (see Table 66) |
| CT scan | 62 | As previously given (see Table 66) |
| Echocardiography scan – transthoracic | 121 | As previously given (see Table 66) |
| MRI scan | 248 | As previously given (see Table 66) |
| Chest radiography | 41 | As previously given (see Table 66) |
| Chest radiography and ultrasonography | 108 | As previously given (see Table 66) |
| Sigmoidoscopy | 164 | NHS Reference Costs 2012/13.45 Procedures in Outpatients. FZ57Z Diagnostic or Therapeutic, Rigid Sigmoidoscopy, 19 years and over. 104 Colorectal Surgery |
| Treatment/action | Unit cost (£) | Reference: sheet from which costs were taken, specific HRG code and/or specialty code from which costs were taken |
|---|---|---|
| Bladder cystoscopy | 129 | NHS Reference Costs 2012/13.45 Procedures in Outpatients. LB15E Minor Bladder Procedures, 19 years and over. 101 Urology |
| Colonoscopy | 257 | NHS Reference Costs 2012/13.45 Procedures in Outpatients. FZ51Z Diagnostic Colonoscopy, 19 years and over. 100 General Surgery |
| Diverticulitis | 686 | NHS Reference Costs 2012/13.45 Elective inpatients. FZ83H Major Oesophageal, Stomach or Duodenum Procedures, 19 years and over with CC Score 4–6. 301 Gastroenterology, with the costs associated with the average LOS reported subtracted at a cost of £265 per day |
| ECG | 477 | NHS Reference Costs 2012/13.45 Day Cases. EA47Z Electrocardiogram Monitoring and stress testing. 320 Cardiology |
| Fasciotomy | 6182 | NHS Reference Costs 2012/13.45 Non-elective inpatients. QZ02D Lower Limb Arterial Surgery with CC Score 6–10. 107 Vascular Surgery, with the costs associated with the average LOS reported subtracted at a cost of £265 per day |
| Gastroscopy | 676 | As endoscopy (see Table 66) |
| Groin scan/procedure of lymphatic system | 3191 | NHS Reference Costs 2012/1345 |
| Oesophagogastroduodenoscopy | 676 | As endoscopy (see Table 66) |
| Leg amputation | 13,353 | NHS Reference Costs 2012/13.45 Non-elective inpatients. QZ11D Amputations with CC Score 8–13. 107 Vascular Surgery |
| Recatheterisation | 1534 | NHS Reference Costs 2012/1345 |
| Stoma bag system | 63.38 | NHS Electronic Drug Tariff.80 Part IXC – Stoma Appliances (Colostomy Sets). Weighted average of all sets |
| Tesio catheter insertion under fluoroscopy | 325 | NHS Reference Costs 2012/1345 |
| Uroscopy | 129 | NHS Reference Costs 2012/13.45 Procedures in Outpatients. LB15E Minor Bladder Procedures, 19 years and over. 101 Urology |
| Resource | Unit cost (£) | Reference: sheet from which costs were taken, specific HRG code and/or specialty code from which costs were taken |
|---|---|---|
| GP at surgery | 34 | Unit Costs of Health and Social Care 2013;49 10.8b, GP – unit costs. Per-patient contact lasting 11.7 minutes. Excluding qualification costs and direct care staff costs |
| GP at home | 85 | Unit Costs of Health and Social Care 2013;49 10.8b, GP – unit costs. Per out-of-surgery visit lasting 23.4 minutes. Excluding qualification costs and direct care staff costs |
| Out-of-hours GP | 34 | As GP at surgery |
| Walk-in centre | 34 | As GP at surgery |
| GP nurse | 11.37 | Unit Costs of Health and Social Care 2013;49 10.6, Nurse (GP practice). £44 per hour of face-to-face contact, excluding qualification costs. Average contact 15.5 minutes |
| District nurse | 39 | Unit Costs of Health and Social Care 2013;49 10.1, Community Nurse. Using data from NHS Reference Costs 2011/12,45 the mean average cost for a face-to-face contact in district nursing services for 2012/2013 was £39, with an IQR of £33 to £46. Costs have been uprated using the HCHS pay and prices inflator |
| Other NHS or social services | ||
| Cardiac rehabilitation/exercise class | 42 | NHS Reference Costs 2012/13;45 Total – Outpatient Attendances. 327 – Cardiac Rehabilitation |
| Cardiac nurse | 70 | NHS Reference Costs 2012/13;45 Community Health Services – Nursing, N11AF, Specialist Nursing – Cardiac Nursing/Liaison, Adult, Face to face |
| Diabetic nurse | 70 | NHS Reference Costs 2012/13;45 Community Health Services – Nursing, N15AF, Specialist Nursing – Diabetic Nursing/Liaison, Adult, Face to face |
| Anticoagulation service | 10 | NHS Reference Costs 2012/13;45 Non Consultant Led Outpatient Attendances; Non-Admitted Non-Face to Face Attendance, Follow-up, 324 – Anticoagulation Service |
| Community pharmacist | 69.64 | Unit Costs of Health and Social Care 2013;49 9.6, Community pharmacist. £127 per hour of direct clinical activities. Contact assumed to be for 32.9 minutesa |
| Cardiac rehabilitation by phone | 50 | NHS Reference Costs 2012/13;45 Non Consultant Led Outpatient Attendances; Non-Admitted Non-Face to Face Attendance, Follow-up, 327 – Cardiac Rehabilitation |
| Dietitian | 71 | NHS Reference Costs 2012/13;45 Community Health Services – Allied Health Professionals. A03 – Dietician |
| Health-care support worker | 16 | Unit Costs of Health and Social Care 2013;49 10.5, Clinical support worker nursing (community). £30 per hour of home visiting. Contact assumed to be for 32.9 minutesa |
| Occupational therapist | 53 | Unit Costs of Health and Social Care 2013;49 13.2, Hospital occupational therapist. Using data from NHS Reference Costs 2011/12, the mean average cost for a non-consultant led (non-admitted) follow-up occupational therapy attendance was £53, with an IQR of £30 to £64. Costs have been uprated using the HCHS pay and prices inflator |
| Physiotherapist | 34 | Unit Costs of Health and Social Care 2013;49 13.1, Hospital physiotherapist. Using data from NHS Reference Costs 2011/12, the mean average cost for a non-consultant-led (non-admitted) follow-up physiotherapy attendance was £34, with an IQR of £28 to £38. Costs have been uprated using the HCHS pay and prices inflator |
| Social worker | 87.19 | Unit Costs of Health and Social Care 2013;49 11.2, Social worker (adult services). £159 per hour of face-to-face contact, excluding qualification costs. Contact assumed to be for 32.9 minutesa |
| Other NHS or social services at home | ||
| Cardiac rehabilitation/nurse | 70 | NHS Reference Costs 2012/13;45 Community Health Services – Nursing. N11AF Specialist Nursing – Cardiac Nursing/Liaison, Adult, Face to face |
| Phone call to cardiology | 49 | NHS Reference Costs 2012/13;45 Outpatients. WF01C, Non-Admitted Non-Face to Face Attendance, Follow-up. 320 – Cardiology |
| Carer | 8.50 | Unit Costs of Health and Social Care 2013;49 11.6, Home care worker. The mean hourly cost of all home care including LA-funded and independent provision was £17. Just over half of local authority funded visits lasted 30 minutes. Sixteen per cent of visits were 15 minutes and 19% of a home care workers’ time was spent travelling. Assume 30-minute visit |
| Community matron | 68 | NHS Reference Costs 2012/13;45 Community Health Services – Nursing. N06AF – Specialist Nursing – Active Case Management (Community Matrons), Adult, Face to face |
| Community mental health team carer | 36 | Unit Costs of Health and Social Care 2013;49 10.2 Nurse (mental health), £65 per hour of face-to-face contact, excluding qualifications. Contact assumed to be for 32.9 minutesa |
| Dietitian | 71 | NHS Reference Costs 2012/13;45 Community Health Services – Allied Health Professionals. A03 – Dietician |
| Occupational therapist | 73 | Unit Costs of Health and Social Care 2013;49 9.2, NHS community occupational therapist. Using data from NHS Reference Costs 2011/12, the mean average cost for a one-to-one contact of occupational therapy services was £73, with an IQR of £50 to £86. Costs have been updated using the HCHS pay and prices inflator |
| Paramedic | 174 | NHS Reference Costs 2012/13;45 Ambulance Services. ASS01 – See and treat or refer |
| Physiotherapist | 47 | Unit Costs of Health and Social Care 2013;49 9.1, Community physiotherapist. Using data from NHS Reference Costs 2011/12, the mean average cost for a one-to-one contact in physiotherapy services was £47, with an IQR of £37 to £52. Costs have been uprated using the HCHS pay and prices inflator |
| Social worker | 87.19 | Unit Costs of Health and Social Care 2013;49 11.2, Social worker (adult services). £159 per hour of face-to-face contact, excluding qualification costs. Contact assumed to be for 32.9 minutesa |
| Nurse specialist | 60 | NHS Reference Costs 2012/13;45 Community Health Services – Nursing, N29AF, Other Specialist Nursing, Adult, Face to face |
| Respiratory nurse | 75 | NHS Reference Costs 2012/13;45 Community Health Services – Nursing, N08AF, Specialist Nursing – Asthma and Respiratory Nursing/Liaison, Adult, Face to face |
| NHS direct call | 13 | NHS Direct annual report 2012/1381 |
| Indoor and outdoor grab rails | 91 | Unit Costs of Health and Social Care 2013;49 7.3.1, social services access improvements |
| Mobile shower chair | 55 | Unit Costs of Health and Social Care 2013;49 7.3.1, social services shower |
A detailed breakdown of total average costs per participant
| Resource use | Randomised to restrictive threshold (n = 1000), mean cost (£) (SE) | Randomised to liberal threshold (n = 1003), mean cost (£) (SE) | Restrictive vs. liberal threshold, mean cost (£) difference (SE) |
|---|---|---|---|
| Red blood cells | |||
| Red blood cells | 257 (12) | 379 (13) | –122 (18) |
| Red blood cell administration | 30 (1) | 48 (1) | –17 (2) |
| Total red blood cells | 287 (13) | 427 (15) | –140 (19) |
| Cardiac procedure | |||
| Initial cardiac surgery | 7309 (18) | 7313 (18) | –4 (26) |
| Blood products | |||
| FFP | 27 (2) | 26 (2) | 1 (2) |
| Platelets | 135 (7) | 134 (7) | 1 (10) |
| Cryoprecipitate | 43 (5) | 39 (4) | 4 (7) |
| Total blood products | 206 (12) | 199 (11) | 7 (16) |
| Inpatient complications | |||
| Primary outcome | |||
| Antibiotics for infectious complication | 21 (5) | 14 (2) | 7 (5) |
| Stroke | 3 (1) | 3 (1) | 0 (1) |
| Suspected MI | 6 (3) | 13 (5) | –7 (6) |
| Gut infarction | 11 (6) | 3 (3) | 8 (6) |
| AKI – stage 3 | 86 (11) | 73 (10) | 13 (15) |
| Other complications | |||
| Reoperation | 636 (70) | 704 (75) | –67 (102) |
| Reintubation | 28 (4) | 29 (4) | –1 (5) |
| Tracheostomy | 182 (32) | 176 (31) | 6 (45) |
| Mask CPAP | 68 (6) | 65 (7) | 3 (9) |
| Pneumothorax requiring chest drainage | 59 (16) | 55 (15) | 4 (22) |
| Pleural effusion requiring drainage | 245 (32) | 248 (36) | –3 (48) |
| Pacing | 946 (48) | 956 (47) | –9 (67) |
| SVT/AF requiring treatment | 2 (0) | 2 (0) | 0 (0) |
| VF/VT requiring intervention | 44 (11) | 16 (6) | 28 (13) |
| Low cardiac output | 33 (3) | 35 (4) | –1 (5) |
| SAEs | 17 (7) | 12 (5) | 5 (9) |
| Other inpatient complications | 296 (54) | 311 (54) | –15 (76) |
| Total complications and SAEs | 2684 (137) | 2714 (146) | –30 (200) |
| Inpatient LOS | |||
| CICU | 1359 (138) | 1330 (158) | 29 (210) |
| HDU | 1916 (72) | 1890 (74) | 25 (104) |
| Ward | 2221 (59) | 2287 (68) | –66 (90) |
| ICU | 8 (8) | 17 (12) | –9 (14) |
| Another unit/hospital | 351 (51) | 368 (51) | –17 (72) |
| Total LOS | 5854 (201) | 5892 (221) | –38 (299) |
| Blood saving techniques | |||
| Tranexamic acid | 13 (0) | 13 (0) | 0 (0) |
| Trasylol | 13 (2) | 11 (2) | 2 (3) |
| Intraoperative cell salvage | 85 (3) | 88 (3) | –4 (4) |
| Post-operative cell salvage | 10 (1) | 8 (1) | 2 (2) |
| Beriplex | 22 (3) | 20 (3) | –2 (4) |
| Factor VIIa | 17 (7) | 12 (6) | 5 (9) |
| Total blood saving techniques | 159 (9) | 152 (8) | 7 (12) |
| Fluids in theatre/CICU/HDU | |||
| Inotropes | 36 (1) | 35 (1) | 1 (1) |
| Gelofusine | 7 (0) | 7 (0) | 0 (0) |
| HES | 9 (1) | 9 (1) | 0 (1) |
| Other fluids | 3 (0) | 3 (0) | 0 (0) |
| Total fluids | 55 (1) | 55 (1) | 0 (2) |
| Readmissions to hospital | |||
| LOS (ward and ICU) | 446 (58) | 447 (50) | –1 (76) |
| Complications and SAEs | 271 (38) | 259 (40) | 12 (55) |
| Readmission via ED and/or ambulance | 52 (4) | 47 (4) | 5 (6) |
| Total readmissions | 770 (85) | 753 (78) | 17 (116) |
| ED attendances | |||
| Total ED visits | 9 (1) | 8 (1) | 1 (1) |
| Ambulance to ED | 7 (1) | 4 (1) | 3 (2) |
| Total ED | 16 (2) | 12 (2) | 4 (3) |
| Outpatient appointments | |||
| Cardiac surgery outpatient visits | 131 (5) | 151 (6) | –20 (8) |
| Cardiology outpatient visits | 37 (2) | 33 (2) | 4 (3) |
| Other outpatient visits | 32 (4) | 30 (4) | 2 (5) |
| Ambulance to appointment | 1 (0) | 2 (1) | 0 (1) |
| Total outpatients | 202 (6) | 216 (7) | –14 (9) |
| Other health and social care contacts | |||
| GP at surgery | 68 (2) | 71 (2) | –3 (3) |
| GP at home | 37 (3) | 32 (2) | 5 (4) |
| Practice nurse | 18 (1) | 18 (1) | 0 (2) |
| District nurse | 96 (8) | 86 (7) | 10 (11) |
| Other contacts | 160 (9) | 160 (12) | 0 (15) |
| Total other contacts | 378 (14) | 366 (16) | 12 (21) |
| Total costs | 17,945 (332) | 18,127 (357) | –182 (488) |
Changes in the use of regular medications between admission to and discharge from the cardiac surgery unit
Information on regular medications taken by participants was recorded on CRF Form D2 for two time points (1) on admission to the cardiac surgery unit (baseline) and (2) at discharge from the cardiac surgery unit. In the main costing analyses, we assumed that any medications participants were on at discharge, they took for the 3-month follow-up and were costed for 3 months.
In this separate analysis, we summarised the number of participants on each medication at baseline and at discharge, and the change in the use of medications. We then costed the medications participants were on at baseline and at discharge for a period of 1 week to get an insight into the costs of their regular use. Comparisons were then made between the costs of these medications taken at baseline and at discharge from hospital, to determine whether or not there were significant changes before and after surgery. There was very little missing data for the use of regular medications at baseline and at discharge. Complete information was available for all drugs at both time points for 1990 of the 2003 participants and, therefore, a complete case analysis was performed.
Table 75 shows the number of participants on each medication at baseline and at discharge, and the change between the time points for each transfusion group. There was quite a lot of change in the use of medications before and after surgery. The use of diuretics, aspirin, warfarin and antiarrhythmics increased considerably in both groups from baseline to discharge, whereas the use of calcium antagonists, oral nitrates and angiotensin-converting enzyme (ACE) inhibitors reduced after surgery in both groups. The use of FeSO4 increased after surgery, but to a much greater extent in the restrictive group than the liberal group.
| Drug name | Restrictive threshold, n = 992 | Liberal threshold, n = 998 | ||||
|---|---|---|---|---|---|---|
| Baseline, frequency (%) | Discharge, frequency (%) | Discharge vs. baseline, frequency (%) difference | Baseline, frequency (%) | Discharge, frequency (%) | Discharge vs. baseline, frequency (%) difference | |
| Digoxin | 44 (4) | 38 (4) | –6 (–1) | 57 (6) | 54 (5) | –3 (–0) |
| Diuretics | 336 (34) | 634 (64) | 298 (30) | 360 (36) | 652 (65) | 292 (29) |
| Beta blockers | 590 (59) | 668 (67) | 78 (8) | 588 (59) | 671 (67) | 83 (8) |
| Calcium antagonists | 228 (23) | 101 (10) | –127 (–13) | 247 (25) | 106 (11) | –141 (–14) |
| Aspirin | 597 (60) | 754 (76) | 157 (16) | 606 (61) | 777 (78) | 171 (17) |
| Oral nitrates | 222 (22) | 27 (3) | –195 (–20) | 217 (22) | 26 (3) | –191 (–19) |
| Angiotensin 2 blockers | 116 (12) | 55 (6) | –61 (6) | 114 (11) | 60 (6) | –54 (–5) |
| ACE inhibitors | 453 (46) | 338 (34) | –115 (–12) | 425 (43) | 328 (33) | –97 (10) |
| Warfarin | 108 (11) | 263 (27) | 155 (16) | 119 (12) | 251 (25) | 132 (13) |
| Clopidogrel | 187 (19) | 141 (14) | –46 (–5) | 162 (16) | 152 (15) | –10 (–1) |
| Statins | 713 (72) | 726 (73) | 13 (1) | 720 (72) | 757 (76) | 37 (4) |
| Anti-arrhythmic | 24 (2) | 284 (29) | 260 (26) | 29 (3) | 273 (27) | 244 (24) |
| Heparin | 33 (3) | 33 (3) | 0 (0) | 54 (5) | 33 (3) | –21 (–2) |
| Intravenous glyceryl trinitrate | 23 (2) | 2 (0) | –21 (–2) | 21 (2) | 9 (1) | –12 (–1) |
| FeSO4 | 29 (3) | 154 (16) | 125 (13) | 37 (4) | 63 (6) | 26 (3) |
Table 76 shows the mean weekly costs per participant in each group for each of the medications at baseline and at discharge. The daily unit cost for each of the drugs is also shown and most are very inexpensive. Costs have reduced after surgery – mean total costs are similar in both groups, approximately £4 at baseline and £2 at discharge. The reduction in costs after surgery is driven by the reduced costs of oral nitrates and intravenous glyceryl trinitrate in both groups, and also by the reduced cost of heparin in the liberal group.
| Restrictive threshold, n= 992 | Liberal threshold, n = 998 | ||||||
|---|---|---|---|---|---|---|---|
| Drug name | Daily unit cost (£) | Baseline, mean cost (£) | Discharge, mean cost (£) | Discharge vs. baseline, mean cost (£) difference | Baseline, mean cost (£) | Discharge, mean cost (£) | Discharge vs. baseline, mean cost (£) difference |
| Digoxin | 0.04 | 0.01 | 0.01 | 0.00 | 0.02 | 0.02 | 0.00 |
| Diuretics | 0.03 | 0.07 | 0.13 | 0.06 | 0.08 | 0.14 | 0.06 |
| Beta-blockers | 0.03 | 0.12 | 0.14 | 0.02 | 0.12 | 0.14 | 0.02 |
| Calcium antagonists | 0.03 | 0.05 | 0.02 | –0.03 | 0.05 | 0.02 | –0.03 |
| Aspirin | 0.03 | 0.13 | 0.16 | 0.03 | 0.13 | 0.16 | 0.04 |
| Oral nitrates | 0.98 | 1.54 | 0.19 | –1.35 | 1.49 | 0.18 | –1.31 |
| Angiotensin 2 blockers | 0.04 | 0.03 | 0.02 | –0.02 | 0.03 | 0.02 | –0.02 |
| ACE inhibitors | 0.04 | 0.13 | 0.10 | –0.03 | 0.12 | 0.09 | –0.03 |
| Warfarin | 0.03 | 0.02 | 0.06 | 0.03 | 0.03 | 0.05 | 0.03 |
| Clopidogrel | 0.06 | 0.08 | 0.06 | –0.02 | 0.07 | 0.06 | 0.00 |
| Statins | 0.04 | 0.20 | 0.20 | 0.00 | 0.20 | 0.21 | 0.01 |
| Antiarrhythmic | 0.06 | 0.01 | 0.12 | 0.11 | 0.01 | 0.11 | 0.10 |
| Heparin | 2.27 | 0.53 | 0.53 | 0.00 | 0.86 | 0.53 | –0.33 |
| Intravenous glyceryl trinitrate | 6.49 | 1.05 | 0.09 | –0.96 | 0.96 | 0.41 | –0.55 |
| FeSO4 | 0.11 | 0.02 | 0.12 | 0.10 | 0.03 | 0.05 | 0.02 |
| Total cost | 4.00 | 1.95 | –2.05 | 4.19 | 2.19 | –1.99 | |
The costs of these medications are low but it is important to bear in mind that these are weekly costs, whereas participants are likely to be on these medications for a long time. Furthermore, people on long-term medication are likely to have additional health-care appointments, for example people on warfarin have regular monitoring checks, so costs are incurred to the NHS beyond the drug costs.
Non-NHS costs: did these differ between trial groups?
The primary perspective of the evaluation was that of the UK NHS and Personal Social Services. However, data were collected on some types of non-NHS costs and if resource use differed between the trial groups for these non-NHS costs, we planned to include these costs in a wider perspective in a sensitivity analysis. The main non-NHS resource collected was participants’ means of travel to hospital for readmissions or visits after discharge. We investigated whether or not resource use for travel to hospital differed between trial groups, to determine whether or not it was important to conduct a sensitivity analysis around this.
On the follow-up questionnaire, participants were asked to record how they travelled to hospital for each readmission, ED visit and outpatient appointment. The means of transport was recorded for 315 of the 418 readmissions recorded on the CRFs that were included in the costings for 112 of the 144 ED visits recorded and for 1387 of the outpatient appointments recorded. These responses are shown in Table 77.
| Means of transport | Randomised to restrictive threshold, frequency (%) | Randomised to liberal threshold, frequency (%) | Restrictive vs. liberal threshold, % difference |
|---|---|---|---|
| Transport to readmission | n = 153 | n = 162 | |
| Ambulance | 77 (50) | 79 (49) | 1 |
| Hospital provided transport | 5 (3) | 8 (5) | –2 |
| Hospital transport | 4 (3) | 8 (5) | –2 |
| Taxi (hospital paid) | 1 (1) | 0 (0) | 1 |
| Transport at private expense | 67 (44) | 75 (46) | –2 |
| Friend/relative in car | 62 (41) | 68 (42) | –1 |
| Self-driven in car | 2 (1) | 1 (1) | 0 |
| Taxi (self-paid) | 3 (2) | 5 (3) | –1 |
| Public transport | 0 (0) | 1 (1) | –1 |
| Other | 4 (3)a | 0 (0) | 3 |
| Transport to ED | n = 59 | n = 53 | |
| Ambulance | 24 (41) | 11 (21) | 20 |
| Hospital provided transport | 1 (2) | 0 (0) | 2 |
| Hospital transport | 1 (2) | 0 (0) | 2 |
| Taxi (hospital paid) | 0 (0) | 0 (0) | 0 |
| Transport at private expense | 34 (57) | 42 (79) | –22 |
| Friend/relative in car | 23 (39) | 34 (64) | –25 |
| Self-driven in car | 3 (5) | 6 (11) | –6 |
| Taxi (self-paid) | 3 (5) | 1 (2) | 3 |
| Public transport | 5 (8) | 1 (2) | 6 |
| Transport to OP appointment | n = 654 | n = 733 | |
| Ambulance | 4 (1) | 6 (1) | 0 |
| Hospital provided transport | 19 (3) | 28 (4) | –1 |
| Hospital transport | 14 (2) | 22 (3) | –1 |
| Taxi (hospital paid) | 5 (1) | 6 (1) | 0 |
| Transport at private expense | 624 (95) | 691 (94) | 1 |
| Friend/relative in car | 395 (60) | 455 (62) | –2 |
| Self-driven in car | 141 (22) | 119 (16) | 6 |
| Taxi (self-paid) | 24 (4) | 40 (5) | –1 |
| Public transport | 64 (10) | 77 (11) | –1 |
| Other | 7 (1)b | 8 (1)c | 0 |
For readmissions to hospital, approximately 50% of participants were taken by ambulance, the majority of other participants travelled at their own expense, most frequently being taken by a friend or relative by car. The proportion of participants travelling by each means is very similar between the trial groups. Relatively few ED visits were recorded by participants, so while the proportion of participants travelling by different means looks to vary between the groups, the numbers are small. For outpatient appointments, 95% of participants travelled at private expense, the majority being taken by a friend or relative by car or driving himself or herself. The proportion of participants travelling by each means is very similar between the trial groups.
Means of transport to hospital did not appear to differ between the trial groups; therefore, a sensitivity analysis taking a wider perspective was not conducted.
Sensitivity analyses
Sensitivity analyses around unit costs
| Sensitivity analysis | Resource/complication | Unit costs used in base-case analysis | Alternative strategies for sensitivity analysis |
|---|---|---|---|
| 1 | Ward stay in cardiac unit (first admission) | £392 | £265 (cost used for ward stay beyond index cardiac admission) |
| 2 | Ward stay beyond index cardiac admission (further stay in another unit/hospital or readmission) | £265 | £392 (cost used for ward stay in cardiac unit, first admission) |
| 3 | Bed-days in first admission | £1608 general ICU £1190 CICU £619 HDU £392 cardiac ward £265 another unit/hospital ward (£1168 if known to be ICU) |
Alter bed-day costs in first admission by ± 25% and 50% |
| 4 | Bed-days in readmissions | £1168 ICU £265 ward |
Alter readmission ICU/ward costs by ± 25% and 50% |
| 5 | Stroke | £139 for physiotherapy and diagnostics as recorded (CT scan £62; MRI scan £248) | £705 (taken from Reference Costs,45 see Table 66) |
| 6 | Wound dehiscence | Covered by reoperation if the two dates are the same; otherwise £161 unless VAC therapy is stated then £3501 | Assume VAC therapy for those without reoperations (£3501) |
| 7 | Low cardiac output | £313; IABP not included | Add cost of IABP £2776 |
| 8 | Chest drain | £4177 | –50%: £2088.50 |
| 9 | Pacing | £3073 | ± 25% and 50% |
| 10 | Tracheostomy | £5354 (includes one radiograph) | ± 25% and 50% |
| 11 | Reoperations | £6608 if operation takes < 3 hours and £8298 if ≥ 3 hours. Reoperations in readmissions costed at £6608 + £1421 for blood products | Cost all reoperations at the lower (£6608) and higher figures (£8298). Include £1421 for blood products for reoperations in readmissions |
| 12 | Antibiotics | eMIT47 when available, otherwise BNF;48 see Table 63 | Increase costs by 100% |
| 13 | Antibiotics | eMIT47 when available, otherwise BNF;48 see Table 63 | Cost most common antibiotics (those received by 20 or more participants) using BNF;48 see Table 62 |
| 14 | Antibiotics | eMIT47 when available, otherwise BNF;48 see Table 63 | For antibiotics participants receive orally or intravenously, cost all as oral |
| 15 | Antibiotics | eMIT47 when available, otherwise BNF;48 see Table 63 | For antibiotics participants receive orally or intravenously, cost all as intravenous |
| 16 | Outpatient visits | See Tables 70 and 71 | ± 25% and 50% |
| Sensitivity analysis | Randomised to restrictive threshold (n = 1000), mean cost (£) (SE) | Randomised to liberal threshold (n = 1003), mean cost (£) (SE) | Restrictive vs. liberal threshold, mean cost (£) difference (SE) |
|---|---|---|---|
| Base case | 17,945 (332) | 18,127 (357) | –182 (488) |
| SA1 (ward stay, cardiac unit) | 17,226 (327) | 17,386 (352) | –161 (480) |
| SA2 (ward stay, beyond index cardiac admission) | 18,267 (346) | 18,476 (370) | –208 (507) |
| SA3 (bed-days, first admission) | |||
| + 25% | 19,409 (377) | 19,600 (408) | –191 (556) |
| –25% | 16,482 (289) | 16,654 (308) | –173 (422) |
| + 50% | 20,872 (423) | 21,073 (460) | –201 (625) |
| –50% | 15,018 (248) | 15,181 (261) | –163 (360) |
| SA4 (bed-days, readmissions) | |||
| + 25% | 18,057 (336) | 18,239 (360) | –182 (493) |
| –25% | 17,834 (329) | 18,016 (355) | –182 (484) |
| + 50% | 18,168 (341) | 18,350 (363) | –182 (498) |
| –50% | 17,722 (326) | 17,904 (353) | –182 (481) |
| SA5 (stroke) | 17,954 (333) | 18,137 (358) | –182 (489) |
| SA6 (wound dehiscence) | 18,022 (337) | 18,200 (361) | –178 (494) |
| SA7 (low cardiac output) | 18,248 (341) | 18,439 (369) | –192 (502) |
| SA8 (chest drain) | 17,712 (324) | 17,905 (348) | –193 (475) |
| SA9 (pacing) | |||
| +25% | 18,182 (336) | 18,366 (361) | –184 (493) |
| –25% | 17,709 (329) | 17,888 (355) | –180 (484) |
| +50% | 18,419 (340) | 18,605 (364) | –187 (498) |
| –50% | 17,472 (326) | 17,649 (352) | –177 (480) |
| SA10 (tracheostomy) | |||
| +25% | 17,991 (337) | 18,171 (362) | –180 (495) |
| –25% | 17,900 (327) | 18,083 (352) | –183 (481) |
| +50% | 18,036 (342) | 18,215 (367) | –179 (502) |
| –50% | 17,854 (323) | 18,039 (348) | –185 (474) |
| SA11 (reoperations) | |||
| £6608 | 17,930 (331) | 18,115 (356) | –185 (486) |
| £8298 | 18,092 (339) | 18,296 (366) | –203 (499) |
| SA12 (antibiotics) | 17,968 (334) | 18,143 (358) | –175 (490) |
| SA13 (antibiotics, BNF) | 18,004 (335) | 18,190 (361) | –186 (492) |
| SA14 (antibiotics, oral) | 17,943 (332) | 18,126 (357) | –182 (488) |
| SA15 (antibiotics, i.v.) | 17,948 (332) | 18,131 (358) | –183 (488) |
| SA16 (outpatient visits) | |||
| +25% | 17,995 (332) | 18,181 (357) | –185 (488) |
| –25% | 17,895 (332) | 18,074 (357) | –178 (488) |
| +50% | 18,045 (332) | 18,234 (357) | –189 (488) |
| –50% | 17,845 (333) | 18,020 (358) | –175 (488) |
Costs from randomisation
Costs for 3 months from randomisation rather than from surgery have been calculated for each participant. Events that occurred before randomisation were excluded: the cardiac procedure and blood saving techniques in theatre [tranexamic acid, aprotinin (Trasylol, The Nordic group) and cell salvage]. Costs associated with post-operative cell saver were included only if participants were randomised within 4 hours of surgery. The costs of red blood cells given pre-randomisation and complications occurring pre-randomisation were excluded. For events which may have started before randomisation but extended beyond randomisation, such as LOS and intubations, durations were calculated from the time of randomisation.
Resource use for other blood products (FFP, platelets, cryoprecipitate) and activated factor VII and Beriplex was captured for the hospital stay. It was not possible to determine if these resources were used pre or post randomisation. The costs of these products have been included in this analysis. It was not possible to determine if fluids given in theatre, CICU or HDU were given pre or post randomisation and the costs of these products were excluded.
Any events occurring within 3 months of randomisation rather than of surgery were included in the analysis. Given that randomisation occurs after surgery, slightly more post-discharge resource use was included in this analysis than the costs to 3 months from surgery.
Tables 80 and 81 present the mean resource use and mean costs to 3 months from randomisation. Participants in the restrictive group received on average one less unit of red blood cells than participants in the liberal group; other resource use was similar between the groups. In terms of costs, the reduced use of red blood cells in the restrictive group resulted in a cost difference of –£141 in red blood cells between the groups, which is largely what drives the difference in total costs between the groups of –£134.
| Resource use component | Randomised to restrictive threshold (n = 1000), frequency (%) or mean (SE) | Randomised to liberal threshold (n = 1003), frequency (%) or mean (SE) | Restrictive vs. liberal threshold, % or mean (SE) difference |
|---|---|---|---|
| Red blood cells, number of units/participant | 1.49 (0.08) | 2.49 (0.09) | –1.00 (0.12) |
| Blood products, number of units/participant | |||
| FFP | 1.00 (0.06) | 0.95 (0.06) | 0.05 (0.08) |
| Platelets | 0.65 (0.03) | 0.64 (0.03) | 0.01 (0.05) |
| Cryoprecipitate | 0.23 (0.03) | 0.21 (0.02) | 0.02 (0.04) |
| Inpatient complications | |||
| Primary outcome, number (%) of participants | |||
| Antibiotics for infectious complication | 319 (32%) | 322 (32%) | 0% |
| Stroke | 11 (1%) | 14 (1%) | 0% |
| Suspected MI | 2 (0%) | 4 (0%) | 0% |
| Gut infarction | 5 (1%) | 1 (0%) | 0% |
| AKI, stage 3 | 47 (5%) | 45 (4%) | 0% |
| Other complications, number of events/participant | |||
| Reoperation | 0.07 (0.01) | 0.08 (0.01) | –0.01 (0.01) |
| Reintubation | 0.05 (0.01) | 0.06 (0.01) | –0.01 (0.01) |
| Tracheostomy | 0.03 (0.01) | 0.03 (0.01) | 0.00 (0.01) |
| Mask CPAP | 0.10 (0.01) | 0.09 (0.01) | 0.01 (0.02) |
| Pneumothorax requiring chest drainage | 0.01 (0.00) | 0.01 (0.00) | 0.00 (0.00) |
| Pleural effusion requiring drainage | 0.06 (0.01) | 0.06 (0.01) | 0.00 (0.01) |
| Pacing | 0.09 (0.01) | 0.06 (0.01) | 0.03 (0.01) |
| SVT/AF requiring treatment | 0.35 (0.02) | 0.33 (0.02) | 0.02 (0.03) |
| VF/VT requiring intervention | 0.02 (0.01) | 0.01 (0.00) | 0.01 (0.01) |
| Low cardiac output | 0.03 (0.01) | 0.03 (0.01) | 0.01 (0.01) |
| Inpatient LOS, days/participant | |||
| CICU | 0.89 (0.11) | 0.86 (0.13) | 0.03 (0.17) |
| HDU | 2.75 (0.12) | 2.71 (0.12) | 0.04 (0.17) |
| Ward | 5.49 (0.15) | 5.68 (0.17) | –0.19 (0.22) |
| Another unit/hospital | 1.26 (0.18) | 1.36 (0.21) | –0.09 (0.28) |
| Blood saving techniques, number (%) of participants | |||
| Post-operative cell salvagea | 24 (2%) | 20 (2%) | 0% |
| Readmissions to hospital | |||
| LOS, days/participant | 1.39 (0.15) | 1.48 (0.16) | –0.09 (0.22) |
| ED attendances | |||
| Total ED visits, number/participant | 0.08 (0.01) | 0.07 (0.01) | 0.01 (0.01) |
| Outpatient appointments, number/participant | |||
| Cardiac surgery outpatient visits | 0.44 (0.02) | 0.51 (0.02) | –0.07 (0.03) |
| Cardiology outpatient visits | 0.28 (0.02) | 0.26 (0.02) | 0.02 (0.03) |
| Other outpatient visits | 0.17 (0.02) | 0.17 (0.02) | –0.01 (0.03) |
| Other health-care contacts, number/participant | |||
| GP at surgery | 1.99 (0.07) | 2.10 (0.08) | –0.11 (0.10) |
| GP at home | 0.43 (0.05) | 0.38 (0.03) | 0.05 (0.06) |
| Practice nurse | 1.55 (0.15) | 1.57 (0.13) | –0.02 (0.18) |
| District nurse | 2.40 (0.22) | 2.18 (0.21) | 0.22 (0.30) |
| Cost component | Randomised to restrictive threshold (n = 1000), mean cost (£) (SE) | Randomised to liberal threshold (n = 1003), mean cost (£) (SE) | Restrictive versus liberal threshold, mean cost (£) difference (SE) |
|---|---|---|---|
| Red blood cells | 208 (11) | 349 (13) | –141 (17) |
| Inpatient episode | |||
| Other blood products | 206 (12) | 199 (11) | 7 (16) |
| Complications and SAEs | 1694 (120) | 1663 (128) | 31 (175) |
| LOSa | 5274 (198) | 5318 (219) | –45 (295) |
| Blood saving techniques | 43 (8) | 36 (7) | 7 (10) |
| Regular medications | 26 (2) | 29 (2) | –3 (3) |
| Total | 7243 (286) | 7245 (322) | –2 (430) |
| Post discharge | |||
| Readmissions | 780 (87) | 765 (79) | 15 (117) |
| ED visits | 16 (2) | 12 (2) | 4 (3) |
| Outpatient appointments | 202 (6) | 219 (7) | –17 (9) |
| Other medical/social care | 376 (13) | 369 (17) | 7 (21) |
| Total | 1374 (92) | 1365 (83) | 9 (124) |
| Total costs | 8825 (310) | 8959 (340) | –134 (460) |
Inpatient resource use for red blood cells, LOS and complications reduces when we consider resource use from randomisation rather than from surgery (see Table 80 and Chapter 6, Table 37). The differences between the groups are similar for both analyses, but units of red blood cells transfused reduce by 0.6 units when we consider resource use from randomisation rather than from surgery, and CICU stay reduces by 0.25 days and HDU stay by 0.34 days. The number of complications experienced by participants also reduces, particularly for pacing, supraventricular tachycardia/atrial fibrillation requiring treatment, and low cardiac output.
Subgroup analyses
| ‘Low-risk’ stratum | ‘High-risk’ stratum | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Restrictive | Liberal | Restrictive vs. liberal | Restrictive | Liberal | Restrictive vs. liberal | ||||||||
| Mean costs (SE) | Mean QALYs (SE) | Mean costs (SE) | Mean QALYs (SE) | Mean cost difference (SE) | Mean QALY difference (SE) | ICER | Mean costs (SE) | Mean QALYs (SE) | Mean costs (SE) | Mean QALYs (SE) | Mean cost difference (SE) | Mean QALY difference (SE) | ICER |
| Isolated CABG | Other operation types | ||||||||||||
| £14,663 (£356), n = 408 | 0.1853 (0.0023) | £15,218 (£406), n = 408 | 0.1819 (0.0022) | –£555 (£540) | 0.0034 (0.0031) | Restrictive dominant (–£161,423) | £20,208 (£483), n = 592 | 0.1765 (0.0020) | £20,122 (£519), n = 595 | 0.1784 (0.0021) | £86 (£709) | –0.0019 (0.0029) | Liberal dominant (–£44,221) |
| < 75 years | ≥ 75 years | ||||||||||||
| £17,146 (£367), n = 714 | 0.1813 (0.0018) | £17,290 (£437), n = 680 | 0.1796 (0.0019) | –£144 (£571) | 0.0017 (0.0025) | Restrictive dominant (–£86,221) | £19,940 (£702), n = 286 | 0.1774 (0.0029) | £19,889 (£609), n = 323 | 0.1800 (0.0029) | £51 (£929) | –0.0026 (0.0040) | Liberal dominant (–£19,818) |
| No diabetes | Diet, oral medication or insulin-controlled diabetes | ||||||||||||
| £17,365 (£352), n = 802 | 0.1827 (0.0016) | £17,701 (£363), n = 802 | 0.1815 (0.0018) | –£336 (£506) | 0.0012 (0.0024) | Restrictive dominant (–£277,188) | £20,296 (£869), n = 198 | 0.1699 (0.0039) | £19,826 (£1035), n = 201 | 0.1729 (0.0039) | £469 (£1351) | –0.0029 (0.0056) | Liberal dominant (–£160,777) |
| No lung diseasea | Chronic pulmonary disease or asthmaa | ||||||||||||
| £17,648 (£330), n = 889 | 0.1833 (0.0016) | £18,150 (£399), n = 861 | 0.1806 (0.0017) | –£502 (£518) | 0.0028 (0.0022) | Restrictive dominant (–£181,346) | £20,325 (£1389), n = 111 | 0.1564 (0.0048) | £17,987 (£715), n = 142 | 0.1736 (0.0041) | £2338 (£1562) | –0.0172 (0.0064) | Liberal dominant (–£135,981) |
| eGFR > 60 ml/minute | eGFR ≤ 60 ml/minute | ||||||||||||
| £17,342 (£381), n = 715 | 0.1820 (0.0017) | £17,293 (£400), n = 698 | 0.1824 (0.0018) | £48 (£552) | –0.0004 (0.0024) | Liberal dominant (–£117,537) | £19,460 (£661), n = 285 | 0.1757 (0.0031) | £20,077 (£729), n = 303 | 0.1735 (0.0032) | –£617 (£984) | 0.0022 (0.0044) | Restrictive dominant (–£275,536) |
| Males | Females | ||||||||||||
| £17,982 (£421), n = 693 | 0.1836 (0.0019) | £18,367 (£468), n = 680 | 0.1823 (0.0020) | –£386 (£629) | 0.0012 (0.0026) | Restrictive dominant (–£314,941) | £17,863 (£519), n = 307 | 0.1728 (0.0026) | £17,622 (£511), n = 323 | 0.1741 (0.0028) | £241 (£728) | –0.0013 (0.0039) | Liberal dominant (–£183,713) |
| Good ventricular function | Moderate or poor ventricular function | ||||||||||||
| £17,667 (£371), n = 787 | 0.1831 (0.0016) | £17,582 (£404), n = 771 | 0.1827 (0.0017) | £85 (£549) | 0.0004 (0.0022) | £210,032 | £18,854 (£706), n = 204 | 0.1711 (0.0039) | £20,287 (£785), n = 221 | 0.1700 (0.0041) | –£1432 (£1056) | 0.0011 (0.0054) | Restrictive dominant (–£1,332,993) |
Appendix 4 Transfusion Indication Threshold Reduction case report forms
Appendix 5 Statistical analysis plan
For further information on the statistical analysis plan, please see Pike et al. 34
List of abbreviations
- AE
- adverse event
- AKI
- acute kidney injury
- ARDS
- acute respiratory distress syndrome
- ASEPSIS
- additional treatment, serious discharge, erythema, purulent exudate, separation of deep tissues, isolation of bacteria, stay duration as inpatient
- BNF
- British National Formulary
- CABG
- coronary artery bypass graft
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CICU
- cardiac intensive care unit
- CPAP
- continuous positive airway pressure
- CPB
- cardiopulmonary bypass
- CRF
- case report form
- CT
- computerised tomography
- DMEC
- Data Monitoring and Ethics Committee
- ECG
- echocardiogram
- ED
- emergency department
- eGFR
- estimated glomerular filtration rate
- eMIT
- electronic marketing information tool
- EQ-5D-3L
- European Quality of Life-5 Dimensions-3 Level
- EuroSCORE
- European System for Cardiac Operative Risk Evaluation
- FFP
- fresh frozen plasma
- GI
- gastrointestinal
- GMR
- geometric mean ratio
- GP
- general practitioner
- HCHS
- Hospital and Community Health Services
- HDU
- high-dependency unit
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IQR
- interquartile range
- ITT
- intention to treat
- IV
- instrumental variable
- LOS
- length of stay
- MedDRA
- Medical Dictionary for Regulatory Activities
- MI
- myocardial infarction
- MRI
- magnetic resonance imaging
- NHSBT
- NHS Blood and Transplant
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PIL
- patient information leaflet
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- risk ratio
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SE
- standard error
- TITRe2
- Transfusion Indication Threshold Reduction
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale