Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 09/119/01. The protocol was agreed in July 2014. The assessment report began editorial review in May 2015 and was accepted for publication in October 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
We would like to acknowledge that Stephen Marks received grants from Astellas and Novartis for immunosuppressive randomised controlled studies in paediatric renal transplant recipients during the conduct of the study. In addition, Jan Dudley was a member of an expert review panel in January 2008 and developed consensus recommendation on the optimal use of CellCept® (Roche Products) in paediatric renal transplantation and received an honorarium for Roche for this work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Haasova et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
The aim of this assessment is to review and update the evidence of the clinical effectiveness and cost-effectiveness of immunosuppressive regimens for renal transplantation in children and adolescents [a review of National Institute for Health and Care Excellence (NICE) guidance TA99]. 1 Two therapy stages are assessed: induction therapy [regimens including basiliximab (BAS) (Simulect,® Novartis Pharmaceuticals) or rabbit antihuman thymocyte immunoglobulin (r-ATG) (Thymoglobuline,® Sanofi)] and maintenance therapy [regimens including immediate-release tacrolimus (TAC-IR) [Adoport® (Sandoz); Capexion® (Mylan), Modigraf® (Astellas Pharma); Perixis® (Accord Healthcare); Prograf® (Astellas Pharma); Tacni® (Teva); Vivadex® (Dexcel Pharma)], prolonged-released tacrolimus (TAC-PR) (Advagraf,® Astellas Pharma), belatacept (BEL) (Nulojix,® Bristol-Myers Squibb), mycophenolate mofetil (MMF) [Arzip® (Zentiva), CellCept® (Roche Products), Myfenax® (Teva), generic MMF is manufactured by Accord Healthcare, Actavis, Arrow Pharmaceuticals, Dr Reddy’s Laboratories, Mylan, Sandoz and Wockhardt], mycophenolate sodium (MPS) (Myfortic,® Novartis Pharmaceuticals), sirolimus (SRL) (Rapamune,® Pfizer) and everolimus (EVL) (Certican,® Novartis Pharmaceuticals), alone or in combination].
The systematic review and economic evaluation developed to support the current NICE guidance TA99 was published by Yao et al. in 2006. 2 This assessment incorporated relevant evidence presented in the previous report and report new evidence.
Description of health problem
End-stage renal disease
Chronic kidney disease (CKD) in childhood leads to lifelong health complications, often resulting in the need of a kidney transplant. 3 In 2013, 891 children and adolescents < 18 years of age were receiving treatment at paediatric nephrology centres for end-stage renal disease (ESRD). 4 ESRD is a long-term irreversible decline in kidney function, for which renal replacement therapy (RRT) is required if the individual is to survive. ESRD is often the result of an acute kidney injury or primarily a progression from CKD, which describes abnormal kidney function and/or structure. Although RRT can take a number of forms (kidney transplantation, haemodialysis and peritoneal dialysis), the preferred option for people with ESRD is kidney transplantation, rather than dialysis, owing to improved duration and quality of life with transplantation compared with dialysis. 5
Transplantation
Kidney transplantation is the transfer of a healthy kidney from a donor to a recipient. Kidneys for transplantation may be obtained via living donation (related or unrelated), donation after brain death (DBD; those with deceased heart-beating who are maintained on a ventilator in an intensive care unit, with death diagnosed using brain stem tests) or donation after circulatory death [DCD; non-heart-beating donors who cannot be diagnosed as brainstem dead but whose death is verified by the absence of a heart beat (cardiac arrest)].
Children and adolescents represent a distinct group of transplant recipients and can differ from adults in several important aspects, including the cause of established renal failure, the complexity of the surgical procedure, the metabolism and pharmacokinetic properties of immunosuppressants, the developing immune system and immune response following organ transplantation, the measures of success of the transplant procedure, the number and the degree of comorbid conditions, the susceptibility to post-transplant complications, and the degree of adherence to treatment. 6,7 The metabolism of many immunosuppressive medications substantially differs in young children compared with adults and drug metabolism changes as children grow and develop.
Following kidney transplantation, major clinical concerns for children and adolescents are acute kidney rejection, graft loss and diminished growth. Acute kidney rejection occurs when the immune response of the graft recipient attempts to destroy the graft as the graft is deemed foreign tissue. 5 Therefore, immunosuppressive therapy is implemented to reduce the risk of kidney rejection and prolong survival of the graft. Prior to renal transplantation, growth retardation in children and adolescents with CKD may already be an issue owing to a combination of inadequate nutritional intake, acidosis, renal osteodystrophy and alterations to the growth hormone insulin-like growth factor. 8 However, post transplant, the steroidal therapy often included in immunosuppression regimens can affect longitudinal growth and calcium/phosphorous metabolism. 9,10
Aetiology, pathology and prognosis
Aetiology
In children, ESRD is usually due to innate structural abnormalities or genetic causes or is acquired in childhood through glomerulonephritis. 11 Figure 1 displays the causative diagnoses for children and adolescents (< 16 years old) with primary renal disease in 2013.
FIGURE 1.
Causative diagnoses for children and adolescents; primary renal disease percentage in incident and prevalent children and adolescents with established renal failure patients < 16 years old in 2013. Reproduced with permission from UK Renal Registry 17th Annual Report (figure 4.3, p. 99). 4 The data reported here have been supplied by the UK Renal Registry of the Renal Association. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the UK Renal Registry or the Renal Association.

Pathology
Table 1 displays the distribution of the UK primary renal diagnosis for end-stage renal failure over time, reported from 1999 to 2003, 2004 to 2008 and 2008 to 2013 in children and adolescents aged < 16 years. Renal dysplasia, which is abnormal tissue development in the kidney, is the primary renal disease diagnosis in approximately one-third of all children and adolescents with ESRD.
| Primary renal diagnosis | 1999–2003 | 2004–8 | 2009–13 | 1999–2013 | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | % change | |
| Renal dysplasia + reflux | 157 | 29.1 | 191 | 33.7 | 182 | 33.7 | 4.6 |
| Obstructive uropathy | 80 | 14.8 | 75 | 13.3 | 97 | 18 | 3.1 |
| Glomerular disease | 130 | 24.1 | 112 | 19.8 | 83 | 15.4 | –8.7 |
| Tubulointerstitial diseases | 42 | 7.8 | 46 | 8.1 | 41 | 7.6 | –0.2 |
| Congenital nephrotic syndrome | 27 | 5 | 33 | 5.8 | 35 | 6.5 | 1.5 |
| Metabolic | 29 | 5.4 | 25 | 4.4 | 31 | 5.7 | 0.4 |
| Uncertain aetiology | 12 | 2.2 | 32 | 5.7 | 29 | 5.4 | 3.1 |
| Renovascular disease | 23 | 4.3 | 19 | 3.4 | 19 | 3.5 | –0.7 |
| Polycystic kidney disease | 16 | 3 | 19 | 3.4 | 19 | 3.5 | 0.6 |
| Malignancy and associated disease | 10 | 1.9 | 9 | 1.6 | 4 | 0.7 | –1.1 |
| Drug nephrotoxicity | 14 | 2.6 | 5 | 0.9 | 0 | 0 | –2.6 |
When chronic renal failure occurs, children and adolescents may experience malaise, nausea, loss of appetite, change in mental alertness, bone pain, headaches, stunted growth, change in urine outputs, urinary incontinence, pale skin, bad breath, poor muscle tone, tissue swelling and hearing deficit. Treatment of chronic renal failure depends on the degree of kidney function that remains and the age of the child/adolescent. Treatment may include dialysis, kidney transplantation, diet restrictions, diuretic therapy and medications (to help with growth and prevent bone density losses). 12
Acute rejection
In patients who survive transplantation, acute rejection (AR) may occur when the immune response of the host attempts to destroy the graft as the graft is identified as foreign tissue. 5 AR is treated by modifying the immunosuppressive regimen (increasing doses or switching treatments). Untreated AR will ultimately result in destruction of the graft; however, high levels of immunosuppression may also increase the risk of other infections and malignancy. 5 AR is primarily measured following a biopsy and graded according to Banff criteria (grades I–III, for which grade III indicates the most severe). The Banff classification13 is:
-
Banff grade I: tubulointerstitial inflammation only.
-
Banff grade IA: interstitial inflammation moderate–severe and/or tubulitis moderate.
-
Banff grade IB: tubulitis severe.
-
Banff grade II: intimal arteritis.
-
Banff grade IIA: intimal arteritis mild–moderate.
-
Banff grade IIB: intimal arteritis severe.
-
Banff grade III: transmural arteritis and/or fibrinoid necrosis.
Although the incidence of AR following a transplant is included in this appraisal, its treatment is outside the scope. In addition to AR affecting the survival of the graft, other reasons which may instigate graft loss include blood clots, narrowing of an artery, fluid retention around the kidney, side effects of other medications and recurrent kidney disease. 14
It is important to note that failing to stay on the immunosuppression regime prescribed following a kidney transplant will also significantly increase the risk of AR and/or graft loss. 15 If the kidney is lost, ultimately the patient will need to return/start on dialysis, for which the quality of life is reduced and overall costs are higher. 5
Graft function
Glomerular filtration rate (GFR) describes the flow rate of filtered fluid through the kidney. GFR is expressed in terms of volume filtered per unit time [sometimes this is also expressed per average surface area (1.73 m2)]. There are various methods used to calculate GFR [estimated glomerular filtration rate (eGFR)] from serum creatinine levels, age, sex and ethnic group (e.g. Modification of Diet in Renal Disease, Cockcroft–Gault, and Nankivell). Different methods are used for children and adolescents (e.g. Schwartz and Counahan–Barratt equations). Levels of eGFR represent the level of kidney function and Table 2 presents the NICE cut-off values for classification of CKD (NICE guidelines CG182). 16 These values apply to children aged > 2 years and up to (and including) adulthood. 17
| GFR category | GFR (ml/minute/1.73 m2) | Terms |
|---|---|---|
| 1 | > 90 | Normal or high |
| 2 | 60–89 | Mildly decreased |
| 3a | 45–59 | Mildly to moderately decreased |
| 3b | 30–44 | Moderately to severely decreased |
| 4 | 15–29 | Severely decreased |
| 5 | < 15 | Kidney failure |
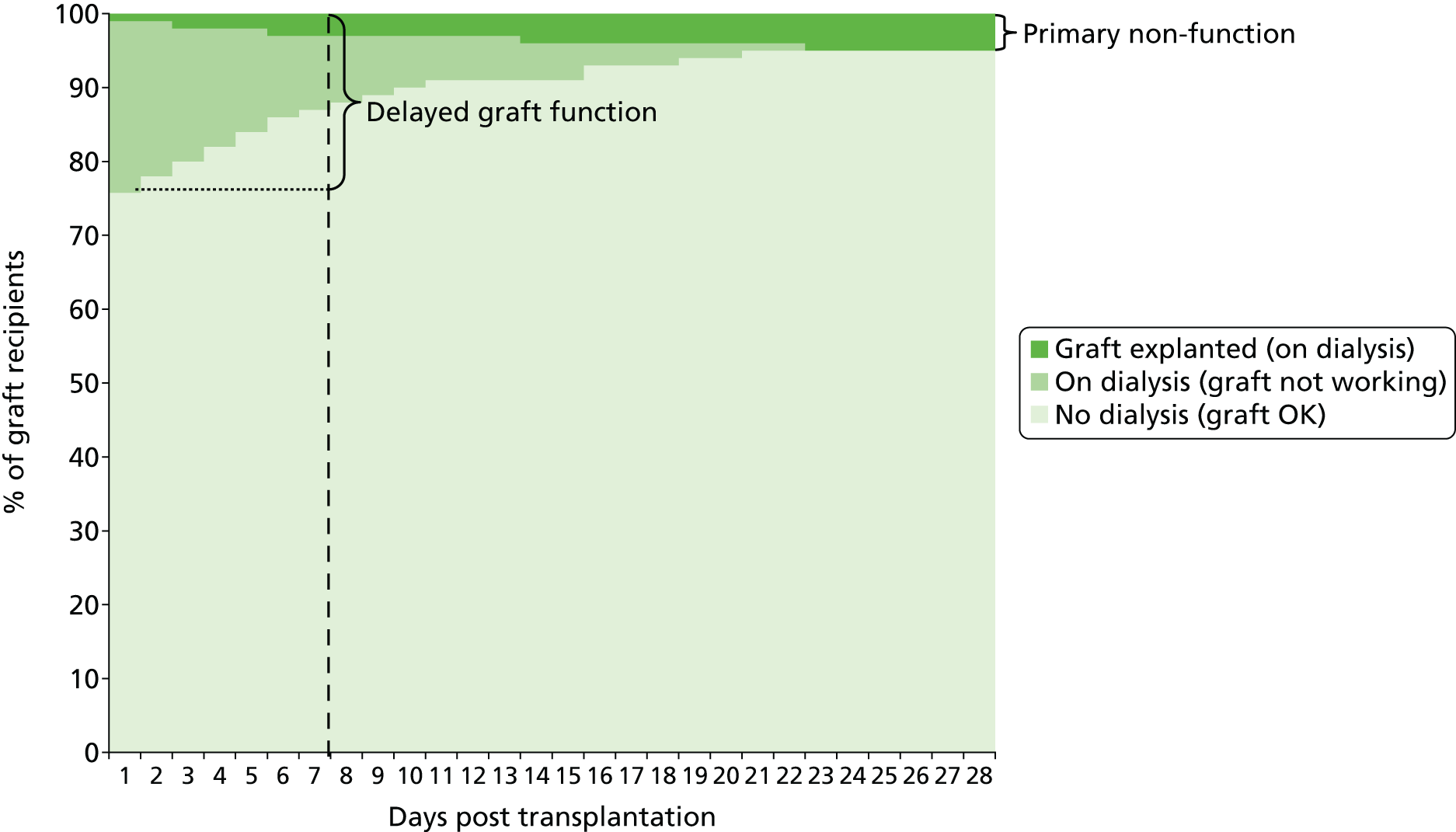
Some children and adolescents may experience delayed graft function (DGF) after transplantation and Figure 2 shows a hypothetical graph to explain the relationship between normally functioning grafts, DGF and primary non-functioning (PNF) grafts. At 7 days post transplant, some of the children and adolescents who need dialysis and whose grafts are therefore classified as DGF will have grafts that never function. When this has been established, these grafts are classified as PNF.
FIGURE 2.
Hypothetical graph to explain graft function, DGF and primary non-functioning graft. Reproduced with permission from Bond et al. 18 Contains information licensed under the Non-Commercial Government Licence v1.0.
Growth
Normal growth is often affected in children and adolescents with ESRD; short stature is diagnosed if the height standard deviation score (SDS) is < 2.5 of the target height. 19 There are three main factors that may impact post-transplant growth:
-
Age at transplantation. Following a transplant, post-transplantation catch-up growth is not uncommon; however, it is unlikely to be sufficient to compensate for the pre-transplant accrued deficit. 20 Data from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) indicated that children < 6 years of age exhibit catch-up growth whereas children > 6 years at the time of transplantation exhibit limited to no catch-up growth.
-
Allograft function. An increase of 1.0 mg/dl in serum creatinine level (indicating a decrease in kidney function) has been associated with a decrease of 0.17 in SDS. 21
-
Corticosteroid (CCS) dose. For example, reducing steroids to every other day22 and withdrawing or avoiding steroids23 have been associated with improved growth. Similarly, Grenda et al. 24 reported an increase of 0.13 in SDS in a group of primarily pre-pubertal children who withdrew from steroids on day 5 compared with those in whom the dose was tapered to 10 mg/m2.
UK data are not available on growth changes following kidney transplant in children and adolescents; however, data from the NAPRTCS are available. The NAPRTCS 2010 annual report indicates that at transplantation, the mean height deficits for all children and adolescents is –1.75 SDS (–1.78 for boys and –1.70 for girls). 25 For children and adolescents who have reached their adult height following kidney transplant (n = 2867), the average SDS is –1.40, with 25% having a SDS of –2.2 or worse and 10% are > 3.24 SDS below the population average. 25 In addition, German data reported by Nissel et al. ,26 who followed 37 children for a mean duration of 8.5 years to monitor their growth, showed that those children who received their transplant before the start of puberty attained an adult height that was on average 5.2 cm (boys) and 13.0 cm (girls) lower than predicted while those who received their transplant after the onset of puberty had a final adult height that was on average 12.6 cm (both boys and girls) lower than the target.
Prognosis
Data collected for survival rates of children and adolescents < 16 years of age starting RRT between 1999 and 2012 were collected from UK paediatric centres. 4 The median follow-up time was 3.5 years (ranging from 1 day to 15 years). There were a total of 99 deaths reported. Table 3 shows the survival hazard ratios (HRs) (following adjustment for age at start of RRT, sex and RRT modality) and highlights that children starting RRT at < 2 years of age, compared with 12- to 16-year-olds starting RRT, had a worse survival outcome with a HR of 5.0.
| Hazard ratio | Confidence interval | p-value | |
|---|---|---|---|
| Age | |||
| 0–< 2 years | 5.0 | 2.8–8.8 | < 0.0001 |
| 2–< 4 years | 2.9 | 1.4–5.7 | 0.003 |
| 4–< 8 years | 2.2 | 1.3–4 | 0.006 |
| 8–< 12 years | 1.4 | 0.7–2.9 | 0.400 |
| 12–< 16 years | 1.0 | – | – |
| Sex | |||
| Female | 1.2 | 0.7–1.9 | 0.5 |
| Male | 1.0 | – | – |
| Modality | |||
| Dialysis | 7.1 | 4.7–10.7 | < 0.0001 |
| Transplant | 1.0 | – | – |
Various factors may influence survival following a kidney transplant. A study of 1189 child/adolescent kidney transplants in England between April 2001 and March 2012 found that 33 children and adolescents did not survive. 27 The most common causes of these 33 deaths were renal (n = 8; classified as ESRD, renal dysplasia and disorder of kidney/ureter), infections (n = 6) and malignancy (n = 5). 27 The age of the recipient was not found to significantly impact patient survival: age 0–1 years (100% survival), age 2–5 years (96% survival), age 6–12 years (97.5% survival) and age 13–18 years (97.4% survival). 27
Important prognostic factors
A number of important factors have been identified within the research literature that may influence overall survival and graft survival. These factors are summarised below:
-
Age: both the age of the recipient and the age of the donor will influence the survival of the transplant. The number of kidney transplants performed is much lower in infants and small children than in older children. This has been attributed to some centres keeping a child on dialysis until they reach an arbitrary age when they are deemed suitable for a transplant. 28
-
Recipient ethnicity: black patients tend to have worse graft function, shorter graft survival and higher rates of chronic allograft nephropathy than white patients. 29 Racial differences have also been indicated in American children, with poorer outcomes in black children following a kidney transplant than in white or Hispanic children. 30
-
Waiting time to transplant: the longer a person is on dialysis waiting for a kidney transplant, the poorer their outcomes post transplantation. 31
-
Cold ischaemia time: the shorter this time (≤ 20 hours), the better the immediate and long-term outcomes. 32
-
Donor type: receiving a donated kidney from a live donor will probably result in better outcomes than receiving a kidney from a deceased donor. 29 Similarly, receiving a kidney from extended criteria donors (donors who may for example be older, have a history of diabetes mellitus or hypertension or have an increased risk of passing on an infection or malignancy) will have inferior graft survival rates and increased incidences of AR when compared with receiving a standard donated kidney. 33
-
Immunological risk, to include human leucocyte antigen (HLA) and blood group incompatibility: if the number of mismatches from the donor to the recipient are higher, there is an increased likelihood of AR and graft loss. 29
-
Comorbidities, for example diabetes mellitus, cancer and cardiovascular disease (CVD): the higher a patient score on the Charlson Comorbidity Index, the lower the patient and graft survival is likely to be. AR is not significantly correlated to the Charlson Comorbidity Index. 34
Incidence and/or prevalence
In 2013, 891 children and young people < 18 years of age were receiving treatment for ESRD at UK paediatric nephrology centres, of whom 80.2% had a functioning kidney transplant, 11.7% were receiving haemodialysis and 8.1% were receiving peritoneal dialysis. 4 When comparing RRT data from the most recent 5-year period (2009–13) with the two previous periods (1999–2003 and 2004–8), a sustained increase in the number of younger children (aged 0 to < 8 years when starting RRT) can be seen, while the number of older children (8 to < 16 years when starting RRT) has decreased. Consequently, the total number of children starting RRT has remained relatively constant; 546 children between 1999 and 2003, 575 children between 2004 and 2008, and 560 children between 2008 and 2013. 4
Table 4 presents the number of children and adolescents commencing RRT in 2013 with data presented by age and by sex.
| Age group | All patients n (pmarp) | Male n (pmarp) | Female n (pmarp) | M : F ratio |
|---|---|---|---|---|
| 0–< 2 years | 19 (11.8) | 13 (15.7) | 6 (7.6) | 2.1 |
| 2–< 4 years | 17 (10.6) | 11 (13.4) | 6 (7.6) | 1.7 |
| 4–< 8 years | 14 (4.5) | 4 (2.5) | 10 (6.6) | 0.4 |
| 8–< 12 years | 31 (11.0) | 20 (13.9) | 11 (8.0) | 1.7 |
| 12–< 16 years | 31 (10.7) | 12 (8.1) | 19 (13.4) | 0.6 |
| Under 16 years | 112 (9.3) | 60 (9.7) | 52 (8.8) | 1.1 |
Although the number of children and adolescents starting RRT has not changed significantly, the number of children and adolescents actively waiting for a kidney transplant fell from 112 in 2005 to 70 in 2014. Figure 3 displays the number of children and adolescents on the transplant list both active and suspended over time from 2005 to March 2014 (when suspension from the list may occur if the transplant cannot go ahead, e.g. further medical problems making the operation unsafe).
FIGURE 3.
Children and adolescents on the kidney-only transplant waiting list at March 2013. Reproduced with permission from NHS Blood and Transplant. 32

One hundred and twenty five kidney transplant operations were performed on children and adolescents in the UK between April 2013 and March 2014. 32 The total number of transplants in children and adolescents and the graft type (living, DBD and DCD) performed each year from 2004 to 2014 are displayed in Figure 4. In children and adolescents, most donated kidneys are from living and DBD donors, with very few kidneys being form DCD donors.
FIGURE 4.
Kidney-only transplants in children and adolescents 2004–2014. Reproduced with permission from NHS Blood and Transplant. 32

Overall survival reported in children and adolescents following kidney transplants from deceased and living donors is similar at both 1- and 5-year follow-up; however, graft survival at 5 years is improved if the donors are living (Table 5). 32
| Kidney graft survival | Patient survival | |||
|---|---|---|---|---|
| 1 year,a % (95% CI) | 5 years,b % (95% CI) | 1 year,a % (95% CI) | 5 years,b % (95% CI) | |
| Deceased donors | 96 (93 to 98) | 84 (79 to 88) | 99 (97 to 100) | 99 (96 to 100) |
| Living donors | 95 (92 to 97) | 94 (89 to 96) | 99 (97 to 100) | 99 (96 to 100) |
Data on incidence and prevalence of AR in children and adolescents are not available for the UK. However, they are likely to be similar to those reported in the NAPRTCS, which indicates that for transplants occurring between 1987 and 2010, the prevalence in children and adolescents of at least one episode of AR following a kidney transplant is 46% (41% in live donors and 51% in deceased donors). 25
Impact of kidney transplantation
Significance for patients
Living with ESRD may substantially challenge the well-being of children and adolescents. Not only will the disease impact physical health, but mental and social health may also be affected owing to increased hospital visits and the child or adolescent’s inability to take part in the same activities as their peers. 35 However, having a kidney transplant will improve the symptoms associated with ESRD and dialysis and reduce the time spent in hospital. 36 The median wait time for a child/adolescent requiring a kidney transplant in the UK is 342 days. 32
Kidney transplantation requires a lifelong regimen of immunosuppressive medication. Immunosuppressants may produce unpleasant side effects (including possible skin cancer, crumbling bones, fatigue, body hair growth, swollen gums and weight gain). 37 Nevertheless, favourable social and professional outcomes have been observed from a long-term follow-up (15.6 years ± 3 years) of people who had a kidney transplant as a child (aged 10 years ± 5 years). 38 Adherence to post-transplant immunosuppressive regimens is important for favourable clinical outcomes in children and adolescents39 and has been suggested as a core strategy to improve clinical outcomes. 40 In addition, failing to follow treatment may result in an increase in medical costs. 41
Acute rejection is common in the first year after kidney transplantation and treatment of AR involves a more intensive drug treatment than standard maintenance regimens, which in turn increases the possibility of adverse events (AEs). Should a graft be lost, the child/adolescent will face another wait for transplantation (if appropriate) and will need to undergo dialysis while waiting for transplantation (although a pre-emptive transplantation may be available), or need to undergo dialysis for life where transplantation is not possible.
The impact on a child/adolescent returning to or starting dialysis (of the psychological burden of graft failure and going back to a previous treatment) is little researched, but necessarily includes the impact of being on dialysis per se: dialysis is time-consuming and may affect education and normal family life and require changes in diet and fluid intake. Common side effects of dialysis (either haemodialysis or peritoneal dialysis) include fatigue, low blood pressure, invasive staphylococcal infections, muscle cramps, itchy skin, peritonitis, hernia and weight gain. 42
Finally, growth retardation in children and adolescents with ESRD is thought to be a combination of inadequate nutritional intake, acidosis, renal osteodystrophy and alterations to the growth hormone insulin-like growth factor. 8 Ensuring optimal growth or optimisation of final height is a major concern for children and adolescents with ESRD, as short stature may have an impact on social development, self-esteem and quality of life and is associated with an increase in the number of hospitalisations and behavioural and cognitive disorders, and a decrease in the level of education and employment in adulthood. 20,43–45
Unfortunately, data relating specifically to quality of life are currently available only in the adult population, among whom there are clear quality-of-life improvements from having a functioning kidney transplant compared with being on dialysis. 46–52
Significance for the NHS
Treatment for ESRD is considered resource-intensive for the NHS because current costs have been estimated to use 1–2% of the total NHS budget to treat 0.05% of the population (both adult and child/adolescent). 53 Based on data from the Department of Health, it is estimated that in 2008/9, the total expenditure on ‘renal problems’ in England was £1.3B, representing 1.4% of the NHS expenditure. 53 An economic evaluation of treatments for ESRD by de Wit et al. 54 showed that transplantation is the most cost-effective form of RRT with increased quality of life and independence for an individual.
There are no apparent reasons why RRT demand may dramatically increase in children and adolescents. However, it is projected that an increasingly overweight population will increase the demand for RRT, with a consequent increase in pressure on services from renal units and other health-care providers dealing with comorbidities. Increased resources may be needed for dialysis, surgery, pathology, immunology, tissue typing, histopathology, radiology, pharmacy and hospital beds. Demand is likely to be particularly significant in areas where there are large South Asian, African and African Caribbean communities and in areas of social deprivation, where people are more susceptible to kidney disease. 4
Measurement of disease
The outcome of kidney transplants (and of the success of immunosuppressive regimens) can be measured in a variety of ways. These include:
Short term
-
Immediate graft function: the graft works immediately following transplantation, removing the need for further dialysis.
-
Delayed graft function: the graft does not work immediately and dialysis is required during the first week post transplant. Dialysis has to continue until graft function recovers sufficiently to make it unnecessary. This period may last up to 12 weeks in some cases.
-
Primary non-function: the graft never works after transplantation.
Long term
-
Rejection rates: the percentage of grafts that are rejected by the recipients’ bodies; rejection can be acute or chronic.
-
Graft survival: the length of time that a graft functions in the recipient.
-
Graft function: a measure of the efficiency of the graft by various markers, for example GFR and serum creatinine levels.
-
Patient survival: how long the recipient survives.
-
Quality of life: how a person’s well-being is affected by the transplant.
Current service provision
Management of end-stage kidney disease
End-stage renal disease is primarily managed by RRT. The patient pathway leading to RRT for those with ESRD can be seen in Figure 5. Once a child/adolescent has been diagnosed with ESRD, the RRT options are a transplant (from a living or deceased donor) or dialysis (haemodialysis and peritoneal dialysis). If suitable, the option of a pre-emptive kidney transplant (when transplantation is performed without the child/adolescent spending any time on dialysis) is also available.
FIGURE 5.
The care pathway for RRT. Reproduced with permission from The National Service Framework for Renal Services. Part One: Dialysis and Transplantation. 11 © Crown Copyright 2004. Contains public sector information licensed under the Open Government Licence v3.0.

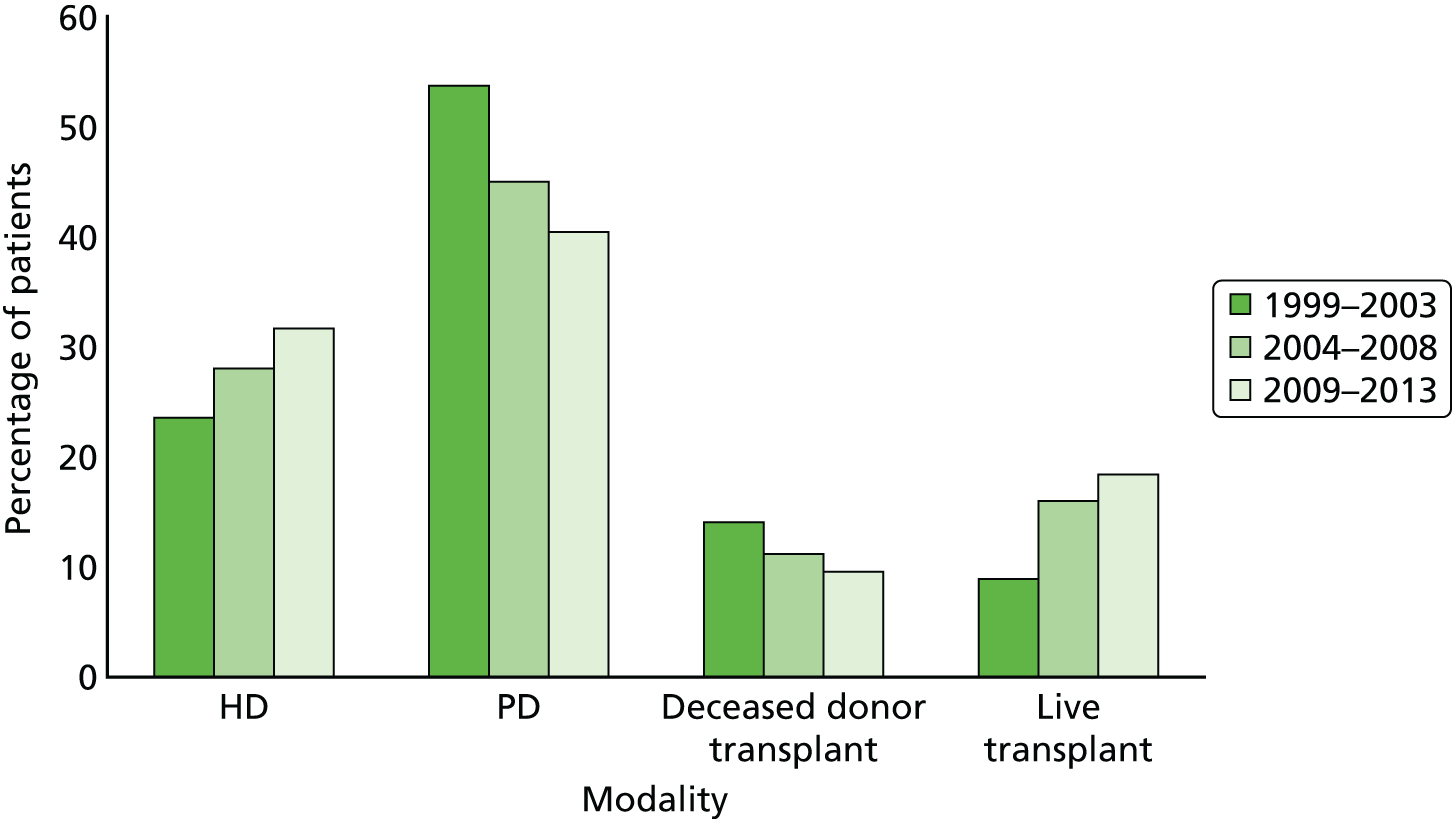
The form of treatment modality at the start of RRT changed from 1999 to 2013 (Figure 6). The primary changes are an increase in the number of kidney transplants from living donors and a simultaneous decrease in donations from deceased donors. In addition, an increase in haemodialysis and a concurrent decrease in peritoneal dialysis are seen (see Figure 6).
FIGURE 6.
Type of treatment at start of RRT for incident children and adolescents < 16 years old by 5-year time period. HD, haemodialysis; PD, peritoneal dialysis. Reproduced with permission from UK Renal Registry 17th Annual Report (figure 4.4. p. 102). 4 The data reported here have been supplied by the UK Renal Registry of the Renal Association. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the UK Renal Registry or the Renal Association.
The 2013 data suggest that most children and adolescents receive a kidney transplant (78%) and that the proportion of living and deceased kidney donations is equal: 50% and 50%, respectively (Figure 7).
FIGURE 7.
Renal replacement therapy treatment used by prevalent children and adolescents < 16 years old in 2013. HD, haemodialysis; PD, peritoneal dialysis. Reproduced with permission from UK Renal Registry 17th Annual Report (figure 4.1. p. 98). 4 The data reported here have been supplied by the UK Renal Registry of the Renal Association. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the UK Renal Registry or the Renal Association.

Management of kidney transplants
If transplantation is the chosen method for RRT for a child/adolescent with ESRD then there are three main service provision steps required for the management of the transplant.
The first of these is organ procurement, which includes the identification and management of potential donors and assessment of donor suitability. HLAs are carried on cells within the body, enabling the body to distinguish between its ‘self’ or to recognise ‘non-self’ that should be attacked. The closer the HLA matching, the less vigorously the body will attack the foreign transplant and, consequently, the chances of graft survival are improved. HLA mismatch refers to the number of mismatches between the donor and the recipient at the A, B and DR loci, with a maximum of two mismatches at each locus. 32 Therefore, a match would have a score of zero and a complete mismatch would have a score of six. However, it should be noted that with the improvements in immunosuppressants, the significance of HLA matching has diminished. 55
The second step is the provision of immunosuppressive therapy. Immunosuppressants are the drugs taken around the time of, and following, an organ transplant. They are aimed at reducing the body’s ability to reject the transplant and thus at increasing patient and graft survival and preventing acute and/or chronic rejection (while minimising associated toxicity, infection and malignancy). Immunosuppressants are required in some form for all kidney transplant recipients (KTRs), except potentially when the donor is an identical twin.
The final service provision step is short- and long-term follow-up following transplantation. This step involves looking for indications of any kidney graft dysfunction and other complications. Complications fall into four categories.
-
Medical follow-ups to include rejections, nephrotoxicity of calcineurin inhibitors (CNIs) and recurrence of the native kidney diseases.
-
Anatomic complications of surgery to include renal artery thrombosis, renal artery stenosis, urine leaks from disruption of the anastomosis, ureteral stenosis and obstruction and lymphocele.
-
Other complications include infection, malignancy, new-onset of diabetes mellitus, liver disease, hypertension, CVD.
-
Ensuring growth is not impeded and maximal ‘catch-up’ growth is achieved. The 2010 NAPRTCS report suggests that the average final adult height of a renal transplant recipient has increased significantly from –1.93 SDS between 1987 and 1991 to –0.94 SDS between 2002 and 2010. 25
If the kidney loses its function, many of the physiological changes that occur mimic those seen with progressive renal diseases from other causes. Therefore, these symptoms should be managed in a similar way to the non-transplant population. However, it should be noted that the loss of a kidney transplant carries increased susceptibility to bruising and infection compared with pre-transplant kidney failure. 56
Once the kidney is confirmed to have been lost, the graft may or may not need to be surgically removed. The decision of whether or not the graft is removed is often made on a case-by-case basis taking into consideration all perceived benefits and risks. The immunosuppression regimen can then be tapered and withdrawn while the patient returns to dialysis and waits for a new kidney to become available.
Current service cost
The overall cost of CKD to the NHS in England was estimated as £1.45B in 2009–10, with more than half of total estimated expenditure for RRT. 57 The costs of RRT can be divided into costs associated with the transplantation and costs associated with dialysis. Transplantation costs can include the cost of workup for transplantation (assessing recipient suitability), maintaining and co-ordinating the waiting list, obtaining donor kidneys (harvesting, storage and transport for deceased donors; nephrectomy procedure for living donors), cross-matching for donor–recipient compatibility, the transplantation procedure, induction immunosuppression, hospital inpatient stay following procedure, initial and long-term maintenance immunosuppression, prophylaxis and monitoring for infections, monitoring of graft function and general health, adjustment of immunosuppressant dosages, treatment of AR, and treatment of associated AEs. Should the kidney be lost, the costs of restarting dialysis (dialysis costs, the cost of treatment for AEs attributable to dialysis and the cost of dialysis access surgery) would be incurred.
Data from the NHS Reference Costs 2013 to 2014 indicated that the cost of kidney transplantation in those < 19 years of age is, on average, £20,576. 58 Paediatric nephrology outpatient clinics are, on average, £249 and the cost of haemodialysis and peritoneal dialysis is, on average, £79,807 and £41,382, respectively. 58
Variation in services
There are currently 13 paediatric renal centres in the UK, nine that offer dialysis and perform transplantations [Birmingham, Bristol, Glasgow, Leeds, London (Guys and Great Ormond Street), Nottingham, Belfast and Manchester] and four that offer renal care but not transplantations (Cardiff, Liverpool, Newcastle and Southampton).
After kidney transplantation, recipients are prescribed an immunosuppression regimen consisting of both induction and maintenance therapy. Following this, they are offered check-up appointments with their clinic (consultant nephrologist) to monitor general health, kidney function, immunosuppressive drugs, infections (prophylaxis and treatment) and to address any social or psychological concerns. The Renal Association Guidelines59 suggest the following frequency of clinic appointments:59
-
two to three times weekly for the first month after transplantation
-
one to two times weekly for months 2 to 3 after transplantation
-
every 1 to 2 weeks for months 4 to 6 after transplantation
-
every 4 to 6 weeks for months 6 to 12 after transplantation
-
once every 3–6 months thereafter
-
detailed annual post-operative reviews.
Clinician estimations of average frequency of outpatient visits have been reported as 34.3, 6.3 and 4.7 visits for the first, second and third years post transplant, respectively, with UK database figures suggesting 39.7, 11.0 and 9.2 visits for the first, second and third years post transplant, respectively. 60
Service provision (clinic appointments or other services) is likely to increase if AR occurs (possibly requiring hospital admission and escalating treatment) and when there is declining graft function (which might necessitate more regular clinic visits, blood tests and other investigations and changes to treatment regimens). Patients may also present to their general practitioner (GP) or accident and emergency department with AEs related to kidney transplantation or immunosuppressive regimen and this may be followed by an additional referral to the consultant nephrologist or other appropriate specialist (e.g. renal dietitian), followed by management as required (e.g. additional prescribing and monitoring).
In addition to these services, The Renal Association Guidelines59 also recommend that recipients of a transplant should have the following:59
-
online access to their results via the ‘Renal Patient View’ service
-
open access to the renal transplant outpatient service
-
an established point of contact for enquiries
-
access to patient information (which should be available in both written and electronic formats).
Current National Institute for Health and Care Excellence guidance
Current NICE guidance on immunosuppressive therapy for renal transplantation in children and adolescents (NICE technology appraisal guidance, TA99) has the following recommendations for induction and maintenance therapy. 1
Induction therapy
Basiliximab or daclizumab (DAC), used as part of a ciclosporin (CSA)-based immunosuppressive regimen, is recommended as an option for induction therapy in the prophylaxis of acute organ rejection in children and adolescents undergoing renal transplantation, irrespective of immunological risk. The induction therapy (BAS or DAC) with the lowest acquisition cost should be used, unless it is contraindicated. 1 The marketing authorisation for DAC has been withdrawn at the request of the manufacturer.
Maintenance therapy
Tacrolimus (TAC) is recommended as an alternative option to CSA when a CNI is indicated as part of an initial or a maintenance immunosuppressive regimen for renal transplantation in children and adolescents. The initial choice of TAC or CSA should be based on the relative importance of their side effect profiles for the individual patient. 1
Mycophenolate mofetil is recommended as an option as part of an immunosuppressive regimen for child and adolescent renal transplant recipients only when:
-
there is proven intolerance to CNIs, particularly nephrotoxicity which could lead to risk of chronic allograft dysfunction or
-
there is a very high risk of nephrotoxicity necessitating the minimisation or avoidance of a CNI until the period of high risk has passed. 1
The use of MMF in CCS reduction or withdrawal strategies for child and adolescent renal transplant recipients is recommended only within the context of randomised clinical trials. 1
Mycophenolate sodium is currently not recommended for use as part of an immunosuppressive regimen in child or adolescent renal transplant recipients. 1
Sirolimus is not recommended for children or adolescents undergoing renal transplantation except when proven intolerance to CNIs (including nephrotoxicity) necessitates the complete withdrawal of these treatments. 1
As a consequence of following this guidance, some medicines may be prescribed outside the terms of their UK marketing authorisation. Health-care professionals prescribing these medicines should ensure that children and adolescents receiving renal transplants and/or their legal guardians are aware of this and that they consent to the use of these medicines in these circumstances. 1
Description of technology under assessment
Summary of intervention
This technology assessment report considers nine pharmaceutical interventions. Two are used as induction therapy and seven are used as a part of maintenance therapy in renal transplantation. The two interventions considered for induction therapy are BAS and r-ATG. The seven interventions considered for maintenance therapy are TAC-IR and TAC-PR, MMF, MPS, BEL, SRL and EVL.
Induction therapy
Basiliximab (Simulect,® Novartis Pharmaceuticals) is a monoclonal antibody which acts as an interleukin 2 receptor antagonist. It has a UK marketing authorisation for prophylaxis of AR in allogeneic renal transplantation in children (aged 1–17 years). The summary of product characteristics (SPC) states it is to be used concomitantly with CSA for microemulsion- and CCS-based immunosuppression, in patients with panel reactive antibodies < 80%, or in a triple maintenance immunosuppressive regimen containing CSA for microemulsion, CCSs and either azathioprine (AZA) or MMF. 7
Rabbit antihuman thymocyte immunoglobulin is a gamma immunoglobulin. It has a UK marketing authorisation for the prevention of graft rejection in renal transplantation. The SPC states it is usually used in combination with other immunosuppressive drugs. It is administered intravenously. The UK marketing authorisation is not restricted to adults only. 7
Maintenance therapy
Tacrolimus is a CNI that is available in an immediate-release formulation (Adoport,® Sandoz; Capexion,® Mylan; Modigraf,® Astellas Pharma; Perixis,® Accord Healthcare; Prograf,® Astellas Pharma; Tacni,® Teva; Vivadex,® Dexcel Pharma). All of these formulations of TAC have UK marketing authorisations for prophylaxis of transplant rejection in kidney allograft recipients. The marketing authorisations include adults and children. 7 TAC (Modigraf®, Astellas Pharma) is available in a granule form which can be suspended in liquid and may be more suitable for those who struggle swallowing pills.
Tacrolimus is also available in a prolonged-release formulation (Advagraf,® Astellas Pharma). It has a UK marketing authorisation for prophylaxis of transplant rejection in kidney allograft recipients. The marketing authorisation is restricted to adults. The Commission on Human Medicines advises that all oral TAC (including both TAC-IR and TAC-PR) medicines in the UK should be prescribed and dispensed by brand name only. 7
Belatacept is designed to selectively inhibit CD28-mediated co-stimulation of T-cells. BEL has a UK marketing authorisation for prophylaxis of graft rejection in adults receiving a renal transplant, in combination with CCSs and a mycophenolic acid (MPA; Myfortic,® Novartis Pharmaceuticals). The SPC recommends that an interleukin 2 receptor antagonist for induction therapy is added to this BEL-based regimen. The SPC states that the safety and efficacy of BEL in children and adolescents aged 0–18 years have not yet been established. This formulation does not have a UK marketing authorisation for the prophylaxis of transplant rejection in renal transplantation in children and adolescents. 7
Mycophenolate mofetil is a prodrug of MPA which acts as an antiproliferative agent (Arzip,® Zentiva; CellCept,® Roche Products; Myfenax,® Teva); generic MMF is manufactured by Accord Healthcare, Actavis, Arrow Pharmaceuticals, Dr Reddy’s Laboratories, Mylan, Sandoz and Wockhardt). It has a UK marketing authorisation for use in combination with CSA and CCSs for the prophylaxis of acute transplant rejection in people undergoing kidney transplantation. The UK marketing authorisation is not restricted to adults (dosage recommendations for children aged 2–18 years are included in the SPC). 7
Mycophenolate sodium is an enteric-coated formulation of MPA. This formulation has the same UK marketing authorisation as MMF; however, this is restricted to adults. This formulation does not have a UK marketing authorisation for the prophylaxis of transplant rejection in renal transplantation in children and adolescents. 7
Sirolimus (Rapamune,® Pfizer) is an antiproliferative with a non-calcineurin-inhibiting action. It has a UK marketing authorisation for the prophylaxis of organ rejection in adult patients at low to moderate immunological risk receiving a renal transplant. It is recommended to be used initially in combination with CSA and CCSs for 2–3 months. It may be continued as maintenance therapy with CCSs only if CSA can be progressively discontinued. This formulation does not have a UK marketing authorisation for the prophylaxis of transplant rejection in renal transplantation in children and adolescents. 7
Everolimus (Certican,® Novartis Pharmaceuticals) is a proliferation signal inhibitor and is an analogue of SRL. EVL does not currently have a UK marketing authorisation for immunosuppressive treatment in kidney transplantation in children and adolescents. 7
Current usage in the NHS
There is a variation in the use of induction and maintenance therapy in the UK. Table 6 provides an overview of immunosuppression regimens for low-risk first renal transplants (e.g. blood group and HLA compatible) in the 10 paediatric transplant centres in the UK. Four out of the 10 centres use BAS as a part of induction therapy. Apart from the use of antibody induction, all centres use a single dose of methylprednisolone at the time of transplantation. The table also illustrates the difference in the use of the two proliferative agents (MMF and AZA), the agreement in the use of CNI across all centres (TAC; usually Adoport), and the use of steroids as a part of maintenance therapy. The current NICE guidelines are followed by using TAC + AZA + CCS ± BAS regimens. 1 However, the use of MMF is not limited to proven intolerance to CNIs, or to a very high risk of nephrotoxicity necessitating a temporary minimisation or avoidance of CNI (see Current National Institute for Health and Care Excellence guidance for more details).
| Hospital | Antibody used for induction therapy | Maintenance therapy |
|---|---|---|
| Birmingham Children’s Hospital | BAS | TWIST protocol: TAC + MMF + CCS |
| Bristol Children’s Hospital | Nonea | Triple therapy: TAC + AZA + CCS |
| Glasgow, Yorkhill | BAS | TWIST protocol: TAC + MMF + CCS |
| Leeds, Paediatric Unitb | Nonec | Triple therapy: TAC + AZA + CCSd |
| London, Evelina Children’s Hospital | BAS | Triple therapy: TAC + AZA + CCSe |
| London, Great Ormond Street | None | Triple therapy: TAC + AZA + CCS |
| Newcastle Great North Children’s Hospital | None | Triple therapy: TAC + AZA + CCS |
| Nottingham Children’s Unit | Nonef | Triple therapy: TAC + AZA + CCSg |
| Royal Belfast Hospital for Sick Children | Nonec | Triple therapy: TAC + MMF + CCSh |
| Royal Manchester’s Children’s Hospital | BAS | TWIST protocol: TAC + MMF + CCS |
Anticipated costs associated with intervention
The cost of the intervention (immunosuppressive regimen) is determined primarily by the choice and combination of the drugs and their dosages. Indicative costs for different immunosuppressive agents are given in Table 7. Caution should be exercised in interpreting these as dosages are commonly titrated and may differ from those indicated.
| Compound | Unit cost | Recommended dose | Estimated weekly cost for 31.5 kg body weight, surface area 1.1 m2 (10-year-old male)a |
|---|---|---|---|
| AZA | Hospital pharmacy: 0.1 p per mgb | 1–3 mg/kg per day, adjusted according to responsec | Hospital pharmacy: 22.05 p to 66.15 p |
| Community pharmacy: 0.1 p per mgd | Community pharmacy: 22.05 p to 66.15 p | ||
| BAS | £75.87 per mg (10-mg vial) and £42.12 per mg (20-mg vial)c | Child > 1 year, body weight < 35 kg, 10 mg within 2 hours before transplant surgery and 10 mg 4 days after surgery | Cost calculated based on recommended dose: |
| Child body weight ≥ 35 kg, 20 mg within 2 hours before transplant surgery and 20 mg 4 days after surgeryc | Child < 35 kg: £1517.38 (induction period only) | ||
| Child ≥ 35 kg: £842.38 (induction period) | |||
| BEL | £1.42 per mgc | Not licensed for use in childrenc | £55.83 (adult, weight-based dose) |
| Adult dose 5 mg/kg per 4 weeks | |||
| CSA | Hospital pharmacy: 1.65 p per mgb | 8–12 mg/kg/daye | Hospital pharmacy: £29.10–43.66 |
| Community pharmacy: 2.55 p per mgc | Community pharmacy: £44.98–67.47 | ||
| CCSs | Hospital pharmacy: 0.3 p per mgb | Methylprednisolone: 10–20 mg/kg or 400–600 mg/m2 (maximum 1 g) once daily for 3 daysc | Hospital pharmacy: £2.83–5.67 |
| Community pharmacy: 0.9 p per mgd | Prednisolone: consult local treatment protocols for details.c An example: 60 mg/m2/day during first week, eventually weaned down to < 10 mg/m2 on alternate days | Community pharmacy: £8.49–17.01 | |
| EVL | £9.90 per mgf | Not licensed for use in childrenc | £103.95 (adult non-weight-based dose) |
| Adult dose of 1.5 mg per dayg | |||
| TAC-IR | Hospital pharmacy: 52.0 p per mgb | 150 µg/kg twice daily, adjusted according to whole blood concentration | Hospital pharmacy: £34.40 |
| Community pharmacy: 118.6 p per mgc,d | Community pharmacy: £78.45 | ||
| MMF | Hospital pharmacy: 37.74 p per gb | 300 mg/m2 twice daily (maximum 2 g) if in addition with TAC and CCSsc | Hospital pharmacy: £1.74 |
| Community pharmacy: 40.44 p per gd | 600 mg/m2 twice daily (maximum 2 g) if in addition with CSA and CCSsc | Community pharmacy: £1.86 | |
| Hospital pharmacy: £3.48 | |||
| Community pharmacy: £3.73 | |||
| MPS | 0.5 p per mgc | Not licensed for use in childrenc | £50.4 (adult non-weight-based dose) |
| Adult dose 1440 mg per dayc | |||
| TAC-PR | 106.8 p per mgc | Not licensed for use in childrenc | £47.10 (adult weight-based dose) |
| Adult dose 0.2 mg/kg per day | |||
| r-ATG | £6.35 per mgc | Not licensed for use in childrenc | £2100.52 (induction period only) |
| 1.5 mg/kg/day administered by intravenous infusion for 7–14 daysh | |||
| SRL | £2.88 per mgc,d | Not licensed for use in childrenc Adult dose 2 mg per dayc |
£40.36 (adult non-weight-based dose) |
In addition, drug administration costs are also incurred for some maintenance agents. CSA, TAC, SRL and EVL are routinely titrated using therapeutic drug monitoring, which is estimated to cost approximately £26 per test (testing frequency is reduced as patients become stabilised in dosage), and BEL requires intravenous (i.v.) infusion, entailing catheterisation and nursing time. The cost of this is difficult to estimate but estimates range from £15466 to £320. 11
Chapter 2 Definition of the decision problem
Decision problem
The purpose of this assessment is to answer the following question:
What is the clinical effectiveness and cost-effectiveness of the following immunosuppressive therapies in renal transplantation in children and adolescents:
-
Basiliximab and r-ATG as an induction therapy, and
-
TAC-IR, TAC-PR, MMF, MPS, BEL, SRL, and EVL as a maintenance therapy
-
including a review of TA99.
The project was undertaken based on a published scope7 and in accordance with a protocol. 67
Interventions
A total of nine interventions are considered, two for induction therapy and seven for initial and long-term maintenance therapy.
The two induction treatments are:
-
BAS
-
r-ATG.
The seven maintenance treatments are:
-
TAC-PR formulation (Advagraf,® Astellas Pharma)
-
TAC-IR formulations [Adoport® (Sandoz); Capexion® (Mylan); Modigraf® (Astellas Pharma); Perixis® (Accord Healthcare); Prograf® (Astellas Pharma); Tacni® (Teva); Vivadex® (Dexcel Pharma)]
-
BEL MMF
-
MPS SRL
-
EVL.
These treatments are described in Chapter 1, Summary of Intervention. Several of the drugs being assessed are used in the NHS outside the terms of their UK marketing authorisation, for example in children and adolescents, or in high-risk people, or in unlicensed drug combinations. Specifically EVL, TAC-PR, BEL, MPS and SRL are not currently licensed for the prophylaxis of transplant rejection in renal transplantation in children and adolescents.
Under an exceptional directive from the Department of Health, the Appraisal Committee may consider making recommendations about the use of drugs outside the terms of their existing marketing authorisation when there is compelling evidence of their safety and effectiveness. Accordingly, the review included controlled studies that used drugs outside the terms of their marketing authorisations.
Populations including subgroups
The population being assessed are children and adolescents aged 0–18 years (inclusive) undergoing kidney transplantation. Patients receiving multiorgan transplants and those who have received transplants and immunosuppression previously were excluded.
If data allow, the following subgroups were considered:
-
different age groups
-
level of immunological risk (including HLA compatibility and blood group compatibility)
-
people at high risk of rejection within the first 6 months
-
people who have had a retransplant within 2 years
-
previous AR
-
people at high risk of complications from immunosuppression (including new-onset diabetes mellitus).
Relevant comparators
For induction therapy, the treatments are to be compared with each other, as data permit, or with other regimens that do not include monoclonal or polyclonal antibodies. For maintenance therapy, each treatment or regimen (combination of treatments) is to be compared with the other treatments or regimens as data permit, or with a CNI with or without an antiproliferative agent and/or CCSs.
Outcomes
The health-related outcomes to be included in this technology assessment are:
-
patient survival
-
graft survival
-
graft function
-
time to and incidence of AR
-
severity of AR
-
growth
-
adverse effects of treatment
-
health-related quality of life (HRQoL).
Key issues
A number of factors may influence the survival and function of transplanted kidney and the survival of the recipient.
The viability of the kidney may depend on the type of donor (living related, living unrelated, DBD, DCD or expanded criteria donor), the age of the donor, whether or not they had comorbidities such as diabetes mellitus, and the length of cold ischaemia. Furthermore, the age, sex, ethnicity and health of the recipient, and the length of time the recipient is on dialysis prior to transplantation may affect the outcome of transplantation. These issues have been discussed in more detail in Chapter 1, Important prognostic factors.
Overall aims and objectives of assessment
This assessment reviewed and updated the evidence for the clinical effectiveness and cost-effectiveness of immunosuppressive therapies in children and adolescents renal transplantation. This was to be done by conducting a systematic review of clinical effectiveness studies and a model-based economic evaluation of induction and maintenance immunosuppressive regimens to update the current guidance (TA99). We have incorporated relevant evidence presented in this previous report and report new evidence. This included a new decision-analytic model of kidney transplantation outcomes to investigate which regimen is the most cost-effective option.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
This systematic review was commissioned by NICE to update the previous guidance (TA99). 1 The systematic review and economic evaluation developed to support current NICE guidance TA99, was published by Yao et al. in 2006. 2 The differences between the remit of the previous review and the protocol of the current one are discussed in The previous assessment report.
There was one departure from the protocol:67 the age of population eligibility criterion was changed from < 18 years (a common definition of children and adolescents) to ≤ 18 years [the age inclusion criterion applied by the three eligible randomised controlled trial (RCTs)].
The aim was to systematically review the clinical effectiveness of immunosuppressive therapies in child and adolescent (≤ 18 years) renal transplantation; that is to determine their effect on patient survival, graft survival, graft function, time to and incidence of AR, severity of AR and quality of life, growth, and their impact on AEs.
Identification of studies
Bibliographic literature database searching was conducted on 14 April 2014 and updated on 7 January 2015. The searches for individual effectiveness studies (RCTs and controlled clinical trials) took the following form: (terms for kidney or renal transplant or kidney or renal graft) AND (terms for the interventions under review) AND [a study design limit to randomised control trials (RCT) or controlled trials]. In order to update the previous assessment,2 the searches were date limited (2002–current). These searches were not limited by language or to human-only studies because such a limit may have blocked retrieval of includable studies for R-ATG (line 8 of the MEDLINE search). The following databases were searched: MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid), EMBASE (via Ovid), Cochrane Central Register of Controlled Trials (CENTRAL) (via Wiley Online Library) and Web of Science [via Institute for Scientific Information (ISI) – including conference proceedings]. In addition, the following trials registries were hand-searched in January 2015: Current Controlled Trials, ClinicalTrials.gov, FDA website, EMA website (European Public Assessment Reports).
Separate searches were undertaken to identify systematic reviews of RCTs and non-randomised studies. These searches took the following form: (terms for kidney or renal transplant or kidney or renal graft) AND (terms for the interventions under review) AND (a pragmatic limit to systematic reviews). The same population and intervention search terms were used as in the individual studies search. A pragmatic, methodological search filter was used to limit by study design. No other limits (e.g. language) were applied to this search. The search was run from database inception in the following databases: MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid), EMBASE (via Ovid), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE) and Health Technology Assessment (HTA) (The Cochrane Library via Wiley Online Library) and Health Management Information Consortium (HMIC) (via Ovid).
The search strategies are recorded in Appendix 1.
The database search results were exported to, and deduplicated using, EndNote (X5) (Thomson Reuters, CA, USA). Deduplication was also performed manually.
Furthermore, the following websites were searched for background information.
Renal societies (UK)
-
British Renal Society (www.britishrenal.org/).
-
Renal Association (www.renal.org/).
-
UK Renal Registry (www.renalreg.com/).
-
Kidney Research UK (www.kidneyresearchuk.org/).
-
British Kidney Patient Association (www.britishkidney-pa.co.uk/).
-
National Kidney Federation (www.kidney.org.uk/).
Renal societies (international)
-
American Society of Nephrology (www.asn-online.org/).
-
American Association of Kidney Patients (www.aakp.org/).
-
National Kidney Foundation (US; www.kidney.org/).
-
Canadian Society of Nephrology (www.csnscn.ca/).
-
Kidney Foundation of Canada (www.kidney.ca/).
-
Australian and New Zealand Society of Nephrology (www.nephrology.edu.au/).
-
Kidney Health Australia (www.kidney.org.au/).
-
Kidney Society Auckland (www.kidneysociety.co.nz/).
Previous Health Technology Assessment review
Studies included in the previous HTA review (Yao et al. 2) were screened using the inclusion criteria for the Peninsula Technology Assessment Group (PenTAG) review (Inclusion and exclusion criteria).
Reference lists
Reference lists of included guidelines, systematic reviews, company submissions and clinical trials were scrutinised in order to identify additional studies.
Ongoing trials
Searches for ongoing trials were also undertaken. Terms for the intervention and condition of interest were used to search the following trial registers for ongoing trials: ClinicalTrials.gov and Controlled Trials (ISRCTN). Trials that did not relate to immunosuppressive therapies for kidney transplantation in children and adolescents were removed by hand-sorting. All searches for ongoing trials were carried out in January 2015. The search strategies can be found in Appendix 1.
Adult randomised controlled trial evidence
In addition, as specified in the review protocol, all child/adolescents RCT and non-RCT evidence included in this review was compared with adult evidence identified from parallel HTA 09/46/01 appraisal. 68 This parallel HTA was conducted by PenTAG to inform the ongoing technology appraisal of immunosuppressive therapy for kidney transplantation in adults (review of technology appraisal guidance 85; NICE appraisal ID 456). The NIHR Evaluation, Trials and Studies Coordinating Centre reference for the adult report is 09/46/01 (www.nice.org.uk/guidance/indevelopment/gid-tag348/documents).
Inclusion and exclusion criteria
Studies retrieved from the literature searches were selected for inclusion according to the inclusion/exclusion criteria specified below. Studies available only as abstracts were included provided sufficient methodological details were reported to allow critical appraisal of study quality. We also contacted authors for additional data.
Study design
The clinical effectiveness review included:
-
eligible studies – RCTs in children and adolescents (≤ 18 years), RCTs of adults and children/adolescents in which a subgroup analysis of children and adolescents is reported, and non-randomised controlled studies (comparative quasi-experimental and observational studies were considered)
-
search strategy – databases were searched to identify RCTs, systematic reviews of RCTs and systematic reviews of non-randomised controlled studies. Individual non-randomised controlled studies were identified via the bibliographies of systematic reviews (i.e. individual non-randomised controlled studies were not searched for directly).
For the purpose of this review, a systematic review was defined as one that has:
-
a focused research question
-
explicit search criteria that are available to review, either in the document or on application
-
explicit inclusion/exclusion criteria, defining the population(s), intervention(s), comparator(s), and outcome(s) of interest
-
a critical appraisal of included studies, including consideration of internal and external validity of the research
-
a synthesis of the included evidence, whether narrative or quantitative.
Interventions
Studies evaluating the use of the following immunosuppressive therapies for renal transplantation were included.
Induction therapy
-
Basiliximab.
-
Rabbit antihuman thymocyte immunoglobulin.
Maintenance therapy
-
TAC-PR formulation.
-
TAC-IR formulations.
-
Belatacept.
-
MMF (generic MMF manufactured by Accord Healthcare, Actavis, Arrow Pharmaceuticals, Dr Reddy’s Laboratories, Mylan, Sandoz and Wockhardt).
-
Mycophenolate sodium.
-
Sirolimus.
-
Everolimus.
All treatments are described in detail in Chapter 1, Summary of intervention.
In addition (as evidence allows), adherence to treatment and the use of treatments in conjunction with either CCS or CNI reduction or withdrawal strategies is considered. To achieve this, only studies that meet the inclusion criteria are examined. As such, studies in which the intervention is identical in both study arms, but dose reduction or withdrawal of CCSs or CNIs occurs in one arm, were excluded.
Comparator
Studies using the following comparators were included.
Induction therapy
-
Regimens without monoclonal or polyclonal antibodies, for example regimens that include methylprednisolone or placebo (PBO).
-
Interventions should also be compared with each other.
Maintenance therapy
-
A CNI with or without an antiproliferative agent and/or CCSs.
-
Interventions should also be compared with each other.
In addition, when appropriate, the interventions were appraised as part of combination regimens.
Population
The population is children and adolescents aged ≤ 18 years undergoing kidney transplantation. The kidney donor may be living related, living unrelated or deceased. Patients receiving multiorgan transplants and those who have received transplants and immunosuppression previously were excluded.
Outcomes
The outcome measures to be considered are:
-
patient survival
-
graft survival
-
graft function
-
time to and incidence of AR
-
severity of AR
-
growth
-
adverse effects (AE) of treatment
-
HRQoL.
Screening
All records were dual screened. First, titles and abstracts returned by the search strategy were screened for inclusion. The screening was distributed across a team of five researchers (TJ-H, LC, MHa, MB and HC). Update searches were screened by two reviewers (MHa and JV-C) and disagreements were resolved by discussion, with involvement of a third reviewer (TJ-H or MHa) if necessary. Full texts of identified studies were obtained and screened in the same way. Studies reported only as abstracts were included provided sufficient methodological details were reported to allow critical appraisal of study quality. In addition, studies included in the review conducted by Yao et al. 2 were screened for inclusion.
As specified in the review protocol, the searches for systematic reviews were separately screened to identify systematic reviews of non-randomised studies and these in turn were screened to identify non-randomised studies for inclusion in the review.
Data extraction
Information from new studies (not included in TA99) was extracted and tabulated; information included details of the study design and methodology, baseline characteristics of participants and results including HRQoL and any AEs if reported (see Appendix 1). All included studies (including those in TA99) were quality appraised.
If we identified several publications for one study, we evaluated the effectiveness data from the most recent publication and amended this with information from other publications. For quality appraisal purposes, all publications relating to a study were assessed together.
Critical appraisal strategy
Randomised control trials
Four reviewers (LC, MHa, HC and TJ-H) independently assessed the quality of all studies included in the clinical effectiveness review. The internal and external validity of RCTs was assessed according to criteria based on Centre for Reviews and Dissemination (CRD) guidance69 (Table 8).
| Bias | Criteria for assessment of risk of bias |
|---|---|
| Treatment allocation | 1. Was the assignment to the treatment groups really random? 2. Was treatment allocation concealed? |
| Similarity of groups | 3. Were the groups similar at baseline in terms of prognostic factors? |
| Implementation of masking | 4. Were the care providers blinded to the treatment allocation? 5. Were the outcome assessors blinded to the treatment allocation? 6. Were the participants blinded to the treatment allocation? |
| Outcomes | 7. Were all a priori outcomes reported? 8. Were complete data reported, e.g. was attrition and exclusion (including reasons) reported for all outcomes? 9. Did the analyses include an ITT analysis? |
| Generalisability | 10. Are there any specific limitations which might limit the applicability of this study’s findings to the current NHS in England? |
Non-randomised control trials
There is no agreed recommended appraisal tool for the assessment of non-randomised studies. 70 The CRD handbook suggests considering the study design, risk of bias, other issues related to study quality, choice of outcome measure, statistical issues, quality of reporting, quality of the intervention and generalisability. 69 Therefore, the internal and external validity of non-RCTs was assessed according to criteria based on CRD guidance69 (Table 9).
| Bias | Criteria for assessment of risk of bias |
|---|---|
| Treatment allocation | 1. Was the method of allocation reported? 2. Is the allocation to groups or to the study a source of selection bias? |
| Similarity of groups | 3. Were the groups similar at baseline in terms of prognostic factors? |
| Implementation of masking | 4. Were the care providers blinded to the treatment allocation? 5. Were the outcome assessors blinded to the treatment allocation? 6. Were the participants blinded to the treatment allocation? |
| Outcomes | 7. Was follow-up long enough for outcomes to occur? 8. Were complete data reported, e.g. was attrition and exclusion (including reasons) reported for all outcomes? 9. Were statistical analyses adjusted to account for any between-group differences? |
| Generalisability | 10. Was the group(s) representative of NHS renal transplant patients? |
Methods of data synthesis
Data were tabulated and discussed in a narrative review. The subgroups defined in Chapter 2, Populations including subgroups, were considered in the analyses.
Meta-analyses
When data permitted, the results of individual studies comparing the same regimens were pooled using the methods described below.
A random-effects model was assumed for all meta-analyses. For binary data, an odds ratio (OR) was used as a measure of treatment effect and the DerSimonian–Laird method was used for pooling. 71 For continuous data (e.g. graft function), mean differences were calculated if the outcome was measured on the same scale in all trials. If applicable, publication bias was assessed using funnel plots, the Harbord test was used for binary outcomes [OR, log-standard error (SE)] and the Egger test for continuous data. All analyses were performed in Stata 13 (StataCorp LP, College Station, TX, USA).
For studies with more than one intervention arm (that were separately compared with the same control arm), the number of events and the total sample size in the control arm were divided equally across the comparisons, and when pooling mean differences the total sample size in the control arm was adjusted and divided equally across the comparisons. However, if only one experimental arm was eligible for the analysis, all participants and events assigned to the control arm were included. If the number of events was zero in one of the studies arms, a value of 0.5 was added to all study arms to allow for statistical analyses.
Results of the systematic review
Quantity and quality of research available
The current review summarises both randomised and non-randomised controlled evidence. The assessment of clinical effectiveness is reported separately for induction and maintenance regimens.
Randomised control trials
Our searches returned 5079 unique titles and abstracts, with 784 papers retrieved for detailed consideration. To ensure the inclusion of trials with mixed child/adolescent and adult populations that reported separate results for children and adolescents, the searches and title and abstract screening were not limited to children and adolescents. Update searches conducted on 7 January 2015 returned 416 unique titles and abstracts. Forty papers were retrieved for detailed consideration.
Of the 824 full-text papers retrieved, 793 were excluded (a list of these records with reasons for their exclusion can be found in Appendix 2, Table 135). Although RCTs in mixed populations were identified, none included subgroup analysis by age – providing separate results for children/adolescents and adults – and were therefore excluded from the review (a list of these records can be found in Appendix 2, Table 136). Three RCTs (published in one abstract72 and seven papers73–79) met the inclusion criteria.
Only one abstract72 was included in the review. This abstract included new data related to Offner et al. 73 and sufficient methodological information to inform the quality appraisal. In addition, there were 23 articles that were systematic reviews and all eligible systematic reviews were tabulated (see Appendix 3, Table 137).
The process is illustrated in detail in Figure 8.
FIGURE 8.
Clinical effectiveness review: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. SR, systematic review.
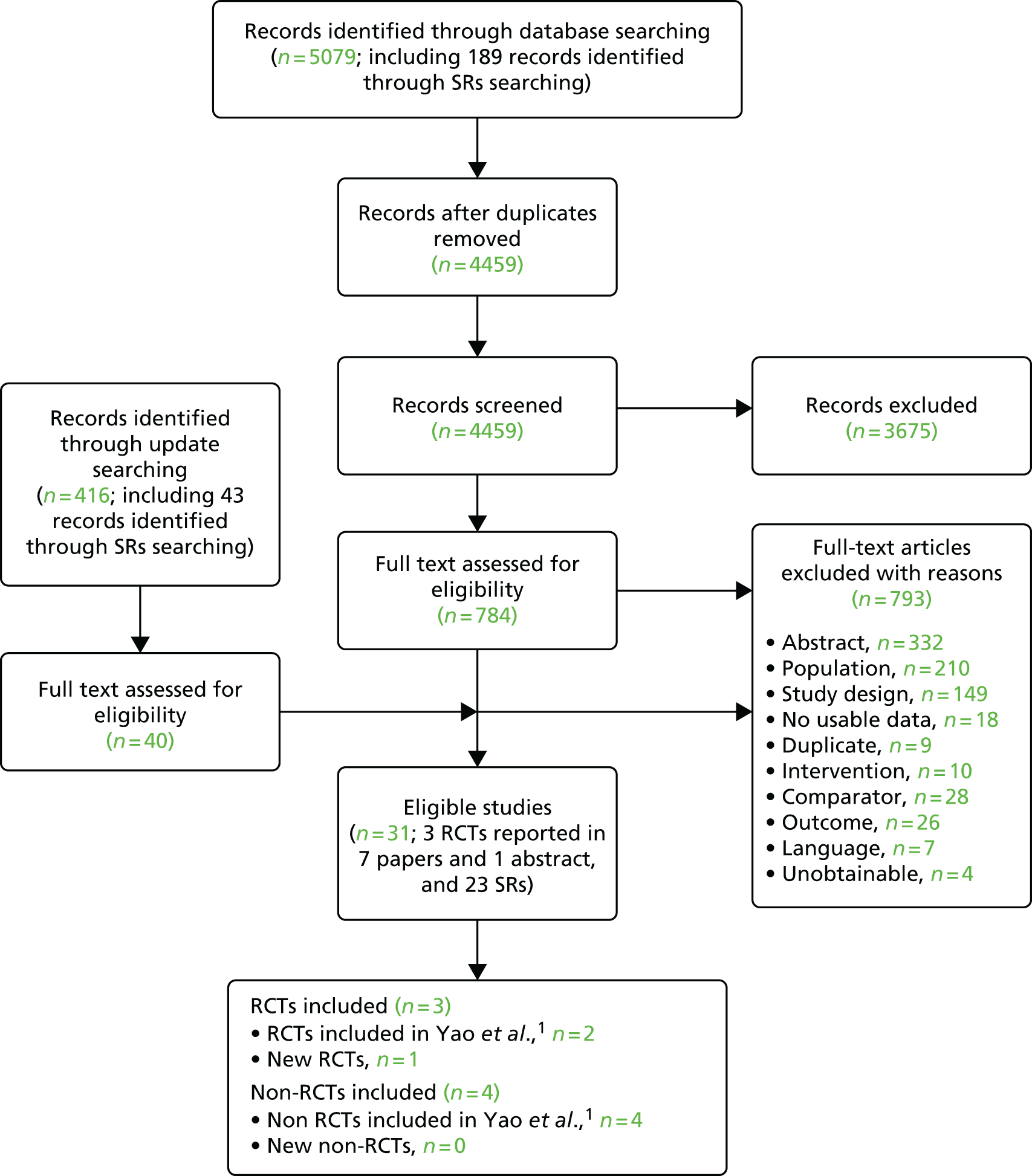
In summary, three RCTs (published in seven papers73–79 and one abstract72) were found eligible and are included in this review (Table 10).
| Study | n a | Agent (n) | Control (n) | Outcomes | Multiple publications |
|---|---|---|---|---|---|
| Induction therapy | |||||
| Offner et al.73 | 192 | BAS + CSA + MMF + CCS (100) | PBO + CSA + MMF + CCS (92) | Mortality, graft loss, graft function, BPAR, AE | Höcker et al.,74 Jungraithmayr et al.72 |
| Grenda et al.75 | 192 | BAS + TAC + AZA + CCS (99) | NI + TAC + AZA + CCS (93) | Mortality, graft loss, graft function, BPAR, AE | Webb et al.76 |
| Maintenance therapy | |||||
| Trompeter et al.77 | 196 | TAC + AZA + CCS (103) | CSA + AZA + CCS (93) | Mortality, graft loss, graft function, BPAR, AE | Filler et al.,78 Filler et al.79 |
Non-randomised trials
The systematic reviews were used to identify non-RCTs. We screened the titles and abstracts of 226 unique references identified by the PenTAG systematic review searches (including 43 records from update searches) and retrieved 38 papers for detailed consideration. All eligible systematic reviews were tabulated (see Appendix 3, Table 137).
In total, four non-RCTs met the inclusion criteria and were considered eligible for inclusion (Table 11). All of these were included in the previous HTA by Yao et al. 2 so no new non-RCTs were identified. However, in 2007 one of the four non-RCT studies83 published 5-year follow-up data85 that were not included in the previous HTA.
| Study | n a | Treatment | Outcomes | Multiple publications |
|---|---|---|---|---|
| Induction and maintenance therapy | ||||
| Garcia et al80 | 24 | BAS + TAC + AZA + CCS vs. BAS + CSA + MMF + CCS | Mortality, graft loss, graft function, BPAR, AE | N/A |
| Maintenance therapy | ||||
| Antoniadis et al.81 | 14 | CSA + MMF + CCS vs. CSA + AZA + CCSb | Graft function, BPAR, AE | N/A |
| cBenfield et al.82 | 67 | (OKT3 or CSA) + MMF + CCS vs. (OKT3 or CSA) + AZA + CCS | Mortality, graft loss, graft function, BPAR | N/A |
| Staskewitz et al.83 | 139d | CSA + MMF + CCSe vs. CSA + AZA + CCS | Mortality, graft loss, graft function, BPAR, AE | Jungraithmayr et al.,84 Jungraithmayr et al.85 |
Ongoing studies
Eleven ongoing trials were considered relevant to this review and were investigated further. An overview of the 11 trials with reasons for inclusion/exclusion in PenTAG review is provided in Appendix 4, Table 138. Only one of these ongoing trials was identified as potentially eligible for inclusion. The methods and design of this trial (A2314) were reported as conference abstracts. 86–89 This international trial investigates the efficacy, tolerability and safety of early introduction of EVL, reduced CNIs and early steroid elimination compared with standard CNI, MMF and steroid regimen in paediatric renal transplant recipients and is sponsored by Novartis. The estimated date of completion is December 2016, so it was not included in this review. The search of ongoing studies in trial registries did not identify any additional RCTs for inclusion in the PenTAG systematic review.
The previous assessment report
The assessment report published as Yao et al. 2 informed the current NICE guidance TA99. 1 The aim of the previous HTA was to establish the clinical effectiveness (harms and benefits) and cost-effectiveness of four of the newer immunosuppressive drugs for renal transplantation, namely BAS, DAC, TAC and mycophenolate (mofetil and sodium), and of SRL in children and adolescents.
The previous HTA review adopted the following approach of three evidence levels:
-
Level 1 evidence: findings from RCTs carried out in children and adolescents with kidney transplants. This could include RCTs undertaken solely in children and adolescents, or RCTs where a subgroup analysis in children and adolescents was reported.
-
Level 2 evidence: when level 1 evidence was not available, use of findings from RCTs undertaken in adults with kidney transplants.
-
Level 3 evidence: findings from non-randomised comparative evidence collected in children and adolescents with kidney transplants. Level 3 evidence was used to complement and check the consistency of level 2 evidence (if level 1 evidence was not available).
The current PenTAG systematic review aims to establish the clinical effectiveness and cost-effectiveness of immunosuppressive regimens including BAS and r-ATG as an induction therapy in renal transplantation in children and adolescents, and of immunosuppressive regimens including TAC-IR, TAC-PR, MMF, MPS, BEL, SRL, and EVL as a maintenance therapy in renal transplantation in children and adolescents (including review of TA99).
The current PenTAG review included:
-
RCTs in children and adolescents (≤ 18 years), and RCTs of adults and children and adolescents in which a subgroup analysis of children and adolescents is reported
-
systematic reviews which include non-randomised studies evaluating the interventions of interest in children and adolescents (≤ 18 years).
In addition, the PenTAG review compares results in children and adolescents with those from the parallel HTA 09/46/01 appraisal ‘Immunosuppressive therapy for kidney transplantation in adults’. 68
In the sections below we summarise the evidence included in TA99 and highlight the differences between the PenTAG review and the previous review.
Randomised control trials
Children and adolescents
The previous TA991 included three paediatric RCTs: the unpublished Wyeth 0468E1–217-US study (Wyeth submission 2005), Trompeter et al. ,77 and an abstract by Grenda et al. 90 (Table 12). The Wyeth submission 2005 compared an addition of SRL to a CNI maintenance therapy [(CSA or TAC) + CCS], with a triple maintenance therapy [(CSA or TAC) + (MMF or AZA) + CCS] in children and adolescents (≤ 20 years of age) who experienced one or more episodes of AR or chronic rejection after kidney transplantation. 2 Because of the trial design (treatment combinations were allowed) and population characteristics (age and time from transplantation) this study is not eligible to be included in the current review. The other two paediatric RCTs included in Yao et al. 2 are included in the PenTAG review. 77,90 Additional publications of Grenda et al. 90 were identified in our searches (the previous HTA included only 6-month follow-up data; see Table 12). We identified one new RCT73 that was not included in Yao et al. 2
| Number | Study | Multiple publications | Treatments | Published | Included in PenTAG (reason) |
|---|---|---|---|---|---|
| 1 | Grenda et al.90 | Fijusawa/Astellas 2005 | BAS vs. PBO | Abstract only; full trial provided in Fujusawa/Astellas’ submission | Yes, trial was published as Grenda et al.75 and Webb et al.76 |
| 2 | Trompeter et al.77 | Filler et al.78,79 | TAC vs. CSA | Yes | Yes |
| 3 | Wyeth submission 2005 | 0468E1–217-US, NCT00005113 (study was terminated) | Addition of SRL | No; full trial provided in Fujusawa/Astellas’ submission | No (population, design) |
Non-randomised studies
An overview of the nine non-randomised studies included in Yao et al. 2 with reasons for inclusion/exclusion in the current review is provided in Table 13. Five studies were excluded from the PenTAG review (see Table 13):
-
Duzova et al. 91 (compared BAS with no induction) administered triple therapy of (CSA or TAC) + (AZA or MMF) + CCS; however, a breakdown of the numbers (and results) in each combination was not reported and the mean recipient age was 14.9 ± 3.6 years (range 7–21 years).
-
Pape et al. 92 recruited a child with a combined kidney–liver transplantation.
-
Swiatecka-Urban et al. 93 included children, adolescents and adults (inclusion criteria aged < 21 years).
-
Neu et al. 94 included children, adolescents and adults (inclusion criteria aged > 2 years and < 21 years) and the use of induction therapy varied in the study.
-
Steffen et al. 95 was published as an abstract only and did not include enough information to allow critical appraisal.
| Number | ID | n a | Treatments | Included in PenTAG (reason) |
|---|---|---|---|---|
| Induction therapy | ||||
| 1 | Duzova et al.91 | 43 | BAS + (CSA or TAC) + (AZA or MMF) + CCS vs. (CSA or TAC) + (AZA or MMF) + CCS | No (design and population) |
| 2 | Pape et al.92 | 77 | BAS + CSA + CCS vs. CSA + CS | No (population)b |
| 3 | Swiatecka-Urban et al.93 | 32 | BAS + TAC + CCS vs. TAC + CCSc | No (population) |
| Maintenance therapy | ||||
| 4 | Garcia et al.80 | 24 | BAS + TAC + AZA + CCS vs. BAS + CSA + MMF + CCS | Yes |
| 5 | Neu et al.94 | 986 | TAC + MMF + CCS vs. CSA + MMF + CS | No (population) |
| 6 | Antoniadis et al.81 | 14 | CSA + MMF + CCS vs. CSA + AZA + CCSd | Yes |
| 7 | Steffen et al.95 | NR | No (abstract) | |
| 8 | Staskewitz et al.83 (Jungraithmayr et al.84) | 120 | CSA + MMF + CCSe vs. CSA + AZA + CCS | Yes |
| 9 | fBenfield et al.82 | 678 | (OKT3 or CSA) + MMF + CCS vs. (OKT3 or CSA) + AZA + CCS | Yes |
In summary, four non-randomised studies were included in the PenTAG review and all were also included in the previous HTA review by Yao et al. 2 No new non-randomised studies were identified in PenTAG systematic review searches.
Adults
The previous TA99 included evidence from 25 adult RCTs. In comparison, the updated HTA 09/46/01 appraisal ‘Immunosuppressive therapy for kidney transplantation in adults’ included 86 trials: 11 induction studies, 73 maintenance studies and two studies of both induction and maintenance treatment. An overview of the 25 adult RCTs included in Yao et al. 2 with reasons for inclusion/exclusion in the parallel HTA review68 is provided in Appendix 5 (see Table 139).
If relevant, the adult evidence from the HTA 09/46/01 appraisal was summarised and compared with child/adolescent evidence included in the PenTAG review.
Quality of included studies
We appraised both newly identified trials and those included in the previous HTA review. 2 The reasons for reappraising trials were first to ensure consistency with appraisal of the new study and, second, because we have access to new information from papers published after the inclusion date for the previous review. Only primary research studies were appraised (i.e. not systematic reviews). If a trial was reported in multiple publications, only one quality assessment of the trial was conducted (all publications for that trial were assessed together).
Randomised controlled trials
In total, three RCTs were assessed: two induction studies and one maintenance study. 73,75,77
Overall assessment
For all three RCTs, fewer than half of the items constituting the quality appraisal assessment were rated as being of ‘adequate’ quality (Table 14). All of these trials either did not report, or lacked clarity on, at least 5 out of the 10 quality appraisal items. It is possible that items that were not clearly reported in the papers were in fact adequately conducted in the trials. Nevertheless, all three RCTs were rated as ‘inadequate’ for at least one item of the quality appraisal assessment.
| Study | Was the assignment to the treatment groups really random? | Was the treatment allocation concealed? | Were the groups similar at baseline in terms of prognostic factors? | Were the care providers blinded to the treatment allocation? | Were the outcome assessors blinded to the treatment allocation? | Were the participants blinded to the treatment allocation? | Were all a priori outcomes reported? | Were complete data reported, e.g. was attrition and exclusion (including reasons) reported for all outcomes? | Did the analyses include an ITT analysis? | Are there any specific limitations that might limit the applicability of this study’s findings to the current NHS in England? |
|---|---|---|---|---|---|---|---|---|---|---|
| Offner et al.73 | Adequate | Unclear | Adequate | Adequate | Unclear | Adequate | Unclear | Unclear | Inadequate | Adequate |
| Grenda et al.75 | Unclear | Unclear | Adequate | Inadequate | NR | Inadequate | Unclear | Unclear | Unclear | Adequate |
| Trompeter et al.77 | Unclear | Adequate | Partiala | Inadequate | NR | Inadequate | Unclear | Unclear | Inadequate | Adequate |
Treatment allocation
Random allocation: the method of random allocation, including the method of sequence generation, was clearly stated and adequate in only one trial73 and unclear in the other two trials. 75,77
Concealment of allocation: the method of concealment of allocation was clearly reported in only one trial77 and unclear in the other two trials. 73,75
Similarity of groups
Baseline characteristics: all three RCTs stated that baseline characteristics were similar between treatments arms on a range of prognostic factors (see Table 16 for a summary of baseline characteristics). However, one trial appeared to have a higher percentage of males in the PBO arm than the BAS arm (67.4% vs. 56%, respectively). 73
Implementation of masking
Treatment allocation masked from providers: the method was clearly stated and adequate in only one trial. 73 In the other two trials,75,77 care providers were not blinded to treatment allocation.
Treatment allocation masked from outcome assessors: none of the three trials clearly reported whether or not treatment allocation was masked from outcome assessors. 73,75,77
Treatment allocation masked from participants: the method was clearly stated and adequate in only one trial. 73 In the other two trials, participants were not blinded to treatment allocation.
Completeness of trials
In all three studies,73,75,77 it was not clear whether or not all reported outcomes were the same as those in the trial protocol and the reporting of loss to follow-up, withdrawals and dropouts was also not clearly reported.
Intention-to-treat (ITT) analysis: none of the trials was rated as adequate. One induction trial investigating the effectiveness of BAS excluded eight participants who received a ‘commercially available formulation of the drug instead of the blinded study drug Simulect’ and was, therefore, rated as ‘inadequate’ for this item of the quality appraisal assessment. 73 Similarly, one study excluded participants who did not receive study medication and excluded an additional four participants because of reporting issues and so was also rated as ‘inadequate’ for this item. 77 The remaining study75 did not clearly report the initial number of participants who were randomised, so it was unclear whether or not all randomised and transplanted participants were included in the analyses.
Applicability of trials to the NHS
Applicability to the current NHS in England: all three studies were considered to be applicable to the NHS because no specific limitations with regards applicability were found in the study. 73,75,77 All three trials were conducted in Europe, patient and donor characteristics were largely representative of the NHS in England and doses of the drug under investigation were similar to current recommended doses, although Trompeter et al. 77 administered 10 mg of BAS for participants who were < 40 kg, and 20 mg for participants who were ≥ 40 kg, whereas the recommended cut-off for increasing the dose from 10 mg to 20 mg is currently 35 kg.
Overall assessment
For all four non-randomised studies, fewer than half of the items constituting the quality appraisal assessment were adequately addressed (Table 15). However, for all studies, at least 5 out of the 10 quality appraisal items were either not applicable (owing to study design), not reported, or not clearly reported. It is possible that items that were not clearly reported in the papers were in fact adequately conducted in the studies.
| Study | Design | Was the allocation to group(s) reported? | Is the allocation to groups or to the study a source of selection bias? | Were the groups similar at baseline in terms of prognostic factors? | Were the care providers blinded to the treatment allocation? | Were the outcome assessors blinded to the treatment allocation? | Were the participants blinded to the treatment allocation? | Was follow-up long enough for outcomes to occur? | Were complete data reported, e.g. was attrition and exclusion (including reasons) reported for all outcomes? | Were statistical analyses adjusted to account for any between-group differences? | Was the group(s) representative of NHS renal transplant patients? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antoniadis et al.81 | Non-RCT | NR | Unclear | Unclear | NR | NR | NR | Adequate | Adequate | NR | Inadequate |
| Benfield et al.82 | Historically controlled studya | Adequate | Unclear | Unclear | N/A | N/A | N/A | Adequate | Adequate | Inadequate | Unclear |
| Garcia et al.80 | Retrospective cohort study | Adequate | Unclear | Inadequateb | N/A | N/A | N/A | Partial | Adequate | NR | Unclear |
| Staskewitz et al.83 | Historically controlled study | Adequate | Unclear | Partialc | N/A | N/A | N/A | Adequate | Adequate | Inadequate | Inadequate |
Treatment allocation
Allocation to groups: three of the non-randomised studies adequately described what the treatment and control groups were and the general basis for allocating participants to a particular treatment. 80,82,83 In two studies,82,83 allocation to groups was dictated by changes to the treatment protocol in the study centres (i.e. they were historically controlled studies). One study compared two retrospective cohorts (for which treatment allocation was unrelated to the study design). 80 Despite being a prospective non-randomised, controlled trial, the remaining study did not report the basis for allocation to treatment groups. 81
Avoidance of selection bias: none of the four studies provided evidence that selection bias (to the study overall and to treatment groups) was minimised within the context of the study design. All four studies were rated as ‘unclear’ with regards minimisation of selection bias. Two studies did not confirm whether or not all eligible participants were recruited for either group. 82,83 The other two studies did state that all transplanted children and adolescents were included in the study but did not clearly describe how participants were allocated to treatment groups, so the extent of possible selection bias to groups is not clear. 80,81
Similarity of groups
Baseline characteristics: three out of the four studies did not clearly report whether or not treatment groups were similar at baseline on a range of prognostic factors and they omitted descriptive statistical information (see Table 17 for a summary of baseline characteristics). 80–82 In two studies,80,83 the age of participants statistically significantly differed between treatment groups. In addition, although the groups were reported not to be significantly different for gender, the percentages of males appeared to be different (6/12, 50% and 8/12, 66.7%, respectively) in one small study. 83
Implementation of masking
None of the four non-randomised studies reported whether or not treatment allocation was masked from treatment providers, outcome assessors or participants. However, for three of the studies this was not applicable, because blinding could not be reasonably expected given the study design. 80,82,83 The remaining study was a prospective non-RCT, therefore, masking of care providers, outcome assessors (by using independent assessors) and participants could be done but was not reported. 81
Length of follow-up
Three of the non-randomised studies had an adequate length of follow-up, with all participants followed for at least 6 months. 81–83 The remaining study was rated as ‘partial’ because not all participants were followed for at least 6 months but DGF was included as an outcome (this outcome would usually be assessed within the first month of transplantation). 80
Completeness of trials
All four of the non-randomised studies adequately described the completeness of the study, either by describing withdrawals or drop-outs (including reasons) or by making it clear that all enrolled participants completed the study.
Adjustment for bias in non-randomised studies
This item of the quality appraisal assessment was applicable to all four studies. However, two of the studies did not perform any adjustment for bias in their analyses. 82,83 For the other two studies, analyses were not fully reported, so this could not be assessed. 80,81
Applicability of trials to the current NHS in England
None of the non-randomised studies was considered to be clearly applicable to the NHS in England. Two studies were rated as inadequate because the study population was not representative of the current NHS in England. In one of these studies, all kidneys were from living related donors81 and in the other, > 90% of kidneys were from cadaveric donors. 83 The other two studies were both rated as unclear because the populations were not recruited from the European Union, but it was not clear to what extent the population characteristics could generalise to the NHS in England. 80,82
Baseline characteristics
Randomised controlled studies
Baseline characteristics of the three included RCTs73,75,77 are summarised in Table 16. All three studies were conducted over multicentres in Europe. Only Offner et al. 73 reported the countries involved (Germany, France and Switzerland). 73 Mean age across the studies’ arms ranged from 10.1 years to 11.5 years. The proportion of adolescents (with 12 or 13 years old being the cut-off point for adolescence in the three studies; see Table 16 for details) is 36.6% to 54.4% across the study arms. Boys represented 56.0–67.4% of participants. Two studies had a high proportion of white participants (87–95%),73,77 with one trial not reporting ethnicity. 75 The proportion of living donors across the study arms ranges from 15.5% to 35.8%. The proportion of first transplants is high, ranging from 85% to 96% across the arms. Finally, HLA antigen mismatch ranges from 2.3 to 2.7 across the three trials. A close antigen match is no longer considered critical owing to the more effective immunosuppressive therapy, but a better HLA match may lead to longer graft survival.
| Study | Induction | Maintenance | n a | Mean age, years (SD) | Adolescents n/N, % | First transplant n/N, % | Male n/N, % | Donor type n/N, % | Ethnic groupb n/N, % | Mean HLA mismatches Mean (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Living | Deceased | ||||||||||
| Offner et al.73 | BAS | CSA + MMF + CCS | 100 | 10.7 (4.6) | 43/100, 43c | 96/100, 96 | 56/100, 56.0 | 30/100, 30 | 70/100, 70 | 95/100 white, 95 | 2.6 (1.2) |
| 5/100 other, 5 | |||||||||||
| PBO | 92 | 10.8 (4.9) | 43/92, 46.7c | 88/92, 96 | 62/92, 67.4 | 32/92, 34.8 | 60/92, 65.2 | 84/92, white 91.3 | 2.2 (1.0) | ||
| 8/92, other 8.7 | |||||||||||
| Grenda et al.75 | BAS | TAC + AZA + CCS | 99 | 11.5 (4.1) | 53/99 53.5d | 95/99, 96 | 62/99, 62.6 | 20/99 20.2 | 79/99 79.8 | NR | 2.5 (NR) |
| NI | 93 | 11.3 (4.0) | 51/93 54.4d | 87/93, 93.5 | 57/93, 61.3 | 16/93 17.2 | 77/93 82.8 | NR | 2.3 (NR) | ||
| Trompeter et al.77 | Methylprednisolone | TAC + AZA + CCS | 103 | 10.5 (4.6) | 41/103 39.8e | 94/103, 91 | 64/103, 62.1 | 16/103, 15.5 | 87/103, 84.5 | 90/103, white 87.4 | 2.5 (NR) |
| 1/103, black 1 | |||||||||||
| 1/103, oriental 1 | |||||||||||
| 11/103, other 10.7 | |||||||||||
| CSA + AZA + CCS | 93 | 10.1 (4.5) | 34/93, 36.6e | 79/93, 85 | 56/93, 60.2 | 15/93, 16.1 | 78/93, 83.1 | 82/92, white 88.2 | 2.7 (NR) | ||
| 0/92, black 0 | |||||||||||
| 3/92, oriental 3.2 | |||||||||||
| 8/92, other 8.6 | |||||||||||
Non-randomised studies
Similarly, baseline characteristics of the four included non-randomised studies [Antoniadis et al. 81 (non-RCT), Benfield et al. 82 (historically controlled study), Garcia et al. 80 (retrospective cohort study) and Staskewitz et al. 200183 (historically controlled study)] are summarised in Table 17. 80–83 The Antoniadis et al. 81 study was conducted in one Greek centre, the Benfield et al. 82 study was conducted in two centres in the USA and the Staskewitz et al. 83 study was conducted in 12 German centres. Garcia et al. 80 did not report where or within how many centres their study was performed, but the authors are all based in Brazil and, therefore, it is likely that this study was completed in Brazil. Not surprisingly, the baseline characteristics of the non-RCTs vary not only across the studies, but also within the studies. Mean age across the study arms ranges from 9.0 years to 11.5 years; however, none of the non-RCTs reports the proportion of adolescents included. Boys represented 50.0–66.7% of participants. Two studies had a high proportion of white participants (75–100%),80,83 one study reported that between 19% and 25% of participants were black (dependent on treatment group),82 while one study did not report ethnicity. 81 Most studies included a high proportion of living donors (75 –100%). However, one study reported only 6% living donors in one treatment group and 9% in the other treatment group. 83 This was the only study reporting mean HLA mismatches (2.69–2.89). 83
| Study | Induction | Maintenance therapy | N a | Mean age, years (SD) | Adolescents n/N, % | First transplant n/N, % | Male n/N, % | Donor type n/N, % | Ethnic group n/N, % | Mean HLA mismatches (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Living | Deceased | ||||||||||
| Antoniadis et al.81 | Methylprednisolone | CSA + MMF + CCS | 7 | 10 (4–12)b | NR | NR | NR | 7/7, 100 | N/A | NR | NR |
| CSA + AZA + CCS | 7 | NR | NR | NR | 7/7, 100 | N/A | NR | NR | |||
| cBenfield et al.82 | OKT3 | CSA + MMF + CCS | 17 | 10.7 (5.3) | NR | NR | 20/36, 55 | 25/36, 69 | 11/36, 31 | 9/36, 25 black | NR |
| CSA | CSA + MMF + CCS | 19 | NR | NR | NR | ||||||
| OKT3 | CSA + AZA + CCS | 17 | 9.4 (5.1) | NR | NR | 19/31, 61 | 24/31, 77 | 12/31, 39 | 6/31, 19 black | NR | |
| CSA | CSA + AZA + CCS | 14 | NR | NR | NR | ||||||
| Garcia et al.80 | BAS | TAC + AZA + CCS | 12 | 11.3 (9.3) | NR | NR | 6/12, 50% | 8/12, 66.7 | 4/12, 33.3 | 11/12, 91.7 | NR |
| CSA + MMF + CCS | 12 | 9.0 (6) | NR | NR | 8/12, 66.7 | 7/12, 58.3 | 5/12, 41.7 | 9/12, 75 | NR | ||
| Staskewitz et al.83 | Prednisone/methylprednisolone | CSA + MMF + CCS | 85d | 11.5 (3.6) | NR | 61/65, 94 | 42/65, 65 | 4/65, 6 | 61/65, 94 | 65/65, 100 Caucasian | 2.69 (0.87) |
| NR | CSA + AZA + CCS | 54 | 9.9 (4.7) | NR | 53/54, 98 | 32/54, 59 | 5/54, 9 | 49/54, 91 | 54/54, 100 Caucasian | 2.89 (0.96) | |
Results of the included studies
No studies were identified that evaluated growth or HRQoL in the use of induction immunosuppression therapy in renal transplantation in children and adolescents. In addition, no studies that would allow analyses of adherence to treatment and the use of treatments in conjunction with either CCS or CNI reduction or withdrawal strategies were identified.
A summary comparing our results with those of the adult kidney transplant population (using evidence from parallel HTA appraisal ‘Immunosuppressive therapy for kidney transplantation in adults’68) is made at the end of this section. Briefly, 11 induction trials, 73 maintenance trials and two trials of both induction and maintenance were included in the parallel HTA.
Induction therapy
Two RCTs of induction therapy73,75 (reported in four publications73–76 and one abstract72) in children and adolescents were identified in the review; the population characteristics are summarised in Table 16. Offner et al. 73 compared BAS induction therapy with PBO: BAS + CSA + MMF + CCS versus PBO + CSA + MMF + CCS. Grenda et al. 75 compared BAS induction therapy with no induction: BAS + TAC + AZA + CCS versus TAC + AZA + CCS. No RCTs were identified that evaluated r-ATG in children and adolescents.
No non-RCTs in the child/adolescent population evaluated induction therapies.
Mortality
Both RCTs73,75 provided data on mortality for BAS versus no induction or PBO (Table 18). Grenda et al. 75 reported the longest follow-up data at 2 years post transplant. No evidence of a statistically significant difference in overall survival between BAS and comparator arms was reported at any time point.
| Study | Treatment | 3 months | 6 months | 1 year | 2 years | ||||
|---|---|---|---|---|---|---|---|---|---|
| n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | ||
| aOffner et al.73 | BAS + CSA + MMF + CCS | 1/100, 1 | 2.79 (0.11 to 69.31) | 2/100, 2 | 4.69 (0.22; 99.10) | 3/100, 3 | 6.64 (0.34 to 130.33) | NR | N/A |
| PBO + CSA + MMF + CCS | 0/92, 0 | 0/92, 0 | 0/92, 0 | NR | |||||
| Grenda et al.75 | BAS + TAC + AZA + CCS | NR | N/A | 0/99, 0 | N/A | NR | N/A | 0/99, 0 | 0.33 (0.01 to 8.20) |
| NI + TAC + AZA + CCS | NR | 0/93, 0 | NR | 1/93, 1 | |||||
Summary
In summary, there was no evidence that BAS improved survival when compared with PBO or no induction. This is similar to the conclusions of the previous HTA. 2
Graft loss
Both RCTs73,75 provided data on graft loss for BAS versus no induction or PBO (Table 19). Grenda et al. 75 reported the longest follow-up data of 2 years. No evidence of a significant difference between the BAS and control arms was reported for any data point.
| Study | Treatment | 6 months | 1 year | 2 years | |||
|---|---|---|---|---|---|---|---|
| n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | ||
| Offner et al.73 | BAS + CSA + MMF + CCS | 1/100, 1 | 0.92 (0.06 to 14.92) | 1/100, 1 | 0.92 (0.06 to 14.92) | NR | N/A |
| PBO + CSA + MMF + CCS | 1/92, 1 | 1/92, 1 | NR | ||||
| Grenda et al.75 | BAS + TAC + AZA + CCS | 5/99, 5 | 0.94 (0.26 to 3.34) | NR | N/A | 5/99, 5 | 0.50 (0.16 to 1.54) |
| NI + TAC + AZA + CCS | 5/93, 5 | NR | 9/93, 10 | ||||
The pooled results at 6-month follow-up did not find any significant difference between BAS and control arms for graft loss [OR = 93 favours BAS; 95% confidence interval (CI) 0.29 to 2.97, I2 = 0%, τ2 = 0; Figure 9].
Summary
In summary, there was no evidence that BAS lowered graft loss when compared with PBO or no induction. This is similar to the conclusions of the previous HTA. 2
Graft function
Both RCTs73,75 reported graft function estimated using the Schwartz equation (ml/minute/1.73 m2; Table 20). There were no statistically significant differences between BAS and control arms at any data point (between 6 months and 2 years). Both RCTs reported 6-month and 2-year follow-ups, no standard deviation (SD) was reported at 2 years by Offner et al. 73 and no SD was reported at 6 months or 2 years by Grenda et al. 75
| Study | Treatment | 6 months | 1 year | 2 years | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | t-test (p-value) | Mean (SD) | t-test (p-value) | Mean (SD) | t-test (p-value) | ||
| aOffner et al.73 | BAS + CSA + MMF + CCS | 80 (27) | –1.73 (0.08) | 79 (23) | –0.88 (0.38) | 80 (NR) | –0.92 (0.36) |
| PBO + CSA + MMF + CCS | 87 (29) | 82 (24) | 84 (NR) | ||||
| bGrenda et al.75 | BAS + TAC + AZA + CCS | 77.6 (NR) | –0.48 (0.63) | NR | N/A | 66.7 (NR) | 0.22 (0.82) |
| NI + TAC + AZA + CCS | 79.4 (NR) | NR | 65.8 (NR) | ||||
To allow for combining the results at 6-month and 2-year follow-ups, a SD of 26 ml/minute/1.73 m2 was used (‘average’ SD calculated from SD available at 6-month and 2-year follow-ups; Figure 10). The pooled results do not suggest any difference for eGFR between BAS and control arms: weighted mean difference (WMD) = –4.20 (favours controls; 95% CI –9.60 to 1.20, I2 = 0%) at 6 months and WMD = –1.38 (favours controls; 95% CI –7.20 to 4.44, I2 = 0%) at 2 years. Grenda et al. 75 also reported incidences of DGF (defined as requiring dialysis for more than 1 day during the first study week). The rate of DGF was not statistically significantly different between the two arms: 11 out of 99 participants (11%) and 5 out of 93 participants (5%) in BAS and no induction arms, respectively (p-value was not reported). 75
Summary
In summary, there was no evidence that BAS lowered graft function when compared with PBO or no induction. The child/adolescent RCT evidence identified in the previous HTA review2 concluded that BAS did not increase serum creatinine levels at 1-year follow-up when compared with no induction.
Acute rejection
Both RCTs73,75 provided data on biopsy-proven acute rejection (BPAR) for BAS versus no induction or PBO (Table 21). Grenda et al. 75 reported the longest follow-up data of 2 years. No evidence of a statistically significant difference between the BAS and the comparators arms was reported for any data point. The pooled results at 6 months did not find any difference between BAS and control arms for BPAR: OR = 0.71 (favours BAS; 95% CI 0.40 to 1.27, I2 = 15.7%, τ2 = 0.03; Figure 11).
| Study ID | Treatment | 3 months | 6 months | 1 year | 2 years | ||||
|---|---|---|---|---|---|---|---|---|---|
| n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | ||
| Offner et al.73 | BAS + CSA + MMF + CCS | 6/100, 6 | 0.39 (0.14 to 1.07) | 11/100, 11 | 0.51 (0.23 to 1.14) | 13/100, 13 | 0.51 (0.24; 1.08) | NR | N/A |
| PBO + CSA + MMF + CCS | 13/92, 14 | 18/92, 20 | 21/92, 23 | NR | |||||
| Grenda et al.75 | BAS + TAC + AZA + CCS | NR | N/A | 19/99, 19 | 0.93 (0.46 to 1.87) | NR | N/A | 23/99, 23 | 0.74 (0.39 to 1.40) |
| NI + TAC + AZA + CCS | NR | 19/93, 20 | NR | 27/93, 29 | |||||
In addition, Grenda et al. 75 also reported BPAR separately for younger and older age groups (< 12 years and ≥ 12 years, respectively). The incidence of BPAR was lower in the patients < 12 years in the no induction arm (4/42, 10%) than the same age group with BAS (6/46, 13%), although this difference was not statistically significant (Wilcoxon–Gehan test, p-value was not reported). Conversely, incidences of BPAR were higher for the patients ≥ 12 years with no induction (15/51, 29%) than the same age group with BAS (13/53, 25%); however, again, this difference was not statistically significant (Wilcoxon–Gehan test, p-value was not reported).
Finally, the data from Offner et al. 75 of 79 BAS and 65 PBO on study participants (reported in an abstract by Jungraithmayr et al. 72) found a cumulative AR rate of 33% versus 35% in the BAS and PBO arms, respectively, at 2 years and a cumulative AR rate of 41% versus 45% in the BAS and PBO arms, respectively, at 5 years. Results were not statistically significant at either data point. 72
Time to BPAR (Table 22) was only reported by Grenda et al. 75 The median time to BPAR appears to be similar between the two arms (p-values were not reported in the study). 75 Time to first BPAR episode or treatment failure within the first 6 months post transplant was the primary efficacy end point in Offner et al. 73 The proportion of children and adolescents (Kaplan–Meier estimates) achieving this efficacy point was 16.7% in the BAS arm and 21.7% in the PBO arm. The difference was not statistically significant; HR of 0.72 (favours BAS; 95% CI 0.42 to 1.26). 73
| Study | Treatment | Time to AR median (range), days |
|---|---|---|
| Grenda et al.75 | BAS + TAC + AZA + CCS | 41 (2–176) |
| NI + TAC + AZA + CCS | 43 (1–150) |
Severity of BPAR was reported by Offner et al. 73 and Grenda et al. 75 (Table 23). All BPAR episodes in BAS treated patients were mild (grade IA or IB), whereas 8 out of 18 episodes in the PBO group were moderate (grade IIA) in Offner et al. 73 Similarly, there seemed to be more moderate BPAR episodes (Banff 2) in the no induction group than the BAS group in Grenda et al. 75 However, Offner et al. 73 also performed biopsies in children who had not recently experienced clinical signs of rejection or undergone biopsy (at 6 months, n = 64 and n = 60 in BAS and PBO groups, respectively) to identify subclinical rejections. The rate (p = 0.055) and severity (p-value not reported) of subclinical rejections was higher in the BAS group (25.0%) than in the PBO group (11.7%). 73
| Study | BAS + CSA + MMF + CCS n events/N participants (%) | PBO/NI + CSA + MMF + CCS n events/N participants (%) | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BPAR | Banff 1 | Banff 2 | Banff 3 | BPAR | Banff 1 | Banff 2 | Banff 3 | ||
| Grenda et al.75 | 19/99 (19.2) | 15/99 (15) | 3/99 (3) | 1/99 (1) | 19/93 (20.4) | 11/93 (12) | 7/93 (8) | 1/93 (1) | NR |
| aOffner et al.73 | 11/100 (11) | Grade IA: 8/100 (8) | Grade IIA: 0/100 (0) | 0/100 (0) | 18/92 (19.6) | Grade IA: 9/92 (10) | Grade IIA: 8/92 (9) | 0/92 (0) | 0.308b |
| Grade IB: 3/100 (3) | Grade IIB: 0/100 (0) | Grade IB: 1/92 (1) | Grade IIB: 0/92 (0) | ||||||
Summary
In summary, there was no evidence that BAS reduced incidences of, severity and time to BPAR when compared with PBO or no induction. This is similar to the conclusions of the previous HTA; no significant differences in BPAR for BAS versus no therapy in children were found. 2
Adverse events
Two RCTs73,75 provided data on AEs for BAS versus no induction or PBO. Offner et al. 73 reported AEs that occurred in at least 10% of the safety population. Grenda et al. 75 reported AEs that occurred in at least 10% in either treatment arm. The AEs reported in these trials are summarised in Table 24.
| AE | Follow-up | aOffner et al.73 | bGrenda et al.75 | ||||
|---|---|---|---|---|---|---|---|
| BAS n events/N participants, % | PBO n events/N participants, % | OR (95% CI) | BAS n events/N participants, % | NI n events/N participants, % | OR (95% CI) | ||
| Any infections | 1 year | 104/109, 95 | 84/93, 90 | 2.23 (1.03 to 4.68) | NR | NR | N/A |
| 1–2 years | 13/79, 16 | 12/65, 12 | 0.87 (0.37 to 2.06) | NR | NR | N/A | |
| Serious infections | 1 year | 58/109, 53 | 45/93, 48 | 1.21 (0.72 to 2.05) | NR | NR | N/A |
| 2 years | NR | NR | N/A | NR | NR | N/A | |
| UTI | 6 months | NR | NR | N/A | 19/99, 19 | 26/93, 28 | 0.61 (0.31 to 1.20) |
| 1 year | 38/109, 29 | 21/93, 23 | 1.84 (0.99 to 3.40) | NR | NR | N/A | |
| Bacterial infections | 6 months | NR | NR | N/A | 32/99, 32 | 30/93, 32 | 1.00 (0.55 to 1.81) |
| 2 years | NR | NR | N/A | 47/99, 45 | 45/93, 48 | 0.96 (0.56 to 1.65) | |
| Viral infections | 6 months | NR | NR | N/A | 15/99, 15 | 15/93, 16 | 0.93 (0.43 to 2.02) |
| 2 years | NR | NR | N/A | 26/99, 26 | 24/93, 26 | 1.02 (0.54 to 1.93) | |
| CMV infections | 6 months | NR | NR | N/A | 7/99, 7 | 2/93, 2 | 3.46 (0.70 to 17.11) |
| 1 year | 14/109, 13 | 8/93, 9 | 1.57 (0.63 to 3.92) | NR | NR | N/A | |
| EBV infections | 1 year | 10/109, 9 | 11/93, 12 | 0.75 (0.30 to 1.86) | NR | NR | N/A |
| Solid tumour | 6 months | NR | NR | N/A | 0/99, 0 | 0/93, 0 | N/A |
| 1 year | 1/109, 1 | 0/93, 0 | 2.58 (0.10 to 64.19) | NR | NR | N/A | |
| PTLD | 6 months | NR | NR | N/A | 0/99, 0 | 2/93, 2 | 0.18 (0.01 to 3.88) |
| 1 year | 2/109, 2 | 5/93, 5 | 0.33 (0.06 to 1.74) | NR | NR | N/A | |
| 2 years | NR | NR | N/A | 1/99, 1 | 2/93, 2 | 0.46 (0.04 to 5.21) | |
| Hypertension | 6 months | NR | NR | N/A | 34/99, 34 | 36/93, 39 | 0.83 (0.47 to 1.47) |
| Any AE | 6 months | NR | NR | N/A | 91/99, 92 | 84/93, 90 | 1.22 (0.58 to 2.57) |
| 1 year | 108/109, 99 | 92/93, 99 | 1.17 (0.16, 8.59) | NR | NR | N/A | |
In one trial,73 more infections were found with BAS than with PBO (OR = 2.23; favours PBO; 95% CI 1.03 to 4.68). 73 In Grenda et al. 75 toxic nephropathy was higher in the BAS arm than in the no induction arm (14.1% vs. 4.3%, respectively; p = 0.03). Similarly, abdominal pain was higher in the BAS arm than no induction (11.1% vs. 2.2%, respectively; p = 0.02). 75
Grenda et al. 75 also reported changes in glucose metabolism disorders. None of the children and adolescents had a glucose metabolism disorder {described as glucose tolerance decreased, hyperglycaemia or diabetes mellitus using the modified coding symbols for a thesaurus of adverse reaction terms [The Coding Symbols for a Thesaurus of Adverse Reaction Terms (COSTART) dictionary]} at baseline. However, during the study, 13 patients (13.1%) in the BAS arm and 10 patients (10.8%) in the no induction arm developed a glucose metabolism disorder within the first 6 months. One new case of impaired glucose metabolism was noted at 1 year; this new case resolved at 2 years.
Summary
In summary, more infections were found with BAS than with PBO (OR = 2.23, favours PBO; 95% CI 1.03 to 4.68). 73 In addition, Grenda et al. 75 found that toxic nephropathy and abdominal pain were higher in the BAS arm than in the no induction arm (p = 0.03 and p = 0.02, respectively). The previous HTA reported post-transplant diabetes mellitus in only one study90 and the rest of the data were confidential and were, therefore, omitted from the report.
Maintenance therapy
One RCT77 and four non-RCTs80–83 of maintenance therapy in children and adolescents were included in the review. RCT evidence evaluating TAC and non-RCT evidence on the use of TAC and MMF was identified.
The population characteristics from the one RCT of maintenance treatment identified in the review are summarised in Table 16. Trompeter et al. 77 compared the use of TAC + AZA + CCS with CSA + AZA + CCS. 77 No RCTs evaluated TAC-PR, MMF, MPS, EVL, SRL and BEL in children and adolescents.
The population characteristics from the four non-RCTs of maintenance treatment identified in the review80–83 are summarised in Table 17. Garcia et al. 80 compared the use of BAS + TAC + AZA + CC with BAS + CSA + MMF + CCS in a retrospective cohort study. Antoniadis et al. 81 compared the use of CSA + MMF + CCS with CSA + AZA + CCS in a non-RCT. Benfield et al. 82 reported retrospective analyses of a randomised, multicentre trial of OKT3 (a murine monoclonal antibody muromonab-CD3) versus CSA induction therapy with two types of maintenance therapies, but only the comparison of CSA + MMF + CCS with CSA + AZA + CCS was included in this review. Finally, Staskewitz et al. 83 compared the use of CSA + MMF + CCS with CSA + AZA + CCS in a historically controlled study. No non-randomised evidence was identified regarding the use of TAC-PR, MPS, EVL, SRL and BEL in the child/adolescent population.
Mortality
Randomised controlled trials
Trompeter et al. 77 compared the use of TAC + AZA + CCS with CSA + AZA + CCS. The trial reported similar survival rates in both arms, which were not significantly different at 6 months, 1 year, 2 years or 4 years (Table 25).
| Follow-up | Trompeter et al.77 | ||
|---|---|---|---|
| TAC + AZA + CCS n events/N participants, % | CSA + AZA + CCS n events/N participants, % | OR (95% CI) | |
| 6 months | 3/103, 3 | 3/93, 3 | 0.90 (0.18 to 4.58) |
| 1 year | 3/103, 3 | 3/93, 3 | 0.90 (0.18 to 4.58) |
| 2 years | 3/103, 3 | 4/93, 4 | 0.67 (0.15 to 3.07) |
| 4 years | 5/103, 5 | 4/93, 4 | 1.14 (0.30 to 4.36) |
Non-randomised controlled trials
Three non-RCTs80,81,83 provided data on mortality (Table 26) and two trials compared MMF with AZA. 81,83 The remaining study80 compared TAC + AZA with CSA + MMF. Staskewitz et al. 83 reported long-term follow-up of up to 5 years, but no further deaths were recorded in either arm. No statistically significant difference in child/adolescent survival between MMF and AZA and between TAC + AZA and CSA + MMF was reported.
| Study | Treatment | 3 months | 6 months | 1 year | |||
|---|---|---|---|---|---|---|---|
| n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | ||
| Garcia et al.80 | BAS + TAC + AZA + CCS | 0/12, 0 | N/A | NR | N/A | NR | N/A |
| BAS + CSA + MMF + CCS | 0/12, 0 | NR | NR | ||||
| Antoniadis et al.81 | CSA + MMF + CCS | NR | N/A | NR | N/A | 0/7, 0 | N/A |
| CSA + AZA + CCS | NR | NR | 0/7, 0 | ||||
| aStaskewitz et al.83 | CSA + MMF + CCS | NR | N/A | 0/86, 0 | 0.20 (0.008 to 5.14) | 0/86, 0 | 0.08 (0.004 to 1.67) |
| CSA + AZA + CCS | NR | 1/54, 2 | 3/54, 6 | ||||
Summary
In summary, no difference in survival was found between TAC and CSA from the child/adolescent RCT. In addition, no difference was found between TAC and CSA, and between MMF and AZA, in the child/adolescent non-RCT evidence. This is similar to the conclusions of the previous HTA. 2
Graft loss
Randomised controlled trials
Trompeter et al. 77 compared the use of TAC + AZA + CCS with CSA + AZA + CCS. Graft loss appeared to be higher in the CSA arm than in the TAC arm, especially at the longer follow-up (2–4 years), but the difference was not statistically significant (Table 27).
| Follow-up | Trompeter et al.77 | ||
|---|---|---|---|
| TAC + AZA + CCS n events/N participants, % | CSA + AZA + CCS n events/N participants, % | OR (95% CI) | |
| 6 months | 6/103, 6 | 13/93, 14 | 0.38 (0.14 to 1.05) |
| 1 year | 8/103, 8 | 15/93, 16 | 0.44 (0.18 to 1.09) |
| 2 years | 8/103, 8 | 16/93, 17 | 0.41 (0.16 to 1.00) |
| 4 years | 9/103, 9 | 17/93, 18 | 0.43 (0.18 to 1.01) |
Non-randomised controlled trials
Three non-RCTs80,81,83 provided data on graft loss (Table 28). Two trials compared MMF with AZA. 81,83 The remaining study80 compared TAC + AZA with CSA + MMF. Staskewitz et al. 83 found better graft survival in MMF than with AZA in up to a 5-year follow-up,83 while Antoniadis et al. 81 did not find a statistically significant difference in graft loss between MMF and AZA. No statistically significant difference in graft loss between TAC + AZA and CSA + MMF regimens was reported. 80
| Study | Treatment | 3 months | 1 year | 2 years | 3 years | 4 years | 5 years | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | ||
| Garcia et al.80 | BAS + TAC + AZA + CCS | 0/12, 0 | 0.30 (0.01 to 8.30) | NR | N/A | NR | N/A | NR | N/A | NR | N/A | NR | N/A |
| BAS + CSA + MMF + CCS | 1/12, 8 | NR | NR | NR | NR | NR | |||||||
| Antoniadis et al.81 | CSA + MMF + CCS | NR | N/A | 0/7, 0 | N/A | NR | N/A | NR | N/A | NR | N/A | NR | N/A |
| CSA + AZA + CCS | NR | 0/7, 0 | NR | NR | NR | NR | |||||||
| Staskewitz et al.83 | CSA + MMF + CCS | NR | N/A | 2/86, 2 | 0.14 (0.03 to 0.68) | 4/86, 5 | 0.24 (0.07 to 0.84) | 4/86, 5 | 0.15 (0.05 to 0.51) | 7/86, 8 | 0.25 (0.09 to 0.69) | 8/86, 9 | 0.24 (0.09 to 0.63) |
| CSA + AZA + CCS | NR | 8/54, 15 | 9/54, 17 | 13/54, 24 | 14/54, 26 | 16/54, 30 | |||||||
Summary
In summary, no statistically significant difference was found between TAC and CSA for graft loss. However, the RCT child/adolescent evidence identified in the previous HTA review2 concluded that TAC lowered graft loss at 2- (10/103 vs. 19/93; p = 0.03) and 4-year follow-ups (11/103 vs. 20/93; p = 0.03). This discrepancy in result is because we have excluded graft loss due to death from our analyses. This was, first, to avoid double counting with another key outcome (mortality) and, second, because death-censored graft survival is a well-established clinical outcome to which death with functioning graft (DWFG) is intrinsically related, just as mortality is to overall survival. It should be noted that after the removal of graft loss due to death from the analyses, the evidence from Trompeter et al. 77 suggested borderline statistically non-significantly lower graft loss in TAC than CSA (OR = 0.41, 95% CI 0.16 to 1.00, and OR = 0.43, 95% CI 0.18 to 1.01 at 2- and 4-year follow-ups, respectively). In addition, the current review and the previous HTA2 found better graft survival in MMF than in AZA (up to 5-year follow-up) in one non-RCT. 83
Graft function
Randomised controlled trials
Trompeter et al. 77 compared the use of TAC + AZA + CCS with CSA + AZA + CCS and reported graft function estimated using the Schwartz equation (ml/minute/1.73 m2). Significantly higher graft function in the TAC arm than in the AZA arm was reported (Table 29). No data on DGF were reported. 77
| Follow-up | Trompeter et al.77 | ||
|---|---|---|---|
| TAC + AZA + CCS, mean (SD), n participants | CSA + AZA + CCS, mean (SD), n participants | t-test (p-value) | |
| 6 months | 65.6 (19.9), 91 | 61.2 (15.8), 86 | 1.62 (0.11) |
| 1 yeara | 64.9 (20.7), 84 | 57.8 (21.9), 77 | 2.11 (0.04) |
| 2 years | 64.9 (19.8), 71 | 51.7 (20.3), 66 | 3.85 (< 0.01) |
| 3 years | 66.7 (26.4), 81 | 53.0 (23.3), 55 | 3.11 (< 0.01) |
| 4 years | 71.5 (22.9), 51 | 53.0 (21.6), 44 | 4.03 (< 0.01) |
Non-randomised controlled trials
Only one non-RCT provided data on graft function. Garcia et al. 80 compared TAC + AZA with CSA + MMF and reported graft function at a 3-month follow-up (Table 30). There were no significant differences between the arms for graft function [eGFR, creatinine clearance (ml/minute)]. Garcia et al. 80 also reported incidences of DGF. The same rate of DGF was reported in the two arms [1/12 (8%) and 1/12 (8%), respectively]. 80
| Study ID | Treatment | 3 months | |
|---|---|---|---|
| Mean (SD) | t-test (p-value) | ||
| Garcia et al. 200280 | BAS + TAC + AZA + CCS | 71 (23) | –1.28 (0.21) |
| BAS + CSA + MMF + CCS | 82 (19) | ||
Summary
In summary, lower graft function was associated with TAC compared with CSA in the child/adolescent RCT. This is similar to the conclusions of the previous HTA. 2 In addition, no difference in graft function between TAC + AZA and CSA + MMF regimens was reported in the one non-RCT. 80 However, the previous HTA included a non-RCT by Neu et al. 94 which found significantly better graft function at 1- and 2-year follow-ups (p < 0.01).
Acute rejection
Randomised controlled trials
Trompeter et al. 77 compared the use of TAC + AZA + CCS with CSA + AZA + CCS, reporting statistically significantly higher BPAR at a 6-month follow-up, and AR (which was not biopsy proven) at 6-month and 1-year follow-ups in the CSA arm compared with the TAC arm (Table 31). In addition, 2- and 4-year follow-up data are available for Trompeter et al. 77 in Filler et al. 79 However, these analyses do not take into account those who were lost to follow-up and those who died. In the second year of the trial, 7 out of 77 patients in the TAC group and 9 out of 71 patients in the CSA group experienced AR (p = 0.6041, Fisher’s exact test). 79 In the third year, 2 out of 70 patients in the TAC group and 6 out of 57 patients in the CSA group experienced AR (p = 0.1454, Fisher’s exact test). 79 Finally, in the fourth year, 2 out of 57 patients in the TAC group and 6 out of 42 patients in the CSA group experienced AR (p = 0.1359, Fisher’s exact test). 79 Rejection episodes frequently occurred in the same patients that experienced AR previously. Although overall treatment group differences were maintained after the first year, the annual differences in AR were not statistically significant for years 2, 3 and 4. 79 Time to, and severity of, AR were not reported in Trompeter et al. 77
| Study ID | Acute rejection | Treatment | 6 months | 1 yeara | ||
|---|---|---|---|---|---|---|
| n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | |||
| Trompeter et al. 200277 | BPARb | TAC + AZA + CCS | 17/94, 18 | 0.29 (0.15 to 0.57) | NR | N/A |
| CSA + AZA + CCS | 37/86, 43 | NR | ||||
| AR | TAC + AZA + CCS | 38/103, 37 | 0.40 (0.23 to 0.71) | 42/103, 41 | 0.43 (0.25 to 0.76) | |
| CSA + AZA + CCS | 55/93, 59 | 57/93, 62.3 | ||||
Non-randomised controlled trials
Four non-RCTs80–83 provided data on BPAR (Table 32)80–83 and three studies compared MMF with AZA. 81–83 The remaining study80 compared TAC + AZA with CSA + MMF. No statistically significant difference in BPAR was found between the MMF arm and AZA arms, and between TAC + AZA and CSA + MMF.
| Study | Treatment | 3 months | 6 months | ||
|---|---|---|---|---|---|
| n events/N participants, % | OR (95% CI) | n events/N participants, % | OR (95% CI) | ||
| Garcia et al. 200280 | BAS + TAC + AZA + CCS | 1/12, 8 | 0.45 (0.04 to 5.78) | NR | N/A |
| BAS + CSA + MMF + CCS | 2/12, 17 | NR | |||
| Antoniadis et al. 199881 | CSA + MMF + CCS | NR | N/A | 0/7, 0 | 0.08 (0.003 to 1.94) |
| CSA + AZA + CCS | NR | 3/7, 43 | |||
| Staskewitz et al. 200183 | CSA + MMF + CCS | NR | N/A | 10/65, 15 | 0.52 (0.21 to 1.29) |
| CSA + AZA + CCS | NR | 14/54, 26 | |||
| Benfield et al. 199982 | CSA + MMF + CCS | NR | N/A | 4/17, 24a | 0.56 (0.13 to 2.47) |
| CSA + AZA + CCS | NR | 6/17, 35 | |||
The pooled results at a 6-month follow-up suggested borderline statistically non-significantly lower BPAR in MMF compared with AZA (OR = 0.48, 95% CI 0.23 to 1.02, I2 = 0%, τ2 = 0; Figure 12).
In addition, Garcia et al. 80 reported the severity of AR (Table 33); one Banff 3 episode was reported in TAC + AZA and two Banff 1 episodes were reported in CSA + MMF. No study reported time to BPAR.
| Study | Treatment | 3 month n events/N participants, % | ||
|---|---|---|---|---|
| Banff 1 | Banff 2 | Banff 3 | ||
| Garcia et al. 200280 | BAS + TAC + AZA + CCS | 0/12, 0 | 0/12, 0 | 1/12, 8 |
| BAS + CSA + MMF + CCS | 2/12, 17 | 0/12, 0 | 0/12, 0 | |
Summary
In summary, higher rates of BPAR were found in CSA than TAC in the one included child/adolescent RCT with 6-month data. 77 The RCT child/adolescent evidence identified in the previous HTA review2 also concluded more BPAR in the CSA arm than the TAC. 77 However, the limited longer follow-up data from this study did not find statistically significant differences in AR between TAC and CSA at 2- and 4-year follow-ups. 79 In addition, no statistically significant difference in BPAR was found between the MMF arm and AZA arms, and between TAC + AZA and CSA + MMF arms in the non-randomised evidence. The pooled non-RCT child/adolescent evidence identified in the previous HTA review suggested less BPAR with MMF compared with AZA [relative risk (RR) = 0.39 favours MMF; 95% CI 0.19 to 0.79]. Similarly, our analyses suggested borderline statistically non-significantly lower BPAR in MMF than AZA at 6-month follow-up (OR = 0.48, 95% CI 0.23; 1.02, I2 = 0%, τ2 = 0).
Adverse events
Randomised controlled trials
One child/adolescent RCT77 provided data on AE for maintenance treatments. This study compared the use of TAC + AZA + CCS with CSA + AZA + CCS and reported no statistically significant differences between TAC and CSA for a range of AEs (Table 34). In addition, the incidence of new-onset diabetes mellitus after transplantation (NODAT) (defined as insulin use for > 30 consecutive days in previously non-diabetic patients) was not significantly different between TAC and CSA; NODAT was reported for 3 out of 100 children and adolescents (3.0%) in the TAC group and 2 out of 93 children and adolescents (2.2%) in the CSA group. 77 The proportion of children and adolescents withdrawing owing to AEs was 10% (10/103) in TAC and 15% (14/93) in CSA arms (OR = 0.61; favours TAC; 95% CI 0.25 to 1.44). Finally, Trompeter et al. 77 reported that a deficiency of magnesium in the blood and diarrhoea were more common with TAC than with CSA [34.0% compared with 12.9% (p = 0.001) and 13.6% compared with 3.2% (p = 0.011), respectively], while excessive hair growth, flu syndrome and swollen gums were less common with TAC than with CSA [0.0% compared with 7.5% (p = 0.005), 0.0% compared with 5.4% (p = 0.023) and 0.0% compared with 5.4% (p = 0.023), respectively]. 77
| Adverse events | AE n events/N participants, % | ||
|---|---|---|---|
| aTrompeter et al.77 | |||
| TAC + AZA + CCS | CSA + AZA + CCS | OR (95% CI) | |
| Any infections | 71/103, 69 | 60/93, 65 | 0.88 (0.45 to 1.67) |
| UTI | 30/103, 30 | 31/93, 33 | 0.82 (0.45 to 1.49) |
| Bacterial infections | 43/103, 42 | 38/93, 41 | 1.04 (0.60 to 1.80) |
| Viral infections | 23/103, 22 | 23/93, 25 | 0.88 (0.45 to 1.69) |
| PTLD | 1/103, 1 | 2/93, 2 | 0.45 (0.04 to 5.01) |
| Solid tumour | 1/103, 1 | 0/93, 0 | 2.73 (0.11 to 67.99) |
| Hypertension | 71/103, 69 | 57/93, 61 | 1.40 (0.83 to 2.36) |
| Any AE | 98/103, 95 | 93/93, 100 | 0.10 (0.01 to 1.57) |
Non-randomised controlled trials
Three non-RCTs provided data on AEs (Table 35)80,81,83 and two trials compared MMF with AZA. 81,83 The remaining study80 compared TAC + AZA with CSA + MMF. 80 Staskewitz et al. 83 reported AEs only for the MMF group and not for the historic control AZA group. No statistically significant between-group differences in AEs were found (see Table 35) in the non-RCTs that did compare treatment groups.
| Adverse events | Follow-up | AE n events/N participants, % | ||||||
|---|---|---|---|---|---|---|---|---|
| Garcia et al.80 | Antoniadis et al.81 | Staskewitz et al.83 | ||||||
| TAC + AZA | CSA + MMF | OR (95% CI) | MMF | AZA | OR (95% CI) | MMF | ||
| UTI | 3 months | NR | NR | N/A | NR | NR | N/A | 13/65, 20 |
| 6 months | NR | NR | N/A | 2/7, 28 | 5/7, 71 | 0.16 (0.02 to 1.55) | 14/65, 22 | |
| CMV infections | 3 months | 4/12, 33.3 | 0/12, 0 | 13.80 (0.67 to 286.1) | NR | NR | N/A | 9/65, 14 |
| 6 months | NR | NR | N/A | 3/7, 43 | 5/7, 71 | 0.30 (0.04 to 2.51) | 10/65, 15 | |
| Respiratory infections | 3 months | NR | NR | N/A | NR | NR | N/A | 15/65, 23 |
| 6 months | NR | NR | N/A | 1/7, 14 | 3/7, 42 | 0.22 (0.02 to 2.92) | 20/65, 31 | |
| Herpes simplex | 3 months | NR | NR | N/A | NR | NR | N/A | 6/65, 9 |
| 6 months | NR | NR | N/A | 2/7, 28 | 1/7, 14 | 2.40 (0.17 to 33.52) | 8/65, 12 | |
| Oral thrush | 3 months | NR | NR | N/A | NR | NR | N/A | 2/65, 3 |
| 6 months | NR | NR | N/A | 1/7, 14 | 1/7, 14 | N/A | 2/65, 3 | |
| Diarrhoea | 3 months | NR | NR | N/A | NR | NR | N/A | 11/65, 17 |
| 6 months | NR | NR | N/A | 1/7, 14 | 0/7, 0 | 3.55 (0.12 to 103.51) | 13/65, 20 | |
| Abdominal pain | 3 months | NR | NR | N/A | NR | NR | N/A | 14/65, 22 |
| 6 months | NR | NR | N/A | NR | NR | N/A | 16/65, 25 | |
| NODAT | 3 months | 1/12, 8.3 | 0/12, 0 | 3.29 (0.12 to 89.20) | NR | NR | N/A | NR |
In addition, Staskewitz et al. 83 reported AEs up to 5 years of follow-up for the MMF group (see Appendix 5, Table 140). 84,85
Summary
The RCT results suggested no statistically significant differences between TAC and CSA for a range of AEs [any infections, urinary tract infections (UTIs), bacterial infections, viral infections, post-transplant lymphoproliferative disease (PTLD), solid tumour, hypertension, any AE and NODAT]. 77 This is similar to the conclusions of the previous HTA. 2 In addition, no statistically significant differences between MMF and AZA for UTI, cytomegalovirus (CMV) infections, respiratory infections, herpes simplex, oral thrush and diarrhoea were identified in the non-randomised evidence. 81 Similarly, no statistically significant differences between TAC + AZA and CSA + MMF in CMV infections and NODAT were identified in the non-randomised evidence. 80 In contrast, the previous HTA found significantly more CMV infection in TAC + AZA than CSA + MMF (4/12 vs. 0/12, respectively; p = 0.04) in the same non-RCT. 80 This discrepancy in results is due to different statistical analyses used as the current review calculated OR (OR = 13.80, favours CSA + MMF; 95% CI 0.67 to 286.10). This inconsistency highlights the small size of this study80 (n = 24) and the uncertainties of its results.
Comparing children and adolescents, and adult evidence
The results from the current review are contrasted with those from the parallel HTA appraisal ‘Immunosuppressive therapy for kidney transplantation in adults’. 68
Induction therapy
The current review identified two RCTs73,75 evaluating BAS induction therapy in children and adolescents. Offner et al. 73 compared BAS induction therapy with PBO and Grenda et al. 75 compared BAS induction therapy with no induction.
Mortality
Adult randomised controlled trial evidence
In the adult evidence identified by the parallel HTA, three RCTs comparing BAS and no induction reported mortality97–99 and four studies compared BAS with PBO. 100–103 Six studies reported results at 1-year follow-up. 98–103 The pooled results at 1 year with four studies98,100–102 suggest no difference between BAS and PBO or no induction: OR = 0.95 (favours BAS; 95% CI 0.49 to 1.87, I2 = 0.7%, τ2 = 0.004);98,100–102 two studies reported zero events in both arms. 99,103
Summary
In summary, there was no evidence that BAS improved survival when compared with PBO or no induction in the adult evidence. The child/adolescent RCT evidence is consistent with the adult RCT evidence identified in the parallel HTA.
Graft loss
Adult randomised controlled trial evidence
In the adult evidence identified by the parallel HTA, three studies comparing BAS and no induction reported graft loss97–99 and four studies compared BAS with PBO. 100–103 Six studies reported results at 1-year follow-up. 98–103 The pooled results at 1 year with five studies98,100–103 suggest no difference between BAS and PBO or no induction: OR = 0.82 (favours BAS; 95% CI 0.56 to 1.21, I2 = 0.0%, τ2 = 0.0);98,100–103 one study reported zero events in both arms. 99
Summary
In summary, there was no evidence that BAS lowered graft loss when compared with PBO or no induction in the adult evidence. The child/adolescent RCT evidence is consistent with the adult RCT evidence identified in the parallel HTA.
Graft function
Adult randomised controlled trial evidence
In the adult evidence identified by the parallel HTA, graft function was reported by four studies at 1 year comparing BAS with PBO. 99–102 The pooled analysis for graft function implied no beneficial effect of BAS compared with controls: WMD = 1.93 (favours BAS; 95% CI –0.97 to 4.83, I2 = 23.9%). 99–102 One study comparing BAS and no induction reported data on graft function from 1 year to 10 years. 99 It was summarised that up to 7 years, graft function appeared to be slightly better for participants who received BAS; however, the effect reduced over time and the reverse was true at 10 years. Furthermore, the difference across all time points was not statistically significant. 99
Summary
In summary, there was no significant evidence that BAS increased graft function when compared with PBO or no induction in the adult evidence. The child/adolescent RCT evidence is consistent with the adult RCT evidence identified in the parallel HTA.
Acute rejection
Adult randomised controlled trial evidence
In the adult evidence identified by the parallel HTA, three studies comparing BAS and no induction97–99 and four studies comparing BAS with PBO reported AR. 100–103 The pooled results at 1 year with five studies98,100–103 suggest less BPAR in BAS than PBO or no induction (OR = 0.53; favours BAS; 95% CI 0.40 to 0.70, I2 = 0.0%, τ2 = 0.0). Furthermore, Sheashaa et al. 99 reported BPAR at 10 years, at which time BAS continues to show a beneficial effect compared with no induction (OR = 0.41, 95% CI 0.18 to 0.96).
In addition, six studies reported severity of BPAR. 97,99–103 The results do not suggest that BAS is associated with more severe BPAR than no induction or PBO (Table 36).
| Study | Time point (years) | BAS | PBO/no induction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff 1 | Banff 2 | Banff 3 | n | BPAR | Banff 1 | Banff 2 | Banff 3 | ||
| Albano et al. 201397 | 0.5 | 283 | 36 | 16 | 18 | 2 | 302 | 31 | 13 | 15 | 3 |
| aLawen et al. 2003103 | 0.5 | 59 | 9 | 5 | 1 | 2 | 64 | 17 | 4 | 11 | 1 |
| Nashan et al. 1997101 | 0.5 | 190 | 51 | 20 | 26 | 5 | 186 | 73 | 31 | 31 | 11 |
| Ponticelli et al. 2001102 | 0.5 | 168 | 31 | 15 | 12 | 4 | 172 | 49 | 16 | 25 | 8 |
| Kahan et al. 1999100 | 1 | 173 | 61 | 26 | 31 | 4 | 173 | 85 | 38 | 37 | 10 |
| bSheashaa et al. 200399 | 1 | 50 | 29 | 27 | 2 | 50 | 45 | 35 | 10 | ||
| bSheashaa et al. 200399 | 5 | 50 | 27 | 24 | 3 | 50 | 36 | 25 | 11 | ||
| bSheashaa et al. 200399 | 7 | 50 | 41 | 3 | 2 | 50 | 55 | 44 | 11 | ||
| bSheashaa et al. 200399 | 10 | 50 | 41 | 3 | 2 | 50 | 55 | 44 | 11 | ||
Summary
In summary, the adult evidence suggested less BPAR in BAS than PBO or no induction, but no difference in severity of BPAR was found. Similarly, there was no evidence that BAS reduced incidences of, severity and time to BPAR when compared with PBO or no induction in the child/adolescent RCTs. 73,75
Adverse events
Adult randomised controlled trial evidence
Five adult RCTs comparing BAS with PBO or no induction identified by the parallel HTA reported AEs at 1-year follow-up. 98,100,101,103,104 No significant differences in NODAT, PTLD, malignancy, infections and CMV infections were found between BAS and PBO or no induction arms (Table 37).
| AE | Studies | OR | 95% CI | I 2 | t 2 |
|---|---|---|---|---|---|
| NODATa | Kyllönen et al. 200798 | 3.79 | 0.43 to 33.64 | N/A | N/A |
| Malignancyb | Kahan et al. 1999100 | 0.62 | 0.22 to 1.76 | 0% | 0 |
| Kyllönen et al. 200798 | |||||
| Nashan et al. 1997101 | |||||
| PTLDb | Nashan et al. 1997101 | 0.98 | 0.06 to 15.77 | N/A | N/A |
| Infectionsa | Kahan et al. 1999100 | 0.98 | 0.80 to 1.20 | 0% | 0 |
| Nashan et al. 1997101 | |||||
| Lawen et al. 2003103 | |||||
| CMVa | Kahan et al. 1999100 | 0.8 | 0.56 to 1.13 | 0% | 0 |
| Kyllönen et al. 200798 | |||||
| Nashan et al. 1997101 | |||||
| Lawen et al. 2003103 |
Summary
In summary, the adult RCT evidence identified in the parallel HTA did not find any significant differences in NODAT, PTLD, malignancy, infections and CMV infections between BAS and PBO or no induction conditions. However, the child/adolescent RCTs found more infections with BAS than with PBO in one study (OR = 2.23, favours PBO; 95% CI 1.03 to 4.68). 73
Maintenance therapy
The current review identified one RCT77 and four non-RCTs80–83 evaluating maintenance therapy in children and adolescents. Trompeter et al. 77 compared the use of TAC and CSA. Garcia et al. 80 compared the use of TAC+AZA and CSA+MMF. Antoniadis et al. ,81 Benfield et al. 82 and Staskewitz et al. 200183 compared the use of MMF and AZA.
Mortality
Parallel Health Technology Assessment adult randomised controlled trial evidence
Ten adult RCTs comparing TAC + AZA with CSA + AZA identified by the parallel HTA reported mortality. 105–114 The pooled results at 1 year with eight studies106–111,113,114 found no statistically significant difference between TAC and CSA (OR = 1.51; favours CSA; 95% CI 0.75 to 3.06, I2 = 14.8%). One study107 reported mortality up to 5 years, but the results are consistent with earlier time points and indicated no statistically significant difference between arms (OR 1.20; favours CSA; 95% CI 0.69 to 2.07). 107
Seven adult RCTs comparing MMF + CSA and AZA + CSA identified by the parallel HTA reported mortality. 114–120 The pooled results at 1 year with five studies114,116–119 suggest no significant difference between MMF and AZA (OR = 1.19; favours AZA; 95% CI 0.47 to 3.02, I2 = 0%, τ2 = 0). In addition, two studies reported mortality at a 3-year follow-up, suggesting no difference between MMF and AZA (OR = 0.56 favours MMF; 95% CI 0.23 to 1.23, I2 = 0%, τ2 = 0). 115,118 The study reported by Tuncer et al. 118 provided data at 5 years, which also indicated no preference for either MMF or AZA (OR 0.73, 95% CI 0.15 to 3.50).
Summary
In summary, no difference in survival was found between TAC and CSA and between MMF and AZA in the adult evidence. The child/adolescent RCT and child/adolescent non-RCT evidence is consistent with the adult RCT evidence identified in the parallel HTA.
Graft loss
Parallel Health Technology Assessment adult randomised controlled trial evidence
Eleven adult RCTs comparing TAC + AZA with CSA + AZA identified by the parallel HTA reported graft loss. 105–114,121 The pooled results at 1 year with eight studies107–111,113,114,121 found no significant difference between TAC and CSA (OR = 0.83; favours TAC; 95% CI 0.54 to 1.27, I2 = 12.3%; in addition, one study reported zero events in both arms108). As with mortality, the results for graft loss suggest no statistically significant difference between TAC and CSA. This lack of preference for either treatment remained at 2- (OR 0.71, 95% CI 0.40 to 1.25)107,121, 4- (OR 0.96, 95% CI 0.62 to 1.48)107 and 5-year follow-ups (OR 0.92, 95% CI 0.61 to 1.40). 107 However, the pooling of two trials at 6 months gives an OR of 0.45 with 95% CI 0.24 to 0.84, which is statistically significant in favour of TAC. 110,112
Five adult RCTs comparing MMF + CSA with AZA + CSA identified by the parallel HTA reported graft loss. 114–117,120 The pooled results at 1 year with four studies114–117 suggest no significant difference between MMF and AZA (OR = 0.76; favours MMF; 95% CI 0.38 to 1.50, I2 = 32.3%, τ2 = 0.120).
Summary
In summary, 1-year follow-up data found no statistically significant difference in graft loss between TAC and CSA and between MMF and AZA in the adult evidence. Similarly, no statistically significant difference was found between TAC and CSA for graft loss in the child/adolescent RCT evidence. However, it should be noted that the evidence from Trompeter et al. 77 suggested borderline statistically non-significantly lower in graft loss with TAC compared with CSA (OR = 0.41, 95% CI 0.16 to 1.00, and OR = 0.43, 95% CI 0.18 to 1.01 at 2- and 4-year follow-ups, respectively). In addition, the current review found better graft survival in MMF than in AZA in a 5-year follow-up from one child/adolescent non-RCT. 83
Graft function
Parallel Health Technology Assessment adult randomised controlled trial evidence
Four adult RCTs comparing TAC with CSA identified by the parallel HTA reported graft function. 105,110,122,123 No meta-analysis was conducted because the results were presented in a number of ways and were not appropriate for pooling. One study110 suggested lower graft function for TAC, as opposed to CSA at 1- and 2-year follow-ups, but not at a 3-year follow-up. Another study122 did not find statistically significant difference between TAC and CSA at 1-year follow-up. Conflicting results were reported by all four trials across all time points (1 month to 3 years).
Summary
In summary, conflicting adult evidence was reported in the parallel HTA across all time points (1 month to 3 years) and it is not clear if there is any difference between TAC and CSA with regard to graft function. In contrast, better graft function was associated with TAC compared with CSA in the one child/adolescent RCT. 77 In addition, no difference in graft function between TAC + AZA and CSA + MMF regimens was reported in the one non-RCT. 80
Acute rejection
Parallel Health Technology Assessment adult randomised controlled trial evidence
Nine adult RCTs comparing TAC with CSA identified by the parallel HTA reported AR at 1 year. 107–111,113,114,121,124 The pooled results at 1 year with all nine studies found significantly higher BPAR in the CSA arm than the TAC arm (OR = 0.50; favours TAC; 95% CI 0.39 to 0.64, I2 = 8.1%). 107–111,113,114,121,124 In addition, Mayer et al. 107 reported BPAR at 4 years, for which the beneficial effect of TAC appeared to be maintained (OR 0.38 favours TAC, 95% CI 0.25 to 0.57).
Time to first BPAR was reported by two studies109,121 which suggested that BPAR may occur quicker for participants receiving TAC (35 days, SD 13) than CSA (59 days, SD 38); however, no statistical tests were reported. 121 Campos et al. 109 reported that the mean time to BPAR was comparable between the TAC and CSA groups (14.5 days, SD 47.3, and 12.0 days, SD 21.0, respectively).
Severity of BPAR was reported by four studies (Table 38). 110,112,113,121 At 6 months, Charpentier et al. 112 report the proportion of people with BPAR classified as Banff 3 as 10.7% for TAC and 15.4% for CSA and by 2 years Margreiter et al. 110 report 6.4% and 16.8% of people with BPAR experiencing Banff 3, for TAC and CSA, respectively.
| Study | Time point (years) | TAC + AZA | CSA + AZA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff 1 | Banff 2 | Banff 3 | n | BPAR | Banff 1 | Banff 2 | Banff 3 | ||
| Margreiter et al. 2002 110 | 0.5 | 286 | 56 | 21 | 31 | 4 | 271 | 101 | 34 | 49 | 18 |
| Charpentier et al. 2003112 | 0.5 | 186 | 28 | 18 | 7 | 3 | 184 | 39 | 14 | 19 | 6 |
| Baboolal et al. 2002121 | 1 | 27 | 5 | 3 | 2 | 0 | 24 | 8 | 5 | 3 | 0 |
| Hardinger et al. 2005113 | 1 | 134 | 6 | 3 | 3 | 0 | 66 | 4 | 1 | 3 | 0 |
| aMargreiter et al. 2002110 | 1 | 286 | 60 | 23 | 33 | 4 | 271 | 111 | 39 | 54 | 18 |
| aMargreiter et al. 2002110 | 2 | 286 | 62 | 23 | 35 | 4 | 271 | 113 | 40 | 54 | 19 |
Six adult RCTs comparing MMF and AZA identified by the parallel HTA reported BPAR. 114–117,119,120 The pooled results from three studies115,119,120 at 6-month follow-up suggested less BPAR in the MMF than the AZA arm (OR = 0.50; favours MMF; 95% CI 0.35 to 0.72, I2 = 35.1%, τ2 = 0.036), while pooled results of four RCTs114–117 at 1-year follow-up suggested no statistically significant between-group differences for BPAR (OR = 0.67; 95% CI 0.37 to 1.22, I2 = 58.3%, τ2 = 0.198).
In addition, three RCTs identified by the parallel HTA reported severity of BPAR (Table 39). 115,117,120 Overall, at 0.5 years, the more severe classification of Banff 3 appears to be more likely in the AZA arm for people with BPAR (CSA 9.1%, AZA 15.9% for Sollinger et al. 120 and CSA 5.9%, AZA 11.9% for the Tricontinental Group 1996115).
| Study | Time point (years) | MMF + CSA | AZA + CSA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff 1 | Banff 2 | Banff 3 | n | BPAR | Banff 1 | Banff 2 | Banff 3 | ||
| Sollinger et al. 1995120 | 0.5 | 167 | 33 | 18 | 12 | 3 | 166 | 63 | 29 | 24 | 10 |
| Tricontinental study 1996115 | 0.5 | 173 | 34 | 16 | 16 | 2 | 166 | 59 | 26 | 26 | 7 |
| aMerville et al. 2004117 | 1 | 37 | 5 | 4 | 1 | 0 | 34 | 7 | 2 | 3 | 2 |
Insufficient data were provided for time to BPAR to allow pooled analysis as only Merville et al. 117 reported time to BPAR as 48.5 days for MMF and 43.7 days for AZA.
Summary
In summary, pooled results of nine adult RCTs identified by the parallel HTA at 1-year follow-up suggested less BPAR with TAC compared with CSA. Similarly, higher rates of BPAR were found in CSA compared with TAC in the one included child/adolescent RCT at a 6-month follow-up. 77 No statistically significant differences were found between TAC and CSA in the adult RCT evidence with regard to time to BPAR, and severity of BPAR. No child/adolescent evidence on severity and time to BPAR was identified.
In addition, pooled results of three adult RCTs identified by the parallel HTA at 6-months follow-up suggested less BPAR with MMF than AZA (OR = 0.50; favours MMF; 95% CI 0.35 to 0.72, I2 = 35.1%); however, the pooled results of four adult RCTs at 1-year follow-up suggested no statistical significance between-group differences (OR = 0.67; 95% CI 0.37 to 1.22, I2 = 58.3%). Similarly in the child/adolescent non-randomised evidence, no statistically significant differences in BPAR were found between the MMF and AZA arms, and between TAC + AZA and CSA + MMF.
Adverse events
Parallel Health Technology Assessment adult randomised controlled trial evidence
Ten adult RCTs comparing TAC with CSA identified by the parallel HTA reported AEs at 1-year follow-up,106–109,113,114,121,125–127 six studies compared TAC + AZA + CCS with CSA + AZA + CCS regimens,106–109,113,121 two studies compared TAC + MMF + CCS with CSA + MMF + CCS regimens,114,125 one study compared TAC + SRL + CCS with CSA + SRL + CCS regimens,126 and one study (Symphony study comparing four regimens127) compared low TAC + MMF + CCS with low CSA + MMF + CCS regimens. 127 No difference in PTLD, malignancy, infections and CMV infection was found between TAC and CSA regimens at 1-year follow-up. The meta-analysis (including eight studies106–109,113,125–127) suggested more cases of NODAT in TAC regimens compared with CSA (OR = 2.22; favours CSA; 95% CI 1.42 to 3.46, I2 = 0%). All meta-analyses are summarised in Table 40.
| AE | Study | OR | 95% CI | I 2 | t 2 |
|---|---|---|---|---|---|
| NODAT | Laskow et al. 1996106 | 2.22 | 1.42 to 3.46 | 0% | 0 |
| Mayer et al. 1997107 | |||||
| Jarzembowski et al. 2005108 | |||||
| Campos et al. 2002109 | |||||
| Hardinger et al. 2005113 | |||||
| Yang et al. 1999 125 | |||||
| Symphony127 | |||||
| Chen et al. 2008126 | |||||
| Malignancy | Mayer et al. 1997107 | 1.36 | 0.54 to 3.39 | 0% | 0.57 |
| Hardinger et al. 2005113 | |||||
| Yang et al. 1999125 | |||||
| Symphony127 | |||||
| Infections | Mayer et al. 1997107 | 1.12 | 0.84 to 1.49 | 0% | 0.46 |
| Chen et al. 2008126 | |||||
| Yang et al. 1999125 | |||||
| Symphony127 | |||||
| CMV | Baboolal et al. 2002121 | 0.8 | 0.59 to 1.09 | 0% | 0.6 |
| Mayer et al. 1997107 | |||||
| Jarzembowski et al. 2005108 | |||||
| Weimer et al. 2006114 | |||||
| Symphony127 | |||||
| Yang et al. 1999125 | |||||
| Hardinger et al. 2005113 |
Three adult RCTs that compared MMF with AZA reported AEs; one study compared MMF + CSA + CCS with AZA + CSA + CCS regimens,117 and two three-arm studies compared MMF + CSA + CCS with AZA + CSA + CCS regimens. 114,116 No difference in infections and CMV infection were found between MMF and AZA regimens at 1-year follow-up. However, only two studies114,117 reported CMV infection and only one study reported infections. 116
Summary
The result suggested no difference between TAC and CSA for mortality, graft loss and AEs, although more BPAR and AR, and worse graft function was reported in CSA compared with TAC. 77 The child/adolescent RCT found no statistically significant differences between TAC and CSA for a range of AEs including NODAT (e.g. any infections, UTIs, bacterial infections, viral infections, PTLD and solid tumour).
Summary
Three RCTs are included in the clinical effectiveness systematic review presented in this report: one new RCT73 and two RCTs from the previous assessment. 75,77
Four non-RCTs are included in our review. All of these were also included in the previous assessment by Yao et al. 2 No new non-randomised studies were identified in our searches.
Induction therapy
Two RCTs of induction therapy (reported in four publications and one abstract) evaluating BAS in children and adolescents were identified in the review. 73,75 No RCTs were identified that evaluated r-ATG in children and adolescents.
No non-RCTs in the child and adolescents population evaluated induction therapies.
We found no significant difference in survival, graft loss, graft function, incidences of BPAR, severity of BPAR and time to BPAR between BAS and PBO/no induction. 73,75
Comparison with the previous Health Technology Assessment and the parallel Health Technology Assessment in adults
The results of the current review are similar to the previous HTA. 2
In addition, the child RCT evidence is similar to the conclusions of the parallel HTA in adults. However, the adult evidence found less BPAR in BAS than PBO or no induction (OR = 0.53; favours BAS; 95% CI 0.40 to 0.70, I2 = 0.0%, τ2 = 0.0; pooled results at 1-year follow-up with five studies).
The comparison of the child/adolescent RCT evidence with the previous HTA and the parallel HTA in adults is summarised in Table 41.
| Outcome | Follow-up | PenTAG RCTs BAS vs. control | Yao et al.2 RCTs BAS vs. control | Parallel HTA adult RCTs BAS vs. control (meta-analysis at 1-year follow-up) |
|---|---|---|---|---|
| OR (95% CI) | RR (95% CI) | OR (95% CI) | ||
| Mortality | 3 months | 2.79 (0.11 to 69.31)73 | ||
| 6 months | 4.69 (0.22 to 99.10)73 No deaths in either arm75 |
No deaths in either arm75 | ||
| 1 year | 6.64 (0.34 to 130.33)73 | 0.95 (0.49 to 1.87); I2 = 0.7%98,100–102 No deaths in either arm99,103 |
||
| 2 years | 0.33 (0.01 to 8.20)75 | |||
| Graft Loss | 6 months | 0.93 (0.29 to 2.97); I2 = 0%73,75 | 0.93 (95% CI 0.28 to 3.12)75 | |
| 1 year | 0.92 (0.06 to 14.92)73 | 0.82 (0.56 to 1.21); I2 = 0%98,100–103 No deaths in either arm99 |
||
| 2 years | 0.50 (0.16 to 1.54)75 | |||
| BPAR | 3 months | 0.39 (0.14 to 1.07)73 | ||
| 6 months | 0.71 (0.40 to 1.27); I2 = 15.7%73,75 | 0.93 (95% CI 0.53 to 1.65)75 | ||
| 1 year | 0.51 (0.24 to 1.08)73 | 0.53 (0.40 to 0.70); I2 = 0%98,100–103 | ||
| 2 years | 0.74 (0.39 to 1.40)75 | |||
| eGFR | 6 months | WMDa –4.20 (–9.60 to 1.20); I2 = 0%73,75 | WMDb 4.5 (95% CI –6.26 to 5.26)75 | |
| 1 year | Mean (SD)a: 79(23) vs. 82 (24); p = 0.38d,73 | WMDc 1.93 (–0.97 to 4.83); I2 = 23.9%99–102 | ||
| 2 years | WMDa –1.38 (–7.20 to 4.44); I2 = 0%73,75 |
Maintenance therapy
Randomised controlled trial evidence
One RCT of maintenance therapy (reported in three publications) evaluating TAC (compared with CSA) in children and adolescents was identified. 77 No RCTs were identified that evaluated TAC-PR, MMF, MPA, SRL, EVL or BEL in children and adolescents.
From the RCTs, we found no significant difference in survival or graft loss between TAC and CSA. 77 However, a significantly higher graft function (mean eGFR of 71.5 ml/minute/1.73 m2, SD 22.9 ml/minute/1.73 m2, in TAC vs. mean eGFR of 53.0 ml/minute/1.73 m2, SD 21.6 ml/minute/1.73 m2, in CSA; t-test = 4.03; p < 0.01 at 4-year follow-up), and less BPAR (OR = 0.29, favours TAC, 95% CI 0.15 to 0.57 at 6-month follow-up) was found in TAC compared with CSA. 77
Comparison with the previous Health Technology Assessment and the parallel Health Technology Assessment in adults
The results of the current review for survival, graft function and BPAR are similar to the previous HTA. 2 However, the RCT child and adolescent evidence identified in the previous HTA review2 concluded that TAC lowered graft loss at 2- and 4-year follow-ups. The difference in these results is because we excluded graft loss due to death from all analyses. This was, first, to avoid double counting with another key outcome (mortality) and, second, because death-censored graft survival is a well-established clinical outcome, to which DWFG is intrinsically related. After the removal of graft loss due to death from the analyses, the evidence from Trompeter et al. 77 suggested a borderline (statistically non-significant) lower graft loss with TAC than CSA (OR = 0.41, 95% CI 0.16 to 1.00, and OR = 0.43, 95% CI 0.18 to 1.01 at 2- and 4-year follow-ups, respectively). In addition, while there were statistically significant treatment group differences in BPAR and AR at 6 months, the annual differences in AR were not statistically significant for years 2, 3 and 4. 77,79
In addition, the child RCT evidence is similar to the conclusions of the parallel HTA in adults. The pooled result of nine studies at 1-year follow-up found less BPAR in TAC than CSA (OR = 0.50, favours TAC; 95% CI 0.39 to 0.64, I2 = 8.1%). The comparison of the child/adolescent RCT evidence with the previous HTA and the parallel HTA in adults is summarised in Table 42.
| Outcome | Follow-up | PenTAG RCTs TAC vs. CSA | Yao et al.2 RCTs TAC vs. CSA | Parallel HTA adult RCTs TAC vs. CSA (meta-analysis at 1 year follow-up) |
|---|---|---|---|---|
| OR (95% CI) | RR (95% CI) | OR (95% CI) | ||
| Mortality | 6 months | 0.9 (0.18 to 4.58)77 | 0.9 (0.21 to 3.84)77 | |
| 1 year | 0.9 (0.18 to 4.58)77 | n/N: 3/103 vs. 3/93 (p = 0.90)77 | 1.51 (0.75 to 3.06); I2 = 14.8%106–111,113,114 | |
| 2 years | 0.67 (0.15 to 3.07)77 | n/N: 3/103 vs. 4/93 (NS)77 | ||
| 4 years | 1.14 (0.30 to 4.36)77 | n/N: 5/103 vs. 4/93 (p = 0.90) | ||
| Graft lossa | 6 months | 0.38 (0.14 to 1.05)77 | 0.48 (0.22 to 1.08)77 | |
| 1 year | 0.44 (0.18 to 1.09)77 | n/N: 10/103 vs. 17/93 (p = 0.082)77 | 10.83 (0.542 to 1.27); I2 = 12.3%106,107,109–111,113,114,121 | |
| 2 years | 0.41 (0.16 to 1.00)77 | n/N: 10/103 vs. 19/93 (p = 0.03)77 | ||
| 4 years | 0.43 (0.18 to 1.01)77 | n/N: 11/103 vs. 20/93 (p = 0.03)77 | ||
| BPAR | 6 months | 0.29 (0.15 to 0.57)77 | 0.42 (0.26 to 0.69)77 | |
| 1 year | 0.50 (0.39 to 0.64);I2 = 8.1%107–111,113,114,121,124 | |||
| eGFRb | 6 months | Mean (SD):c 65.6 (19.9) vs. 61.2(15.8); dp = 0.1177 | Mean (SD):c 90.91 (34.2) vs. 86.09 (26.8)77; dp = 0.0977 | No meta-analysis was performed; conflicting results were reported by all four trials across all time points (1 month to 3 years)99–102 |
| 1 year | Mean (SD):c 64.9 (20.7) vs. 57.8 (21.9); dp = 0.0477 | Mean (SD):c 62.5 vs. 56.4; dp < 0.0177 | ||
| 2 years | Mean (SD):c 64.9 (19.8) vs. 51.7 (20.3); dp < 0.0177 | Mean (SD):c 64.9 vs. 51.7; dp < 0.0177 | ||
| 3 years | Mean (SD):c 66.7 (26.4) vs. 53.0 (23.3); dp < 0.0177 | |||
| 4 years | Mean (SD):c 71.5 (22.9) vs. 53.0 (21.6); dp < 0.0177 | Mean (SD):c 71.5 vs. 53.0; dp < 0.0177 |
Non-randomised controlled trial evidence
Three non-RCTs evaluating MMF (compared with AZA) in children and adolescents were identified. 81–83 One non-RCT compared TAC + AZA with CSA + MMF. 80 No non-RCTs were identified that evaluated TAC-PR, MPA, SRL, EVL or BEL in children and adolescents.
Tacrolimus versus ciclosporin
We found no statistically significant difference in survival between MMF and AZA in the non-RCTs. 81,83 Similarly, no statistically significant difference in BPAR between MMF and AZA in the non-RCTs was identified. 81–83 A significantly lower graft loss was found in MMF compared with AZA at 1- to 5- year follow-ups in one of the two non-RCTs83 (OR = 0.24 at 5-year follow-up; favours MMF; 95% CI 0.09 to 0.63). However, this was not confirmed by the other non-RCT at 1-year follow-up. 81 Graft function (eGFR) was not measured in the three included non-RCTs comparing MMF and AZA. 81–83
In addition, conflicting evidence was found in the parallel HTA in adults. No difference in graft loss was found between MMF and AZA in the adult evidence; OR = 0.76 (favours MMF; 95% CI 0.38 to 1.50, I2 = 32.3%, τ2 = 0.120; pooled results of four studies a 1-year follow-up). 114–117,120 The pooled results of three adult RCTs at 6-month follow-up suggested less BPAR with MMF than AZA (OR = 0.50; favours MMF; 95% CI 0.35 to 0.72, I2 = 35.1%);115,119,120 however, the pooled results of four adult RCTs at 1-year follow-up suggested no statistically significant between-group differences (OR = 0.67; 95% CI 0.37 to 1.22, I2 = 58.3%). 114,116–119 Finally no significant difference in survival between MMF and AZA was found in the adult evidence (OR = 1.19; favours AZA; 95% CI 0.47 to 3.02, I2 = 0%, τ2 = 0; pooled results of five studies at 1-year follow-up). 114,116–119
Tacrolimus ± azathioprine versus ciclosporin ± mycophenolate mofetil
We found no statistically significant difference in survival, graft loss, BPAR, graft function and DGF between TAC + AZA and CSA + MMF in the non-RCT. 80
No adult evidence comparing TAC + AZA and CSA + MMF was identified in the parallel HTA in adults.
Adverse events
Induction
More infections were found in children treated with BAS than in those treated with PBO (OR = 2.23, favours PBO; 95% CI 1.03 to 4.68). 73 In addition, Grenda et al. 75 found that toxic nephropathy and abdominal pain was higher in the BAS arm than no induction (p = 0.03 and p = 0.02, respectively). 75 The previous HTA reported only post-transplant diabetes mellitus90 and the rest of the data were confidential and were omitted from the report. 2
In addition, the child RCT evidence is largely similar to the conclusions of the parallel HTA in adults. The adult 1-year follow-up RCT evidence identified in the parallel HTA did not find any significant differences in NODAT, PTLD, malignancy, infections and CMV infections between BAS and PBO or no induction. 98,100,101,103,104
Maintenance therapy
There were no statistically significant differences between TAC and CSA for a range of AEs (any infections, UTIs, bacterial infections, viral infections, PTLD, solid tumour, hypertension, any AEs and NODAT). 77 This is similar to the conclusions of the previous HTA. 2 However, Trompeter et al. 77 also reported that a deficiency of magnesium in the blood and diarrhoea were more common with TAC than with CSA, while excessive hair growth, flu syndrome and swollen gums were less common with TAC than with CSA. 77 In addition, there were no statistically significant differences between MMF and AZA for UTI, CMV infections, respiratory infections, herpes simplex, oral thrush and diarrhoea identified in the non-randomised evidence. 81 Similarly, no statistically significant differences between TAC + AZA and CSA + MMF in CMV infections and NODAT were identified in the non-randomised evidence. 80
However, the parallel HTA in adults found more cases of NODAT in TAC than CSA (OR = 2.22; favours CSA; 95% CI 1.42 to 3.46, I2 = 0%; pooled results of eight studies at 1-year follow-up). 106–109,113,125–127 In addition, no difference in CMV infections114,117 and infection116 were found between MMF and AZA regimens in the adult evidence at 1-year follow-up.
Companies’ reviews of clinical effectiveness
One submission (Astellas) was presented summarising evidence on the effectiveness of immunosuppressive therapies in child/adolescent renal transplantation.
Astellas submitted a systematic review summarising evidence on the clinical effectiveness and safety of TAC-IR therapy compared with current alternative treatments [TAC-PR (Advagraf), CSA, SRL, BEL, and EVL] as primary immunosuppressive therapies in patients undergoing renal transplantation. The submission did not address the study question in full.
The literature searches were conducted in the key bibliographic databases: MEDLINE, EMBASE, The Cochrane Library and Cochrane NHS Economic Evaluation Database (NHS EED). The literature search was limited from 2002 to June 2014. The literature searches use minimal free-text search terms without the use of truncation or controlled indexing and selective synonyms are used for the interventions/comparators. This reflects poor sensitivity and, combined with the fact that searching has been conducted on only the abstracts of potential studies, it is possible that studies may have been missed. In addition, although the submission states that evidence will be assessed from RCTs and non-RCTs, a RCT study design filter was applied. It is unclear from the search strategies provided how the referenced non-RCT data would have been captured.
Only one child/adolescent RCT77 and two child/adolescent non-RCTs80,94 were included in the company submission. In addition, adult RCT evidence was summarised; an overview of adult RCTs included in Astellas’ submission with reasons for inclusion/exclusion in the PenTAG parallel review is provided in Appendix 6, Table 141.
Tacrolimus versus ciclosporin
Trompeter et al. 77 is the only child/adolescent RCT comparing TAC with CSA that is included both in the Astellas submission and in the PenTAG review. Astellas reported a significantly higher graft function, BPAR and better graft survival in TAC than AZA. 77 However, we have excluded graft loss due to death from our analyses. This was, first, to avoid double counting with another key outcome (mortality) and, second, because death-censored graft survival is a well-established clinical outcome to which DWFG is intrinsically related, just as mortality is to overall survival. After the removal of graft loss due to death from the analyses, the evidence from Trompeter et al. 77 suggested borderline statistically non-significantly lower graft loss in TAC than in CSA (OR = 0.41, 95% CI 0.16 to 1.00, and OR = 0.43, 95% CI 0.18 to 1.01, at 2- and 4-year follow-ups, respectively).
Astellas’ clinical effectiveness results from adult RCTs suggest less AR and more NODAT for TAC than for CSA. The findings from the adult RCTs were similar to the conclusions in the parallel HTA: more BPAR and more NODAT were found for TAC than for CSA, but it was not clear whether or not TAC improved graft function when compared with CSA.
Tacrolimus versus sirolimus
No child/adolescent evidence comparing TAC and SRL was identified. Astellas’ clinical effectiveness results from adult RCTs suggest better graft survival and less AR with TAC compared with SRL, but they included a trial comparing TAC and no induction-based regimen with SRL + r-ATG induction regimen. 128 The parallel PenTAG review found fewer incidences of BPAR for TAC compared with SRL. In addition, Astellas pooled results from studies comparing SRL with MMF in TAC-based regimens and significantly more drug discontinuations were found in the SRL + TAC regimen than in the MMF + TAC regimen.
Immediate-release tacrolimus versus prolonged-release tacrolimus
No child/adolescent evidence comparing immediate-release TAC and prolonged-release TAC formulations was identified. Astellas’ clinical effectiveness results from adult RCTs suggest no difference between TAC and TAC-PR. The results do not conflict with conclusions in the parallel HTA review. 68
Tacrolimus versus belatacept
No child/adolescent evidence comparing TAC and BEL was identified. In addition, no adult RCTs comparing TAC and BEL were identified. Astellas performed an indirect treatment comparison to compare Advagraf with Prograf, with more intensive and less intensive BEL regimens. Evidence of less AR with Prograf compared with both BEL regimens was presented. In addition, better graft survival was found with Prograf compared with the more intensive BEL regimen, and better survival was found with Prograf compared with the less intensive BEL regimen. Finally, evidence of less AR with Advagraf compared with the less intensive BEL regimen was presented. However, it was not clear what TAC evidence was included and the results presented seem to be conflicted. The parallel HTA network meta-analyses results suggested that BEL + MMF may be more effective at reducing the odds of mortality than TAC + MMF and SRL + MMF. In addition, a study directly comparing BEL and TAC regimens was identified in the parallel HTA. 129
Tacrolimus versus everolimus
No child/adolescent evidence comparing TAC and EVL was identified. In addition, no adult RCTs comparing TAC and EVL were identified. Astellas performed an indirect treatment comparison to compare TAC with EVL. It is not clear what TAC evidence was included and why the results were not reported separately for TAC and TAC-PR (as they were presented in the TAC vs. BEL comparison). No statistically significant differences between TAC and EVL were identified in the submission. The parallel HTA network meta-analyses results did not find any difference between TAC and EVL regimens for clinical effectiveness outcomes.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
The purpose of this section of the report is to review existing evidence on the cost-effectiveness of immunosuppressive regimens [BAS and r-ATG as induction therapies, and TAC-IR, TAC-PR, MMF, MPS, SRL, EVL and BEL as maintenance therapies (including a review of TA99)] in renal transplantation in children and adolescents.
Methods
Searches
Bibliographic literature searching was conducted on 8 April 2014. The searches took the following form: (terms for kidney or renal transplant or kidney or renal graft) AND (terms for the interventions under review) AND (a costs or economic literature search filter). The search was date limited 2002–current in line with the previous assessment and the searches were updated on 15 January 2015. The search was not limited by language and it was not limited to human-only studies.
The following databases were searched: MEDLINE and MEDLINE In-Process (via Ovid), EMBASE (via Ovid), NHS EED (via Wiley Online Library), Web of Science (ISI – including conference proceedings), Health Economic Evaluations Database (HEED) (via Wiley) and EconLit (via EBSCOhost). The search strategies are recorded in Appendix 1.
Screening
Inclusion and exclusion criteria were the same as for the clinical effectiveness systematic review (see Chapter 3, Inclusion and exclusion criteria), with the following exceptions (as specified in the appraisal protocol):
-
Non-randomised studies were included (e.g. decision model-based analyses, or analyses of patient-level cost and effectiveness data alongside observational studies).
-
Full cost-effectiveness analyses, cost–utility analyses and cost–benefit analyses were included (economic evaluations that report only average cost-effectiveness ratios were included only if the incremental ratios can be easily calculated from the published data).
-
Studies that measure only costs but not health benefits were excluded except for stand-alone cost analyses from the perspective of the UK NHS.
-
Only economic evaluations from the UK, the USA, Canada, Australia and western Europe were included as these settings may include data generalisable to the UK.
All records were dual screened. Titles and abstracts were screened for relevance by two reviewers (RMM and LC), with disagreements resolved by discussion. Full texts were retrieved for references judged to be relevant and were screened for eligibility by the same reviewers, with disagreements resolved by discussion.
The bibliographies of review articles not judged eligible for inclusion were examined by one reviewer (LC) to identify other potentially relevant references. These references were retrieved and checked for eligibility in the same way as full texts from database searches.
Quality assessment
Studies meeting the criteria for inclusion were assessed by one reviewer (RMM) using the checklist developed by Evers et al. 130 When studies were based on decision models they were also quality assessed using the checklist developed by Philips et al. 131,132
Synthesis
Economic studies were summarised and synthesised using tabulated data and narrative synthesis.
Results
Identified studies
The electronic database search for cost-effectiveness evidence, including update searches conducted on 18 November 2014, identified 2090 records. After deduplication 1378 records remained, all of which were screened by title and abstract. Of these, 86 full texts were assessed for eligibility. Thirty-eight full texts were deemed to meet the eligibility criteria for the review. The process is illustrated in detail in Figure 13.
FIGURE 13.
Cost-effectiveness review: PRISMA flow diagram. CEA, cost-effectiveness analyses; CUA, cost–utility analyses. a, The previous HTA review by Yao et al. ;2 includes child, adolescent and adult evidence.
Only one study2 was identified that met the inclusion criteria. This was the HTA report of the previous NICE appraisal on the topic in children or adolescent patients. The rest of the subsection is devoted to reviewing this study.
Yao et al. 2 reports the methods and results of economic analyses submitted to the previous NICE appraisal on the topic by three sponsoring companies. All of these analyses used an equation estimated from regression analysis (meta-model) of child/adolescent simulation outcomes of immunosuppressive regimens derived from a model originally developed by one company (Novartis) for informing its submission to the corresponding NICE review on adult patients. The adult metamodel was developed by the Technology Assessment Group at Birmingham and the individual companies adapted it to children and adolescents. After critically appraising the evidence submitted by the companies, the group at Birmingham then produced its own analysis by adapting the metamodel to children and adolescents.
Briefly, the Birmingham model was a Markov model spanning a 10-year horizon after the initial transplant. It consisted of three states: functioning graft, graft failed (dialysis) and death. In common with models in this clinical area, surrogate outcomes were used to extrapolate beyond the end of follow-up in the RCT evaluating the relative effects of immunosuppressive regimens in terms of BPAR. The model used a HR of 1.41 for graft failure up to 7 years post transplant for children and adolescents (≤ 18 years) treated for an AR before discharge versus those not treated. The Birmingham group then used this surrogate relationship to translate 12-month differences in BPAR rates between immunosuppressive regimens from RCT studies in children and adolescents for therapies other than MMF and DAC, for which adult RCT data were used, into 10-year graft survival differences. The study also adjusted the resource use and costs for age–weight immunosuppressive doses in children and adolescents.
Table 43 presents the characteristics of the analysis by Yao et al. 2 Results were presented for two pairwise comparisons of induction regimens and two pairwise comparisons of initial and maintenance immunosuppressive regimens. In the comparisons of induction therapy regimens, BAS was found to result in lower total costs and higher quality-adjusted life-years (QALYs) than no induction in patients managed with either TAC or CSA in a CNI-containing triple immunosuppressive therapy including AZA and steroids. In terms of the initial and maintenance immunosuppressive regimens, TAC was found to have an incremental cost per QALY gained of £145,540 relative to CSA, while the corresponding figure for MMF relative to AZA was £194,559 when these therapies were combined with CSA and steroids. It is worth noting that the latter comparison was based on efficacy data from studies on MMF use in adults. Table 44 summarises the base-case results. However, altering the hazard (risk) ratio of graft loss with AR from 1.41 (which was based on a single observational study in children and adolescents) to a HR of 1.96 (derived from a pooled analysis of adult observational studies) and arbitrarily increasing the cost of dialysis from the base-case value of £21,000 (which was estimated from data on adults) to £50,000, as a way of accounting for the higher staff-to-patient ratios in children and adolescents, resulted in a cost per QALY gained of £34,000. 2
| Author and country | Regimens | Population | Study type | Perspective | Outcomes considered | Horizon | Model based? | Sponsor |
|---|---|---|---|---|---|---|---|---|
| Yao et al.,2 UK | Induction: BAS + CSA + AZA + CCS vs. CSA + AZA + CCS BAS + TAC + AZA + CCS vs. TAC + AZA + CCS Initial and maintenance: TAC + AZA + CCS vs. CSA + AZA + CCS CSA + MMF + CCS vs. CSA + AZA + CCS |
Children and adolescents with renal transplant | Cost–utility analysis | NHS and PSS | QALYs | 10 years | Yes | Adapted model by independent Technology Assessment Group from model originally developed by Novartis for adult patients |
| Regimens compared | BTAS vs. TAS | BCAS vs. CAS | TAS vs. CAS | CMS vs. CAS |
|---|---|---|---|---|
| Initial age range | 3–13 years | |||
| Time horizon | 10 years | |||
| Discounted incremental QALYs | 0.038 | 0.074 | 0.090 | 0.049 |
| Discounted incremental costs (£) | –451 | –1103 | 13,716 | 9543 |
| ICER, incremental cost per QALY gained | Dominant | Dominant | 145,540 | 194,559 |
| Notes | Costs discounted at 6%; QALYs discounted at 1.5%, costs are in 2005 prices | Cost discounted at 6%, QALYS 1.5%. Efficacy data were based on meta-analysis that included studies of MMF in adults | ||
The technology assessment review team at Birmingham developed these analyses after considering evidence submitted by three companies using the Birmingham original model, which related to adult patients. The companies had found their sponsored drugs to result in lower total costs and higher QALYs, when compared with the triple therapy of CSA, AZA and steroids (CSA + AZA + CCS). Although the independent assessment by the Birmingham group confirmed the companies’ finding that BAS induction was expected to reduce total costs and increase QALYs, its results for initial and maintenance immunosuppression were contrary to those obtained by the companies, as TAC, AZA and steroids had an incremental cost-effectiveness ratio (ICER) above £30,000 relative to CAS and the same was found for CSA with MMF and steroids. Moreover, the technology assessment team at Birmingham found these results robust to uncertainty in the hazard rate used to extrapolate differences in AR rates to long-term estimates of health benefit.
These analyses represent the only available evidence about the costs and benefits of immunosuppressive regimens in recipients of kidney transplants aged ≤ 18. However, this evidence is based on regimens that may no longer represent routine practice in terms of therapies used (MMF has become part of standard immunosuppressive therapy) and dosages (lower doses of TAC are being used as they are perceived to have a better efficacy and safety profile).
As for the methodology behind this evidence, the assessment was based on a meta-analysis of the evidence on AR rates, although for MMF this included studies in adult patients. The study did not account for costs and HRQoL effects of changes in graft function and omitted the effect of differences between regimens in terms of the graft function on longer-term prognosis. Recent evidence from studies in adults suggest that quality of life133 and costs60 do vary significantly with renal function and this casts some doubt on the conclusion by the Birmingham group that small QALY differences are generally found between regimens. It is also questionable whether or not the surrogate relationship between AR and graft survival was validly implemented, because the estimated HR used to predict graft survival was estimated from AR rates occurring before discharge post transplantation, while the efficacy data used to model treatment differences were based on 12-month outcomes post transplantation. In addition, lack of data prevented the analysis from accounting for side effect differences between regimens, to which results were found to be sensitive. The quality assessment of these analyses are summarised in Table 45.
| Item | Yao et al.2 |
|---|---|
| 1. Is the study population clearly described? | Y |
| 2. Are competing alternatives clearly described? | Y |
| 3. Is a well-defined research question posed in answerable form? | Y |
| 4. Is the economic study design appropriate to the stated objective? | Y |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | N |
| 6. Is the actual perspective chosen appropriate? | Y |
| 7. Are all important and relevant costs for each alternative identified? | Y |
| 8. Are all costs measured appropriately in physical units? | ? |
| 9. Are costs valued appropriately? | ? |
| 10. Are all important and relevant outcomes for each alternative identified? | N |
| 11. Are all outcomes measured appropriately? | ? |
| 12. Are outcomes valued appropriately? | ? |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | Y |
| 14. Are all future costs and outcomes discounted appropriately? | Y |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | N |
| 16. Do the conclusions follow from the data reported? | Y |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | N |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | Y |
| 19. Are ethical and distributional issues discussed appropriately? | N |
Critical appraisal of company submissions
Astellas’ submission
The submission compared
-
twice-daily TAC-IR (Prograf) with
-
once-daily TAC-PR,
and, using a different modelled relationship between efficacy and effectiveness to that used by the previous comparison, it separately compared
-
twice-daily TAC-IR (Prograf) with
-
Modigraf (TAC granules for oral solution – for 3 years, then switch to Prograf)
-
TAC specials (oral suspensions)
-
EVL
-
BEL
-
SRL with low-dose CSA (CNI minimisation)
-
SRL with MMF (CNI avoidance).
Prograf was considered to be the standard treatment of choice in adult renal transplantation immunosuppression based on its UK market share, while the comparators investigated were deemed to be used infrequently. The submission cites evidence of improved outcomes for Advagraf relative to the current standard regimen, Prograf, since the former became available in 2009. In addition, as requested by the NICE scope, EVL, BEL and SRL were included in the evaluation despite their lack of market authorisation in the UK.
Astellas’ analysis found that Prograf was cost-effective compared with all comparators, except SRL (avoidance), which the company argues is not a treatment option that is routinely considered of use for children and adolescents in general. Further, Advagraf was considered cost-effective relative to Prograf and recommended by the company to be adopted as the new standard of care. Owing to limited information on children and adolescents, the model was populated with information from adult KTRs from a meta-analysis and network meta-analysis of evidence on short-term outcomes from comparative clinical studies in adults.
The submission pointed to evidence on the relationship between adherence, acute and long-term graft rejection, and graft failure. In particular, it is stated that adherence to immunosuppressant regimens positively affects graft survival by preventing the development of de novo donor specific antibodies, which have been associated with a reduction in 10-year graft survival. 134 This is the stated justification for translating the observed improvement in adherence with once-daily TAC relative to twice-daily TAC135 into graft and patient survival benefits in the Astellas model. 135 In addition, the company claims that once-daily TAC-PR has a better pharmacokinetic profile than twice-daily TAC (lower intra-patient variability,136 which results in a lower risk of long-term graft failure137). The company also cites analyses from the Collaborative Transplant Study for Europe (2011–13 data) presented at the 2014 World Transplant Congress, which shows that Advagraf-treated patients had higher patient and graft survival rates than Prograf-treated patients over the 12 months following renal transplantation. However, this observation was not robust to the adjustment for multiple confounders (HR 0.76; p = 0.14, 95% CI was not stated).
The submission also cites the results of a meta-analysis pointing to increased risk of NODAT with TAC (RR at 12 months 1.72, 95% CI 1.17 to 2.52; RR at 36 months 2.71, 95% CI 1.61 to 4.57) relative to CSA and acknowledges the evidence on the association between NODAT and reduced graft survival (RR 1.63, 95% CI 1.46 to 1.84). 138 The company argues that these estimates may have been the result of patients treated with high doses of TAC relative to current practice. To support this claim, the submission cites the results of a Phase III study comparing Advagraf with Prograf,139 which used lower doses of TAC and found lower incidence rates of NODAT than those in the studies included in the meta-analysis report. 139 However, it is noted that the Krämer et al. 139 evidence is not relevant to the meta-analysis finding of a higher RR of NODAT with TAC than CSA.
Review of economic models and their results in the submission
The submission provides an overview of model structures and conclusions of previous cost-effectiveness analyses of renal transplantation immunosuppressive regimes. From searches of electronic databases (NHS EED, The Cochrane Library, MEDLINE and other sources not specified) it identified and included 12 studies in its review (although the Astellas submission states that 11 studies were included). No details were provided about the inclusion criteria for the review of economic studies but all of the reviewed studies were conducted in adults.
One of the included studies compared TAC-IR with TAC-PR (US study140); four studies compared TAC with CSA (two in continental Europe,141,142 one in the UK143 and the remaining study was from the USA and measured only costs of medication113); seven studied SRL in CNI avoidance or minimisation strategies versus TAC (one from the USA,144 another from the UK,145 two more from Germany146,147 and three studies from Colombia, Mexico and Poland148–150).
The submission briefly described the main results of these studies without critically assessing their validity and applicability to a UK setting, although it mentions the limited transferability of results from non-UK studies (10 out of the 12). It concludes that the evidence supports the view that TAC is cost-effective when compared with CSA, but that it is ambiguous in relation to the comparison against SRL in a CNI avoidance or minimisation strategy. The submission also includes a section where three published models are described. 144,148,149 No assessment of their strengths and weakness was presented. These models are all of adult patient populations and are therefore not included in the review of cost-effectiveness studies of this monograph. 144,148,149
Economic evaluation by the company
The cost-effectiveness analysis submitted by Astellas is an adaptation of a published Markov model-based assessment of the cost-effectiveness of TAC, in either its extended release formulation, Advagraf, or the current standard therapy of immediate-release (Prograf151) in adult KTRs. The model describes the annual transitions between four health states starting from kidney-only transplantation: functioning graft without history of AR, functioning graft having experienced AR, graft failure (dialysis) and death (Table 46). Owing to the lack of child/adolescent data, the Astellas submission is based on a review of short-term safety and efficacy outcomes of immunosuppression in adults, reported by RCTs published study until June 2014. These were then extrapolated using registry data on child/adolescent graft and patient survival. The base-case analyses submitted by the company discount costs and QALY outcomes at an annual rate of 3.5%.
| Population | Comparators (initial and maintenance therapy) | Horizon | Model structure | Surrogates to model long term | Health states/events modelled | Risk factors | AEs | Model drivers (sensitivity analysis) | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Age 8 years (minimum 2 years); 26.0 kg (female); 25.6 kg (male) starting weight); England and Wales |
|
10 years (maximum 16 years; i.e. for starting age 2 years: analysis ended at age 18 years in all cases) | Markov model of annual cycles with tunnel states extrapolation of 1 year trial outcomes | AR | Functioning graft – no previous BPAR Functioning graft – previous BPAR Failed graft (dialysis) Functioning regraft – no previous BPAR Functioning regraft –previous BPAR Death |
BPAR | Malignancies CMV infections NODAT Wound-healing disorders Anaemia HMGCoA Hypertension |
Improved adherence with PR medication TAC-IR vs. SRL: Graft survival (scenario with graft survival in Symphony trial [CNI minimisation] with DAC induction) |
Assumes that BPAR only occur in the first 12 months. Graft and patient survival were estimated from UK 5-year survival statistics in children and adolescents with renal transplant (UK NHSBT Report 2012–13) extrapolated to 10 years post-transplant by exponential and linear function of time, respectively. Survival in dialysis was estimated from 10-year UK survival statistics in adults, extrapolated by exponential function. Utility values of AEs not accounted for. Model has flaws of implementation, especially in relation to retransplants |
Efficacy data
The model accounts for differences in outcomes between regimens that originate in their differing impact on BPAR at 12 months post transplant. These differences in BPAR between the regimens evaluated were estimated from RCTS of adult KTRs (Table 47). The model was based on the assumption that the effects of treatment on this surrogate outcome lasted for only the first year post transplantation. In fact, the model allowed BPAR to occur only in the first 12 months post transplantation. This assumption was combined with (1) the estimated RR of graft failure for a functioning graft with previous BPAR versus no previous BPAR and (2) the 1-year post-transplant BPAR frequency, both from estimates reported by Opelz et al. ,153 to derive the graft survival curves for grafts without prior AR and grafts with history of AR from the child 5-year graft survival profile in UK registry data (including graft survival rates for years 3 and 4 derived by linear interpolation32). The model extrapolation was complemented by using exponential survival curves to extend graft survival from 5 years up to 16 years post transplantation.
| Product | Rate, % | Comment |
|---|---|---|
| Prograf (base comparator) | 12.6 | Silva et al.,152 Albano et al.,97 Krämer et al.139 |
| Modigraf/TAC specials | 12.6 | Assumed the same as Prograf owing to lack of data |
| Advagraf | 14.6 | Silva et al.,152 Albano et al.,97 Krämer et al.139 and meta-analysis |
| BEL | 30.7 | Silva et al.,152 Albano et al.,97 Krämer et al.139 and meta-analysis |
| EVL (CNI minimisation) | 18.0 | Silva et al.,152 Albano et al.,97 Krämer et al.139 and meta-analysis |
| SRL (CNI minimisation) | 16.5 | Silva et al.,152 Albano et al.,97 Krämer et al.139 and meta-analysis |
| SRL (CNI avoidance) | 28.7 | Silva et al.,152 Albano et al.,97 Krämer et al.139 and meta-analysis |
With regard to patient survival, the model used the 1-, 2- and 5-year post-transplantation survival rates in children and adolescents from the NHS Blood and Transplant (NHSBT) Report 2013–201432 as the estimated survival rates with a functioning graft. To populate survival probabilities in the state of graft failure, the model used annual survival rates of adult patients on dialysis followed for 10 years from the UK Renal Registry. 154 The patient survival rates were extrapolated until 18 years of age (i.e. 10 years post transplant in the base case) by linear extrapolation of the available data, projecting survival rates from the last observed rate. There is no mention in the submission about adjusting survival for increases in background mortality as the cohort in the model ages. For patients in the state of graft failure, which was assumed to be associated with the use of dialysis, the probability of receiving a retransplant was populated with data from adults treated at a centre in Cardiff, Wales. 155
In addition to the difference in efficacy, measured in terms of AR rates (see Table 47), the model allowed for differences in effectiveness between the TAC arms through the differences in adherence associated with the once daily, prolonged-release formulations of the drug (Advagraf) versus the twice-daily immediate-release formulations of the drug (Prograf). The model employed comparative estimates on adherence with Advagraf versus Prograf of 88.2% versus 78.8% from a published randomised study135 and combined them with an estimated RR of graft failure in non-adherent versus adherent patients of 3.47 derived from a meta-analysis15 to obtain a RR of graft failure of 0.848, which was applied to the graft survival curves (until year 5 and, by exponential curve extrapolation, thereafter) that were common to all other immunosuppressive treatment strategies in the model. 15,135
Adverse events
The model allows for seven types of AE following transplantation: malignancy, diabetes mellitus, anaemia, CMV infection, hypertension, wound-healing disorders and the need for 3-hydroxy-3-methylglutaryl-coenzyme A. These events were assigned costs (except for the last type of event which had zero cost and, thus, was effectively omitted from the analysis) but no disutility. The AE incidence rates used in the model, reproduced in Table 48, differed across immunosuppressant treatment arms, although these had no influence on the probability of graft failure and patient death. Such differences only affected the costs differences between the treatments.
| Product | AE | Year 1 | Year 2 | Year 3 and later |
|---|---|---|---|---|
| Advagraf/Prograf/Modigraf/TAC specials | Malignancies | 0.00 | 0.00 | 0.43 |
| CMV infections | 3.62 | 3.62 | 0.04 | |
| NODAT | 6.07 | 6.07 | 6.27 | |
| Wound-healing disorders | 4.12 | 4.12 | 0.00 | |
| Anaemia | 14.71 | 14.71 | 14.71 | |
| HMGCoA | 13.84 | 13.84 | 3.46 | |
| Hypertension | 9.17 | 9.17 | 9.17 | |
| EVL | Malignancies | 2.43 | 2.43 | 0.64 |
| CMV infections | 3.19 | 3.19 | 0.04 | |
| NODAT | 5.58 | 5.58 | 5.77 | |
| Wound-healing disorders | 10.72 | 10.72 | 0.00 | |
| Anaemia | 27.30 | 27.30 | 27.30 | |
| HMGCoA | 29.47 | 29.47 | 7.37 | |
| Hypertension | 31.63 | 31.63 | 31.63 | |
| SRL (CNI minimisation and avoidance regimens) | Malignancies | 0.20 | 0.20 | 0.05 |
| CMV infections | 2.11 | 2.11 | 0.03 | |
| NODAT | 5.88 | 5.88 | 6.07 | |
| Wound-healing disorders | 10.72 | 10.72 | 0.00 | |
| Anaemia | 18.68 | 18.68 | 18.68 | |
| HMGCoA | 21.77 | 21.77 | 5.44 | |
| Hypertension | 15.08 | 15.08 | 15.08 | |
| BEL | Malignancies | 2.32 | 2.32 | 0.61 |
| CMV infections | 7.65 | 7.65 | 0.09 | |
| NODAT | 4.00 | 4.00 | 4.19 | |
| Wound-healing disorders | 4.12 | 4.12 | 0.00 | |
| Anaemia | 14.71 | 14.71 | 14.71 | |
| HMGCoA | 18.88 | 18.88 | 18.88 | |
| Hypertension | 31.12 | 31.12 | 31.12 |
The incidence rates of AEs were derived from a systematic review and meta-analysis published in 2006,156 the values adopted by the published economic model for adults in Germany by Jurgensen et al. 146 and trial outcomes from the BENEFIT and BENEFIT-EXT trials. 157,158
The rates of AEs were assumed to be the same with Advagraf and Prograf and for the two SRL regimens (CNI avoidance and CNI minimisation). According to the incidence rates figures in this model, TAC has the lowest annual incidence of malignancy (except for SRL from the third post-transplantation year onwards), CMV, anaemia (except for BEL which had the same annual incidence rates as those of TAC), dyslipidaemia and hypertension, but was associated with an excess incidence of NODAT over the other options.
Utilities
Health-related quality of life and QALY outcomes were calculated from time spent in the graft functioning state and the graft failure state, which involved dialysis. Based on published European Quality of Life-5 Dimensions (EQ-5D) estimates,159 the functioning state was associated with a utility value of 0.71, regardless of any prior experience of AR, and the graft failure state was associated with a utility of 0.459, which was equal to the weighted average of the utility of haemodialysis (0.44), experienced by 82% of dialysis patients, and peritoneal dialysis (0.53), received by the rest. 159
Retransplantation
The model allows for the occurrence and effects of retransplantation, using the time to retransplantation data reported by McEwan et al. 145,155 for adult patients. However, the states following the first retransplantation (i.e. functioning graft with prior AR on the current retransplant, functioning graft without prior AR on the current retransplant – regardless of AR of any previous transplant and graft failure) face the same transition probabilities, utility values and costs as the corresponding states before retransplantation. This is likely biasing the analysis in favour of treatments with higher rejection rates in the model (as higher AR rates imply higher graft failure rates in this model) and may be interpreted as a conservative assumption of the relative effectiveness and incremental costs advantage of TAC over the comparators.
Resource utilisation and unit costs
The amount of drug use for TAC was age dependent and imputed according to weight by age distributions in observational data by associating body surface area with mean weight by age statistics from UK growth charts. 160,161 Dosages per kg of body weight for all medications were based on adult dosages as detailed in the British National Formulary (BNF)162 and the corresponding Summary Product Characteristics, with the exception of MMF, which was based on body surface area parameters, and EVL, which was based on data from a study in children and adolescents. 163
The model used BNF prices for both interventions and comparators. The cost per mg of Advagraf used was 23% lower than that of Prograf, based on the BNF list prices and information on the market share of pack sizes for Prograf. [The authors present sensitivity analyses of discounts on TAC list prices limited to the first (commercial-in-confidence information has been removed) days post transplantation.] Prices for other immunosuppressant regimens were based on BNF prices. Table 49 reproduces table 38 in the submission, which details the prices used by the Astellas model. The submission says that TAC prices were not available in the electronic market information tool (eMIT), apparently to justify its deviation from the NICE methods guide (section 5.5.2)165 which specifies that, if available, reduced prices should be used in the reference case, that is, eMIT prices reflecting the prices paid by NHS trusts. The submission does not give any further reason for their using list prices for TAC and all the other drug regimens.
| Variable | Value | Comment |
|---|---|---|
| Cost per mg: Simulect® | £42.12 | Injection, powder for reconstitution, BAS, net price 10-mg vial = £758.69, 20-mg vial = £842.38 (both with water for injections). For i.v. infusion |
| Cost per mg: Prograf® | £1.62 | Concentrate for i.v. infusion, 5 mg/ml of TAC, net price 1-ml ampoule = £58.45. Capsules, TAC (as monohydrate) 500 µg (yellow), net price 50-capsule pack = £61.88; 1 mg (white), 50-capsule pack = £80.28, 100-capsule pack = £160.54; 5 mg (greyish-red), 50-capsule pack = £296.58 and using market distribution of pack sizes |
| Cost per mg: Advagraf® | £1.24 | Capsules, m/r, TAC (as monohydrate) 500 µg (yellow/orange), net price 50-capsule pack = £35.79; 1 mg (white/orange), 50-capsule pack = £71.59, 100-capsule pack = £143.17; 3 mg (orange), 50-capsule pack = £214.76; 5 mg (red/orange), 50-capsule pack = £266.92 |
| Cost per mg: BEL | £1.42 | i.v. infusion, powder for reconstitution, BEL, net price 250-mg vial = £354.52 |
| Cost per mg: EVL | £5.87 | No UK price available price at the time of this submission. Estimated price of EVL based on the price of Afinitor® (EVL) white-yellow, EVL, 5 mg, net price 30-tablet pack = £2250.00; 10 mg, 30-tablet pack = £2970.00 and assuming use of cheapest in terms of cost per mg |
| Cost per mg: Modigraf | £7.22 | Granules, TAC (as monohydrate), 200 µg, net price 50-sachet pack = £71.30; 1 mg, 50-sachet pack = £356.65 |
| Cost per mg: specials | £3.83 | TAC 2.5 mg/5 ml oral suspension, 100 ml = £232.44; TAC 5 mg/5 ml oral suspension, 100 ml = £301.96164 |
| Cost per mg: SRL (Rapamune®) | £3.45 | Tablets, coated, SRL 500 µg (tan), net price 30-tablet pack = £69.00; 1 mg (white), 30-tablet pack = £86.49; 2 mg (yellow), 30-tablet pack = £172.98 |
| Cost per mg: BEL (Nulojix®) | £1.42 | i.v. infusion, powder for reconstitution, BEL, net price 250-mg vial = £354.52 |
| Cost per mg: Neoral | £0.03 | Capsules, 10 mg of CSA (yellow/white), net price 60-capsule pack = £19.40; 25 mg (blue/grey), 30-capsule pack = £19.52; 50 mg (yellow/white), 30-capsule pack = £38.23; 100 mg (blue/grey), 30-capsule pack = £72.57 |
| Cost per mg: CellCept | £0.003 | Capsules, blue/brown, MMF 250 mg, net price 100-capsule pack = £82.26 |
| Cost per mg: Thymoglobuline® | £6.35 | i.v. infusion, powder for reconstitution, r-ATG, net price 25-mg vial = £158.77 |
Treatment of AR was assigned costs of i.v. steroids and, for the 20% of steroid-resistant BPAR cases, a regimen of r-ATG and the cost of an inpatient hospital stay for acute kidney injury without complications (£1737 overall mean cost). This assumed zero medical management costs for the 80% of patients with steroid-sensitive AR, which ignores any follow-up costs to monitor treatment efficacy. The cost per year of dialysis was £31,806 and the cost of retransplant was £26,639. Although the latter was based on UK NHS Reference Costs 2013 to 2014,58 the former was based on a microcosting study in seven hospital units in the UK. 166 The study measured the average costs of dialysis per year for a ‘typical patient’, who is likely to be an adult. These costs were measured from the service provider’s perspective and included direct costs and the costs of transport and medication usage. They excluded the costs of access surgery and managing dialysis complications. In addition, capital costs of the hospital building were not included. The costs of AEs adopted are presented in Table 50, which reproduces table 35 in the Astellas submission to NICE. The major elements of costs are summarised in Table 51.
| Variable | Value | Comment |
|---|---|---|
| Malignancies | £1388 to £4452 depending on body surface area (m2) | PTLD/skin/non-Hodgkin’s lymphoma. Mabthera concentrate for i.v. infusion, 10 mg/ml of rituximab, net price 10-ml vial = £174.63, 50-ml vial = £873.15. No costs included of other treatment modalities |
| CMV infections | £221 to £1151 depending on weight (kg) | i.v. ganciclovir 14–21 days then maintenance for 8 weeks. Cymevene® i.v. infusion, powder for reconstitution, ganciclovir (as sodium salt). Net price 500-mg vial = £29.77 |
| NODAT | £17.38 | Tablets, coated, metformin hydrochloride 500 mg, net price 28-tablet pack = 87p, 84-tablet pack = £1.00; 850 mg, 56-tablet pack = £1.36 |
| Wound-healing disorders | £0.00 | – |
| Anaemia | £16.88/kg | Binocrit® injection maintenance dose 17–33 units/kg three times weekly, prefilled syringe, epoetin alfa, net price 1000 units = £4.33; 2000 units = £8.65; 3000 units = £12.98; 4000 units = £17.31; 5000 units = £21.64; 6000 units = £25.96; 8000 units = £40.73; 10,000 units = £43.27 |
| LDL cholesterol | £235.03 | Zocor® tablets, all f/c, simvastatin 10 mg (peach), net price 28-tablet pack = £18.03; 20 mg (tan), 28-tablet pack = £29.69; 40 mg (red), 28-tablet pack = £29.69; 80 mg (red), 28-tablet pack = £29.69 |
| Hypertension | £15.51 | Capsules, Ramipril 1.25 mg, net price 28-capsule pack = 99p; 2.5 mg, 28-capsule pack = £1.05; 5 mg, 28-capsule pack = £1.12; 10 mg, 28-capsule pack = £1.19 |
| Cost elements | Astellasa |
|---|---|
| TAC-IR therapy (per year) | 1559 (first year) 1366 (second year+)b |
| TAC-PR therapy (per year) | 1322 (first year) 1112 (second year+) |
| Modigraf | 13,654 (first year) 13,580 (second year+) |
| TAC administration | 0 |
| MMF therapy (per year) | 1326c |
| CSA therapy | N/Ad |
| EVL (per year) | 5086 |
| EVL administration | 0 |
| SRL (per year) | 2536 (first year) 2522 (second year+) |
| SRL administration | 0 |
| BEL (per year) | 4018 (first year) 2374 (second year+) |
| BEL administration | 0 |
| CCSs | 176 (first year) 139 (second year+) |
| AR (event) | 889e |
| Dialysis (per year) | 31,806f |
| Retransplantation | 26,639g |
| Retransplantation: organ procurement | 0 |
Results
The base-case results presented by Astellas are displayed in Table 52. The expected discounted (at 3.5%) QALYs (censored after 10 years) were 5.569 for TAC-IR (Prograf), 5.565 for SRL CNI minimisation, 5.564 for EVL, 5.553 for SRL CNI avoidance, and 5.551 for BEL, in a cohort of patients of mean age 8 years. For TAC once-daily prolonged-release formulation (Advagraf), discounted QALYs were 5.569. The Modigraf and TAC specials regimens were assumed to result in the same health outcomes as Prograf.
| Submission | Regimens compared | Patient characteristics | Time horizon (years) | Life-years (undiscounted) | Discounted costs (£) | Discounted QALYs | ICER incremental cost per QALY |
|---|---|---|---|---|---|---|---|
| Astellas 2003 | TAC-IR (Prograf) | Mean age 8 years Weight 11.3–12.2 |
10 | 9.472 | 58,471 | 5.569 | Prograf vs. SRL: 1,576,937 |
| TAC (Modigraf) | 9.472 | 88,915 | 5.569 | ||||
| TAC specials | 9.472 | 72,945 | 5.569 | ||||
| SRL I | 9.468 | 52,339 | 5.565 | ||||
| EVL | 9.467 | 90,168 | 5.564 | ||||
| SRL II | 9.456 | 61,490 | 5.553 | ||||
| BEL | 9.455 | 75,726 | 5.551 | ||||
| TAC-PR (Advagraf) | 9.502 | 53,395 | 5.604 | Advagraf dominates | |||
| TAC-IR (Prograf) | 9.472 | 58,471 | 5.569 |
In the base-case results, results comparing TAC immediate-release (Prograf) with non-TAC immunosuppressive regimens, Prograf produced more QALYs than any of the comparators and lower costs than BEL and EVL, SRL avoidance, Modigraf and TAC specials whereas it had higher cost than the SRL minimisation regimen. The ICER against SRL CNI minimisation strategy was in excess of £1M. In the comparison of TAC regimens, Advagraf dominated Prograf, given its lower costs and higher QALYs (both discounted and undiscounted).
The results were found to be sensitive to the starting age (base case started at 8 years, while sensitivity analyses started at 2, 10 and 13 years) and the discount rate, AEs and half-cycle corrections. The results against SRL were found to change significantly when graft survival parameters in the model were populated with data from the Symphony study127 instead of the NHSBT Service data32 used in the base-case analyses: low dose TAC was found to dominate SRL as CNI avoidance regimen when both were given with DAC induction, 2 g of MMF and steroids. In discussing these findings, the authors note that the Symphony study127 has reported outcomes up to 3 years and is the largest prospective study in the de novo kidney transplantation to date, which showed TAC to result in lower AR, better renal function and graft survival outcomes at 1 year than the SRL regimen.
On the basis of these results, the company submission concludes that TAC is cost-effective and that Advagraf should become the standard of care as it produces lower costs and better health outcomes than Prograf. The latter statement is further supported, the submission claims, by the expected benefits (not accounted for in the Astellas model) arising from the improved pharmacokinetic profile of Advagraf relative to Prograf. Despite the apparent cost-effectiveness of its CNI minimisation mode, the submission states that the results of the Symphony trial have discouraged the general use of SRL and that BEL’s high cost and high AR rate may do likewise, citing a report by the All Wales Medicines Strategy Group168 as supportive evidence for this assertion.
Critical appraisal
The analysis presented by Astellas (Table 53 shows the quality checklist) covers a number of appropriate comparators, including new regimens BEL and regimens with modes of action different from that of CNIs (i.e. EVL and SRL), as well alternative TAC formulations that are believed by the company to be used in routine practice (i.e. Modigraf and specials). However, it omits one relevant comparator: CSA. There is no justification in the submission as to why this drug regimen was not considered. This suggests that the results presented may be misleading owing to the exclusion of a relevant comparator. In addition, all of the regimens analysed by Astellas were evaluated in combination with MMF. This seems to contradict the assertion in the company’s submission that ‘Most children in the UK receive triple immunosuppression therapy with a CNI (CSA or TAC), a DNA proliferation inhibitor (usually azathioprine), and a CCS following kidney transplantation’ (Astellas’ submission, page 1). Astellas also reported the results of sensitivity analyses that varied the mean starting age of patients in the cohort modelled, but as the analysis was censored/stopped at age 18 years, it is difficult to assign any meaningful interpretation to their findings that the results were sensitive to such variation.
| Item | Astellas’ submission |
|---|---|
| 1. Is the study population clearly described? | Y |
| 2. Are competing alternatives clearly described? | Y |
| 3. Is a well-defined research question posed in answerable form? | Y |
| 4. Is the economic study design appropriate to the stated objective? | Y |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | Y |
| 6. Is the actual perspective chosen appropriate? | Y |
| 7. Are all important and relevant costs for each alternative identified? | Y |
| 8. Are all costs measured appropriately in physical units? | Y |
| 9. Are costs valued appropriately? | Y |
| 10. Are all important and relevant outcomes for each alternative identified? | N |
| 11. Are all outcomes measured appropriately? | Y |
| 12. Are outcomes valued appropriately? | Y |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | Y |
| 14. Are all future costs and outcomes discounted appropriately? | Y |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | Y |
| 16. Do the conclusions follow from the data reported? | Y |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | N |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | N |
| 19. Are ethical and distributional issues discussed appropriately? | N |
There are two logical concerns with the Astellas model-based analysis. First, by accounting for the advantages in adherence of Advagraf in its comparison with Prograf, it makes the comparison of outcomes of Advagraf with those of other immunosuppressive regimens in the model invalid, as no allowance was made for any effects of adherence on graft survival for the other regimens analysed in the model. Indeed this undermines the fundamental assumption in the model that all significant differences in any drug regimen comparison may be accounted for by the effect through the surrogate, in this case the rate of AR. 169 Thus, regardless of the validity of the comparative analysis of Advagraf and Prograf, indirect comparisons of model results between advagraf and SRL, and EVL and BEL are invalid. Second, although the model was adjusted to include the effect of adherence on graft survival in the Advagraf versus Prograf comparison, the patient survival curves (for the functioning and failed graft states) were left unchanged, thus the same set of patient survival curves was applied to all immunosuppressive options analysed. This implies the empirically questionable assumption that improvements in graft survival, such as those obtained with Advagraf relative to Prograf (and indeed relative to all other model arms), do not translate in direct patient survival benefits. This inconsistent logic in turn leads to underestimating the benefits of Advagraf and overestimating its costs.
Inspection of the Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA) model spreadsheets revealed that the TAC drug regimen options (Advagraf and Prograf) and EVL were the only treatment arms populated by actual data on immunosuppressive drug use from the RCT sample that served as the source for the respective efficacy data; drug consumption values for BEL and SRL regimens were based on treatment guidelines (BNF or SPC). Adult dosages (per kg body weight) of these treatments were used to estimate costs in the model. The only therapies for which child-specific doses were used in calculating resource utilisation in the analysis were MMF and EVL. There are important distinctions with adults that are likely to cast doubt on these drug dosage values. In particular, as acknowledged by the authors in relation to TAC pharmacokinetic studies, children and adolescents appear to eliminate the drug more rapidly than older adults. Further, in relation to steroids, there are concerns about the effects of the medication on growth, which are likely to lead to its more limited use in children and adolescents than in adults.
There is inadequate use of the registry data used to extrapolate short-term efficacy outcomes from RCT in the model. The model used the data from the NHSBT report 2013–1432 on patient survival rates for kidney-only transplant recipients in the UK (table 28, p. 35, Astellas’ submission) to populate the patient survival parameters of patients with a functioning graft, ignoring the fact that such data on survival rates were likely to include deaths from both patients with a functioning and a failed graft. Instead, the probability of death in the graft functioning state should have been calculated as the remainder of the annual probability of death from the NHSBT patient survival data minus the product of probability of mortality in the graft failure state and the proportion of patients with a failed graft. In other words, the Astellas model is likely to overestimate mortality in the functioning graft states, which, in turn, underestimates the benefits of gains in efficacy (i.e. reductions in AR in the model) that any regimen may have over another, for example TAC over the comparators. Thus, the results reported by Astellas in the submission may be treated as conservative estimates of the costs and benefits of its TAC regimes. In relation to the evidence presented in support of Advagraf, its quality is limited by the omission of CSA as a comparator therapy and the fact that the Advagraf versus Prograf comparison is based on what is, in effect, a different model of the outcomes of renal transplantation from that used to compare Prograf with all the other regimens. In fact, the model used for comparing Advagraf with Prograf contradicts the fundamental premise of the model used to compare Prograf with all regimens other than Advagraf: that AR captures all important drivers of clinically meaningful outcomes.
One other issue relates to the way the model was structured. Although the model allowed repeat transplantation to occur for a given individual, only for the first transplantation were the costs and HRQoL of subsequent dialysis accounted for. Although the proportion of patients with more than one retransplantation may be small, this assumption could have been important to the conclusions derived from the comparison with CSA, had such comparator been included.
In addition, Astellas chose to use values of time to retransplantation for patients on dialysis that were obtained from adult studies, whose mean wait for a retransplant was 3 years. 155 This was in contradiction with the company’s submission, which stated that ‘Children tend to be prioritised in deceased donor organ allocation systems: the median wait for a kidney in the UK during 2003–2006 for patients aged < 18 years was 277 days’. 170
There is also an anomaly with regards to the timing of transplantation. Markov models typically imply that transitions occur at the end of the period represented by each cycle. In the present case, the cycle length was 1 year and the authors of the Astellas model correctly decided on using half-cycle corrections to reduce the inaccuracy in expected costs and QALY calculations arising from more frequent average state transitions. However, the model assumed that a proportion of patients undergo retransplantation in the very first cycle and that these made a transition from the failed graft state to a functioning graft post retransplantation state as if the retransplant had occurred at the start of the period so that they spent the whole cycle length (6 months, owing to the half-cycle correction) with a functioning graft after retransplantation in the first cycle. However, this is incorrect as in a cohort of de novo kidney transplant patients, the discrete Markov process transition from a functioning first graft to a functioning retransplant requires two sequential intervening events to occur (i.e. graft failure and retransplantation) and a minimum of two cycles, one for each event.
In terms of the values used to populate the model, the costs of dialysis – one of the most influential parameters in the analysis – was derived from a microcosting study of the treatment pathway of a typical (i.e. adult) patient at six hospital units. This study166 sought to inform the introduction of Payment by Results in the NHS. 171 It did not include the costs of access surgery, managing dialysis complications and capital building costs. Reference costs for dialysis are now available that may reflect more representative data. 58 On this basis of this feature and the observation that children and adolescents tend to require higher staff-to-patient ratios than adults,2 it is expected that the costs of dialysis have been underestimated by the Astellas analysis.
The analysis does not account for discounts in price paid by hospitals for TAC-IR (Prograf), MMF, steroids and CSA (in the SRL CNI minimisation regimen), which were found to be one-third, one-tenth, one-tenth and a half of the list prices, respectively (see Tables 49 and 97). The implications of these differences are further explored in the next section (see Chapter 5).
Chapter 5 Independent economic assessment
Introduction
The objective of this independent economic assessment was to answer the following study question in line with the NICE reference case:165
What is the cost-effectiveness of immunosuppressive regimens in renal transplantation in children and adolescents, of BAS and r-ATG as an induction therapy and TAC-IR, TAC-PR, MMF, MPS, SRL, EVL and BEL as a maintenance therapy?
We are aware of only one published economic evaluation that partially addresses the study question, which is the economic evaluation conducted to support current NICE guidance TA99, published by Yao et al. 2 This evaluation did not include the interventions rabbit antihuman thymocyte, EVL or BEL. Astellas submitted an economic evaluation which also does not address the study question in full.
No economic evaluation has independently addressed the full study question in line with the NICE reference case and, therefore, a new economic assessment was required.
The economic assessment was conducted in parallel with an economic assessment of the same study question in the adult population (review of NICE guidance TA85) and the decision-analytic model developed in Microsoft Excel for the parallel assessment was used as the basis for answering the study question in this assessment in a cost–utility analysis with modifications to make it more relevant to the child/adolescent population.
Methods
Summary of changes from Peninsula Technology Assessment Group model for adults
This economic assessment was conducted using an economic model originally developed by PenTAG to evaluate the cost-effectiveness of immunosuppressive agents in adult KTRs. A summary of changes is provided here as a reference for readers familiar with the original model for adult KTRs (Table 54).
| Type of change | Description | Detailed description and justification in Chapter 5 |
|---|---|---|
| Structural | Addition of two new arms: BAS + TAC + AZA and r-ATG + TAC + AZA | Interventions and comparators |
| Change of assumed baseline regimen from BAS + TAC + MMF to BAS + TAC + AZA | Model structure | |
| Removal of DCD and living unrelated donors for first graft | Graft survival , Baseline | |
| Addition of extra retransplantation | Markov model | |
| Inclusion of six new arms (three pairs), based on child/adolescent RCTs identified in Chapter 3 (summarised in Table 10) | Decision tree | |
| Inclusion of body weight and surface area as age-dependent variables affecting doses | Target population and subgroups | |
| Natural history parameters | Baseline graft survival re-estimated for those under 18 years and according to age group (< 6 years, 6–12 years, > 12 years) | Graft survival , Baseline |
| Increased rate of retransplantation while < 18 years | Interventions and comparators | |
| Surrogate relationship between eGFR and graft survival re-estimated from a child/adolescent study | Graft survival , Graft function at 12 months | |
| Baseline eGFR at 12 months re-estimated from a child/adolescent study | Graft survival , Graft function at 12 months | |
| Probability of pre-emptive retransplantation at loss of first graft set to 20% | Graft survival , Use of graft survival in the model | |
| Re-estimated baseline risks of AR, cytomegalovirus infection and NODAT | Adverse events | |
| Re-estimated risk profiles for cytomegalovirus and Epstein–Barr virus | Tables 91 and 93 | |
| Mortality rate while receiving dialysis estimated for those < 18 years | Overall surviva l, Mortality after graft loss | |
| Cost parameters (resource use) | Dosages for TAC-IR, CSA, MMF, AZA and prednisolone updated with estimates from child/adolescent studies | Resource use , Maintenance therapy |
| Cytomegalovirus prophylaxis resource use updated | Resource use , Infection prophylaxis | |
| Post-transplant monitoring resource use updated | Resource use , Monitoring | |
| Mix of haemodialysis and peritoneal dialysis estimated for those < 18 years | Resource use , Dialysis | |
| Cost parameters (unit costs) | Cost of temporary access for haemodialysis estimated for those < 19 yearsa | Unit cost, Dialysis |
| Ongoing costs of haemodialysis and peritoneal dialysis updated for those < 19 years | Unit cost, Dialysis | |
| Cost of 10 mg of BAS dose added for KTRs who weigh < 35 kg | Unit cost, Induction | |
| Costs estimated for differing severity of AR (spontaneously resolving, steroid sensitive and steroid resistant) | Unit costs , Acute rejection | |
| Cost of PTLD estimated | Unit costs , Post-transplant lymphoproliferative disease | |
| Costs of hypertension and hypomagnesaemia estimated | Unit costs, Hypomagnesaemia and Hypertension | |
| Costs of explant surgery estimated for those < 19 years | Unit costs , Explant surgery | |
| Costs of pre-transplant workup and transplantation estimated for those < 19 years | Unit costs , Subsequent transplant |
Modelling approach
Target population and subgroups
The target population was children and adolescents undergoing kidney-only transplantation (i.e. people receiving multiorgan transplants are not included). The upper age limit for the population ‘children and adolescents’ is not always clear as young people aged 16–18 years may receive their treatment in child/adolescent or adult centres. 172 Although some data sets include only young people aged < 16 years, the population for the economic assessment is children and adolescents aged < 18 years. The vast majority of transplant kidneys for this population come from DBD and living related donors (UK Transplant Registry standard data set, see Appendix 10 for further details) (Cathy Hopkinson, NHSBT, 15 October 2014, personal communication).
The UK Transplant Registry standard data set contains data on all solid organ transplants in the UK between 1995 and 2012. It allows linkage of multiple transplants for a single recipient and includes graft and patient survival (measured in days). A total of 34,803 records refer to kidney-only transplants, of which 29,759 recorded both graft and patient survival, 4937 recorded graft survival only (although it may be inferred that the patient survived at least as long as the graft), 24 recorded patient survival only, and 83 recorded neither graft nor patient survival.
The population modelled is incident KTRs and did not include prevalent KTRs (i.e. people who received a kidney transplant in the past) or those suffering from AR (although a number of the interventions separately have marketing authorisation for the treatment of AR).
To explore the impact of age at time of transplantation on cost-effectiveness, subgroups were identified by age (Table 55). In addition to this, the average cost-effectiveness of interventions was calculated by determining weighted average total discounted costs and QALYs for each year of age. It was assumed that the same number of transplants would be conducted in 16- and 17-year-olds as for 15-year-olds in order to estimate the cost-effectiveness for those under 18 years. No other subgroups were analysed as there was no evidence from child/adolescent RCTs identified in the systematic review of clinical effectiveness to support economic evaluation of these subgroups.
| Age (years) | Number of transplants (2000–13) | Proportion of transplants (2000–13) |
|---|---|---|
| 1 | 30 | 2.2% |
| 2 | 77 | 5.5% |
| 3 | 89 | 6.4% |
| 4 | 83 | 6.0% |
| 5 | 80 | 5.8% |
| 6 | 66 | 4.7% |
| 7 | 65 | 4.7% |
| 8 | 80 | 5.8% |
| 9 | 84 | 6.0% |
| 10 | 91 | 6.5% |
| 11 | 97 | 7.0% |
| 12 | 120 | 8.6% |
| 13 | 117 | 8.4% |
| 14 | 151 | 10.9% |
| 15 | 161 | 11.6% |
The weight and body surface area of child/adolescent KTRs are important for dosing and are highly dependent on age. It was assumed that the weight of child/adolescent KTRs would follow the median weight of UK children and adolescents160,161 (Figure 14). In scenario analyses it was assumed instead that the weight of child/adolescent KTRs would follow the ninth centile weight of UK children and adolescents to reflect the possibility that child/adolescent KTRs may have had their growth impaired by renal failure.
FIGURE 14.
Median weight of UK children and adolescents according to age.
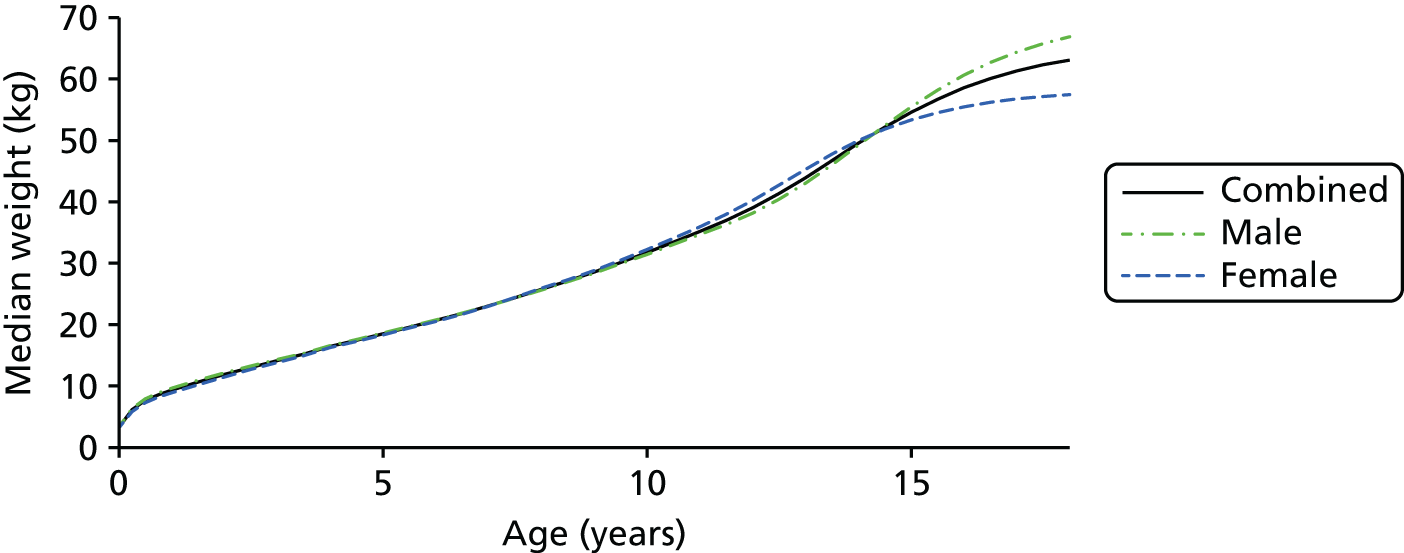
Body surface area was then calculated from weight based on the table for body surface area estimation in the BNF for Children,173,174 as shown in Table 56.
| Weight (kg) | BSA (m2) |
|---|---|
| 1.0 | 0.10 |
| 1.5 | 0.13 |
| 2.0 | 0.16 |
| 2.5 | 0.19 |
| 3.0 | 0.21 |
| 3.5 | 0.24 |
| 4.0 | 0.26 |
| 4.5 | 0.28 |
| 5.0 | 0.30 |
| 5.5 | 0.32 |
| 6.0 | 0.34 |
| 6.5 | 0.36 |
| 7.0 | 0.38 |
| 7.5 | 0.40 |
| 8.0 | 0.42 |
| 8.5 | 0.44 |
| 9.0 | 0.46 |
| 9.5 | 0.47 |
| 10.0 | 0.49 |
| 11.0 | 0.53 |
| 12.0 | 0.56 |
| 13.0 | 0.59 |
| 14.0 | 0.62 |
| 15.0 | 0.65 |
| 16.0 | 0.68 |
| 17.0 | 0.71 |
| 18.0 | 0.74 |
| 19.0 | 0.77 |
| 20.0 | 0.79 |
| 21.0 | 0.82 |
| 22.0 | 0.85 |
| 23.0 | 0.87 |
| 24.0 | 0.90 |
| 25.0 | 0.92 |
| 26.0 | 0.95 |
| 27.0 | 0.97 |
| 28.0–29.0 | 1.00 |
| 30.0–34.0 | 1.10 |
| 35.0–38.0 | 1.20 |
| 39.0–43.0 | 1.30 |
| 44.0–48.0 | 1.40 |
| 49.0–53.0 | 1.50 |
| 54.0–58.0 | 1.60 |
| 59.0–64.0 | 1.70 |
| 65.0–69.0 | 1.80 |
| 70.0–75.0 | 1.90 |
| 76.0–81.0 | 2.00 |
| 82.0–87.0 | 2.10 |
| 88.0–90.0 | 2.20 |
Setting and location
The NHS in England (although some data sources have been UK-wide, particularly the UK Renal Registry and the UK Transplant Registry standard data set).
Study perspective
In line with the NICE reference case,165 the perspective adopted on outcomes was all direct health effects for patients (and, when relevant, carers) and the perspective adopted on costs was that of the NHS and Personal Social Services.
Interventions and comparators
As the immunosuppressive agents are used in combination and in sequence, we used treatment regimens as interventions and comparators rather than individual agents, although the cost-effectiveness of an individual agent versus another individual agent can then be evaluated by considering the cost-effectiveness of regimens which are identical but for the use of the intervention agent or the comparator.
Regimens were included as interventions or comparators if they were in current use in the NHS or if they would plausibly be used in the NHS and there was sufficient clinical evidence to estimate the costs and outcomes for KTRs receiving those regimens. It was necessary to include regimens that are not in current clinical practice to allow all the interventions being appraised to have their cost-effectiveness appraised. The only regimen which is a pure ‘comparator regimen’ (in that it contains no agents listed as interventions in the scope) is CSA + AZA.
Two regimens were included which were not included in the economic assessment for adults: BAS + TAC + AZA and r-ATG + TAC + AZA. The first was added as it is in common use in the NHS and the second was added to allow comparison of BAS and r-ATG in combination with TAC-IR and AZA.
Table 57 presents the regimens considered in this analysis as well as an indication of whether or not the Assessment Group believes the regimen to be a licensed combination for children and adolescents (however, no warranty or representation is given as to the correctness of the information presented in this regard, which reflects the Assessment Group’s understanding of the marketing authorisation as stated in the summaries of product characteristics; this understanding has not been confirmed by a clinician or pharmacist and, therefore, its accuracy cannot be guaranteed, particularly as regards drug combinations).
| Identifier | Induction therapy | Maintenance therapya | Licensed |
|---|---|---|---|
| CSA + MMF | None | CSA and MMF | Y |
| TAC + MMF | None | TAC-IR and MMF | U |
| CSA + AZA | None | CSA and AZA | Y |
| TAC + AZA | None | TAC-IR and AZA | Y |
| CSA + EVL | None | CSA and EVL | N |
| TAC + SRL | None | TAC-IR and SRL | N |
| TAC-PR + MMF | None | TAC-PR and MMF | N |
| BAS + CSA + MMF | BAS | CSA and MMF | Y |
| BAS + TAC + MMF | BAS | TAC-IR and MMF | U |
| BAS + CSA + AZA | BAS | CSA and AZA | Y |
| BAS + TAC + AZA | BAS | TAC-IR and AZA | U |
| BAS + SRL + MMF | BAS | SRL and MMF | U |
| BAS + BEL + MMF | BAS | BEL and MMF | N |
| BAS + CSA + MPS | BAS | CSA and MPS | N |
| r-ATG + CSA + MMF | R-ATG | CSA and MMF | Y |
| r-ATG + TAC + MMF | R-ATG | TAC-IR and MMF | U |
| r-ATG + CSA + AZA | R-ATG | CSA and AZA | Y |
| r-ATG + TAC + AZA | R-ATG | TAC-IR and AZA | Y |
In its submission, Astellas also included the following regimens, which we have not modelled:
-
SRL and CSA (with BAS induction) – note that we have modelled SRL and TAC without BAS induction (although the SPC for SRL specifies it is to be used in combination with CSA, we found significantly more RCT evidence in the adult population for which it was used in combination with TAC)
-
EVL and CSA (with BAS induction) – note that we have modelled this without BAS induction because there were slightly more patients in adult RCTs receiving this regimen without induction
-
TAC-IR (‘specials’ for first 3 years followed by Prograf for remaining life of graft) and MMF (with BAS induction)
-
TAC-IR (Modigraf for first 3 years followed by Prograf for remaining life of graft) and MMF (with BAS induction).
The last two regimens are for children and adolescents who are unable to swallow Prograf capsules (although, inconsistently, they are assumed to be able to swallow MMF capsules and prednisolone tablets) and able to swallow Modigraf suspension (our expert advisory group has suggested some children cannot swallow Modigraf suspension and require fully liquid formulations, which can be purchased from specialist manufacturers rather than being prepared as specials by pharmacists or carers).
Time horizon
The time horizon was 50 years for consistency with the parallel HTA in adults and to ensure that all important differences in costs or outcomes between the technologies are included.
Discount rate
In line with the NICE reference case, the discount rate for costs and health effects was 3.5% per annum. 165
Choice of health outcomes
The primary health outcome of the independent economic assessment was QALYs for each comparator regimen, in line with the NICE reference case. 165
Secondary outcomes included:
-
undiscounted life-years (life expectancy)
-
undiscounted life-years with a functioning graft
-
undiscounted life-years on dialysis
-
likelihood of experiencing at least one episode of AR
-
likelihood of developing NODAT
-
likelihood of receiving a second, third or fourth transplant.
Model structure
Owing to the paucity of RCT evidence in the child/adolescent kidney transplant population it was decided that two types of analyses would be conducted.
The first type of analysis was based on actual RCT evidence in the child/adolescent kidney transplant population meeting the inclusion criteria for our systematic review of clinical effectiveness evidence (see Chapter 3, Inclusion and exclusion criteria). For each RCT, a decision tree was used to model the expected costs incurred and QALYs accrued for the duration of the trial (see Decision tree), followed by extrapolation using the Markov model (see Markov model), as shown in Figure 15. These analyses allow for an estimation of the cost-effectiveness of the interventions BAS and TAC-IR while relying on as little evidence from the adult population as possible, but do not allow for estimation of the cost-effectiveness of other interventions.
FIGURE 15.
Simplified diagram of decision tree used for economic analyses based on child/adolescent RCTs.
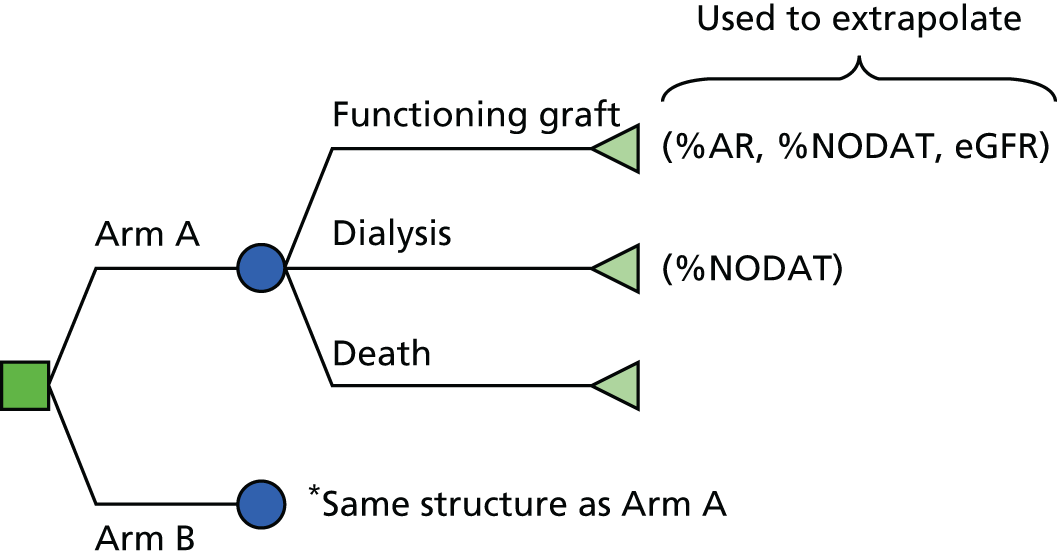
The second type of analysis was conducted using the Markov model only (see Markov model) and by assuming effectiveness estimates from adults (relating to death within 12 months, graft loss within 12 months, AR within 12 months, eGFR at 12 months, NODAT within 12 months, CMV infection and dyslipidaemia within 12 months) apply to children directly. This analysis allows the cost-effectiveness of all interventions and comparators to be evaluated, but relies on a strong assumption that the effectiveness estimates will not be biased when applied to a different population.
We do not present either type of analysis as a preferred base case because both have deficiencies. We attempt to draw conclusions by comparing the results of both types of analyses.
All analyses were constructed in Microsoft Excel 2010.
Decision tree
For each of the three RCTs in children and adolescents, a decision tree was created which calculated the following outcomes for each arm:
-
costs (discounted and undiscounted) of immunosuppression, AR and AEs during the trial duration
-
life-years up to the trial duration with functioning graft and with dialysis
-
QALYs (discounted and undiscounted) during the trial duration
-
for extrapolation using the Markov model
-
proportion of KTRs alive with functioning graft at the end of the trial duration
-
proportion of KTRs who are dialysis-dependent at the end of the trial duration
-
probability of AR within 12 months
-
probability of NODAT within 12 months
-
graft function (mean eGFR) at 12 months.
-
The discounted costs and QALYs from the decision tree and from the Markov model extrapolation were then combined. Cost-effectiveness results were presented both with ICERs and with incremental net health benefit figures (calculated at £20,000 and £30,000 per QALY). Cost-effectiveness results were also calculated by restricting the time horizon to the trial duration, that is, without extrapolating using the Markov model.
For simplicity, it was assumed that no KTRs losing their graft would be retransplanted within the trial duration. For Offner et al. ,73 with follow-up of only 1 year, this is likely to be a very reasonable assumption. For Grenda et al. 75 and Trompeter et al. ,77 with follow-up of 2 and 5 years, respectively, this may result in a bias against the arm with greater graft loss.
Methods for estimating costs
Resource use as reported in the RCTs was used to estimate costs during the trial duration. When the resource use for certain components was not reported in RCTs, either assumptions were made to extrapolate from RCT evidence in adults, or if these cost components were small and/or unlikely to vary between arms, these components were excluded from the analysis.
Immunosuppression resource use was frequently reported as dose per kg body weight or per m2 body surface area, so these were estimated and were modelled to increase over the course of the trial duration in line with child/adolescent growth curves. If baseline body weight was not reported, it was estimated based on age at baseline.
Methods for estimating life-years
For each RCT, we estimated the numbers and times of KTRs losing their grafts (any cause, including DWFG) and the numbers and times of KTRs dying. It was then assumed that all KTRs not losing their graft or dying were censored at the end of the trial duration. Restricted mean survival was calculated (restricted to the trial duration) as shown in Table 58. The estimated life-years with functioning graft was then the restricted mean graft survival (not censored for DWFG). Restricted mean patient survival minus restricted mean graft survival gave the estimated life-years on dialysis.
| Trial | Trompeter et al.77 | Grenda et al.75 | Offner et al.73 | |||
|---|---|---|---|---|---|---|
| Arm | TAC + AZA | CSA + AZA | TAC + AZA | BAS + TAC + AZA | BAS + CSA + MMF | CSA + MMF |
| Overall survival | ||||||
| T max | 4 | 2 | 1 | |||
| E[T] | 3.921 | 3.852 | 1.996 | 2.000 | 0.984 | 1.000 |
| SE[T] | 0.0383 | 0.0733 | 0.0018 | 0.0057 | ||
| Graft survival | ||||||
| T max | 4 | 2 | 1 | |||
| E[T] | 3.769 | 3.609 | 1.840 | 1.884 | 0.975 | 0.994 |
| SE[T] | 0.0748 | 0.1030 | 0.0550 | 0.0503 | 0.0123 | 0.0055 |
For the probabilistic sensitivity analyses (PSAs), the restricted mean survivals were estimated by fitting a gamma random variable to the difference between follow-up and restricted mean survival using the method of moments. More specifically, if Tdiff is the difference between the follow-up duration (Tmax) and the restricted mean survival (T):
These gamma random variables were sampled separately for each arm and for graft survival and patient survival. In the event that graft survival was sampled as longer than patient survival (an impossibility) in one or both arms, graft survival was compressed in both arms by the same factor such that graft survival was equal to or less than patient survival.
If there were no events in one arm, the SE of restricted mean survival in the total population was assumed for both arms, and a small constant was added to E[Tdiff] for both arms.
Outcomes for extrapolation
Overall survival (Kaplan–Meier) as reported by the RCTs was used to estimate the proportion of children and adolescents dead at the end of the trial duration, that is, at the start of extrapolation using the Markov model (Table 59). Kaplan–Meier graft survival (this time censored for DWFG) was used to estimate the proportion of those alive who would still have a functioning graft (see Table 59).
| Trial | Trompeter et al.77 | Grenda et al.75 | Offner et al.73 | |||
|---|---|---|---|---|---|---|
| Arm | TAC + AZA | CSA + AZA | TAC + AZA | BAS + TAC + AZA | BAS + CSA + MMF | CSA + MMF |
| Kaplan–Meier overall survival | 0.94 | 0.92 | 0.989 | 1.000 | 0.972 | 1.000 |
| Kaplan–Meier graft survival (censored for DWFG) | 0.954 | 0.792 | 0.896 | 0.949 | 0.981 | 0.989 |
| AR within 12 monthsa | 0.43 | 0.62 | 0.26 | 0.24 | 0.13 | 0.23 |
| NODAT within 12 months | 0.019 | 0.011 | 0.011 | 0.040 | 0.0 | 0.0 |
| eGFR at 12 months (ml/minute/1.73 m2) | 64.9 | 57.8 | 74.9 | 74.0 | 79 | 82 |
Markov model
A Markov model structure was used with three main states: functioning graft, graft loss and death.
The KTRs start in the functioning graft unless they suffer PNF, in which case they start in the graft loss state. Transitions can occur from functioning graft to graft loss, reflecting disease progression; transitions are not permitted in the opposite direction except through retransplantation. Up to three retransplantations are possible and, therefore, there are four substates for functioning graft and graft loss reflecting the graft number (1–4). As with the initial graft, it is possible that PNF will occur and, therefore, transitions can occur directly to graft loss following second, third or fourth graft. Pre-emptive retransplantation can occur from the original functioning graft state, but not from functioning graft states 2–4. Death can occur from any state but the rate of mortality is greater in the graft loss state (see Overall survival, Mortality after graft loss) and increases with age.
Irrespective of the regimen used for immunosuppression in the first graft, a common regimen was used for subsequent grafts (BAS + TAC + MMF), as this was judged the most likely regimen for kidney transplantation in adults (and most retransplantations are expected to occur after KTRs reach adulthood).
Figure 16 gives the model diagram showing the nine states in the model. Self-links are omitted from all states in both figures for clarity (there are no tunnel states).
FIGURE 16.
Markov model diagram. Note that green arrows indicate pre-emptive retransplantation while dashed arrows signify PNF of a subsequent retransplantation. FR, functioning graft; GL, graft loss.
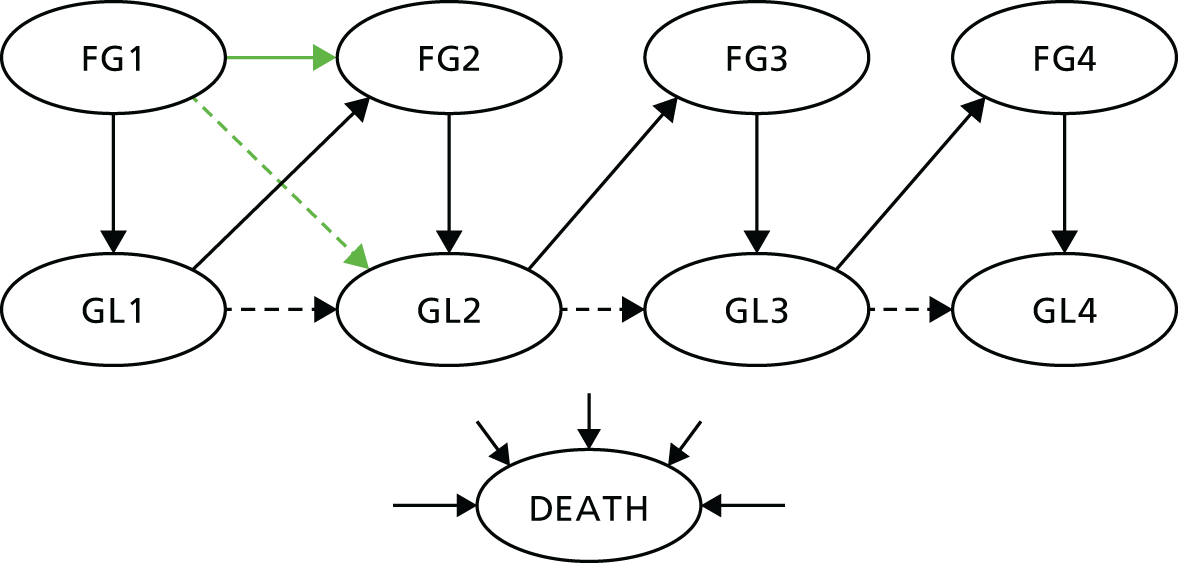
In addition to these health states, for each regimen the incidence of AR, CMV infection, dyslipidaemia and NODAT was estimated.
For each allowable transition, a transition rate was modelled. The probability of each transition was then calculated using the following formula:
where ri is the hazard rate of the specific transition, R is the sum of allowable transition rates (including ri) and Δt is the time step (cycle length).
Table 60 gives a summary of how the transition rates were dependent on factors such as age, AR and NODAT. BAS + TAC + AZA was assumed to be the baseline regimen for the initial graft, for the following reasons:
-
Only two of the four regimens in current use in the NHS (TAC + AZA and BAS + TAC + AZA) are consistent with current NICE guidance TA99. 1
-
Although the most common regimen in use is TAC + AZA, this is also expected to result in worse outcomes than BAS + TAC + AZA, TAC + MMF and BAS + TAC + MMF (except death within 12 months, for which it is expected to be superior to TAC + MMF, and eGFR at 12 months, for which it is expected to be superior to TAC + MMF and BAS + TAC + MMF) according to network meta-analyses of adult RCT evidence, and so TAC + AZA may not be as close to average UK outcomes as BAS + TAC + AZA.
| Transition | Corresponding clinical outcome | Dependent on |
|---|---|---|
| Functioning graft to graft loss (first graft) | Disease progression (graft loss/survival) | First year |
| Time since transplantation | ||
| Regimen-specific OR of graft loss within 12 months | ||
| Subsequent years | ||
| Time since transplantation | ||
| BPAR within 12 months | ||
| NODAT within 12 months | ||
| eGFR at 12 months | ||
| Functioning graft to graft loss (subsequent graft) | Disease progression (graft loss/survival) | (Constant) |
| Functioning graft to death (first graft) | DWFG | First year |
| Time since transplantation | ||
| Regimen-specific HR based on OR of patient death within 12 months | ||
| Subsequent years | ||
| Time since transplantation | ||
| Age | ||
| NODAT | ||
| Functioning graft to death (subsequent graft) | DWFG | Age |
| NODAT | ||
| Graft loss to subsequent functioning graft | Retransplantation | Age |
| Graft loss to death | Mortality while receiving dialysis | Age |
Factors included in the model
Overall survival
Overall survival was not explicitly included as an input to the model and, therefore, emerges from the two modelled rates of mortality (see Overall survival, Death with functioning graft and Mortality after graft loss).
The exception to this is that the rate of DWFG in the first year was adjusted using an individual HR for each regimen to achieve the desired OR of patient mortality as derived from the mixed-treatment comparison (MTC) and head-to-head comparisons.
Although it would be possible to use numerical methods (e.g. Solver add-in for Microsoft Excel) to achieve exact patient mortality, it was felt it would add significant computational burden, create significant opportunity for human error (forgetting to rerun Solver every time relevant parameters were changed) and would greatly slow down PSAs.
Therefore, a regression approach was used instead, by running different parameter values through the model and recording the resulting odds of mortality within 12 months. The two factors driving patient survival at 12 months that could vary between regimens were identified as the OR of graft loss (after returning to dialysis the mortality rate increases) and the HR of DWFG. The OR of patient mortality within 12 months was plotted against the HR of DWFG for various different ORs of graft loss, and was found to be linearly dependent on a log-log plot (Figure 17).
FIGURE 17.
Odds ratio of patient mortality is dependent on HR of DWFG and OR of DCGL. DCGL, death-censored graft loss.

For each OR of graft loss, linear regression of ln(odds of patient mortality) versus ln(HR of DWFG) was performed and the values of the linear regression coefficients were found to be linearly dependent on the OR of graft loss (Figure 18).
FIGURE 18.
Linear regression coefficients for ln(OR of patient death) versus ln(HR of DWFG) plotted versus OR of graft loss.
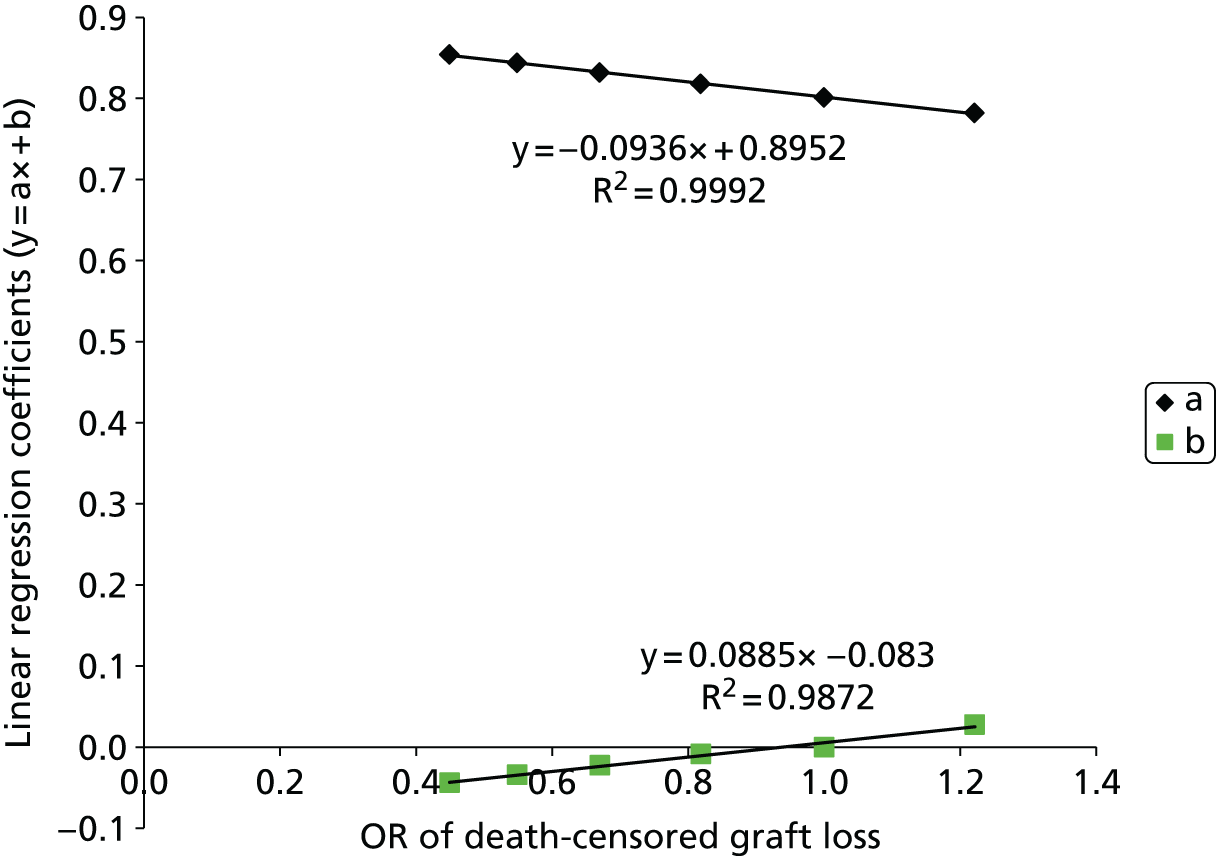
The appropriate HR for DWFG to achieve a desired OR of patient mortality is therefore derived as follows (where ORDCGL,i is the OR of graft loss, HRDWFG,i is the HR of DWFG and ORPD,i is the OR of patient death):
As can be seen in Table 61, the regression formulae perform well in most instances.
| Regimen | HR for DWFG from regression | HR for DWFG from solver |
|---|---|---|
| CSA + MMF | 0.724 | 0.717 |
| TAC + MMF | 1.302 | 1.295 |
| CSA + AZA | 0.745 | 0.739 |
| TAC + AZA | 1.129 | 1.127 |
| CSA + EVL | 1.186 | 1.183 |
| TAC + SRL | 1.106 | 1.105 |
| TAC-PR + MMF | 1.739 | 1.696 |
| BAS + CSA + MMF | 0.641 | 0.629 |
| BAS + TAC + MMF | 1.143 | 1.142 |
| BAS + CSA + AZA | 0.661 | 0.649 |
| BAS + SRL + MMF | 1.308 | 1.299 |
| BAS + BEL + MMF | 0.284 | 0.227 |
| BAS + CSA + MPS | 0.388 | 0.349 |
| r-ATG + CSA + MMF | 0.429 | 0.395 |
| r-ATG + TAC + MMF | 0.764 | 0.760 |
| r-ATG + CSA + AZA | 0.439 | 0.402 |
| r-ATG + TAC + AZA | 0.655 | 0.642 |
Death with functioning graft
In adult KTRs, DWFG is a significant cause of graft loss. It is a less significant cause of graft loss for children and adolescents because their life expectancy is much greater.
More KTRs die from infection and malignancy than dialysis recipients and the risk of both is increased by greater immunosuppression. 175 CVD is also a significant cause of mortality in people who have transplants. As with members of the general population, the mortality rate increases with age, plus there are a number of additional risks factors affecting patient survival that are adjusted for when comparing survival across different centres. 96
Crude estimates of DWFG will vary according to immunological risk and donor kidney type (i.e. living donor, DCD, DBD) because of differences in baseline demographics (living donor KTRs tend to be younger) and in immunosuppression (KTRs at greater immunological risk tend to receive greater immunosuppression, which increases the risk of infection and malignancy). 176 The use of steroids is also linked to increased risk of death from CVD and infection. 177
There is also evidence to suggest that the risks of cardiovascular and infectious causes of death are elevated in KTRs with reduced graft function at 1 year post transplantation. 177
The modelling framework employed allowed flexibility in the rate of DWFG in the first graft modelled but less flexibility for subsequent grafts, for which it could not be dependent on time since transplantation.
The baseline rate of DWFG for the first graft was estimated from the UK Transplant Registry standard data set for each donor type (DBD, DCD, living related, living unrelated) after adjusting for transplant period (adjusted to 2007–12) and age group (adjusted to 31–50 years). The Kaplan–Meier survival function was directly used for the first 19 years, followed by an extrapolation based on the estimated rate of DWFG from 9–19 years. The baseline survivor function is shown in Figure 19.
FIGURE 19.
Baseline survivor function for DWFG.
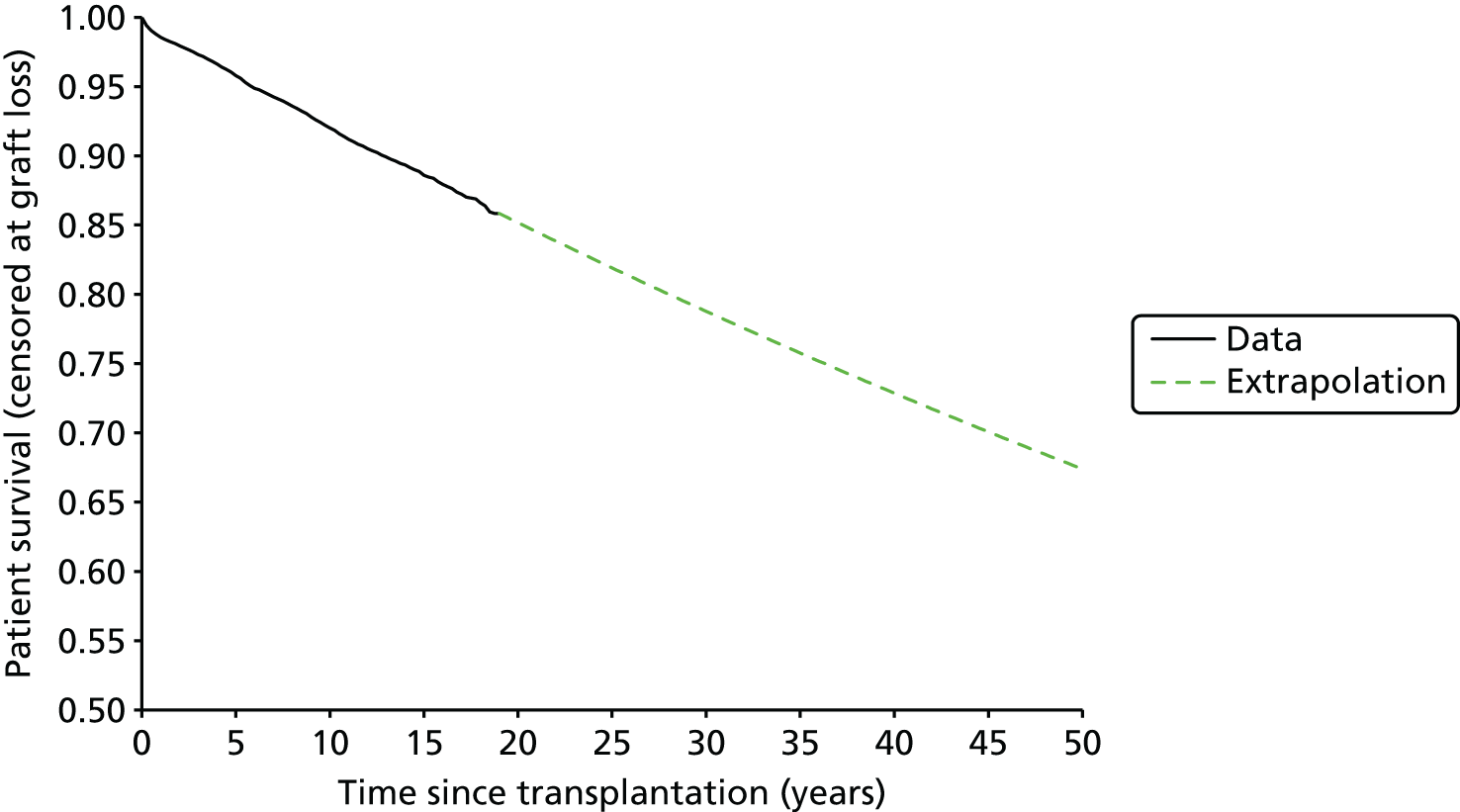
The rate of DWFG was then adjusted by sex, donor type and age based on a Cox proportional-hazards analysis of the UK Transplant Registry data set (Table 62 and see Appendix 10). For the first 12 months an individual HR was applied for each regimen to achieve a target OR of patient mortality (see Overall survival) and thereafter a HR for NODAT was applied according to Cole et al. 178
| Covariate | HR |
|---|---|
| NODAT | 1.41 |
| Sex: female | 0.865 |
| Donor type | |
| DBD | 1 |
| DCD | 1.083 |
| Living related | 0.551 |
| Living unrelated | 0.703 |
| Age (years) | |
| < 18 | 0.377 |
| 18–30 | 0.369 |
| 31–40 | 0.712 |
| 41–50 | 1 |
| 51–60 | 2.140 |
| 61–70 | 4.128 |
| 71–75 | 7.583 |
| 76–80 | 8.576 |
| 81–85 | 13.751 |
| > 85 | 23.552 |
Mortality after graft loss
Following graft loss, in the absence of an available kidney for pre-emptive retransplantation, KTRs will be placed on dialysis. Some KTRs will be waitlisted for retransplantation while others will be judged not fit for retransplantation owing to unsuitability for surgery or prohibitively great immunological risk. The mortality rate for dialysis recipients is known to be significantly greater than that for age-matched members of the general population. 154
It was assumed that mortality rates following graft loss would be the same as mortality rates for dialysis recipients and dependent on age group (Table 63). It is notable that the rate of mortality for children and adolescents on dialysis is higher than the rates for KTRs aged 18–49 years.
| Age group (years) | Hazard rate of mortality (SE) |
|---|---|
| < 18 | 0.034 (0.011) |
| 18–24 | 0.010 (0.003) |
| 25–29 | 0.012 (0.003) |
| 30–34 | 0.009 (0.002) |
| 35–39 | 0.015 (0.002) |
| 40–44 | 0.021 (0.002) |
| 45–49 | 0.027 (0.002) |
| 50–54 | 0.041 (0.003) |
| 55–59 | 0.053 (0.003) |
| 60–64 | 0.079 (0.004) |
| 65–69 | 0.107 (0.005) |
| 70–74 | 0.149 (0.006) |
| 75–79 | 0.211 (0.007) |
| 80–84 | 0.275 (0.011) |
| > 85 | 0.408 (0.019) |
For the PSA, the SE of mortality rate in each group was estimated by dividing the square root of the number of observed deaths by the estimated exposure.
Graft survival
Graft survival is a key measure of the clinical effectiveness of an immunosuppressive regimen and is critical also for cost-effectiveness as graft loss necessitates expensive dialysis treatment, which has a detrimental impact on HRQoL or retransplantation (a costly procedure).
Use of graft survival in the model
In the model regimen-specific graft survival drives transitions from functioning graft to graft loss states for the first graft, whereas for subsequent grafts a constant rate of graft loss was assumed across all regimens (see Subsequent grafts).
The transitions for the first graft are calculated by first estimating a graft survival curve (censored for DWFG) for each regimen, then multiplying this with a curve estimating patient survival (censored for graft loss) to obtain an estimate for how many KTRs should be alive and in the functioning graft state in each cycle. The rate of graft loss for cycle, i, is then calculated as:
where S(ti) is the product of survival curves for the start of cycle i and Δt = ti+ 1 – ti is the cycle length.
The details for how the survival curves are estimated were given earlier (see Overall survival), but briefly:
-
Graft survival censored for DWFG is estimated by adjusting baseline graft survival from the UK Transplant Registry standard data set in the first year according to the OR of graft loss within 12 months and thereafter according to a surrogate relationship based on AR within 12 months, NODAT within 12 months and eGFR at 12 months.
-
Death with functioning graft is estimated by adjusting baseline patient survival estimated from the UK Transplant Registry standard data set in the first year according to the OR of patient death within 12 months and thereafter according to a surrogate relationship based on NODAT within 12 months.
To account for the possibility of pre-emptive retransplantation, the rate of graft loss is partitioned between transitions from first functioning graft to graft loss following first graft; first functioning graft to second functioning graft (successful pre-emptive retransplantation); and first functioning graft to graft loss following second graft (unsuccessful pre-emptive retransplantation). It was assumed that 20% would receive pre-emptive retransplantation,179 of which 1.6% would result in PNF (based on the UK Transplant Registry standard data set, see Appendix 10).
Estimation of graft survival
It has been established in adults that AR, NODAT and graft function measured at 12 months are predictive of graft survival. 178,180–184
For children and adolescents we identified far fewer studies estimating the relationship between the potentially predictive attributes identified for adults (AR, NODAT and graft function at 12 months) and graft survival.
Muscheites et al. 185 considered a number of potentially predictive factors for death-censored graft loss in 104 children and adolescents receiving kidney transplants in one out of four German centres: recipient age (< 6 years, 6–12 years, > 12 years); recipient gender; donor type; number of HLA mismatches; number of rejection episodes; underlying renal disease; transplant period (1989–95, 1996–2000); change in GFR (between 30 days and 12 months; between 6 and 12 months); and GFR at 30 days, 6 months and 12 months. KTRs with graft survival of < 1 year were excluded and the mean follow-up was 8.3 years. They found that in univariate Cox analyses only the absolute GFR values at 30 days, 6 months and 12 months were predictive of graft survival with a significance level of 0.05. Furthermore, when considering a multivariate Cox analysis only GFR at 12 months was predictive of long-term graft survival. This study concludes that AR is not predictive (in univariate or multivariate analyses, significance level 0.05), but does not report any central estimates for the HR due to AR. It is possible that the study was insufficiently powered to estimate the effect of AR on graft survival with precision and it is also possible that excluding patients with graft survival < 1 year would also limit the predictive power of AR. The study also does not include NODAT as a covariate.
Tejani and Sullivan186 considered the relationship between AR and ‘chronic rejection graft loss’ (which accounted for 30.8% of failed grafts). Although they found that AR is a significant predictor of chronic rejection graft loss, they do not report the relationship between AR and graft loss overall.
It was decided that the relationship between eGFR and graft survival would be estimated based on the results of Muscheites et al. 185 as these appear to be in the relevant population and estimated using appropriate statistical methodology. It was decided that for AR and NODAT, the same relationship as used for the adult population would be used, as this is consistent with TA991 (where the Committee in their consideration of the evidence accepted an AR surrogate relationship based on adult evidence).
It could be argued that as no statistically significant evidence for a relationship between AR and graft survival was found by Muscheites et al. ,185 that no such relationship should be included in the model, but it was felt that if two regimens were predicted to result in the same eGFR but one regimen was predicted to reduce the rate of AR, that this should be reflected in the predicted graft survival. In addition, as Muscheites et al. 185 did not report the central estimate for the HR according to AR, it is possible that the central estimate may not be too different from the HR for adults.
It may also be noted that the HR of graft loss (for KTRs experiencing BPAR in the first 12 months versus KTRs not experiencing BPAR) assumed in this model (1.60 on the basis of adult evidence) is less than the HR assumed to inform TA85 and TA99 (1.96), although it is greater than a HR proposed by the Assessment Group for TA99 and rejected by the NICE Appraisal Committee at that time (a value of 1.41).
Throughout this section it should be noted that graft survival (and the underlying event, graft failure) does not include DWFG, that is, only considering people who are alive and who become dependent on dialysis or require retransplantation.
Baseline graft survival for the first year was estimated from the UK Transplant Registry standard data set using the Kaplan–Meier method, restricting to the first graft for each recipient and adjusting to the year 2012 (using Cox proportional hazards on transplant year). Graft survival was estimated separately for DBD and living related donors (DCD and living unrelated donors are very rare in child/adolescent transplantation). KTRs with graft failure on the day of transplant were assumed to have PNF and were excluded. Any KTRs dying with a functioning graft were censored at the time of death. Figure 20 gives the baseline graft survival.
FIGURE 20.
Graft survival in first year according to donor type. LRD, living related donors.
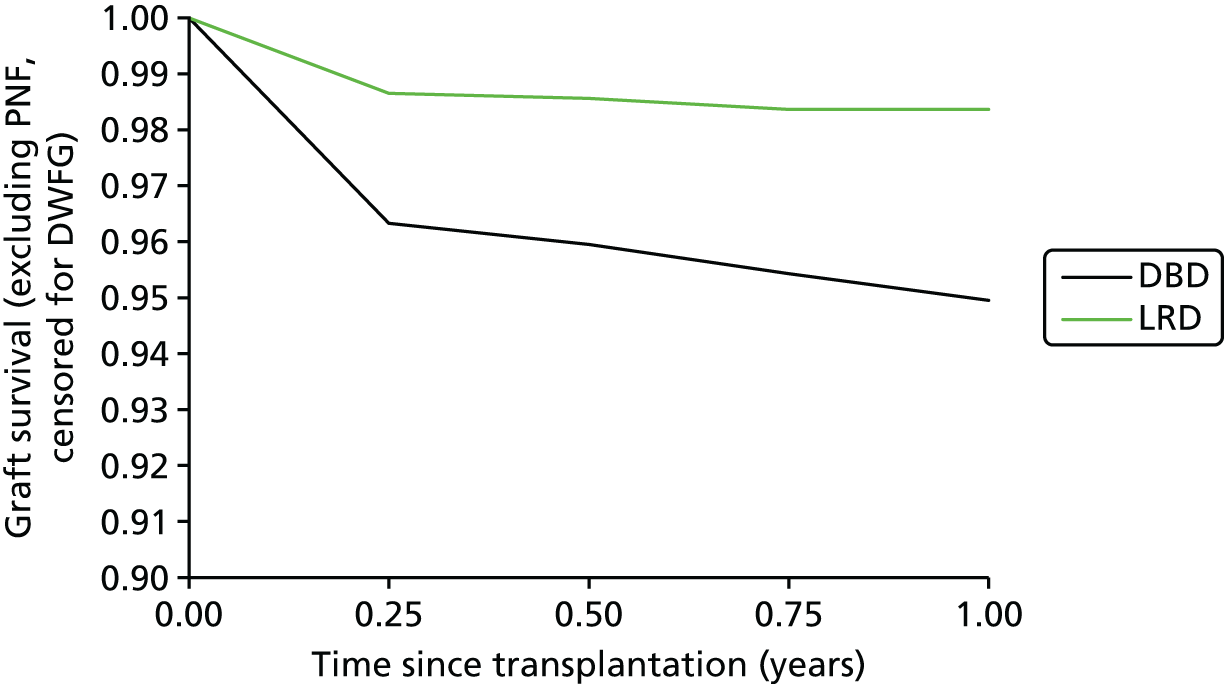
Baseline graft survival was extrapolated by fitting a Weibull curve to conditional survival from 1 year for first graft (i.e. fitted to KTRs whose first grafts survived at least 1 year), with proportional hazards covariates for donor type and transplant year. The fit of this Weibull curve was verified with a graphical test of the Cox–Snell residuals (Figure 21), which demonstrated that the fit was good as there was little deviation from the diagonal except for long follow-up (when censoring tends to cause such deviations).
FIGURE 21.
Graphical verification of the fit to graft survival.
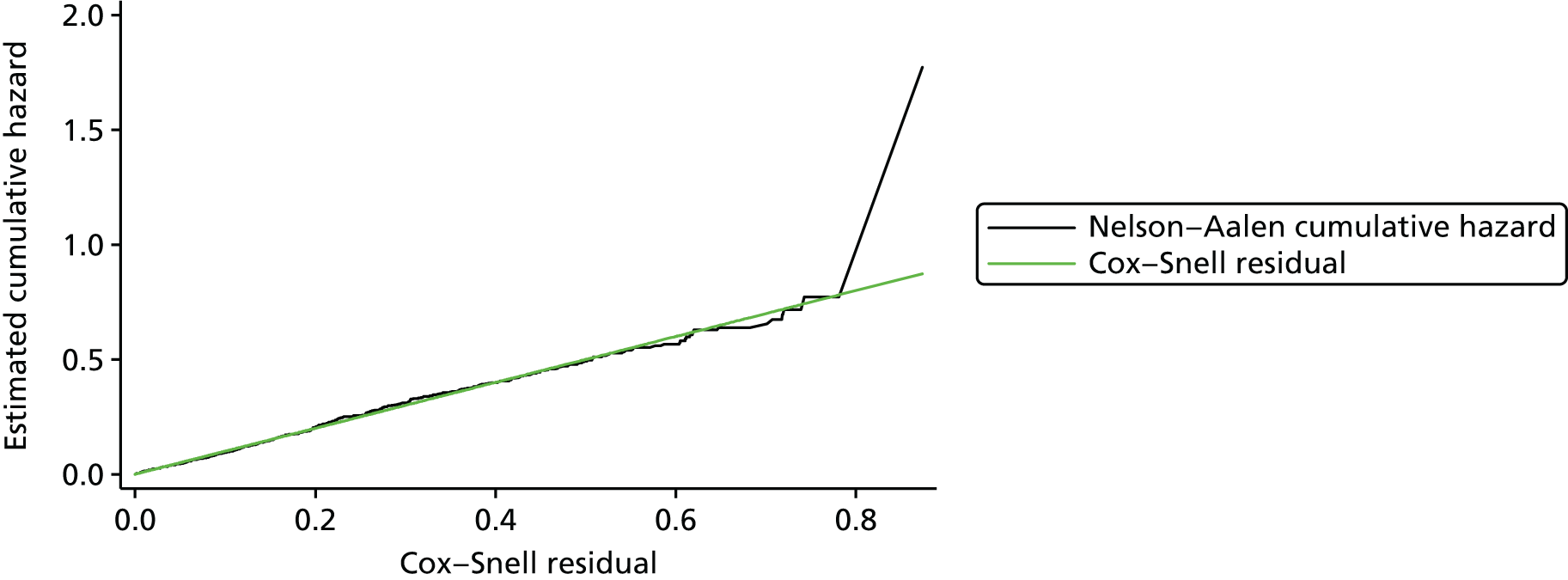
Other parametric survival distributions were not explored owing to the adequacy of the Weibull fit and for consistency with the parallel HTA (in which a Weibull curve was further indicated owing to the need to apply HRs derived from a separate Weibull fit reported by Levy et al. 182).
The baseline model for conditional graft survival from 1 year is then:
where t is time after 1 year, λ is the rate parameter and γ is the shape parameter (with a value of 1.103, implying increasing hazard rate with time).
A different rate parameter is obtained for different covariate values (proportional hazards model), the baseline rate parameter was obtained by assuming the following covariate values: donor type = [(DBD, 0.638), (living related, 0.362)]; transplant year = 2012. These led to a baseline rate parameter value of 0.02187.
The resulting baseline graft survival in the PenTAG model is shown in Figure 22.
FIGURE 22.
Baseline graft survival in the PenTAG model.

Results presented by Hudson and Collett187 at the British Transplantation Society Congress (February 2014) suggest that for deceased donors the median graft survival (death censored) for DBD grafts is 21–22 years (and higher for grafts from living donors), while estimated 30-year graft survival is 36% for DBD grafts (and expected to be higher for living donor grafts). These results serve as external validation of the extrapolation in the PenTAG model.
Graft survival for the first year was adjusted using the proportional odds method such that for each regimen the ORs of graft loss (excluding death and PNF) throughout the first year matched the ORs of graft loss as detailed in Based on adult randomised controlled trial evidence.
Graft survival for the first graft after the first year was modelled using the surrogate end points renal function at 12 months, AR within 12 months and NODAT within 12 months.
The surrogate relationship was implemented using proportional hazards and summarised in Table 64 and expanded in sections below. The rate parameters for all regimens (after adjusting according to the surrogate relationship) are given in Table 65. The resulting graft survival (excluding DWFG) at 1, 3, 5 and 10 years for each regimen is given in Table 66.
| Relationship | HR | Source |
|---|---|---|
| AR within 12 months | 1.60 | Cole et al.178 |
| Renal function (eGFR ml/minute/1.73m2) at 12 months | 1 for eGFR > 80 1.59 for 45 < eGFR ≤ 80 55.9 for eGFR ≤ 45 |
Muscheites et al.185 |
| NODAT within 12 months | 1.12 | Cole et al.178 |
| Regimen | Rate parameter (λ) |
|---|---|
| CSA + MMF | 0.0391 |
| TAC + MMF | 0.0300 |
| CSA + AZA | 0.0461 |
| TAC + AZA | 0.0269 |
| CSA + EVL | 0.0331 |
| TAC + SRL | 0.0424 |
| TAC-PR + MMF | 0.0303 |
| BAS + CSA + MMF | 0.0323 |
| BAS + TAC + MMF | 0.0247 |
| BAS + CSA + AZA | 0.0375 |
| BAS + TAC + AZA | 0.0219 |
| BAS + SRL + MMF | 0.0286 |
| BAS + BEL + MMF | 0.0210 |
| BAS + CSA + MPS | 0.0272 |
| r-ATG + CSA + MMF | 0.0346 |
| r-ATG + TAC + MMF | 0.0267 |
| r-ATG + CSA + AZA | 0.0397 |
| r-ATG + TAC + AZA | 0.0236 |
| Regimen | Graft survival (excluding DWFG and PNF) (%) | |||
|---|---|---|---|---|
| 1 year | 3 years | 5 years | 10 years | |
| CSA + MMF | 97.01 | 89.19 | 80.97 | 62.34 |
| TAC + MMF | 97.24 | 91.16 | 84.65 | 69.27 |
| CSA + AZA | 96.02 | 86.97 | 77.62 | 57.06 |
| TAC + AZA | 95.47 | 90.10 | 84.30 | 70.42 |
| CSA + EVL | 97.51 | 90.81 | 83.69 | 67.09 |
| TAC + SRL | 95.37 | 87.06 | 78.40 | 59.06 |
| TAC-PR + MMF | 96.70 | 90.60 | 84.07 | 68.66 |
| BAS + CSA + MMF | 97.47 | 90.94 | 83.98 | 67.69 |
| BAS + TAC + MMF | 97.66 | 92.61 | 87.14 | 73.88 |
| BAS + CSA + AZA | 96.63 | 89.15 | 81.27 | 63.27 |
| BAS + TAC + AZA | 96.16 | 91.74 | 86.92 | 75.11 |
| BAS + SRL + MMF | 96.52 | 90.76 | 84.56 | 69.84 |
| BAS + BEL + MMF | 97.91 | 93.59 | 88.87 | 77.26 |
| BAS + CSA + MPS | 97.81 | 92.25 | 86.26 | 71.92 |
| r-ATG + CSA + MMF | 97.67 | 90.66 | 83.23 | 66.04 |
| r-ATG + TAC + MMF | 97.85 | 92.39 | 86.49 | 72.36 |
| r-ATG + CSA + AZA | 96.88 | 88.96 | 80.66 | 61.88 |
| r-ATG + TAC + AZA | 96.45 | 91.69 | 86.51 | 73.91 |
The average graft function (eGFR) at 12 months for each regimen was estimated by estimating the baseline average eGFR at 12 months. We were unable to find these figures in the UK Renal Registry annual reports; the best available estimate is 82 ml/minute/1.73 m2 (SD 27 ml/minute/1.73 m2) from a German multicentre observational study. 185 This study, by Muscheites et al. ,185 also informs the surrogate relationship between graft function at 12 months and graft survival. Dividing eGFR into three categories (< 45 ml/minute/1.73 m2, 45–80 ml/minute/1.73 m2 and > 80 ml/minute/1.73 m2) the authors found that compared with KTRs in the highest eGFR category at 12 months, those in the lowest had significantly worse graft survival (HR 55.9, 95% CI 5.29 to 591), and those in the middle category had worse graft survival, but this was not shown to be statistically significant (HR 1.59, 95% CI 0.52 to 4.87).
The regimen-specific proportion of KTRs in each eGFR category at 12 months was estimated by first calculating the expected mean eGFR for the regimen by adding the regimen-specific mean eGFR difference (see Based on adult randomised controlled trial evidence) to the baseline mean eGFR, then assuming a normal distribution with a SD of 27 ml/minute/1.73 m2.
Acute rejection rates within 12 months were estimated using effectiveness estimates as described in Based on adult randomised controlled trial evidence and a baseline AR rate for BAS + TAC + AZA.
The baseline AR rate for BAS + TAC + AZA was estimated as 19 out of 99 = 19.2% from Grenda et al. 75
The effect of AR on graft survival after the first year was estimated using the HR of 1.60 from Cole et al. 178 A regimen-specific raw HR was then calculated according to the weighted average of the HRs for AR (1.60) and no rejection (1.00) with the weights equal to the AR rate for each regimen. These were then normalised to give HRs versus the baseline (BAS + TAC + AZA), as shown in Table 67.
| Regimen | AR rate | Raw HR | HR vs. baseline |
|---|---|---|---|
| CSA + MMF | 27.83% | 1.167 | 1.046 |
| TAC + MMF | 24.57% | 1.147 | 1.029 |
| CSA + AZA | 44.98% | 1.270 | 1.139 |
| TAC + AZA | 32.09% | 1.193 | 1.069 |
| CSA + EVL | 27.19% | 1.163 | 1.043 |
| TAC + SRL | 23.89% | 1.143 | 1.025 |
| TAC-PR + MMF | 24.11% | 1.145 | 1.026 |
| BAS + CSA + MMF | 16.24% | 1.097 | 0.984 |
| BAS + TAC + MMF | 14.07% | 1.084 | 0.972 |
| BAS + CSA + AZA | 29.13% | 1.175 | 1.053 |
| BAS + TAC + AZA (baseline) | 19.19% | 1.115 | 1.000 |
| BAS + SRL + MMF | 15.22% | 1.091 | 0.979 |
| BAS + BEL + MMF | 24.88% | 1.149 | 1.031 |
| BAS + CSA + MPS | 22.37% | 1.134 | 1.017 |
| r-ATG + CSA + MMF | 11.98% | 1.072 | 0.961 |
| r-ATG + TAC + MMF | 10.31% | 1.062 | 0.952 |
| r-ATG + CSA + AZA | 22.40% | 1.134 | 1.017 |
| r-ATG + TAC + AZA | 14.30% | 1.086 | 0.974 |
The methods for estimating the incidence of NODAT within the first 12 months since transplantation are described in the section Diabetes mellitus.
The effect of NODAT on graft survival after the first year was estimated using the HR of 1.12 from Cole et al. 178 (based on the adult population) and incorporated using the same methodology as for graft function and AR. Table 68 demonstrates that the impact of NODAT on graft survival is fairly small, which is to be expected given the conclusions of Cole et al. 178 that NODAT primarily increases the rate of DWFG, which is not considered here.
| Regimen | Incidence of NODAT | Raw HR | HR vs. baseline |
|---|---|---|---|
| CSA + MMF | 1.83% | 1.002 | 0.997 |
| TAC + MMF | 4.04% | 1.005 | 1.000 |
| CSA + AZA | 1.83% | 1.002 | 0.997 |
| TAC + AZA | 4.04% | 1.005 | 1.000 |
| CSA + EVL | 1.74% | 1.002 | 0.997 |
| TAC + SRL | 6.33% | 1.008 | 1.003 |
| TAC-PR + MMF | 4.75% | 1.006 | 1.001 |
| BAS + CSA + MMF | 1.83% | 1.002 | 0.997 |
| BAS + TAC + MMF | 4.04% | 1.005 | 1.000 |
| BAS + CSA + AZA | 1.83% | 1.002 | 0.997 |
| BAS + TAC + AZA (baseline) | 4.04% | 1.005 | 1.000 |
| BAS + SRL + MMF | 3.22% | 1.004 | 0.999 |
| BAS + BEL + MMF | 0.79% | 1.001 | 0.996 |
| BAS + CSA + MPS | 1.71% | 1.002 | 0.997 |
| r-ATG + CSA + MMF | 1.83% | 1.002 | 0.997 |
| r-ATG + TAC + MMF | 4.04% | 1.005 | 1.000 |
| r-ATG + CSA + AZA | 1.83% | 1.002 | 0.997 |
| r-ATG + TAC + AZA | 4.04% | 1.005 | 1.000 |
Adverse events
Synthesis of AE data is rarely conducted across studies owing to typically low incidence (resulting in low statistical power to detect differences) and heterogeneity of reporting. The challenge of synthesising such data is impossible in the case of child/adolescent kidney transplantation owing to the paucity of RCT evidence. Even so, for this model and in the model for the adult population it was judged important to consider the possible impact of different regimens on AE rates because the profile of AEs is considered highly clinically relevant.
Owing to the lack of RCT evidence in children and adolescents, it was decided that in the analysis for which effectiveness estimates are drawn from adult RCT evidence, the impact of regimens on AEs should also be drawn from those adult RCTs. However, in the analyses based on child/adolescent RCTs, estimates of incidence were taken from those child/adolescent RCTs when possible, even when this meant a different set of AEs was included.
In this section and subsections we describe how the incidences of NODAT, CMV infection, dyslipidaemia and anaemia are estimated in the analysis based on adult RCT evidence.
Cytomegalovirus infection is assumed to be a one-off event occurring in the first year, whereas NODAT, dyslipidaemia and anaemia are chronic conditions modelled for the full time horizon while patients are alive. All AEs incur costs while NODAT additionally results in a utility decrement (see Disutility due to diabetes mellitus).
Diabetes mellitus
The incidence of diabetes mellitus in individuals receiving dialysis is higher than that in the general population, at around 6% per year, with incidence marginally higher in individuals receiving haemodialysis. 188 Kidney transplantation appears to result in a significant increase in the incidence of diabetes mellitus in the first year post transplant (and especially in the first 6 months), after which incidence falls to similar levels to those seen in people on dialysis (see figure 2 of Woodward et al. 188). TAC has been repeatedly associated with the development of NODAT5,178 and the same incidence pattern is observed of significantly elevated incidence in the first year post transplant. 188
Pre-existing diabetes mellitus in the cohort was not modelled, only NODAT within 12 months. Based on a visual inspection of figure 1 of Woodward et al. ,188 it was assumed that 75% of NODAT in the first year would occur within the first 6 months. Incidence of NODAT after the first year was not modelled, as the results of Woodward et al. 188 suggest that after the first year the incidence of diabetes mellitus returns to pre-transplantation levels.
As in the model for adult KTRs, we assume that after the first year there is no change in the prevalence of NODAT in the population.
Baseline 12-month incidence of NODAT for BAS + TAC + AZA was estimated to be 4.0% from Grenda et al. 75
In the model for adult KTRs it was assumed that the effect of changing regimen from baseline (BAS + TAC + AZA) could be estimated by multiplying the effects of changing the agents TAC and AZA. In fact, no RCTs were identified comparing MMF and AZA which reported NODAT and, therefore, it was assumed that AZA and MMF would lead to the same incidence of NODAT.
Tables 69 and 70 list the studies (RCTs from the systematic review of clinical effectiveness in adults) informing the impact of replacing TAC-IR and MMF, respectively, on 12-month NODAT incidence. The corresponding network diagrams are given in Figures 23 and 24.
| Study | Compares | NODAT in 12 months |
|---|---|---|
| Ciancio et al. 2008189 | MMF vs. MPS | 7/61 vs. 6/55 |
| aFerguson et al. 2011129 | MMF vs. SRL | 0/33 vs. 2/26 |
| Takahashi et al. 2013190 | MMF vs. EVL | 3/61 vs. 7/61 |
| Tedesco Silva et al. 2010191 | MMF vs. EVL | 19/273 vs. 14/274 |
| Anil Kumar et al. 2005192 | MMF vs. SRL | 2/75 vs. 2/75 |
| Gonwa et al. 2003193 | MMF vs. SRL | 9/176 vs. 10/185 |
| Sampaio et al. 2008194 | MMF vs. SRL | 6/50 vs. 12/50 |
| Study | Compares | NODAT in 12 months |
|---|---|---|
| Laskow et al. 1996106 | TAC vs. CSA | 12/67 vs. 1/20 |
| Mayer et al. 1997107 | TAC vs. CSA | 17/303 vs. 3/145 |
| Campos and Abbud Filho 2002109 | TAC vs. CSA | 10/85 vs. 3/81 |
| Hardinger et al. 2005113 | TAC vs. CSA | 5/134 vs. 1/66 |
| Raofi et al. 1999195 | TAC vs. CSA | 3/14 vs. 4/21 |
| Yang et al. 1999125 | TAC vs. CSA | 1/24 vs. 1/21 |
| Krämer et al. 2010139 | TAC vs. TAC PR | 20/336 vs. 22/331 |
| Tsuchiya et al. 2013196 | TAC vs. TAC PR | 0/52 vs. 1/50 |
| aVincenti et al. 2005197 | CSA vs. BEL | 6/73 vs. 1/71 |
| aBENEFIT198 | CSA vs. BEL | 16/221 vs. 7/226 |
| aBENEFIT-EXT199 | CSA vs. BEL | 11/184 vs. 7/175 |
| bFerguson et al. 2011129 | TAC vs. BEL | 1/30 vs. 0/33 |
| Lebranchu et al. 2009200 | CSA vs. SRL | 2/97 vs. 3/96 |
| Buchler et al. 2007201 | CSA vs. SRL | 3/74 vs. 9/71 |
| Kreis et al. 2000202 | CSA vs. SRL | 1/38 vs. 1/40 |
| Guba et al. 2010203 | CSA vs. SRL | 4/71 vs. 5/69 |
| Martinez-Mier et al. 2006204 | CSA vs. SRL | 1/21 vs. 1/20 |
| Schaefer et al. 2006205 | TAC vs. SRL | 5/39 vs. 6/41 |
| Groth et al. 1999206 | CSA vs. SRL | 1/42 vs. 1/41 |
| Chen et al. 2008126 | TAC vs. CSA | 1/21 vs. 1/20 |
| Symphony127 | TAC vs. CSA vs. SRL | 34/403 vs. 17/408 vs. 25/380 |
FIGURE 23.
Network diagram for network meta-analysis estimating the impact on NODAT incidence of replacing MMF.
FIGURE 24.
Network diagram for network meta-analysis estimating the impact on NODAT incidence of replacing TAC-IR.

Mixed-treatment comparisons were conducted for both and in both cases a fixed-effects model was considered to be more appropriate owing to a lower deviance information criterion (DIC) (58.28 vs. 60.39 and 25.52 vs. 27.04). The results of the MTCs are presented in Tables 71 and 72.
| Agent | OR vs. baseline (natural logarithmic scale) | OR vs. baseline (linear scale) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | 95% CrI | Median | 95% CrI | |||
| TAC | (Baseline) | |||||||
| TAC-PR | 0.1694 | 0.3199 | 0.1687 | –0.4546 | 0.8003 | 1.184 | 0.635 | 2.226 |
| CSA | –0.8162 | 0.2086 | –0.8136 | –1.231 | –0.4129 | 0.443 | 0.292 | 0.662 |
| BEL | –1.671 | 0.381 | –1.665 | –2.431 | –0.9394 | 0.189 | 0.088 | 0.391 |
| SRL | –0.2345 | 0.2239 | –0.2339 | –0.6734 | 0.2016 | 0.791 | 0.510 | 1.223 |
| Agent | OR vs. baseline (natural logarithmic scale) | OR vs. baseline (linear scale) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | 95% CrI | Median | 95% CrI | |||
| MMF | (Baseline) | |||||||
| MPS | –0.07041 | 0.6122 | –0.0656 | –1.291 | 1.126 | 0.937 | 0.275 | 3.083 |
| SRL | 0.4739 | 0.3318 | 0.4719 | –0.1688 | 1.131 | 1.603 | 0.845 | 3.099 |
| EVL | –0.05221 | 0.3194 | –0.05309 | –0.6831 | 0.5742 | 0.948 | 0.505 | 1.776 |
The mean log-ORs were combined from the MTCs to estimate an overall OR for each regimen, as shown in Table 73, which when combined with the baseline incidence for BAS + TAC + MMF resulted in the estimated 12-month incidence of NODAT for each regimen, as shown in Table 74.
| Regimen | Replace TAC | OR | Replace MMF | OR | Overall OR |
|---|---|---|---|---|---|
| CSA + MMF | CSA | 0.442 | – | 1.000 | 0.442 |
| TAC + MMF | – | 1.000 | – | 1.000 | 1.000 |
| CSA + AZA | CSA | 0.442 | AZA | 1.000 (assumed) | 0.442 |
| TAC + AZA | – | 1.000 | AZA | 1.000 (assumed) | 1.000 |
| CSA + EVL | CSA | 0.442 | EVL | 0.949 | 0.420 |
| TAC + SRL | – | 1.000 | SRL | 1.606 | 1.606 |
| TAC-PR + MMF | TAC-PR | 1.185 | – | 1.000 | 1.185 |
| BAS + CSA + MMF | CSA | 0.442 | – | 1.000 | 0.442 |
| BAS + TAC + MMF | – | 1.000 | – | 1.000 | 1.000 |
| BAS + CSA + AZA | CSA | 0.442 | AZA | 1.000 (assumed) | 0.442 |
| BAS + TAC + AZA | – | 1.000 | AZA | 1.000 (assumed) | 1.000 |
| BAS + SRL + MMF | SRL | 0.791 | – | 1.000 | 0.791 |
| BAS + BEL + MMF | BEL | 0.188 | – | 1.000 | 0.188 |
| BAS + CSA + MPS | CSA | 0.442 | MPS | 0.932 | 0.412 |
| r-ATG + CSA + MMF | CSA | 0.442 | – | 1.000 | 0.442 |
| r-ATG + TAC + MMF | – | 1.000 | – | 1.000 | 1.000 |
| r-ATG + CSA + AZA | CSA | 0.442 | AZA | 1.000 (assumed) | 0.442 |
| r-ATG + TAC + AZA | – | 1.000 | AZA | 1.000 (assumed) | 1.000 |
| Regimen | NODAT incidence (%) |
|---|---|
| CSA + MMF | 1.83 |
| TAC + MMF | 4.04 |
| CSA + AZA | 1.83 |
| TAC + AZA | 4.04 |
| CSA + EVL | 1.74 |
| TAC + SRL | 6.33 |
| TAC-PR + MMF | 4.75 |
| BAS + CSA + MMF | 1.83 |
| BAS + TAC + MMF | 4.04 |
| BAS + CSA + AZA | 1.83 |
| BAS + TAC + AZA | 4.04 |
| BAS + SRL + MMF | 3.22 |
| BAS + BEL + MMF | 0.79 |
| BAS + CSA + MPS | 1.71 |
| r-ATG + CSA + MMF | 1.83 |
| r-ATG + TAC + MMF | 4.04 |
| r-ATG + CSA + AZA | 1.83 |
| r-ATG + TAC + AZA | 4.04 |
Cytomegalovirus infection
Cytomegalovirus infection was judged on the basis of examining the incidence of CMV infection in RCTs included in the systematic review in the adult population and on the basis of the Cochrane systematic reviews of maintenance immunosuppression by Webster et al. 156,207 that CMV infection could be affected by the use of mammalian/mechanistic target of rapamycin inhibitor (mTOR-I) (SRL and EVL) and that the impact could vary depending on whether replacing a CNI or antimetabolite in the ‘standard triple-therapy’.
Table 75 lists the studies (RCTs from the systematic review of clinical effectiveness) that could inform the estimate of the impact on CMV infection incidence of using mTOR-I. The corresponding network diagram for these studies is given in Figure 25.
| Study | Compares | CMV infection within 12 months |
|---|---|---|
| Vitko et al. 2004208 | No mTOR-I vs. mTOR-I replacing antimetabolite | 38/196 vs. 10/194 |
| Takahashi et al. 2013190 | No mTOR-I vs. mTOR-I replacing antimetabolite | 21/61 vs. 3/61 |
| Tedesco Silva et al. 2010191 | No mTOR-I vs. mTOR-I replacing antimetabolite | 16/273 vs. 2/274 |
| Chadban et al. 2013209 | No mTOR-I vs. mTOR-I replacing antimetabolite | 2/47 vs. 4/30 |
| Sampaio et al. 2008194 | No mTOR-I vs. mTOR-I replacing antimetabolite | 6/50 vs. 6/50 |
| Mjörnstedt et al. 2012210 | No mTOR-I vs. mTOR-I replacing CNI | 13/100 vs. 9/102 |
| Flechner et al. 2002211 | No mTOR-I vs. mTOR-I replacing CNI | 2/30 vs. 3/31 |
| Lebranchu et al. 2009200 | No mTOR-I vs. mTOR-I replacing CNI | 6/97 vs. 4/96 |
| Büchler et al. 2007201 | No mTOR-I vs. mTOR-I replacing CNI | 17/74 vs. 4/71 |
| Kreis et al. 2000202 | No mTOR-I vs. mTOR-I replacing CNI | 8/38 vs. 2/40 |
| Guba et al. 2010203 | No mTOR-I vs. mTOR-I replacing CNI | 20/71 vs. 5/69 |
| Martinez-Mier et al. 2006204 | No mTOR-I vs. mTOR-I replacing CNI | 0/21 vs. 1/20 |
| Symphony127 | No mTOR-I vs. No mTOR-I vs. mTOR-I replacing CNI | 39/403 vs. 45/408 vs. 23/380 |
FIGURE 25.
Network diagram for network meta-analysis estimating the impact on CMV incidence of mTOR-I use.

Fixed-effects and random-effects MTCs were conducted and the random-effects model was judged to be superior on the basis of DIC (54.02 vs. 59.54 for fixed-effects model). The results of the random-effects MTC are shown in Table 76.
| mTOR-I use | OR vs. baseline (natural logarithmic scale) | OR vs. baseline (linear scale) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | 95% CrI | Median | 95% CrI | |||
| No mTOR-I | (Baseline) | |||||||
| mTOR-I replacing CNI | –0.7981 | 0.3889 | –0.806 | –1.558 | 0.01047 | 0.447 | 0.211 | 1.011 |
| mTOR-I replacing antimetabolite | –1.153 | 0.4916 | –1.175 | –2.091 | –0.1184 | 0.309 | 0.124 | 0.888 |
| σ (random effects parameter) | 0.7915 | 0.4085 | 0.7538 | 0.08925 | 1.705 | |||
The baseline incidence of CMV infection was estimated from Jongsma et al. 212 who found that 25.8% of transplantations in 159 Dutch children and adolescents were followed by CMV infection within 1 year. The typical regimens were CSA + MMF and BAS + CSA + MMF.
Combining the baseline incidence with the treatment effects results in the incidence rates for each regimen as shown in Table 77.
| Regimen | CMV incidence within 12 months (%) |
|---|---|
| CSA + EVL | 9.88 |
| TAC + SRL | 9.88 |
| BAS + SRL + MMF | 13.53 |
| No mTOR-I | 25.79 |
Dyslipidaemia
Dyslipidaemia was judged on the basis of examining the incidence of CMV infection in RCTs in the adult population and on the basis of the Cochrane systematic reviews of maintenance immunosuppression by Webster et al. 156,207 that the incidence of dyslipidaemia could be increased by the use of mTOR-I in the immunosuppressive regimen. It was considered that it was not necessary to separately estimate the risk whether used in combination with a CNI or with an antimetabolite. Therefore, to increase statistical power the effect of mTOR-I use on dyslipidaemia incidence was estimated as the OR of dyslipidaemia incidence for mTOR-I use versus no mTOR-I use.
Table 78 details the adult population RCTs that compared regimens with and without mTOR-I and that reported dyslipidaemia. The direction of effect is consistent across the studies and the corresponding network diagram of these studies is given in Figure 26. Fixed- and random-effects meta-analyses were conducted and it was judged on the basis of DIC (28.267 vs. 29.897) that a fixed-effects analysis was appropriate. The results of the fixed-effects meta-analysis are shown in Table 79.
| Study | Compares | Dyslipidaemia within 12 months |
|---|---|---|
| Vitko et al. 2004208 | No mTOR-I vs. mTOR-I use | 24/196 vs. 51/194 |
| Takahashi et al. 2013190 | No mTOR-I vs. mTOR-I use | 19/61 vs. 28/61 |
| Tedesco Silva et al. 2010191 | No mTOR-I vs. mTOR-I use | 43/273 vs. 57/274 |
| Sampaio et al. 2008194 | No mTOR-I vs. mTOR-I use | 8/50 vs. 11/50 |
| Mjörnstedt et al. 2012210 | No mTOR-I vs. mTOR-I use | 9/100 vs. 13/102 |
| Flechner et al. 2002211 | No mTOR-I vs. mTOR-I use | 16/30 vs. 20/31 |
| Lebranchu et al. 2009200 | No mTOR-I vs. mTOR-I use | 4/97 vs. 8/96 |
| Büchler et al. 2007201 | No mTOR-I vs. mTOR-I use | 38/74 vs. 50/71 |
| Guba et al. 2010203 | No mTOR-I vs. mTOR-I use | 5/71 vs. 14/69 |
| Symphony127 | No mTOR-I vs. mTOR-I use | 91/811 vs. 60/380 |
FIGURE 26.
Network diagram for network meta-analysis estimating the impact on dyslipidaemia incidence of mTOR-I use.

| mTOR-I use | OR vs. baseline (natural logarithmic scale) | OR vs. baseline (linear scale) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | 95% CrI | Median | 95% CrI | |||
| No mTOR-I | (Baseline) | |||||||
| mTOR-I use | 0.5566 | 0.1005 | 0.5555 | 0.3604 | 0.7533 | 1.743 | 1.434 | 2.124 |
The baseline incidence of dyslipidaemia (without mTOR-I use) was estimated by Bonthuis et al. 213 based on European registry data for child/adolescent RRT recipients. The incidence of dyslipidaemia was 55.5% (313/564) for transplant recipients, versus 85.1% and 76.1% for haemodialysis and peritoneal dialysis recipients, respectively. This study also highlighted that SRL was associated with significantly increased lipid levels versus TAC and CSA. The incidence of dyslipidaemia with mTOR-I use was therefore estimated as 68.5%.
Anaemia
Anaemia is an AE that affects KTRs and people on dialysis. As reference costs for dialysis already include anaemia costs, only anaemia in people with functioning grafts was modelled. It was assumed that there would be no difference in the prevalence of anaemia between different immunosuppressive regimens. The prevalence of anaemia requiring treatment with erythropoiesis-stimulating agents (ESAs) was estimated as 5.2%, based on a study by Vanrenterghem et al. 214 This prevalence was assumed to be the same regardless of time since transplantation, age, or other factors.
Retransplantation
In the parallel HTA to evaluate the cost-effectiveness of immunosuppressive agents for adult KTRs,68 the rate of retransplantation was estimated for those under 65 years as 0.1037 from the UK Transplant Registry standard data set. To estimate the rate of retransplantation specifically for children and adolescents (who generally receive priority in DBD allocation) this rate was multiplied by 3.422 for those under 18 years, to reflect that median waiting time for adults is 3.422 times greater than median waiting time for children and adolescents (1160 days vs. 339 days).
Pre-emptive retransplantations were also included, as described in Use of graft survival in the model.
Subsequent grafts
Owing to limitations of Markov modelling imposed by the memoryless assumption, there is reduced flexibility in the modelling of costs and outcomes for subsequent grafts. It was assumed that the hazard rates of all transitions, costs and utilities are dependent only on time in the model and the arm under consideration.
Comprehensive information on immunosuppressive regimens used does not appear to be collected;215,216 the UK Renal Registry data set does not include BAS induction and the UK Transplant Registry does not include any data on immunosuppressive regimens employed.
It was assumed that the same immunosuppressive regimen would be used for all subsequent grafts, regardless of the immunosuppressive regimen used for the first graft. BAS + TAC + MMF was chosen as the immunosuppressive regimen for subsequent grafts as it is believed to be the most common immunosuppressive regimen in use in the UK. People receiving subsequent grafts are more likely to receive monoclonal or polyclonal antibody induction as they are likely to be at higher immunological risk. People can become sensitised to r-ATG if received as induction for first graft or for treatment of steroid-resistant AR, thus it was judged to be less likely to be used as induction than BAS.
Assuming the same immunosuppressive regimen for subsequent grafts for all regimens has the effect that the cost-effectiveness of regimens is primarily driven by outcomes for the first graft. Table 80 summarises the parameters affecting subsequent grafts.
| Parameter | Value | Source |
|---|---|---|
| Natural history | ||
| Baseline rate of DWFG | 0.00780 | Assumed to be the same as long-running rate of DWFG for first graft |
| Rate of graft loss | 0.03589 | Exponential distribution fitted to UK Transplant Registry standard data set (see Appendix 10) (first graft and PNF excluded) |
| Resource use | ||
| TAC dosage | 0.10 mg/kg/day | Assumed to be somewhat higher than the long-running dosage for first graft (0.08 with AZA/MMF, 0.07 with SRL) due to increased risk of rejection |
| MMF dosage | 2 g/day | Recommended daily dose |
| Prednisolone dosage | 16.3 mg/day | Assumed to be same as first graft |
| Monitoring (clinic, TAC TDM, blood test, renal profile, liver function tests) | Once monthly | Assumption |
Effectiveness estimates
The key effectiveness parameters driving cost-effectiveness in the model are:
-
graft loss within 12 months
-
patient death within 12 months
-
AR within 12 months
-
graft function at 12 months
-
NODAT at 12 months
-
CMV infection within 12 months
-
dyslipidaemia at 12 months.
As explained in Model structure, it was not possible to estimate these for all interventions based on RCT evidence in the child/adolescent kidney transplant population. Therefore, it was decided that separate analyses would be conducted based on adult RCT evidence (allowing comparison of all interventions) and on child/adolescent RCT evidence (only allowing a very limited number of comparisons).
The analyses based on child/adolescent RCT evidence differ somewhat from the analyses based on adult RCT evidence as they utilise a decision tree to estimate costs and QALYs in the trial duration followed by extrapolation with the Markov model. As such, graft loss and patient death are estimated at the study end and additionally the restricted mean survival of the patient and the graft are estimated (restricted to the trial duration), as described in Decision tree.
Based on adult randomised controlled trial evidence
Graft loss, patient death, AR and graft function were primarily estimated from network meta-analyses of adult RCT evidence for induction and maintenance regimens, assuming independence of treatment effects (i.e. that the clinical effectiveness for a complete regimen can be decomposed into the effectiveness for the induction therapy and the maintenance regimen).
Some arms were included in the network meta-analyses which do not correspond to regimens in the model and the results for these arms were not included, but the arms were not dropped from the network meta-analyses as they could still contribute indirect effect estimates. The mean treatment effects from the network meta-analyses are summarised in Table 81.
| Arm | Mortality within 12 monthsa (lower is better) | Graft loss within 12 monthsa (lower is better) | eGFR at 12 monthsb (higher is better) | BPAR within 12 monthsb (lower is better) |
|---|---|---|---|---|
| Induction (vs. no induction) | ||||
| BAS | –0.1168 | –0.1712 | +2.615 | –0.6878 |
| R-ATG | –0.4605 | –0.2534 | +0.7524 | –1.041 |
| Maintenance (vs. CSA + AZA) | ||||
| TAC + AZA | +0.3234 | +0.1353 | +9.304 | –0.5484 |
| CSA + MPA | –0.0569 | –0.2971 | +1.609 | –0.7516 |
| TAC + MPA | +0.4218 | –0.3788 | +6.531 | –0.9205 |
| BEL + MPA | –0.7630 | –0.4915 | +10.55 | –0.2159 |
| CSA + EVL | +0.3330 | –0.4843 | +4.863 | –0.7835 |
| TAC + SRL | +0.3248 | +0.1587 | –0.3523 | –0.9574 |
| SRL + MPA | +0.5416 | +0.0321 | +3.846 | –0.8283 |
Head-to-head comparisons for TAC-PR versus TAC-IR and for MPS versus MMF were additionally used to identify any differences in effectiveness between these agents. In the network meta-analysis, MMF and MPS were assumed to be the same agent to simplify the analysis and increase the statistical power. The head-to-head comparisons did not identify any statistically significant differences in clinical effectiveness. The effectiveness of MMF was assumed to be that of mycophenolate in the network meta-analysis and the effectiveness of MPS was estimated by combining the network meta-analysis and head-to-head effectiveness estimates (yMPA and yMPS – MMF, respectively) as follows (on the appropriate scale, i.e. log-odds for dichotomous outcomes, linear scale for eGFR):
The effectiveness of TAC-PR was similarly estimated:
The effectiveness estimates were combined with the following estimated baseline values (for BAS + TAC + AZA): mortality within 12 months (odds) = 0.0052 (based on the model with baseline graft loss and DWFG rates); graft loss within 12 months (odds) = 0.0400 (based on UK Transplant Registry standard data set); eGFR at 12 months (ml/minute/1.73 m2) = 82 (based on Muscheites et al. 185); AR within 12 months (odds) = 0.2375 (based on Grenda et al. 75). The resulting absolute effectiveness estimates are given in Table 82.
| Regimen | Mortality within 12 months (odds) | Graft loss within 12 months (odds) | Mean eGFR (ml/minute/1.73 m2) | BPAR within 12 months (odds) |
|---|---|---|---|---|
| CSA + MMF | 0.0039 | 0.0245 | 71.7 | 0.386 |
| TAC + MMF | 0.0063 | 0.0225 | 76.6 | 0.326 |
| CSA + AZA | 0.0041 | 0.0329 | 70.1 | 0.818 |
| TAC + AZA | 0.0058 | 0.0376 | 79.4 | 0.472 |
| CSA + EVL | 0.0058 | 0.0203 | 74.9 | 0.373 |
| TAC + SRL | 0.0057 | 0.0384 | 69.7 | 0.314 |
| TAC-PR + MMF | 0.0082 | 0.0270 | 76.4 | 0.318 |
| BAS + CSA + MMF | 0.0035 | 0.0206 | 74.3 | 0.194 |
| BAS + TAC + MMF | 0.0056 | 0.0190 | 79.2 | 0.164 |
| BAS + CSA + AZA | 0.0037 | 0.0277 | 72.7 | 0.411 |
| BAS + TAC + AZA | 0.0052 | 0.0317 | 82.0 | 0.238 |
| BAS + SRL + MMF | 0.0064 | 0.0286 | 76.5 | 0.180 |
| BAS + BEL + MMF | 0.0020 | 0.0170 | 83.2 | 0.331 |
| BAS + CSA + MPS | 0.0024 | 0.0178 | 78.2 | 0.288 |
| r-ATG + CSA + MMF | 0.0026 | 0.0190 | 72.4 | 0.136 |
| r-ATG + TAC + MMF | 0.0040 | 0.0175 | 77.4 | 0.115 |
| r-ATG + CSA + AZA | 0.0028 | 0.0256 | 70.8 | 0.289 |
| r-ATG + TAC + AZA | 0.0037 | 0.0292 | 80.1 | 0.167 |
The effectiveness estimates for the other outcomes (NODAT, CMV infection and dyslipidaemia) are also estimated from the RCTs identified in the systematic review of clinical effectiveness (see sections Diabetes mellitus, Cytomegalovirus infection and Dyslipidaemia in Adverse events).
Health measurement and valuation
The EQ-5D (3-level version) is the preferred instrument to measure HRQoL in the NICE reference case,165 but it is designed for use in adults. An adapted version of EQ-5D, the European Quality of Life-5 Dimensions Youth version (EQ-5D-Y), has been developed for children and adolescents (aged 8–17 years), but there is currently no method to value states measured in EQ-5D-Y (except naively applying the EQ-5D value set which is cautioned against). 217 Furthermore, we attempted to systematically identify any HRQoL studies in the child/adolescent kidney transplant population and did not find any.
In the absence of any studies measuring HRQoL in the child/adolescent population, it was assumed that the formula estimating the utility of general population health, the utility decrements for the different methods of RRT and the utility decrement for diabetes mellitus would be the same as for the adult population, as follows.
Utility was estimated for KTRs by first estimating age-dependent baseline utility for the general population, then applying a utility decrement according to whether KTRs were in the functioning graft or graft loss state. In addition, the proportion of the population with NODAT was estimated and a utility decrement was applied to both functioning graft and graft loss states to reflect the decreased HRQoL for KTRs with NODAT.
In the PSA utility decrements were drawn from gamma distributions to ensure that they did not result in increased utility.
With the exception of the source for baseline utility (see Utility of general population), sources of utility estimates were obtained from sources found through a systematic bibliographic search of the relevant literature. This search combined established terms and synonyms for identifying studies of utility and HRQoL, with population search terms for renal transplant, dialysis and ESRD. No study design filter was used.
The search yielded 1311 titles and abstracts, which were screened by an experienced HTA researcher (RA). Only 99 were studies that yielded or used EQ-5D scores (the preferred preference-based measure for informing NICE technology assessments). Studies were sought which yielded EQ-5D derived health state scores (using UK general population valuations), for health states or clinical events of relevance in our provisional model structure: functioning renal graft, failing renal graft, chronic allograft injury, acute kidney rejection, NODAT, malignancy following renal transplant and infection following renal transplant.
Utility of general population
Baseline utility was modelled using the following equation:
where male is equal to 1 for men and 0 for women. This equation was derived from the Health Survey for England (2012)218 using the well-established methodology of Ara and Brazier. 219 The data set includes 16- and 17-year-olds but does not appear to include utility estimates for younger individuals (all of whom had utility recorded as exactly 1) and, therefore, this is an extrapolation.
Utility with dialysis
A systematic review and meta-analysis by Liem et al. 220 reported pooled estimates of utility for various health states of people undergoing RRT. It reported random-effects meta-analyses of six studies159,221–224 which had produced EQ-5D index scores (either explicitly based on the UK utility tariff or assumed to be so by the authors) for haemodialysis (range 0.44–0.62) and of four studies159,221,223,224 for peritoneal dialysis (range 0.53–0.65). The estimates used in our model are shown in Table 83.
| Type of dialysis | Pooled mean (95% CI) | Number of studies | Number of people |
|---|---|---|---|
| Haemodialysis | 0.56 (0.49 to 0.62) | 6 | 1315 |
| Peritoneal dialysis | 0.58 (0.50 to 0.67) | 4 | 192 |
These estimates were then converted into utility decrements from baseline age-related general health (assuming age 60.4 years and 58% male for haemodialysis, and age 57.9 and 55% male for peritoneal dialysis) in order that the utility of those on dialysis would always be lower than in people in the general population of the same age and sex.
The estimated utility decrements were [mean (SE)]: haemodialysis [0.277 (0.034)] and peritoneal dialysis [0.264 (0.044)].
Utility with functioning graft
The same systematic review and meta-analysis by Liem et al. 220 reported pooled estimates of utility for people living with a functioning renal graft. It reported a random-effects meta-analysis of five studies159,223,225–227 that had produced EQ-5D index scores (either explicitly based on the UK utility tariff or assumed to be so by the authors) for people living with a functioning renal graft (range of means, some medians, 95% CI 0.71 to 0.86; Table 84).
| Health state | Pooled mean (95% CI) | Number of studies | Number of people |
|---|---|---|---|
| Functioning graft | 0.81 (0.72 to 0.90) | 5 | 673 |
It was assumed that the HRQoL for KTRs would not exceed that of members of the general population (aged 51.4 years and 60% male), so this absolute estimate was converted into a utility decrement from baseline of 0.053 (SE 0.049).
Disutility due to diabetes mellitus
Our literature search for utilities revealed one study looking specifically at disutility of NODAT in renal transplantation patients. 228 This is a recent study in the adult RRT population and reports EQ-5D utility data, with an estimated disutility of 0.06 associated with NODAT. This figure does not adjust for people with CVD complications and, therefore, is appropriate to how we model NODAT. We note that the study was conducted in only one hospital in USA and the valuation set for the utility values is US based229 so the outcomes may not be generalisable to the UK population. It has been demonstrated by Johnson et al. 230 that US-valued health states are statistically higher than the UK-valued health states for 31 out of 42 valued EQ-5D health states and that extreme health states are most notably different. However, this does not necessarily reflect the differences between health states and we believe that having utility data from a relevant patient population is the most important factor in choosing this value. For example, one alternative would be to use diabetes mellitus compared with general population using Health Survey for England data. 218 This would be a broader population of comparison and is unlikely to reflect the true utility impact of diabetes mellitus on someone who has received a kidney transplant.
In their submission to the parallel technology appraisal to evaluate the cost-effectiveness of immunosuppressive agents for adult KTRs,68 Bristol-Myers Squibb incorporated disutility of 0.041 for NODAT citing Currie et al. 231 as its source, which is a study looking at costs. We believe Bristol-Myers Squibb intended to cite the other Currie et al. paper from 2005,232 but it is still not clear how it calculated this value. In its model, the deterministic value for disutility of NODAT appears to be 0.06, which corresponds with our chosen value.
Astellas (in its submission to this technology appraisal) reports the findings of Wyld et al. 233 which does report utilities, deriving a disutility of 0.10 between no diabetes mellitus and diabetes mellitus groups of people with CKD. However, this is not restricted to the renal transplant population and it is not clear which utility elicitation method is used.
Estimating resources and costs
Costs are incurred in the model either in the form of events (e.g. induction therapy, AR, CMV infection, retransplantation) or in the form of ongoing costs (e.g. maintenance therapy, NODAT, dialysis).
The following costs are incurred exclusively in the functioning graft state (ongoing unless otherwise stated):
-
induction therapy (event)
-
maintenance therapy
-
monitoring
-
infection prophylaxis
-
AR (event)
-
CMV infection (event)
-
anaemia.
The following costs are incurred exclusively in the graft loss state:
-
dialysis.
The following costs are incurred in both the functioning graft and graft loss states:
-
NODAT
-
dyslipidaemia.
The following costs are incurred only when transitioning between states:
-
from functioning graft to graft loss: explant surgery, dialysis access surgery
-
from graft loss to functioning graft (and other retransplantation transitions): retransplantation.
Currency, price date and conversion
Costs are all in 2014/15 pounds sterling. Costs in earlier financial years are inflated based on the Hospital and Community Health Services pay and prices index (Table 85). 234
| Year | HCHS pay and prices index | Inflation factor |
|---|---|---|
| 2008/9 | 267.0 | 1.106 |
| 2009/10 | 268.6 | 1.099 |
| 2010/11 | 276.7 | 1.067 |
| 2011/12 | 282.5 | 1.045 |
| 2012/13 | 287.3 | 1.028 |
| 2013/14 | 290.5 | 1.016 |
| 2014/15 | 295.3 (projected based on previous 3 years) | 1.000 |
No costs were included in different currencies so conversion was not necessary.
Resource use
Induction therapy
Basiliximab can be administered by i.v. infusion or i.v. injection but it was assumed that it would be administered by i.v. infusion in accordance with Brennan et al. 235 As i.v. infusion is a more costly method of administration than i.v. injection, this may overestimate the costs of BAS administration.
Rabbit anti-human thymocyte immunoglobulin is administered only by i.v. infusion and it was assumed it would be administered as in Brennan et al. ,235 which was conducted in adults. We found no RCT evidence in children or adolescents for r-ATG to inform dosages. We assumed no wastage of r-ATG, which may result in the costs being underestimated.
The dosage for BAS is 10 mg if the recipient’s weight is < 35 kg and 20 mg if the recipient’s weight is ≥ 35 kg. 63 This cut-off was used by Offner et al. ,73 while a higher cut-off of 40 kg was used by Grenda et al. 75 Table 86 describes resource use for induction therapy.
| Parameter | Value | Source | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAS induction | ||||||||||||||||||||
| BAS 10 mg doses | 1.964 | Brennan et al.235 | ||||||||||||||||||
| BAS 20 mg doses | 0 | (Weight < 35 kg) | ||||||||||||||||||
| Administration (i.v. infusion) | 1.964 | Brennan et al.235 | ||||||||||||||||||
| R-ATG induction | ||||||||||||||||||||
| R-ATG mg/kg | 6.5 | Brennan et al.235 | ||||||||||||||||||
| Administration (i.v. infusion) | 4.525 | Assumption based on Brennan et al.235 Number of dosesPeople12263104245976171Actual breakdown not reported but given that 87.9% were initiated before reperfusion, 68.8% received the intended five doses, one patient received six doses, also one patient received six doses. At least four doses were received by 87.2% of people |
Number of doses | People | 1 | 2 | 2 | 6 | 3 | 10 | 4 | 24 | 5 | 97 | 6 | 1 | 7 | 1 | Actual breakdown not reported but given that 87.9% were initiated before reperfusion, 68.8% received the intended five doses, one patient received six doses, also one patient received six doses. At least four doses were received by 87.2% of people | |
| Number of doses | People | |||||||||||||||||||
| 1 | 2 | |||||||||||||||||||
| 2 | 6 | |||||||||||||||||||
| 3 | 10 | |||||||||||||||||||
| 4 | 24 | |||||||||||||||||||
| 5 | 97 | |||||||||||||||||||
| 6 | 1 | |||||||||||||||||||
| 7 | 1 | |||||||||||||||||||
| Actual breakdown not reported but given that 87.9% were initiated before reperfusion, 68.8% received the intended five doses, one patient received six doses, also one patient received six doses. At least four doses were received by 87.2% of people | ||||||||||||||||||||
In the base case, recipients are aged 10 years with expected body weight 32 kg and, therefore, they receive 10 mg doses rather than 20 mg doses.
Maintenance therapy
Dosages for those under 18 years were estimated from child/adolescent RCTs when possible. When this was not possible, dosing guidelines for adults were followed when they were already weight based. When they were not weight based, it was assumed that the dose for children and adolescents would be lower and would be proportional to their weight or body surface area. Table 87 describes resource use for maintenance therapy.
| Parameter | Value | Source | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAC-IR | ||||||||||||||||
| With AZA | Those < 18 years TimeDosage (mg/m2/day)0–6 months7.576–12 months5.61Thereafter4.89 |
Time | Dosage (mg/m2/day) | 0–6 months | 7.57 | 6–12 months | 5.61 | Thereafter | 4.89 | Trompeter et al.77 | ||||||
| Time | Dosage (mg/m2/day) | |||||||||||||||
| 0–6 months | 7.57 | |||||||||||||||
| 6–12 months | 5.61 | |||||||||||||||
| Thereafter | 4.89 | |||||||||||||||
| Those > 18 years TimeDosage (mg/kg/day)12–36 months0.09Thereafter0.08 |
Time | Dosage (mg/kg/day) | 12–36 months | 0.09 | Thereafter | 0.08 | Margreiter110 | |||||||||
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 12–36 months | 0.09 | |||||||||||||||
| Thereafter | 0.08 | |||||||||||||||
| With MMF | Those < 13 years: 0.18 mg/kg/day | Grenda et al.24 (assumed no higher than AZA) | ||||||||||||||
| Those 13–17 years: 0.13 mg/kg/day | ||||||||||||||||
| Those > 18 years: 0.08 mg/kg/day | ||||||||||||||||
| With SRL | TimeDosage (mg/kg/day)0–1 month0.1751–3 months0.1103–6 months0.1046–12 months0.08012+ months0.070 | Time | Dosage (mg/kg/day) | 0–1 month | 0.175 | 1–3 months | 0.110 | 3–6 months | 0.104 | 6–12 months | 0.080 | 12+ months | 0.070 | Starting dose from Gonwa et al.193 (0–1 month); assumed no higher than with MMF (1–6 months); Gonwa et al.,193 Anil Kumar et al.236 (6+ months) | ||
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–1 month | 0.175 | |||||||||||||||
| 1–3 months | 0.110 | |||||||||||||||
| 3–6 months | 0.104 | |||||||||||||||
| 6–12 months | 0.080 | |||||||||||||||
| 12+ months | 0.070 | |||||||||||||||
| TAC-PR | ||||||||||||||||
| With MMF | As for TAC-IR plus 0.015 mg/kg/day for 12 months | Wlodarczyk et al.,237 Krämer et al.,139 Tsuchiya et al.196 and Oh et al.238 | ||||||||||||||
| CSA | ||||||||||||||||
| With AZA | < 18 years TimeDosage (mg/m2/day)0–6 months2516–12 months192Thereafter180 |
Time | Dosage (mg/m2/day) | 0–6 months | 251 | 6–12 months | 192 | Thereafter | 180 | Trompeter et al.77 | ||||||
| Time | Dosage (mg/m2/day) | |||||||||||||||
| 0–6 months | 251 | |||||||||||||||
| 6–12 months | 192 | |||||||||||||||
| Thereafter | 180 | |||||||||||||||
| > 18 years TimeDosage (mg/kg/day)12–36 months2.93Thereafter2.84 |
Time | Dosage (mg/kg/day) | 12–36 months | 2.93 | Thereafter | 2.84 | Margreiter110 | |||||||||
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 12–36 months | 2.93 | |||||||||||||||
| Thereafter | 2.84 | |||||||||||||||
| With MMF or MPS | < 18 years (with induction) TimeDosage (mg/kg/day)0–3 months7.803–6 months7.156–12 months6.65Thereafter6.20 |
Time | Dosage (mg/kg/day) | 0–3 months | 7.80 | 3–6 months | 7.15 | 6–12 months | 6.65 | Thereafter | 6.20 | Offner et al.73 | ||||
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–3 months | 7.80 | |||||||||||||||
| 3–6 months | 7.15 | |||||||||||||||
| 6–12 months | 6.65 | |||||||||||||||
| Thereafter | 6.20 | |||||||||||||||
| < 18 years (no induction) TimeDosage (mg/kg/day)0–3 months7.673–6 months6.856–12 months6.20Thereafter5.90 |
Time | Dosage (mg/kg/day) | 0–3 months | 7.67 | 3–6 months | 6.85 | 6–12 months | 6.20 | Thereafter | 5.90 | ||||||
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–3 months | 7.67 | |||||||||||||||
| 3–6 months | 6.85 | |||||||||||||||
| 6–12 months | 6.20 | |||||||||||||||
| Thereafter | 5.90 | |||||||||||||||
| > 18 years: 2.82 mg/kg/day | Rowshani et al.239 | |||||||||||||||
| With EVL | TimeDosage (mg/kg/day)0–12 months3.912+ months2.1 | Time | Dosage (mg/kg/day) | 0–12 months | 3.9 | 12+ months | 2.1 | Vitko et al.208 | ||||||||
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–12 months | 3.9 | |||||||||||||||
| 12+ months | 2.1 | |||||||||||||||
| AZA | ||||||||||||||||
| With TAC | < 18 years: 1.80 mg/kg/day | Trompeter et al.77 | ||||||||||||||
| > 18 years: 1.20 mg/kg/day | Laskow et al.106 | |||||||||||||||
| With CSA | < 18 years: 1.80 mg/kg/day | (Assumed equal to TAC) | ||||||||||||||
| > 18 years: 1.22 mg/kg/day | Vacher-Coponat et al.240 | |||||||||||||||
| MMF | ||||||||||||||||
| With TAC | < 13 years: 0.54 g/m2/day | Grenda et al.24 | ||||||||||||||
| 13–17 years: 0.60 g/m2/day | ||||||||||||||||
| > 18 years: 1.47 g/day | Ekberg et al.127 | |||||||||||||||
| With CSA | < 18 years (with induction) TimeDosage (g/m2/day)0–3 months1.063–6 months1.016–12 months0.95Thereafter0.93 |
Time | Dosage (g/m2/day) | 0–3 months | 1.06 | 3–6 months | 1.01 | 6–12 months | 0.95 | Thereafter | 0.93 | Offner et al.73 | ||||
| Time | Dosage (g/m2/day) | |||||||||||||||
| 0–3 months | 1.06 | |||||||||||||||
| 3–6 months | 1.01 | |||||||||||||||
| 6–12 months | 0.95 | |||||||||||||||
| Thereafter | 0.93 | |||||||||||||||
| < 18 years (no induction) TimeDosage (g/m2/day)0–3 months1.043–6 months0.936–12 months0.83Thereafter0.82 |
Time | Dosage (g/m2/day) | 0–3 months | 1.04 | 3–6 months | 0.93 | 6–12 months | 0.83 | Thereafter | 0.82 | ||||||
| Time | Dosage (g/m2/day) | |||||||||||||||
| 0–3 months | 1.04 | |||||||||||||||
| 3–6 months | 0.93 | |||||||||||||||
| 6–12 months | 0.83 | |||||||||||||||
| Thereafter | 0.82 | |||||||||||||||
| > 18 years: 1.67 g/day | Ekberg et al.127 | |||||||||||||||
| With SRL | TimeDosage (g/m2/day)0–3 months1.163–12 months1.00Thereafter0.85 | Time | Dosage (g/m2/day) | 0–3 months | 1.16 | 3–12 months | 1.00 | Thereafter | 0.85 | Ekberg et al.127 (assuming adult body surface area 1.73 m2) | ||||||
| Time | Dosage (g/m2/day) | |||||||||||||||
| 0–3 months | 1.16 | |||||||||||||||
| 3–12 months | 1.00 | |||||||||||||||
| Thereafter | 0.85 | |||||||||||||||
| With BEL | 1.16 g/m2/day | Vincenti et al.198 (assuming adult body surface area 1.73 m2) | ||||||||||||||
| MPS | ||||||||||||||||
| With CSA | TimeDosage (mg/kg/day)0–3 months22.83–9 months19.29+ months17.5 | Time | Dosage (mg/kg/day) | 0–3 months | 22.8 | 3–9 months | 19.2 | 9+ months | 17.5 | Mjörnstedt et al.210 (assuming adult body weight 63 kg) | ||||||
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–3 months | 22.8 | |||||||||||||||
| 3–9 months | 19.2 | |||||||||||||||
| 9+ months | 17.5 | |||||||||||||||
| SRL | ||||||||||||||||
| With TAC | TimeDosage (mg/kg/day)0–12 months0.05912–60 months0.044Thereafter0.029 | Time | Dosage (mg/kg/day) | 0–12 months | 0.059 | 12–60 months | 0.044 | Thereafter | 0.029 | Anil Kumar et al.236 (assuming adult body weight 63 kg) | ||||||
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–12 months | 0.059 | |||||||||||||||
| 12–60 months | 0.044 | |||||||||||||||
| Thereafter | 0.029 | |||||||||||||||
| With MMF | TimeDosage (mg/kg/day)0–3 months0.0823–6 months0.0716–9 months0.0559–12 months0.05112–48 months0.04648+ months0.041 | Time | Dosage (mg/kg/day) | 0–3 months | 0.082 | 3–6 months | 0.071 | 6–9 months | 0.055 | 9–12 months | 0.051 | 12–48 months | 0.046 | 48+ months | 0.041 | Lebranchu et al.200 (assuming adult body weight 63 kg) |
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–3 months | 0.082 | |||||||||||||||
| 3–6 months | 0.071 | |||||||||||||||
| 6–9 months | 0.055 | |||||||||||||||
| 9–12 months | 0.051 | |||||||||||||||
| 12–48 months | 0.046 | |||||||||||||||
| 48+ months | 0.041 | |||||||||||||||
| EVL | ||||||||||||||||
| With CSA | TimeDosage (mg/kg/day)0–3 months0.0473–6 months0.0446–9 months0.0409–12 months0.04112–24 months0.04124+ months0.032 | Time | Dosage (mg/kg/day) | 0–3 months | 0.047 | 3–6 months | 0.044 | 6–9 months | 0.040 | 9–12 months | 0.041 | 12–24 months | 0.041 | 24+ months | 0.032 | Tedesco Silva et al.191 and Lorber et al.241 (assuming adult body weight 63 kg) |
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–3 months | 0.047 | |||||||||||||||
| 3–6 months | 0.044 | |||||||||||||||
| 6–9 months | 0.040 | |||||||||||||||
| 9–12 months | 0.041 | |||||||||||||||
| 12–24 months | 0.041 | |||||||||||||||
| 24+ months | 0.032 | |||||||||||||||
| BEL | ||||||||||||||||
| Drug acquisition | (Round up to nearest 250 mg) TimeDoses per quarter year10 mg/kg5 mg/kg0–3 months503–6 months12Thereafter03.26 |
Time | Doses per quarter year | 10 mg/kg | 5 mg/kg | 0–3 months | 5 | 0 | 3–6 months | 1 | 2 | Thereafter | 0 | 3.26 | Dosing schedule: 10 mg/kg on days 1 and 5, weeks 2, 4, 8 and 12, then 5 mg/kg every 4 weeks thereafter | |
| Time | Doses per quarter year | |||||||||||||||
| 10 mg/kg | 5 mg/kg | |||||||||||||||
| 0–3 months | 5 | 0 | ||||||||||||||
| 3–6 months | 1 | 2 | ||||||||||||||
| Thereafter | 0 | 3.26 | ||||||||||||||
| Drug administration (i.v. infusion) | TimeInfusions per quarter0–3 months53–6 months3Thereafter3.26 | Time | Infusions per quarter | 0–3 months | 5 | 3–6 months | 3 | Thereafter | 3.26 | |||||||
| Time | Infusions per quarter | |||||||||||||||
| 0–3 months | 5 | |||||||||||||||
| 3–6 months | 3 | |||||||||||||||
| Thereafter | 3.26 | |||||||||||||||
| Prednisolone | ||||||||||||||||
| With CSA | < 18 years TimeDosage (mg/kg/day)0–6 months2.4Thereafter0.3 |
Time | Dosage (mg/kg/day) | 0–6 months | 2.4 | Thereafter | 0.3 | Trompeter et al.77 | ||||||||
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–6 months | 2.4 | |||||||||||||||
| Thereafter | 0.3 | |||||||||||||||
| Without CSA | < 18 years TimeDosage (mg/kg/day)0–6 months2.1Thereafter0.3 |
Time | Dosage (mg/kg/day) | 0–6 months | 2.1 | Thereafter | 0.3 | |||||||||
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–6 months | 2.1 | |||||||||||||||
| Thereafter | 0.3 | |||||||||||||||
| All maintenance regimens | > 18 years: 16.3 mg/day | Ekberg et al.127 | ||||||||||||||
Tacrolimus, SRL, EVL and CSA are titrated to achieve target whole blood trough concentrations, as numerous factors can affect their absorption and removal from the bloodstream and therapeutic windows can be narrow.
Belatacept is administered intravenously according to a prescribed schedule. It was assumed that the ‘less intensive’ regimen from the BENEFIT198 (Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial) and BENEFIT-EXT199 (Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial-Extended criteria donors) studies would be used. We were advised that vial sharing would most likely not be feasible and, therefore, we assumed full wastage of excess BEL.
Dialysis
Access surgery is required for long-term dialysis. In the case of haemodialysis, the creation of an arteriovenous fistula is common, which requires time to heal and mature after surgery before use. It was therefore assumed that all people on haemodialysis would also incur the cost of one temporary tunnelled central venous catheter.
The mix of haemodialysis and peritoneal dialysis is known to vary over time, with younger people generally considered better suited to peritoneal dialysis (Table 88). The haemodialysis mix was reflected in incident and prevalent people on dialysis, but conversion costs (between dialysis modes) were not included.
| Age group (years) | Proportion receiving haemodialysis (%) |
|---|---|
| 0–1 | 45.5 |
| 2–3 | 46.4 |
| 4–7 | 55.6 |
| 8–11 | 64.5 |
| 12–15 | 70.5 |
| 16–17 | 62.5 |
| 18–24 | 79.1 |
| 25–34 | 80.4 |
| 35–44 | 84.5 |
| 45–54 | 84.3 |
| 55–64 | 85.2 |
| 65–74 | 85.8 |
| 75–84 | 89.0 |
| 85+ | 91.5 |
Acute rejection
The number of KTRs suffering at least one AR episode was derived as detailed in Acute rejection within 12 months and Based on adult randomised controlled trial evidence.
To account for the fact that some KTRs may experience more than one AR episode, a study112 was identified that gave both the number of people experiencing at least one AR episode and the total number of episodes. From this, it was estimated that there would be 1.19 ARs expected per person suffering at least one AR event.
Grenda et al. 75 and Trompeter et al. 77 report ARs in the first 6 months according to their response to treatments as either ‘spontaneously resolving’ (i.e. not requiring changes to treatment), ‘steroid sensitive’ (i.e. resolving after a short course of high-dose CCSs) or ‘steroid resistant’ (i.e. not resolving after a short course of high-dose CCSs). ARs between 6 and 24 months were not reported by those categories, so it was assumed that 80% were steroid sensitive and 20% steroid resistant. Table 89 gives the numbers of ARs in the RCTs in children and adolescents.
| Trial | Trompeter et al.77 | Grenda et al.75 | Offner et al.73 | |||
|---|---|---|---|---|---|---|
| Arm | TAC + AZA (n = 103) | CSA + AZA (n = 93) | TAC + AZA (n = 93) | BAS + TAC + AZA (n = 99) | BAS + CSA + MMF (n = 100) | CSA + MMF (n = 92) |
| 0–6 months | 11 | 19 | ||||
| Spontaneously resolving | 2 | 0 | 2 | 1 | ||
| Steroid sensitive | 45 | 65 | 14 | 15 | ||
| Steroid resistant | 8 | 26 | 3 | 3 | ||
| 6–12 months | 4 | 2 | 8 | 4 | 2 | 3 |
| 12–24 months | 7 | 9 | ||||
| 24–36 months | 2 | 6 | ||||
| 36–48 months | 2 | 6 | ||||
Infection prophylaxis
Cytomegalovirus prophylaxis was included for KTRs at high risk of CMV infection (D+/R–; i.e. donor is seropositive, recipient is seronegative) following the Birmingham Children’s Hospital Renal Unit protocol (Fiona Gamston, Birmingham Children’s Hospital, 15 July 2014, personal communication). It was assumed that all high-risk patients would receive valganciclovir (Valcyte®, Roche Products Ltd) at a once-daily dose calculated using the formula:
Doses are rounded to 450 mg or 900 mg (whichever is nearest). For example, a KTR with body surface area of 1.2 m2 and eGFR 40 ml/minute/1.73 m2 would have a target dose of 336 mg, rounded up to 450 mg.
According to the Birmingham protocol, prophylaxis is for 3 months, followed by a month at half dose if quantitative polymerase chain reaction (PCR) at 3 months is negative, followed by discontinuation if quantitative PCR at 4 months is negative. Relevant data on the proportions having negative PCR at 3 or 4 months were not available and were therefore estimated.
Humar et al. 243 report a comparison of 100-day and 200-day CMV prophylaxis in adults (aged ≥ 16 years). Figure 3 of Humar et al. 243 suggests that, at 90 days, approximately 10% of patients have developed CMV viraemia and in the month after discontinuation (100-day arm), approximately 14% of patients developed CMV viraemia. It was therefore assumed that 10% would receive 3 months’ prophylaxis plus 2 months’ pre-emptive treatment (at the same dose), 76% of patients would receive 4 months’ planned prophylaxis while the remaining 14% would receive 4 months’ planned prophylaxis plus 2 months’ pre-emptive treatment at the full target dose (Table 90).
| Proportion of CMV high-risk patients (%) | Time at full dose | Time at half dose |
|---|---|---|
| 10 | 5 months | None |
| 76 | 3 months | 1 month |
| 14 | 5 months | 1 month |
Half dosage was implemented assuming that alternate day dosing was acceptable, meaning the effective target daily dose was rounded to 225 mg, 450 mg or 900 mg (whichever is nearest).
Cytomegalovirus prophylaxis was not included for intermediate- or low-risk KTRs (Table 91), except in the case of intermediate-risk KTRs receiving r-ATG, who were assumed to receive 3 months’ CMV prophylaxis (based on the Royal and Exeter protocol for adults244).
| CMV risk category | Proportion of child/adolescent KTRs |
|---|---|
| High risk (D+/R–) | 54/209 = 25.8% |
| Intermediate risk (D±/R+) | 84/209 = 40.2% |
| Low risk (D–/R–) | 71/209 = 34.0% |
Pneumocystis jirovecii pneumonia (PJP) and UTI prophylaxis was assumed to be 480 mg of co-trimoxazole (Septrin®, Aspen Pharma Trading Ltd) daily for 3 months.
Monitoring
The KTRs receive monitoring on a frequent basis after transplantation, which is gradually tapered for KTRs with stable grafts.
The following monitoring was included:
-
full blood count
-
renal profile
-
liver function tests
-
therapeutic drug monitoring (TAC, CSA, SRL and EVL)
-
viral quantitative PCR [CMV, BK virus (BKV), Epstein–Barr virus (EBV)].
In addition, KTRs attend regular outpatient clinics. KTRs with degraded or deteriorating graft function receive more intensive monitoring to maximise graft survival.
It was assumed that children and adolescents would attend clinics and receive monitoring according to the Birmingham protocol (Fiona Gamston, Birmingham Children’s Hospital, 15 July 2014, personal communication), and this was assumed to taper after a number of years to quarterly visits (Table 92).
| Time | Visits per month |
|---|---|
| Month 1 | 12 |
| Month 2 | 8 |
| Month 3 | 4 |
| Months 4–6 | 2 |
| Months 7–12 | 1 |
| Year 2 | 1 (assumed) |
| Year 3 | 2/3 (assumed) |
| Thereafter | 1/3 (assumed) |
Kidney transplant recipients at high risk of CMV infection (D+/R–; i.e. donor is seropositive, recipient is seronegative) were assumed to receive monthly CMV quantitative PCR for 4 months and CMV serology at 3 months, following the Birmingham protocol (Fiona Gamston, Birmingham Children’s Hospital, 15 July 2014, personal communication).
According to the Birmingham protocol, all CMV seronegative patients (high risk and low risk) should receive annual CMV serology until they are seropositive. It was assumed that, on average, this would require two annual tests for high-risk patients (50.9% of high-risk adult patients in Humar et al. 243 were PCR positive at 12 months) and five annual tests for low-risk patients.
It was also assumed that intermediate-risk patients would receive weekly CMV quantitative PCR for 3 months [based on the Bristol Royal Hospital for Children and the Royal Devon and Exeter protocols244 (Jan Dudley, Bristol Royal Hospital for Children, 25 June 2014, personal communication)] unless they received induction with r-ATG, in which case they would receive CMV prophylaxis for 3 months.
The BKV quantitative PCR was assumed to be conducted for all children and adolescents at 3, 6 and 12 months (based on the Royal Devon and Exeter protocol244).
The EBV quantitative PCR was assumed to be conducted for children and adolescents at high risk of EBV infection (Table 93) monthly for months 1–6, then at 9 months and 12 months (based on the Royal Devon and Exeter protocol244).
| EBV risk category | Proportion of child/adolescent KTRs |
|---|---|
| High risk (D+/R–) | 28/82 = 34.1% |
| Intermediate risk (D±/R+) | 48/82 = 58.5% |
| Low risk (D–/R–) | 6/82 = 7.3% |
Explant surgery
Not all grafts are explanted on failure, with the likelihood of nephrectomy decreasing with time since transplantation. NHSBT provided data on the probability of nephrectomy as a function of time since transplantation for the PenTAG assessment report for NICE guidance TA165,18 which we have reproduced in Table 94 and used to estimate resource use of explant surgery following failure of the initial graft.
| Time since transplantation | Proportion of grafts explanted (%) |
|---|---|
| 0–3 months | 41 |
| 3–12 months | 23 |
| 12–24 months | 9 |
| 24+ months | 4 |
| Subsequent grafts | 5.9 |
For the subsequent graft it was estimated that 5.9% would be explanted on failure by applying the proportions of grafts explanted for the first graft to the exponential graft survival curve for subsequent grafts.
Subsequent retransplantation
Based on the NHS Reference Costs 2013 to 2014,58 it was estimated that there would be 1.44 ‘workups for retransplantation’ for each actual retransplantation (which can include a number of tests for fitness for transplant surgery, fitness for long-term immunosuppression, immunological assessment and assessment of risk factors for graft and patient survival) and that living donor costs would be incurred in 34.9% of retransplantations and deceased donor costs in 65.1%.
Diabetes mellitus medication
It was assumed that KTRs with NODAT would receive three 500-mg metformin tablets daily. Although this may not be a sophisticated or accurate estimate of the cost of diabetes mellitus medication, it is considered that the costs of complications incurred in and out of hospital will significantly exceed the cost of diabetes mellitus medication.
Dyslipidaemia
It was assumed that 60% of people with dyslipidaemia would receive fluvastatin as the evidence base for this with regards to safety is greatest according to clinical advice. A dosage of 40 mg per day was assumed as this is the starting dose in Riella et al. 246
It was assumed that 30% of people would receive pravastatin as the evidence base for safety is smaller. A dosage of 20 mg per day was assumed, again as this is the starting dose in Riella et al. 246
It was assumed that 10% of people would receive simvastatin as there have been safety warnings with respect to CSA. A dosage of 10 mg per day was assumed, again as this is the starting dose in Riella et al. 246
Medical management for dyslipidaemia was assumed to be one dietetics outpatient attendance per year and one GP appointment per year.
Post-transplant lymphoproliferative disease
Post-transplant lymphoproliferative disease was not included in the analyses based on adult effectiveness estimates, but was reported as an outcome in all three paediatric RCTs (Table 95).
Hypomagnesaemia
Trompeter et al. 77 reported hypomagnesaemia as an AE occurring significantly more frequently in the TAC arm than in the CSA arm. Hypomagnesaemia requiring medication occurred within 6 months in 42 out of 103 TAC patients and in 21 out of 93 CSA patients.
Hypomagnesaemia was assumed to last from incidence for the trial duration (4 years).
Hypertension
Hypertension was the most frequent AE reported by Trompeter et al. ,77 with 91 out of 103 TAC patients and 81 out of 93 CSA patients requiring antihypertensive medication within 6 months.
Hypertension was assumed to last from incidence for the trial duration (4 years).
Anaemia
According to Vanrenterghem et al. ,214 207 out of 3969 (5.2%) adult KTRs required ESA treatment for anaemia, with a mean weekly dose of 5832 IU. Therefore, it was assumed that child and adolescent KTRs would, on average, receive 3967 IU of ESA per quarter-year cycle while they were not dependent on dialysis.
The NHS Reference Costs 2013 to 201458 indicates that the costs of ESA treatment for anaemia (and of drug treatments for bone mineral disorders) should be included in Healthcare Resource Group (HRG) costs. Therefore, it was assumed that additional ESA therapy would not be included for people in the graft loss state.
Unit costs
The following sources were used to identify unit costs for drug acquisition:
-
Commercial Medicines Unit eMIT62
-
BNF Volume 68 (January 2015 online update)63
-
BNF for Children Volume 68 (January 2015 online update). 173
The eMIT national database was the preferred source as it represents the average cost actually paid by NHS hospitals, including any negotiated discounts.
For procedures, the NHS Reference Costs 2013 to 201458 (inflated to 2014/15 prices) were the preferred source of unit costs. When unit costs could not be found within the NHS reference costs, a pragmatic search of England- and UK-wide sources was conducted.
Induction
Drug acquisition costs for induction therapy are given in Table 96.
Maintenance immunosuppression
Although historically the prescribing of maintenance immunosuppression has, in some cases, been transferred to primary care physicians through shared care arrangements and dispensing in the community, at present paediatric KTRs are not being transferred out of hospital care and hospital prescribing and KTRs previously transferred out are being repatriated (Fiona Gamston, Birmingham Children’s Hospital, 10 March 2015, personal communication). A similar process is under way for adult KTRs. As a result, in this analysis it is assumed that hospital prescribing and dispensing is appropriate and, therefore, eMIT costs are preferred when available.
For TAC-PR, there is a significant difference in unit price between 5-mg capsules (£1.07 per mg) and smaller 1-mg and 3-mg capsules (£1.43 per mg). In the absence of data on relative quantities purchased, it was assumed that virtually all KTRs receiving TAC-PR would receive one 5-mg capsule daily, with some KTRs also taking one or more lower dose capsules to achieve their target daily dose. The appropriate unit cost would therefore lie between £1.07 and £1.43 per mg. It was further considered that there may be scope for negotiated discounts on the more expensive capsules. Therefore, it was assumed that the lower unit price (£1.07 per mg) would be used in the base-case analyses. Drug acquisition costs for maintenance therapy are given in Table 97.
| Agent | Pack details | Units | Unit cost | Source |
|---|---|---|---|---|
| TAC-IR | 50 × 1 mg = £28.81 | mg | £0.5201 (based on eMIT market share) | CMU eMIT62 |
| 100 × 1 mg = £55.05 | ||||
| 50 × 0.5 mg = £24.90 | ||||
| 50 × 5 mg = £88.57 | ||||
| TAC-PR | 50 × 0.5 mg = £35.79 | mg | £1.0677 (based on 50 × 5-mg pack) | BNF 6863 |
| 50 × 1 mg = £71.59 | ||||
| 100 × 1 mg = £143.17 | ||||
| 50 × 3 mg = £214.76 | ||||
| 50 × 5 mg = £266.92 | ||||
| CSA | 30 × 100 mg = £46.15 | mg | £0.0165 (based on eMIT market share) | CMU eMIT62 |
| 60 × 10 mg = £16.61 | ||||
| 30 × 25 mg = £14.55 | ||||
| 30 × 50 mg = £25.26 | ||||
| MMF | 50 × 500 mg = £9.17 | g | £0.3774 (based on eMIT market share) | CMU eMIT62 |
| 100 × 250 mg = £10.94 | ||||
| MPS | 120 × 180 mg = £96.72 | mg | £0.004478 (based on 120 × 180-mg pack) | BNF 6863 |
| 120 × 360 mg = £193.43 | ||||
| AZA | 28 × 25 mg = £1.63 | mg | £0.001075 (based on eMIT market share) | CMU eMIT62 |
| 100 × 25 mg = £9.43 | ||||
| 56 × 50 mg = £2.53 | ||||
| 100 × 50 mg = £5.03 | ||||
| SRL | 30 × 0.5 mg = £69.00 | mg | £2.8830 (based on 30 × 2-mg pack) | BNF 6863 |
| 30 × 1 mg = £86.49 | ||||
| 30 × 2 mg = £172.98 | ||||
| EVL | 60 × 0.25 mg = £148.50 | mg | £9.9000 | Novartis’ submission |
| BEL | Single 250-mg vial = £354.52 | Vial | £354.52 | BNF 6863 |
| Prednisolone | 28 × 1 mg = £0.15 | mg | £0.003286 (based on eMIT market share) | CMU eMIT62 |
| 30 × 2.5 mg = £1.65 | ||||
| 100 × 2.5 mg = £5.33 | ||||
| 30 × 5 mg = £1.61 | ||||
| 100 × 5 mg = £5.41 | ||||
| 28 × 5 mg = £0.39 |
Dialysis
Costs of haemodialysis and peritoneal dialysis are broken down in NHS Reference Costs by mode (haemodialysis, peritoneal dialysis), age (≥ 19 years, ≤ 18 years), location for haemodialysis (hospital, satellite, home), access method for haemodialysis (haemodialysis catheter, arteriovenous fistula or graft), complications for haemodialysis (blood-borne virus, no blood-borne virus), specific modality for peritoneal dialysis (continuous ambulatory, automated, assisted automated) and overall location (at base, away from base). There are 40 Healthcare Resource Group version 4 (HRG4) codes (and corresponding currencies in the NHS Reference Costs) for dialysis in total (including four for acute kidney injury).
The costs of haemodialysis and peritoneal dialysis were estimating by dividing the HRG4s currencies by mode and age, making assumptions about the number of currency units per week and then calculating a weighted average cost based on activity.
Haemodialysis was assumed to be performed three times weekly unless at home, in which case it was assumed to be performed 3.23 times per week on average (based on inspection of reported average number of sessions per week after removing clearly erroneous outliers). Peritoneal dialysis is explicitly costed per day according to the Reference Costs Guidance247 and, therefore, was assumed to be performed seven times weekly.
The currencies for acute kidney injury were included but these make up a vanishingly small proportion of activity and do not have a significant impact on overall cost estimates.
It was estimated for adults (in 2013/14 prices) that haemodialysis would cost £459.59 per week and peritoneal dialysis £452.57 per week. These costs correspond to £6093 and £6000 per quarter-year cycle, in 2014/15 prices, for haemodialysis and peritoneal dialysis, respectively.
It was estimated for children and adolescents (in 2013/14 prices) that haemodialysis would cost £1529.53 per week and peritoneal dialysis £793.09 per week. These costs correspond to £20,278 and £10,515 per quarter-year cycle, in 2014/15 prices, for haemodialysis and peritoneal dialysis, respectively.
Dialysis access costs were estimated per procedure from NHS Reference Costs 2013 to 2014 and inflated to 2014/15 prices (Table 98).
| Procedure | Unit cost (< 19 years) | Unit cost (≥ 19 years) |
|---|---|---|
| Temporary access for haemodialysis | £1747 | £823 |
| Long-term access for haemodialysis | £1946 | £1946 |
| Long-term access for peritoneal dialysis | £1101 | £1101 |
Acute rejection
The only estimates of the cost of treating AR in children and adolescents are:
-
Yao et al. :2 £4644 (price year not stated), which appears to be based on an amalgamation of the company submitted costs for TA85 (i.e. for the adult population).
-
Astellas (estimate for TA99):2 ‘around £1000’ (price year not reported).
-
Astellas (estimate for current appraisal): £889 [£38.40 for steroid-sensitive AR (80% of cases), £4292 for steroid-resistant AR (20% of cases)] (presumed 2012/13 prices).
It was decided that none of these estimates were appropriate because they were not recent, in the wrong patient population or omitted important cost components (such as the cost of administration and hospitalisation for steroid-sensitive AR in the more recent estimate by Astellas). In the absence of any appropriate costs for children and adolescents, it was decided that the cost estimated by Bristol–Myers Squibb in its submission to the parallel technology appraisal to update NICE guidance TA85 (kidney transplantation in adults) would be used, as it was judged the most appropriate cost for the PenTAG assessment in that technology appraisal. The cost of AR was estimated as £3217 in 2009 Great British pounds, which was inflated to £3557 in 2014/15 prices.
It is possible that the cost of treating AR could be greater in children and adolescents than in adults because often hospitalisation costs are greater in children and adolescents. On the other hand, it may be that reduced drug costs (owing to reduced dosage requirements) counter this. Furthermore, it may be that some expensive treatments are also deemed to be inappropriate for children and adolescents. Nevertheless, £3557 is deemed to be an appropriate central estimate for the cost of treating AR in children and adolescents.
Grenda et al. 75 and Trompeter et al. 77 report ARs in the first 6 months according to their response to treatments, as either ‘spontaneously resolving’ (i.e. not requiring changes to treatment), ‘steroid sensitive’ (i.e. resolving after a short course of high-dose CCSs), or ‘steroid resistant’ (i.e. not resolving after a short course of high-dose CCSs).
We assumed that the cost of spontaneously resolving AR would be £145 (the cost of a clinic visit) and that the cost of steroid-sensitive AR could be approximated by HRG4 currency LA07P (acute kidney injury without treatment complication and comorbidity score 0–3),48 as the cost of high-dose CCSs is not significant (in 2014/15 prices, this is £1274).
We assumed that steroid-resistant AR would be treated by a course of 7 days’ r-ATG infusion at 1.5 mg/kg, plus the cost of steroid-sensitive AR. The total medical management cost for steroid-resistant AR was estimated to be £3456 and the drug acquisition cost to be £44.46 per kg body weight. This may be an underestimate of the true cost of AR.
New-onset diabetes mellitus after transplantation
To our knowledge the only estimated costs for NODAT are:
-
Astellas/Fujisawa, in their submission for NICE guidance TA99, proposed a one-off cost of £533 for diabetes mellitus followed by treatment switching (although notably this switching was mostly from CSA + AZA to TAC + AZA or from TAC + AZA to TAC + MMF). 2
-
Yao et al. 2 did not specifically cost for NODAT, but do include a one-off cost for side effects (including NODAT) of £200 followed by treatment switching.
-
Astellas, in its submission for this appraisal, propose a yearly cost of £17.38 for NODAT, comprising metformin tablets only.
We considered that the costs estimated for NICE guidance TA99 are not appropriate as sources are not given and the costs are not recent. We also considered that the costs estimated by Astellas for this appraisal are not appropriate as they do not include any possible complications resulting from NODAT.
We assumed that the costs estimated for NODAT in the adult population could be a reasonable approximation to costs in children and adolescents. Although these costs would be likely to include certain costs that are unlikely to be incurred in young patients (particularly cardiovascular complications), there would also be likely to be increased costs of medical management for children and adolescents with NODAT and greater costs in the event of any complications requiring hospitalisation. The cost of diabetes mellitus in adults in the general population was estimated as £2028 per year (£1352 inpatient costs, £676 non-inpatient costs). 248 This was inflated to £2084 per year in 2014/15 prices.
Dyslipidaemia
Statin acquisition costs for the treatment of dyslipidaemia are given in Table 99 and medical management costs are given in Table 100.
| Statin | Pack details | Units | Unit cost | Source |
|---|---|---|---|---|
| Fluvastatin | 28 × 20 mg = £1.59 | mg | £0.002216 (weighted by eMIT market share) | CMU eMIT62 |
| 28 × 40 mg = £1.79 | ||||
| Pravastatin | 28 × 10 mg = £4.32 | mg | £0.002561 (weighted by eMIT market share) | CMU eMIT62 |
| 28 × 20 mg = £1.85 | ||||
| 28 × 40 mg = £0.79 | ||||
| Simvastatin | 28 × 10 mg = £0.15 | mg | £0.000339 (weighted by eMIT market share) | CMU eMIT62 |
| 28 × 20 mg = £0.24 | ||||
| 28 × 40 mg = £0.34 |
| Attendance | Source | Unit cost | |
|---|---|---|---|
| 2013/14 prices | 2014/15 prices | ||
| Dietetics outpatient | NHS Reference Costs 2013 to 2014:58 dietetics outpatients service (service code 654) | £61.69 | £62.70 |
| General practice | PSSRU unit costs 2014:234 GP (excluding direct care staff costs, without qualification costs, per 17.2-minute clinic) | £50.00 | £50.82 |
Infection prophylaxis
Drug acquisition costs for infection prophylaxis are given in Table 101. Costs for CMV prophylaxis (valganciclovir) are clearly much higher than costs for PJP and UTI prophylaxis.
Cytomegalovirus infection treatment
In the parallel HTA to inform the update to NICE guidance TA85,68 Bristol–Myers Squibb submitted a microcosting study249 in which the cost of CMV infection was estimated to be £2271 in 2009 prices. This was inflated to £3009 in 2014/15 prices.
Astellas, in its submission for this appraisal, proposes a cost of £221–1151 depending on body weight. This cost includes drug acquisition [ganciclovir (Cymevene®, Roche Products Ltd)] but does not include any other costs, including drug administration and other medical management (e.g. hospitalisation costs).
It was decided that the costs derived from adults would be more appropriate because, if anything, the costs of treating CMV infection could be greater in children and adolescents than in adults.
Post-transplant lymphoproliferative disease
Post-transplant lymphoproliferative disease was assumed to incur £1206 in drug administration (four i.v. infusions) and £3040/m2 body surface area in drug acquisition [four × 375 mg/m2 rituximab (Mabthera®, Roche Products Ltd), £1.7463/mg].
Hypomagnesaemia
The cost of hypomagnesaemia requiring treatment was estimated to be £290.18 per year (one sachet of Magnaspartate daily, £0.80 per sachet). 173
Hypertension
The annual cost of hypertension requiring medication was estimated to be £120.10 (Table 102), based on resource use in John and Domingo. 250
| Item | Resource use | Unit cost | Item cost (per year) |
|---|---|---|---|
| Dietetics clinic | 1 per year | £62.70 | £62.70 |
| Amlodipine | 5 mg per day | £0.0071 per mg | £13.04 |
| Bendroflumethiazide | 1 tablet per day | £0.0344 per 2.5-mg tablet | £12.56 |
| Captopril | 25 mg per day | £0.0035 per mg | £31.81 |
| Total | £120.10 |
Anaemia
Costs of ESA therapy were estimated assuming that the ESA with lowest acquisition cost would be used (following NICE guidance TA323 which relates to cancer-treatment induced anaemia; Table 103). Based on the BNF list prices epoetin alfa (Binocrit®, Sandoz) is the cheapest ESA, although it is possible that local pharmacy negotiations may result in reduced costs to the NHS in practice.
| Agent | Pack details | Units | Unit cost | Source |
|---|---|---|---|---|
| Epoetin alfa (Binocrit) | 1000 IU = £4.33 | Per 1000 IU | £4.33 (based on 1000-IU prefilled syringe) | BNF 6863 |
| 2000 IU = £8.65 | ||||
| 3000 IU = £12.98 | ||||
| 4000 IU = £17.31 | ||||
| 5000 IU = £21.64 | ||||
| 6000 IU = £25.96 | ||||
| 8000 IU = £40.73 | ||||
| 10,000 IU = £43.27 |
Drug administration
All maintenance agents except BEL are administered orally (unless people are unable to take medication orally) and this was assumed to not incur any cost.
Basiliximab is administered by i.v. infusion or injection and r-ATG is administered by i.v. infusion. BAS is administered on the day of transplantation and 4 days after transplantation. It is very likely that KTRs will still be inpatients for the latter administration. R-ATG is administered by i.v. infusion for 3–9 days. It is likely that KTRs will be inpatients for all of these infusions (a typical adult patient is estimated to require 10 days’ inpatient stay251 and children and adolescents are unlikely to require significantly shorter duration).
Belatacept is administered by i.v. infusion in an outpatient setting after the KTR is discharged from hospital. It is possible that there would be some efficiency savings by combining administration attendances with regular attendances for monitoring and clinics in early months but, thereafter, administrations are likely to be more frequent than other visits.
The NHS reference costs do not estimate a cost of i.v. infusion for inpatients as it is assumed to be a part of standard care and costs assigned to procedures taking precedence (e.g. kidney transplant). Nevertheless it was considered important to estimate the cost of administration separately for induction therapies to enable fair comparison against no induction and potential future comparisons against other induction with alternative modes of administration.
We believe that the most appropriate HRG4 currencies for i.v. administration of BAS, r-ATG and BEL are SB12Z (deliver simple parenteral chemotherapy at first attendance) and SB15Z (deliver subsequent elements of a chemotherapy cycle), which when inflated to 2014/15 prices have unit costs of £228.95 and £325.59, respectively.
Kidney transplant recipient follow-up
The unit cost of follow-up clinics was estimated from outpatient attendance costs in the nephrology service, using a weighted average of the different types of attendance (with weights based on national activity). When inflated to 2014/15 prices, the unit cost of a follow-up clinic was estimated to be £145.27 (Table 104). First face-to-face attendances were included as well as follow-up clinics on the basis that some people receive follow-up at a different centre to where they received their transplant and the relative weight of these clinics in calculating the average is small.
| Type of attendance | Number of attendances | National average unit cost (2013/14 prices) | ||
|---|---|---|---|---|
| Consultant led | Non-admitted face to face | First | 85,206 | £185.95 |
| Follow-up | 652,678 | £146.59 | ||
| Non-admitted non-face to face | First | 1124 | £143.13 | |
| Follow-up | 3033 | £109.24 | ||
| Non-consultant led | Non-admitted face to face | First | 7770 | £140.42 |
| Follow-up | 109,174 | £94.15 | ||
| Non-admitted non-face to face | First | 246 | £60.38 | |
| Follow-up | 5810 | £42.06 | ||
| Weighted average | £142.93 | |||
| (In 2014/15 prices) | £145.27 | |||
Monitoring
The unit cost of viral quantitative PCR was assumed to be the same for CMV, EBV and BKV. The most appropriate recent cost estimate that could be found was from University College London Hospitals provider-to-provider service 2013/14 tariff. 252 This is a recent cost from an NHS provider. The tariffs are likely to be slightly higher than the costs of in-house laboratory tests but this was assumed to be a small effect and it was also considered that some centres might not have in-house quantitative PCR facilities. The tariff for CMV quantitative PCR was £46 in 2013/14 prices and this was inflated to £46.75 in 2014/15 prices for use in the model. The cost of CMV serology was estimated from the same source which, when inflated to 2014/15 prices, is £18.29.
The unit costs of therapeutic drug monitoring were estimated from the Department of Biochemistry and Immunology, University Hospital of Wales, therapeutic drug monitoring test repertoire. CSA, TAC and SRL therapeutic drug monitoring all incurred charges of £26.28, which was inflated to £26.71 in 2014/15 prices for use in the model. The cost of therapeutic drug monitoring was assumed to be the same as that for SRL.
Other tests (full blood count, renal profile and liver function tests) were estimated based on the costing template produced by NHS Kidney Care to assist in the costing of renal transplantation,251 as shown in Table 105.
| Test | Unit cost (2008/9 prices) | Unit cost (2014/15 prices) |
|---|---|---|
| Full blood count | £4.57 | £5.05 |
| Renal profile | £4.11 | £4.54 |
| Liver function test | £4.20 | £4.64 |
Explant surgery
The cost of explant surgery was estimated using NHS Reference Costs 2013 to 2014. The appropriate HRG4 currencies were identified using the 2013/14 Reference Cost Grouper Code to Group workbook,253 by mapping from NHS Classification of Interventions and Procedures (OPCS-4) code M026 (excision of rejected transplanted kidney) to groups LB61, LB62 and LB63.
The average cost (weighted by activity) for adults (from HRGs LB61 and LB62) was £4886 in 2013/14 prices (£4966 in 2014/15 prices). The average cost (weighted by activity) for children and adolescents (from HRG LB63) was £4751 in 2013/14 prices (£4829 in 2014/15 prices).
Subsequent transplant
Living donor costs fall under three HRG4 currencies:
-
LA10Z: live donor kidney screening
-
LA11Z: kidney pre-transplantation workup of live donor
-
LB46Z: live donation of kidney.
The total living donor costs per live kidney donation were calculated by dividing the total cost for each currency by the activity for actual live donation, resulting in a combined cost of £8770.60 per live kidney donation in 2013/14 prices (Table 106).
| HRG4 currency | Frequency | Unit cost | Total cost |
|---|---|---|---|
| LA10Z: live kidney donor screening | 801 | £659.61 | £528,351 |
| LA11Z: kidney pre-transplantation workup of live donor | 1524 | £477.95 | £728,398 |
| LB46Z: live donation of kidney | 805 | £7209.43 | £5803,587 |
| Total cost | £7060,337 | ||
| (Per live donation of kidney) | £8770.60 |
Deceased donor costs comprise the cost of retrieval, which may be divided into staffing, consumables and transport. NHSBT performed a service evaluation of the National Organ Retrieval Service and reported various costs. 254 Staffing costs were reported separately for abdominal retrieval teams and these were used to estimate the staffing cost of retrieval at £6093.49 in 2012/13 prices (Table 107). The average cost of consumables per retrieval was reported as £1770.30, although it should be noted that this also included cardiothoracic retrievals. The total cost of transport was reported as £4,098,473.94 and this was divided by the total number of retrievals (abdominal and cardiothoracic) for a unit cost of £2005.12 per retrieval. The total cost of retrieval was therefore estimated to be £9869 in 2012/13 prices, which was inflated to £10,142 in 2014/15 prices for the model. The average cost of retransplantation was estimated as £20,576 (Table 108) and Table 109 gives a summary of all costs relating to subsequent retransplantation.
| Abdominal retrieval team | Number of retrievals | Average staffing cost per retrieval |
|---|---|---|
| University Hospitals Birmingham NHS Foundation Trust | 215 | £4440.56 |
| Cambridge University Hospitals NHS Foundation Trust | 245 | £4082.34 |
| University Hospital of Wales | 72 | £5979.36 |
| King’s College Hospital NHS Foundation Trust | 246 | £2865.03 |
| Leeds Teaching Hospitals NHS Trust/Central Manchester and Manchester Children’s Foundation Hospitals NHS Trust | 251 | £8645.29 |
| Newcastle upon Tyne NHS Foundation Trust | 179 | £5158.09 |
| Oxford Radcliffe Hospitals NHS Trust | 126 | £6912.76 |
| Royal Free Hampstead NHS Trust | 122 | £10,800.90 |
| Royal Infirmary of Edinburgh (SORT) | 117 | £10,366.39 |
| Average | £6093.49 |
| Procedure | HRG4 currency | Unit cost | |
|---|---|---|---|
| 2013/14 prices | 2014/15 prices | ||
| Recipient workup | LA12 A: Kidney Pre-Transplantation Workup of Recipient, 19 years and over | Adults: £835.06 | Adults: £848.72 |
| LA12B: Kidney Pre-Transplantation Workup of Recipient, 18 years and under | Children and adolescents: £496.61 | Children and adolescents: £504.73 | |
| Living donor costs | See Table 106 | £8,770.60 | £8,914.05 |
| Deceased donor costs | See Unit costs, Subsequent transplant | £9,868.92 | £10,142.05 |
| Transplant surgery | See Table 108 | Adults: £15,772.38 Children and adolescents: £20,576.15 |
Adults: £16,030.35 Children and adolescents: £20,912.68 |
| HRG4 currency | Activity | Unit cost | Total cost |
|---|---|---|---|
| LA01A: kidney transplant, 19 years and over, from cadaver non-heart-beating donor | 553 | £13,603.01 | £7,522,463 |
| LA02A: kidney transplant, 19 years and over, from cadaver heart-beating donor | 991 | £15,520.53 | £15,380,850 |
| LA03A: kidney transplant, 19 years and over, from live donor | 826 | £17,526.91 | £14,477,231 |
| Average (adults) | £15,772.38 | ||
| LA01B: kidney transplant, 18 years and under, from cadaver non-heart-beating donor | 11 | £27,496.72 | £302,464 |
| LA02B: kidney transplant, 18 years and under, from cadaver heart-beating donor | 47 | £18,502.00 | £869,594 |
| LA03B: kidney transplant, 18 years and under, from live donor | 55 | £20,964.49 | £1,153,047 |
| Average (children and adolescents) | £20,576.15 | ||
Summary of model parameters
See Appendix 7 for base-case values and PSA distributions for the parameters in the model.
Model verification
The decision model was tested by an independent academic decision modeller (AS). Extreme value testing and other black box testing techniques were applied to ensure the model performed as expected. 185
Results
Summary cost-effectiveness results are presented in the following form throughout, with regimens sorted in order of ascending effectiveness (total discounted QALYs):
-
total costs
-
incremental costs versus previous regimen
-
total QALYs
-
incremental QALYs versus previous regimen
-
ICER (vs. the previous regimen on the cost-effectiveness frontier unless the regimen is dominated or extended dominated)
-
incremental net health benefit at £20,000 and £30,000 per QALY versus the referent regimen (the regimen on the cost-effectiveness frontier with the lowest total QALYs)
-
for probabilistic cost-effectiveness results the following is also presented:
-
the probability that each regimen is cost-effective (i.e. gives the greatest net health benefit of all regimens being compared) at £20,000 and £30,000 per QALY
-
Based on child/adolescent randomised controlled trials
Trompeter et al.77
In the deterministic analysis based on Trompeter et al. 77 we found that TAC-IR dominated CSA whether restricting attention to the reported duration of the trial (4 years) or additionally extrapolating to a maximum time horizon of 50 years using the Markov decision model (Table 110).
| Regimen | TAC + AZA | CSA + AZA |
|---|---|---|
| Trial duration (4 years) | ||
| Discounted costs | £17,731 | £25,550 |
| Discounted QALYs | 3.3290 | 3.2530 |
| ICER (cost/QALY) | Dominant | – |
| INHB at £20,000/QALY | 0.4669 | – |
| INHB at £30,000/QALY | 0.3366 | – |
| Extrapolation (46 years) | ||
| Discounted costs | £159,214 | £195,939 |
| Discounted QALYs | 13.3895 | 12.9169 |
| Combined (50 years) | ||
| Discounted costs | £176,946 | £221,489 |
| Discounted QALYs | 16.7185 | 16.1698 |
| ICER (cost/QALY) | Dominant | – |
| INHB at £20,000/QALY | 2.7758 | – |
| INHB at £30,000/QALY | 2.0334 | – |
During the trial period, costs were predicted to be lower in the TAC arm owing to significant savings in dialysis costs (£5897 savings) as well as in the costs of immunosuppression and AR (£638 and £1508 savings, respectively), offset in part by increased costs of AEs (£225 greater). Table 111 gives further details.
| Regimen | TAC + AZA | CSA + AZA |
|---|---|---|
| Undiscounted costs | ||
| Immunosuppression | £5965 | £6652 |
| AR | £1232 | £2756 |
| AEs | £1158 | £921 |
| Dialysis | £10,710 | £17,167 |
| Total | £19,065 | £27,496 |
| Discounted costs | ||
| Immunosuppression | £5650 | £6288 |
| AR | £1219 | £2728 |
| AEs | £1082 | £857 |
Costs were also predicted to be lower in the TAC arm during the extrapolation period, mainly owing to savings in dialysis (Table 112).
| Regimen | TAC + AZA | CSA + AZA |
|---|---|---|
| Maintenance immunosuppression (initial graft) | £8277 | £5914 |
| Monitoring (initial graft) | £5145 | £3096 |
| Dialysis | £105,979 | £136,719 |
| Retransplantation | £14,703 | £18,717 |
| Maintenance immunosuppression (subsequent grafts) | £8684 | £11,220 |
| Monitoring (subsequent grafts) | £13,122 | £16,973 |
| Other costs | £3304 | £3299 |
| Total | £159,214 | £195,939 |
Discounted QALYs were predicted to be greater in the TAC arm in both the trial duration and extrapolation periods, due, in part, to extended life expectancy (3.92 and 39.51 years with 4- and 50-year time horizons, respectively, vs. 3.85 and 38.68 years for CSA). Increased graft survival also contributed to QALY gains for TAC versus CSA.
Probabilistic analysis
When the average costs and QALYs from the probabilistic analysis are considered, as in the deterministic analysis TAC-IR is dominant over CSA (Table 113). Costs are predicted to be lower with TAC-IR, particularly those of dialysis, and QALYs are predicted to be greater.
| Regimen | TAC + AZA | CSA + AZA |
|---|---|---|
| Trial duration (4 years) | ||
| Discounted costs | £17,979 | £25,749 |
| Discounted QALYs | 3.3267 | 3.2512 |
| ICER (cost/QALY) | Dominant | – |
| INHB at £20,000/QALY | 0.4640 | – |
| INHB at £30,000/QALY | 0.3345 | – |
| Extrapolation (46 years) | ||
| Discounted costs | £156,878 | £192,962 |
| Discounted QALYs | 13.3755 | 12.8957 |
| Combined (50 years) | ||
| Discounted costs | £174,857 | £218,711 |
| Discounted QALYs | 16.7022 | 16.1469 |
| ICER (cost/QALY) | Dominant | – |
| INHB at £20,000/QALY | 2.7480 | – |
| INHB at £30,000/QALY | 2.0171 | – |
As shown in the scatter cloud (Figure 27), the vast majority of probabilistic simulations predict that TAC-IR is cost saving when compared with CSA, and a significant number also predict that TAC-IR results in greater QALYs. TAC-IR is predicted to be cost-effective at £20,000 per QALY in 100.0% of simulations and at £30,000 per QALY in 100.0% of simulations (Figure 28).
FIGURE 27.
Probabilistic sensitivity analysis scatter cloud for Trompeter et al. 77 (TAC vs. CSA). Note: dashed line indicates £20,000 per QALY threshold; points to south-east of this line indicate that TAC is cost-effective vs. CSA at £20,000 per QALY. The black dot indicates mean incremental costs and QALYs.
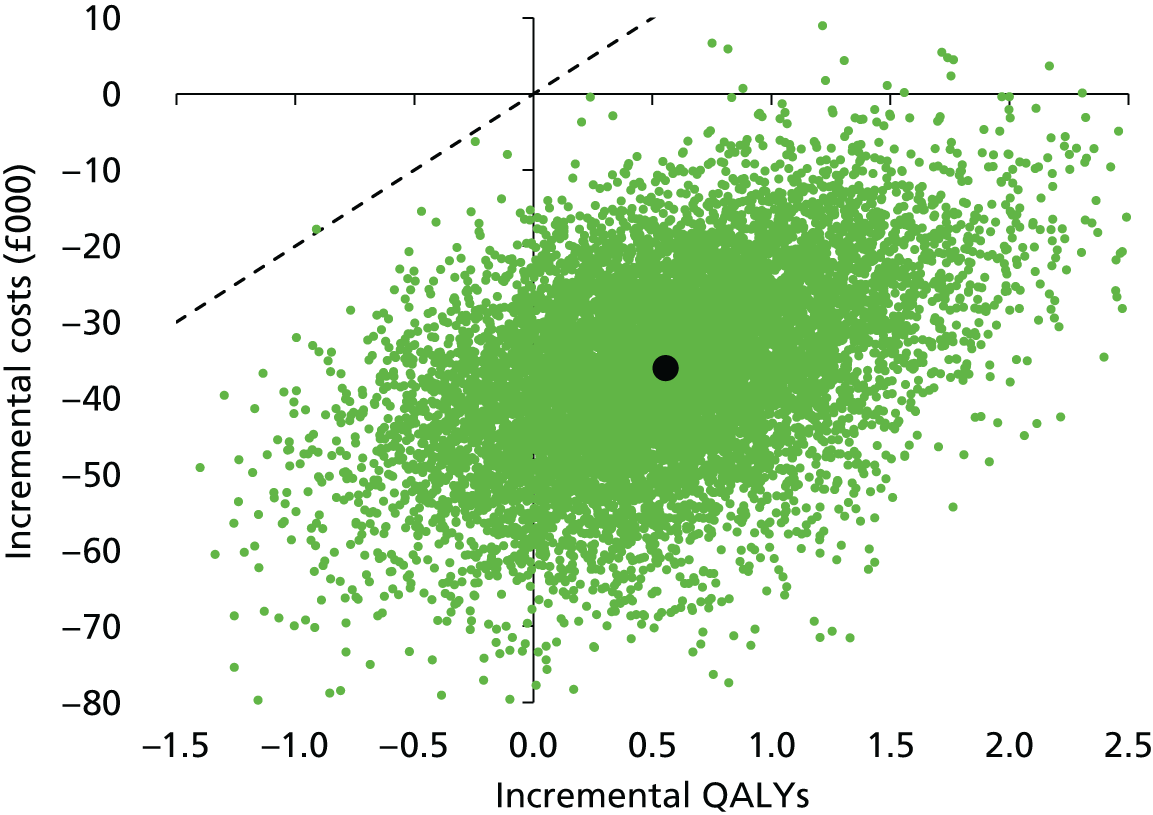
Scenario analyses
Assuming that body weight in the extrapolation period follows the ninth centile for age (rather than the median) results in marginally reduced costs of maintenance immunosuppression in both arms.
Immediate-release tacrolimus remains dominant over CSA. The incremental net health benefit for TAC-IR versus CSA is marginally increased at £20,000 and £30,000 per QALY (2.7773 and 2.0344, respectively).
When the surrogate relationship between AR and graft survival is removed (leaving eGFR at 12 months as the dominant determinant of graft survival), TAC-IR continues to dominate CSA in the deterministic analysis.
Trial duration outcomes are not affected (as the surrogate relationship is only used for extrapolation). The effect of removing the surrogate relationship is to increase the extrapolated graft survival in both arms, but more so for the CSA arm. This consequently leads to reduced total costs and increased QALYs in both arms.
The incremental net health benefit for TAC-IR versus CSA is reduced but remains positive at £20,000 and £30,000 per QALY (2.6762 and 1.9665, respectively).
Grenda et al.75
In the deterministic analysis based on Grenda et al. ,75 we found that induction with BAS was more effective and less costly than no induction, whether looking at just the trial duration (2 years) or extrapolating to a 50-year time horizon. BAS dominated no induction with a 2- or 50-year time horizon (Table 114).
| Regimen | TAC + AZA | BAS + TAC + AZA |
|---|---|---|
| Trial duration (2 years) | ||
| Discounted costs | £13,757 | £13,631 |
| Discounted QALYs | 1.7319 | 1.7436 |
| ICER (cost/QALY) | – | Dominant |
| INHB at £20,000/QALY | – | 0.0179 |
| INHB at £30,000/QALY | – | 0.0159 |
| Extrapolation (48 years) | ||
| Discounted costs | £127,256 | £121,684 |
| Discounted QALYs | 15.7609 | 15.9309 |
| Combined (50 years) | ||
| Discounted costs | £141,012 | £135,315 |
| Discounted QALYs | 17.4928 | 17.6745 |
| ICER (cost/QALY) | – | Dominant |
| INHB at £20,000/QALY | – | 0.4665 |
| INHB at £30,000/QALY | – | 0.3716 |
The additional £2481 cost of induction in the BAS arm (and the £269 additional cost of AEs) in the trial duration are marginally outweighed by savings (£2776 from dialysis and £99 from AR costs), as shown in Table 115.
| Regimen | TAC + AZA | BAS + TAC + AZA |
|---|---|---|
| Undiscounted costs | ||
| Immunosuppression | £2266 | £4758 |
| AR | £531 | £428 |
| AEs | £242 | £515 |
| Dialysis | £11,264 | £8361 |
| Total | £14,304 | £14,063 |
| Discounted costs | ||
| Immunosuppression | £2220 | £4702 |
| AR | £525 | £426 |
| AEs | £240 | £508 |
| Dialysis | £10,772 | £7996 |
| Total | £13,757 | £13,631 |
Cost savings are also realised in the extrapolation period by reducing future expenditure on dialysis and subsequent grafts, partially offset by increased cumulative immunosuppression costs for the initial graft and increased costs associated with NODAT (Table 116).
| Regimen | TAC + AZA | BAS + TAC + AZA |
|---|---|---|
| Maintenance immunosuppression (initial graft) | £13,334 | £14,021 |
| Monitoring (initial graft) | £9167 | £9630 |
| Dialysis | £75,689 | £69,730 |
| Retransplantation | £10,567 | £9799 |
| Maintenance immunosuppression (subsequent grafts) | £6121 | £5640 |
| Monitoring (subsequent grafts) | £9279 | £8538 |
| NODAT | £424 | £1611 |
| Other costs | £2676 | £2715 |
| Total | £127,256 | £121,684 |
Basiliximab was predicted to give greater QALYs in the trial duration owing to better graft survival (overall survival was very similar in both arms). In the extrapolation, BAS was predicted to give greater QALYs and greater life expectancy.
Probabilistic analysis
When the average costs and QALYs from the probabilistic analysis are considered, as in the deterministic analysis BAS is dominant over no induction (Table 117).
| Regimen | TAC + AZA | BAS + TAC + AZA |
|---|---|---|
| Trial duration (2 years) | ||
| Discounted costs | £13,751 | £13,636 |
| Discounted QALYs | 1.7302 | 1.7419 |
| ICER (cost/QALY) | – | Dominant |
| INHB at £20,000/QALY | – | 0.0174 |
| INHB at £30,000/QALY | – | 0.0155 |
| Extrapolation (48 years) | ||
| Discounted costs | £129,696 | £124,073 |
| Discounted QALYs | 15.6259 | 15.8008 |
| Combined (50 years) | ||
| Discounted costs | £143,447 | £137,708 |
| Discounted QALYs | 17.3562 | 17.5427 |
| ICER (cost/QALY) | – | Dominant |
| INHB at £20,000/QALY | – | 0.4734 |
| INHB at £30,000/QALY | – | 0.3778 |
As shown in the scatter cloud (Figure 29), the majority of probabilistic simulations predict that BAS results in greater QALYs than no induction and 59% of simulations predicting cost savings with BAS. BAS is predicted to be cost-effective at £20,000 per QALY in 67.4% of simulations and at £30,000 per QALY in 69.7% of simulations (Figure 30).
FIGURE 29.
Probabilistic sensitivity analysis scatter cloud for Grenda et al. 75 (BAS vs. no induction). Note: dashed line indicates £20,000 per QALY threshold; points to south-east of this line indicate that BAS is cost-effective versus no induction at £20,000 per QALY. The black dot indicates mean incremental costs and QALYs.
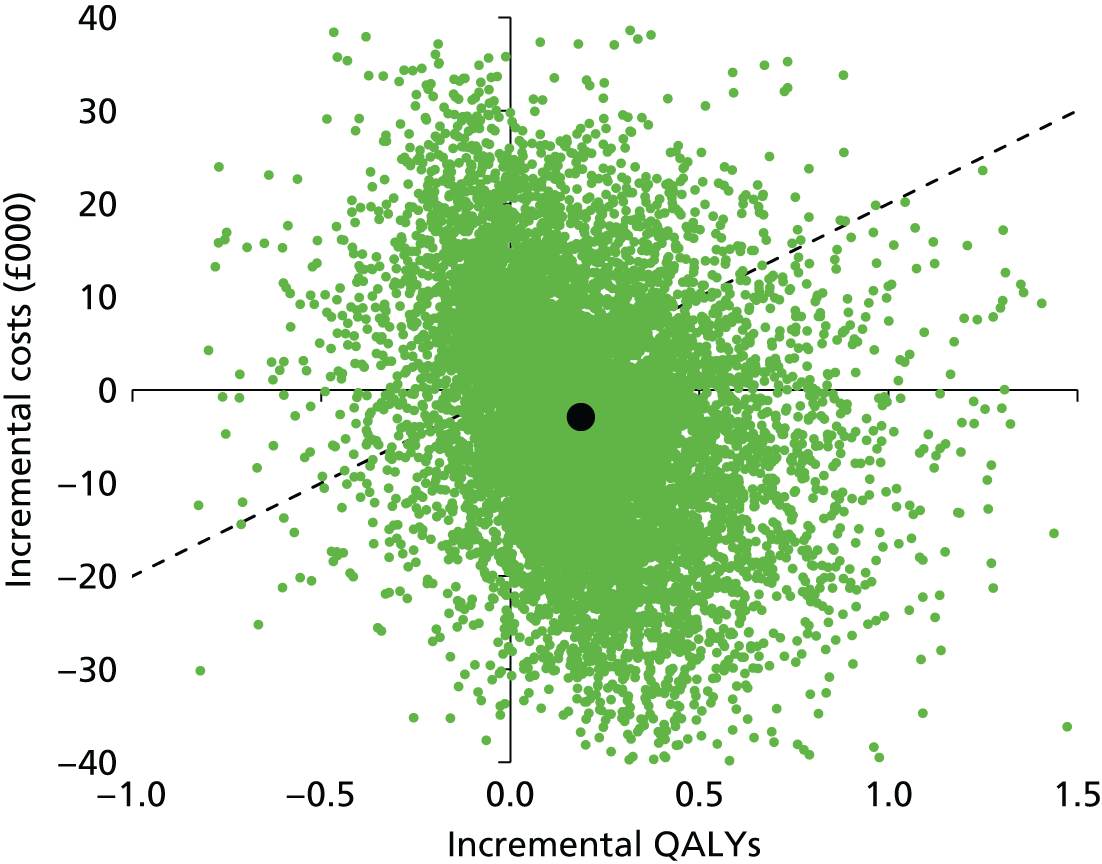
Scenario analyses
Assuming that body weight follows the ninth centile for age (as opposed to the median) results in reduced costs of immunosuppression in both arms.
Basiliximab remains dominant over no induction in the deterministic analysis. The incremental net health benefit for BAS versus no induction increases slightly at £20,000 and £30,000 per QALY (0.4725 and 0.3755, respectively).
Removing the surrogate relationship between AR and graft survival marginally increases graft survival in both arms, reducing costs and increasing QALYs.
Basiliximab remains dominant over no induction in the deterministic analysis. The incremental net health benefit for BAS versus no induction decreases slightly at £20,000 and £30,000 per QALY (0.4446 and 0.3559, respectively).
Offner et al.73
Contrary to analyses based on Grenda et al. ,75 analyses based on Offner et al. 73 suggest that BAS is more costly and less effective than no induction, whether with a time horizon of 1 year (trial duration) or 50 years (Table 118).
| Regimen | BAS + CSA + MMF | CSA + MMF |
|---|---|---|
| Trial duration (2 years) | ||
| Discounted costs | £5408 | £3297 |
| Discounted QALYs | 0.8839 | 0.8992 |
| ICER (cost/QALY) | Dominated | – |
| INHB at £20,000/QALY | –0.1208 | – |
| INHB at £30,000/QALY | –0.0857 | – |
| Extrapolation (48 years) | ||
| Discounted costs | £129,804 | £123,387 |
| Discounted QALYs | 16.9461 | 17.4765 |
| Combined (50 years) | ||
| Discounted costs | £135,212 | £126,684 |
| Discounted QALYs | 17.8300 | 18.3757 |
| ICER (cost/QALY) | Dominated | – |
| INHB at £20,000/QALY | –0.9721 | – |
| INHB at £30,000/QALY | –0.8299 | – |
During the trial duration BAS was predicted to result in lower AR costs (saving of £387) but also increased costs of immunosuppression, AEs and dialysis (increases of £2203, £19 and £276, respectively), as shown in Table 119.
| Regimen | BAS + CSA + MMF | CSA + MMF |
|---|---|---|
| Undiscounted costs | ||
| Immunosuppression | £3795 | £1591 |
| AR | £462 | £851 |
| AEs | £500 | £481 |
| Dialysis | £683 | £401 |
| Total | £5441 | £3323 |
| Discounted costs | ||
| Immunosuppression | £3778 | £1575 |
| AR | £461 | £849 |
| AEs | £500 | £481 |
| Dialysis | £669 | £393 |
| Total | £5408 | £3297 |
When extrapolated beyond the trial duration, BAS was expected to result in greater costs of dialysis and costs associated with retransplantation (Table 120).
| Regimen | BAS + CSA + MMF | CSA + MMF |
|---|---|---|
| Maintenance immunosuppression (initial graft) | £15,715 | £16,481 |
| Monitoring (initial graft) | £9807 | £10,606 |
| Dialysis | £73,825 | £68,017 |
| Retransplantation | £11,706 | £10,770 |
| Maintenance immunosuppression (subsequent grafts) | £6003 | £5522 |
| Monitoring (subsequent grafts) | £9933 | £9093 |
| Other costs | £2815 | £2899 |
| Total | £129,804 | £123,387 |
In the trial duration, BAS is predicted to give worse graft survival and overall survival, resulting in fewer QALYs. When extrapolated to 50 years, BAS is still expected to give fewer QALYs and reduced life expectancy (40.6 years vs. 41.8 years for no induction).
Probabilistic analysis
Results from the probabilistic analysis are consistent with the deterministic analysis; BAS is still expected to be dominated by no induction (Table 121).
| Regimen | BAS + CSA + MMF | CSA + MMF |
|---|---|---|
| Trial duration (2 years) | ||
| Discounted costs | £5423 | £3301 |
| Discounted QALYs | 0.8789 | 0.8941 |
| ICER (cost/QALY) | Dominated | – |
| INHB at £20,000/QALY | –0.1212 | – |
| INHB at £30,000/QALY | –0.0859 | – |
| Extrapolation (48 years) | ||
| Discounted costs | £130,442 | £124,886 |
| Discounted QALYs | 16.8328 | 17.3400 |
| Combined (50 years) | ||
| Discounted costs | £135,865 | £128,187 |
| Discounted QALYs | 17.7117 | 18.2341 |
| ICER (cost/QALY) | Dominated | – |
| INHB at £20,000/QALY | –0.9062 | – |
| INHB at £30,000/QALY | –0.7783 | – |
As shown in the scatter cloud (Figure 31), BAS is predicted to result in QALY loss in a significant majority of simulations; it is also predicted to increase costs in the majority of simulations. BAS is predicted to be cost-effective in 10.3% and 7.4% of simulations at £20,000 and £30,000 per QALY, respectively (Figure 32).
FIGURE 31.
Probabilistic sensitivity analysis scatter cloud for Offner et al. 73 (BAS vs. no induction). Note: dashed line indicates £20,000 per QALY threshold; points to south-east of this line indicate that BAS is cost-effective versus no induction at £20,000 per QALY. The black dot indicates mean incremental costs and QALYs.
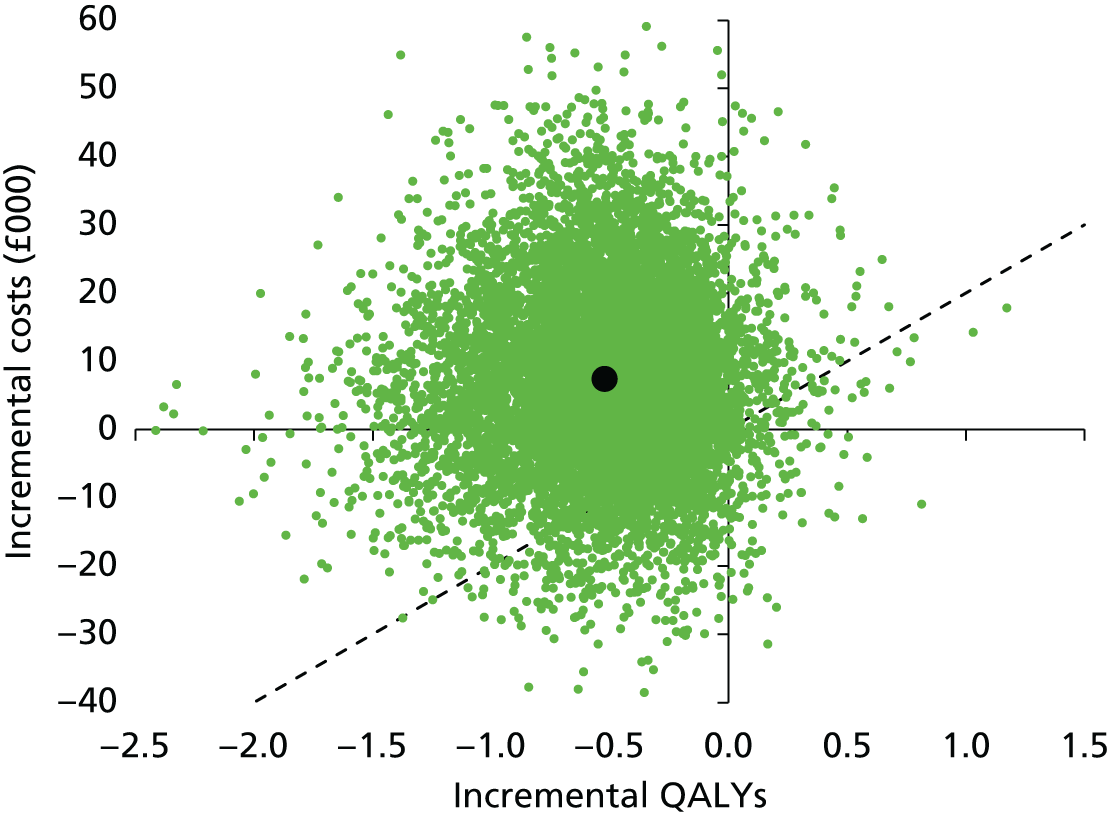
Scenario analyses
Assuming that body weight follows the ninth centile for age (as opposed to the median) results in reduced costs of immunosuppression in both arms.
Basiliximab remains dominated by no induction in the deterministic analysis. The incremental net health benefit for BAS versus no induction decreases slightly at £20,000 and £30,000 per QALY (–0.9743 and –0.8314, respectively).
Removing the surrogate relationship between AR and graft survival marginally decreases graft survival in the BAS arm, increasing costs and reducing QALYs, while increasing graft survival in the no induction arm.
Basiliximab remains dominated by no induction in the deterministic analysis. The incremental net health benefit for BAS versus no induction decreases at £20,000 and £30,000 per QALY (–1.1409 and –0.9474, respectively).
Summary of results from analyses based on child/adolescent randomised controlled trials
The analysis based on Trompeter et al. 77 suggested that TAC-IR would be cost-effective versus CSA at £20,000 or £30,000 per QALY as it was more effective and cost-saving both in the trial duration and when extrapolated.
The analyses based on Grenda et al. 75 and Offner et al. 73 produced contradictory results for the cost-effectiveness of BAS versus no induction. The analyses based on Grenda et al. 75 suggested that BAS would result in reduced costs and increased QALYs (i.e. BAS was dominant) while the analyses based on Offner et al. 73 suggested that BAS would result in increased costs and decreased QALYs (i.e. BAS was dominated). These results were robust to scenario analyses.
Using effectiveness estimates from adult studies
Further results for these analyses are given in Appendix 9.
Deterministic results
Induction agents
Basiliximab and r-ATG were both simultaneously compared with no induction with four different maintenance combinations (CSA + MMF, TAC + MMF, CSA + AZA and TAC + AZA).
Basiliximab was found to be less costly and more effective (and, therefore, dominant) over no induction and r-ATG in all comparisons (Table 122). R-ATG was also found to be more costly and effective than no induction (i.e. no induction dominated r-ATG).
| Induction agent | Discounted costs | Discounted QALYs | ICER (cost per QALY) | Incremental net health benefit | |||
|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | ||
| With CSA + AZA | vs. BAS | ||||||
| R-ATG | £216,114 | – | 17.9721 | – | Dominated | –1.0123 | –0.7278 |
| No induction | £210,097 | –£6017 | 18.0031 | +0.0310 | Dominated | –0.6804 | –0.4962 |
| BAS | £199,042 | –£11,055 | 18.1308 | +0.1277 | – | – | – |
| With CSA + MMF | vs. BAS | ||||||
| R-ATG | £209,097 | – | 18.0702 | – | Dominated | –1.0887 | –0.7846 |
| No induction | £199,910 | –£9188 | 18.1269 | +0.0567 | Dominated | –0.5726 | –0.4217 |
| BAS | £190,856 | –£9053 | 18.2468 | +0.1200 | – | – | – |
| With TAC + AZA | vs. BAS | ||||||
| R-ATG | £183,191 | – | 18.2468 | – | Dominated | –1.1228 | –0.8082 |
| No induction | £174,989 | –£8202 | 18.2970 | +0.0502 | Dominated | –0.6625 | –0.4846 |
| BAS | £164,316 | –£10,673 | 18.4259 | +0.1288 | – | – | – |
| With TAC + MMF | vs. BAS | ||||||
| R-ATG | £189,637 | – | 18.1763 | – | Dominated | –1.1560 | –0.8317 |
| No induction | £179,719 | –£9918 | 18.2398 | +0.0635 | Dominated | –0.5966 | –0.4377 |
| BAS | £170,182 | –£9537 | 18.3596 | +0.1198 | – | – | – |
The differences in QALYs from r-ATG to no induction and from no induction to BAS are explained by increased life expectancy overall and by more projected time with functioning graft and less projected time dependent on dialysis (Table 123). Graft life expectancy for the first graft was greater for BAS than for r-ATG and no induction. The gains in graft survival for the first graft do not fully translate to gains in projected time with functioning graft or life expectancy because when a graft is lost later in life there is less time to achieve retransplantation and the mortality rate while on dialysis is greater.
| Induction agent | Graft life expectancy (first graft; years) | Life expectancy (years) | Projected years with functioning graft | Projected years receiving dialysis | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | Total | Incremental | Total | Incremental | |
| With CSA + AZA | ||||||||
| R-ATG | 14.421 | – | 43.136 | – | 33.557 | – | 9.578 | – |
| No induction | 15.098 | +0.677 | 43.175 | +0.039 | 33.784 | +0.226 | 9.391 | –0.187 |
| BAS | 17.229 | +2.131 | 43.378 | +0.203 | 34.490 | +0.706 | 8.888 | –0.503 |
| With CSA + MMF | ||||||||
| R-ATG | 15.983 | – | 43.294 | – | 34.059 | – | 9.236 | – |
| No induction | 17.110 | +1.126 | 43.374 | +0.080 | 34.445 | +0.386 | 8.929 | –0.306 |
| BAS | 19.171 | +2.062 | 43.566 | +0.191 | 35.159 | +0.714 | 8.407 | –0.523 |
| With TAC + AZA | ||||||||
| R-ATG | 20.068 | – | 43.582 | – | 35.474 | – | 8.109 | – |
| No induction | 21.263 | +1.194 | 43.649 | +0.067 | 35.925 | +0.451 | 7.724 | –0.385 |
| BAS | 23.597 | +2.334 | 43.858 | +0.209 | 36.785 | +0.860 | 7.073 | –0.651 |
| With TAC + MMF | ||||||||
| R-ATG | 18.881 | – | 43.467 | – | 34.994 | – | 8.473 | – |
| No induction | 20.304 | +1.423 | 43.553 | +0.086 | 35.502 | +0.508 | 8.051 | –0.422 |
| BAS | 22.449 | +2.145 | 43.746 | +0.193 | 36.286 | +0.784 | 7.460 | –0.591 |
Finally, we compared these analyses to the analyses based on Grenda et al. 75 and Offner et al. 73 (reported in Based on child/adolescent RCTs). The analyses based on Grenda et al. 75 suggested that BAS (with TAC + AZA) was dominant compared with no induction regimen, while the analyses based on Offner et al. 73 suggested that BAS (with CSA + MMF) was dominated by no induction regimen. In summary, the deterministic results based on adult data were consistent with the analyses based on Grenda et al. 75 but not with the analyses based on Offner et al. 73
Maintenance agents
Table 124 shows the summary of cost-effectiveness results for maintenance agents. It shows that TAC-IR is dominant over CSA, TAC-PR and SRL, but is less effective and less costly than BEL. Because the ICER of BEL versus TAC-IR is > £500,000 per QALY, only TAC-IR is cost-effective in these comparisons at £20,000 and £30,000 per QALY. Comparing these results to the results based on Trompeter et al. ,77 the regimen of TAC + AZA (vs. TAC + CSA) was dominant in both analyses (see Tables 110 and 124).
| Maintenance agent | Discounted costs | Discounted QALYs | ICER (cost per QALY) | Incremental net health benefit | |||
|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | ||
| With MMF | vs. TAC | ||||||
| CSA | £199,910 | – | 18.1269 | – | Dominated | –1.1224 | –0.7859 |
| TAC-PR | £196,165 | –£3744 | 18.1854 | +0.0586 | Dominated | –0.8767 | –0.6026 |
| TAC | £179,719 | –£16,446 | 18.2398 | +0.0544 | – | – | – |
| With AZA | vs. TAC | ||||||
| CSA | £210,097 | – | 18.0031 | – | Dominated | –2.0494 | –1.4642 |
| TAC | £174,989 | –£35,108 | 18.2970 | +0.2940 | – | – | – |
| With BAS + MMF | vs. TAC | ||||||
| SRL | £198,631 | – | 18.2423 | – | Dominated | –1.5397 | –1.0655 |
| CSA | £190,856 | –£7,775 | 18.2468 | +0.0045 | Dominated | –1.1464 | –0.8019 |
| TAC | £170,182 | –£20,674 | 18.3596 | +0.1127 | – | – | – |
| BEL | £293,175 | +£122,993 | 18.5901 | +0.2306 | £533,449 | –5.9191 | –3.8692 |
| With BAS + AZA | vs. TAC | ||||||
| CSA | £199,042 | – | 18.1308 | – | Dominated | –2.0314 | –1.4526 |
| TAC | £164,316 | –£34,726 | 18.4259 | +0.2951 | – | – | – |
| With r-ATG + MMF | vs. TAC | ||||||
| CSA | £209,097 | – | 18.0702 | – | Dominated | –1.0791 | –0.7548 |
| TAC | £189,637 | –£19,460 | 18.1763 | +0.1061 | – | – | – |
| With r-ATG + AZA | vs. TAC | ||||||
| CSA | £216,114 | – | 17.9721 | – | Dominated | –1.9209 | –1.3722 |
| TAC | £183,191 | –£32,923 | 18.2468 | +0.2748 | – | – | – |
| With CSA | vs. MMF | ||||||
| AZA | £210,097 | – | 18.0031 | – | Dominated | –0.6332 | –0.4634 |
| MMF | £199,910 | –£10,188 | 18.1269 | +0.1238 | – | – | – |
| EVL | £259,327 | +£59,417 | 18.2209 | +0.0940 | £632,246 | –2.8769 | –1.8866 |
| With TAC | vs. AZA | ||||||
| SRL | £222,300 | – | 17.9553 | – | Dominated | –2.7073 | –1.9187 |
| MMF | £179,719 | –£42,581 | 18.2398 | +0.2844 | Dominated | –0.2938 | –0.2149 |
| AZA | £174,989 | –£4730 | 18.2970 | +0.0572 | – | – | – |
| With BAS + CSA | vs. MMF | ||||||
| AZA | £199,042 | – | 18.1308 | – | Dominated | –0.5254 | –0.3889 |
| MMF | £190,856 | –£8186 | 18.2468 | +0.1161 | – | – | – |
| MPS | £198,303 | +£7447 | 18.3907 | +0.1438 | £51,770 | –0.2285 | –0.1044 |
| With BAS + TAC | vs. AZA | ||||||
| MMF | £170,182 | – | 18.3596 | – | Dominated | –0.3596 | –0.2618 |
| AZA | £164,316 | –£5866 | 18.4259 | +0.0663 | – | – | – |
| With r-ATG + CSA | vs. MMF | ||||||
| AZA | £216,114 | – | 17.9721 | – | Dominated | –0.4490 | –0.3321 |
| MMF | £209,097 | –£7017 | 18.0702 | +0.0982 | – | – | – |
| With r-ATG + TAC | vs. AZA | ||||||
| MMF | £189,637 | – | 18.1763 | – | Dominated | –0.3928 | –0.2853 |
| AZA | £183,191 | –£6446 | 18.2468 | +0.0705 | – | – | – |
Table 124 also shows that when considering AZA, MMF, MPS, EVL and SRL, the results are less simple. SRL is dominated by MMF and AZA, but EVL and MPS are both the most effective and most costly treatments in their comparisons. The ICER for EVL is > £600,000 per QALY and, therefore, EVL is not predicted to be cost-effective at £20,000 or £30,000 per QALY, while the ICER for MPS is slightly > £50,000 per QALY. The cost-effectiveness of MMF appears to be dependent on the concomitant treatments: when MMF is used in combination with CSA it is dominant over AZA (and cost-effective at £20,000 and £30,000 per QALY), while when it is used in combination with TAC-IR, AZA is dominant (and MMF is therefore not cost-effective at £20,000 or £30,000 per QALY).
Table 125 gives further details in terms of projected life-years (overall and in certain health states).
| Maintenance agent | Graft life expectancy (first graft; years) | Life expectancy (years) | Projected years with functioning graft | Projected years receiving dialysis | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | Total | Incremental | Total | Incremental | |
| With MMF | ||||||||
| CSA | 17.110 | – | 43.374 | – | 34.445 | – | 8.929 | – |
| TAC-PR | 20.038 | +2.929 | 43.452 | +0.077 | 35.370 | +0.925 | 8.082 | –0.848 |
| TAC | 20.304 | +0.266 | 43.553 | +0.102 | 35.502 | +0.132 | 8.051 | –0.031 |
| With AZA | ||||||||
| CSA | 15.098 | – | 43.175 | – | 33.784 | – | 9.391 | – |
| TAC | 21.263 | +6.164 | 43.649 | +0.474 | 35.925 | +2.141 | 7.724 | –1.667 |
| With BAS + MMF | ||||||||
| SRL | 20.376 | – | 43.534 | – | 35.533 | – | 8.001 | – |
| CSA | 19.171 | –1.204 | 43.566 | +0.032 | 35.159 | –0.374 | 8.407 | +0.406 |
| TAC | 22.449 | +3.277 | 43.746 | +0.180 | 36.286 | +1.127 | 7.460 | –0.947 |
| BEL | 24.625 | +2.176 | 44.125 | +0.379 | 37.236 | +0.950 | 6.889 | –0.571 |
| With BAS + AZA | ||||||||
| CSA | 17.229 | – | 43.378 | – | 34.490 | – | 8.888 | – |
| TAC | 23.597 | +6.367 | 43.858 | +0.480 | 36.785 | +2.295 | 7.073 | –1.815 |
| With r-ATG + MMF | ||||||||
| CSA | 15.983 | – | 43.294 | – | 34.059 | – | 9.236 | – |
| TAC | 18.881 | +2.898 | 43.467 | +0.173 | 34.994 | +0.935 | 8.473 | –0.763 |
| With r-ATG + AZA | ||||||||
| CSA | 14.421 | – | 43.136 | – | 33.557 | – | 9.578 | – |
| TAC | 20.068 | +5.647 | 43.582 | +0.447 | 35.474 | +1.916 | 8.109 | –1.470 |
| With CSA | ||||||||
| AZA | 15.098 | – | 43.175 | – | 33.784 | – | 9.391 | – |
| MMF | 17.110 | +2.011 | 43.374 | +0.200 | 34.445 | +0.661 | 8.929 | –0.462 |
| EVL | 19.183 | +2.074 | 43.499 | +0.124 | 35.118 | +0.673 | 8.380 | –0.549 |
| With TAC | ||||||||
| SRL | 15.862 | – | 43.139 | – | 33.979 | – | 9.160 | – |
| MMF | 20.304 | +4.442 | 43.553 | +0.415 | 35.502 | +1.524 | 8.051 | –1.109 |
| AZA | 21.263 | +0.959 | 43.649 | +0.096 | 35.925 | +0.423 | 7.724 | –0.327 |
| With BAS + CSA | ||||||||
| AZA | 17.229 | – | 43.378 | – | 34.490 | – | 8.888 | – |
| MMF | 19.171 | +1.942 | 43.566 | +0.188 | 35.159 | +0.669 | 8.407 | –0.481 |
| MPS | 21.364 | +2.193 | 43.810 | +0.244 | 35.983 | +0.824 | 7.827 | –0.579 |
| With BAS + TAC | ||||||||
| MMF | 22.449 | – | 43.746 | – | 36.286 | – | 7.460 | – |
| AZA | 23.597 | +1.148 | 43.858 | +0.111 | 36.785 | +0.498 | 7.073 | –0.387 |
| With r-ATG + CSA | ||||||||
| AZA | 14.421 | – | 43.136 | – | 33.557 | – | 9.578 | – |
| MMF | 15.983 | +1.562 | 43.294 | +0.159 | 34.059 | +0.501 | 9.236 | –0.343 |
| With r-ATG + TAC | ||||||||
| MMF | 18.881 | – | 43.467 | – | 34.994 | – | 8.473 | – |
| AZA | 20.068 | +1.187 | 43.582 | +0.115 | 35.474 | +0.480 | 8.109 | –0.365 |
Immediate-release tacrolimus was compared with CSA (six comparisons), TAC-PR (one comparison), SRL (one comparison) and BEL (one comparison).
Immediate-release tacrolimus was found to be less costly and more effective than all comparators except BEL in all comparisons. BEL was predicted to be more costly and more effective than TAC-IR with an ICER of > £500,000 per QALY.
As demonstrated in Table 125, TAC-IR is predicted to result in prolonged survival of the initial graft by 3.2–6.4 years compared with CSA, as well as to prolong overall survival by 0.2–0.5 years. TAC-IR is predicted to give greater graft and overall survival than CSA, TAC-PR and SRL, but reduced graft and overall survival than BEL.
Prolonged-release tacrolimus was compared with CSA and TAC-IR, in combination with MMF and CCSs.
Prolonged-release tacrolimus was predicted to be less costly and more effective than CSA but was also predicted to be more costly and less effective than TAC-IR and was therefore dominated and not cost-effective at any cost-effectiveness threshold.
BEL was compared with CSA, TAC-IR and SRL, in combination with BAS induction, MMF and CCSs.
Belatacept was predicted to be more costly and more effective than all comparators. As CSA and SRL were predicted to be dominated by TAC-IR, the relevant comparator for BEL is TAC-IR. The ICER of BEL was predicted to be > £500,000 per QALY.
Mycophenolate mofetil was compared with AZA (six comparisons), MPS (one comparison), SRL (one comparison) and EVL (one comparison).
When used in combination with CSA (three comparisons), MMF was predicted to be less costly and more effective than AZA. However, when used in combination with TAC-IR (three comparisons), MMF was predicted to be more costly and less effective than AZA. To summarise, MMF was dominant when used in combination with CSA but was dominated when used in combination with TAC-IR.
When compared with EVL in combination with CSA and CCSs, MMF was predicted to be less costly and less effective, with the ICER of EVL predicted to be > £600,000 per QALY.
When compared with SRL in combination with TAC and CCSs, MMF was predicted to be less costly and more effective than SRL, but was itself dominated by AZA in this comparison.
When compared with MPS in combination with BAS induction, CSA and CCSs, MMF was predicted to be less costly and less effective, with the ICER of MPS predicted to be > £50,000 per QALY.
At a cost-effectiveness threshold between £20,000 and £30,000 per QALY, MMF is predicted to be cost-effective in regimens containing CSA but not in regimens containing TAC-IR.
Mycophenolate sodium was compared with AZA and MMF in combination with BAS induction, CSA and CCSs. It was found to dominate AZA and was predicted to be more costly and more effective than MMF with an ICER of > £50,000 per QALY.
Sirolimus was compared with CSA, TAC-IR and BEL, in combination with BAS induction, MMF and CCSs, and was also compared with AZA and MMF, in combination with TAC-IR and CCSs.
When compared with CSA, TAC-IR and BEL, SRL was predicted to be dominated by CSA and TAC-IR.
When compared with AZA and MMF, SRL was predicted to be dominated by AZA and MMF.
Everolimus was compared with AZA and MMF in combination with CSA and CCSs. EVL was predicted to be more costly and more effective than AZA and mycophenolate, with the appropriate ICER of EVL (vs. MMF) predicted to be > £600,000 per QALY.
Regimens
When all 18 regimens were simultaneously compared, all regimens were predicted to be dominated by BAS + TAC + AZA, except for BAS + BEL + MMF, which was predicted to have an ICER of > £700,000 per QALY (Table 126).
| Regimen | Discounted total costs | Discounted total QALYs | ICER (cost per QALY) | INHB at £20,000/QALY | INHB at £30,000/QALY |
|---|---|---|---|---|---|
| BAS + TAC + AZA | £164,316 | 18.4259 | – | – | – |
| BAS + BEL + MMF | £293,175 | 18.5901 | £784,515 | –6.2787 | –4.1310 |
Summary
At cost-effectiveness thresholds between £20,000 and £30,000 per QALY, BAS was predicted to be cost-effective when compared with no induction and to r-ATG.
At cost-effectiveness thresholds between £20,000 and £30,000 per QALY, TAC-IR was predicted to be cost-effective when compared with CSA, TAC-PR, SRL and BEL.
At cost-effectiveness thresholds between £20,000 and £30,000 per QALY, AZA was predicted to be cost-effective (vs. MMF and SRL) when used in combination with TAC while MMF was predicted to be cost-effective (vs. AZA, MPS and EVL) when used in combination with CSA.
At cost-effectiveness thresholds between £20,000 and £30,000 per QALY, the only regimen predicted to be cost-effective when compared with all other regimens was BAS + TAC + AZA, which dominated all other regimens except BAS + BEL + MMF (which was more costly and more effective with an ICER of > £700,000 per QALY).
Probabilistic results
Probabilistic results were obtained after running 10,000 iterations. As demonstrated in Figure 33 (which compares the discounted costs for each regimen) there is good agreement between deterministic and probabilistic total discounted costs, with no significant non-linearities observed. Figure 34 suggests that total discounted QALYs overall are slightly lower when estimated in probabilistic analyses. Two regimens appear to have dropped more QALYs than the others in the probabilistic analyses: TAC-PR + MMF and BAS + TAC + MMF.
FIGURE 33.
Comparison of deterministic and probabilistic total discounted costs.
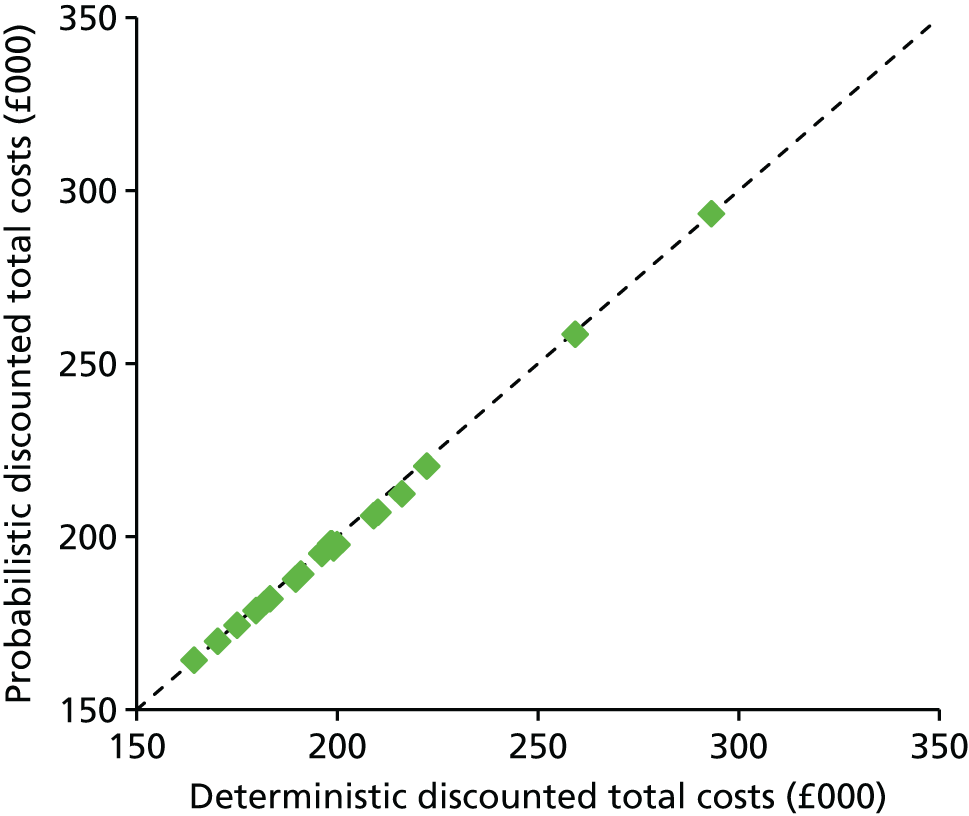
FIGURE 34.
Comparison of deterministic and probabilistic total discounted QALYs.

Induction agents
Summary cost-effectiveness results are shown in Table 127. In all four comparisons BAS is expected to dominate no induction, which is in turn expected to dominate r-ATG. The same pattern was observed in deterministic analyses.
| Induction agent | Discounted costs | Discounted QALYs | ICER (cost per QALY) | Incremental net health benefit | Probability cost-effective | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | £20,000/QALY | £30,000/QALY | ||
| With CSA + AZA | vs. BAS | ||||||||
| R-ATG | £212,166 | – | 17.9509 | – | Dominated | –0.9361 | –0.6747 | 8.1% | 8.2% |
| No induction | £206,855 | –£5310 | 17.9751 | +0.0242 | Dominated | –0.6463 | –0.4735 | 0.5% | 0.4% |
| BAS | £196,484 | –£10,371 | 18.1029 | +0.1278 | – | – | – | 91.4% | 91.4% |
| With CSA + MMF | vs. BAS | ||||||||
| R-ATG | £205,769 | – | 18.0414 | – | Dominated | –1.0072 | –0.7275 | 6.8% | 7.0% |
| No induction | £197,421 | –£8347 | 18.0896 | +0.0482 | Dominated | –0.5416 | –0.4011 | 1.5% | 1.3% |
| BAS | £188,991 | –£8430 | 18.2097 | +0.1201 | – | – | – | 91.8% | 91.8% |
| With TAC + AZA | vs. BAS | ||||||||
| R-ATG | £181,824 | – | 18.2064 | – | Dominated | –1.0652 | –0.7696 | 7.4% | 7.7% |
| No induction | £174,153 | –£7671 | 18.2538 | +0.0473 | Dominated | –0.6343 | –0.4666 | 0.7% | 0.6% |
| BAS | £164,088 | –£10,065 | 18.3848 | +0.1311 | – | – | – | 91.9% | 91.7% |
| With TAC + MMF | vs. BAS | ||||||||
| R-ATG | £187,494 | – | 18.1220 | – | Dominated | –1.0751 | –0.7760 | 6.7% | 6.9% |
| No induction | £178,415 | –£9079 | 18.1765 | +0.0545 | Dominated | –0.5667 | –0.4188 | 1.4% | 1.3% |
| BAS | £169,546 | –£8870 | 18.2997 | +0.1232 | – | – | – | 91.9% | 91.9% |
There is limited uncertainty predicted in the cost-effectiveness results as a result of parameter uncertainty. The probability of BAS being cost-effective at £20,000–30,000 per QALY is predicted to range from 91.4% to 91.9%. It is predicted that it is possible (though much less likely) that r-ATG could be cost-effective at £20,000–30,000 per QALY. It is predicted to be very unlikely that no induction could be cost-effective.
Maintenance agents
Table 128 shows the summary cost-effectiveness results for maintenance agents in the probabilistic analysis.
| Maintenance agent | Discounted costs | Discounted QALYs | ICER (cost per QALY) | Incremental net health benefit | Probability cost-effective | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | £20,000/QALY | £30,000/QALY | ||
| With MMF | vs. TAC | ||||||||
| CSA | £197,421 | – | 18.0896 | – | Dominated | –1.0372 | –0.7204 | 0.5% | 0.8% |
| TAC-PR | £194,861 | –£2560 | 18.0765 | –0.0130 | Dominated | –0.9222 | –0.6481 | 0.1% | 0.3% |
| TAC | £178,415 | –£16,446 | 18.1765 | +0.0999 | – | – | – | 99.4% | 98.9% |
| With AZA | vs. TAC | ||||||||
| CSA | £206,855 | – | 17.9751 | – | Dominated | –1.9138 | –1.3688 | 0.0% | 0.0% |
| TAC | £174,153 | –£32,702 | 18.2538 | +0.2787 | – | – | – | 100.0% | 100.0% |
| With BAS + MMF | vs. TAC | ||||||||
| SRL | £197,730 | – | 18.1774 | – | Dominated | –1.5315 | –1.0617 | 0.0% | 0.0% |
| CSA | £188,991 | –£8739 | 18.2097 | +0.0323 | Dominated | –1.0623 | –0.7382 | 0.4% | 0.6% |
| TAC | £169,546 | –£19,445 | 18.2997 | +0.0900 | – | – | – | 99.6% | 99.3% |
| BEL | £293,117 | +£123,571 | 18.5326 | +0.2330 | £530,421 | –5.9456 | –3.8861 | 0.0% | 0.0% |
| With BAS + AZA | vs. TAC | ||||||||
| CSA | £196,484 | – | 18.1029 | – | Dominated | –1.9018 | –1.3618 | 0.0% | 0.0% |
| TAC | £164,088 | –£32,396 | 18.3848 | +0.2820 | – | – | – | 100.0% | 100.0% |
| With r-ATG + MMF | vs. TAC | ||||||||
| CSA | £205,769 | – | 18.0414 | – | Dominated | –0.9943 | –0.6897 | 0.4% | 0.7% |
| TAC | £187,494 | –£18,275 | 18.1220 | +0.0806 | – | – | – | 99.6% | 99.3% |
| With r-ATG + AZA | vs. TAC | ||||||||
| CSA | £212,166 | – | 17.9509 | – | Dominated | –1.7727 | –1.2670 | 0.0% | 0.0% |
| TAC | £181,824 | –£30,342 | 18.2064 | +0.2556 | – | – | – | 100.0% | 100.0% |
| With CSA | vs. MMF | ||||||||
| AZA | £206,855 | – | 17.9751 | – | Dominated | –0.5862 | –0.4290 | 0.1% | 0.1% |
| MMF | £197,421 | –£9434 | 18.0896 | +0.1145 | – | – | – | 99.9% | 99.9% |
| EVL | £258,260 | +£60,839 | 18.1562 | +0.0666 | £912,988 | –2.9753 | –1.9613 | 0.0% | 0.0% |
| With TAC | vs. AZA | ||||||||
| SRL | £220,087 | – | 17.8930 | – | Dominated | –2.6574 | –1.8918 | 0.0% | 0.0% |
| MMF | £178,415 | –£41,672 | 18.1765 | +0.2834 | Dominated | –0.2904 | –0.2194 | 25.9% | 25.1% |
| AZA | £174,153 | –£4262 | 18.2538 | +0.0773 | – | – | – | 74.1% | 74.9% |
| With BAS + CSA | vs. MMF | ||||||||
| AZA | £196,484 | – | 18.1029 | – | Dominated | –0.4815 | –0.3566 | 0.2% | 0.2% |
| MMF | £188,991 | –£7493 | 18.2097 | +0.1068 | – | – | – | 74.0% | 69.8% |
| MPS | £197,722 | +£8730 | 18.2768 | +0.0671 | £130,080 | –0.3694 | –0.2239 | 25.9% | 30.1% |
| With BAS + TAC | vs. AZA | ||||||||
| MMF | £169,546 | – | 18.2997 | – | Dominated | –0.3581 | –0.2671 | 20.4% | 19.9% |
| AZA | £164,088 | –£5458 | 18.3848 | +0.0852 | – | – | – | 79.6% | 80.1% |
| With r-ATG + CSA | vs. MMF | ||||||||
| AZA | £212,166 | – | 17.9509 | – | Dominated | –0.4104 | –0.3038 | 0.4% | 0.3% |
| MMF | £205,769 | –£6397 | 18.0414 | +0.0905 | – | – | – | 99.6% | 99.7% |
| With r-ATG + TAC | vs. AZA | ||||||||
| MMF | £187,494 | – | 18.1220 | – | Dominated | –0.3680 | –0.2735 | 18.3% | 18.0% |
| AZA | £181,824 | –£5670 | 18.2064 | +0.0845 | – | – | – | 81.7% | 82.0% |
| With CSA | vs. MMF | ||||||||
| AZA | £209,016 | – | 17.9481 | – | Dominated | –0.5872 | –0.4292 | 0.1% | 0.1% |
| MMF | £199,539 | –£9477 | 18.0614 | +0.1133 | – | – | – | 99.9% | 99.9% |
| EVL | £259,701 | +£60,162 | 18.1244 | +0.0630 | £954,838 | –2.9451 | –1.9424 | 0.0% | 0.0% |
| With TAC | vs. AZA | ||||||||
| SRL | £221,807 | – | 17.8558 | – | Dominated | –2.6408 | –1.8824 | 0.0% | 0.0% |
| MMF | £180,529 | –£41,278 | 18.1350 | +0.2792 | Dominated | –0.2977 | –0.2273 | 24.9% | 23.9% |
| AZA | £176,305 | –£4,224 | 18.2215 | +0.0865 | – | – | – | 75.1% | 76.2% |
| With BAS + CSA | vs. MMF | ||||||||
| AZA | £197,127 | – | 18.1019 | – | Dominated | –0.4830 | –0.3575 | 0.2% | 0.2% |
| MMF | £189,597 | –£7530 | 18.2083 | +0.1065 | – | – | – | 75.0% | 71.1% |
| MPS | £198,660 | +£9063 | 18.2739 | +0.0656 | £138,196 | –0.3876 | –0.2365 | 24.8% | 28.8% |
| With BAS + TAC | vs. AZA | ||||||||
| MMF | £170,179 | – | 18.2944 | – | Dominated | –0.3602 | –0.2696 | 20.0% | 19.4% |
| AZA | £164,746 | –£5433 | 18.3829 | +0.0885 | – | – | – | 80.0% | 80.6% |
| With r-ATG + CSA | vs. MMF | ||||||||
| AZA | £201,211 | – | 18.0837 | – | Dominated | –0.4273 | –0.3173 | 0.4% | 0.3% |
| MMF | £194,609 | –£6602 | 18.1809 | +0.0972 | – | – | – | 99.6% | 99.7% |
| With r-ATG + TAC | vs. AZA | ||||||||
| MMF | £175,703 | – | 18.2763 | – | Dominated | –0.3816 | –0.2823 | 17.9% | 17.8% |
| AZA | £169,739 | –£5963 | 18.3598 | +0.0835 | – | – | – | 82.1% | 82.2% |
As in the deterministic analysis, it is predicted that TAC-IR dominates CSA (as well as TAC-PR and SRL), but is less costly and less effective than BEL (ICER £530,421 per QALY).
In addition, matching the results of the deterministic analysis it is again predicted that MMF is cost-effective when used in combination with CSA, but not when used in combination with TAC-IR.
Mycophenolate sodium is still not predicted to be cost-effective and, in fact, its estimated ICER is £130,080 per QALY in the probabilistic analysis, compared with £51,770 per QALY in the deterministic analysis.
Sirolimus is still not predicted to be cost-effective. As in the deterministic analyses, SRL is dominated by CSA and TAC-IR when used in combination with BAS and MMF, and is dominated by MMF and AZA when used in combination with TAC-IR.
Everolimus is still not predicted to be cost-effective. It is predicted to be more expensive and more effective than MMF and AZA when in combination with CSA with an ICER > £900,000 per QALY (compared with an ICER of > £600,000 per QALY in the deterministic analysis).
Cost-effectiveness acceptability curves
Cost-effectiveness acceptability curves show, for each regimen, the probability that regimen is cost-effective at various thresholds. In this context, the probability of a regimen being cost-effective is the proportion of PSA iterations in which the regimen gives the greatest net health benefit.
No crossovers are observed in the cost-effectiveness acceptability curves and it was verified that in all cases the regimen with the greatest probability of being cost-effective at each threshold also gave the greatest expected net health benefit.
All treatment combinations with BAS induction were predicted to be cost-effective at £20,000 per QALY in at least 91.4% of the simulations and at £30,000 per QALY in at least 91.4% of simulations (Figures 35–38).
FIGURE 35.
Cost-effectiveness acceptability curves for induction agents in combination with CSA and AZA.
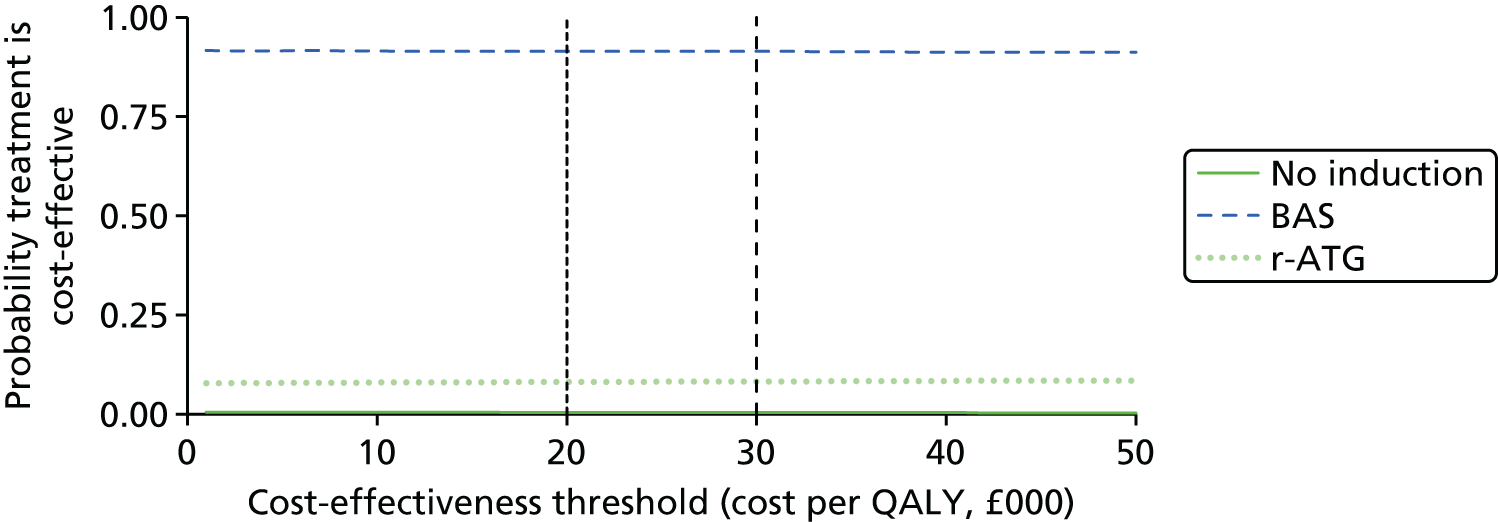
FIGURE 36.
Cost-effectiveness acceptability curves for induction agents in combination with CSA and MMF.

FIGURE 37.
Cost-effectiveness acceptability curves for induction agents in combination with TAC-IR and AZA.
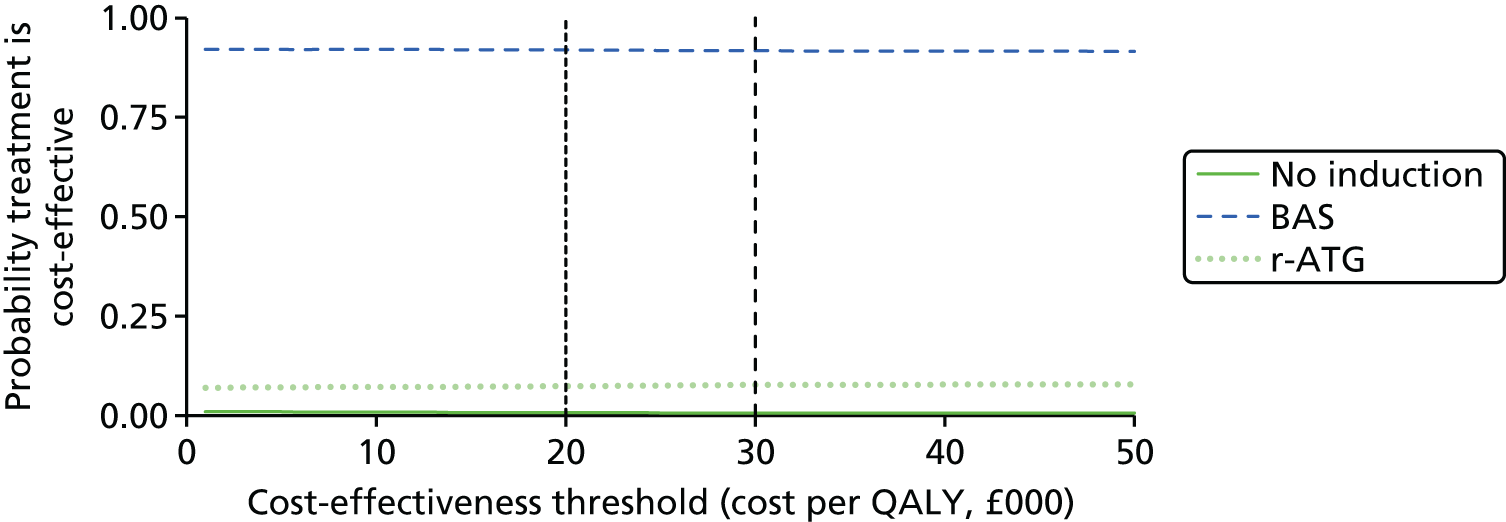
FIGURE 38.
Cost-effectiveness acceptability curves for induction agents in combination with TAC-IR and MMF.

All treatment combinations with TAC were predicted to be cost-effective at £20,000 per QALY in over 99% of the simulations and at £30,000 per QALY in over 98% of simulations (Figures 39–44).
FIGURE 39.
Cost-effectiveness acceptability curves for maintenance agents in combination with MMF.

FIGURE 40.
Cost-effectiveness acceptability curves for maintenance agents in combination with AZA.
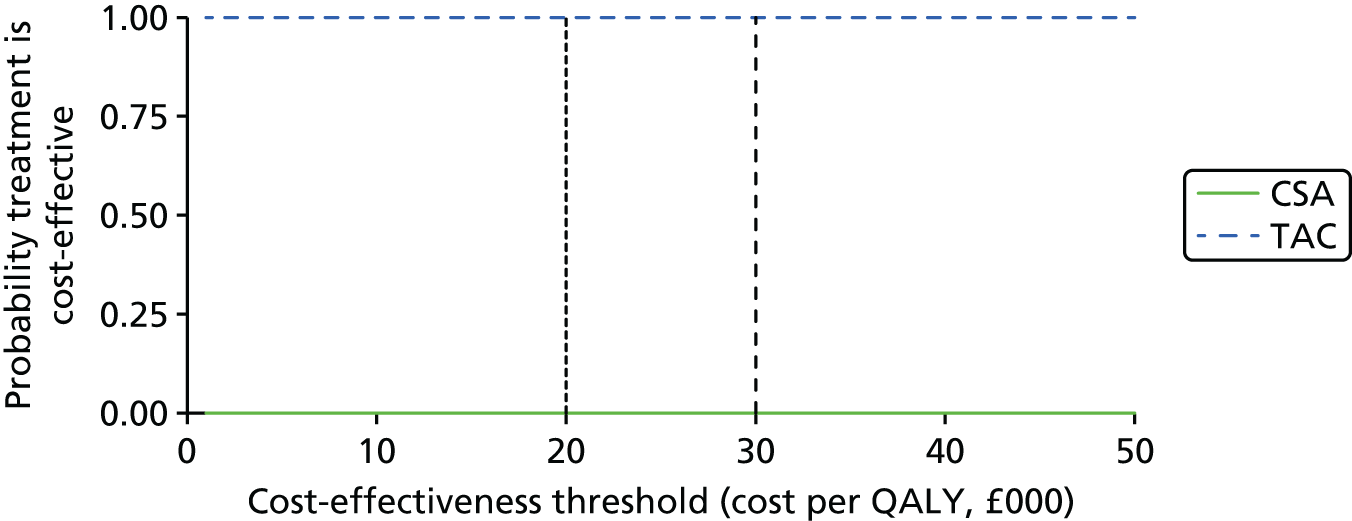
FIGURE 41.
Cost-effectiveness acceptability curves for maintenance agents in combination with BAS and MMF.
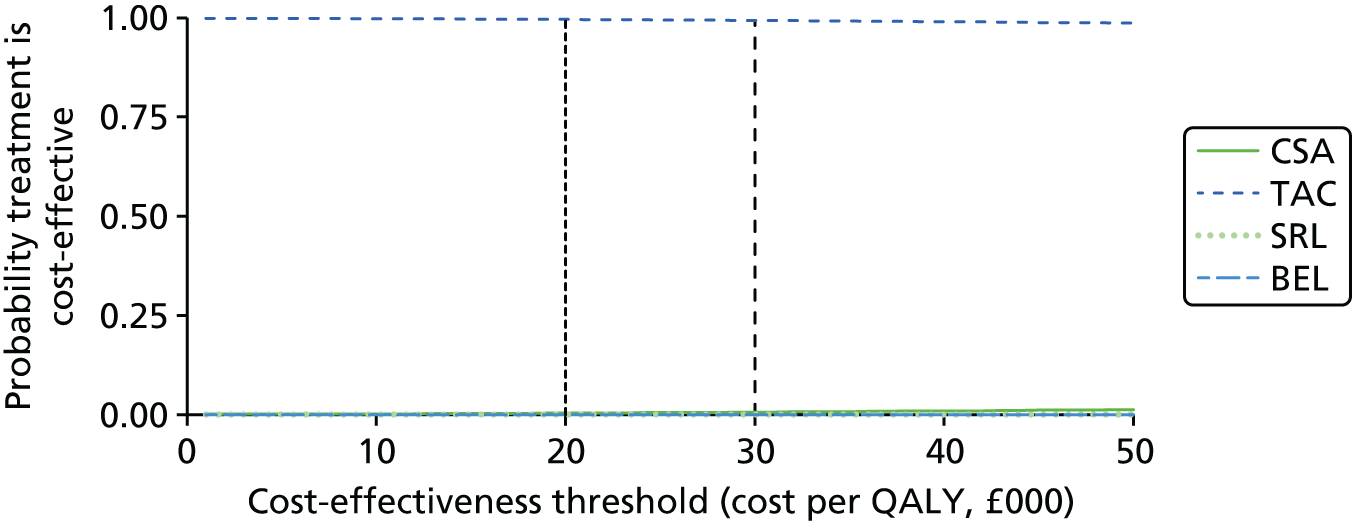
FIGURE 42.
Cost-effectiveness acceptability curves for maintenance agents in combination with BAS and AZA.
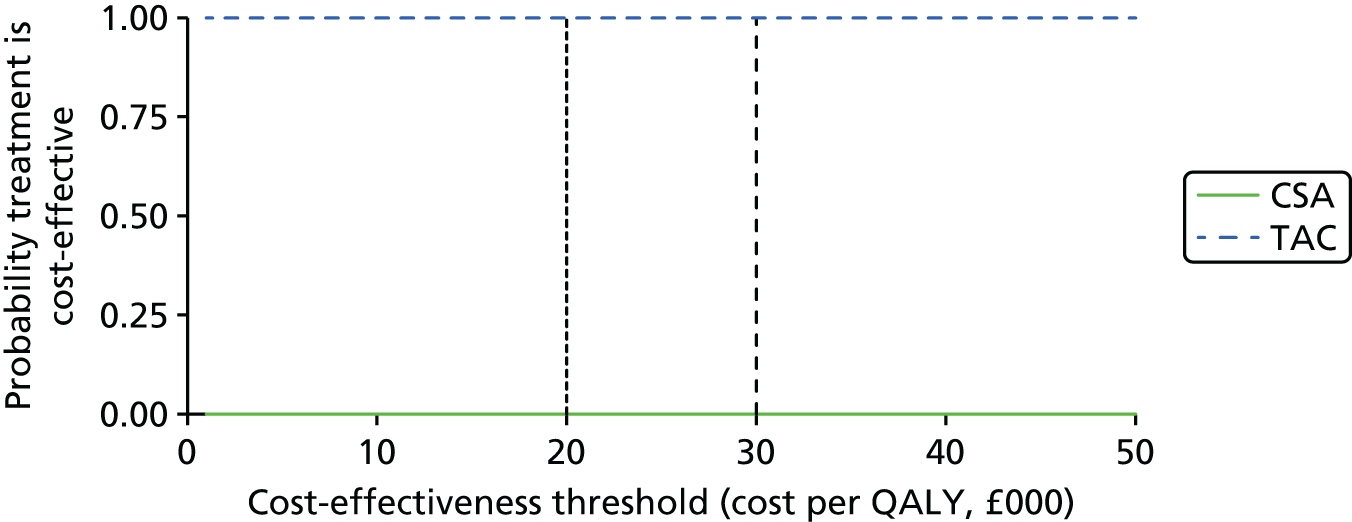
FIGURE 43.
Cost-effectiveness acceptability curves for maintenance agents in combination with r-ATG and MMF.
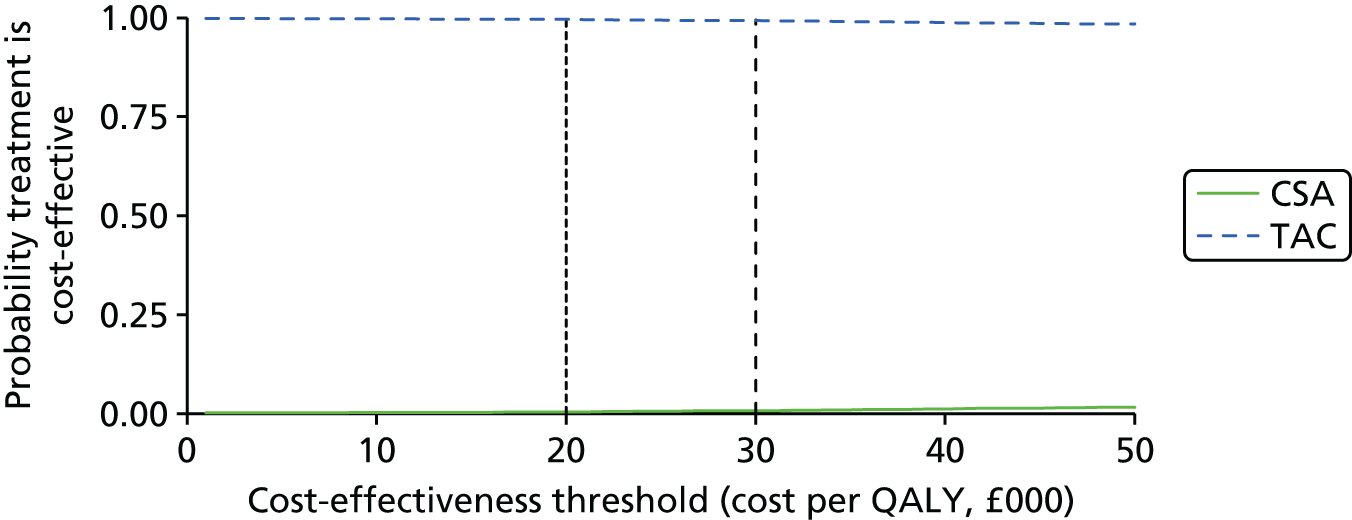
FIGURE 44.
Cost-effectiveness acceptability curves for maintenance agents in combination with r-ATG and AZA.

Mycophenolate mofetil (in combination with CSA and no induction) was predicted to be cost-effective in 99.9% of the simulations at £20,000 and £30,000 per QALY (Figure 45).
FIGURE 45.
Cost-effectiveness acceptability curves for maintenance agents in combination with CSA.
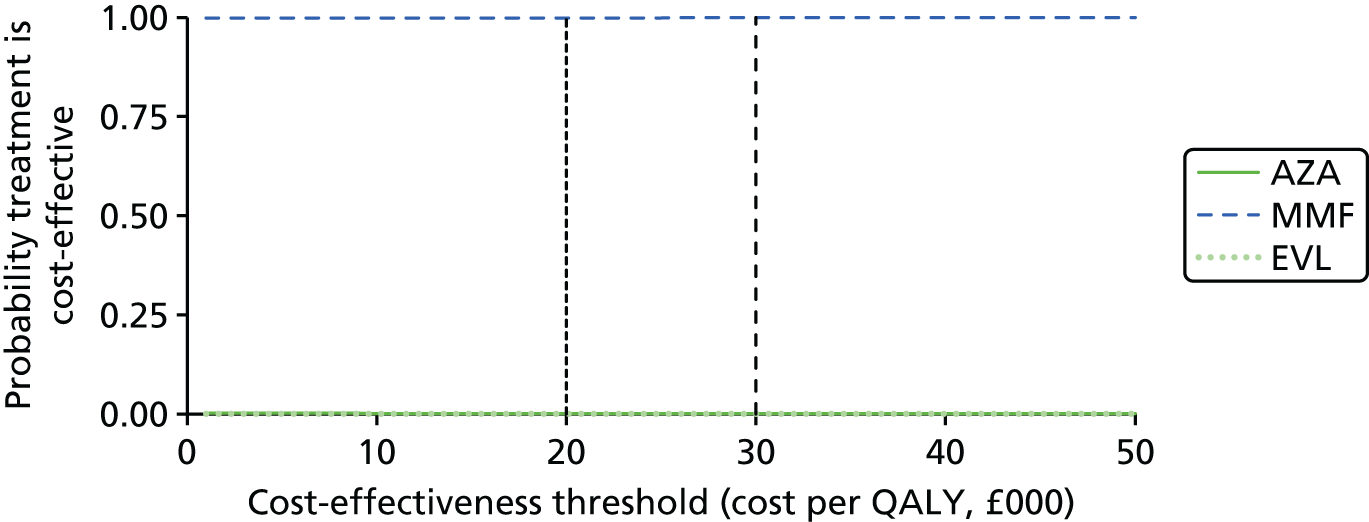
Azathioprine (in combination with TAC and no induction) was predicted to be cost-effective at £20,000 per QALY in 74.1% of the simulations and at £30,000 per QALY in 74.9% of simulations (Figure 46).
FIGURE 46.
Cost-effectiveness acceptability curves for maintenance agents in combination with TAC-IR.

Mycophenolate mofetil (in combination with BAS and CSA) was predicted to be cost-effective at £20,000 per QALY in 74.0% of the simulations and at £30,000 per QALY in 69.8% of simulations (Figure 47).
FIGURE 47.
Cost-effectiveness acceptability curves for maintenance agents in combination with BAS and CSA.
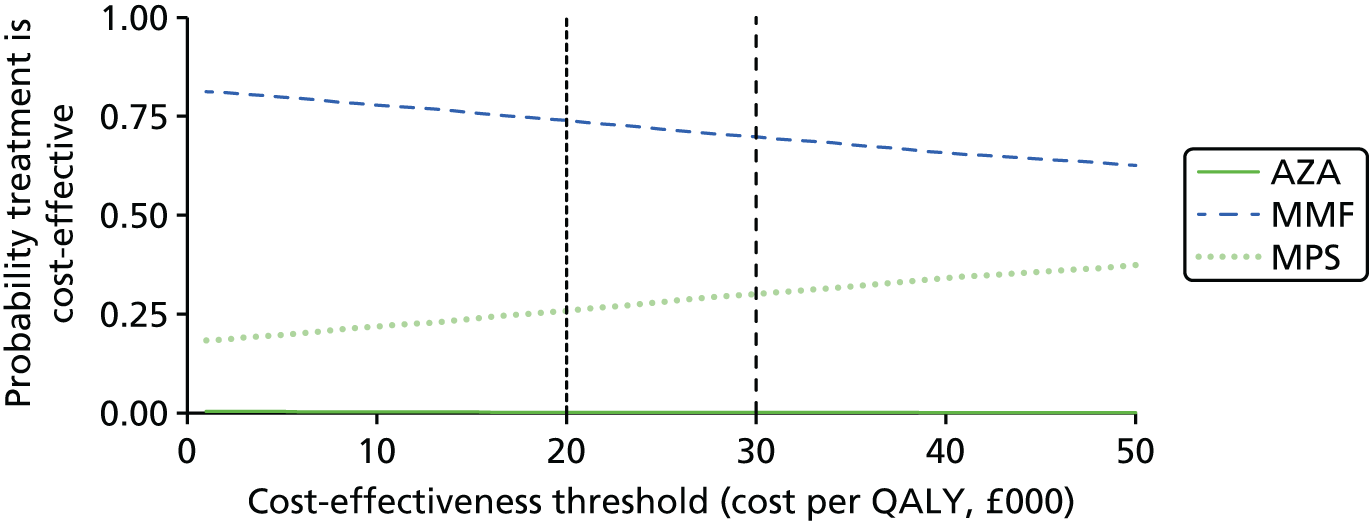
Azathioprine (in combination with BAS and TAC) was predicted to be cost-effective at £20,000 per QALY in 79.6% of the simulations and at £30,000 per QALY in 80.1% of simulations (Figure 48).
FIGURE 48.
Cost-effectiveness acceptability curves for maintenance agents in combination with BAS and TAC-IR.
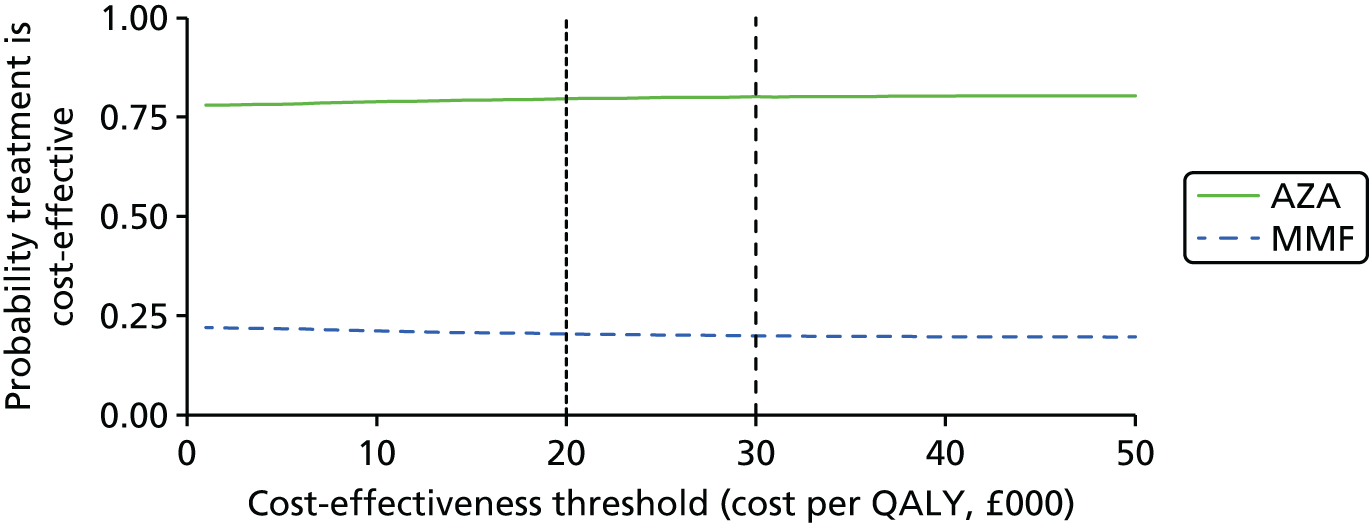
Azathioprine (in combination with r-ATG and CSA) was predicted to be cost-effective at £20,000 per QALY in 99.6% of the simulations and at £30,000 per QALY in 99.7% of simulations (Figure 49).
FIGURE 49.
Cost-effectiveness acceptability curves for maintenance agents in combination with r-ATG and CSA.
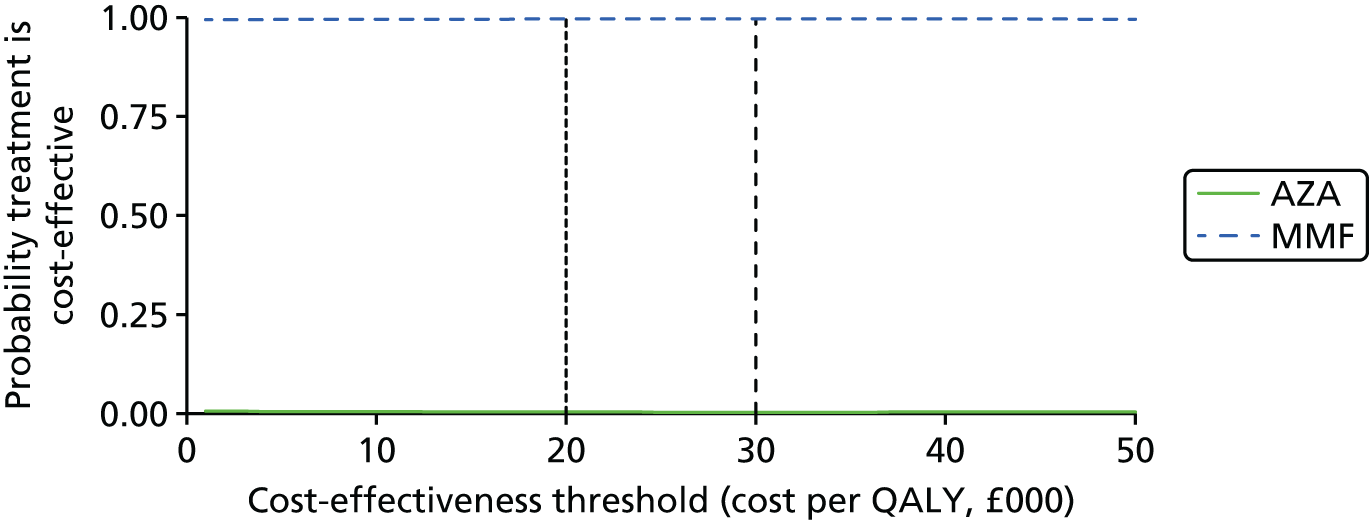
Azathioprine (in combination with r-ATG and TAC) was predicted to be cost-effective at £20,000 per QALY in 81.7% of the simulations and at £30,000 per QALY in 82.0% of simulations (Figure 50).
FIGURE 50.
Cost-effectiveness acceptability curves for maintenance agents in combination with r-ATG and TAC-IR.
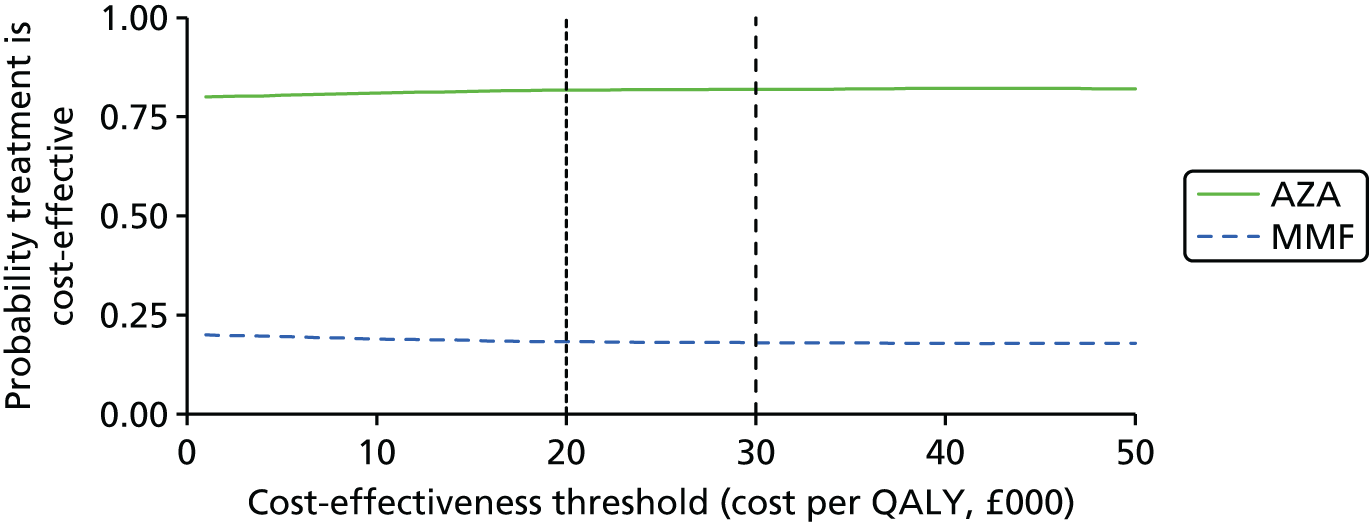
Scenario analyses
Below average weight for kidney transplant recipients
When body weight was assumed to follow the ninth centile for age (rather than the median) the immunosuppression costs of most arms decreased. QALYs were unaffected.
The incremental net health benefits at £20,000 per QALY did not change sign (i.e. no agents previously not cost-effective became cost-effective or vice versa). At £30,000 per QALY the incremental net health benefit for MPS became positive, suggesting that in this scenario MPS is cost-effective at £30,000 per QALY (but not at £20,000 per QALY). The ICER for MPS in this scenario is £27,006 per QALY.
Surrogate relationship between acute rejection and graft survival removed
When the surrogate relationship between AR and graft survival was removed, the result was increased graft survival for all regimens except BAS + CSA + MMF, BAS + TAC + MMF, BAS + SRL + MMF, r-ATG + CSA + MMF, r-ATG + TAC + MMF, and r-ATG + TAC + AZA (for which graft survival was decreased). Increased graft survival usually results in reduced overall costs and increased QALYs and this was observed across regimens as expected.
No incremental net health benefits changed sign at £20,000 or £30,000 per QALY, although the ICER for MPS dropped to £33,157 per QALY.
Subgroup analyses
The only subgroup analyses which were conducted were based on the age of KTRs. The age at time of transplantation was varied from 2 years to 17 years.
For most regimens, discontinuities in total discounted costs were observed at age 6 years and age 13 years, which are explained by the HRs for graft survival according to age, taken from Muscheites et al. ,185 in which graft survival was predicted to be worse for children aged 6–12 years at the time of transplantation than for younger children or older adolescents. Reduced graft survival results in greater total costs as more recipients lose their grafts earlier and require dialysis.
For all regimens, the total discounted QALYs decreased with increasing age, except at age 13 years, when discounted QALYs were greater than for age 12 years (due to the changing HR for graft survival indicated above). The cause of decreasing total discounted QALYs is likely to be greater exposure to higher rates of DWFG.
The total discounted costs and QALYs are shown for BAS, TAC-IR and AZA in Figure 51. Across the age range, BAS + TAC + AZA was the most cost-effective regimen at £20,000 and £30,000 per QALY (Figures 52 and 53). When the weighted average total discounted costs and QALYs (weighted by number of KTRs at each age) are calculated, BAS + TAC + AZA is the cost-effective regimen at £20,000 and £30,000 per QALY (Table 129).
FIGURE 51.
Total discounted costs and QALYs for regimen of BAS, TAC-IR and AZA as age at transplantation is varied.

FIGURE 52.
Rank of net health benefit at £20,000 per QALY for all regimens as the age at time of transplantation is varied.
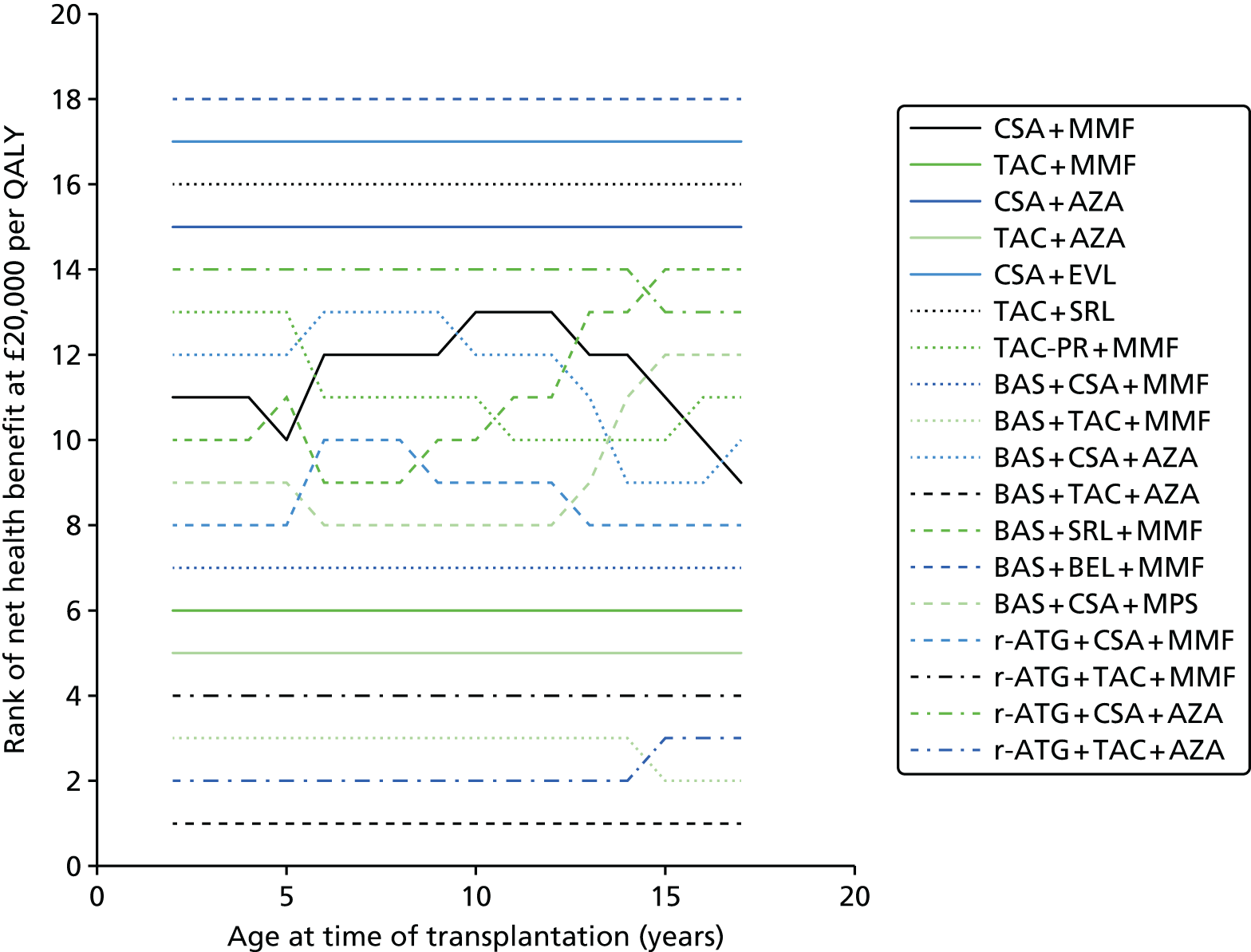
FIGURE 53.
Rank of net health benefit at £30,000 per QALY for all regimens as the age at time of transplantation is varied.

| Regimen | Net health benefit | |
|---|---|---|
| £20,000 per QALY | £30,000 per QALY | |
| CSA + MMF | 9.08 | 12.11 |
| TAC + MMF | 9.99 | 12.74 |
| CSA + AZA | 8.32 | 11.55 |
| TAC + AZA | 10.25 | 12.93 |
| CSA + EVL | 5.60 | 9.81 |
| TAC + SRL | 7.50 | 10.99 |
| TAC-PR + MMF | 9.10 | 12.13 |
| BAS + CSA + MMF | 9.59 | 12.49 |
| BAS + TAC + MMF | 10.65 | 13.23 |
| BAS + CSA + AZA | 9.08 | 12.11 |
| BAS + TAC + AZA | 10.95 | 13.45 |
| BAS + SRL + MMF | 9.02 | 12.10 |
| BAS + BEL + MMF | 2.33 | 7.76 |
| BAS + CSA + MPS | 9.23 | 12.29 |
| r-ATG + CSA + MMF | 9.28 | 12.28 |
| r-ATG + TAC + MMF | 10.34 | 13.02 |
| r-ATG + CSA + AZA | 8.83 | 11.94 |
| r-ATG + TAC + AZA | 10.69 | 13.27 |
Summary of results from analyses based on extrapolating effectiveness estimates from adults
Basiliximab was predicted to be cost-effective at £20,000–30,000 per QALY.
R-ATG and no induction were not predicted to be cost-effective at £20,000–30,000 per QALY compared with BAS.
Immediate-release tacrolimus was predicted to be cost-effective at £20,000–30,000 per QALY.
Prolonged-release tacrolimus, SRL, BEL and CSA were not predicted to be cost-effective at £20,000–30,000 per QALY compared with TAC-IR and each other.
MMF was predicted to be cost-effective at £20,000–30,000 per QALY when used in combination with CSA, but not when used in combination with TAC-IR.
Azathioprine was predicted to be cost-effective at £20,000–30,000 per QALY when used in combination with TAC-IR, but not when used in combination with CSA.
Mycophenolate sodium was not predicted to be cost-effective at £20,000–30,000 per QALY compared with MMF and AZA, but was cost-effective at £30,000 per QALY in a scenario analysis in which body weight followed the ninth centile rather than median weight for age.
Sirolimus and EVL were not predicted to be cost-effective at £20,000–30,000 per QALY compared with MMF and AZA.
Summary of results from Peninsula Technology Assessment Group economic assessment
Basiliximab was predicted to be cost-effective at £20,000–30,000 per QALY compared with no induction in one analysis based on a RCT in children and adolescents,75 but was not predicted to be cost-effective in an analysis based on another RCT in children and adolescents. 73 BAS was predicted to be cost-effective at £20,000–30,000 per QALY compared with no induction and r-ATG in analyses based on extrapolating effectiveness estimates from the adult population.
Rabbit anti-human thymocyte immunoglobulin was not predicted to be cost-effective at £20,000–30,000 compared with BAS in analyses based on extrapolating effectiveness estimates from the adult population.
Immediate-release tacrolimus was predicted to be cost-effective at £20,000–30,000 per QALY compared with CSA in an analysis based on a RCT in children and adolescents77 and was also predicted to be cost-effective compared with CSA, TAC-PR, SRL and BEL in analyses based on extrapolating effectiveness estimates from the adult population.
Mycophenolate mofetil was predicted to be cost-effective at £20,000–30,000 per QALY when used in combination with CSA in analyses based on extrapolating effectiveness estimates from the adult population, but was not predicted to be cost-effective when used in combination with TAC-IR.
Prolonged-release tacrolimus, SRL, BEL, MPS and EVL were not predicted to be cost-effective at £20,000–30,000 per QALY compared with TAC-IR in analyses based on extrapolating effectiveness estimates from the adult population.
Comparison of the Peninsula Technology Assessment Group, Astellas and previous Assessment Group’s model-based analyses
In this section, we compare the model-based analysis of maintenance regimens by the independent Assessment Group (PenTAG) with relevant analyses in the company submission (from Astellas) and with the previous analyses2 which informed NICE’s current guidance1 on these technologies. Table 130 shows which specific immunosuppression agents have been evaluated by the three models.
| Agent | TA99 | PenTAG | Astellas |
|---|---|---|---|
| BAS | Y | Y | N |
| R-ATG | N | Y | N |
| (No induction) | Y | Y | N |
| TAC-IR | Y | Y | Y |
| TAC-PR | N | Y | Y |
| MMF | Y | Y | N |
| MPS | Y | Y | N |
| SRL | Y | Y | Y |
| EVL | N | Y | Y |
| BEL | N | Y | Y |
| (CSA) | Y | Y | N |
| (AZA) | Y | Y | N |
Table 131 summarises which combination regimens have been compared by the PenTAG and Astellas models in the child/adolescent kidney transplant populations. The Astellas submission did not provide cost-effectiveness analysis of induction therapies and only one comparison in the previous technology assessment for NICE compared induction therapies (BAS vs. no induction).
| PenTAG | Astellas |
|---|---|
| TAC (+ AZA) vs. CSA (+ AZA) (based on one child/adolescent RCT) | TAC (granules for oral solution) vs. TAC ‘specials’ (liquid preparations) vs. BEL vs. EVL vs. SRL + low-dose CSA (= CNI minimisation) vs. SRL + MMF (= CNI avoidance) |
| In addition, based on adult RCT evidence following BAS induction: TAC (+ MMF) vs. CSA (+ MMF) vs. SRL (+ MMF) vs. BEL (+ MMF) |
|
| TAC vs. PR-TAC (based on adult RCT) | TAC vs. PR-TAC |
Fully explaining the differences between the different model cost-effectiveness outputs is more challenging than usual, because
-
the main assumptions in the Astellas model are different in very many respects, including:
-
10-year time horizon versus 50 years in PenTAG analyses
-
basing effectiveness differences only on BPAR at 12 months post transplant
-
omission of CSA as a relevant comparator for maintenance therapies
-
large difference between the assumed utility of living with a functioning graft (0.71) and being on dialysis (haemodialysis 0.44, peritoneal dialysis 0.53)
-
drug unit costs were all based on BNF list prices in the Astellas analyses, whereas in the PenTAG analyses we used prices from the eMIT database, when possible, to reflect nationally available discounted prices (i.e. for TAC-IR, CSA, AZA, MMF, prednisolone)
-
drug consumption values for SRL regimens were based on treatment guidelines rather than trial evidence of actual dosage intensity.
-
-
the Yao et al. 2 model assumptions and parameters are not fully described in any one report (and we were also unable to obtain the model files to assess it). The model used in the Yao et al. 2 analysis is:
-
a child-/adolescent-adapted version of an adult post-transplant immunosuppression model, which was based on:
-
a ‘meta-model’ developed for the previous technology assessment for NICE of immunosuppression following kidney transplantation255 which was, in turn, based on:
-
the Novartis model submitted to the previous technology appraisal process for these drugs.
-
-
-
Therefore, it was not possible to know with certainty what the input parameters and other main assumptions were in the Yao et al. 2 model. In addition, the incremental cost-effectiveness analyses produced by the Yao et al. 2 model used different discount rates for costs (6% per year) and QALYs (1.5% per year), according to the NICE methods guidance at that time. 256 Like the current Astellas model, it also had a limited time horizon of 10 years. Without access to the original model, and no reporting of the model outputs for each comparator or as undiscounted costs or QALYs, it is impossible to adjust for these differences. The results, which are most different between the Yao et al. 2 and PenTAG modelling, are those that relied on adult RCT data – and for which the PenTAG has substantially updated the effectiveness estimates from more recent trials (Table 132). In contrast, the cost-effectiveness result for BAS versus no induction – which does use available child/adolescent RCT evidence in both models – arrives at the same conclusion as Yao et al. 2 did in 2006, that is, that BAS is both more effective and cheaper than no induction.
| Compared regimens | Table 56 in Yao et al.2 | PenTAGa | ||
|---|---|---|---|---|
| Estimateb | ICER (£ per QALY)b | Estimateb | ICER (£ per QALY)b | |
| CAS vs. TAS (= CSA + AZA vs. TAC + AZA) | ||||
| Incremental costs (£) | 13,716 | 145,540 | –35,267 | TAS dominant |
| Incremental QALYs | 0.09 | +0.2888 | ||
| CAS vs. CMS (= CSA + AZA vs. CSA + MMF) | ||||
| Incremental costs (£) | 9543 | 194,559 | –10,202 | CMS dominant |
| Incremental QALYs | 0.049 | +0.1232 | ||
| CAS vs. BCAS (= CSA + AZA vs. BAS + CSA + AZA) | ||||
| Incremental costs (£) | –1103 | BCAS dominant | –12,726 | BCAS dominant |
| Incremental QALYs | 0.074 | +0.1522 | ||
| CAS vs. DCAS (= CSA + AZA vs. DAC + CSA + AZA)c | ||||
| Incremental costs (£) | –417 | DCAS dominant | N/A | |
| Incremental QALYs | 0.05 | N/A | ||
| TAS vs. BTAS (= TAC + AZA vs. BAS + TAC + AZA) | ||||
| Incremental costs (£) | –451 | BTAS dominant | –12,335 | BTAS dominant |
| Incremental QALYs | 0.038 | +0.1584 | ||
For reference, three larger tables in Appendix 8 compare the main cost parameters, effectiveness parameters and main cost and effectiveness results for the three models, where they are known (see Tables 142–144). These show, for example, that the PenTAG model assumptions tended to include fuller costing of the administration of the maintenance therapies. In addition, although applied differently in the models, the utility difference between living with a functioning graft and living on dialysis was greater in the Astellas model (difference of between ≈0.25 and ≈0.3) than in the PenTAG and Yao et al. 2 models (≈0.2 difference).
Peninsula Technology Assessment Group’s and Astellas’ model-based analyses compared
Table 133 shows the company’s and the Assessment Group’s analysis of the cost-effectiveness of the two types of TAC. While the Astellas analysis estimates that TAC-PR dominates TAC-IR (estimating it to be > £5000 cheaper over 10 years and to generate 0.035 extra discounted QALYs, the PenTAG analysis produces the opposite result – based on effectiveness evidence from adult RCTs), TAC-PR is dominated by both TAC-IR and CSA. In the PenTAG analysis, TAC-PR is > £18,000 more costly than TAC-IR and generates 0.06 fewer discounted QALYs (both over a time horizon of 50 years).
| Agent | Discounted costs | Discounted QALYs | ICER | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| PenTAG | |||||
| CSA | £202,424 | – | 18.1018 | – | Dominated |
| TAC-PR | £198,433 | –£3992 | 18.1503 | +0.0485 | Dominated |
| TAC | £182,163 | –£16,270 | 18.2085 | +0.0581 | – |
| Astellas | |||||
| TAC-PR | £53,395 | – | 5.604 | – | |
| TAC | £58,471 | +£5,076 | 5.569 | –0.035 | Dominated |
This opposite result in incremental QALYs mostly arises because of the different trial data used within the two models and the fact that long-term outcomes in the Astellas model are driven entirely by rates of AR. For informing the effectiveness parameters of the drugs on BPAR, mortality, graft loss and renal function, the PenTAG analysis uses meta-analysis of two direct head-to-head trials of the two comparators. 139,196 None of the pooled ORs is statistically significant and all except the comparison for BPAR favour the TAC-IR. In contrast, the Astellas review reports using three trials;97,139,152 including two meta-analyses of BPAR (each including two unspecified trials) which they conclude show the two types of TAC to be of ‘similar efficacy and safety’. However, in their model, these data sources are then used to justify TAC-IR having a 2 percentage point higher rate of AR than TAC-PR, which then drives differences in long-term graft survival (and costs). In their modelling, they also factor in greater adherence to treatment with TAC-PR, which departs from the ITT analysis of the trials.
Table 134 shows the company’s and the Assessment Group’s analysis of the cost-effectiveness of TAC, BEL, SRL and CSA. In particular, it shows the impact of the very different time horizons of the two models on the accumulated costs and QALYs. The other main differences are that in the Astellas model BEL is the least effective treatment (but the most effective in the PenTAG model) and only about £20,000 more expensive than TAC (compared with £153,000 more expensive in the PenTAG model). The omission of CSA from the Astellas modelling does not invalidate comparisons between the two analyses, because in the PenTAG model the CSA regime is dominated (less effective and more costly) than TAC – and so effectively ruled out of further consideration.
| Agent | Discounted costs | Discounted QALYs | ICER | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| PenTAG (all with BAS + MMF) | |||||
| SRL | £199,145 | – | 18.2423 | – | Dominated |
| CSA | £191,679 | –£7466 | 18.2468 | +0.0045 | Dominated |
| TAC | £170,915 | –£20,763 | 18.3596 | +0.0485 | – |
| BEL | £324,708 | +£153,792 | 18.5901 | +0.0581 | £667,031 |
| Astellas | vs. TAC | ||||
| SRL I (CNI minimisation) | £52,339 | –£6132 | 5.565 | –0.004 | £1,576,937 |
| SRL II (CNI avoidance) | £61,490 | +£3019 | 5.553 | –0.016 | Dominated by TAC |
| TAC | £58,471 | – | 5.569 | – | – |
| TAC ‘specials’ | £72,945 | +£14,474 | 5.564 | –0.001 | Higher cost similar QALYs |
| BEL | £75,726 | +£17,255 | 5.551 | –0.014 | Dominated by TAC |
Despite these substantial differences in assumptions and included comparators, in both model-based analyses TAC (immediate release) is found to be the most cost-effective regimen.
Chapter 6 Discussion
Statement of principal findings
Aim
The remit for this report was to review and update the evidence used to inform the current NICE guidance (TA99)1 on the clinical effectiveness and cost-effectiveness of immunosuppressive therapies in renal transplantation in children and adolescents. The systematic review and economic evaluation developed to support current NICE guidance TA99 was published by Yao et al. 2 in 2006. We have incorporated relevant evidence presented in this previous report and report new evidence. This includes a new decision analytic model of kidney transplantation outcomes to investigate which regimen is the most cost-effective option.
In this section, we do not restate the previous evidence, but assume that the discussion is read in the context of the previous evidence summaries and the decisions which followed from them. The conclusions focus on implications of the new clinical effectiveness and cost-effectiveness evidence for service provision.
Clinical effectiveness systematic review
Three RCTs are included in the clinical effectiveness systematic review presented in this report: one new RCT73 and two RCTs from the previous assessment. 75,77
Four non-RCTs are included in our review. 80–83 All of these were also included in the previous assessment by Yao et al. 2006. 2 No new non-randomised studies were identified in our searches.
Induction therapy
Two RCTs of induction therapy (reported in four publications and one abstract) evaluating BAS in children and adolescents were identified in the review. 73,75 No RCTs were identified that evaluated r-ATG in children and adolescents.
No non-RCTs in the child and adolescents population evaluated induction therapies.
We found no significant difference in survival, graft loss, graft function, incidence of BPAR or time to BPAR between BAS and PBO/no induction. 73,75
The results of the current review are similar to those of the previous HTA. 2
Maintenance therapy
Randomised controlled trial evidence
One RCT of maintenance therapy (reported in three publications) evaluating TAC (compared with CSA) in children and adolescents was identified. 77 No RCTs were identified that evaluated TAC-PR, MMF, MPA, SRL, EVL or BEL in children and adolescents.
From the RCT, we found no significant difference in survival or graft loss between TAC and CSA. 77 However, a significantly higher graft function [mean eGFR of 71.5 ml/minute/1.73 m2 (SD 22.9 ml/minute/1.73 m2) in TAC vs. mean eGFR of 53.0 ml/minute/1.73 m2 (SD 21.6 ml/minute/1.73 m2) in CSA; t-test = 4.03; p < 0.01 at 4-year follow-up), and less BPAR (OR = 0.29, favours TAC, 95% CI 0.15 to 0.57 at 6-month follow-up)] was found in TAC compared with AZA at up to 4 years’ follow-up. 77
The results of the current review for survival, graft function and BPAR are similar to those of the previous HTA. 2 However, the RCT child and adolescent evidence identified in the previous HTA review2 concluded that TAC lowered graft loss at 2- and 4-year follow-ups. The difference in these results is because we excluded graft loss due to death from all analyses. This was, first, to avoid double counting with another key outcome (mortality) and, second, because death-censored graft survival is a well-established clinical outcome, to which DWFG is intrinsically related. After the removal of graft loss due to death from the analyses, the evidence from Trompeter et al. 77 suggested a borderline (statistically non-significant) lower graft loss with TAC than CSA (OR = 0.41, favours TAC; 95% CI 0.16 to 1.00; and OR = 0.43, favours TAC; 95% CI 0.18 to 1.01 at 2- and 4-year follow-ups, respectively). In addition, although there were statistically significant treatment group differences in BPAR and AR at 6 months, the annual differences in AR were not statistically significant for years 2, 3 and 4. 77,79
Non-randomised controlled trial evidence
Three non-RCTs evaluating MMF (compared with AZA) in children and adolescents were identified. 81–83 One non-RCT compared TAC + AZA with CSA + MMF. 80 No non-RCTs were identified that evaluated TAC-PR, MPA, SRL, EVL or BEL in children and adolescents.
We found no statistically significant difference in survival between MMF and AZA in the non-RCTs. 81,83 Similarly, no statistically significant difference in BPAR between MMF and AZA in the non-RCTs was identified. 81–83 A significantly lower graft loss was found in MMF than AZA at 1- to 5-year follow-ups in one of the two non-RCTs83 (OR = 0.24 at 5-year follow-up; favours MMF; 95% CI 0.09 to 0.63). However, this was not confirmed by the other non-RCT at 1-year follow-up. 81 In addition, we found no statistically significant difference in survival, graft loss, BPAR, graft function and DGF between TAC + AZA and CSA + MMF in the non-RCTs. 80
Adverse events
Induction
More infections were found in children treated with BAS than in those treated with PBO (OR = 2.23, favours PBO; 95% CI 1.03 to 4.68). 73 In addition, Grenda et al. 75 found that toxic nephropathy and abdominal pain were higher in the BAS arm than no induction (p = 0.03 and p = 0.02, respectively). 75 The previous HTA reported only post-transplant diabetes mellitus,90 the rest of the data they found were confidential and excluded from the report. 2
Maintenance therapy
There were no statistically significant differences between TAC and CSA for a range of AEs (any infections, UTIs, bacterial infections, viral infections, PTLD, solid tumour, hypertension, any AE and NODAT). 77 This is similar to the conclusions of the previous HTA. 2 In addition, there were no statistically significant differences between MMF and AZA for UTI, CMV infections, respiratory infections, herpes simplex, oral thrush and diarrhoea identified in the non-randomised evidence. 81 Similarly, no statistically significant differences between TAC + AZA and CSA + MMF in CMV infections and NODAT were identified in the non-randomised evidence. 80
Previous technology assessment
The previous assessment (TA99) in 20061 found scarce RCT evidence on the clinical effectiveness of immunosuppressive agents in renal transplantation in children and adolescents. Only three child and adolescent RCTs were identified,77,90 including the Wyeth submission 2005. Child and adolescent RCT evidence was identified for TAC,77 BAS90 and SRL (Wyeth submission 2005). Only non-RCT evidence was identified for MMF. 81,83,95 Finally, no child and adolescent evidence was identified for MPS and DAC (since the previous assessment, the marketing authorisation of DAC has been withdrawn at the request of the manufacturer). In addition, three non-RCTs were identified for BAS,91–93 one non-RCT for TAC,94 and one non-RCT compared TAC + AZA with MMF + CSA. 80
The addition of induction therapy (BAS) was not found to be beneficial. The only child and adolescent induction therapy RCT found that the addition of BAS failed to significantly improve BPAR, graft function, graft loss, mortality and AE. Similarly, a meta-analysis of adult RCTs, found no significant difference in graft loss, mortality or AE. In general, compared with a triple regimen of CSA + AZA + CCS, the newer immunosuppressive agents were found to lead to lower rates of BPAR. One included child and adolescent RCT found that TAC led to lower BPAR at 6-month follow-up (RR = 0.42, favours TAC; 95% CI 0.26 to 0.69) and higher eGFR at 1-year follow-up (p = 0.003; 6-month follow-up data were not statistically significantly different) than CSA. This lower rate of BPAR with TAC was also shown in the meta-analysis of six adult RCTs at 1-year follow-up (RR = 0.61, favours TAC; 95% CI 0.53 to 0.71). The total level of withdrawal in children and adolescents was reduced in those receiving TAC compared with CSA (RR = 0.61, favours TAC; 95% CI 0.39 to 0.96). Pooled results of two adult RCTs found that compared with AZA, SRL reduced BPAR (RR = 0.60, favours SRL; 95% CI 0.45 to 0.80), improved eGFR (MD = 28.7, favours SRL; 95% CI 18.8 to 38.5) and increased the level of hyperlipidaemia (RR = 1.57, favours AZA; 95% CI 1.19 to 2.07). 257,258
In summary, important gaps in the evidence concerning the impact of the newer immunosuppressants on AEs, long-term outcomes (including graft loss and survival), growth and overall health-related quality were identified by the previous technology assessment.
Published economic evaluations
Only one previous cost-effectiveness study of immunosuppressive regimens in children and adolescents was identified. 2 It was conducted by the Technology Assessment Group at the University of Birmingham as part of the previous NICE technology appraisal process. The study evaluated the cost-effectiveness of adding BAS induction to CNI maintenance therapy with TAC or CSA combined with AZA and steroids. The study also compared CSA with TAC when given in combination with AZA and steroids, and separately, MMF compared with AZA as part of the triple therapy containing CSA and steroids.
The analysis was conducted using a Markov model of a cohort with starting age ranging between 3 years and 13 years and a 10-year horizon. The study found that BAS induction resulted in higher costs and more QALYs than the alternative of no induction in both the TAC- and CSA-containing regimens. TAC was found to have a base-case ICER (incremental cost per QALY) of £145,000 relative to CSA, while MMF had an ICER of £195,000 relative to AZA when given as part of CSA-containing triple therapy. Although some of the methodological details were not provided in the study report,2 the sensitivity analysis showed that these results were subject to a high degree of uncertainty. In particular, when the costs of dialysis were increased to reflect high possible levels of staff requirements of dialysis treatment in children and adolescents and the estimated treatment effects on AR based on data from adults were used, the ICER for the comparison of TAC compared with CSA triple therapy reduced to £35,000. This uncertainty, and the fact that the underlying model used in this analysis accounted for BPAR only as the surrogate measure of effectiveness (ignoring the role of renal function), suggests that new evidence on the cost-effectiveness of immunosuppressive regimens in children and adolescents is warranted.
Independent economic assessment
The PenTAG economic assessment included two types of analyses.
The first type of analysis used effectiveness estimates only from RCTs in children and adolescents and, therefore, can only evaluate the cost-effectiveness of BAS (vs. no induction) and TAC-IR (vs. CSA).
The second type of analysis extrapolated effectiveness estimates from RCTs in adults and allows for the cost-effectiveness of all interventions to be evaluated. Although effectiveness estimates in these analyses were restricted to adults, a significant amount of evidence from children and adolescents was used, including baseline characteristics, costs, baseline graft and overall survival, and the relationship between graft function and graft survival. The analysis produced different results to those in the parallel HTA for adults to inform an update of NICE guidance TA85.
Neither type of analysis is presented as a preferred base case because both have their deficiencies.
Induction agents
Using effectiveness estimates from randomised controlled trials in children and adolescents
Analyses based on evidence from RCTs in children and adolescents led to contradictory conclusions regarding the cost-effectiveness of BAS versus no induction.
In the analysis based on Grenda et al. ,75 BAS was predicted to be more effective and less costly than no induction (in combination with TAC-IR and AZA) using either a 2-year time horizon (corresponding to the trial follow-up) or 50-year time horizon. BAS was therefore dominant over no induction using a 2- or 50-year time horizon. The probability of BAS being cost-effective at £20,000–30,000 per QALY was 67.3–69.3% (50-year time horizon).
In the analysis based on Offner et al. ,73 BAS was predicted to be more costly and less effective than no induction (in combination with CSA and MMF) using either a 1-year time horizon (corresponding to the trial follow-up) or 50-year time horizon. BAS was therefore dominated by no induction at either time horizon. The probability of BAS being cost-effective at £20,000–30,000 per QALY was 6.7–9.4% (50-year time horizon).
The results of both analyses were robust to scenario analyses in which the surrogate relationship between AR and graft survival was removed, and the ninth centile for body weight for age was used (instead of median weight).
No economic analyses of r-ATG could be conducted based on RCTs in children and adolescents because no such RCTs were identified.
Using effectiveness estimates from randomised controlled trials in adults
Analyses based on evidence from RCTs in the adult population suggested that BAS induction is likely to be cost-effective at £20,000–30,000 per QALY compared with no induction and r-ATG induction.
Depending on the maintenance regimen used, the probability of BAS being cost-effective at £20,000–30,000 per QALY was 67.6–72.8%, while the probability of r-ATG being cost-effective at £20,000–30,000 per QALY was 27.0–32.4%. The probability of no induction being cost-effective at £20,000–30,000 per QALY was 0.0–0.2%.
Results were robust to removal of the surrogate relationship between AR and graft survival and/or assuming ninth centile weight according to age rather than median weight.
Maintenance agents
Using effectiveness estimates from randomised controlled trials in children and adolescents
An analysis based on a RCT in children and adolescents suggested that TAC-IR is likely to be cost-effective at £20,000–30,000 per QALY. In the analysis based on Trompeter et al. ,77 TAC-IR in combination with AZA was predicted to be more effective and less costly than CSA, whether using a 4-year time horizon (corresponding to the trial follow-up) or a 50-year time horizon. The probability of BAS being cost-effective at £20,000–30,000 per QALY was over 99.9% (50-year time horizon).
Results were robust to removal of the surrogate relationship between AR and graft survival, and to assuming ninth centile weight according to age rather than median weight.
No economic analyses of TAC-PR, MMF, MPS, SRL, EVL or BEL could be conducted based on RCTs in children and adolescents because no such RCTs were identified.
Using effectiveness estimates from RCTs in adults
Analyses using effectiveness estimates from RCTs in adults suggested that:
-
TAC-IR is likely to be cost-effective at £20,000–30,000 per QALY (99.3–100.0% of PSA simulations)
-
TAC-PR is unlikely to be cost-effective at £20,000–30,000 per QALY (expected to be dominated by TAC-IR and cost-effective in only 0.2–0.3% of PSA simulations)
-
MMF is likely to be cost-effective at £20,000–30,000 per QALY when used with or without induction and in combination with CSA (cost-effective in 71.1–99.9% of PSA simulations)
-
MMF is unlikely to be cost-effective at £20,000–30,000 per QALY when used with or without induction and in combination with TAC-IR (expected to be dominated by AZA and cost-effective in only 17.8–24.9% of PSA simulations)
-
MPS is unlikely to be cost-effective at £20,000–30,000 per QALY when used in combination with BAS induction and CSA (ICER over £50,000 per QALY and cost-effective in 24.8–28.8% of PSA simulations)
-
SRL is unlikely to be cost-effective at £20,000–30,000 per QALY when used in combination with BAS induction and MMF (expected to be dominated by CSA and TAC-IR and cost-effective in only 0.1% of PSA simulations)
-
SRL is unlikely to be cost-effective at £20,000–30,000 per QALY when used in combination with TAC-IR (expected to be dominated by MMF and AZA and cost-effective in 0.0% of PSA simulations)
-
EVL is unlikely to be cost-effective at £20,000–30,000 per QALY when used in combination with CSA (ICER over £600,000 per QALY and cost-effective in 0.0% of PSA simulations)
-
BEL is unlikely to be cost-effective at £20,000–30,000 per QALY when used in combination with BAS induction and MMF (ICER over £600,000 per QALY and cost-effective in 0.0% of PSA simulations).
If ninth centile weight according to age is assumed (instead of median weight), in the deterministic analysis MPS becomes cost-effective in the deterministic analysis at £30,000 per QALY but not at £20,000 per QALY (ICER £27,000 per QALY). However, the assumed weight–dose relationship may not be accurate (the relationship was assumed to be directly proportional, e.g. patients weighing 50% of median adult weight would require 50% of the average adult dose). In addition, this assumes that kidney transplant patients do not move from the ninth centile of weight.
Results are robust to removal of the surrogate relationship between AR and graft survival, although the deterministic ICER for MPS is lowered to £33,000 per QALY.
Company submissions
The only cost-effectiveness analysis submitted by pharmaceutical companies was that of Astellas, the sponsor of two TAC-IR formulations (Prograf and Modigraf) and TAC-PR (Advagraf). It compared TAC-IR (Prograf) with TAC oral solutions (specials), SRL with MMF (CNI avoidance regimen), SRL with CSA (CNI minimisation regimen), EVL and BEL. TAC-IR was found to have an ICER relative to SRL CNI minimisation of £1,600,000. However, the company concluded that, given the minimal use of SRL in maintenance immunosuppression for kidney transplantations in England and Wales since the publication of the Symphony study, SRL is not a relevant comparator in these countries. As TAC dominated all other regimens it was deemed to be cost-effective. In a separate analysis, TAC-IR (Prograf) was compared with TAC-PR (Advagraf) by modelling the effects of the different adherence profiles between the two regimens on BPAR and, independently, on graft survival. Advagraf was found to result in lower costs and more QALYs than Prograf and was therefore recommended as the cost-effective treatment option.
Although these analyses were set out to meet the specification of the NICE reference case, they are subject to limitations that question the validity of the results and conclusions derived from them. The most important problem is that the model uses efficacy data from RCTs conducted in adult patients. The triple regimen of CSA + MMF + CCS was an important omission from the list of comparators and for which no reason was given in the submission. The unit cost values adopted for the analysis reflect drug list prices as opposed to prices actually paid by hospitals at a discount, as evidenced from eMIT data. Moreover, the drug dosages used for regimens other than MMF and EVL in the cost analysis were derived from those specified by national prescribing guidelines for adults (BNF). In addition, by truncating the analysis at age 18 years, the sensitivity analysis conducted by Astellas based on starting age becomes meaningless. The model ignored important recent evidence about renal graft function as an important outcome for both costs and HRQoL. Further, the Markov model structure used by Astellas was based on annual cycles and assumed that within the first year after transplantation some patients would experience graft failure and retransplantation. Although some patients may experience this in reality, the way the model implemented this effectively assumed that all such patients would experience failure and retransplantation on day 1. This suggests that the cycle length chosen by Astellas inadequately reflected the patient experience that it sought to model. These limitations cast more uncertainty on the results than seems justified by the available data and knowledge of the disease, and suggest that more evidence addressing some of those limitations would benefit NICE recommendations in this area.
Comparison of the Peninsula Technology Assessment Group, Astellas and previous assessment group’s model-based analyses
We attempted to compare and explain the main differences in cost, clinical effectiveness and cost-effectiveness estimates between the three models. In the case of the Astellas analyses this was hampered by the substantial number of important differences in modelling assumptions [such as the much shorter time horizon (10 years) and reliance on data from different trials and different outcome measures from those trials to drive effectiveness differences].
For comparing TAC-IR with TAC-PR, the PenTAG and Astellas analyses arrive at opposite conclusions (the Astellas analysis in favour of TAC-PR). This is primarily because of reliance on BPAR at 12 months post transplant as the main surrogate outcome driving QALY differences, different unit cost sources, and using outcome data from different trials to those on which the PenTAG analysis is based. The other analysis by Astellas, comparing a larger range of maintenance therapies (but omitting CSA), showed that SRL would be the most cost-effective treatment (although its report does not highlight this) whereas the PenTAG analysis shows TAC-IR to be the most cost-effective. However, there is considerable uncertainty and the Astellas analysis is based on very small differences in estimated QALYs.
It was virtually impossible to compare our model-based analyses with those by Yao et al. ,2 which informed NICE’s current guidance on these drugs for children and adolescents (TA99). 1 This is because the Yao et al. 2 model is not fully described in a single report, the model itself is not available and even the results were reported only at the level of incremental costs and QALYs (i.e. no separately reported total costs and QALYs by model comparator). Their cost-effectiveness results also reflect differential discounting of future QALYs (1.5% per year) and costs (6%), and a limited 10-year time horizon. Despite these major differences, the findings in favour of the use of BAS as an induction therapy were similar between the Yao et al. 2 and current PenTAG analyses. In contrast, based on more adult RCT evidence and a 50-year time horizon, the PenTAG analysis found that TAC (with AZA) was more effective and less costly than CSA, and that MMF (with CSA) was more effective and less costly than AZA.
Strengths and limitations
Systematic review of studies of clinical effectiveness
Strengths
-
The systematic review is conducted by an independent research team using the latest evidence.
-
The literature searches were not restricted to child/adolescent populations so as to preserve the sensitivity of the searches and enable identifying RCTs for which mixed populations may have been recruited, but outcomes were reported according to age.
Limitations
-
The number of included RCTs is low; child/adolescent-specific evidence was identified only for BAS and TAC-IR. No RCT evidence from children or adolescents was identified for r-ATG, TAC-PR, MMF, MPS, SRL, EVL and BEL.
-
Databases were searched to identify systematic reviews of non-RCTs; however, individual non-RCTs were not searched for directly. It is likely that some non-RCT comparative evidence was missed. In addition, results from non-randomised studies may differ from RCT evidence. It can be argued that large, prospective and comprehensive case series may achieve high external validity, but we did not search for such studies.
-
There is a possibility of spuriously positive tests for statistical significance arising from conducting multiple tests; we did not formally make adjustments for multiple testing. In addition, owing to a small number of included studies, publication bias were not assessed.
-
For all included studies, less than half of the items constituting the quality appraisal assessment were adequately addressed in the research articles.
-
No studies reporting on quality of life, adherence and growth were identified.
-
No RCTs were found to support the subgroup analyses specified in the review protocol.
In addition, this report highlights some methodological issues. Some of the newer immunosuppressive drugs, such as EVL and SRL, would normally be given to children and adolescents after an initial maintenance therapy that consists of more conventional drugs. This makes it challenging to compare the clinical effectiveness of such regimens as only children and adolescents who are well maintained on their initial maintenance therapy would be given such drugs.
Economic model by the Peninsula Technology Assessment Group
Strengths
-
This is an analysis conducted by an independent academic group, adhering to the NICE reference case when possible.
-
All interventions and relevant allowable comparators are included and evaluated for cost-effectiveness.
-
The natural history of disease is based on UK data, either published by the UK Renal Registry or from new analyses of the UK Transplant Registry standard data set.
-
Important differences in the costs of dialysis between those under 19 years of age and adults have been included.
-
Analyses have been conducted based on all available RCTs in children and adolescents eligible for inclusion.
-
Additional analyses have been conducted based on a systematic review and network meta-analysis of RCTs in the adult population to allow comparison of all interventions even when no relevant RCTs in children and adolescents were identified.
-
The surrogate relationship between graft function (eGFR) at 12 months and graft survival has been estimated from a study of children and adolescents.
-
Pre-emptive retransplantations are included for a minority of KTRs following failure of the initial graft (avoiding dialysis which is costly and reduces HRQoL).
-
Unit costs are those relevant to the NHS (e.g. CMU eMIT costs were used when available).
-
Dosages for those under 18 years of age are based, when possible, on RCTs in children and adolescents, while dosages for those over 18 years of age are estimated from RCTs in adults.
-
Probabilistic sensitivity analyses are presented to reflect the possible impact of parameter uncertainty.
Limitations
-
Graft function has not been modelled over time, but is only estimated at 12 months in order to estimate graft survival thereafter. Reduced graft function can have an impact on HRQoL, but this is a limited effect until graft function is significantly reduced. For most regimens this would slightly reduce total QALYs (owing to reduced utility near the end of the graft life). For non-CNI regimens, it is possible that graft function would be better sustained and these regimens would not suffer a QALY loss and would therefore become more cost-effective (but this would be unlikely to lead to them being cost-effective at £20,000–30,000 per QALY).
-
The cost-effectiveness of reducing or eliminating CCSs has not been evaluated. Some regimens (particularly those with antibody induction) could make it more possible to reduce or eliminate CCSs and the side effects associated with them. Given that significant effort is already invested to minimise maintenance dosage of CCSs, and that the cost of CCSs is minimal, it may be that avoidance would have only a small impact on cost-effectiveness.
-
The cost of NHS-funded transport for haemodialysis patients has not been included. Kerr et al. 57 estimated transport costs of £2792 per year per haemodialysis patient (almost certainly mainly estimated from adults – costs may be higher for children as they may be more likely to be reimbursed and transport for parents is also reimbursed). Including transport costs would improve cost-effectiveness for regimens with better graft survival, as this delays and reduces time on dialysis.
-
Treatment discontinuation and treatment switching are not modelled except in the events of graft failure (treatment discontinuation) and retransplantation (treatment switched to BAS + TAC + MMF regardless of previous treatment). Given the uncertainty about which treatments would be switched it is difficult to predict the effect of this on cost-effectiveness.
-
Independence of AR, NODAT and eGFR at 12 months was assumed when predicting graft survival. It is possible that there would be correlation between these outcomes and that the proportion of patients with particularly unfavourable outcomes at 12 months (e.g. AR, NODAT and low eGFR) is underestimated, and likewise the proportion of patients with favourable 12-month outcomes. The impact of this on cost-effectiveness is uncertain.
-
The surrogate relationships from AR and NODAT to graft survival are based on the adult population. It is not possible to estimate the impact of this on cost-effectiveness.
-
Continuing immunosuppression following graft loss was not modelled, although it may occur in clinical settings. This would lead to slightly increased total costs, particularly for more expensive immunosuppressive agents, and would probably improve cost-effectiveness for less expensive agents.
-
A proportional hazards assumption was made for the graft survival surrogate relationship. As a Weibull model was used, this is also equivalent to an accelerated failure time assumption. Alternative assumptions could have the HR being time dependent. We have not estimated the impact of alternative assumptions for graft survival on cost-effectiveness.
-
No attempt was made to explicitly model adherence to immunosuppressive agents owing to the absence of evidence on this outcome in identified RCTs; it is thought that non-adherence is a significant cause of late AR and graft loss, but any gains in clinical effectiveness owing to improved adherence attributable to any individual agent or regimen are considered speculative. If any regimen robustly demonstrated improved long-term graft survival owing to improved adherence, this would result in improved cost-effectiveness for that regimen.
-
It was assumed that there would be no treatment interactions between induction and maintenance therapies affecting clinical effectiveness outcomes; however, it is known that there is a pharmacokinetic interaction between BAS and MMF that results in prolonged BAS half-life (and similar interactions may exist between other induction and maintenance therapies). It is not possible to estimate the impact of such interactions on cost-effectiveness.
-
Owing to inconsistent reporting of AEs in RCTs included in our systematic review, a limited range of AEs were modelled: NODAT, CMV infection, dyslipidaemia and anaemia (of these, anaemia was assumed not to vary between regimens). Malignancy, PTLD, proteinuria, hypertension, EBV infection, BKV infection, other infections and other AEs were not modelled. In addition, induction agents were assumed not to affect the incidence of AEs. Cost-effectiveness has been overestimated for regimens with increased risk of AEs (as these generally increase costs and lower quality of life).
-
No drug wastage (e.g. part used packs/vials) was assumed for any intervention except BEL; the other agent for which wastage may be likely to occur is r-ATG. The cost-effectiveness of r-ATG may have been somewhat overestimated, but given the uncertainty in dosages in children and adolescents it is unlikely to be very significant.
-
The generalisability of cost-effectiveness results hinges on the generalisability of the clinical effectiveness evidence. Most of the interventions being considered (except BAS and TAC-IR) have not been evaluated in RCTs of children and adolescents, but only in adults.
Areas of uncertainty
This technology assessment was conducted by an independent academic group, builds on existing secondary research and economic evaluations and adheres to the NICE reference case when possible. However, there are some important sources of uncertainty that impact on the conclusions:
-
Most of the interventions being considered (except BAS and TAC-IR) have not been evaluated in published RCTs in children and adolescents.
-
Follow-up in RCTs is limited and, therefore, it has not been possible to externally validate predicted survival differences between regimens.
-
Randomised controlled trials have not provided evidence to support pre-specified subgroup analyses.
-
There was no evidence to support analyses of the cost-effectiveness of interventions for children and adolescents unable to swallow tablets, for whom the following may or may not be appropriate:
-
TAC-IR oral suspension (Modigraf)
-
TAC-IR liquid (from specials manufacturers)
-
CSA solution (Neoral)
-
SRL solution (Rapamune)
-
AZA oral suspension (from specials manufacturers)
-
MMF oral suspension (CellCept).
-
-
The costs for diabetes mellitus are highly uncertain, especially as the costs relate to the general adult diabetic population.
-
It is not known whether or not NHS hospitals might secure discounts from list prices when these were assumed in the model (i.e. for BAS, r-ATG, TAC-PR, MPS, SRL, EVL and BEL).
-
Combinations of immunosuppressive agents other than those considered could be used in clinical practice (the PenTAG model can be extended to include additional combinations).
Chapter 7 Conclusion
Cost-effectiveness estimates for immunosuppressive agents in children and adolescents based on effectiveness estimates in children and adolescents are available only for BAS and TAC-IR. For TAC-IR, the economic analysis based on one RCT77 suggests that TAC-IR is cost-effective (vs. CSA, in combination with AZA) at £20,000–30,000 per QALY. For BAS, the analysis based on one RCT75 found BAS to be dominant, while the analysis based on the other RCT73 found BAS to be dominated.
Consideration of the cost-effectiveness of immunosuppressive agents in children and adolescents by extrapolating effectiveness estimates from the adult population (when there is considerable RCT evidence) suggests that, at a cost-effectiveness threshold of £20,000–30,000 per QALY, BAS and TAC-IR are cost-effective in all considered combinations, while MMF is cost-effective only if used in combination with CSA. BAS induction, TAC-IR and AZA were predicted to be cost-effective at £20,000–30,000 per QALY when all regimens were compared.
Implications for service provision
Basiliximab is used regularly as induction therapy for child/adolescent kidney transplant patients in the NHS, but is not routinely used in all centres. BAS is recommended as an option for induction therapy by current NICE guidance (TA99). 1 Conflicting results from the new economic analyses conducted mean that it is not possible to conclude whether induction with BAS is more or less costly than no induction, but the magnitude of the cost difference is unlikely to be great because induction therapy is administered only at the time of transplantation and is not an ongoing cost.
Rabbit anti-human thymocyte immunoglobulin is not currently used routinely in the NHS and was not considered by current NICE guidance TA99. 1 Economic analyses based on extrapolation from adult effectiveness estimates suggest that induction with r-ATG is more costly than induction with BAS, but less costly than no induction.
For maintenance therapy, TAC-IR is the current standard of care in the NHS and was recommended as an option for maintenance therapy by current NICE guidance TA99. 1 If TAC-PR, SRL or BEL were to be used in place of TAC-IR this would be likely to increase costs. It is also predicted that if CSA were to be used in place of TAC-IR this would lead to increased costs.
Azathioprine and MMF are both widely and routinely used in the NHS, although current NICE guidance (TA99) recommended only MMF as an option for maintenance therapy in a restricted population. 1 Economic analyses based on extrapolation from adult effectiveness estimates suggest that MMF is likely to be more costly than AZA in combination with TAC-IR. These analyses also suggest that replacing AZA or MPS with SRL, EVL or MMF would lead to increased costs.
Belatacept, which is administered intravenously, would be expected to add an extra burden to service providers although, given the limited number of children and adolescents receiving kidney transplantation, the additional burden of drug administration may be able to be accommodated without significant changes to staffing levels.
Suggested research priorities
It is recommended that high-quality primary research into the effectiveness of immunosuppressive agents for kidney transplantation in children and adolescents is conducted. This could be experimental or observational research.
In particular, a prospective study using the UK Renal Registry data set would be beneficial. Such a study would ideally include longitudinal recording of immunosuppression (combination and doses, reflecting changes as soon as they are made), as well as recording AR episodes and regular graft function measurements. A study would also need to ensure that all covariates for effectiveness outcomes (especially potential confounders) were recorded. Such a study could also include HRQoL measurements, preferably using a generic instrument validated in the child and adolescent population such as EQ-5D-Y or Child Health Utility 9 dimensions (CHU9D), and measurements of growth.
In addition, given the perceived importance of adherence to immunosuppression, it may also be desirable to establish an objective and practical measure of adherence so that any differences in adherence between regimens can be identified, as well as any effect this has on outcomes.
Finally, although limitations of non-RCT evidence were noted above, a systematic review of non-RCTs (not limited to search for systematic reviews of non-RCTs) to map all available child and adolescents’ evidence on this topic is needed.
Acknowledgements
We would like to thank Fiona Gamston, Renal Transplant Sister Birmingham Children’s Hospital; Dr Paul Tappenden, Deputy Technical Director, ScHARR Technology Assessment Group; Dr Jaime Peters, Research Fellow in Health Economic Modelling, PenTAG, University of Exeter; Martin Hoyle, Associate Professor in Health Economics, PenTAG, University of Exeter; and Mr Jacob Akoh, Consultant General and Transplant Surgeon, Plymouth Hospitals NHS Trust.
We would also like to acknowledge the help of Andy Salmon, for model checking, and Sue Whiffin for her administrative support, both from the University of Exeter Medical School.
Contributions of authors
Marcela Haasova provided overall project management and led the systematic review of clinical effectiveness, including assessment of all abstracts and titles for possible inclusion and meta-analysis for clinical effectiveness outcomes. Drafted or edited all sections of the report.
Tristan Snowsill led the design, development and execution of the economic model and wrote the sections on the design and results of the economic model. Contributed to the critique of the submission from Astellas and to the writing of the general discussion and conclusions.
Tracey Jones-Hughes assessed abstracts and titles for inclusion and contributed to the writing and editing of the report.
Louise Crathorne assessed titles and abstracts for inclusion in the effectiveness and cost-effectiveness review. Contributed to writing and editing of the cost-effectiveness systematic review.
Chris Cooper led the literature searching and contributed to writing and editing the report.
Jo Varley-Campbell assessed abstracts and titles for inclusion and contributed to the writing and editing of the report.
Ruben Mujica-Mota led the systematic review of economic evaluations and provided advice on design of the model.
Helen Coelho assessed titles and abstracts for inclusion and exclusion. Contributed to writing and editing the report.
Nicola Huxley assisted with identification of model parameters and contributed to writing and editing of the report.
Jenny Lowe critiqued and wrote summaries of the literature searches for the company submissions.
Jan Dudley provided clinical input into the design of the model, and advised on clinical matters.
Stephen Marks provided clinical input into the design of the model and advised on clinical matters.
Chris Hyde extracted data for inclusion in the clinical effectiveness systematic review.
Mary Bond had oversight of project management and the clinical effectiveness systematic review and contributed to the editing of the report.
Rob Anderson contributed to the interpretation and comparison of cost-effectiveness results and the writing and editing of the report. Overall director of the project and guarantor of the report.
Data sharing statement
This is a systematic review; therefore, there are no primary data to share. Further information can be obtained from the lead author if needed.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Immunosuppressive Therapy for Renal Transplantation in Children and Adolescents; NICE Technology Appraisal Guidance 99. London: NICE; 2006.
- Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al. A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10490.
- Camilla R, Magnetti F, Barbera C, Bignamini E, Riggi C, Coppo R, et al. Children with chronic organ failure possibly ending in organ transplantation: a survey in an Italian region of 5,000,000 inhabitants. Acta Paediatr 2008;97:1285-91. http://dx.doi.org/10.1111/j.1651-2227.2008.00854.x.
- Pruthi R, Hamilton AJ, O’Brien C, Casula A, Braddon F, Inward C, et al. UK Renal Registry 17th Annual Report: Chapter 4 Demography of the UK Paediatric Renal Replacement Therapy Population in 2013. Nephron 2015;129:87-98. http://dx.doi.org/10.1159/000370274.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group . KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9:S1-157. http://dx.doi.org/10.1111/j.1600-6143.2009.02834.x.
- Final Scope: Immunosuppressive Therapy for Kidney Transplantation in Adults (Review of Technology Appraisal Guidance 85). London: NICE; 2014.
- Final Scope: Immunosuppressive Therapy for Kidney Transplantation in Children and Adolescents (Review of Technology Appraisal Guidance 99). London: NICE; 2014.
- Fine RN. Management of growth retardation in pediatric recipients of renal allografts. Nat Clin Pract Nephrol 2007;3:318-24. http://dx.doi.org/10.1038/ncpneph0502.
- Salas P, Pinto V, Rodriguez J, Zambrano MJ, Mericq V. Growth retardation in children with kidney disease. Int J Endocrinol 2013;2013. http://dx.doi.org/10.1155/2013/970946.
- Mericq V, Salas P, Pinto V, Cano F, Reyes L, Brown K, et al. Steroid withdrawal in pediatric kidney transplant allows better growth, lipids and body composition: a randomized controlled trial. Horm Res Paediatr 2013;79:88-96. http://dx.doi.org/10.1159/000347024.
- The National Service Framework for Renal Services. Part One: Dialysis and Transplantation. London: Department of Health; 2004.
- Berry J, Sohrabi F. Overview of Renal Failure in Children. Health Encyclopaedia: University of Rochester Medical Centre; n.d.
- Bhowmik DM, Dinda AK, Mahanta P, Agarwal SK. The evolution of the Banff classification schema for diagnosing renal allograft rejection and its implications for clinicians. Indian J Nephrol 2010;20:2-8. http://dx.doi.org/10.4103/0971-4065.62086.
- Tushla L. When A Transplant Fails. National Kidney Foundation n.d. www.kidney.org/transplantation/transaction/TC/summer09/TCsm09_TransplantFails (accessed 9 February 2015).
- Butler JA, Peveler RC, Roderick P, Smith PWF, Horne R, Mason JC. Modifiable risk factors for non-adherence to immunosuppressants in renal transplant recipients: a cross-sectional study. Nephrol Dial Transplant 2004;19:3144-9. http://dx.doi.org/10.1093/ndt/gfh505.
- Chronic Kidney Disease in Adults: Assessment and Management. London: NICE; 2014.
- Whyte DA, Fine RN. Chronic kidney disease in children. Pediatr Rev 2008;29:335-41. http://dx.doi.org/10.1542/pir.29-10-335.
- Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R. The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13380.
- Oostdijk W, Grote FK, de Muinck Keizer-Schrama SM, Wit JM. Diagnostic approach in children with short stature. Horm Res 2009;72:206-17. http://dx.doi.org/10.1159/000236082.
- Harambat J, Cochat P. Growth after renal transplantation. Pediatr Nephrol 2009;24:1297-306. http://dx.doi.org/10.1007/s00467-008-0787-0.
- Tejani A, Fine R, Alexander S, Harmon W, Stablein D. Factors predictive of sustained growth in children after renal transplantation. The North American Pediatric Renal Transplant Cooperative Study. J Pediatr 1993;122:397-402. http://dx.doi.org/10.1016/S0022-3476(05)83423-7.
- Jabs K, Sullivan EK, Avner ED, Harmon WE. Alternate-day steroid dosing improves growth without adversely affecting graft survival or long-term graft function. A report of the North American Pediatric Renal Transplant Cooperative Study. Transplantation 1996;61:31-6. http://dx.doi.org/10.1097/00007890-199601150-00008.
- Sarwal MM, Yorgin PD, Alexander S, Millan MT, Belson A, Belanger N, et al. Promising early outcomes with a novel, complete steroid avoidance immunosuppression protocol in pediatric renal transplantation. Transplantation 2001;72:13-21. http://dx.doi.org/10.1097/00007890-200107150-00006.
- Grenda R, Watson A, Trompeter R, Tonshoff B, Jaray J, Fitzpatrick M, et al. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. Am J Transplant 2010;10:828-36. http://dx.doi.org/10.1111/j.1600-6143.2010.03047.x.
- North American Pediatric Renal Trials and Collaborative Studies . NAPRTCS 2010 Annual Transplant Report 2010. https://web.emmes.com/study/ped/annlrept/2010_Report.pdf (accessed 9 February 2015).
- Nissel R, Brazda I, Feneberg R, Wigger M, Greiner C, Querfeld U, et al. Effect of renal transplantation in childhood on longitudinal growth and adult height. Kidney Int 2004;66:792-800. http://dx.doi.org/10.1111/j.1523-1755.2004.00805.x.
- Farrugia D, Cheshire J, Mahboob S, Begaj I, Khosla S, Ray D, et al. Mortality after pediatric kidney transplantation in England – a population-based cohort study. Pediatr Transplant 2014;18:16-22. http://dx.doi.org/10.1111/petr.12173.
- Chavers B, Najarian JS, Humar A. Kidney transplantation in infants and small children. Pediatr Transplant 2007;11:702-8. http://dx.doi.org/10.1111/j.1399-3046.2007.00768.x.
- Gordon EJ, Ladner DP, Caicedo JC, Franklin J. Disparities in kidney transplant outcomes: a review. Semin Nephrol 2010;30:81-9. http://dx.doi.org/10.1016/j.semnephrol.2009.10.009.
- Patzer RE, Mohan S, Kutner N, McClellan WM, Amaral S. Racial and ethnic disparities in pediatric renal allograft survival in the United States. Kidney Int 2015;87:584-92. http://dx.doi.org/10.1038/ki.2014.345.
- Meier-Kriesche HU, Schold JD, Srinivas TR, Howard RJ, Fujita S, Kaplan B. Sirolimus in combination with tacrolimus is associated with worse renal allograft survival compared to mycophenolate mofetil combined with tacrolimus. Am J Transplant 2005;5:2273-80. http://dx.doi.org/10.1111/j.1600-6143.2005.01019.x.
- Annual Report On Kidney Transplantation, Report for 2013/2014. London: NHS; 2014.
- Metzger RA, Delmonico FL, Feng S, Port FK, Wynne JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant 2003;3:114-25. http://dx.doi.org/10.1034/j.1600-6143.3.s4.11.x.
- Wu FL, Tsai MK, Chen RR, Sun SW, Huang JD, Hu RH, et al. Effects of calcineurin inhibitors on sirolimus pharmacokinetics during staggered administration in renal transplant recipients. Pharmacotherapy 2005;25:646-53. http://dx.doi.org/10.1592/phco.25.5.646.63593.
- Assadi F. Psychological impact of chronic kidney disease among children and adolescents: not rare and not benign. J Nephropathol 2013;2:1-3. http://dx.doi.org/10.5812/nephropathol.8968.
- Nicholas DB, Picone G, Selkirk EK. The lived experiences of children and adolescents with end-stage renal disease. Qual Health Res 2011;21:162-73. http://dx.doi.org/10.1177/1049732310382789.
- Orr A, Willis S, Holmes M, Britton P, Orr D. Living with a kidney transplant – a qualitative investigation of quality of life. J Health Psychol 2007;12:653-62. http://dx.doi.org/10.1177/1359105307078172.
- Morel P, Almond PS, Matas AJ, Gillingham KJ, Chau C, Brown A, et al. Long-term quality-of-life after kidney-transplantation in childhood. Transplantation 1991;52:47-53. http://dx.doi.org/10.1097/00007890-199107000-00010.
- Dobbels F, Van Damme-Lombaert R, Vanhaecke J, De Geest S. Growing pains: non-adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatr Transplant 2005;9:381-90. http://dx.doi.org/10.1111/j.1399-3046.2005.00356.x.
- Bartosh SM, Ryckman FC, Shaddy R, Michaels MG, Platt JL, Sweet SC. A national conference to determine research priorities in pediatric solid organ transplantation. Pediatr Transplant 2008;12:153-66. http://dx.doi.org/10.1111/j.1399-3046.2007.00811.x.
- Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant 2009;9:2597-606. http://dx.doi.org/10.1111/j.1600-6143.2009.02798.x.
- Dialysis – Risks and Side Effects. NHS Choices. n.d.
- Furth SL, Hwang W, Yang C, Neu AM, Fivush BA, Powe NR. Growth failure, risk of hospitalization and death for children with end-stage renal disease. Pediatr Nephrol 2002;17:450-5. http://dx.doi.org/10.1007/s00467-002-0838-x.
- Qvist E, Jalanko H, Holmberg C. Psychosocial adaptation after solid organ transplantation in children. Pediatr Clin North Am 2003;50:1505-19. http://dx.doi.org/10.1016/S0031-3955(03)00128-7.
- Broyer M, Le Bihan C, Charbit M, Guest G, Tete MJ, Gagnadoux MF, et al. Long-term social outcome of children after kidney transplantation. Transplantation 2004;77:1033-7. http://dx.doi.org/10.1097/01.TP.0000120947.75697.8B.
- Apostolou T, Hutchison AJ, Boulton AJM, Chak W, Vileikyte L, Uttley L, et al. Quality of life in CAPD, transplant, and chronic renal failure patients with diabetes. Renal Failure 2007;29:189-97. http://dx.doi.org/10.1080/08860220601098862.
- Balaska A, Moustafellos P, Gourgiotis S, Pistolas D, Hadjiyannakis E, Vougas V, et al. Changes in health-related quality of life in Greek adult patients 1 year after successful renal transplantation. Exp Clin Transplant 2006;4:521-4.
- Dale PL, Hutton J, Elgazzar H. Utility of health states in chronic kidney disease: a structured review of the literature. Curr Med Res Opin 2008;24:193-206. http://dx.doi.org/10.1185/030079908X253410.
- Nourbala MH, Hollisaaz MT, Nasiri M, Bahaeloo-Horeh S, Najafi M, Araghizadeh H, et al. Pain affects health-related quality of life in kidney transplant recipients. Transplant Proc 2007;39:1126-9. http://dx.doi.org/10.1016/j.transproceed.2007.03.004.
- Seedat YK, Macintosh CG, Subban JV. Quality-of-life for patients in an end-stage renal-disease program. S Afr Med J 1987;71:500-4.
- Sureshkumar KK, Patel BM, Markatos A, Nghiem DD, Marcus RJ. Quality of life after organ transplantation in type 1 diabetics with end-stage renal disease. Clin Transplant 2006;20:19-25. http://dx.doi.org/10.1111/j.1399-0012.2005.00433.x.
- Overbeck I, Bartels M, Decker O, Harms J, Hauss J, Fangmann J. Changes in quality of life after renal transplantation. Transplant Proc 2005;37:1618-21. http://dx.doi.org/10.1016/j.transproceed.2004.09.019.
- Kidney Disease: Key Facts and Figures. London: NHS Kidney Care; 2010.
- de Wit GA, Ramsteijn PG, Charro FT. Economic evaluation of end stage renal disease treatment. Health Policy 1998;44:215-32. http://dx.doi.org/10.1016/S0168-8510(98)00017-7.
- Su XM, Zenios SA, Chakkera H, Milford EL, Chertow GM. Diminishing significance of HLA matching in kidney transplantation. Am J Transplant 2004;4:1501-8. http://dx.doi.org/10.1111/j.1600-6143.2004.00535.x.
- Working Party of The British Transplantation Society . United Kingdom Guidelines: Management of The Failing Kidney Transplant. The British Transplantation Society 2013. www.bts.org.uk/Documents/Guidelines/Active/Failing%20Graft%20Guideline%20-%20Draft%20-%20under%20consultation%20until%2030%20May%202014.pdf (accessed 9 February 2015).
- Kerr M, Bray B, Medcalf J, O’Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012;27:73-80. http://dx.doi.org/10.1093/ndt/gfs269.
- NHS Reference Costs 2013 to 2014. London: Department of Health; 2014.
- Baker R, Jardine A, Andrews P. Renal association clinical practice guideline on post-operative care of the kidney transplant recipient. Nephron Clinical Practice 2011;118:C311-47. http://dx.doi.org/10.1159/000328074.
- Chamberlain G, Baboolal K, Bennett H, Pockett RD, McEwan P, Sabater J, et al. The Economic Burden of Posttransplant Events in Renal Transplant Recipients in Europe. Transplantation 2014;97:854-61. http://dx.doi.org/10.1097/01.tp.0000438205.04348.69.
- Boyd E. Surface Area of the Human Body. Minneapolis, MN: University of Minnesota Press; 1935.
- eMIT National Database (2014/06). Drugs and Pharmaceutical Electronic Market Information (eMIT). London: Department of Health; 2014.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- Drugs.com . Cyclosporine Dosage Guide With Precautions - Drugs.Com 2015. www.drugs.com/dosage/cyclosporine.html (accessed 25 February 2015).
- Drugs.com . Anti-Thymocyte Globulin (Rabbit) Dosage Guide With Precautions – Drugs.Com 2015. www.drugs.com/dosage/anti-thymocyte-globulin-rabbit.html#Usual_Paediatric_Dose_for_Renal_Transplant (accessed 25 February 2015).
- Bristol Myers Squibb . Immunosuppressive Therapy for Kidney Transplantation in Adults (review of Technology Appraisal Guidance 85); Belatacept Submission of Evidence 2014.
- Protocol: Immunosuppressive Therapy for Kidney Transplantation in Children and Adolescents (Review of Technology Appraisal Guidance TA99). London: NICE; 2014.
- Jones-Hughes T, Snowsill T, Haasova M, Coelho H, Crathorne L, Cooper C, et al. Immunosuppressive therapy for kidney transplantation in adults (review of technology appraisal guidance 85); a systematic review and economic model. Health Technol Assess 2016.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Healthcare. York: Centre for Reviews and Dissemination, University of York; 2009.
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7. http://dx.doi.org/10.3310/hta7270.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
- Jungraithmayr TC, Grossmann A, Cochat P, Doetsch J, Gschaidmair H, Hoecker B, et al. Long-term results after induction therapy with Basiliximab in pediatric renal transplantation. Pediatr Transplant 2009;13.
- Offner G, Toenshoff B, Höcker B, Krauss M, Bulla M, Cochat P, et al. Efficacy and safety of basiliximab in pediatric renal transplant patients receiving cyclosporine, mycophenolate mofetil, and steroids. Transplantation 2008;86:1241-8. http://dx.doi.org/10.1097/TP.0b013e318188af15.
- Höcker B, Kovarik JM, Daniel V, Opelz G, Fehrenbach H, Holder M, et al. Pharmacokinetics and immunodynamics of basiliximab in pediatric renal transplant recipients on mycophenolate mofetil comedication. Transplantation 2008;86:1234-40. http://dx.doi.org/10.1097/TP.0b013e318188ae18.
- Grenda R, Watson A, Vondrak K, Webb NJ, Beattie J, Fitzpatrick M, et al. A prospective, randomized, multicenter trial of tacrolimus-based therapy with or without basiliximab in pediatric renal transplantation. Am J Transplant 2006;6:1666-72. http://dx.doi.org/10.1111/j.1600-6143.2006.01367.x.
- Webb NJ, Prokurat S, Vondrak K, Watson AR, Hughes DA, Marks SD, et al. Multicentre prospective randomised trial of tacrolimus, azathioprine and prednisolone with or without basiliximab: two-year follow-up data. Pediatric Nephrology 2009;24:177-82. http://dx.doi.org/10.1007/s00467-008-0931-x.
- Trompeter R, Filler G, Webb NJA, Watson AR, Milford DV, Tyden G, et al. Randomized trial of tracolimus versus cyclosporin microemulsion in renal transplantation. Pediatric Nephrology 2002;17:141-9. http://dx.doi.org/10.1007/s00467-001-0795-9.
- Filler G, Trompeter R, Webb NJ, Watson AR, Milford DV, Tyden G, et al. One-year glomerular filtration rate predicts graft survival in pediatric renal recipients: a randomized trial of tacrolimus vs cyclosporine microemulsion. Transplant Proc 2002;34:1935-8. http://dx.doi.org/10.1016/S0041-1345(02)03128-7.
- Filler G, Webb NJ, Milford DV, Watson AR, Gellermann J, Tyden G, et al. Four-year data after pediatric renal transplantation: a randomized trial of tacrolimus vs. cyclosporin microemulsion. Pediatr Transplant 2005;9:498-503. http://dx.doi.org/10.1111/j.1399-3046.2005.00334.x.
- Garcia CD, Schneider L, Barros VR, Guimaraes PC, Garcia VD. Pediatric renal transplantation under tacrolimus or cyclosporine immunosuppression and basiliximab induction. Transplant Proc 2002;34:2533-4. http://dx.doi.org/10.1016/S0041-1345(02)03475-9.
- Antoniadis A, Papachristou F, Gakis D, Takoudas D, Sotiriou I. Comparison between mycophenolate mofetil and azathioprine based immunosuppression in pediatric renal transplantation from living related donors. Transplant Proc 1998;30:4085-6. http://dx.doi.org/10.1016/S0041-1345(98)01350-5.
- Benfield MR, Symons JM, Bynon S, Eckhoff D, Herrin J, Harmon W, et al. Mycophenolate mofetil in pediatric renal transplantation. Pediatr Transplant 1999;3:33-7. http://dx.doi.org/10.1034/j.1399-3046.1999.00003.x.
- Staskewitz A, Kirste G, Tonshoff B, Weber LT, Boswald M, Burghard R, et al. Mycophenolate mofetil in pediatric renal transplantation without induction therapy: results after 12 months of treatment. German Pediatric Renal Transplantation Study Group. Transplantation 2001;71:638-44. http://dx.doi.org/10.1097/00007890-200103150-00010.
- Jungraithmayr T, Staskewitz A, Kirste G, Boswald M, Bulla M, Burghard R, et al. Pediatric renal transplantation with mycophenolate mofetil-based immunosuppression without induction: results after three years. Transplantation 2003;75:454-61. http://dx.doi.org/10.1097/01.TP.0000045748.95874.64.
- Jungraithmayr TC, Wiesmayr S, Staskewitz A, Kirste G, Bulla M, Fehrenbach H, et al. Five-year outcome in pediatric patients with mycophenolate mofetil-based renal transplantation. Transplantation 2007;83:900-5. http://dx.doi.org/10.1097/01.tp.0000258587.70166.87.
- Gupta D. Design of a randomized study evaluating everolimus in pediatric renal transplant recipients. Transplant International 2013;26.
- Langer RM, Pape L, Tonshoff B, Dello Strologo L, Ettenger R, Niaudet P, et al. Evaluation of safety and efficacy of everolimus with reduced tacrolimus: design of a randomized, multicenter, open-label study in pediatric renal transplant recipients. Pediatr Transplant 2013;17.
- Tonshoff B, Pape L, Strologo LD, Ettenger R, Niaudet P, Martzloff ED, et al. Design of crad001a2314: a randomised study evaluating everolimus in paediatric renal transplantation. Transplant International 2013;26.
- Tonshoff B, Pape L, Ettenger R, Dello Strologo L, Niaudet P, Martzloff D, et al. Early conversion of calcineurin inhibitor to everolimus in de novo paediatric renal transplant recipients and its impact on efficacy and renal function; design of an open-label, randomised, multi-centre study. Transplantation 2012;94. http://dx.doi.org/10.1097/00007890-201211271-02397.
- Grenda R, Watson A, Vondrak K, Webb NJ, Beattie J. Tacrolimus Triple Therapy With or Without Monoclonal Antibody Administration: A Multicentre, Randomised Study in Paediatric Kidney Transplantation n.d.
- Duzova A, Buyan N, Bakkaloglu M, Dalgic A, Soylemezoglu O, Besbas N, et al. Triple immunosuppression with or without basiliximab in pediatric renal transplantation: acute rejection rates at one year. Transplant Proc 2003;35:2878-80. http://dx.doi.org/10.1016/j.transproceed.2003.10.087.
- Pape L, Strehlau J, Henne T, Latta K, Nashan B, Ehrich JH, et al. Single centre experience with basiliximab in paediatric renal transplantation. Nephrol Dial Transplant 2002;17:276-80. http://dx.doi.org/10.1093/ndt/17.2.276.
- Swiatecka-Urban A, Garcia C, Feuerstein D, Suzuki S, Devarajan P, Schechner R, et al. Basiliximab induction improves the outcome of renal transplants in children and adolescents. Pediatr Nephrol 2001;16:693-6. http://dx.doi.org/10.1007/s004670100642.
- Neu AM, Ho PLM, Fine RN, Furth SL, Fivush BA. Tacrolimus vs. cyclosporine A as primary immunosuppression in pediatric renal transplantation: a NAPRTCS study. Pediatr Transplant 2003;7:217-22. http://dx.doi.org/10.1034/j.1399-3046.2003.00079.x.
- Steffen B, Gotz V, Chu A, Gordon R, Morris J. Mycophenolate mofetil (MMF) versus azathioprine (AZA) in a large registry of pediatric renal transplant patients. J Am Soc Nephrol 2003;14.
- Statistical Methodology and Risk-adjustment for Survival Rate Estimation. Hertfordshire: NHS Blood and Transplant; 2011.
- Albano L, Banas B, Klempnauer JL, Glyda M, Viklicky O, Kamar N. OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation 2013;96:897-903. http://dx.doi.org/10.1097/TP.0b013e3182a203bd.
- Kyllönen LE, Eklund BH, Pesonen EJ, Salmela KT. Single bolus antithymocyte globulin versus basiliximab induction in kidney transplantation with cyclosporine triple immunosuppression: efficacy and safety. Transplantation 2007;84:75-82. http://dx.doi.org/10.1097/01.tp.0000268084.64888.f3.
- Sheashaa HA, Bakr MA, Ismail AM, Sobh MA, Ghoneim MA. Basiliximab reduces the incidence of acute cellular rejection in live-related-donor kidney transplantation: a three-year prospective randomized trial. J Nephrol 2003;16:393-8.
- Kahan BD, Rajagopalan PR, Hall M, Grp USRS. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti-interleukin-2-receptor monoclonal antibody. Transplantation 1999;67:276-84. http://dx.doi.org/10.1097/00007890-199901270-00016.
- Nashan B, Moore R, Amlot P, Schmidt AG, Abeywickrama K, Soulillou JP. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. Lancet 1997;350:1193-8. http://dx.doi.org/10.1016/S0140-6736(97)09278-7.
- Ponticelli C, Yussim A, Cambi V, Legendre C, Rizzo G, Salvadori M, et al. A randomized, double-blind trial of basiliximab immunoprophylaxis plus triple therapy in kidney transplant recipients. Transplantation 2001;72:1261-7. http://dx.doi.org/10.1097/00007890-200110150-00014.
- Lawen JG, Davies EA, Mourad G, Oppenheimer F, Molina MG, Rostaing L, et al. Randomized double-blind study of immunoprophylaxis with basiliekimab, a chimeric anti-interleukin-2 receptor monoclonal antibody, in combination with mycophenolate mofetil-containing triple therapy in renal transplantation. Transplantation 2003;75:37-43. http://dx.doi.org/10.1097/00007890-200301150-00007.
- Bingyi S, Yeyong Q, Ming C, Chunbai M, Wenqiang Z. Randomised trial of simulect versus placebo for control of acute rejection in renal allograft recipients. Transplant Proc 2003;35:192-4. http://dx.doi.org/10.1016/S0041-1345(02)03769-7.
- Schleibner S, Krauss M, Wagner K, Erhard J, Christiaans M, van Hooff J, et al. FK 506 versus cyclosporin in the prevention of renal allograft rejection--European pilot study: six-week results. Transpl Int 1995;8:86-90.
- Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation 1996;62:900-5. http://dx.doi.org/10.1097/00007890-199610150-00005.
- Mayer AD, Dmitrewski J, Squifflet JP, Besse T, Grabensee B, Klein B, et al. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation 1997;64:436-43. http://dx.doi.org/10.1097/00007890-199708150-00012.
- Jarzembowski T, Panaro F, Raofi V, Dong G, Testa G, Sankary H, et al. Long-term results of a prospective randomized trial comparing tacrolimus versus cyclosporine in African-American recipients of primary cadaver renal transplant. Transplant Int 2005;18:419-22. http://dx.doi.org/10.1111/j.1432-2277.2004.00055.x.
- Campos HH, Abbud Filho M. One-year follow-up of a Brazilian randomized multicenter study comparing tacrolimus versus cyclosporine in kidney transplantation. Transplant Proc 2002;34:1656-8. http://dx.doi.org/10.1016/S0041-1345(02)02968-8.
- Margreiter R. Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: a randomised multicentre study. Lancet 2002;359:741-6. http://dx.doi.org/10.1016/S0140-6736(02)07875-3.
- Waller JR, Murphy GJ, Metcalfe MS, Sandford RM, Pattenden CJ, Nicholson ML. Primary immunosuppression with tacrolimus is associated with a reduction in renal allograft fibrosis compared with neoral therapy. Transplant Proc 2002;34:1587-8. http://dx.doi.org/10.1016/S0041-1345(02)03033-6.
- Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, et al. A three-arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation 2003;75:844-51. http://dx.doi.org/10.1097/01.TP.0000056635.59888.EF.
- Hardinger KL, Bohl DL, Schnitzler MA, Lockwood M, Storch GA, Brennan DC. A randomized, prospective, pharmacoeconomic trial of tacrolimus versus cyclosporine in combination with thymoglobulin in renal transplant recipients. Transplantation 2005;80:41-6. http://dx.doi.org/10.1097/01.TP.0000162980.68628.5A.
- Weimer R, Susal C, Yildiz S, Staak A, Pelzl S, Renner F, et al. Post-transplant sCD30 and neopterin as predictors of chronic allograft nephropathy: impact of different immunosuppressive regimens. Am J Transplant 2006;6:1865-74. http://dx.doi.org/10.1111/j.1600-6143.2006.01407.x.
- Tricontinental MMF renal study . A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation 1996;61:1029-37. http://dx.doi.org/10.1097/00007890-199604150-00008.
- Sadek S, Medina J, Arias M, Sennesael J, Squifflet JP, Vogt B. Short–term combination of mycophenolate mofetil with cyclosporine as a therapeutic option for renal transplant recipients: a prospective, multicenter, randomized study. Transplantation 2002;74:511-7. http://dx.doi.org/10.1097/00007890-200208270-00013.
- Merville P, Berge F, Deminiere C, Morel D, Chong G, Durand D, et al. Lower incidence of chronic allograft nephropathy at 1 year post-transplantation in patients treated with mycophenolate mofetil. Am J Transplant 2004;4:1769-75. http://dx.doi.org/10.1111/j.1600-6143.2004.00533.x.
- Tuncer M, Gürkan A, Erdogan O, Demirba A, Süleymanlar G, Ersoy FF, et al. Mycophenolate mofetil in renal transplantation: five years experience. Transplant Proc 2002;34:2087-8. http://dx.doi.org/10.1016/S0041-1345(02)02861-0.
- Remuzzi G, Cravedi P, Costantini M, Lesti M, Ganeva M, Gherardi G, et al. Mycophenolate mofetil versus azathioprine for prevention of chronic allograft dysfunction in renal transplantation: the MYSS follow-up randomized, controlled clinical trial. J Am Soc Nephrol 2007;18:1973-85. http://dx.doi.org/10.1681/ASN.2006101153.
- Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation 1995;60:225-32. http://dx.doi.org/10.1097/00007890-199508000-00003.
- Baboolal K, Jones GA, Janezic A, Griffiths DR, Jurewicz WA. Molecular and structural consequences of early renal allograft injury. Kidney Int 2002;61:686-96. http://dx.doi.org/10.1046/j.1523-1755.2002.00149.x.
- Waller J, Murphy G, Bicknell G, Sandford R, Nicholson M. Primary immunosuppression with tacrolimus is associated with reduction in renal allograft fibrosis compared with neoral therapy. Nephrol Dial Transplant 2002;17:177-8. http://dx.doi.org/10.1016/s0041-1345(02)03033-6.
- van Duijnhoven EM, Christiaans MH, Boots JM, Nieman FH, Wolffenbuttel BH, van Hooff JP. Glucose metabolism in the first 3 years after renal transplantation in patients receiving tacrolimus versus cyclosporine-based immunosuppression. J Am Soc Nephrol 2002;13:213-20.
- Radermacher J, Meiners M, Bramlage C, Kliem V, Behrend M, Schlitt HJ, et al. Pronounced renal vasoconstriction and systemic hypertension in renal transplant patients treated with cyclosporin A versus FK 506. Transpl Int 1998;11:3-10. http://dx.doi.org/10.1111/j.1432-2277.1998.tb00948.x.
- Yang HC, Holman MJ, Langhoff E, Ulsh PJ, Dellock CA, Gupta M, et al. Tacrolimus/‘low-dose’ mycophenolate mofetil versus microemulsion cyclosporine/‘low-dose’ mycophenolate mofetil after kidney transplantation–1-year follow-up of a prospective, randomized clinical trial. Transplant Proc 1999;31:1121-4. http://dx.doi.org/10.1016/S0041-1345(98)01929-0.
- Chen KH, Tsai MK, Lai IR, Lin Wu FL, Hu RH, Lee PH. Favorable results of concomitant tacrolimus and sirolimus therapy in Taiwanese renal transplant recipients at 12 months. J Formos Med Assoc 2008;107:533-9. http://dx.doi.org/10.1016/S0929-6646(08)60166-7.
- Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007;357:2562-75. http://dx.doi.org/10.1056/NEJMoa067411.
- Glotz D, Charpentier B, Abramovicz D, Lang P, Rostaing L, Rifle G, et al. Thymoglobulin induction and sirolimus versus tacrolimus in kidney transplant recipients receiving mycophenolate mofetil and steroids. Transplantation 2010;89:1511-7. http://dx.doi.org/10.1097/TP.0b013e3181db09e4.
- Ferguson R, Grinyó J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, et al. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant 2011;11:66-7. http://dx.doi.org/10.1111/j.1600-6143.2010.03338.x.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Philips Z, Ginelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics 2006;24:355-71. http://dx.doi.org/10.2165/00019053-200624040-00006.
- Neri L, McEwan P, Sennfalt K, Baboolal K. Characterizing the relationship between health utility and renal function after kidney transplantation in UK and US: a cross-sectional study. Health Qual Life Outcomes 2012;10. http://dx.doi.org/10.1186/1477-7525-10-139.
- Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 2012;12:1157-67. http://dx.doi.org/10.1111/j.1600-6143.2012.04013.x.
- Kuypers DR, Peeters PC, Sennesael JJ, Kianda MN, Vrijens B, Kristanto P, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation 2013;95:333-40. http://dx.doi.org/10.1097/TP.0b013e3182725532.
- Wu MJ, Cheng CY, Chen CH, Wu WP, Cheng CH, Yu DM, et al. Lower Variability of Tacrolimus Trough Concentration After Conversion From Prograf to Advagraf in Stable Kidney Transplant Recipients. Transplantation 2011;92:648-52. http://dx.doi.org/10.1097/TP.0b013e3182292426.
- Borra LC RJ, Kal JA, Mathot RA, Weimar W, van Gelder T. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant 2010;25:2757-63. http://dx.doi.org/10.1093/ndt/gfq096.
- Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 2003;3:178-85. http://dx.doi.org/10.1034/j.1600-6143.2003.00010.x.
- Krämer BK, Charpentier B, Bäckman L, Silva HT, Mondragon-Ramirez G, Cassuto-Viguier E, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant 2010;10:2632-43. http://dx.doi.org/10.1111/j.1600-6143.2010.03256.x.
- Abecassis MM, Seifeldin R, Riordan ME. Patient outcomes and economics of once-daily tacrolimus in renal transplant patients: results of a modeling analysis. Transplant Proc 2008;40:1443-5. http://dx.doi.org/10.1016/j.transproceed.2008.03.090.
- Craig AM, McKechnie T, McKenna M, Klein W, Schindler TM. A cost-effectiveness analysis of tacrolimus versus cyclosporine microemulsion following kidney transplantation. Transplant Proc 2002;34:1646-8. http://dx.doi.org/10.1016/S0041-1345(02)02964-0.
- Lazzaro C, McKechnie T, McKenna M. Tacrolimus versus cyclosporin in renal transplantation in Italy: cost-minimisation and cost-effectiveness analyses. J Nephrol 2002;15:580-8.
- Orme ME, Jurewicz WA, Kumar N, McKechnie TL. The cost effectiveness of tacrolimus versus microemulsified cyclosporin: a 10-year model of renal transplantation outcomes. Pharmacoeconomics 2003;21:1263-76. http://dx.doi.org/10.2165/00019053-200321170-00003.
- Earnshaw SR, Graham CN, Irish WD, Sato R, Schnitzler MA. Lifetime cost-effectiveness of calcineurin inhibitor withdrawal after de novo renal transplantation. J Am Soc Nephrol 2008;19:1807-16. http://dx.doi.org/10.1681/ASN.2007040495.
- McEwan P, Dixon S, Baboolal K, Conway P, Currie CJ. Evaluation of the cost effectiveness of sirolimus versus tacrolimus for immunosuppression following renal transplantation in the UK. Pharmacoeconomics 2006;24:67-79. http://dx.doi.org/10.2165/00019053-200624010-00006.
- Jurgensen JS, Arns W, Hass B. Cost-effectiveness of immunosuppressive regimens in renal transplant recipients in Germany: a model approach. Eur J Health Econ 2010;11:15-2. http://dx.doi.org/10.1007/s10198-009-0148-3.
- Jurgensen JS, Ikenberg R, Greiner RA, Hosel V. Cost-effectiveness of modern mTOR inhibitor based immunosuppression compared to the standard of care after renal transplantation in Germany. Eur J Health Econ 2015;16:377-90. http://dx.doi.org/10.1007/s10198-014-0579-3.
- Gamboa O, Montero C, Mesa L, Benavides C, Reino A, Torres RE, et al. Cost-effectiveness analysis of the early conversion of tacrolimus to mammalian target of rapamycin inhibitors in patients with renal transplantation. Transplant Proc 2011;43:3367-76. http://dx.doi.org/10.1016/j.transproceed.2011.09.092.
- Rely K, Alexandre PK, Garcia-Garcia EG, Mucino-Ortega E, Salinas-escudero G, Galindo-Suarez RM. Cost-utility assessment of sirolimus versus tacrolimus for primary prevention of graft rejection in renal transplant recipients in Mexico. Value Health 2012;15. http://dx.doi.org/10.1016/j.jval.2012.03.840.
- Niemczyk M, Nowak M, Pilecki T, Wyzgal J, Ziolkowski J, Zygier D, et al. Economic evaluation of sirolimus-based immunosuppressive regimens in kidney graft recipients. Transplant Proc 2006;38:74-7. http://dx.doi.org/10.1016/j.transproceed.2005.11.092.
- Muduma G, Shaw J, Hart WM, Odeyemi A, Odeyemi I. Cost utility analysis of immunosuppressive regimens in adult renal transplant recipients in England and Wales. Patient Prefer Adherence 2014;8:1537-46.
- Silva HT, Yang HC, Abouljoud M, Kuo PC, Wisemandle K, Bhattacharya P, et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant 2007;7:595-608. http://dx.doi.org/10.1111/j.1600-6143.2007.01661.x.
- Opelz G, Dohler B. Collaborative Transplant Study Report. Influence of time of rejection on long-term graft survival in renal transplantation. Transplantation 2008;85:661-6. http://dx.doi.org/10.1097/TP.0b013e3181661695.
- Pruthi R, Steenkamp R, Feest T. UK Renal Registry 16th Annual Report: chapter 8 Survival and Cause of Death of UK Adult Patients on Renal Replacement Therapy in 2012: National and Centre-specific Analyses. Nephron Clinical Practice 2013;125:139-70. http://dx.doi.org/10.1159/000360027.
- McEwan P, Baboolal K, Conway P, Currie CJ. Evaluation of the cost-effectiveness of sirolimus versus cyclosporin for immunosuppression after renal transplantation in the United Kingdom. Clin Ther 2005;27:1834-46. http://dx.doi.org/10.1016/j.clinthera.2005.11.002.
- Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients. Cochrane Database System Rev 2006;2. http://dx.doi.org/10.1002/14651858.cd004290.pub2.
- Vincenti F, Blancho G, Durrbach A, Friend P, Grinyo J, Halloran PF, et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol 2010;21:1587-96. http://dx.doi.org/10.1681/ASN.2009111109.
- Durrbach A, Larsen CP, Medina Pestana J, Vanrenterghem Y, Vincenti F, Florman S, et al. Belatacept vs cyclosporine in ECD kidney transplants: two-year outcomes from the BENEFIT-EXT study. NDT Plus 2010;3. http://dx.doi.org/10.1097/00007890-201007272-00303.
- Lee AJ, Morgan CL, Conway P, Currie CJ. Characterisation and comparison of health-related quality of life for patients with renal failure. Curr Med Res Opin 2005;21:1777-83. http://dx.doi.org/10.1185/030079905X65277.
- Royal College of Paediatrics and Child Health . School Age Charts and Resources Boys 2–18 Years n.d. www.rcpch.ac.uk/system/files/protected/page/NEW%20Boys%202-18yrs%20(4TH%20JAN%202013).pdf (accessed 25 February 2015).
- Royal College of Paediatrics and Child Health . School Age Charts and Resources, Girls 2–18 Years n.d. www.rcpch.ac.uk/system/files/protected/page/NEW%20Girls%202-18yrs(4TH%20JAN%202012).pdf (accessed 25 February 2015).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- Ettenger R, Hoyer PF, Grimm P, Webb N, Loirat C, Mahan JD, et al. Multicenter trial of everolimus in pediatric renal transplant recipients: results at three year. Pediatr Transplant 2008;12:456-63. http://dx.doi.org/10.1111/j.1399-3046.2007.00832.x.
- NHS . NHS Electronic Drug Tariff 2014 n.d. www.drugtariff.nhsbsa.nhs.uk/#/00261650-FA/FA00261203/Home (accessed 25 February 2015).
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Baboolal K, McEwan E, Sondhi S, Spiewanowski P, Wechowski J, Wilson K. The cost of renal dialysis in a UK setting--a multicentre study. Nephrol Dial Transplant 2008;23:1982-9. http://dx.doi.org/10.1093/ndt/gfm870.
- NHS . NHS Reference Costs – Renal Transplant and Dialysis 2013.
- All Wales Medicines Strategy Group . Belatacept (Nulojix®) 2012. www.awmsg.org/awmsgonline/app/appraisalinfo/430 (accessed 25 February 2015).
- Taylor RS, Elston J. The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13080.
- Williams A. Paediatric renal transplantation. Paediatr Child Health 2012;22:346-50. http://dx.doi.org/10.1016/j.paed.2012.04.001.
- Health and Social Care Information Centre . Introduction to Payment by Results n.d. www.hscic.gov.uk/article/2047/Introduction-to-Payment-by-Results (accessed 25 February 2015).
- Pruthi R, O’Brien C, Casula A, Braddon F, Lewis M, Maxwell H, et al. UK Renal Registry 16th annual report: chapter 7 demography of the UK paediatric renal replacement therapy population in 2012. Nephron Clinical Practice 2013;125:127-38. http://dx.doi.org/10.1159/000360026.
- British National Formulary for Children. London: BMJ Group and Pharmaceutical Press; 2014.
- Sharkey I, Boddy AV, Wallace H, Mycroft J, Hollis R, Picton S, et al. Body surface area estimation in children using weight alone: application in paediatric oncology. Br J Cancer 2001;85:23-8. http://dx.doi.org/10.1054/bjoc.2001.1859.
- Pruthi R, Casula A, MacPhee I. UK Renal Registry 16th Annual Report: chapter 3 Demographic and biochemistry profile of kidney transplant recipients in the UK in 2012: national and centre-specific analyses. Nephron Clinical Practice 2013;125:55-80. http://dx.doi.org/10.1159/000360022.
- Opelz G, Dohler B. Association of HLA mismatch with death with a functioning graft after kidney transplantation: a collaborative transplant study report. Am J Transplant 2012;12:3031-8. http://dx.doi.org/10.1111/j.1600-6143.2012.04226.x.
- Opelz G, Dohler B. Association between steroid dosage and death with a functioning graft after kidney transplantation. Am J Transplant 2013;13:2096-105. http://dx.doi.org/10.1111/ajt.12313.
- Cole EH, Johnston O, Rose CL, Gill JS. Impact of acute rejection and new-onset diabetes on long-term transplant graft and patient survival. Clin J Am Soc Nephrol 2008;3:814-21. http://dx.doi.org/10.2215/CJN.04681107.
- Johnston O, Rose CL, Gill JS, Gill JS. Risks and benefits of preemptive second kidney transplantation. Transplantation 2013;95:705-10. http://dx.doi.org/10.1097/TP.0b013e31827a938f.
- Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int 2002;62:311-18. http://dx.doi.org/10.1046/j.1523-1755.2002.00424.x.
- Kasiske BL, Andany MA, Danielson B. A thirty per cent chronic decline in inverse serum creatinine is an excellent predictor of late renal allograft failure. Am J Kidney Dis 2002;39:762-8. http://dx.doi.org/10.1053/ajkd.2002.31996.
- Levy AR, Briggs AH, Johnston K, Maclean JR, Yuan Y, L’Italien GJ, et al. Projecting long-term graft and patient survival after transplantation. Value Health 2014;17:254-60. http://dx.doi.org/10.1016/j.jval.2014.01.001.
- Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation 2003;75:1291-5. http://dx.doi.org/10.1097/01.TP.0000061602.03327.E2.
- Salvadori M, Rosati A, Bock A, Chapman J, Dussol B, Fritsche L, et al. Estimated one-year glomerular filtration rate is the best predictor of long-term graft function following renal transplant. Transplantation 2006;81:202-6. http://dx.doi.org/10.1097/01.tp.0000188135.04259.2e.
- Muscheites J, Wigger M, Drueckler E, Klaassen I, John U, Wygoda S, et al. Estimated one-yr glomerular filtration rate is an excellent predictor of long-term graft survival in pediatric first kidney transplants. Pediatr Transplant 2009;13:365-70. http://dx.doi.org/10.1111/j.1399-3046.2008.00976.x.
- Tejani A, Sullivan EK. The impact of acute rejection on chronic rejection: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant 2000;4:107-11. http://dx.doi.org/10.1034/j.1399-3046.2000.00091.x.
- Hudson A, Collett D. Estimating Long-Term Kidney Graft and Patient Survival Estimates Using Period Analysis 2014. www.odt.nhs.uk/pps/estimating_kidney_survival_using_period_analysis.ppt (accessed 25 February 2015).
- Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, et al. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant 2003;3:590-8. http://dx.doi.org/10.1034/j.1600-6143.2003.00082.x.
- Ciancio G, Burke GW, Gaynor JJ, Roth D, Sageshima J, Kupin W, et al. Randomized trial of mycophenolate mofetil versus enteric-coated mycophenolate sodium in primary renal transplant recipients given tacrolimus and daclizumab/thymoglobulin: one year follow-up. Transplantation 2008;86:67-74. http://dx.doi.org/10.1097/TP.0b013e3181734b4a.
- Takahashi K, Uchida K, Yoshimura N, Takahara S, Teraoka S, Teshima R, et al. Efficacy and safety of concentration-controlled everolimus with reduced-dose cyclosporine in Japanese de novo renal transplant patients: 12-month results. Transplantation Res 2013;2. http://dx.doi.org/10.1186/2047-1440-2-14.
- Tedesco-Silva H, Cibrik D, Johnston T, Lackova E, Mange K, Panis C, et al. Everolimus plus reduced-exposure CsA versus mycophenolic acid plus standard-exposure CsA in renal-transplant recipients. Am J Transplant 2010;10:1401-13. http://dx.doi.org/10.1111/j.1600-6143.2010.03129.x.
- Anil Kumar MS, Heifets M, Fyfe B, Saaed MI, Moritz MJ, Parikh MH, et al. Comparison of steroid avoidance in tacrolimus/mycophenolate mofetil and tacrolimus/sirolimus combination in kidney transplantation monitored by surveillance biopsy. Transplantation 2005;80:807-14. http://dx.doi.org/10.1097/01.tp.0000173378.28790.0b.
- Gonwa T, Mendez R, Yang HC, Weinstein S, Jensik S, Steinberg S. Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 months. Transplantation 2003;75:1213-20. http://dx.doi.org/10.1097/01.TP.0000062837.99400.60.
- Sampaio EL, Pinheiro-Machado PG, Garcia R, Felipe CR, Park SI, Casarini DE, et al. Mycophenolate mofetil vs. sirolimus in kidney transplant recipients receiving tacrolimus-based immunosuppressive regimen. Clinical Transplantation 2008;22:141-9.
- Raofi V, Holman DM, Coady N, Vazquez E, Dunn TB, Bartholomew AM, et al. A prospective randomized trial comparing the efficacy of tacrolimus versus cyclosporine in black recipients of primary cadaveric renal transplants. Am J Surg 1999;177:299-302. http://dx.doi.org/10.1016/S0002-9610(99)00042-2.
- Tsuchiya T, Ishida H, Tanabe T, Shimizu T, Honda K, Omoto K, et al. Comparison of pharmacokinetics and pathology for low-dose tacrolimus once-daily and twice-daily in living kidney transplantation: prospective trial in once-daily versus twice-daily tacrolimus. Transplantation 2013;96:198-204. http://dx.doi.org/10.1097/TP.0b013e318296c9d5.
- Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med 2005;353:770-81. http://dx.doi.org/10.1056/NEJMoa050085.
- Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010;10:535-46. http://dx.doi.org/10.1111/j.1600-6143.2009.03005.x.
- Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 2010;10:547-57. http://dx.doi.org/10.1111/j.1600-6143.2010.03016.x.
- Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, et al. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant 2009;9:1115-23. http://dx.doi.org/10.1111/j.1600-6143.2009.02615.x.
- Büchler M, Caillard S, Barbier S, Thervet E, Toupance O, Mazouz H, et al. Sirolimus versus cyclosporine in kidney recipients receiving thymoglobulin, mycophenolate mofetil and a 6-month course of steroids. Am J Transplant 2007;7:2522-31. http://dx.doi.org/10.1111/j.1600-6143.2007.01976.x.
- Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D, et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation 2000;69:1252-60. http://dx.doi.org/10.1097/00007890-200004150-00009.
- Guba M, Pratschke J, Hugo C, Krämer BK, Nohr-Westphal C, Brockmann J, et al. Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short-term calcineurin inhibitor-based quadruple therapy in de novo renal transplant patients: one-year analysis of a randomized multicenter trial. Transplantation 2010;90:175-83. http://dx.doi.org/10.1097/TP.0b013e3181e11798.
- Martinez-Mier G, Mendez-Lopez MT, Budar-Fernandez LF, Estrada-Oros J, Franco-Abaroa R, George-Micelli E, et al. Living related kidney transplantation without calcineurin inhibitors: initial experience in a Mexican center. Transplantation 2006;82:1533-6. http://dx.doi.org/10.1097/01.tp.0000235823.09788.f6.
- Schaefer HM, Kizilisik AT, Feurer I, Nylander WA, Langone AJ, Helderman JH, et al. Short-term results under three different immunosuppressive regimens at one center. Transplant Proc 2006;38:3466-7. http://dx.doi.org/10.1016/j.transproceed.2006.10.098.
- Groth CG, Backman L, Morales JM, Calne R, Kreis H, Lang P, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation 1999;67:1036-42. http://dx.doi.org/10.1097/00007890-199904150-00017.
- Webster A, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database System Rev n.d.;19. http://dx.doi.org/10.1002/14651858.cd003961.pub2.
- Vitko S, Margreiter R, Weimar W, Dantal J, Viljoen HG, Li Y, et al. Everolimus (certican) 12-month safety and efficacy versus mycophenolate mofetil in de Novo renal transplant recipients. Transplantation 2004;78:1532-40. http://dx.doi.org/10.1097/01.TP.0000141094.34903.54.
- Chadban S, Eris J, Russ G, Campbell S, Chapman J, Pussell B, et al. Enteric-coated mycophenolate sodium in combination with full dose or reduced dose cyclosporine, basiliximab and corticosteroids in Australian de novo kidney transplant patients. Nephrology 2013;18:63-70. http://dx.doi.org/10.1111/nep.12004.
- Mjörnstedt L, Sørensen SS, Zur Mühlen B, Jespersen B, Hansen JM, Bistrup C, et al. Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. Am J Transplant 2012;12:2744-53. http://dx.doi.org/10.1111/j.1600-6143.2012.04162.x.
- Flechner SM, Goldfarb D, Modlin C, Feng JY, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporin. Transplantation 2002;74:1070-6. http://dx.doi.org/10.1097/00007890-200210270-00002.
- Jongsma H, Bouts AH, Cornelissen EAM, Beersma MFC, Cransberg K. Cytomegalovirus prophylaxis in pediatric kidney transplantation: the Dutch experience. Pediatr Transplant 2013;17:510-17. http://dx.doi.org/10.1111/petr.12115.
- Bonthuis M, van Stralen KJ, Jager KJ, Baiko S, Jahnukainen T, Laube GF, et al. Dyslipidaemia in children on renal replacement therapy. Nephrol Dial Transplant 2014;29:594-603. http://dx.doi.org/10.1093/ndt/gft429.
- Vanrenterghem Y, Ponticelli C, Morales JM, Abramowicz D, Baboolal K, Eklund B, et al. Prevalence and management of anemia in renal transplant recipients: a European survey. Am J Transplant 2003;3:835-45. http://dx.doi.org/10.1034/j.1600-6143.2003.00133.x.
- Fuggle SV, Allen JE, Johnson RJ, Collett D, Mason PD, Dudley C, et al. Factors affecting graft and patient survival after live donor kidney transplantation in the UK. Transplantation 2010;89:694-701. http://dx.doi.org/10.1097/TP.0b013e3181c7dc99.
- Summers DM, Johnson RJ, Allen J, Fuggle SV, Collett D, Watson CJ, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet 2010;376:1303-11. http://dx.doi.org/10.1016/S0140-6736(10)60827-6.
- Wille N, Badia X, Bonsel G, Burstrom K, Cavrini G, Devlin N, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res 2010;19:875-86. http://dx.doi.org/10.1007/s11136-010-9648-y.
- Health Survey for England 2012. London: Health and Social Care Information Centre; 2013.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Liem YS, Bosch JL, Myriam Hunink MG. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health 2008;11:733-41. http://dx.doi.org/10.1111/j.1524-4733.2007.00308.x.
- Manns B, Johnson JA, Taub K, Mortis G, Ghali WA, Donaldson C. Quality of life in patients treated with hemodialysis or peritoneal dialysis: what are the important determinants?. Clinical Nephrology 2003;60:341-51. http://dx.doi.org/10.5414/CNP60341.
- Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al. An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9240.
- Sennfalt K, Magnusson M, Carlsson P. Comparison of hemodialysis and peritoneal dialysis --a cost-utility analysis. Peritoneal Dialysis International 2002;22:39-47.
- Wasserfallen JB, Halabi G, Saudan P, Perneger T, Feldman HI, Martin PY, et al. Quality of life on chronic dialysis: comparison between haemodialysis and peritoneal dialysis. Nephrology Dialysis Transplantation 2004;19:1594-9. http://dx.doi.org/10.1093/ndt/gfh175.
- Cleemput I, Kesteloot K, De Geest S, Dobbels F, Vanrenterghem Y. Health professionals’ perceptions of health status after renal transplantation: a comparison with transplantation candidates’ expectations. Transplantation 2003;76:176-82. http://dx.doi.org/10.1097/01.TP.0000072807.46212.FA.
- Greiner W, Obermann K, Schulenburg JM. Socio-economic evaluation of kidney-transplantation in Germany. Arch Hellenic Med 2001;18:147-55.
- Moons P, Vanrenterghem Y, Hooff JP, Squifflet JP, Margodt D, Mullens M, et al. Health-related quality of life and symptom experience in tacrolimus-based regimens after renal transplantation: a multicentre study. Transplant International 2003;16:653-64. http://dx.doi.org/10.1111/j.1432-2277.2003.tb00366.x.
- Dukes JL, Seelam S, Lentine KL, Schnitzler MA, Neri L. Health-related quality of life in kidney transplant patients with diabetes. Clin Transplant 2013;27:E554-62. http://dx.doi.org/10.1111/ctr.12198.
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203-20. http://dx.doi.org/10.1097/00005650-200503000-00003.
- Johnson JA, Luo N, Shaw JW, Kind P, Coons SJ. Valuations of EQ-5D health states: are the United States and United Kingdom different?. Med Care 2005;43:221-8. http://dx.doi.org/10.1097/00005650-200503000-00004.
- Currie CJ, Morgan CL, Dixon S, McEwan P, Marchant N, Bearne A, et al. The financial costs of hospital care for people with diabetes who have single and multiple macrovascular complications. Diabetes Res Clin Pract 2005;67:144-51. http://dx.doi.org/10.1016/j.diabres.2004.01.002.
- Currie CJ, McEwan P, Peters JR, Patel TC, Dixon S. The routine collation of health outcomes data from hospital treated subjects in the Health Outcomes Data Repository (HODaR): descriptive analysis from the first 20,000 subjects. Value Health 2005;8:581-90. http://dx.doi.org/10.1111/j.1524-4733.2005.00046.x.
- Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLOS Med 2012;9. http://dx.doi.org/10.1371/journal.pmed.1001307.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Brennan DC, Daller JA, Lake KD, Cibrik D, Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 2006;355:1967-77. http://dx.doi.org/10.1056/NEJMoa060068.
- Anil Kumar MS, Irfan Saeed M, Ranganna K, Malat G, Sustento-Reodica N, Kumar AM, et al. Comparison of four different immunosuppression protocols without long-term steroid therapy in kidney recipients monitored by surveillance biopsy: five-year outcomes. Transplant Immunology 2008;20:32-4. http://dx.doi.org/10.1016/j.trim.2008.08.005.
- Wlodarczyk Z, Squifflet JP, Ostrowski M, Rigotti P, Stefoni S, Citterio F, et al. Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: a randomized, open-label trial. Am J Transplant 2009;9:2505-13. http://dx.doi.org/10.1111/j.1600-6143.2009.02794.x.
- Oh CK, Huh KH, Lee JS, Cho HR, Kim YS. Safety and efficacy of conversion from twice-daily tacrolimus to once-daily tacrolimus one month after transplantation: randomized controlled trial in adult renal transplantation. Yonsei Med J 2014;55:1341-7. http://dx.doi.org/10.3349/ymj.2014.55.5.1341.
- Rowshani AT, Scholten EM, Bemelman F, Eikmans M, Idu M, Roos-van Groningen MC, et al. No difference in degree of interstitial Sirius red-stained area in serial biopsies from area under concentration-over-time curves-guided cyclosporine versus tacrolimus-treated renal transplant recipients at one year. J Am Soc Nephrol 2006;17:305-12. http://dx.doi.org/10.1681/ASN.2005030249.
- Vacher-Coponat H, Moal V, Indreies M, Purgus R, Loundou A, Burtey S, et al. A randomized trial with steroids and antithymocyte globulins comparing cyclosporine/azathioprine versus tacrolimus/mycophenolate mofetil (CATM2) in renal transplantation. Transplantation 2012;93:437-43. http://dx.doi.org/10.1097/TP.0b013e31824215b7.
- Lorber MI, Mulgaonkar S, Butt KM, Elkhammas E, Mendez R, Rajagopalan PR, et al. Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: a 3-year randomized, multicenter, phase III study. Transplantation 2005;80:244-52. http://dx.doi.org/10.1097/01.TP.0000164352.65613.24.
- Shaw C, Pitcher D, Pruthi R, Fogarty D. UK Renal Registry 16th Annual Report: chapter 2 UK RRT Prevalence in 2012: national and Centre-Specific Analyses. Bristol: UK Renal Registry; 2013.
- Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, et al. The efficacy and safety of 200 days Valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant 2010;10:1228-37. http://dx.doi.org/10.1111/j.1600-6143.2010.03074.x.
- Moore J. Kidney Transplant Protocol. Exeter: Royal Devon and Exeter NHS Foundation Trust; 2012.
- Hocker B, Fickenscher H, Delecluse HJ, Bohm S, Kusters U, Schnitzler P, et al. Epidemiology and morbidity of Epstein–Barr virus infection in pediatric renal transplant recipients: a multicenter, prospective study. Clin Infect Dis 2013;56:84-92. http://dx.doi.org/10.1093/cid/cis823.
- Riella LV, Gabardi S, Chandraker A. Dyslipidemia and its therapeutic challenges in renal transplantation. Am J Transplant 2012;12:1975-82. http://dx.doi.org/10.1111/j.1600-6143.2012.04084.x.
- Department of Health . Reference Costs Guidance 2013–14 n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/289224/reference_costs_collection_2013-14_2.pdf (accessed 25 February 2015).
- Alva ML, Gray A, Mihaylova B, Leal J, Holman RR. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabet Med 2015;32:459-66. http://dx.doi.org/10.1111/dme.12647.
- Ling C, Pandit P, Bennett H. Belatacept Micro-Costing Model – UK. Cardiff: Cardiff Research Consortium; 2011.
- John EG, Domingo LT. Hypertension and obesity after pediatric kidney transplantation: management based on pathophysiology: a mini review. Int J Prev Med 2014;5:S25-38.
- Developing Robust Reference Costs for Kidney Transplants – Update. London: NHS Kidney Care; 2011.
- University College London Hospitals NHS Foundation Trust . Provider to Provider Services 2013–2014 Tariff n.d. www.uclh.nhs.uk/aboutus/wwd/Documents/Provider%20to%20Provider%20Tariff%202013-14.pdf (accessed 25 February 2015).
- HRG4 + Reference Costs Code to Group. Health and Social Care Information Centre; 2014.
- National Organ Retrieval Service: service Evaluation. London: NHS Blood and Transplant; 2013.
- Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al. Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9210.
- Guide to the Methods of Technology Appraisal. London: NICE; 2004.
- Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet 2000;356:194-202. http://dx.doi.org/10.1016/S0140-6736(00)02480-6.
- Machado PGP, Felipe CR, Hanzawa NM, Park SI, Garcia R, Alfieri F, et al. An open-label randomized trial of the safety and efficacy of sirolimus vs. azathioprine in living related renal allograft recipients receiving cyclosporine and prednisone combination. Clin Transplant 2004;18:28-3. http://dx.doi.org/10.1111/j.1399-0012.2004.00113.x.
- Kahan BD. Two-year results of multicenter phase III trials on the effect of the addition of sirolimus to cyclosporine-based immunosuppressive regimens in renal transplantation. Transplant Proc 2003;35:37S-51S. http://dx.doi.org/10.1016/S0041-1345(03)00353-1.
- Almeida CC, Silveira MR, Araujo VE, Lemos LLP, Oliveira Costa J, Reis CAL, et al. Safety of immunosuppressive drugs used as maintenance therapy in kidney transplantation: a systematic review and meta-analysis. Pharmaceuticals 2013;6:1170-94. http://dx.doi.org/10.3390/ph6101170.
- Andrassy J, Hoffmann VS, Rentsch M, Stangl M, Habicht A, Meiser B, et al. Is cytomegalovirus prophylaxis dispensable in patients receiving an mTOR inhibitor-based immunosuppression? A systematic review and meta-analysis. Transplantation 2012;94:1208-17. http://dx.doi.org/10.1097/TP.0b013e3182708e56.
- Brooks RJ, Higgins GY, Webster AC. Systematic review of randomized controlled trial quality in pediatric kidney transplantation. Pediatric Nephrology 2010;25:2383-92. http://dx.doi.org/10.1007/s00467-010-1595-x.
- Ho ET, Wong G, Craig JC, Chapman JR. Once-daily extended-release versus twice-daily standard-release tacrolimus in kidney transplant recipients: a systematic review. Transplantation 2013;95:1120-8. http://dx.doi.org/10.1097/TP.0b013e318284c15b.
- Kasiske BL, De Mattos A, Flechner SM, Gallon L, Meier-Kriesche HU, Weir MR, et al. Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am J Transplant 2008;8:1384-92. http://dx.doi.org/10.1111/j.1600-6143.2008.02272.x.
- Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation 2009;87:785-94. http://dx.doi.org/10.1097/TP.0b013e3181952623.
- Liu Y, Zhou P, Han M, Xue CB, Hu XP, Li C. Basiliximab or antithymocyte globulin for induction therapy in kidney transplantation: a meta-analysis. Transplant Proc 2010;42:1667-70. http://dx.doi.org/10.1016/j.transproceed.2010.02.088.
- Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database System Rev 2014;11. http://dx.doi.org/10.1002/14651858.cd010699.pub2.
- Moore J, Middleton L, Cockwell P, Adu D, Ball S, Little MA, et al. Calcineurin inhibitor sparing with mycophenolate in kidney transplantation: a systematic review and meta-analysis. Transplantation 2009;87:591-605. http://dx.doi.org/10.1097/TP.0b013e318195a421.
- Mulay AV, Cockfield S, Stryker R, Fergusson D, Knoll GA. Conversion from calcineurin inhibitors to sirolimus for chronic renal allograft dysfunction: a systematic review of the evidence. Transplantation 2006;82:1153-62. http://dx.doi.org/10.1097/01.tp.0000237101.58974.43.
- Peddi VR, Wiseman A, Chavin K, Slakey D. Review of combination therapy with mTOR inhibitors and tacrolimus minimization after transplantation. Transplant Rev 2013;27:97-107. http://dx.doi.org/10.1016/j.trre.2013.06.001.
- Pengel LH, Liu LQ, Morris PJ. Do wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors? A systematic review of randomized controlled trials. Transpl Int 2011;24:1216-30. http://dx.doi.org/10.1111/j.1432-2277.2011.01357.x.
- Su VCH, Greanya ED, Ensom MHH. Impact of mycophenolate mofetil dose reduction on allograft outcomes in kidney transplant recipients on tacrolimus-based regimens: a systematic review. Ann Pharmacother 2011;45:248-57. http://dx.doi.org/10.1345/aph.1p456.
- Webster AC, Playford EG, Higgins G, Chapman JR, Craig JC. Interleukin 2 receptor antagonists for renal transplantation recipients: a meta-analysis of randomized trials. Transplantation 2004;77:166-76. http://dx.doi.org/10.1097/01.TP.0000109643.32659.C4.
- Webster AC, Playford EG, Higgins G, Chapman JR, Craig J. . Cochrane Database System Rev 2004;1. http://dx.doi.org/10.1002/14651858.cd003897.pub2.
- Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ 2005;331:810-14. http://dx.doi.org/10.1136/bmj.38569.471007.AE.
- Webster AC, Lee VWS, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation 2006;81:1234-48. http://dx.doi.org/10.1097/01.tp.0000219703.39149.85.
- Webster AC, Ruster LP, McGee R, Matheson SL, Higgins GY, Willis NS, et al. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database System Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd003897.pub3.
- Yan HL, Zong HT, Cui YS, Li N, Zhang Y. Calcineurin inhibitor avoidance and withdrawal for kidney transplantation: a systematic review and meta-analysis of randomized controlled trials. Transplant Proc 2014;46:1302-13. http://dx.doi.org/10.1016/j.transproceed.2014.02.010.
- Oh CK, Kim SJ, Kim JH, Lee JH. Prospective controlled protocol for three months steroid withdrawal with tacrolimus, basiliximab, and mycophenolate mofetil in renal transplant recipients. J Korean Med Sci 2012;27:337-42. http://dx.doi.org/10.3346/jkms.2012.27.4.337.
- Benfield MR, Bartosh S, Ikle D, Warshaw B, Bridges N, Morrison Y, et al. A randomized double-blind, placebo controlled trial of steroid withdrawal after pediatric renal transplantation. Am J Transplant 2010;10:81-8. http://dx.doi.org/10.1111/j.1600-6143.2009.02767.x.
- Flechner SM, Gurkan A, Hartmann A, Legendre CM, Russ GR, Campistol JM, et al. A randomized, open-label study of sirolimus versus cyclosporine in primary de novo renal allograft recipients. Transplantation 2013;95:1233-41. http://dx.doi.org/10.1097/TP.0b013e318291a269.
- Sarwal MM, Ettenger RB, Dharnidharka V, Benfield M, Mathias R, Portale A, et al. Complete steroid avoidance is effective and safe in children with renal transplants: a multicenter randomized trial with three-year follow-up. Am J Transplant 2012;12:2719-29. http://dx.doi.org/10.1111/j.1600-6143.2012.04145.x.
- Gelder T, Silva HT, Fijter JW, Budde K, Kuypers D, Tyden G, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation 2008;86:1043-51. http://dx.doi.org/10.1097/TP.0b013e318186f98a.
- Cransberg K, Cornelissen M, Lilien M, Hoeck K, Davin JC, Nauta J. Maintenance immunosuppression with mycophenolate mofetil and corticosteroids in pediatric kidney transplantation: temporary benefit but not without risk. Transplantation 2007;83:1041-7. http://dx.doi.org/10.1097/01.tp.0000260146.57898.9c.
- Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med 1998;338:161-5. http://dx.doi.org/10.1056/NEJM199801153380304.
- Vincenti F, Nashan B, Bumgardner G, Hardie I, Pescovitz M, Johnson RWG, et al. Three year outcome of the phase III clinical trials with Daclizumab. Transplantation 2000;69. http://dx.doi.org/10.1097/00007890-200004271-00577.
- Hengster P, Pescovitz MD, Hyatt D, Margreiter R. Roche Study Group . Cytomegalovirus infections after treatment with daclizumab, an anti IL-2 receptor antibody, for prevention of renal allograft rejection. Transplantation 1999;68:310-13. http://dx.doi.org/10.1097/00007890-199907270-00028.
- Bumgardner GL, Hardie I, Johnson RW, Lin A, Nashan B, Pescovitz MD, et al. Results of 3-year phase III clinical trials with daclizumab prophylaxis for prevention of acute rejection after renal transplantation. Transplantation 2001;72:839-45. http://dx.doi.org/10.1097/00007890-200109150-00017.
- Ponticelli C, Yussim A, Cambi V, Legendre C, Rizzo G, Salvadori M, et al. Basiliximab significantly reduces acute rejection in renal transplant patients given triple therapy with azathioprine. Transplant Proc 2001;33:1009-10. http://dx.doi.org/10.1016/S0041-1345(00)02307-1.
- Folkmane I, Bicans J, Amerika D, Chapenko S, Murovska M, Rosentals R. Low rate of acute rejection and cytomegalovirus infection in kidney transplant recipients with basiliximab. Transplant Proc 2001;33:3209-10. http://dx.doi.org/10.1016/S0041-1345(01)02366-1.
- Folkmane I, Bicans J, Chapenko S, Murovska M, Rosentals R. Results of renal transplantation with different immunosuppressive regimens. Transplant Proc 2002;34:558-9. http://dx.doi.org/10.1016/S0041-1345(01)02845-7.
- Shapiro R, Jordan M, Scantlebury V, Fung J, Jensen C, Tzakis A, et al. FK 506 in clinical kidney transplantation. Transplant Proc 1991;23:3065-7.
- Mayer AD. Four-year follow-up of the European Tacrolimus Multicenter Renal Study. Transplant Proc 1999;31:27S-28S. http://dx.doi.org/10.1016/S0041-1345(99)00789-7.
- Mayer AD. Chronic rejection and graft half-life: five-year follow-up of the European Tacrolimus Multicenter Renal Study. Transplant Proc 2002;34:1491-2. http://dx.doi.org/10.1016/S0041-1345(02)02942-1.
- Mayer D. Tacrolimus vs cyclosporin in renal transplantation: five-year follow-up of the European Multicentre Study. Am J Transplant 2002;2.
- Jurewicz WA. Immunological and nonimmunological risk factors with tacrolimus and Neoral in renal transplant recipients: an interim report. Transplant Proc 1999;31:64S-66S. http://dx.doi.org/10.1016/S0041-1345(99)00798-8.
- Jurewicz WA. Tacrolimus versus ciclosporin immunosuppression: long-term outcome in renal transplantation. Nephrol Dial Transplant 2003;18:i7-11. http://dx.doi.org/10.1093/ndt/gfg1028.
- Sperschneider H. A large, multicentre trial to compare the efficacy and safety of tacrolimus with cyclosporine microemulsion following renal transplantation. Transplant Proc 2001;33:1279-81. http://dx.doi.org/10.1016/S0041-1345(00)02477-5.
- Krämer BK, Zulke C, Kammerl MC, Schmidt C, Hengstenberg C, Fischereder M, et al. Cardiovascular risk factors and estimated risk for CAD in a randomized trial comparing calcineurin inhibitors in renal transplantation. Am J Transplant 2003;3:982-7. http://dx.doi.org/10.1034/j.1600-6143.2003.00156.x.
- Dietl KH. Oral dosing of tacrolimus and cyclosporine microemulsion – Results from a large multicenter study in renal transplantation. Transplant Proc 2002;34:1659-60. http://dx.doi.org/10.1016/S0041-1345(02)02969-X.
- Töz H, Sen S, Sezi M, Duman S, Ozkahya M, Ozbek S, et al. Comparison of tacrolimus and cyclosporin in renal transplantation by the protocol biopsies. Transplant Proc 2004;36:134-6. http://dx.doi.org/10.1016/j.transproceed.2003.11.056.
- Murphy GJ, Waller JR, Sandford RS, Furness PN, Nicholson ML. Randomized clinical trial of the effect of microemulsion cyclosporin and tacrolimus on renal allograft fibrosis. Br J Surg 2003;90:680-6. http://dx.doi.org/10.1002/bjs.4134.
- Mathew TH. A blinded, long-term, randomized multicenter study of mycophenolate mofetil in cadaveric renal transplantation: results at three years. Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation 1998;65:1450-4. http://dx.doi.org/10.1097/00007890-199806150-00007.
- Miladipour AH, Ghods AJ, Nejadgashti H. Effect of mycophenolate mofetil on the prevention of acute renal allograft rejection. Transplant Proc 2002;34:2089-90. http://dx.doi.org/10.1016/S0041-1345(02)02863-4.
- Baltar J, Ortega F, Rebollo P, Gomez E, Laures A, Alvarez-Grande J. Changes in health-related quality of life in the first year of kidney transplantation. Nefrologia 2002;22:262-8.
- Salvadori M, Holzer H, De Mattos A, Sollinger H, Arns W, Oppenheimer F, et al. Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant 2004;4:231-36. http://dx.doi.org/10.1046/j.1600-6143.2003.00337.x.
- Johnson RW, Kreis H, Oberbauer R, Brattstrom C, Claesson K, Eris J. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation 2001;72:777-86. http://dx.doi.org/10.1097/00007890-200109150-00007.
- Abou-Jaoude MM, Irani-Hakime N, Ghantous I, Najm R, Afif C, Almawi WY. Cyclosporine microemulsion (Neoral) versus tacrolimus (FK506) as maintenance therapy in kidney transplant patients. Transplant Proc 2003;35:2748-9. http://dx.doi.org/10.1016/j.transproceed.2003.09.036.
- Abou-Jaoude MM, Najm R, Shaheen J, Nawfal N, Abboud S, Alhabash M, et al. Tacrolimus (FK506) versus cyclosporine microemulsion (neoral) as maintenance immunosuppression therapy in kidney transplant recipients. Transplant Proc 2005;37:3025-8. http://dx.doi.org/10.1016/j.transproceed.2005.08.040.
- Busque S, Shoker A, Landsberg D, McAlister V, Halloran P, Shapiro J, et al. Canadian multicentre trial of tacrolimus/azathioprine/steroids versus tacrolimus/mycophenolate mofetil/steroids versus neoral/mycophenolate mofetil/steroids in renal transplantation. Transplant Proc 2001;33:1266-7. http://dx.doi.org/10.1016/S0041-1345(00)02471-4.
- Johnson C, Ahsan N, Gonwa T, Halloran P, Stegall M, Hardy M, et al. Randomized trial of tacrolimus (Prograf) in combination with azathioprine or mycophenolate mofetil versus cyclosporine (Neoral) with mycophenolate mofetil after cadaveric kidney transplantation. Transplantation 2000;69:834-41. http://dx.doi.org/10.1097/00007890-200003150-00028.
- Garcia DM, Gago JM, Mendiluce A, Gordillo R, Bustamente J. Tacrolimus-basiliximab versus cyclosporine-basiliximab in renal transplantation ‘de novo’: acute rejection and complications. Transplant Proc 2003;35:1694-6. http://dx.doi.org/10.1016/S0041-1345(03)00576-1.
- Morris-Stiff GJ, Quiroga H, Stockdill H, Jones G, Janeciz A, Lord R, et al. Neoral® in cadaveric renal transplantation: 189 patients with a minimum 1-year follow up. Br J Surg 2000;87.
- Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 2007;7:1506-14. http://dx.doi.org/10.1111/j.1600-6143.2007.01749.x.
- Wang XH, Tang XD, Xu D. Tacrolimus vs CyA Neoral in combination with MMF and steroids after cadaveric renal transplantation. Transplant Proc 2000;32:1702-3. http://dx.doi.org/10.1016/S0041-1345(00)01408-1.
- White SA, Jain S, Williams ST, Doughman T, Hayes P, Murphy G, et al. Randomized trial comparing neoral and tacrolimus immunosuppression for recipients of renal transplants procured from different donor groups. Transplant Proc 2000;32. http://dx.doi.org/10.1016/S0041-1345(00)00910-6.
- Williams ST, White SA, Doughman T. A randomised trial comparing Neoral (ciclosporin) and tacrolimus immunosuppression for recipients of renal transplants procured from different donor groups. Br J Surg 1999;86.
- Flechner SM, Glyda M, Cockfield S, Grinyó J, Legendre C, Russ G, et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant 2011;11:1633-44. http://dx.doi.org/10.1111/j.1600-6143.2011.03573.x.
- Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant 2006;6:514-22. http://dx.doi.org/10.1111/j.1600-6143.2005.01177.x.
- Bertoni E, Larti A, Rosso G, Zanazzi M, Maria L, Salvadori M. Good outcomes with cyclosporine very low exposure with everolimus high exposure in renal transplant patients. J Nephrol 2011;24:613-8. http://dx.doi.org/10.5301/JN.2011.6247.
- Ciancio . A randomized long-term trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (Neoral) and sirolimus in renal transplantation. 1. Drug interactions and rejection at one year. Transplantation 2004;77.
- Mendez R, Gonwa T, Yang HC, Weinstein S, Jensik S, Steinberg S. A prospective, randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 1 year. Transplantation 2005;80:303-9. http://dx.doi.org/10.1097/01.tp.0000167757.63922.42.
Appendix 1 Literature searching strategies
Clinical effectiveness searches
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to present.
Date searched: Wednesday 7 January 2015.
Hits: 95.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | Kidney Transplantation/ | 81,673 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 34,747 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 41,731 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 36,959 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 46,496 |
| 6 | 1 or 2 or 3 or 4 or 5 | 115,157 |
| 7 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 1080 |
| 8 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 6436 |
| 9 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 17,526 |
| 10 | Tacrolimus/ | 13,172 |
| 11 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 228 |
| 12 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 28,566 |
| 13 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 22,525 |
| 14 | Sirolimus/ | 14,642 |
| 15 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 3203 |
| 16 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 75,480 |
| 17 | 6 and 16 | 9696 |
| 18 | Randomized Controlled Trial.pt. | 405,805 |
| 19 | (random$ or RCT or placebo$ or single blind$ or double blind$ or triple blind$).ti,ab,ot. | 863,332 |
| 20 | clinical trial.pt. | 503,357 |
| 21 | (“controlled trial$” or “clinical trial$”).ti,ab,ot. | 356,127 |
| 22 | 18 or 19 or 20 or 21 | 1,343,010 |
| 23 | 6 and 16 and 22 | 2481 |
| 24 | limit 23 to yr=“2014 -Current” | 95 |
Notes: N/A.
File: N/A.
Database: EMBASE
Host: Ovid.
Data parameters: 1974 to 5 January 2015.
Date searched: Wednesday 7 January 2015.
Hits: 272.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | kidney transplantation/ | 97,857 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 51,138 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 56,254 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 52,314 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 66,083 |
| 6 | 1 or 2 or 3 or 4 or 5 | 154,370 |
| 7 | basiliximab/ | 6754 |
| 8 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 2323 |
| 9 | thymocyte antibody/ | 20,451 |
| 10 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 8932 |
| 11 | tacrolimus/ | 54,178 |
| 12 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 26,496 |
| 13 | belatacept/ | 1003 |
| 14 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 555 |
| 15 | mycophenolic acid/ | 10,124 |
| 16 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 36,223 |
| 17 | rapamycin/ | 36,866 |
| 18 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 29,130 |
| 19 | everolimus/ | 14,653 |
| 20 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 7135 |
| 21 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 | 149,906 |
| 22 | 6 and 21 | 25,851 |
| 23 | randomized controlled trial/ | 358,007 |
| 24 | (random$ or placebo$ or single blind$ or double blind$ or triple blind$).ti,ab,ot. | 1,039,570 |
| 25 | (“controlled trial$” or “clinical trial$”).ti,ab,ot. | 434,667 |
| 26 | 23 or 24 or 25 | 1,314,663 |
| 27 | 22 and 26 | 3526 |
| 28 | limit 27 to yr=“2014 -Current” | 272 |
Notes: N/A.
File: N/A.
Database: Cochrane CENTRAL
Host: Wiley Online Library.
Data parameters: Issue 12 of 12, December 2014.
Date searched: Wednesday 7 January 2015.
Hits: 75.
| # | Searches | Results |
|---|---|---|
| 1 | MeSH descriptor: [Kidney Transplantation] this term only | 3313 |
| 2 | (Kidney* near/3 transplant*) | 5959 |
| 3 | (Renal near/3 transplant*) | 4492 |
| 4 | ((kidney or renal) near/3 (recipient* or dono* or donation* or replac*)) | 3839 |
| 5 | ((graft* or allograft* or homograft* or allogeneic) and (kidney* or renal)) | 5192 |
| 6 | #1 or #2 or #3 or #4 or #5 | 9188 |
| 7 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”) | 522 |
| 8 | ((rabbit* near/3 Anti-thymocyte*) or (rabbit* near/3 Antithymocyte*) or (rabbit* near/3 thymocyte*) or (rabbit* near/3 polyclonal) or (rabbit* and ATG) or RATG or thymoglobulin*) | 364 |
| 9 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”) | 2587 |
| 10 | MeSH descriptor: [Tacrolimus] this term only | 1181 |
| 11 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”) | 87 |
| 12 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep* or Myfenax or Myfortic or Mofetil) | 3477 |
| 13 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”) | 2199 |
| 14 | MeSH descriptor: [Sirolimus] this term only | 1071 |
| 15 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”) | 939 |
| 16 | #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 | 7471 |
| 17 | #6 and #16 Publication Year from 2014 to 2015 | 102 |
Notes: this search strategy represents the whole of The Cochrane Library but only CENTRL was downloaded in this instance (CENTRAL 75, EED 2, Groups 2, CDSR 20, DARE 3).
File: N/A.
Database: Web of Science
Host: ISI Thompson Reuters.
Data parameters: 1900–2014.
Date searched: Wednesday 7 January 2015.
Hits: 183.
| # | Results | Searches |
|---|---|---|
| 16 | 183 | #14 AND #13 Refined by: PUBLICATION YEARS: ( 2014 ) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 15 | 2,702 | #14 AND #13 Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 14 | 1,421,223 | TOPIC: ((((random* or rct* or “controlled trial*” or “clinical trial*”)))) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 13 | 13,127 | #12 AND #5 Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 12 | 142,824 | #11 OR #10 OR #9 OR #8 OR #7 OR #6 Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 11 | 5570 | TOPIC: (((Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”))) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 10 | 111,240 | TOPIC: (((“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep* or Myfenax or Myfortic or Mofetil))) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 9 | 486 | TOPIC: (((Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”))) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 8 | 23,942 | TOPIC: (((Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”))) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 7 | 6468 | TOPIC: ((((rabbit* near/3 Anti-thymocyte*) or (rabbit* near/3 Antithymocyte*) or (rabbit* near/3 thymocyte*) or (rabbit* near/3 polyclonal) or (rabbit* and ATG) or RATG or thymoglobulin*))) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 6 | 1475 | TOPIC: (((Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”))) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 5 | 125,548 | #4 OR #3 OR #2 OR #1 Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 4 | 53,666 | TOPIC: ((((graft* or allograft* or homograft* or allogeneic) and (kidney* or renal)))) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 3 | 50,443 | TOPIC: ((((kidney or renal) near/3 (recipient* or dono* or donation* or replac*)))) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 2 | 60,478 | TOPIC: (((Renal near/3 transplant*))) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
| 1 | 47,055 | TOPIC: (((Kidney* near/3 transplant*))) Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH Timespan=All years |
Notes: auto suggest was turned off. No records for 2015 on date of search.
File: N/A.
Database: Health Management Information Consortium (HMIC)
Host: Ovid.
Data parameters: 1979 to November 2014.
Date searched: Wednesday 7 January 2015.
Hits: 0.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | Kidney Transplantation/ | 121 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 84 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 81 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 152 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 28 |
| 6 | 1 or 2 or 3 or 4 or 5 | 314 |
| 7 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 2 |
| 8 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 1 |
| 9 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 8 |
| 10 | Tacrolimus/ | 0 |
| 11 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 0 |
| 12 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 23 |
| 13 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 11 |
| 14 | Sirolimus/ | 0 |
| 15 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 2 |
| 16 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 33 |
| 17 | 6 and 16 | 3 |
| 18 | Randomized Controlled Trial.pt. | 0 |
| 19 | (random$ or RCT or placebo$ or single blind$ or double blind$ or triple blind$).ti,ab,ot. | 10,914 |
| 20 | clinical trial.pt. | 0 |
| 21 | (“controlled trial$” or “clinical trial$”).ti,ab,ot. | 5640 |
| 22 | 18 or 19 or 20 or 21 | 12,174 |
| 23 | 6 and 16 and 22 | 1 |
| 24 | limit 23 to yr=“2014 -Current” | 0 |
Notes: N/A.
File: N/A.
Systematic reviews search strategy: clinical effectiveness searches
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to present.
Date searched: Thursday 8 January 2015.
Hits: 10.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | Kidney Transplantation/ | 81,679 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 34,743 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 41,731 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 36,952 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 46,489 |
| 6 | 1 or 2 or 3 or 4 or 5 | 115,148 |
| 7 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 1080 |
| 8 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 6435 |
| 9 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 17,524 |
| 10 | Tacrolimus/ | 13,170 |
| 11 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 228 |
| 12 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 28,558 |
| 13 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 22,498 |
| 14 | Sirolimus/ | 14,646 |
| 15 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 3201 |
| 16 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 75,448 |
| 17 | 6 and 16 | 9694 |
| 18 | (systematic adj3 review$).ti,ab,kw,ot. | 67,562 |
| 19 | 17 and 18 | 50 |
| 20 | limit 19 to yr=“2014 -Current” | 10 |
Notes: N/A.
File: N/A.
Database: EMBASE
Host: Ovid.
Data parameters: 1974 to 7 January 2015.
Date searched: Thursday 8 January 2015.
Hits: 19.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | kidney transplantation/ | 97,867 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 51,145 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 56,258 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 52,323 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 66,091 |
| 6 | 1 or 2 or 3 or 4 or 5 | 154,387 |
| 7 | basiliximab/ | 6757 |
| 8 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 2323 |
| 9 | thymocyte antibody/ | 20,454 |
| 10 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 8933 |
| 11 | tacrolimus/ | 54,192 |
| 12 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 26,500 |
| 13 | belatacept/ | 1004 |
| 14 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 555 |
| 15 | mycophenolic acid/ | 10,128 |
| 16 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 36,231 |
| 17 | rapamycin/ | 36,874 |
| 18 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 29,138 |
| 19 | everolimus/ | 14,659 |
| 20 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 7137 |
| 21 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 | 149,945 |
| 22 | 6 and 21 | 25,858 |
| 23 | (systematic adj3 review$).ti,ab,kw,ot. | 79,043 |
| 24 | 22 and 23 | 127 |
| 25 | limit 24 to yr=“2014 -Current” | 19 |
Notes: N/A.
File: N/A.
Database: Cochrane CDSR and Database of Abstracts of Review of Effects
Host: Wiley Online Library.
Data parameters: CDSR Issue 1 of 12, January 2015, DARE and HTA Issue 4 of 4, October 2014.
Date searched: Thursday 8 January 2015.
Hits: 23 (102 in total – CDSR 20, DARE 3, CENTRAL 75, NHS EED 2, Groups 2, HTA 0).
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | MeSH descriptor: [Kidney Transplantation] this term only | 3313 |
| 2 | (Kidney* near/3 transplant*) | 5959 |
| 3 | (Renal near/3 transplant*) | 4492 |
| 4 | ((kidney or renal) near/3 (recipient* or dono* or donation* or replac*)) | 3839 |
| 5 | ((graft* or allograft* or homograft* or allogeneic) and (kidney* or renal)) | 5192 |
| 6 | #1 or #2 or #3 or #4 or #5 | 9188 |
| 7 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”) | 522 |
| 8 | ((rabbit* near/3 Anti-thymocyte*) or (rabbit* near/3 Antithymocyte*) or (rabbit* near/3 thymocyte*) or (rabbit* near/3 polyclonal) or (rabbit* and ATG) or RATG or thymoglobulin*) | 364 |
| 9 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”) | 2587 |
| 10 | MeSH descriptor: [Tacrolimus] this term only | 1181 |
| 11 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”) | 87 |
| 12 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep* or Myfenax or Myfortic or Mofetil) | 3477 |
| 13 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”) | 2200 |
| 14 | MeSH descriptor: [Sirolimus] this term only | 1071 |
| 15 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”) | 940 |
| 16 | #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 | 7472 |
| 17 | #6 and #16 Publication Year from 2014 to 2015 | 102 |
Notes: the search strategy represents the whole of The Cochrane Library. CDSR and DARE results downloaded but not CENTRAL or NHS EEDS as hits/results would have been picked up in the effectiveness and cost-effectiveness searches.
File: N/A.
Database: Health Management Information Consortium (HMIC)
Host: Ovid.
Data parameters: 1979 to November 2014.
Date searched: Thursday 8 January 2015.
Hits: 0.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | Kidney Transplantation/ | 121 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 84 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 81 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 152 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 28 |
| 6 | 1 or 2 or 3 or 4 or 5 | 314 |
| 7 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 2 |
| 8 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 1 |
| 9 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 8 |
| 10 | Tacrolimus/ | 0 |
| 11 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 0 |
| 12 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 23 |
| 13 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 11 |
| 14 | Sirolimus/ | 0 |
| 15 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 2 |
| 16 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 33 |
| 17 | 6 and 16 | 3 |
| 18 | 16 and 17 | 3 |
| 19 | limit 18 to yr=“2014 -Current” | 0 |
Notes: N/A.
File: N/A.
Ongoing studies
(Basiliximab OR Basiliximabum OR Simulect OR “interleukin 2 receptor antibody”) AND (kidney* OR renal)
((rabbit AND Anti-thymocyte*) OR (rabbit AND Antithymocyte*) OR (rabbit AND thymocyte*) OR (rabbit* AND polyclonal) OR (rabbit* AND ATG) OR RATG OR thymoglobulin*) AND (kidney* OR renal)
(Tacrolimus OR Fujimycin OR Prograf OR Advagraf OR Adoport OR Capexion OR Modigraf OR Perixis OR Tacni OR Vivadex OR Protopic OR Tsukubaenolide OR “FK 506” OR “FK-506” OR “FK506” OR “fr-900506”) AND (kidney* OR renal)
(Belatacept OR Nulojix OR “lea29y” OR “lea 29y” OR “bms 224818”) AND (kidney* OR renal)
(“Mycophenolic acid” OR MPA OR Mycophenolate OR Arzip OR CellCep* OR Myfenax OR Myfortic OR Mofetil) AND (kidney* OR renal)
(Sirolimus OR Rapamune OR Rapamycin OR “ay 22-989”) AND (kidney* OR renal)
(Everolimus OR Zortress OR Certican OR Afinitor OR Evertor OR “SDZ RAD”) AND (kidney* OR renal)
Cost-effectiveness searches
Database: MEDLINE
Host: Ovid.
Data parameters: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to present.
Date searched: Thursday 15 January 2015.
Hits: 34.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | Kidney Transplantation/ | 79,778 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 34,082 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 40,996 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 35,985 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 45,333 |
| 6 | 1 or 2 or 3 or 4 or 5 | 112,264 |
| 7 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 1054 |
| 8 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 6278 |
| 9 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 16,989 |
| 10 | Tacrolimus/ | 12,817 |
| 11 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 217 |
| 12 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 27,735 |
| 13 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 20,509 |
| 14 | Sirolimus/ | 13,403 |
| 15 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 3038 |
| 16 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 71,697 |
| 17 | 6 and 16 | 9482 |
| 18 | Economics/ | 26,539 |
| 19 | exp Economics, Pharmaceutical/ | 2535 |
| 20 | exp Economics, Medical/ | 13,480 |
| 21 | exp Economics, Hospital/ | 19,774 |
| 22 | (pharmacoeconomic* or socioeconomics or economic$).ti,ab,kw. | 180,610 |
| 23 | ec.fs. | 339,974 |
| 24 | exp “Costs and Cost Analysis”/ | 183,530 |
| 25 | Cost of Illness/ | 18,219 |
| 26 | (cost* or cba or cea or cua or (value adj2 money) or pric$ or fiscal or funding or financial or finance or budget$ or (expenditure$ not Energy)).ti,ab,kw. | 517,055 |
| 27 | 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 | 872,822 |
| 28 | 17 and 27 | 431 |
| 29 | limit 28 to yr=“2014 -Current” | 34 |
Notes: N/A.
File: N/A.
Database: EMBASE
Host: Ovid.
Data parameters: EMBASE 1974 to 14 January 2015.
Date searched: Thursday 15 January 2015.
Hits: 139.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | kidney transplantation/ | 97,901 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 51,174 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 56,282 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 52,361 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 66,121 |
| 6 | 1 or 2 or 3 or 4 or 5 | 154,466 |
| 7 | basiliximab/ | 6765 |
| 8 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 2325 |
| 9 | thymocyte antibody/ | 20,465 |
| 10 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 8936 |
| 11 | tacrolimus/ | 54,246 |
| 12 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 26,521 |
| 13 | belatacept/ | 1006 |
| 14 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 555 |
| 15 | mycophenolic acid/ | 10,141 |
| 16 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 36,267 |
| 17 | rapamycin/ | 36,926 |
| 18 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 29,195 |
| 19 | everolimus/ | 14,696 |
| 20 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 7151 |
| 21 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 | 150,139 |
| 22 | 6 and 21 | 25,879 |
| 23 | exp Economics/ | 220,609 |
| 24 | models, economic/ | 105,274 |
| 25 | exp health economics/ | 636,555 |
| 26 | exp “Costs and Cost Analysis”/ | 263,409 |
| 27 | Cost of illness/ | 14,621 |
| 28 | resource allocation/ | 15,767 |
| 29 | pe.fs. | 62,540 |
| 30 | (cost$ or cba or cea or cua or (value adj2 money) or pric$ or fiscal or funding or financial or finance or budget$ or (expenditure$ not Energy)).ti,ab,kw. | 673,305 |
| 31 | 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 | 1,300,678 |
| 32 | 22 and 31 | 1475 |
| 33 | limit 32 to yr=“2014 -Current” | 139 |
Notes: N/A.
File: N/A.
Database: Cochrane NHS EED
Host: Wiley Online Library.
Data parameters: Issue 4 of 4, October 2014.
Date searched: Thursday 15 January 2015.
Hits: 2.
Search strategy
| ID | Search | Hits |
|---|---|---|
| 1 | MeSH descriptor: [Kidney Transplantation] this term only | 3313 |
| 2 | (Kidney* near/3 transplant*) | 5959 |
| 3 | (Renal near/3 transplant*) | 4493 |
| 4 | ((kidney or renal) near/3 (recipient* or dono* or donation* or replac*)) | 3839 |
| 5 | ((graft* or allograft* or homograft* or allogeneic) and (kidney* or renal)) | 5193 |
| 6 | #1 or #2 or #3 or #4 or #5 | 9189 |
| 7 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”) | 522 |
| 8 | ((rabbit* near/3 Anti-thymocyte*) or (rabbit* near/3 Antithymocyte*) or (rabbit* near/3 thymocyte*) or (rabbit* near/3 polyclonal) or (rabbit* and ATG) or RATG or thymoglobulin*) | 364 |
| 9 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”) | 2587 |
| 10 | MeSH descriptor: [Tacrolimus] this term only | 1181 |
| 11 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”) | 87 |
| 12 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep* or Myfenax or Myfortic or Mofetil) | 3477 |
| 13 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”) | 2200 |
| 14 | MeSH descriptor: [Sirolimus] this term only | 1071 |
| 15 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”) | 941 |
| 16 | #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 | 7473 |
| 17 | #6 and #16 Publication Year from 2014 to 2015 | 102 |
Notes: this search strategy represents the whole of The Cochrane Library (NHS EED 2, Groups 2, CENTRAL 75, CDSR 20, DARE 3).
File: N/A.
Database: Web of Science
Host: ISI Thompson Reuters.
Data parameters: 1900–current.
Date searched: Thursday 15 January 2015.
Hits: 55.
Search strategy
| # | Results | Searches |
|---|---|---|
| 16 | 55 | #14 AND #13 Refined by: PUBLICATION YEARS: ( 2014 ) Timespan=All years Search language=Auto |
| 15 | 697 | #14 AND #13 Timespan=All years Search language=Auto |
| 14 | Approximately 3,354,783 | TOPIC: (((pharmacoeconomic* or socioeconomics or economic* or pric* or cost* or cba or cea or cua or “health utilit*” or “value for money”))) Timespan=All years Search language=Auto |
| 13 | Approximately 30,726 | #12 AND #5 Timespan=All years Search language=Auto |
| 12 | Approximately 261,400 | #11 OR #10 OR #9 OR #8 OR #7 OR #6 Timespan=All years Search language=Auto |
| 11 | Approximately 12,458 | TOPIC: (((Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”))) Timespan=All years Search language=Auto |
| 10 | Approximately 175,118 | TOPIC: (((“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep* or Myfenax or Myfortic or Mofetil))) Timespan=All years Search language=Auto |
| 9 | 554 | TOPIC: (((Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”))) Timespan=All years Search language=Auto |
| 8 | Approximately 65,143 | TOPIC: (((Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”))) Timespan=All years Search language=Auto |
| 7 | Approximately 21,632 | TOPIC: ((((rabbit* near/3 Anti-thymocyte*) or (rabbit* near/3 Antithymocyte*) or (rabbit* near/3 thymocyte*) or (rabbit* near/3 polyclonal) or (rabbit* and ATG) or RATG or thymoglobulin*))) Timespan=All years Search language=Auto |
| 6 | 2,283 | TOPIC: (((Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”))) Timespan=All years Search language=Auto |
| 5 | Approximately 332,469 | #4 OR #3 OR #2 OR #1 Timespan=All years Search language=Auto |
| 4 | Approximately 158,169 | TOPIC: ((((graft* or allograft* or homograft* or allogeneic) and (kidney* or renal)))) Timespan=All years Search language=Auto |
| 3 | Approximately 122,313 | TOPIC: ((((kidney or renal) near/3 (recipient* or dono* or donation* or replac*)))) Timespan=All years Search language=Auto |
| 2 | Approximately 145,513 | TOPIC: (((Renal near/3 transplant*))) Timespan=All years Search language=Auto |
| 1 | Approximately 163,622 | TOPIC: (((Kidney* near/3 transplant*))) Timespan=All years Search language=Auto |
Notes: auto suggest was turned off.
File: N/A.
Database: EconLit
Host: EBSCOhost.
Data parameters: 1886–current.
Date searched: Thursday 15 January 2015.
Hits: 0.
Search strategy
(Basiliximab or Basiliximabum or Simulect or Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or Belatacept or Nulojix or “Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep or Myfenax or Myfortic or Mofetil or Sirolimus or Rapamune or Rapamycin or Everolimus or Zortress or Certican or Afinitor or Evertor) AND (kidney or renal)
Notes: N/A.
File: N/A.
Database: Health Economic Evaluations Database (HEED)
Host: via The Cochrane Library.
Date searched: Monday 14 April 2014.
Hits: 35.
(Basiliximab or Basiliximabum or Simulect or Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or Belatacept or Nulojix or “Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep or Myfenax or Myfortic or Mofetil or Sirolimus or Rapamune or Rapamycin or Everolimus or Zortress or Certican or Afinitor or Evertor) AND (kidney or renal)
Notes: the search recorded here was our initial search. HEED had closed by the time we updated the searches, so we were unable to update our HEED searches.
File: N/A.
Searches for utility data: search strategy
The searches for utility data are recorded below. These searches took the following form: (terms for kidney or renal transplant or kidney or renal graft or renal dialysis) AND (terms for utility questionnaires such as SF36 or CHU 9D) and were run from database inception.
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to present.
Date searched: 3 September 2014.
Volume: 714.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | Kidney Transplantation/ | 79,870 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw. | 33,553 |
| 3 | (Renal adj3 transplant$).ti,ab,kw. | 40,747 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw. | 35,663 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw. | 45,183 |
| 6 | 1 or 2 or 3 or 4 or 5 | 112,067 |
| 7 | Renal Dialysis/ | 73,812 |
| 8 | Peritoneal Dialysis/ | 14,950 |
| 9 | ((kidney or renal or peritoneal) and (dialysis or dialyses)).ti,ab,kw. | 48,847 |
| 10 | 7 or 8 or 9 | 107,010 |
| 11 | 6 or 10 | 201,694 |
| 12 | (euroqol or euro qol or eq5d or eq 5d or EQ-5D or EQ-5D-Y).ti,ab,kw. | 4481 |
| 13 | (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,kw. | 1391 |
| 14 | (sf10 or sf 10 or short form 10 or shortform 10 or sf ten or sften or shortform ten or short form ten).ti,ab,kw. | 77 |
| 15 | (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve).ti,ab,kw. | 3016 |
| 16 | (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab,kw. | 24 |
| 17 | (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty).ti,ab,kw. | 341 |
| 18 | (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirstysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,kw. | 17,026 |
| 19 | (health utilities index$ or (hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3)).ti,ab,kw. | 1172 |
| 20 | (“time trade off” or “time tradeoff” or TTO).ti,ab,kw. | 1234 |
| 21 | standard gamble$.ti,ab,kw. | 697 |
| 22 | (CHU9D or CHU 9D or “Child Health Utility”).ti,ab,kw. | 13 |
| 23 | “discrete choice”.ti,ab,kw. | 713 |
| 24 | (AQoL or “Assessment of Quality of Life”).ti,ab,kw. | 1274 |
| 25 | 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 | 28,980 |
| 26 | 11 and 25 | 766 |
| 27 | limit 26 to english language | 714 |
Notes: N/A.
File name: MEDLINE.txt.
Database: EMBASE
Host: Ovid.
Data parameters: 1974 to 2014 week 34.
Date searched: 3 September 2014.
Volume: 915
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | kidney transplantation/ | 96,703 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw. | 50,181 |
| 3 | (Renal adj3 transplant$).ti,ab,kw. | 55,376 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw. | 51,117 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw. | 64,806 |
| 6 | 1 or 2 or 3 or 4 or 5 | 151,605 |
| 7 | renal replacement therapy/ | 36,722 |
| 8 | peritoneal dialysis/ | 23,371 |
| 9 | ((kidney or renal or peritoneal) and (dialysis or dialyses)).ti,ab,kw. | 64,637 |
| 10 | 7 or 8 or 9 | 97,785 |
| 11 | 6 or 10 | 224,149 |
| 12 | (euroqol or euro qol or eq5d or eq 5d or EQ-5D or EQ-5D-Y).ti,ab,kw. | 7316 |
| 13 | (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,kw. | 1533 |
| 14 | (sf10 or sf 10 or short form 10 or shortform 10 or sf ten or sften or shortform ten or short form ten).ti,ab,kw. | 109 |
| 15 | (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve).ti,ab,kw. | 4428 |
| 16 | (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab,kw. | 35 |
| 17 | (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty).ti,ab,kw. | 333 |
| 18 | (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirstysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,kw. | 23,918 |
| 19 | Short Form 36/ | 12,496 |
| 20 | (health utilities index$ or (hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3)).ti,ab,kw. | 1547 |
| 21 | (“time trade off” or “time tradeoff” or TTO).ti,ab,kw. | 1599 |
| 22 | standard gamble$.ti,ab,kw. | 812 |
| 23 | (CHU9D or CHU 9D or “Child Health Utility”).ti,ab,kw. | 13 |
| 24 | “discrete choice”.ti,ab,kw. | 958 |
| 25 | (AQoL or “Assessment of Quality of Life”).ti,ab,kw. | 1812 |
| 26 | 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 | 43,846 |
| 27 | 11 and 26 | 991 |
| 28 | limit 27 to english language | 915 |
Notes: N/A.
File name: EMBASE.txt.
Database: The Cochrane Library (CENTRAL, HTA and NHS EED)
Host: Wiley Online Library.
Data parameters: CENTRAL Issue 8 of 12, August 2014; HTA and NHS EED Issue 3 of 4 July 2014.
Date searched: 3 September 2014.
Volume: 174.
Search strategy
| ID | Search | Hits |
|---|---|---|
| 1 | MeSH descriptor: [Kidney Transplantation] this term only | 3298 |
| 2 | (Kidney* near/2 transplant*) | 5497 |
| 3 | (Renal near/2 transplant*) | 3841 |
| 4 | ((kidney or renal) near/2 (recipient* or dono* or donation* or replac*)) | 3399 |
| 5 | ((graft* or allograft* or homograft* or allogeneic) and (kidney* or renal)) | 4785 |
| 6 | #1 or #2 or #3 or #4 or #5 | 8307 |
| 7 | MeSH descriptor: [Renal Dialysis] this term only | 3496 |
| 8 | MeSH descriptor: [Peritoneal Dialysis] this term only | 417 |
| 9 | ((kidney or renal or peritoneal) and (dialysis or dialyses)) | 8888 |
| 10 | #7 or #8 or #9 | 8888 |
| 11 | #6 or #10 | 15,502 |
| 12 | (euroqol or euro qol or eq5d or eq 5d or EQ-5D or EQ-5D-Y) | 2221 |
| 13 | (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six) | 11,746 |
| 14 | (sf10 or sf 10 or short form 10 or shortform 10 or sf ten or sften or shortform ten or short form ten) | 12,533 |
| 15 | (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve) | 9569 |
| 16 | (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen) | 6668 |
| 17 | (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty) | 7393 |
| 18 | (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirstysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six) | 9081 |
| 19 | (health utilities index* or (hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3)) | 6541 |
| 20 | (“time trade off” or “time tradeoff” or TTO) | 512 |
| 21 | standard gamble* | 521 |
| 22 | (CHU9D or CHU 9D or “Child Health Utility”) | 3 |
| 23 | “discrete choice” | 47 |
| 24 | (AQoL or “Assessment of Quality of Life”) | 302 |
| 25 | #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 | 22,511 |
| 26 | #11 and #25 | 847 |
Notes: N/A.
File name: Cochrane.txt.
Resource: ScHARRHUD
URL: (http://update-sbs.update.co.uk/scharr11/index.php?recordsN1&m=search).
Date searched: 3 September 2014.
Volume: 9.
Search strategy
kidney* or renal or dialysis
Notes: N/A.
File name: N/A.
Resource: EuroQoL website
URL: www.euroqol.org/eq-5d-references/reference-search.html.
Date searched: 3 September 2014.
Volume: 24.
Search strategy
kidney or renal or dialysis
Notes: 5 out of 24 were unique when de-duplicated against the EMBASE search.
File name: N/A.
Resource: HERC database of mapping studies
URL: www.herc.ox.ac.uk/downloads/mappingdatabase.
Date searched: 3 September 2014.
Volume: 0.
Search strategy
A hand-search of the Excel database was performed.
Notes: Dakin H. Review of studies mapping from quality of life or clinical measures to EQ-5D: an online database. Health and Quality of Life Outcomes. 11:151. HERC database of mapping studies, Version 3.0 (Last updated: 26th June 2014). 2013. URL: www.herc.ox.ac.uk/downloads/mappingdatabase.
Appendix 2 Excluded studies
| Study | Reason |
|---|---|
| Health Technology Assessment database. Immunosuppressive therapy for renal transplantation in children and adolescents. In NICE 2006. HTA database Accession Number: 32006000316 | Abstract |
| Health Technology Assessment database. Belatacept for prophylaxis of organ rejection in renal transplantation. In National Horizon Scanning Centre 2008. HTA database Accession Number: 32010000604 | Abstract |
| Health Technology Assessment database. Everolimus (Certican) for prophylaxis of organ rejection in renal or cardiac transplantation. In National Horizon Scanning Centre 2008. HTA database Accession Number: 32010000590 | Abstract |
| Health Technology Assessment database. Rapid HTA on the use of everolimus to prevent renal transplant rejection. In Department of Science and Technology - Brazilian Health Technology Assessment General Coordination (DECIT-CGATS) 2009. HTA database Accession Number: 32011000271 | Abstract |
| Health Technology Assessment database. Tacrolimus (Advagraf®) for the prophylaxis of transplantrejection in adult kidney or liver allograft recipients and the treatment of allograft rejection resistant to treatment with other immunosuppressive medicinal products in adult patients. In All Wales Therapeutics and Toxicology Centre (AWTTC), secretariat of the All Wales Medicines Strategy Group (AWMSG) 2009. HTA database Accession Number: 32012000410 | Abstract |
| Health Technology Assessment database. Tacrolimus (Advagraf®). In All Wales Therapeutics and Toxicology Centre (AWTTC) 2011. HTA database Accession Number: 32012000361 | Abstract |
| Health Technology Assessment database. Belatacept (Nulojix®). In All Wales Therapeutics and Toxicology Centre (AWTTC) 2012. HTA database Accession Number: 32012000600) | Abstract |
| Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet 2011;377:837–47. [Erratum published in Lancet 2012;380:1994.] | No data |
| Albano L, Banas B, Klempnauer JL, Glyda M, Viklicky O, Kamar N, Optimising immunoSuppression After Kidney transplantation with ADVAGRAF Study Group. OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation 2013;96:897–903 [Erratum published in Transplantation 2014;97:e38.] | No data |
| Silva AP, Tonato E, Durao Jr M, Requiao-Moura L, Arruda E, Chinen R, et al. A randomized clinical trial of early conversion from tacrolimus to everolimus in deceased donor kidney transplantation. Transpl Int 2013;26:277–78 | Abstract |
| Abou-Jaoude MM, Ghantous I, Almawi WY. Tacrolimus (FK506) versus cyclosporin A microemulsion (Neoral) maintenance immunosuppression: effects on graft survival and function, infection, and metabolic profile following kidney transplantation (KT). Mol Immunol 2003;39:1095–100 | Population |
| Abou-Jaoude MM, Irani-Hakime N, Ghantous I, Najm R, Afif C, Almawi WY. Cyclosporine microemulsion (Neoral) versus tacrolimus (FK506) as maintenance therapy in kidney transplant patients. Transplant Proc 2003;35:2748–9 | Study design |
| Abou-Jaoude MM, Najm R, Shaheen J, Nawfal N, Abboud S, Alhabash M, et al. Tacrolimus (FK506) versus cyclosporine microemulsion (neoral) as maintenance immunosuppression therapy in kidney transplant recipients. Transplant Proc 2005;37:3025–8 | Study design |
| Abramowicz D, Del Carmen Rial M, Vitko S, del Castillo D, Manas D, Lao M, et al. Cyclosporine withdrawal from a mycophenolate mofetil-containing immunosuppressive regimen: results of a five-year, prospective, randomized study. J Am Soc Nephrol 2005;16:2234–40 | Population |
| Adu D, Cockwell P, Ives NJ, Shaw J, Wheatley K. Interleukin-2 receptor monoclonal antibodies in renal transplantation: meta-analysis of randomised trials. BMJ 2003;326:789 | Study design |
| Agha IA, Brennan DC. BK virus and current immunosuppressive therapy. Graft 2002;5:S65–72 | Study design |
| Ahlenstiel-Grunow T, Koch A, Großhennig A, Frömke C, Sester M, Sester U, et al. A multicenter, randomized, open-labeled study to steer immunosuppressive and antiviral therapy by measurement of virus (CMV, ADV, HSV)-specific T cells in addition to determination of trough levels of immunosuppressants in pediatric kidney allograft recipients (IVIST01-trial): study protocol for a randomized controlled trial. Trials 2014;15:324 | Study design |
| Ahsan N, Holman MJ, Jarowenko MV, Razzaque MS, Yang HC. Limited dose monoclonal IL-2R antibody induction protocol after primary kidney transplantation. Am J Transplant 2002;2:568–73 | Intervention |
| Akalin E, Ames S, Sehgal V, Murphy B, Bromberg JS, Fotino M, Friedlander R. Intravenous immunoglobulin and thymoglobulin induction treatment in immunologically high-risk kidney transplant recipients. Transplantation 2005;79:742 | Abstract |
| Al Najjar A, Etienne I, Le Pogamp P, Bridoux F, Le Meur Y, Toupance O, et al. Long-term results of monoclonal anti-Il2-receptor antibody versus polyclonal antilymphocyte antibodies as induction therapy in renal transplantation. Transplant Proc 2006;38:2298–9 | Abstract |
| Al Najjar A, Etienne I, Toupance O, Westeel PF, Hurault De Ligny B, Rerolle JP, et al. Long term follow-up of a multicenter randomized trial comparing a CNI-free regimen with sirolimus (SRL) to a cyclosporine based regimen: the spiesser study. Am J Transplant 2010;10:505 | Abstract |
| Albano L, Alamartine E, Toupance O, Moulin B, Merville P, Rerolle JP, et al. Conversion from everolimus with low-exposure cyclosporine to everolimus with mycophenolate sodium maintenance therapy in kidney transplant recipients: a randomized, open-label multicenter study. Ann Transplant 2012;17:58–67 | Population |
| Albano L, Banas B, Kamar N. Safety and renal function in tacrolimus prolonged release vs tacrolimus immediate release-based therapy in renal transplantation – The OSAKA study. Am J Transplant 2011;11:125 | Abstract |
| Albano L, Banas B, Kamar N. Outcomes with tacrolimus-based immunosuppression after kidney transplantation with standard-or extendedcriteria donor organsthe osaka study. Transpl Int 2013;26:59 | Abstract |
| Albano L, Banas B, Rostaing L. Efficacy and optimised dosing in tacrolimus prolonged release vs tacrolimus immediate release-based therapy in renal transplantation – The OSAKA study. Am J Transplant 2011;11:125 | Abstract |
| Albano L, Banas B, Klempnauer JL, Glyda M, Viklicky O, Kamar N, Optimising immunoSuppression After Kidney transplantation with ADVAGRAF Study Group. OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation 2013;96:897–903 | Population |
| Alberú J, Pascoe MD, Campistol JM, Schena FP, Rial Mdel C, Polinsky M, et al. Lower malignancy rates in renal allograft recipients converted to sirolimus-based, calcineurin inhibitor-free immunotherapy: 24-month results from the CONVERT trial. Transplantation 2011;92:303–10 | Population |
| Alemi M, Samadzadeh B, Bardideh A, Heidarnejadiyan J, Torkaman Asadi F. The effect of preoperative induction therapy with mycophenolate mofetil in early outcomes of living-donor renal allograft transplantation. Int J Urol 2012;19:163 | Abstract |
| Alloway R, Mulgaonkar S, Ueda K, Cohen D, Kaplan B. A Phase 2 randomized study of the pharmacokinetics, safety and efficacy of LCP-Tacro tablets once-a-day vs Prograf capsules twice-a-day in de novo kidney transplants. Am J Transplant 2011;11:355 | Abstract |
| Alloway R, Steinberg S, Khalil K, Gourishankar S, Miller J, Norman D, et al. Conversion of stable kidney transplant recipients from a twice daily Prograf-based regimen to a once daily modified release tacrolimus-based regimen. Transplant Proc 2005;37:867–70 | Study design |
| Alloway RR, Mulgaonkar S, Bowers VD, Stevenson KRU, Cohen DJ, Katz E, et al. A phase 2b, open-label, multi-center, prospective, randomized study to compare the pharmacokinetics and safety of lcp-tacro (TM) tablets once-a-day to prograf (R) capsules twice-a-day in de novo kidney transplant patients. Am J Transplant 2009;9:414 | Abstract |
| Alloway RR, Sadaka B, Trofe-Clark J, Wiland A, Bloom RD. Pharmacokinetic comparison of generic tacrolimus (hecoria (TM)) versus prograf (R) in stable kidney transplant recipients: a randomized, crossover study. Am J Transplant 2012;12:406 | Abstract |
| Alpay N. Conversion from calcineurin inhibitors to everolimus resulted in decrease of serum TGF-beta and urinary ngal in renal transplant recipients. Nephrol Dial Transplant 2013;28:i500–1 | Abstract |
| Alvarado A, Chhabra D, Wang E, Najafian N, Friedewald J, Ho B, et al. Prospective randomized study to evaluate the feasability of CNI elimination with conversion to sirolimus in prednisone-free immunosuppressive regimen. Am J Transplant 2012;12:42 | Abstract |
| Andrassy J, Hoffmann VS, Rentsch M, Stangl M, Habicht A, Meiser B, et al. Is cytomegalovirus prophylaxis dispensable in patients receiving an mTOR inhibitor-based immunosuppression? a systematic review and meta-analysis. Transplantation 2012;94:1208–17 | Duplicate |
| Andres A, Bloom R, Bunnapradist S, Cassuto E, Chan L, Hart M, et al. Randomized, multicenter study on the safety and efficacy of enteric-coated mycophenolate sodium combined with basiliximab and low-or standard dose of tacrolimus in de novo renal transplant patients. Transpl Int 2007;20:217 | Abstract |
| Andrés A, Budde K, Clavien PA, Becker T, Kessler M, Pisarski P, et al. A randomized trial comparing renal function in older kidney transplant patients following delayed versus immediate tacrolimus administration. Transplantation 2009;88:1101–8 | Study design |
| Andres A, del Castillo D, Gainza FJ, Purroy A, Bustamante J, Rengel M, et al. Comparison of a secuential therapy with tacrolimus versusa standar triple therapy in aged kidney transplantation with aged donors: results of a multicenter, prospective and randomized trial (Estrella Study). Am J Transplant 2007;7:443 | Abstract |
| Andrés A, Delgado-Arranz M, Morales E, Dipalma T, Polanco N, Gutierrez-Solis E, et al. Extended-release tacrolimus therapy in de novo kidney transplant recipients: single-center experience. Transplant Proc 2010;42:3034–7 | Study design |
| Andres I, Font B, Mora S, Lahoz R, Ortega F. Quality of life of enteric-coated mycophenolate sodium (EC-MPS) in renal transplant recipients with gastrointestinal tract complaints to mycophenolate mofetil (MMF)Myvida study. Value Health 2009;12:A311 | Abstract |
| Anil Kumar MS, Heifets M, Fyfe B, Saaed MI, Moritz MJ, Parikh MH, Kumar A. Comparison of steroid avoidance in tacrolimus/mycophenolate mofetil and tacrolimus/sirolimus combination in kidney transplantation monitored by surveillance biopsy. Transplantation 2005;80:807–14 | Population |
| Anil Kumar MS, Irfan Saeed M, Ranganna K, Malat G, Sustento-Reodica N, Kumar AM, Meyers WC. Comparison of four different immunosuppression protocols without long-term steroid therapy in kidney recipients monitored by surveillance biopsy: five-year outcomes. Transpl Immunol 2008;20:32–42 | Population |
| Anonymous. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ 2014;349:g7543 | No data |
| Perez-Simon J, Sr., Martino R, Parody R, Cabrero M, Lopez-Corral L, Valcarcel D, et al. The combination of siromilus plus tacrolimus (SiTac) improves the results of cyclosporine plus mycophenolate mofetil (CsAMMF) after reduced intensity conditioning (RIC) unrelated donor allogeneic transplantation. Blood 2011;118:406–7 | Abstract |
| Araki M, Flechner SM, Ismail HR, Flechner LM, Zhou L, Derweesh IH, et al. Posttransplant diabetes mellitus in kidney transplant recipients receiving calcineurin or mTOR inhibitor drugs. Transplantation 2006;81:335–41 | Study design |
| Arns W, Breuer S, Choudhury S, Taccard G, Lee J, Binder V, et al. Enteric-coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophenolate mofetil. Clin Transplant 2005;19:199–206 | Outcome |
| Arns W, Neumayer HH, Lehner F, Witzke O, Sommerer C, Kliem V, et al. Herakles at month 24: follow-up results on efficacy and safety of three different treatment regimens in de novo renal transplant patients demonstrate options for individualized immunsosuppression. Transpl Int 2013;26:21 | Abstract |
| Arns W, Sommerer C, Witzke O, Lehner F, Zeier M, Neumayer HH, et al. Efficacy and safety of three different treatment regimens in de novo renal transplant patients: results of the herakles trial. Transplantation 2012;94:995 | Abstract |
| Arora S, Tangirala B, Osadchuk L, Sureshkumar KK. Belatacept : a new biological agent for maintenance immunosuppression in kidney transplantation. Expert Opin Biol Ther 2012;12:965–79 | Study design |
| Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Hené RJ, et al. Randomized conversion from cyclosporine to tacrolimus in renal transplant patients: improved lipid profile and unchanged plasma homocysteine levels. Transplant Proc 2002;34:1793–4 | Population |
| Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Improved cardiovascular risk profile and renal function in renal transplant patients after randomized conversion from cyclosporine to tacrolimus. J Am Soc Nephrol 2003;14:1880–8 | Population |
| Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Conversion from cyclosporine to tacrolimus improves quality-of-life indices, renal graft function and cardiovascular risk profile. Am J Transplant 2004;4:937–45 | Population |
| Åsberg A, Apeland T, Reisaeter AV, Foss A, Leivestad T, Heldal K, et al. Long-term outcomes after cyclosporine or mycophenolate withdrawal in kidney transplantation - results from an aborted trial. Clin Transpl 2013;27:E151–6 | Population |
| Asberg A, Midtvedt K, Line PD, Narverud J, Holdaas H, Jenssen T, et al. Calcineurin inhibitor avoidance with daclizumab, mycophenolate mofetil, and prednisolone in DR-matched de novo kidney transplant recipients. Transplantation 2006;82:62–8 | Comparator |
| Baas MC, Gerdes VE, Ten Berge IJ, Heutinck KM, Florquin S, Meijers JC, Bemelman FJ. Treatment with everolimus is associated with a procoagulant state. Thromb Res 2013;132:307–11 | Outcome |
| Baas MC, Kers J, Florquin S, de Fijter JW, van der Heide JJ, van den Bergh Weerman MA, et al. Cyclosporine versus everolimus: effects on the glomerulus. Clin Transplant 2013;27:535–40 | Study design |
| Baas MC, Kers J, Florquin S, Van Den Bergh Weerman MA, Ten Berge IJM, Bemelman FF. Prolonged treatment with everolimus does not induce podocyte damage and leaves the glomerular basement membrane intact. Am J Transplant 2011;11:317 | Abstract |
| Baboolal K, Zaiac M, Zamauskaite A, Newstead C. This multicentre, randomised study comparing conversion from calcineurin inhibitors (CNIs) to sirolimus versus standard therapy in renal allograft recipients showed a lower rate of development of subsequent malignant disease in the group receiving sirolimus. Am J Transplant 2009;9:238 | Abstract |
| Baczkowska T, Perkowska-Ptasińska A, Sadowska A, Lewandowski Z, Nowacka-Cieciura E, Cieciura T, et al. Serum TGF-beta1 correlates with chronic histopathological lesions in protocol biopsies of kidney allograft recipients. Transplant Proc 2005;37:773–5 | Intervention |
| Bakker RC, Hollander AA, Mallat MJ, Bruijn JA, Paul LC, de Fijter JW. Conversion from cyclosporine to azathioprine at three months reduces the incidence of chronic allograft nephropathy. Kidney Int 2003;64:1027–34 | Intervention |
| Bakr MA, Gheith OA, Ismael AM, Baz ME, Shehab El-Dein AB, Ghoneim MA. Rescue immunosuppressive therapies in living-related renal allotransplant: a long-term prospective randomized evaluation. Exp Clin Transplant 2008;6:48–53 | Population |
| Balbontin FG, Kiberd B, Belistky P, Singh D, Fraser A, Lawen JG. One year randomized study comparing cyclosporine microemulsion with C2 monitoring and tacrolimus in de novo kidney transplantation. Am J Transplant 2004;4:236–7 | Abstract |
| Balbontin FG, Kiberd B, Belitsky P, Singh D, Fraser A, Lawen JG. Six month randomized study comparing cyclosporine microemulsion with C2 monitoring and tacrolimus in de novo kidney transplantation. J Urol 2004;171:515 | Outcome |
| Banas B, Albano L, Cassuto E, Glyda M, Klempnauer J, Lehner F, et al. The impact of acute rejection on renal function-perspectives from the OSAKA study. Transplantation 2012;94:983 | Abstract |
| Banas B, Boger CA, Lehner F. Efficacy, safety and optimised dosing in tacrolimus prolonged release vs tacrolimus immediate release-based therapy in renal transplantation-the Osaka study. Transpl Int 2011;24:35 | Abstract |
| Banas B, Cassuto E, Glyda M, Kamar N, Klempnauer J, Lehner F, et al. Selection of appropriate composite endpoints is critical for assessing efficacy failure-perspectives from the OSAKA study. Transplantation 2012;94:3 | Abstract |
| Banas B, Kamar N, Lehner F, Albano L, Glyda M, Viklicky O. Acute rejection in renal transplantation recipients treated with tacrolimus prolonged release-and immediate release-based therapy – The osaka study (optimizing immunosuppression after kidney transplantationwith advagraf). Transpl Int 2011;24:38–9 | Abstract |
| Banas B, Kruger B, Viklicky O. Tacrolimus prolonged release optimises exposure during the immediate postoperative period. Transplantation 2012;94:81–2 | Abstract |
| Bansal D, Yadav AK, Kumar V, Minz M, Sakhuja V, Jha V. Deferred pre-emptive switch from calcineurin inhibitor to sirolimus leads to improvement in GFR and expansion of T regulatory cell population: a randomized, controlled trial. PLOS ONE 2013;8:e75591 | Study design |
| Barsoum RS, Morsey AA, Iskander IR, Morgan MM, Fayad TM, Atalla NT, et al. The Cairo kidney center protocol for rapamycin-based sequential immunosuppression in kidney transplant recipients: 2-year outcomes. Exp Clin Transplant 2007;5:649–57 | Population |
| Bataille S, Moal V, Gaudart J, Indreies M, Purgus R, Dussol B, et al. Cytomegalovirus risk factors in renal transplantation with modern immunosuppression. Transpl Infect Dis 2010;12:480–8 | Outcome |
| Becker LE, Xue Y, Gross ML, Waldherr R, Schwenger V, Zeier M, et al. Evolution of allograft fibrosis and related markers in kidney transplant patients under treatment with cyclosporine and everolimus. NDT Plus 2010;3:iii527 | Abstract |
| Bemelman FJ, de Maar EF, Press RR, van Kan HJ, ten Berge IJ, Homan van der Heide JJ, de Fijter HW. Minimization of maintenance immunosuppression early after renal transplantation: an interim analysis. Transplantation 2009;88:421–8 | Population |
| Benfield MR, Tejani A, Harmon WE, McDonald R, Stablein DM, McIntosh M, Rose S, CCTPT Study Group. A randomized multicenter trial of OKT3 mAbs induction compared with intravenous cyclosporine in pediatric renal transplantation. Pediatr Transplant 2005;9:282–92 | Study design |
| Bertoni E, Carta P, Salvadori M. Cyclosporine very low dose with everolimus high dose is associated with excellent outcomes in renal transplant patients. Transpl Int 2011;24:112 | Abstract |
| Bertoni E, Larti A, Rosso G, Zanazzi M, Di Maria L, Salvadori M. Good outcomes with cyclosporine very low exposure with everolimus high exposure in renal transplant patients. J Nephrol 2011;24:613–8 | Population |
| Birnbaum LM, Lipman M, Paraskevas S, Chaudhury P, Tchervenkov J, Baran D, et al. Management of chronic allograft nephropathy: a systematic review. Clin J Am Soc Nephrol 2009;4:860–5 | Population |
| Blydt-Hansen TD, Gibson IW, Birk PE. Histological progression of chronic renal allograft injury comparing sirolimus and mycophenolate mofetil-based protocols. A single-center, prospective, randomized, controlled study. Pediatr Transplant 2010;14:909–18 | No data |
| Boggi U, Danesi R, Vistoli F, Del Chiaro M, Signori S, Marchetti P, et al. A benefit-risk assessment of basiliximab in renal transplantation. Drug Saf 2004;27:91–106 | Study design |
| Bolin P, Shihab FS, Mulloy L, Henning AK, Gao J, Bartucci M, et al. Optimizing tacrolimus therapy in the maintenance of renal allografts: 12-month results. Transplantation 2008;86:88–95 | Study design |
| Borda B, Lengyel C, Várkonyi T, Kemény E, Ottlakán A, Kubik A, et al. Side effects of the calcineurin inhibitor, such as new-onset diabetes after kidney transplantation. Acta Physiol Hung 2014;101:388–94 | Population |
| Bouwes Bavinck J. Prevention of skin cancer in organ transplant recipients. Br J Dermatol 2012;167:e2 | Abstract |
| Bowman LJ, Edwards A, Brennan DC. The role of rabbit antithymocyte globulin in renal transplantation. Exp Opin Orphan Drug 2014;2:971–87 | Study design |
| Brar JE, Nader ND. Immune minimization strategies in renal transplantation. Immunol Invest 2014;43:807–18 | Study design |
| Brennan DC, Koch MJ. Is mycophenolate mofetil really necessary in renal transplantation? A review of the MYSS follow-up study. Nat Clin Pract Nephrol 2007;3:602–3 | Abstract |
| Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant 2005;5:582–94 | Population |
| Brennan DC, Daller JA, Lake KD, Cibrik D, Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 2006;355:1967–77 | Population |
| Bresnahan B, Vincenti F, Grinyo J, Charpentier B, Russo GD, Garg P, et al. Renal benefit of belatacept versus cyclosporine in kidney transplant patients is not impacted by acute rejection (BENEFIT study). Am J Transplant 2010;10:14 | Abstract |
| Brian Stevens R, Skorupa JY, Rigley TH, Sandoz JP, Kellogg A, Miller N, et al. Calcineurin-inhibitor withdrawalvs. Minimization after kidney transplantation is safe but does not improve renalfunction; 5-year results of a prospective, randomized trial. Am J Transplant 2010;10:505 | Abstract |
| Budde K, Arns W, Sommerer C, Reinke P, Eisenberger U, Fischer W, et al. Improved renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 2 years follow-up of the zeus trial. Am J Transplant. 2010;10:503 | Abstract |
| Budde K, Arns W, Sommerer C, Reinke P, Eisenberger U, Vogel EM, et al. Improved renal function of an Everolimus/Enteric-Coated Mycophenolate Sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 3 years follow-up of the ZEUS trial. Am J Transplant 2011;11:66 | Abstract |
| Budde K, Arns W, Sommerer C, Lehner F, Zeier M, Neumayer H, et al. Superior renal function in an everolimus-based calcineurin inhibitor free regimen compared to standard cyclosporine/mycophenolate and low cyclosporine/everolimus: follow-up of the herakles study at month 24. Am J Transplant 2013;13:310–1 | Abstract |
| Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Analysis of renal function in everolimus/enteric-coated mycophenolate sodium treated de novo renal transplant recipients after calcineurin inhibitor withdrawal: the ZEUS study. Am J Transplant 2009;9:259 | Abstract |
| Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet 2011;377:837–47 | Population |
| Budde K, Bunnapradist S, Rostaing L. A phase III randomized trial of conversion to once-daily extended release meltdose tacrolimus tablets (LCP-tacro) from twice-daily tacrolimus capsules (prograf): efficacy results from an analysis of specific patient sub-populations. Transplantation 2012;94:984 | Abstract |
| Budde K, Bunnapradist S, Grinyo JM, Ciechanowski K, Denny JE, Silva HT, Rostaing L, Envarsus study group. Novel once-daily extended-release tacrolimus (LCPT) versus twice-daily tacrolimus in de novo kidney transplants: one-year results of Phase III, double-blind, randomized trial. Am J Transplant 2014;14:2796–806 | Population |
| Budde K, Curtis J, Knoll G, Chan L, Neumayer HH, Seifu Y, Hall M, ERL B302 Study Group. Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant 2004;4:237–43 | Population |
| Budde K, Glander P, Diekmann F, Dragun D, Waiser J, Fritsche L, et al. Enteric-coated mycophenolate sodium: safe conversion from mycophenolate mofetil in maintenance renal transplant recipients. Transpl Proc 2004;36:524S–7S | Population |
| Budde K, Knoll G, Curtis J, Kahana L, Pohanka E, Seifu Y, Neumayer HH. Safety and efficacy after conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium: results of a 1-year extension study. Transplant Proc 2005;37:912–5 | Study design |
| Budde K, Knoll G, Curtis J, Chan L, Pohanka E, Gentil M, et al. [Long-term safety and efficacy after conversion of maintenance renal transplant recipients from mycophenolate mofetil (MMF) to enteric-coated mycophenolate sodium (EC-MPA, myfortic).] Nieren- und Hochdruckkrankheiten 2006;35:454–64 | Study design |
| Budde K, Knoll G, Curtis J, Chan L, Pohanka E, Gentil M, et al. Long-term safety and efficacy after conversion of maintenance renal transplant recipients from mycophenolate mofetil (MMF) to enteric-coated mycophenolate sodium (EC-MPA, myfortic). Clin Nephrol 2006;66:103–11 | Language |
| Budde K, Lehner F, Arns W, Reinke P, Eisenberger U, Paulus EM, et al. Improved renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 4 years follow-up of the zeus trial. Am J Transplant 2012;12:298 | Abstract |
| Budde K, Lehner F, Sommerer C, Arns W, Reinke P, Eisenberger U, et al. Conversion from cyclosporine to everolimus at 4.5 months posttransplant: 3-year results from the randomized ZEUS study. Am J Transplant 2012;12:1528–40 | Population |
| Budde K, Sommerer C, Haller H, Arns W, Kramer S, Vogel EM, et al. Renal function of an Everolimus based therapy after Calcineurin Inhibitor withdrawal in maintenance renal transplant recipients: 2 year data of the APOLLO trial. Am J Transplant 2011;11:411 | Abstract |
| Budde K, Sommerer C, Haller H, Suwelack B, May C, Paulus EM, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 3 year data of the apollo trial. Am J Transplant 2012;12:298 | Abstract |
| Budde K, Sommerer C, Reinke P, Haller H, Arns W, Witzke O, et al. Outcome on renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 4 year data of the apollo trial. Am J Transplant 2013;13:311–2 | Abstract |
| Budde K, Witzke O, Sommerer C, Reinke P, Eisenberger U, Paulus E, et al. Improved renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 5 years follow-up of the zeus trial. Am J Transplant 2013;13:35–6 | Abstract |
| Budde K, Zeier M, Haller H, Arns W, Kramer S, E MV, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients. Am J Transplant 2010;10:504 | Abstract |
| Büchler M, Caillard S, Barbier S, Thervet E, Toupance O, Mazouz H, et al. Sirolimus versus cyclosporine in kidney recipients receiving thymoglobulin, mycophenolate mofetil and a 6-month course of steroids. Am J Transplant 2007;7:2522–31 | Population |
| Bunnapradist S, Danovitch GM. Minimizing ciclosporin in renal transplant recipients on daclizumab, mycophenolate and steroids. Nat Clin Pract Nephrol 2007;3:426–7 | Abstract |
| Bunnapradist S, Ciechanowski K, West-Thielke P, Mulgaonkar S, Rostaing L, Vasudev B, Budde K, MELT investigators. Conversion from twice-daily tacrolimus to once-daily extended release tacrolimus (LCPT): the phase III randomized MELT trial. Am J Transplant 2013;13:760–9 | Population |
| Burke GW. Randomized Trial of 2 Antibody Induction Steroid Avoidance Protocols Accompanied by Maintenance Therapy with Prograf and Myfortic. URL: clinicaltrials.gov/ct2/show/NCT01172418 (accessed 25 July 2014) | Comparator |
| Burke GW, Ciancio C, Blomberg BB, Rosen A, Suzart K, Roth D, et al. Randomized trial of three different immunosuppressive regimens to prevent chronic renal allograft rejection. Transplant Proc 2002;34:1610–1 | Comparator |
| Burkhalter F, Oettl T, Descoeudres B, Bachmann A, Guerke L, Mihatsch MJ, et al. High incidence of rejection episodes and poor tolerance of sirolimus in a protocol with early steroid withdrawal and calcineurin inhibitor-free maintenance therapy in renal transplantation: experiences of a randomized prospective single-center study. Transpl Proc 2012;44:2961–5 | Study design |
| Busque S, Cantarovich M, Mulgaonkar S, Gaston R, Gaber AO, Mayo PR, et al. The PROMISE study: a phase 2b multicenter study of voclosporin (ISA247) versus tacrolimus in de novo kidney transplantation. Am J Transplant 2011;11:2675–84 | Outcome |
| Cabello M, García P, González-Molina M, Díez de los Rios MJ, García-Sáiz M, Gutiérrez C, et al. Pharmacokinetics of once- versus twice-daily tacrolimus formulations in kidney transplant patients receiving expanded criteria deceased donor organs: a single-center, randomized study. Transplant Proc 2010;42:3038–40 | Abstract |
| Cabello-Diaz M, Gutierrez-Vilchez E, Gonzalez-Molina M, Hidalgo-Guzman P, Diez-de los Rios MJ, Garcia-Saiz M, et al. Pharmacokinetics of the two tacrolimus formulations in older patients who receive a cadaveric kidney graft from an expanded criteria donor. Randomized single-centre study. Basic Clinical Pharmacology and Toxicology 2011;109:32 | Population |
| Campbell S, Walker R, Pilmore H, Kanellis J, Russ G, Hutchison B. Wound healing events are dose related: a multicenter, Prospective study on everolimus in renal transplantation. Immunol Cell Biol 2011;89:A16–7 | Abstract |
| Campbell SB, Walker R, Tai SS, Jiang Q, Russ GR. Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am J Transplant 2012;12:1146–56 | Population |
| Campistol JM, Holt DW, Epstein S, Gioud-Paquet M, Rutault K, Burke JT. Bone metabolism in renal transplant patients treated with cyclosporine or sirolimus. Transpl Int 2005;18:1028–35 | Study design |
| Campos HH, Abbud Filho M, Brazilian Tacrolimus Study Group. One-year follow-up of a Brazilian randomized multicenter study comparing tacrolimus versus cyclosporine in kidney transplantation. Transplant Proc 2002;34:1656–8 | Population |
| Cantarovich D, Rostaing L, Kamar N, Ducloux D, Saint-Hillier Y, Mourad G, et al. Early corticosteroid avoidance in kidney transplant recipients receiving ATG-F induction: 5-year actual results of a prospective and randomized study. Am J Transplant 2014;14:2556–64 | Population |
| Cantarovich M, Durrbach A, Hiesse C, Ladouceur M, Benoit G, Charpentier B. 20-year follow-up results of a randomized controlled trial comparing antilymphocyte globulin induction to no induction in renal transplant patients. Transplantation 2008;86:1732–7 | Study design |
| Cao X, Colombel JF. A systematic review of de novo IBD in solid organ transplant recipient. J Gastroenterol Hepatol 2013;28:590 | Intervention |
| Carmellini M, Pattison J, Riad H, Yaqoob M, Vergara M, Witte S, et al. Renal function in renal transplant recipients after 24 months of immunosuppression with concentration-controlled everolimus plus reduced cyclosporine exposure: update from the A2309 study. Transpl Int 2011;24:57 | Abstract |
| Carmellini M, Todeschini P, Manzia TM, Valerio F, Messina M, Sghirlanzoni MC, et al. Twelve-month outcomes from evidence trial (everolimus once-a-day regimen with cyclosporine versus corticosteroid elimination) in adult kidney transplant recipients. Transpl Int 2013;26:100 | Abstract |
| Carmellini M, Yaqoob M, Pattison J, Riad H, Wang Z, Cornu-Artis HC, et al. Correlation of everolimus exposure with efficacy and safety outcomes in renal transplant recipients: 24-month update. Transpl Int 2011;24:248 | Abstract |
| Carroll RP, Hester J, Wood KJ, Harden PN. Conversion to sirolimus in kidney transplant recipients with squamous cell cancer permits potential protective changes in immune phenotype. Transplantation 2012;94:167 | Abstract |
| Carroll RP, Hester J, Wood KJ, Harden PN. Conversion to sirolimus in kidney transplant recipients with squamous cell cancer and changes in immune phenotype. Nephrol Dial Transplant 2013;28:462–5 | Population |
| Cataneo-Davila A, Zuniga-Varga J, Correa-Rotter R, Alberu J. Renal function outcomes in kidney transplant recipients after conversion to everolimus-based immunosuppression regimen with CNI reduction or elimination. Transpl Proc 2009;41:4138–46 | Population |
| Cerezo O, Bravo MG, Jimenez Aranda P, Lemus EA. Clinical benefits of immunosuppression therapy in renal trasplant Patients. Systematic review and meta-analysis. Value Health 2013;16:A697 | Abstract |
| Cerezo O, Bravo MG, Jimenez Aranda P, Lemus EA. Clinical benefits of immunosuppression therapy in renal trasplant Patients. Systematic review and meta-analysis. Value Health 2013;16:A697 | Duplicate |
| Chadban S, Campbell S, Russ G, Walker R, Chapman J, Pussell B, et al. A one-year, randomised, open label, parallel group study to investigate the safety and efficacy of enteric-coated Mycophenolate sodium (EC-MPS) in combination with full dose or reduced dose cyclosporine microemulsion (CSA-ME), basiliximab and steroids in de novo kidney transplantation. Immunol Cell Biol 2006;84:A6–A | Abstract |
| Chadban S, Eris J, Pilmore H, Lee P, Woodcock C, Kurstjens N, et al. Socrates-steroid or cyclosporin removal after transplantation using everolimus: histological analysis. Transplantation 2012;94:977 | Abstract |
| Chadban SJ, Eris JM, Kanellis J, Pilmore H, Lee PC, Lim SK, et al. A randomized, controlled trial of everolimus-based dual immunosuppression versus standard of care in de novo kidney transplant recipients. Transpl Int 2014;27:302–11 | Population |
| Chan L, Greenstein S, Hardy MA, Hartmann E, Bunnapradist S, Cibrik D, et al. Multicenter, randomized study of the use of everolimus with tacrolimus after renal transplantation demonstrates its effectiveness. Transplantation 2008;85:821–6 | Comparator |
| Charpentier B. A three arm study comparing immediate tacrolimus therapy with ATG induction therapy followed by either tacrolimus or cyclosporine in adult renal transplant recipients. Transpl Proc 2002;34:1625–6 | Population |
| Charpentier B, Grinyo J, Medina Pestana JO, Vanrenterghem Y, Vincenti F, Dong Y, et al. 3-Year Safety profile of belatacept in kidney transplant recipients from the benefit and BENEFIT-EXT studies. Transpl Int 2011;24:68–9 | Abstract |
| Charpentier B, Groth CG, Bäckman L, Morales JM, Calne R, Kreis H, et al. Bicêtre hospital experience with sirolimus-based therapy in human renal transplantation: the Sirolimus European Renal Transplant Study. Transplant Proc 2003;35(Suppl. 3):58–61 | Population |
| Charpentier B, Medina Pestana JO, Del C Rial M, Rostaing L, Grinyó J, Vanrenterghem Y, et al. Long-term exposure to belatacept in recipients of extended criteria donor kidneys. Am J Transplant 2013;13:2884–91 | Population |
| Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, et al. A three-arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation 2003;75:844–51 | Population |
| Charpentier B, Vincenti F, Rice K, Budde K, Campistol J, Duan T, et al. Three-year outcomes in patients with delayed graft function in phase iii studies of belatacept vs cyclosporine in kidney transplantation (benefit and benefit-ext). Transplantation 2012;94:996 | Abstract |
| Chen KH, Tsai MK, Lai IR, Lin Wu FL, Hu RH, Lee PH. Favorable results of concomitant tacrolimus and sirolimus therapy in Taiwanese renal transplant recipients at 12 months. J Formos Med Assoc 2008;107:533–9 | Population |
| Cheung CY, Chan HW, Liu YL, Chau KF, Li CS. Long-term graft function with tacrolimus and cyclosporine in renal transplantation: paired kidney analysis. Nephrology 2009;14:758–63 | Study design |
| Cheung CY, Wong KM, Chan HW, Liu YL, Chan YH, Wong HS, et al. Paired kidney analysis of tacrolimus and cyclosporine microemulsion-based therapy in Chinese cadaveric renal transplant recipients. Transpl Int 2006;19:657–66 | Study design |
| Chhabra D, Alvarado A, Dalal P, Leventhal J, Wang C, Sustento-Reodica N, et al. Impact of calcineurin-inhibitor conversion to mTOR inhibitor on renal allograft function in a prednisone-free regimen. Am J Transplant 2013;13:2902–11 | Population |
| Chhabra D, Skaro AI, Leventhal JR, Dalal P, Shah G, Wang E, Gallon L. Long-term kidney allograft function and survival in prednisone-free regimens: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. Clin J Am Soc Nephrol 2012;7:504–12 | Population |
| Chisholm MA, Middleton MD. Modified-release tacrolimus. Ann Pharmacother 2006;40:270–5 | Study design |
| Christian M, Bjerre A, Wennberg L, Ettenger R, Pape L, Tonshoff B, et al. Design and baseline characteristics of CRADLE: a study evaluating the efficacy and safety of everolimus to reduce CNI exposure and to withdraw steroids in pediatric renal transplant recipients. Pediatr Nephrol 2014;29:1755 | Abstract |
| Chun DXY, Alexandre H, Sandrine GS, Olivier T, Isabelle E, Christophe L, et al. The phenotype of tubular epithelial cells does not recover after a conversion from cyclosporine a to siroliumus. Nephrol Dial Transpl 2012;27:ii517 | Abstract |
| Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized long-term trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate mofetil versus cyclosporine (NEORAL)/sirolimus in renal transplantation. II. Survival, function, and protocol compliance at 1 year. Transplantation 2004;77:252–8 | Study design |
| Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized long-term trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (NEORAL) and sirolimus in renal transplantation. I. Drug interactions and rejection at one year. Transplantation 2004;77:244–51 | Duplicate |
| Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized long-term trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (NEORAL) and sirolimus in renal transplantation. I. Drug interactions and rejection at one year. Transplantation 2004;77:244–51 | Duplicate |
| Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized long-term trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (NEORAL) and sirolimus in renal transplantation. I. Drug interactions and rejection at one year. Transplantation 2004;77:244–51 | Duplicate |
| Ciancio G, Burke GW, Gaynor JJ, Ruiz P, Roth D, Kupin W, et al. A randomized long-term trial of tacrolimus/sirolimus versus tacrolimums/mycophenolate versus cyclosporine/sirolimus in renal transplantation: three-year analysis. Transplantation 2006;81:845–52 | Population |
| Ciancio G, Burke GW, Gaynor JJ, Roth D, Sageshima J, Kupin W, et al. Randomized trial of mycophenolate mofetil versus enteric-coated mycophenolate sodium in primary renal transplant recipients given tacrolimus and daclizumab/thymoglobulin: one year follow-up. Transplantation 2008;86:67–74 | Population |
| Ciancio G, Gaynor JJ, Guerra G, Sageshima J, Chen L, Mattiazzi A, et al. Randomized trial of three induction antibodies in kidney transplantation: long-term results. Transplantation 2014;97:1128–38 | Population |
| Ciancio G, Gaynor JJ, Zarak A, Sageshima J, Guerra G, Roth D, et al. Randomized trial of mycophenolate mofetil versus enteric-coated mycophenolate sodium in primary renal transplantation with tacrolimus and steroid avoidance: four-year analysis. Transplantation 2011;91:1198–205 | Population |
| Ciancio G, Miller J, Gonwa TA. Review of major clinical trials with mycophenolate mofetil in renal transplantation. Transplantation 2005;80(Suppl. 2):191–200 | Study design |
| Cibrik D, Johnston T, Kim Y, Walker R, Zibari G, Cornu-Artis C, et al. Everolimus exposure and relationship to efficacy and safety: results from a multicenter study in renal transplantation using reduced CsA exposure. Am J Transplant 2010;10:567–8 | Abstract |
| Cibrik D, Johnston T, Kim YS, Walker R, Zibari G, Mange K, et al. Everolimus allows for around 60% reduction in CsA exposure over 12 months: results from a multicenter, prospective study in renal transplantation. Am J Transplant 2010;10:511 | Abstract |
| Cibrik D, Kim YS, Johnston T, Walker R, Zibari G, Mange K, et al. Benefits of everolimus with reduced CSA exposure on renal function: a multicenter, prospective study in renal transplantation. Am J Transplant 2010;10:151–2 | Abstract |
| Cibrik D, Kim YS, Johnston T, Walker R, Zibari GB, Cornu-Artis C, et al. Renal function stability in renal transplant recipients receiving concentration-controlled everolimus with reduced cyclosporine exposure: 24 month results from the A2309 study. Am J Transplant 2011;11:406–7 | Abstract |
| Cibrik D, Silva HT, Vathsala A, Lackova E, Cornu-Artis C, Walker RG, et al. Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation 2013;95:933–42 | Study design |
| Citterio F, Scatà MC, Romagnoli J, Pozzetto U, Nanni G, Castagneto M. Conversion to tacrolimus immunosuppression in renal transplant recipients: 12-month follow-up. Transplant Proc 2002;34:1685–6 | Population |
| Citterio F, Scolari MP, Salvadori M, Castagneto M, Rigotti P, Albertazzi A, et al. A randomized trial comparing standard everolimus plus cyclosporine with higher blood everolimus levels plus very low cyclosporine levels in renal transplant recipients: preliminary results of the everest study. Transpl Int 2007;20:124 | Abstract |
| Claes K, Meier-Kriesche HU, Schold JD, Vanrenterghem Y, Halloran PF, Ekberg H. Effect of different immunosuppressive regimens on the evolution of distinct metabolic parameters: evidence from the Symphony study. Nephrol Dial Transplant 2012;27:850–7 | Population |
| Clayton P, McDonald S, Chapman J, Chadban S. Mycophenolate vs azathioprine for kidney transplantation: 15 year follow-up of a randomized trial. Nephrology 2011;16:69 | Abstract |
| Clayton PA, McDonald SP, Chapman JR, Chadban SJ. Mycophenolate versus azathioprine for kidney transplantation: a 15-year follow-up of a randomized trial. Transplantation 2012;94:152–8 | Population |
| Cransberg K, Cornelissen M, Lilien M, Hoeck K, Davin JC, Nauta J. Maintenance immunosuppression with mycophenolate mofetil and corticosteroids in pediatric kidney transplantation: temporary benefit but not without risk. Transplantation 2007;83:1041–7 | Population |
| Cristelli M, Felipe C, Oliveira N, Gusukuma L, Ferreira A, Sandes-Freitas T, et al. De novo everolimus (EVR) versus mycophenolate (MPA) in kidney transplant recipients receiving tacrolimus (TAC). Transplantation 2014;98:141 | Abstract |
| Cruzado JM, Bestard O, Riera L, Torras J, Gil-Vernet S, Serón D, et al. Immunosuppression for dual kidney transplantation with marginal organs: the old is better yet. Am J Transplant 2007;7:639–44 | Study design |
| Dalal P, Xu L, Joseph L, Shah G, Chhabra D, Wang E, et al. Prospective randomized study to evaluate the long term impact on graft survivaland function of two pred-free, cnibased maintenance immunosuppressions: FK/MMF vs. FK/SRL. Am J Transplant 2010;10:512 | Abstract |
| Dantal J, Berthoux F, Moal MC, Rostaing L, Legendre C, Genin R, et al. Efficacy and safety of de novo or early everolimus with low cyclosporine in deceased-donor kidney transplant recipients at specified risk of delayed graft function: 12-month results of a randomized, multicenter trial. Transpl Int 2010;23:1084–93 | Population |
| Dantal J, Berthoux F, Moal MC, Rostaing L, Legendre C, Genin R, et al. Efficacy and safety of de novo or early everolimus with low cyclosporine in deceased-donor kidney transplant recipients at specified risk of delayed graft function: 12-month results of a randomized, multicenter trial. Transpl Int 2010;23:1084–93 [Erratum published in Transpl Int 2012;25:138] | Duplicate |
| David-Neto E, Cocuzza CS, Pereira LM, de Castro MCR, Fadel LM, Prado ES, et al. A prospective, randomized, controlled study using oral GTT to diagnose impaired glucose metabolism in renal transplant patients under cyclosporin and tacrolimus. Am J Transplant 2005;5:408 | Abstract |
| De Fijter JW, Ewe SH, Den Hartigh J, Ng ACT, Delgado V, Mallat MJK, et al. Beneficial effects of late concentration-controlled CNI withdrawal in renal transplant recipients. Am J Transplant 2011;11:406 | Abstract |
| De Fijter JW, Hoogendijk-Van Den Akker JM, Harden PN, Hoitsma AJ, Proby C, Wolterbeek R, et al. Reduced cutaneous squamous cell carcinoma after conversion to sirolimus: a 2-year prospective open-label multicenter trial. Am J Transplant 2012;12:161 | Abstract |
| De Simone P, Detry O, Kintmalm G, Goss J, McCormick P, Rossi M, et al. Superior renal function sustained for 24 months through early everolimus-facilitated reduction of tacrolimus versus standard tacrolimus in de novo liver transplant recipients: results of a randomized trial. Am J Transplant 2013;13:169–70 | Abstract |
| Dean PG, Grande JP, Sethi S, Park WD, Griffin MD, Cosio FG, et al. Kidney transplant histology after one year of continuous therapy with sirolimus compared with tacrolimus. Transplantation 2008;85:1212–5 | Study design |
| Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 2004;77:1555–61 | Outcome |
| Del Castillo D, Franco A, Tabernero JM, Errasti P, Valdes F, Garcia C, et al. Prospective, multicenter, randomized, open-label study of myfortic (EC-MPS) with steroid withdrawal vs Myfortic (TM) (EC-MPS) with standard steroid regimen to prevent acute rejection in de novo kidney transplantation. Am J Transplant 2005;5:191 | Abstract |
| Demirbas A, Hugo C, Grinyó J, Frei U, Gürkan A, Marcén R, et al. Low toxicity regimens in renal transplantation: a country subset analysis of the Symphony study. Transpl Int 2009;22:1172–81 | Population |
| Dharnidharka VR, Fiorina P, Harmon WE. Kidney transplantation in children. N Engl J Med 2014;371:549–58 | Study design |
| Diekmann F, Gutiérrez-Dalmau A, López S, Cofán F, Esforzado N, Ricart MJ, et al. Influence of sirolimus on proteinuria in de novo kidney transplantation with expanded criteria donors: comparison of two CNI-free protocols. Nephrol Dial Transplant 2007;22:2316–21 | Population |
| Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant 2010;14:603–13 | Study design |
| Dobbels F, Wong S, Joo S, Kalsekar A. Health-related quality of life after kidney transplantation: results from belatacept clinical trials. Am J Transplant 2011;11:352–3 | Abstract |
| Dobbels F, Wong S, You M, Kalsekar A. Patient reports of immunosuppressant related side-effects after kidney transplantation: results from the belatacept phase III clinical trial (BENEFIT). Am J Transplant 2011;11:353 | Abstract |
| Duboix-xu Y, Lebranchu Y, De Ligny BH, Thervet E, Mazouz H, Lepogamp P, et al. Conversion from cyclosporine to Sirolimus at M3 after renal transplantation does not reduce the score of epithelialto mesenchymaltransition at M12: ancillary study of the concept study. Am J Transplant 2010;10:510-1 | Abstract |
| Dudley C, Pohanka E, Riad H, Dedochova J, Wijngaard P, Sutter C, Silva HT, Mycophenolate Mofetil Creeping Creatinine Study Group. Mycophenolate mofetil substitution for cyclosporine a in renal transplant recipients with chronic progressive allograft dysfunction: the “creeping creatinine” study. Transplantation 2005;79:466–75 | Population |
| Duerr M, Naik M, Schmidt D, Neumayer H, Budde K. Higher rates of acute rejections despite enhanced rates of regulatory T cells under mtor inhibitor therapy in renal transplant patients. Am J Transplant 2012;12:301 | Abstract |
| Duerr M, Nolting J, Naik M, Neumayer HH, Budde K. Higher frequency of regulatory T-cells after conversion from cyclosporine to everolimus in a prospective randomized trial in renal allograft recipients. Am J Transplant 2011;11:66 | Abstract |
| Durlik M, Paczek L, Rutkowski B, Lewandowska D, Debska-Slizien A, Chamienia A, et al. The efficacy and safety of ciclosporin (Equoral®) capsules after renal transplantation: a multicentre, open-label, phase IV clinical trial. Ann Transplant 2010;15:51–9 | Study design |
| Durrbach A, Florman S, Larsen C, Pestana JM, Vanrenterghem Y, Vincente F, et al. Primary outcomes from a randomized, phase III study of belatacept versus cyclosporine in ECD kidney transplants (BENEFIT-EXT study). Am J Transplant 2010;10:7 | Abstract |
| Durrbach A, Florman S, Zhang R, Becker T, Grinyo J, Lang P, et al. Four-year outcomes by donor type from the long-term extension of the belatacept BENEFIT and BENEFIT-EXT studies. Am J Transplant 2012;12:407 | Abstract |
| Durrbach A, Florman S, Zhang R, Lang P, Lehner F, Massari P, et al. Five-year outcomes by donor type from the long-term extension of the belatacept BENEFIT-EXT study. Am J Transplant 2013;13:311 | Abstract |
| Durrbach A, Larsen C, Medina-Pestana JD, Vanrenterghem Y, Vincenti F, Florman S, et al. Primary outcomes from a randomized, Phase III study of belatacept vs cyclosporine in ECD kidney transplants (BENEFIT-EXT Study). Am J Transplant 2009;9:199 | Abstract |
| Durrbach A, Larsen CP, Medina Pestana J, Vanrenterghem Y, Vincenti F, Florman S, et al. Belatacept vs cyclosporine in ECD kidney transplants: two-year outcomes from the BENEFIT-EXT study. NDT Plus 2010;3:iii262 | Abstract |
| Durrbach A, Medina-Pestana JO, Rostaing L, Bresnahan B, Helderman JH, Rice K, et al. Improving or maintaining renal function with belatacept: 5-year benefit long-term extension results. Transpl Int 2013;26:92 | Abstract |
| Durrbach A, Medina-Pestana JO, Vanrenterghem Y, Rial M, Charpentier B, Matas A, et al. Improving or maintaining renal function over 5 years with belatacept in recipients of extended-criteria donor kidneys. Transpl Int 2013;26:44 | Abstract |
| Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 2010;10:547–57 | Population |
| Durrbach A, Rostaing L, Tricot L, Ouali N, Wolf P, Pouteil-Noble C, et al. Prospective comparison of the use of sirolimus and cyclosporine in recipients of a kidney from an expanded criteria donor. Transplantation 2008;85:486–90 | Population |
| Ekberg H, Bernasconi C, Nöldeke J, Yussim A, Mjörnstedt L, Erken U, et al. Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the Symphony study. Nephrol Dial Transplant 2010;25:2004–10 | Population |
| Ekberg H, Grinyó J, Nashan B, Vanrenterghem Y, Vincenti F, Voulgari A, et al. Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR Study. Am J Transplant 2007;7:560–70 | Population |
| Ekberg H, Mamelok RD, Pearson TC, Vincenti F, Tedesco-Silva H, Daloze P. The challenge of achieving target drug concentrations in clinical trials: experience from the Symphony study. Transplantation 2009;87:1360–6 | Population |
| Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Klempnauer J, Guerkan A, et al. 2-year results of the symphony study: comparing standard immunosuppression against low-dose cyclosporine, tacrolimus or sirolimus associated with MMF, daclizumab and corticosteroids in de-novo renal transplantation. Transpl Int 2007;20:2 | Abstract |
| Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007;357:2562–75 | Intervention |
| El-Agroudy AE, El-Dahshan KF, Wafa EW, Sheashaa HA, Gad ZA, Ismail AM, et al. Safe conversion of mycophenolate mofetil to azathioprine in kidney transplant recipients with sirolimus-based immunosuppression. Nephrology 2009;14:255–61 | Population |
| El-Sabrout R, Delaney V, Qadir M, Butt F, Hanson P, Butt KMH. Sirolimus in combination with tacrolimus or mycophenolate mofetil for minimizing acute rejection risk in renal transplant recipients - A single center experience. Transpl Proc 2003;35:89S–94S | Study design |
| Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med 2012;367:329–39 | Study design |
| Facundo C, Diaz JM, Guirado L, Duran F, Herreros MA, Diaz M, Sola R. Results of a triple induction regime with tacrolimus, mycophenolate mofetil, and prednisone in renal transplantation. Transplant Proc 2002;34:98 | Study design |
| Favi E, Citterio F, Spagnoletti G, Gargiulo A, Delreno F, Romagnoli J, Castagneto M. Prospective clinical trial comparing two immunosuppressive regimens, tacrolimus and mycophenolate mofetil versus everolimus and low-dose cyclosporine, in de novo renal transplant recipients: results at 6 months follow-up. Transplant Proc 2009;41:1152–5 | Study design |
| Favi E, Citterio F, Spagnoletti G, Gargiulo A, Romagnoli J, Castagneto M. A prospective clinical trial comparing tacrolimus-mmf to cyclosporine-everolimus in de novo renal transplant recipients: 2 years results. Transpl Int 2009;22:241 | Abstract |
| Favi E, Silvestrini N, Pedroso J, Salerno M, Spagnoletti G, Bianchi V, et al. Extended-release tacrolimus plus everolimus vs extended-release tacrolimus plus micophenolate mofetil in primary deceased donor kidney transplant recipients: 1-year results of an open label, randomized phase 2 clinical trial. Am J Transpl 2013;13:316 | Abstract |
| Favi E, Silvestrini N, Pedroso JA, Salerno MP, Spagnoletti G, Romagnoli J, et al. Er-tacrolimus plus everolimus vs ertacrolimus plus MMF in primary deceased donor kidney transplantation: 1-year results of single center, open label, prospective, randomized clinical trial. Transpl Int 2013;26:241 | Abstract |
| Favi E, Silvestrini N, Salerno MP, Romagnoli J, Citterio F. Extended-release tacrolimus plus everolimus or micophenolate mofetil in deceased donor kidney transplant recipients: 6-month results of a prospective randomized clinical trial. Am J Transpl 2012;12:42–3 | Abstract |
| Favi E, Silvestrini N, Spagnoletti G, Castagneto M, Citterio F. Thymoglobulin and basiliximab vs basiliximab as induction therapy in deceased donor kidney transplantation: 1-year results of a prospective clinical trial. Am J Transpl 2011;11:147 | Abstract |
| Favi E, Silvestrini N, Valente I, Salerno MP, Castagneto M, Citterio F. Lower acute rejection with basiliximab and short course, low dose thymoglobulin vs basiliximab as induction therapy in deceased donor renal transplant recipients: 6-month results of a prospective clinical trial. Am J Transpl 2010;10:321 | Abstract |
| Favi E, Spagnoletti G, Salerno MP, Pedroso JA, Romagnoli J, Citterio F. Tacrolimus plus mycophenolate mofetil vs. cyclosporine plus everolimus in deceased donor kidney transplant recipients: three-yr results of a single-center prospective clinical trial. Clin Transpl 2013;27:E359–67 | Study design |
| Favi E, Spagnoletti G, Silvestrini N, Salerno MP, Pedroso JA, Romagnoli J, et al. Thymoglobulin plus basiliximab versus basiliximab induction in deceased donor kidney transplant recipients treated with tacrolimus and MMF: 1-year results of a prospective clinical trial. Transpl Int 2013;26:83 | Abstract |
| Favi E, Spagnoletti G, Silvestrini N, Salerno M, Pedroso J, Romagnoli J, et al. Thymoglobulin plus basiliximab vs basiliximab as induction therapy in deceased donor kidney transplant recipients treated with tacrolimus and mycophenolate mofetil: 1-year results of a prospective clinical trial. Am J Transpl 2013;13:426 | Abstract |
| Felix M, Felipe C, Tedesco H, Medina-Pestana J. Safety profile after planned conversion from tacrolimus (TAC) to sirolimus (SRL) based immunosuppressive therapy in kidney transplant recipients (KTR). Transplantation 2014;98:544–5 | Abstract |
| Fellstrom B, Holdas H, Holme I, Jardine A, Soveri I. Cardiovascular risk calculator for renal transplant recipients: applications to BENEFIT and BENEFIT-EXT trials. Am J Transpl 2012;12:409–10 | Abstract |
| Feng XF, Min M, Zuo FJ, Zhou MS, Wang LM. Conversion from tacrolimus to cyclosporine A improves new-onset diabetes mellitus after transplantation. Chinese Journal of Tissue Engineering Research 2013;17:9176–81 | Language |
| Ferguson R, Grinyó J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, et al. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant 2011;11:66–76 | Population |
| Ferguson R, Vincenti F, Kaufman DB, Woodle ES, Marder BA, Citterio F, et al. Immunosuppression with belatacept-based, CNI-avoiding and steroid-avoiding regimens vs a tacrolimus-based, steroid-avoiding regimen in kidney transplant patients: results of a 1-year, randomized study. Am J Transpl 2010;10:150 | Abstract |
| Ferrer F, Machado S, Alves R, Macário F, Bastos C, Roseiro A, Mota A. Induction with basiliximab in renal transplantation. Transplant Proc 2010;42:467–70 | Study design |
| Filipe R, Mota A, Alves R, Bastos C, Macário F, Figueiredo A, et al. Kidney transplantation with corticosteroid-free maintenance immunosuppression: a single center analysis of graft and patient survivals. Transplant Proc 2009;41:843–5 | Study design |
| Filler G. Randomised clinical trial in paediatric renal transplantation: tacrolimus (tac) vs cyclosporine neoral (cya) - 3-year data [abstract]. JASN 2003;14:65a | Abstract |
| Filler G. Finding the optimal therapeutic window for tacrolimus. Pediatr Transplant 2014;18:783–5 | Study design |
| Fisher G, Rocha V, dos Santos M, Devergie A, Robin M, de Latour RP, et al. Myeophenolate mofetil (MMF) with or without tracolimus (FK506) as a second line treatment for steroid-resistant acute graft-versus-host disease. The experience of Saint Louis Hospital. Blood 2006;108:819A–A | Abstract |
| Flechner S, Friend P, Campistol J, Weir M, Diekmann F, Russ G. De novo immunosuppression with mammalian target of rapamycin inhibitors and posttransplantation malignancy in focus. Transplant Proc 2009;41(Suppl. 6):42–4 | Study design |
| Flechner S, Glyda M, Steinberg S, Harler MB, Invest OT. A randomized, open-label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (TAC) and mycophenolate mofetil (MMF) regimen in de novo renal allograft recipients: renal function results from the Orion study. Transplant Int 2007;20:25 | Abstract |
| Flechner S, Glyda M, Steinberg S, Harler MB, Investigators OT. A randomized, open-label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (TAC) and mycophenolate mofetil regimen (MMF) in De novo renal allograft recipients: acute rejection and graft survival results from the orion study. Transplant Int 2007;20:209–10 | Abstract |
| Flechner SM, Cockfield S, Grinyo J, Russ G, Wissing KM, Legendre C, et al. A randomized, open-label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with tacrolimus (TAC) plus mycophenolate mofetil (MMF) in De Novo renal allograft recipients: preliminary 2-year safety results from the ORION trial. Am J Transplant 2008;8:582 | Abstract |
| Flechner SM, Glyda M, Tai SS. Delayed graft function (DGF) in two sirolimus (SRL)-based regimens compared with tacrolimus (TAC) and mycophenolate mofetil (MMF) in de novo renal allograft recipients. Am J Transplant 2009;9:277–8 | Abstract |
| Flechner SM, Glyda M, Cockfield S, Grinyó J, Legendre Ch, Russ G, et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant 2011;11:1633–44 | Population |
| Flechner SM, Goldfarb D, Modlin C, Feng JY, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporin. Transplantation 2002;74:1070–6 | Population |
| Flechner SM, Goldfarb D, Solez K, Modlin CS, Mastroianni B, Savas K, et al. Kidney transplantation with sirolimus and mycophenolate mofetil-based immunosuppression: 5-year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation 2007;83:883–92 | Population |
| Flechner SM, Gurkan A, Hartmann A, Legendre CM, Russ GR, Campistol JM, et al. A randomized, open-label study of sirolimus versus cyclosporine in primary de novo renal allograft recipients. Transplantation 2013;95:1233–41 | Population |
| Flechner SM, Gurkan A, Tai SS, Schulman SL. Incidence of delayed graft function (DGF) in a sirolimus (SRL)-based versus cyclosporine (CsA)-based regimen in de novo renal allograft recipients Am J Transplant 2009;9:278 | Abstract |
| Flechner SM, Kurian SM, Solez K, Cook DJ, Burke JT, Rollin H, et al. De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. Am J Transplant 2004;4:1776–85 | Population |
| Florman S, Becker T, Bresnahan B, Chevaile-Ramos A, Carvalho D, Muehibacher F, et al. Three year outcomes by donor type in phase III studies of belatacept vs cyclosporine in kidney transplantation (benefit and benefit-EXT). Transplant Int 2011;24:51 | Abstract |
| Florman S, Becker T, Bresnahan B, Chevaile-Ramos A, DeCarvalho D, Muehlbacher F, et al. Three-year outcomes by donor type in phase III studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT & BENEFIT-EXT). Am J Transplant 2011;11:100 | Abstract |
| Florman S, Bresnahan B, Chan L, Helderman H, Dong Y, Harler MB, et al. Three year outcomes in Black/African American kidney transplant recipients from the BENEFIT and BENEFIT-EXT studies. Am J Transplant 2011;11:350 | Abstract |
| Florman S, Durrbach A, Grinyo J, Pestana JOM, Rial MDC, Vitko S, et al. 4-year results from the long-term extension of the belatacept BENEFIT-EXT study. Am J Transplant 2012;12:82 | Abstract |
| Florman S, Durrbach A, Larsen C, Pestana JM, Vanrenterghem Y, Vincenti F, et al. Outcomes as a function of donor criteria from a phase III study of belatacept vs cyclosporine in kidney transplantation (benefitext). Am J Transplant 2010;10:150 | Abstract |
| Florman S, Rice K, Chan L, Steinberg S, Pearson T, Duan T, et al. Four-year outcomes in black/African American kidney transplant recipients from the long-term extension of the belatacept BENEFIT and BENEFIT-EXT studies. Am J Transplant 2012;12:404 | Abstract |
| Florman S, Rice K, Chan L, Zhang R, Abouljoud M, Steinberg S, et al. Outcomes at five years in black/African-American kidney transplant recipients from the long-term extension of the belatacept benefit and BENEFIT-EXT studies. Am J Transplant 2013;13:311 | Abstract |
| Foroncewicz B, Mucha K, Ciszek M, Małkowski P, Durlik M, Szmidt J, et al. A comparison between two tacrolimus-based immunosuppression regimens in renal transplant recipients: 7-year follow-up. Ann Transplant 2013;18:384–92 | Study design |
| Forsythe J. A phase II open label single centre randomized study of tacrolimus plus sirolimus and corticosteroids compared with tacrolimus plus azathioprine and corticosteroids in de novo renal allografts recipients. 2002. National Research Register, UK. URL: www.nrr.nhs.uk/ (accessed 25 July 2014) | Unobtainable |
| Franz S, Regeniter A, Hopfer H, Mihatsch M, Dickenmann M. Tubular toxicity in sirolimus- and cyclosporine-based transplant immunosuppression strategies: an ancillary study from a randomized controlled trial. Am J Kidney Dis 2010;55:335–43 | Study design |
| Frei U, Daloze P, Vítko S, Klempnauer J, Reyes-Acevedo R, Titiz I, et al. Acute rejection in low-toxicity regimens: clinical impact and risk factors in the Symphony study. Clin Transplant 2010;24:500–9 | Population |
| Friend PJ. Thymoglobulin induction and steroid-free immunosuppression in kidney transplantation from deceased donors after cardiac death – an open label randomised controlled trial to evaluate the role of thymoglobulin as induction immunosuppression in kidney transplants from deceased donors after cardiac death. 2011. URL: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/711/CN-00796711/frame.html (accessed 25 July 2014) | No data |
| Frimat L, Cassuto-Viguier E, Charpentier B, Noël C, Provôt F, Rostaing L, et al. Impact of cyclosporine reduction with MMF: a randomized trial in chronic allograft dysfunction. The ‘reference’ study. Am J Transplant 2006;6:2725–34 | Population |
| Frimat L, Cassuto-Viguier E, Provôt F, Rostaing L, Charpentier B, Akposso K, et al. Long-Term Impact of Cyclosporin Reduction with MMF Treatment in Chronic Allograft Dysfunction: REFERENECE Study 3-Year Follow Up. J Transplant 2010;2010:402750 | Population |
| Gaber AO, Kahan BD, Van Buren C, Schulman SL, Scarola J, Neylan JF, Sirolimus High-Risk Study Group. Comparison of sirolimus plus tacrolimus versus sirolimus plus cyclosporine in high-risk renal allograft recipients: results from an open-label, randomized trial. Transplantation 2008;86:1187–95 | Population |
| Gallon L, Monica G, Friedewald J, Cabral B, Miller J, Najafaian N, et al. Prospective randomized study to evaluate feasibility of conversion of CNI to SRL in a pred-free immunosuppressive regimen. Impact on treg generation. Am J Transplant 2009;9:260 | Abstract |
| Gallon L, Perico N, Dimitrov BD, Winoto J, Remuzzi G, Leventhal J, et al. Long-term renal allograft function on a tacrolimus-based, pred-free maintenance immunosuppression comparing sirolimus vs. MMF. Am J Transplant 2006;6:1617–23 | Population |
| Gamboa O, Montero C, Mesa L, Benavides C, Reino A, Torres RE, Castillo JS. Cost-effectiveness analysis of the early conversion of tacrolimus to mammalian target of rapamycin inhibitors in patients with renal transplantation. Transplant Proc 2011;43:3367–76 | Population |
| Martin Garcia D, Martin Gago J, Mendiluce A, Gordillo R, Bustamente J. Tacrolimus-Basiliximab versus Cyclosporine-Basiliximab in renal transplantation “de novo”: acute rejection and complications. Transplant Proc 2003;35:1694–6 | Study design |
| Garcia I, Spanish-Italian Tacrolimus Study Group. Efficacy and safety of dual versus triple tacrolimus-based therapy in kidney transplantation: two-year follow-up. Transplant Proc 2002;34:1638–9 | Comparator |
| Garcia R, Machado PG, Felipe CR, Park SI, Spinelli GA, Franco MF, et al. Exploratory calcineurin inhibitor-free regimens in living-related kidney transplant recipients. Braz J Med Biol Res 2007;40:457–65 | Study design |
| van Gelder T, Silva HT, de Fijter H, Budde K, Kuypers D, Mamelok RD, et al. How delayed graft function impacts exposure to mycophenolic acid in patients after renal transplantation. Ther Drug Monit 2011;33:155–64 | Population |
| van Gelder T, Silva HT, de Fijter JW, Budde K, Kuypers D, Tyden G, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation 2008;86:1043–51 | Comparator |
| van Gelder T, Tedesco Silva H, de Fijter JW, Budde K, Kuypers D, Arns W, et al. Renal transplant patients at high risk of acute rejection benefit from adequate exposure to mycophenolic acid. Transplantation 2010;89:595–9 | Comparator |
| Gelder T, ter Meulen CG, Hené R, Weimar W, Hoitsma A. Oral ulcers in kidney transplant recipients treated with sirolimus and mycophenolate mofetil. Transplantation 2003;75:788–91 | Study design |
| Gelens MA, Christiaans MH, van Heurn EL, van den Berg-Loonen EP, Peutz-Kootstra CJ, van Hooff JP. High rejection rate during calcineurin inhibitor-free and early steroid withdrawal immunosuppression in renal transplantation. Transplantation 2006;82:1221–3 | Population |
| Gheith O, Al-Otaibi T, Mansour H. Next-generation calcineurin inhibitors in development for the prevention of organ rejection. Transplant Research and Risk Management 2014;6:23–30 | Study design |
| Glotz D, Charpentier B, Abramovicz D, Lang P, Rostaing L, Rifle G, et al. 6 months preliminary results of a randomized trial comparing sirolimus (SRL) versus tacrolimus (FK) in 141 transplant patients receiving a cadaveric renal graft. Am J Transplant 2005;5:460 | Study design |
| Glotz D, Charpentier B, Abramovicz D, Lang P, Rostaing L, Rifle G, et al. Thymoglobulin induction and sirolimus versus tacrolimus in kidney transplant recipients receiving mycophenolate mofetil and steroids. Transplantation 2010;89:1511–7 | Abstract |
| Gonwa T, Johnson C, Ahsan N, Alfrey EJ, Halloran P, Stegall M, et al. Randomized trial of tacrolimus plus mycophenolate mofetil or azathioprine versus cyclosporine plus mycophenolate mofetill after cadaveric kidney transplantation: results at three years. Transplantation 2003;75:2048–53 | Population |
| Gonwa T, Mendez R, Yang HC, Weinstein S, Jensik S, Steinberg S, Prograf Study Group. Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 months. Transplantation 2003;75:1213–20 | Population |
| Gonzalez F, Espinoza M, Herrera P, Rocca X, Reynolds E, Lorca E, et al. Everolimus versus azathioprine in a cyclosporine and ketoconazole-based immunosuppressive therapy in kidney transplant: 3-year follow-up of an open-label, prospective, cohort, comparative clinical trial. Transplant Proc 2010;42:270–2 | Study design |
| Gonzalez Molina M, Morales JM, Marcen R, Campistol JM, Oppenheimer F, Serón D, et al. Renal function in patients with cadaveric kidney transplants treated with tacrolimus or cyclosporine. Transplant Proc 2007;39:2167–9 | Study design |
| Graeme R, Mamta A, Thomas B, Bresnahan B, Campistol JM, Darji P, et al. Belatacept associated with preserved renal function and structure compared with cyclosporine (CSA) in kidney transplant patients. Immunol Cell Biol 2010;88(6):A11–12 | Abstract |
| Graeme R, Steve C, Scott C, Brian H, John K, Philip O, et al. Everolimus plus reduced-dose cyclosporine: results from a randomized, phase iii study in 833 De-novo renal transplant recipients. Immunol Cell Biol 2010;88:A22 | Study design |
| Grafals M. Low Dose Thymoglobulin as Induction Agent on Prednisone-Free Regimens of Renal Transplant Recipients. 2011. URL: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/457/CN-00794457/frame.html (accessed 25 July 2014) | Comparator |
| Grannas G, Richter N, Klempnauer J, Lehner F. 10 years’ experience with belatacept (nulojix). Transplantation 2012;94:964 | Abstract |
| Grannas G, Schrem H, Klempnauer J, Lehner F. Ten years experience with belatacept-based immunosuppression after kidney transplantation. J Clin Med Res 2014;6:98–110 | Study design |
| Gregoor P, De Sevaux RGL, Ligtenberg G, Hoitsma AJ, Hene RJ, Weimar W, et al. Withdrawal of cyclosporine or prednisone six months after kidney transplantation in patients on triple drug therapy: a randomized, prospective, multicenter study. J Am Soc Nephrol 2002;13:1365–73 | Study design |
| Grinyo J, Abouljoud M, Germain M, Manfro R, Morales J, Legendre C, et al. Improving or sustaining renal function over 3 years with belatacept or cyclosporine a (CSA): insights from the benefit study. Transplant Int 2011;24:250 | Abstract |
| Grinyo J, Abouljoud M, Germain M, Manfro R, Morales J, Legendre C, et al. Likelihood of improving or sustaining renal function over three years with belatacept or CsA: insights from the BENEFIT study. Am J Transplant 2011;11:349 | Abstract |
| Grinyo J, Alberu J, Contieri FL, Manfro RC, Mondragon G, Nainan G, et al. Improvement in renal function in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: 2-year results from the long-term extension of a phase II study. Transplant Int 2012;25:1059–64 | Population |
| Grinyo J, Charpentier B, Medina Pestana J, Vanrenterghem Y, Vincenti F, Shi R, et al. Safety profile of belatacept in kidney transplant recipients from a pooled analysis of phase II and phase III studies. NDT Plus 2010;3:iii270 | Abstract |
| Grinyo J, Durrbach A, Rostaing L, Bresnahan B, Helderman J, Rice K, et al. Likelihood of improving or maintaining renal function over five years with belatacept or CSA: insights from the benefit long-term extension study. Am J Transplant 2013;13:182 | Abstract |
| Grinyo J, Florman S, Medina Pestana JO, Del Carmen Rial M, Muehlbacher F, Durrbach A, et al. Long-term extension of the belatacept BENEFIT-EXT study: results at month 48. Transplantation 2012;94:974 | Abstract |
| Grinyo J, Nainan G, Del Carmen Rial M, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from cyclcosporine or tacrolimus to belatacept: results from the long-term extension of a phase II study. Transplant Int 2011;24:70 | Abstract |
| Grinyo J, Nainan G, Rial M, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: results: from the long-term extension of a phase II study. Am J Transplant 2011;11:99 | Abstract |
| Grinyo J, Pestana JM, Becker T, Rial MC, Dong Y, Block A, et al. Likelihood of improving or sustaining renal function over three years with belatacept or CsA: insights from the BENEFIT-EXT study. Am J Transplant 2012;12:82 | Abstract |
| Grinyo J, Rial M, Alberu J, Steinberg S, Manfro R, Nainan G, et al. Outcomes of switching to belatacept from a calcineurin inhibitor in kidney transplant recipients: 3 year results from the long-term extension of a phase ii study. Am J Transplant 2013;13:182 | Abstract |
| Grinyo J, Vanrenterghem Y, Durrbach A, Rial M, Charpentier B, Matas A, et al. Likelihood of improving or maintaining renal function in recipients of extended-criteria donor kidneys over five years with belatacept or CsA (benefit-ext long-term extension study). Am J Transplant 2013;13:310 | Abstract |
| Grinyo JM, Campistol JM, Paul J, García-Martínez J, Morales JM, Prats D, et al. Pilot randomized study of early tacrolimus withdrawal from a regimen with sirolimus plus tacrolimus in kidney transplantation. Am J Transplant 2004;4:1308–14 | Study design |
| Grinyo JM, Ekberg H, Mamelok RD, Oppenheimer F, Sanchez-Plumed J, Gentil MA, et al. The pharmacokinetics of mycophenolate mofetil in renal transplant recipients receiving standard-dose or low-dose cyclosporine, low-dose tacrolimus or low-dose sirolimus: the Symphony pharmacokinetic substudy. Nephrol Dial Transplant 2009;24:2269–76 | Population |
| Grinyo JM, Marks W, Vincenti F, Kaufman DB, Marder BA, Woodle S, et al. Immunosuppression with belatacept-based, CNI-free, steroid-avoiding regimens in kidney transplant recipients: 6 month, interim results. Am J Transplant 2009;9:382 | Abstract |
| Grinyo JM, Mondragon-Ramirez G, Darji P, Bresnahan B, Pearson T, Di Russo GB, et al. Belatacept is associated with preservation of renal function and structure at 1 year compared to cyclosporine in kidney transplant patients (BENEFIT Study). Am J Transplant 2009;9:258–9 | Abstract |
| Grinyo JM, Paul J, Novoa P, Errasti P, Franco A, Aldana G, et al. Better renal function in renal-transplant recipients treated with everolimus plus cyclosporine elimination compared with cyclosporine minimisation. Am J Transplant 2010;10:503 | Abstract |
| Grushkin C, Mahan JD, Mange KC, Hexham JM, Ettenger R. De novo therapy with everolimus and reduced-exposure cyclosporine following pediatric kidney transplantation: a prospective, multicenter, 12-month study. Pediatr Transplant 2013;17:237–43 | Population |
| Gu YH, Du JX, Ma ML. Sirolimus and Non-melanoma Skin Cancer Prevention After Kidney Transplantation: A Meta-analysis (Provisional Abstract). DARE; 2012. DARE Accession Number: 12013033631. URL: http://www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=12013033631&UserID=0 (accessed 25 July 2014) | Population |
| Gu YH, Du JX, Ma ML. Sirolimus and non-melanoma skin cancer prevention after kidney transplantation: a meta-analysis. Asian Pac J Cancer Prev 2012;13:4335–9 | Population |
| Guba M, Pratschke J, Hugo C, Kraemer B, Burmeister D, Brockmann J, et al. A randomized multicenter trial of early conversion to sirolimus/mycophenolate/steroids versus cyclosporine/mycophenolate/steroids in renal transplantation: one-year analysis (SMART-study). Am J Transplant 2009;9:497 | Abstract |
| Guba M, Pratschke J, Hugo C, Kraemer B, Nohr-Westphal C, Brockmann J, et al. Renal function, efficacy and safety of sirolimus and mycophenolat mofetil therapy after early calcineurin-inhibitor withdrawal in de novo renal transplant patients: one-year analysis of a randomized multicenter trial. Transplant Int 2009;22:78 | Abstract |
| Guba M, Pratschke J, Hugo C, Krämer BK, Nohr-Westphal C, Brockmann J, et al. Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short-term calcineurin inhibitor-based quadruple therapy in de novo renal transplant patients: one-year analysis of a randomized multicenter trial. Transplantation 2010;90:175–83 | Population |
| Guba M, Witzke O, Lehner F, Arns W, Sommerer C, Neumayer HH, et al. The herakles study at 24 month: superior renal function in an everolimus-based cnifree regimen. Transplant Int 2013;26:110 | Abstract |
| Guerra G, Ciancio G, Gaynor JJ, Zarak A, Brown R, Hanson L, et al. Randomized trial of immunosuppressive regimens in renal transplantation. JASN 2011;22:1758–68 | Study design |
| Guerra G, Gaynor JJ, Ciancio G, Zarak A, Sageshima J, Roth D, et al. Randomized trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate versus cyclosporine (neoral (r))/sirolimus in renal transplantation: seven year results. Am J Transplant 2009;9:325 | Abstract |
| Gupta D. Design of a randomized study evaluating everolimus in pediatric renal transplant recipients. Transplant Int 2013;26:328 | Abstract |
| Gürkan A, Kaçar S, Erdogdu U, Varilsüha C, Kandemir G, Karaca C, et al. The effect of sirolimus in the development of chronic allograft nephropathy. Transplant Proc 2008;40:114–6 | Population |
| Hakemi M, Shahebrahimi K, Ganji MR, Najafi I, Broumand B. Side effects of mycophenolate mofetil versus azathioprine in iranian renal transplant recipients (single-center experience). Transplant Proc 2002;34:2091–2 | Study design |
| Hamdy AF, Bakr MA, Ghoneim MA. Long-term efficacy and safety of a calcineurin inhibitor-free regimen in live-donor renal transplant recipients. J Am Soc Nephrol 2008;19:1225–32 | Population |
| Hamdy AF, Bakr MA, Ghoneim MA. Proteinuria among primarily sirolimus treated live-donor renal transplant recipients' long-term experience. Exp Clin Transplant 2010;8:283–91 | Population |
| Hamdy AF, El-Agroudy AE, Bakr MA, Mostafa A, El-Baz M, El-Shahawy el-M, Ghoneim MA. Comparison of sirolimus with low-dose tacrolimus versus sirolimus-based calcineurin inhibitor-free regimen in live donor renal transplantation. Am J Transplant 2005;5:2531–8 | Population |
| Han D, Kim Y-S, Park KT, Kim S-J, Ha J-W, Kim H-C, et al. A Phase III, Randomized, open-label, comparative, multicenter study to assess the safety and efficacy of prograf (R) (tacrolimus) and extended release (XL) Tacrolimus in asian de novo kidney transplants from living donors: 6 month results. Am J Transplant 2009;9:413 | Abstract |
| Han DJ, Park JB, Kim YS, Kim SJ, Ha J, Kim HC, et al. A 39-month follow-up study to evaluate the safety and efficacy in kidney transplant recipients treated with modified-release tacrolimus (FK506E)-based immunosuppression regimen. Transplant Proc 2012;44:115–7 | Study design |
| Han F, Wu J, Huang H, Zhang X, He Q, Wang Y, et al. Conversion from cyclosporine to sirolimus in chronic renal allograft dysfunction: a 4-year prospective study. Exp Clin Transplant 2011;9:42–9 | Population |
| Hanaway M, Woodle ES, Mulgaonkar S, Peddi R, Harrison G, Vandeputte K, et al. 12 month results of a multicenter, randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation. Am J Transplant 2008;8:215 | Abstract |
| Hardinger KL, Bohl DL, Schnitzler MA, Lockwood M, Storch GA, Brennan DC. A randomized, prospective, pharmacoeconomic trial of tacrolimus versus cyclosporine in combination with thymoglobulin in renal transplant recipients. Transplantation 2005;80:41–6 | Population |
| Harold Y. A phase III, randomized, open-label, comparative, multi-center study to assess the safety and efficacy of Prograf (R) (Tacrolimus)/MMF, extended release (XL) Tacrolimus/MMF and Neoral (R) (Cyclosporine)/MMF in de novo kidney transplant recipients: 2 year results. Am J Transplant 2007;7:183 | Abstract |
| Havenith SH, Yong SL, Donselaar-van der Pant KA, Lier RA, Berge IJ, Bemelman FJ. Everolimus-treated renal transplant recipients have a more robust CMV-specific CD8+ T-cell response compared with cyclosporine- or mycophenolate-treated patients. Transplantation 2013;95:184–91 | Study design |
| Hazzan M, Buob D, Labalette M, Provot F, Glowacki F, Hoffmann M, et al. Assessment of the risk of chronic allograft dysfunction after renal transplantation in a randomized cyclosporine withdrawal trial. Transplantation 2006;82:657–62 | Outcome |
| Hazzan M, Labalette M, Copin MC, Glowacki F, Provôt F, Pruv FR, Noël C. Predictive factors of acute rejection after early cyclosporine withdrawal in renal transplant recipients who receive mycophenolate mofetil: results from a prospective, randomized trial. J Am Soc Nephrol 2005;16:2509–16 | Outcome |
| Heilman RL, Cortese C, Geiger XJ, Younan K, Wadei HM, Mai ML, et al. Impact of early conversion from tacrolimus to sirolimus on chronic allograft changes in kidney recipients on rapid steroid withdrawal. Transplantation 2012;93:47–53 | Population |
| Heilman RL, Younan K, Wadei HM, Mai ML, Reddy KS, Chakkera HA, Gonwa TA. Results of a prospective randomized trial of sirolimus conversion in kidney transplant recipients on early corticosteroid withdrawal. Transplantation 2011;92:767–73 | Population |
| Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant 2004;4:583–95 | Population |
| Heller T, Gelder T, Budde K, Fijter JW, Kuypers D, Arns W, et al. Plasma concentrations of mycophenolic acid acyl glucuronide are not associated with diarrhea in renal transplant recipients. Am J Transplant 2007;7:1822–31 | Outcome |
| Hernández D, Miquel R, Porrini E, Fernández A, González-Posada JM, Hortal L, et al. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine-based immunosuppression. Transplantation 2007;84:706–14 | Population |
| Hertig A, Kamar N, Anglicheau D, Moulin B, Hazzan M, Hurault De Ligny B, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: the certitem trial. Transplant Int 2013;26:2 | Abstract |
| Hest RM, Gelder T, Vulto AG, Mathot RA. Population pharmacokinetics of mycophenolic acid in renal transplant recipients. Clin Pharmacokinet 2005;44:1083–96 | Study design |
| Hirsch HH, Vincenti F, Friman S, Wiecek A, Pescovitz MD, Jenssen T, et al. Prospective study of polyomavirus BK viruria and viremia in De Novo renal transplantation comparing cyclosporine and tacrolimus: a multivariate analysis. Am J Transplant 2009;9:337 | Abstract |
| Hirsch HH, Vincenti F, Friman S, Tuncer M, Citterio F, Wiecek A, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant 2013;13:136–45 | Outcome |
| Ho ETL, Wong G, Chapman JR, Craig J. Once daily extended release versus twice daily standard release tacrolimus in kidney transplant recipients: a systematic review. Transplantation 2012;94:989 | Abstract |
| Hoerning A, Köhler S, Jun C, Lu J, Fu J, Tebbe B, et al. Cyclosporin but not everolimus inhibits chemokine receptor expression on CD4+ T cell subsets circulating in the peripheral blood of renal transplant recipients. Clin Exp Immunol 2012;168:251–9 | Outcome |
| Holdaas H, Rostaing L, Serón D, Cole E, Chapman J, Fellstrøm B, et al. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation 2011;92:410–8 | Duplicate |
| Holdaas H, Rostaing L, Serón D, Cole E, Chapman J, Fellstrøm B, et al. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation 2011;92:410–8 | Duplicate |
| Holdaas H, Rostaing L, Serón D, Cole E, Chapman J, Fellstrøm B, et al. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation 2011;92:410–8 | Population |
| Holdaas H, Rostaing L, Serón D, Cole E, Chapman J, Fellstrøm B, et al. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation 2011;92:410–8 | Duplicate |
| Homan Van Der Heide JJ, De Fijter JW, Ten Berge I, De Maar EF, Bemelman FJ. Mecano: mycophenolate sodium vs everolimus or ciclosporin with allograft nephropathy as outcome study: clinical results. Transplant Int 2011;24:85 | Abstract |
| Hooff J, Walt I, Kallmeyer J, Miller D, Dawood S, Moosa MR, et al. Pharmacokinetics in stable kidney transplant recipients after conversion from twice-daily to once-daily tacrolimus formulations. Therapeutic Drug Monitoring 2012;34:46–52 | Study design |
| van Hooff JP, Squifflet JP, Vanrenterghem Y. Benelux experience with a combination of tacrolimus and mycophenolate mofetil: 4-year results. Transplant Proc 2002;34:1591–3 | Comparator |
| Hooff JP, Squifflet JP, Wlodarczyk Z, Vanrenterghem Y, Paczek L. A prospective randomized multicenter study of tacrolimus in combination with sirolimus in renal-transplant recipients. Transplantation 2003;75:1934–9 | Comparator |
| Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, Proby CM, Wolterbeek R, Bouwes Bavinck JN, de Fijter JW. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol 2013;31:1317–23 | Study design |
| Howell M, Yeo R, Tong A, Craig JC, Howard K, Wong G. Adverse events of maintenance immunosuppression following kidney transplantation reported in randomised controlled trials: a systematic review. URL: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/360/CN-01009360/frame.html (accessed 25 July 2014) | Abstract |
| Huang HF, Yao X, Chen Y, Xie WQ, Shen-Tu JZ, Chen JH. Cyclosporine A and tacrolimus combined with enteric-coated mycophenolate sodium influence the plasma mycophenolic acid concentration - a randomised controlled trial in Chinese live related donor kidney transplant recipients. Int J Clin Pract Suppl 2014;68:4–9 | Outcome |
| Huh W, Lee K, Lee K, Kim S, Joh J, Oh H. Randomized trial of tacrolimus versus cyclosporine in steroid withdrawal regimen after living kidney transplantation. Clin Pharmacol Ther 2003;73:P26 | Abstract |
| Iaria G, Pisani F, Iorio B, Lucchesi C, De Luca L, Ielpo B, et al. Long-term results of kidney transplantation with cyclosporine- and everolimus-based immunosuppression. Transplant Proc 2006;38:1018–9 | Study design |
| Ibrahim H, Issa N, Spong R, Kukla A, Kandaswamy R, Dunn T, et al. CNI reduction vs. mTOR based immunosuppression after prednisone discontinuation: four year preliminary results from a large randomized trial. Am J Transplant 2012;12:302 | Abstract |
| Ireland R. Transplantation: early switch from calcineurin inhibitors to mTOR inhibitors leads to improved renal graft function. Nat Rev Nephrol 2011;7:243 | Study design |
| Nicholson M. A prospective randomised trial of the use of cellcept to allow early tacrolimus withdrawal in live donor kidney transplantation. ISRCTN. URL: www.controlled-trials.com/ISRCTN63298320 (accessed 25 July 2014) | No data |
| Hammad A. A randomised prospective trial of daclizumab induction followed by sirolimus in association with mycophenolate mofetil and steroids versus standard cyclosporin based triple therapy for rejection prophylaxis in renal transplantation. ISRCTN. URL: www.controlled-trials.com/ISRCTN74336394 (accessed 25 July 2014) | No data |
| Jarzembowski T, Panaro F, Raofi V, Dong G, Testa G, Sankary H, Benedetti E. Long-term results of a prospective randomized trial comparing tacrolimus versus cyclosporine in African-American recipients of primary cadaver renal transplant. Transpl Int 2005;18:419–22 | Population |
| Jesky MD, Sharif A, Borrows RJ. Does conversion from cyclosporine to tacrolimus as secondary prevention provide better outcomes in renal allograft recipients? A meta-analysis. Am J Transplant 2011;11:410 | Abstract |
| Jevnikar A, Arlen D, Barrett B, Boucher A, Cardella C, Cockfield SM, et al. Five-year study of tacrolimus as secondary intervention versus continuation of cyclosporine in renal transplant patients at risk for chronic renal allograft failure. Transplantation 2008;86:953–60 | Population |
| Joannides R, Etienne I, Iacob M, Hurault de Ligny B, Barbier S, Bellien J, et al. Comparative effects of sirolimus and cyclosporin on conduit arteries endothelial function in kidney recipients. Transpl Int 2010;23:1135–43 | Population |
| Joannidès R, Monteil C, de Ligny BH, Westeel PF, Iacob M, Thervet E, et al. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. Am J Transplant 2011;11:2414–22 | Population |
| Johari Y, Bryson D, Nicholson M. A randomised controlled trial comparing switching to rapamune based immunosuppression with tacrolimus minimisation for renal transplantation. Br J Surg 2010;97:68–9 | Abstract |
| Johari Y, Bryson D, Barlow A, Nicholson M. Cyclosporine micro-emulsion versus tacrolimus for renal transplantation: 10-year follow-up for single centre randomised controlled trial. Br J Surg 2010;97:32–3 | Abstract |
| Johari Y, Bryson D, Medcalf J, Nicholson M. Cyclosporine versus tacrolimus for renal transplantation: 10 year follow up of a randomised controlled trial. Br J Surg 2010;97:37 | Abstract |
| Jose M, Caring for Australians with Renal Impairment (CARI). The CARI guidelines. Calcineurin inhibitors in renal transplantation: adverse effects. Nephrology 2007;12(Suppl. 1):66–74 | Study design |
| Joss N, Rodger RS, McMillan MA, Junor BJ. Randomized study comparing cyclosporine with azathioprine one year after renal transplantation-15-year outcome data. Transplantation 2007;83:582–7 | Population |
| Junge G, De Simone P, Fung J, Kohler S, Saliba F. Urinary protein excretion in non-renal transplant patients-does mTOR-inhibitor treatment matter? Am J Transplant 2013;13:531–2 | Abstract |
| Junge G, Tufveson G, Riad H, Cibrik D, Tedesco H, Schwende H, et al. Better renal allograft function with everolimus facilitated CNI reduction - Graft type, donor criteria and gender analysis. NDT Plus 2010;3:iii540 | Abstract |
| Jungraithmayr TC, Wiesmayr S, Staskewitz A, Kirste G, Bulla M, Fehrenbach H, et al. Five-year outcome in pediatric patients with mycophenolate mofetil-based renal transplantation. Transplantation 2007;83:900–5 | Study design |
| Jurewicz WA. Tacrolimus versus ciclosporin immunosuppression: long-term outcome in renal transplantation. Nephrol Dial Transplant 2003;18:i7–i11 | Population |
| Kaabak M, Babenko N, Zokoyev A, Schekaturov S, Sandrikov V. Eculizumab for prevention and treatment of kidney graft reperfusion injury, preliminary results of RCT. Transplantation 2014;98:257–8 | Abstract |
| Kahan BD. Two-year results of multicenter phase III trials on the effect of the addition of sirolimus to cyclosporine-based immunosuppressive regimens in renal transplantation. Transplant Proc 2003;35(Suppl. 3):37–51 | Population |
| Kalil AC, Florescu DF, Sun J. Induction immunosuppression: what is the difference in the risk of serious infections between interleukin-2RA and polyclonal antibodies? Am J Transplant 2009;9:283 | Abstract |
| Kalil AC, Florescu MC, Grant W, Miles C, Morris M, Stevens RB, et al. Risk of serious opportunistic infections after solid organ transplantation: interleukin-2 receptor antagonists versus polyclonal antibodies. A meta-analysis. Expert Rev Anti Infect Ther 2014;12:881–96 | Study design |
| Kamar N, Allard J, Ribes D, Durand D, Ader JL, Rostaing L. Assessment of glomerular and tubular functions in renal transplant patients receiving cyclosporine A in combination with either sirolimus or everolimus. Clin Nephrol 2005;63:80–6 | Study design |
| Kamar N, Lehner F, Banas B, Viklicky O, Albano L, Glyda M. Efficacy and safety of tacrolimus prolonged release and immediate release in de novo renal transplantation – the osaka study (optimizing immunosuppression after kidney transplantation with advagraf). Transplant Int 2011;24:39 | Abstract |
| Kamar N, Rial M, Alberu J, Steinberg SM, Manfro R, Nainan G, et al. 3-year outcomes after switching to belatacept from a calcineurin inhibitor in stable kidney transplant recipients. Transplant Int 2013;26:44 | Abstract |
| Kamar N, Rial M, Alberu J, Steinberg S, Manfro R, Nainan G, et al. Three-years outcomes after switching to belatacept from calcineurin inhibitor in stable kidney transplant recipients. Transplant Int 2013;26:22 | Abstract |
| Kamar N, Rostaing L, Cassuto E, Villemain F, Moal MC, Ladrière M, et al. A multicenter, randomized trial of increased mycophenolic acid dose using enteric-coated mycophenolate sodium with reduced tacrolimus exposure in maintenance kidney transplant recipients. Clin Nephrol 2012;77:126–36 | Population |
| Kandaswamy R, Melancon JK, Dunn T, Tan M, Casingal V, Humar A, et al. A prospective randomized trial of steroid-free maintenance regimens in kidney transplant recipients – an interim analysis. Am J Transplant 2005;5:1529–36 | Population |
| Kang MH, Kim HJ, Ko RK, Ko SK. A systematic review of immunosuppressive regimens in lower immunological risk renal transplant recipients. Value Health 2010;13:A473–4 | Abstract |
| Kang MH, Kim HJ, Ko RK, Ko SK. A systematic review of immunosuppressive regimens in lower immunological risk renal transplant recipients. Value Health 2010;13:A473–4 | Duplicate |
| Karpe Krishna M, Talaulikar Girish S, Walters G. Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database System Rev 2007;4:CD006750 | Study design |
| Kasiske BL, de Mattos A, Flechner SM, Gallon L, Meier-Kriesche HU, Weir MR, Wilkinson A. Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am J Transplant 2008;8:1384–92 | Study design |
| Keown P, Balshaw R, Khorasheh S, Chong M, Marra C, Kalo Z, Korn A. Meta-analysis of basiliximab for immunoprophylaxis in renal transplantation. BioDrugs 2003;17:271–9 | Population |
| Ke-Pu L, Xiao-Min Y, Shuai-Jun M, Zhi-Bin L, Geng Z, Jian-Lin Y. Effects of tacrolimus and cyclosporine A on inflammatory cytokines and blood lipid after renal transplantation. Journal of Clinical Rehabilitative Tissue Engineering Research 2011;15:5769–72 | Language |
| Keven K, Sahin M, Kutlay S, Sengul S, Erturk S, Ersoz S, Erbay B. Immunoglobulin deficiency in kidney allograft recipients: comparative effects of mycophenolate mofetil and azathioprine. Transpl Infect Dis 2003;5:181–6 | Outcome |
| Khosroshahi HT, Tubbs RS, Shoja MM, Ghafari A, Noshad H, Ardalan MR. Effect of prophylaxis with low-dose anti-thymocyte globulin on prevention of acute kidney allograft rejection. Transplant Proc 2008;40:137–9 | Population |
| Khwaja K, Asolati M, Harmon J, Melancon JK, Dunn T, Gillingham K, et al. Outcome at 3 years with a prednisone-free maintenance regimen: a single-center experience with 349 kidney transplant recipients. Am J Transplant 2004;4:980–7 | Study design |
| Kihm LP, Hinkel UP, Michael K, Sommerer C, Seckinger J, Morath C, et al. Contrast enhanced sonography shows superior microvascular renal allograft perfusion in patients switched from cyclosporine A to everolimus. Transplantation 2009;88:261–5 | Population |
| Knight SR, Morris PJ. Does the evidence support the use of mycophenolate mofetil therapeutic drug monitoring in clinical practice? A systematic review. Transplantation 2008;85:1675–85 | Study design |
| Knoll GA, Kokolo MB, Mallick R, Beck A, Buenaventura CD, Ducharme R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ 2014;349:g6679 | Duplicate |
| Knoll GA, Kokolo MB, Mallick R, Beck A, Buenaventura CD, Ducharme R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ 2014;349:g6679 | Study design |
| Kobashigawa J, Ross H, Kfoury AG, Van Bakel A, Ewald G, Burton J, et al. CMV infections are less frequent in de novo heart transplant recipients receiving immunosuppression with everolimus plus reduced CsA compared to MMF and standard CsA. Am J Transplant 2011;11:131–2 | Abstract |
| Koch M, Becker T, Lueck R, Neipp M, Klempnauer J, Nashan B. Basiliximab induction therapy in kidney transplantation: benefits for long term allograft function after 10 years? Biologics 2009;3:51–6 | Study design |
| Koukoulaki M, Grispou U, Pistolas D, Balaska K, Apostolou T, Anagnostopoulou M, et al. Monitoring of BK polyoma virus in renal transplant recipients. Preliminary results of a prospective study. Nephrol Dial Transplant 2005;20:V177–V | Abstract |
| Kovac D, Kotnik V, Kandus A. Basiliximab and mycophenolate mofetil in combination with low-dose cyclosporine and methylprednisolone effectively prevent acute rejection in kidney transplant recipients. Transplant Proc 2005;37:4230–4 | Study design |
| Kramer B. Significantly better freedom from acute rejection with tacrolimus vs. cyclosporine-based immunosuppression in renal transplant recipients at 7-year follow-up. Am J Transplant 2010;10:568 | Abstract |
| Kramer B, Kruger B, Banas B, Tomlinson P. Early post-transplant blood levels in de novo renal recipients on tacrolimus prolonged release (TACQD) versus tacrolimus immediate release (TACBD) in A phase III double-blind double-dummy study. Transplant Int 2011;24:54 | Abstract |
| Kramer BK. Better tolerability and significantly higher freedom from acute rejection at 7 years with tacrolimus vs. cyclosporine-based immunosuppression in renal transplant recipients. NDT Plus 2010;3:iii284 | Abstract |
| Krämer BK, Böger C, Krüger B, Marienhagen J, Pietrzyk M, Obed A, et al. Cardiovascular risk estimates and risk factors in renal transplant recipients. Transplant Proc 2005;37:1868–70 | Population |
| Krämer BK, Del Castillo D, Margreiter R, Sperschneider H, Olbricht CJ, Ortuño J, et al. Efficacy and safety of tacrolimus compared with ciclosporin A in renal transplantation: three-year observational results. Nephrol Dial Transplant 2008;23:2386–92 | Population |
| Krämer BK, Charpentier B, Bäckman L, Silva HT, Mondragon-Ramirez G, Cassuto-Viguier E, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant 2010;10:2632–43 | Population |
| Krämer BK, Klinger M, Vítko Š, Glyda M, Midtvedt K, Stefoni S, et al. Tacrolimus-based, steroid-free regimens in renal transplantation: 3-year follow-up of the ATLAS trial. Transplantation 2012;94:492–8 | Comparator |
| Krämer BK, Klinger M, Wlodarczyk Z, Ostrowski M, Midvedt K, Stefoni S, et al. Tacrolimus combined with two different corticosteroid-free regimens compared with a standard triple regimen in renal transplantation: one year observational results. Clin Transplant 2010;24:E1–9 | Study design |
| Krämer BK, Montagnino G, Castillo D, Margreiter R, Sperschneider H, Olbricht CJ, et al. Efficacy and safety of tacrolimus compared with cyclosporin A microemulsion in renal transplantation: 2 year follow-up results. Nephrology, Dialysis, Transplantation 2005;20:968–73 | Study design |
| Kramer BK, Zulke C, Kammerl MC, Schmidt C, Hengstenberg C, Fischereder M, et al. Cardiovascular risk factors and estimated risk for CAD in a randomized trial comparing calcineurin inhibitors in renal transplantation. Am J Transplant 2003;3:982–7 | Outcome |
| Kreis H. Worse renal transplant outcomes with sirolimus-mycophenolate than with calcineurin inhibitor regimens. Nat Clin Pract Nephrol 2007;3:424–5 | Study design |
| Krischock L, Marks SD. Induction therapy: why, when, and which agent? Pediatr Transplant 2010;14:298–313 | Study design |
| Kumar A, Zaman W, Chaurasia D, Gupta A, Sharma RK, Gulati S. Prospective randomized trial to evaluate the efficacy of single low dose ATG induction in renal transplant recipient with spousal kidney. Indian J Urol 2002;19:58–62 | Study design |
| Kumar N, Manimaran R, Williams C, Ravanan R. Tacrolimus preserves renal function better than cyclosporin at 10 years – long term results of a randomised controlled trial. Am J Transplant 2009;9:200 | Abstract |
| Kwon O, Cho JH, Choi JY, Park SH, Kim YL, Kim HK, et al. Long-term outcome of azathioprine versus mycophenolate mofetil in cyclosporine-based immunosuppression in kidney transplantation: 10 years of experience at a single center. Transplant Proc 2013;45:1487–90 | Study design |
| Kyllönen LE, Eklund BH, Pesonen EJ, Salmela KT. Single bolus antithymocyte globulin versus basiliximab induction in kidney transplantation with cyclosporine triple immunosuppression: efficacy and safety. Transplantation 2007;84:75–82 | Population |
| Langer RM, Hené R, Vitko S, Christiaans M, Tedesco-Silva H, Ciechanowski K, et al. Everolimus plus early tacrolimus minimization: a phase III, randomized, open-label, multicentre trial in renal transplantation. Transpl Int 2012;25:592–602 | Study design |
| Langer RM, Pape L, Tonshoff B, Dello Strologo L, Ettenger R, Niaudet P, et al. Evaluation of safety and efficacy of everolimus with reduced tacrolimus: design of a randomized, multicenter, open-label study in pediatric renal transplant recipients. Pediatr Transplant 2013;17:80 | Abstract |
| Langone AJ, Chan L, Bolin P, Cooper M. Enteric-coated mycophenolate sodium versus mycophenolate mofetil in renal transplant recipients experiencing gastrointestinal intolerance: a multicenter, double-blind, randomized study. Transplantation 2011;91:470–8 | Population |
| Larsen C, Alberu J, Massari P, Acevedo RR, Kamar N, Lin CS, et al. 4-Year results from the long-term extension of the belatacept BENEFIT study. Am J Transplant 2012;12:82 | Abstract |
| Larsen C, Vincenti F, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension (LTE) of the belatacept evaluation of nephroprotection and efficacy as first-line immunosuppression trial (benefit) study. Am J Transplant 2013;13:312 | Abstract |
| Larsen C, Vincenti F, Grinyo JM, Charpentier B, Di Russo GB, Garg P, et al. Renal benefit of belatacept vs cyclosporine in kidney transplant patients is not impacted by acute rejection (BENEFIT Study). Am J Transplant 2009;9:220 | Abstract |
| Larsen CP, Bray R, Gebel H, Ganguly B, Kulbokas E, Brickman D, et al. Evaluation of donor-specific antibodies in kidney transplant patients treated with belatacept-or cyclosporine-based immunosuppression in benefit and BENEFIT-EXT. Transplant Internat 2011;24:69 | Abstract |
| Larsen CP, Grinyo J, Charpentier B, Medina Pestana J, Kamar N, Vanrenterghem Y, et al. Belatacept vs cyclosporine in kidney transplant recipients: two-year outcomes from the benefit study. NDT Plus 2010;3:iii262 | Abstract |
| Larsen CP, Grinyó J, Medina-Pestana J, Vanrenterghem Y, Vincenti F, Breshahan B, et al. Belatacept-based regimens versus a cyclosporine A-based regimen in kidney transplant recipients: 2-year results from the BENEFIT and BENEFIT-EXT studies. Transplantation 2010;90:1528–35 | Population |
| Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant 2006;6:514–22 | Population |
| Lawen JG, Davies EA, Mourad G, Oppenheimer F, Molina MG, Rostaing L, et al. Randomized double-blind study of immunoprophylaxis with basiliximab, a chimeric anti-interleukin-2 receptor monoclonal antibody, in combination with mycophenolate mofetil-containing triple therapy in renal transplantation. Transplantation 2003;75:37–43 | Population |
| Lebranchu Y, Bridoux F, Büchler M, Le Meur Y, Etienne I, Toupance O, et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am J Transplant 2002;2:48–56 | Population |
| Lebranchu Y, Buchler M, Etienne I, Toupance O, Westel PF, Legendre C, et al. 12 month results of a randomized trial comparing sirolimus (SRL) versus cyclosporine (CsA) in 150 transplant patients receiving a cadaveric renal graft. Am J Transplant 2005;5:540 | Abstract |
| Lebranchu Y, Etienne I, Toupance O, Westeel PF, de Ligny BH, Rerolle JP, et al. Cni avoidance and steroid withdrawal in renal transplantation. Results at three years of a prospective multicenter randomized trial comparing sirolimus (srl) and cyclosporine (csa): the spiesser study. Transplant Internat 2009;22:244 | Abstract |
| Lebranchu Y, Legendre C, Merville P, Durrbach A, Rostaing L, Thibault G, et al. Comparison of interleukin-2 (Il-2) Blockade in kidney transplant patients randomized to 40mg or 80mg basiliximab (BSX) with cyclosporine (CsA) or 80mg BSX with everolimus (EVR). Transplantation 2014;98:581 | Abstract |
| Lebranchu Y, Snanoudj R, Toupance O, Weestel PF, Hurault de Ligny B, Buchler M, et al. Five-year results of a randomized trial comparing de novo sirolimus and cyclosporine in renal transplantation: the SPIESSER study. Am J Transplant 2012;12:1801–10 | Population |
| Lebranchu Y, Thierry A, Thervet E, Büchler M, Etienne I, Westeel PF, et al. Efficacy and safety of early cyclosporine conversion to sirolimus with continued MMF-four-year results of the Postconcept study. Am J Transplant 2011;11:1665–75 | Abstract |
| Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, et al. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant 2009;9:1115–23 | Population |
| Lebranchu Y, Touchard G, Buchler M, Thervet E, Etienne I, Westeel PF, et al. Efficacy and safety of early cyclosporine (CSA) conversion to sirolimus (SRL) with mycophenolate mofetil (MMF): 5-year results of the post-concept study. Transplant Internat 2011;24:57 | Population |
| Lebranchu Y, Toupance O, Touchard G, Thervet E, Etienne I, Mazouz H, et al. Impact on renal function of early conversion at 3 months from cyclosporine (CsA) to sirolimus (SRL) in association with mycophenolate mofetil (MMF) in kidney transplantation: 30-months follow up of a multicenter randomized controlled trial: the concept study. Am J Transplant 2009;9:260 | Abstract |
| Lebranchu Y, Toupance O, Touchard G, Thervet E, Etienne I, Westeel PF, et al. Impact of early conversion at 3 months from cyclosporine (CSA) to sirolimus (SRL) in association with mycophenolate mofetil (MMF) on renal function – “Results at 48 months of follow up of a multicenter randomized controlled trial: the concept study”. Am J Transplant 2010;10:151 | Abstract |
| Lee YJ, Kim B, Lee JE, Kim YG, Kim DJ, Kim SJ, et al. Randomized trial of cyclosporine and tacrolimus therapy with steroid withdrawal in living-donor renal transplantation: 5-year follow-up. Transpl Int 2010;23:147–54 | Population |
| Legendre C, Campistol JM, Squifflet JP, Burke JT, Sirolimus European Renal Transplant Study Group. Cardiovascular risk factors of sirolimus compared with cyclosporine: early experience from two randomized trials in renal transplantation. Transplant Proc 2003;35(Suppl. 3):151–3 | Study design |
| Legendre C, Srinivas TR, Pascual J, Chadban S, Citterio F, Henry M, et al. The transform trial design: a large randomized, multicenter, open-label study of everolimus with reduced calcineurin inhibitors in de novo renal transplantation. Transplant Internat 2013;26:23–4 | Abstract |
| Lehner F, Banas B. Influence of donor related factors on outcomes with tacrolimus-based immunosuppression after kidney transplantation – the Osaka study. Transplant Internat 2011;24:21 | Abstract |
| Lehner F, Anns W, Witzke O, Budde K, Sommerer C, Eisenber-Ger U, et al. Three years follow-up of the zeus trial: maintained better renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients. Transplant Internat 2011;24:50 | Abstract |
| Lehner F, Arns W, Reinke P, Eisenberger U, Paulus EM, Scheidl S, et al. Renal function in everolimus/enteric-coated mycophenolate sodium treated de novo living renal transplant recipients after calcineurin inhibitor withdrawal: subgroup analysis of the zeus study. Transplant Internat 2011;24:50–1 | Abstract |
| Lehner F, Banas B, Kamar N, Glyda M, Viklicky O, Albano L. Influence of donor related factors on outcomes with tacrolimus-based immunosuppression after kidney transplantation – the osaka study (optimizing immunosuppression after kidney transplantationwith Advagraf). Transplant Internat 2011;24:164-5 | Abstract |
| Lehner F, Budde K, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Improved renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 3 year follow-up of the zeus trial. Transplant Internat 2011;24:57 | Abstract |
| Lehner F, Guba M, Arns W, Sommerer C, Neumayer HH, Jacobi J, et al. Follow-up data from herakles study at month 24: maintained superior renal function in patients on an everolimus-based calcineurin inhibitor free regimen compared to standard cyclosporine/mycophenolate and low cyclosporine/everolimus. Transplant Internat 2013;26:28 | Abstract |
| Lehner F, Sommerer C, Arns W, Eisenberger U, Reinke P, Pressmar K, et al. A post hoc analysis of 2 prospective, open-label, multicenter, randomized trials: onset and progression of diabetes in kidney transplant patients receiving everolimus or cyclosporine. Results from ZEUS and HERAKLES. Transplant Internat 2013;26:21 | Abstract |
| Lehner F, Sommerer C, Arns W, Reinke P, Eisenberger U, Wuthrich RP, et al. Post HOC subgroup analysis from ZEUS: outcome on renal function, efficacy and safety in livingdonor kidney transplant recipients after conversion from a calcineurin inhibitor to an everolimus based regimen. Transplant Internat 2013;26:8 | Abstract |
| Lehner F, Sommerer C, Reinke P, Arns W, Eisenberger U, Paulus EM, et al. 5-year follow-up on the ZEUS KTX trial: everolimus conversion after CNI withdrawal. Transplant Internat 2013;26:81 | Abstract |
| Lehner F, Sommerer C, Witzke O, Arns W, Kliem V, Neumayer HH, et al. Herakles at month 24: efficacy and safety of 3 different regimens in de novo renal transplant patients. Transplant Internat 2013;26:82 | Abstract |
| Lezaic VD, Marinkovic J, Ristic S, Dokic ZM, Basta Jovanovic G, Radivojevic DM, et al. Conversion of azathioprine to mycophenolate mofetil and chronic graft failure progression. Transplant Proc 2005;37:734–6 | Population |
| Libetta C, Canevari M, Margiotta E, Martinelli C, Borettaz I, Esposito P, et al. Preliminary data of controlled randomized study (ever twist) on tolerance induction. Transplant Internat 2013;26:20 | Abstract |
| Libetta C, Margiotta E, Borettaz I, Canevari M, Martinelli C, Lainu E, et al. Everolimus and lowdose of tacrolimus combined with thymoglobulin induction induces regulatory t cells expansion in de novo kidney transplant recipients: preliminary data of controlled randomized study (ever twist). Nephrol Dial Transplant 2013;28:i277 | Abstract |
| Liefeldt L, Brakemeier S, Glander P, Waiser J, Lachmann N, Schönemann C, et al. Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant 2012;12:1192–8 | Population |
| Lim W, Eris J, Kanellis J, Pussell B, Wiid Z, Witcombe D, et al. Conversion from calcineurin-inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients: a systematic review and meta-analysis of randomised trials. Nephrology 2013;18:44–5 | Abstract |
| Lim W, Eris J, Kanellis J, Pussell B, Wiid Z, Witcombe D, et al. Conversion from calcineurin-inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients: a systematic review and meta-analysis of randomised trials. Nephrology 2013;18:44–5 | Duplicate |
| Lim WH, Eris J, Kanellis J, Pussell B, Wiid Z, Witcombe D, et al. A systematic review of conversion from calcineurin inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients. Am J Transplant 2014;14:2106–19 | Population |
| Lin CC, Chuang FR, Lee CH, Wang CC, Chen YS, Liu YW, et al. The renal-sparing efficacy of basiliximab in adult living donor liver transplantation. Liver Transpl 2005;11:1258–64 | Study design |
| Liu B, Lin ZB, Ming CS, Zhang WJ, Chen ZS, Sha B, et al. Randomized trial of tacrolimus in combination with mycophenolate mofetil versus cyclosporine with mycophenolate mofetil in cadaveric renal transplant recipients with delayed graft function. Transplant Proc 2003;35:87–8 | Study design |
| Liu M, Zhang W, Gu M, Yin C, Zhang WY, Lv Q, Xu D. Protective effects of sirolimus by attenuating connective tissue growth factor expression in human chronic allograft nephropathy. Transplant Proc 2007;39:1410–5 | Outcome |
| Liu Y, Yang MS, Yuan JY. Immunosuppressant utilization and cardiovascular complications among Chinese patients after kidney transplantation: a systematic review and analysis. Int Urol Nephrol 2013;45:885–92 | Study design |
| Liu Y, Yang MS, Yuan JY. Immunosuppressant utilization and cardiovascular complications among Chinese patients after kidney transplantation: a systematic review and analysis. Int Urol Nephrol 2013;45:885–92 | Duplicate |
| Liu Y, Zhou P, Han M, Xue CB, Hu XP, Li C. Basiliximab or antithymocyte globulin for induction therapy in kidney transplantation: a meta-analysis. Transplant Proc 2010;42:1667–70 | Study design |
| Ljuca F, Imamović S, Mesić D, Hasukić SH, Omerović S, Bazardzanović M, Iljazagić-Halilović F. Micophenolat Mofetil versus Azathioprine: effects on renal graft function in early posttransplant period. Bosn J Basic Med Sci 2009;9:156–60 | Study design |
| Lo A, Egidi MF, Gaber LW, Amiri HS, Vera S, Nezakatgoo N, Gaber AO. Comparison of sirolimus-based calcineurin inhibitor-sparing and calcineurin inhibitor-free regimens in cadaveric renal transplantation. Transplantation 2004;77:1228–35 | Study design |
| Lorber MI, Mulgaonkar S, Butt KM, Elkhammas E, Mendez R, Rajagopalan PR, et al. Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: a 3-year randomized, multicenter, phase III study. Transplantation 2005;80:244–52 | Population |
| Loriga G, Ciccarese M, Pala PG, Satta RP, Fanelli V, Manca ML, et al. De novo everolimus-based therapy in renal transplant recipients: effect on proteinuria and renal prognosis. Transplant Proc 2010;42:1297–302 | Population |
| Lou HX, Vathsala A. Conversion from mycophenolate mofetil to azathioprine in high-risk renal allograft recipients on cyclosporine-based immunosuppression. Transplant Proc 2004;36:2090–1 | Population |
| Luan FL, Zhang H, Schaubel DE, Miles CD, Cibrik D, Norman S, Ojo AO. Comparative risk of impaired glucose metabolism associated with cyclosporine versus tacrolimus in the late posttransplant period. Am J Transplant 2008;8:1871–7 | Outcome |
| Maamoun H, Khashab S, Belal D, Soliman AR. Azathioprine increases cyclosporine-induced hyperuricemia in renal transplant recipient. Transplantation 2012;94:969 | Abstract |
| Machado PG, Felipe CR, Hanzawa NM, Park SI, Garcia R, Alfieri F, et al. An open-label randomized trial of the safety and efficacy of sirolimus vs. azathioprine in living related renal allograft recipients receiving cyclosporine and prednisone combination. Clin Transplant 2004;18:28–38 | Population |
| Maiorano A, Stallone G, Schena A, Infante B, Pontrelli P, Schena FP, Grandaliano G. Sirolimus interferes with iron homeostasis in renal transplant recipients. Transplantation 2006;82:908–12 | Population |
| Marchetti P, Vincenti F, Friman S, Scheuermann E, Grp DS. New-onset diabetes impaired fasting glucose after renal transplantation: results of a prospective, randomised trial comparing cyclosporine versus tacrolimus. Diabetologia 2006;49:500–1 | Abstract |
| Margreiter R, European Tacrolimus vs Ciclosporin Microemulsion Renal Transplantation Study Group. Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: a randomised multicentre study. Lancet 2002;359:741–6 | Population |
| Margreiter R. Tacrolimus vs ciclosporin microemulsion in renal transplantation. A randomized multicentre study. Chirurgische Praxis. 2002;60:611–2 | Abstract |
| Margreiter R, Pohanka E, Sparacino V, Sperschneider H, Kunzendorf U, Huber W, et al. Open prospective multicenter study of conversion to tacrolimus therapy in renal transplant patients experiencing ciclosporin-related side-effects. Transpl Int 2005;18:816–23 | Study design |
| Marks WH, Ilsley JN, Dharnidharka VR. Posttransplantation lymphoproliferative disorder in kidney and heart transplant recipients receiving thymoglobulin: a systematic review. Transplant Proc 2011;43:1395–404 | Study design |
| Martínez-Castelao A, Sarrias X, Bestard O, Gil-Vernet S, Serón D, Cruzado JM, et al. Arterial elasticity measurement in renal transplant patients under anticalcineurin immunosuppression. Transplant Proc 2005;37:3788–90 | Population |
| Mas V, Maluf D, Scian M, Chalasani G, Sustento-Reodica N, Leventhal J, et al. Differential impact of calcineurin and mammalian target of rapamycin inhibition on immune, inflammation and antigen presentation genes expression in renal allograft biopsies. Am J Transplant 2012;12:40 | Abstract |
| Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev 2014;11:CD010699 | Duplicate |
| Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev 2014;11:CD010699 | Study design |
| Masson P, Henderson LK, Craig J, Webster AC. Belatacept for kidney transplant recipients: a systematic review and meta-analysis. Transplantation 2012;94:968–9 | Abstract |
| Masson P, Henderson LK, Craig J, Webster AC. Belatacept for kidney transplant recipients: a systematic review and meta-analysis. Transplantation 2012;94:968–9 | Duplicate |
| Matas A, Gillingham K. Prospective randomized study of low level CNI vs SRL @ 6 mos posttx, while pred (P)-free. Transplantation 2014;98:542 | Abstract |
| Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant 2004;18:446–9 | Study design |
| Mayer AD. Chronic rejection and graft half-life: five-year follow-up of the European Tacrolimus Multicenter Renal Study. Transplant Proc 2002;34:1491–2 | Population |
| Medina Pestana J, Grinyo J, Vanrenterghem Y, Becker T, Florman S, Lang P, et al. Belatacept compared with cyclosporine in renal allograft recipients of extended criteria donor kidneys: 3-year outcomes from the phase III benefit-EXT trial. Transplant Int 2011;24:51 | Abstract |
| Medina-Pestana JO, Garcia VD, David-Neto E, Carvalho DBM, Contieri F, Abbud-Filho M, et al. Conversion from tacrolimus to sirolimus-based immunosuppressive regimen in kidney transplant recipients. Preliminary results. Am J Transplant 2011;11:462 | Abstract |
| Meier M, Bode W, Nitschke M, Wong W, Kramer J, Lehnert H, et al. Low dose tacrolimus versus mycophenolate-mofetil in ‘old for old’ kidney transplantation: a one year prospective multicenter randomized controlled trial. Am J Transplant 2009;9:498 | Abstract |
| Meier M, Nitschke M, Weidtmann B, Jabs WJ, Wong W, Suefke S, et al. Slowing the progression of chronic allograft nephropathy by conversion from cyclosporine to tacrolimus: a randomized controlled trial. Transplantation 2006;81:1035–40 | Population |
| Meier-Kriesche HU, Davies NM, Grinyó J, Heading R, Mamelok R, Wijngaard P, et al. Mycophenolate sodium does not reduce the incidence of GI adverse events compared with mycophenolate mofetil. Am J Transplant 2005;5:1164 | Study design |
| Mendez R, Gonwa T, Yang HC, Weinstein S, Jensik S, Steinberg S. A prospective, randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 1 year. Transplantation 2005;80:303–9 | Population |
| Merville P, Bergé F, Deminière C, Morel D, Chong G, Durand D, et al. Lower incidence of chronic allograft nephropathy at 1 year post-transplantation in patients treated with mycophenolate mofetil. Am J Transplant 2004;4:1769–75 | Population |
| Metcalfe MS, Jain S, Waller JR, Saunders RN, Bicknell GR, Nicholson ML. A randomized trial of mycophenolate mofetil versus azathioprine as calcineurin inhibitor sparing agents in the treatment of chronic allograft nephropathy. Transplant Proc 2002;34:1812–4 | Population |
| Mjornstedt L, Schwartz Sorensen S, Von Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Renal function three years after early conversion from a calcineurin inhibitor to everolimus: results from a randomized trial in kidney transplantation. Transplant Int 2014;28:42–51 | Population |
| Mjornstedt L, Sorensen SS, Von Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Improved renal function by overnight switch from cyclosporine to everolimus at week 7 after renal transplantation. One year results from a randomized, controlled trial. Transplant Int 2011;24:94 | Abstract |
| Mjörnstedt L, Sørensen SS, Zur Mühlen B, Jespersen B, Hansen JM, Bistrup C, et al. Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. Am J Transplant 2012;12:2744–53 | Population |
| Monaco AP, Morris PJ. Everolimus and long-term outcomes in renal transplantation: seeking an optimal strategy for immunosuppression. Transplantation 2011;92(Suppl. 3):1–2 | Study design |
| Montagnino G, Krämer BK, Arias M, European Tacrolimus vs Cyclosporin Microemulsion Renal Transplantation Study Group. Efficacy and safety of tacrolimus compared with cyclosporine microemulsion in kidney transplantation: twelve-month follow-up. Transplant Proc 2002;34:1635–7 | Abstract |
| Montagnino G, Sandrini S, Casciani C, Schena FP, Carmellini M, Civati G, et al. A randomized trial of steroid avoidance in renal transplant patients treated with everolimus and cyclosporine. Transplant Proc 2005;37:788–90 | Comparator |
| Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care 2002;25:583–92 | Population |
| Moore R. New-onset diabetes after renal transplantation: comparing ciclosporin and tacrolimus. Nature Clinical Practice Nephrology 2008;4:20–1 | Comparator |
| Morales JM, Andrés A, Dominguez-Gil B, Arriola M, Gutiérrez MJ, Hernández E, et al. Ten years of treatment with tacrolimus is related to an excellent renal function, allowing monotherapy in a large proportion of cases: unicentric results of the tacrolimus versus cyclosporine: a European multicentric study in kidney transplant patients. Transplant Proc 2005;37:3738-42 | Study design |
| Morales JM, Campistol JM, Kreis H, Mourad G, Eris J, Schena FP, et al. Sirolimus-based therapy with or without cyclosporine: long-term follow-up in renal transplant patients. Transplant Proc 2005; 37:693–6 | Language |
| Morales JM, Grinyó JM, Campistol JM, García-Martínez J, Arias M, Paul J, et al. Improved renal function, with similar proteinuria, after two years of early tacrolimus withdrawal from a regimen of sirolimus plus tacrolimus. Transplantation 2008;86:620–2 | Study design |
| Morales JM, Hartmann A, Walker R, Arns W, Senatorski G, Grinyó JM, et al. Similar lipid profile but improved long-term outcomes with sirolimus after cyclosporine withdrawal compared to sirolimus with continuous cyclosporine. Transplant Proc 2009;41:2339–44 | Outcome |
| Morales JM, Tedesco-Silva H, Peddi VR, Russ GR, Marder BA, Hahn CM, et al. Planned transition from tacrolimus to sirolimus versus continued tacrolimus in renal allograft patients. Transplant Int 2013;26:81 | Abstract |
| Moscarelli L, Caroti L, Antognoli G, Zanazzi M, Di Maria L, Carta P, Minetti E. Everolimus leads to a lower risk of BKV viremia than mycophenolic acid in de novo renal transplantation patients: a single-center experience. Clin Transplant 2013;27:546–54 | Study design |
| Mourad G, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. Sequential protocols using basiliximab versus antithymocyte globulins in renal-transplant patients receiving mycophenolate mofetil and steroids. Transplantation 2004;78:584–90 | Population |
| Mourer JS, Hartigh J, Zwet EW, Mallat MJ, Dubbeld J, Fijter JW. Randomized trial comparing late concentration-controlled calcineurin inhibitor or mycophenolate mofetil withdrawal. Transplantation 2012;93:887–94 | Study design |
| Mucha K, Foroncewicz B, Durlik M, Chmura A, Szmidt J, Paczek L. Seven-year follow-up of 77 renal transplant reciepients (RTRs) treated with tacrolimus-based immunosuppression (IS). NDT Plus 2010;3:iii 268–9 | Abstract |
| Mucha K, Foroncewicz B, Paczek L, Pazik J, Lewandowska D, Krawczyk A, et al. 36-month follow-up of 75 renal allograft recipients treated with steroids, tacrolimus, and azathioprine or mycophenolate mofetil. Transplant Proc 2003;35:2176–8 | Abstract |
| Muehlbacher F, Becker T, Campistol JM, Carvalho DBM, Florman S, Lang P, et al. Donor sub-type analysis of three-year outcomes from a phase iii study of belatacept in recipients of extended criteria donor kidneys (benefit-ext trial). Transplant Int 2011;24:221–2 | Abstract |
| Muhlbacher F, Florman S, Zhang R, Lang P, Lehner F, Massari P, et al. 5-year outcomes by donor type from the longterm extension of the belatacept benefit-ext study. Transplant Int 2013;26:92 | Abstract |
| Mühlbacher F, Neumayer HH, del Castillo D, Stefoni S, Zygmunt AJ, Budde K, European Rapamune Cyclosporine Minimization Study Group. The efficacy and safety of cyclosporine reduction in de novo renal allograft patients receiving sirolimus and corticosteroids: results from an open-label comparative study. Transpl Int 2014;27:176–86 | Population |
| Mulay AV, Cockfield S, Stryker R, Fergusson D, Knoll GA. Conversion from calcineurin inhibitors to sirolimus for chronic renal allograft dysfunction: a systematic review of the evidence. Transplantation 2006;82:1153–62 | Population |
| Mulay AV, Hussain N, Fergusson D, Knoll GA. Calcineurin inhibitor withdrawal from sirolimus-based therapy in kidney transplantation: a systematic review of randomized trials. Am J Transplant 2005;5:1748–56 | Duplicate |
| Mulay AV, Hussain N, Fergusson D, Knoll GA. Calcineurin inhibitor withdrawal from sirolimus-based therapy in kidney transplantation: a systematic review of randomized trials. Am J Transplant 2005;5:1748–56 | Population |
| Murakami N, Riella LV, Funakoshi T. Risk of metabolic complications in kidney transplantation after conversion to mTOR inhibitor: a systematic review and meta-analysis. Am J Transplant 2014;14:2317–27 | Population |
| Murbraech K, Holdaas H, Massey R, Undset LH, Aakhus S. Cardiac response to early conversion from calcineurin inhibitor to everolimus in renal transplant recipients: an echocardiographic substudy of the randomized controlled CENTRAL trial. Transplantation 2014;97:184–8 | Outcome |
| Murphy GJ, Waller JR, Sandford R, Nicholson ML. De novo tacrolimus-based immunosuppression reduces renal allograft fibrosis compared to neoral: a prospective randomized clinical trial. Br J Surg 2002;89:7 | Outcome |
| Murphy GJ, Waller JR, Sandford RS, Furness PN, Nicholson ML. Randomized clinical trial of the effect of microemulsion cyclosporin and tacrolimus on renal allograft fibrosis. Br J Surg 2003;90:680–6 | Population |
| Nafar M, Alipour B, Ahmadpoor P, Pour-Reza-Gholi F, Samadian F, Samavat S, Farhangi S. Sirolimus versus calcineurin inhibitor-based immunosuppressive therapy in kidney transplantation: a 4-year follow-up. Iran J Kidney Dis 2012;6:300–6 | Population |
| Nashan B, Ivens K, Suwelack B, Arns W, Abbud Filho M, myPROMS DE02 Study Group. Conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in maintenance renal transplant patients: preliminary results from the myfortic prospective multicenter study. Transplant Proc 2004;36(Suppl. 2):521–3 | Population |
| NCT. A Randomized, Open-Label, Comparative Evaluation of Conversion from Calcineurin Inhibitors to Sirolimus Versus Continued Use of Calcineurin Inhibitors in Renal Allograft Recipients. URL: www.clinicaltrials.gov/ct/show/NCT00038948 (accessed 25 July 2014) | No data |
| NCT. Phase II/III, Open-Label, Randomized, Controlled, Multiple-Dose Study of Efficacy and Safety of BMS-224818 as Part of A Quadruple Drug Regimen in First Renal Transplant Recipients. URL: www.clinicaltrials.gov/ct2/show/NCT00035555 (accessed 25 July 2014) | No data |
| NCT. A Multi-Centre, Randomised, Open-Label Study to Compare Conversion from Cyclosporin to Rapamune (Sirolimus) Versus Standard Therapy in Established Renal Allograft Recipients on Maintenance Therapy with Mild to Moderate Renal Insufficiency (UK-RAP-09). URL: www.clinicaltrials.gov/ct2/show/NCT00273871 (accessed 25 July 2014) | No data |
| NCT. A Phase III, Randomized, Open-Label, Comparative, Multi-Center Study to Assess the Safety and Efficacy of Prograf (tacrolimus)/MMF, Modified Release (MR) Tacrolimus/MMF and Neoral (cyclosporine)/MMF in de novo Kidney Transplant Recipients. URL: www.clinicaltrials.gov/ct2/show/NCT00064701 (accessed 25 July 2014) | No data |
| NCT. An Open-Label, Concentration Controlled, Randomized, 12 Month Study of Prograf + Rapamune + Cor [Study Evaluating Sirolimus in End Stage Renal Disease in High Risk Kidney Transplant Recipients]. URL: www.clinicaltrials.gov/ct2/show/NCT00044720 (accessed 25 July 2014) | Study design |
| Nguyen C, Shapiro R. New immunosuppressive agents in pediatric transplantation. Clinics 2014;69(Suppl. 1):8–16 | Study design |
| Nichelle L, Canet S, Garrigue V, Chong G, Mourad G. Arterial hypertension in renal transplant recipients treated with tacrolimus or cyclosporine-Neoral. Transplant Proc 2002;34:2824–5 | Intervention |
| Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database of Syst Rev 2014:CD000011 | Population |
| Novoa PA, Grinyó JM, Ramos FJ, Errasti P, Franco A, Aldana G, et al. De novo use of everolimus with elimination or minimization of cyclosporine in renal transplant recipients. Transplant Proc 2011;43:3331–9 | Comparator |
| Noyola-Villalobos H, Martinez-Calva I, Gomez Vazquez A, Mendiola Fernandez R, Jimenez Chavarria E, Rendon Dosal H, et al. Randomized controlled trial of early conversion from calcineurin inhibitor to everolimus in adult renal allograft patients at a single transplant center in Mexico. Transplantation 2012;94:910. | Abstract |
| Oberbauer R. Calcineurin inhibitor withdrawal from sirolimus-based therapy in kidney transplantation: a systematic review of randomized trials. Am J Transplant 2005;5:3023 | Outcome |
| Oberbauer R, Hutchison B, Eris J, Arias M, Claesson K, Mota A, et al. Health-related quality-of-life outcomes of sirolimus-treated kidney transplant patients after elimination of cyclosporine A: results of a 2-year randomized clinical trial. Transplantation 2003;75:1277–85 | Comparator |
| Oberbauer R, Segoloni G, Campistol JM, Kreis H, Mota A, Lawen J, et al. Early cyclosporine withdrawal from a sirolimus-based regimen results in better renal allograft survival and renal function at 48 months after transplantation. Transpl Int 2005;18:22–8 | Study design |
| O'Connell P, Fassett R, Pilmore H, Chapman J, Hutchison B, Russ G, et al. Long-term post transplantation switch to an everolimus-based therapy with cni elimination/minimization does not overall impact graft function: the ascertain study. Immunol Cell Biol 2011;89:A5 | Abstract |
| O'Connell P, Fassett R, Pilmore H, Chapman J, Hutchison B, Russ G, et al. Post-HOC analysis of the ascertain trial: everolimus based therapy with CNI elimination improves renal function in select populations. Immunol Cell Biol 2011;89:A5 | Abstract |
| Oh C, Huh K, Lee J, Lee J, Cho H, Kim Y. Multicenter randomized clinical investigation for the safety and efficacy of advagraf (R) (extended-release tacrolimus) vs. prograf (R) (twice-daily tacrolimus) in de novo korean adult kidney recipients. Am J Transplant 2013;13:317 | Abstract |
| Oh CK, Huh KH, Ha J, Kim YH, Kim YL, Kim YS. Safety and efficacy of the early introduction of everolimus with reduced-exposure cyclosporine a in de novo kidney recipients. Transplantation 2015;99:180–6 | Population |
| Oppenheimer F, Rebollo P, Grinyo JM, Ortega F, Sanchez-Plumed J, Gonzalez-Molina M, et al. Health-related quality of life of patients receiving low-toxicity immunosuppressive regimens: a substudy of the Symphony Study. Transplantation 2009;87:1210–3 | Intervention |
| Ortega F, Sanchez-Fructuoso A, Cruzado JM, Gomez-Alamillo JC, Alarcon A, Pallardo M, et al. Quality of life and tolerability of enteric-coated mycophenolate sodium (EC-MPS) in renal transplant recipients with gastrointestinal tract complaints to mycophenolate mofetil (MMF): a multicenter, randomized, open-label, controlled trial. Am J Transplant 2009;9:408–9 | Abstract |
| Ortega F, Sanchez-Fructuoso A, Cruzado JM, Gomez-Alamillo JC, Alarcon A, Pallardo L, et al. The use of higher doses of mycophenolic acid (MPA) is not associated with worse gastrointestinaltolerability in renal transplant patients converted from mycophenolate mofetil (MMF) to enteric-coated mycophenolate sodium (EC-MPS). Am J Transplant 2010;10:512 | Abstract |
| Ortega F, Sanchez-Fructuoso A, Cruzado JM, Gomez-Alamillo JC, Alarcon A, Pallardo LL, et al. A high glomerular filtration rate (GFR) and the use of an enteric-coated formulation of mycophenolic acid predict less gastrointestinal complaints in renal transplant patients. Transplant Int 2011;24:220 | Abstract |
| Ortega F, Sánchez-Fructuoso A, Cruzado JM, Gómez-Alamillo JC, Alarcón A, Pallardó L, et al. Gastrointestinal quality of life improvement of renal transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium drugs or agents: mycophenolate mofetil and enteric-coated mycophenolate sodium. Transplantation 2011;92:426–32 | Outcome |
| Otukesh H. Basiliximab induction therapy in pediatric renal transplantation, a double blind clinical trial. Pediatr Nephrol 2013;28:1533 | Abstract |
| Özdemir BH, Özdemir AA, Erdal R, Özdemir FN, Haberal M. Rapamycin prevents interstitial fibrosis in renal allografts through decreasing angiogenesis and inflammation. Transplant Proc 2011;43:524–6 | Study design |
| Painter PL, Topp KS, Krasnoff JB, Adey D, Strasner A, Tomlanovich S, Stock P. Health-related fitness and quality of life following steroid withdrawal in renal transplant recipients. Kidney Int 2003;63:2309–16 | Comparator |
| Pankewycz O, Leca N, Kohli R, Weber-Shrikant E, Said M, Alnimri M, et al. Conversion to low-dose tacrolimus or rapamycin 3 months after kidney transplantation: a prospective, protocol biopsy-guided study. Transplant Proc 2011;43:519–23 | Abstract |
| Pankewycz O, Leca N, Said M, Feng L, Patel S, Alnimri M, et al. Tacrolimus minimization or sirolimus conversion at 3 months provides equivalent 1 year renal allograft function and histology in low-risk patients with normal protocol biopsies. Am J Transplant 2011;11:408 | Abstract |
| Pankewycz O, Leca N, Said M, Feng L, Patel S, Kohli R, et al. A protocol biopsy directed randomized trial comparing tacrolimus minimization to sirolimus conversion at 3 months results in an equivalent degree of histological injury at 1 year and equivalent renal function at 2 years. Am J Transplant 2012;12:304 | Abstract |
| Pankewycz O, Leca N, Said M, Feng L, Patel S, Kohli R, et al. A protocol biopsy directed randomized trial comparing tacrolimus minimization to sirolimus conversion at 3 months results in an equivalent degree of histological injury at 1 year yet equivalent renal function at 2 years. Transplantation 2012;94:967 | Abstract |
| Pankewycz O, Leca N, Wallace P, Said M, Feng L, Patel S, et al. Rabbit anti-thymocyte globulin (rATG) induction therapy followed by tacrolimus conversion to sirolimus at 3 months does not expand treg cells. Transplantation 2012;94:771 | Abstract |
| Pankewycz O, Leca N, Wallace P, Said M, Feng L, Patel S, et al. Rabbit anti-thymocyte globulin (rATG) induction therapy followed by tacrolimus conversion to sirolimus at 3 months does not increase treg cells. Am J Transplant 2012;12:448 | Abstract |
| Pankewycz O, Said M, Feng L, Patel S, Alnimri M, Kohli R, et al. Conversion to low dose tacrolimus or rapamycin 3 months after kidney transplant: a prospective, protocolbiopsy guided study. Am J Transplant 2010;10:509 | Abstract |
| Pankewycz OG, Wallace PK, Said M, Leca N, Feng L, Patel S, et al. Low dose rabbit anti-thymocyte globulin induction therapy selectively depletes blood lymphocytes but does not promote Treg expansion. Am J Transplant 2011;11:177–8 | Abstract |
| Paoletti E, Marsano L, Bellino D, Cassottana P, Cannella G. Effect of everolimus on left ventricular hypertrophy of de novo kidney transplant recipients: a 1 year, randomized, controlled trial. Transplantation 2012;93:503–8 | Study design |
| Paoletti E, Marsano L, Bellino D, Cassottana P, Rolla D, Di Maio G. Everolimus for regression of left ventricular hypertrophy of renal transplant recipients: a randomized controlled trial. Am J Transplant 2012;12:31 | Abstract |
| Park JB, Kim SJ, Oh HY, Han YS, Kim DJ, Park JW, et al. Steroid withdrawal in living donor renal transplant recipients using tacrolimus and cyclosporine: a randomized prospective study. Transplant Int 2006;19:478–84 | Population |
| Parrott NR, Hammad AQ, Watson CJ, Lodge JP, Andrews CD. Multicenter, randomized study of the effectiveness of basiliximab in avoiding addition of steroids to cyclosporine a monotherapy in renal transplant recipients. Transplantation 2005;79:344–8 | Comparator |
| Pascual J, Ortuño J, Spanish and Italian Tacrolimus Study Group. Simple tacrolimus-based immunosuppressive regimens following renal transplantation: a large multicenter comparison between double and triple therapy. Transplant Proc 2002;34:89–91 | Study design |
| Pascual J, Del Castillo D, Cabello M, Pallardo L, Grinyo JM, Fernandez AM, et al. Tacrolimus (Tac)-Everolimus (EVL) combination for kidney transplantation (KT): a phase II dose comparison randomized pharmacokinetic (PK). Am J Transplant 2008;8:585 | Abstract |
| Pascual J, Galeano C, Royuela A, Zamora J. A systematic review on steroid withdrawal between 3 and 6 months after kidney transplantation. Transplantation 2010;90:343–9 | Comparator |
| Pascual J, Hene R, Langer R, Christiaans M, Ciechanowski K, Vilatoba M, et al. Preservation of renal function with everolimus and very low tacrolimus exposure in de novo renal transplant recipients (RTXR) at 12 months: the asset study. Am J Transplant 2010;10:502 | Abstract |
| Pascual J, Hooff JP, Salmela K, Lang P, Rigotti P, Budde K. Three-year observational follow-up of a multicenter, randomized trial on tacrolimus-based therapy with withdrawal of steroids or mycophenolate mofetil after renal transplant. Transplantation 2006;82:55–61 | Study design |
| Pascual J, Segoloni G, Gonzalez Molina M, Castillo D, Capdevila L, Arias M, et al. Comparison between a two-drug regimen with tacrolimus and steroids and a triple one with azathioprine in kidney transplantation: results of a European trial with 3-year follow up. Transplant Proc 2003;35:1701–3 | Population |
| Pascual J, Zamora J, Galeano C, Royuela A, Quereda C. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev 2009;1:CD005632 | Study design |
| Pavlakis M. Mycophenolate mofetil versus sirolimus as an adjunct to calcineurin inhibition after renal transplantation. Nat Clin Pract Nephrol 2006;2:558–9 | Outcome |
| Pearson T, Vincenti F, Grinyo J, Charpentier B, Pestana JM, Rostaing L, et al. Primary outcomes from a randomized, phase III study of belatacept versus cyclosporine in kidney transplant recipients (BENEFIT study). Am J Transplant 2010;10:6 | Abstract |
| Peddi R, Hanaway M, Woodle S, Mulgaonkar S, Harrison G, Vandeputte K, et al. Final 36 month results of a randomized trial comparing three induction agents (Alemtuzumab, Thymoglobulin and Basiliximab) with tacrolimus, mycophenolate mofetil and rapid steroid withdrawal in renal transplantation. Am J Transplant 2010;10:49 | Abstract |
| Perkins J, Alsina M, Anasetti C, Ayala E, Fernandez HF, Kharfan-Dabaja M, et al. A randomized, controlled trial of graft-versus-host disease (GVHD) prophylaxis comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate: analysis of GVHD, relapse and survival. Blood 2008;112:779 | Abstract |
| Pescovitz MD, El-Shahawy M, Vincenti F. Incidence of glucose metabolism disorders at six months after kidney transplantation in non-white patients randomized to cyclosporine or tacrolimus: results of a multicenter study. Am J Transplant 2008;8:525 | Abstract |
| Pescovitz MD, Vincenti F, Hart M, Melton L, Whelchel J, Mulgaonkar S, et al. Pharmacokinetics, safety, and efficacy of mycophenolate mofetil in combination with sirolimus or ciclosporin in renal transplant patients. Br J Clin Pharmacol 2007;64:758–71 | Intervention |
| Pestana JO, Grinyo JM, Vanrenterghem Y, Becker T, Campistol JM, Florman S, et al. Three-year outcomes from BENEFIT-EXT: a phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant 2012;12:630–9 | Population |
| Picard N. Does tacrolimus, in comparison with sirolimus, increase mycophenolic acid exposure in kidney transplant recipients? Clin Pharmacol Ther 2010;87:650–1 | Study design |
| Pietruck F, Budde K, Salvadori M, Sollinger H, Bourbigot B, Gentil MA, Oppenheimer F. Efficacy and safety of enteric-coated mycophenolate sodium in renal transplant patients with diabetes mellitus: post hoc analyses from three clinical trials. Clin Transplant 2007;21:117–25 | Study design |
| Pilch NA, Taber DJ, Moussa O, Thomas B, Denmark S, Meadows HB, et al. Prospective randomized controlled trial of rabbit antithymocyte globulin compared with IL-2 receptor antagonist induction therapy in kidney transplantation. Ann Surg 2014;259:888–93 | Study design |
| Plischke M, Riegersperger M, Steiner S, Seidinger D, Winkelmayer WC, Sunder-Plassmann G. Short-term renal function in long-term kidney transplant recipients after conversion from cyclosporine a to tacrolimus. A randomized controlled trial. Am J Transplant 2012;12:204 | Abstract |
| Pliszczynski J, Kahan BD. Better actual 10-year renal transplant outcomes of 80% reduced cyclosporine exposure with sirolimus base therapy compared with full cyclosporine exposure without or with concomittant sirolimus treatment. Transplant Proc 2011;43:3657–68 | Population |
| Pliszczynski J, Abraham JBA, Schoenberg L, Kahan BD. Fullor 80% reduced cyclosporine (CSA) exposure improves 1 but not 10 or 5 year renal transplant outcomes with sirolimus (SRL) base therapy. Am J Transplant 2010;10:507 | Abstract |
| Polvino WJ, Melkus TC, Nigro V. Reduction in tacrolimus c-max by conversion from twice-daily tacrolimus capsules (prograf (R)) to once-daily extended release meltdose (R) tacrolimus tablets (LCP-Tacro (TM)): phase ii randomized trial in stable kidney transplant patients. Am J Transplant 2012;12:407–8 | Abstract |
| Ponticelli C. The pros and the cons of mTOR inhibitors in kidney transplantation. Expert Rev Clin Immunol 2014;10:295–305 | Study design |
| Ponticelli C, Salvadori M, Scolari MP, Citterio F, Rigotti P, Veneziano A, Bartezaghi M, EVEREST Study. Everolimus and minimization of cyclosporine in renal transplantation: 24-month follow-up of the EVEREST study. Transplantation 2011;91:e72–3 | Comparator |
| Prokopenko E, Scherbakova E, Vatazin A, Pasov S, Budnikova N, Agafonova S. Does mycophenolate mofetil increase the incidence of infections in renal transplant recipients? Drugs Exp Clin Res 2005;31:199–205 | Study design |
| Pussell B, Russ G, Walker R, Campbell S, O'Connell P, Kanellis J, et al. Conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients: 18-month efficacy and safety results from a large, randomized, open-label, comparative trial. Immunol Cell Biol 2006;84:A19–20 | Abstract |
| Reinke P, Haller H, Rath T, Arns W, Paulus EM, Scheidf S, et al. Two year data of the apollo trial: renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients. Transplant Int 2011;24:50 | Abstract |
| Reinke P, Lehner F, Witzke O, Sommerer C, Eisenberger U, Arns W, et al. 5 Years follow-up on renal function-ZEUS trial: improved renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients. Transplant Int 2013;26:21 | Abstract |
| Remuzzi G, Cravedi P, Costantini M, Lesti M, Ganeva M, Gherardi G, et al. Mycophenolate mofetil versus azathioprine for prevention of chronic allograft dysfunction in renal transplantation: the MYSS follow-up randomized, controlled clinical trial. J Am Soc Nephrol 2007;18:1973–85 | Population |
| Remuzzi G, Lesti M, Gotti E, Ganeva M, Dimitrov BD, Ene-Iordache B, et al. Mycophenolate mofetil versus azathioprine for prevention of acute rejection in renal transplantation (MYSS): a randomised trial. Lancet 2004;364:503–12 | Population |
| Renner FC, Dietrich H, Bulut N, Celik D, Gaertner ND, Karoui S, et al. The development of BK viremia after renal transplantation is associated with a reduced CD8 cell IL-2 response. Transplant Int 2011;24:56 | Abstract |
| Renner FC, Dietrich H, Bulut N, Celik D, Freitag E, Gaertner N, et al. The risk of polyomavirus-associated graft nephropathy is increased by a combined suppression of CD8 and CD4 cell-dependent immune effects. Transplant Proc 2013;45:1608–10 | No data |
| Rhat T, Sommerer C, Haller H, Reinke P, Witzke O, Suwelack B, et al. Outcome on renal function of everolimus conversion in maintenance KTX patients: 4 years apollo trial. Transplant Int 2013;26:240 | Abstract |
| Rice K, Vanrenterghem Y, Merville P, Muehlbacher F, Zhang R, Duan T, et al. Three-year outcomes in elderly kidney transplant recipients treated with belatacept vs cyclosporine in BENEFIT-EXT. Am J Transplant 2012;12:403 | Abstract |
| Richard MG, Angela W, Ruster Lorenn P, Matheson Sandra L, Higgins Gail Y, Willis Narelle S, et al. Interleukin-2 receptor antagonists versus atg for kidney transplant recipients: an updated cochrane review. Immunol Cell Biol 2010;88:A21 | Abstract |
| Riegersperger M, Plischke M, Sengoelge G, Steiner S, Seidinger D, Winkelmayer WC, et al. Effect of conversion from cyclosporine to tacrolimus on endothelial progenitor cells in stable long-term kidney transplant recipients A Randomized Controlled Trial. Am J Transplant 2012;12:203 | Population |
| Riegersperger M, Plischke M, Steiner S, Seidinger D, Sengoelge G, Winkelmayer WC, Sunder-Plassmann G. Effect of conversion from ciclosporin to tacrolimus on endothelial progenitor cells in stable long-term kidney transplant recipients. Transplantation 2013;95:1338–45 | Abstract |
| Roodnat J, Hilbrands LB, Hene RJ, De Sevaux RGL, Gregoor PJHS, Van Gestel JAK, et al. 15 year follow-up of a multicentre, randomised, calcineurin inhibitor (CNI) withdrawal study in kidney transplantation. Transplant Int 2013;26:83–4 | Abstract |
| Roodnat JI, Hilbrands LB, Hené RJ, de Sévaux RG, Smak Gregoor PJ, Kal-van Gestel JA, et al. 15-year follow-up of a multicenter, randomized, calcineurin inhibitor withdrawal study in kidney transplantation. Transplantation 2014;98:47–53 | Population |
| Rostaing L, Budde K, Bunnapradist S. A phase 3, double-blind, multi-center, non-inferiority, randomized study to examine the efficacy and safety of lcp-tacro (TM) tablets, once daily, compared to prograf (R) capsules, twice daily, in combination with mycophenolate mofetil in de novo adult kidney transplantation: baseline characteristics. Am J Transplant 2013;13:339 | Abstract |
| Rostaing L, Budde K, Ciechanowski K, Bunnapradist S, Silva H, Grinyi JM. Once-daily LCP-tacro demonstrates comparable efficacy and safety to twice daily prograf: a phase 3 study for prevention of acute allograft rejection in de novo adult kidney transplant recipients. Transplant Int 2013;26:171 | Abstract |
| Rostaing L, Ciechanowski K, Bunnapradist S, Mulgaonkar S. Conversion from tacrolimus capsules twice daily to tacrolimus tablets once daily in stable kidney transplant patients: efficacy results from a phase iii, open-label, multicenter, prospective, randomized study. Transplant Int 2011;24:227 | Abstract |
| Rostaing L, Fassett R, Dantal J, Binet I, O'Connell P, MacHein U, et al. Risk factor analysis for renal function outcome in maintenance renal transplant recipients from the ASCERTAIN study. Am J Transplant 2011;11:44–5 | Abstract |
| Rostaing L, Massari P, Garcia VD, Mancilla-Urrea E, Nainan G, del Carmen Rial M, et al. Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: a randomized phase II study. Clin J Am Soc Nephrol 2011;6:430–9 | Population |
| Rostaing L, Mourad G, Legendre C. Sustainable tolerability effects of myfortic (R) in combination with Neoral (R) and steroids at 12 months, in de novo kidney transplantation: a randomized, multicentre, open, prospective controlled study. Am J Transplant 2005;5:190 | Abstract |
| Rostaing L, Nainan G, Del Carmen Rial M, Steinberg S, Vincenti F, Shi R, et al. Switch from a CNI-to a belatacept-based immunosuppressive regimen in kidney transplant recipients is safe and results in better renal function: 12 month results from a phase II study. NDT Plus 2010;3:iii285 | Abstract |
| Rostaing L, Neumayer HH, Reyes-Acevedo R, Bresnahan B, Florman S, Vitko S, et al. Belatacept-versus cyclosporine-based immunosuppression in renal transplant recipients with pre-existing diabetes. Clin J Am Soc Nephrol 2011;6:2696–704 | Population |
| Rostaing L, Reyes-Acevedo R, Neumayer HH, Vitko S, Xing J, Thomas D, et al. Outcomes at 3 years in kidney transplant recipients with pre-transplant diabetes from two phase 3 belatacept studies. Transplant Int 2011;24:69 | Abstract |
| Rostaing L, Vincenti F, Grinyó J, Rice KM, Bresnahan B, Steinberg S, et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. Am J Transplant 2013;13:2875–83 | Population |
| Ruggenenti P, Codreanu I, Cravedi P, Perna A, Gotti E, Remuzzi G. Basiliximab combined with low-dose rabbit anti-human thymocyte globulin: a possible further step toward effective and minimally toxic T cell-targeted therapy in kidney transplantation. Clin J Am Soc Nephrol 2006;1:546–54 | Comparator |
| Ruggenenti P, Perico N, Gotti E, Cravedi P, D'Agati V, Gagliardini E, et al. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation 2007;84:956–64 | Population |
| Ruiz JC, Alonso A, Arias M, Campistol JM, González Molina M, González Posada JM, et al. Conversion to sirolimus. Nefrologia 2006;26(Suppl. 2):52–63 | Study design |
| Ruiz JC, Campistol JM, Sanchez-Fructuoso A, Mota A, Grinyo JM, Paul J, et al. Early sirolimus use with cyclosporine elimination does not induce progressive proteinuria. Transplant Proc. 2007;39:2151–2 | Abstract |
| Ruiz JC, Sanchez Fructuoso A, Hernandez D, Sanchez Plumed J, Fernandez A, Pastor Rodriguez A, et al. Better renal function with early everolimus (EVL) introduction and calcineurin inhibitor (CNI) withdrawal at third month in kidney recipients at month 12: results of the eric study. Transplant Int 2011;24:112 | Abstract |
| Ruiz JC, Sanchez Fructuoso A, Hernandez D, Sanchez Plumed J, Fernandez A, Pastor Rodriguez A, et al. Better renal function with early everolimus introduction and calcineurin inhibitor withdrawal at third month in kidney recipients at month 12: results of the the ERIC study. Am J Transplant 2011;11:407 | Abstract |
| Rush DN, Cockfield SM, Nickerson PW, Arlen DJ, Boucher A, Busque S, et al. Factors associated with progression of interstitial fibrosis in renal transplant patients receiving tacrolimus and mycophenolate mofetil. Transplantation 2009;88:897–903 | Study design |
| Russ G, Durrbach A, Larsen CP, Medina Pestana J, Vanrenterghem Y, Vincenti F, et al. Benefit-ext study two year outcomes: belatacept vs cyclosporine (CSA) in extended criteria donor (ECD) kidney transplants. Immunol Cell Biol 2011;89:A2 | Abstract |
| Russ G, Eris J, Kanellis J, Hutchison B, Hibberd A, Pilmore H, et al. Multicentre rct of early switch to everolimus plus steroids or everolimus plus csa versus csa, mpa and steroids in de novo kidney transplant recipients: 12 month analysis. Immunol Cell Biol 2012;90:A30–A | Abstract |
| Russ G, Jamieson N, Oberbauer R, Arias M, Murgia MG, Blancho G, et al. Three-year health-related quality-of-life outcomes for sirolimus-treated kidney transplant patients after elimination of cyclosporine. Transpl Int 2007;20:875–83 | Study design |
| Russ G, Segoloni G, Oberbauer R, Legendre C, Mota A, Eris J, et al. Superior outcomes in renal transplantation after early cyclosporine withdrawal and sirolimus maintenance therapy, regardless of baseline renal function. Transplantation 2005;80:1204–11 | Comparator |
| Russ G, Walker R, Pilmore H, Kanellis J, Hutchison B, Chadban S, et al. Lower incidence of cytomegalovirus and BK virus with everolimus versus mycophenolate in DE novo renal transplant patients: results from a multicenter, prospective study. Immunol Cell Biol 2011;89:A23–4 | Abstract |
| Russ G, Walker R, PilmoreH, Kanellis J, Hutchison B, Chadban S, et al. Everolimus plus reduced csa exposure: efficacy results from a multicenter, randomized prospective study in renal transplantation. Immunol Cell Biol 2011;89:A1–2 | Study design |
| Saddadi F, Sedghipour M, Tabatabaei A, Kamal Hedaiat D, Alatab S. Comparison of the effects of sirolimus and cyclosporine on left ventricular hypertrophy in kidney transplant recipients, a 1-year single center prospective cohort study in Dr. Shariati hospital Tehran, Iran. Iranian J Kidney Dis 2011;5:62–3 | Abstract |
| Sadek S, Medina J, Arias M, Sennesael J, Squifflet JP, Vogt B, Neo Int-05 Study group. Short-term combination of mycophenolate mofetil with cyclosporine as a therapeutic option for renal transplant recipients: a prospective, multicenter, randomized study. Transplantation 2002;74:511–7 | Population |
| Saito K, Uchida K, Takahara S, Yoshimura N, Teraoka S, Cornu-Artis C, et al. Efficacy of everolimus with reduced cyclosporine in japanese de novo renal transplant recipients: 24-month, randomized, multicenter study. Am J Transplant 2013;13:314 | Abstract |
| Salmela K, Vitko S, Wlodarczyk Z, Czajkowski Z, Margreiter R, Grp TS. Tacrolimus with MMF or two different doses of sirolimus in kidney transplantation: a large randomised multicentre study. Am J Transplant 2005;5:571 | Abstract |
| Salvadori M, Holzer H, Civati G, Sollinger H, Lien B, Tomlanovich S, et al. Long-term administration of enteric-coated mycophenolate sodium (EC-MPS; myfortic) is safe in kidney transplant patients. Clin Nephrol 2006;66:112–9 | Study design |
| Salvadori M, Holzer H, De Mattos A, Sollinger H, Arns W, Oppenheimer F, et al. Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant 2004;4:231–6 | Population |
| Salvadori M, Scolari MP, Bertoni E, Citterio F, Rigotti P, Cossu M, et al. Everolimus with very low-exposure cyclosporine a in de novo kidney transplantation: a multicenter, randomized, controlled trial. Transplantation 2009;88:1194–202 | Study design |
| Samadzadeh B, Alemi M, Heidarnejadiyan J, Torkamanasadi F. Prophylactic effect of mycophenolate mofetil on early outcomes of living donor kidney transplantation. Iran J Kidney Dis 2012;6:63–8 | Population |
| Sampaio EL, Pinheiro-Machado PG, Garcia R, Felipe CR, Park SI, Casarini DE, et al. Mycophenolate mofetil vs. sirolimus in kidney transplant recipients receiving tacrolimus-based immunosuppressive regimen. Clin Transplant 2008;22:141–9 | Population |
| Samsel R, Pliszczyński J, Chmura A, Korczak G, Włodarczyk Z, Cieciura T, et al. Safety and efficacy of high dose ATG bolus administration on rewascularization in kidney graft patients – long term results. Ann Transplant 2008;13:32–9 | Population |
| Sanchez-Fructuoso A, Ruiz JC, Hernandez D, Sanchez-Plumed J, Fernandez A, Pastor Rodriguez A, et al. Early everolimus introduction and calcineurin inhibitor withdrawal in renal transplant patients: a multicenter, randomized, open-label study (the eric study). Am J Transplant 2010;10:506 | Abstract |
| Sánchez-Fructuoso AI. Everolimus: an update on the mechanism of action, pharmacokinetics and recent clinical trials. Expert Opin Drug Metab Toxicol 2008;4:807–19 | Comparator |
| Sandes Freitas TV, Harada KM, Felipe CR, Galante NZ, Sampaio EL, Ikehara E, et al. Steroid or tacrolimus withdrawal in renal transplant recipients using sirolimus. Int Urol Nephrol 2011;43:1221–8 | Abstract |
| Sandes-Freitas T, Felipe C, Campos E, Soares M, Tedesco H, Franco M, et al. Incidence of subclinical rejection and de novo donor specific antibodies in calcineurin sparing regimens. Am J Transplant 2013;13:35 | Study design |
| Sarvary E, Wagner L, Telkes G, Gaman G, Varga M, Gaal I, et al. De novo Prograf versus de novo Advagraf: are trough level profile curves similar? Transplant Proc 2014;46:2164–7 | Population |
| Saturnino Luciana TM, Ceccato Maria GB, Cherchiglia Mariangela L, Andrade Eli lola G, Giordano Luiz Flavio C, Acurcio Francisco A. Target of rapamycin inhibitors (TORi) as maintenance immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev 2012;3:CD009637 | Study design |
| Schaefer HM, Kizilisik AT, Feurer I, Nylander WA, Langone AJ, Helderman JH, Shaffer D. Short-term results under three different immunosuppressive regimens at one center. Transplant Proc 2006;38:3466–7 | Population |
| Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation 2009;87:233–42 | Population |
| Schena FP, Wali RK, Pascoe MD, Alberu J, Rial MD, Sirolimus Renal Conversion Trial S. A randomized, open-label, comparative evaluation of conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients. Am J Transplant 2005;5:413 | Abstract |
| Schnuelle P, van der Heide JH, Tegzess A, Verburgh CA, Paul LC, van der Woude FJ, de Fijter JW. Open randomized trial comparing early withdrawal of either cyclosporine or mycophenolate mofetil in stable renal transplant recipients initially treated with a triple drug regimen. J Am Soc Nephrol 2002;13:536–43 | Study design |
| Schwarz C, Mayerhoffer S, Berlakovich G, Steininger R, Soliman T, Watschinger B, et al. Belatacept in de novo kidney transplant recipients-10-year experience in a single center. European Surgery – Acta Chirurgica Austriaca 2011;43:12–13 | Abstract |
| Sellarés J, Moreso F, Ruiz JC, Seron D. Mean glomerular volume after renal transplantation in patients receiving sirolimus and cyclosporine a compared with elimination of cyclosporine a at 3 months. Transplantation 2011;91:e5–6 | Comparator |
| Sellars D. A phase 4, randomised open-label, controlled, single centre study of induction with basiliximab, mycophenolate mofetil and tacrolimus with rapid steriod withdrawal and randomisation to either continuation with mycophenolate mofetil and tacromlimus or switch to sirolimus and mycophenolate mofetil maintenance in renal transplant recipeints. 2004. National Research Register, UK. URL: www.nrr.nhs.uk (accessed 25 July 2014) | Unobtainable |
| Servais A, Meas-Yedid V, Toupance O, Lebranchu Y, Thierry A, Moulin B, et al. Interstitial fibrosis quantification in renal transplant recipients randomized to continue cyclosporine or convert to sirolimus. Am J Transplant 2009;9:2552–60 | Population |
| Shah G, Xu L, Dalal P, Chhabra D, Friedewald J, Ho B, et al. Conversion from CNI to SRL in a pred-free immunosuppressive regimen: interim report of a prospective randomized study. Am J Transplant 2010;10:504 | Abstract |
| Shamseddin MK, Gupta A. Sirolimus: not so sparing in the Spare-the-Nephron trial. Kidney Int 2011;79:1379 | Language |
| Sharif A, Shabir S, Chand S, Cockwell P, Ball S, Borrows R. Meta-analysis of calcineurin-inhibitor-sparing regimens in kidney transplantation. J Am Soc Nephrol 2011;22:2107–18 | Study design |
| Sheashaa HA, Bakr MA, Ismail AM, Gheith OE, El-dahshan KF, Sobh MA, Ghoneim MA. Long-term evaluation of basiliximab induction therapy in live donor kidney transplantation: a five-year prospective randomized study. Am J Nephrol 2005;25:221–5 | Population |
| Sheashaa HA, Bakr MA, Ismail AM, Mahmoud KM, Sobh MA, Ghoneim MA. Basiliximab induction therapy for live donor kidney transplantation: a long-term follow-up of prospective randomized controlled study. Clin Exper Nephrol 2008;12:376–81 | Population |
| Sheashaa HA, Bakr MA, Ismail AM, Sobh MA, Ghoneim MA. Basiliximab reduces the incidence of acute cellular rejection in live-related-donor kidney transplantation: a three-year prospective randomized trial. J Nephrol 2003;16:393–8 | Population |
| Sheashaa HA, Bakr MA, Rashad RH, Ismail AM, Sobh MA, Ghoneim MA. Ten-year follow-up of basiliximab induction therapy for live-donor kidney transplant: a prospective randomized controlled study. Exp Clin Transplant 2011;9:247–51 | Population |
| Sheashaa HA, Hamdy AF, Bakr MA, Abdelbaset SF, Ghoneim MA. Long-term evaluation of single bolus high dose ATG induction therapy for prophylaxis of rejection in live donor kidney transplantation. Int Urol Nephrol 2008;40:515–20 | Population |
| Shehata M, Bhandari S, Venkat-Raman G, Moore R, D'Souza R, Riad H, et al. Effect of conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium on maximum tolerated dose and gastrointestinal symptoms following kidney transplantation. Transpl Int 2009;22:821–30 | Study design |
| Shehata M, Bhandari S, Venkat-Raman G, Moore R, D’Souza R. Health-related quality of life maintained despite increase in mycophenolic acid (mpa) dose following conversion from mycophenolate mofetil (mmf) to enteric-coated mycophenolate sodium (ec-mps): a randomized, multicenter trial in kidney transplant recipients. Transplant Int 2009;22:110 | Abstract |
| Shihab F, Christians U, Smith L, Wellen JR, Kaplan B. Focus on mTOR inhibitors and tacrolimus in renal transplantation: pharmacokinetics, exposure-response relationships, and clinical outcomes. Transpl Immunol 2014;31:22–32 | Study design |
| Shihab F, Tedesco-Silva H, Johnston T, Kim YS, Zibari GB, Walker R, et al. Lower incidence of cytomegalovirus and BK virus adverse events with everolimus versus mycophenolate was maintained over 24 months in de novo renal transplant recipients. Am J Transplant 2011;11:45 | Abstract |
| Shihab FS, Cibrik D, Chan L, Kim YS, Carmellini M, Walker R, et al. Association of clinical events with everolimus exposure in kidney transplant patients receiving reduced cyclosporine. Clin Transplant 2013;27:217–26 | Study design |
| Shihab FS, Waid TH, Conti DJ, Yang H, Holman MJ, Mulloy LC, et al. Conversion from cyclosporine to tacrolimus in patients at risk for chronic renal allograft failure: 60-month results of the CRAF Study. Transplantation 2008;85:1261–9 | Population |
| Shun CS, Hao JW, Sun J, Yang DA. A comparison between the therapeutic effects of mycophenolate mofetil and azathioprine in the management of patients after renal transplantation. Herald of Medicine 2002;21:544–6 | Language |
| Sidhu M, Odeyemi AO, Hart WM, Dada BR. Belatacept versus tacrolimus: results of an indirect analysis from a systematic review of immunosuppressive therapies for kidney transplant recipients. Value Health 2011;14:A330 | Abstract |
| Sid Sidhu M, Odeyemi AO, Hart WM, Dada BR. Belatacept versus tacrolimus: results of an indirect analysis from a systematic review of immunosuppressive therapies for kidney transplant recipients. Value Health 2011;14:A330 | Dupliocate |
| Silva H. A phase III, randomized, open-label, comparative, multi-center study to assess the safety and efficacy of Prograf (Tacrolimus)/MMF, modified release (MR) Tacrolimus/MMF and Neoral (Cyclosporine)/MMF in de novo kidney transplant recipients: 12 month results. Am J Transplant 2006;6(Suppl. 2):318 | Abstract |
| Silva HT, Yang HC, Abouljoud M, Kuo PC, Wisemandle K, Bhattacharya P, et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant 2007;7:595–608 | Population |
| Silva HT, Yang HC, Meier-Kriesche HU, Croy R, Holman J, Fitzsimmons WE, First MR. Long-term follow-up of a phase III clinical trial comparing tacrolimus extended-release/MMF, tacrolimus/MMF, and cyclosporine/MMF in de novo kidney transplant recipients. Transplantation 2014;97:636–41 | Population |
| Silva HT, Felipe CR, Garcia VD, Neto ED, Filho MA, Contieri FL, et al. Planned randomized conversion from tacrolimus to sirolimus-based immunosuppressive regimen in de novo kidney transplant recipients. Am J Transplant 2013;13:3155–63 | Population |
| Smith MP, Newstead CG, Ahmad N, Lewington AJ, Tibble S, Lodge JP, et al. Poor tolerance of sirolimus in a steroid avoidance regimen for renal transplantation. Transplantation 2008;85:636–9 | Study design |
| Sola R, Diaz JM, Guirado L, Sainz Z, Gich I, Picazo M, et al. Tacrolimus in induction immunosuppressive treatment in renal transplantation: comparison with cyclosporine. Transplant Proc 2003;35:1699–700 | Study design |
| Soleimani AR, Kamkar I, Nikoueinejad H, Moraweji AR. Comparison of cyclosporine and sirolimus effects on serum creatinine level over five years after kidney transplantation. Transplant Proc 2013;45:1644–7 | Population |
| Sollinger H. Enteric-coated mycophenolate sodium: therapeutic equivalence to mycophenolate mofetil in de novo renal transplant patients. Transplant Proc 2004;36:517S–20S | Comparator |
| Sommerer C, Budde K, Becker T, Arns W, Reinke P, Eisenberger U, et al. New onset diabetes after transplantation and mTOR inhibitors: results of the ZEUS trial. Am J Transplant 2011;11:412–13 | Abstract |
| Sommerer C, Rath T, Budde K, Haller H, Arns W, Scheidl S, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 2 year follow-up data of the apollo trial. Transplant Int 2011;24:180 | Abstract |
| Sommerer C, Rath T, Haller H, Arns W, Suwelack B, Reinke P, et al. 4 Year data of the apollo trial: outcome on renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients. Transplant Int 2013;26:21 | Abstract |
| Squifflet JP, Vanrenterghem Y, Hooff JP, Salmela K, Rigotti P. Safe withdrawal of corticosteroids or mycophenolate mofetil: results of a large, prospective, multicenter, randomized study. Transplant Proc 2002;34:1584–6 | Study design |
| SRCTN. Mycophenolate sodium versus Everolimus or Cyclosporine with Allograft Nephropathy as Outcome. URL: www.controlled-trials.com/ISRCTN69188731 (accessed 25 July 2014) | No data |
| Stallone G, Di Paolo S, Schena A, Infante B, Battaglia M, Ditonno P, et al. Addition of sirolimus to cyclosporine delays the recovery from delayed graft function but does not affect 1-year graft function. J Am Soc Nephrol 2004;15:228–33 | Population |
| Stallone G, Infante B, Schena A, Battaglia M, Ditonno P, Loverre A, et al. Rapamycin for treatment of chronic allograft nephropathy in renal transplant patients. JASN 2005;16:3755–62 | Population |
| Stegall MD, Larson TS, Prieto M, Gloor J, Textor S, Nyberg S, et al. Kidney transplantation without calcineurin inhibitors using sirolimus. Transplant Proc 2003;35:125S–7S | Population |
| Stevens RB, Foster KW, Lane JT, Miles CD, Kalil AC, Sandoz JP, et al. Significantly reduced renal allograft histopathology after single-dose rATG induction and calcineurin-inhibitor withdrawal vs. minimization: final report from a prospective, randomized clinical trial. Am J Transplant 2011;11:209–10 | Abstract |
| Stoves J, Newstead CG, Baczkowski AJ, Owens G, Paraoan M, Hammad AQ. A randomized controlled trial of immunosuppression conversion for the treatment of chronic allograft nephropathy. Nephrol Dial Transplant 2004;19:2113–20 | Population |
| Strologo LD, Tonshoff B, Pape L, Ettenger R, Niaudet P, Martzloff ED, et al. Rationale and design of a study evaluating the efficacy and safety of early conversion of calcineurin inhibitor to everolimus in paediatric renal transplant recipients. Pediatr Nephrol 2012;27:1816 | Abstract |
| Su L, Tam N, Deng R, Chen P, Li H, Wu L. Everolimus-based calcineurin-inhibitor sparing regimens for kidney transplant recipients: a systematic review and meta-analysis. Int Urol Nephrol 2014;46:2035–44 | Population |
| Sułowicz W, Bachleda P, Rydzewski A, Rutkowski B, Szakály P, Asztalos L, et al. Discontinuation of mycophenolate mofetil from a tacrolimus-based triple regimen 2 months after renal transplantation: a comparative randomized, multicentre study. Transpl Int 2007;20:230–7 | Population |
| Suszynski TM, Gillingham KJ, Rizzari MD, Dunn TB, Payne WD, Chinnakotla S, et al. Prospective randomized trial of maintenance immunosuppression with rapid discontinuation of prednisone in adult kidney transplantation. Am J Transplant 2013;13:961–70 | Population |
| Suwelack B, Gerhardt U, Kobelt V, Hillebrand U, Matzkies F, Hohage H. Design and preliminary results of a randomized study on the conversion of treatment with calcineurin inhibitors to mycophenolate mofetil in chronic renal graft failure: effect, on serum cholesterol levels. Transplant Proc 2002;34:1803–5 | Study design |
| Taber D, Bratton C, Al Manasra A, Pilch N, Meadows H, McGillicuddy J, et al. The impact of induction therapy on clinical outcomes and quality of life in aged kidney transplant recipients. Am J Transplant 2013;13:429 | Abstract |
| Taber DJ, Pilch NA, Meadows HB, Denmark S, McGillicuddy JW, Bratton CF, et al. Prospective comparative efficacy of induction therapy in a high-risk kidney transplant (KTX) population. Am J Transplant 2012;12:57 | Abstract |
| Takahara S, Uchida K, Yoshimura N, Teraoka S, Kobayashi E, Teshima R, et al. Efficacy and safety of concentration controlled everolimus with reduced dose cyclosporine in japanese adult de-novo renal transplant patients: 12 month results. Am J Transplant 2012;12:300 | Abstract |
| Takahashi K, Uchida K, Yoshimura N, Takahara S, Teraoka S, Teshima R, et al. Efficacy and safety of concentration-controlled everolimus with reduced-dose cyclosporine in Japanese de novo renal transplant patients: 12-month results. Transplant Res 2013;2:14 | Population |
| Tan J, Yang S, Wu W. Basiliximab (Simulect) reduces acute rejection among sensitized kidney allograft recipients. Transplant Proc 2005;37:903–5 | Comparator |
| Tanabe K, Tsuchiya T, Ishida H, Tanabe T, Shimizu T, Omoto K, et al. An open label, prospective randomized controlled study comparing tacrolimus once-daily and twice-daily in de novo kidney transplantation: pharmacokinetics and pathological analysis by protocol biopsy. Am J Transplant 2012;12:55 | Abstract |
| Tang SC, Chan KW, Tang CS, Lam MF, Leung CY, Tse KC, et al. Conversion of ciclosporin A to tacrolimus in kidney transplant recipients with chronic allograft nephropathy. Nephrol Dial Transplant 2006;21:3243–51 | Study design |
| Tedesco H. Efficacy and Safety of Induction Strategies Combined with Low Tacrolimus Exposure in Kidney Transplant Recipients Receiving Everolimus or Sodium Mycophenolate. 2011. URL: www.clinicaltrials.gov/ct2/show/NCT01354301 (accessed 25 July 2014) | No data |
| Tedesco H, Felipe C, Franco M, Sandes T, Campos E, Pestana JOM. High incidence of subclinical acute rejection in low risk kidney transplant recipients on tacrolimus-based immunosuppressive regime. Transplantation 2012;94:329 | Abstract |
| Tedesco H, Felipe C, Sandes T, Cristelli M, Rodrigues C, Pestana JOM. A prospective randomized trial aimed to reduce the incidence of cytomegalovirus (CMV) infection in kidney transplant recipients. Transplantation 2012;94:4 | Abstract |
| Tedesco H, Felipe C, Wang L, Rodrigues C, Sandes T, Cristelli M, et al. A prospective randomized trial aimed to reduce the incidence of cytomegalovirus (CMV) infection in kidney transplant (KT) recipients. Am J Transplant 2013;13:56 | Abstract |
| Tedesco H, Garcia V, David-Neto E, Contieri F, Carvalho D, Abbud M, et al. Conversion from tacrolimus (TAC) to sirolimus (SRL)-based immunosuppressive regimen in kidney transplant recipients: 1 year results. Am J Transplant 2012;12:299 | Abstract |
| Tedesco H, Kim YS, Lackova E, Johnston T, Zibari G, Panis C, et al. Everolimus with reduced-dose cyclosporine as a strategy for optimizing long-term renal function: results from a randomized study in 833 de-novo renal-transplant recipients. Transplant Int 2009;22:186–7 | Abstract |
| Tedesco H, Neto E, Garcia V, Continieri F, Carvalho D, Abbud M, et al. Conversion from tacrolimus (TAC) to sirolimus (SRL)-based immunosuppressive regimen in kidney transplant recipients: 2 years results. Am J Transplant 2013;13:313 | Abstract |
| Tedesco Silva H, Cibrik D, Johnston T, Lackova E, Mange K, Panis C, et al. Everolimus plus reduced-exposure CsA versus mycophenolic acid plus standard-exposure CsA in renal-transplant recipients. Am J Transplant 2010;10:1401–13 | Study design |
| Tedesco-Silva H, Bernhardt P, Dong G, Escrig C. Search for new endpoints for clinical trials of immunosuppressive drugs in kidney transplantation. Transplant Int 2013;26:248 | Abstract |
| Tedesco-Silva H, Kim YS, Johnston T, Walker R, Zibari GB, Cornu-Artis C, et al. Concentration-controlled everolimus with reduced cyclosporine concentration in de novo renal transplant recipients: efficacy results at 24 months. Am J Transplant 2011;11:46 | Abstract |
| Tedesco-Silva H, Peddi R, Russ G, Marder B, Hahn C, Li H, et al. Open-label study of planned transition from tacrolimus to sirolimus vs continued tacrolimus in renal allograft recipients: demographics and interim safety results. Am J Transplant 2013;13:337 | Abstract |
| Tedesco-Silva H, Peddi VR, Sanchez-Fructoso A, Russ G, Marder B, Hahn C, et al. Interim results from an open-label study of planned transition from tacrolimus to sirolimus vs continued tacrolimus in renal allograft recipients: cardiovascular safety. Transplantation 2012;94:142 | Abstract |
| Tedesco-Silva H, Vitko S, Pascual J, Eris J, Magee JC, Whelchel J, et al. 12-month safety and efficacy of everolimus with reduced exposure cyclosporine in de novo renal transplant recipients. Transpl Int 2007;20:27–36 | Comparator |
| Teh LK, Dom SHM, Zakaria ZA, Salleh MZ. A systematic review of the adverse effects of tacrolimus in organ transplant patients. African J Pharmacy Pharmacol 2011;5:764–71 | Population |
| Thervet E, Durrbach A, Rostaing L, Ouali N, Wolf P, Pouteil-Noble C, et al. Use of sirolimus as initial therapy after renal transplantation: preliminary results of a randomized pilot study in patient receiving marginal kidneys. Am J Transplant 2004;4(Suppl. 8):345 | Abstract |
| Thierry A, Pourreau F, Jollet I, Abou-Ayache R, Bridoux F, Touchard G. Minimization of immunosuppression: long-term impact on HLA allo-immunisation and graft outcome. Am J Transplant 2012;12:302 | Abstract |
| Thurston S, Kalsekar A, G.J LI, Sennfalt K. Mixed treatment comparisons of immunosuppressants following renal transplant. Value Health 2011;14:A331 | Abstract |
| Thurston S, Kalsekar A, G.J LI, Sennfalt K. Mixed treatment comparisons of immunosuppressants following renal transplant. Value Health 2011;14:A331 | Duplicate |
| Tian JH, Wang X, Yang KH, Liu AP, Luo XF, Zhang J. Induction with and without antithymocyte globulin combined with cyclosporine/tacrolimus-based immunosuppression in renal transplantation: a meta-analysis of randomized controlled trials. Transplant Proc 2009;41:3671–6 | Population |
| Tischer SM, Pilch NA, Taber DJ, Krisl JC, Meadows HB, Byrns JS, et al. Does RATG induction therapy increase the risk of severe infection in kidney transplant recipients? Am J Transplant 2012;12:317 | Abstract |
| Tischer SM, Taber DJ, Pilch NA, Krisl JC, Meadows HB, McGillicuddy JW, et al. Critical analysis of BK infection in kidney transplant recipients with modern immunosuppression. Am J Transplant 2012;12:346 | Abstract |
| Toenshoff B, Weber L, Hoecker B. Prospective randomized multicenter trial on withdrawal of steroids in pediatric renal transplant recipients with stable graft function on cyclosporin a (CsA) and mycophe-nolate mofetil (MMF). Pediatr Nephrol 2007;22:1429 | Abstract |
| Tonshoff B, Pape L, Ettenger R, Dello Strologo L, Niaudet P, Martzloff D, et al. Early conversion of calcineurin inhibitor to everolimus in de novo paediatric renal transplant recipients and its impact on efficacy and renal function; design of an open-label, randomised, multi-centre study. Transplantation 2012;94:1208 | Abstract |
| Tonshoff B, Pape L, Strologo LD, Ettenger R, Niaudet P, Martzloff ED, et al. Design of crad001a2314: a randomised study evaluating everolimus in paediatric renal transplantation. Transplant Int 2013;26:328 | Abstract |
| Touchard G, Mourad G, Lebranchu Y, Rostaing L, Villemain F, Heng A-E, et al. Intensified dose of enteric-coated mycophenolate sodium (EC-MPS) for steroids avoidance, in combination with ciclosporine micro-emulsion (CSA-ME): multicenter, randomized, open label, comparative study in de novo kidney transplantation (DOMINOS). Transplant Int 2009;22:232–3 | Abstract |
| Touchard G, Mourad G, Lebranchu Y, Rostaing L, Villemain F. Multicenter, randomized, comparative, open-labelstudy to evaluate efficacy and safety a combination of anti-IL2R, intensified dose of enteric-coated mycophenolate sodium (EC-MPS) for 6 weeks, ciclosporine micro-emulsion (CSA-ME), with or without steroids, in adult kidney de novo transplant recipients (TxR). Am J Transplant 2010;10:515 | Abstract |
| Töz H, Sen S, Sezi M, Duman S, Ozkahya M, Ozbek S, et al. Comparison of tacrolimus and cyclosporin in renal transplantation by the protocol biopsies. Transplant Proc 2004;36:134–6 | Population |
| Trofe-Clark J, Goral S, Shaw L, Figurski M, Abt PL, Bloom RD. Comparative study of gastrointestinal(GI) events in african american kidney transplant recipients treated with mycophenolate mofetil (MMF) versus enteric coated mycophenolate sodium (ECMS). Am J Transplant 2010;10:470 | Abstract |
| Trompeter R, Filler G, Webb NJA, Watson AR, Milford DV, Tyden G, et al. Randomized trial of tracolimus versus cyclosporin microemulsion in renal transplantation. Pediatr Nephrol 2002;17:141–9 | Duplicate |
| Tsuchiya T, Ishida H, Tanabe T, Shimizu T, Honda K, Omoto K, Tanabe K. Comparison of pharmacokinetics and pathology for low-dose tacrolimus once-daily and twice-daily in living kidney transplantation: prospective trial in once-daily versus twice-daily tacrolimus. Transplantation 2013;96:198–204 | Population |
| Tullius SG, Pratschke J, Strobelt V, Kahl A, Reinke P, May G, et al. ATG versus basiliximab induction therapy in renal allograft recipients receiving a dual immunosuppressive regimen: one-year results. Transplant Proc 2003;35:2100–1 | Abstract |
| Turconi A, Rilo LR, Goldberg J, de Boccardo G, Garsd A, Otero A. Open-label, multicenter study on the safety, tolerability, and efficacy of Simulect in pediatric renal transplant recipients receiving triple therapy with cyclosporin, mycophenolate, and corticosteroids. Transplant Proc 2005;37:672–4 | No data |
| Urbizu JM, Amenabar JJ, Gomez-Ullate P, Zarraga S, Lampreabe I. Immunosuppression using tacrolimus/mycophenolate versus neoral/mycophenolate following kidney transplantation: a single-center experience. Transplant Proc 2002;34:87–8 | Study design |
| Vacher-Coponat H, Brunet C, Moal V, Loundou A, Bonnet E, Lyonnet L, et al. Tacrolimus/mycophenolate mofetil improved natural killer lymphocyte reconstitution one year after kidney transplant by reference to cyclosporine/azathioprine. Transplantation 2006;82:558–66 | Outcome |
| Vacher-Coponat H, Moal V, Indreies M, Purgus R, Loundou A, Burtey S, et al. A randomized trial with steroids and antithymocyte globulins comparing cyclosporine/azathioprine versus tacrolimus/mycophenolate mofetil (CATM2) in renal transplantation. Transplantation 2012;93:437–43 | Population |
| Van Der Giet M, Brakemeier S, Liefeldt L, Glander P, Diekmann F, Hohne M, et al. The impact of everolimus versus CNI-based immuno suppression on cardiovascular function and stiffness after renal transplantation. Am J Transplan. 2010;10:506 | Abstract |
| Van Der Heide JJH, De Fijter JW, De Maar EF, Ten Berge I, Bemelman FJ. Low acute rejection rate and superior renal function 2 years after early CsA with drawal and overnight switch to everolimus. Am J Transplant 2010;10:508 | Abstract |
| Van Doesum W, Gard L, Van Son WJ, Sanders JSF, Riezebos A, Niesters BGM, et al. Incidence and outcome of BK infection in a randomized controlled multicenter study with renal transplant patients receiving duo-therapy. Transplant Int 2013;26:166 | Abstract |
| Van Gurp E, Bustamante J, Franco A, Rostaing L, Becker T, Rondeau E, et al. Comparable Renal Function at 6 Months with Tacrolimus Combined with Fixed-Dose Sirolimus or MMF: results of a Randomized Multicenter Trial in Renal Transplantation. J Transplant 2010;2010:731426 | Population |
| Vanrenterghem Y, Bresnahan B, Campistol J, Durrbach A, Grinyó J, Neumayer HH, et al. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies). Transplantation 2011;91:976–83 | Outcome |
| Vanrenterghem Y, Hooff JP, Squifflet JP, Salmela K, Rigotti P, Jindal RM, et al. Minimization of immunosuppressive therapy after renal transplantation: results of a randomized controlled trial. Am J Transplant 2005;5:87–95 | Study design |
| Vathsala A, Schena FP, Wali RK, Pascoe MD, Alberu J, Del Carmen Rial M, et al. Conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients: a randomized, open-label, comparative trial. Nephrology 2005;10:A217–A | Abstract |
| Vester U, Kranz B, Wehr S, Boger R, Hoyer PF, RAD B 351 Study Group. Everolimus (Certican) in combination with neoral in pediatric renal transplant recipients: interim analysis after 3 months. Transplant Proc 2002;34:2209–10 | Study design |
| Vincenti F, Blancho G, Durrbach A, Friend P, Grinyo J, Halloran PF, et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol 2010;21:1587–96 | Population |
| Vincenti F, Charpentier B, Rostaing L, Reyes-Acevedo R, Massari P, Vitko S, et al. Long-term extension of the belatacept benefit study: result’s at month 48. Transplantation 2012;94:958 | Abstract |
| Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010;10:535–46 | Population |
| Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 2007;7:1506–14 | Study design |
| Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, et al. DIRECT (diabetes incidence after renal transplantation: neoral (R) C2 monitoring versus tacrolimus) investigators (2007) results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus (vol 7, pg 1506, 2007). Am J Transplant 2008;8:908 | Study design |
| Vincenti F, Grinyo JM, Charpentier B, Medina-Pestana JD, Rostaing L, Vanrenterghem Y, et al. Primary outcomes from a randomized, Phase III study of belatacept vs cyclosporine in kidney transplant recipients (BENEFIT Study). Am J Transplant 2009;9:191–2 | Abstract |
| Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation 2002;73:775–82 | Population |
| Vincenti F, Larsen C, Alberu J, Garcia V, Rostaing L, Rice K, et al. Three-year outcomes from benefit: a phase III-study of belatacept vs cyclosporine in kidney transplant recipients. Transplant Int 2011;24:21 | Abstract |
| Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med 2005;353:770–81 | Population |
| Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant 2012;12:210–7 | Population |
| Vincenti F, Pescovitz MD, El-Shahawy M. Glucose metabolism disorders in non-white renal transplant patients receiving cyclosporine or tacrolimus in an international, randomized trial. Transplant Int 2007;20:115 | Abstract |
| Vincenti F, Rostaing L, DIRECT (Diabetes Incidence after REnal Transplantation: Neoral C2 monitoring versus Tacrolimus) investigators. Rationale and design of the DIRECT study: a comparative assessment of the hyperglycemic effects of tacrolimus and cyclosporine following renal transplantation. Contemp Clin Trials 2005;26:17–24 | No data |
| Vincenti F, Tuncer M, Castagneto M, Klinger M, Friman S, Scheuermann EH, et al. Prospective, multicenter, randomized trial to compare incidence of new-onset diabetes mellitus and glucose metabolism in patients receiving cyclosporine microemulsion versus tacrolimus after de novo kidney transplantation. Transplant Proc 2005;37:1001–4 | Duplicate |
| Vítko S, Klinger M, Salmela K, Wlodarczyk Z, Tydèn G, Senatorski G, et al. Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation 2005;80:1734–41 | Comparator |
| Vítko S, Klinger M, Salmela K, Wlodarczyk Z, Tydèn G, Senatorski G, et al. Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation 2005;80:1734–41 | Study design |
| Vítko S, Margreiter R, Weimar W, Dantal J, Viljoen HG, Li Y, et al. Everolimus (Certican) 12-month safety and efficacy versus mycophenolate mofetil in de novo renal transplant recipients. Transplantation 2004;78:1532–40 | Population |
| Vítko S, Margreiter R, Weimar W, Dantal J, Kuypers D, Winkler M, et al. Three-year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. Am J Transplant 2005;5:2521–30 | Population |
| Vondrak K, Grenda R, Watson A, Janda J, Simkova E, Seeman T, et al. Immunosupression with triple combination with tacrolimus with or without monoclonal antibody induction: a multicentric randomized study in children after kidney transplantation. Kidney Blood Press Res 2006;29:381 | Abstract |
| Vondrak K, Grenda R, Watson AR, Webb NJA, Beattie J, Pediat Tacrolimus Study G. Tacrolimus triple therapy with or without monoclonal antibody administration: a multicentre, randomized study in pediatric kidney transplantation. Am J Transplant 2005;5:401–2 | Abstract |
| Wagner M, Balk EM, Webster AC, et al. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev 2009;2:CD007746 | No data |
| Waid T. Tacrolimus as secondary intervention vs. cyclosporine continuation in patients at risk for chronic renal allograft failure. Clin Transplant 2005;19:573–80 | Intervention |
| Walker R, Vathsala A, Zibari GB, Kim YS, Cibrik D, Johnston T, et al. Class related adverse events in renal transplant recipients treated with everolimus: 24 month results from the A2309 study. Am J Transplant 2011;11:407 | Abstract |
| Walker RG, Cottrell S, Sharp K, Tripodi R, Nicholls KM, Fraser I, et al. Conversion of cyclosporine to tacrolimus in stable renal allograft recipients: quantification of effects on the severity of gingival enlargement and hirsutism and patient-reported outcomes. Nephrology 2007;12:607–14 | Outcome |
| Walker RG, Rostaing L, Nainan G, Del CRM, Steinberg S, Vincenti F, et al. A switch to belatacept-based immunosuppresive regimen in kidney transplant recipients from calcineurin inhibitors (CNI) has a favourable safety profile and results in improved renal function: 12-month results from a phase II study. Immunol Cell Biol 2011;89:A3 | Abstract |
| Waller JR, Murphy GJ, Metcalfe MS, Sandford RM, Pattenden CJ, Nicholson ML. Primary immunosuppression with tacrolimus is associated with a reduction in renal allograft fibrosis compared with neoral therapy. Transplant Proc 2002;34:1587–8 | Population |
| Wang K, Zhang H, Li Y, Wei Q, Li H, Yang Y, Lu Y. Efficacy of mycophenolate mofetil versus azathioprine after renal transplantation: a systematic review. Transplant Proc 2004;36:2071–2 | Population |
| Wang K, Zhang H, Li Y, Wei Q, Li H, Yang Y, Lu Y. Safety of mycophenolate mofetil versus azathioprine in renal transplantation: a systematic review. Transplant Proc 2004;36:2068–70 | Population |
| Wang R, Xu Y, Wu J, Wang Y, He Q, Chen J. Reduced-dose cyclosporine with mycophenolate mofetil and prednisone significantly improves the long-term glomerular filtration rate and graft survival. Intern Med 2013;52:947–53 | Study design |
| Warejko JK, Hmiel SP. Single-center experience in pediatric renal transplantation using thymoglobulin induction and steroid minimization. Pediatr Transplant 2014;18:816–21 | Study design |
| Watorek E, Szymczak M, Boratynska M, Patrzalek D, Klinger M. Cardiovascular risk in kidney transplant recipients receiving mammalian target of rapamycin inhibitors. Transplant Proc 2011;43:2967–9 | Comparator |
| Watorek E, Szymczak M, Boratynska M, Patrzalek D, Klinger M. Cardiovascular risk in kidney transplant recipients receiving mTOR inhibitors. Transplant Int 2011;24:118 | Abstract |
| Watson AR, Grenda R, Vondrak K, European Multicentre Tacrolimus S. A multicentre, randomised trial of tacrolimus triple therapy with or without basiliximab in paediatric kidney transplantation. Pediatr Transplant 2005;9:56 | Abstract |
| Watson CJ, Firth J, Williams PF, Bradley JR, Pritchard N, Chaudhry A, et al. A randomized controlled trial of late conversion from CNI-based to sirolimus-based immunosuppression following renal transplantation. Am J Transplant 2005;5:2496–503 | Population |
| Weimer R, Süsal C, Yildiz S, Streller S, Pelzl S, Staak A, et al. sCD30 and neopterin as risk factors of chronic renal transplant rejection: impact of cyclosporine A, tacrolimus, and mycophenolate mofetil. Transplant Proc 2005;37:1776–8 | Population |
| Weimer R, Süsal C, Yildiz S, Staak A, Pelzl S, Renner F, et al. Post-transplant sCD30 and neopterin as predictors of chronic allograft nephropathy: impact of different immunosuppressive regimens. Am J Transplant 2006;6:1865–74 | Population |
| Weir M. Long-term assessment of function in patients completing the spare-the-nephron study with a functioning graft. Am J Transplant 2013;13:36 | Abstract |
| Weir M, Mulgaonkar S, Pearson T, Patel A, Patel D, Shidban H, et al. Mycophenolate Mofetil/Sirolimus maintenance therapy after calcineurin inhibitor withdrawal in renal transplant recipients: 2-Year Outcomes of the spare-the-nephron (STN) Trial. Am J Transplant 2009;9:200–1 | Abstract |
| Weir MR, Mulgaonkar S, Chan L, Shidban H, Waid TH, Preston D, et al. Mycophenolate mofetil-based immunosuppression with sirolimus in renal transplantation: a randomized, controlled Spare-the-Nephron trial. Kidney Int 2011;79:897–907 | Abstract |
| Welberry Smith MP, Cherukuri A, Newstead CG, Lewington AJ, Ahmad N, Menon K, et al. Alemtuzumab induction in renal transplantation permits safe steroid avoidance with tacrolimus monotherapy: a randomized controlled trial. Transplantation 2013;96:1082–8 | Population |
| West-Thielke PM, Bodziak KA, Cohen DJ. Conversion to once-daily extended release meltdose (R) tacrolimus tablets (lcp-tacro (TM)) from twice-daily tacrolimus capsules (prograf (R)) is safe and efficacious in african american kidney transplant recipients: results from a phase iii randomized trial. Am J Transplant 2012;12:405–6 | Abstract |
| Williams P. An open label randomised study of sirolimus in patients with impaired renal function following renal transplantation. National Research Register, UK. URL: www.nrr.nhs.uk/ (accessed 25 July 2014) | Unobtainable |
| Wiseman AC, McCague K, Kim Y, Geissler F, Cooper M. The effect of everolimus versus mycophenolate upon proteinuria following kidney transplant and relationship to graft outcomes. Am J Transplant 2013;13:442–9 | Outcome |
| Wissing KM, Pipeleers L. Obesity, metabolic syndrome and diabetes mellitus after renal transplantation: prevention and treatment. Transplant Rev 2014;28:37–46 | Study design |
| Wissing KM, Fomegné G, Broeders N, Ghisdal L, Hoang AD, Mikhalski D, et al. HLA mismatches remain risk factors for acute kidney allograft rejection in patients receiving quadruple immunosuppression with anti-interleukin-2 receptor antibodies. Transplantation 2008;85:411–6 | Study design |
| Wissing KM, Kuypers D, Abramowicz D, Weekers L, Budde KMD, Rath T, et al. Conversion from tacrolimus to cyclosporine a improves glucose metabolism in patients with new onset diabetes after renal transplantation: interim analysis of a prospective and randomized study. Transplant Int 2013;26:37 | Abstract |
| Wlodarczyk Z, Ostrowski M, Mourad M, Krämer BK, Abramowicz D, Oppenheimer F, et al. Tacrolimus pharmacokinetics of once- versus twice-daily formulations in de novo kidney transplantation: a substudy of a randomized phase III trial. Ther Drug Monit 2012;34:143–7 | Population |
| Wlodarczyk Z, Squifflet JP, Ostrowski M, Rigotti P, Stefoni S, Citterio F, et al. Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: a randomized, open-label trial. Am J Transplant 2009;9:2505–13 | Population |
| Włodarczyk Z, Wałaszewski J, Perner F, Vitko S, Ostrowski M, Bachleda P, et al. Freedom from rejection and stable kidney function are excellent criteria for steroid withdrawal in tacrolimus-treated kidney transplant recipients. Ann Transplant 2002;7:28–31 | Population |
| Wlodarczyk Z, Walaszewski J, Perner F, Vitko S, Ostrowski M, Bachleda P, et al. Steroid withdrawal at 3 months after kidney transplantation: a comparison of two tacrolimus-based regimens. Transpl Int 2005;18:157–62 | Population |
| Woestenburg AT, Peeters P, Sennesael J, Abramowicz D, Wissing KM, Geers C, et al. Interstitial fibrosis and fibrous intimal thickening in de novo renal allografts under sirolimus or cyclosporine: results of a randomised, controlled trial (FIBRASIC). Transplant Int 2009;22:79 | Abstract |
| Wohlfahrtova M, Viklicky O. Recent trials in immunosuppression and their consequences for current therapy. Curr Opin Organ Transplant 2014;19:387–94 | Study design |
| Woodle ES, Grp TS. A randomized, prospective, multicenter study of thymoglobulin in renal transplantation for induction and minimization of steroids (TRIMS). Am J Transplant 2005;5:571 | Abstract |
| Woodside KJ, Thomas PG, Lappin JA, Vaidya S, Rajaraman S, Gugliuzza KK. An open label, randomized, controlled trial of a tolerogenic induction protocol using alemtuzumab (Campath 1H) and tacrolimus monotherapy versus thymoglobulin induction with triple drug therapy in high immunological risk renal transplantation. Am J Transplant 2007;7:522 | Abstract |
| Wu B, Wu FB, Yu L, Li TP, Tang Y. Effectiveness and safety of calcineurin inhibitor withdrawal from target-of-rapamycin-inhibitor-based immunosuppression in kidney transplantation: a meta analysis (Provisional abstract). Chinese Journal of Evidence-Based Medicine 2010;10:33–9 | Study design |
| Wu FL, Tsai MK, Chen RR, Sun SW, Huang JD, Hu RH, et al. Effects of calcineurin inhibitors on sirolimus pharmacokinetics during staggered administration in renal transplant recipients. Pharmacotherapy 2005;25:646–53 | Study design |
| Wyrley-Birch H, Kanwar A, Vijayanand D, Navarro A, Reddy M, Wilson C, et al. A prospective randomised paired trial of sirolimus versus tacrolimus as primary immunosuppression following non heart beating donor kidney transplantation after anti-il2 monoclonal antibody induction. Transplant Int 2010;23:16 | Abstract |
| Xue W, Zhang Q, Xu Y, Wang W, Zhang X, Hu X. Effects of tacrolimus and cyclosporine treatment on metabolic syndrome and cardiovascular risk factors after renal transplantation: a meta-analysis. Chin Med J 2014;127:2376–81 | Population |
| Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al. A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children. Health Technol Assess 2006;10(49) | Duplicate |
| Yaqoob M, Pattison J, Riad H, Cornu-Artis C, Wang Z, Shihab F. Cytomegalovirus and BK virus infections are less frequent with everolimus versus mycophenolate immunosuppression: 24-month update from the 2309 study in de novo renal transplant recipients. Transplant Int 2011;24:40–1 | Unobtainable |
| Yaqoob M, Riad H, Pattison J, Cornu-Artis C, Wang Z, Tedesco Silva H. Efficacy and safety of 24 months immunosuppression with concentration-controlled everolimus and reduced cyclosporine in de novo renal transplant recipients. Transplant Int 2011;24:39 | Abstract |
| Yoshimura N, Uchida K, Takahara S, Teraoka S, Kobayashi E, Teshima R, et al. Concentration-controlled everolimus with reduced cyclosporine concentration in Japanese de novo renal transplant recipients: efficacy and safety results at 12 months: Japanese multicenter study. Transplantation 2012;94:990 | Abstract |
| Zachariah M, Nader ND, Brar J, Singh N, Venuto R, Patel S, et al. Alemtuzumab and minimization immunotherapy in kidney transplantation: long-term results of comparison with rabbit anti-thymocyte globulin and standard triple maintenance therapy. Transplant Proc 2014;46:94–100 | Study design |
| Zadrazil J, Horak P, Strebl P, Krejci K, Kajabova M, Schneiderka P, et al. In vivo oxidized low-density lipoprotein (ox-LDL) aopp and tas after kidney transplantation: a prospective, randomized one year study comparing cyclosporine A and tacrolimus based regiments. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2012;156:14–20 | Population |
| Zeier M, Budde K, Arns W, Guba M, Sommerer C, Neumayer H, et al. Efficacy and safety of three different treatment regimens in de novo renal transplant patients: follow-up results of the herakles trial at month 24. Am J Transplant 2013;13:183 | Abstract |
| Zhang YG, Teng DH, Wang L, et al. Effectiveness and safety of rapamycin-based immunosuppression regimen with or without CsA in renal transplantation: a systematic review. Chinese Journal of Evidence-Based Medicine 2006;6:94–106 | Study design |
| Zhong J-y, Qu L-x, Zhang M, Jiao Z, Lu F-m. Application of basiliximab in prevention of acute allograft rejection in kidney transplantation recipients. Zhongguo Xinyao yu Linchuang Zazhi 2005;24:468–71 | Language |
| Zhu QG, Zhao YK, Liu W, Luo H, Qiu Y, Gao ZZ. Two-year observation of a randomized trial on tacrolimus-based therapy with withdrawal of steroids or mycophenolate mofetil after renal transplantation. Chinese Med Sci J 2008;23:244–8 | Study design |
| Study | Treatment comparisons (number of participants in each arm) | Eligibility criteria | Age mean (SD), median [range] (years) |
|---|---|---|---|
| Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized long-term trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (Neoral) and sirolimus in renal transplantation. I. Drug interactions and rejection at one year. Transplantation 2004;77:244–251 | TAC + SRL (50) vs. TAC + MMF (50) vs. CSA + SRL (50) | ≥ 13 years | 50 (13) vs. 47 (16) vs. 44 (16) |
| Flechner SM, Gurkan A, Hartmann A, Legendre CM, Russ GR, Campistol JM, et al. A randomized, open-label study of sirolimus versus cyclosporine in primary de novo renal allograft recipients. Transplantation 2013;95:1233–41 | SRL (314) vs. CSA (161) | ≥ 13 years | 42.9 (SE 0.8) vs. 42.7 (SE 1.1) |
| Gaber AO, Kahan BD, Buren C, Schulman SL, Scarola J, Neylan JF. Comparison of sirolimus plus tacrolimus versus sirolimus plus cyclosporine in high-risk renal allograft recipients: results from an open-label, randomized trial. Transplantation 2008;86:1187–95 | TAC (224) vs. CSA (224) | ≥ 13 years | 46.4 [15–73] vs. 44.4 [15–80] |
| Kahan BD for The Rapamune US Study Group. Efficacy of sirolimus compared with azathoprine for reduction of acute renal allograft rejection: a randomised multicentre study. Lancet 2000;356:194–202a | SRL 2 mg (284) vs. SRL 5 mg (274) vs. AZA (161) | ≥ 13 yearsb | 44.9 (13.6) vs. 46.8 (13.0) vs. 45.6 (13.0) |
| MacDonald AS for The Rapamune US Study Group. A worldwide, phase III ranomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation 2001;71:271–80a | SRL 2 mg (227) vs. SRL 5 mg (219) vs. PBO (130) | Included participants aged 15–71 yearsc | 45.6 (12.3) [15–71] vs. 45.1 (12.2) [17–68] vs. 46 (13.1) [16–72] |
| Lee YJ, Kim B, Lee JE, Kim YG, Kim DJ, Kim SJ, et al. Randomized trial of cyclosporine and tacrolimus therapy with steroid withdrawal in living-donor renal transplantation: 5-year follow-up. Transplant Int 2010;23:147–54 | CSA (55) vs. TAC (62) | > 15 years | 38.5 (9.5) vs. 38.8 (9.2) |
| Machado PG, Felipe CR, Hanzawa NM, Park SI, Garcia R, Alfieri F, et al. An open-label randomized trial of the safety and efficacy of sirolimus vs. azathioprine in living related renal allograft recipients receiving cyclosporine and prednisone combination. Clin Transplant 2004;18:28–38 | SRL (35) vs. AZA (35) | ≥ 13 years | 35.8 (10.5) vs. 32.7 (10.4) |
| Wu FL, Tsai MK, Chen RR, Sun SW, Huang JD, Hu RH, et al. Effects of calcineurin inhibitors on sirolimus pharmacokinetics during staggered administration in renal transplant recipients. Pharmacotherapy 2005;25:646–53 | TAC (11) vs. CSA (10) | 13–65 yearsd | 40.4 (10.4) vs. 36.9 (8.1) |
| Silva HT Jr, Yang HC, Abouljoud M, Kuo PC, Wisemandle K, Bhattacharya P, et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant 2007;7:595–608e | TAC PR (214) vs. TAC (212) vs. CSA (212) | ≥ 12 years | 47.8 (13), 48 [17–77] vs. 48.6 (12.9), 50.5 [19–74] vs. 47.6 (13), 48.5 [17–77] |
Appendix 3 Systematic reviews
| Trial | Aim | Identified RCTs | Identified non-RCTs |
|---|---|---|---|
| Almeida et al. 2013260 | To evaluate the safety of the most commonly used immunosuppressive regimens | 0 | 0 |
| Andrassy et al. 2012261 | To summarise clinical trials after solid organ transplantation and describe potential mechanisms involved in the antiCMV effect of mTOR-inhibitors | 0 | 0 |
| Brooks et al. 2010262 | To evaluate the quality of reporting of transplantation trials in children published in contemporary biomedical literature | 2 | 0 |
| Ho et al. 2013263 | To evaluate the benefits and harms of sustained-release daily dosing formulation compared with standard twice daily TAC in KTRs | 0 | 0 |
| Kasiske et al. 2008264 | To conduct a systematic review of RCTs to critically examine the incidence and type of dyslipidemia associated with mTOR-Is | 0 | 0 |
| Knight et al. 2009265 | To identify whether or not MMF improves outcomes compared with AZA in renal transplant recipients, particularly in incidence of acute rejection, patient and graft survival, and toxicity | 0 | 0 |
| Liu et al. 2010266 | To compare the efficacy and safety of BAS with antithymocyte globulin for induction therapy | 0 | 0 |
| Masson et al. 2014267 | To synthesise data from RCTs that compared belatacept with other primary maintenance immunosuppression regimens | 0 | 0 |
| Moore et al. 2009268 | To assess transplant outcomes after CNI sparing with mycophenolate as sole adjunctive immunosuppression | 0 | 0 |
| Mulay et al. 2006269 | To systematically review all clinical studies that evaluated CNI conversion to SRL in patients with chronic nephropathy | 0 | 0 |
| Peddi et al. 2013270 | To evaluate the efficacy and safety of immunosuppressive regimens containing a mTOR-I with TAC minimisation therapy in solid organ transplant recipients | 0 | 0 |
| Pengel et al. 2011271 | To evaluate the occurrence of wound complications and lymphoceles in solid organ transplant recipients receiving mTOR-Is from the time of transplantation compared with patients not receiving mTOR-I | 0 | 0 |
| Su et al. 2011272 | To evaluate clinical consequences of and MMF dose reduction in renal transplant recipients on TAC based regimens | 0 | 0 |
| Webster et al. 2004273 | To systematically identify and summarise the effects of IL-2Ra as induction agents, as an addition to standard therapy, or as an alternative to other antibody therapies in common use (antithymocyte globulins, antilymphocyte globulins, monomurab-CD3) | 0 | 0 |
| Webster et al. 2004274 | To systematically identify and summarise the effects of using an IL2Ra, as an addition to standard therapy, or as an alternative to other antibody therapy | 0 | 0 |
| Webster et al. 2005207 | To systematically review randomised controlled trials in which TAC had been compared with ciclosporin as initial immunosuppressive therapy in the treatment of KTRs | 0 | 0 |
| Webster et al. 2005275 | To compare the effects of TAC with ciclosporin as primary therapy for KTRs | 0 | 0 |
| Webster et al. 2006156 | To identify systematically and summarise the current available evidence of the short- and long-term benefits and harms of SRL and everolimus when used in primary immunosuppressive regimens for KTRs | 0 | 0 |
| Webster et al. 2006276 | To investigate the benefits and harms of immunosuppressive regimens containing TOR-I when compared with other regimens as initial therapy for KTRs | 0 | 0 |
| Webster et al. 2010277 (update of Webster et al. 2004274) | To systematically identify and summarise the effects of using an IL2Ra, as an addition to standard therapy, or as an alternative to another immunosuppressive induction strategy | 0 | 0 |
| Woodroffe et al. 2005255 | To examine the clinical effectiveness and cost-effectiveness of the newer immunosuppressive drugs for renal transplantation: BAS, daclizumab, TAC, mycophenolate (mofetil and sodium) and SRL | 1 | 0 |
| Yan et al. 2014278 | To evaluate the efficacy and safety of CNI avoidance, CNI withdrawal, and CNI regimens on postoperative patient and graft survival, acute rejection, renal function and adverse events | 0 | 0 |
| Yao et al. 20062 | To establish the clinical effectiveness (harms and benefits) and cost-effectiveness of four of the newer immunosuppressive drugs for renal transplantation, namely BAS, daclizumab, TAC and MMF and sodium) and of SRL in children | 2 | 4 |
Appendix 4 Ongoing trials
| Study | Sponsor/collaborators | Trial name | n | Status | Included in PenTAG (reason) |
|---|---|---|---|---|---|
| NCT01791491 | Bristol-Myers Squibb | Phase II Pharmacokinetics, Efficacy, and Safety of Belatacept in Paediatric Renal Transplant Recipients | 54 | Recruiting | N/A |
| NCT01544491 A2314; Gupta et al. 2013,86 Langer et al. 2013,87 Tonshoff et al. 201289 and Tonshoff et al. 201388 |
Novartis Pharmaceuticals | Efficacy, Tolerability and Safety of Early Introduction of Everolimus, Reduced Calcineurin Inhibitors and Early Steroid Elimination Compared to Standard CNI, Mycophenolate Mofetil and Steroid Regimen in Paediatric Renal Transplant Recipients | 106 | Recruiting | N/A |
| NCT01550445 Oh et al. 2012279 |
Ajou University School of Medicine | Steroid Withdrawal Immunosuppression After Renal Transplantation | 30 | Unknown | Not included (design) |
| NCT00023244, Study 315 (mentioned in Yao et al. 20062 as ongoing; Benfield et al. 2010280) |
National Institute of Allergy and Infectious Diseases (NIAID), Cooperative Clinical Trials in Paediatric Transplantation; Pfizer (formerly Wyeth) | Steroid Withdrawal in Pediatric Kidney Transplant Recipients | 274 | Terminated | Not included (steroid withdrawal) |
| NCT00137345 Flechner et al. 2013281 |
Pfizer (formerly Wyeth) | Study Comparing Sirolimus With Cyclosporine in a Calcineurin Inhibitor (CNI)-Free Regimen in Kidney Transplant Recipients | 500 | Terminated | Not included (population) |
| NCT00005113 (included in Yao et al. 20062; 0468E1–217-US) |
Children’s Hospital Boston; Pfizer (formerly Wyeth) | A Study to Compare Treatment With Sirolimus Versus Standard Treatment in Patients Who Have Received a Kidney Transplant | 213 | Terminated | Not included (no data available and population) |
| NCT00228020 Offner et al. 200873 |
Novartis | Study of Safety and Efficacy of a Basiliximab, Mycophenolate Mofetil, Cyclosporine Microemulsion and Prednisone Combination Treatment Regimen in Pediatric Renal Allograft Recipients | 212 | Completed | Included |
| NCT00141037 Sarwal et al. 2012282 |
National Institute of Allergy and Infectious Diseases (NIAID) Astellas Pharma Inc. Hoffmann-La Roche | Steroid-Free Versus Steroid-Based Immunosuppression in Pediatric Renal (Kidney) Transplantation | 130 | Completed | Not included |
| NCT00296348 | Astellas Pharma Inc. | Comparing Efficacy and Safety of Steroid Withdrawal With Tacrolimus and MMF With Induction in Children After Kidney Transplantation (TWIST) | 198 | Completed | Not included |
| NCT00166244 van Gelder et al. 2008283 |
Erasmus Medical Hoffmann-La Roche Center | Fixed Dose MMF vs. Concentration Controlled MMF After Renal Transplantation | 901 | Completed | Not included (population) |
| ISRCTN89278733 Cransberg et al. 2007284 |
Erasmus Medical Center | Safety and efficacy of mycophenolate mofetil in pediatric renal transplantation | 44 | Completed | Not included (design) |
Appendix 5 Clinical effectiveness: additional information
| Study | Multiple publications | Treatments | Included in PenTAG (reason) |
|---|---|---|---|
| Vincenti et al. 1998285 | Vincenti et al. 1998286 Hengster et al. 1999287 Bumgarden et al. 2001288 | DAC vs. PBO | No (treatment) |
| Bingyi et al. 2003104 | N/A | BAS vs. PBO | Yes |
| Ponticelli et al. 2001102 | Ponticelli et al. 2001289 | BAS vs. PBO | Yes |
| Sheashaa et al. 200399 | N/A | BAS vs. NI | Yes |
| Folkmane et al. 2001290 | Folkmane et al. 2002291 (a) | BAS vs. NI and MMF vs AZA | No (design) |
| Shapiro et al. 1991292 | N/A | TAC vs. CSA | No (design) |
| Mayer et al. 1997107 | Mayer et al. 1999293 Mayer et al. 2002294 Mayer et al. 2002295 European Tacrolimus Multicentre Renal Study | TAC vs. CSA | Yes |
| Radermacher et al. 1998124 | N/A | TAC vs. CSA | No (design) |
| Van Duijnhoven et al. 2002123 | N/A | TAC vs. CSA | Yes |
| Jurewicz et al. 1999296 | Baboolal et al. 2002121 Jurewicz et al. 2003297 Welsh Transplant Research group | TAC vs. CSA | Yes |
| Sperschneider et al. 2001298 | Krämer et al. 2003299 Dietl et al. 2002300 Margreiter et al. 2002110 | TAC vs. CSA | Yes |
| Töz et al. 2004301 | N/A | TAC vs. CSA | Yes |
| Campost et al. 2003109 | Brazilian TAC study | TAC vs. CSA | Yes |
| Murphy et al. 2003302 | N/A | TAC vs. CSA | Yes |
| Mathew et al. 1998303 | Tricontinental Mycophenolate Mofetil Renal Transplantation Study 1996 | MMF vs. AZA | Yes |
| Miladipour et al. 2002304 | N/A | MMF vs. AZA | No (design) |
| Sadek et al. 2002116 | N/A | MMF vs. AZA | Yes |
| Tuncer et al. 2002118 | N/A | MMF vs. AZA | Yes |
| Sollinger et al. 1995120 | MMF Acute Renal transplantation Study Group 1996 | MMF vs. AZA | Yes |
| Baltar et al. 2002305 | N/A | MMF vs. AZA | No (language) |
| Salvadori et al. 2004306 | N/A | MPS vs. MMF | Yes |
| Kahan 2000257 | Rapamune US study | SRL vs. AZA | No (design) |
| Machado et al. 2004258 | N/A | SRL vs. AZA | No (design) |
| Groth et al. 1999206 | Sirolimus European Renal transplantation Study group | SRL vs. CSA | Yes |
| Johnson et al. 2001307 | Rapamune Maintenance Regimen (RMR) study | Addition of SRL and CSA removal | No (design) |
| AE | Follow-up | CSA + MMF + CCS | ||
|---|---|---|---|---|
| n events | n participants | % | ||
| Respiratory infections | 1 year | 24 | 69 | 35 |
| 1–2 years | 6 | 57 | 11 | |
| 2–3 years | 4 | 44 | 9 | |
| UTIs | 1 year | 14 | 69 | 20 |
| 1–2 years | 6 | 57 | 11 | |
| 2–3 years | 4 | 44 | 9 | |
| CMV infections | 1 year | 11 | 69 | 16 |
| 1–2 years | 2 | 57 | 4 | |
| 2–3 years | 0 | 44 | 0 | |
| 3–5 years | 2 | 44 | 5 | |
| EBV infections | 1 year | 2 | 69 | 3 |
| 1–2 years | 8 | 57 | 14 | |
| 2–3 years | 2 | 44 | 5 | |
| 3–5 years | 3 | 78 | 4 | |
| Solid tumour | 1 year | 0 | 69 | 0 |
| 1–2 years | 0 | 57 | 0 | |
| 2–3 years | 1 | 44 | 2 | |
| 3–5 years | 0 | 78 | 0 | |
| PTLD | 1 year | 1 | 69 | 1 |
| 1–2 years | 0 | 57 | 0 | |
| 2–3 years | 0 | 44 | 0 | |
| 3–5 years | 0 | 78 | 0 | |
| Herpes simplex | 1 year | 11 | 69 | 16 |
| 1–2 years | 4 | 57 | 7 | |
| 2–3 years | 0 | 44 | 0 | |
| 3–5 years | 8 | 78 | 10 | |
| HPV6 | 1 year | 1 | 69 | 1 |
| 1–2 years | 2 | 57 | 4 | |
| 2–3 years | 1 | 44 | 2 | |
| 3–5 years | 3 | 78 | 4 | |
| Oral thrush | 1 year | 3 | 69 | 4 |
| 1–2 years | 2 | 57 | 4 | |
| 2–3 years | 0 | 44 | 0 | |
| Diarrhoea | 1 year | 37 | 69 | 54 |
| 1–2 years | 9 | 57 | 16 | |
| 2–3 years | 3 | 44 | 7 | |
| Abdominal pain/nausea | 1 year | 12 | 69 | 17 |
| 1–2 years | 5 | 57 | 9 | |
| 2–3 years | 3 | 44 | 7 | |
Appendix 6 Astellas’ submission
| Study | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Parallel Adult HTA (reason) |
|---|---|---|---|---|---|
| Ekberg et al. 2007127 | CSA + MMF + CCS | DAC + LOW CSA + MMF + CCS | DAC + LOW TAC + MMF + CCS | DAC + LOW SRL + MMF + CCS | Included |
| Abou-Jaoude et al. 2003308 | DAC/r-ATG/NON + TAC + AZA + CCS | DAC/r-ATG/NON + CSA + AZA + CCS | N/A | N/A | Excluded (study design) |
| Abou-Jaoude et al. 2005309 | DAC/ZENA/NONE + TAC + AZA/MMF + CCS | DAC/ZENA/NONE + CSA + AZA/MMF + CCS | N/A | N/A | Excluded (study design) |
| Busque et al. 2001310 | TAC + MMF + CCS | TAC + AZA + CCS | CSA + MMF + CCS | N/A | Excluded (study design) |
| Campos et al. 2002109 | TAC + AZA + CCS | CSA + AZA + CCS | N/A | N/A | Included |
| Hardinger et al. 2005113 | r-ATG + TAC + AZA + CCS | r-ATG + CSA + AZA + CCS | N/A | N/A | Included |
| Johnson et al. 2000311 | TAC + AZA + CCS | CSA + MMF + CCS | TAC + MMF + CCS | N/A | Excluded (population) |
| Margreiter et al. 2002110 | TAC + AZA + CCS | CSA + AZA + CCS | N/A | N/A | Included |
| Garcia et al. 2003312 | CSA + CCS | BAS + CSA + CCS | BAS + TAC + CCS | N/A | Excluded (study design) |
| Morris-Stiff et al. 2000313 | TAC + AZA + CCS | CSA + AZA + CCS | N/A | N/A | Excluded (population) |
| Murphy et al. 2003302 | TAC + AZA + CCS | CSA + AZA + CCS | N/A | N/A | Included |
| Raofi et al. 1999195 | OKT3 + TAC + CCS | OKT3 + CSA + CCS | N/A | N/A | Included |
| Silva et al. 2007152 | BAS + TAC PR + MMF + CCS | BAS + TAC + MMF + CS | BAS + CSA + MMF + CCS | N/A | Excluded (population) |
| Toz et al. 2004301 | TAC + AZA + CCS | CSA + AZA + CCS | N/A | N/A | Included |
| Vincenti et al. 2007314 | BAS + TAC + MMF/MPS + CCS | BAS + CSA + MMF/MPS + CCS | N/A | N/A | Excluded (intervention) |
| Wang et al. 2000315 | TAC + MMF + CCS | CSA + MMF + CCS | N/A | N/A | Excluded (abstract) |
| White et al. 2000316 | TAC + CCS | CSA + CCS | N/A | N/A | Excluded (abstract) |
| Williams et al. 1999317 | TAC + CCS | CSA + CCS | N/A | N/A | Excluded (abstract) |
| Yang et al. 1999125 | TAC + MMF + CCS | CSA + MMF + CCS | N/A | N/A | Included |
| Flechner et al. 2011318 | DAC + TAC + SRL + CCS | DAC + MMF + SRL + CCS | N/A | N/A | Included |
| Glotz et al. 2010128 | TAC + MMF + CCS | r-ATG + SRL + MMF + CCS | N/A | N/A | Excluded (intervention) |
| Larson et al. 2006319 | r-ATG + TAC + MMF + CCS | r-ATG + SRL + MMF + CCS | N/A | N/A | Included |
| Vincenti et al. 2010198 | BAS + BEL LOW + MMF + CCS | BAS + BEL HIGH + MMF + CCS | BAS + CSA + MMF + CCS | N/A | Included |
| Durrbach et al. 2010199 | BAS + BEL LOW + MMF + CCS | BAS + BEL HIGH + MMF + CCS | BAS + CSA + MMF + CCS | N/A | Included |
| Bertoni et al. 2011320 | BAS + EVL + CSA + CCS | BAS + MPS + CSA + CCS | N/A | N/A | Included |
| Tedesco Silva et al. 2010191 | BAS + EVL LOW + CSA + CCS | BAS + EVL HIGH + CSA + CCS | BAS + MPA + CSA + CCS | N/A | Included |
| Albano et al. 201397 | TAC + MMF + CCS | TAC(0.2 MG) + MMF + CCS | TAC PR (0.3 MG) + MMF + CCS | BAS + TAC PR + MMF + CCS | Included |
| Krämer et al. 2010139 | TAC + MMF + CCS | TAC + MMF + CCS | N/A | N/A | Included |
| Ciancio et al. 2004321 | SRL + TAC + CCS | MMF + TAC + CCS | SRL + CSA + CCS | N/A | Excluded (population) |
| Gonwa et al. 2003193 | SRL + TAC + CCS | MMF + TAC + CCS | N/A | N/A | Included |
| Mendez et al. 2005322 | SRL + TAC + CCS | MMF + TAC + CCS | N/A | N/A | Included |
Appendix 7 Summary of model parameters
| Parameter | Value | PSA distribution |
|---|---|---|
| Study characteristics (clinical effectiveness estimates from adult RCTs) | ||
| Patient age (years) | 10 | Not varied |
| Patient weight (kg) | 31.8 | Not varied |
| Proportion male | 0.598 | Not varied |
| Donor type (first graft) | ||
| DBD | 0.645 | Not varied |
| Living related | 0.355 | Not varied |
| Donor type (subsequent grafts) | ||
| DBD | 0.833 | Not varied |
| Living related | 0.167 | Not varied |
| Study characteristics (Trompeter et al.77) | ||
| Patient age (years) | 10.3 | Normal(10.31, 0.325) |
| Patient weight (kg) | 32.6 | Normal(32.58, 1.159) |
| Proportion male | 0.612 | Beta(120, 76) |
| Study characteristics (Grenda et al.75) | ||
| Patient age (years) | 11.4 | Normal(11.40, 0.292) |
| Proportion male | 0.620 | Beta(119, 73) |
| Study characteristics (Offner et al.73) | ||
| Patient age (years) | 10.7 | Normal(10.75, 0.342) |
| Proportion male | 0.615 | Beta(118, 74) |
| Surrogate relationships | ||
| Graft survival (censored for DWFG) | ||
| AR | 1.60 | Log-normal(0.47, 0.037) |
| NODAT | 1.12 | Log-normal(0.113, 0.061) |
| eGFR (ml/minute/1.73 m2) | ||
| ≥ 80 | 1 | Not varied |
| 45–80 | 1.59 | Log-normal(0.463, 0.571) |
| < 45 | 55.9 | Log-normal(4.024, 1.203) |
| DWFG | ||
| NODAT | 1.41 | Log-normal(0.113, 0.061) |
| Sex = female | 0.865 | Log-normal(–0.145, 0.036) |
| Donor type | ||
| DBD | 1 | Not varied |
| Living related | 0.551 | Log-normal(–0.595, 0.071) |
| Age (years) | ||
| 0–17 | 0.377 | Log-normal(–0.975, 0.186) |
| 18–30 | 0.369 | Log-normal(–0.996, 0.117) |
| 31–40 | 0.712 | Log-normal(–0.339, 0.091) |
| 41–50 | 1 | Not varied |
| 51–60 | 2.140 | Log-normal(0.761, 0.059) |
| 61–70 | 4.128 | Log-normal(1.418, 0.053) |
| Effectiveness estimates from adult RCTs | ||
| Mortality within 12 months [ln(OR)] | ||
| Induction agents (vs. no induction) | Multivariate normal | |
| BAS | –0.117 | |
| R-ATG | –0.461 | |
| Maintenance regimens (vs. CSA + AZA) | Multivariate normal | |
| TAC + AZA | 0.323 | |
| CSA + MMF | –0.057 | |
| TAC + MMF | 0.422 | |
| BEL + MMF | –0.763 | |
| CSA + EVL | 0.333 | |
| TAC + SRL | 0.325 | |
| SRL + MMF | 0.542 | |
| Head to head | ||
| MPS vs. MMF | –0.435 | Normal(–0.435, 1.231) |
| TAC-PR vs. TAC | 0.245 | Normal(0.245, 0.481) |
| Graft loss within 12 months [ln(OR)] | ||
| Induction agents (vs. no induction) | Multivariate normal | |
| BAS | –0.171 | |
| R-ATG | –0.253 | |
| Maintenance regimens (vs. CSA + AZA) | Multivariate normal | |
| TAC + AZA | 0.135 | |
| CSA + MMF | –0.297 | |
| TAC + MMF | –0.379 | |
| BEL + MMF | –0.492 | |
| CSA + EVL | –0.484 | |
| TAC + SRL | 0.159 | |
| SRL + MMF | 0.032 | |
| Head to head | ||
| MPS vs. MMF | –0.148 | Normal(–0.148, 0.524) |
| TAC-PR vs. TAC | 0.183 | Normal(0.183, 0.290) |
| BPAR within 12 months [ln(OR)] | ||
| Baseline (BAS + TAC + AZA) | 0.192 | Beta(19, 80) |
| Induction agents (vs. no induction) | Multivariate normal | |
| BAS | –0.688 | |
| R-ATG | –1.041 | |
| Maintenance regimens (vs. CSA + AZA) | Multivariate normal | |
| TAC + AZA | –0.548 | |
| CSA + MMF | –0.752 | |
| TAC + MMF | –0.921 | |
| BEL + MMF | –0.216 | |
| CSA + EVL | –0.784 | |
| TAC + SRL | –0.957 | |
| SRL + MMF | –0.828 | |
| Head to head | ||
| MPS vs. MMF | 0.396 | Normal(0.396, 0.678) |
| TAC-PR vs. TAC | –0.025 | Normal(–0.025, 0.383) |
| Graft function at 12 months [mean difference (ml/minute/1.73 m2)] | ||
| Baseline (BAS + TAC + AZA) | 82 (SD 27) | Not varied |
| Induction agents (vs. no induction) | Multivariate normal | |
| BAS | 2.615 | |
| R-ATG | 0.752 | |
| Maintenance regimens (vs. CSA + AZA) | Multivariate normal | |
| TAC + AZA | 9.304 | |
| CSA + MMF | 1.609 | |
| TAC + MMF | 6.531 | |
| BEL + MMF | 10.550 | |
| CSA + EVL | 4.863 | |
| TAC + SRL | –0.352 | |
| SRL + MMF | 3.846 | |
| Head to head | ||
| MPS vs. MMF | 3.9 | Normal(3.9, 2.9) |
| TAC-PR vs. TAC | –0.211 | Normal(–0.211, 1.302) |
| Effectiveness estimates (Trompeter et al.77) | ||
| Mortality within 4 years | ||
| TAC + AZA | 0.06 | Beta(6, 97) |
| CSA + AZA | 0.08 | Beta(7, 86) |
| Graft loss (excluding DWFG) within 4 years | ||
| TAC + AZA | 0.046 | Beta(5, 98) |
| CSA + AZA | 0.208 | Beta(19, 74) |
| AR within 12 months | ||
| TAC + AZA | 0.43 | Beta(44, 58) |
| CSA + AZA | 0.62 | Beta(58, 35) |
| eGFR at 12 months (ml/minute/1.73 m2) | ||
| TAC + AZA | 64.9 | Normal(64.9, 2.17) |
| CSA + AZA | 57.8 | Normal(57.8, 2.27) |
| Effectiveness estimates (Grenda et al.75) | ||
| Mortality within 48 months | ||
| TAC + AZA | 0.011 | Beta(1.5, 92.5) |
| BAS + TAC + AZA | 0.000 | Beta(0.5, 99.5) |
| Graft loss (excluding DWFG) within 48 months | ||
| TAC + AZA | 0.104 | Beta(10.2, 83.8) |
| BAS + TAC + AZA | 0.051 | Beta(5.5, 94.5) |
| AR within 12 months | ||
| TAC + AZA | 0.26 | Beta(24, 69) |
| BAS + TAC + AZA | 0.24 | Beta(23.5, 75.5) |
| eGFR at 12 months (ml/minute/1.73 m2) | ||
| TAC + AZA | 74.9 | Normal(74.9, 2.04) |
| BAS + TAC + AZA | 74.0 | Normal(74.0, 1.98) |
| Effectiveness estimates (Offner et al.73) | ||
| Mortality within 48 months | ||
| BAS + CSA + MMF | 0.028 | Beta(3.3, 97.7) |
| CSA + MMF | 0.000 | Beta(0.5, 92.5) |
| Graft loss (excluding DWFG) within 48 months | ||
| BAS + CSA + MMF | 0.019 | Beta(1.9, 98.1) |
| CSA + MMF | 0.011 | Beta(1.0, 91.0) |
| AR within 12 months | ||
| BAS + CSA + MMF | 0.13 | Beta(13, 87) |
| CSA + MMF | 0.23 | Beta(21, 71) |
| eGFR at 12 months (ml/minute/1.73 m2) | ||
| BAS + CSA + MMF | 79 | Normal(79, 2.3) |
| CSA + MMF | 82 | Normal(82, 2.5) |
| NODAT within 12 months | ||
| Based on adult evidence | ||
| Baseline | 0.040 | Beta(4, 95) |
| Maintenance agents (vs. TAC) [ln(OR)] | Multivariate normal | |
| TAC-PR | 0.169 | |
| CSA | –0.816 | |
| BEL | –1.671 | |
| SRL | –0.234 | |
| Maintenance agents (vs. MMF) [ln(OR)] | Multivariate normal | |
| MPS | –0.070 | |
| SRL | 0.474 | |
| EVL | –0.052 | |
| Trompeter et al.77 | ||
| TAC + AZA | 0.019 | Beta(2,101) |
| CSA + AZA | 0.011 | Beta(1, 92) |
| Grenda et al.75 | ||
| TAC + AZA | 0.011 | Beta(1, 92) |
| BAS + TAC + AZA | 0.040 | Beta(4, 95) |
| Offner et al.73 | ||
| BAS + CSA + MMF | 0.0 | Beta(0.5, 100.5) |
| CSA + MMF | 0.0 | Beta(0.5, 92.5) |
| AEs | ||
| CMV | ||
| Based on adult evidence | ||
| Baseline | 0.258 | Beta(41, 118) |
| Maintenance agents (vs. no mTOR-I) [ln(OR)] | Multivariate normal | |
| mTOR-I replacing CNI | –0.798 | |
| mTOR-I replacing antimetabolite | –1.153 | |
| Grenda et al.75 | ||
| TAC + AZA | 0.022 | Beta(2, 91) |
| BAS + TAC + AZA | 0.071 | Beta(7, 92) |
| Offner et al.73 | ||
| BAS + CSA + MMF | 0.128 | Beta(14, 95) |
| CSA + MMF | 0.086 | Beta(8, 85) |
| Dyslipidaemia | ||
| Based on adult evidence | ||
| Baseline | 0.555 | Beta(313, 251) |
| Maintenance agents (vs. no mTOR-I) [ln(OR)] | ||
| mTOR-I | 0.557 | Normal(0.557, 0.100) |
| PTLD | ||
| Trompeter et al.77 | ||
| TAC + AZA | 0.029 | Beta(3, 100) |
| CSA + AZA | 0.032 | Beta(3, 90) |
| Grenda et al.75 | ||
| TAC + AZA | 0.022 | Beta(2, 91) |
| BAS + TAC + AZA | 0.010 | Beta(1, 98) |
| Offner et al.73 | ||
| BAS + CSA + MMF | 0.028 | Beta(3, 106) |
| CSA + MMF | 0.054 | Beta(5, 88) |
| Toxic nephropathy | ||
| Grenda et al.75 | ||
| TAC + AZA | 0.043 | Beta(4, 89) |
| BAS + TAC + AZA | 0.141 | Beta(14, 85) |
| Abdominal pain | ||
| Grenda et al.75 | ||
| TAC + AZA | 0.022 | Beta(2, 91) |
| BAS + TAC + AZA | 0.111 | Beta(11, 88) |
| DGF | ||
| Grenda et al.75 | ||
| TAC + AZA | 0.054 | Beta(5, 88) |
| BAS + TAC + AZA | 0.111 | Beta(11, 88) |
| Hypertension | ||
| Trompeter et al.77 | ||
| TAC + AZA | 0.883 | Beta(91, 12) |
| CSA + AZA | 0.871 | Beta(81, 12) |
| Hypomagnesaemia | ||
| Trompeter et al.77 | ||
| TAC + AZA | 0.408 | Beta(42, 61) |
| CSA + AZA | 0.226 | Beta(21, 72) |
| Anaemia | ||
| Based on adult evidence | 0.052 | Beta(207, 3762) |
| Retransplantation | ||
| Probability of pre-emptive retransplantation on loss of first graft | 0.2 | Beta(3, 12) |
| Rate of retransplantation (by age) | ||
| < 18 years (HR) | 3.422 | Normal(3.422, 0.397) |
| 18–64 years | 0.104 | Normal(0.104, 0.0023) |
| (Rate declines after 65 years) | ||
| Baseline rate of DWFG (subsequent grafts) | 0.0078 | Log-normal(–4.853, 0.472) |
| Baseline rate of graft loss (subsequent grafts) | 0.0359 | Log-normal(–3.327, 0.084) |
| Mortality | ||
| Rate of death on dialysis following graft loss (by age in years) | ||
| 0–17 | 0.034 | Normal(0.034, 0.010) |
| 18–24 | 0.010 | Normal(0.010, 0.003) |
| 25–29 | 0.012 | Normal(0.012, 0.003) |
| 30–34 | 0.009 | Normal(0.009, 0.002) |
| 35–39 | 0.015 | Normal(0.015, 0.002) |
| 40–44 | 0.021 | Normal(0.021, 0.002) |
| 45–49 | 0.027 | Normal(0.027, 0.002) |
| 50–54 | 0.041 | Normal(0.041, 0.003) |
| 55–59 | 0.053 | Normal(0.053, 0.003) |
| 60–64 | 0.079 | Normal(0.079, 0.004) |
| 65–69 | 0.107 | Normal(0.107, 0.005) |
| Other natural history parameters | ||
| Probability of PNF | ||
| DBD | 0.014 | Beta(21, 1456) |
| Living related | 0.019 | Beta(15, 755) |
| Proportion of NODAT in first 6 months | 0.75 | Beta(75, 25) |
| Risk stratification for CMV infection | Dirichlet(54, 84, 71) | |
| High risk (D+/R–) | 0.258 | |
| Intermediate risk (D±/R+) | 0.402 | |
| Low risk (D–/R–) | 0.340 | |
| Risk stratification for EBV infection | Dirichlet(28, 48, 6) | |
| High risk (D+/R–) | 0.341 | |
| Intermediate risk (D±/R+) | 0.585 | |
| Low risk (D–/R–) | 0.073 | |
| Utilities | ||
| Baseline utility | Multivariate normal | |
| Constant | 0.9679812 | |
| Coefficient for age | –0.001807 | |
| Coefficient for age2 | –0.00000971 | |
| Coefficient for sex (male) | 0.0232887 | |
| Disutilities | ||
| Functioning graft | 0.053 | Gamma(1.179, 0.045) |
| Haemodialysis | 0.277 | Gamma(66.90, 0.004) |
| Peritoneal dialysis | 0.264 | Gamma(35.73, 0.007) |
| Resource use | ||
| Induction therapy | ||
| BAS (10 mg if weight < 35 kg; 20 mg if weight ≥ 35 kg) | 1.964 | 1 + beta(95, 4) |
| R-ATG drug acquisition (mg/kg) | 6.5 | Normal(6.5, 0.126) |
| R-ATG i.v. administration | 4.525 | Normal(4.525, 0.079) |
| Maintenance therapy | ||
| See Table 87 | Unless SE reported or could be calculated, a log-normal distribution was fitted using the method of moments and assuming coefficient of variation of 10% with following exceptions: coefficient of variation = 50% for TAC-PR vs. TAC resource use coefficient of variation = 2% for BEL resource use |
|
| Trompeter et al.77 | ||
| TAC (with AZA) (mg/m2/day) | X1 ∼ normal(8.80, 0.240) X2 ∼ normal(6.33, 0.292) X3 ∼ normal(4.89, 0.329) |
|
| 0–6 months | 7.565 | (X1 +X2)/2 |
| 6–12 months | 5.610 | (X2 +X3)/2 |
| Thereafter | 4.890 | X3 |
| CSA (with AZA) (mg/m2/day) | X1 ∼ normal(299.4, 10.4) X2 ∼ normal(203.3, 5.1) X3 ∼ normal(180.0, 6.6) |
|
| 0–6 months | 251.35 | (X1 +X2)/2 |
| 6–12 months | 191.65 | (X2 +X3)/2 |
| Thereafter | 180.00 | X3 |
| AZA (mg/kg/day) | 1.80 | Normal(1.80, 0.04) |
| Prednisolone (mg/kg/day) | X1 ∼ normal(3.9, 0.19) X2 ∼ normal(4.5, 0.37) X3 ∼ normal(0.3, 0.02) |
|
| 0–6 months (with TAC) | 2.1 | (X1 +X3)/2 |
| 0–6 months (with CSA) | 2.4 | (X2 +X3)/2 |
| Thereafter (with TAC or CSA) | 0.3 | X3 |
| Grenda et al.75 | ||
| TAC (with MMF) (mg/kg/day) | ||
| Throughout (prepubertal) | 0.180 | Normal(0.180, 0.014) |
| Throughout (pubertal) | 0.130 | Normal(0.130, 0.010) |
| MMF (with TAC) (g/m2/day) | ||
| Throughout (prepubertal) | 0.54 | Normal(0.54, 0.002) |
| Throughout (pubertal) | 0.60 | Normal(0.60, 0.003) |
| Offner et al.73 | ||
| CSA (with BAS+MMF) (mg/kg/day) | ||
| 0–3 months | 7.80 | Normal(7.80, 0.34) |
| 3–6 months | 7.15 | Normal(7.15, 0.33) |
| 6–12 months | 6.65 | Normal(6.65, 0.29) |
| Thereafter | 6.20 | Normal(6.20, 0.27) |
| CSA (with MMF) (mg/kg/day) | ||
| 0–3 months | 7.67 | Normal(7.67, 0.34) |
| 3–6 months | 6.85 | Normal(6.85, 0.30) |
| 6–12 months | 6.20 | Normal(6.20, 0.28) |
| Thereafter | 5.90 | Normal(5.90, 0.26) |
| MMF (with BAS+CSA) (g/m2/day) | X1 ∼ normal(1.06, 0.03) X2 ∼ normal(1.06, 0.03) X3 ∼ normal(0.96, 0.04) X4 ∼ normal(0.93, 0.04) |
|
| 0–3 months | 1.06 | (X1 + 2×X2)/3 |
| 3–6 months | 1.01 | (X2 +X3)/2 |
| 6–12 months | 0.95 | (X3 +X4)/2 |
| Thereafter | 0.93 | X4 |
| MMF (with CSA) (g/m2/day) | X1 ∼ normal(1.11, 0.03) X2 ∼ normal(1.00, 0.04) X3 ∼ normal(0.85, 0.04) X4 ∼ normal(0.82, 0.04) |
|
| 0–3 months | 1.04 | (X1 + 2×X2)/3 |
| 3–6 months | 0.93 | (X2 +X3)/2 |
| 6–12 months | 0.83 | (X3 +X4)/2 |
| Thereafter | 0.82 | X4 |
| Graft loss | ||
| Proportion of failed grafts explanted by time since transplantation | ||
| 0–3 months | 0.41 | Beta(1.95, 2.81) |
| 3–12 months | 0.23 | Beta(2.85, 9.54) |
| 12–24 months | 0.09 | Beta(3.55, 35.9) |
| 24+ months | 0.04 | Beta(3.80, 91.2) |
| Proportion of failed grafts explanted (subsequent grafts) | 0.056 | Linear combination of above |
| Subsequent transplantation | ||
| Workup for retransplantation | 1.44 | Normal(3423, 58.5)/2370 |
| Living donor costs | 0.349 | Beta(826, 1544) |
| Deceased donor costs | 0.651 | 1 minus above |
| Maintenance immunosuppression | ||
| TAC (mg/kg/day) | 0.1 | Log-normal(–2.31, 0.1) |
| MMF (g/day) | 2 | Log-normal(0.688, 0.1) |
| Prednisolone (mg/day) | 16.3 | Log-normal(2.79, 0.1) |
| Infection prophylaxis | ||
| Co-trimoxazole (PJP and UTI prophylaxis): Septrin (480-mg tablets in first three months) | 90 | Log-normal(4.49, 0.1) |
| Valganciclovir (CMV prophylaxis) (proportion of affected patients multiplied by time) | ||
| Full dose 0–3 months | 1 | Not varied |
| Half dose 3–6 months | 0.3 | Beta(3, 7) |
| Full dose 3–6 months | 0.16 | Beta(1.6, 8.4) |
| Valganciclovir dosage according to target dose | (Daily only/alternate days allowed) | |
| 0–337.5 | 450/225 | |
| 337.5–675 | 450/450 | |
| 675+ | 900/900 | |
| GFR for target dose calculation | 80 | Normal(80, 2) |
| AR | ||
| Expected number of AREs per patient experiencing 1 + ARE | 1.193 | Normal(136, 11.7)/114 |
| CMV infection treatment | ||
| Expected number of CMV infections per patient experiencing 1 + CMV infection | 1 | Not varied |
| Diabetes mellitus | ||
| Antidiabetic medication: metformin 500-mg tablets per 3 months | 273.9 | Log-normal(5.61, 0.1) |
| Complications (inpatient) | 0.25 | Not varied |
| Complications (non-inpatient) | 0.25 | Not varied |
| Dyslipidaemia | ||
| Statins (mg per cycle per affected patient) | ||
| Fluvastatin | 2191 | Log-normal(7.66, 0.25) |
| Pravastatin | 548 | Log-normal(6.28, 0.25) |
| Simvastatin | 91 | Log-normal(4.48, 0.25) |
| Medical management (attendances per cycle per affected patient) | ||
| Dietetics outpatients | 0.25 | Log-normal(–1.42, 0.25) |
| GP | 0.25 | Log-normal(–1.42, 0.25) |
| Anaemia | ||
| Proportion requiring ESA treatment | 0.052 | Beta(207, 3762) |
| Mean weekly dose | 5.832 | Normal(5.832, 0.067) |
| Monitoring | ||
| Clinics (first 3 months) | 26.1 | Log-normal(3.26, 0.05) |
| Blood tests (first 3 months) | 26.1 | Log-normal(3.26, 0.05) |
| Clinics + bloods (per cycle) | ||
| 3–6 months | 6.5 | Log-normal(1.87, 0.1) |
| 6–12 months | 3 | Log-normal(1.09, 0.1) |
| 12–24 months | 3 | Log-normal(1.09, 0.1) |
| 24–36 months | 2 | Log-normal(0.69, 0.1) |
| 36 + months | 1 | Log-normal(1.87, 0.1) |
| Subsequent grafts | 3 | Log-normal(1.07, 0.25) |
| Viral PCR | ||
| 0–3 months (CMV) (if no r-ATG) | 6.02 | Log-normal(1.76, 0.25) |
| 0–3 months (CMV) (with r-ATG) | 1.98 | Log-normal(0.65, 0.25) |
| 3–6 months (CMV) | 0.26 | Log-normal(–1.38, 0.25) |
| 0–6 months (BKV) | 1 | Log-normal(–0.03, 0.25) |
| 6–12 months (BKV) | 0.5 | Log-normal(–0.72, 0.25) |
| 0–6 months (EBV) | 1.02 | Log-normal(–0.01, 0.25) |
| 6–12 months (EBV) | 0.34 | Log-normal(–1.10, 0.25) |
| Viral serology (per cycle) | ||
| 0–3 months (CMV) | 0.26 | Log-normal(–1.38, 0.25) |
| At 12 and 24 months (CMV) | 0.60 | Log-normal(–0.54, 0.25) |
| At 36, 48 and 60 months (CMV) | 0.34 | Log-normal(–1.11, 0.25) |
| Dialysis | ||
| Proportion of dialysis patients receiving haemodialysis (by age in years) | ||
| 0–1 | 0.455 | Beta(10, 12) |
| 2–3 | 0.464 | Beta(13, 15) |
| 4–7 | 0.556 | Beta(15, 12) |
| 8–11 | 0.645 | Beta(20, 11) |
| 12–15 | 0.705 | Beta(31, 13) |
| 16–17 | 0.625 | Beta(15, 9) |
| 18–24 | 0.791 | Beta(276, 73) |
| 25–34 | 0.804 | Beta(913, 223) |
| 35–44 | 0.845 | Beta(1853, 340) |
| 45–54 | 0.843 | Beta(3358, 624) |
| 55–64 | 0.852 | Beta(4408, 768) |
| 65–74 | 0.858 | Beta(5824, 967) |
| 75–84 | 0.890 | Beta(5533, 681) |
| 85 + | 0.915 | Beta(1246, 116) |
| Access surgery | ||
| Temporary access (for HD) | 1 | Not varied |
| Long-term access (for HD) | 1 | Not varied |
| Long-term access (for PD) | 1 | Not varied |
| Unit costs | ||
| Dialysis | ||
| Access surgery | ||
| Long-term access for HD | £1946 | Normal(1946, 98) |
| Temporary access for HD (years) | ||
| < 19 | £1747 | Normal(1747, 113) |
| ≥ 19 | £823 | Normal(823, 40) |
| Long-term access for PD | £1101 | Normal(1101, 120) |
| Ongoing costs (per cycle) | ||
| Haemodialysis | ||
| < 19 | £20,278 | Normal(20,278, 3134) |
| ≥ 19 | £6093 | Normal(6093, 164) |
| Peritoneal dialysis | ||
| < 19 | £10,515 | Normal(10,515, 881) |
| ≥ 19 | £6000 | Normal(6000, 183) |
| Induction agents | ||
| BAS and r-ATG | See Table 96 | Not varied |
| Maintenance agents | ||
| TAC-PR, MPS, SRL, EVL and BEL | See Table 96 | Not varied |
| TAC-IR, CSA, MMF, AZA and prednisolone | See Table 97 | Mixture models |
| AR treatment | ||
| AR (per episode) | £3557 | Log-normal(8.15, 0.25) |
| Spontaneously resolving | £145 | Log-normal(4.97, 0.1) |
| Steroid sensitive | £1274 | Log-normal(7.14, 0.1) |
| Steroid resistant (medical management) | £3456 | Log-normal(8.12, 0.25) |
| Steroid resistant (drug acquisition per kg) | £44.46 | Log-normal(0.64, 0.25) |
| Infection prophylaxis | ||
| Septrin (per 480-mg tablet) | £0.16 | Not varied |
| Valcyte (per 450-mg tablet) | £18.02 | Not varied |
| Infection treatment | ||
| CMV infection | £3,009 | Log-normal(7.98, 0.25) |
| Anaemia | ||
| Binocrit (per 1000 IU) | £4.33 | Not varied |
| Diabetes mellitus | ||
| Metformin (per 500-mg tablet) | £0.0054 | Normal(0.0054, 0.00001) |
| Complications (annual cost) | ||
| Inpatient | 1389 | Normal(1389, 99) |
| Non-inpatient | 695 | Normal(695, 19) |
| Dyslipidaemia | ||
| Statins (per mg) | ||
| Fluvastatin | £0.0022 | Mixture model |
| Pravastatin | £0.0026 | Mixture model |
| Simvastatin | £0.0003 | Mixture model |
| Medical management | ||
| Dietetics | £62.70 | Normal(62.70, 2.76) |
| GP | £50.82 | Normal(50.82, 5.38) |
| PTLD | ||
| MabThera (per mg) | £1.75 | Not varied |
| Hypertension | ||
| Amlodipine (per mg) | £0.0071 | Not varied |
| Bendroflumethiazide (per 2.5-mg tablet) | £0.0344 | Not varied |
| Captopril (per mg) | £0.0035 | Not varied |
| Hypomagnesaemia | ||
| Magnaspartate (per sachet) | £0.80 | Not varied |
| Drug administration | ||
| i.v. infusion (first) | £228.95 | Normal(228.95, 15.54) |
| i.v. infusion (subsequent) | £325.59 | Normal(325.59, 45.74) |
| Monitoring | ||
| Clinic | £145 | Log-normal(4.97, 0.1) |
| Viral PCR (CMV, EBV, BKV) | £46.75 | Log-normal(3.81, 0.25) |
| CMV serology | £18.29 | Log-normal(2.88, 0.25) |
| Therapeutic drug monitoring (CSA, TAC, SRL, EVL) | £26.71 | Log-normal(3.25, 0.25) |
| Full blood count | £5.05 | Log-normal(1.62, 0.1) |
| Renal profile | £4.54 | Log-normal(1.51, 0.1) |
| Liver profile | £4.64 | Log-normal(1.53, 0.1) |
| Explant | ||
| < 19 | £4829 | Normal(4829, 483) |
| ≥ 19 | £4966 | Normal(4966, 497) |
| Subsequent retransplantation | ||
| Recipient workup | ||
| < 19 | £505 | Normal(505, 50) |
| ≥ 19 | £849 | Normal(849, 84) |
| Living donor costs | £8914 | Normal(8914, 891) |
| Deceased donor costs | £10,142 | Normal(10142, 1014) |
| Transplant surgery | ||
| < 19 | £20,913 | Normal(20913, 2091) |
| ≥ 19 | £16,030 | Normal(16030, 1603) |
Appendix 8 Comparison of the Peninsula Technology Assessment Group, Astellas and previous Assessment Group’s model-based analyses
| Cost parameter | Previous Assessment Group model2 (£) | PenTAGa (£) | Astellasb (£) |
|---|---|---|---|
| TAC therapy (per year) | (£1.70/mg) 3909 | With MMF
|
1559 (first year) 1366 (second year) |
| TAC administration | 0 | 1031 (first year) 321 (second year) 214 (third year) 107 (fourth year +) |
0 |
| MMF therapy (per year) | 2737 | With TAC
|
1326 |
| CSA therapy (per year) | 1368 | With MMF
|
N/A |
| CSA administration | 0 | 1031 (first year) 321 (second year) 214 (third year) 107 (fourth year+) |
N/Ac |
| BEL (per year) | N/A | 7276 (first year) 4624 (thereafter for weight ≤ 50 kg) 9249 (thereafter for weight > 50 kg) |
4018 (first year) 2374 (second year+) |
| BEL administration | N/A | 4632 (first year) 4247 (thereafter) |
0 |
| CCSs | 0 | 46 (first year) 13–20 (thereafter) |
176 (first year) 139 (second year+) |
| AR (event) | 4644 | 3557 (4244 per patient experiencing AR) | 2536 (first year) 2522 (second year+) |
| Dialysis (per year) | 21,060 | < 19 years
|
0 |
| Retransplantation | N/A | < 19 years
|
5086 |
| Retransplantation: organ procurement | N/A | 9714 | 0 |
| Effectiveness parameter | Previous Assessment Group model2 | PenTAG | Astellas |
|---|---|---|---|
| Time to graft failure (median) | NR | (To nearest 0.25 years, excluding DWFG) CSA + MMF: 14.00 TAC + MMF: 17.50 CSA + AZA: 12.00 TAC + AZA: 18.75 CSA + EVL: 16.25 TAC + SRL: 12.75 TAC-PR + MMF: 17.25 BAS + CSA + MMF: 16.50 BAS + TAC + MMF: 21.00 BAS + CSA + AZA: 14.50 BAS + TAC + AZA: 22.75 BAS + SRL + MMF: 18.00 BAS + BEL + MMF: 24.25 BAS + CSA + MPS: 19.25 r-ATG + CSA + MMF: 15.75 r-ATG + TAC + MMF: 19.50 r-ATG + CSA + AZA: 13.75 r-ATG + TAC + AZA: 21.50 |
Time to 15% failure (median not achieved within model horizon) Without BCAR at 12 months: 7 years With BCAR at 12 months: 6 yearsa |
| Time to transplantation from graft failure (mean unless otherwise stated) | NR | Mean time to transplantation or death following failure of initial graft: 4.86 years (range 4.39–5.17 years) | 3.5 years (median) |
| Annual change in GFR | N/A | N/A | N/A |
| Utility of functioning graft –first transplant | 0.84 (NR, assumed is same as Woodroffe et al.255) |
0.909 (age 10 years) 0.888 (age 20 years) 0.866 (age 30 years) 0.841 (age 40 years) 0.815 (age 50 years) 0.786 (age 60 years) |
0.712 |
| Utility of functioning graft –second + transplants | 0.84 (NR, assumed is same as Woodroffe et al.255) |
As first | 0.712 |
| Utility of dialysis state | 0.65 (NR, assumed is same as Woodroffe et al.255) |
0.691 (age 10 years) 0.668 (age 20 years) 0.645 (age 30 years) 0.619 (age 40 years) 0.592 (age 50 years) 0.564 (age 60 years) |
0.483 |
| Model | Regimens compared | Functioning first graft (years) | Functioning graft (years) | Years with graft loss/dialysis | Life-years | QALYsa | Costs (£)a | ICER incremental cost per QALY |
|---|---|---|---|---|---|---|---|---|
| Astellas | TAC (+ MMF + CCS) SRL I (+ MMF + CCS) EVL (+ MMF + CCS) SRL II (+ MMF + CCS) BEL (+ MMF + CCS) |
NR | NR | NR | 9.472 9.468 9.467 9.456 9.455 |
5.569 5.565 5.564 5.553 5.551 |
58,471 52,339 90,168 61,490 75,726 |
TAC TD vs. SRL I: £1,576,937 (other options are dominated by TAC TD) |
| TAC TDb (+ MMF + CCS) TAC ODb (+ MMF + CCS) |
NR | NR | NR | 9.472 9.502 |
5.569 5.604 |
58,471 53,395 |
TAC OD dominates | |
| Assessment Group (PenTAG) | TAC (+ MMF) TAC (+ BAS + MMF) SRL I (+ BAS + MMF) BEL (+ BAS + MMF) CSA (+ AZA) CSA (+ MMF) TAC (+ AZA) TAC-PR (+ MMF) |
19.94 22.45 20.38 24.62 14.80 16.79 20.91 19.68 |
35.35 36.29 35.53 37.24 33.67 34.32 35.77 35.21 |
8.14 7.46 8.00 6.89 9.46 9.01 7.82 8.17 |
43.49 43.75 43.53 44.12 43.13 43.33 43.59 43.38 |
18.21 18.36 18.24 18.59 17.98 18.10 18.27 18.15 |
182,163 170,915 199,144 324,708 212,626 202,424 177,360 198,433 |
CSA vs. TAC: TAC dominates AZA vs. MMF: AZA dominates (with TAC) MMF dominates (with CSA) SRL vs. TAC: TAC dominates BEL vs. TAC: £667,031 |
Appendix 9 Additional results from the Peninsula Technology Assessment Group model
| Regimen | Induction (first graft) | Maintenance immunosuppression (first graft) | AR (first graft) | Graft loss (first graft) | Infection prophylaxis (first graft) | CMV infection (first graft) | Monitoring (first graft) |
|---|---|---|---|---|---|---|---|
| CSA + MMF | £0 | £17,911 | £1128 | £164 | £550 | £760 | £17,101 |
| TAC + MMF | £0 | £16,460 | £994 | £146 | £550 | £760 | £18,176 |
| CSA + AZA | £0 | £14,302 | £1834 | £189 | £547 | £760 | £16,307 |
| TAC + AZA | £0 | £15,517 | £1302 | £170 | £546 | £760 | £18,335 |
| CSA + EVL | £0 | £88,084 | £1102 | £147 | £551 | £291 | £20,309 |
| TAC + SRL | £0 | £28,250 | £967 | £195 | £545 | £291 | £18,809 |
| TAC-PR + MMF | £0 | £31,223 | £976 | £155 | £549 | £760 | £18,032 |
| BAS + CSA + MMF | £2019 | £19,892 | £675 | £147 | £551 | £760 | £17,845 |
| BAS + TAC + MMF | £2019 | £17,594 | £585 | £129 | £552 | £760 | £18,902 |
| BAS + CSA + AZA | £2019 | £15,552 | £1211 | £168 | £549 | £760 | £17,118 |
| BAS + TAC + AZA | £2019 | £16,586 | £798 | £148 | £548 | £760 | £19,132 |
| BAS + SRL + MMF | £2019 | £34,225 | £633 | £155 | £548 | £399 | £18,141 |
| BAS + BEL + MMF | £2019 | £155,131 | £1034 | £117 | £553 | £760 | £16,891 |
| BAS + CSA + MPS | £2019 | £39,558 | £930 | £132 | £552 | £760 | £18,595 |
| r-ATG + CSA + MMF | £2676 | £17,820 | £494 | £158 | £1194 | £760 | £16,579 |
| r-ATG + TAC + MMF | £2676 | £15,744 | £425 | £142 | £1194 | £760 | £17,584 |
| r-ATG + CSA + AZA | £2676 | £13,952 | £925 | £178 | £1190 | £760 | £15,948 |
| r-ATG + TAC + AZA | £2676 | £15,045 | £590 | £159 | £1187 | £760 | £17,863 |
| Regimen | Retransplantation | Immunosuppression (subsequent grafts) | Monitoring (subsequent grafts) | Graft loss (subsequent grafts) | Dialysis | NODAT | Anaemia | Dyslipidaemia |
|---|---|---|---|---|---|---|---|---|
| CSA + MMF | £16,885 | £9842 | £14,995 | £68 | £116,778 | £813 | £1238 | £1677 |
| TAC + MMF | £14,706 | £8493 | £12,939 | £59 | £101,680 | £1802 | £1272 | £1681 |
| CSA + AZA | £18,376 | £10,766 | £16,414 | £75 | £126,832 | £810 | £1214 | £1671 |
| TAC + AZA | £14,305 | £8235 | £12,570 | £57 | £98,419 | £1805 | £1283 | £1683 |
| CSA + EVL | £15,418 | £8936 | £13,610 | £62 | £106,710 | £774 | £1260 | £2074 |
| TAC + SRL | £17,837 | £10,414 | £15,887 | £72 | £122,944 | £2806 | £1221 | £2061 |
| TAC-PR + MMF | £14,882 | £8597 | £13,105 | £60 | £102,768 | £2115 | £1267 | £1677 |
| BAS + CSA + MMF | £15,467 | £8966 | £13,654 | £62 | £107,059 | £816 | £1261 | £1682 |
| BAS + TAC + MMF | £13,324 | £7653 | £11,656 | £53 | £92,165 | £1808 | £1296 | £1686 |
| BAS + CSA + AZA | £16,827 | £9803 | £14,939 | £68 | £116,300 | £813 | £1239 | £1677 |
| BAS + TAC + AZA | £12,804 | £7330 | £11,186 | £51 | £88,144 | £1811 | £1310 | £1689 |
| BAS + SRL + MMF | £14,707 | £8490 | £12,944 | £59 | £101,528 | £1437 | £1272 | £2074 |
| BAS + BEL + MMF | £12,101 | £6918 | £10,537 | £48 | £83,688 | £354 | £1325 | £1698 |
| BAS + CSA + MPS | £14,072 | £8111 | £12,350 | £56 | £97,426 | £765 | £1287 | £1690 |
| r-ATG + CSA + MMF | £17,617 | £10,307 | £15,694 | £71 | £122,014 | £812 | £1225 | £1675 |
| r-ATG + TAC + MMF | £15,581 | £9037 | £13,758 | £63 | £107,937 | £1801 | £1256 | £1679 |
| r-ATG + CSA + AZA | £18,810 | £11,048 | £16,833 | £77 | £130,031 | £810 | £1206 | £1670 |
| r-ATG + TAC + AZA | £14,966 | £8646 | £13,182 | £60 | £103,299 | £1804 | £1270 | £1682 |
| Regimen | Life expectancy | Undiscounted life-years with functioning graft | Undiscounted life-years on dialysis | AR within 12 months | NODAT within 12 months | Proportion receiving second transplant | Proportion receiving third transplant | Proportion receiving fourth transplant |
|---|---|---|---|---|---|---|---|---|
| CSA + MMF | 43.37 | 34.44 | 8.93 | 0.271 | 0.018 | 0.780 | 0.326 | 0.081 |
| TAC + MMF | 43.55 | 35.50 | 8.05 | 0.239 | 0.040 | 0.715 | 0.284 | 0.069 |
| CSA + AZA | 43.17 | 33.78 | 9.39 | 0.441 | 0.018 | 0.812 | 0.352 | 0.090 |
| TAC + AZA | 43.65 | 35.92 | 7.72 | 0.313 | 0.040 | 0.691 | 0.274 | 0.067 |
| CSA + EVL | 43.50 | 35.12 | 8.38 | 0.265 | 0.017 | 0.739 | 0.299 | 0.073 |
| TAC + SRL | 43.14 | 33.98 | 9.16 | 0.232 | 0.063 | 0.797 | 0.341 | 0.087 |
| TAC-PR + MMF | 43.45 | 35.37 | 8.08 | 0.235 | 0.048 | 0.717 | 0.287 | 0.070 |
| BAS + CSA + MMF | 43.57 | 35.16 | 8.41 | 0.162 | 0.018 | 0.741 | 0.300 | 0.073 |
| BAS + TAC + MMF | 43.75 | 36.29 | 7.46 | 0.141 | 0.040 | 0.669 | 0.258 | 0.062 |
| BAS + CSA + AZA | 43.38 | 34.49 | 8.89 | 0.291 | 0.018 | 0.777 | 0.324 | 0.081 |
| BAS + TAC + AZA | 43.86 | 36.78 | 7.07 | 0.192 | 0.040 | 0.638 | 0.245 | 0.059 |
| BAS + SRL + MMF | 43.53 | 35.53 | 8.00 | 0.152 | 0.032 | 0.711 | 0.283 | 0.069 |
| BAS + BEL + MMF | 44.12 | 37.24 | 6.89 | 0.249 | 0.008 | 0.623 | 0.234 | 0.055 |
| BAS + CSA + MPS | 43.81 | 35.98 | 7.83 | 0.224 | 0.017 | 0.698 | 0.273 | 0.066 |
| r-ATG + CSA + MMF | 43.29 | 34.06 | 9.24 | 0.119 | 0.018 | 0.801 | 0.341 | 0.086 |
| r-ATG + TAC + MMF | 43.47 | 34.99 | 8.47 | 0.102 | 0.040 | 0.746 | 0.302 | 0.074 |
| r-ATG + CSA + AZA | 43.14 | 33.56 | 9.58 | 0.222 | 0.018 | 0.824 | 0.361 | 0.093 |
| r-ATG + TAC + AZA | 43.58 | 35.47 | 8.11 | 0.142 | 0.040 | 0.720 | 0.288 | 0.071 |
| Regimen | Total discounted costs | Total discounted QALYs | ||||
|---|---|---|---|---|---|---|
| Base case | Scenario 1 | Scenario 2 | Base case | Scenario 1 | Scenario 2 | |
| CSA + MMF | £199,910 | £197,252 | £195,685 | 18.1269 | 18.1554 | 18.1269 |
| TAC + MMF | £179,719 | £178,138 | £175,974 | 18.2398 | 18.2569 | 18.2398 |
| CSA + AZA | £210,097 | £202,041 | £206,583 | 18.0031 | 18.0868 | 18.0031 |
| TAC + AZA | £174,989 | £171,128 | £171,604 | 18.2970 | 18.3387 | 18.2970 |
| CSA + EVL | £259,327 | £258,260 | £242,849 | 18.2209 | 18.2474 | 18.2209 |
| TAC + SRL | £222,300 | £221,113 | £215,947 | 17.9553 | 17.9696 | 17.9553 |
| TAC-PR + MMF | £196,165 | £194,866 | £189,774 | 18.1854 | 18.2008 | 18.1854 |
| BAS + CSA + MMF | £190,856 | £191,872 | £186,442 | 18.2468 | 18.2358 | 18.2468 |
| BAS + TAC + MMF | £170,182 | £171,903 | £166,389 | 18.3596 | 18.3407 | 18.3596 |
| BAS + CSA + AZA | £199,042 | £195,682 | £195,463 | 18.1308 | 18.1663 | 18.1308 |
| BAS + TAC + AZA | £164,316 | £164,316 | £160,885 | 18.4259 | 18.4259 | 18.4259 |
| BAS + SRL + MMF | £198,631 | £199,792 | £191,707 | 18.2423 | 18.2277 | 18.2423 |
| BAS + BEL + MMF | £293,175 | £292,935 | £283,749 | 18.5901 | 18.6097 | 18.5901 |
| BAS + CSA + MPS | £198,303 | £197,393 | £190,327 | 18.3907 | 18.4023 | 18.3907 |
| r-ATG + CSA + MMF | £209,097 | £211,660 | £204,555 | 18.0702 | 18.0432 | 18.0702 |
| r-ATG + TAC + MMF | £189,637 | £192,841 | £185,673 | 18.1763 | 18.1422 | 18.1763 |
| r-ATG + CSA + AZA | £216,114 | £215,070 | £212,374 | 17.9721 | 17.9827 | 17.9721 |
| r-ATG + TAC + AZA | £183,191 | £184,933 | £179,583 | 18.2468 | 18.2283 | 18.2468 |
Appendix 10 UK Transplant Registry standard national organ transplant data set
The UK Transplant Registry maintains a standard data set, which is available on request without the need for prior approval (URL: www.odt.nhs.uk/uk-transplant-registry/data/; Cathy Hopkinson, Statistics and Clinical Studies, NHSBT, 15 October 2014, personal communication). The data set contains details of all solid organ transplants (kidney, liver, pancreas, intestine, heart, lung and multiorgan) between 1995 and 2012. The data set contains limited information about the donor, recipient and match between them.
Key variables in the data set which have been used in analyses supporting the economic modelling:
-
RECIP_ID – allows subsequent retransplantations to be identified and graft number to be estimated
-
DTYPE (DBD; DCD; living related; living unrelated; domino; living – relationship unspecified; living unrelated – pooled; living unrelated – altruistic) – classification of donor type (it was assumed that relationships from domino onwards are living unrelated)
-
RAGE_GRP (< 18; 18–30; 31–50; 51–60; 61–70; > 70) – recipient age group
-
RSEX (male; female) – recipient sex
-
TY_YR (1995; 1996; . . .; 2012) – transplant year
-
TX_TYPE (kidney only; . . .) – used to restrict to kidney-only transplants
-
KID_GSURV – kidney (graft) survival (days since transplantation)
-
KID_GCENS – 0 if graft survival was censored; 1 if graft failed
-
KID_PSURV – patient survival following kidney transplant (days since transplantation)
-
KID_PCENS – 0 if patient survival was censored; 1 if patient died.
Glossary
- Acute rejection
- Process by which the graft recipient’s immune system attempts to destroy the graft, usually within the first 3 months of transplantation.
- Cadaveric transplant
- A transplant kidney removed from someone who has died.
- Calcineurin inhibitor
- Ciclosporin or tacrolimus.
- Cytomegalovirus
- A virus that normally causes only a mild ‘flu-like’ illness. In people with a kidney transplant, cytomegalovirus can cause a more serious illness, affecting the lungs, liver and blood.
- Donation after brain death
- A donation from people in whom the heart is still beating after brain death has occurred (heart-beating donors). Most, but not all, cadaveric transplants. The extended criteria donor kidneys include donations from heart-beating donors who would not normally meet the criteria for transplantation and are likely to have a lower chance of long-term success.
- Donation after circulatory death
- A donation from people who cannot be diagnosed as brainstem dead but whose death is verified by the absence of a heart beat (non-heart-beating donors).
- Donor
- A person who donates an organ to another person (the recipient).
- Glomerular filtration rate
- Flow rate of filtered fluid through the kidney, measured directly by injecting a harmless chemical (e.g. inulin) into the blood and then measuring how much of the chemical is filtered in a given unit of time.
- Graft function
- A measure of the efficiency of the graft by various markers, for example glomerular filtration rate and serum creatinine levels.
- Graft loss
- Absence of kidney function occurring any time after transplantation requiring chronic dialysis and/or retransplantation (excluding loss caused by death).
- Haemodialysis
- Removal of waste products by passing blood out of the body, through a filtering system (dialyser) and then back to the body.
- 1-Haplotype identical
- Human leucocyte antigens are inherited as a set called a ‘haplotype’ from one or both parents. 1-Haplotype identical is not a ‘perfect’ human leucocyte antigen match; a 2-haplotype identical is a perfect human leucocyte antigen match.
- Living related transplant
- A kidney donated by a living relative of the recipient. A well-matched living related transplant is likely to last longer than either a living unrelated transplant or a cadaveric transplant.
- Living unrelated transplant
- A kidney transplant from a living person who is biologically unrelated to the recipient.
- Mycophenolic acid
- Mycophenolate mofetil or mycophenolate sodium.
- Nephritis
- A general term for inflammation of the kidneys. This is also used as an abbreviation for glomerulonephritis.
- OKT3
- A murine monoclonal antibody muromonab-CD3.
- Peritoneal dialysis
- Removal of waste products using the peritoneum as a filter. Dialysis fluid is pumped into the peritoneal cavity and waste products and excess fluid are moved from the blood into the dialysis fluid, which is then drained from the cavity.
- Recipient
- In the context of transplantation, a person who receives an organ from another person (the donor).
- Rejection
- The process whereby a patient’s immune system recognises a transplant kidney as foreign and tries to destroy it. Rejection can be acute or chronic.
- Renal replacement therapy
- Dialysis or kidney transplantation.
List of abbreviations
- AE
- adverse event
- AR
- acute rejection
- AZA
- azathioprine
- BAS
- basiliximab
- BEL
- belatacept
- BKV
- BK virus
- BNF
- British National Formulary
- BPAR
- biopsy-proven acute rejection
- CCS
- corticosteroid
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CHU9D
- Child Health Utility 9 dimensions
- CI
- confidence interval
- CKD
- chronic kidney disease
- CMV
- cytomegalovirus
- CNI
- calcineurin inhibitor
- CRD
- Centre for Reviews and Dissemination
- CSA
- ciclosporin
- CVD
- cardiovascular disease
- DAC
- daclizumab
- DARE
- Database of Abstracts of Reviews of Effect
- DBD
- donation after brain death
- DCD
- donation after circulatory death
- DGF
- delayed graft function
- DIC
- deviance information criterion
- DWFG
- death with functioning graft
- EBV
- Epstein–Barr virus
- eGFR
- estimated glomerular filtration rate
- eMIT
- electronic market information tool
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-Y
- European Quality of Life-5 Dimensions Youth version
- ESA
- erythropoiesis-stimulating agent
- ESRD
- end-stage renal disease
- EVL
- everolimus
- GFR
- glomerular filtration rate
- GP
- general practitioner
- HEED
- Health Economic Evaluations Database
- HLA
- human leucocyte antigen
- HMIC
- Health Management Information Consortium
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRG4
- Healthcare Resource Group version 4
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ISI
- Institute for Scientific Information
- ITT
- intention to treat
- i.v.
- intravenous
- KTR
- kidney transplant recipient
- MMF
- mycophenolate mofetil
- MPA
- mycophenolic acid
- MPS
- mycophenolate sodium
- MTC
- mixed-treatment comparison
- MTOR-I
- mammalian/mechanistic target of rapamycin inhibitor
- NAPRTCS
- North American Pediatric Renal Trials and Collaborative Studies
- NHSBT
- NHS Blood and Transplant
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NODAT
- new onset diabetes mellitus after transplantation
- OR
- odds ratio
- PBO
- placebo
- PCR
- polymerase chain reaction
- PenTAG
- Peninsula Technology Assessment Group
- PJP
- Pneumocystis jirovecii pneumonia
- PNF
- primary non-function
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- PTLD
- post-transplant lymphoproliferative disease
- QALY
- quality-adjusted life-year
- r-ATG
- rabbit antihuman thymocyte immunoglobulin
- RCT
- randomised controlled trial
- RR
- relative risk
- RRT
- renal replacement therapy
- SD
- standard deviation
- SDS
- standard deviation score
- SE
- standard error
- SPC
- summary of product characteristics
- SRL
- sirolimus
- TAC
- tacrolimus
- TAC-IR
- immediate-release tacrolimus
- TAC-PR
- prolonged-release tacrolimus
- UTI
- urinary tract infection
- WMD
- weighted mean difference
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed commercial-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence data removed and replaced by the statement ‘commercial-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.