Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 09/46/01. The protocol was agreed in May 2014. The assessment report began editorial review in April 2015 and was accepted for publication in September 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jason Moore has received sponsorship from Astellas UK for conference attendance.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Jones-Hughes et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the health problem
End-stage renal disease
End-stage renal disease (ESRD) is a long-term irreversible decline in kidney function, for which renal replacement therapy (RRT) is required if the individual is to survive. ESRD is often the result of an acute kidney injury (AKI) or primarily a progression from chronic kidney disease (CKD), which describes abnormal kidney function and/or structure. CKD is common, frequently unrecognised and often exists together with other conditions [e.g. cardiovascular disease (CVD) and diabetes mellitus]. An estimated 4% of people in the UK with CKD progress to ESRD over a 5.5-year follow-up period. 1
Although RRT can take a number of forms [kidney transplantation, haemodialysis (HD) and peritoneal dialysis (PD)], the preferred option for people with ESRD is kidney transplantation, rather than dialysis. This is as a result of improved duration and quality of life with transplantation compared with dialysis. 2
Transplantation: patient survival, acute rejection and graft loss
Kidney transplantation is the transfer of a healthy kidney from a donor to a recipient. Kidneys for transplantation may be obtained via living donation (related or unrelated), donation after brain death (DBD) (deceased heart-beating donors, who are maintained on a ventilator in an intensive care unit, with death diagnosed using brainstem tests) or donation after circulatory death (DCD) [non-heart-beating donors who cannot be diagnosed as brainstem dead but whose death is verified by the absence of a heart beat (cardiac arrest)]. Most kidneys are primarily obtained from DBD donors, with the donor pool being extended by using DCD donors, and extended criteria donors (ECDs) (people who aged > 60 years without comorbidities; aged > 50 years with hypertension or death from cerebrovascular accident; or donors with terminal serum creatinine levels of > 1.5 mg/dl).
Following kidney transplantation, major clinical concerns are acute kidney rejection and graft loss. Acute kidney rejection occurs when the immune response of the host attempts to destroy the graft, as the graft is deemed to be foreign tissue. 2 Following renal transplantation, immunosuppressive therapy is implemented to reduce the risk of kidney rejection and prolong survival of the graft.
Aetiology, pathology and prognosis
Renal disease
Most diseases that cause renal failure fall into five categories: systemic disease, glomerulonephritis, hypertension, obstruction and genetic disease (Table 1), with diabetes mellitus causing around 20% of all renal disease. 3
| Category | Description |
|---|---|
| Systemic disease | Diabetes mellitus, autoimmune conditions (e.g. systemic lupus erythematosus and vasculitis), amyloidosis and multiple myeloma |
| Glomerulonephritis | There are many different causes of glomerulonephritis. Some types are relatively benign and unlikely to progress to established renal failure, whereas other forms are more aggressive and can have an impact on disease progression and the development of established renal failure |
| Hypertension | Accelerated hypertension causes CKD; however, early recognition and treatment of high blood pressure can have a positive effect on the disease. Hypertension is a common cause of renal failure in people of African origin |
| Obstruction | Any pathology that obstructs the free flow of urine through the urinary system can cause CKD. Most often obstruction is secondary to enlargement of the prostate gland in elderly men, but other causes include kidney stones, bladder tumours and congenital abnormalities of the renal tract |
| Genetic disease | Genetic disease accounts for about 8% of all kidney failure in the UK. Polycystic kidney disease is the most common genetic disease causing CKD |
When established renal failure is reached, people become tired, nauseated, lose their appetite and cope less well both physically and mentally. The signs of established renal failure include fluid retention (shown as swollen ankles or breathlessness), itching, pallor and raised blood pressure. These symptoms are accompanied by falling haemoglobin levels and abnormality of biochemical markers, for example serum urea, serum creatinine and potassium. When someone reaches this point they will need RRT within weeks or months to prevent death. Treatment will continue for the rest of their lives.
Survival, acute rejection and graft loss after transplantation
Various factors may influence patient survival after kidney transplantation (including factors related to the donor and to the patient). For example, the type of donor can influence patient survival, with recipients of a kidney transplant from an ECD having inferior survival outcomes compared with recipients of standard criteria donor kidneys. However, those from an ECD will still have significantly better survival outcomes than people on waiting lists who remain on HD. 4,5
In people who survive transplantation, acute rejection (AR) may occur when the immune response of the host attempts to destroy the graft, as the graft is deemed foreign tissue. 2 AR is treated using changes to the immunosuppressive regimen (increasing doses or switching treatments). Untreated AR will ultimately result in destruction of the graft. However, high levels of immunosuppression may also increase the risk of other infections and malignancy. 2 AR is primarily measured after a biopsy and is graded according to Banff criteria (grades I–III). The gradings are as follows: grade I, moderate to severe mononuclear cell interstitial infiltrate and moderate tubulitis; grade II, severe tubulitis and/or intimal arteritis; and grade III, transmural arteritis. 6 Incidences of ARs after a transplant are included in this appraisal; however, the treatment for AR is outside the scope of this appraisal.
In addition to ARs affecting the survival of the graft, other reasons that may facilitate graft loss include blood clots, narrowing of an artery, fluid retention around the kidney, side effects of other medications and recurrent kidney disease (www.kidney.org). A major cause of long-term graft loss is chronic allograft nephropathy, an ill-defined process, characterised clinically by progressive deterioration in graft function (GRF), proteinuria and hypertension, and pathologically by changes on biopsy. Chronic allograft nephropathy is a consequence of immunological and non-immunological injury. Immunological factors include human leucocyte antigen (HLA) matching, episodes of AR and suboptimal immunosuppression. Important non-immunological factors implicated are donor organ characteristics, delayed graft function (DGF), recipient-related factors, hypertension and hyperlipidaemia. Recently, the acute and chronic toxicity of calcineurin inhibitors (CNIs) has also been implicated. 7 People with high titres of preformed circulating anti-HLA antibodies – which may come about as a result of underlying illness, previous transplantation, previous pregnancy or multiple blood transfusions – are at high risk of chronic rejection. 8
It is important to note that failing to adhere (or comply) with the immunosuppression regimen prescribed after a kidney transplant will also significantly increase the risk of an ARs and/or graft loss. 9 If the kidney is lost then, ultimately, the patient will need to return to dialysis where quality of life is lower and overall costs are higher. 2
Incidence and prevalence in the UK
The most recent report by the UK NHS regarding kidney disease stated that there were 1,739,443 people aged ≥ 18 years in England in 2008–9 who were registered with CKD (stages 3–5). This represents an overall crude (not adjusted for age) proportion of 4.1% of the UK population in the ≥ 18 years age group. 10 Figure 1 presents the prevalence of people who have detected and registered CKD around England in 2008–9. 10 The actual prevalence that would include those undetected and unregistered would be much higher.
FIGURE 1.
Chronic kidney disease prevalence by primary care trust, England 2008–9. Source: Kidney Disease: Key Facts and Figures, NHS Kidney Care, September 2010. 10 Produced by EMPHO on behalf of Department of Health. Based on Ordinance Survey Material. Do not reproduce, © Crown Copyright 2010. All rights reserved. Department of Health 100020290.

In 2013, the incidence rate of RRT in the UK was stable, at 109 per million population, reflecting RRT initiation for 7006 new cases per year. 3 There were 56,940 adults receiving RRT in the UK on 31 December 2013, an absolute increase of 4.0% from 2012, although the number of people with a functioning transplant increased to 7.1%. The UK adult-only prevalence of RRT was 888 per million population. 3 Table 2 displays the prevalence of adults in the UK who are receiving HD, PD or living with a transplant split for age (< 65 years and ≥ 65 years).
| Country | < 65 years old | ≥ 65 years old | ||||
|---|---|---|---|---|---|---|
| HD | PD | Transplant | HD | PD | Transplant | |
| England | 9121 | 1720 | 19,766 | 10,952 | 1457 | 5016 |
| Northern Ireland | 261 | 38 | 676 | 389 | 43 | 139 |
| Scotland | 888 | 115 | 2050 | 972 | 111 | 428 |
| Wales | 430 | 91 | 1158 | 648 | 91 | 359 |
| UK | 10,700 | 1964 | 23,650 | 12,961 | 1702 | 5942 |
Between April 2013 and March 2014 2464 adult kidney transplant operations were performed in England: 97 in Northern Ireland, 112 in Wales and 242 in Scotland. 11 Figure 2 shows the total number of adult kidney only transplants performed in the last 10 years, by type of donor. 11 The number of adult transplants from DCD has been steadily increasing over the time period to 779 in the last financial year. The number of adult transplants from DBD has increased in the last couple of years to 1101 in 2013–14 after remaining fairly constant for the previous four financial years. The number of adult living kidney transplants performed has also increased over the time period, and 1049 were performed in the last financial year. 11
FIGURE 2.
Kidney transplant rates in the UK. Source: Annual Report on Kidney Transplantation, Report for 2013/2014, NHS Blood and Transplant. 11 Reproduced with permission.

The NHS Blood and Transplant11 annual report (NHSBT) on kidney transplantation reported kidney and patient survival following a kidney transplant over 1 and 5 years, split for deceased and living donors (Table 3).
| Donor | Kidney graft survival: % (95% CI) | Patient survival: % (95% CI) | ||
|---|---|---|---|---|
| 1 yeara | 5 yearsb | 1 yeara | 5 yearsb | |
| Deceased | 93 (93 to 94) | 86 (85 to 87) | 96 (95 to 96) | 89 (88 to 90) |
| Living | 97 (96 to 97) | 91 (89 to 92) | 99 (98 to 99) | 95 (95 to 96) |
Acute rejection following a kidney transplant is likely to be reported in approximately one-third of recipients (www.kidney.org). However, the incidences are variable depending on both patient and donor characteristics, as well as the immunosuppression regimen allocated.
Impact of health problem
Significance for patients
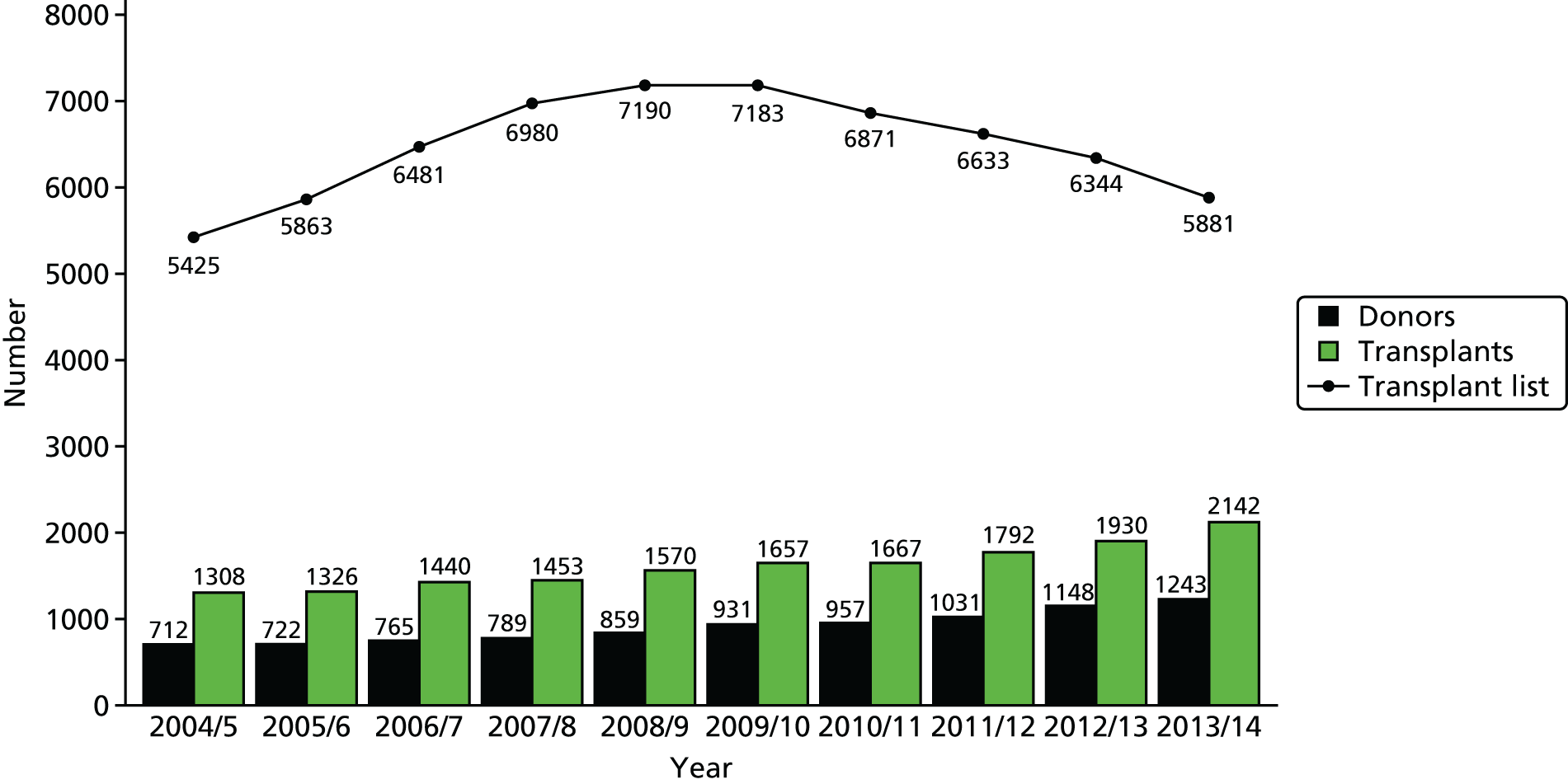
To a person suffering from ESRD the opportunity to have a kidney transplant is literally a matter of life or death. In the year 2013–14, in the UK, 239 people died while on the active and suspended waiting lists for kidney transplantation; 518 people were removed from the list because they were no longer fit enough, most of whom would go on to die. 12 Encouragingly, over the last 5 years there has been a decline in the number of people waiting for a kidney transplant (Figure 3). This decline has primarily been attributed to an increase in the number of transplants being performed each year, as the number of people joining the list each year has remained relatively stable. 12 Although this is encouraging, figures from people registered between April 2007 and March 2011 indicated that the median wait time for a kidney-only transplant in the UK was over 3 years (1114 days) with a 95% confidence interval (CI) of 1091 to 1137 days. 13
FIGURE 3.
Number of donors, transplants and people on the active transplant list from 1 April 2004 to 31 March 2014. Decreased donor kidney programme in the UK, 1 April 2004 to 31 March 2014. Number of donors, transplant and patients on the active transplant list at 31 March. Source: Organ Donation and Transplantation Activity Report 2013/2014, NHSBT. 12 Reproduced with permission.
Although kidney transplantation relieves the person with ESRD from lengthy dialysis, the strict regimen of immunosuppressant medication required may produce unpleasant side effects, including possible skin cancer, crumbling bones, fatigue, body hair growth, swollen gums and weight gain. 14 Nevertheless, a large number of studies have similarly documented, using a variety of instruments, the clear quality-of-life improvements of having a functioning kidney transplant compared with being on dialysis. 15–27 Overbeck et al. ,26 for example, compared the quality of life of those who had received a kidney transplant with those dialysing and on the waiting list, and they found that, when measured with the Short Form questionnaire-36 items (SF-36), people who had received a transplant reported better physical functioning, perception of general health, social functioning and overall physical component than those still dialysing, although these scores did not match those of the general population (Table 4).
| Population | Physical functioning (p ≤ 0.001) | Bodily pain (p = 0.062) | General health (p ≤ 0.01) | Social functioning (p ≤ 0.01) | Physical well-being summary (p ≤ 0.001) |
|---|---|---|---|---|---|
| Dialysis (n = 65) | 62.7 | 62.8 | 39.7 | 71.0 | 38.9 |
| Transplant (n = 76) | 77.0 | 73.5 | 51.0 | 83.9 | 45.6 |
| General population | 84.8 | 77.7 | 68.5 | 89.0 | 50.2 |
Acute rejection is common in the first year after kidney transplantation, and treatment of AR involves a more intensive drug treatment than standard maintenance regimens, which, in turn, increases the possibility of unpleasant side effects. The treatment for AR is outside the scope of this appraisal. Should a graft be lost, people face another wait for transplantation (if appropriate), which may be even longer owing to sensitisation to the mismatched HLA on the failed donor kidney. Furthermore, they will need to undergo dialysis while waiting for transplantation or for life when transplantation is not possible. This, in effect, means that people may be in a worse position from when they started their treatment, but with the added psychological and physical burden from having undergone transplantation. Indeed, many people will develop depression following the loss of a graft. 28
The impact on people of returning to dialysis (with regards psychological burden of graft failure and going back to a previous treatment modality) is scarcely documented, but necessarily includes the impact of being on dialysis per se: dialysis is time-consuming and may affect employment, education, normal family life and require changes in diet and fluid intake. Common side effects to dialysis (either HD or PD) include fatigue, low blood pressure, invasive staphylococcal infections, muscle cramps, itchy skin, peritonitis, hernia and weight gain (www.nhs.uk). Quality of life is lower on dialysis than the general population29 and declines over time as the patient remains on dialysis. 30
Significance for the NHS
Treatment for ESRD has been deemed resource intensive for the NHS, as current costs have been estimated to utilise 1–2% of the total NHS budget to treat 0.05% of the population. 10 Data from the Department of Health estimated that in 2008–9 the total expenditure on ‘renal problems’ in England was £1.3B, representing 1.4% of the NHS expenditure. An economic evaluation of treatments for ESRD by de Wit et al. 31 showed that transplantation is the most cost-effective form of RRT with increased quality of life and independence for people.
It is projected that with an increasingly elderly and overweight population the demand for RRT will increase, with a consequent pressure on services providing renal units and other health-care providers dealing with comorbidities. Increased resources may be needed for dialysis, surgery, pathology, immunology, tissue typing, histopathology, radiology, pharmacy and hospital beds. Demand is likely to be particularly significant in areas where there are large South Asian, African and African Caribbean communities, and in areas of social deprivation, in which people are more susceptible to kidney disease. 32
Data from the NHS Standard Contract for Adult Kidney Transplant Service indicated that the cost for the first year of care following a kidney transplant is approximately £17,000 and then £5000 for every subsequent year. Conversely, the cost of dialysis is approximately £30,800 per year. 33 However, should a graft be lost following a transplant, the NHS would incur increased costs from either the patient returning to dialysis or requiring a replacement renal transplant (in comparison with successful maintenance of the kidney graft). Similarly, each AR episode would incur increased costs because of the changes made to the immunosuppression regimen to treat the rejection.
Measurement of disease
The outcome of kidney transplants (and of the success of immunosuppressive regimens) can be measured in a variety of ways. These include:
Short term:
-
Immediate GRF – the graft works immediately after transplantation, removing the need for further dialysis.
-
DGF – the graft does not work immediately and dialysis is required during the first week post transplant. Dialysis has to continue until GRF recovers sufficiently to make it unnecessary. This period may last up to 12 weeks in some cases.
-
Primary non-function (PNF) – the graft never works after transplantation.
Long term:
-
Graft survival – the length of time that a GRFs in the recipient.
-
GRF – a measure of the efficiency of the graft by various markers, for example glomerular filtration rate (GFR) and serum creatinine levels (Table 5). Measuring serum creatinine concentrations is a simple method for estimating GFR. Estimated glomerular filtration rate (eGFR) is calculated from serum creatinine levels, age, sex and race, and provides information on creatinine clearance (CRC). There are various methods used to calculate eGFR [Modification of Diet in Renal Disease (MDRD), Cockcroft–Gault, Nankivell methods], although no formula has been shown to be consistently more superior to another. 35
-
Rejection rates – the percentage of grafts that are rejected by the recipients’ bodies; these can be acute or chronic.
-
Patient survival – how long the recipient survives with the transplanted kidney.
-
Quality of life – how a person’s well-being is affected by the transplant.
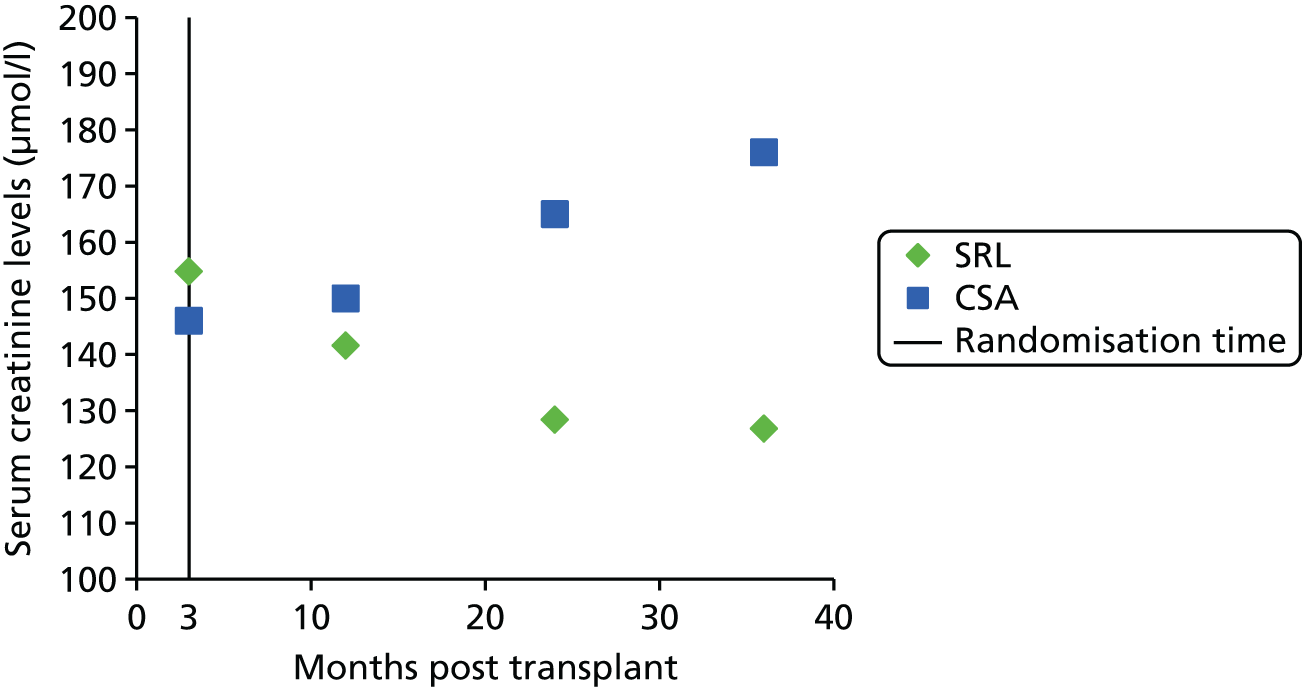
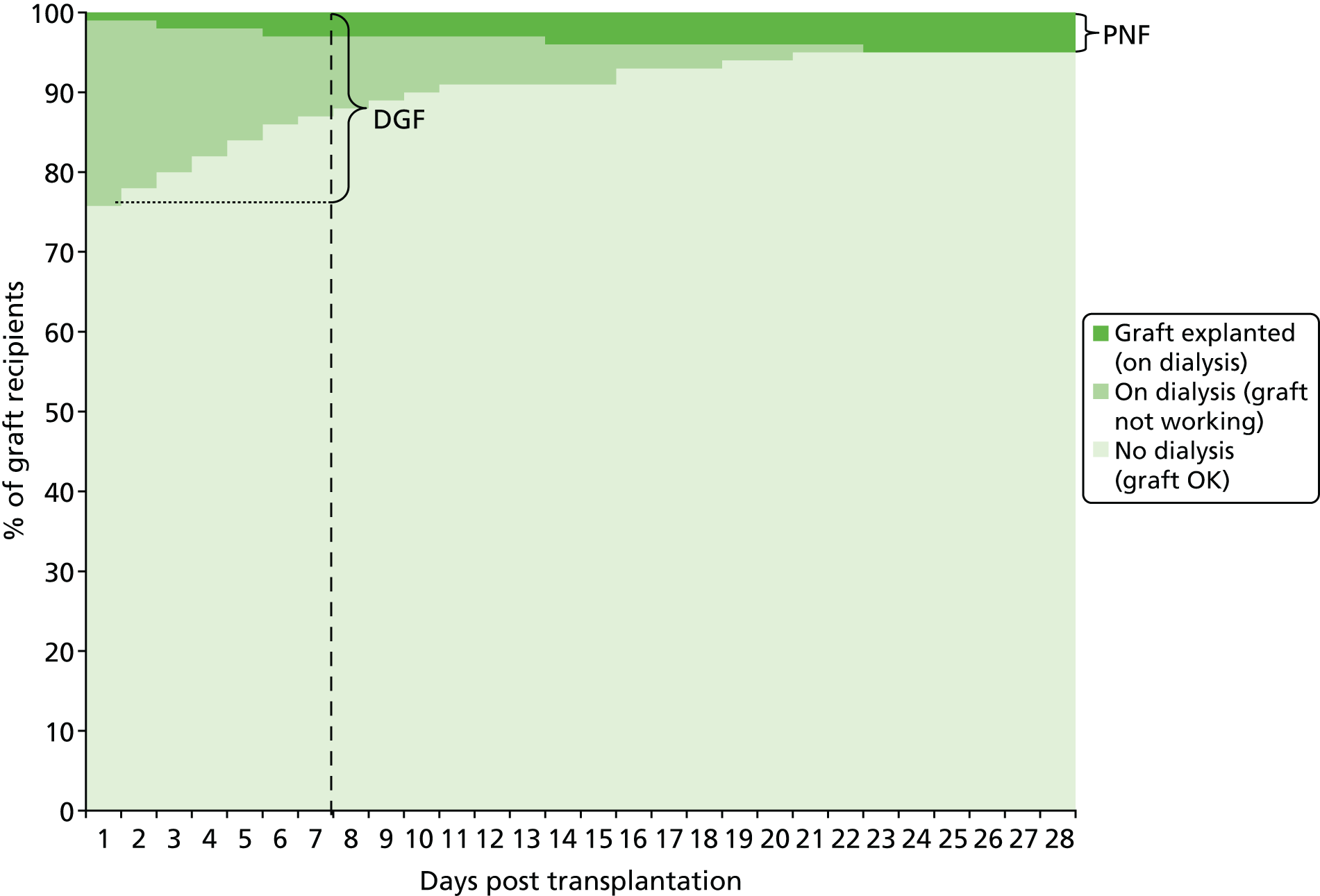
Figure 4 shows a hypothetical graph to explain the relationship between DGF and PNF. At 7 days post transplant, some of the people who have needed to dialyse, and whose grafts are therefore classified as DGF, will, in fact, have grafts that never function. When this has been established, these grafts are classified as PNF.
FIGURE 4.
Hypothetical graph to explain the relationship between DGF and PNF.
Current service provision
Management of disease
Management of end-stage renal disease
End-stage renal disease is primarily managed by RRT. The patient pathway leading to RRT for those with ESRD can be seen in Figure 5. The distribution of people on differing RRTs in the UK as of 31 December 2012 is shown in Figure 6.
FIGURE 5.
The care pathway for RRT. Source: The National Service Framework for Renal Services – Part 1: Dialysis and Transplantation. 36

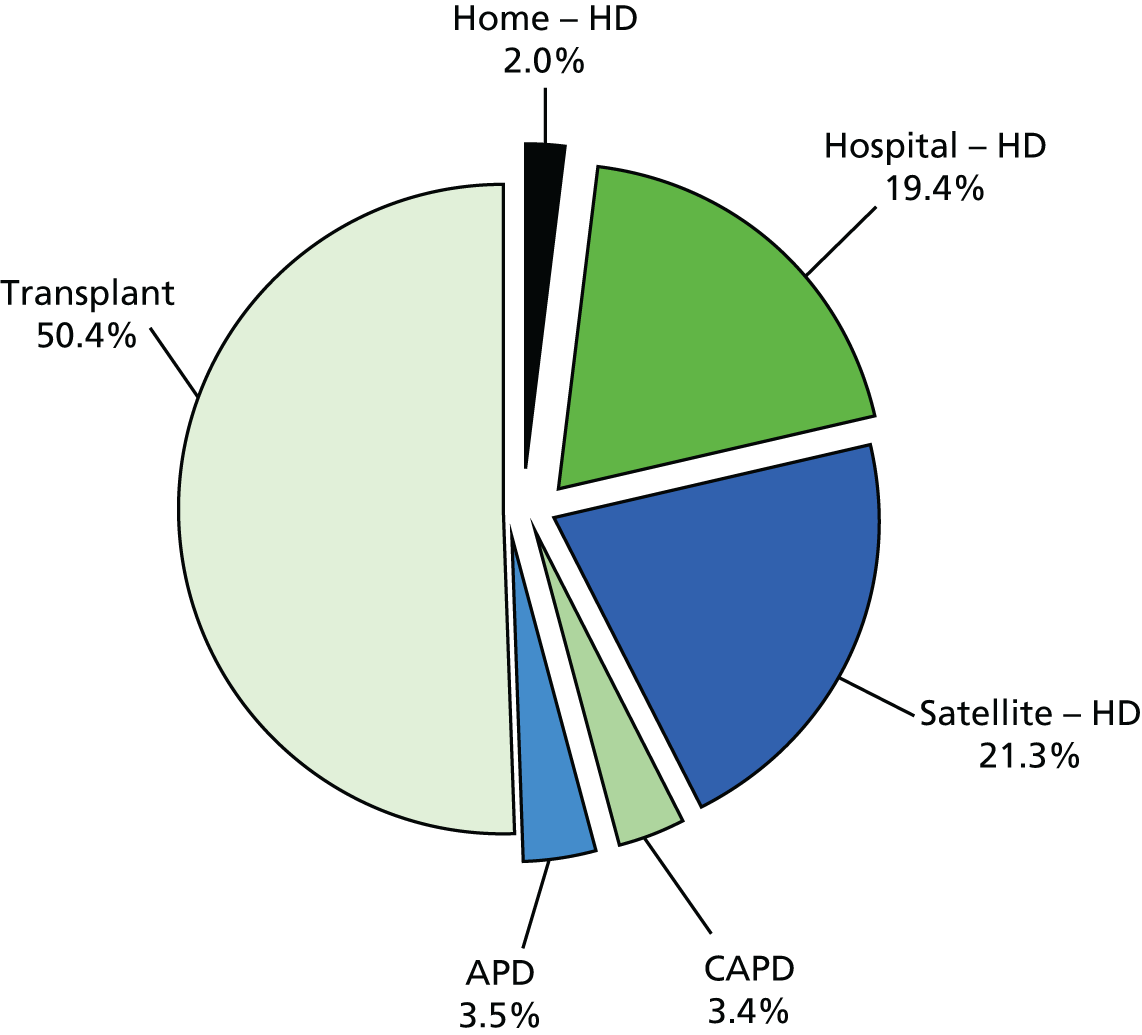
FIGURE 6.
Treatment modality in prevalent RRT adults on 31 December 2012 in the UK. Source: The Sixteenth Annual report from the UK Renal Registry. 3 APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis. Source: Annual Report on Kidney Transplantation, Report for 2013/2014, NHS Blood and Transplant. 11 Reproduced with permission. The data reported here have been supplied by the UK Renal Registry of the Renal Association. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the UK Renal Registry or the Renal Association.
Management of kidney transplant
If transplantation is the chosen method of RRT for a patient with ESRD then, from the perspective of person receiving the transplant, there are three main service provision steps required for the management of the transplant.
The first of these steps is organ procurement, which includes the identification of potential donors, assessment of donor suitability, determination of donor brain death (where applicable) and medical management of the donor. Donor–recipient compatibility includes an assessment on HLA matching. HLAs are carried on cells within the body, enabling the body to distinguish between ‘itself’ and ‘non-self’, which should be attacked. The closer the HLA matching, the less vigorously the body will attack the foreign transplant; consequently, the chances of graft survival are improved. HLA mismatch refers to the number of mismatches between the donor and the recipient at the A, B and DR loci, with a maximum of two mismatches at each locus. 11 However, it should be noted that because of improvements in immunosuppressants, the significance of HLA matching has diminished. 37
The second step is the provision of immunosuppressive therapy. Immunosuppressants are the drugs taken around the time of, and following, an organ transplant. They are aimed at reducing the body’s ability to reject the transplant, and thus at increasing patient and graft survival and preventing acute and/or chronic rejection (while minimising associated toxicity, infection and malignancy). Immunosuppressants are required in some form for all kidney transplant recipients (KTRs) except, potentially, when the donor is an identical twin. The immunosuppressive drugs can be divided into induction and maintenance drugs. Induction drugs are powerful antirejection drugs that are taken at the time of transplantation and close after, when the risk of rejection is highest. Maintenance drugs are less powerful antirejection drugs that are used as both initial and long-term maintenance therapy.
The final service provision step is short- and long-term follow-up following transplantation. This step involves looking for indications of any kidney graft dysfunction and/or other complications. Complications fall into three categories:
-
medical follow-ups to monitor for, and treat, rejections; nephrotoxicity of CNIs; and recurrence of the native kidney diseases
-
anatomical complications of surgery, including renal artery thrombosis, renal artery stenosis, urine leaks from disruption of the anastomosis, ureteral stenosis and obstruction, and lymphocele
-
other complications, including infection, malignancy, new onset of diabetes mellitus, liver disease, hypertension and CVD.
Management of graft loss
As the kidney loses its function, many of the physiological changes that occur mimic those seen with progressive renal diseases from other aetiologies. Therefore, these symptoms should be managed in a similar way to the non-transplant population, although it should be noted that the loss of a kidney transplant carries increased susceptibility to bruising and infection compared with pretransplant kidney failure. 28
Once the kidney is confirmed to have been lost, the graft may or may not need to be surgically removed. The decision as to whether or not the graft is removed is often made on a case-by-case basis, taking into consideration all perceived benefits and risks. The immunosuppression regimen can then be tapered and withdrawn while the patient returns to dialysis and waits for a new kidney to become available. However, in cases when people have not already formed antibodies to donor HLA, immunosuppression may be continued to allow access to a wider pool of potential donors. Success rates of a subsequent kidney transplant are equivocal. Some report that a subsequent transplant will generally be as good as the first,28 whereas others report inferior graft survival for those receiving their second38 or third39 transplant in comparison with those receiving their first transplant.
Management of graft loss will also include management of the psychological impact of the loss; owing to an increased risk for depression following the loss of a graft, it is recommended that depressive symptoms should be actively investigated and managed along conventional lines. 28
Current service cost
The overall cost of CKD to the NHS in England was estimated as £1.45B in 2009–10, with more than half of total estimated expenditure going on RRT. 40 The costs of RRT can be divided into the costs associated with transplantation and the costs associated with dialysis. Transplantation costs can include the cost of work-up for transplantation (assessing recipient suitability), maintaining and co-ordinating the waiting list, obtaining donor kidneys (harvesting, storage and transport for deceased donors; nephrectomy procedure for living donors), cross-matching for donor–recipient compatibility, the transplantation procedure, induction immunosuppression, hospital inpatient stay following procedure, initial and long-term maintenance immunosuppression, prophylaxis and monitoring for infections, monitoring of GRF and general health, adjustment of immunosuppressant dosages, treatment of AR and treatment of associated adverse events (AEs). Should the kidney be lost, the costs of restarting dialysis (dialysis costs, the cost of treatment for AEs attributable to dialysis and the cost of dialysis access surgery) would be incurred.
Variation in services
Currently, 71 adult renal centres are operating in the UK (five renal centres in Wales, five in Northern Ireland, nine in Scotland, 52 in England) offering various levels of renal care. This includes 23 adult transplant centres in the UK (one in Wales, one in Northern Ireland, two in Scotland, 19 in England). There is some variation across the services provided between these 71 centres; however, information describing how the services differ is not readily available.
After kidney transplantation, recipients are prescribed an immunosuppression regimen consisting of both induction and maintenance therapy. Following this, they are offered check-up appointments with their clinic (consultant nephrologist) to monitor general health, kidney function, immunosuppressive drugs, infections (prophylaxis and treatment) and to address any social or psychological concerns. The following frequency of clinic appointments is suggested for an uncomplicated patient. 41
-
two or three times weekly for the first month after transplantation
-
once or twice weekly for months 2–3 after transplantation
-
every 1–2 weeks for months 4–6 after transplantation
-
every 4–6 weeks for months 6–12 after transplantation
-
3- to 6-monthly thereafter
-
detailed annual postoperative reviews.
Clinician estimations of average frequency of outpatient visits have been reported as 34.3, 6.3 and 4.7 visits, respectively, for the first, second and third years post transplant, with figures from the Cardiff Transplant Unit suggesting 39.7, 11.0 and 9.2 visits, respectively, for the first, second and third years post transplant. 42
Service provision (clinic appointments or other services) is likely to increase if AR occurs (possibly requiring hospital admission and escalating treatment), and, where there is declining GRF (which might necessitate more regular clinic visits, blood tests and other investigations and changes to treatment regimens). People may also present to their general practitioner (GP) or accident and emergency department with AEs related to kidney transplantation or immunosuppressive regimen and this may be followed by an additional referral to the consultant nephrologist or other appropriate specialist (e.g. renal dietitian), followed by management as required (e.g. additional prescribing and monitoring).
In addition to these services, all people should have the following:41
-
online access to their results via the ‘Renal Patient View’ service (http://rixg.org/patientview2/patientview-2-2-released/)
-
open access to the renal transplant outpatient service
-
an established point of contact for enquiries
-
access to patient information (which should be available in both written and electronic formats).
Current National Institute for Health and Care Excellence guidance
Current National Institute for Health and Care Excellence (NICE) guidance on ‘Immunosuppressive Therapy for Renal Transplantation in Adults’ (NICE technology appraisal guidance 85, TA85) has the following recommendations for induction and maintenance therapy. 43
Induction therapy
-
Basiliximab (BAS) (Simulect®, Novartis Pharmaceuticals UK Ltd) or daclizumab (DAC), used as part of a CNI-based immunosuppressive regimen, are recommended as options for induction therapy in the prophylaxis of acute organ rejection in adults who are undergoing renal transplantation. The induction therapy (BAS or DAC) with the lowest acquisition cost should be used. 43
Maintenance therapy
-
Tacrolimus (TAC) (Adoport®, Sandoz; Capexion®, Mylan; Modigraf®, Astellas Pharma; Perixis®, Accord Healthcare; Prograf®, Astellas Pharma; Tacni®, Teva; Vivadex®, Dexcel Pharma) is an alternative to ciclosporin (CSA) when a CNI is indicated as part of an initial or a maintenance immunosuppressive regimen in renal transplantation for adults. The initial choice of TAC or CSA should be based on the relative importance of their side effect profiles for individual people. 43
-
Mycophenolate mofetil (MMF) (Arzip®, Zentiva; CellCept®, Roche Products; Myfenax®, Teva) is recommended for adults as an option as part of an immunosuppressive regimen only:
-
where there is proven intolerance to CNIs, particularly nephrotoxicity, leading to risk of chronic allograft dysfunction, or
-
in situations in which there is a very high risk of nephrotoxicity necessitating minimisation or avoidance of a CNI. 43
-
-
Sirolimus (SRL) (Rapamune®, Pfizer) is recommended for adults as an option as part of an immunosuppressive regimen only in cases of proven intolerance to CNIs (including nephrotoxicity) necessitating complete withdrawal of these treatments. 43
As a consequence of following this guidance, some medicines may be prescribed outside the terms of their UK marketing authorisation. Clinicians prescribing these drugs should ensure that people are aware of this, and that they consent to their use in such circumstances. 43
Since the publication of the current guidance in 2004,43 the marketing authorisation for DAC has been withdrawn. In addition, new technologies have received marketing authorisations for induction therapy [rabbit anti-human thymocyte immunoglobulin (rATG) (Thymoglobulin®, Sanofi)] and maintenance therapy [belatacept (BEL) (Nulojix®, Bristol-Myers Squibb); a prolonged-release formulation of TAC (TAC-PR) (Advagraf®, Astellas Pharma); and an oral suspension of immediate-release TAC]. In addition, another new technology [everolimus (EVL) (Certican®, Novartis Pharmaceuticals UK Ltd)] has been studied as an immunosuppressant in renal transplantation. EVL received UK marketing authorisation in this therapy area in November 2014.
Description of technology under assessment
Summary of intervention
This technology assessment report considers nine pharmaceutical interventions. Two are used as induction therapy and seven are used as a part of maintenance therapy in renal transplantation. The two interventions considered for induction therapy are BAS and rATG. The seven interventions considered for maintenance therapy are immediate-release TAC and TAC-PR, MMF, mycophenolate sodium (MPS) (Myfortic®, Novartis Pharmaceuticals UK Ltd), BEL, SRL and EVL.
Induction therapy
Basiliximab is a monoclonal antibody that acts as an interleukin-2 receptor antagonist. It has a UK marketing authorisation for the prophylaxis of acute organ rejection in de novo allogeneic renal transplantation in adults. The Summary of Product Characteristics states that it is to be used concomitantly with CSA for microemulsion (ME)- and corticosteroid (CCS)-based immunosuppression in people with a panel reactive antibody (PRA) score of < 80%, or in a triple maintenance immunosuppressive regimen containing CSA for ME, CCSs and either azathioprine (AZA) or MMF. Higher PRA scores indicate higher immunological risk. BAS is administered intravenously.
Rabbit anti-human thymocyte immunoglobulin is a gamma immunoglobulin generated by immunising rabbits with human thymocytes. It has a UK marketing authorisation for the prevention of graft rejection in renal transplantation. The Summary of Product Characteristics states that it is usually used in combination with other immunosuppressive drugs and is administered intravenously.
Maintenance therapy
Tacrolimus is a CNI. It is available in a prolonged-release formulation and immediate-release formulations. All of these formulations (see Current National Institute for Health and Care Excellence guidance, above) have UK marketing authorisations for the prophylaxis of transplant rejection in adults who are undergoing kidney transplantation, and all are administered orally. Prograf® can also be administered intravenously. The Commission on Human Medicines advises that all oral TAC medicines in the UK should be prescribed and dispensed by brand name only.
Belatacept is a soluble fusion protein that is designed to selectively inhibit CD28-mediated co-stimulation of T cells. BEL has a UK marketing authorisation for prophylaxis of graft rejection in adults who are receiving a renal transplant, in combination with CCSs and a mycophenolic acid (MPA). The Summary of Product Characteristics recommends that an interleukin-2 receptor antagonist is added to this BEL-based regimen. BEL is administered intravenously.
Mycophenolate mofetil is a prodrug of MPA that acts as an antiproliferative agent; generic MMF is manufactured by Accord Healthcare, Actavis, Arrow Pharmaceuticals, Dr Reddy’s Laboratories, Mylan, Sandoz and Wockhardt.
Mycophenolate sodium. Mycophenolate is also available as an enteric-coated formulation: mycophenolate sodium (EC-MPS).
(Mycophenolate mofetil and MPS have UK marketing authorisations for use in combination with CSA and CCSs for the prophylaxis of acute transplant rejection in people undergoing kidney transplantation. Both drugs can be administered orally; MMF can also be administered intravenously.)
Sirolimus is a non-calcineurin-inhibiting immunosuppressant and acts as an antiproliferative agent. It has a UK marketing authorisation for the prophylaxis of organ rejection in adults – at low to moderate immunological risk – who are receiving a renal transplant. It is recommended to be used initially in combination with CSA and CCSs for 2–3 months. It may be continued as maintenance therapy with CCSs only if CSA can be progressively discontinued. It is administered orally.
Everolimus is an analogue of SRL and therefore is a non-calcineurin-inhibiting immunosuppressant which acts as an antiproliferative. EVL has recently (November 2014) received UK marketing authorisation for immunosuppressive treatment in kidney transplantation. It has been studied in clinical trials in numerous regimens containing one or more additional immunosuppressant (including CSA, TAC, anti-thymocyte immunoglobulin, mycophenolate, CCSs and BAS) and compared with various alternative immunosuppressive regimens in adults undergoing kidney transplantation. EVL is administered orally.
Important prognostic factors
A number of important factors that may influence both patient and graft survival have been identified:
-
Age – both the age of the recipient and the age of the donor will influence the survival of the transplant. Graft survival decreases as the age of the recipient or the donor increases. 44
-
Sex – women have a better graft survival rate than men, whereas men have better patient survival than women. 44
-
Recipient ethnicity – black people have worse GRF, shorter graft survival and higher rates of chronic allograft nephropathy than white people. 44
-
Waiting time to transplant – the longer a patient is on dialysis, waiting for a kidney transplant, the poorer his/her outcomes are post transplantation. 45
-
Cold ischaemia time – the shorter this time (≤ 20 hours), the better the immediate and long-term outcomes. 11
-
Donor type – adults receiving donated kidneys from live donors have a better outcome than those who are receiving kidneys from deceased donors. 44 Similarly, people receiving a kidney from ECDs (donors who may, for example, be older or have a history of diabetes mellitus or hypertension) will have inferior graft survival rates and increased incidences of AR compared with patients who are receiving a standard donated kidney. 46
-
Immunological risk, to include HLA and blood group incompatibility – when the number of mismatches from the donor to the recipient is higher, there is an increased likelihood of AR and graft loss. 44
-
Comorbidities, for example diabetes mellitus, cancer and CVD – the higher a patient scores on the Charlson Comorbidity Index, the lower the patient and graft survival is likely to be. AR is not significantly correlated to the Charlson Comorbidity Index. 47
There is also evidence to suggest that African American people will require a higher dose of TAC,48 MMF49 and SRL50 to achieve the target levels than white people. However, how the prescription of the immunosuppression regimen offered in the UK differs between subgroups is not readily available.
Current usage in the NHS
Although the combination of TAC + mycophenolate (MMF or Myfortic) + prednisolone is widely used, immunosuppressive regimens tend to vary according to renal centre (thus the use of the drugs under consideration varies across centres). Some examples of immunosuppressive regimens in the UK are given below in Table 6, but this is by no means exhaustive, as there are so many possible combinations of treatments.
| Hospital | Treatment |
|---|---|
| Royal Devon and Exeter Hospital, Exetera | Variable baseline immunosuppression depending on transplant centre. Typically, all kidney-alone transplant patients should have BAS on days 1 and 4 in the transplant centre. Everyone will receive a combination of prednisolone, CNI (either CSA or TAC) and/or antiproliferative agent (either AZA or mycophenolate). As an alternative, people may be offered an mTOR inhibitor (either SRL or EVL) |
| Derriford Hospital, Plymoutha | ‘SYMPHONY study’51 regimen using triple therapy irrespective of immunological risk or DGF risk with TAC, MMF or MPS, and a reducing course of prednisolone |
| Nottingham University Hospitals NHS Trust52 | Standard immunological risk: BAS induction therapy. TAC, AZA and prednisolone maintenance therapy |
| Oxford Transplant Centre53 | Recipients receive alemtuzumab induction Maintenance immunosuppression is steroid free with TAC-PR and MMF or MPS |
| Royal Infirmary of Edinburgh54 | Methyl prednisolone 500 mg intravenously just prior to releasing clamps, and again at 24 hours Standard immunosuppression is TAC-led triple therapy with prednisolone and AZA |
Anticipated costs associated with the interventions
The cost of the intervention (immunosuppressive regimen) is determined primarily by the choice and combination of the drugs and their respective dosages. Indicative costs for different immunosuppressive agents are given in Table 7. Caution should be exercised in interpreting these, as dosages are commonly titrated and may differ from those indicated.
| Compound | Unit cost (pence) | For 70-kg patient | |
|---|---|---|---|
| Estimated weekly dosage | Estimated weekly cost (£) | ||
| CSA | Hospital pharmacy 1.65 per mg;a community pharmacy 2.55 per mgb | 4 mg/kg per dayb = 1960 mg | Hospital pharmacy 32.28; community pharmacy 49.95 |
| Immediate-release TAC | Hospital pharmacy 52.0 per mg;a community pharmacy 118.6 per mga,c | 0.2 mg/kg per dayd = 98 mg | Hospital pharmacy 50.98; community pharmacy 116.26 |
| TAC-PR | 106.8 per mgb | 0.2 mg/kg per dayd = 98 mg | 52.31 |
| AZA | Hospital pharmacy 0.1 per mg;a community pharmacy 0.1 per mgc | 1.75 mg/kg per dayb = 858 mg | Hospital pharmacy 0.92; community pharmacy 0.98 |
| MMF | Hospital pharmacy 37.7 per g;a community pharmacy 40.4 per gc | 2 g per dayb = 14 g | Hospital pharmacy 5.28; community pharmacy 5.66 |
| MPS | 0.5 per mgb | 1,440 mg per dayb = 705,600 mg | 45.14 |
| SRL | 288.3 per mgb,c | 2 mg per dayb = 14 mg | 40.36 |
| EVL | 990.0 per mge | 2 mg per daye = 14 mg | 138.60 |
| BEL | 141.8 per mgb | 5 mg/kg per 4 weeksf,g = 125 mg | 177.25 |
| CCSs | Hospital pharmacy 0.3 per mg;a community pharmacy 0.9 per mgc | 15 mg/dayb = 105 mg | Hospital pharmacy 0.35; community pharmacy 0.92 |
In addition, drug administration costs are also incurred for some maintenance agents: CSA, TAC, SRL and EVL are routinely titrated using therapeutic drug monitoring, which are estimated to cost approximately £26 per test (testing frequency is reduced as people become stabilised in dosage); BEL requires intravenous (i.v.) infusion, entailing catheterisation and nursing time. The cost of this is difficult to estimate but estimates range from £15463 to £320. 64 Costs are considered in greater detail in Chapter 7.
Chapter 2 Definition of the decision problem
Decision problem
Interventions
A total of nine interventions are being considered, two for induction therapy and seven for initial and long-term maintenance therapy.
The two induction treatments are:
-
BAS
-
rATG.
The seven maintenance treatments are:
-
TAC-PR
-
TAC immediate-release formulations
-
BEL
-
MMF
-
MPS
-
SRL
-
EVL.
These treatments are summarised in Chapter 1 (see Summary of intervention). The maintenance treatments will be appraised as part of combination regimens where appropriate. Under an exceptional directive from the Department of Health, the Appraisal Committee may consider making recommendations about the use of drugs outside the terms of their existing marketing authorisation when there is compelling evidence of their safety and effectiveness. Accordingly, the review will include studies that used drugs outside the terms of their marketing authorisations.
Populations
The population being assessed is adults undergoing kidney transplantation from a living–related donor, living–unrelated donor or deceased donor. People receiving multiorgan transplants, and those who have received transplants and immunosuppression previously, will be excluded. When data allow, the following subgroups will be considered: level of immunological risk (including HLA compatibility and blood group compatibility), people at high risk of rejection within the first 6 months, people who have had a retransplant within 2 years, previous AR and people at high risk of complications from immunosuppression (including new-onset diabetes mellitus).
Relevant comparators
For induction therapy, the treatments are to be compared with each other as data permit, or with other regimens that do not include monoclonal or polyclonal antibodies. For maintenance therapy, each treatment or regimen (combination of treatments) is to be compared with the other treatments or regimens as data permit, or with a CNI with or without an antiproliferative agent and/or CCSs.
Outcomes
The health-related outcomes to be included in this report are:
-
patient survival
-
graft survival
-
GRF (eGFR, which is a measure of the kidney’s ability to filter and remove waste products)
-
time to and incidence of AR
-
severity of AR
-
adverse effects of treatment
-
health-related quality of life (HRQoL).
Key issues
A number of factors may influence the survival and function of a donated kidney and the survival of the recipient.
The viability of the kidney may depend on the type of donor (living–related, living–unrelated, DBD, DCD or ECD), the age of the donor, whether or not he/she had comorbidities (such as diabetes mellitus) and the length of cold ischaemia. Furthermore, the age, sex, ethnicity and health of the recipient, and the length of time the recipient is on dialysis prior to transplantation, may affect the outcome of transplantation.
Overall aims and objectives of assessment
The aim of this assessment is to review and update the evidence for the clinical effectiveness and cost-effectiveness of immunosuppressive therapies in adult renal transplantation. This will be done by conducting a systematic review of clinical effectiveness studies and a model-based economic evaluation of induction and maintenance immunosuppressive regimens to update the current guidance (TA85). 43 The current guidance was primarily based on research evidence presented to NICE in the assessment report by Woodroffe et al. 65 We have incorporated relevant evidence that was presented in this previous report and we report new evidence from 2002 to the present. This will include a new decision-analytic model of kidney transplantation outcomes to investigate which regimen is the most cost-effective option.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
The project was undertaken in accordance with a predefined protocol. There were no major departures from this protocol.
The aim was to systematically review the effectiveness of immunosuppressive therapies in adult renal transplantation and determine the effect on patient survival; graft survival; GRF; time to, and incidence of, AR; severity of AR; the effectiveness in improving HRQoL and the impact of AEs. The review was undertaken following the principles published by the NHS Centre for Reviews and Dissemination. 66
Identification of studies
Bibliographic literature searching was conducted on 14 April 2014. The effectiveness searches took the following form: (terms for kidney or renal transplant or kidney or renal graft) AND (terms for the interventions under review) AND (a study design limit to RCTs or controlled trials). The search was date limited to 2002 to current, in line with the previous assessment, and the searches were updated on 18 November 2014. The search was not limited by language or human-only studies to ensure that records were not missed in error. Instead, these exclusion criteria were implemented during the screening process.
The following databases were searched for randomised controlled trials (RCTs) MEDLINE (via Ovid), EMBASE (via Ovid), Cochrane Central Register of Controlled Trials (via Wiley Online Library) and Web of Science (via ISI; including conference proceedings). The following trials registries were hand-searched: ClinicalTrials.Gov (https://clinicaltrials.gov/) and Controlled Trials (www.controlled-trials.com/). The search strategies (including web-searching) are recorded in Appendix 1.
A separate search was undertaken to identify systematic reviews. These searches took the following form: (terms for kidney or renal transplant or kidney or renal graft) AND (terms for the interventions under review) AND (a pragmatic limit to systematic reviews). The search was run from database inception in the following databases: MEDLINE (via Ovid), EMBASE (via Ovid), Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects and Health Technology Assessment (HTA; The Cochrane Library via Wiley Online Library) and Health Management Information Consortium (via Ovid). The search was not limited by language and it was not limited to human-only studies. The search strategies are recorded in Appendix 1.
In addition, the following websites were searched for background information:
Renal societies (UK)
-
British Renal Society www.britishrenal.org/.
-
Renal Association www.renal.org/.
-
UK Renal Registry www.renalreg.com/.
-
Kidney Research UK www.kidneyresearchuk.org/.
-
British Kidney Patient Association www.britishkidney-pa.co.uk/.
-
National Kidney Federation www.kidney.org.uk/.
Renal societies (international)
-
American Society of Nephrology www.asn-online.org/.
-
American Association of Kidney Patients www.aakp.org/.
-
National Kidney Foundation (USA) www.kidney.org/.
-
Canadian Society of Nephrology www.csnscn.ca/.
-
Kidney Foundation of Canada www.kidney.ca/.
-
Australian and New Zealand Society of Nephrology www.nephrology.edu.au/.
-
Kidney Health Australia www.kidney.org.au/.
-
Kidney Society Auckland www.kidneysociety.co.nz/.
The database search results were exported to, and deduplicated using EndNote (X5) (Thomson Routers, CA, USA). Deduplication was also performed using manual checking. The search strategies and the numbers retrieved for each database are detailed in Appendix 1. After the reviewers completed the screening process, the bibliographies of included papers were scrutinised for further potentially includable studies.
Studies included in the previous adult and child HTA reviews65,67 were screened against the inclusion criteria for the Peninsula Technology Assessment Group (PenTAG) review for includable studies. Reference lists of included guidelines, systematic reviews and clinical trials were scrutinised for additional studies.
Ongoing studies
A search for ongoing trials was also undertaken. The terms used to search the ClinicalTrials.gov and Controlled Trials [International Standard Randomised Controlled Trial Number (ISRCTN)] trial registers for the interventions are included in Appendix 1.
Trials that did not relate to immunosuppressive therapies for kidney transplantation in adults were removed by hand-sorting. Finally, duplicates, identified via their study identification numbers, where possible, were removed. Searches were carried out on 19 September 2014.
Inclusion and exclusion criteria
Study design
Only RCTs were included. Systematic reviews of RCTs were also included in order to ensure all relevant clinical trials were identified.
Population
Adults who were undergoing kidney transplantation only, and receiving immunosuppressive therapy, were included in this review. Multiorgan transplantation, the treatment of episodes of AR and individuals who have previously received a renal transplant and immunosuppression (i.e. individuals who were not undergoing the process of a new renal transplant) are outside the scope of this appraisal.
Interventions
Studies evaluating the use of the following immunosuppressive therapies for renal transplantation were included (further details in Chapter 1, Induction therapy and Maintenance therapy).
Induction therapy regimens containing:
-
BAS
-
rATG.
Maintenance therapy regimens containing:
-
MMF
-
MPS – EC-MPS
-
immediate-release TAC
-
TAC-PR
-
BEL
-
SRL
-
EVL.
Under an exceptional directive from the Department of Health, these interventions can be assessed outside their existing marketing authorisation (to reflect their use in clinical practice) where there was compelling evidence of safety and effectiveness.
Comparators
The comparators of interest for induction therapies were regimens without monoclonal or polyclonal antibodies or one of the other interventions under consideration.
For maintenance therapies, the comparators were a CNI with or without an antiproliferative agent and/or CCSs or a regimen including one of the other interventions under consideration.
Outcomes
Outcomes sought from the studies fell into four main categories: mortality, graft-related outcomes, AEs data and HRQoL outcomes. Owing to the variability in evidence available and in order to ensure consistency with the modelling, measurements were restricted as follows:
-
Mortality
-
Graft-related outcomes:
-
graft survival – when graft loss is defined as return to chronic dialysis, retransplant, graft removal or death
-
GRF – (estimated) eGFR, which is an estimate of actual GFR; a number of formulae are available for eGFR, which may require age, weight, sex and serum creatinine level
-
time to, and incidence of, biopsy-proven acute rejection (BPAR)
-
severity of AR according to the Banff classification (grades I–III).
-
-
AEs:
-
malignancy and post-transplant lymphoproliferative disorder (PTLD)
-
diabetes mellitus
-
infections
-
cytomegalovirus (CMV).
-
-
HRQoL, including data on validated quality-of-life measures, for example the European Quality of Life-5 Dimensions (EQ-5D), the SF-36 and the Kidney Transplant Questionnaire (KTQ-25).
Selection of studies
Studies retrieved from the searches were selected for inclusion according to the inclusion/exclusion criteria specified in Inclusion and exclusion criteria. Initially, titles and abstracts returned by the search strategy were screened for inclusion independently by two researchers, with TJ-H as first reviewer and LC, MHa, MB or HC as second reviewer. Disagreements were resolved by discussion, with involvement of a third reviewer (MHa or HC). Full texts of identified studies were obtained and screened in the same way.
In addition, studies included in the reviews conducted by Woodroffe et al. 65 and Yao et al. 67 were screened for inclusion against the eligibility criteria for this review.
Data extraction strategy
Included full papers were split between five reviewers (TJ-H, MHa, LC, MB and HC), with TJ-H as first reviewer for the purposes of data extraction using a standardised data extraction form, and checked independently by another reviewer. Discrepancies were resolved by discussion with the involvement of an additional review team member (MHa or HC) if necessary. Information extracted and tabulated included details of the study’s design and methodology, baseline characteristics of participants, and results, including HRQoL and any AEs, if reported.
If several publications were identified for one study, the data were extracted from the most recent publication and supplemented with information from other publications.
For studies comparing both induction and maintenance, we assigned a separate reference for each study arm, with the author and publication year of the main publication, and added the suffixes ‘a’ and ‘b’.
Critical appraisal strategy
Four reviewers (TJH, MHa, MB and HC) independently assessed quality for the newly identified studies (2002 onwards) according to criteria based on Centre for Reviews and Dissemination guidance (Table 8). 66
| Criteria | Assessment question |
|---|---|
| Treatment allocation |
|
| Similarity of groups |
|
| Implementation of masking |
|
| Completeness of trial |
|
| Generalisability |
|
Methods of data synthesis
Where data permitted the results of individual studies were pooled using Stata SE 13.1 (StataCorp LP, College Station, TX, USA) to investigate:
-
estimation of overall treatment effect
-
assessment of heterogeneity
-
subgroup analysis
-
assessment of publication bias.
Owing to the heterogeneity of population and study characteristics, a random-effects model was assumed for all meta-analyses. For binary data, odds ratio (OR) was used as a measure of treatment effect and the DerSimonian–Laird method was used for pooling. For continuous data (eGFR), mean differences (MDs) were calculated if the outcome was measured on the same scale in all trials.
If a study had two intervention arms that were separately compared with the control arm, when pooling ORs the number of events and the total sample size in the control arm were divided equally across the comparisons, and when pooling MDs the total sample size in the control arm was adjusted and divided equally across the comparisons. However, if only one experimental arm was eligible for the analysis then all participants assigned to the control arm were included.
A narrative synthesis accompanies all included data.
Network meta-analyses
Network meta-analyses (NMAs) were undertaken within a Bayesian framework in WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK). Where prior distributions were required, they were intended to be vague.
For all NMAs assessing the effectiveness of induction therapy, the reference treatment was no induction/placebo (PBO). For networks evaluating the effectiveness of maintenance therapy, the reference treatment was CSA + AZA. For the outcomes graft loss, mortality and BPAR, fixed- and random-effects models having a binomial likelihood with logit link were used (see code in Appendix 6). For the outcome of GRF, models with a normal likelihood and identify link were used (see code in Appendix 6). All models account for the fact that some RCTs have more than two arms. 68
Trials reporting zero events for all arms for a particular outcome were excluded from the analysis, as these trials would not contribute information to the network. Where a trial had a zero event in at least one, but not all, treatment arms, 0.5 was added to all cells to allow the model to run within WinBUGS version 14 (MRC Biostatistics Unit, Cambridge, UK). 68
Analyses were run with three chains, a burn-in of 40,000 iterations followed by an additional 100,000 iterations, with thinning of every fifth iteration to help convergence. Convergence of the models was assessed by visual inspection of autocorrelation and trace plots for all monitored variables.
Fixed- and random-effects NMAs were analysed and compared using the deviance information criteria (DIC). Models with the lowest DIC were assumed to have a better fit to the data. The posterior medians and 95% credibility intervals (CrIs) are reported.
To assess inconsistency in the network, the inconsistency degrees of freedom (ICDF) were calculated (reflecting the number of independent loops in the network) and inconsistency networks (where only direct evidence for a comparison between treatments is used) were modelled. 69 Results from the inconsistency models were compared with those from the consistency models (where direct and indirect evidence were combined) to help identify inconsistencies within the network. The model with the lowest DIC was assumed to be a better fit to the data.
The NMAs that have been conducted to satisfy relevant items on the Decision Support Unit’s Evidence Synthesis Checklist. 70
Systematic review results
Owing to the number of regimens for both the interventions and comparators, the assessment of effectiveness will be reported separately for induction and maintenance. All RCT evidence identified for each intervention is presented.
Identified research for induction and maintenance therapies
We screened the titles and abstracts of 5079 unique references identified by the searches, with 750 papers retrieved for detailed consideration. As highlighted in Figure 7, a total of 715 papers were excluded (a list of these, with reasons for their exclusion, can be found in Appendix 2). Overall. 107 studies met the inclusion criteria. At both stages, initial disagreements were easily resolved by consensus.
FIGURE 7.
Flow chart: clinical effectiveness review. SR, systematic review.

We then reassessed included studies from the review conducted by Woodroffe et al. 65 (43 studies) (TA85). Of these, 20 studies were considered eligible for inclusion in the update review. 71–90 The scope for the adult review by Woodroffe et al. 65 differed from the final scope issued by NICE; the induction therapy originally included DAC [European Union (EU) marketing authorisation withdrawn in January 2009] and not rATG, the maintenance therapy did not include BEL or EVL, and treatment of AR was included but is outside the scope of this appraisal. Reasons for exclusion from this review include data that were available only in abstract format, population (either participants receiving multiorgan transplant or mixed population of age groups) or duplicate (studies also retrieved in the update searches).
Citations of the included systematic reviews were also searched by two reviewers (HC and MHa). This process revealed an additional two papers.
Update searches were conducted on 18 November 2014 using the same methodology as described earlier. A total of 375 records were screened by three reviewers (TJH, HC and MHa) and 99 records were selected for full-text retrieval. Four papers were judged to be eligible on full-text appraisal. A list of these items, with reasons for their exclusion, can be found in Appendix 2.
The process is illustrated in detail in Figure 7. Note, for the sake of clarity, the figures for the initial and update searches have been combined.
Quality of included studies
We appraised the newly identified trials and those included in the previous HTA review. The reason for reappraising trials from the previous HTA review were twofold: first, to ensure consistency with appraisal of the newer studies, and, second, because we have access to new information from papers that were published after the inclusion date for the previous review. Only primary studies were appraised. Secondary analyses of previously published data were not assessed. Similarly, if a trial was reported in multiple publications, only one quality assessment of the trial was conducted (all publications for that trial were assessed together). In total, 86 trials were assessed (11 induction studies, 73 maintenance studies and two studies of both induction and maintenance treatment). Quality assessments of included trials are presented in Appendix 4. The two trials of both induction and maintenance treatment are repeated in both of these tables.
Overall assessment
The 86 included RCTs49,51,58,59,71–152 were of variable quality, but all appear to be flawed. However, as a result of reporting omissions, for most of the trials it was difficult to make a general assessment regarding quality. The quality appraisal should, therefore, be noted with caution. In fact, six72,73,95–98 of the 14 induction trials, 4075–85,91–94,99–122,153 of the 74 maintenance trials, and one123 of the two trials of both induction and maintenance either did not report, or lacked clarity on, at least five of the 10 items constituting the quality appraisal assessment.
Only four induction studies71–74 and six maintenance studies58,124–127,150 adequately addressed five or more of the 10 items of the quality appraisal assessment. However, even the reports of these trials omitted important information relating to quality, with six71–74,124,125 of the seven failing to clearly describe the procedure used for allocation concealment, and one58 failing to include an intention-to-treat (ITT) analysis.
Seven of the maintenance studies75,76,78,91–94 and two of the induction studies95,96 did not adequately address any of the items in the quality appraisal assessment. Further details of the quality of included studies, according to individual quality appraisal items, are described as follows.
Treatment allocation
Random allocation
The method of random allocation,71,86,128 including the method of sequence generation, was clearly stated and adequate in only two induction studies71,128 and 18 maintenance studies,86,103,110,112,119,122,124,126,127,129–136,150 whereas 65 studies (nine induction studies72–74,87,95,98,137 and 54 maintenance studies51,58,59,75–85,88,89,91–94,99–102,104–109,111,113–118,120,121,125,138–147,152–155) and both of the studies of induction and maintenance treatment123,148 did not clearly specify the method used. The remaining maintenance study149 used a minimisation technique that included a random element.
Concealment of allocation
The method of concealment of allocation was clearly reported in 12 trials (two induction studies,97,128 nine maintenance studies,58,114,129,130,133,140,147,150,152 and one study136 of both induction and maintenance treatment). Fifty-four trials51,72–74,76–79,81–85,87–89,91–93,95,96,98–100,102–106,108–113,115–120,124,127,131,134,135,139,141,143–145,153–155 did not report any information on allocation concealment, whereas 20 trials71,75,80,86,94,101,107,121–123,125,126,132,136–138,142,146,149,156 provided some information pertaining to allocation concealment but lacked sufficient detail or clarity to demonstrate that allocation was adequately concealed.
Similarity of groups
Baseline characteristics
Fifty-seven trials (48 maintenance studies,51,58,77,80–82,84,86,94,99,100,102,104–109,113–117,119–121,124–127,131,132,134,138,139,141–147,149,150,152,154,156,157 eight induction studies71,72,74,87,97,128,137 and one study123 for induction and maintenance) fully reported baseline characteristics. Nine trials (eight maintenance studies88,89,92,110–112,122,148 and one study148 of both induction and maintenance) reported significant baseline between-group differences for key factors, including PRA grade, number of previous transplants, patient age, pretransplant diabetes mellitus, HLA mismatches and ECD donor kidneys. A further six maintenance studies91,101,130,133,140,155 were rated as ‘partial’ because they reported a baseline difference in patient sex.
The remaining trials (four induction studies,71,95,96,98 26 maintenance studies59,75–80,83–85,93,94,103,107,114,115,118,126,127,129,131,132,142,150,152,153 and one study123 of both induction and maintenance) did not provide sufficient information for a judgement to be made about baseline similarity of groups, either by omitting to report sufficient statistical information, by reporting on a very limited range of patient baseline characteristics or by not reporting any patient baseline characteristics.
Implementation of masking
Treatment allocation masked from participants
Five induction studies,87,96,98,128,137 47 maintenance studies51,59,76,78–80,82–84,86,88,92–94,103,105–108,111,113,116,118,125,126,129–135,138–142,144–149,151–153,155 and both of the studies of induction and maintenance treatment123,148 did not blind participants to treatment allocation.
Only two maintenance studies89,124 and four induction studies71–74 made clear that the participants were blinded to treatment allocation. A further four maintenance studies58,77,143,150 were rated as ‘partial’ because it was reported that participants were blinded for a limited period of time only (until 24 weeks for one study58 and until 12 months for the other three studies. 77,143,150
One further induction study95 was rated as ‘unclear’ because, despite being PBO controlled, no further details were reported about blinding. The remaining trials (one induction study97 and 20 maintenance studies75,81,85,91,99–102,104,109,110,112,114,115,117,119–122,127) did not report any information about blinding participants to treatment allocation.
Treatment allocation masked from clinicians
All of the trials that did not blind participants from treatment allocation also failed to mask treatment allocation from clinicians. 51,59,76,78–80,82–84,86–88,92–94,96,98,103,105–108,111,113,116,118,123,125,126,128–135,137–142,144–149,151–153,155 An additional induction study97 also stated that treatment allocation was not masked from clinicians (participant blinding was not reported). Similarly, the four induction studies71–74 and two maintenance studies89,124 that reported blinding participants to treatment allocation also masked treatment allocation from clinicians. Again, four maintenance studies58,77,143,150 were rated as ‘partial’ for clinician blinding because blinding occurred for only a limited time, and one induction study95 was rated as ‘unclear’ because, although it was a PBO-controlled trial, no further details were reported about blinding. The other 20 maintenance studies75,81,85,91,99–102,104,109,110,112,114,115,117,119–122,127 did not report any details about clinician blinding.
Treatment allocation masked from outcome assessors
The majority of trials (52 maintenance studies,51,75–77,79–84,86,89,91–94,99–102,104–106,108,109,111–114,116–122,130,131,133,138,140,141,144–149,151–153,155 nine induction studies,71–73,87,95–98,128 and both of the studies123,148 of induction and maintenance treatment) did not report whether outcome assessors were blind to treatment allocation.
One induction study137 and five maintenance studies78,132,134,135,139 made it clear that the outcome assessors were not blinded to treatment allocation. For fifteen trials58,59,74,85,88,103,107,110,115,124–127,129,142 (one induction study74 and 14 maintenance studies58,59,85,88,103,107,110,115,124–127,129,142) it was clear that outcome assessors were blinded for at least one outcome, and a further two maintenance studies143,150 were given a ‘partial’ rating because the outcome assessors were blinded for the first 12 months of the study.
Completeness of trials
Reporting of all a priori outcomes
All trials were rated as ‘unclear’ with regard to reporting of a priori outcomes. 51,58,59,71–89,91–135,137–153,155 This was because the trial reports failed to explicitly state whether or not all outcomes defined in the study protocol were reported.
Reporting of loss to follow-up, withdrawals and dropouts
Fifty-four trials adequately reported loss to follow-up, withdrawals and dropouts (by providing numbers and reasons by treatment group). Of these, 45 were maintenance studies,51,58,59,80,81,83,84,88,102,104,106–108,111–114,116,118–120,124–127,130–135,138,139,141,142,144–152,155 eight were induction studies,71–74,87,98,128,137 and one148 was a study of both induction and maintenance treatment. In 22 trials (20 maintenance studies76,85,86,91–94,99–101,103,105,109,110,115,121,122,129,140,143 and two induction studies95,96), the reporting of loss to follow-up, withdrawals and dropouts was inadequate, with key information omitted. A further four trials75,79,97,123 (one induction study,97 two maintenance studies75,79 and one study of both induction and maintenance treatment123) were rated as ‘unclear’. For the study of both induction and maintenance, this was because, despite all of the relevant information being provided, the numbers did not appear to tally. For the other three trials,75,79,97 this was because of the fact that all participants appeared to complete the study but this was not explicitly stated. For the remaining six maintenance studies,77,78,82,89,117,153 information regarding loss to follow-up, withdrawals and dropouts was not reported.
Intention-to-treat analysis
Primarily, a strict definition of ITT was used (all randomised and transplanted participants). According to this definition, 48 trials (seven induction studies71–74,87,98,137 and 41 maintenance studies51,59,77,79,80,84,86,88,89,100–102,104,106–108,110,113,115,117,120,121,124–127,129–131,134,135,139,141–143,146,149–153) were rated as adequately performing an ITT analysis, with 19 trials (three induction studies,128,158,159 14 maintenance studies,58,83,91,114,119,132,133,138,140,144145,147,148,155 and both studies123,148 of induction and maintenance treatment) not performing an adequate ITT analysis. In 16 cases (two induction studies96,97 and 14 maintenance studies75,76,81,82,92–94,99,103,105,109,111,112,116) there was a lack of clarity regarding whether or not an ITT analysis had been conducted. The other five trials (one induction95 and four maintenance studies78,85,118,122) did not report any relevant information regarding whether or not an ITT analysis had been conducted.
A secondary definition of ITT analysis was also used (all randomised and transplanted participants or < 10% excluded). When this definition was applied, 13 of the trials previously rated as inadequate were instead rated as adequate (11 maintenance studies58,83,114,119,132,133,138,140,147,148,155 and both of the studies123,148 of induction and maintenance treatment). Thus, only four trials91,128,144,145 did not perform an adequate ITT analysis. The number of trials rated as ‘unclear’ or ‘not reported’ did not change when this definition of ITT was used.
Applicability of trials to the NHS
Applicability to the current NHS in England
Only 11 trials (one induction study,74 nine maintenance studies51,58,86,114,124,125,132,133,155 and one study123 of both induction and maintenance) were adequately applicable to the current NHS in England. The majority of trials (seven induction studies,71,87,95,97,98,128,137 41 maintenance studies,59,75,77–82,84,85,88,89,93,94,99,101,109,112,115–118,120,129–131,134,135,138,139,141,142,144–152 and one study148 of both induction and maintenance) were limited in some way with regard to applicability to the current NHS in England. In all except one of these trials this was primarily as a result of the fact that patients, donors or organ characteristics were not representative of the current NHS in England (e.g. > 90% deceased donors or ‘suboptimal transplants’ or ‘high risk of rejection population’). In the other trial135 this was primarily owing to a lack of statistical power.
The remaining three induction studies72,73,96 and 23 maintenance studies76,83,91,92,100,102–108,110,111,113,119,121,122,126,127,140,143,153 were rated as ‘unclear’ regarding applicability to the current NHS in England. The primary reason for this was as follows: the study lacked clarity regarding key demographic or patient–donor characteristics (two induction studies73,96 and 10 maintenance studies76,83,91,92,102–104,107,113,140); the study was based on a non-EU population (two induction studies72,159 and 13 maintenance studies100,105,106,108,110,111,119,121,122,126,127,143,153).
Study characteristics
Induction therapies
Thirteen studies71–74,87,95–98,123,128,137,148 were identified focusing on induction therapies.
Details of study characteristics can be found in Appendix 5.
The majority of trials report outcomes up to 1 year, with the period of induction therapy generally continued for up to 14 days. No data for HRQoL were identified. It should be noted that, for some studies, the dose no longer reflects clinical practice; however, there were insufficient data for further analysis. Where a higher and lower dose was used in the RCT, the lower dose was selected for investigation.
Overall, no new evidence has been identified for BAS vs. PBO and additional data has been added to both rATG vs. no induction and BAS vs. no induction (Table 9). 96,148,158,160 All data for rATG compared with no induction has been identified by the PenTAG search.
| Study | Induction therapy | Included in TA85 | Update review | n a | Maintenance used |
|---|---|---|---|---|---|
| Bingyi 200395 | BAS vs. PBO | ✓b | 12 | CSA + AZA + CCSs | |
| Kahan 199972 | ✓ | 346 | CSA + CCSs | ||
| Lawen 200374 | ✓c | 123 | CSA + MMF + CCSs | ||
| Nashan 199771 | ✓ | 380 | CSA + CCSs | ||
| Ponticelli 200173 | ✓ | 340 | CSA + AZA + CCSs | ||
| Albano 2013123 | BAS vs. no induction | ✓ | 1251 | CSA + MMF + CCSs | |
| Sheashaa 200397 | ✓b | 100 | CSA + AZA + CCSs | ||
| Kyllönen 2007128 | ✓ | 102 | CSA + AZA + CCSs | ||
| Charpentier 200196,158 | rATG vs. no induction | ✓ | 309 | TAC + AZA + CCSs | |
| Charpentier 2003148,160 | ✓ | 371 | TAC + AZA + CCSs | ||
| Brennan 2006137 | BAS vs. rATG | ✓ | 278 | CSA + MMF + CCSs | |
| Lebranchu 200287 | ✓c | 100 | CSA + MMF + CCSs | ||
| Mourad 200498,159 | ✓ | 105 | CSA + MMF + CCSs |
Maintenance therapies
Seventy-five studies were identified focusing on a combination of 30 maintenance therapy comparisons (Table 10). Details of study characteristics can be found in Appendix 5.
| Study (multiple publications) | Maintenance therapy | Included in TA85 | Update review | n |
|---|---|---|---|---|
| Schleibner 199579 | TAC + AZA vs. CSA + AZA | ✓ | 47 | |
| Laskow 199680 (Vincenti 1996161) | ✓ | 120 | ||
| Mayer 199788 (Mayer 1999,162 2002163) | ✓ | 448 | ||
| Radermacher 199881 | ✓ | 41 | ||
| Jarzembowski 200599 | ✓ | 35 | ||
| Baboolal 200282 | ✓ | 51 | ||
| Campos 200283 | ✓ | 166 | ||
| Margreiter 200284 (Krämer 2005,164 2008165) | ✓ | 560 | ||
| Van Duijnhoven 200275 | ✓ | 23 | ||
| Waller 200276 (Murphy 2003166) | ✓ | 102 | ||
| Charpentier 2003148 | ✓ | 555 | ||
| Töz 200485 | ✓ | 35 | ||
| Hardinger 2005100 (Brennan 2005167) | ✓ | 200 | ||
| Sollinger 199577 | CSA + MMF low vs. CSA + AZA vs. CSA + MMF | ✓ | 499 | |
| Tricontinental MMF renal study 199689 (Mathew 1998,168 Clayton 2012169) | ✓ | 497 | ||
| Sadek 200286 | CSA + MMF vs. CSA + AZA | ✓ | 477 | |
| Tuncer 200278 | ✓ | 76 | ||
| Merville 2004138 | ✓ | 71 | ||
| Remuzzi 2007101 (The MYSS trial, Remuzzi 2004170) | ✓ | 336 | ||
| Wlodarczyk 2005139 (Wlodarczyk 2002171) | TAC + MMF vs. CSA + AZA | ✓ | 489 | |
| Vacher-Coponat 2012129 | ✓ | 289 | ||
| Zadrazil 2012102 | TAC + MMF vs. CSA + MMF | ✓ | 53 | |
| Hernández 2007130 | ✓ | 240 | ||
| Rowshani 2006103 | ✓ | 126 | ||
| Yang 199990 (Ulsh 1999153) | ✓ | 60 | ||
| Weimer 2006104 (Weimer 2005172) | TAC + AZA vs. CSA + AZA vs. CSA + MMF | ✓ | 81 | |
| Wlodarczyk 2009140 | TAC + MMF vs. TAC-PR + MMF | ✓ | 122 | |
| Krämer 201058 | ✓ | 667 | ||
| Tsuchiya 2013141 | ✓ | 102 | ||
| Oh 2014105 | ✓ | 104 | ||
| Albano 2013123 (OSAKA trial) | TAC + MMF vs. TAC-PR 0.2 mg/kg/day+ MMF vs. TAC-PR 0.3 mg/kg/day | ✓ | 1251 | |
| Ciancio 2008106 (Ciancio 2011173) | MMF + TAC vs. MPS + TAC | ✓ | 150 | |
| Salvadori 2004124 | MMF + CSA vs. MPS + CSA | ✓ | 423 | |
| Vincenti 2005125 (Vincenti 2010156) | BEL low + MMF vs. BEL high + MMF vs. CSA + MMF | ✓ | 218 | |
| BENEFIT (Vincenti 2010,59 Larsen 2010,60 Vincenti 2012,61 Rostaing 201362) | ✓ | 686 | ||
| BENEFIT-EXT (Durrbach 2010,142 Medina Pestana 2012,174 Charpentier 2013,175 Larsen 201060) | ✓ | 578 | ||
| Ferguson 2011126 | BEL + MMF vs. BEL + SRL vs. TAC + MMF | ✓ | 89 | |
| Lorber 2005143 | EVL low + CSA vs. EVL high + CSA vs. MMF + CSA | ✓ | 583 | |
| ATLAS Vítko 2005150 (Vítko 2004,176 2005177) | ✓ | 588 | ||
| Takahashi 2013131 | ✓ | 122 | ||
| Chadban 2013152 (SOCRATES) | EVL vs. EVL + CSA vs. CSA + MPS | ✓ | 126 | |
| Tedesco-Silva 2010107 | EVL low + CSA vs. EVL high + CSA vs. MPA + CSA | ✓ | 783 | |
| Bertoni 2011144 | EVL + CSA vs. MPS + CSA | ✓ | 106 | |
| Budde 2011132 (Budde 2012,178 Liefeldt 2012179) | EVL + MPS vs. CSA + MPS | ✓ | 300 | |
| Mjörnstedt 2012133 | ✓ | 202 | ||
| Barsoum 2007108 | SRL + CSA vs. MMF + CSA | ✓ | 113 | |
| Stallone 2004109 | ✓ | 90 | ||
| Anil Kumar 2005110 | SRL + TAC vs. MMF + TAC | ✓ | 150 | |
| Mendez 2005111 (Gonwa 2003180) | ✓ | 361 | ||
| Sampaio 2008112 | ✓ | 100 | ||
| Gelens 2006113 | ✓ | 54 | ||
| Gallon 2006145 (Chhabra 2012181) | ✓ | 83 | ||
| Van Gurp 2010114 | ✓ | 634 | ||
| Flechner 2002127 (Flechner 2004,182 2007183) | SRL + MMF vs. CSA + MMF | ✓ | 61 | |
| Noris 2007115 (Ruggenenti 2007184) | ✓ | 21 | ||
| Lebranchu 2009149 (Servais 2009,185 Lebranchu 2011,186 Joannides 2011187) | ✓ | 192 | ||
| Büchler 2007134 (Lebranchu 2012,188 Joannides 2010189) | ✓ | 145 | ||
| Soleimani 201391 | ✓ | 88 | ||
| Durrbach 2008146 | ✓ | 69 | ||
| Kreis 2000116 – identified from Campistol 2005190 | ✓ | 78 | ||
| Guba 2010147 | ✓ | 140 | ||
| Martinez-Mier 2006117 | ✓ | 41 | ||
| Nafar 2012118 | ✓ | 100 | ||
| Larson 2006151 (Stegall 2003191) | TAC + MMF vs. SRL + MMF | ✓ | 162 | |
| Schaefer 200692 | ✓ | 80 | ||
| Heilman 2011135 (Heilman 2012157) | ✓ | 122 | ||
| Smith 200893 | ✓ | 51 | ||
| Silva 2013119 | TAC + MPS vs. SRL + MPS | ✓ | 204 | |
| Hamdy 2005120 (Hamdy 2008,192 Hamdy 2010193) | TAC + SRL vs. MMF + SRL | ✓ | 132 | |
| Charpentier 2003136 (Groth 1999194) | SRL + AZA vs. CSA + AZA | ✓ | 83 | |
| Chen 2008121 | TAC + SRL vs. CSA + SRL | ✓ | 41 | |
| Vítko 200694 | SRL low + TAC vs. SRL high + TAC vs. MMF + TAC | ✓ | 977 | |
| Flechner 2011155 (ORION study) | SRL + TAC vs. SRL + MMF vs. MMF + TAC | ✓ | 450 | |
| Grinyo 200951 (SYMPHONY study, Ekberg 2009,195 2010,196 Demirbas 2009,197 Frei 2010,198 Claes 2012199) | MMF + CSA vs. MMF + low CSA vs. MMF + low TAC vs. MMF low SRL (one study) | ✓ | 1529 | |
| Anil Kumar 2008122 (Anil Kumar 2005110) | TAC + MMF vs. TAC + SRL vs. CSA + MMF vs. CSA + SRL | ✓ | 200 |
Outcomes are reported up to a maximum of 5 years, although the majority of data available is reported at 1 year. No data for HRQoL were identified. As for induction therapy RCTs, in some cases the dose no longer reflects clinical practice; however, there were insufficient data for further analysis. When a higher and lower dose was used in the RCT, the lower dose was selected for investigation.
Other than for the TAC + AZA against CSA + AZA combination, the majority of data were identified by the PenTAG search.
Population characteristics
Induction therapies
Baseline characteristics of trial participants for induction therapy are summarised in Table 11.
| Study | Maintenance therapy | Arm | n | Mean age, years (SD) | Male (%) | Donor type (%) | Race (%) | Mean HLA mismatches (SD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Living | DBD | DCD | ECD | Cadaveric | ||||||||
| BAS vs. PBO (five studies) | ||||||||||||
| Bingyi 200395 | CSA + AZA + CCSs | BAS | 6 | 35–59 (range) | 4 (67) | NR | NR | NR | NR | NR | NR | NR |
| PBO | 6 | 36–54 (range) | 5 (83) | NR | NR | NR | NR | NR | NR | NR | ||
| Kahan 199972 | CSA + CCSs | BAS | 173 | 44.9 (11.79) | 111 (64) | 54 (31) | 0 | 0 | 0 | 119 (69) | White 117 (68) African American 47 (27) Asian 0 (0) Other 9 (5) |
4.0 (1.44) |
| PBO | 173 | 46.2 (12.0) | 108 (62) | 51 (29) | 0 | 0 | 0 | 122 (71) | White 106 (61) African American 59 (34) Asian 5 (3) Other 3 (52) |
3.9 (1.37) | ||
| Lawen 200374 | CSA + MMF + CCSs | BAS | 59 | 45.4 (13.1) | 45 (76.3) | 16 (27.1) | 0 | 0 | 0 | 43 (72.9) | White 52 (88.1) Black 6 (10.2) Asian 1 (1.7) |
3.0 (1.5) |
| PBO | 64 | 45.9 (12.1) | 41 (64.1) | 14 (21.9) | 0 | 0 | 0 | 50 (78.1) | White 58 (90.6) Black 6 (4.7) Asian 1 (4.7) |
3.3 (1.5) | ||
| Nashan 199771 | CSA + CCSsa | BAS | 193 | 49.0 (median) 18–74 (range) | 126 (66.3) | NR | NR | NR | NR | 190 (100) | White 179 (94.2) Black 3 (1.6) Other 8 (4.2) |
3.2 (1.2) |
| PBO | 187 | 48.0 (median) 18–73 (range) | 118 (63.4) | NR | NR | NR | NR | 186 (100) | White 179 (96.2) Black 1 (0.5) Other 6 (3.2) |
3.0 (1.2) | ||
| Ponticelli 200173 | CSA + AZA + CCSs | BAS | 168 | 44.2 (13.5) | 110 (65.5) | 27 (16.1) | 0 | 0 | 0 | 141 (83.9) | White 146 (86.9) Black 1 (0.6%) Oriental 1 (0.6%) Other 20 (11.9%) |
2.9 (1.4) |
| PBO | 172 | 44.2 (13.0) | 118 (68.8) | 32 (18.6) | 0 | 0 | 0 | 140 (81.4) | White 150 (87.2) Black 2 (1.2%) Oriental 2 (1.2%) Other 18 (10.5%) |
2.9 (1.4) | ||
| BAS vs. no induction (three studies) | ||||||||||||
| Albano 2013123 | TAC + MMF + CCSsb | BAS | 283 | 49.3 (13.5) | 185 (65.4) | 36 (12.7) | 0 | 5 (1.8) | 158 (55.8) | 247 (87.3) | White 265 (93.6) Black 11 (3.9) Asian, other 7 (2.5) |
3.0 |
| No induction | 302 | 50.7 (13.0) | 206 (68.2) | 34 (11.3) | 0 | 3 (1.0) | 155 (51.3) | 268 (88.7) | White 284 (94.0) Black 14 (4.6) Asian, other 4 (1.3) |
3.1 | ||
| Sheashaa 200397 | CSA + AZA + CCSs | BAS | 50 | 32.9 (9.9) | 44 (88) | 50 (100) | 0 | 0 | 0 | 0 | NR | < 3; n = 9 3; n = 34 ≥ 4; n = 7 |
| No induction | 50 | 32.5 (10.8) | 41 (82) | 50 (100) | 0 | 0 | 0 | 0 | NR | < 3; n = 9 3; n = 31 ≥ 4; n = 10 |
||
| Kyllönen 2007128 | CSA + AZA + CCSs | rATG | 53 | 47.8 (22–64), range | 14 (26) | 0 | NR | NR | NR | 53 (100) | NR | 2.13 |
| BAS | 58 | 45.5 (22–65), range | 27 (46) | 0 | NR | NR | NR | 58 (100) | NR | 2.19 | ||
| No induction | 44 | 47.5 (28–64), range | 15 (34) | 0 | NR | NR | NR | 44 (100) | NR | 2.48 | ||
| rATG vs. no induction (two studies) | ||||||||||||
| Charpentier 200196 | TAC + AZA + CCSs | rATG | 151 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| No induction | 158 | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Charpentier 2003148 | TAC + AZA + CCSsc | rATG | 186 | 44.7 (11.4) | 118 (63.4) | 0 | NR | NR | NR | 186 (100) | White 169 (90.9) Black 7 (3.8) Other 10 (5.4) |
2.8 |
| No induction | 185 | 44.5 (11.0) | 121 (65.4) | 0 | NR | NR | NR | 185 (100) | White 170 (91.9) Black 5 (2.7) Other 10 (5.4) |
2.9 | ||
| BAS vs. rATG (three studies) | ||||||||||||
| Brennan 2006137 | CSA + MMF + CCSs | BAS | 137 | 49.7 (13.0) | 82 (59.9) | 0 | NR | 6 (4.4) | NR | 82 (100) | White 89 (65.0) Black 39 (28.5) American Indian 0 Asian 3 (2.2) Other 6 (4.4) |
NR |
| rATG | 141 | 51.3 (13.1) | 79 (56.0) | 0 | NR | 7 (5.0) | NR | 79 (100) | White 85 (60.3) Black 41 (29.1) American Indian 1 (0.7) Asian 4 (2.8) Other 10 (7.1) |
NR | ||
| Lebranchu 200287 | CSA + MMF + CCSs | BAS | 50 | 44.1 (11.5) | 36 (72.0) | 0 | NR | NR | NR | 50 (100) | White 46 (92.0) Other 4 (8.0%) |
3.5 |
| rATG | 50 | 45.8 (10.8) | 32 (64.0) | 0 | NR | NR | NR | 50 (100) | White 47 (94.0%) Other 3 (6.0%) |
3.5 | ||
| Mourad 200498 | CSA + MMF + CCSs | BAS | 52 | 45.3 (12.4) | 30 (28.6) | 2 (3.8) | NR | NR | NR | 50 (96.2) | NR | NR |
| rATG | 53 | 45.4 (12.7) | 32 (30.5) | 1 (1.8) | NR | NR | NR | 52 (98.2) | NR | NR | ||
Mean age across studies ranges from 30.3 to 51.3 years. Men generally represented a higher proportion of the participants (57.5–76.3%) other than in the study reported by Mourad et al. ,98 in which men constituted 28.6% and 30.5% of the BAS and rATG arms, respectively.
Earlier papers tended to record cadaveric donors, with no further details; however, newer trials report deceased donors as DCD, DBD and ECD. Four studies71,87,128,148 used only cadaveric donors and one study97 used only living donors. In the remainder of the studies, the donors were either mixed or not reported.
The majority of studies had a high proportion of white participants: 60.3–96.2%. Brennan et al. 167 and Kahan et al. 72 report a comparatively high percentage of black participants in the BAS and rATG arms, respectively (28.5% and 29.1%; 27% and 34%, respectively).
The mismatching of HLAs ranges from 2.13 to 4 (see Chapter 1, Management of kidney transplant). Although a close antigen match is no longer considered to be critical because immunosuppressive therapy is more effective, a better HLA match may lead to longer the graft survival.
Maintenance therapies
Baseline characteristics of trial participants for maintenance therapy are summarised in Table 12.
| Study (multiple publications) | Included in TA85 | Induction therapy | Arm | n | Age (years) | Male (%) | Donor type (%) | Race (%) | HLA mismatches (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Living | DBD | DCD | ECD | Cadaveric | |||||||||
| TAC + AZA vs. CSA + AZA (13 studies) | |||||||||||||
| Schleibner 199579 | ✓ | CCSs | TAC | 31 | 46.1 | NR | NR | NR | NR | NR | NR | NR | NR |
| CSA | 16 | 45.1 | NR | NR | NR | NR | NR | NR | NR | NR | |||
| Laskow 199680 | ✗ | rATG + CCSs | Low TAC | 33 | 44.0 | 24 (73) | 0 | 0 | 0 | 0 | 33 (100) | White 17 (51.5) African American 7 (21.2) Asian 6 (18.2) Hispanic 3 (9.1) Other 0 |
NR |
| Medium TAC | 30 | 44.3 | 15 (50) | 0 | 0 | 0 | 0 | 30 (100) | White 11 (36.7) African American 11 (36.7) Asian 4 (13.3) Hispanic 4 (13.3) Other 0 |
NR | |||
| High TAC | 29 | 44.1 | 21 (72) | 0 | 0 | 0 | 0 | 29 (100) | White 19 (65.5) African American 6 (20.7) Asian 1 (3.4) Hispanic 1 (3.4) Other 2 (6.9) |
NR | |||
| CSA | 28 | 46.6 | 22 (79) | 0 | 0 | 0 | 0 | 28 (100) | White 15 (53.6) African American 6 (21.4) Asian 2 (7.1) Hispanic 3 (17.9) Other 0 |
NR | |||
| Mayer 199788 (Mayer 2002,163 1999162) | ✓ | CCSs | TAC | 303 | 46.6 | 196 (64.7) | 0 | 0 | 0 | 0 | 303 (100) | NR | NR |
| CSA | 145 | 45.8 | 92 (63.4) | 0 | 0 | 0 | 0 | 145 (100) | NR | NR | |||
| Radermacher 199881 | ✓ | CCSs | TAC | 28 | 41.3 | 63 | 0 | 0 | 0 | 0 | 28 (100) | NR | HLA (loci A) match 0.81 HLA (loci B) match 0.89 HLA (loci DR) match 0.35 |
| CSA | 13 | 47.1 | 50 | 0 | 0 | 0 | 0 | 13 (100) | NR | HLA (loci A) match 0.85 HLA (loci B) match 0.77 HLA (loci DR) match 0.39 |
|||
| Jarzembowski 200599 | ✗ | OKT3 + CCSs | TAC | 14 | 44 | 8 (57.1) | 0 | 0 | 0 | 0 | 14 (100) | African American: 14 (100) | 3.8 |
| CSA | 21 | 46 | 16 (76.2) | 0 | 0 | 0 | 0 | 21 (100) | African American: 21 (100) | 4.5 | |||
| Baboolal 200282 | ✓ | CCSs | TAC | 27 | 41 | 49 | 0 | 0 | 0 | 0 | 27 (100) | NR | 2.4 |
| CSA | 24 | 42 | 48 | 0 | 0 | 0 | 0 | 24 (100) | NR | 2.5 | |||
| Campos 200283 | ✓ | CCSs | TAC | 85 | 40.5 | 41 (48) | 46 (54) | 0 | 0 | 0 | 39 (46) | NR | NR |
| CSA | 81 | 40.9 | 45 (56) | 39 (48) | 0 | 0 | 0 | 42 (52) | NR | NR | |||
| Margreiter 200284 (Krämer 2005,164 2008165) | ✓ | CCSs | TAC | 287 | 42.4 | 200 (69.9) | 13 (4.5) | 0 | 0 | 0 | 273 (95.5) | White 283 (99.0) Black 0 (0) Oriental 3 (1.0) |
Loci A: 0.83 Loci B: 0.99 Loci DR: 0.66 |
| CSA | 273 | 43.8 | 171 (63.1) | 8 (3.0) | 0 | 0 | 0 | 263 (97.0) | White 270 (99.6) Black 1 (0.4) Oriental 0 (0) |
Loci A: 0.86 Loci B: 1.00 Loci DR: 0.68 |
|||
| Van Duijnhoven 200275 | ✓ | CCSs | TAC | 11 | 45.4 | 8 (72.7) | 0 | 0 | 0 | 0 | 11 (100) | White 11 (100) | NR |
| CSA | 12 | 46.8 | 9 (75.0) | 0 | 0 | 0 | 0 | 12 (100) | White 12 (100) | NR | |||
| Waller 200276 (Murphy 2003166) | ✓ | CCSs | TAC | 52 | 45 | 32 (61.5) | 9 (17.3) | 0 | 21 (40.4) | 0 | 22 (42.3) | NR | (A, B, DR loci) 0: 4 (8) 1: 4 (8) 2: 10 (20) 3: 16 (32) 4: 13 (26) 5: 3 (6) 6: 0 (0) |
| CSA | 50 | 45 | 35 (70) | 8 (16) | 0 | 21 (42) | 0 | 21 (42) | NR | (A, B, DR loci) 0: 7 (13) 1: 2 (4) 2: 8 (15) 3: 16 (31) 4: 16 (31) 5: 3 (6) 6: 0 (0) |
|||
| Charpentier 2003148 | ✗ | rATG + CCSs | TAC | 186 | 44.7 | 118 (63.4) | 0 | 0 | 0 | 0 | 186 (100) | White 169 (90.9) Black 7 (3.8) Other 10 (5.4) |
2.8 |
| CSA | 184 | 43.6 | 116 (63.0) | 0 | 0 | 0 | 0 | 184 (100) | White 162 (88.8) Black 11 (6.0) Other 11 (6.0) |
2.7 | |||
| CCSs | TAC | 185 | 44.5 | 121 (65.4) | 0 | 0 | 0 | 0 | 185 (100) | White 170 (91.9) Black 5 (2.7) Other 10 (5.4) |
2.9 | ||
| Töz 200485 | ✓ | CCSs | TAC | 17 | 35 | 10 (58.8) | 12 (70.6) | 0 | 0 | 0 | 5 (29.4) | NR | NR |
| CSA | 18 | 30 | 12 (66.7) | 14 (77.8) | 0 | 0 | 0 | 4 (22.2) | NR | NR | |||
| Hardinger 2005100 (Brennan 2005167) | ✗ | rATG + CCSs | TAC | 134 | 44 | 86 (64) | 55 (41) | 0 | 0 | 0 | 79 (59) | White 106 (79) African American 24 (18) Other 4 (3) |
2.28 |
| CSA | 66 | 46 | 40 (61) | 32 (48) | 0 | 0 | 0 | 34 (52) | White 52 (79) African American 12 (18) Other 2 (3) |
2.48 | |||
| CSA + MMF low vs. CSA + AZA vs. CSA + MMF (two studies) | |||||||||||||
| Sollinger 199577 | ✓ | rATG + CCSs | MMF low | 167 | 45.1 | 95 (57) | 0 | 0 | 0 | 0 | 167 (100) | White 101 (60.5) Black 44 (26.3) Hispanic 15 (9.0) Asian 2 (1.2) Other 5 (3.0) |
0: 11 (7) 1: 4 (2) 2: 17 (10) 3: 35 (21) 4: 48 (29) 5: 31 (19) 6: 1 |
| MMF high | 166 | 46.1 | 98 (59) | 0 | 0 | 0 | 0 | 166 (100) | White 118 (71.1) Black 33 (19.9) Hispanic 11 (6.6) Asian 3 (1.8) Other 1 (0.6) |
0: 10 (6) 1: 5 (3) 2: 17 (10) 3: 39 (23) 4: 49 (30) 5: 34 (20) 6: 0 |
|||
| AZA | 166 | 45.9 | 95 (57) | 0 | 0 | 0 | 0 | 100 | White 103 (62.0) Black 40 (24.1) Hispanic 14 (8.4) Asian 6 (3.6) Other 3 (1.8) |
0: 14 (8) 1: 6 (4) 2: 12 (7) 3: 40 (24) 4: 42 (25) 5: 40 (24) 6: 11 (7) |
|||
| Tricontinental MMF renal study 199689 (Matthew 1998,168 Clayton 2012169) | ✓ | CCSs | MMF low | 173 | 46 | 93 (53.8) | 0 | 0 | 0 | 0 | 173 (100) | NR | NR |
| MMF high | 164 | 46 | 98 (59.8) | 0 | 0 | 0 | 0 | 164 (100) | NR | NR | |||
| AZA | 166 | 47 | 111 (66.9) | 0 | 0 | 0 | 0 | 166 (100) | NR | NR | |||
| CSA + MMF vs. CSA + AZA (four studies) | |||||||||||||
| Sadek 200286 | ✓ | MMF | 162 | 43.9 | 115 (71) | NR | NR | NR | NR | 139 (86) | White 148 (91.4) Black 3 (1.2) Asian 4 (2.5) Other 8 (4.9) |
NR | |
| CCSs | AZA | 157 | 43.9 | 94 (59.9) | NR | NR | NR | NR | 137 (87) | White 142 (90.4) Black 5 (3.2) Asian 5 (3.2) Other 5 (3.2) |
NR | ||
| MMF/AZA | 158 | 44.7 | 102 (64.6) | NR | NR | NR | NR | 136 (86) | White 142 (89.9) Black 7 (4.4) Asian 6 (3.8) Other 3 (1.9) |
NR | |||
| Tuncer 200278 | ✓ | rATG + CCSs | MMF | 38 | 34.8 | 27 (71.1) | 32 (84.2) | 0 | 0 | 0 | 6 (15.8) | NR | 2.5 |
| AZA | 38 | 41.4 | 28 (73.7) | 29 (76.3) | 0 | 0 | 0 | 9 (23.7) | NR | 2.7 | |||
| Merville 2004138 | ✗ | rATG + CCSs | MMF | 37 | 44 | 26 (78.4) | 0 | 0 | 0 | 0 | 37 (100) | NR | 2.7 |
| AZA | 34 | 47 | 23 (58.8) | 0 | 0 | 0 | 0 | 34 (100) | NR | 2.8 | |||
| Remuzzi 2007101 (The MYSS trial, Remuzzi 2004170) | ✗ | CCSs | MMF | 168 | 43.3 | 119 (71) | 0 | 0 | 0 | 0 | 168 (100) | NR | 0: 3 (2) 1: 42 (25) 2: 71 (42) 3: 45 (27) Missing: 7 (4%) |
| AZA | 168 | 45.9 | 100 (60) | 0 | 0 | 0 | 0 | 168 (100) | NR | 0: 6 (4) 1: 40 (24) 2: 82 (49) 3: 33 (20) Missing: 7 (4) |
|||
| TAC + MMF vs. CSA + AZA (two studies) | |||||||||||||
| Wlodarczyk 2005139 (Wlodarczyk 2002171) | ✗ | CCSs | TAC + MMF | 243 | 43.8 | 156 (64.2) | 9 (3.7) | 0 | 0 | 0 | 234 (96.3) | NR | 2.8 |
| TAC + AZA | 246 | 42.1 | 157 (63.8) | 11 (4.5) | 0 | 0 | 0 | 235 (95.5) | NR | 2.6 | |||
| Vacher-Coponat 2012129 | ✗ | rATG + CCSs | TAC + MMF | 143 | 46 | 87 (61) | 0 | 0 | 0 | 0 | 143 (100) | NR | 2.83 |
| CSA + AZA | 146 | 47 | 89 (61) | 0 | 0 | 0 | 0 | 146 (100) | NR | 2.84 | |||
| TAC + MMF vs. CSA + MMF (four studies) | |||||||||||||
| Zadrazil 2012102 | ✗ | CCSs | TAC | 24 | 52.9 | 18 (75.0) | NR | NR | NR | NR | NR | NR | NR |
| CSA | 29 | 54.4 | 16 (55.2) | NR | NR | NR | NR | NR | NR | NR | |||
| Hernández 2007130 | ✗ | BAS + rATG + CCSs | TAC + MMF | 80 | 47 | 44 (55) | 0 | 0 | 0 | 0 | 80 (100) | White (100) | 3.8 |
| CSA + MMF | 80 | 48 | 50 (62.5) | 0 | 0 | 0 | 0 | 80 (100) | White (100) | 3.7 | |||
| CSA + AZA | 80 | 47 | 59 (73.8) | 0 | 0 | 0 | 0 | 80 (100) | White (100) | 3.4 | |||
| Rowshani 2006103 | ✗ | BAS + CCSs | TAC | 63 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| CSA | 63 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||
| Yang 199990 (Ulsh 1999153) | ✓ | CCSs | TAC | 30 | 46.5 | 16 (52) | NR | NR | NR | NR | 19 (62.9) | White 24 (81) | DR (19) A/B 21/23 |
| CSA | 30 | 46.8 | 21 (69) | NR | NR | NR | NR | 23 (76.9) | White 28 (92) | DR (16) A/B 19/22 |
|||
| TAC + AZA vs. CSA + AZA vs. CSA + MMF (one study) | |||||||||||||
| Weimer 2006104 (Weimer 2005172) | ✗ | rATG | TAC + AZA | 28 | 45 | 18 (64.3) | 7 (25) | 0 | 0 | 0 | 21 (75) | NR | HLA-A, B, DR: 2.5 HLA-B, DR: 1.6 |
| CSA + AZA | 25 | 50 | 13 (52.0) | 4 (16) | 0 | 0 | 0 | 21 (84) | NR | HLA-A, B, DR: 2.2 HLA-B, DR: 1.6 |
|||
| CSA + MMF | 28 | 44 | 9 (29.0) | 9 (32) | 0 | 0 | 0 | 19 (68) | NR | HLA-A, B, DR: 2.7 HLA-B, DR: 2.1 |
|||
| TAC + MMF vs. TAC-PR + MMF (four studies) | |||||||||||||
| Wlodarczyk 2009140 | ✗ | CCSs | TAC | 59 | 43.6 | 44 (74.6) | NR | NR | NR | NR | NR | White 59 (100) | NR |
| TAC-PR | 63 | 44.0 | 36 (56.7) | NR | NR | NR | NR | NR | White 61 (96.7) Black (0) Asian (0) Other 2 (3.3) |
NR | |||
| Krämer 201058 | ✗ | CCSs | TAC | 336 | 45.5 | 215 (64) | 92 (27.4) | 0 | 0 | 0 | 244 (72.6) | White 273 (81.6) Black 19 (5.7) Asian 7 (2.1) Other 37 (11) |
Mean A: 1.0 Mean B: 1.2 Mean DR: 0.8 |
| TAC-PR | 331 | 44.9 | 204 (61.6) | 89 (26.9) | 0 | 0 | 0 | 242 (73.1) | White 277 (83.7) Black 14 (4.2) Asian 5 (1.5) Other 35 (10.6) |
Mean A: 1.0 Mean B: 1.1 Mean BR: 0.9 |
|||
| Tsuchiya 2013141 | ✗ | BAS + CCSs | TAC | 52 | 46.1 | 35 (67.3) | NR | NR | NR | NR | NR | NR | 2.6 |
| TAC-PR | 50 | 47.5 | 34 (68.0) | NR | NR | NR | NR | NR | NR | 2.9 | |||
| Oh 2014105 | ✗ | BAS + CCSs | TAC | 31 | 46.9 | 16 (57.1) | 16 (51.6) | 0 | 0 | 0 | 15 (48.4) | NR | 0–2: 6 (19.4) 3–4: 16 (51.6) 5–6: 9 (29.0) |
| TAC-PR | 29 | 44.5 | 17 (58.6) | 17 (58.6) | 0 | 0 | 0 | 12 (41.4) | NR | 0–2: 6 (20.7) 3–4: 13 (44.8) 5–6: 10 (34.5) |
|||
| TAC + MMF vs. TAC-PR 0.2 + MMF vs. TAC-PR 0.3 (one study) | |||||||||||||
| Albano 2013123 (OSAKA trial) | ✗ | CCSs | TAC | 320 (309) | 50.8 | 211 (68.3) | 41 (13.3) | 0 | 0 | 0 | 268 (86.7) | White 296 (95.8) Black 7 (2.3) Other 6 (1.9) |
3.1 |
| TAC-PR low | 316 (302) | 50.7 | 206 (68.2) | 34 (11.3) | 0 | 0 | 0 | 268 (88.7) | White 284 (94.0) Black 14 (4.6) Other 4 (1.3) |
3.1 | |||
| TAC-PR high | 317 (304) | 50.2 | 204 (67.1) | 33 (10.9) | 0 | 0 | 0 | 271 (89.1) | White 291 (95.7) Black 7 (2.3) Other 6 (2.0) |
3.2 | |||
| TAC-PR low + BAS | 298 (283) | 49.3 | 185 (65.4) | 36 (12.7) | 0 | 0 | 0 | 247 (87.3) | White 265 (93.6) Black 11 (3.9) Other 7 (2.5) |
3.0 | |||
| MMF + TAC vs. MPS + TAC (one study) | |||||||||||||
| Ciancio 2008106 (Ciancio 2011173) | ✗ | rATG + DAC + CCSs | MMF | 75 | 49.7 | 50 (66.7) | 14 (18.7) | 0 | 2 (2.7) | 1 (1.3) | 65.3 [+ 2 (2.7) paediatric en bloc and 7 (9.3) double kidneys] | White 30 (40.0) Hispanic 22 (29.3) African American 20 (26.7) Other 3 (4.0) |
3.87 |
| MPS | 75 | 51.1 | 25 (74.7) | 8 (10.7) | 0 | 3 (4.0) | 4 (6.7) | 65.3 [+ 2 (2.7) paediatric en bloc and 8 (10.7) double kidneys] | White 24 (32.0) Hispanic 23 (30.7) African American 24 (32.0) Other 4 (5.3) |
3.95 | |||
| MMF + CSA vs. MPS + CSA (one study) | |||||||||||||
| Salvadori 2004124 | ✗ | CCSs | MMF | 210 | 47.2 | 142 (67.6) | 37 (17.6) | 0 | 0 | 0 | 173 (82.4) | White 187 (89.0) Black 13 (6.2) Oriental 2 (1.0) Other 8 (3.8) |
0–3: 60.0 4–6: 38.6 |
| MPS | 213 | 47.1 | 137 (64.3) | 32 (15) | 0 | 0 | 0 | 181 (85) | White 187 (87.8) Black 17 (8.0) Oriental 3 (1.4) Other 6 (2.8) |
0–3: 62.0 4–6: 37.1 |
|||
| BEL low + MMF vs. BEL high + MMF vs. CSA + MMF (three studies) | |||||||||||||
| Vincenti 2005125 (Vincenti 2010156) | ✗ | BAS + CCSs | BEL low | 71 | 42.1 | 48 (68) | NR | NR | NR | NR | 52 (73) | White 57 (80) Black 6 (9) Other 8 (11) |
> 3: 41 |
| BEL high | 74 | 46.5 | 54 (73) | NR | NR | NR | NR | 51 (69) | White 64 (86) Black 6 (8) Other 6 (6) |
> 3: 42 | |||
| CSA | 73 | 46.1 | 57 (78) | NR | NR | NR | NR | 57 (78) | White 59 (81) Black 6 (8) Other 8 (11) |
> 3: 40 | |||
| BENEFIT (Vincenti 2010, 2012,59,61 Larsen 2010,60,61 Rostaing 201362) | ✗ | BAS + CCSs | BEL low | 226 | 42.6 | 65 | NR | NR | NR | NR | NR | White (59) Black (10) Asian (13) Other (18) |
NR |
| BEL high | 219 | 43.6 | 69 | NR | NR | NR | NR | NR | White (60) Black (7) Asian (12) Other (21) |
NR | |||
| CSA | 221 | 43.5 | 75 | NR | NR | NR | NR | NR | White (63) Black (8) Asian (12) Other (17) |
NR | |||
| BENEFIT-EXT (Durrbach 2010,142 Medina Pestana 2012,174 Charpentier 2013,175 Larsen 201060) | ✗ | BAS + CCSs | BEL low | 175 | 56.1 | 74 | 0 | 0 | 0 | 175 (100) | 0 | White (77) Black (14) Other (10) |
> 3: 50 |
| BEL high | 184 | 56.7 | 65 | 0 | 0 | 0 | 184 (100) | 0 | White (75) Black (14) Other (12) |
> 3: 51 | |||
| CSA | 184 | 55.7 | 63 | 0 | 0 | 0 | 184 (100) | 0 | White (75) Black (12) Other (14) |
> 3: 58 | |||
| BEL + MMF vs. BEL + SRL vs. TAC + MMF (one study) | |||||||||||||
| Ferguson 2011126 | ✗ | rATG + CCSs | BEL + MMF | 33 | 49.2 | 25 (76) | 16 (48) | 0 | 0 | 0 | 17 (52) | White 24 (73) Black 8 (24) Other 1 (3) |
NR |
| BEL + SRL | 26 | 52.7 | 20 (77) | 15 (57) | 0 | 0 | 0 | 11 (42) | White 23 (89) Black 3 (12) Other 0 (0) |
NR | |||
| TAC + MMF | 30 | 53.6 | 22 (73) | 13 (43) | 0 | 0 | 0 | 17 (57) | White 23 (77) Black 5 (17) Other 2 (7) |
NR | |||
| EVL low + CSA vs. EVL high + CSA vs. MMF + CSA (three studies) | |||||||||||||
| Lorber 2005143 | ✗ | CCSs | EVL low | 193 | 43.3 | 110 (57.0) | 94 (48.7) | 94 (48.7) | 5 (2.6) | 0 | 0 | White 133 (70.5) Black 29 (15.0) Hispanic 20 (10.4) Asian 3 (3.7) Other 8 (4.1) |
< 3: 23.8 ≥ 3: 76.2 |
| EVL high | 194 | 43.7 | 123 (63.4) | 94 (48.4) | 93 (47.9) | 7 (3.6) | 0 | 0 | White 123 (63.4) Black 36 (18.6) Hispanic 14 (7.2) Asian 6 (3.1) Other 15 (7.7) |
< 3: 27.8 ≥ 3: 72.2 |
|||
| MMF | 196 | 43.4 | 132 (67.3) | 106 (54.1) | 85 (43.4) | 5 (2.6) | 0 | 0 | White 129 (65.8) Black 33 (16.8) Hispanic 24 (12.2) Asian 2 (1.0) Other 8 (4.1) |
< 3: 28.6 ≥ 3: 71.4 |
|||
| ATLAS Vítko 2005150 (Vítko 2004,176 2005177) | ✗ | CCSs | EVL low | 194 | 45.2 | 114 (58.8) | NR | NR | NR | NR | > 90 | White 181 (93.3) Black 4 (2.1) Oriental 4 (2.1) Other 5 (2.6) |
NR |
| EVL high | 198 | 44.1 | 127 (64.1) | NR | NR | NR | NR | > 90 | White 177 (89.4) Black 9 (4.5) Oriental 5 (2.5) Other 7 (3.5) |
NR | |||
| MMF | 196 | 46.1 | 139 (70.9) | NR | NR | NR | NR | > 90 | White 171 (87.2) Black 11 (5.6) Oriental 6 (3.1) Other 8 (4.1) |
NR | |||
| Takahashi 2013131 | ✗ | BAS + CCSs | EVL | 61 | 42.5 | 46 (75.4) | 60 (98.3) | 1 (1.6) | 0 | 0 | 0 | NR | 1: 11.5 2: 14.8 3: 41.0 < 3: 26.2 ≥ 3: 73.8 |
| MMF | 61 | 38.6 | 37 (60.7) | 60 (98.4) | 0 | 1 (1.6) | 0 | 0 | NR | 1: 3.3 2: 26.2 3: 39.5 < 3: 29.5 ≥ 3: 70.5 |
|||
| EVL vs. EVL + CSA vs. CSA + MPS (one study) | |||||||||||||
| Chadban 2013152 (SOCRATES) | ✗ | BAS + CCSs | EVL | 49 | 48.8 | 32 (65.3) | 27 (55.1) | 20 (40.8) | 2 (4.1) | 0 | 0 | White 26 (53.1) Black 0 Asian 19 (38.8) Pacific Islander 0 Other 4 (8.2) |
0: 3 (6.1) 1: 8 (16.3) 2: 9 (18.4) > 2: 27 (55.1) Missing: 2 (4.1) |
| EVL + CSA | 30 | 43.5 | 24 (80) | 16 (53.3) | 13 (43.3) | 1 (3.3) | 0 | 0 | White 13 (43.3) Black 1 (3.3) Asian 14 (46.7) Pacific Islander 1 (3.3) Other 1 (3.3) |
0: 2 (6.7) 1: 0 (0) 2: 3 (10.0) > 2: 24 (80.0) Missing: 1 (3.3) |
|||
| CSA + MPS | 47 | 45.8 | 34 (72.3) | 31 (65.9) | 15 (31.9) | 1 (2.1) | 0 | 0 | White 25 (53.2) Black 0 Asian 19 (40.4) Pacific Islander 3 (6.4) Other 0 |
0: 6 (12.8) 1: 5 (10.6) 2: 6 (12.8) > 2: 27 (57.4) Missing: 3 (6.4) |
|||
| EVL low + CSA vs. EVL high + CSA vs. MPA + CSA (one study) | |||||||||||||
| Tedesco-Silva 2010107 | ✗ | BAS + CCSs | EVL low | 277 | 45.7 | 176 (63.5) | 147 (53) | 128 (46.2) | 2 (0.7) | 0 | [Missing 1 (0.4)] | White 193 (69.7) | 0: 10 (3.0) 1: 19 (6.9) 2: 37 (13.4) ≥ 3: 210 (75.8) |
| EVL high | 279 | 45.3 | 191 (68.5) | 151 (54.1) | 126 (45.2) | 0 | 0 | White 180 (64.5) | 0: 15 (5.4) 1: 18 (6.5) 2: 51 (18.3) ≥ 3: 194 (69.5) |
||||
| MPA | 277 | 47.2 | 189 (68.6) | 148 (53.5) | 127 (45.8) | 1 (0.4) | 0 | [Missing 1 (0.4)] | White 190 (68.6) | 0: 15 (5.4) 1: 19 (6.9) 2: 40 (14.4) ≥ 3: 202 (72.9) |
|||
| EVL + CSA vs. MPS + CSA (one study) | |||||||||||||
| Bertoni 2011144 | ✗ | BAS + CCSs | EVL | 56 | 45.7 | NR | NR | NR | NR | NR | NR | NR | 3.364 |
| MPS | 50 | 49.75 | NR | NR | NR | NR | NR | NR | NR | 3.5 | |||
| EVL + MPS vs. CSA + MPS (two studies) | |||||||||||||
| Budde 2011132 (Budde 2012,178 Liefeldt 2012179) | ✗ | BAS + CCSs | EVL + CSA | 155 | 46.9 | 102 (66) | 32 (27) | 0 | 0 | 0 | 113 (73) | White 152 (98.1) Asian 2 (1.3) Other 1 (0.6) |
DR 0: 59 (38) 1: 68 (44) 2: 28 (18) |
| CSA | 145 | 46.7 | 86 (59) | 38 (27) | 0 | 0 | 0 | 107 (74) | White 152 (98.1) Asian 2 (1.3) Other 1 (0.6) |
DR 0: 59 (38) 1: 68 (44) 2: 28 (18) |
|||
| Mjörnstedt 2012133 | ✗ | BAS + CCSs | EVL | 102 | 55.5 | 70 (68.6) | NR | NR | NR | NR | 73 (71.6) | White 99 (97.1) | A: 14/100 (14) B: 11/100 (11) DR: 26/99 (26.3) |
| CSA | 100 | 53.8 | 74 (74) | NR | NR | NR | NR | 71 (71.0) | White 100 (100) | A: 24/99 (24.2) B: 14/99 (14.1) DR: 23/99 (23.3) |
|||
| SRL + CSA vs. MMF + CSA (two studies) | |||||||||||||
| Barsoum 2007108 | ✗ | CCSs | SRL | 76 | 45 | 47 (61.8) | NR | NR | NR | NR | NR | NR | 3.1 |
| MMF | 37 | 44 | 27 (73.0) | NR | NR | NR | NR | NR | NR | 2.8 | |||
| Stallone 2004109 | ✗ | BAS + CCSs | SRL | 42 | 50.4 | NR | NR | NR | NR | NR | NR | NR | 3.25 |
| MMF | 48 | 51.8 | NR | NR | NR | NR | NR | NR | NR | 3.14 | |||
| SRL + TAC vs. MMF + TAC (six studies) | |||||||||||||
| Anil Kumar 2005110 | ✗ | BAS + CCSs | SRL | 75 | 55 | 54 (72) | NR | NR | NR | NR | 65 (87) | African American 44 (59) | 4.8 |
| MMF | 75 | 49 | 51 (68) | NR | NR | NR | NR | 67 (89) | African American 45 (60) | 4.3 | |||
| Mendez 2005111 (Gonwa 2003180) | ✗ | CCSs | SRL | 185 | 45.3 | 123 (66.5) | 68 (36.8) | 0 | 0 | 0 | 117 (63.2) | White 94 (50.8) African American 51 (27.6) Hispanic 28 (15.1) Other 12 (6.5) |
3.4 |
| MMF | 176 | 47.8 | 123 (69.9) | 63 (35.8) | 0 | 0 | 0 | 113 (64.2) | White 95 (54.0) African American 43 (24.4) Hispanic 24 (13.6) Other 14 (8.0) |
3.6 | |||
| Sampaio 2008112 | ✗ | CCSs | SRL | 50 | 37.4 | 31 (62) | 38 (76) | 0 | 0 | 0 | 12 (24) | White 21 (42) Black 23 (46) Other 6 (12) |
3.4 |
| MMF | 50 | 42.6 | 38 (76) | 38 (76) | 0 | 0 | 0 | 12 (24) | White 27 (54) Black 16 (32) Other 7 (14) |
3.3 | |||
| Gelens 2006113 | ✗ | CCSs | SRL + TAC | 18 | 59.3 | 12 (67) | 3 (17) | 4 (22) | 11 (61) | 0 | 0 | NR | Number of A mismatches 11 (61) Number of B mismatches 6 (33) Number of DR mismatches 9 (50) |
| SRL + MMF | 18 | 57.1 | 12 (67) | 3 (17) | 6 (33) | 9 (50) | 0 | 0 | NR | Number of A mismatches 6 (33) Number of B mismatches 6 (33) Number of DR mismatches 6 (33) |
|||
| MMF + TAC | 18 | 47.6 | 13 (72) | 3 (17) | 10 (56) | 5 (28) | 0 | 0 | NR | Number of A mismatches: 5 (28) Number of B mismatches: 4 (22) Number of DR mismatches: 5 (28) |
|||
| Gallon 2006145 (Chhabra 2012181) | ✗ | BAS + CCSs | SRL | 37 | 45.7 | 22 (59.5) | 27 (73) | 0 | 0 | 0 | 10 (27.0) | White 25 (67.6) African American 10 (27.0) Hispanic 1 (2.7) Asian 1 (2.7) |
3.1 |
| MMF | 46 | 42.3 | 28 (62.2) | 30 (66.7) | 0 | 0 | 0 | 15 (33.3) | White 30 (66.7) African American 11 (24.4) Hispanic 1 (2.2) Asian 3 (6.7) |
3.6 | |||
| Van Gurp 2010114 | ✗ | CCSs | SRL | 318 | 44.3 | 204 (64.2) | 41 (12.9) | 0 | 0 | 0 | 277 (87.1) | White 299 (94) Black 10 (3.1) Oriental 7 (2.2) Other 2 (0.6) |
2.9 |
| MMF | 316 | 44.9 | 204 (64.6) | 32 (10.1) | 0 | 0 | 0 | 284 (89.9) | White 303 (95.9) Black 7 (2.2) Oriental 4 (1.3) Other 2 (0.6) |
3.0 | |||
| SRL + MMF vs. CSA + MMF (10 studies) | |||||||||||||
| Flechner 2002127 (Flechner 2004,182 2007183) | ✗ | BAS + CCSs | SRL | 31 | 48.4 | 21 (67.7) | 11 (35.5) | 0 | 0 | 0 | 20 (64.5) | White 20 (64.5) Black 8 (25.8) Asian 3 (9.7) |
3.04 |
| CSA | 30 | 46.7 | 19 (63.3) | 10 (33.3) | 0 | 0 | 0 | 20 (66.7) | White 21 (70.0) Black 7 (23.3) Asian 2 (6.7) |
2.82 | |||
| Noris 2007115 (Ruggenenti 2007184) | ✗ | Alemtuzumab + CCSs | SRL | 11 | 51 | 6 (70) | 0 (0) | 0 | 0 | 0 | 11 (100) | NR | 4.0 |
| CSA | 10 | 47 | 7 (70) | 2 (20) | 0 | 0 | 0 | 8 (80) | NR | 4.0 | |||
| Lebranchu 2009149 (Servais 2009,185 Lebranchu 2011,186 Joannides 2011187) | ✗ | DAC + CCSs | SRL + CSA | 95 | 46.5 | 67 (70.5) | 0 | 25 (26.3) | 46 (48.4) | 24 (25.3) | 0 | NR | 3.9 |
| CSA | 97 | 47.3 | 70 (72.2) | 0 | 22 (22.7) | 43 (44.3) | 32 (33.0) | 0 | NR | 3.7 | |||
| Büchler 2007134 (Lebranchu 2012,188 Joannides 2010189) | ✗ | rATG + CCSs | SRL | 71 | 45.6 | 44 (62.0) | 0 | 0 | 0 | 0 | 71 (100) | White 67 (94.4) | 3.52 |
| CSA | 74 | 41.3 | 45 (60.80) | 0 | 0 | 0 | 0 | 74 (100) | White 71 (95.9) | 3.39 | |||
| Soleimani 201391 | ✗ | CCSs | SRL | 29 | 46.72 | 24 (82.8) | NR | NR | NR | NR | NR | NR | NR |
| CSA | 59 | 41.93 | 32 (54.2) | NR | NR | NR | NR | NR | NR | NR | |||
| Durrbach 2008146 | ✗ | CCSs | SRL | 33 | 52.6 | NR | NR | NR | NR | NR | NR | NR | 3.68 |
| CSA | 36 | 57.1 | NR | NR | NR | NR | NR | NR | NR | 3.5 | |||
| Kreis (2000)116 – identified from Campistol 2005190 | ✗ | CCSs | SRL | 40 | 43.5 | 28 (70) | 0 | 0 | 0 | 0 | 40 (100) | White 38 (95) Black 1 (3) Oriental 1 (3) Other 0 |
Match 0: 1 (3) 1: 5 (13) 2: 10 (25) 3: 13 (33) 4: 8 (20) 5: 3 (8) 6: 0 |
| CSA | 38 | 42.9 | 27 (71) | 0 | 0 | 0 | 0 | 38 (100) | White 35 (92) Black 0 Oriental 1 (3) Other 2 (5) |
Match 0: 2 (5) 1: 6 (16) 2: 11 (29) 3: 11 (29) 4: 5 (13) 5: 2 (5) 6: 1 (3) |
|||
| Guba 2010147 | ✗ | rATG + CCSs | SRL + CSA | 69 | 47.0 | 45 (65.2) | 8 (11.6) | 61 (88.4) | 0 | 0 | 0 | White 68 (98.6) Asian 1 (1.4) |
2.8 |
| CSA | 71 | 47.1 | 50 (70.4) | 7 (9.9) | 64 (90.1) | 0 | 0 | 0 | White 70 (98.6) Asian 1 (1.4) |
2.9 | |||
| Martinez-Mier 2006117 | ✗ | BAS + CCSs | SRL | 21 | 29.6 | 12 (57) | 21 (100) | 0 | 0 | 0 | 0 | NR | 2.7 |
| CSA | 20 | 31.2 | 12 (60) | 20 (100) | 0 | 0 | 0 | 0 | NR | 2.9 | |||
| Nafar 2012118 | ✗ | CCSs | SRL + CSA/MMF | 50 | 38.5 | 29 (58) | NR | NR | NR | NR | NR | NR | NR |
| CSA + MMF | 50 | 42.5 | 26 (52) | NR | NR | NR | NR | NR | NR | NR | |||
| TAC + MMF vs. SRL + MMF (four studies) | |||||||||||||
| Larson 2006151 (Stegall 2003191) | ✗ | rATG + CCSs | TAC | 82 | 48 | 44 (53.7) | 71 (85) | 0 | 0 | 0 | 0 | White 79 (94) | NR |
| SRL | 80 | 50 | 45 (56.3) | 65 (81) | 0 | 0 | 0 | 0 | White 78 (98) | NR | |||
| Schaefer 200692 | ✗ | rATG | TAC | 39 | NR | NR | NR | NR | NR | NR | NR | NR | 3.4 |
| SRL | 41 | NR | NR | NR | NR | NR | NR | NR | NR | 3.8 | |||
| TAC | 39 | NR | NR | NR | NR | NR | NR | NR | NR | 2.7 | |||
| Heilman 2011135 (Heilman 2012157) | ✗ | rATG + CCSs | SRL + TAC | 62 | 51.7 | 40 (65) | NR | NR | NR | 1 (1.6) | 29 (46.8) | African American 6 (10) Hispanic 9 (15) |
3.4 |
| TAC | 60 | 54.1 | 36 (60) | NR | NR | NR | 1 (1.7) | 33 (55) | African American 5 (8) Hispanic 7 (12) |
3.2 | |||
| Smith 200893 | ✗ | BAS | TAC→ SRL | 10 | 42 | 7 | 1 (10) | 9 (90) | 0 | 0 | 0 | White 9 (90) Other 1 (10) |
Mean mismatch A: 0.8 B: 1.3 DR: 0.2 |
| TAC→ SRL | 13 | 49 | 10 | 4 (30.8) | 8 (61.5) | 0 | 0 | 0 | White 13 (100) Other 0 |
Mean mismatch A: 0.8 B: 0.9 DR: 0.5 |
|||
| TAC | 28 | 50 | 19 | 4 (14.3) | 23 (82.1) | 0 | 0 | 0 | White 28 (100) Other 0 |
Mean mismatch A: 1.0 B: 0.9 DR: 0.5 |
|||
| TAC + MPS vs. SRL + MPS (one study) | |||||||||||||
| Silva 2013119 | ✗ | CCSs | SRL | 97 | 44.5 | 66 (68) | 50 (52) | 47 (48) | 0 | 0 | 0 | White 52 (54) Black 11 (11) Mixed 29 (30) Other 5 (5) |
A: 1.2 B: 1.2 DR: 0.9 |
| TAC | 107 | 43.9 | 72 (67) | 61 (57) | 46 (43) | 0 | 0 | 0 | White 60 (56) Black 11 (10) Mixed 28 (26) Other 8 (8) |
A: 1.2 B: 1.1 DR: 0.9 |
|||
| TAC + SRL vs. MMF + SRL (one study) | |||||||||||||
| Hamdy 2005120 (Hamdy 2008,192 2010193) | ✗ | CCSs | SRL + TAC | 65 | 32 | 52 (80) | 65 (100) | 0 | 0 | 0 | 0 | NR | 0: 11 1: 8 2: 36 3: 8 4: 2 |
| SRL + MMF | 67 | 31.8 | 47 (70.1) | 67 (100) | 0 | 0 | 0 | 0 | NR | 0: 7 1: 8 2: 43 3: 7 4: 2 |
|||
| SRL + AZA vs. CSA + AZA (one study) | |||||||||||||
| Charpentier 2003136 (Groth 1999194) | ✓ | CCSs | SRL | 41 | 47.54 | 29 (71) | 0 | 0 | 0 | 0 | 42 (100) | White 40 (98) Black 0 Oriental 0 Other 1 (2) |
Matches 0: 6 (15) 1: 7 (17) 2: 11 (27) 3: 7 (17) 4: 6 (15) 5: 4 (10) 6: 0 |
| CSA | 42 | 41.67 | 25 (60) | 0 | 0 | 0 | 0 | 42 (100) | White 37 (88) Black 1 (2) Oriental 3 (7) Other 1 (2) |
Matches 0: 5 (12) 1: 7 (17) 2: 9 (21) 3: 15 (36) 4: 2 (5) 5: 3 (7) 6: 1 (2) |
|||
| TAC + SRL vs. CSA + SRL (one study) | |||||||||||||
| Chen 2008121 | ✗ | CCSs | TAC | 21 | 42.7 | 5 (23.8) | 8 (38.1) | 0 | 0 | 0 | 13 (61.9) | NR | 3.3 |
| CSA | 20 | 40.2 | 7 (35) | 7 (35) | 0 | 0 | 0 | 13 (65) | NR | 2.8 | |||
| SRL low + TAC vs. SRL high + TAC vs. MMF + TAC (one study) | |||||||||||||
| Vítko 200694 | ✗ | CCSs | SRL low | 325 | 44.6 | 210 (64.6) | 30 (9.2) | NR | NR | NR | NR | White 316 (97.2) Black 4 (1.2) Oriental 3 (0.9) Other 2 (0.6) |
2.8 |
| SRL high | 325 | 47.3 | 196 (60.3) | 36 (11.1) | NR | NR | NR | NR | White 317 (97.5) Black 2 (0.6) Oriental 2 (0.6) Other 4 (1.2) |
2.9 | |||
| MMF | 327 | 46.0 | 218 (66.7) | 27 (8.3) | NR | NR | NR | NR | White 319 (97.6) Black 3 (0.9) Oriental 3 (0.9) Other 2 (0.6) |
2.9 | |||
| SRL + TAC vs. SRL + MMF vs. MMF + TAC (one study) | |||||||||||||
| Flechner 2011155 (the ORION study) | ✗ | DAC + CCSs | SRL + TAC | 155 | 47.9 | 109 (71.7) | 60 (40) | 0 | 0 | 0 | 92 (60) | White 114 (75) Black 14 (9) Asian 6 (4) Other 18 (11.8) |
3.38 |
| SRL + MMF | 155 | 50.4 | 110 (72.4) | 56 (37) | 0 | 0 | 0 | 96 (63) | White 117 (77) Black 17 (11) Asian 4 (2.6) Other 14 (9.2) |
3.36 | |||
| TAC + MMF | 140 | 48.4 | 81 (58.3) | 50 (36) | 0 | 0 | 0 | 89 (64) | White 102 (73) Black 15 (11) Asian 5 (3.6) Other 17 (12.2) |
3.32 | |||
| MMF + CSA vs. MMF + low CSA vs. MMF + low TAC vs. MMF low SRL (one study) | |||||||||||||
| Grinyo 2009,51 (Ekberg 2009,195 2010;196 Demirbas 2009;197 Frei 2010;198 Claes 2012199) | ✗ | DAC + CCSs | CSA | 390 | 45.9 | 148 (38) | 134 (34.4) | 0 | 0 | 0 | 256 (65.6) | White 359 (92.1) Black 8 (2.1) Asian 5 (1.3) Other 18 (4.6) |
2: 70 (18) |
| Low CSA | 339 | 47.2 | 115 (34) | 121 (35.6) | 0 | 0 | 0 | 218 (64.2) | White 312 (92.2) Black 8 (2.3) Asian 3 (0.8) Other 16 (4.8) |
2: 64 (19) | |||
| Low TAC | 401 | 45.5 | 136 (34) | 148 (36.9) | 0 | 0 | 0 | 252 (62.8) | White 377 (94.0) Black 4 (1.0) Asian 3 (0.7) Other 17 (4.2) |
2: 72 (18) | |||
| Low SRL | 399 | 44.8 | 132 (33) | 143 (35.9) | 0 | 0 | 0 | 256 (64.2) | White 376 (94.2) Black 5 (1.3) Asian 2 (0.5) Other 16 (4.0) |
2: 64 (16) | |||
| TAC + MMF vs. TAC + SRL vs. CSA + MMF vs. CSA + SRL (one study) | |||||||||||||
| Anil Kumar 2008122 (Kumar 2006,200 2005110) | ✗ | BAS + CCSs | CSA + MMF | 50 | 51 | 35 (70) | 0 | 0 | 0 | 12 (24) | 41 (82) | African American 25 (50) | 4.0 |
| CSA + SRL | 50 | 56 | 37 (74) | 0 | 0 | 0 | 11 (22) | 43 (86) | African American 25 (50) | 4.1 | |||
| TAC + MMF | 50 | 48 | 34 (68) | 0 | 0 | 0 | 11 (22) | 44 (88) | African American 27 (54) | 4.0 | |||
| TAC + SRL | 50 | 59 | 34 (68) | 0 | 0 | 0 | 13 (26) | 43 (86) | African American 26 (52) | 4.1 | |||
Mean age across studies ranges from 29.6 to 57.1 years. Men represented 50–80% of participants for the bulk of the studies. The studies by Baboolal et al. 82 and Campos et al. 83 fell slightly below this, with men at 48–49%, whereas Chen et al. 121 recruited only 24% and 35% in treatment arms and Grinyo et al. 51 recruited 33% and 38%.
As for induction therapies, earlier papers tended to record cadaveric donors, with no further details. Fifteen studies75,77,80–82,88,89,99,116,129,130,134,136,138,148 used only cadaveric donors and no studies used only living. For the remainder of the studies, the donors were either mixed or not reported.
The majority of studies had a high proportion of white participants; however, Jarzembowski et al. 99 recruited all African American participants, Ciancio et al. 106 recruited Hispanic (29.3% and 30.7%) and African American (26.7% and 32.0%) participants, Chadban et al. 152 reported Asian participants to be 38.8%, 46.7% and 40.4% in each arm, Anil Kumar et al. 110 recruited 59% and 60% African American participants, and Anil Kumar et al. 122 recruited 50–54% African American participants in each arm.
For the maintenance studies, HLA is reported in a variety of formats, making any comparisons between studies difficult. As previously mentioned, the matching of HLAs is no longer considered critical, but may have an impact on graft survival.
Study results
The following outcomes have been addressed for each combination of therapies for both induction and maintenance, with meta-analysis performed where possible:
-
mortality
-
graft loss
-
BPAR
-
GRF
-
time to BPAR
-
severity of BPAR
-
adverse effects of treatment
-
HRQoL.
We also sought HRQoL outcome data from included RCTs. However, none was reported, so we do not have a section for this outcome.
Furthermore, because of an insufficient number of RCTs within each comparison for induction and maintenance therapies (i.e. 10 or more, as recommended by the Cochrane Handbook201), publication bias has not been investigated with funnel plots.
For severity of BPAR, reporting is generally very poor and it is unclear if all of the people with BPAR have received a Banff classification. Therefore, the results as reported are presented with no further analysis.
Induction therapies
BAS compared with PBO/no induction
The 2005 review identified four RCTs71–74 investigating the effectiveness of BAS compared with PBO. One RCT95 was identified in the review by Yao et al. 67
No additional studies were identified in the PenTAG search. No data were identified for HRQoL and time to BPAR.
For BAS compared with no induction, one RCT97 was identified in TA99 and two further RCTs123,128 were identified by the PenTAG search.
Mortality
Participant mortality was recorded at 6 months by three studies. 73,74,123 Six studies71–74,97,128 report mortality at 1 year.
As displayed in Table 13 and Figure 8, the OR at 0.5 years for the studies by Ponticelli et al. ,73 Albano et al. 123 and Lawen et al. 74 indicates that BAS is associated with lower odds of mortality, although the results are not statistically significant (OR 0.36, 95% CI 0.13 to 1.01).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Albano 2013,123 Ponticelli 2001,73 Lawen 200374 | 0.5 | 3a | 0.36 | 0.13 to 1.01 | 0.0 | 0 |
| Kyllönen 2007,128 Kahan 1999,72 Nashan 1997,71 Ponticelli 2001,73 Lawen 2003,74 Sheashaa 200397 | 1 | 6b | 0.95 | 0.49 to 1.87 | 0.0 | 0 |
| Sheashaa 200397 | 3 | 1 | 0.33 | 0.01 to 8.21 | NA | |
| 5 | 0.19 | 0.01 to 4.10 | ||||
| 7 | 1.00 | 0.24 to 4.24 | ||||
| 10 | 0.78 | 0.20 to 3.10 |
FIGURE 8.
Forest plot: mortality for BAS vs. PBO/no induction.
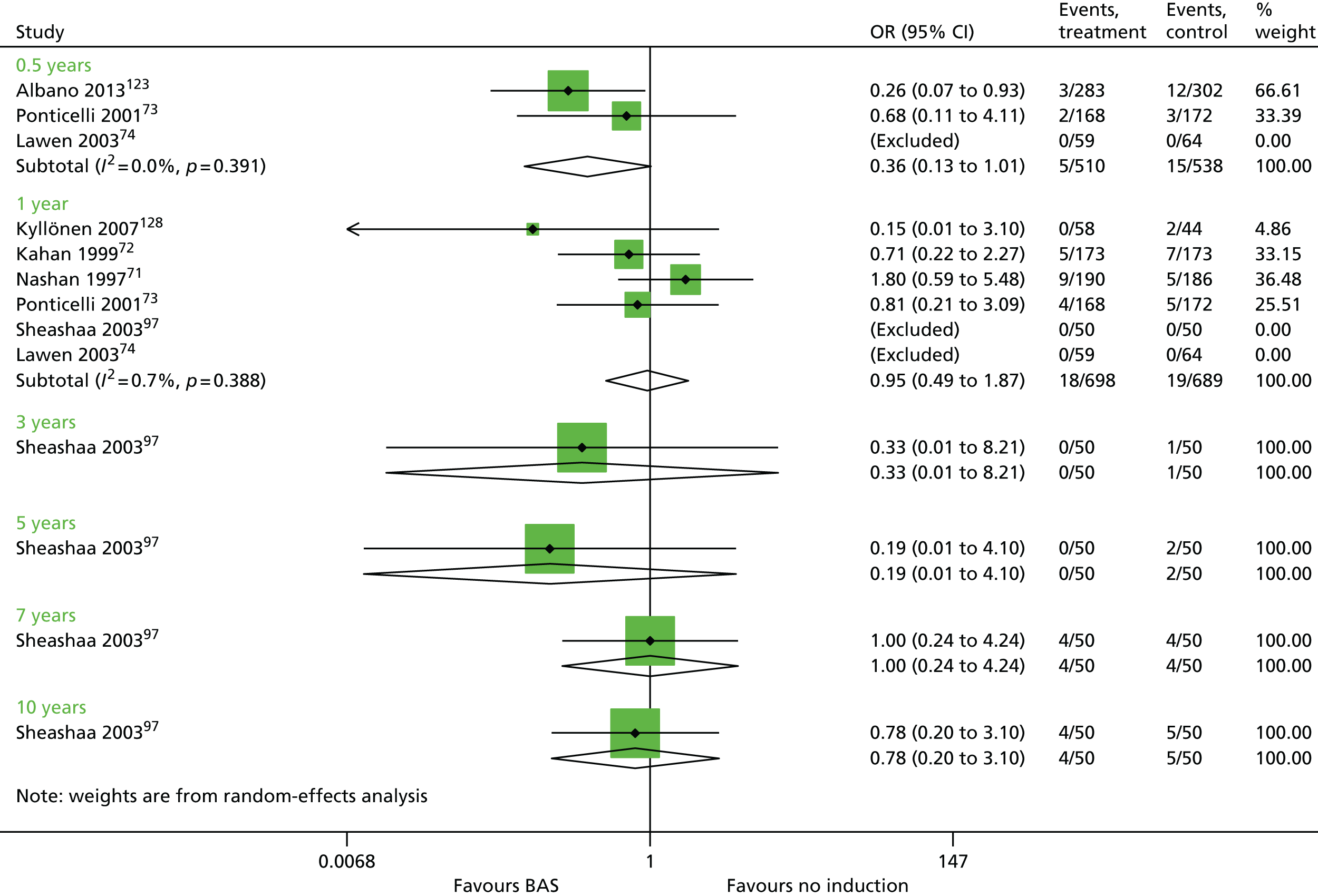
Pooled results at 1 year for the studies by Lawen et al. 74 and Sheashaa et al. 97 also display no statistically significant difference between BAS and PBO/no induction up to 1 year, which is in agreement with the previous HTA65 (OR 0.95, 95% CI 0.49 to 1.87). The effect estimate for the Sheashaa et al. 97 study at 3, 5, 7 and 10 years also shows no difference between arms.
Graft loss
Of the seven studies in this group,71–74,97,123,128 three studies73,74,123 recorded graft loss at 6 months and six studies71–74,97,128 at 1 year (Table 14 and Figure 9).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Albano 2013,123 Ponticelli 2001,73 Lawen 200374 | 0.5 | 3 | 0.78 | 0.50 to 1.22 | 0.0 | 0.0 |
| Kyllönen 2007,128 Kahan 1999,72 Nashan 1997,71 Ponticelli 2001,73 Lawen 2003,74 Sheashaa 200397 | 1 | 6a | 0.82 | 0.56 to 1.21 | 0.0 | 0.0 |
| Sheashaa 200397 | 3 | 1 | 3.06 | 0.12 to 76.95 | NA | |
| 5 | 5.21 | 0.24 to 111.24 | ||||
| 7 | 1.00 | 0.24 to 4.24 | ||||
| 10 | 0.78 | 0.20 to 3.10 |
FIGURE 9.
Forest plot: graft loss for BAS vs. PBO/no induction.

At both time points the OR may indicate some benefit of BAS compared with PBO or no induction in reducing graft loss (0.5 years: OR 0.78, 95% CI 0.50 to 1.22; 1 year: OR 0.82, 95% CI 0.56 to 1.21). However, this estimate must be treated with caution because of the wide CIs indicating a lack of statistical significance.
The one study97 reporting results at 3, 5, 7 and 10 years showed no statistically significant difference between arms (see Table 14).
Graft function
Pooled analysis for GRF measured as CRC (Table 15 and Figure 10) implies no beneficial effect of BAS compared with PBO [0.5 years: weighted mean difference (WMD) –1.38 ml/minute/1.73 m2, 95% CI –5.96 to 3.20 ml/minute/1.73 m2; 1 year: WMD 1.93 ml/minute/1.73 m2, 95% CI –0.97 to 4.83 ml/minute/1.73 m2]. 71–73,97,123 In particular, results for 0.5 years must be treated with caution because of the substantial heterogeneity across studies (I2 = 83.4%). It should also be noted that, at 1 year, the study reported by Kahan et al. ,72 which indicates an improved GRF for participants on BAS, had a higher percentage of African American participants (34% and 27%) who generally exhibit poor long-term graft survival compared with other ethnic groups. 72
| Study | Time point (years) | Trials | WMD (ml/minute/1.73 m2) | 95% CI (ml/minute/1.73 m2) | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Albano 2013,123 Ponticelli 2001,73 Nashan 199771 | 0.5 | 3 | –1.38 | –5.96 to 3.20 | 83.4 | 0.06 |
| Kyllönen 2007,128 Kahan 1999,72 Nashan 1997,71 Ponticelli 2001,73 Lawen 2003,74 Sheashaa 200397 | 1 | 4 | 1.93 | –0.97 to 4.83 | 23.9 | 5.75 |
FIGURE 10.
Forest plot: GRF for BAS vs. PBO/no induction. SD, standard deviation; WMD, weighted mean difference.

Data up to 10 years reported by Sheashaa et al. 97 (Table 16) indicate no statistically significant difference between BAS and no induction.
| Study | Time point (years) | BAS, mean ml/minute/1.73 m2 (SD) | No induction, mean ml/minute/1.73 m2 (SD) | MD (ml/minute/1.73 m2) | 95% CI (ml/minute/1.73 m2) | p-value (t-test) |
|---|---|---|---|---|---|---|
| Sheashaa 200397 | 1 | 75.0 (14.1) | 72.0 (12.9) | 3.00 | –2.30 to 8.30 | 0.2697 |
| 3 | 76.6 (12.9) | 72.3 (13.7) | 4.34 | –0.88 to 9.56 | 0.1094 | |
| 5 | 73.4 (16.2) | 71.3 (12.3) | 2.19 | –3.44 to 7.82 | 0.4671 | |
| 7 | 71.2 (14.5) | 68.6 (14.4) | 2.60 | –3.06 to 8.26 | 0.3705 | |
| 10 | 64.1 (15.2) | 65.5 (15.1) | –1.40 | –7.15 to 4.35 | 0.6451 |
Biopsy-proven acute rejection
The results of BPAR at 0.5 years are inconclusive because of the substantial heterogeneity across studies (I2 = 80.7%). 71,73,74,123 In contrast, at 1 year, BAS statistically significantly reduced BPAR compared with PBO/no induction (OR 0.53, 95% CI 0.40 to 0.70, I2 = 0.0%) (Table 17 and Figure 11). 72–74,97,128 Furthermore, the report by Sheashaa et al. 97 indicates this effect is maintained up to 10 years (OR 0.41, 95% CI 0.18 to 0.96). 97
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Albano 2013,123 Ponticelli 2001,73 Lawen 2003,74 Nashan 199771 | 0.5 | 4 | 0.59 | 0.31 to 1.10 | 80.7 | 0.064 |
| Kyllönen 2007,128 Kahan 1999,72 Ponticelli 2001,73 Lawen 2003,74 Sheashaa 200397 | 1 | 5 | 0.53 | 0.40 to 0.70 | 0.0 | 0.0 |
FIGURE 11.
Forest plot: BPAR for BAS vs. PBO.
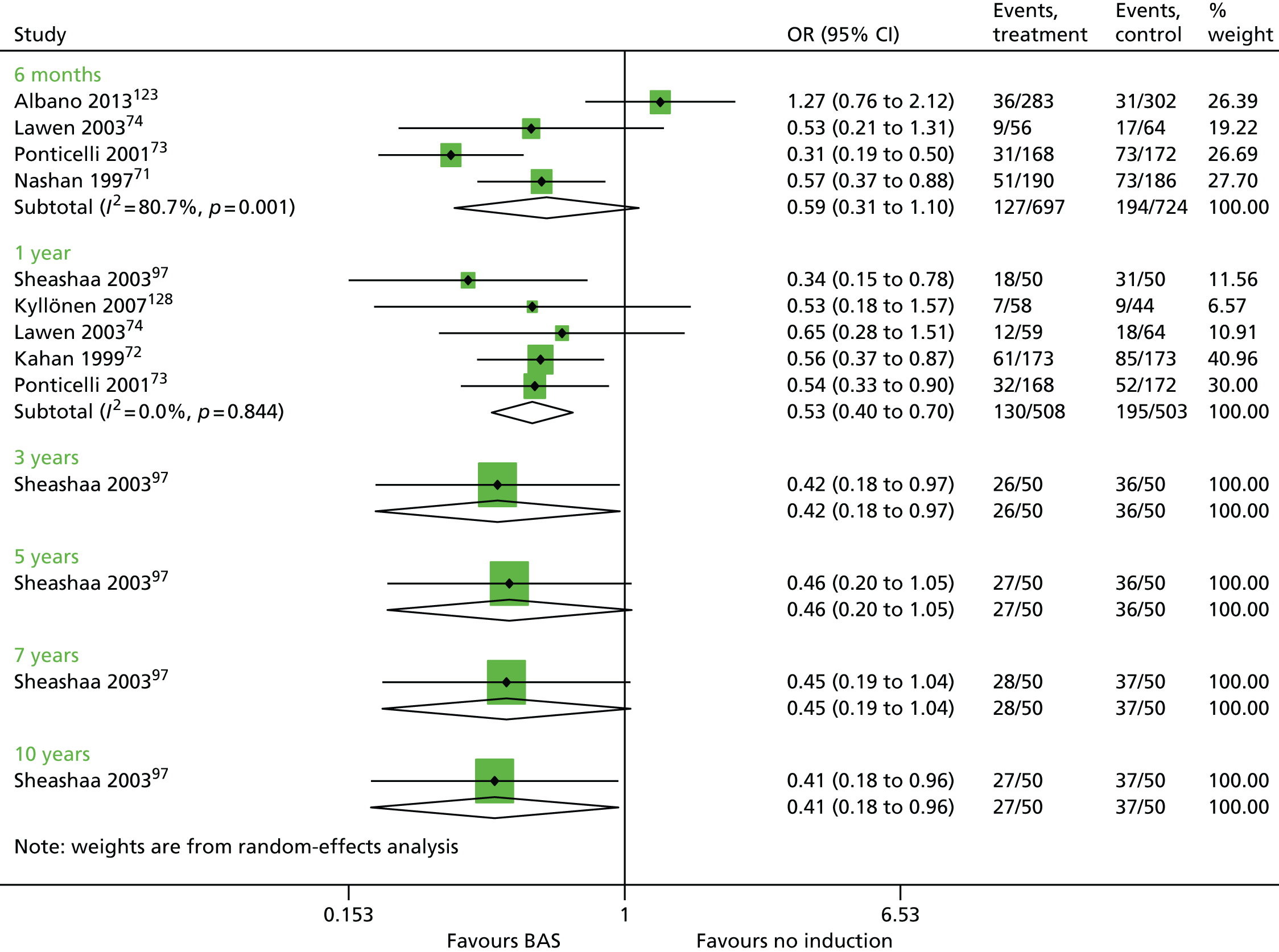
Severity of biopsy-proven acute rejection
Six studies71–74,97,123 report severity of BPAR (Table 18). Overall, Table 18 indicates that BAS may be associated with less severe exacerbations of BPAR.
| Study | Time point (years) | BAS | PBO/no induction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff classification | n | BPAR | Banff classification | ||||||
| I | II | III | I | II | III | ||||||
| Albano 2013123 | 0.5 | 283 | 36 | 16 | 18 | 2 | 302 | 31 | 13 | 15 | 3 |
| aLawen 200374 | 0.5 | 59 | 9 | 5 | 1 | 2 | 64 | 17 | 4 | 11 | 1 |
| Nashan 199771 | 0.5 | 190 | 51 | 20 | 26 | 5 | 186 | 73 | 31 | 31 | 11 |
| Ponticelli 200173 | 0.5 | 168 | 31 | 15 | 12 | 4 | 172 | 49 | 16 | 25 | 8 |
| Kahan 199972 | 1 | 173 | 61 | 26 | 31 | 4 | 173 | 85 | 38 | 37 | 10 |
| bSheashaa 200397 | 1 | 50 | 29 | 27 | 2 | 50 | 45 | 35 | 10 | ||
| 5 | 50 | 27 | 24 | 3 | 50 | 36 | 25 | 11 | |||
| 7 | 50 | 41 | 3 | 2 | 50 | 55 | 44 | 11 | |||
| 10 | 50 | 41 | 3 | 2 | 50 | 55 | 44 | 11 | |||
Time to biopsy-proven acute rejection
Only one study128 reported time to BPAR (Table 19). In general, the results seem similar between arms, although no induction has a broader range (BAS 35–267 days, no induction 10–364 days).
| Study | BAS | No induction | Statistical test (p-value) | ||||
|---|---|---|---|---|---|---|---|
| n | BPAR | Time to BPAR, days | n | BPAR | Time to BPAR, days | ||
| Kyllönen 2007128 | 58 | 7 | Mean 97, median 46, range 35–267 | 44 | 9 | Mean 101, median 35, range 10–364 | NR |
Summary of results for BAS compared with PBO/no induction
Pooled results indicate no statistically significant difference between BAS and PBO/no induction for mortality up to 1 year (six studies71–74,97,128) (OR 0.95, 95% CI 0.49 to 1.87).
The effect estimate for the Sheashaa et al. 97 study at 3, 5, 7 and 10 years also shows no difference between arms. 97
No statistically significant difference is found between BAS and PBO/no induction for graft loss (six studies71–74,97,128) (0.5 years OR 0.78, 95% CI 0.50 to 1.22; 1 year OR 0.82, 95% CI 0.56 to 1.21). This is also the case for the single study when follow-up continues up to 10 years. 97
Pooled analysis for GRF measured as CRC implies no beneficial effect of BAS compared with PBO (0.5 years, WMD –1.38 ml/minute/1.73 m2, 95% CI –5.96 to 3.20 ml/minute/1.73 m2; 1 year, 1.93, 95% CI –0.97 to 4.83 ml/minute/1.73 m2). 71–73,97,123
The results of BPAR at 0.5 years are inconclusive because of the substantial heterogeneity across studies71,73,74,123 (I2 = 80.7%). In contrast, at 1 year, BAS statistically significantly reduced BPAR compared with PBO/no induction (OR 0.53, 95% CI 0.40 to 0.70; I2 = 0.0%). 72–74,97,128 Furthermore, the report by Sheashaa et al. 97 indicates that this effect is maintained up to 10 years (OR 0.41, 95% CI 0.18 to 0.96). 97 In general, severity of BPAR appeared reduced with BAS.
rATG vs. no induction
Both RCTs for this comparison were identified via the PenTAG search. 96,148
Mortality
Two trials96,148 provided data on mortality for rATG vs. no induction (Table 20). Follow-up data are provided to only 1 year. 96 No clear evidence of a difference between arms is visible, as the OR is close to ‘1’ and the CIs are wide.
Graft loss
Two trials96,148 provide graft loss data for rATG vs. no induction (Table 21). For both studies,96,148 CIs are extremely wide, crossing an OR of 1, indicating no statistical difference between arms.
Graft function
No studies reported GRF.
Biopsy-proven acute rejection
Two studies96,148 report on BPAR for rATG vs. no induction for 0.5 years and 1 year (Table 22). The data at 1 year suggest a statistically significant beneficial effect for rATG (OR 0.41, 95% CI 0.24 to 0.52). 96
Severity of biopsy-proven acute rejection
One study148 reports severity of BPAR at 0.5 years (Table 23). For people identified with BPAR, the occurrence of the most severe classification was 10.7% for rATG and 6.4% for no induction. For Banff classification II, there is a greater association with no induction (rATG 25%, no induction 36.2%).
| Study | Time point (years) | rATG | No induction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff classification | n | BPAR | Banff classification | ||||||
| I | II | III | I | II | III | ||||||
| Charpentier 2003148 | 0.5 | 186 | 28 | 18 | 7 | 3 | 185 | 47 | 27 | 17 | 3 |
Time to biopsy-proven acute rejection
Time to BPAR is reported by one study96 (Table 24), in which more participants experience BPAR at 7–10 days with no induction than with rATG.
Summary of results for rATG vs. no induction
Only two studies96,148 report rATG vs. no induction. No statistically significant difference was seen for mortality, graft loss or GRF. For BPAR, the data at 1 year suggest a statistically significant beneficial effect for rATG (OR 0.41, 95% CI 0.24 to 0.52) and for severity of BPAR; at Banff classification II, there are greater odds of association with no induction (1 year: OR 0.09, 95% CI 0.01 to 0.73).
BAS vs. rATG
The RCT reported by Lebranchu et al. 87 was identified in the 2005 review. The PenTAG search retrieved a further two RCTs: Brennan et al. 137 and Mourad et al. 98 All three RCTs87,98,137 had a maintenance therapy comprising CSA, MMF and CCSs.
Mortality
The comparison between BAS and rATG for mortality is reported by three studies87,98,137 (Table 25 and Figure 12). Two studies are pooled with 1-year results where no statistically significant effect is seen between arms (OR 1.03, 95% CI 0.35 to 3.00). 98,137
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Lebranchu 200287 | 0.5 | 1 | 3.06 | 0.12 to 76.95 | NA | NA |
| Mourad 2004,98 Brennan 2006137 | 1 | 2 | 1.03 | 0.35 to 3.00 | 0.0 | 0.0 |
FIGURE 12.
Forest plot: mortality for BAS vs. rATG.
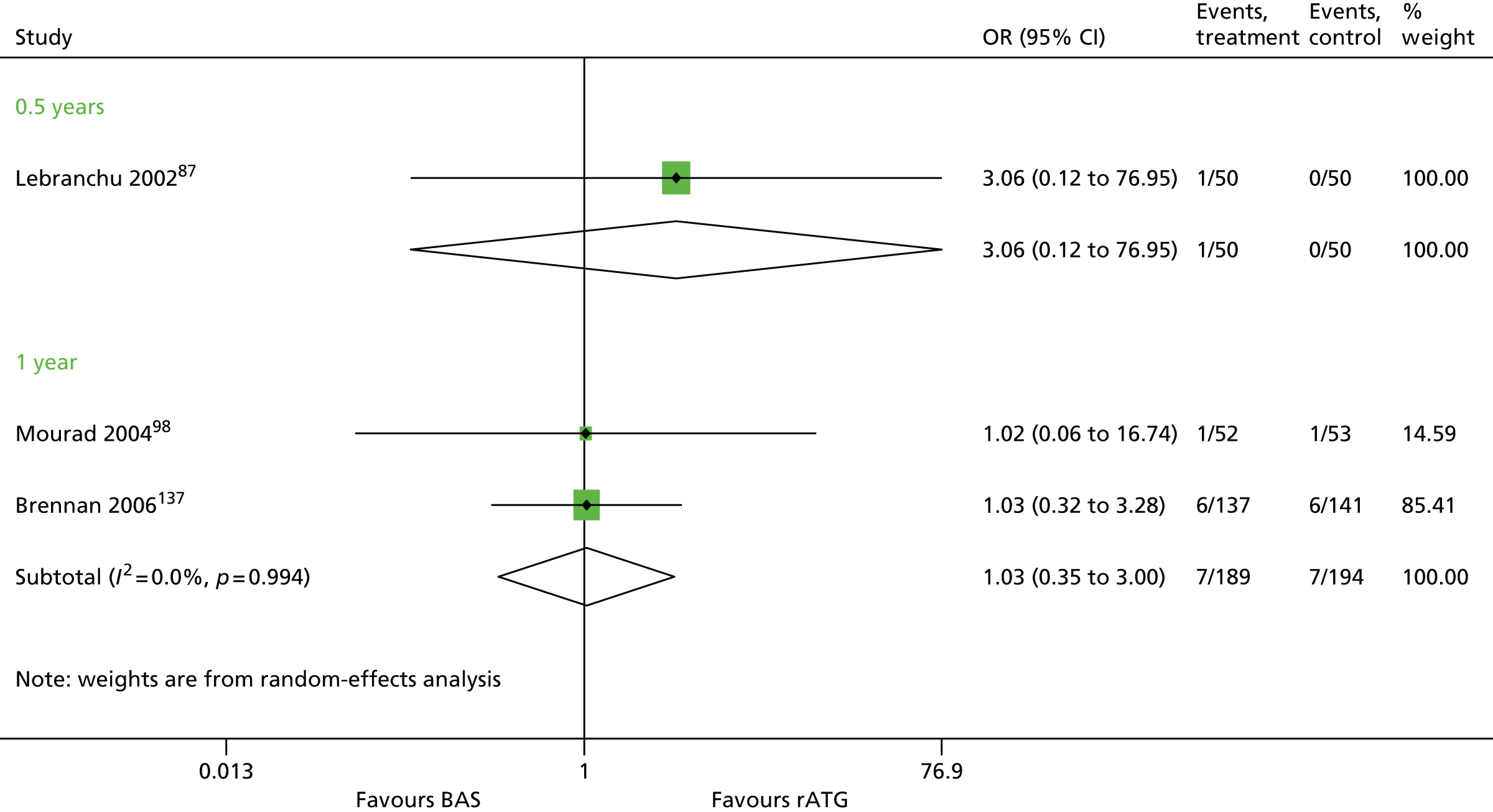
Graft loss
Data from three trials87,98,137 were pooled at the 1-year time point (Table 26 and Figure 13). Although the OR indicates lower odds of graft loss associated with rATG, the effect is not statistically significant (OR 1.36, 95% CI 0.61 to 3.03). There was no evidence of heterogeneity across studies. For the individual study87 at 0.5 years there was no statistically significant effect for BAS or rATG.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Lebranchu 200287 | 0.5 | 1 | 1.00 | 0.14 to 7.39 | NA | NA |
| Lebranchu 2002,87 Mourad 2004,98 Brennan 2006137 | 1 | 3 | 1.36 | 0.61 to 3.03 | 0.0 | 0.0 |
FIGURE 13.
Forest plot: graft loss for BAS vs. rATG.

Graft function
Only Lebranchu et al. 87 report GRF, with results at 0.5 years and 1 year (Table 27). The MD for CRC of 6.10 ml/minute/1.73 m2 at 1 year in favour of BAS is not statistically significant (p = 0.1103).
| Study | Time point (years) | BAS, mean ml/minute/1.73 m2 (SD) | rATG, mean ml/minute/1.73 m2 (SD) | MD (ml/minute/1.73 m2) | 95% CI (ml/minute/1.73 m2) | p-value (t-test) |
|---|---|---|---|---|---|---|
| Lebranchu 200287 | 0.5 | 63 (14.7) | 59.1 (20.3) | 3.90 | –3.13 to 10.93 | 0.2739 |
| 1 | 66.5 (17.9) | 60.4 (19.9) | 6.10 | –1.42 to 13.612 | 0.1103 |
Biopsy-proven acute rejection
A total of three studies87,98,137 report on BPAR for BAS vs. rATG (Table 28 and Figure 14). At both 0.5 years and 1 year, the 95% CIs imply a lack of statistically significant difference between treatments (0.5 years, OR 1.00, 95% CI 0.24 to 4.24; 1 year, OR 1.57, 95% CI 0.95 to 2.61). For Brennan et al. ,137 as a much larger study with narrower CIs, rATG appears to reduce BPAR, although this effect is lost when pooled with the smaller studies.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Lebranchu 200287 | 0.5 | 1 | 1.00 | 0.24 to 4.24 | 0.0 | 0.0 |
| Lebranchu 2002,87 Mourad 2004,98 Brennan 2006137 | 1 | 3 | 1.57 | 0.95 to 2.61 | 0.0 | 0.0 |
FIGURE 14.
Forest plot: BPAR for BAS vs. rATG.
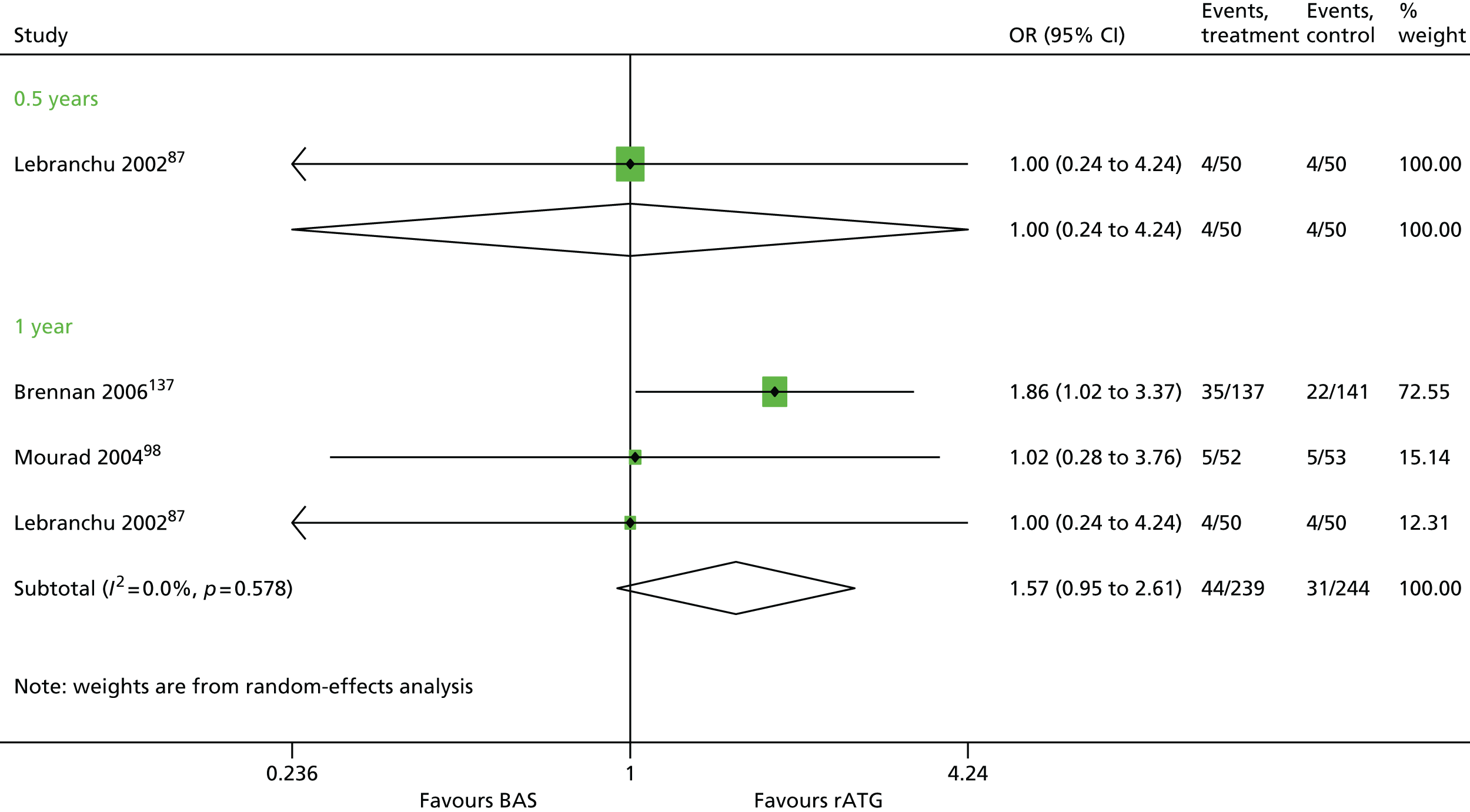
Severity of biopsy-proven acute rejection
Two studies87,98 report on severity of BPAR, although results are not provided for all Banff classifications (Table 29). No difference is seen between treatments.
Time to biopsy-proven acute rejection
Time to BPAR is reported by two studies87,98 (Table 30). Neither of the studies87,98 revealed a statistically significant difference between BAS and rATG, despite the study by Mourad et al. 98 reporting a mean time for BAS of 155 days (SD 196.27 days) and for rATG of 35 days (SD 30.19 days).
| Study | BAS | rATG | χ2 (p-value)a | ||||
|---|---|---|---|---|---|---|---|
| n | BPAR | Mean time to BPAR, days (SD) | n | BPAR | Mean time to BPAR, days (SD) | ||
| bLebranchu 200287 | 50 | 4 | 48.5 (29.8) | 50 | 4 | 35 (29.7) | 0.00 (0.98) |
| cMourad 200498 | 52 | 5 | 155 (196.3) | 53 | 5 | 35 (30.2) | 0.08 (0.77) |
Maintenance therapies
TAC + AZA vs. CSA + AZA
Fourteen studies75,76,79–85,88,99,100,104,148 were identified using this combination. Where possible, meta-analysis has been performed. Results are presented for all outcomes, other than HRQoL where no evidence was reported.
Mortality
Ten studies76,79,80,83,84,88,99,100,104,148 report mortality, with meta-analysis possible at the 0.5- and 1-year time points (Table 31 and Figure 15). All studies76,79,80,83,84,88,99,100,104,148 are presented graphically on the forest plot to provide a visual overview (see Figure 15). At 0.5 years, pooled results of only two studies84,148,164,165 generate an OR of 0.54 (95% CI 0.18 to 1.62), indicating lower odds of mortality for TAC; however, the large CIs indicate a low level of precision, and, as they all overlap, the null value (OR = 1) there is unlikely to be a significant difference between treatments. Although the OR at 1 year, which includes eight studies,76,80,83,84,88,99,100,104 has shifted to 1.51, indicating reduced odds of mortality in the CSA arm, the 95% CI of 0.75 to 3.06 also suggests no significant difference between treatments. Heterogeneity across studies for the 1-year time point is low and may not be important at this level according to the Cochrane Handbook201 (I2 = 14.8%). Mayer et al. 88 report mortality up to 5 years; however, the results are consistent with earlier time points and indicate no difference between arms (OR 1.20, 95% CI 0.69 to 2.07).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Schleibner 199579 | 0.08 | 1a | NA | NA | NA | NA |
| Margreiter 2002,84 Krämer 2005,164 2008,165 Charpentier 2003148 | 0.5 | 2 | 0.54 | 0.18 to 1.62 | 0.0 | 0.0 |
| Laskow 1996;80 Vincenti 1996;161 Mayer 1997,88 1999,162 2002;163 Jarzembowski 2005;99 Campos 2002;83 Margreiter 2002;84 Krämer 2005,164 2008;165 Waller 2002;76 Murphy 2003;166 Hardinger 2005;100 Brennan 2005;167 Weimer 2005,172 2006104 | 1 | 8b | 1.51 | 0.75 to 3.06 | 14.8 | 0.13 |
| Margreiter 2002;84 Krämer 2005,164 2008165 | 2 | 1 | 0.53 | 0.15 to 1.85 | NA | NA |
| Mayer 1997,88 1999,162 2002163 | 4 | 1 | 1.23 | 0.68 to 2.21 | NA | NA |
| 5 | 1 | 1.20 | 0.69 to 2.07 | NA | NA |
FIGURE 15.
Forest plot: mortality for TAC + AZA vs. CSA + AZA.
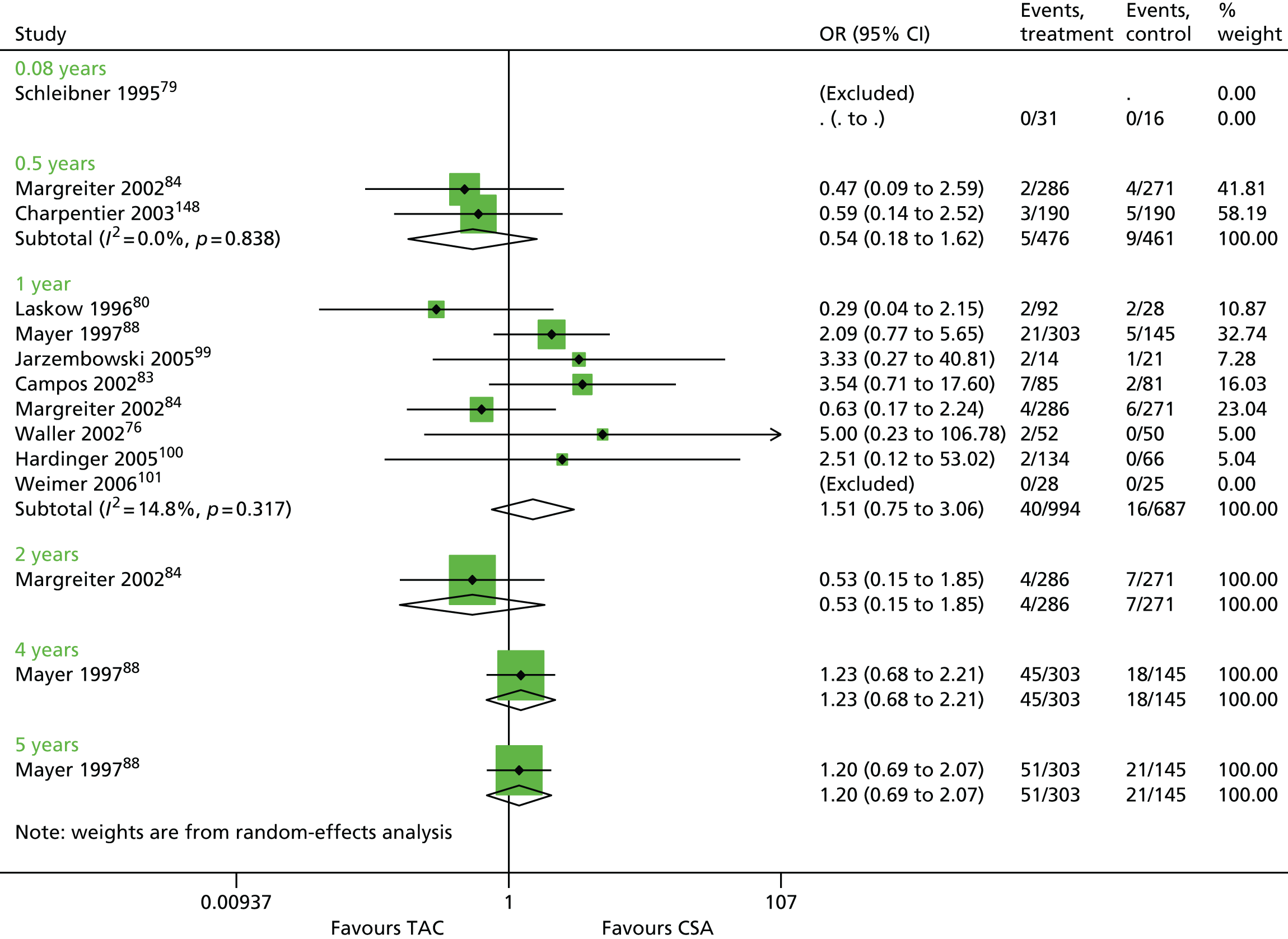
Graft loss check
Graft loss is reported for 10 trials76,79,80,83,84,88,99,100,104,148 (Table 32 and Figure 16). Results were pooled for the 0.5-, 1- and 2-year time points. The pooling of trials reported by Margreiter et al. 84 and Charpentier et al. 148 at 0.5 years gives an OR of 0.45 (95% CI 0.24 to 0.84), which is statistically significant in favour of TAC. 84,148 The 1-year time point is more reliable, at which seven studies are pooled (see Table 32), generating an OR of 1.18 (95% CI 0.72 to 1.93). However, as with mortality, the results for graft loss suggest no difference between TAC and CSA. This lack of statistical significance for either treatment remains at 5 years (OR 0.92, 95% CI 0.61 to 1.40).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Schleibner 199579 | 0.08 | 1 | 0.16 | 0.01 to 4.26 | NA | NA |
| Margreiter 2002;84 Krämer 2005,164 2008;165 Charpentier 2003148 | 0.5 | 2 | 0.45 | 0.24 to 0.84 | 0.0 | 0.0 |
| Mayer 1997,88 1999,162 2002;163 Jarzembowski 2005;99 Campos 2002;83 Margreiter 2002;84 Krämer 2005,164 2008;165 Waller 2002;76 Murphy 2003;166 Hardinger 2005;100 Brennan 2005;167 Weimer 2005,172 2006104 | 1 | 7a | 1.18 | 0.72 to 1.93 | 0.0 | 0.0 |
| Baboolal 2002;82 Margreiter 2002;84 Krämer 2005,164 2008165 | 2 | 2 | 0.71 | 0.40 to 1.25 | 0.0 | 0.0 |
| Mayer 1997,88 1999,162 2002163 | 4 | 1 | 0.96 | 0.62 to 1.48 | NA | NA |
| 5 | 1 | 0.92 | 0.61 to 1.40 | NA | NA |
FIGURE 16.
Forest plot: graft loss for TAC + AZA vs. CSA + AZA.
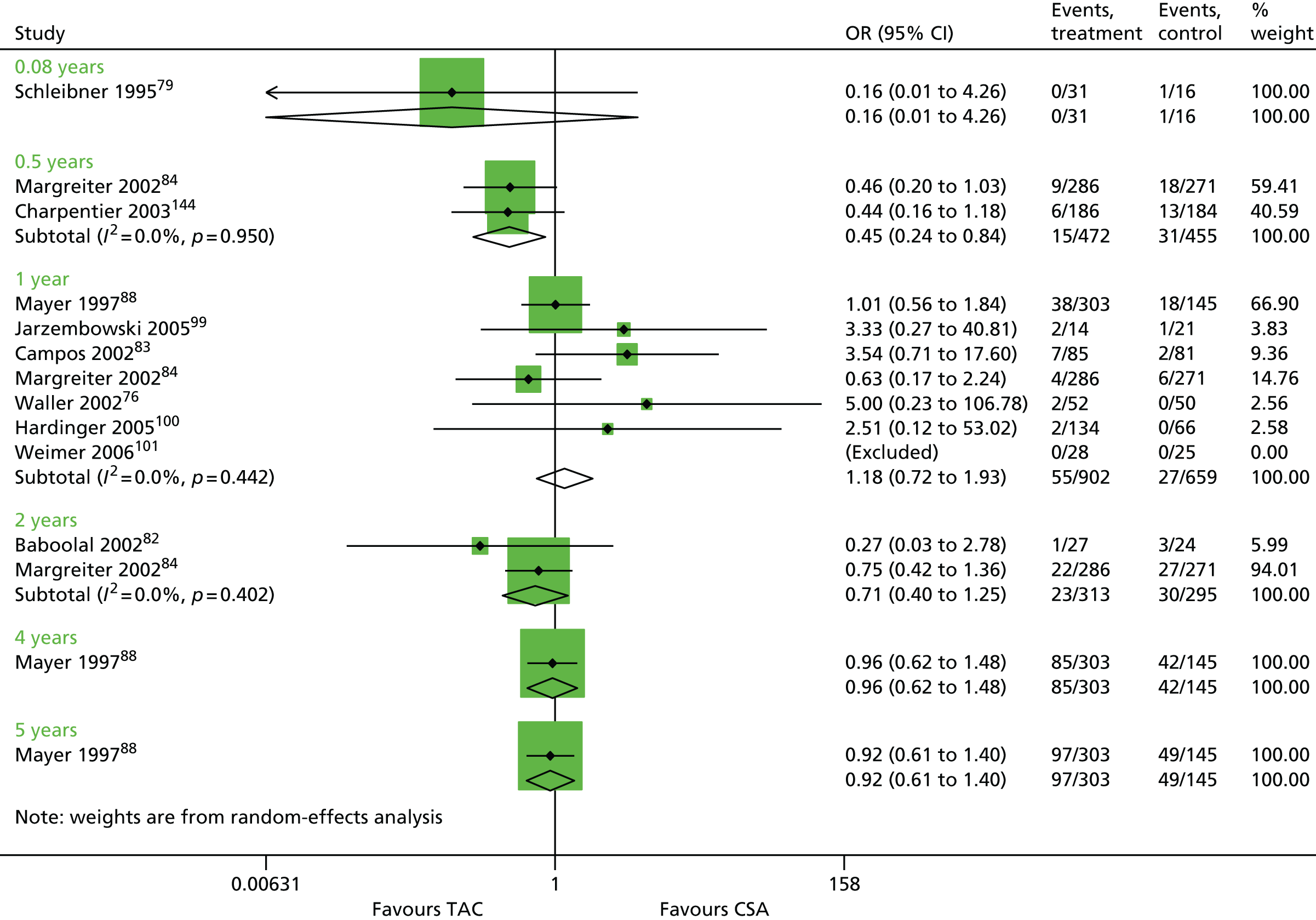
Graft function
Graft function was measured and reported by four studies,75,76,79,84 with effects measured from 0.08 to 3 years. No meta-analysis is provided for GRF, as the results are presented in a number of ways and are not appropriate for pooling. In general, Table 33 shows some variation between arms with large SDs; for example, results presented by Margreiter et al. 84 at 1 year imply an improved GRF for TAC as opposed to CSA [68.9 (SD 23.2) ml/minute/1.73 m2 and 61.8 (SD 23.2) ml/minute/1.73 m2, respectively], which is in contrast with the study of Van Duijnhoven et al. ,75 who report 60.2 ml/minute/1.73 m2 (range 11.5–86.2 ml/minute/1.73 m2) and 64.9 ml/minute/1.73 m2 (range 29.5–84.5 ml/minute/1.73 m2), respectively. This conflict between studies is seen at all time points.
| Study | Time point (years) | TAC, mean ml/minute/1.73 m2 (SD) | CSA, mean ml/minute/1.73 m2 (SD) | MD (ml/minute/1.73 m2) | 95% CI (ml/minute/1.73 m2) | p-value (t-test) |
|---|---|---|---|---|---|---|
| aLaskow 199680 | 0.08 | 50.3 (16.25) | 48.52 (22.5) | 0.0959 | –0.5078 to 0.6995 | 0.3114 |
| bVan Duijnhoven 200275 | 0.25 | 41.7 (13.5–100.2) | 60.5 (26.8–74.5) | NA | NA | NA |
| bMargreiter 200284 | 0.5 | 44.8 (13.6–106.1) | 65.1 (29.6–84.2) | NA | NA | NA |
| Margreiter 200284 | 1 | 68.9 (23.2) | 61.8 (23.2) | 0.3106 | 0.1434 to 0.4777 | 0.003 |
| bVan Duijnhoven 200275 | 60.2 (11.5–86.2) | 64.9 (29.5–84.5) | NA | NA | NA | |
| cWaller 200276 | 47 (14) | 47 (18) | 0 | –0.392 to 0.392 | 1.000 | |
| Margreiter 200284 | 2 | 68.9 (23.2) | 61.8 (23.2) | 0.3106 | 0.1434 to 0.4777 | 0.003 |
| bVan Duijnhoven 200275 | 60.6 (10.0–99.2) | 57.1 (18.8–79.2) | NA | NA | NA | |
| Margreiter 200284 | 3 | 67.3 (23.6) | 64.0 (23.9) | 0.139 | –0.0274 to 0.3053 | 0.1017 |
| bVan Duijnhoven 200275 | 64.0 (38.9–97.9) | 66.9 (9.5–94.2) | NA | NA | NA |
Biopsy-proven acute rejection
All time points from 0.08 to 4 years reveal ORs of < 1 for BPAR, indicating that TAC is more effective than CSA in reducing this outcome (Table 34 and Figure 17). 76,79–84,88,99,100,104,148 BPAR outcomes were reported by nine studies76,81–84,88,99,100,104 at 1 year, where pooled analysis gives an OR of 0.50 and 95% CI 0.39 to 0.64. Minimal heterogeneity is indicated across the studies at year 1 (I2 = 8.1%). Mayer et al. 88 report BPAR at 4 years, when the beneficial effect of TAC appears to be maintained (OR 0.38, 95% CI 0.25 to 0.57).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Schleibner 1995,79 Laskow 199680 | 0.08 | 2 | 0.67 | 0.26 to 1.70 | 0.0 | 0.00 |
| Margreiter 2002;84 Krämer 2005,164 2008;165 Charpentier 2003148 | 0.5 | 2 | 0.50 | 0.32 to 0.79 | 50.1 | 0.06 |
| Mayer 1997,88 1999,162 2002;163 Radermacher 1998;81 Jarzembowski 2005;99 Baboolal 2002;82 Campos 2002;83 Margreiter 2002;84 Krämer 2005,164 2008;165 Waller 2002;76 Murphy 2003;166 Hardinger 2005;100 Brennan 2005;167 Weimer 2005,172 2006104 | 1 | 9 | 0.50 | 0.39 to 0.64 | 8.1 | 0.01 |
| Baboolal 2002;82 Margreiter 2002;84 Krämer 2005,164 2008165 | 2 | 1 | 0.39 | 0.27 to 0.56 | NA | NA |
| Mayer 1997,88 1999,162 2002163 | 3 | 1 | 0.74 | 0.52 to 1.07 | NA | NA |
| 4 | 1 | 0.38 | 0.25 to 0.57 | NA | NA |
FIGURE 17.
Forest plot: BPAR for TAC + AZA vs. CSA + AZA. Note: only the low-dose TAC arm is used for Laskow et al. ,80 as this is closest to the dose used in practice.

Severity of biopsy-proven acute rejection
Four trials82,84,100,148 report on severity of BPAR from 6 months to 2 years (Table 35). For the studies by Baboolal et al. 82 and Hardinger et al. ,100 at 1 year, no participants with BPAR experienced Banff grade III for either arm. 82,100 At 6 months, Charpentier et al. 148 report the proportion of people with BPAR classified as Banff III as 10.7% for TAC and 15.4% for CSA and by 2 years Margreiter et al. 84 report 6.4% and 16.8% of people with BPAR experiencing Banff III, for TAC and CSA, respectively.
| Study | Time point (years) | TAC + AZA | CSA + AZA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff classification | N | BPAR | Banff classification | ||||||
| I | II | III | I | II | III | ||||||
| Margreiter 200284 | 0.5 | 286 | 56 | 21 | 31 | 4 | 271 | 101 | 34 | 49 | 18 |
| Charpentier 2003148 | 0.5 | 186 | 28 | 18 | 7 | 3 | 184 | 39 | 14 | 19 | 6 |
| Baboolal 200282 | 1 | 27 | 5 | 3 | 2 | 0 | 24 | 8 | 5 | 3 | 0 |
| Hardinger 2005100 | 1 | 134 | 6 | 3 | 3 | 0 | 66 | 4 | 1 | 3 | 0 |
| aMargreiter 200284 | 1 | 286 | 60 | 23 | 33 | 4 | 271 | 111 | 39 | 54 | 18 |
| 2 | 286 | 62 | 23 | 35 | 4 | 271 | 113 | 40 | 54 | 19 | |
Time to biopsy-proven acute rejection
Time to first BPAR is reported by only two studies,82,83 with contrasting results (Table 36). The results reported by Baboolal et al. 82 indicate that BPAR is achieved more quickly for participants receiving TAC (35 days, SD 13 days) rather than CSA (59 days, SD 38 days).
| Study | TAC + AZA | CSA + AZA | Statistical test (p-value) | ||||
|---|---|---|---|---|---|---|---|
| n | BPAR | Mean time to BPAR, days (SD) | n | BPAR | Mean time to BPAR, days (SD) | ||
| Baboolal 200282 | 27 | 8 | 35 (13) | 24 | 5 | 59 (38) | NR |
| Campos 200283 | 85 | 29 | 14.5 (47.3) | 81 | 31 | 12.0 (21.0) | NRa |
Summary of results for TAC + AZA vs. CSA + AZA
-
Ten studies76,79,80,83,84,88,99,100,104,148 report mortality, with meta-analysis possible at the 0.5- and 1-year time points. At 0.5 years, pooled results of only two studies84,148 generates an OR of 0.54, 95% CI 0.18 to 1.62, indicating lower odds of mortality for TAC; however, the large CIs overlap the null value (OR = 1) therefore there is unlikely to be a significant difference between treatments. Although the OR at 1 year, which includes eight studies,76,80,83,84,88,99,100,104 has shifted to 1.51, indicating reduced odds of mortality in the CSA arm, the 95% CI of 0.75 to 3.06 also suggest no significant difference between treatments. Heterogeneity across studies for the 1-year time point is low and may not be important at this level according to the Cochrane Handbook201 (I2 = 14.8%).
-
Graft loss is reported for 10 trials. 76,79,80,83,84,88,99,100,104,148 Results were pooled for the 0.5-, 1- and 2-year time points. The pooling of trials reported by Margreiter et al. 84 and Charpentier et al. 148 at 0.5 years give an OR of 0.45 (95% CI 0.24 to 0.84), which is statistically significant in favour of TAC. 84,148 The 1-year time point, where seven studies76,83,84,88,99,100,104 are pooled, generates an OR of 1.18 and a 95% CI 0.72 to 1.93, which is not statistically significant, and this remains the case at 5 years.
-
Graft function was measured and reported by four studies,75,80,84,98 with effects measured from 0.08 to 3 years. No meta-analysis is possible, as the results are presented in a number of ways and are not appropriate for pooling. In general, there is some variation between arms with large SDs, for example results presented by Margreiter et al. 84 at 1 year imply an improved GRF for TAC as opposed to CSA [68.9 ml/minute/1.73 m2 (SD 23.2 ml/minute/1.73 m2) and 61.8 ml/minute/1.73 m2 (SD 23.2 ml/minute/1.73 m2), respectively], which is in contrast with Van Duijnhoven et al. 2002, who report 60.2 ml/minute/1.73 m2 (range 11.5–86.2 ml/minute/1.73 m2) and 64.9 ml/minute/1.73 m2 (range 29.5–84.5 ml/minute/1.73 m2), respectively. This conflict between studies is seen at all time points.
-
All time points from 0.08 to 4 years reveal ORs of < 1 for BPAR, indicating that TAC is more effective than CSA in reducing this outcome. BPAR outcomes were reported by nine studies76,81–84,88,99,100,104 at 1 year, where pooled analysis gives an OR of 0.50 and a 95% CI of 0.39 to 0.64. Low heterogeneity is indicated across the studies at year 1 (I2 = 8.1%). Mayer et al. 88 report BPAR at 4 years, where the beneficial effect of TAC appears to be maintained (OR 0.38, 95% CI 0.25 to 0.57).
-
Four trials82,84,100,148 report on severity of BPAR from 6 months to 2 years. For the studies by Baboolal et al. 82 and Hardinger et al. ,100 at 1 year, no participants with BPAR experienced Banff grade III for either arm. 82,100 At 6 months, Charpentier et al. 148 report the proportion of people with BPAR classified as Banff III as 10.7% for TAC and 15.4% for CSA and, by 2 years, Margreiter et al. 84 report 6.4% and 16.8% of people with BPAR experiencing Banff III, for TAC and CSA, respectively.
-
Time to first BPAR is reported by only two studies, with contrasting results. However, the difference between arms for Campos et al. 83 is not statistically significant (p = 0.6631). The results reported by Baboolal et al. 82 indicate that BPAR is achieved more quickly for participants receiving TAC (35 days, SD 13 days) rather than CSA (59 days, SD 38 days).
CSA + MMF vs. CSA + AZA
Seven studies77,78,86,89,101,104,138 report on this combination of immunosuppressive therapies, with a follow-up of 5 years. All outcomes have been reported other than HRQoL.
Mortality
Seven studies77,78,86,89,101,104,138 report on mortality for CSA + MMF compared with CSA + AZA. Pooling results of five studies78,86,101,104,138 for this combination imply no difference between arms at 1 year, with no evidence of heterogeneity across studies (Table 37 and Figure 18). The ORs switch from > 1 to < 1 for the pooled results at 1 and 3 years; however, the CIs cross ‘OR = 1’ in both cases, suggesting that there may be no difference between MMF and AZA (OR 1.19, 95% CI 0.47 to 3.02 and OR 0.56, 95% CI 0.26 to 1.23, respectively). The study reported by Tuncer et al. 78 provides data at 5 years, which also indicates no preference for either MMF or AZA (OR 0.73, 95% CI 0.15 to 3.50).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Sollinger 1995,77 Tricontinental 1996,89 Remuzzi 2007101 | 0.5 | 3 | 1.00 | 0.42 to 2.35 | 0 | 0 |
| Sadek 2002,86 Tuncer 2002,78 Merville 2004,138 Remuzzi 2007,101 Weimer 2006104 | 1 | 5a | 1.19 | 0.47 to 3.02 | 0 | 0 |
| Tricontinental 1996,89 Tuncer 200278 | 3 | 2 | 0.56 | 0.26 to 1.23 | 0 | 0 |
| 5 | 1 | 0.73 | 0.15 to 3.50 | NA | NA |
FIGURE 18.
Forest plot: mortality for CSA + MMF vs. CSA + AZA.

Graft loss
Five studies77,86,89,104,138 report on graft loss, with results pooled at 0.5- and 1-year time points (Table 38 and Figure 19). However, the 0.5-year time point has only two studies77,89 and a substantial level of heterogeneity (I2 = 72.2%), therefore the OR of 0.58 (95% CI 0.04 to 0.59), which indicates that MMF is more effective at reducing graft loss, must be treated with caution. 201 The results for 1 year suggest no difference between arms (OR 0.76, 95% CI 0.38 to 1.50). Merville et al. 138 appear to show more of an effect in favour of MMF; however, the population is much smaller than that for the Tricontinental study89 and Sadek et al. 86 and Weimer et al. 104 found no evidence of graft loss in either arm.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Sollinger 1995,77 Tricontinental 199689 | 0.5 | 2 | 0.58 | 0.04 to 0.59 | 72.2 | 1.2684 |
| Tricontinental 1996,89 Sadek 2002,86 Merville 2004,138 Weimer 2006104 | 1 | 4 | 0.76 | 0.38 to 1.50 | 32.3 | 0.1203 |
| Tricontinental 199689 | 3 | 1 | 0.94 | 0.51 to 1.71 | NA | NA |
FIGURE 19.
Forest plot: graft loss for CSA + MMF vs. CSA + AZA.

Graft function
Only Merville et al. 138 reported on this outcome. At both 6 months and 1 year there was no statistically significant difference in mean GRF (0.5 years; p = 0.7236 and 1 year; p = 0.6584) (Table 39).
| Study | Time | MMF, mean (SD) | AZA, mean (SD) | MD | 95% CI | p-value (t-test) |
|---|---|---|---|---|---|---|
| Merville 2004138 | 0.5 | 60.4 (17.3) | 58.5 (27.1) | 0.08 | –0.38 to 0.55 | 0.72 |
| 1 | 61.3 (15.8) | 63.1 (16.8) | –0.11 | –0.58 to 0.35 | 0.66 |
Biopsy-proven acute rejection
Six studies77,86,89,101,104,138 report on BPAR. Unlike mortality and graft loss, BPAR analysis reveals that MMF is more beneficial than AZA at 0.5 and 1 year (0.5 years OR 0.50, 95% CI 0.35 to 0.72; 1 year OR 0.47, 95% CI 0.36 to 0.62) (Table 40 and Figure 20). 104
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Sollinger 1995,77 Tricontinental 1996,89 Remuzzi 2007101 | 0.5 | 3 | 0.50 | 0.35 to 0.72 | 35.1 | 0.0361 |
| Tricontinental 1996,89 Sadek 2002,86 Merville 2004,138 Weimer 2006104 | 1 | 4 | 0.47 | 0.36 to 0.62 | 0.0 | 0.00 |
FIGURE 20.
Forest plot: BPAR for CSA + MMF vs. CSA + AZA.

Severity of biopsy-proven acute rejection
Two studies were available for 0.5 years77,89 and one study138 for 1 year, although sample numbers are low for this study (Table 41). Overall, at 0.5 years the more severe classification of Banff III appears to be more likely in the AZA arm for people with BPAR (CSA 9.1%, AZA 15.9% for Sollinger et al. ;77 CSA 5.9%, AZA 11.9% for the Tricontinental group 199689).
| Study | Time point (years) | CSA + MMF | CSA + AZA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff classification | n | BPAR | Banff classification | ||||||
| I | II | III | I | II | III | ||||||
| Sollinger 199577 | 0.5 | 167 | 33 | 18 | 12 | 3 | 166 | 63 | 29 | 24 | 10 |
| Tricontinental 199689 | 0.5 | 173 | 34 | 16 | 16 | 2 | 166 | 59 | 26 | 26 | 7 |
| aMerville 2004138 | 1 | 37 | 5 | 4 | 1 | 0 | 34 | 7 | 2 | 3 | 2 |
Time to biopsy-proven acute rejection
Insufficient data are provided for analysis on this outcome. Merville et al. 138 report 48.5 days for MMF and 43.7 days for AZA. 138
Summary of results for CSA + MMF vs. CSA + AZA
-
Seven studies77,78,86,101,104,138,202 report on mortality for CSA + MMF vs. CSA + AZA. Pooling results of five studies78,86,101,104,138 for this combination imply no difference between arms at 1 year, with no evidence of heterogeneity across studies. The ORs switch from > 1 to < 1, for the pooled results at 1 and 3 years; however, the CIs cross ‘OR = 1’ in both cases, suggesting that there may be no difference between MMF and AZA (OR 1.19, 95% CI 0.47 to 3.02; and 0.56, 95% CI 0.26 to 1.23). The study reported by Tuncer et al. 78 provides data at 5 years, which also indicate no preference for either MMF or AZA (OR 0.73, 95% CI 0.15 to 3.50).
-
Five studies77,86,104,138,202 report on graft loss, with results pooled at 0.5- and 1-year time points. However, the 0.5-year time point has only two studies77,89 and a substantial level of heterogeneity (I2 = 72.2%); therefore, the OR of 0.58 (95% CI 0.04 to 0.59), which indicates that MMF is more effective at reducing graft loss, must be treated with caution. 201 The results for 1 year suggest no difference between arms (OR 0.76, 95% CI 0.38 to 1.50). The study by Merville et al. 138 appears to show more of an effect in favour of MMF; however, the population is much smaller than that for the Tricontinental study89 and Sadek et al. 86
-
Only Merville et al. 138 reported on this outcome: at 6 months the mean GRF was greater for the MMF arm; however, this was reversed at 1 year, when AZA had greater GRF. 138 There is no significant difference between arms (0.5 years, p = 0.7236; 1 year, p = 0.6584).
-
Six studies report on BPAR. 77,86,101,104,138,202 Unlike mortality and graft loss, BPAR analysis reveals that MMF is more beneficial than AZA at 0.5 and 1 year [0.5 years, OR 0.50 (95% CI 0.35 to 0.72); 1 year, OR 0.47 (95% CI 0.36 to 0.62)].
-
Two studies were available for 0.5 years77,89 and one study138 for 1 year. Overall, at 0.5 years the more severe classification of Banff III appears to be more likely in the AZA arm for people with BPAR (CSA 9.1%, AZA 15.9% for Sollinger et al. ;77 CSA 5.9%, AZA 11.9% for the Tricontinental group89). Insufficient data are provided for analysis on time to BPAR. Merville et al. 138 report a slightly more rapid rate of 48.5 days for MMF and 43.7 days for AZA.
TAC + MMF vs. CSA + AZA
Two studies129,139 compared these combinations. GRF and time to BPAR are not reported.
Mortality
Wlodarczyk et al. 139 report mortality at 0.5 years and Vacher-Caponat et al. 129 report at 1 year (Table 42). At 1 year, there are twice as many deaths for TAC + MMF as for CSA + AZA; however, although the OR is > 1, the wide 95% CIs imply no statistically significant difference between arms.
Graft loss
As with mortality, there is only one study for each time point of 0.5 years139 and 1 year129 (Table 43). The wide CIs highlight the low precision and indicate no difference between arms.
Biopsy-proven acute rejection for TAC + MMF vs. CSA + AZA
Only two studies129,139 have reported BPAR: one study at 0.5 years139 and one study at the 1-year time point129 (Table 44). In both cases the OR is < 1, indicating that TAC + MMF is associated with lower odds of BPAR (OR 0.64, 95% CI 0.42 to 0.98; OR 0.35, 95% CI 0.15 to 0.83, respectively).
Severity of biopsy-proven acute rejection
This outcome is reported only by Vacher-Caponat et al. ,129 with no participants experiencing Banff II and III in the TAC + MMF arm, but with 14.3% and 4.8% reported in the CSA + AZA arm, respectively (Table 45).
| Study | Time point (years) | TAC + MMF | CSA + AZA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff classification | n | BPAR | Banff classification | ||||||
| I | II | III | I | II | III | ||||||
| aVacher-Caponat 2012129 | 1 | 143 | 8 | 9 | 0 | 0 | 146 | 21 | 19 | 3 | 1 |
Summary for TAC + MMF vs. CSA + AZA
-
Wlodarczyk et al. 139 report mortality at 0.5 years and Vacher-Caponat et al. 129 report mortality at 1 year. In both cases the OR is > 1, indicating that TAC + MMF is associated with greater odds of mortality; however, the 95% CIs cross ‘OR = 1’, implying no statistically significant difference between arms.
-
Only one study reporting on graft loss at 0.5 years139 and 1 year. 129 No significant difference is evident between treatments.
-
Only two studies have reported BPAR: one study at 0.5 years139 and one study at the 1-year time point. 129 In both cases the OR is < 1, indicating that TAC + MMF is associated with lower odds of BPAR (OR 0.64, 95% CI 0.42 to 0.98; OR 0.35, 95% CI 0.15 to 0.83, respectively).
-
Severity of BPAR is reported by only one study,129 with the greater proportion of people experiencing Banff II and III in the CSA + AZA arm.
TAC + MMF vs. CSA + MMF
This combination of immunosuppressive therapy was identified in five RCTs,51,102,103,130,203 with all outcomes other than HRQoL reported. The RCT reported by Grinyo et al. 51 is also known as the SYMPHONY study.
Mortality
The effect estimate of five pooled studies51,102,103,130,203 at 1 year suggests that TAC + MMF is associated with higher odds of mortality (OR 1.62, 95% CI 0.77 to 3.44; Table 46 and Figure 21). However, although there is no evidence of heterogeneity across studies (I2 = 0.0%), the CIs are wide and cross ‘OR = 1’, indicating low precision and a lack of statistical significance. Results for 2 years and 5 years also demonstrate no statistically significant difference between treatments.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Hernández 2007,130 Rowshani 2006,103 Kumar 2009,203 Grinyo 2009,51 Zadrazil 2012102 | 1 | 5a | 1.62 | 0.77 to 3.44 | 0.0 | 0.0 |
| Hernández 2007130 | 2 | 1 | 2.11 | 0.61 to 7.32 | NA | NA |
| Kumar 2009203 | 5 | 1 | 0.87 | 0.31 to 2.47 | NA | NA |
FIGURE 21.
Forest plot: mortality for TAC + MMF vs. CSA + MMF.
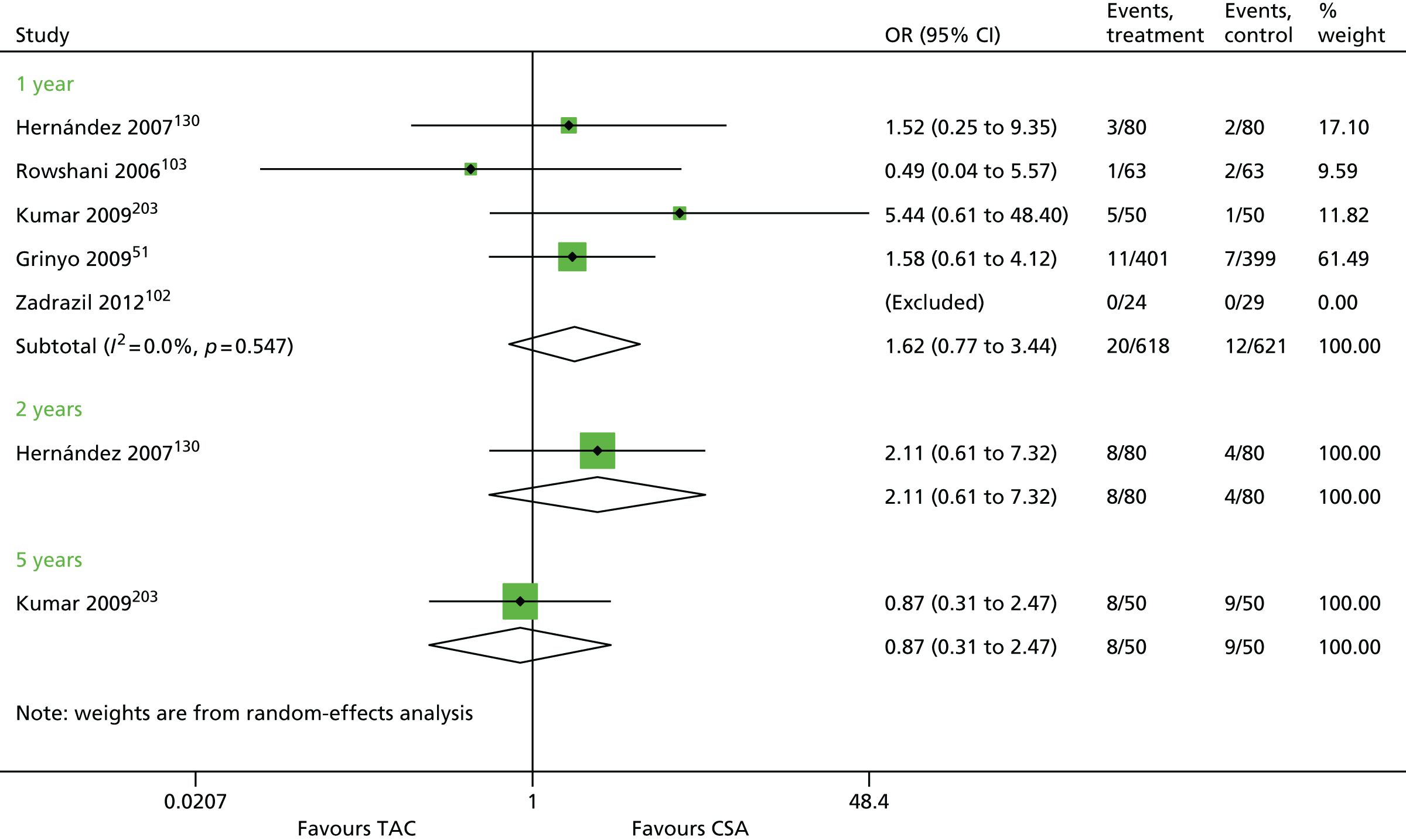
Graft loss
Graft loss is reported for five studies. 51,102,103,130,153,203 The OR for pooled results at 1 year and 2 years (1.43 and 1.63, respectively) implies greater odds of graft loss for TAC + MMF; however, the CIs cross ‘OR = 1’, indicating no difference between arms (Table 47 and Figure 22).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Rowshani 2006,103 Ulsh 1999,153 Kumar 2009,203 Grinyo 2009,51 Zadrazil 2012102 | 1 | 5a | 1.43 | 0.37 to 5.52 | 11.4 | 0.17 |
| Hernández 2007,130 Anil Kumar 2008122 | 2 | 2 | 1.63 | 0.73 to 3.65 | 0.0 | 0.0 |
| Anil Kumar 2008122 | 3 | 1 | 1.11 | 0.45 to 2.75 | NA | NA |
| 4 | 1 | 1.10 | 0.46 to 2.62 | NA | NA | |
| 5 | 1 | 1.19 | 0.53 to 2.69 | NA | NA |
FIGURE 22.
Forest plot: graft loss for TAC + MMF vs. CSA + MMF.

Kumar et al. 203 report graft loss up to 5 years, with similar results of no difference between arms.
Graft function
Graft function as CRC is reported by three studies51,103,130 up to 3 years (Table 48 and Figure 23). Pooling of results for 1- and 2-year data demonstrated a statistically significant difference in GRF in favour of TAC (WMD 4.22 ml/minute/1.73 m2, 95% CI 1.23 to 7.20 ml/minute/1.73 m2; WMD 5.75, 95% CI 2.76 to 8.74 ml/minute/1.73 m2, respectively). There is low evidence of heterogeneity across the 1-year studies (I2 = 9.8%).
| Study | Time point (years) | Trials | WMD (ml/minute/1.73 m2) | 95% CI (ml/minute/1.73 m2) | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Hernández 2007130 | 0.5 | 1 | 4.00 | –2.14 to 10.14 | NA | NA |
| Hernández 2007,130 Rowshani 2006,103 Grinyo 200951 | 1 | 3 | 4.22 | 1.23 to 7.20 | 9.8 | 0.77 |
| Hernández 2007,130 Grinyo 200951 | 2 | 2 | 5.75 | 2.76 to 8.74 | 0.0 | 0.0 |
| Grinyo 200951 | 3 | 1 | 4.60 | 1.35 to 7.85 | NA | NA |
FIGURE 23.
Forest plot: GRF for TAC + MMF vs. CSA + MMF.

Biopsy-proven acute rejection for TAC + MMF vs. CSA + MMF
Biopsy-proven acute rejection was reported by five studies. 51,102,103,153,203 One-year outcomes provided by four of these studies51,103,153,203 were pooled (Table 49 and Figure 24). The study at 0.5 years by Kumar et al. 203 indicates that lower odds of BPAR are associated with TAC. This is in agreement with the pooled results at 1 year, although some heterogeneity is noted across studies (OR 0.59, 95% CI 0.37 to 0.94; I2 = 19.3%). The study reported by Hernández et al. 130 at 2 years does not demonstrate a statistical difference between arms (OR 1.22, 95% CI 0.51 to 2.91).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Grinyo 200951 | 0.5 | 1 | 0.45 | 0.31 to 0.67 | NA | NA |
| Ulsh 1999,153 Rowshani 2006,103 Kumar 2009,203 Grinyo 200951 | 1 | 4 | 0.59 | 0.37 to 0.94 | 19.3 | 0.06 |
| Hernández 2007130 | 2 | 1 | 1.22 | 0.51 to 2.91 | NA | NA |
FIGURE 24.
Forest plot: BPAR for TAC + MMF vs. CSA + MMF.

Severity of biopsy-proven acute rejection
Two studies51,130 report severity of BPAR separately at 1 and 2 years (Table 50). For year 1, results indicate that for people with BPAR, TAC + MMF and CSA + MMF have a similar proportion experiencing Banff III (TAC + MMF 7.8%; CSA + MMF 7.1%). 51 The study by Hernández et al. 130 indicates no clear difference for between arms for all three classifications. 130
| Study | Time point (years) | TAC + MMF | CSA + MMF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff classification | n | BPAR | Banff classification | ||||||
| I | II | III | I | II | III | ||||||
| aGrinyo 200951 | 1 | 399 | 102 | 55 | 39 | 8 | 401 | 42 | 24 | 15 | 3 |
| Hernández 2007130 | 2 | 80 | 13 | 7 | 4 | 2 | 80 | 11 | 6 | 4 | 1 |
Time to biopsy-proven acute rejection
Mean time to BPAR was reported by Ulsh et al. 153 in favour of TAC (Table 51).
| Study | TAC + MMF | CSA + MMF | Statistical test (p-value) | ||||
|---|---|---|---|---|---|---|---|
| n | BPAR | Mean time to BPAR, days (SD) | n | BPAR | Mean time to BPAR, days (SD) | ||
| Ulsh 1999153 | 30 | 4 | 88.7 (32.3) | 30 | 4 | 42 (35.3) | NS |
Summary of results for TAC + MMF vs. CSA + MMF
-
The effect estimate of five pooled studies51,102,103,130,203 at 1 year suggests that TAC + MMF is associated with higher odds of mortality (OR 1.62, 95% CI 0.77 to 3.44). However, although there is no evidence of heterogeneity across studies (I2 = 0.0%), the CIs are wide and cross ‘OR = 1’, indicating low precision and a lack of statistical significance. Results for 2 years and 5 years also demonstrate no statistically significant difference between treatments.
-
Graft loss is reported for five studies. 51,102,103,122,153 The OR for pooled results at 1 and 2 years (1.43 and 1.63, respectively) implies greater odds of graft loss for TAC + MMF; however, the CIs cross ‘OR = 1’, indicating no statistically significant difference between arms. The lack of difference remains at 5 years for the study reported by Kumar et al. 203
-
GRF is reported by three studies up to 3 years. 51,103,130 Pooling of results for 1- and 2-year data demonstrated a statistically significant difference in GRF in favour of TAC (WMD 4.22 ml/minute/1.73 m2, 95% CI 1.23 to 7.20 ml/minute/1.73 m2; WMD 5.75 ml/minute/1.73 m2, 95% CI 2.76 to 8.74 ml/minute/1.73 m2, respectively).
-
BPAR was reported by five studies,51,102,103,122,153 with four studies51,103,122,153 reporting at 1 year as being suitable for meta-analysis. The study at 0.5 years by Kumar et al. 203 indicates that lower odds of BPAR are associated with TAC. This is in agreement with the pooled results at 1 year, although some heterogeneity is noted across studies (OR 0.59, 95% CI 0.37 to 0.94; I2 = 19.3%). Two studies51,130 report severity of BPAR separately at 1 year and 2 years with no clear difference in proportion of people with Banff III grading.
-
Time to BPAR was reported by Ulsh et al. ,153 with a difference in favour of TAC of 88.7 days (p = 0.0001).
TAC + MMF vs. TAC-PR + MMF
Four studies105,123,141,204 are reported investigating all outcomes other than time to BPAR and HRQoL for TAC (immediate release) + MMF vs. TAC-PR (prolonged release) + MMF.
Mortality
Four studies105,123,141,204 report on mortality: two studies report at 0.5 years105,123 and two studies report at 1 year141,204 (Table 52 and Figure 25). At each time point, one of the studies had no deaths in either arm and both ORs indicate no statistical difference (0.5 years, OR 0.65, 95% CI 0.23 to 1.84; 1 year, OR 0.78, 95% CI 0.31 to 2.01).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Albano 2013,123 Oh 2014105 | 0.5 | 2a | 0.65 | 0.23 to 1.84 | NA | NA |
| Krämer 2010,204 Tsuchiya 2013141 | 1 | 2a | 0.78 | 0.31 to 2.01 | NA | NA |
FIGURE 25.
Forest plot: mortality for TAC + MMF vs. TAC-PR + MMF.

Graft loss
Four studies105,123,141,204 report on graft loss: two studies report at 0.5 years105,123 and two studies report at 1 year. 141,204 As illustrated by the forest plot (Table 53 and Figure 26), no clear benefit is seen for either immediate-release or TAC-PR with regard to graft loss at 6 months and 1 year. ORs for both are identical and < 1; however, CIs cross ‘OR = 1’, indicating no statistical difference between arms (OR 0.83, 95% CIs 0.30 to 2.30 and 0.47 to 1.47).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Oh 2014,105 Albano 2013123 | 0.5 | 2 | 0.83 | 0.30 to 2.30 | 0.0 | 0 |
| Krämer 2010,204 Tsuchiya 2013141 | 1 | 2a | 0.83 | 0.47 to 1.47 | NA | NA |
FIGURE 26.
Forest plot: graft loss for TAC + MMF vs. TAC-PR + MMF.
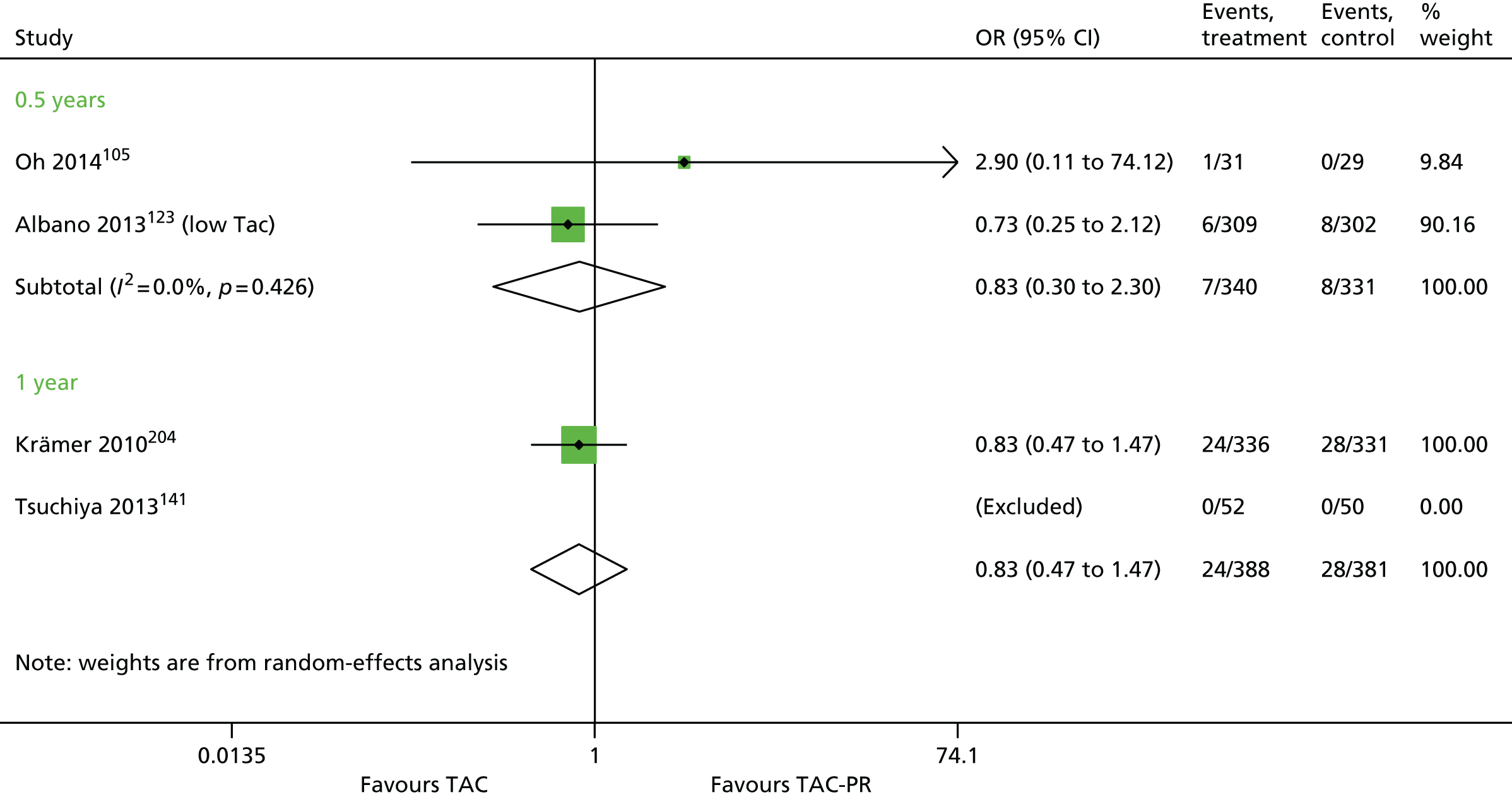
Graft function
Graft function is reported by three studies:123,141,204 one study123 for 0.5 years and two studies141,204 for 1 year (Table 54 and Figure 27). Pooling of results at 1 year demonstrated no statistically significant difference in GRF (WMD 0.21 ml/minute/1.73 m2, 95% CI –2.10 to 2.53 ml/minute/1.73 m2); however, the single study by Albano et al. 123 suggests immediate-release TAC to be more effective than TAC-PR for GRF (WMD 1.90 ml/minute/1.73 m2, 95% CI to 5.40 ml/minute/1.73 m2).
| Study | Time point (years) | Trials | WMD (ml/minute/1.73 m2) | 95% CI (ml/minute/1.73 m2) | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Albano 2013123 | 0.5 | 1 | 1.90 | –1.60 to 5.40 | NA | NA |
| Krämer 2010,204 Tsuchiya 2013141 | 1 | 2 | 0.21 | –2.10 to 2.53 | 0.0 | 0.0 |
FIGURE 27.
Forest plot: GRF for TAC + MMF vs. TAC-PR + MMF.

Biopsy-proven acute rejection for TAC + MMF vs. TAC-PR + MMF
Three studies105,123,204 report BPAR at 0.5 years and two studies141,204 report at 1 year (Table 55 and Figure 28). Pooling of results at both time points shows no significant difference between arms (OR 1.37 95% CI 1.00 to 1.87; OR 1.03, 95% CI 0.48 to 2.17). Furthermore, moderate heterogeneity exists across studies (I2 = 34.8% and 44.4%). 201
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Krämer 2010,204 Oh 2014,105 Albano 2013123 | 0.5 | 3 | 1.37 | 1.00 to 1.87 | 34.8 | 0.04 |
| Krämer 2010,204 Tsuchiya 2013141 | 1 | 2 | 1.03 | 0.48 to 2.17 | 44.4 | 0.16 |
FIGURE 28.
Forest plot: BPAR for TAC + MMF vs. TAC-PR + MMF.
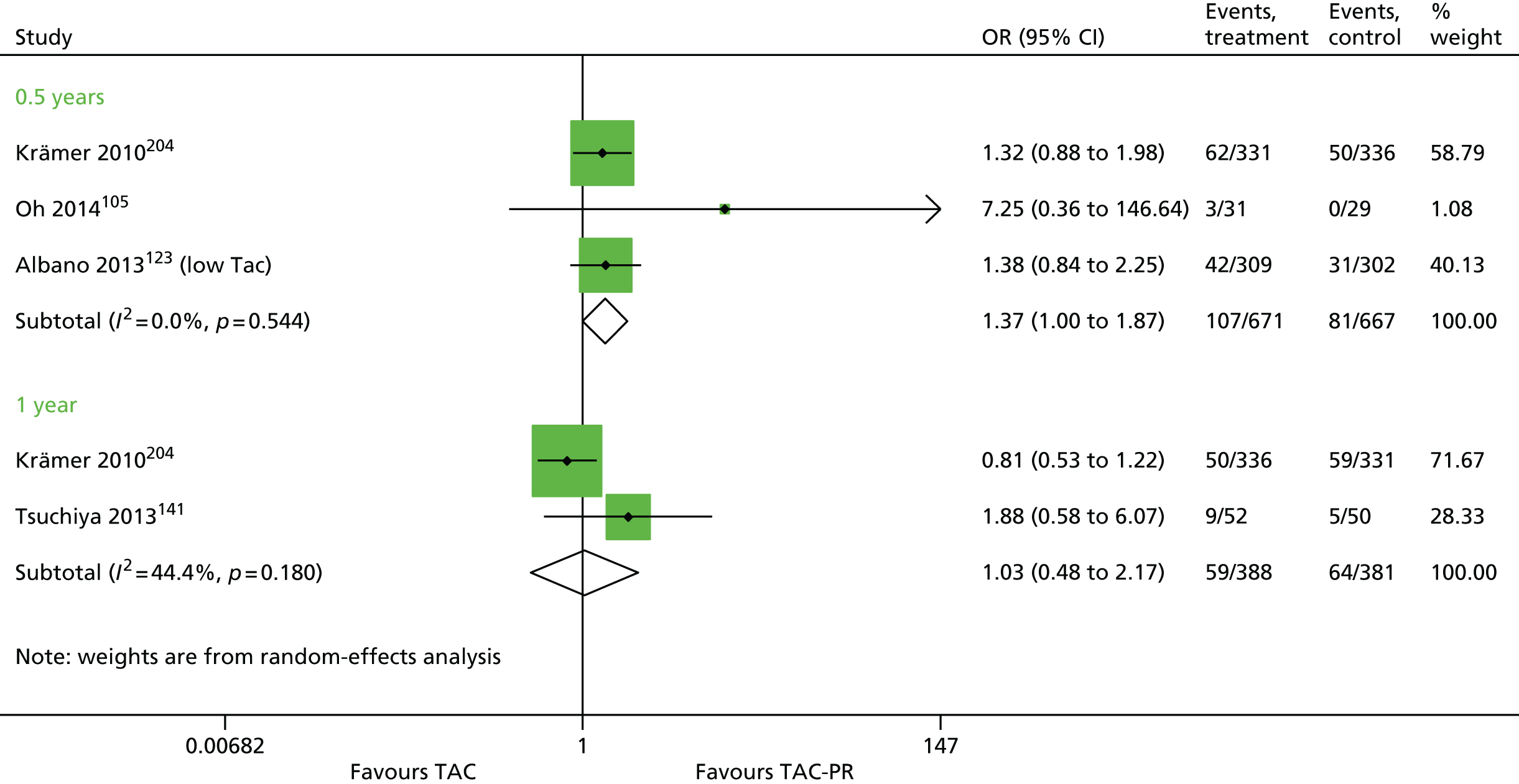
Severity of biopsy-proven acute rejection
Two studies123,204 report severity of BPAR, both of which indicate that, for people with BPAR, the severity may be reduced with immediate TAC (Table 56).
Summary for TAC + MMF vs. TAC-PR + MMF
-
Four studies105,123,141,204 report on mortality: two studies report at 0.5 years123,204 and two studies report at 1 year. 105,141 At each time point, one of the studies had no deaths in either arm and both ORs indicate no statistical difference (0.5 years, OR 0.65, 95% CI 0.23 to 1.84; 1 year, OR 0.78, 95% CI 0.31 to 2.01).
-
Four studies105,123,141,204 report on graft loss: two studies report at 0.5 years105,123 and two studies report at 1 year. 141,204 No clear benefit is seen for either immediate-release TAC or TAC-PR with regard to graft loss at 6 months and 1 year. ORs for both are identical and < 1; however, CIs cross ‘OR = 1’, indicating no statistical difference between arms (OR 0.83, 95% CI 0.30 to 2.30; and 95% CI 0.47 to 1.47). GRF is reported by three studies:123,141,205 one study for 0.5 years123 and two studies for 1 year. 141,205 Pooling of results at 1 year demonstrated no statistically significant difference in GRF (WMD 0.21 ml/minute/1.73 m2, 95% CI –2.10 to 2.53 ml/minute/1.73 m2); however, the single study by Albano et al. 123 suggests that TAC is more effective than TAC-PR for GRF (WMD 1.90 ml/minute/1.73 m2, 95% CI 1.70 to 2.10 ml/minute/1.73 m2).
-
Three studies105,123,204 report BPAR at 0.5 years and two studies report141,204 at 1 year. Pooling of results at both time points shows no significant difference between arms (OR 1.37, 95% CI 1.00 to 1.87; OR 1.03, 95% CI 0.48 to 2.17).
-
Two studies123,204 report severity of BPAR, both of which indicate that, for people with BPAR, the severity may be reduced with immediate.
MMF + TAC vs. MPS + TAC
As only one trial106 reported outcomes for this combination, results are presented in summary tables (Tables 57 and 58).
| Study | Outcome | Time point (years) | MMF | MPS | OR | 95% CI |
|---|---|---|---|---|---|---|
| Ciancio 2008106 | Mortality, n/N (%) | 1 | 0/75 (0) | 1/75 (1) | NA | NA |
| 4 | 2/75 (3) | 3/75 (4) | 0.6575 | 0.1067 to 4.0524 | ||
| Graft loss, n/N (%) | 1 | 2/75 (3) | 2/75 (3) | NA | NA | |
| 4 | 6/75 (8) | 8/75 (11) | 0.5059 | 0.1768 to 1.4476 | ||
| BPAR, n/N (%) | 1 | 2/75 (3) | 7/75 (9) | 0.2661 | 0.0534 to 1.3259 | |
| 2 | 8/75 (11) | 7/75 (9) | 1.1599 | 0.3983 to 3.3783 | ||
| 4 | 14/75 (19) | 13/75 (17) | 1.0946 | 0.4756 to 2.5192 | ||
| Banff classification, n/BPAR | ||||||
| I | 1 | 1/2 | 6/7 | NA | ||
| II | 1/2 | 0/7 | ||||
| III | 0/2 | 1/7 | ||||
| Study | Time point (years) | MMF (SE) | MPS (SE) | MD | 95% CI | p-value (t-test) |
|---|---|---|---|---|---|---|
| Ciancio 2008106 | 0.5 | 63.3 (2.1) | 66.0 (2.0) | –1.3167 | –1.67 to 0.96 | < 0.0001 |
| 1 | 62.10 (2.0) | 66.0 (2.1) | –1.9019 | –2.29 to 1.52 | < 0.0001 | |
| 2 | 63.7 (2.2) | 64.10 (2.4) | –0.1737 | –0.49 to 0.15 | 0.2891 | |
| 3 | 71.3 (3.0) | 69.8 (2.7) | 0.5256 | 0.20 to 0.85 | 0.0016 |
In contrast with other outcomes, GRF displays a significant difference in favour of MPS at 0.5 years and 1 year (0.5 years, MD –1.317; 1 year, MD –1.9019; p < 0.0001) (see Table 58). This effect is lost at later time points.
Overall, there appears to be no discernible difference between arms, as all CIs are wide and cross ‘OR = 1’. Time to BPAR is not reported.
Summary for MMF + CSA vs. MPS + TAC
Only one study106 was identified for this combination. No difference was identified between interventions, other than for GRF, where a statistically significant difference in favour of MPS at 0.5 years and 1 year (p < 0.0001) was noted. This effect is lost at later time points.
MMF + CSA vs. MPS + CSA
Only one trial using this combination is reported by Salvadori et al. ;124 therefore, all outcomes are included in a summary table up to 1 year (Table 59). Overall, the OR indicates that MPS is associated with lower mortality (OR 4.12, 95% CI 0.46 to 37.14); however, the CIs are wide and the effect is not statistically significant. Graft loss initially has better odds for MPS at 0.5 years; however, this reverses at 1 year. Again, CIs imply no statistical significance. BPAR and severity of BPAR show no difference between interventions. GRF and time to BPAR are not reported.
| Study | Outcome | Time | MMF | MPS | OR | 95% CI |
|---|---|---|---|---|---|---|
| Salvadori 2004124 | Mortality, n/N (%) | 0.5 | 2/210 (1) | 1/213 (0) | 2.04 | 0.18 to 22.65 |
| 1 | 4/210 (2) | 1/213 (0) | 4.12 | 0.45 to 37.14 | ||
| Graft loss, n/N (%) | 0.5 | 9/210 (4) | 7/213 (3) | 1.32 | 0.48 to 3.61 | |
| 1 | 6/210 (3) | 15/213 (7) | 0.39 | 0.15 to 1.02 | ||
| BPAR, n/N (%) | 0.5 | 48/210 (23) | 46/213 (22) | 1.08 | 0.68 to 1.70 | |
| 1 | 51/210 (24) | 48/213 (22) | 1.10 | 0.70 to 1.73 | ||
| Banff Classification, n/BPAR | ||||||
| I | 1 | 31/48 | 33/46 | NA | ||
| II | 14/48 | 12/46 | ||||
| III | 3/48 | 2/46 | ||||
Summary for MMF + CSA vs. MPS + CSA
-
Only one trial reported by Salvadori et al. 124 uses this combination. GRF and time to BPAR are not reported. All other results indicate no significant difference between MMF and MPS.
BEL + MMF vs. CSA + MMF
Three studies60,206,207 report on this combination of therapies. Time to BPAR and HRQoL are not reported
Mortality
Three studies60,125,206 report 1-year outcomes, with the Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial (BENEFIT)60 and the BENEFIT–Extended Criteria Donors (BENEFIT-EXT)142 providing data for up to 5 years. The ORs generally fall at < 1 for all time points, indicating that BEL has a lower association with mortality than CSA (Table 60 and Figure 29). However, the CIs indicate that this is not statistically significant.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) |
|---|---|---|---|---|---|
| Vincenti 2005,125 BENEFIT 2010,60 BENEFIT-EXT 2010142 | 1 | 3 | 0.47 | 0.20 to 1.08 | 0.0 |
| BENEFIT 2010,60 BENEFIT-EXT 2010142 | 2 | 2 | 0.76 | 0.41 to 1.41 | 0.0 |
| 3 | 2 | 0.80 | 0.49 to 1.29 | 0.0 | |
| 5 | 2 | 0.71 | 0.40 to 1.29 | 13.85 |
FIGURE 29.
Forest plot: mortality for BEL + MMF vs. CSA + MMF.
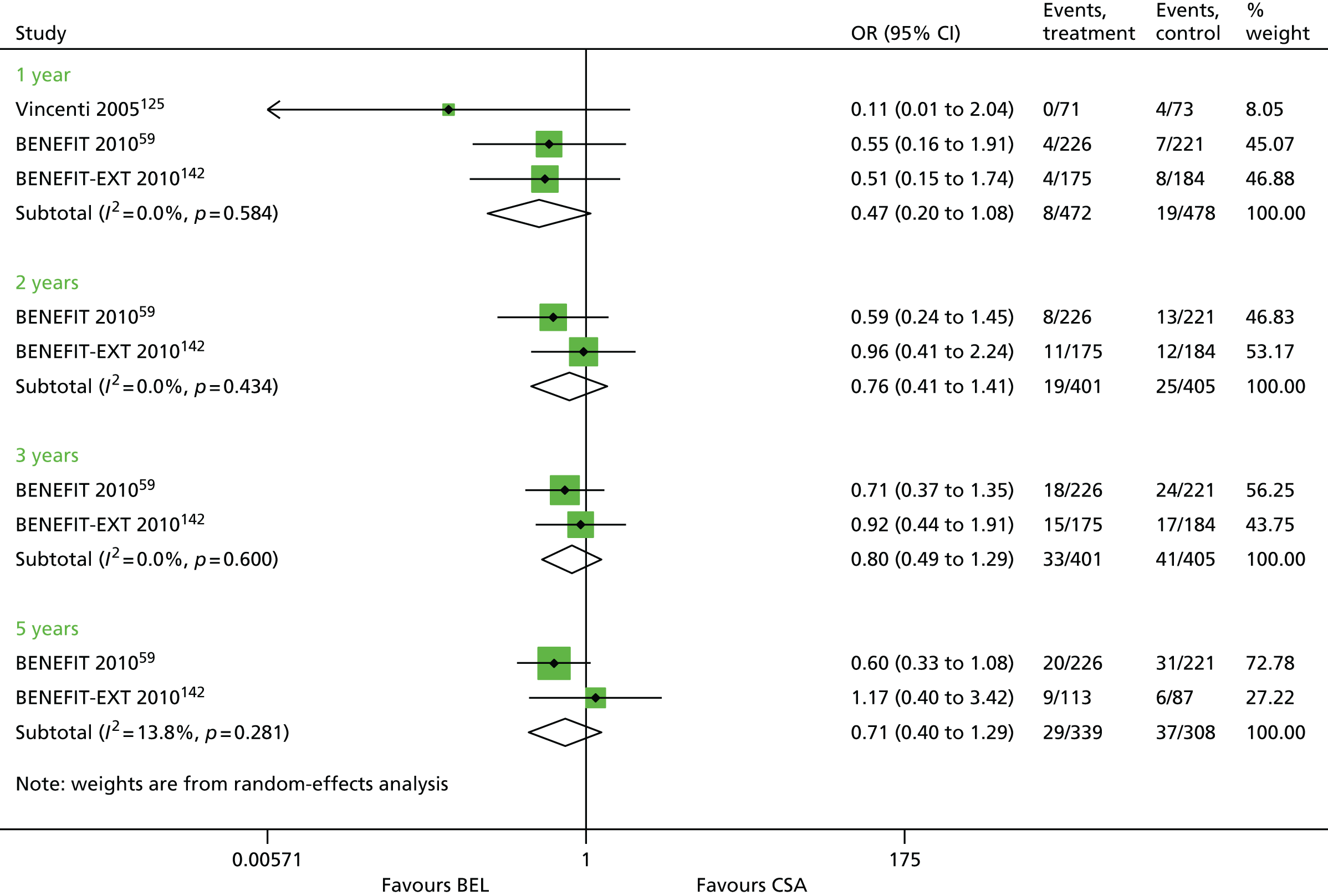
Graft loss
The OR for graft loss is also reported by three studies60,125,206 up to 5 years. Pooled results indicate that BEL may be preferable to CSA, although the results are not statistically significant (1 year, OR 0.74, 95% CI 0.42 to 1.31) (Table 61 and Figure 30). However, at 5 years, there may be more confidence that this effect is true (OR 0.40, 95% CI 0.19 to 0.87).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Vincenti 2005,125 BENEFIT 2010,60 BENEFIT-EXT 2010142 | 1 | 3 | 0.74 | 0.42 to 1.31 | 0.0 | 0.0 |
| BENEFIT 2010,60 BENEFIT-EXT 2010142 | 2 | 2 | 0.85 | 0.49 to 1.49 | 0.0 | 0.0 |
| 3 | 2 | 0.79 | 0.48 to 1.32 | 0.0 | 0.0 | |
| 5 | 2 | 0.40 | 0.19 to 0.87 | 0.0 | 0.0 |
FIGURE 30.
Forest plot: graft loss for BEL + MMF vs. CSA + MMF.

Graft function
Graft function is reported by three studies60,125,206 up to 5 years (Table 62 and Figure 31). The results must be treated with caution because of substantial heterogeneity across studies, which may be caused by variations in methods of calculation and measurement of GRF (I2 = 73.6–91.2%). Pooling of results for 1- and 3-year data demonstrated a statistically significant difference for GRF in favour of BEL (WMD 7.83 ml/minute/1.73 m2, 95% CI 1.57 to 4.10 ml/minute/1.73 m2, and WMD 16.08 ml/minute/1.73 m2, 95% CI 5.59 to 26.56 ml/minute/1.73 m2, respectively).
| Study | Time point (years) | Trials | WMD (ml/minute/1.73 m2) | 95% CI (ml/minute/1.73 m2) | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| aVincenti 2005,125 bBENEFIT 2010,60 bBENEFIT-EXT 2010142 | 1 | 3 | 7.83 | 1.57 to 14.10 | 73.6 | 21.96 |
| bBENEFIT 2010,60 bBENEFIT-EXT 2010142 | 2 | 2 | 11.06 | –1.38 to 23.51 | 91.2 | 73.58 |
| 3 | 2 | 16.08 | 5.59 to 26.56 | 89.5 | 51.23 | |
| bBENEFIT 201060 | 5 | 1 | 23.40 | 20.04 to 26.76 | NA | NA |
FIGURE 31.
Forest plot: GRF for BEL + MMF vs. CSA + MMF.

Biopsy-proven acute rejection
The results for BPAR indicate substantial heterogeneity for the 1-, 2- and 3-year time points (I2 = 58.7%, 38.4% and 62.2%, respectively) (Table 63). 60,206,207 Overall, participants in the CSA arm appear to be less likely to experience BPAR at between 1 and 5 years, as opposed to those in the BEL arm (1 year, OR 1.53, 95% CI 0.78 to 3.02).
Severity of biopsy-proven acute rejection
Three studies60,125,142 report severity of BPAR at 1 year (Table 64). Overall, there is no clear difference between arms in the proportion of people with BPAR experiencing Banff II or III classification. 201,206
| Study | Time point (years) | BEL + MMF | CSA + MMF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff classification | n | BPAR | Banff classification | ||||||
| I | II | III | I | II | III | ||||||
| Vincenti 2005125 | 0.5 | 71 | 4 | 0 | 4 | 0 | 73 | 6 | 2 | 4 | 0 |
| BENEFIT 201060 | 1 | 226 | 39 | 12 | 26 | 1 | 221 | 16 | 8 | 8 | 0 |
| aBENEFIT-EXT 2010142 | 175 | 31 | 6 | 25 | 0 | 184 | 26 | 4 | 22 | 0 | |
| Vincenti 2005125 | 71 | 4 | 0 | 4 | 0 | 73 | 6 | 2 | 4 | 0 | |
Summary for BEL + MMF vs. CSA + MMF
-
Three studies60,125,142 report 1-year outcomes, with two studies60,142 providing data up to 5 years. The ORs generally fall to < 1 for all time points, indicating that BEL has a lower association with mortality than CSA. However, the CIs indicate that this is not statistically significant.
-
The OR for graft loss up is also reported by three studies60,125,142 up to 4 years. Pooled results indicate that BEL may be preferable to CSA, although the results are not statistically significant (1 year, OR 0.74, 95% CI 0.42 to 1.31). However, at 5 years, there may be more confidence that this effect is true (OR 0.40, 95% CI 0.19 to 0.87).
-
GRF is reported by three studies60,125,142 up to 5 years. The results must be treated with caution because of substantial heterogeneity across studies, which may be caused by variations in methods of calculation and measurement of GRF (I2 = 73.6–91.2%). Pooling of results for 1- and 3-year data demonstrated a statistically significant difference for GRF in favour of BEL (WMD 7.83 ml/minute/1.73 m2, 95% CI 1.57 to 14.10 ml/minute/1.73 m2 and WMD 16.08 ml/minute/1.73 m2, 95% CI 5.59 to 26.56 ml/minute/1.73 m2, respectively).
-
In contrast with previous outcomes, results for BPAR are more clear for the three studies. 60,125,142 However, there is substantial heterogeneity across studies at the 1-, 2- and 3-year time points (I2 = 58.7%, 38.4% and 62.2%, respectively). 60,125,142 Overall, participants in the CSA arm appear to be less likely to experience BPAR at between 1 and 5 years, as opposed to those in the BEL arm (1 year, OR 1.53, 95% CI 0.78 to 3.02). Three studies60,125,142 report severity of BPAR at 1 year. Overall, there is no clear difference between arms in the proportion of people with BPAR experiencing Banff II or III classification. 60,125,142
BEL + MMF vs. BEL + SRL vs. TAC + MMF
This combination is reported only in the Ferguson et al. ,126 study therefore results are summarised in below (Table 65). Time to BPAR is not reported. Analysis indicates no statistical difference between arms for any outcome; however, the sample size is relatively low (n = 26 and n = 30).
| Study | Time point (years) | Outcomes | BEL + MMF, n/N | BEL + SRL, n/N | TAC + MMF, n/N | Chi-squared |
|---|---|---|---|---|---|---|
| Ferguson 2011126 | 0.5 | BPAR | 4/33 | 1/26 | 1/30 | 2.0751; p = 0.354 |
| Banff classification I | 0/33 | 0/26 | 0/30 | NA | ||
| Banff classification II | 4/33 | 1/26 | 1/30 | 2.0751; p = 0.354 | ||
| Banff classification III | 0/33 | 0/26 | 0/30 | NA | ||
| 1 | Mortality | 1/33 | 0/26 | 0/30 | 1.6656; p = 0.435 | |
| Graft loss | 2/33 | 2/26 | 0/30 | 2.0675; p = 0.356 | ||
| BPAR | 5/33 | 1/26 | 1/30 | 3.2067; p = 0.201 |
EVL + CSA vs. MMF + CSA
Three RCTs131,143,150 investigating this combination of immunosuppressive therapies were identified. All outcomes other than time to BPAR were reported.
Mortality
Mortality is reported at 0.5,150 1131 and 3143 years (Table 66 and Figure 32). Results are pooled for the 1- and 2-year time points, where the OR is > 1, indicating a preference in favour of MMF; however, this is not statistically significant (OR 1.83, 95% CI 0.80 to 4.20; OR 1.06, 95% CI 0.60 to 1.85, respectively). This trend is reflected at 0.5 years and 3 years.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| ATLAS 2004150 | 0.5 | 1 | 3.13 | 0.83 to 11.74 | NA | NA |
| Lorber 2005,143 ATLAS 2004,150 Takahashi 2013131 | 1 | 3 | 1.83 | 0.80 to 4.20 | 0.0 | 0.0 |
| Lorber 2005,143 ATLAS 2004150 | 3 | 2 | 1.06 | 0.60 to 1.85 | 0.0 | 0.0 |
FIGURE 32.
Forest plot: mortality for EVL + CSA vs. MMF + CSA.

Graft loss
Three RCTs131,143,150 report graft loss for this combination (Table 67 and Figure 33). There is considerable heterogeneity across studies for 1 and 3 years (I2 = 80.0% and 74.3%, respectively) therefore results must be treated with caution. The study reported by Lorber et al. ,143 which favours MMF, appears to be in contrast with the ATLAS study;150 however, there is no statistically significant difference between arms for either trial.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| ATLAS 2004150 | 0.5 | 1 | 0.45 | 0.08 to 1.13 | NA | NA |
| Lorber 2005,143 ATLAS 2004,150 Takahashi 2013131 | 1 | 3 | 0.93 | 0.26 to 3.39 | 80.0 | 0.6944 |
| Lorber 2005,143 ATLAS 2004150 | 3 | 2 | 1.07 | 0.40 to 2.85 | 74.3 | 0.3700 |
FIGURE 33.
Forest plot: graft loss for EVL + CSA vs. MMF + CSA.

Graft function
Lorber et al. 143 provide a median and range for GRF rather than a SD; therefore, results could not be pooled with the ATLAS study150 (Table 68). Overall, there is no significant difference in GRF between EVL + CSA and MMF + CSA (p = 0.1989 to 0.3703).
| Study | Time point (years) | EVL, mean ml/minute/1.73 m2 (SD) | MMF, mean ml/minute/1.73 m2 (SD) | MD, ml/minute/1.73 m2 | 95% CI, ml/minute/1.73 m2 | p-value (t-test) |
|---|---|---|---|---|---|---|
| aLorber 2005143 | 1 | 58 (7–124) | 69 (8–153) | NA | NA | NA |
| ATLAS 2004150 | 52 (21) | 54 (18) | –0.1023 | –0.30 to 0.10 | 0.3131 | |
| aLorber 2005143 | 2 | 60 (5–141) | 71 (6–412) | NA | NA | NA |
| ATLAS 2004150 | 55 (24) | 58 (22) | –0.1303 | –0.33 to 0.07 | 0.1989 | |
| aLorber 2005143 | 3 | 57 (4–140) | 70 (8–157) | NA | NA | NA |
| ATLAS 2004150 | 55 (23) | 57 (21) | –0.0908 | –0.29 to 0.11 | 0.3703 |
Biopsy-proven acute rejection
The pooled and unpooled ORs of < 1 for this outcome all suggest that EVL is associated with lower odds of BPAR; however, the CIs indicate a lack of statistical significance (Table 69 and Figure 34). 131,143,150 There is no evidence of heterogeneity across studies.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| ATLAS 2004150 | 0.5 | 1 | 0.90 | 0.56 to 1.45 | NA | NA |
| Lorber 2005,143 ATLAS 2004,150 Takahashi 2013131 | 1 | 3 | 0.84 | 0.60 to 1.16 | 0.0 | 0.0 |
| Lorber 2005,143 ATLAS 2004150 | 3 | 2 | 0.91 | 0.66 to 1.26 | 0.0 | 0.0 |
FIGURE 34.
Forest plot: BPAR for EVL + CSA vs. MMF + CSA.
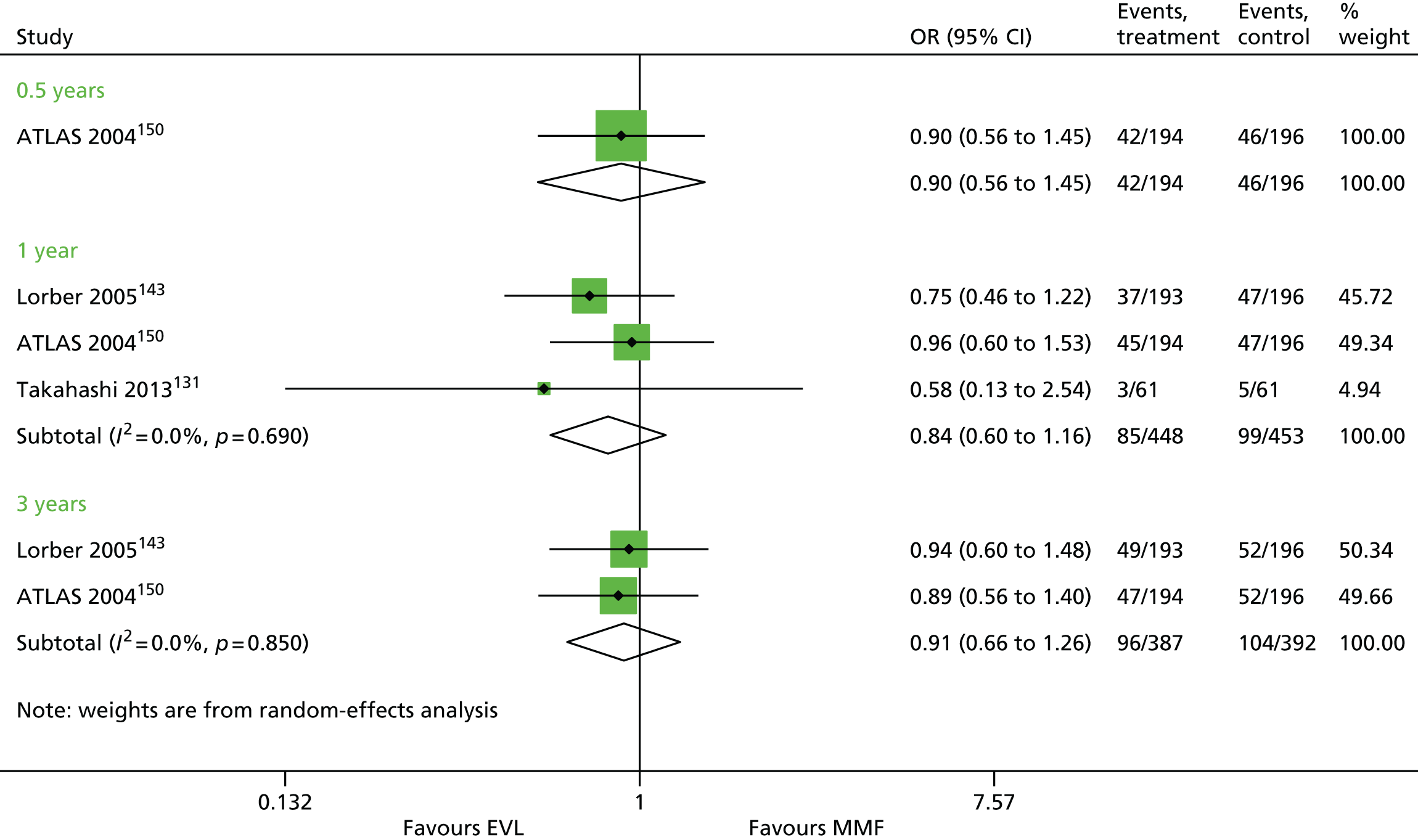
Severity of biopsy-proven acute rejection
Severity of BPAR is reported by only Takahashi et al. 131 at 1 year (Table 70). No occurrences of Banff II or III classification were reported.
| Study | Time point (years) | Banff classification | EVL, n/BPAR | MMF, n/BPAR |
|---|---|---|---|---|
| Takahashi 2013131 | 1 | None/borderline | 2/3 | 3/5 |
| I | 1/3 | 2/5 |
Summary for EVL + CSA vs. MMF + CSA
-
Results for mortality are pooled for three studies131,143,150 at the 1-year time point. The OR is > 1, indicating a preference in favour of MMF; however, this is not statistically significant (OR 1.83, 95% CI 0.80 to 4.20). This trend is reflected at 0.5 years and 3 years.
-
Three RCTs131,143,150 report graft loss for this combination; however, there is significant heterogeneity across studies for 1 and 3 years (I2 = 80.0% and 74.3%, respectively). The study reported by Lorber et al. ,143 which favours MMF, appears to be in contrast with the ATLAS study,150 which favours EVL; however, there is no statistical difference between arms for either trial.
-
Lorber et al. 143 provide a median and range for GRF, rather than a SD; therefore, results could not be pooled with the ATLAS study. 150 Overall, there is no significant difference in GRF between EVL + CSA and MMF + CSA (p = 0.1989 to 0.3703).
-
The pooled and unpooled ORs of < 1 for BPAR all suggest that EVL is associated with lower odds; however, the CIs indicate a lack of statistical significance. 131,143,150 There is no evidence of heterogeneity across studies. Severity of BPAR is reported only by Takahashi et al. 131 at 1 year, when no occurrences of Banff II or III classifications are reported. 131
EVL + CSA vs. MPS + CSA
Three RCTs107,144,152 were identified reporting on this combination. All outcomes other than time to BPAR and HRQoL are reported.
Mortality
Pooled analysis of three studies107,144,152 at 1 year for mortality indicates no significant difference between EVL + CSA and MPS + CSA (OR 1.02, 95% CI 0.42 to 2.45; Table 71 and Figure 35). No heterogeneity was evident across studies.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Chadban 2014,152 Tedesco-Silva 2010,107 Bertoni 2011144 | 1 | 3 | 1.02 | 0.42 to 2.45 | 0.0 | 0.0 |
FIGURE 35.
Forest plot: mortality for EVL + CSA vs. MPS + CSA.

Graft loss
The OR for graft loss is generated from three pooled studies,107,144,152 which indicates that EVL may be preferable in reducing graft loss; however, this result is not statistically significant (OR 0.65, 95% CI 0.15 to 2.87) (Table 72 and Figure 36). Furthermore, moderate heterogeneity is noted across studies.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Chadban 2014,152 Tedesco-Silva 2010,107 Bertoni 2011144 | 1 | 3 | 0.65 | 0.15 to 2.87 | 34.8 | 0.7158 |
FIGURE 36.
Forest plot: graft loss for EVL + CSA vs. MPS + CSA.

Graft function
Two studies107,144 report GRF; however, although results are pooled, the heterogeneity between them is extremely high (I2 = 91.2%) (Table 73 and Figure 37). As such, the evidence is unclear as to which treatment may be beneficial.
| Study | Time point (years) | Trials | WMD | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Tedesco-Silva 2010,107 Bertoni 2011144 | 1 | 2 | 8.56 | –10.66 to 27.77 | 91.2 | 176.12 |
FIGURE 37.
Forest plot: GRF for EVL + CSA vs. CSA + MPS.
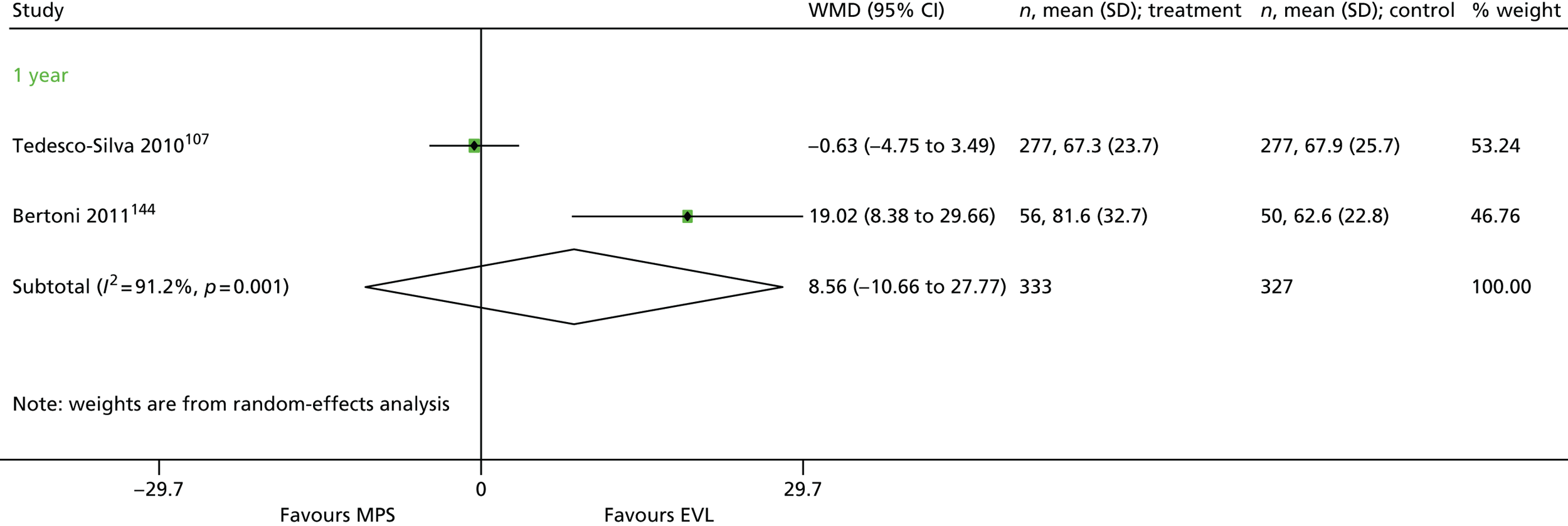
Biopsy-proven acute rejection
Biopsy-proven acute rejection is reported by three studies107,144,152 at 1 year. Pooling of results indicates no statistically significant difference between EVL + CSA vs. MPS + CSA (OR 1.01, 95% CI 0.68 to 1.48) (Table 74 and Figure 38).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Chadban 2014,152 Tedesco-Silva 2010,107 Bertoni 2011144 | 1 | 3 | 1.01 | 0.68 to 1.48 | 0.0 | 0.0 |
FIGURE 38.
Forest plot: BPAR for EVL + CSA vs. MPS + CSA.
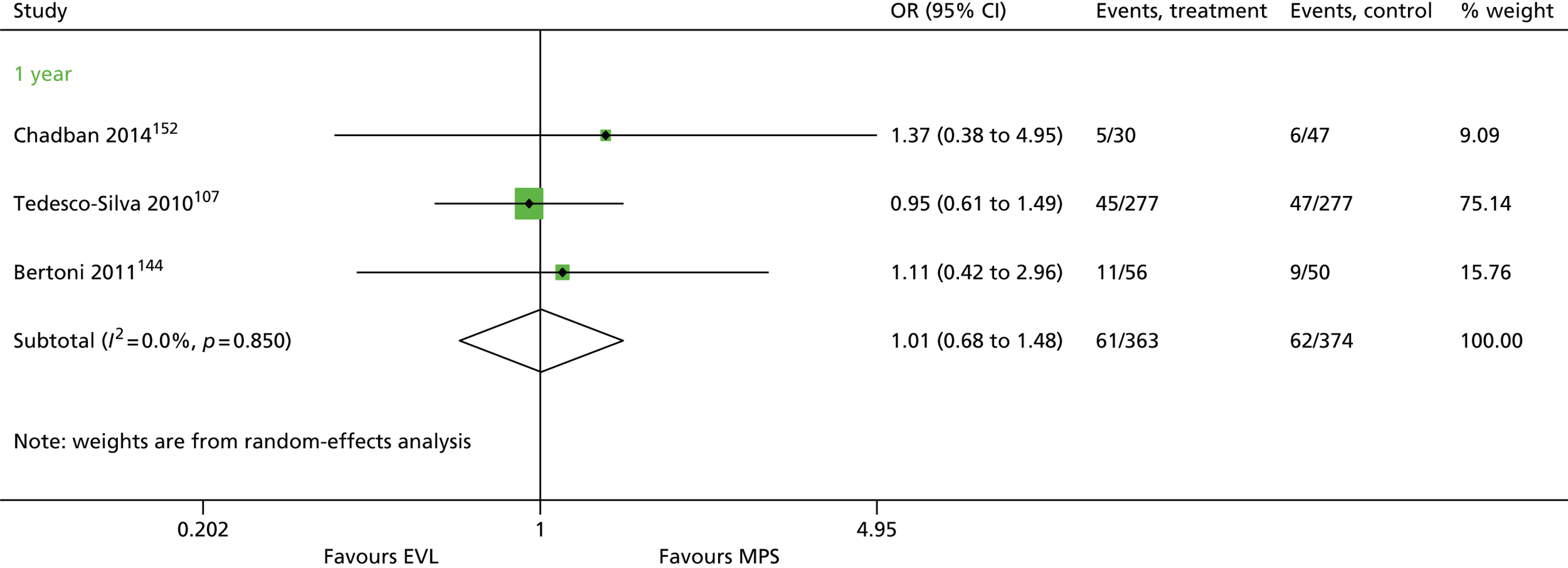
Severity of biopsy-proven acute rejection
The study reported by Tedesco-Silva et al. 107 suggests that more people with BPAR receiving MPS experienced Banff II classification; however, there was no difference for Banff III (Table 75). There were no Banff II or III episodes reported in the EVL treatment for Chadban et al. ,152 with only one episode among those receiving MPS treatment; however, the sample size is small.
| Study | Time point (years) | EVL + CSA | MPS + CSA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff classification | n | BPAR | Banff classification | ||||||
| I | II | III | I | II | III | ||||||
| aChadban 2014152 | 1 | 30 | 5 | 5 | 0 | 0 | 47 | 6 | 7 | 1 | 1 |
| bTedesco-Silva 2010107 | 1 | 277 | 47 | 32 | 8 | 1 | 277 | 50 | 29 | 17 | 1 |
Summary for EVL + CSA vs. MPS + CSA
-
Pooled analysis of three studies107,144,152 at 1 year indicates no significant difference between EVL + CSA and MPS + CSA (OR 1.02, 95% CI 0.42 to 2.45). No heterogeneity was evident across studies.
-
The OR for graft loss is generated from three pooled studies,107,144,152 which indicates that EVL may be preferable in reducing graft loss; however, this result is not statistically significant (OR 0.648, 95% CI 0.146 to 2.870). Furthermore, moderate heterogeneity is noted across studies.
-
BPAR is reported by three studies107,144,152 at 1 year. Pooling of results indicates no statistically significant difference between EVL + CSA compared with MPS + CSA (OR 1.01, 95% CI 0.68 to 1.48).
-
The study reported by Tedesco-Silva et al. 107 suggests that more people with BPAR receiving MPS experienced Banff II grading; however, there was no difference for Banff III (see Table 78). There were no Banff II or III episodes reported in the EVL treatment for Chadban et al. ,152 with only one episode among those receiving MPS treatment; however, the sample size is small. 107
EVL + MPS vs. CSA + MPS
Only the study reported by Mjörnstedt et al. 133 investigated this combination of therapies. Therefore, outcomes are summarised in Table 76. Time to BPAR is not reported. Data are provided at 1 year, when there is no statistical difference between arms for mortality or graft loss. There is evidence to indicate greater odds of BPAR associated with EVL + MPS (OR 19.31, 95% CI 9.09 to 41.04). There is no significant difference in severity of BPAR.
| Study | Time point (years) | Outcome | EVL + MPS | CSA + MPS | OR | 95% CI |
|---|---|---|---|---|---|---|
| Mjörnstedt 2012133 | 1 | Mortality, n/N (%) | 2/102 (98) | 2/100 (98) | 1.00 | 0.14 to 7.24 |
| Graft loss, n/N (%) | 0/102 (0) | 0/100 (0) | NA | NA | ||
| BPAR, n/N (%) | 28/102 (27) | 11/100 (11) | 3.06 | 1.43 to 6.56 | ||
| BPAR – no Banff, n/N (%) | 31/102 (30) | 6/100 (6) | 6.84 | 2.71 to 17.28 | ||
| BPAR – Banff I, n/N (%) | 5/102 (5) | 7/100 (7) | 0.68 | 0.21 to 2.23 | ||
| BPAR – Banff II, n/N (%) | 0/102 (0) | 0/100 (0) | NA | NA |
SRL + CSA vs. MMF + CSA
Three RCTs108,109,122 were identified for this combination of therapies. No time to BPAR or severity of BPAR was reported.
Mortality
Two studies109,122 were available for pooling at 1 year; however, one of the studies109 had no deaths in either arm (Table 77 and Figure 39). The ORs appear to indicate lower odds associated with mortality for SRL; however, this is not statistically significant (1 year, OR 0.49, 95% CI 0.04 to 5.59). The 2- and 5-year time points also show no statistically significant difference (2 years, OR 0.31, 95% CI 0.05 to 1.92; 5 years, OR 1.0, 95% CI 0.36 to 2.77).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Anil Kumar 2008,122 aStallone 2004109 | 1 | 2 | 0.49 | 0.04 to 5.59 | NA | NA |
| Barsoum 2007108 | 2 | 1 | 0.31 | 0.05 to 1.92 | NA | NA |
| Anil Kumar 2008122 | 5 | 1 | 1.0 | 0.36 to 2.77 | NA | NA |
FIGURE 39.
Forest plot: mortality for SRL + CSA vs. MMF + CSA.
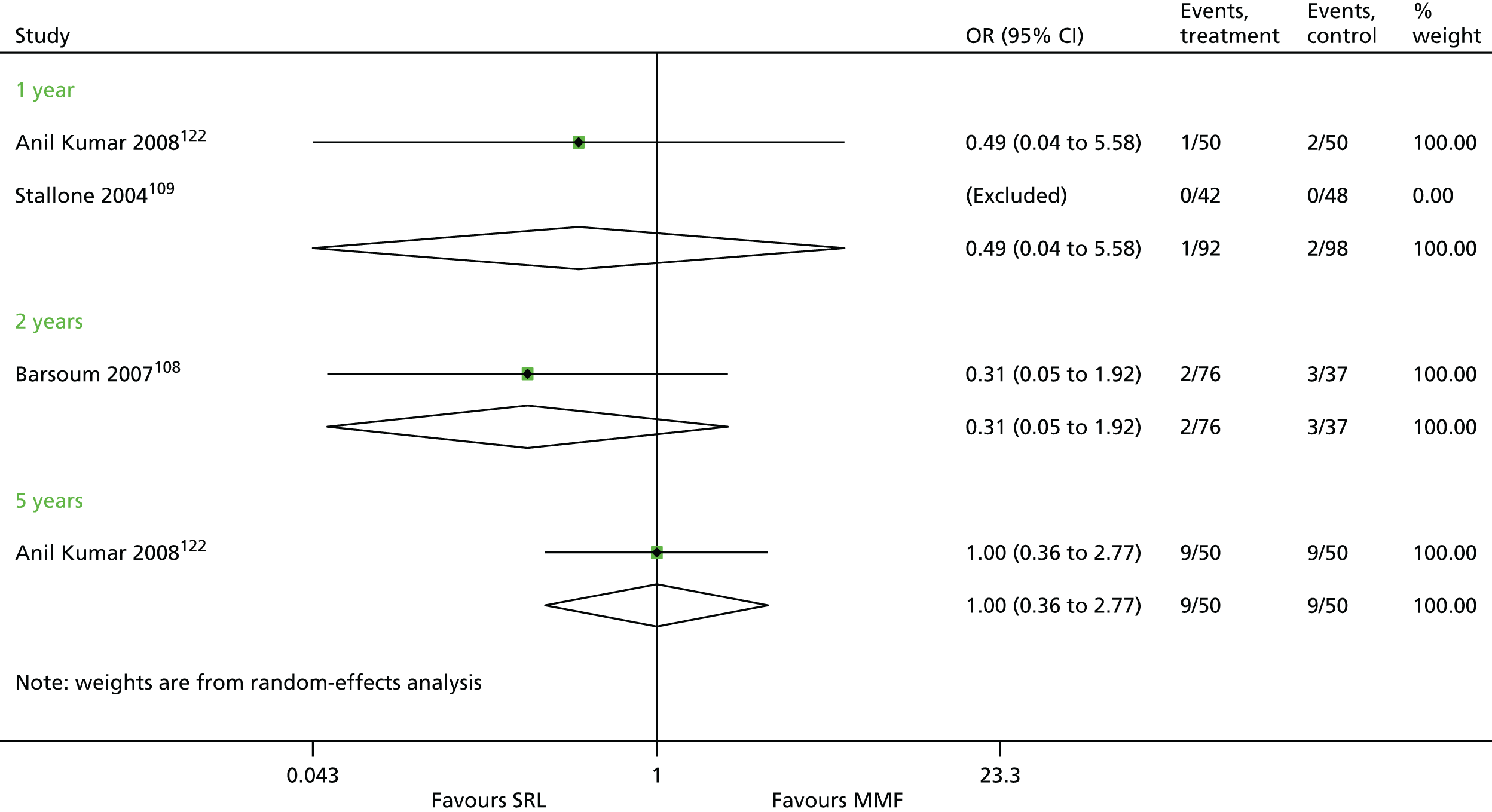
Graft loss
Three studies108,109,122 report on graft loss for SRL + CSA vs. MMF + CSA from 1 to 5 years (Table 78 and Figure 40). 108,109,122 The ORs up to 4 years slightly favour MMF; however, there is no statistically significant effect overall. At 5 years, the OR becomes ‘1’, indicating no benefit for either treatment.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Anil Kumar 2008,122 Stallone 2004109 | 1 | 2 | 1.53 | 0.24 to 9.59 | NA | NA |
| Barsoum 2007,108 Anil Kumar 2008122 | 2 | 2 | 1.20 | 0.42 to 3.45 | 0.0 | 0.0 |
| Anil Kumar 2008122 | 3 | 1 | 1.36 | 0.56 to 3.30 | NA | NA |
| 4 | 1 | 1.32 | 0.57 to 3.10 | NA | NA | |
| 5 | 1 | 1.0 | 0.36 to 2.77 | NA | NA |
FIGURE 40.
Forest plot: graft loss for SRL + CSA vs. MMF + CSA.
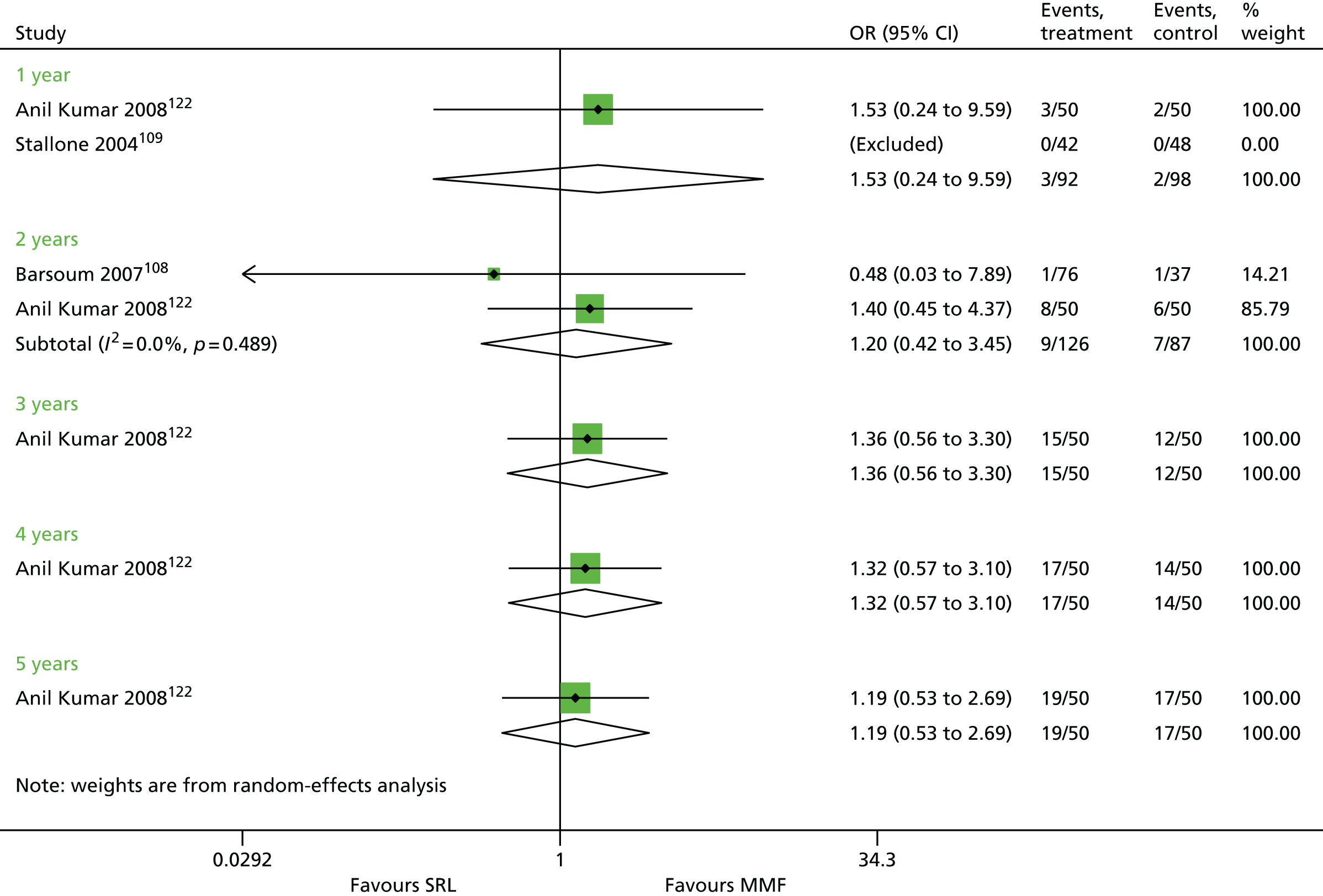
Graft function
Graft function is monitored by one study109 at 1 year (Table 79). No statistical difference is apparent between SRL and MMF (WMD 0.11 ml/minute/1.73 m2; p = 0.5708).
| Study | SRL, n (SD) | MMF, n (SD) | WMD (ml/minute/1.73 m2) | 95% CI (ml/minute/1.73 m2) | p-value (t-test) |
|---|---|---|---|---|---|
| Stallone 2003109 | 61.5 (11) | 60.3 (9) | 0.11 | –0.28 to 0.51 | 0.5708 |
Biopsy-proven acute rejection
The study by Anil Kumar et al. 122 reporting on BPAR at 1 year a similar percentage of events in both arms and therefore no difference between treatments (Table 80). 122 At 2 years, Barsoum et al. 108 report more favourable outcomes for SRL; however, this is not statistically significant (OR 0.65, 95% CI 0.22 to 1.87).
Summary for SRL + CSA vs. MMF + CSA
-
Two studies109,122 were available for pooling at 1 year; however, one of the studies109 had no deaths in either arm. The ORs appear to indicate lower odds associated with mortality for SRL; however, this is not statistically significant (1 year, OR 0.49, 95% CI 0.04 to 5.59). The 2- and 5-year time points also show no statistically significant difference (2 years, OR 0.31, 95% CI 0.05 to 1.92; 5 years, OR 1.0, 95% CI 0.36 to 2.77).
-
Three studies108,122,208 report on graft loss for SRL + CSA compared with MMF + CSA from 1 to 5 years. ORs slightly favour MMF, but the effect is not statistically significant (1 year, OR 1.53, 95% CI 0.24 to 9.59).
-
GRF is monitored by one study109 at 1 year. No statistical difference is apparent between SRL and MMF (WMD 0.11 ml/minute/1.73 m2; p = 0.5708).
-
The study by Anil Kumar et al. 122 reporting on BPAR at 1 year had eight events in both arms and therefore no difference between treatment. At 2 years, Barsoum et al. 108 report more favourable outcomes for SRL; however, this is not statistically significant (OR 0.65, 95% CI 0.22 to 1.87).
SRL + TAC vs. MMF + TAC
A total of eight RCTs94,110,112,114,122,145,155,180 were identified investigating SRL + TAC vs. MMF + TAC with all outcomes other than HRQoL reported.
Mortality
Eight RCTs94,110,112,114,122,145,155,180 report mortality from 0.08 years to 3 years (Table 81 and Figure 41). The ORs vary from < 1 at 0.08 years to > 1 at 3 years; however, the CIs are wide and cross ‘OR = 1’, indicating no statistical significance at any time point.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Anil Kumar 2008122 | 0.08 | 1 | 0.38 | 0.07 to 2.03 | NA | NA |
| Van Gurp 2010114 Vítko 200694 | 0.5 | 2 | 0.83 | 0.19 to 3.59 | 6.9 | 0.08 |
| Anil Kumar 2005,110 Gonwa 2003,180 Sampaio 2008,112 Gallon 2006,145 Flechner 2011155 | 1 | 5 | 0.98 | 0.47 to 2.02 | 0.0 | 0.0 |
| Anil Kumar 2005,110 Flechner 2011155 | 2 | 2 | 1.03 | 0.37 to 2.89 | 10.8 | 0.07 |
| Gallon 2006145 | 3 | 1 | 3.74 | 0.15 to 94.55 | NA | NA |
FIGURE 41.
Forest plot: mortality for SRL + TAC vs. MMF + TAC.

Graft loss
Five RCTs112,114,122,145,180 were identified reporting graft loss (Table 82 and Figure 42). Four RCTs112,122,145,180 are pooled at 1 year, at which increased odds of graft loss are associated with SRL. However, the effect is not statistically significant (OR 1.43, 95% CI 0.44 to 4.66). There may also be moderate heterogeneity across studies following pooling (I2 = 38.8%). The study by Anil Kumar et al. 122 provides follow-up to 5 years, with the OR of < 1 favouring SRL; however, the results are not statistically significant.
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Van Gurp 2010114,180 | 0.5 | 1 | 0.33 | 0.03 to 3.18 | NA | NA |
| Gonwa 2003,180 Sampaio 2008,112 Gallon 2006,145 Anil Kumar 2008122 | 1 | 4 | 1.43 | 0.44 to 4.66 | 38.8 | 0.54 |
| Anil Kumar 2008122 | 2 | 1 | 0.72 | 0.23 to 2.24 | NA | NA |
| Gallon 2006,145 Anil Kumar 2008122 | 3 | 2 | 1.59 | 0.13 to 19.23 | 77.2 | 2.55 |
| Anil Kumar 2008122 | 4 | 1 | 0.58 | 0.23 to 1.46 | NA | NA |
| 5 | 1 | 0.70 | 0.30 to 1.61 | NA | NA |
FIGURE 42.
Forest plot: graft loss SRL + TAC vs. MMF + TAC.

Graft function
Three RCTs111,114,145 were identified reporting GRF; however, because of the different time points, only two RCTs111,114 could be pooled at 0.5 years (Tables 83 and 84; Figure 43). The results indicate no statistical difference between arms (WMD –1.875 ml/minute/1.73 m2, 95% CI –8.425 to 4.675 ml/minute/1.73 m2). Furthermore, substantial heterogeneity across studies is evident (I2 = 81.6%).
| Study | Time point | Trials | WMD (ml/minute/1.73 m2) | 95% CI (ml/minute/1.73 m2) | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Mendez 2005,111 Van Gurp 2010114 | 6 months | 2 | –1.875 | –8.425 to 4.675 | 81.6 | 18.86 |
| Study | Time point (years) | Trials | GRF, mean ml/minute/1.73 m2 | |
|---|---|---|---|---|
| SRL | MMF | |||
| Mendez 2005111 | 1 | 1 | 54.3 | 58.4 |
| Gallon 2006145 | 3 | 1 | 36.9 | 58.3 |
| 8.5 | 23.5 | 54.1 | ||
FIGURE 43.
Forest plot: GRF for SRL + TAC vs. MMF + TAC.

Biopsy-proven acute rejection
Biopsy-proven acute rejection is reported in four studies,112,122,145,180 with three studies112,122,145 pooled at 1 year (Table 85 and Figure 44). The ORs for 0.5 years and 1 year suggest that MMF + TAC has lower odds of BPAR; however, the effect is not statistically significant (1 year, OR 1.16, 95% CI 0.56 to 2.60). There is also a low level of heterogeneity (I2 = 27.8%).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Gonwa 2003180 | 0.5 | 1 | 1.16 | 0.62 to 2.19 | NA | NA |
| Anil Kumar 2008,122 Sampaio 2008,112 Gallon 2006145 | 1 | 3 | 1.21 | 0.56 to 2.60 | 27.8 | 0.13 |
FIGURE 44.
Forest plot: BPAR for SRL + TAC vs. MMF + TAC.
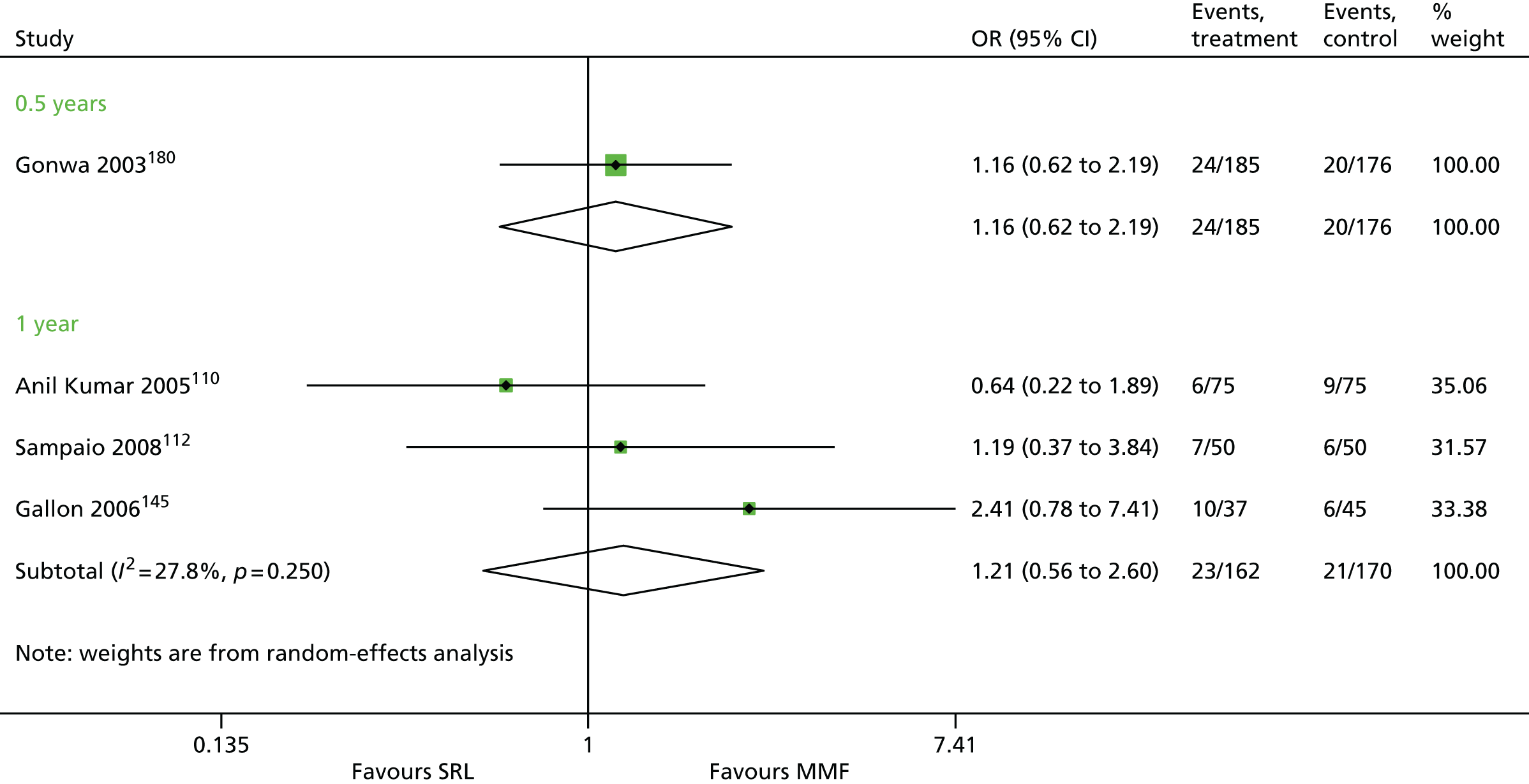
Severity of biopsy-proven acute rejection
Four studies94,112,114,155 report severity of BPAR (Table 86). No clear difference is apparent at either time point for Banff II or III classification between SRL and MMF.
| Study | Time point (years) | SRL + TAC | MMF + TAC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff classification | n | BPAR | Banff classification | ||||||
| I | II | III | I | II | III | ||||||
| aVan Gurp 2010114 | 0.5 | 318 | 48 | 30 | 17 | 1 | 316 | 39 | 20 | 17 | 2 |
| Vítko 200694 | 0.5 | 325 | 82 | 77 | 5 | 327 | 73 | 71 | 2 | ||
| aFlechner 2011155 | 1 | 152 | 22 | 17 | 5 | 0 | 139 | 11 | 5 | 6 | 0 |
| Sampaio 2008112 | 1 | 50 | 7 | 4 | 3 | 0 | 50 | 6 | 2 | 4 | 0 |
| aFlechner 2011155 | 2 | 152 | 25 | 20 | 5 | 0 | 139 | 16 | 10 | 6 | 0 |
Time to biopsy-proven acute rejection
Time to BPAR is reported by Sampaio et al. ,112 which appears to favour MMF (Table 87).
Summary for SRL + TAC vs. MMF + TAC
-
Eight RCTs94,110,112,114,122,145,155,180 report mortality from 0.08 years to 3 years. The ORs vary from < 1 at 0.08 years to > 1 at 3 years; however, the CIs are wide and cross ‘OR = 1’, indicating no statistical significance at any time point.
-
Five RCTs were identified reporting graft loss. 112,114,122,145,180 Four RCTs are pooled at 1 year where increased odds of graft loss are associated with SRL; however, the effect is not statistically significant (OR 1.43, 95% CI 0.44 to 4.66). There may also be moderate heterogeneity across studies following pooling (I2 = 38.8%). The study by Anil Kumar et al. 122 provides follow-up to 5 years, with the OR of < 1 favouring SRL; however, the results are not statistically significant.
-
Three RCTs111,114,145 were identified reporting GRF; however, because of the different time points, only two RCTs111,114 could be pooled at 0.5 years. The results indicate no statistical difference between arms (WMD –1.875 ml/minute/1.73 m2, 95% CI –8.425 to 4.675 ml/minute/1.73 m2). Furthermore, substantial heterogeneity across studies is evident (I2 = 81.6%).
-
BPAR is reported in four studies,112,122,145,180 with three studies112,122,145 pooled at 1 year. The ORs for 0.5 years and 1 year suggest that MMF + TAC has lower odds of BPAR; however, the effect is not statistically significant (1 year, OR 1.16, 95% CI 0.56 to 2.60). There is also a low level of heterogeneity (I2 = 27.8%).
-
Four studies94,112,114,155 report severity of BPAR. No clear difference is apparent for Banff II or III classification between SRL and MMF. Time to BPAR is reported by Sampaio et al. ,112 with a statistically significant difference demonstrated in favour of MMF (MD 48.6 days; p = 0.0017).
SRL + MMF vs. CSA + MMF
Ten studies91,115–118,127,134,146,147,149 were identified investigating SRL + MMF compared with CSA + MMF.
Mortality
Eight studies115–117,127,134,146,147,149 report on mortality, with seven pooled at 1 year (Table 88 and Figure 45). No statistically significant difference was evident at this time point (1 year, OR 0.98, 95% CI 0.28 to 3.42). Data are available up to 5 years; however, the effect is also not statistically significant (5 years, OR 1.15, 95% CI 0.42 to 3.13). 115,209
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Durrbach 2008146 | 0.5 | 1 | 3.37 | 0.13 to 85.63 | NA | NA |
| Flechner 2002,127 Noris 2007,115 Lebranchu 2009,149 Büchler 2007,134 Kreis 2000,116 Guba 2010,147 Martinez-Mier 2006117 | 1 | 7a | 0.98 | 0.28 to 3.42 | 0.0 | 0 |
| Flechner 2002,127 Noris 2007115 | 2 | 2 | 4.02 | 0.42 to 38.31 | 0.0 | 0 |
| Lebranchu 2009149 | 4 | 1 | 1.11 | 0.15 to 8.05 | NA | NA |
| Flechner 2002,127 Büchler 2007134 | 5 | 2 | 1.15 | 0.42 to 3.13 | 0.0 | 0 |
FIGURE 45.
Forest plot: mortality for SRL + MMF vs. CSA + MMF.
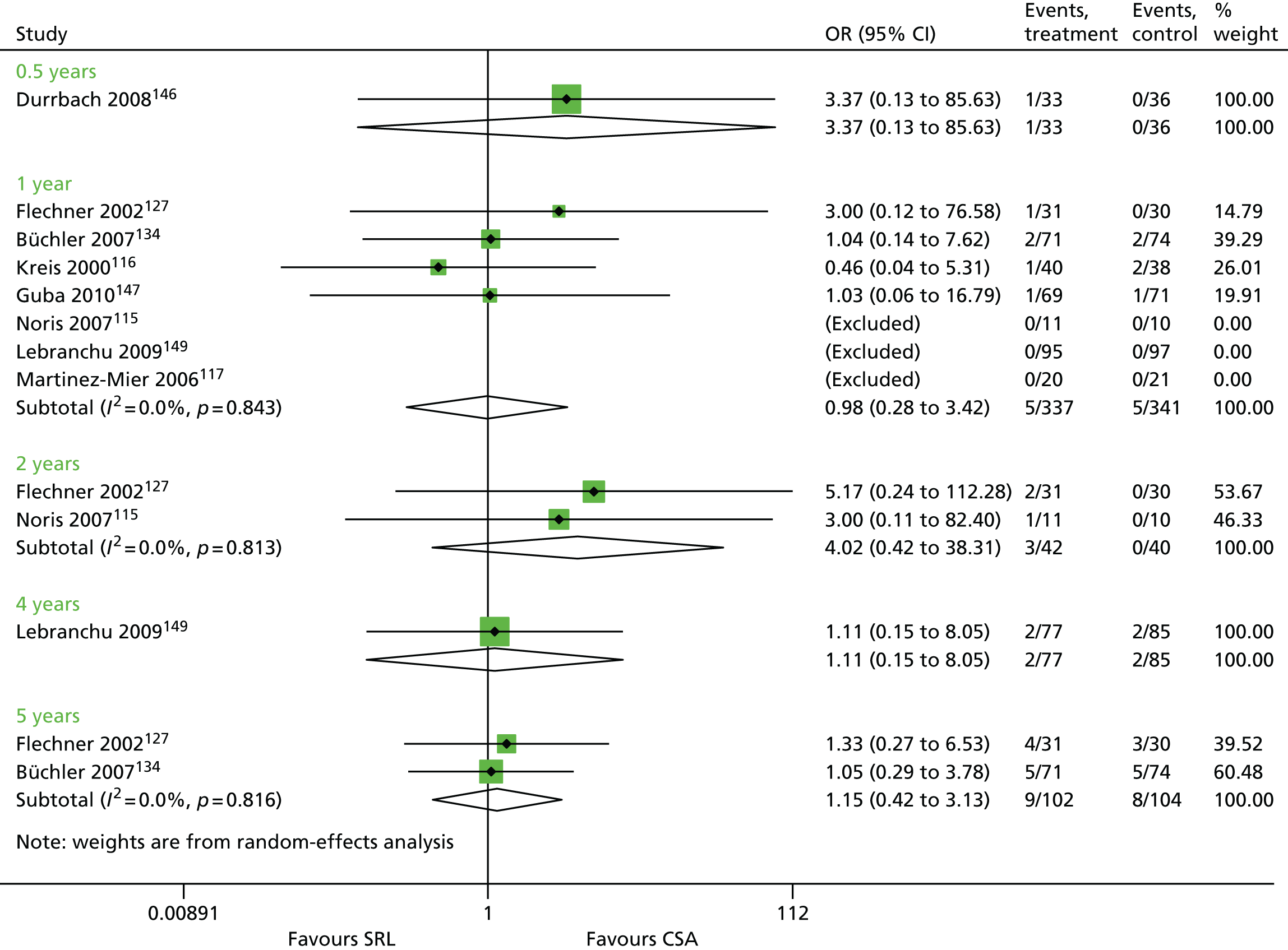
Graft loss
Eight studies115–117,127,134,146,147,149 report on graft loss from 0.5 years to 5 years (Table 89 and Figure 46). Seven studies115–117,127,134,147,149 are pooled at 1 year; however, there is no statistically significant difference between SRL + MMF and CSA + MMF (1 year, OR 1.06, 95% CI 0.44 to 2.56). Flechner et al. 127 and Büchler et al. 134 report graft loss at 5 years; however, again, there is no statistically significant difference and heterogeneity across studies is substantial (5 years, OR 0.57, 95% CI 0.05 to 7.25, I2 = 76.6%).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Durrbach 2008146 | 0.5 | 1 | 4.83 | 0.51 to 45.62 | NA | NA |
| Flechner 2002,127 Lebranchu 2009,149 Büchler 2007,134 Kreis 2000,116 Guba 2010,147 Martinez-Mier 2006,117 Noris 2007115 | 1 | 7a | 1.06 | 0.44 to 2.56 | 0.0 | 0 |
| Flechner 2002,127 Noris 2007115 | 2 | 2 | 0.18 | 0.01 to 3.93 | NA | NA |
| Lebranchu 2009149 | 4 | 1 | 5.66 | 0.27 to 119.81 | NA | NA |
| Flechner 2002,127 Büchler 2007134 | 5 | 2 | 0.57 | 0.05 to 7.25 | 76.6 | 2.6195 |
FIGURE 46.
Forest plot: graft loss for SRL + MMF vs. CSA + MMF.

Graft function
Six studies117,118,127,134,146,149 report GRF (note, this includes Lebranchu et al. ,67 with 68.9 ml/minute/1.73 m2 for SRL and 64.4 ml/minute for CSA; however, a SD is not provided). Pooled analysis for 0.5 years and 1 year suggests that improved GRF is associated with CSA, although this effect is not statistically significant (0.5 year, WMD 6.99 ml/minute/1.73 m2, 95% CI 0.45 to 13.53 ml/minute/1.73 m2; 1 year, WMD 9.41 ml/minute/1.73 m2, 95% CI –1.28 to 20.09 ml/minute/1.73 m2) (Table 90 and Figure 47). The individual studies for 2, 3, 4 and 5 years all have OR of < 1 and are statistically significant, therefore CSA appears beneficial in terms of GRF.
| Study | Time point (years) | Trials | WMD (ml/minute/1.73 m2) | 95% CI (ml/minute/1.73 m2) | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Durrbach 2008,146 Flechner 2002,127 Martinez-Mier 2006117 | 0.5 | 3 | 6.99 | 0.45 to 13.53 | 30.8 | 10.47 |
| Flechner 2002,127 Büchler 2007,134 Martinez-Mier 2006117 | 1 | 3 | 9.41 | –1.28 to 20.09 | 72.7 | 64.39 |
| Flechner 2002127 | 2 | 1 | 17.00 | 9.72 to 24.28 | NA | NA |
| Nafar 2012118 | 3 | 1 | 10.00 | 1.38 to 18.62 | NA | NA |
| 4 | 1 | 9.50 | 0.50 to 18.50 | NA | NA | |
| Büchler 2007134 | 5 | 1 | 9.10 | 1.68 to 16.52 | NA | NA |
FIGURE 47.
Forest plot: GRF for SRL + MMF vs. CSA + MMF.

Biopsy-proven acute rejection
Eight studies115–117,127,134,146,147,149 report on BPAR from 0.5 years to 5 years (Table 91 and Figure 48). Seven studies115–117,127,134,147,149 are pooled at 1 year; however, there is no statistically significant difference between arms, although the OR falls in favour of CSA + MMF (1 year, OR 1.29, 95% CI 0.81 to 2.04). Flechner et al. 127 and Büchler et al. 134 report BPAR at 5 years; however, again, there is no statistically significant difference and heterogeneity across studies is substantial (5 years, OR 0.77, 95% CI 0.37 to 1.63).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Durrbach 2008146 | 0.5 | 1 | 1.52 | 0.31 to 7.35 | NA | NA |
| Flechner 2002,127 Lebranchu 2009,149 Büchler 2007,134 Kreis 2000,116 Guba 2010,147 Martinez-Mier 2006,117 Noris 2007115 | 1 | 7 | 1.29 | 0.81 to 2.04 | 0.0 | 0 |
| Flechner 2002127 | 2 | 1 | 0.34 | 0.06 to 1.94 | NA | NA |
| Lebranchu 2009149 | 4 | 1 | 1.11 | 0.15 to 8.05 | NA | NA |
| Flechner 2002,127 Büchler 2007134 | 5 | 2 | 0.77 | 0.37 to 1.63 | 0.0 | 0 |
FIGURE 48.
Forest plot: BPAR for SRL + MMF vs. CSA + MMF.

Severity of biopsy-proven acute rejection
Severity of BPAR is reported by three studies116,127,134 at 1 year (Table 92). Flechner et al. 127 also report results for 5 years. Sample sizes are relatively low, with similar proportions of people with BPAR experiencing Banff II and III classification.
| Study | Time point (years) | SRL + MMF | CSA + MMF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | BPAR | Banff classification | n | BPAR | Banff classification | ||||||
| I | II | III | I | II | III | ||||||
| Büchler 2007134 | 1 | 71 | 9 | 5 | 2 | 2 | 74 | 6 | 2 | 2 | 2 |
| aFlechner 2002127 | 1 | 31 | 2 | 1 | 0 | 0 | 30 | 5 | 2 | 1 | 0 |
| Kreis 2000116 | 1 | 40 | 11 | 1 | 10 | 0 | 38 | 7 | 2 | 4 | 1 |
| bFlechner 2002127 | 5 | 31 | 4 | 2 | 0 | 0 | 30 | 7 | 3 | 2 | 0 |
Time to biopsy-proven acute rejection
Time to BPAR is reported by three studies127,134,146 (Table 93). A statistically significant difference is seen by Durrbach et al. 146 (SRL 56 days, SD 57 days; CSA 94 days, SD 47 days; p = 0.0035). 146 The studies reported by Büchler et al. 134 and Flechner et al. 127 show no statistical difference between treatments (p = 0.3858 and p = 0.982, respectively).
| Study | SRL + MMF | CSA + MMF | Statistical test (p-value) | ||||
|---|---|---|---|---|---|---|---|
| n | BPAR | Mean time to BPAR, days (SD) | n | BPAR | Mean time to BPAR, days (SD) | ||
| aDurrbach 2008146 | 33 | 4 | 56 (57) | 36 | 3 | 94 (47) | NR (0.399) |
| Büchler 2007134 | 71 | 12 | 75 (82) | 74 | 6 | 87 (84) | NS |
| bFlechner 2002127 | 31 | 4 | 481 (471) | 30 | 7 | 471 (534) | χ2 = 1.01 (p = 0.31) |
Summary of results for SRL + MMF vs. CSA + MMF
-
Eight studies115–117,127,134,146,147,149 report on mortality, with seven studies115–117,127,134,147,149 pooled at 1 year. No statistically significant difference was evident at this time point (1 year, OR 0.98, 95% CI 0.28 to 3.42) or up to 5 years (5 years, OR 1.15, 95% CI 0.42 to 3.13). 115,209
-
Eight studies115–117,127,134,146,147,149 report on graft loss from 0.5 years to 5 years. Seven studies115–117,127,134,147,149 are pooled at 1 year; however, there is no statistically significant difference between SRL + MMF and CSA + MMF (1 year, OR 1.06, 95% CI 0.44 to 2.56). Flechner et al. 127 and Büchler et al. 134 report graft loss at 5 years; however, again, there is no statistically significant difference and heterogeneity across studies is substantial (5 years, OR 0.57, 95% CI 0.05 to 7.25, I2 = 76.6%).
-
Six studies117,118,127,134,146,149 report GRF (note, this includes Lebranchu et al. ,149 with 68.9 ml/minute/1.73 m2 for SRL and 64.4 ml/minute/1.73 m2 for CSA; however, a SD is not provided). Pooled analysis for 0.5 years and 1 year suggests that improved GRF is associated with TAC, although this effect is not statistically significant (0.5 year, WMD 6.99 ml/minute/1.73 m2 95% CI 0.45 to 13.53 ml/minute/1.73 m2; 1 year, WMD 9.41 ml/minute/1.73 m2, 95% CI –1.28 to 20.09 ml/minute/1.73 m2). The individual studies for 2, 3, 4 and 5 years all have OR of < 1 and are statistically significant, therefore TAC appears to be beneficial in terms of GRF.
-
Eight studies115–117,127,134,146,147,149 report on BPAR from 0.5 years to 5 years. Seven studies115–117,127,134,147,149 are pooled at 1 year; however, there is no statistically significant difference between arms, although the OR falls in favour of CSA + MMF (1 year, OR 1.29, 95% CI 0.81 to 2.04). Flechner et al. 127 and Büchler et al. 134 report BPAR at 5 years; however, again, there is no statistically significant difference and heterogeneity across studies is substantial (5 years, OR 0.77, 95% CI 0.37 to 1.63).
-
Severity of BPAR is reported by three studies116,127,134 at 1 year. Sample sizes are relatively low, with similar proportions of people with BPAR experiencing Banff II and III classification. Time to BPAR is reported by three studies. 127,134,146 A statistically significant difference is seen by Durrbach et al. 146 (SRL 56 days, SD 57 days; CSA 94 days, SD 47 days; p = 0.0035). The studies reported by Büchler et al. 134 and Flechner et al. 127 show no statistical difference between treatments (p = 0.3858 and p = 0.982, respectively).
TAC + MMF vs. SRL + MMF
Four studies92,93,135,154 report outcomes for this combination of treatments. No time to BPAR or HRQoL is reported.
Mortality
Four studies92,93,135,154 are pooled with 1-year results for mortality; however, two of these studies93,135 had no deaths for either TAC + MMF or SRL + MMF (Table 94 and Figure 49). Furthermore, analysis suggests no statistically significant difference between TAC + MMF and SRL + MMF (OR 0.80, 95% CI 0.13 to 4.99). Heilman et al. 135 also present results at 2 years (see Table 99). Again, results are not statistically significant (OR 2.10, 95% 0.19 to 23.83).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Larson 2006,154 Schaefer 2006,92 Heilman 2011,135 Smith 200893 | 1 | 4a,b | 0.80 | 0.13 to 4.99 | 19.2 | 0.39 |
| Heilman 2011135 | 2 | 1 | 2.10 | 0.19 to 23.83 | NA | NA |
FIGURE 49.
Forest plot: mortality for TAC + MMF vs. SRL + MMF.
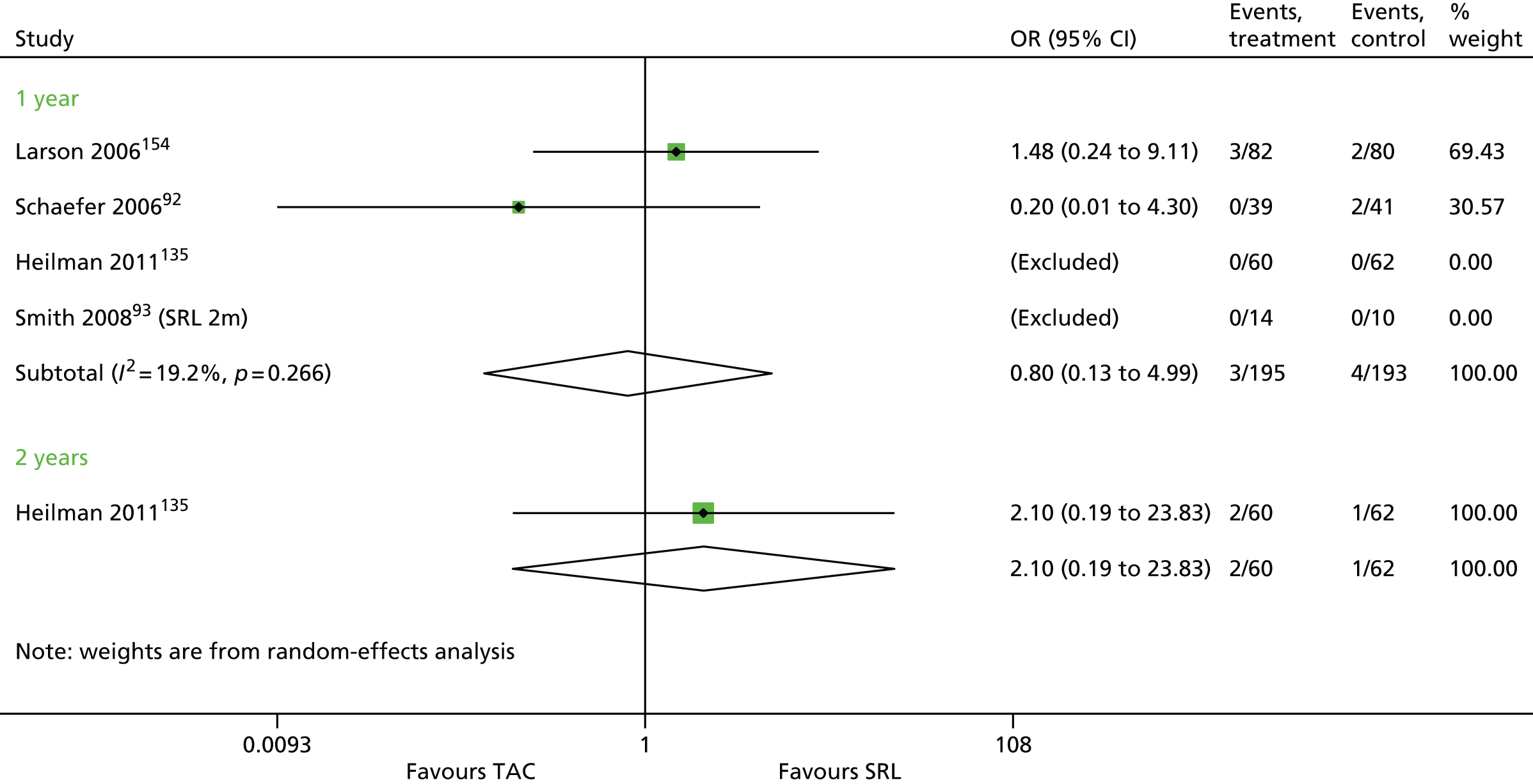
Graft loss
Four studies92,93,135,154 are pooled with 1-year results for graft loss (Table 95 and Figure 50). Again, two of these studies93,135 had no graft loss in either arm. Although the OR implies that reduced graft loss is associated with TAC, this is not statistically significant (OR 0.68, 95% CI 0.18 to 2.58).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Larson 2006,154 Schaefer 2006,92 Heilmann 2011,135 Smith 200893 | 1 | 4a | 0.68 | 0.18 to 2.58 | 0.0 | 0.0 |
| Heilman 2011135 | 2 | 1b | NA | NA | NA | NA |
FIGURE 50.
Forest plot: graft loss for TAC + MMF vs. SRL + MMF.

Graft function
Two studies135,154 report GRF at 1 year and 2 years (Table 96 and Figure 51). The pooled ORs for both time points indicate no statistically significant difference between TAC + MMF and SRL + MMF (1 year, WMD –2.50 ml/minute/1.73 m2, 95% CI –6.85 to 1.85 ml/minute/1.73 m2).
| Study | Time point (years) | Trials | WMD (ml/minute/1.73 m2) | 95% CI (ml/minute/1.73 m2) | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Larson 2006,154 Heilman 2011135 | 1 | 2 | –2.50 | –6.85 to 1.85 | 0.0 | 0.0 |
| 2 | 2 | 0.57 | –3.70 to 4.55 | 0.0 | 0.0 |
FIGURE 51.
Forest plot: GRF for TAC + MMF vs. SRL + MMF.
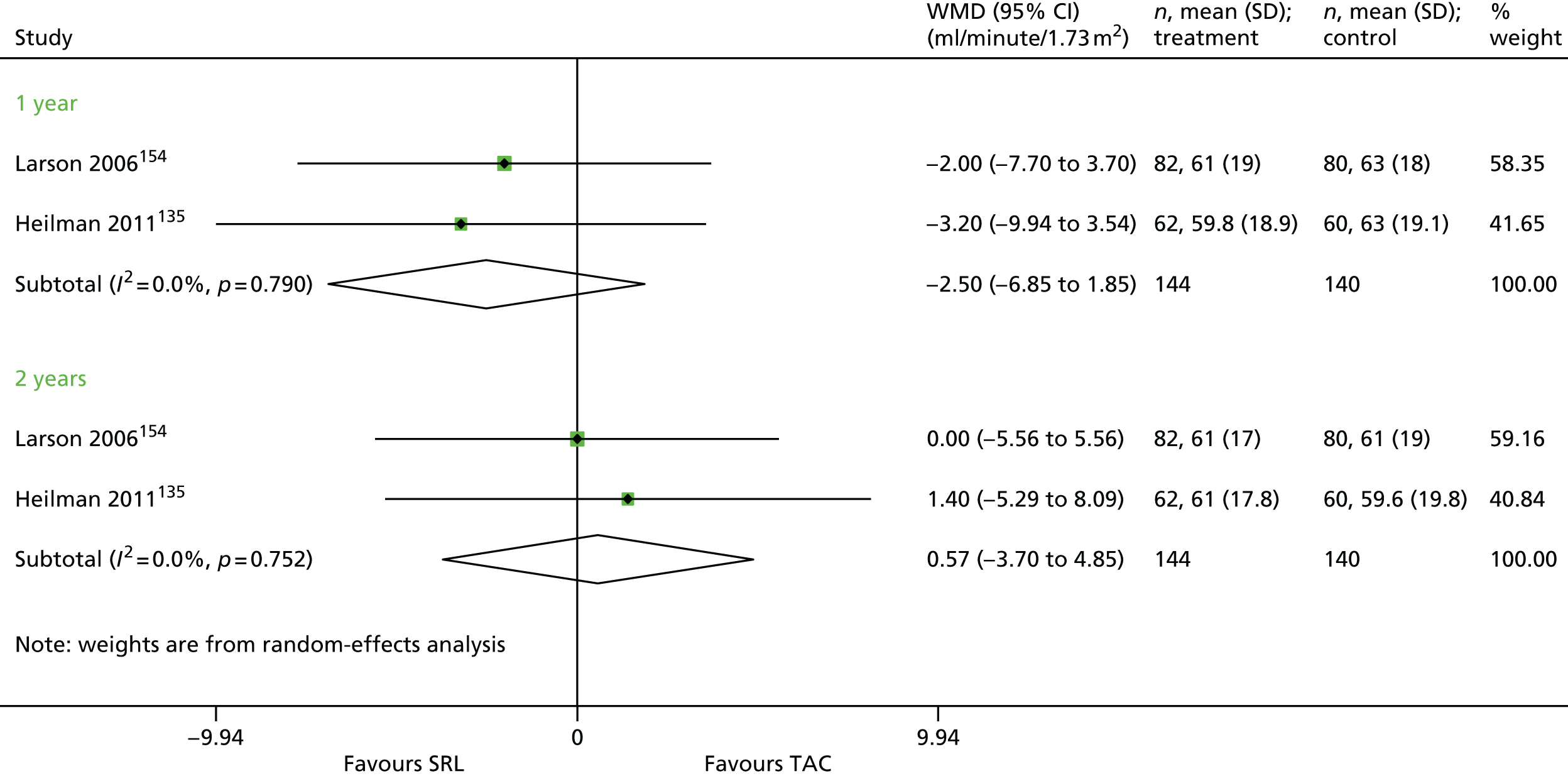
Biopsy-proven acute rejection
Biopsy-proven acute rejection is reported by three studies92,93,135 (Table 97 and Figure 52). Pooled results indicate that there are lower odds of BPAR associated with TAC at 1 year (OR 0.32, 95% CI 0.12 to 0.87). There does not appear to be any evidence of heterogeneity across studies (I2 = 0.0%).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Schaefer 2006,92 Heilman 2011,135 Smith 200893 | 1 | 3 | 0.32 | 0.12 to 0.87 | 0.0 | 0.0 |
FIGURE 52.
Forest plot: BPAR for TAC + MMF vs. SRL + MMF

Severity of biopsy-proven acute rejection
Only one study93 reports on severity of BPAR (Table 98). For Banff classification II, there is no difference at 1 year between TAC + MMF and SRL + MMF; however, the sample size is very small.
| Study | Time point (years) | Banff classification | TAC + MMF, n/BPAR | SRL + MMF, n/BPAR |
|---|---|---|---|---|
| Smith 200893 | 1 | I | 2/3 | 1/3 |
| II | 1/1 | 0/1 |
Summary of results for TAC + MMF vs. SRL + MMF
-
Four studies92,93,135,154 are pooled with 1-year results for mortality; however, two of these studies had no deaths for either TAC + MMF or SRL + MMF. Furthermore, analysis suggests no significant difference between TAC + MMF and SRL + MMF (OR 0.80, 95% CI 0.13 to 4.99) Heilman et al. 135 also present results at 2 years of mortality for TAC + MMF vs. SRL + MMF. Again, results are not statistically significant (OR 2.10, 95% 0.19 to 23.83).
-
Four studies92,93,135,154 are pooled with 1-year results for graft loss. Again, two of these studies93,135 had no graft loss in either arm. Although the OR implies reduced graft loss associated with TAC, this is not statistically significant (OR 0.68, 95% CI 0.18 to 2.58).
-
Two studies135,154 report GRF at 1 year and 2 years. The pooled ORs for both time points indicate no statistically significant difference between TAC + MMF and SRL + MMF (1 year, WMD –2.50 ml/minute/1.73 m2, 95% CI –6.85 to 1.85 ml/minute/1.73 m2).
-
BPAR is reported by three studies. 92,93,135 Pooled results indicate that there are lower odds of BPAR associated with TAC at 1 year (OR 0.32, 95% CI 0.12 to 0.87). There does not appear to be any evidence of heterogeneity across studies (I2 = 0.0%). Only one study93 reports on severity of BPAR. Banff classification I and II demonstrate no difference at 1 year between TAC + MMF and SRL + MMF; however, the sample size is very small.
TAC + MPS vs. SRL + MPS
The study by Silva et al. 119 is the only one to report on this combination; therefore, a summary of outcomes at 2 years is presented in Table 99. The OR for BPAR appears to favour TAC (OR 0.63, 95% CI 0.3482 to 1.1397); however, this is not statistically significant. All other outcomes also show no statistically significant difference between arms.
| Study | Time point (years) | Outcome | TAC + MPS | SRL + MPS | OR | 95% CI |
|---|---|---|---|---|---|---|
| Silva 2013119 | 2 | Patient survival, n/N (%) | 3/107 (97) | 3/97 (97) | 0.9038 | 0.17 to 4.59 |
| Graft survival, n/N (%) | 1/107 (99) | 1/97 (99) | 0.9057 | 0.06 to 14.68 | ||
| BPAR, n/N (%) | 29/107 (27) | 36/97 (37) | 0.63 | 0.35 to 1.14 | ||
| Banff classification none/borderline, n/N (%) | 5/107 (5) | 8/97 (8) | 0.5576 | 0.18 to 1.77 | ||
| Banff classification I, n/N (%) | 16/107 (15) | 17/97(17) | 0.8274 | 0.39 to 1.74 | ||
| Banff classification II, n/N (%) | NR | NR | ||||
| Banff classification III, n/N (%) | NR | NR |
TAC + SRL vs. MMF + SRL
Hamdy et al. 120 is the only study to report on this combination; therefore, a summary of outcomes at 1–5 years is presented in Table 100. The OR for mortality at 3 years appears to favour MMF (OR 4.39, 95% CI 0.48 to 40.39); however, this is not statistically significant. All other outcomes also show no statistical difference between arms.
| Study | Time point (years) | Outcome | TAC + SRL | MMF + SRL | OR | 95% CI |
|---|---|---|---|---|---|---|
| Hamdy 2005120 | 1 | Mortality, n/N (%) | 2/65 (1.5) | 0/67 (0) | NA | NA |
| BPAR, n/N (%) | 12/65 (18) | 9/67 (13) | 1.4591 | 0.57 to 3.74 | ||
| GRF, mean (ml/minute/1.73 m2) (SD) | 89 (30) | 93 (25.2) | p = 0.4078 | |||
| 2 | Mortality, n/N (%) | 2/65 (1.5) | 0/67 (0) | NA | NA | |
| GRF, mean (ml/minute/1.73 m2) (SD) | 79.6 (25.5) | 94.9 (28.9) | p = 0.0016 | |||
| 3 | Mortality, n/N (%) | 4/65 (6.1) | 1/67 (1.5) | 4.3934 | 0.48 to 40.39 | |
| BPAR, n/N (%) | 12/65 (18) | 9/67 (13) | 1.4591 | 0.57 to 3.74 | ||
| GRF, mean (ml/minute/1.73 m2) (SD) | 76.1a | 88a | NA | |||
| 5 | Graft loss, n/N (%) | 7/65 (11) | 7/67 (11) | 1.0345 | 0.34 to 3.13 |
SRL + AZA vs. CSA + AZA
One trial148 reported investigating SRL + AZA vs. CSA + AZA, and a summary of outcomes at 0.5 years and 1 year is presented (Table 101). There is a statistically significant difference between both arms at 0.5 years and 1 year in favour of SRL + AZA (p < 0.0001) for GRF. There is no statistically significant difference between arms for other outcomes.
| Study | Time point (years) | Outcome | SRL + AZA (%) | CSA + AZA (%) | OR | 95% CI (p-value) |
|---|---|---|---|---|---|---|
| Charpentier 2003148 | 0.5 | BPAR, n/N (%) | 17/41 (41) | 16/42 (38) | 1.151 | 0.4776 to 2.7742 |
| GRF, mean (ml/minute/1.73 m2) (SD) | 67 (4) | 59 (3) | (p < 0.0001) | |||
| Banff classification I, n/N (%) | 6/41 (15) | 9/42 (21) | 0.6286 | 0.2016 to 1.9599 | ||
| Banff classification II, n/N (%) | 9/41 (22) | 6/42 (14) | 1.6875 | 0.5411 to 5.2631 | ||
| Banff classification III, n/N (%) | 2/41 (5) | 1/42 (2) | 2.1026 | 0.1832 to 4.1267 | ||
| 1 | Patient survival, n/N (%) | 41/41 (100) | 41/42 (98) | NA | NA | |
| Graft survival, n/N (%) | 40/41 (98) | 39/42 (93) | 0.325 | 0.0324 to 3.2603 | ||
| GRF, mean (ml/minute/1.73 m2) (SD) | 69.5 (4.1) | 58.7 (3.6) | (p < 0.0001) |
TAC + SRL vs. CSA + SRL
Two studies121,122 reported this combination, presenting outcomes at 1 year and 5 years. No severity or time to AR reported.
Mortality
At both 1 year and 5 years there is no statistically significant difference between TAC + SRL and CSA + SRL for mortality (Table 102). 121,122 Notably, for Anil Kumar et al. 122 there are no deaths in either arm at 1 year.
Graft loss
Two studies121,122 report graft loss, with pooled result at 1 year and individual results up to 5 years (Table 103 and Figure 53). Results are consistent across all time points for lower odds being associated with graft loss for TAC + SRL; however, the effect is not statistically significant (1 year, OR 0.68, 95% CI 0.16 to 2.90).
| Study | Time point (years) | Trials | OR | 95% CI | I2 (%) | τ2 |
|---|---|---|---|---|---|---|
| Anil Kumar 2008,122 Chen 2008121 | 1 | 2 | 0.68 | 0.16 to 2.90 | 0.0 | 0.0 |
| Anil Kumar 2008122 | 2 | 1 | 0.72 | 0.23 to 2.24 | NA | NA |
| 3 | 1 | 0.44 | 0.17 to 1.17 | NA | NA | |
| 4 | 1 | 0.49 | 0.20 to 1.20 | NA | NA | |
| 5 | 1 | 0.70 | 0.30 to 1.61 | NA | NA |
FIGURE 53.
Forest plot: graft loss for TAC + SRL vs. CSA + SRL.
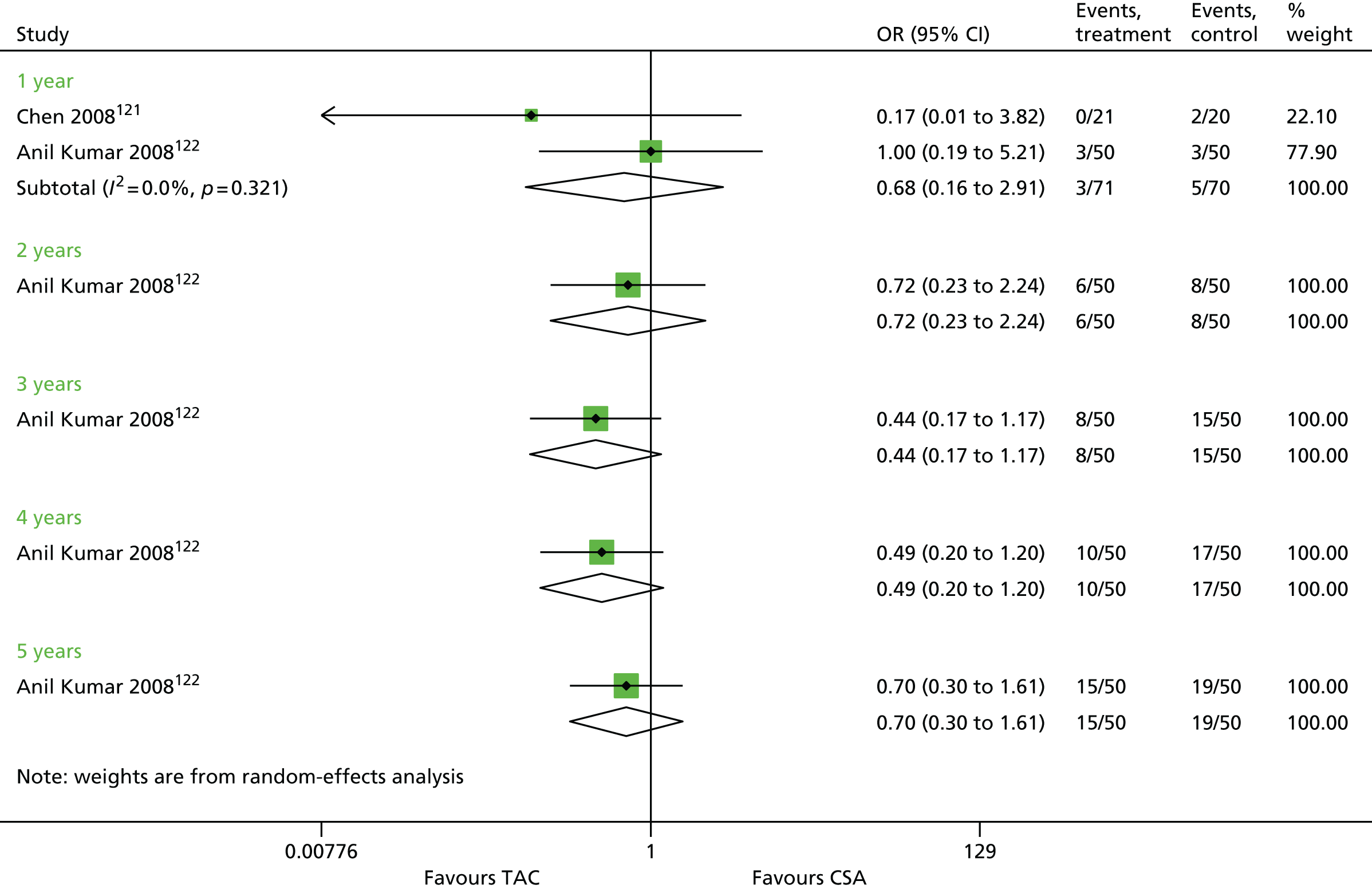
Graft function
Chen et al. 121 report GRF at 0.5 years and 1 year (Table 104), which appears to be statistically significantly greater for TAC + SRL at 0.5 years and 1 year (p < 0.0001 and p = 0.0004, respectively).
| Study | Time point (years) | TAC + SRL, ml/minute/1.73 m2 (SE) | CSA + SRL, ml/minute/1.73 m2 (SE) | MD | p-value |
|---|---|---|---|---|---|
| Chen 2008121 | 0.5 | 52.77 (3.86) | 46.42 (3.95) | 6.35 | < 0.0001 |
| 1 | 52.04 (4.38) | 46.79 (4.38) | 5.25 | 0.0004 |
Biopsy-proven acute rejection
This is reported only by Anil Kumar et al. 122 at 1 year (Table 105). The OR implies BPAR to be more likely for CSA + SRL; however, this is not statistically significant (OR 0.48, 95% CI 0.08 to 2.74).
| Study | Time point (years) | TAC + SRL, n/N (%) | CSA + SRL, n/N (%) | OR | 95% CI |
|---|---|---|---|---|---|
| Anil Kumar 2008122 | 1 | 2/50 (4) | 4/50 (8) | 0.48 | 0.08 to 2.74 |
Summary of results for TAC + SRL vs. CSA + SRL
-
Owing to the same number events in either arm at both time points, there is no difference between TAC + SRL and CSA + SRL for mortality. 121,122
-
Two studies121,122 report graft loss, with pooled result at 1 year and individual results up to 5 years. Results are consistent across all time points in showing that lower odds are associated with graft loss for TAC + SRL; however, the effect is not statistically significant (1 year, OR 0.68, 95% CI 0.16 to 2.90).
-
Chen et al. 121 report GRF at 0.5 years and 1 year, which appears to be statistically significantly greater for TAC + SRL at 0.5 years and 1 year (p < 0.0001 and p = 0.0004, respectively).
-
BPAR is reported only by Anil Kumar et al. 122 at 1 year. The OR implies BPAR to be more likely for CSA + SRL; however, this is not statistically significant (OR 0.48, 95% CI 0.08 to 2.74).
Induction therapy results
Network meta-analysis was performed for all induction studies reporting graft loss, mortality, BPAR and eGFR at 1-year follow-up. Figure 54 displays the network for included induction studies.
FIGURE 54.
Network diagram for all included induction studies. Note: circles denote number of studies.
Graft loss
Ten RCTs71–74,87,95–98,137 informing the effectiveness of three treatments (no induction/PBO, BAS and rATG) were included in the network for graft loss (Figure 55).
FIGURE 55.
Network diagram for induction studies reporting graft loss. Note: circles denote number of studies.

The DIC suggested little difference between the fit of the fixed- and random-effects models, with the fixed effects being the slightly better fit; thus, only the results of the fixed-effects model are shown in Table 106.
| Treatment comparison | Graft loss | Mortality | BPAR |
|---|---|---|---|
| BAS vs. PBO/no treatment | 0.82 (0.56 to 1.18) | 0.99 (0.53 to 1.85) | 0.52 (0.41 to 0.65) |
| rATG vs. PBO/no treatment | 0.77 (0.39 to 1.47) | 0.84 (0.33 to 2.07) | 0.36 (0.24 to 0.54) |
| rATG vs. BAS | 0.94 (0.50 to 1.75) | 0.84 (0.36 to 1.96) | 0.70 (0.47 to 1.03) |
From these analyses there is little evidence to suggest that BAS and rATG are more effective than no induction/PBO in reducing graft loss, as the 95% CrIs include an OR of ‘1’. Furthermore, there is little evidence to suggest that rATG is more effective than BAS. Of the three treatments analysed in this network, rATG was estimated as having a 57% probability of being the most effective treatment, with BAS having a 38% probability of being the most effective treatment. Analyses suggested that there was little evidence of inconsistency within this network.
Mortality
Ten RCTs71–74,87,95–98,137 informing the effectiveness of three treatments (no induction/PBO, BAS and rATG) were included in the network for mortality (Figure 56).
FIGURE 56.
Network diagram for induction studies reporting mortality. Note: circles denote number of studies.

The DIC suggested little difference between the fit of the fixed- and random-effects models, with the fixed effects being the slightly better fit, thus only the results of the fixed-effects model are shown in Table 106.
From these analyses there is little evidence to suggest that BAS and rATG are more effective than no induction/PBO in reducing mortality, as the 95% CrIs include an OR of ‘1’ (see Table 106), and there is little evidence to suggest that rATG is more effective than BAS. Of the three treatments analysed in this network, rATG was estimated as having a 54% probability of being the most effective treatment, with BAS having a 22% probability of being the most effective treatment. Analyses suggested that there was little evidence of inconsistency within this network.
Biopsy-proven acute rejection
Nine RCTs71–74,87,96–98,137 informing the effectiveness of three treatments (no induction/PBO, BAS and rATG) were included in the network for mortality (Figure 57).
FIGURE 57.
Network diagram for induction studies reporting BPAR. Note: circles denote number of studies.
The DIC suggested little difference between the fit of the fixed- and random-effects models, with the fixed effects being the slightly better fit, and so only the results of the fixed-effects model are shown in Table 106.
From these analyses, evidence suggests that BAS and rATG are more effective than no induction/PBO in reducing BPAR and that rATG is more effective than BAS. Of the three treatments analysed in this network, rATG was estimated as having a 96% probability of being the most effective treatment, with BAS having a 3% probability of being the most effective treatment. Analyses suggested that there was little evidence of inconsistency within this network.
Graft function
Five RCTs71–73,87,97 informing the effectiveness of three treatments (no induction/PBO, BAS and rATG) were included in the network for GRF (Figure 58).
FIGURE 58.
Network diagram for induction studies reporting GRF. Note: circles denote number of studies.
The DIC suggested very little difference between the fit of the fixed- and random-effects models. For comparison with the above outcomes, the results of the fixed-effects model are shown in Table 107.
| Treatment comparison | GRF (ml/minute/1.73 m2) |
|---|---|
| BAS vs. PBO/no treatment | 2.11 (–0.45 to 4.68) |
| rATG vs. PBO/no treatment | –3.95 (–11.80 to 3.94) |
| rATG vs. BAS | –6.06 (–13.46 to 1.37) |
There is no evidence to suggest that BAS or rATG is more effective than PBO/no induction, and no evidence to suggest that one treatment is more effective than the other. BAS has a 89% probability of being the most effective treatment, whereas rATG has a 5% probability of being the most effective treatment. Analyses suggested that there was little evidence of inconsistency within this network.
Maintenance therapy results
Network meta-analysis was performed for all maintenance studies reporting graft loss, mortality, BPAR and eGFR at 1-year follow-up. Figure 59 displays the network for included induction studies.
FIGURE 59.
Network diagram for all included maintenance studies reporting graft loss. Note: circles denote number of studies.
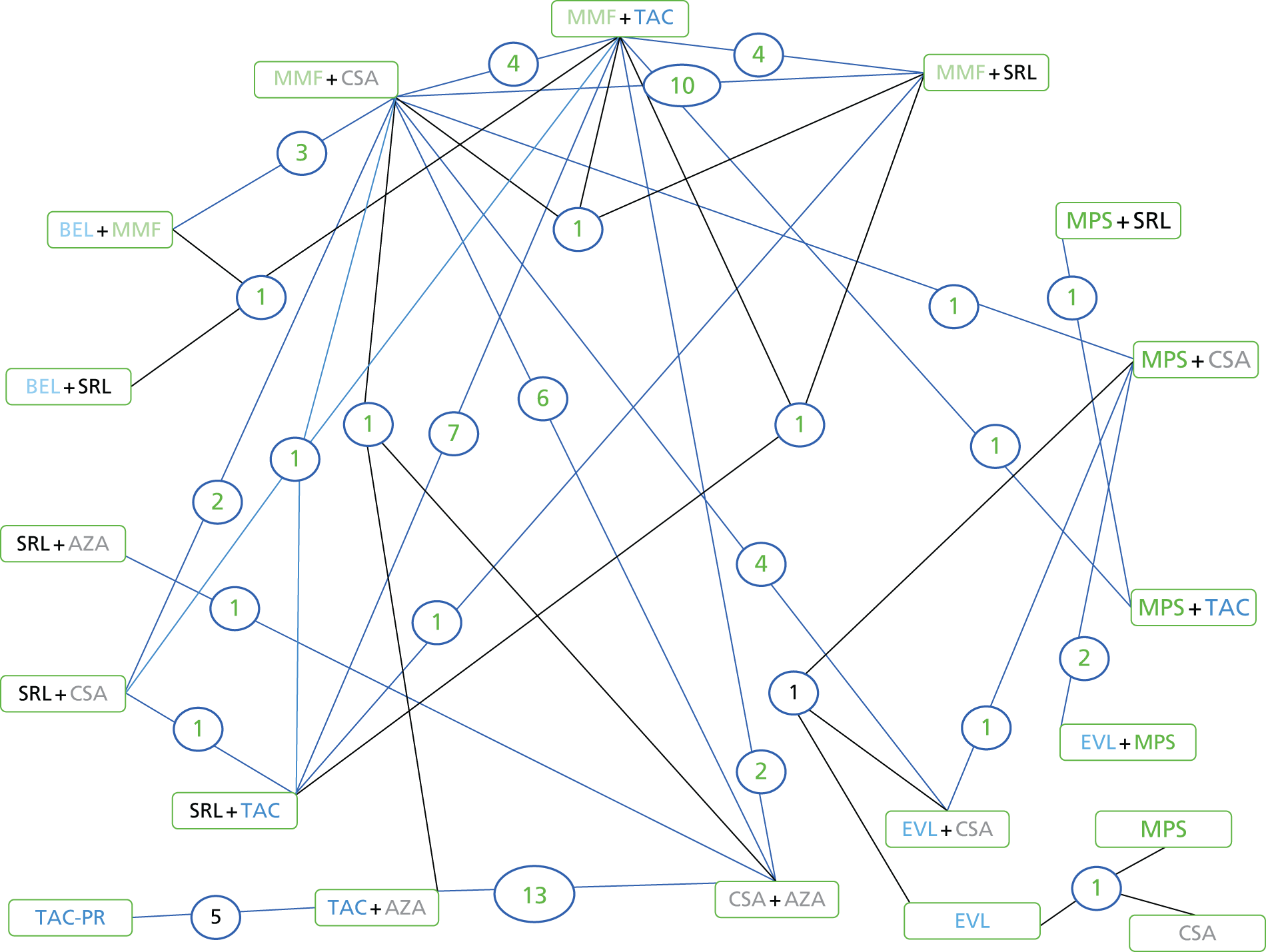
Data on 13 treatments from 49 studies51,59,76,80,82–84,86,88–90,92,93,100,102–104,107–112,115,117,118,121,122,125–127,129,131,133–136,138,142–145,147,149–152,155,210 were potentially includable in the NMA (Figure 60). However, 11 studies had zero events in all treatment arms, so would not contribute information to the NMA; therefore, they were excluded from the NMA. Owing to the exclusion of these studies, the treatment EVL + MPS could not be included in the network. Therefore, data from 40 studies51,59,76,80,82–84,86,88–90,92,100,103,104,107,108,110–112,117,118,122,125–127,129,134,136,138,142–145,147,149–152,155 (including five three-arm studies51,104,126,152,155 and one four-arm study122) on the effectiveness of 12 treatments to reduce graft loss informed the NMA. Thirteen82,90,100,104,112,126,127,138,144,145,147,149,152 of the 40 studies had at least one treatment arm with no graft loss events; therefore, 0.5 was added to each cell.
FIGURE 60.
Network diagram for maintenance studies reporting graft loss. Note: circles denote number of studies.

The DIC indicated that the random-effects model was a slightly better fit to the data than the fixed-effects model (154.4 vs. 157.5), and so results from only the random-effects models are presented here. The results of the fixed-effects models are given in Appendix 6. The probabilities that each treatment was the most effective in reducing graft loss compared with all other treatments are shown in Table 108.
| Treatment | Probability of being ‘best’ treatment (%) |
|---|---|
| EVL | 60 |
| SRL + AZA | 29 |
| SRL + CSA | 6 |
| BEL + SRL | 2 |
| BEL + MMF | 2 |
| EVL + CSA | 1 |
| CSA + AZA | < 1 |
| TAC + AZA | < 1 |
| MMF + CSA | < 1 |
| TAC + MMF | < 1 |
| SRL + TAC | < 1 |
| SRL + MMF | < 1 |
Although the results suggest that EVL has a 60% probability of being the most effective treatment for reducing graft loss compared with all other treatments (with SRL + AZA having a 29% probability), there is little evidence to suggest that treatment with EVL reduces graft loss compared with other treatments. The posterior median ORs for EVL compared with all of the other treatments are < 1, indicating a reduction in the odds of having a graft loss; however, the upper 95% CrIs limits are > 1, suggesting that EVL could increase the odds of a graft loss compared with all other treatments (Table 109). In fact, there is little evidence from the NMA to suggest that any treatment is more effective at reducing graft loss than any other treatment.
| Intervention treatment | Comparator treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSA + AZA | TAC + AZA | MMF + CSA | TAC + MMF | BEL + SRL | BEL + MMF | EVL + CSA | SRL + TAC | SRL + CSA | SRL + MMF | SRL + AZA | |
| TAC + AZA | 1.13 (0.67 to 2.15) | ||||||||||
| MMF + CSA | 0.76 (0.35 to 1.44) | 0.67 (0.24 to 1.50) | |||||||||
| TAC + MMF | 0.69 (0.28 to 1.55) | 0.61 (0.19 to 1.56) | 0.92 (0.48 to 1.77) | ||||||||
| BEL + SRL | 1.41 (0.14 to 13.14) | 1.24 (0.11 to 12.02) | 1.89 (0.20 to 16.49) | 2.05 (0.22 to 18.01) | |||||||
| BEL + MMF | 0.62 (0.20 to 1.78) | 0.55 (0.14 to 1.72) | 0.82 (0.35 to 1.97) | 0.89 (0.32 to 2.53) | 0.43 (0.05 to 3.94) | ||||||
| EVL + CSA | 0.63 (0.20 to 1.58) | 0.56 (0.14 to 1.58) | 0.84 (0.39 to 1.63) | 0.91 (0.33 to 2.27) | 0.44 (0.04 to 4.47) | 1.02 (0.31 to 2.95) | |||||
| SRL + TAC | 1.19 (0.38 to 3.35) | 1.05 (0.28 to 3.27) | 1.57 (0.64 to 3.93) | 1.71 (0.80 to 3.69) | 0.83 (0.08 to 8.57) | 1.92 (0.56 to 6.48) | 1.88 (0.62 to 6.32) | ||||
| SRL + CSA | 0.54 (0.10 to 2.56) | 0.48 (0.07 to 2.42) | 0.73 (0.15 to 3.10) | 0.79 (0.16 to 3.36) | 0.38 (0.03 to 5.31) | 0.88 (0.15 to 4.66) | 0.87 (0.16 to 4.54) | 0.46 (0.09 to 2.05) | |||
| SRL + MMF | 1.06 (0.38 to 2.43) | 0.94 (0.27 to 2.45) | 1.40 (0.72 to 2.58) | 1.52 (0.74 to 2.92) | 0.74 (0.08 to 7.09) | 1.71 (0.56 to 4.70) | 1.67 (0.66 to 4.40) | 0.89 (0.34 to 2.15) | 1.92 (0.41 to 9.74) | ||
| SRL + AZA | 0.25 (0.01 to 3.10) | 0.22 (0.01 to 2.86) | 0.33 (0.01 to 4.71) | 0.36 (0.01 to 5.39) | 0.17 (0.01 to 5.68) | 0.40 (0.01 to 6.53) | 0.40 (0.01 to 6.52) | 0.21 (0.01 to 3.45) | 0.46 (0.01 to 9.83) | 0.24 (0.01 to 3.68) | |
| EVL | 0.09 (0.01 to 2.15) | 0.08 (0.01 to 1.96) | 0.13 (0.01 to 2.67) | 0.14 (0.01 to 3.12) | 0.06 (0.01 to 3.00) | 0.15 (0.01 to 3.65) | 0.15 (0.01 to 3.34) | 0.08 (0.01 to 1.96) | 0.17 (0.01 to 5.60) | 0.09 (0.01 to 2.09) | 0.36 (0.01 to 41.00) |
There is no evidence to suggest that this network is affected by inconsistencies between the direct and indirect evidence (see Appendix 6). The DICs were very similar between the consistency and inconsistency models (154.4 vs. 153.7) and the 95% CrIs based on the direct evidence overlapped those based on the direct and indirect evidence.
Mortality
Data on 13 treatments from 52 studies51,59,76,78,80,83,84,86,88–90,92,93,100,102–104,107–112,115–118,120–122,125–127,129–131,133–136,138,142,145,147,149–151,155,210 were potentially includable in the NMA (Figure 61). However, 10 trials93,102,104,109,115,117,121,131,135,149 had zero events in all arms and were excluded from the NMA, resulting in 42 trials51,59,76,78,80,83,84,86,88–90,92,100,103,107,108,110–112,116,118,120,122,125–127,129,130,133,134,136,138,142–145,147,150–152,155,210 contributing to the NMA (including four three-arm trials51,104,122,152,155 and one four-arm trial122). Twelve59,76,78,90,92,100,120,126,127,136,145,152 of the 42 included trials had zero events in at least one treatment arm and so 0.5 was added to all cells in those trials.
FIGURE 61.
Network diagram for maintenance studies reporting mortality. Note: circles denote number of studies.

Although the DIC indicated that the fixed-effects model was a slightly better fit to the data than the random-effects model (137.7 vs. 139.5), the random-effects results are presented here and used in the economic model for consistency, as the remaining maintenance treatment analyses indicated the random-effects model to be the best-fitting model. The results of the fixed-effects models are given in Appendix 6. The probabilities that each treatment was the most effective in reducing graft loss compared with all other treatments are shown in Table 110.
| Treatment | Probability of being ‘best’ treatment (%) |
|---|---|
| SRL + AZA | 34 |
| EVL | 30 |
| BEL + SRL | 27 |
| EVL + MPS | 4 |
| BEL + MMF | 3 |
| SRL + CSA | 3 |
| CSA + AZA | < 1 |
| TAC + AZA | < 1 |
| MMF + CSA | < 1 |
| EVL + CSA | < 1 |
| SRL + TAC | < 1 |
| SRL + MMF | < 1 |
| TAC + MMF | 0 |
The regimens SRL + AZA (34%), EVL (30%) and BEL + SRL (27%) were estimated to have the greatest probabilities of being the most effective treatments to reduce mortality compared with all others, with the remaining treatments having a very low probability of being the best treatment. This reflects the findings presented below (see Table 120), which show that SRL + AZA, EVL and BEL + SRL are consistently estimated to have posterior median ORs of < 1 compared with all treatments, but, as the upper 95% CrI limits are > 1, there is the possibility that these treatments could increase mortality compared with other treatments.
The NMA suggests that BEL + MMF is more effective than TAC + MMF and SRL + MMF at reducing mortality. However, there is a great deal of uncertainty associated with many of the results presented (Table 111), especially for BEL + SRL.
| Intervention treatment | Comparator treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSA + AZA | TAC + AZA | MMF + CSA | TAC + MMF | BEL + SRL | BEL + MMF | EVL + MPS | EVL + CSA | SRL + TAC | SRL + CSA | SRL + MMF | SRL + AZA | |
| TAC + AZA | 1.38 (0.74 to 2.60) | |||||||||||
| MMF + CSA | 0.94 (0.45 to 1.95) | 0.68 (0.26 to 1.78) | ||||||||||
| TAC + MMF | 1.53 (0.63 to 3.71) | 1.10 (0.37 to 3.28) | 1.61 (0.89 to 3.00) | |||||||||
| BEL + SRL | 0.31 (0.01 to 8.78) | 0.22 (0.46 to 6.65) | 0.34 (0.01 to 8.57) | 0.21 (0.01 to 5.21) | ||||||||
| BEL + MMF | 0.47 (0.15 to 1.38) | 0.34 (0.09 to 1.18) | 0.50 (0.21 to 1.11) | 0.31 (0.11 to 0.83) | 1.49 (0.05 to 729.6) | |||||||
| EVL + MPS | 0.94 (0.08 to 10.78) | 0.68 (0.06 to 8.29) | 1.00 (0.09 to 10.09) | 0.62 (0.05 to 6.73) | 3.24 (0.05 2374) | 2.03 (0.16 to 24.24) | ||||||
| EVL + CSA | 1.40 (0.52 to 3.65) | 1.01 (0.32 to 3.20) | 1.47 (0.77 to 2.84) | 0.91 (0.37 to 2.21) | 4.47 (0.16 2219) | 2.98 (1.04 to 8.75) | 1.48 (0.13 to 17.37) | |||||
| SRL + TAC | 1.38 (0.49 to 3.88) | 1.00 (0.30 to 3.32) | 1.46 (0.65 to 3.23) | 0.91 (0.48 to 1.70) | 4.40 (0.16 2217) | 2.95 (0.96 to 9.45) | 1.46 (0.13 to 17.68) | 0.99 (0.36 to 2.76) | ||||
| SRL + CSA | 0.62 (0.14 to 2.70) | 0.45 (0.09 to 2.24) | 0.66 (0.17 to 2.37) | 0.41 (0.10 to 1.53) | 2.03 (0.06 1055) | 1.33 (0.27 to 6.22) | 0.66 (0.04 to 9.51) | 0.44 (0.10 to 1.88) | 0.45 (0.10 to 1.80) | |||
| SRL + MMF | 1.72 (0.68 to 4.31) | 1.24 (0.41 to 3.78) | 1.81 (0.98 to 3.42) | 1.13 (0.62 to 2.01) | 5.48 (0.21 2627) | 3.65 (1.35 to 10.62) | 1.81 (0.17 to 20.70) | 1.23 (0.50 to 3.05) | 1.24 (0.58 to 2.67) | 2.75 (0.70 to 11.71) | ||
| SRL + AZA | 0.19 (0.01 to 6.03) | 0.14 (0.01 to 4.51) | 0.20 (0.01 to 6.91) | 0.13 (0.01 to 4.39) | 0.66 (0.01 to 634.1) | 0.41 (0.01 to 15.87) | 0.19 (0.01 to 14.58) | 0.14 (0.01 to 4.89) | 0.14 (0.01 to 5.11) | 0.30 (0.01 to 13.73) | 0.11 (0.01 to 3.91) | |
| EVL | 0.25 (0.01 to 6.20) | 0.18 (0.01 to 4.84) | 0.27 (0.01 to 5.96) | 0.17 (0.01 to 3.92) | 0.81 (0.01 to 759.8) | 0.54 (0.01 to 13.82) | 0.25 (0.01 to 13.72) | 0.18 (0.01 to 4.11) | 0.18 (0.01 to 4.55) | 0.40 (0.01 to 12.89) | 0.15 (0.01 to 3.52) | 1.27 (0.01 to 11.84) |
There is no evidence to suggest that this network is affected by inconsistencies between the direct and indirect evidence (see Appendix 6). The DICs were slightly lower for the consistency model than for the inconsistency model (139.5 vs. 143.9) and the 95% CrIs that were based on the direct evidence overlapped those based on the direct and indirect evidence.
Biopsy-proven acute rejection
Thirteen treatments and 42 studies51,59,76,81–83,86,88–90,92,93,100,103,107,110,112,115–118,120,121,125–127,129,131,133–136,142,144,145,147,149,150,152,210 (including three three-arm studies51,126,152 and one four-arm study122) contribute to this NMA (Figure 62).
FIGURE 62.
Network diagram for maintenance studies reporting BPAR. Note: circles denote number of studies.
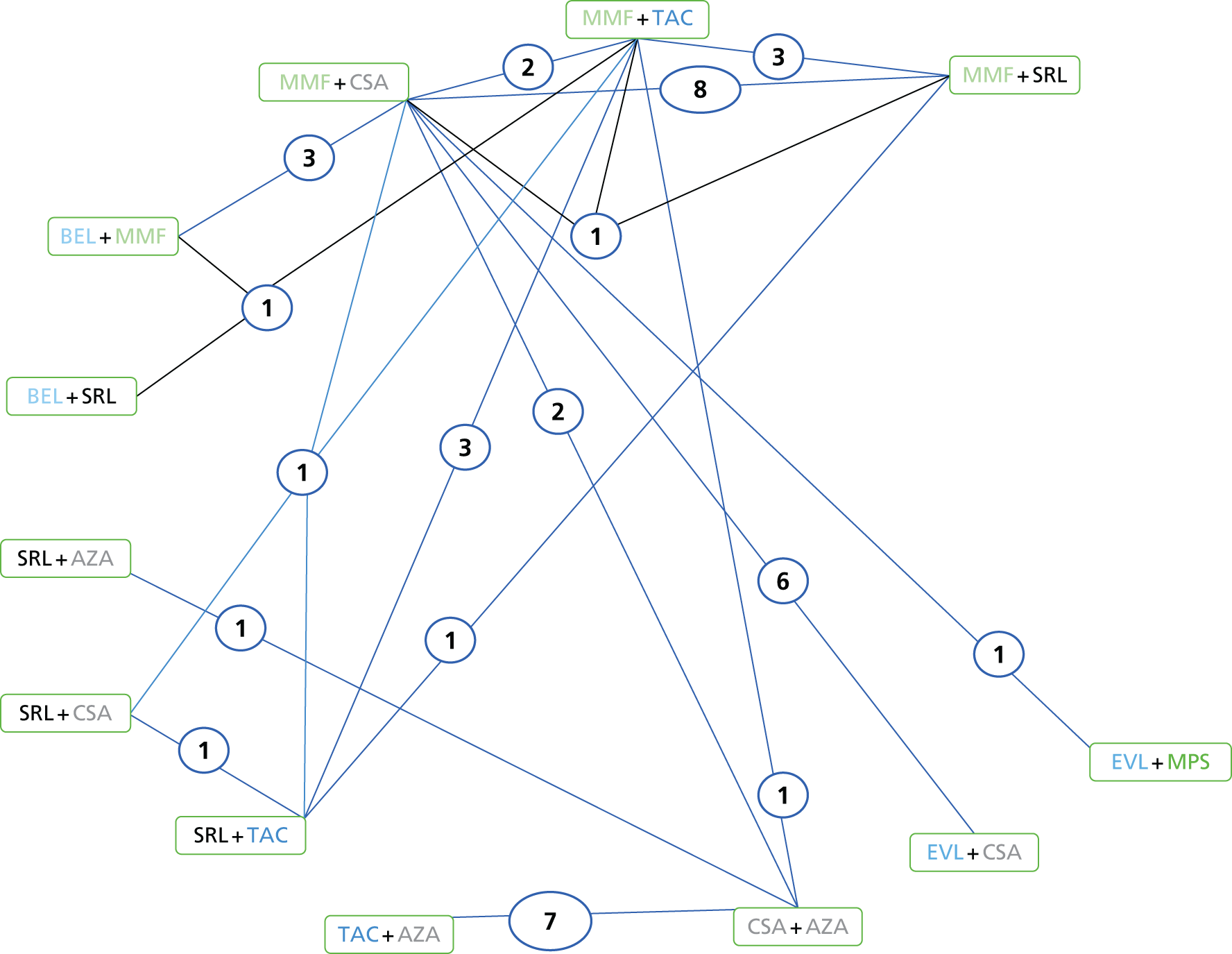
The DIC for the random-effects models was lower than that for the fixed-effects model (156.3 vs. 170.8) and so the random-effects model results are reported here (see Appendix 6 for fixed-effects results). The probabilities that each treatment was the most effective in reducing graft loss compared with all other treatments are shown in Table 112.
| Treatment | Probability of being ‘best’ treatment (%) |
|---|---|
| BEL + SRL | 58 |
| SRL + CSA | 27 |
| SRL + TAC | 5 |
| TAC + MMF | 2 |
| EVL + CSA | 2 |
| SRL + MMF | 2 |
| TAC + AZA | 1 |
| MMF + CSA | < 1 |
| BEL + MMF | < 1 |
| EVL + MPS | < 1 |
| SRL + AZA | < 1 |
| EVL | < 1 |
| CSA + AZA | 0 |
The regimen BEL + SRL has the highest probability (58%) of being the most effective treatment compared with all other treatments for reducing BPAR; however, there is no evidence that BEL + SRL is any more effective than the other treatments (Table 113). CSA + AZA has a 0% probability of being the best treatment and there is evidence to suggest that many treatments are more effective than CSA + AZA (see Table 112). The results from the NMA also indicate that MMF + CSA, TAC + MMF and SRL + TAC are all more effective than EVL + MPS at reducing BPAR. However, as with the other NMAs for maintenance therapy, there is a great deal of uncertainty associated with the estimated ORs. Therefore, apart from CSA + AZA and EVL + MPS performing poorly in some comparisons, it is difficult to say that any one treatment is more effective than another, as the 95% CrIs are so wide.
| Intervention treatment | Comparator treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSA + AZA | TAC + AZA | MMF + CSA | TAC + MMF | BEL + SRL | BEL + MMF | EVL + MPS | EVL + CSA | SRL + TAC | SRL + CSA | SRL + MMF | SRL + AZA | |
| TAC + AZA | 0.58 (0.36 to 0.93) | |||||||||||
| MMF + CSA | 0.47 (0.25 to 0.88) | 0.81 (0.37 to 1.80) | ||||||||||
| TAC + MMF | 0.40 (0.19 to 0.79) | 0.69 (0.29 to 1.60) | 0.85 (0.52 to 1.35) | |||||||||
| BEL + SRL | 0.17 (0.01 to 1.74) | 0.30 (0.01 to 3.18) | 0.37 (0.01 to 3.40) | 0.43 (0.01 to 4.08) | ||||||||
| BEL + MMF | 0.81 (0.34 to 1.94) | 1.39 (0.51 to 3.80) | 1.71 (0.91 to 3.20) | 2.02 (0.96 to 4.38) | 4.64 (0.52 to 150.5) | |||||||
| EVL + MPS | 1.48 (0.40 to 5.54) | 2.56 (0.65 to 10.40) | 3.14 (1.01 to 10.10) | 3.71 (1.10 to 13.26) | 8.77 (0.69 to 333.80) | 1.84 (0.50 to 6.96) | ||||||
| EVL + CSA | 0.46 (0.21 to 0.99) | 0.79 (0.32 to 1.97) | 0.97 (0.61 to 1.54) | 1.14 (0.60 to 2.26) | 2.64 (0.27 to 89.39) | 0.57 (0.26 to 1.24) | 0.31 (0.09 to 1.05) | |||||
| SRL + TAC | 0.38 (0.16 to 0.93) | 0.67 (0.24 to 1.82) | 0.82 (0.40 to 1.64) | 0.96 (0.51 to 1.80) | 2.24 (0.22 to 76.12) | 0.48 (0.19 to 1.20) | 0.26 (0.07 to 0.98) | 0.84 (0.36 to 1.94) | ||||
| SRL + CSA | 0.28 (0.06 to 1.08) | 0.48 (0.10 to 2.04) | 0.59 (0.15 to 2.03) | 0.70 (0.18 to 2.38) | 1.63 (0.12 to 62.23) | 0.34 (0.08 to 1.37) | 0.19 (0.03 to 1.01) | 0.61 (0.14 to 2.28) | 0.72 (0.18 to 2.52) | |||
| SRL + MMF | 0.43 (0.22 to 0.92) | 0.75 (0.32 to 1.85) | 0.92 (0.61 to 1.44) | 1.09 (0.67 to 1.89) | 2.53 (0.26 to 84.18) | 0.54 (0.26 to 1.17) | 0.29 (0.09 to 1.02) | 0.95 (0.52 to 1.84) | 1.13 (0.57 to 2.38) | 1.57 (0.45 to 6.39) | ||
| SRL + AZA | 1.16 (0.34 to 3.96) | 2.00 (0.53 to 7.50) | 2.45 (0.62 to 9.71) | 2.89 (0.71 to 12.11) | 6.88 (0.49 to 272.60) | 1.43 (0.32 to 6.48) | 0.78 (0.13 to 4.66) | 2.53 (0.59 to 10.83) | 3.00 (0.66 to 13.93) | 4.19 (0.67 to 28.50) | 2.66 (0.62 to 10.91) | |
| EVL | 1.26 (0.33 to 4.81) | 2.18 (0.53 to 9.08) | 2.67 (0.83 to 8.77) | 3.16 (0.90 to 11.48) | 7.47 (0.58 to 289.90) | 1.56 (0.41 to 6.02) | 0.85 (0.16 to 4.43) | 2.76 (0.84 to 9.21) | 3.28 (0.84 to 13.15) | 4.58 (0.83 to 27.72) | 2.91 (0.81 to 10.03) | 1.09 (0.18 to 6.71) |
There is no evidence to suggest that this network is affected by evidence inconsistencies (see Appendix 6). The DIC was slightly lower for the consistency model than for the inconsistency model (156.3 vs. 159.7) and the 95% CrIs that were based on the direct evidence overlapped those based on the direct and indirect evidence.
Graft function
Twelve treatments and 35 studies51,59,60,76,82,84,102–104,107,109,115,117,118,120,121,125,126,129–131,133–136,138,142,144,145,147,149,150,152,155 (including four three-arm studies51,104,126,155) contribute to this NMA (Figure 63).
FIGURE 63.
Network diagram for maintenance studies reporting BPAR. Note: circles denote number of studies.
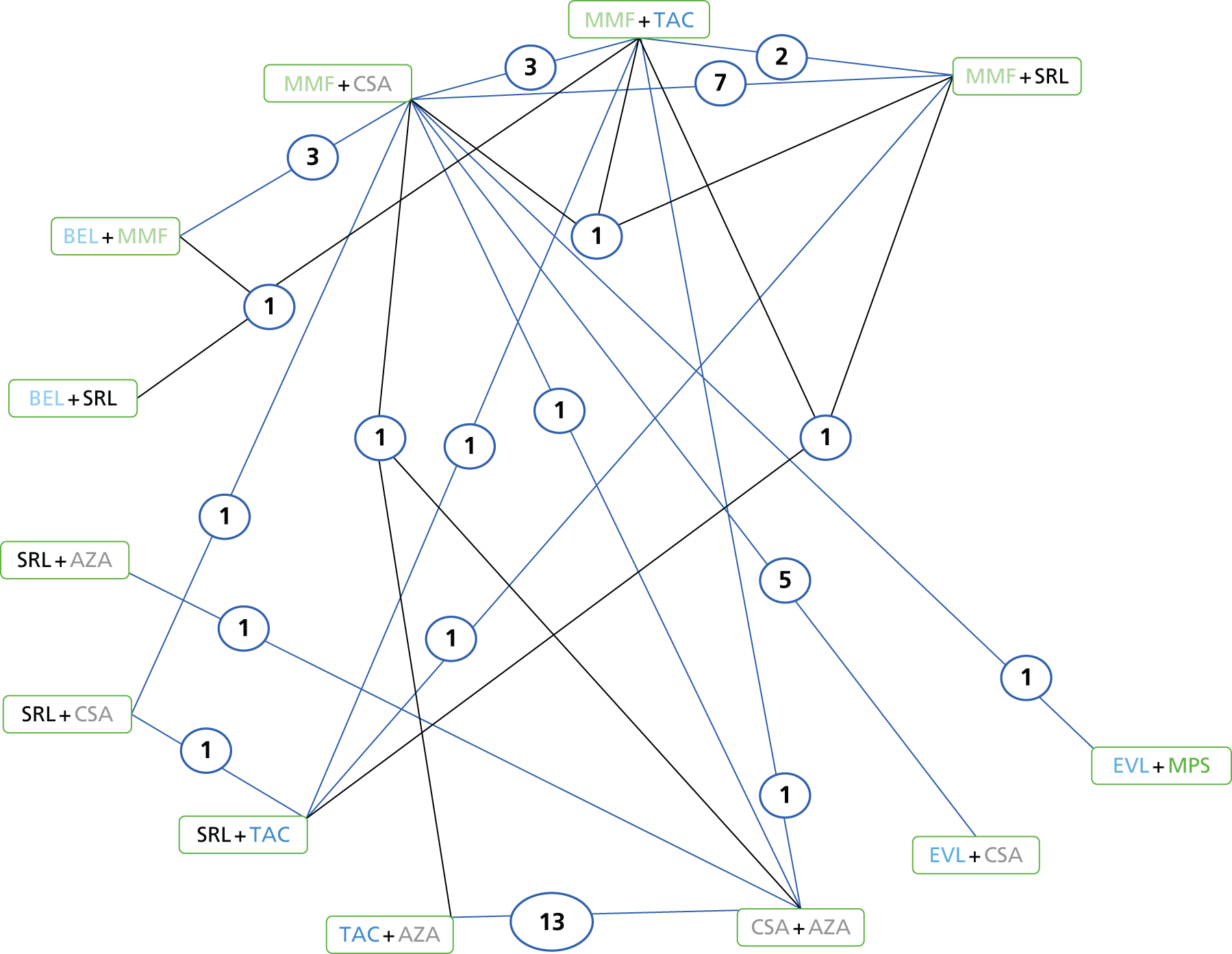
The DIC was lower for the random-effects model than for the fixed-effects model (147.8 vs. 323.7), suggesting a better fit to the data for the random-effects model. Therefore, the random-effects model results are reported (see Appendix 6 for fixed-effects model results). The treatment with the highest probability of being the most effective (Table 114) is BEL + SRL (44% probability), with SRL + AZA having a 28% probability. The results in Table 115 suggest that a number of treatments (TAC + AZA, TAC + MMF, BEL + MMF and SRL + AZA) are more effective than CSA + AZA, and also that TAC + AZA, TAC + MMF and BEL + MMF are more effective than SRL + TAC. However, because of the limited direct evidence informing many of the comparisons, the 95% CrIs are very wide for a number of comparisons, limiting conclusions to be made on the effectiveness of one treatment over another.
| Treatment | Probability of being ‘best’ treatment (%) |
|---|---|
| BEL + SRL | 44 |
| SRL + AZA | 28 |
| BEL + MMF | 17 |
| TAC + AZA | 9 |
| EVL + MPS | 1 |
| TAC + MMF | < 1 |
| EVL + CSA | < 1 |
| SRL + TAC | < 1 |
| SRL + CSA | < 1 |
| SRL + MMF | < 1 |
| CSA + AZA | 0 |
| MMF + CSA | 0 |
| Intervention treatment | Comparator treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSA + AZA | TAC + AZA | MMF + CSA | TAC + MMF | BEL + SRL | BEL + MMF | EVL + MPS | EVL + CSA | SRL + TAC | SRL + CSA | SRL + MMF | |
| TAC + AZA | 9.31 (4.32 to 14.28) | ||||||||||
| MMF + CSA | 1.61 (–4.16 to 7.41) | –7.70 (–14.53 to -0.86) | |||||||||
| TAC + MMF | 6.53 (0.38 to 12.68) | –2.78 (–10.08 to 4.54) | 4.92 (0.87 to 8.98) | ||||||||
| BEL + SRL | 12.33 (–3.97 to 28.60) | 3.01 (–13.75 to 19.72) | 10.71 (–4.81 to 26.20) | 5.79 (–9.53 to 21.06) | |||||||
| BEL + MMF | 10.54 (2.47 to 18.66) | 1.24 (–7.65 to 10.19) | 8.94 (3.13 to 14.79) | 4.02 (–2.72 to 10.73) | –1.76 (–17.52 to 13.94) | ||||||
| EVL + MPS | 0.33 (–12.22 to 12.96) | –8.98 (–22.07 to 4.18) | –1.27 (–12.45 to 9.93) | –6.19 (–18.06 to 5.70) | –12.01 (–31.12 to 7.20) | –10.21 (–22.81 to 2.44) | |||||
| EVL + CSA | 4.85 (–2.84 to 12.58) | –4.44 (–12.97 to 4.08) | 3.26 (–1.82 to 8.34) | –1.66 (–8.19 to 4.84) | –7.47 (–23.76 to 8.87) | –5.69 (–13.44 to 2.08) | 4.52 (–7.80 to 16.81) | ||||
| SRL + TAC | –0.34 (–8.53 to 7.85) | –9.66 (–18.68 to -0.59) | –1.96 (–8.35 to 4.43) | –6.88 (–13.01 to -0.75) | –12.67 (–29.08 to 3.69) | –10.90 (–19.40 to -2.43) | –0.68 (–13.59 to 12.15) | –5.22 (–13.35 to 2.94) | |||
| SRL + CSA | –1.63 (–11.13 to 7.96) | –10.93 (–21.14 to -0.63) | –3.23 (–11.07 to 4.64) | –8.16 (–16.34 to 0.09) | –13.95 (–31.08 to 3.24) | –12.18 (–21.86 to -2.43) | –1.95 (–15.66 to 11.69) | –6.49 (–15.83 to 2.85) | –1.26 (–8.97 to 6.45) | ||
| SRL + MMF | 3.84 (–2.72 to 10.43) | –5.47 (–13.02 to 2.12) | 2.24 (–1.55 to 6.05) | –2.69 (–6.92 to 1.57) | –8.47 (–24.16 to 7.24) | –6.71 (–13.52 to 0.12) | 3.50 (–8.29 to 15.31) | –1.02 (–7.35 to 5.33) | 4.20 (–2.02 to 10.41) | 5.47 (–2.72 to 13.67) | |
| SRL + AZA | 10.78 (1.07 to 20.44) | 1.47 (–9.41 to 12.35) | 9.17 (–2.13 to 20.47) | 4.24 (–7.23 to 15.73) | –1.52 (–20.45 to 17.46) | 0.24 (–12.40 to 12.84) | 10.43 (–5.48 to 26.36) | 5.93 (–6.47 to 18.29) | 11.12 (–1.55 to 23.81) | 12.41 (–1.20 to 25.99) | 6.93 (–4.77 to 18.61) |
For the random-effects model, there was little evidence of inconsistency within the network (see Appendix 6).
Summary for network meta-analysis
Induction therapy
-
There is no evidence to suggest that BAS or rATG is more effective than PBO/no induction, or each other, in reducing the odds of graft loss, mortality or CRC-GFR.
-
rATG and BAS are both estimated to be more effective than PBO/no induction at reducing BPAR, but the evidence does not suggest a difference between the two treatments.
-
Evidence suggests that although no treatment effect is seen for rATG, BAS is estimated to be more effective than PBO/no induction for increasing CRC-GFR.
Maintenance therapy
None of the maintenance regimens performed consistently well on all four outcomes. An overview of probability ranking on the four outcomes is presented (Table 116). However, because the analyses included 12 or 13 treatment regimens for each of the four outcomes, the results should be treated with great caution. 211 In addition, differences between treatments in probability of being best of < 90% cannot be given much credence. 211
| Treatment | Probability of being ‘best’ treatment (%) | |||
|---|---|---|---|---|
| Mortality | Graft loss | BPAR | GFR | |
| SRL + AZA | 34 | 29 | < 1 | 28 |
| EVL | 30 | 60 | < 1 | < 1 |
| BEL + SRL | 27 | 2 | 58 | 44 |
| EVL + MPS | 4 | NA | < 1 | NA |
| BEL + MMF | 3 | 2 | < 1 | 17 |
| SRL + CSA | 3 | 6 | 27 | < 1 |
| TAC + MMF | 0 | < 1 | 2 | < 1 |
| MMF + CSA | < 1 | < 1 | < 1 | 0 |
| SRL + TAC | < 1 | < 1 | 5 | < 1 |
| SRL + MMF | < 1 | < 1 | 2 | < 1 |
| EVL + CSA | < 1 | 1 | 2 | 1 |
| TAC + AZA | < 1 | < 1 | 1 | 9 |
| CSA + AZA | < 1 | < 1 | 0 | 0 |
In all NMAs for maintenance therapy there is a great deal of heterogeneity:
-
There is no evidence to suggest that one treatment is any more effective at reducing the odds of graft loss than any other treatment.
-
There is evidence to suggest that BEL + MMF is more effective at reducing the odds of mortality than TAC + MMF and SRL + MMF, but no other treatments are estimated to be any more effective at reducing mortality than any other treatment.
-
MMF + CSA, TAC + MMF and SRL + TAC are estimated to be more effective than CSA + AZA and EVL + MPS at reducing the odds of BPAR. In addition, TAC + AZA and EVL + CSA are also estimated to be more effective than CSA + AZA at reducing the odds of BPAR. However, apart from CSA + AZA and EVL + MPS performing poorly in some comparisons, it is difficult to say that any one treatment is more effective than another as the 95% CrIs are very wide.
-
Similarly, a number of treatments, TAC + AZA, TAC + MMF and BEL + MMF, are estimated to be more effective than CSA + AZA and MMF + CSA at increasing GRF. In addition, SRL + AZA is estimated to be more effective than CSA + AZA at increasing GRF. However, because of a lack of direct evidence, the 95% CrIs are wide for a number of comparisons. As a result, conclusions on the effectiveness of one treatment over another are limited.
Adverse events
Adverse events for each study are presented below. We conducted numerous comparisons and meta-analyses of the adverse effects of treatment reported in included RCTs at 1 year, as other time points had insufficient data for pooling. All of the meta-analyses (and associated forest plots) can be found in Appendix 7, rather than the main body of the report; however, the results are summarised as follows:
-
Some evidence suggested more CMV infections in rATG regimens compared with BAS regimens,212 and in rATG regimens than with no induction (study by Charpentier96). 128
-
The meta-analysis comparing TAC and CSA regimens (including eight studies51,80,83,88,90,100,121,210) suggested more cases of new-onset diabetes after transplant/transplantation (NODAT) in TAC regimens than in CSA regimens.
-
The meta-analyses comparing BEL with CSA regimens (including three studies59,125,142) suggested more cases of NODAT in CSA regimens than in BEL regimens.
-
The meta-analyses comparing SRL and CSA regimens (including seven studies116,117,134,147,149,194,195) suggested more cases of NODAT in CSA regimens than in SRL regimens.
-
The meta-analysis comparing MMF and EVL (including three studies107,131,177) suggested more cases of CMV infections in MMF regimens than in EVL regimens.
Induction therapy
All 13 induction studies71–74,87,95–98,123,128,137,148 reported some AE data. The time of follow-up varied from 6 months to 7 years in the individual studies (Table 117). Most studies reported a 1-year follow-up, although the AEs reported varied across the studies. The following AEs are summarised below: NODAT, PTLD, malignancy (including PTLD), any infections and CMV.
| Number | Study | n | Maintenance used | Time point |
|---|---|---|---|---|
| BAS vs. PBO (five studies) | ||||
| 1 | Bingyi 200395 | 12 | CSA + AZA + CCSs | 1 year |
| 2 | Kahan 199972 | 346 | CSA + CCSs | 1 year |
| 3 | Lawen 200374 | 123 | CSA + MMF + CCSs | 6 months |
| 4 | Nashan 199771 | 380 | CSA + CCSs | 1 year |
| 5 | Ponticelli 200173 | 340 | CSA + AZA + CCSs | 6 months |
| BAS vs. no induction (three studies) | ||||
| 6 | Albano 2013123 | 1251 | CSA + MMF + CCSs | 6 months |
| 7 | Sheashaa 200397 | 100 | CSA + AZA + CCSs | 3, 5 and 7 years |
| 8 | Kyllönen 2007128 | 155 | CSA + AZA + CCSs | 1 year |
| rATG vs. no induction (two studies) | ||||
| 9 | Charpentier 200196 | 309 | TAC + AZA + CCSs + CSA + MMF | 1 year |
| 10 | Charpentier 2003148 | 555 | TAC + AZA + CCSs | 6 months |
| BAS vs. rATG (three studies) | ||||
| 11 | Brennan 2006137 | 278 | CSA + MMF + CCSs | 1 year |
| 12 | Lebranchu 200287 | 100 | CSA + MMF + CCSs | 6 months, 1 year |
| 13 | Mourad 200498 | 105 | CSA + MMF + CCSs | 1 year |
New-onset diabetes mellitus
Seven studies87,95–97,123,128,148 reported NODAT events and their frequencies are shown in Table 118. The studies that reported NODAT events showed frequencies ranging from 0 to 5 out of 58 (9%). None of the comparisons suggests a statistically significant difference.
| Number | Study | 6 months | 1 year | 3 years | 5 years | 7 years |
|---|---|---|---|---|---|---|
| BAS vs. PBO (five studies) | ||||||
| 1 | Bingyi 200395 | NR | 0/6 vs. 0/6 | NR | NR | NR |
| 2 | Kahan 199972 | NR | NR | NR | NR | NR |
| 3 | Lawen 200374 | NR | NR | NR | NR | NR |
| 4 | Nashan 199771 | NR | NR | NR | NR | NR |
| 5 | Ponticelli 200173 | NR | NR | NR | NR | NR |
| BAS vs. no induction (three studies) | ||||||
| 6 | Albano 2013123 | 31/247 vs. 35/265 | NR | NR | NR | NR |
| 7 | Sheashaa 200397 | NR | NR | 4/50 vs. 7/50 | 4/50 vs. 7/50 | 4/50 vs. 7/50 |
| 8 | Kyllönen 2007128 | NR | 5/58 vs. 1/44 | NR | NR | NR |
| rATG vs. no induction (two studies) | ||||||
| 9 | Charpentier 200196 | NR | 5/145 vs. 7/154 | NR | NR | NR |
| 10 | Charpentier 2003148 | 13/177 vs. 7/173 | NR | NR | NR | NR |
| BAS vs. rATG (three studies) | ||||||
| 11 | Brennan 2006137 | NR | NR | NR | NR | NR |
| 12 | Lebranchu 200287 | NR | 1/51 vs. 1/50 | NR | NR | NR |
| 13 | Mourad 200498 | NR | NR | NR | NR | NR |
Malignancy and post-transplant lymphoproliferative disorder
Ten studies71–73,87,95,97,123,128,137,148 reported malignancy, including PTLD. The frequency of these events can be seen in Table 119. Frequencies ranged from 0 to 3/168 (2%). No statistically significant differences between treatments were noted.
| Number | Study | 6 months | 1 year | 3 years | 5 years | 7 years |
|---|---|---|---|---|---|---|
| BAS vs. PBO (five studies) | ||||||
| 1 | Bingyi 200395 | NR | 0/6 vs. 0/6 | NR | NR | NR |
| 2 | Kahan 199972 | NR | 2/173 vs. 6/173 | NR | NR | NR |
| 3 | Lawen 200374 | No malignancy data (0/59 vs. 0/64) | NR | NR | NR | NR |
| 4 | Nashan 199771 | NR | 3/190 vs. 2/186 | NR | NR | NR |
| 5 | Ponticelli 200173 | NR | 3/168 vs. 6/172a | NR | NR | NR |
| BAS vs. no induction (three studies) | ||||||
| 6 | Albano 2013123 | 3/283 vs. 2/302 | NR | NR | NR | NR |
| 7 | Sheashaa 200397 | NR | NR | 1/50 vs. 1/50 | 1/50 vs. 2/50 | 1/50 vs. 3/50 |
| 8 | Kyllönen 2007128 | NR | 0/58 vs. 1/44 | NR | NR | NR |
| rATG vs. no induction (two studies) | ||||||
| 9 | Charpentier 200196 | NR | NR | NR | NR | NR |
| 10 | Charpentier 2003148 | 4/186 vs. 1/185 | NR | NR | NR | NR |
| BAS vs. rATG (three studies) | ||||||
| 11 | Brennan 2006137 | NR | 1/137 vs. 5/141 | NR | NR | NR |
| 12 | Lebranchu 200287 | 0/51 vs. 0/50 | 0/51 vs. 0/50 | NR | NR | NR |
| 13 | Mourad 200498 | NR | NR | NR | NR | NR |
Infections
Ten studies reported71–74,95,97,98,123,137,148 infections related to the induction therapies (Table 120). Frequencies ranged from 0 to 129 out of 173 (75%). At 6 months and 1 year, a statistically significant difference in favour of BAS is indicated.
| Number | Study | 6 months | 1 year | 3 years | 5 years | 7 years |
|---|---|---|---|---|---|---|
| BAS vs. PBO (five studies) | ||||||
| 1 | Bingyi 200395 | NR | 0/6 vs. 0/6 | NR | NR | NR |
| 2 | Kahan 199972 | NR | 129/173 vs. 127/173 | NR | NR | NR |
| 3 | Lawen 200374 | 37/59 vs. 45/64 | NR | NR | NR | NR |
| 4 | Nashan 199771 | NR | 161/190 vs. 161/186 | NR | NR | NR |
| 5 | Ponticelli 200173 | 110/168 vs. 113/172 | NR | NR | NR | NR |
| BAS vs. no induction (three studies) | ||||||
| 6 | Albano 2013123 | 74/287 vs. 76/309 | NR | NR | NR | NR |
| 7 | Sheashaa 200397 | NR | NR | NRa | NRa | NRa |
| 8 | Kyllönen 2007128 | NR | NR | NR | NR | NR |
| rATG vs. no induction (two studies) | ||||||
| 9 | Charpentier 200196 | NR | NRa | NR | NR | NR |
| 10 | Charpentier 2003148 | 126/186 vs. 108/185 | NR | NR | NR | NR |
| BAS vs. rATG (three studies) | ||||||
| 11 | Brennan 2006137 | NR | 103/137 vs. 121/141b | NR | NR | NR |
| 12 | Lebranchu 200287 | 33/51 vs. 43/50b | NR | NR | NR | NR |
| 13 | Mourad 200498 | NR | 22/52 vs. 28/53 | NR | NR | NR |
Cytomegalovirus
Thirteen studies71–74,87,95–98,123,128,137,148 reported CMV events in induction therapies (Table 121). Frequencies ranged from 0 to 49 out of 151 (32%), with a statistically significant difference noted for BAS vs. rATG (three studies). For Lebranchu et al. 87 and Mourad et al. 98 a reduced occurrence of CMV is seen for the BAS arm, whereas for the study reported by Brennan et al. ,137 fewer occurrences are seen for rATG.
| Number | Study | 6 months | 1 year | 3 years | 5 years | 7 years |
|---|---|---|---|---|---|---|
| BAS vs. PBO (five studies) | ||||||
| 1 | Bingyi 200395 | NR | 0/6 vs. 0/6 | NR | NR | NR |
| 2 | Kahan 199972 | NR | 12/173 vs. 16/173 | NR | NR | NR |
| 3 | Lawen 200374 | 8/59 vs. 12/64 | NR | NR | NR | NR |
| 4 | Nashan 199771 | NR | 39/190 vs. 50/186 | NR | NR | NR |
| 5 | Ponticelli 200173 | 29/168 vs. 25/172 | NR | NR | NR | NR |
| BAS vs. no induction (three studies) | ||||||
| 6 | Albano 2013123 | 9/287 vs. 12/309 | NR | NR | NR | NR |
| 7 | Sheashaa 200397 | NR | NR | 3/50 vs. 3/50 | 3/50 vs. 4/50 | 4/50 vs. 4/50 |
| 8 | Kyllönen 2007128 | NR | 9/58 vs. 9/53 vs. 5/44 | NR | NR | NR |
| rATG vs. no induction (two studies) | ||||||
| 9 | Charpentier 200196 | NR | 49/151 vs. 30/158a | NR | NR | NR |
| 10 | Charpentier 2003148 | 45/186 vs. 29/185a | NR | NR | NR | NR |
| BAS vs. rATG (three studies) | ||||||
| 11 | Brennan 2006137 | NR | 24/137 vs. 11/141a | NR | NR | NR |
| 12 | Lebranchu 200287 | 6/51 vs. 19/50a | NR | NR | NR | NR |
| 13 | Mourad 200498 | NR | 11/52 vs. 22/53a | NR | NR | NR |
Maintenance therapy
Most of the 75 maintenance studies (Table 122) reported some AE data. The time of follow-up varied from 6 months to 10 years. Most studies reported 1-year follow-up, although the AE reported varied across the studies. The following AEs are summarised below: NODAT, PTLD, malignancy (including PTLD), any infections and CMV. All AEs are tabulated and narratively described in the sections below.
| Number | Study | AEs |
|---|---|---|
| TAC + AZA vs. CSA + AZA (13 studies) | ||
| 1 | Schleibner 199579 | NR |
| 2 | Laskow 199680 | 1 year |
| 3 | Mayer 199788 | 1 year, 4 years, 5 years |
| 4 | Radermacher 199881 | 1 year |
| 5 | Jarzembowski 200599 | 1 year |
| 6 | Baboolal 200282 | 1 year |
| 7 | Campos 200283 | 1 year |
| 8 | Margreiter 200284 | 6 months, 2 years, 3 years |
| 9 | Van Duijnhoven 200275 | NR |
| 10 | Waller 200276 | 1 year |
| 11 | Charpentier 2003148 | 6 months |
| 12 | Töz 200485 | NR |
| 13 | Hardinger 2005100 | 1 year |
| CSA + MMF low vs. CSA + AZA vs. CSA + MMF (two studies) | ||
| 14 | Sollinger 199577 | 6 months |
| 15 | Tricontinental MMF renal study 199689 | 6 months, 1 year, 3 years |
| CSA + MMF vs. CSA + AZA (four studies) | ||
| 16 | Sadek 200286 | 1 year |
| 17 | Tuncer 200278 | NR |
| 18 | Merville 2004138 | 1 year |
| 19 | Remuzzi 2007101 | 6 months, 5 years |
| TAC + MMF vs. CSA + AZA (two studies) | ||
| 20 | Wlodarczyk 2002171 | 6 months |
| 21 | Vacher-Coponat 2012129 | 1 year, 3 years |
| TAC + MMF vs. CSA + MMF (four studies) | ||
| 22 | Zadrazil 2012102 | NR |
| 23 | Hernández 2007130 | 2 years |
| 24 | Rowshani 2006103 | NR |
| 25 | Ulsh 1999153 | 1 year |
| TAC + AZA vs. CSA + AZA vs. CSA + MMF (one study) | ||
| 26 | Weimer 2005172 | 1 year |
| TAC + MMF vs. TAC-PR + MMF (four studies) | ||
| 27 | Wlodarczyk 2009140 | NR |
| 28 | Krämer 201058 | 1 year |
| 29 | Tsuchiya 2013141 | 1 year |
| 30 | Oh 2014105 | NR |
| TAC + MMF vs. TAC-PR 0.2 + MMF vs. TAC-PR 0.3 (one study) | ||
| 31 | Albano 2013123 | 6 months |
| MMF + TAC vs. MPS + TAC (one study) | ||
| 32 | Ciancio 2008106 | 1 year, 4 years |
| MMF + CSA vs. MPS + CSA (one study) | ||
| 33 | Salvadori 2004124 | 1 year |
| BEL low + MMF vs. BEL high + MMF vs. CSA + MMF (three studies) | ||
| 34 | Vincenti 2005125 | 1 year, 2 years, 3 years, 4 years, 5 years |
| 35 | BENEFIT60 | 1 year, 2 years, 3 years, 5 years |
| 36 | BENEFIT-EXT142 | 1 year, 2 years, 3 years, 5 years |
| BEL + MMF vs. BEL + SRL vs. TAC + MMF (one study) | ||
| 37 | Ferguson 2011126 | 1 year |
| EVL low + CSA vs. EVL high + CSA vs. MMF + CSA (three studies) | ||
| 38 | Lorber 2005143 | 3 years |
| 39 | ATLAS150 | 1 year, 3 years |
| 40 | Takahashi 2013131 | 1 year |
| EVL vs. EVL + CSA vs. CSA + MPS (one study) | ||
| 41 | Chadban 2013152 | 1 year |
| EVL low + CSA vs. EVL high + CSA vs. MPA + CSA (one study) | ||
| 42 | Tedesco-Silva 2010107 | 1 year |
| EVL + CSA vs. MPS + CSA (one study) | ||
| 43 | Bertoni 2011144 | 1 year |
| EVL + MPS vs. CSA + MPS (two studies) | ||
| 44 | Budde 2011132 | 1 year, 2 years, 3 years |
| 45 | Mjörnstedt 2012133 | 1 year |
| SRL + CSA vs. MMF + CSA (two studies) | ||
| 46 | Barsoum 2007108 | 2 years |
| 47 | Stallone 2004109 | NR |
| SRL + TAC vs. MMF + TAC (six studies) | ||
| 48 | Anil Kumar 2005110 | 1 year |
| 49 | Gonwa 2003180 | 6 months, 1 year |
| 50 | Sampaio 2008112 | 1 year |
| 51 | Gelens 2006113 | NR |
| 52 | Gallon 2006145 | 3 years, 8.5 years |
| 53 | Van Gurp 2010114 | 6 months |
| SRL + MMF vs. CSA + MMF (10 studies) | ||
| 54 | Flechner 2002127 | 1 year, 5 years |
| 55 | Noris 2007115 | 2 years |
| 56 | Lebranchu 2009149 | 1 year, 4 years |
| 57 | Büchler 2007134 | 1 year, 5 years |
| 58 | Soleimani 201391 | 5 years |
| 59 | Durrbach 2008146 | 6 months |
| 60 | Kreis 2000116 | 1 year |
| 61 | Guba 2010147 | 1 year |
| 62 | Martinez-Mier 2006117 | 1 year |
| 63 | Nafar 2012118 | NR |
| TAC + MMF vs. SRL + MMF (four studies) | ||
| 64 | Stegall 2003191 | NR |
| 65 | Schaefer 200692 | 1 year |
| 66 | Heilman 2011135 | 1 year |
| 67 | Smith 200893 | NR |
| TAC + MPS vs. SRL + MPS (one study) | ||
| 68 | Silva 2013119 | 2 years |
| TAC + SRL vs. MMF + SRL (one study) | ||
| 69 | Hamdy 2005120 | 1 year, 2 years, 5 years |
| SRL + AZA vs. CSA + AZA (one study) | ||
| 70 | Groth 1999194 | 1 year |
| TAC + SRL vs. CSA + SRL (one study) | ||
| 71 | Chen 2008121 | 1 year |
| SRL low + TAC vs. SRL high + TAC vs. MMF + TAC (one study) | ||
| 72 | Vítko 200694 | 6 months |
| SRL + TAC vs. SRL + MMF vs. MMF + TAC (one study) | ||
| 73 | Flechner 2011155 | 1 year, 2 years |
| MMF + CSA vs. MMF + low CSA vs. MMF + low TAC vs. MMF low SRL (one study) | ||
| 74 | Grinyo 200951 | 1 year, 3 years |
| TAC + MMF vs. TAC + SRL vs. CSA + MMF vs. CSA + SRL (one study) | ||
| 75 | Anil Kumar 2005110 | 5 years |
New-onset diabetes mellitus
Only one study148 out of 13 found statistically significant difference for TAC + AZA vs. CSA + AZA at the 6-month time point in favour of CSA (Table 123). Vincenti et al. 125 found CSA + MMF to have a statistically significant difference to BEL + MMF, but, again, only at 6 months. There is a statistically significant increase in NODAT for SRL high + TAC at 6 months when compared with SRL low + TAC and MMF + TAC. 94 Two other studies51,122 show an increase in NODAT: Grinyo et al. 51 for MMF + low TAC and Anil Kumar et al. 122 for TAC + MMF.
| Number | Study | 6 months | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 8.5 |
|---|---|---|---|---|---|---|---|---|
| TAC + AZA vs. CSA + AZA (13 studies) | ||||||||
| 1 | Schleibner 199579 | NR | NR | NR | NR | NR | NR | NR |
| 2 | Laskow 199680 | NR | 12/92 vs. 1/28a | NR | NR | NR | NR | NR |
| 3 | Mayer 199788 | NR | 17/303 vs. 3/145 | NR | NR | 17/303 vs. 3/145b | NR | NR |
| 4 | Radermacher 199881 | NR | NR | NR | NR | NR | NR | NR |
| 5 | Jarzembowski 200599 | NR | 3/14 vs. 4/21 | NR | NR | NR | NR | NR |
| 6 | Baboolal 200282 | NR | NR | NR | NR | NR | NR | NR |
| 7 | Campos 200283 | NR | 10/85 vs. 3/81 | NR | NR | NR | NR | NR |
| 8 | Margreiter 200284 | 13/286 vs. 5/271 | NR | 8/286 vs. 4/271 | NR | NR | NR | NR |
| 9 | Van Duijnhoven 200275 | NR | NR | NR | NR | NR | NR | NR |
| 10 | Waller 200276 | NR | NR | NR | NR | NR | NR | NR |
| 11 | Charpentier 2003148 | 13/177 vs. 2/177c | NR | NR | NR | NR | NR | NR |
| 12 | Töz 200485 | NR | NR | NR | NR | NR | NR | NR |
| 13 | Hardinger 2005100 | NR | 5/134 vs. 1/66 | NR | NR | NR | NR | NR |
| CSA + MMF low vs. CSA + AZA vs. CSA + MMF (two studies) | ||||||||
| 14 | Sollinger 199577 | NR | NR | NR | NR | NR | NR | NR |
| 15 | Tricontinental MMF renal study 199689 | NR | NR | NR | NR | NR | NR | NR |
| CSA + MMF vs. CSA + AZA (four studies) | ||||||||
| 16 | Sadek 200286 | NR | NR | NR | NR | NR | NR | NR |
| 17 | Tuncer 200278 | NR | NR | NR | NR | NR | NR | NR |
| 18 | Merville 2004138 | NR | NR | NR | NR | NR | NR | NR |
| 19 | Remuzzi 2007101 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. CSA + AZA (two studies) | ||||||||
| 20 | Wlodarczyk 2002171 | 27/243 vs. 27/246 | NR | NR | NR | NR | NR | NR |
| 21 | Vacher-Coponat 2012129 | NR | 8/128 vs. 11/137 | NR | 21/143 vs. 17/146 | NR | NR | NR |
| TAC + MMF vs. CSA + MMF (four studies) | ||||||||
| 22 | Zadrazil 2012102 | NR | NR | NR | NR | NR | NR | NR |
| 23 | Hernández 2007130 | NR | NR | 15/55 vs. 9/58 | NR | NR | NR | NR |
| 24 | Rowshani 2006103 | NR | NR | NR | NR | NR | NR | NR |
| 25 | Ulsh 1999153 | NR | 1/24 vs. 1/21 | NR | NR | NR | NR | NR |
| TAC + AZA vs. CSA + AZA vs. CSA + MMF (one study) | ||||||||
| 26 | Weimer 2005172 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. TAC-PR + MMF (four studies) | ||||||||
| 27 | Wlodarczyk 2009140 | NR | NR | NR | NR | NR | NR | NR |
| 28 | Krämer 201058 | NR | 17/298 vs. 18/284 | NR | NR | NR | NR | NR |
| 29 | Tsuchiya 2013141 | NR | NR | NR | NR | NR | NR | NR |
| 30 | Oh 2014105 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. TAC-PR 0.2 + MMF vs. TAC-PR 0.3 (one study) | ||||||||
| 31 | Albano 2013123 | 44/274 vs. 35/265 vs. 49/268 | NR | NR | NR | NR | NR | NR |
| MMF + TAC vs. MPS + TAC (one study) | ||||||||
| 32 | Ciancio 2008106 | NR | 7/61 vs. 6/55 | NR | NR | 13/61 vs. 8/55 | NR | NR |
| MMF + CSA vs. MPS + CSA (one study) | ||||||||
| 33 | Salvadori 2004124 | NR | NR | NR | NR | NR | NR | NR |
| BEL low + MMF vs. BEL high + MMF vs. CSA + MMF (three studies) | ||||||||
| 34 | Vincenti 2005125 | NR | 1/71 vs. 1/74 vs. 6/73c | 7/102 vs. 2/26 | 8/102 vs. 2/26 | 8/102 vs. 2/26 | 9/102 vs. 2/26 | NR |
| 35 | BENEFIT60 | NR | 7/226 vs. 11/219 vs. 16/221 | NR | NR | NR | NR | NR |
| 36 | BENEFIT-EXT142 | NR | 7/175 vs. 3/184 vs. 11/184 | NR | 18/175 vs. 9/184 vs. 17/184 | NR | NR | NR |
| BEL + MMF vs. BEL + SRL vs. TAC + MMF (one study) | ||||||||
| 37 | Ferguson 2011126 | NR | 0/33 vs. 2/26 vs. 1/30 | NR | NR | NR | NR | NR |
| EVL low + CSA vs. EVL high + CSA vs. MMF + CSA (three studies) | ||||||||
| 38 | Lorber 2005143 | NR | NR | NR | NR | NR | NR | NR |
| 39 | ATLAS150 | NR | NR | NR | 13/194 vs. 25/198 vs. 11/196 | NR | NR | NR |
| 40 | Takahashi 2013131 | NR | 7/61 vs. 3/61 | NR | NR | NR | NR | NR |
| EVL vs. EVL + CSA vs. CSA + MPS (one study) | ||||||||
| 41 | Chadban 2013152 | NR | 8/49 vs. 12/30 vs. 13/47 | NR | NR | NR | NR | NR |
| EVL low + CSA vs. EVL high + CSA vs. MPA + CSA (one study) | ||||||||
| 42 | Tedesco-Silva 2010107 | NR | 14/274 vs. 22/278 vs. 19/273 | NR | NR | NR | NR | NR |
| EVL + CSA vs. MPS + CSA (one study) | ||||||||
| 43 | Bertoni 2011144 | NR | NR | NR | NR | NR | NR | NR |
| EVL + MPS vs. CSA + MPS (two studies) | ||||||||
| 44 | Budde 2011132 | NR | 2/155 vs. 3/145 | NR | NR | NR | NR | NR |
| 45 | Mjörnstedt 2012133 | NR | NR | NR | NR | NR | NR | NR |
| SRL + CSA vs. MMF + CSA (two studies) | ||||||||
| 46 | Barsoum 2007108 | NR | NR | 3/76 vs. 3/37 | NR | NR | NR | NR |
| 47 | Stallone 2004109 | NR | NR | NR | NR | NR | NR | NR |
| SRL + TAC vs. MMF + TAC (six studies) | ||||||||
| 48 | Anil Kumar 2005110 | NR | 2/75 vs. 2/75 | NR | NR | NR | NR | NR |
| 49 | Gonwa 2003180 | 10/132 vs. 9/117 | 10/132 vs. 9/117d | NR | NR | NR | NR | NR |
| 50 | Sampaio 2008112 | NR | 12/50 vs. 6/50 | NR | NR | NR | NR | NR |
| 51 | Gelens 2006113 | NR | NR | NR | NR | NR | NR | NR |
| 52 | Gallon 2006145 | NR | NR | NR | 2/37 vs. 1/45 | NR | NR | 9/37 vs. 6/45 |
| 53 | Van Gurp 2010114 | 25/318 vs. 32/316 | NR | NR | NR | NR | NR | NR |
| SRL + MMF vs. CSA + MMF (10 studies) | ||||||||
| 54 | Flechner 2002127 | NR | NR | NR | NR | NR | 1/31 vs. 2/30 | NR |
| 55 | Noris 2007115 | NR | NR | 1/11 vs. 2/10 | NR | NR | NR | NR |
| 56 | Lebranchu 2009149 | NR | 3/96 vs. 2/97 | NR | NR | 7/96 vs. 2/97 | NR | NR |
| 57 | Büchler 2007134 | NR | 9/71 vs. 3/74 | NR | NR | NR | 2/63 vs. 4/68e | NR |
| 58 | Soleimani 201391 | NR | NR | NR | NR | NR | NR | NR |
| 59 | Durrbach 2008146 | NR | NR | NR | NR | NR | NR | NR |
| 60 | Kreis 2000116 | NR | 1/40 vs. 1/38 | NR | NR | NR | NR | NR |
| 61 | Guba 2010147 | NR | 5/69 vs. 4/71 | NR | NR | NR | NR | NR |
| 62 | Martinez-Mier 2006117 | NR | 1/20 vs. 1/21 | NR | NR | NR | NR | NR |
| 63 | Nafar 2012118 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. SRL + MMF (four studies) | ||||||||
| 64 | Stegall 2003191 | Mean follow-up 33 months (17–47 months) | ||||||
| 65 | Schaefer 200692 | NR | 5/39 vs. 6/41 | NR | NR | NR | NR | NR |
| 66 | Heilman 2011135 | NR | NR | NR | NR | NR | NR | NR |
| 67 | Smith 200893 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MPS vs. SRL + MPS (one study) | ||||||||
| 68 | Silva 2013107 | NR | NR | NR | NR | NR | NR | NR |
| TAC + SRL vs. MMF + SRL (one study) | ||||||||
| 69 | Hamdy 2005120 | NR | 18/65 vs. 13/65 | NR | NR | 19/65 vs. 15/67 | NR | NR |
| SRL + AZA vs. CSA + AZA (one study) | ||||||||
| 70 | Groth 1999194 | NR | 1/41 vs. 1/42 | NR | NR | NR | NR | NR |
| TAC + SRL vs. CSA + SRL (one study) | ||||||||
| 71 | Chen 2008121 | NR | 1/21 vs. 1/20 | NR | NR | NR | NR | NR |
| SRL low + TAC vs. SRL high + TAC vs. MMF + TAC (one study) | ||||||||
| 72 | Vítko 200694 | 20/296 vs. 44/290 vs. 28/295b | NR | NR | NR | NR | NR | NR |
| SRL + TAC vs. SRL + MMF vs. MMF + TAC (one study) | ||||||||
| 73 | Flechner 2011155 | NR | 27/120 vs. 7/117 vs. 12/110b | NR | NR | NR | NR | NR |
| MMF + CSA vs. MMF + low CSA vs. MMF + low TAC vs. MMF low SRL (one study) | ||||||||
| 74 | Grinyo 200951 | NR | 23/384 vs. 17/408 vs. 34/403 vs. 25/380 | NR | 19/233 vs. 12/248 vs. 30/249 vs. 18/228b | NR | NR | NR |
| TAC + MMF vs. TAC + SRL vs. CSA + MMF vs. CSA + SRL (one study) | ||||||||
| 75 | Anil Kumar 2008122 | NR | NR | NR | NR | NR | 12/50 vs. 8/50 vs. 0/50 vs. 8/50b | NR |
Malignancy and post-transplant lymphoproliferative disorder
For all combinations reporting malignancy and PTLD, no statistically significant difference was seen between arms (Table 124).
| Number | Study | 6 months | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 8.5 |
|---|---|---|---|---|---|---|---|---|
| TAC + AZA vs. CSA + AZA (13 studies) | ||||||||
| 1 | Schleibner 199579 | NR | NR | NR | NR | NR | NR | NR |
| 2 | Laskow 199680 | NR | NR | NR | NR | NR | NR | NR |
| 3 | Mayer 199788 | NR | 6/303 vs. 3/145 | NR | NR | NR | 21/303 vs. 11/145 | NR |
| 4 | Radermacher 199881 | NR | NR | NR | NR | NR | ||
| 5 | Jarzembowski 200599 | NR | (no cases reported) | NR | NR | NR | NR | NR |
| 6 | Baboolal 200282 | NR | NR | NR | NR | NR | NR | NR |
| 7 | Campos 200283 | NR | NR | NR | NR | NR | NR | NR |
| 8 | Margreiter 200284 | NR | NR | 3/237 vs. 1/222 | 7/231 vs. 5/217 | NR | NR | NR |
| 9 | Van Duijnhoven 200275 | NR | NR | NR | NR | NR | NR | NR |
| 10 | Waller 200276 | NR | NR | NR | NR | NR | NR | NR |
| 11 | Charpentier 2003148 | 2/185 vs. 4/184 | NR | NR | NR | NR | NR | NR |
| 12 | Töz 200485 | NR | NR | NR | NR | NR | NR | NR |
| 13 | Hardinger 2005100 | NR | 2/134 vs. 0/66 | NR | NR | NR | NR | NR |
| CSA + MMF low vs. CSA + AZA vs. CSA + MMF (two studies) | ||||||||
| 14 | Sollinger 199577 | 8/165 vs. 2/164 vs. 3/166 | NR | NR | NR | NR | NR | NR |
| 15 | Tricontinental MMF renal study 199689 | NR | 18/171 vs. 12/162 vs. 14/164 | NR | 25/171 vs. 29/162 vs. 19/164 | NR | NR | NR |
| CSA + MMF vs. CSA + AZA (four studies) | ||||||||
| 16 | Sadek 200286 | NR | NR | NR | NR | NR | NR | NR |
| 17 | Tuncer 200278 | NR | NR | NR | NR | NR | NR | NR |
| 18 | Merville 2004138 | NR | NR | NR | NR | NR | NR | NR |
| 19 | Remuzzi 2007101 | NR | NR | NR | NR | NR | 8/124 vs. 13/124 | NR |
| TAC + MMF vs. CSA + AZA (two studies) | ||||||||
| 20 | Wlodarczyk 2002171 | NR | NR | NR | NR | NR | NR | NR |
| 21 | Vacher-Coponat 2012129 | NR | 3/143 vs. 5/146 | NR | 3/143 vs. 6/146 | NR | NR | NR |
| TAC + MMF vs. CSA + MMF (four studies) | ||||||||
| 22 | Zadrazil 2012102 | NR | NR | NR | NR | NR | NR | NR |
| 23 | Hernández 2007130 | NR | NR | 2/80 vs. 2/80 | NR | NR | NR | NR |
| 24 | Rowshani 2006103 | NR | NR | NR | NR | NR | NR | NR |
| 25 | Ulsh 1999153 | NR | 0/24 vs. 1/21 | NR | NR | NR | NR | NR |
| TAC + AZA vs. CSA + AZA vs. CSA + MMF (one study) | ||||||||
| 26 | Weimer 2005172 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. TAC-PR + MMF (four studies) | ||||||||
| 27 | Wlodarczyk 2009140 | NR | NR | NR | NR | NR | NR | NR |
| 28 | Krämer 201058 | NR | 8/336 vs. 6/331 | NR | NR | NR | NR | NR |
| 29 | Tsuchiya 2013141 | NR | 0/50 vs. 1/50 | NR | NR | NR | NR | NR |
| 30 | Oh 2014105 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. TAC-PR 0.2 + MMF vs. TAC-PR 0.3 (one study) | ||||||||
| 31 | Albano 2013123 | 1/309 vs. 2/302 vs. 3/304 | NR | NR | NR | NR | NR | NR |
| MMF + TAC vs. MPS + TAC (one study) | ||||||||
| 32 | Ciancio 2008106 | NR | 0/61 vs. 0/55 | NR | NR | 2/61 vs. 1/55 | NR | NR |
| MMF + CSA vs. MPS + CSA (one study) | ||||||||
| 33 | Salvadori 2004124 | NR | 5/210 vs. 5/213 | NR | NR | NR | NR | NR |
| BEL low + MMF vs. BEL high + MMF vs. CSA + MMF (three studies) | ||||||||
| 34 | Vincenti 2005125 | NR | 0/71 vs. 2/74 vs. 2/73 | NR | NR | NR | 14/102 vs. 3/26 | NR |
| 35 | BENEFIT60 | NR | 4/226 vs. 5/219 vs. 1/221 | 9/226 vs. 18/219 vs. 11/221 | 10/226 vs. 18/219 vs. 12/221 | NR | 10/165 vs. 9/155 vs. 12/136 | NR |
| 36 | BENEFIT-EXT142 | NR | 4/175 vs. 4/184 vs. 6/184 | 14/175 vs. 17/184 vs. 15/185 | 15/175 vs. 16/184 vs. 19/184 | NR | 8/113 vs. 10/104 vs. 9/87 | NR |
| BEL + MMF vs. BEL + SRL vs. TAC + MMF (one study) | ||||||||
| 37 | Ferguson 2011126 | NR | 0/33 vs. 1/26 vs. 1/30 | NR | NR | NR | NR | NR |
| EVL low + CSA vs. EVL high + CSA vs. MMF + CSA (three studies) | ||||||||
| 38 | Lorber 2005143 | NR | NR | NR | 9/193 vs. 10/194 vs. 12/196 | NR | NR | NR |
| 39 | ATLAS150 | NR | NR | NR | 10/194 vs. 9/198 vs. 9/196 | NR | NR | NR |
| 40 | Takahashi 2013131 | NR | 2/61 vs. 0/61 | NR | NR | NR | NR | NR |
| EVL vs. EVL + CSA vs. CSA + MPS (one study) | ||||||||
| 41 | Chadban 2013152 | NR | 2/49 vs. 0/30 vs. 1/47 | NR | NR | NR | NR | NR |
| EVL low + CSA vs. EVL high + CSA vs. MPA + CSA (one study) | ||||||||
| 42 | Tedesco-Silva 2010107 | NR | NR | NR | NR | NR | NR | NR |
| EVL + CSA vs. MPS + CSA (one study) | ||||||||
| 43 | Bertoni 2011144 | NR | 0/56 vs. 2/50 | NR | NR | NR | NR | NR |
| EVL + MPS vs. CSA + MPS (two studies) | ||||||||
| 44 | Budde 2011132 | NR | NR | NR | 5/155 vs. 7/145 | NR | NR | NR |
| 45 | Mjörnstedt 2012133 | NR | 2/102 vs. 2/100 | NR | NR | NR | NR | NR |
| SRL + CSA vs. MMF + CSA (two studies) | ||||||||
| 46 | Barsoum 2007108 | NR | NR | 4/76 vs. 0/37 | NR | NR | NR | NR |
| 47 | Stallone 2004109 | NR | NR | NR | NR | NR | NR | NR |
| SRL + TAC vs. MMF + TAC (six studies) | ||||||||
| 48 | Anil Kumar 2005110 | NR | NR | NR | NR | NR | NR | NR |
| 49 | Gonwa 2003180 | 0/185 vs. 0/176 | 2/185 vs. 1/176 | NR | NR | NR | NR | NR |
| 50 | Sampaio 2008112 | NR | 0/50 vs. 0/50 | NR | NR | NR | NR | NR |
| 51 | Gelens 2006113 | NR | NR | NR | NR | NR | NR | NR |
| 52 | Gallon 2006145 | NR | NR | NR | NR | NR | NR | 2/37 vs. 0/45 |
| 53 | Van Gurp 2010114 | 2/318 vs. 2/316 | NR | NR | NR | NR | NR | NR |
| SRL + MMF vs. CSA + MMF (10 studies) | ||||||||
| 54 | Flechner 2002127 | NR | NR | NR | NR | NR | 3/31 vs. 6/30 | NR |
| 55 | Noris 2007115 | NR | NR | NR | NR | NR | NR | NR |
| 56 | Lebranchu 2009149 | NR | 2/96 vs. 0/97 | NR | NR | 6/96 vs. 9/97 | NR | NR |
| 57 | Büchler 2007134 | NR | 1/71 vs. 3/74 | NR | NR | NR | 4/63 vs. 9/68 | NR |
| 58 | Soleimani 201391 | NR | NR | NR | NR | NR | NR | NR |
| 59 | Durrbach 2008146 | 0/33 vs. 4/36 | NR | NR | NR | NR | NR | NR |
| 60 | Kreis 2000116 | NR | 0/40 vs. 0/38 | NR | NR | NR | NR | NR |
| 61 | Guba 2010147 | NR | 0/69 vs. 4/71 | NR | NR | NR | NR | NR |
| 62 | Martinez-Mier 2006117 | NR | NR | NR | NR | NR | NR | NR |
| 63 | Nafar 2012118 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. SRL + MMF (four studies) | ||||||||
| 64 | Stegall 2003191 | Mean follow-up 33 months (17–47 months) | ||||||
| 65 | Schaefer 200692 | NR | NR | NR | NR | NR | NR | NR |
| 66 | Heilman 2011135 | NR | NR | NR | NR | NR | NR | NR |
| 67 | Smith 200893 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MPS vs. SRL + MPS (one study) | ||||||||
| 68 | Silva 2013107 | NR | NR | 2/142 vs. 2/141 | NR | NR | NR | NR |
| TAC + SRL vs. MMF + SRL (one study) | ||||||||
| 69 | Hamdy 2005120 | NR | NR | 0/65 vs. 0/65 | NR | NR | 0/65 vs. 0/67 | NR |
| SRL + AZA vs. CSA + AZA (one study) | ||||||||
| 70 | Groth 1999194 | NR | 0/41 vs. 2/42 | NR | NR | NR | NR | NR |
| TAC + SRL vs. CSA + SRL (one study) | ||||||||
| 71 | Chen 2008121 | NR | NR | NR | NR | NR | NR | NR |
| SRL low + TAC vs. SRL high + TAC vs. MMF + TAC (one study) | ||||||||
| 72 | Vítko 200694 | 0/325 vs. 2/325 vs. 0/327 | NR | NR | NR | NR | NR | NR |
| SRL + TAC vs. SRL + MMF vs. MMF + TAC (one study) | ||||||||
| 73 | Flechner 2011155 | NR | NR | 7/152 vs. 5/152 vs. 5/139 | NR | NR | NR | NR |
| MMF + CSA vs. MMF + low CSA vs. MMF + low TAC vs. MMF low SRL (one study) | ||||||||
| 74 | Grinyo 200951 | NR | 5/384 vs. 4/408 vs. 8/403 vs. 9/380 | NR | 8/233 vs. 7/248 vs. 8/249 vs. 7/228 | NR | NR | NR |
| TAC + MMF vs. TAC + SRL vs. CSA + MMF vs. CSA + SRL (one study) | ||||||||
| 75 | Anil Kumar 2008122 | NR | NR | NR | NR | NR | 10/50 vs. 2/50 vs. 9/50 vs. 2/50 | NR |
Infections
Maintenance therapy studies that reported infection rates gave frequencies of 9 out of 237 (4%) to 85 out of 85 (100%; Table 125). Despite the relatively common occurrence of infections, only one study150 displayed a statistically significant difference between arms in favour of SRL low + TAC, as opposed to SRL high + TAC and MMF + TAC.
| Number | Study | 6 months | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 8.5 |
|---|---|---|---|---|---|---|---|---|
| TAC + AZA vs. CSA + AZA (13 studies) | ||||||||
| 1 | Schleibner 199579 | NR | NR | NR | NR | NR | NR | NR |
| 2 | Laskow 199680 | NR | NRa | NR | NR | NR | NR | NR |
| 3 | Mayer 199788 | NR | 229/303 vs. 109/145 | NR | NR | NR | NR | NR |
| 4 | Radermacher 199881 | NR | NRa | NR | NR | NR | NR | NR |
| 5 | Jarzembowski 200599 | NR | NR | NR | NR | NR | NR | NR |
| 6 | Baboolal 200282 | NR | NR | NR | NR | NR | NR | NR |
| 7 | Campos 200283 | NR | 85/85 vs. 81/81 | NR | NR | NR | NR | NR |
| 8 | Margreiter 200284 | NR | NR | 9/237 vs. 9/222b | 9/231 vs. 10/217b | NR | NR | NR |
| 9 | Van Duijnhoven 200275 | NR | NR | NR | NR | NR | NR | NR |
| 10 | Waller 200276 | NR | NR | NR | NR | NR | NR | NR |
| 11 | Charpentier 2003148 | 126/186 vs. 138/184 | NR | NR | NR | NR | NR | NR |
| 12 | Töz 200485 | NR | NR | NR | NR | NR | NR | NR |
| 13 | Hardinger 2005100 | NR | NR | NR | NR | NR | NR | NR |
| CSA + MMF low vs. CSA + AZA vs. CSA + MMF (two studies) | ||||||||
| 14 | Sollinger 199577 | 74/165 vs. 75/164 vs. 78/166 | NR | NR | NR | NR | NR | NR |
| 15 | Tricontinental MMF renal study 199689 | NRa | NR | NR | NRa | NR | NR | NR |
| CSA + MMF vs. CSA + AZA (four studies) | ||||||||
| 16 | Sadek 200286 | NR | 122/162 vs. 103/157 | NR | NR | NR | NR | NR |
| 17 | Tuncer 200278 | NR | NR | NR | NR | NR | NR | NR |
| 18 | Merville 2004138 | NR | NR | NR | NR | NR | NR | NR |
| 19 | Remuzzi 2007101 | NR | NR | NR | NR | NR | 79/124 vs. 89/124 | NR |
| TAC + MMF vs. CSA + AZA (two studies) | ||||||||
| 20 | Wlodarczyk 2002171 | NR | NR | NR | NR | NR | NR | NR |
| 21 | Vacher-Coponat 2012129 | NR | NRa | NR | NR | NR | NR | NR |
| TAC + MMF vs. CSA + MMF (four studies) | ||||||||
| 22 | Zadrazil 2012102 | NR | NR | NR | NR | NR | NR | NR |
| 23 | Hernández 2007130 | NR | NR | NRa | NR | NR | NR | NR |
| 24 | Rowshani 2006103 | NR | NR | NR | NR | NR | NR | NR |
| 25 | Ulsh 1999153 | NR | 11/30 vs. 5/30 | NR | NR | NR | NR | NR |
| TAC + AZA vs. CSA + AZA vs. CSA + MMF (one study) | ||||||||
| 26 | Weimer 2005172 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. TAC-PR + MMF (four studies) | ||||||||
| 27 | Wlodarczyk 2009140 | NR | NR | NR | NR | NR | NR | NR |
| 28 | Krämer 201058 | NR | NRa | NR | NR | NR | NR | NR |
| 29 | Tsuchiya 2013141 | NR | NR | NR | NR | NR | NR | NR |
| 30 | Oh 2014105 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. TAC-PR 0.2 + MMF vs. TAC-PR 0.3 (one study) | ||||||||
| 31 | Albano 2013123 | 79/311 vs. 76/309 vs. 72/307 | NR | NR | NR | NR | NR | NR |
| MMF + TAC vs. MPS + TAC (one study) | ||||||||
| 32 | Ciancio 2008106 | NR | 10/75 vs. 11/75 | NR | NR | 23/75 vs. 29/75 | NR | NR |
| MMF + CSA vs. MPS + CSA (one study) | ||||||||
| 33 | Salvadori 2004124 | NR | 154/210 vs. 148/213 | NR | NR | NR | NR | NR |
| BEL low + MMF vs. BEL high + MMF vs. CSA + MMF (three studies) | ||||||||
| 34 | Vincenti 2005125 | NR | 52/71 vs. 54/74 vs. 55/73 | NR | NR | NR | NRa | NR |
| 35 | BENEFIT60 | NR | 158/226 vs. 152/219 vs. 157/221 | 181/226 vs. 173/219 vs. 175/221 | 185/226 vs. 175/219 vs. 176/221 | NR | 25/165 vs. 26/155 vs. 26/136 (months 36–60) | NR |
| 36 | BENEFIT-EXT142 | NR | NR | 144/175 vs. 147/184 vs. 147/184 | 144/175 vs. 145/184 vs. 151/184 | NR | NRa | NR |
| BEL + MMF vs. BEL + SRL vs. TAC + MMF (one study) | ||||||||
| 37 | Ferguson 2011126 | NR | 26/33 vs. 20/26 vs. 20/30 | NR | NR | NR | NR | NR |
| EVL low + CSA vs. EVL high + CSA vs. MMF + CSA (three studies) | ||||||||
| 38 | Lorber 2005143 | NR | NR | NR | NRa | NR | NR | NR |
| 39 | ATLAS150 | NR | NR | NR | NRa | NR | NR | NR |
| 40 | Takahashi 2013131 | NR | 50/61 vs. 57/61 | NR | NR | NR | NR | NR |
| EVL vs. EVL + CSA vs. CSA + MPS (one study) | ||||||||
| 41 | Chadban 2013152 | NR | 33/49 vs. 18/30 vs. 34/47 | NR | NR | NR | NR | NR |
| EVL low + CSA vs. EVL high + CSA vs. MPA + CSA (one study) | ||||||||
| 42 | Tedesco-Silva 2010107 | NR | 169/274 vs. 178/278 vs. 185/273 | NR | NR | NR | NR | NR |
| EVL + CSA vs. MPS + CSA (one study) | ||||||||
| 43 | Bertoni 2011144 | NR | NR | NR | NR | NR | NR | NR |
| EVL + MPS vs. CSA + MPS (two studies) | ||||||||
| 44 | Budde 2011132 | NR | 96/155 vs. 75/145 | 35/155 vs. 30/145 | 31/155 vs. 29/145 | NR | NR | NR |
| 45 | Mjörnstedt 2012133 | NR | 59/102 vs. 52/100 | NR | NR | NR | NR | NR |
| SRL + CSA vs. MMF + CSA (two studies) | ||||||||
| 46 | Barsoum 2007108 | NR | NR | NR | NR | NR | NR | NR |
| 47 | Stallone 2004109 | NR | NR | NR | NR | NR | NR | NR |
| SRL + TAC vs. MMF + TAC (six studies) | ||||||||
| 48 | Anil Kumar 2005110 | NR | NR | NR | NR | NR | NR | NR |
| 49 | Gonwa 2003180 | NR | NR | NR | NR | NR | NR | NR |
| 50 | Sampaio 2008112 | NR | NR | NR | NR | NR | NR | NR |
| 51 | Gelens 2006113 | NR | NR | NR | NR | NR | NR | NR |
| 52 | Gallon 2006145 | NR | NR | NR | NR | NR | NR | 9/37 vs. 11/45 |
| 53 | Van Gurp 2010114 | 149/318 vs. 162/316 | NR | NR | NR | NR | NR | NR |
| SRL + MMF vs. CSA + MMF (10 studies) | ||||||||
| 54 | Flechner 2002127 | NR | NR | NR | NR | NR | 14/31 vs. 16/30 | NR |
| 55 | Noris 2007115 | NR | NR | NRa | NR | NR | NR | NR |
| 56 | Lebranchu 2009149 | NR | NR | NR | NR | 4/96 vs. 4/97 | NR | NR |
| 57 | Büchler 2007134 | NR | NR | NR | NR | NR | NR | NR |
| 58 | Soleimani 201391 | NR | NR | NR | NR | NR | NR | NR |
| 59 | Durrbach 2008146 | NR | NR | NR | NR | NR | NR | NR |
| 60 | Kreis 2000116 | NR | NR | NR | NR | NR | NR | NR |
| 61 | Guba 2010147 | NR | 36/69 vs. 43/71 | NR | NR | NR | NR | NR |
| 62 | Martinez-Mier 2006117 | NR | NRa | NR | NR | NR | NR | NR |
| 63 | Nafar 2012118 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. SRL + MMF (four studies) | ||||||||
| 64 | Stegall 2003191 | Mean follow-up 33 months (17–47 months) | ||||||
| 65 | Schaefer 200692 | NR | NR | NR | NR | NR | NR | NR |
| 66 | Heilman 2011135 | NR | NR | NR | NR | NR | NR | NR |
| 67 | Smith 200893 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MPS vs. SRL + MPS (one study) | ||||||||
| 68 | Silva 2013107 | NR | NR | NR | NR | NR | NR | NR |
| TAC + SRL vs. MMF + SRL (one study) | ||||||||
| 69 | Hamdy 2005120 | NR | NRa | NR | NR | NR | NRa | NR |
| SRL + AZA vs. CSA + AZA (one study) | ||||||||
| 70 | Groth 1999194 | NR | NRa | NR | NR | NR | NR | NR |
| TAC + SRL vs. CSA + SRL (one study) | ||||||||
| 71 | Chen 2008121 | NR | 4/21 vs. 3/20 | NR | NR | NR | NR | NR |
| SRL low + TAC vs. SRL high + TAC vs. MMF + TAC (one study) | ||||||||
| 72 | Vítko 200694 | 124/325 vs. 149/325 vs. 160/327c | NR | NR | NR | NR | NR | NR |
| SRL + TAC vs. SRL + MMF vs. MMF + TAC (one study) | ||||||||
| 73 | Flechner 2011155 | NR | NR | 93/152 vs. 97/152 vs. 93/139 | NR | NR | NR | NR |
| MMF + CSA vs. MMF + low CSA vs. MMF + low TAC vs. MMF low SRL (one study) | ||||||||
| 74 | Grinyo 200951 | NR | Severe infection only: 58/384 vs. 57/408 vs. 60/403 vs. 78/380 | NR | 184/233 vs. 171/248 vs. 177/249 vs. 169/228 | NR | NR | NR |
| TAC + MMF vs. TAC + SRL vs. CSA + MMF vs. CSA + SRL (one study) | ||||||||
| 75 | Anil Kumar 2008122 | NR | NR | NR | NR | NR | NR | NR |
Cytomegalovirus
Studies that reported the frequencies of CMV showed that this ranged from 0 to 7 out of 27 (26%) (Table 126).
| Number | Study | 6 months | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 8.5 |
|---|---|---|---|---|---|---|---|---|
| TAC + AZA vs. CSA + AZA (13 studies) | ||||||||
| 1 | Schleibner 199579 | NR | NR | NR | NR | NR | NR | NR |
| 2 | Laskow 199680 | NR | NR | NR | NR | NR | NR | NR |
| 3 | Mayer 199788 | NR | 41/303 vs. 24/145 | NR | NR | NR | NR | NR |
| 4 | Radermacher 199881 | NR | NR | NR | NR | NR | NR | NR |
| 5 | Jarzembowski 200599 | NR | 0/14 vs. 0/21 | NR | NR | NR | NR | NR |
| 6 | Baboolal 200282 | NR | 7/27 vs. 7/24 | NR | NR | NR | NR | NR |
| 7 | Campos 200283 | NR | NR | NR | NR | NR | NR | NR |
| 8 | Margreiter 200284 | NR | NR | NR | NR | NR | NR | NR |
| 9 | Van Duijnhoven 200275 | NR | NR | NR | NR | NR | NR | NR |
| 10 | Waller 200276 | NR | NR | NR | NR | NR | NR | NR |
| 11 | Charpentier 2003148 | 45/186 vs. 52/184 | NR | NR | NR | NR | NR | NR |
| 12 | Töz 200485 | NR | NR | NR | NR | NR | NR | NR |
| 13 | Hardinger 2005100 | NR | 5/134 vs. 4/66 | NR | NR | NR | NR | NR |
| CSA + MMF low vs. CSA + AZA vs. CSA + MMF (two studies) | ||||||||
| 14 | Sollinger 199577 | 15/165 vs. 10/164 vs. 18/166a | NR | NR | NR | NR | NR | NR |
| 15 | Tricontinental MMF renal study 199689 | NR | 12/171 vs. 18/164 vs. 10/162a | NR | 12/171 vs. 11/164 vs. 18/162a | NR | NR | NR |
| CSA + MMF vs. CSA + AZA (four studies) | ||||||||
| 16 | Sadek 200286 | NR | 32/162 vs. 17/157b | NR | NR | NR | NR | NR |
| 17 | Tuncer 200278 | NR | NR | NR | NR | NR | NR | NR |
| 18 | Merville 2004138 | NR | 11/37 vs. 17/34 | NR | NR | NR | NR | NR |
| 19 | Remuzzi 2007101 | 43/168 vs. 42/168 | NR | NR | NR | NR | 39/124 vs. 45/124 | NR |
| TAC + MMF vs. CSA + AZA (two studies) | ||||||||
| 20 | Wlodarczyk 2002171 | 12/243 vs. 14/246 | NR | NR | NR | NR | NR | NR |
| 21 | Vacher-Coponat 2012129 | NR | 25/143 vs. 28/146 | NR | NR | NR | NR | NR |
| TAC + MMF vs. CSA + MMF (four studies) | ||||||||
| 22 | Zadrazil 2012102 | NR | NR | NR | NR | NR | NR | NR |
| 23 | Hernández 2007130 | NR | NR | 20/80 vs. 16/80 | NR | NR | NR | NR |
| 24 | Rowshani 2006103 | NR | NR | NR | NR | NR | NR | NR |
| 25 | Ulsh 1999153 | NR | 3/30 vs. 0/30 | NR | NR | NR | NR | NR |
| TAC + AZA vs. CSA + AZA vs. CSA + MMF (one study) | ||||||||
| 26 | Weimer 2005172 | NR | 7/28 vs. 11/25 vs. 13/31 | NR | NR | NR | NR | NR |
| TAC + MMF vs. TAC-PR + MMF (four studies) | ||||||||
| 27 | Wlodarczyk 2009140 | NR | NR | NR | NR | NR | NR | NR |
| 28 | Krämer 201058 | NR | 19/336 vs. 33/331b | NR | NR | NR | NR | NR |
| 29 | Tsuchiya 2013141 | NR | 7/52 vs. 4/50 | NR | NR | NR | NR | NR |
| 30 | Oh 2014105 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. TAC-PR 0.2 + MMF vs. TAC-PR 0.3 (one study) | ||||||||
| 31 | Albano 2013123 | 21/311 vs. 12/309 vs. 17/307 | NR | NR | NR | NR | NR | NR |
| MMF + TAC vs. MPS + TAC (one study) | ||||||||
| 32 | Ciancio 2008106 | NR | 1/75 vs. 0/75 | NR | NR | 0/75 vs. 1/75 | NR | NR |
| MMF + CSA vs. MPS + CSA (one study) | ||||||||
| 33 | Salvadori 2004124 | NR | 43/210 vs. 46/213 | NR | NR | NR | NR | NR |
| BEL low + MMF vs. BEL high + MMF vs. CSA + MMF (three studies) | ||||||||
| 34 | Vincenti 2005125 | NR | 11/71 vs. 10/74 vs. 13/73 | NR | NR | NR | 1/102 vs. 1/26 | NR |
| 35 | BENEFIT60 | NR | 10/226 vs. 9/219 vs. 6/221 | 12/226 vs. 12/219 vs. 7/221 | 26/226 vs. 22/219 vs. 25/221 | NR | NR | NR |
| 36 | BENEFIT-EXT142 | NR | 24/175 vs. 21/184 vs. 24/184 | 16/175 vs. 17/184 vs. 12/184 | 27/175 vs. 32/184 vs. 31/184 | NR | 4/113 vs. 4/104 vs. 3/87 | NR |
| BEL + MMF vs. BEL + SRL vs. TAC + MMF (one study) | ||||||||
| 37 | Ferguson 2011126 | NR | 1/33 vs. 1/26 vs. 2/30 | NR | NR | NR | NR | NR |
| EVL low + CSA vs. EVL high + CSA vs. MMF + CSA (three studies) | ||||||||
| 38 | Lorber 2005143 | NR | NR | NR | 10/196 vs. 8/194 vs. 12/196 | NR | NR | NR |
| 39 | ATLAS150 | NR | 10/194 vs. 15/198 vs. 38/196b | NR | 11/194 vs. 16/198 vs. 40/196c | NR | NR | NR |
| 40 | Takahashi 2013131 | NR | 3/61 vs. 21/61b | NR | NR | NR | NR | NR |
| EVL vs. EVL + CSA vs. CSA + MPS (one study) | ||||||||
| 41 | Chadban 2013152 | NR | 2/49 vs. 2/30 vs. 4/47 | NR | NR | NR | NR | NR |
| EVL low + CSA vs. EVL high + CSA vs. MPA + CSA (one study) | ||||||||
| 42 | Tedesco-Silva 2010107 | NR | 2/274 vs. 4/278 vs. 16/273b | NR | NR | NR | NR | NR |
| EVL + CSA vs. MPS + CSA (one study) | ||||||||
| 43 | Bertoni 2011144 | NR | NR | NR | NR | NR | NR | NR |
| EVL + MPS vs. CSA + MPS (two studies) | ||||||||
| 44 | Budde 2011132 | NR | 10/155 vs. 14/145 | NR | NR | NR | NR | NR |
| 45 | Mjörnstedt 2012133 | NR | 9/102 vs. 13/100 | NR | NR | NR | NR | NR |
| SRL + CSA vs. MMF + CSA (two studies) | ||||||||
| 46 | Barsoum 2007108 | NR | NR | NR | NR | NR | NR | NR |
| 47 | Stallone 2004109 | NR | NR | NR | NR | NR | NR | NR |
| SRL + TAC vs. MMF + TAC (six studies) | ||||||||
| 48 | Anil Kumar 2005110 | NR | NR | NR | NR | NR | NR | NR |
| 49 | Gonwa 2003180 | NR | NR | NR | NR | NR | NR | NR |
| 50 | Sampaio 2008112 | NR | 6/50 vs. 6/50 | NR | NR | NR | NR | NR |
| 51 | Gelens 2006113 | NR | NR | NR | NR | NR | NR | NR |
| 52 | Gallon 2006145 | NR | NR | NR | 1/37 vs. 1/45 | NR | NR | NR |
| 53 | Van Gurp 2010114 | 9/318 vs. 38/316b | NR | NR | NR | NR | NR | NR |
| SRL + MMF vs. CSA + MMF (10 studies) | ||||||||
| 54 | Flechner 2002127 | NR | 3/31 vs. 2/30 | NR | NR | NR | 2/31 vs. 3/30 | NR |
| 55 | Noris 2007115 | NR | NR | 0/11 vs. 4/10 | NR | NR | NR | NR |
| 56 | Lebranchu 2009149 | NR | 4/96 vs. 6/97 | NR | NR | NR | NR | NR |
| 57 | Büchler 2007134 | NR | 4/71 vs. 17/74b | NR | NR | NR | NR | NR |
| 58 | Soleimani 201391 | NR | NR | NR | NR | NR | 14/29 vs. 16/59 | NR |
| 59 | Durrbach 2008146 | 1/33 vs. 1/36 | NR | NR | NR | NR | NR | NR |
| 60 | Kreis 2000116 | NR | 2/40 vs. 8/38b | NR | NR | NR | NR | NR |
| 61 | Guba 2010147 | NR | 5/69 vs. 20/71 | NR | NR | NR | NR | NR |
| 62 | Martinez-Mier 2006117 | NR | 1/20 vs. 0/21 | NR | NR | NR | NR | NR |
| 63 | Nafar 2012118 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MMF vs. SRL + MMF (four studies) | ||||||||
| 64 | Stegall 2003191 | Mean follow-up 33 months (17–47 months) | ||||||
| 65 | Schaefer 200692 | NR | NR | NR | NR | NR | NR | NR |
| 66 | Heilman 2011135 | NR | 8/62 vs. 8/60 | NR | NR | NR | NR | NR |
| 67 | Smith 200893 | NR | NR | NR | NR | NR | NR | NR |
| TAC + MPS vs. SRL + MPS (one study) | ||||||||
| 68 | Silva 2013107 | NR | NR | 4/107 vs. 5/97 | NR | NR | NR | NR |
| TAC + SRL vs. MMF + SRL (one study) | ||||||||
| 69 | Hamdy 2005120 | NR | NR | NR | NR | NR | NR | NR |
| SRL + AZA vs. CSA + AZA (one study) | ||||||||
| 70 | Groth 1999194 | NR | 6/41 vs. 5/42 | NR | NR | NR | NR | NR |
| TAC + SRL vs. CSA + SRL (one study) | ||||||||
| 71 | Chen 2008121 | NR | NR | NR | NR | NR | NR | NR |
| SRL low + TAC vs. SRL high + TAC vs. MMF + TAC (one study) | ||||||||
| 72 | Vítko 200694 | 16/325 vs. 13/325 vs. 26/327 | NR | NR | NR | NR | NR | NR |
| SRL + TAC vs. SRL + MMF vs. MMF + TAC (one study) | ||||||||
| 73 | Flechner 2011155 | NR | NR | NR | NR | NR | NR | NR |
| MMF + CSA vs. MMF + low CSA vs. MMF + low TAC vs. MMF low SRL (one study) | ||||||||
| 74 | Grinyo 200951 | NR | 55/384 vs. 45/408 vs. 39/403 vs. 23/380b | NR | Yes | NR | NR | NR |
| TAC + MMF vs. TAC + SRL vs. CSA + MMF vs. CSA + SRL (one study) | ||||||||
| 75 | Anil Kumar 2008122 | NR | NR | NR | NR | NR | 1/50 vs. 0/50 vs. 1/50 vs. 0/50 | NR |
The CSA + MMF arm of the following trials displayed a statistically significant difference, in terms of increased episodes of CMV: Sadek et al. ,86 Vítko et al. ,94 Takahashi et al. ,131 Büchler et al. ,134 Kreise et al. ,116 Tedesco-Silva et al. 107 and Grinyo et al. 51 Krämer et al. 58 reported a statistically significant difference for TAC-PR + MMF vs. TAC + MMF and Van Gurp et al. 114 found increased events for TAC + MMF as opposed to SRL + TAC.
Summary of clinical effectiveness
Summary of pairwise comparisons
Overall, we found that, despite the volume of evidence, there is little impact on effectiveness conclusions from the head-to-head comparisons, particularly for graft loss and mortality. However, this may be a reflection of the lack of long-term data, as very few studies reported all outcomes beyond 1 year, and also the frequently substantial level of heterogeneity across studies. Furthermore, the quality of trials was variable and, as a result of reporting omissions, it was difficult to make a general assessment regarding quality.
Induction
-
We found no evidence to suggest BAS or rATG are more effective than PBO, no induction or each other in reducing the odds of mortality. Similarly, for graft loss, we found no evidence of a statistically significant difference for BAS or rATG vs. PBO, no induction or each other.
-
Three RCTs98,137,149 were identified for BAS vs. rATG. No statistically significant difference was seen for any of the outcomes.
-
For the head-to-head comparisons, we found evidence to suggest that rATG and BAS are more effective than PBO or no induction at reducing BPAR (rATG at 1 year, OR 0.41, 95% CI 0.24 to 0.52 BAS at 1 year, OR 0.53, 95% CI 0.40 to 0.70). However, there is no statistically significant difference between BAS and rATG.
-
Time to BPAR is reported only for rATG vs. no induction and BAS vs. rATG. The one study96 for rATG vs. no induction found that more participants experienced BPAR at 7–10 days with no induction than with rATG (seven participants for rATG vs. 30 participants for no induction). There was no statistically significant difference between interventions for BAS vs. rATG.
Maintenance
-
We found no evidence that any maintenance therapies were preferable to others in terms of mortality.
-
For graft loss outcomes reported by maintenance studies, we found evidence that at 5 years BEL + MMF may be superior to CSA + MMF (OR 0.40, 95% CI 0.19 to 0.87, I2 = 0.0%). The 0.5-year time point has only two studies and a substantial level of heterogeneity (I2 = 72.2%); therefore, the OR of 0.58 and 95% CI 0.09 to 3.59, which indicates that MMF is more effective at reducing graft loss, must be treated with caution. 201 The results for 1 year suggest no difference between arms (OR 0.76, 95% CI 0.38 to 1.50). The Merville et al. 138 study appears to show more of an effect in favour of MMF; however, the population is much smaller than that for the Tricontinental study89 and the Sadek et al. 86 study. Weimer et al. 104 found no evidence of graft loss in either arm.
-
Several treatments showed a beneficial effect with regard to reducing BPAR, although this varied across time points. For all the following combinations, the arm containing TAC displayed lower odds associated with BPAR:
-
TAC + AZA vs. CSA + AZA (0.5 years, OR 0.50, 95% CI 0.32 to 0.79; I2 = 50.1%; 1 year, OR 0.50, 95% CI 0.39 to 0.64; I2 = 8.1%; 4 years, OR 0.38, 95% CI 0.25 to 0.57)
-
TAC + MMF vs. CSA + AZA (0.5 years, OR 0.64, 95% CI 0.41 to 0.98; 1 year, OR 0.35, 95% CI 0.15 to 0.82)
-
TAC + MMF vs. CSA + MMF (1 year, OR 0.59, 95% CI 0.37 to 0.94, I2 = 19.3%)
-
TAC + MMF vs. SRL + MMF (1 year, OR 0.32, 95% CI 0.12 to 0.87, I2 = 0.0%)
-
TAC + SRL vs. TAC + MMF (0.5 years, OR 0.65, 95% CI 0.44 to 0.96).
-
-
For CSA + MMF vs. CSA + AZA, at 0.5 years and 1 year, there is statistically significant evidence to suggest that MMF is more effective (0.5 years, OR 0.50, 95% CI 0.35 to 0.72, I2 = 35.1%).
-
TAC is also associated with lower odds of reduced GRF for:
-
TAC + MMF vs. CSA + MMF (3 years, WMD 4.60 ml/minute/1.73 m2, 95% CI 1.35 to 7.85 ml/minute/1.73 m2)
-
TAC + MMF vs. TAC-PR + MMF (0.5 years, WMD 1.90 ml/minute/1.73 m2, 95% CI 1.70 to 2.10 ml/minute/1.73 m2)
-
TAC + SRL vs. CSA + SRL (0.5 years, MD 6.35 ml/minute/1.73 m2, p < 0.0001; 1 year, MD 5.25, p = 0.0004).
-
-
For MMF + TAC vs. MPS + TAC, MPS at 1 year and 3 years is more effective (1 year, MD 1.9 ml/minute/1.73 m2, p < 0.0001; 3 years MD 0.5 ml/minute/1.73 m2, p = 0.0016). BEL appears more effective at 1 year and 3 years for BEL + MMF vs. CSA + MMF (1 year, WMD 7.83 ml/minute/1.73 m2, 95% CI 1.57 to 14.10 ml/minute/1.73 m2; I2 = 73.6%; 3 years, WMD 16.08 ml/minute/1.73 m2, 95% CI 5.59 to 26.56 ml/minute/1.73 m2; I2 = 89.5%); however, heterogeneity across studies is substantial. Where there are two comparisons involving SRL and CSA, the regimen including MMF suggests CSA to be more beneficial up to 5 years (5 years, WMD 9.10 ml/minute/1.73 m2, 95% CI 1.68 to 16.52 ml/minute/1.73 m2), yet, in contrast, the regimen including AZA suggests SRL to be more effective (1 year, MD 10.8 ml/minute/1.73 m2, p < 0.0001).
-
Time to BPAR is generally poorly reported and therefore it is challenging to form a conclusion. Again, TAC + AZA vs. CSA + AZA shows conflicting results for two studies; however, the statistically significant result suggests that BPAR is achieved more quickly for participants receiving TAC rather than CSA (MD 24 days; p = 0.0033). This is also true for TAC + MMF vs. CSA + MMF (MD 46.7 days; p < 0.0001). When SRL + TAC and MMF + TAC are compared, a reduced time to BPAR is seen for MMF (MD 48.6 days; p = 0.0017). For SRL + MMF vs. CSA + MMF, one146 of three studies127,134,146 demonstrates a statistically significant difference in favour of CSA (MD 38 days; p = 0.0035); however, the other two studies127,134 show no difference.
-
For TAC + AZA vs. CSA + AZA, there may be lower odds of the more severe BPAR for the arm containing TAC. Similarly, for TAC + MMF vs. TAC-PR + MMF, TAC has a lower proportion of people experiencing the more severe BPAR of Banff III classification.
Summary for network meta-analysis
Induction therapy
-
There is no evidence to suggest BAS or rATG are more effective than PBO/no induction or each other in reducing the odds of graft loss or mortality, which is in agreement with the pairwise comparisons.
-
rATG and BAS are both estimated to be more effective than PBO/no induction, with rATG being more effective than BAS at reducing BPAR.
-
Evidence suggests that although no treatment effect is seen for rATG, BAS is estimated to be more effective than PBO/no induction for increasing CRC-GFR.
Maintenance therapy
-
For all NMAs for maintenance therapy there is a great deal of heterogeneity.
-
There is no evidence to suggest that one treatment is any more effective at reducing the odds of graft loss than any other treatment.
-
There is evidence to suggest that BEL + MMF is more effective at reducing the odds of mortality than TAC + MMF and SRL + MMF, but no other treatments are estimated to be any more effective at reducing mortality than any other treatment.
-
A number of treatments are estimated to be more effective than CSA + AZA and EVL + MPS at reducing the odds of BPAR, and CSA + AZA and SRL + TAC at increasing GFR, but no other treatments are estimated to be any more effective at reducing the odds of BPAR or increasing GFR than any other treatment.
Comparison between clinical effectiveness analyses
Induction
Network meta-analysis and pairwise comparisons were in agreement for all comparable outcomes other than GRF, for which NMA suggested that BAS may be more effective than PBO/no induction.
Maintenance
-
Pairwise comparisons found no evidence that any maintenance therapies were preferable to others in terms of mortality; however, NMA found evidence to suggest that BEL + MMF is more effective at reducing the odds of mortality than TAC + MMF and SRL + MMF.
-
Following NMA, there is no evidence to suggest that one treatment is any more effective at reducing the odds of graft loss than any other treatment. For pairwise comparisons, there is some evidence that BEL + MMF may be superior to CSA + MMF; CSA + MMF may be superior to CSA + AZA; and TAC + AZA may be superior to CSA + AZA.
-
A number of treatments were estimated to be more effective than CSA + AZA and EVL + MPS at reducing the odds of BPAR by the NMA, but no treatments found to be any more effective than any other. As for the pairwise comparisons, the arm containing TAC displayed lower odds associated with BPAR for TAC + AZA vs. CSA + AZA; TAC + MMF vs. CSA + AZA;TAC + MMF vs. CSA + MMF and TAC + MMF vs. SRL + MMF.
-
The NMA found evidence that CSA + AZA and SRL + TAC were effective at increasing GFR, but no other treatments were estimated to be any more effective than any other treatment. Although the pairwise comparison found that TAC was generally associated with lower odds of reduced GRF for TAC + MMF vs. CSA + MMF; TAC + MMF vs. TAC-PR + MMF; TAC + SRL vs. CSA + SRL. For MMF + TAC vs. MPS + TAC, MPS was more effective.
Current assessment (Technology Assessment 85)
Relevant to this review, the current assessment (TA85) found that BAS, TAC and MMF consistently reduced the incidence of short-term (1-year) AR compared with conventional immunosuppressive therapy. The independent use of BAS, TAC and MMF was associated with a similar absolute reduction in 1-year acute rejection rate (ARR) (approximately 15%).
The trials did not assess how the improvement in trials, the impact of the newer immunosuppressants on long-term graft loss and patient survival remain uncertain.
The absence of both long-term outcome and quality of life from trial data makes assessment of the clinical effectiveness challenging.
Ongoing studies
Searches of ClinicalTrials.gov and Controlled Trials were conducted (see Appendix 1 for the search strategy used). All searches were carried out in January 2015. A total of 256 trials were considered to be relevant to this review and were investigated further. Sixty-nine studies were identified as ongoing (active not recruiting, n = 16; not yet recruiting, n = 7) or recruiting (n = 46). In 26 trials the current status was recorded as ‘unknown’. Twenty-three trials had terminated, two had been suspended and three had been withdrawn; of these, five had results available. Finally, 133 studies were completed. A summary of the trials is provided in Table 127. The search of ongoing studies did not identify any additional RCTs for inclusion in PenTAG systematic review; 18 studies were already considered in PenTAG review. An overview of these trials is provided in Appendix 8.
| Trial status (N) | n included in PenTAG | n excluded (reason) |
|---|---|---|
| Active, not recruiting (16) | 3 | 13 (7 – no publication, 1 – no data, 1 – mixed transplants, 4 – not relevant) |
| Not yet recruiting (7) | 0 | 7 (no data) |
| Recruiting (46) | 0 | 46 (no data) |
| Unknown (26) | 2 | 24 (12 – no publication, 2 – mixed population, 2 – no data, 1 – dosing studies, 7 – not relevant) |
| Suspended (2) | 0 | 2 (2 – no publication) |
| Withdrawn (3) | 0 | 3 (1 – no publication, 2 – not relevant) |
| Terminated (23) | 0 | 23 [2 – no publication, 1 – mixed population, 6 – treatment (dosing or conversion), 14 – not relevant] |
| Completed (133) | 13 | 120 [60 – no publication, 6 – mixed population, 6 – no data, 15 – treatment (dosing or conversion), 33 – not relevant] |
Critique of company submissions’ search strategies
Submissions from four companies were presented, summarising evidence on the effectiveness of immunosuppressive therapies in renal transplantation: Sandoz, Astellas, Bristol-Myers Squibb and Novartis.
Sandoz
The company’s literature search is primarily focused on finding studies that report on Adoport®, Sandoz’s licensed version of TAC. The searches presented by Sandoz are transparent, replicable and consistent with the aims of the company’s submission, which is a systematic review of Adoport with no economic model.
Sandoz’s literature searches have been conducted in a range of bibliographic databases, including MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials and NHS Economic Evaluation Database (NHS EED). These searches have been supplemented with an unreported search of Sandoz’s internal databases.
We believe these searches to be adequate but we are unable to exclude the possibility of reporting bias. The search strategies are geared to locate studies that include the brand name (Adoport) or drug name (TAC) and company name (Sandoz). It is feasible that a title/abstract might merely mention the drug name without a brand or company stated and, if such a study existed, this would be missed by the company’s literature searches. The nature of RCT reporting makes this unlikely for trial data but, for AEs or economic literature, it is a possibility. However, as the manufacturer made an unreported search of its own databases it is unlikely it would have missed one of its own trials.
Sandoz’s submission summarised the evidence on Adoport and compared Adoport with Prograft®, the Astellas-licensed version of TAC. It identified 26 papers: one RCT (reported in two papers) and 24 non-randomised studies (non-RCTs). The RCT was a pharmacokinetics study and had no clinical effectiveness data. None of the included studies is considered in PenTAG systematic review (Table 128).
| Study | Included in PenTAG review | Reason for exclusion |
|---|---|---|
| Alloway 2012213 | ✗ | Study design |
| Bloom 2013214 | ✗ | Study design |
| Connor 2012215 | ✗ | Study design |
| Connor 2013216 | ✗ | Study design |
| Heavner 2013217 | ✗ | Study design |
| Marfo 2013218 | ✗ | Study design |
| McDevitt-Potter 2011219 | ✗ | Study design |
| Richards 2014220 | ✗ | Study design |
| Rosenborg 2014221 | ✗ | Study design |
| Spence 2012222 | ✗ | Study design |
| Babu 2013223 | ✗ | Abstract |
| Betmouni 2012224 | ✗ | Abstract |
| Chiu 2012225 | ✗ | Abstract |
| Crowther 2012226 | ✗ | Abstract |
| Dick 2011227 | ✗ | Abstract |
| Heldenbrand 2012228 | ✗ | Abstract |
| Jogia 2013229 | ✗ | Abstract |
| Kendrew 2013230 | ✗ | Abstract |
| Qazi 2012231 | ✗ | Abstract |
| Sharma 2013232 | ✗ | Abstract |
| Shiu 2013233 | ✗ | Abstract |
| Siddiqi 2011234 | ✗ | Abstract |
| Storey 2013235 | ✗ | Abstract |
| Venkataramanan 2012236 | ✗ | Abstract |
| Wilcock 2013237 | ✗ | Abstract |
| Marsen 2012238 | ✗ | Study design |
In summary, the results of Sandoz’s submission are not comparable with the results of the current HTA review.
Astellas
The literature searches have been conducted in the key bibliographic databases, MEDLINE, EMBASE, The Cochrane Library and Cochrane NHS EED.
The literature searches used minimal free-text search terms without the use of truncation or controlled indexing, and selected synonyms were used for the interventions/comparators. This reflects poor sensitivity and, combined with the fact that searching has been conducted on only the abstracts of potentially includable studies, it is possible that some studies may have been missed.
The submission set out to compare the efficacy and safety of TAC (Prograf) therapy with the efficacy and safety of current alternative treatments [TAC-PR (Advagraf), CSA, SRL and BEL] in addition to EVL, as primary immunosuppressive therapies in people undergoing renal transplantation.
Thirty-eight RCTs were identified: 19 studies comparing TAC and CSA regimens, 10 studies comparing SRL and TAC regimens [CNI avoidance (six studies), CNI avoidance and steroids withdrawal (one study), CNI minimisation (three studies)], three trials comparing TAC-PR and TAC regimens, two studies reporting on BEL and six studies reporting on EVL. Two studies239,240 included information for two comparisons. No head-to-head studies comparing TAC with BEL, and TAC with EVL, were identified (Table 129). Two separate NMAs were performed: one comparing TAC with EVL, and another comparing TAC with BEL.
| Study | Included in PenTAG review | Reason for exclusion |
|---|---|---|
| Ekberg 2007240 | ✓ | |
| Abou-Jaoude 2003241 | ✗ | Study design |
| Abou-Jaoude 2005242 | ✗ | Study design |
| Busque 2001243 | ✗ | Study design |
| Campos 200283 | ✓ | |
| Hardinger 2005100 | ✓ | |
| Johnson 2000244 | ✗ | Population |
| Margreiter 200284 | ✓ | |
| Martin Garcia 2003245 | ✗ | Study design |
| Morris-Stiff 1998246 | ✗ | Population |
| Murphy 2003166 | ✓ | |
| Raofi 1999210 | ✓ | |
| Silva 2007239 | ✗ | Population |
| Töz 200485 | ✓ | |
| Vincenti 2007247 | ✗ | Study design |
| Wang 2000248 | ✗ | Abstract |
| White 2000249 | ✗ | Abstract |
| Williams 1999250 | ✗ | Abstract |
| Yang 199990 | ✓ | |
| Flechner 2011155 | ✓ | |
| Glotz 2010251 | ✗ | Study design |
| Larson 2006154 | ✓ | |
| Chhabra 2013252 | ✗ | Study design |
| Lo 2004253 | ✗ | Study design |
| Hamdy 2005120 | ✓ | |
| Ciancio 2004 2004254,255 | ✗ | Population |
| Gonwa 2003180 | ✓ | |
| Mendez 2005111 | ✓ | |
| Vincenti 201059 | ✓ | |
| Durrbach 2010142 | ✓ | |
| Bertoni 2011144 | ✓ | |
| Tedesco-Silva 2010107 | ✓ | |
| Albano 2013123 | ✓ | |
| Krämer 2010204 | ✓ | |
| Langer 2012256 | ✗ | Study design |
| Chan 2008257 | ✗ | Study design |
| Favi 2012258 | ✗ | Abstract |
| Ruiz 2011259 | ✗ | Abstract |
In summary, Astellas’ results suggest no significant differences between TAC and EVL regimens, and less BPAR in BEL than in TAC. In the head-to-head comparisons, no differences between TAC and TAC-PR were identified. In addition, more AR episodes were identified in CSA than in TAC and in SRL than in TAC.
In comparison, the PenTAG NMA found evidence to suggest that BEL + MMF is more effective at reducing the odds of mortality than TAC + MMF and SRL + MMF, but no other treatments were estimated to be any more effective at reducing mortality than any other treatment. In addition, BEL + MMF are estimated to be more effective than CSA + AZA and MMF + CSA at increasing GRF. The head-to-head comparisons suggested that the clinical effectiveness of TAC-PR and TAC are similar, with TAC having a lower proportion of people experiencing the more severe BPAR of Banff III classification (OR 0.11, 95% CI 0.01 to 0.87; I2 = 0.0%). We also found some benefits to using TAC regimens compared with CSA regimens. For a full summary of head-to-head comparisons see Summary of pairwise comparisons, above.
Bristol-Myers Squibb
The literature searching used for this submission is not sufficient to provide a systematic and transparent review of BEL. The literature searching takes the following structure: (terms for TAC) AND (a methodological search filter to limit to RCTs). The literature search does not include any search terms for BEL, the intervention under submission by the company, or CSA.
In practice, this means that the searches will pick up studies of BEL only if BEL is in comparison with TAC. The company states that BEL has not been compared with TAC in head-to-head RCTs, noting that, in the case of BENEFIT59 and BENEFIT-EXT,142 CSA was the main licensed treatment used in clinical practice. This statement further confuses the rationale for using TAC as the named intervention in the literature search for this submission. It is therefore likely that includable trials have been missed (Table 130).
| First author and year | Included in PenTAG review | Reason for exclusion |
|---|---|---|
| Abou-Jaoude 2003260 | ✗ | Study design |
| Busque 2001243 | ✗ | Study design |
| Campos 200283 | ✓ | |
| Charpentier 2003148 | ✓ | |
| Chen 2008121 | ✓ | |
| Cheung 2006261 | ✗ | Study design |
| Egfjord 2002262 | ✗ | Abstract |
| Ekberg 2007240 | ✓ | |
| El Haggan 2002263 | ✗ | Abstract |
| Hardinger 2005100 | ✓ | |
| Hernández 2007130 | ✓ | |
| Liu 2003264 | ✗ | Population |
| Margreiter 200284 | ✓ | |
| Mayer 199788 | ✓ | |
| Murphy 2003166 | ✓ | |
| Radermacher 199881 | ✓ | |
| Rowshani 2006103 | ✓ | |
| Töz 200485 | ✓ | |
| Tsinalis 2000265 | ✗ | Abstract |
| Van Duijnhoven 200275 | ✓ | |
| Vincenti 1996161 | ✓ | |
| Vincenti 2007247 | ✗ | Study design |
| Wang 2000248 | ✗ | Abstract |
| Yang 199990 | ✓ | Included |
| Yu 2000266 | ✗ | Abstract |
| Nichelle 2002267 | ✗ | Study design |
| Heering 1998268 | ✗ | Data |
| Ichimaru 2001269 | ✗ | Study design |
| Anil Kumar 2008122 | ✓ | |
| BENEFIT59 | ✓ | |
| BENEFIT-EXT142 | ✓ | |
| Vincenti 2005206 | ✓ |
In summary, because of the issues with the literature searches in Bristol-Myers Squibb’s submission, Bristol-Myers Squibb’s conclusions are not comparable with the results of the current HTA review (see Table 130).
Novartis
The company’s literature search for this submission is systematic, robust and transparent. The company has searched all of the required databases and made an exhaustive attempt to locate published and unpublished studies. The submission compared the efficacy and safety of MPS and EVL, as primary immunosuppressive therapies in people undergoing renal transplantation. A total of seven RCTs, three open-label extension studies of RCTs, as well as three non-RCTs with MPS regimen were identified in the systematic review. A total of 14 studies (25 publications and two unpublished clinical study reports) with EVL regimen were identified in the systematic review; eight RCTs, five prospective studies and one observational study (Table 131).
| First author and year | Included in PenTAG review | Reason for exclusion |
|---|---|---|
| Salvadori 2001,270 2004,124 2006271 | ✓ | |
| Budde 2004,272 2005,273 2006274 | ✗ | Intervention |
| Shehata 2009275 | ✗ | Study design |
| Ortega 2011276 | ✗ | Study design |
| Langone 2013,277 Chan 2013278 | ✗ | Study design |
| Shah 2013279 | ✗ | Study design |
| Ciancio 2008,106 2011173 | ✓ | |
| Langone 2011280 | ✗ | Study design |
| Chan 2006281 | ✗ | Study design |
| Hwang 2010282 | ✗ | Study design |
| Novartis CSR, Tedesco-Silva 2010,283 Cibrik 2013284 | ✓ | |
| Takahashi 2013,131 Takahara 2012,285 Saito 2013286 | ✓ | |
| Paoletti 2012,287 2012288 | ✗ | Study design |
| Favi 2009,289 2009,290 2010,291 2013292 | ✗ | Study design |
| Gonzalez 2010293 | ✗ | Study design |
| Miserlis 2008294 | ✗ | Study design |
| Watarai 2013295 | ✗ | Study design |
| Loriga 2010296 | ✗ | Study design |
| Vítko 2005,150 Dantal 2002,297 Vítko 2005,177 Oppenheimer 2003298 | ✓ | |
| Lorber 2005143 | ✓ | |
| Novartis CSR, NCT01025817; CRAD001AUS92299 | ✗ | Data |
| Tedesco 2012,300 2013301 | ✗ | Abstract |
| Favi 2012,258 2013302 | ✗ | Abstract |
| Kamar 2005,303 Rostaing 2001304 | ✗ | Design |
In summary, Novartis’ results suggests that MMF and MPS are comparable. Similar conclusions were made in the current HTA review in head-to-head studies. In addition, the submission suggested the use of EVL in early CNI minimisation. The NMA results of the current HTA review did not suggest that EVL regimens were better in reducing mortality or graft loss and improving GRF than all other treatments. However, the EVL + MPS regimen was estimated to be less effective than the MMF + CSA regimen in reducing the odds of BPAR. In addition, the EVL + CSA regimen was estimated to be more effective than the CSA + AZA regimen in reducing the odds of BPAR. However, apart from the CSA + AZA and EVL + MPS regimens performing poorly in some comparisons, it is difficult to say that any one treatment is more effective than another, as the 95% CIs are very wide.
Chapter 4 Assessment of cost-effectiveness
Review of cost-effectiveness evidence
The purpose of this section of the report is to review existing evidence on the cost-effectiveness of immunosuppressive regimens [BAS and rATG as induction therapies, and immediate-release TAC, TAC-PR, MMF, MPS, SRL, EVL and BEL as maintenance therapies (including a review of TA85)], in renal transplantation in adults.
Methods
Searches
Bibliographic literature searching was conducted on 8 April 2014. The searches took the following form: (terms for kidney or renal transplant or kidney or renal graft) AND (terms for the interventions under review) AND (a costs or economic literature search filter). The search was date limited 2002–current, in line with the previous assessment, and the searches were updated on 18 November 2014. The search was not limited by language and it was not limited to human-only studies.
The following databases were searched: MEDLINE (via Ovid), EMBASE (via Ovid), NHS EED (via Wiley Online Library), Web of Science (via ISI – including conference proceedings), HEED (via Wiley Online Library) and EconLit (via EBSCOhost). The search strategies are recorded in Appendix 1.
Screening
Inclusion and exclusion criteria were the same as for the clinical effectiveness systematic review (see Chapter 3, Inclusion and exclusion criteria), with the following exceptions (as specified in the appraisal protocol):
-
Non-randomised studies were included (e.g. decision-model-based analyses, or analyses of patient-level cost and effectiveness data alongside observational studies).
-
Full cost-effectiveness analyses (CEAs), cost–utility analyses and cost–benefit analyses were included. (Economic evaluations that report only average cost-effectiveness ratios were included only if the incremental ratios can be easily calculated from the published data.)
-
Studies that measure only costs but not health benefits were excluded except for stand-alone cost analyses from the perspective of the NHS.
-
Only economic evaluations from the UK, USA, Canada, Australia and Western Europe were included, as these settings may include data that are generalisable to the UK.
Titles and abstracts were screened for relevance by two reviewers (RMM and LC), with disagreements resolved by discussion. Full texts were retrieved for references that were judged to be relevant, and screened for eligibility by the same reviewers, with disagreements resolved by discussion.
The bibliographies of review articles that were not judged to be eligible for inclusion were examined by one reviewer (LC) to identify other potentially relevant references. These references were retrieved and checked for eligibility in the same way as full texts from database searches.
Quality assessment
Studies meeting the criteria for inclusion were assessed by one reviewer (RMM) using the checklist developed by Evers et al. 305 (Table 132). When studies are based on decision models they will be further quality assessed using the checklist developed by Philips et al. 322,323
| Number | Item | Jürgensen 2010,306 2015307 | Earnshaw 2008308 | Orme 2003309 | McEwan 2005,310 2006311 | Woodroffe 200565 | Crompton 2003312 | Emparan 2003,313 2006314 | Chilcott 2002315 | Walters 2003316 | Popat 2014317 | Muduma 2014318 | Craig 2002,319 Lazzaro 2002320 | Abecassis 2008321 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I&M | I&M | I&M | I&M | I&M | Ind | Ind | Ind | Ind | I&M | I&M | I&M | I&M | ||
| 1. | Is the study population clearly described? | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N |
| 2. | Are competing alternatives clearly described? | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 3. | Is a well-defined research question posed in answerable form? | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| 4. | Is the economic study design appropriate to the stated objective? | N | Y | N | Y | Y | N | N | N | N | N | Y | N | N |
| 5. | Is the chosen time horizon appropriate to include relevant costs and consequences? | N | N | N | Y | N | N | N | N | N | N | Y | N | N |
| 6. | Is the actual perspective chosen appropriate? | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | ? |
| 7. | Are all important and relevant costs for each alternative identified? | N | N | Y | N | Y | N | N | Y | Y | N | ? | N | N |
| 8. | Are all costs measured appropriately in physical units? | Y | N | N | Y | ? | ? | ? | N | Y | N | ? | ? | N |
| 9. | Are costs valued appropriately? | Y | ? | Y | Y | ? | N | ? | ? | Y | ? | ? | ? | N |
| 10. | Are all important and relevant outcomes for each alternative identified? | N | N | N | N | N | N | N | N | N | N | ? | N | N |
| 11. | Are all outcomes measured appropriately? | N | N | N | N | ? | N | N | Y | Y | N | ? | N | N |
| 12. | Are outcomes valued appropriately? | N | X | N | N | ? | N | N | N | N | N | ? | N | N |
| 13. | Is an incremental analysis of costs and outcomes of alternatives performed? | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y/N | Y | Y |
| 14. | Are all future costs and outcomes discounted appropriately? | Y | Y | N | Y | ? | NA | NA | NA | NA | NA | Y | NA | Y |
| 15. | Are all important variables, the values of which are uncertain, appropriately subjected to sensitivity analysis? | Y | Y | Y | Y | N | N | N | Y | Y | N | ? | N | ? |
| 16. | Do the conclusions follow from the data reported? | Y | Y | Y | Y | Y | Y | N | Y | N | Y | ? | N | Y |
| 17. | Does the study discuss the generalisability of the results to other settings and patient/client groups? | N | N | N | N | N | N | N | Y | Y | N | N | N | N |
| 18. | Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | N | N | N | N | Y | N | N | N | N | Y | N | N | N |
| 19. | Are ethical and distributional issues discussed appropriately? | N | N | N | N | N | N | N | N | N | N | N | N | N |
Synthesis
Economic studies were summarised and synthesised using tabulated data and narrative synthesis.
Results
Identified studies
The electronic database search for cost-effectiveness evidence identified 2241 records. After deduplication, 1378 records remained, all of which were screened by title and abstract. Of these, 86 full texts were assessed for eligibility. Nineteen full texts were deemed to meet the eligibility criteria for the review. The study selection process is detailed in Figure 64.
FIGURE 64.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart. CUA, cost–utility analysis. a, Includes studies reporting UK costs and effects without economic evaluation, and stand-alone cost analyses based in the NHS.
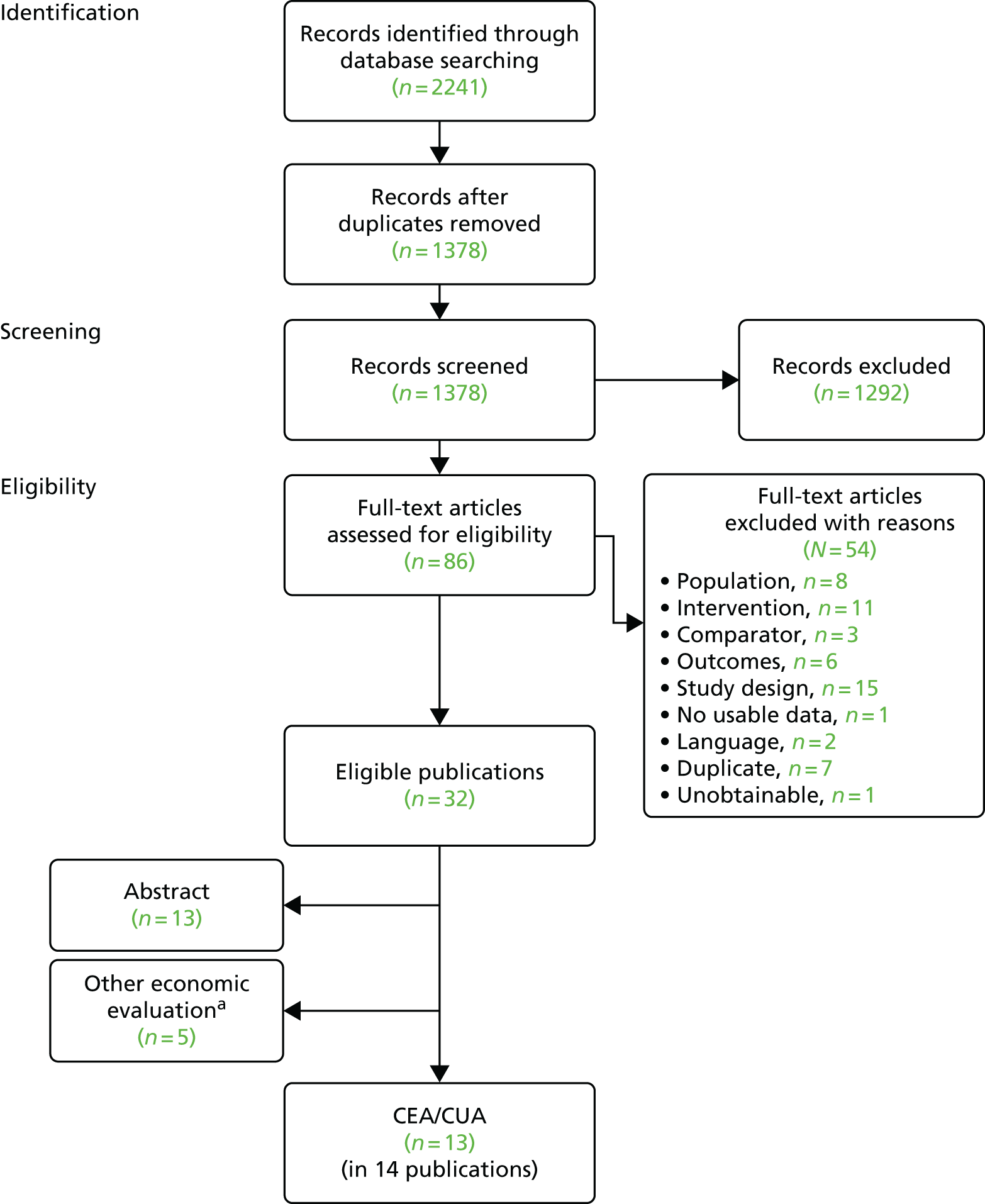
Twelve economic evaluations were included in the review (published in 14 publications65,306,308–316,319–321). Update searches, conducted on 18 November 2014, yielded an additional six reports42,307,318,324,325 on economic evaluations eligible for inclusion in the review. Of these, one report307 was an update on a study identified by the original search, and another three reports318,324,325 constituted multiple reports on a newly identified study.
Thirteen studies65,307–310,312,314–319,321 were included in this review. Five were studies of induction regimens,312,314–317 three of which were studies of UK adults,315–317 and 10 were studies of initial and maintenance immunosuppression,65,307–310,318,319,321 four of which were of UK adults. 65,309,310,318 In what follows, studies of induction regimens are reviewed before reviewing studies of initial and maintenance immunosuppressive regimens, by country setting (UK vs. other). Table 133 describes the characteristics of included studies of induction regimens. Table 134 describes the characteristics of included studies of initial and maintenance regimens. All studies but one were sponsored by the industry or co-authored by an individual person who was affiliated with a company manufacturing or commercialising one of the evaluated treatments.
| Author | Country | Regimens | Population | Study type | Perspective | Outcomes considered | Horizon | Model based? | Sponsor |
|---|---|---|---|---|---|---|---|---|---|
| Chilcott 2002315 | Seven countries (EU), including UK, and presents results by country | BAS + CSA + CCSs vs. PBO + CSA + CCSs | Adult renal transplant recipients (mean age 47.4 and 47.0 years) | Cost (alongside trial) analysis | Hospital | Aggregate mean total cost of resources per patient Cost per suspected rejection episode |
1 year | No | Funded by Novartis |
| Crompton 2003312 | USA | BAS vs. no BAS (given with CSA + AZA + CCSs) | Adult renal transplant recipients | CEA | Not stated | AR; graft and patient survival; GRF; incidence of infection, malignancy | 1 year | No | NR |
| Emparan 2003,313 2006314 | Spain | BAS + CSA BAS + CSA + MMF TAC + MMF (CCSs tapering for all) |
Old-to-old renal transplant recipients (mean age 69.3 and 68.2 years) | CEA | Not stated | GRF; rejection at 1 year; survival at 1 year; dialysis required; CRC; cost difference | 1 year | Unclear | NR |
| Popat 2014317 | UK | IL2Mab (BAS or DAC) vs. ATG (given with CSA + MMF + CCSs; a minority given TAC + MMF + CCSs) | Adult renal transplant recipients from donors after cardiac death (mean age 48 and 54 years) | CEA | Hospital | Patient survival Death censored graft survival |
1 year | No | Supported by Genzyme |
| Walters 2003316 | UK | BAS + CSA + CCSs vs. PBO + CSA + CCSs | Adult renal transplant recipients, adults aged 18–70 years | Cost (alongside trial) | NHS | Aggregate mean total cost of resources per patient Cost per treatment failure avoided |
6 months | No | Funded by Novartis |
| Author | Country | Regimens | Population | Study type | Perspective | Outcomes considered | Horizon | Model based? | Sponsor |
|---|---|---|---|---|---|---|---|---|---|
| Abecassis 2008321 | USA | TAC b.i.d. + MMF vs. TAC o.d. + MMF | Renal transplant recipients – no age reported but the Vincenti et al. 2002 paper,326 on which this is based, has adults | CEA | Not stated | Incidence of AR; graft survival; costs (drug cost; graft loss; transplantation; mortality); total costs | 5 years | Yes | NR – of note, one author Astellas Pharma |
| Juergensen 2010306 and 2015307 | Germany | SRL + CCSs (CSA withdrawal) SRL (CSA minimisation) EVE (CSA minimisation) TAC (low-dose) MMF + CCSs |
Renal transplant recipients – age not stated | CEA | SHI perspective | Cost per life-year gained Cost per with functioning graft gained |
24 months, 120 months | Yes | Funding source not reported (COI are reported) |
| Lazzaro 2002;320 Craig 2002319 | Austria, Belgium, Germany, Italy, Luxembourg, Spain and Switzerland | TAC + AZA + CCSs CSA + AZA + CCSs |
Adult renal transplant recipients | CEA | Italian hospital perspective | Cost per patient with a functioning graft Cost per surviving patient |
12 months | No | Supported by an unrestricted grant from Fujisawa GmbH Munich Germany |
| McEwan 2005,310 2006311 | UK | SRL vs. TAC SRL vs. CSA |
Renal transplant recipients – mean age 45.9 years | CUA, CEA | NHS and PSS | Mean time to graft failure and mean life expectancy converted to health utility Cost/QALY |
10 years, 20 years | Yes | NR – of note, one author employee Wyeth Laboratories |
| Muduma 2014318 | UK | TAC (Advagraf), TAC (Prograf), BEL, CSA SRL CNI minimisation SRL CNI avoidance (all given with MMF + CCSs) |
Renal transplant recipients – age 45 years | CUA | NHS and PSS | BPAR Retransplants Life-years Cost/QALY |
25 years | Yes | Funded by Astellas |
| Orme 2003309 | UK | CSA + AZA + CCSs vs. TAC + AZA + CCSs Given with induction TAC or CSA pretransplant and methylprednisolone + AZA perioperatively |
Adult renal transplant recipients – based on Jurewicz 2003327 | CEA | UK Transplant Unit | Cumulative cost Cost per survivor Cost per patient with functioning graft Cost per patient rejection free |
10 years | Yes | Funded by Fujisawa |
| Earnshaw 2008308 | USA | SRL + CCSs; MMF + CSA + CCSs; MMF + TAC + CCSs | Adult renal transplant recipients – mean age 45.89 years | CUA CEA |
Not stated | Serum creatinine; immunosuppressive drug and other medical costs; life-years gained; QALYs | Lifetime | Yes | Wyeth Pharmaceuticals |
| Woodroffe 200565 | UK | TAC vs. CSA with: a. AZA + CCSs b. MMF + CCSs MMF vs. AZA with: a. TAC + CCSs b. CCSs + CCSs |
Adult renal transplant recipients | CUA | NHS and PSS | Costs QALYs |
10 years | Yes | NIHR HTA programme – NICE |
Induction therapy
UK studies
Walters et al. 2003
In a multi-European country RCT, BAS induction was compared with PBO in people who were given triple therapy with CSA, AZA and steroids. 316 Information on costs of immunosuppressant drugs, hospitalisations, procedures, outpatient visits, laboratory tests, renal biopsies, concomitant medications, dialysis and nephrectomy was prospectively collected for the trial follow-up period of 6 months. Retransplantation costs were not included. A cost-effectiveness analysis (CEA) conducted alongside the trial included all costs up to 6 months and the costs of dialysis up to 12 months. This analysis adopted a NHS hospital perspective; it pooled the data on clinical outcomes and resource utilisation from all countries and people involved in the trial (n = 340) but evaluated resource use using UK national and local unit costs (1997–9 prices).
Basiliximab was found to reduce the incidence of first confirmed AR episodes by 6 months (absolute risk reduction 0.14). The rate of graft failure with BAS was 11% and 18% in the PBO arm (p = 0.24). The mortality rate was 2% and 3%, respectively (p = 1.00). In terms of the number of people with AEs or infections reported as serious, the comparisons had p ≥ 0.65.
In terms of costs, hospitalisations were the largest element of the total, followed by dialysis and AR. Comparisons by resource-use category between arms had all p ≥ 0.05. Over the 6-month period post transplantation, BAS had an incremental cost of £231 (95% CI –£1983 to £2446). Including the 6–12 months costs of dialysis, the BAS had an incremental total costs of –£30 (95% CI –£2326 to £2686). In the 6-month period post transplantation, the incremental costs per case of treatment failure (i.e. no AR, graft failure or death) avoided with BAS was £1650.
The authors found that, despite the fears of increased AEs from overimmunosuppression, BAS given with triple therapy resulted in fewer ARs and no difference in costs relative to PBO in the first 6 months.
The study provides valuable evidence of data on resource use and short-term outcomes of induction therapy with BAS. For our present purposes, the main limitation of this study is the lack of relevant comparators, such as induction with rATG. Further, as the authors point out, the use of these regimens in combination with triple-therapy immunosuppressive regimens commonly used in recent years, in particular a CNI with MMF and steroids, would have added relevance to the study.
The authors do not include the costs of retransplantation in their 1-year analysis, despite including the costs of dialysis. They also do not provide any evidence of the impact of induction on HRQoL. In addition, an attempt to investigate the potential long-term implications of ARR prevention with BAS is warranted, using the framework linking biomarkers to longer-term patient and graft survival outcomes using a predictive model.
A major limitation of the study is the fact that the quantities of resource utilisation were derived from a sample of people being treated in the UK and 11 other countries. 316 The authors acknowledge that important differences may exist between these countries, as evidenced by the length of hospital stay such that ‘whereas prevention of early episodes of AR may save a readmission in the US, this would not necessarily lead to an earlier hospital discharge following transplantation in some of the countries involved in this study (e.g. Israel, Poland, Turkey)’ (Walters et al. 316). This limits the validity of the results of this study, which was designed from an English NHS perspective.
Chilcott et al. 2002
In a separate study of a similar design to that used in the study by Walters et al. ,316 Chilcott et al. 315 compared the costs of renal immunosuppression in centres in Canada and six European countries, including the UK. The study followed people for 12 months and, unlike the study by Walters et al.,316 which calculated costs for the UK using pooled resource utilisation data from all countries, only resource utilisation data from each country were used to estimate the respective costs. Country-specific unit costs were adjusted for purchasing power parity (PPP) to reflect the actual opportunity costs of health-care resources in each country. 315
The study involved 376 people (BAS, n = 190; PBO, n = 186) and, as Walters et al. 316 had found for 6-month post-transplantation outcomes, observed that BAS reduced the rate of (suspected) ARs (BAS 37%, PBO 54.8%; absolute risk difference (ARD) –16.9, 95% CI –29 to –4] without affecting graft loss (ARD –1.3, 95% CI –8.1 to 5.4) and patient survival (ARD 2.0, 95% CI –1.8 to 5.9) at 12 months. The authors report that no retransplantations were recorded in any group over the 12-month post-transplantation period studied.
Tests of differences in resource quantities used between the trial arms were all associated with p > 0.05. The costs estimates were reported in terms of PPP US dollars (US$,1996 prices). After converting them back to PPP pounds sterling (£) using the £0.4 = US$1 conversion rate provided by the Chilcott et al. 315 study (see Table 141),315 the mean total per-patient cost in the BAS arm was £19,174 and £18,510 in the PBO arm (difference £664, 95% CI –£1660 to £2944). The incremental cost per suspected case of AR avoided at 12 months post transplantation was £3929. In addition, and unlike the similar study by Walters et al. ,316 the study by Chilcott et al. 315 presents total cost estimates for the subgroup of UK adults (n = 37) in the trial. (The report presents these figures only in chart form; Chilcott et al. ,315 figure 4). The total incremental cost of BAS over 12 months is approximately £3500. This implies an incremental cost of £8284 per suspected case of AR avoided. Despite the sampling uncertainty in the subgroup analysis by country, results presented in figure 4 of the report by Chilcott et al. 315 suggest heterogeneous findings across countries.
A similar critique applies to this report as that formulated above for the report by Walters et al. ,316 with a couple of qualifications. First, Chilcott et al. 315 present results for the subgroup of UK adults. Although these results are based on small numbers, they suggest possible heterogeneity of findings across countries, as the point estimate of incremental costs of BAS range from almost US$0 in Germany and France to US$3500 in the UK, to US$10,000 in Belgium and Switzerland (Chilcott et al. ,315 figure 4). A second strength of the Chilcott et al. 315 study relative to the Walters et al. 316 study lies in its longer period of follow-up, during which information on all costs was collected, 12 months post transplantation, compared with the 6-month period of Walters et al. 316 study (the latter also included costs for a 6-month extension period, but only for dialysis).
Popat et al. 2014
This recent study317 reports evidence of costs and health outcomes that were associated with two immunosuppressive induction therapies given to recipients of renal transplants from DCD in a single centre in London. This was a before-and-after comparison of 1-year outcomes after transplantation, between a anti-interleukin-2 receptor monoclonal antibody (IL2Mab) induction regimen (BAS or DAC) given to people receiving a renal transplant from January 2007 to July 2008 and induction with ATG given to renal transplantation people starting from the time of its adoption at the centre in August 2008 to August 2009.
The study included 24 adults in the old induction arm (IL2Mab 2 mg/kg) who had a mean age of 54.3 years compared with 48.0 years in the new (ATG 3.75 mg/kg) induction group of 21 adults. There was some imbalance in terms of sex and race, as 71% in the IL2Mab group were male compared with 38% in those given ATG, and 62% in the former group were white compared with 33% in the latter group. Forty-two out of 45 people were given standard immunosuppression with CSA, MMF and prednisolone, and 3 out of 45 were given TAC, MMF and prednisolone. At 1 year post transplantation, 91.7% of people in the IL2Mab group were alive, whereas at 3 years 83.4% survived. In the ATG group all people were alive at both time points. In terms of graft survival (censored by death), all people in both groups had a functioning graft at 1 year, whereas 95.8% had a functioning graft at 3 years in the IL2Mab group compared with 95.2% in the ATG group. The authors interpreted these results as evidence of no significant differences in patient and graft survival.
The study also looked at DGF, the duration of DGF measured by the number of HD sessions, the rate of BPAR and incidence of infections requiring hospital admission. ATG resulted in 42.8% of people having DGF and 62.5% of people treated with IL2Mab experienced such outcome (p = 0.08). More people required HD sessions, experienced BPAR, had infections requiring admission, were readmitted and had experienced CMV infections in the latter group than in the former group (p ≤ 0.03 for all of these comparisons).
The study reported a cost analysis associated with observed outcomes up to 12 months post transplantation, using local NHS unit costs for hospital bed-day and HD sessions and British National Formulary (BNF) drug prices for induction and maintenance immunosuppression, which were applicable at the time people received the transplant. Their results are converted to per-patient costs and presented in Table 135.
| Cost category | IL2Mab arm (£) | ATG arm (£) |
|---|---|---|
| Immunosuppression (acquisition costs) | 1729 | 2250 |
| Inpatient bed-days post transplantation | 6967 | 4552 |
| Inpatient bed-days for readmission | 2867 | 933 |
| HD sessions | 836 | 494 |
| CMV prophylaxis and treatment | 1954 | 2229 |
| Clinic visits | 6967 | 4465 |
| Total cost per patient at 1 year post transplanta | 18,929 | 14,904 |
Antithymocyte (immune)globulin was found to result in savings in inpatient bed-days post transplantation and those due to readmissions, as well as HD costs and clinic visits, whereas the additional costs of ATG induction (£479 per patient, calculated by PenTAG) were not found to be statistically significant. The drivers of the cost savings by ATG were found in the inpatient bed-days after transplantation and clinic visits.
The main contribution of this study is to provide evidence on health and economic outcomes in a comparison of two active induction regimens. Owing to its small size, the results may be influenced by outliers, thus limiting the validity of the reported findings. In addition, lack of power is of concern for statistical inference of differences in health outcomes, and more so for inference on costs, which tends to require larger samples than those required by studies of clinical effects. 328 Moreover, results may be confounded by the fact that the IL2Mab arm was treated at an earlier date than the ATG arm; some of the difference in costs may be because of different discharge practice across the two periods, as opposed to an effect of the induction regimen.
The importance of clinic visits as a driver of total costs found in this study is consistent with evidence submitted to NICE by the company sponsoring one of the drugs being evaluated for this appraisal (Bristol-Myers Squibb), on post-transplantation costs in standard practice from the renal transplant database in Cardiff, Wales. The same finding is analysed in an international context in a published report42 of the same evidence. Nevertheless, evidence from a larger study is required to confirm the findings reported by Popat et al. ,317 in which induction regimens are given in combination with current triple therapy, that is, low-dose TAC with MMF and steroids, and relevant outcomes not measured in their study, especially HRQoL outcomes, are measured.
Non-UK studies
In a US study by Crompton et al. ,329 54 living donor transplant recipients were randomised in a 1 : 1 ratio to receive BAS induction or no induction, and all were given triple immunosuppressive therapy with CSA ME, AZA and CCSs. At 12 months post transplantation, the rate of AR episodes in the induction intervention arm was 22% compared with 15% in the control (p > 0.05). Differences between arms in serum creatinine measured at 1, 2, 3, 6 and 12 months all had p > 0.05, and no AEs were associated with BAS. Four graft losses occurred during follow-up, all in the intervention arm; it was stated that only one was immunological but no additional information was reported. The study329 evaluated differences in resource use, using charges as opposed to economic costs of the resources consumed. BAS provided no clear clinical benefit or evidence of being cost-effective in this low-risk patient population. However, insufficient numbers of people were included in the study to allow one to derive conclusive findings. Another limitation is its use of BAS in people receiving triple therapy of CSA with AZA and steroids, instead of current standard regimens combining CNI, MMF and steroids.
A study330,331 from Spain investigated two regimens of BAS induction: (1) a CNI-avoidance regimen (CSA 8 mg/kg daily was introduced when the creatinine level reached a value of < 3 mg/dl) and (2) a CNI-minimisation regimen (CSA 4 mg/kg daily with MMF 500 mg/12 hours from day 1). The regimens were compared against a TAC (Prograf 0.3 mg/kg daily with a trough level of 8–12 ng/ml) with MMF (500 mg/12 hours) and steroids regimen in elderly people. The reports identified for this study330,331 provided Markov model simulated costs and health outcomes for eight people in each of options ‘1’ and ‘2’, and 15 people for the TAC comparator up to 1 year post transplantation, but were only in summary form and lacked information on methodology related to model structure, cost definition, sources and values of unit costs, and effectiveness parameters to allow critical appraisal of the reported cost difference relative to TAC arm (–€8355 for option ‘1’ and –€5695 for option ‘2’).
Initial and maintenance immunosuppression studies
UK studies
Orme et al. 2003
Orme et al. 309 compared the costs and clinical outcomes of TAC (Prograf) vs. CSA ME given in triple-therapy regimens including AZA and CCSs. Their study309 was based on data from the direct comparison of these regimens in a RCT that was conducted at a single centre in Wales, in which clinical and resource-use data were collected prospectively for each patient over a median follow-up of 2.7 years (maximum 4 years). People in the trial had undergone renal transplantation between 1996 and 2000 (CSA, n = 89; TAC, n = 90). The resource items for which data were recorded in the study included number of days in specialised wards (transplant/nephrology and intensive care unit during the initial admissions and subsequent readmissions), number of dialysis sessions required in cases of a DGF, number of diagnostic tests (e.g. transplant biopsy, ultrasound scan and other radiological investigations), and minor surgical procedures and operations for complications. The economic evaluation adopted a 10-year analytical horizon and extrapolated the trial outcomes from 5 to 10 years using patient and graft survival data from the UK Transplant Support Service Authority Audit. During the extrapolated period, the rates of change in patient and graft survival rates were assumed to be the same between the TAC and CSA immunosuppressant regimens. The analysis also assumed that ARRs changed by the same rates as graft survival rates for the extrapolation phase of the analysis. The per-patient costs for years 4–10 were extrapolated using an average of annual costs with functioning graft and costs with graft failure (dialysis) in the trial, weighted by the proportion of people surviving with a function graft at the end of the year.
According to ITT analysis at 4 years, 89% of people survived in the TAC arm and 80% survived in the CSA arm. In terms of graft survival, the figures were 81% and 71%, respectively. The proportion of people who were rejection free was observed to decline annually for the first 4 years of CSA by 48 percentage points in year 1, 5 in year 2, 2 in year 3 and 1 in year 4, and by 37 percentage points in year 1, 4 in year 2, 1 in year 3 and 4 in year 4 with TAC. In terms of costs, the observed per-patient costs in the first year post transplant were £9990 under TAC compared with £9783 under CSA. In the observed years 2–4, the TAC arm had lower per-patient costs, from £133 to £350 less than in the CSA arm as a result of the higher proportion of people with a failed graft and receiving dialysis in the latter. The study309 presented results in terms of incremental cost per additional survivor, per extra patient with a functioning graft, and per rejection-free patient. Although the number of years of life achieved after transplantation under each treatment was not presented, PenTAG approximated them by numerical integration using Newton–Cotes methods (Simpson’s rule332) from the percentages of people alive at the end of each of the 10 years of analysis reported by the study. This yielded an estimated 8.28 life-years under TAC and 7.61 life-years under CSA. The information provided in the paper also allowed us to adjust the cost discounting to convert results from the 6% annual rate used by the study309 to the current NICE recommended rate of 3.5%. Similarly, methods were used to approximate discounted life-years at that rate. The resulting discounted incremental cost per life-year gained by TAC over CSA was £1457.
This study309 reported detailed unit cost information, although quantities of resource utilisation were not provided, which limits the ability to assess the generalisability of results to England. This is regrettable, as this is one of the studies with the longest prospective follow-up of health-care use and health outcomes in KTRs, and thus a potential source of longitudinal data on quantities of resource use and their interpatient variability. Further, the study did not account for HRQoL effects of immunosuppression and did not consider the importance of outcomes in terms of renal function for costs and benefits. In particular, there is emerging evidence that CKD stage not only matters for current costs and HRQoL experienced by the patient, but also has an important role as a prognostic factor and determinant of graft survival. 333 It is also noted that the time horizon of the analysis may now be too short to estimate cost adequately. Despite the inadequate measure used to synthesise cost-effectiveness in the study report, our calculations suggest that in the sample studied by Orme et al. ,309 TAC is well within the NICE threshold of cost-effectiveness. Although we did not adjust prices to current levels, these are unlikely to rise beyond £5000 the incremental cost-effectiveness ratio (ICER) per quality-adjusted life-year (QALY) gained in this sample of TAC compared with CSA.
Woodroffe et al. 2005 (Assessment Group for NICE Technology Appraisal Guidance 85)
Based on its review of models submitted by four sponsoring companies for the NICE TA85, the assessment group at Birmingham University performed an analysis based on the model submitted by Novartis, based on the information in the industry submissions and its own systematic review of the published evidence on effectiveness and cost-effectiveness. 65 The Novartis model simulated the experience of individual people after renal transplantation, represented by transitions between health states defined by AR, no AR, hospital dialysis, PD and death. It included a model component that captured the effects on clinical outcomes of NODAT, which allowed accounting for the clinical implications of the high incidence of NODAT with TAC that the company found in its systematic review. The model also accounted for cause-specific mortality risks from five comorbidities that were associated with diabetes mellitus or other causes. Costs and utilities were specific to each health state. TAC was found to have incremental costs per QALY ratios in the range of £59,548–166,112 relative to CSA when evaluated as candidate components of triple therapy containing AZA and CCSs. Larger ICERs were found for the comparison in the context of triple therapy comprising MMF and CCSs. For the comparison of MMF and AZA, the ICER ranged from £39,297 to dominated, when evaluated alongside TAC and CCSs, and from £52,166 to £109,549 as part of triple therapy containing CSA and steroids. The authors refer to these ranges as 95% CIs but, as these did not account for the variation in costs, they are likely to misrepresent uncertainty.
Difficulties encountered by the Birmingham Assessment Group in implementing its analysis thus prevented it from satisfactorily accounting for uncertainty. 65 The group could obtain 95% CI for incremental QALYs but not for costs, and thus the degree of uncertainty in its results was left unaddressed. A more fundamental problem arises, however, with the use of a model, such as that of Novartis, which assumes that the main clinical outcomes, that is, the years of patient life and with a functioning graft gained, are adequately predicted by short-term ARRs and post-transplant diabetes mellitus (PTDM). In recent years, evidence has emerged suggesting that renal function is a predictor of clinically and economically significant outcomes, and that AR may be less relevant once CKD stage is accounted for. 333–335 CEAs published since the Birmingham Assessment Group’s review was conducted, and reviewed in the rest of this chapter, reflect these methodological developments, as summarised in Table 133. At the time of the Birmingham review, the evidence was ambiguous about the prognostic predictive power of renal function relative to AR and, as the group acknowledges, its analysis reflects this (Woodroffe et al. 65). 333–335
McEwan et al. 2005, 2006
In two papers, McEwan et al. 310,311 evaluate the cost–utility of SRL against CSA, and SRL against TAC, for maintenance immunosuppression from the NHS perspective using a discrete event simulation model of individual patient evolution from the time of kidney transplantation until 20 years post transplant. 310 This study310,311 was one of the first to account for renal function as a predictor of transplant outcomes. It simulated the monthly evolution of a patient’s health status by transitions between three mutually exclusive health states: (1) patient with a functioning graft; (2) patient with failed graft (dialysis); and (3) death. In addition, AR events were accounted for. The model allowed for retransplants and different probabilities of experiencing an AR, patient death, graft failure and transplant after graft failure, depending on the number of transplants that the patient had received at each point in time. Movements between health states were associated with changes in costs and HRQoL, whereas the occurrence of transplant, graft failure, and ARs and graft failure was associated only with costs.
The effects of SRL and CSA on clinical outcomes were assumed to occur through their effects on renal function, which determined long-term clinical outcomes independently of treatment. The relative efficacy of SRL compared with CSA was derived from a single trial involving 430 people from 57 centres in Europe, Canada and Australia (the Rapamune Maintenance Regimen Study, Oberbauer et al. 336). People included in this trial were given the same immunosuppression regimen (CSA + SRL + steroids) for the first 3 months after transplantation and then randomised to continue on the regimen or switch to a regimen of once-daily SRL and steroids. Serum creatinine values in each trial arm at the time of randomisation, that is, 3 months post transplantation, and at 1, 2 and 3 years, were used as inputs (surrogate measures) in estimated equations for predicting the risk of long-term clinical events (Figure 65). The authors also assumed that in 50% of subjects treated with SRL, graft survival ‘would prevail for the entire time horizon’. 310
The surrogate relationship between renal function and clinical events defining transitions between health states in the model was estimated from analysis of longitudinal data on outcomes experienced by 937 transplant patients up to 20 years post transplantation in routine practice, recorded at the University Hospital of Wales, Cardiff (see Appendix 9 for details). People were treated over the period 1982–2001, during most of which CSA was the standard immunosuppressant therapy. 310
The authors found that SRL regimen would cost the NHS £62,120 per patient over 20 years, whereas CSA would cost £69,525 (at 2003 prices and 6.5% annual discount rate). SRL was found to result in more discounted years with a functioning graft and in 0.16 additional discounted life-years per patient; it also resulted in more QALYs than those achieved with CSA. These results were based on the assumption that 50% of SRL patients would maintain their graft survival over the entire modelled period; when this value was set to 0%, the incremental cost per QALY gained by SRL was £51,778 under the 10-year horizon and £11,161 under the 20-year horizon. The same analysis was performed for the comparison of SRL and TAC,311 using the creatinine levels observed in people receiving CSA in the Rapamune trial336 as proxies for creatinine levels in people receiving TAC in the model, and replacing the price of CSA with that of TAC. The results were qualitatively similar, with SRL both saving costs and producing health benefits relative to TAC.
The main strength of the study310 is its account for the effect of renal function on long-term outcomes and use of probabilities of clinical events from observational data of people treated in routine practice. Further, it is the only study to have accounted for the temporal variation in risk factors for those events over a 20-year period. However, the internal validity of the results is questionable because of the differences between the trial population on which the efficacy data were based and the patient population of the model, as detailed in Appendix 9. In addition, this study310 did not account for the incidence of clinical conditions, such as malignancy, cardiovascular events and NODAT. This is an important limitation in the light of the expected benefits of SRL on malignancy. Most important, however, are the safety concerns (increased death risk) associated with the drug, which suggest that SRL may not be justified in people who have kidney transplants other than those who are at high risk of cancer. 337 It must also be noted that, although the study accounts for the role of renal function as a predictor of long-term outcomes, it does not allow for its impact on costs42 and HRQoL. 338
Muduma et al. 2014
In a recent study,318 the current UK standard treatment for adults, twice-daily immediate-release TAC (Prograf), was compared with current options, namely CSA ME, SRL with CNI minimisation, SRL without CNI, BEL and 1-day TAC-PR (Advagraf), in terms of cost-effectiveness from the perspective of the NHS. The analysis considered each of these treatment options as part of a regimen that also included MMF and CCSs, and BAS induction (consisting, in the base case, of 20 mg 2 hours before surgery and 20 mg 4 days after surgery; an alternative scenario considered additional doses during the first few days after transplantation). The study found that, although Prograf resulted in more efficient use of health-care resources relative to CSA ME and BEL, it was not cost-effective relative to SRL. Although Advagraf produced lower costs and higher benefits than Prograf, its cost-effectiveness ratio against SRL (CNI minimisation regimen) was £58,350. These results were found to be sensitive to the time horizon and the effect of adherence.
Costs and health benefits were accumulated according to a Markov model of annual cycles that represented the evolution of the patient heath status following a successful transplant for up to 25 years. The model included four health states: (1) functioning graft without a history of BPAR; (2) functioning graft with a history of BPAR; (3) non-functioning graft; and (4) death. The occurrence of repeat transplantation was modelled using a tunnel state. The model assigned an excess risk of graft loss for the state of functioning graft with prior BPAR relative to the functioning graft without prior BPAR state, using estimates derived from the literature. The model was specified so that BPAR could occur only in the first year after transplantation, which the authors justified on pragmatic grounds, given the limited data available from the literature on BPAR outcomes beyond 1 year.
This study318 did not report adequate information on the methods and results of that review, the primary study sources for the probabilities of AR used, or the actual values used for these parameters. The treatment-specific outcome data reported related to the advantage of Advagraf over Prograf in terms of adherence to treatment schedule. The differences in 1-year ARRs were used to predict patient and graft survival for the first 5 years post transplantation using data from UK renal transplant summary statistics12 and patient survival for the first 10 years after the start of the spell on dialysis were populated using UK data; the probabilities of retransplantation while in dialysis were obtained from data reported by McEwan et al. ,310,311 reviewed in this chapter. 339 Exponential curves were used to extrapolate patient and graft survival curves and survival time on dialysis to 25 years. Further details of this study are discussed in Appendix 9.
Despite its stated aim to comply with the NICE reference case specifications, this study318 faced limitations in terms of the availability of data to do so, the adopted model structure, issues of model implementation and the quality of reporting. The model assumed that the cost-effectiveness was driven by the differences in the rate of AR between treatment regimens, and that these fundamental differences occurred only during the first year post transplant. The validity of this assumption and the results of this study hinge on the quality of the evidence on the relationship between AR and graft and patient survival. In any case, it is difficult to defend the extrapolation of 1-year surrogate measures to clinical outcomes 25 years into the future, as generated by the statistical model of AR and graft survival in this study. Another problem with this report is its lack of any information on the values of the parameters driving the results, that is, the relative differences in the risk of AR between regimens. This fact makes it impossible to replicate the results reported by the paper. Third, based on the information provided, it appears that the amount of immunosuppressant use in the model might not have reflected the actual total use of the medications that brought about the AR outcomes which were used to populate the effectiveness model parameters. The authors do not report any attempt to derive mean daily drug use or dose intensity from the RCT data from which the AR estimates were derived for populating the model. Another issue arises with the way in which transition probabilities were derived from the registry data on transplant and patient survival. As this issue is discussed for one of the industry submissions, which used the same data and model, the reader is referred to that section (see Chapter 5, Astellas’ submission).
Non-UK studies
Three identified reports investigated the cost-effectiveness of SRL regimens: one report in the USA308 and two in Germany. 307,311 Two studies319,320 evaluated TAC compared with CSA ME in European countries. One study321 investigated once-daily TAC compared with twice-daily TAC in the USA. 321 These studies are discussed in detail in Appendix 9. Their model characteristics and results are presented in Tables 136–138.
| Study | Population | Comparators | Horizon | Model structure | Surrogates to model long term | Health states/events modelled | Risk factors | AEs | Key factors (sensitivity analysis) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Abecassis 2008321 | Adult, USA | TAC b.i.d. + MMF vs. TAC o.d. + MMF | 5 years | Markov – stochastic state-transition model | Graft loss |
|
Retransplant – reduction in five-graft survival relative to original graft | None | Rate of relative non-adherence between once-and twice-daily TAC | Renal transplant recipients – no age reported but triala on which clinical parameters are based was conducted in adults |
| Woodroffe 200565 | Adult, UK | DAC, BAS, TAC, MMF, MPS, SRL | 10 years | Meta-model of simulation model outputs | AR impact on graft loss PTDM impact on survival |
|
Diabetes mellitus on graft loss Comorbidities on death: diabetic nephropathy, retinopathy, neuropathy, CHD, CVD |
PTDM | ARR | Assumes TAC is twice the PTDM rate of other drugs |
| McEwan 2005 2006310,311 | Adult, UK | SRL vs. TAC vs. CSA All with AZA + CCSs |
10 and 20 years | DES model; monthly cycles | Serum creatinine levels at 3 months, and 1, 2 and 3 years |
|
Number of transplants Diabetes mellitus Age (for patient survival) |
Switch from SRL to TAC/CSA (intolerance) | Percentage with GRF for entire 10 years Percentage switching from SRL Percentage low-dose CSA |
Includes costs of: antihypertensive drugs, prophylaxis CMV ±, CV, bone loss, anaemia, bone loss, OKT3 |
| Earnshaw 2008308 | Adult, de novo, 45.89 years, USA | SRL + CCSs MMF + CSA + CCSs MMF + TAC + CCSs |
Life-time | Decision tree of first + Markov (may return to decision tree for a subsequent transplant) | Serum creatinine 12 months (Based on Hariharan et al. 2002340) |
|
Donor type (baseline) Transplant number |
Increased triglyceride and/or cholesterol levelsb Diabetes mellitus incidence (at 3, 12 and 36 months) |
Discount rate Diabetes mellitus-related parameters: incidence, excess death, and costs Serum creatinine (by design) |
|
| Orme 2003309 | Adults, UK | Induction: TAC or CSA pretransplant and CCSs + AZA perioperatively Maintenance: CSA + AZA + CCSs vs. TAC + AZA + CCSs |
10 | Extrapolation from patient and graft survival outcomes | None |
|
None | NR |
|
The rates of change of rejection rates were assumed equal to those for graft loss, which were based on data from the UK renal audit data and may bias results against TAC |
| Jurgensen 2010,306 2015307 | Adults, Germany (age not statedd) | 1. SRL + CNI minimisation + CCSs 2. SRL CNI + CCSs 3. EVE + CNI minimisation + CCSs 4. CSA + MMF + CCSs 5. TAC + MMF + CCSs |
2 and 10 years | Markov to extrapolate two outcomes; monthly cycles |
|
|
None | Malignancies; CMV infections; PT diabetes mellitus; anaemia; dyslipidaemia; hypertension; wound-healing disorders |
|
Allowed unrestricted number of retransplants Data for the first 2 years from systematic review of RCTsd Extrapolation from 2 to 10 years using registry datae Malignancy data for CSA and TAC up to 6 years |
| Emparan 2003,314 2005313 | Old-to-old transplant recipient (68–69 years), Spain | BAS + CSA BAS + CSA + MMF TAC + MMF CCSs tapering for all |
1 year | Markov simulation Monte Carlo; cycle duration was not stated | NA | CRC (day 7) Dialysis requirements (first month); rejection, infections; GRF; patient survival |
None | Infections (30 days) | NR | Inadequate reporting of methods prevents assessment of study quality |
| Muduma 2014318 | Adults age ≥ 18 years, UK | TAC (a, Advagraf; b, Prograf) + MMF + CCSs SRL (a, without CNI; b, CNI minimisation) + MMF ME + CCSs CSA + MMF ME + CCSs BEL+ MMF ME + CCSs |
5 and 25 years | Markov; annual cycles with tunnel states for functioning graft with previous BPAR and for retransplantations | BPAR (effects lasted only for the first) | Functioning graft without previous BPAR; functioning graft with previous BPAR; graft failure; death | None | AEs were referred to as accounted for in the model but no further information was provided | Time horizon Effect of increased adherence (TAC a vs. b) Costs and utilities of dialysis (HD and CAPD) Inclusion of AEs (for the comparison TAC vs. CSA |
Did not provide any information on sources and values for the relative efficacy parameter (in terms of BPAR) The drug resource-use estimates were not derived from the samples of relative efficacy parameter estimates The authors implemented the mortality risks so that only the maximum of the background and risk with a functioning/failed graft, applied at any one time |
| Study | Regimens compared | Patient characteristics | Time horizon | Years with a functioning graft | Life-years (undiscounted) | Discounted incremental costs (£) | ICER: incremental cost per QALY (£) | Notes on ICER |
|---|---|---|---|---|---|---|---|---|
| Orme 2003309 | TAC | Mean age 44–48 years | 10 years | 7.09 | 795 | 1457a | Costs and ICER are adjusted to 3.5% discounting of costs and life-years gained, and are in 1999 prices | |
| CSA ME | Diabetes mellitus 7–9% BMI 24–26 kg/m2 |
6.54 | ||||||
| McEwan 2005,310 2006311 | SRL | Mean age 43 years Weight 77 kg Diabetes mellitus 7% |
10 years, 20 years | 14.27 | 15.37 | 62,120 | SRL dominant | Cost discounting 6%, QALYs 3.5% Source of difference in effectiveness between TAC and CSA unclear: identical parameter values and methods were used for them |
| CSA | 12.35 | 15.18 | 69,525 | |||||
| TAC | 12.09 | NR | 75,265 | |||||
| Woodroffe 200565 | TAC vs. CSA with:
|
NR | 10 years | NR | NR | TAC vs. CSA:
|
TAC vs. CSA:
|
Cost discounting 6%, QALYs 3.5% Results of a meta-regression of outputs from patient simulation model submitted to NICE by Novartis, as a function of ARR and PTDM (TAC was given 14% vs. 7% rate for other regimens). Figures within parentheses reflect ranges of incremental QALY associated with 95% CI of ARRs in systematic review by Woodroffe |
MMF vs. AZA with:
|
MMF vs. AZA:
|
MMF vs. AZA:
|
||||||
| Muduma 2014318 | TAC:
BEL + MMF ME + CCSs |
45 years (range 18–65 years) Weight 70.3 kg |
25 | NR | NR | Relative to Prograf: CSA: 10,928 SRL:
BEL: 33,521 |
Relative to Prograf: CSA: 21,244 SRL:
BEL: dominated |
Discounting at 3.5% of costs and QALYs In 2013 prices |
| Relative to Advagraf: Prograf: 10,928 |
Relative to Advagraf: Prograf dominated |
| Study and country | Regimens compared | Patient characteristics | Time horizon | Years with a functioning graft | Life-years (undiscounted) | Discounted incremental costs | ICER: incremental cost per QALY or per life-year (if QALYs not available) | Notes on ICER |
|---|---|---|---|---|---|---|---|---|
| Earnshaw 2008,308 USA | SRL + CCSs (CNI withdrawal) | Mean age 46 years | Lifetime | NR | 11.43 | US$472,799 | SRL dominant | Cost and QALYs discounted at 3%, QALYS 3.5% Model based on 1-year post-transplantation serum creatinine values and graft survival by donor type Third-party payer perspective |
| CSA + MMF + CCSs | 11.37 | US$484,020 | ||||||
| TAC + MMF + CCSs | 11.13 | US$505,420 | ||||||
| Jurgensen 2015,307 2010,306 Germany | SRL + CCSs (CSA withdrawal) | Not stated | 10 years | 4.99 | 5.64 | €145,788 | TAC regimen vs. SRL (CSA minimisation): €387,684 (other regimens are dominated) | Incremental cost per life-year gained; QALYs were not calculated Statutory health insurance perspective Study evaluates low-dose TAC in accordance with ELiTE SYMPHONY trial Use of MTC in NMA Detailed account of AE probabilities and costs |
| SRL (CSA minimisation) | 5.83 | 6.47 | €107,246 | |||||
| EVL (CSA minimisation) | 5.19 | 5.98 | €154,822 | |||||
| TAC (low dose) MMF + CCSs | 5.90 | 6.49 | €114,612 | |||||
| Abecassis 2008,321 USA | TAC o.d. + MMF | Not stated | 5 years | 4.30 | 4.53 | US$228,734 | TAC o.d. is dominant | Year 2006 prices Discounted costs at 5% |
| TAC b.i.d. + MMF | 4.19 | 4.52 | US$238,144 |
In common with the UK study by McEwan et al. 310,311 discussed before, the US study by Earnshaw et al. 308 evaluated SRL + steroids after CNI withdrawal, but, in this case, it compared it against triple therapy of TAC or CSA combined with MMF and steroids. 308,310,311 Applying a decision-analytic model extending over the lifetime of a 46-year-old first-transplant patient, it found that the regimen was the dominant treatment for the adult renal transplantation population in general. Its use resulted in 0.30 extra years of life relative to TAC-containing triple therapy and 0.06 extra years of life relative to triple therapy containing CSA. In terms of discounted (at 3% per annum) QALYs, the results were 0.30 and 0.12, respectively. SRL CNI withdrawal produced a cost saving of US$33,000 relative to TAC, and of US$11,000 when compared with CSA. The same qualitative results were found for the subgroup analysis by donor type (living, deceased non-ECD and deceased ECD).
The study by Earnshaw et al. 308 is different from other reports on the same topic in its attempt to provide evidence on cost-effectiveness across different donor types. In common with other studies evaluating SRL, it found the regimen to be cost-effective, in this case relative to current standard triple therapy containing a CNI. Similar criticisms as those made above to the UK reports by McEwan et al. , in relation to the current perception of SRL as having a restricted use because of issues about safety, may be applied to this study. 310,311 In terms of its methodology, this study used a model to predict long-term graft survival from 1-year renal function outcomes that were specific to the three regimens, accounting for graft survival differences between donor types. Although the use of renal function as driving clinical outcomes is supported by recent statistical evidence in samples of people treated in routine practice,333 the model structure adopted by Earnshaw et al. 308 relies on a simplistic assumption of constant (instantaneous) probability (hazard rates) of graft failure over time, which more recent studies find to be inconsistent with the data. 333,334 In addition, the study does not account for the direct effects of renal function on costs and HRQoL. Thus, important differences between therapies might not have been captured with this model as patients accumulated time in the functioning graft state. 42,338
One study307,311 presents the results of a Markov model of 10-year outcomes representing the transition across health states experienced by people after renal transplantation in Germany. The model compares SRL CSA avoidance with SRL CSA minimisation and low-dose TAC triple therapy with MMF and steroids. The latter was included in acknowledgement of the changes in immunosuppressant treatment practice following the publication of results from the SYMPHONY trials. 195,196,199 The analysis was conducted from the perspective of the German statutory health insurance. The study307,311 found that low-dose TAC in triple therapy with MMF and steroids has a cost per life-year gained in excess of €100,000, relative to the SRL CSA minimisation regimen. All other comparators were found irrelevant for identifying the cost-effective treatment option, as they were dominated by these two regimens.
The study provides new evidence about the cost-effectiveness of low-dose TAC regimens favoured at present by current practice, which has emerged following the publication of the SYMPHONY trial results. 195,196,199 One of the strengths of this analysis is the attempt to derive comparative evidence for the effects of the different regimens from evidence synthesis based on indirect comparisons, through NMA. Another is its account for AEs including graft failure, malignancies, CMV infections, PTDM, wound-healing disorders, and post-transplant anaemia, 3-hydroxy-3-methylglutaryl-coenzyme A (HMGCoA) and hypertension treatments. However, the value of this study from an English NHS decision-making point of view is diminished by their choice of comparators, which excludes CSA-based triple therapy and other new treatments such as BEL. The study also has limited information use for informing NICE recommendations, as it did not account for HRQoL outcomes. The model itself is not amenable to account for available evidence on HRQoL and costs associated with the effects of immunosuppressive regimens on renal function, as the renal function plays no role in the health status of people in the model or indeed has no prognostic effect on long-term graft or patient survival outcomes, which were assumed to be driven by 2-year differences in the rate of AR between model arms.
A study321 co-authored by an affiliate of Astellas’ Pharma US modelled the expected costs and clinical outcomes of once-daily TAC-PR and twice-daily immediate-release TAC, each given in combination with MMF, for transplant recipients in the USA. The study used a stochastic state-transition Markov model extending 5 years post transplantation to predict the amount of time that people with a functioning graft were alive, receiving dialysis as a result of graft failure, or dead. The total discounted (5% annually) costs per patient were US$228,734 with once-daily TAC and US$238,144 with twice-daily TAC. The low quality of reporting by this article321 prevents assessment of its validity. The sources of values for some model parameters or the methods used to identify them were not reported. Moreover, the values of some parameters were not provided, preventing the replication of results by the reader.
The remaining study319,320 compared the resource-use costs and health outcomes over 6 months post transplantation of people who were randomised to receive TAC (n = 286) and CSA ME (n = 287), as part of triple immunosuppressive therapy with AZA and steroids. This was a multicountry trial, in which TAC was given at an initial daily dose of 0.3 mg/kg, whereas the starting dose of CSA ME was 8–10 mg/kg per day. The study retrospectively measured resource-use quantities and costs of immunosuppressant drugs, concomitant medications, hospitalisation, dialysis and rejection episodes from the 50 centres in seven Western European countries that participated in the trial. ITT analysis revealed per-patient cost savings achieved by TAC, ranging from €1776 in Italy to €524 in Spain (figures in year 2000 prices). The authors attribute part of the variation to the higher cost of hospitalisation in Italy than in the other countries. Most of the savings with TAC were a result of fewer days in hospital for the initial stay and readmissions (Italian case 50%), lower costs of immunosuppressive medication for graft rejection (37%) and incidence of dialysis (13%). 320
The length of follow-up in this study319,320 was insufficient to capture important clinical events, such as graft and patient survival or AEs such as PTDM, with which TAC immunosuppression has been associated. In addition, the study319,320 did not report any results in terms of changes in renal function, which has been observed to be associated with costs and HRQoL, as well as a prognostic factor of graft and patient survival. Moreover, the detailed report on the Italian case found that differences in costs were statistically insignificant (i.e. p > 0.05), suggesting that the overall reduction in costs may have been due to chance alone. In any case, the study319,320 may have had insufficient power to perform statistical inference on cost effects. 328 Therefore, the conclusion that ‘the overall costs of treating a patient with TAC during the 6-month post-transplantation period are substantially lower [than that for CSA ME]’ may not be supported by the results of the study.
Chapter 5 Critical appraisal of company submissions
Three companies submitted economic models to NICE: Astellas, Novartis and Bristol-Myers Squibb.
Astellas’ submission
Overview
The submission compared twice-daily immediate-release TAC (Prograf) with once-daily TAC-PR (Advagraf), and against BEL, EVL and SRL. Immediate-release TAC was considered to be the standard treatment of choice in adult renal transplantation immunosuppression, based on its UK market share, whereas the comparators investigated were deemed to be used infrequently. The submission cites evidence of improved outcomes for TAC-PR relative to the current standard regimen, immediate-release TAC, since the former became available in 2009. In addition, EVL was included in the evaluation despite its lack of market authorisation in the UK, as requested by the NICE scope.
The analysis found that immediate-release TAC resulted in reduced total costs and health benefits relative to the comparators, EVL and BEL; it was concluded that TAC-PR is cost-effective and should be the new standard of care. Although the health benefits of immediate-release TAC were found insufficient to compensate for its increased cost relative to SRL, the latter regimen was consider to apply to only a selected subgroup of adults receiving a kidney transplant.
The submission pointed to evidence on the relationship between treatment adherence and acute and long-term graft rejection, and graft failure as surrogate markers of outcomes. In particular, it stated that adherence to immunosuppressant regimens positively affects graft survival by preventing the development of de novo donor-specific antibodies, which have been associated with a reduction in 10-year graft survival. 342 This is then used to translate the observed improvement in adherence with PR TAC relative to immediate-release TAC into graft and patient survival benefits. 343 In addition, the company claims that TAC-PR has a better pharmacokinetic profile than twice-daily TAC (lower intrapatient variability,344 which results in a lower risk of long-term graft failure. 345 The company also cites analyses from the Collaborative Transplant Study for Europe presented at the 2014 World Transplant Congress, which shows that people who are treated with TAC-PR had higher patient and graft survival rates than people treated with immediate-release TAC over 12 months, following renal transplantation, in Collaborative Transplant Study data for 2011–13. However, this observation was not robust to the adjustment for multiple confounders [hazard ratio (HR) 0.76; p = 0.14; 95% CI were not stated].
The submission also cites the results of a meta-analysis pointing to increased risk of PTDM with TAC [relative risk (RR) at 12 months 1.72, 95% CI 1.17 to 2.52; RR at 36 months 2.71, 95% CI 1.61 to 4.57] relative to CSA, and acknowledges the evidence on the association between PTDM and reduced graft survival (RR 1.63, 95% CI 1.46 to 1.84). 346 The company argues that these estimates may have been the result of people treated with high doses of TAC relative to current practice. To support this claim the submission cites the results of a Phase III study204 comparing TAC-PR with immediate-release TAC, which used lower doses of TAC and found lower incidence rates of PTDM than those in the studies included in the meta-analysis report. It is noted, however, that the latter evidence had no bearing on the meta-analysis finding of a higher RR of PTDM with TAC than CSA.
Efficacy and effectiveness evidence
The submission reports a systematic review of the RCT evidence of effectiveness of immunosuppression after first kidney-only transplant. The review involved an electronic search of bibliographic databases covering studies published during the period 2002–June 2014, and was complemented by relevant studies from two published reviews. 65,347
Based on 6-month and 1-year pooled data from 19 RCTs including 3796 people, immediate-release TAC had a lower rate of BPAR than CSA ME (RR 0.69, 95% CI 0.57 to 0.82). However, based on data from 10 studies that reported the outcome in 1859 people, immediate-release TAC resulted in higher incidence of PTDM (1.57, 95% CI 1.16 to 2.12). In terms of other outcomes (graft survival, patient survival and death-censored graft survival) differences were found not to be statistically significant at the 5% level.
Pooled-effect estimates for immediate-release TAC, compared with SRL given as a CNI avoidance regimen, were obtained from four RCTs of 6–12 months’ follow-up involving 1397 people. Neither patient survival nor PTDM differed in statistically significant manner between the arms, whereas SRL produced a higher risk of developing AR (RR 2.28, 95% CI 1.37 to 3.79) and lower survival probability (RR 0.95, 95% CI 0.92 to 0.98). In the SRL CNI minimisation regimen, two studies were found, involving 461 people in the comparison of immediate-release TAC/SRL and steroids with immediate-release TAC/MMF and steroids. No differences were found in patient and graft survival, ARs and PTDM at 6–12 months post transplant, whereas more discontinuations were found in the former arm.
For the comparison between TAC-PR and CSA ME, the submission cites one multicentre study that compared these two options and an immediate-release TAC option, all in combination with MMF and steroids. The study found similar efficacy across the three treatment arms in terms of patient and graft survival and AR but there is no measure of uncertainty reported alongside the event rates presented.
Astellas presents results from its own meta-analysis of two studies comparing immediate-release TAC with TAC-PR for de novo kidney transplantation in terms of BPAR stratified for people with (RR 1.16, 95% CI 0.82 to 1.63) and without (RR 1.28, 95% CI 0.98 to 1.68) induction. It cites results of a published meta-analysis that included observational data348 as consistent with the claim that TAC-PR is as effective as immediate-release TAC in preventing BPAR and graft failure at 12 months post kidney transplantation.
For the TAC minimisation versus EVL comparison, no difference in patient and graft survival at 6–12 months was found in three studies involving 358 people (RR 1.01). The submission also cites results from the ASSET trial256 regarding a higher 12-month rate of BPAR (RR 2.19, 95% CI 0.20 to 23.77) with a low-dose TAC with EVL regimen versus standard-dose TAC with EVL regimen (both regimens were given from 3 months post transplantation after an initial 3-month regimen of standard TAC). 256 For the comparison of TAC withdrawal with EVL introduction versus the continuation of an initial 3-month regimen of TAC, MPS and steroids, one study259 was cited as reporting no graft failure or patient death in either group at 12 months; renal function, as measured by eGFR of 53.38 ml/minute/1.73 m2 in the TAC continuation group and 57.27 ml/minute/1.73 m2 in the EVL group (p = 0.25); and no BPAR case in the TAC group and 17.5% incidence in the EVL group (RR 0.05, 95% CI 0.00 to 0.79). Given the absence of RCTs of TAC compared with EVL, Astellas estimated their relative effects indirectly from head-to-head studies of EVL plus low-dose CSA compared with standard CSA (two studies, reporting RR ratios between 0.98 and 1.01 for AR, graft and patient survival outcomes at 3–12 months) and studies of TAC compared with CSA.
Likewise for TAC compared with BEL, estimates were obtained from indirect comparisons, through studies of each of these regimens against CSA. The TAC studies have been described in this section. As for BEL, data from two Phase III trials with 3-year follow-up data were used for the indirect comparison: one included adults receiving a living donor or standard criteria deceased donor kidney (BENEFIT study59) and the other was a study of similar design but included ECDs (BENEFIT-EXT study142). The company presented separate and combined results of analyses of 1-year data from both trials stratified by a more-intensive and a less-intensive BEL regimen. In general, BEL was found to have higher BPAR rates, less chronic allograft nephropathy (for the more intensive BEL regimen) and improved renal function over CSA. BEL also reduced the incidence of NODAT.
Combining up to 1-year results from BENEFIT59 and BENEFIT-EXT,142 the meta-analysis of immediate-release TAC compared with CSA (number of studies: AR 19, graft survival 11, patient survival 10, WMD in GFR, 2), and outcomes of TAC-PR compared with CSA from the Phase III trial reported by Silva et al. ,239 TAC-PR was found to result in a lower ARR (RR 0.24, 95% CI 0.12 to 0.51) and lower WMDs in GFR (MD –10.50, 95% CI –16.57 to –4.43) than both the more-intensive and less-intensive BEL regimens. 349 The company also cites the results of an indirect comparative analysis conducted by Bristol-Myers Squibb, which showed ‘no significant difference’ between BEL and TAC for mortality, graft loss or GFR at 12 and 36 months (All Wales Medicines Strategy Group 2012)350 and higher ARR and lower incidence of NODAT for BEL than for TAC.
Another indirect comparison by Astellas produced estimates of AR, graft survival and patient survival for immediate-release TAC relative to EVL. The RR ratios were, respectively, 0.70 (95% CI 0.48 to 1.03), 0.97 (95% CI 0.93 to 1.03) and 0.98 (95% CI 0.95 to 1.02).
Review of economic models and their results in the submission
The submission provides an overview of model structures and conclusions of previous CEAs of renal transplantation immunosuppressive regimens. From searches of electronic databases (NHS EED, The Cochrane Collaboration, MEDLINE and other database not specified) Astellas identified and included in its review 12 ‘representative studies because they met the inclusion criteria’ (Astellas’ submission, p. 28, chapter 8, Review of economic studies – it states that 11 studies were included in the review but 12 are actually cited). No details were provided about the inclusion criteria for the review of economic studies; such criteria, therefore, presumably refer to criteria employed for the effectiveness review in the submission. One of the included studies compared immediate-release TAC with TAC-PR (this study is reviewed in section 1.2 of the company’s submission). 321 Four studies compared TAC with CSA (three309,319,320 of which met the criteria for inclusion in the review of section 1.2; the remaining study100 was excluded from the review of section 1.2 because it measured costs only for medication) and seven studies306–308,311,351–353 examined SRL in CNI avoidance or minimisation strategies compared with TAC (four studies307,308,311 included in the review of section 1.2) and three studies351–353 that were excluded from it as a result of the country to which they apply.
The submission briefly described the main results of these studies without critically assessing their validity and applicability to a UK setting, although a warning is issued about limited transferability of results from non-UK (10 out of the 12) studies. It concludes that the evidence supports the view that TAC is cost-effective relative to CSA, but that it is ambiguous in relation to the comparison against SRL in a CNI avoidance or minimisation strategy. The submission also includes a section in which three published models are described. No assessment of their strengths and weakness was presented. These models308,351,352 share the characteristics of models described and discussed in Assessment of cost-effectiveness (one of them308 is reviewed in that section).
Economic evaluation by the company
The CEA submitted by Astellas is an update of a published Markov model-based assessment of the cost-effectiveness of TAC, in either its prolonged-release formulation, TAC-PR, or the current standard therapy of immediate release (immediate-release TAC) by Muduma et al. ,318 reviewed in Chapter 4 (see Identified studies). The model describes the annual transitions between four health states, starting from kidney-only transplantation: functioning graft without history of AR; functioning graft having experienced AR; graft failure (dialysis); and death. The submission extends the effectiveness review for the model from June 2013, the cut-off date of the published study,318 to June 2014. In addition, the analysis in the submission to NICE adds EVL in a CNI minimisation regimen to the list of treatments evaluated in the published paper.
Efficacy data used in the model
The model represents differences in outcomes between regimens as caused by their impact on BPAR. The model was based on the assumption that the effects of treatment on this surrogate outcome lasted for only the first year post transplantation. This assumption was combined with (1) the estimated RR of graft failure for a functioning graft with previous BPAR compared with no previous BPAR and (2) the 1-year post-transplant BPAR frequency, both from estimates reported by Opelz et al. ,354 to derive the graft survival curves for grafts without prior AR and grafts with history of AR from the 5-year graft survival profile in UK registry data (NHSBT) 2013355 (Table 139). The model extrapolation was complemented by exponential survival curves to extend survival from 5 years up to 25 years post transplantation.
| Product | Rate (%) | Comment |
|---|---|---|
| Immediate-release TAC (base comparator) | 12.6 | 123,204,239 |
| TAC-PR | 14.6 | 123,204,239 and meta-analysis (section 2 of company submission) |
| BEL | 30.7 | 123,204,239 and meta-analysis (sections 2 and 3) |
| EVL (CNI minimisation) | 18.0 | 123,204,239 and meta-analysis (sections 2 and 3) |
| SRL (CNI minimisation) | 16.5 | 123,204,239 and meta-analysis (section 2) |
| SRL (CNI avoidance) | 28.7 | 123,204,239 and meta-analysis (section 2) |
With regard to patient survival, the model used the 1-, 2- and 5-year post-transplantation survival rates from the NHSBT report 2012–13355 as the estimated survival rates with a functioning graft. To populate survival probabilities in the state of graft failure, the model used annual survival rates of people on dialysis followed for 10 years from the UK Renal Registry. 3 The graft and patient survival rates were extrapolated to 25 years by estimating an exponential curve on the available data (including graft survival rates for years 3 and 4 derived by linear interpolation) and projecting survival rates from the last observed rate with the estimated curve. There is no mention in the submission about adjusting for increases in background mortality as the cohort in the model ages.
In addition to the difference in efficacy, measured in terms of ARRs, the model allowed for differences in effectiveness between the TAC arms through the differences in adherence induced by the once-daily, prolonged-release (Adagraf) compared with the twice-daily immediate-release formulations of the drug (immediate-release TAC). The model utilised comparative estimates of adherence with TAC-PR with immediate-release TAC of 88.2% vs. 78.8% from a published study343 and combined them with an estimated RR of graft failure in non-adherent versus adherent people of 3.47 derived from a meta-analysis,356 to obtain a RR of graft failure of 0.848, which was applied to the graft survival curves (until year 5, and by exponential curve extrapolation thereafter) that were common to all of the other immunosuppressive treatment strategies in the model.
There are two logical inconsistencies with this modelling procedure. First, accounting for the advantages in adherence with TAC-PR over immediate-release TAC makes comparison of TAC-PR with other immunosuppressive regimens in the model invalid, as no allowance was made for any effects of adherence on graft survival for the other regimens analysed in the model. Indeed, this undermines the fundamental assumption in the model that all significant differences in any drug regimen comparison may be accounted for by the effect through the surrogate, in this case the rate of AR. 357 Thus, regardless of the validity of the comparative analysis of TAC-PR and immediate-release TAC, the indirect comparisons of model results between TAC-PR and SRL, EVL and BEL are then invalid.
Second, although the model was adjusted to include the effect of adherence on graft survival in the comparison of TAC-PR with immediate-release TAC, the patient survival curves (for the functioning and failed graft states) were left unchanged, so that the same set of patient survival curves was applied to all immunosuppressive options analysed. This implies the questionable assumption that improvements in graft survival, such as those obtained with TAC-PR relative to immediate-release TAC (and indeed relative to all other model arms), do not translate in direct patient survival benefits. This inconsistent logic in turn leads to underestimating the benefits of TAC-PR and overestimating its costs.
Inspection of the Microsoft Excel® 2010 version 14 (Microsoft Corporation, Redmond, WA, USA) model spreadsheets revealed that the TAC drug regimen options (TAC-PR and immediate-release TAC) and EVL were the only treatment arms populated by data on actual immunosuppressive drug use (from the RCT sample on which the efficacy for the regimen was estimated); drug consumption values for BEL and SRL regimens were based on treatment guidelines (BNF or Summary of Product Characteristics).
Adverse events
The model allows for seven types of AE following transplantation: malignancy, diabetes mellitus, anaemia, CMV infection, hypertension, HMGCoA and wound-healing disorders. These events were assigned costs (except for the last type of event which had zero cost) but no disutility. The AE incidence rates in the model, reproduced in Table 140, differed across immunosuppressant treatment arms, although these had no influence on the probability of graft failure and patient death. Such differences only affected the costs differences between the treatments.
| Product | AE | Year 1 | Year 2 | Year 3 and later |
|---|---|---|---|---|
| TAC-PR/immediate-release TAC | Malignancies | 0.00 | 0.00 | 0.43 |
| CMV infections | 3.62 | 3.62 | 0.04 | |
| PTDM | 6.07 | 6.07 | 6.27 | |
| Wound-healing disorders | 4.12 | 4.12 | 0.00 | |
| Anaemia | 14.71 | 14.71 | 14.71 | |
| HMGCoA | 13.84 | 13.84 | 3.46 | |
| Hypertension | 9.17 | 9.17 | 9.17 | |
| EVL | Malignancies | 2.43 | 2.43 | 0.64 |
| CMV infections | 3.19 | 3.19 | 0.04 | |
| PTDM | 5.58 | 5.58 | 5.77 | |
| Wound-healing disorders | 10.72 | 10.72 | 0.00 | |
| Anaemia | 27.30 | 27.30 | 27.30 | |
| HMGCoA | 29.47 | 29.47 | 7.37 | |
| Hypertension | 31.63 | 31.63 | 31.63 | |
| SRL (CNI minimisation and avoidance regimens) | Malignancies | 0.20 | 0.20 | 0.05 |
| CMV infections | 2.11 | 2.11 | 0.03 | |
| PTDM | 5.88 | 5.88 | 6.07 | |
| Wound-healing disorders | 10.72 | 10.72 | 0.00 | |
| Anaemia | 18.68 | 18.68 | 18.68 | |
| HMGCoA | 21.77 | 21.77 | 5.44 | |
| Hypertension | 15.08 | 15.08 | 15.08 | |
| BEL | Malignancies | 2.32 | 2.32 | 0.61 |
| CMV infections | 7.65 | 7.65 | 0.09 | |
| PTDM | 4.00 | 4.00 | 4.19 | |
| Wound-healing disorders | 4.12 | 4.12 | 0.00 | |
| Anaemia | 14.71 | 14.71 | 14.71 | |
| HMGCoA | 18.88 | 18.88 | 18.88 | |
| Hypertension | 31.12 | 31.12 | 31.12 |
The incidence rates of AEs were derived from a systematic review and meta-analysis published in 2006,341 the values adopted by the published economic model for Germany by Jurgensen et al. 306 reviewed in section 1.2 of the company’s submission and trial outcomes from the BENEFIT and BENEFIT-EXT trials. 207
The rates of AEs were assumed to be the same with TAC-PR and immediate-release TAC and for the two SRL regimens (CNI avoidance and CNI minimisation). According to the incidence rates in this model, TAC has the lowest annual incidence of malignancy (except for SRL from the third post-transplantation year onwards), CMV, anaemia (except for BEL, which had the same annual incidence rates as those of TAC), dyslipidaemia and hypertension, but was associated with an excess incidence of PTDM over the other options.
Health-related quality of life and QALY outcomes were calculated from time spent in the graft functioning state and the graft failure state, which involved dialysis. Based on published estimates,358 the functioning state was associated with a utility value of 0.71, regardless of any prior experience of AR, and the graft failure state was associated with a utility of 0.459, which was equal to the weighted average of the utility of HD (0.44), experienced by 82% of people on dialysis, and PD (0.53) received by the rest. 358
The model allows for the occurrence and effects of retransplantation, using the time to retransplantation data reported by McEwan et al. ,310,311 which is reviewed above (see Chapter 4, Review of cost-effectiveness evidence). However, the states following the first retransplantation (i.e. functioning graft with prior AR on the current retransplant, functioning graft without prior AR on the current retransplant – regardless of AR of any previous transplant – and graft failure) face the same transition probabilities, utility values and costs as the corresponding states before retransplantation. 310,311 This is likely to bias the analysis in favour of treatments with higher rejection rates in the model (as higher ARRs imply higher graft failure rates in this model) and may be interpreted as a conservative assumption of the relative effectiveness and incremental costs advantage of TAC over the comparators.
In addition, one incorrect calculation was identified in the Excel spreadsheets of the model submitted by Astellas. The problem was that the model used the data from the NHSBT from 2012 to 2013, on patient survival rates for kidney-only transplant recipients in the UK (p. 35, table 25, in the submission by Astellas) to populate the patient survival parameters of people with a functioning graft, ignoring the fact that such data on survival rates were likely to include deaths of both people with a functioning and those with a failed graft. Instead, the probability of death in the graft functioning state should have been calculated as the remainder of the annual probability of death from the NHSBT patient survival data minus the product of probability of mortality in the graft failure state and the proportion of people with a failed graft. In other words, the Astellas model is likely to overestimate mortality in the functioning graft states, which, in turn, underestimates the benefits of any gains in efficacy (i.e. reductions in AR in the model) that any regimen may have over another (e.g. TAC over the comparators).
Unit costs
The cost per mg of TAC-PR used was 23% lower than that of immediate-release TAC. (The authors present sensitivity analyses of discounts on TAC list prices limited to the first 90 days post transplantation.) Prices for other immunosuppressant regimens were based on BNF prices.
Treatment of ARs was assigned costs of i.v. steroids plus, for the 20% of steroid-resistant BPAR cases, the treatment costs of a regimen of rATG and an inpatient hospital stay for AKI without complications (£1737 overall mean cost). This assumed zero medical management costs for the 80% of people with steroid-sensitive AR and ignores any costs of follow-up to monitor treatment efficacy. The cost per year of dialysis was £38,387 and the cost of retransplant was £25,953. The costs of AEs adopted are presented in Table 141 (which reproduces table 35 in the Astellas submission).
| Variable | Value (£) | Comment |
|---|---|---|
| Malignancies | 8801 | Skin/non-Hodgkin’s lymphoma Mabthera concentrate for i.v. infusion, rituximab 10 mg/ml, net price 10-ml vial = £174.63, 50-ml vial = £873.15 |
| CMV infections | 1863 | i.v. ganciclovir (Cymevene®, Roche Products Ltd) 14–21 days then maintenance for 8 weeks Ganciclovir; i.v. infusion, powder for reconstitution, ganciclovir (as sodium salt). Net price 500-mg vial = £29.77 |
| PTDM | 17.38 | Tablets, coated, metformin hydrochloride 500 mg Net price 28-tablet pack = 87p, 84-tablet pack = £1.00; 850 mg, 56-tablet pack = £1.36 |
| Wound-healing disorders | 0.00 | – |
| Anaemia | 1186.61 | Epoetin alfa (Binocrit®, Sandoz Ltd) injection maintenance dose 17–33 units/kg three times weekly, pre-filled syringe Net price 1000 units = £4.33; 2000 units = £8.65; 3000 units = £12.98; 4000 units = £17.31; 5000 units = £21.64; 6000 units = £25.96; 8000 units = £40.73; 10,000 units = £43.27 |
| LDL cholesterol | 235.03 | Simvastatin (Zocor®, Merck & Co.) tablets, all f/c, simvastatin 10 mg (peach) Net price 28-tablet pack = £18.03; 20 mg (tan), 28-tablet pack = £29.69; 40 mg (red), 28-tablet pack = £29.69; 80 mg (red), 28-tablet pack = £29.69 |
| Hypertension | 15.51 | Capsules, ramipril 1.25 mg Net price 28-capsule pack = 99p; 2.5 mg, 28-capsule pack = £1.05; 5 mg, 28-capsule pack = £1.12; 10 mg, 28-capsule pack = £1.19 |
Results
The Astellas submission produces life expectancies (censored after 25 years) of 16.60 for TAC (immediate-release TAC), 16.57 for SRL CNI minimisation, 16.56 for EVL, 16.48 for SRL CNI avoidance, and 16.47 for BEL in a cohort of people of mean age 45 years, 37% of whom are women. The expected discounted (at 3.5%) QALYs were 8.01, 7.99, 7.99, 7.94 and 7.94, respectively. For TAC once-daily prolonged-release formulation (TAC-PR), total life expectancy was 16.96 and discounted QALY was 8.21.
In the base-case results, immediate-release TAC produced more QALYs than any of the comparators and lower costs than BEL and EVL, whereas it had higher cost against the SRL regimens. The ICER against SRL CNI minimisation strategy was in excess of £1M and the ICER against SRL CNI avoidance strategy was £174,842. In the comparison of TAC regimens, TAC-PR dominated immediate-release TAC, given its lower costs and higher QALYs (both discounted and undiscounted).
The results were found to be similar after changing assumptions, including the time horizon, from the base case of 25 years to 10, 15 and 20 years, the exclusion of discounting, AEs and half-cycle corrections. The results against SRL were found to change significantly when graft survival parameters in the model were populated with data from the SYMPHONY trial instead of the NHSBT data used in the base-case analyses: TAC-PR was found to dominate SRL as CNI avoidance regimen when both were given with DAC induction, 2 g MMF and steroids. In discussing these findings, the authors note that SYMPHONY trial has reported outcomes up to 3 years and is the largest prospective study in the de novo kidney transplantation to date, which showed TAC to result in lower AR, better renal function and graft survival outcomes at 1 year than the SRL regimen.
On the basis of these results, the company concludes that TAC is cost-effective and that TAC-PR should become the standard of care, as it produces lower costs and better health outcomes than immediate-release TAC. The latter statement is further supported, the submission claims, by the expected benefits, not accounted for in the Astellas model, arising from the improved pharmacokinetic profile of TAC-PR relative to immediate-release TAC. In addition, the authors argue that the results of the SYMPHONY trial have discouraged use of SRL, and that BEL’s high cost and high ARR may do likewise, citing a report by the All Wales Medicines Strategy Group350 as supportive evidence for this assertion.
Critical appraisal
The analysis presented by Astellas covers a number of appropriate comparators, including new regimens, BEL, and regimens with modes of action different from that of CNIs, that is, EVL and SRL. However, it omits one relevant comparator: CSA. There is no justification in the submission as to why this drug regimen option was not considered in the analysis. Muduma et al. 318 present the results of the same analysis based on data from the literature recorded in electronic databases up to 1 year earlier than the review in the Astellas submission (i.e. June 2013 vs. June 2014, respectively). The results reported by Muduma et al. ,318 who acknowledge employment by Astellas in the publication, are very similar to those presented by the Astellas submission for those drug regimens that were common to both reports (i.e. TAC-PR, immediate-release TAC, BEL, SRL CNI minimisation and SRL CNI avoidance). Unlike the Astellas submission, Muduma et al. 318 report results for CSA. The ICER of immediate-release TAC against CSA was £21,244 (table 1, base-case results318) and the cost-effectiveness acceptability curve for the comparison showed that the TAC option had a 59.5% probability of being cost-effective at the £30,000 willingness to pay for a QALY threshold. The sensitivity analysis showed that the result of this comparison was sensitive to the inclusion of the AE costs, that is, when omitting them altogether the ICER for TAC increased to £35,446.
This evidence casts doubt on the robustness of the cost-effectiveness results and conclusions in the Astellas submission, and suggests that the results presented may be misleading owing to the exclusion of a relevant comparator. It is unfortunate that the submission did not include CSA, given the previous published degree of uncertainty in the cost-effectiveness of TAC.
There is use of inadequate data within the model. As discussed above, the estimates of patient survival in the functioning graft state may have been underestimated. This works against the more efficacious treatments, such as TAC, which had the lowest ARRs of all the regimens compared. Thus, the results reported by Astellas in the submission may be treated as conservative estimates of the costs and benefits of its TAC regimens. In relation to the evidence presented in support of TAC-PR, this may suffer from the previous criticism about the incomplete set of comparators, and the fact that the TAC-PR versus immediate-release TAC comparison is based on what is in effect a different model of the outcomes of renal transplantation from that used to compare immediate-release TAC against all the other regimens. In fact, the model used for comparing TAC-PR with immediate-release TAC contradicts the fundamental premise of the model used to compare immediate-release TAC with all regimens other than TAC-PR: that AR captures all important drivers of clinically meaningful outcomes.
One other issue relates to the way the model was structured. Although the model allowed repeat transplantation to occur for a given individual, the costs and HRQoL of subsequent dialysis were accounted for only the first transplantation. Although the proportion of people with more than one retransplantation may be small, this assumption could have been important to the conclusions derived from the comparison with CSA, had such comparator been included.
Another concern relates to how the timing of transplantation was implemented in the model. Markov models imply that transitions occur at the end of the period represented by each cycle. In the present case, the cycle length was 1 year and the authors of the Astellas model rightly decided on using half-cycle corrections to reduce the inaccuracy of calculation of expected costs and benefits that arise from having a long cycle length given the frequency of state transitions. The model, however, assumed that the proportion of people who undergo retransplantation in the very first cycle made a transition from the failed graft state to a functioning graft post-retransplantation state, as if the retransplant had occurred at the start of the period, so that they spent the whole cycle length (6 months owing to the half-cycle correction) with a functioning graft after retransplantation in the first cycle. This is wrong, as in a cohort of people with de novo kidney transplants, the discrete Markov process transition from a functioning first graft to a functioning retransplant requires two sequential intervening events to occur, that is, graft failure and retransplantation (i.e. a minimum of two cycles, one for each event, is required).
In summary, the main limitations of the Astellas economic analyses are:
-
Omission of CSA as a relevant comparator (without justification).
-
Patient survival estimates in the functioning graft state may have been underestimated, which works against treatments with low rates of AR, such as TAC. The underestimation is, in part, because of an error in using UK registry data on survival rates of both people with functioning and those with failed grafts to inform the survival rates for those in the model with a functioning graft.
-
The analyses comparing the TAC-PR regimens with other non-TAC regimens are invalid, as the two TAC regimens incorporate differences in treatment adherence and this is not accounted for in the other regimens.
-
Drug dosage levels for BEL and SRL were based on treatment guidelines, whereas for other regimens they were based on actual trial data.
-
The cost and HRQoL of dialysis were not included for recipients of second or subsequent transplantations.
-
The analysis does not account for the role of GRF in (1) long-term graft survival outcomes and (2) current costs and utilities.
Novartis’ submission
Novartis, the company that produces EVL, submitted a simulation model of an individual patient’s health experience for the lifetime remaining after renal transplantation in the English NHS. The following treatments were evaluated for a group of simulated people of mean age 45.7 years (SD 12.7 years), mean weight 70 kg (SD 10 kg), 68.5% of whom were male, and mean MDRD eGFR 9.03 ml/minute/1.73 m2 (SD 7.9 ml/minute/1.73 m2):
-
EVL + reduced-dose CSA + steroids versus:
-
TAC + MMF + CCSs
-
Standard-dose CSA + MMF + steroids
-
-
EC-MPS + standard-dose CSA + steroids versus:
-
Standard-dose CSA + MMF + steroids.
-
The model was specified as monthly transitions between six health states:
-
stable post-transplant state (functioning graft)
-
AR
-
graft failure
-
dialysis
-
retransplantation and
-
death (from CKD or other causes).
Moving between these states is associated with changes in direct health-care costs, whereas HRQoL (utility) changes are accounted for transitions between the states of having a functioning graft to a failed graft, and from any of these to the absorbing state of death. In addition, the model accounts for the changes in mortality risks, utilities and monitoring costs (outpatient specialist visit) with renal function. Although the costs associated with AEs emerging following transplantation were measured for six type of events [proteinuria, BK virus (BKV) infection, CMV infection, hyperlipidaemia, wound and hypertension], only for two of these was the loss of utility measured in the analysis (proteinuria and hypertension).
The model assumes that AR may happen up to 3 years after a transplant, and applies the same probabilities of this type of event to first and subsequent transplantations. The probability of chronic rejection (i.e. graft failure) is independent of renal function in the model. Once a patient’s graft fails, dialysis is started and given until the time a new transplant is received, which is determined by a random normal distribution process with mean of 36 months (SD 12 months). This feature of the model is what gives it its discrete event simulation nature.
The model allows different rates of change in renal function (eGFR) between the first year (during which they are specific to the immunosuppressive treatments) and the second, third and subsequent years, when the rate of eGFR change is common to all treatment arms in the model.
The model parameters for the EVL and MPA regimens were populated with efficacy and safety outcomes at 12 months from the study by Tedesco-Silva et al. ,359 a multicountry trial that compared EVL 1.5 mg/day with mycophenolate acid 1.44 g/day in people receiving a primary kidney-only transplant in the period October 2005–October 2008. The values for the TAC regimen were obtained from a trial reported by Larson et al. ,154 which compared TAC with SRL in people receiving a kidney-only transplant (79% of whom were primary transplants in the TAC arm) in the period April 2001–January 2004 in the USA. The source of the efficacy and safety data for the MMF regimen was the multinational trial report by Vítko et al. ,177 which compared EVL with MMF in primary transplant patients who were recruited between August 1998 and August 1999.
The indirect nature of the relative efficacy data used as inputs to the cost-effectiveness model of the three comparisons submitted by Novartis presents some problems for valid estimation. In addition to the different dates when the trials were conducted and the type of transplant (primary only or mixed) for the EVL–TAC comparison, there were differences between the two studies in terms of the use of induction. Tedesco-Silva et al. 360 reported that participants in their trial of EVL were administered two BAS 20-mg doses: one within 2 hours before transplantation and the other at 4 days post transplantation ‘or according to local practice’,360 whereas Larson et al. 154 reported that all people received thymoglobulin 1.5 mg/kg/day on days 0, 1, 2, 4 and 6 post transplant. The sample of TAC participants was also slightly older but more balanced in terms of sex, and had a higher proportion of living donor transplants. The major issue, however, is the fact that the actual amount of TAC use in the efficacy trial was different from the dose used to cost the same regimen in the model. Larson et al. 154 report that the TAC was started at a 3 mg twice daily. The estimated mean daily dosing at 1 year, separately reported for the first 59 people randomised to TAC, was 6.3 mg per day (SD 0.9 mg per day). 361 The model, however, applied costs to the TAC arm at a quoted BNF recommended dose of 0.25 mg/kg/day for a group of individuals of 70 kg mean weight, thus resulting in a mean daily dose of 17.5 mg, which is considerably higher than the actual drug use that corresponds to the efficacy outcomes used by the model. The dose behind the TAC drug acquisition costs used in the Novartis submission is also larger than the mean daily doses for immediate-release TAC reported by Tedesco-Silva et al. ,349 which Astellas adopted in its submission, and which are consistent with the report of Dean et al. 361
In relation to the data sources for the comparison of EVL with the MMF + CSA regimen, the trial samples differ in terms of the period covered by the study and the country mix. The proportion of cadaveric donors transplant recipients was 46.6% in the EVL group compared with > 90% in the MMF + CSA regimen. 150 Moreover, the MMF regimen was given without induction therapy, in contrast with the trial that provided the outcome data for the EVL model arm. 359 The same issues applied to the comparison of MPA with MMF + CSA, as the data source for MPA was the same trial as that for EVL. 107
Costs
Immunosuppressive costs of the MPS + EVL treatment regimens were based on the dosing protocols of the individual trial that was the source of efficacy data, whereas the costs of drug acquisition for the comparators, that is, the TAC and MMF + CSA regimen, were based on BNF-recommended starting dosages. Other health-care costs included the costs of monitoring GP visits, which increased with higher CKD state. The cost of an AR event was taken from that reported by McEwan et al. 310 The annual costs of dialysis, £22,877, were obtained from a 2011 NICE costing report362 on organ donation for transplantation. Retransplantation involved an estimated cost of £17,736, a weighted average of NHS reference costs 2012/201364 for transplant procedures for varying ages and donor types.
Utilities
Estimates of utilities were derived from the study by Neri et al. ,363 who reported EQ-5D health states measured in a cross-sectional study of people with kidney-only transplants in the UK, valued using UK tariffs, as a function of CKD states. As renal function deteriorated so did the HRQoL (utility) values experienced by the simulated patient in the model. The model accounted for negative impacts on HRQoL (disutilities) of two adverse effects, proteinuria (reduced utility by 0.043) and hypertension (reduced utility by 0.010).
Results
EVL + reduced CSA vs. TAC + MMF
Novartis reports a life expectancy at transplantation in a patient group of mean age 45.7 years (SD 12.7 years) of 25.71 life-years under the EVL immunosuppression compared with 23.39 life-years under TAC, and discounted QALYs of 8.86 and 7.37, respectively (Novartis’ submission, table 5.18, Base-case analysis – deterministic ICERs). Given the discounted costs per patient that result under these options, £135,358 for EVL and £140,972 for TAC, EVL was found to be the preferred option, as it is less costly and more effective than TAC.
Further results accounting for uncertainty in model inputs relating to uncertain parameters (ARRs, chronic rejection rates, rate of change in eGFR after 12 months post transplant, health-state utilities and event costs) confirmed that the probability of EVL being cost-effective was 100% at thresholds ranging from £0 to £200,000 per QALY.
EVL + reduced CSA vs. MMF + standard-dose CSA
The EVL regimen was found to produce 1.76 extra years of life over the MMF with CSA regimen in the base case of a cohort of mean age 45.7 years. This corresponded to 0.99 extra discounted QALYs (Novartis’ submission, table 5.18, Base-case analysis – deterministic ICERs). The EVL containing triple therapy was also associated with £59,354 extra discounted costs over the MMF + CSA regimen, and a practically identical ICER figure, given the 0.99 discounted QALY benefit with EVL.
In probabilistic sensitivity analysis (PSA) accounting for the uncertain parameters (as listed for the results of the EVL vs. TAC comparison), the EVL had a 0% probability of being cost-effective relative to MMF for cost-effectiveness thresholds ranging from £0 to approximately £86,000 willingness to pay per QALY, and was still < 15% at £200,000 per QALY.
The fact that the PSA yielded a willingness to pay per QALY threshold at which EVL had a 50% chance of being cost-effective (> £200,000 per QALY), which was more than three times its deterministic ICER of £59,354, indicates that the model has important non-linearities and that using the deterministic values for decision-making is incorrect. Although this warning would not have made any difference to a decision based on a £30,000 per QALY threshold (i.e. both determinist and probabilistic results led to the same conclusion) for this comparison or the previous one discussed (i.e. EVL vs. TAC), the distinction does matter for interpreting the results of the third comparison presented by Novartis – of EC-MPS vs. MMF, discussed next.
EC-MPS vs. MMF + standard-dose CSA
In the deterministic base-case analysis, the mycophenolate regimen was found to result in 25.48 life-years, and 8.69 discounted QALYs per patient (table 5.18, Base-case analysis – deterministic ICERs). Mycophenolate acid had an extra 1.31 life-years and 0.80 discounted QALYs per treated patient relative to MMF. Given its additional discounted costs of £10,588, EVL had an ICER of £13,209 per QALY relative to MMF with CSA.
In the PSA that accounted for the effect of uncertain parameter estimates (as listed in the results of EVL relative to TAC), mycophenolate acid had a 50% chance of being cost-effective at a threshold value of around £28,000 willingness to pay per QALY.
Although the deterministic ICER for MPA is below the lower cost-effectiveness threshold adopted by NICE (£20,000), the willingness-to-pay threshold corresponding to the 50% probability of mycophenolate acid being cost-effective in the PSA is ≈ £28,000) suggesting that EVL may be borderline cost-effective, in relation to the £30,000 maximum acceptable amount NICE is willing to pay for a QALY. This comparison shows that the deterministic results are potentially misleading for informing decisions or deriving model predictions about treatment outcomes in this model.
Critique
The Novartis model uses a patient simulation model of monthly cycles to calculate the costs and health outcomes of immunosuppressant regimens over the remaining lifetime (i.e. 50 years post transplantation). The main strength of the model is its account of the occurrence of clinical events that determine health status, that is, AR, and graft and patient survival, as well as the effect of renal function on costs and HRQoL.
The study failed to conduct adequate evidence synthesis, as their methods of identification of relevant evidence on efficacy was not systematic, as acknowledged by the authors. The model analyses were based on data from single trials, and their analyses were restricted to undertake pairwise indirect comparisons of the treatments investigated in each of those individual trials. This led to results that were at odds with findings from the systematic review of the clinical evidence undertaken by PenTAG (see Chapter 3, Summary of pairwise comparisons) which found no statistically significant improvement in efficacy outcomes (AR, graft failure, death) of EC-MPS compared with MMF, whereas the Novartis model-based analysis produced an extra 1.31 life-years for EC-MPS. Therefore, the results by Novartis are likely to be biased, and consideration of additional efficacy evidence from direct and indirect comparisons would have allowed the company to provide a more reliable technology assessment.
Some errors were identified in the calculation of unit costs of immunosuppression for the CSA component of the EVL regimen, which was common to two other comparators, but is not part of the current standard clinical practice in England. This had the effect of underestimating costs for the CSA-containing regimens.
The model accounted for some important AEs, but omitted one of the most important determinants of patient and graft survival: PTDM.
A major flaw in the model is the assumption that graft failure occurs independently of the GRF or the occurrence of AR. The probability of graft failure (labelled chronic rejection in the submission) is based on 12-month post-transplantation trial data for each regimen, which, given that this probability is constant over the 50-year time horizon of the model, casts serious doubt about the validity of the findings.
In summary, the main strength of the Novartis analysis is its account for the effect of differences in GRF between treatment arms on current costs and utilities. Its main limitations are:
-
The use of treatment effectiveness data from single selected RCTs, not systematic reviews or meta-analysis, and based on pairwise indirect comparisons of those trials. The estimated effectiveness of EC-MPS compared with MMF is therefore substantially greater than that estimated from the assessment group’s systematic review and meta-analysis.
-
The model structure contains the assumption that graft failure occurs independently of GRF or the occurrence of AR. Instead, the probability of graft failure is based on the trial-derived rates at 1 year post transplant, which are then assumed to remain constant throughout the modelled period.
-
Regimens involving CSA (including the EVL regimen) had incorrect unit costs for CSA; this would underestimate the cost of those regimens.
-
The estimate of the annual cost of dialysis is from an unusual source, and substantially lower than current costs as in the NHS reference costs.
-
The AE PTDM is not included in the model (despite others being included).
Bristol-Myers Squibb’s submission
The following regimens, all following BAS induction, were compared in the Bristol-Myers Squibb submission:
-
BEL (less-intensive dosing) + MMF + steroids vs. CSA + MMF + steroids
-
BEL (less-intensive dosing) + MMF + steroids vs. TAC (immediate release) + MMF + steroids.
Two patient populations were studied, namely standard criteria donor recipients, and the ECDs recipients of de novo renal transplants. In addition, the submission presented subgroup analyses for people of weight of > 90 kg.
In its review of the effectiveness evidence, the company justifies its exclusion of SRL from the analysis arguing that, in practice, its use ‘is generally restricted to treating renal transplant patients whose renal function is steadily declining on TAC or CSA, and in whom other measures (such as dose adjustment) have not been successful’ (Bristol-Myers Squibb’s submission, chapter 3, efficacy section). As for TAC-PR, the company argued that there was insufficient direct or indirect evidence to include it as a comparator. EVL was excluded from the analysis because it lacks UK marketing authorisation. As for MMF and MPS, the company states that they were not included as comparators because they are required to be given with CCSs as part of triple therapy containing BEL, TAC or CSA.
The evidence used to populate the efficacy and safety parameters in the model used in the Bristol-Myers Squibb analysis was derived from the BENEFIT59) and BENEFIT-EXT142 trials, which compared BEL with CSA. The efficacy and safety parameter values for BEL relative to immediate-release TAC were obtained from indirect comparisons in a NMA of 32 studies, 29 of which compared TAC with CSA and three studies (including BENEFIT59 and BENEFIT-EXT142) of BEL compared with CSA.
In making the case for BEL the submission argues that the i.v. mode of administration is likely to result in increased adherence to treatment relative to TAC and CSA, which are administered orally and require routine monitoring to drug exposure and dose adjustment. The company claims that this would be expected to result in improved outcomes with BEL over the CNI comparators. Further, in setting the context of the economic evaluation (Bristol-Myers Squibb’s submission, chapter 6, Cost-effectiveness of BEL) the company states that the drivers of the evaluation were the acquisition cost of BEL, the number of years of functioning graft and the costs and utility (HRQoL) of dialysis following graft failure, which led it to perform subgroup analyses in those whose expected graft survival is short. Therefore, because ‘post-transplant renal function is a well-established predictor of graft survival this analysis focused on people with a post-transplant eGFR < 30ml/minute/1.73m2 as these people represent those for whom improved post-transplant renal function is most likely to have significant health and cost benefits’.
The analysis is based on the 3-year outcomes from the pooled data from BENEFIT59 and BENEFIT-EXT,142 including renal function (eGFR), and the cumulative incidence of NODAT, AR, PTLD, graft failure and death, where eGFR of < 15 ml/minute/1.73m2 was assumed to identify people with graft failure. The Markov model developed by Levy et al. 334 was then used to extrapolate these outcomes to the long term. To avoid repeating the description in Chapter 4 (see Identified studies), the main features of this model are summarised here.
The model represents annual transitions among the following health states:
-
functioning graft (including distinguishing four categories of renal function according to National Kidney Foundation Kidney Disease Outcomes Quality Initiative)
-
GFR stage 2 (GFR2) = ≥ 60 ml/minute/1.73 m2
-
GFR3a = 45 ml/minute/1.73 m2 ≤ GFR < 60 ml/minute/1.73 m2
-
GFR3b = 30 ml/minute/1.73 m2 ≤ GFR < 45 ml/minute/1.73 m2
-
GFR4 = 15 ml/minute/1.73 m2 ≤ GFR < 30 ml/minute/1.73 m2
-
-
graft failure/dialysis defined as:
-
GFR5 = GFR < 15 ml/minute/1.73 m2
-
-
functioning re-graft/retransplantation
-
death.
The probabilities of transitions between these states were populated by time to event models estimated by Levy et al. 334 using US registry data. The survival models were the following:
-
Weibull time to event models for graft survival [two models: (1) graft failure 1–4 years after transplant and (2) graft failure > 4 years]
-
Weibull time to event model for patient survival [two models: (1) death with a functioning graft 1–4 years after transplant and (2) death with functioning graft (DWTG) of > 4 years]
-
exponential survival model of time from graft failure to retransplant
-
exponential survival model of time from retransplant to graft failure
-
exponential patient survival on dialysis (after graft failure)
-
exponential patient survival after retransplant.
The Weibull survival model adjusted for covariates including patient age, sex, baseline eGFR, weight, NODAT, AR events, PTLD, donor type and other, calendar year, and patient and donor characteristics. 334 The conditioning of these models’ predictions on baseline eGFR allowed the derivation of separate survival curves for the different starting (i.e. at 3 years post transplant) renal functioning health states in the model. In order to assign costs and utilities for each starting eGFR group, the total time spent with a functioning graft predicted from the survival models (adjusted for death risks) was allocated to different eGFR categories by assuming that eGFR declined linearly over time from its starting level (the midpoint of the starting eGFR stage) until reaching graft failure, which was associated with an eGFR level of 15 ml/minute/1.73 m2. Thus, for example, the group of people who entered the Markov model in GFR2 (at 3 years post transplant) at the midpoint GFR level of 67.5 ml/minute/1.73 m2; those in these groups who experienced graft failure, say on the fifth annual cycle (i.e. 8 years post transplant), would be assumed to have traversed from eGFR2 to eGFR5 at an annual rate of 10.5 ml/minute/1.73 m2 [ = (67.5 – 15)/5 ml/minute/1.73 m2]. Thus, the members of this illustrative group of modelled people would have made a transition from GFR2 to GFR3a in the first year (at the end of which they would reach a GFR level of 57 ml/minute/1.73 m2), remain in eGFR during the second year (to finish it at a GFR level of 46.5 ml/minute/1.73 m2), then make a transition to, and end the third year in, GFR3b (at a GFR level of 36 ml/minute/1.73 m2), make a transition to GFR4 in the fourth year (to end the year at GFR level of 25.5 ml/minute/1.73 m2) and experience graft failure at the end of the fifth year (GFR level of 15 ml/minute/1.73 m2). In the model, some people die without graft failure, and they were assumed to have remained in the same eGFR stage as that in which they entered the model [on the basis of regression analysis of United States Renal Data System (USRDS) data on which the survival models were estimated].
After calculating expected costs and outcomes in the Markov model for each starting eGFR stage over 37 years (which, added to the initial 3-year period, amounts to the modelled horizon of 40 years adopted in the base case), the expected costs and outcomes for the whole population were calculated by a weighted average of the expected costs and QALYs across starting model stages. The proportions were the frequency distributions of people at 3 years post transplant across functioning graft stages (approximated by a normal distribution using mean and SD of eGFR values), dialysis stage and death. Finally, the expected costs and QALYs over the extrapolated Markov phase were added to costs and QALYs associated with the observed trial outcomes in the trial to calculate total QALYs and costs over 40 years for each trial arm in BENEFIT59 and BENEFIT-EXT. 142
Efficacy parameter estimates
The main inputs for the model were those estimated from the NMA at 36 months. These are presented in Table 142, which reproduces table in the industry submission (Bristol-Myers Squibb’s submission, section 6.1, Model inputs, table 28). In the model, the effect of NODAT on graft and patient survival curves is accounted for by applying HRs from the literature. 365 PTLD and CVD were accounted for in the model by assigning a 50% chance of death to each of them. The sources of these estimates were not given.
| Outcome | OR (95% CI) | |
|---|---|---|
| TAC vs. CSA | BEL vs. CSA | |
| Graft lossa | 0.86 (0.63 to 1.17) | 0.92 (0.44 to 1.93) |
| Patient deatha | 1.27 (0.88 to 1.89) | 0.77 (0.37 to 1.55) |
| AR event | 0.63 (0.50 to 0.81) | 1.57 (0.80 to 3.03) |
| Difference in true mean value (95% CI) | ||
| eGFRa | 6.20 (0.64 to 12.47) | 16.04 (6.19 to 25.53) |
According to the Bristol-Myers Squibb submission, the distribution of the patient cohort at the start of the Markov model for each of the three regimens evaluated – BEL, TAC and CSA – was calculated from the pooled BENEFIT59 and BENEFIT-EXT142 trial data on GFR outcomes at 36 months post transplant. They assumed that GFR level followed a normal distribution to derive the distribution across functioning graft states, and used the observed means of 38.6 and SD of 22.93 for CSA, 54.64 for BEL (from the BENEFIT59 trials) and 44.8 for TAC (from NMA relative to CSA). But the assumption of normally distributed GFR is problematic, as it implies that in the CSA arm, 4.6% of people at the end of the trial phase (and therefore at the start the Markov model phase) have a negative GFR value. However, inspection of the model’s Excel spreadsheets revealed that these values were not used in the model, but rather a mean of 50.80 and SD of 21.80 for CSA, which implies that 0.9% of people have a negative GFR value at 3 years post transplant. The means for TAC and BEL were, in turn, 58.47 and 66.96, and they also applied the SD 21.80 for CSA (these imply negative GFR values for < 0.4% of people).
To validate the survival curves underpinning its Markov model, which were estimated from US data, the company compared the predictions from its Weibull survival models with UK data from the NHSBT 2013 report355 (these have been discussed in relation to the model submitted by Astellas, submission section 6.1). The predicted survival curves from the Bristol-Myers Squibb model by type of donor (DBD and DCD) are compared with the corresponding UK data points at year 1, 2 and 5 post transplant. Owing to the difficulty of visualising the chart presented by Bristol-Myers Squibb (Bristol-Myers Squibb’s submission, figure 22), the 5-year survival curves reported by the NHSBT 2013 report are reproduced in Figure 66, alongside the corresponding predictions in the survival model informing the Markov model in the company’s submission. It shows that the model predictions for the DBD graft survival (DBD predictions based on USRDS) converge towards actual UK data for the corresponding donor type. The model predictions based on the DCD patient population, however, appear to diverge from the trend observed in UK data for each donor type. This is of concern, as predictions from this model were used to extrapolate 3-year trial outcomes for 37 years.
FIGURE 66.
Validation of first adult kidney-only graft survival predictions of the Bristol-Myers Squibb model (based on US data from the USRDS) with NHS data (NHSBT) by donor type.
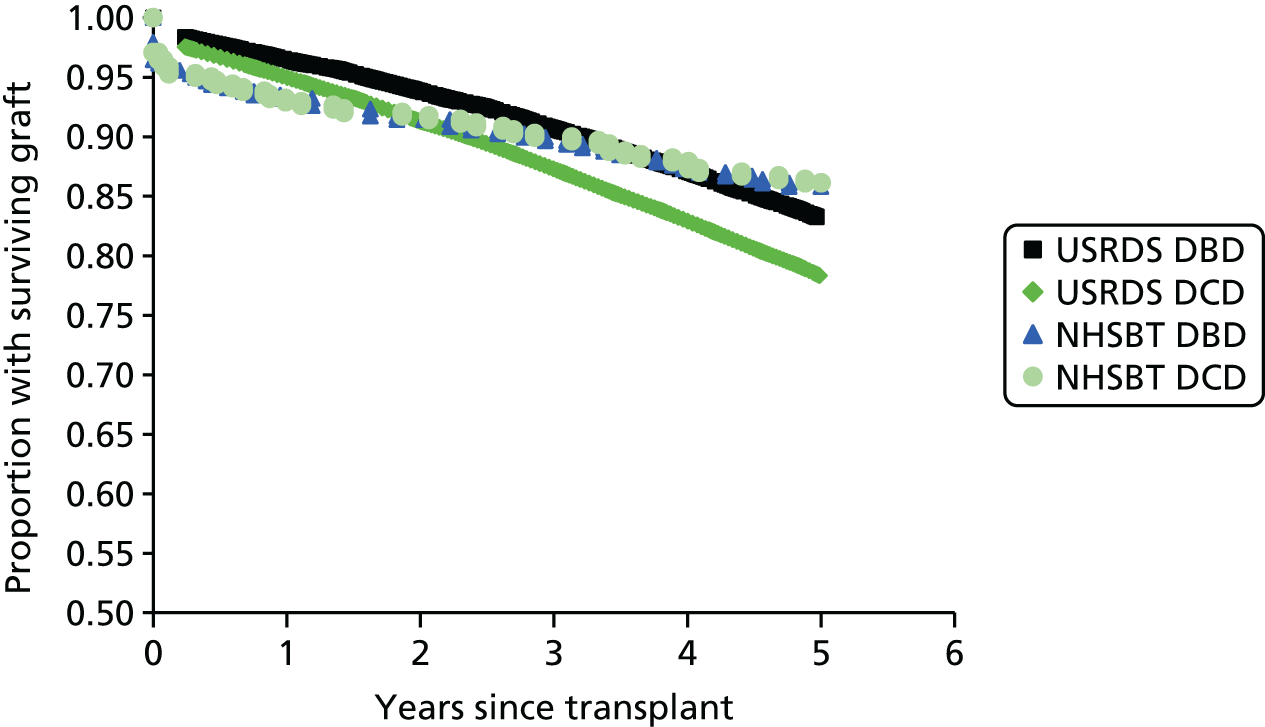
Changes in eGFR stages were associated with changes in utilities and costs. Utilities were derived from a cross-sectional study of UK renal transplant patients. 338 AEs including AR, NODAT and PTLD were given estimated annual utility losses of 0.50, 0.06 and 0.44, respectively, reported from the literature.
Costs
The submission provides actual data on estimated costs of clinical events following transplantation in standard practice at a single centre in Wales (Table 143). The analysis has been published as part of a multinational study report (described in Chamberlain et al. 42 Assessment of cost-effectiveness, Results), which shows some common and divergent practice between this site and other European centres. Briefly, costs were estimated in a retrospective analysis of computerised records from the Cardiff Renal Transplant Database, related to all individuals aged ≥ 18 years who received a kidney-only transplant recorded between January 1998 and December 2005. They were followed up to 3 years, and the analysis included those in whom data were recorded for at least 12 months after transplant and whose data included their most recent transplant in the studied period.
| Functioning graft | Costs (£)a | Utilitiesb | ||
|---|---|---|---|---|
| BEL | TAC | CSA | ||
| GFR2, year 1 | 5580 | 5677 | 5600 | 0.64 |
| GFR3a, year 1 | 5637 | 5735 | 5657 | 0.58 |
| GFR3b, year 1 | 7800 | 7897 | 7820 | 0.58 |
| GFR4, year 1 | 8132 | 8230 | 8152 | 0.49 |
| GFR2, year 2 | 1562 | 1659 | 1582 | 0.64 |
| GFR3a, year 2 | 1850 | 1947 | 1870 | 0.58 |
| GFR3b, year 2 | 3073 | 3170 | 3093 | 0.58 |
| GFR4, year 2 | 4102 | 4200 | 4122 | 0.49 |
| GFR2, year 3+ | 1570 | 1668 | 1590 | 0.64 |
| GFR3a, year 3+ | 1922 | 2019 | 1942 | 0.58 |
| GFR3b, year 3+ | 3366 | 3433 | 3355 | 0.58 |
| GFR4, year 3+ | 4258 | 4356 | 4278 | 0.49 |
| Dialysis | 43,650 | 43,748 | 43,670 | 0.28 |
| Functioning regraft | 7190 | 7,288 | 7210 | TAC: 0.59c BEL or CSA: 0.60c |
| One-time cost of graft failure | ||||
| Year 1 | 1384 | |||
| Year 2 | 431 | |||
| Year 3+ | 191 | |||
| One time costs/disutility of PTLD | 4890 | 0.44 | ||
| One time costs/disutility of AR | 3483.28 | 0.50 | ||
The study provided evidence that was previously unavailable for the UK on actual costs of post-transplantation care and events stratified by GFR at 1 year post transplant. The sample for analysis included 370 people in whom a variety of treatment regimens were used. Of the 20 different treatments used in this period, triple therapy with TAC steroids and AZA was the most frequent (19%), followed by triple therapy with TAC, steroids and MMF (18%). The next most frequently used regimens were double therapy with TAC and AZA or TAC with MMF (9% each). By the second year the proportion of people on these TAC triple regimens had declined (to 14% and 12% of the sample), whereas the proportion of people on the double therapy TAC had increased (to 14% and 13%). The same observation was made from 24 months to the 24+ months’ follow-up point.
Another aspect of this data source is the observed number of TAC immunosuppressant doses used over the follow-up period in this sample. Although, the dose of TAC, given as part of triple therapy alongside MMF and steroids, was continually reduced over the first year from the mean of 10.31 mg at month 1 to 6.36 mg at month 12, and was 5.73 mg and 5.71 mg at month 24 and month 24+, respectively, the dose was kept at 11.23 mg throughout the observation period in the triple regimen that included AZA (Bristol-Myers Squibb’s submission, appendix 5, Preliminary report PORTRAIT database study Cardiff).
On the basis of the resource-use estimates from the PORTRAIT study report, the TAC drug regimen and the CSA regimen costs were estimated. Drug use was valued at BNF 67369 prices [for TAC, the average price of immediate-release TAC 1 mg of 50- and 100-capsule packs was used; for CSA, the average prices of Capimune® (Mylan), Capsorin® (Morningside Pharmaceuticals Ltd), Deximune® (Dexcel Pharma Ltd) and Neoral® (Novartis), 30-capsule packs, were used]. Administration costs included one laboratory test per outpatient appointment to determine CNI level, and accounted for the observed number of outpatient appointments in years 1, 2 and 2+. The costs of BEL administration included the costs of i.v. infusion, which were obtained from a previous HTA report on abatacept (from which BEL was derived, and that has the same method and frequency of administration). Thus, the annual drug acquisition and administration costs of the regimens in the first year of the model for a 75-kg patient were £13,472 for BEL, £3937 for TAC (immediate-release TAC) and £1972 for CSA. These costs were smaller in the second and subsequent years by about 30%, 25% and 15% in the BEL, TAC and CSA arms, respectively.
Results of Bristol-Myers Squibb’s analyses
In the base-case results for a cohort of people with a starting average age of 43 years, at 40 years post initial transplant 11% of people would be alive under BEL, whereas that would be 8.8% under TAC and 7.4% under CSA. By that point, in 75.6% of people the graft would have failed under BEL, whereas that would have happened in 73.8% of people under TAC and 76.9% under CSA. Correspondingly, 19.3% of people received retransplantation under BEL, 19.2% under TAC and 20.6% under CSA.
When comparing total discounted costs, BEL resulted in incremental costs of £91,001 over TAC and £92,216 over CSA. In turn, the incremental discounted QALYs were 0.62 relative to TAC and 0.97 relative to CSA. The incremental cost per additional QALY of BEL relative to TAC was £147,334, whereas that for TAC relative to CSA was £3375.
These results were driven by the higher costs of BEL immunosuppression, which, despite its associated savings in dialysis costs relative to the other regimens (£15,469 relative to CSA and £2248 relative to TAC), incurred seven and three times the cost of immunosuppression of the CSA (additional costs £109,402) and TAC (£95,159 difference) regimens, respectively. These results were confirmed by PSAs and deterministic sensitivity analyses, which showed the ICER to be insensitive to variation in uncertain parameters.
The submission presented additional analyses for a special group of people with a shorter expected graft survival than that for the overall patient population. This is referred to as ‘subgroup analysis’ by the company, and implemented by defining the group as those people with GFR of < 30 ml/minute/1.73 m2 at 1 year post transplant. They implement a post-hoc adjustment to the model so that the effect of eGFR improvements within that range may be accounted for in the model, which originally was specified in discrete eGFR categories and thus restricted all people entering the model in the same category to having the same benefits. The company found that, in these people, BEL results in higher benefits (0.46 extra QALYs in both comparisons) and lower costs (–£1478 relative to CSA and –£4166 relative to TAC).
However, this analysis suffers from a logical flaw. It assumes that those people whom the company claims to have identified as able to benefit from their drug regimen may be identified with precision. In fact they may not. The meaningful definition of subgroup analyses in a setting where risk and uncertainty influence the outcomes of treatment such as this, so that the outputs of a decision model are mathematic expectations of cost and benefits, identifies a selected group of people for special management on the basis of observable characteristics defined at the outset. The defining characteristic of the selected group of people in the subgroup analysis by Bristol-Myers Squibb is an outcome of treatment, and thus not known at the time of transplant (which would be required for sound decision-making analysis about choice of maintenance treatment).
A subgroup analysis presented by Bristol-Myers Squibb finds that BEL may be cost-effective in people with body weight of approximately 90 kg and more. At this body weight, BEL use incurs minimal vial wastage, thus maximising effectiveness for the given cost.
Critique
The model captures all the most important clinical outcomes and AEs arising post transplantation, and accounts for the role of renal function as a prognostic factor for long-term graft survival and its contemporaneous effects on HRQoL and costs. It also accounts for the effect of short-term AR on longer-term graft and patient survival.
A major strength of the evidence presented by Bristol-Myers Squibb is the cost study used to populate the costs of immunosuppressant drug use and administration in the model and the costs associated with renal function. This evidence has been reported as part of a wider study42 in a peer-reviewed publication.
The major limitation of this study is the questionable generalisability of the values used to populate the transition probabilities of the model used to extrapolate short-term trial outcomes to 40 years. The survival models that inform the transition probabilities to the key events, that is, graft failure after transplant, time to retransplantation after graft failure, and possibly patient survival with a functioning graft, may reflect the experience of a patient population that does not correspond to that of the UK.
Another issue is the use of efficacy differences between regimens at 3 years post transplant to populate the entire initial 3 years, as if these differences had occurred from day 1 and remained constant until the end of the third year post transplantation, which we know was not the case, and bias the analysis in favour of BEL, the company’s drug. In fact, inspection of the model spreadsheet reveals that discounting was not applied to the first 3-year costs and benefits.
A methodological limitation is the assumed linear, constant decline in eGFR, which was the driver of the Markov model used to extrapolate outcomes beyond 3 years, in order to estimate quality of life over the graft survival period conditional on initial eGFR value. This, in turn, reflected the limited information available on renal function from registry data; studies using multicentre cohorts could potentially address this issue by measuring, rather than imputing, renal function periods of longer than 2–3 years, which are typically found in the experimental literature.
In summary, the Bristol-Myers Squibb model has numerous strengths, but has the following main limitations:
-
The use of US data to extrapolate the survival data for key transition probabilities to 40 years (graft failure, time-to-retransplantation after failure).
-
The use of efficacy differences between regimens at 3 years post transplant to invalidly calculate benefit differences throughout the first 3 years in the cost-effectiveness model, which favours the company’s drug, BEL.
-
Lack of accounting for the costs of concomitant regimens used in the triple-therapy regimens investigated by the RCTs, which served as the source of efficacy values in the model (discussed in Comparison between the model submissions).
-
Lack of discounting of costs and QALYs in the first 3 years of the analysis, which invalidly raises the benefits of BEL proportionally more than it increases its incremental costs.
-
The assumed linear decline in eGFR 3 years post transplant at a rate with no validation or sensitivity analysis of this assumption.
-
A ‘subgroup analysis’ based on people with poor GRF at 1 year, but who would not be identifiable at the time of starting maintenance immunosuppression (and therefore also outside the scope of this technology assessment)
-
Another subgroup analysis, of those with a body weight of 90 kg, should be disregarded, as this subgroup is based only on the cost differences that would be affected by the patient’s weight.
Comparison between the model submissions
Besides the treatment comparisons, the company submissions also differ in terms of the models used to evaluate those treatments (Table 144). Table 145 highlights how useful the evidence provided in each of the economic evaluations may be to inform the decision-making. Given the necessity to extrapolate short-term outcomes reported in trials with typical follow-ups of 1–3 years, the main differences between extrapolating models used by the three companies are reflected in the choice of surrogate outcome used to drive the disease course in people with renal transplantation and the duration of any relative effects of treatments.
| Study | Population | Comparators: initial and maintenance | Horizon (years) | Model structure | Surrogates to model long term | Health states/events modelled | Risk factors | AEs | Model drivers (sensitivity analysis) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Astellas | Age 45 years 70.3 kg England and Wales |
|
25 | Markov model of annual cycles with tunnel states extrapolation of one-trial outcomes | AR Adherence (for analysis of immediate-release TAC vs. extended-release TAC only) |
Functioning graft – no previous BPAR Functioning graft – previous BPAR Failed graft (dialysis), Functioning regraft – no previous BPAR Functioning regraft – previous BPAR Death |
BPAR | Malignancies CMV infections PTDM Wound-healing disorders Anaemia HMGCoA Hypertension |
Improved adherence with extended-release medication Immediate-release TAC vs. SRL: Graft survival [scenario with graft survival in SYMPHONY trial (CNI minimisation) with DAC induction] |
Assumes that BPAR occurs only in the first 12 months Graft and patient survival were estimated from UK transplant 5-year survival statistics (UK NHSBT report 2012–13) extrapolated to 25 by exponential function of time Survival in dialysis was estimated from 10-year UK survival statistics, extrapolated by exponential function Utility values of AEs not accounted for Model has flaws of implementation, especially in relation to retransplants |
| Bristol-Myers Squibb | Age 43 years 69% male 75 kg BENEFIT trial59 (low risk) Reduced kidney function (GFR) BENEFIT-EXT142 trial (ECD) |
BEL CSA TAC |
40 | Markov model of annual cycles extrapolation (Levy et al.334 model) of three trial outcomes | AR GFRs |
Functioning graft stratified by level of renal function (eGFR of ≥ 60 ml/minute/1.73 m2 45 ml/minute/1.73 m2 ≤ eGFR < 60 ml/minute/1.73 m2, 30 ml/minute/1.73 m2 ≤ eGFR < 45 ml/minute/1.73 m2, 15 ml/minute/1.73 m2 ≤ eGFR < 30 ml/minute/1.73 m2 Failed graft (eGFR < 15 ml/minute/1.73 m2), functioning regraft, death |
Renal function AR, NODAT (separate from main model) Donor and recipient characteristics |
NODAT AR PTLD |
Price of IS (acquisition costs of BEL) Number of years with functioning graft Cost and utility of dialysis |
Based on observational study of resource utilisation of 3-year follow-up Based on surrogate clinical outcome model estimated from US patient population BEL not cost-effective for renal transplant population Conclusion that it is ‘likely cost-effective in ECD recipients, or in those anticipated to have low kidney function (GFR) post-transplantation and short graft survival’ is flawed Case made for use in higher weight categories/those requiring higher doses of IS Includes costs of IS administration |
| Novartis | Age 45.7 years eGFR 9.03 ml/minute/1.73 m2 Weight 70 kg 68% male England and Wales |
EVL + CSA (low dose) vs
|
50 | Individual patient, discrete event simulation model | GFRs (annual rate of change) |
|
None | Proteinuria BKV CMV Hyperlipidaemia Delayed wound healing Hypertension |
EVL vs. TAC and EVL vs. MMF:
|
eGFR (CKD stage) drives patient mortality; graft survival is an independent event, based on treatment-specific first-year post-transplant probabilities All costs of AEs measured; only disutilities of proteinuria and hypertension were measured CKD monitoring costs were included Interpretation of results of EC-MPS vs. MMF comparison is flawed: model is non-linear in uncertain parameters and PSA results provide correct base-case results, i.e. EC-MPS ICER falls between £20,000 and £30,000 Mistake found in calculation of CSA costs |
| Item | Astellas’ submission | Novartis’ submission | Bristol-Myers Squibb’s submission | |
|---|---|---|---|---|
| I&M | I&M | I&M | ||
| 1. | Is the study population clearly described? | Y | Y | Y |
| 2. | Are competing alternatives clearly described? | Y | Y | Y |
| 3. | Is a well-defined research question posed in answerable form? | Y | Y | Y |
| 4. | Is the economic study design appropriate to the stated objective? | Y | Y | Y |
| 5. | Is the chosen time horizon appropriate to include relevant costs and consequences? | Y | Y | Y |
| 6. | Is the actual perspective chosen appropriate? | Y | Y | Y |
| 7. | Are all important and relevant costs for each alternative identified? | Y | N | Y |
| 8. | Are all costs measured appropriately in physical units? | Y | Y | Y |
| 9. | Are costs valued appropriately? | Y | Y | N |
| 10. | Are all important and relevant outcomes for each alternative identified? | N | N | Y |
| 11. | Are all outcomes measured appropriately? | Y | Y | Y |
| 12. | Are outcomes valued appropriately? | Y | Y | Y |
| 13. | Is an incremental analysis of costs and outcomes of alternatives performed? | Y | Y | Y |
| 14. | Are all future costs and outcomes discounted appropriately? | Y | Y | Y |
| 15. | Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | Y | Y | N |
| 16. | Do the conclusions follow from the data reported? | Y | Y | N |
| 17. | Does the study discuss the generalisability of the results to other settings and patient/client groups? | N | Y | N |
| 18. | Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | N | N | N |
| 19. | Are ethical and distributional issues discussed appropriately? | N | N | N |
The submission by Astellas uses a Markov structure to model the disease evolution and the effects of treatment in the relevant cohort of people. In this model the occurrence of BPAR in the first year post transplant (for the first transplant and any second transplant occurring in the first year of the model) affects the probability of graft failure in subsequent years. Renal function plays no role in this model. In contrast, differences in eGFR changes between the triple-therapy regimens in the first year drive the modelled outcomes of subsequent years in the model by Novartis. Although the risk, costs and HRQoL consequences associated with ARs are accounted for in this model, these events do not affect graft survival. Graft failure is thus as likely to occur while individuals are at CKD stages 1 and 2 as when they are at CKD stage 5, and any state in between those two extremes for that matter. The model by Bristol-Myers Squibb, unlike that by Novartis, assumes that eGFR at the end of year 1 determines graft survival. However, unlike Astellas and similarly to Novartis, the Bristol-Myers Squibb model allows for the costs and consequences of changes in eGFR over time in the functioning graft state and for the effect of eGRF on the probability of patient death. An additional advantage of the Bristol-Myers Squibb analysis over that of Novartis is its allowance for the effects of AR in the first year post transplant to affect patient and graft survival thereafter, as the analysis by Astellas does for the graft survival only.
The figures adopted by the Novartis submission seem to underestimate the costs of twice-daily immediate-release TAC doses. Their cost per mg for TAC is £0.82, whereas the weighted average figure for the market share of the different presentations used by Astellas is £1.618. On the other hand, the mean daily dose at 70 kg body weight for TAC in the Novartis submission is 17.5 mg, whereas the average daily dose for the first year used by Astellas is 7.17 mg. This results in an average maintenance monthly cost of TAC that is 24% higher in the model by Novartis than in the model by Astellas (i.e. £438 vs. £353 per month).
Other differences were found in terms of the unit costs of the MMF therapy. Novartis used a £9.65 price per pack of 50 tablets of 500 mg each, obtained from market data [Commercial Medicines Unit (CMU) Electronic Market Information Tool (eMit) 2014370], whereas Astellas used a price almost 10 times higher: £82.26 per pack of 50 capsules of 500 mg, citing BNF 2014. 56 The effect of the chosen MMF price is also different across the submitted analyses, as MMF is a concomitant medication across all immunosuppressive regimens analysed in the evaluation by Astellas, whereas in the Novartis analysis MMF is not part of the regimens involving the company’s own therapies (i.e. EVL and EC-MPS). Thus, although across submissions the treatment regimens that include the companies’ drugs may be associated with increased effectiveness, a higher MMF price has different implications across the submissions: it makes it less attractive for the NHS to adopt such a regimen (as people live longer and incur higher drug costs) in the Astellas analysis, whereas the opposite occurs in the Novartis case (as only the cost of comparator regimens increases).
Although the three models submitted to NICE for this assessment varied in terms of the way the health course of an individual evolved and the use of immunosuppression affected such path, accounting of costs was similar in some aspects once the cycle length of models was taken into account. Table 146 presents the most important costs for those elements that were common across the models.
| Company | Astellasa | Bristol-Myers Squibbb,c | Novartisa,c |
|---|---|---|---|
| TAC therapy (per year) | 4255d | 3937 (first) 2821 (second plus)e |
5283 |
| TAC administration | 0 | 386 (first) 89 (second)e |
0 |
| MMF therapy (per year) | 2402f | 0g | 282h |
| CSA therapy | NAi | 1971 (first) 1562 (second plus)e |
839 (first) 694 (second plus) |
| CSA administration | 0 | 386 (first) 90 (second)e |
0 |
| BEL (per year) | 10,966 (first) 6480 (second-plus) |
13,472 (first) 9217 (second plus) |
NA |
| BEL administration | 0 | 2457 (first) 1996 (second plus) |
NA |
| CCSs | 178 | 0g | 285 |
| AR (event) | 1738 | 3483 | 1725 |
| Dialysis (per year) | 38,387j | 43,586k | 22,877l |
| Retransplantation | 25,953 | 25,908 | 17,736 |
| Retransplantation: organ procurement | 0 | 12,954 | 0 |
Although the acquisition costs of TAC are comparable across the three industry submissions, only the one by Bristol-Myers Squibb reports any estimates of drug administration, which have the merit of being based on observed data as opposed to assumptions about compliance with dosing guidelines or protocols. With respect to immunosuppression costs, it may be noted that Bristol-Myers Squibb did not account for costs of other concomitant drugs that are part of triple-therapy immunosuppression (e.g. MMF + CCSs, which were given in BENEFIT59 and BENEFIT-EXT142).
More importantly for the results is the observation that Bristol-Myers Squibb used an estimate of dialysis costs372 that was twice the size of the estimate adopted by Novartis (NICE costing guideline 2011374) and almost 13% higher than that of Astellas. 371 Given the driving influence of dialysis costs for cost-effectiveness and an issue to be discussed next in relation to the time spent on dialysis in the models, the quality of evidence gained by the Bristol-Myers Squibb model in estimating immunosuppression-related costs and event costs may have been partly offset by an overestimation of the cost savings to be obtained from reducing the time for which people experienced dialysis.
In Table 147, the key features of the effectiveness elements of the analyses performed by the companies are presented. A salient aspect of the comparison model specifications is the longer expected time to retransplantation at the time dialysis starts for those people whose graft fails in the Bristol-Myers Squibb model. It is noted that this estimate was derived from an exponential survival model from an older patient sample in the USA (Medicare-covered transplant-only people). This model has a hazard (instantaneous probability) of receiving a transplant that is constant over time and that is predicted according to donor and patient characteristics (Levy et al. 334). In the Bristol-Myers Squibb model these characteristics are fixed over time and result in the constant annual probability of 4% of receiving a transplant while on dialysis. This means that the expected waiting time for a retransplant in a US sample with the Bristol-Myers Squibb’s model characteristics (which match the BENEFIT59 and BENEFIT-EXT142 sample characteristics), as detailed in the Bristol-Myers Squibb submission, is 16.5 years at the start of dialysis. This waiting time is clearly longer than the waiting time currently expected in the UK, which may be closer to the values adopted by Astellas and Novartis in their models.
| Company | Astellasa | Bristol-Myers Squibbb | Novartisc | |
|---|---|---|---|---|
| Time to graft failure (median) | Without BPAR at 12 months: 23 | Initial GFR2 15.0 | EVL: 15.8 | |
| Initial GFR3a 11.5 | EC-MPS: 21.3 | |||
| With BPAR at 12 months: > 25c | Initial GFR3b 7.0 | MMF + CSA: 7.2 | ||
| Initial GFR4 2.5 | TAC + CSA: 8.3 | |||
| Time to transplantation from graft failure (mean unless otherwise stated) | 3.5 (median) | 16.5d | 3 (SD 1) | |
| Annual change in GFR | NA | –3 (fourth plus) | –1.66 (second) –2.68 (third plus) |
|
| Utility of functioning state | First transplant | 0.71 | 0.49–0.64 (depending on GFR stage) | 0.49–0.64 (depending on GFR stage) |
| Second-plus transplants | 0.71 | 0.59 | 0.49–0.64 (depending on GFR stage) | |
| Utility of dialysis state | 0.459 | 0.28 | 0.28 | |
In any case, the median time to retransplant may also be unrealistic for the USA, even after considering issues about socioeconomic barriers to access and related features of that system. After inspection of the estimated coefficients of the exponential model reported by Levy et al. 334 (supplementary material, file 1, and reproduced by the Bristol-Myers Squibb submission as appendix 4, table 1), the age covariate (which remains fixed at 40.3 years throughout the 40 annual cycles of the Markov model, so that those proportions of the cohort who experience graft failure early in the model have the same probability of receiving a retransplant in any given cycle as that people who experience graft failure in the latter part of the modelled time horizon) is positively associated with the probability of retransplant, which means that those who start dialysis at older ages have shorter expected waits for a retransplant and suggests that the model was estimated in a cohort of much older people than the Bristol-Myers Squibb’s modelled age of 40 years (e.g. for graft failure at age 70 years the model yields an expected wait of approximately 10 years to receive a retransplant).
The overestimation of time to retransplant in the Bristol-Myers Squibb model that was just described has the implication of overestimating the time on dialysis with its associated costs and loss in quality of life. This, in turn, means that the model is likely to overestimate the benefits of any advantages in terms of graft survival that BEL has over its comparators, TAC and CSA. Likewise, this probably exaggerates the costs savings and quality-of-life gains of TAC over CSA, which suggest that its ICER (£3375; this was not stated in the Bristol-Myers Squibb submission but implicit in their numbers and calculated from them by PenTAG) is an underestimate. Table 148 presents a summary of model outputs for the three industry model submissions.
| Submission | Regimen compared | Patient characteristics | Life-years | QALYs (discounted) | Discounted costs (£) | ICER: incremental cost per QALY |
|---|---|---|---|---|---|---|
| Astellas | TAC b.i.d. | Mean age 45 years Weight 70.3 kg |
17.88 | 8.01 | 130,118 | TAC vs. SRL I: £1,651,801 TAC vs. SRL II: £170,681 |
| SRL I | 17.82 | 7.99 | 104,905 | |||
| EVL | 17.80 | 7.99 | 142,995 | |||
| SRL II | 17.73 | 7.94 | 119,371 | |||
| BEL | 11.72 | 7.94 | 163,740 | |||
| TAC o.d. | 18.19 | 8.21 | 118,907 | TAC o.d. dominates | ||
| TAC b.i.d. | 17.88 | 8.01 | 130,118 | |||
| Bristol-Myers Squibb | BEL | Mean age 43 years Weight 75 kg |
19.53 | 7.14 | 296,503 | BEL vs. TAC: £149,182 TAC vs. CSA: £3375 |
| TAC | 18.02 | 6.53 | 205,502 | |||
| CSA | 17.38 | 6.17 | 204,287 | |||
| Novartis | EVL + CSA (low dose) | Mean age 45.7 years (SD 12.7 years) Weight 70 kg (SD 10 kg) Mean eGFR 9.03 ml/minute/1.73 m2 (SD 7.9 ml/minute/1.73 m2) |
25.71 | 8.86 | 135,358 | EVL dominant |
| TAC+ MMF | 23.39 | 7.37 | 140,972 | |||
| EVL + CSA (low dose) | 25.80 | 8.89 | 136,180 | MM + CSA vs. EVE + CSA: > £200,000 | ||
| MMF + CSA | 24.04 | 7.89 | 76,826 | |||
| EC-MPS + MMF | 25.48 | 8.69 | 87,359 | EC-MPS vs. MMF + CSA: £29,000 | ||
| MMF + CSA | 24.17 | 7.89 | 76,771 | |||
Chapter 6 Peninsula Technology Assessment Group economic assessment
Summary
Methods
A de novo economic model was developed to address the decision problem in a cost–utility analysis. A discrete time-state transition model (semi-Markov) was used, in which transition probabilities were dependent on age and time since initial transplantation. A cycle length of a quarter year was used, and transitions were assumed to occur mid-cycle. A time horizon of 50 years was adopted. Costs were included from a NHS and Personal Social Services (PSS) perspective. Health effects were measured in QALYs and were calculated by assuming health state-specific utility decrements from a baseline utility that was age dependent and derived from the Health Survey for England 2012. 375 The utility decrements were based on a published systematic review and meta-analysis of preference-based quality-of-life studies in patients undergoing RRT, with the European Quality of Life-5 Dimensions, three-level version (EQ-5D-3L) used for measurement and most likely valued using the UK valuation tariff based on a representative sample of the general population (see Estimating resources and costs). 376 Costs and QALYs were discounted at 3.5% per annum and costs were inflated as necessary to 2014–15 prices.
Interventions and comparators
The following induction agents were included:
-
BAS
-
rATG.
Regimens not including induction by monoclonal or polyclonal antibodies were also included.
The following maintenance agents were included:
-
immediate-release TAC
-
TAC-PR
-
MMF
-
MPS
-
SRL
-
EVL
-
BEL.
Regimens including CSA and/or AZA were also included. CCSs were assumed to be used in all regimens, but at a tapered dose.
Sixteen regimens were modelled in total:
-
CSA + MMF
-
TAC + MMF
-
CSA + AZA
-
TAC + AZA
-
CSA + EVL
-
TAC + SRL
-
TAC-PR + MMF
-
BAS + CSA + MMF
-
BAS + TAC + MMF
-
BAS + CSA + AZA
-
BAS + SRL + MMF
-
BAS + BEL + MMF
-
BAS + CSA + MPS
-
rATG + CSA + MMF
-
rATG + TAC + MMF
-
rATG + CSA + AZA.
Model structure
Kidney transplant recipients were assumed to be in one of three health states at any time: functioning graft, graft loss or death (see Finalised structure, and Figure 67). In the functioning graft state, KTRs were not dependent on dialysis, whereas in the graft loss state, KTRs were dialysis dependent. In addition to these health states, for each regimen the incidence of AR, CMV infection, dyslipidaemia and NODAT was estimated, with corresponding costs (during the first year for AR and CMV infection; ongoing for dyslipidaemia and NODAT). NODAT was also associated with a utility decrement based on EQ-5D measurements from kidney transplant patients in a US clinic, valued according to a US valuation tariff (see Disutility due to diabetes mellitus). 7 The incidence of AR and NODAT were also used as surrogate determinants of graft survival and DWFG (NODAT only).
Up to two retransplantations were modelled, which could take place from the graft loss state or from the functioning graft state (for the initial graft only) corresponding to pre-emptive retransplantation. KTRs would transition to the next functioning graft state if the retransplantation was successful or to the next graft loss state if it was unsuccessful (i.e. in the event of PNF). The rate of retransplantations was assumed to reduce with age > 65 years, reaching zero by age 80 years (see Retransplantation).
Transitions out of the functioning graft state correspond to the clinical outcome of graft loss/survival and are either DWFG or graft loss excluding DWFG (i.e. dependence on dialysis or pre-emptive retransplantation). The baseline rates of these transitions from functioning graft were calculated from data from the UK Transplant Registry standard data set. 377 The rate of mortality following graft loss was based on UK data published in the UK Renal Registry 16th Annual Report339 (see Factors included in the model).
Baseline death-censored graft survival was taken directly for the first year from Kaplan–Meier analysis, and from the first year onwards a Weibull curve was fitted, which was demonstrated to fit the data well.
Death-censored graft survival at 1 year was estimated for each regimen, based on the ORs of graft loss within 12 months. This was incorporated into the model by applying a proportional odds assumption to death-censored graft survival in the first year.
A surrogate relationship between AR, NODAT and GRF (eGFR) at 12 months and graft survival was modelled, based on applying a HR to the Weibull curve after the first year (see Graft survival). The HR for AR was 1.6,378 for NODAT, 1.12,378 and for eGFR, 1–5.80, depending on the eGFR interval. 334
Patient survival at 1 year was estimated for each regimen based on the OR of mortality within 12 months. This was incorporated into the model by applying a regimen-specific HR of DWFG within the first year.
A surrogate relationship between NODAT and DWFG after the first year was also modelled, with a HR of 1.41. 378
Source of effectiveness estimates
The ORs for the incidence of BPAR, graft loss and patient mortality, and the absolute difference in eGFR, were primarily estimated from the NMAs of clinical effectiveness evidence. The results for induction agents and maintenance regimens were chained assuming independence. The results for TAC-PR + MMF and BAS + CSA + MPS were based on results for TAC + MMF and BAS + CSA + MMF with additional adjustment based on head-to-head comparisons (see Effectiveness estimates for further details).
The incidences of NODAT, CMV and dyslipidaemia were also estimated using NMAs of RCTs from the systematic review of clinical effectiveness, although some simplifying assumptions were made to overcome the limited amount of evidence.
Costs
See Estimating resources and costs for further details.
Drug acquisition costs were average NHS acquisition costs where these could be estimated (from the CMU eMit database370) or the list prices (BNF 6856) otherwise.
Drug administration costs included i.v. administration for BAS, rATG and BEL (estimated from NHS reference costs 2013–14),64 and therapeutic drug monitoring for TAC, SRL, EVL and CSA (estimated from a price list for NHS patients from University Hospital of Wales).
Costs of procedures and dialysis were estimated from NHS reference costs 2013–14,64 where available, or from UK sources otherwise.
The costs of AR and CMV infection were taken from a microcosting study commissioned by Bristol-Myers Squibb. 379
The significant costs of NODAT were estimated from a recent publication based on the UK Prospective Diabetes Study (UKPDS),380 which was conducted in the general population with type 2 diabetes mellitus.
The costs of KTR follow-up and monitoring were estimated based on a database study commissioned by Bristol-Myers Squibb.
Infection prophylaxis costs were estimated based on the kidney transplant protocol of a UK hospital. Additional CMV prophylaxis costs for regimens containing rATG induction.
Uncertainty analyses
A PSA was conducted to estimate the joint effect of parameter estimation uncertainty on cost-effectiveness. Structural sensitivity analyses relating to graft survival were conducted. A scenario analysis in which list prices were adopted for all drug acquisition costs was performed, and a two-way threshold analysis was conducted relating to the costs of BEL.
Results
Base-case analyses
See Base-case analysis for further details.
In the base-case deterministic and probabilistic analyses, the following agents were predicted to be cost-effective at £20,000 and £30,000 per QALY:
-
BAS
-
immediate-release TAC
-
MMF
Relevant ICERs do not exist for these agents because they dominated other agents or were less costly and less effective than other agents, with ICERs significantly > £30,000 per QALY.
When all regimens were simultaneously compared, only BAS + TAC + MMF was predicted to be cost-effective at £20,000 and £30,000 per QALY.
Deterministic and probabilistic cost-effectiveness results for other agents were:
-
No induction (three comparisons) – Dominated in deterministic and probabilistic analyses.
-
rATG (three comparisons) – Dominated in deterministic and probabilistic analyses.
-
CSA (four comparisons) – Deterministic ICERs of £131,000–205,000 per QALY (three comparisons) or dominated (one comparison); probabilistic ICERs of £202,000–303,000 per QALY (three comparisons) or dominated (one comparison)
-
TAC-PR (one comparison) – Dominated in deterministic and probabilistic analyses.
-
AZA (four comparisons) – Dominated in deterministic and probabilistic analyses.
-
MPS (one comparison) – Deterministic ICER of £144,000 per QALY; dominated in probabilistic analysis.
-
SRL (two comparisons) – Dominated in deterministic and probabilistic analyses.
-
EVL (one comparison) – Deterministic ICER of £1,532,000 per QALY; probabilistic ICER of £3,260,000 per QALY.
-
BEL (one comparison) – Deterministic ICER of £424,000 per QALY; probabilistic ICER of £446,000 per QALY.
Scenario analyses
See the Results section (Scenario analyses, below) for further details.
In a scenario analysis investigating the impact of structural uncertainty in the surrogate effect of AR, NODAT and GRF at 12 months on graft survival, it was found that if the surrogate effect was weakened (by limiting its duration) then no induction and CSA became cost-effective at £20,000 and £30,000 per QALY compared with BAS induction and immediate-release TAC, respectively, in some combinations. When used in combination with immediate-release TAC and MMF, no induction became cost-effective if the duration of the surrogate effect was limited to 1–2 years. In combination with CSA and MMF the duration had to be further limited, and in combination with CSA and AZA no induction was not cost-effective even when the surrogate effect was eliminated entirely. The duration of surrogate effect had to be limited to 3–7 years or less (depending on the comparison) for CSA to be cost-effective compared with immediate-release TAC at £20,000 or £30,000 per QALY.
A second structural uncertainty analysis considered the possibility that CNI-free regimens could result in prolonged graft survival by avoiding the nephrotoxic effects of CNIs. The graft survival for the SRL-containing regimen BAS + SRL + MMF had to be markedly different from the base case for SRL to become cost-effective at £20,000 or £30,000 per QALY and the BEL-containing regimen BAS + BEL + MMF was not cost-effective at £20,000 or £30,000 per QALY at any point in the analysis.
When list prices were adopted instead of average NHS acquisition costs for drug acquisition costs, CSA and AZA became cost-effective at £20,000–30,000 per QALY in some combinations, with immediate-release TAC and MMF remaining cost-effective at £20,000–30,000 per QALY in other comparisons.
Belatacept was not found to be cost-effective at £20,000–30,000 per QALY, even at zero price, or at list price with zero administration cost.
Introduction
The objective of this independent economic assessment was to answer the following study question in line with the NICE reference case:381
What is the cost-effectiveness of immunosuppressive regimens in renal transplantation in adults, of basiliximab and rabbit anti-human thymocyte immunoglobulin as an induction therapy and immediate-release tacrolimus, prolonged-release tacrolimus, mycophenolate mofetil, mycophenolate sodium, sirolimus, everolimus and belatacept as a maintenance therapy?
Although there have been a number of economic evaluations that partially address the study question (see Chapter 4), none has independently addressed the full study question in line with the NICE reference case381 and therefore a new economic assessment was required.
A decision-analytic model was developed in Excel to address the study question in a cost–utility analysis.
Methods
Modelling approach
Target population and subgroups
The target population was adults undergoing kidney-only transplantation (i.e. people receiving multiorgan transplants are not included). The donor may be living–related, living–unrelated or deceased (following brain death or cardiac death).
The population included only incident KTRs, and did not include prevalent KTRs (i.e. people who received a kidney transplant in the past), even those suffering from AR (although a number of the interventions separately have marketing authorisation for the treatment of AR).
In the base-case analysis, KTRs were assumed to be aged 50 years (the median age of incident KTRs in 2012 was 50.5 years382) and 62% were men (UK Transplant Registry standard data set 2007–12).
The mean weight of KTRs was estimated by identifying RCTs included in the systematic review of clinical effectiveness (see Chapter 3) which reported weight as a baseline characteristic. A random-effects model was used, which resulted in estimated mean [standard error (SE)] weight of 70.2 kg (1.2 kg).
Setting and location
The NHS in England and Wales.
Study perspective
In line with the NICE reference case,381 the perspective adopted on outcomes was all direct health effects for patients and other people, and the perspective adopted on costs was that of the NHS and PSS.
Comparators
As the immunosuppressive agents are used in combination and in sequence, we used treatment regimens as comparators rather than individual agents. However, the cost-effectiveness of an individual agent compared with another individual agent can be evaluated by considering the cost-effectiveness of regimens that are identical except for the use of the intervention or comparator of interest. Regimens were included as comparators if they were in current use in the NHS or if they would plausibly be used in the NHS (as advised by a number of clinical experts) and there was sufficient clinical evidence to estimate the costs and outcomes for KTRs receiving those regimens.
Table 149 presents the regimens considered in this analysis, as well as an indication of whether or not the assessment group believes the regimen to be a licensed combination (although no warranty or representation is given as to the correctness of the information presented in this regard).
| Identifier | Induction therapy | Maintenance therapya | Licensed |
|---|---|---|---|
| CSA + MMF | None | CSA + MMF | Y |
| TAC + MMF | None | Immediate-release TAC + MMF | U |
| CSA + AZA | None | CSA + AZA | Y |
| TAC + AZA | None | Immediate-release TAC + AZA | Y |
| CSA + EVL | None | CSA + EVL | Y |
| TAC + SRL | None | Immediate-release TAC + SRL | N |
| TAC-PR + MMF | None | TAC-PR + MMF | U |
| BAS + CSA + MMF | BAS | CSA + MMF | Y |
| BAS + TAC + MMF | BAS | Immediate-release TAC + MMF | U |
| BAS + CSA + AZA | BAS | CSA + AZA | Y |
| BAS + SRL + MMF | BAS | SRL + MMF | U |
| BAS + BEL + MMF | BAS | BEL + MMF | Ub |
| BAS + CSA + MPS | BAS | CSA + MPS | U |
| rATG + CSA + MMF | rATG | CSA + MMF | Y |
| rATG + TAC + MMF | rATG | Immediate-release TAC + MMF | U |
| rATG + CSA + AZA | rATG | CSA + AZA | Y |
Astellas, in their submission, included the following regimens which we have not modelled:
-
BAS + CSA + SRL (although we have modelled TAC + SRL)
-
BAS + CSA + EVL (although we have modelled CSA + EVL).
Bristol-Myers Squibb and Novartis did not present any regimens that we have not modelled.
Time horizon
The time horizon was 50 years or age 100 years, whichever is earlier. The median age of incident KTRs in 2012 was 50.5 years. 382
Discount rate
In line with the NICE reference case381 the discount rate for costs and health effects was 3.5% per annum.
Choice of health outcomes
The primary health outcome of the independent economic assessment was QALYs for each comparator regimen, in line with the NICE reference case. 381 Secondary outcomes included:
-
undiscounted life-years (life expectancy)
-
undiscounted life-years with a functioning graft
-
undiscounted life-years on dialysis
-
likelihood of experiencing at least one episode of AR
-
likelihood of developing NODAT
-
likelihood of receiving a second or third transplant.
Model structure
Conceptualisation
We followed the approach to model conceptualisation described by Kalthenthaler et al. 383 in the NICE Decision Support Unit Technical Support Document 13.
Several meetings were held with Dr Jason Moore (Consultant Nephrologist; the Kidney Unit, Royal Devon & Exeter NHS Foundation Trust), during which problem-oriented conceptual models for various disease processes and service pathways were discussed and refined. The problem-oriented conceptual models were then circulated to the expert advisory group, recruited for the assessment, who made comments and suggestions. A design-oriented conceptual model was then developed, based heavily on the kidney logic conceptual model, and this formed the basis for the final model structure.
Finalised structure
In the final model structure, KTRs were assumed at all times to be in one of three principal health states:
-
functioning graft (not dialysis dependent)
-
graft loss (dependent on dialysis)
-
death.
Kidney transplant recipients start in the functioning graft unless they suffer PNF, in which case they start in the graft loss state. Transitions can occur from functioning graft to graft loss, reflecting disease progression; transitions are not permitted in the opposite direction, except through retransplantation. Up to two retransplantations are possible and therefore there are three substates for functioning graft and graft loss, reflecting the graft number (1–3). As with the initial graft, it is possible that PNF will occur and therefore transitions can occur directly to graft loss following second or third graft. Pre-emptive retransplantation can occur from the original functioning graft state. Death can occur from any state but the rate of mortality is greater in the graft loss state (see Mortality) and increases with age.
Irrespective of the regimen used for immunosuppression in the first graft, a common regimen was used for subsequent grafts (BAS + TAC + MMF). See below (Retransplantation) for our justification of this approach.
Figure 67 gives the model diagram showing the seven states in the model. Self-links are omitted from all states in both figures for clarity (there are no tunnel states).
FIGURE 67.
Model diagram. FG, functioning graft; GL, graft loss; dashed arrows indicated PNF; green arrows indicate pre-emptive retransplantation.

A Markov cohort model was used, such that individual KTRs were not simulated. The model was constructed using Excel.
In addition to these health states, for each regimen the incidence of AR, CMV infection, dyslipidaemia and NODAT was estimated.
For each allowable transition, a transition rate was modelled. The probability of each transition was then calculated using the following formula:
where ri is the hazard rate of the specific transition, R is the sum of allowable transition rates (including ri) and Δt is the time step (cycle length).
In some cases the transition rate was engineered to achieve a desired change in state membership, but in all cases a transition rate was calculated.
Table 150 gives a summary of how the transition rates were dependent on factors such as age, AR and NODAT. BAS + TAC + MMF was assumed to be the baseline regimen that was most close to current UK practice and outcomes.
| Transition | Corresponding clinical outcome | Dependent on |
|---|---|---|
| Functioning graft to graft loss (first graft) | Disease progression (graft loss/survival) | First
|
Subsequent
|
||
| Functioning graft to graft loss (subsequent graft) | Disease progression (graft loss/survival) | (Constant) |
| Functioning graft to death (first graft) | DWFG | First
|
Subsequent
|
||
| Functioning graft to death (subsequent graft) | DWFG | Age NODAT |
| Graft loss to subsequent functioning graft | Retransplantation | Age |
| Graft loss to death | Mortality while receiving dialysis | Age |
Factors included in the model
Overall survival
Overall survival was not explicitly included as an input to the model and therefore emerges from the two modelled rates of mortality: DWFG (see Mortality) and mortality after graft loss (see Mortality after graft loss).
The exception to this is that the rate of DWFG in the first year was adjusted using an individual HR for each regimen to achieve the desired OR of patient mortality as derived from the mixed-treatment comparison (MTC) and head-to-head comparisons.
Although it would be possible to use numerical methods (e.g. Solver add-in for Excel) to achieve exact patient mortality, it was felt that it would add significant computational burden, create significant opportunity for human error (forgetting to re-run Solver every time relevant parameters were changed) and greatly slow down PSAs.
Therefore, a regression approach was used instead. The two factors driving patient survival at 12 months, which could vary between regimens, were identified as the OR of graft loss (after returning to dialysis the mortality rate increases) and the HR of DWFG. The OR of patient mortality within 12 months was plotted against the HR of DWFG for various different ORs of graft loss, and was found to be linearly dependent on a log–log plot (Figure 68).
FIGURE 68.
Odds ratio of patient mortality is dependent on HR of DWFG and OR of death-censored graft loss. OR[DCGL], OR of graft loss.
For each OR of graft loss, linear regression of ‘ln(odds of patient mortality)’ compared with ‘ln(HR of DWFG)’ was performed, and the values of the linear regression coefficients were found to be linearly dependent on the OR of graft loss (Figure 69).
FIGURE 69.
Linear regression coefficients for ln(OR of patient death) vs. ln(HR of DWFG) plotted vs. OR of graft loss.

The appropriate HR for DWFG to achieve a desired OR of patient mortality is therefore derived as follows (where ORDCGL,i is the OR of graft loss, HRDWFG,i is the HR of DWFG and ORPD,i is the OR of patient death):
As can be seen in Table 151, the regression formulae perform well in most instances.
| Regimen | HR for DWFG | |
|---|---|---|
| From regression | Using Solver | |
| CSA + MMF | 0.581 | 0.571 |
| TAC + MMF | 0.998 | 1.002 |
| CSA + AZA | 0.606 | 0.596 |
| TAC + AZA | 0.870 | 0.873 |
| CSA + EVL | 0.907 | 0.910 |
| TAC + SRL | 0.870 | 0.873 |
| TAC-PR + MMF | 1.307 | 1.306 |
| BAS + CSA + MMF | 0.584 | 0.575 |
| BAS + TAC + MMF | 0.997 | 1.000 |
| BAS + CSA + AZA | 0.611 | 0.602 |
| BAS + SRL + MMF | 1.125 | 1.129 |
| BAS + BEL + MMF | 0.271 | 0.233 |
| BAS + CSA + MPS | 0.364 | 0.337 |
| rATG + CSA + MMF | 0.484 | 0.468 |
| rATG + TAC + MMF | 0.826 | 0.827 |
| rATG + CSA + AZA | 0.506 | 0.489 |
Graft survival
Graft survival is a key measure of the clinical effectiveness of an immunosuppressive regimen and is critical also for cost-effectiveness, as graft loss necessitates expensive dialysis treatment, which has a detrimental impact on HRQoL or retransplantation (a costly procedure).
Use of graft survival in the model
In the model, graft survival drives transitions from functioning graft to graft loss states for the first graft, whereas for subsequent grafts a constant rate of graft loss was assumed across all regimens (see Subsequent grafts).
The transitions for the first graft are calculated by first estimating a graft survival curve (censored for DWFG) for each regimen then multiplying this with a curve estimating patient survival (censored for graft loss) to obtain an estimate for how many KTRs should be alive and in the functioning graft state in each cycle. The rate of graft loss for cycle i is then calculated as:
where S(ti) is the product of survival curves for the start of cycle i and Δt = ti + 1 − ti is the cycle length.
The details for how the survival curves are estimated are given later in this section and in the later section Death with functioning graft, but, briefly:
-
Graft survival censored for DWFG is estimated by adjusting survival estimated from the UK Transplant Registry standard data set in the first year according to the OR of graft loss within 12 months and thereafter according to a surrogate relationship based on AR within 12 months, NODAT within 12 months and eGFR at 12 months.
-
DWFG is estimated by adjusting survival estimated from the UK Transplant Registry standard data set in the first year according to the OR of patient death within 12 months and thereafter according to a surrogate relationship based on NODAT within 12 months.
To account for the possibility of pre-emptive retransplantation the rate of graft loss is partitioned between transitions from first functioning graft to graft loss following first graft; first functioning graft to second functioning graft (successful pre-emptive retransplantation); and first functioning graft to graft loss following second graft (unsuccessful pre-emptive retransplantation). The split between these transitions is age dependent (as the likelihood of pre-emptive retransplantation decreases with advancing age; Table 152). The probability that a KTR in each age range is suitable for retransplantation was taken from table 32 of Bond et al. ,384 which was, in turn, estimated from a figure in chapter 5 of the UK Renal Registry Eighth Annual Report. 385 It was then assumed that 20% of these KTRs would receive a pre-emptive retransplantation. 386
| Age group (years) | FG1→GL1, % | FG1→FG2, % | FG1→GL2, % |
|---|---|---|---|
| 18–34 | 89.2 | 10.5 | 0.3 |
| 35–44 | 90.2 | 9.6 | 0.2 |
| 45–54 | 92.4 | 7.4 | 0.2 |
| 55–64 | 94.6 | 5.3 | 0.1 |
| ≥ 65 | 98.0 | 2.0 | 0.0 |
Estimation of graft survival
Graft survival for most people is now so long that most clinical trials do not follow up or maintain randomisation sufficiently long to obtain mature estimates for graft survival. AR became the primary end point in most clinical trials and was treated as a surrogate marker by three of four economic analyses submitted by companies for the current guidance, TA85. 43
Subsequently, there have been analyses confirming that AR and NODAT are predictors of graft loss,378 as well as seemingly contradictory findings that immunosuppressive agents achieving lower ARRs do not deliver improvements in graft survival. 387 In addition, several analyses have suggested that renal function at 1 year post transplant is a good predictor of long-term graft survival. 334,340,388–390
Throughout this section it should be noted that graft survival and failure does not include DWFG, that is, considering only people who are alive and who become dependent on dialysis or require retransplantation.
Baseline
Baseline graft survival for the first year (Figure 70) was estimated from the UK Transplant Registry standard data set using the Kaplan–Meier method, restricting to first graft for each patient and only transplants since 2007; survival was calculated separately for four different donor types (DBD, DCD, living–related, living–unrelated). Graft survival was then calculated as the weighted average according to the donor type distribution. KTRs with graft failure on the day of transplantation were assumed to have PNF and were also excluded. Any KTRs dying with a functioning graft were censored at the time of death.
FIGURE 70.
Graft survival in first year according to graft type. LRD, living–related donor; LURD, living–unrelated donor.

Baseline graft survival was extrapolated by fitting a Weibull curve to conditional survival from 1 year (i.e. fitted to KTRs whose grafts survived at least 1 year), with proportional hazards covariates for graft number, donor type and transplant period (1995–2000, 2001–6, 2007–12). The fit of this Weibull curve was verified with a graphical test of the Cox–Snell residuals (Figure 71), which demonstrated that the fit was good, as there was little deviation from the diagonal except for long follow-up (when censoring tends to cause such deviations).
FIGURE 71.
Graphical verification of the fit to graft survival.

The baseline model for conditional graft survival from 1 year is then:
where t is time after 1 year, λ is the rate parameter and γ is the shape parameter (with a value of 1.105, implying increasing hazard rate with time).
A different rate parameter is obtained for different covariate values (proportional hazards model), the baseline rate parameter was obtained by assuming the following covariate values: graft number = 1; donor type = (DBD 0.659), (DCD 0.078), (living–related 0.195), (living–unrelated 0.068)}; transplant period = 2007–12. These led to a baseline rate parameter value of 0.01809.
Baseline graft survival in the PenTAG model is shown in Figure 72.
FIGURE 72.
Baseline graft survival in the PenTAG model.
Adjustments during the first year
Graft survival for the first year was adjusted using the proportional odds method, such that for each regimen the ORs of graft loss (excluding death and PNF) throughout the first year matched the ORs of graft loss as detailed below (see Effectiveness estimates).
Adjustments after the first year
Graft survival for the first graft after the first year was modelled using the surrogate end points renal function at 12 months, AR within 12 months and NODAT within 12 months, which are all predictors of graft loss. 334,378
The surrogate relationship was implemented using proportional hazards and summarised in Table 153 and expanded in the sections below. The rate parameters for all regimens (after adjusting according to the surrogate relationship) are given in Table 154. The resulting graft survival (excluding DWFG) at 1, 3, 5 and 10 years for each regimen are given in Table 155.
| Relationship | HR | Source |
|---|---|---|
| AR within 12 months | 1.60 | Cole 2008378 |
| Renal function (eGFR) at 12 months | eGFR ≥ 60 ml/minute/1.73 m2: 1 45 ml/minute/1.73 m2 ≤ eGFR < 60 ml/minute/1.73 m2: 1.409 30 ml/minute/1.73 m2 ≤ eGFR < 45 ml/minute/1.73 m2: 2.406 15 ml/minute/1.73 m2 ≤ eGFR < 30 ml/minute/1.73 m2: 5.801 |
Levy 2014334 |
| NODAT within 12 months | 1.12 | Cole 2008378 |
| Regimen | Rate parameter (λ) |
|---|---|
| CSA + MMF | 0.0233 |
| TAC + MMF | 0.0201 |
| CSA + AZA | 0.0264 |
| TAC + AZA | 0.0193 |
| CSA + EVL | 0.0212 |
| TAC + SRL | 0.0244 |
| TAC-PR + MMF | 0.0202 |
| BAS + CSA + MMF | 0.0208 |
| BAS + TAC + MMF | 0.0181 |
| BAS + CSA + AZA | 0.0232 |
| BAS + SRL + MMF | 0.0196 |
| BAS + BEL + MMF | 0.0169 |
| BAS + CSA + MPS | 0.0192 |
| rATG + CSA + MMF | 0.0240 |
| rATG + TAC + MMF | 0.0210 |
| rATG + CSA + AZA | 0.0264 |
| Regimen | Graft survival, % (excluding DWFG and PNF) | |||
|---|---|---|---|---|
| 1 year | 3 years | 5 years | 10 years | |
| CSA + MMF | 95.37 | 90.71 | 85.62 | 73.23 |
| TAC + MMF | 95.72 | 91.66 | 87.20 | 76.18 |
| CSA + AZA | 93.87 | 88.69 | 83.07 | 69.58 |
| TAC + AZA | 93.04 | 89.26 | 85.08 | 74.73 |
| CSA + EVL | 96.13 | 91.84 | 87.14 | 75.57 |
| TAC + SRL | 92.89 | 88.13 | 82.96 | 70.41 |
| TAC-PR + MMF | 94.90 | 90.86 | 86.41 | 75.43 |
| BAS + CSA + MMF | 96.19 | 91.97 | 87.34 | 75.94 |
| BAS + TAC + MMF | 96.48 | 92.79 | 88.73 | 78.58 |
| BAS + CSA + AZA | 94.93 | 90.31 | 85.27 | 72.97 |
| BAS + SRL + MMF | 94.78 | 90.87 | 86.57 | 75.92 |
| BAS + BEL + MMF | 96.84 | 93.38 | 89.54 | 79.92 |
| BAS + CSA + MPS | 96.69 | 92.77 | 88.45 | 77.73 |
| rATG + CSA + MMF | 96.42 | 91.56 | 86.27 | 73.41 |
| rATG + TAC + MMF | 96.69 | 92.42 | 87.73 | 76.19 |
| rATG + CSA + AZA | 95.25 | 89.99 | 84.30 | 70.61 |
Graft function at 12 months
The average GRF (eGFR) at 12 months for each regimen was estimated by first estimating the baseline average eGFR at 12 months in the UK. Pruthi et al. 382 report (in text and in figures 3.5a–c) the median and interquartile range (IQR) of eGFR at 12 months between 2005 and 2011 by donor type (DBD, DCD, living). For each donor type, a normal distribution was fitted by setting the normal distribution mean (µ) to the median and setting the SD (σ) to IQR/1.349, as shown in Table 156.
| Donor type | Reported | Fitted normal distribution | ||
|---|---|---|---|---|
| Median | IQR | µ | σ | |
| Living | 56.4 | 22.1 | 56.4 | 16.40 |
| DBD | 52.7 | 25.8 | 52.7 | 19.11 |
| DCD | 49.4 | 25.7 | 49.4 | 19.06 |
To validate the fit, the predicted quartiles were plotted against the reported quartiles (Figure 73). The scatter points are very close to the dashed line, indicating equality.
FIGURE 73.
Comparison of reported eGFR quartiles and modelled eGFR quartiles. Q, quartile.

To estimate the overall average eGFR (weighted according to the frequency of different donor types), a mixture distribution was created from the three normal distributions and the following formulae were used to calculate the mean and variance of the resulting mixture distribution.
Acute rejection within 12 months
Acute rejection rates within 12 months were estimated using effectiveness estimates as described below (see Effectiveness estimates) and a baseline ARR for BAS + TAC + MMF.
The baseline ARR was estimated from Rowshani et al. 103 and Tsuchiya et al. ,141 as these were the only studies with the exact regimen of BAS + TAC + MMF. Simple pooling was used for the deterministic estimate of the ARR, resulting in an estimate of 12.17%.
The effect of AR on graft survival after the first year was then estimated using the HR of 1.60 from Cole et al. 378 As for GRF, a raw HR was then calculated according to the weighted average of the HRs for AR and no rejection (1.00) with the weights equal to the ARR for each regimen. These were then normalised to give HRs compared with the baseline (BAS + TAC + MMF).
Table 157 summarises the calculations and results for the effect of AR on graft survival.
| Regimen | Graft survival (excluding DWFG and PNF) | |||
|---|---|---|---|---|
| 1 year | 3 years | 5 years | 10 years | |
| CSA + MMF | 95.37 | 90.71 | 85.62 | 73.23 |
| TAC + MMF | 95.72 | 91.66 | 87.20 | 76.18 |
| CSA + AZA | 93.87 | 88.69 | 83.07 | 69.58 |
| TAC + AZA | 93.04 | 89.26 | 85.08 | 74.73 |
| CSA + EVL | 96.13 | 91.84 | 87.14 | 75.57 |
| TAC + SRL | 92.89 | 88.13 | 82.96 | 70.41 |
| TAC-PR + MMF | 94.90 | 90.86 | 86.41 | 75.43 |
| BAS + CSA + MMF | 96.19 | 91.97 | 87.34 | 75.94 |
| BAS + TAC + MMF | 96.48 | 92.79 | 88.73 | 78.58 |
| BAS + CSA + AZA | 94.93 | 90.31 | 85.27 | 72.97 |
| BAS + SRL + MMF | 94.78 | 90.87 | 86.57 | 75.92 |
| BAS + BEL + MMF | 96.84 | 93.38 | 89.54 | 79.92 |
| BAS + CSA + MPS | 96.69 | 92.77 | 88.45 | 77.73 |
| rATG + CSA + MMF | 96.42 | 91.56 | 86.27 | 73.41 |
| rATG + TAC + MMF | 96.69 | 92.42 | 87.73 | 76.19 |
| rATG + CSA + AZA | 95.25 | 89.99 | 84.30 | 70.61 |
New-onset diabetes after transplant/transplantation within 12 months
The methods for estimating the incidence of NODAT within the first 12 months since transplantation are described below (see Diabetes mellitus).
The effect of NODAT on graft survival after the first year was estimated using the HR of 1.12 from Cole et al. ,378 and incorporated using the same methodology as for GRF and AR. Table 158 demonstrates that the impact of NODAT on graft survival is fairly small, which is to be expected, given that the conclusions of Cole et al. 378 that NODAT primarily increases the rate of DWFG, which is not considered here.
| Regimen | Incidence of NODAT (%) | Raw HR | HR vs. baseline |
|---|---|---|---|
| CSA + MMF | 4.98 | 1.006 | 0.993 |
| TAC + MMF | 10.60 | 1.013 | 1.000 |
| CSA + AZA | 4.98 | 1.006 | 0.993 |
| TAC + AZA | 10.60 | 1.013 | 1.000 |
| CSA + EVL | 4.74 | 1.006 | 0.993 |
| TAC + SRL | 16.00 | 1.019 | 1.006 |
| TAC-PR + MMF | 12.32 | 1.015 | 1.002 |
| BAS + CSA + MMF | 4.98 | 1.006 | 0.993 |
| BAS + TAC + MMF | 10.60 | 1.013 | 1.000 |
| BAS + CSA + AZA | 4.98 | 1.006 | 0.993 |
| BAS + SRL + MMF | 8.57 | 1.010 | 0.998 |
| BAS + BEL + MMF | 2.18 | 1.003 | 0.990 |
| BAS + CSA + MPS | 4.66 | 1.006 | 0.993 |
| rATG + CSA + MMF | 4.98 | 1.006 | 0.993 |
| rATG + TAC + MMF | 10.60 | 1.013 | 1.000 |
| rATG + CSA + AZA | 4.98 | 1.006 | 0.993 |
Mortality
Death with functioning graft
In adult KTRs, DWFG is a significant cause of graft loss. Compared with dialysis recipients, more KTRs die from infection and malignancy, the risk of both being increased by greater immunosuppression. 382 CVD is also a significant cause of mortality in people who have transplants. As with members of the general population, the mortality rate increases with age, plus there are a number of additional risks factors affecting patient survival which are adjusted for when comparing survival across different centres. 391
Crude estimates of DWFG will vary according to immunological risk and donor kidney type (i.e. living donor, DCD, DBD) because of differences in baseline demographics (living donor KTRs tend to be younger) and in immunosuppression (KTRs at greater immunological risk tend to receive greater immunosuppression which increases the risk of infection and malignancy). 392 The use of steroids is also linked to increased risk of death from CVD and infection. 393
There is also evidence to suggest that the risks of cardiovascular and infectious causes of death are elevated in KTRs with reduced GRF at 1 year post transplantation. 393
The modelling framework employed allowed flexibility in the rate of DWFG in the first graft modelled but less flexibility for subsequent grafts, for which it could not be dependent on time since transplantation.
The baseline rate of DWFG for the first graft was estimated from the UK Transplant Registry standard data set for each donor type (DBD, DCD, living–related, living–unrelated) after adjusting for transplant period (adjusted to 2007–12) and age group (adjusted to 31–50 years). The Kaplan–Meier survival function was directly used for the first 19 years, followed by an extrapolation based on the estimated rate of DWFG from 9 to 19 years. The baseline survivor function is shown in Figure 74.
FIGURE 74.
Baseline survivor function for DWFG.
The rate of DWFG was then adjusted by sex, donor type and age based on a Cox proportional hazards analysis of the UK Transplant Registry data set (Table 159). For the first 12 months an individual HR was applied for each regimen to achieve a target OR of patient mortality (see Overall survival), and thereafter a HR for NODAT was applied according to Cole et al. 378
| Covariate | HR |
|---|---|
| NODAT | 1.41 |
| Sex: female | 0.865 |
| Donor type | |
| DBD | 1 |
| DCD | 1.083 |
| Living | |
| Related | 0.551 |
| Unrelated | 0.703 |
| Age (years) | |
| < 18 | 0.377 |
| 18–30 | 0.369 |
| 31–40 | 0.712 |
| 41–50 | 1 |
| 51–60 | 2.140 |
| 61–70 | 4.128 |
| 71–75 | 7.583 |
| 76–80 | 8.576 |
| 81–85 | 13.751 |
| > 85 | 23.552 |
Mortality after graft loss
Following graft loss, in the absence of an available kidney for pre-emptive retransplantation, KTRs will be placed on dialysis. Some KTRs will be waitlisted for retransplantation, whereas others will be judged not fit for retransplantation as a result of unsuitability for surgery or prohibitively great immunological risk. The mortality rate for dialysis recipients is known to be significantly greater than that for age-matched members of the general population. 339 An analysis by Webb et al. 394 demonstrated that people waiting for retransplantation following graft loss experience a greater mortality rate than incident dialysis recipients waitlisted for transplantation for at least 3 years when adjusted for age. It is not clear, however, that mortality across all dialysis recipients will differ according to whether the recipient has previously lost a graft.
As it was not possible to incorporate any temporary increase in mortality rate immediately following graft loss and there was not sufficient evidence to suggest it should be included, it was assumed that mortality rates following graft loss would be the same as mortality rates for dialysis recipients and dependent on age group (Table 160).
| Age group (years) | Hazard rate of mortality (SE) |
|---|---|
| 20–24 | 0.010 (0.003) |
| 25–29 | 0.012 (0.003) |
| 30–34 | 0.009 (0.002) |
| 35–39 | 0.015 (0.002) |
| 40–44 | 0.021 (0.002) |
| 45–49 | 0.027 (0.002) |
| 50–54 | 0.041 (0.003) |
| 55–59 | 0.053 (0.003) |
| 60–64 | 0.079 (0.004) |
| 65–69 | 0.107 (0.005) |
| 70–74 | 0.149 (0.006) |
| 75–79 | 0.211 (0.007) |
| 80–84 | 0.275 (0.011) |
| 85+ | 0.408 (0.019) |
For the PSA, the SE of mortality rate in each group was estimated by dividing the square root of the number of observed deaths by the estimated exposure.
Adverse events
Synthesis of AE data is rarely conducted across studies because of typically low incidence (resulting in low statistical power to detect differences) and heterogeneity of reporting. For this model it was judged important to consider the possible impact of different regimens on AE rates because the profile of AEs is considered highly clinically relevant. For example, the current NICE guidance TA8543 recommends that ‘The initial choice between [immediate-release] TAC and CSA should be based on the relative importance of their side effect profiles for individual people’.
Given the heterogeneity of reporting of AEs it was felt to be unlikely to be useful to model many AEs but, instead, to focus where there was established clinical opinion that was also supported by RCTs in our systematic review (see Chapter 3, Systematic review results). Diabetes (NODAT) was considered very important to include (and has been included in previous economic evaluations, see Chapter 5), and CMV infection and dyslipidaemia were judged suitable for inclusion as they had been identified by a recent Cochrane review8,341 as being linked to mammalian target of rapamycin complex 1 (mTOR-I) use (decreasing CMV infection incidence and increasing dyslipidaemia).
Anaemia was also included as an AE, as it has been included in previous economic evaluations and is seen as an important cost relating to RRT, but it was assumed not to vary between regimens.
Cytomegalovirus infection is assumed to be a one-off event occurring in the first year, whereas NODAT, dyslipidaemia and anaemia are chronic conditions that are modelled for the full time horizon, while patients are alive. All AEs incur costs, but NODAT additionally results in a utility decrement (see Adverse events).
Diabetes mellitus
The incidence of diabetes mellitus in individuals receiving dialysis is higher than that in the general population, at around 6% per year, with incidence marginally higher in individuals receiving HD. 395 Kidney transplantation appears to result in a significant increase in the incidence of diabetes mellitus in the first year post transplant (and especially in the first 6 months), after which incidence falls to similar levels to those seen in people on dialysis (see figure 2 of Woodward et al. 395). TAC has been repeatedly associated with the development of NODAT2,378 and the same incidence pattern is observed of significantly elevated incidence in the first year post transplant. 395
Pre-existing diabetes mellitus in the cohort was not modelled, only NODAT within 12 months. Based on a visual inspection of figure 1 of Woodward et al. ,395 it was assumed that 75% of NODAT in the first year would occur within the first 6 months. Incidence of NODAT after the first year was not modelled.
Two competing factors will affect the proportion of people with diabetes mellitus after the first year. First, additional incidence of diabetes mellitus will occur at a greater rate than that in the general population. Second, individuals with diabetes mellitus will face a greater mortality rate than those without diabetes mellitus. For simplicity we assume that these factors approximately cancel each other out and we maintain the same prevalence of NODAT from 1 year onwards.
Baseline 12-month incidence of NODAT for BAS + TAC + MMF was estimated to be 10.6%, based on the results of the SYMPHONY study. 196
We did not find significant evidence to suggest that induction therapies affected the incidence of NODAT, so the incidence of NODAT was modelled independently of induction agent.
As all modelled maintenance regimens are triple-therapy regimens, and to maximise statistical power, it was assumed that the incidence of NODAT in each regimen could be estimated by combining independent estimates for replacing immediate-release TAC and/or MMF in the baseline regimen.
Tables 161 and 162 list the studies (RCTs from the systematic review of clinical effectiveness) informing the impact of replacing immediate-release TAC and MMF, respectively, on 12-month NODAT incidence.
| Study | Compares | NODAT in 12 months |
|---|---|---|
| Laskow 199680 | TAC vs. CSA | 12/67 vs. 1/20 |
| Mayer 199788 | TAC vs. CSA | 17/303 vs. 3/145 |
| Campos 200283 | TAC vs. CSA | 10/85 vs. 3/81 |
| Hardinger 2005100 | TAC vs. CSA | 5/134 vs. 1/66 |
| Raofi 1999210 | TAC vs. CSA | 3/14 vs. 4/21 |
| Yang 199990 | TAC vs. CSA | 1/24 vs. 1/21 |
| Krämer 2010204 | TAC vs. TAC-PR | 20/336 vs. 22/331 |
| Tsuchiya 2013141 | TAC vs. TAC-PR | 0/52 vs. 1/50 |
| aVincenti 2005206 | CSA vs. BEL | 6/73 vs. 1/71 |
| aBENEFIT59 | CSA vs. BEL | 16/221 vs. 7/226 |
| aBENEFIT-EXT142 | CSA vs. BEL | 11/184 vs. 7/175 |
| bFerguson 2011126 | TAC vs. BEL | 1/30 vs. 0/33 |
| Lebranchu 2009149 | CSA vs. SRL | 2/97 vs. 3/96 |
| Büchler 2007134 | CSA vs. SRL | 3/74 vs. 9/71 |
| Kreis 2000116 | CSA vs. SRL | 1/38 vs. 1/40 |
| Guba 2010147 | CSA vs. SRL | 4/71 vs. 5/69 |
| Martinez-Mier 2006117 | CSA vs. SRL | 1/21 vs. 1/20 |
| Schaefer 200692 | TAC vs. SRL | 5/39 vs. 6/41 |
| Groth 1999194 | CSA vs. SRL | 1/42 vs. 1/41 |
| Chen 2008121 | TAC vs. CSA | 1/21 vs. 1/20 |
| SYMPHONY240 | TAC vs. CSA vs. SRL | 34/403 vs. 17/408 vs. 25/380 |
| Study | Compares | NODAT in 12 months |
|---|---|---|
| Ciancio 2008106 | MMF vs. MPS | 7/61 vs. 6/55 |
| aFerguson 2011126 | MMF vs. SRL | 0/33 vs. 2/26 |
| Takahashi 2013131 | MMF vs. EVL | 3/61 vs. 7/61 |
| Tedesco-Silva 2010107 | MMF vs. EVL | 19/273 vs. 14/274 |
| Anil Kumar 2005110 | MMF vs. SRL | 2/75 vs. 2/75 |
| Gonwa 2003180 | MMF vs. SRL | 9/176 vs. 10/185 |
| Sampaio 2008112 | MMF vs. SRL | 6/50 vs. 12/50 |
Mixed-treatment comparisons were conducted for both; in both cases a fixed-effects model was considered to be more appropriate as a result of a lower DIC (58.28 vs. 60.39 and 25.52 vs. 27.04). The results of the MTCs are presented in Tables 163 and 164.
| Agent | OR vs. baseline (natural logarithmic scale) | |||
|---|---|---|---|---|
| Mean | SD | Median | 95% CrI | |
| TAC | (Baseline) | |||
| TAC-PR | 0.1694 | 0.3199 | 0.1687 | −0.4546 to 0.8003 |
| CSA | −0.8162 | 0.2086 | −0.8136 | −1.231 to −0.4129 |
| BEL | −1.671 | 0.381 | −1.665 | −2.431 to −0.9394 |
| SRL | −0.2345 | 0.2239 | −0.2339 | −0.6734 to 0.2016 |
| Agent | OR vs. baseline (natural logarithmic scale) | |||
|---|---|---|---|---|
| Mean | SD | Median | 95% CrI | |
| MMF | (Baseline) | |||
| MPS | −0.07041 | 0.6122 | −0.0656 | −1.291 to 1.126 |
| SRL | 0.4739 | 0.3318 | 0.4719 | −0.1688 to 1.131 |
| EVL | −0.05221 | 0.3194 | −0.05309 | −0.6831 to 0.5742 |
The mean log-ORs were combined from the MTCs to estimate an overall OR for each regimen, as shown in Table 165, which, when combined with the baseline incidence for BAS + TAC + MMF, resulted in the estimated 12-month incidence of NODAT for each regimen as shown in Table 166.
| Regimen | Replace TAC | OR | Replace MMF | OR | Overall OR |
|---|---|---|---|---|---|
| CSA + MMF | CSA | 0.442 | – | 1 | 0.442 |
| TAC + MMF | – | 1 | – | 1 | 1 |
| CSA + AZA | CSA | 0.442 | AZA | 1 (assumed) | 0.442 |
| TAC + AZA | – | 1 | AZA | 1 (assumed) | 1 |
| CSA + EVL | CSA | 0.442 | EVL | 0.949 | 0.420 |
| TAC + SRL | – | 1 | SRL | 1.606 | 1.606 |
| TAC-PR + MMF | TAC-PR | 1.185 | – | 1 | 1.185 |
| BAS + CSA + MMF | CSA | 0.442 | – | 1 | 0.442 |
| BAS + TAC + MMF | – | 1 | – | 1 | 1 |
| BAS + CSA + AZA | CSA | 0.442 | AZA | 1 (assumed) | 0.442 |
| BAS + SRL + MMF | SRL | 0.791 | – | 1 | 0.791 |
| BAS + BEL + MMF | BEL | 0.188 | – | 1 | 0.188 |
| BAS + CSA + MPS | CSA | 0.442 | MPS | 0.932 | 0.412 |
| rATG + CSA + MMF | CSA | 0.442 | – | 1 | 0.442 |
| rATG + TAC + MMF | – | 1 | – | 1 | 1 |
| rATG + CSA + AZA | CSA | 0.442 | AZA | 1 (assumed) | 0.442 |
| Regimen | NODAT incidence (%) |
|---|---|
| CSA + MMF | 4.98 |
| TAC + MMF | 10.60 |
| CSA + AZA | 4.98 |
| TAC + AZA | 10.60 |
| CSA + EVL | 4.74 |
| TAC + SRL | 16.00 |
| TAC-PR + MMF | 12.32 |
| BAS + CSA + MMF | 4.98 |
| BAS + TAC + MMF | 10.60 |
| BAS + CSA + AZA | 4.98 |
| BAS + SRL + MMF | 8.57 |
| BAS + BEL + MMF | 2.18 |
| BAS + CSA + MPS | 4.66 |
| rATG + CSA + MMF | 4.98 |
| rATG + TAC + MMF | 10.60 |
| rATG + CSA + AZA | 4.98 |
Cytomegalovirus infection
It was judged, on the basis of examining the incidence of CMV infection in RCTs included in the systematic review, and on the basis of the Cochrane systematic reviews of maintenance immunosuppression by Webster et al. ,8,341 that CMV infection could be affected by the use of mTOR-I (SRL and EVL) and that the impact could vary depending on whether replacing a CNI or antimetabolite in the ‘standard triple therapy’.
Table 167 lists the studies (RCTs from the systematic review of clinical effectiveness) that could inform the estimate of the impact on CMV infection incidence of using mTOR-I.
| Study | Compares | CMV infection within 12 months |
|---|---|---|
| Vítko 2005150 | No mTOR-I vs. mTOR-I replacing antimetabolite | 38/196 vs. 10/194 |
| Takahashi 2013131 | No mTOR-I vs. mTOR-I replacing antimetabolite | 21/61 vs. 3/61 |
| Tedesco-Silva 2010107 | No mTOR-I vs. mTOR-I replacing antimetabolite | 16/273 vs. 2/274 |
| Chadban 2013152 | No mTOR-I vs. mTOR-I replacing antimetabolite | 2/47 vs. 4/30 |
| Sampaio 2008112 | No mTOR-I vs. mTOR-I replacing antimetabolite | 6/50 vs. 6/50 |
| Mjörnstedt 2012133 | No mTOR-I vs. mTOR-I replacing CNI | 13/100 vs. 9/102 |
| Flechner 2002127 | No mTOR-I vs. mTOR-I replacing CNI | 2/30 vs. 3/31 |
| Lebranchu 2009149 | No mTOR-I vs. mTOR-I replacing CNI | 6/97 vs. 4/96 |
| Büchler 2007134 | No mTOR-I vs. mTOR-I replacing CNI | 17/74 vs. 4/71 |
| Kreis 2000116 | No mTOR-I vs. mTOR-I replacing CNI | 8/38 vs. 2/40 |
| Guba 2010147 | No mTOR-I vs. mTOR-I replacing CNI | 20/71 vs. 5/69 |
| Martinez-Mier 2006117 | No mTOR-I vs. mTOR-I replacing CNI | 0/21 vs. 1/20 |
| SYMPHONY240 | No mTOR-I vs. no mTOR-I vs. mTOR-I replacing CNI | 39/403 vs. 45/408 vs. 23/380 |
Fixed- and random-effects MTCs were conducted, and the random-effects model was judged to be superior on the basis of DIC (54.02 vs. 59.54 for fixed-effects model). The results of the random-effects MTC are shown in Table 168.
| mTOR-I use | OR vs. baseline (natural logarithmic scale) | |||
|---|---|---|---|---|
| Mean | SD | Median | 95% CrI | |
| No mTOR-I | (Baseline) | |||
| mTOR-I replacing CNI | −0.7981 | 0.3889 | −0.806 | −1.558 to 0.01047 |
| mTOR-I replacing antimetabolite | −1.153 | 0.4916 | −1.175 | −2.091 to −0.1184 |
| σ (random effects parameter) | 0.7915 | 0.4085 | 0.7538 | 0.08925 to 1.705 |
The baseline incidence of CMV infection (i.e. for no mTOR-I use) was estimated by fitting a logistic model to the absolute incidence of CMV infection in all RCT arms not using mTOR-I and reporting CMV infection incidence within 12 months (Table 169) with study-level random intercepts. The estimated average baseline CMV incidence is 10.72% (95% CI 1.87% to 43.09%).
| Study | CMV infection within 12 months |
|---|---|
| Mayer 199788 | TAC + AZA: 41/303; CSA + AZA: 24/145 |
| Hardinger 2005100 | TAC + AZA: 5/134; CSA + AZA: 4/66 |
| Raofi 1999210 | TAC + AZA: 0/14; CSA + AZA: 0/24 |
| Baboolal 200282 | TAC + AZA: 7/27; CSA + AZA: 7/24 |
| Merville 2004138 | CSA + MMF: 11/37; CSA + AZA: 17/34 |
| Vacher-Coponat 2012129 | TAC + MMF: 25/143; CSA + AZA: 28/146 |
| Yang 199990 | TAC + MMF: 3/30; CSA + MMF: 0/30 |
| Weimer 2006104 | TAC + AZA: 7/28; CSA + AZA: 11/25; CSA + MMF: 13/31 |
| Krämer 2010204 | TAC + MMF: 19/336; TAC-PR + MMF: 33/331 |
| Tsuchiya 2013141 | TAC + MMF: 7/52; TAC-PR + MMF: 4/50 |
| Ciancio 2008106 | TAC + MMF: 1/75; TAC + MPS: 0/75 |
| Salvadori 2004124 | CSA + MMF: 43/210; CSA + MPS: 46/213 |
| Vincenti 2005206 | BEL + MMF: 11/71; CSA + MMF: 13/73 |
| BENEFIT59 | BEL + MMF: 10/226; CSA + MMF: 6/221 |
| BENEFIT-EXT142 | BEL + MMF: 24/175; CSA + MMF: 24/184 |
| Ferguson 2011126 | BEL + MMF: 1/33; TAC + MMF: 2/30 |
| Vítko 2005150 | CSA + MMF: 38/196 |
| Takahashi 2013131 | CSA + MMF: 21/61 |
| Tedesco-Silva 2010107 | CSA + MPS: 16/273 |
| Chadban 2013152 | CSA + MPS: 2/47 |
| Mjörnstedt 2012133 | CSA + MPS: 13/100 |
| Sampaio 2008112 | TAC + MMF: 6/50 |
| Flechner 2002127 | CSA + MMF: 2/30 |
| Lebranchu 2009149 | CSA + MMF: 6/97 |
| Büchler 2007134 | CSA + MMF: 17/74 |
| Kreis 2000116 | CSA + MMF: 8/38 |
| Guba 2010147 | CSA + MMF: 20/71 |
| Martinez-Mier 2006117 | CSA + MMF: 0/21 |
| SYMPHONY240 | CSA + MMF: 45/408; TAC + MMF: 39/403 |
Combining the baseline incidence with the treatment effects results in the incidence rates for each regimen as shown in Table 170.
| Regimen | CMV incidence (%) within 12 months |
|---|---|
| CSA + EVL | 3.65 |
| TAC + SRL | 3.65 |
| BAS + SRL + MMF | 5.13 |
| No mTOR-I | 10.72 |
Dyslipidaemia
It was judged, on the basis of examining the incidence of CMV infection in RCTs included in the systematic review, and on the basis of the Cochrane systematic reviews of maintenance immunosuppression by Webster et al. ,8,341 that the incidence of dyslipidaemia could be increased by the use of mTOR-I in the immunosuppressive regimen. It was considered that it was not necessary to separately estimate the risk, whether used in combination with a CNI or with an antimetabolite, and therefore to increase statistical power the effect of mTOR-I use on dyslipidaemia incidence was estimated as the OR of dyslipidaemia incidence for mTOR-I use compared with no mTOR-I use.
Table 171 details the RCTs from our systematic review (see Chapter 3, Systematic review results) that compared regimens with and without mTOR-I and which reported dyslipidaemia. The direction of effect is consistent across the studies.
| Study | Incidence of dyslipidaemia within 12 months | |
|---|---|---|
| No mTOR-I | mTOR-I | |
| Vítko 2005150 | 24/196 | 51/194 |
| Takahashi 2013131 | 19/61 | 28/61 |
| Tedesco-Silva 2010107 | 43/273 | 57/274 |
| Mjörnstedt 2012133 | 9/100 | 13/102 |
| Sampaio 2008112 | 8/50 | 11/50 |
| Flechner 2002127 | 16/30 | 20/31 |
| Lebranchu 2009149 | 4/97 | 8/96 |
| Büchler 2007134 | 38/74 | 50/71 |
| Guba 2010147 | 5/71 | 14/69 |
| SYMPHONY240 | 91/811 | 60/380 |
Fixed- and random-effects meta-analyses were conducted and it was judged on the basis of DIC (28.267 vs. 29.897) that a fixed-effects analysis was appropriate. The results of the fixed-effects meta-analysis are shown in Table 172.
| mTOR-I use | OR vs. baseline (natural logarithmic scale) | |||
|---|---|---|---|---|
| Mean | SD | Median | 95% CrI | |
| No mTOR-I | (Baseline) | |||
| mTOR-I | 0.5566 | 0.1005 | 0.5555 | 0.3604 to 0.7533 |
To estimate the baseline incidence of dyslipidaemia (without mTOR-I use), we identified all of the RCTs in our systematic review which reported dyslipidaemia and considered at least one regimen without mTOR-I use (Table 173). A logistic model was fitted, as for CMV incidence, and the average dyslipidaemia incidence for no mTOR-I use was estimated to be 20.17% (95% CI 3.56% to 63.37%). On this basis, the incidence of dyslipidaemia for regimens including mTOR-I was estimated to be 30.59%.
| Study | Dyslipidaemia incidence within 12 months |
|---|---|
| Hardinger 2005100 | TAC + AZA: 40/134; CSA + AZA: 26/66 |
| Vacher-Coponat 2012129 | TAC + MMF: 54/128; CSA + AZA: 78/137 |
| Vincenti 2005206 | BEL + MMF: 9/71; CSA + MMF: 6/73 |
| Ferguson 2011126 | BEL + MMF: 12/33; TAC + MMF: 12/30 |
| Vítko 2005150 | CSA + MMF: 24/196 |
| Takahashi 2013131 | CSA + MMF: 19/61 |
| Tedesco-Silva 2010107 | CSA + MPS: 43/273 |
| Mjörnstedt 2012133 | CSA + MPS: 9/100 |
| Sampaio 2008112 | TAC + MMF: 8/50 |
| Flechner 2002127 | CSA + MMF: 16/30 |
| Lebranchu 2009149 | CSA + MMF: 4/97 |
| Büchler 2007134 | CSA + MMF: 38/74 |
| Guba 2010147 | CSA + MMF: 5/71 |
| SYMPHONY240 | CSA + MMF: 51/408; TAC + MMF: 40/403 |
Anaemia
Anaemia is an AE that affects KTRs and people on dialysis. As reference costs for dialysis already include anaemia costs, only anaemia in people with functioning grafts was modelled. It was assumed that there would be no difference in the prevalence of anaemia between different immunosuppressive regimens. The prevalence of anaemia requiring treatment with erythropoiesis-stimulating agents (ESAs) was estimated as 5.2%, based on a study by Vanrenterghem et al. 396 This prevalence was assumed to be the same regardless of time since transplantation, age or other factors.
Retransplantation
The baseline rate of retransplantation following graft loss was estimated from the UK Transplant Registry standard data set in the following way:
-
Data cleaning was performed.
-
Living (relationship unspecified), domino, altruistic and unrelated pooled donors were all reclassified as living–unrelated donors.
-
Transplant recipients who were missing codes for sex or age group were removed.
-
Transplant recipients whose earliest transplant in the data set was not ‘kidney only’ were removed.
-
-
Transplant recipients whose first graft was still functioning, who were lost to follow-up or who died with a functioning graft, were removed.
-
The total number of recipients whose first transplant was recorded as ‘failed’ and who had no subsequent transplant recorded was calculated as N1 = 5085.
-
Recipients whose first transplant failed and had no subsequent transplant were removed if patient survival was not recorded or if patient survival (actual or censored at follow-up) was not greater than graft survival, leaving N1* = 1567 recipients with only one transplant recorded and failed.
-
The total time for which those not receiving a subsequent transplant were followed was estimated as:(5){[sum(patient survival in days) − sum(graft survival in days)]/365.2425 × [N1/N1*]} = 13,627.61 years.
-
The total time between graft failure and retransplantation for those with a subsequent transplant was estimated as {sum(year of second transplant) – [sum(year of first transplant) + sum(first graft survival in days)/365.2425]} = 5955.05 years.
-
The total follow-up time was therefore estimated as 13,627.61 + 5681.06 = 19,582.66 years;
-
The number of retransplants was calculated by counting the number of recipients with two or more transplants recorded, N> 1 = 2031.
-
The rate of retransplantation was estimated as 0.1037 (SE 0.0023).
It was then assumed that the rate of retransplantation would reduce after age 65 years and reach zero by age 80 years, and that the rate would decline linearly between these ages. This assumption was corroborated with our evidence advisory group.
Pre-emptive retransplantations were also modelled from the first functioning graft state in the event of graft loss, as described above (see Graft survival).
Subsequent grafts
Owing to limitations of Markov modelling imposed by the memoryless assumption, there is reduced flexibility in the modelling of costs and outcomes for subsequent grafts. It must be assumed that the hazard rates of all transitions, costs and utilities are dependent only on time in the model and the arm under consideration.
Comprehensive information on immunosuppressive regimens used does not appear to be collected;397,398 the UK Renal Registry data set does not include BAS induction and the UK Transplant Registry does not include any data on immunosuppressive regimens utilised.
It was assumed that the same immunosuppressive regimen would be used for all subsequent grafts, regardless of the immunosuppressive regimen used for the first graft. BAS + TAC + MMF was chosen as the immunosuppressive regimen for subsequent grafts, as it is believed to be the most common immunosuppressive regimen in use in the UK. People receiving subsequent grafts are more likely to receive monoclonal or polyclonal antibody induction, as they are likely to be at higher immunological risk. People can become sensitised to rATG if received as induction for first graft or for treatment of steroid-resistant AR so it was judged to be less likely to be used as induction compared with BAS.
Assuming the same immunosuppressive regimen for subsequent grafts for all regimens has the effect that the cost-effectiveness of regimens is primarily driven by outcomes for the first graft.
Table 174 summarises the parameters affecting subsequent grafts.
| Parameter | Value | Source |
|---|---|---|
| Natural history | ||
| Baseline rate of DWFG | 0.00780 | Assumed to be the same as long-running rate of DWFG for first graft |
| Rate of graft loss | 0.03589 | Exponential distribution fitted to UK Transplant Registry standard data set (first graft and PNF excluded) |
| Resource use | ||
| TAC dosage | 0.10 mg/kg/day | Assumed to be somewhat higher than the long-running dosage for first graft (0.08 with AZA/MMF, 0.07 with SRI) because of increased risk of rejection |
| MMF dosage | 2 g/day | Recommended daily dose |
| Prednisolone dosage | 16.3 mg/day | Assumed to be same as first graft |
| Monitoring (clinic, TAC TDM, blood test, renal profile, LFT) | Once monthly | Assumption |
Effectiveness estimates
The key effectiveness parameters driving cost-effectiveness in the model are:
-
graft loss within 12 months
-
patient death within 12 months
-
AR within 12 months
-
GRF at 12 months
-
NODAT at 12 months
-
CMV infection within 12 months
-
dyslipidaemia at 12 months.
Graft loss, patient death, AR and GRF were primarily estimated from the NMAs for induction and maintenance regimens (see Chapter 3, Network meta-analyses), assuming independence of treatment effects (i.e. that the effectiveness for a complete regimen can be decomposed into the effectiveness for the induction therapy and the maintenance regimen).
Some arms were included in the NMAs that do not correspond to regimens in the model and the results for these arms were not included but the arms were not dropped from the NMAs, as they could still contribute indirect effect estimates.
The mean treatment effects from the NMAs are summarised in Table 175.
| Arm | Mortality within 12 monthsa | Graft loss within 12 monthsa | eGFR at 12 monthsb | BPAR within 12 monthsb |
|---|---|---|---|---|
| Lower is better | Lower is better | Higher is better | Lower is better | |
| Induction (vs. no induction) | ||||
| BAS | −0.0067 | −0.2021 | 2.113 | −0.6523 |
| rATG | −0.1788 | −0.2687 | −3.942 | −1.0147 |
| Maintenance (vs. CSA + AZA) | ||||
| TAC + AZA | 0.3234 | 0.1353 | 9.304 | −0.5484 |
| CSA + MPA | −0.0569 | −0.2971 | 1.609 | −0.7516 |
| TAC + MPA | 0.4218 | −0.3788 | 6.531 | −0.9205 |
| BEL + MPA | −0.7630 | −0.4915 | 10.55 | −0.2159 |
| CSA + EVL | 0.3330 | −0.4843 | 4.863 | −0.7835 |
| TAC + SRL | 0.3248 | 0.1587 | −0.3523 | −0.9574 |
| SRL + MPA | 0.5416 | 0.0321 | 3.846 | −0.8283 |
Head-to-head comparisons for TAC-PR compared with immediate-release TAC, and for MPS compared with MMF, were additionally used to identify any differences in effectiveness between these agents. In the NMA, MMF and MPS were assumed to be the same agent to simplify the analysis and increase the statistical power. The head-to-head comparisons did not identify any statistically significant differences in effectiveness. The effectiveness of MMF was assumed to be that of mycophenolate in the NMA and the effectiveness of MPS was estimated by combining the NMA and head-to-head effectiveness estimates.
The effectiveness estimates were combined with the following estimated baseline values (for BAS + TAC + MMF): mortality within 12 months (odds) = 0.0153; graft loss within 12 months (odds) = 0.0365; eGFR at 12 months (ml/minute/1.73 m2) = 53.4; and AR within 12 months (odds) = 0.139. The resulting absolute effectiveness estimates are given in Table 176.
| Regimen | Mortality within 12 months (odds) | Graft loss within 12 months (odds) | Mean eGFR (ml/minute/1.73 m2) | BPAR within 12 months (odds) |
|---|---|---|---|---|
| CSA + MMF | 0.0097 | 0.0485 | 46.4 | 0.315 |
| TAC + MMF | 0.0154 | 0.0446 | 51.3 | 0.266 |
| CSA + AZA | 0.0103 | 0.0652 | 44.8 | 0.668 |
| TAC + AZA | 0.0140 | 0.0746 | 54.1 | 0.386 |
| CSA + EVL | 0.0141 | 0.0402 | 49.6 | 0.305 |
| TAC + SRL | 0.0140 | 0.0764 | 44.4 | 0.256 |
| TAC-PR + MMF | 0.0198 | 0.0536 | 51.1 | 0.260 |
| BAS + CSA + MMF | 0.0097 | 0.0396 | 48.5 | 0.164 |
| BAS + TAC + MMF | 0.0153 | 0.0365 | 53.4 | 0.139 |
| BAS + CSA + AZA | 0.0102 | 0.0533 | 46.9 | 0.348 |
| BAS + SRL + MMF | 0.0173 | 0.0550 | 50.7 | 0.152 |
| BAS + BEL + MMF | 0.0052 | 0.0326 | 57.4 | 0.280 |
| BAS + CSA + MPS | 0.0065 | 0.0342 | 52.4 | 0.244 |
| rATG + CSA + MMF | 0.0083 | 0.0371 | 42.4 | 0.114 |
| rATG + TAC + MMF | 0.0129 | 0.0341 | 47.4 | 0.096 |
| rATG + CSA + AZA | 0.0087 | 0.0499 | 40.8 | 0.242 |
The effectiveness estimates for the other outcomes (NODAT, CMV infection and dyslipidaemia) are also estimated from the RCTs that were identified in the systematic review of clinical effectiveness, as described above (see Diabetes mellitus, Cytomegalovirus infection and Dyslipidaemia).
Measurement and valuation of preference-based outcomes
Utility was estimated for KTRs by first estimating age-dependent baseline utility for the general population then applying a utility decrement according to whether KTRs were in the functioning graft or graft loss state. In addition, the proportion of the population with NODAT was estimated and a utility decrement was applied to both functioning graft and graft loss states to reflect the decreased HRQoL for KTRs with NODAT.
In the PSA, utility decrements were drawn from gamma distributions to ensure that they did not result in increased utility.
With the exception of the source for baseline utility (following section), sources of utility estimates were obtained from sources found through a systematic bibliographic search of the relevant literature. This search combined established terms and synonyms for identifying studies of utility and HRQoL, with population search terms for renal transplant, dialysis and ESRD (see syntax for full search strategy in Appendix 1). No study design filter was used.
The search yielded 1311 titles and abstracts, which were screened by an experienced health technology assessment researcher (RA). Only 99 of these were studies that yielded or used EQ-5D scores (the preferred preference-based measure for informing NICE technology assessments). Studies that yielded EQ-5D-derived health state scores (using UK general population valuations) were sought for health states or clinical events of relevance in our provisional model structure: functioning renal graft, failing renal graft, chronic allograft injury, acute kidney rejection, NODAT, malignancy following renal transplant and infection following renal transplant.
Baseline utility
Baseline utility was modelled using the following equation:
This equation was derived from the Health Survey for England – 2012,399 using the well-established methodology of Ara and Brazier. 400
Utility with dialysis
A systematic review and meta-analysis by Liem et al. 401 reported pooled estimates of utility for various health states of people undergoing RRT. It reported random-effects meta-analyses of six studies that had produced EQ-5D index scores for HD (range 0.44–0.62) and four studies for PD (range 0.53–0.65). The estimates used in our model are shown in Table 177.
| Type of dialysis | Pooled mean (95% CI) | Number of studies | Number of people |
|---|---|---|---|
| HD | 0.56 (0.49 to 0.62) | 6 | 1315 |
| PD | 0.58 (0.50 to 0.67) | 4 | 192 |
These estimates were then converted into utility decrements from baseline age-related general health in order that the utility of those on dialysis would always be lower than people in the general population of the same age and sex.
The estimated utility decrements were [mean (SE)]: HD 0.277 (0.034); PD 0.264 (0.044).
Disutility due to established renal failure treated with transplantation (i.e. functioning graft)
The same systematic review and meta-analysis by Liem et al. 401 reported pooled estimates of utility for people living with a functioning renal graft (Liem meta-analysis). It reported a random-effects meta-analysis of five studies, which had produced EQ-5D index scores for people living with a functioning renal graft (range of means, some medians, 0.71–0.86; Table 178).
| Health state | Pooled mean (95% CI) | Number of studies | Number of people |
|---|---|---|---|
| Functioning graft | 0.81 (0.72 to 0.90) | 5 | 673 |
It was assumed that the HRQoL for KTRs would not exceed that of members of the general population, so this absolute estimate was converted into a utility decrement from baseline of 0.053 (SE 0.049).
Disutility due to diabetes mellitus
Our literature search for utilities revealed one study looking specifically at disutility of NODAT in renal transplantation patients. 402 This is a recent study402 in the relevant patient population and reports EQ-5D utility data, with an estimated disutility of 0.06 associated with NODAT. This figure does not adjust for people with CVD complications and therefore is appropriate to how we model NODAT. We note that the study402 was conducted in only one hospital in USA and the valuation set for the utility values is US based,403 so the outcomes may not be generalisable to the UK population. It has been demonstrated by Johnson et al. 404 that US-valued health states are statistically higher than the UK-valued health states for 31 out of 42 valued EQ-5D health states and that extreme health states are most notably different. 404 However, this does not necessarily reflect the differences between health states and we believe that having utility data from a relevant patient population is the most important factor in choosing this value.
For example, one alternative would be to use diabetes mellitus compared with the general population using Health Survey for England data. This would be a broader population of comparison and unlikely to reflect the true utility impact of diabetes mellitus on someone who has received a kidney transplant.
Bristol-Myers Squibb incorporated disutility of 0.041 for NODAT citing Currie et al. 366 as their source, which is a study looking at costs. We believe they intended to cite the other Currie et al. paper,405 but it is still not clear how they calculated this value. In their model, the deterministic value for disutility of NODAT appears to be 0.06, which corresponds with our chosen value.
Astellas reports the findings of Wyld et al. ,406 which does report utilities, deriving a disutility of 0.10 between ‘no diabetes’ and ‘diabetes’ groups of people with CKD. However, this is not restricted to only renal transplant population and it is not clear which utility elicitation method is used.
Estimating resources and costs
Costs are incurred in the model either in the form of events (e.g. induction therapy, AR, CMV infection, retransplantation) or in the form of ongoing costs (e.g. maintenance therapy, NODAT, dialysis).
The following costs are incurred exclusively in the functioning graft state (ongoing unless otherwise stated):
-
induction therapy (event)
-
maintenance therapy
-
monitoring
-
infection prophylaxis
-
AR (event)
-
CMV infection (event)
-
anaemia.
The following costs are incurred exclusively in the graft loss state:
-
dialysis.
The following costs are incurred in both the functioning graft and graft loss states:
-
NODAT
-
dyslipidaemia.
The following costs are incurred only when transitioning between states:
-
from functioning graft to graft loss: explant surgery, dialysis access surgery
-
from graft loss to functioning graft (and other retransplantation transitions): retransplantation.
Currency, price date, and conversion
Costs are all in 2014–15 pounds sterling (£; GBP). Costs in earlier financial years are inflated based on the Hospital and Community Health Services pay and prices index (Table 179). 407
| Year | Hospital and Community Health Services pay and prices index | Inflation factor |
|---|---|---|
| 2008–9 | 267.0 | 1.106 |
| 2009–10 | 268.6 | 1.099 |
| 2010–11 | 276.7 | 1.067 |
| 2011–12 | 282.5 | 1.045 |
| 2012–13 | 287.3 | 1.028 |
| 2013–14 | 290.5 | 1.016 |
| 2014–15 | 295.3 (projected based on previous three) | 1 |
No costs were included in different currencies, so conversion was not necessary.
Resource use
Induction therapy
Basiliximab can be administered by i.v. infusion or i.v. injection, but it was assumed that it would be administered by i.v. infusion in accordance with Brennan et al. (Table 180). 137 Intravenous infusion is a more costly method of administration than i.v. injection, so this may overestimate the costs of BAS administration.
| Parameter | Value | Source | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAS induction | ||||||||||||||||||||
| BAS, 20-mg doses | 1.964 | Brennan 2006137 | ||||||||||||||||||
| Administration (i.v. infusion) | 1.964 | |||||||||||||||||||
| rATG induction | ||||||||||||||||||||
| rATG, mg/kg | 6.5 | Brennan 2006137 | ||||||||||||||||||
| Administration (i.v. infusion) | 4.525 | Assumption based on Brennan 2006137 Number of dosesPeople12263104245976171Actual breakdown not given but, given that 87.9% initiated before reperfusion, 68.8% received the intended five doses, one patient received six doses. At least four doses were received by 87.2% of people. |
Number of doses | People | 1 | 2 | 2 | 6 | 3 | 10 | 4 | 24 | 5 | 97 | 6 | 1 | 7 | 1 | Actual breakdown not given but, given that 87.9% initiated before reperfusion, 68.8% received the intended five doses, one patient received six doses. At least four doses were received by 87.2% of people. | |
| Number of doses | People | |||||||||||||||||||
| 1 | 2 | |||||||||||||||||||
| 2 | 6 | |||||||||||||||||||
| 3 | 10 | |||||||||||||||||||
| 4 | 24 | |||||||||||||||||||
| 5 | 97 | |||||||||||||||||||
| 6 | 1 | |||||||||||||||||||
| 7 | 1 | |||||||||||||||||||
| Actual breakdown not given but, given that 87.9% initiated before reperfusion, 68.8% received the intended five doses, one patient received six doses. At least four doses were received by 87.2% of people. | ||||||||||||||||||||
Rabbit ATG is administered only by i.v. infusion and it was assumed it would be administered as in Brennan et al. 137
Maintenance therapy
Tacrolimus, SRL, EVL and CSA are titrated to achieve target whole blood trough concentrations, as numerous factors can affect their absorption and removal from the bloodstream and therapeutic windows can be narrow.
The target whole blood concentrations are usually higher initially to ensure adequate immunosuppression and are then lowered to reduce the likelihood and impact of AEs (including nephrotoxicity for CNIs).
There is a substantial body of evidence that the dosage required to achieve target whole blood concentrations is affected by concomitant treatments and, as such, the model includes different dosage schedules for each agent according to concomitant treatment.
It was not possible to estimate the impact of different induction therapies on the required dosage in the early days and weeks, but this is unlikely to have a significant impact on overall costs.
Belatacept is administered intravenously in accordance with a prescribed schedule. It was assumed that the ‘less-intensive’ regimen from the BENEFIT59 and BENEFIT-EXT142 studies would be used. We were advised that vial sharing would most likely not be feasible and therefore we assumed full wastage of excess BEL.
Mean weight of KTRs was estimated by identifying RCTs included in the systematic review of clinical effectiveness that reported weight as a baseline characteristic. A random-effects model was used, which resulted in estimated mean (SE) weight of 70.2 kg (1.2 kg). The SD of weight of KTRs was estimated by pooling the SDs reported, resulting in a SD of 14.8 kg. A normal distribution was then assumed to calculate the expected number of vials required for 10-mg/kg and 5-mg/kg doses. It was estimated that 3.31 vials would be required for a 10-mg/kg dose and 1.91 vials for a 5-mg/kg dose (Table 181).
| Number of vials | Dose | |
|---|---|---|
| 10 mg/kg | 5 mg/kg | |
| 1 | 0.1% | 24.7% |
| 2 | 8.5% | 59.6% |
| 3 | 54.2% | 15.3% |
| 4 | 35.0% | 0.3% |
| 5 | 2.2% | 0.0% |
| Expected | 3.31 | 1.91 |
Overall resource use for maintenance therapy is detailed in Table 182.
| Parameter | Value | Source | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immediate-release TAC | ||||||||||||||||
| With AZA | TIme (months)Dosage (mg/kg/day)0–10.2251–30.1753–60.1356–120.11012–360.09036+0.080 | TIme (months) | Dosage (mg/kg/day) | 0–1 | 0.225 | 1–3 | 0.175 | 3–6 | 0.135 | 6–12 | 0.110 | 12–36 | 0.090 | 36+ | 0.080 | Margreiter 200284 |
| TIme (months) | Dosage (mg/kg/day) | |||||||||||||||
| 0–1 | 0.225 | |||||||||||||||
| 1–3 | 0.175 | |||||||||||||||
| 3–6 | 0.135 | |||||||||||||||
| 6–12 | 0.110 | |||||||||||||||
| 12–36 | 0.090 | |||||||||||||||
| 36+ | 0.080 | |||||||||||||||
| With MMF | TimeDosage (mg/kg/day)0–2 weeks0.1682–6 weeks0.1766–12 weeks0.1103–6 months0.1046–12 months0.08612+ months0.080 | Time | Dosage (mg/kg/day) | 0–2 weeks | 0.168 | 2–6 weeks | 0.176 | 6–12 weeks | 0.110 | 3–6 months | 0.104 | 6–12 months | 0.086 | 12+ months | 0.080 | Rowshani 2006103 for 0–12 months; assumed no higher than with AZA for 12+ months |
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–2 weeks | 0.168 | |||||||||||||||
| 2–6 weeks | 0.176 | |||||||||||||||
| 6–12 weeks | 0.110 | |||||||||||||||
| 3–6 months | 0.104 | |||||||||||||||
| 6–12 months | 0.086 | |||||||||||||||
| 12+ months | 0.080 | |||||||||||||||
| With SRL | Time (months)Dosage (mg/kg/day)0–10.1751–30.1103–60.1046–120.08012+0.070 | Time (months) | Dosage (mg/kg/day) | 0–1 | 0.175 | 1–3 | 0.110 | 3–6 | 0.104 | 6–12 | 0.080 | 12+ | 0.070 | Starting dose from Gonwa 2003180 (0–1 month); assumed no higher than with MMF (1–6 months); Gonwa 2003,180 Anil Kumar 2008122 (6+ months) | ||
| Time (months) | Dosage (mg/kg/day) | |||||||||||||||
| 0–1 | 0.175 | |||||||||||||||
| 1–3 | 0.110 | |||||||||||||||
| 3–6 | 0.104 | |||||||||||||||
| 6–12 | 0.080 | |||||||||||||||
| 12+ | 0.070 | |||||||||||||||
| TAC-PR | ||||||||||||||||
| With MMF | As for immediate-release TAC plus 0.015 mg/kg/day for 12 months | Wlodarczyk 2009,140 Krämer 2010,204 Tsuchiya 2013,141 Oh 2014105 | ||||||||||||||
| CSA | ||||||||||||||||
| With AZA | Time (months)Dosage (mg/kg/day)0–16.381–34.533–63.776–123.3812–362.9336+2.84 | Time (months) | Dosage (mg/kg/day) | 0–1 | 6.38 | 1–3 | 4.53 | 3–6 | 3.77 | 6–12 | 3.38 | 12–36 | 2.93 | 36+ | 2.84 | Margreiter 200284 |
| Time (months) | Dosage (mg/kg/day) | |||||||||||||||
| 0–1 | 6.38 | |||||||||||||||
| 1–3 | 4.53 | |||||||||||||||
| 3–6 | 3.77 | |||||||||||||||
| 6–12 | 3.38 | |||||||||||||||
| 12–36 | 2.93 | |||||||||||||||
| 36+ | 2.84 | |||||||||||||||
| With MMF or MPS | TimeDosage (mg/kg/day)0–2 weeks7.622–6 weeks5.726–12 weeks3.063–62.866–122.8212+2.82 | Time | Dosage (mg/kg/day) | 0–2 weeks | 7.62 | 2–6 weeks | 5.72 | 6–12 weeks | 3.06 | 3–6 | 2.86 | 6–12 | 2.82 | 12+ | 2.82 | Rowshani 2006103 |
| Time | Dosage (mg/kg/day) | |||||||||||||||
| 0–2 weeks | 7.62 | |||||||||||||||
| 2–6 weeks | 5.72 | |||||||||||||||
| 6–12 weeks | 3.06 | |||||||||||||||
| 3–6 | 2.86 | |||||||||||||||
| 6–12 | 2.82 | |||||||||||||||
| 12+ | 2.82 | |||||||||||||||
| With EVL | Time (months)Dosage (mg/kg/day)0–123.912+2.1 | Time (months) | Dosage (mg/kg/day) | 0–12 | 3.9 | 12+ | 2.1 | Vítko 2005150 | ||||||||
| Time (months) | Dosage (mg/kg/day) | |||||||||||||||
| 0–12 | 3.9 | |||||||||||||||
| 12+ | 2.1 | |||||||||||||||
| AZA | ||||||||||||||||
| With TAC | Time (months)Dosage (mg/kg/day)0–61.506+1.20 | Time (months) | Dosage (mg/kg/day) | 0–6 | 1.50 | 6+ | 1.20 | Starting dose 1–2 mg/kg/day; Laskow 199680 | ||||||||
| Time (months) | Dosage (mg/kg/day) | |||||||||||||||
| 0–6 | 1.50 | |||||||||||||||
| 6+ | 1.20 | |||||||||||||||
| With CSA | Time (months)Dosage (mg/kg/day)0–66–1212–3636+ | Time (months) | Dosage (mg/kg/day) | 0–6 | 6–12 | 12–36 | 36+ | Starting dose 1–2 mg/kg/day; Sadek 2002,86 Vacher-Coponat 2012;129 assumed | ||||||||
| Time (months) | Dosage (mg/kg/day) | |||||||||||||||
| 0–6 | ||||||||||||||||
| 6–12 | ||||||||||||||||
| 12–36 | ||||||||||||||||
| 36+ | ||||||||||||||||
| MMF | ||||||||||||||||
| With TAC | Time (months)Dosage (g/day)0–32.003–121.7412+1.47 | Time (months) | Dosage (g/day) | 0–3 | 2.00 | 3–12 | 1.74 | 12+ | 1.47 | Starting dose 2 g/day; SYMPHONY240 | ||||||
| Time (months) | Dosage (g/day) | |||||||||||||||
| 0–3 | 2.00 | |||||||||||||||
| 3–12 | 1.74 | |||||||||||||||
| 12+ | 1.47 | |||||||||||||||
| With CSA | Time (months)Dosage (g/day)0–32.003–121.8412+1.67 | Time (months) | Dosage (g/day) | 0–3 | 2.00 | 3–12 | 1.84 | 12+ | 1.67 | Starting dose 2 g/day; SYMPHONY240 | ||||||
| Time (months) | Dosage (g/day) | |||||||||||||||
| 0–3 | 2.00 | |||||||||||||||
| 3–12 | 1.84 | |||||||||||||||
| 12+ | 1.67 | |||||||||||||||
| With SRL | Time (months)Dosage (g/day)0–32.003–121.7312+1.47 | Time (months) | Dosage (g/day) | 0–3 | 2.00 | 3–12 | 1.73 | 12+ | 1.47 | Starting dose 2 g/day; SYMPHONY240 | ||||||
| Time (months) | Dosage (g/day) | |||||||||||||||
| 0–3 | 2.00 | |||||||||||||||
| 3–12 | 1.73 | |||||||||||||||
| 12+ | 1.47 | |||||||||||||||
| With BEL | TimeDosage (g/day)Throughout2.00 | Time | Dosage (g/day) | Throughout | 2.00 | Starting dose 2 g/day; BENEFIT59 | ||||||||||
| Time | Dosage (g/day) | |||||||||||||||
| Throughout | 2.00 | |||||||||||||||
| MPS | ||||||||||||||||
| With CSA | Time (months)Dosage (mg/day)0–314403–912119+1107 | Time (months) | Dosage (mg/day) | 0–3 | 1440 | 3–9 | 1211 | 9+ | 1107 | Starting dose; Mjörnstedt 2012133 | ||||||
| Time (months) | Dosage (mg/day) | |||||||||||||||
| 0–3 | 1440 | |||||||||||||||
| 3–9 | 1211 | |||||||||||||||
| 9+ | 1107 | |||||||||||||||
| SRL | ||||||||||||||||
| With TAC | Time (months)Dosage (mg/day)0–123.7012–602.7560+1.80 | Time (months) | Dosage (mg/day) | 0–12 | 3.70 | 12–60 | 2.75 | 60+ | 1.80 | Anil Kumar 2008122 | ||||||
| Time (months) | Dosage (mg/day) | |||||||||||||||
| 0–12 | 3.70 | |||||||||||||||
| 12–60 | 2.75 | |||||||||||||||
| 60+ | 1.80 | |||||||||||||||
| With MMF | Time (months)Dosage (mg/day)0–35.203–64.456–93.509–123.2512–482.9048+2.60 | Time (months) | Dosage (mg/day) | 0–3 | 5.20 | 3–6 | 4.45 | 6–9 | 3.50 | 9–12 | 3.25 | 12–48 | 2.90 | 48+ | 2.60 | Lebranchu 2009149 |
| Time (months) | Dosage (mg/day) | |||||||||||||||
| 0–3 | 5.20 | |||||||||||||||
| 3–6 | 4.45 | |||||||||||||||
| 6–9 | 3.50 | |||||||||||||||
| 9–12 | 3.25 | |||||||||||||||
| 12–48 | 2.90 | |||||||||||||||
| 48+ | 2.60 | |||||||||||||||
| EVL | ||||||||||||||||
| With CSA | Time (months)Dosage (mg/day)0–32.943–62.756–92.539–122.6012–242.6024+2.00 | Time (months) | Dosage (mg/day) | 0–3 | 2.94 | 3–6 | 2.75 | 6–9 | 2.53 | 9–12 | 2.60 | 12–24 | 2.60 | 24+ | 2.00 | Tedesco-Silva 2010,107 Lorber 2005143 |
| Time (months) | Dosage (mg/day) | |||||||||||||||
| 0–3 | 2.94 | |||||||||||||||
| 3–6 | 2.75 | |||||||||||||||
| 6–9 | 2.53 | |||||||||||||||
| 9–12 | 2.60 | |||||||||||||||
| 12–24 | 2.60 | |||||||||||||||
| 24+ | 2.00 | |||||||||||||||
| BEL (with MMF) | ||||||||||||||||
| Drug acquisition | Time (months)Dosage (vials/quarter)0–316.533–67.136+6.24 | Time (months) | Dosage (vials/quarter) | 0–3 | 16.53 | 3–6 | 7.13 | 6+ | 6.24 | Dosing schedule: 10 mg/kg on days 1 and 5, weeks 2, 4, 8 and 12, then 5 mg/kg every 4 weeks thereafter | ||||||
| Time (months) | Dosage (vials/quarter) | |||||||||||||||
| 0–3 | 16.53 | |||||||||||||||
| 3–6 | 7.13 | |||||||||||||||
| 6+ | 6.24 | |||||||||||||||
| Drug administration (i.v. infusion) | Time (months)Infusions per quarter0–353–636+3.26 | Time (months) | Infusions per quarter | 0–3 | 5 | 3–6 | 3 | 6+ | 3.26 | |||||||
| Time (months) | Infusions per quarter | |||||||||||||||
| 0–3 | 5 | |||||||||||||||
| 3–6 | 3 | |||||||||||||||
| 6+ | 3.26 | |||||||||||||||
| Prednisolone | ||||||||||||||||
| (All maintenance regimens) | TimeDosage (mg/day)Throughout16.3 | Time | Dosage (mg/day) | Throughout | 16.3 | SYMPHONY240 | ||||||||||
| Time | Dosage (mg/day) | |||||||||||||||
| Throughout | 16.3 | |||||||||||||||
Dialysis
Access surgery is required for long-term dialysis. In the case of HD, the creation of an arteriovenous fistula is common, which requires time to heal and mature after surgery before use. It was therefore assumed that all people on HD would also incur the cost of one temporary tunnelled central venous catheter.
The mix of HD and PD is known to vary over time, with younger people generally considered better suited to PD (Table 183). The HD mix was reflected in incident and prevalent people on dialysis, but conversion costs (between dialysis modes) were not included.
| Age group (years) | Proportion (%) receiving HD |
|---|---|
| 18–24 | 79.1 |
| 25–34 | 80.4 |
| 35–44 | 84.5 |
| 45–54 | 84.3 |
| 55–64 | 85.2 |
| 65–74 | 85.8 |
| 75–84 | 89.0 |
| 85+ | 91.5 |
Acute rejection
The number of KTRs suffering at least one AR episode was derived as detailed above (see Acute rejection within 12 months and Effectiveness estimates).
To account for the fact that some KTRs may experience more than one AR episode, a study148 was identified which gave both the number of people experiencing at least one AR episode and the total number of episodes. From this it was estimated that there would be 1.19 ARs expected per person suffering at least one AR event.
Infection prophylaxis
Infection prophylaxis was based on the Royal Devon and Exeter transplant protocol. 408
Cytomegalovirus prophylaxis is 200 days’ valganciclovir for high-risk KTRs (donor seropositive and recipient seronegative). Intermediate- and low-risk KTRs do not receive prophylaxis for CMV, with the exception of intermediate-risk KTRs receiving rATG, who receive 4.5 months’ CMV prophylaxis. 408 The dosage of valganciclovir is adjusted based on Cockcroft–Gault CRC, being 900 mg daily for KTRs with CRC of > 60 ml/minute/1.73 m2; 450 mg daily for KTRs with CRC of 40–59 ml/minute/1.73 m2; 450 mg on alternate days for KTRs with CRC of 25–39 ml/minute/1.73 m2; and 450 mg twice weekly for CRC of 10–24 ml/minute/1.73 m2. It was assumed that KTRs in the functioning graft state were split equally in the 25–39, 40–59 and > 60 ml/minute/1.73 m2 bands, and that KTRs in the chronic allograft injury state were all in the 10–24 ml/minute/1.73 m2 band. In the model, 23.2% of KTRs were assumed to be at high risk of CMV infection, based on Harvala et al. 409
Pneumocystis jirovecii pneumonia (PCP) and urinary tract infection (UTI) prophylaxis was assumed to be co-trimoxazole, 480 mg daily for 3 months.
Monitoring
Kidney transplant recipients receive monitoring on a frequent basis after transplantation, which is gradually tapered for KTRs with stable grafts.
The following monitoring was included:
-
full blood count
-
renal profile
-
liver function tests
-
therapeutic drug monitoring (TAC, CSA, SRL and EVL)
-
viral quantitative polymerase chain reaction (PCR) [CMV, BKV, Epstein–Barr virus (EBV)].
In addition KTRs attend regular outpatient clinics.
Kidney transplant recipients with degraded GRF receive more intensive monitoring to maximise graft survival.
A retrospective observational study was conducted by Ling and Chamberlain410 and submitted by Bristol-Myers Squibb, which detailed the post-transplant outpatient tests conducted according to the Cardiff Renal Transplant Database.
It was assumed that every monitoring visit would involve full blood count, renal profile, liver function test and therapeutic drug monitoring (if appropriate) and therefore the test performed the most number of times in each time period was assumed to be representative of monitoring visits.
The data from the observational study410 clearly show that, when patients are stratified by their eGFR at 12 months, their monitoring is more intensive for lower eGFR ranges, but also that even for the lowest eGFR groups there is a decrease in monitoring over time. The maximum follow-up in the study is to 36 months and therefore extrapolation methods should be considered carefully. Increased monitoring for KTRs with lower eGFR at 12 months is caused by, in part, the absolute level of GRF but also to the trajectory of GRF. KTRs with rapidly declining GRF will receive more monitoring and clinics in an attempt to slow the rate of decline. It is therefore quite unlikely that costs associated with low eGFR in the first 36 months will be representative of costs in much later years for patients who eventually reach the same eGFR on a slower trajectory.
This, plus the paucity of data on the evolving eGFR distribution of KTRs over time, is a compelling reason to avoid having absolute eGFR levels driving costs to the extent that is observed in short-term follow-up.
We decided to use the data from the observational study for the first 36 months but thereafter to assume four clinics and blood tests a year, based on the Royal Devon and Exeter transplant protocol,408 which suggests that KTRs with stable GRF should have monitoring tapered to every 3–6 months.
Table 184 details the monitoring visits assumed in the model.
| Time, month(s) | Number of monitoring visits | Rate of monitoring visits (number per year) |
|---|---|---|
| 0–1 | 13.07 | 157 |
| 1–2 | 6.75 | 81 |
| 2–3 | 4.95 | 59 |
| 3–6 | 8.99 | 36 |
| 6–12 | 7.93 | 16 |
| 12–24 | 10.77 | 11 |
| 24–36 | 14.00 | 14 |
| 36+ | 4 per (based on 3- to 6-monthly clinic plus bloods in Royal Devon and Exeter protocol) | |
Clinics were assumed to be as frequent as monitoring visits, except for the first 3 months, when they were assumed to be once weekly on the basis of the Royal Devon and Exeter protocol.
Viral quantitative PCR was modelled based on the Royal Devon and Exeter protocol, in which KTRs at intermediate risk of CMV infection (i.e. seropositive recipients) receive CMV quantitative PCR once weekly for 3 months. In the model, 41.5% of KTRs were assumed to be at intermediate risk of CMV infection, based on Harvala et al. 409
All KTRs receive BKV quantitative PCR at 3, 6 and 12 months.
Kidney transplant recipients at high risk of EBV disease (i.e. seronegative recipients from seropositive donors) receive monthly quantitative PCR to 6 months, followed by tests at 9 and 12 months. The proportion of KTRs at high risk of EBV disease was estimated from the Cavallo et al. 411 study, in which 289 out of 290 recipients were EBV seropositive and 51 out of 55 donors were EBV seropositive. Assuming that donor–recipient matching is independent of EBV risk, the chance of a KTR being EBV high risk is (1/290) × (51/55) = 0.32%.
Explant surgery
Not all grafts are explanted on failure, with the likelihood of nephrectomy decreasing with time since transplantation. NHSBT provided data on the probability of nephrectomy as a function of time since transplantation for the PenTAG assessment report for NICE guidance TA165,384 which we have reproduced in Table 185, and used to estimate resource use of explant surgery following failure of the initial graft.
| Time since transplantation | Proportion (%) of grafts explanted |
|---|---|
| 0–3 months | 4 |
| 3–12 months | 23 |
| 12–24 months | 9 |
| 24+ months | 4 |
| Subsequent grafts | 5.9 |
For the subsequent graft it was estimated that 5.9% would be explanted on failure by applying the proportions of grafts explanted for the first graft to the exponential graft survival curve for subsequent grafts.
Subsequent retransplantation
Based on the Department for Health reference costs 2013–14,412 it was estimated that there would be 1.44 ‘workups for retransplantation’ for each actual retransplantation (which can include a number of tests for fitness for transplant surgery, fitness for long-term immunosuppression, immunological assessment and assessment of risk factors for graft and patient survival) and that living donor costs would be incurred in 34.9% of retransplantations and deceased donor costs in 65.1%.
Diabetes mellitus medication
It was assumed that KTRs with NODAT would receive three 500-mg metformin tablets daily. Although this may not be a sophisticated or accurate estimate of the cost of diabetes mellitus medication, it is considered that the costs of complications incurred in and out of hospital will significantly exceed the cost of diabetes mellitus medication.
Dyslipidaemia
It was assumed that 60% of people with dyslipidaemia would receive fluvastatin, as the evidence base for this with regard to safety is greatest according to clinical advice. A dosage of 40 mg per day was assumed, as this is the starting dose in the Riella et al. 413 study.
It was assumed that 30% of people would receive pravastatin, as the evidence base for safety is smaller. A dosage of 20 mg per day was assumed, again, as this is the starting dose in the Riella et al. 413 study.
It was assumed that 10% of people would receive simvastatin, as there have been safety warnings with respect to CSA. A dosage of 10 mg per day was assumed, again as this is the starting dose in the Riella et al. study. 413
Medical management for dyslipidaemia was assumed to be one dietetics outpatient attendance per year and one GP appointment per year.
Anaemia
According to Vanrenterghem et al. ,396 207 out of 3969 (5.2%) of KTRs required ESA treatment for anaemia, with a mean weekly dose of 5832 IU. It was assumed, therefore, that KTRs would, on average, receive 3967 IU of ESA per quarter-year cycle while they were not dependent on dialysis.
The NHS reference costs guidance 2013–14412 indicates that the costs of ESA treatment for anaemia (and of drug treatments for bone mineral disorders) should be included in Healthcare Resource Group (HRG) costs. It was therefore assumed that additional ESA therapy would not be included for people in the graft loss state.
Unit costs
The following sources were used to identify unit costs for drug acquisition:
The eMit national database was the preferred source, as it represents the average cost actually paid by NHS hospitals, including any negotiated discounts.
For procedures the NHS reference costs 2013–1464 (inflated to 2014–15 prices) were the preferred source of unit costs. When unit costs could not be found within the NHS reference costs then a pragmatic search of England and UK-wide sources was conducted.
Induction
Drug acquisition costs for induction therapy are given in Table 186.
| Agent | Pack details | Units | Unit cost (£) | Source |
|---|---|---|---|---|
| BAS | Single 20-mg vial = £842.38 | 20-mg doses | 842.38 | BNF 6856 |
| rATG | Single 25-mg vial = £158.77 | mg | 6.35 |
Maintenance immunosuppression
Historically, the prescribing of maintenance immunosuppression has, in some cases (when people have stable dosing requirements), been transferred to primary care physicians with dispensing in the community. The NICE reference case states that for medicines predominantly prescribed in primary care, prices should be based on the Drug Tariff. Recently, however, the NHS England and the Welsh Health Specialised Services Committee has directed that prescribing of immunosuppressants should be repatriated to secondary care on the grounds of patient safety. 33,414 As a result, in this analysis it is assumed that hospital prescribing and dispensing is appropriate for costing and therefore eMit costs are preferred when available, followed by BNF costs (Table 187).
| Agent | Pack details | Units | Unit cost | Source |
|---|---|---|---|---|
| Immediate-release TAC | 50 × 1 mg = £28.81 100 × 1 mg = £55.05 50 × 0.5 mg = £24.90 50 × 5 mg = £88.57 |
mg | £0.5201 (based on market share) | CMU eMit |
| TAC-PR | 50 × 0.5 mg = £35.79 50 × 1 mg = £71.59 100 × 1 mg = £143.17 50 × 3 mg = £214.76 50 × 5 mg = £266.92 |
mg | £1.0677 (based on 50 × 5-mg pack) | BNF 68 |
| CSA | 30 × 100 mg = £46.15 60 × 10 mg = £16.61 30 × 25 mg = £14.55 30 × 50 mg = £25.26 |
mg | £0.0165 (based on market share) | CMU eMit |
| MMF | 50 × 500 mg = £9.17 100 × 250 mg = £10.94 |
g | £0.3774 (based on market share) | CMU eMit |
| MPS | 120 × 180 mg = £96.72 120 × 360 mg = £193.43 |
mg | £0.004478 (based on 120 × 180-mg pack) | BNF 68 |
| AZA | 28 × 25 mg = £1.63 100 × 25 mg = £9.43 56 × 50 mg = £2.53 100 × 50 mg = £5.03 |
mg | £0.001075 (based on market share) | CMU eMit |
| SRL | 30 × 0.5 mg = £69.00 30 × 1 mg = £86.49 30 × 2 mg = £172.98 |
mg | £2.8830 (based on 30 × 2-mg pack) | BNF 68 |
| EVL | 60 × 0.25 mg = £148.50 | mg | £9.9000 | Novartis’ submission |
| BEL | Single 250-mg vial = £354.52 | Vial | £354.52 | BNF 68 |
| Prednisolone | 28 × 1 mg = £0.15 30 × 2.5 mg = £1.65 100 × 2.5 mg = £5.33 30 × 5 mg = £1.61 100 × 5 mg = £5.41 28 × 5 mg = £0.39 |
mg | £0.003286 (based on market share) | CMU eMit |
For TAC-PR there is a significant difference in unit price between 5-mg capsules (£1.07 per mg) and smaller capsules (£1.43 per mg). In the absence of data on relative quantities purchased, it was assumed that virtually all KTRs receiving TAC-PR would receive one 5-mg capsule daily, with some KTRs also taking one or more lower-dose capsules to achieve their target daily dose. The appropriate unit cost would therefore lie between £1.07 and £1.43 per mg. It was further considered that there may be scope for negotiated discounts on the more expensive capsules. Therefore, it was assumed that the lower unit price (£1.07 per mg) would be used in the base-case analyses.
Dialysis
Dialysis access surgery costs were estimated per procedure (Table 188) and ongoing dialysis costs (i.e. the cost of dialysis sessions) were estimated per quarter-year cycle.
| Procedure | HRG4 currency | Unit cost (£) | |
|---|---|---|---|
| 2013–14 prices | 2014–15 prices | ||
| HD access surgery | YQ42Z: Open Arteriovenous Fistula, Graft or Shunt Procedures | 1915 | 1946 |
| HD temporary access surgery | YR41A: Insertion of Tunnelled Central Venous Catheter, 19 and over | 810 | 823 |
| PD access surgery | LA05Z: Renal Replacement PD Associated Procedures | 1083 | 1101 |
Costs of HD and PD are broken down in NHS reference costs by mode (HD, PD), age (≥ 19 years, ≤ 18 years), location for HD (hospital, satellite, home), access method for HD (HD catheter, arteriovenous fistula or graft), complications for HD (blood-borne virus, no blood-borne virus), specific modality for PD (continuous ambulatory, automated, assisted automated) and overall location (at base, away from base). There are 40 HRG4 currencies for dialysis in total (including four for AKI).
The costs of HD and PD were estimating by dividing the HRG4 currencies by mode and age, making assumptions about the number of currency units per week and then calculating a weighted average cost based on activity.
Haemodialysis was assumed to be performed three times weekly unless at home, in which case it was assumed to be performed 3.23 times per week on average (based on inspection of reported average number of sessions per week after removing clearly erroneous outliers). PD is explicitly costed per day according to the reference costs guidance and therefore was assumed to be performed seven times weekly.
The currencies for AKI were included, but these make up a vanishingly small proportion of activity and do not have a significant impact on overall cost estimates.
It was estimated for adults (in 2013–14 prices) that HD would cost £459.59 per week and PD £452.57 per week. These correspond to £6093 and £6000 per quarter-year cycle in 2014–15 prices for HD and PD, respectively.
Acute rejection
Costing AR is challenging because, although the initial treatment pathway for T-cell-mediated AR (which is the most common) is fairly standardised (bolus i.v. methylprednisolone and reassessment of immunosuppressive agent dosage) there is a great amount of variation in treatment if the AR is steroid resistant and/or antibody mediated. It is also not clear how many AR episodes require hospitalisation and/or dialysis.
A microcosting study was conducted by Ling et al. 379 for Bristol-Myers Squibb, in which 11 UK renal consultants from nine centres completed a questionnaire estimating resource use for an average transplant patient. This study379 was submitted by Bristol-Myers Squibb as part of the technology appraisal.
With regard to AR, a unit cost was estimated by considering the following possible costs:
-
inpatient stay
-
additional clinic visits
-
laboratory tests
-
first-line therapies
-
methylprednisolone
-
prednisolone
-
-
second-line therapies
-
rATG
-
i.v. immunoglobulin
-
OKT3 (a murine monoclonal Ig2a anti-T cell antibody)
-
plasma exchange
-
rituximab.
-
The estimated cost for an AR episode was £3217 in 2009 GBP, of which £615 was first-line treatment (all people), £798 was second-line treatment (significantly more expensive but required by only a small proportion of people), £797 was extra clinic visits and £1007 was hospitalisation.
This unit cost was inflated to £3557 in 2014–15 prices for use in the model.
Alternative unit costs were considered as follows:
-
Astellas assumed that people with steroid-sensitive AR (80%) would receive 4 days of therapy with i.v. methylprednisolone (500 mg/day) at a cost of £38.40, whereas people with steroid-resistant AR (20%) would receive 10 days of rATG at a dose of 1.5 mg/kg/day and incur the cost ‘Acute kidney injury without [comorbidities or complications]’ from NHS reference costs (total cost for steroid-resistant AR = £8535). The average cost of an AR episode was therefore estimated to be £1738. It was judged that the cost of treating steroid-sensitive AR had likely been underestimated, as there were no costs included for diagnosis, hospitalisation or i.v. administration and, as such, the estimated average cost of £1738 may be underestimated.
-
Novartis assumed a cost of £1725 based on inflating the cost of AR in the McEwan et al. 310 study from 2003 to 2013 costs. The original cost included 2 days’ hospitalisation for all people, increased immunosuppression using TAC, MMF and methylprednisolone for 33% of people and muromonab-CD3 (Orthoclone OKT3, Janssen–Cilag) for 5% of people. Given how old the cost estimate is, and that more therapies are used now beyond muromonab-CD3 for steroid-resistant AR, it was judged that this cost estimate might not be applicable to current practice.
New-onset diabetes after transplantation
Recent studies of the costs of diabetes mellitus to the NHS – such as Hex et al. ,415 or cost–utility studies such as Davies et al. 416 and Gillies et al. 417 – demonstrate that the costs of complications associated with diabetes mellitus far outweigh the direct treatment costs. Therefore, we believe it is important to include these costs within the model, particularly as this allows us to capture the additional costs of CVD associated with diabetes mellitus.
In its submission, Astellas costs annually for metformin, applied only to those with a functioning graft. By comparing this figure to the dose recommendations in the BNF, this value forms a good basis for treatment costs. Treatment costs for diabetes mellitus are also likely to increase as more people become insulin dependent, but the data on how many people become insulin dependent and when are poor. Furthermore, the total cost of diabetes mellitus must include both treatment and complications costs. As the cost of complications far outweighs the costs of treatment for diabetes mellitus, we believe the inclusion of an insulin cost would not make a significant difference to the cost-effectiveness results and we therefore do not account for it in the model.
Bristol-Myers Squibb used the annual cost of diabetes mellitus of £1174, taken from Currie et al. ,418 and inflated to 2014 prices. This reflects the annual per-patient cost of all prescriptions and consultations accrued by the diabetic population. It is not clear whether or not this includes renal costs. It is also reflective of cost to the NHS per year, as opposed to annual per-patient cost, reflective of their lifetime costs. We therefore considered alternative sources for our diabetes mellitus costs.
One possible source is Gillies et al. ,417 who calculate the annual cost of clinically detected type 2 diabetes mellitus to be £2756 (2006 costs). This value comes from the UKPDS data reported in Clarke et al. 380 and inflated to 2006 prices. These costs seem to be outdated and the authors did not explicitly state whether or not renal transplantation costs are included, so we identified a more recent paper419 on the costs of complications associated with diabetes mellitus from the UKPDS via personal communication with Professor Alistair Gray of the University of Oxford. This study follows the original UKPDS cohort, since the closing of the intervention in 1997, to 2007, and includes 10 years of follow-up of over 3000 people with type 2 diabetes mellitus. The list of complications did not include renal disease, but it did include several complications associated with CVD. The average age of the population is slightly higher than that of the people in our model (63 as opposed to 50 years) and, as they are no longer newly diagnosed people, this may make costs higher than expected for the first few cycles of the model. However, given the size of the trial and the recentness of the data, we believe this source to be appropriate. From the supplementary tables 4 and 5, the average annual per-patient costs of complications across the study period were given at 2012 prices as £1352 for inpatient costs (SD £5364) and £676 (SD £1081) for non-inpatient costs. This demonstrates both the size of these costs compared with the cost of treatment of diabetes mellitus and also the variation in the cost of diabetes mellitus complications.
Dyslipidaemia
Statin acquisition costs for the treatment of dyslipidaemia are given in Table 189 and medical management costs are given in Table 190.
| Statin | Pack details | Units | Unit cost (£) | Source |
|---|---|---|---|---|
| Fluvastatin | 28 × 20 mg = £1.59 28 × 40 mg = £1.79 |
mg | 0.002216 (weighted by market share) | CMU eMit |
| Pravastatin | 28 × 10 mg = £4.32 28 × 20 mg = £1.85 28 × 40 mg = £0.79 |
mg | 0.002561 (weighted by market share) | |
| Simvastatin | 28 × 10 mg = £0.15 28 × 20 mg = £0.24 28 × 40 mg = £0.34 |
mg | 0.000339 (weighted by market share) |
Infection prophylaxis
Drug acquisition costs for infection prophylaxis are given in Table 191. Costs for CMV prophylaxis (valganciclovir) are clearly much higher than costs for PCP and UTI prophylaxis.
| Agent | Pack details | Units | Unit cost (£) | Source |
|---|---|---|---|---|
| Co-trimoxazole (Septrin®) | 100 × 480 mg = £15.52 | Per 480-mg tablet | 0.1552 | BNF 6856 |
| Valganciclovir (Valcyte®) | 60 × 450 mg = £1081.46 | Per 450-mg tablet | 18.02 |
Cytomegalovirus infection treatment
Ling et al. 379 (in the microcosting study referred to above: see Acute rejection) estimated the cost of CMV infection treatment to be £2721 in 2009 GBP. This was inflated to £3009 in 2014–15 prices for use in the model.
Alternative unit costs were considered as follows:
-
Astellas assumes a unit cost of £1863 based on i.v. ganciclovir induction for 14–21 days followed by i.v. ganciclovir maintenance for 8 weeks. It appears to have included only drug acquisition costs for this schedule and not administration costs, which would be substantial. It is possible that oral valganciclovir could be used for maintenance instead of i.v. ganciclovir, reducing the administration costs in this period, but there would still be 14–21 days of administration costs excluded from this estimate. It was judged that £1863 is likely to be an underestimate of the true cost of CMV infection.
-
Novartis assumes a unit cost of £45 based on a GP visit on presentation of symptoms. This appears to be a significant underestimation of the true cost of CMV infection.
Anaemia
Costs of ESA therapy were estimated assuming that the ESA with lowest acquisition cost would be used (following NICE guidance TA323,420 which relates to cancer treatment-induced anaemia) (Table 192). Based on the BNF list prices, Binocrit® is the cheapest ESA, although it is possible that local pharmacy negotiations may result in reduced costs to the NHS in practice.
| Agent | Pack details | Units | Unit cost (£) | Source |
|---|---|---|---|---|
| Epoetin alfa (Binocrit®) | 1000 IU = £4.33 2000 IU = £8.65 3000 IU = £12.98 4000 IU = £17.31 5000 IU = £21.64 6000 IU = £25.96 8000 IU = £40.73 10,000 IU = £43.27 |
Per 1000 IU | 4.33 (based on 1000 pre-filled syringes) | BNF 6856 |
Drug administration
All maintenance agents except BEL are administered orally (unless people are unable to take medication orally) and this was assumed to not incur any cost.
Basiliximab is administered by i.v. infusion or injection and rATG is administered by i.v. infusion. BAS is administered on the day of transplantation and 4 days after transplantation. It is very likely that KTRs will still be inpatients for the latter administration. rATG is administered by i.v. infusion for 3–9 days. It is likely that KTRs will be inpatients for all of these infusions (a typical patient is estimated to require 10 days’ inpatient stay). 421
Belatacept is administered by i.v. infusion in an outpatient setting after the KTR is discharged from hospital. It is possible that there would be some efficiency savings by combining administration attendances with regular attendances for monitoring and clinics in early months but thereafter administrations are likely to be more frequent than other visits.
The NHS reference costs do not estimate a cost of i.v. infusion for inpatients as it is assumed to be a part of standard care and costs assigned to procedures taking precedence (e.g. kidney transplant). Nevertheless it was considered important to estimate the cost of administration separately for induction therapies to enable fair comparison against no induction and potential future comparisons against other induction with alternative modes of administration.
We believe that the most appropriate HRG4 currencies for i.v. administration of BAS and rATG are SB12Z (Deliver simple parenteral chemotherapy at first attendance) and SB15Z (Deliver subsequent elements of a chemotherapy cycle), which, when inflated to 2014–15 prices, have unit costs of £228.95 and £325.59, respectively.
For BEL, we believe that the most appropriate HRG4 currency is SB12Z, in the outpatient setting, which, when inflated to 2014–15 prices, has the unit cost of £167.50.
Kidney transplant recipient follow-up
The unit cost of follow-up clinics was estimated from outpatient attendance costs in the nephrology service, using a weighted average of the different types of attendance (with weights based on national activity). When inflated to 2014–15 prices the unit cost of a follow-up clinic was estimated to be £145.27 (Table 193). First face-to-face attendances were included, as well as follow-up clinics on the basis that some people receive follow-up at a different centre to where they received their transplant and the relative weight of these clinics in calculating the average is small.
| Type of attendance | Number of attendances | National average unit cost (2013–14 prices, £) | ||
|---|---|---|---|---|
| Consultant led | Non-admitted face to face | First | 85,206 | 185.95 |
| Follow-up | 652,678 | 146.59 | ||
| Non-admitted non-face to face | First | 1124 | 143.13 | |
| Follow-up | 3033 | 109.24 | ||
| Non-consultant led | Non-admitted face to face | First | 7770 | 140.42 |
| Follow-up | 109,174 | 94.15 | ||
| Non-admitted non-face to face | First | 246 | 60.38 | |
| Follow-up | 5810 | 42.06 | ||
| Weighted average | 142.93 | |||
| (In 2014–15 prices) | 145.27 | |||
Monitoring
The unit cost of viral quantitative PCR was assumed to be the same for CMV, EBV and BKV. The most appropriate recent cost estimate that could be found was from University College London Hospitals provider-to-provider service 2013–14 tariff. 422 This is a recent cost from a NHS provider. The tariffs are likely to be slightly higher than the costs of in-house laboratory tests, but this was assumed to be a small effect and it was also considered that some centres might not have in-house quantitative PCR facilities. The tariff for CMV quantitative PCR was £46 in 2013–14 prices and this was inflated to £46.75 in 2014–15 prices for use in the model.
The unit costs of therapeutic drug monitoring were estimated from the Department of Biochemistry and Immunology, University Hospital of Wales, therapeutic drug monitoring test repertoire. 423 CSA, TAC and SRL therapeutic drug monitoring all incurred charges of £26.28, which was inflated to £26.71 in 2014–15 prices for use in the model. The cost of therapeutic drug monitoring was assumed to be the same as that for SRL.
Other tests (full blood count, renal profile and liver function tests) were estimated based on the costing template produced by NHS Kidney Care to assist in the costing of renal transplantation,421 as shown in Table 194.
| Test | Unit cost (2008–9 prices, £) | Unit cost (2014–15 prices, £) |
|---|---|---|
| Full blood count | 4.57 | 5.05 |
| Renal profile | 4.11 | 4.54 |
| Liver function test | 4.20 | 4.64 |
Explant surgery
The cost of explant surgery was estimated using NHS reference costs 2013 to 2014. 64 The appropriate HRG4 currencies were identified using the 2013–14 Reference Cost Grouper Code to Group workbook,424 by mapping from OPCS-4 code M026 (Excision of rejected transplanted kidney) to groups LB60, LB61, LB62 and LB63 (Table 195). The average cost (weighted by activity) was £4886 in 2013–14 prices, which was inflated to £4966 in 2014–15 prices for the model.
| HRG4 | Activity | Unit cost (2013–14 prices, £) | Total cost (2013–14 prices, £) |
|---|---|---|---|
| LB61C: Major, Open or Percutaneous, Kidney or Ureter Procedures, 19 and over, with CC Score 10+ | 697 | 8175.72 | 5,698,474 |
| LB61D: Major, Open or Percutaneous, Kidney or Ureter Procedures, 19 and over, with CC Score 7–9 | 796 | 5593.30 | 4,452,263 |
| LB61E: Major, Open or Percutaneous, Kidney or Ureter Procedures, 19 and over, with CC Score 4–6 | 1661 | 4984.97 | 8,280,041 |
| LB61F: Major, Open or Percutaneous, Kidney or Ureter Procedures, 19 and over, with CC Score 2–3 | 2391 | 4123.49 | 9,859,272 |
| LB61G: Major, Open or Percutaneous, Kidney or Ureter Procedures, 19 and over, with CC Score 0–1 | 3947 | 3694.03 | 14,580,351 |
| LB62C: Major Laparoscopic, Kidney or Ureter Procedures, 19 and over, with CC Score 3+ | 962 | 6445.46 | 6,200,531 |
| LB62D: Major Laparoscopic, Kidney or Ureter Procedures, 19 and over, with CC Score 0–2 | 3860 | 5404.85 | 20,862,707 |
Subsequent transplant
Living donor costs fall under three HRG4 currencies:
-
LA10Z – Live Donor Kidney Screening
-
LA11Z – Kidney Pretransplantation Work-up of Live Donor
-
LB46Z – Live Donation of Kidney.
The total living donor costs per live kidney donation were calculated by dividing the total cost for each currency by the activity for actual live donation, resulting in a combined cost of £8770.60 per live kidney donation in 2013–14 prices (Table 196). Reference costs and unit costs of transplant surgery and subsequent transplants can be found in Tables 197 and 198.
| HRG4 currency | Activity | Unit cost (£) | Total cost (£) |
|---|---|---|---|
| LA10Z: Live Kidney Donor Screening | 801 | 659.61 | 528,351 |
| LA11Z: Kidney Pretransplantation Work-up of Live Donor | 1524 | 477.95 | 728,398 |
| LB46Z: Live Donation of Kidney | 805 | 7209.43 | 5,803,587 |
| Total cost | 7,060,337 | ||
| (Per live donation of kidney) | 8770.60 | ||
| HRG4 currency | Activity | Unit cost (£) | Total cost (£) |
|---|---|---|---|
| LA01A: Kidney Transplant, 19 and over, from Cadaver Non Heart-Beating Donor | 553 | 13,603.01 | 7,522,463 |
| LA02A: Kidney Transplant, 19 and over, from Cadaver Heart-Beating Donor | 991 | 15,520.53 | 15,380,850 |
| LA03A: Kidney Transplant, 19 and over, from Live Donor | 826 | 17,526.91 | 14,477,231 |
| Average | 15,772.38 | ||
| Procedure | HRG4 currency | Unit cost (£) | |
|---|---|---|---|
| 2013–14 prices | 2014–15 prices | ||
| Recipient work-up | LA12A: Kidney Pretransplantation Work-up of Recipient, 19 and over | 835.06 | 848.72 |
| Living donor costs | See Table 204 | 8770.60 | 8914.05 |
| Deceased donor costs | See Subsequent transplant | 9868.92 | 10,142.05 |
| Transplant surgery | See Table 205 | 15,772.38 | 16,030.35 |
Deceased donor costs comprise the cost of retrieval, which may be divided into staffing, consumables and transport. NHSBT performed a service evaluation of the National Organ Retrieval Service and reported various costs. 355 Staffing costs were reported separately for abdominal retrieval teams and these were used to estimate the staffing cost of retrieval at £6093.49 in 2012–13 prices (Table 199). The average cost of consumables per retrieval was reported as £1770.30, although it should be noted that this included cardiothoracic retrievals also. The total cost of transport was reported as £4,098,473.94 and this was divided by the total number of retrievals (abdominal and cardiothoracic) for a unit cost of £2005.12 per retrieval. The total cost of retrieval was therefore estimated to be £9869 in 2012–13 prices, which was inflated to £10,142 in 2014–15 prices for the model.
| Abdominal retrieval team | Number of retrievals | Average staffing cost per retrieval (£) |
|---|---|---|
| University Hospitals Birmingham NHS FT | 215 | 4440.56 |
| Cambridge University Hospitals NHS FT | 245 | 4082.34 |
| University Hospital of Wales | 72 | 5979.36 |
| King’s College Hospital NHS FT | 246 | 2865.03 |
| Leeds Teaching Hospitals NHS Trust/Central Manchester and Manchester Children’s Foundation Hospitals NHS Trust | 251 | 8645.29 |
| Newcastle-upon-Tyne NHS FT | 179 | 5158.09 |
| Oxford Radcliffe Hospitals NHS Trust | 126 | 6912.76 |
| Royal Free Hampstead NHS Trust | 122 | 10,800.90 |
| Royal Infirmary of Edinburgh (SORT) | 117 | 10,366.39 |
| Average | 6093.49 | |
Summary of model parameters
Appendix 11 details base-case values, sources and PSA distributions for parameters in the model.
Model verification
The decision model was tested by an independent academic decision modeller (Andy Salmon) twice, once following development of the deterministic base case and once following the addition of the probabilistic analyses. Extreme value testing and other black-box testing techniques were applied to ensure the model performed as expected. The testing checklist was also applied by TS following the addition of the probabilistic analyses as an additional check on correct implementation.
Results
We first present the base-case analysis, which we believe to be closest to the NICE reference case. Deterministic results for the base-case analysis are given below (see Deterministic results), as are probabilistic results (see Probabilistic results).
Next we present scenario analyses that explore structural and other uncertainties in the economic assessment. Structural uncertainty in the extrapolation of graft survival is explored in two scenario analyses below (see Graft survival structural scenario analyses). Although it is believed that unit costs for drug acquisition have been identified appropriately and in line with the reference case, we also explore the impact of using list prices for all drugs, and conduct a two-way threshold analysis on costs relating to BEL (see Cost-related scenario analyses).
Summary cost-effectiveness results are presented in the following form throughout, with regimens sorted in order of ascending effectiveness (total QALYs):
-
total costs
-
incremental costs compared with the previous regimen
-
total QALYs
-
incremental QALYs compared with the previous regimen
-
ICER (compared with the previous regimen on the cost-effectiveness frontier unless the regimen is dominated or extended dominated)
-
incremental net health benefit (INHB) at £20,000 and £30,000 per QALY compared with the referent regimen (the regimen on the cost-effectiveness frontier with the lowest total QALYs).
For probabilistic cost-effectiveness results the following are also presented:
-
the probability that each regimen is cost-effective (i.e. gives the greatest net health benefit of all regimens being compared) at £20,000 and £30,000 per QALY.
Note that, throughout, costs and ICERs are reported rounded to the nearest £1 and QALYs are reported to four decimal places. This should not be taken as an indication of the precision of these estimates, but to allow for third-party checking of the accuracy of calculations.
Base-case analysis
Deterministic results
Induction agents
We present the cost-effectiveness of induction agents BAS and rATG and the comparator of no induction in the context of three different maintenance regimens:
-
CSA, AZA and CCSs
-
CSA, MMF and CCSs
-
TAC, MMF and CCSs.
Note that although other regimens including BAS are modelled (BAS + SRL + MMF, BAS + BEL + MMF, BAS + CSA + MPS) these cannot be meaningfully compared with any other regimens to estimate the cost-effectiveness of BAS.
Summary cost-effectiveness results are given in Table 200.
| Induction agent | Discounted costs (£) | Discounted QALYs | ICER (cost per QALY, £) | INHB | |||
|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | ||
| With CSA + AZA | |||||||
| vs. BAS | |||||||
| No induction | 101,595 | – | 10.7711 | – | Dominated | –0.2994 | –0.2436 |
| rATG | 104,570 | + 2975 | 10.8182 | + 0.0471 | Dominated | –0.4011 | –0.2956 |
| BAS | 98,244 | –6326 | 10.9029 | + 0.0848 | – | – | – |
| With CSA + MMF | |||||||
| vs. BAS | |||||||
| No induction | 97,429 | – | 10.9145 | – | Dominated | –0.2207 | –0.1838 |
| rATG | 101,940 | + 4511 | 10.9281 | + 0.0135 | Dominated | –0.4327 | –0.3206 |
| BAS | 95,219 | –6720 | 11.0247 | + 0.0966 | – | – | – |
| With TAC + MMF | |||||||
| vs. BAS | |||||||
| No induction | 92,226 | – | 10.8884 | – | Dominated | –0.1906 | –0.1603 |
| rATG | 97,146 | + 4920 | 10.9047 | + 0.0163 | Dominated | –0.4203 | –0.3080 |
| BAS | 90,405 | –6741 | 10.9880 | + 0.0832 | – | – | – |
BAS
Basiliximab was compared with no induction and with rATG in three comparisons. In all three comparisons, BAS was predicted to dominate no induction and rATG. Therefore, BAS is predicted to be cost-effective at £20,000 and £30,000 per QALY.
rATG
Rabbit ATG was compared with no induction and with BAS in three comparisons. In all three comparisons, rATG was predicted to be dominated by BAS. Therefore, rATG is not predicted to be cost-effective at £20,000 and £30,000 per QALY.
As shown in Appendix 10 (see Table 230), rATG induction results in greater induction therapy costs than BAS and greater costs of infection prophylaxis (as KTRs at intermediate risk of CMV require prophylaxis if receiving rATG induction). These cost increases are partially offset by a reduction in costs of AR treatment (owing to reduced incidence of AR).
Summary
In all comparisons, BAS was dominant over no induction and rATG and was the only cost-effective induction agent.
Maintenance agents
We present the cost-effectiveness results for the following maintenance agents:
-
immediate-release TAC
-
TAC-PR
-
MMF
-
MPS
-
SRL
-
EVL
-
BEL.
These are compared with each other as appropriate and also with CSA or AZA. All maintenance agents were modelled with concomitant treatment, which would be CCSs plus MMF, AZA, CSA or immediate-release TAC according to the evidence base, plus optional induction therapy (BAS or rATG). Comparisons are made holding all concomitant treatments equal. Summary results are given in Table 201.
| Maintenance agent | Discounted costs (£) | Discounted QALYs | ICER (cost per QALY, £) | INHB | |||
|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | ||
| With MMF | |||||||
| vs. TAC | |||||||
| TAC-PR | 106,529 | – | 10.7920 | – | Dominated | –0.8116 | –0.5732 |
| TAC | 92,226 | –14,303 | 10.8884 | +0.0964 | – | – | – |
| CSA | 97,429 | +5203 | 10.9145 | +0.0261 | 199,118 | –0.2340 | –0.1473 |
| With AZA | |||||||
| vs. TAC | |||||||
| CSA | 101,595 | – | 10.7711 | – | Dominated | –0.5124 | –0.3745 |
| TAC | 93,319 | –8276 | 10.8696 | +0.0986 | – | – | – |
| With BAS + MMF | |||||||
| vs. TAC | |||||||
| SRL | 114,549 | – | 10.9010 | – | Dominated | –1.2941 | –0.8917 |
| TAC | 90,405 | –24,144 | 10.9880 | +0.0869 | – | – | – |
| CSA | 95,219 | +4815 | 11.0247 | +0.0367 | 131,035 | –0.2040 | –0.1237 |
| BEL | 209,409 | +114,189 | 11.2941 | +0.2694 | 423,890 | –5.6441 | –3.6607 |
| With rATG + MMF | |||||||
| vs. TAC | |||||||
| TAC | 97,146 | – | 10.9047 | – | – | – | – |
| CSA | 101,940 | +4794 | 10.9281 | +0.0234 | 205,214 | –0.2163 | –0.1364 |
| With CSA | |||||||
| vs. MMF | |||||||
| AZA | 101,595 | – | 10.7711 | – | Dominated | –0.3518 | –0.2824 |
| MMF | 97,429 | –4166 | 10.9145 | +0.1435 | – | – | – |
| EVL | 176,154 | +78,725 | 10.9659 | +0.0514 | 1,532,379 | –3.8849 | –2.5728 |
| With TAC | |||||||
| vs. MMF | |||||||
| SRL | 125,539 | – | 10.6023 | – | Dominated | –1.9518 | –1.3966 |
| AZA | 93,319 | –32,220 | 10.8696 | +0.2674 | Dominated | –0.0734 | –0.0552 |
| MMF | 92,226 | –1093 | 10.8884 | +0.0188 | – | – | – |
| With BAS + CSA | |||||||
| vs. MMF | |||||||
| AZA | 98,244 | – | 10.9029 | – | Dominated | –0.2730 | –0.2226 |
| MMF | 95,219 | –3025 | 11.0247 | +0.1218 | – | – | – |
| MPS | 111,540 | +16,321 | 11.1377 | +0.1130 | 144,449 | –0.7030 | –0.4310 |
| With rATG + CSA | |||||||
| vs. MMF | |||||||
| AZA | 104,570 | – | 10.8182 | – | Dominated | –0.2414 | –0.1976 |
| MMF | 101,940 | –2631 | 10.9281 | +0.1099 | – | – | – |
Immediate-release TAC
Immediate-release TAC was compared with CSA (four comparisons), TAC-PR (one comparison), SRL (one comparison) and BEL (one comparison).
When used in combination with MMF and CCSs, immediate-release TAC dominated TAC-PR and was less costly and less effective than CSA. The ICER of CSA compared with immediate-release TAC was £199,118 per QALY and therefore immediate-release TAC was the only cost-effective agent in this comparison at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY.
When used in combination with AZA and CCSs, immediate-release TAC dominated CSA.
When used in combination with BAS induction, MMF and CCSs, immediate-release TAC dominated SRL and was less costly and less effective than CSA and BEL. The ICERs for CSA and BEL in this comparison were £131,035 and £423,890 per QALY, respectively, and therefore immediate-release TAC was the only cost-effective agent in this comparison at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY.
When used in combination with rATG induction, MMF and CCSs, immediate-release TAC was less costly and less effective than CSA. The ICER of CSA was £255,592 per QALY and therefore immediate-release TAC was the only cost-effective agent in this comparison at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY.
In three comparisons (all with MMF), immediate-release TAC was predicted be less effective than CSA. In all comparisons, however, immediate-release TAC was predicted to result in greater life expectancy and more years with functioning graft (see Appendix 10, Table 231). The QALY loss arises because of the reduction in HRQoL in KTRs who develop NODAT; 10.6% of KTRs are predicted to develop NODAT with immediate-release TAC, compared with 5.0% of KTRs for CSA. If the utility decrement for NODAT is removed (and NODAT therefore only affects costs, graft survival and DWFG) then immediate-release TAC is more effective than CSA in all comparisons and therefore is dominant (see Appendix 10, Table 235).
TAC-PR
Prolonged-release tacrolimus was compared with CSA and immediate-release TAC in combination with MMF and CCSs. TAC-PR was dominated by both CSA and immediate-release TAC in this comparison.
MMF
Mycophenolate mofetil was compared with AZA (four comparisons), SRL (one comparison), EVL (one comparison) and MPS (one comparison).
Mycophenolate mofetil dominated (i.e. was more effective and less costly than) AZA in all four comparisons.
When used in combination with immediate-release TAC and CCSs, MMF dominated SRL.
When used in combination with CSA and CCSs, MMF was less costly and less effective than EVL. The ICER of EVL was £1,532,379 per QALY and therefore MMF was the only cost-effective agent in this comparison at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY.
When used in combination with BAS induction, CSA and CCSs, MMF was less costly and less effective than MPS. The ICER of MPS was £144,449 per QALY and therefore MMF was the only cost-effective agent in this comparison at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY.
MPS
Mycophenolate sodium was compared with AZA and MMF in combination with BAS induction, CSA and CCSs. MPS was more costly and more effective than AZA and MMF. The ICER of MPS was £144,449 per QALY and therefore MPS was not cost-effective at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY.
Mycophenolate sodium was considerably more costly than MMF, with discounted maintenance immunosuppression costs more than double those of MMF, although there were some predicted savings in dialysis expenditure (see Appendix 10, Table 230). MPS was predicted to lead to increased time with functioning graft and increased life expectancy compared with MMF, which is why it was predicted to give increased QALYs (see Appendix 10, Table 231).
SRL
Sirolimus was compared with CSA, immediate-release TAC and BEL in one comparison (in combination with BAS induction, MMF and CCSs) and with AZA and MMF in one comparison (in combination with immediate-release TAC and CCSs).
Sirolimus was dominated by CSA and TAC in the first comparison, and was dominated by AZA and MMF in the second comparison.
EVL
Everolimus was compared with AZA and MMF in combination with CSA and CCSs. EVL was more costly and more effective than AZA and MMF. The ICER of EVL was £1,532,739 per QALY and therefore EVL was not cost-effective at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY.
BEL
Belatacept was compared with CSA, immediate-release TAC and SRL in combination with BAS induction, MMF and CCSs. BEL was more costly and more effective than all comparators. The ICER of BEL was £423,890 per QALY and therefore BEL was not cost-effective at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY.
Summary
Only immediate-release TAC and MMF were cost-effective at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY.
Prolonged-release tacrolimus and SRL were dominated in their relevant comparisons, whereas MPS, EVL and BEL were all the most costly and most effective treatment in their relevant comparisons, but with ICERs of significantly greater than £30,000 per QALY.
Comparing all regimens
When all regimens are simultaneously compared, the following regimens are dominated or extended dominated (if indicated):
-
TAC + SRL
-
TAC-PR + MMF
-
CSA + AZA
-
TAC + AZA
-
TAC + MMF
-
CSA + MMF
-
BAS + SRL + MMF
-
BAS + CSA + AZA
-
rATG + CSA + AZA
-
CSA + EVL
-
rATG + TAC + MMF (extended dominated)
-
rATG + CSA + MMF (extended dominated).
Four regimens were neither dominated nor extended dominated and therefore lay on the cost-effectiveness frontier, and the cost-effectiveness results for these are presented in Table 202. BAS + TAC + MMF was predicted to be the only cost-effective regimen at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY.
| Regimen | Discounted costs (£) | Discounted QALYs | ICER (£) | INHB (£) | |||
|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | lncremental | 20,000 | 30,000 | ||
| BAS + TAC + MMF | 90,405 | – | 10.9880 | – | – | – | – |
| BAS + CSA + MMF | 95,219 | +4815 | 11.0247 | +0.0367 | 131,035 | –0.2040 | –0.1237 |
| BAS + CSA + MPS | 111,540 | +16,321 | 11.1377 | +0.1130 | 144,449 | –0.9070 | –0.5548 |
| BAS + BEL + MMF | 209,409 | +97,869 | 11.2941 | +0.1564 | 625,761 | –5.6441 | –3.6607 |
Additional results
Additional results for the deterministic base case (including disaggregated discounted costs and additional clinical outcomes) can be found in Appendix 10.
Probabilistic results
The PenTAG model was run for 10,000 PSA iterations. Non-linearities in models often manifest in substantially different results between probabilistic and deterministic analyses. Figure 75 demonstrates that there are no significant discrepancies in terms of total costs for each regimen. Figure 76 indicates that there are some discrepancies in terms of total QALYs for each regimen between the probabilistic and deterministic analyses, but there appears to be no systemic bias.
FIGURE 75.
Comparison of deterministic and probabilistic costs in the PenTAG model.
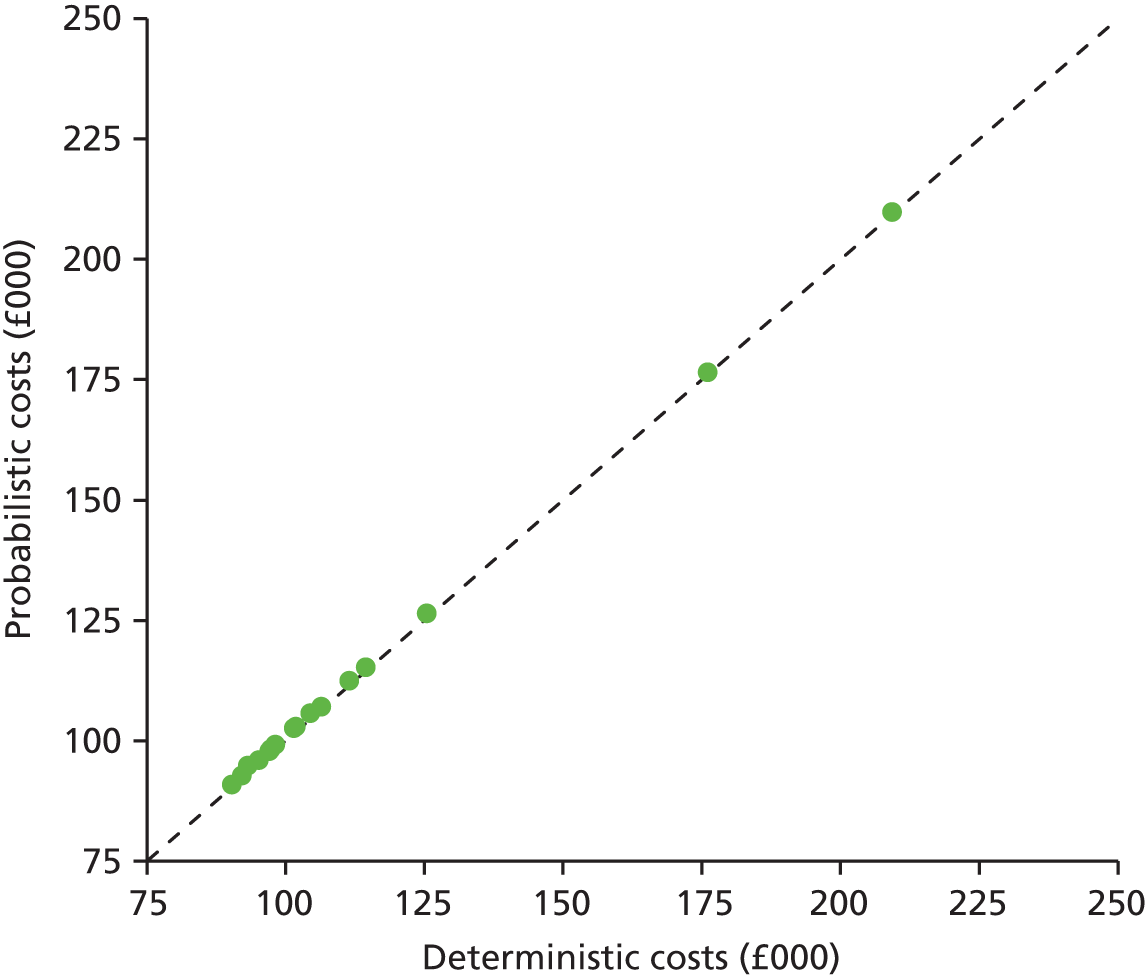
FIGURE 76.
Comparison of deterministic and probabilistic QALYs in the PenTAG model.
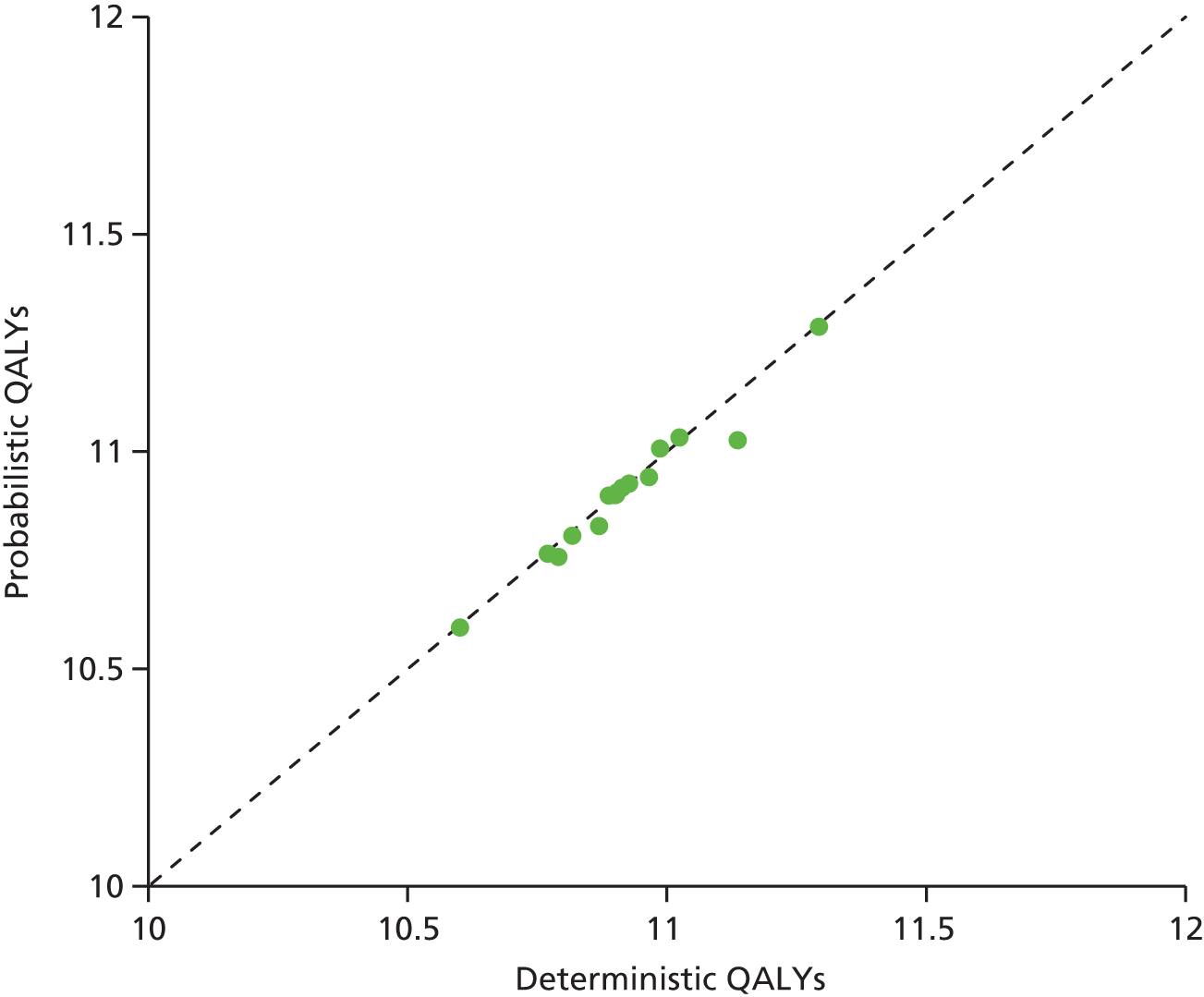
The most significant outlier appears to be BAS + CSA + MPS + CCSs, which is predicted to result in 11.1377 QALYs in the deterministic analysis, but only 11.0244 in the probabilistic analysis. It was ascertained that this outlier effect is caused by the significant uncertainty in the probability of mortality within the first 12 months for this regimen: the 95% CI of the OR of mortality for MPS compared with MMF is 0.058 to 7.23. When the probability of mortality drawn from the PSA distribution is extremely low, the regression formulae for estimating the appropriate HR for DWFG perform badly and, in some cases, even a HR of zero results in above-target mortality as a result of the mortality following graft loss. Noting that, in the deterministic base base, MPS was not cost-effective at £20,000 or £30,000 per QALY (the ICER of MPS vs. MMF was > £100,000 per QALY), we have not attempted to compensate for this discrepancy in our analyses.
Induction agents
Probabilistic cost-effectiveness results for induction agents (Table 203) were not significantly altered from the deterministic results (see Table 199). No induction and rATG continued to be dominated by BAS in all three comparisons.
| Induction agent | Discounted costs (£) | Discounted QALYs | ICER (£) | INHB | Probability cost-effective (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | £20,000/QALY | £30,000/QALY | ||
| With CSA + AZA | |||||||||
| vs. BAS | |||||||||
| No induction | 102,504 | – | 10.7634 | – | Dominated | –0.3028 | –0.2471 | 0.68 | 0.55 |
| rATG | 105,683 | +3179 | 10.8048 | +0.0415 | Dominated | –0.4203 | –0.3115 | 5.78 | 6.83 |
| BAS | 99,159 | –6524 | 10.8989 | +0.0941 | – | – | – | 93.54 | 92.62 |
| With CSA + MMF | |||||||||
| vs. BAS | |||||||||
| No induction | 98,219 | – | 10.9155 | – | Dominated | –0.2294 | –0.1914 | 1.81 | 1.34 |
| rATG | 102,831 | +4613 | 10.9250 | +0.0094 | Dominated | –0.4506 | –0.3357 | 3.29 | 4.10 |
| BAS | 95,938 | –6893 | 11.0309 | +0.1059 | – | – | – | 94.90 | 94.56 |
| With TAC + MMF | |||||||||
| vs. BAS | |||||||||
| No induction | 92,660 | – | 10.8972 | – | Dominated | –0.2013 | –0.1703 | 2.68 | 2.30 |
| rATG | 97,750 | +5090 | 10.9047 | +0.0075 | Dominated | –0.4482 | –0.3324 | 2.84 | 3.99 |
| BAS | 90,802 | –6948 | 11.0055 | +0.1008 | – | – | – | 94.48 | 93.71 |
BAS
Basiliximab was predicted to dominate no induction and rATG in all three comparisons (as in the deterministic results). BAS was cost-effective at £20,000 per QALY in 93.5–94.9% of PSA iterations across comparisons and at £30,000 per QALY in 92.6–94.6% of iterations.
rATG
Rabbit ATG was predicted to dominate by BAS in all three comparisons (as in the deterministic results). Rabbit ATG was cost-effective at £20,000 per QALY in 2.8–5.8% of PSA iterations across comparisons and at £30,000 per QALY in 4.0–6.8% of iterations.
Cost-effectiveness acceptability curves
Cost-effectiveness acceptability curves are shown in Figures 77–79 for the three comparisons. Although these have not been presented as cost-effectiveness acceptability frontiers (in which only the regimen with the greatest expected net health benefit is shown for each cost-effectiveness threshold), the only effect this would have would be to remove the curves for no induction and rATG, as BAS is predicted to give the greatest expected net health benefit across the cost-effectiveness threshold range explored (£1000–50,000 per QALY).
FIGURE 77.
Cost-effectiveness acceptability curves for induction agents in combination with CSA, AZA and CCSs.
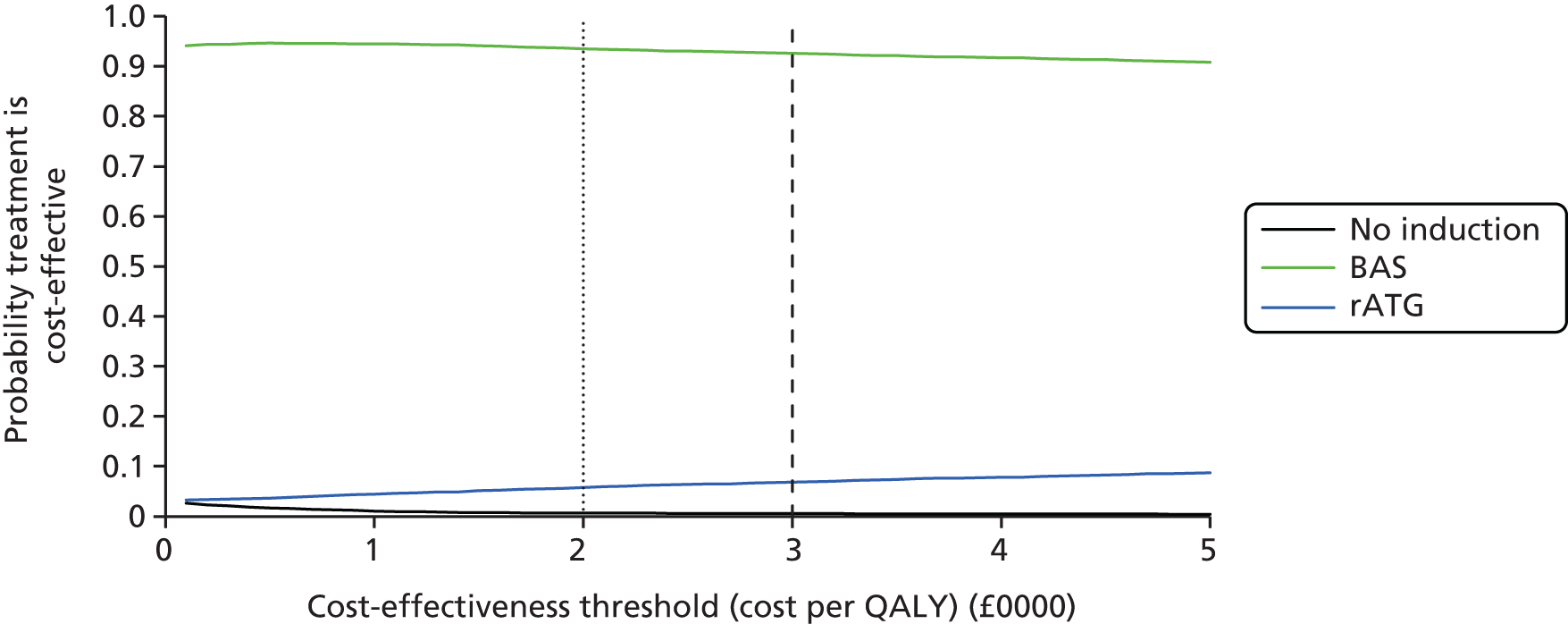
FIGURE 78.
Cost-effectiveness acceptability curves for induction agents in combination with CSA, MMF and CCSs.
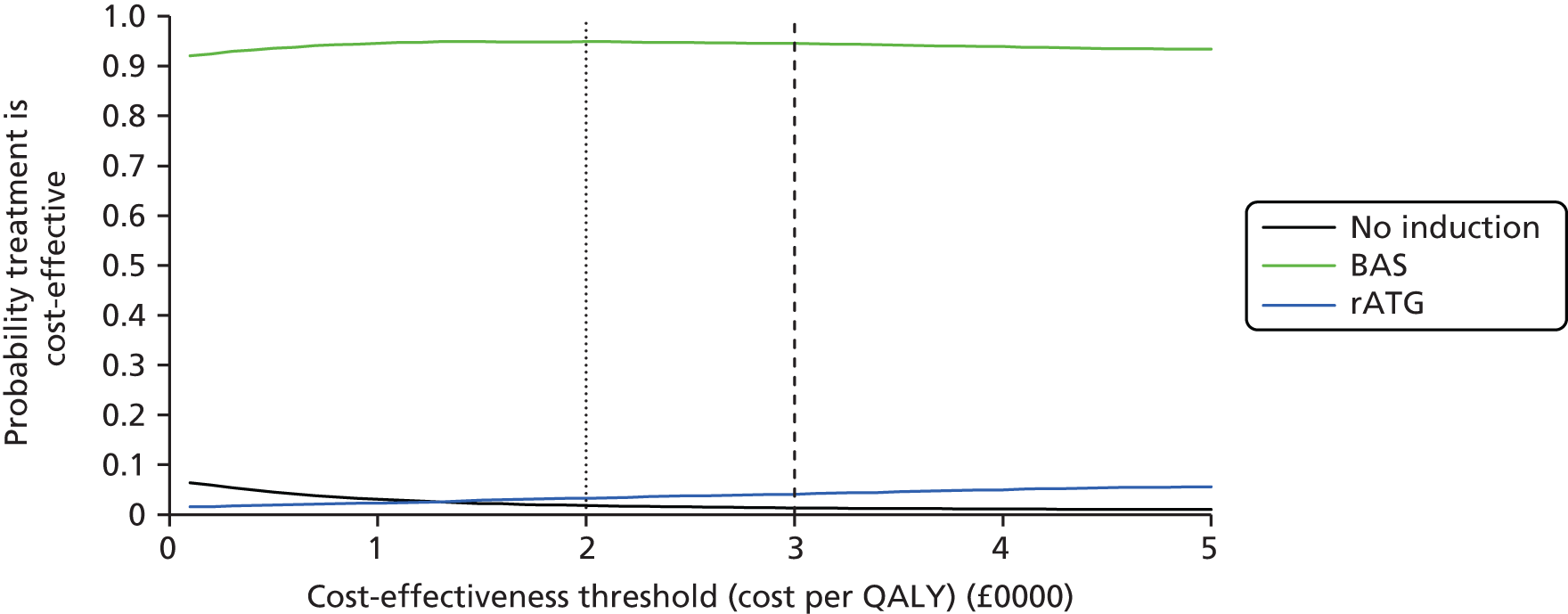
FIGURE 79.
Cost-effectiveness acceptability curves for induction agents in combination with immediate-release TAC, MMF and CCSs.

Summary
Basiliximab is predicted to be cost-effective with an error probability of 5.1–6.5% (cost-effectiveness threshold of £20,000 per QALY) to 5.4–7.4% (cost-effectiveness threshold of £30,000 per QALY). ‘No induction’ and rATG are predicted not to be cost-effective.
Maintenance agents
A summary of cost-effectiveness results in the probabilistic analysis is given in Table 204. All of the treatments that were dominated in the deterministic analysis remain dominated in the probabilistic analysis. In addition, BAS + CSA + MPS is now predicted to be dominated by BAS + CSA + MMF, whereas, in the deterministic analysis, it was more costly and more effective, with an ICER of > £100,000 per QALY. The treatment that was cost-effective at £20,000 and £30,000 per QALY in each comparison in the deterministic analysis remains cost-effective in the probabilistic analysis.
| Maintenance agent | Discounted costs (£) | Discounted QALYs | ICER (cost per QALY, £) | INHB | Probability cost-effective (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | £20,000/QALY | £30,000/QALY | ||
| With MMF | |||||||||
| vs. TAC | |||||||||
| TAC-PR | 106,985 | – | 10.7557 | – | Dominated | –0.8577 | –0.6189 | 0.00 | 0.01 |
| TAC | 92,660 | –14,325 | 10.8972 | +0.1414 | – | – | – | 88.21 | 83.17 |
| CSA | 98,219 | +5558 | 10.9155 | +0.0184 | 302,909 | –0.2596 | –0.1669 | 11.79 | 16.82 |
| With AZA | |||||||||
| vs. TAC | |||||||||
| CSA | 102,504 | – | 10.7634 | – | Dominated | –0.4497 | –0.3210 | 6.29 | 8.25 |
| TAC | 94,783 | –7721 | 10.8270 | +0.0636 | – | – | – | 93.71 | 91.75 |
| With BAS + MMF | |||||||||
| vs. TAC | |||||||||
| SRL | 115,212 | – | 10.8988 | – | Dominated | –1.3273 | –0.9204 | 0.00 | 0.00 |
| TAC | 90,802 | –24,411 | 11.0055 | +0.1067 | – | – | – | 87.06 | 81.78 |
| CSA | 95,938 | +5137 | 11.0309 | +0.0254 | 202,358 | –0.2314 | –0.1458 | 12.94 | 18.22 |
| BEL | 209,677 | +113,738 | 11.2855 | +0.2547 | 446,594 | –5.6637 | –3.6824 | 0.00 | 0.00 |
| With rATG + MMF | |||||||||
| vs. TAC | |||||||||
| TAC | 97,750 | – | 10.9047 | – | – | – | – | 87.59 | 82.57 |
| CSA | 102,831 | +5082 | 10.9250 | +0.0203 | 250,785 | –0.2338 | –0.1491 | 12.41 | 17.43 |
| With CSA | |||||||||
| vs. MMF | |||||||||
| AZA | 102,504 | – | 10.7634 | – | Dominated | –0.3664 | –0.2950 | 9.13 | 8.18 |
| MMF | 98,219 | –4286 | 10.9155 | +0.1522 | – | – | – | 90.87 | 91.82 |
| EVL | 176,463 | +78,245 | 10.9395 | +0.0240 | 3,260,294 | –3.8882 | –2.5842 | 0.00 | 0.00 |
| With TAC | |||||||||
| vs. MMF | |||||||||
| SRL | 126,339 | – | 10.5931 | – | Dominated | –1.9880 | –1.4267 | 0.00 | 0.00 |
| AZA | 94,783 | –31,557 | 10.8270 | +0.2339 | Dominated | –0.1763 | –0.1409 | 35.68 | 35.64 |
| MMF | 92,660 | –2123 | 10.8972 | +0.0702 | – | – | – | 64.32 | 64.36 |
| With BAS + CSA | |||||||||
| vs. MMF | |||||||||
| AZA | 99,159 | – | 10.8989 | – | Dominated | –0.2930 | –0.2393 | 12.52 | 11.30 |
| MPS | 112,360 | +13,200 | 11.0244 | +0.1255 | Dominated | –0.8275 | –0.5538 | 0.11 | 0.62 |
| MMF | 95,938 | –16,421 | 11.0309 | +0.0065 | – | – | – | 87.37 | 88.08 |
| With rATG + CSA | |||||||||
| vs. MMF | |||||||||
| AZA | 105,683 | – | 10.8048 | – | Dominated | –0.2627 | –0.2152 | 14.47 | 13.13 |
| MMF | 102,831 | –2852 | 10.9250 | +0.1201 | – | – | – | 85.53 | 86.87 |
Immediate-release TAC
Immediate-release TAC was compared with CSA (four comparisons), TAC-PR (one comparison), SRL (one comparison) and BEL (one comparison).
In all comparisons immediate-release TAC was the least costly intervention. It dominated TAC-PR when used in combination with MMF; it dominated CSA when used in combination with AZA; and it dominated SRL when used in combination with BAS + MMF. When used in combination with MMF or BAS + MMF or rATG + MMF, immediate-release TAC was less effective than CSA but the ICERs of CSA compared with immediate-release TAC were > £200,000 per QALY. Immediate-release TAC was less costly and less effective than BEL when used in combination with BAS + MMF, but the relevant ICER of BEL (vs. CSA) was > £400,000 per QALY.
In all comparisons, immediate-release TAC was predicted to be cost-effective at £20,000–30,000 per QALY. The probability of immediate-release TAC being cost-effective (i.e. giving the greatest net health benefit in each comparison) at £20,000 and £30,000 per QALY ranged from 81.8% to 93.7%.
TAC-PR
Prolonged-release tacrolimus was compared with immediate-release TAC and CSA in combination with MMF. TAC-PR was predicted to be dominated by immediate-release TAC and CSA, and therefore not predicted to be cost-effective at any cost-effectiveness threshold. The probability of TAC-PR being cost-effective was 0.0% at both £20,000 and £30,000 per QALY.
MMF
Mycophenolate mofetil was compared with AZA (four comparisons), MPS (one comparison), EVL (one comparison) and SRL (one comparison).
Mycophenolate mofetil was predicted to dominate AZA in all comparisons, and to dominate SRL when used in combination with TAC, and to dominate MPS when used in combination with BAS + CSA. MMF was predicted to be less costly and less effective than EVL when used in combination with CSA, but the ICER of EVL (vs. MMF) was > £3,000,000 per QALY and therefore MMF was predicted to be cost-effective at £20,000–30,000 per QALY.
In all comparisons, MMF was predicted to be cost-effective at £20,000–30,000 per QALY. The probability of MMF being cost-effective at £20,000 and £30,000 per QALY ranged from 64.3% to 91.8% across comparisons.
MPS
Mycophenolate sodium was compared with MMF and AZA in combination with BAS + CSA. MPS was predicted to be dominated by MMF and therefore was not predicted to be cost-effective at any cost-effectiveness threshold. The probability of MPS being cost-effective was 0.1% at £20,000 per QALY, and 0.6% and £30,000 per QALY.
SRL
Sirolimus was compared with immediate-release TAC, BEL and CSA in combination with BAS + MMF. SRL was predicted to be dominated by immediate-release TAC and CSA and therefore not predicted to be cost-effective at any cost-effectiveness threshold. The probability of SRL being cost-effective in combination with BAS + MMF was 0.0% at both £20,000 and £30,000 per QALY.
Sirolimus was also compared with MMF and AZA in combination with TAC. SRL was predicted to be dominated by MMF and AZA, and therefore not predicted to be cost-effective at any cost-effectiveness threshold. The probability of SRL being cost-effective in combination with TAC was 0.0% at both £20,000 and £30,000 per QALY.
EVL
Everolimus was compared with MMF and AZA in combination with CSA. EVL was predicted to be more costly and more effective than all comparators. The relevant ICER for EVL (vs. MMF) was > £3,000,000 per QALY and, therefore, EVL was not predicted to be cost-effective at £20,000–30,000 per QALY. The probability of EVL being cost-effective was 0.0% at both £20,000 and £30,000 per QALY.
BEL
Belatacept was compared with immediate-release TAC, SRL and CSA in combination with BAS + MMF. BEL was predicted to be more costly and more effective than all comparators. The relevant ICER for BEL (vs. CSA) was > £400,000 per QALY and therefore BEL was not predicted to be cost-effective at £20,000–30,000 per QALY. The probability of BEL being cost-effective was 0.0% at both £20,000 and £30,000 per QALY.
Cost-effectiveness acceptability curves
Figures 80–87 show the cost-effectiveness acceptability curves for maintenance agents in the probabilistic analysis. As for induction agents, we have not presented these as cost-effectiveness acceptability frontiers because the agent with the highest probability of being cost-effective also gives the greatest expected net health benefit in the range explored.
FIGURE 80.
Cost-effectiveness acceptability curves for maintenance agents (CSA, TAC and TAC-PR) in combination with MMF.
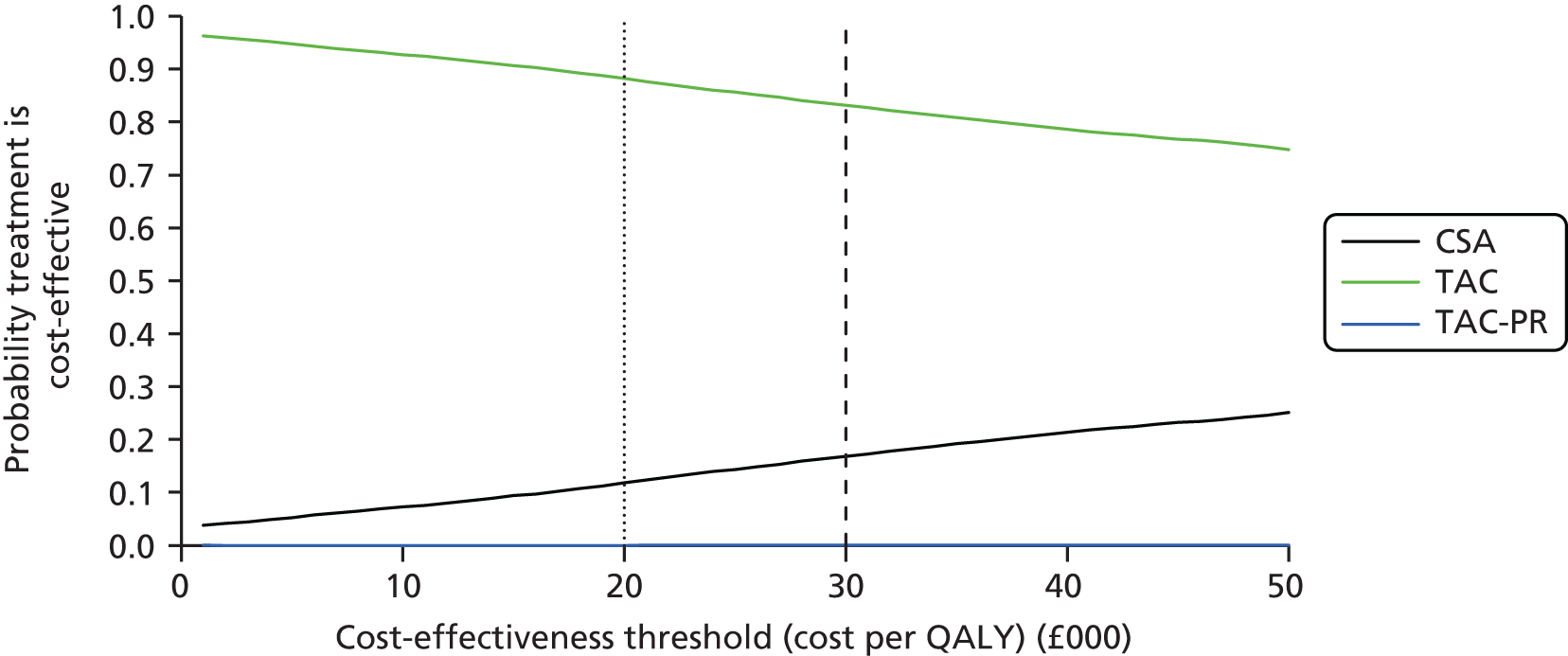
FIGURE 81.
Cost-effectiveness acceptability curves for maintenance agents (CSA and TAC) in combination with AZA.
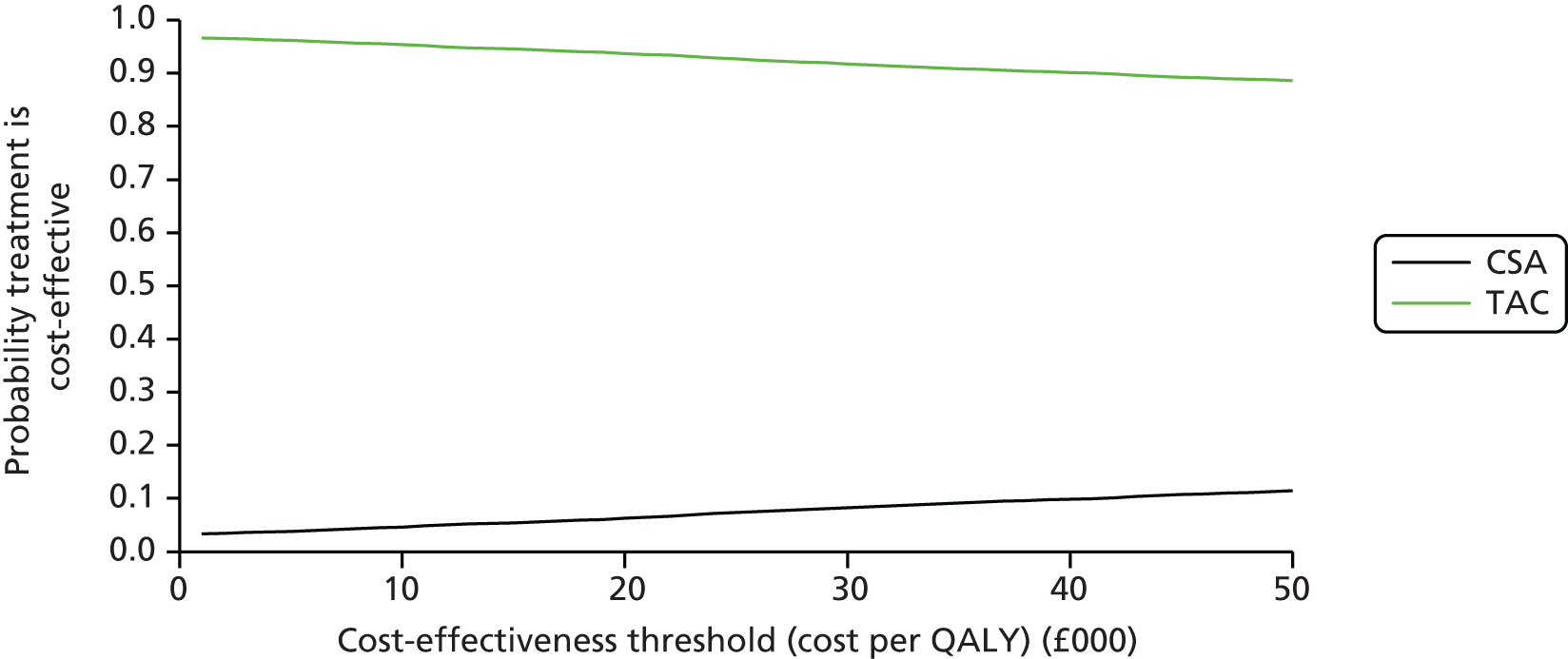
FIGURE 82.
Cost-effectiveness acceptability curves for maintenance agents (CSA, TAC, SRL and BEL) in combination with BAS + MMF.

FIGURE 83.
Cost-effectiveness acceptability curves for maintenance agents (CSA and TAC) in combination with rATG + MMF.

FIGURE 84.
Cost-effectiveness acceptability curves for maintenance agents (AZA, MMF and EVL) in combination with CSA.

FIGURE 85.
Cost-effectiveness acceptability curves for maintenance agents (AZA, MMF and SRL) in combination with TAC.

FIGURE 86.
Cost-effectiveness acceptability curves for maintenance agents (AZA, MMF and MPS) in combination with BAS + CSA.

FIGURE 87.
Cost-effectiveness acceptability curves for maintenance agents (AZA and MMF) in combination with rATG + CSA.
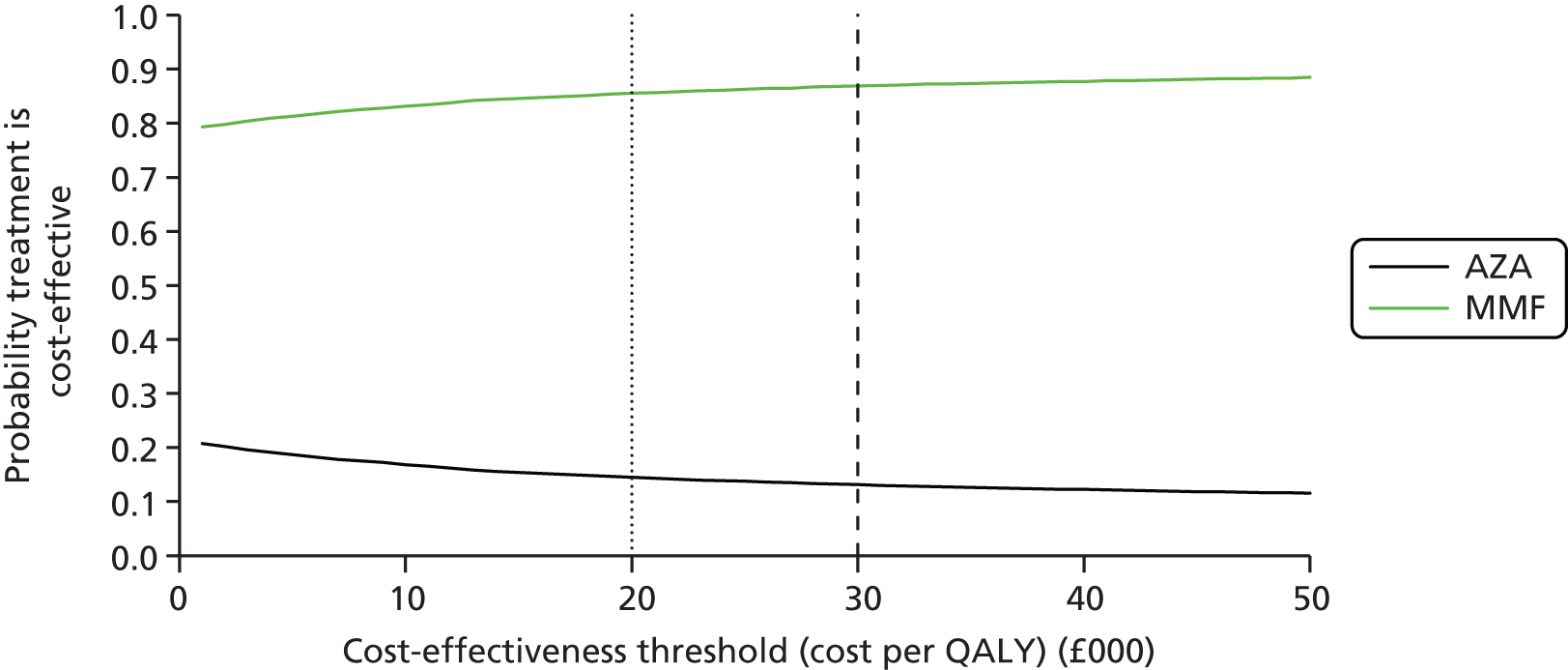
Summary
As in the deterministic analysis, only immediate-release TAC and MMF were cost-effective at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY.
Prolonged-release tacrolimus, MPS and SRL were dominated in their relevant comparisons, whereas EVL and BEL were always the most costly and most effective treatment in their relevant comparisons but with ICERs that were significantly greater than £30,000 per QALY.
Comparing all regimens
When all regimens are compared simultaneously, all regimens are dominated or extended dominated (rATG + TAC + MMF, rATG + CSA + MMF) except for BAS + TAC + MMF, BAS + CSA + MMF and BAS + BEL + MMF, which lie on the cost-effectiveness frontier. BAS + CSA + MPS is not predicted to be on the cost-effectiveness frontier in the probabilistic analysis, whereas it was in the deterministic analysis. As explained above (see Probabilistic results) this may be because of a downwards bias on probabilistic QALYs compared with deterministic QALYs for this regimen due to non-linearities. The cost-effectiveness results for the regimens on the cost-effectiveness frontier are given in Table 205.
| Regimen | Discounted costs (£) | Discounted QALYs | ICER (£) | INHB | Probability cost-effective (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | £20,000/QALY | £30,000/QALY | ||
| Regimens on the cost-effectiveness frontier | |||||||||
| BAS + TAC + MMF | 90,802 | – | 11.0055 | – | – | – | – | 68.97 | 65.32 |
| BAS + CSA + MMF | 95,938 | +5137 | 11.0309 | +0.0254 | 202,358 | –0.2314 | –0.1458 | 8.97 | 13.43 |
| BAS + BEL + MMF | 209,677 | +113,738 | 11.2855 | +0.2547 | 446,594 | –5.6637 | –3.6824 | 0.00 | 0.00 |
| Probability a regimen on the cost-effectiveness frontier is cost-effective | 77.94 | 78.75 | |||||||
| Regimens not on the cost-effectiveness frontier | |||||||||
| TAC + SRL | 126,339 | +35,538 | 10.5931 | –0.4124 | Dominated | –2.1893 | –1.5970 | 0.00 | 0.00 |
| TAC-PR + MMF | 106,985 | +16,184 | 10.7557 | –0.2498 | Dominated | –1.0589 | –0.7892 | 0.00 | 0.00 |
| CSA + AZA | 102,504 | +11,703 | 10.7634 | –0.2421 | Dominated | –0.8273 | –0.6322 | 0.00 | 0.00 |
| rATG + CSA + AZA | 105,683 | +14,882 | 10.8048 | –0.2006 | Dominated | –0.9447 | –0.6967 | 0.08 | 0.11 |
| TAC + AZA | 94,783 | +3981 | 10.8270 | –0.1785 | Dominated | –0.3775 | –0.3112 | 16.86 | 14.94 |
| TAC + MMF | 92,660 | +1859 | 10.8972 | –0.1083 | Dominated | –0.2013 | –0.1703 | 1.55 | 1.21 |
| BAS + SRL + MMF | 115,212 | +24,411 | 10.8988 | –0.1067 | Dominated | –1.3273 | –0.9204 | 0.00 | 0.00 |
| BAS + CSA + AZA | 99,159 | +8357 | 10.8989 | –0.1066 | Dominated | –0.5244 | –0.3851 | 0.96 | 1.26 |
| rATG + TAC + MMF | 97,750 | +6948 | 10.9047 | –0.1008 | Dominated | –0.4482 | –0.3324 | 2.18 | 2.97 |
| CSA + MMF | 98,219 | +7417 | 10.9155 | –0.0900 | Dominated | –0.4608 | –0.3372 | 0.09 | 0.12 |
| rATG + CSA + MMF | 102,831 | +12,030 | 10.9250 | –0.0805 | Dominated | –0.6820 | –0.4815 | 0.33 | 0.51 |
| CSA + EVL | 176,463 | +85,662 | 10.9395 | –0.0660 | Dominated | –4.3491 | –2.9214 | 0.00 | 0.00 |
| BAS + CSA + MPS | 112,360 | +21,558 | 11.0244 | +0.0189 | Dominated | –1.0590 | –0.6997 | 0.01 | 0.13 |
| Probability a regimen not on the cost-effectiveness frontier is cost-effective | 22.06 | 21.25 | |||||||
These results indicate that there is a 78.0–78.8% probability that a regimen on the cost-effectiveness frontier gives the maximum net health benefit at £20,000–30,000 per QALY. The probability that BAS + TAC + MMF gives the maximum net health benefit is 69.0% at £20,000 per QALY and 65.3% at £30,000 per QALY.
Table 205 also presents the cost-effectiveness results for regimens that are not on the cost-effectiveness frontier. All incremental costs and QALYs and INHBs are compared with BAS + TAC + MMF. All of these regimens are, by definition, dominated or extended dominated, although not in every case by BAS + TAC + MMF. Interestingly, at £20,000 per QALY there is a regimen that is not on the cost-effectiveness frontier (TAC + AZA), which is predicted to be more likely to be cost-effective than BAS + CSA + MMF and BAS + BEL + MMF (which are both on the frontier).
It is known that when the cost-effectiveness of an intervention is highly uncertain it can result in a flatteringly high probability of being cost-effective. A graphical representation that helps to identify this phenomenon is the rankogram,425 which plots the probability distribution for the rank of an intervention according to a certain measure. We present rankograms of the net health benefit at £20,000 per QALY for all 16 regimens in Figure 88. These suggest that the ranks of CSA + AZA, CSA + EVL, TAC + SRL, TAC-PR + MMF, BAS + TAC + MMF, BAS + SRL + MMF and BAS + BEL + MMF are fairly well, or extremely well, estimated (little dispersion in rank probability distribution), whereas the ranks for other regimens are less well estimated. The mean rank can also be calculated and is also presented in Table 205, demonstrating that the regimen with the greatest expected rank is BAS + TAC + MMF.
FIGURE 88.
Rankograms of net health benefit at £20,000 per QALY for each regimen. (a) CSA + MMF (5.8); (b) TAC + MMF (3.2); (c) proportion of simulations in PSA; (c) CSA + AZA (9.2); (d) TAC + AZA (4.8); (e) CSA + EVL (15.0); (f) TAC + SRL (14.0); (g) TAC-PR + MMF (10.7); (h) BAS + CSA + MMF (3.3); (i) BAS + TAC + MMF (1.5); (j) BAS + CSA + AZA (6.1); (k) BAS + SRL + MMF (12.2); (l) BAS + BEL + MMF (16.0); (m) BAS + CSA + MPS (10.5); (n) rATG + CSA + MMF (7.9); (o) rATG + TAC + MMF (5.5); and (p) rATG + CSA + AZA (10.2).
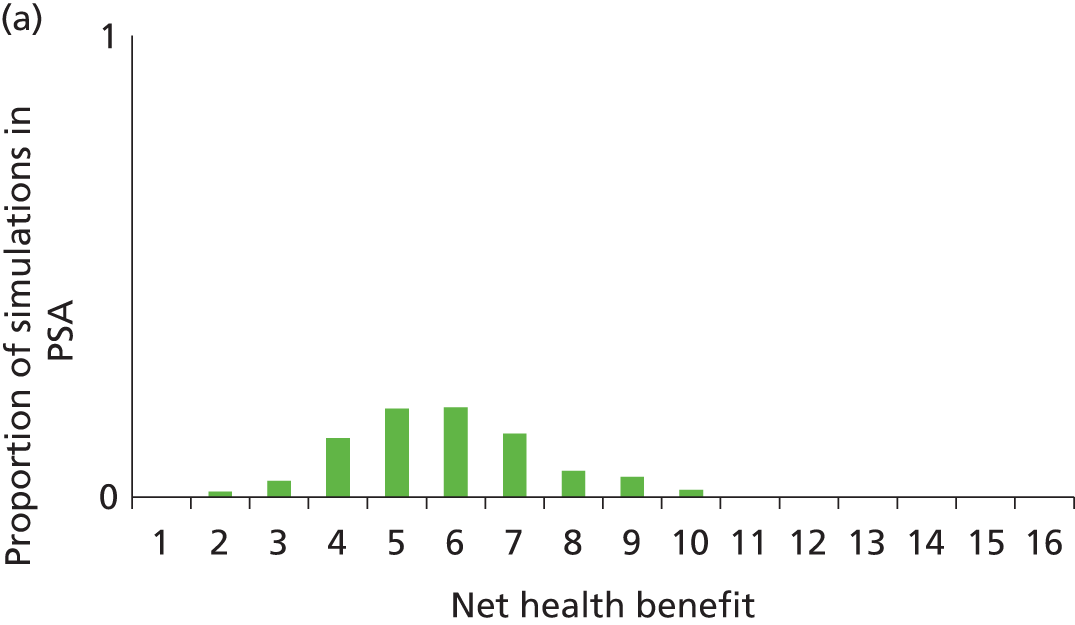


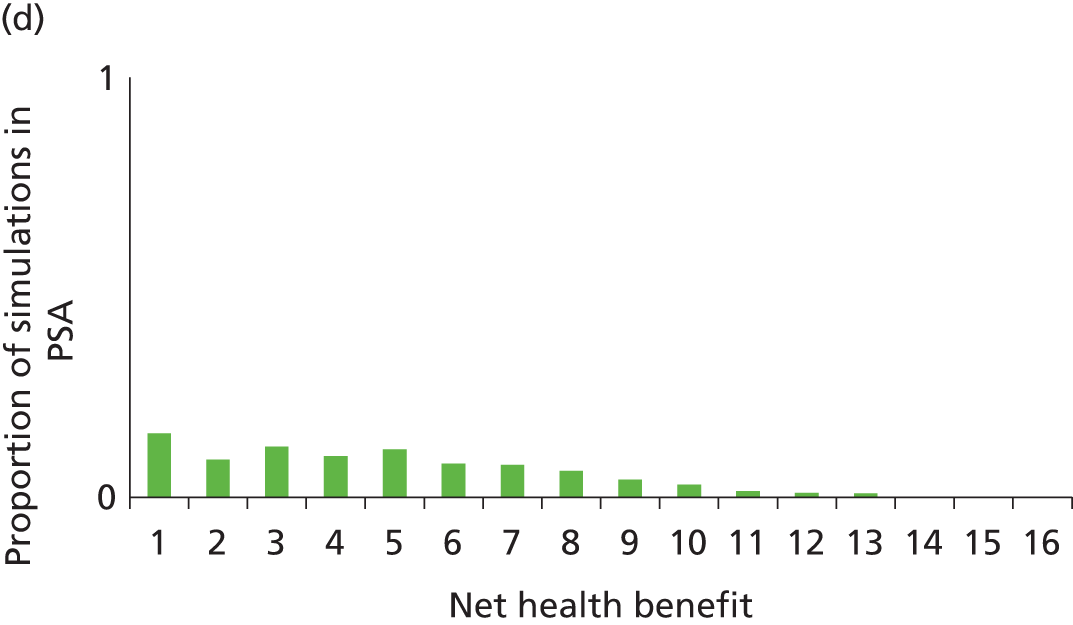

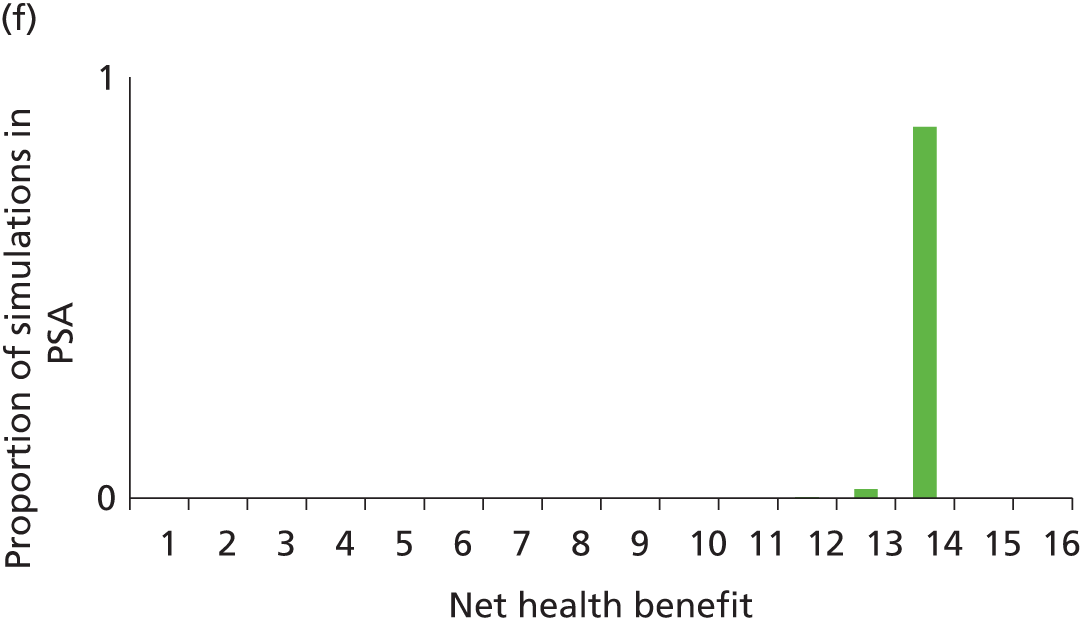
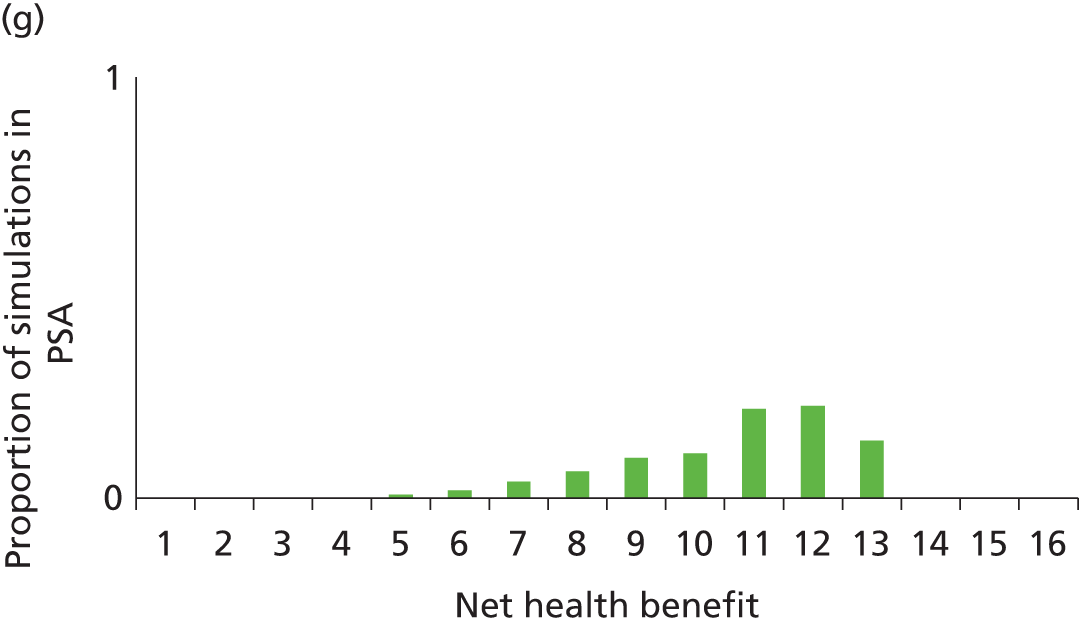

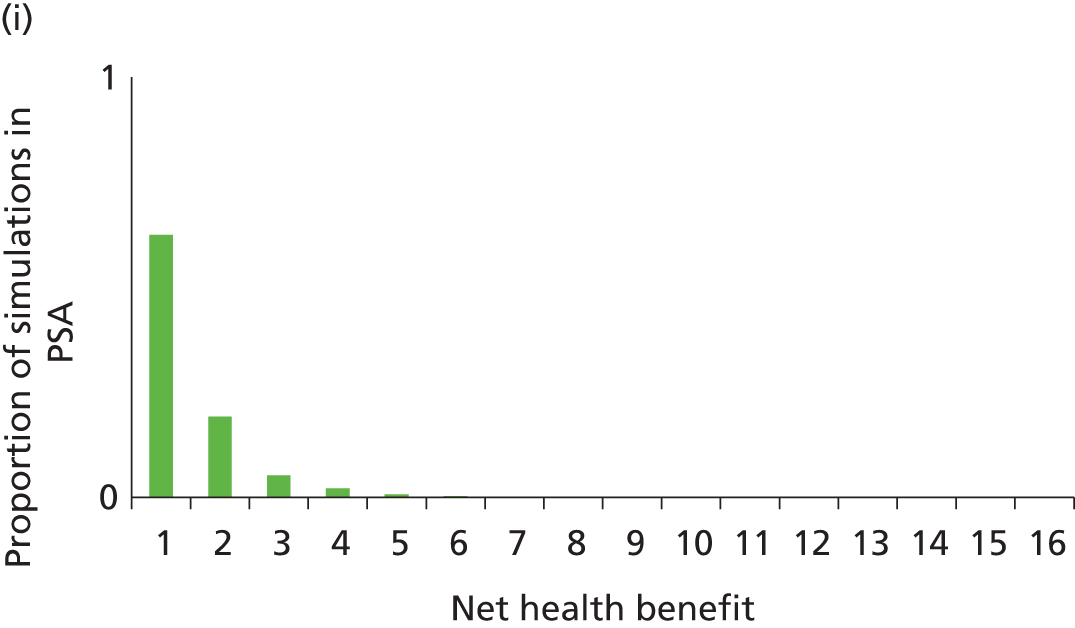
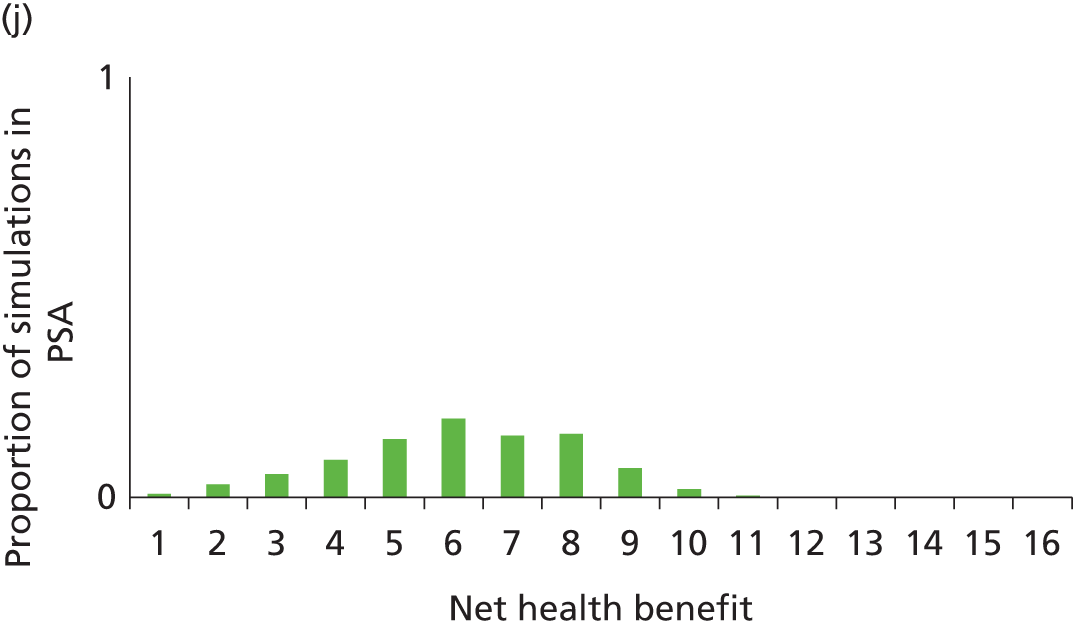
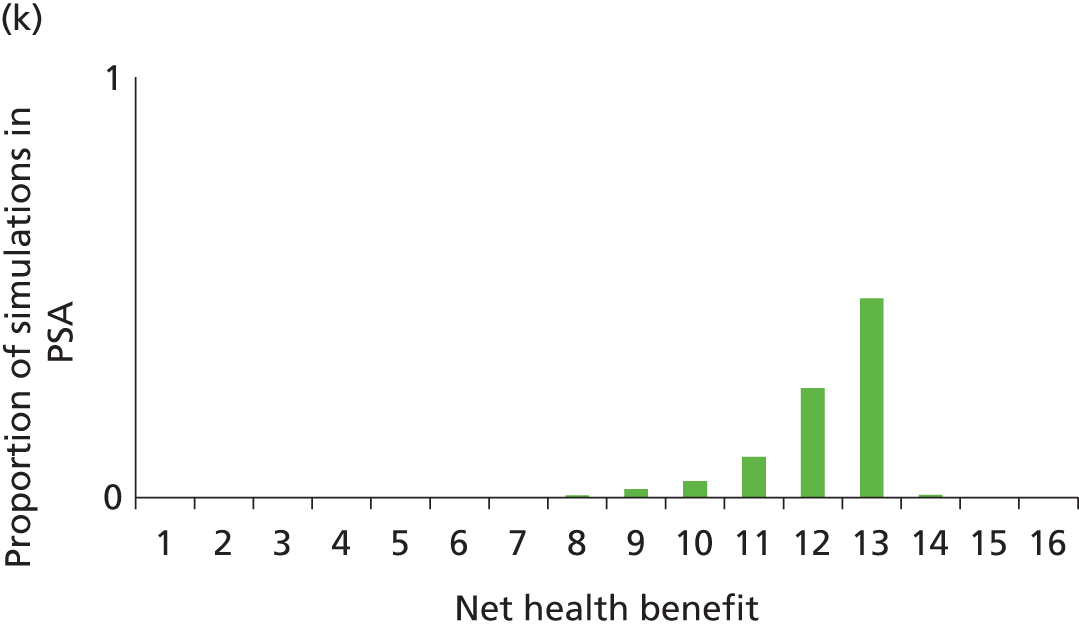
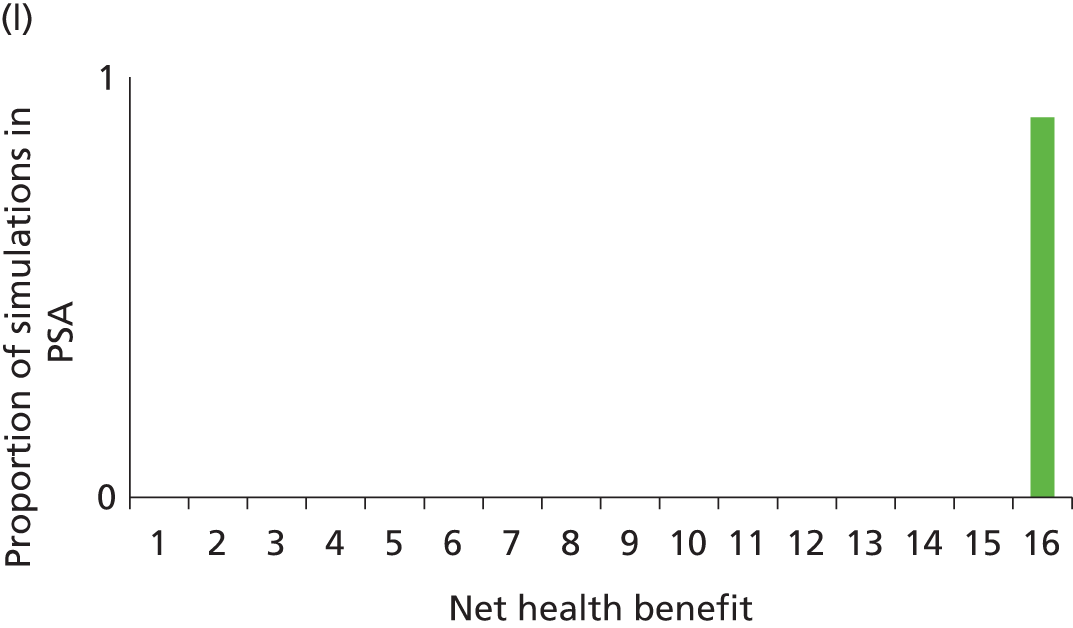

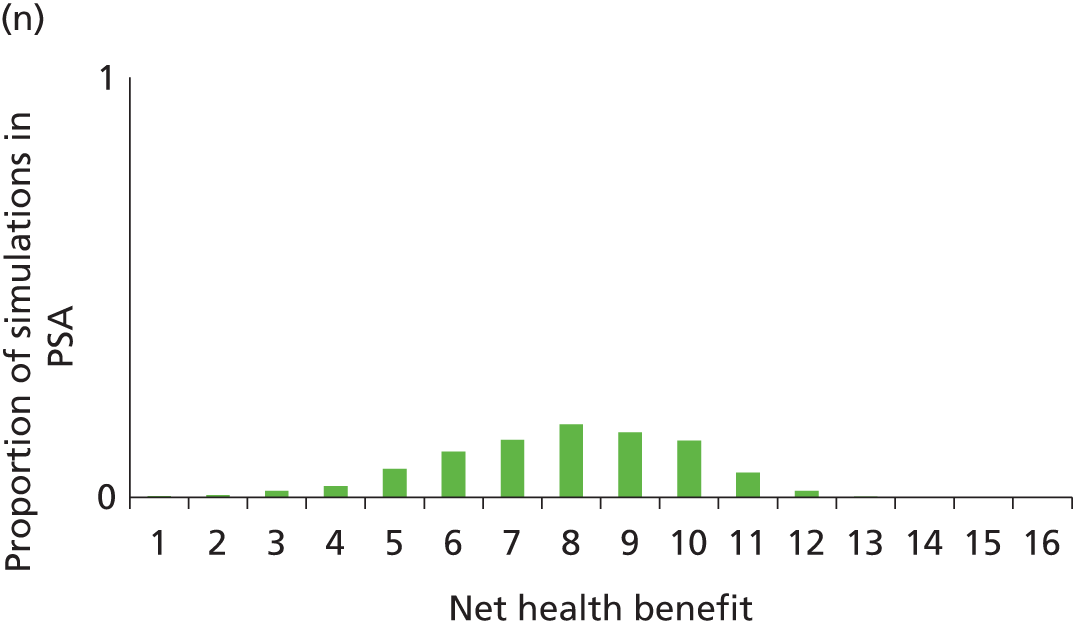
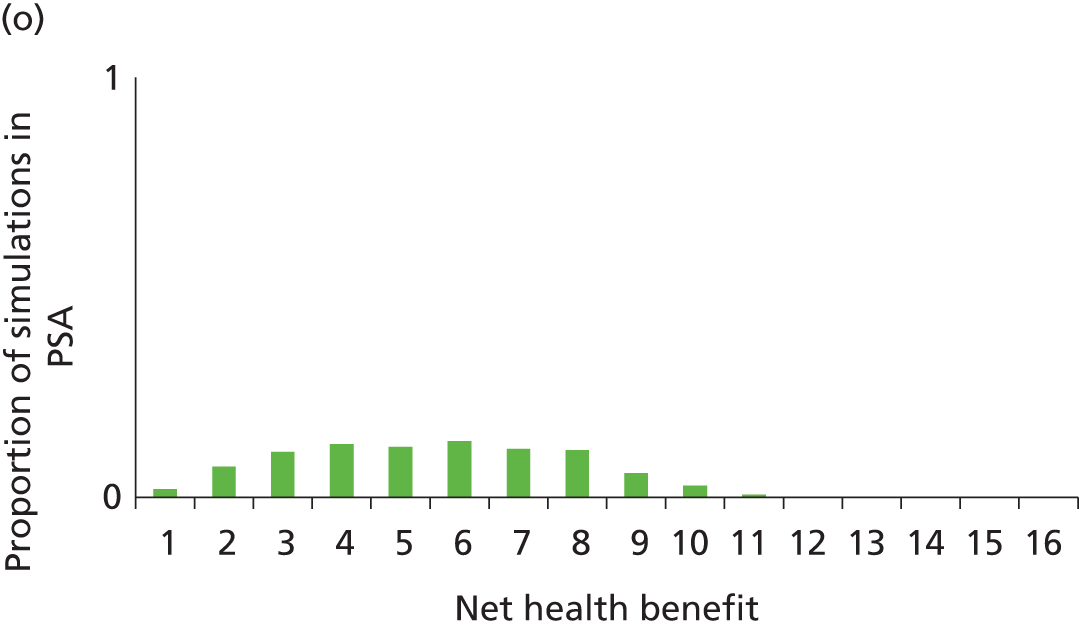

Scenario analyses
Graft survival structural scenario analyses
Eliminating graft survival differences after a certain time
To explore what impact the model for death-censored graft survival had on cost-effectiveness, a scenario analysis was conducted in which after n years the hazard rate of death-censored graft loss was equalised for all regimens (set equal to the baseline hazard function). This is equivalent to the conditional graft survival from time n years being identical across the regimens.
The ‘n years’ was varied from 1 to 20; the base case is effectively n = 50. When n = 1 it is therefore assumed that AR, eGFR and NODAT do not affect graft survival after 1 year and that long-term graft survival is determined solely by graft survival at 1 year. As n increases, the surrogate relationship from AR, eGFR and NODAT to graft survival is strengthened towards the base case.
Figure 89 shows the net health benefit of all regimens as n is varied from 1 to 20. Figure 90 shows a close-up of the regimens with high net health benefit (BAS + CSA + MPS, TAC-PR + MMF, BAS + SRL + MMF, TAC + SRL, CSA + EVL and BAS + BEL + MMF are not visible in this figure).
FIGURE 89.
Net health benefit of regimens as duration of surrogate effect on graft survival is varied.

FIGURE 90.
Net health benefit of regimens as duration of surrogate effect on graft survival is varied (close-up).

Tables 206 and 207, respectively, indicate the ranges of n for which induction and maintenance agents are cost-effective (i.e. give the greatest net health benefit in each comparison).
| Induction agent | Range of n for which induction agent is cost-effective | |
|---|---|---|
| £20,000/QALY | £30,000/QALY | |
| With CSA + AZA | ||
| No induction | NA | NA |
| BAS | 1–20 | 1–20 |
| rATG | NA | NA |
| With CSA + MMF | ||
| No induction | 1–2 | 1 |
| BAS | 3–20 | 2–20 |
| rATG | NA | NA |
| With TAC + MMF | ||
| No induction | 1–3 | 1–2 |
| BAS | 4–20 | 3–20 |
| rATG | NA | NA |
| Maintenance agent | Range of n for which maintenance agent is cost-effective | |
|---|---|---|
| £20,000/QALY | £30,000/QALY | |
| With MMF | ||
| TAC-PR | NA | NA |
| TAC | 5–20 | 8–20 |
| CSA | 1–4 | 1–7 |
| With AZA | ||
| CSA | 1–4 | 1–5 |
| TAC | 5–20 | 6–20 |
| With BAS + MMF | ||
| SRL | NA | NA |
| TAC | 6–20 | 9–20 |
| CSA | 1–5 | 1–8 |
| BEL | NA | NA |
| With rATG + MMF | ||
| TAC | 5–20 | 8–20 |
| CSA | 1–4 | 1–7 |
| With CSA | ||
| AZA | NA | NA |
| MMF | 1–20 | 1–20 |
| EVL | NA | NA |
| With TAC | ||
| SRL | NA | NA |
| AZA | NA | NA |
| MMF | 1–20 | 1–20 |
| With BAS + CSA | ||
| AZA | NA | NA |
| MMF | 1–20 | 1–20 |
| MPS | NA | NA |
| With rATG + CSA | ||
| AZA | NA | NA |
| MMF | 1–20 | 1–20 |
Table 207 indicates that TAC-PR, SRL, BEL, EVL and MPS were not cost-effective at £20,000 or £30,000 per QALY for any n from 1 to 20. MMF was cost-effective at £20,000 and £30,000 per QALY for all n from 1 to 20. For lower values of n (up to 4–8), CSA was cost-effective at £20,000 or £30,000 per QALY, whereas for higher values (towards the base case), TAC was cost-effective at £20,000 and £30,000 per QALY.
As can be seen in Figure 90, once n is ≥ 6, BAS + TAC + MMF gives the greatest net health benefit. When n is < 6, BAS + CSA + MMF, CSA + MMF, BAS + CSA + AZA, CSA + AZA and TAC + MMF give greater net health benefit than BAS + TAC + MMF for some n, although only BAS + CSA + MMF or CSA + MMF gives the greatest net health benefit for n < 6. Base-case graft survival curves for CSA + MMF, BAS + CSA + MMF and BAS + TAC + MMF are shown in Figures 91 and 92.
FIGURE 91.
Death-censored graft survival for CSA + MMF, BAS + CSA + MMF and BAS + TAC + MMF (base case).
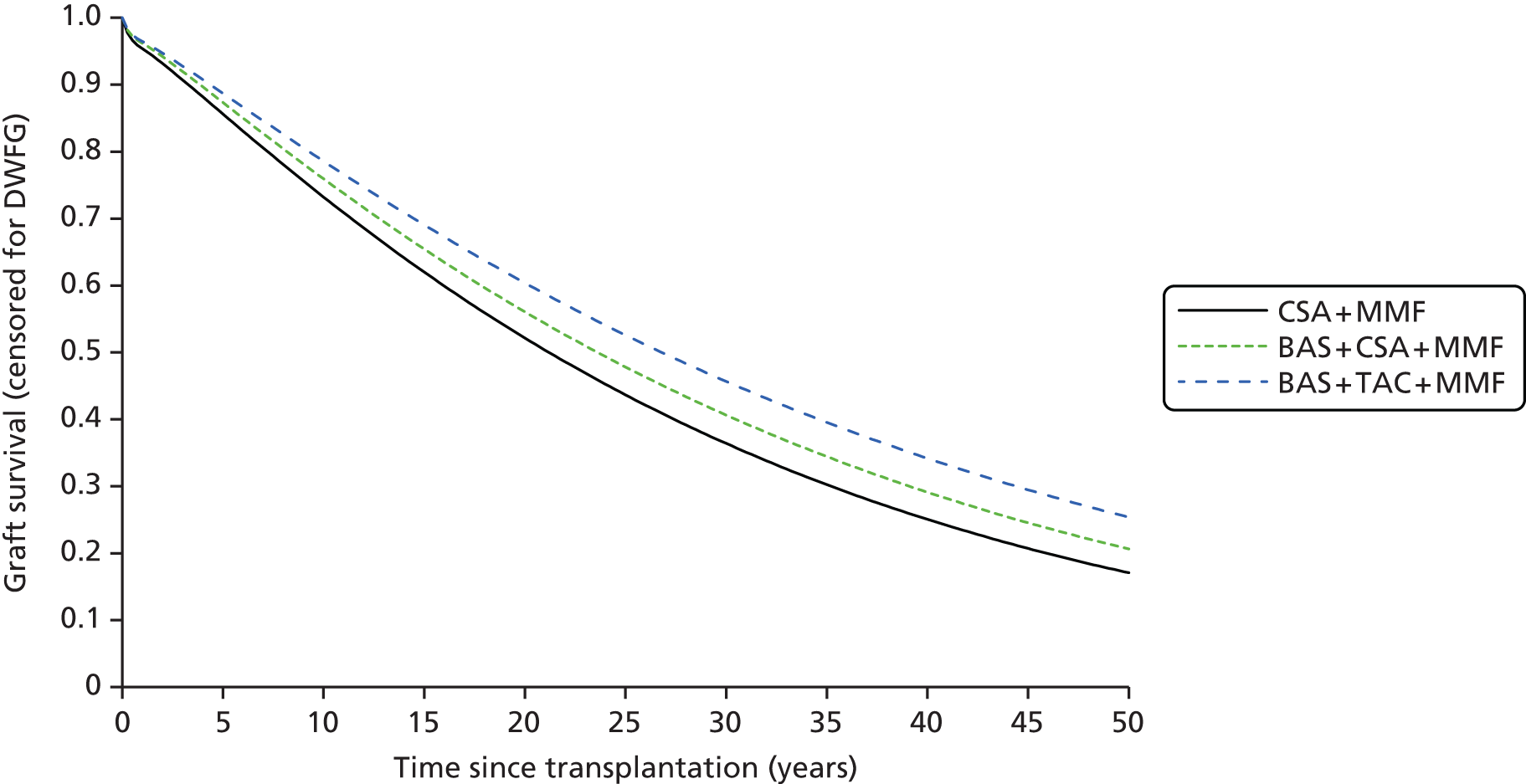
FIGURE 92.
Death-censored graft survival for CSA + MMF, BAS + CSA + MMF and BAS + TAC + MMF (base case; close-up 0–10 years).
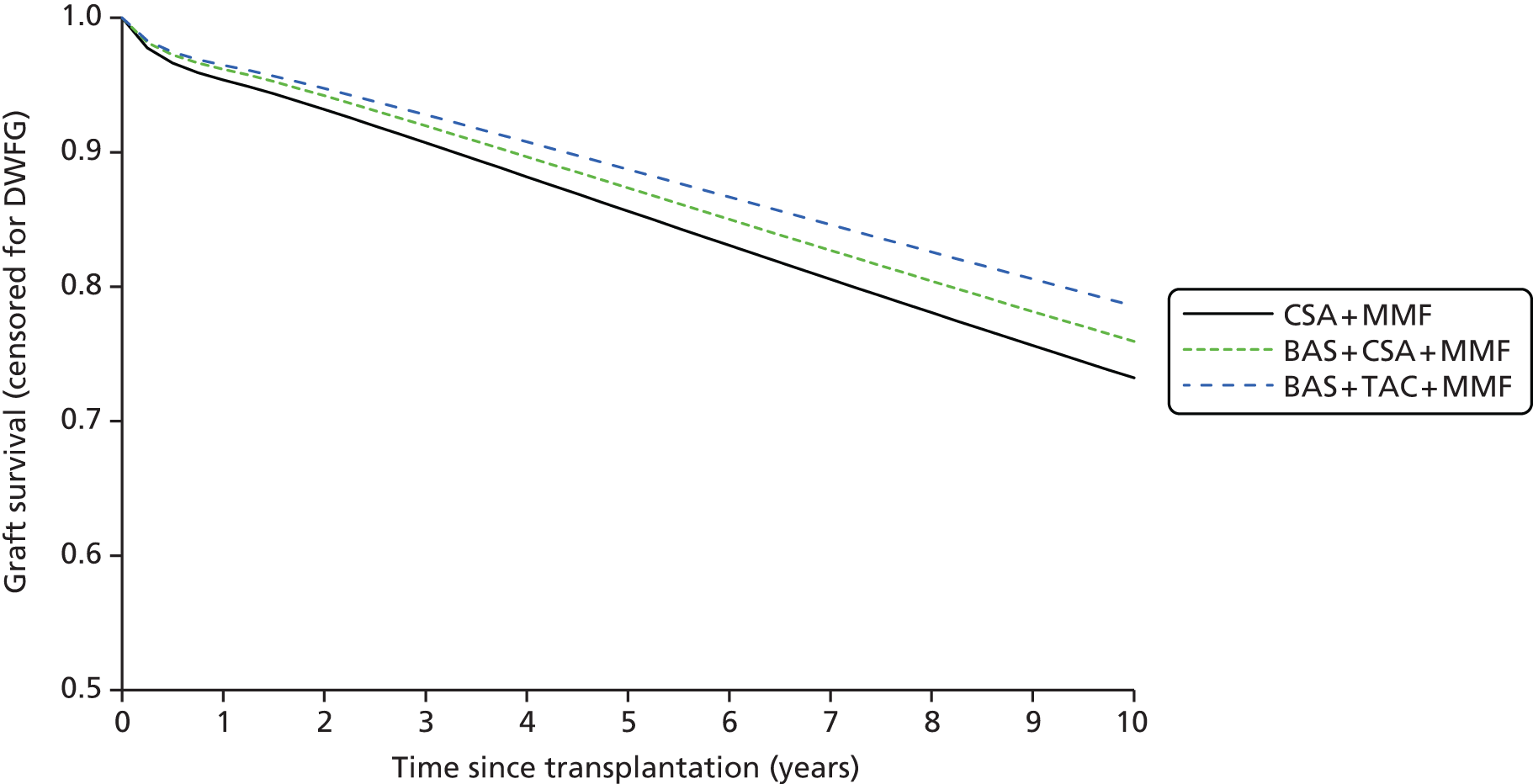
When n = 5, BAS + CSA + MMF gives the greatest net health benefit and the graft survival for CSA + MMF, BAS + CSA + MMF and BAS + TAC + MMF is shown in Figures 93 and 94. As expected, by reducing the duration of the surrogate effect, the graft survival curves diverge significantly less than in the base case.
FIGURE 93.
Death-censored graft survival for CSA + MMF, BAS + CSA + MMF and BAS + TAC + MMF (n = 5).
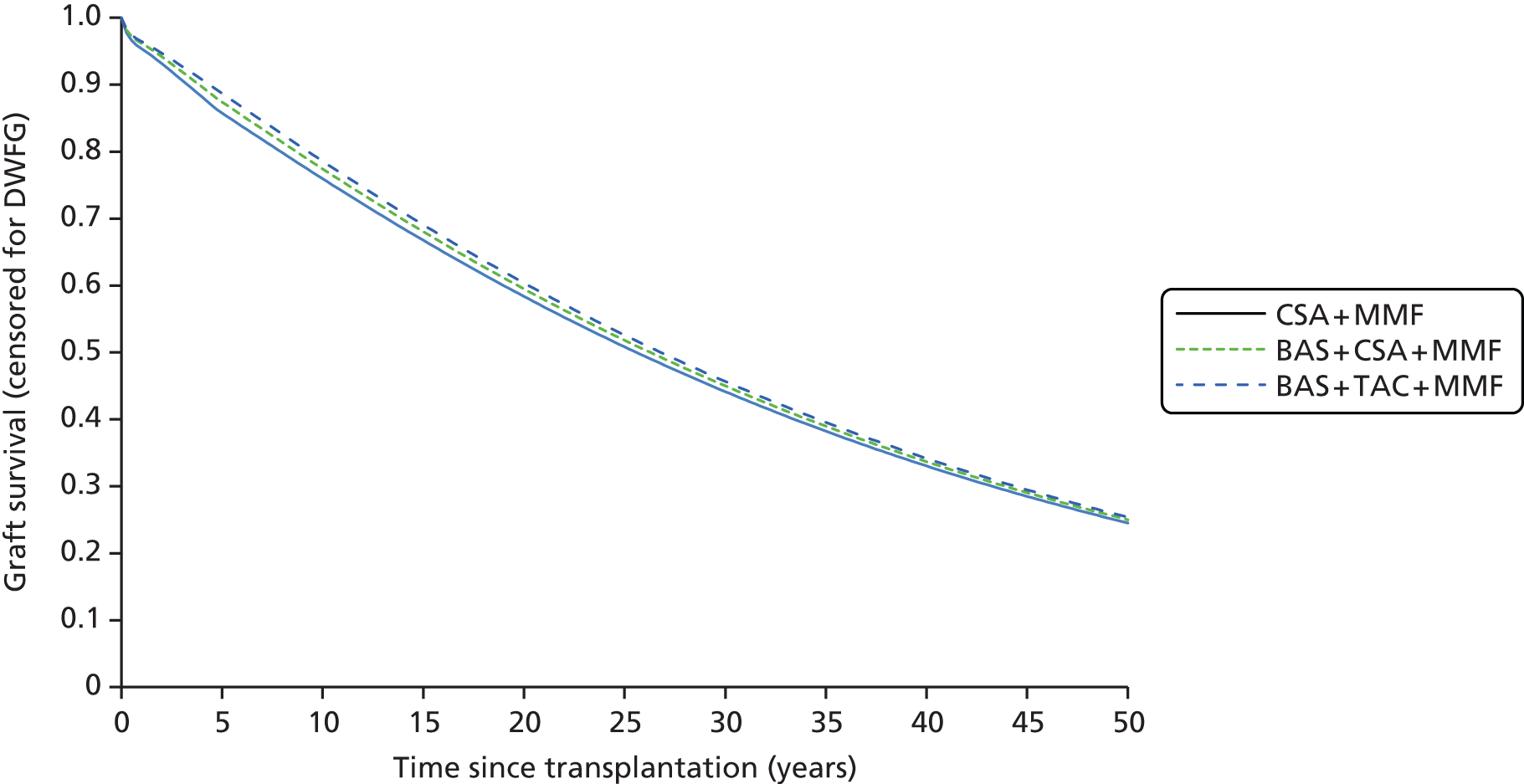
FIGURE 94.
Death-censored graft survival for CSA + MMF, BAS + CSA + MMF and BAS + TAC + MMF (n = 5; close-up 0–10 years).
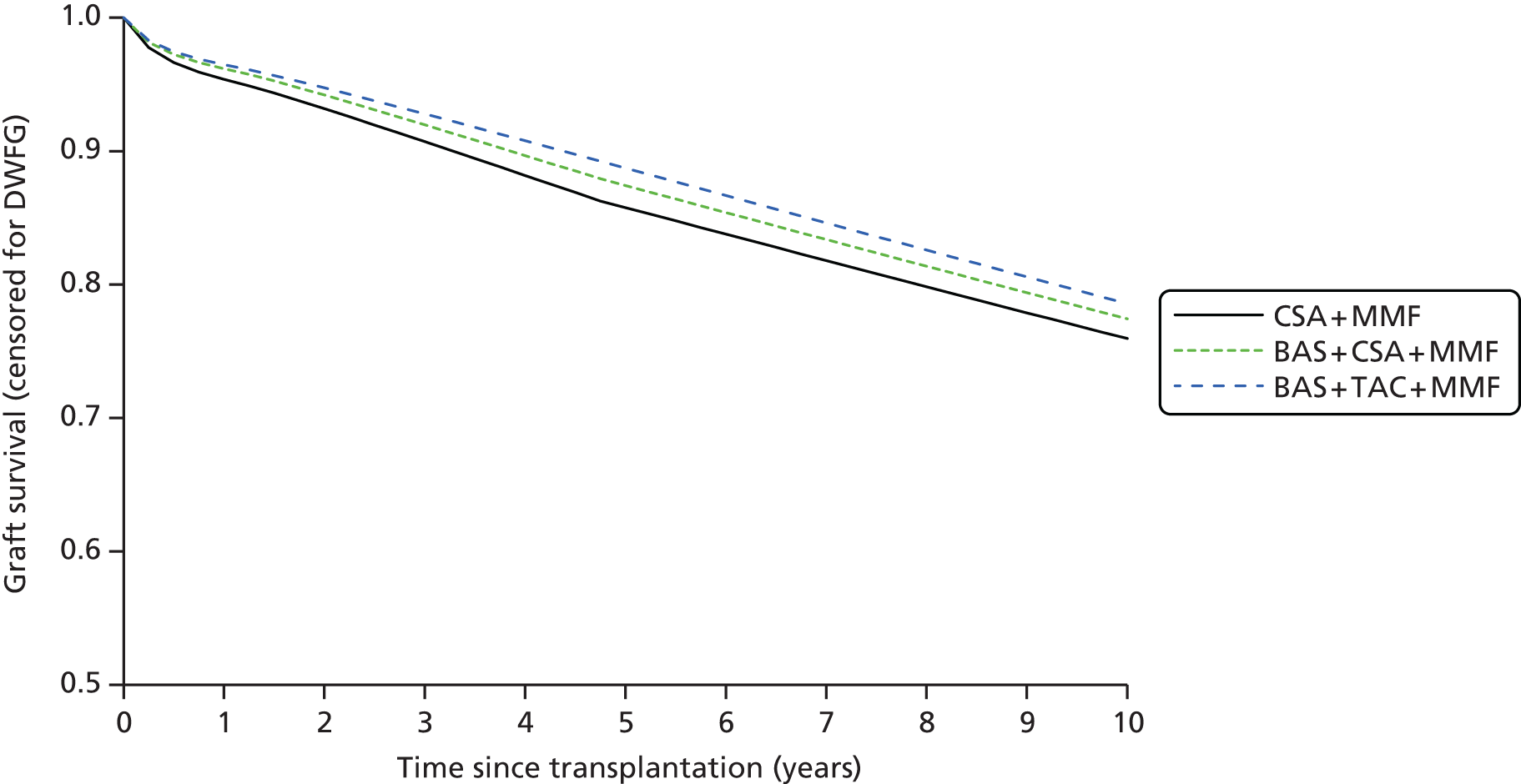
When n = 2, CSA + MMF gives the greatest net health benefit and the graft survival for CSA + MMF, BAS + CSA + MMF and BAS + TAC + MMF is shown in Figures 95 and 96. As there is now only 1 year of graft survival difference extrapolated according to the surrogate relationship, the graft survival curves are virtually identical. In this scenario CSA + MMF gives the greatest net health benefit but it is noteworthy that the net health benefit of CSA + MMF is quite sensitive to n and, even in this scenario, only four regimens are predicted to give greater net health benefit than BAS + TAC + MMF: CSA + MMF, TAC + MMF, BAS + CSA + MMF and BAS + CSA + AZA.
FIGURE 95.
Death-censored graft survival for CSA + MMF, BAS + CSA + MMF and BAS + TAC + MMF (n = 2).
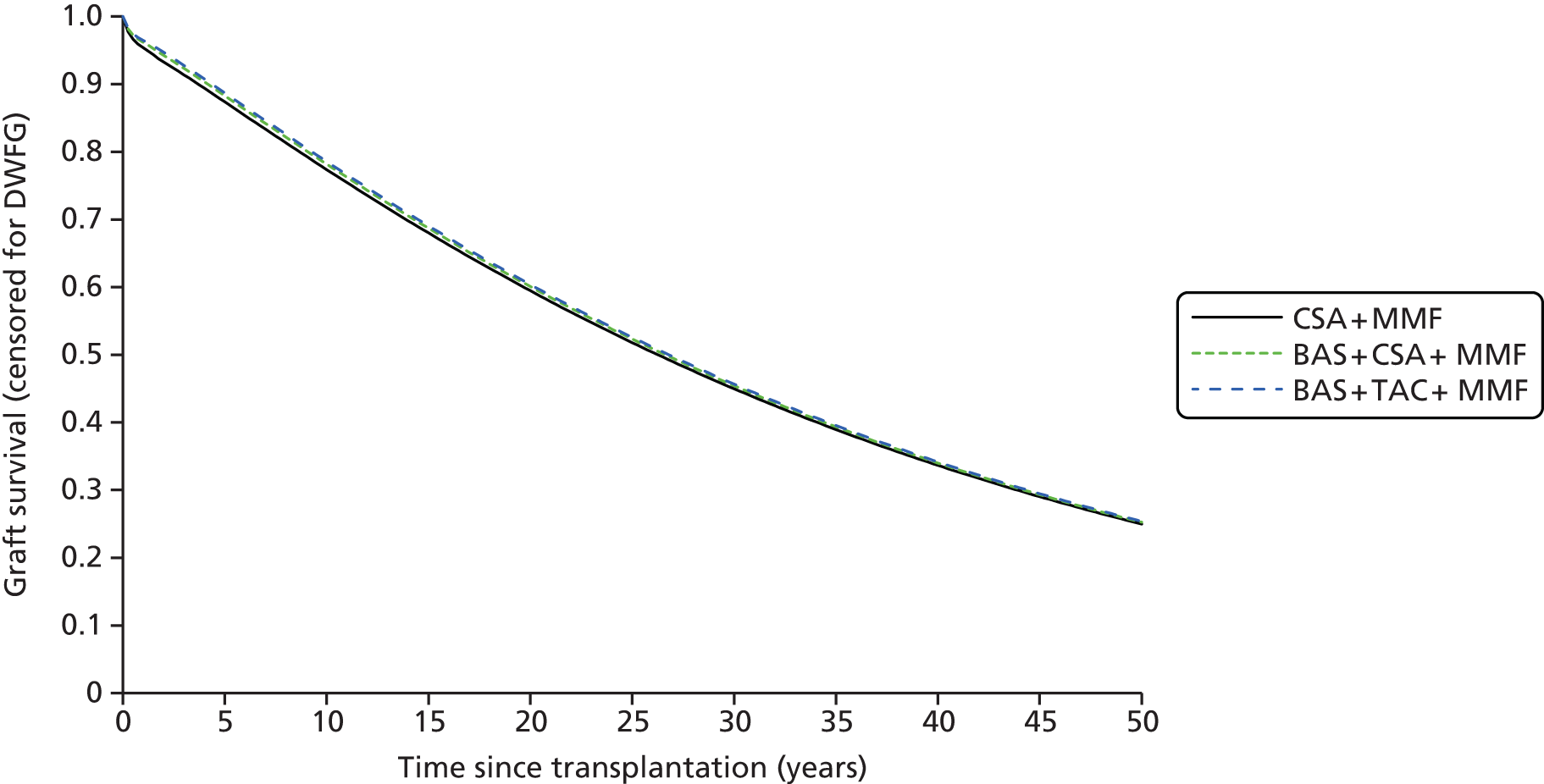
FIGURE 96.
Death-censored graft survival for CSA + MMF, BAS + CSA + MMF and BAS + TAC + MMF (n = 2; close-up 0–10 years).
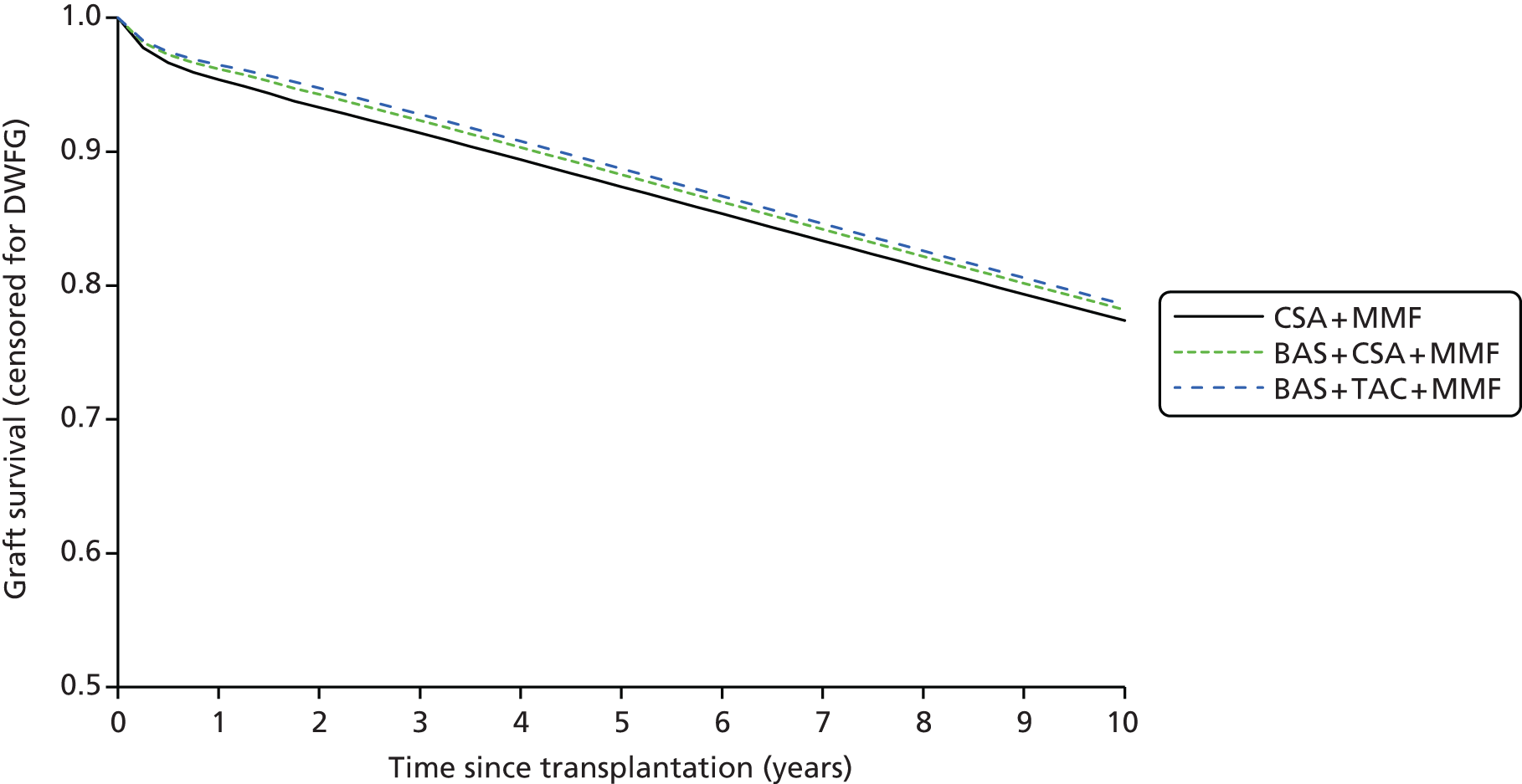
Different gamma parameter for calcineurin inhibitor-free regimens
It may be plausible that avoiding CNIs will prolong long-term graft survival by avoiding CNI nephrotoxicity. This possibility was investigated by reducing the gamma (γ) parameter in the Weibull model for graft survival (death censored) for regimens without CNI, that is, for BAS + SRL + MMF and BAS + BEL + MMF.
An offset was included for ln(γ) of between −2 and 0 (equivalent to the base case). The INHB for BAS + SRL + MMF and BAS + BEL + MMF compared with BAS + TAC + MMF was calculated (as TAC was predicted to be the only cost-effective agent in combination with BAS + MMF at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY). The INHB was calculated at both £20,000 and £30,000 per QALY. As shown in Figures 97 and 98, there is a crossover for SRL but not for BEL across the range explored, suggesting that SRL could be cost-effective at £20,000–30,000 per QALY if long-term graft survival were significantly better than extrapolated in the base case.
FIGURE 97.
Incremental net health benefit (at £20,000 per QALY) of SRL and BEL vs. TAC as gamma parameter of graft survival is varied.
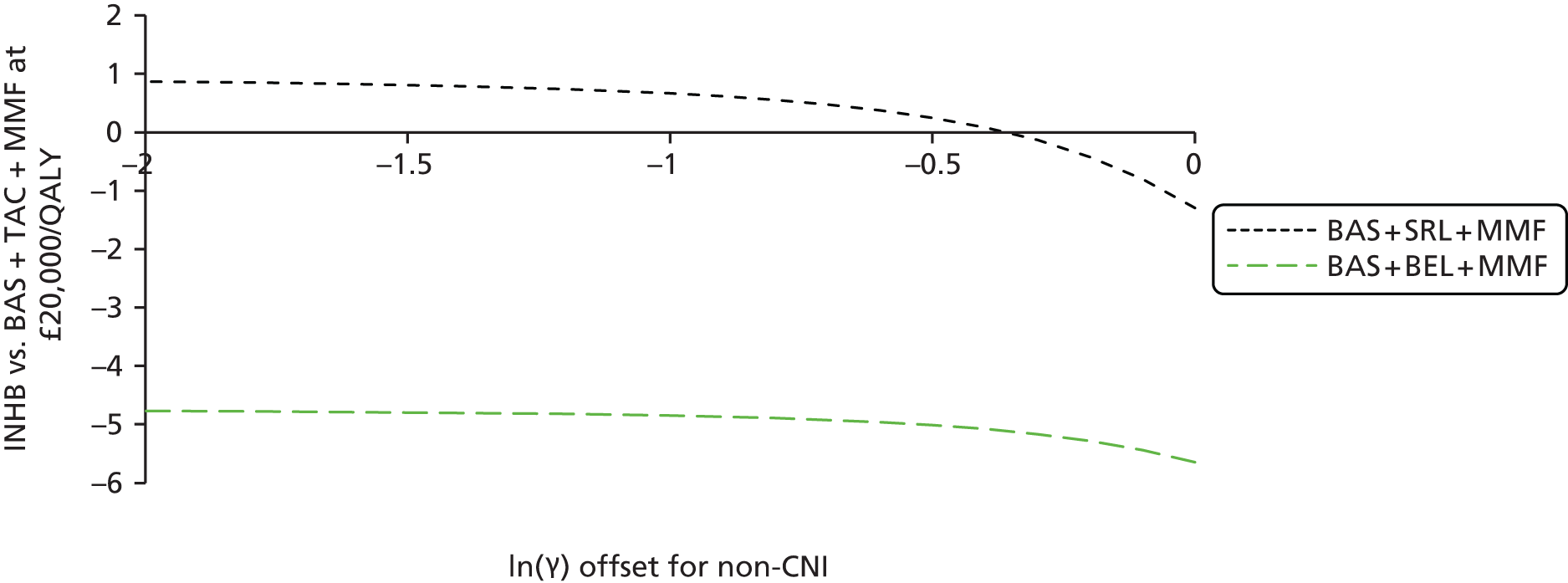
FIGURE 98.
Incremental net health benefit (at £30,000 per QALY) of SRL and BEL vs. TAC as gamma parameter of graft survival is varied.

Crossover at £20,000 per QALY occurs for SRL with a ln(γ) offset of −0.3582 (corresponding to γ = 0.773), which leads to a reduction in total discounted costs from £114,549 to £99,859 and an increase in total discounted QALYs from 10.9010 to 11.4607. Death-censored graft survival in this scenario is shown in Figure 99. In this scenario, TAC and SRL are equally cost-effective at £20,000 per QALY but BEL is not cost-effective.
FIGURE 99.
Death-censored graft survival when non-CNI gamma parameter for graft survival is 0.773 for SRL and BEL vs. 1.105 for TAC.

Crossover at £30,000 per QALY occurs for SRL with a ln(γ) offset of −0.2767 (corresponding to γ = 0.838), which leads to a reduction in total discounted costs of £102,065 and an increase in total discounted QALYs to 11.3766. Death-censored graft survival in this scenario is shown in Figure 100. In this scenario, TAC and SRL are equally cost-effective at £30,000 per QALY but BEL is not cost-effective.
FIGURE 100.
Death-censored graft survival when non-CNI gamma parameter for graft survival is 0.838 for SRL and BEL vs. 1.105 for TAC.
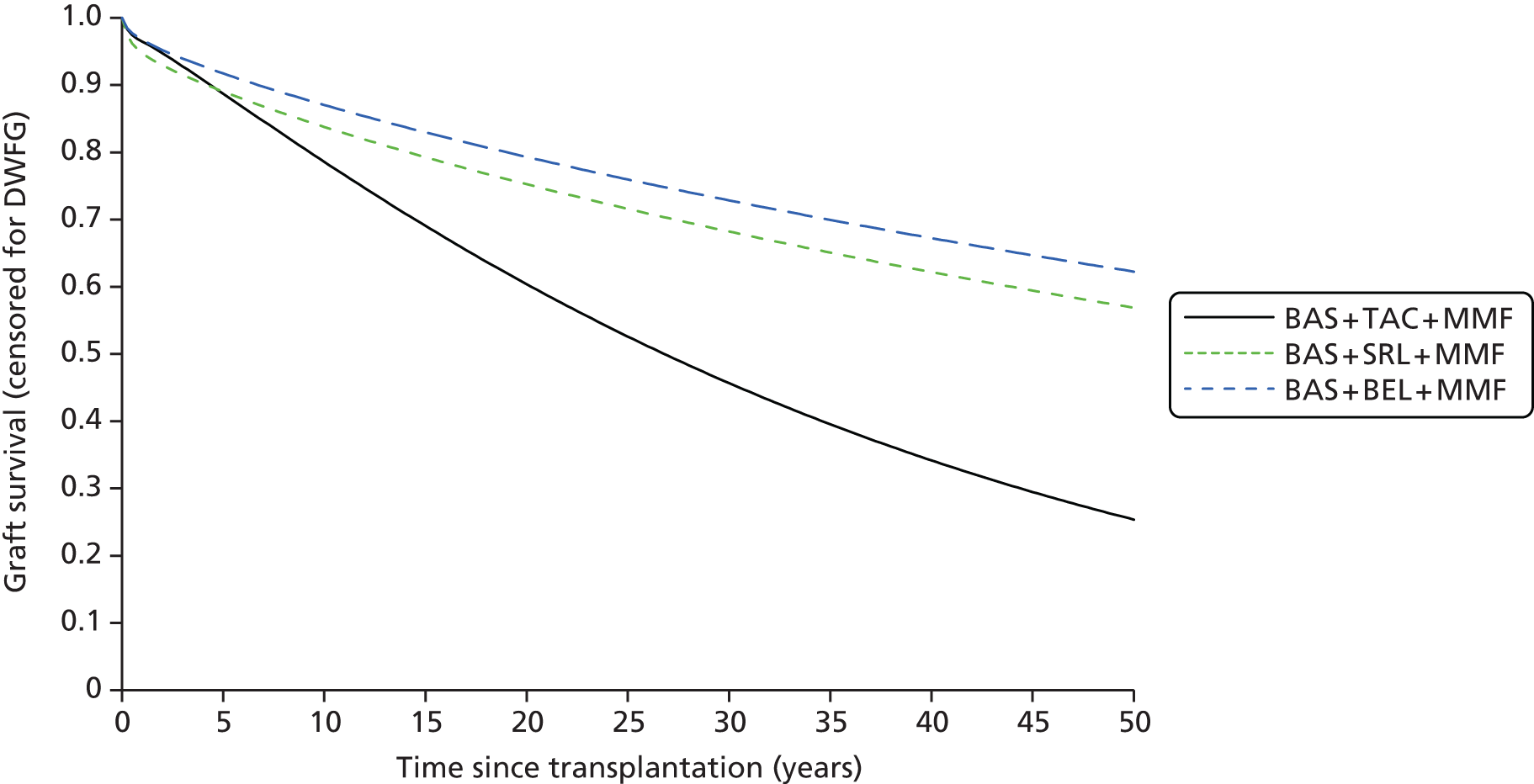
Cost-related scenario analyses
List prices for drug acquisition costs
A scenario analysis was conducted in which the drug acquisition costs (for immunosuppression, NODAT and dyslipidaemia) were taken from list prices (BNF 6856) rather than the CMU eMit database.
Unit costs for CSA, TAC, AZA and MMF increased, which, as expected, increased the total costs for all regimens (as there were no regimens not including at least one of CSA, TAC, AZA and MMF).
The cost-effectiveness results for induction agents were only marginally affected (Table 208). No induction and rATG continued to be dominated by BAS.
| Induction agent | Discounted costs (£) | Discounted QALYs | ICER (cost per QALY, £) | INHB | |||
|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | ||
| With CSA + AZA | |||||||
| vs. BAS | |||||||
| No induction | 104,711 | – | 10.7711 | – | Dominated | –0.3077 | –0.2491 |
| rATG | 107,627 | +2916 | 10.8182 | +0.0471 | Dominated | –0.4064 | –0.2992 |
| BAS | 101,194 | –6433 | 10.9029 | +0.0848 | – | – | – |
| With CSA + MMF | |||||||
| vs. BAS | |||||||
| No induction | 103,302 | – | 10.9145 | – | Dominated | –0.2218 | –0.1846 |
| rATG | 107,807 | +4504 | 10.9281 | +0.0135 | Dominated | –0.4335 | –0.3212 |
| BAS | 101,069 | –6738 | 11.0247 | +0.0966 | – | – | – |
| With TAC + MMF | |||||||
| vs. BAS | |||||||
| No induction | 104,443 | – | 10.8884 | – | Dominated | –0.1815 | –0.1542 |
| rATG | 109,376 | +4933 | 10.9047 | +0.0163 | Dominated | –0.4119 | –0.3023 |
| BAS | 102,803 | –6573 | 10.9880 | +0.0832 | – | – | – |
The cost-effectiveness results for maintenance agents showed some marked differences from the reference case analysis (Table 209). In general, the INHB (at £20,000 per QALY) of TAC compared with CSA decreased, in some cases causing it to become negative. Likewise, in general, the INHB of MMF compared with AZA decreased, in some cases causing it to become negative. The cost-effectiveness of TAC-PR, SRL, EVL and MPS improved marginally but still none was predicted to be cost-effective in the range of £20,000–30,000 per QALY. The cost-effectiveness of BEL was virtually unchanged, with an ICER of > £400,000 per QALY.
| Maintenance agent | Discounted costs (£) | Discounted QALYs | ICER (cost per QALY, £) | INHB | |||
|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | ||
| With MMF | |||||||
| vs. CSA | |||||||
| TAC-PR | 111,581 | – | 10.7920 | – | Dominated | –0.5365 | –0.3985 |
| TAC | 104,443 | –7139 | 10.8884 | +0.0964 | Dominated | –0.0831 | –0.0641 |
| CSA | 103,302 | –1140 | 10.9145 | +0.0261 | – | – | – |
| With AZA | |||||||
| vs. TAC | |||||||
| CSA | 104,711 | – | 10.7711 | – | Dominated | –0.1744 | –0.1491 |
| TAC | 103,195 | –1515 | 10.8696 | +0.0986 | – | – | – |
| With BAS + MMF | |||||||
| vs. CSA | |||||||
| SRL | 119,577 | – | 10.9010 | – | Dominated | –1.0491 | –0.7406 |
| TAC | 102,803 | –16,773 | 10.9880 | +0.0869 | Dominated | –0.1235 | –0.0946 |
| CSA | 101,069 | –1734 | 11.0247 | +0.0367 | – | – | – |
| BEL | 215,325 | +114,256 | 11.2941 | +0.2694 | 424,137 | –5.4434 | –3.5391 |
| With CSA | |||||||
| vs. MMF | |||||||
| AZA | 104,711 | – | 10.7711 | – | Dominated | –0.2139 | –0.1904 |
| MMF | 103,302 | –1408 | 10.9145 | +0.1435 | – | – | – |
| EVL | 178,788 | +75,486 | 10.9659 | +0.0514 | 1,469,322 | –3.7229 | –2.4648 |
| With TAC | |||||||
| vs. AZA | |||||||
| SRL | 134,088 | – | 10.6023 | – | Dominated | –1.8120 | –1.2971 |
| AZA | 103,195 | –30,893 | 10.8696 | +0.2674 | – | – | – |
| MMF | 104,443 | +1247 | 10.8884 | +0.0188 | 66,470 | –0.0436 | –0.0228 |
| With BAS + CSA | |||||||
| vs. MMF | |||||||
| AZA | 101,194 | – | 10.9029 | – | Dominated | –0.1280 | –0.1260 |
| MMF | 101,069 | –125 | 11.0247 | +0.1218 | – | – | – |
| MPS | 114,174 | +13,105 | 11.1377 | +0.1130 | 115,991 | –0.5423 | –0.3239 |
| With rATG + CSA | |||||||
| vs. AZA | |||||||
| AZA | 107,627 | – | 10.8182 | – | – | – | – |
| MMF | 107,807 | +180 | 10.9281 | +0.1099 | 1633 | 0.1009 | 0.1039 |
With a cost-effectiveness threshold in the range of £20,000–30,000 per QALY, the following changes were observed in cost-effectiveness:
-
CSA, instead of TAC, was cost-effective in combination with MMF, BAS + MMF and rATG + MMF (TAC remained cost-effective in combination with AZA)
-
AZA, instead of MMF, was cost-effective in combination with TAC (MMF remained cost-effective in combination with CSA, BAS + CSA and rATG + CSA).
Threshold analysis on costs associated with BEL
A two-way threshold analysis was conducted on the two costs associated with BEL: drug administration and drug acquisition. It was found that the total discounted costs for BAS + BEL + MMF were exactly linearly dependent on both costs according to the following formula:
This formula was used to calculate the ICER of BAS + BEL + MMF compared with BAS + TAC + MMF. ICER isolines (lines of constant ICER) are straight lines in the two-dimensional plot of the costs of i.v. administration and BEL vials, as shown in Figure 101.
FIGURE 101.
Threshold analysis on costs associated with BEL.

The threshold analysis indicated that if the administration cost in the base case is assumed to be correct, BAS + BEL + MMF is not predicted to be cost-effective at £20,000–30,000 per QALY, even at zero acquisition cost. As the acquisition and administration costs are both NHS costs, and are intrinsically related to treating the condition of interest with BEL, both of these costs should be included in the reference-case analysis. The administration cost associated with BEL is a genuine incremental cost associated with BEL and not with other available treatments. 426 Even if administration costs are excluded for BEL, BAS + BEL + MMF is not predicted to be cost-effective at £20,000–30,000 per QALY, based on the current list price for drug acquisition. Bristol-Myers Squibb argues for a cost of administration for BEL of £153.57. At this cost of administration, BAS + BEL + MMF is still not predicted to be cost-effective at £20,000 per QALY, even at zero acquisition cost.
Comparison of Peninsula Technology Assessment Group’s model-based results with those in company submissions
Below, we compare the main deterministic analyses from three of the company submissions with those produced by the independent Assessment Group (PenTAG). These have been selected to include the main maintenance treatments produced and evaluated by the three companies that provided model-based cost-effectiveness studies: TAC-PR compared with immediate-release TAC (Astellas), EVL (Novartis), EC-MPS (Novartis) and BEL (Bristol-Myers Squibb). Although some of the PenTAG analyses contained a larger set of comparator treatments, they are generally comparable, especially after dominated comparators are excluded from the PenTAG analyses.
Overall, for comparisons with the above treatments and equivalent concomitant drugs, the PenTAG model led to lower estimations of discounted incremental costs (between 25% and 40% lower) than the company’s analyses. This largely reflects the lower estimates of incremental graft survival that resulted from our systematic review and NMA. And all of the models utilised different assumptions to extrapolate from short-term trial outcomes to the long term (25–50 years, depending on the model).
For reference, three larger tables at the end of this section (see Tables 214–216) compare the main cost parameters, effectiveness parameters, and main cost and effectiveness results for the three companies’ models and the PenTAG model. These show, for example, that the PenTAG model assumptions tended to include fuller costing of the administration of the maintenance therapies, and more realistic (NHS reference cost) relatively lower annual costs of dialysis (except Novartis, which used similar costs for dialysis). In addition, although applied differently in the models, the approximate utility difference between living with a functioning graft and living on dialysis was greater in the three companies’ analyses (typical difference of between ≈ 0.25 to ≈ 0.3) than in the PenTAG model (≈ 0.2 difference). Overall, these particular differences in the companies’ models will tend to magnify the impact on QALYs of any incremental effectiveness differences that affect long-term graft survival, and also reduce their associated incremental cost.
Peninsula Technology Assessment Group’s and Astellas’ analysis of immediate-release TAC compared with TAC-PR
Table 210 shows the company’s and the assessment group’s analysis of the cost-effectiveness of prolonged-release compared with immediate-release TAC. The Astellas analysis estimates TAC-PR to be both cheaper and more effective than immediate-release TAC (i.e. prolonged-release ‘dominates’ immediate-release TAC). This is the opposite result to the PenTAG analysis.
| Maintenance agent | Discounted costs (£) | Discounted QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| PenTAG (assessment group) | |||||
| TAC-PR (+ MMF) | 111,499 | – | 10.6172 | – | Dominated |
| TAC (+ MMF) | 92,827 | –18,672 | 10.8595 | +0.2423 | – |
| CSA (+ MMF) | 98,157 | +5330 | 10.8925 | +0.0330 | 161,408 |
| Astellas | |||||
| TAC-PR | 118,907 | –11,211 | 8.2100 | +0.2000 | – |
| TAC | 130,118 | – | 8.0100 | – | Dominated |
| CSA | Missing from Astellas’ comparators | ||||
This opposite result in incremental QALYs mostly arises because of the different trial data used within the two models and the fact that long-term outcomes in the Astellas model are driven entirely by rates of AR. For informing the effectiveness parameters of the drugs on BPAR, mortality, graft loss and renal function, the PenTAG analysis uses meta-analysis of two direct head-to-head trials of the two comparators. 141,204 All of the pooled ORs are not statistically significant and all, except the comparison for BPAR, favour the immediate-release TAC. In contrast, the Astellas review reports using three trials123,204,239 and one meta-analysis and concludes that the two types of TAC are of ‘similar efficacy and safety’. In their model, however, these data sources are then used to justify immediate-release TAC having a 2 percentage-point higher rate of AR than TAC-PR, which then drives differences in long-term graft survival (and costs). In its modelling it also factors in greater adherence to treatment with TAC-PR, which departs from the ITT analysis of the trials.
Peninsula Technology Assessment Group’s and Novartis’ analysis of EVL and of EC-MPS
Table 211 shows the company’s and the assessment group’s analysis of the cost-effectiveness of EVL and relevant comparators. Novartis conducted two analyses, with different comparators and doses of CSA, and estimated that EVL either dominates TAC or, when compared with MMF, has an ICER of £59,696 per QALY. The PenTAG analysis (comparison with MMF shown) produces an ICER of > £1.7M per QALY. As AZA is dominated in the PenTAG analysis, and omitted from the Novartis analysis, both of these ICERs are relative to the next most effective and cheaper treatment – MMF.
| Agent | Discounted costs (£) | Discounted QALYs | ICER | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| PenTAG | |||||
| AZA | 102,320 | – | 10.7486 | – | Dominated |
| MMF | 98,157 | –4163 | 10.8925 | +0.1439 | – |
| EVL | 176,788 | +78,631 | 10.9376 | +0.0451 | 1,743,739 |
| Novartis | |||||
| AZA | Missing from Novartis’ comparators | ||||
| MMF | 76,826 | 7.8900 | |||
| EVL | 136,180 | +59,354 | 8.8900 | +1.0000 | 59,354 |
There is a modest difference in the incremental costs between the two analyses, with the Novartis analysis estimating the incremental cost of EVL over MMF to be 25% lower than the PenTAG analysis (£59,354 vs. £78,631). However, most of the difference in the ICER is explained by the Novartis analysis estimating a 20-fold higher incremental QALYs between the two treatments (1 QALY vs. 0.045 QALYs in the PenTAG analysis).
This large difference in incremental QALYs will be the combined result of a large number of differences in the parameter values and structural assumptions within each of the models, which led to differences in incremental graft survival and incremental life-years. The undiscounted incremental time lived with a functioning graft between EVL and MMF is 0.32 years from the PenTAG analysis and 5.17 years from the Novartis analysis. Correspondingly, the incremental overall survival (life-years) is 0.09 years from the PenTAG analysis but 1.76 years from the Novartis analysis. These differences in incremental graft and overall survival are, in turn, likely to be mainly caused by the use by Novartis of rates of acute and chronic rejection from single arms of different individual trials (Tedesco-Silva et al. 107 for EVL, Vítko et al. 150 for chronic rejection) compared with less clear evidence of such large effect differences for AR or graft survival from the PenTAG MTC).
Table 212 shows the Novartis and the PenTAG’s analysis of the cost-effectiveness of MPS and relevant comparators. Although the Novartis analysis estimates at a favourable ICER for its own product, of £13,235 per QALY, our analysis produces an ICER of £145,072 per QALY. As, again, AZA is dominated in the PenTAG analysis, and omitted from the Novartis analysis, both of these ICERs are relative to the next most effective and cheaper treatment – MMF.
| Agent | Discounted costs (£) | Discounted QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| PenTAG | |||||
| AZA | 98,667 | – | 10.9029 | – | Dominated |
| MMF | 95,654 | –3013 | 11.0247 | +0.1218 | – |
| MPS | 112,045 | +16,391 | 11.1377 | +0.1130 | 145,072 |
| Novartis | |||||
| AZA | Missing from Novartis’ comparators | ||||
| MMF | 76,771 | – | 7.89 | – | – |
| MPS | 87,359 | +10,588 | 8.69 | +0.8000 | 13,235 |
There is a modest difference in the incremental costs between the two analyses, with the Novartis analysis estimating the incremental cost of MPS over MMF to be 35% lower than the PenTAG analysis (£10,588 vs. £16,391). However, most of the difference in the ICER is explained by the Novartis analysis estimating a sevenfold higher incremental QALYs between the two treatments (0.80 vs. 0.113 QALYs).
This large difference in incremental QALYs will be the combined result of a large number of differences in the parameter values and structural assumptions within each of the models, which led to differences in incremental graft survival and incremental life-years. The undiscounted incremental time lived with a functioning graft between MPS and MMF is 0.4 years from the PenTAG analysis and 4.66 years from the Novartis analysis. Similarly, the incremental overall survival (life-years) is 0.24 years from the PenTAG analysis but 4.66 years from the Novartis analysis.
For informing the effectiveness of the drugs on BPAR, mortality, graft loss and renal function, the PenTAG analysis uses meta-analysis of direct head-to-head trials of the two comparators. 106,107,150,270
Peninsula Technology Assessment’s and Bristol-Myers Squibb’s analysis of BEL
Table 213 shows the companies’ and the assessment group’s analysis of the cost-effectiveness of BEL and relevant comparators. Although the Bristol-Myers Squibb analysis estimates an ICER for BEL, of £95,068 per QALY (compared with TAC), our analysis produces an ICER of £519,094 per QALY (compared with CSA).
| Agent | Discounted costs (£) | Discounted QALYs | ICER (£) | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| PenTAG | |||||
| SRL | 114,554 | – | 10.9010 | – | Dominated |
| TAC | 90,794 | –23,760 | 10.9880 | +0.0869 | – |
| CSA | 95,654 | +4860 | 11.0247 | +0.0367 | 132,272 |
| BEL | 235,490 | +139,836 | 11.2941 | +0.2694 | 519,094 |
| Bristol-Myers Squibb | |||||
| TAC | 205,502 | +1215 | 6.53 | 0.36 | 3375 |
| CSA | 204,287 | – | 6.17 | ||
| BEL | 296,503 | +92,216 | 7.14 | 0.97 | 95,068 |
There is a large absolute difference in the incremental costs between the two analyses, with the Bristol-Myers Squibb analysis estimating the incremental cost of BEL to be £47,620 (34%) lower than the PenTAG analysis (£92,216 vs. £139,836). This will be owing, in part, to the PenTAG model using costs for the i.v. administration of BEL approximately twice those of the Bristol-Myers Squibb analysis, and the Bristol-Myers Squibb model using an unusually high annual cost for dialysis (£43,586 – about £19,000 more than the NHS reference cost). However, most of the difference in the ICER is explained by the Bristol-Myers Squibb analysis estimating a nearly fourfold higher incremental QALYs between the relevant treatments (0.97 vs. 0.269 QALYs).
This difference in incremental QALYs will be the combined result of a large number of differences in the parameter values and structural assumptions within each of the models, which led to differences in incremental graft survival and incremental life-years. The undiscounted incremental time lived with a functioning graft between BEL and TAC/CSA is 0.95 years from the PenTAG analysis and 1.51 years from the Bristol-Myers Squibb analysis. Similarly, the incremental overall survival (life-years) is 0.57 years from the PenTAG analysis and 1.51 years from the Bristol-Myers Squibb analysis. These differences in incremental graft and overall survival are, in turn, likely to be a result of the Bristol-Myers Squibb analysis relying on a much longer assumed time between graft failure and retransplantation [16.5 years vs. 5 years time to retransplantation (or death in the PenTAG analysis)], assumed linear changes in GFR within the functioning graft state determining long-term outcomes and long-term transition probabilities being based on US cohort data (not UK registry data, as in the PenTAG analysis).
The following three tables (Tables 214–216) compare the main cost parameters, effectiveness parameters, and main cost and effectiveness results for the three companies’ models and the PenTAG model.
| Cost parameter | Astellasa | Bristol-Myers Squibbb,c | Novartisa,c | PenTAG |
|---|---|---|---|---|
| TAC therapy (per year) | 4255d | 3937 (first year) 2821 (second year+)e | 5283 | With AZA 1816 (first year) 1196 (second and third year) 1063 (fourth year+) With MMF 1378 (first year) 1063 (second year+) |
| TAC administration | 0 | 386 (first year), 89 (second year)e | 0 | 1114 (first year) 374 (second year) 107 (third year+) |
| MMF therapy (per year) | 2402f | 0g | 282h | With TAC 249 (first year) 202 (second year+) With CSA 259 (first year) 230 (second year+) With SRL 248 (first year) 202 (second year+) With BEL 276 |
| CSA therapy | NAi | 1971 (first year) 1562 (second year+)e | 839 (first year), 694 (second year+) | With AZA 1649 (first year) 1233 (second and third year) 1195 (fourth year+) With MMF/MPS 1374 (first year) 1187 (second year+) |
| CSA administration | 0 | 386 (first year), 90 (second year)e | 0 | 1114 (first year) 374 (second year) 107 (third year+) |
| BEL (per year) | 10,966 (first year) 6480 (second year+) | 13,472 (first year) 9217 (second year+) | NA | 12,812 (first year) 8849 (second year+) |
| BEL administration | 0 | 2457 (first year) 1996 (second year+) | NA | 4728 (first year) 4246 (second year+) |
| CCSs | 178 | 0g | 285 | 20 |
| AR (event) | 1738 | 3483 | 1725 | 3557 |
| Dialysis (per year) | 38,387j | 43,586k | 22,877l | 24,372 (HD) 24,000 (PD) 24,314 (mix, age 45–54 years) |
| Retransplantation | 25,953 | 25,908 | 17,736 | 16,030 (procedure) 1226 (work-up) |
| Retransplantation: organ procurement | 0 | 12,954 | 0 | 8914 (live donor) 10,142 (deceased donor) |
| Effectiveness parameter | Astellasa | Bristol-Myers Squibbb | Novartisc | Assessment group (PenTAG) |
|---|---|---|---|---|
| Time to graft failure (median) (years) | Without BPAR at 12 months: 23 With BPAR at 12 months: > 25c |
Initial GFR2: 15.0 Initial GFR3a: 11.5 Initial GFR3b: 7.0 Initial GFR4: 2.5 |
EVL: 15.8 MPS: 21.3 MMF + CSA: 7.2 TAC + CSA: 8.3 |
(To nearest 0.25) CSA + MMF: 13.75 years TAC + MMF: 14.75 years CSA + AZA: 12.75 years TAC + AZA: 14.50 years CSA + EVL: 14.50 years TAC + SRL: 12.75 years TAC-PR + MMF: 13.25 years BAS + CSA + MMF: 14.75 years BAS + TAC + MMF: 15.50 years BAS + CSA + AZA: 13.75 years BAS + SRL + MMF: 14.75 years BAS + BEL + MMF: 16.50 years BAS + CSA + MPS: 15.50 years rATG + CSA + MMF: 14.75 years rATG + TAC + MMF: 15.50 years rATG + CSA + AZA: 13.75 years |
| Time to transplantation from graft failure (mean unless otherwise stated) (years) | 3.5 (median) | 16.5d | 3 (SD 1) | Mean time to transplantation or death following failure of initial graft 4.97 (range 4.87–5.06) |
| Annual change in GFR | NA | –3 (fourth+) | –1.66 (second), –2.68 (third+) | NA |
| Utility of functioning graft: first transplant | 0.71 | 0.49–0.64 (depending on GFR stage) | 0.49–0.64 (depending on GFR stage) | 0.815 (age 50 years) 0.786 (age 60 years) 0.755 (age 70 years) 0.723 (age 80 years) |
| Utility of functioning graft: second+transplants | 0.71 | 0.59 | 0.49–0.64 (depending on GFR stage) | As first |
| Utility of dialysis state | 0.459 | 0.28 | 0.28 | HD:
|
| Model | Regimens compared | Functioning first graft (years) | Functioning graft (years) | Years with graft loss/dialysis | Life-years | QALYsa | Costs (£)a | ICER: incremental cost (£) per QALY |
|---|---|---|---|---|---|---|---|---|
| Astellas | TAC b.i.d. (+ MMF + CCSs) | 15.10 | 15.40 | 2.44 | 17.88 | 8.01 | 130,118 | TAC vs. SRL I: 1,651,801 TAC vs. SRL II: 170,681 |
| SRL I (+ MMF + CCSs) | 15.05 | 15.36 | 2.46 | 17.82 | 7.99 | 104,905 | ||
| EVL (+ MMF + CCSs) | 15.03 | 15.34 | 2.46 | 17.80 | 7.99 | 142,995 | ||
| SRL II (+ MMF + CCSs) | 14.90 | 15.22 | 2.51 | 17.73 | 7.94 | 119,371 | ||
| BEL (+ MMF + CCSs) | 14.88 | 15.20 | 2.52 | 11.72 | 7.94 | 163,740 | ||
| TAC b.i.d.b (+ MMF + CCSs) | 15.76 | 16.03 | 2.16 | 18.19 | 8.21 | 118,907 | TAC b.i.d. dominates | |
| TAC o.d.b (+ MMF + CCSs) | 15.10 | 15.40 | 2.44 | 17.88 | 8.01 | 130,118 | ||
| Assessment group (PenTAG) | TAC b.i.d. (+ MMF + CCSs) | 16.49 | 19.32 | 3.03 | 22.36 | 10.86 | 92,827 | No PenTAG analysis compared EVL with BEL |
| EVL (+ CSA + CCSs) | 16.39 | 19.32 | 3.13 | 22.44 | 10.94 | 176,788 | ||
| BEL (BAS + MMF + CCSs) | 18.01 | 20.50 | 2.70 | 23.21 | 11.29 | 235,490 | ||
| TAC o.d.b (+ MMF + CCSs) | 16.49 | 19.32 | 3.03 | 22.36 | 10.86 | 92,827 | TAC o.d. dominates | |
| TAC b.i.d.b (+ MMF + CCSs) | 15.24 | 18.46 | 3.39 | 21.85 | 10.62 | 111,499 | ||
| Bristol-Myers Squibb | BEL + ? (not stated) | 13.39 | 14.53 | 5.00 | 19.53 | 7.14 | 296,503 | BEL vs. TAC: 149,182 TAC vs. CSA 3375 |
| TAC + ? (not stated) | 11.89 | 13.04 | 4.98 | 18.02 | 6.53 | 205,502 | ||
| CSA + ? (not stated) | 10.80 | 12.05 | 5.33 | 17.38 | 6.17 | 204,287 | ||
| Assessment group (PenTAG) | BEL + (MMF + CCSs) | 18.01 | 20.50 | 2.70 | 23.21 | 11.29 | 235,490 | BEL vs. TAC: 472,708c CSA vs. TAC 132,272 |
| TAC + (MMF + CCSs) | 17.28 | 19.85 | 2.79 | 22.64 | 10.99 | 90,794 | ||
| CSA + (MMF + CCSs) | 16.67 | 19.55 | 3.08 | 22.64 | 11.02 | 95,654 | ||
| Novartisc | EVL + CSA (low dose) | 14.28 | 14.98 | 10.73 | 25.71 | 8.86 | 135,358 | EVL dominant |
| TAC + MMF | 9.92 | 9.94 | 13.45 | 23.39 | 7.37 | 140,972 | ||
| EVL + CSA (low dose) | 13.91 | 14.34 | 11.46 | 25.80 | 8.89 | 136,180 | MMF + CSA vs. EVE + CSA: 59,696 (deterministic), > 200,000 (probabilistic) | |
| MMF + CSA | 9.03 | 9.17 | 15.01 | 24.04 | 7.89 | 76,826 | ||
| MPS + CSA | 15.97 | 16.01 | 9.47 | 25.48 | 8.69 | 87,359 | MPS + CSA vs. MMF + CSA: 13,209 (deterministic), ≈ 29,000 (probabilistic) | |
| MMF + CSA | 9.43 | 9.35 | 14.77 | 24.17 | 7.89 | 76,771 | ||
| Assessment group (PenTAG) | EVL + CSA (low dose) | 16.39 | 19.32 | 3.13 | 22.44 | 10.9376 | 176,788 | EVE + CSA vs. MMF + CSA: 1,743,739 |
| TAC + MMF | 15.82 | 19.00 | 3.35 | 22.44 | 10.8925 | 98,157 | ||
| EC-MPS + MMF | 17.24 | 19.55 | 3.13 | 22.88 | 11.1377 | 112,045 | MPS vs. MMF + CSA: 145,072 | |
| MMF + CSA | 16.67 | 19.95 | 2.84 | 22.64 | 11.0247 | 95,654 | ||
Chapter 7 Discussion
Statement of principal findings
Aim
The remit for this report was to review and update the evidence used to inform the current NICE guidance (TA85) on the clinical effectiveness and cost-effectiveness of immunosuppressive therapies in adult renal transplantation. The current guidance is Woodroffe et al. 65 We have incorporated relevant evidence presented in this previous report and reported new evidence from 2002 to the present. This includes a new decision-analytic model of kidney transplantation outcomes to investigate which regimen is the most cost-effective option.
Clinical effectiveness systematic review
Previous technology assessment for the National Institute for Health and Care Excellence
The previous assessment (TA85) in 200243 found that BAS, TAC and MMF consistently reduced the incidence of short-term (1-year) AR compared with conventional immunosuppressive therapy (e.g. dual- or triple-combination therapy for induction and/or maintenance including CSA, AZA and CCSs). The independent use of BAS, TAC and MMF was associated with a similar absolute reduction in 1-year ARR (approximately 15%). However, the effects of these drugs did not appear to be additive (e.g. benefit of TAC with adjuvant MMF was a 5% reduction in ARR, compared with a 15% reduction with adjuvant AZA). Thus, the addition of one of these drugs to a baseline immunosuppressant regimen was likely to affect adversely the incremental cost-effectiveness of the addition of another.
Important gaps in the evidence were identified concerning the impact of the newer immunosuppressants on long-term graft loss and patient survival. The absence of both long-term outcome and quality of life from trial data makes assessment of the clinical effectiveness and cost-effectiveness on the newer immunosuppressants contingent on modelling based on extrapolations from short-term trial outcomes.
Updated systematic review
In total, 67 new RCTs49,51,58,59,74,87,91–135,137–152 were included in the clinical effectiveness review presented in this report, with an additional 19 RCTs71–73,75–86,88–90,136 meeting our inclusion criteria from the previous assessment.
For the head-to-head comparisons of induction therapies, from 0.5 years to 10 years post transplant, we found no evidence to suggest that BAS or rATG is more effective than PBO, no induction or each other in reducing the odds of mortality (overall survival). Similarly, for graft loss, we found no evidence of a statistically significant difference for BAS or rATG versus PBO, no induction or each other.
We found evidence to suggest that rATG and BAS are more effective than PBO or no induction at reducing BPAR (rATG at 1 year, OR 0.34, 95% CI 0.22 to 0.52, I2 = 8.9%; BAS at 1 year, OR 0.53, 95% CI 0.40 to 0.70, I2 = 0.0%). A statistically significant difference was found for the severity of BPAR, comparing BAS versus rATG, whereas BAS was associated with lower odds of Banff III classification, the most severe classification of AR (1 year, OR 0.04, 95% CI 0.00 to 0.65).
We found no evidence that any maintenance therapies were preferable to others in terms of mortality.
For graft loss outcomes reported by maintenance studies, we found evidence that at 5 years BEL + MMF may be superior to CSA + MMF (OR 0.40, 95% CI 0.19 to 0.87; I2 = 0.0%). At 0.5 years, the odds of reduced graft loss are greater for CSA + MMF than for CSA + AZA (OR 0.58, 95% CI 0.04 to 0.59; I2 = 72.2%).
Several treatments showed a beneficial effect with regard to reducing BPAR, although this varied across time points. For all the following comparisons, the arm containing TAC displayed lower odds of BPAR:
-
TAC + AZA versus CSA + AZA (0.5 years, OR 0.50, 95% CI 0.32 to 0.79, I2 = 50.1%; 1 year, OR 0.50, 95% CI 0.39 to 0.64, I2 = 8.1%; 4 years, OR 0.38, 95% CI 0.25 to 0.57)
-
TAC + MMF vs. CSA + AZA (0.5 years, OR 0.64, 95% CI 0.41 to 0.98; 1 year, OR 0.35, 95% CI 0.15 to 0.82)
-
TAC + MMF vs. CSA + MMF (1 year, OR 0.59, 95% CI 0.37 to 0.94, I2 = 19.3%)
-
TAC + MMF vs. SRL + MMF (1 year, OR 0.32, 95% CI 0.12 to 0.87, I2 = 0.0%)
-
TAC + SRL vs. TAC + MMF (0.5 years, OR 0.65, 95% CI 0.44 to 0.96).
For CSA + MMF versus CSA + AZA, at 0.5 years and 1 year, there is statistically significant evidence to suggest that MMF is more effective (0.5 years, OR 0.50, 95% CI 0.35 to 0.72, I2 = 35.1%).
Tacrolimus is also associated with lower odds of reduced GRF for the following regimens:
-
TAC + MMF versus CSA + MMF (at 3 years, eGFR WMD 4.60 ml/minute/1.73 m2, 95% CI 1.35 ml/minute/1.73 m2 to 7.85 ml/minute/1.73 m2)
-
TAC + MMF versus TAC-PR + MMF (at 0.5 years, eGFR WMD 1.90 ml/minute/1.73 m2, 95% CI 1.70 to 2.10 ml/minute/1.73 m2)
-
TAC + SRL versus CSA + SRL (at 0.5 years, eGFR MD 6.35 ml/minute/1.73 m2, p < 0.0001; 1 year MD 5.25 ml/minute/1.73 m2, p = 0.0004).
For MMF + TAC versus MPS + TAC, MPS at 1 year and 3 years is more effective (1 year, MD 1.9 ml/minute/1.73 m2, p < 0.0001; 3 years, eGFR MD 0.5 ml/minute/1.73 m2, p = 0.0016). BEL appears more effective at 1 year and 3 years for BEL + MMF vs. CSA + MMF (1 year, eGFR WMD 7.83 ml/minute/1.73 m2, 95% CI 1.57 to 14.10 ml/minute/1.73 m2, I2 = 73.6%; 3 years, WMD 16.08 ml/minute/1.73 m2, 95% CI 5.59 to 26.56 ml/minute/1.73 m2, I2 = 89.5%); however, heterogeneity across studies is substantial. Where there are two comparisons involving SRL and CSA, the regimen including MMF suggests CSA to be more beneficial up to 5 years (5 years, eGFR WMD 9.10 ml/minute/1.73 m2, 95% CI 1.68 to 16.52 ml/minute/1.73 m2), yet, in contrast, the regimen including AZA suggests SRL to be more effective (1 year, eGFR MD 10.8 ml/minute/1.73 m2, p < 0.0001).
Time to BPAR is generally poorly reported and therefore it is challenging to form a conclusion. Again, TAC + AZA versus CSA + AZA shows conflicting results for two studies; however, the statistically significant result in one of the two studies suggests that BPAR is achieved more quickly for participants receiving TAC rather than CSA (MD 24 days, p = 0.0033). This is also true for TAC + MMF versus CSA + MMF (MD 46.7 days, p < 0.0001). When SRL + TAC and MMF + TAC are compared, a reduced time to BPAR is seen for MMF (MD 48.6 days, p = 0.0017). For SRL + MMF compared with CSA + MMF, one of three studies demonstrates a statistically significant difference in favour of CSA (MD 38 days, p = 0.0035); however, the other two studies show no difference.
Regarding BPAR severity, for TAC + AZA versus CSA + AZA, there are lower odds of the more severe BPAR for the arm containing TAC, although there is substantial heterogeneity across studies (Banff III, OR 0.28, 95% CI 0.12 to 0.66). Similarly, for TAC + MMF compared with TAC-PR + MMF, TAC has a lower proportion of people experiencing the more severe BPAR of Banff III classification (OR 0.11, 95% CI 0.01 to 0.87, I2 = 0.0%).
Following NMA for induction therapy, there is no evidence to suggest that BAS or rATG is more effective than PBO/no induction or each other in reducing the odds of graft loss or mortality. rATG and BAS were both estimated to be more effective than PBO/no induction, with rATG being more effective than BAS at reducing BPAR. There is evidence to suggest that BAS is more effective than PBO/no induction for increasing GRF.
With regard to maintenance therapy, the NMA showed that none of the maintenance regimens performed consistently well on all four outcomes and a great deal of heterogeneity was noted.
-
No evidence was found to suggest that one treatment was any more effective at reducing the odds of graft loss than any other treatment.
-
There is evidence to suggest that BEL + MMF is more effective at reducing the odds of mortality than TAC + MMF and SRL + MMF, but no other treatments are estimated to be any more effective at reducing mortality than any other treatment.
-
MMF + CSA, TAC + MMF and SRL + TAC are estimated to be more effective than CSA + AZA and EVL + MPS at reducing the odds of BPAR. In addition, TAC + AZA and EVL + CSA are estimated to be more effective than CSA + AZA at reducing the odds of BPAR. However, apart from CSA + AZA and EVL + MPS performing poorly in some comparisons, it is difficult to say that any one treatment is more effective at reducing BPAR than another, as the 95% CIs are very wide.
-
Similarly, a number of treatments (TAC + AZA, TAC + MMF and BEL + MMF) are estimated to be more effective than CSA + AZA and MMF + CSA at increasing GRF. In addition, SRL + AZA is estimated to be more effective than CSA + AZA at increasing GRF. However, as a result of the limited direct evidence informing many of the comparisons and the 95% CIs being very wide, we can conclude only that CSA + AZA and MMF + CSA are performing poorly in some comparisons.
Analysis of AEs revealed the following:
-
Some evidence suggested that there were more CMV infections with rATG regimens than with BAS regimens,212 and with rATG regimens than with no induction. 96 The meta-analysis comparing TAC and CSA regimens (including eight studies) suggested more cases of NODAT with TAC regimens compared with CSA regimens.
-
The meta-analyses comparing BEL with CSA regimens (including three studies) suggested more cases of NODAT with CSA regimens than with BEL regimens (including three studies).
-
The meta-analyses comparing SRL and CSA regimens (including seven studies) suggested more cases of NODAT with CSA regimens than with SRL.
-
The meta-analysis comparing MMF and EVL (including three studies) suggested more cases of CMV infections in MMF regimens compared with EVL.
Overall, we found that, despite the volume of evidence, there is little impact on effectiveness conclusions from the head-to-head comparisons, particularly for graft loss and mortality. However, this may be a reflection of the lack of long-term data, because very few studies reported all outcomes beyond 1 year, and also there was frequently a substantial level of heterogeneity across studies. The quality of trials was variable and, owing to reporting omissions, it was difficult to make a general assessment regarding quality. Furthermore, all results must be interpreted with caution as multiple testing increases chances of type 1 errors and no corrections for multiple tests were executed.
Economic evaluations
Published economic evaluations
-
There is limited evidence on costs and benefits of induction regimens, as studies are typically economic evaluations conducted alongside single-centre RCTs of 1 year duration or less, involving small samples and reporting insufficient data in order to evaluate their generalisability.
-
Studies of initial and maintenance immunosuppression are all sponsored by the industry or conducted by a person affiliated to them (except for the analysis by the Birmingham assessment group, which reviewed the evidence on behalf of NICE during the previous appraisal on the topic).
-
Studies of initial and maintenance immunosuppression typically use a biomarker as a surrogate to extrapolate outcomes from RCTs of 1–3 years’ duration to the long term (i.e. 10–50 years after initial transplantation).
-
Since the previous NICE appraisal, the main development in economic evaluation modelling of immunosuppressive regimens is the use of renal function as a surrogate outcome in addition to AR for extrapolating trial efficacy outcomes to long-term graft and patient survival.
-
In addition, new evidence has emerged that changes in renal function directly impact on current HRQoL and costs and this is now recognised by the more recently published models.
-
In the UK, however, only one study of initial and maintenance immunosuppression has accounted for these methodological developments but it suffers from a lack of a systematic approach to evidence synthesis on the efficacy of relevant UK treatments in routine use.
-
Evidence from other countries is of questionable generalisability because of inadequate reporting of the regimens being compared.
-
A new study would fill a gap the evidence base required to inform NHS decision-making by adopting a systematic approach to evidence synthesis on all relevant comparators, from an independent standpoint and incorporating the latest methodological developments and evidence on the topic.
Company submissions
-
Three companies developed models of initial and maintenance immunosuppression in adult patients were submitted to NICE: Astellas, Novartis and Bristol-Myers Squibb.
-
The analysis by Astellas compared TAC (Prograf) with SRL CNI avoidance, SRL CNI minimisation, BEL and EVL. In addition, it presented a comparison of TAC once-daily extended release (Advagraf) and twice-daily immediate release (Prograf formulations).
-
The study found that Prograf is cost-effective against BEL and EVL, but it was not cost-effective relative to the SRL regimens, against which it found ICERs of > £100,000 per QALY. In turn, Advagraf was found to cost less and generate more QALYs than Prograf.
-
The analysis by Astellas was found to be flawed owing to the structure and the implementation of the model used to extrapolate short-term efficacy differences between the regimens compared; that is, the model did not account for the effect of regimens on renal function, and the Markov model included errors in the way the incidence of retransplantations was modelled.
-
In addition, it is questionable whether or not the SRL regimens apply to the general kidney transplant patient population modelled by Astellas.
-
Novartis presented the results of pairwise comparisons between EVL (in combination with reduced dose CSA and steroids) and TAC or CSA (each combined with MMF and steroids). In addition, it presented an analysis of EC-MPS (combined with standard-dose CSA and steroids) versus CSA (with MMF and steroids). Outcomes were modelled over a 50-year time horizon.
-
Novartis found that EVL was cost-effective against TAC and CSA. However, when results accounted for uncertainty in parameter estimates, EVL was borderline cost-effective (as evidenced by the ICER against CSA being in the vicinity of £30,000 per QALY).
-
The analysis of MPS found it not to be cost-effective relative to CSA.
-
The analyses by Novartis were likely to be biased because of the lack of a systematic approach to the identification of evidence on efficacy, and also because of the assumptions built in the model used to predict long-term graft and patient survival from short-term efficacy outcomes; the differences in efficacy between the regimens compared were derived from indirect comparisons of outcomes in trial arms from single studies. The model assumed that the rate of chronic rejection at 12 months post transplant for each therapy applied throughout the modelled time horizon, independently of AR and renal function outcomes.
-
Bristol-Myers Squibb compared BEL with TAC and CSA, over a 40-year time horizon, using MTC to estimate the efficacy of each regimen at 36 months. A model was then used to extrapolate from this end point to 40 years.
-
The analyses found that BEL was not cost-effective, and the company produced additional ‘subgroup analyses’ by selecting a group of patients at high risk of short graft survival for which BEL may be more economically attractive. Selecting patients in this way may be impractical in routine practice, as it is by definition outcome dependent (unknown immediately after transplant). The company also performed subgroup analysis based on patient weight; in patients with body weight of > 90 kg BEL was found to be cost-effective.
-
The analysis by Bristol-Myers Squibb was strengthened by the use of observational data on resource utilisation data, which was analysed as a function of renal function.
-
Although Bristol-Myers Squibb adopted the more advanced techniques to model long-term graft and patient survival, including information on renal function and AR in a prognostic model, its analyses were found to be biased because of the use of surrogate-based models of patient and graft survival estimated from US data; these were found to differ from graft survival outcomes in the UK kidney transplant patient population. There were other limitations that related to how the impact on HRQoL and costs of changes in renal function were measured, as well as how the surrogate long-term outcome model was used to derive the transition probabilities in the model.
-
Owing to the listed limitations of the industry analyses, an independent de novo analysis is warranted, which synthesises the evidence base on effectiveness outcomes and combines them with observational routinely available data on long-term outcomes of UK kidney transplant patients with a decision analysis model from the NHS and PSS perspective.
Peninsula Technology Assessment Group economic assessment
Previous appraisal
The previous appraisal (TA85)43 considered the cost-effectiveness of BAS, DAC, TAC (immediate release), MMF and SRL. Briefly, the Appraisal Committee considered the following:
-
BAS and TAC (immediate release) would probably be cost-effective (vs. no induction and CSA, respectively).
-
MMF was unlikely to be cost-effective in the general setting (vs. AZA) but was likely to be cost-effective in settings in which a reduction in CSA dose is required.
-
SRL in combination with CCSs should be considered as an option when proven intolerance to CNIs necessitates their complete withdrawal.
Update
In this update review we have a slightly different set of interventions under consideration because of the removal of DAC and the addition of rATG as induction, TAC-PR, MPS, EVL and BEL.
We have constructed an independent economic model that incorporates current costs, evidence published since the previous appraisal and an updated surrogate relationship that additionally takes into account GRF following transplantation.
We present our principal findings for each intervention separately, summarising the findings from deterministic and probabilistic analyses and relevant scenario analyses.
Induction agents
BAS
Basiliximab is predicted to be cost-effective at £20,000–30,000 per QALY in the deterministic analysis and the probabilistic analysis. BAS was cost-effective at £20,000 per QALY in 77.2–85.6% of PSA iterations across comparisons and at £30,000 per QALY in 72.7–80.6% of iterations.
When the duration of the surrogate effect on graft survival was reduced, BAS gradually became less cost-effective. When in combination with CSA and AZA, BAS remained cost-effective compared with no induction at £20,000 and £30,000 per QALY. When followed by CSA or immediate-release TAC and MMF, BAS was no longer cost-effective at £20,000 per QALY when the duration of surrogate effect was limited to 0 or 1 year, but was cost-effective at £30,000 per QALY unless the surrogate effect was eliminated.
Adopting list prices for drug acquisition instead of average NHS acquisition costs (from the CMU eMit database) did not materially affect the cost-effectiveness of BAS.
rATG
Rabbit ATG is not predicted to be cost-effective at £20,000–30,000 per QALY in the deterministic analysis or the probabilistic analysis. rATG was cost-effective at £20,000 per QALY in 13.7–22.6% of PSA iterations across comparisons and at £30,000 per QALY in 19.1–27.2% of iterations.
When the duration of surrogate effect on graft survival was varied from 0 to 19 years, at no point was rATG cost-effective at £20,000–30,000 per QALY in any of the three comparisons.
Adopting list prices for drug acquisition instead of average NHS acquisition costs did not materially affect the cost-effectiveness of rATG.
Summary for induction agents
Basiliximab is predicted to be cost-effective at £20,000–30,000 per QALY, whereas rATG is not.
Maintenance agents
Immediate-release TAC
Immediate-release TAC is predicted to be cost-effective at £20,000–30,000 per QALY in the deterministic and PSAs across all comparisons. The probability of immediate-release TAC being cost-effective at £20,000 and £30,000 per QALY ranged from 81.8% to 94.6%.
When the duration of surrogate effect on graft survival was reduced either immediate-release TAC or CSA was cost-effective at £20,000 or £30,000 per QALY. CSA was cost-effective when the surrogate effect was shorter, whereas immediate-release TAC was cost-effective when the surrogate effect lasted longer.
Adopting list prices instead of average NHS acquisition costs resulted in immediate-release TAC no longer being cost-effective at £20,000 or £30,000 per QALY when used in combination with MMF (CSA was instead cost-effective) but remaining cost-effective when used in combination with AZA.
TAC-PR
Prolonged-release tacrolimus is not predicted to be cost-effective at £20,000 or £30,000 per QALY in any analyses (including scenario analyses). The probability of TAC-PR being cost-effective was 0.0% at £20,000 and £30,000 per QALY.
MMF
Mycophenolate mofetil is predicted to be cost-effective at £20,000 and £30,000 per QALY in the deterministic and probabilistic analyses. The probability of MMF being cost-effective at £20,000 and £30,000 per QALY ranged from 63.2% to 92.2% across comparisons.
The cost-effectiveness of MMF was robust to structural scenario analyses.
Adopting list prices instead of average NHS acquisition costs resulted in MMF no longer being cost-effective at £20,000 or £30,000 per QALY when used in combination with immediate-release TAC (AZA instead was cost-effective) but remaining cost-effective when used in combination with CSA.
MPS
Mycophenolate sodium is not predicted to be cost-effective at £20,000 or £30,000 per QALY in any analyses (including scenario analyses). The probability of MPS being cost-effective was 0.1% at £20,000 per QALY and 0.8% at £30,000 per QALY.
SRL
Sirolimus is not predicted to be cost-effective at £20,000 or £30,000 per QALY in the deterministic or probabilistic analyses whether in combination with immediate-release TAC or in combination with BAS induction and MMF. The probability of SRL being cost-effective in either combination was 0.0% at £20,000 and £30,000 per QALY.
A threshold analysis was conducted in which the gamma parameter of the Weibull distribution for death-censored graft survival was allowed to vary independently for regimens not including CNIs. SRL was included in one of the two affected regimens (BAS + SRL + MMF). The threshold analysis indicated that there are values for gamma for which SRL is cost-effective at £20,000 or £30,000 per QALY, but these result in markedly different survival curves for SRL compared with immediate-release TAC, for which we are aware of no supporting high-quality evidence.
Other scenario analyses did not lead to SRL becoming cost-effective at £20,000 or £30,000 per QALY.
EVL
Everolimus is not predicted to be cost-effective at £20,000 or £30,000 per QALY in any analyses (including scenario analyses). The probability of EVL being cost-effective was 0.0% at £20,000 and £30,000 per QALY.
BEL
Belatacept is not predicted to be cost-effective at £20,000 or £30,000 per QALY in the deterministic or probabilistic analyses. The probability of BEL being cost-effective was 0.0% at £20,000 and £30,000 per QALY.
A threshold analysis was conducted in which the gamma parameter of the Weibull distribution for death-censored graft survival was allowed to vary independently for regimens not including CNIs. BEL was included in one of the two affected regimens (BAS + BEL + MMF). The threshold analysis suggested that no value of gamma would enable BEL to be cost-effective at £20,000 or £30,000 per QALY.
Another threshold analysis was conducted to investigate the impact of the administration and acquisition costs of BEL on cost-effectiveness. With the base-case cost of administration BEL is not cost-effective at £20,000 or £30,000 per QALY, even at zero acquisition cost. With the list price for acquisition cost, BEL is similarly not cost-effective at £20,000 or £30,000 per QALY, even at zero administration cost.
Other scenario analyses did not lead to BEL being cost-effective at £20,000 or £30,000 per QALY.
Summary for maintenance agents
Base-case deterministic and probabilistic results suggest that at cost-effectiveness thresholds of between £20,000 and £30,000 per QALY, only BAS, immediate-release TAC and MMF are likely to be cost-effective.
When structural uncertainty about the surrogate relationship for graft survival was explored it was found that when the surrogate relationship was weakened, no induction became cost-effective instead of BAS, and CSA became cost-effective instead of immediate-release TAC. MMF remained cost-effective throughout.
Another structural uncertainty analysis investigating the possibility that CNI-free regimens could prolong graft survival found that a regimen containing SRL could become cost-effective at £20,000 or £30,000 per QALY but required potentially implausible gains in graft survival. The analysis also found that BEL could not become cost-effective at £20,000 or £30,000 per QALY despite the same potentially implausible gains in graft survival.
When list prices were adopted instead of average NHS acquisition costs (despite this being considered a deviation from the reference case), CSA was cost-effective instead of TAC in some comparisons and AZA was cost-effective instead of mycophenolate in some comparisons.
Prespecified subgroup analyses were not possible, based on the RCTs included in the systematic review of clinical effectiveness, and therefore have not been conducted.
Comparison between the PenTAG and company models
We compared the main deterministic analyses from three of the company submissions with those produced by the independent assessment group (PenTAG). These assessed the cost-effectiveness of TAC-PR compared with immediate-release TAC (Astellas), EVL (Novartis), EC-MPS (Novartis) and BEL (Bristol-Myers Squibb). Although some of the PenTAG analyses contained a larger set of comparator treatments, they were generally comparable after dominated comparators were excluded from the PenTAG analyses.
Overall, the PenTAG analyses of cost-effectiveness were considerably less favourable than the companies’ analyses of their own products. This could mostly be attributed to the companies’ analyses basing their effectiveness assumptions on the results of specific RCTs (rather than meta-analysis), combined with using different surrogate end points and/or US cohort data to extrapolate long-term outcomes such as graft survival.
The economic modelling by PenTAG tended to include fuller costing of the administration of the maintenance therapies, and more realistic, relatively lower, annual costs of dialysis (except Novartis). In addition, the utility difference between living with a functioning graft and living on dialysis was generally greater in the three companies’ analyses (typical difference of between ≈ 0.25 and ≈ 0.3) than in the PenTAG model (≈ 0.2 difference). Overall, these differences in the company’s models will tend to magnify the impact on QALYs of any incremental effectiveness differences that affect long-term graft survival, and also reduce their associated incremental cost.
Strengths and limitations
Systematic review of studies of effectiveness
The strengths of this systematic review are that is was conducted by an independent, experienced research team using the latest evidence and working to a prespecified protocol (PROSPERO CRD42014013189), which follows a robust methodology.
There are a number of limitations.
-
Owing to the level of reporting detail, we were unable to perform subgroup analysis according to donor or HLA matching.
-
Study design and participant characteristics varied widely across studies, leading to substantial heterogeneity.
-
The 86 included RCTs were of variable quality, but all appear to be flawed. However, because of reporting omissions for most trials, for example on random allocation or a priori outcomes, it was difficult to make a general assessment regarding quality.
-
Very few trials reported longer-term follow-up, with the majority reporting data at 1 year.
Economic modelling by Peninsula Technology Assessment Group
Strengths
-
This is an analysis conducted by an independent academic group, adhering to the NICE reference case where possible.
-
All interventions and relevant comparators allowable are included and evaluated for cost-effectiveness (Table 217).
-
The natural history of disease (e.g. graft survival, DWFG, mortality while receiving dialysis) is based on UK data, either published by the UK Renal Registry in its annual reports or from new analyses of the UK Transplant Registry data set.
-
Relative effectiveness parameters are taken directly from the results of the systematic review of clinical effectiveness when possible (including for key outcomes of graft survival, patient survival, post-transplantation GRF and AR) and when not possible are synthesised from data reported in RCTs included in the systematic review.
-
The prognostic significance of AR, post-transplantation GRF and NODAT on outcomes is incorporated into the analysis.
-
Pre-emptive retransplantations are included for a minority of KTRs following failure of the initial graft (avoiding dialysis which is costly and reduces HRQoL).
-
Unit costs are those relevant to the NHS (e.g. CMU eMit costs were used where available).
-
Dosing of immunosuppressive agents is based on recent RCTs and for many included tapering to low levels as would be targeted in clinical practice.
-
A PSA is presented to reflect the potential impact of parameter uncertainty.
-
Structural uncertainty in the modelling of graft survival is addressed through scenario analyses.
| Agent | PenTAG | Astellas | Bristol-Myers Squibb | Novartis | TA8543 |
|---|---|---|---|---|---|
| BAS | Y | N | N | N | Y |
| rATG | Y | N | N | N | N |
| (No induction) | Y | N | N | N | Y |
| Immediate-release TAC | Y | Y | Y | P | Y |
| TAC-PR | Y | Y | N | N | N |
| MMF | Y | N | N | Y | Y |
| MPS | Y | N | N | Y | N |
| SRL | Y | Y | N | N | Y |
| EVL | Y | Y | N | Y | N |
| BEL | Y | Y | Y | N | N |
| CSA | Y | N | Y | P | Y |
| AZA | Y | N | N | N | Y |
Limitations
-
We have not modelled eGFR for regimens except at 12 months; the Novartis and Bristol-Myers Squibb analyses both estimated eGFR over time and used CKD stages (defined by eGFR intervals) to drive certain costs and HRQoL; the Bristol-Myers Squibb analysis in particular predicts significantly greater costs in more advanced CKD stages, although it is considered likely that both the absolute eGFR and the trajectory of eGFR for a patient will determine the level of monitoring and, therefore, the level of monitoring for CKD stage 4 patients in the 24–36 months after transplantation may not be a good reflection of the level of monitoring for patients reaching CKD stage 4 much later (with a much shallower trajectory); in the absence of evidence that any agent or regimen leads to greater time in higher or lower eGFR ranges other than by extension of graft survival, we consider that our model adequately incorporates the clinical importance of eGFR through the surrogate relationship with graft survival and that modelling eGFR further in the model would be rather speculative and unlikely to lead to significant differences in cost-effectiveness.
-
We have not included any analysis of the cost-effectiveness of reducing or eliminating CCSs, although in many studies informing the model the CCS dose was heavily tapered for long-term maintenance; as the cost of CCSs is minimal, this would be very unlikely to affect cost-effectiveness results.
-
We did not include NHS-funded transport costs for HD, which may constitute around 10% of the total cost of HD provision; inclusion of transport costs would increase the overall cost of HD and make regimens with less time dependent on dialysis more cost-effective.
-
We did not include any treatment discontinuation or switching except following graft loss; published RCTs suggest that treatment switching is usually towards immediate-release TAC and MMF.
-
We did not differentiate between different severity of AR, that is, if any regimen results in less-severe AR (but no fewer) episodes then this will not be reflected and the cost-effectiveness will be underestimated.
-
We applied HRs for graft survival based on eGFR at 12 months, which were intended for extrapolation to only 4 years, although justifications are given for not using the HRs intended for further extrapolation.
-
We assumed independence of AR, NODAT and eGFR at 12 months within each regimen; if, for example, patients experiencing AR in the first 12 months are likely to have a lower eGFR at 12 months than patients who are not experiencing AR then there will be second-order error in the estimated HR for each regimen (in this example an over-representation of patients with AR and high eGFR, and patients without AR and with low eGFR, and an under-representation of patients with AR and low eGFR, and patients without AR and with high eGFR); at the aggregate level AR, NODAT and eGFR were estimated according to RCTs included in the systematic review and therefore correlation of these at the aggregate level across regimens would be possible and would be represented in the model.
-
We did not include continuing immunosuppression following graft loss (which may happen in clinical settings).
-
We combined estimates of incremental renal function between comparators, based on different measurements of GRF (measured GFR, MDRD eGFR, Cockcroft–Gault CRC and measured CRC).
-
We assumed that a proportional hazards model for graft survival is appropriate when it is possible that certain regimens may result in qualitatively different survival curves, for example owing to absence of CNI nephrotoxicity in CNI-sparing regimens; we conducted a scenario analysis that demonstrated that markedly (and perhaps implausibly) different survival curves would be required for cost-effectiveness to be demonstrated.
-
We modelled de novo SRL with BAS induction and MMF rather than including initial CSA medication and delayed SRL initiation, although this may be common in clinical practice while surgical wounds heal; including delayed SRL initiation would slightly reduce costs and improve cost-effectiveness of the BAS + SRL + MMF regimen.
-
We made no attempt to explicitly model adherence to immunosuppressive medication owing to the absence of evidence on this outcome in RCTs included in the systematic review of clinical effectiveness; there is some evidence that non-adherence is a cause of late AR and graft loss, but at this time any gains in clinical effectiveness due to improved adherence attributable to any individual agent or regimen are considered to be speculative.
-
It was assumed that there would be no treatment interactions between induction and maintenance therapies affecting clinical effectiveness outcomes. It is, known, however, for example, that there is a pharmacokinetic interaction between BAS and MMF, which results in prolonged BAS half-life.
-
Owing to inconsistent reporting of AEs in RCTs included in our systematic review only a few AEs were modelled: NODAT, CMV infection, dyslipidaemia and anaemia. Of these, anaemia was assumed not to vary between regimens. Induction agents were assumed not to affect the incidence of AEs. Malignancy, PTLD, proteinuria, hypertension, EBV infection, BKV infection, other infections and other AEs were not modelled. CVD was included as a potential sequela of NODAT (inpatient and non-inpatient costs and increased rate of DWFG) but was not included otherwise (including as a sequelae of dyslipidaemia).
Economic modelling in the company submissions
Uncertainties
-
Long-term outcomes from RCTs are seldom reported so it has not been possible to externally validate the predicted survival differences between regimens.
-
No evidence has been identified on the influence of the induction or maintenance therapies on HRQoL.
-
RCTs identified in the systematic review have not provided evidence to support subgroup analyses.
-
The costs for diabetes mellitus are highly uncertain, especially as the costs relate to the general diabetic population rather than transplant recipients with NODAT.
-
It is not known whether or not NHS hospitals might secure discounts from list prices where these were assumed in the model (i.e. for BAS, rATG, TAC-PR, MPS, SRL, EVL and BEL).
Chapter 8 Conclusions
The additional clinical effectiveness evidence identified in this updated systematic review suggests that there is little impact on effectiveness conclusions from the head-to-head comparisons, particularly for graft loss and mortality. Following NMA for induction therapy, there is no evidence to suggest that BAS or rATG is more effective than PBO/no induction or each other in reducing the odds of graft loss or mortality. rATG and BAS were both estimated to be more effective than PBO/no induction, with rATG being more effective than BAS at reducing BPAR. There is evidence to suggest that BAS is more effective than PBO/no induction for increasing GRF.
With regard to maintenance therapy, the NMA showed that none of the maintenance regimens performed consistently well on all four outcomes, and a great deal of heterogeneity was noted.
As for cost-effectiveness, the analyses conducted and reported here suggest that only a regimen of BAS induction followed by maintenance with immediate-release TAC and MMF would be cost-effective at £20,000–30,000 per QALY. If only these interventions were to be recommended then we believe there would be very little implication for service provision.
Implication for service provision
It is believed that the immunosuppressive regimen of BAS induction, followed by maintenance with immediate-release TAC and MMF (with or without CCSs), is in common use at present.
Suggested research priorities
New research in the following areas could reduce the uncertainty noted:
-
Good-quality, well-reported, longer-term RCTs up to 10 years for both induction and maintenance, with adequate sample sizes and clear randomisation are essential.
-
RCTs to include HRQoL as an outcome, and sufficiently powered for subgroup analysis by sex, donor type, ethnicity and HLA matching.
-
Improved reporting of trials would be beneficial, in particular the reporting of randomisation methods and withdrawal, dropouts and loss to follow-up.
-
RCTs comparing clinically relevant doses of immunosuppressive therapy would be beneficial.
-
Use of real-world data, such as the UK Renal Registry data set, may provide a more sensitive and specific understanding of immunosuppression and renal transplant outcomes.
Acknowledgements
We would like to thank Dr David Game, Consultant Nephrologist, Guy’s Hospital and Mr Jacob Akoh, Consultant General and Transplant Surgeon, Plymouth Hospitals NHS Trust.
We would also like to acknowledge the help of Andy Salmon for model checking, and Sue Whiffin for her administrative support, both from the University of Exeter Medical School.
Data sharing statement
This is a systematic review, therefore, there are no data to share. Further information can be obtained from the lead author.
Contribution of authors
Tracey Jones-Hughes provided overall project management; led the systematic review of clinical effectiveness, including assessment of all abstracts and titles for possible inclusion and meta-analysis for clinical effectiveness outcomes; and drafted or edited all sections of the report.
Tristan Snowsill led the design, development and execution of the economic model; wrote the sections on the design and results of the economic model; and contributed to the critique of industry submissions.
Marcela Haasova assessed abstracts and titles for inclusion, and contributed to the writing and editing of the report and contributed to the NMA.
Helen Coelho assessed titles and abstracts for inclusion and exclusion; conducted the quality appraisal of the effectiveness systematic review; contributed to other parts of the effectiveness systematic review, and to the writing and editing the report.
Louise Crathorne assessed titles and abstract for inclusion in the effectiveness and cost-effectiveness review, and contributed to writing and editing of the cost-effectiveness systematic review.
Chris Cooper led the literature searching, and contributed to writing and editing the report.
Ruben Mujica-Mota led the systematic review of economic evaluations and provided advice on design of the model.
Jaime Peters led the NMA, and contributed to writing and editing the report.
Jo Varley-Campbell assessed abstracts and titles for inclusion, and contributed to the writing and editing of the report.
Nicola Huxley assisted with identification of model parameters, and contributed to writing and editing of the report.
Jason Moore provided clinical input into the design of the model, and advised on clinical matters.
Matt Allwood contributed to the writing and editing of the report.
Jenny Lowe critiqued and wrote summaries of the literature searches for the company submissions.
Chris Hyde extracted data for inclusion in the clinical effectiveness systematic review.
Martin Hoyle provided advice on model structure and identified parameters, and contributed to writing and editing of the report.
Mary Bond had oversight of project management and the clinical effectiveness systematic review, and contributed to the editing of the report.
Rob Anderson contributed to the cost-effectiveness review and the writing and editing of the report. Overall Director of the project and Guarantor of the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 2003;42:677-84. http://dx.doi.org/10.1016/S0272-6386(03)00916-8.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group . KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am J Transplant 2009;9:S1-157. http://dx.doi.org/10.1111/j.1600-6143.2009.02834.x.
- UK Renal Registry 16th Annual Report: Chapter 2 UK RRT Prevalence in 2012: National and Centre-Specific Analyses. Bristol: UKRR; 2013.
- Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 2005;294:2726-33. http://dx.doi.org/10.1001/jama.294.21.2726.
- Ojo AO, Hanson JA, Meier-Kriesche HU, Okechukwu CN, Wolfe RA, Leichtman AB, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol 2001;12:589-97.
- Bhowmik DM, Dinda AK, Mahanta P, Agarwal SK. The evolution of the Banff classification schema for diagnosing renal allograft rejection and its implications for clinicians. Indian J Nephrol 2010;20:2-8. http://dx.doi.org/10.4103/0971-4065.62086.
- Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Medical progress: strategies to improve long-term outcomes after renal transplantation. N Engl J Med 2002;346:580-90. http://dx.doi.org/10.1056/NEJMra011295.
- Webster A, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev 2005;4. http://dx.doi.org/10.1002/14651858.cd003961.pub2.
- Butler J, Roderick P, Mullee M, Mason J, Peveler R. Frequency and impact of nonadherence to immunosuppression after renal transplantation: a systematic review. Transplantation 2004;77:769-89. http://dx.doi.org/10.1097/01.TP.0000110408.83054.88.
- Kidney Disease: Key Facts and Figures. NHS Kidney Care; 2010.
- Annual Report on Kidney Transplantation Report for 2013/2014 (1 April 2004 to 31 March 2014). Watford: NHSBT; 2014.
- Organ Donation and Transplantation Annual Activity Report 2012–2013. Watford: NHSBT; n.d.
- Organ Donation and Transplantation: Activity Figures for the UK as at 12 April 2013. Watford: NHSBT; 2014.
- Orr A, Willis S, Holmes M, Britton P, Orr D. Living with a kidney transplant: a qualitative investigation of quality of life. J Health Psychol 2007;12:653-62. http://dx.doi.org/10.1177/1359105307078172.
- Apostolou T, Hutchison AJ, Boulton AJM, Chak W, Vileikyte L, Uttley L, et al. Quality of life in CAPD, transplant, and chronic renal failure patients with diabetes. Ren Fail 2007;29:189-97. http://dx.doi.org/10.1080/08860220601098862.
- Balaska A, Moustafellos P, Gourgiotis S, Pistolas D, Hadjiyannakis E, Vougas V, et al. Changes in health-related quality of life in Greek adult patients 1 year after successful renal transplantation. Exp Clin Transplant 2006;4:521-4.
- Bremer BA, Mccauley CR, Wrona RM, Johnson JP. Quality of life in end-stage renal-disease: a reexamination. Am J Kidney Dis 1989;13:200-9. http://dx.doi.org/10.1016/S0272-6386(89)80053-8.
- Dale PL, Hutton J, Elgazzar H. Utility of health states in chronic kidney disease: a structured review of the literature. Curr Med Res Opin 2008;24:193-206. http://dx.doi.org/10.1185/030079908X253410.
- Evans RW, Manninen DL, Garrison LP, Hart LG, Blagg CR, Gutman RA, et al. The quality of life of patients with end-stage renal-disease. N Engl J Med 1985;312:553-9. http://dx.doi.org/10.1056/NEJM198502283120905.
- Morris PL, Jones B. Transplantation versus dialysis: a study of quality of life. Transplant Proc 1988;20:23-6.
- Morris PL, Jones B. Life satisfaction across treatment methods for patients with end-stage renal-failure. Med J Aust 1989;150:428-32.
- Nourbala MH, Hollisaaz MT, Nasiri M, Bahaeloo-Horeh S, Najafi M, Araghizadeh H, et al. Pain affects health-related quality of life in kidney transplant recipients. Transplant Proc 2007;39:1126-9. http://dx.doi.org/10.1016/j.transproceed.2007.03.004.
- Seedat YK, Macintosh CG, Subban JV. Quality-of-life for patients in an end-stage renal-disease program. S Afr Med J 1987;71:500-4.
- Simmons RG, Anderson CR, Abress LK. Quality-of-life and rehabilitation differences among 4 end-stage renal-disease therapy groups. Scand J Urol Nephrol 1990;131:7-22.
- Sureshkumar KK, Patel BM, Markatos A, Nghiem DD, Marcus RJ. Quality of life after organ transplantation in type 1 diabetics with end-stage renal disease. Clin Transplant 2006;20:19-25. http://dx.doi.org/10.1111/j.1399-0012.2005.00433.x.
- Overbeck I, Bartels M, Decker O, Harms J, Hauss J, Fangmann J. Changes in quality of life after renal transplantation. Transplant Proc 2005;37:1618-21. http://dx.doi.org/10.1016/j.transproceed.2004.09.019.
- Yildirim A. The importance of patient satisfaction and health-related quality of life after renal transplantation. Transplant Proc 2006;38:2831-4. http://dx.doi.org/10.1016/j.transproceed.2006.08.162.
- Management of the Failing Kidney Transplant. London: The British Transplantation Society; 2013.
- Lamping DL, Constantinovici N, Roderick P, Normand C, Henderson L, Harris S, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: a prospective cohort study. Lancet 2000;356:1543-50. http://dx.doi.org/10.1016/S0140-6736(00)03123-8.
- Bakewell AB, Higgins RM, Edmunds ME. Quality of life in peritoneal dialysis patients: decline over time and association with clinical outcomes. Kidney Int 2002;61:239-48. http://dx.doi.org/10.1046/j.1523-1755.2002.00096.x.
- de Wit AG, Ramsteijn PG, Charro FT. Economic evaluation of end stage renal disease treatment. NHS Econ Eval Database 1998;44:215-32.
- UK Renal Registry 17th Annual Report. Appendix C Renal Services Descried for Non-Physicians. Bristol: UKRR; 2014.
- NHS Standard Contract for Adult Kidney Transplant Service. London: NHS England; 2013.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3:1-150.
- White CA HD, Akbari A, Garland J, Knoll GA. Performance of creatinine-based estimates of GFR in kidney transplant recipients: a systematic review. Am J Kidney Dis 2008;51:1005-15. http://dx.doi.org/10.1053/j.ajkd.2008.02.308.
- The National Service Framework for Renal Services. Part One: Dialysis and Transplantation. London: DH; 2004.
- Su XM, Zenios SA, Chakkera H, Milford EL, Chertow GM. Diminishing significance of HLA matching in kidney transplantation. Am J Transplant 2004;4:1501-8. http://dx.doi.org/10.1111/j.1600-6143.2004.00535.x.
- Trebern-Launay K, Foucher Y, Giral M, Legendre C, Kreis H, Kessler M, et al. Poor long-term outcome in second kidney transplantation: a delayed event. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0047915.
- Redfield RR, Gupta M, Rodriguez E, Wood A, Abt PL, Levine MH. Graft and patient survival outcomes of a third kidney transplant. Transplantation 2015;99:416-23. http://dx.doi.org/10.1097/TP.0000000000000332.
- Kerr M, Bray B, Medcalf J, O’Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012;27:73-80. http://dx.doi.org/10.1093/ndt/gfs269.
- Baker R, Jardine A, Andrews P. Renal association clinical practice guideline on post-operative care of the kidney transplant recipient. Nephron Clin Pract 2011;118:C311-47. http://dx.doi.org/10.1159/000328074.
- Chamberlain G, Baboolal K, Bennett H, Pockett RD, McEwan P, Sabater J, et al. The economic burden of posttransplant events in renal transplant recipients in Europe. Transplantation 2014;97:854-61. http://dx.doi.org/10.1097/01.tp.0000438205.04348.69.
- Immunosuppressive Therapy for Renal Transplantation in Adults. London: NICE; 2004.
- Gordon EJ, Ladner DP, Caicedo JC, Franklin J. Disparities in kidney transplant outcomes: a review. Semin Nephrol 2010;30:81-9. http://dx.doi.org/10.1016/j.semnephrol.2009.10.009.
- Meier-Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA, Cibrik DM, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000;58:1311-17. http://dx.doi.org/10.1046/j.1523-1755.2000.00287.x.
- Metzger RA, Delmonico FL, Feng S, Port FK, Wynne JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant 2003;3:114-25. http://dx.doi.org/10.1034/j.1600-6143.3.s4.11.x.
- Wu C, Evans I, Joseph R, Shapiro R, Tan H, Basu A, et al. Comorbid conditions in kidney transplantation: association with graft and patient survival. J Am Soc Nephrol 2005;16:3437-44. http://dx.doi.org/10.1681/ASN.2005040439.
- Laftavi MR, Patel SK, Feng L, Said MI, Ryan MR, Laftavi H, et al. African American (AA) renal transplant recipients (RTR) require higher tacrolimus (TAC) doses to achieve target levels compared to white (W) RTR: does clotrimazole help?. Am J Transplant 2012;12:299-300.
- Neylan JF. Immunosuppressive therapy in high-risk transplant patients: dose-dependent efficacy of mycophenolate mofetil in African-American renal allograft recipients. Transplantation 1997;64:1277-82. http://dx.doi.org/10.1097/00007890-199711150-00008.
- Neylan J. Effect of race on efficacy and safety of sirolimus vs. azathioprine + standard immunotherapy in renal transplantation. Transplantation 1999;67. http://dx.doi.org/10.1097/00007890-199904150-00948.
- Grinyo JM, Ekberg H, Mamelok RD, Oppenheimer F, Sanchez-Plumed J, Gentil MA, et al. The pharmacokinetics of mycophenolate mofetil in renal transplant recipients receiving standard-dose or low-dose cyclosporine, low-dose tacrolimus or low-dose sirolimus: the SYMPHONY pharmacokinetic substudy. Nephrol Dial Transplant 2009;24:2269-76. http://dx.doi.org/10.1093/ndt/gfp162.
- Kidney Transplant Protocol. Nottingham: Nottingham University Hospitals NHS Trust; 2013.
- Muthusamya AS, Vaidhya AC, Sinha S, Roy D, Elker DE, Friend PJ. Alemtuzumab induction and steroid-free maintenance immunosuppression in pancreas transplantation. Am J Transplant 2008;8:2126-31. http://dx.doi.org/10.1111/j.1600-6143.2008.02373.x.
- Royal Infirmary of Edinburgh . EdRen Handbook: Renal Transplant Protocols 2007. www.edren.org (accessed 2 December 2015).
- Commercial Medicines Unit n.d. www.cmu.nhs.uk/ (accessed December 2014).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- NHS Business Services Authority . Drug Tariff n.d. www.nhsbsa.nhs.uk/PrescriptionServices/4940.aspx (accessed February 2015).
- Krämer BK, Klinger M, Wlodarczyk Z, Ostrowski M, Midvedt K, Stefoni S, et al. Tacrolimus combined with two different corticosteroid-free regimens compared with a standard triple regimen in renal transplantation: 1 year observational results. Clin Transplant 2010;24:E1-9. http://dx.doi.org/10.1111/j.1399-0012.2009.01162.x.
- Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010;10:535-46. http://dx.doi.org/10.1111/j.1600-6143.2009.03005.x.
- Larsen CP, Grinyo J, Medina-Pestana J, Vanrenterghem Y, Vincenti F, Breshahan B, et al. Belatacept-based regimens versus a cyclosporine A-based regimen in kidney transplant recipients: 2-year results from the BENEFIT and BENEFIT-EXT studies. Transplantation 2010;90:1528-35. http://dx.doi.org/10.1097/TP.0b013e3181ff87cd.
- Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant 2012;12:210-17. http://dx.doi.org/10.1111/j.1600-6143.2011.03785.x.
- Rostaing L, Vincenti F, Grinyo J, Rice KM, Bresnahan B, Steinberg S, et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. Am J Transplant 2013;13:2875-83. http://dx.doi.org/10.1111/ajt.12460.
- Bristol-Myers Squibb . Immunosuppressive Therapy for Kidney Transplantation in Adults (Review of Technology Appraisal Guidance 85). Belatacept Submission of Evidence 2014.
- NHS Reference Costs 2013 to 2014. London: DH; 2014.
- Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al. Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9210.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Healthcare. York: NHS CRD; 2009.
- Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al. A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10490.
- Dias SW, Sutton NA, Ades A. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-analysis of Randomised Controlled Trials. London: NICE; 2011.
- Dias SW, Sutton NA, Caldwell D, Lu G, Ades A. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. London: NICE; 2011.
- Ades AC, Reken D, Welton S, Sutton N, Dias AS. NICE DSU Technical Support Document 7: Evidence Synthesis of Treatment Efficacy in Decision making: A Reviewer’s Checklist. London: NICE; 2012.
- Nashan B, Moore R, Amlot P, Schmidt AG, Abeywickrama K, Soulillou JP. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. Lancet 1997;350:1193-8. http://dx.doi.org/10.1016/S0140-6736(97)09278-7.
- Kahan BD, Rajagopalan PR, Hall M. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti-interleukin-2-receptor monoclonal antibody. Transplantation 1999;67:276-84. http://dx.doi.org/10.1097/00007890-199901270-00016.
- Ponticelli C, Yussim A, Cambi V, Legendre C, Rizzo G, Salvadori M, et al. A randomized, double-blind trial of basiliximab immunoprophylaxis plus triple therapy in kidney transplant recipients. Transplantation 2001;72:1261-7. http://dx.doi.org/10.1097/00007890-200110150-00014.
- Lawen JG, Davies EA, Mourad G, Oppenheimer F, Molina MG, Rostaing L, et al. Randomized double-blind study of immunoprophylaxis with basiliximab, a chimeric anti-interleukin-2 receptor monoclonal antibody, in combination with mycophenolate mofetil-containing triple therapy in renal transplantation. Transplantation 2003;75:37-43. http://dx.doi.org/10.1097/00007890-200301150-00007.
- Van Duijnhoven EM, Christiaans MH, Boots JM, Nieman FH, Wolffenbuttel BH, van Hooff JP. Glucose metabolism in the first 3 years after renal transplantation in patients receiving tacrolimus versus cyclosporine-based immunosuppression. J Am Soc Nephrol 2002;13:213-20.
- Waller JR, Murphy GJ, Metcalfe MS, Sandford RM, Pattenden CJ, Nicholson ML. Primary immunosuppression with tacrolimus is associated with a reduction in renal allograft fibrosis compared with neoral therapy. Transplant Proc 2002;34:1587-8. http://dx.doi.org/10.1016/S0041-1345(02)03033-6.
- Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation 1995;60:225-32. http://dx.doi.org/10.1097/00007890-199508000-00003.
- Tuncer M, Gürkan A, Erdoan O, Demirba A, Süleymanlar G, Ersoy FF, et al. Mycophenolate mofetil in renal transplantation: five years experience. Transplant Proc 2002;34:2087-8. http://dx.doi.org/10.1016/S0041-1345(02)02861-0.
- Schleibner S, Krauss M, Wagner K, Erhard J, Christiaans M, van Hooff J, et al. FK 506 versus cyclosporin in the prevention of renal allograft rejection: European pilot study six-week results. Transplant Int 1995;8:86-90.
- Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation 1996;62:900-5. http://dx.doi.org/10.1097/00007890-199610150-00005.
- Radermacher J, Meiners M, Bramlage C, Kliem V, Behrend M, Schlitt HJ, et al. Pronounced renal vasoconstriction and systemic hypertension in renal transplant patients treated with cyclosporin A versus FK 506. Transplant Int 1998;11:3-10. http://dx.doi.org/10.1111/j.1432-2277.1998.tb00948.x.
- Baboolal K, Jones GA, Janezic A, Griffiths DR, Jurewicz WA. Molecular and structural consequences of early renal allograft injury. Kidney Int 2002;61:686-96. http://dx.doi.org/10.1046/j.1523-1755.2002.00149.x.
- Campos HH, Abbud Filho M. One-year follow-up of a Brazilian randomized multicenter study comparing tacrolimus versus cyclosporine in kidney transplantation. Transplant Proc 2002;34:1656-8. http://dx.doi.org/10.1016/S0041-1345(02)02968-8.
- Margreiter R. Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: a randomised multicentre study. Lancet 2002;359:741-6. http://dx.doi.org/10.1016/S0140-6736(02)07875-3.
- Töz H, Sen S, Sezi M, Duman S, Ozkahya M, Ozbek S, et al. Comparison of tacrolimus and cyclosporin in renal transplantation by the protocol biopsies. Transplant Proc 2004;36:134-6. http://dx.doi.org/10.1016/j.transproceed.2003.11.056.
- Sadek S, Medina J, Arias M, Sennesael J, Squifflet JP, Vogt B. Short-term combination of mycophenolate mofetil with cyclosporine as a therapeutic option for renal transplant recipients: a prospective, multicenter, randomized study. Transplantation 2002;74:511-17. http://dx.doi.org/10.1097/00007890-200208270-00013.
- Lebranchu Y, Bridoux F, Büchler M, Le Meur Y, Etienne I, Toupance O, et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am J Transplant 2002;2:48-56. http://dx.doi.org/10.1034/j.1600-6143.2002.020109.x.
- Mayer AD, Dmitrewski J, Squifflet JP, Besse T, Grabensee B, Klein B, et al. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation 1997;64:436-43. http://dx.doi.org/10.1097/00007890-199708150-00012.
- The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group . A blinded, randomised clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation 1996;61:1029-37. http://dx.doi.org/10.1097/00007890-199604150-00008.
- Yang HC, Holman MJ, Langhoff E, Ulsh PJ, Dellock CA, Gupta M, et al. Tacrolimus/‘low-dose’ mycophenolate mofetil versus microemulsion cyclosporine/‘low-dose’ mycophenolate mofetil after kidney transplantation: 1-year follow-up of a prospective, randomized clinical trial. Transplant Proc 1999;31:1121-4. http://dx.doi.org/10.1016/S0041-1345(98)01929-0.
- Soleimani AR, Kamkar I, Nikoueinejad H, Moraweji AR. Comparison of cyclosporine and sirolimus effects on serum creatinine level over five years after kidney transplantation. Transplant Proc 2013;45:1644-7. http://dx.doi.org/10.1016/j.transproceed.2013.01.060.
- Schaefer HM, Kizilisik AT, Feurer I, Nylander WA, Langone AJ, Helderman JH, et al. Short-term results under three different immunosuppressive regimens at one center. Transplant Proc 2006;38:3466-7. http://dx.doi.org/10.1016/j.transproceed.2006.10.098.
- Smith MP, Newstead CG, Ahmad N, Lewington AJ, Tibble S, Lodge JP, et al. Poor tolerance of sirolimus in a steroid avoidance regimen for renal transplantation. Transplantation 2008;85:636-9. http://dx.doi.org/10.1097/TP.0b013e3181613e65.
- Vítko S, Wlodarczyk Z, Kyllönen L, Czajkowski Z, Margreiter R, Backman L, et al. Tacrolimus combined with two different dosages of sirolimus in kidney transplantation: results of a multicenter study. Am J Transplant 2006;6:531-8. http://dx.doi.org/10.1111/j.1600-6143.2005.01193.x.
- Bingyi S, Yeyong Q, Ming C, Chunbai M, Wenqiang Z. Randomised trial of simulect versus placebo for control of acute rejection in renal allograft recipients. Transplant Proc 2003;35:192-4. http://dx.doi.org/10.1016/S0041-1345(02)03769-7.
- Charpentier B. Induction versus noninduction protocols in anti-calcineurin-based immunosuppression. Transplant Proc 2001;33:3334-6. http://dx.doi.org/10.1016/S0041-1345(01)02435-6.
- Sheashaa HA, Bakr MA, Ismail AM, Sobh MA, Ghoneim MA. Basiliximab reduces the incidence of acute cellular rejection in live-related-donor kidney transplantation: a three-year prospective randomized trial. J Nephrol 2003;16:393-8.
- Mourad G, Rostaing L, Legendre C. Assessment of two strategies of neoral® administration, early versus delayed, on renal function and efficacy in de novo renal transplant patients receiving myfortic®, steroids and anti-il2r antibodies: 6 months interim results. Transplantation 2004;78. http://dx.doi.org/10.1097/00007890-200407271-01219.
- Jarzembowski T, Panaro F, Raofi V, Dong G, Testa G, Sankary H, et al. Long-term results of a prospective randomized trial comparing tacrolimus versus cyclosporine in African-American recipients of primary cadaver renal transplant. Transplant Int 2005;18:419-22. http://dx.doi.org/10.1111/j.1432-2277.2004.00055.x.
- Hardinger KL, Bohl DL, Schnitzler MA, Lockwood M, Storch GA, Brennan DC. A randomized, prospective, pharmacoeconomic trial of tacrolimus versus cyclosporine in combination with thymoglobulin in renal transplant recipients. Transplantation 2005;80:41-6. http://dx.doi.org/10.1097/01.TP.0000162980.68628.5A.
- Remuzzi G, Cravedi P, Costantini M, Lesti M, Ganeva M, Gherardi G, et al. Mycophenolate mofetil versus azathioprine for prevention of chronic allograft dysfunction in renal transplantation: the MYSS follow-up randomized, controlled clinical trial. JASN 2007;18:1973-85. http://dx.doi.org/10.1681/ASN.2006101153.
- Zadrazil J, Horak P, Strebl P, Krejci K, Kajabova M, Schneiderka P, et al. In vivo oxidized low-density lipoprotein (ox-LDL) aopp and tas after kidney transplantation: a prospective, randomized 1 year study comparing cyclosporine A and tacrolimus based regiments. Biomed Papo Med Fac Univ Palacký 2012;156:14-20. http://dx.doi.org/10.5507/bp.2012.008.
- Rowshani AT, Scholten EM, Bemelman F, Eikmans M, Idu M, Roos-van Groningen MC, et al. No difference in degree of interstitial Sirius red-stained area in serial biopsies from area under concentration-over-time curves-guided cyclosporine versus tacrolimus-treated renal transplant recipients at 1 year. JASN 2006;17:305-12. http://dx.doi.org/10.1681/ASN.2005030249.
- Weimer R, Susal C, Yildiz S, Staak A, Pelzl S, Renner F, et al. Post-transplant sCD30 and neopterin as predictors of chronic allograft nephropathy: impact of different immunosuppressive regimens. Am J Transplant 2006;6:1865-74. http://dx.doi.org/10.1111/j.1600-6143.2006.01407.x.
- Oh CK, Huh KH, Lee JS, Cho HR, Kim YS. Safety and efficacy of conversion from twice-daily tacrolimus to once-daily tacrolimus one month after transplantation: randomized controlled trial in adult renal transplantation. Yonsei Med J 2014;55:1341-7. http://dx.doi.org/10.3349/ymj.2014.55.5.1341.
- Ciancio G, Burke GW, Gaynor JJ, Roth D, Sageshima J, Kupin W, et al. Randomized trial of mycophenolate mofetil versus enteric-coated mycophenolate sodium in primary renal transplant recipients given tacrolimus and daclizumab/thymoglobulin: 1 year follow-up. Transplantation 2008;86:67-74. http://dx.doi.org/10.1097/TP.0b013e3181734b4a.
- Tedesco-Silva H, Cibrik D, Johnston T, Lackova E, Mange K, Panis C, et al. Everolimus plus reduced-exposure CsA versus mycophenolic acid plus standard-exposure CsA in renal-transplant recipients. Am J Transplant 2010;10:1401-13. http://dx.doi.org/10.1111/j.1600-6143.2010.03129.x.
- Barsoum RS, Morsey AA, Iskander IR, Morgan MM, Fayad TM, Atalla NT, et al. The Cairo Kidney Center protocol for rapamycin-based sequential immunosuppression in kidney transplant recipients: 2-year outcomes. Exp Clin Transplant 2007;5:649-57.
- Stallone G, Di Paolo S, Schena A, Infante B, Battaglia M, Ditonno P, et al. Addition of sirolimus to cyclosporine delays the recovery from delayed graft function but does not affect 1-year graft function. J Am Soc Nephrol 2004;15:228-33. http://dx.doi.org/10.1097/01.ASN.0000102469.32182.8C.
- Anil Kumar MS, Heifets M, Fyfe B, Saaed MI, Moritz MJ, Parikh MH, et al. Comparison of steroid avoidance in tacrolimus/mycophenolate mofetil and tacrolimus/sirolimus combination in kidney transplantation monitored by surveillance biopsy. Transplantation 2005;80:807-14. http://dx.doi.org/10.1097/01.tp.0000173378.28790.0b.
- Mendez R, Gonwa T, Yang HC, Weinstein S, Jensik S, Steinberg S. A prospective, randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 1 year. Transplantation 2005;80:303-9. http://dx.doi.org/10.1097/01.tp.0000167757.63922.42.
- Sampaio EL, Pinheiro-Machado PG, Garcia R, Felipe CR, Park SI, Casarini DE, et al. Mycophenolate mofetil vs. sirolimus in kidney transplant recipients receiving tacrolimus-based immunosuppressive regimen. Clin Transplant 2008;22:141-9. http://dx.doi.org/10.1111/j.1399-0012.2007.00756.x.
- Gelens MA, Christiaans MH, Heurn EL, Berg-Loonen EP, Peutz-Kootstra CJ, Hooff JP. High rejection rate during calcineurin inhibitor-free and early steroid withdrawal immunosuppression in renal transplantation. Transplantation 2006;82:1221-3. http://dx.doi.org/10.1097/01.tp.0000232688.76018.19.
- Van Gurp E, Bustamante J, Franco A, Rostaing L, Becker T, Rondeau E, et al. Comparable renal function at 6 months with tacrolimus combined with fixed-dose sirolimus or MMF: results of a randomized multicenter trial in renal transplantation. J Transplant 2010. http://dx.doi.org/10.1155/2010/731426.
- Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. JASN 2007;18:1007-18. http://dx.doi.org/10.1681/ASN.2006101143.
- Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramawicz D, et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation 2000;69:1252-60. http://dx.doi.org/10.1097/00007890-200004150-00009.
- Martinez-Mier G, Mendez-Lopez MT, Budar-Fernandez LF, Estrada-Oros J, Franco-Abaroa R, George-Micelli E, et al. Living related kidney transplantation without calcineurin inhibitors: initial experience in a Mexican center. Transplantation 2006;82:1533-6. http://dx.doi.org/10.1097/01.tp.0000235823.09788.f6.
- Nafar M, Alipour B, Ahmadpoor P, Pour-Reza-Gholi F, Samadian F, Samavat S, et al. Sirolimus versus calcineurin inhibitor-based immunosuppressive therapy in kidney transplantation: a 4-year follow-up. Iran J Kidney Dis 2012;6:300-6.
- Silva HT, Felipe CR, Garcia VD, Neto ED, Filho MA, Contieri FLC, et al. Planned randomized conversion from tacrolimus to sirolimus-based immunosuppressive regimen in de novo kidney transplant recipients. Am J Transplant 2013;13:3155-63. http://dx.doi.org/10.1111/ajt.12481.
- Hamdy AF, El-Agroudy AE, Bakr MA, Mostafa A, El-Baz M, El-Shahawy el M, et al. Comparison of sirolimus with low-dose tacrolimus versus sirolimus-based calcineurin inhibitor-free regimen in live donor renal transplantation. Am J Transplant 2005;5:2531-8. http://dx.doi.org/10.1111/j.1600-6143.2005.01064.x.
- Chen KH, Tsai MK, Lai IR, Lin Wu FL, Hu RH, Lee PH. Favorable results of concomitant tacrolimus and sirolimus therapy in Taiwanese renal transplant recipients at 12 months. J Formos Med Assoc 2008;107:533-9. http://dx.doi.org/10.1016/S0929-6646(08)60166-7.
- Anil Kumar MS, Irfan Saeed M, Ranganna K, Malat G, Sustento-Reodica N, Kumar AM, et al. Comparison of four different immunosuppression protocols without long-term steroid therapy in kidney recipients monitored by surveillance biopsy: five-year outcomes. Transpl Immunol 2008;20:32-4. http://dx.doi.org/10.1016/j.trim.2008.08.005.
- Albano L, Banas B, Klempnauer JL, Glyda M, Viklicky O, Kamar N. OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation 2013;96:897-903. http://dx.doi.org/10.1097/TP.0b013e3182a203bd.
- Salvadori M, Holzer H, De Mattos A, Sollinger H, Arns W, Oppenheimer F, et al. Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant 2004;4:231-6. http://dx.doi.org/10.1046/j.1600-6143.2003.00337.x.
- Vincenti F. Costimulation blockade with belatacept in renal transplantation: reply. N Engl J Med 2005;353. http://dx.doi.org/10.1056/NEJM200511103531919.
- Ferguson R, Grinyó J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, et al. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant 2011;11:66-7. http://dx.doi.org/10.1111/j.1600-6143.2010.03338.x.
- Flechner SM, Goldfarb D, Modlin C, Feng JY, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporin. Transplantation 2002;74:1070-6. http://dx.doi.org/10.1097/00007890-200210270-00002.
- Kyllönen LE, Eklund BH, Pesonen EJ, Salmela KT. Single bolus antithymocyte globulin versus basiliximab induction in kidney transplantation with cyclosporine triple immunosuppression: efficacy and safety. Transplantation 2007;84:75-82. http://dx.doi.org/10.1097/01.tp.0000268084.64888.f3.
- Vacher-Coponat H, Moal V, Indreies M, Purgus R, Loundou A, Burtey S, et al. A randomized trial with steroids and antithymocyte globulins comparing cyclosporine/azathioprine versus tacrolimus/mycophenolate mofetil (CATM2) in renal transplantation. Transplantation 2012;93:437-43. http://dx.doi.org/10.1097/TP.0b013e31824215b7.
- Hernández D, Miquel R, Porrini E, Fernández A, González-Posada JM, Hortal L, et al. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine-based immunosuppression. Transplantation 2007;84:706-14. http://dx.doi.org/10.1097/01.tp.0000282872.17024.b7.
- Takahashi K, Uchida K, Yoshimura N, Takahara S, Teraoka S, Teshima R, et al. Efficacy and safety of concentration-controlled everolimus with reduced-dose cyclosporine in Japanese de novo renal transplant patients: 12-month results. Transplant Res 2013;2. http://dx.doi.org/10.1186/2047-1440-2-14.
- Budde K, Becker T, Arns W. Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet 2011;377:837-47. http://dx.doi.org/10.1016/S0140-6736(10)62318-5.
- Mjörnstedt L, Sørensen SS, Zur Mühlen B, Jespersen B, Hansen JM, Bistrup C, et al. Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. Am J Transplant 2012;12:2744-53. http://dx.doi.org/10.1111/j.1600-6143.2012.04162.x.
- Büchler M, Caillard S, Barbier S, Thervet E, Toupance O, Mazouz H, et al. Sirolimus versus cyclosporine in kidney recipients receiving thymoglobulin, mycophenolate mofetil and a 6-month course of steroids. Am J Transplant 2007;7:522-31. http://dx.doi.org/10.1111/j.1600-6143.2007.01976.x.
- Heilman RL, Younan K, Wadei HM, Mai ML, Reddy KS, Chakkera HA, et al. Results of a prospective randomized trial of sirolimus conversion in kidney transplant recipients on early corticosteroid withdrawal. Transplantation 2011;92:767-73. http://dx.doi.org/10.1097/TP.0b013e31822805d7.
- Charpentier B, Groth CG, Bäckman L, Morales JM, Calne R, Kreis H, et al. Bicêtre hospital experience with sirolimus-based therapy in human renal transplantation: the Sirolimus European Renal Transplant Study. Transplant Proc 2003;35:S58-61. http://dx.doi.org/10.1016/S0041-1345(03)00213-6.
- Brennan DC, Daller JA, Lake KD, Cibrik D, Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 2006;355:1967-77. http://dx.doi.org/10.1056/NEJMoa060068.
- Merville P, Berge F, Deminiere C, Morel D, Chong G, Durand D, et al. Lower incidence of chronic allograft nephropathy at 1 year post-transplantation in patients treated with mycophenolate mofetil. Am J Transplant 2004;4:1769-75. http://dx.doi.org/10.1111/j.1600-6143.2004.00533.x.
- Wlodarczyk Z, Walaszewski J, Perner F, Vítko S, Ostrowski M, Bachleda P, et al. Steroid withdrawal at 3 months after kidney transplantation: a comparison of two tacrolimus-based regimens. Transpl Int 2005;18:157-62. http://dx.doi.org/10.1111/j.1432-2277.2004.00011.x.
- Wlodarczyk Z, Squifflet JP, Ostrowski M, Rigotti P, Stefoni S, Citterio F, et al. Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: a randomized, open-label trial. Am J Transplant 2009;9:2505-13. http://dx.doi.org/10.1111/j.1600-6143.2009.02794.x.
- Tsuchiya T, Ishida H, Tanabe T, Shimizu T, Honda K, Omoto K, et al. Comparison of pharmacokinetics and pathology for low-dose tacrolimus once-daily and twice-daily in living kidney transplantation: prospective trial in once-daily versus twice-daily tacrolimus. Transplantation 2013;96:198-204. http://dx.doi.org/10.1097/TP.0b013e318296c9d5.
- Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 2010;10:547-57. http://dx.doi.org/10.1111/j.1600-6143.2010.03016.x.
- Lorber MI, Mulgaonkar S, Butt KM, Elkhammas E, Mendez R, Rajagopalan PR, et al. Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: a 3-year randomized, multicenter, phase III study. Transplantation 2005;80:244-52. http://dx.doi.org/10.1097/01.TP.0000164352.65613.24.
- Bertoni E, Larti A, Rosso G, Zanazzi M, Maria L, Salvadori M. Good outcomes with cyclosporine very low exposure with everolimus high exposure in renal transplant patients. J Nephrol 2011;24:613-18. http://dx.doi.org/10.5301/JN.2011.6247.
- Gallon L, Perico N, Dimitrov BD, Winoto J, Remuzzi G, Leventhal J, et al. Long-term renal allograft function on a tacrolimus-based, pred-free maintenance immunosuppression comparing sirolimus vs. MMF. Am J Transplant 2006;6:1617-23. http://dx.doi.org/10.1111/j.1600-6143.2006.01340.x.
- Durrbach A, Rostaing L, Tricot L, Ouali N, Wolf P, Pouteil-Noble C, et al. Prospective comparison of the use of sirolimus and cyclosporine in recipients of a kidney from an expanded criteria donor. Transplantation 2008;85:486-90. http://dx.doi.org/10.1097/TP.0b013e318160d3c9.
- Guba M, Pratschke J, Hugo C, Krämer BK, Nohr-Westphal C, Brockmann J, et al. Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short-term calcineurin inhibitor-based quadruple therapy in de novo renal transplant patients: one-year analysis of a randomized multicenter trial. Transplantation 2010;90:175-83. http://dx.doi.org/10.1097/TP.0b013e3181e11798.
- Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, et al. A three-arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation 2003;75:844-51. http://dx.doi.org/10.1097/01.TP.0000056635.59888.EF.
- Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, et al. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant 2009;9:1115-23. http://dx.doi.org/10.1111/j.1600-6143.2009.02615.x.
- Vítko S, Klinger M, Salmela K, Wlodarczyk Z, Tydèn G, Senatorski G, et al. Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: results of the atlas study. Transplantation 2005;80:1734-41. http://dx.doi.org/10.1097/01.tp.0000188300.26762.74.
- Larsen C, Alberu J, Massari P, Acevedo RR, Kamar N, Lin CS, et al. 4-year results from the long-term extension of the belatacept BENEFIT study. Am J Transplant 2012;12.
- Chadban SJ, Eris JM, Kanellis J, Pilmore H, Lee PC, Lim SK, et al. A randomized, controlled trial of everolimus-based dual immunosuppression versus standard of care in de novo kidney transplant recipients. Transpl Int 2014;27:302-11. http://dx.doi.org/10.1111/tri.12252.
- Ulsh PJ, Yang HC, Holman MJ, Ahsan N. New strategies using ‘low-dose’ mycophenolate mofetil to reduce acute rejection in patients following kidney transplantation. J Transpl Coord 1999;9:114-18. http://dx.doi.org/10.7182/prtr.1.9.2.t4l566l63m0g1126.
- Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant 2006;6:514-22. http://dx.doi.org/10.1111/j.1600-6143.2005.01177.x.
- Flechner SM, Glyda M, Cockfield S, Grinyó J, Legendre C, Russ G, et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant 2011;11:1633-44. http://dx.doi.org/10.1111/j.1600-6143.2011.03573.x.
- Vincenti F, Blancho G, Durrbach A, Friend P, Grinyo J, Halloran PF, et al. Five-year safety and efficacy of belatacept in renal transplantation. JASN 2010;21:1587-96. http://dx.doi.org/10.1681/ASN.2009111109.
- Heilman RL, Cortese C, Geiger XJ, Younan K, Wadei HM, Mai ML, et al. Impact of early conversion from tacrolimus to sirolimus on chronic allograft changes in kidney recipients on rapid steroid withdrawal. Transplantation 2012;93:47-53. http://dx.doi.org/10.1097/TP.0b013e3182394cb3.
- Samsel R, Pliszczyski J, Chmura A, Korczak G, Wodarczyk Z, Cieciura T, et al. Safety and efficacy of high dose ATG bolus administration on rewascularization in kidney graft patients – long term results. Ann Transplant 2008;13:32-9.
- Sollinger H, Kaplan B, Pescovitz MD, Philosophe B, Roza A, Brayman K, et al. Basiliximab versus antithymocyte globulin for prevention of acute renal allograft rejection. Transplantation 2001;72:1915-19. http://dx.doi.org/10.1097/00007890-200112270-00008.
- Sheashaa HA, Hamdy AF, Bakr MA, Abdelbaset SF, Ghoneim MA. Long-term evaluation of single bolus high dose ATG induction therapy for prophylaxis of rejection in live donor kidney transplantation. Int Urol Nephrol 2008;40:515-20. http://dx.doi.org/10.1007/s11255-007-9242-6.
- Vincenti F, Laskow DA, Neylan JF, Mendez R, Matas AJ. One-year follow-up of an open-label trial of FK506 for primary kidney transplantation. A report of the U.S. Multicenter FK506 Kidney Transplant Group. Transplantation 1996;61:1576-81. http://dx.doi.org/10.1097/00007890-199606150-00005.
- Mayer AD. Four-year follow-up of the European Tacrolimus Multicenter Renal Study. Transplant Proc 1999;31:27-8. http://dx.doi.org/10.1016/S0041-1345(99)00789-7.
- Mayer AD. Chronic rejection and graft half-life: five-year follow-up of the European Tacrolimus Multicenter Renal Study. Transplant Proc 2002;34:1491-2. http://dx.doi.org/10.1016/S0041-1345(02)02942-1.
- Krämer BK, Montagnino G, Castillo D, Margreiter R, Sperschneider H, Olbricht CJ, et al. Efficacy and safety of tacrolimus compared with cyclosporin A microemulsion in renal transplantation: 2 year follow-up results. Nephrol Dial Transplant 2005;20:968-73. http://dx.doi.org/10.1093/ndt/gfh739.
- Krämer BK, Castillo D, Margreiter R, Sperschneider H, Olbricht CJ, Ortuno J, et al. Efficacy and safety of tacrolimus compared with ciclosporin A in renal transplantation: three-year observational results. Nephrol Dial Transplant 2008;23:2386-92. http://dx.doi.org/10.1093/ndt/gfn004.
- Murphy GJ, Waller JR, Sandford RS, Furness PN, Nicholson ML. Randomized clinical trial of the effect of microemulsion cyclosporin and tacrolimus on renal allograft fibrosis. Br J Surg 2003;90:680-6. http://dx.doi.org/10.1002/bjs.4134.
- Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant 2005;5:582-94. http://dx.doi.org/10.1111/j.1600-6143.2005.00742.x.
- Mathew T. A blinded, long-term, randomized multicentre study of mycophenolate mofetil in cadaveric renal transplantation: results at three years. Transplantation 1998;65:1450-4. http://dx.doi.org/10.1097/00007890-199806150-00007.
- Clayton PA, McDonald SP, Chapman JR, Chadban SJ. Mycophenolate versus azathioprine for kidney transplantation: a 15-year follow-up of a randomized trial. Transplantation 2012;94:152-8. http://dx.doi.org/10.1097/TP.0b013e31825475a3.
- Remuzzi G, Lesti M, Gotti E, Ganeva M, Dimitrov BD, Ene-Iordache B, et al. Mycophenolate mofetil versus azathioprine for prevention of acute rejection in renal transplantation (MYSS): a randomised trial. Lancet 2004;364:503-12. http://dx.doi.org/10.1016/S0140-6736(04)16808-6.
- Wlodarczyk Z, Walaszewski J, Perner F, Vítko S, Ostrowski M, Bachleda P, et al. Freedom from rejection and stable kidney function are excellent criteria for steroid withdrawal in tacrolimus-treated kidney transplant recipients. Ann Transplant 2002;7:28-31.
- Weimer R, Susal C, Yildiz S, Streller S, Pelzl S, Staak A, et al. sCD30 and neopterin as risk factors of chronic renal transplant rejection: impact of cyclosporine A, tacrolimus, and mycophenolate mofetil. Transplant Proc 2005;37:1776-8. http://dx.doi.org/10.1016/j.transproceed.2005.02.088.
- Ciancio G, Gaynor JJ, Zarak A, Sageshima J, Guerra G, Roth D, et al. Randomized trial of mycophenolate mofetil versus enteric-coated mycophenolate sodium in primary renal transplantation with tacrolimus and steroid avoidance: four-year analysis. Transplantation 2011;91:1198-205. http://dx.doi.org/10.1097/TP.0b013e3182003d76.
- Pestana JO, Grinyo JM, Vanrenterghem Y, Becker T, Campistol JM, Florman S, et al. Three-year outcomes from BENEFIT-EXT: a phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant 2012;12:630-9. http://dx.doi.org/10.1111/j.1600-6143.2011.03914.x.
- Charpentier B, Medina Pestana JO, Rial M del C, Rostaing L, Grinyo J, Vanrenterghem Y, et al. Long-term exposure to belatacept in recipients of extended criteria donor kidneys. Am J Transplant 2013;13:2884-91. http://dx.doi.org/10.1111/ajt.12459.
- Vítko S, Margreiter R, Weimar W, Dantal J, Viljoen HG, Li Y, et al. Everolimus (certican) 12-month safety and efficacy versus mycophenolate mofetil in de novo renal transplant recipients. Transplantation 2004;78:1532-40. http://dx.doi.org/10.1097/01.TP.0000141094.34903.54.
- Vítko S, Margreiter R, Weimar W, Dantal J, Kuypers D, Winkler M, et al. Three-year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. Am J Transplant 2005;5:2521-30. http://dx.doi.org/10.1111/j.1600-6143.2005.01063.x.
- Budde K, Lehner F, Sommerer C, Arns W, Reinke P, Eisenberger U, et al. Conversion from cyclosporine to everolimus at 4.5 months posttransplant: 3-year results from the randomized ZEUS study. Am J Transplant 2012;12:1528-40. http://dx.doi.org/10.1111/j.1600-6143.2012.03994.x.
- Liefeldt L, Brakemeier S, Glander P, Waiser J, Lachmann N, Schönemann C, et al. Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant 2012;12:1192-8. http://dx.doi.org/10.1111/j.1600-6143.2011.03961.x.
- Gonwa T, Mendez R, Yang HC, Weinstein S, Jensik S, Steinberg S. Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 months. Transplantation 2003;75:1213-20. http://dx.doi.org/10.1097/01.TP.0000062837.99400.60.
- Chhabra D, Skaro AI, Leventhal JR, Dalal P, Shah G, Wang E, et al. Long-term kidney allograft function and survival in prednisone-free regimens: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. Clin J Am Soc Nephrol 2012;7:504-12. http://dx.doi.org/10.2215/CJN.06940711.
- Flechner SM, Kurian SM, Solez K, Cook DJ, Burke JT, Rollin H, et al. De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. Am J Transplant 2004;4:1776-85. http://dx.doi.org/10.1111/j.1600-6143.2004.00627.x.
- Flechner SM, Goldfarb D, Solez K, Modlin CS, Mastroianni B, Savas K, et al. Kidney transplantation with sirolimus and mycophenolate mofetil-based immunosuppression: 5-year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation 2007;83:883-92. http://dx.doi.org/10.1097/01.tp.0000258586.52777.4c.
- Ruggenenti P, Perico N, Gotti E, Cravedi P, D’Agati V, Gagliardini E, et al. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation 2007;84:956-64. http://dx.doi.org/10.1097/01.tp.0000284808.28353.2c.
- Servais A, Meas-Yedid V, Toupance O, Lebranchu Y, Thierry A, Moulin B, et al. Interstitial fibrosis quantification in renal transplant recipients randomized to continue cyclosporine or convert to sirolimus. Am J Transplant 2009;9:2552-60. http://dx.doi.org/10.1111/j.1600-6143.2009.02803.x.
- Lebranchu Y, Thierry A, Thervet E, Büchler M, Etienne I, Westeel PF, et al. Efficacy and safety of early cyclosporine conversion to sirolimus with continued MMF-four-year results of the postconcept study. Am J Transplant 2011;11:1665-75. http://dx.doi.org/10.1111/j.1600-6143.2011.03637.x.
- Joannidès R, Monteil C, Ligny BH, Westeel PF, Iacob M, Thervet E, et al. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. Am J Transplant 2011;11:2414-22. http://dx.doi.org/10.1111/j.1600-6143.2011.03697.x.
- Lebranchu Y, Snanoudj R, Toupance O, Weestel PF, Hurault de Ligny B, Büchler M, et al. Five-year results of a randomized trial comparing de novo sirolimus and cyclosporine in renal transplantation: the spiesser study. Am J Transplant 2012;12:1801-10. http://dx.doi.org/10.1111/j.1600-6143.2012.04036.x.
- Joannides R, Etienne I, Iacob M, Ligny BH, Barbier S, Bellien J, et al. Comparative effects of sirolimus and cyclosporin on conduit arteries endothelial function in kidney recipients. Transpl Int 2010;23:1135-43. http://dx.doi.org/10.1111/j.1432-2277.2010.01122.x.
- Campistol JM, Holt DW, Epstein S, Gioud-Paquet M, Rutault K, Burke JT. Bone metabolism in renal transplant patients treated with cyclosporine or sirolimus. Transpl Int 2005;18:1028-35. http://dx.doi.org/10.1111/j.1432-2277.2005.00163.x.
- Stegall MD, Larson TS, Prieto M, Gloor J, Textor S, Nyberg S, et al. Kidney transplantation without calcineurin inhibitors using sirolimus. Transplant Proc 2003;35. http://dx.doi.org/10.1016/S0041-1345(03)00226-4.
- Hamdy AF, Bakr MA, Ghoneim MA. Long-term efficacy and safety of a calcineurin inhibitor-free regimen in live-donor renal transplant recipients. JASN 2008;19:1225-32. http://dx.doi.org/10.1681/ASN.2007091001.
- Hamdy AF, Bakr MA, Ghoneim MA. Proteinuria among primarily sirolimus treated live-donor renal transplant recipients’ long-term experience. Exp Clin Transplant 2010;8:283-91.
- Groth CG, Backman L, Morales JM, Calne R, Kreis H, Lang P, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation 1999;67:1036-42. http://dx.doi.org/10.1097/00007890-199904150-00017.
- Ekberg H, Mamelok RD, Pearson TC, Vincenti F, Tedesco-Silva H, Daloze P. The challenge of achieving target drug concentrations in clinical trials: experience from the symphony study. Transplantation 2009;87:1360-6. http://dx.doi.org/10.1097/TP.0b013e3181a23cb2.
- Ekberg H, Bernasconi C, Nöldeke J, Yussim A, Mjörnstedt L, Erken U, et al. Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the Symphony study. Nephrol Dial Transplant 2010;25:2004-10. http://dx.doi.org/10.1093/ndt/gfp778.
- Demirbas A, Hugo C, Grinyó J, Frei U, Gürkan A, Marcén R, et al. Low toxicity regimens in renal transplantation: a country subset analysis of the Symphony study. Transpl Int 2009;22:1172-81. http://dx.doi.org/10.1111/j.1432-2277.2009.00937.x.
- Frei U, Daloze P, Vítko S, Klempnauer J, Reyes-Acevedo R, Titiz I, et al. Acute rejection in low-toxicity regimens: clinical impact and risk factors in the Symphony study. Clin Transplant 2010;24:500-9. http://dx.doi.org/10.1111/j.1399-0012.2009.01093.x.
- Claes K, Meier-Kriesche HU, Schold JD, Vanrenterghem Y, Halloran PF, Ekberg H. Effect of different immunosuppressive regimens on the evolution of distinct metabolic parameters: evidence from the Symphony study. Nephrol Dial Transplant 2012;27:850-7. http://dx.doi.org/10.1093/ndt/gfr238.
- Kumar MS, Heifets M, Moritz MJ, Saeed MI, Khan SM, Fyfe B, et al. Safety and efficacy of steroid withdrawal two days after kidney transplantation: analysis of results at three years. Transplantation 2006;81:832-9. http://dx.doi.org/10.1097/01.tp.0000203558.34739.c6.
- Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons Ltd; 2008.
- Clayton P, McDonald S, Chapman J, Chadban S. Mycophenolate vs. azathioprine for kidney transplantation: 15 year follow-up of a randomized trial. Nephrology 2011;16.
- Kumar N, Manimaran R, Williams C, Ravanan R. Tacrolimus preserves renal function better than cyclosporin at 10 years: long term results of a randomised controlled trial. Am J Transplant 2009;9.
- Krämer BK, Charpentier B, Bäckman L, Tedesco-Silva HT, Mondragon-Ramirez G, Cassuto-Viguier E, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant 2010;10:2632-43. http://dx.doi.org/10.1111/j.1600-6143.2010.03256.x.
- Krämer BK. NDT Plus 2010;3.
- Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med 2005;353:770-81. http://dx.doi.org/10.1056/NEJMoa050085.
- Durrbach A, Larsen CP, Medina Pestana J, Vanrenterghem Y, Vincenti F, Florman S, et al. Belatacept vs. cyclosporine in ECD kidney transplants: two-year outcomes from the BENEFIT-EXT study. NDT Plus 2010;3. http://dx.doi.org/10.1097/00007890-201007272-00303.
- Stallone G, Di Paolo S, Schena A, Infante B, Grandaliano G, Battaglia M, et al. Early withdrawal of cyclosporine A improves 1-year kidney graft structure and function in sirolimus-treated patients. Transplantation 2003;75:998-1003. http://dx.doi.org/10.1097/01.TP.0000057240.95073.35.
- Flechner SM, Friend PJ, Brockmann J, Ismail HR, Zilvetti M, Goldfarb D, et al. Alemtuzumab induction and sirolimus plus mycophenolate mofetil maintenance for CNI and steroid-free kidney transplant immunosuppression. Am J Transplant 2005;5:3009-14. http://dx.doi.org/10.1111/j.1600-6143.2005.01123.x.
- Raofi V, Holman DM, Coady N, Vazquez E, Dunn TB, Bartholomew AM, et al. A prospective randomized trial comparing the efficacy of tacrolimus versus cyclosporine in black recipients of primary cadaveric renal transplants. Am J Surg 1999;177:299-302. http://dx.doi.org/10.1016/S0002-9610(99)00042-2.
- Dias SW, Sutton N, Ades A. NICE DSU Technical Support Document 1: Introduction to Evidence Synthesis for Decision making. London: NICE; 2011.
- Mourad G, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. Sequential protocols using basiliximab versus antithymocyte globulins in renal-transplant patients receiving mycophenolate mofetil and steroids. Transplantation 2004;78:584-90. http://dx.doi.org/10.1097/01.TP.0000129812.68794.CC.
- Alloway RR, Sadaka B, Trofe-Clark J, Wiland A, Bloom RD. A randomized pharmacokinetic study of generic tacrolimus versus reference tacrolimus in kidney transplant recipients. Am J Transplant 2012;12:2825-31. http://dx.doi.org/10.1111/j.1600-6143.2012.04174.x.
- Bloom RD, Trofe-Clark J, Wiland A, Alloway RR. A randomized, crossover pharmacokinetic study comparing generic tacrolimus vs. the reference formulation in subpopulations of kidney transplant patients. Clin Transplant 2013;27:E685-93. http://dx.doi.org/10.1111/ctr.12256.
- Connor A, Prowse A, MacPhee I, Rowe PA. Generic tacrolimus in renal transplantation: trough blood concentration as a surrogate for drug exposure. Transplantation 2012;93:e45-6. http://dx.doi.org/10.1097/TP.0b013e318256dd13.
- Connor A, Prowse A, Newell P, Rowe PA. A single-centre comparison of the clinical outcomes at 6 months of renal transplant recipients administered Adoport or Prograf preparations of tacrolimus. Clin Kidney J 2013;6:21-8. http://dx.doi.org/10.1093/ckj/sfs154.
- Heavner MS, Tichy EM, Yazdi M, Formica RN, Jr, Kulkarni S, Emre S. Clinical outcomes associated with conversion from brand-name to generic tacrolimus in hospitalized kidney transplant recipients. Am J Health-Syst Pharm 2013;70:1507-12. http://dx.doi.org/10.2146/ajhp120783.
- Marfo K, Aitken S, Akalin E. Clinical outcomes after conversion from brand-name tacrolimus (prograf) to a generic formulation in renal transplant recipients: a retrospective cohort study. P&Amp;T 2013;38:484-8.
- McDevitt-Potter LM, Sadaka B, Tichy EM, Rogers CC, Gabardi S. A multicenter experience with generic tacrolimus conversion. Transplantation 2011;92:653-7. http://dx.doi.org/10.1097/TP.0b013e31822a79ad.
- Richards KR, Hager D, Muth B, Astor BC, Kaufman D, Djamali A. Tacrolimus trough level at discharge predicts acute rejection in moderately sensitized renal transplant recipients. Transplantation 2014;97:986-91. http://dx.doi.org/10.1097/TP.0000000000000149.
- Rosenborg S, Nordstrom A, Almquist T, Wennberg L, Barany P. Systematic conversion to generic tacrolimus in stable kidney transplant recipients. Clin Kidney J 2014;7:151-5. http://dx.doi.org/10.1093/ckj/sfu015.
- Spence MM, Nguyen LM, Hui RL, Chan J. Evaluation of clinical and safety outcomes associated with conversion from brand-name to generic tacrolimus in transplant recipients enrolled in an integrated health care system. Pharmacotherapy 2012;32:981-7. http://dx.doi.org/10.1002/phar.1130.
- Babu A, Ravanan R, Udayara. Adoport-V-Prograf in De-Novo Renal Transplants: Single Centre Experience n.d.
- Betmouni R, Bedi R, Duncan N, Galliford J, Goodall D, Owen J, et al. Switching Prograf® to Generic Tacrolimus (Adoport®) Is Safe and Cost Effective in Renal and Pancreas Transplants n.d.
- Chiu C, Miyashiro S. Pharmacist-managed conversion of Prograf to generic tacrolimus in kidney and liver transplant patients with stable allograft function. Hawaii J Med Public Health 2012;7:148-50.
- Crowther BR, Dobie H, Brady R, Hall R, Maxwell P. Conversion of Prograf® to generic tacrolimus in stable renal transplant recipients. Am J Transplant 2012;12.
- Dick TB, Raines AA, Van der Werf W, Alonso D, Fujita S, Stinson JB. Comparison of dose requirements of Sandoz™ generic tacrolimus with brand Prograf® in kidney transplant recipients. Am J Transplant 2011;11.
- Heldenbrand S, Jones GD, Bornhorst J, Payakachat N. Extended comparison of therapeutic treatment outcomes of de novo liver and kidney transplant recipients with generic tacrolimus (Sandoz™ or Brand Name (Prograf®). Am J Transplant 2012;12.
- Jogia P, Oskiera D, Booth S, McKane W. Generic switch of tacrolimus in prevalent kidney transplant recipients. Am J Transplant 2013;13.
- Kendrew P, Edey M, Bhandan S. An Investigation into the Differences Between Adoport® and Prograf® in Kidney Transplant Recipients n.d.
- Qazi YA, Bolonesi R, Monis T, Smogorzewski M, Sheikh R, Alexopoulos S, et al. Effect of generic tacrolimus on the incidence of acute rejection in kidney transplant recipients: a single center experience. Am J Transplant 2012;12:205-6.
- Sharma H, El-Bakry A, Wong C. Assessment of Post Transplantation Results of Adoport Compared to Prograf: A Short Sample Study n.d.
- Shiu K, Rezk T, Henry J. Programmed Switching of Renal Transplant Recipients from Branded to Generic Tacrolimus Is Safe, Well-Tolerated and Cost-Effective n.d.
- Siddiqi N, Lu A, Jones T, Akalin E, Marfo K. Clinical and economic outcomes: de novo use of FDA-approved bioequivalent formulation of generic tacrolimus versus brand tacrolimus (Prograf). Am J Transplant 2011;11.
- Storey R, Hossain MA, Shrivastava S, Popoola J, Heap S, MacPhee I. Tacrolimus dosing in renal transplant recipients following introduction of a generic preparation. Transpl Int 2013;26.
- Venkataramanan R, Raghu V, Momper J, Schonder K, Shapiro R, Humar A. The effect of generic substitution of tacrolimus in liver and kidney transplant recipients. Transplantation 2012;94. http://dx.doi.org/10.1097/00007890-201211271-01465.
- Wilcock M, Self P, Dickinson S, Johnston P, Stratton J, Parry R. Switching branded immunosuppressants in a stable renal transplant population: a single centre experience. Int J Pharm Pract 2013;21.
- Marsen T. How safe is conversion from tacrolimus to its generic drug? A single center experience. Open. J Nephrol 2012;2:72-7. http://dx.doi.org/10.4236/ojneph.2012.24012.
- Silva HT, Yang HC, Abouljoud M, Kuo PC, Wisemandle K, Bhattacharya P, et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant 2007;7:595-608. http://dx.doi.org/10.1111/j.1600-6143.2007.01661.x.
- Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007;357:2562-75. http://dx.doi.org/10.1056/NEJMoa067411.
- Abou-Jaoude MM, Irani-Hakime N, Ghantous I, Najm R, Afif C, Almawi WY. Cyclosporine microemulsion (Neoral) versus tacrolimus (FK506) as maintenance therapy in kidney transplant patients. Transplant Proc 2003;35:2748-9. http://dx.doi.org/10.1016/j.transproceed.2003.09.036.
- Abou-Jaoude MM, Najm R, Shaheen J, Nawfal N, Abboud S, Alhabash M, et al. Tacrolimus (FK506) versus cyclosporine microemulsion (neoral) as maintenance immunosuppression therapy in kidney transplant recipients. Transplant Proc 2005;37:3025-8. http://dx.doi.org/10.1016/j.transproceed.2005.08.040.
- Busque S, Shoker A, Landsberg D, McAlister V, Halloran P, Shapiro J, et al. Canadian multicentre trial of tacrolimus/azathioprine/steroids versus tacrolimus/mycophenolate mofetil/steroids versus neoral/mycophenolate mofetil/steroids in renal transplantation. Transplant Proc 2001;33:1266-7. http://dx.doi.org/10.1016/S0041-1345(00)02471-4.
- Johnson C, Ahsan N, Gonwa T, Halloran P, Stegall M, Hardy M, et al. Randomized trial of tacrolimus (Prograf) in combination with azathioprine or mycophenolate mofetil versus cyclosporine (Neoral) with mycophenolate mofetil after cadaveric kidney transplantation. Transplantation 2000;69:834-41. http://dx.doi.org/10.1097/00007890-200003150-00028.
- Garcia DM, Gago JM, Mendiluce A, Gordillo R, Bustamente J. Tacrolimus-basiliximab versus cyclosporine-basiliximab in renal transplantation ‘de novo’: acute rejection and complications. Transplant Proc 2003;35:1694-6. http://dx.doi.org/10.1016/S0041-1345(03)00576-1.
- Morris-Stiff G, Singh J, Ostrowski K, Balaji V, Moore R, Darby C, et al. Prospective randomized study comparing FK 506 (Prograf) and cyclosporine a (Neoral) as primary immunosuppression in cadaveric renal transplants at a single institution: interim report of the first 80 cases. Transplant Proc 1998;30:1295-6. http://dx.doi.org/10.1016/S0041-1345(98)00248-6.
- Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 2007;7:1506-14. http://dx.doi.org/10.1111/j.1600-6143.2007.01749.x.
- Wang XH, Tang XD, Xu D. Tacrolimus vs. CyA neoral in combination with MMF and steroids after cadaveric renal transplantation. Transplant Proc 2000;32:1702-3. http://dx.doi.org/10.1016/S0041-1345(00)01408-1.
- White SA, Jain S, Williams ST, Doughman T, Hayes P, Murphy G, et al. Randomized trial comparing neoral and tacrolimus immunosuppression for recipients of renal transplants procured from different donor groups. Transplant Proc 2000;32. http://dx.doi.org/10.1016/S0041-1345(00)00910-6.
- Williams S, White S, Jain S, Doughman T, Hayes P, Knight A. A randomised trial comparing Neoral (ciclosporin) and tacrolimus immunosuppression for recipients of renal transplants procured from different donor groups. Br J Surg 1998;86.
- Glotz D, Charpentier B, Abramovicz D, Lang P, Rostaing L, Rifle G, et al. Thymoglobulin induction and sirolimus versus tacrolimus in kidney transplant recipients receiving mycophenolate mofetil and steroids. Transplantation 2010;89:1511-17. http://dx.doi.org/10.1097/TP.0b013e3181db09e4.
- Chhabra D, Alvarado A, Dalal P, Leventhal J, Wang C, Sustento-Reodica N, et al. Impact of calcineurin-inhibitor conversion to mTOR inhibitor on renal allograft function in a prednisone-free regimen. Am J Transplant 2013;13:2902-11. http://dx.doi.org/10.1111/ajt.12437.
- Lo A, Egidi MF, Gaber LW, Amiri HS, Vera S, Nezakatgoo N, et al. Comparison of sirolimus-based calcineurin inhibitor-sparing and calcineurin inhibitor-free regimens in cadaveric renal transplantation. Transplantation 2004;77:1228-35. http://dx.doi.org/10.1097/01.TP.0000121504.69676.5E.
- Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized long-term trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (neoral) and sirolimus in renal transplantation. I. Drug interactions and rejection at 1 year. Transplantation 2004;77:244-51. http://dx.doi.org/10.1097/01.TP.0000101290.20629.DC.
- Ciancio . A randomized long-term trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (Neoral) and sirolimus in renal transplantation. 1. Drug interactions and rejection at 1 year. Transplantation 2004;77.
- Langer RM, Hené R, Vítko S, Christiaans M, Tedesco-Silva H, Ciechanowski K, et al. Everolimus plus early tacrolimus minimization: a phase III, randomized, open-label, multicentre trial in renal transplantation. Transpl Int 2012;25:592-60. http://dx.doi.org/10.1111/j.1432-2277.2012.01465.x.
- Chan L, Greenstein S, Hardy MA, Hartmann E, Bunnapradist S, Cibrik D, et al. Multicenter, randomized study of the use of everolimus with tacrolimus after renal transplantation demonstrates its effectiveness. Transplantation 2008;85:821-6. http://dx.doi.org/10.1097/TP.0b013e318166927b.
- Favi E, Silvestrini N, Salerno MP, Romagnoli J, Citterio F. Extended-release tacrolimus plus everolimus or micophenolate mofetil in deceased donor kidney transplant recipients: 6-month results of a prospective randomized clinical trial. Am J Transplant 2012;12:42-3.
- Ruiz JC, Sanchez Fructuoso A, Hernández D, Sanchez Plumed J, Fernandez A, Pastor Rodriguez A, et al. Better renal function with early everolimus (EVL) introduction and calcineurin inhibitor (CNI) withdrawal at third month in kidney recipients at month 12: results of the ERIC study. Transpl Int 2011;24.
- Abou-Jaoude MM, Ghantous I, Almawi WY. Tacrolimus (FK506) versus cyclosporin A microemulsion (Neoral) maintenance immunosuppression: effects on graft survival and function, infection, and metabolic profile following kidney transplantation (KT). Mol Immunol 2003;39:1095-100. http://dx.doi.org/10.1016/S0161-5890(03)00070-1.
- Cheung CY, Wong KM, Chan HW, Liu YL, Chan YH, Wong HS, et al. Paired kidney analysis of tacrolimus and cyclosporine microemulsion-based therapy in Chinese cadaveric renal transplant recipients. Transpl Int 2006;19:657-66. http://dx.doi.org/10.1111/j.1432-2277.2006.00335.x.
- Egfjord M, Ladefoged J, Olgaard K. Similar Frequency of Acute Rejection, Graft-and Patient Survival in Quadruple Therapy With Tacrolimus Versus Cyclosporin in Combination With Prednisone, Mycophenolate Mofetil, and ATGAM After Renal Allotransplantation n.d.
- El Haggan W, Barthe N, Vendrely B, Chauveau P, Berger F, Aparicio M, et al. One year evolution of bone mineral density in kidney transplant recipients receiving tacrolimus versus cyclosporine. Transplant Proc 2002;34:1817-18. http://dx.doi.org/10.1016/S0041-1345(02)03094-4.
- Liu B, Lin ZB, Ming CS, Zhang WJ, Chen ZS, Sha B, et al. Randomized trial of tacrolimus in combination with mycophenolate mofetil versus cyclosporine with mycophenolate mofetil in cadaveric renal transplant recipients with delayed graft function. Transplant Proc 2003;35:87-8. http://dx.doi.org/10.1016/S0041-1345(02)04003-4.
- Tsinalis D, Binet I, Dickenmann M, Steiger J, Brunner F, Thiel G. Cost of Medical Care After Renal Transplantation Comparing Cyclosporine-Mycophenolate to Tacrolimus-Azathioprine: A Randomised Controlled Study n.d.
- Yu L, Wang Y, Fu SJ, Cheng XJ. Clinical experience with prograf (Tacrolimus, FK 506) in Chinese patients after renal transplantation. Transplant Proc 2000;32:1709-10. http://dx.doi.org/10.1016/S0041-1345(00)01405-6.
- Nichelle L, Canet S, Garrigue V, Chong G, Mourad G. Arterial hypertension in renal transplant recipients treated with tacrolimus or cyclosporine-neoral. Transplant Proc 2002;34:2824-5. http://dx.doi.org/10.1016/S0041-1345(02)03530-3.
- Heering P, Ivens K, Aker S, Grabensee B. Distal tubular acidosis induced by FK506. Clin Transplant 1998;12:465-71.
- Ichimaru N, Takahara S, Kokado Y, Wang JD, Hatori M, Kameoka H, et al. Changes in lipid metabolism and effect of simvastatin in renal transplant recipients induced by cyclosporine or tacrolimus. Atherosclerosis 2001;158:417-23. http://dx.doi.org/10.1016/S0021-9150(01)00438-5.
- Salvadori M. Therapeutic equivalence of mycophenolate sodium versus mycophenolate mofetil in de novo renal transplant recipients. Transplant Proc 2001;33:3245-7. http://dx.doi.org/10.1016/S0041-1345(01)02379-X.
- Salvadori M, Holzer H, Civati G, Sollinger H, Lien B, Tomlanovich S, et al. Long-term administration of enteric-coated mycophenolate sodium (EC-MPS; myfortic) is safe in kidney transplant patients. Clin Nephrol 2006;66:112-19.
- Budde K, Curtis J, Knoll G, Chan L, Neumayer HH, Seifu Y, et al. Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant 2004;4:237-43. http://dx.doi.org/10.1046/j.1600-6143.2003.00321.x.
- Budde K, Knoll G, Curtis J, Kahana L, Pohanka E, Seifu Y, et al. Safety and efficacy after conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium: results of a 1-year extension study. Transplant Proc 2005;37:912-15. http://dx.doi.org/10.1016/j.transproceed.2004.12.048.
- Budde K, Knoll G, Curtis J, Chan L, Pohanka E, Gentil M, et al. Long-term safety and efficacy after conversion of maintenance renal transplant recipients from mycophenolate mofetil (MMF) to enteric-coated mycophenolate sodium (EC-MPA, myfortic). Nieren Hochdruck 2006;35:454-64.
- Shehata M, Bhandari S, Venkat-Raman G, Moore R, D’Souza R, Riad H, et al. Effect of conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium on maximum tolerated dose and gastrointestinal symptoms following kidney transplantation. Transpl Int 2009;22:821-30. http://dx.doi.org/10.1111/j.1432-2277.2009.00877.x.
- Ortega F, Sánchez-Fructuoso A, Cruzado JM, Gómez-Alamillo JC, Alarcón A, Pallardó L, et al. Gastrointestinal quality of life improvement of renal transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium drugs or agents: mycophenolate mofetil and enteric-coated mycophenolate sodium. Transplantation 2011;92:426-32. http://dx.doi.org/10.1097/TP.0b013e31822527ca.
- Langone A, Doria C, Greenstein S, Narayanan M, Ueda K, Sankari B, et al. Does reduction in mycophenolic acid dose compromise efficacy regardless of tacrolimus exposure level? An analysis of prospective data from the Mycophenolic Renal Transplant (MORE) Registry. Clin Transplant 2013;27:15-24. http://dx.doi.org/10.1111/j.1399-0012.2012.01694.x.
- Chan L, Shihab F, Pankewycz O, Doria C, Wiland A, McCague K, et al. Mycophenolic acid (MPA) dosing: effect on efficacy to 4 years after kidney transplantation in the mycophenolic acid observational renal transplant (MORE) study. Am J Transplant 2013;13.
- Shah T, Vu D, Cho Y, Mateo R, Huang E, Hutchinson C. Drug tolerability and outcomes in kidney transplant recipients treated with two formulations of mycophenolic acid. J Pharma Drug Develop 2013;1.
- Langone AJ, Chan L, Bolin P, Cooper M. Enteric-coated mycophenolate sodium versus mycophenolate mofetil in renal transplant recipients experiencing gastrointestinal intolerance: a multicenter, double-blind, randomized study. Transplantation 2011;91:470-8. http://dx.doi.org/10.1097/tp.0b013e318205568c.
- Chan L, Mulgaonkar S, Walker R, Arns W, Ambühl P, Schiavelli R. Patient-reported gastrointestinal symptom burden and health-related quality of life following conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium. Transplantation 2006;81:1290-7. http://dx.doi.org/10.1097/01.tp.0000209411.66790.b3.
- Hwang HS, Hyoung BJ, Kim S, Oh HY, Kim YS, Kim JK, et al. Improved gastrointestinal symptoms and quality of life after conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in renal transplant patients receiving tacrolimus. J Korean Med Sci 2010;25:1759-65. http://dx.doi.org/10.3346/jkms.2010.25.12.1759.
- Tedesco-Silva H, Johnston T, Kim YS, Zibari G, Walker R. Everolimus-treated renal transplant patients have a lower incidence of CMV and BKV: results from a multicenter, prospective study [abstract: 1659]. Am J Transplant 2010;10.
- Cibrik D, Silva HT, Vathsala A, Lackova E, Cornu-Artis C, Walker RG, et al. Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation 2013;95:933-42. http://dx.doi.org/10.1097/TP.0b013e3182848e03.
- Takahara S, Uchida K, Yoshimura N, Teraoka S, Kobayashi E, Teshima R, et al. Efficacy and safety of concentration controlled everolimus with reduced dose cyclosporine in Japanese adult de-novo renal transplant patients: 12 month results. Am J Transplant 2012;12.
- Saito K, Uchida K, Takahara S, Yoshimura N, Teraoka S, Cornu-Artis C, et al. Efficacy of everolimus with reduced cyclosporine in Japanese de novo renal transplant recipients: 24-month, randomized, multicenter study. Am J Transplant 2013;13.
- Paoletti E, Marsano L, Bellino D, Cassottana P, Rolla D, Di Maio G. Everolimus for regression of left ventricular hypertrophy of renal transplant recipients: a randomized controlled trial. Am J Transplant 2012;12.
- Paoletti E, Marsano L, Bellino D, Cassottana P, Cannella G. Effect of everolimus on left ventricular hypertrophy of de novo kidney transplant recipients: a 1 year, randomized, controlled trial. Transplantation 2012;93:503-8. http://dx.doi.org/10.1097/TP.0b013e318242be28.
- Favi E, Citterio F, Spagnoletti G, Gargiulo A, Delreno F, Romagnoli J, et al. Prospective clinical trial comparing two immunosuppressive regimens, tacrolimus and mycophenolate mofetil versus everolimus and low-dose cyclosporine, in de novo renal transplant recipients: results at 6 months follow-up. Transplant Proc 2009;41:1152-5. http://dx.doi.org/10.1016/j.transproceed.2009.03.019.
- Favi E, Citterio F, Spagnoletti G, Gargiulo A, Romagnoli J, Castagneto M. A prospective clinical trial comparing tacrolimus-MMF to cyclosporine-everolimus in de novo renal transplant recipients: 2 years results. Transpl Int 2009;22.
- Favi E, Spagnoletti G, Gargiulo A, Romagnoli J, Castagneto M. The combination of everolimus and low dose cyclosporine allows similar results as the standard tacrolimus and MMF regimen: 3 year results of a prospective clinical trial in renal transplant recipients. Am J Transplant 2010;10.
- Favi E, Spagnoletti G, Silvestrini N, Salerno M, Pedroso J, Romagnoli J, et al. Thymoglobulin plus basiliximab vs. basiliximab as induction therapy in deceased donor kidney transplant recipients treated with tacrolimus and mycophenolate mofetil: 1-year results of a prospective clinical trial. Am J Transplant 2013;13.
- Gonzalez F, Espinoza M, Herrera P, Rocca X, Reynolds E, Lorca E, et al. Everolimus versus azathioprine in a cyclosporine and ketoconazole based immunosuppressive therapy in kidney transplantation: 1-year follow-up of a comparative clinical trial. Transpl Int 2007;20.
- Miserlis G, Papanikolaou V, Vergoulas G, Antoniadis N, Fouzas I, Vrochidis D, et al. Efficacy and safety of everolimus with low dose cyclosporine A compared with mycophenolate mofetil and full dose cyclosporine A in de novo renal transplant recipients. Transplantation 2008;86. http://dx.doi.org/10.1097/01.tp.0000331145.10647.c0.
- Watarai Y, Yamamoto T, Tsujita M, Hiramitsu T, Nanmoku K, Goto N, et al. Efficacy and safety of everolimus based immunosuppression on de novo kidney transplantation with 4 years follow-up, especially in protocol biopsy findings and donor specific antibody production. Transpl Int 2013;26.
- Loriga G, Ciccarese M, Pala PG, Satta RP, Fanelli V, Manca ML, et al. De novo everolimus-based therapy in renal transplant recipients: effect on proteinuria and renal prognosis. Transplant Proc 2010;42:1297-302. http://dx.doi.org/10.1016/j.transproceed.2010.03.120.
- Dantal J, Vítko S, Margreiter R, Weimar W, Viljoen H, Somberg K. Everolimus (certican™, RAD) is associated with a reduced incidence of CMV infection following renal transplantation. Am J Transplant 2002;2.
- Oppenheimer F, Oyen O, Viljoen H, Vítko S, Falcone A, Cremer M. 36-month results of an international study with everolimus for the prevention of allograft rejection in de novo kidney transplant recipients. Am J Transplant 2003;3.
- Novartis . RAD001/Everolimus:/A/12/Month,/Multi-Center,/Randomized,/Open-Label/Non-Inferiority/Study/Comparing/the/Safety/and/Efficacy/of/Concentration-Controlled/Everolimus/With/Low/Dose/Tacrolimus/to/CellCept/(Mycophenolate/Mofetil)/With/Standard/Dose/Tacrolimus/in/De/Novo/Renal/Transplant/Recipients 2014.
- Tedesco H, Felipe C, Sandes T, Cristelli M, Rodrigues C, Pestana JOM. A prospective randomized trial aimed to reduce the incidence of cytomegalovirus (CMV) infection in kidney transplant recipients. Transplantation 2012;94. http://dx.doi.org/10.1097/00007890-201211271-00006.
- Tedesco H, Felipe C, Wang L, Rodrigues C, Sandes T, Cristelli M, et al. A prospective randomized trial aimed to reduce the incidence of cytomegalovirus (CMV) infection in kidney transplant (KT) recipients. Am J Transplant 2013;13.
- Favi E, Silvestrini N, Pedroso J, Salerno M, Spagnoletti G, Bianchi V, et al. Extended-release tacrolimus plus everolimus vs. extended-release tacrolimus plus micophenolate mofetil in primary deceased donor kidney transplant recipients: 1-year results of an open label, randomized phase 2 clinical trial. Am J Transplant 2013;13.
- Kamar N, Allard J, Ribes D, Durand D, Ader JL, Rostaing L. Assessment of glomerular and tubular functions in renal transplant patients receiving cyclosporine A in combination with either sirolimus or everolimus. Clin Nephrol 2005;63:80-6. http://dx.doi.org/10.5414/CNP63080.
- Rostaing L, Tran Van T, Cointault O, Lavayssiere L, Durand D, Ader J. Assessment of renal function in de novo renal transplant patients receiving either sirolimus or everolimus in addition to cyclosporine A. J Am Soc Nephrol 2001;12.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Jürgensen JS, Arns W, Hass B. Cost-effectiveness of immunosuppressive regimens in renal transplant recipients in Germany: a model approach. Eur J Health Econ 2010;11:15-2. http://dx.doi.org/10.1007/s10198-009-0148-3.
- Jürgensen JS, Ikenberg R, Greiner RA, Hosel V. Cost-effectiveness of modern mTOR inhibitor based immunosuppression compared to the standard of care after renal transplantation in Germany. Eur J Health Econ 2015;16:377-90. http://dx.doi.org/10.1007/s10198-014-0579-3.
- Earnshaw SR, Graham CN, Irish WD, Sato R, Schnitzler MA. Lifetime cost-effectiveness of calcineurin inhibitor withdrawal after de novo renal transplantation. J Am Soc Nephrol 2008;19:1807-16. http://dx.doi.org/10.1681/ASN.2007040495.
- Orme ME, Jurewicz WA, Kumar N, McKechnie TL. The cost effectiveness of tacrolimus versus microemulsified cyclosporin: a 10-year model of renal transplantation outcomes. Pharmacoeconomics 2003;21:1263-76. http://dx.doi.org/10.2165/00019053-200321170-00003.
- McEwan P, Baboolal K, Conway P, Currie CJ. Evaluation of the cost-effectiveness of sirolimus versus cyclosporin for immunosuppression after renal transplantation in the United Kingdom. Clin Ther 2005;27:1834-46. http://dx.doi.org/10.1016/j.clinthera.2005.11.002.
- McEwan P, Dixon S, Baboolal K, Conway P, Currie CJ. Evaluation of the cost effectiveness of sirolimus versus tacrolimus for immunosuppression following renal transplantation in the UK. Pharmacoeconomics 2006;24:67-79. http://dx.doi.org/10.2165/00019053-200624010-00006.
- Crompton JA, Somerville T, Smith L, Corbett J, Nelson E, Holman J, et al. Lack of economic benefit with basiliximab induction in living related donor adult renal transplant recipients. Pharmacotherapy 2003;23:443-50. http://dx.doi.org/10.1592/phco.23.4.443.32119.
- Emparan C, Wolters H, Laukotter M, Dame C, Senninger N. Cost-effectiveness analysis of basixilimab induction and calcineurin-sparing protocols in ‘old to old’ programs using Markov models. Transplant Proc 2003;35:1324-5. http://dx.doi.org/10.1016/S0041-1345(03)00378-6.
- Emparan C. Economic evaluation of new immunosuppressive drugs in renal transplantation. Expert Rev Clin Immunol 2006;2:183-6. http://dx.doi.org/10.1586/1744666X.2.2.183.
- Chilcott JB, Holmes MW, Walters S, Akehurst RL, Nashan B. The economics of renal transplantation with basiliximab (simulect) in preventing acute rejection in renal transplantation. Transplant Int 2002;15:486-93. http://dx.doi.org/10.1111/j.1432-2277.2002.tb00204.x.
- Walters SJ, Whitfield M, Akehurst RL, Chilcott JB. Economic implications of the use of basiliximab in addition to triple immunosuppressive therapy in renal allograft recipients: a UK perspective. Pharmacoeconomics 2003;21:129-38. http://dx.doi.org/10.2165/00019053-200321020-00005.
- Popat R, Syed A, Puliatti C, Cacciola R. Outcome and cost analysis of induction immunosuppression with IL2Mab or ATG in DCD kidney transplants. Transplantation 2014;97:1161-5. http://dx.doi.org/10.1097/01.tp.0000442505.10490.20.
- Muduma G, Shaw J, Hart WM, Odeyemi A, Odeyemi I. Cost utility analysis of immunosuppressive regimens in adult renal transplant recipients in England and Wales. Patient Prefer Adher 2014;8:1537-46. http://dx.doi.org/10.2147/PPA.S69461.
- Craig AM, McKechnie T, McKenna M, Klein W, Schindler TM. A cost-effectiveness analysis of tacrolimus versus cyclosporine microemulsion following kidney transplantation. Transplant Proc 2002;34:1646-8. http://dx.doi.org/10.1016/S0041-1345(02)02964-0.
- Lazzaro C, McKechnie T, McKenna M. Tacrolimus versus cyclosporin in renal transplantation in Italy: cost-minimisation and cost-effectiveness analyses. J Nephrol 2002;15:580-8.
- Abecassis MM, Seifeldin R, Riordan ME. Patient outcomes and economics of once-daily tacrolimus in renal transplant patients: results of a modeling analysis. Transplant Proc 2008;40:1443-5. http://dx.doi.org/10.1016/j.transproceed.2008.03.090.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics 2006;24:355-71. http://dx.doi.org/10.2165/00019053-200624040-00006.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Muduma G, Odeyemi I, Pollock RF. A UK analysis of the cost of switching renal transplant patients from an immediate-release to a prolonged-release formulation of tacrolimus based on differences in trough concentration variability. J Med Econ 2014;17:520-6. http://dx.doi.org/10.3111/13696998.2014.916713.
- Muduma G, Odeyemi I, Smith-Palmer J, Pollock RF. Budget impact of switching from an immediate-release to a prolonged-release formulation of tacrolimus in renal transplant recipients in the UK based on differences in adherence. Patient Prefer Adher 2014;8:391-9. http://dx.doi.org/10.2147/PPA.S60213.
- Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation 2002;73:775-82. http://dx.doi.org/10.1097/00007890-200203150-00021.
- Jurewicz WA. Tacrolimus versus ciclosporin immunosuppression: long-term outcome in renal transplantation. Nephrol Dial Transplant 2003;18:i7-11. http://dx.doi.org/10.1093/ndt/gfg1028.
- Drummond M, O’Brien B, Stoddart GL, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford Medical Publications; 1987.
- Crompton JA, Sundberg A, Smith L, Somerville T, Corbett J, Nelson E, et al. Economic analysis of basiliximab and mycophenolate mofetil in living-related donor renal transplant program. Pharmacotherapy 2003;23.
- Emparan C, Wolters H, Laukotte M, Senninger N. The cost-effectiveness of basiliximab induction in ‘old-to-old’ kidney transplant programs: Bayesian estimation, simulation, and uncertainty analysis. Transplant Proc 2005;37:2069-71. http://dx.doi.org/10.1016/j.transproceed.2005.03.008.
- Emparan C, Laukotter M, Wolters H, Dame C, Heidenreich S, Senninger N. Calcineurin-free protocols with basiliximab induction allow patients included in ‘old to old’ programs achieve standard kidney transplant function. Transplant Proc 2003;35:1326-7. http://dx.doi.org/10.1016/S0041-1345(03)00379-8.
- An Introduction to Numerical Analysis. Toronto, ON: John Wiley & Sons; 1989.
- Schnitzler M, Johnston K, Axelrod D, Gheorghian A, Lentine KL. Associations of renal function at 1-year after kidney transplantation with subsequent return to dialysis, mortality and healthcare costs. Transplantation 2011;91:1347-56. http://dx.doi.org/10.1097/TP.0b013e31821ab993.
- Levy AR, Briggs AH, Johnston K, Maclean JR, Yuan Y, L’Italien GJ, et al. Projecting long-term graft and patient survival after transplantation. Value Health 2014;17:254-60. http://dx.doi.org/10.1016/j.jval.2014.01.001.
- Barnieh L, Yilmaz S, McLaughlin K, Hemmelgarn BR, Klarenbach S, Manns BJ, et al. The cost of kidney transplant over time. Prog Transplant 2014;24:257-62. http://dx.doi.org/10.7182/pit2014710.
- Oberbauer R, Kreis H, Campistol JM, Mota A, Daloze P, Ruiz JC, et al. Renal function improves significantly after early cyclosporine withdrawal in sirolimus-treated renal transplant recipients: 3-year results of the Rapamune Maintenance Regimen (RMR) trial. JASN 2003;14.
- Knoll GA, Kokolo MB, Mallick R, Beck A, Buenaventura CD, Ducharme R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ 2014;349. http://dx.doi.org/10.1136/bmj.g6679.
- Neri L, McEwan P, Sennfalt K, Baboolal K. Characterizing the relationship between health utility and renal function after kidney transplantation in UK and US: a cross-sectional study. Health Qual Life Outcomes 2012;10. http://dx.doi.org/10.1186/1477-7525-10-139.
- Pruthi R, Steenkamp R, Feest T. UK Renal Registry 16th annual report. Chapter 8. Survival and cause of death of UK adult patients on renal replacement therapy in 2012: national and centre-specific analyses. Nephron Clin Pract 2013;125:139-70. http://dx.doi.org/10.1159/000360027.
- Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int 2002;62:311-18. http://dx.doi.org/10.1046/j.1523-1755.2002.00424.x.
- Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients. Cochrane Database Syst Rev 2006;2. http://dx.doi.org/10.1002/14651858.cd004290.pub2.
- Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 2012;12:1157-67. http://dx.doi.org/10.1111/j.1600-6143.2012.04013.x.
- Kuypers DR, Peeters PC, Sennesael JJ, Kianda MN, Vrijens B, Kristanto P, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation 2013;95:333-40. http://dx.doi.org/10.1097/TP.0b013e3182725532.
- Wu MJ, Cheng CY, Chen CH, Wu WP, Cheng CH, Yu DM, et al. Lower variability of tacrolimus trough concentration after conversion from Prograf to Advagraf in stable kidney transplant recipients. Transplantation 2011;92:648-52. http://dx.doi.org/10.1097/TP.0b013e3182292426.
- Borra LC RJ, Kal JA, Mathot RA, Weimar W, van Gelder T. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant 2010;25:2757-63. http://dx.doi.org/10.1093/ndt/gfq096.
- Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 2003;3:178-85. http://dx.doi.org/10.1034/j.1600-6143.2003.00010.x.
- Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ 2005;331:810-14. http://dx.doi.org/10.1136/bmj.38569.471007.AE.
- Ho ET, Wong G, Craig JC, Chapman JR. Once-daily extended-release versus twice-daily standard-release tacrolimus in kidney transplant recipients: a systematic review. Transplantation 2013;95:1120-8. http://dx.doi.org/10.1097/TP.0b013e318284c15b.
- Tedesco-Silva H, Vítko S, Pascual J, Eris J, Magee JC, Whelchel J, et al. 12-month safety and efficacy of everolimus with reduced exposure cyclosporine in de novo renal transplant recipients. Transpl Int 2007;20:27-36. http://dx.doi.org/10.1111/j.1432-2277.2006.00414.x.
- Secretariat Assessment Report: Advice No. 1712 Belatacept (Nulojix®). New York, NY: Bristol-Myers Squibb; 2012.
- Gamboa O, Montero C, Mesa L, Benavides C, Reino A, Torres RE, et al. Cost-effectiveness analysis of the early conversion of tacrolimus to mammalian target of rapamycin inhibitors in patients with renal transplantation. Transplant Proc 2011;43:3367-76. http://dx.doi.org/10.1016/j.transproceed.2011.09.092.
- Rely K, Alexandre PK, Garcia-Garcia EG, Mucino-Ortega E, Salinas-Escudero G, Galindo-Suarez RM. Cost-utility assessment of sirolimus versus tacrolimus for primary prevention of graft rejection in renal transplant recipients in Mexico. Value Health 2012;15. http://dx.doi.org/10.1016/j.jval.2012.03.840.
- Niemczyk M, Nowak M, Pilecki T, Wyzgal J, Ziolkowski J, Zygier D, et al. Economic evaluation of sirolimus-based immunosuppressive regimens in kidney graft recipients. Transplant Proc 2006;38:74-7. http://dx.doi.org/10.1016/j.transproceed.2005.11.092.
- Opelz G, Dohler B. Collaborative Transplant Study Report. Influence of time of rejection on long-term graft survival in renal transplantation. Transplantation 2008;85:661-6. http://dx.doi.org/10.1097/TP.0b013e3181661695.
- NHS Blood and Transplant (NHSBT) . National Organ Retrieval Service: Service Evaluation 2013. www.nhsbt.nhs.uk/download/board_papers/sept13/National_Organ_Retrieval_Service_Service_Evaluation.pdf (accessed 2 December 2014).
- Butler JA, Peveler RC, Roderick P, Smith PWF, Horne R, Mason JC. Modifiable risk factors for non-adherence to immunosuppressants in renal transplant recipients: a cross-sectional study. Nephrol Dial Transplant 2004;19:3144-9. http://dx.doi.org/10.1093/ndt/gfh505.
- Taylor RS, Elston J. The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technol Assessment reports. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13080.
- Lee AJ, Morgan CL, Conway P, Currie CJ. Characterisation and comparison of health-related quality of life for patients with renal failure. Curr Med Res Opin 2005;21:1777-83. http://dx.doi.org/10.1185/030079905X65277.
- Tedesco-Silva H, Garcia VD, Contieri FL, Boni Monteiro de Carvalho D, Noronha IL, Gonçalves RT, et al. Comparison of the safety and efficacy of cyclosporine minimization versus cyclosporine elimination in de novo renal allograft patients receiving sirolimus. Transplant Proc 2010;42:1659-66. http://dx.doi.org/10.1016/j.transproceed.2010.02.083.
- Tedesco-Silva H, Kim YS, Johnston T, Walker R, Zibari GB, Cornu-Artis C, et al. Concentration-controlled everolimus with reduced cyclosporine concentration in de novo renal transplant recipients: efficacy results at 24 months. Am J Transplant 2011;11.
- Dean PG, Larson TS, Rea DJ, Griffin MD, Textor SC, Schwab TR, et al. The effects of calcineurin inhibitor avoidance on renal function and graft histology after kidney transplantation: a prospective, randomized comparison of tacrolimus and sirolimus. Transplantation 2004;78. http://dx.doi.org/10.1097/00007890-200407271-00241.
- Organ Donation for Transplantation: Costing Report. Implementing NICE Guidance. London: NICE; 2011.
- Neri L, Dukes J, Brennan DC, Salvalaggio PR, Seelam S, Desiraju S, et al. Impaired renal function is associated with worse self-reported outcomes after kidney transplantation. Qual Life Res 2011;20. http://dx.doi.org/10.1007/s11136-011-9905-8.
- Goring SM, Levy AR, Ghement I, Kalsekar A, Eyawo O, L’Italien GJ, et al. A network meta-analysis of the efficacy of belatacept, cyclosporine and tacrolimus for immunosuppression therapy in adult renal transplant recipients. Curr Med Res Opin 2014;30:1473-87. http://dx.doi.org/10.1185/03007995.2014.898140.
- Levy A, Johnston K, Kalsekar A, Schnitzler M, L’Italien G, Kasiske B. Modeled long term projections of clinical outcomes from BENEFIT and BENEFIT-EXT. Am J Transplant 2012;12:406-7.
- Currie CJ, Morgan CL, Dixon S, McEwan P, Marchant N, Bearne A, et al. The financial costs of hospital care for people with diabetes who have single and multiple macrovascular complications. Diabetes Res Clin Pract 2005;67:144-51. http://dx.doi.org/10.1016/j.diabres.2004.01.002.
- Beckwith J, Nyman JA, Flanagan B, Schrover R, Schuurman HJ. A health-economic analysis of porcine islet xenotransplantation. Xenotransplantation 2010;17:233-42. http://dx.doi.org/10.1111/j.1399-3089.2010.00586.x.
- Morton RL, Howard K, Webster AC, Wong G, Craig JC. The cost-effectiveness of induction immunosuppression in kidney transplantation. Nephrol Dial Transplant 2009;24:2258-69. http://dx.doi.org/10.1093/ndt/gfp174.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- eMit National Database (2014/06): Drugs and Pharmaceutical Electronic Market Information (eMit). London: Department of Health; 2014.
- Beaudet A, Palmer JL, Timlin L, Wilson B, Bruhn D, Boye KS, et al. Cost-utility of exenatide once weekly compared with insulin glargine in patients with type 2 diabetes in the UK. J Med Econ 2011;14:357-66. http://dx.doi.org/10.3111/13696998.2011.579213.
- Baboolal K, McEwan P, Sondhi S, Spiewanowski P, Wechowski J, Wilson K. The cost of renal dialysis in a UK setting: a multicentre study. Nephrol Dial Transplant 2008;23:1982-9. http://dx.doi.org/10.1093/ndt/gfm870.
- Organ Donation for Transplantation: Improving Donor Identification and Consent Rates for Deceased Organ Donation. London: NICE; 2011.
- Organ Donation for Transplantation. Costing Report. Implementing NICE Guidance. NICE; 2011.
- Craig R, Mindell J. Health Survey for England 2012. London: The Health and Social Care Information Centre; 2013.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- NHS Blood and Transplant . The UK Transplant Registry Standard Data Set (2007–2012) n.d. www.odt.nhs.uk/uk-transplant-registry/data/ (accessed December 2014).
- Cole EH, Johnston O, Rose CL, Gill JS. Impact of acute rejection and new-onset diabetes on long-term transplant graft and patient survival. Clin J Am Soc Nephrol 2008;3:814-21. http://dx.doi.org/10.2215/CJN.04681107.
- Ling C, Pandit P, Bennett H. Belatacept Micro-costing Model. UK. Cardiff: Cardiff Research Consortium; 2011.
- Clarke PM, Gray AM, Briggs A, Stevens RJ, Matthews DR, Holman RR. Cost-utility analyses of intensive blood glucose and tight blood pressure control in type 2 diabetes (UKPDS 72). Diabetologia 2005;48:868-77. http://dx.doi.org/10.1007/s00125-005-1717-3.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Pruthi R, Casula A, MacPhee I. UK Renal Registry 16th Annual Report. Chapter 3. Demographic and biochemistry profile of kidney transplant recipients in the UK in 2012: national and centre-specific analyses. Nephron Clin Pract 2013;125:55-80. http://dx.doi.org/10.1159/000360022.
- Kaltenthaler E, Tappnden P, Paisley S, Squires H. Technical Support Document 13: Identifying and Reviewing Evidence to Inform the Conceptualisation and population of Cost-Effectiveness Models. London: NICE; 2011.
- Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R. The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13380.
- Ansell D, Feest T, Williams A, Winearls C. UK Renal Registry Report 2005: 8th Annual Report of the Renal Association. Bristol: UK Renal Registry; 2005.
- Johnston O, Rose CL, Gill JS. Risks and benefits of preemptive second kidney transplantation. Transplantation 2013;95:705-10. http://dx.doi.org/10.1097/TP.0b013e31827a938f.
- Opelz G, Doehler B, Collaborative Transplant S. Influence of immunosuppressive regimens on graft survival and secondary outcomes after kidney transplantation. Transplantation 2009;87:795-802. http://dx.doi.org/10.1097/TP.0b013e318199c1c7.
- Kasiske BL, Andany MA, Danielson B. A thirty per cent chronic decline in inverse serum creatinine is an excellent predictor of late renal allograft failure. Am J Kidney Dis 2002;39:762-8. http://dx.doi.org/10.1053/ajkd.2002.31996.
- Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation 2003;75:1291-5. http://dx.doi.org/10.1097/01.TP.0000061602.03327.E2.
- Salvadori M, Rosati A, Bock A, Chapman J, Dussol B, Fritsche L, et al. Estimated one-year glomerular filtration rate is the best predictor of long-term graft function following renal transplant. Transplantation 2006;81:202-6. http://dx.doi.org/10.1097/01.tp.0000188135.04259.2e.
- Statistical Methodology and Risk-Adjustment for Survival Rate Estimation. Watford: NHS BT; 2011.
- Opelz G, Dohler B. Association of HLA mismatch with death with a functioning graft after kidney transplantation: a collaborative transplant study report. Am J Transplant 2012;12:3031-8. http://dx.doi.org/10.1111/j.1600-6143.2012.04226.x.
- Opelz G, Dohler B. Association between steroid dosage and death with a functioning graft after kidney transplantation. Am J Transplant 2013;13:2096-105. http://dx.doi.org/10.1111/ajt.12313.
- Webb L, Casula A, Tomson C, Ben-Shlomo Y. Survival after renal transplant failure: a UK Renal Registry analysis. Nephrol Dial Transplant 2012;27.
- Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, et al. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant 2003;3:590-8. http://dx.doi.org/10.1034/j.1600-6143.2003.00082.x.
- Vanrenterghem Y, Ponticelli C, Morales JM, Abramowicz D, Baboolal K, Eklund B, et al. Prevalence and management of anemia in renal transplant recipients: a European survey. Am J Transplant 2003;3:835-45. http://dx.doi.org/10.1034/j.1600-6143.2003.00133.x.
- Fuggle SV, Allen JE, Johnson RJ, Collett D, Mason PD, Dudley C, et al. Factors affecting graft and patient survival after live donor kidney transplantation in the UK. Transplantation 2010;89:694-701. http://dx.doi.org/10.1097/TP.0b013e3181c7dc99.
- Summers DM, Johnson RJ, Allen J, Fuggle SV, Collett D, Watson CJ, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet 2010;376:1303-11. http://dx.doi.org/10.1016/S0140-6736(10)60827-6.
- Health Survey for England – 2012. London: HSCIC; 2013.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Liem YS, Bosch JL, Myriam Hunink MG. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health 2008;11:733-41. http://dx.doi.org/10.1111/j.1524-4733.2007.00308.x.
- Dukes JL, Seelam S, Lentine KL, Schnitzler MA, Neri L. Health-related quality of life in kidney transplant patients with diabetes. Clin Transplant 2013;27:E554-62. http://dx.doi.org/10.1111/ctr.12198.
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203-20. http://dx.doi.org/10.1097/00005650-200503000-00003.
- Johnson JA, Luo N, Shaw JW, Kind P, Coons SJ. Valuations of EQ-5D health states: are the United States and United Kingdom different?. Med Care 2005;43:221-8. http://dx.doi.org/10.1097/00005650-200503000-00004.
- Currie CJ, McEwan P, Peters JR, Patel TC, Dixon S. The routine collation of health outcomes data from hospital treated subjects in the Health Outcomes Data Repository (HODaR): descriptive analysis from the first 20,000 subjects. Value Health 2005;8:581-90. http://dx.doi.org/10.1111/j.1524-4733.2005.00046.x.
- Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLOS Med 2012;9. http://dx.doi.org/10.1371/journal.pmed.1001307.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Moore J. Kidney Transplant Protocol. Exeter: Royal Devon and Exeter NHS Foundation Trust; 2012.
- Harvala H, Stewart C, Muller K, Burns S, Marson L, MacGilchrist A, et al. High risk of cytomegalovirus infection following solid organ transplantation despite prophylactic therapy. J Med Virol 2013;85:893-8. http://dx.doi.org/10.1002/jmv.23539.
- Ling C, Chamberlain G. Preliminary Report: PORTRAIT Database Study: Cardiff. Cardiff: Cardiff Research Consortium; 2011.
- Cavallo R, Elia M, Gruosso V, Curtoni A, Costa C, Bergallo M. Molecular epidemiology of Epstein–Barr virus in adult kidney transplant recipients. Transplant Proc 2010;42:2527-30. http://dx.doi.org/10.1016/j.transproceed.2010.05.151.
- Department of Health (DH) . Reference Costs Guidance 2013–14 2014. www.gov.uk/government/publications/nhs-reference-costs-collection-guidance-for-2013-to-2014.
- Riella LV, Gabardi S, Chandraker A. Dyslipidemia and its therapeutic challenges in renal transplantation. Am J Transplant 2012;12:1975-82. http://dx.doi.org/10.1111/j.1600-6143.2012.04084.x.
- Immunosuppression Following Renal Transplantation. Caerphilly: Welsh Health Specialised Services Committee; 2012.
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012;29:855-62. http://dx.doi.org/10.1111/j.1464-5491.2012.03698.x.
- Davies MJ, Chubb BD, Smith IC, Valentine WJ. Cost-utility analysis of liraglutide compared with sulphonylurea or sitagliptin, all as add-on to metformin monotherapy in type 2 diabetes mellitus. Diabet Med 2012;29:313-20. http://dx.doi.org/10.1111/j.1464-5491.2011.03429.x.
- Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ 2008;336:1180-5. http://dx.doi.org/10.1136/bmj.39545.585289.25.
- Currie CJ, Gale EA, Poole CD. Estimation of primary care treatment costs and treatment efficacy for people with Type 1 and Type 2 diabetes in the United Kingdom from 1997 to 2007*. Diabet Med 2010;27:938-48. http://dx.doi.org/10.1111/j.1464-5491.2010.03040.x.
- Alva ML, Gray A, Mihaylova B, Leal J, Holman RR. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabet Med 2015;32:459-66. http://dx.doi.org/10.1111/dme.12647.
- Erythropoiesis Stimulating Agents (Epoetin and Darbepoetin) for Treating Anaemia in People with Cancer Having Chemotherapy. TA323. London: NICE; 2014.
- Developing Robust Reference Costs for Kidney Transplants: Update. NHS Kidney Care; 2011.
- Provider-to-Provider Services 2013–2014 Tariff. London: University College London Hospitals; 2014.
- Department of Biochemistry and Immunology, University Hospital of Wales . Therapeutic Drug Monitoring Test Repertoire n.d. www.cardiffandvaleuhb.wales.nhs.uk/sitesplus/documents/1143/PD-BIO-TDMRepertoire.pdf (accessed February 2015).
- National Casemix Office (NCO) . HRG4 + Reference Costs Code to Group 2014. www.hscic.gov.uk/casemix/costing.
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. http://dx.doi.org/10.1016/j.jclinepi.2010.03.016.
- Davis S. Assessing Technologies That are Not Cost-effective at a Zero Price. Sheffield: NICE Decision Support Unit; 2014.
- Salvadori M. Long term administration of enteric-coated mycophenolate sodium (EC-MPS, myfortic) is safe in kidney transplant patients. Transplantation 2004;78. http://dx.doi.org/10.1097/00007890-200407271-00690.
- Stevenson MD, Oakley J, Chilcott JB. Gaussian process modelling in conjunction with individual patient simulation modelling: a case study describing the calculation of cost-effectiveness ratios for the treatment of osteoporosis. Med Decis Mak 2004;24:89-100. http://dx.doi.org/10.1177/0272989X03261561.
- Irish W, Sherrill B, Brennan DC, Lowell J, Schnitzler M. Three-year posttransplant graft survival in renal-transplant patients with graft function at 6 months receiving tacrolimus or cyclosporine microemulsion within a triple-drug regimen. Transplantation 2003;76:1686-90. http://dx.doi.org/10.1097/01.TP.0000090865.20886.B7.
- Kaplan B, Schold JD, Meier-Kriesche HU. Long-term graft survival with neoral and tacrolimus: a paired kidney analysis. J Am Soc Nephrol 2003;14:2980-4. http://dx.doi.org/10.1097/01.ASN.0000095250.92361.D5.
- Bunnapradist S, Daswani A, Takemoto SK. Graft survival following living-donor renal transplantation: a comparison of tacrolimus and cyclosporine microemulsion with mycophenolate mofetil and steroids. Transplantation 2003;76:10-5. http://dx.doi.org/10.1097/01.TP.0000079965.62765.1A.
- UK Renal Registry 15th Annual Report. Bristol: UKRR; 2012.
- Johnson RW, Kreis H, Oberbauer R, Brattstrom C, Claesson K, Eris J. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation 2001;72:777-86. http://dx.doi.org/10.1097/00007890-200109150-00007.
- 2004 Annual Report, Transplant Data 1994–2003. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, and Richmond, United Network for Organ Sharing, and Ann Arbor, MI, University Renal Research and Education Association; 2004.
- Rapamune Dosing Study [data on file]. Plymouth Meeting, PA: Surveillance Data Inc.; 2005.
- Matas AJ, Schnitzler M. Payment for living donor (vendor) kidneys: a cost-effectiveness analysis. Am J Transplant 2004;4:216-21. http://dx.doi.org/10.1046/j.1600-6143.2003.00290.x.
- Churchill DN, Torrance GW, Taylor DW, Barnes CC, Ludwin D, Shimizu A, et al. Measurement of quality of life in end-stage renal disease: the time trade-off approach. Clin Invest Med 1987;10:14-20.
- Weng FL, Israni AK, Joffe MM, Hoy T, Gaughan CA, Newman M, et al. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J Am Soc Nephrol 2005;16:1839-48. http://dx.doi.org/10.1681/ASN.2004121059.
- Tanriover B, Stone PW, Mohan S, Cohen DJ, Gaston RS. Future of Medicare immunosuppressive drug coverage for kidney transplant recipients in the United States. Clin J Am Soc Nephrol 2013;8:1258-66. http://dx.doi.org/10.2215/CJN.09440912.
Appendix 1 Literature searching strategies
Clinical effectiveness
The following search strategies were used to identify studies of intervention effectiveness for this appraisal. They were first run on 14 April 2014 and the same strategy was used on 18 November 2014 to update the literature base: this most recent search is recorded below. The effectiveness searches take the following form: (terms for kidney or renal transplant or kidney or renal graft) AND (terms for the interventions under review) AND (a study design limit to RCTs or controlled trials). The search was not limited by language and it was not limited to human-only studies because such a limit would have blocked retrieval of includable studies for rATG (line 8 of the Medline search). The effectiveness searches were combined with the systematic review searches in our update searches.
Search annex
Database: Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE
Host: Ovid
Data parameters: 1946 to present.
Date searched: 18 November 2014.
Searcher: Chris.
Checked by: Simon/Jenny.
Hits: 73.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | Kidney Transplantation/ | 81,142 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 34,392 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 41,464 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 36,554 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 46,102 |
| 6 | 1 or 2 or 3 or 4 or 5 | 114,277 |
| 7 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 1063 |
| 8 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 6382 |
| 9 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 17,331 |
| 10 | Tacrolimus/ | 13,055 |
| 11 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 219 |
| 12 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 28,176 |
| 13 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 21,975 |
| 14 | Sirolimus/ | 14,369 |
| 15 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 3088 |
| 16 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 74,259 |
| 17 | 6 and 16 | 9593 |
| 18 | Randomized Controlled Trial.pt. | 400,000 |
| 19 | (random$ or RCT or placebo$ or single blind$ or double blind$ or triple blind$).ti,ab,ot. | 850,201 |
| 20 | clinical trial.pt. | 501,246 |
| 21 | (“controlled trial$” or “clinical trial$”).ti,ab,ot. | 348,859 |
| 22 | 18 or 19 or 20 or 21 | 1,324,400 |
| 23 | (systematic adj3 review$).ti,ab,kw,ot. | 65,381 |
| 24 | 22 or 23 | 1,361,806 |
| 25 | 17 and 24 | 2456 |
| 26 | limit 25 to yr=“2014 -Current” | 73 |
Notes: not applicable.
File: not applicable.
Database: EMBASE
Host: Ovid.
Data parameters: 1974 to 17 November 2014.
Date searched: 18 November 2014.
Searcher: Chris.
Hits: 259.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | kidney transplantation/ | 97,441 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 50,853 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 55,991 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 51,947 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 65,675 |
| 6 | 1 or 2 or 3 or 4 or 5 | 153,480 |
| 7 | basiliximab/ | 6681 |
| 8 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 2311 |
| 9 | thymocyte antibody/ | 20,236 |
| 10 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 8854 |
| 11 | tacrolimus/ | 53,638 |
| 12 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 26,290 |
| 13 | belatacept/ | 989 |
| 14 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 547 |
| 15 | mycophenolic acid/ | 9985 |
| 16 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 35,917 |
| 17 | rapamycin/ | 36,443 |
| 18 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 28,739 |
| 19 | everolimus/ | 14,356 |
| 20 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 6988 |
| 21 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 | 148,218 |
| 22 | 6 and 21 | 25,662 |
| 23 | randomized controlled trial/ | 355,008 |
| 24 | (random$ or placebo$ or single blind$ or double blind$ or triple blind$).ti,ab,ot. | 1,028,637 |
| 25 | (“controlled trial$” or “clinical trial$”).ti,ab,ot. | 428,701 |
| 26 | 23 or 24 or 25 | 1,300,553 |
| 27 | (systematic adj3 review$).ti,ab,kw,ot. | 77,376 |
| 28 | 26 or 27 | 1,343,995 |
| 29 | 22 and 28 | 3537 |
| 30 | limit 29 to yr=“2014 -Current” | 259 |
Notes: not applicable
File: not applicable
Database: Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects and Cochrane Central Register of Controlled Trials
Host: Wiley Online Library.
Data parameters: Issue 11 of 12, November 2014, Issue 4 of 4, October 2014, Issue 10 of 12, October 2014
Date searched: 18 November 2014
Searcher: Chris
Hits: 64 (CDSR 10; DARE 3; CENTRAL 51)
ID, Search, Hits
Search strategy
#1 MeSH descriptor: [Kidney Transplantation] this term only (3311)
#2 (Kidney* near/3 transplant*) (5789)
#3 (Renal near/3 transplant*) (4385)
#4 ((kidney or renal) near/3 (recipient* or dono* or donation* or replac*)) (3706)
#5 ((graft* or allograft* or homograft* or allogeneic) and (kidney* or renal)) (4956)
#6 #1 or #2 or #3 or #4 or #5 8481 (7509)
#7 (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”) (486)
#8 ((rabbit* near/3 Anti-thymocyte*) or (rabbit* near/3 Antithymocyte*) or (rabbit* near/3 thymocyte*) or (rabbit* near/3 polyclonal) or (rabbit* and ATG) or RATG or thymoglobulin*) (346)
#9 (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”) (2463)
#10 MeSH descriptor: [Tacrolimus] this term only (1180)
#11 (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”) (58)
#12 (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep* or Myfenax or Myfortic or Mofetil) (3315)
#13 (Sirolimus or Rapamune or Rapamycin or “ay 22-989”) (2034)
#14 MeSH descriptor: [Sirolimus] this term only (1067)
#15 (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”) (724)
#16 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 (7002)
#17 #6 and #16 Publication Year from 2014 (67)
Notes: not applicable
File: not applicable
Database: Web of Science
Host: ISI Thompson Reuters.
Data parameters: 1900–2014.
Date searched: 18 November 2014.
Searcher: Chris.
Hits: 2290.
-
TOPIC: ((Kidney* near/3 transplant*))
-
TOPIC: ((Renal near/3 transplant*))
-
TOPIC: (((kidney or renal) near/3 (recipient* or dono* or donation* or replac*)))
-
TOPIC: (((graft* or allograft* or homograft* or allogeneic) and (kidney* or renal)))
-
#4 OR #3 OR #2 OR #1
-
TOPIC: ((Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”))
-
TOPIC: (((rabbit* near/3 Anti-thymocyte*) or (rabbit* near/3 Antithymocyte*) or (rabbit* near/3 thymocyte*) or (rabbit* near/3 polyclonal) or (rabbit* and ATG) or RATG or thymoglobulin*))
-
TOPIC: ((Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”))
-
TOPIC: ((Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”))
-
TOPIC: ((“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep* or Myfenax or Myfortic or Mofetil))
-
TOPIC: ((Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”))
-
#12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6
-
#13 AND #5 (Refined by: PUBLICATION YEARS: (2005 OR 2009 OR 2011 OR 2007 OR 2010 OR 2006 OR 2008 OR 2013 OR 2012 OR 2014))
-
TOPIC: (((random* or rct* or “controlled trial*” or “clinical trial*”)))
-
#16 AND #15
Notes: auto-suggest was turned off.
File: not applicable.
Database: Health Management Information Consortium
Host: Ovid
Data parameters: 1979 to September 2014.
Date searched: Tuesday, 18 November 2014.
Searcher: Chris.
Hits: 0.
Search strategy:
| # | Searches | Results |
|---|---|---|
| 1 | Kidney Transplantation/ | 120 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 83 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 81 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 152 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 28 |
| 6 | 1 or 2 or 3 or 4 or 5 | 313 |
| 7 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 2 |
| 8 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 1 |
| 9 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 8 |
| 10 | Tacrolimus/ | 0 |
| 11 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 0 |
| 12 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 23 |
| 13 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 10 |
| 14 | Sirolimus/ | 0 |
| 15 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 2 |
| 16 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 32 |
| 17 | 6 and 16 | 3 |
| 18 | Randomized Controlled Trial.pt. | 0 |
| 19 | (random$ or RCT or placebo$ or single blind$ or double blind$ or triple blind$).ti,ab,ot. | 10,838 |
| 20 | clinical trial.pt. | 0 |
| 21 | (“controlled trial$” or “clinical trial$”).ti,ab,ot. | 5592 |
| 22 | 18 or 19 or 20 or 21 | 12,088 |
| 23 | (systematic adj3 review$).ti,ab,kw,ot. | 3692 |
| 24 | 22 or 23 | 14,553 |
| 25 | 17 and 24 | 2 |
| 26 | limit 25 to yr=“2014 -Current” | 0 |
Notes: not applicable.
File: not applicable.
Trials registries
The following search strategies were used in ClinicalTrials.gov and the International Standard Randomised Controlled Trial Number (ISRCTN) Registry, Controlled Trials. These were hand-searched on 19 October 2014 via: https://clinicaltrials.gov/ and www.controlled-trials.com/, respectively.
(Basiliximab OR Basiliximabum OR Simulect OR “interleukin 2 receptor antibody”) AND (kidney* OR renal)
((rabbit AND Anti-thymocyte*) OR (rabbit AND Antithymocyte*) OR (rabbit AND thymocyte*) OR (rabbit* AND polyclonal) OR (rabbit* AND ATG) OR RATG OR thymoglobulin*) AND (kidney* OR renal)
(Tacrolimus OR Fujimycin OR Prograf OR Advagraf OR Adoport OR Capexion OR Modigraf OR Perixis OR Tacni OR Vivadex OR Protopic OR Tsukubaenolide OR “FK 506” OR “FK-506” OR “FK506” OR “fr-900506”) AND (kidney* OR renal)
(Belatacept OR Nulojix OR “lea29y” OR “lea 29y” OR “bms 224818”) AND (kidney* OR renal)
(“Mycophenolic acid” OR MPA OR Mycophenolate OR Arzip OR CellCep* OR Myfenax OR Myfortic OR Mofetil) AND (kidney* OR renal)
(Sirolimus OR Rapamune OR Rapamycin OR “ay 22-989”) AND (kidney* OR renal)
(Everolimus OR Zortress OR Certican OR Afinitor OR Evertor OR “SDZ RAD”) AND (kidney* OR renal)
Web searches
The following websites were hand-searched:
Renal societies (UK)
British Renal Society: www.britishrenal.org/
Renal Association: www.renal.org/
UK Renal Registry: www.renalreg.com/
Kidney Research: UK www.kidneyresearchuk.org/
British Kidney Patient Association: www.britishkidney-pa.co.uk/
National Kidney Federation: www.kidney.org.uk/
Renal societies (international)
American Society of Nephrology: www.asn-online.org/
American Association of Kidney Patients: www.aakp.org/
National Kidney Foundation (US): www.kidney.org/
Canadian Society of Nephrology: www.csnscn.ca/
Kidney Foundation of Canada: www.kidney.ca/
Australian and New Zealand Society of Nephrology: www.nephrology.edu.au/
Kidney Health Australia: www.kidney.org.au/
Kidney Society Auckland: www.kidneysociety.co.nz/
Ongoing trials
The following terms were used to search the ClinicalTrials.gov and Controlled Trials (ISRCTN) trial registers for the interventions:
(Basiliximab OR Basiliximabum OR Simulect OR “interleukin 2 receptor antibody”) AND (kidney* OR renal)
((rabbit AND Anti-thymocyte*) OR (rabbit AND Antithymocyte*) OR (rabbit AND thymocyte*) OR (rabbit* AND polyclonal) OR (rabbit* AND ATG) OR RATG OR thymoglobulin*) AND (kidney* OR renal)
(Tacrolimus OR Fujimycin OR Prograf OR Advagraf OR Adoport OR Capexion OR Modigraf OR Perixis OR Tacni OR Vivadex OR Protopic OR Tsukubaenolide OR “FK 506” OR “FK-506” OR “FK506” OR “fr-900506”) AND (kidney* OR renal)
(Belatacept OR Nulojix OR “lea29y” OR “lea 29y” OR “bms 224818”) AND (kidney* OR renal)
(“Mycophenolic acid” OR MPA OR Mycophenolate OR Arzip OR CellCep* OR Myfenax OR Myfortic OR Mofetil) AND (kidney* OR renal)
(Sirolimus OR Rapamune OR Rapamycin OR “ay 22-989”) AND (kidney* OR renal)
(Everolimus OR Zortress OR Certican OR Afinitor OR Evertor OR “SDZ RAD”) AND (kidney* OR renal)
Cost-effectiveness searches
The following search strategies were used to identify studies reporting cost or economic data. They were first run on 8 April 2014 and the same strategy was used on 18 November 2014 to update the literature base. The searches took the following form: (terms for kidney or renal transplant or kidney or renal graft) AND (terms for the interventions under review) AND (a costs or economic literature search filter). The search was not limited by language and it was not limited to human-only studies because such a limit would have blocked retrieval of includable studies for rATG (line 8 of the Medline search). Searching was date limited 2002 to current, in line with the previous assessment.
Search annex
Database: MEDLINE
Host: Ovid
Data parameters: 1946 to present
Date searched: 18 November 2014
Searcher: Chris
Hits: 27
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | Kidney Transplantation/ | 81,142 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 34,392 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 41,464 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 36,554 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 46,102 |
| 6 | 1 or 2 or 3 or 4 or 5 | 114,277 |
| 7 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 1063 |
| 8 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 6382 |
| 9 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 17,331 |
| 10 | Tacrolimus/ | 13,055 |
| 11 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 219 |
| 12 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 28,176 |
| 13 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 21,975 |
| 14 | Sirolimus/ | 14,369 |
| 15 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 3088 |
| 16 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 74,259 |
| 17 | 6 and 16 | 9593 |
| 18 | Economics/ | 27,421 |
| 19 | exp Economics, Pharmaceutical/ | 2601 |
| 20 | exp Economics, Medical/ | 13,982 |
| 21 | exp Economics, Hospital/ | 20,161 |
| 22 | (pharmacoeconomic* or socioeconomics or economic$).ti,ab,kw. | 183,564 |
| 23 | ec.fs. | 349,785 |
| 24 | exp “Costs and Cost Analysis”/ | 189,530 |
| 25 | (cost* or cba or cea or cua or (value adj2 money) or pric$ or fiscal or funding or financial or finance or budget$ or (expenditure$ not Energy)).ti,ab,kw. | 530,644 |
| 26 | 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 | 896,638 |
| 27 | 17 and 26 | 440 |
| 28 | limit 27 to yr=“2014 -Current” | 27 |
Notes: not applicable.
File: not applicable.
Database: EMBASE
Host: Ovid.
Data parameters: 1974 to 17 November 2014.
Date searched: 18 November 2014.
Searcher: Chris.
Hits: 131.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | kidney transplantation/ | 97,441 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw,ot. | 50,853 |
| 3 | (Renal adj3 transplant$).ti,ab,kw,ot. | 55,991 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw,ot. | 51,947 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw,ot. | 65,675 |
| 6 | 1 or 2 or 3 or 4 or 5 | 153,480 |
| 7 | basiliximab/ | 6681 |
| 8 | (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”).ti,ab,kw,ot. | 2311 |
| 9 | thymocyte antibody/ | 20,236 |
| 10 | ((rabbit$ adj3 Anti-thymocyte$1) or (rabbit$ adj3 Antithymocyte$1) or (rabbit$ adj3 thymocyte$1) or (rabbit$ adj3 polyclonal) or (rabbit$ and ATG) or RATG or thymoglobulin$2).ti,ab,kw,ot. | 8854 |
| 11 | tacrolimus/ | 53,638 |
| 12 | (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”).ti,ab,kw,ot. | 26,290 |
| 13 | belatacept/ | 989 |
| 14 | (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”).ti,ab,kw,ot. | 547 |
| 15 | mycophenolic acid/ | 9985 |
| 16 | (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep$1 or Myfenax or Myfortic or Mofetil).ti,ab,kw,ot. | 35,917 |
| 17 | rapamycin/ | 36,443 |
| 18 | (Sirolimus or Rapamune or Rapamycin or “ay 22-989”).ti,ab,kw,ot. | 28,739 |
| 19 | everolimus/ | 14,356 |
| 20 | (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”).ti,ab,kw,ot. | 6988 |
| 21 | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 | 148,218 |
| 22 | 6 and 21 | 25,662 |
| 23 | exp Economics/ | 220,356 |
| 24 | models, economic/ | 104,606 |
| 25 | exp health economics/ | 630,542 |
| 26 | exp “Costs and Cost Analysis”/ | 260,530 |
| 27 | Cost of illness/ | 14,509 |
| 28 | resource allocation/ | 15,619 |
| 29 | pe.fs. | 61,812 |
| 30 | (cost$ or cba or cea or cua or (value adj2 money) or pric$ or fiscal or funding or financial or finance or budget$ or (expenditure$ not Energy)).ti,ab,kw. | 665,827 |
| 31 | 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 | 1,288,868 |
| 32 | 22 and 31 | 1464 |
| 33 | limit 32 to yr=“2014 -Current” | 131 |
Notes: not applicable.
File: not applicable.
Database: Cochrane NHS Economic Evaluation Database
Host: Wiley Online Library.
Data parameters: Issue 4 of 4, October 2014.
Date searched: 18 November 2014.
Searcher: Chris.
Hits: 29.
ID, Search, Hits
Search strategy
#1 MeSH descriptor: [Kidney Transplantation] this term only (3274)
#2 (Kidney* near/3 transplant*) (5590)
#3 (Renal near/3 transplant*) (4265)
#4 ((kidney or renal) near/3 (recipient* or dono* or donation* or replac*)) (3480)
#5 ((graft* or allograft* or homograft* or allogeneic) and (kidney* or renal)) (4701)
#6 #1 or #2 or #3 or #4 or #5 8481)
#7 (Basiliximab or Basiliximabum or Simulect or “interleukin 2 receptor antibody”) (457)
#8 ((rabbit* near/3 Anti-thymocyte*) or (rabbit* near/3 Antithymocyte*) or (rabbit* near/3 thymocyte*) or (rabbit* near/3 polyclonal) or (rabbit* and ATG) or RATG or thymoglobulin*) (330)
#9 (Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or “FK 506” or “FK-506” or “FK506” or “fr-900506”) (2328)
#10 MeSH descriptor: [Tacrolimus] this term only (1168)
#11 (Belatacept or Nulojix or “lea29y” or “lea 29y” or “bms 224818”) (52)
#12 (“Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep* or Myfenax or Myfortic or Mofetil) (3143)
#13 (Sirolimus or Rapamune or Rapamycin or “ay 22-989”) (1881)
#14 MeSH descriptor: [Sirolimus] this term only (1037)
#15 (Everolimus or Zortress or Certican or Afinitor or Evertor or “SDZ RAD”) (602)
#16 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 (6587)
#17 #6 and #16 Publication Date from 2005 to 2014 (1273)
Notes: not applicable.
File: not applicable.
Database: Web of Science
Host: ISI Thompson Reuters.
Data parameters: 1900–2014.
Date searched: 18 November 2014.
Searcher: Chris.
Hits: 40.
Lines 1–13 of the WOS Effectiveness search was used, combined with:
TOPIC: ((pharmacoeconomic* or socioeconomics or economic* or pric* or cost* or cba or cea or cua or “health utilit*” or “value for money”))
Notes: not applicable.
File: not applicable.
Database: EconLit
Host: EBSCOhost.
Data parameters: 1886–2014.
Date searched: 18 November 2014.
Searcher: Chris.
Hits: 0.
(Basiliximab or Basiliximabum or Simulect or Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or Belatacept or Nulojix or “Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep or Myfenax or Myfortic or Mofetil or Sirolimus or Rapamune or Rapamycin or Everolimus or Zortress or Certican or Afinitor or Evertor) AND (kidney or renal)
Notes: not applicable.
File: not applicable.
Database: Health Economic Evaluations Database (HEED)
Host: via The Cochrane Library.
Date searched: Tuesday, 18 November 2014.
Searcher: Chris.
Hits: 3.
(Basiliximab or Basiliximabum or Simulect or Tacrolimus or Fujimycin or Prograf or Advagraf or Adoport or Capexion or Modigraf or Perixis or Tacni or Vivadex or Protopic or Tsukubaenolide or Belatacept or Nulojix or “Mycophenolic acid” or MPA or Mycophenolate or Arzip or CellCep or Myfenax or Myfortic or Mofetil or Sirolimus or Rapamune or Rapamycin or Everolimus or Zortress or Certican or Afinitor or Evertor) AND (kidney or renal)
Notes: not applicable
File: not applicable
Searches for utility data
The searches for utility data are recorded below. These searches took the following form:
(terms for kidney or renal transplant or kidney or renal graft or renal dialysis) AND (terms for utility questionnaires such as SF36 or CHU 9D) and were run from database inception.
Search annex
Database: Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE
Host: Ovid
Data parameters: 1946 to present.
Date searched: 3 September 2014.
Volume: 714.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | Kidney Transplantation/ | 79,870 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw. | 33,553 |
| 3 | (Renal adj3 transplant$).ti,ab,kw. | 40,747 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw. | 35,663 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw. | 45,183 |
| 6 | 1 or 2 or 3 or 4 or 5 | 112,067 |
| 7 | Renal Dialysis/ | 73,812 |
| 8 | Peritoneal Dialysis/ | 14,950 |
| 9 | ((kidney or renal or peritoneal) and (dialysis or dialyses)).ti,ab,kw. | 48,847 |
| 10 | 7 or 8 or 9 | 107,010 |
| 11 | 6 or 10 | 201,694 |
| 12 | (euroqol or euro qol or eq5d or eq 5d or EQ-5D or EQ-5D-Y).ti,ab,kw. | 4481 |
| 13 | (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,kw. | 1391 |
| 14 | (sf10 or sf 10 or short form 10 or shortform 10 or sf ten or sften or shortform ten or short form ten).ti,ab,kw. | 77 |
| 15 | (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve).ti,ab,kw. | 3016 |
| 16 | (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab,kw. | 24 |
| 17 | (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty).ti,ab,kw. | 341 |
| 18 | (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirstysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,kw. | 17,026 |
| 19 | (health utilities index$ or (hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3)).ti,ab,kw. | 1172 |
| 20 | (“time trade off” or “time tradeoff” or TTO).ti,ab,kw. | 1234 |
| 21 | standard gamble$.ti,ab,kw. | 697 |
| 22 | (CHU9D or CHU 9D or “Child Health Utility”).ti,ab,kw. | 13 |
| 23 | “discrete choice”.ti,ab,kw. | 713 |
| 24 | (AQoL or “Assessment of Quality of Life”).ti,ab,kw. | 1274 |
| 25 | 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 | 28,980 |
| 26 | 11 and 25 | 766 |
| 27 | limit 26 to english language | 714 |
Notes: not applicable.
File name: MEDLINE.txt.
Database: EMBASE
Host: Ovid.
Data parameters: 1974 to 2014, week 34.
Date searched: 3 September 2014.
Volume: 915.
Search strategy
| # | Searches | Results |
|---|---|---|
| 1 | kidney transplantation/ | 96,703 |
| 2 | (Kidney$ adj3 transplant$).ti,ab,kw. | 50,181 |
| 3 | (Renal adj3 transplant$).ti,ab,kw. | 55,376 |
| 4 | ((kidney or renal) adj3 (recipient$ or dono$ or donation$ or replac$)).ti,ab,kw. | 51,117 |
| 5 | ((graft$ or allograft$ or homograft$ or allogeneic) and (kidney$ or renal)).ti,ab,kw. | 64,806 |
| 6 | 1 or 2 or 3 or 4 or 5 | 151,605 |
| 7 | renal replacement therapy/ | 36,722 |
| 8 | peritoneal dialysis/ | 23,371 |
| 9 | ((kidney or renal or peritoneal) and (dialysis or dialyses)).ti,ab,kw. | 64,637 |
| 10 | 7 or 8 or 9 | 97,785 |
| 11 | 6 or 10 | 224,149 |
| 12 | (euroqol or euro qol or eq5d or eq 5d or EQ-5D or EQ-5D-Y).ti,ab,kw. | 7316 |
| 13 | (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,kw. | 1533 |
| 14 | (sf10 or sf 10 or short form 10 or shortform 10 or sf ten or sften or shortform ten or short form ten).ti,ab,kw. | 109 |
| 15 | (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve).ti,ab,kw. | 4428 |
| 16 | (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab,kw. | 35 |
| 17 | (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty).ti,ab,kw. | 333 |
| 18 | (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirstysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,kw. | 23,918 |
| 19 | Short Form 36/ | 12,496 |
| 20 | (health utilities index$ or (hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3)).ti,ab,kw. | 1547 |
| 21 | (“time trade off” or “time tradeoff” or TTO).ti,ab,kw. | 1599 |
| 22 | standard gamble$.ti,ab,kw. | 812 |
| 23 | (CHU9D or CHU 9D or “Child Health Utility”).ti,ab,kw. | 13 |
| 24 | “discrete choice”.ti,ab,kw. | 958 |
| 25 | (AQoL or “Assessment of Quality of Life”).ti,ab,kw. | 1812 |
| 26 | 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 | 43,846 |
| 27 | 11 and 26 | 991 |
| 28 | limit 27 to english language | 915 |
Notes: not applicable.
File Name: EMBASE.txt.
Database: The Cochrane Library (Cochrane Central Register of Controlled Trials, Health Technology Assessment and NHS Economic Evaluation Database)
Host: Wiley Online Library.
Data parameters: CENTRAL Issue 8 of 12, August 2014; HTA and NHS EED issue 3 of 4 July 2014.
Date searched: 3 September 2014.
Volume: 174.
Search strategy:
ID, Search, Hits
Search strategy
#1 MeSH descriptor: [Kidney Transplantation] this term only (3298)
#2 (Kidney* near/2 transplant*) (5497)
#3 (Renal near/2 transplant*) (3841)
#4 ((kidney or renal) near/2 (recipient* or dono* or donation* or replac*)) (3399)
#5 ((graft* or allograft* or homograft* or allogeneic) and (kidney* or renal)) (4785)
#6 #1 or #2 or #3 or #4 or #5 (8307)
#7 MeSH descriptor: [Renal Dialysis] this term only (3496)
#8 MeSH descriptor: [Peritoneal Dialysis] this term only (417)
#9 ((kidney or renal or peritoneal) and (dialysis or dialyses)) (8888)
#10 #7 or #8 or #9 (8888)
#11 #6 or #10 (15,502)
#12 (euroqol or euro qol or eq5d or eq 5d or EQ-5D or EQ-5D-Y) (2221)
#13 (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six) (11,746)
#14 (sf10 or sf 10 or short form 10 or shortform 10 or sf ten or sften or shortform ten or short form ten) (12,533)
#15 (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve) (9569)
#16 (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen) (6668)
#17 (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty) (7393)
#18 (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirstysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six) (9081)
#19 (health utilities index* or (hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3)) (6541)
#20 (“time trade off” or “time tradeoff” or TTO) (512)
#21 standard gamble* (521)
#22 (CHU9D or CHU 9D or “Child Health Utility”) (3)
#23 “discrete choice” (47)
#24 (AQoL or “Assessment of Quality of Life”) (302)
#25 #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 (22,511)
#26 #11 and #25 (847)
Notes: N/A.
File name: Cochrane.txt.
Resource: School of Health and Related Research (ScHARR) Health Utilities Database (HUD)
URL: (http://update-sbs.update.co.uk/scharr11/index.php?recordsN1&m=search)
Date searched: 3 September 2014.
Volume: 9.
Search strategy: kidney* or renal or dialysis
Notes:
File name:
Resource: Euroqol website
URL: www.euroqol.org/eq-5d-references/reference-search.html
Date searched: 3 September 2014.
Volume: 24.
Search strategy: kidney or renal or dialysis.
Notes: 5/24 were unique when deduplicated against the EMBASE search.
File name:
Resource: Higher Education Recruitment Consortium (HERC) database of mapping studies
URL: www.herc.ox.ac.uk/downloads/mappingdatabase
Date searched: 3 September 2014.
Volume: 0.
Search strategy: A hand-search of the Excel database was performed.
Notes: Dakin H. Review of studies mapping from quality of life or clinical measures to EQ-5D: an online database. Health Qual Life Outcomes 2013;11:151. HERC database of mapping studies, version 3.0 (last updated 26 June 2014). URL: www.herc.ox.ac.uk/downloads/mappingdatabase.
Appendix 2 Excluded studies
| Study | Rationale |
|---|---|
| Abou-Jaoude MM, Ghantous I, Almawi WY. Tacrolimus (FK506) versus cyclosporin A microemulsion (Neoral) maintenance immunosuppression: effects on graft survival and function, infection, and metabolic profile following kidney transplantation (KT). Mol Immunol 2003;39:1095–100 | Population |
| Abramowicz D, Carmen Rial M, Vítko S, Castillo D, Manas D, Lao M, et al. Ciclosporin withdrawal from a mycophenolate mofetil-containing immunosuppressive regimen: results of a five-year, prospective, randomized study. J Am Soc Nephrol 2005;16:2234–40 | Population |
| Adu D, Cockwell P, Ives NJ, Shaw J, Wheatley K. Interleukin-2 receptor monoclonal antibodies in renal transplantation: meta-analysis of randomised trials. BMJ 2003;326:789 | Study design |
| Agha I, Brennan D. BK virus and current immunosuppressive therapy. Graft 2002;5:S65 | Study design |
| Ahsan N, Holman MJ, Jarowenko MV, Razzaque MS, Yang HC. Limited dose monoclonal IL-2R antibody induction protocol after primary kidney transplantation. Am J Transplant 2002;2:568–73 | Intervention |
| Albano L, Alamartine E, Toupance O, Moulin B, Merville P, Rerolle JP, et al. Conversion from everolimus with low-exposure cyclosporine to everolimus with mycophenolate sodium maintenance therapy in kidney transplant recipients: a randomized, open-label multicenter study. Ann Transplant 2012;17:58–67 | Population |
| Alberú J, Pascoe MD, Campistol JM, Schena FP, Rial Mdel C, Polinsky M, et al. Lower malignancy rates in renal allograft recipients converted to sirolimus-based, calcineurin inhibitor-free immunotherapy: 24-month results from the CONVERT trial. Transplantation 2011;92:303–10 | Population |
| Alloway R, Steinberg S, Khalil K, Gourishankar S, Miller J, Norman D, et al. Conversion of stable kidney transplant recipients from a twice daily Prograf®-based regimen to a once daily modified release tacrolimus-based regimen. Transplant Proc 2005;37:867–70 | Study design |
| Andrassy J, Hoffmann VS, Rentsch M, Stangl M, Habicht A, Meiser B, et al. Is cytomegalovirus prophylaxis dispensable in patients receiving an motor inhibitor-based immunosuppression? A systematic review and meta-analysis. Transplantation 2012;94:1208–17 | Population |
| Andres A, Delgado-Arranz M, Morales E, Dipalma T, Polanco N, Gutierrez-Solis E, et al. Extended-release tacrolimus therapy in de novo kidney transplant recipients: single-center experience. Transplant Proc 2010;42:3034–7 | Study design |
| Araki M, Flechner SM, Ismail HR, Flechner LM, Zhou LM, Derweesh IH, et al. Posttransplant diabetes mellitus in kidney transplant recipients receiving calcineurin or mTOR inhibitor drugs. Transplantation 2006;81:335–41 | Study design |
| Arns W, Breuer S, Choudhury S, Taccard G, Lee J, Binder V, et al. Enteric-coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophenolate mofetil. Clin Transplant 2005;19:199–206 | Outcome |
| Arora S, Tangirala B, Osadchuk L, Sureshkumar KK. Belatacept: a new biological agent for maintenance immunosuppression in kidney transplantation. Expert Opin Biol Ther 2012;12:965–79 | Study design |
| Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Hené RJ, et al. Randomized conversion from cyclosporine to tacrolimus in renal transplant patients: improved lipid profile and unchanged plasma homocysteine levels. Transplant Proc 2002;34:1793–4 | Population |
| Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Improved cardiovascular risk profile and renal function in renal transplant patients after randomized conversion from cyclosporine to tacrolimus. J Am Soc Nephrol 2003;14:1880–8 | Population |
| Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Conversion from cyclosporine to tacrolimus improves quality-of-life indices, renal graft function and cardiovascular risk profile. Am J Transplant 2004;4:937–45 | Population |
| Åsberg A, Apeland T, Reisaeter AV, Foss A, Leivestad T, Heldal K, et al. Long-term outcomes after cyclosporine or mycophenolate withdrawal in kidney transplantation: results from an aborted trial. Clin Transplant 2013;27:E151–6 | Population |
| Baas MC, Gerdes VEA, Berge IJM, Heutinck KM, Florquin S, Meijers JCM, et al. Treatment with everolimus is associated with a procoagulant state. Thromb Res 2013;132:307–11 | Outcome |
| Baczkowska T, Perkowska-Ptasińska A, Sadowska A, Lewandowski Z, Nowacka-Cieciura E, Cieciura T, et al. Serum TGF-beta1 correlates with chronic histopathological lesions in protocol biopsies of kidney allograft recipients. Transplant Proc 2005;37:773–5 | Intervention |
| Bakker RC, Hollander AA, Mallat MJ, Bruijn JA, Paul LC, de Fijter JW. Conversion from cyclosporine to azathioprine at three months reduces the incidence of chronic allograft nephropathy. Kidney Int 2003;64:1027–34 | Intervention |
| Bataille S, Moal V, Gaudart J, Indreies M, Purgus R, Dussol B, et al. Cytomegalovirus risk factors in renal transplantation with modern immunosuppression. Transpl Infect Dis 2010;12:480–8 | Outcome |
| Bemelman FJ, de Maar EF, Press RR, van Kan HJ, ten Berge IJ, Homan van der Heide JJ, et al. Minimization of maintenance immunosuppression early after renal transplantation: an interim analysis. Transplantation 2009;88:421–8 | |
| Blydt-Hansen TD, Gibson IW, Birk PE. Histological progression of chronic renal allograft injury comparing sirolimus and mycophenolate mofetil-based protocols. A single-center, prospective, randomized, controlled study. Pediatr Transplant 2010;14:909–18 | No data |
| Birnbaum LM, Lipman M, Paraskevas S, Chaudhury P, Tchervenkov J, Baran D, et al. Management of chronic allograft nephropathy: a systematic review. Clin J Am Soc Nephrol 2009;4:860–5 | Outcome |
| Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant 2005;5:582–594 | Duplicate |
| Budde K, Glander P, Diekmann F, Dragun D, Waiser J, Fritsche L, et al. Enteric-coated mycophenolate sodium: safe conversion from mycophenolate mofetil in maintenance renal transplant recipients. Transplant Proc 2004;36:S524–7 | Population |
| Budde K, Curtis J, Knoll G, Chan L, Neumayer HH, Seifu Y, et al. Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant 2004;4:237–43 | Population |
| Budde K, Knoll G, Curtis J, Kahana L, Pohanka E, Seifu Y, et al. Safety and efficacy after conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium: results of a 1-year extension study. Transplant Proc 2005;37:912–15 | Study design |
| Budde, K, Knoll, G, Curtis, J, Chan, L, Pohanka, E, Gentil, M, et al. Long-term safety and efficacy after conversion of maintenance renal transplant recipients from mycophenolate mofetil (MMF) to enteric-coated mycophenolate sodium (EC-MPA, myfortic). Nieren Hochdruck 2006;35:454–64 | Language |
| Budde K, Knoll G, Curtis J, Chan L, Pohanka E, Gentil M, et al. Long-term safety and efficacy after conversion of maintenance renal transplant recipients from mycophenolate mofetil (MMF) to enteric-coated mycophenolate sodium (EC-MPA, myfortic). Clin Nephrol 2006;66:103–11 | Study design |
| Bunnapradist S, Ciechanowski K, West-Thielke P, Mulgaonkar S, Rostaing L, Vasudev B, et al. Conversion from twice-daily tacrolimus to once-daily extended release tacrolimus (LCPT): the phase III randomized MELT trial. Am J Transplant 2013;13:760–9 | Population |
| Burke GW. Randomized trial of 2 antibody induction steroid avoidance protocols accompanied by maintenance therapy with Prograf ® and Myfortic. 2001. URL: http://clinicaltrials.gov/ct2/show/NCT01172418 (accessed 18 July 2014) | Comparator |
| Busque S, Cantarovich M, Mulgaonkar S, Gaston R, Gaber AO, Mayo PR, et al. The PROMISE study: a phase 2b multicenter study of voclosporin (ISA247) versus tacrolimus in de novo kidney transplantation. Am J Transplant 2011;11:2675–84 | Outcome |
| Cabello M, García P, González-Molina M, Díez de los Rios MJ, García-Sáiz M, Gutiérrez, C, et al. Pharmacokinetics of once- versus twice-daily tacrolimus formulations in kidney transplant patients receiving expanded criteria deceased donor organs: a single-center, randomized study. Transplant Proc 2010;42:3038–40 | Population |
| Study design | |
| Campbell SB, Walker R, Tai SS, Jiang Q, Russ GR. Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am J Transplant 2012;12:1146–56 | Population |
| Carroll RP, Hester J, Wood KJ, Harden PN. Conversion to sirolimus in kidney transplant recipients with squamous cell cancer and changes in immune phenotype. Nephrol Dial Transplant 2013;28:462–5 | Population |
| Cataneo-Davila A, Zuniga-Varga J, Correa-Rotter R, Alberu J. Renal function outcomes in kidney transplant recipients after conversion to everolimus-based immunosuppression regimen with CNI reduction or elimination. Transplant Proc 2009;41:4138–46 | Population |
| Chadban SJ, Eris JM, Kanellis J, Pilmore H, Lee PC, Lim SK, et al. A randomized, controlled trial of everolimus-based dual immunosuppression versus standard of care in de novo kidney transplant recipients. Transpl Int 2014;27:302–11 | Language |
| Chan L, Greenstein S, Hardy MA, Hartmann E, Bunnapradist S, Cibrik D, et al. Multicenter, randomized study of the use of everolimus with tacrolimus after renal transplantation demonstrates its effectiveness. Transplantation 2008;85:821–6 | Comparator |
| Chhabra D, Alvarado A, Dalal P, Leventhal J, Wang C, Sustento-Reodica N, et al. Impact of calcineurin-inhibitor conversion to mTOR inhibitor on renal allograft function in a prednisone-free regimen. Am J Transplant 2013;13:2902–11 | Population |
| Chisholm MA, Middleton MD. Modified-release tacrolimus. Ann Pharmacother 2006;40:270–5 | Study design |
| Ciancio G, Miller J, Gonwa TA. Review of major clinical trials with mycophenolate mofetil in renal transplantation. Transplantation 2005;80:S191–200 | Study design |
| Citterio F, Scatà MC, Romagnoli J, Pozzetto U, Nanni G, Castagneto M. Conversion to tacrolimus immunosuppression in renal transplant recipients: 12-month follow-up. Transplant Proc 2002;34:1685–6 | Population |
| Cransberg K, Cornelissen M, Lilien M, Hoeck K, Davin JC, Nauta J. Maintenance immunosuppression with mycophenolate mofetil and corticosteroids in pediatric kidney transplantation: temporary benefit but not without risk. Transplantation 2007;83:1041–7 | Population |
| Cruzado JM, Bestard O, Riera L, Torras J, Gil-Vernet S, Seron D, et al. Immunosuppression for dual kidney transplantation with marginal organs: the old is better yet. Am J Transplant 2007;7:639–44 | Study design |
| Dantal J, Berthoux F, Moal MC, Rostaing L, Legendre C, Genin R, et al. Efficacy and safety of de novo or early everolimus with low cyclosporine in deceased-donor kidney transplant recipients at specified risk of delayed graft function: 12-month results of a randomized, multicenter trial. Transpl Int 2010;23:1084–93. [Erratum published in Transpl Int 2012;25:138.] | Duplicate |
| Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 2004;77:1555–61 | Outcome |
| Diekmann F, Gutierrez-Dalmau A, Lopez S, Cofan F, Esforzado N, Ricart MJ, et al. Influence of sirolimus on proteinuria in de novo kidney transplantation with expanded criteria donors: comparison of two CNI-free protocols. Nephrol Dial Transplant 2004;22:2316–21 | Population |
| Dudley C, Pohanka E, Riad H, Dedochova J, Wijngaard P, Sutter C, et al. Mycophenolate mofetil substitution for cyclosporine a in renal transplant recipients with chronic progressive allograft dysfunction: the ‘creeping creatinine’ study. Transplantation 2005;79:466–75 | Population |
| Durlik M, Paczek L, Rutkowski B, Lewandowska D, Debska-Slizien A, Chamienia A, et al. The efficacy and safety of ciclosporin (Equoral®) capsules after renal transplantation: a multicentre, open-label, phase IV clinical trial. Ann Transplant 2010;15:51–9 | Study design |
| Ekberg H, Grinyó J, Nashan B, Vanrenterghem Y, Vincenti F, Voulgari A, et al. Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR study. Am J Transplant 2007;7:560–70 | Comparator |
| Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007;357:2562–75 | Intervention |
| El-Agroudy AE, El-Dahshan KF, Wafa EW, Sheashaa HA, Gad ZA, Ismail AM, et al. Safe conversion of mycophenolate mofetil to azathioprine in kidney transplant recipients with sirolimus-based immunosuppression. Nephrology 2009;14:255–61 | Population |
| El-Sabrout R, Delaney V, Qadir M, Butt F, Hanson P, Butt KM. Sirolimus in combination with tacrolimus or mycophenolate mofetil for minimizing acute rejection risk in renal transplant recipients: a single center experience. Transplant Proc 2003;35:S89–94 | Study design |
| Facundo C, Diaz JM, Guirado L, Duran F, Herreros MA, Diaz M, et al. Results of a triple induction regime with tacrolimus, mycophenolate mofetil, and prednisone in renal transplantation. Transplant Proc 2002;34:98 | Study design |
| Favi E, Citterio F, Spagnoletti G, Gargiulo A, Delreno F, Romagnoli J, et al. Prospective clinical trial comparing two immunosuppressive regimens, tacrolimus and mycophenolate mofetil versus everolimus and low-dose cyclosporine, in de novo renal transplant recipients: results at 6 months follow-up. Transplant Proc 2009;41:1152–5 | Study design |
| Favi E, Spagnoletti G, Salerno MP, Pedroso JA, Romagnoli J, Citterio F. Tacrolimus plus mycophenolate mofetil vs. cyclosporine plus everolimus in deceased donor kidney transplant recipients: three-year results of a single-center prospective clinical trial. Clin Transplant 2013;27:E359–67 | Study design |
| Ferguson R, Grinyó J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, et al. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant 2011;11:66–76 | Population |
| Ferrer F, Machado S, Alves R, Macario F, Bastos C, Roseiro A, et al. Induction with basiliximab in renal transplantation. Transplant Proc 2010;42:467–70 | Study design |
| Filipe R, Mota A, Alves R, Bastos C, Macario F, Figueiredo A, et al. Kidney transplantation with corticosteroid-free maintenance immunosuppression: a single center analysis of graft and patient survivals. Transplant Proc 2009;41:843–5 | Study design |
| Filler G, Webb NJ, Milford DV, Watson AR, Gellermann J, Tyden G, et al. Four-year data after pediatric renal transplantation: a randomized trial of tacrolimus vs. cyclosporin microemulsion. Pediatr Transplant 2005;9:498–503 | Outcome |
| Flechner S, Friend P, Campistol J, Weir M, Diekmann F, Russ G. De novo immunosuppression with mammalian target of rapamycin inhibitors and posttransplantation malignancy in focus. Transplant Proc 2009;41:S42–4 | Study design |
| Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 2002;74:1070–6 | Population |
| Friend PJ. Thymoglobulin Induction and Steroid-free Immunosuppression in Kidney Transplantation from Deceased Donors after Cardiac Death: An Open Label Randomised Controlled Trial to Evaluate the Role of Thymoglobulin as Induction Immunosuppression in Kidney Transplants from Deceased Donors after Cardiac Death. 2014. URL: http://clinicaltrials.gov/ct2/show/NCT01239563 (accessed 25 July 2014) | No data |
| Frimat L, Cassuto-Viguier E, Charpentier B, Noël C, Provôt F, Rostaing L, et al. Impact of cyclosporine reduction with MMF: a randomized trial in chronic allograft dysfunction. The ‘reference’ study. Am J Transplant 2006;6:2725–34 | Population |
| Frimat L, Cassuto-Viguier E, Provot F, Rostaing L, Charpentier B, Akposso K, et al. Long-term impact of cyclosporin reduction with MMF treatment in chronic allograft dysfunction: REFERENCE study 3-year follow up. J Transplant 2010;2010:402750 | Population |
| Foroncewicz B, Mucha K, Ciszek M, Malkowski P, Durlik M, Szmidt J, et al. A comparison between two tacrolimus-based immunosuppression regimens in renal transplant recipients: 7-year follow-up. Ann Transplant 2013;18:384–92 | Study design |
| Gaber AO, Kahan BD, Buren C, Schulman SL, Scarola J, Neylan JF. Comparison of sirolimus plus tacrolimus versus sirolimus plus cyclosporine in high-risk renal allograft recipients: results from an open-label, randomized trial. Transplantation 2008;86:1187–95 | Population |
| Garcia I. Efficacy and safety of dual versus triple tacrolimus-based therapy in kidney transplantation: two-year follow-up. Transplant Proc 2002;34:1638–9 | Comparator |
| Gonzalez F, Espinoza M, Herrera P, Rocca X, Reynolds E, Lorca E, et al. Everolimus versus azathioprine in a cyclosporine and ketoconazole-based immunosuppressive therapy in kidney transplant: 3-year follow-up of an open-label, prospective, cohort, comparative clinical trial. Transplant Proc 2010;42:270–2 | Study design |
| van Gelder T, Silva HT, de Fijter JW, Budde K, Kuypers D, Tyden G, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation 2008;86:1043–51 | Comparator |
| van Gelder T, Tedesco-Silva H, Fijter JW, Budde K, Kuypers D, Arns W, et al. Renal transplant patients at high risk of acute rejection benefit from adequate exposure to mycophenolic acid. Transplantation 2010;89:595–599 | Comparator |
| van Gelder T, Silva HT, de Fijter H, Budde K, Kuypers D, Mamelok RD, et al. How delayed graft function impacts exposure to mycophenolic acid in patients after renal transplantation. Ther Drug Monit 2011;33:155–64 | Population |
| Gonwa T, Johnson C, Ahsan N, Alfrey EJ, Halloran P, Stegall M, et al. Randomized trial of tacrolimus plus mycophenolate mofetil or azathioprine versus cyclosporine plus mycophenolate mofetil after cadaveric kidney transplantation: results at three years. Transplantation 2003;75:2048–59 | Population |
| Gonzalez Molina M, Morales JM, Marcen R, Campistol JM, Oppenheimer F, Seron D, et al. Renal function in patients with cadaveric kidney transplants treated with tacrolimus or cyclosporine. Transplant Proc 2007;39:2167–9 | Study design |
| Gürkan A, Kaçar S, Erdoğdu U, Varilsüha C, Kandemir G, Karaca C, et al. The effect of sirolimus in the development of chronic allograft nephropathy. Transplant Proc 2008;40:114–16 | Population |
| Grafals M. Low Dose Thymoglobulin as Induction Agent on Prednisone-free Regimens of Renal Transplant Recipients. 2011. URL: http://clinicaltrials.gov/ct2/show/NCT01280617 (accessed 25 July 2014) | Comparator |
| Grinyo J, Alberu J, Contieri FL, Manfro RC, Mondragon G, Nainan G, et al. Improvement in renal function in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: 2-year results from the long-term extension of a phase II study. Transpl Int 2012;25:1059–64 | Population |
| Grushkin C, Mahan JD, Mange KC, Hexham JM, Ettenger R. De novo therapy with everolimus and reduced-exposure cyclosporine following pediatric kidney transplantation: a prospective, multicenter, 12-month study. Pediatr Transplant 2013;17:237–43 | Population |
| Study design | |
| Hakemi M, Shahebrahimi K, Ganji MR, Najafi I, Broumand B. Side effects of mycophenolate mofetil versus azathioprine in Iranian renal transplant recipients (single-center experience). Transplant Proc 2002;34:2091–2 | Study design |
| Han DJ, Park JB, Kim YS, Kim SJ, Ha J, Kim HC, et al. A 39-month follow-up study to evaluate the safety and efficacy in kidney transplant recipients treated with modified-release tacrolimus (FK506E)-based immunosuppression regimen. Transplant Proc 2012;44:115–17 | Study design |
| Han F, Wu J, Huang H, Zhang X, He Q, Wang Y, et al. Conversion from cyclosporine to sirolimus in chronic renal allograft dysfunction: a 4-year prospective study. Exp Clin Transplant 2011;9:42–9 | Population |
| Hazzan M, Labalette M, Copin MC, Glowacki F, Provôt F, Pruv FR, et al. Predictive factors of acute rejection after early cyclosporine withdrawal in renal transplant recipients who receive mycophenolate mofetil: results from a prospective, randomized trial. J Am Soc Nephrol 2005;16:2509–16 | Outcome |
| Heller T, van Gelder T, Budde K, de Fijter JW, Kuypers D, Arns W, et al. Plasma concentrations of mycophenolic acid acyl glucuronide are not associated with diarrhea in renal transplant recipients. Am J Transplant 2007;7:1822–31 | Outcome |
| Van Hest RM, van Gelder T, Vulto AG, Mathot RA. Population pharmacokinetics of mycophenolic acid in renal transplant recipients. Clin Pharmacokinet 2005;44:1083–96 | Study design |
| Hirsch HH, Vincenti F, Friman S, Tuncer M, Citterio F, Wiecek A, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant 2013;13:136–45 | Outcome |
| Höcker B, Kovarik JM, Daniel V, Opelz G, Fehrenbach H, Holder M, et al. Pharmacokinetics and immunodynamics of basiliximab in pediatric renal transplant recipients on mycophenolate mofetil comedication. Transplantation 2008;86:1234–40 | Comparator |
| Holdaas H, Rostaing L, Serón D, Cole E, Chapman J, Fellstrøm B, et al. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation 2011;92:410–18 | Population |
| Duplicate | |
| Van Hooff JP, Squifflet JP, Wlodarczyk Z, Vanrenterghem Y, Paczek L. A prospective randomized multicenter study of tacrolimus in combination with sirolimus in renal-transplant recipients. Transplantation 2003;75:1934–9 | Comparator |
| Van Hooff J, Walt I, Kallmeyer J, Miller D, Dawood S, Moosa MR, et al. Pharmacokinetics in stable kidney transplant recipients after conversion from twice-daily to once-daily tacrolimus formulations. Ther Drug Monit 2012;34:46–52 | Study design |
| Huang HF, Yao X, Chen Y, Xie WQ, Shen-Tu JZ, Chen JH, et al. Cyclosporine A and tacrolimus combined with enteric-coated mycophenolate sodium influence the plasma mycophenolic acid concentration: a randomised controlled trial in Chinese live related donor kidney transplant recipients. Int J Clin Pract Suppl 2014;68:4–9 | Outcome |
| Iaria G, Pisani F, Iorio B, Lucchesi C, De Luca L, Ielpo B. Long-term results of kidney transplantation with cyclosporine- and everolimus-based immunosuppression. Transplant Proc 2006;38:1018–19 | Study design |
| Ireland R. Early switch from calcineurin inhibitors to mTOR inhibitors leads to improved renal graft function. Nat Rev Nephrol 2011;7:243 | Study design |
| ISRCT. A Randomised Prospective Trial of Daclizumab Induction Followed by Sirolimus in Association with Mycophenolate Mofetil and Steroids versus Standard Cyclosporin based Triple Therapy for Rejection Prophylaxis in Renal Transplantation. 2011. URL: http://controlled-trials.com/ISRCTN74336394 (accessed 25 July 2014) | No data |
| ISRCT. A Prospective Randomised Trial of the Use of Cellcept to Allow Early Tacrolimus Withdrawal in Live Donor Kidney Transplantation. 2004. URL: http://controlled-trials.com/ISRCTN63298320 (accessed 25 July 2014) | No data |
| ISRCT. Mycophenolate Sodium versus Everolimus or Cyclosporine with Allograft Nephropathy as Outcome. 2006. URL: http://controlled-trials.com/ISRCTN69188731 (accessed 25 July 2014) | No data |
| Jevnikar A, Arlen D, Barrett B, Boucher A, Cardella C, Cockfield SM, et al. Five-year study of tacrolimus as secondary intervention versus continuation of cyclosporine in renal transplant patients at risk for chronic renal allograft failure. Transplantation 2008:86:953–60 | Population |
| Jose M. Calcineurin inhibitors in renal transplantation: adverse effects. Nephrology 2007;12:S66–74 | Study design |
| Joss N, Rodger RS, McMillan MA, Junor BJ. Randomized study comparing cyclosporine with azathioprine one year after renal transplantation: 15-year outcome data. Transplantation 2007;83:582–7 | Population |
| Jungraithmayr TC, Wiesmayr S, Staskewitz A, Kirste G, Bulla M, Fehrenbach H, et al. Five-year outcome in pediatric patients with mycophenolate mofetil-based renal transplantation. Transplantation 2007;83:900–5 | Study design |
| Jurewicz WA. Tacrolimus versus cyclosporin immunosuppression: long-term outcome in renal transplantation. Nephrol Dial Transplant 2003;1:i7–11 | Population |
| Kahan BD. Two-year results of multicenter phase III trials on the effect of the addition of sirolimus to cyclosporine-based immunosuppressive regimens in renal transplantation. Transplant Proc 2003;35:S37–51 | Population |
| Kamar N, Allard J, Ribes D, Durand D, Ader JL, Rostaing L. Assessment of glomerular and tubular functions in renal transplant patients receiving cyclosporine A in combination with either sirolimus or everolimus. Clin Nephrol 2005;63:80–6 | Study design |
| Kamar N, Rostaing L, Cassuto E, Villemain F, Moal MC, Ladrière M, et al. A multicenter, randomized trial of increased mycophenolic acid dose using enteric-coated mycophenolate sodium with reduced tacrolimus exposure in maintenance kidney transplant recipients. Clin Nephrol 2012;77:126–36 | Population |
| Kandaswamy R, Melancon JK, Dunn T, Tan M, Casingal V, Humar A, et al. A prospective randomized trial of steroid-free maintenance regimens in kidney transplant recipients: an interim analysis. Am J Transplant 2005;5:1529–36 | Population |
| Ke-Pu L, Xiao-Min Y, Shuai-Jun M, Zhi-Bin L, Geng Z, Jian-Lin Y. Effects of tacrolimus and cyclosporine A on inflammatory cytokines and blood lipid after renal transplantation. J Clin Rehabil Tissue Eng Res 2011;15:5769–72 | Language |
| Khwaja K, Asolati M, Harmon J, Melancon JK, Dunn T, Gillingham K, et al. Outcome at 3 years with a prednisone-free maintenance regimen: a single-center experience with 349 kidney transplant recipients. Am J Transplant 2004;4:980–7 | Study design |
| Kihm LP, Hinkel UP, Michael K, Sommerer C, Seckinger J, Morath C, et al. Contrast enhanced sonography shows superior microvascular renal allograft perfusion in patients switched from cyclosporine A to everolimus. Transplantation 2009;88:261–5 | Population |
| Koch M, Becker T, Lueck R, Neipp M, Klempnauer J, Nashan B. Basiliximab induction therapy in kidney transplantation: benefits for long term allograft function after 10 years? Biologics 2009;3:51–6 | Study design |
| Khosroshahi HT, Tubbs RS, Shoja MM, Ghafari A, Noshad H, Ardalan MR. Effect of prophylaxis with low-dose anti-thymocyte globulin on prevention of acute kidney allograft rejection. Transplant Proc 2008;40:137–9 | Population |
| Kovac D, Kotnik V, Kandus A. Basiliximab and mycophenolate mofetil in combination with low-dose cyclosporine and methylprednisolone effectively prevent acute rejection in kidney transplant recipients. Transplant Proc 2005;37:4230–4 | Study design |
| Krämer BK, Zülke C, Kammerl MC, Schmidt C, Hengstenberg C, Fischereder M, et al. Cardiovascular risk factors and estimated risk for CAD in a randomized trial comparing calcineurin inhibitors in renal transplantation. Am J Transplant 2003;3:982–7 | Outcome |
| Krämer BK, Böger C, Krüger B, Marienhagen J, Pietrzyk M, Obed A, et al. Cardiovascular risk estimates and risk factors in renal transplant recipients. Transplant Proc 2005;37:1868–70 | Outcome |
| Krämer BK, Klinger M, Vítko S, Glyda M, Midtvedt K, Stefoni S, et al. Tacrolimus-based, steroid-free regimens in renal transplantation: 3-year follow-up of the ATLAS trial. Transplantation 2012;94:492–8 | Comparator |
| Kreis H. Worse renal transplant outcomes with sirolimus-mycophenolate than with calcineurin inhibitor regimens. Nat Clin Pract Nephrol 2007;3:424–5 | Study design |
| Krischock L, Marks SD. Induction therapy: why, when, and which agent? Pediatr Transplant 2010;14:298–313 | Study design |
| Kwon O, Cho JH, Choi JY, Park SH, Kim YL, Kim HK, et al. Long-term outcome of azathioprine versus mycophenolate mofetil in cyclosporine-based immunosuppression in kidney transplantation: 10 years of experience at a single center. Transplant Proc 2013;45:1487–90 | Study design |
| Kumar A, Zaman W, Chaurasia D, Gupta A, Sharma RK, Gulati S. Prospective randomized trial to evaluate the efficacy of single low dose ATG induction in renal transplant recipient with spousal kidney. Indian J Urol 2002;19:58–62 | Study design |
| Langone AJ, Chan L, Bolin P, Cooper M. Enteric-coated mycophenolate sodium versus mycophenolate mofetil in renal transplant recipients experiencing gastrointestinal intolerance: a multicenter, double-blind, randomized study. Transplantation 2011;91:470–8 | Population |
| Lezaic VD, Marinkovic J, Ristic S, Dokic ZM, Basta Jovanovic G, Radivojevic DM, et al. Conversion of azathioprine to mycophenolate mofetil and chronic graft failure progression. Transplant Proc 2005;37:734–6 | Population |
| Lin CC, Chuang FR, Lee CH, Wang CC, Chen YS, Liu YW, et al. The renal-sparing efficacy of basiliximab in adult living donor liver transplantation. Liver Transpl 2005;11:1258–64 | Study design |
| Liu B, Lin ZB, Ming CS, Zhang WJ, Chen ZS, Sha B. Randomized trial of tacrolimus in combination with mycophenolate mofetil versus cyclosporine with mycophenolate mofetil in cadaveric renal transplant recipients with delayed graft function. Transplant Proc 2003;35:87–8 | Study design |
| Liu M, Zhang W, Gu M, Yin C, Zhang WY, Lv Q, et al. Protective effects of sirolimus by attenuating connective tissue growth factor expression in human chronic allograft nephropathy. Transplant Proc 2007;39:1410–15 | Outcome |
| Liu Y, Zhou P, Han M, Xue CB, Hu XP, Li C. Basiliximab or antithymocyte globulin for induction therapy in kidney transplantation: a meta-analysis. Transplant Proc 2010;42:1667–70 | Study design |
| Liu Y, Yang MS, Yuan JY. Immunosuppressant utilization and cardiovascular complications among Chinese patients after kidney transplantation: a systematic review and analysis. Int Urol Nephrol 2013;45:885–92 | Study design |
| Ljuca F, Imamovic S, Mesic D, Hasukic SH, Omerovic S, Bazardzanovic M. Mycophenolate mofetil versus azathioprine: effects on renal graft function in early posttransplant period. Bosn J Basic Med Sci 2009;9:156–60 | Study design |
| Lou HX, Vathsala A. Conversion from mycophenolate mofetil to azathioprine in high-risk renal allograft recipients on cyclosporine-based immunosuppression. Transplant Proc 2004;36:2090–1 | Population |
| Loriga G, Ciccarese M, Pala PG, Satta RP, Fanelli V, Manca ML, et al. De novo everolimus-based therapy in renal transplant recipients: effect on proteinuria and renal prognosis. Transplant Proc 2010;42:1297–302 | Study design |
| Population | |
| Luan FL, Zhang H, Schaubel DE, Miles CD, Cibrik D, Norman S, et al. Comparative risk of impaired glucose metabolism associated with cyclosporine versus tacrolimus in the late posttransplant period. Am J Transplant 2008;8:1871–7 | Study design |
| Outcome | |
| Maiorano A, Stallone G, Schena A, Infante B, Pontrelli P, Schena FP, et al. Sirolimus interferes with iron homeostasis in renal transplant recipients. Transplantation 2006;82:908–12 | Population |
| Martínez-Castelao A, Sarrias X, Bestard O, Gil-Vernet S, Serón D, Cruzado JM, et al. Arterial elasticity measurement in renal transplant patients under anticalcineurin immunosuppression. Transplant Proc 2005;37:3788–90 | Population |
| Study design | |
| Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant 2004;18:446–9 | Study design |
| Meier M, Nitschke M, Weidtmann B, Jabs WJ, Wong W, Suefke S, et al. Slowing the progression of chronic allograft nephropathy by conversion from cyclosporine to tacrolimus: a randomized controlled trial. Transplantation 2006;81:1035–40 | Study design |
| Language | |
| Meier-Kriesche HU, Davies NM, Grinyo J, Heading R, Mamelok R, Wijngaard P, et al. Mycophenolate sodium does not reduce the incidence of GI adverse events compared with mycophenolate mofetil. Am J Transplant 2005;5:1164 | Study design |
| Metcalfe MS, Jain S, Waller JR, Saunders RN, Bicknell GR, Nicholson ML. A randomized trial of mycophenolate mofetil versus azathioprine as calcineurin inhibitor sparing agents in the treatment of chronic allograft nephropathy. Transplant Proc 2002;34:1812–14 | Population |
| Monaco AP, Morris PJ. Everolimus and long-term outcomes in renal transplantation: seeking an optimal strategy for immunosuppression. Transplantation 2011;92:S1–2 | Study design |
| Montagnino G, Sandrini S, Casciani C, Schena FP, Carmellini M, Civati G, et al. A randomized trial of steroid avoidance in renal transplant patients treated with everolimus and cyclosporine. Transplant Proc 2005;37:788–90 | Comparator |
| Moore R. New-onset diabetes after renal transplantation: comparing ciclosporin and tacrolimus. Nat Clin Pract Nephrol 2008;4:20–1 | Comparator |
| Study design | |
| Morales JM, Campistol JM, Kreis H, Mourad G, Eris J, Schena FP, et al. Sirolimus-based therapy with or without cyclosporine: long-term follow-up in renal transplant patients. Transplant Proc 2005;37:693–6 | Study design |
| Language | |
| Morales JM, Hartmann A, Walker R, Arns W, Senatorski G, Grinyo JM, et al. Similar lipid profile but improved long-term outcomes with sirolimus after cyclosporine withdrawal compared to sirolimus with continuous cyclosporine. Transplant Proc 2009;41:2339–44 | Outcome |
| Moscarelli L, Caroti L, Antognoli G, Zanazzi M, Di Maria L, Carta P, et al. Everolimus leads to a lower risk of BKV viremia than mycophenolic acid in de novo renal transplantation patients: a single-center experience. Clin Transplant 2013;27:546–54 | Study design |
| Mulay AV, Hussain N, Fergusson D, Knoll GA. Calcineurin inhibitor withdrawal from sirolimus-based therapy in kidney transplantation: a systematic review of randomized trials. Am J Transplant 2005;5:1748–56 | No data |
| Nashan B, Ivens K, Suwelack B, Arns W, Abbud FM. Conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in maintenance renal transplant patients: preliminary results from the myfortic prospective multicenter study. Transplant Proc 2004;36:S521–3 | Population |
| NCT. Phase II/III, Open-label, Randomized, Controlled, Multiple-dose Study of Efficacy and Safety of BMS-224818 as Part of a Quadruple Drug Regimen in First Renal Transplant Recipients. 2002. URL: http://clinicaltrials.gov/show/NCT00035555 (accessed 25 July 2014) | No data |
| NCT. A Phase III, Randomized, Open-label, Comparative, Multi-center Study to Assess the Safety and Efficacy of Prograf (tacrolimus)/MMF, Modified Release (MR) tacrolimus/MMF and Neoral (cyclosporine)/MMF in de novo Kidney Transplant Recipients. 2004. URL: http://clinicaltrials.gov/show/NCT00064701 (accessed 25 July 2014) | No data |
| NCT. A Multi-centre, Randomized, Open-label, Study to Compare Conversion from Calcineurin Inhibitors to Rapamune versus Standard Therapy in Established Renal Allograft Recipients on Maintenance Therapy with Mild to Moderate Renal Insufficiency. 2004. URL: http://clinicaltrials.gov/ct2/show/NCT00273871 (accessed 25 July 2014) | No data |
| NCT. A Randomized, Open-label, Comparative Evaluation of Conversion from Calcineurin Inhibitors to Sirolimus versus Continued Use of Calcineurin Inhibitors in Renal Allograft Recipients. 2002. URL: http://clinicaltrials.gov/ct2/show/NCT00038948 (accessed 25 July 2014) | No data |
| Nichelle L, Canet S, Garrigue V, Chong G, Mourad G. Arterial hypertension in renal transplant recipients treated with tacrolimus or cyclosporine-neoral. Transplant Proc 2002;34:2824–5 | Intervention |
| Novoa PA, Grinyó JM, Ramos FJ, Errasti P, Franco A, Aldana G, et al. De novo use of everolimus with elimination or minimization of cyclosporine in renal transplant recipients. Transplant Proc 2011;43:3331–9 | Comparator |
| Oberbauer R. Calcineurin inhibitor withdrawal from sirolimus-based therapy in kidney transplantation: a systematic review of randomized trials. Am J Transplant 2005;5:3023 | Study design |
| Outcome | |
| Oberbauer R, Hutchison B, Eris J, Arias M, Claesson K, Mota A, et al. Health-related quality-of-life outcomes of sirolimus-treated kidney transplant patients after elimination of cyclosporine A: results of a 2-year randomized clinical trial. Transplantation 2003;75:1277–85 | Comparator |
| Oppenheimer F, Rebollo P, Grinyo JM, Ortega F, Sanchez-Plumed J, Gonzalez-Molina M, et al. Health-related quality of life of patients receiving low-toxicity immunosuppressive regimens: a substudy of the symphony study. Transplantation 2009;87:1210–13 | Intervention |
| Ortega F, Sánchez-Fructuoso A, Cruzado JM, Gómez-Alamillo JC, Alarcón A, Pallardó L, et al. Gastrointestinal quality of life improvement of renal transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium drugs or agents: mycophenolate mofetil and enteric-coated mycophenolate sodium. Transplantation 2011;92:426–32 | Outcome |
| Ozdemir BH, Ozdemir AA, Erdal R, Ozdemir FN, Haberal M. Rapamycin prevents interstitial fibrosis in renal allografts through decreasing angiogenesis and inflammation. Transplant Proc 2011;43:524–6 | Study design |
| Painter PL, Topp KS, Krasnoff JB, Adey D, Strasner A, Tomlanovich S, et al. Health-related fitness and quality of life following steroid withdrawal in renal transplant recipients. Kidney Int 2003;63:2309–16 | Comparator |
| Parrott NR, Hammad AQ, Watson CJ, Lodge JP, Andrews CD. Multicenter, randomized study of the effectiveness of basiliximab in avoiding addition of steroids to cyclosporine a monotherapy in renal transplant recipients. Transplantation 2005;79:344–8 | Comparator |
| Pascual J, Segoloni G, Gonzalez Molina M, del Castillo D, Capdevila L, Arias M, et al. Comparison between a two-drug regimen with tacrolimus and steroids and a triple one with azathioprine in kidney transplantation: results of a European trial with 3-year follow up. Transplant Proc 2003;35:1701–3 | Population |
| Pascual J, Galeano C, Royuela A, Zamora J. A systematic review on steroid withdrawal between 3 and 6 months after kidney transplantation. Transplantation 2010;90:343–9 | Comparator |
| Pavlakis M. Mycophenolate mofetil versus sirolimus as an adjunct to calcineurin inhibition after renal transplantation. Nat Clin Pract Nephrol 2006;2:558–9 | Outcome |
| Study design | |
| Pescovitz MD, Vincenti F, Hart M, Melton L, Whelchel J, Mulgaonkar S, et al. Pharmacokinetics, safety, and efficacy of mycophenolate mofetil in combination with sirolimus or ciclosporin in renal transplant patients. Br J Clin Pharmacol 2007;64:758–71 | Intervention |
| Picard N. Does tacrolimus, in comparison with sirolimus, increase mycophenolic acid exposure in kidney transplant recipients? Clin Pharmacol Ther 2010;87:650–1 | Study design |
| Pliszczynski J, Kahan BD. Better actual 10-year renal transplant outcomes of 80% reduced cyclosporine exposure with sirolimus base therapy compared with full cyclosporine exposure without or with concomitant sirolimus treatment. Transplant Proc 2011;43:3657–68 | Population |
| Study design | |
| Ponticelli C, Salvadori M, Scolari MP, Citterio F, Rigotti P, Veneziano A, et al. Everolimus and minimization of cyclosporine in renal transplantation: 24-month follow-up of the EVEREST study. Transplantation 2011;91:e72–3 | Comparator |
| Prokopenko E, Scherbakova E, Vatazin A, Pasov S, Budnikova N, Agafonova S. Does mycophenolate mofetil increase the incidence of infections in renal transplant recipients? Drugs Exp Clin Res 2005;31:199–205 | Study design |
| Renner FC, Dietrich H, Bulut N, Celik D, Freitag E, Gaertner N, et al. The risk of polyomavirus-associated graft nephropathy is increased by a combined suppression of CD8 and CD4 cell-dependent immune effects. Transplant Proc 2013;45:1608–10 | No data |
| Riegersperger M, Plischke M, Steiner S, Seidinger D, Sengoelge G, Winkelmayer WC, et al. Effect of conversion from ciclosporin to tacrolimus on endothelial progenitor cells in stable long-term kidney transplant recipients. Transplantation 2013;95:1338–45 | Population |
| Rostaing L, Massari P, Garcia VD, Mancilla-Urrea E, Nainan G, Carmen Rial M, et al. Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: a randomized phase II study. Clin J Am Soc Nephrol 2011;6:430–9 | Population |
| Ruggenenti P, Codreanu I, Cravedi P, Perna A, Gotti E, Remuzzi G. Basiliximab combined with low-dose rabbit anti-human thymocyte globulin: a possible further step toward effective and minimally toxic T cell-targeted therapy in kidney transplantation. Clin J Am Soc Nephrol 2006;1:546–54 | Comparator |
| Ruiz JC, Alonso A, Arias M, Campistol JM, Gonzalez Molina M, Gonzalez Posada JM, et al. Conversion to sirolimus. Nefrologia 2006;26:52–63 | Study design |
| Rush DN, Cockfield SM, Nickerson PW, Arlen DJ, Boucher A, Busque S, et al. Factors associated with progression of interstitial fibrosis in renal transplant patients receiving tacrolimus and mycophenolate mofetil. Transplantation 2009;88:897–903 | Study design |
| Russ G, Segoloni G, Oberbauer R, Legendre C, Mota A, Eris J, et al. Superior outcomes in renal transplantation after early cyclosporine withdrawal and sirolimus maintenance therapy, regardless of baseline renal function. Transplantation 2005;80:1204–11 | Comparator |
| Samadzadeh B, Alemi M, Heidarnejadiyan J, Torkamanasadi F. Prophylactic effect of mycophenolate mofetil on early outcomes of living donor kidney transplantation. Iran J Kidney Dis 2012;6:63–8 | Population |
| Sanchez-Fructuoso AI. Everolimus: an update on the mechanism of action, pharmacokinetics and recent clinical trials. Expert Opin Drug Metab Toxicol 2008;4:807–19 | Comparator |
| Study design | |
| Schena FP, Pascoe MD, Alberu J, Carmen Rial M, Oberbauer R, Brennan DC, et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation 2009;87:233–42 | Population |
| Sellares J, Moreso F, Carlos Ruiz J, Seron D. Mean glomerular volume after renal transplantation in patients receiving sirolimus and cyclosporine a compared with elimination of cyclosporine A at 3 months. Transplantation 2011;91:E5–6 | Comparator |
| Shamseddin MK, Gupta A. Sirolimus: not so sparing in the spare-the-nephron trial. Kidney Int 2011;79:1379 | Language |
| Shihab FS, Waid TH, Conti DJ, Yang H, Holman MJ, Mulloy LC, et al. Conversion from cyclosporine to tacrolimus in patients at risk for chronic renal allograft failure: 60-month results of the CRAF study. Transplantation 2008;85:1261–9 | Population |
| Silva HT, Yang HC, Abouljoud M, Kuo PC, Wisemandle K, Bhattacharya P, et al. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant 2007;7:595–608 | Population |
| Silva HT, Yang HC, Meier-Kriesche HU, Croy R, Holman J, Fitzsimmons WE, et al. Long-term follow-up of a phase III clinical trial comparing tacrolimus extended-release/MMF, tacrolimus/MMF, and cyclosporine/MMF in de novo kidney transplant recipients. Transplantation 2014;97:636–41 | Population |
| Solà R, Díaz JM, Guirado L, Sainz Z, Gich I, Picazo M, García R, et al. Tacrolimus in induction immunosuppressive treatment in renal transplantation: comparison with cyclosporine. Transplant Proc 2003;35:1699–700 | Study design |
| Sollinger H. Enteric-coated mycophenolate sodium: therapeutic equivalence to mycophenolate mofetil in de novo renal transplant patients. Transplant Proc 2004;36:S517–20 | Study design |
| Comparator | |
| Stallone G, Infante B, Schena A, Battaglia M, Ditonno P, Loverre A, et al. Rapamycin for treatment of chronic allograft nephropathy in renal transplant patients. J Am Soc Nephrol 2005;16:3755–62 | Population |
| Stoves J, Newstead CG, Baczkowski AJ, Owens G, Paraoan M, Hammad AQ. A randomized controlled trial of immunosuppression conversion for the treatment of chronic allograft nephropathy. Nephrol Dial Transplant 2004;19:2113–20 | Population |
| Su VCH, Greanya ED, Ensom MHH. Impact of mycophenolate mofetil dose reduction on allograft outcomes in kidney transplant recipients on tacrolimus-based regimens: a systematic review. Ann Pharmacother 2011;45:248–57 | Study design |
| Sun CS, Hao JW, Sun J. A comparison between the therapeutic effects of mycophenolate mofetil and azathioprine in the management of patients after renal transplantation. Herald Med 2002;21:544 | Language |
| Suszynski TM, Gillingham KJ, Rizzari MD, Dunn TB, Payne WD, Chinnakotla S, et al. Prospective randomized trial of maintenance immunosuppression with rapid discontinuation of prednisone in adult kidney transplantation. Am J Transplant 2013;13:961–70 | Population |
| Suwelack B, Gerhardt U, Kobelt V, Hillebrand U, Matzkies F, Hohage H. Design and preliminary results of a randomized study on the conversion of treatment with calcineurin inhibitors to mycophenolate mofetil in chronic renal graft failure: effect, on serum cholesterol levels. Transplant Proc 2002;34:1803–5 | Study design |
| Takahashi K, Uchida K, Yoshimura N, Takahara S, Teraoka S, Teshima R, et al. Efficacy and safety of concentration-controlled everolimus with reduced-dose cyclosporine in Japanese de novo renal transplant patients: 12-month results. Transplant Res 2013;2:14 | Intervention |
| Tan J, Yang S, Wu W. Basiliximab (Simulect) reduces acute rejection among sensitized kidney allograft recipients. Transplant Proc 2005;37:903–5 | Comparator |
| Tedesco H. Efficacy and Safety of Induction Strategies Combined with Low Tacrolimus Exposure in Kidney Transplant Recipients Receiving Everolimus or Sodium Mycophenolate. 2011. URL: http://clinicaltrials.gov/ct2/show/NCT01354301 (accessed 25 July 2014) | No data |
| Tedesco-Silva H, Vítko S, Pascual J, Eris J, Magee JC, Whelchel J, et al. 12-month safety and efficacy of everolimus with reduced exposure cyclosporine in de novo renal transplant recipients. Transpl Int 2007;20:27–36 | Comparator |
| Trompeter R, Filler G, Webb NJ, Watson AR, Milford DV, Tyden G, et al. Randomized trial of tacrolimus versus cyclosporin microemulsion in renal transplantation. Pediatr Nephrol 2002;17:141–9 | No data |
| Turconi A, Rilo LR, Goldberg J, de Boccardo G, Garsd A, Otero A. Open-label, multicenter study on the safety, tolerability, and efficacy of Simulect in pediatric renal transplant recipients receiving triple therapy with cyclosporin, mycophenolate, and corticosteroids. Transplant Proc 2005;37:672–4 | No data |
| Study design | |
| Urbizu JM, Amenabar JJ, Gomez-Ullate P, Zarraga S, Lampreabe I. Immunosuppression using tacrolimus/mycophenolate versus neoral/mycophenolate following kidney transplantation: a single-center experience. Transplant Proc 2002;34:87–8 | Study design |
| Vacher-Coponat H, Brunet C, Moal V, Loundou A, Bonnet E, Lyonnet L, et al. Tacrolimus/mycophenolate killer lymphocyte recon kidney transplant mofetil improved natural titution one year after by reference to cyclosporine/azathioprine. Transplantation 2006;82:558–66 | Outcome |
| Vester U, Kranz B, Wehr S, Boger R, Hoyer PF. Everolimus (Certican) in combination with neoral in pediatric renal transplant recipients: interim analysis after 3 months. Transplant Proc 2002;34:2209–10 | Study design |
| Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation 2002;73:775–82. [Erratum appears in Transplantation 2002;73:1370.] | Population |
| Vincenti F, Rostaing L. Rationale and design of the DIRECT study: a comparative assessment of the hyperglycemic effects of tacrolimus and cyclosporine following renal transplantation. Contemp Clin Trials 2005;26:17–24 | No data |
| Vincenti F, Tuncer M, Castagneto M, Klinger M, Friman S, Scheuermann EH, et al. Prospective, multicenter, randomized trial to compare incidence of new-onset diabetes mellitus and glucose metabolism in patients receiving cyclosporine microemulsion versus tacrolimus after de novo kidney transplantation. Transplant Proc 2005;37:1001–4 | Study design |
| Duplicate | |
| Vítko S, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, Senatorski G, et al. Corticosteroid-free regimens: Tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil in comparison with a standard triple regimen in renal transplantation: results of the atlas study. Transplantation 2005;80:1734–41 | Comparator |
| Waid T. Tacrolimus as secondary intervention vs. cyclosporine continuation in patients at risk for chronic renal allograft failure. Clin Transplant 2005;19:573–80 | Intervention |
| Walker RG, Cottrell S, Sharp K, Tripodi R, Nicholls KM, Fraser I, et al. Conversion of cyclosporine to tacrolimus in stable renal allograft recipients: quantification of effects on the severity of gingival enlargement and hirsutism and patient-reported outcomes. Nephrology 2007;12:607–14 | Outcome |
| Wang K, Zhang H, Li Y, Wei Q, Li H, Yang Y, et al. Efficacy of mycophenolate mofetil versus azathioprine after renal transplantation: a systematic review. Transplant Proc 2004;36:2071–2 | Study design |
| Wang R, Xu Y, Wu J, Wang Y, He Q, Chen J. Reduced-dose cyclosporine with mycophenolate mofetil and prednisone significantly improves the long-term glomerular filtration rate and graft survival. Intern Med 2013;52:947–53 | Study design |
| Watorek E, Szymczak M, Boratynska M, Patrzalek D, Klinger M. Cardiovascular risk in kidney transplant recipients receiving mammalian target of rapamycin inhibitors. Transplant Proc 2011;43:2967–9 | Study design |
| Comparator | |
| Watson CJ, Firth J, Williams PF, Bradley JR, Pritchard N, Chaudhry A, et al. A randomized controlled trial of late conversion from CNI-based to sirolimus-based immunosuppression following renal transplantation. Am J Transplant 2005;5:2496–503 | Population |
| Wissing KM, Fomegne G, Broeders N, Ghisdal L, Hoang AD, Mikhalski D, et al. HLA mismatches remain risk factors for acute kidney allograft rejection in patients receiving quadruple immunosuppression with anti-interleukin-2 receptor antibodies. Transplantation 2008;85:411–16 | Study design |
| Wlodarczyk Z, Ostrowski M, Mourad M, Krämer BK, Abramowicz D, Oppenheimer F, et al. Tacrolimus pharmacokinetics of once- versus twice-daily formulations in de novo kidney transplantation: a substudy of a randomized phase III trial. Ther Drug Monit 2012;34:143–7 | Population |
| Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al. A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children. Health Technol Assess 2006;10(49) | Comparator |
| Zhong JY, Qu LX, Zhang M, Jiao Z, Lu FM. Application of basiliximab in prevention of acute allograft rejection in kidney transplantation recipients. Zhongguo Xinyao yu Linchuang Zazhi 2005;24:468–71 | Language |
Appendix 3 Abstracts
| Akalin E, Ames S, Sehgal V, Murphy B, Bromberg JS, Fotino M, et al. Intravenous immunoglobulin and thymoglobulin induction treatment in immunologically high-risk kidney transplant recipients. Transplantation 2005;79:742 |
| Al Najjar A, Etienne I, Le Pogamp P, Bridoux F, Le Meur Y, Toupance O, et al. Long-term results of monoclonal anti-IL-2-receptor antibody versus polyclonal antilymphocyte antibodies as induction therapy in renal transplantation. Transplant Proc 2006;38:2298–9 |
| Al Najjar A, Etienne I, Toupance O. Long term follow-up of a multicenter randomized trial comparing a CNI-free regimen with sirolimus (SRL) to a cyclosporine based regimen: the Spiesser study. Am J Transplant 2010;10:505 |
| Albano L, Banas B, Kamar N. Outcomes with tacrolimus-based immunosuppression after kidney transplantation with standard-or extended criteria donor organs: the Osaka study. Transpl Int 2013;26:59 |
| Albano L, Banas B, Kamar N. Safety and renal function in tacrolimus prolonged release vs tacrolimus immediate release-based therapy in renal transplantation: the OSAKA study. Am J Transplant 2011;11:125 |
| Alemi M, Samadzadeh B, Bardideh A, Heidarnejadiyan J, Torkaman Asadi F. The effect of preoperative induction therapy with mycophenolate mofetil in early outcomes of living-donor renal allograft transplantation. Int J Urol 2012;19:163 |
| Alloway RR, Mulgaonkar S, Bowers VD, Stevenson KRU, Cohen DJ, Katz E, et al. A Phase 2b, open-label, multi-center, prospective, randomized study to compare the pharmacokinetics and safety of LCP-Tacro™ tablets once-a-day to Prograf® capsules twice-a-day in de novo kidney transplant patients. Am J Transplant 2009;9;414 |
| Alloway RR, Mulgaonkar S, Ueda K, Cohen D, Kaplan B. A Phase 2 randomized study of the pharmacokinetics, safety and efficacy of LCP-Tacro™ tablets once-a-day vs Prograf® capsules twice-a-day in de novo kidney transplants. Am J Transplant 2011;11:355 |
| Alloway RR, Sadaka B, Trofe-Clark J, Wiland A, Bloom RD. Pharmacokinetic comparison of generic Tacrolimus (Hecoria™) versus Prograf® in stable kidney transplant recipients: a randomized, crossover study. Am J Transplant 2012;12:406 |
| Alpay N. Conversion from calcineurin inhibitors to everolimus resulted in decrease of serum TGF-beta and urinary NGAL in renal transplant recipients. Nephrol Dial Transplant 2013;28:i500–1 |
| Alvarado A, Chhabra D, Wang E, Najafian N, Friedewald J, Ho B, et al. Prospective randomized study to evaluate the feasibility of CNI elimination with conversion to sirolimus in prednisone-free immunosuppressive regimen. Am J Transplant 2012;12:42 |
| Andres A, Bloom R, Bunnapradist S, Cassuto E, Chan L, Hart M. Randomized, multicenter study on the safety and efficacy of enteric-coated mycophenolate sodium combined with basiliximab and low-or standard dose of tacrolimus in de novo renal transplant patients. Transpl Int 2007;20:217 |
| Andres A, del Castillo D, Gainza FJ, Purroy A, Bustamante J, Rengel M. Comparison of a sequential therapy with tacrolimus versus a standard triple therapy in aged kidney transplantation with aged donors: results of a multicenter, prospective and randomized trial (Estrella Study). Am J Transplant 2007;7:443 |
| Andres I, Font B, Mora S, Lahoz R, Ortega F. Quality of life of enteric-coated mycophenolate sodium (EC-MPS) in renal transplant recipients with gastrointestinal tract complaints to mycophenolate mofetil (MMF): Myvida study. Value Health 2009;12:A311 |
| Antonio Perez-Simon J, Sr, Martino R, Parody R. The combination of sirolimus plus tacrolimus (SITAC) improves the results of cyclosporine plus mycophenolate mofetil (CsAMMF) after reduced intensity conditioning (RIC) unrelated donor allogeneic transplantation. Blood 2011;118:406–7 |
| Arns W, Neumayer HH, Lehner F, Witzke O, Sommerer C, Kliem V. Herakles at month 24: follow-up results on efficacy and safety of three different treatment regimens in de novo renal transplant patients demonstrate options for individualized immunosuppression. Transpl Int 2013;26:21 |
| Arns W, Sommerer C, Witzke O, Lehner F, Zeier M, Neumayer HH. Efficacy and safety of three different treatment regimens in de novo renal transplant patients: results of the Herakles trial. Transplantation 2012;94:995 |
| Baas MC, Kers J, Florquin S, Van Den Bergh Weerman MA, Ten Berge IJM, Bemelman FF. Prolonged treatment with everolimus does not induce podocyte damage and leaves the glomerular basement membrane intact. Am J Transplant 2011;11:317 |
| Baboolal K, Zaiac M, Zamauskaite A, Newstead C. This multicentre, randomised study comparing conversion from calcineurin inhibitors (CNIs) to sirolimus versus standard therapy in renal allograft recipients showed a lower rate of development of subsequent malignant disease in the group receiving sirolimus. Am J Transplant 2009;9:238 |
| Balbontin FG, Kiberd B, Belistky P, Singh D, Fraser A, Lawen JG. One year randomized study comparing cyclosporine microemulsion with C2 monitoring and tacrolimus in de novo kidney transplantation. Am J Transplant 2004;4:236–7 |
| Banas B, Albano L, Cassuto E, Glyda M, Klempnauer J, Lehner F. The impact of acute rejection on renal function-perspectives from the OSAKA study. Transplantation 2012;94:983 |
| Banas B, Boger CA, Lehner F. Efficacy, safety and optimised dosing in tacrolimus prolonged release vs tacrolimus immediate release-based therapy in renal transplantation-the Osaka study. Transpl Int 2011;24:35 |
| Banas B, Cassuto E, Glyda M, Kamar N, Klempnauer J, Lehner F, et al. Selection of appropriate composite endpoints is critical for assessing efficacy failure-perspectives from the OSAKA study. Transplantation 2012;94:3 |
| Banas B, Kamar N, Lehner F, Albano L, Glyda M, Viklicky O. Acute rejection in renal transplantation recipients treated with tacrolimus prolonged release-and immediate release-based therapy: the Osaka study (optimizing immunosuppression after kidney transplantation with Advagraf). Transpl Int 2011;24:38–9 |
| Banas B, Kruger B, Viklicky O. Tacrolimus prolonged release optimises exposure during the immediate postoperative period. Transplantation 2012;94:81–2 |
| Becker LE, Xue Y, Gross ML, Waldherr R, Schwenger V, Zeier M. Evolution of allograft fibrosis and related markers in kidney transplant patients under treatment with cyclosporine and everolimus. NDT Plus 2010;3:iii527 |
| Bertoni E, Carta P, Salvadori M. Cyclosporine very low dose with everolimus high dose is associated with excellent outcomes in renal transplant patients. Transpl Int 2011;24:112 |
| Bouwes Bavinck J. Prevention of skin cancer in organ transplant recipients. Br J Dermatol 2012;167:e2 |
| Brennan DC, Koch MJ. Is mycophenolate mofetil really necessary in renal transplantation? A review of the MYSS follow-up study. Nat Clin Pract Nephrol 2007;3:602–3 |
| Bresnahan B, Vincenti F, Grinyo J, Charpentier B, Russo GD, Garg P. Renal benefit of belatacept versus cyclosporine in kidney transplant patients is not impacted by acute rejection (BENEFIT study). Am J Transplant 2010;10:14 |
| Brian Stevens R, Skorupa JY, Rigley TH, Sandoz JP, Kellogg A, Miller N. Calcineurin-inhibitor withdrawal vs. minimization after kidney transplantation is safe but does not improve renal function; 5-year results of a prospective, randomized trial. Am J Transplant 2010;10:505 |
| Budde K, Arns W, Sommerer C, Lehner F, Zeier M, Neumayer H, et al. Superior renal function in an everolimus-based calcineurin inhibitor free regimen compared to standard cyclosporine/mycophenolate and low cyclosporine/everolimus: follow-up of the Herakles study at month 24. Am J Transplant 2013;13:310–11 |
| Budde K, Arns W, Sommerer C, Reinke P, Eisenberger U, Fischer W, et al. Improved renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 2 years follow-up of the ZEUS trial. Am J Transplant 2010;10:503 |
| Budde K, Arns W, Sommerer C, Reinke P, Eisenberger U, Vogel EM, et al. Improved renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 3 years follow-up of the ZEUS trial. Am J Transplant 2011;11:66 |
| Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, et al. Analysis of renal function in everolimus/enteric-coated mycophenolate sodium treated de novo renal transplant recipients after calcineurin inhibitor withdrawal: the ZEUS study. Am J Transplant 2009;9:259 |
| Budde K, Bunnapradist S, Rostaing L. A phase III randomized trial of conversion to once-daily extended release MeltDose tacrolimus tablets LCP-Tacro™ from twice-daily tacrolimus capsules Prograf®: efficacy results from an analysis of specific patient sub-populations. Transplantation 2012;94:984 |
| Budde K, Lehner F, Arns W, Reinke P, Eisenberger U, Paulus EM, et al. Improved renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 4 years follow-up of the ZEUS trial. Am J Transplant 2012;12:298 |
| Budde K, Sommerer C, Haller H, Arns W, Krämer S, Vogel EM, et al. Renal function of an Everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 2 year data of the APOLLO trial. Am J Transplant 2011;11:411 |
| Budde K, Sommerer C, Haller H, Suwelack B, May C, Paulus EM, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 3 year data of the APOLLO trial. Am J Transplant 2012;12:298 |
| Budde K, Sommerer C, Reinke P, Haller H, Arns W, Witzke O, et al. Outcome on renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 4 year data of the APOLLO trial. Am J Transplant 2013;13:311–12 |
| Budde K, Witzke O, Sommerer C, Reinke P, Eisenberger U, Paulus E, et al. Improved renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 5 years follow-up of the ZEUS trial. Am J Transplant 2013;13:35–6 |
| Budde K, Zeier M, Haller H, Arns W, Krämer S, Vogel EM, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients. Am J Transplant 2010;10:504 |
| Bunnapradist S, Danovitch GM. Minimizing ciclosporin in renal transplant recipients on daclizumab, mycophenolate and steroids. Nat Clin Pract Nephrol 2007;3:426–7 |
| Cabello M, García P, González-Molina M, Díez de los Rios MJ, García-Sáiz M, Gutiérrez C, et al. Pharmacokinetics of once- versus twice-daily tacrolimus formulations in kidney transplant patients receiving expanded criteria deceased donor organs: a single-center, randomized study. Transplant Proc 2010;42:3038–40 |
| Campbell S, Walker R, Pilmore H, Kanellis J, Russ G, Hutchison B. Wound healing events are dose related: a multicenter, prospective study on everolimus in renal transplantation. Immunol Cell Biol 2011;89:A16–17 |
| Carmellini M, Pattison J, Riad H, Yaqoob M, Vergara M, Witte S, et al. Renal function in renal transplant recipients after 24 months of immunosuppression with concentration-controlled everolimus plus reduced cyclosporine exposure: update from the A2309 study. Transpl Int 2011;24:57 |
| Carmellini M, Todeschini P, Manzia TM, Valerio F, Messina M, Sghirlanzoni MC, et al. Twelve-month outcomes from evidence trial (everolimus once-a-day regimen with cyclosporine versus corticosteroid elimination) in adult kidney transplant recipients. Transpl Int 2013;26:100 |
| Carmellini M, Yaqoob M, Pattison J, Riad H, Wang Z, Cornu-Artis HC, et al. Correlation of everolimus exposure with efficacy and safety outcomes in renal transplant recipients: 24-month update. Transpl Int 2011;24:248 |
| Carroll RP, Hester J, Wood KJ, Harden PN. Conversion to sirolimus in kidney transplant recipients with squamous cell cancer permits potential protective changes in immune phenotype. Transplantation 2012;94:167 |
| Cerezo O, Bravo MG, Jimenez Aranda P, Lemus EA. Clinical benefits of immunosuppression therapy in renal transplant Patients. Systematic review and meta-analysis. Value Health 2013;16:A697 |
| Chadban S, Campbell S, Russ G, Walker R, Chapman J, Pussell B, et al. A one-year, randomised, open label, parallel group study to investigate the safety and efficacy of enteric-coated Mycophenolate sodium (EC-MPS) in combination with full dose or reduced dose cyclosporine microemulsion (CSA-ME), basiliximab and steroids in de novo kidney transplantation. Immunol Cell Biol 2006;84:A6 |
| Chadban S, Campbell S, Russ G, Walker R, Chapman J, Pussell B, et al. Socrates-steroid or cyclosporin removal after transplantation using everolimus: Histological analysis. Transplantation 2012;94:977 |
| Charpentier B, Grinyo J, Medina Pestana JO, Vanrenterghem Y, Vincenti F, Dong Y, et al. 3-year safety profile of belatacept in kidney transplant recipients from the benefit and BENEFIT-EXT studies. Transpl Int 2011;24:68–9 |
| Charpentier B, Vincenti F, Rice K, Budde K, Campistol J, Duan T, et al. Three-year outcomes in patients with delayed graft function in phase iii studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT and BENEFIT-EXT). Transplantation 2012;94:996 |
| Christian M, Bjerre A, Wennberg L, Ettenger R, Pape E, Tonshoff B, et al. Design and baseline characteristics of CRADLE: a study evaluating the efficacy and safety of everolimus to reduce CNI exposure and to withdraw steroids in pediatric renal transplant recipients. Pediatr Nephrol 2014;29:1755. |
| Chun DXY, Alexandre H, Sandrine GS, Olivier T, Isabelle E, Christophe L, et al. The phenotype of tubular epithelial cells does not recover after a conversion from cyclosporine a to sirolimus. Nephrol Dial Transplant 2012;27:ii517 |
| Cibrik D, Johnston T, Kim Y, Walker R, Zibari G, Cornu-Artis C, et al. Everolimus exposure and relationship to efficacy and safety: results from a multicenter study in renal transplantation using reduced CsA exposure. Am J Transplant 2010:10:567–8 |
| Cibrik D, Johnston T, Kim YS, Walker R, Zibari. Everolimus allows for around 60% reduction in CsA exposure over 12 months: results from a multicenter, prospective study in renal transplantation. Am J Transplant 2010;10:511 |
| Cibrik D, Kim YS, Johnston T, Walker R, Zibari G. Benefits of everolimus with reduced CSA exposure on renal function: a multicenter, prospective study in renal transplantation. Am J Transplant 2010;10:151–2 |
| Cibrik D, Kim YS, Johnston T, Walker R, Zibari GB, Cornu-Artis C, et al. Renal function stability in renal transplant recipients receiving concentration-controlled everolimus with reduced cyclosporine exposure: 24 month results from the A2309 study. Am J Transplant 2011;11:406–7 |
| Citterio F, Scolari MP, Salvadori M, Castagneto M, Rigotti P, Albertazzi A, et al. A randomized trial comparing standard everolimus plus cyclosporine with higher blood everolimus levels plus very low cyclosporine levels in renal transplant recipients: preliminary results of the Everest study. Transpl Int 2007;20:124 |
| Clayton P, McDonald S, Chapman J, Chadban S. Mycophenolate vs azathioprine for kidney transplantation: 15 year follow-up of a randomized trial. Nephrology 2011;16:69 |
| Cristelli MP, Tedesco-Silva H, Medina-Pestana JO, Franco MF. De novo everolimus (EVR) versus mycophenolate (MPA) in kidney transplant recipients receiving tacrolimus (TAC). Transplantation 2014;98:141 |
| Dalal P, Xu L, Joseph L, Shah G, Chhabra D. Prospective randomized study to evaluate the long term impact on graft survival and function of two pred-free, CNI based maintenance immunosuppressions: FK/MMF vs. FK/SRL. Am J Transplant 2010;10:512 |
| David-Neto E, Cocuzza CS, Pereira LM, Castro MCR, Fadel LM, Prado ES, et al. A prospective, randomized, controlled study using oral GTT to diagnose impaired glucose metabolism in renal transplant patients under cyclosporin and tacrolimus. Am J Transplant 2005;5:408 |
| De Fijter JW, Ewe SH, Den Hartigh J, Ng ACT, Delgado V, Mallat MJK, et al. Beneficial effects of late concentration-controlled CNI withdrawal in renal transplant recipients. Am J Transplant 2011;11:406 |
| De Fijter JW, Hoogendijk-Van Den Akker JM, Harden PN, Hoitsma AJ, Proby C, Wolterbeek R, et al. Reduced cutaneous squamous cell carcinoma after conversion to sirolimus: a 2-year prospective open-label multicenter trial. Am J Transplant 2012;12:161 |
| De Simone P, Detry O, Kintmalm G, Goss J, McCormick P, Rossi M, et al. Superior renal function sustained for 24 months through early everolimus-facilitated reduction of tacrolimus versus standard tacrolimus in de novo liver transplant recipients: results of a randomized trial. Am J Transplant 2013;13:169–70 |
| Del Castillo D, Franco A, Tabernero JM, Errasti P, Valdes F, Garcia C, et al. Prospective, multicenter, randomized, open-label study of myfortic (EC-MPS) with steroid withdrawal vs Myfortic™ (EC-MPS) with standard steroid regimen to prevent acute rejection in de novo kidney transplantation. Am J Transplant 2005;5:191 |
| Dobbels F, Wong S, Joo S, Kalsekar A. Health-related quality of life after kidney transplantation: results from belatacept clinical trials. Am J Transplant 2011;11:352–3 |
| Dobbels F, Wong S, You M, Kalsekar A. Patient reports of immunosuppressant related side-effects after kidney transplantation: results from the belatacept phase III clinical trial (BENEFIT). Am J Transplant 2011;11:353 |
| Duboix-xu Y, Lebranchu Y, De Ligny BH, Thervet E, Mazouz H, Lepogamp P, et al. Conversion from cyclosporine to Sirolimus at M3 after renal transplantation does not reduce the score of epithelial to mesenchymal transition at M12: ancillary study of the Concept study. Am J Transplant 2010;10:510–11 |
| Duerr M, Naik M, Schmidt D, Neumayer H, Budde K. Higher rates of acute rejections despite enhanced rates of regulatory T cells under mTOR inhibitor therapy in renal transplant patients. Am J Transplant 2012;12:301 |
| Duerr M, Nolting J, Naik M, Neumayer HH, Budde K. Higher frequency of regulatory T-cells after conversion from cyclosporine to everolimus in a prospective randomized trial in renal allograft recipients. Am J Transplant 2011;11:66 |
| Durrbach A, Florman S, Larsen C, Pestana JM, Vanrenterghem Y, Vincente F, et al. Primary outcomes from a randomized, phase III study of belatacept versus cyclosporine in ECD kidney transplants (BENEFIT-EXT study). Am J Transplant 2010;10:7 |
| Durrbach A, Florman S, Zhang R, Becker T, Grinyo J, Lang P, et al. Four-year outcomes by donor type from the long-term extension of the belatacept BENEFIT and BENEFIT-EXT studies. Am J Transplant 2012;12:407 |
| Durrbach A, Florman S, Zhang R, Lang P, Lehner F, Massari P, et al. Five-year outcomes by donor type from the long-term extension of the belatacept BENEFIT-EXT study. Am J Transplant 2013;13:311 |
| Durrbach A, Larsen C, Medina-Pestana JD, Vanrenterghem Y, Vincenti F, Florman S, et al. Primary outcomes from a randomized, phase III study of belatacept vs cyclosporine in ECD kidney transplants (BENEFIT-EXT Study). Am J Transplant 2009;9:199 |
| Durrbach A, Larsen CP, Medina Pestana J, Vanrenterghem Y, Vincenti F, Florman S, et al. Belatacept vs cyclosporine in ECD kidney transplants: two-year outcomes from the BENEFIT-EXT study. NDT Plus 2010;3:iii262 |
| Durrbach A, Medina-Pestana JO, Rostaing L, Bresnahan B, Helderman JH, Rice K, et al. Improving or maintaining renal function with belatacept: 5-year benefit long-term extension results. Transpl Int 2013;26:92 |
| Durrbach A, Medina-Pestana JO, Vanrenterghem Y, Rial M, Charpentier B, Matas A, et al. Improving or maintaining renal function over 5 years with belatacept in recipients of extended-criteria donor kidneys. Transpl Int 2013;26:44 |
| Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Klempnauer J, Guerkan A, et al. 2-year results of the symphony study: comparing standard immunosuppression against low-dose cyclosporine, tacrolimus or sirolimus associated with MMF, daclizumab and corticosteroids in de-novo renal transplantation. Transpl Int 2007;20:25 |
| Favi E, Citterio F, Spagnoletti G, Gargiulo A, Romagnoli J, Castagneto M. A prospective clinical trial comparing tacrolimus-MMF to cyclosporine-everolimus in de novo renal transplant recipients: 2 years results. Transpl Int 2009;22:241 |
| Favi E, Citterio F, Spagnoletti G, Gargiulo A, Romagnoli J, Castagneto M. ER-tacrolimus plus everolimus vs ER-tacrolimus plus MMF in primary deceased donor kidney transplantation: 1-year results of single center, open label, prospective, randomized clinical trial. Transpl Int 2013;26:241 |
| Favi E, Silvestrini N, Pedroso J, Salerno M, Spagnoletti G, Bianchi V. Extended-release tacrolimus plus everolimus vs extended-release tacrolimus plus mycophenolate mofetil in primary deceased donor kidney transplant recipients: 1-year results of an open label, randomized phase 2 clinical trial. Am J Transplant 2013;13:316 |
| Favi E, Silvestrini N, Salerno MP, Romagnoli J, Citterio F. Extended-release tacrolimus plus everolimus or mycophenolate mofetil in deceased donor kidney transplant recipients: 6-month results of a prospective randomized clinical trial. Am J Transplant 2012;12:42–3 |
| Favi E, Silvestrini N, Spagnoletti G, Castagneto M, Citterio F. Thymoglobulin and basiliximab vs basiliximab as induction therapy in deceased donor kidney transplantation: 1-year results of a prospective clinical trial. Am J Transplant 2011;11:147 |
| Favi E, Silvestrini N, Valente I, Salerno MP, Castagneto M, Citterio F. Lower acute rejection with basiliximab and short course, low dose thymoglobulin vs basiliximab as induction therapy in deceased donor renal transplant recipients: 6-month results of a prospective clinical trial. Am J Transplant 2010;10:321 |
| Favi E, Spagnoletti G, Silvestrini N, Salerno M, Pedroso J, Romagnoli J, et al. Thymoglobulin plus basiliximab vs basiliximab as induction therapy in deceased donor kidney transplant recipients treated with tacrolimus and mycophenolate mofetil: 1-year results of a prospective clinical trial. Am J Transplant 2013;13:426 |
| Favi E, Spagnoletti G, Silvestrini N, Salerno MP, Pedroso JA, Romagnoli J, et al. Thymoglobulin plus basiliximab versus basiliximab induction in deceased donor kidney transplant recipients treated with tacrolimus and MMF: 1-year results of a prospective clinical trial. Transpl Int 2013;26:83 |
| Felix M, Felipe C, Tedesco H, Medina-Pestana J. Safety profile after planned conversion from tacrolimus (TAC) to sirolimus (SRL) based immunosuppressive therapy in kidney transplant recipients (KTR). Transplantation 2014;98:544–5 |
| Fellstrom B, Holdas H, Holme I, Jardine A, Soveri I. Cardiovascular risk calculator for renal transplant recipients: applications to BENEFIT and BENEFIT-EXT trials. Am J Transplant 2012;12:409–10 |
| Ferguson R, Vincenti F, Kaufman DB, Woodle ES, Marder BA, Citterio F, et al. Immunosuppression with belatacept-based, CNI-avoiding and steroid-avoiding regimens vs a tacrolimus-based, steroid-avoiding regimen in kidney transplant patients: results of a 1-year, randomized study. Am J Transplant 2010;10:150 |
| Filler G, Webb N. Randomised clinical trial in paediatric renal transplantation: tacrolimus (TAC) vs cyclosporine neoral (CYA): 3-year data. J Am Soc Nephrol 2003;14:65a |
| Fisher G, Rocha V, dos Santos M, Devergie A, Robin M, de Latour RP, et al. Mycophenolate mofetil (MMF) with or without tacrolimus (FK506) as a second line treatment for steroid-resistant acute graft-versus-host disease. The experience of Saint Louis Hospital. Blood 2006;108:819A |
| Flechner S, Glyda M, Steinberg S, Harler MB, Invest OT. A randomized, open-label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (TAC) and mycophenolate mofetil (MMF) regimen in de novo renal allograft recipients: renal function results from the Orion study. Transpl Int 2007;20:25 |
| Flechner S, Glyda M, Steinberg S, Harler MB, Investigators OT. A randomized, open-label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (TAC) and mycophenolate mofetil regimen (MMF) in de novo renal allograft recipients: acute rejection and graft survival results from the Orion study. Transpl Int 2007;20:209–10 |
| Flechner SM, Cockfield S, Grinyo J Flechner SM, Cockfield S, Grinyo J, Russ G, Wissing KM, Legendre C, et al. A randomized, open-label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with tacrolimus (TAC) plus mycophenolate mofetil (MMF) in de novo renal allograft recipients: preliminary 2-year safety results from the ORION trial. Am J Transplant 2008;8:582 |
| Flechner SM, Glyda M, Tai SS. Delayed graft function (DGF) in two sirolimus (SRL)-based regimens compared with tacrolimus (TAC) and mycophenolate mofetil (MMF) in de novo renal allograft recipients. Am J Transplant 2009;9:277–8 |
| Flechner SM, Gurkan A, Tai SS, Schulman S.L. Incidence of delayed graft function (DGF) in a sirolimus (SRL)- based versus cyclosporine (CsA)-based regimen in de novo renal allograft recipients. Am J Transplant 2009;9:278 |
| Florman S, Becker T, Bresnahan B, Chevaile-Ramos A, Carvalho D, Muehibacher F, et al. Three year outcomes by donor type in phase III studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT and BENEFIT-EXT). Transpl Int 2011;24:51 |
| Florman S, Becker T, Bresnahan B, Chevaile-Ramos A, DeCarvalho D, Muehlbacher F, et al. Three-year outcomes by donor type in phase III studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT & BENEFIT-EXT). Am J Transplant 2011;11:100 |
| Florman S, Bresnahan B, Chan L, Helderman H, Dong Y, Harler MB, et al. Three year outcomes in Black/African American kidney transplant recipients from the BENEFIT and BENEFIT-EXT studies. Am J Transplant 2011;11:350 |
| Florman S, Durrbach A, Grinyo J, Pestana JOM, Rial MDC, Vítko S, et al. 4-year results from the long-term extension of the belatacept BENEFIT-EXT study. Am J Transplant 2012;12:82 |
| Florman S, Durrbach A, Larsen C, Pestana JM, Vanrenterghem Y, Vincenti F, et al. Outcomes as a function of donor criteria from a phase III study of belatacept vs cyclosporine in kidney transplantation (BENEFIT-EXT). Am J Transplant 2010;10:150 |
| Florman S, Rice K, Chan L, Steinberg S, Pearson T, Duan T, et al. Four-year outcomes in black/African American kidney transplant recipients from the long-term extension of the belatacept BENEFIT and BENEFIT-EXT studies. Am J Transplant 2012;12:404 |
| Florman S, Rice K, Chan L, Zhang R, Abouljoud M, Steinberg S, et al. Outcomes at five years in black/African-American kidney transplant recipients from the long-term extension of the belatacept benefit and BENEFIT-EXT studies. Am J Transplant 2013;13:311 |
| Gallon L, Monica G, Friedewald J, Cabral B, Miller J, Najafaian N, et al. Prospective randomized study to evaluate feasibility of conversion of CNI to SRL in a pred-free immunosuppressive regimen. Impact on Treg generation. Am J Transplant 2009;9:260 |
| Glotz D, Charpentier B, Abramovicz D, Lang P, Rostaing L, Rifle G, et al. Thymoglobulin induction and sirolimus versus tacrolimus in kidney transplant recipients receiving mycophenolate mofetil and steroids. Transplantation 2010;89:1511–17 |
| Graeme R, Mamta A, Thomas B, Bresnahan B, Campistol JM, Darji P, et al. Belatacept associated with preserved renal function and structure compared with cyclosporine (CSA) in kidney transplant patients. Immunol Cell Biol 2010;88:A11–12 |
| Grannas G, Richter N, Klempnauer J, Lehner F. 10 years’ experience with belatacept (Nulojix). Transplantation 2012;94:964 |
| Grinyo J, Abouljoud M, Germain M, Manfro R, Morales J, Legendre C, et al. Improving or sustaining renal function over 3 years with belatacept or cyclosporine a (CSA): insights from the benefit study. Transpl Int 2011;24:250 |
| Grinyo J, Charpentier B, Medina Pestana J, Vanrenterghem Y, Vincenti F, et al. Safety profile of belatacept in kidney transplant recipients from a pooled analysis of phase II and phase III studies. NDT Plus 2010;3:iii270 |
| Grinyo J, Durrbach A, Rostaing L, Bresnahan B, Helderman J, Rice K, et al. Likelihood of improving or sustaining renal function over three years with belatacept or CsA: insights from the BENEFIT study. Am J Transplant 2013;13:182 |
| Grinyo J, Durrbach A, Rostaing L, Bresnahan B, Helderman J, Rice K, et al. Likelihood of improving or maintaining renal function over five years with belatacept or CSA: insights from the benefit long-term extension study. Am J Transplant 2013;13:182 |
| Grinyo J, Nainan G, Del Carmen Rial M, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: results from the long-term extension of a phase II study. Transpl Int 2011;24:70 |
| Grinyo J, Nainan G, Rial M, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: results from the long-term extension of a phase II study. Am J Transplant 2011;11:99 |
| Grinyo J, Pestana JM, Becker T, Rial MC, Dong Y, Block A, et al. Likelihood of improving or sustaining renal function over three years with belatacept or CsA: insights from the BENEFIT-EXT study. Am J Transplant 2012;12:82 |
| Grinyo J, Pestana JM, Becker T, Rial MC, Dong Y, Block A, et al. Long-term extension of the belatacept BENEFIT-EXT study: results at month 48. Transplantation 2012;94:974 |
| Grinyo J, Rial M, Alberu J, Steinberg S, Manfro R, Nainan G, et al. Outcomes of switching to belatacept from a calcineurin inhibitor in kidney transplant recipients: 3 year results from the long-term extension of a phase ii study. Am J Transplant 2013;13:182 |
| Grinyo J, Vanrenterghem Y, Durrbach A, Rial M, Charpentier B, Matas A, et al. Likelihood of improving or maintaining renal function in recipients of extended-criteria donor kidneys over five years with belatacept or CsA (BENEFIT-EXT long-term extension study). Am J Transplant 2013;13:310 |
| Grinyo JM, Marks W, Vincenti F, Kaufman DB, Marder BA, Woodle S, et al. Immunosuppression with belatacept-based, CNI-free, steroid-avoiding regimens in kidney transplant recipients: 6 month, interim results. Am J Transplant 2009;9:382 |
| Grinyo JM, Mondragon-Ramirez G, Darji P, Bresnahan B, Pearson T, Di Russo GB, et al. Belatacept is associated with preservation of renal function and structure at 1 year compared to cyclosporine in kidney transplant patients (BENEFIT Study). Am J Transplant 2009;9:258–9 |
| Grinyo JM, Paul J, Novoa P, Errasti P, Franco A, Aldana G, et al. Better renal function in renal-transplant recipients treated with everolimus plus cyclosporine elimination compared with cyclosporine minimisation. Am J Transplant 2010;10:503 |
| Guba M, Pratschke J, Hugo C, Kraemer B, Burmeister D, Brockmann J. A randomized multicenter trial of early conversion to sirolimus/mycophenolate/steroids versus cyclosporine/mycophenolate/steroids in renal transplantation: one-year analysis (SMART-Study). Am J Transplant 2009;9:497 |
| Guba M, Pratschke J, Hugo C, Kraemer B, Nohr-Westphal C, Brockmann J, et al. Renal function, efficacy and safety of sirolimus and mycophenolate mofetil therapy after early calcineurin-inhibitor withdrawal in de novo renal transplant patients: one-year analysis of a randomized multicenter trial. Transpl Int 2009;22:78 |
| Guba M, Witzke O, Lehner F, Arns W, Sommerer C, Neumayer HH, et al. The HERAKLES study at 24 month: superior renal function in an everolimus-based CNI free regimen. Transpl Int 2013;26:110 |
| Guerra G, Gaynor JJ, Ciancio G, Zarak A, Sageshima J, Roth D. Randomized trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate versus cyclosporine (neoral/sirolimus in renal transplantation: seven year results. Am J Transplant 2009;9:325 |
| Gupta D. Design of a randomized study evaluating everolimus in pediatric renal transplant recipients. Transpl Int 2013;26:328 |
| Han D, Kim Y-S, Park KT, Kim S-J, Ha J-W, Kim H-C, et al. A phase III, randomized, open-label, comparative, multicenter study to assess the safety and efficacy of Prograf® (tacrolimus) and extended release (XL) tacrolimus in Asian de novo kidney transplants from living donors: 6 month results. Am J Transplant 2009;9:413 |
| Hanaway M, Woodle ES, Mulgaonkar S, Peddi R, Harrison G, Vandeputte K, et al. 12 month results of a multicenter, randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation. Am J Transplant 2008;8:215 |
| Harold Y. A phase III, randomized, open-label, comparative, multi-center study to assess the safety and efficacy of Prograf® (Tacrolimus)/MMF, extended release (XL) Tacrolimus/MMF and Neoral® (Cyclosporine)/MMF in de novo kidney transplant recipients: 2 year results. Am J Transplant 2007;7:183 |
| Hertig A, Kamar N, Anglicheau D, Moulin B, Hazzan M, Hurault De Ligny B, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: the CERTITEM trial. Transpl Int 2013;26:2 |
| Hirsch HH, Vincenti F, Friman S, Wiecek A, Pescovitz MD, Jenssen T, et al. Prospective study of polyomavirus BK viruria and viremia in de novo renal transplantation comparing cyclosporine and tacrolimus: a multivariate analysis. Am J Transplant 2009;9:337 |
| Ho ETL, Wong G, Chapman JR, Craig J. Once daily extended release versus twice daily standard release tacrolimus in kidney transplant recipients: a systematic review. Transplantation 2012;94:989 |
| Holdaas H, Rostaing L, Seron D. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation 2011;92:410–18. [Erratum appears in Transplantation. 2011;92:e61. Note: multiple investigator names added.] |
| Holdaas H, Rostaing L, Seron D. Erratum: Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation 2011;92:e61 |
| Howell M, Yeo R, Tong A, Craig JC, Howard K, Wong G. Adverse events of maintenance immunosuppression following kidney transplantation reported in randomised controlled trials: a systematic review. Nephrology 2014;50:9–16 |
| Huh W, Lee K, Lee K, Kim S, Joh J, Oh H. Randomized trial of tacrolimus versus cyclosporine in steroid withdrawal regimen after living kidney transplantation. Clin Pharmacol Ther 2003;73:26 |
| Ibrahim H, Issa N, Spong R, Kukla A, Kandaswamy R, Dunn T, et al. CNI reduction vs. mTOR based immunosuppression after prednisone discontinuation: four year preliminary results from a large randomized trial. Am J Transplant 2012;12:302 |
| Jesky MD, Sharif A, Borrows RJ. Does conversion from cyclosporine to tacrolimus as secondary prevention provide better outcomes in renal allograft recipients? A meta-analysis. Am J Transplant 2011;11:410 |
| Johari Y, Bryson D, Barlow A, Nicholson M. Cyclosporine micro-emulsion versus tacrolimus for renal transplantation: 10-year follow-up for single centre randomised controlled trial. Br J Surg 2010;97:32–3 |
| Johari Y, Bryson D, Medcalf J, Nicholson M. Cyclosporine versus tacrolimus for renal transplantation: 10 year follow up of a randomised controlled trial. Br J Surg 2010;97:37 |
| Johari Y, Bryson D, Nicholson M. A randomised controlled trial comparing switching to rapamune based immunosuppression with tacrolimus minimisation for renal transplantation. Br J Surg 2010;97:68–9 |
| Junge G, De Simone P, Fung J, Kohler S, Saliba F. Urinary protein excretion in non-renal transplant patients-does mTOR-inhibitor treatment matter? Am J Transplant 2013;13:531–2 |
| Junge G, Tufveson G, Riad H, Cibrik D, Tedesco H, Schwende H, et al. Better renal allograft function with everolimus facilitated CNI reduction – graft type, donor criteria and gender analysis. NDT Plus 2010;3:iii540 |
| Kaabak M, Babenko N, Zokoyev A, Schekaturov S, Sandrikov V. Eculizumab for prevention and treatment of kidney graft reperfusion injury, preliminary results of RCT. Transplantation 2014;98:257–8 |
| Kalil AC, Florescu DF, Sun J. Induction immunosuppression: what is the difference in the risk of serious infections between interleukin-2RA and polyclonal antibodies? Am J Transplant 2009;9:283 |
| Kamar N, Lehner F, Banas B, Viklicky O, Albano L, Glyda M. Efficacy and safety of tacrolimus prolonged release and immediate release in de novo renal transplantation: the OSAKA study (optimizing immunosuppression after kidney transplantation with advagraf). Transpl Int 2011;24:39 |
| Kamar N, Rial M, Alberu J, Steinberg SM, Manfro R, Nainan G, et al. 3-year outcomes after switching to belatacept from a calcineurin inhibitor in stable kidney transplant recipients. Transpl Int 2013;26:44 |
| Kamar N, Rial M, Alberu J, Steinberg SM, Manfro R, Nainan G, et al. Three-years outcomes after switching to belatacept from calcineurin inhibitor in stable kidney transplant recipients. Transpl Int 2013;26:22 |
| Kang MH, Kim HJ, Ko RK, Ko SK. A systematic review of immunosuppressive regimens in lower immunological risk renal transplant recipients. Value Health 2010;13:A473–4 |
| Kobashigawa J, Ross H, Kfoury AG, Van Bakel A, Ewald G, Burton J, et al. CMV infections are less frequent in de novo heart transplant recipients receiving immunosuppression with everolimus plus reduced CsA compared to MMF and standard CsA. Am J Transplant 2011;11:131–2 |
| Koukoulaki M, Grispou U, Pistolas D, Balaska K, Apostolou T, Anagnostopoulou M, et al. Monitoring of BK polyoma virus in renal transplant recipients. Preliminary results of a prospective study. Nephrol Dial Transplant 2005;20:V177 |
| Krämer B, Kruger B, Banas B, Tomlinson P. Early post-transplant blood levels in de novo renal recipients on tacrolimus prolonged release (TACQD) versus tacrolimus immediate release (TACBD) in a phase III double-blind double-dummy study. Transpl Int 2011;24:54 |
| Krämer B. Significantly better freedom from acute rejection with tacrolimus vs. cyclosporine-based immunosuppression in renal transplant recipients at 7-year follow-up. Am J Transplant 2010;10:568 |
| Krämer BK. Better tolerability and significantly higher freedom from acute rejection at 7 years with tacrolimus vs. cyclosporine-based immunosuppression in renal transplant recipients. NDT Plus 2010;3:iii284 |
| Kumar N, Manimaran R, Williams C, Ravanan R. Tacrolimus preserves renal function better than cyclosporin at 10 years: long term results of a randomised controlled trial. Am J Transplant 2009;9:200 |
| Langer RM, Pape L, Tonshoff B, Dello Strologo L, Ettenger R, Niaudet P, et al. Evaluation of safety and efficacy of everolimus with reduced tacrolimus: design of a randomized, multicenter, open-label study in pediatric renal transplant recipients. Pediatr Transplant 2013;17:80 |
| Larsen C, Alberu J, Massari P, Acevedo RR, Kamar N, Lin CS, et al. 4-Year results from the long-term extension of the belatacept BENEFIT study. Am J Transplant 2012;12:82 |
| Larsen C, Vincenti F, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension (LTE) of the belatacept evaluation of nephroprotection and efficacy as first-line immunosuppression trial (BENEFIT) study. Am J Transplant 2013;13:312 |
| Larsen C, Vincenti F, Grinyo JM, Charpentier B, Di Russo GB, Garg P, et al. Renal benefit of belatacept vs cyclosporine in kidney transplant patients is not impacted by acute rejection (BENEFIT Study). Am J Transplant 2009;9:220 |
| Larsen CP, Bray R, Gebel H, Ganguly B, Kulbokas E, Brickman D, et al. Evaluation of donor-specific antibodies in kidney transplant patients treated with belatacept-or cyclosporine-based immunosuppression in benefit and BENEFIT-EXT. Transpl Int 2011;24:69 |
| Larsen CP, Grinyo J, Charpentier B, Medina Pestana J, Kamar N, Vanrenterghem Y, et al. Belatacept vs cyclosporine in kidney transplant recipients: two-year outcomes from the BENEFIT study. NDT Plus 2010;3:iii262 |
| Lebranchu Y, Büchler M, Etienne I, Toupance O, Westel PF, Legendre C, et al. 12 month results of a randomized trial comparing sirolimus (SRL) versus cyclosporine (CsA) in 150 transplant patients receiving a cadaveric renal graft. Am J Transplant 2005;5:540 |
| Lebranchu Y, Etienne I, Toupance O, Westeel PF, de Ligny BH, Rerolle JP, et al. CNI avoidance and steroid withdrawal in renal transplantation. results at three years of a prospective multicenter randomized trial comparing sirolimus (SRL) and cyclosporine (CsA): the SPIESSER study. Transpl Int 2009;22:244 |
| Lebranchu Y, Legendre C, Merville P, Durrbach A, Rostaing L, Thibault G, et al. Comparison of interleukin-2 (il-2) blockade in kidney transplant patients randomized to 40 mg or 80 mg basiliximab (BSX) with cyclosporine (CsA) or 80 mg BSX with everolimus (EVR). Transplantation 2014;98:581 |
| Lebranchu Y, Thierry A, Thervet E, Büchler M, Etienne I, Westeel PF, et al. Efficacy and safety of early cyclosporine conversion to sirolimus with continued MMF-four-year results of the Postconcept study. Am J Transplant 2011;11:1665–75 |
| Lebranchu Y, Toupance O, Touchard G, Thervet E, Etienne I, Mazouz H. Impact on renal function of early conversion at 3 months from cyclosporine (CsA) to sirolimus (SRL) in association with mycophenolate mofetil (MMF) in kidney transplantation: 30-months follow up of a multicenter randomized controlled trial: the CONCEPT study. Am J Transplant 2009;9:260 |
| Lebranchu Y, Toupance O, Touchard G, Thervet E, Etienne I, Westeel PF, et al. Impact of early conversion at 3 months from cyclosporine (CSA) to sirolimus (SRL) in association with mycophenolate mofetil (MMF) on renal function: ‘results at 48 months of follow up of a multicenter randomized controlled trial: the CONCEPT study’. Am J Transplant 2010;10:151 |
| Legendre C, Srinivas TR, Pascual J, Chadban S, Citterio F, Henry M, et al. The transform trial design: a large randomized, multicenter, open-label study of everolimus with reduced calcineurin inhibitors in de novo renal transplantation. Transpl Int 2013;26:23–4 |
| Lehner F, Arns W, Reinke P. Renal function in everolimus/enteric-coated mycophenolate sodium treated de novo living renal transplant recipients after calcineurin inhibitor withdrawal: subgroup analysis of the ZEUS study. Transpl Int 2011;24:50–1 |
| Lehner F, Arns W, Witzke O. Three years follow-up of the Zeus trial: maintained better renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients. Transpl Int 2011;24:50 |
| Lehner F, Banas B, Kamar N, Glyda M, Viklicky O, Albano L. Influence of donor related factors on outcomes with tacrolimus-based immunosuppression after kidney transplantation: the OSAKA study (optimizing immunosuppression after kidney transplantation with Advagraf). Transpl Int 2011;24:164–5 |
| Lehner F, Banas B. Influence of donor related factors on outcomes with tacrolimus-based immunosuppression after kidney transplantation: the OSAKA study. Transpl Int 2011;24:21 |
| Lehner F, Budde K, Arns W, et al. Improved renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 3 year follow-up of the ZEUS trial. Transpl Int 2011;24:57 |
| Lehner F, Guba M, Arns W, Sommerer C, Neumayer HH, Jacobi J, et al. Follow-up data from Herakles study at month 24: Maintained superior renal function in patients on an everolimus-based calcineurin inhibitor free regimen compared to standard cyclosporine/mycophenolate and low cyclosporine/everolimus. Transpl Int 2013;26:28 |
| Lehner F, Sommerer C, Arns W, Reinke P, Eisenberger U, Wuthrich RP, et al. A post hoc analysis of 2 prospective, open-label, multicenter, randomized trials: Onset and progression of diabetes in kidney transplant patients receiving everolimus or cyclosporine. Results from ZEUS and HERAKLES. Transpl Int 2013;26:21 |
| Lehner F, Sommerer C, Arns W, Reinke P, Eisenberger U, Wuthrich RP, et al. Post HOC subgroup analysis from ZEUS: outcome on renal function, efficacy and safety in living donor kidney transplant recipients after conversion from a calcineurin inhibitor to an everolimus based regimen. Transpl Int 2013;26:8 |
| Lehner F, Sommerer C, Reinke P, Arns W, Eisenberger U, Paulus EM et al. 5-year follow-up on the ZEUS KTX trial: everolimus conversion after CNI withdrawal. Transpl Int 2013;26:81 |
| Lehner F, Sommerer C, Witzke O, Arns W, Kliem V, Neumayer HH, et al. HERAKLES at month 24: efficacy and safety of 3 different regimens in de novo renal transplant patients. Transpl Int 2013;26:82 |
| Libetta C, Canevari M, Margiotta E, Martinelli C, Borettaz I, Esposito P, et al. Preliminary data of controlled randomized study (ever twist) on tolerance induction. Transpl Int 2013;26:20 |
| Libetta C, Margiotta E, Borettaz I, Canevari M, Martinelli C, Lainu E, et al. Everolimus and low dose of tacrolimus combined with thymoglobulin induction induces regulatory t cells expansion in de novo kidney transplant recipients: preliminary data of controlled randomized study (EVER TWIST). Nephrol Dial Transplant, 2013;28:i277 |
| Lim W, Eris J, Kanellis J, Pussell B, Wiid Z, Witcombe D, et al. Conversion from calcineurin-inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients: a systematic review and meta-analysis of randomised trials. Nephrology 2013;18:44–5 |
| Maamoun H, Khashab S, Belal D, Soliman AR. Azathioprine increases cyclosporine-induced hyperuricemia in renal transplant recipient. Transplantation 2012;94:969 |
| Marchetti P, Vincenti F, Friman S, Scheuermann E. New-onset diabetes impaired fasting glucose after renal transplantation: results of a prospective, randomised trial comparing cyclosporine versus tacrolimus. Diabetologia 2006;49:500–1 |
| Margreiter R. Tacrolimus vs ciclosporin microemulsion in renal transplantation. A randomized multicentre study. Chirurgische Praxis 2002:60:611–12 |
| Mas V, Maluf D, Scian M, Chalasani G, Sustento-Reodica N, Leventhal J, et al. Differential impact of calcineurin and mammalian target of rapamycin inhibition on immune, inflammation and antigen presentation genes expression in renal allograft biopsies. Am J Transplant 2012;12:40 |
| Masson P, Henderson LK, Craig J, Webster AC. Belatacept for kidney transplant recipients: a systematic review and meta-analysis. Transplantation 2012;94:968–9 |
| Matas A, Gillingham K. Prospective randomized study of low level CNI vs SRL @ 6 mos posttx, while pred (P)-free. Transplantation 2014;98:542 |
| Medina Pestana J, Grinyo J, Vanrenterghem Y, Becker T, Florman S, Lang P, et al. Belatacept compared with cyclosporine in renal allograft recipients of extended criteria donor kidneys: 3-year outcomes from the phase III BENEFIT-EXT trial. Transpl Int 2011;24:51 |
| Medina-Pestana JO, Garcia VD, David-Neto E, Carvalho DBM, Contieri F, Abbud-Filho M, et al. Conversion from tacrolimus to sirolimus-based immunosuppressive regimen in kidney transplant recipients. Preliminary results. Am J Transplant 2011;11:462 |
| Meier M, Bode W, Nitschke M, Wong W, Krämer J, Lehnert H, et al. Low dose tacrolimus versus mycophenolate-mofetil in ‘old for old’ kidney transplantation: a one year prospective multicenter randomized controlled trial. Am J Transplant 2009;9:498 |
| Mjörnstedt L, Sorensen SS, Von Zur Muhlen B. Improved renal function by overnight switch from cyclosporine to everolimus at week 7 after renal transplantation. One year results from a randomized, controlled trial. Transpl Int 2011;24:94 |
| Montagnino G, Krämer BK, Arias M. Efficacy and safety of tacrolimus compared with cyclosporine microemulsion in kidney transplantation: twelve-month follow-up. Transplant Proc 2002;34:1635–7 |
| Morales JM, Tedesco-Silva H, Peddi VR, Russ GR, Marder BA, Hahn CM, et al. Planned transition from tacrolimus to sirolimus versus continued tacrolimus in renal allograft patients. Transpl Int 2013;26:81 |
| Mucha K, Foroncewicz B, Durlik M, Chmura A, Szmidt J, Paczek L. Seven-year follow-up of 77 renal transplant recipients (RTRs) treated with tacrolimus-based immunosuppression (IS). NDT Plus 2010;3:iii268–9 |
| Mucha K, Foroncewicz B, Paczek L, Pazik J, Lewandowska D, Krawczyk A, et al. 36-month follow-up of 75 renal allograft recipients treated with steroids, tacrolimus, and azathioprine or mycophenolate mofetil. Transplant Proc 2003;35:2176–8 |
| Muehlbacher F, Becker T, Campistol JM, Carvalho DBM, Florman S, Lang P, et al. Donor sub-type analysis of three-year outcomes from a phase III study of belatacept in recipients of extended criteria donor kidneys (BENEFIT-EXT trial). Transpl Int 2011;24:221–2 |
| Muhlbacher F, Florman S, Zhang R, Lang P, Lehner F, Massari P, et al. 5-year outcomes by donor type from the long-term extension of the belatacept BENEFIT-EXT study. Transpl Int 2013;26:92 |
| Noyola-Villalobos H, Martinez-Calva I, Vazquez GA, Fernandez MR, Chavarria JE, Dosal RH, et al. Randomized controlled trial of early conversion from calcineurin inhibitor to everolimus in adult renal allograft patients at a single transplant center in Mexico. Transplantation 2012;94:910 |
| O’Connell P, Fassett R, Pilmore H, Chapman J, Hutchison B, Russ G, et al. Long-term post transplantation switch to an everolimus-based therapy with CNI elimination/minimization does not overall impact graft function: the ASCERTAIN study. Immunol Cell Biol 2011;89:A5 |
| O’Connell P, Fassett R, Pilmore H, Chapman J, Hutchison B, Russ G, et al. Post-hoc analysis of the ascertain trial: everolimus based therapy with CNI elimination improves renal function in select populations. Immunol Cell Biol 2011:89:A5 |
| Oh C, Huh K, Lee J, Lee J, Cho H, Kim Y. Multicenter randomized clinical investigation for the safety and efficacy of advagraf (extended-release tacrolimus) vs. Prograf® (twice-daily tacrolimus) in de novo Korean adult kidney recipients. Am J Transplant 2013;13:317 |
| Ortega F, Sanchez-Fructuoso A, Cruzado JM, Gomez-Alamillo JC, Alarcon A, Pallardo M, et al. Quality of life and tolerability of enteric-coated mycophenolate sodium (EC-MPS) in renal transplant recipients with gastrointestinal tract complaints to mycophenolate mofetil (MMF): a multicenter, randomized, open-label, controlled trial. Am J Transplant 2009;9:408–9 |
| Ortega F, Sanchez-Fructuoso A, Cruzado JM, Gomez-Alamillo JC, Alarcon A, Pallardo L, et al. The use of higher doses of mycophenolic acid (MPA) is not associated with worse gastrointestinal tolerability in renal transplant patients converted from mycophenolate mofetil (MMF) to enteric-coated mycophenolate sodium (EC-MPS). Am J Transplant 2010;10:512 |
| Ortega F, Sanchez-Fructuoso A, Cruzado JM, Gomez-Alamillo JC, Alarcon A, Pallardo LL, et al. A high glomerular filtration rate (GFR) and the use of an enteric-coated formulation of mycophenolic acid predict less gastrointestinal complaints in renal transplant patients. Transpl Int 2011;24:220 |
| Otukesh H. Basiliximab induction therapy in pediatric renal transplantation, a double blind clinical trial. Pediatr Nephrol 2013;28:1533 |
| Pankewycz O, Leca N, Kohli R, Weber-Shrikant E, Said M, Alnimri M, et al. Conversion to low-dose tacrolimus or rapamycin 3 months after kidney transplantation: a prospective, protocol biopsy-guided study. Transplant Proc 2011;43:519–23 |
| Pankewycz O, Leca N, Kohli R, Weber-Shrikant E, Said M, Alnimri M, et al. Conversion to low dose tacrolimus or rapamycin 3 months after kidney transplant: a prospective, protocol biopsy guided study. Am J Transplant 2010;10:509 |
| Pankewycz O, Leca N, Said M, Feng L, Patel S, Alnimri M, et al. Tacrolimus minimization or sirolimus conversion at 3 months provides equivalent 1 year renal allograft function and histology in low-risk patients with normal protocol biopsies. Am J Transplant 2011;11:408 |
| Pankewycz O, Leca N, Said M, Feng L, Patel S, Kohli R, et al. A protocol biopsy directed randomized trial comparing tacrolimus minimization to sirolimus conversion at 3 months results in an equivalent degree of histological injury at 1 year and equivalent renal function at 2 years. Am J Transplant 2012;12:304 |
| Pankewycz O, Leca N, Said M, Feng L, Patel S, Kohli R, et al. A protocol biopsy directed randomized trial comparing tacrolimus minimization to sirolimus conversion at 3 months results in an equivalent degree of histological injury at 1 year yet equivalent renal function at 2 years. Transplantation 2012;94:967 |
| Pankewycz O, Leca N, Wallace P, Said M, Feng L, Patel S, et al. Rabbit anti-thymocyte globulin (rATG) induction therapy followed by tacrolimus conversion to sirolimus at 3 months does not increase Treg cells. Am J Transplant 2012;12:448 |
| Pankewycz O, Leca N, Wallace P, Said M, Feng L, Patel S, et al. Rabbit anti-thymocyte globulin (rATG) induction therapy followed by tacrolimus conversion to sirolimus at 3 months does not expand Treg cells. Transplantation 2012;94:771 |
| Pankewycz OG, Wallace PK, Said M, Leca N, Feng L, Patel S, et al. Low dose rabbit anti-thymocyte globulin induction therapy selectively depletes blood lymphocytes but does not promote Treg expansion. Am J Transplant 2011;11:177–8 |
| Paoletti E, Marsano L, Bellino D, Cassottana P, Rolla D, Di Maio G. Everolimus for regression of left ventricular hypertrophy of renal transplant recipients: a randomized controlled trial. Am J Transplant 2012;12:31 |
| Pascual J, Del Castillo D, Cabello M, Pallardo L, Grinyo JM, Fernandez AM, et al. Tacrolimus (Tac)-Everolimus (EVL) combination for kidney transplantation (KT): a phase II dose comparison randomized pharmacokinetic (PK). Am J Transplant 2008;8:585 |
| Pascual J, Hene R, Langer R, Christiaans M, Ciechanowski K, Vilatoba M, et al. Preservation of renal function with everolimus and very low tacrolimus exposure in de novo renal transplant recipients (RTXR) at 12 months: the ASSET study. Am J Transplant 2010;10:502 |
| Pearson T, Vincenti F, Grinyo J, Charpentier B, Pestana JM, Rostaing L, et al. Primary outcomes from a randomized, phase III study of belatacept versus cyclosporine in kidney transplant recipients (BENEFIT study). Am J Transplant 2010;10:6 |
| Peddi R, Hanaway M, Woodle S, Mulgaonkar S, Harrison G, Vandeputte K, et al. Final 36 month results of a randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and rapid steroid withdrawal in renal transplantation. Am J Transplant 2010;10:49 |
| Perkins J, Alsina M, Anasetti C, Ayala E, Fernandez HF, Kharfan-Dabaja M, et al. A randomized, controlled trial of graft-versus-host disease (GVHD) prophylaxis comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate: analysis of GVHD, relapse and survival. Blood 2008;112:779 |
| Pescovitz MD, El-Shahawy M, Vincenti F. Incidence of glucose metabolism disorders at six months after kidney transplantation in non-white patients randomized to cyclosporine or tacrolimus: results of a multicenter study. Am J Transplant 2008;8:525 |
| Plischke M, Riegersperger M, Steiner S, Seidinger D, Winkelmayer WC, Sunder-Plassmann G. Short-term renal function in long-term kidney transplant recipients after conversion from cyclosporine a to tacrolimus. A randomized controlled trial. Am J Transplant 2012;12:204 |
| Pliszczynski J, Abraham JBA, Schoenberg L, Kahan BD. Fullor 80% reduced cyclosporine (CSA) exposure improves 1 but not 10 or 5 year renal transplant outcomes with sirolimus (SRL) base therapy. Am J Transplant 2010;10:507 |
| Polvino WJ, Melkus TC, Nigro V. Reduction in tacrolimus c-max by conversion from twice-daily tacrolimus capsules (Prograf®) to once-daily extended release MeltDose® tacrolimus tablets (LCP-Tacro™): phase II randomized trial in stable kidney transplant patients. Am J Transplant 2012;12:407–8 |
| Pussell B, Russ G, Walker R, Campbell S, O’Connell P, Kanellis J, et al. Conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients: 18-month efficacy and safety results from a large, randomized, open-label, comparative trial. Immunol Cell Biol 2006;84:A19–20 |
| Reinke P, Haller H, Rath T, Arns W, Paulus EM, Scheidf S, et al. Two year data of the Apollo trial: renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients. Transpl Int 2011;24:50 |
| Reinke P, Lehner F, Witzke O, Sommerer C, Eisenberger U, Arns W, et al. 5 Years follow-up on renal function-ZEUS trial: improved renal function of an everolimus/enteric-coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients. Transpl Int 2013;26:21 |
| Renner FC, Dietrich H, Bulut N, Celik D, Gaertner ND, Karoui S, et al. The development of BK viremia after renal transplantation is associated with a reduced CD8 cell IL-2 response. Transpl Int 2011;24:56 |
| Rhat T, Sommerer C, Haller H, Reinke P, Witzke O, Suwelack B, et al. Outcome on renal function of everolimus conversion in maintenance KTX patients: 4 years APOLLO trial. Transpl Int 2013;26:240 |
| Rice K, Vanrenterghem Y, Merville P, Muehlbacher F, Zhang R, Duan T, et al. Three-year outcomes in elderly kidney transplant recipients treated with belatacept vs cyclosporine in BENEFIT-EXT. Am J Transplant 2012;12:403 |
| Richard MG, Angela W, Ruster Lorenn P, Matheson Sandra L, Higgins Gail Y, Willis Narelle S, et al. Interleukin-2 receptor antagonists versus ATG for kidney transplant recipients; an updated Cochrane review. Immunol Cell Biol 2010;88:A21 |
| Riegersperger M, Plischke M, Steiner S, Seidinger D, Sengoelge G, Winkelmayer WC, et al. Effect of conversion from ciclosporin to tacrolimus on endothelial progenitor cells in stable long-term kidney transplant recipients. Transplantation 2013;95:1338–45 |
| Roodnat J, Hilbrands LB, Hene RJ, De Sevaux RGL, Gregoor PJHS, Van Gestel JAK, et al. 15 year follow-up of a multicentre, randomised, calcineurin inhibitor (CNI) withdrawal study in kidney transplantation. Transpl Int 2013;26:83–4 |
| Rostaing L, Budde K, Bunnapradist S. A phase 3, double-blind, multi-center, non-inferiority, randomized study to examine the efficacy and safety of LCP-Tacro™ tablets, once daily, compared to Prograf® capsules, twice daily, in combination with mycophenolate mofetil in de novo adult kidney transplantation: baseline characteristics. Am J Transplant 2013;13:339 |
| Rostaing L, Ciechanowski K, Bunnapradist S, Mulgaonkar S. Conversion from tacrolimus capsules twice daily to tacrolimus tablets once daily in stable kidney transplant patients: efficacy results from a phase III, open-label, multicenter, prospective, randomized study. Transpl Int 2011;24:227 |
| Rostaing L, Fassett R, Dantal J, Binet I, O’Connell P, MacHein U, et al. Risk factor analysis for renal function outcome in maintenance renal transplant recipients from the ASCERTAIN study. Am J Transplant 2011;11:44–5 |
| Rostaing L, Mourad G, Legendre C. Sustainable tolerability effects of Myfortic® in combination with Neoral® and steroids at 12 months, in de novo kidney transplantation: a randomized, multicentre, open, prospective controlled study. Am J Transplant 2005;5:190 |
| Rostaing L, Nainan G, Del Carmen Rial M, Steinberg S, Vincenti F, Shi R, et al. Switch from a CNI-to a belatacept-based immunosuppressive regimen in kidney transplant recipients is safe and results in better renal function: 12 month results from a phase II study. NDT Plus 2010;3:iii285 |
| Rostaing L, Reyes-Acevedo R, Neumayer HH, Vítko S, Xing J, Thomas D, et al. Outcomes at 3 years in kidney transplant recipients with pre-transplant diabetes from two phase 3 belatacept studies. Transpl Int 2011;24:69 |
| Ruiz JC, Campistol JM, Sanchez-Fructuoso A, Mota A, Grinyo JM, Paul J, et al. Early sirolimus use with cyclosporine elimination does not induce progressive proteinuria. Transplant Proc 2007;39:2151–2 |
| Ruiz JC, Sanchez Fructuoso A, Hernández D, Sanchez Plumed J, Fernandez A, Pastor Rodriguez A, et al. Better renal function with early everolimus (EVL) introduction and calcineurin inhibitor (CNI) withdrawal at third month in kidney recipients at month 12: results of the ERIC study. Transpl Int 2011;24:112 |
| Ruiz JC, Sanchez Fructuoso A, Hernández D, Sanchez Plumed J, Fernandez A, Pastor Rodriguez A, et al. Better renal function with early everolimus introduction and calcineurin inhibitor withdrawal at third month in kidney recipients at month 12: results of the ERIC study. Am J Transplant 2011;11:407 |
| Russ G, Durrbach A, Larsen CP, Medina Pestana J, Vanrenterghem Y, Vincenti F, et al. BENEFIT-EXT study two year outcomes: belatacept vs cyclosporine (CSA) in extended criteria donor (ECD) kidney transplants. Immunol Cell Biol 2011;89:A2 |
| Russ G, Eris J, Kanellis J, Hutchison B, Hibberd A, Pilmore H, et al. Multicentre RCT of early switch to everolimus plus steroids or everolimus plus CSA versus CSA, MPA and steroids in de novo kidney transplant recipients: 12 month analysis. Immunol Cell Biol 2012;90:A30 |
| Russ G, Walker R, Pilmore H, Kanellis J, Hutchison B, Chadban S, et al. Lower incidence of cytomegalovirus and BK virus with everolimus versus mycophenolate in de novo renal transplant patients: results from a multicenter, prospective study. Immunol Cell Biol 2011;89:A23–4 |
| Saddadi F, Sedghipour M, Tabatabaei A, Kamal Hedaiat D, Alatab S. Comparison of the effects of sirolimus and cyclosporine on left ventricular hypertrophy in kidney transplant recipients, a 1-year single center prospective cohort study in Dr Shariati Hospital, Tehran, Iran. Iran J Kidney Dis 2011;5:62–3 |
| Saito K, Uchida K, Takahara S, Yoshimura N, Teraoka S, Cornu-Artis C, et al. Efficacy of everolimus with reduced cyclosporine in Japanese de novo renal transplant recipients: 24-month, randomized, multicenter study. Am J Transplant 2013;13:314 |
| Salmela K, Vítko S, Wlodarczyk Z, Czajkowski Z, Margreiter R. Tacrolimus with MMF or two different doses of sirolimus in kidney transplantation: a large randomised multicentre study. Am J Transplant 2005;5:571 |
| Sanchez-Fructuoso A, Ruiz JC, Hernández D, Sanchez-Plumed J, Fernandez A, Pastor Rodriguez A, et al. Early everolimus introduction and calcineurin inhibitor withdrawal in renal transplant patients: a multicenter, randomized, open-label study (the ERIC study). Am J Transplant 2010;10:506 |
| Sandes Freitas TV, Harada KM, Felipe CR, Galante NZ, Sampaio EL, Ikehara E, et al. Steroid or tacrolimus withdrawal in renal transplant recipients using sirolimus. Int Urol Nephrol 2011;43:1221–8 |
| Schena FP, Wali RK, Pascoe MD, Alberu J, Rial MD, Sirolimus Renal Conversion Trial S. A randomized, open-label, comparative evaluation of conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients. Am J Transplant 2005;5:413 |
| Schwarz C, Mayerhoffer S, Berlakovich G, Steininger R, Soliman T, Watschinger B, et al. Belatacept in de novo kidney transplant recipients: 10-year experience in a single center. Eur Surg 2011;43:12–13 |
| Shah G, Xu L, Dalal P, Chhabra D, Friedewald J, Ho B, et al. Conversion from CNI to SRL in a pred-free immunosuppressive regimen: interim report of a prospective randomized study. Am J Transplant 2010;10:504 |
| Shehata M, Bhandari S, Venkat-Raman G, Moore R, D’Souza R. Health-related quality of life maintained despite increase in mycophenolic acid (MPA) dose following conversion from mycophenolate mofetil (MMF) to enteric-coated mycophenolate sodium (EC-MPS): a randomized, multicenter trial in kidney transplant recipients. Transpl Int 2009;22:110 |
| Shihab F, Tedesco-Silva H, Johnston T, Kim YS, Zibari GB, Walker R, et al. Lower incidence of cytomegalovirus and BK virus adverse events with everolimus versus mycophenolate was maintained over 24 months in de novo renal transplant recipients. Am J Transplant 2011;11:45 |
| Sidhu M, Odeyemi AO, Hart WM, Dada BR. Belatacept versus tacrolimus: results of an indirect analysis from a systematic review of immunosuppressive therapies for kidney transplant recipients. Value Health 2011;14:A330 |
| Silva AP, Tonato E, Durao M, Jr, Requiao-Moura L, Arruda E, Chinen R, et al. A randomized clinical trial of early conversion from tacrolimus to everolimus in deceased donor kidney transplantation. Transpl Int 2013;26:277–8 |
| Silva H. A phase III, randomized, open-label, comparative, multi-center study to assess the safety and efficacy of Prograf® (Tacrolimus)/MMF, modified release (MR) Tacrolimus/MMF and Neoral® (Cyclosporine)/MMF in de novo kidney transplant recipients: 12 month result. Am J Transplant 2006;318:A748 |
| Sommerer C, Budde K, Becker T, Arns W, Reinke P, Eisenberger U, et al. New onset diabetes after transplantation and mTOR inhibitors: results of the ZEUS trial. Am J Transplant 2011;11:412–13 |
| Sommerer C, Rath T, Budde K, Haller H, Arns W, Scheidl S, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 2 year follow-up data of the APOLLO trial. Transpl Int 2011;24:180 |
| Sommerer C, Rath T, Haller H, Arns W, Suwelack B, Reinke P, et al. 4 Year data of the Apollo trial: outcome on renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients. Transpl Int 2013;26:21 |
| Stevens RB, Foster KW, Lane JT, Miles CD, Kalil AC, Sandoz JP, et al. Significantly reduced renal allograft histopathology after single-dose rATG induction and calcineurin-inhibitor withdrawal vs. minimization: final report from a prospective, randomized clinical trial. Am J Transplant 2011;11:209–10 |
| Strologo LD, Tonshoff B, Pape L, Ettenger R, Niaudet P, Martzloff ED, et al. Rationale and design of a study evaluating the efficacy and safety of early conversion of calcineurin inhibitor to everolimus in paediatric renal transplant recipients. Pediatr Nephrol 2012;27:1816 |
| Taber D, Bratton C, Al Manasra A, Pilch N, Meadows H, McGillicuddy J, et al. The impact of induction therapy on clinical outcomes and quality of life in aged kidney transplant recipients. Am J Transplant 2013;13:429 |
| Taber DJ, Pilch NA, Meadows HB, Denmark S, McGillicuddy JW, Bratton CF, et al. Prospective comparative efficacy of induction therapy in a high-risk kidney transplant (KTX) population. Am J Transplant 2012;12:57 |
| Takahara S, Uchida K, Yoshimura N, Teraoka S, Kobayashi E, Teshima R, et al. Efficacy and safety of concentration controlled everolimus with reduced dose cyclosporine in Japanese adult de-novo renal transplant patients: 12 month results. Am J Transplant 2012;12:300 |
| Tanabe K, Tsuchiya T, Ishida H, Tanabe T, Shimizu T, Omoto K, et al. An open label, prospective randomized controlled study comparing tacrolimus once-daily and twice-daily in de novo kidney transplantation: pharmacokinetics and pathological analysis by protocol biopsy. Am J Transplant 2012;12:55 |
| Tedesco H, Felipe C, Franco M, Sandes T, Campos E, Pestana JOM. High incidence of subclinical acute rejection in low risk kidney transplant recipients on tacrolimus-based immunosuppressive regime. Transplantation 2012;94:329 |
| Tedesco H, Felipe C, Sandes T, Cristelli M, Rodrigues C, Pestana JOM. A prospective randomized trial aimed to reduce the incidence of cytomegalovirus (CMV) infection in kidney transplant recipients. Transplantation 2012;94:4 |
| Tedesco H, Felipe C, Wang L. A prospective randomized trial aimed to reduce the incidence of cytomegalovirus (CMV) infection in kidney transplant (KT) recipients. Am J Transplant 2013;13:56 |
| Tedesco H, Garcia V, David-Neto E, Contieri F, Carvalho D, Abbud M, et al. Conversion from tacrolimus (TAC) to sirolimus (SRL)-based immunosuppressive regimen in kidney transplant recipients: 1 year results. Am J Transplant 2012;12:299 |
| Tedesco H, Kim YS, Lackova E, Johnston T, Zibari G, Panis C, et al. Everolimus with reduced-dose cyclosporine as a strategy for optimizing long-term renal function: results from a randomized study in 833 de-novo renal-transplant recipients. Transpl Int 2009;22:186–7 |
| Tedesco H, Neto E, Garcia V, Continieri F, Carvalho D, Abbud M, et al. Conversion from tacrolimus (TAC) to sirolimus (SRL)-based immunosuppressive regimen in kidney transplant recipients: 2 years results. Am J Transplant 2013;13:313 |
| Tedesco-Silva H, Bernhardt P, Dong G, Escrig C. Search for new endpoints for clinical trials of immunosuppressive drugs in kidney transplantation. Transpl Int 2013;26:248 |
| Tedesco-Silva H, Kim YS, Johnston T, Walker R, Zibari GB, Cornu-Artis C, et al. Concentration-controlled everolimus with reduced cyclosporine concentration in de novo renal transplant recipients: efficacy results at 24 months. Am J Transplant 2011;11:46 |
| Tedesco-Silva H, Peddi R, Russ G, Marder B, Hahn C, Li H, et al. Open-label study of planned transition from tacrolimus to sirolimus vs continued tacrolimus in renal allograft recipients: demographics and interim safety results. Am J Transplant 2013;13:337 |
| Tedesco-Silva H, Peddi VR, Sanchez-Fructoso A, Russ G, Marder B, Hahn C, et al. Interim results from an open-label study of planned transition from tacrolimus to sirolimus vs continued tacrolimus in renal allograft recipients: cardiovascular safety. Transplantation 2012;94:142 |
| Thervet E, Durrbach A, Rostaing L, Ouali N, Wolf P, Pouteil-Noble C, et al. Use of sirolimus as initial therapy after renal transplantation: preliminary results of a randomized pilot study in patient receiving marginal kidneys. Am J Transplant 2004;345:A686 |
| Thierry A, Pourreau F, Jollet I, Abou-Ayache R, Bridoux F, Touchard G. Minimization of immunosuppression: long-term impact on HLA allo-immunisation and graft outcome. Am J Transplant 2012;12:302 |
| Thurston S, Kalsekar A, Sennfalt K. Mixed treatment comparisons of immunosuppressants following renal transplant. Value Health 2011;14:A331 |
| Tischer SM, Pilch NA, Taber DJ, Krisl JC, Meadows HB, Byrns JS, et al. Does RATG induction therapy increase the risk of severe infection in kidney transplant recipients? Am J Transplant 2012;12:317 |
| Tischer SM, Taber DJ, Pilch NA, Krisl JC, Meadows HB, McGillicuddy JW, et al. Critical analysis of BK infection in kidney transplant recipients with modern immunosuppression. Am J Transplant 2012;12:346 |
| Tönshoff B, Pape L, Ettenger R, Dello Strologo L, Niaudet P, Martzloff D, et al. Early conversion of calcineurin inhibitor to everolimus in de novo paediatric renal transplant recipients and its impact on efficacy and renal function; design of an open-label, randomised, multi-centre study. Transplantation 2012;94:1208 |
| Tönshoff B, Pape L, Dello Strologo L, Ettenger R, Niaudet P, Martzloff ED, et al. Design of crad001a2314: a randomised study evaluating everolimus in paediatric renal transplantation. Transpl Int 2013;26:328 |
| Tönshoff B, Weber L, Hoecker B. Prospective randomized multicenter trial on withdrawal of steroids in pediatric renal transplant recipients with stable graft function on cyclosporin a (CsA) and mycophenolate mofetil (MMF). Pediatr Nephrol 2007;22:1429 |
| Touchard G, Mourad G, Lebranchu Y, et al. Intensified dose of enteric-coated mycophenolate sodium (EC-MPS) for steroids avoidance, in combination with ciclosporine micro-emulsion (CsA-ME): multicenter, randomized, open label, comparative study in de novo kidney transplantation (DOMINOS). Transpl Int 2009;22:232 |
| Touchard G, Mourad G, Lebranchu Y, Rostaing L, Villemain F. Multicenter, randomized, comparative, open-label study to evaluate efficacy and safety a combination of anti-IL2R, intensified dose of enteric-coated mycophenolate sodium (EC-MPS) for 6 weeks, ciclosporine micro-emulsion (CSA-ME), with or without steroids, in adult kidney de novo transplant recipients (TxR). Am J Transplant 2010;10:515 |
| Trofe-Clark J, Goral S, Shaw L, Figurski M, Abt PL, Bloom RD. Comparative study of gastrointestinal (GI) events in African American kidney transplant recipients treated with mycophenolate mofetil (MMF) versus enteric coated mycophenolate sodium (ECMS). Am J Transplant 2010;10:470 |
| Tullius SG, Pratschke J, Strobelt V, Kahl A, Reinke P, May G, et al. ATG versus basiliximab induction therapy in renal allograft recipients receiving a dual immunosuppressive regimen: one-year results. Transplant Proc 2003;35:2100–1 |
| Van Der Giet M, Brakemeier S, Liefeldt L, Glander P, Diekmann F, Hohne M, et al. The impact of everolimus versus CNI-based immunosuppression on cardiovascular function and stiffness after renal transplantation. Am J Transplant 2010;10:506 |
| Van der Heide JJ, de Fijter JW, ten Berge I, de Maar EF, Bemelman FJ. Mecano: mycophenolate sodium vs everolimus or ciclosporin with allograft nephropathy as outcome study: clinical results. Transpl Int 2011;24:85 |
| Van der Heide JJH, de Fijter JW, de Maar EF, ten Berge I, Bemelman FJ. Low acute rejection rejection rate and superior renal function 2 years after early CsA withdrawal and overnight switch to everolimus. Am J Transplant 2010;10:508 |
| Van Doesum W, Gard L, Van Son WJ, Sanders JSF, Riezebos A, Niesters BGM, et al. Incidence and outcome of BK infection in a randomized controlled multicenter study with renal transplant patients receiving duo-therapy. Transpl Int 2013;26:166 |
| Vathsala A, Schena F, Wali RK, Pascoe MD, Alberu J, Carmen Rial M. Conversion from calcineurin inhibitors to sirolimus versus continued use of calcineurin inhibitors in renal allograft recipients: a randomized, open-label, comparative trial. Nephrology 2005;10:A217 |
| Vincenti F, Charpentier B, Rostaing L, Reyes-Acevedo R, Massari P, Vítko S, et al. Long-term extension of the belatacept BENEFIT study: result’s at month 48. Transplantation 2012;94:958 |
| Vincenti F, Grinyo JM, Charpentier B, Medina-Pestana JD, Rostaing L, Vanrenterghem Y, et al. Primary outcomes from a randomized, phase III study of belatacept vs cyclosporine in kidney transplant recipients (BENEFIT Study). Am J Transplant 2009;9:191–2 |
| Vincenti F, Larsen C, Alberu J, Garcia V, Rostaing L, Rice K, et al. Three-year outcomes from BENEFIT: a phase III-study of belatacept vs cyclosporine in kidney transplant recipients. Transpl Int 2011;24:21 |
| Vincenti F, Pescovitz MD, El-Shahawy M. Glucose metabolism disorders in non-white renal transplant patients receiving cyclosporine or tacrolimus in an international, randomized trial. Transpl Int 2007;20:115 |
| Vondrak K, Grenda R, Watson A, Janda J, Simkova E, Seeman T, et al. Immunosuppression with triple combination with tacrolimus with or without monoclonal antibody induction: a multicentric randomized study in children after kidney transplantation. Kidney Blood Press Res 2006;29:381 |
| Vondrak K, Grenda R, Watson AR, Webb NJA, Beattie J, Pediatric Tacrolimus Study Group. Tacrolimus triple therapy with or without monoclonal antibody administration: a multicentre, randomized study in pediatric kidney transplantation. Am J Transplant 2005;5:401–2 |
| Walker R, Vathsala A, Zibari GB, Kim YS, Cibrik D, Johnston T, et al. Class related adverse events in renal transplant recipients treated with everolimus: 24 month results from the A2309 study. Am J Transplant 2011;11:407 |
| Walker RG, Rostaing L, Nainan G, Del CRM, Steinberg S, Vincenti F. A switch to belatacept-based immunosuppressive regimen in kidney transplant recipients from calcineurin inhibitors (CNI) has a favourable safety profile and results in improved renal function: 12-month results from a phase II study. Immunol Cell Biol 2011;89:A3 |
| Watorek E, Szymczak M, Boratynska M, Patrzalek D, Klinger M. Cardiovascular risk in kidney transplant recipients receiving mTOR inhibitors. Transpl Int 2011;24:118 |
| Watson AR, Grenda R, Vondrak K, European Multicentre Tacrolimus S. A multicentre, randomised trial of tacrolimus triple therapy with or without basiliximab in paediatric kidney transplantation. Pediatr Transplant 2005;9:56 |
| Weir M, Mulgaonkar S, Pearson T, Patel A, Patel D, Shidban H, et al. Mycophenolate mofetil/sirolimus maintenance therapy after calcineurin inhibitor withdrawal in renal transplant recipients: 2-year outcomes of the spare-the-nephron (STN) trial. Am J Transplant 2009;9:200–1 |
| Weir M. Long-term assessment of function in patients completing the Spare-The-Nephron study with a functioning graft. Am J Transplant 2013;13:36 |
| Weir MR, Mulgaonkar S, Chan L, Shidban H, Waid TH, Preston D,, et al. Mycophenolate mofetil-based immunosuppression with sirolimus in renal transplantation: a randomized, controlled spare-the-nephron trial. Kidney Int 2011;79:897–907 |
| West-Thielke PM, Bodziak KA, Cohen DJ. Conversion to once-daily extended release MeltDose® tacrolimus tablets (LCP-Tacro™) from twice-daily tacrolimus capsules (Prograf®) is safe and efficacious in African American kidney transplant recipients: results from a phase III randomized trial. Am J Transplant 2012;12:405–6 |
| Wissing KM, Kuypers D, Abramowicz D, Weekers L, Budde KMD, Rath T, et al. Conversion from tacrolimus to cyclosporine a improves glucose metabolism in patients with new onset diabetes after renal transplantation: interim analysis of a prospective and randomized study. Transpl Int 2013;26:37 |
| Woestenburg AT, Peeters P, Sennesael J, Abramowicz D, Wissing KM, Geers C, et al. Interstitial fibrosis and fibrous intimal thickening in de novo renal allografts under sirolimus or cyclosporine: results of a randomised, controlled trial (FIBRASIC). Transpl Int 2009;22:79 |
| Woodle ES. A randomized, prospective, multicenter study of thymoglobulin in renal transplantation for induction and minimization of steroids (TRIMS). Am J Transplant 2005;5:571 |
| Woodside KJ, Thomas PG, Lappin JA, Vaidya S, Rajaraman S, Gugliuzza KK. An open label, randomized, controlled trial of a tolerogenic induction protocol using alemtuzumab (Campath 1H) and tacrolimus monotherapy versus thymoglobulin induction with triple drug therapy in high immunological risk renal transplantation. Am J Transplant 2007;7:522 |
| Wyrley-Birch H, Kanwar A, Vijayanand D, Navarro A, Reddy M, Wilson C, et al. A prospective randomised paired trial of sirolimus versus tacrolimus as primary immunosuppression following non heart beating donor kidney transplantation after anti-IL-2 monoclonal antibody induction. Transpl Int 2010;23:16 |
| Yaqoob M, Riad H, Pattison J, Cornu-Artis C, Wang Z, Tedesco-Silva H. Efficacy and safety of 24 months immunosuppression with concentration controlled everolimus and reduced cyclosporine in de novo renal transplant recipients. Transpl Int 2011;24:39 |
| Yoshimura N, Uchida K, Takahara S, Teraoka S, Kobayashi E, Teshima R, et al. Concentration-controlled everolimus with reduced cyclosporine concentration in Japanese de novo renal transplant recipients: efficacy and safety results at 12 months: Japanese multicenter study. Transplantation 2012;94:990 |
| Zeier M, Budde K, Arns W, Guba M, Sommerer C, Neumayer H, et al. Efficacy and safety of three different treatment regimens in de novo renal transplant patients: follow-up results of the Herakles trial at month 24. Am J Transplant 2013;13:183 |
Appendix 4 Quality assessment
Induction therapies
| Study, year | Random allocation | Allocation concealment | Baseline similarity | Care providers blinded | Outcome assessors blinded | Patients blinded | All a priori outcomes reported | Complete data reported | ITT | Limitations to applicability |
|---|---|---|---|---|---|---|---|---|---|---|
| Bingyi 200395 | Unclear | NR | NR | Uncleara | NR | Uncleara | Unclear | Inadequate | NR | Inadequate |
| Kahan 199972 | Unclear | NR | Adequate | Adequate | NR | Adequate | Unclear | Adequate | Adequate | Unclearb |
| Lawen 200374 | Unclear | NR | Adequate | Adequate | Adequate | Adequate | Unclear | Adequate | Adequate | Adequate |
| Nashan 199771 | Adequate | Unclear | Adequate | Adequate | NR | Adequate | Unclear | Adequate | Adequate | Inadequate |
| Ponticelli 200173 | Unclear | NR | Adequate | Adequate | NR | Adequate | Unclear | Adequate | Adequate | Unclearc |
| dAlbano 2013123 (OSAKA trial; NCT00717470) | Unclear | Unclear | Adequate | Inadequate | NR | Inadequate | Unclear | Uncleare | Inadequate | Adequate |
| Sheashaa 200397 (Sheashaa 2005, 2008160 and 2011) | Unclear | Adequate | Adequate | Inadequate | NR | NR | Unclear | Unclearf | Unclear | Inadequate |
| Charpentier 200196 | Unclear | NR | NR | Inadequate | NR | Inadequate | Unclear | Inadequate | Unclear | Unclearc |
| dCharpentier 2003148 | Unclear | Adequate | Inadequateg | Inadequate | NR | Inadequate | Unclear | Adequate | Inadequate | Inadequate |
| Brennan 2006137 | Unclear | Unclear | Adequate | Inadequate | Inadequate | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Lebranchu 200287 | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Mourad 200498 | Unclear | NR | Partialh | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Kyllönen 2007128 | Adequate | Adequate | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Inadequate | Inadequate |
Maintenance therapies
| Study, year | Random allocation | Allocation concealment | Baseline similarity | Care providers blinded | Outcome assessors blinded | Patients blinded | All a priori outcomes reported | Complete data reported | ITT | Limitations to applicability |
|---|---|---|---|---|---|---|---|---|---|---|
| Schleibner 199579 | Unclear | NR | NR | Inadequate | NR | Inadequate | Unclear | Uncleara | Adequate | Inadequate |
| Laskow 199680 (Vincenti 1996161) | Unclear | Unclear | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Mayer 199788 (Mayer 1999,162 2002163) | Unclear | NR | Inadequateb | Inadequate | Adequate | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Radermacher 199881 | Unclear | NR | Adequate | NR | NR | NR | Unclear | Adequate | Unclear | Inadequate |
| Jarzembowski 200599 | Unclear | NR | Adequate | NR | NR | NR | Unclear | Inadequate | Unclear | Inadequate |
| Baboolal 200282 | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | NR | Unclear | Inadequate |
| Campos 200283 | Unclear | NR | Partialc | Inadequate | NR | Inadequate | Unclear | Adequate | Inadequate | Uncleard |
| Margreiter 200284 (Krämer 2005,164 2008165) | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Van Duijnhoven 200275 | Unclear | Unclear | Unclear | NR | NR | NR | Unclear | Uncleara | Unclear | Inadequate |
| Waller 200276 (Murphy 2003166) | Unclear | NR | Partiale | Inadequate | NR | Inadequate | Unclear | Inadequate | Unclear | Uncleard |
| fCharpentier 2003148 | Unclear | Adequate | Inadequateg | Inadequate | NR | Inadequate | Unclear | Adequate | Inadequate | Inadequate |
| Töz 200485 | Unclear | NR | Partialc | NR | Adequate | NR | Unclear | Inadequate | NR | Inadequate |
| Hardinger 2005100 (Brennan 2005167) | Unclear | NR | Adequate | NR | NR | NR | Unclear | Inadequate | Adequate | Unclearh |
| Sollinger 199577 | Unclear | NR | Adequate | Partiali | NR | Partiali | Unclear | NR | Adequate | Inadequate |
| Tricontinental MMF renal study 199689 (Mathew 1998,168 Clayton 2012169) | Unclear | NR | Inadequatej | Adequate | NR | Adequate | Unclear | NR | Adequate | Inadequate |
| Sadek 200286 | Adequate | Unclear | Adequate | Inadequate | NR | Inadequate | Unclear | Inadequate | Adequate | Adequate |
| Tuncer 200278 | Unclear | NR | Partiale | Inadequate | Inadequate | Inadequate | Unclear | NR | NR | Inadequate |
| Merville 2004138 | Unclear | Unclear | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Inadequate | Inadequate |
| Remuzzi 2007101 (The MYSS trial, Remuzzi 2004170) | Unclear | Unclear | Partialk | NR | NR | NR | Unclear | Inadequate | Adequate | Inadequate |
| Wlodarczyk 2005139 (Wlodarczyk 2002171) | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Inadequate | Adequate | Inadequate |
| Vacher-Coponat 2012129 | Adequate | Adequate | Partiale | Inadequate | Adequate | Inadequate | Unclear | Inadequate | Adequate | Inadequate |
| Zadrazil 2012102 | Unclear | NR | Adequate | NR | NR | NR | Unclear | Adequate | Adequate | Uncleard |
| Hernández 2007130 | Adequate | Adequate | Partialk | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Rowshani 2006103 | Adequate | NR | Unclear | Inadequate | Adequate | Inadequate | Unclear | Inadequate | Unclear | Uncleard |
| Ulsh 1999153 (Yang 199990) | Unclear | NR | Partiale | Inadequate | NR | Inadequate | Unclear | NR | Adequate | Unclearh |
| Weimer 2006104 (Weimer 2005172) | Unclear | NR | Adequate | NR | NR | NR | Unclear | Adequate | Adequate | Uncleard |
| Wlodarczyk 2009140 | Unclear | Adequate | Partialk | Inadequate | Inadequate | Inadequate | Unclear | Adequate | Inadequate | Uncleard |
| Krämer 201058 (NCT00189839) | Unclear | Adequate | Adequate | Partiall | Adequate | Partiall | Unclear | Adequate | Inadequate | Adequate |
| Tsuchiya 2013141 | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Oh 2014105 | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Inadequatem | Unclear | Unclearh |
| Albano 2013123 (NCT00717470, fOSAKA Trial) | Unclear | Unclear | Adequate | Inadequate | NR | Inadequate | Unclear | Uncleark | Inadequate | Adequate |
| Ciancio 2008106 (Ciancio 2011,173 3016, R01DK25243–25) | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Unclearh |
| Salvadori 2004124 | Adequate | NR | Adequate | Adequate | Adequate | Adequate | Unclear | Adequate | Adequate | Adequate |
| Vincenti 2005125 (Vincenti 2010156) | Unclear | Unclear | Adequate | Inadequate | Adequate | Inadequate | Unclear | Adequate | Adequate | Adequate |
| BENEFIT (Vincenti 2010,59 Larsen 2010,60 Vincenti 2012,61 Rostaing 201362) | Unclear | Unclear | Adequate | Inadequate | Adequate | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| BENEFIT-EXT (Durrbach 2010,142 Medina Pestana 2012,174 Charpentier 2013,175 Larsen 201060) | Unclear | Unclear | Adequate | Inadequate | Adequate | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Ferguson 2011126 | Adequate | Unclear | Adequate | Inadequate | Adequate | Inadequate | Unclear | Adequate | Adequate | Unclearh |
| Lorber 2005143 | Unclear | NR | Adequate | Partiali | Partiali | Partiali | Unclear | Inadequate | Adequate | Unclearh |
| ATLAS Vítko 2005150 (Vítko 2004,176 2005177) | Adequate | Adequate | Adequate | Partiali | Partiali | Partiali | Unclear | Adequate | Adequate | Inadequate |
| Takahashi 2013131 | Adequate | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Chadban 2014152 (SOCRATES) | Unclear | Adequate | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Tedesco-Silva 2010107 | Unclear | Unclear | Adequate | Inadequate | Adequate | Inadequate | Unclear | Adequate | Adequate | Uncleard |
| Bertoni 2011144 | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Inadequate | Inadequate |
| Budde 2011132 (Budde 2012,178 Liefeldt 2012,179 NCT00154310) | Adequate | Unclear | Adequate | Inadequate | Inadequate | Inadequate | Unclear | Adequate | Inadequate | Adequate |
| Mjörnstedt 2012133 (NCT00634920) | Adequate | Adequate | Partialk | Inadequate | NR | Inadequate | Unclear | Adequate | Inadequate | Adequate |
| Barsoum 2007108 | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Unclearh |
| Stallone 2004109 | Unclear | NR | Adequate | NR | NR | NR | Unclear | Inadequate | Unclear | Inadequate |
| Anil Kumar 2005110 | Adequate | NR | Inadequaten | NR | Adequate | NR | Unclear | Inadequate | Adequate | Unclearh |
| Mendez 2005111 (Gonwa 2003180) | Unclear | NR | Inadequateo | Inadequate | NR | Inadequate | Unclear | Adequate | Unclear | Unclearh |
| Sampaio 2008112 | Adequate | NR | Inadequateo | NR | NR | NR | Unclear | Adequate | Unclear | Inadequate |
| Gelens 2006113 | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Uncleard |
| Gallon 2006145 (Chhabra 2012181) | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Inadequate | Inadequate |
| Van Gurp 2010114 | Unclear | Adequate | Adequate | NR | NR | NR | Unclear | Adequate | Inadequate | Adequate |
| Flechner 2002127 (Flechner 2004,182 2007183) | Adequate | NR | Adequate | NR | Adequate | NR | Unclear | Adequate | Adequate | Unclearh |
| Noris 2007115 (Ruggenenti 2007184) | Unclear | NR | Adequate | NR | Adequate | NR | Unclear | Inadequate | Adequate | Inadequate |
| Lebranchu 2009149 (Servais 2009,185 Lebranchu 2011,186 Joannides 2011,187 2004–002987–62) | Partialp | Unclear | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Büchler 2007134 (Lebranchu 2012,188 Joannides 2010189) | Adequate | NR | Adequate | Inadequate | Inadequate | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Soleimani 201391 | Unclear | NR | Partialk | NR | NR | NR | Unclear | Inadequate | Inadequate | Uncleard |
| Durrbach 2008146 (0468E1–100969) | Unclear | Unclear | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Kreis (2000)116 – identified from Campistol 2005190 | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Unclear | Inadequate |
| Guba 2010147 | Unclear | Adequate | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Inadequate | Inadequate |
| Martinez-Mier 2006117 | Unclear | NR | Adequate | NR | NR | NR | Unclear | NR | Adequate | Inadequate |
| Nafar 2012118 (IRCT138804333049N7) | Unclear | NR | Partialc | Inadequate | NR | Inadequate | Unclear | Adequate | NR | Inadequate |
| Larson 2006151 (Stegall 2003191) | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Schaefer 200692 | Unclear | NR | Inadequateq | Inadequate | NR | Inadequate | Unclear | Inadequate | Unclear | Uncleard |
| Heilman 2011135 (Heilman 2012,157 NCT00170053) | Adequate | NR | Adequate | Inadequate | Inadequate | Inadequate | Unclear | Adequate | Adequate | Inadequate |
| Smith 200893 | Unclear | NR | NR | Inadequate | NR | Inadequate | Unclear | Inadequate | Unclear | Inadequate |
| Silva 2013119 (NCT01802268) | Adequate | NR | Adequate | NR | NR | NR | Unclear | Adequate | Inadequate | Unclearh |
| Hamdy 2005120 (Hamdy 2008,192 2010193) | Unclear | NR | Adequate | NR | NR | NR | Unclear | Adequate | Adequate | Inadequate |
| Charpentier 2003136 (Groth 1999194) | Adequate | Unclear | Inadequateo | Inadequate | NR | Inadequate | Unclear | Adequate | Inadequate | Inadequate |
| Chen 2008121 | Unclear | Unclear | Adequate | NR | NR | NR | Unclear | Inadequate | Adequate | Unclearh |
| Vítko 200694 | Unclear | Unclear | Adequate | Inadequate | NR | Inadequate | Unclear | Inadequate | Unclear | Inadequate |
| Flechner 2011155 (ORION study, NCT00266123) | Unclear | NR | Partialk | Inadequate | NR | Inadequate | Unclear | Adequate | Inadequate | Adequate |
| Grinyo 200951 (SYMPHONY study, Ekberg 2009,195 Demirbas 2009,197 Ekberg 2010,196 Frei 2010,198 Claes 2012199) | Unclear | NR | Adequate | Inadequate | NR | Inadequate | Unclear | Adequate | Adequate | Adequate |
| Anil Kumar 2008122 (Anil Kumar 2005,110 CRG110600009) | Adequate | Unclear | Inadequater | NR | NR | NR | Unclear | Inadequate | NR | Unclearh |
Appendix 5 Study characteristics
Induction
| Study (multiple publications) | Previous MTA | n | Maintenance used | Patients survival | Graft survival | BPAR | Time to BPAR | Severity of BPAR | GRF (CRC) | Serum creatinine | AEs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BAS vs. PBO (five studies) | |||||||||||
| Bingyi 200395 | ✓a | 12 | CSA + AZA + CCSs | 1 year | 1 year | 6 months, 1 year | 1 year | ||||
| Kahan 199972 | ✓ | 346 | CSA + CCSs | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | ||
| Lawen 200374 | ✓b | 123 | CSA + MMF + CCSs | 6 months, 1 year | 6 months, 1 year | 6 months, 1 year | 6 months,c 1 year | 6 months | 1 yeard | 6 months | |
| Nashan 199771 | ✓ | 380 | CSA + CCSs | 6 months | 6 months | 6 months, 1 year | 1 year | ||||
| Ponticelli 200173 | ✓ | 340 | CSA + AZA + CCSs | 6 months | 6 months | 6 months | 6 monthsc | 1 month, 3 months, 6 months, 1 year | 1 month, 3 months, 6 months, 1 year | 6 months | |
| BAS vs. no induction (two studies) | |||||||||||
| Albano 2013123 (dOSAKA trial, NCT00717470) | ✗ | 1251 | CSA + MMF + CCSs | 6 months | 6 months | 6 months | 6 months | 6 months | 6 months | 6 months | |
| Sheashaa 200397 (Sheashaa 2005, 2008160 and 2011) | ✓a | 100 | CSA + AZA + CCSs | 1 year, 3 years, 5 years, 7 years, 10 years | 1 year,c 3 years, 5 years, 7 years, 10 years | 1 year, 3 years, 5 years, 7 years, 10 years | 1 year, 3 years, 5 years, 7 years, 10 years | 1 year, 3 years, 5 years, 7 years, 10 years | 3 years, 5 years, 7 years, 10 years | ||
| rATG vs. no induction (four studies) | |||||||||||
| Charpentier 200196 | ✗ | 309 | TAC + AZA + CCSs | 1 year | 1 year | 1 year | 1 yeare | 1 year | 1 year | ||
| Samsel 2008158 | ✗ | 79 | CSA + MMF (converted to AZA) + CCSs | 1 year, 2 years, 3 years, 4 years, 5 years | 1 year, 2 years, 3 years, 4 years, 5 years | 1 year | 1 year | 1 yeard | 6 months, 1 year, 2 years, 3 years, 4 years, 5 years | 5 years | |
| Sheashaa 2008160 | ✗ | 80 | CNI + prolif + CCSen | 5 years | 5 years | 1 year, 5 years | 1 year | 1 year,e 5 yearse | 5 years | ||
| dCharpentier 2003148 | ✗ | 555 | TAC + AZA + CCSs | 6 months | 6 months | 6 months | 6 months | 6 months | 6 months | ||
| BAS vs. rATG (four studies) | |||||||||||
| Brennan 2006137 | ✗ | 278 | CSA + MMF + CCSs | 1 year | 1 year | 1 year | 1 yeard | 1 year | 1 year | ||
| Lebranchu 200287 | ✓b | 100 | CSA + MMF + CCSs | 6 months, 1 year | 6 months, 1 year | 6 months, 1 year | 6 months, 1 year | 6 months, 1 year | 6 months,d 1 yeard | 6 months, 1 year | 6 months |
| Mourad 200498 | ✗ | 105 | CSA + MMF + CCSs | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | ||
| Sollinger 2001159 | ✓ | 135 | CSA + MMF + CCSs | 1 year | 1 year | 6 months, 1 year | 1 year | 1 year | 1 year | ||
| BAS vs. rATG vs. no induction (one study) | |||||||||||
| Kyllönen 2007128 | ✗ | 155 | CSA + AZA + CCSs | 1 year, 5 years | 1 year, 5 years | 1 year | 1 year | 1 year, 2 years,f 3 years,f 4 years,f 5 yearsf | 1 year | 1 year, 5 years | |
Maintenance
| Study (multiple publications) | Previous MTA | n | Patients survival | Graft survival | BPAR | Time to BPAR | Severity of BPAR | GRF (eGFR) | Serum creatinine | AEs |
|---|---|---|---|---|---|---|---|---|---|---|
| TAC + AZA vs. CSA + AZA (13 studies) | ||||||||||
| Schleibner 199579 | ✓ | 47 | 6 weeks | 6 weeks | 6 weeks | 6 weeks | ||||
| Laskow 199680 (Vincenti 1996161) | ✗ | 120 | 1 year | 1 year | 42 days | 42 days, 1 year | Day 42, 1 year | |||
| Mayer 199788 (Mayer 1999,162 2002163) | ✓ | 448 | 1 year, 5 years | 1 year, 5 years | 1 year, 4 years | 1 year, 4 years | 1 year | |||
| Radermacher 199881 | ✓ | 41 | 1 year | 1 year, 4 years | ||||||
| Jarzembowski 200599 | ✗ | 35 | 1 year | 1 year | 1 year | 1 month, 6 months, 1 year, 3 years, 5 years | ||||
| Baboolal 200282 | ✓ | 51 | 1 year, 2 years | 1 year | 1 year | 1 year | 1 yeara | |||
| Campos 200283 | ✓ | 166 | 1 year | 1 year | 1 year | 1 year | 1 year | |||
| Margreiter 200284 (Krämer 2005,164 2008165) | ✓ | 560 | 6 months, 1 year, 2 years, 3 years | 6 months, 1 year, 3 years | 6 months, 1 year, 2 years, 3 years | 6 months, 1 year, 2 years | 2 years, 3 years | 6 months, 1 year, 2 years | 6 months, 2 years | |
| Van Duijnhoven 200275 | ✓ | 23 | 1 year | 3 months, 6 months, 1 year, 2 years, 3 years | ||||||
| Waller 200276 (Murphy 2003166) | ✓ | 102 | 1 year | 1 year | 1 yeara | |||||
| Charpentier 2003148 | ✗ | 555 | 6 months | 6 months | 6 months | 6 months | 6 months | |||
| Töz 200485 | ✓ | 35 | ||||||||
| Hardinger 2005100 (Brennan 2005167) | ✗ | 200 | 1 year | 1 year | 1 year | 1 year | 1 yeara | 6 months, 1 year | 1 year | |
| CSA + MMF low vs. CSA + AZA vs. CSA + MMF (two studies) | ||||||||||
| Sollinger 199577 | ✓ | 499 | 6 months | 6 months | 6 months | 6 months | 6 months | 6 months | ||
| Tricontinental MMF renal study 199689 (Mathew 1998,168 Clayton 2012169) | ✓ | 497 | 6 months, 1 year, 3 years | 6 months, 1 year, 3 years | 6 months | 6 months | 6 months, 1 year, 3 years | 6 months, 3 years | ||
| CSA + MMF vs. CSA + AZA (four studies) | ||||||||||
| Sadek 200286 | ✓ | 477 | 1 year | 1 year | 1 year | 1 year | ||||
| Tuncer 200278 | ✓ | 76 | 1 year, 3 years, 5 years | 1 year | ||||||
| Merville 2004138 | ✗ | 71 | 1 year | 1 year | 1 year | 1 year | 1 year | 6 months, 1 year | 6 months, 1 year | 1 year |
| Remuzzi 2007101 (the MYSS trial, Remuzzi 2004170) | ✗ | 336 | 6 months, 1 year, 5 years | 5 years | 6 months, 1 year, 5 years | 6 months,b 5 years | 6 months | 6 months, 1 year, 5 years | ||
| TAC + MMF vs. CSA + AZA (two studies) | ||||||||||
| Wlodarczyk 2005139 (Wlodarczyk 2002171) | ✗ | 489 | 6 months | 6 months | 3 months, 6 months | 3 monthsa | 6 months | 6 months | ||
| Vacher-Coponat 2012129 | ✗ | 289 | 1 year, 3 years | 1 year, 3 years | 1 year | 1 year | 1 year,b 3 years | 1 year, 3 years | ||
| TAC + MMF vs. CSA + MMF (four studies) | ||||||||||
| Zadrazil 2012102 | ✗ | 53 | 1 year | 1 year | 6 months, 1 year | 6 months | ||||
| Hernández 2007130 | ✗ | 240 | 1 year, 2 years | 2 years | 2 years | 2 years | 6 months,b 1 yearb | 1 year | 2 years | |
| Rowshani 2006103 | ✗ | 126 | 1 year | 1 year | 1 year | 1 year | 1 year | |||
| Ulsh 1999153 (Yang 199990) | ✓ | 60 | 1 year | 1 year | 1 year | 1 year | 1 yeara | 1 year | 1 year | |
| TAC + AZA vs. CSA + AZA vs. CSA + MMF (one study) | ||||||||||
| Weimer 2006104 (Weimer 2005172) | ✗ | 81 | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | ||
| TAC + MMF vs. TAC-PR + MMF (four studies) | ||||||||||
| Wlodarczyk 2009140 | ✗ | 122 | ||||||||
| Krämer 201058 (NCT00189839) | ✗ | 667 | 1 year | 1 year | 6 months, 1 year | 1 year | 1 yearb | 1 year | 1 year | |
| Tsuchiya 2013141 | ✗ | 102 | 1 year | 1 year | 1 year | 1 year | 1 year | |||
| Oh 2014105 | ✗ | 104 | 6 months | 6 months | 6 months | 6 months | ||||
| TAC + MMF vs. TAC-PR 0.2 + MMF vs. TAC-PR 0.3 (one study) | ||||||||||
| Albano 2013123 (NCT00717470, OSAKA Triald) | ✗ | 1251 | 6 months | 6 months | 6 months | 6 months | 6 months | 6 months | ||
| MMF + TAC vs. MPS + TAC (one study) | ||||||||||
| Ciancio 2008106 (Ciancio 2011,173 3016, R01DK25243–25) | ✗ | 150 | 1 year, 4 years | 1 year, 4 years | 1 year, 2 years, 4 years | 1 year | 1 month, 3 months, 6 months, 1 year, 2 years, 3 years, 4 years | 1 month, 3 months, 6 months, 1 year, 2 years, 3 years, 4 years | 1 year, 4 years | |
| MMF + CSA vs. MPS + CSA (one study) | ||||||||||
| Salvadori 2004124 | ✗ | 423 | 6 months, 1 year | 6 months, 1 year | 6 months, 1 year | 6 months | 6 m, 1 year | |||
| BEL low + MMF vs. BEL high + MMF vs. CSA + MMF (three studies) | ||||||||||
| Vincenti 2005125 (Vincenti 2010156) | ✗ | 218 | 1 year | 1 year | 6 months, 1 year | 6 months, 1 year, 5 years | 1 year | 1 year, 5 years | ||
| BENEFIT (Vincenti 2010,59 Larsen 2010,60 Vincenti 2012,61 Rostaing 201362) | ✗ | 686 | 1 year, 2 years, 3 years, 5 years | 1 year, 2 years, 3 years, 5 years | 1 year, 2 years, 3 years, 4 years, 5 years | 1 year | 1 year, 2 years, 3 years, 5 years | 1 year, 2 years, 3 years, 5 years | ||
| BENEFIT-EXT (Durrbach 2010,142 Medina Pestana 2012,174 Charpentier 2013,175 Larsen 201060) | ✗ | 578 | 1 year, 2 years, 3 years, 5 years | 1 year, 2 years, 3 years, 5 years | 1 year, 2 years, 3 years, 5 years | 1 year, 5 years | 1 year, 2 years, 3 years, 5 years | 1 year, 2 years, 3 years, 5 years | ||
| BEL + MMF vs. BEL + SRL vs. TAC + MMF (one study) | ||||||||||
| Ferguson 2011126 | ✗ | 89 | 1 year | 1 year | 6 months, 1 year | 6 months | 1 year | 1 year | ||
| EVL low + CSA vs. EVL high + CSA vs. MMF + CSA (three studies) | ||||||||||
| Lorber 2005143 | ✗ | 583 | 1 year, 3 years | 1 year, 3 years | 1 year, 3 years | 1 year, 2 years, 3 years | 1 year, 3 years | 3 years | ||
| ATLAS Vítko 2005150 (Vítko 2004,176 2005177) | ✗ | 588 | 6 months, 1 year, 3 years | 6 months, 1 year, 3 years | 6 months, 1 year, 3 years | 1 year, 2 years, 3 years | 1 year, 2 years, 3 years | 1 year, 3 years | ||
| Takahashi 2013131 | ✗ | 122 | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | ||
| EVL vs. EVL + CSA vs. CSA + MPS (one study | ||||||||||
| Chadban 2014152 (SOCRATES) | ✗ | 126 | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | ||
| EVL low + CSA vs. EVL high + CSA vs. MPA + CSA (one study) | ||||||||||
| Tedesco-Silva 2010107 | ✗ | 783 | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | |
| EVL + CSA vs. MPS + CSA (one study) | ||||||||||
| Bertoni 2011144 | ✗ | 106 | 1 year | 1 year | 1 year | 1 yearb | ||||
| EVL + MPS vs. CSA + MPS (two studies) | ||||||||||
| Budde 2011132 (Budde 2012,178 Liefeldt 2012,179 NCT00154310) | ✗ | 300 | ||||||||
| Mjörnstedt 2012133 (NCT00634920) | ✗ | 202 | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | |
| SRL + CSA vs. MMF + CSA (two studies) | ||||||||||
| Barsoum 2007108 | ✗ | 113 | 2 years | 2 years | 2 years | 1 year, 2 years | 2 years | |||
| Stallone 2004109 | ✗ | 90 | 1 year | 1 year | 6 months, 1 yearb | 1 year | ||||
| SRL + TAC vs. MMF + TAC (six studies) | ||||||||||
| Anil Kumar 2005110 | ✗ | 150 | 1 year, 2 years | 1 year | 1 year | 1 year | 1 year | |||
| Mendez 2005111 (Gonwa 2003180) | ✗ | 361 | 6 months, 1 year | 6 months, 1 year | 6 months, 1 year | 6 months,b 1 year | 6 months, 1 year | 6 months, 1 year | ||
| Sampaio 2008112 | ✗ | 100 | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year |
| Gelens 2006113 | ✗ | 54 | ||||||||
| Gallon 2006145 (Chhabra 2012181) | ✗ | 83 | 3 years, 8.5 years | 3 years, 8.5 years | 1 year,b 3 years,b 8.5 years | 3 years | ||||
| Van Gurp 2010114 | ✗ | 634 | 6 months | 6 months | 6 months | 6 months | 6 monthsb | 6 months | ||
| SRL + MMF vs. CSA + MMF (10 studies) | ||||||||||
| Flechner 2002127 (Flechner 2004,182 2007183) | ✗ | 61 | 1 year, 2 years, 5 years | 1 year, 2 years, 5 years | 1 year, 2 years, 5 years | 1 year, 5 years | 1 year, 5 years | 1 month, 3 months, 6 months, 1 year, 2 years, 5 years | 1 month, 3 months, 6 months, 1 year, 2 years, 5 years | 1 year, 2 years, 5 years |
| Noris 2007115 (Ruggenenti 2007184) | ✗ | 21 | 1 year, 2 years | 1 year, 2 years | 1 year, 2 years | 1 year, 2 years | 2 years | |||
| Lebranchu 2009149 (Servais 2009,185 Lebranchu 2011,186 Joannides 2011,187 2004–002987–62) | ✗ | 192 | 1 year, 4 years | 1 year, 4 years | 1 year, 4 years | 6 months,a 1 year,b 4 years | 6 months, 1 year | 1 year, 4 years | ||
| Büchler 2007134 (Lebranchu 2012,188 Joannides 2010189) | ✗ | 145 | 1 year, 5 years | 1 year, 5 years | 1 year, 5 years | 1 year | 1 year | 1 year,b 5 years | 5 years | 1 year, 5 years |
| Soleimani 201391 | ✗ | 88 | 1 month, 1 year, 3 years, 4 years, 5 years | |||||||
| Durrbach 2008146 (0468E1–100969) | ✗ | 69 | 6 months | 6 months | 6 months | 6 months | 6 monthsb | 6 months | 6 months | |
| Kreis 2000116 – identified from Campistol 2005190 | ✗ | 78 | 1 year | 1 year | 1 year | 1 year | 1 yearb | 6 months, 1 year | 1 year | |
| Guba 2010147 | ✗ | 140 | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | ||
| Martinez-Mier 2006117 | ✗ | 41 | 1 year | 1 year | 1 year | 6 months, 1 year | 6 months, 1 year | |||
| Nafar 2012118 (IRCT138804333049N7) | ✗ | 100 | 1 year, 2 years, 3 years, 4 years | 1 year, 2 years, 3 years, 4 years | 1 year | 1 year, 2 years, 3 years, 4 years | 1 year, 2 years, 3 years, 4 years | |||
| TAC + MMF vs. SRL + MMF (four studies) | ||||||||||
| Larson 2006151 (Stegall 2003191) | ✗ | 162 | 1 year | 1 year | 1 year, 2 yearsb | |||||
| Schaefer 200692 | ✗ | 80 | 1 year | 1 year | 1 year | 1 year | ||||
| Heilman 2011135 (Heilman 2012,157 NCT00170053) | ✗ | 122 | 1 year, 2 years | 1 year, 2 years | 1 year | 1 year,b 2 years | 1 year | 2 years | ||
| Smith 200893 | ✗ | 51 | 1 year | 1 year | 1 year | 1 year | 1 year | 1 year | ||
| TAC + MPS vs. SRL + MPS (one study) | ||||||||||
| Silva 2013119 (NCT01802268) | ✗ | 204 | 2 years | 2 years | 2 years | 2 years | 2 years | 2 years | ||
| TAC + SRL vs. MMF + SRL (one study) | ||||||||||
| Hamdy 2005120 (Hamdy 2008,192 2010193) | ✗ | 132 | 1 year, 2 years, 3 years, 4 years, 5 years | 2 years, 3 years, 4 years, 5 years | 1 year, 3 years | 1 year, 2 years, 3 years, 4 years | 2 years | 2 years, 4 years | ||
| SRL + AZA vs. CSA + AZA (one study) | ||||||||||
| Charpentier 2003136 (Groth 1999194) | ✓ | 83 | 1 year | 1 year | 6 months | 6 months | 6 months, 1 year | 6 months, 1 year | 1 year | |
| TAC + SRL vs. CSA + SRL (one study) | ||||||||||
| Chen 2008121 | ✗ | 41 | 1 year | 1 year | 1 year | 1 year | 6 months, 1 yearb | 1 year | ||
| SRL low + TAC vs. SRL high + TAC vs. MMF + TAC (one study) | ||||||||||
| Vítko 200694 | ✗ | 977 | 6 months | 6 months | 6 months | 6 months | 6 months | 6 months | ||
| SRL + TAC vs. SRL + MMF vs. MMF + TAC (one study) | ||||||||||
| Flechner 2011155 (ORION study, NCT00266123) | ✗ | 450 | 1 year, 2 years | 2 years | 1 year, 2 years | 1 year, 2 years | 1 year,b 2 years | 2 years | ||
| MMF + CSA vs. MMF + low CSA vs. MMF + low TAC vs. MMF low SRL (one study) | ||||||||||
| Grinyo 200951 (SYMPHONY study, Ekberg 2009,195 Demirbas 2009,197 Ekberg 2010,196 Frei 2010,198 Claes 2012199) | ✗ | 1529 | 1 year | 1 year | 6 months, 1 year | 1 year | 1 year | 1 year,b 2 years, 3 years | 1 year, 3 years | |
| TAC + MMF vs. TAC + SRL vs. CSA + MMF vs. CSA + SRL (one study) | ||||||||||
| Anil Kumar 2008122 (Anil Kumar 2005,110 CRG110600009) | ✗ | 200 | 5 years | 1 year, 2 years, 3 years, 4 years, 5 years | 1 year | 1 year,b 2 years, 3 years, 4 years, 5 years | 1 year, 2 years, 3 years, 4 years, 5 years | 1 year, 5 years | ||
Appendix 6 Network meta-analysis
Induction therapy results
Graft loss
Results of random-effects model and consistency analyses
As there are direct data for three comparisons and three treatments, but a three-arm trial, there are no ICDF for this network. Comparing the DIC between the consistency and inconsistency models (Table 218) suggests that the consistency models provide a slightly better fit to the data for both the fixed- and random-effects models. Furthermore, the posterior median estimates are similar between the consistency and inconsistency models and the 95% CrIs overlap considerably.
| Treatment comparison | Fixed effects | Random effects | ||
|---|---|---|---|---|
| Consistency | Inconsistency | Consistency | Inconsistency | |
| OR (BAS vs. PBO/no treatment) | 0.84 (0.59 to 1.21) | 0.81 (0.55 to 1.19) | 0.84 (0.55 to 1.30) | 0.81 (0.49 to 1.29) |
| OR (rATG vs. PBO/no treatment) | 0.78 (0.45 to 1.34) | 0.89 (0.43 to 1.95) | 0.78 (0.42 to 1.43) | 0.90 (0.40 to 2.03) |
| OR (rATG vs. BAS) | 0.92 (0.53 to 1.59) | 0.80 (0.38 to 1.66) | 0.93 (0.51 to 1.69) | 0.80 (0.40 to 1.80) |
| Estimate of between-study heterogeneity | 0.15 (0.01 to 0.63) | 0.16 (0.01 to 0.70) | ||
| Total residual deviance | 19.56 | 20.26 | 20.44 | 21.16 |
| Relative number of model parameters | 14.63 | 15.60 | 15.71 | 16.66 |
| DIC | 34.19 | 35.86 | 36.15 | 37.82 |
Mortality
Results of random-effects model and consistency analyses
As there are direct data for three comparisons and three treatments, but a three-arm trial, there are no ICDF for this network. Comparing the DIC between the consistency and inconsistency models suggests that the consistency models provide a better fit to the data for both the fixed- and random-effects models. Furthermore, the posterior median estimates are similar between the consistency and inconsistency models and the 95% CrIs overlap considerably (Table 219).
| Treatment comparison | Fixed effects | Random effects | ||
|---|---|---|---|---|
| Consistency | Inconsistency | Consistency | Inconsistency | |
| OR (BAS vs. PBO/no treatment) | 0.89 (0.49 to 1.62) | 0.91 (0.47 to 1.74) | 0.82 (0.28 to 1.77) | 0.81 (0.19 to 1.99) |
| OR (rATG vs. PBO/no treatment) | 0.68 (0.28 to 1.39) | 0.59 (0.17 to 1.79) | 0.56 (0.14 to 0.14) | 0.51 (0.07 to 2.01) |
| OR (rATG vs. BAS) | 0.72 (0.34 to 1.47) | 0.74 (0.29 to 1.79) | 0.68 (0.23 to 1.73) | 0.68 (0.15 to 2.32) |
| Estimate of between-study heterogeneity | 0.39 (0.02 to 1.74) | 0.46 (0.02 to 2.20) | ||
| Total residual deviance | 25.08 | 26.12 | 24.66 | 25.53 |
| Relative number of model parameters | 13.35 | 14.28 | 15.41 | 16.49 |
| DIC | 38.43 | 40.40 | 40.07 | 42.02 |
Biopsy-proven acute rejection
Results of random-effects model and consistency analyses
As there are direct data for three comparisons and three treatments, but a three-arm trial, there are no ICDF for this network. Comparing the DIC between the consistency and inconsistency models suggests that the consistency models provide a better fit to the data for both the fixed- and random-effects models. Furthermore, the posterior median estimates are similar between the consistency and inconsistency models and the 95% CrIs overlap considerably (Table 220).
| Treatment comparison | Fixed effects | Random effects | ||
|---|---|---|---|---|
| Consistency | Inconsistency | Consistency | Inconsistency | |
| OR (BAS vs. PBO/no treatment) | 0.50 (0.40 to 0.62) | 0.51 (0.40 to 0.64) | 0.50 (0.37 to 0.64) | 0.50 (0.37 to 0.71) |
| OR (rATG vs. PBO/no treatment) | 0.35 (0.25 to 0.49) | 0.34 (0.22 to 0.53) | 0.35 (0.24 to 0.51) | 0.33 (0.19 to 0.55) |
| OR (rATG vs. BAS) | 0.70 (0.51 to 0.97) | 0.73 (0.47 to 1.12) | 0.71 (0.49 to 1.04) | 0.75 (0.46 to 1.26) |
| Estimate of between-study heterogeneity | 0.12 (0.01 to 0.46) | 0.13 (0.01 to 0.52) | ||
| Total residual deviance | 21.00 | 21.96 | 21.08 | 21.83 |
| Relative number of model parameters | 14.01 | 15.00 | 15.84 | 16.96 |
| DIC | 35.01 | 36.96 | 36.92 | 38.79 |
Graft function
Results of random-effects model and consistency analyses
As there are direct data for three comparisons and three treatments, the ICDF for this network is 1. Comparing the DIC between the consistency and inconsistency models suggests very little difference between the models; however, the mean effect for rATG from the direct evidence (the inconsistency model) is much larger than that when both direct and indirect evidence are used (the consistency model): 3.44 (95% CrI –2.49 to 9.36) vs. 0.75 (95% CrI –3.99 to 5.48) from the fixed-effects model. Nevertheless, the 95% CrIs overlap considerably (Table 221).
| Treatment comparison | Fixed effects | Random effects | ||
|---|---|---|---|---|
| Consistency | Inconsistency | Consistency | Inconsistency | |
| OR (BAS vs. PBO/no induction) | 2.62 (0.13 to 5.08) | 2.11 (–0.46 to 4.67) | 2.60 (–1.00 to 6.19) | 2.00 (–1.79 to 5.64) |
| OR (rATG vs. PBO/no induction) | 0.75 (–3.99 to 5.48) | 3.44 (–2.49 to 9.36) | 0.54 (–5.82 to 6.65) | 3.41 (–4.50 to 11.36) |
| OR (rATG vs. BAS) | –1.86 (–6.72 to 3.00) | –6.05 (–13.46 to 1.34) | –2.03 (–8.53 to 4.19) | –6.04 (–15.13 to 3.05) |
| Estimate of between-study heterogeneity | 2.27 (0.12 to 4.80) | 2.14 (0.11 to 4.78) | ||
| Total residual deviance | 14.28 | 13.11 | 12.38 | 11.95 |
| Relative number of model parameters | 7.98 | 8.99 | 9.85 | 10.41 |
| DIC | 22.26 | 22.10 | 22.23 | 22.36 |
Maintenance therapy results
Graft loss
Fixed-effects model results
| Intervention treatment | Comparator treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSA + AZA | TAC + AZA | MMF + CSA | TAC + MMF | BEL + SRL | BEL + MMF | EVL + CSA | SRL + TAC | SRL + CSA | SRL + MMF | SRL + AZA | |
| TAC + AZA | 1.01 (0.71 to 1.44) | ||||||||||
| MMF + CSA | 0.83 (0.53 to 1.29) | 0.83 (0.47 to 1.44) | |||||||||
| TAC + MMF | 0.73 (0.41 to 1.27) | 0.72 (0.37 to 1.40) | 0.87 (0.56 to 1.36) | ||||||||
| BEL + SRL | 1.46 (0.19 to 10.34) | 1.45 (0.18 to 10.58) | 1.75 (0.23 to 11.93) | 2.01 (0.27 to 13.62) | |||||||
| BEL + MMF | 0.67 (0.33 to 1.35) | 0.66 (0.30 to 1.45) | 0.80 (0.46 to 1.39) | 0.92 (0.45 to 1.84) | 0.46 (0.07 to 3.37) | ||||||
| EVL + CSA | 0.76 (0.41 to 1.43) | 0.76 (0.37 to 1.55) | 0.92 (0.58 to 1.44) | 1.05 (0.56 to 1.97) | 0.52 (0.07 to 4.09) | 1.15 (0.56 to 2.35) | |||||
| SRL + TAC | 1.26 (0.58 to 2.72) | 1.25 (0.54 to 2.91) | 1.52 (0.77 to 2.97) | 1.73 (0.97 to 3.14) | 0.87 (0.12 to 6.90) | 1.90 (0.80 to 4.52) | 1.65 (0.74 to 3.71) | ||||
| SRL + CSA | 0.59 (0.14 to 2.12) | 0.59 (0.13 to 2.21) | 0.71 (0.17 to 2.39) | 0.82 (0.20 to 2.72) | 0.40 (0.04 to 4.09) | 0.88 (0.20 to 3.38) | 0.77 (0.18 to 2.83) | 0.47 (0.11 to 1.61) | |||
| SRL + MMF | 1.24 (0.69 to 2.23) | 1.23 (0.62 to 2.42) | 1.49 (0.98 to 2.28) | 1.71 (1.10 to 2.67) | 0.85 (0.12 to 6.46) | 1.86 (0.93 to 3.74) | 1.62 (0.88 to 3.02) | 0.98 (0.50 to 1.91) | 2.09 (0.61 to 8.77) | ||
| SRL + AZA | 0.25 (0.01 to 2.47) | 0.25 (0.01 to 2.54) | 0.30 (0.01 to 3.12) | 0.35 (0.01 to 3.66) | 0.17 (0.01 to 3.77) | 0.38 (0.01 to 4.19) | 0.33 (0.01 to 3.53) | 0.20 (0.01 to 2.25) | 0.42 (0.01 to 6.49) | 0.20 (0.01 to 2.17) | |
| EVL | 0.10 (0.01 to 1.96) | 0.10 (0.01 to 2.00) | 0.13 (0.01 to 2.27) | 0.14 (0.01 to 2.72) | 0.07 (0.01 to 2.55) | 0.16 (0.01 to 3.03) | 0.14 (0.01 to 2.50) | 0.08 (0.01 to 1.64) | 0.17 (0.01 to 4.52) | 0.08 (0.01 to 1.58) | 0.41 (0.01 to 36.99) |
Consistency analysis results
There are direct data for 18 comparisons and 12 treatments in the network; however, four independent loops are informed by multiarm trials only and so the ICDF, reflecting the number of independent loops in the network, is 18 – (12 – 1) – 4 = 3. Comparing the DIC between the consistency and inconsistency models suggests that there is little difference between the random-effects models (154.4 vs. 153.6). Furthermore, the posterior median estimates are similar between the consistency and inconsistency models and the 95% CrIs overlap considerably (Table 223).
| Comparison | Fixed effects | Random effects | ||
|---|---|---|---|---|
| Consistency | Inconsistency | Consistency | Inconsistency | |
| TAC + AZA vs. CSA + AZA | 1.01 (0.71 to 1.44) | 1.00 (0.71 to 1.43) | 1.13 (0.67 to 2.15) | 1.11 (0.65 to 2.11) |
| MMF + CSA vs. CSA + AZA | 0.83 (0.53 to 1.29) | 0.69 (0.42 to 1.10) | 0.76 (0.35 to 1.44) | 0.59 (0.24 to 1.24) |
| TAC + MMF vs. CSA + AZA | 0.73 (0.41 to 1.27) | 1.79 (0.64 to 5.51) | 0.69 (0.28 to 1.55) | 1.79 (0.40 to 8.47) |
| SRL + AZA vs. CSA + AZA | 0.25 (0.01 to 2.47) | 0.25 (0.01 2047) | 0.25 (0.01 to 3.10) | 0.25 (0.01 to 3.17) |
| TAC + MMF vs. MMF + CSA | 0.87 (0.56 to 1.35) | 0.85 (0.50 to 1.42) | 0.92 (0.48 to 1.77) | 1.14 (0.51 to 3.11) |
| BEL + MMF vs. MMF + CSA | 0.80 (0.45 to 1.39) | 0.73 (0.41 to 1.30) | 0.82 (0.35 to 1.97) | 0.70 (0.27 to 1.71) |
| EVL + CSA vs. MMF + CSA | 0.92 (0.59 to 1.44) | 0.92 (0.59 to 1.44) | 0.84 (0.39 to 1.63) | 0.84 (0.38 to 1.64) |
| SRL + TAC vs. MMF + CSA | 1.52 (0.77 to 2.97) | 0.55 (0.11 to 2.03) | 0.57 (0.64 to 3.93) | 0.76 (0.11 to 5.21) |
| SRL + CSA vs. MMF + CSA | 0.71 (0.17 to 2.39) | 0.55 (0.13 to 1.85) | 0.73 (0.15 to 3.10) | 0.69 (0.13 to 3.73) |
| SRL + MMF vs. MMF + CSA | 1.49 (0.98 to 2.28) | 1.42 (0.91 to 2.23) | 1.40 (0.72 to 2.58) | 1.13 (0.49 to 2.23) |
| EVL vs. CSA + AZA | 0.13 (0.01 to 2.27) | 0.13 (0.01 to 2.28) | 0.13 (0.01 to 2.67) | 0.12 (0.01 to 2.69) |
| BEL + SRL vs. TAC + MMF | 2.01 (0.27 to 13.63) | 11.82 (0.59 to 5642.03) | 2.05 (0.22 to 18.01) | 12.33 (0.48 to 6727.78) |
| BEL + MMF vs. TAC + MMF | 0.92 (0.45 to 1.84) | 9.13 (0.46 to 4429.31) | 0.89 (0.32 to 2.53) | 9.55 (0.38 to 5014.05) |
| SRL + TAC vs. TAC + MMF | 1.73 (0.97 to 3.14) | 2.48 (1.22 to 5.31) | 1.71 (0.80 to 3.69) | 2.59 (1.05 to 6.95) |
| SRL + MMF vs. TAC + MMF | 1.71 (1.10 to 2.67) | 2.34 (0.95 to 5.97) | 1.52 (0.74 to 2.91) | 2.43 (0.78 to 8.17) |
| Total residual deviance | 107.6 | 103.8 | 93.64 | 90.14 |
| Relative number of model parameters | 49.868 | 53.499 | 60.791 | 63.518 |
| DIC | 157.498 | 157.299 | 154.431 | 153.658 |
Mortality
Fixed-effects model results
| Intervention treatment | Comparator treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSA + AZA | TAC + AZA | MMF + CSA | TAC + MMF | BEL + SRL | BEL + MMF | EVL + MPS | EVL + CSA | SRL + TAC | SRL + CSA | SRL + MMF | SRL + AZA | |
| TAC + AZA | 1.40 (0.80 to 2.55) | |||||||||||
| MMF + CSA | 0.95 (0.49 to 1.85) | 0.67 (0.28 to 1.62) | ||||||||||
| TAC + MMF | 1.53 (0.68 to 3.48) | 1.08 (0.40 to 2.96) | 1.61 (0.92 to 2.88) | |||||||||
| BEL + SRL | 0.32 (0.01 to 8.29) | 0.22 (0.01 to 6.20) | 0.34 (0.01 to 8.25) | 0.21 (0.01 to 4.96) | ||||||||
| BEL + MMF | 0.47 (0.16 to 1.30) | 0.33 (0.10 to 1.07) | 0.50 (0.22 to 1.07) | 0.31 (0.12 to 0.78) | 1.48 (0.06 to 746.80) | |||||||
| EVL + MPS | 0.93 (0.09 to 9.81) | 0.66 (0.06 to 7.43) | 0.98 (0.10 to 9.46) | 0.61 (0.06 to 6.29) | 3.15 (0.06 2029) | 1.97 (0.18 to 21.70) | ||||||
| EVL + CSA | 1.41 (0.57 to 3.46) | 1.00 (0.34 to 2.91) | 1.48 (0.82 to 2.73) | 0.92 (0.40 to 2.11) | 4.43 (0.17 2261) | 2.98 (1.13 to 8.21) | 1.51 (0.15 to 16.00) | |||||
| SRL + TAC | 1.39 (0.53 to 3.66) | 0.99 (0.32 to 3.04) | 1.47 (0.69 to 3.13) | 0.91 (0.50 to 1.64) | 4.35 (0.17 2178) | 2.95 (1.02 to 8.84) | 1.50 (0.14 to 16.46) | 0.99 (0.37 to 2.59) | ||||
| SRL + CSA | 0.62 (0.14 to 2.48) | 0.44 (0.09 to 1.98) | 0.66 (0.17 to 2.25) | 0.41 (0.10 to 1.43) | 2.00 (0.06 1052) | 1.32 (0.29 to 5.75) | 0.67 (0.05 to 8.92) | 0.44 (0.10 to 1.73) | 0.45 (0.11 to 1.68) | |||
| SRL + MMF | 1.74 (0.75 to 4.12) | 1.24 (0.44 to 3.46) | 1.84 (1.04 to 3.33) | 1.14 (0.67 to 1.95) | 5.45 (0.22 2730) | 3.70 (1.44 to 9.99) | 1.88 (0.18 to 19.72) | 1.24 (0.54 to 2.87) | 1.25 (0.62 to 2.57) | 2.79 (0.77 to 11.44) | ||
| SRL + AZA | 0.19 (0.01 to 6.02) | 0.14 (0.01 to 4.51) | 0.20 (0.01 to 6.82) | 0.13 (0.01 to 4.39) | 0.62 (0.01 to 641.3) | 0.41 (0.01 to 15.10) | 0.19 (0.01 to 13.93) | 0.14 (0.01 to 4.87) | 0.14 (0.01 to 5.03) | 0.30 (0.01 to 13.27) | 0.11 (0.01 to 3.86) | |
| EVL | 0.24 (0.01 to 6.09) | 0.17 (0.01 to 4.58) | 0.26 (0.01 to 5.99) | 0.16 (0.01 to 3.89) | 0.75 (0.01 to 729.2) | 0.51 (0.01 to 13.38) | 0.24 (0.01 to 13.20) | 0.17 (0.01 to 4.02) | 0.17 (0.01 to 4.53) | 0.38 (0.01 to 12.10) | 0.14 (0.01 to 3.46) | 1.23 (0.01 1232) |
Consistency analysis results
There are direct data for 20 comparisons and 13 treatments in the network; however, four independent loops are informed by multiarm trials only and so the ICDF, reflecting the number of independent loops in the network, is 20 – (13 – 1) – 4 = 4. Comparing the DIC between the consistency and inconsistency models suggests that the consistency model provides a better fit to the data (139.5 vs. 143.9). Furthermore, the posterior median estimates are similar between the consistency and inconsistency models and the 95% CrIs overlap considerably (Table 225).
| Comparison | Fixed effects | Random effects | ||
|---|---|---|---|---|
| Consistency | Inconsistency | Consistency | Inconsistency | |
| TAC + AZA vs. CSA + AZA | 1.40 (0.80 to 2.54) | 1.40 (0.80 to 2.55) | 1.38 (0.74 to 2.60) | 1.38 (0.73 to 2.61) |
| MMF + CSA vs. CSA + AZA | 0.95 (0.49 to 1.85) | 0.89 (0.43 to 1.83) | 1.06 (0.45 to 1.95) | 0.88 (0.40 to 1.93) |
| TAC + MMF vs. CSA + AZA | 1.53 (0.68 to 3.48) | 2.26 (0.40 to 18.76) | 1.53 (0.63 to 3.71) | 2.32 (0.38 to 6.89) |
| SRL + AZA vs. CSA + AZA | 0.19 (0.01 to 6.02) | 0.20 (0.1 to 5.98) | 0.20 (0.01 to 6.03) | 0.20 (0.01 to 6.60) |
| TAC + MMF vs. MMF + CSA | 1.61 (0.92 to 2.88) | 1.84 (0.95 to 3.57) | 1.61 (0.89 to 3.00) | 1.89 (0.93 to 735.09) |
| BEL + MMF vs. MMF + CSA | 0.50 (0.22 to 1.07) | 0.42 (0.17 to 0.93) | 0.50 (0.21 to 1.11) | 0.41 (0.16 to 0.98) |
| EVL + MPS vs. MMF + CSA | 0.98 (0.10 to 9.46) | 0.98 (0.10 to 9.62) | 1.00 (0.09 to 10.08) | 0.98 (0.10 to 10.43) |
| EVL + CSA vs. MMF + CSA | 1.48 (0.82 to 2.73) | 1.48 (0.82 to 2.73) | 1.48 (0.77 to 2.83) | 1.46 (0.76 to 2.87) |
| SRL + TAC vs. MMF + CSA | 1.47 (0.69 to 3.13) | 0.77 (0.10 to 3.71) | 1.46 (0.65 to 3.23) | 0.82 (0.10 to 4.48) |
| SRL + CSA vs. MMF + CSA | 0.66 (0.17 to 2.25) | 0.63 (0.17 to 2.17) | 0.66 (0.17 to 2.37) | 0.65 (0.16 to 2.41) |
| SRL + MMF vs. MMF + CSA | 1.83 (1.04 to 3.33) | 1.88 (0.99 to 3.63) | 1.81 (0.98 to 3.42) | 1.84 (0.90 to 3.82) |
| EVL vs. MMF + CSA | 0.26 (0.01 to 5.99) | 0.26 (0.01 to 6.05) | 0.27 (0.01 to 5.96) | 0.24 (0.01 to 6.00) |
| BEL + SRL vs. TAC + MMF | 0.21 (0.01 to 4.96) | 1.15 (0.01 to 740.26) | 0.21 (0.01 to 5.21) | 1.17 (0.01 to 753.70) |
| BEL + MMF vs. TAC + MMF | 0.31 (0.12 to 0.78) | 4.85 (0.16 2421.16) | 0.31 (0.11 to 0.83) | 4.94 (0.16 2457.75) |
| SRL + TAC vs. TAC + MMF | 0.91 (0.50 to 1.64) | 0.95 (0.47 to 1.88) | 0.91 (0.48 to 1.70) | 0.94 (0.44 to 1.94) |
| SRL + MMF vs. TAC + MMF | 1.14 (0.67 to 1.95) | 1.54 (0.63 to 3.79) | 1.13 (0.62 to 2.01) | 1.53 (0.58 to 4.06) |
| Total residual deviance | 85.74 | 85.85 | 85.17 | 85.32 |
| Relative number of model parameters | 51.958 | 56.274 | 54.343 | 58.586 |
| DIC | 137.698 | 142.124 | 139.513 | 143.906 |
Biopsy-proven acute rejection
Fixed-effects model results
| Intervention treatment | Comparator treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSA + AZA | TAC + AZA | MMF + CSA | TAC + MMF | BEL + SRL | BEL + MMF | EVL + MPS | EVL + CSA | SRL + TAC | SRL + CSA | SRL + MMF | SRL + AZA | |
| TAC + AZA | 0.55 (0.41 to 0.73) | |||||||||||
| MMF + CSA | 0.47 (0.34 to 0.66) | 0.86 (0.55 to 1.34) | ||||||||||
| TAC + MMF | 0.43 (0.29 to 0.63) | 0.78 (0.48 to 1.28) | 0.90 (0.70 to 1.17) | |||||||||
| BEL + SRL | 0.18 (0.01 to 1.39) | 0.32 (0.01 to 2.60) | 0.38 (0.01 to 2.85) | 0.41 (0.01 to 3.16) | ||||||||
| BEL + MMF | 0.83 (0.50 to 1.39) | 1.52 (0.84 to 2.73) | 1.75 (1.20 to 2.59) | 1.94 (1.23 to 3.07) | 4.67 (0.62 to 137.00) | |||||||
| EVL + MPS | 1.48 (0.65 to 3.54) | 2.70 (1.13 to 6.77) | 3.12 (1.48 to 7.01) | 3.45 (1.56 to 8.05) | 8.48 (0.94 to 266.2) | 1.78 (0.77 to 4.3) | ||||||
| EVL + CSA | 0.46 (0.30 to 0.70) | 0.84 (0.50 to 1.40) | 0.97 (0.76 to 1.25) | 1.07 (0.75 to 1.54) | 2.59 (0.33 to 77.35) | 0.55 (0.35 to 0.87) | 0.31 (0.13 to 0.68) | |||||
| SRL + TAC | 0.39 (0.21 to 0.70) | 0.70 (0.36 to 1.37) | 0.82 (0.49 to 1.36) | 0.90 (0.55 to 1.46) | 2.18 (0.27 to 66.47) | 0.46 (0.24 to 0.88) | 0.26 (0.10 to 0.65) | 0.84 (0.47 to 1.48) | ||||
| SRL + CSA | 0.28 (0.08 to 0.81) | 0.50 (0.14 to 1.54) | 0.58 (0.18 to 1.63) | 0.64 (0.19 to 1.79) | 1.56 (0.15 to 51.73) | 0.33 (0.09 to 0.99) | 0.18 (0.04 to 0.67) | 0.60 (0.18 to 1.73) | 0.71 (0.21 to 2.04) | |||
| SRL + MMF | 0.32 (0.21 to 0.48) | 0.59 (0.36 to 0.97) | 0.68 (0.53 to 0.87) | 0.75 (0.57 to 0.99) | 1.82 (0.24 to 54.07) | 0.39 (0.25 to 0.61) | 0.22 (0.09 to 0.48) | 0.70 (0.49 to 1.00) | 0.84 (0.50 to 1.40) | 1.17 (0.41 to 3.92) | ||
| SRL + AZA | 1.15 (0.47 to 2.81) | 2.10 (0.82 to 5.38) | 2.44 (0.94 to 6.33) | 2.69 (1.02 to 7.14) | 6.61 (0.68 to 213.7) | 1.39 (0.50 to 3.86) | 0.78 (0.22 to 2.62) | 2.51 (0.94 to 6.73) | 2.99 (1.02 to 8.74) | 4.22 (1.03 to 19.13) | 3.57 (1.34 to 9.52) | |
| EVL | 1.26 (0.49 to 3.35) | 2.30 (0.86 to 6.36) | 2.66 (1.10 to 6.67) | 2.94 (1.17 to 7.65) | 7.25 (0.76 to 231.7) | 1.52 (0.58 to 4.09) | 0.85 (0.26 to 2.78) | 2.74 (1.12 to 6.92) | 3.27 (1.17 to 9.35) | 4.61 (1.17 to 20.58) | 3.90 (1.55 to 10.09) | 1.09 (0.30 to 4.10) |
Consistency analysis
There are direct data for 21 comparisons and 13 treatments in the network; however, three independent loops are informed by multiarm trials only and so the ICDF, reflecting the number of independent loops in the network, is 21 – (13 – 1) – 3 = 6. Comparing the DIC between the consistency and inconsistency random-effects models suggests that the consistency model has a slightly better fit to the data (156.3 vs. 159.7). Furthermore, the posterior median estimates are similar between the consistency and inconsistency models and the 95% CrIs overlap considerably (Table 227).
| Comparison | Fixed effects | Random effects | ||
|---|---|---|---|---|
| Consistency | Inconsistency | Consistency | Inconsistency | |
| TAC + AZA vs. CSA + AZA | 0.55 (0.41 to 0.74) | 0.55 (0.41 to 0.73) | 0.58 (0.36 to 0.93) | 0.58 (0.35 to 0.94) |
| MMF + CSA vs. CSA + AZA | 0.47 (0.34 to 0.66) | 0.49 (0.34 to 0.71) | 0.47 (0.25 to 0.88) | 0.49 (0.24 to 1.01) |
| TAC + MMF vs. CSA + AZA | 0.43 (0.29 to 0.64) | 0.34 (0.14 to 0.78) | 0.40 (0.19 to 0.79) | 0.34 (0.10 to 1.14) |
| SRL + AZA vs. CSA + AZA | 1.15 (0.47 to 2.81) | 1.15 (0.47 to 2.82) | 1.16 (0.34 to 3.96) | 1.15 (0.33 to 4.00) |
| TAC + MMF vs. MMF + CSA | 0.91 (0.70 to 1.17) | 0.99 (0.75 to 1.31) | 0.85 (0.52 to 1.35) | 0.79 (0.41 to 1.43) |
| BEL + MMF vs. MMF + CSA | 1.75 (1.20 to 2.59) | 1.68 (1.15 to 2.50) | 1.71 (0.91 to 3.20) | 1.56 (0.79 to 3.01) |
| EVL + MPS vs. MMF + CSA | 3.12 (1.48 to 7.01) | 3.13 (1.49 to 7.02) | 3.14 (1.01 to 10.09) | 3.15 (1.00 to 10.19) |
| EVL + CSA vs. MMF + CSA | 0.97 (0.76 to 1.25) | 0.97 (0.76 to 1.25) | 0.97 (0.61 to 1.54) | 0.97 (0.60 to 1.56) |
| SRL + TAC vs. MMF + CSA | 0.81 (0.49 to 1.36) | 0.19 (0.02 to 0.75) | 0.82 (0.40 to 1.64) | 0.16 (0.02 to 0.89) |
| SRL + CSA vs. MMF + CSA | 0.58 (0.18 to 1.63) | 0.43 (0.11 to 1.29) | 0.59 (0.15 to 2.03) | 0.50 (0.08 to 1.62) |
| SRL + MMF vs. MMF + CSA | 0.68 (0.53 to 0.87) | 0.69 (0.54 to 0.34) | 0.92 (0.62 to 1.44) | 1.05 (0.67 to 1.74) |
| EVL + MMF + CSA | 2.66 (1.10 to 6.67) | 2.66 (1.09 to 6.67) | 2.67 (0.82 to 8.77) | 2.79 (0.79 to 10.27) |
| BEL + SRL vs. TAC + MMF | 0.41 (0.01 to 3.16) | 1.15 (0.03 to 44.21) | 0.43 (0.01 to 4.08) | 1.16 (0.03 to 50.80) |
| BEL + MMF vs. TAC + MMF | 1.94 (1.23 to 3.07) | 6.96 (0.88 to 196.37) | 2.02 (0.01 to 4.37) | 7.08 (0.73 to 227.01) |
| SRL + TAC vs. TAC + MMF | 0.90 (0.55 to 1.46) | 1.21 (0. 63 to 2.32) | 0.96 (0.51 to 1.80) | 1.22 (0.54 to 2.78) |
| SRL + MMF vs. TAC + MMF | 0.75 (0.57 to 0.99) | 1.07 (0.45 to 2.55) | 1.09 (0.67 to 1.89) | 1.14 (0.41 to 3.20) |
| SRL + CSA vs. SRL + TAC | 0.71 (0.21 to 2.04) | 1.05 (0.03 to 42.95) | 0.72 (0.18 to 2.52) | 1.05 (0.02 to 46.43) |
| SRL + MMF vs. SRL + TAC | 0.84 (0.50 to 1.40) | 0.68 (0.25 to 1.75) | 1.113 (0.57 to 2.38) | 0.68 (0.18 to 2.43) |
| Total residual deviance | 117 | 115.9 | 88.44 | 87.91 |
| Relative number of model parameters | 53.843 | 59.588 | 67.828 | 71.836 |
| DIC | 170.843 | 175.488 | 156.268 | 159.746 |
Graft function
Fixed-effects model results
| Intervention treatment | Comparator treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSA + AZA | TAC + AZA | MMF + CSA | TAC + MMF | BEL + SRL | BEL + MMF | EVL + MPS | EVL + CSA | SRL + TAC | SRL + CSA | SRL + MMF | |
| TAC + AZA | 12.54 (11.17 to 13.90) | ||||||||||
| MMF + CSA | 4.34 (3.12 to 5.57) | –8.19 (–9.73 to – 6.66) | |||||||||
| TAC + MMF | 5.37 (5.07 to 5.68) | –7.17 (–8.56 to – 5.77) | 1.03 (–0.21 to 2.27) | ||||||||
| BEL + SRL | 12.68 (0.02 to 25.32) | 0.14 (–12.57 to 12.87) | 8.34 (–4.35 to 21.04) | 7.31 (–5.34 to 19.96) | |||||||
| BEL + MMF | 13.02 (9.95 to 16.10) | 0.49 (–2.73 to 3.70) | 8.68 (5.83 to 11.53) | 7.65 (4.58 to 10.73) | 0.34 (–12.49 to 13.21) | ||||||
| EVL + MPS | 3.08 (–3.18 to 9.33) | –9.45 (–15.79 to – 3.14) | –1.26 (–7.41 to 4.86) | –2.30 (–8.56 to 3.97) | –9.59 (–23.69 to 4.49) | –9.94 (–16.75 to – 3.18) | |||||
| EVL + CSA | 6.01 (3.30 to 8.73) | –6.53 (–9.41 to – 3.66) | 1.67 (–0.76 to 4.09) | 0.64 (–2.09 to 3.36) | –6.67 (–19.60 to 6.24) | –7.01 (–10.77 to – 3.25) | 2.93 (–3.65 to 9.52) | ||||
| SRL + TAC | 1.12 (–1.79 to 4.02) | –11.42 (–14.57 to – 8.28) | –3.22 (–6.16 to – 0.30) | –4.25 (–7.16 to – 1.35) | –11.56 (–24.52 to 1.40) | –11.90 (–15.98 to – 7.83) | –1.97 (–8.76 to 4.85) | –4.89 (–8.69 to – 1.08) | |||
| SRL + CSA | –1.39 (–4.53 to 1.77) | –13.93 (–17.27 to – 10.59) | –5.73 (–8.82 to – 2.64) | –6.76 (–9.90 to – 3.61) | –14.07 (–27.08 to – 1.05) | –14.42 (–18.6 to – 10.21) | –4.47 (–11.34 to 2.41) | –7.40 (–11.32 to – 3.49) | –2.51 (–4.93 to – 0.09) | ||
| SRL + MMF | 1.94 (0.01 to 3.87) | –10.60 (–12.84 to – 8.36) | –2.40 (–4.28 to – 0.52) | –3.43 (–5.36 to – 1.50) | –10.74 (–23.53 to 2.05) | –11.08 (–14.50 to – 7.69) | –1.14 (–7.54 to 5.27) | –4.07 (–7.15 to – 1.01) | 0.82 (–2.29 to 3.94) | 3.33 (–0.04 to 6.69) | |
| SRL + AZA | 10.80 (8.40 to 13.20) | –1.74 (–4.50 to 1.02) | 6.45 (3.76 to 9.15) | 5.43 (3.01 to 7.85) | –1.87 (–14.78 to 11.02) | –2.22 (–6.12 to 1.67) | 7.73 (1.03 to 14.44) | 4.79 (1.17 to 8.42) | 9.69 (5.90 to 13.45) | 12.20 (8.22 to 16.14) | 8.86 (5.78 to 11.94) |
Consistency analysis results
There are direct data for 18 comparisons and 12 treatments in the network; however, two independent loops are informed by multiarm trials only and so the ICDF, reflecting the number of independent loops in the network, is 18 – (12 – 1) – 2 = 5. Comparing the DIC between the consistency and inconsistency random-effects models suggests that the consistency model has a slightly better fit to the data (147.8 vs. 150.0). Furthermore, the posterior median estimates are similar between the consistency and inconsistency models and the 95% CrIs overlap considerably (Table 229).
| Treatment comparison | Fixed effects | Random effects | ||
|---|---|---|---|---|
| Consistency | Inconsistency | Consistency | Inconsistency | |
| TAC + AZA vs. CSA + AZA | 12.54 (11.17 to 13.9) | 13.09 (11.7 to 14.48) | 9.31 (4.32 to 14.28) | 9.78 (4.65 to 14.87) |
| MMF + CSA vs. CSA + AZA | 4.34 (3.12 to 5.57) | 6.00 (4.53 to 7.47) | 1.61 (–4.16 to 7.41) | 3.60 (–3.88 to 11.09) |
| TAC + MMF vs. CSA + AZA | 5.37 (5.07 to 5.68) | 5.30 (4.99 to 5.61) | 6.53 (0.38 to 12.68) | 5.29 (–4.13 to 14.71) |
| SRL + AZA vs. CSA + AZA | 10.80 (8.40 to 13.20) | 10.80 (8.40 to 13.20) | 10.78 (1.07 to 20.44) | 10.77 (1.10 to 20.48) |
| TAC + MMF vs. MMF + CSA | 1.03 (–0.21 to 2.27) | 5.20 (2.56 to 7.84) | 4.92 (0.87 to 8.98) | 4.98 (–0.75 to 10.70) |
| BEL + MMF vs. MMF + CSA | 8.68 (5.83 to 11.53) | 8.52 (5.55 to 11.48) | 8.94 (3.13 to 14.79) | 7.83 (1.48 to 14.18) |
| EVL + MPS vs. MMF + CSA | –1.26 (–7.41 to 4.86) | –1.26 (–7.41 to 4.87) | –1.27 (–12.45 to 9.93) | –1.25 (–12.49 to 9.91) |
| EVL + CSA vs. MMF + CSA | 1.67 (–0.76 to 4.09) | 1.67 (–0.75 to 4.09) | 3.26 (–1.82 to 8.34) | 3.25 (–1.82 to 8.34) |
| SRL + CSA vs. MMF + CSA | –5.73 (–8.82 to -2.64) | 1.20 (–3.08 to 5.47) | –3.23 (–11.07 to 4.64) | 1.19 (–9.14 to 11.52) |
| SRL + MMF vs. MMF + CSA | –2.40 (–4.28 to -0.52) | –2.66 (–4.92 to -0.41) | 2.24 (–1.55 to 6.05) | 2.00 (–2.34 to 6.39) |
| BEL + SRL vs. TAC + MMF | 7.31 (–5.35 to 19.96) | 7.80 (–5.02 to 20.63) | 5.79 (–9.53 to 21.06) | 7.76 (–8.18 to 23.79) |
| BEL + MMF vs. TAC + MMF | 7.65 (4.58 to 10.73) | 9.58 (–1.03 to 20.20) | 4.02 (–2.72 to 10.73) | 9.60 (–4.61 to 23.70) |
| SRL + TAC vs. TAC + MMF | –4.25 (–7.16 to -1.35) | –8.36 (–12.11 to -4.60) | –6.88 (–13.01 to -0.75) | –9.87 (–17.58 to -2.18) |
| SRL + MMF vs. TAC + MMF | –3.43 (–5.36 to –1.50) | –2.14 (–5.45 to 1.15) | –2.69 (–6.92 to 1.57) | –0.61 (–7.01 to 5.82) |
| SRL + CSA vs. SRL + TAC | –2.51 (–4.93 to –0.09) | –5.24 (–7.92 to –2.57) | –1.26 (–8.97 to 6.45) | –5.22 (–15.03 to 4.55) |
| SRL + MMF vs. SRL + TAC | 0.82 (–2.29 to 3.94) | 4.14 (–5.31 to 13.59) | 4.20 (–2.02 to 10.41) | 4.08 (–9.18 to 17.46) |
| Total residual deviance | 277.7 | 245.7 | 82.75 | 83.42 |
| Relative number of model parameters | 45.987 | 50.949 | 65.058 | 66.594 |
| DIC | 323.687 | 296.649 | 147.808 | 150.014 |
Appendix 7 Adverse events
Adverse events: meta-analyses at 1-year follow-up
When data permitted, the 1-year follow-up results of individual studies were pooled using meta-analyses; NODAT, PTLD, malignancy (including PTLD), any infections and CMV were considered. The DerSimonian–Laird random-effects method was used for pooling. OR was used as a measure of treatment effect.
The number of studies included in the individual meta-analyses was between two and eight, therefore, we did not investigate publication bias; tests for funnel plot asymmetry should be used only when there are at least 10 studies included in the meta-analysis, when there are fewer studies the power of the tests is too low to distinguish chance from real asymmetry (Cochrane Handbook 2008). 201 In addition, no corrections for multiple comparisons were executed. Therefore, any meta-analyses results presented in this section must be interpreted with caution.
Induction regimens
Nine studies71,72,74,87,95,96,98,128,137 reported some AEs at 1-year follow-up. Four studies71,72,74,95 compared BAS with PBO or no induction, three studies87,98,137 compared BAS and rATG, one study96 compared rATG with no induction,96 and one study128 compared BAS, ATG-Fresenius® and no induction (only the comparison of BAS and no induction was considered in the analyses).
All AEs are summarised in the sections below according to induction therapy used. Similarly to the clinical effectiveness outcomes, studies comparing BAS with PBO, and BAS with no induction, were combined.
BAS compared with placebo and no induction
New-onset diabetes after transplant/transplantation, malignancy, PTLD, infections and CMV infections were reported in studies comparing BAS with PBO, and BAS with no induction (results from studies comparing BAS with PBO, and BAS with no induction were combined). No differences between BAS and control arms were identified for any AE. The NODAT (Figure 102), malignancy (Figure 103), PTLD (Figure 104), infections (Figure 105) and CMV results (Figure 106) are presented below. In summary, no differences in NODAT, PTLD, malignancy, infections and CMV infections were found between BAS and control arms.
FIGURE 102.
New-onset diabetes after transplant/transplantation: BAS vs. PBO and no induction. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; studies with no events in both arms were excluded from the analysis.
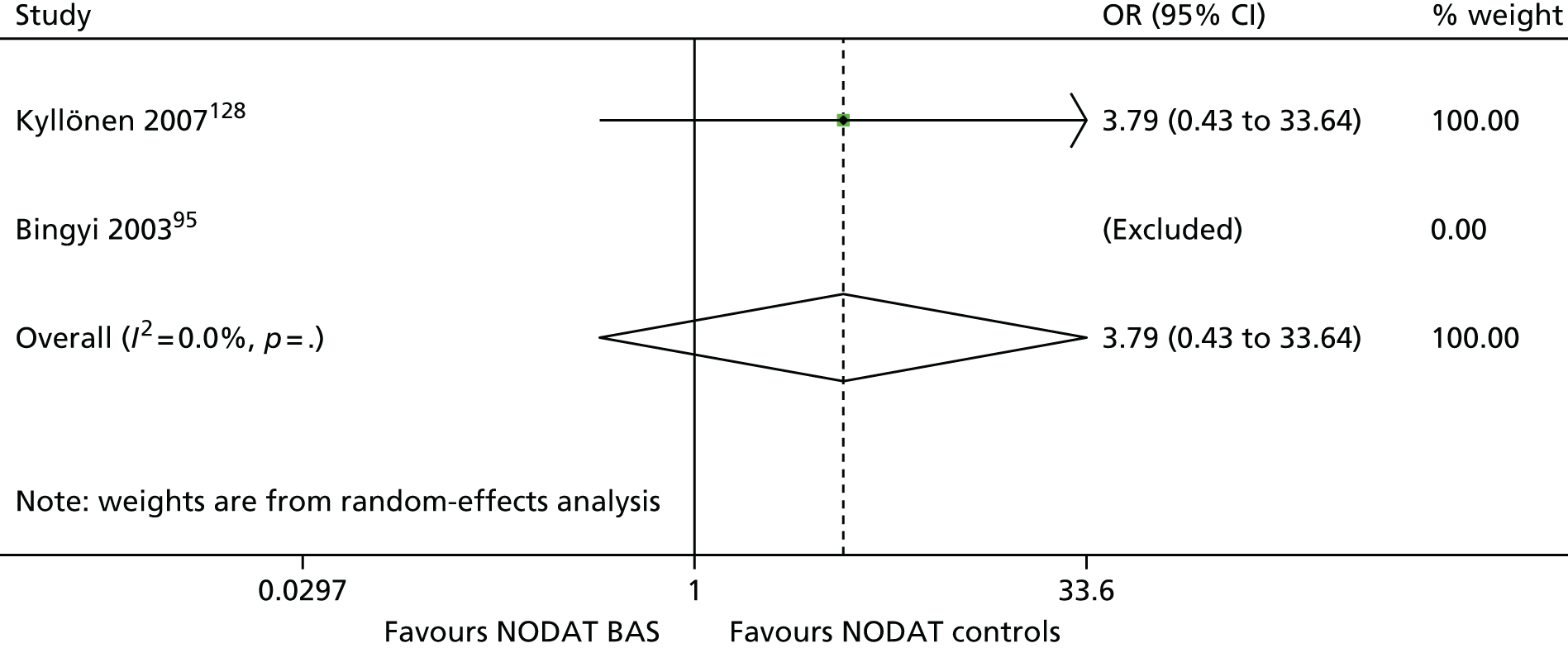
FIGURE 103.
Malignancy: BAS vs. PBO and no induction. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; studies with no events in both arms were excluded from the analysis; the estimate of between-study variance, τ2, was 0.000.
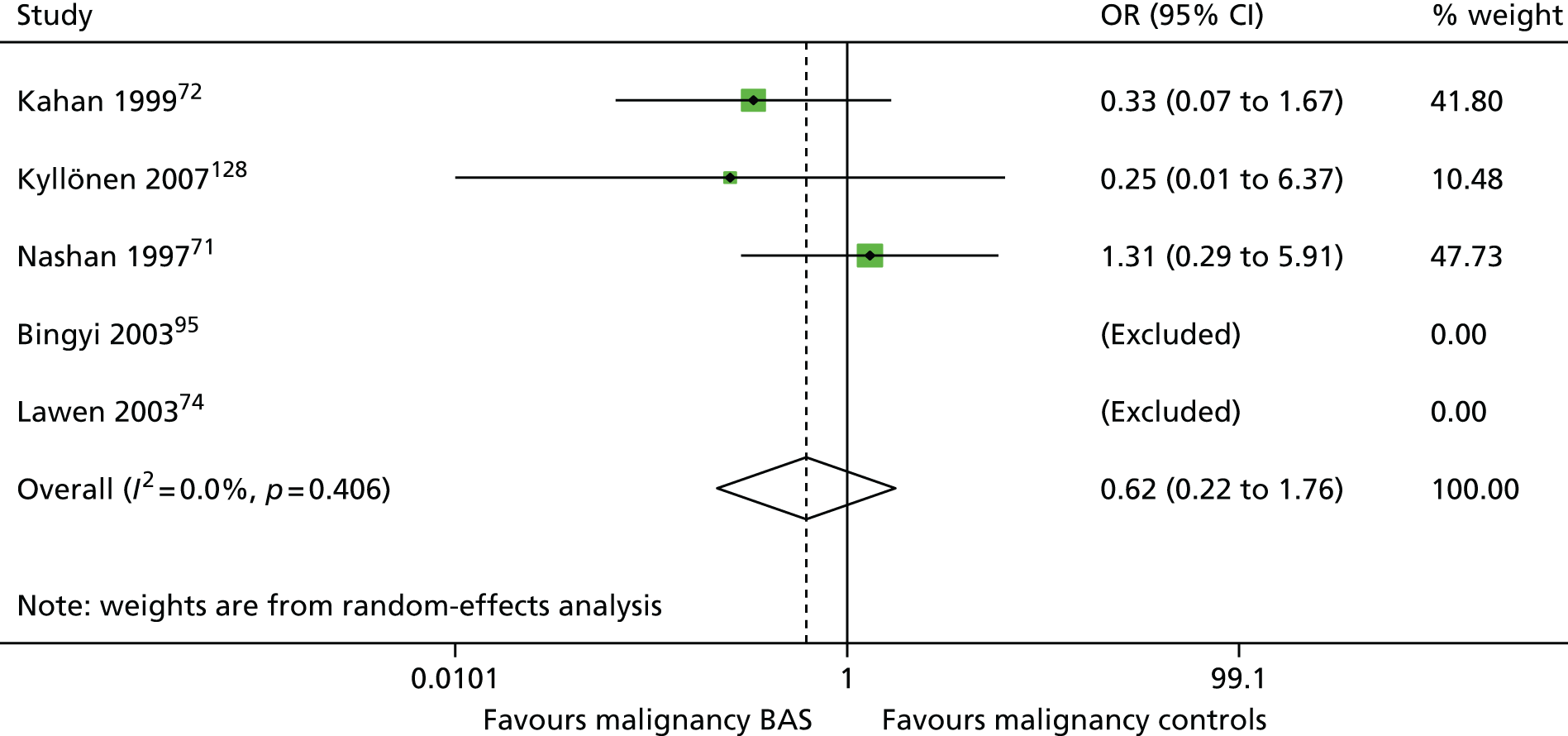
FIGURE 104.
Post-transplant lymphoproliferative disorder: BAS vs. PBO and no induction. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; studies with no events in both arms were excluded from the analysis.

FIGURE 105.
Infections: BAS vs. PBO and no induction. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; studies with no events in both arms were excluded from the analysis; the estimate of between-study variance, τ2, was 0.000.
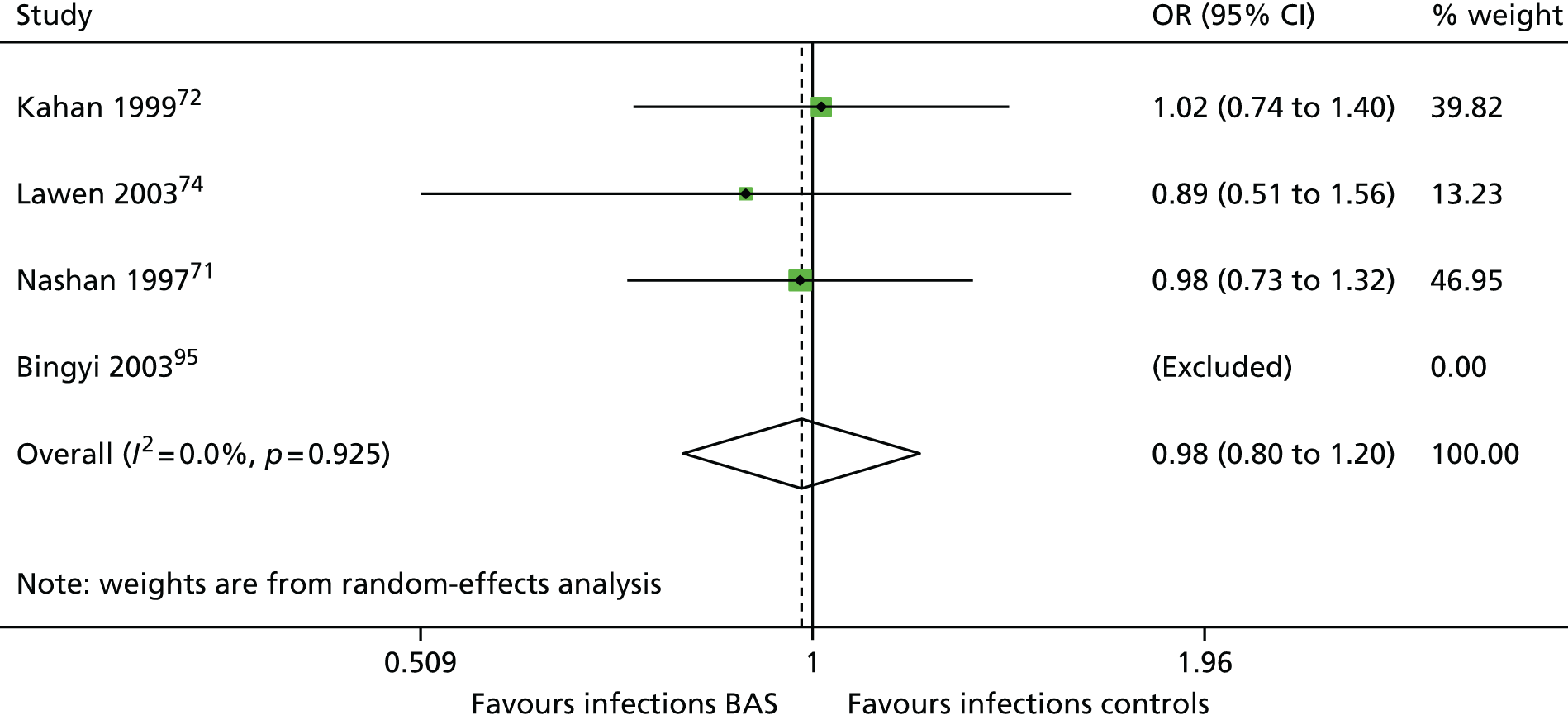
FIGURE 106.
Cytomegalovirus: BAS vs. PBO and no induction. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; studies with no events in both arms were excluded from the analysis; the estimate of between-study variance, τ2, was 0.000.

BAS compared with rATG
Three studies87,98,137 comparing BAS with rATG reported AEs; NODAT, PTLD, malignancy, infections and CMV infections were reported. No difference in NODAT was found in one study87 [OR 0.98 (favours BAS, 95% CI 0.06 to 16.11)]. Malignancy (Figure 107), PTLD (Figure 108) infections (Figure 109) and CMV results (Figure 110) are presented below.
In summary, no difference in NODAT, PTLD, malignancy and infections were found between the two induction regimens, rATG and BAS. One study137 suggested more CMV infections with rATG regimens than with BAS regimens [OR 2.25 (favours rATG, 95% CI 1.06 to 4.76)].
FIGURE 107.
Malignancy: BAS vs. rATG. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.
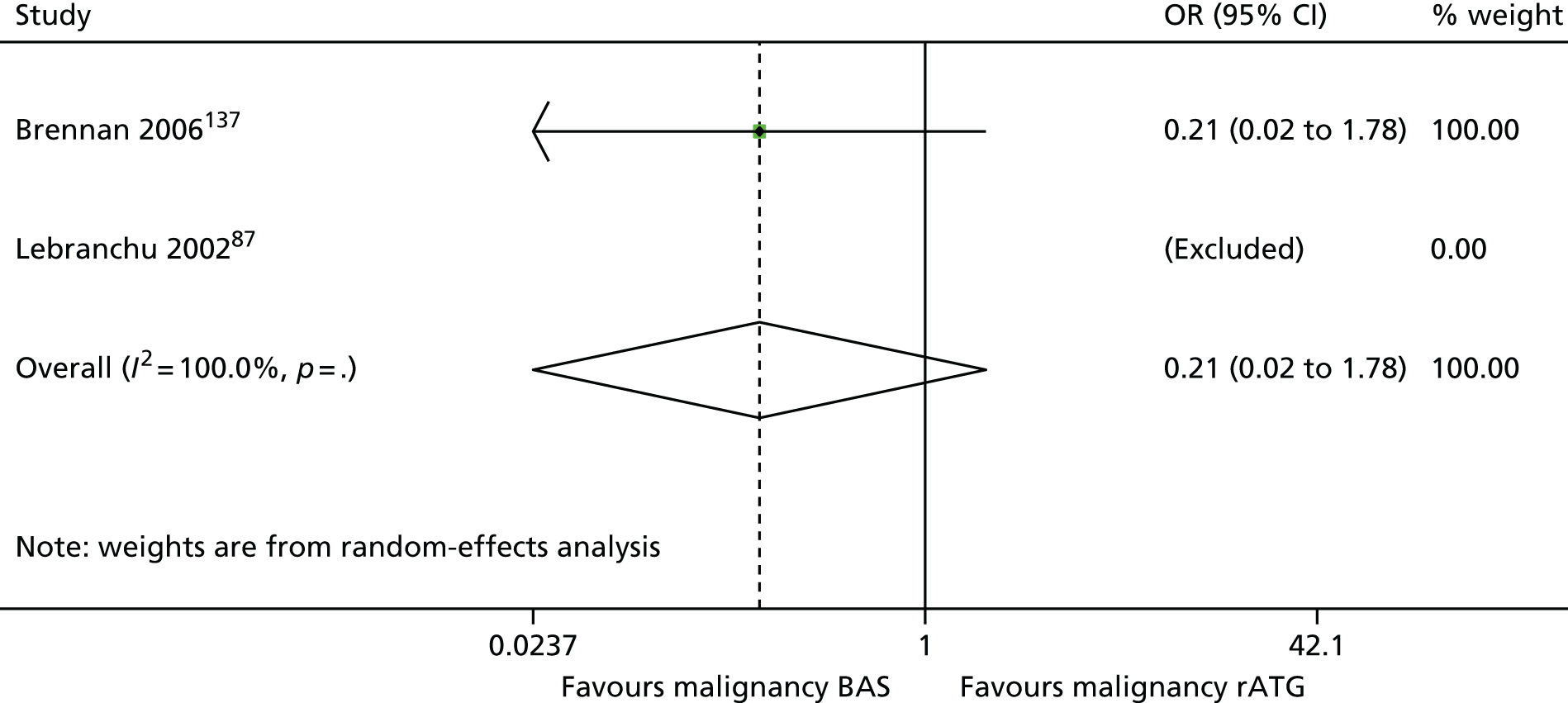
FIGURE 108.
Post-transplant lymphoproliferative disorder: BAS vs. rATG. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; studies with no events in both arms were excluded from the analysis.
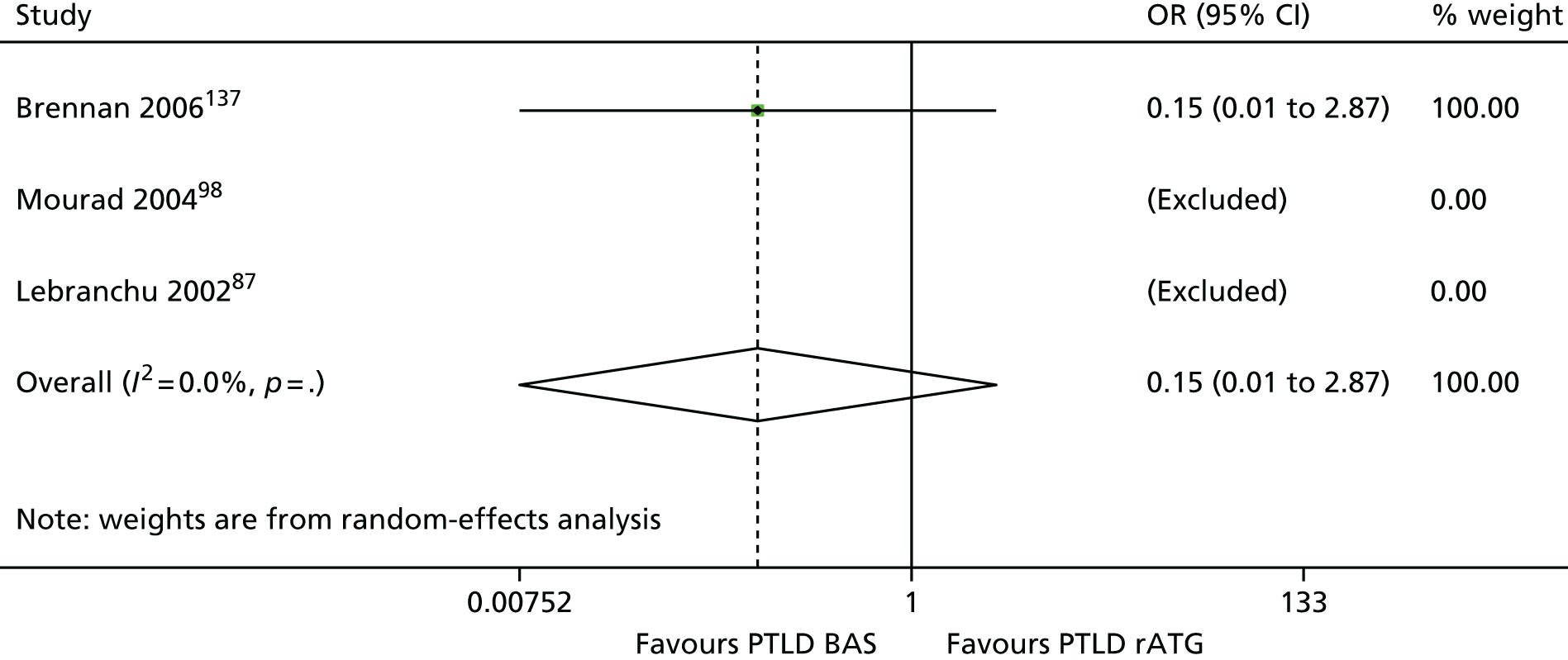
FIGURE 109.
Infections: BAS vs. rATG. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.
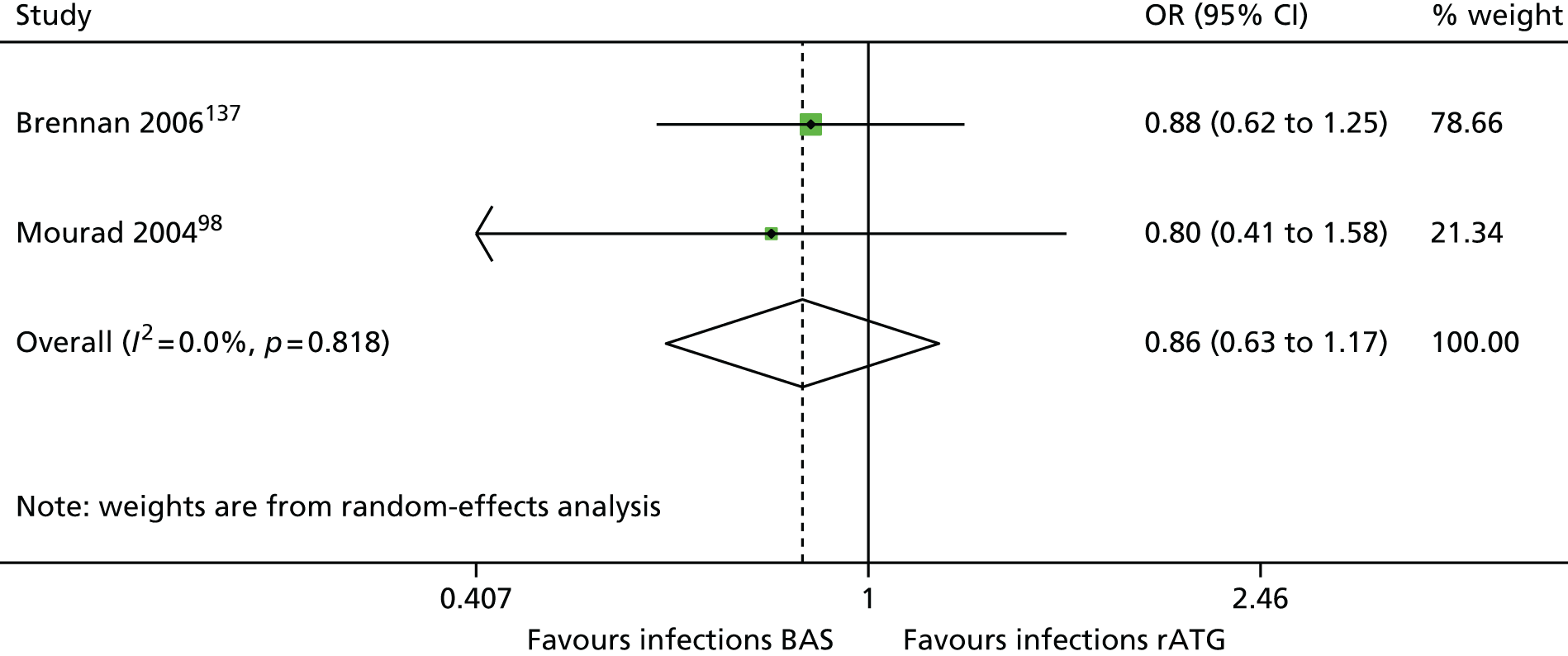
FIGURE 110.
Cytomegalovirus: BAS vs. rATG. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.251.
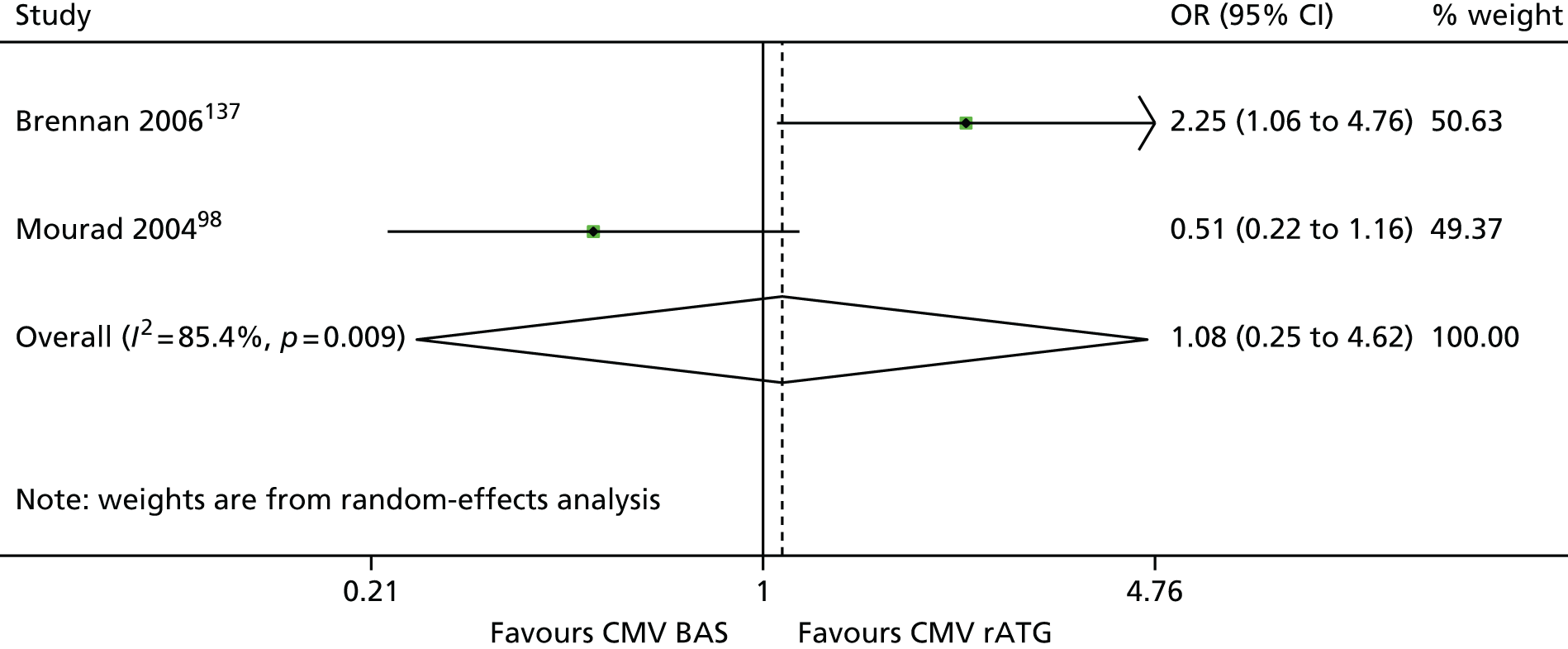
rATG compared with no induction
New-onset diabetes after transplant/transplantation and CMV infections were reported in one study96 comparing rATG with no induction. More CMV infections were reported in the rATG arm than in controls: (OR 2.11, favours no induction, 95% 1.26 to 3.52) and no difference in NODAT was found between rATG and no induction (OR 0.75, favours rATG, 95% CI 0.23 to 2.42).
In summary, one study96 suggested more CMV infections with rATG regimens than with no induction.
Maintenance regimens
Thirty-nine studies60,80,82,83,86,88,90,92,100,104,106,107,110–112,116,117,120,121,126,127,131,133,134,136,138,141,144,147,149,152,177,194,195,204,206,207,210,427 (of the 76 maintenance studies) reported some AEs at 1-year follow-up. Twenty-seven studies reported NODAT,51,58,59,80,83,88,90,92,100,107,110,112,116,117,120,121,125,126,131,134,141,142,147,149,180,194,210 22 studies reported malignancy,51,58,59,88,90,100,106,112,116,124–126,131,133,134,142,144,147,149,152,180,194 nine studies reported PTLD,60,100,112,125,134,142,180,195,210 15 studies reported infections51,59,86,88,90,92,100,106,107,124,125,131,133,147,152 and 28 studies reported CMV infections. 51,58,59,82,88,90,100,104,106,107,112,116,117,124–126,131,133,134,138,141,142,147,149,150,152,155,210
Ferguson et al. 126 compared three regimens: BEL + MMF, TAC + MMF and BEL + SRL; however, only BEL + MMF and TAC + MMF results were used in meta-analyses. Similarly, one study by Chadban et al. 152 compared EVL + CSA and MPS + CSA and EVL; however, only results of EVL + CSA and MPS + CSA arms were used in meta-analyses. Finally, the SYMPHONY trial195 compared low CSA + MMF, low TAC + MMF, SRL + MMF and CSA + MMF; however, only the results of low CSA + MMF, low TAC + MMF and SRL + MMF were used in meta-analyses. In addition, one study129 reported AEs at 1-year follow-up, but the study did not use comparable concomitant therapies and therefore the results of this study could not be included in meta-analyses.
Tacrolimus compared with ciclosporin
Ten studies80,82,83,88,90,99,100,104,121,195,210 comparing TAC with CSA reported AEs. Six studies80,82,83,88,90,99,100,104,210 used TAC + AZA + CCS and CSA + AZA + CCS regimens, two studies90,104 compared TAC + MMF + CCS and CSA + MMF + CCS regimens, one study121 compared TAC + SRL + CCS and CSA + SRL + CCS regimens and one study (SYMPHONY comparing four regimens)195 compared low TAC + MMF + CCS and low CSA + MMF + CCS regimens.
The meta-analyses suggested more cases of NODAT in TAC regimens compared with CSA (Figure 111), no difference for malignancy (Figure 112), no difference for infections (Figure 113) and no difference for CMV infections (Figure 114). Three studies100,195,210 reported no PTLD cases in both arms. In summary, no difference in PTLD, malignancy, infections and CMV infection were found between TAC and CSA regimens at 1-year follow-up. The meta-analysis (including eight studies) suggested more cases of NODAT in TAC regimens than with CSA.
FIGURE 111.
New-onset diabetes after transplant/transplantation: TAC vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.

FIGURE 112.
Malignancy: TAC vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.

FIGURE 113.
Infections: TAC vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.
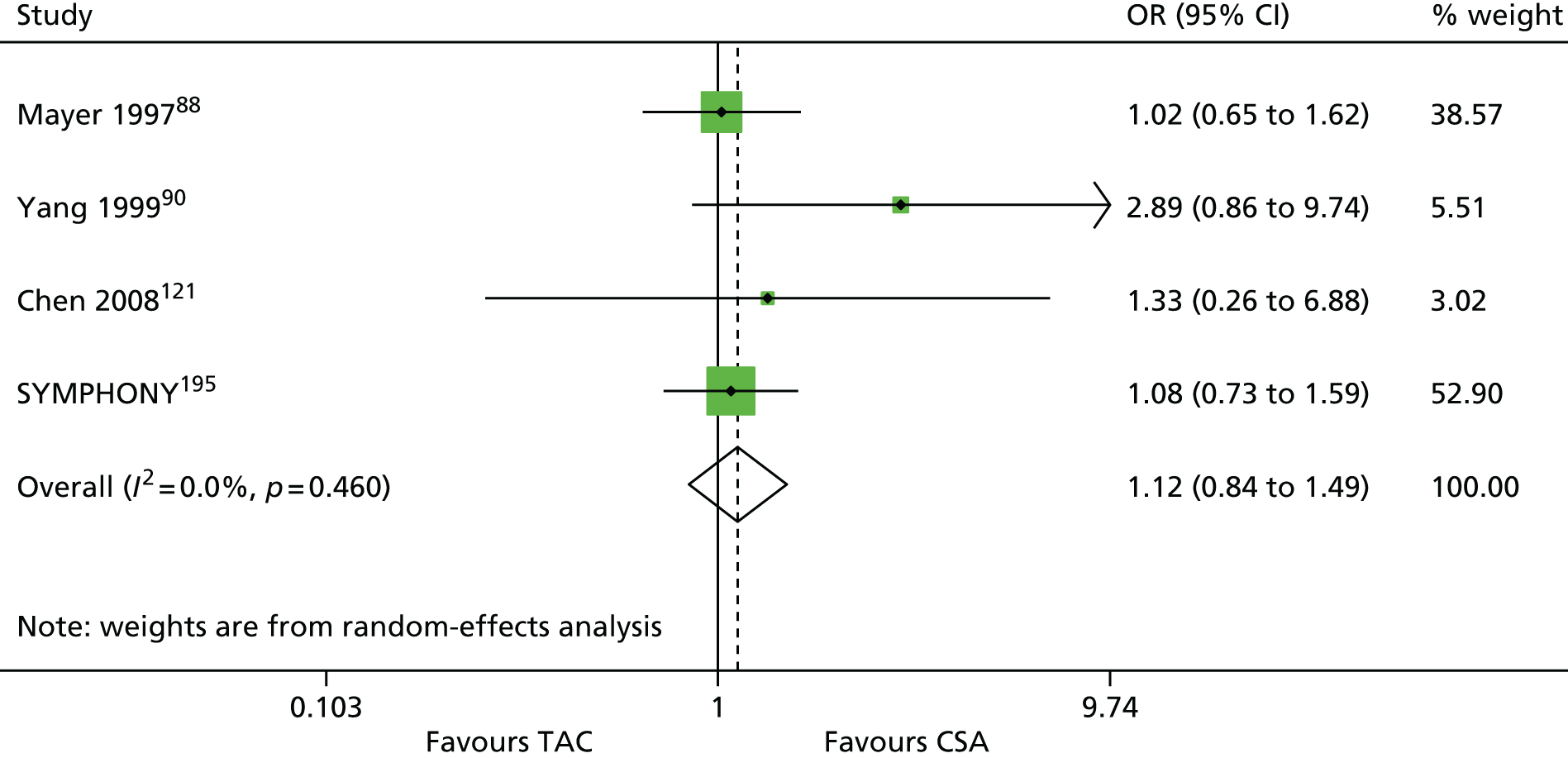
FIGURE 114.
Cytomegalovirus: TAC vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; studies with no events in both arms were excluded from the analysis; the estimate of between-study variance, τ2, was 0.000. Raofi et al. 210 reported 0 out of 14 and 0 out of 24 CMV infections in TAC and CSA arms, respectively.
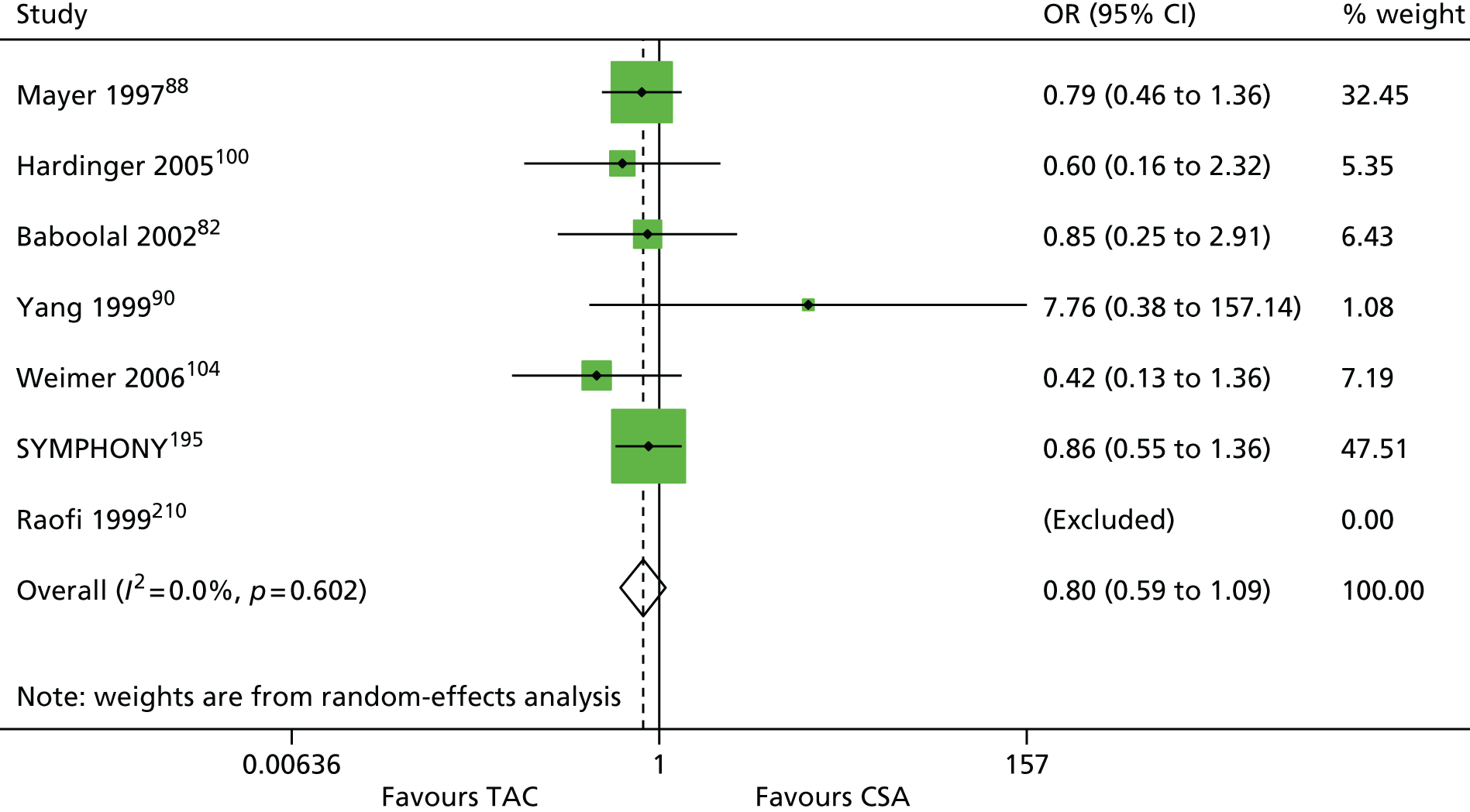
MMF versus CSA
One three-arm study86 comparing MMF with CSA reported AE; this study86 used the following regimens: MMF + AZA + CCS and CSA + AZA + CCS. No difference was found between the two arms for infections [OR = 0.86 (favours CSA, 95% CI 0.56 to 1.30)]. No other AEs were reported in this study.
In summary, no difference in infections was found between MMF and CSA regimens at 1-year follow-up. However, only one study86 reported infection.
BEL versus CSA
Three studies60,206,207 comparing BEL with CSA reported AEs. All three studies60,206,207 used BEL + MMF + CCS and CSA + MMF + CCS regimens. In addition, two studies60,207 used two BEL regimens: low and high BEL doses. Only the results of the low-BEL arms (closer to the licence dose) were used in the analyses.
The meta-analyses suggested more cases of NODAT with CSA regimens than with BEL regimens (Figure 115); no difference for malignancy (Figure 116), no difference for PTLD (Figure 117), no difference for infections (Figure 118) and CMV infections (Figure 119) between BEL and CSA regimens were identified. In summary, no difference in malignancy, PTLD, infections and CMV infection were found between BEL and CSA regimens at 1-year follow-up. The meta-analysis (including three studies) suggested more cases of NODAT with CSA regimens than with BEL regimens.
FIGURE 115.
New-onset diabetes after transplant/transplantation: BEL vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.

FIGURE 116.
Malignancy: BEL vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.472.

FIGURE 117.
Post-transplant lymphoproliferative disorder: BEL vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000. Vincenti et al. 206 reported 0 out of 71 and 0 out of 73 PTLD cases in BEL and CSA arms, respectively.
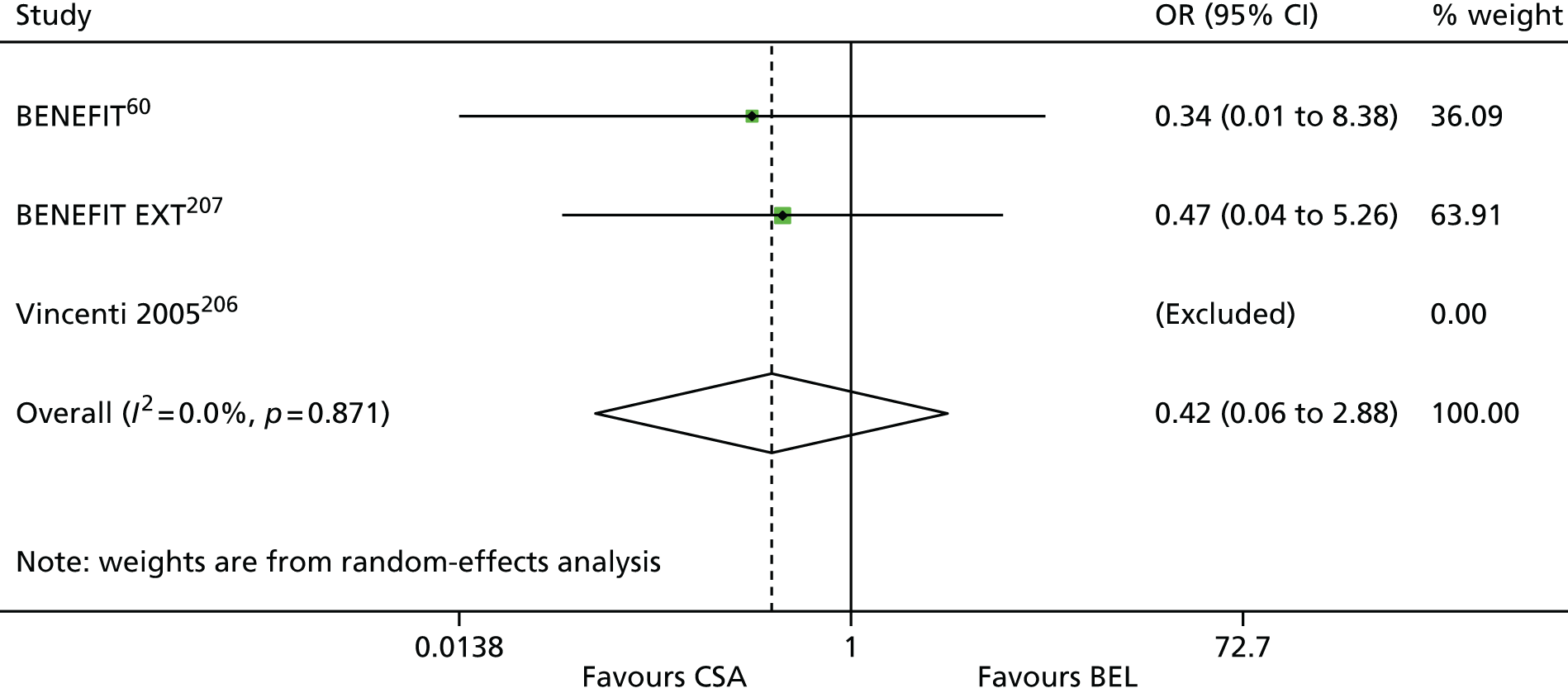
FIGURE 118.
Infections: BEL vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.

FIGURE 119.
Cytomegalovirus: BEL vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.

EVL compared with CSA
One study133 comparing EVL with CSA reported AEs; this study133 used the following regimens: EVL + MPS + CCS and CSA + MPS + CCS. No difference was found between the two arms for malignancy [OR = 1.02 (favours EVL, 95% CI 0.14 to 7.39)], for infections [OR = 0.79 (favours CSA, 95% CI 0.47 to 1.32)] and for CMV infections, [OR = 1.54 (favours EVL, 95% CI 0.63 to 3.78)]. PTLD and NODAT were not reported in this study.
In summary, no differences in malignancy, infections and CMV infection were found between EVL and CSA regimens at 1-year follow-up. However, only one study133 reported malignancy, infections and CMV infection.
SRL versus CSA
Eight studies116,117,127,134,136,147,149,194,195 comparing SRL with CSA reported AEs. Six studies116,117,127,134,147,149 used SRL + MMF + CCS and CSA + MMF + CCS regimens. One study136,194 used SRL + AZA + CCS and CSA + AZA + CCS regimens, and one study (SYMPHONY comparing four regimens)195 compared SRL + MMF + CCS and CSA + MMF + CCS regimens.
The meta-analyses suggested more cases of NODAT with SRL regimens than with CSA (Figure 120), no difference in malignancy (Figure 121), no difference in PTLD (Figure 122), no difference for infections (Figure 123) and more cases of no difference for infections CMV in CSA than with SRL regimen (Figure 124). In summary, no difference in malignancy, PTLD, infections or CMV infections were found between SRL and CSA regimens at 1-year follow-up. The meta-analysis (including seven studies) suggested more cases of NODAT with CSA regimens than with SRL.
FIGURE 120.
New-onset diabetes after transplant/transplantation: SRL vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.

FIGURE 121.
Malignancy: SRL vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; studies with no events in both arms were excluded from the analysis; the estimate of between-study variance, tau-squared, was 0.000. Kreis et al. 116 reported 0 out of 40 and 0 out of 38 malignancy cases in SRL and CSA arms, respectively.
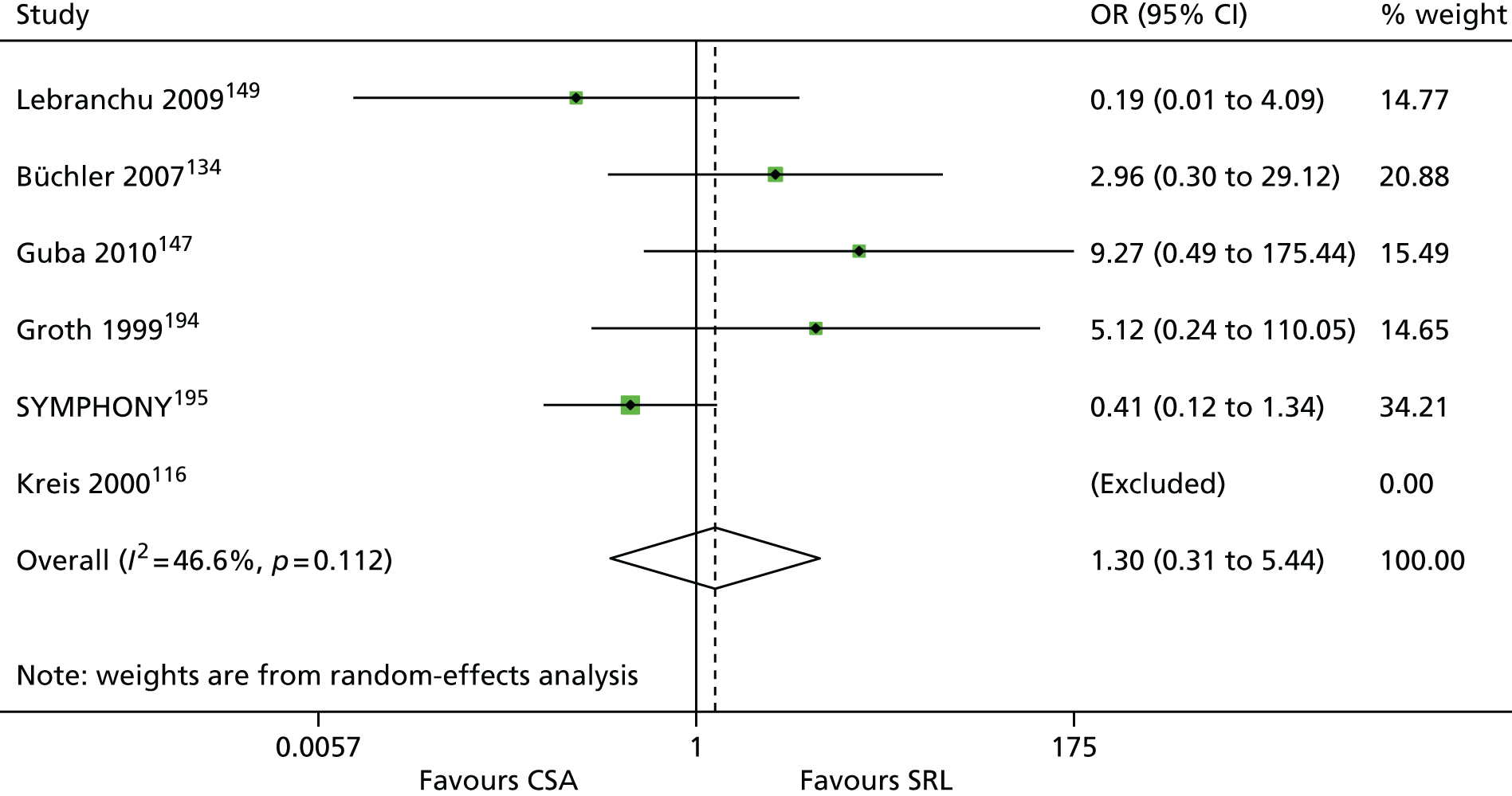
FIGURE 122.
Post-transplant lymphoproliferative disorder: SRL vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.
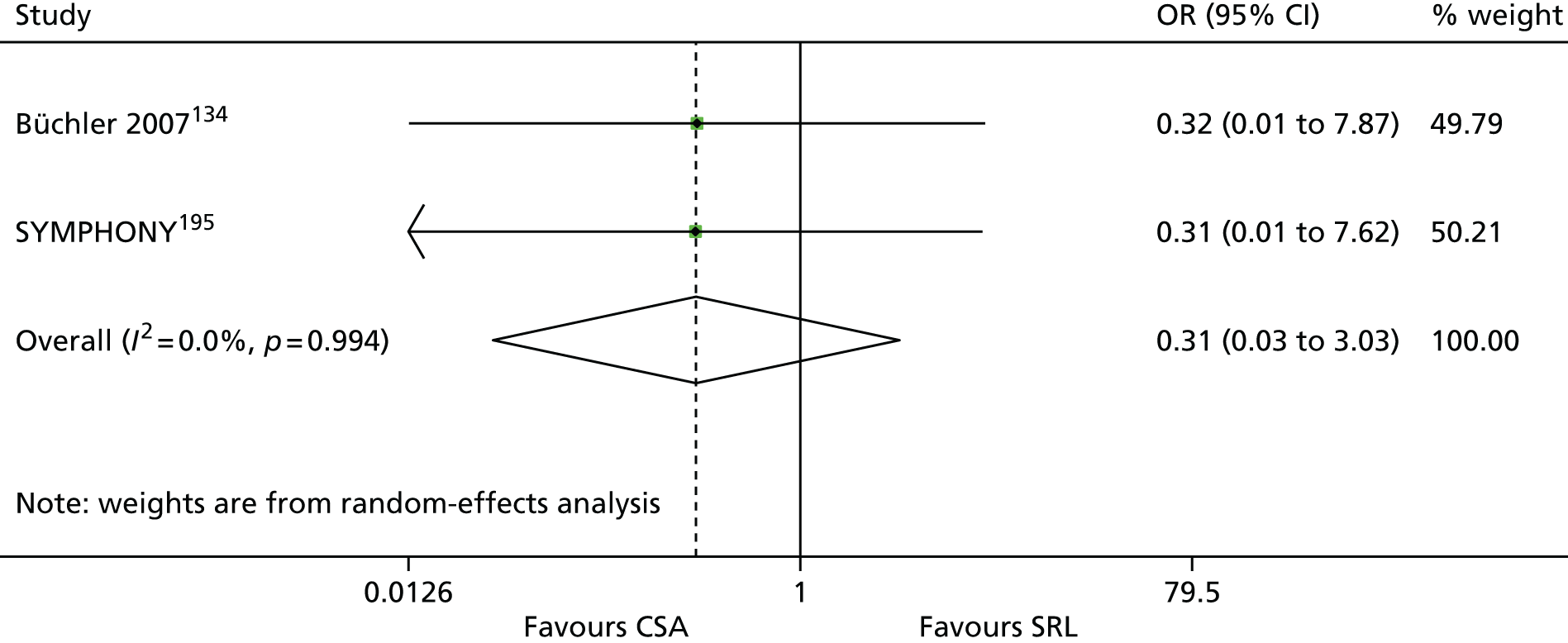
FIGURE 123.
Infections: SRL vs. CSA. ID, identification. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.248.
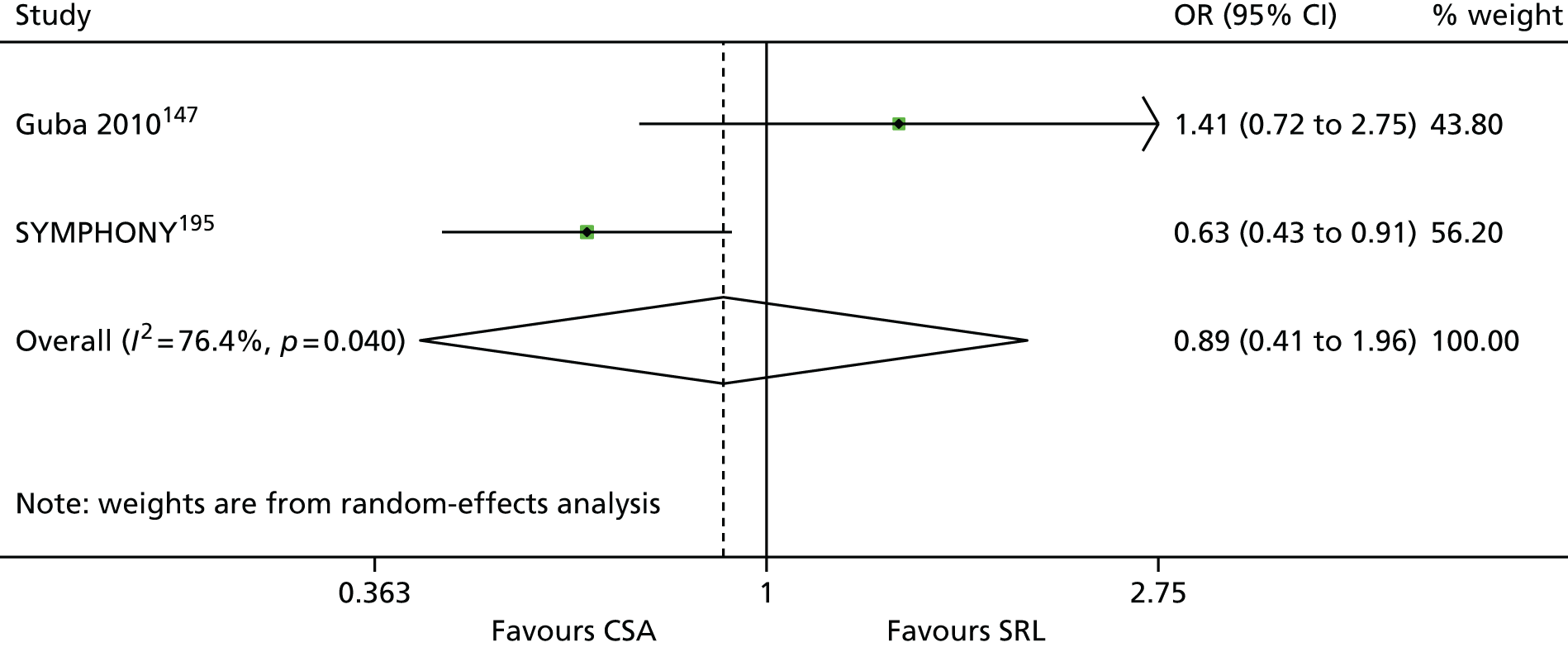
FIGURE 124.
Cytomegalovirus: SRL vs. CSA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.
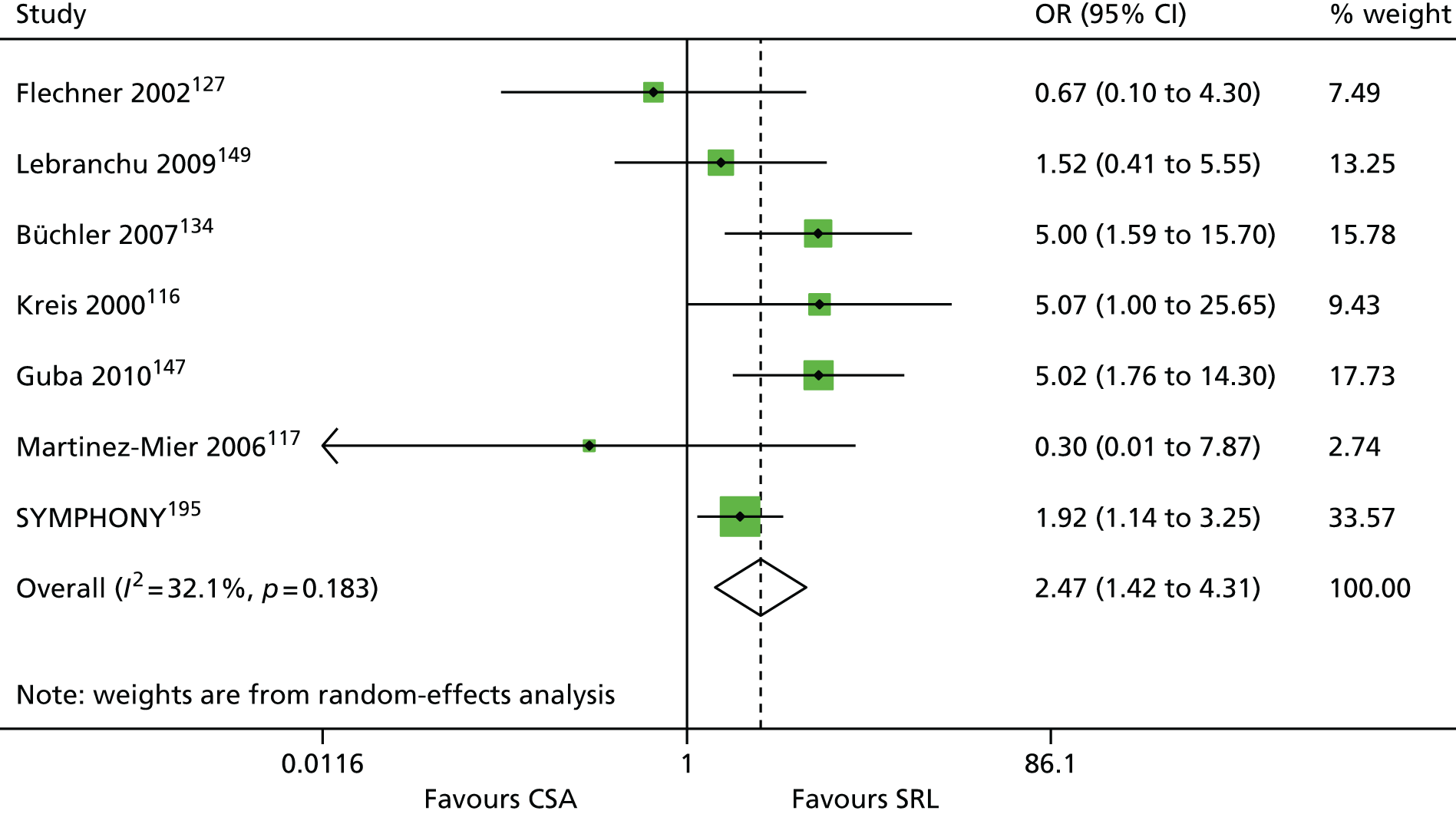
TAC (short release) compared with TAC-PR
Two studies141,204 comparing TAC with TAC-PR reported AEs; both studies141,204 used TAC + MMF + CCS and TAC-PR + MMF + CCS regimens.
The meta-analyses suggested no differences for NODAT (Figure 125) and no differences for CMV (Figure 126). In addition, no difference was found between the two arms in one study204 for malignancy [OR = 1.32 (favours TAC-PR, 95% CI 0.45 to 3.85)]. No results for PTLD were reported. 204 In summary, no difference in NODATs and CMV infection were found between TAC and TAC-PR regimens at 1-year follow-up. However, only two studies141,204 reported NODATs and CMV infection.
FIGURE 125.
New-onset diabetes after transplant/transplantation: TAC vs. TAC-PR. ID, identification; TAC QD, once-daily TAC-PR. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.

FIGURE 126.
Cytomegalovirus: TAC vs. TAC-PR. TAC QD, once-daily TAC-PR. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.452.

MMF compared with TAC
One study120 comparing MMF with TAC reported AEs; this study used the following regimens: MMF + SRL + CCS and TAC + SRL + CCS. No difference was found between the two arms for NODAT [OR = 1.59 (favours MMF, 95% CI 0.72 to 3.53)]. No other AEs were reported in this study. 120
In summary, no difference in NODAT was found between MMF and TAC regimens at 1-year follow-up. However, only one study120 reported NODAT.
BEL compared with TAC
One three-arm study126 comparing BEL with TAC reported AEs; this study126 used BEL + MMF + CCS and TAC + MMF + CCS regimens. No difference was found between the two arms for NODAT [OR = 3.42 (favours BEL, 95% CI 0.13 to 87.10)], for malignancy [OR = 3.42 (favours BEL, 95% CI 0.13 to 87.10)] or for CMV infections [OR = 2.29 (favours BEL, 95% CI 0.20 to 26.47)]. PTLD and infections were not reported in this study.
In summary, no difference in NODAT, malignancy, infections or CMV infections were found between BEL and TAC regimens at 1-year follow-up. However, only one study126 reported NODAT, malignancy, infections and CMV infections.
SRL versus TAC
Two studies92,195 comparing SRL with TAC reported AEs; one study92 used SRL + MMF + CCS and TAC + MMF + CCS regimens and one study (SYMPHONY comparing four regimens)195 compared SRL + MMF + CCS and TAC + MMF + CCS regimens.
The meta-analysis suggested no difference for NODAT (Figure 127). However, publication bias was not explored and the number of pooled studies is small; therefore, the result must be interpreted with caution. No difference was found between the two arms in one study (SYMPHONY)195 for malignancy [OR = 0.83 (favours TAC, 95% CI 0.32 to 2.19)], for PTLD [OR = 0.31 (favours TAC, 95% CI 0.01 to 7.72)] and for CMV infections [OR = 1.66 (favours SRL, 95% CI 0.97 to 2.84)]. 195 More infections were found in the SRL arm than in the TAC arm for infections [OR = 0.68 (favours TAC, 95% CI 0.47 to 0.98)].
FIGURE 127.
New-onset diabetes after transplant/transplantation: SRL vs. TAC. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.

In summary, no difference in NODAT, PTLD, malignancy or CMV infections was found between SRL and TAC regimens at 1-year follow-up. One study (SYMPHONY)195 found statistically significantly more infections in the SRL arm than in the TAC arm. 195 However, only two studies92,195 reported NODATs, and only one study195 reported PTLD, malignancy, infections and CMV infections.
EVL compared with MMF
Three studies107,131,177 comparing EVL with MMF reported AEs; all studies107,131,177 used EVL + CSA + CCS and MMF + CSA + CCS regimens. Tedesco-Silva et al. 107 reported using MPA; it was assumed that MMF was used.
The meta-analyses suggested no differences for NODAT (Figure 128) and infections (Figure 129); conversely, a significant difference was found for CMV infections (Figure 130); more CMV infections were found with MMF than with EVL. No difference was found between the two arms in one study131 for malignancy [OR = 0.19 (favours MMF, 95% CI 0.01 to 4.11)]. PTLD was not reported in these studies. In summary, no differences in NODAT, PTLD, malignancy or infection were found between EVL and MMF regimens at 1-year follow-up. The meta-analysis (including three studies) suggested more cases of CMV infections with MMF regimens than with EVL.
FIGURE 128.
New-onset diabetes after transplant/transplantation: EVL vs. MMF. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.456.
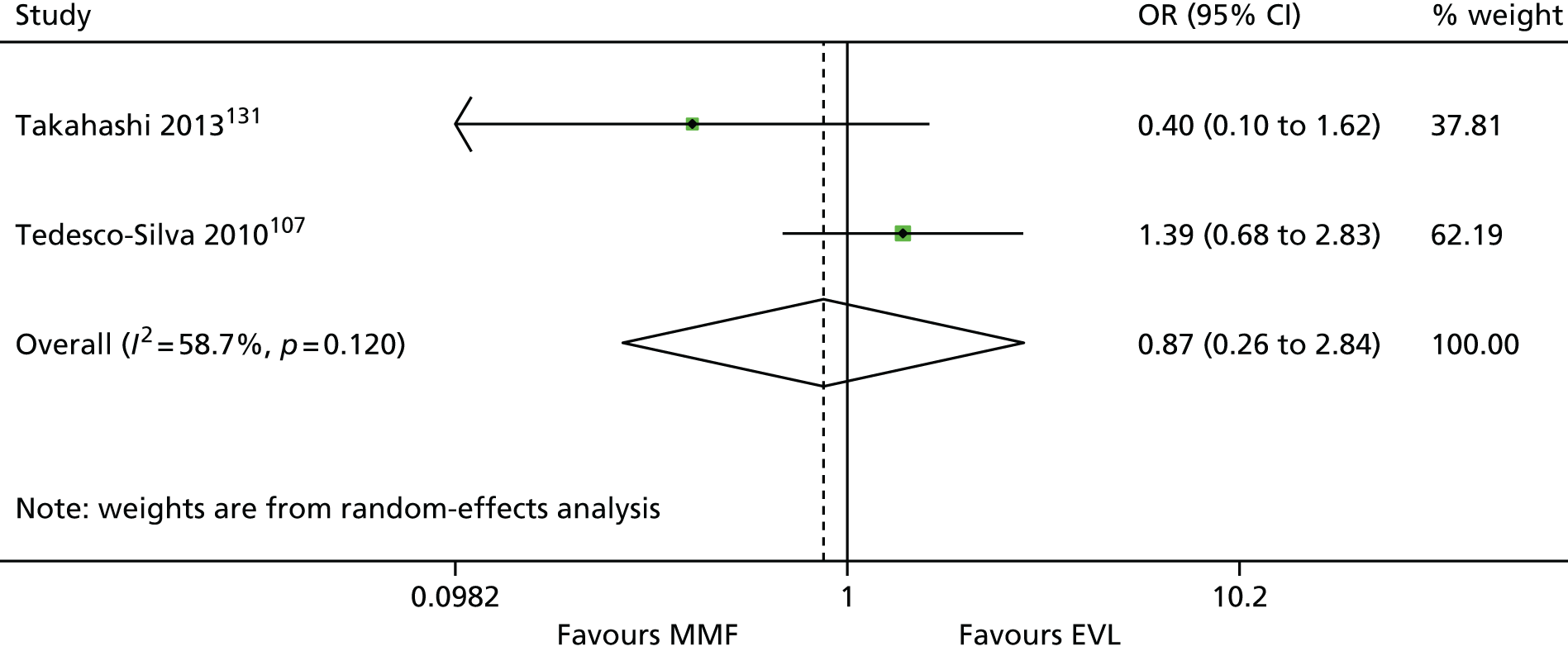
FIGURE 129.
Infection: EVL vs. MMF. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.178.
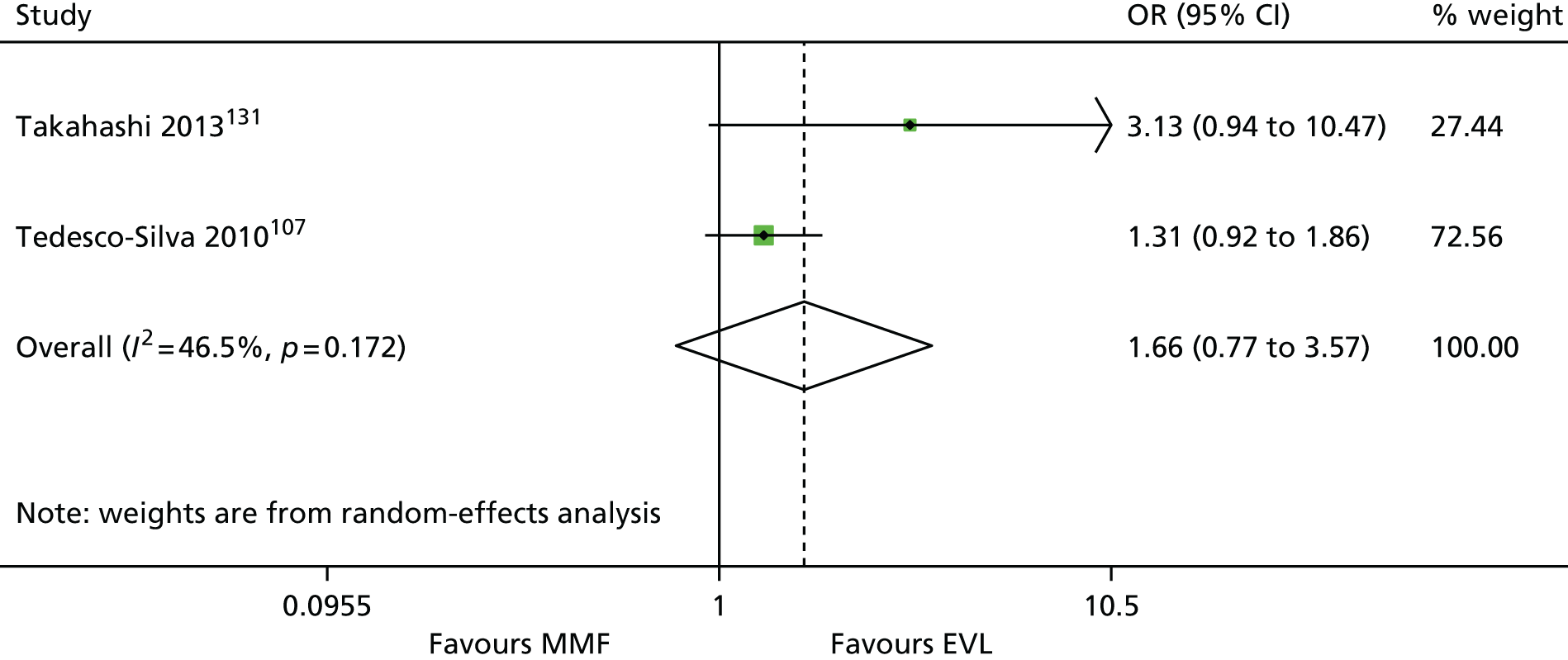
FIGURE 130.
Cytomegalovirus: EVL vs. MMF. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.
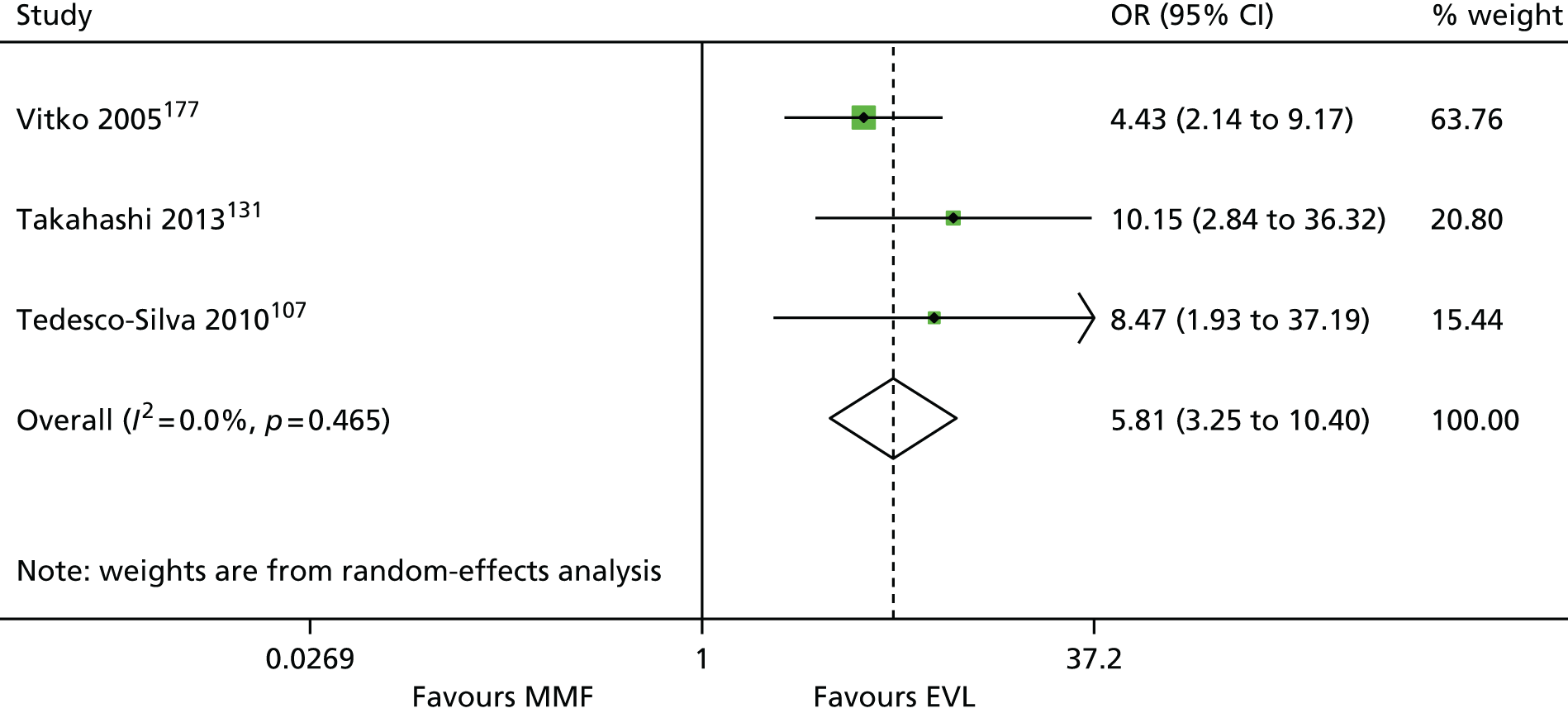
SRL compared with MMF
Three studies110–112 comparing SRL with MMF reported AEs; all studies110–112 used SRL + TAC + CCS and MMF + TAC + CCS regimens.
The meta-analyses suggested no differences for NODAT (Figure 131), malignancy (Figure 132) or PTLD (Figure 133). However, publication bias was not explored and the number of pooled studies is small; therefore, all results must be interpreted with caution. No difference was found between the two arms in one study112 for CMV infections: 6 out of 50 (12%) and 6 out of 50 (12%), respectively. Infections were not reported in these studies.
FIGURE 131.
New-onset diabetes after transplant/transplantation: SRL vs. MMF. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.
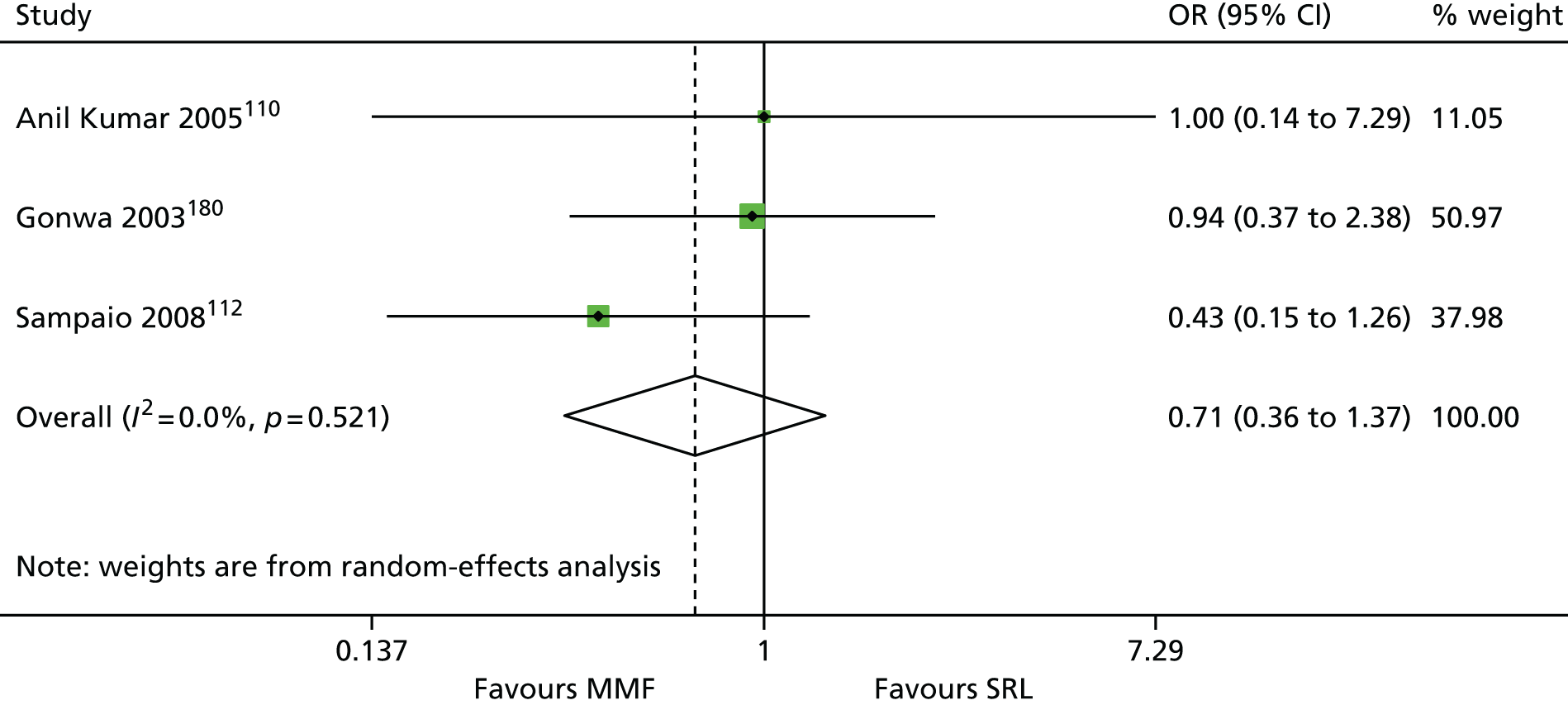
FIGURE 132.
Malignancy: SRL vs. MMF. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; studies with no events in both arms were excluded from the analysis. Sampaio et al. 112 reported 0 out of 0 and 0 out of 50 malignancy cases in SRL and MMF arms, respectively.

FIGURE 133.
Post-transplant lymphoproliferative disorder: SRL vs. MMF. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data. Sampaio et al. 112 reported 0/50 and 0/50 PTLD cases in SRL and MMF arms, respectively.
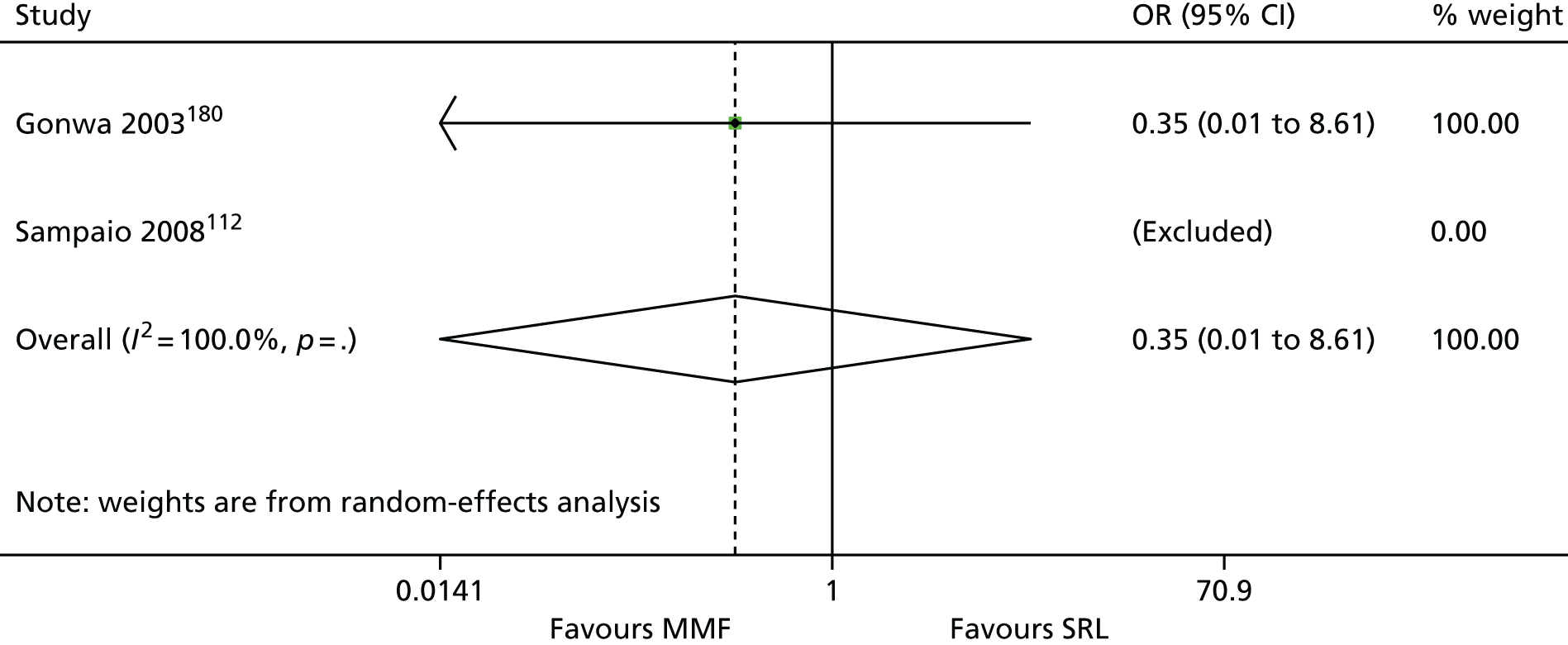
In summary, no differences in NODAT, PTLD, malignancy or CMV infections were found between SRL and MMF regimens at 1-year follow-up. However, only three studies110–112 reported NODAT and PTLD; two studies reported malignancy;111,112 and only one study112 reported CMV infections.
MMF versus MPS
Two studies comparing MMF with MPS reported AEs; one study106 used MMF + TAC + CCS and MPS + TAC + CCS regimens and one study427 used MMF + CSA + CCS and MPS + CSA + CCS regimens.
The meta-analyses suggested no differences for malignancy (Figure 134), infections (Figure 135) or CMV infections (Figure 136). However, publication bias was not explored and the number of pooled studies is small; therefore, all results must be interpreted with caution. No difference was found between the two arms106 for NODAT [OR = 1.06 (favours MPS, 95% CI 0.33 to 3.36)]. In summary, no difference in NODAT, malignancy, infections or CMV infections was found between MMF and MPS regimens at 1-year follow-up. However, only two studies106,427 reported malignancy, infections and CMV infections, and only one study106 reported NODAT.
FIGURE 134.
Malignancy: MMF vs. MPS. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; studies with no events in both arms were excluded from the analysis. Ciancio et al. 106 reported 0 out of 61 and 0 out of 55 malignancy cases in MMF and MPS arms, respectively.

FIGURE 135.
Infections: MMF vs. MPS. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.

FIGURE 136.
Cytomegalovirus: MMF vs. MPS. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.

MMF versus AZA
Three studies86,104,138 comparing MMF with AZA reported AEs; one study138 used MMF + CSA + CCS and AZA + CSA + CCS regimens, and two three-arm studies86,104 used MMF + CSA + CCS and AZA + CSA + CCS regimens.
The meta-analyses suggested no differences for CMV infections (Figure 137). However, publication bias was not explored and the number of pooled studies is small; therefore, all results must be interpreted with caution. No difference was found between the two arms for infections in one study86 [OR = 1.60 (favours MMF, 95% CI 0.98–2.60)]. 86 NODAT, malignancy and PTLD were not reported in these studies. In summary, no differences in infections and CMV infection were found between MMF and AZA regimens at 1-year follow-up. However, only three studies86,104,138 reported CMV infections, and only one study86 reported infections.
FIGURE 137.
Cytomegalovirus: MMF vs. AZA. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.030.
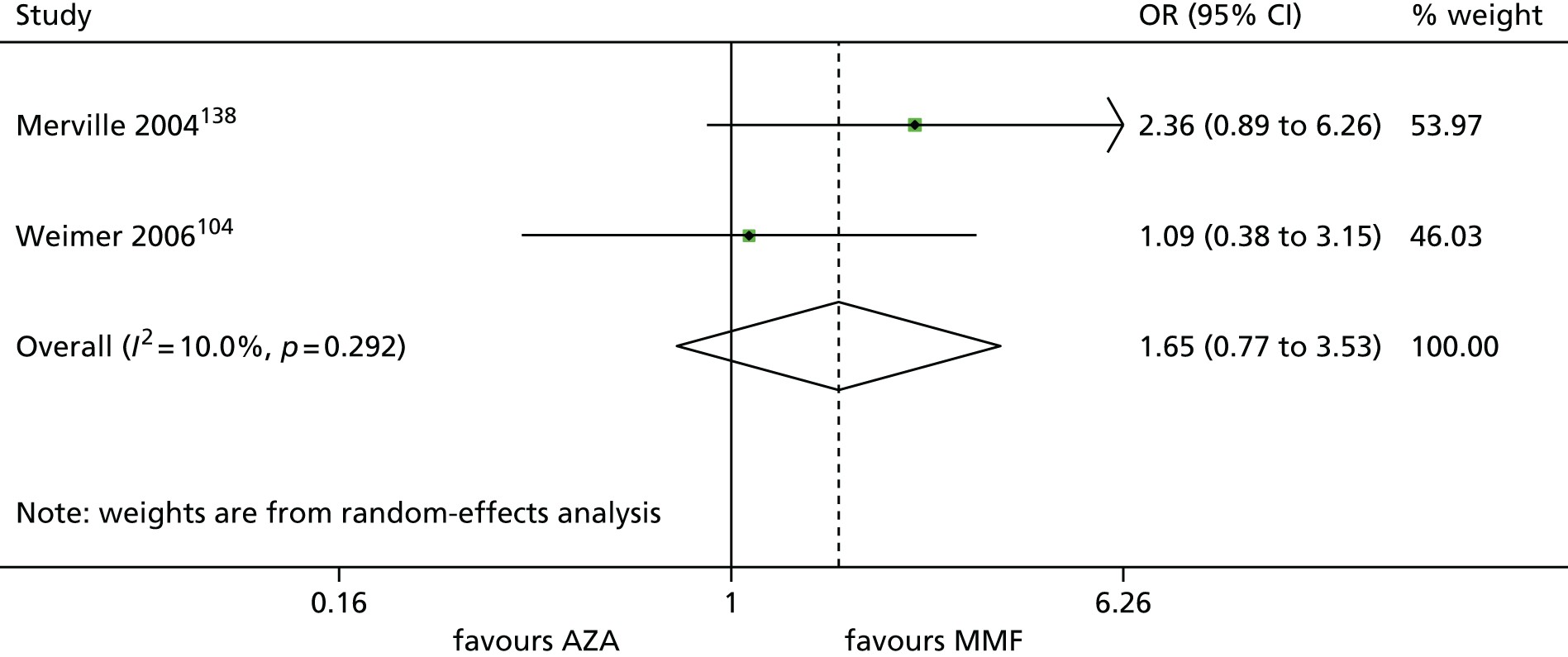
EVL versus MPS
Two studies144,152 comparing EVL with MPS reported AE; one study144 used EVL + CSA + CCS and MPS + CSA + CCS regimens and one three-arm study145 also used EVL + CSA + CCS and MPS + CSA + CCS regimens.
The meta-analyses suggested no differences for malignancy (Figure 138). However, publication bias was not explored and the number of pooled studies is small; therefore, all results must be interpreted with caution. No difference was found between the two arms in one study152 for NODAT [OR = 0.45 (favours MPS, 95% CI 0.17 to 1.20)], infections [OR = 1.74 (favours MMF, 95% CI 0.72 to 4.20)] or CMV infections [OR = 0.29 (favours MPS, 95% CI 0.05 to 1.71)]. 152 PTLD was not reported in either of the two studies. 144,152 In summary, no differences in NODAT, malignancy, infections and CMV infections were found between EVL and MPS regimens at 1-year follow-up. However, only two studies144,152 reported malignancy, and only one study152 reported NODAT, infections and CMV infections.
FIGURE 138.
Malignancy: EVL vs. MPS. Note: DerSimonian and Laird random-effects meta-analysis with 1-year follow-up data; the estimate of between-study variance, τ2, was 0.000.

Summary
Induction regimens
No difference in NODAT, PTLD, malignancy and infections were found between the two induction regimens, rATG and BAS, when compared with each other or with no induction (and/or PBO) regimens at 1-year follow-up. One study96 suggested more CMV infections with rATG regimens than with no induction. One study88 suggested more CMV infections in rATG regimens than in BAS regimens, but the results were not confirmed by other study. 98 In addition, publication bias was not explored and the number of pooled studies is small, therefore all results must be interpreted with caution.
Maintenance regimens
The meta-analyses of AEs at 1-year follow-up suggested significant differences in AEs for the following regimens. The meta-analysis comparing TAC and CSA regimens (including eight studies51,80,83,88,90,100,121,210) suggested more cases of NODAT with TAC regimens than with CSA regimens. The meta-analyses comparing BEL with CSA regimens (including three studies59,125,142) suggested more cases of NODAT with CSA regimens than with BEL regimens (including three studies). The meta-analyses comparing SRL with CSA regimens suggested more cases of NODAT with SRL than with CSA (including seven studies51,116,117,134,147,149,194) and more CMV infections with CSA than with SRL (including seven studies51,116,117,127,134,147,149). The meta-analysis comparing MMF and EVL (including three studies107,131,177) suggested more cases of CMV infections with MMF regimens than with EVL. However, publication bias was not explored and the number of pooled studies is small; therefore, all results must be interpreted with caution.
Appendix 8 Ongoing trials
Ongoing studies: identified trials
| n | Study | Sponsor/collaborators | Trial name | Sample size | Status |
|---|---|---|---|---|---|
| 1 | NCT01780844 | Astellas Pharma Global Development Inc., Kyowa Hakko Kirin Company Ltd | A Study to Assess the Efficacy and Safety of ASKP1240 in de Novo Kidney Transplant Recipients | 149 | Active, not recruiting |
| 2 | NCT01817322 | Samsung Medical Center | Kidney Graft Function Under the Immunosuppression Strategies (MyLowCSA) | 140 | |
| 3 | NCT01354301 | Hospital do Rim e Hipertensão | Efficacy and Safety of Induction Strategies Combined With Low Tacrolimus Exposure in Kidney Transplant Recipients Receiving Everolimus or Sodium Mycophenolate | 300 | |
| 4 | NCT00494741 | Mario Negri Institute for Pharmacological Research, Agenzia Italiana del Farmaco | MMF vs. AZA for Kidney Transplantation (ATHENA) | 224 | |
| 5 | NCT00782821 | University of Cincinnati Millennium Pharmaceuticals, Inc., Genzyme, a Sanofi Company | Randomized Trial of Induction Therapies in High Immunological Risk Kidney Transplant Recipients | 40 | |
| 6 | NCT00693446 | Nantes University Hospital | A Study To Compare Sirolimus Versus Tacrolimus In De Novo Simultaneous Pancreas- Kidney Allograft Recipients Receiving An Induction Therapy With Antithymocyte Globulin Plus Mycophenolate Mofetil Plus Corticosteroids | 118 | |
| 7 | NCT01114529 | Novartis | Efficacy, Safety and Evolution of Cardiovascular Parameters in Renal Transplant Recipients (ELEVATE) | 717 | |
| 8 | NCT00256750 | Bristol-Myers Squibb | Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression (BENEFIT) | 660 | |
| 9 | NCT00114777 | Bristol-Myers Squibb | Study of Belatacept in Subjects Who Are Undergoing a Renal Transplant (BENEFIT-EXT) | 600 | |
| 10 | NCT00514514 | Novartis | Study Investigating a Standard Regimen in de Novo Kidney Transplant Patients Versus a Calcineurin Inhibitor (CNI)-Free Regimen and a CNI Low Dose Regimen | 450 | |
| 11 | NCT00533442 | University of Miami, Astellas Pharma Inc. | Rapamycin Versus Mycophenolate Mofetil in Kidney-Pancreas Recipients | 190 | |
| 12 | NCT01005706 | Medical University of South Carolina, Pfizer (formerly Wyeth) | Sirolimus Conversions in African-American Renal Transplant Recipients | 40 | |
| 13 | NCT01878786 | Matthew Cooper | A Pilot Study Comparing the Safety and Efficacy of Everolimus With Other Medicines in Recipients of ECD/DCD Kidneys (Evered) | 50 | |
| 14 | NCT01187953 | Veloxis Pharmaceuticals | Double-Blind, Double-Dummy, Effic/Safety, LCP-Tacro™ Vs Prograf®, Prevention Rejection, De Novo Adult Kidney Tx (LCPTacro3002) | 540 | |
| 15 | NCT01053221 | University of Wisconsin, Madison | Mycophenolic Acid Monotherapy in Recipients of HLA-Identical Living-Related Transplantation | 30 | |
| 16 | NCT01062555 | University of Minnesota - Clinical and Translational Science Institute Roche Pharma AG, Pfizer (formerly Wyeth), Genzyme, a Sanofi Company | Calcineurin Inhibitor Sparing After Kidney Transplantation (CNI-Sparing) | 600 | |
| 17 | NCT01239563 | University of Oxford, Oxford University Hospitals NHS Trust Genzyme, a Sanofi Company | Thymoglobulin Induction in Kidney Transplant Recipients (TIKT) | 40 | Not yet recruiting |
| 18 | NCT01837043 | Nair, Vinay, DO, Mount Sinai School of Medicine, Bristol-Myers Squibb | Early Conversion From CNI to Belatacept in Renal Transplant Recipients With Delayed and Slow Graft Function | 90 | |
| 19 | NCT02137239 | Bristol-Myers Squibb | Evaluation of Acute Rejection Rates in de Novo Renal Transplant Recipients Following Thymoglobulin Induction, CNI-free, Nulojix (Belatacept)-Based Immunosuppression | 240 | |
| 20 | NCT01875224 | University of Arizona, Bristol-Myers Squibb | Comparison of NODAT in Kidney Transplant Patients Receiving Belatacept Versus Standard Immunosuppression | 32 | |
| 21 | NCT01822483 | Irmandade Santa Casa de Misericórdia de Porto Alegre, Novartis | A Prospective Study to Investigate Mycophenolic Acid (MPA) Exposure Through Area Under the Curve (AUC) in Renal Transplants Recipients Treated With Mycophenolate Mofetil (MMF) and After Conversion to Mycophenolate Sodium (EC-MPS) (AUC-MPA) | 100 | |
| 22 | NCT02058875 | University of Saskatchewan Novartis Pharmaceuticals, Canada Inc. | Cardiovascular Risk Following Conversion to Full Dose Myfortic® and Neoral® Two-Hour Post Level Monitoring (COBACAM) | 100 | |
| 23 | NCT01895049 | Helio Tedesco Silva Junior, Novartis, Sanofi | Comparison Between Two Tacrolimus-Based Immunosuppressant Regimens and Induction With Thymoglobulin in Kidney Transplants From Deceased Donors With Expanded Criteria | 200 | |
| 24 | NCT02056938 | Nantes University Hospital | ATG Versus Basiliximab in Kidney Transplant Displaying Low Immunological Risk But High Susceptibility to DGF (PREDICT-DGF) | 460 | Recruiting |
| 25 | NCT01856257 | National Institute of Allergy and Infectious Diseases (NIAID), Clinical Trials in Organ Transplantation | Safety and Efficacy of a Steroid-Free, Calcineurin Inhibitor-Free, Belatacept-Based Immunosuppressive Regimen | 180 | |
| 26 | NCT01560572 | University Medical Centre Groningen, Leiden University Medical Center, Academisch Medisch Centrum – Universiteit van Amsterdam (AMC-UvA) | Steroid Free Immunosuppression or Calcineurin Inhibitor Minimization After Basiliximab Induction Therapy in Kidney Transplantation: Comparison With a Standard Quadruple Immunosuppressive Regimen (Allegro) | 300 | |
| 27 | NCT00903188 | University Hospital, Antwerp, Novartis Pharmaceuticals Erasme University Hospital, University Hospital Ghent University Hospital of Liege Universitair Ziekenhuis Brussel |
Calcineurin Inhibitor (CNI) Versus Steroid Cessation in Renal Transplantation (CISTCERT) | 152 | |
| 28 | NCT01950819 | Novartis Pharmaceuticals | Advancing Renal Transplant Efficacy and Safety Outcomes With an Everolimus-Based Regimen (TRANSFORM) | 2040 | |
| 29 | NCT01649427 | Novartis Pharmaceuticals | Comparison of a Tacrolimus Hexal® Based Regimen Versus a Prograf® Based Regimen in de Novo Renal Transplant Recipients (Spartacus) | 326 | |
| 30 | NCT02083991 | Vastra Gotaland Region | Trial of Steroid Avoidance and Low-Dose CNI by ATG-Induction in Renal Transplantation (SAILOR) | 200 | |
| 31 | NCT01680861 | Gaetano Ciancio | Tacrolimus/Everolimus Versus Tacrolimus/Enteric-Coated Mycophenolate Sodium | 50 | |
| 32 | NCT01265537 | University of British Columbia, Astellas Pharma Canada Inc. | A Pilot Study Comparing the Use of Low-Target Versus Conventional Target Advagraf (Astellas) | 30 | |
| 33 | NCT01663805 | MARIO ABBUD FILHO | Effects of the Use of ‘de novo’ Everolimus in Renal Transplant Population | 80 | |
| 34 | NCT01541176 | Nantes University Hospital | Impact of the Absence of Steroids on the Evolution of Renal Function and on the Progression of Graft Fibrosis, Quantified by Numerical Method, in Patients With Renal Transplant (Astronef) | 186 | |
| 35 | NCT01656135 | University of Regensburg, European Commission | Reference Group Trial for The ONE Study | 60 | |
| 36 | NCT02102854 | The Methodist Hospital System | Single Dose rATG for Renal Allograft Rejection | 30 | |
| 37 | NCT00906204 | Wright State University Sanofi University of Arizona Wake Forest School of Medicine University of Nebraska, The Methodist Hospital System |
Safety Trial of Single Versus Multiple Dose Thymoglobulin Induction in Kidney Transplantation (STAT) | 165 | |
| 38 | NCT01729494 | University of Cincinnati | Belatacept Early Steroid Withdrawal Trial | 315 | |
| 39 | NCT02152345 | Columbia University | Belatacept Compared with Tacrolimus in Deceased Donor Renal Transplant Recipients | 100 | |
| 40 | NCT01653847 | Northwestern University, Novartis | Impact of Two Prednisone-Free Maintenance Immunosuppressive Regimens With Reduced Dose FK506 + Everolimus vs. Standard Dose Tacrolimus (FK506) + Mycophenolate Mofetil (MMF) on Subpopulation of T and B Cells, Renal Allograft Function and Gene Expression Profiles in Renal Allograft Biopsies at 12 Months Post-transplant. Prospective Single Center Study in Recipients of Renal Transplant Allograft | 88 | |
| 41 | NCT01631058 | University of Sao Paulo General Hospital | Immunosuppression in Renal Transplantation in The Elderly: Time to Rethink – nEverOld Study | 90 | |
| 42 | NCT00866879 | Northwestern University, Pfizer (formerly Wyeth) | Randomized Conversion of Calcineurin-Inhibitors in Renal Allograft Recipients | 275 | |
| 43 | NCT02062892 | University of Colorado, Denver, Novartis Pharmaceuticals | Differentiating Everolimus Versus Sirolimus in Combination With Calcineurin Inhibitors in Kidney Transplant Patients (DESIRE) | 150 | |
| 44 | NCT00896012 | University at Buffalo, Novartis University of Washington |
Kidney Biopsy Controlled Trial of Calcineurin Inhibitor Withdrawal | 120 | Recruiting (invitation) |
| 45 | NCT01860183 | Clinical Hospital Merkur University Medical Centre Ljubljana Clinical Hospital Centre Osijek University Hospital Rijeka |
Effect of 3 g versus 2 g MMF in Combination With Tacrolimus on Progression of Renal Allograft Interstitial Fibrosis | 80 | Recruiting |
| 46 | NCT01820572 | Bristol-Myers Squibb | A Study in Maintenance Kidney Transplant Recipients Following Conversion to Nulojix® (Belatacept)-Based | 600 | |
| 47 | NCT02213068 | Lorenzo Gallon Bristol-Myers Squibb | Belatacept 3 Month Post Transplant Conversion Study | 51 | |
| 48 | NCT01790594 | National Institute of Allergy and Infectious Diseases (NIAID) Clinical Trials in Organ Transplantation | Optimization of NULOJIX® (Belatacept) Usage as a Means of Minimizing CNI Exposure in Simultaneous Pancreas and Kidney Transplantation | 60 | |
| 49 | NCT01921218 | Andrew B Adams, MD, PhD, Bristol-Myers Squibb | Belatacept Therapy for the Failing Renal Allograft (IM103-133) | 72 | |
| 50 | NCT02134288 | Von Visger, Jon, MD Bristol-Myers Squibb | Belatacept for Renal Transplant Recipients With Delayed Graft Function | 40 | |
| 51 | NCT01595984 | Centre Hospitalier Universitaire, Amiens Novartis | Comparison of Efficacy and Safety of Treatment With a Calcineurin Inhibitor (CNI) versus a CNI-free Treatment in Renal Transplantation (CIME) | 134 | |
| 52 | NCT02221583 | University of Cincinnati, Astellas Pharma Inc. | Pharmacokinetics of Immunosuppressants in Renal Transplant Candidates Who Have Undergone Laparoscopic Sleeve Gastrectomy | 24 | |
| 53 | NCT01935128 | University of Toledo Health Science Campus, Novartis Pharmaceuticals | Calcineurin-inhibitor Elimination/Reduction Randomized to Everolimus/Myfortic® vs. Everolimus/Reduced Tacrolimus in Renal Transplant Recipients Following Campath® Induction | 50 | |
| 54 | NCT01169701 | Novartis | 24 Months Follow-up, Two Arm Study to Compare the Cardiovascular Profile in a Regimen With Everolimus + Mycophenolic Acid (MPA) versus (vs.) a Regimen of CNI + MPA in Maintenance Renal Transplant Recipients (EVITA) | 80 | |
| 55 | NCT01544491 | Novartis Pharmaceuticals | Efficacy, Tolerability and Safety of Early Introduction of Everolimus, Reduced Calcineurin Inhibitors and Early Steroid Elimination Compared with Standard CNI, Mycophenolate Mofetil and Steroid Regimen in Paediatric Renal Transplant Recipients | 106 | |
| 56 | NCT01842269 | Chong Kun Dang Pharmaceutical | Evaluate the Efficacy and Safety of My-Rept® Tablet Versus My-Rept® Capsule in Combination With Tacrolimus in Kidney Transplant Patients (My-Rept®_KT_P4) | 156 | |
| 57 | NCT01410448 | Novartis Pharmaceuticals | Everolimus in de Novo Kidney Transplant Recipients (NEVERWOUND) | 396 | |
| 58 | NCT02036554 | Seoul St. Mary’s Hospital, Novartis | Evaluate Efficacy Study of Combination Therapy of Everolimus and Low Dose Tacrolimus in Renal Allograft Recipients (PROTECT) | 234 | |
| 59 | NCT02077556 | National Taiwan University Hospital | Effect of Everolimus on the Pharmacokinetics of Tacrolimus in Renal Transplant Patients | 70 | |
| 60 | NCT01843348 | Novartis Pharmaceuticals | 12 Month Athena Study: Everolimus vs. Standard Regimen in de Novo Kidney Transplant Patients (ATHENA) | 612 | |
| 61 | NCT02096107 | Medical University of South Carolina Novartis | Novartis Everolimus Transition | 60 | |
| 62 | NCT01680952 | Yonsei University | Study to Evaluate the Safety and Efficacy of Extended Release Tacrolimus (Advagraf®) + Sirolimus (Rapamune®), Versus Extended Release Tacrolimus (Advagraf®) + Mycophenolate Mofetil in Kidney Transplant Patients | 60 | |
| 63 | NCT01801280 | Klemens Budde Novartis Pharmaceuticals | Influence of Pantoprazole to the Bioavailability of Myfortic® and CellCept® | 24 | |
| 64 | NCT01612299 | University of Kentucky | Effects of Zortress® + Tacrolimus vs. Standard Immunosuppression on Progression of Coronary Artery Calcifications and Bone Disease in de Novo Renal Transplant Recipients | 60 | |
| 65 | NCT02208791 | University of Sao Paulo General Hospital | Effects of the Quadruple Immunosuppression on Peripheral Blood Lymphocytes and Development of Anti-HLA Antibodies in Kidney Transplant | 45 | |
| 66 | NCT02084446 | Ronaldo de Matos Esmeraldo, MD, Novartis Pharmaceuticals | Everolimus + Very Low Tacrolimus vs. Enteric-coated Mycophenolate Sodium + Low Tacrolimus in de Novo Renal Transplant | 120 | |
| 67 | NCT01276834 | Dianet Dialysis Centers, UMC Utrecht | Comparison of Immunosuppression on Progression of Arteriosclerosis in Renal Transplantation (NOCTX-2) | 80 | |
| 68 | NCT01976390 | Dr Ronald Pelletier, Novartis | Comparing Everolimus and Sirolimus in Renal Transplant Recipients | 60 | |
| 69 | ISRCTN88894088 | University of Oxford | Campath, Calcineurin inhibitor reduction and Chronic allograft nephropathy | 800 | |
| NCT01120028 | |||||
| 70 | NCT00724022 | University Hospital Freiburg, Roche Pharma AG, Astellas Pharma GmbH, Genzyme, a Sanofi Company | Phase IV Study to Evaluate Calcineurin Inhibitor Reduced, Steroid Free Immunosuppression After Renal Transplantation (Harmony) | 600 | Unknown |
| 71 | NCT01550445(a) | Ajou University School of Medicine | Steroid Withdrawal Immunosuppression After Renal Transplantation | 30 | |
| 72 | NCT00302497 | McGill University Health Center | EXTEND Protocol for Transplanted Patient to Evaluate Kidney Function | 50 | |
| 73 | NCT00199667 | University Hospital, Limoges, Hoffmann-La Roche | Concentration Controlled Versus Fixed Dose of MMF in Kidney Transplant Recipients | 137 | |
| 74 | NCT00556933 | University of Nebraska, Genzyme, a Sanofi Company | Improved Induction and Maintenance Immunosuppression in Kidney Transplantation | 180 | |
| 75 | NCT00807144 | Hammersmith Hospitals NHS Trust | Standard versus Prolonged-Release Tacrolimus Monotherapy After Alemtuzumab Induction in Kidney Transplantation | 100 | |
| 76 | NCT00296296 | Stanford University | Immunosuppression Impact on the Metabolic Control of First Kidney Transplant Recipients With Pre-Existing Type 2 Diabetes (DM) | 40 | |
| 77 | NCT01239472 | Andre Barreto Pereira, Novartis | Cytokines Evaluation in Early Calcineurin Inhibitors Withdrawn on Renal Transplant | 30 | |
| 78 | NCT00707759 | Maria Angela Delucchi Bicocchi, University of Chile, Fondo Nacional de Desarrollo Científico y Tecnológico, Chile | Steroid Withdrawal in Pediatric Renal Transplant Immunosuppression: Impact on Growth, Bone Metabolism and Acute Rejection | 70 | |
| 79 | NCT01334333 | University of British Columbia, Simon Fraser University, Astellas Pharma Canada, Inc. | Comparison of Medication Adherence Between Once and Twice Daily Tacrolimus in Stable Renal Transplant Recipients | 100 | |
| 80 | NCT01399242 | Hospital Universitário São José | Efficacy of Certican® in Combination With Myfortic® in Renal (HUSJ1) | 40 | |
| 81 | NCT00737659 | Rabin Medical Center | CellCept® Dose Adjustment Versus Fixed Dose (Standard Care) in Renal Transplant Recipients (MMF) | 138 | |
| 82 | NCT00309218 | Klinik für Kinder- und Jugendmedizin Hoffmann-La Roche | Steroid Withdrawal in Pediatric Renal Transplant Recipients Under Cyclosporine (CyA) and Mycophenolate Mofetil (MMF) | 40 | |
| 83 | NCT00166712 | Northwestern University Northwestern Memorial Hospital | A Trial of Two Steroid-Free Approaches Toward Mycophenolate Mofetil-Based Monotherapy Immunosuppression | 40 | |
| 84 | NCT00733733 | Radboud University Erasmus Medical Centre Maastricht University Leiden University Medical Centre University Medical Centre Utrecht University Medical Centre Groningen Academisch Medisch Centrum – Universiteit van Amsterdam (AMC-UvA) |
Anti-T-Lymphocyte Globulin (ATG) in Renal Transplantation of Kidneys With a Non-Heart-Beating (NHB) Donor | 180 | |
| 85 | NCT01159080 | Asan Medical Center Seoul National University Hospital, Samsung Medical Center | Treatment of the Optimum Dose of Calcineurin Inhibitor and Mycophenolate Sodium in Kidney Recipients (OPTIMUM) | 350 | |
| 86 | NCT01014234 | IRCCS Policlinico S Matteo | Rapamycin and Regulatory T Cells in Kidney Transplantation | 56 | |
| 87 | NCT00223678 | Vanderbilt University | Mycophenolate Mofetil and Rapamycin as Secondary Intervention vs. Continuation of Calcineurin Inhibitors in Patients at Risk for Chronic Renal Allograft Failure | 30 | |
| 88 | NCT01455649 | Deise de Boni Monteiro de Carvalho | Study to Evaluate the Safety and Efficacy of Switching Calcineurin Inhibitor to Everolimus After Kidney Transplantation in Adults | 30 | |
| 89 | NCT00166829 | National Taiwan University Hospital | The Effect of Sirolimus on the Pharmacokinetics of Tacrolimus | 40 | |
| 90 | NCT00541814 | University Hospital Birmingham, Novartis | Calcineurin Inhibitor Minimisation in Renal Transplant Recipients With Stable Allograft Function (CNIM-SRT) | 90 | |
| ISRCTN60081949 | |||||
| 91 | NCT01640743 | IRCCS Policlinico S Matteo | Effect of Different Therapeutic Strategies on Regulatory T Cells in Kidney Transplantation (EVERTWIST) | 58 | |
| 92 | NCT00290069 | Sociedad Andaluza de Trasplantes de Organos y Tejidos | Renal Function Optimization With Mycophenolate Mofetil (MMF) Immunosuppressor Regimens (ALHAMBRA) | 94 | |
| 93 | NCT00252655 | Wayne State University | Use of Sirolimus vs. Tacrolimus For African-American Renal Transplant Recipients | 40 | |
| 94 | NCT00141804 | University Hospital Muenster, Proverum GmbH KKS Netzwerk, Fujisawa GmbH |
Efficacy and Safety of Sirolimus in Combination With Tacrolimus | 190 | |
| 95 | NCT00166816 | National Taiwan University Hospital | The Pharmacokinetics of Sirolimus When Combined With Cyclosporine or Tacrolimus in Renal Transplant Patients | 40 | |
| 96 | NCT01436305 | National Institute of Allergy and Infectious Diseases (NIAID) | Optimization of NULOJIX® (Belatacept) Usage As A Means of Avoiding Calcineurin Inhibitor (CNI) and Steroids in Renal Transplantation | 19 | Suspended |
| 97 | NCT01244659 | EMS | A Randomized Study Assess the Safety and Efficacy of Tacrolimus vs. Prograf® in Renal Transplantation Treatment | 60 | |
| 98 | NCT00729768 | Genentech | A Study to Evaluate Efalizumab Compared with Cyclsporine as an Immunosuppressant Regimen in De Novo Renal Transplantation | 200 | Withdrawn |
| 99 | NCT01149993 | Georgetown University, Novartis | Attenuating Ischemia Reperfusion Injury After Living Donor Renal Transplantation | 0 | |
| 100 | NCT01038505 | University of Miami, Pfizer (formerly Wyeth) | Comparison of Tacrolimus and Myfortic Versus Tacrolimus and Sirolimus | 0 | |
| 101 | NCT00956293 | Novartis Pharmaceuticals | Study to Evaluate the Efficacy, Safety and Tolerability of Everolimus in de Novo Renal Transplant Recipients Participating in the Eurotransplant Senior Program (Senator) | 207 | Terminated |
| 102 | NCT00284921 | Novartis Pharmaceuticals | MYPROMS-ES02: Safety and Efficacy of Basiliximab, Cyclosporine Microemulsion and Enteric-coated Mycophenolate Sodium (EC-MPS) versus EC-MPS and Steroid Therapy in Kidney Transplant Recipients Who Are Hepatitis C Positive | 60 | |
| 103 | NCT00928811 | Drexel University College of Medicine, Novartis | Study to Evaluate the Safety of Chronic Administration of Simulect to Subjects Receiving a First Kidney Transplant | 5 | |
| 104 | NCT00137345(a) | Pfizer (formerly Wyeth) | Study Comparing Sirolimus With Cyclosporine in a Calcineurin Inhibitor (CNI)-Free Regimen in Kidney Transplant Recipients | 500 | |
| 105 | NCT01387659 | The University of Texas, Galveston, Novartis Pharmaceuticals | Evaluate Tolerability of Myfortic®/Simulect® and Tacrolimus Without Steroids in Three Patient Populations | 4 | |
| 106 | NCT00522548 | University of Pennsylvania, Novartis Pharmaceuticals | Study of Gastrointestinal Side Effects in African American Kidney Transplant Recipients Treated With CellCept or Myfortic | 37 | |
| 107 | NCT00235781 | Washington University School of Medicine | Single Dose Thymoglobulin for Induction in Adult Renal Allograft Recipients | 90 | |
| 108 | NCT00332839 | Novartis Pharmaceuticals | Comparison of CNI-based Regimen versus CNI-free Regimen in Kidney Transplant Recipients | 93 | |
| 109 | NCT00217152 | Mayo Clinic, Roche Pharma AG | A Kidney Transplant Study to Look at the Effects of Taking Fixed Doses of CellCept Versus Taking Doses of CellCept Based on the Concentration of CellCept in the Blood When Taking Full or Reduced Dose Calcineurin Inhibitors | 12 | |
| 110 | NCT01324934 | Neovii Biotech, Eurotrials, Scientific Consultants, Recerca Clínica SL, PsyConsult | Efficacy and Safety of ATG-Fresenius Following a Renal Transplantation, Without Corticosteroids | 40 | |
| 111 | NCT00596947 | University of Pennsylvania | Prednisone Withdrawal Versus Prednisone Maintenance After Kidney Transplant | 18 | |
| 112 | NCT00311311 | Pfizer | Study Comparing Effect On Carotid Atherosclerosis Following Conversion From Tacrolimus To Sirolimus Post-Transplant In Kidney Transplant Patients | 72 | |
| 113 | NCT00434590 | Novartis Pharmaceuticals | Efficacy and Tolerability of Full Dose Enteric-coated Mycophenolate Sodium, in Addition to Cyclosporine for Microemulsion Reduced Dose, in Maintenance Renal Transplant Recipients | 10 | |
| 114 | NCT00148252 | University of Oslo School of Pharmacy | Lowering Total Immunosuppressive Load in Renal Transplant Recipients More Than 12 Months Posttransplant | 298 | |
| 115 | NCT00204230 | University Hospital Muenster, Hoffmann-La Roche | MMF and Calcineurin Inhibitor Withdrawal in CAN | 86 | |
| 116 | NCT01609673 | Helady Pinheiro, MD, PhD, Novartis | Study of Everolimus in de Novo Renal Transplant Recipients | 1 | |
| 117 | NCT01213394 | Ramesh Prasad Hoffmann-La Roche | Mycophenolate Mofetil for Reducing Cardiovascular Risk in Renal Transplant Recipients (MMCR) | 2 | |
| 118 | NCT00991510 | Teva Pharmaceutical Industries, Parexel | Comparative Bioavailability of Myfenax® and CellCept® in Kidney Transplant Patients | 43 | |
| 119 | NCT00658333 | Novartis Pharmaceuticals | A Study designed to Compare the Tolerability of an Increased Dose of Enteric-coated Mycophenolate Acid (MPA) in Renal Transplant Patients Whose Dose of Mycophenolate Mofetil (MMF) Was Reduced Due to Gastrointestinal Symptoms | 30 | |
| 120 | NCT00133172 | Astellas Pharma Inc. Astellas Pharma Canada, Inc. | Effect of Rapid Steroid Withdrawal on Subclinical Markers of Rejection | 85 | |
| 121 | NCT00752479 | Mario Negri Institute for Pharmacological Research | Mesenchymal Stem Cells Under Basiliximab/Low Dose rATG to Induce Renal Transplant Tolerance | 4 | |
| 122 | NCT00928811 | Drexel University College of Medicine Novartis | Study to Evaluate the Safety of Chronic Administration of Simulect to Subjects Receiving a First Kidney Transplant | 5 | |
| 123 | NCT00452361 | Pfizer (formerly Wyeth) | Study Evaluating of Calcineurin Inhibitors Versus Sirolimus in Renal Allograft Recipient | 31 | |
| 124 | NCT00658320 | Novartis | Concentration Controlled Everolimus With Reduced Dose Cyclosporine Versus Mycophenolate Mofetil With Standard Dose Cyclosporine in de Novo Renal Transplant Adult Recipients Treated With Basiliximab and Corticosteroids | 122 | Completed |
| 125 | NCT00113269 | Astellas Pharma Inc. | Safety/Efficacy of Induction Agents With Tacrolimus, MMF, and Rapid Steroid Withdrawal in Renal Transplant Recipients (INTAC) | 501 | |
| 126 | NCT00235300 | Genzyme, a Sanofi Company | An Open-Label, Prospective, Randomized, Multi-center, Phase II Comparative Trial of Thymoglobulin Versus Simulect for the Prevention of Delayed Graft Function and Acute Allograft Rejection in Renal Allograft Recipients. | 240 | |
| 127 | NCT00965094 | Novartis Pharmaceuticals | Efficacy and Safety of Everolimus + EC-MPS After Early CNI Elimination vs. EC-MPS + Tacrolimus in Renal Transplant Recipients | 36 | |
| 128 | NCT00154284 | Novartis | Everolimus in a Cyclosporine Microemulsion-free Regimen Compared with a Low-dose Cyclosporine Microemulsion Regimen, in de Novo Kidney Transplant Patients (CERTES02) | 114 | |
| 129 | NCT01079143 | Novartis Pharmaceuticals | Progression of Renal Interstitial Fibrosis/Tubular Atrophy (IF/TA) According to Epithelial-Mesenchymal Transition (EMT) and Immunosuppressive Regimen (Everolimus Based versus CNI Based) in de Novo Renal Transplant Recipients (CERTITEM) | 194 | |
| 130 | NCT00251004 | Novartis | Efficacy and Safety Study of Everolimus Plus Reduced Cyclosporine Versus Mycophenolic Acid Plus Cyclosporine in Kidney Transplant Recipients | 833 | |
| 131 | NCT00543569 | Astellas Pharma Inc. | A Study to Assess the Safety and Efficacy of Alefacept in Kidney Transplant Recipients | 323 | |
| 132 | NCT01304836 | Astellas Pharma Inc. | A Study Looking at Diabetes in Kidney Transplant Recipients Receiving Immunosuppressive Regimen With or Without Steroids (ADVANCE) | 1166 | |
| 133 | NCT00369161 | Novartis | A Twelve-Month, Multicenter, Open-label, Randomized Study of the Safety, Tolerability and Efficacy of Everolimus With Basiliximab, Corticosteroids and Two Different Exposure Levels of Tacrolimus in de Novo Renal Transplant Recipients | 228 | |
| 134 | NCT00284947 | Novartis | Safety and Efficacy of Basiliximab in Calcineurin Inhibitor Intolerant Long-term Kidney Transplant Recipients Treated With Mycophenolic Acid and Steroids | 7 | |
| 135 | NCT00239031 | Novartis | Study of Enteric-Coated Mycophenolate Sodium (EC-MPS) Plus Reduced-Dose Cyclosporine Microemulsion (CSA-ME) Compared with EC-MPS Plus Standard Dose CSA-ME in Elderly de Novo Renal Transplant Recipients Treated With Basiliximab and Short-Term Steroids | 117 | |
| 136 | NCT00492869 | Novartis Pharmaceuticals | Efficacy and Safety of AEB071 versus Tacrolimus in Combination With Mycophenolate Acid Sodium, Basiliximab and Steroids in Preventing Acute Rejection After Kidney Transplantation | 124 | |
| 137 | NCT01596062 | Novartis Pharmaceuticals | Pharmacodynamics, Efficacy and Safety of Basiliximab 40 or 80 mg in Combination With Ciclosporine Microemulsion or Everolimus, in Adult Low Risk de Novo Renal Transplant Recipients (IDEALE Study) | 16 | |
| 138 | NCT00154232 | Novartis Pharmaceuticals | Study to Evaluate the Combination of Enteric-coated Mycophenolate Sodium (EC-MPS), Basiliximab, and C2-monitored Cyclosporine in de Novo Renal Transplant Recipients at Potential High Risk of Delayed Graft Function (DGF) | 46 | |
| 139 | NCT00634920 | Novartis Pharmaceuticals | Evaluation of Early Conversion to Everolimus From Cyclosporine in de Novo Renal Transplant Recipients | 204 | |
| 140 | NCT00717470 | Astellas Pharma Inc. | A Study in Kidney Transplant Subjects to Investigate the Optimal Suppression of Immunity to Help Prevent Kidney Rejection (OSAKA) | 1252 | |
| 141 | NCT00170833 | Novartis | Safety, Tolerability and Efficacy of Everolimus With Lower Versus Higher Levels of Tacrolimus in de Novo Renal Transplant Patients | 80 | |
| 142 | NCT00308425 | Novartis | Safety and Efficacy of Enteric-coated Mycophenolate Sodium (EC-MPS) Plus Valsartan in Patients With Kidney Transplants (MYTHOS) | 119 | |
| 143 | NCT00610961 | University of Florida, Novartis Pharmaceuticals | Induction Related BK Viremia in Renal Transplant Patients | 60 | |
| 144 | NCT00842699 | Brigham and Women’s Hospital, Genzyme, a Sanofi Company | Characterization of Immunological Profile of Renal Transplant Patients Undergoing Induction Treatment With Thymoglobulin vs. IL-2 Receptor Antagonist Basiliximab | 40 | |
| 145 | NCT00229138 | Novartis Pharmaceuticals | Efficacy and Safety of Enteric-Coated Mycophenolate Sodium (EC-MPS) in Kidney Transplant Recipients | 291 | |
| 146 | NCT00101738 | Novartis Pharmaceuticals | Freedom Study: Myfortic in Kidney Transplant Patients | 342 | |
| 147 | NCT00820911 | Novartis Pharmaceuticals | Efficacy and Safety of AEB071 Versus Cyclosporine in de Novo Renal Transplant Recipients | 175 | |
| 148 | NCT00167947 | Pfizer (formerly Wyeth) | Study Evaluating Sirolimus in Kidney Transplant Recipients. | 150 | |
| 149 | NCT00504543 | Novartis Pharmaceuticals | Efficacy, Safety and Tolerability of AEB071 Versus Cyclosporine in the Novo Renal Transplant Recipients | 311 | |
| 150 | NCT00403416 | Novartis Pharmaceuticals | Efficacy and Safety of AEB071 Plus Tacrolimus (Converted to Mycophenolic Acid After 3 Months), in Renal Transplant Patients | 215 | |
| 151 | NCT00531440 | Novartis Pharmaceuticals | This is a 2-year Follow-up Study to Evaluate the Long-term Effects in Patients Who Completed the Study CRAD001A2307 | 256 | |
| 152 | NCT00106639 | Pfizer | A 6-Month Study Of CP-690,550 versus Tacrolimus In Kidney Transplant Patients | 61 | |
| 153 | NCT01336296 | University of Cincinnati, Novartis Pharmaceuticals | Evaluate Effects and Safety of Pre-load Myfortic® in Transplant Patients | 61 | |
| 154 | NCT00552201 | Centre Hospitalier Universitaire, Amiens, Roche Pharma AG, Astellas Pharma Inc. | Tacrolimus in Renal Transplantation: Individualization by Pharmacogenetic | 280 | |
| 155 | NCT01028092 | University Hospital, Brest, Novartis, Roche Pharma AG, Genzyme, a Sanofi Company, Ministry of Health, France | mTor-inhibitor (Everolimus) Based Immunosuppressive Strategies for CNI Minimisation in OLD for Old Renal Transplantation (EVEROLD) | 327 | |
| 156 | NCT01435291 | Centre Hospitalier Universitaire de Nice | AADAPT – Analysis of Advagraf Dose Adaptation Post Transplantation | 45 | |
| 157 | NCT00771875 | University of Cincinnati | Randomized Trial for Mixed Acute Rejection | 30 | |
| 158 | NCT00261820 | Pfizer (formerly Wyeth) | Study Comparing Two Immunosuppressive Regimens in De Novo Renal Allograft Recipients | 160 | |
| 159 | NCT00771745 | University of Cincinnati, Genzyme, a Sanofi Company | Prospective Pilot Study of Pre-Transplant Thymoglobulin Administration in Living Donor Renal Transplant Recipients | 11 | |
| 160 | NCT00076570 | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | Combination Drug Therapy Followed by Single Drug Steroid Free Therapy to Prevent Organ Rejection in Kidney Transplantation | 31 | |
| 161 | NCT00089947 | Genzyme, a Sanofi Company | A Study to Evaluate the Effect of Thymoglobulin and Reduced Doses of Steroids to Prevent Renal Transplant Rejection | 150 | |
| 162 | NCT00007787 | National Institute of Allergy and Infectious Diseases (NIAID) | Antibody and Delayed Cyclosporine Versus Initial Cyclosporine Alone in Patients Receiving Kidney Transplants | 350 | |
| 163 | NCT00284934 | Novartis | Enteric-coated Mycophenolate Sodium (EC-MPS) With Reduced-dose Tacrolimus Versus EC-MPS With Standard-dose Tacrolimus in Stable Kidney Transplant Recipients (OLYMPE) | 94 | |
| 164 | NCT00266123 | Pfizer (formerly Wyeth) | Study Comparing Two Sirolimus Regimens vs. Tacrolimus and Mycophenolate Mofetil Regimen in Kidney Transplant Recipients | 420 | |
| 165 | NCT00765661 | Veloxis Pharmaceuticals, CTI Clinical Trial and Consulting Services, Aptuit Inc. | Pharmacokinetics of LCP-Tacro(TM) Once Daily And Prograf® Twice A Day in Adult De Novo Kidney Transplant Patients | 63 | |
| 166 | NCT01363752 | Astellas Pharma Inc. | A Study Looking at Kidney Function in Kidney Transplant Recipients Who Are Taking Anti-rejection Medication Including Tacrolimus and With or Without Sirolimus. (ADHERE) | 853 | |
| 167 | NCT00297765 | Astellas Pharma Inc. | Optimizing Prograf® Therapy in Renal Transplant Patients (OPTIMA) | 323 | |
| 168 | NCT00213590 | University Hospital, Rouen | Renal Function Evaluation After Reduction of Cyclosporine A Dose in Renal Transplant Patients (DICAM) | 208 | |
| 169 | NCT00273871 | Pfizer (formerly Wyeth) | Study Comparing Conversion From Calcineurin Inhibitors to Rapamune versus Standard Therapy in Established Renal Allograft Recipients | 190 | |
| 170 | NCT00369382 | Pfizer (formerly Wyeth) | Study Of The Safety And Efficacy Of Conversion From a CNI to Sirolimus In Renally-Impaired Heart Transplant Recipients | 121 | |
| 171 | NCT00717379 | Astellas Pharma Inc. | Study of Tacrolimus Immunosuppressive Therapy After Kidney Transplantation | 50 | |
| 172 | NCT00496483 | Veloxis Pharmaceuticals, CTI Clinical Trial and Consulting Services | Pharmacokinetics of LCP-Tacro in Stable Kidney Transplant Patients | 60 | |
| 173 | NCT01802268 | Helio Tedesco Silva Junior, Pfizer | Planned Conversion From TAC to SRL-based Regimen in de Novo Kidney Transplant Recipients | 320 | |
| 174 | NCT00296309 | Astellas Pharma Inc., Astellas Pharma Europe BV | Comparing Efficacy & Safety of Tacrolimus & MMF With/Without Induction in the Elderly Following Kidney Transplantation. | 267 | |
| 175 | NCT00402168 | Bristol-Myers Squibb | A Study of BMS-224818 (Belatacept) in Patients Who Have Undergone a Kidney Transplant and Are Currently on Stable Cyclosporine or Tacrolimus Regimen With or Without Corticosteroids | 171 | |
| 176 | NCT00035555 | Bristol-Myers Squibb | Study Comparing the Safety and Efficacy of Belatacept With That of Cyclosporine in Patients With a Transplanted Kidney | 230 | |
| 177 | NCT00455013 | Bristol-Myers Squibb | A Phase II Study of Belatacept (BMS-224818) With a Steroid-free Regimen in Subjects Undergoing Kidney Transplantation | 93 | |
| 178 | NCT00183248 | University of Miami Immune Tolerance Network (ITN) | Using Donor Stem Cells and Alemtuzumab to Prevent Organ Rejection in Kidney Transplant Patients | 9 | |
| 179 | NCT00284934 | Novartis | Enteric-coated Mycophenolate Sodium (EC-MPS) With Reduced-Dose Tacrolimus Versus EC-MPS With Standard-Dose Tacrolimus in Stable Kidney Transplant Recipients (OLYMPE) | 94 | |
| 180 | NCT00369278 | Novartis Pharmaceuticals | Intensified vs. Standard Dose Therapy With Mycophenolate Sodium Plus Cyclosporin Microemulsion and Corticosteroid Combination in Patients With de Novo Renal Transplant Patients | 128 | |
| 181 | NCT00419926 | Novartis | Evaluation of the Therapeutic Benefit of an Initial Intensified Dosing Regimen of Mycophenolate Sodium Versus a Standard Regimen in Renal Transplant Patients | 313 | |
| 182 | NCT00812123 | University Hospital, Basel, Switzerland, Pfizer (formerly Wyeth) | Calcineurin Free Immunosuppression in Renal Transplant Recipients | 127 | |
| 183 | NCT00154310 | Novartis | Efficacy and Safety of Everolimus With Enteric-Coated Mycophenolate Sodium (EC-MPS) in a Cyclosporine Microemulsion-free Regimen Compared with Standard Therapy in de Novo Renal Transplant Patients | 300 | |
| 184 | NCT00170846 | Novartis Pharmaceuticals | ASCERTAIN: Assessment of Everolimus in Addition to Calcineurin Inhibitor Reduction in the Maintenance of Renal Transplant Recipients | 394 | |
| 185 | NCT00425308 | Novartis Pharmaceuticals | Efficacy and Safety of Everolimus in Combination With Cyclosporine Microemulsion Versus Everolimus in Combination With Enteric-coated Mycophenolate Sodium (EC-MPS), in Adult Renal Transplant Patients in Maintenance | 30 | |
| 186 | NCT01064791 | Novartis Pharmaceuticals | Efficacy, Safety, Tolerability, and Pharmacokinetics of Sotrastaurin Combined With Tacrolimus vs. a Mycophenolic Acid-Tacrolimus Regimen in Renal Transplant Patients | 298 | |
| 187 | NCT00149903 | Novartis Pharmaceuticals | Study of Enteric-coated Mycophenolate Sodium Versus Mycophenolate Mofetil in Adult de Novo Renal Transplant Patients | 300 | |
| 188 | NCT00275535 | Mayo Clinic, Pfizer (formerly Wyeth), Genzyme, a Sanofi Company, Roche Pharma AG | The Comparison of Tacrolimus and Sirolimus Immunosuppression Based Drug Regimens in Kidney Transplant Recipients | 165 | |
| 189 | NCT00371826 | Novartis Pharmaceuticals | SOCRATES: Steroid or Cyclosporine Removal After Transplantation Using Everolimus | 126 | |
| 190 | NCT00239057 | Novartis | Study of Enteric-coated Mycophenolate Sodium Maintenance Therapy in Patients With Renal Transplant Receiving Cyclosporine Microemulsion and Steroids | 23 | |
| 191 | NCT00811915 | University Hospital, Rouen | Study to Compare the Safety and Efficacy of Sirolimus (Rapamune) to Tacrolimus (Advagraf) Associated to Mycophenolate Mofetil (CellCept) Between 12 and 36 Months After Kidney Transplantation (EPARGNE) | 65 | |
| 192 | NCT00461825 | Poitiers University Hospital | Maintenance Neoral Monotherapy Compared with Bitherapy in Renal Transplantation | 207 | |
| 193 | NCT01742624 | Astellas Pharma Korea, Inc. | Study to Evaluate the Safety and Efficacy of Advagraf vs. Prograf in Kidney Transplantation Patients 1 Month After the Transplantation (AdProCISE) | 60 | |
| 194 | NCT00200551 | Nantes University Hospital | A Study of Mycophenolate Mofetil and Cyclosporin, Without Concomitant Corticosteroids, After a First Renal Transplant | 200 | |
| 195 | NCT00483756Yes | Pfizer | Study of a JAK3 Inhibitor for the Prevention of Acute Rejection in Kidney Transplant Patients | 338 | |
| 196 | NCT00138970 | University of Oslo School of Pharmacy | Calcineurin Inhibitor-Free Immunosuppression in Renal Transplant Recipients at Low Immunogenic Risk | 70 | |
| 197 | NCT00912678 | University of Luebeck, Astellas Pharma GmbH | Minimizing Immunosuppression in Old for Old Kidney Transplantation (ESP-CNI) | 90 | |
| 198 | NCT00533624 | University of Miami, Novartis | Myfortic vs. Cellcept in Kidney Transplant Recipients | 150 | |
| 199 | NCT00413920 | Novartis | Efficacy and Safety of Enteric-coated Mycophenolate Sodium and Cyclosporine in Combination With and Without Steroids, in Adult Renal Transplant Recipients | 222 | |
| 200 | NCT01025817 CRAD001AUS92 |
Novartis Pharmaceuticals | Non-inferiority Study of Safety and Efficacy of Everolimus With Low Dose Tacrolimus to Mycophenolate Mofetil With Standard Dose Tacrolimus in Kidney Transplant Recipients | 613 | |
| 201 | NCT00650468 | Astellas Pharma Inc. | A Study to Compare Early Steroid Withdrawal and Long-Term Steroid Maintenance Therapy in Kidney Transplant Patients | 397 | |
| 202 | NCT00087581 | Hoffmann-La Roche | Study of Therapeutic Monitoring of CellCept (Mycophenolate Mofetil) After Kidney Transplantation | 717 | |
| 203 | NCT00374803 | University of Cincinnati, Novartis | Study of Myfortic in Combination With Tacrolimus and Thymoglobulin in Early Corticosteroid Withdrawal | 45 | |
| 204 | NCT00693381 | Astellas Pharma Inc | Mycophenolate Mofetil (MMF) Discontinuation From a Tacrolimus/MMF/Steroid Triple Regimen After Kidney Transplantation (DISTAMP) | 152 | |
| 205 | NCT00195273 | Pfizer (formerly Wyeth) | Study Evaluating Sirolimus in Kidney Transplant Recipients | 61 | |
| 206 | NCT00239083 | Novartis | Efficacy and Safety of Enteric-Coated Mycophenolate Sodium (EC-MPS) in Renal Transplant Patients | 40 | |
| 207 | NCT00885820 | Astellas Pharma Inc Astellas Pharma Canada, Inc. | Benefit of Early Protocol Biopsy and Treatment of Subclinical Rejection | 240 | |
| 208 | NCT00400647 | Novartis | Gastrointestinal and Health-related Quality of Life in Kidney Transplant Patients Treated With Mycophenolate Mofetil | 136 | |
| 209 | NCT00296361 | Astellas Pharma Inc. | To Compare the Efficacy and Safety of a Therapy of Tacrolimus With Sirolimus or MMF in Kidney Transplantation (RESTORE) | 634 | |
| 210 | NCT00238992 | Novartis Pharmaceuticals | Study of Enteric-coated Mycophenolate Sodium (EC-MPS) With Steroid Withdrawal vs. EC-MPS With Standard Steroid Regimen in de Novo Renal Transplant Recipients | 144 | |
| 211 | NCT00817687 | Hoffmann-La Roche | A Study of the Impact of an Early Biopsy in Patients Treated With CellCept (Mycophenolate Mofetil) After Kidney Transplantation | 66 | |
| 212 | NCT00321113 | Astellas Pharma Inc. | Comparison of Two Tacrolimus Based Immunosuppressive Regimens in Recipients Receiving Marginal Donor Kidneys (TIGRE) | 142 | |
| 213 | NCT00064701 | Astellas Pharma Inc. | Comparative Study of Modified Release (MR) Tacrolimus/Mycophenolate Mofetil (MMF) in de Novo Kidney Transplant Recipients | 668 | |
| 214 | NCT00788567 | Hoffmann-La Roche | CLEAR Study – A Study of CellCept (Mycophenolate Mofetil) in Recipients of Kidney Transplants | 136 | |
| 215 | NCT00182559 | Medical University of Vienna | The Vienna Prograf and Endothelial Progenitor Cell Study | 148 | |
| 216 | NCT00681213 | University of Miami, Wyeth-Ayesrst Pharmaceuticals, Roche Laboratories and Fujusawa Healthcare, Inc. | Tacrolimus/Sirolimus Versus Tacrolimus/Mycophenolate Mofetil (MMF) Versus Neoral/Sirolimus in Adult, Primary Kidney Transplantation | 150 | |
| 217 | NCT00166244(b) | Erasmus Medical Hoffmann-La Roche Center | Fixed Dose MMF vs. Concentration Controlled MMF After Renal Transplantation | 901 | |
| 218 | NCT00240955 | Novartis | Extension Study of Enteric-coated Mycophenolate Sodium With Short-term or No Steroid Use Compared With Enteric-coated Mycophenolate Sodium With Standard Steroid Therapy in de Novo Kidney Recipients | 79 | |
| 219 | NCT01706471 | Yonsei University | Safety and Efficacy of the Early Introduction of Everolimus (Certican®) With Low Dose of Cyclosporine in de Novo Kidney Recipients After 1 Month of Transplantation | 60 | |
| 220 | NCT00400400 | Novartis Pharmaceuticals | Enteric-Coated Mycophenolate Sodium (EC-MPS) and Mycophenolate Mofetil (MMF) in Renal Transplant Patients With Gastrointestinal (GI) Intolerance | 400 | |
| 221 | NCT00121810 | Hoffmann-La Roche | Kidney Spare the Nephron (STN) Study - A Study of CellCept (Mycophenolate Mofetil) and Rapamune (Sirolimus) in Kidney Transplant Recipients | 305 | |
| 222 | NCT00189839 | Astellas Pharma Inc. | A Study to Evaluate the Safety and Efficacy of FK506E (MR4) in Patients Undergoing Primary Kidney Transplantation | 699 | |
| 223 | NCT02005562 | Hoffmann-La Roche | OPERA Study: A Study of Two Dosing Regimens of CellCept (Mycophenolate Mofetil) in Kidney Transplant Patients | 263 | |
| 224 | NCT00758602 | Hoffmann-La Roche | A Study of CellCept (Mycophenolate Mofetil) Combined With Tacrolimus and Corticosteroids in Kidney Transplant Patients | 210 | |
| 225 | NCT00717678 | Astellas Pharma Taiwan, Inc. | A Randomized Study to Assess the Safety and Efficacy of Prograf vs. Prograf-XL in de Novo Kidney Transplant Recipients | 73 | |
| 226 | NCT00275522 | Mayo Clinic, Pfizer (formerly Wyeth) | The Comparison of Three Different Immunosuppressant Regimens in Kidney Transplant Recipients | 16 | |
| 227 | NCT00337493 | Hoffmann-La Roche | Pharmacogenetic Study of CellCept (Mycophenolate Mofetil) in Kidney Transplant Patients | 155 | |
| 228 | NCT00305396 | Vanderbilt University, Genzyme, a Sanofi Company | Calcineurin Inhibitor Avoidance With Thymoglobulin and Sirolimus in Kidney Transplantation | 80 | |
| 229 | NCT00187941 | University of Florida Hoffmann-La Roche | MPA PK Monitoring Strategy With MMF/FK Based Immunosuppression | 22 | |
| 230 | NCT01280617 | Lahey Clinic Brigham and Women’s Hospital | Low Dose Thymoglobin in Renal Transplant Patients | 58 | |
| 231 | NCT00777933 | Samsung Medical Center | Randomized Trial of Cyclosporine and Tacrolimus Therapy With Steroid Withdrawal in Living-Donor Renal Transplantation | 131 | |
| 232 | NCT01601821 | Pfizer | Open Label Comparative Study of de Novo Renal Allograft Recipients Receiving CSA + MMF + Corticosteroids versus CSA + Rapamune + Corticosteroids | 245 | |
| 233 | NCT00585468 | University of Utah | Pharmacokinetic Profile of Myfortic (Enteric Coated Mycophenolate Sodium) in a Rapid Steroid Withdrawal Protocol | 24 | |
| 234 | NCT01183247 | University Hospital, Basel, Switzerland Novartis | An Open, Single Centre, Randomised, Parallel Group Study to Investigate Three Different Immunosuppressive Regimens (SterFreePlus) | 63 | |
| 235 | NCT00248313 | Pfizer (formerly Wyeth) | Study Comparing Cyclosporin Dose Reduction With Cyclosporin Elimination in Kidney Transplant Recipients Taking Sirolimus | 470 | |
| 236 | NCT00170885 | Novartis | Everolimus in Combination With Cyclosporine Microemulsion in de Novo Renal Transplant Recipients | NR | |
| 237 | NCT00895583 | Pfizer | Study Evaluating A Planned Transition From Tacrolimus To Sirolimus In Kidney Transplant Recipients | 254 | |
| 238 | NCT00428064 | Pfizer (formerly Wyeth) | Study Evaluating Sirolimus and Cyclosporine in Kidney Transplant Recipients | 408 | |
| 239 | NCT00195429 | Pfizer (formerly Wyeth) | A Study Comparing the Withdrawal of Steroids or Tacrolimus in Kidney Transplant Recipients | 47 | |
| 240 | NCT00195468 | Pfizer (formerly Wyeth) | Study Comparing Cyclosporine Dose Reduction vs. Cyclosporine Elimination in Kidney Transplant Recipients Taking Sirolimus | 280 | |
| 241 | NCT00306397 | University Hospital, Basel, Switzerland | Pilot Study to Investigate a Steroid Free Immunosuppressive Regimen for Renal Transplant Recipients | 100 | |
| 242 | NCT01023815 | Novartis | Once-a-Day Regimen With Everolimus, Low Dose Cyclosporine and Steroids in Comparison With Steroid Withdrawal or Twice-a-Day Regimen With Everolimus, Low Dose Cyclosporine and Steroids. (EVIDENCE) | 184 | |
| 243 | NCT00518375 | Pfizer (formerly Wyeth) | Study Comparing Graft Function in Renal Allograft Recipients Receiving Reduced or Standard Dose CSA With Sirolimus | 250 | |
| 244 | NCT00309270 | Mario Negri Institute for Pharmacological Research | Low Dose Sirolimus or CSA-Based Maintenance Immunosuppression After Induction With Campath-1 in Kidney Transplantation | 21 | |
| 245 | NCT00507793 | Pfizer (formerly Wyeth) | Study Evaluating the Efficacy and Safety of Cyclosporine Reduction in Kidney Transplant Recipients Receiving Sirolimus | 385 | |
| 246 | NCT00519116 | Pfizer (formerly Wyeth) | Study Comparing Standard Dose and Reduced Dose Tacrolimus With Sirolimus in Renal Transplant Patients | 150 | |
| 247 | NCT00518271 | Pfizer (formerly Wyeth) | Study Comparing Standard Dose and Reduced Dose Tacrolimus + Sirolimus + Corticosteroids in Renal Allograft Recipients | 120 | |
| 248 | NCT00254709 | Pfizer (formerly Wyeth) | Study Evaluating Two Different Sirolimus-based Immunosuppressive Regimens in Elderly Kidney Transplant Recipients | 66 | |
| 249 | NCT00038948 | Pfizer (formerly Wyeth) | Study Comparing Conversion to Sirolimus vs. Continued Use of Calcineurin Inhibitors in Kidney Transplant Recipients | 830 | |
| 250 | NCT00470665 | Pfizer (formerly Wyeth) | Study Comparing Sirolimus/Prograf vs. Sirolimus/CSA in High-Risk Renal Transplant Recipients | 460 | |
| 251 | ISRCTN87678078 | Hospital Universitario de Canarias | Efficacy and Security of Low Toxicity Immunosuppressive Regimen Using Basiliximab, Mycophenolate Mofetil, Neoral or Tacrolimus and Corticosteroids versus Full Doses of Neoral, Thymoglobulin, Azathioprine and Corticosteroids | 240 | |
| 252 | ISRCTN94424606 | Leeds Teaching Hospitals NHS Trust (UK) | Steroid Avoidance in Leeds with Alemtuzumab or Mycophenolate Mofetil (MMF) Immunosuppression | 120 | |
| 253 | ISRCTN76390219 | University Hospitals of Leicester NHS Trust | A randomised controlled trial comparing the use of sirolimus based biphasic immunosuppression with myfortic to allow early Calcineurin Inhibitor (CNI) withdrawal in renal transplantation | 42 | |
| 254 | ISRCTN55817881 | Leiden University Medical Centre (LUMC) | Calcineurin-inhibitor Nephrotoxicity and Efficacy Study | 126 | |
| 255 | ISRCTN74429508 | University of Munich - Department of Surgery | A randomized multicenter trial to assess the efficacy of a combined therapy with Sirolimus (Rapamune®), MMF (Cellsept®) and corticosteroids after early elimination of cyclosporin compared with a standard immunosuppression with cyclosporin, MMF and corticosteroids in patients after kidney transplantation | 140 | |
| 256 | ISRCTN69188731 | Academic Medical Center (AMC), Renal Transplant Unit (The Netherlands) | Mycophenolate sodium versus Everolimus or Cyclosporine with Allograft Nephropathy as Outcome | 255 | |
Appendix 9 Detailed narrative review of cost-effectiveness evidence
Induction regimens
UK studies
Walters et al. 2003
In a multi-European country RCT, BAS induction was compared with PBO in patients who were given triple therapy with CSA, AZA and steroids. 316 Information on costs of immunosuppressant drugs, hospitalisations, procedures, outpatient visits, laboratory tests, renal biopsies, concomitant medications, dialysis and nephrectomy was prospectively collected for the trial follow-up period of 6 months. Retransplantation costs were not included. A CEA, conducted alongside the trial, included all costs up to 6 months and the costs of dialysis up to 12 months. This analysis adopted a NHS hospital perspective; it pooled the data on clinical outcomes and resource utilisation from all countries and patients involved in the trial (n = 340), but evaluated resource use using UK national and local unit costs (1997–99 prices).
Basiliximab was found to reduce the incidence of first confirmed AR episodes by 6 months (absolute risk reduction 0.14). The rate of graft failure with BAS was 11% and 18%, respectively, in the PBO arm (p = 0.24). The mortality rate was 2% and 3%, respectively (p = 1.00). In terms of the number of patients with AEs reported as serious, infections reported as serious, and AEs or infections reported as serious, the comparisons had p ≥ 0.65.
The distribution of costs in each trial arm was as presented in Figure 139. Hospitalisation costs were the largest element of total costs, followed by dialysis and AR.
Comparisons by resource-use category between arms had all p ≥ 0.05. Over the 6-month period post-transplantation BAS had an incremental cost of £231 (95% CI –£1983 to £2446). [Including the 6–12 months costs of dialysis, the BAS had an incremental total costs of –£30 (95% CI –£2326 to £2686.)] In the 6-month period post transplantation, the incremental cost per case of treatment failure (i.e. no AR, graft failure or death) avoided with BAS was £1650.
The authors conclude by stating that, despite the fears of increased AEs from overimmunosuppression, BAS given with triple therapy resulted in fewer ARs and no difference in costs relative to PBO in the first 6 months.
Critique
The study provides valuable evidence of data on resource use and short-term outcomes of induction therapy with BAS. For our present purposes, the main limitation of this study is the lack of relevant comparators such as induction with rATG. Further, as the authors point out, the use of these regimens in combination with triple-therapy immunosuppressive regimens commonly used in recent years, in particular a CNI with MMF and steroids, would have added relevance to the study.
The authors do not include the costs of retransplantation in their 1-year analysis, despite including the costs of dialysis. Inclusion of retransplantation costs incurred even within this study’s short time horizon (1 year) would have provided an indication of the rate at which the most relevant costs elements accrue for the present decision problem.
The study does not provide any evidence of the impact of induction on HRQoL. This prevented an adequate representation of the cost and benefits balance of BAS. In addition, an attempt to investigate the potential long-term implications of ARR prevention with BAS is warranted, using the framework linking biomarkers to longer-term patient and graft survival outcomes using a predictive model.
A major limitation of the study316 is the fact that the quantities of resource utilisation were derived from a sample of patients being treated in the UK and 11 other countries. The authors316 acknowledge that important differences may exist between these countries, as evidenced by the length of hospital stay, such that ‘whereas prevention of early episodes of AR may save a readmission in the US, this would not necessarily lead to an earlier hospital discharge following transplantation in some of the countries involved in this study (e.g. Israel, Poland, Turkey)’ (p. 136). This limits the validity of the results of this study, which was designed from an English NHS perspective.
Chilcott et al. 2002
A separate study315 of a similar design to that of Walters et al. ,316 described above, was conducted in centres from Canada and six European countries, including the UK. The study315 followed patients for 12 months and, unlike the study by Walters et al. ,315 valued resource utilisation using country-specific prices adjusted for PPP to reflect the actual opportunity costs of health-care resources in each country.
The study involved 376 patients (BAS, n = 190; PBO, n = 186) and, as Walters et al. 316 had found for 6-month post-transplantation outcomes, observed that BAS reduced the rate of (suspected) ARs (BAS 37%, PBO 54.8%; ARD –16.9%, 95% CI –29% to –4%), without affecting graft loss (ARD –1.3%, 95% CI –8.1% to 5.4%) and patient survival (ARD 2.0%, 95% CI –1.8% to 5.9%) at 12 months. The authors report that no retransplantations were recorded in any group over the 12-month post-transplantation period studied.
The report315 presents the results of statistical tests of differences in resource quantities used between the trial arms, which were all associated with p-values of > 0.05. The costs estimates were reported in terms of PPP US$ (1996 prices). Here we present the results of this report after converting them back to PPP (£) using the £0.4 = US$1 conversion rate provided by the report315 (table 1). The mean total per-patient cost was £19,174 in the BAS arm and £18,510 in the PBO arm (difference £664, 95% CI –£1660 to £2944). The incremental cost per suspected case of AR avoided at 12 months post transplantation was £3929.
Unlike the study by Walters et al. ,316 with which it shares many design features, the study by Chilcott et al. 315 presents total cost estimates for the subgroup of UK patients (n = 37) in the trial. (The report presents these figures only in chart form; see figure 4. 315) The total incremental cost of BAS over 12 months is approximately £3500. This implies an incremental cost per suspected case of AR avoided of £8284. Although the estimates for the UK subgroup are more susceptible to random sampling variation than those for the overall sample, these results, and those presented in figure 4 of the report,315 which compare results across country of origin patient subgroups, hint at heterogeneous findings across countries.
Critique
A similar critique applies to this report as that formulated above for the report by Walters et al. ,316 with a couple of qualifications. First, Chilcott et al. 315 present results for the subgroup of UK patients. Although these results are based on small numbers, they suggest possible heterogeneity of findings across countries, as the point estimate of incremental costs of BAS range from almost US$0 in Germany and France to US$3500 in the UK, to US$10,000 in Belgium and Switzerland (see figure 4315). A second strength of the Chilcott study315 relative to the that by Walters et al. 316 lies in its longer period of follow-up, during which information on all costs was collected 12 months post transplantation, compared with the 6-month period of the Walters et al. 316 study (the latter also included costs for a 6-month extension period, but only for dialysis).
Popat et al. 2014
A recent study317 reports evidence of costs and health outcomes associated with two immunosuppressive induction therapies given to recipients of renal transplants from DCD in a single centre in London. This was a before-and-after comparison of 1-year outcomes after transplantation, between a IL2Mab induction regimen (BAS or DAC) given to patients receiving a renal transplant from January 2007 to July 2008 and induction with ATG given to renal transplantation patients starting from the time of its adoption at the centre in August 2008 to August 2009.
The study included 24 patients in the old induction arm (IL2Mab 2 mg/kg) who had a mean age of 54.3 years compared with 48.0 years in the new (ATG 3.75 mg/kg) induction group of 21 patients. There was some imbalance in terms of sex and race, as 71% in the IL2Mab group were male, compared with 38% of those given ATG, and 62% of the former group were white, compared with 33% of the latter. Forty-two of 45 patients were given standard immunosuppression with CSA, MMF and prednisolone, and 3 out of 45 were given TAC, MMF and prednisolone.
At 1 year post transplantation, 91.7% of patients in the IL2Mab group were alive, whereas at 3 years 83.4% survived. In the ATG group all patients were alive at both time points. In terms of graft survival (censored by death), all patients in both groups had a functioning graft at 1 year, whereas 95.8% in the IL2Mab group had a functioning graft at 3 years, compared with 95.2% with ATG. The authors interpreted these results as evidence of no significant differences in patient and graft survival.
The study also looked at DGF, the duration of DGF measured by the number of HD sessions, the rate of BPAR, and incidence of infections requiring hospital admission. ATG resulted in 42.8% of patients having DGF, and 62.5% of patients treated with IL2Mab experienced such an outcome (p = 0.08). More patients in the latter group required HD sessions, experienced BPAR, had infections requiring admission, were readmitted and had experienced CMV infections than in the former group (p ≤ 0.03 of differences for all of these outcomes).
The study reported a cost analysis associated with observed outcomes up to 12 months post transplantation, using local NHS unit costs for hospital bed-day and HD sessions, and BNF drug prices for induction and maintenance immunosuppression applicable at the time that patients received the transplant.
Anti-thymocyte globulin was found to result in savings in inpatient bed-days post transplantation and those caused by readmissions, as well as HD costs and clinic visits, whereas the additional costs of ATG induction were not found to be statistically significant. It is unclear how this statistical test was performed, as the report presents the difference in the group total acquisition costs of immunosuppression therapy only between the two arms, which had different numbers of patients, rather than the correct corresponding total per-patient cost estimates. At 1 year, total per-patient costs were £18,929 and £14,904 in the IL2Mab and ATG arms, respectively (p = 0.002). The drivers of the cost savings by ATG were found in the inpatient bed-days after transplantation and clinic visits.
Critique
The main contribution of this study is to provide evidence on health and economic outcomes in a comparison of two active induction regimens. Owing to its small size, the results may be influenced by outliers, thus limiting the validity of the reported findings. In addition, lack of power is of concern for statistical inference of differences in health outcomes and more so for inference on costs, which tends to require larger samples than those required by studies of clinical effects. 328
The importance of clinic visits as a driver of total costs found in this study is consistent with evidence submitted to NICE by the company sponsoring one of the drugs being evaluated for this appraisal (Bristol-Myers Squibb), on post-transplantation costs in standard practice from the renal transplant database in Cardiff Wales. The same finding is analysed in an international context in a published report of the same evidence. 42
Further research is warranted to confirm the findings of the present study, in which induction regimens are given in combination with current triple therapy (i.e. low-dose TAC with MMF and steroids), involving larger samples, and collecting information on relevant outcomes not measured in this study, especially HRQoL outcomes.
Non-UK studies
Crompton et al. 2003
In a US study,312 54 living donor transplant recipients were randomised in a 1 : 1 ratio to receive BAS induction or no induction, and all were given triple immunosuppressive therapy with CSA ME, AZA and CCSs. At 12 months post transplantation, the rate of AR episodes in the induction intervention arm was 22%, compared with 15% in the control (p > 0.05). Differences between arms in serum creatinine measured at 1, 2, 3, 6 and 12 months all had p > 0.05, and no AEs were associated with BAS. Four graft losses occurred during follow-up, all in the intervention arm; only one was immunological.
The study312 evaluated differences in resource use using charges as opposed to economic costs of the resources consumed. Payments for readmissions were derived from DRG (diagnosis-related group) tariffs. Infections were assigned drug treatments costs, and the unit costs of drugs were derived from wholesale prices. Mean initial hospitalisation charges in prices of the year 2000 were US$68,094 in the intervention group versus US$51,970 in the control (p > 0.05). A lower frequency of readmissions was observed in the intervention arm (52%) than in the control arm (67%; p = 0.33), although admissions in the former were associated with a shorter length of stay (4.5 vs. 5.0; p > 0.05) than those occurring in the control. The average charge per readmission was US$21,953 compared with US$10,148, respectively (p > 0.05). The authors note that these differences in mean charges were influenced by an outlier who experienced steroid-resistant rejection.
The authors conclude that BAS did not provide clear clinical benefit or evidence of being cost-effective in this patient population. In discussing findings from previous studies, they note that lack of rejection rate reduction within the 12-month period of analysis explained their contradictory finding of lack of clinical and economic benefit.
Critique
This study investigates the clinical and economic benefit of BAS in a low-risk patient population (living-donor kidney recipients). The results appear to suggest that BAS may not be justified in this type of patient. However, as the authors recognise, an insufficient number of patients was included in the study to allow one to derive conclusive findings. They also note the susceptibility of their results to outliers.
This study offers limited value for informing NHS decisions as a result of the following caveats relating to its design: (1) BAS was tested in patients receiving triple-therapy immunosuppression that combined CSA with AZA and steroids, which reflects the practice from the time these patients received their transplant (period 1997–2000) – today regimens combining CNI, MMF and steroids are standard; (2) the small sample studied, as discussed; (3) the use of charges to approximate economic costs, as the former are likely to deviate from the latter due to hospital market power exercised through mark-ups in prices for their services; and (4) the omission of any measure of HRQoL effects.
Other studies with limited data
Another study313,330 investigated two regimens of BAS induction – (a) a CNI-free regimen (CSA 8 mg/kg daily was introduced as soon as the creatinine level reached a value of < 3 mg/dl) and (b) a CNI-minimisation regimen (CSA 4 mg/kg daily with MMF 500 mg/12 hours from day 1) – and compared them against a TAC (Prograf 0.3 mg/kg daily with a trough level of 8–12 ng/ml) with MMF (500 mg/12 hours) and steroids regimen in elderly patients.
Although the study was presented as a Markov model of costs and health benefits up to 1 year post transplantation, its two identified reports313,330 were in summary form, and provided no information on methodology related to model structure, source and values of unit costs and effectiveness parameters. Mean simulated results at 1 year were presented for eight patients in option (a), eight patients in option (b) and 15 patients in the TAC comparator arm, for CRC (39.6, 37.4 and 31.2 ml/minute/1.73 m2, respectively), the mean hospital stay, rate of rejection (12.5%, 12.5% and 13.2%), patient survival (100%, 100% and 93%), GRF, and cost difference relative to TAC arm [–€8355 for option (a); –€5695 for option (b)]. However, as these outcome measures or their constituent elements were not defined, their interpretation is too uncertain to warrant any further comment.
Critique
This quality of reporting of this study prevented its critical assessment. The most obvious limitations of this study are its short length of follow-up (1 year), the lack of measures of the patient HRQoL impact of the therapeutic options, and the very low patient numbers that are simulated (≤ 15 per arm), as a result of which we were unable to reliably estimate interarm cost differences.
Initial and maintenance immunosuppression studies
UK studies
Orme et al. 2003
Orme et al. 309 compared the costs and clinical outcomes of TAC (Prograf) versus CSA ME given in triple-therapy regimens including AZA and CCSs. At the time of the study the latter was the standard treatment in the UK. The study was based on data from the direct comparison of these regimens in a RCT conducted at a single centre in Wales, in which clinical and resource-use data were collected prospectively for each patient over a median follow-up of 2.7 years (maximum 4 years). Patients in the trial had undergone renal transplantation between 1996 and 2000 (CSA, n = 89; TAC arm, n = 90). The authors of the study state that the clinical results of that trial were comparable to those of other published studies of the two therapies at the time (before 2003).
The resource items for which data were recorded in the study included number of days in specialised wards (transplant/nephrology and intensive care unit during the initial admissions and subsequent readmissions), number of dialysis sessions required in cases of a DGF, number of diagnostic tests (e.g. transplant biopsy, ultrasound scan and other radiological investigations), and minor surgical procedures and operations for complications.
The use of medication was estimated based on daily dosages during the entire trial follow-up. The number of HD sessions and continuous ambulatory PD days observed as a result of graft failure were also measured, as were concomitant medications such as MMF, ATG, OKT3 and ganciclovir.
The economic evaluation adopted a 10-year analytical horizon and extrapolated the trial outcomes from 5 to 10 years using patient and graft survival data from the UK Transplant Support Service Authority Audit. During the extrapolated period, the rates of change in patient survival rates were assumed to be the same between the TAC and CSA immunosuppressant regimens (change from years 4 to 5 = –3 percentage points; and from years 5 to 10 = –3.4 percentage points). The same procedure was applied to the extrapolation of graft survival outcomes in the trial (change from years 4 to 5 = –3.5 percentage points; and from years 5 to 10 = –2.4 percentage points). The analysis also assumed that ARRs changed by the same rates as graft survival rates for the extrapolation phase of the analysis.
The per-patient costs for years 4–10 were extrapolated by the weighted average formula: per-patient costs in year = [pf × annual costs with functioning graft + (ps – pf) × annual costs with graft failure (dialysis)]/ps, where ‘pf’ is the proportion of patients with a function graft at the end of the year and ‘ps’ is the patient survival rate at the same time point. The annual costs with functioning graft and with graft failure were estimated from the trial data in the relevant patient subsamples.
Total costs reported by the study reflect unit costs collected by the local NHS Trust in Cardiff and corresponded to 1999 prices. Costs were discounted as the 6% annual rate and health outcomes at the 3.5% rate, in accordance with the NICE recommendations that were effective at the time of publication.
For the observed trial phase, ITT results were as follows. At 4 years, 89% of patients survived on TAC and 80% did on the CSA arm. In terms of graft survival, the figures were 81% and 71%. The proportion of patients who were rejection free was observed to decline annually for the first 4 years by 48, 5, 2, 1 percentage points with CSA, and by 37, 4, 1, 4 percentage points with TAC. In terms of costs, the observed per-patient costs in the first year post transplant were £9990 under TAC compared with £9783 under CSA. In the subsequently observed years 2–4, the TAC arm had lower per-patient costs – from £133 to £350 less – than the CSA arm due to the higher proportion of patients with a failed graft and receiving dialysis in the latter.
By the end of 10 years’ follow-up, the model predicted that the cumulative (discounted at 6%) costs would be £23,803 and £23,204 per patient under the TAC regimen and CSA regimen, respectively. In terms of clinical outcomes, the model predicted that 64% and 56% of patients receiving TAC and CSA would be alive, respectively, and that 61% of TAC-treated patients would survive with a functioning graft, compared with 52% under CSA.
The study presented results in terms of incremental cost per additional survivor, per extra patient with a functioning graft and per rejection-free patient. Although the number of years of life achieved after transplantation under each treatment was not presented, the Evidence Review Group approximated them by numerical integration using Newton–Cotes methods (Simpson’s rule) from the percentages of patients alive at the end of each of the 10 years of analysis reported by the study. This yielded an estimated 8.28 life-years under TAC and 7.61 life-years under CSA. The information provided in the paper also allows adjustment to be made to the cost discounting to convert results from the 6% annual rate used by the study to the current NICE-recommended rate of 3.5%. Similarly discounted life-years were approximated by applying the discount rate to the end-of-year survival rates provided by the study before applying the numerical method just described for undiscounted life-years. The resulting discounted incremental cost per life-year gained by TAC over CSA was £1457.
The authors found that the parameters that affected costs the most were the cost of hospitalisation per patient and the costs of immunosuppression per patient. The authors used trial information to account for uncertainty in these and health outcome parameters and performed PSA. In conclusion, TAC was found to be cost-effective, in terms of numbers of survivors, patients with functioning graft and rejection-free patients.
Critique
This study had detailed unit cost information reported, although quantities of resource utilisation were not provided, which limits the value of this study to other researchers who might be interested in replicating the analysis by applying their local prices or to generalise the results to England. As this is one of the studies with the longest prospective follow-ups of health-care use and health outcomes by patients, its value to research activity was also diminished by the lack of information on longitudinal results in terms of quantities of resource use and interpatient variability.
The study309 did not account for HRQoL effects of immunosuppression, which limited the value of this study to inform resource allocation decisions. The model does not consider the importance of outcomes in terms of renal function for costs and benefits. In particular, there is emerging evidence that not only does CKD stage matter for current costs and HRQoL experienced by the patient, but it also has an important role as a prognostic factor and determinant of graft survival. 333
The time horizon of the analysis may now be too short to estimate cost adequately, especially as the paper adopted a higher discount rate (6%) than that currently recommended by NICE (3.5%). This means that at a £1 of extra costs with TAC costs in 10 years post transplant are now worth £0.71 in present value terms, as opposed to £0.56 when discounting at the 6% rate.
Owing to the lack of reporting of an ICER in terms of life-year gained, we derived this from the information provided in the report and adjusted the discounting applied originally to obtain the ICER at the 3.5% discount rate currently recommended by NICE. This suggests that, in the sample studied by Orme et al.,309 TAC is well within the NICE threshold of cost-effectiveness. Although we did not adjust prices to current levels, in this sample of TAC versus CSA these are unlikely to raise the ICER per QALY gained beyond £5000.
Woodroffe et al. 2005
The Evidence Review Group at Birmingham reviewed the models submitted by sponsoring companies to the previous NICE appraisal process on the topic. Woodroffe et al. 65 reviewed and critically appraised the economic evaluation results from four models developed by the sponsoring companies. They then developed their own analysis based on their preferred model, based on the information in the industry submissions, and their own systematic review of the published evidence on effectiveness and cost-effectiveness. They chose to use one of the submitted models, the one developed by Novartis, to produce their analysis with some minor modifications. 65
The Novartis model simulated the experience of individual patients after renal transplantation, represented by transitions between five health states: AR, no AR, hospital dialysis, PD and death. A PTDM model component captured the effects on clinical outcomes of PTDM. (This allowed accounting for the clinical implications of the high incidence of PTDM with TAC that the company found in its systematic review.) The model accounted for cause-specific mortality risks from five comorbidities associated with diabetes mellitus or other causes (not specified). Costs were specific to each health state, allowing for different costs of dialysis (37.4% of which was ambulatory peritoneal), and severity of AR (steroid responsive vs. resistant; no details were given) and utilities distinguished between death (0), successful transplant (i.e. functioning graft, 0.84 utility) and dialysis (i.e. failed graft, 0.65 utility) states.
The Novartis model was driven by a model linking ARR to graft and patient survival outcomes, so that, conditional on the level of ARR (and PTDM rate), an immunosuppressant drug had no independent effects on those outcomes. The Birmingham group thus estimated a metamodel of the results of repeatedly running the Novartis model, each time with different input values for the rate of AR, and covering the range of values found in its own systematic review of the literature. They carried out a set of runs with the 1-year PTDM rate fixed at 14% and another set at 7%, to reflect the values differences in PTDM outcomes between TAC and other regimens. By fitting linear regressions to the QALY model outputs against the AR inputs, the metamodel for QALYs was estimated. This process was also conducted for costs, although this required carrying out separate sets of model runs for the different levels of monthly immunosuppression costs corresponding to the different regimens being evaluated.
A summary of the findings reported by the Birmingham group is presented in the tables of Chapter 5 in the main text. TAC was found to have incremental costs per QALY ratios in the range of £59,548 to £166,112 relative to CSA when evaluated as candidate components of triple therapy containing AZA and CCSs. Larger ICERs were found for the comparison in the context of triple therapy constituted by MMF and CCSs.
For the comparison of MMF with AZA, the ICER ranged from £39,297 to dominated when evaluated alongside TAC and CCSs, and from £52,166 to £109,549 as part of triple therapy containing CSA and steroids. The authors refer to these ranges as 95% CIs but, as these did not account for the variation in costs, they are likely to misrepresent uncertainty.
Critique of Birmingham analysis
The metamodel just described is an efficient way to derive measures of central tendency for costs and benefits in models that extrapolate short-term surrogate outcomes to long-term clinical health benefits and costs (Stevenson et al. 2004428). The difficulties encountered by the Birmingham group in implementing such a meta-model as described in its report65 prevented it from solving satisfactorily the problem that is common to patient simulation models with many parameters, namely that running them is costly – in the Novartis case, that means requiring several hours to run each time new values for ARR are adopted, which means that, at the number of patient simulations that in these models may be run in the available time, results vary from one run to another despite using identical parameter values and model specifications. They could obtain 95% CI for incremental QALYs but not for costs, and thus the degree of uncertainty in their results was left unaddressed.
A more fundamental problem arises, however, with the use of a model such as that of Novartis, which assumes that the main clinical outcomes, that is, years of the patient’s life and with a functioning graft gained, are adequately predicted by short-term ARRs and PTDM. In recent years, evidence has emerged suggesting that renal function is a predictor of clinically and economically significant outcomes, and that AR may be less relevant once CKD stage is accounted for. 333,334,338 At the time of the Birmingham review, the evidence was ambiguous about the prognostic predictive power of renal function relative to AR and, as they acknowledge, their analysis reflects this (Woodroffe et al. ,65 p. 52).
McEwan et al. 2005, 2006
A couple of papers by McEwan et al. 310,311 evaluate the cost–utility of SRL against CSA, and SRL against TAC, for maintenance immunosuppression, from the NHS perspective, using a discrete event simulation model of individual patient evolution from the time of kidney transplantation until 20 years post transplant. The authors justify their choice of analytical time horizon on the need to account for the longer-term implications of improved renal function on graft survival. In particular, they notice that the mean graft survival is > 12 years and argue that a ‘10-year horizon would fail to capture the majority of benefits that patients within the simulation would gain if extended graft survival is attained’. 311
The model represented a contribution to the literature at the time it was published because of its account of renal function as a predictor of transplant outcomes. The model simulated the evolution of a patient’s health status by transitions between mutually exclusive health states occurring in monthly cycles. Three health states were included in the model: (1) patient with a functioning graft; (2) patient with failed graft (dialysis); and (3) death. In addition, AR events were accounted for. The model allowed for retransplants and, as described below, different probabilities of experiencing an AR, patient death, graft failure and transplant after graft failure, depending on the number of transplants that the patient had received at each point in time. Movements between health states were associated with changes in costs and HRQoL, whereas the occurrence of transplant, graft failure, and ARs and graft failure were associated only with costs.
The effects of SRL and CSA on clinical outcomes were assumed to occur through their effects on renal function, which determined long-term clinical outcomes independently of treatment. The relative efficacy of SRL compared with CSA was derived from a single trial involving 430 patients from 57 centres in Europe, Canada and Australia (the Rapamune Maintenance Regimen Study, Oberbauer et al. 336). Patients included in this trial were given the same immunosuppression regimen (CSA + SRL + CCSs) for the first 3 months after transplantation and then randomised to continue on the regimen or switch to a regimen of once-daily SRL and steroids. Serum creatinine values in each trial arm at the time of randomisation, that is, 3 months post transplantation, and at 1, 2 and 3 years, were used as inputs (surrogate measures) in estimated equations for predicting the risk of long-term clinical events.
The serum creatinine outcomes from the Rapamune Maintenance Regimen Study336 used in the model as drivers of differences in effectiveness between immunosuppression regimens are illustrated in Figure 140. The authors also assumed that for 50% of subjects treated with SRL, graft survival ‘would prevail for the entire time horizon’. 310 The authors further state that ‘supporting evidence for this assertion was the increasing difference and the stability of mean serum creatinine in these subjects within a clinical trial’, referring to the data in Figure 140.
The surrogate relationship between renal function and clinical events defining transitions between health states in the model was estimated from analysis of longitudinal data on outcomes experienced by 937 transplant patients up to 20 years post transplantation in routine practice, recorded at the University Hospital of Wales, Cardiff. The patients were treated over the period 1982–2001, during most of which time CSA was the standard immunosuppressant therapy. 310 Baseline survival curves and Cox proportional hazards (predictive) models were fitted to individual data on time to AR and time to graft failure from transplant, time from graft failure to retransplant, and time from first transplant to death. Separate estimates were obtained from the estimated Cox proportional hazard models for the additional risk (HR) of graft failure in the first year, years 1–2, years 2–3 and year 3 onwards post transplant for the number of transplants and creatinine value (respectively, at 3 months, 1 year, 2 years and 3 years post transplant). Similarly, separate estimates of the HR of death for the different periods after first transplant were made as a function of age at first transplant, diabetes mellitus and creatinine values. The graft failure HRs for one extra previous transplant varied from 7.11 (95% CI 5.53 to 9.14) in the first year to 5.44 (95% CI 3.71 to 7.97) in years 3+ post transplant. The HR for a 100-mol/l increase in creatinine increased monotonically over time from 1.36 (95% CI 1.29 to 1.44) in the first year to 3.74 (95% CI 3.54 to 3.96) in years 3+ post transplant. Diabetes mellitus and age at first transplant had estimated constant HRs of death after first transplant of 2.81 (95% CI 1.88 to 4.19) and 1.05 (95% CI 1.04 to 1.06), whereas the HR for creatinine increased after the second year post transplant.
Costs
The costs of SRL daily doses included an initial 3-month period at 2 mg, 6 mg for months 4–12 and 4 mg thereafter. The costs of CSA included daily doses of 6 mg/kg for the first 6 months and 4 mg/kg thereafter. The costs of AZA and prednisolone in standard doses were added to these regimens. Costs for other drugs included prophylaxis regimens given for the first 6 months to CMV-positive and CMV-negative patients at baseline, as well as antihypertensive medication and cardiovascular treatment with blood pressure therapy and lipid-lowering drugs for all patients. The cost of treatment for anaemia and bone disease was assigned to simulated patients who reached creatinine values of ≥ 300 µmol/l. The model also included the costs of treating AR events, for which a 2-day hospital stay was assumed to occur by expert opinion, and the costs of retransplants, graft loss and HD. Drug-use quantities were valued at BNF prices. Unit costs of procedures were derived from NHS reference costs, apart from the hospital charge for AR, which was derived from local hospital costs at the University Hospital of Wales.
Utilities
A utility value difference of 0.27 was used by this study to account for differences in HRQoL between patients with a functioning graft and those with a failed graft and on dialysis. No account was made of any effects of clinical events on HRQoL. 311
Results
The authors found that SRL regimen would cost the NHS £62,120 per patient over 20 years, whereas CSA would cost £7405 more, that is, £69,525 (at 2003 prices and 6.5% annual discount rate). SRL was found to result in more discounted years with a functioning graft and in 0.16 additional discounted life-years per patient; it also resulted in more QALYs than those achieved with CSA (no figures were reported). Extending the horizon of analysis from 10 to 20 years increased savings achieved by SRL 26 times (from £276 to £7405) and discounted life-years gained twofold (from 0.06 to 0.16). These results were most sensitive to the critical assumption that 50% of SRL patients would maintain their graft survival over the entire modelled period. When 0% of patients had their ‘graft survival prevail for the entire horizon’310 or ‘graft survival sustained over the full time horizon of the model’311 the incremental cost per QALY gained by SRL was 51,778 under the 10-year horizon and £11,161 under the 20-year horizon.
The same analysis was performed for the comparison of SRL with TAC. 311 In implementing this analysis, the authors assumed that ‘as TAC and CSA are equivalent in terms of renal function, the creatinine levels observed in patients receiving CSA in the Rapamune Maintenance Regimen Study trial336 were used as proxies for creatinine levels in patients receiving TAC within the model. To better reflect outcomes in normal clinical practice in the UK, the creatinine profile from the Cardiff cohort (i.e. the sample used to estimate the patient and graft survival equations as a function of creatinine surrogates) was also used as the basis for an alternative set of TAC analyses. The authors then used the BNF price of TAC as they had used the price for CSA in the analysis previously described. The results were qualitatively similar with SRL both saving costs and producing health benefits relative to TAC.
Critique of the model
The model strength lies in its account for the effect of renal function on long-term outcomes. Moreover, the model derives probability of clinical events from observational data of patients treated in routine practice and distinguishes the temporal variation in the effect of risk factors for those events over a 20-year period.
As for its weaknesses, the study310,311 does not account for the incidence of clinical conditions, such as malignancy, cardiovascular events and NODAT. This is an important limitation in the light of the expected benefits of SRL on malignancy.
Most important, however, are the safety concerns associated with the drug. A recent systematic review of RCTs comparing SRL-containing regimens with other immunosuppressants found that although SRL reduced the incidence of malignancy it also increased the risk of mortality, which led researchers to conclude that use of the treatment may not be justified in kidney transplant patients other than those at high risk of cancer. 337
Although the study310,311 accounts for the role of renal function as a predictor of long-term outcomes, it does not allow for its impact on costs and HRQoL. For example, recent evidence suggests that CKD stage is significantly associated with costs42 and HRQoL. 338
The analysis relies on a single trial of SRL versus CSA which started 3 months after renal transplantation, following an initial immunosuppression regimen that combined SRL, CSA and steroids. In addition, the trial compared a regimen different from that represented in the costs analysis of the model: the trial compared SRL with CNI withdrawal after 3 months with SRL CNI minimisation, yet the cost analysis in the model included costs of AZA for both regimens. 310,311
In addition to the strengths and flaws just described, the analysis comparing SRL with TAC suffered from two problems. First, it assumed that TAC and CSA ‘are equivalent in terms of renal function’, citing three sources,244,429,430 despite having acknowledged in the introduction that the results of these studies had been contradicted by other studies favouring one or the other regimen. 180,327,431 In fact, the authors acknowledge that ‘the main advantage of tacrolimus over cyclosporine is that it is associated with a reduction in the incidence and severity of AR’,311 yet the analysis does not account for this difference as it uses data from the Cardiff cohort that includes primarily CSA-treated patients (TAC had only ‘become available until very recently’ in the period covered by the McEwan et al. 311 cohort) to derive the probabilities of AR for the TAC arm of the model.
The second problem relates to the use of serum creatinine values from the Cardiff cohort to populate surrogate outcomes used in the model ‘to better reflect outcomes in normal practice in the UK’. This ignores the issue that the Cardiff cohort comprised, primarily, CSA patients, as just discussed.
In fact there is no information in the paper311 that permits the reader to discern the source of variation in outcomes between the TAC arm of the model and the results previously reported for CSA,310 as all parameter inputs related to health benefits in the model are the same for the TAC and CSA arms (for the analysis based on the Rapamune serum creatinine outcome data336).
Muduma et al. 2014
In a recent study306,308–311,313,314,316,318,320,324,325 the current UK standard treatment for adults, twice-daily immediate-release TAC, Prograf, was compared with current options, namely CSA ME, SRL with CNI minimisation, SRL without CNI, BEL and 1-day TAC-PR, Advagraf, in terms of cost-effectiveness from the perspective of the NHS. The analysis considered each of these treatment options as part of a regimen that also included MMF and CCSs, and BAS induction (consisting, in the base case, of 20 mg 2 hours before surgery, and 20 mg 4 days after surgery; an alternative scenario considered additional doses during the first few days after transplantation). The study found that although Prograf resulted in more efficient use of health-care resources relative to CSA ME and BEL, it was not cost-effective relative to SRL. Although Advagraf produced lower costs and higher benefits than Prograf, its cost-effectiveness ratio against SRL (CNI minimisation regimen) was £58,350. These results were found to be sensitive to the time horizon and the effect of adherence.
Costs and health benefits were accumulated according to a Markov model of annual cycles that represented the evolution of the patient heath status following a successful transplant for up to 25 years. The model included four health states: (1) functioning graft without a history of BPAR; (2) functioning graft with a history of BPAR; (3) non-functioning graft; and (4) death. The occurrence of repeat transplantation was modelled using a tunnel state. The model assigned an excess risk of graft loss for the state of functioning graft with prior BPAR relative to the functioning graft without prior BPAR state, using estimates derived from the literature. The model was specified so that BPAR could occur only in the first year after transplantation, which the authors justified on pragmatic grounds given the limited data available from the literature on BPAR outcomes beyond 1 year.
Although the authors report that relative differences in ARRs between the treatment regimens under comparison were obtained from a systematic review of the effectiveness literature, the study did not report any information on the methods and results of that review (apart from stating that the review included studies published in the period January 2002 to June 2013 and that direct and indirect comparisons were used), the primary study sources for the probabilities of AR used, or the actual values used for these parameters. The treatment-specific outcome data reported related to the advantage of Advagraf over Prograf in terms of adherence to treatment schedule.
The remaining details relating to effectiveness parameter values applied equally to all regimens in the comparison: graft and patient survival for the first 5 years post transplantation were obtained from UK renal transplant summary statistics;13 survival parameters for the first 10 years after the start of the spell on dialysis were populated using UK data;432 the probabilities of retransplantation while in dialysis were obtained from data by McEwan et al. ,310 reviewed in this chapter. Exponential curves were used to extrapolate modelled graft and patient survival and survival on time on dialysis beyond the 5- and 10-year periods covered by their data sources.
The costs of immunosuppression regimens were based on the doses reported by a previous study239 (0.12, 0.10 and 0.08 mg/kg, respectively, at 1, 6 and 12 months for Prograf; 0.14, 0.11 and 0.09 mg/kg, respectively, for Advagraf). Mean daily doses for the other components of immunosuppressive triple therapy were based on BNF recommendations. The costs of dialysis (a weighted average of the costs of ambulatory peritoneal and HD), transplantation and AR, allowing for the excess costs of steroid-resistant BPAR including the costs of 4 days, hospitalisation (i.e. the NHS reference costs figure for uncomplicated AKI), completed the list of costs measured by this study. Utility values for the functioning graft state of 0.71 for HD and 0.44 for PD were obtained from a previous study. 358
Critique
Despite its stated aim to comply with the NICE reference case specifications, this study310,311 faced limitations in terms of the availability of data to do so, the adopted model structure, issues of model implementation, and the quality of reporting. The model assumed that the cost-effectiveness was driven by the differences in the rate of AR between-treatment regimens, and that these fundamental differences occurred only during the first year post transplant. The validity of this assumption and the results of this study hinge on the quality of the evidence on the relationship between AR and graft and patient survival in the study that estimated the empirical relationship supporting the present model. 354 In any case, it is difficult to defend extrapolating results from 1 year surrogate measures to clinical outcomes 25 years into the future, as this study310,311 has done with the statistical model of AR and graft survival.
Another problem with this report is its lack of any information on the values of the parameters driving the results, that is, the relative differences in the risk of AR between regimens. This fact makes it impossible to replicate the results reported by the paper.
Third, there are at least two problems with the way the model was populated or implemented. Although no information was given on the values and sources used to populate the efficacy parameter values, the information that is provided suggests that the amount of immunosuppressant use in the model might not have reflected the actual total use of the medications that brought about the AR outcomes that were used to populate the effectiveness model parameters. The authors do not report any attempt to derive mean daily drug use or dose intensity from the RCT data from which the AR estimates were derived for populating the model; the only statement in this regard is ‘Immunosuppression doses of Prograf and Advagraf were based on a study by Silva et al. For BEL, CSA and SRL, mean daily dosing was based on the latest version of the BNF16 as were the daily doses of the concomitant medications MMF and corticosteroids’. A separate issue was also identified in the statement that ‘The model employed an algorithm in which, for a given patient of a given age, the greatest probability of mortality was selected from the three possible mortality causes captured by the model: increased mortality with a functioning graft or dialysis, or the natural mortality of the general population’. In this regard it is difficult to think of a situation in which the general population of the same age (and presumably, sex) would face higher risks of death than the average patient on dialysis or a functioning graft. It would have been more natural instead to account for excess mortality risks for patients after renal transplantation over the background mortality risks, using registry data such as those utilised by the authors.
Another issue arises with the way transition probabilities were derived from the registry data on transplant and patient survival. As this issue is discussed for one of the industry submissions that used the same data and model, the reader is referred to that section (see Chapter 5, Astellas’ submission).
Non-UK studies
Three identified reports investigated the cost-effectiveness of SRL regimens, one in the USA308 and two in Germany. 306,307 Two studies evaluated TAC compared with CSA ME in European countries. 319,320 One study321 investigated once-daily TAC compared with twice-daily TAC in the USA.
Earnshaw et al. 2008
As in the UK studies by McEwan et al. ,310,311 this US study308 evaluated SRL + CCSs after CNI withdrawal but, in this case, it compared it against triple therapy of TAC or CSA combined with MMF and steroids. The cost–utility of these regimens over the lifetime of a 46-year-old first-transplant patient was investigated for the adult renal transplantation population in general and specific patient groups defined by donor type (living, deceased non-ECD, deceased ECD).
This study308 used a decision tree model for the first year post transplantation, followed by a Markov model of annual cycles of health-state transitions between four health states: functioning graft, functioning graft with AR, failed graft (dialysis while awaiting retransplant) and death. The model allowed for the occurrence of one retransplant, which was followed by a return to the first year post transplantation decision tree. The differences in long-term health outcomes between the regimens were driven by their relative efficacy in terms of AR and renal function, defined in terms of serum creatinine values. A model of long-term graft survival as a function of serum creatinine at 12 months was used to populate the transition probabilities of the Markov model phase.
The model implied that the assumption that no induction regimen was used. Estimates of first-year ARRs were obtained from the literature for the different regimens and used to populate the first-year model phase. These ARRs were adjusted to subtract the effect of variable use of induction immunoprophylaxis across trials of the different immunosuppressive regimens, based on efficacy estimates observed in clinical trials of the induction agents concerned. The authors acknowledge that the adjustments involved the simplifying assumption that the use of induction agents did not affect long-term clinical outcomes.
Differences in AR between regimens were assumed to last only until the first year post transplantation. The probability of AR in the first year of the Markov phase, year 2 post transplantation, was assumed to be the same across model arms and to decline linearly to a 0% probability over a 10-year time horizon. The model assumed the same rate of graft loss in the first year post transplant across treatment regimens. Graft loss in subsequent years was predicted by renal function as measured by creatinine levels at 12 months, based on the relationship estimated by Hariharan et al. 340 Means and SDs of serum creatinine reported in the trials for the different regimens at 12 months127,254,433 were used to derive the distribution of the patient cohort across serum creatinine categories at the start of the Markov phase of the model. The probability of graft survival was derived from half-life graft survival rates for the different serum creatinine groups and donor types and by assuming that graft survival followed an exponential curve, so that the probabilities of graft failure were constant over time within each regimen arm of the Markov model.
The annual probability of receiving a second transplant after graft failure was estimated from a median waiting time of 5.08 years, assuming an exponential curve of time to retransplantation from graft failure. This figure was based on US registry data from 1993 to 2003 (US Organ Procurement and Transplantation Network 2004434).
The model also accounted for the increased cumulative incidence of diabetes mellitus up to 3 years after transplantation with TAC-containing regimens (22.1% vs. 14.2% in the first year, 28.2% vs. 19.1% in the second year and 31.8 vs. 21.0% in the third year), assuming no further incidence of diabetes mellitus after year 3. This was associated with an annual medical cost of US$14,966 (in 2005 prices), based on a report by Woodward et al. ,395 and also with a RR of 1.46 for graft failure in the model. The model also accounted for the costs of increased triglyceride and cholesterol levels, as represented by the 12-month proportion of patients on statins in each model arm, which was assumed to remain constant until either graft loss or patient death (Earnshaw et al. ,308 p. 1813).
Different age-specific mortality rates were adopted according to organ type and for patients on dialysis. In addition, excess mortality risk with diabetes mellitus was accounted for by means of a RR of 1.87.
The costs of immunosuppressants were derived from the daily allowable consumption for each regimen from the Surveillance Data Inc. 435 data set of March 2005, valued at wholesale acquisition costs. The cost of patients on statins was derived from the prices for a generic medication. No additional detail was provided in terms of the unit costs used by the model, apart from stating that they were obtained mainly from a previous CEA,436 which reported Medicare-based health state costs from the USRDS.
Utility values only varied between the graft functioning state, which received a 0.84 utility, graft failure state, at 0.44, and death, valued at 0, based on time trade-off estimates from a study published in 1987. 437 The graft failure state was calculated by the weighted average HRQoL experienced by patients across dialysis types.
In common with the UK studies of SRL CNI withdrawal discussed in this review, Earnshaw et al. 308 found that a dual SRL plus steroids (CNI withdrawal) regimen was the dominant treatment regimen. Its use resulted in 0.30 extra years of life relative to TAC-containing triple therapy, and 0.06 extra years of life relative to triple therapy containing CSA. In terms of discounted (at 3% per annum) QALYs, the results were 0.30 and 0.12, respectively. SRL CNI withdrawal produced a cost savings of US$33,000 relative to TAC, and US$11,000 when compared against CSA. The same qualitative results were found for the subgroup analysis by donor type.
Critique
This study308 is different from other reports on the same topic in its attempt to provide evidence on cost-effectiveness across different donor types. In common with other studies evaluating SRL, it found the regimen to be cost-effective, in this case relative to current standard triple therapy containing a CNI.
Similar criticisms as those made above to the UK reports by McEwan et al. ,310,311 in relation to the current perception of SRL as having a restricted use due to issues about safety, may be applied to this study.
In terms of its methodology, this study308 used a model to predict long-term graft survival from 1-year renal function outcomes specific to the three regimens, accounting for graft survival differences between donor types. Although the use of renal function as driving clinical outcomes is supported by recent statistical evidence in samples of patients treated in routine practice,333 the model structure adopted by Earnshaw et al. 308 relies on a simplistic assumption of constant (instantaneous) probability (hazard rates) of graft failure over time, which more recent studies find to be inconsistent with the data. 334
In addition, the study does not account for the direct effects of renal function on costs and HRQoL. Thus, important differences between therapies might not have been captured with this model as patients accumulated time in the functioning graft state. 42,338
A technical issue was found in the way this study implemented the distribution of patients between serum creatinine categories at the start of the Markov phase (1 year post transplantation). As the authors assumed that serum creatinine was normally distributed and the mean and SD values adopted for the TAC arms were, respectively, 1.20 and 1.40, the model implies that 19% of serum creatinine values would be ≤ 0. Therefore, the assumed distribution is likely to underestimate the proportion of patients found in the higher creatinine value categories at 12 months and, as a result of the role of serum creatinine in the prediction of graft survival, the amount of time patients were expected to live with a functioning graft in the TAC arm.
Jurgensen et al. 2010, 2014
A couple of reports306,307 present the results of a Markov model representing the transition across health states experienced by patients after renal transplantation in Germany. The model compares SRL CSA avoidance with SRL CSA minimisation and low-dose TAC triple therapy with MMF and steroids. The latter was included in acknowledgement of the changes in immunosuppressant treatment practice following the publication of results from the SYMPHONY trials. The analysis was conducted from the perspective of the German statutory health insurance.
The model is designed as monthly cycles across five health states: functioning graft, AR, graft failure, dialysis (waiting on retransplant) and death. The time horizon of the analysis was 10 years post transplantation, and long-term survival outcomes were assumed to be driven by 2-year differences in the rate of AR between model arms estimated from direct and indirect comparisons of RCT outcome data.
One of the strengths of this analysis is its attempt to derive comparative evidence for the effects of the different regimens from evidence synthesis based on indirect comparisons, through NMA. The study306,307 provides details account of the probability of AEs including graft failure, malignancies, CMV infections, PTDM, wound-healing disorders and post-transplant anaemia, HMGCoA and hypertension treatments. The evidence synthesis reported by this study306,307 was used by one of the companies to populate the parameters of its model (see Abecassis et al. 2008, below – the section on the Astellas model).
The study found that low-dose TAC in triple therapy with MMF and steroids has a cost per life-year gained in excess of €100,000, relative to the SRL CSA minimisation regimen. All other comparators were found to be irrelevant for identifying the cost-effective treatment option, as they were dominated by these two regimens.
Critique
The study306,307 provides valuable new evidence about the cost-effectiveness of low-dose TAC regimens currently favoured by current practice, which has emerged following the publication of the SYMPHONY trial results. However, the value of this study from an English NHS decision-making point of view is diminished by the choice of comparators, which excludes CSA-based triple therapy and other new treatments such as BEL.
The study306,307 also has limited information use for informing NICE recommendations, as it did not account for HRQoL outcomes. The model itself is not amenable to account for available evidence on important HRQoL and costs effects associated with the effects of immunosuppressive regimens on renal function, as the renal function plays no role in the health status of patients in the model.
Abecassis et al. 2008
This study, co-authored by an affiliate of Astellas Pharma US, modelled the expected costs and clinical outcomes of once-daily TAC-PR and twice-daily immediate-release TAC, each given in combination with MMF, for transplant recipients in the USA. A stochastic state-transition Markov model extending 5 years post transplantation was used for that purpose. The model was used to predict the amount of time patients were alive with a functioning graft, receiving dialysis as a result of graft failure or dead.
The baseline for this model comprised the rate of adherence, incidence of ARs and graft loss up to 5 years post transplantation in the twice-daily TAC arm of the FK506 trial reported by Vincenti et al. 326 To project the effect of TAC once-daily relative to the baseline, the improved adherence with once-daily relative to twice-daily immunosuppressant regimens438 was combined with estimates of effect of non-adherence on graft survival from a systematic review. 9
The source of values for other parameters determining clinical events in the model (i.e. incidence of late AR requiring antibody treatment with once-daily TAC, reduction of 5-year graft survival for retransplanted organs relative to original graft) was not reported. Moreover, the information reported was insufficient to allow the reader to replicate the reported findings. For example, values of patient survival rates under dialysis were not reported or could not be calculated from the reported information. HRQoL outcomes were not accounted for by the model.
Immunosuppressant drug use and the resource utilisation parameters associated with the occurrence of clinical events were populated with cost data for medical procedures and hospitalisations from Medicare and the USRDS, and Medicare Average Sales Price year 2006 prices for drugs. The analysis included the costs of TAC and MMF immunosuppressant drugs, retransplantations, antibody rejection treatment, dialysis, graft loss costs other than dialysis, and mortality costs. The analysis applied the same price per milligram to both regimens for estimating their costs.
The study report 5-year predicted survival rate with once-daily TAC of 69.1%, as opposed to the estimated rate of 63.0% with TAC twice daily. The amount of time spent alive with a functioning graft was predicted to be 51.6 months and 50.3 months, respectively; the time spent in dialysis (with a failed graft) was 2.8 and 3.9 months, respectively. The total time alive was the sum of time with a functioning graft and time on dialysis (i.e. 54.4 months for once-daily TAC and 54.2 months for twice-daily TAC).
Once-daily TAC generated discounted (5% annually) total costs per patient of US$228,734. This amount US$9,411 less than the corresponding costs of twice-daily TAC – US$238,144. The authors report that ‘sensitivity analysis were conducted around key model inputs’ and that ‘throughout all sensitivities tested, once-daily extended release TAC remained dominant in terms of cost-effectiveness’, but they provide no other information on these analyses.
Critique
The low quality of reporting in this article prevents the assessment of its validity. The sources of values for some model parameters or the methods used to identify them were not reported. Moreover, the values of some parameters were not provided, preventing the replication of results by the reader.
In terms of the patient population, this article stated that the 5-year trial outcomes reported by Vincenti et al. 326 served as the baseline. It is unclear whether event costs, which were derived from an older population (Medicare), would correspond to the baseline population.
The authors do not discuss their results or their implications for routine patient management. An explanation for some of their findings seems warranted. In particular, the figures produced imply that, over 5 years, the benefits of improved adherence with once-daily TAC are manifested primarily in terms of quality of life (i.e. 1.3 extra months with a functioning graft), as the patient life expectancy is improved by only 0.2 months. Moreover, by the end of the analytical time horizon, 5 years post transplantation, the graft survival curves of the two regimens show a continuing diverging trend that had started at 1 year after transplantation. This suggests that the time horizon of the analysis may be insufficient to capture relevant clinical events, including presumably those relating to patient survival.
The analysis did not account for uncertainty in model parameters. This is a serious limitation owing to the small differences in graft and patient survival outcomes between the regimens, and more so for estimating their corresponding QALY difference.
Other studies not meeting the inclusion criteria
Levy et al. 334 developed a Markov model to extrapolate short-term trial outcomes (at 36 months) to 20 years, using transition probabilities across health states defined by eGFR ranges. These probabilities were estimated from Weibull time to event models of graft and patient survival for each initial (3 year) eGFR category separately estimated from USRDS data; exponential models were also estimates for time to death and time to retransplantation following graft failure, and for graft failure and death after retransplantation using the same data. The effect of NODAT model on graft and patient survival was accounted for separately on the basis of excess risk parameter using values from the literature, as data on NODAT were not available from ESRDS. The estimated graft survival and patient survival were calibrated by comparing the model-predicted survival to Kaplan–Meier survival curves fitted to USRDS data over the first 5 years. The model estimated the time patients spent in the functioning health states by assuming a constant linear decrease in eGFR until graft failure (eGFR < 15 ml/minute/1.73 m2) from the initial, 36-month eGFR level. Utility weights derived from the literature were then assigned to health states to estimate expected QALYs over a 20-year horizon for each initial eGFR state; the results were then aggregated by applying weights corresponding to the distribution across eGFR categories at 36 months. The authors illustrated their model application using 3-year follow-up data from the BENEFIT59 trial.
As the authors claim, Levy et al. 334 provide a valuable framework to translate trial outcomes for any immunosuppressive regimen to the long term. In principle this would allow us to subject all analyses to a common model that was based on observational data from current US practice. On the other hand, however, the model reflects the experience of a representative sample from the Medicare population in the USA, practically all of whom are ≥ 65 years old. This raises questions about the applicability of this model’s outcomes to the UK and other patient populations with access to greater coverage of immunosuppressive therapy439 and younger patient populations.
Motivated by the observed lack of gains in graft survival over recent years, Barnieh et al. 335 provide evidence of increasing costs of maintenance immunosuppression over time in a single Canadian centre. The study335 analysed the change of costs of immunosuppression for first adult kidney-only transplant patients between the periods of 1998–2001 and 2002–6, which were divided by change from CSA to TAC as the CSA agent of standard choice in triple therapy with antimetabolite (MMF) and steroid (prednisone) immunosuppression regimen and from non-routine use of BAS to the routine use of DAC for induction immunosuppression. Direct costs including medications, laboratory tests, pretransplant diagnostic imaging, outpatient services (day surgery, ambulatory care and emergency department visits), diagnostic imaging, hospitalisations, and physician services incurred for these patients, during transplant admission and up to year 3 after transplant were measured. These were economic costs paid by the provincial government (the sole funder of hospital and physician services for hospitalised patients) expressed in constant prices of a single year.
Before-and-after differences in health outcomes were not significant (i.e. before vs. after period, ARR 28% vs. 20%, p = 0.08; total graft failure, 89% vs. 92%, p = 0.73; mean survival over extended follow-up of 7 years, 6.1 years vs. 6.2 years, p = 0.57). On the other hand, MD in cumulative costs up to 3 years was CAN$45,011 (95% CI CAN$30,985 to CAN$59,037; p < 0.001) and was driven by the difference in immunosuppressant costs during the first year associated with the frequency of use of DAC induction therapy, which was more expensive than BAS induction therapy. Although DAC is no longer in use in Canada, the authors argue that results are relevant because of the use of other high-cost immunosuppressive agents in current general use. In addition, the study found differences in terms of outpatient services, which the authors suggest may have resulted from increased use of dialysis and increased use of day medicine facilities for infusions.
Chamberlain et al. 42 provide key evidence that direct health care costs vary with renal function using data from patient cohorts from nine European countries. Specifically, 3-year post-transplantation costs differ by GFR at 1 year post transplantation. Patients with GFR ≥ 60 ml/minute/1.73 m2 at 1 year had total costs that were 35% lower than those of patients with 30 ml/minute/1.73 m2 ≥ GFR ≥ 15 ml/minute/1.73 m2.
The study reported by Lazzaro et al. 320 and Craig et al. 319 compared the resource used, costs and health outcomes over 6 months post transplantation of patients randomised to receive TAC (n = 286) or CSA ME (n = 287), as part of triple immunosuppressive therapy with AZA and steroids. This was a multicountry trial, for which TAC was given at an initial daily dose of 0.3 mg/kg, whereas the starting dose of CSA ME was 8–10 mg/kg per day.
The study retrospectively measured resource-use quantities and costs of immunosuppressant drugs, concomitant medications, hospitalisation, dialysis, and rejection episodes from the 50 centres in seven Western European countries that participated in the trial. One report320 presents a CEA from the Italian hospital perspective, whereas a separate article undertakes the same analysis for Germany and Italy, and compares the results for the three countries, that is, of using country-specific unit costs in each of them to value total costs on the pooled trial data across all countries.
Patients in the study had an average age of 43 years, mean weight of 69 kg and 99% were classified as Caucasian. Thirty-eight (6.8%) of the 557 patients included in the trial had already had one (n = 37) or two (n = 1) previous transplants. These characteristics were balanced across trial arms.
The costs of immunosuppressants and treatment of AEs were based on hospital prices, which in the Italian analysis reportedly included a 50% discount on drug retail prices. 320 The costs of concomitant medication were based on the lowest generic price.
By the end of the 6-month post-transplantation period, the incidence of AR was 32.5% in the TAC arm and 51.3% in the CSA arm. The proportion of patients who switched to the alternative immunosuppressant regimens, as a result of treatment failure or AEs, was 2.8% and 19.0%, respectively. Differences in both patient (99.3% vs. 98.5%) and graft survival (94.8% vs. 91.9%) had p > 0.10.
Intention-to-treat analysis resulted in lower total per-patient costs with TAC than with CSA in all three countries. The per-patient cost savings achieved by TAC ranged from €1776 in Italy to €524 in Spain (figures in year 2000 prices). The authors attribute part of the variation to the higher cost of hospitalisation in Italy than in the other countries.
Most of the savings with TAC were as a result of fewer days in hospital for the initial stay and readmissions (Italian case 50%), lower costs of immunosuppressive medication for graft rejection (37%) and incidence of dialysis (13%). 320 According to the Italian perspective, €400 (12%) out of the €3200 per-patient costs of immunosuppressant therapy incurred by the TAC trial arm in the first 6 months post transplantation were caused by switching regimens as a result of treatment failure or AEs. The corresponding figures in the CSA arm were €1000 (37%) of €2700. 320
Critique
The length of follow-up in this study may have allowed it to capture differences in the terms of outcome measures that serve as surrogates for clinical outcomes, but was sufficient to capture important clinical events such as graft and patient survival. In addition, the study did not report any results in terms of changes in renal function, which has been observed to be associated with costs and HRQoL, as well as serving as a prognostic predictor of graft and patient survival.
In particular, the study may have failed to capture important AEs such as the incidence of PTDM, with which TAC immunosuppression has been found to be associated. The detailed report on the Italian case found that differences in costs were statistically insignificant (i.e. p > 0.05), suggesting that the overall reduction in costs may have been due to chance alone. In any case, in common with many economic and cost evaluations alongside randomised trials, the study may have been insufficiently powered to enable statistical inference on cost effects to be performed. 328
Therefore, the conclusion that ‘the overall costs of treating a patient with TAC during the 6-month post-transplantation period are substantially lower [than that for CSA ME]’ may not be warranted.
Appendix 10 Additional results from the Peninsula Technology Assessment Group’s economic model
Disaggregated discounted costs
| Regimen | Induction therapy (first graft) | Maintenance immunosuppress-ion (first graft) | AR (first graft) | Infection prophylaxis (first graft) | CMV infection (first graft) | Monitoring (first graft) | Retransplantation | |
|---|---|---|---|---|---|---|---|---|
| CSA + MMF | 0 | 15,970 | 989 | 761 | 313 | 16,112 | 4882 | |
| TAC + MMF | 0 | 14,884 | 867 | 761 | 313 | 16,365 | 4392 | |
| CSA + AZA | 0 | 13,519 | 1653 | 755 | 313 | 15,622 | 5454 | |
| TAC + AZA | 0 | 13,347 | 1149 | 751 | 313 | 16,099 | 4652 | |
| CSA + EVL | 0 | 96,482 | 965 | 762 | 107 | 18,891 | 4495 | |
| TAC + SRL | 0 | 34,841 | 842 | 751 | 107 | 17,977 | 5309 | |
| TAC-PR + MMF | 0 | 27,838 | 850 | 757 | 313 | 16,176 | 4499 | |
| BAS + CSA + MMF | 2188 | 16,558 | 582 | 764 | 313 | 16,466 | 4454 | |
| BAS + TAC + MMF | 2188 | 15,358 | 502 | 763 | 313 | 16,684 | 4010 | |
| BAS + CSA + AZA | 2188 | 14,143 | 1065 | 759 | 313 | 16,057 | 4925 | |
| BAS + SRL + MMF | 2188 | 35,557 | 544 | 757 | 150 | 16,283 | 4439 | |
| BAS + BEL + MMF | 2188 | 140,512 | 904 | 767 | 313 | 14,426 | 3838 | |
| BAS + CSA + MPS | 2188 | 35,617 | 809 | 766 | 313 | 16,744 | 4181 | |
| rATG + CSA + MMF | 4255 | 16,026 | 423 | 1,692 | 313 | 16,200 | 4847 | |
| rATG + TAC + MMF | 4255 | 14,914 | 363 | 1,691 | 313 | 16,442 | 4388 | |
| rATG + CSA + AZA | 4255 | 13,728 | 804 | 1,683 | 313 | 15,823 | 5284 | |
| Regimen | Immunosuppression (subsequent grafts) | Monitoring (subsequent grafts) | Dialysis | NODAT | Anaemia | Dyslipidaemia | Graft loss | Total |
| CSA + MMF | 2686 | 3686 | 49,145 | 1465 | 865 | 408 | 147 | 97,429 |
| TAC + MMF | 2403 | 3297 | 44,413 | 3113 | 877 | 407 | 133 | 92,226 |
| CSA + AZA | 3011 | 4132 | 54,264 | 1452 | 842 | 404 | 175 | 101,595 |
| TAC + AZA | 2567 | 3522 | 46,358 | 3113 | 871 | 407 | 169 | 93,319 |
| CSA + EVL | 2469 | 3388 | 45,572 | 1397 | 877 | 619 | 130 | 176,154 |
| TAC + SRL | 2908 | 3991 | 52,561 | 4623 | 838 | 608 | 183 | 125,539 |
| TAC-PR + MMF | 2463 | 3381 | 45,244 | 3592 | 868 | 404 | 144 | 106,529 |
| BAS + CSA + MMF | 2445 | 3355 | 45,195 | 1476 | 883 | 410 | 129 | 95,219 |
| BAS + TAC + MMF | 2189 | 3004 | 40,840 | 3134 | 893 | 409 | 117 | 90,405 |
| BAS + CSA + AZA | 2713 | 3723 | 49,469 | 1464 | 864 | 407 | 152 | 98,244 |
| BAS + SRL + MMF | 2441 | 3350 | 44,684 | 2518 | 876 | 617 | 145 | 114,549 |
| BAS + BEL + MMF | 2111 | 2897 | 39,350 | 658 | 917 | 418 | 110 | 209,409 |
| BAS + CSA + MPS | 2293 | 3146 | 42,660 | 1391 | 898 | 414 | 118 | 111,540 |
| rATG + CSA + MMF | 2657 | 3646 | 49,005 | 1467 | 867 | 408 | 134 | 101,940 |
| rATG + TAC + MMF | 2391 | 3282 | 44,581 | 3118 | 878 | 408 | 122 | 97,146 |
| rATG + CSA + AZA | 2907 | 3989 | 52,916 | 1456 | 849 | 405 | 156 | 104,570 |
Additional outcomes
| Regimen | Mean undiscounted life years (life expectancy) | Undiscounted life years with functioning graft | Undiscounted life years on dialysis | AR (%) | NODAT (%) | Proportion receiving | |
|---|---|---|---|---|---|---|---|
| Second transplant | Third transplant | ||||||
| CSA + MMF | 22.397 | 19.070 | 3.326 | 24.0 | 5.0 | 23.8 | 2.7 |
| TAC + MMF | 22.421 | 19.407 | 3.014 | 21.0 | 10.6 | 21.4 | 2.4 |
| CSA + AZA | 22.102 | 18.471 | 3.631 | 40.1 | 5.0 | 26.3 | 3.0 |
| TAC + AZA | 22.430 | 19.342 | 3.088 | 27.9 | 10.6 | 22.2 | 2.6 |
| CSA + EVL | 22.509 | 19.404 | 3.105 | 23.4 | 4.7 | 22.0 | 2.4 |
| TAC + SRL | 21.886 | 18.395 | 3.491 | 20.4 | 16.0 | 25.4 | 3.0 |
| TAC-PR + MMF | 22.248 | 19.198 | 3.051 | 20.6 | 12.3 | 21.8 | 2.5 |
| BAS + CSA + MMF | 22.636 | 19.554 | 3.082 | 14.1 | 5.0 | 21.8 | 2.4 |
| BAS + TAC + MMF | 22.640 | 19.850 | 2.790 | 12.2 | 10.6 | 19.6 | 2.2 |
| BAS + CSA + AZA | 22.380 | 19.041 | 3.339 | 25.8 | 5.0 | 23.9 | 2.7 |
| BAS + SRL + MMF | 22.448 | 19.434 | 3.014 | 13.2 | 8.6 | 21.4 | 2.4 |
| BAS + BEL + MMF | 23.206 | 20.502 | 2.704 | 21.9 | 2.2 | 18.8 | 2.1 |
| BAS + CSA + MPS | 22.877 | 19.953 | 2.923 | 19.6 | 4.7 | 20.5 | 2.3 |
| rATG + CSA + MMF | 22.403 | 19.065 | 3.338 | 10.3 | 5.0 | 23.8 | 2.6 |
| rATG + TAC + MMF | 22.432 | 19.385 | 3.046 | 8.8 | 10.6 | 21.6 | 2.4 |
| rATG + CSA + AZA | 22.178 | 18.609 | 3.570 | 19.5 | 5.0 | 25.7 | 2.9 |
Using Solver instead of flexible regression to match mortality at 12 months
| Regimen | Total discounted costs (£) | Total discounted QALYs | Net health benefit | |
|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | |||
| CSA + MMF | 97,441 | 10.9160 | 6.0440 | 7.6680 |
| TAC + MMF | 92,222 | 10.8879 | 6.2768 | 7.8138 |
| CSA + AZA | 101,607 | 10.7724 | 5.6921 | 7.3855 |
| TAC + AZA | 93,315 | 10.8692 | 6.2034 | 7.7586 |
| CSA + EVL | 176,148 | 10.9655 | 2.1581 | 5.0939 |
| TAC + SRL | 125,534 | 10.6018 | 4.3251 | 6.4173 |
| TAC-PR + MMF | 106,530 | 10.7920 | 5.4656 | 7.2411 |
| BAS + CSA + MMF | 95,230 | 11.0261 | 6.2646 | 7.8517 |
| BAS + TAC + MMF | 90,401 | 10.9875 | 6.4674 | 7.9741 |
| BAS + CSA + AZA | 98,254 | 10.9042 | 5.9915 | 7.6291 |
| BAS + SRL + MMF | 114,544 | 10.9005 | 5.1733 | 7.0824 |
| BAS + BEL + MMF | 209,510 | 11.2998 | 0.8244 | 4.3162 |
| BAS + CSA + MPS | 111,576 | 11.1417 | 5.5629 | 7.4225 |
| rATG + CSA + MMF | 101,959 | 10.9304 | 5.8325 | 7.5318 |
| rATG + TAC + MMF | 97,145 | 10.9045 | 6.0473 | 7.6664 |
| rATG + CSA + AZA | 104,590 | 10.8205 | 5.5910 | 7.3342 |
| Regimen | Total discounted costs (£) | Total discounted QALYs | ICER (cost per QALY) (£) | INHB | |
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| BAS + TAC + MMF | 90,401 | 10.9875 | – | – | – |
| BAS + CSA + MMF | 95,230 | 11.0261 | 125,110 | –0.2028 | –0.1224 |
| BAS + CSA + MPS | 111,576 | 11.1417 | 141,349 | –0.9045 | –0.5516 |
| BAS + BEL + MMF | 209,510 | 11.2998 | 619,299 | –5.6431 | –-3.6579 |
Removing disutility for NODAT
| Induction agent | Discounted costs (£) | Discounted QALYs | ICER (cost per QALY) (£) | INHB | |||
|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | ||
| With CSA + AZA | |||||||
| vs. BAS | |||||||
| No induction | 101,595 | – | 10.8127 | – | Dominated | –0.2998 | –0.2439 |
| rATG | 104,570 | 2975 | 10.8600 | 0.0472 | Dominated | –0.4013 | –0.2959 |
| BAS | 98,244 | –6326 | 10.9450 | 0.0850 | – | – | – |
| With CSA + MMF | |||||||
| vs. BAS | |||||||
| No induction | 97,429 | – | 10.9566 | – | Dominated | –0.2210 | –0.1841 |
| rATG | 101,940 | 4511 | 10.9702 | 0.0136 | Dominated | –0.4329 | –0.3209 |
| BAS | 95,219 | –6720 | 11.0671 | 0.0969 | – | – | – |
| With TAC + MMF | |||||||
| vs. BAS | |||||||
| No induction | 92,226 | – | 10.9778 | – | Dominated | –0.1912 | –0.1609 |
| rATG | 97,146 | 4920 | 10.9942 | 0.0165 | Dominated | –0.4208 | –0.3084 |
| BAS | 90,405 | –6741 | 11.0779 | 0.0837 | – | – | – |
| Maintenance agent | Discounted costs (£) | Discounted QALYs | ICER (cost per QALY) (£) | INHB | |||
|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | ||
| With MMF | |||||||
| vs. TAC | |||||||
| TAC-PR | 106,529 | – | 10.8952 | – | Dominated | –0.7978 | –0.5594 |
| CSA | 97,429 | –9100 | 10.9566 | 0.0614 | Dominated | –0.2813 | –0.1946 |
| TAC | 92,226 | –5203 | 10.9778 | 0.0212 | – | – | – |
| With AZA | |||||||
| vs. TAC | |||||||
| CSA | 101,595 | – | 10.8127 | – | Dominated | –0.5601 | –0.4222 |
| TAC | 93,319 | –8276 | 10.9590 | 0.1463 | – | – | – |
| With BAS + MMF | |||||||
| vs. TAC | |||||||
| SRL | 114,549 | – | 10.9733 | – | Dominated | –1.3118 | –0.9094 |
| CSA | 95,219 | –19,329 | 11.0671 | 0.0938 | Dominated | –0.2516 | –0.1713 |
| TAC | 90,405 | –4815 | 11.0779 | 0.0109 | – | – | – |
| BEL | 209,409 | 119,004 | 11.3130 | 0.2350 | 506,309 | –5.7152 | –3.7318 |
| With rATG + MMF | |||||||
| vs. TAC | |||||||
| CSA | 101,940 | – | 10.9702 | – | Dominated | –0.2637 | –0.1838 |
| TAC | 97,146 | –4794 | 10.9942 | 0.0241 | – | – | – |
| With CSA | |||||||
| vs. MMF | |||||||
| AZA | 101,595 | – | 10.8127 | – | Dominated | –0.3522 | –0.2827 |
| MMF | 97,429 | –4166 | 10.9566 | 0.1439 | – | – | – |
| EVL | 176,154 | 78,725 | 11.0060 | 0.0494 | 1,593,185 | –3.8869 | –2.5748 |
| With TAC | |||||||
| vs. MMF | |||||||
| SRL | 125,539 | – | 10.7350 | – | Dominated | –1.9084 | –1.3532 |
| AZA | 93,319 | –32,220 | 10.9590 | 0.2240 | Dominated | –0.0734 | –0.0552 |
| MMF | 92,226 | –1093 | 10.9778 | 0.0188 | – | – | – |
| With BAS + CSA | |||||||
| vs. MMF | |||||||
| AZA | 98,244 | – | 10.9450 | – | Dominated | –0.2733 | –0.2229 |
| MMF | 95,219 | –3025 | 11.0671 | 0.1221 | – | – | – |
| MPS | 111,540 | 16,321 | 11.1776 | 0.1106 | 147,616 | –0.7055 | –0.4335 |
| With rATG + CSA | |||||||
| vs. MMF | |||||||
| AZA | 104,570 | – | 10.8600 | – | Dominated | –0.2417 | –0.1979 |
| MMF | 101,940 | –2631 | 10.9702 | 0.1102 | – | – | – |
Using 2007–12 donor type distribution
| Induction agent | Discounted costs (£) | Discounted QALYs | ICER (cost per QALY) (£) | INHB | |||
|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | ||
| With CSA + AZA | |||||||
| vs. BAS | |||||||
| No induction | 99,452 | – | 10.9491 | – | Dominated | –0.2958 | –0.2421 |
| rATG | 102,558 | 3106 | 10.9934 | 0.0443 | Dominated | –0.4068 | –0.3014 |
| BAS | 96,233 | –6325 | 11.0839 | 0.0905 | – | – | – |
| With CSA + MMF | |||||||
| vs. BAS | |||||||
| No induction | 95,517 | – | 11.0949 | – | Dominated | –0.2166 | –0.1818 |
| rATG | 100,114 | 4598 | 11.1051 | 0.0103 | Dominated | –0.4363 | –0.3248 |
| BAS | 93,428 | –6686 | 11.2071 | 0.1020 | – | – | – |
| With TAC + MMF | |||||||
| vs. BAS | |||||||
| No induction | 90,413 | – | 11.0735 | – | Dominated | –0.1853 | –0.1571 |
| rATG | 95,394 | 4980 | 11.0866 | 0.0131 | Dominated | –0.4212 | –0.3101 |
| BAS | 88,724 | –6670 | 11.1743 | 0.0877 | – | – | – |
| Maintenance agent | Discounted costs (£) | Discounted QALYs | ICER (cost per QALY) (£) | INHB | |||
|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | £20,000/QALY | £30,000/QALY | ||
| With MMF | |||||||
| vs. TAC | |||||||
| TAC-PR | 105,133 | – | 10.9784 | – | Dominated | –0.8311 | –0.5857 |
| TAC | 90,413 | –14,720 | 11.0735 | 0.0951 | – | – | – |
| CSA | 95,517 | 5103 | 11.0949 | 0.0214 | 238,659 | –0.2338 | –0.1487 |
| With AZA | |||||||
| vs. TAC | |||||||
| CSA | 99,452 | – | 10.9491 | – | Dominated | –0.5164 | –0.3802 |
| TAC | 91,278 | –8175 | 11.0568 | 0.1077 | – | – | – |
| With BAS + MMF | |||||||
| vs. TAC | |||||||
| SRL | 113,366 | – | 11.0876 | – | Dominated | –1.3188 | –0.9081 |
| TAC | 88,724 | –24,642 | 11.1743 | 0.0867 | – | – | – |
| CSA | 93,428 | 4704 | 11.2071 | 0.0328 | 143,420 | –0.2024 | –0.1240 |
| BEL | 211,416 | 117,987 | 11.4794 | 0.2723 | 433,299 | –5.8295 | –3.7846 |
| With rATG + MMF | |||||||
| vs. TAC | |||||||
| TAC | 95,394 | – | 11.0866 | – | – | – | – |
| CSA | 100,114 | 4721 | 11.1051 | 0.0186 | 253,976 | –0.2174 | –0.1388 |
| With CSA | |||||||
| vs. MMF | |||||||
| AZA | 99,452 | – | 10.9491 | – | Dominated | –0.3426 | –0.2770 |
| MMF | 95,517 | –3936 | 11.0949 | 0.1458 | – | – | – |
| EVL | 176,982 | 81,466 | 11.1500 | 0.0551 | 1,477,730 | –4.0182 | –2.6604 |
| With TAC | |||||||
| vs. MMF | |||||||
| SRL | 124,216 | – | 10.7817 | – | Dominated | –1.9819 | –1.4186 |
| AZA | 91,278 | –32,938 | 11.0568 | 0.2751 | Dominated | –0.0599 | –0.0455 |
| MMF | 90,413 | –864 | 11.0735 | 0.0167 | – | – | – |
| With BAS + CSA | |||||||
| vs. MMF | |||||||
| AZA | 96,233 | – | 11.0839 | – | Dominated | –0.2634 | –0.2167 |
| MMF | 93,428 | –2805 | 11.2071 | 0.1232 | – | – | – |
| MPS | 110,393 | 16,965 | 11.3211 | 0.1140 | 148,867 | –0.7343 | –0.4515 |
| With rATG + CSA | |||||||
| vs. MMF | |||||||
| AZA | 102,558 | – | 10.9934 | – | Dominated | –0.2339 | –0.1932 |
| MMF | 100,114 | –2444 | 11.1051 | 0.1118 | – | – | – |
Appendix 11 Summary of parameters in the Peninsula Technology Assessment Group’s economic model
| Parameter | Value | Source | PSA distribution |
|---|---|---|---|
| Study characteristics | |||
| Patient age (years) | 50 | Pruthi 2013382 | N/A |
| Patient weight (kg) | |||
| Mean | 70.18 | Multiple RCTs | Normal(70.18, 1.118) |
| SD | 14.79 | Multiple RCTs | N/A |
| Proportion male | 0.617 | UK Transplant Registry standard data set (2007–12) | N/A |
| Donor type (first graft) | UK Transplant Registry standard data set | ||
| DBD | 0.664 | N/A | |
| DCD | 0.079 | N/A | |
| Living–related | 0.191 | N/A | |
| Living–unrelated | 0.066 | N/A | |
| Donor type (subsequent graft) | UK Transplant Registry standard data set | ||
| DBD | 0.630 | N/A | |
| DCD | 0.083 | N/A | |
| Living–related | 0.198 | N/A | |
| Living–unrelated | 0.089 | N/A | |
| Surrogate relationships | |||
| Graft survival (censored for DWFG) | |||
| AR | 1.60 | Cole 2008378 | Log-normal(0.47, 0.037) |
| NODAT | 1.12 | Cole 2008378 | Log-normal(0.113, 0.061) |
| eGFR | Levy 2014334 | Multivariate Log-normal | |
| 45–60 ml/minute/1.73 m2 | 1.409 | ||
| 30–45 ml/minute/1.73 m2 | 2.406 | ||
| 15–30 ml/minute/1.73 m2 | 5.801 | ||
| DWFG | |||
| NODAT | 1.41 | Cole 2008378 | Log-normal(0.344, 0.061) |
| Sex: female | 0.865 | UK Transplant Registry standard data set | Log-normal(–0.145, 0.036) |
| Donor type (vs. DBD) | UK Transplant Registry standard data set | ||
| DCD | 1.083 | Log-normal(0.08, 0.061) | |
| Living–related | 0.551 | Log-normal(–0.595, 0.071) | |
| Living–unrelated | 0.703 | Log-normal(–0.353, 0.081) | |
| Age (years) | UK Transplant Registry standard data set | ||
| 0–17 | 0.377 | Log-normal(–0.975, 0.186) | |
| 18–30 | 0.369 | Log-normal(–0.996, 0.117) | |
| 31–40 | 0.712 | Log-normal(–0.339, 0.091) | |
| 41–50 | 1 | N/A | |
| 51–60 | 2.140 | Log-normal(0.761, 0.059) | |
| 61–70 | 4.128 | Log-normal(1.418, 0.053) | |
| 71–75 | 7.583 | Log-normal(2.026, 0.072) | |
| 76–80 | 8.576 | Log-normal(2.149, 0.089) | |
| 81–85 | 13.751 | Log-normal(2.621, 0.144) | |
| 86–90 | 23.552 | Log-normal(3.159, 0.362) | |
| Effectiveness estimates | |||
| Mortality within 12 months [ln(OR)] | |||
| Induction agents (vs. no induction) | NMA | Multivariate normal | |
| BAS | –0.117 | ||
| rATG | –0.461 | ||
| Maintenance agents (vs. CSA + AZA) | NMA | Multivariate normal | |
| TAC + AZA | 0.323 | ||
| CSA + MPA | –0.057 | ||
| TAC + MPA | 0.422 | ||
| BEL + MPA | –0.763 | ||
| CSA + EVL | 0.333 | ||
| TAC + SRL | 0.325 | ||
| SRL + MPA | 0.542 | ||
| Head to head | |||
| MPS vs. MMF | –0.435 | Random-effects meta-analysis of Ciancio 2008106 and Salvadori 2001270 | Normal(–0.435, 1.231) |
| TAC-PR vs. TAC | 0.245 | Krämer 2010204 | Normal(0.245, 0.481) |
| Graft loss within 12 months [ln(OR)] | |||
| Induction agents (vs. no induction) | NMA | Multivariate normal | |
| BAS | –0.171 | ||
| rATG | –0.253 | ||
| Maintenance agents (vs. CSA + AZA) | NMA | Multivariate normal | |
| TAC + AZA | 0.135 | ||
| CSA + MPA | –0.297 | ||
| TAC + MPA | –0.379 | ||
| BEL + MPA | –0.492 | ||
| CSA + EVL | –0.484 | ||
| TAC + SRL | 0.159 | ||
| SRL + MPA | 0.032 | ||
| Head to head | |||
| MPS vs. MMF | –0.148 | Fixed-effects meta-analysis of Ciancio 2008106 and Salvadori 2001270 | Normal(–0.148, 0.524) |
| TAC-PR vs. TAC | 0.183 | Krämer 2010204 | Normal(0.183, 0.29) |
| BPAR within 12 months [ln(OR)] | |||
| Induction agents (vs. no induction) | NMA | Multivariate normal | |
| BAS | –0.688 | ||
| rATG | –1.041 | ||
| Maintenance agents (vs. CSA + AZA) | NMA | Multivariate normal | |
| TAC + AZA | –0.548 | ||
| CSA + MPA | –0.752 | ||
| TAC + MPA | –0.921 | ||
| BEL + MPA | –0.216 | ||
| CSA + EVL | –0.784 | ||
| TAC + SRL | –0.957 | ||
| SRL + MPA | –0.828 | ||
| Head to head | |||
| MPS vs. MMF | 0.396 | Random-effects meta-analysis of Ciancio 2008106 and Salvadori 2001270 | Normal(0.396, 0.678) |
| TAC-PR vs. TAC | –0.025 | Random-effects meta-analysis of Krämer 2010204 and Tsuchiya 2013141 | Normal(–0.025, 0.383) |
| GRF (eGFR) at 12 months [MD (ml/minute/1.73 m2)] | |||
| Induction agents (vs. no induction) | NMA | Multivariate normal | |
| BAS | 2.615 | ||
| rATG | 0.752 | ||
| Maintenance agents (vs. CSA + AZA) | NMA | Multivariate normal | |
| TAC + AZA | 9.304 | ||
| CSA + MPA | 1.609 | ||
| TAC + MPA | 6.531 | ||
| BEL + MPA | 10.550 | ||
| CSA + EVL | 4.863 | ||
| TAC + SRL | –0.352 | ||
| SRL + MPA | 3.846 | ||
| Head to head | |||
| MPS vs. MMF | 3.900 | Ciancio 2008106 | Normal(0.396, 0.678) |
| TAC-PR vs. TAC | –0.211 | Fixed-effects meta-analysis of Krämer 2010204 and Tsuchiya 2013141 | Normal(–0.025, 0.383) |
| Baseline effectiveness (BAS + TAC + MMF) | |||
| Graft loss within 12 months | 0.035 | UK Transplant Registry standard data set | N/A |
| BPAR within 12 months | 0.122 | Rowshani 2006103 and Tsuchiya 2013141 | Beta(14, 101) |
| GRF (eGFR) at 12 months (ml/minute/1.73 m2) | Pruthi 2013382 | ||
| Mean | 53.4 | N/A | |
| SD | 18.5 | N/A | |
| AEs | |||
| NODAT within 12 months | |||
| Baseline (BAS + TAC + MMF) | 0.106 | ||
| Maintenance agents (vs. TAC) [ln(OR)] | NMA | Multivariate normal | |
| TAC-PR | 0.169 | ||
| CSA | –0.816 | ||
| BEL | –1.671 | ||
| SRL | –0.234 | ||
| Maintenance agents (vs. MMF) [ln(OR)] | NMA | Multivariate normal | |
| MPS | –0.070 | ||
| SRL | 0.474 | ||
| EVL | –0.052 | ||
| CMV infection within 12 months | |||
| Baseline (BAS + TAC + MMF) | 0.107 | Multiple RCTs | Logit-normal(–2.12, 0.94) |
| mTOR-I use (vs. no use) [ln(OR)] | NMA | Multivariate normal | |
| As CNI | –0.798 | ||
| As antimetabolite | –1.153 | ||
| Dyslipidaemia within 12 months | |||
| Baseline (BAS + TAC + MMF) | 0.202 | Multiple RCTs | Logit-normal(–1.376, 0.982) |
| mTOR-I use (vs. no use) [ln(OR)] | 0.557 | Fixed-effects meta-analysis | Normal(0.557, 0.101) |
| Anaemia requiring ESA therapy | 0.052 | Vanrenterghem 2003396 | Beta(207 3762) |
| Retransplantation | |||
| Probability of pre-emptive retransplantation on loss of first graft | Bond 2009384 and Johnston 2013386 | ||
| Aged 18–34 years | 0.108 | Beta(3.46, 28.58) | |
| Aged 35–44 years | 0.098 | Beta(3.51, 32.31) | |
| Aged 45–54 years | 0.076 | Beta(3.62, 44.01) | |
| Aged 55–64 years | 0.054 | Beta(3.73, 65.34) | |
| Aged 65+ years | 0.020 | Beta(3.9, 191.1) | |
| Rate of retransplantation | |||
| Aged < 65 years (rate declines linearly from age 65 to 80 years after which no retransplantation) | 0.104 | UK Transplant Registry standard data set | Normal(0.104, 0.0023) |
| Baseline rate of DWFG (subsequent grafts) | 0.0078 | UK Transplant Registry standard data set | Log-normal(–4.965, 0.472) |
| Baseline rate of graft loss (subsequent grafts) | 0.0359 | UK Transplant Registry standard data set | Log-normal(–3.327, 0.084) |
| Mortality | |||
| Rate of death on dialysis following graft loss (by age, years) | Pruthi 2013339 | ||
| 20–24 | 0.01 | Normal(0.01, 0.0032) | |
| 25–29 | 0.012 | Normal(0.018, 0.0042) | |
| 30–34 | 0.009 | Normal(0.018, 0.0042) | |
| 35–39 | 0.015 | Normal(0.043, 0.0066) | |
| 40–44 | 0.021 | Normal(0.089, 0.0094) | |
| 45–49 | 0.027 | Normal(0.141, 0.0119) | |
| 50–54 | 0.041 | Normal(0.226, 0.015) | |
| 55–59 | 0.053 | Normal(0.284, 0.0169) | |
| 60–64 | 0.079 | Normal(0.437, 0.0209) | |
| 65–69 | 0.107 | Normal(0.553, 0.0235) | |
| 70–74 | 0.149 | Normal(0.682, 0.0261) | |
| 75–79 | 0.211 | Normal(0.792, 0.0281) | |
| 80–84 | 0.275 | Normal(0.652, 0.0255) | |
| 85+ | 0.408 | Normal(0.452, 0.0213) | |
| Other natural history parameters | |||
| Probability of PNF | UK Transplant Registry standard data set | ||
| DBD | 0.026 | Beta(147 5489) | |
| DCD | 0.033 | Beta(99 2858) | |
| Living–related | 0.015 | Beta(53 3541) | |
| Living–unrelated | 0.012 | Beta(27 2149) | |
| Proportion of NODAT in first 6 months | 0.75 | Woodward 2003395 | Beta(75, 25) |
| Risk stratification for CMV infection | Harvala 2013409 | Dirichlet(52, 93, 79) | |
| High risk (D+/R–) | 0.232 | ||
| Intermediate risk (D+/R+ or D–/R+) | 0.415 | ||
| Low risk (D–/R–) | 0.353 | ||
| Risk stratification for EBV infection | Cavallo 2010411 | ||
| Seropositive donors | 0.927 | Beta(51, 4) | |
| Seropositive recipients | 0.997 | Beta(289, 1) | |
| Utilities | |||
| Baseline utility | Health Survey for England 2012399 | Multivariate normal | |
| Constant | 0.9679812 | ||
| Coefficient for age | –0.001807 | ||
| Coefficient for age2 | –9.71× 106 | ||
| Coefficient for sex = male | 0.0232887 | ||
| Disutilities | Liem 2008401 | ||
| Functioning graft | 0.053 | Gamma(1.179, 0.0453) | |
| HD | 0.277 | Gamma(66.9, 0.0041) | |
| PD | 0.264 | Gamma(35.73, 0.0074) | |
| Resource use | |||
| Immunosuppression (first transplant) | |||
| Induction therapy | Brennan 2006137 | ||
| BAS (20 mg dose + i.v. administration) | 1.964 | Normal(1.964, 0.016) | |
| rATG | |||
| Drug acquisition ( mg/kg) | 6.5 | Normal(6.5, 0.126) | |
| Intravenous administration | 4.525 | Normal(4.525, 0.079) | |
| Maintenance therapy: | |||
| TAC (with AZA; mg/kg/day) | Margreiter 200284 | ||
| 0–1 month | 0.225 | Log-normal(–1.497, 0.0998) | |
| 1–3 months | 0.175 | Log-normal(–1.748, 0.0998) | |
| 3–6 months | 0.135 | Log-normal(–2.007, 0.0998) | |
| 6–12 months | 0.11 | Log-normal(–2.212, 0.0998) | |
| 12–36 months | 0.09 | Log-normal(–2.413, 0.0998) | |
| 36+ months | 0.08 | Log-normal(–2.531, 0.0998) | |
| TAC (with MMF; mg/kg/day) | Rowshani 2006103 | ||
| 0–2 weeks | 0.168 | Log-normal(–1.789, 0.0998) | |
| 2–6 weeks | 0.176 | Log-normal(–1.742, 0.0998) | |
| 6–12 weeks | 0.11 | Log-normal(–2.212, 0.0998) | |
| 3–6 months | 0.104 | Log-normal(–2.268, 0.0998) | |
| 6–12 months | 0.086 | Log-normal(–2.458, 0.0998) | |
| 12+ months | 0.08 | Log-normal(–2.531, 0.0998) | |
| TAC (with SRL; mg/kg/day) | Gonwa 2003,180 Anil Kumar 2008122 | ||
| 0–1 month | 0.175 | Log-normal(–1.748, 0.0998) | |
| 1–3 months | 0.11 | Log-normal(–2.212, 0.0998) | |
| 3–6 months | 0.104 | Log-normal(–2.268, 0.0998) | |
| 6–12 months | 0.08 | Log-normal(–2.531, 0.0998) | |
| 12+ months | 0.07 | Log-normal(–2.664, 0.0998) | |
| TAC-PR (with MMF) | |||
| As TAC plus 0.015 mg/kg/day for first 12 months | 0.015 | Wlodarczyk 2009,140 Krämer 2010,204 Tsuchiya 2013,141 Oh 2014105 | Normal(0.015, 0.0075) |
| CSA (with AZA; mg/kg/day) | Margreiter 200284 | ||
| 0–1 month | 6.375 | Log-normal(1.847, 0.0998) | |
| 1–3 months | 4.525 | Log-normal(1.505, 0.0998) | |
| 3–6 months | 3.765 | Log-normal(1.321, 0.0998) | |
| 6–12 months | 3.375 | Log-normal(1.211, 0.0998) | |
| 12–36 months | 2.93 | Log-normal(1.07, 0.0998) | |
| 36+ months | 2.84 | Log-normal(1.039, 0.0998) | |
| CSA (with MMF/MPS; mg/kg/day) | Rowshani 2006103 | ||
| 0–2 weeks | 7.62 | Log-normal(2.026, 0.0998) | |
| 2–6 weeks | 5.72 | Log-normal(1.739, 0.0998) | |
| 6–12 weeks | 3.06 | Log-normal(1.113, 0.0998) | |
| 3–6 months | 2.86 | Log-normal(1.046, 0.0998) | |
| 6–12 months | 2.82 | Log-normal(1.032, 0.0998) | |
| 12+ months | 2.82 | Log-normal(1.032, 0.0998) | |
| CSA (with EVL; mg/kg/day) | Vítko 2005150 | ||
| 0–12 months | 3.9 | Log-normal(1.356, 0.0998) | |
| 12+ months | 2.1 | Log-normal(0.737, 0.0998) | |
| AZA (with TAC; mg/kg/day) | Laskow 199680 | ||
| 0–6 months | 1.5 | Log-normal(0.4, 0.0998) | |
| 6+ months | 1.2 | Log-normal(0.177, 0.0998) | |
| AZA (with CSA; mg/kg/day) | Sadek 200286 and Vacher-Coponat 2012129 | ||
| 0–6 months | 1.5 | Log-normal(0.4, 0.0998) | |
| 6–12 months | 1.4 | Log-normal(0.331, 0.0998) | |
| 12–36 months | 1.215 | Log-normal(0.19, 0.0998) | |
| 36+ months | 1.215 | Log-normal(0.19, 0.0998) | |
| MMF (with TAC; g/day) | SYMPHONY240 | ||
| 0–3 months | 2 | Log-normal(0.688, 0.0998) | |
| 3–12 months | 1.736 | Log-normal(0.547, 0.0998) | |
| 12+ months | 1.472 | Log-normal(0.382, 0.0998) | |
| MMF (with CSA; g/day) | SYMPHONY240 | ||
| 0–3 months | 2 | Log-normal(0.688, 0.0998) | |
| 3–12 months | 1.836 | Log-normal(0.603, 0.0998) | |
| 12+ months | 1.672 | Log-normal(0.509, 0.0998) | |
| MMF (with SRL; g/day) | SYMPHONY240 | ||
| 0–3 months | 2 | Log-normal(0.688, 0.0998) | |
| 3–12 months | 1.7335 | Log-normal(0.545, 0.0998) | |
| 12+ months | 1.467 | Log-normal(0.378, 0.0998) | |
| MMF (with BEL; g/day) | BENEFIT59 | ||
| Throughout | 2 | Log-normal(0.688, 0.0998) | |
| MPS (with CSA; mg/day) | |||
| 0–3 months | 1440 | Log-normal(7.267, 0.0998) | |
| 3–9 months | 1211 | Log-normal(7.094, 0.0998) | |
| 9+ months | 1107 | Log-normal(7.004, 0.0998) | |
| SRL (with TAC; mg/day) | Anil Kumar 2008122 | ||
| 0–12 months | 3.7 | Log-normal(1.303, 0.0998) | |
| 12–60 months | 2.75 | Log-normal(1.007, 0.0998) | |
| 60+ months | 1.8 | Log-normal(0.583, 0.0998) | |
| SRL (with MMF; mg/day) | Lebranchu 2009149 | ||
| 0–3 months | 5.2 | Log-normal(1.644, 0.0998) | |
| 3–6 months | 4.45 | Log-normal(1.488, 0.0998) | |
| 6–9 months | 3.5 | Log-normal(1.248, 0.0998) | |
| 9–12 months | 3.25 | Log-normal(1.174, 0.0998) | |
| 12–48 months | 2.9 | Log-normal(1.06, 0.0998) | |
| 48+ months | 2.6 | Log-normal(0.951, 0.0998) | |
| EVL (with CSA; mg/day) | Tedesco-Silva 2010107 and Lorber 2005143 | ||
| 0–3 months | 2.937 | Log-normal(1.072, 0.0998) | |
| 3–6 months | 2.75 | Log-normal(1.007, 0.0998) | |
| 6–9 months | 2.533 | Log-normal(0.925, 0.0998) | |
| 9–12 months | 2.6 | Log-normal(0.951, 0.0998) | |
| 12–24 months | 2.6 | Log-normal(0.951, 0.0998) | |
| 24+ months | 2 | Log-normal(0.688, 0.0998) | |
| BEL (with MMF) | Dosing schedule | ||
| Drug acquisition (250-mg vials per quarter) | |||
| 0–3 months | 16.53 | Log-normal(2.805, 0.02) | |
| 3–6 months | 7.13 | Log-normal(1.964, 0.02) | |
| 6+ months | 6.24 | Log-normal(1.83, 0.02) | |
| Drug administration (per quarter) | |||
| 0–3 months | 5 | Log-normal(1.609, 0.02) | |
| 3–6 months | 3 | Log-normal(1.098, 0.02) | |
| 6+ months | 3.26 | Log-normal(1.182, 0.02) | |
| Prednisolone (mg/day) | SYMPHONY240 | ||
| Throughout | 16.3 | Log-normal(2.786, 0.0998) | |
| Subsequent transplants | |||
| Proportion of failed grafts explanted (time since transplantation) | Bond 2009384 | ||
| 0–3 months | 0.41 | Beta(1.95, 2.81) | |
| 3–12 months | 0.23 | Beta(2.85, 9.54) | |
| 12–24 months | 0.09 | Beta(3.55, 35.9) | |
| 24+ months | 0.04 | Beta(3.8, 91.2) | |
| Subsequent graft | 0.059 | ||
| Subsequent retransplantation | |||
| Workup for retransplantation | 1.444 | NHS reference costs 2013–1464 | Normal(1.444, 0.025) |
| Living donor costs | 0.349 | NHS reference costs 2013–1464 | Normal(0.349, 0.012) |
| Deceased donor costs | 0.651 | NHS reference costs 2013–1464 | 1 – living donor costs |
| Maintenance immunosuppression | |||
| TAC ( mg/kg/day) | 0.1 | Assume somewhat higher than for original graft because of increased risk of rejection | Log-normal(–2.308, 0.0998) |
| MMF (g/day) | 2 | Recommended daily dose | Log-normal(0.688, 0.0998) |
| Prednisolone (mg/day) | 16.3 | SYMPHONY240 | Log-normal(2.786, 0.0998) |
| Infection prophylaxis | |||
| Co-trimoxazole (PCP and UTI prophylaxis) | |||
| Septrin (480-mg tablets in first 3 months) | 90 | Log-normal(4.495, 0.0998) | |
| Valganciclovir (CMV prophylaxis): Valcyte 450-mg tablets | |||
| Full dose 0–3 months (D+/R– or D[+/–]/R + with rATG) | 182.6 | N/A | |
| Full dose 3–6 months (D+/R–) | 182.6 | N/A | |
| Full dose 3–6 months (D[+/–]/R+ with rATG) | 91.3 | Uniform(0, 182.6) | |
| Full dose 6–9 months (D+/R–) | 34.8 | N/A | |
| Dose adjustment for renal function | 0.473 | Log-normal(–0.779, 0.246) | |
| Adverse events | |||
| Expected number of AR events per patient experiencing 1+ AR events | 1.193 | Charpentier 2003148 | Normal(1.193, 0.102) |
| Antidiabetic medication: metformin 500-mg tablets per 3 months | 273.9 | Log-normal(5.608, 0.0998) | |
| Dyslipidaemia | |||
| Statins | Riella 2012413 | ||
| Fluvastatin (mg per cycle for affected patient) | 2191 | Log-normal(7.662, 0.246) | |
| Pravastatin (mg per cycle for affected patient) | 548 | Log-normal(6.276, 0.246) | |
| Simvastatin (mg per cycle for affected patient) | 91.3 | Log-normal(4.484, 0.246) | |
| Medical management | |||
| Dietetics outpatient attendance (number per cycle) | 0.25 | Log-normal(–1.417, 0.246) | |
| GP appointment (# per cycle) | 0.25 | Log-normal(–1.417, 0.246) | |
| Anaemia requiring ESA therapy | |||
| Mean weekly dose (× 1000 IU) | 5.832 | Vanrenterghem 2003396 | Normal(5.832, 0.067) |
| Monitoring | |||
| Clinic (per cycle) | |||
| 0–3 months | 13.0 | Log-normal(2.567, 0.05) | |
| Thereafter as for blood tests (below) | |||
| Subsequent grafts | 3 | Log-normal(1.068, 0.246) | |
| Blood tests | Ling and Chamberlain 2011410 | ||
| 0–1 months | 13.07 | Normal(13.07, 0.259) | |
| 1–2 months | 6.75 | Normal(6.75, 0.186) | |
| 2–3 months | 4.95 | Normal(4.95, 0.159) | |
| 3–6 months | 8.99 | Normal(8.99, 0.215) | |
| 6–12 months | 3.97 | Normal(7.93, 0.202) | |
| 12–24 months | 2.69 | Normal(10.77, 0.235) | |
| 24–36 months | 3.5 | Normal(14, 0.268) | |
| ≥ 36 months | 1 | Log-normal(–0.03, 0.246) | |
| Subsequent grafts | 3 | Log-normal(1.068, 0.246) | |
| Viral PCR (per cycle) | |||
| 0–3 months (CMV) | 5.42 | Log-normal(2.538, 0.246) | |
| 0–6 months (BKV) | 1 | Log-normal(–0.03, 0.246) | |
| 6–12 months (BKV) | 0.5 | Log-normal(–0.723, 0.246) | |
| 0–6 months (EBV) | 0.0096 | Log-normal(1.068, 0.246) | |
| 6–12 months (EBV) | 0.0032 | Log-normal(–0.03, 0.246) | |
| Dialysis | |||
| Proportion receiving HD by age (years) | UK Renal Registry 16th Annual Report (figure 2.7)3 | ||
| 18–24 | 0.791 | Beta(276, 73) | |
| 25–34 | 0.804 | Beta(913, 223) | |
| 35–44 | 0.845 | Beta(1853, 340) | |
| 45–54 | 0.843 | Beta(3358, 624) | |
| 55–64 | 0.852 | Beta(4408, 768) | |
| 65–74 | 0.858 | Beta(5824, 967) | |
| 75–84 | 0.890 | Beta(5533, 681) | |
| ≥ 85 | 0.915 | Beta(1246, 116) | |
| Unit costs | |||
| Dialysis | NHS reference costs 2013–1464 | ||
| HD | |||
| Access surgery | £1946.32 | Normal(1946.32, 97.81) | |
| Temporary access | £823.25 | Normal(823.25, 40.43) | |
| Per quarter | £6093.11 | Normal(6093.11, 163.99) | |
| PD | |||
| Access surgery | £1100.71 | Normal(1100.71, 119.76) | |
| Per quarter | £6000.00 | Normal(6000, 183.24) | |
| Induction agents | |||
| BAS | |||
| Simulect (per 20 mg) | £842.38 | BNF 6856 | N/A |
| rATG | |||
| Thymoglobulin (per mg) | £6.35 | BNF 6856 | N/A |
| Maintenance agents | |||
| TAC (immediate-release capsules) | |||
| NHS acquisition cost (per mg) | £0.5201 | eMit370 | Mixture model |
| CSA (immediate-release capsules) | |||
| NHS acquisition cost (per mg) | £0.0165 | eMit370 | Mixture model |
| MMF | |||
| NHS acquisition cost (per g) | £0.3774 | eMit370 | Mixture model |
| MPS | |||
| Myfortic (per mg) | £0.0045 | BNF 6856 | N/A |
| AZA | |||
| NHS acquisition cost (per mg) | £0.0011 | eMit370 | Mixture model |
| SRL | |||
| Rapamune (per mg) | £2.883 | BNF 6856 | N/A |
| EVL | |||
| Certican (per mg) | £9.90 | Novartis’ submission | N/A |
| BEL | |||
| Nulojix (per 250-mg vial) | £354.52 | BNF 6856 | N/A |
| Prednisolone | |||
| NHS acquisition cost (per mg) | £0.0033 | eMit370 | Mixture model |
| AR (per episode) | £3557.39 | Ling 2011379 | Log-normal(8.146, 0.246) |
| Infection prophylaxis | |||
| Co-trimoxazole (PCP and UTI prophylaxis) | |||
| Septrin (per 480-mg tablet) | £0.155 | BNF 6856 | N/A |
| Valganciclovir (CMV prophylaxis) | |||
| Valcyte (per 450-mg tablet) | £18.02 | BNF 6856 | N/A |
| CMV infection | £3008.91 | Ling 2011379 | Log-normal(7.979, 0.246) |
| Anaemia requiring ESA therapy | |||
| Erythropoietin | |||
| Binocrit (per 1000 IU) | £4.33 | BNF 6856 | N/A |
| NODAT | |||
| Anti-diabetic treatment | |||
| Metformin (per 500-mg tablet) | £0.0054 | eMit370 | Normal(0.0054, 0.00001) |
| Annual cost of complications | Alva 2014419 | ||
| Inpatient | £1388.92 | Normal(1388.92, 99.42) | |
| Non-inpatient | £694.92 | Normal(694.92, 18.54) | |
| Dyslipidaemia | |||
| Statins | |||
| Fluvastatin (per mg) | £0.0022 | eMit370 | Mixture model |
| Pravastatin (per mg) | £0.0026 | eMit370 | Mixture model |
| Simvastatin (per mg) | £0.0003 | eMit370 | Mixture model |
| Medical management | |||
| Dietetics outpatient attendance | £62.70 | NHS reference costs 2013–1464 | Normal(62.7, 2.66) |
| GP appointment | £50.82 | PSSRU Unit Costs 2014407 | Normal(50.82, 5.08) |
| Drug administration | |||
| Intravenous infusion | |||
| First infusion | £228.95 | NHS reference costs 2013–1464 | Normal(228.95, 15.83) |
| Subsequent infusions | £325.59 | NHS reference costs 2013–1464 | Normal(325.59, 45.79) |
| BEL | £167.50 | NHS reference costs 2013–1456 | Normal(167.50, 11.58) |
| Monitoring | |||
| Clinic | £145.27 | NHS reference costs 2013–1464 | |
| Viral PCR | University College London Hospitals NHS Foundation Trust | ||
| Provider to provider services: 2013–14 tariff. 2013 | |||
| EBV | £46.75 | Equal to CMV PCR | |
| CMV | £46.75 | Log-normal(3.815, 0.246) | |
| BKV | £46.75 | Equal to CMV PCR | |
| Therapeutic drug monitoring (TDM) | Dept of Medical Biochemistry and Immunology, University Hospital of Wales Therapeutic drug monitoring test repertoire 2013/2014. 2013 |
||
| CSA TDM | £26.71 | Log-normal(3.255, 0.246) | |
| TAC TDM | £26.71 | Equal | |
| SRL TDM | £26.71 | Equal | |
| EVL TDM | £26.71 | Equal | |
| General tests | NHS Kidney Care 2011421 | ||
| Full blood count | £5.05 | Log-normal(1.615, 0.0998) | |
| Renal profile | £4.54 | Log-normal(1.509, 0.0998) | |
| Liver profile | £4.64 | Log-normal(1.531, 0.0998) | |
| Explant surgery | £4965.59 | NHS reference costs 2013–1464 | Normal(4965.59, 496.56) |
| Subsequent retransplantation | |||
| Recipient work-up | £848.72 | NHS reference costs 2013–1464 | Normal(848.72, 84.87) |
| Living donor costs | £8914.05 | NHS reference costs 2013–1464 | Normal(8914.05, 891.41) |
| Deceased donor costs | £10,142.05 | NHSBT 2013355 | Normal(10,142.05, 1014.21) |
| Transplant surgery | £16,030.35 | NHS reference costs 2013–1464 | Normal(16,030.35, 1603.04) |
Glossary
- Acute kidney rejection
- When the immune response of the host attempts to destroy the graft, as the graft is deemed foreign tissue.
- Adverse events
- Any untoward medical occurrence in a patient or clinical investigation subject who is administered a pharmaceutical product.
- Banff
- Criteria used to grade the severity of an acute rejection following a biopsy of the kidney, with ‘grade I’ being least severe and ‘grade III’ being most severe.
- Biopsy-proven acute rejection
- When an acute kidney rejection is confirmed through a biopsy and correspondence with the Banff criteria.
- Calcineurin inhibitor
- Ciclosporin or tacrolimus.
- Chronic kidney disease
- Abnormal kidney function and/or structure.
- Cold ischaemia time
- Period during which a donated kidney is transported in ice from donor to recipient. Duration is related to extent of kidney damage.
- Creatinine clearance
- One method of determining glomerular filtration rate. Urine is collected (usually for 24 hours) to determine the amount of creatinine that was removed from the blood over a given time interval.
- Cytomegalovirus
- A virus that normally causes only a mild ‘flu-like’ illness. In people with a kidney transplant, cytomegalovirus can cause a more serious illness, affecting the lungs, liver and blood.
- Deceased donor transplant
- A transplant kidney removed from someone who has died.
- Delayed graft function
- When the graft does not work immediately and dialysis is required during the first week post transplant. Dialysis has to continue until graft function recovers sufficiently to make it unnecessary. This period may last for up to 12 weeks in some cases.
- Donation after brain death
- Deceased heart-beating donors who are maintained on a ventilator in an intensive care unit, with death diagnosed using brainstem tests.
- Donation after circulatory death
- Non-heart-beating donors who cannot be diagnosed as brainstem dead but whose death is verified by the absence of a heart beat (cardiac arrest).
- Donor
- A person who donates an organ to another person (the recipient).
- End-stage renal disease
- A long-term irreversible decline in kidney function.
- Extended criteria donors
- People who are aged > 60 years without comorbidities, aged > 50 years, with hypertension or death from cerebrovascular accident, or donors with terminal serum creatinine level of > 1.5 mg/dl.
- Glomerular filtration rate
- A test that is used to check how well the kidneys are working. Specifically, it estimates how much blood passes through the glomeruli each minute. Glomeruli are tiny filters in the kidneys, which filter waste from the blood.
- Graft
- Surgical transplant of living tissue, in this case the kidney.
- Graft loss
- Loss of function from the transplanted organ.
- Haemodialysis
- An extracorporeal removal of waste products from the blood when the kidneys are in a state of renal failure.
- Human leucocyte antigen
- The locus of genes that encode for proteins on the surface of cells, which are responsible for regulation of the immune system in humans.
- Immunosuppressant
- Drugs given to lower the body’s ability to reject a transplanted organ.
- Induction drugs
- Powerful anti-rejection drugs that are taken at the time of transplantation, and close after, when the risk of rejection is highest.
- Kidney transplant
- Transfer of a healthy kidney from a donor to a recipient.
- Living–related transplant
- A kidney donated by a living relative of the recipient. A well-matched living–related transplant is likely to last longer than either a living–unrelated transplant or a deceased donor transplant.
- Living–unrelated transplant
- A kidney transplant from a living person who is biologically unrelated to the recipient.
- Maintenance drugs
- Less powerful antirejection drugs compared with induction drugs, which are used as both initial and long-term maintenance therapy.
- Mycophenolic acid
- Mycophenolate mofetil or mycophenolate sodium.
- Nephritis
- A general term for inflammation of the kidneys. Also used as an abbreviation for glomerulonephritis.
- Peritoneal dialysis
- Dialysis that uses the patient’s peritoneum in the abdomen as a membrane across which fluids and dissolved substances (electrolytes, urea, glucose, albumin and other small molecules) are exchanged from the blood.
- Post-transplant lymphoproliferative disorder
- A B-cell proliferation owing to therapeutic immunosuppression after organ transplantation. Patients with post-transplant lymphoproliferative disorder may develop infectious mononucleosis-like lesions or polyclonal polymorphic B-cell hyperplasia. Some of these B cells may undergo mutations that will render them malignant, giving rise to a lymphoma.
- Recipient
- In the context of transplantation, a person who receives an organ from another person (the donor).
- Rejection
- The process whereby a patient’s immune system recognises a transplant kidney as foreign and tries to destroy it. Rejection can be acute or chronic.
- Renal replacement therapy
- Dialysis or kidney transplantation.
List of abbreviations
- AE
- adverse event
- AKI
- acute kidney injury
- AR
- acute rejection
- ARD
- absolute risk difference
- ARR
- acute rejection rate
- ATG
- anti-human thymocyte/antithymocyte (immune)globulin
- AZA
- azathioprine
- BAS
- basiliximab
- BEL
- belatacept
- BENEFIT
- Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial
- BENEFIT-EXT
- BENEFIT–Extended Criteria Donors
- BKV
- BK virus
- BNF
- British National Formulary
- BPAR
- biopsy-proven acute rejection
- CCS
- corticosteroid
- CEA
- cost-effectiveness analysis
- CI
- confidence interval
- CKD
- chronic kidney disease
- CMU
- Commercial Medicines Unit
- CMV
- cytomegalovirus
- CNI
- calcineurin inhibitor
- CRC
- creatinine clearance
- CrI
- credibility interval
- CSA
- ciclosporin
- CVD
- cardiovascular disease
- DAC
- daclizumab
- DBD
- donation after brain death
- DCD
- donation after circulatory death
- DGF
- delayed graft function
- DIC
- deviance information criterion
- DWFG
- death with functioning graft
- EBV
- Epstein–Barr virus
- EC-MPS
- enteric-coated mycophenolate sodium
- ECD
- extended criteria donor
- eGFR
- estimated glomerular filtration rate
- eMit
- Electronic Market Information Tool
- EQ-5D
- European Quality of Life-5 Dimensions (EuroQoL instrument)
- ESA
- erythropoiesis-stimulating agent
- ESRD
- end-stage renal disease
- EU
- European Union
- EVL
- everolimus
- GFR
- glomerular filtration rate
- GP
- general practitioner
- GRF
- graft function
- HD
- haemodialysis
- HLA
- human leucocyte antigen
- HMGCoA
- 3-hydroxy-3-methylglutanyl-coenzyme A
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICDF
- inconsistency degrees of freedom
- ICER
- incremental cost-effectiveness ratio
- INHB
- incremental net health benefit
- IQR
- interquartile range
- IL2Mab
- anti-interleukin-2 receptor monoclonal antibody
- ITT
- intention to treat
- KTR
- kidney transplant recipient
- MD
- mean difference
- MDRD
- Modification of Diet in Renal Disease
- ME
- microemulsion
- MMF
- mycophenolate mofetil
- MPA
- mycophenolic acid
- MPS
- mycophenolate sodium
- MTC
- mixed-treatment comparison
- mTOR-I
- mammalian target of rapamycin complex 1
- NHSBT
- NHS Blood and Transplant
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NODAT
- new-onset diabetes after transplant/transplantation
- OR
- odds ratio
- PBO
- placebo
- PCP
- Pneumocystis jirovecii pneumonia
- PCR
- polymerase chain reaction
- PD
- peritoneal dialysis
- PenTAG
- Peninsula Technology Assessment Group
- PNF
- primary non-function
- PPP
- purchasing power parity
- PRA
- panel reactive antibody
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PTDM
- post-transplant diabetes mellitus
- PTLD
- post-transplant lymphoproliferative disorder
- QALY
- quality-adjusted life-year
- rATG
- rabbit anti-human thymocyte immunoglobulin
- RCT
- randomised controlled trial
- RR
- relative risk
- RRT
- renal replacement therapy
- SE
- standard error
- SRL
- sirolimus
- TAC
- tacrolimus
- TAC-PR
- prolonged-release tacrolimus
- UKPDS
- UK Prospective Diabetes Study
- USRDS
- United States Renal Data System
- UTI
- urinary tract infection
- WMD
- weighted mean difference