Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 05/501/04. The contractual start date was in September 2009. The draft report began editorial review in January 2015 and was accepted for publication in November 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
The probiotic and placebo used in this trial were manufactured and transported to the UK free of charge by the Yakult Honsha Co. Ltd, Tokyo, Japan. The company had no involvement in the trial design or conduct or in the analysis and interpretation of the data, nor has the chief investigator had any direct contact with the company. Edmund Juszczak has been a member of the Health Technology Assessment (HTA) Commissioning Board since November 2013. Michael Millar was a member of the Diagnostic and Screening panel of the HTA throughout the trial. Peter Brocklehurst has been chairperson of the HTA Maternal, Neonatal and Child Health panel since December 2014. He received money from Oxford Analytica for consultancy and as chairperson of the Medical Research Council Methodology Research Programme panel; his Institution received money from the National Institute for Health and Care Excellence for his role as lead for maternal health review updates and for evidence updates of National Institute for Health and Care Excellence guidance during the conduct of the trial. He also reports that his institution received money for numerous Medical Research Council, National Institute for Health Research Health Services and Delivery Research and National Institute for Health Research HTA programme grants.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Costeloe et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
This is a multicentre, double-blind, randomised, placebo-controlled trial to study possible benefits of the early administration of the probiotic Bifidobacterium breve strain BBG-001 to babies born before 31 weeks’ gestation and recruited within 48 hours of birth. The primary end points are late-onset bloodstream infection diagnosed on a sample drawn after 72 hours, necrotising enterocolitis (NEC) and death. The trial aimed to recruit 1300 babies from approximately 20 UK neonatal units.
Chapter 2 Scientific background
Acquired infection and necrotising enterocolitis in preterm babies
Hospital-acquired infection is reported in about 25% of babies with birthweight < 1500 g who survive the first 3 days,1 and it contributes to the high mortality and morbidity in this population.
Necrotising enterocolitis is the most common serious gastrointestinal complication of preterm birth and has high mortality and morbidity. 2,3 The pathogenesis of NEC is multifactorial,4 related to immaturity of the immunological and barrier functions and involving bacterial invasion of the intestinal mucosa. There is no agreed international diagnostic case definition for NEC; the system most widely used is the ‘modified’ Bell classification,5 which involves a range of clinical, haematological and radiological criteria. Bell stage 1 is very non-specific, whereas Bell stages 2 and 3 have more objective radiological features and are often considered interchangeable with the terms ‘proven, confirmed or serious’ NEC used in some publications. Estimates of the incidence of ‘confirmed’ NEC in babies with a birthweight of < 1500 g vary between about 6% and 10%.
The microbiome in the newborn baby and its relation to late-onset sepsis and necrotising enterocolitis
The microbiome is the term used to describe the population of micro-organisms with which individual humans co-exist, predominantly within the bowel. Healthy breastfed term infants become colonised early in life with a wide range of bacteria dominated by bifidobacteria and lactobacilli acquired during and after birth from close contact with the mother;6 these microbes are believed to confer a range of health benefits. By comparison, preterm infants nursed in neonatal units become colonised with a more limited range of bacteria and fungi. 7–9 The pattern of colonisation reflects the micro-organisms found in the ‘antibiotic-rich’ environment of the neonatal unit and is dominated by members of the Enterobacteriaceae family, Pseudomonas, enterococci, yeasts, staphylococci and clostridia that are potentially pathogenic and may cause infection in the colonised infant or may spread and cause disease in other infants.
The specific mechanisms by which anaerobic lactobacilli and bifidobacteria protect against infection with pathogenic organisms are believed to involve increased secretion of immunoglobulin A and upregulation of immunoglobulin A receptor sites, strengthening of epithelial tight junctions, lowering the intraluminal pH through acid fermentation and modification of intestinal inflammatory responses through preferential stimulation of T-helper cells, all resulting in reduced bacterial translocation. 10 This subject has been the focus of a number of reviews. 11–13
The extent to which abnormal patterns of early colonisation of the intestine have deleterious effects on later health is also incompletely understood. A number of recent studies have shown changes in the patterns of stool bacterial colonisation in the period preceding the clinical onset of NEC and late-onset sepsis,14–17 but whether or not these changes are causative or part of the disease process is unclear.
For the context of this trial a probiotic is defined as a live microbial supplement that colonises the gut and improves health. 18
The extent to which intestinal colonisation with probiotic bacteria can be achieved in the preterm newborn baby is unclear. However, the concept of active management of the bowel flora to prevent hospital-acquired infection and NEC is an attractive therapeutic option that seems likely to have a good safety profile.
Probiotics and the prevention of late-onset sepsis and necrotising enterocolitis
Randomised controlled trials
When the Probiotics in Preterm infantS (PiPS) trial was designed, we believed that only one randomised controlled trial (RCT) had been published that reported the effect of probiotics on late-onset sepsis and/or NEC in preterm babies. This was an Italian trial published in 2002 and involving 585 babies below 33 weeks’ gestational age19 treated with a product containing Lactobacillus rhamnosus GG. It failed to show a significant reduction in NEC incidence or blood culture-positive episodes of late-onset sepsis (probiotic vs. placebo: NEC, 1.4% vs. 2.7%; septic episodes, 4.1% vs. 4.7%). The results are difficult to interpret, as the analysis was not by intention to treat; babies dying in the first 2 weeks were excluded and only septic episodes and episodes of NEC with onset at least 7 days after commencement of the intervention were considered in the analysis. This probably accounts for the low reported rates of adverse outcomes.
When recruitment to the PiPS trial began in 2010, four20–23 further trials with clinical primary outcomes had been published.
The first two trials were reported in 2005 and showed a reduction in the incidence of NEC in infants given probiotic mixtures; both studies recruited at a single site. The first was from a hospital in Taiwan,20 in which a mixture of L. acidophilus and B. infantis or placebo was given in breast milk twice daily until discharge from hospital to 367 babies of birthweight < 1500 g, who had survived beyond 7 days and were clinically stable with umbilical lines removed and commencing milk feeds. Reductions in incidence were seen for NEC, from 5.3% to 1.1% (p = 0.04); blood culture-positive late-onset sepsis, from 19.3% to 12.2% (p = 0.03); death, from 10.7% to 3.9% (p = 0.009); and for the combined outcome of NEC, late-onset sepsis or death from 32.1% to 17.2% (p = 0.009). The second study21 recruited 145 babies at an Israeli hospital, who were randomised to receive a product containing three probiotic strains (B. infantis, Streptococcus thermophilus and B. bifidus) or unsupplemented milk feeds given once a day. The median age at commencement of the intervention was 3 days and the intervention was continued until 36 weeks’ postmenstrual age. There was no difference in episodes of blood culture-positive infection or of death, but episodes of NEC appeared to be reduced in the intervention arm (16.4% vs. 4.0%; p = 0.03) and it was reported that there was a reduction in the severity of the illness. These two studies, the first to report prevention of NEC, were subject to considerable interest and extensive review. 24–26
In 2007 a meta-analysis27 was published including these three trials19–21 together with four others designed to study different outcomes, one of which involved a fungal rather than a bacterial intervention. 28 A total of 1393 babies were involved, and the conclusion was that there was evidence that probiotic interventions might reduce the incidence of NEC and all-cause mortality, apparently without adverse effects, but that there were important outstanding questions about choice of probiotic product and dosing.
The third and fourth RCTs with clinical primary outcomes were published in 2008 and 2009, a single-site trial from a hospital in India22 and a multicentre trial from Taiwan. 23 In the trial from India, a combination of B. infantis, B. bifidum, B. longum and L. acidophilus given with breast milk twice daily to 186 babies of < 32 weeks’ gestation and birthweight < 1500 g was compared with breast milk alone. Participants were clinically stable and, as in the previous studies, receiving milk feeds. The end points included feed tolerance, length of stay and serious neonatal morbidities. Babies dying from causes other than late-onset sepsis or NEC were excluded and no power calculation was given. A significant reduction in time to achieve full feeds and length of stay was reported to be associated with probiotic use. There was an overall reduction in all stages of NEC from 15.8% in the control group to 5.5% in the probiotic group (p = 0.04), but no significant reduction in the incidence of NEC of Bell stage ≥ 2. There was a significant reduction in culture-positive late-onset sepsis, from 29.5% to 14.3% (p = 0.02), and of death, from 14.7% to 4.4% (p = 0.04).
The multicentre trial from Taiwan23 recruited a total of 434 babies of birthweight < 1500 g and gestational age < 4 weeks from seven centres. A product containing B. bifidum and L. acidophilus was added to the milk feed; babies entirely fed with formula were excluded, as were babies in whom feeds had not been started by 3 weeks of age. There was a composite primary outcome, death or NEC Bell stage ≥ 2, which was significantly lower in the intervention group (1.8% vs. 9.2%; p = 0.002). In addition, there were more babies with late-onset sepsis in the intervention group (18.4% vs. 11.1%.) A large number of infants died (98 out of a potential 580 eligible infants) without ever achieving the study entry criteria.
In the period leading up to the start of recruitment to the PiPS trial in 2010 and during recruitment there have been sharp divisions in the paediatric literature about the use of probiotics. Some authors have strongly advocated a change of practice to routine use29 because of the apparent association with a reduction in NEC and death, as suggested in a series of meta-analyses,30–32 whereas others have recommended caution because of the heterogeneity of the participants and of the interventions and the methodological failings of some trials. 33,34
At the time of writing, 11 RCTs designed to study the efficacy of a bacterial probiotic intervention, with late-onset sepsis and/or NEC and/or death as the primary outcome, have been published in English. These trials account for 4396 of the 5529 (80%) babies randomised in 20 trials included in the most recent Cochrane review of probiotics to prevent NEC in preterm babies. 35 These trials are characterised by a range of inclusion and exclusion criteria and by varying exposure to maternal breast milk. This may, in part, explain the wide reported ranges of rates of late-onset sepsis, NEC and death. Of those studies reporting such data, mortality and NEC rates among excluded infants are in some cases high (Table 1). The extent to which reviews such as this may be subject to publication bias is difficult to assess owing to the inclusion of a large number of trials. Many of which are small and not designed to study clinical outcomes.
| Reference | Eligibility criteria | Exclusions (other than congenital malformations and lack of consent) and their outcomes if available | Intervention including whether or not in milk | Placebo | Blind | Age at starting intervention | Rates of late-onset sepsis, NEC and death in non-intervention group |
|---|---|---|---|---|---|---|---|
| Dani et al. 200219 | < 33 weeks old and < 1500 g in weight; randomised, n = 585 | Death within 2 weeks of birth, n = 29 | Lactobacillus GG with milk | Yes | Yes | Mean 3.4 days (SD 3.7 days) | After 7 days of intervention: late-onset sepsis, 12 of 290; NEC, 8 of 290; death, N/A |
| Bin-Nun et al. 200521 | < 1500 g in weight; randomised, n = 148 | N/A | B. bifidus, B. infantis and S. thermophilus with milk | No | Yes | Mean 2.7 daysa (SD 2.3 days) | Late-onset sepsis, 24 of 73; NEC, 12 of 73; death, 8 of 73 |
| bLin et al. 200520 | > 7 days old, < 1500 g in weight, central lines removed > 24 hours; randomised, n = 367 | 50 of 417 potential recruits died or had NEC < 7 days | L. acidophilus and B. infantis with breast milk | No | Unclear | N/A, but after 7 days | Late-onset sepsis, 36 of 187; NEC, 10 of 187; death, 20 of 187 |
| Lin et al. 200823 | < 34 weeks old, < 1500 g in weight ‘who survived to feed enterally’; randomised, n = 443 | Exclusive formula feeds, nil by mouth > 3 weeks, 98 of 580 assessed for eligibility died before milk started | L. acidophilus and B. bifidum with breast milk | No | Unclear | Mean 4.5 daysa (SD 3.0 days) | Late-onset sepsis, 24 of 217; NEC, 14 of 217; death, 9 of 217 |
| cSamanta et al. 200922 | < 32 weeks old, < 1500 g in weight, survived 48 hours; randomised, n = 186 | Died from ‘other’ illnesses | B. infantis, B. bifidum, B. longum and L. acidophilus with breast milk | No | Not known | Mean 6.0 daysd (SD 1.4 days) | Late-onset sepsis, 28 of 95; NEC, 15 of 95; death, 14 of 95 |
| Mihatsch et al. 201036 | < 30 weeks old; randomised, n = 183 | None | B. lactis BB12 with milk | Yes | Yes | Mean 5 days (SD 2.7 days) | Late-onset sepsis, 40 of 89; NEC, 4 of 89; death, 1 of 89 |
| Sari et al. 201137 | < 33 weeks old; < 1500 g in weight;e randomised, n = 242 | None | L. sporogenes with milk | No | Unclear | Mean 2 daysd | Late-onset sepsis, 26 of 111; NEC, 10 of 111; death, 4 of 111 |
| fBraga et al. 201138 | 750–1499 g in weight;g randomised, n = 243 | Congenital infection | B. breve and L. casei with donor breast milk | No | Yes | Day 2 | Late-onset sepsis, 42 of 112; NEC, 4 of 112; death, 27 of 112 |
| Rojas et al. 201239 | ≤ 48 hours old, < 2000 g in weight, haemodynamically stable;h randomised, n = 750 | None | L. reuteri DSM 17938i | Yes | Yes | Day 2 | Late-onset sepsis, 40 of 378; NEC, 15 of 378; death, 28 of 378 |
| Fernández-Carrocera et al. 201340 | < 1500 g in weight, randomised, n = 150 | Apgar score of < 6 at 5 minutes, NEC Bell stage 1 | L. acidophilus, L. rhamnosus, L. casei, L. plantarum, B. infantis and S. thermophilus with milk | No | Unclear | Median 5 days (range 1–23 days) | Late-onset sepsis, 44 of 75; NEC, 12 of 75; death, 7 of 75 |
| jJacobs et al. 201341 | < 32 weeks old, < 1500 g in weight, randomised < 72 hours;k randomised, n = 1099 | Likely to die within 72 hours, mother taking non-dietary probiotics | B. infantis, B. lactis and S. thermophilus with milk | Yes | Yes | Median 5 days (IQR 4–7 days) | Late-onset sepsis, 89 of 551; NEC, 24 of 551; death, 28 of 551 |
| jOncel et al. 201442 | < 33 weeks old, ≤ 1500 g in weight; randomised, n = 424l | L. reuteri DSM 17938i | Yes | Yes | Median 1 day (range 1–5 days) | Late-onset sepsis, 25 of 200; NEC, 10 of 200; death, 20 of 200 |
With the exception of a multicentre trial published in 2012 and recruiting babies of birthweight up to 2000 g,39 the probiotic intervention was given either in milk or, in one study,42 separately but coincident with the start of feeding. This suggests that babies with perceived contraindications to starting feeds, who are likely to be those babies at highest risk of NEC, might be excluded or have deferred entry to the trials. The majority of the trials were not placebo controlled and relied on the responsible clinical staff being blind to the allocation through the use of unsupplemented milk as the comparator (see Table 1).
None of the trials was designed with statistical power to study NEC or death rates as separate outcomes.
In contrast to NEC and death, the various meta-analyses do not suggest a protective effect of probiotics for late-onset sepsis. The assessment of efficacy to reduce late-onset sepsis is complicated by the lack of a standardised definition of the outcome.
This problem was addressed by the multicentre Australasian ProPrems trial,41 which is the largest of the previously published trials. The results were presented in 2012 but were not published until after the completion of PiPS trial recruitment. A rigorous definition of late-onset sepsis was used and the trial was statistically powered to show a reduction from 23% to 16%. The event rates of both late-onset sepsis and NEC in this trial were lower than predicted and a non-statistically significant reduction in late-onset sepsis, from 16.2% to 13.1%, was observed.
It is generally held that, in order for a probiotic intervention to be effective, it should ‘colonise’ the intestine and the administered bacteria should multiply within it. Successful colonisation is likely to be influenced by local factors such as feeding and antimicrobial use. Human breast milk contains oligosaccharides known to promote colonisation by bifidobacteria while many probiotic strains are sensitive to commonly administered antimicrobials, for example bifidobacteria are sensitive to penicillins. Manufacturers of probiotics inevitably select strains that readily colonise the intestine and, theoretically, these strains might be particularly likely to spread between babies, especially in hospitals where cots are close together or the nurse-to-baby ratio is low. In this respect, the efficacy of a probiotic intervention might be expected to vary more between different institutions than a standard chemical drug intervention. Of the 20 RCTs included in the most recent Cochrane review,35 the only trial to report colonisation by allocated group in detail is the single-site study reported by Kitajima et al. ,43 which involved the administration of a single-strain probiotic product containing B. breve YIT4010 (BBG-001). Of 45 babies in the probiotic group, 73% were colonised at 2 weeks and 91% at 6 weeks, whereas, of the 46 babies given placebo, 12% were colonised at 2 weeks and 44% at 6 weeks. This adds a level of complexity to the interpretation of all data from trials of probiotics, particularly for those using polymicrobial products, as it is likely that different components will colonise babies in both groups so that at different time points in their clinical course babies might be colonised with anywhere between none or all of the administered strains.
Observational studies/historic comparisons
Despite the frequent calls for probiotics to be used routinely for preterm babies, there are few published accounts of their impact in routine use.
An early report44 of a trial in a tertiary hospital in Colombia, in which a product containing L. acidophilus and B. infantis was given for 1 year to all admitted newborn infants, reported a decrease in all stages of NEC compared with the previous year, from 6.6% (n = 1237) to 3.0% (n = 1282); all other aspects of care were unchanged.
There have been more recent reports of use targeted towards preterm babies.
In 2010 Luoto et al. ,45 reported on 12 years’ experience in five tertiary neonatal units in Finland. In one neonatal unit, following an outbreak of NEC, administration of Lactobacillus GG was introduced for all babies of birthweight < 1500 g. In three other neonatal units, the same product was administered to babies with gastrointestinal problems and the final neonatal unit used no probiotic. The standard of care in all hospitals was to use donor breast milk in the absence of maternal milk. The authors did not find a protective effect in the hospital using ‘prophylactic’ probiotics when the incidence remained higher than in the other hospitals or any effect on the clinical course of NEC in those hospitals in which probiotics were given to symptomatic babies.
A further three retrospective cohort studies have been published. 46–48
A report from a single site in the USA46 compared the incidence of NEC in 79 babies (of birthweight ≤ 1000 g) born between 2009 and 2011 in whom L. reuteri was routinely administered and babies born in the previous 5 years; detailed feeding data were not reported and infants who died in the first week were excluded. A reduction in NEC Bell stage ≥ 2 from 15.1% to 2.5% was reported (p = 0.0475); there were baseline differences in the characteristics of the babies and between-year variation in NEC incidence.
In a study in France,47 during a 3-year period from 2008, babies born between 24 and 31 weeks’ gestation (n = 347) and starting feeds within 48 hours of birth on a tertiary neonatal unit were administered L. casei rhamnosus, Lcr35 strain from the beginning of feeding, their outcomes were compared with those of unsupplemented babies born in the previous 5 years (n = 783). Babies dying in the first week were excluded from the analysis. 47 In the second period, the incidence of late-onset sepsis was reduced from 16.6% to 10.7% [odds ratio (OR) 0.60, 95% confidence interval (CI) 0.40 to 0.89], the incidence of NEC Bell stage ≥ 2 was reduced from 5.3% to 1.2% (OR 0.23, 95% CI 0.08 to 0.69) and the mortality rate fell from 4.8% to 2.3% (OR 0.46, 95% CI 0.21 to 1.00).
A Canadian study48 published in 2014 reported NEC Bell stage ≥ 2 and the composite outcome NEC or death of babies born before 32 weeks’ gestation for 17-month periods before (n = 317) and after (n = 294) the introduction of a product containing B. breve, B. longum, B. bifidum, B. infantis and Lactobacillus GG was given with the first feed at a single site. The incidence of NEC decreased from 9.8% to 5.5% (p < 0.02) and the incidence of death or NEC fell from 17.0% to 10.5% (p < 0.05). There was a non-significant reduction in death as a separate outcome. After adjustment for gestational age, intrauterine growth restriction and sex, the OR for NEC in the second period was 0.51 (95% CI 0.26 to 0.98) and for death or NEC was 0.56 (95% CI 0.33 to 0.93).
Safety
None of the published RCTs or non-randomised studies, including a summary of 6 years’ use of Lactobacillus GG across two neonatal units in northern Italy49 that contains no efficacy data, reports any complications of probiotic administration; most importantly, no instances were recorded of late-onset sepsis with the administered probiotic strains.
Before the beginning of recruitment to this trial there had been occasional reports, including in the paediatric literature,50,51 of disseminated infection following enteral supplementation with Lactobacillus species, but no reports of late-onset sepsis with Bifidobacterium. In 2010, what we believe to be the first case of a Bifidobacterium septicaemia was reported. 52 This involved a positive blood culture for B. breve strain BBG-001 (the strain used for the PiPS trial) in a full-term baby recovering after surgery for exomphalos. The baby is described as having a mild illness that was treated with standard empirical antibiotic treatment involving ampicillin/sulbactam and amikacin; the child made an uneventful recovery.
In 2012, a second report53 described a twin born at 27 weeks’ gestation, birthweight 600 g, who was fed with maternal breast and in whom a probiotic preparation containing B. infantis and L. acidophilus was instituted on day 8. On day 18 the infant became unwell with abdominal symptoms. A blood culture grew two species, B. infantis and B. longum. She was treated with vancomycin, cefotaxime and metronidazole, and recovered.
There is an anxiety that, theoretically, manipulating the developing microbiome by administering probiotics might modify the immunological function of the intestine or that antibiotic resistance genes might be transferred from the probiotic to pathogenic bacteria, thereby putting the individual at increased short-term risk of infection or possibly of unpredictable long-term health change. 54
Studies on the effect of probiotics on intestinal colonisation with potential pathogens are few, and the results inconsistent. A small study involving 30 babies, with a mean gestational age of 33 weeks and a mean birthweight of 1486 g, was suggestive that B. breve administration might be associated with reduced colonisation with Enterobacteriaceae. 55 However, a more recent placebo-controlled randomised trial56 of formula-fed babies, born before 32 weeks’ gestation, found increased colonisation with Enterobacteriaceae, enterococci and staphylococci in 21 of 47 babies whose feed was supplemented with Lactobacillus GG. This was not associated with increased late-onset sepsis in these babies. Of the 12 clinical trials listed in Table 1, all of which were designed to study clinical outcomes, five reported higher rates of sepsis in the active arm than in the placebo arm,19,21,23,37,39 although only one was statistically significant. 23 These trials use various definitions of late-onset sepsis.
A RCT studying a product with six bacterial strains, four species of Lactobacillus and two of Bifidobacterium, in 296 adult patients with acute pancreatitis reported increased mortality in the active arm, [24/152 (16%) vs. 9/144 (6%) in the placebo arm (p = 0.01)]. 57 The most frequent cause of death was multiorgan failure and there were no reports of probiotic bacteraemias. In 9 of the 24 patients in the active group who died, ischaemic bowel was found at either laparotomy or autopsy; ischaemic bowel was not found in those patients in the placebo group who died. The intervention in this trial was given twice daily directly into the jejunum and represents a huge bacterial load, estimated at 1010 bacteria. The reasons for the increased mortality are unclear. This trial might be interpreted as a reminder that, despite worldwide extensive consumption of probiotics, it should not be assumed that they are safe in extremely ill patients with compromised intestinal function; this would include preterm babies, particularly those with problems establishing enteral nutrition.
The justification for continued recruitment to the PiPS trial was kept under review throughout its progress as reports of more trials of routine use and of probiotic septicaemias became available; at no time was it considered that the accumulating evidence either of efficacy or safety was such that a recommendation to stop the trial early should be made. The overarching consideration was whether or not the findings of the various trials were applicable to the population of preterm babies at risk of late-onset sepsis and NEC in UK neonatal units. Rates of late-onset sepsis and NEC are inversely related to gestational age at birth2,58–60 and become relatively low from around 32 weeks’ gestation; the requirement of clinicians is for a preventative intervention that can safely be given to all babies at risk of late-onset sepsis and NEC.
The choice of probiotic
The ideal probiotic for a clinical trial would have extensive preclinical data, including experience in the preterm newborn infant and information about dosage, supporting its probable efficacy and safety. In addition, it would be available in a stable pure form known to be free of contaminants; a suitable and indistinguishable placebo would be available; and it would be easy to grow and identify the bacterium in the laboratory so that colonisation of participants could be monitored and probiotic infection easily detected. None of the interventions used in the published studies in the newborn infant fulfils these criteria.
There are also choices to be made regarding whether or not a single or multistrain product is used.
There are very few studies comparing different probiotic interventions in the preterm baby. One recent Phase 1 study61 suggested different effects of a B. infantis species compared with B. longum in respect of bacterial diversity and total counts of bifidobacteria, particularly when augmented by administration of maternal milk. A second study62 comparing a product containing a single strain of B. breve with a product providing the same quantity of B. breve together with two species of B. longum suggested that the three-strain product was associated with increased colonisation with B. breve and fewer Enterobacteriaceae species; whether or not these effects are attributable to the diversity or to the greater bacterial load of the three-strain product is unclear.
Of the 12 trials included in Table 1, four used products containing a single strain, three contained different strains of Lactobacillus, one contained B. lactis BB12 and the remaining four studies used combinations of up to six different bacterial strains. In large part, the choice of product appears to have been governed by availability and the ability to mix it with milk.
Although not explicitly stated in the text, in one trial conducted in Israel by Bin-Nun et al. ,21 and reported at a scientific meeting (Dr C Hammerman, Zedek Medical Center, Jerusalem, personal communication), the intervention [ABC Dophilus® (Solgar®), which contained B. infantis, S. thermophilus and B. bifidus], was selected because of anxiety about possible infection with Lactobacillus-containing products. The same product was used in the recent and heretofore the largest published trial, ProPrems, carried out in Australia. 41 When asked about the choice, the author explained that it was not because of the bacterial content but simply that it had been previously evaluated and shown to have efficacy against NEC incidence, was available and could be imported under licence into Australia (Dr SE Jacobs, Royal Women’s Hospital, Melbourne, VIC, personal communication). The formulation of that product has now changed so that it contains Lactobacillus.
Two published meta-analyses34,63 attempt to group trials to study the effects of different organisms and combinations, but they fail to reach clear conclusions and highlight the need for further study.
Quality
Of the 12 trials detailed in Table 1, nine quote the manufacturer’s data describing the content of the product but do not describe the storage conditions or any further testing to confirm the contents, their purity or their stability through the course of trial recruitment. The B. lactis used by Mihatsch et al. 36 was checked monthly to ensure the viability and purity of the product together with the 24-hour stability of the prepared suspension. The six-strain product used by Fernández-Carrocera et al. 40 (four strains of Lactobacillus, one of B. infantis and one of S. thermophilus) was checked twice against the manufacturer’s quality control register and the ABC Dophilus used for the ProPrems trial41 was imported under licence into Australia. Each batch was then subjected to independent confirmation of taxonomy and quality by checking the probiotic content using polymerase chain reaction (PCR) and the purity by culture.
Experience with Bifidobacterium breve BBG-001
Use of B. breve BBG-001, the probiotic strain used for the PiPS trial, was first reported by a Japanese group. 43 Ninety-one infants of birthweight < 1500 g were randomised to receive active product or placebo. The trial commenced with milk feeds, administered twice daily and continued for 28 days, by which time 82% of the active intervention group and 28% of the placebo group were colonised with B. breve BBG-001. Clinical outcomes analysed whether or not the baby was successfully colonised with the probiotic organism. Improved food tolerance, accelerated time to establish full feeds and increased weight gain that was sustained after discontinuation of administration of probiotic were reported. No other clinical outcome was published and none is available; probiotic use with this product became and remains routine in that investigator’s department (Dr Kitajima, Osaka Medical Center and Research Institute for Maternal and Child Health, Osaka, Japan, 2013, personal communication).
A single-site pilot study using the same product, B. breve BBG-001, was undertaken by the current investigators. 64 B. breve BBG-001 was used because at the time the study was designed it was the only probiotic strain reported to confer any clinical benefit in the preterm baby. 43 The primary objectives of the pilot were to study whether or not the intervention was tolerated this early in development and to confirm that colonisation was achieved with a once-daily dosage regimen. The design differed from previous studies in that the study product was commenced within 48 hours of birth, whether or not milk feeds had been started. This was to avoid excluding those babies at greatest risk of adverse outcomes and to maximise the possibility of early colonisation with the probiotic organism, even in babies from whom the responsible clinician might choose to withhold milk feeds because of a perceived high risk of NEC. The products were prepared as described in the report by Kitajima et al. ,43 but only a single 1-ml dose was given, as opposed to the whole content of the sachet given in two or three 1-ml doses. We estimated the dose given to be around 5 × 108 colony-forming units (CFUs) of B. breve BBG-001. The colonisation rates we achieved were similar to those quoted by Kitajima et al. 43 and the numbers of bifidobacteria in the stools of those babies who were colonised were the same whether they were in the active intervention or placebo groups: at 14 days, 12 out of 19 (63%) infants in the active intervention group were colonised with a mean 7.3 [standard deviation (SD) 1.7] log10 CFUs per gram wet weight of stool and 4 out of 17 (24%) infants in the placebo group were colonised with a mean with 7.4 (SD 3.0) CFUs per gram wet weight of stool. These data support the conclusion that B. breve had actively colonised the babies and the same dose was therefore used in the main trial.
Forty infants of birthweight < 1500 g were randomised at a single site (Homerton University Hospital Foundation Trust, London, UK) over a 6-month period in 2004 to receive B. breve BBG-001 or placebo; both products were well tolerated by all babies. Quantitative microbiology was undertaken on stools. Analysis of the stool passed closest to 28 days showed that 79% of the group receiving probiotic and 35% receiving placebo were colonised with B. breve BBG-001; this high cross-contamination rate is comparable with published experience43 and was considered likely to have occurred both in the milk kitchen and between babies in the ward. All babies who commenced enteral feeding did so with maternal breast milk.
Analysed by intention to treat, probiotic supplementation was associated with improved feed tolerance and weight gain, and there were fewer babies with episodes of infection at 28 days (23% vs. 44%). The study was too small to allow any estimate of an impact on the incidence of NEC (nine suspected or proven cases, of whom five were randomised to receive probiotic and four placebo). When outcomes were analysed by whether or not the infant was colonised with the administered probiotic, it was found that colonisation was associated with a reduction in the number of babies with any episode of infection over the entire hospital stay (from 66% to 24%; p = 0.017) and also that fewer colonised babies remained oxygen dependent at 36 weeks’ postmenstrual age (40% vs. 79%; p = 0.038). In addition, there was some evidence of increased microbial diversity in the stools of colonised babies, although the numbers are small. In particular, at 28 days, no stool of non-colonised babies was also colonised with anaerobic bacterial species; in contrast, 66% of those colonised with B. breve BBG-001 were also colonised with anaerobic bacterial species. Colonisation with Gram-negative organisms was high in both groups. When analysed by intention to treat, there was no difference in the duration of antibiotic use in the two groups, but, when analysed by whether or not there was successful colonisation, there was a significant reduction in the number of days on antibiotics over the whole hospital stay in those colonised, from a mean of 39 days to 19 days (p = 0.04).
The intervention was continued for a shorter time period (28 days) in this pilot study than in the subsequently published studies that found a reduction in NEC incidence with probiotic use, all of which continued the intervention to either 36 weeks’ postmenstrual age or discharge from hospital. Two babies randomised to receive probiotic in the pilot study developed proven NEC that was fatal: one at 29 days and one at 30 days (one of these infants was not successfully colonised). When the PiPS trial was designed it was recognised that babies at high risk of developing NEC may do so later than 4 weeks’ postnatal age, it is now known from observational studies that more immature babies develop the disease at an older postnatal age with the peak age at onset around 31–34 weeks’ postmenstrual age. 65,66
Subsequent to the design of the PiPS trial, we are not aware of any published reports of the use of B. breve BBG-001 in the newborn baby.
Regulatory status of probiotics
At the time of the pilot study using B. breve BBG-001 that we conducted in 2004,64 probiotics were considered as food supplements and the trial was not conducted to International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice (ICH-GCP) standard. A product given to prevent such serious complications of prematurity as NEC, late-onset sepsis and death clearly fulfils the definition of a medicinal product used within the European Community: ‘Any substance or combination of substances presented as having properties for treating or preventing disease in human beings’ (Article 1, Directive 2001/83/EC). 67 The regulator, the Medicines and Healthcare products Regulatory Agency (MHRA), required that for this trial the probiotic intervention should be considered as a medicine and the PiPS trial, unlike all other published trials investigating the use of probiotics in newborn babies, was conducted to ICH-GCP standard.
Rationale for the design of the Probiotics in Preterm infantS trial
When the PiPS trial was designed, there had been no published trials, apart from the study of Dani et al. ,19 that reported effects of probiotics on NEC, late-onset sepsis and death, and no trials reporting benefits of probiotic use. Despite this, in the context of the current understanding of the epidemiology and pathogenesis of NEC and late-onset sepsis and the importance of identifying preventative strategies, the probability that a probiotic intervention might be both efficacious and safe seemed high.
Choice of product
When our trial was designed, as far as we were aware the only study to report any benefit associated with probiotic use in the newborn infant was the trial by Kitajima et al. 43 published in 1997, studying nutritional outcome, although the analysis was based on whether or not the baby had been successfully colonised rather than by intention to treat. We were also aware that the product used in that trial, B. breve BBG-001, had been in routine use in Japan for a number of years, seemingly without problems. These observations underpinned our decision to use this product for our pilot study, in which we found it to be well tolerated and confirmed that we could achieve good colonisation rates with a single daily dose.
We were keen to monitor colonisation of all participants, and B. breve BBG-001 had the further advantage that the manufacturer was able to provide a selective strain-specific medium so that it could reliably be cultured and identified.
Population
The study population was selected as being that at greatest risk of NEC. In particular, we were keen to start the intervention as soon as practicable, not only because babies begin to acquire intestinal flora from birth, whether or not fed, but also because as the clinical course progresses and complications arise we believe that it is easier to find reasons not to recruit babies into trials and we thought it essential that we recruit a trial population that was as representative as possible of all preterm admissions so that at trial conclusion we could address the question of whether or not probiotics should be given routinely to this population.
Chapter 3 Methods
This was a multicentre, double-blind RCT.
Objective
The objective of the trial was to determine whether or not early administration of the probiotic B. breve strain BBG-001 to preterm infants reduced the incidence of episodes of infection, NEC and death.
The trial protocol is available on the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC) website at www.nets.nihr.ac.uk.
Participants
Participants were preterm babies born between 23+0 and 30+6 weeks’ gestation and recruited with informed signed parental consent within 48 hours of birth. Babies were eligible for recruitment whether or not they had been born in the recruiting centre. Those with a lethal congenital malformation or any malformation of the gastrointestinal tract detected before 48 hours or who were considered to have no realistic chance of survival were excluded. Receiving antibiotics for proven or suspected infection was not an exclusion criterion.
Trial sites
The trial was conducted at 57 sites. Of these, 24 were recruitment sites and 33 were sites to which participants were transferred for continuing care. A complete list of the recruitment sites is available in Appendix 1.
Interventions
The active intervention was B. breve BBG-001, suspended in one-eighth strength of the infant formula Neocate® (Nutricia Ltd, Trowbridge, UK). The placebo was one-eighth-strength Neocate. A description of Neocate is available at www.neocate.co.uk/uploadedFiles/Neocate/Resources_Library/Documents/Neocate_LCP_data_card.pdf (accessed 26 August 2016).
The probiotic and placebo powders were manufactured and supplied by the Yakult Honsha Co. Ltd (Tokyo, Japan) in identical square foil sachets each containing 1 g of product. The sachets of active product contained B. breve BBG-001 freeze-dried with maize starch and those of placebo contained freeze-dried maize starch alone; the appearance of the powders was identical. The trial was conducted using a single batch of products manufactured specifically for this study, the release criteria for this batch stated that each sachet of the active product contained between 2 × 108 and 2 × 1010 CFUs. After importation to the UK, the sachets were packed at Bilcare Global Clinical Supplies (Europe) Ltd into packages each containing 91 sachets (the maximum number of sachets a baby might require) of either active product or placebo. Each of the 91 sachets and the package was labelled with a unique five-digit alphanumeric identifier.
Product preparation, administration and blinding
The manufacturer’s instructions involved suspending the powders in water, allowing the maize starch to settle for 30 minutes and administering the supernatant within the next 2.5 hours. Prepared in this way, the supernatant of the active product was cloudy and that of the placebo clear. This was overcome by substituting one-eighth-strength Neocate for the water. Occasionally the turbidity of the supernatant still varied slightly and, therefore, to be completely confident that the active intervention and placebo were indistinguishable, they were prepared in specially manufactured amber-coloured bijou bottles (Figure 1).
FIGURE 1.
Prepared product in amber-coloured bijou bottle.

Kitajima et al. ,43 using the same product, prepared it using 2 ml of water. During our preliminary work we found that when using 2 ml that it was sometimes difficult, using a syringe, to withdraw 1 ml without disturbing the maize starch residue. We were keen not to increase the volume that we were administering to the babies but equally keen to ensure, with minimal evidence to guide us, that we gave adequate numbers of bacteria. By a process of trial and error we found that, if we increased the volume used to suspend the powder to 3 ml, then only rarely were we unable easily to withdraw 1 ml. We emphasised to investigators the importance of not disturbing the maize starch and suggested that, if they had any difficulty withdrawing 1 ml, they could simply give less on that day.
The products were prepared in the milk kitchens on the neonatal units of the participating hospitals, usually by one of the nurses engaged in clinical care. In order to minimise the possibility of cross-contamination of the placebo by B. breve BBG-001, members of the trial team provided on-site training with an emphasis on handwashing and decontamination of working surfaces in between preparing each baby’s intervention. This teaching was repeated, on request, for new staff and was supported with detailed guidance on laminated sheets for display in milk kitchens. The guidance sheet for product preparation is available in Appendix 2.
Dosage
The range of values quoted by the manufacturers of products used in published trials is from 106 to 109 CFUs per dose. The dose used in the study of Kitajima et al. 43 is the most relevant for this trial because the same product was used. The babies in the study of Kitajima et al. 43 were given the contents of a whole sachet (estimated in the publication to contain 1 × 109 CFUs) in two or three divided doses (i.e. 3.3 × 108 to 5.0 × 108 CFUs per dose) each 1 ml in volume. In our pilot study we achieved colonisation rates similar to Kitajima et al. 43 with a single dose. The manufacturer stated that each sachet of the batch used for the PiPS trial contained between 2 × 108 and 2 × 1010 CFUs. Preparing the product as we did, using 3 ml of Neocate, suggests that the range of bacterial counts in a 1-ml dose would be between 6.7 × 107 and 6.7 × 109 CFUs of B. breve BBG-001.
A record was kept of doses omitted and of sachets wasted, and was reconciled centrally against the number of unused sachets in the package after it was collected by the trial research nurses when the baby had completed the intervention.
Administration
The intervention was prescribed using the five-digit alphanumeric identifier for the pack allocated for that baby. This was written on the side of the bijou bottle during preparation and checked by the nurses before administration. A feeding syringe was used to withdraw 1 ml of supernatant, which was given to the baby.
Extension of the shelf-life of the interventions: viability counts for B. breve BBG-001 in the active intervention
The manufacturer supplied data documenting the decline of viability and lack of contamination of previous batches of the product extending for 42 months from manufacture. As there was a lack of evidence beyond that time, the stated shelf life for the batches provided for the PiPS trial extended to the end of August 2012 for the active product and September 2012 for the placebo. During 2011 and 2012 it became clear that recruitment would need to continue until mid-2013 and that a second batch of interventions would be needed. It emerged that circumstances had changed and that new batches of intervention, particularly of placebo, could not easily be provided. We knew from work with the previous batch of the active product used for the pilot study and from monitoring undertaken in the early stages of this trial that the counts of viable B. breve BBG-001 were declining only slowly.
In the absence of any other guidance we accepted 2 × 108 (8.3 log10) CFUs of B. breve BBG-001 per sachet (6.7 × 107 or 7.8 log10-CFUs per dose) as the minimum figure that we should accept for this trial.
Analysis of unused sachets returned from centres during the early stages of the PiPS trial had been carried out and corrected to give the viable count per 1-ml dose. A plot of these data (Figure 2) showed a gradual decline in viable counts (solid blue line). The manufacturer provided stability data from two different batches of material for 42 months from manufacture, shown as CFUs per sachet for comparison (see Figure 2, solid black and green lines). All three lines are roughly parallel and extrapolation from the data obtained from the batch being used for the PiPS trial indicated that the number of viable organisms per dose would remain well above 6.7 × 107 or 7.8 log10-CFUs for at least 48 months (i.e. until October 2013) and probably beyond.
FIGURE 2.
Projected decline in dosage of B. breve BBG-001 beyond November 2011 compared with decline in sachet content of two earlier batches supplied by the manufacturer. The y-axis shows the count of B. breve BBG-001 expressed in log10-CFUs. The x-axis shows the actual date for the trial batch of B. breve BBG-001 and time in months since manufacture for all batches. The solid green and black lines were provided by the manufacturer and relate to two earlier batches of the product. The counts are per sachet and extend for 42 months after manufacture. The solid blue line is the count per dose of B. breve BBG-001, as made up for the PiPS trial. The blue triangles represent the mean of all measurements made since the previous triangle. The regression line is extended beyond the final measurement in November 2011 to estimate the likely viability at the projected end of use of intervention in the trial in October 2013. The dashed green line at around 8.7 log10-CFUs represents the estimated single dose used in the trial of Kitajima et al. 43 The dashed blue line at 8.3 log10-CFUs represents the lower cut-off point per sachet of the batch of B. breve BBG-001 provided by the manufacturer for the PiPS trial, when prepared as for the trial this equates to 1 ml providing a dose of 6.7 × 107 or 7.8 log10-CFUs.

On the basis of these data, a successful application was made to the MHRA to extend the shelf life of both probiotic and placebo to the end of October 2013 and all sachets and boxes were relabelled accordingly. It was agreed that we should continue to monitor the counts of viable bacteria in a randomly selected unused sachet from each pack after the baby had completed its course of treatment to confirm both that the rate of decline was not accelerating and that the products remained free of contamination. It was agreed that if the viable counts fell below 2 × 108 CFUs (8.3 log10-CFUs) per sachet, or if any contamination was identified, recruitment would stop.
Outcomes
The rationale and definitions of outcomes are detailed in appendices 1–4 of the trial protocol, which is available on the NETSCC website. 68
Primary outcomes
-
Any baby experiencing an episode of bloodstream infection, with any organism other than a skin commensal, diagnosed on a sample of blood drawn more than 72 hours after birth and before 46 weeks’ postmenstrual age, death or discharge from hospital, whichever is soonest. Skin commensals include coagulase-negative staphylococci and Corynebacterium.
-
NEC, Bell stage 2 or 3.
-
Death before discharge from hospital.
Secondary outcomes
-
Number of babies with the composite outcome of any or a combination of the three primary outcomes.
Secondary microbiological outcomes
Outcomes 2–7 are for samples taken > 72 hours after birth and before 46 weeks’ postmenstrual age, death or discharge home:
-
Number of babies with any positive blood culture with an organism recognised as a skin commensal (e.g. coagulase-negative staphylococci or Corynebacterium).
-
Number of babies with blood cultures taken.
-
Number of blood cultures taken per baby.
-
Number of babies with episodes of bloodstream infection with organisms other than skin commensals by organism, for example Escherichia coli, Klebsiella spp., fungi, and by antibiotic resistance types, specifically meticillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE) and extended-spectrum beta-lactamase (ESBL)-producing Gram-negative bacteria.
-
Number of babies with isolates of organisms other than skin commensals from a normally sterile site other than blood, for example cerebrospinal fluid, suprapubic aspiration of urine, pleural cavity, etc.
-
Number of babies with a positive culture of B. breve BBG-001 from any normally sterile site.
In addition:
-
Total duration of days of antibiotics and/or antifungals administered per baby after 72 hours and until 46 weeks’ postmenstrual age, death or discharge from hospital, whichever is soonest, for treatment of suspected or proven late-onset sepsis, that is, excluding prophylactic use.
-
The number of babies colonised with the administered probiotic strain defined by the isolation of B. breve BBG-001 from stool samples at 2 weeks’ postnatal age and at 36 weeks’ postmenstrual age.
-
Stool flora: the number of babies colonised with MRSA, VRE or ESBL at 2 weeks’ postnatal and at 36 weeks’ postmenstrual age.
Nutritional and gastroenterological outcomes
-
Age at achieving full enteral nutrition (defined as 150 ml/kg/day for 1 day).
-
Change of weight z-score from birth to 36 weeks’ postmenstrual age or discharge from hospital if sooner.
Other clinical outcomes
-
Bronchopulmonary dysplasia.
-
Hydrocephalus and/or intraparenchymal cysts confirmed by cerebral ultrasound scan performed during the baby’s inpatient stay.
-
Worst stage of retinopathy of prematurity in either eye at discharge or death.
-
Length of stay in intensive, high-dependency and special care unit.
Randomisation
Randomisation was performed by health-care staff trained in trial procedures and named on the trial delegation log.
Randomisation to receive either probiotic or placebo used a central web-based service, with telephone back-up, based at the National Perinatal Epidemiological Unit (NPEU), University of Oxford, Oxford, UK. The randomisation program used a minimisation algorithm to ensure balance across site, sex, gestational age at birth (23, 24, 25, 26/27 and 28–30 weeks’ gestation) and whether or not randomisation occurred sooner than 24 hours after birth.
At randomisation the investigator was given a unique five-digit study number for the baby, which was the principal identifier throughout the trial, and a five-digit alphanumeric number for the intervention pack to be used.
Parents, clinicians and outcome assessors were blind to the allocation.
Trial procedures
Consent
Whenever possible, preliminary discussions supported by written information about the trial would be offered to parents before the birth if the baby was likely to be eligible. This happened both at recruiting centres and at local hospitals that routinely referred babies into the recruiting centres. Informed written consent was sought from a parent after the birth only when they had been given a full oral and written explanation of the study. Copies of the parent information leaflet, the consent form and the leaflet provided to investigators summarising the material that should be covered in discussions about the trial are available in Appendices 3–5.
Investigators were encouraged to discuss the trial with parents periodically during the hospitalisation to confirm their continued understanding and willingness to participate.
At all stages it was made clear to the parents that they remained free to withdraw their baby from the study at any time with no need to provide an explanation. When babies were transferred between hospitals, the parents were given written information including the name of the consultant acting as principal investigator (PI) at the receiving hospital.
Parents who did not speak English were approached only if an appropriate adult interpreter was available.
Withdrawal from the trial
When parents requested that their baby be withdrawn from the trial, we completed an additional data form (see form 6, Appendix 6) that clarified whether or not it was only administration of the intervention that was to be discontinued and whether or not the parents were willing for data already collected, for outstanding data and/or stool collection to continue and for those data to be used.
Clinical care of participants
The day-to-day clinical care of participants was entirely at the discretion of the responsible clinical team. Investigators were encouraged to use maternal breast milk, but feeding regimes were not standardised.
Discontinuation of the trial intervention
Whether or not the intervention was discontinued temporarily when babies were unwell was at the discretion of the parents and attending clinical staff. The only circumstance in which clear advice was given to withhold a dose was when intestinal perforation was suspected.
Stool sample collection
Stool samples were collected as close as possible to 14 days’ postnatal and 36 weeks’ postmenstrual age. These times were chosen for practical reasons, the main objective being to gain a snapshot of stool colonisation by B. breve BBG-001 as a marker of intestinal colonisation. It was considered that, at 2 weeks of age, enteral feeds would be established in the majority of babies, who would have received the intervention for over a week, while still being before the time, for this population, of the peak incidence of NEC. Thirty-six weeks was selected, as this is the time at which outcome data describing bronchopulmonary dysplasia and growth were reported. Investigators were asked, if possible, to send three full scoops of stool. Samples were posted for processing to the Microbiology Laboratory at the Royal London Hospital, Barts Health NHS Trust, UK, using the Thermacor transportation system for diagnostic samples (Dyecor Ltd, Hereford, UK) and the Royal Mail.
No other biological samples were collected.
Data collection
With the exception of detailed results of routine microbiological investigations, all trial data were collected onto paper forms, which were posted to the NPEU Clinical Trials Unit for checking and double-entered onto a web-based clinical database, OpenClinica (OpenClinica, LLC, Waltham, MA, USA). Data were entered in accordance with NPEU Clinical Trials Unit OpenClinica data entry conventions. All personal details were entered into a Microsoft Access® 2013 database (version 15, Microsoft Corporation, Redmond, WA, USA).
Form 1: trial entry (see Appendix 7)
Part A of this form had to be completed and the answers available to facilitate randomisation and parts B–F of the form comprised baseline maternal and neonatal information. It was requested that it was posted to the trial office within 1 week of birth.
Form 2: daily data collection (see Appendix 8)
The aim of this form was to collect details of enteral feeds and antimicrobial interventions for the first 14 days of life until the collection of the first stool sample so as to enable later detailed analysis of determinants of colonisation at 14 days with B. breve BBG-001. If the baby was transferred between hospitals during this time, then a copy was retained at the referring hospital and the original form accompanied the baby.
Form 3: clinical details of baby at transfer, discharge or death (see Appendix 9)
This form provided clinical details and was due for completion at discharge from hospital, at death or if the baby was transferred to a different hospital, that is one form was completed for each admission and a baby could accrue multiple forms. If the baby reached 36 weeks’ postmenstrual age during the admission, the details of growth and respiratory support to determine whether or not the baby had bronchopulmonary dysplasia were provided. The form included a question about whether or not the baby had experienced any episode of NEC or other abdominal pathology which, if affirmative, led to completion of form 4.
Form 4: abdominal pathology (see Appendix 10)
This form was completed for any episode of suspected abdominal pathology. Multiple forms, each for a different episode, might be completed during a single admission covered by a single form 3 and if a baby was transferred between hospitals for specialist management of NEC multiple forms might be received from different hospitals for the same episode. The staging of an episode of NEC was primarily based on that provided by the clinician on the form but the form included questions about the clinical characteristics of the episode with the intention that these would later be checked to confirm consistency with the stated NEC staging.
Form 4 review
All cases in which any form 4 had been received were reviewed by Professor Kate Costeloe (chief investigator), Dr Kenny McCormick (consultant neonatologist, PI for the PiPS trial at the John Radcliffe Hospital, Oxford, UK) and Mrs Michele Upton (PiPS research nurse), to determine the number of discrete episodes of NEC, the highest Bell staging of any NEC episode, the age at onset of the first episode of any NEC and of stage 2 or 3 NEC and the agreed diagnosis of episodes of other abdominal pathologies. The review involved scrutiny of all forms 4 together with the associated forms 3 and, when relevant, with postmortem reports and operation notes. Outstanding inconsistencies and queries were resolved together with the PIs with reference to the contemporaneous medical records.
Routine microbiological data
The results of routine microbiological investigations together with the antibiotic sensitivities of cultured bacteria were obtained directly from the staff in the laboratories of participating hospitals on an Microsoft Excel® 2010 spreadsheet (Version 14, Microsoft Corporation, Redmond, WA, USA). They were scrutinised individually by Dr Michael R Millar to ensure that all positive cultures from normally sterile sites were identified. All positive cultures were entered onto the trial database together with the sampling site, species of bacteria and patterns of antibiotic resistance. The accuracy of the trial microbiological data was checked by comparing 20% of trial data entries against the Microsoft Excel-recorded laboratory returns.
Safety and adverse event reporting
Unexpected serious adverse events (SAEs) and suspected unexpected serious adverse reactions (SUSARs) were reported using form 5 (see Appendix 11). Two SUSARs were noted prospectively:
-
intestinal obstruction associated with maize starch
-
bacteraemia with B. breve BBG-001.
Stool samples: microbiology methods
All samples were processed in the microbiology laboratory at Barts Health NHS Trust. When multiple samples were received from the same infant, then the sample collected closest to the appropriate date (14 days postnatal age or 36 weeks’ postmenstrual age) was selected for storage and analysis.
The microbiology laboratory at Barts Health NHS Trust is accredited through Clinical Pathology Accreditation (UK) Ltd (a wholly owned subsidiary of the United Kingdom Accreditation Service). The procedures used in this study, such as those used for culture, identification, antibiotic sensitivity testing of organisms and disposal of waste, were performed in accordance with laboratory standard operating procedures.
On receipt into the laboratory, specimens were weighed and divided in to two equal parts. The study number and date of receipt of each specimen were recorded. Half was frozen and stored at –80 °C; this sample was collected to allow additional chemical, immunological and molecular analyses including the molecular detection of the trial strain (B. breve BBG-001). The other half was diluted 1 : 10 in a cryopreservative broth [brain–heart infusion broth (Oxoid Microbiological Products Ltd, Basingstoke, UK) containing 10% glycerol (weight/volume)], mixed by vortexing for 10 seconds, and then placed in 1-ml aliquots into sterile 1.5-ml Eppendorf tubes (Eppendorf AG, Hamburg, Germany) before freezing at –80 °C.
Detection of Bifidobacterium breve BBG-001, meticillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci and extended-spectrum beta-lactamase-producing Gram-negative bacteria in stool samples by culture
Samples were batch processed. Vials of frozen sample in cryopreservative were allowed to thaw at room temperature, and 100 µl of the faecal broth was serially diluted in phosphate-buffered saline. Aliquots of 100 µl of the neat, 10−1, 10−3 and 10−5 dilutions were inoculated onto the agar medium plates.
Selective media were used for the detection of the trial strain (B. breve BBG-001), MRSA, VRE and ESBL-producing Enterobacteriaceae. The selective medium used for detection of the B. breve BBG-001 was Trypticase® peptone oligosaccharide (TOS) agar (Yakult Honsha Ltd, Japan) containing carbenicillin (10 µg/ml) and streptomycin (50 µg/ml). TOS agar was incubated for 48–72 hours anaerobically. ESBL-producing Enterobacteriaceae were cultured using MacConkey agar (Oxoid Microbiological Products Ltd, Basingstoke, UK) and Brilliance™ ESBL agar (Oxoid Microbiological Products Ltd), MRSA using mannitol salt agar with oxacillin (Oxoid Microbiological Products Ltd) and VRE using Slanetz and Bartley agar with vancomycin (Oxoid Microbiological Products Ltd). Inoculated selective media for MRSA, VRE and ESBL were incubated at 37 °C for 24–36 hours in air.
Identification and enumeration of cultured Bifidobacterium breve
Bifidobacterium breve produces a characteristic white convex colony on TOS agar. The faecal concentration of B. breve was determined by counting the number of colonies of faecal dilutions on TOS agar, allowing estimation of the numbers in the undiluted samples. Cultured colonies were identified using matrix-assisted laser desorption/ionisation time of flight (MALDI-TOF) mass spectrometry (Bruker UK Ltd, Coventry, UK). In the early phase of the study the identification of a proportion of representative colonies was confirmed by 16S ribosomal deoxyribonucleic acid (DNA) sequencing.
Identification and enumeration of antibiotic-resistant bacteria
Bacterial colonies growing on selective agars were enumerated. Isolates were identified using standard laboratory methods including MALDI-TOF mass spectrometry (Bruker UK Ltd, Coventry, UK). MRSA and VRE isolates were identified as MRSA or VRE using British Society for Antimicrobial Chemotherapy methods and interpretive criteria. 69 Gram-negative bacilli which grew on MacConkey or Brilliance ESBL agar were tested for susceptibility to a range of antibiotics using British Society for Antimicrobial Chemotherapy methods. 69 Antibiotics tested included cefuroxime, ceftazidime, cefpodoxime, ampicillin, gentamicin, piperacillin/tazobactam, amoxicillin with clavulanate, tetracycline, trimethoprim, amikacin, tobramycin, imipenem, ertapenem, tigecycline, colistin, ciprofloxacin, aztreonam and chloramphenicol. Antibiotic-resistant isolates were stored by emulsification of colonies into microbank storage vials (Pro-lab Diagnostics, Wirral, UK) and stored at –80 °C.
Molecular detection of Bifidobacterium breve strain BBG-001 in stool samples
Deoxyribonucleic acid was extracted from faecal matter using the QIAamp DNA stool minikit (Qiagen Ltd, Manchester, UK) in accordance with the manufacturer’s instructions with an added bead-beating step. A strain-specific quantitative real-time PCR method previously reported by Fujimoto et al. in 201170 was used to detect B. breve BBG-001. The forward PCR primer sequence was 5′-ATGGCAAAACCGGGCTGAA-3′ and the reverse 5′-CCCACCTCTCATCCGC-3′ to give a 313-bp PCR product. Amplification and detection was carried out in 96-well optical low-profile plates (Anachem Ltd, Luton, UK) on a Bio-Rad CFX 96 real-time PCR machine-C1000 thermal cycler (Bio-Rad, Hercules, CA, USA). The PCR assay was re-optimised for use on the Bio-Rad machine with a fast PCR mix. Several PCR mixes were tested (Bio-Rad, Molzym and Agilent). The annealing temperature was optimised using the temperature gradient function of the Bio-Rad PCR machine. A primer titration was also performed.
Each PCR reaction (final/total volume of 10 µl) contained 1 µl of DNA template, 400 nM of each PCR primer, and 2 × Brilliant III Ultra-Fast QPCR Master Mix (Agilent Technologies, Santa Clara, CA, USA). The 313-bp target sequence was amplified with an initial hold of 3 minutes at 95 °C followed by 40 cycles of 5 seconds at 95 °C and 10 seconds at the optimised annealing temperature of 62 °C. The melt cycle involved a temperature ramp from 65 °C to 95 °C, with a 5-second hold at each 0.5 °C step of the ramp.
Each sample was analysed in triplicate at neat and 1 : 10 concentration to check for PCR inhibition. A no-template control, a faecal extraction-negative control and a faecal extraction-positive control were also included on each PCR run. A sample was scored as positive if there is amplification for two or three out of three replicates with a melt curve at 87 °C, 82 °C or 85 °C, because we found that the strain-specific sequence derived from stool samples did sometimes give an 82 °C or 85 °C melt curve. Purified B. breve BBG-001 DNA produced a melt curve of 87 °C. Serial 10-fold dilution standards were run on each plate using the trial strain DNA at concentrations from 30 ng/µl to 30 fg/µl to allow estimation of the quantity of B. breve BBG-001 in each sample.
Data validation
Validation programs performed a series of range, logic and missing data checks to identify any inconsistencies within and across forms on an ongoing basis. Some queries were resolved at the NPEU according to predefined protocols; those that could not be resolved were reconciled between the staff in the trial office, the PiPS trial research nurses, chief investigator and PI with reference to the clinical record, and documented accordingly.
Sample size
Estimating sample size was difficult because of the paucity of reliable contemporary outcome data by gestational age. Based on data collected for local service appraisals around the year 2000, it was thought that the event rate of each of the primary outcomes might be as high as 15%. At a two-sided significance level of 5%, a trial of 1300 infants would have 90% power to detect a 40% relative risk (RR) reduction from 15% to 9.1%, or a 44% RR reduction from 12% to 6.7% or from 10% to 5.6% for each of the primary outcomes. These reductions were deemed to be of clinical importance by the investigators.
Recruitment targets
The aim was to begin recruitment within 6 months of trial commencement in September 2009, to recruit 300 babies in the first 3 months and accelerating gradually so that thereafter an average of 50 babies were recruited each month, with a total recruitment time of 2 years and 6 months.
Statistical methods
The full statistical analysis plan is available in Appendix 12.
The comparison of primary interest was whether or not there was a difference between the groups of the trial in any of the three primary outcomes. The primary analysis of primary and secondary outcomes was by intention to treat, that is the outcomes were compared across randomised groups for all infants recruited regardless of whether or not, or for how long, they received the allocated PiPS trial interventions.
Adjusted analyses were performed on all comparative analyses adjusting for the variables used in the minimisation algorithm, hospital, sex, gestational age at birth (23, 24, 25, 26/27 and 28–30 weeks’ gestation), and whether or not randomisation occured sooner than 24 hours after birth. The adjusted analyses also took into account correlation of outcomes between participating babies from multiple births.
For binary outcomes, for example whether or not a baby ever had an episode of infection, adjusted RRs and CIs were calculated. For continuous outcomes, for example the number of episodes of infection, adjusted differences in means or unadjusted differences in medians (depending on the distribution of the data) were calculated with CIs. Analysis of time-to-event outcomes, such as reaching full enteral feeding, used survival analysis techniques.
Subgroup analyses included an interaction test and, when appropriate, results are presented as adjusted RRs with 95% CIs.
Prespecified subgroup analyses were performed on the primary outcomes by intention to treat, stratified by:
-
whether or not randomised in the first or second 24 hours after birth
-
gestational age at birth as per minimisation: 23, 24, 25, 26/27 and 28–30 weeks’ gestation
-
male versus female
-
colonised versus not colonised at 2 weeks’ postnatal age
-
gestational age < 28+0 or ≥ 28+0 weeks’ gestation.
An additional subgroup analysis was performed post hoc by birthweight above or below 1 kg to facilitate comparison with data from the published ProPrems trial41 and to address recommendations made in successive systematic reviews30,32,35 concerning routine administration of probiotics in these birthweight categories.
A secondary analysis of all clinical and microbiological outcomes was conducted in those babies for whom stool colonisation data were available at 2 weeks’ postnatal age by whether or not the baby was colonised with B. breve BBG-001 identified by either culture or PCR.
Determinants of successful colonisation at 2 weeks’ postnatal age in those babies analysed to receive probiotics were investigated using forward stepwise regression. The factors assessed were baseline characteristics together with day of first feed, type of milk, use of antacid and administration of antibiotics beyond the fifth day after birth.
Significance levels and multiplicity
The 95% CIs are presented for all analyses on the primary outcomes, and a significance level of 5% (consistent with a 95% CI) used to indicate statistical significance.
Owing to the large number of secondary outcomes, all analyses are presented with 99% CIs and a significance level of 1% (consistent with a 99% CI) is used to indicate statistical significance.
The p-values are not presented for comparative analyses, but are for tests of interaction.
Regulatory approvals and protocol changes
Version 1.0 of the protocol, dated 29 January 2009, was approved by the South Central Oxford A Ethics Committee on 12 May 2009. The name of the trial was changed from PREFER to PiPS (as a condition of approval from the ethics committee) and this resulted in the protocol being amended to version 2.0, dated 18 May 2009.
Two changes to the conduct of the trial resulted in protocol version 3.0 (dated 6 October 2009): the way the investigational medicinal product (IMP) was allocated (from study number to pack number) and the duration of daily data collection of milk feeds and antibiotic/antifungal usage (from ‘until full feeds reached’ to ‘until 2 weeks’ postnatal age’). Minor typographical changes resulted in version 3.1 (dated 3 February 2010), and an update to include a more recent appraisal of the literature on Bifidobacterium use in infants led to version 4.0 (dated 13 April 2010).
Two sections relating to safety reporting and the addition of continuing care sites were updated in March 2010 (version 5.0, dated 17 March 2011). Clarification was made to safety reporting at different ‘levels’ of sites to state that safety will be assessed continuously and reported irrespective of site status. The description of how the addition and set-up of continuing care sites was achieved in practice was updated, and the implementation of the generic site-specific application system and ‘statement of responsibilities’ for gaining approvals of continuing care sites that fell outside recognised clinical pathways for transfers was added. All of these amendments received Research Ethics Committee approval.
A number of changes to the trial protocol resulted in version 6.0 (dated 24 July 2012). The background and rationale section of the protocol was updated with information from the latest publications, and the window for primary outcome data for late-onset sepsis and secondary outcome data collection for microbiological culture and antibiotic/antifungal use was ‘closed’ at 46 weeks’ postmenstrual age for those babies still in hospital. A paragraph was added to appendix 6 of the protocol about the identification of carbapenem (imipenem, meropenem)-resistant Enterobacteriaceae cultures from stool samples taken from the PiPS trial participants and the procedure for alerting sites of this finding. Details of flagging in sections 3.11 and 10.6 of the protocol were modified to reflect changes in the current Medical Research Information Service system and what services could be provided under the current project remit. The lower limit of eligibility for gestational age, which had not been clear in previous versions of the protocol, was explicitly defined. The specified dose range for the IMP was changed from between 2.2 × 109 and 3.2 × 109 CFUs to between 6.7 × 107 and 6.7 × 109 CFUs along with the expiry date for the IMP and placebo in substantial amendment number 5. References to this specification range in the protocol were all updated to reflect this change.
The final version of the protocol (version 6), dated July 2012, is available on the NETSCC website at www.nets.nihr.ac.uk. All other trial documents are listed in Table 2, with details of amendments and version change.
| Study document | Version as of April 2014 | Changes |
|---|---|---|
| Consent form | Version 3.1, 20 January 2010 | Amendments made before the start of recruitment included additional questions for flagging of babies and use of personal identifiable data. Minor amendments to wording of question on confidentiality (to be in line with National Information Governance Board for Health and Social Care recommendations), form design and instructions on how to use it |
| Parent information leaflet | Version 5.1, 14 February 2011 | Amendments made during recruitment included minor formatting and typographical errors changes, updating of text on participant ‘flagging’ and consent for providing primary care trust details (in line with NHS Information Centre recommendations), and an update to the current appraisal of literature on probiotic use in infants |
| General practitioner letter | Version 3.0, 25 July 2012 | Amended at the time of sending to change the trial name from PREFER to PiPS and to add the mother’s name and date of birth |
| Transfer contact sheet | Version 1.0, 21 March 2010 | No amendments. For parents of babies that have been transferred between hospitals. This document will give the contact details of the study team at the receiving hospital |
| Form 1: trial entry | Version 3.0, 20 June 2011 | Amendment during recruitment. Correction of clerical errors, minor formatting of form design, and addition and clarification of instructions |
| Form 2: daily data collection | Version 3.0, 21 March 2011 | Addition during recruitment of a question about stool collection and instructions for form completion. Correction of clerical errors and clarifications |
| Form 3: clinical details of baby at transfer, discharge or death | Version 4.0, 20 June 2011 | Addition during recruitment of questions clarifying stool collection and the date of last dose, removal of redundant questions on respiratory support. Correction of clerical errors, minor formatting to form design and addition of instructions |
| Form 4: abdominal pathology | Version 3.0, 20 June 2011 | Removal during recruitment of a redundant subquestion on NEC, correction of clerical errors, minor formatting to form design and addition of instructions |
| Form 5: SAE/SUSAR reporting | Version 2.0, 30 June 2010 | Correction prior to the beginning of recruitment of clerical errors, minor formatting to form design and addition of instructions |
| Form 6: discontinuation or withdrawal | Version 3.0, 20 June 2011 | Correction during recruitment of clerical errors, minor formatting to form design and addition of instructions |
Monitoring
Central monitoring was performed throughout the trial by the NPEU Clinical Trials Unit co-ordinating centre to ensure that case report forms were complete and to detect unusual patterns and outliers in data. In addition, on-site monitoring was completed for 98% of participating sites, which involved inspection of site files and checks on compliance with trial procedures and good clinical practice (GCP). Site audits and source verification of data were carried out only if ‘triggered’ by central monitoring or from routine site visits undertaken by PiPS research; no such triggers occurred during the trial.
Trial oversight and patient and public involvement
The trial was overseen by an independent Trial Steering Committee and Data Monitoring Committee. The Trial Steering Committee first met during the planning stages of the trial and supported the investigators while clarification was being obtained from the MHRA around the status of probiotics and the requirement for a Clinical Trial Certificate. Thereafter, the committees met annually.
The membership of the Trial Steering Committee included a representative of the preterm baby charity Bliss who represented parents and who advised mainly on the development of the trial and the production of parent information.
The general conduct of the trial was managed by a trial co-investigators group including investigators, trial co-ordinator, research nurses, statisticians and other staff of the NPEU who met every 4–6 weeks.
Results
A total of 1315 infants were recruited from 24 hospitals within 60 miles of London (UK) over 37 months from July 2010. Details of recruitment, by site, are given in Table 3. The start of recruitment was delayed by 3 months so that it began 9 months after the core trial staff came into post; recruitment rates were initially slow, so that after 9 months only 121 babies had been recruited and it was 17 months before 50 babies were recruited in 1 month (Figure 3).
| Hospital | Start date | Total recruited | Consent withdrawn for use of data, n | Allocated to receive probiotic, n (%) | Allocated to receive placebo, n (%) |
|---|---|---|---|---|---|
| Homerton University Hospital, London | 10 June 2010 | 263 | 3 | 126 (19.4) | 134 (20.3) |
| Royal London Hospital, London | 21 July 2010 | 74 | – | 37 (5.7) | 37 (5.6) |
| Whipps Cross University Hospital, London | 4 August 2010 | 28 | – | 14 (2.2) | 14 (2.1) |
| Queen’s Hospital, Romford, London | 20 August 2010 | 60 | – | 29 (4.5) | 31 (4.7) |
| Newham University Hospital, London | 24 September 2010 | 66 | – | 32 (4.9) | 34 (5.2) |
| Medway Maritime Hospital, Kent | 21 October 2010 | 76 | – | 40 (6.2) | 36 (5.5) |
| St Thomas’ Hospital, London | 29 October 2010 | 97 | 2 | 46 (7.1) | 49 (7.4) |
| North Middlesex University Hospital, London | 3 December 2010 | 22 | – | 11 (1.7) | 11 (1.7) |
| St Peter’s Hospital, Chertsey | 8 December 2010 | 96 | – | 46 (7.1) | 50 (7.6) |
| William Harvey Hospital, Ashford, Kent | 9 December 2010 | 61 | – | 29 (4.5) | 32 (4.9) |
| Whittington Hospital | 24 January 2011 | 9 | – | 6 (0.9) | 3 (0.5) |
| King’s College Hospital, London | 11 February 2011 | 23 | – | 12 (1.9) | 11 (1.7) |
| Southend University Hospital, Essex | 11 February 2011 | 21 | – | 11 (1.7) | 10 (1.5) |
| Barnet Hospital, London | 16 February 2011 | 31 | – | 15 (2.3) | 16 (2.4) |
| University College Hospital, London | 16 February 2011 | 93 | – | 45 (6.9) | 48 (7.3) |
| University Hospital, Lewisham, London | 22 February 2011 | 24 | – | 12 (1.9) | 12 (1.8) |
| St George’s Hospital, London | 4 April 2011 | 56 | – | 28 (4.3) | 28 (4.2) |
| Croydon University Hospital, London | 28 April 2011 | 11 | – | 7 (1.1) | 4 (0.6) |
| John Radcliffe Hospital, Oxford | 12 May 2011 | 71 | – | 36 (5.5) | 35 (5.3) |
| Luton and Dunstable University Hospital, Herts | 20 June 2011 | 32 | – | 16 (2.5) | 16 (2.4) |
| Watford General Hospital | 20 June 2011 | 27 | – | 14 (2.2) | 13 (2.0) |
| Tunbridge Wells Hospital at Pembury, Kent | 1 October 2011 | 35 | – | 19 (2.9) | 16 (2.4) |
| Basildon University Hospital, London | 10 November 2011 | 11 | – | 5 (0.8) | 6 (0.9) |
| Royal Sussex County Hospital, Brighton | 4 May 2012 | 28 | – | 14 (2.2) | 14 (2.1) |
| Total | 1315 | 5 | 650 | 660 |
FIGURE 3.
Rates of recruitment from July 2010. The target of 1294 recruits stated in the protocol was reached after 36 months but the project management group, having first confirmed with the Research Ethics Committee, extended recruitment for 1 additional month up to the IMP expiry date, in order to maximise the power of the study.
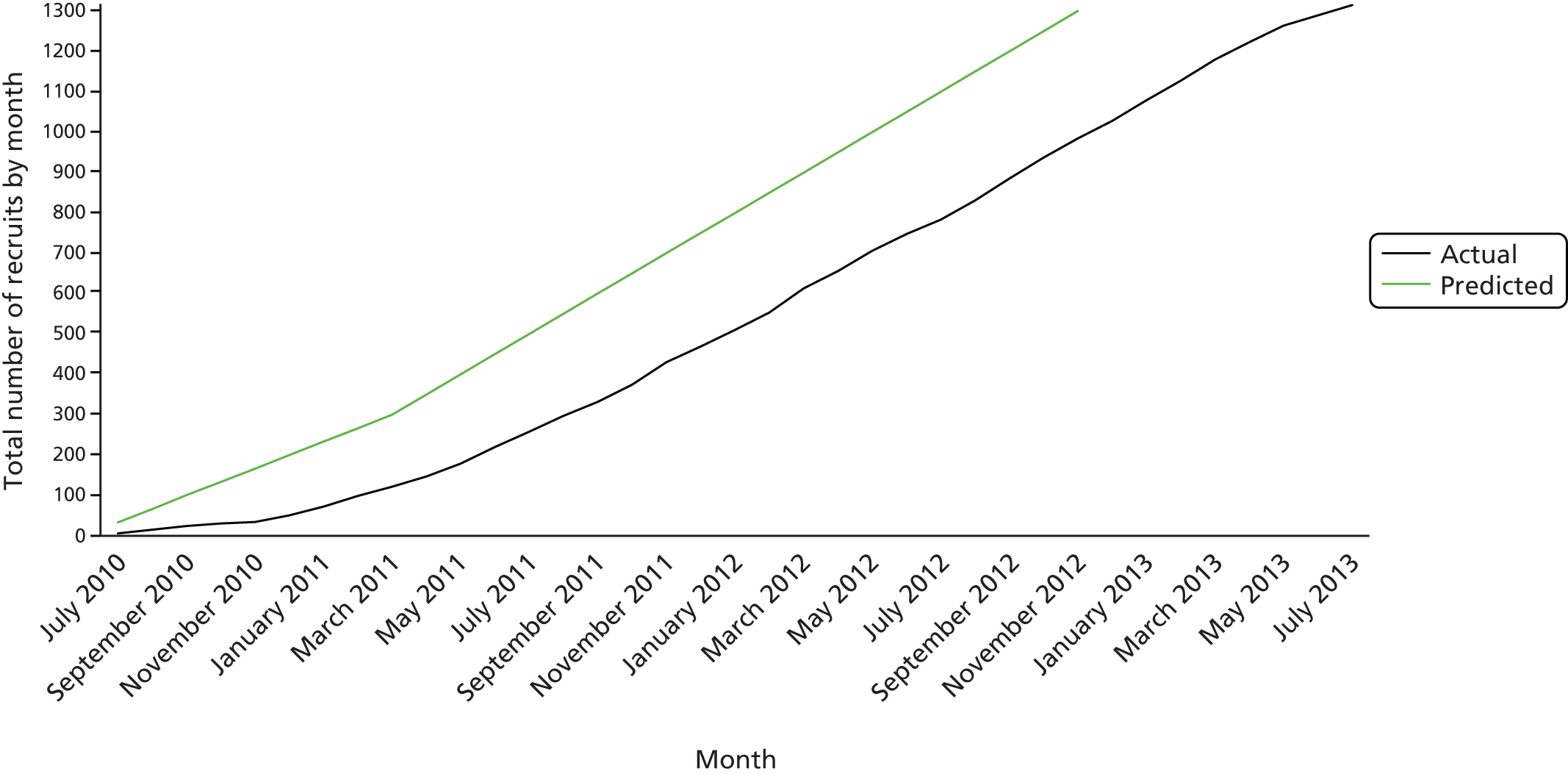
It subsequently emerged that a total of six protocol deviations concerning baseline data items had occurred at randomisation: one infant in each group was over 48 hours old and two in each group were outside the target gestational age range (Table 4).
| Protocol deviation | Trial group | |
|---|---|---|
| Probiotic (n = 650) | Placebo (n = 660) | |
| Randomisation > 48 hours’ postnatal age, n (%) | 1 (0.2) | 1 (0.2) |
| Gestational age < 23+0 weeks, n (%) | 0 | 1 (0.2) |
| Gestational age ≥ 30+6 weeks, n (%) | 2 (0.3) | 1 (0.2) |
There was almost complete retrieval of data entry forms (Table 5).
| Form | Retrieval rate | |
|---|---|---|
| Due | Received (% of total due) | |
| Consent form | 1315 | 1314 (99.9) |
| Form 1: trial entry | 1315 | 1314 (99.9) |
| Form 2: daily data collection | 1315 | 1310 (99.6) |
| Form 3: clinical details of baby at transfer, discharge or death | 2535 | 2530 (99.8) |
| Form 4: abdominal pathology | 609 | 609 (100) |
| Form 5: SAE/SUSAR | 2 | 2 (100) |
| Form 6: parental discontinuation or withdrawal | 37 | 37 (100) |
| Total | 7128 | 7116 (99.8) |
The parents of five babies withdrew consent for all participation, including for the use of data already collected (Figure 4).
FIGURE 4.
Participant flow. a, Of eight babies who received no intervention, seven died within 7 days of birth and one was transferred on the first day after birth to a hospital without the necessary approvals to administer the intervention; b, Two babies were incorrectly allocated packs containing B. breve BBG-001 and received that product throughout their course; c, Five sets of parents, four in the probiotic and one in the placebo group, withdrew from the trial completely and also withdrew consent for the use of data already collected.

Of the 1315 babies randomised, eight never received any intervention (seven of these died within 1 week of birth and one was transferred early to a hospital without the necessary regulatory approvals to administer the intervention). Two babies randomised to receive placebo were wrongly allocated probiotic packs.
Interim analyses
Interim analyses were undertaken when entry data for 371 babies and outcome data for death and NEC were available for 138 babies and again when entry data for 936 babies and outcome data for 598 babies were available. The results were reviewed by the Data Monitoring Committee, which recommended that recruitment should continue with no changes in target numbers.
Chapter 4 Final analysis
Baseline data
Maternal and baby characteristics were similar between the two groups (Tables 6 and 7). Nine per cent of babies were born outside the recruiting centre and 26% were randomised within the first 24 hours. The median age at which the first dose of intervention was administered was 44 hours.
| Characteristic | Trial group | |
|---|---|---|
| Probiotic (n = 650) | Placebo (n = 660) | |
| Ethnic group, n (n/N, %) | ||
| White | 374 (57.8) | 362 (55.4) |
| Indian | 28 (4.3) | 33 (5.0) |
| Pakistani | 17 (2.6) | 20 (3.0) |
| Bangladeshi | 32 (4.9) | 30 (4.6) |
| Black African | 96 (14.8) | 100 (15.3) |
| Black Caribbean | 32 (4.9) | 31 (4.7) |
| Other | 68 (10.5) | 77 (11.8) |
| Missing | 3 | 7 |
| Maternal (years) | ||
| Mean (SD) | 30.6 (6.5) | 30.9 (6.6) |
| Range | 15–58 | 15–58 |
| Missing | 0 | 1 |
| Antenatal steroid use, n (n/N, %) | ||
| Yes, started within 24 hours of birth | 168 (26.1) | 167 (25.5) |
| Yes, started over 24 hours before birth | 412 (63.9) | 440 (67.1) |
| None | 65 (10.1) | 49 (7.5) |
| Missing | 5 | 4 |
| Membrane rupture more than 24 hours before birth, n (n/N, %) | ||
| Yes | 171 (27.2) | 187 (29.1) |
| No | 458 (72.8) | 456 (70.9) |
| Missing | 21 | 17 |
| Chorioamnionitis diagnosed clinically within 24 hours of birth, n (n/N, %) | ||
| Yes | 88 (14.4) | 80 (13.0) |
| No | 523 (85.6) | 537 (87.0) |
| Missing | 39 | 43 |
| Maternal antibiotics within 24 hours of birth, n (n/N, %) | ||
| Yes | 220 (36.5) | 226 (36.1) |
| No | 383 (63.5) | 400 (63.9) |
| Missing | 47 | 34 |
| Characteristic | Trial group | |
|---|---|---|
| Probiotic (n = 650) | Placebo (n = 660) | |
| Postnatal age at randomisation (hours) | ||
| Median | 35.3 | 35.4 |
| IQR | 23.8–43.3 | 23.4–43.6 |
| Range | 0.5–50.5 | 0.7–48.2 |
| < 24 hours, n (%) | 167 (25.7) | 172 (26.1) |
| 24 to ≤ 48 hours, n (%) | 482 (74.2) | 487 (73.8) |
| > 48 hours, n (%) | 1 (0.2) | 1 (0.2) |
| Gestational age at birth (weeks) | ||
| Median | 28.0 | 28.0 |
| IQR | 26.1–29.4 | 26.1–29.6 |
| Range | 23.0–31.6 | 22.6–31.0 |
| < 23 weeks, n (%) | 0 | 1 (0.2) |
| 23 to < 24 weeks, n (%) | 20 (3.1) | 17 (2.6) |
| 24 to < 25 weeks, n (%) | 60 (9.2) | 60 (9.1) |
| 25 to < 26 weeks, n (%) | 69 (10.6) | 73 (11.1) |
| 26 to < 28 weeks, n (%) | 166 (25.5) | 168 (25.5) |
| 28 to < 30 weeks, n (%) | 217 (33.4) | 219 (33.2) |
| ≥ 30 weeks, n (%) | 118 (18.2) | 122 (18.5) |
| Sex | ||
| Male, n (%) | 374 (57.5) | 370 (56.1) |
| Female, n (%) | 276 (42.5) | 290 (43.9) |
| Babies born per pregnancy | ||
| Singleton, n (%) | 457 (70.3) | 459 (69.6) |
| Multiple, n (%) | 193 (29.7) | 201 (30.5) |
| If multiple, babies born, n (% of multiples) | ||
| 1 | 2 (1.0) | 0 |
| 2 | 167 (86.5) | 175 (87.1) |
| 3 | 19 (9.8) | 23 (11.4) |
| 4 | 5 (2.6) | 3 (1.5) |
| Born in enrolling hospital, n (n/N, %) | ||
| Yes | 589 (90.6) | 603 (91.5) |
| No | 61 (9.4) | 56 (8.5) |
| Missing | 0 | 1 |
| Mode of delivery, n (n/N%, ) | ||
| Vaginal birth | 309 (47.5) | 310 (47.0) |
| Caesarean before labour onset | 221 (34.0) | 204 (31.0) |
| Caesarean after labour onset | 120 (18.5) | 145 (22.0) |
| Missing | 0 | 1 |
| Forceps or ventouse used, n (n/N, %) | ||
| Yes | 13 (2.0) | 16 (2.5) |
| No | 634 (98.0) | 638 (97.6) |
| Missing | 3 | 6 |
| Main cause of preterm birth, n (n/N, %) | ||
| Prelabour rupture of membranes | 184 (28.5) | 182 (27.7) |
| Preterm labour | 245 (37.9) | 276 (42.0) |
| Antepartum haemorrhage | 54 (8.4) | 63 (9.6) |
| Pregnancy-induced hypertension | 54 (8.4) | 34 (5.2) |
| Other maternal illness | 66 (10.2) | 54 (8.2) |
| Poor fetal growth (mother well) | 43 (6.7) | 48 (7.3) |
| Missing | 4 | 3 |
| Birthweight (g) | ||
| n | 650 | 660 |
| Mean (SD) | 1039 (311.7) | 1043 (317.0) |
| Range | 450–2200 | 475–1935 |
| Birthweight ≤ 1000 g, n (%) | 317 (48.8) | 327 (49.5) |
| Birthweight > 1000 g, n (%) | 333 (51.2) | 333 (50.5) |
| Birthweight z-score | ||
| n | 649 | 657 |
| Mean (SD) | –0.43 (1.04) | –0.42 (1.05) |
| Range | –3.7 to 3.9 | –3.7 to 4.1 |
| Missing | 1a | 3a |
| Heart rate > 100 b.p.m. 5 minutes after birth, n (n/N, %) | ||
| Yes | 599 (92.4) | 593 (90.4) |
| No | 49 (7.6) | 63 (9.6) |
| Missing | 2 | 4 |
| Apgar score 5 minutes after birth, n (n/N, %) | ||
| 0–3 | 25 (3.9) | 15 (2.3) |
| 4–6 | 86 (13.5) | 96 (15.0) |
| 7–10 | 524 (82.5) | 531 (82.7) |
| Missing | 15 | 18 |
| CRIB II71 | ||
| n | 606 | 622 |
| Mean (SD) | 8.9 (3.5) | 8.8 (3.4) |
| Range | 2–20 | 1–19 |
| Missing | 44 | 38 |
Several of the highest recruiting hospitals are sited in multicultural inner-city areas: this is reflected in the spread of ethnicity, with 57% of babies overall being born to white women, 20% to Afro-Caribbean women and 12% to women whose families were from the Indian subcontinent.
Ninety-one per cent of the babies had been exposed to antenatal corticosteroid, 28% were born following pregnancies with rupture of the placental membranes more than 24 hours previously and 36% had been exposed to maternal antibiotics within 24 hours of birth.
The median gestational age was 28+0 weeks, 48% being born before 28 weeks. Mean birthweight was 1041 g, 49% being born at or below a birthweight of 1000 g.
Other early characteristics
Of 22 babies with major malformations, three in the probiotic and two in the placebo group died before discharge from hospital.
There were 1281 (98%) babies who received enteral nutrition within the first 14 days, of whom 96% received some maternal breast milk. In 48.5% of these, the maternal milk was augmented with either donor breast or formula milk (Table 8). Of the 29 babies who received no milk in the first 14 days, 18 died, 13 in the probiotic group and five in the placebo group. Almost all of the babies received antibiotics in the first 5 days after birth, and around 70% were given more antibiotic between day 6 and day 14. In total, 10.8% received antacid; antacid administration was recorded because of the possibility that raising the gastric pH would impact the microbiome.
| Characteristic | Trial group | |
|---|---|---|
| Probiotic (n = 650) | Placebo (n = 660) | |
| Age at first dose of intervention (hours) | ||
| N | 633 | 638 |
| Median age (hours) | 43.9 | 44.3 |
| IQR | 31.1–52.1 | 32.2–51.1 |
| Congenital malformationsa | ||
| Yes, n/N (%) | 30 (4.6) | 37 (5.6) |
| No, n/N (%) | 620 (95.4) | 622 (94.4) |
| Missing, n | 0 | 1 |
| If Yes | ||
| Minor,b n (% of congenital malformations) | 19 (63.3) | 24 (68.6) |
| Major,b n (% of congenital malformations) | 11 (36.7) | 11 (31.4) |
| Missing, n | 0 | 2 |
| Enteral feeding in the first 14 days,c postnatal age at first feed (days) | ||
| Number fed within 14 days of birth | 634 | 647 |
| Mean age (SD) | 3.2 (1.9) | 3.2 (1.9) |
| Median age (days) | 3 | 3 |
| IQR | 2–4 | 2–4 |
| Range | 1–14 | 1–14 |
| Type of milk received (0–14 days) | ||
| Any maternal breast milk, n (% of those fed in first 14 days) | 602 (92.6) | 625 (94.7) |
| Any donor breast milk, n (% of those fed in first 14 days) | 131 (20.2) | 139 (21.1) |
| Any formula, n (% of those fed in first 14 days) | 223 (34.3) | 226 (34.2) |
| Maternal breast milk only (0–14 days) | ||
| Yes, n (%) | 300 (46.2) | 306 (46.4) |
| No, n (%) | 350 (53.8) | 354 (53.6) |
| Antacids and antibiotic use (0–14 days)d | ||
| Any antacid given, n (%) | 64 (9.9) | 77 (11.7) |
| Antibiotics given in first 5 days, n (%) | 647 (99.5) | 651 (98.6) |
| Antibiotics given between day 6 and day 14, n (%) | 452 (69.5) | 471 (71.4) |
Compliance
A total of 76 (5.8%) babies discontinued the intervention early, 28 at parental request and the others for clinical indications. These include a small number of babies who in the early stages of the trial discontinued the intervention early because they were transferred to a hospital that did not have the regulatory approvals to administer the intervention.
On average, infants received around 87% of the recommended doses between randomisation and 36 weeks’ postmenstrual age or death if sooner (Table 9).
| Trial group | ||
|---|---|---|
| Probiotic (n = 650) | Placebo (n = 660) | |
| Permanent early discontinuation, n (%) | 35 (5.4) | 41 (6.2) |
| Reason for permanent early discontinuation, n (n/N, %) | ||
| Parental request | 14 (2.2) | 14 (2.1) |
| Clinician recommendation | 21 (3.2) | 26 (3.9) |
| Missing | 0 | 1 |
| Per cent recommended dosesa taken between randomisation and 36 weeks’ postmenstrual age, n | 597 | 608 |
| Mean (SD), % | 86.7 (21.3) | 87.8 (19.5) |
| Missing, % | 31 | 34 |
| Data unreliable and set to missing,b % | 22 | 18 |
The number of those born at higher gestation age falling below the ‘whisker’ on Figure 5 is largely because of babies being discharged from hospital before 36 weeks’ postmenstrual age. It is thought likely that the wider range of compliance at extremely low gestation age is because of those babies having more episodes when the clinicians chose to omit doses, although the effect is only apparent in the probiotic and not the placebo group.
FIGURE 5.
Adherence to trial intervention by allocation, gestational age and intention to treat for (a) probiotic group; and (b) placebo group. Box and whisker plot by gestational age and percentage of recommended doses that were given. The whiskers represent the adjacent values (1.5 × interquartile range).
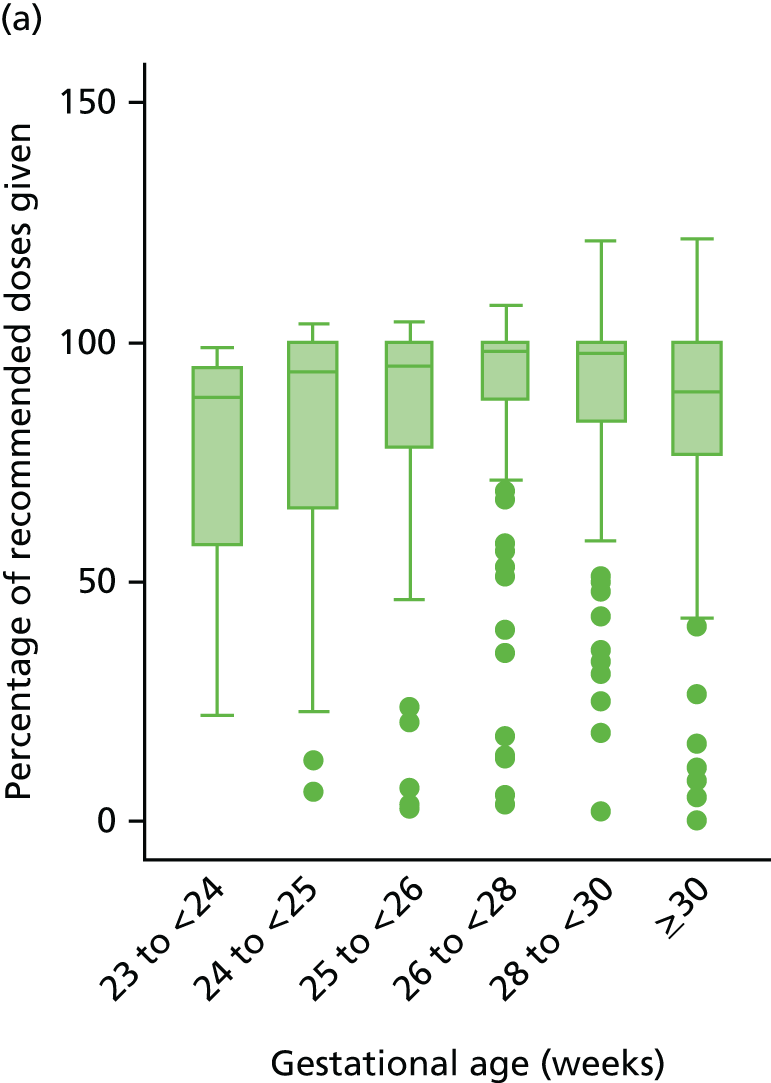

Quality of the interventions
No organisms other than B. breve BBG-001 were grown from any of the sachets returned to the laboratory at Barts Health NHS Trust.
The number of viable B. breve BBG-001 in the returned sachets fell as predicted during the recruitment period (Figure 6). The average number of viable organisms in the sachets measured in November 2013 (1 month after the final dose of intervention was given in the trial) was 1.5 × 108 CFUs with a range from 7.0 × 107 to 1.5 × 108 CFUs. The lowest count remained above the level of 6.7 × 107 CFUs that had been agreed with the MHRA to be the low threshold for dosage.
FIGURE 6.
Viability of B. breve BBG-001 in returned sachets over the recruitment period. The y-axis represents the count of B. breve BBG-001 expressed in log10 CFUs. The x-axis represents actual date for the batch of B. breve BBG-001 used in the PiPS trial and time in months since manufacture for all batches. The green and black lines were provided by the manufacturer and relate to two earlier batches of the product. The counts are per sachet and extend for 42 months after manufacture. The blue line is the count per dose of B. breve BBG-00,1 as made up for the PiPS trial. The blue triangles represent the mean of all measurements made since the previous triangle. The continuous horizontal line at around 8.7 log10-CFUs represents the estimated single dose used in the trial of Kitajima et al. 43 The horizontal interrupted line at 8.3 log10-CFUs represents the lower cut-off point per sachet of the batch of B. breve BBG-001 provided by the manufacturer for the PiPS trial. When prepared as for the trial this equates to 1 ml providing a dose of 6.7 × 107 or 7.8 log10-CFUs.

Primary outcomes by intention to treat
There was no evidence that administration of the probiotic had a beneficial effect on any of the primary outcomes. The proportion of infants who had an episode of NEC Bell stage 2 or 3 was 10.0% in the probiotic group, compared with 9.4% in the placebo group (adjusted RR 0.93, 95% CI 0.68 to 1.27); the corresponding figures for late-onset sepsis were 11.7% and 11.2% (adjusted RR 0.97, 95% CI 0.73 to 1.29) and for death were 8.5% and 8.3% (adjusted RR 0.93, 95% CI 0.67 to 1.30) (Table 10).
| Primary analysis | Trial group | Adjusteda RR (95% CI) | |
|---|---|---|---|
| Probiotic (n = 650), n (%) | Placebo (n = 660), n (%) | ||
| Late-onset sepsisb | 73 (11.2) | 77 (11.7) | 0.97 (0.73 to 1.29) |
| NECc | 61 (9.4) | 66 (10.0) | 0.93 (0.68 to 1.27) |
| Death before discharge from hospital | 54 (8.3) | 56 (8.5) | 0.93 (0.67 to 1.30) |
Subgroup analyses of primary outcomes by intention to treat
The prevalence of infection associated with probiotic administration was reduced (from 7.3% to 2.8%) in the subgroup born at 28 and 29 weeks (adjusted RR 0.39, 99% CI 0.16 to 0.96). There were no other differences for the prespecified subgroup analyses for the three primary outcomes or for exploratory analyses for subgroups with birthweight > 1 kg versus < 1 kg (Figure 7).
FIGURE 7.
Forest plots of subgroup analyses of primary outcomes by intention to treat. (a) NEC; (b) late-onset sepsis; and (c) death at discharge.
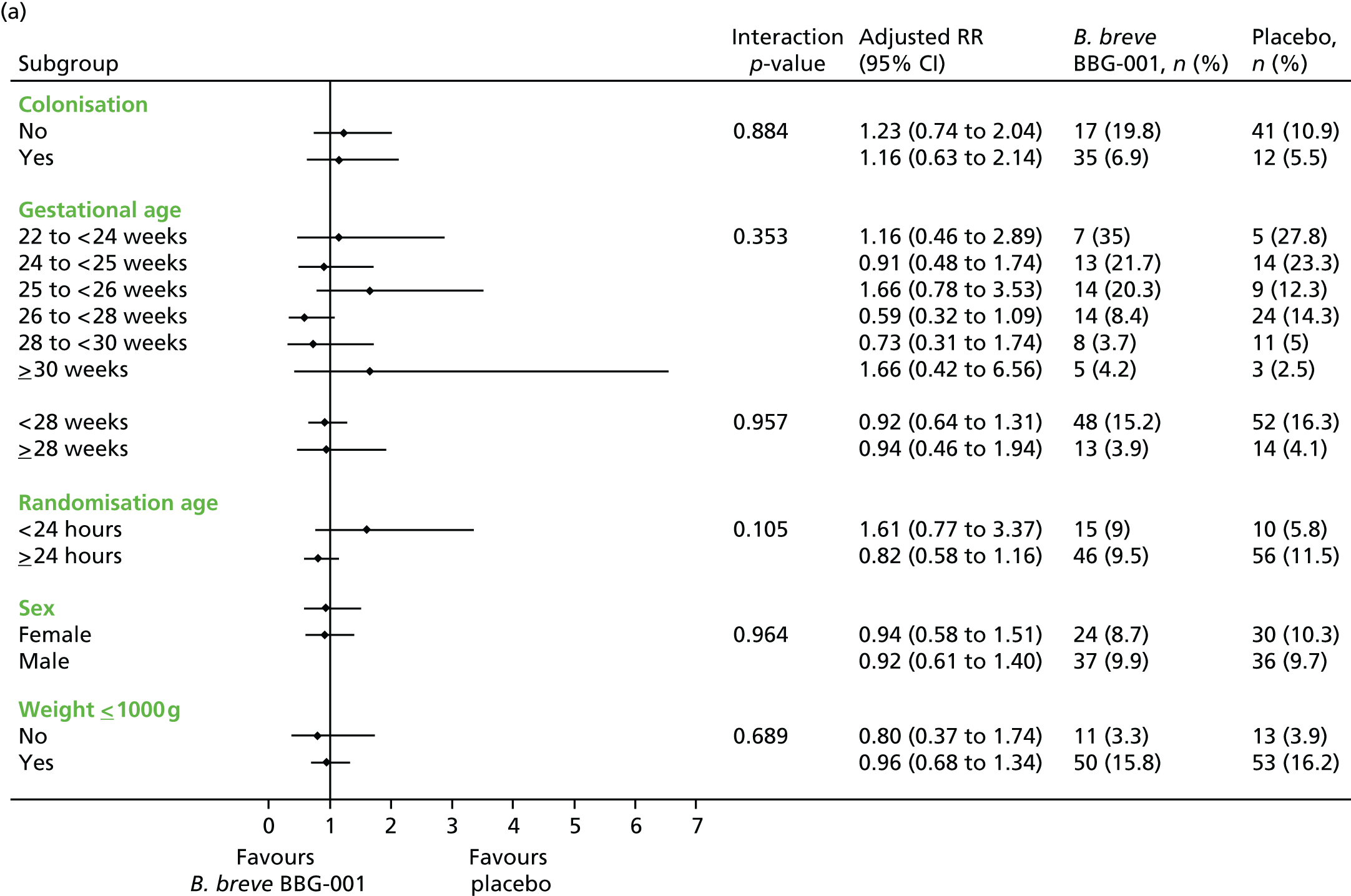

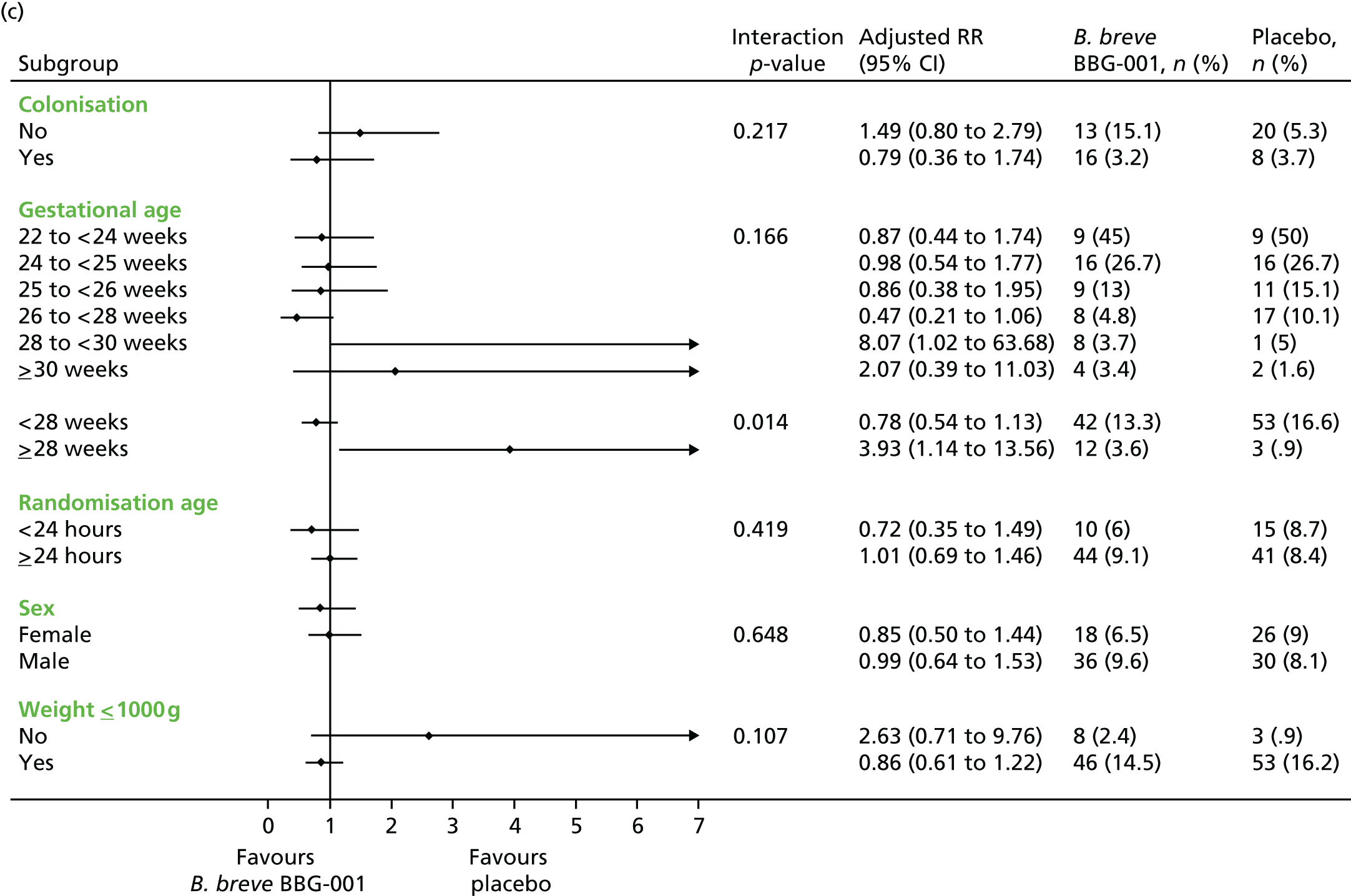
Severity of necrotising enterocolitis
Prespecified exploratory analyses showed no evidence of any differences in the age at onset of NEC (median postnatal age 30 weeks in both groups) or in severity (62% of cases categorised as stage 3 in probiotic vs. 68% placebo). The primary causes of death were also similar, with 21 of 54 deaths in the probiotic group and 24 of 56 in the placebo group being attributed either to late-onset sepsis or to NEC (Table 11).
| Outcome | Trial group | Adjusteda RR (99% CI) | |
|---|---|---|---|
| Probiotic (n = 650), n (%) | Placebo (n = 660), n (%) | ||
| NEC Bell stage 3 | 38 (5.9) | 45 (6.8) | 0.85 (0.51 to 1.41) |
| Surgery for NEC stage ≥ 2 | 35 (5.4) | 39 (5.9) | 0.89 (0.53 to 1.51) |
| Death attributed to NEC stage ≥ 2 | 9 (1.4) | 14 (2.1) | 0.62 (0.21 to 1.84) |
| Postmenstrual age (weeks) at onset of NEC stage ≥ 2, median (IQR) | 30.0 (27.9 to 32.6) | 30.1 (28.0 to 32.1) | –0.71 (–2.4 to 1.0) |
| Spontaneous intestinal perforation | 7 (1.1) | 4 (0.6) | 1.30 (0.71 to 2.3) |
| Deaths attributed to infection, n (%) | 12 (1.8) | 10 (1.5) | 1.10 (0.66 to 1.80) |
Secondary outcomes by intention to treat
There was also no evidence of benefit for any of the secondary outcomes including other measures of late-onset sepsis (Table 12); the proportion of infants with any positive blood culture after 72 hours was 28.6% in the probiotic group and 31.2% in the placebo group. The range of infecting bacteria was similar between the groups, with Enterobacteriaceae or staphylococci being identified in the majority of cases. There were three bloodstream infections attributable to antibiotic-resistant bacteria in the probiotic group and eight in the placebo group.
| Secondary outcomes | Trial group | Adjusteda RR (99% CI) | |
|---|---|---|---|
| Probiotic (n = 650), n (%) | Placebo (n = 660), n (%) | ||
| Late-onset sepsisb, NECc or death at discharge home | 143 (22.0) | 147 (22.3) | 0.99 (0.79 to 1.25) |
| Late-onset sepsis-related and microbiological outcomes | |||
| Positive blood culture for skin commensal | 141 (21.7) | 161 (24.4) | 0.88 (0.69 to 1.13) |
| Any blood culture taken after 72 hours | 490 (75.4) | 519 (78.6) | 0.97 (0.92 to 1.02) |
| Number of blood cultures per infant after 72 hours, median (IQR) | 2 (1 to 4) | 2 (1 to 5) | 0 (0–0) |
| Bloodstream infection by organism | |||
| Enterobacteriaceae | 23 (3.5) | 29 (4.4) | 0.80 (0.41 to 1.59) |
| Enterococcus | 13 (2.0) | 14 (2.1) | 0.92 (0.35 to 2.43) |
| Staphylococcus | 21 (3.2) | 17 (2.6) | 1.26 (0.56 to 2.82) |
| Fungi | 5 (0.8) | 5 (0.8) | 1.00 (0.20 to 5.06) |
| Other non-skin commensals | 22 (3.4) | 22 (3.3) | 0.93 (0.44 to 1.96) |
| Antibiotic-resistant bloodstream infection | |||
| MRSA | 0 | 3 (0.5) | Too few data |
| VRE | 1 (0.2) | 0 | Too few data |
| ESBL-producing Gram-negative bacteria | 1 (0.2) | 5 (0.8) | Too few data |
| Gentamicin resistant | 1 (0.2) | 0 | Too few data |
| Isolates of organisms from other normally sterile sites | |||
| Suprapubic urine | 1 (0.2) | 1 (0.2) | Too few data |
| Cerebrospinal fluid | 5 (0.8) | 6 (0.9) | 0.83 (0.18 to 3.80) |
| Pleural cavity | 1 (0.2) | 0 | Too few data |
| Peritoneum | 13 (2.0) | 10 (1.5) | 1.31 (0.45 to 3.84) |
| Other (joint fluid) | 0 | 1 (0.2) | Too few data |
| B. breve BBG-001 from any normally sterile site | 0 | 0 | Too few data |
| Total days of antibiotics after 72 hours, median (IQR) [range] | 10 (4 to 23) [0 to130] | 11 (4–24) [0 to 202] | 0 (–2 to 1) |
| Total days of antifungals after 72 hours, median (IQR) [range] | 0 (0 to 0) [0 to 154] | 0 (0 to 0) [0 to 79] | 0 (0 to 0) |
| Enteral feeding and growth | |||
| n | 649 | 660 | |
| Reached full feeds, n (%) | 613 (94.5) | 619 (93.8) | 0.91 (0.79 to 1.06)d |
| Died before reaching full feeds, n (%) | 32 (4.9) | 37 (5.6) | |
| Missing, n | 1 | 0 | |
| Postnatal age at first full feed, (150 ml of milk/kg/day), days | |||
| Median | 14 | 14 | |
| 99% CI | 13 to 16 | 13 to 16 | |
| IQR | 10 to 22 | 10 to 22 | |
| Change in weight z-score (from baseline to 36 weeks’ postmenstrual age) | |||
| n | 648 | 657 | |
| Mean (SD) | –1.33 (0.93) | –1.38 (0.92) | 0.03 (–0.08 to 0.15) |
| Range | –4.62 to 1.8 | –6.76 to 2.69 | |
| Other morbidities | |||
| Survivors to 36 weeks’ postmenstrual age, n | 595 | 604 | |
| Bronchopulmonary dysplasia (any O2 at 36 weeks’ postmenstrual age), n (n/N, %) | 239 (40.2) | 223 (36.9) | 1.02 (0.87 to 1.20) |
| Severe bronchopulmonary dysplasia at 36 weeks’ postmenstrual agee | 87 (14.6) | 73 (12.1) | 1.13 (0.80 to 1.60) |
| n | 646 | 657 | |
| Hydrocephalus and/or porencephaly and/or periventricular leucomalacia noted at any timef | 46 (7.1) | 37 (5.6) | 1.23 (0.72 to 2.12) |
| n | 600 | 605 | |
| Worst stage of retinopathy of prematurity ≥ stage 3 in either eye | 23 (3.8) | 25 (4.1) | 0.91 (0.44 to 1.88) |
| Length of stay | |||
| n | 647 | 657 | |
| Total length of hospital stay (days), median (IQR) | 68 (48 to 98) | 66 (46 to 95) | 1 (–4 to 6) |
| n | 649 | 658 | |
| Intensive care stay (days), median (IQR) | 10 (5 to 32) | 12 (5 to 32) | 0 (–1 to 1) |
| High-dependency unit stay (days), median (IQR) | 20 (6 to 34) | 17 (5 to 33) | 1 (–1 to 3) |
Infections that were not bloodstream infections were similar between the groups and there were no reports of growth of Bifidobacterium from any normally sterile site.
Rates of other major neonatal morbidities, administration of antimicrobials, time to establish full enteral feeds, growth up to 36 weeks’ postmenstrual age and length of stay were similar between the groups (see Table 12).
The Kaplan–Meier plot of time to first full feed at 150 ml of milk/kg/day is a Figure 8.
FIGURE 8.
Kaplan–Meier plot of time to first full feed at 150 ml of milk/kg/day by intention to treat. HR, hazard ratio.
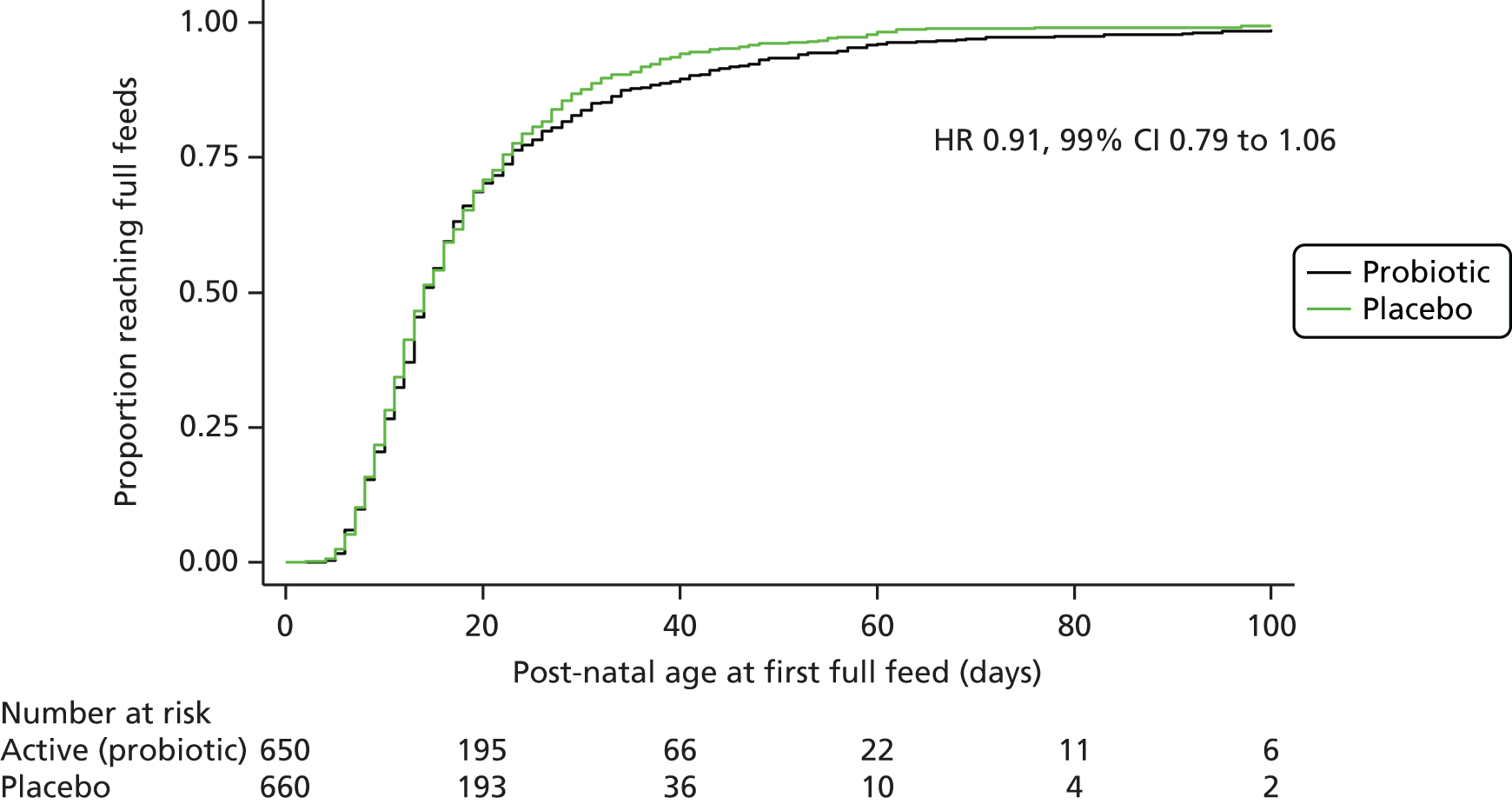
One or both of the stool samples collected from 38 (out of 611, 6.2%) infants in the probiotic and 35 (out of 619, 5.7%) in the placebo group were colonised with antibiotic-resistant bacteria (Table 13).
| Secondary outcome | Trial group | Adjusteda RR (99% CI) | |
|---|---|---|---|
| Probiotic (n = 650), n (%) | Placebo (n = 660), n (%) | ||
| Stool culture at 2 weeks’ postnatal age, 1266 infants alive, 1186 (94%) stool samples received | |||
| B. breve BBG-001, n (% of received) | 436 (73.8) | 122 (20.5) | 3.51 (2.83 to 4.34) |
| MRSA | 1 (0.2) | 2 (0.3) | Too few data |
| VRE | 0 | 1 (0.2) | Too few data |
| ESBL-producing Gram-negative bacteria | 18 (3.0) | 20 (3.4) | 0.76 (0.36 to 1.61) |
| Stool PCR at 2 weeks’ postnatal age | |||
| PCR positive, n (% of tested) | 416 (84) | 177 (35) | 2.42 (2.06 to 2.85) |
| B. breve BBG-001 positive by culture or PCR | 505 (85) | 219 (37) | 2.30 (1.99 to 2.66) |
| Stool culture at 36 weeks’ postmenstrual age, 1245 infants alive, 1043 (84%) stool samples received | |||
| B. breve BBG-001, n (% of received) | 438 (83.6) | 253 (48.7) | 1.69 (1.50 to 1.91) |
| MRSA | 1 (0.2) | 0 | Too few data |
| VRE | 3 (0.6) | 1 (0.2) | 2.97 (0.15 to 57.67) |
| ESBL-producing Gram-negative bacteria | 19 (3.6) | 18 (3.5) | 0.98 (0.44 to 2.18) |
Stool colonisation by Bifidobacterium breve by intention to treat
Stools were received at the microbiology laboratory at Barts Health NHS Trust from 1186 (94%) of the 1266 babies still alive at 2 weeks’ postnatal age and from 1043 (83%) of the 1235 babies still alive at 36 weeks’ postmenstrual age.
By 2 weeks’ postnatal age, B. breve BBG-001 was detected by culture in 73.8% of the stool samples available from babies in the probiotic group and in 20.5% of those from the placebo group, rising to 83.6% and 48.7%, respectively, at 36 weeks’ postnatal age (see Table 13).
At 2 weeks’ postnatal age, 1007 samples contained sufficient stool to also test for the presence of B. breve BBG-001 using PCR. Of the 123 samples from babies in the probiotic group that were culture negative, 69 (56.1%) were positive by PCR, and of the 405 negative samples in the placebo group 94 (24.0%) were positive. A total of 85% of babies in the probiotic and 37% in the placebo group had B. breve BBG-001 detected in their stools using either culture or PCR (see Table 13).
Of the 504 babies in the probiotic group who had stools cultured at both 2 weeks’ postnatal and 36 weeks’ postmenstrual age, 54 (10.7%) were positive for B. breve BBG-001 on only the first occasion, 104 (20.6%) were positive only on the second and 317 (62.9%) on both occasions. In the placebo group, 23 of 495 (4.6%) were positive on only the first occasion, 169 (34.1%) on only the second and 75 (15.2%) on both occasions.
Total colonisation rates by culture were monitored by site during recruitment and ranged at 2 weeks’ postnatal age from 37.5% to 80.3% (Table 14). During the course of the trial those hospitals with higher colonisation rates were often those with smaller numbers of babies and at no time were there hospitals that were clearly high outliers so as to trigger additional training from the trial research nurses.
| Enrolling centre | Colonisation with B. breve BBG-001, n (% at site) | |
|---|---|---|
| Yes (n = 724) | No (n = 462) | |
| Homerton University Hospital | 142 (63.7) | 81 (36.3) |
| University College Hospital | 51 (57.3) | 38 (42.7) |
| St Peter’s Hospital, Chertsey | 54 (60.7) | 35 (39.3) |
| St Thomas’ Hospital, London | 46 (54.8) | 38 (45.2) |
| John Radcliffe Hospital | 34 (50.0) | 34 (50.0) |
| Medway Maritime Hospital | 49 (68.1) | 23 (31.9) |
| The Royal London Hospital | 35 (54.7) | 29 (45.3) |
| St George’s Hospital, London | 35 (66.0) | 18 (34.0) |
| Newham University Hospital | 49 (80.3) | 12 (19.7) |
| William Harvey Hospital, Ashford | 33 (58.9) | 23 (41.1) |
| Royal Sussex County Hospital | 9 (37.5) | 15 (62.5) |
| Queen’s Hospital, Romford | 28 (57.1) | 21 (42.9) |
| Tunbridge Wells Hospital at Pembury | 19 (57.6) | 14 (42.4) |
| Luton and Dunstable Hospital | 17 (54.8) | 14 (45.2) |
| Watford General Hospital | 17 (63.0) | 10 (37.0) |
| Barnet Hospital | 18 (62.1) | 11 (37.9) |
| University Hospital Lewisham | 14 (66.7) | 7 (33.3) |
| Whipps Cross Hospital | 18 (72.0) | 7 (28.0) |
| King’s College Hospital | 15 (68.2) | 7 (31.8) |
| Southend University Hospital | 11 (61.1) | 7 (38.9) |
| North Middlesex University Hospital | 12 (70.6) | 5 (29.4) |
| Basildon Hospital | 8 (72.7) | 3 (27.3) |
| Croydon University Hospital | 5 (45.5) | 6 (54.6) |
| Whittington Hospital | 5 (55.6) | 4 (44.4) |
Determinants of colonisation
The results of the analysis of determinants of colonisation at 2 weeks in the probiotic group are presented in Table 15. In the final model, only increasing gestational age in weeks was statistically significantly associated with increased colonisation (OR 1.36; p < 0.0001), and the administration of any antibiotic beyond the fifth day after birth was significantly associated with less colonisation (OR 0.26; p = 0.027).
| Characteristic | Single factor models | Model 2 | Model 3 | Final model |
|---|---|---|---|---|
| Postnatal age at first dose (hours) | OR 0.99 (p = 0.0659) | |||
| Gestational age at birth (weeks) | OR 1.42 (p < 0.0001a) | OR 1.33 (p < 0.0001) | OR 1.40 (p < 0.0001) | OR 1.36 (p < 0.0001) |
| Birthweight (kg) | OR 1.00b (p < 0.0001) | |||
| Apgar score at 5 minutes | OR 1.15 (p = 0.0122) | |||
| Day of first feed | OR 0.81 (p < 0.0001) | OR 0.90 (p = 0.0725) | ||
| Sex (male, female) | OR 1.40 (p = 0.1614) | |||
| Multiple birth (single/multiple) | OR 0.98 (p = 0.9209) | |||
| Ethnic group | p = 0.4881 | |||
| White vs. Indian | OR 0.73 | |||
| White vs. Pakistani | OR 2.91 | |||
| White vs. Bangladeshi | OR 2.19 | |||
| White vs. black African | OR 0.81 | |||
| White vs. black Caribbean | OR 1.18 | |||
| White vs. other | OR 2.00 | |||
| Antenatal steroids prior to birth (yes/no) | OR 1.00 (p = 0.9913) | |||
| Membrane rupture > 24 hours before birth (yes/no) | OR 1.16 (p = 0.5808) | |||
| Antibiotics in 24 hours before birth (yes/no) | OR 1.44 (p = 0.1635) | OR 1.94 (p = 0.0199) | OR 1.76 (p = 0.0506) | |
| Chorioamnionitis in 24 hours before birth (yes/no) | OR 0.80 (p = 0.5010) | |||
| Delivery mode (caesarean/vaginal delivery) | OR 0.62 (p = 0.0434) | |||
| Formula and breast milk (day 1–14) | OR 2.21 (p = 0.0056) | |||
| Formula only (day 1–14) | OR 1.29 (p = 0.7415) | |||
| Breast milk only (day 1–14) | OR 0.52 (p = 0.0151) | |||
| Antacids (day 1–14) | OR 1.24 (p = 0.6096) | |||
| Antibiotics (day 6–14) | OR 0.14 (p < 0.0001) | OR 0.21 (p = 0.0004) | OR 0.24 (p = 0.0014) | OR 0.26 (p = 0.0027) |
Administration to the mother of antibiotic in the 24 hours preceding birth was marginally associated with an increased OR (1.76; p = 0.0506) and increasing age in days at the start of enteral milk feeds with decreased colonisation (OR 0.90; p = 0.0725).
Secondary analysis by colonisation with Bifidobacterium breve at 2 weeks’ postnatal age
At 2 weeks’ postnatal age, stool colonisation data were available for 1186 babies in 724 (61.0%), of whom B. breve BBG-001 was detected either by culture or by PCR.
Baseline data by colonisation
The baseline characteristics of mothers and babies, together with early clinical data collected after randomisation by colonisation status at 2 weeks are presented in Tables 16–18 and show few differences between the groups other than an under-representation among the colonised babies of those who had received only maternal breast milk and of those who had received any antibiotic after the fifth day of life.
| Characteristic | Colonisation with B. breve BBG-001 | |
|---|---|---|
| Yes (n = 724) | No (n = 462) | |
| Ethnic group | ||
| White, n (%col, %row) | 407 (56.5, 60.1) | 270 (58.7, 39.9) |
| Indian, n (%col, %row) | 31 (4.3, 57.4) | 23 (5.0, 42.6) |
| Pakistani, n (%col, %row) | 26 (3.6, 81.3) | 6 (1.3, 18.8) |
| Bangladeshi, n (%col, %row) | 38 (5.3, 70.4) | 16 (3.5, 29.6) |
| Black African, n (%col, %row) | 99 (13.8, 55.9) | 78 (17.0, 44.1) |
| Black Caribbean, n (%col, %row) | 39 (5.4, 68.4) | 18 (3.9, 31.6) |
| Other, n (%col, %row) | 80 (11.1, 62.0) | 49 (10.7, 38.0) |
| Missing, n | 4 | 2 |
| Mother’s age (years) | ||
| n | 724 | 461 |
| Mean (SD) | 30.7 (6.5) | 31.1 (6.62) |
| Minimum to maximum | 15 to 58 | 16 to 58 |
| Missing | 0 | 1 |
| Antenatal steroid use | ||
| Yes, started within 24 hours of birth, n (%col, %row) | 177 (24.6, 57.8) | 129 (28.2, 42.2) |
| Yes, started over 24 hours before birth, n (%col, %row) | 476 (66.2, 61.6) | 297 (64.9, 38.4) |
| None, n (%col, %row) | 66 (9.2, 67.4) | 32 (7.0, 32.7) |
| Missing, n | 5 | 4 |
| Membrane rupture more than 24 hours before birth | ||
| Yes, n (%col, %row) | 193 (27.4, 59.2) | 133 (29.8, 40.8) |
| No, n (%col, %row) | 511 (72.6, 61.9) | 314 (70.3, 38.1) |
| Missing, n | 20 | 15 |
| Chorioamnionitis diagnosed clinically within 24 hours of birth | ||
| Yes, n (%col, %row) | 87 (12.9, 58.0) | 63 (14.6, 42.0) |
| No, n (%col, %row) | 588 (87.1, 61.4) | 370 (85.5, 38.6) |
| Missing, n | 49 | 29 |
| Antibiotics in 24 hours before birth | ||
| Yes, n (%col, %row) | 251 (37.1, 60.9) | 161 (36.7, 39.1) |
| No, n (%col, %row) | 426 (62.9, 60.5) | 278 (63.3, 39.5) |
| Missing, n | 47 | 23 |
| Characteristic | Colonisation with B. breve BBG-001 | |
|---|---|---|
| Yes (n = 724) | No (n = 462) | |
| Postnatal age at randomisation (hours) | ||
| n | 724 | 462 |
| Median | 35.2 | 35.0 |
| IQR | 22.9 to 43.5 | 24.0 to 43.7 |
| Range | 0.5 to 48.0 | 1.0 to 48.2 |
| ≤ 24 hours, n (%col, %row) | 201 (27.8, 63.6) | 115 (24.9, 36.4) |
| 24 to < 48 hours, n (%col, %row) | 523 (72.2, 60.2) | 346 (74.9, 39.8) |
| > 48 hours, n (%col, %row) | 0 | 1 (0.2, 100) |
| Gestational age at birth (weeks) | ||
| n | 724 | 462 |
| Median | 28.4 | 27.6 |
| IQR | 26.7 to 29.7 | 25.7 to 29.1 |
| Range | 23 to 31.6 | 22.6 to 30.9 |
| < 23 weeks, n (%col, %row) | 0 | 1 (0.2, 100) |
| 23 to < 24 weeks, n (%col, %row) | 13 (1.8, 43.3) | 17 (3.7, 56.7) |
| 24 to < 25 weeks, n (%col, %row) | 47 (6.5, 48.0) | 51 (11.0, 52.0) |
| 25 to < 26 weeks, n (%col, %row) | 70 (9.7, 54.3) | 59 (12.8, 45.7) |
| 26 to < 28 weeks, n (%col, %row) | 172 (23.8, 57.7) | 126 (27.3, 42.3) |
| 28 to < 30 weeks, n (%col, %row) | 272 (37.6, 66.7) | 136 (29.4, 33.3) |
| ≥ 30 weeks, n (%col, %row) | 150 (20.7, 67.6) | 72 (15.6, 32.4) |
| Sex | ||
| Male, n (%col, %row) | 401 (55.4, 59.9) | 268 (58.0, 40.1) |
| Female, n (%col, %row) | 323 (44.6, 62.5) | 194 (42.0, 37.5) |
| Babies born per pregnancy | ||
| Singleton, n (%col, %row) | 496 (68.5, 59.5) | 338 (73.2, 40.5) |
| Multiple, n (%col, %row) | 228 (31.5, 64.8) | 124 (26.8, 35.2) |
| If multiple, babies born, n (%col, %row) | ||
| 1 | 2 (0.9, 100) | 0 |
| 2 | 194 (85.1, 64.5) | 107 (86.3, 35.6) |
| 3 | 26 (11.4, 63.4) | 15 (12.1, 36.6) |
| 4 | 6 (2.6, 75.0) | 2 (1.6, 25.0) |
| Born in enrolling hospital, n (%col, %row) | ||
| Yes | 667 (92.1, 61.3) | 421 (91.1, 38.7) |
| No | 57 (7.9, 58.2) | 41 (8.9, 41.8) |
| Mode of delivery | ||
| Vaginal birth, n (%col, %row) | 332 (45.9, 60.4) | 218 (47.3, 39.6) |
| Caesarean before labour onset, n (%col, %row) | 240 (33.2, 62.8) | 142 (30.8, 37.2) |
| Caesarean after labour onset, n (%col, %row) | 152 (21.0, 60.1) | 101 (21.9, 39.9) |
| Missing, n | 0 | 1 |
| Forceps or ventouse used | ||
| Yes, n (%col, %row) | 18 (2.5, 66.7) | 9 (2.0, 33.3) |
| No, n (%col, %row) | 704 (97.5, 61.1) | 448 (98.0, 38.9) |
| Missing, n | 2 | 5 |
| Main cause of preterm birth | ||
| Prelabour rupture of membranes, n (%col, %row) | 211 (29.3, 62.2) | 128 (27.8, 37.8) |
| Preterm labour, n (%col, %row) | 275 (38.2, 59.4) | 188 (40.9, 40.6) |
| Antepartum haemorrhage, n (%col, %row) | 69 (9.6, 63.9) | 39 (8.5, 36.1) |
| Pregnancy-induced hypertension, n (%col, %row) | 51 (7.1, 67.1) | 25 (5.4, 32.9) |
| Other maternal illness, n (%col, %row) | 68 (9.4, 62.4) | 41 (8.9, 37.6) |
| Poor fetal growth (mother well), n (%col, %row) | 46 (6.4, 54.1) | 39 (8.5, 45.9) |
| Missing, n | 4 | 2 |
| Birthweight (g) | ||
| n | 724 | 462 |
| Mean (SD) | 1084 (312.4) | 1000 (304.9) |
| Range | 450 to 1935 | 475 to 1845 |
| Birthweight ≤ 1000 g, n (%col, %row) | 309 (42.7, 55.0) | 253 (54.8, 45.0) |
| Birthweight > 1000 g, n (%col, %row) | 415 (57.3, 66.5) | 209 (45.2, 33.5) |
| Birthweight z-scorea | ||
| n | 722 | 460 |
| Mean (SD) | –0.39 (1.02) | –0.44 (1.09) |
| Range | –3.69 to 4.09 | –3.65 to 3.92 |
| Missing | 2 | 2 |
| Heart rate > 100 b.p.m. 5 minutes after birth | ||
| Yes, n (%col, %row) | 669 (92.5, 61.7) | 415 (90.6, 38.3) |
| No, n (%col, %row) | 54 (7.5, 55.7) | 43 (9.4, 44.3) |
| Missing, n | 1 | 4 |
| Apgar score 5 minutes after birth | ||
| 0–3, n (%col, %row) | 20 (2.8, 55.6) | 16 (3.6, 44.4) |
| 4–6, n (%col, %row) | 91 (12.8, 59.1) | 63 (14.1, 40.9) |
| 7–10, n (%col, %row) | 598 (84.3, 61.8) | 369 (82.4, 38.2) |
| Missing, n | 15 | 14 |
| CRIB II71 | ||
| n | 676 | 443 |
| Mean (SD) | 8.3 (3.4) | 9.4 (3.5) |
| Range | 1 to 20 | 2 to 19 |
| Missing | 48 | 19 |
| Characteristic | Colonised with B. breve BBG-001 (n = 724) | Not colonised with B. breve BBG-001 (n = 462) |
|---|---|---|
| Enteral feeding in the first 14 daysa | ||
| Number fed within 14 days of birth | 723 | 455 |
| Postnatal age at first feed (days) | ||
| Mean age (SD) | 3.0 (1.6) | 3.5 (2.3) |
| Median age (IQR) | 3 (2–4) | 3 (2–4) |
| Range | (1–12) | (1–14) |
| Type of milk received (0–14 days), n (%) | ||
| Any maternal breast milk | 691 (95.4) | 439 (95.0) |
| Any donor breast milk | 157 (21.7) | 99 (21.4) |
| Any formula | 290 (40.1) | 130 (28.1) |
| Maternal breast milk only (0–14 days), n (%) | ||
| Yes | 307 (42.4) | 238 (51.5) |
| No | 417 (57.6) | 224 (48.5) |
| Antacid and antibiotic use (0–14 days),b n (%) | ||
| Any antacid given | 72 (9.9) | 50 (10.8) |
| Antibiotics given in first 5 days | 717 (99.0) | 457 (98.9) |
| Antibiotics given between days 6 and 14 | 459 (63.4) | 374 (80.1) |
| Total days of antibiotics days 0–14, median (IQR) | 8 (3–18) | 14 (7–30) |
Primary outcomes by colonisation
Despite the three primary outcomes all being less frequent in those babies who were colonised with B. breve BBG-001 at 2 weeks, there was no clear evidence of benefit associated with colonisation. The proportion of infants who had an episode of NEC Bell stage 2 or 3 was 6.5% in the colonised group, compared with 12.6% in the non-colonised group (adjusted RR 0.68, 99% CI 0.43 to 1.09); the corresponding figures for late-onset sepsis were 9.3% and 14.3% (adjusted RR 0.88, 99% CI 0.59 to 1.31) and for death were 3.3% and 7.1% (adjusted RR 0.68, 99% CI 0.35 to 1.29) (Table 19).
| Primary outcome | Colonisation with B. breve BBG-001 | ||
|---|---|---|---|
| Yes (n = 724), n (%) | No (n = 462), n (%) | Adjusteda RR (99% CI) | |
| Late-onset sepsisb | 67 (9.3) | 66 (14.3) | 0.88 (0.59 to 1.31) |
| NECc | 47 (6.5) | 58 (12.6) | 0.68 (0.43 to 1.09) |
| Death | 24 (3.3) | 33 (7.1) | 0.68 (0.35 to 1.29) |
Secondary outcomes by colonisation
Despite trends towards reduced rates of adverse outcomes in those babies who were colonised at 2 weeks, there was no clear evidence of the benefit for any of the secondary outcomes other than the time to full feeds, which was lower in those infants who were colonised (Table 20 and Figure 9).
| Secondary outcomes | Colonised with B. breve BBG-001 (n = 724), n (%) | Not colonised with B. breve BBG-001 (n = 462), n (%) | Adjusteda RR (99% CI) |
|---|---|---|---|
| Late-onset sepsis,b NECc or death at discharge home | 106 (14.6) | 114 (24.7) | 0.79 (0.60 to 1.06) |
| Late-onset sepsis-related and microbiological outcomes | |||
| Positive blood culture for skin commensal | 134 (18.5) | 130 (28.1) | 0.78 (0.60 to 1.01) |
| Any blood culture taken after 72 hours | 517 (71.4) | 396 (85.7) | Not convergedd |
| Number of blood cultures per infant after 72 hours, median (IQR) | 2 (0 to 4) | 3 (1 to 6) | 0 (0 to 0) |
| Bloodstream infection by organism | |||
| Enterobacteriaceae | 23 (3.2) | 23 (5.0) | 0.88 (0.42 to 1.82) |
| Enterococcus | 12 (1.7) | 13 (2.8) | 0.81 (0.29 to 2.22) |
| Staphylococcus | 20 (2.8) | 14 (3.0) | 1.1 (0.45 to 2.64) |
| Fungi | 5 (0.7) | 3 (0.7) | 1.09 (0.17 to 7.13) |
| Other non-skin commensals | 19 (2.6) | 19 (4.1) | 0.83 (0.36 to 1.88) |
| Antibiotic-resistant bloodstream infection | |||
| MRSA | 0 | 2 (0.4) | Too few data |
| VRE | 0 | 1 (0.2) | Too few data |
| ESBL-producing Gram-negative bacteria | 2 (0.3) | 4 (0.9) | 0.30 (0.03 to 2.78) |
| Gentamicin resistant | 1 (0.1) | 0 | Too few data |
| Isolates of organisms from other normally sterile sites | |||
| Suprapubic urine | 0 | 1 (0.2) | Too few data |
| Cerebrospinal fluid | 5 (0.7) | 6 (1.3) | 0.67 (0.14 to 3.27) |
| Pleural cavity | 0 | 0 | |
| Peritoneum | 10 (1.4) | 9 (2.0) | 0.72 (0.22 to 2.33) |
| Other (joint fluid) | 0 | 1 (0.2) | Too few data |
| B. breve BBG-001 from any normally sterile site | 0 | 0 | Too few data |
| Total days of antibiotics after 72 hours, median (IQR) | 8 (3 to 18) | 14 (7 to 30) | 5 (4 to 7) |
| Total days of antifungals after 72 hours, median (IQR) [range] | 0 (0 to 0) [0 to 154] | 0 (0 to 3) [0 to 58] | 0 (0 to 0) |
| Feeding and growth | |||
| Reached full feeds, n (%) | 718 (99.2) | 447 (96.8) | |
| Died before reaching full feeds | 4 (0.6) | 13 (2.8) | |
| Postnatal age at first full feed (150 ml of milk/kg/day), days | |||
| Median | 13 | 17 | 1.36 (1.16 to 1.59)e |
| 99% CI of median | 12 to 14 | 15 to 18 | |
| IQR | 9 to 19 | 11 to 26 | |
| Change in weight z-score (from baseline to 36 weeks’ postmenstrual age) | |||
| n | 721 | 460 | |
| Mean (SD) | –1.33 (0.92) | –1.47 (0.91) | 0.08 (–0.05 to 0.21) |
| Range | –6.76 to 2.69 | –6.02 to 1.23 | |
| Other morbidities | |||
| Survivors to 36 weeks’ postmenstrual age, n | 699 | 428 | |
| Bronchopulmonary dysplasia (any O2 at 36 weeks’ postmenstrual age) | 238 (34.1) | 192 (44.9) | 0.80 (0.67 to 0.95) |
| Severe bronchopulmonary dysplasia at 36 weeks’ postmenstrual agef | 79 (11.3) | 72 (16.8) | 0.94 (0.66 to 1.34) |
| n | 645 | 658 | |
| Hydrocephalus and/or intraparenchymal cysts, n (n/N, %)g | 38 (5.3) | 40 (8.7) | 0.71 (0.41 to 1.24) |
| n | 691 | 441 | |
| Worst stage of retinopathy of prematurity ≥ stage 3 in either eye, n (n/N, %) | 23 (3.3) | 23 (5.2) | 0.65 (0.31 to 1.37) |
| Length of stay | |||
| n | 724 | 459 | |
| Total length of hospital stay (days), median (IQR) | 64 (46 to 91) | 75 (53 to 104) | 9 (4 to 14) |
| n | 724 | 461 | |
| Intensive care stay (days), median (IQR) | 8 (4 to 29) | 18 (5 to 39) | 4 (2 to 7) |
| High-dependency unit stay (days), median (IQR) | 16 (5 to 31) | 23 (9 to 37) | 4 (1 to 7) |
FIGURE 9.
Kaplan–Meier plot of time to first feed by colonisation with B. breve BBG-001 at 2 weeks’ postnatal age. HR, hazard ratio.
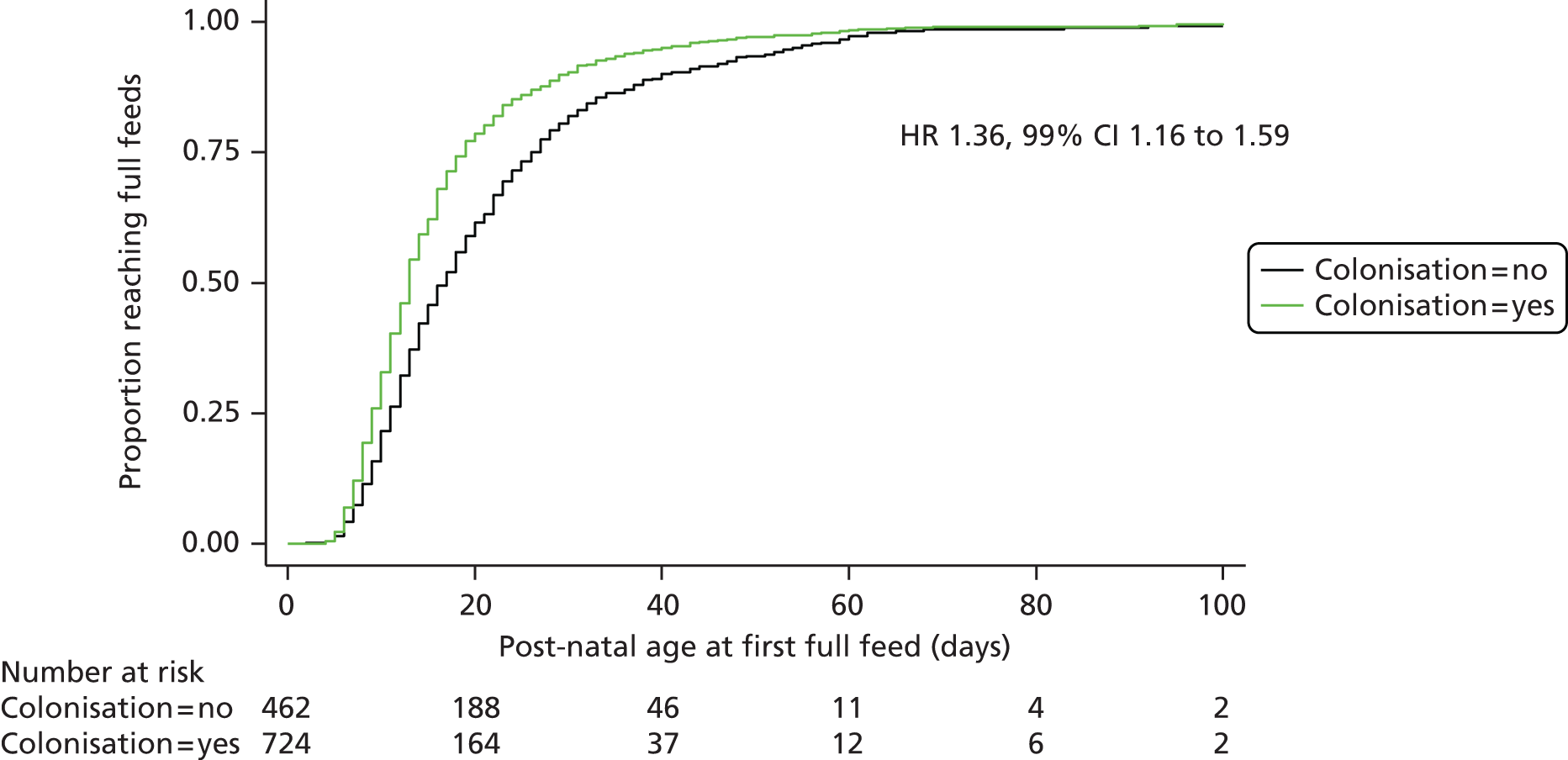
Safety
There were no reports of babies not tolerating the interventions. Although detailed data were not collected, our understanding was that those parents who requested discontinuation of the intervention did so because of intercurrent clinical problems, often suspected or proven NEC. We received no requests to ‘unblind’ the intervention.
There were no reports of positive culture of any bifidobacteria from any normally sterile site.
Analysed by intention to treat, there were no differences in the rates of stool colonisation by antibiotic-resistant bacterial strains either at 2 weeks’ postnatal or 36 weeks’ postmenstrual age. At 2 weeks, 4.1% of stools from babies colonised with B. breve BBG-001 were positive for ESBL-producing Gram-negative bacteria, compared with 1.7% of those not colonised with B. breve BBG-001 (Table 21); this difference is not statistically significant. At 36 weeks’ postmenstrual age, the rate of ESBL-producing Gram-negative bacterial colonisation was 3.8% in the colonised babies and 3.3% in the non-colonised babies.
| Secondary outcome | Colonised with B. breve BBG-001 (n = 724), n (%) | Not colonised with B. breve BBG-001 (n = 462), n (%) | Adjusteda RR (99% CI) |
|---|---|---|---|
| 2 weeks’ postnatal age | |||
| MRSA, n (%) | 3 (0.4) | 0 | Too few data |
| VRE, n (%) | 1 (0.1) | 0 | Too few data |
| ESBL-producing Gram-negative bacteria n (%) | 30 (4.1) | 8 (1.7) | 1.86 (0.72 to 4.80) |
| 36 weeks’ postmenstrual ageb | |||
| MRSA, n (n/N, %) | 1 (0.2) | 0 | Too few data |
| VRE, n (n/N, %) | 1 (0.2) | 3 (0.8) | 0.21 (0.01 to 4.09) |
| ESBL-producing Gram-negative bacteria n (n/N, %) | 23 (3.8) | 13 (3.3) | 1.18 (0.49 to 2.82) |
| Missing | 118 | 69 | |
There were two reports of SAEs (Table 22): one baby allocated to the placebo group suffered fatal toxic epidermal necrolysis and one baby allocated to the probiotic group survived a massive pulmonary haemorrhage that was initially, apparently incorrectly, thought to be associated with a transfusion reaction. Neither SAE was considered likely to be related to the interventions. There were no reports of SUSARs.
| Event | Trial group | |
|---|---|---|
| Probiotic (n = 650) | Placebo (n = 660) | |
| Toxic epidermal necrolysis (fatal), n (%) | 0 | 1 (0.2) |
| Pulmonary haemorrhage, n (%) | 1 (0.2) | 0 |
Chapter 5 Discussion
This is the largest trial to date investigating the potential of a probiotic intervention to prevent NEC, late-onset sepsis and death in preterm babies. The probiotic tested was B. breve strain BBG-001. The trial was undertaken and completed because, despite the publication of a number of randomised trials and meta-analyses together with some strong recommendations for routine use of probiotics, there were ongoing concerns about the quality of a number of the trials, lack of evidence as to what product might be useful and, in particular, a lack of confidence that the babies included in the trials were representative of the population in UK neonatal units.
The strengths of this trial are the use of a product with a single bacterial strain, which simplifies the interpretation of results; the monitoring of colonisation, so that the extent of successful colonisation of the active intervention group and cross-colonisation of the placebo group is known; and the size of the trial, as it has adequate statistical power to give clear answers about the prevention of NEC and late-onset sepsis. The aim was to recruit babies representative of the total English newborn infant population by having minimal exclusions, and by recruiting and starting the intervention soon after birth.
This is the first trial of a probiotic in the newborn infant to be performed to ICH-GCP standards.
As with other published trials of probiotics, there was no evidence of short-term harm but, in contrast to the sole other large published trial, the Australasian ProPrems trial,41 and the conclusions of the most recent Cochrane review of this topic,35 this intervention in this population of babies showed no evidence of benefit; in particular, there was no evidence of prevention of NEC.
This discussion will address the design and conduct of the trial, and the findings in the context of current understanding of the pathogenesis of NEC and severe late-onset sepsis, and it will consider implications for clinical practice and make recommendations for future research.
Trial design and conduct
Duration of trial
This trial relates to the prevention of major complications of preterm birth; the application for funding was made in 2005 with a view to trial staff coming into post in during 2006 with recruitment over 30 months from May 2007. In the event, staff came into post in September 2009, the set-up period was 3 months longer than expected and it took 36 months to complete recruitment. That a trial of such importance for the advancement of neonatal care should be so delayed is important for those planning and funding large trials.
The initial delays were in large part related to regulatory issues around the interventions and were perhaps inevitable. It became clear between the pilot and main trial that the intervention was to be classed as a medicine; this was, we believe, the first application for a probiotic intervention to be granted clinical trial authorisation and there was initially a lack of clarity about the standards that the product should meet.
The delays during the set-up phase and early recruitment related to the NHS research and development (R&D) approval processes and, to a lesser extent, in some hospitals, the lack of staff with GCP training, and are discussed in greater detail in this chapter. Once through these difficult early phases, the rate of recruitment was as had been predicted (see Figure 3).
Choice of product
A major criticism of published trials is the failure of the investigators to choose products based on laboratory evidence and evidence from animal studies and preclinical studies in babies supporting their possible efficacy. A problem has been the urgency felt by clinicians to identify interventions to prevent late-onset sepsis and NEC and the lack of such scientific evidence forcing them to select interventions on pragmatic grounds, testing simply what is available or, slightly preferable, a product used in a previous trial that suggested possible efficacy. 73 Our own choice of product was based on the evidence of nutritional advantage in the trial of Kitajima et al. ,43 the availability of a placebo, the potential to monitor stool colonisation and the knowledge that the product had been used extensively in Japan and was regarded as being safe. When it became clear that the intervention would be classified as a medicine and that a clinical trial certificate would be necessary, we were fortunate that the product is manufactured to high specification by the manufacturer and that data supporting its stability were available. This was particularly important when it became apparent that there would be difficulty obtaining a second batch of product in order to complete recruitment and an application had to be made to the MHRA to extend the use beyond the original shelf life. The granting of the extension was contingent on our continuing to monitor the viability of the product in each pack and to confirm the absence of contamination. The additional B. breve BBG-001 viability data gained (see Figure 6) and the absence of any contaminants strengthens confidence in the quality of this product.
Timing of start of intervention
There were a number of reasons why we were keen to begin the intervention early, the most important being that we were keen to be as inclusive as possible and, anecdotally, were conscious that as the clinical course of preterm babies becomes more complex in the days after birth, with events such as episodes of milk intolerance and suspected sepsis, staff and parents may become more reluctant to recruit babies into trials, resulting in the inadvertent exclusion of babies at greatest risk of complications. In addition, whether or not babies are enterally fed in the days after birth, they begin to acquire flora from birth and subsequently through handling and interventions such as passing feeding tubes. If one of the mechanisms by which probiotics might protect against NEC and late-onset sepsis is by modifying the developing flora and discouraging colonisation with potential pathogens, then it seems likely, although evidence is lacking, that efficacy will be enhanced if given early. Some clinicians still withhold feeds from babies at highest risk of NEC (i.e. those babies with growth restriction and evidence of intrauterine hypoxia); thus, delaying the probiotic until some milk is tolerated will inevitably delay its administration. For example, the protocol for the ProPrems trial74 involved early recruitment within 72 hours of birth but the intervention was not started until at least 1 ml of milk was being tolerated every 4 hours; consequently, the intervention was not started until a median age of 5 days (interquartile range 4–7 days),41 whereas in the two published trials39,42 the intervention was started irrespective of feeding at a median age of 2 days39 and 1 day. 42
We were successful in that the median age at randomisation in the PiPS trial was 35 hours (see Table 7) with the median age at the first dose of intervention being 44 hours; this was earlier than the median age at first feed, which was 3 days.
Not being able to prepare the interventions in whole milk created a difficulty in that, although our powders were completely indistinguishable, the probiotic powder was turbid when made up with water and we had to resort to using dilute milk to prepare them. We are uncertain whether or not the additional precaution of preparing the intervention in amber-coloured bottles was really necessary; none of our investigators ever suggested that they could distinguish between the active intervention and placebo preparations. Although it is not explicit in any of the published trials, several of which used no placebo (see Table 1), it seems likely that in some the reason for giving the probiotic intervention in milk was to achieve masking.
In our case the choice of the elemental formula in which to suspend the intervention, Neocate, was made after discussions with gastroenterology and dietitian colleagues. To have used breast milk, which would have been our first choice, was impracticable because of variable availability and opacity. It seemed inconceivable that 1 ml of one-eighth-strength Neocate could impose such a metabolic or immunological stress on the intestinal epithelium that it could cause a clinical problem. We were concerned, however, that the use of any formula might discourage some parents from agreeing to their baby entering the trial. Detailed data were not collected but we regularly questioned our investigator colleagues on whether or not this was a problem. After reassurance that it was believed to be safe and that it would not interfere with successful establishment of breastfeeding, there seemed to be very few instances in which it was a factor in parents refusing to enrol their baby.
Dosage
The trial published by Kitajima et al. in 199743 is the only other study to have used this product. In that study, the intervention was prepared in 2 ml of water and given in ‘two or three’ divided doses. We were not keen either to impose this workload on staff or to retain the product for long periods of time once made up. We increased the volume used to suspend the powder from 2 ml to 3 ml to make it easier for staff administering the products to avoid disturbing the maize starch residue when drawing up the 1-ml dose. We estimated that the minimum bacterial count we were likely to find even with a more dilute suspension would be around 6.7 × 107 CFUs, which remains within the range used in previously published trials.
This is the first trial to have monitored the numbers of bacteria that were being given to babies throughout recruitment. This was particularly important since the product was administered beyond the shelf life that had been specified by the manufacturer. We had undertaken to stop recruitment should the number of viable bacteria fall below the threshold recommended by the manufacturer of 2.2 × 108 CFUs (8.3 log10-CFUs) per sachet, despite the fact that the evidence for that number is not clear. The data we received from the laboratory during recruitment all suggested that the bacterial counts were declining at a predictable rate and remaining well above the threshold. The active intervention given to the final recruits was inevitably analysed after the end of the trial and the count in a single pack had fallen to the threshold. There is, however, no evidence that rates of successful colonisation reduced during the trial, and we have no evidence to suggest that the dose was inadequate.
The intervention was stopped at 36 weeks’ postmenstrual age rather than continuing it to discharge, as has been done in some trials, as it was felt that parents would prefer it not to be stopped when the baby went home. When babies went home earlier than 36 weeks staff were encouraged, if there was opportunity, to discontinue the intervention a few days before discharge.
Whether or not it is necessary to continue the intervention this long in order to achieve prolonged colonisation has not been systematically studied and is likely to be dependent on the gestational age of the baby, use of antibiotics and feeding. In one small study,75 it was noted that L. paracasei NFBC 338 could still be detected in the stool 2 weeks after a single dose given on day 4 to low-birthweight babies who received no antibiotic subsequent to the probiotic. In the PiPS trial, stools were obtained at just two time points, 2 weeks’ postnatal and 36 weeks’ postmenstrual age, so we have no information about coming and going of colonisation in between the two time points; however, as 9.5% of the 571 babies in the probiotic group who were successfully colonised at 2 weeks were no longer colonised at 36 weeks’ postmenstrual age, we would suggest that in current clinical practice it is probably necessary to continue the intervention at least until the baby is beyond the peak time of risk of NEC, around 32 weeks’ postmenstrual age.
None of the previously published trials gives detailed data about exactly how many doses of the intervention babies received, implying, in the majority, that the intervention was given continuously over the prescribed period. Given the anxiety often experienced by nursing staff about giving milk to babies with feed intolerance or any suggestion of early NEC or sepsis, this seems very unlikely. We left the decision of whether or not doses should be omitted to the local clinicians and suggested definite withholding only in the circumstance of suspected intestinal perforation, although we imagined that if a dose of the probiotic was inadvertently given in that situation it could not do any harm. We did give strong encouragement that after resolution of episodes of NEC, if the intervention had been discontinued, it be recommenced, and we are aware that this was done on multiple occasions. We received no reports whatsoever of problems tolerating the interventions and were pleased that the proportion of recommended doses given was high at 85%.
The use of necrotising enterocolitis and late-onset sepsis as trial end points
We had concerns about the objectivity of both NEC and late-onset sepsis as outcomes in clinical trials. The diagnosis of NEC is made on clinical and radiological features (intestinal intramural gas and/or perforation) supported by haematological markers and, in those who die or come to surgery, on the macroscopic and histological appearance of the bowel. The applicability of Bell staging, which for many years has been the method most frequently used to categorise cases, to the contemporary population of very preterm babies with NEC has been challenged,76 but the method has not been replaced by anything more reliable. The radiological sign of intramural gas can be very difficult to detect, with clinicians disagreeing about its presence; the final diagnosis in non-fatal, non-surgical cases is to a great extent dependent on the total picture and the experience of the clinicians and it was agreed that, for trial purposes, whether or not a baby had the disease and the staging thereof should primarily rest with the attending clinical staff. In practice, this is complicated by the way in which the clinical service is organised with only a small number of neonatal units providing neonatal surgery, which results in babies with suspected or proven NEC frequently moving between hospitals while the clinical picture is evolving. It was for these reasons that we felt that it was essential to review all cases with any abdominal pathology after data collection had ended. This process generated new queries to PIs, particularly when the final diagnosis provided did not fit the detailed data, for example stage 2 NEC with no diagnostic radiological features, and it resulted in changes to the final diagnosis in 10 cases. As a result we are as confident as we feel it is possible to be about the accuracy of the NEC diagnosis, but without this rather labour-intensive process we believe that a number of cases would have been wrongly categorised.
Many of the published trials studying the effect of probiotic interventions on sepsis include in their definition ‘any’ positive blood culture. One of the great difficulties in studying rates of neonatal sepsis has been reliably distinguishing between positive cultures of S. epidermidis that represent true infections and those that arise from contamination from the skin during blood sampling. It is possible that the great range of reported infection rates and the lack of consistency of effect on sepsis is in part attributable to inclusion of non-infected cases with positive blood cultures. The Australasian ProPrems trial,41 for which sepsis was the primary outcome, addressed this problem by using strict criteria to define infection with S. epidermidis involving duplicate positive cultures and duration of antibiotic use. The rationale underpinning the pragmatic approach taken for the PiPS trial, which for the primary outcome excluded all positive cultures of S. epidermidis, is described in appendix 1 of the protocol.
As planned, our microbiological outcome data were collected directly from hospital routine laboratories. Although we still believe that we would have had difficulty collecting reliable data about the antibiotic sensitivities of cultured bacteria from neonatal clinicians, this methodology was very laborious and, although we received a high level of support and co-operation from many hospital microbiologists, the process in some hospitals was complicated because of the subcontracting of pathology services. Although, on another occasion, we would follow the same procedure, we would also ask clinicians, in parallel, to report positive blood cultures, since these are unusual and important events and data are likely to be accurate. Such reports would augment and aid interpretation of the often rather complex data received from laboratories that received numerous samples, often seemingly with inadequate information from clinical staff as to their source and the indication for their collection.
The trial population
The gestational age range of eligible recruits (23+0 to 30+6 weeks’ gestation) was selected in order to capture the babies at highest risk of NEC and late-onset sepsis. The importance of gestational age as a risk factor is apparent within the trial data, with all three primary outcomes falling sharply with increasing gestation age within this target range (see Figure 7), but the greater number of births at higher gestational age means that the need for effective prevention remains high. Cochrane reviews of the role of probiotics to prevent NEC prior to that published in 201430,32 argued that the evidence of efficacy in preterm babies with a birthweight > 1 kg supported routine use. More recently, with publication of the ProPrems trial,41 that recommendation has been extended to include the extremely low-birthweight group with birthweight < 1 kg. In a recent review of the published literature, Abrahamsson et al. 73 are generally critical of the quality of published trials but nonetheless recommend that the most important need is for future trials to address the extremely low-birthweight group. The ProPrems trial41 did indeed find evidence of decreased NEC in a population of babies born before 32 weeks’ gestational age, but the rate of NEC in the placebo group was low, at 4.4%, and the upper 95% CI of the number needed to treat to prevent a single case of NEC was 333. There did appear to be a greater effect in the subgroup with a birthweight > 1 kg (0.3% probiotic vs. 3.2% placebo, compared with 4.3% probiotic vs. 5.9% placebo in the extremely low-birthweight group), but the numbers are small and the interaction p-value was 0.08. We would still argue that the case for routine use in the UK for babies of birthweight > 1 kg is not made and that the population included in the PiPS trial is the one we need to study.
Participating hospitals
The recruiting hospitals in the PiPS trial were all in or close to London, UK. The clinical service is organised into networks, with the care of the majority of babies born before 27 weeks’ gestation being in the larger tertiary centres. The recruiting hospitals included both tertiary and secondary centres in order to ensure a spread of gestational ages within the population. During the course of the trial some hospitals further afield expressed enthusiasm to join, but by that time the organisation of the trial was established and we did not have the capacity to increase the number of recruiting centres. The initial decision to confine the trial to the south-east of England was made for two reasons. The first was that in the early planning stages it was expected that the recruitment period for the PiPS trial would overlap with the randomised trial of oxygen targeting, the Benefits of Oxygen Saturation Targeting, Clinical Trial (BOOST),77 which involved many major neonatal centres but none in London. In the event, the beginning of recruitment to PiPS was delayed and hospitals close to London that had recruited to BOOST joined. The second was that considerable personal support by the team of trial research nurses would clearly be needed to both recruiting and ‘step-down’ centres in making up the interventions. This would have been more difficult and expensive had they been further afield. The alternative would have been to fund research nurses in participating centres but, with an intervention that continued until 36 weeks’ postmenstrual age and a pattern of clinical care that involves babies moving between intensive and lower-dependency units, it was concluded that that was impractical.
Setting the trial up and early recruitment problems
We had planned for a 6-month period from the beginning of the trial until the start of recruitment but required 9 months. The principal reasons for this were delays in gaining NHS R&D approvals and, in a number of hospitals, the lack of anyone on the staff who had received GCP training and could act as PI or take informed consent. Our original plan, because we knew that the babies would move between hospitals during the course of the intervention, was not to begin recruitment at any hospital in a network until all hospitals in that network had the necessary approvals in place. This would unquestionably have been an advantage, but such were the delays at some centres that it was not practicable. When a baby was recruited we asked which hospitals he/she might be transferred to in the hope that we could expedite the gaining of approvals in those places. This, however, was only partially successful, and in the early stages of recruitment a number of babies had to discontinue the intervention prematurely because they were transferred to a non-approved site.
Particularly in smaller hospitals, staff found it difficult to get time off to attend GCP courses when the online course was unavailable. As a consequence, few of the smaller hospitals had more than one person, or possibly two people, who could take consent. This problem was compounded by local NHS trust rules preventing nurses, however experienced and familiar with the trial they were, from taking consent in a trial involving a medicinal intervention.
These problems resulted not only in delay in opening sites (see Table 3) but also in very slow early recruitment (see Figure 3), so that after 6 months it had to be questioned whether or not the trial could be delivered. The possibility of engaging more recruiting sites was rejected and with a programme of constant hands-on encouragement and training from trial staff, coupled with support from the Medicines for Children Network staff based at Great Ormond Street Hospital in identifying research nurses, recruitment accelerated so that the total duration was only 6 months longer than originally planned.
The development of standardised procedures for the governance of research in the NHS over the past 20 years has greatly increased the quality and accountability of much clinical research but the resultant bureaucracy can at times be obstructive and still needs to be honed further. The extreme that we encountered was the situation of a hospital in which the staff were keen to collaborate and act as a continuing care site but who were prevented from doing so by their trust, which, because of the financial implications, would agree only if it could be a recruiting centre. Any baby recruited into the trial who was transferred to that hospital for step-down care had to discontinue the intervention despite the parents having agreed for their baby to receive the intervention until 36 weeks’ postmenstrual age and the willingness of the staff to administer it.
Less dramatic, but more frequent, and equally disruptive to the conduct of the trial, were examples of babies who were transferred to hospitals that could not have been predicted and the trial staff encountering such extremes of obstructive behaviour and seeming lack of insight into the importance of protocol adherence on the part of local R&D staff, who argued that their sole interest was to protect the patients, that approval could not be gained in time for the intervention to be continued smoothly. It was sometimes possible to accelerate things by the chief investigator ringing personally, on occasion to the hospital’s director of R&D, but this really should not be necessary and was not always possible or even effective.
Results
Identification of Bifidobacterium breve BBG-001
This is the first multicentre trial of a probiotic intervention in the newborn infant systematically to have studied the presence of the administered probiotic strain in stools as a marker of intestinal colonisation. It was beyond the scope of this trial to collect stools more frequently and so the data serve to provide a snapshot at the two selected time points. The 2-week time point was selected as the basis for the secondary analysis of primary outcomes by ‘colonisation’ because it was thought that in the majority of cases it would be before the onset of NEC and it was speculated that if the intervention was to provide protection it would perhaps have needed to colonise the intestine by around this time. We have no data for the presence of B. breve in the stools between these two time points, but it is clearly possible that, even within the group given the probiotic, colonisation may come and go as, of those babies with samples at both time points, 54 (63%) of the 86 babies who were not colonised at 36 weeks’ postmenstrual age had been colonised at 2 weeks. The selective medium used for culture was provided by the manufacturer and said to be strain specific; it was validated at species level by MALDI-TOF. It was decided early in the trial, if there was sufficient stool, to augment culture with PCR because of concerns that it was sometimes taking several days for samples to reach the laboratory. The PCR is based on a method reported by the manufacturer and again is described by the manufacturer as specific for the intervention strain. PCR, in the context of this trial, has the advantage not only that it might detect bacteria that have died during any delay in reaching the laboratory but also that it might detect bacteria whose growth is inhibited in culture by antibiotic given to the baby.
The proportion not colonised by culture but found to be colonised by PCR was 38% in the B. breve group and 18% in the placebo group. We have not separately evaluated the PCR in comparison with culture and cannot exclude the possibility that the PCR might have identified some strains inaccurately and be giving a high false-positive rate. In selecting strains of commensal bacteria to develop for commercial use, manufacturers will inevitably choose those that colonise readily. It seems unlikely that the high rates of cross-colonisation we found are confined to B. breve BBG-001 but, in the absence of such detailed colonisation data using any other probiotic products, we cannot comment further.
Successful colonisation by Bifidobacterium breve BBG-001 of babies in the probiotic arm of the trial
Despite the early administration of the probiotic intervention, using both culture and PCR we detected B. breve BBG-001 in the stools of only 85% of babies in the probiotic arm of the trial at 2 weeks’ postnatal age. We did not have the resource to do PCR at 36 weeks’ postmenstrual age; the number of those positive by culture alone had risen from 74% at 2 weeks to 84%.
The technique of PCR appears to be very sensitive and, although we were surprised not to detect higher colonisation at 2 weeks, there are multiple reasons why colonisation may not be complete. Antibiotic use in these babies, at least in the first few days after birth, was almost universal. Furthermore, over 10% received an antacid in the first 2 weeks. We were surprised that this number was so high, and it is likely that this had an impact on the acquisition of bacterial flora but the detail has not been researched. It was encouraging that over 90% of infants received some maternal breast milk, as the bifidogenic properties of breast milk are well recognised; however, the actual volumes received by many babies were likely to have been small. It is interesting that in the univariable analysis of determinants of colonisation (see Table 15) babies were disadvantaged by starting feeds later and seemed to be particularly advantaged, in this respect, if receiving a combination of breast and formula milk. One can speculate that this might be attributable to faster advancement of volumes of feeds or, possibly, to the bifidogenic properties of the breast milk being augmented by the addition of oligosaccharides to preterm formula. None of these feeding effects remained significantly associated with colonisation in the multivariable models, for which the overwhelming effects were the negative impact of prolonged antibiotic use and the advantage of higher gestational age.
The determinants of interactions between probiotic bacteria and the human infant gut are comparatively poorly understood. 78 For example, at the present time the extent to which newborn infants at different gestational ages express the specific epitopes required for binding of probiotics is not known. Adhesion to cell surface-associated structures has been shown to be an important determinant of metabolic and immune interactions for both commensal and pathogenic bacteria in animal models of colonisation and disease. 79,80 Motherway et al. 81 described type IVb tight adherence pilus expression and associated genetic determinants in B. breve UCC2003 using a mouse model of colonisation. The epitopes for pilus adhesion in this mouse model have yet to be identified. Specific human mucus binding pili have been described in the probiotic strain L. rhamnosus GG. 82 It is known that skin structures are poorly developed in infants born before 34 weeks’ gestation, that the skin develops rapidly in the few weeks after birth but that in the most preterm infants a fully functional stratum corneum may not have developed by 4 weeks’ postnatal age. 83 It may be that the lack of benefit associated with enteral supplementation with B. breve strain BBG-001 in this study reflects the immaturity of the gastrointestinal tract of the preterm infant in the early weeks of life. Further research may help to elucidate the importance of adhesion and other types of probiotic interaction in determining preterm infant outcomes.
Colonisation by Bifidobacterium breve BBG-001 of the placebo arm of the trial
In order to minimise the possibility of cross-contamination, local nursing staff received training from the trial staff emphasising the importance of completing preparation of the intervention for each baby, then cleaning all working surfaces before progressing to the next. In addition, staff were reminded of the importance of maintaining strictly routine infection control procedures in clinical areas. Despite these precautions, B. breve BBG-001 was detected by culture in 49% of infants in the placebo group by 36 weeks’ postmenstrual age (Table 13).
Pooled (probiotic- plus placebo-allocated babies) colonisation data by site were monitored by the trial management team throughout recruitment. We were conscious that cross-colonisation was taking place, as from a number of sites around 70% of stool samples were colonised. We were looking out for any sites in which there was a clear excess of colonised babies, but because of the movement of babies between sites and interpretation of small numbers it was never possible to be confident that any individual unit was a ‘high outlier’. Rather than target additional training at specific hospitals, we informed investigators that it was clear from the data that cross-colonisation was occurring and we reminded them of the importance of taking precautions.
The rates we found of colonisation of babies allocated to receive placebo were similar to those reported in the study of Kitajima et al. 43 using the same product (44% at 6 weeks after birth) and in our own pilot study (35% at 4 weeks after birth).
Colonisation data have recently been reported for a subset of babies (five in the probiotic group, seven in the placebo group and 31 non-enrolled babies) from a single site involved in the ProPrems trial. 41,84 The intervention included three bacterial strains, S. thermophilus TH-4, B. infantis BB-02 and B. lactis BB-12; stools were analysed by PCR and the presence of at least two of three strains was classified as cross-colonisation. All of the babies allocated to receive probiotic, one of those allocated to receive placebo and two of the other babies were colonised at postnatal ages ranging from 35 to 174 days. At first sight this rate of cross-colonisation seems low compared with our own, but the numbers are too small to be confident about this and it is not clear why the definition of colonisation involved two rather than one bacterial species.
It is most unlikely that cross-colonisation is unique to the PiPS trial.
The combined effects of incomplete colonisation of the active probiotic with only partial colonisation of the placebo group might lead to underestimation of any potential benefit. The intention-to-treat analysis undertaken for subgroups categorised by colonisation status does not suggest, however, that efficacy in this trial is impacted by cross-colonisation (see Figure 7). Nor, in the analysis of primary outcomes by colonisation at 2 weeks (see Table 19), is there clear evidence of advantage associated with B. breve BBG-001 colonisation.
Probiotics in Preterm infantS trial results: outcomes
We found no evidence of benefit for this probiotic intervention in this population of preterm babies for any of the three primary outcomes or for any of the secondary outcomes, which included measures of the severity and time of onset of NEC, other measures of late-onset sepsis and a range of important neonatal morbidities.
The failure to show a reduction in NEC or mortality is at variance with a number of meta-analyses,27,30–32,35,63 including the most recent Cochrane review, and with the recently published large multicentre ProPrems trial41 that recruited in Australia and New Zealand and which, while failing to find evidence of benefit for either sepsis or mortality, did show a protective effect for NEC (2.0% active intervention group vs. 4.4% placebo group). Interestingly, the time of onset of NEC Bell stage ≥ 2 at a postmenstrual age of 30 weeks is earlier in the PiPS trial than in the data based on a Canadian population, but is similar to preliminary data for time of onset of serious (surgical and/or fatal) NEC at a median postmenstrual age of 30 weeks (interquartile range 28–32 weeks’ postmenstrual age) in a 2-year prospective cohort of 14,294 babies born between 23 and 31 completed weeks of gestation admitted to neonatal units in England in 2012 and 2013. 85
Exposure to maternal corticosteroid and early maternal breast milk, both protective against NEC, was high in both the PiPS and the ProPrems trials, but the rates of NEC and pathogen-related sepsis are higher in in the placebo group in this trial (NEC 10.0% vs. 4.4% and pathogen-related sepsis 11.7% vs. 8.7%). The baseline data suggest that a higher proportion of the babies in the PiPS trial are small for their gestational age, which might account for some of this difference. Variations in rates of major morbidities in preterm populations are, however, well recognised and often difficult to explain, most likely being related to variation in baseline risk factors and characteristics of the clinical service. One comment that has been made in a number of commentaries on the possible routine use of probiotics is that probiotics might have greater effect in populations with ‘higher’ rates of NEC, but this is not supported by our findings.
We undertook subgroup analyses (see Figure 7), as described in the trial statistical analysis plan (see Appendix 12), together with an additional analysis to aid comparison with the ProPrems data,41 with the participants categorised by gestational age < 28 weeks versus ≥ 28 weeks. These subgroup analyses are important because in the most recent Cochrane review35 the previous recommendation,32 which stated that probiotics should be routinely used in those with a birthweight > 1 kg but that more evidence of efficacy was needed at lower birthweight, was revised and extended to suggest that routine use be considered at all birthweights. The ProPrems trial subgroup analyses by birthweight and gestational age suggested evidence of efficacy to reduce both NEC and sepsis in those of birthweight > 1 kg and 28 weeks’ gestation, but not in the smaller, less mature, babies. In contrast to the results of the ProPrems trial, we found no evidence of trends towards a decrease of NEC associated with B. breve BBG-001 with increasing gestation or birthweight. Inspection of the data suggests a trend towards lower rates of sepsis in those allocated to receive probiotic, but this does not reach statistical significance. The subgroup analyses do show two statistically significant effects: decreased rates of sepsis in the probiotic group (2.8%) compared with the placebo group (7.3%) (adjusted RR 0.39, 95% CI 0.16 to 0.96) for those born at 28 or 29 weeks of gestation and increased mortality in the probiotic group (12 cases, 3.6%) compared with the placebo group (three cases, 0.9%) (adjusted RR 3.93, 95% CI 1.14 to 13.56). However, a large number of tests have been performed, and the numbers are small, and these results should be treated with caution. The interaction tests for the subgroup analyses are all statistically underpowered. A possible confounder in the PiPS trial is the high rate of colonisation by B. breve BBG-001 of the placebo group. The intention-to-treat subgroup analysis of the primary outcomes by colonisation status at 2 weeks (see Figure 7) does not suggest, however, that efficacy is impacted by cross-colonisation. This conclusion is supported by the secondary analysis of the primary outcomes in those babies from whom a stool sample was received at 2 weeks, not by intention-to-treat but by colonisation status (see Table 19), which, while showing trends towards lower rates of sepsis, NEC and death in those colonised, fails to reach a significant difference for any outcome. The same is true for the analyses of secondary outcomes by colonisation status at 2 weeks, except for statistically significant results showing earlier achievement of full feeds and suggesting reduction of bronchopulmonary dysplasia associated with successful colonisation (see Table 20).
The conclusion of the logistic regression analysis performed to study determinants of successful colonisation in those infants who were given B. breve BBG-001, that increasing gestation is the most important determinant of successful colonisation and use of antibiotics beyond the fifth day after birth the most important association of failure to colonise, may be interpreted to suggest that in this trial population successful colonisation is perhaps simply a marker of babies at lower clinical risk of adverse outcomes and that this explains the trends towards fewer complications when analysing by colonisation.
Much of the reason for the strong recommendations of some authors for routine probiotic use is the finding of the meta-analyses of significant reduction in all-cause mortality. 35 Together, the ProPrems41 and PiPS trials have recruited over 2400 babies, which is over one-third of all babies recruited into over 20 probiotic trials. That neither ProPrems nor PiPS found any evidence of benefit to reduce mortality in intention-to-treat analyses has to raise serious concerns about the findings of the meta-analyses and consequent recommendations.
The secondary analysis of outcomes by colonisation at 2 weeks was planned because we expected that, because of the previous report of Kitajima et al. 43 and our own pilot study, we would find considerable cross-colonisation of the placebo group and that any therapeutic impact of the intervention would be clear only by studying outcomes in the population successfully colonised irrespective of their treatment allocation. That we found trends towards lower rates of the primary outcome and a range of secondary outcomes but that they still failed to reach statistical significance confirms the lack of evidence of efficacy of this particular probiotic intervention to reduce complications of prematurity in this population.
Safety
As in all other trials of probiotic interventions in the newborn infant, we received no reports of problems administering the intervention and no reports of positive cultures of any bifidobacteria.
We encouraged clinicians to ensure that hospital microbiologists knew that this intervention was being given, but are aware from our contacts with microbiologists to collect culture results that the message was not always relayed around departments. The few reports there have been of bifidobacteria sepsis suggest that the infection is usually very mild and easily treated. It is not clear that anaerobic cultures are performed on all specimens or, if it is grown, if laboratory staff are unaware that a baby is receiving bifidobacteria they might be dismissed as contaminants on a routine culture. It is possible that in this and in other trials bifidobacteraemia is missed and under-reported. Nonetheless, even if this is the case, short-term safety appears to be good.
There is interest in the possibility that probiotics might be useful to reduce carriage of antibiotic-resistant pathogens,86 but, conversely, theoretically, by increasing microbial diversity, they might increase pathogen carriage. Our results were reassuring in this respect. Although, overall, around 6% of babies from whom we received stools were colonised at one or other time point with MRSA, VRE or ESBL, there was no evidence of difference between the groups whether categorised by allocation or colonisation.
Longer-term safety data for probiotic interventions in preterm babies are almost completely lacking. There is a reassuring report of growth and development87 of babies followed up to the age of 3 years from one of the earlier trials conducted in Taiwan20 but no trial has been designed with adequate power to look at important long-term health outcomes including atopic disease. The ProPrems trial41 has a follow-up component that promises to begin to fill this void, but those results are not yet available.
Generalisability of the results
A major objective when designing the PiPS trial was to ensure the representativeness of the trial population. Records of all potentially eligible babies, the precise numbers of parents who were approached and reasons for refusal for their baby to join the trial were not required by the trial protocol. The view of the investigators was that such data are often inaccurate and we were conscious that, for this trial, the overwhelming reason for eligible babies not being recruited would be the lack of a staff member available who was familiar with the trial and who also had GCP training. We aimed to ensure that those who were approached were representative of the total preterm population by minimising exclusions and both recruiting for and beginning the intervention early, making it more difficult to exclude babies for spurious clinical reasons, which, purely anecdotally, we suspect often happens.
The PiPS trial population is strikingly multiracial, with the mothers of 56% of the babies being white, 20% being Afro-Caribbean and 12% with origins in the Indian subcontinent. Race is not recognised as a risk factor for the outcomes of this trial, but the racial mix might be related to the observation that the average birthweight was slightly low for gestational age and it is well recognised that intrauterine growth restriction is a risk factor for both NEC and late-onset sepsis. The gestational age-based mortality of the babies in the trial (see Figure 7) is very similar to that reported nationally for neonatal unit admissions in 2009 in England88 (data from 110 neonatal units with details to discharge or death for 34,635 babies born before 33 weeks’ gestational age): 60% mortality rate at 23 weeks’ gestational age, 38% at 24 weeks’ gestational age, 27% at 25 weeks’ gestational age, 16% at 26 weeks’ gestational age, 11% at 27 weeks’ gestational age, 6% at 28 weeks’ gestational age, 4% at 29 weeks’ gestational age and 2% at 30 weeks’ gestational age. NEC lacks a standard agreed case definition and the incidence is not available from UK routine national data; the recording of episodes of infection is incomplete on the national database. The rates that we found in the trial for NEC and late-onset sepsis in the placebo group (10.0% and 11.7%, respectively) were within the ranges that we had specified in the power calculation that were based on routine data collected for neonatal admissions in and around London in the years preceding the trial and similar to rates quoted in large observational studies. 59,60 On balance, we believe that the trial population is certainly representative of the population in south-east England and we are not aware of any good reasons why these results should not be extended to neonatal populations in the rest of the country.
Why does the probiotic intervention in the Probiotics in Preterm infantS trial show no evidence of benefit? Probiotics in Preterm infantS in the context of other trials in preterm babies
In the context of the variable effects on sepsis and lack of effect in multiple meta-analyses we attempted to clarify the role of B. breve BBG-001 to reduce late-onset sepsis by, for our primary outcome, considering only septicaemias caused by definite pathogens. That we found no benefit is disappointing but not unexpected. It was perhaps more surprising that the ProPrems trial41 showed no evidence of improved mortality associated with the three-strain probiotic intervention. In the context of that trial, which we would argue is the first large well-designed preterm probiotic trial to be published, it is possibly less surprising that we similarly found no evidence of reduced mortality associated with B. breve BBG-001 administration in the PiPS trial. However, despite our criticisms and those of others of the quality of many of the published trials,34,73 we were surprised that we have been able to find no evidence of benefit to reduce NEC in a clinical environment with an incidence of definite NEC (Bell stage ≥ 2) of around 10%. At first sight it might be thought that we have missed an effect because of loss of statistical power through incomplete colonisation of the active intervention group and cross-colonisation of the placebo group, but our intention-to-treat subgroup analyses by colonisation and analyses of outcomes by colonisation do not support this view. Furthermore, we were forewarned of this as a potential problem and went to great lengths to provide training to minimise the effect; it seems most unlikely that eithercross-infection rates in the hospitals involved in the PiPS trial are very different from elsewhere or that this has not also been a characteristic of other trials of probiotics.
The results of the PiPS trial might be negative in the sense of identifying a preventative therapy but they are most important in the story of probiotic trials and the search for a means to prevent NEC and late-onset sepsis.
There are many reasons why this intervention might have failed.
In the context of the wide range of products used in previously published trials34,63,73 it is too simplistic to simply dismiss this result as being because we chose the wrong product. The evidence we have, as discussed earlier, from laboratory, animal and limited human data all suggests that different bacterial strains have different protective roles at the intestinal mucosal surface; this is biologically plausible and explains why we are dependent on such an enormous range of commensals for good health. The increased availability of molecular techniques to study the microbiome has led, over the period since the PiPS trial was designed in 2004–6, to an explosion in knowledge of the microbiome, not only in health but in a range of disease states including NEC and late-onset sepsis in the preterm baby. 15–17,89,90 That explosion of knowledge is not confined to the bacteria that inhabit the intestine but also includes other compartments of the body, most notably for the neonatologist, maternal breast milk91 and the complexity of interactions between the microbiome and the immature intestinal immune system that underpin the pathophysiology of NEC. 4,92,93 Although we remain convinced that the key to unlocking the challenge of preventing these neonatal catastrophes lies in this area of biology, it seems naive from the current vantage point to think that this might be achieved by giving a product with a single or even two or three bacterial strains.
Combining trials of different probiotics in meta-analyses
The very strong and often emotional pressure that has been put on clinicians by a number of authors to provide probiotics routinely for preterm babies has been based on no single well-designed large trial but on a series of meta-analyses, the most recent of which was the Cochrane review35 published in 2014, which included 23 trials and over 5500 babies. Other authors have repeatedly challenged not only the rigour of a number of the trials but also the validity of combining trials using such disparate interventions in meta-analyses. We would argue that the results of the PiPS trial further strengthen the argument that combining trials using different probiotic interventions in meta-analyses should be resisted.
Conclusions
Implications for health care
Necrotising enterocolitis and late-onset sepsis remain among the most important causes of death and life-long morbidity in surviving preterm babies. Rates of preterm birth in the UK are among the highest in the developed world94 and are rising;95 therefore, preterm birth represents a major public health issue and the challenge to find interventions to prevent NEC and late-onset sepsis is urgent. We are aware that there is currently a rise in the use of probiotics on UK neonatal units given to prevent NEC. The data from this trial do not support this practice. The short-term safety profile for probiotic interventions for the individual baby is highly favourable, but there are almost no published outcome data beyond the initial hospitalisation, and the use of probiotics on a neonatal unit will impact not only on the developing microbiome of the babies for whom they are prescribed but also, through cross-colonisation, on that of other babies within that unit.
A number of commentaries have suggested that it is perhaps unethical not to inform parents about the evidence of probiotics to prevent NEC29 and that, when given the evidence, parents would be likely to elect that their baby should be given them. 96 This, however, obviously depends on the interpretation of the data by the responsible clinician, the manner in which he or she presents it to the parents and the availability of a product with adequate quality control. The data from this trial provide no evidence that routine supplementation with B. breve BBG-001 would affect the risk of late-onset sepsis, NEC or death in this population.
Recommendations for research
Regulatory considerations and neonatal trials
Although there has been progress in standardising and centralising procedures to ease the conduct of trials, it remains necessary to gain local hospital approvals for those involving inpatients. This is a particular problem for trials recruiting preterm babies, whose clinical care is organised in such a way that they inevitably move between hospitals, sometimes along unpredictable pathways, and it was a major threat to the success of this trial.
This problem may be eased if GCP training was provided for all medical neonatal trainees and consultants, and for interested nurses, who could additionally be able to act as the PI for trials of IMPs, although some hospitals have local rules preventing this. Furthermore, if a baby is transferred after any intervention is complete and the only requirement is to collect outcome data, the procedures for approved members of the trial team to access case notes could be further simplified.
Standardisation of definitions of outcomes
The diagnosis of both NEC and late-onset sepsis has an element of subjectivity, and neither has an internationally agreed case definition either for epidemiological or for drug regulatory purposes. This complicates the interpretation of individual published neonatal trials and undermines the validity of combining the trials in meta-analyses. The PiPS trial team took a pragmatic approach to this problem, using a definition of late-onset sepsis for the primary outcome that is different from other trials and undertaking an independent review of the categorisation of NEC. The difficulties are not underestimated; nonetheless, the lack of agreed simple practical definitions is a major obstacle to progress and, in conjunction with regulators, needs urgently to be addressed.
The choice of products for probiotic trials
Research into the developmental biology and pathophysiology underlying neonatal NEC and late-onset sepsis progresses and ultimately should point the way to the identification of evidence-based interventions that can be tested in clinical trials to prevent NEC and late-onset sepsis, but that stage has not yet been reached.
The choice of probiotic product for this trial seemed, when made, to have a sound clinical basis but, in retrospect, given progress in understanding the pathogenesis of NEC and late-onset sepsis and the complex biology of the microbiome over the past decade, was perhaps naive.
The conventional route of undertaking large expensive Phase 3 trials only after evidence has been gained from animal and smaller trials in humans supporting efficacy and safety may not be appropriate for probiotic interventions. A bacterium having probiotic properties might behave differently in a different species and its action might vary with the developmental stage. At the very least, however, it would probably be advisable in the future before embarking on large neonatal probiotic trials to complete more formal and bigger ‘pilot’ studies using clinical end points; however, this is fraught with difficulties and risks being misleading. Such studies might be designed to involve increased longitudinal sampling to give an opportunity to study colonisation and in that way throw increased light on issues of dosage particularly in relation to feeding and antibiotic use.
What is undoubtedly important is that product quality is monitored and colonisation rates are documented throughout and not just during the set-up phase of clinical trials.
Randomised controlled trials to evaluate probiotics
We do not consider that efficacy of B. breve BBG-001 to prevent NEC and late-onset sepsis was missed in the PiPS trial because of the complication of cross-colonisation; nonetheless, cross-colonisation is probably a feature of all probiotic trials and complicates their interpretation. The problem could be overcome by adopting a cluster design although even that precaution, in the context of unpredictable patterns of transfer within the UK neonatal service, might not totally overcome the problem and in addition has major organisational implications.
The well-designed RCT is the supreme assessment of efficacy since it provides a clean experiment with all confounders taken into account. To suggest that it might not be the optimal way to evaluate a probiotic and to suggest that the conclusions from a trial of a probiotic might be less likely to translate into clinical practice than those of a trial of a stable chemical entity may seem heretical, but these suggestions are worthy of consideration. We can only speculate, but from what we have found it seems inevitable that if a probiotic is introduced into routine practice it will be incorporated into the bacteriological environment of the neonatal unit. We are unaware of any longitudinal studies of the effects of prolonged routine use, if any, on the microbiome in terms of its diversity and frequency of antibiotic-resistant strains in a population of babies, but this is critically important. It is indeed possible not only that an intervention that seemingly shows efficacy in a trial might lose that efficacy over time but equally that an intervention that shows no evidence of benefit might, over time, promote bacterial diversity and be associated with reduced rates of NEC and late-onset sepsis. The same may be true whether or not the intervention is safe.
Studying probiotic efficacy and safety outside a randomised controlled trials
It is inconceivable that all of the outstanding questions about choice and combinations of probiotic and other bioactive interventions can be answered through RCTs.
The pathogenesis of NEC and of neonatal late-onset sepsis is complex, involving the interplay of the acquisition of a microbiome that is different from that of the full-term healthy baby, with functionally immature intestinal and immune systems, and, superimposed upon that, effects of enteral nutrition and administered medications such as antibiotics and antacids.
There is a strong association between the prolonged use of antibiotics and NEC and it is known that the provision of maternal breast milk affords some protection. Furthermore, there is accumulating evidence that the use of evidence-based care bundles may reduce neonatal adverse outcomes including late-onset sepsis and NEC97,98 and it has also been shown in the UK that, through enthusiastically supported quality improvement programmes, the use of maternal breast milk may be increased. 99 Breast milk itself is a safe means to provide not only commensal bacteria and bifidogenic oligosaccharides, but also a range of other factors including lactoferrin and growth factors.
In England, all neonatal units routinely collect a common clinical data set, an anonymised subset of which is held in the Neonatal Data Analysis Unit at Imperial College London. 100 Those data items, the completeness and accuracy of which are steadily increasing, constitute a kite-marked research data set (ISB1595) that includes both interventions and outcomes. These have been successfully linked to Hospital Episode Statistics and have the potential to link to other child health systems, opening up possibilities for obtaining longer-term outcome data. This rich data source has already been the basis of a number of important publications and has huge potential for investigating the effects and the interactions of interventions such as probiotics, prebiotics and other bioactive products that are likely to need testing and whose effects might change over time. Ensuring the completeness and accuracy and the quality of the analysis of these ‘routine’ data may yield more knowledge about the true impact of introducing probiotics, or any other intervention, than the best-designed RCT.
Summary of research recommendations
-
Further streamline training of research competent clinical and NHS R&D staff to ensure that recruitment is optimised and adherence to trial protocols is not unnecessarily jeopardised by transfer between hospitals.
-
Standardise objective case definitions for NEC and late-onset sepsis suitable for epidemiological, trial and regulatory purposes.
-
Consider undertaking pilot trials with clinical outcomes and embedded longitudinal studies of colonisation before embarking on large Phase 3 trials of probiotics.
-
During probiotic trials, monitor the quality of the intervention and intestinal ‘colonisation’.
-
If designing RCTs of probiotics, consider cluster design to limit the effects of cross-colonisation.
-
If introducing probiotic (or other bioactive) interventions into routine use, set up long-term longitudinal monitoring of the microbiome and of clinical outcomes in the neonatal population whether or not prescribed probiotic.
-
Ensure the completeness and accuracy of routine NHS neonatal data so that it reliably forms the basis for studies of the multiple influences on acquisition of NEC and late-onset sepsis.
Acknowledgements
We are grateful to Yakult Honsha Co. Ltd, which was responsible for the manufacture of the active intervention and placebo in Japan at the Yakult Fujisusono Pharmaceutical Plant. The company had no involvement in trial design, conduct or analysis and interpretation of the data, and the chief investigator has had no direct contact with the company.
We are also grateful to Robert Haslam of Somerset House Consultants Ltd, who inspected the Yakult Honsha Company plant in Japan in 2007 to support our application to the MHRA for a clinical trial certificate.
This trial would not have been possible without huge help from medical, nursing and microbiological colleagues in our collaborating hospitals.
Clinicians leading data collection: Abdul Khader, Narendra Aladangady, Selma Al-Wahab, Olutoyin Banjoko, William Barry, Kathryn Beardsall, Raoul Blumberg, David Booth, Andrew Bush, John Chang, Michele Cruwys, Ramon Fernandez, Sunit Godambe, Charles Godden, Vimala Gopinathan, Abdul Hasib, Ahmed Hassan, Ann Hickey, Angela Huertas-Ceballos, Matthew James, Victoria Jones, Jonathan Kefas, Nigel Kennea, Arfa Khan, Hamudi Kisat, Jauro Kuna, Alison Leaf, Geraint Lee, Dwight Lindo, Abdus Mallik, Kenneth McCormick, Anita Mittal, Samudra Mukherjee, Sankara Narayanan, Mark Peters, Raghaven Prasad, Sanjay Rathi, Peter Reynolds, Gopa Sarkar, Karin Schwarz, Shanthi Shanmugalingam, Ajay Sinha, Aung Soe, Geeta Subramanian, Caroline Sullivan, Fiona Thompson, Richard Thwaites, Sabita Uthaya, Vimal Vasu, Vivienne van Someren, Shaun Walter, Graham Whincup, Timothy Wickham and Salim Yasin.
Designated nurses: Geraldine Banfield, Sheula Barlow, Chris Brooks, Christine Cassell, Joanne Castro, Priscilla Chong, Catherine Collins, Ruth Cousins, Alison De-lara, Ashley Douglas, Andrea Edwards, Karen Few, Vicky Goater, Levy Gomez, Jodie Harrison, Jane Hodgkinson, Nicky Holland, Judy Isaacs, Bernadette Jennings, Lou Mair, Maxwell Masuku, Jessie Mertalla, Yvonne Millar, Eniola Nsirim, Buki Odude, Polly Payne, Annette Pope, Lorna Reid, Vilma Ribao, Jo Schofield, Helen Smith, Sonia Sobowiec Kouman, Jane Talbot, Gill Wallace, Mina Wanti, Carol Warden, Beautine Wester, Debbie Wilson, Rosebel Verdan and Norlita Williams.
Staff leading collection of routine microbiology data: Steve Adcock, Zoe Adhami, Hassan Al-Ghusein, Amit Amin, Julie Andrews, Fatih Awad-El-Kariem, Sheula Barlow, Stephen Barrett, Elaine Bibby, Susan Bragman, Allison Bunkall, Benny Cherian, Pietro Coen, Eric Cowie, Hannah Dabrowski, Martino Dall’Antonia, Jayshree Dave, Paul Dexter, Paul Donaldson, Justin Edwards, Graham Fagg, Amanda Fife, Imtiaz Gillani, John Hartley, Anja Hawkins, Ann Hickey, Peter Hitchcock, John Klein, Sandra Lacey, Geraint Lee, Matthew Longbone, Nitin Mahobia, Paul Michalczyk, Simon Namnyak, Stephanie Paget, Vicky Pantelli, Sabita Parida, Stephen Price, Srinivasulu Reddy, Jeffrey Richards, Giovanni Satta, Aarti Shah, Angela Shaw, Rob Shorten, Sorrush Soleimanian, Matthew Strutt, Michele Upton, Jerry Wigmore, Karen Withell and Rella Workman.
We also need to thank Dr William van’t Hoff, Sharon Barrett and the staff in the office of the Medicines for Children Research Network at Great Ormond Street Hospital for their help, particularly in supporting and identifying nurses in collaborating hospitals; the statistician Clare Nelis and the trial data managers at the NPEU, Anna Hobson and Marketa Laubeova; and the trial nurses employed by Queen Mary University of London and based at Homerton Hospital NHS Foundation Trust, Nicola Lawrence, Michele Upton, Denise Tedder and Ellie MacCamlie.
We would like also to thank the members of the Trial Steering Committee and Data Monitoring Committee for their advice and support:
Trial Steering Committee: David Field, Michael Weindling, Jane Abbott, Tim Cole, Michael Hudson and Andrew Leslie.
Data Monitoring Committee: Diana Elbourne, Jim Gray and Ben Stenson.
Above all we are grateful to the families who generously agreed for their babies to participate in this trial.
Contributions of authors
All authors have been involved in the production of this report and have approved the final manuscript.
Kate Costeloe (Professor of Paediatrics, Queen Mary University of London; PiPS Trial Chief Investigator). Trial design, oversight, analysis and interpretation of results. Responsible for the first draft and co-ordination of the production of this report and is its guarantor.
Ursula Bowler (Senior Trials Manager, NPEU). Trial design, oversight of trial administration and conduct, and regulatory advice.
Peter Brocklehurst (Director for the Institute for Women’s Health, University College London; previously the director for the National Perinatal Epidemiology Unit; PiPS Trial Investigator). Trial design.
Pollyanna Hardy (Senior Trials Statistician, NPEU). Oversight of production of statistical analysis plan, analysis and interpretation of results.
Paul Heal (PiPS Trial Co-ordinator, NPEU). Day-to-day running of trial including regulatory aspects, training of trial staff and data collection and collation.
Edmund Juszczak (Director, NPEU Clinical Trials Unit). Trial design and oversight, statistical advice and interpretation of results.
Andy King (Senior Trials Programmer, NPEU). Design and management of trial data collection forms, randomisation program and databases.
Nicola Panton (Research Assistant, Queen Mary University of London). Laboratory processing, culture and PCR of trial stool samples and bacteriological screening of interventions.
Fiona Stacey (Research Nurse, Queen Mary University of London). Co-ordination of research nurse team, oversight of local training and liaison with local trial staff.
Angela Whiley (Research Assistant, Queen Mary University of London). Laboratory processing and culture of trial stool samples and bacteriological screening of interventions.
Mark Wilks (Principal Microbiologist, Barts Health NHS Trust; PiPS Trial Investigator). Trial design and oversight, oversight of microbiology laboratory trial investigations, interpretation and presentation of microbiological results.
Michael R Millar (Consultant in Infection, Barts Health NHS Trust; PiPS trial investigator). Original hypothesis, trial design and oversight, lead for collection of clinical microbiology and analysis and interpretation of results.
Publications
Costeloe KL, Bowler U, Brocklehurst P, Hardy P, Heal P, Juszczak E, King A, Panton N, Stacey F, Whiley A, Wilks M, Millar MR. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled Phase 3 trial. Lancet 2016;387:649–60.
Millar MR, Seale J, Turton J, Wilks M, Costeloe K, Woodford N, et al. ESBL-producing Enterobacteriaceae in 24 neonatal units and associated networks in the south of England: no clustering of ESBL-producing Escherichia coli in units or networks. J Antimicrob Chemother 2016;71:1174–7.
Data sharing statement
All available data relating to this trial can be obtained from the corresponding author via The Senior Trials Manager, The National Perinatal Epidemiological Unit, Nuffield Department of Population Health, University of Oxford, Old Road Campus, Headington, Oxford OX3 7LF, UK.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Stoll BJ, Gordon T, Korones SB, Shankaran S, Tyson JE, Bauer CR, et al. Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr 1996;129:63-71. http://dx.doi.org/10.1016/S0022-3476(96)70191-9.
- Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 2006;368:1271-83. http://dx.doi.org/10.1016/S0140-6736(06)69525-1.
- Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 2005;115. http://dx.doi.org/10.1542/peds.2004-0569.
- Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255-64. http://dx.doi.org/10.1056/NEJMra1005408.
- Walsh MC, Kliegman RM. Necrotising enterocolitis: treatment based on staging criteria. Pediat Clin North Am 1986;33:179-201.
- Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr 1999;28:19-25. http://dx.doi.org/10.1097/00005176-199901000-00007.
- Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed 1999;80:F167-73. http://dx.doi.org/10.1136/fn.80.3.F167.
- Sakata H, Yoshioka H, Fujita K. Development of the intestinal flora in very low birth weight infants compared to normal full-term newborns. Eur J Pediatr 1985;144:186-90. http://dx.doi.org/10.1007/BF00451911.
- Schwiertz A, Gruhl B, Lobnitz M, Michel P, Radke M, Blaut M. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr Res 2003;54:393-9. http://dx.doi.org/10.1203/01.PDR.0000078274.74607.7A.
- Duffy LC. Interactions mediating bacterial translocation in the immature intestine. J Nutr 2000;130:432S-6S.
- Walker WA. Role of nutrients and bacterial colonization in the development of intestinal host defense. J Pediatr Gastroenterol Nutr 2000;30:2-7. http://dx.doi.org/10.1097/00005176-200000002-00002.
- Kohler H, McCormick BA, Walker WA. Bacterial–enterocyte crosstalk: cellular mechanisms in health and disease. J Pediatr Gastroenterol Nutr 2003;36:175-85. http://dx.doi.org/10.1097/00005176-200302000-00005.
- Martin CR, Walker WA. Probiotics: role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol 2008;32:127-37. http://dx.doi.org/10.1053/j.semperi.2008.01.006.
- Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J 2001;15:1398-403. http://dx.doi.org/10.1096/fj.00-0833hyp.
- Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0020647.
- Stewart CJ, Marrs ECL, Magorrian S, Nelson A, Lanyon C, Perry JD, et al. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr 2012;101:1121-7. http://dx.doi.org/10.1111/j.1651-2227.2012.02801.x.
- Mai V, Torrazza RM, Ukhanova M, Wang X, Sun Y, Li N. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0052876.
- Food and Agriculture Organization of the United Nations (FAO)/World Health Organization . Report of a Joint FAO WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk With Live Lactic Acid Bacteria 2001. www.fao.org/3/a-a0512e.pdf (accessed 25 August 2016).
- Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 2002;82:103-8. http://dx.doi.org/10.1159/000063096.
- Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005;115:1-4.
- Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 2005;147:192-6. http://dx.doi.org/10.1016/j.jpeds.2005.03.054.
- Samanta M, Sarkar M, Ghosh P, Ghosh JK, Sinha MK, Chatterjee S. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr 2009;55:128-31. http://dx.doi.org/10.1093/tropej/fmn091.
- Lin H-C, Hsu C-H, Chen H-L, Chung M-Y, Hsu J-F, Lien R-I, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 2008;122:693-700. http://dx.doi.org/10.1542/peds.2007-3007.
- Neu J, Caicedo R. Probiotics: protecting the intestinal ecosystem?. J Pediatr 2005;147:143-6. http://dx.doi.org/10.1016/j.jpeds.2005.05.033.
- Kliegman RM, Willoughby RE. Prevention of necrotizing enterocolitis with probiotics. Pediatrics 2005;115:171-2.
- Bell EF. Preventing necrotizing enterocolitis: what works and how safe?. Pediatrics 2005;115:173-4.
- Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet 2007;369:1614-20. http://dx.doi.org/10.1016/S0140-6736(07)60748-X.
- Costalos C, Skouteri V, Gounaris A, Sevastiadou S, Triandafilidou A, Ekonomidou C, et al. Enteral feeding of premature neonates with Saccharomyces boulardii. Early Hum Dev 2003;74:89-96. http://dx.doi.org/10.1016/S0378-3782(03)00090-2.
- Tarnow-Mordi WO, Wilkinson D, Trivedi A, Brok J. Probiotics reduce all-cause mortality and necrotizing enterocolitis: it is time to change practice. Pediatrics 2010;125:1068-70. http://dx.doi.org/10.1542/peds.2009-2151.
- AlFaleh KM, Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Sys Rev 2008;1. http://dx.doi.org/10.1002/14651858.cd005496.pub2.
- Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 2010;125:921-30. http://dx.doi.org/10.1542/peds.2009-1301.
- AlFaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Sys Rev 2011;3. http://dx.doi.org/10.1002/14651858.cd005496.pub3.
- Soll RF. Probiotics: are we ready for routine use?. Pediatrics 2010;125:1071-2. http://dx.doi.org/10.1542/peds.2010-0643.
- Mihatsch WA, Braegger CP, Decsic T, Kolacek S, Lanzinger H, Mayer B, et al. Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clin Nutr 2012;31.
- AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Sys Rev 2014;4. http://dx.doi.org/10.1002/ebch.1976.
- Mihatsch WA, Vossbeck S, Eikmanns B, Hoegel J, Pohlandt F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology 2010;98:156-63. http://dx.doi.org/10.1159/000280291.
- Sari FN, Dizdar EA, Oguz S, Erdeve O, Uras N, Dilmen U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr 2011;65:434-9. http://dx.doi.org/10.1038/ejcn.2010.278.
- Braga TD, Pontes da Silva GA, Cabral de Lira PI, de Carvalho Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 2011;93:81-6. http://dx.doi.org/10.3945/ajcn.2010.29799.
- Rojas MA, Lozano JM, Rojas MX, Rodriguez VA, Rondon MA, Jaime A, et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012;130:e1113-20. http://dx.doi.org/10.1542/peds.2011-3584.
- Fernández-Carrocera LA, Solis-Herrera A, Cabanillas-Ayón M, Gallardo-Sarmiento RB, García-Pérez CS, Montaño-Rodríguez R. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2013;98:F5-9. http://dx.doi.org/10.1136/archdischild-2011-300435.
- Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 2013;32:1055-62. http://dx.doi.org/10.1542/peds.2013-1339.
- Oncel MY, Sari FN, Arayici S, Guzoglu N, Erdeve O, Uras N, et al. Lactobacillus reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2014;99:F110-15. http://dx.doi.org/10.1136/archdischild-2013-304745.
- Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama H, Fujimura M. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child 1997;76:F101-7. http://dx.doi.org/10.1136/fn.76.2.F101.
- Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis 1999;3:197-202. http://dx.doi.org/10.1016/S1201-9712(99)90024-3.
- Luoto R, Matomäki J, Isolauri E, Lehtonen L. Incidence of necrotizing enterocolitis in very-low-birth-weight infants related to the use of Lactobacillus GG. Acta Paediatr 2010;99:1135-8. http://dx.doi.org/10.1111/j.1651-2227.2010.01795.x.
- Hunter C, Dimaguila MAVT, Gal P, Wimmer JE, Ransom JL, Carlos RQ, et al. Effect of routine probiotic, Lactobacillus reuteri DSM 17938, use on rates of necrotizing enterocolitis in neonates with birthweight < 1000 grams: a sequential analysis. BMC Pediatrics 2012;12. http://dx.doi.org/10.1186/1471-2431-12-142.
- Bonsante F, Iacobelli S, Gouyon J-B. Routine probiotic use in very preterm infants: retrospective comparison of two cohorts. Am J Perinatol 2013;30:41-6.
- Janvier A, Malo J, Barrington KJ. Cohort study of probiotics in a North American neonatal intensive care unit. J Pediatr 2014;164:980-5. http://dx.doi.org/10.1016/j.jpeds.2013.11.025.
- Manzoni P, Lista G, Gallo E, Marangione P, Priolo C, Fontana P, et al. Routine Lactobacillus rhamnosus GG administration in VLBW infants: a retrospective, 6-year cohort study. Early Hum Dev 2011;87S:S35-8. http://dx.doi.org/10.1016/j.earlhumdev.2011.01.036.
- Kunz AN, Noel JM, Fairchok MP. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J Pediatr Gastroenterol Nutr 2004;38:457-8. http://dx.doi.org/10.1097/00005176-200404000-00017.
- Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005;115:178-81.
- Ohishi A, Takahashi S, Ito Y, Ohishi Y, Tsukamoto K, Nanba Y, et al. Bifidobacterium septicaemia associated with postoperative probiotic therapy in a neonate with omphalocele. J Pediatr 2010;156:679-81. http://dx.doi.org/10.1016/j.jpeds.2009.11.041.
- Jenke A, Ruf E-M, Hoppe T, Heldmann M, Stefan W. Bifidobacterium septicaemia in an extremely low-birthweight infant under probiotic therapy. Arch Dis Child Fetal Neonatal Ed 2012;97:F217-18. http://dx.doi.org/10.1136/archdischild-2011-300838.
- Murguia-Peniche T, Mihatsch WA, Zegarra J, Supapannachart S, Ding Z-Y, Neu J. Intestinal mucosal defense system, part 2. Probiotics and prebiotics. Pediatrics 2013;162:S64-71. http://dx.doi.org/10.1016/j.jpeds.2012.11.055.
- Li Y, Shimizu T, Hosaka A, Kaneko N, Ohtsuka Y, Yamashiro Y. Effects of Bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediatr Int 2004;46:509-15. http://dx.doi.org/10.1111/j.1442-200x.2004.01953.x.
- Chrzanowska-Liszewska D, Seliga-Siwecka J, Kornacka MK. The effect of Lactobacillus rhamnosus GG supplemented enteral feeding on the microbiotic flora of preterm infants-double blinded randomized control trial. Early Hum Dev 2012;88:57-60. http://dx.doi.org/10.1016/j.earlhumdev.2011.07.002.
- Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371:651-9. http://dx.doi.org/10.1016/S0140-6736(08)60207-X.
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late onset sepsis in very low birth weight neonates: the experience of the NICHD neonatal research network. Pediatrics 2002;110:285-91. http://dx.doi.org/10.1542/peds.110.2.285.
- Llanos AR, Moss ME, Pinzòna MC, Dye T, Sinkin RA, Kendiga JW. Epidemiology of neonatal necrotising enterocolitis: a population based study. Paediatr Perinatal Epidemiol 2002;16:342-9. http://dx.doi.org/10.1046/j.1365-3016.2002.00445.x.
- Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol 2008;32:70-82. http://dx.doi.org/10.1053/j.semperi.2008.01.004.
- Underwood MA, Kalanetra KM, Bokulich NA, Lewis ZT, Mirmiran M, Tancredi DJ, et al. A comparison of two probiotic strains of bifidobacteria in premature infants. J Pediatrics 2013;163:1585-91. http://dx.doi.org/10.1016/j.jpeds.2013.07.017.
- Ishizeki S, Sugita M, Takata M, Yaeshima T. Effect of administration of bifidobacteria on intestinal microbiota in low-birth-weight infants and transition of administered bifidobacteria: a comparison between one-species and three-species administration. Anaerobe 2013;23:38-44. http://dx.doi.org/10.1016/j.anaerobe.2013.08.002.
- Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg 2012;47:241-8. http://dx.doi.org/10.1016/j.jpedsurg.2011.09.064.
- Costeloe KL, Jesudass R, Al Nakib L, Whiley A, Wilks M, Millar MR. The early administration of Bifidobacterium breve strain BBG to very low birthweight infants: a pilot study. Pediatr Res 2004;55.
- Neu J. Neonatal necrotizing enterocolitis: an update. Acta Paediatr 2005;94:100-5. http://dx.doi.org/10.1080/08035320510043637.
- Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012;129:e298-304.
- European Parliament . Directive 2001 83 EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use 2001.
- Costeloe K, Bowler U, Brocklehurst P, Hardy P, Heal P, Juszczak E, et al. PiPS: Trial of Probiotic Administered Early to Prevent Infection and Necrotising Enterocolitis 2012. www.nets.nihr.ac.uk/__data/assets/pdf_file/0012/51213/PRO-05-501-04.pdf (accessed 25 August 2016).
- British Society for Antimicrobial Chemotherapy (BSAC) . BSAC Methods for Antimicrobial Susceptibility Testing n.d. http://bsac.org.uk/wp-content/uploads/2012/02/Version-12-Apr-2013_final1.pdf (accessed 25 August 2016).
- Fujimoto J, Tanigawa K, Kudo Y, Makino H, Watanabe K. Identification and quantification of viable Bifidobacterium breve strain Yakult in human faeces by using strain-specific primers and propidium monoazide. J Appl Microbiol 2011;110:209-17. http://dx.doi.org/10.1111/j.1365-2672.2010.04873.x.
- Parry G, Tucker J, Tarnow-Mordi W. CRIB II: an update of the clinical risk for babies score. Lancet 2003;361:1789-91. http://dx.doi.org/10.1016/S0140-6736(03)13397-1.
- Jobe AH, Bancalari E. NICHD/NHLBI/ORD workshop summary: bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723-9. http://dx.doi.org/10.1164/ajrccm.163.7.2011060.
- Abrahamsson TR, Rautava S, Moore AM, Neu J, Sherman PM. The time for a confirmative necrotizing enterocolitis probiotics prevention trial in the extremely low birth weight infant in North America is now!. J Pediatr 2014;165:389-94. http://dx.doi.org/10.1016/j.jpeds.2014.05.012.
- Garland SM, Tobin JM, Pirotta M, Tabrizi SN, Opie G, Donath S, et al. The ProPrems trial: investigating the effects of probiotics on late onset sepsis in very preterm infants. BMC Infect Dis 2011;11:210-19. http://dx.doi.org/10.1186/1471-2334-11-210.
- McGee R, O’Connor PM, Russell D, Dempsey EM, Ryan AC, Ross PR, et al. Prolonged faecal excretion following a single dose of probiotic in low birth weight infants. Acta Paediatr 2010;99:1587-8. http://dx.doi.org/10.1111/j.1651-2227.2010.01878.x.
- Gordon PV, Swanson JR, Attridge JT, Clark R. Emerging trends in acquired neonatal intestinal disease: is it time to abandon Bell’s criteria?. J Perinatol 2007;27:661-71. http://dx.doi.org/10.1038/sj.jp.7211782.
- Protocol and Handbook. Oxford: National Perinatal Epidemiology Unit; 2011.
- Ventura M, Turroni F, Motherway MO, MacSharry J, van Sinderen D. Host–microbe interactions that facilitate gut colonization by commensal bifidobacteria. Trends Microbiol 2012;20:467-76. http://dx.doi.org/10.1016/j.tim.2012.07.002.
- Kline KA, Falker S, Dahlberg S, Normark S, Henriques-Normark B. Bacterial adhesins in host–microbe interactions. Cell Host Microbe 2009;5:580-92. http://dx.doi.org/10.1016/j.chom.2009.05.011.
- Krachler AM, Orth K. Targeting the bacteria–host interface: strategies in anti-adhesion therapy. Virulence 2013;4:284-94. http://dx.doi.org/10.4161/viru.24606.
- Motherway MO, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ, et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc Natl Acad Sci USA 2011;108:11217-22. http://dx.doi.org/10.1073/pnas.1105380108.
- Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human mucus binding protein. Proc Natl Acad Sci USA 2009;106:17193-8. http://dx.doi.org/10.1073/pnas.0908876106.
- Kalia YN, Nonato LB, Lund CH, Guy RH. Development of skin barrier function in premature infants. J Invest Dermatol 1998;111:320-6. http://dx.doi.org/10.1046/j.1523-1747.1998.00289.x.
- Hickey L, Garland SM, Jacobs SE, O’Donnell CPF, Tabrizi SN. Cross-colonization of infants with probiotic organisms in a neonatal unit. J Hosp Infect 2014;88:226e-9. http://dx.doi.org/10.1016/j.jhin.2014.09.006.
- Battersby C, Santhakumaran S, Modi N, Costeloe K. Incidence and outcomes of severe necrotising enterocolitis in infants less than 32 weeks gestation: a prospective population study. Arch Dis Child 2014;99. http://dx.doi.org/10.1136/archdischild-2014-307384.112.
- Gibson MK, Pesesky MW, Dantas G. The yin and yang of bacterial resilience in the human gut microbiota. J Mol Biol 2014;426:3866-76. http://dx.doi.org/10.1016/j.jmb.2014.05.029.
- Chou I-C, Kuo H-T, Chang J-S, Wu S-F, Chiu H-Y, Su B-H, et al. Lack of effects of oral probiotics on growth and neurodevelopmental outcomes in preterm very low birth weight infants. J Pediatrics 2010;156:393-6. http://dx.doi.org/10.1016/j.jpeds.2009.09.051.
- Neonatal Data Analysis Unit . Neonatal Specialist Care in England: Report on Mortality in 2009 2010. www1.imperial.ac.uk/resources/E8F81103-48A7-44D4-A914-424EFF22470F/ndaureportmortality2009.pdf (accessed 1 March 2016).
- Madan JC, Salari RC, Saxena D, Davidson L, O’Toole GA, Moore JH, et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed 2012;97:F456-62. http://dx.doi.org/10.1136/fetalneonatal-2011-301373.
- Carlisle EM, Morowitz MJ. The intestinal microbiome and necrotizing enterocolitis. Curr Opin Pediatr 2013;25:382-7. http://dx.doi.org/10.1097/MOP.0b013e3283600e91.
- Jeurink PV, van Bergenhenegouwen J, Jiménez E, Knippels LMJ, Fernández L, Garssen J, et al. Human milk: a source of more life than we imagine. Benef Microbes 2013;4:17-30. http://dx.doi.org/10.3920/BM2012.0040.
- Cilieborg MS, Boye M, Sangild PT. Bacterial colonization and gut development in preterm neonates. Early Human Dev 2012;88:S41-9. http://dx.doi.org/10.1016/j.earlhumdev.2011.12.027.
- Neal MD, Sodhi CP, Dyer M, Afrazi B, Richardson WM, Beer-Stolz D, et al. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol 2013;190:3541-51. http://dx.doi.org/10.4049/jimmunol.1202264.
- MacDorman MF, Mathews TJ, Mohangoo AD, Zeitlin J. International comparisons of infant mortality and related factors: United States and Europe, 2010. National Vital Statistics Reports 2014;63:1-7.
- Zeitlin J, Mohangoo AD, Delnord M, Cuttini M. The second European Perinatal Health Report: documenting changes over 6 years in the health of mothers and babies in Europe. Epidemiol Community Health 2013;67:983-5. http://dx.doi.org/10.1136/jech-2013-203291.
- Deshpande G, Rao SC, Keil AD, Patole SK. Evidence-based guidelines for use of probiotics in preterm neonates. BMC Medicine 2011;9:92-104. http://dx.doi.org/10.1186/1741-7015-9-92.
- Montgomery-Taylor S, Emery F, Anthony M. Eighteen months of ‘Matching Michigan’ at a UK neonatal intensive care unit. Pediatr Infect Dis J 2014;32:565-7. http://dx.doi.org/10.1097/INF.0b013e3182868389.
- Lee SK, Shah PS, Singhal N, Aziz K, Synnes A, McMillan D, et al. Association of a quality improvement program with neonatal outcomes in extremely preterm infants: a prospective cohort study. CMAJ 2014;186:E485-94. http://dx.doi.org/10.1503/cmaj.140399.
- Battersby C, Santhakumaran S, Upton M, Ladbone L, Birch J, Modi N. The impact of a regional care bundle on maternal breast milk use in preterm infants: outcomes of the East of England quality improvement programme. Arch Dis Child Fetal Neonatal Ed 2014;99:F395-40. http://dx.doi.org/10.1136/archdischild-2013-305475.
- Imperial College . Neonatal Data Analysis Unit n.d. www1.imperial.ac.uk/departmentofmedicine/divisions/infectiousdiseases/paediatrics/neonatalmedicine/ndau/ (accessed 1 March 2016).
Appendix 1 Probiotics in Preterm infantS trial sites
Appendix 2 Guidance sheet 3: preparation and administration of the trial intervention
Appendix 3 Parent information leaflet version 5.1
Appendix 4 Consent form version 3.1
Appendix 5 Leaflet for professionals summarising consent process
Appendix 6 Form 6: intervention discontinuation or trial withdrawal
Appendix 7 Form 1: trial entry
Appendix 8 Form 2: daily data
Appendix 9 Form 3: transfer–discharge
Appendix 10 Form 4: abdominal pathology
Appendix 11 Form 5: for reporting serious adverse events and suspected unexpected serious adverse reactions
Appendix 12 Statistical analysis plan
List of abbreviations
- BOOST
- Benefits of Oxygen Saturation Targeting clinical trial
- CFU
- colony-forming unit
- CI
- confidence interval
- DNA
- deoxyribonucleic acid
- ESBL
- extended-spectrum beta-lactamase
- GCP
- good clinical practice
- ICH-GCP
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice
- IMP
- investigational medicinal product
- MALDI-TOF
- matrix-assisted laser desorption/ionisation time of flight
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MRSA
- meticillin-resistant Staphylococcus aureus
- NEC
- necrotising enterocolitis
- NETSCC
- NIHR Evaluation, Trials and Studies Coordinating Centre
- NPEU
- National Perinatal Epidemiological Unit
- OR
- odds ratio
- PCR
- polymerase chain reaction
- PI
- principal investigator
- PiPS
- Probiotics in Preterm infantS
- R&D
- research and development
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- SD
- standard deviation
- SUSAR
- suspected unexpected serious adverse reaction
- TOS
- Trypticase peptone oligosaccharide
- VRE
- vancomycin-resistant enterococci