Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 14/69/06. The protocol was agreed in April 2015. The assessment report began editorial review in October 2015 and was accepted for publication in March 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Westwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Objective
The overall aim of this project was to summarise the evidence available to inform estimates of the clinical effectiveness and cost-effectiveness of adding multiplex allergen testing to the investigation of people with difficult to manage allergic disease, in secondary or tertiary care settings. Multiplex allergen testing may replace some single immunoglobulin E (IgE) testing, but, where the multiplex testing panel does not include all of the suspected allergens, additional single specific IgE (sIgE) tests may be needed.
We defined the following research objectives to address this aim:
-
To assess the effects on clinical outcomes [e.g. allergy symptoms, incidence of acute exacerbations, mortality, adverse events (AEs) of testing and treatment, health-care presentations or admissions, health-related quality of life (HRQoL)] of adding multiplex allergen testing to the investigation of people with difficult to manage allergic disease.
-
To assess the effects on treatment (e.g. restriction diets, immunotherapy, use of other medications such as corticosteroids, number of allergen challenge tests required) of adding multiplex allergen testing to the investigation of people with difficult to manage allergic disease.
-
To assess the accuracy of multiplex allergen testing in predicting clinical reactivity (response to allergen challenge testing or response to immunotherapy) and to investigate whether or not multiplex allergen testing can provide diagnostic information additional to that provided by current standard care in the UK (clinical history, skin prick tests) and single IgE testing or a combination of these approaches).
-
To assess the cost-effectiveness of adding multiplex allergen testing to the investigation of people with difficult to manage allergic disease in secondary or tertiary care settings.
-
This report contains reference to confidential information provided as part of the National Institute for Health and Care Excellence (NICE) appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Chapter 2 Background and definition of the decision problem(s)
Population
The indication for this assessment is to evaluate the clinical effectiveness and cost-effectiveness of using multiplex allergen testing [ImmunoCAP® Immuno-Solid phase Allergy Chip (ISAC) (Thermo Fisher Scientific/Phadia AB, Uppsala, Sweden) or Microtest (Microtest Matrices Ltd, London, UK)] as an adjunct to current clinical investigations in people with allergy that is difficult to manage (defined as people who are allergic to two or more allergens and/or have allergies to unknown sources).
Multiplex allergen testing is likely to be used in secondary care settings or specialist tertiary care centres, as an addition to allergen challenge testing and in addition to, or in place of, some sIgE antibody testing. Multiplex allergen testing may replace some IgE testing, but where the multiplex testing panel does not include all of the suspected allergens, additional sIgE tests may be needed.
Allergy is a term used to describe immune-mediated hypersensitivity to external stimuli (allergens). Immune-mediated hypersensitivity reactions are divided into two categories: IgE-mediated reactions and non-IgE-mediated reactions. IgE antibodies are normally present in very small amounts in the body, but levels are raised in allergic disease. IgE-mediated immune reactions, also called type 1 hypersensitivity reactions, are typically rapid in onset and can involve extreme acute symptoms as in anaphylaxis or prolonged symptoms (e.g. urticaria or eczema). In an IgE-mediated reaction IgE binds to allergen molecules, which are then taken up by receptors on the surface cells of the immune system, causing the release of biologically active agents and consequent response: vasodilation (widening of blood vessels); increased capillary permeability; mucus hypersecretion; smooth muscle contraction; and tissue inflammation.
Non-IgE-mediated reactions are less well understood and are mediated by other components of the immune system. They are typically delayed in onset, and occur 4–28 hours after exposure.
This assessment will focus on IgE-mediated hypersensitivity.
Sensitisation describes the process at the start of the immune response. Exposure to an allergen [e.g. house dust mite (HDM) or pollen] initiates a complex set of cellular events within the human body, leading to the production of a specific IgE antibody to a specific allergen. At this point there is no clinical reaction (rash, sneezing). Upon re-exposure, the allergen can bind to the specific antibody that orchestrates the immune system to initiate a more aggressive and rapid response, resulting in an inflammatory response with clinical symptoms. However, many ‘sensitised’ individuals do not experience clinical reactions upon subsequent exposure to allergen, a situation known as tolerance.
The term polysensitisation usually refers to sensitisation to two or more allergen sources, and the term paucisensitisation has been used to describe sensitisation to between two and four allergens. Clinical reactivity can be difficult to diagnose in polysensitised patients because of problems distinguishing between sensitisation to cross-reactive allergens. Cross-reactivity occurs when the molecular structure/shape of two different antigens is very similar and the antibody recognises the two different antigens as the same antigen; for example, an IgE antibody that recognises and causes an allergic reaction to Bet v 1 in birch pollen can also trigger an allergic response to Cor a1 in hazelnut. In nature there are many molecules with similar molecular structures/shapes and this translates into the clinic as an obstacle when trying to identify all potential allergens that might cause an allergic reaction in a given patient. Currently, patients undergo allergy testing to identify the allergens to which they are sensitive. This is based on skin prick testing or identifying the presence of individual antibodies in the bloodstream using single IgE tests. For both methods, it is difficult to identify multiple cross-reactive allergens for patients who appear to be polysensitised or have difficult to diagnose allergic disease. It has been claimed that multiplex allergen testing may provide improved information about the sensitisation profile in polysensitised patients. This assessment will summarise the available data on information provided by multiplex allergen testing, which is additional to that obtained from single IgE tests and/or skin prick or allergen challenge tests.
It is difficult to obtain reliable statistics on allergy prevalence in the UK. The charity Allergy UK states, on its website, that there are an estimated 21 million adults in the UK who have at least one allergy and that an estimated 10 million of these have two or more allergies;1 however, these figures appear to be taken from a 2010 report on allergy and allergy remedies from the market research company Mintel. Data from the QRESEARCH project, a database containing the psuedoanonymised health records of over 13 million people, from 950 UK general practices,2 can provide some information on the prevalence of allergy symptoms and diagnoses seen in primary care and on changing patterns over time. At the end of 2005, QRESEARCH data indicated that approximately one in nine people had a recorded diagnosis of ‘any allergic disease’ (including asthma, hay fever, eczema, anaphylaxis or peanut allergy); this figure represented a 27.7% increase over a 4-year period. 3 Increases in the incidence of eczema and allergic rhinitis were reported for the same time period; the age- and sex-standardised incidence of eczema was 9.58 per 1000 patient-years in 2001, rising to 13.58 per 1000 patient-years in 2005,4 with the corresponding figures for allergic rhinitis being 5.57 per 1000 patient-years and 7.41 per 1000 patient-years, respectively. 5 QRESEARCH data also indicate that the incidence of multiple allergic disorders is increasing. The age- and sex-standardised incidence of multiple allergic disorders was 4.72 per 1000 patient-years in 2001, rising to 6.28 per 1000 patient-years in 2005. 6 Alongside data on increasing incidence of allergic disease, QRESEARCH reports also record increases in the number of allergy-related prescriptions and general practice consultations, which are indicative of an increasing burden upon the UK NHS. 4–6 There are no QRESEARCH publications that specifically report on food allergy. NICE Clinical Guideline 1167 (food allergy in children and young people) reports an estimated prevalence for self-reported food allergy of between 3% and 35% for individual foods. However, the guideline also notes that only 25–40% of self-reported food allergy is confirmed by oral food challenge (OFC) testing. 7
Allergic disease can present as a severe, life-threatening reaction (anaphylaxis). The National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network have recommended that anaphylaxis be defined as ‘a serious allergic reaction that is rapid in onset and may cause death’ and is likely to be the diagnosis when there is involvement of skin or mucosal tissue (e.g. hives, angioedema) and airway compromise (wheezing, dyspnoea) and/or reduced blood pressure or associated symptoms (hypotonia, syncope), along with a temporal relationship (minutes to several hours) to a potential causative agent. 8 There are limited data on the incidence of anaphylaxis in the UK. Hospital Episode Statistics record ‘allergy (including anaphylaxis)’ as the primary diagnosis associated with Accident and Emergency attendance for around 70,000 cases (approximately 0.4% of all reports) in both 2013 and 2014; however, no separate statistics are recorded for anaphylaxis. 9 A 2010 study,10 based on the Health Improvement Network database, estimated the UK incidence of anaphylaxis at 21.3 [95% confidence interval (CI) 17.6 to 25.4] per 100,000 patient-years. This study included 382 cases of anaphylaxis and the causes were listed as drug (27%); food (24%); insect (12%); latex (0.8%); idiopathic (27%); and no information (10%). 10 NICE Clinical Guideline 13411,12 (anaphylaxis assessment) reports an estimate of 20 UK deaths per year from anaphylaxis from a study conducted in 2000. A study published in 2015,13 which analysed data from 1992 to 2012, shows that mortality has not risen, despite an increase in hospitalisations.
Where data are available, this assessment will focus on studies conducted in the people with allergy that is difficult to manage. If data are lacking for this population, studies conducted in patients with specific allergic disease (e.g. peanut allergy) will not be excluded and all potential clinical applications of multiplex allergen testing will be considered.
Intervention technologies
ImmunoCAP Immuno-Solid phase Allergy Chip
The ImmunoCAP ISAC is a miniaturised immunoassay platform (multiple allergen components immobilised on a slide) that is intended to assess the presence of multiple antibodies in a single blood test. IgE antibodies from the patient’s blood sample bind to the immobilised allergen components on the slide, and allergen-bound IgE antibodies are then detected using fluorescence-labelled anti-IgE antibodies. Slides are read using a separate microarray scanner and image analysis software. Using these technologies may provide more detailed information about individual sensitisation profiles than single IgE testing. ImmunoCAP ISAC is intended for use in complex allergy cases, such as those with inconsistent case histories/unsatisfactory response to treatment, those who are polysensitised and patients with idiopathic anaphylaxis. These are people with severe or unclear allergic disease who test positive to a range of allergens but in whom the true cause of symptoms can be difficult to identify. It is claimed that using the ImmunoCAP ISAC test could improve health outcomes by improving allergy management, more appropriately targeting specific immunotherapy, and reducing the number of investigative diagnostic tests. These improvements could also lead to potential savings to the NHS from reducing the number of tests and avoiding the use of unnecessary immunotherapy.
ImmunoCAP ISAC 112 is a molecular diagnostic test that can simultaneously test for IgE antibodies to 112 components from 51 allergen sources. The ISAC is a miniaturised immunoassay platform that uses a single sample (30 μl) of serum, plasma or capillary blood to test for IgE antibodies to multiple allergens. ImmunoCAP ISAC is a two-step assay. IgE antibodies from the patient sample bind to immobilised allergen components spotted in triplets on polymer-coated slides. Each slide contains four microarrays, giving results for four samples per slide. The results are measured using a biochip scanner [confocal laser scanning devices, in particular the CapitalBio LuxScan 10k microarray scanner (Capitalbio, San Diego, CA, USA), are recommended] and evaluated using proprietary software produced by the same company, Phadia Microarray Image Analysis software (MIA) (Phadia AB, Uppsala, Sweden). ImmunoCAP ISAC is a semi-quantitative test and results are reported in International Standard Units (ISUs), giving indications of specific IgE antibody levels; the operating range is 0.3–100 ISU for IgE (ISU-E). This range approximately corresponds to a concentration range of 0.3–100 kilo international units of allergen-specific-antibody per unit volume of sample (kUA/l) of IgE (1 kUA/l is equal to 2.4 ng/ml). The assay takes a total of 4 hours, including sample processing and incubation time.
Microtest
The Microtest Instrument is a CE (Conformité Européenne)-marked automated immunoassay platform that uses microarrays to simultaneously test for 26 allergen components. It is designed for processing and reading protein microarrays of allergens printed in the biochips. The Microtest instrument can simultaneously process up to five Microtest biochips, each containing a different serum sample, in each run. The process is fully automated. When the test is completed, the Microtest Instrument uses a fluorimeter to read the microarrays and the results are semi-quantitative, reported on an allergy risk scale of 0–4. The user can print or export the reports as appropriate. Microtest is intended for use in any patient (infants, children and adults) presenting with allergy symptoms.
Table 1 summarises the key characteristics of the multiplex allergen tests ImmunoCAP ISAC and Microtest, compared with comparator tests that are currently used in the standard diagnostic work-up of patients with difficult to manage allergic disease.
| ISAC 112 | Microtest | Skin prick | Specific IgE | OFC | |
|---|---|---|---|---|---|
| Time to perform assay/test | 3.45 hours for immunoassay + time to scan + time to interpret results + time to consult with patient |
5 minutes to load samples and reagents ∼ 4 hours to run Microtest machine and generate results + time to consult with patient |
15–20 minutes | 3.45 hours for immunoassay + time to interpret results + time to consult with patient |
Up to 5 hours |
| No. of allergens tested | 51 allergens per chip (4 chips per slide) 4 patient samples can be analysed at once of 51 allergens each |
26 allergens per chip 5 chips can be run at once 5 patient samples can be run at once of 26 allergens each |
3–25 | 1 (variable but up to 650 allergens available on Phadia website) | 1 |
| Staff required | Trained laboratory professionals and physicians | Any trained operator (laboratory professionals not required) | Trained practitioners (for assay and resuscitation) will do the test and give the result | Trained laboratory professionals and physicians | Trained practitioners (for test and resuscitation) |
| Method summary |
|
|
|
|
|
| Controls conducted | Internal positive and negative controls For each component analysed there are three dots and two must be positive to record a positive result Calibration curves must be generated from samples in the kit plus a chip, at least every 30 days |
Internal positive and negative controls. These adjust a stored calibration curve (from international standards) | Positive and negative control | Positive and negative controls | None |
| Quantitative results | Semi-quantitative: 0 < 0.3 ISU-E 1 ≥ 0.3 = < 1 (low) 2 ≥ 1 = < 15 (moderate) 3 ≥ 15 (high) |
Semi-quantitative: 0 < 0.35 kU/l 1 0.35–1 kU/l (low) 2 1.01–15 kU/l (moderate) 3 ≥ 15 kU/l (high) |
None | ||
| Special considerations | Not recommended for investigation of isolated venom allergies, as these patients may have very low levels of IgE, below the detection limit of ImmunoCAP ISAC | Not for use if patient taking antihistamines Emergency equipment must be available (antihistamine, adrenaline, hydrocortisone) |
Immunohistochemical kits and imaging equipment and likely to be variable, between different hospital sites | Stop antihistamine medicines Challenge tests are always undertaken in hospital under close medical supervision where resuscitation equipment and emergency medication are available in case a severe reaction occurs |
|
| Equipment required | ImmunoCAP ISAC 112 IgE kit Laser scanner Computer to run analyser software |
Microtest allergy biochip Microtest allergy cartridges and reagents Microtest instrument Computer and analysis software |
SPT kit | Specific IgE kit Automated analyser |
None |
| References | PHADIA_ISAC-DfU_IgE – Extracted English version.pdfa 45_Phadia_MIA_User_manual_v1.2_EN.pdfa immunocap_isac_112_technical-brochure.pdfa |
Microtest Users Manual 2015.pdfa Microtest Instructions for Use MAN-IFU-SYS-01–03.pdfa |
www.allergyuk.org/diagnosis--testing-of-allergy/skin-testing (accessed 30 August 2016) www.bsaci.org/_literature_121183/Paediatric_skin_prick_testing_guideline (accessed 30 August 2016) |
www.phadia.com/en-GB/5/Products/ImmunoCAP-Assays/1/ (accessed 30 August 2016) | www.allergyuk.org/diagnosis–testing-of-allergy/allergy-challenge (accessed 30 August 2016) www.northumbria.nhs.uk/sites/default/files/images/Oral_Food_Challenge_Test.PDF (accessed 30 August 2016) www.ruh.nhs.uk/patients/patients_leaflets/paediatrics/PAE041_Allergy_food_Challenge_tests.pdf (accessed 30 August 2016) |
There are a number of poorly understood factors that influence whether or not clinical symptoms manifest at a certain IgE level, including inhibitory allergen-specific antibodies of non-IgE subclass. Furthermore, other factors, for example age, patient population, concomitant exposure to other allergens, other clinical conditions such as infections, etc., can also affect the degree of symptoms which may occur following allergen exposure. Thus it is not possible to establish general cut-off values valid for all patients at all times. However, when combined with clinical history, the results of multiplex allergen testing may aid the clinician in the diagnosis of allergy. Multiplex allergen testing should always be used in conjunction with allergy-focused clinical history and may be used in addition to, or in place of, single IgE antibody tests and/or skin prick testing.
Comparator
The comparator for this assessment will be current standard care, which should always include allergy-focused clinical history and can additionally involve tests of IgE antibody status (single IgE antibody testing), tests of clinical reactivity such as skin prick testing or allergen challenge testing, or a combination of these approaches.
Single immunoglobulin E testing
Allergen-specific IgE antibody assays are designed to detect and quantify circulating IgE antibodies to one allergen. The choice of which antibodies to test for is based on the clinical history of the patient, and several single IgE tests and/or a stepwise strategy which tests for the most likely causative agents first may be required.
The single IgE test process involves incubation of a blood sample with specific IgE antibodies. Allergen-specific IgE in the patient’s sample binds to the allergen, and unbound antibodies and excess sample are then removed by washing. Anti-IgE antibody, labelled to enable detection (e.g. fluorescently labelled anti-IgE antibody), is then added. The amount of bound allergen-specific IgE is calculated via a standard calibration curve, which is linked to the World Health Organization (WHO) IgE standard and reported in arbitrary mass units (kUA/l).
Higher levels of IgE are considered to be associated with allergy, but the amount of IgE is not predictive of the severity of reaction. Not all patients with a positive specific IgE test will have clinically manifest allergic reaction when exposed to that allergen. Unlike IgE antibody testing, skin prick tests (SPTs) and allergen challenge tests can provide direct information about clinical reactivity to a given allergen.
Skin prick testing
Skin prick testing is a method used to assist in the diagnosis of IgE-mediated allergic disease in patients with rhinoconjunctivitis, asthma, urticaria, anaphylaxis, atopic eczema or gastrointestinal symptoms that are suspected (based on clinical history) to be caused by type 1 (immediate) allergic reaction. It provides evidence for sensitisation in the form of reaction to allergenic stimulus.
The test involves putting a drop of liquid allergen onto the skin, followed by a gentle pin prick through the drop. SPT interpretation utilises the presence and degree of skin reactivity as a marker for sensitisation. When relevant allergens are introduced into the skin, an IgE-mediated immune response occurs. This produces a ‘weal and flare’ response, which can be quantified. Many different allergens can be tested simultaneously because the resultant reaction to a specific allergen is localised to the immediate area of the SPT.
One potential advantage of SPTs compared with in vitro measurement of IgE antibodies is that the test can be interpreted within 15–20 minutes after the reagent is applied to the skin, and therefore results can potentially be given to the patient in the same consultation. SPT results provide evidence of IgE in skin-resident mast cells which may, but does not always, correlate with clinical reactivity. SPTs can also be utilised to test less common allergens, (e.g. medications, and fresh fruits and vegetables) where no specific IgE antibody assays are available. As with any test, the results of SPTs must be interpreted in the context of medical history, clinical symptoms and, where appropriate, other test results. It has been suggested that skin prick testing is an inexpensive option. However, whilst the test materials may be relatively inexpensive, any estimation of costs should consider the staff time needed to perform these tests in an appropriate and safe health-care setting.
Skin prick testing has the following limitations:
-
Skin reactivity might be affected by previous ingestion of antihistamines or other drugs.
-
Children may not tolerate multiple skin needle pricks.
-
Prior or coexisting dermatological conditions, such as eczema, may preclude the performance of skin tests.
-
The potency of antigen extracts needs to be maintained.
-
Systemic reactions, although very rare, may occur.
-
SPTs alone are not sufficient as a confirmatory test.
Allergen challenge testing
Oral food challenges or inhalant challenges are indicated where there is a discrepancy between clinical history and other test results, and can be useful in establishing the identity of specific triggers. The most rigorous method for allergen challenge tests is double blinded and placebo controlled, thus requiring two separate visits. Therefore, single (patient)-blind and open challenges are more frequently performed because only one visit is required. An open challenge describes a challenge in which the patient can recognise the target trigger and there is no attempt at blinding; this is the least time-intensive type of challenge test, but may produce less reliable results as there is the potential for the result to be influenced by either the patient’s anxiety about a particular trigger and/or the health-care professional’s expectations. The general methodology of any challenge test is to administer the trigger in gradually increasing doses in a medical setting. Allergen challenge tests should be performed in a setting that is fully equipped for emergency treatment if an episode of anaphylaxis occurs.
Care pathway
There are a number of National Institute for Health and Care Excellence (NICE) guidelines that consider elements of the diagnosis, management and treatment of allergy. 7,11,14,15
Diagnosis
Clinical guidelines consistently emphasise the importance of obtaining a clinical history and asking specific, allergy-focused questions. 7,15,16 NICE Clinical Guideline 1167 (food allergy in children and young people) states that this can be done by general practitioners or other primary health-care professionals with the appropriate competencies. According to the guidelines, the following should be included when taking a clinical history:
-
Any personal history of atopic disease (asthma, eczema or allergic rhinitis).
-
Any individual and family history of atopic disease (such as asthma, eczema or allergic rhinitis) or food allergy in parents or siblings.
-
Details of any foods that are avoided and the reasons why.
-
An assessment of presenting symptoms and other symptoms that may be associated with food allergy, including questions about:
-
the age of the child or young person when symptoms first started
-
speed of onset of symptoms following food contact
-
duration of symptoms
-
severity of reaction
-
frequency of occurrence
-
setting of reaction (e.g. at school or home)
-
reproducibility of symptoms on repeated exposure
-
what food and how much exposure to it causes a reaction.
-
-
Cultural and religious factors that affect the foods they eat.
-
Who has raised the concern and suspects the food allergy.
-
What the suspected allergen is.
-
The child or young person’s feeding history, including the age at which they were weaned and whether they were breastfed or formula fed – if the child is currently being breastfed, consider the mother’s diet.
-
Details of any previous treatment, including medication, for the presenting symptoms and the response to this.
-
Any response to the elimination and reintroduction of foods.
The NICE Clinical Guideline 5715 (atopic eczema in children) recommends that health-care professionals should seek to identify potential trigger factors during clinical assessment including:
-
irritants
-
skin infections
-
contact allergens
-
food allergens
-
inhalant allergens.
The Royal College of Paediatrics and Child Health also provides advice on allergy-focused questions to be used when taking a clinical history. An initial screening set of questions is recommended to identify patients, in community settings, for whom a more detailed allergy history may need to be taken. If allergy is suspected, further questions are grouped into six areas:
-
general history questions asking about general health, current medications, previous allergy testing, lifestyle and general home conditions
-
general allergy history questions
-
food-related questions
-
respiratory-related questions
-
ear-, nose- and throat-related questions
-
skin-related questions.
If IgE-mediated allergy is suspected, based on the results of allergy-focused clinical history, NICE Clinical Guideline 1167 (food allergy in children and young people) recommends that the child or young person should be offered a SPT and/or blood tests for specific IgE antibodies to the suspected foods and likely co-allergens. It further recommends that these tests should be undertaken only by health-care professionals with the appropriate competencies to select, perform and interpret them, and should be undertaken only where there are facilities to deal with an anaphylactic reaction. 7 The guideline also states that the patient should be given information on when, where and how an OFC or food reintroduction procedure may be undertaken. However, these tests should not be performed in primary care. 7
Management
The management of allergy is dependent upon type and severity and many allergies can be managed and treated in primary care settings. More severe allergies and more complex patients may require additional management and referral on to specialist services.
The NICE Clinical Guideline 1167 (food allergy in children and young people) recommends referral to secondary or specialist care when the child or young person has:
-
faltering growth in combination with one or more gastrointestinal symptoms
-
not responded to a single-allergen elimination diet
-
had one or more acute systemic reactions
-
had one or more severe delayed reactions
-
confirmed IgE-mediated food allergy and concurrent asthma
-
significant atopic eczema where multiple or cross-reactive food allergies are suspected by the parent or carer.
-
Or there is:
-
persisting parental suspicion of food allergy (especially in children or young people with difficult or perplexing symptoms) despite a lack of supporting history
-
strong clinical suspicion of IgE-mediated food allergy but allergy test results are negative
-
clinical suspicion of multiple food allergies.
-
The NICE Clinical Guideline 57 (atopic eczema in children) and NICE Quality Standard 44 (atopic eczema in children)14,15 both recommend that children with a suspected food allergy should be referred for specialist investigation and management by a paediatric allergist or paediatric dermatologist.
With respect to management following a severe acute episode, NICE Clinical Guideline 13411 (anaphylaxis assessment) recommends that prior to discharge a health-care professional with the appropriate skills and competencies should offer the following:
-
information about anaphylaxis, including the signs and symptoms of an anaphylactic reaction
-
information about the risk of a biphasic reaction
-
information on what to do if an anaphylactic reaction occurs (use the adrenaline injector and call emergency services)
-
a demonstration of the correct use of the adrenaline injector and when to use it
-
advice about how to avoid the suspected trigger (if known)
-
information about the need for referral to a specialist allergy service and the referral process
-
information about patient support groups.
Treatment
Mild allergies can be treated using over-the-counter medications, such as antihistamines, and simple avoidance of the identified allergen(s).
The NICE Clinical Guideline 1167 (food allergy in children and young people) recommends that, once an allergy is suspected, based on clinical history, information should be provided to the patient about:
-
type of allergy suspected
-
risk of severe allergic reaction
-
potential impact of the suspected allergy on other health-care issues, including vaccination.
If a food elimination diet is advised information should be provided on:
-
what foods and drinks to avoid
-
how to interpret food labels
-
alternative sources of nutrition to ensure adequate nutritional intake
-
the safety and limitations of an elimination diet
-
the proposed duration of the elimination diet
-
when, where and how an OFC or food reintroduction procedure may be undertaken.
The NICE Clinical Guideline 5715 (atopic eczema in children) recommends that health-care professionals should use a stepped approach for managing atopic eczema in children and should tailor the treatment step to the severity of the atopic eczema. Emollients should form the basis of atopic eczema management and should always be used, even when the atopic eczema is clear. Management can then be stepped up or down, according to the severity of symptoms, with the addition of the other treatments such as mild-potency topical corticosteroids (for mild eczema), moderate-potency topical corticosteroids (for moderate eczema), tacrolimus, bandages (for moderate or severe eczema), and potent topical corticosteroids, phototherapy and systemic therapy (for severe eczema only). Very potent topical corticosteroids should not be used without specialist dermatological advice.
In selected patients allergen immunotherapy may be appropriate. It involves the repeated administration, either subcutaneously or sublingually, of allergen extracts. The potential outcomes of immunotherapy are:
-
reducing allergy symptoms on subsequent allergen exposure
-
improving quality of life (QoL)
-
inducing long-term tolerance.
Immunotherapy is time-consuming and expensive, and there is a risk of a severe allergic reaction or anaphylaxis during administration. According to the British Society for Allergy & Clinical Immunology guidelines,17 the main indications for immunotherapy in the UK are:
-
IgE-mediated seasonal pollen-induced rhinitis, if symptoms have not responded adequately to optimal pharmacotherapy
-
systemic reactions caused by hymenoptera venom allergy
-
selected patients with animal dander or HDM allergy in whom rigorous allergen avoidance and reasonable pharmacotherapy fail to control symptoms.
The selection, initiation and monitoring of all patients for immunotherapy should be supervised by specialists in allergy. Immunotherapy should be administered only by physicians and nurses with specialist knowledge of allergy and specific immunotherapy. Immunotherapy is an attractive option for the treatment of food allergies, as its goal is to induce tolerance in the person. With desensitisation, the treated person manifests a decreased response to the allergen. 17
Regarding treatment following severe acute episodes, NICE Clinical Guideline 13411 (anaphylaxis assessment) recommends that after emergency treatment for suspected anaphylaxis patients should be offered an appropriate adrenaline injector as an interim measure before the specialist allergy service appointment. An adrenaline autoinjector is a medical device for injecting a measured dose or doses of epinephrine (adrenaline), by means of autoinjector technology. It is most often used for the treatment of anaphylaxis. Most individuals with a severe IgE-mediated food allergy are advised to carry an autoinjector in case of accidental exposure. There are many barriers to the successful use of an autoinjector, including the ability to recognise the symptoms of anaphylaxis, the availability and understanding of how to use the autoinjector, and anxiety associated with its use.
Patient issues and preferences
Allergic reactions can have a daily impact on the QoL of the individual, and can affect their ability to participate in everyday and social activities, perform work-related duties, undertake examinations and pursue their career of choice. The effect of allergies is described in two reports produced by Allergy UK. The Stolen Lives survey found that for 28.4% of respondents allergies had a serious effect on how they planned important life events, and for 26% their allergy severely affected their everyday life. 18 The report The Disturbing Impact of Skin Allergy and Sensitivity in the UK report19 states that 78% of respondents suffered from reactions to their skin allergy all year round, and for 62% their condition had stopped them from going out socially and carrying out day-to-day activities.
Where food allergy is diagnosed, implementing special diets for children can also be difficult for families to manage, particularly where there are multiple dietary requirements in one family. A 201020 review on the psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families reported that non-allergic siblings often adopted the restricted diet that the allergic child followed. The same review20 highlighted the effect of allergy on the QoL of patients and caregivers. It reported that allergy heightened patients’ and caregivers’ anxiety because of the need for constant vigilance, particularly in new situations. It also showed that parents tended to be overprotective of children with allergy, particularly those who have had anaphylaxis. There can also be anxiety for a parent or caregiver associated with administering an adrenaline injection. 20
Chapter 3 Assessment of clinical effectiveness
A systematic review was conducted to summarise the evidence on the clinical effectiveness and cost-effectiveness of ImmunoCAP ISAC and Microtest for multiplex allergen testing in people with allergic disease. Systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care21 and the NICE Diagnostic Assessment Programme Manual. 22
Systematic review methods
Search strategy
Development of search strategies followed the recommendations of the CRD guidance for undertaking reviews in health care. 21 Strategies were based on the technologies of interest.
Candidate search terms were identified from target references, browsing database thesauri (e.g. MEDLINE MeSH and EMBASE Emtree) and from existing reviews identified during the initial scoping searches. These scoping searches were used to generate test sets of target references, which informed text mining analysis of high-frequency subject indexing terms using EndNote X7 reference management software (Thomson Reuters, CA, USA). Strategy development involved an iterative approach testing candidate text and indexing terms across a sample of bibliographic databases, aiming to reach a satisfactory balance of sensitivity and specificity. Search strategies were developed specifically for each database.
The following databases were searched for relevant studies from 2005 to April 2015:
-
MEDLINE (via OvidSP): 1946–week 2 April 2015
-
MEDLINE In-Process Citations (via OvidSP): up to 15 April 2015
-
MEDLINE Daily Update (via OvidSP): up to 15 April 2015
-
PubMed [National Library of Medicine (NLM)] (internet) up to 22 April 2015*
-
EMBASE (via OvidSP): 1974–14 April 2015
-
Cochrane Database of Systematic Reviews (CDSR) (via Wiley): 2015/April/Iss4
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via Wiley): Cochrane Library 2015/March/Iss3
-
Database of Abstracts of Reviews of Effects (DARE) (via Wiley): Cochrane Library 2015/January/Iss1
-
Health Technology Assessment database (HTA) (via Wiley): Cochrane Library 2015/January/Iss1
-
Science Citation Index (SCI) (via Web of Science): 1970–21 April 2015
-
Conference Proceedings Citation Index – Science (CPCI-S) (Web of Science): 1990–21 April 2015
-
BIOSIS Previews (via Web of Science): 1956–21 April 2015
-
Literature in the Health Sciences in Latin America and the Caribbean (LILACS) (internet; http://lilacs.bvsalud.org/en/): 1982–22 April 2015
-
National Institute for Health Research (NIHR) Health Technology Assessment programme (internet; www.hta.ac.uk/): up to 23 April 2015
-
US Food and Drug Administration (FDA) (internet; www.fda.gov): up to 23 April 2015.
*An additional companion PubMed search was undertaken in tandem with MEDLINE via OvidSP; this approach aims to detect the latest ‘ahead of print’ and ‘online first’ electronic content promoted by many leading journals.
A supplementary search was undertaken on the following resource to identify grey literature:
-
OpenGrey (internet; www.opengrey.eu): up to 22 April 2015
Completed and ongoing trials were identified by searches of the following resources:
-
National Institutes of Health (NIH) ClinicalTrials.gov (internet; www.clinicaltrials.gov/): up to 22 April 2015
-
WHO International Clinical Trials Registry Platform (ICTRP) (internet; www.who.int/ictrp/en/): up to 22 April 2015
-
International Standard Randomised Controlled Trial Number (ISRCTN) Registry (internet; www.isrctn.com): up to 22 April 2015.
The following key conference proceedings, were identified in consultation with clinical experts, and were screened for the last 5 years where available:
-
American Academy of Allergy, Asthma and Immunology (AAAAI) Annual Meeting
-
European Academy of Allergy and Clinical Immunology (EAACI)
-
British Society for Allergy & Clinical Immunology (BSACI)
-
Food Allergy and Anaphylaxis Meeting (FAAM)
-
International Symposium on Molecular Allergology (ISMA)
-
American Academy of Dermatology (AAD) Meeting
-
British Association of Dermatologists (BAD).
No restrictions on language or publication status were applied. Searches took into account generic and other product names for the intervention. See Appendix 1 for all search strategies. The main EMBASE strategy for each search was independently peer reviewed by a second Information Specialist, using the Canadian Agency for Drugs and Technologies in Health (CADTH) Peer Review checklist. 23 Identified references were downloaded in EndNote X7 software for further assessment and handling. References in retrieved articles were checked for additional studies.
Inclusion and exclusion criteria
Population
Adults and children with difficult to manage allergic disease who are being assessed in secondary or tertiary care settings. Owing to the paucity of available data, studies conducted in populations not specified as polysensitised or having difficult to manage allergic disease were also included. All presentations of allergic disease (respiratory, skin, gastrointestinal, anaphylaxis) were eligible for inclusion. Difficult to mange disease was defined as people who are allergic to two or more allergens and/or have allergies to unknown sources.
Intervention/index test
Multiplex allergen testing:
-
ImmunoCAP ISAC 112 and previous generations of ImmunoCAP ISAC (Thermo Fisher Scientific/Phadia AB)
-
Microtest (Microtest Matrices).
Comparator
The comparator for this assessment was current standard care, which included allergy-focused clinical history, alternative tests of IgE antibody status (single IgE antibody testing), tests of clinical reactivity (such as skin prick testing or allergen challenge testing) or a combination of these approaches.
Outcomes
-
Clinical outcomes (e.g. allergy symptoms, incidence of acute exacerbations, mortality, AEs of testing and treatment, health-care presentations or admissions, HRQoL, patient anxiety/preferences).
-
Change to management, that is, change to treatment or treatment plan (e.g. restriction diets, immunotherapies, use of other medications such as corticosteroids, number of allergen challenge tests required).
-
Additional diagnostic information – accuracy (sensitivity and specificity) for the prediction of clinical reactivity, as defined by SPTs, allergen challenge tests or response to immunotherapy, plus numbers of participants for whom multiplex allergen testing provided additional information (e.g. allergens component-specific information, cross-reactivities, information on multiple sensitisation), diagnostic yield (number of participants with a definitive diagnosis).
Study design
There were no restrictions on study design. Randomised controlled trials (RCTs), controlled clinical trials, other comparative studies (e.g. ‘before-and-after’ studies) and diagnostic test accuracy studies were eligible for inclusion. Observational study designs were eligible for inclusion only if they reported measures of additional diagnostic information provided by multiplex allergen testing; studies that assessed only concordance between multiplex allergen testing and single IgE antibody testing or other tests were not included.
Protocol change The protocol stated that diagnostic accuracy studies would be included only if they reported both the accuracy (sensitivity and specificity) of multiplex allergen testing for the prediction of clinical reactivity, as defined by SPTs, allergen challenge tests or response to immunotherapy, and the numbers and details of participants for whom multiplex allergen testing provided additional information. No studies of this type were identified. The inclusion criteria were expanded to allow studies that reported direct comparisons of diagnostic accuracy between single IgE testing and multiplex allergen testing, using SPTs or allergen challenge tests as the reference standard. These studies do not address the primary aim of the project, ‘to assess the clinical effectiveness and cost-effectiveness of adding multiplex allergen testing to the investigation of people with difficult to manage allergic disease in secondary or tertiary care settings’, because they provide no information on any additional benefit conferred by the use of multiplex testing. Studies of this type were included with the aim of providing some indication of the performance of multiplex allergen testing, compared with current single IgE antibody testing practice, for predicting clinical response. Data of this type may inform the question of whether or not multiplex testing might, in some circumstances, replace single IgE testing as well as helping to guide possible future research recommendations.
Inclusion screening and data extraction
Two reviewers (MW and SL) independently screened the titles and abstracts of all reports identified by searches and any discrepancies were discussed and resolved by consensus. Full copies of all studies deemed potentially relevant were obtained and the same two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus. Details of studies excluded at the full paper screening stage are presented in Appendix 3.
The principal investigators of completed trials (identified through searches of clinical trials registries) that appeared to meet our inclusion criteria but for which no publication could be identified were contacted and asked to provide publication details or unpublished data.
Data were extracted on the following: study details (including study design, country and funding); stated objective of the study; inclusion and exclusion criteria; participant characteristics (age, gender, primary presentation and previous allergy-related history); details of the multiplex allergen testing method used; details of the tests included in the standard care comparator (e.g. SPT, OFC, single IgE); details of the reference standard test (diagnostic accuracy studies only); outcome measures (included change to treatment or treatment plan, e.g. restriction diets, immunotherapies), change to diagnosis or number of participants with a definitive diagnosis, and comparative accuracy (sensitivity and specificity) of multiplex allergen testing and single IgE for the prediction of clinical reactivity, as defined by SPTs, allergen challenge tests. Data were extracted by one reviewer, using a piloted, standard data extraction form, and checked by a second (MW and SL); any disagreements were resolved by consensus. Full data extraction tables are provided in Appendix 2.
Quality assessment
We planned to use the Cochrane risk-of-bias tool to assess the methodological quality of RCTs;24 however, no RCTs or non-RCTs were identified. The methodological quality of studies providing comparative accuracy data was assessed using QUADAS-2. 25 Observational studies that used a ‘before-and-after’ type of study design to assess the effects of adding information from multiplex allergen testing to the standard diagnostic work-up in the same group of participants were assessed using a review-specific tool designed by the authors (MW, SL and NA). This tool has been designed to focus on elements of study design that we considered relevant to this specific study type, and is based upon the structure of the QUADAS-2 tool. The Critical Appraisal Skills Programme (CASP) cohort risk-of-bias tool was used to assess other observational studies. 26 A narrative description of the potential limitations of any other included studies is provided. The results of the quality assessment have been used for descriptive purposes to provide an evaluation of the overall quality of the included studies and to provide a transparent method of recommendation for design of any future studies. Quality assessment was undertaken by one reviewer and checked by a second reviewer (MW and SL) and any disagreements were resolved by consensus. The applicability of studies to current UK practice was also considered and a narrative description of potential applicability issues is provided. The results of the risk-of-bias assessments are summarised and presented in tables and graphs in the results of the systematic review (see Study quality) and are presented in full, by study, in Appendix 4.
Methods of analysis/synthesis
We planned to use a bivariate/hierarchical summary receiver operating characteristic random-effects model to generate summary estimates and a summary receiver operating characteristic (SROC) curve for test accuracy data,27–29 and a DerSimonian and Laird random-effects model to generate summary estimates of treatment effects. However, because the review identified a small number of studies with between-study variations in participant characteristics (allergy history), multiplex allergen testing methods, allergens tested for, standard care comparators, and outcomes assessed, we did not consider meta-analyses to be appropriate and have provided a structured narrative synthesis. The results of studies included in this review are summarised by outcome type (clinical, change to management and diagnostic accuracy) and are further stratified by allergen type (food and aeroallergens). The results of individual studies are summarised in text and tables. The results of studies providing comparative accuracy data are also illustrated in receiver operating characteristic (ROC) space plots.
Results of the assessment of clinical effectiveness assessment
The searches of bibliographic databases and conference abstracts identified 8619 references. After initial screening of titles and abstracts, 169 were considered to be potentially relevant and ordered for full paper screening; of these, 20 were included in the review30–49 and one50 could not be obtained. All potentially relevant studies cited in documents supplied by the test manufacturers had already been identified by bibliographic database searches. Additional data, relating to the study by Hermansson et al. ,33,34 were obtained through contact with the authors. Figure 1 shows the flow of studies through the review process, and Appendix 3 provides details, with reasons for exclusions, of all publications excluded at the full paper screening stage.
FIGURE 1.
Flow of studies through the review process.
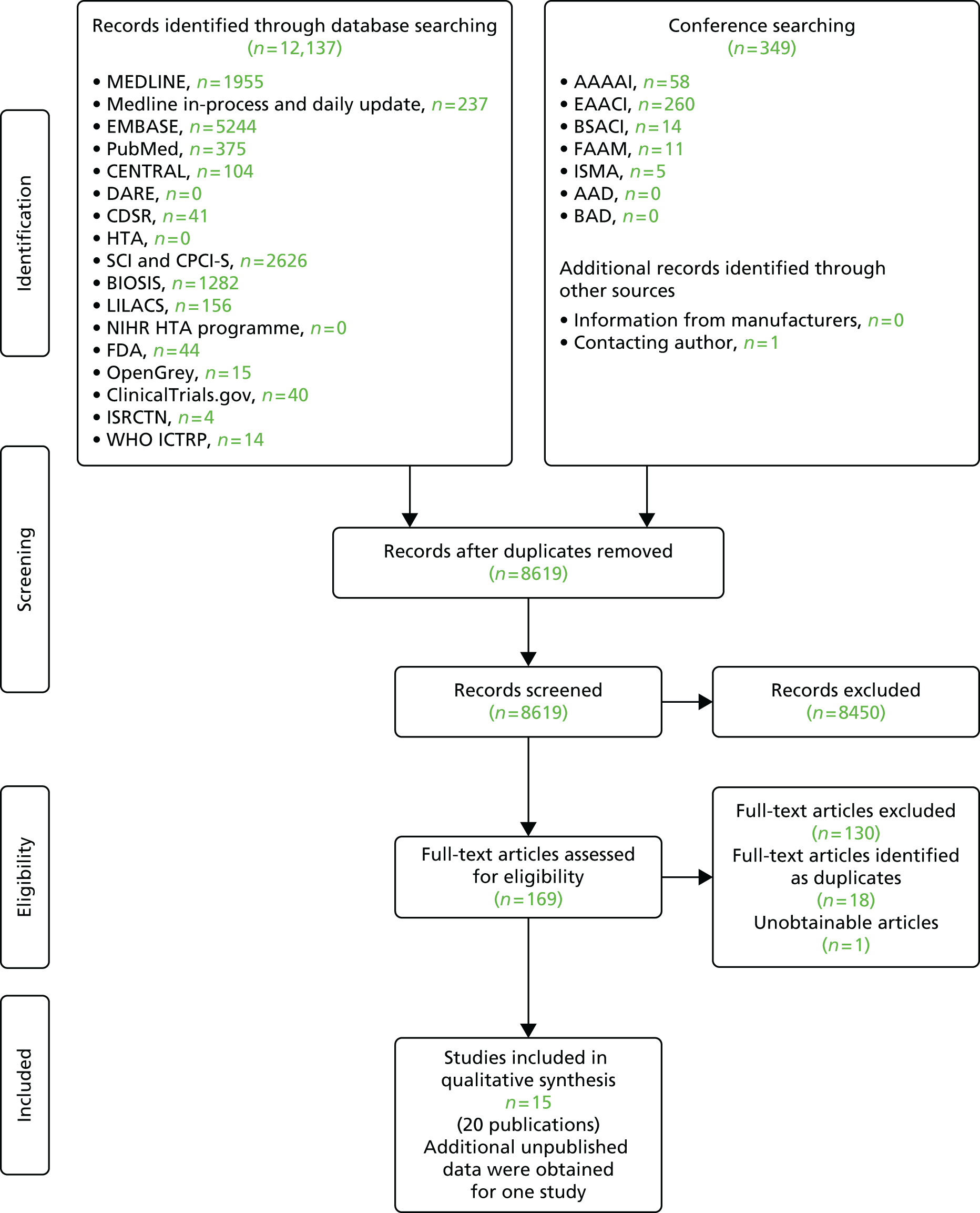
Overview of included studies
Based on the searches and inclusion screening described above (see Search strategy and Inclusion and exclusion criteria, above), 20 publications,30–49 of 15 studies, were included in the review; the results section of this report cites studies using the primary publication and, where this is different, the publication in which the referenced data were reported.
Two of the included studies39,40 were conducted in the UK and, where reported, the remaining studies were conducted in other European countries; one study47 did not report location. Of the 15 included studies, four were funded by,38,46 or received reagents and consumables39 or testing services45 from, the manufacturer. Five studies were publicly funded32,42–44,49 and six studies33,36,37,40,41,47 did not report funding sources. Full details of funding are reported in the baseline study details tables (see Appendix 2, Tables A–C).
All of the included studies evaluated versions of ImmunoCAP ISAC; one study33 evaluated ImmunoCAP ISAC 112, five studies37–39,42,43 evaluated ImmunoCAP ISAC 103, four studies32,44,45,49 evaluated other versions of ImmunoCAP ISAC and five studies36,40,41,46,47 did not specify the version used. We did not identify any studies of Microtest which met the inclusion criteria for this review.
We did not identify any studies that reported clinical outcomes (i.e. allergy symptoms, incidence of acute exacerbations, mortality, AEs of testing and treatment, health-care presentations or admissions, HRQoL, patient anxiety/preferences).
Five studies32,33,37,38,40 assessed the effects on patient management of adding ImmunoCAP ISAC to the standard diagnostic work-up (SPT/single IgE); two studies33,40 reported data on discontinuation or potential discontinuation of food avoidance diets, two studies32,38 assessed changes to immunotherapy prescriptions, and one study37 reported information on clinicians’ judgement of the utility of ImmunoCAP ISAC results in informing patient management. One study33 assesed ImmunoCAP ISAC 112, two studies37,38 assessed ImmunoCAP ISAC 103, one study32 assessed ImmunoCAP ISAC 96 and the remaining study40 did not specify the version used. None of the five studies32,33,37,38,40 reported the inclusion of patients with difficult to manage allergic disease; one study40 reported inclusion criteria which may have been consistent with this classification (moderate to severe eczema and multiple food allergies); however, this study40 was reported only as a conference abstract and hence provided very limited details of participants. One study40 was conducted in the UK, two studies32,37 were conducted in Spain, and one study was conducted in each of Finland33 and Italy. 38
Two studies38,39 assessed the effects on clinical diagnosis of adding ImmunoCAP ISAC 103 to the standard diagnostic work-up: one study39 reported data on new sensitisations identified in patients with idiopathic anaphylaxis and assessed their clinical relevance and the other study38 reported changes to the diagnostic classification made by clinicians following access to ImmunoCAP ISAC results and was conducted in patients with allergic rhinitis, with or without concomitant food allergy. One additional study36 assessed the relationship between change in IgE levels, measured by ImmunoCAP single IgE and an unspecified version of ImmunoCAP ISAC before and after a 3-year course of allergen-specific immunotherapy (SIT), and the clinicians’ evaluation of the benefit of SIT. None of these studies reported test accuracy data. One study39 was conducted in the UK, one study38 was conducted in Italy and one study36 did not report location.
Eight studies41–47,49 compared the diagnostic accuracy of ImmunoCAP ISAC to that of alternative investigations (single IgE testing or SPT) to predict clinical reactivity as defined by SPT or OFC testing (the reference standard). Six studies41,42,44,46,47,49 investigated people with food allergies and two studies43,45 investigated people with allergic rhinitis/respiratory symptoms. None of the eight studies41–47,49 reported the inclusion of patients with difficult to diagnose and manage allergic disease, or described inclusion criteria that could be considered consistent with this classification (e.g. polysensitised patients). One study41 included patients with birch allergy, one study42 included patients with suspected egg allergy, two studies44,49 included patients with suspected cow’s milk and/or hen’s egg allergy, one study46 included patients with cow’s milk allergy, one study47 included patients with hazelnut allergy, one study45 included patients with symptoms of allergic rhinitis and one study43 included patients with pollen allergy. None of the studies was conducted in the UK, seven studies41–46,49 were European and one study47 was unreported. Two studies investigated ISAC 103,42,43 one study44 investigated ISAC 89, one study49 investigated ISAC 59 and one study45 investigated ISAC 50, whereas three studies41,46,47 used unspecified ISAC.
Full details of the characteristics of study participants, study inclusion and exclusion criteria, and intervention and comparator or reference standard are reported in the data extraction tables presented in Appendix 2 (see Tables A–E).
Excluded studies
One hundred and forty-eight full-text articles were retrieved: 18 were identified as duplicates and 130 were subsequently excluded. In all but two cases,51,52 these studies reported no relevant outcomes. Further details of the 131 excluded full papers and the reasons for exclusion can be found in Appendix 4. One study50 could not be obtained.
Study quality
Studies of changes to management, treatment or diagnosis
Seven studies investigated changes to treatment or management outcomes. 32–34,36–40 One very small cohort study,36 with nine participants, assessed the relationship between change in IgE levels (measured by ImmunoCAP single IgE and an unspecified version of ImmunoCAP ISAC before and after a 3-year course of SIT) and the clinicians’ evaluation of the benefit of SIT. The methodological quality of this study36 was assessed using the CASP cohort tool. The remaining studies used a ‘diagnostic before-and-after’ type study design, which compared clinicians’ views and decisions on management, treatment or diagnosis in a single group of patients, before and after access to the results of multiplex allergen testing. The methodological quality of these studies was assessed using a tool designed specifically for this review, which was based on the structure of QUADAS-2. Risk of bias and concerns regarding applicability are summarised in Tables 2 and 3 and Figure 2; full assessments for each study are provided in Appendix 4.
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Comparator | Flow and timing | Patient | Index test | Reference standard | |
| Heaps 201439 | ? | + | + | + | + | + | + |
| Hermansson 201433,34 | – | ? | ? | ? | ? | + | ? |
| Noimark 201240 | ? | ? | ? | + | + | + | + |
| Luengo 201037 | ? | ? | ? | ? | ? | + | + |
| Passalacqua 201338 | ? | + | + | – | – | + | ? |
| Sastre 201232 | ? | + | + | + | – | + | ? |
| Study | A. Are the results of the study valid? | B. What are the results? | C. Will the results help locally? | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Did the study address a clearly focused issue? | 2. Was the cohort recruited in an acceptable way? | 3. Was the exposure accurately measured to minimise bias? | 4. Was the outcome accurately measured to minimise bias? | 5. (a) Have the authors identified all important confounding factors? | (b) Have they taken account of the confounding factors in the design and/or analysis? | 6. (a) Was the follow-up of subjects complete enough? | (b) Was the follow-up of subjects long enough? | 7. What are the results of this study? | 8. How precise are the results? | 9. Do you believe the results? | 10. Can the results be applied to the local population? | 11. Do the results of this study fit with other available evidence? | 12. What are the implications of this study for practice? | |
| Gay-Crosier 201036 | + | ? | ? | ? | ? | – | ? | + | + | ? | ? | ? | ? | ? |
FIGURE 2.
Risk of bias across included ‘diagnostic before-and-after’ studies (change to management or treatment).
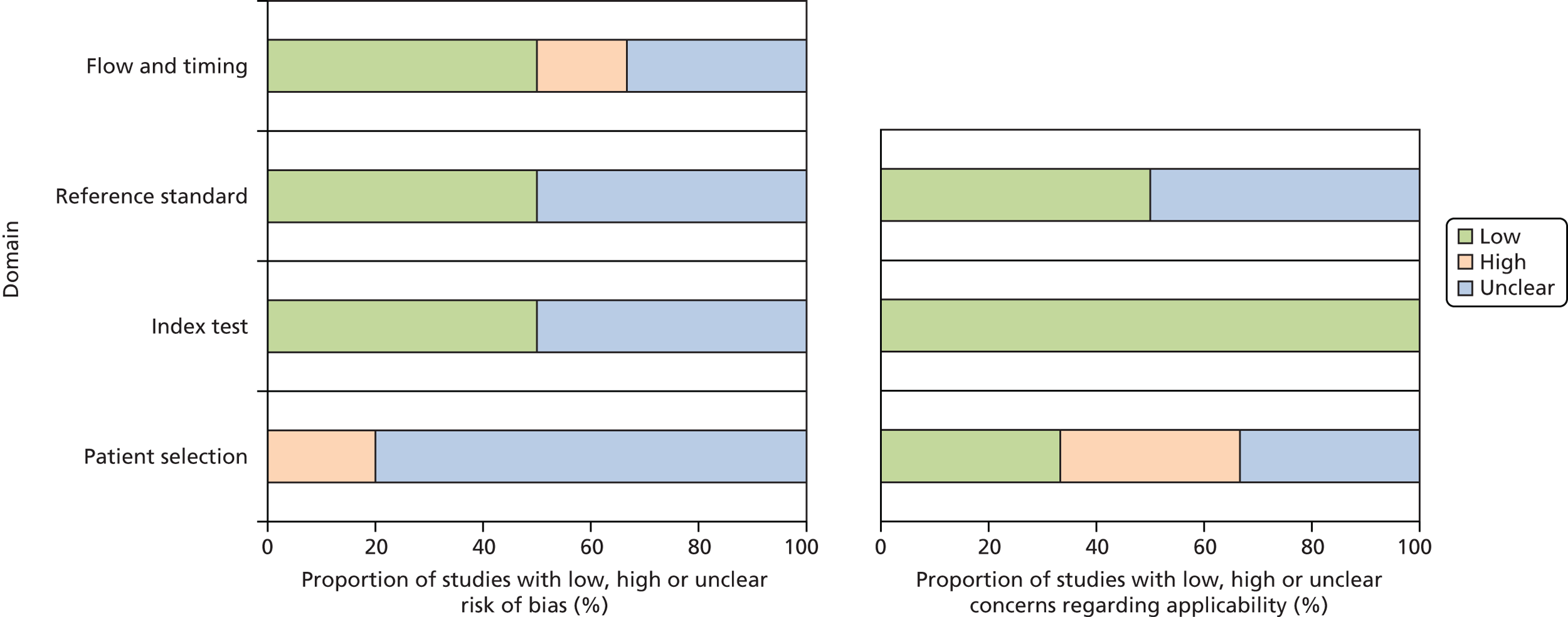
The ‘diagnostic before-and-after studies’ were generally poorly reported, resulting in a high number of ‘unclear’ ratings, with all studies rated as ‘unclear’ risk of bias on at least one domain; four of the studies were published as conference abstracts only. 33,34,36,37,40 Two studies33,38 were rated as ‘high’ risk of bias. One study33 was rated as ‘high’ risk of bias for patient selection; participants were selected from a database of children who were receiving special diets in school catering, and reasons for exclusion included ‘no longer allergic’ (according to self-report or nurse interview) and unwillingness to participate because of, for example, fear of needles, lack of trust in tests and multiple previous testing. The second study38 was rated as ‘high’ risk of bias for flow and timing because the standard care comparator differed between participants; all participants received SPT, and single IgE testing was used ‘as required.’ Although this is likely to be representative of standard practice, it remains a potential source of bias when estimating test performance.
Although this review included patients with any allergy, the primary objective was to assess the clinical effectiveness of multiplex allergen testing in people with complex or difficult to manage allergies, in UK health-care settings. Studies that did not specify that they included participants with difficult to manage allergic disease, or describe inclusion criteria which could be considered consistent with this classification (e.g. polysensitised patients), were therefore rated as having ‘high’ concerns regarding applicability. Studies that were conducted in non-UK settings and which assessed allergens considered unlikely to be relevant to UK populations (e.g. aeroallergens associated with Mediterranean countries) were also rated as having ‘high’ concerns regarding applicability. Two studies39,40 were rated as having ‘low’ concerns regarding participant applicability, and in two studies33,37 insufficient reporting details prevented a judgement and therefore they were rated ‘unclear’. The remaining two studies32,38 were rated as having ‘high’ concerns regarding participant applicability: for both this was because the patients were not from the UK and in one study38 patients did not have difficult to manage disease.
The small observational study36 that was assessed using the CASP cohort tool for risk of bias was reported only as conference abstract; therefore, risk of bias was largely unclear owing to lack of study details.
Studies of diagnostic accuracy
Eight studies41–47,49 compared the diagnostic accuracy of ImmunoCAP ISAC to that of alternative investigations (single IgE testing or SPT) to predict clinical reactivity as defined by SPT or OFC testing (the reference standard). The methodological quality of these studies was assessed using the QUADAS-2 tool (summarised in Table 4 and Figure 3). The full QUADAS-2 assessments for each study are provided in Appendix 4.
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Albarini 201347 | ? | ? | + | ? | – | ? | + |
| Alessandri 201242 | ? | – | ? | – | – | + | + |
| Cabrera–Freitag 201143 | – | – | + | ? | – | + | + |
| De Swert 201241 | – | – | + | – | – | + | + |
| D’Urbano 201044 | – | – | ? | + | – | + | + |
| Ott 200849 | ? | – | ? | ? | – | + | + |
| Sokolova 200946 | – | ? | + | ? | – | + | + |
| Wöhrl 200645 | – | – | + | ? | – | + | + |
FIGURE 3.
Risk of bias across included diagnostic studies (accuracy).
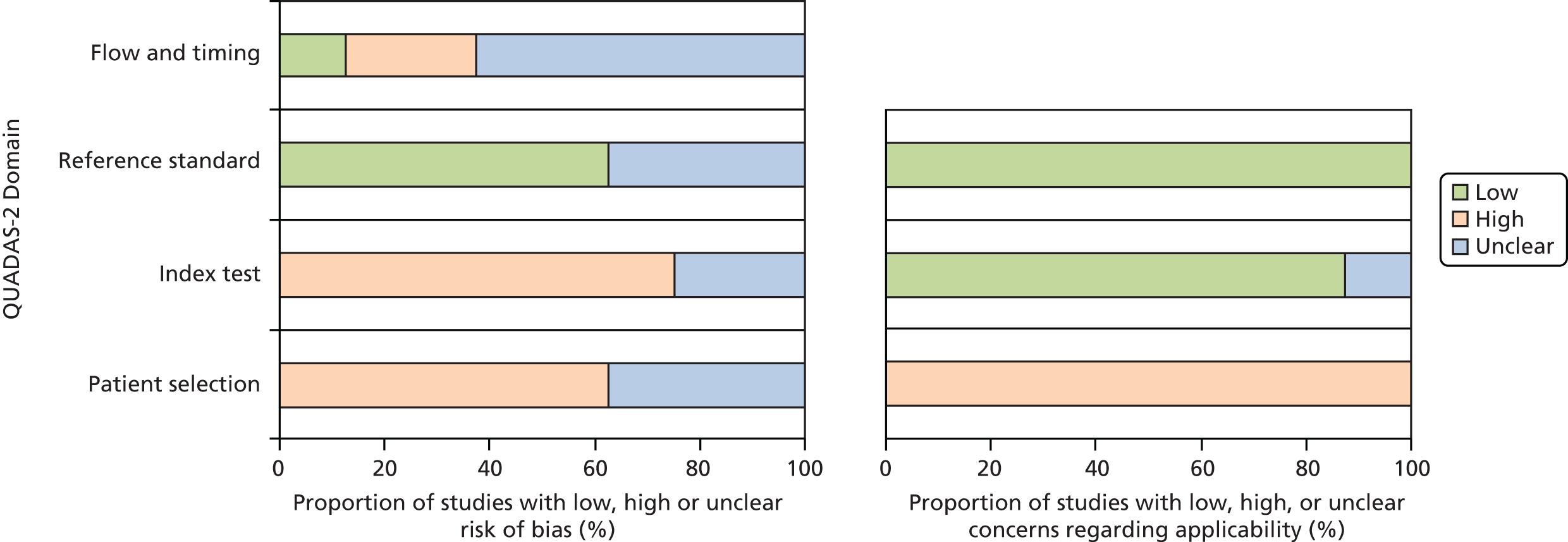
The comparative accuracy studies were generally poorly reported; seven42–47,49 of the eight studies were rated as ‘unclear’ risk of bias on at least one QUADAS-2 domain. One study47 was reported only as a conference abstract.
Seven studies41–46,49 were rated as ‘high’ risk of bias on at least one domain. The main potential sources of bias were in relation to participant selection and application of the index test. Five studies41,43–46 were rated as ‘high’ risk of bias for participant selection because they used a diagnostic case–control design, a design likely to produce inflated estimates of test performance, or applied inappropriate exclusion criteria (patients with eczema,44 high levels of single IgE45 or complex allergy43). Five studies41,43–46 were rated as ‘high’ risk of bias for the index test. In all cases this was because diagnostic thresholds were not prespecified, but were optimised using ROC analyses in the same population that was used to assess test performance, an approach that is likely to result in inflated estimates of test performance. Two of these studies41,42 were also rated as ‘high’ risk of bias for flow and timing, because not all the participants were included in the analysis41 or because not all participants received the same reference standard. 42
As was the case for studies of change to management, treatment or diagnosis, studies that did not specify that they included participants with difficult to manage allergic disease or describe inclusion criteria that could be considered consistent with this classification (e.g. polysensitised patients) were therefore rated as having ‘high’ concerns regarding applicability, and studies that were conducted in non-UK settings and which assessed allergens considered unlikely to be relevant to UK populations (e.g. aeroallergens associated with Mediterranean countries) were also rated as having ‘high’ concerns regarding applicability. All eight comparative accuracy studies41–47,49 were rated as having ‘high’ concerns regarding participant applicability because they did not include people with difficult to manage allergic disease, and three of these studies41,43,45 also focused on allergens that were considered unlikely to be fully applicable to the UK.
Effects on management, treatment and diagnostic classification of adding multiplex allergen testing to the diagnostic work-up of people with difficult to manage allergic disease
Study details
Six studies32,33,37–40 assessed the effects of adding multiplex allergen testing to the standard diagnostic work-up (SPT/single IgE) on the management, treatment or diagnosis of patients. One study assessed ImmunoCAP ISAC 112,33 three studies assessed ImmunoCAP ISAC 103,37–39 one study assessed ImmunoCAP ISAC 9632 and the remaining study used an unspecified version of ImmunoCAP ISAC. 40 All six studies32,33,37–40 used a ‘diagnostic before-and-after’ type study design to assess the effects of adding ImmunoCAP ISAC results to the information available to clinicians on their judgements regarding the management, treatment or diagnosis of a given group of patients.
Change to management or treatment
Two studies investigated the use of ImmunoCAP ISAC to guide decisions on the discontinuation of restrictive diets in children with food allergies. 33,40 Both studies were reported as conference abstracts only and hence provided only limited study details and results. Hermansson et al. 33 used a database to identify 199 school children in Härkätie, Finland, receiving special diets in school catering; (confidential information has been removed) (e-mail from Johannes Savolainen, University of Turku, Finland, to Shona Lang, 23 June 2015, personal communication). (Confidential information has been removed) (Johannes Savolainen, personal communication). The Hermansson study33 did not report any information on clinical outcomes following changes to dietary management. Noimark and Harnik40 investigated 12 children selected from patients attending an East London allergy clinic (no details of the selection criteria were reported). Participants were investigated using SPT and/or single IgE, and an unspecified version of ImmunoCAP ISAC. The authors reported that ISAC enabled potential food reintroductions (peanut n = 4, soy n = 2, wheat n = 4), additional to that indicated by single IgE alone; the numbers of potential reintroductions based on standard diagnostic work-up (SPT and/or single IgE) were not reported. No details were reported of which single IgE/SPTs were conducted or which ISAC components were assessed. Noimark and Harnik40 did not report the number of food reintroductions that occurred following testing or clinical outcomes of any changes to dietary management.
Two studies37,38 assessed the views of clinicians on whether or not ImmunoCAP ISAC testing provided information useful in the management of patients. Luengo et al. 37 performed ImmunoCAP ISAC 103 testing in 55 well-characterised, polysensitised patients (as assessed by SPTs and single IgE tests) with various allergies; no details were reported of which ISAC components were assessed or how these were interpreted. Participating clinicians judged that ImmunoCAP ISAC 103 provided new information useful in the management of the patient in 50 (91%) cases. 37 The added value was in the ability of ImmunoCAP ISAC to differentiate between protein homologues and hence to aid in the discrimination of allergens that were cross-immunoreactive rather than those that were responsible for sensitisation. In 34 (62%) cases the clinicians considered that it would have been useful to perform ImmunoCAP ISAC 103 testing before SPT, as several protein homologues can be investigated at once using ImmunoCAP ISAC. 37 Passalacqua et al. 38 investigated 318 consecutive polysensitised (at least two positive SPTs) patients with respiratory allergy in six allergy units in Italy. Participants were initially investigated using clinical history, SPT and single IgE testing (including mites, grass, olive, Parietaria, birch, cypress, ragweed, mugwort, cat and dog dander, Alternaria and Aspergillus) and were assessed using ImmunoCAP ISAC 103 (no details reported of components assessed or interpretation, but cross-immunoreactive allergens were considered); treating clinicians were required to review their diagnosis/treatment based on the ImmunoCAP ISAC 103 results and provide a judgement of the value of any additional information provided. 38 New information was classified as ‘remarkable’ if it could not be obtained using standard diagnostic work-up and could impact upon accuracy of diagnosis or SIT prescription; new information related to patient management was classified as ‘remarkable’ in 299 (95%) cases and ‘to some extent’ (not defined) in 232 (73%) cases. 38
Two studies32,38 investigated the effect on SIT prescriptions of adding ImmunoCAP ISAC testing to the standard diagnostic work-up of people with respiratory allergy. Passalacqua et al. 38 (described above) reported that a SIT prescription was made for 85 new patients, following testing with ImmunoCAP ISAC 103, who would not have received SIT based on standard diagnostic work-up (SPT/single IgE) alone. In addition, the existing SIT prescription was changed in a further three patients, following ImmunoCAP ISAC 103 testing. 38 Sastre et al. 32 investigated 141 people with respiratory allergy (with or without concomitant food allergy) in one allergy outpatient clinic in Spain. SIT indications were initially assessed based on clinical history and SPT (Olea e, Platanus a, Cupressus a, grass mix, Cynodon d, Phragmites c, Artemisia v, Salsola k and Plantago I), blind to the results of ImmunoCAP 96 testing (Ole e1, Cup s1, Cry j1, Pla a1, Pla a2, Phl p1, Phl p5, Phl p4, Phl p6, rPhl p11, Phl p12, Cyn d1, Sal k1, Aln g1, Bet v1, Cor a1.0101, Amb a1, Art v1, Art v3 and Par j2). 32 Clinicians then reassessed SIT indications based on all diagnostic information, including ImmunoCAP ISAC 96 results. 32 Disagreements on the SIT prescription based on standard diagnostic work-up and that based on all information, including ImmunoCAP ISAC, occurred for 79 (54%) study participants; details are reported in Table 5. 32 Neither study reported details of which SIT prescriptions were actually used, or any subsequent clinical outcomes.
| Study details | Known/suspected allergy (n) | Multiplex allergen test | Standard care | Outcome measure | Component | No. with outcome based on standard care | No. with outcome based on standard care + multiplex allergen test | No. with change in outcome | Additional information |
|---|---|---|---|---|---|---|---|---|---|
| Hermansson 201433 (Johannes Savolainen, personal communication) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Luengo 201037 | Multisensitised allergic patients (55) | ImmunoCAP ISAC 103 | Clinical history SPT and sIgE | Clinicians’ judgement on value of information added by ISAC | New information useful in the management of the patient | NA | 50 | NA | |
| New/more/faster information meaning that it would have been useful to perform ISAC before SPT | NA | 34 | NA | ||||||
| Noimark 201240 | Children with moderate to severe eczema and multiple food allergies (12) | ImmunoCAP ISAC (unspecified version) | SPT and/or specific sIgE | Potential food introduction | Peanut introduction | NR | 4 | NR | The authors concluded that more foods need to be represented on the chip, to allow the introduction of foods which might be avoided in children with multiple food allergies |
| Soy introduction | NR | 2 | NR | ||||||
| Wheat introduction | NR | 4 | NR | ||||||
| Passalacqua 201338 | Polysensitised (at least two positive SPTs) patients with respiratory allergy (318) Healthy controls (91) |
ImmunoCAP ISAC 103 | Clinical history and SPT, followed by specific sIgE assay(s) as required | New prescription of SIT | NR | 32 | 117 | 85 | 31 new prescriptions of a single extract and 54 of two or more extracts |
| Change to prescription of SIT | NR | NA | NA | 3 | Prescriptions were changed in three patients after ISAC | ||||
| Clinicians’ judgement on relevance of information added by ISAC | New information related to management | NA | ‘To some extent’: 232 ‘Remarkable’: 299 |
NA | Clinicians judged that a more confident therapeutic approach was achieved in approximately one-third of cases | ||||
| More confident in management | NA | ‘To some extent’: 232 ‘Remarkable’: 286 |
NA | ||||||
| Sastre 201232 | Patients with allergic rhinoconjunctivitis and/or asthma who were sensitised to pollen, with or without concomitant food allergy (141) | ImmunoCAP ISAC 96 | Clinical history, taking into consideration the time of year of respiratory symptoms and European Academy of Allergy and Clinical Immunology guidelines, + SPT | Prescription of SIT (based on agreement of three blinded authors) | Grass | 17 | 10 | 44a | Agreement in SIT indication before and after ImmunoCAP ISAC results occurred in 46% of participants. The authors concluded that this value makes the case for the usefulness of ISAC, at least in areas of complex sensitisation to pollen, to facilitate accurate prescription |
| Olive | 1 | 1 | 9a | ||||||
| Grass + olive | 4 | 1 | 40a | ||||||
| Grass + cypress | 0 | 1 | 9a | ||||||
| Grass + plane | 0 | 1 | 8a | ||||||
| Olive + cypress | 0 | 2 | 0a | ||||||
| Other extracts | 3 | 4 | 12a | ||||||
| Total | 25 | 20 | 79a |
Change to diagnostic classification
Two studies investigated the effect on diagnostic classification of adding ImmunoCAP ISAC 103 testing to the standard diagnostic work-up of people with allergic disease. 38,39 Heaps et al. 39 investigated 110 patients, from five UK specialist allergy centres, who had a diagnosis of idiopathic anaphylaxis [based on clinical assessment, SPT, single IgE testing and mast cell tryptase (MCT)]. Study participants were reassessed using ImmunoCAP ISAC 103 and clinicians were asked to score the additional information provided. Information provided by ImmunoCAP ISAC 103 was given the highest score (new heat- and digestion-stable sensitisations found, which were thought to have a strong association with anaphylaxis) for 22 (20%) of participants; however, large numbers of sensitisations that were not thought to be associated with anaphylaxis were also identified (see Table 6 for full details). 39 In addition, for a further 35 (32%) of participants the information provided by ImmunoCAP ISAC was deemed to have identified only additional sensitisations which were not thought to be associated with anaphylaxis. 39 Passalacqua et al. 38 (described above; see Change to management or treatment) reported clinicians’ ratings of the value of additional diagnostic information provided by ImmunoCAP ISAC 103 (see Table 6). In addition, this study38 reported detailed information on changes to diagnostic category using five classifications (see Table 6); the addition of ImmunoCAP ISAC 103 testing resulted in increases in the numbers of people classified as ‘poly sensitised with suspected cross-reactivity’ and the number of people diagnosed with both inhalant and food allergies, as well as facilitating a diagnosis for eight previously unclassifiable patients. 38 Full details are provided in Table 6.
| Study details | Known/suspected allergy (n) | Multiplex allergen test | Standard care | Outcome measure | Component | No. with outcome based on standard care | No. with outcome based on standard care + multiplex allergen test | No. with change in outcome | Additional information |
|---|---|---|---|---|---|---|---|---|---|
| Heaps 201439 | Idiopathic anaphylaxis (110) | ImmunoCAP ISAC 103 | Clinical history, SPT, specific sIgE, MCT | Clinicians’ judgement on relevance of information added by ISAC | ISAC score I: no new sensitisation found | NA | 53 | NA | 39 patients had blank reactions, 14 patients had positive ISAC to known sensitisations |
| ISAC score II: new allergen sensitisations found, but these were not thought to be associated with the anaphylaxis | NA | 35 | NA | 322 new sensitisations, including: pollens (24 patients); HDM (22 patients); animal danders (15 patients) | |||||
| ISAC score III: new sensitisations (heat and digestion stable) found, which were thought to have a strong association with the anaphylaxis | NA | 22 (11 substantiated on recall by additional clinical information, SPT, self-challenge, controlled clinical challenge, or accidental exposure) | NA | 203 new sensitisations, of which 35 were thought to be highly likely to be responsible for the anaphylaxis, including components of wheat, shrimp, peanut, soy bean, latex, fish, peach, hazelnut, kiwi, egg, cow’s milk and beef meat The authors concluded that, in these patients, the new information could lead to targeted risk reduction and less uncertainty 8 patients had more than one potential anaphylaxis trigger identified |
|||||
| Passalacqua 201338 | Polysensitised (at least two positive SPTs) patients with respiratory allergy (318) Healthy controls (91) |
ImmunoCAP ISAC 103 | Clinical history and SPT, followed by specific sIgE assay(s) as required | Diagnostic category | A: Polysensitised with only one clinically relevant sensitisation | 56 | 33 | A→B: 32 A→C: 4 A→D: 7 A→E: 0 |
There were no patients for whom a diagnosis could not be reached following ISAC |
| B: True polysensitised with more than one clinically relevant sensitisation | 176 | 117 | B→A: 15 B→C: 54 B→D: 35 B→E: 0 |
||||||
| C: Polysensitised with suspected cross-reactivity | 44 | 99 | C→A: 0 C→B: 6 C→D: 6 C→E: 0 |
||||||
| D: Sensitised to inhalants and foods | 34 | 69 | D→A: 2 D→B: 5 D→C: 9 D→E: 0 |
||||||
| E: Non-classifiable | 8 | 0 | E→A: 3 E→B: 2 E→C: 0 E→D: 3 |
||||||
| Clinicians’ judgement on relevance of information added by ISAC | New information related to diagnosis | NA | ‘To some extent’: 220 ‘Remarkable’: 87 |
NA | Clinicians judged that a more confident diagnostic approach was achieved in approximately one-third of cases | ||||
| More confident in diagnosis | NA | ‘To some extent’: 227 ‘Remarkable’: 295 |
NA |
Other
We identified one additional study36 that assessed the relationship between change in IgE levels measured by ImmunoCAP single IgE and change in IgE levels measured by an unspecified version of ImmunoCAP ISAC before and after a 3-year course of SIT, and the clinicians’ evaluation of the benefit of SIT. This study36 included only nine participants who received a total of 31 courses of SIT (no details of diagnosis were reported). The median specific IgE levels, measured by ISAC, decreased from 5.6 ISU/ml at the beginning of SIT to 0.01 ISU/ml at the end of SIT, and this change correlated with clinical benefit of SIT (evaluated by clinicians) (Spearman’s r = 0.46; p = 0.02). 36 Conversely, allergen-specific single IgE measurements did not show a decrease from the beginning to the end of SIT. 36
Summary
The results of studies in this section provide some indication that the addition of ImmunoCAP ISAC to standard diagnostic work-up can change clinicians’ views on the diagnosis, management and treatment of patients. There was some indication that the use of ImmunoCAP ISAC testing may guide decisions on the discontinuation of restrictive diets, the content of SIT prescriptions, and whether or not patients should receive SIT. However, importantly, none of the studies that we identified reported any information on clinical outcomes subsequent to changes in treatment or management based on ImmunoCAP ISAC. Three studies report the usefulness of ImmunoCAP ISAC for discriminating allergens that are structurally similar and are recognised by the same IgE antibody (cross-immunoreactive); this discrimination appears to be particularly useful for identifying the cause of food allergies. The UK-based study on the use of ImmunoCAP ISAC to investigate idiopathic anaphylaxis indicated that the addition of ImmunoCAP ISAC to standard diagnostic work-up may identify a potentially causative agent in previously undiagnosed patients. However, it should be noted that the addition of ImmunoCAP ISAC also resulted in the identification of large numbers of sensitisations that were not considered to be associated with the anaphylaxis, that is, large numbers of clinically false-positive test results or sensitisations associated with other allergic conditions such as rhinitis.
Diagnostic accuracy of ImmunoCAP Immuno Solid-phase Allergen Chip, compared with other testing options, for the prediction of allergic response
Study details
Six studies41,42,44,46,47,49 were identified which compared the accuracy of ImmunoCAP ISAC to existing diagnostic tests (SPT or single IgE tests) in people with food allergies; two studies43,45 were identified of people with allergies to aeroallergens. None of the studies looked at ISAC 112, two investigated ISAC 103,42,43 one investigated ISAC 89,44 two investigated ISAC 50/5145,49 and three investigated unknown ISAC versions. 41,46,47 The results of the comparative diagnostic accuracy studies are summarised in Table 7.
| Study ID | No. of participants, suspected allergy | Reference standard | Index test or comparator | Component/allergen (cut-off point) | TP | FN | FP | TN | Sensitivity% (95% CI) | Specificity% (95% CI) | Additional information |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Food | |||||||||||
| Albarini 201347 | 35, hazelnut allergy | DBPCFC | ISAC-NR | Cor.a.1.1010 (NR) | 9 | 7 | 5 | 14 | 56.3 (29.9 to 80.2)a | 73.7 (48.8 to 90.9)a | The authors concluded that the association among symptoms and sIgE profile should be carefully investigated considering the natural history and evident food allergy |
| Cor.a.1.0401 (NR) | 7 | 9 | 3 | 16 | 43.8 (19.8 to 70.1)a | 84.2 (60.4 to 96.6)a | |||||
| Cor.a.8 (NR) | 2 | 14 | 2 | 17 | 12.5 (1.6 to 38.3)a | 89.5 (66.9 to 98.7)a | |||||
| Cor.a.9 (NR) | 1 | 15 | 0 | 19 | 6.3 (0.2 to 30.2)a | 100 (82.4 to 100)a | |||||
| sIgE | Hazelnut (0.35 kUI/l) | 16 | 0 | 15 | 4 | 100 (79.4 to 100)a | 21.1 (6.1 to 45.6)a | ||||
| SPT | NR (> 3 mm diameter) | 16 | 0 | 9 | 10 | 100 (79.4 to 100)a | 52.6 (28.9 to 75.6)a | ||||
| Alessandri 201142 | 68, egg | Double-blind food challenge boiled eggs | ISAC 103 | Gal d1 (0) | NRb | NRb | NRb | NRb | 61 (42.1 to 77.1) | 97 (85.1 to 99.9) | The authors reported that evaluation of 103 allergenic molecules by means of the ISAC microarray approach allowed detection of significant clinical sensitisations to other important allergenic sources, confirming that hen’s egg sensitisation is often associated with other sensitisations to food and inhalants |
| Gal d2 (0.21) | NRb | NRb | NRb | NRb | 40 (22.9 to 57.9) | 94 (80.8 to 99.3) | |||||
| Gal d3 (0.06) | NRb | NRb | NRb | NRb | 18 (7 to 35.5) | 100 (90 to 100) | |||||
| sIgE | Egg white (1.23) | NRb | NRb | NRb | NRb | 79 (61.1 to 91) | 71 (53.7 to 85.4) | ||||
| Egg yolk (0.11) | NRb | NRb | NRb | NRb | 64 (45.1 to 79.6) | 88 (72.5 to 96.7) | |||||
| SPT | Egg white extract (8) | NRb | NRb | NRb | NRb | 84 (67.2 to 94.7) | 77 (59.9 to 89.6) | ||||
| Raw egg white (48) | NRb | NRb | NRb | NRb | 88 (71.8 to 96.6) | 80 (63.1 to 91.6) | |||||
| Boiled egg white (11.6) | NRb | NRb | NRb | NRb | 85 (68.1 to 94.9) | 94 (80.8 to 99.3) | |||||
| Egg yolk extract (0) | NRb | NRb | NRb | NRb | 70 (51.3 to 84.4) | 83 (66.4 to 93.4) | |||||
| Raw egg yolk (8.4) | NRb | NRb | NRb | NRb | 79 (61.1 to 91) | 80 (63.1 to 91.6) | |||||
| Boiled egg yolk (4.3) | NRb | NRb | NRb | NRb | 45 (28.1 to 63.6) | 94 (80.8 to 99.3) | |||||
| Double-blind food challenge raw eggs | ISAC 103 | Gal d1 (0) | NRb | NRb | NRb | NRb | 84 (60.4 to 96.6) | 90 (80.4 to 97.7) | |||
| Gal d2 (0) | NRb | NRb | NRb | NRb | 53 (28.9 to 75.6) | 84 (70.3 to 92.7) | |||||
| Gal d3 (0.41) | NRb | NRb | NRb | NRb | 21 (6.1 to 45.6) | 98 (89.1 to 99.7) | |||||
| sIgE | Egg white (2.25) | NRb | NRb | NRb | NRb | 84 (60.4 to 96.6) | 75 (61.1 to 86.7) | ||||
| Egg yolk (0.11) | NRb | NRb | NRb | NRb | 84 (60.4 to 96.6) | 81 (67.4 to 91.1) | |||||
| SPT | Egg white extract (11) | NRb | NRb | NRb | NRb | 89 (66.9 to 98.7) | 73 (58.9 to 85.1) | ||||
| Raw Egg white (71.2) | NRb | NRb | NRb | NRb | 79 (54.4 to 93.9) | 90 (77.8 to 96.6) | |||||
| Boiled egg white (23.3) | NRb | NRb | NRb | NRb | 95 (74 to 99.9) | 86 (72.8 to 94.1) | |||||
| Egg yolk extract (10.7) | NRb | NRb | NRb | NRb | 58 (33.5 to 79.7) | 86 (72.8 to 94.1) | |||||
| Raw egg yolk (8.4) | NRb | NRb | NRb | NRb | 68 (43.4 to 87.4) | 59 (44.2 to 73) | |||||
| Boiled egg yolk (4) | NRb | NRb | NRb | NRb | 53 (28.9 to 75.6) | 86 (72.8 to 94.1) | |||||
| De Swert 201241 | 15 patients, (with birch pollen allergy), soy | Open challenge with Alpro® (Alpro, Ghent, Belgium) soya natural drink | ISAC – NR | rGly m4 (1 ISU) | 6 | 1 | 1 | 4 | 86 (42 to 100)a | 80 (28 to 100) | Levels reported for nGly m5 and nGly m6, for both sIgE and ISAC but no cut-off values to allow determination of sensitivity and specificity |
| sIgE | rGly m4 (17.6 kU/l) | 6 | 2 | 0 | 7 | 75 (35 to 100)a | 100 (59 to 100) | ||||
| SPT | Soy flour (7 mm) | 6 | 2 | 0 | 6 | 75 (35 to 100)a | 100 (54 to 100) | ||||
| D’Urbano 201044 | 104, cow’s milk 58 or hen’s egg 46 | Open food challenge | ISAC 89 | Bos d8 (> 0.60 ISU) | 25 | 1 | 7 | 25 | 78 (60 to 91) | 96 (80 to 99) | |
| Gal d1 (> 0.86 ISU) | 16 | 1 | 6 | 23 | 73 (50 to 89) | 96 (79 to 99) | |||||
| sIgE | Cow’s milk (≥ 16.6 kU/l) | 13 | 1 | 19 | 25 | 41 (24 to 60) | 96 (80 to 99) | ||||
| Egg white (> 25.3 kU/l) | 6 | 1 | 16 | 23 | 27 (11 to 50) | 96 (79 to 99) | |||||
| Serial testing, where ISAC results are considered only in sIgE-negative participants | Cow’s milk (≥ 16.6 kU/l on sIgE or < 16.6 kU/l on sIgE and (> 0.60 ISU on ISAC) | 27 | 2 | 5 | 24 | 84 (67 to 95) | 92 (75 to 99) | ||||
| Hen’s egg (≥ 25.3 kU/l on sIgE or < 25.3 kU/l on sIgE and (> 0.86 ISU on ISAC) | 16 | 2 | 6 | 22 | 73 (50 to 89) | 92 (73 to 99) | |||||
| Ott 200849 | 130, cow’s milk 85, hen’s egg 60 | Double-blind food challenge, or open food challenge in young infants | ISAC 51 | αα casein (0.1) | 11a | 31a | 1a | 42a | 26.2 (13.9 to 42) | 97.7 (87.7 to 99.6) | The authors concluded that allergen microarrays provide a new tool to diagnose symptomatic cow’s milk and hen’s egg allergy. They show performance characteristics comparable to the current diagnostic tests and may be indicated in small children in whom only small blood volumes are obtainable However, they are not capable of replacing double-blind, placebo-controlled food challenges in most cases |
| ββ casein (0.1) | 11a | 31a | 3a | 40a | 26.2 (13.9 to 42) | 93 (89.9 to 98.5) | |||||
| κκ casein (0.2) | 16a | 26a | 6a | 38a | 38.1 (23.6 to 54.4) | 88.4 (74.9 to 96.1) | |||||
| Bos d4 (0.1) | 21a | 21a | 3a | 40a | 50 (34.2 to 65.8) | 93 (80.9 to 98.5) | |||||
| Bos d5 (0.1) | 10a | 32a | 2a | 41a | 23.9 (12.1 to 39.5) | 95.3 (84.2 to 99.3) | |||||
| Gal d1 (> 0) | 26a | 21a | 2a | 13a | 57.8 (42.2 to 72.3) | 86.7 (59.5 to 98) | |||||
| Gal d2 (> 0) | 26a | 21a | 3a | 12a | 57.8 (42.2 to 72.3) | 80 (51.9 to 95.4) | |||||
| Gal d4 (> 0) | 8a | 37a | 0a | 15a | 17.8 (8 to 32.1) | 100 (100) | |||||
| sIgE | Cow’s milk extract (8.1) | 22a | 20a | 8a | 35a | 51.2 (35.5 to 66.7) | 81.4 (66.6 to 91.6) | ||||
| Hen’s egg extract (2.9) | 32a | 13a | 2a | 13a | 71.1 (55.7 to 83.6) | 86.7 (59.5 to 98) | |||||
| SPT | Native cow’s milk (3) | 39a | 3a | 22a | 21a | 93.6 (78.5 to 99) | 48.2 (28.7 to 68) | ||||
| Native hen’s egg (9) | 27a | 18a | 0a | 15a | 60.7 (54.6 to 78.5) | 100 (71.3 to 100) | |||||
| Sokolova 200946 | 37 (CMPA), 4 controls (no history of allergy and drank milk daily) | Oral challenge test | ISAC – NR | At least one cow’s milk allergen component positive: α-lactalbumin (Bos d4), bovine serum albumin (Bos d6), IgG heavy chain (Bos d7), casein (Bos d8) and its fractions (α-S1, β and κ), lactoferrin (Bos d lactoferrin) and β-lactoglobulin (Bos d5.0101 (NR) | 17 | 0 | 2 | 22 | 100a (80.5 to 100) | 91.7a (73 to 99) | The authors concluded that the characterisation of patient sensitisation profiles before and after acquisition of tolerance to cow’s milk protein may contribute to the identification of possible indicators of prognosis of this food allergy |
| sIgE | At least one cow’s milk allergen component positive: whole milk, α-lactoalbumin, β-lactoglobulin and casein (NR) | 17 | 0 | 15 | 9 | 100a (80.5 to 100) | 37.5a (18.8 to 59.4) | ||||
| Aeroallergens | |||||||||||
| Cabrera-Freitag 201143 | 173 patients, (43 grass pollen plus 26 controls and 12 cypress pollen plus 92 controls) | Clinical history and SPT | ISAC – 103 | At least one grass pollen component positive: rPhl p1, rPhl p2, nPhl p4, rPhl p5, rPhl p6, rPhl p7, rPhl p11, rPhl p12 (0.3) | 42a | 1a | 2a | 24a | 97.7 (87.7 to 99.9) | 92.3 (74.9 to 99.0) | |
| At least one grass pollen component positive: rPhl p1, rPhl p2, nPhl p4, rPhl p5, rPhl p6, rPhl p7, rPhl p11, rPhl p12 (0.4) | 41a | 2a | 1a | 25a | 95.3 (84.2 to 99.4) | 96.1 (80.4 to 99.9) | |||||
| Cypress pollen, nCup a1 (0.3) | 11a | 1a | 8a | 84a | 91.7 (61.5 to 99.8) | 91.3 (85.5 to 97.1) | |||||
| Cypress pollen nCup a1 (0.82) | 11a | 1a | 4a | 88a | 91.7 (61.5 to 99.8) | 95.6 (91.5 to 99.8) | |||||
| sIgE | Phleum pratense (0.35) | 41a | 2a | 1a | 25a | 95.3 (84.2 to 99.4) | 96.1 (80.4 to 99.9) | ||||
| P. pratense (0.33) | 41a | 2a | 1a | 25a | 95.3 (84.2 to 99.3) | 96.1 (80.3 to 99.4) | |||||
| Cupressus arizonica (0.35) | 11a | 1a | 18a | 74a | 91.7 (61.5 to 99.8) | 80.4 (72.3 to 88.5) | |||||
| C. arizonica (0.66) | 11a | 1a | 10a | 82a | 91.7 (61.5 to 98.6) | 89.1 (80.9 to 94.7) | |||||
| Wöhrl 200645 | 120 patients with symptoms of allergic rhinitis | Clinical history and SPT | ISAC 50 | At least one HDM allergen component positive: Der p1 and Der p2 (NR) | 18a | 8a | 11a | 85a | 69.2 (48.2 to 86.6) | 90.4 (82.6 to 95.5) | |
| Cat: Fel d1 (NR) | 18a | 5a | 9a | 88a | 78.3 (56.3 to 92.5) | 90.7 (83.1 to 95.7) | |||||
| At least one birch pollen component positive: rBet v1a, rBet v1b, and rBet v2 (NR) | 27a | 4a | 9a | 80a | 87.1 (70.1 to 96.3) | 89.9 (81.7 to 95.3 | |||||
| At least one grass pollen component positive: rPhl p1, 2, 5, 6, 7 (NR) | 42a | 5a | 7a | 66a | 89.4 (76.9 to 96.4) | 90.4 (81.2 to 96.0 | |||||
| Mugwort pollen: rArt v1 (> 0 kUA/l) | 8a | 9a | 2a | 101a | 47.1 (23.0 to 72.1) | 98.1 (93.1 to 99.7) | |||||
| sIgE | HDM: whole allergen extract (NR) | 23a | 3a | 9a | 85a | 88.5 (69.8 to 97.4 | 90.4 (82.6 to 95.5) | ||||
| Cat: whole allergen extract (NR) | 20a | 3a | 6a | 88a | 87.0 (66.4 to 97.1) | 90.7 (83.1 to 95.7) | |||||
| Birch pollen: whole allergen extract (NR) | 24a | 7a | 10a | 79a | 77.4 (58.9 to 90.4) | 88.8 (80.3 to 94.5) | |||||
| Grass pollen: whole allergen extract (NR) | 41a | 6a | 7a | 66a | 87.2 (74.2 to 95.1) | 90.4 (81.2 to 96.0) | |||||
| Mugwort pollen: whole allergen extract (NR) | 15a | 2a | 11a | 92a | 88.2 (63.5 to 98.2) | 89.3 (81.7 to 94.5) | |||||
Diagnosis of food allergy
De Swert et al. 41 investigated soy flour allergy. The diagnostic accuracy of an unknown ISAC version to measure the soy flour component rGly m4 was compared with the single IgE test for the same component and to a SPT for soy flour. Cut-off values were reported separately for each test and OFC testing was used as the reference standard. ISAC had the highest sensitivity, 86% (95% CI 42% to 100%), but the lowest specificity, 80% (95% CI 28% to 100%). The single IgE test and SPT had similar sensitivity (75%) and specificity (100%).
Alessandri et al. 42 investigated allergy to boiled or raw egg. The diagnostic accuracy of ISAC 103, when used to measure three individual egg components (Gal d1 or Gal d2 or Gal d3), was compared with the accuracy of single IgE tests (egg yolk or egg white) and compared with the accuracy of SPTs (egg white extract or raw egg white or boiled egg white or egg yolk extract or raw egg yolk or boiled egg yolk). Cut-off values were reported separately for each test and OFC testing was used as the reference standard. SPT had the highest sensitivity for prediction of allergic response to raw egg white, 88% (95% CI 71.8% to 96.6%), whereas Gal d3 measured using ISAC 103 had the highest specificity, 100% (95% CI 90% to 100%). Results for raw egg were similar to those for boiled egg. In general, single IgE performed similarly to SPT (both measured whole extracts), whereas ISAC 103 gave much more variable results for the three different components measured. No measure of the overall diagnostic performance of ISAC 103 (all components combined) was reported.
Two studies44,49 investigated allergy to cow’s milk and hen’s egg. D’Urbano et al. 44 compared the accuracy of ISAC 89, used to measure two individual components (Gal d1 or Bos d8), to the accuracy of single IgE tests (egg white or cow’s milk). Cut-off values were reported separately for each test and OFC testing was used as the reference standard. Specificity was consistent (96%) for both ISAC 89 components and for cow’s milk and egg white single IgE. Sensitivity values were higher for ISAC 89 components (78% for Bos d8 and 73% for Gal d1) than for the corresponding whole-allergen single IgE tests (41% for cow’s milk and 27% for egg white). When whole-allergen single IgE tests and ISAC 89 were used in series (i.e. ISAC 89 results were considered only in single IgE-negative participants), the combined sensitivity was greater than that for single IgE alone (84% compared with 41% for cow’s milk allergy, and 73% compared with 27% for hen’s egg allergy); specificity was 92% in both cases. Ott et al. 49 compared the accuracy of ISAC 51, used to measure eight individual components (α-casein, β-casein, κ-casein, Bos d4, Bos d5, Gal d1, Gal d2, Gal d4), with the accuracy of single IgE tests (hen’s egg or cow’s milk extract) and to the accuracy of SPTs (native hen’s egg or native cow’s milk). Cut-off values were reported separately for each test and OFC testing was used as the reference standard. The results were very variable between tests. SPT had the highest sensitivity for cow’s milk allergy, 93.6% (95% CI 78.5% to 99%). The ISAC 51 components all had low sensitivity for cow’s milk allergy (ranging from 23.9% to 50% for the five components assessed). Conversely, all five ISAC 51 components had high specificity for cow’s milk allergy (ranging from 88.4% to 97.7%), whereas SPT had low specificity, 48.2% (95% CI 28.7% to 68%). Single IgE testing had the highest sensitivity for hen’s egg allergy, 71.1% (95% CI 55.7% to 83.6%). All three ISAC 51 components had low sensitivity (ranging from 17.8% to 57.8%) and high specificity for hen’s egg allergy; the individual specificities of the ISAC 51 components were 100% for Gal d4, 86.7% for Gal d1 and 80% for Gal d2. Single IgE testing and skin prick testing had comparable specificity (86.7% and 100%, respectively). No measure of the overall diagnostic performance of ISAC 51 (all relevant components combined) was reported for either cow’s milk or hen’s egg allergy.
Sokolova et al. 46 investigated milk allergy. The diagnostic accuracy of an unknown ISAC version, used to measure nine individual components (Bos d 4, Bos d 6, Bos d 7, Bos d 8, casein α-S1, casein β and casein κ, Bos d lactoferrin, Bos d 5.0101), was compared with the accuracy of single IgE tests for four allergens (whole milk, α-lactoalbumin, ββ-lactoglobulin and casein). For both methods, a positive result was defined as positive for at least one component or whole allergen; the cut-off values used to define positivity for individual components and allergens were not reported. OFC testing was used as the reference standard. Both combined ISAC testing and combined single IgE testing had 100% sensitivity; however, ISAC testing had much higher specificity, 91.7% (95% CI 73% to 99%), than the single IgE testing, 37.5% (95% CI 18.8% to 59.4%).
Albarini et al. 47 investigated hazelnut allergy. The diagnostic accuracy of an unknown ISAC version, used to measure four individual components (Cor.a.1.1010, Cor.a.1.0401, Cor.a.8, Cor.a.9), was compared to the accuracy of single IgE tests (hazelnut) and to SPT. Cut-off values were not reported for the ISAC test. OFC testing was used as the reference standard. Both the SPT and the single IgE test had 100% sensitivity, whereas the ISAC components generally had low sensitivity (ranging from 6.3% to 56.3%). However, the ISAC components had higher specificity (ranging from 73.7% to 100%) than either single IgE (21.1%) or skin prick testing (52.6%).
Diagnosis of aeroallergy
Wöhrl 200645 investigated five different aeroallergens (HDM, cat dander, birch pollen, grass pollen and mugwort pollen). The diagnostic accuracy of ISAC 50, used to measure the presence of one or more aeroallergens (up to five), was compared with the accuracy of single IgE tests of whole allergens. Where multiple ISAC components were assessed, a positive result was defined as positive for at least one component. The cut-off points for each test were not reported. Skin prick testing was used as the reference standard. The specificity of ISAC 50 was high for all aeroallergens investigated, regardless of whether a single component or multiple components were assessed (range 89.9–98.1%), and, with the exception of mugwort pollen, was comparable to the specificity estimate for the corresponding whole allergen single IgE test for all aeroallergens investigated (see Table 7). The sensitivity of ISAC 50 was lower than that of single IgE tests for HDM, cat and mugwort pollen. The sensitivities and specificities of the individual components ISAC 50 components were not reported.
Cabrera-Freitag et al. 43 investigated two different pollens (grass pollen or Phleum pratense and cypress pollen or C. arizonica). Two cut-off points (manufacturers’ recommended and ROC optimised) were reported per test and SPT was used as the reference standard. The diagnostic accuracy of ISAC 103, when used to measure the eight components for grass pollen (rPhl p1, rPhl p2, nPhl p4, rPhl p5, rPhl p6, rPhl p7, rPhl p11, rPhl p12), was compared with the accuracy of a single IgE test to measure P. pratense; a positive result was defined as positive for at least one component. The sensitivity and specificity for ISAC 103 and single IgE were similar, irrespective of the cut-off point used. Sensitivity and specificity estimates for individual grass pollen ISAC 103 components were not reported. In addition, the accuracy of ISAC 103 was used to measure the presence of a one component for cypress pollen (nCup a1) in comparison with the accuracy of single IgE tests to measure C. arizonica. The sensitivity estimates for the two tests were equal at both cut-off points (91.7%); however, specificity was higher for ISAC 103 at both cut-off points (91.3% and 95.6%) than for the single IgE test (range 80.4–89.1%).
Summary
The diagnostic performance of ImmunoCAP ISAC in comparison with other tests (single IgE and SPT) varied considerably between studies, according to the allergens investigated and the way in which ISAC testing was applied. In general, individual ISAC components tended to have high specificity, but low sensitivity, relative to whole-allergen single IgE tests or SPT for the prediction of allergic response. The relatively low sensitivities of individual ISAC components are likely to be indicative of the proportions of patients in whom each component is associated with the observed allergic response. Conversely, a high specificity is indicative of a strong association between ISAC positivity for the individual component and an allergic response to whole allergen. However, when ISAC was used to measure the same component as single IgE testing or to measure multiple components (homologous proteins), with a positive test defined as any component positive, it appeared that equivalent sensitivities could be achieved without corresponding loss of specificity. The ability of ImmunoCAP ISAC to discriminate between allergens that are structurally similar and are recognised by the same IgE antibody (cross-immunoreactive) may represent clinically useful additional information (see Effects on management, treatment and diagnostic classification of adding multiplex allergen testing to the diagnostic work-up of people with difficult to manage allergic disease, above). Therefore, if the focused use of groups of ISAC components can achieve equivalent sensitivity and specificity to that of single IgE testing, ISAC testing may be preferred.
The results of the only study to investigate serial testing suggested that use of ImmunoCAP ISAC after single IgE testing only in participants who were negative on single IgE could increase sensitivity relative to single IgE alone without any loss in specificity. None of the comparative diagnostic accuracy studies included in this review was conducted in people with difficult to manage allergic disease, and all studies investigated the diagnostic performance of a limited range of ISAC components of a specified allergen. These studies are therefore unable to provide any information on the specificity of the whole ISAC panel when used to investigate people with difficult to manage allergic disease, that is, the extent to which the multiplex allergen testing may produce ‘false-positive’ results by detecting sensitisations that are not clinically relevant. This indicates the importance of using confirmatory tests after the array and that the current array cannot wholly replace OFC or SPT as a diagnostic procedure.
Chapter 4 Assessment of cost-effectiveness
This chapter explores the cost-effectiveness of multiplex allergen testing compared with current clinical assessment in patients referred for specialist allergy investigation in secondary or tertiary care settings. More specifically, the following research question will be addressed:
-
What is the cost-effectiveness of adding multiplex allergen testing to the investigation of people with difficult to manage IgE-mediated allergic disease in secondary or tertiary care settings?
Review of economic analyses of multiplex allergen testing
Search strategy
Searches were undertaken to locate relevant economic evaluations on adults and children undergoing specialist allergy investigation in secondary or tertiary care settings.
The following databases were searched for relevant studies from 2005 to May 2015:
-
NHS Economic Evaluation Database (NHS EED) (via Wiley): 2005–Issue 2 of 2015/May/Iss2
-
IDEAS via Research Papers in Economics (REPEC) (internet; http://repec.org/): 2005–26 May 2015
-
EconLIT (via EBSCOhost): 2005–21 May 2015
-
EMBASE (via OvidSP): 1974–21 May 2015
-
MEDLINE (via OvidSP): 1946–May Week 3 2015
-
MEDLINE In-Process and Daily Update (via OvidSP): up to 20 May 2015.
Inclusion criteria
Studies reporting outcomes of a full cost-effectiveness analysis, with (at least) one of the comparators including multiplex allergen testing, were eligible for inclusion.
Quality assessment
Included studies are appraised using a quality checklist based on that of Drummond et al. 53
Results
The literature search identified 311 records from bibliographic database searches and supplementary searching (e.g. reference/citation checking, additional database searches including the database search for the assessment of clinical effectiveness). After removing duplicates, and title and abstract screening, 15 records were considered to be potentially relevant; after full-text screening four studies (nine publications, all abstracts) were considered eligible for inclusion (Figure 4). All four included studies are authored by Hermansson (as either first or second author), an employee of Thermo Fisher Scientific. Three studies, reported in six publications,33,34,54–57 considered multiplex allergen testing for children with suspected food allergy (specifically peanut allergy in two studies) and one study, reported in three publications,58–60 considered multiplex allergen testing for patients sensitised to pollen. These studies are described in more detail below and summarised in Table 8. The results of the quality assessment are shown in Table 9.
FIGURE 4.
Flow chart (review of economic analyses). a, Reasons for exclusion: did not report outcomes of a full cost-effectiveness analysis (n = 6).

| Hermansson 201433,34 | Hermansson 2013,54 201255,56 | Glaumann 201357 | Mascialino 2013,58,59 Hermansson 201260 | |
|---|---|---|---|---|
| Population | Finnish suspected food-allergic school children | Children with suspected peanut allergy | Children with suspected peanut allergy | Spanish patients with allergic rhinoconjunctivitis and/or asthma sensitised to pollen from a complex pollen area |
| Setting | Primary care | Primary care | Primary care | NR |
| Time horizon | NR | 5 years | 5 years | 9 years |
| Objective | To evaluate the health-economic benefit of ImmunoCAP ISAC | To demonstrate that MA for peanut allergy at the general practitioner level could increase QALYs and have a considerable economic impact | To compare different diagnostic methods: MA, SPT, OFC and DBPCFC for children with a suspected peanut allergy and to evaluate the patients’ QoL and the economic impact for the health-care system in Sweden | To analyse the cost-effectiveness of MA for SIT indication and QoL |
| Source of effectiveness information | Database from Primary Care Unit (n = 24 children agreed to participate) | Literature | Literature | Database of 141 patients with allergic rhinoconjunctivitis and/or asthma sensitised to pollen from a complex pollen area32 |
| Comparators | Traditional diagnostic algorithm with and without ImmunoCAP ISAC added | Different diagnostic approaches including DBPCFC, SPT and/or MA | Different diagnostic approaches including DBPCFC, OC, SPT and/or MA | SPT and MA vs. SPT |
| Costs items | NR | General practitioner visit, specialist visitor, tests, allergic reaction, allergy treatment (including EpiPen and antihistamine), indirect costs (for sensitivity analysis) | Doctor visits, tests, allergic reaction, allergy treatment (including EpiPen and antihistamine), indirect costs (for sensitivity analysis) | General practitioner visit, nurse visit, specialist visitor, emergency visit, tests, SIT, symptomatic treatment, indirect costs |
| Main measure of benefit | Unnecessary diets | QALYs | QALYs | QALYs |
| Assumptions | NR | NR | NR | Patients would get 6 years of ‘sustained effect’ (i.e. remain healthy) after 3 years of SIT |
| Perspective | NR | Health care | Health care | NR |
| Discount rate | NR | NR | NR | NR |
| Uncertainty around cost-effectiveness ratio expressed | No | No | No | No |
| Sensitivity analysis | No | No | No | No |
| Monetary outcomes | € | SEK (Sweden), USD (USA), RMB (China) | SEK (Sweden) | € |
| Outcomes per comparator | QALYs: NR Other outcomes: adding ImmunoCAP ISAC could identify 63% of the patients as having an unnecessary diet (this was 70% on the posters) Costs: NR |
MA vs. DBPCFC vs. SPT: QALYs: 3.97 vs. 2.54 vs. 3.86 Costs:
|
MA vs. DBPCFC vs. OC vs. SPT: QALYs: 4.34 vs. 3.22 vs. 2.23 vs. 3.66 Costs: 11,267 SEK vs. 24,278 SEK vs. 33,031 SEK vs. 44,851 SEK The results presented on the poster differed but the order of the cost and effects of the comparators remained the same |
MA reduces SIT by at least 20% SPT and MA vs. SPT: QALY: 7.03 vs. 6.88 Costs: €2538 vs. €2608 The costs for SPT and MA presented on the poster were slightly higher (€2583) Moreover, the results presented on the presentation slides differed but the order of the cost and effects of the comparators remained the same |
| Summary of incremental analysis | Adding ImmunoCAP ISAC resulted in a cost per avoided unnecessary diet of €480 (€15 was reported on the poster) | MA is both more effective and less expensive than alternative diagnostic strategies | MA is both more effective and less expensive than alternative diagnostic strategies | SPT and MA combined is both more effective and less expensive than SPT only |
| Hermansson 201433,34 | Hermansson 2013,54 201255,56 | Glaumann 201357 | Mascialino 2013,58,59 Hermansson 201260 | |
|---|---|---|---|---|
| Study design | ||||
| The research question is stated | ✗ | ✗ | ✗ | ✗ |
| The economic importance of the research question is stated | ✗ | ✗ | ✗ | ✗ |
| The viewpoint(s) of the analysis are clearly stated and justified | ✗ | ✗ | ✗ | ✗ |
| The rationale for choosing alternative programmes or interventions compared is stated | ✗ | ✗ | ✗ | ✗ |
| The alternatives being compared are clearly described | ✗ | ✗ | ✗ | ✗ |
| The form of economic evaluation used is stated | ✗ | ✓ | ✓ | ✓ |
| The choice of form of economic evaluation is justified in relation to the questions addressed | ✗ | ✗ | ✗ | ✗ |
| Data collection | ||||
| The source(s) of effectiveness estimates used are stated | ✓ | ✗ | ✗ | ✓ |
| Details of the design and results of effectiveness study are given (if based on a single study) | ✗ | ✗ | ✗ | ✗ |
| Details of the methods of synthesis or meta-analysis of estimates are given (if based on a synthesis of a number of effectiveness studies) | NAa | ✗ | ✗ | NAa |
| The primary outcome measure(s) for the economic evaluation are clearly stated | ✗ | ✗ | ✗ | ✗ |
| Methods to value benefits are stated | NA | ✗ | ✗ | ✗ |
| Details of the subjects from whom valuations were obtained were given | NA | ✗ | ✗ | ✗ |
| Productivity changes (if included) are reported separately | ✗ | ✗ | ✗ | ✗ |
| The relevance of productivity changes to the study question is discussed | ✗ | ✗ | ✗ | ✗ |
| Quantities of resource use are reported separately from their unit costs | ✗ | ✗ | ✗ | ✗ |
| Methods for the estimation of quantities and unit costs are described | ✗ | ✗ | ✗ | ✗ |
| Currency and price data are recorded | ✗ | ✗ | ✗ | ✗ |
| Details of currency of price adjustments for inflation or currency conversion are given | ✗ | ✗ | ✗ | ✗ |
| Details of any model used are given | NAa | ✗ | ✗ | ✗ |
| The choice of model used and the key parameters on which it is based are justified | NAa | ✗ | ✗ | ✗ |
| Analysis and interpretation of results | ||||
| Time horizon of costs and benefits is stated | NAa | ✓ | ✓ | ✓ |
| The discount rate(s) is stated | ✗ | ✗ | ✗ | ✗ |
| The choice of discount rate(s) is justified | ✗ | ✗ | ✗ | ✗ |
| An explanation is given if costs and benefits are not discounted | ✗ | ✗ | ✗ | ✗ |
| Details of statistical tests and CIs are given for stochastic data | NA | NA | NA | NA |
| The approach to sensitivity analysis is given | NA | NA | NA | NA |
| The choice of variables for sensitivity analysis is justified | NA | NA | NA | NA |
| The ranges over which the variables are varied are justified | NA | NA | NA | NA |
| Relevant alternatives are compared | ✓ | ✓ | ✓ | ✓ |
| Incremental analysis is reported | ✓ | ✗ | ✗ | ✗ |
| Major outcomes are presented in a disaggregated as well as aggregated form | ✗ | ✗ | ✗ | ✗ |
| The answer to the study question is given | ✗ | ✗ | ✗ | ✗ |
| Conclusions follow from the data reported | ✗b | ✓ | ✓ | ✓ |
| Conclusions are accompanied by the appropriate caveats | ✗ | ✗ | ✗ | ✗ |
Hermansson 2014
Hermansson et al. 33,34 considered the cost-effectiveness of ImmunoCAP ISAC in addition to a standard diagnostic work-up compared with standard diagnostic work-up without ImmunoCAP ISAC for Finnish school children with a restricted diet as a result of suspected food allergy (community setting). The analysis was informed by 24 children from a larger database (including a total of 2317 school children). The results indicated an unnecessary restricted diet for 63% of the children, resulting in a cost per avoided unnecessary diet of €480 for ImmunoCAP ISAC.
Hermansson 2013 and Hermansson 2012
Another study by Hermansson et al. 54–56 examined the cost-effectiveness of ImmunoCAP ISAC compared with double-blind placebo-controlled food challenge (DBPCFC) and skin prick testing for children with suspected peanut allergy. For this purpose, a Markov model was constructed with a 5-year time horizon. Health states included non-allergic and allergic, and mild and severe allergic reactions were modelled as events. The costs were considered for Sweden, the USA and China. The results indicated that ImmunoCAP ISAC is least expensive, whereas SPT is most expensive, in all three countries. Moreover, ImmunoCAP ISAC was also found to be most effective, leading to 3.97 quality-adjusted life-years (QALYs), whereas the DBPCFC strategy is least effective (2.54 QALYs). Consequently, ImmunoCAP ISAC dominated both the SPT and DBPCFC strategies.
Glaumann 2013
Glaumann et al. 57 examined the cost-effectiveness of ImmunoCAP ISAC compared with DBPCFC, open OFC and SPT for children with suspected peanut allergy in Sweden. A Markov model with a 5-year time horizon was constructed for this purpose. Health states included non-allergic and allergic, and mild and severe allergic reactions were modelled as events. The results indicated that ImmunoCAP ISAC is least expensive, whereas SPT is most expensive. Furthermore, ImmunoCAP ISAC was also found to be most effective, leading to 4.34 QALYs, whereas the OFC strategy was considered least effective (2.23 QALYs). Consequently, ImmunoCAP ISAC dominated all three alternative strategies.
Mascialino 2013 and Hermansson 2012
Mascialino et al. 58,59 and Hermansson et al. 60 examined the cost-effectiveness of ImmunoCAP ISAC with SPT compared with SPT only for Spanish patients sensitised to pollen in a complex pollen area. The analysis was based on a Markov model with a 9-year time horizon and the assumption that patients on specific immunotherapy (SIT) continue this treatment for 3 years and remain healthy for the subsequent 6 years or discontinue SIT and move to symptom management treatment until year 9. The analysis was informed by a data set of 141 patients with allergic rhinoconjunctivitis and/or asthma sensitised to pollen. 32 The results indicated that the addition of ImmunoCAP ISAC to SPT reduces SIT prescriptions and hence results in cost savings compared with SPT only (€2538 vs. €2608). ImmunoCAP ISAC with SPT was also found to be more effective (7.03 QALYs) than SPT only (6.88 QALYs); hence ImmunoCAP ISAC with SPT dominated SPT only.
Quality assessment and summary of studies in the cost-effectiveness review
All four studies33,34,54–60 reported benefits associated with adding ImmunoCAP ISAC to the diagnostic work-up (increased effectiveness), and three out of four studies also showed cost savings when using ImmunoCAP ISAC. However, as all included studies were reported only as conference abstracts, the methods and assumptions used were largely unclear; this severely hampered the assessment of the validity of the results. It was often unclear precisely which diagnostic strategies were examined. The lack of information about these studies is illustrated in Table 9 (study quality checklist for included papers). Besides this transparency issue, the credibility of the sources used in these studies may be questionable. Fundamental inputs of the model were based on expert opinion, inaccessible references, or no references were provided (information was still lacking after full retrieving of copies of the posters and a presentation supplied by the authors). For example, the numbers of true positives, false positives, false negatives and true negatives for ImmunoCAP ISAC appeared to be based on expert opinion in most cases. 33,34,54–57,59,60 In addition, two assessments from this group54–57 focused on the same population, both using a Markov model with a 5-year time horizon, but the reported QALYs and outcomes differed substantially (see Table 8). In conclusion, the available economic assessments indicate that the addition of ImmunoCAP ISAC will increase effectiveness and can be cost-saving. However, given the lack of detail on how these results were produced and the use of expert opinion for key inputs, these findings should be interpreted with extreme caution.
Overview of potentially relevant excluded studies
In addition to the included studies described above, one potentially relevant study61 that considered the incremental costs of multiplex allergen testing was excluded, as it did not report effectiveness outcomes and, as a result, was not considered to be a full cost-effectiveness analysis. For completeness, the results of this study61 (also reported only as a conference abstract) are summarised below (despite efforts in contacting the authors, the full copy of the poster could not be retrieved).
The study by Rodriguez-Ferran et al. 61 considered the costs of SPT, Phadiatop and ImmunoCAP Rapid for screening respiratory allergy in children in primary care. Their results showed that SPT is least expensive (€10–15), followed by ImmunoCAP® RAPID (Thermo Fisher Scientific/Phadia AB, Uppsala, Sweden; €30) and Phadiatop® (Phadia AB, Uppsala, Sweden; €36–67). The authors stated that they believe SPT is cost-effective.
Review of health-related quality of life studies
Search strategy
Searches were undertaken to locate relevant utility studies on adults and children with allergic conditions.
The following databases were searched for relevant studies from database inception date to July 2015:
-
MEDLINE (via OvidSP): 1946–June Week 3 2015
-
MEDLINE In-Process Citations and Daily Update (via OvidSP): up to 29 June 2015
-
EMBASE (via OvidSP): 1974 to 29 June 2015
-
Patient-Reported Outcome and Quality Of Life Instruments Database (PROQOLID) (internet; www.proqolid.org/): up to 1 July 2015
-
NHS EED (via Wiley): 2005–Issue 2, April 2015
-
Cost-Effectiveness Analysis (CEA) Registry (internet; www.ceareregistry.org): up to 1 July 2015.
Inclusion criteria
Studies were required to include utility values obtained using a preference-based instrument. Also, studies were limited to a population with an allergic condition associated with food or pollen. This limitation was applied in order to be pragmatic and focus on allergies for which there was at least some evidence, albeit limited, from the clinical effectiveness review. Non-English-language studies were excluded.
Results
Searches identified 1300 (1074 after removing duplicates) potentially relevant publications, of which 1028 were excluded at the abstract screening stage. Full texts were obtained for the 46 publications that were potentially relevant. Thirty-one publications were excluded at the full-text screening stage because no utility values were reported (n = 29) or the study was not written in English (n = 2). Four of the excluded publications were reviews. The studies included in these reviews were all present in the search results. Three additional publications were identified through reference checking of the included studies. The four studies33,34,54–59 identified in the review of economic analyses of multiplex allergen testing were also identified in this review, but excluded because no original utility data were provided. Seventeen publications were included,62–77 describing 14 studies (Figure 5).
FIGURE 5.
Flow chart (review of HRQoL studies). a, Reasons for exclusion: no utility values (n = 30), non-English (n = 2).
Fourteen studies reporting health-state utilities for allergic conditions were found. Ten studies, reported in 13 publications, used the EuroQol instrument, and reported either the European Quality of Life-5 Dimensions (EQ-5D) utility score62–67 or the visual analogue scale (VAS) score. 68–73 One study reported utilities obtained by the HUI Mark III instrument. 74 Three studies used a direct utility elicitation technique. 75–77 The 10 studies reported on 28 populations: 14 studies with rhinitis/rhinosinusitis/rhinoconjunctivitis/asthma,62–66,68–73,78 11 studies with eczema,67,73,75–77 two studies with food allergy73,74 and one study with mixed allergies except food allergies. 74 Utility values ranged from 0.5000 for patients with allergic rhinitis receiving allergy vaccination72 to 0.970 for persons with mild eczema. 76 Patients who sought help from a specialised allergy clinic (to receive allergy vaccination) and patients currently exposed to allergens seemed to have lower utility scores than the other populations. Only two studies reported on the relationship between the severity of allergic symptoms and utility value75,76 (Table 10).
| Source | Population | N | Country | Instrument | Health-state utility or value | |
|---|---|---|---|---|---|---|
| Mean | SD or 95% CI | |||||
| Remenschneider 201562 | Chronic rhinosinusitis | 242 | USA | EQ-5D | 0.8100 | 0.1300 |
| Pitt 200468 | Seasonal allergic conjunctivitis | 310 | UK | EUROQOL-VAS | 0.8169a | 0.1489 |
| Poole 2014,63 Canonica 2007,64 Bachert 2007,65 Currie 201463 | Seasonal allergic rhinoconjunctivitis | 634 | Europe, including UK | EQ-5D | 0.9380 | 0.9320 to 0.9430 |
| Smith 200569 | Seasonal allergic rhinoconjunctivitis | 200 | Spain | EUROQOL-VAS | 0.8009 | 0.1524 |
| Wasserfallen 199970 | Allergic rhinitis | 21 | USA | EUROQOL-VAS | 0.6250b | 0.1300 |
| Petersen 201366 | Allergic rhinoconjunctivitis or asthma | 248 | Denmark | EQ-5D | 0.7000b | 0.2000 |
| Grass pollen induced | 169 | 0.7000b | 0.1800 | |||
| HDM induced | 25 | 0.6000b | 0.3000 | |||
| Grass and HDM induced | 54 | 0.7200b | 0.1800 | |||
| Egert-Schmidt 2014 71 | Rhinitis, conjunctivitis, asthma | 753 | Germany | EUROQOL-VAS | 0.7000c | NR |
| Petersen 201172 | Allergic rhinitis patients receiving allergy vaccination | 366 | Denmark | EUROQOL-VAS | 0.5000 | 0.2000 |
| Covaciu 201373 | Any allergic disease | 3137 | Sweden | EUROQOL-VAS | 0.9260 | 0.0860 |
| Asthma | 3220 | 0.8990 | 0.0100 | |||
| Rhinitis | 3214 | 0.9230 | 0.0860 | |||
| Food hypersensitivity | 3218 | Sweden | EUROQOL-VAS | 0.9220 | 0.0870 | |
| Eczema | 3156 | Sweden | EUROQOL-VAS | 0.9220 | 0.0910 | |
| Mittmann 199974 | Food allergy | 1075 | Canada | HUI Mark III | 0.8500 | 0.1700 |
| Other allergies | 3102 | 0.8800 | 0.1500 | |||
| Moberg 200967 | Hand eczema | 25247 | Sweden | EQ-5D | 0.7820 | 0.7720 to 0.7920 |
| Lundberg 199977 | Atopic eczema | 132 | Sweden | VAS | 0.7300 | NR |
| TTO | 0.9300 | NR | ||||
| SG | 0.9800 | NR | ||||
| Stephens 200575 | Mild atopic eczema in children | 150 | UK | SG | 0.8625 | NR |
| Moderate atopic eczema in children | 0.6900 | NR | ||||
| Severe atopic eczema in children | 0.5900 | NR | ||||
| Friedman 200476 | Mild atopic eczema | 3539 | USA | VAS converted to utilities using 2.4 in the power function | 0.9970 | NR |
| Mild to moderate atopic eczema | 0.9876 | NR | ||||
| Moderate atopic eczema | 0.9571 | NR | ||||
| Moderate to severe atopic eczema | 0.8971 | NR | ||||
| Severe atopic eczema | 0.8052 | NR | ||||
Six studies,63,67–69,73,78 describing 10 populations, compared health-state utility scores for persons with and without allergic conditions. The largest difference was observed for persons with asthma (–0.0530, EQ-5D) and eczema [–0.0660, EuroQol Visual Analogue Scale (Euroqol-VAS)]. For the other populations (food and airway allergies) the differences ranged between –0.0240 and –0.0330 (Table 11).
| Source | Population | N | Country | Instrument | Health-state utility or value with allergy | Health-state utility or value without allergy | Difference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD or 95% CI | Mean | SD or 95% CI | Mean | SD | |||||
| Pickard 201378 | Hay fever | 79 | USA | EQ-5D | NR | NR | NR | NR | –0.0240 | 0.0520 |
| Pitt 200468 | Seasonal allergic conjunctivitis | 310 | UK | EUROQOL-VAS | 0.8169 | 0.1489 | 0.8492 | 0.1254 | –0.0323a,b | NR |
| Poole 201463 | Seasonal allergic rhinoconjunctivitis | 634 | Europe, including UK | EQ-5D | 0.9380 | 0.9320 to 0.9430 | 0.9140 | 0.9070 to 0.9210 | 0.0240b | NR |
| Smith 200569 | Seasonal allergic rhinoconjunctivitis | 200 | Spain | EUROQOL-VAS | 0.8009 | 0.1524 | 0.8334 | 0.1186 | –0.0325b | NR |
| Moberg 200967 | Hand eczema | 25247 | Sweden | EQ-5D | 0.7820 | 0.7720 to 0.7920 | 0.8480 | 0.8450 to 0.8510 | –0.0660b | NR |
| Covaciu 201373 | Allergic diseases | 3137 | Sweden | EUROQOL-VAS | 0.9260 | 0.0860 | 0.9590 | 0.0650 | –0.0330b | NR |
| Asthma | 3220 | 0.8990 | 0.0100 | 0.9520 | 0.0710 | –0.0530b | NR | |||
| Rhinitis | 3214 | 0.9230 | 0.0860 | 0.9520 | 0.0710 | –0.0290b | NR | |||
| Eczema | 3156 | 0.9220 | 0.0910 | 0.9540 | 0.0690 | –0.0320b | NR | |||
| Food hypersensitivity | 3218 | 0.9220 | 0.0870 | 0.9530 | 0.0710 | –0.0310b | NR | |||
Of the excluded studies, three are worth mentioning because they were conducted in UK health-care settings:
-
Armstrong et al. 79 investigated the cost-effectiveness of a specialist allergy service and adrenaline injectors for those who had suffered anaphylaxis. In this study79 the impact of anaphylactic shock on QoL was, in absence of utility evidence, assumed by the authors to be equal to zero utility for a duration of 9 days at maximum.
-
Meadows et al. 80 investigated immunotherapy in adults and children with seasonal allergic rhinitis. They used mapping to obtain EQ-5D change scores from changes in scores on the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ). 81 The RQLQ scale ranges from 0 (best) to 6 (worst). It was assumed that the top end of the scale maps to the EQ-5D state representing no problems in any of the five dimensions. The bottom end of the RQLQ scale was mapped to the EQ-5D state representing maximum problems with usual activities, pain/discomfort and anxiety/depression, but no problems with mobility or self-care, which were assumed to be unaffected by seasonal allergic rhinitis. This state has a QoL score of –0.07 on the standard UK tariff. As a result, going from worst to best was a 6-point reduction in RQLQ and a 1.07-point increase in the EQ-5D score. Each unit decrease (improvement) in RQLQ was assumed to map to a 0.178-point increase in QoL score (assuming that a unit decrease has the same value at all points on the scale). The authors state that it could be argued that mapping the RQLQ to the whole range of three of the five dimensions of the EQ-5D scale could have led to an overestimation of utility gains, as the bottom score of –0.07 on the EQ-5D (representing a state worse than death) would not be equivalent to the worst score on the RQLQ.
-
Garside et al. 82 investigated the effectiveness of treatments for atopic eczema. In the absence of utility values in the literature they used a UK Utility Panel (n = 15 laypeople) to estimate utilities for three severity stages of atopic eczema using the standard gamble. They used the Dermatology Life Quality Index83 to develop scenarios and obtained valuations of these using SG. The median results were 0.985 for mild eczema, 0.875 for moderate eczema and 0.675 for severe eczema.
Methodology
The aim of this assessment was to compare the cost-effectiveness of adding multiplex allergen testing to current clinical practice with current clinical practice alone for people with difficult to manage IgE-mediated allergic disease in secondary or tertiary care settings. In this setting, multiplex allergen testing might be used to inform clinical decisions (e.g. to perform a food challenge and/or to initiate SIT) through aiding allergy diagnosis, predicting the probability of allergic reactions and/or predicting response to SIT. However, given the paucity of data on the clinical effectiveness of multiplex allergen testing (see Chapter 3), no long-term cost-effectiveness model is developed. This is in accordance with the published protocol for this assessment (PROSPERO registration no. CRD42015019739). More specifically, the lack of data on the clinical consequences of adding multiplex allergen testing to current clinical practice renders the development of a long-term economic model unusable to inform health policy decision-making.
Instead of developing a long-term cost-effectiveness model, the following sections aim to inform research decisions and support future model-based economic evaluations and include the following components:
-
relevant cost-effectiveness analyses are identified and reviewed (see Review of economic analyses of multiplex allergen testing, above)
-
available health-state utility studies are identified and reviewed (see Review of health-related quality of life studies, above)
-
the current clinical diagnostic pathway as well as the potential place for multiplex allergen testing are examined (see Current clinical diagnostic pathways, below)
-
a concept model structure is developed (see Model structure, below)
-
a survey is performed to retrieve the proportions of patients receiving each test (see Model parameters, below)
-
test costs were calculated (see Model parameters, below), and
-
cost analyses were performed to examine the short-term costs of diagnostic pathways with ImmunoCAP ISAC vs. with Microtest vs. without either (standard diagnostic pathway) (see Cost analyses, below).
Current clinical diagnostic pathways
Current clinical diagnostic pathways for patients referred for specialist allergy investigation in secondary or tertiary care settings may include SPT, single IgE testing and an OFC test where appropriate, combined with clinical history. SPT is often the first investigation performed in allergy diagnostics. 84,85 Based on consultations with clinical experts, it is assumed that single IgE testing will be performed in cases where the results of the SPT are not consistent with the clinical history of a patient (e-mail from Roisin Fitzsimons, Guys and St Thomas’ NHS Foundation Trust, 15 July 2015, personal communication). Inconsistency can occur if the SPT for the most likely allergen (based on clinical history) is negative or if a SPT is positive for an allergen that does not seem to explain the symptoms completely. Additionally, an OFC test is usually performed to confirm or rule out allergy to a specific food-related allergen or allergens. 85–87 If SPT is not considered acceptable/practical (e.g. in children with atopic eczema), single IgE testing might be the first-line investigation, using confirmatory OFC or SPT as necessary. Moreover, it might be possible to proceed to OFC based on SPT (and patient history) alone. Figure 6 provides an overview of the possible diagnostic pathways with and without SPT. It should be noted that it is unclear whether or not this theoretical diagnostic pathway (based on clinical expertise and literature) is representative of current UK clinical practice in all secondary or tertiary care settings.
FIGURE 6.
Current diagnostic pathways. In these pathways it is assumed that no further testing will be performed if IgE-mediated allergic response can be ruled out as an explanation for the observed symptoms. In all other cases it is assumed that further testing will be performed.

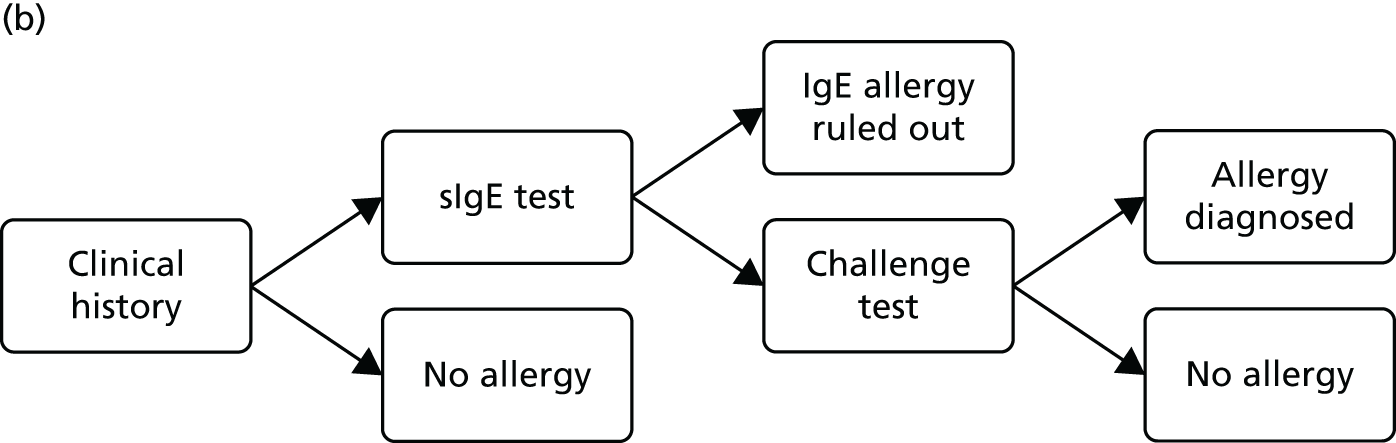
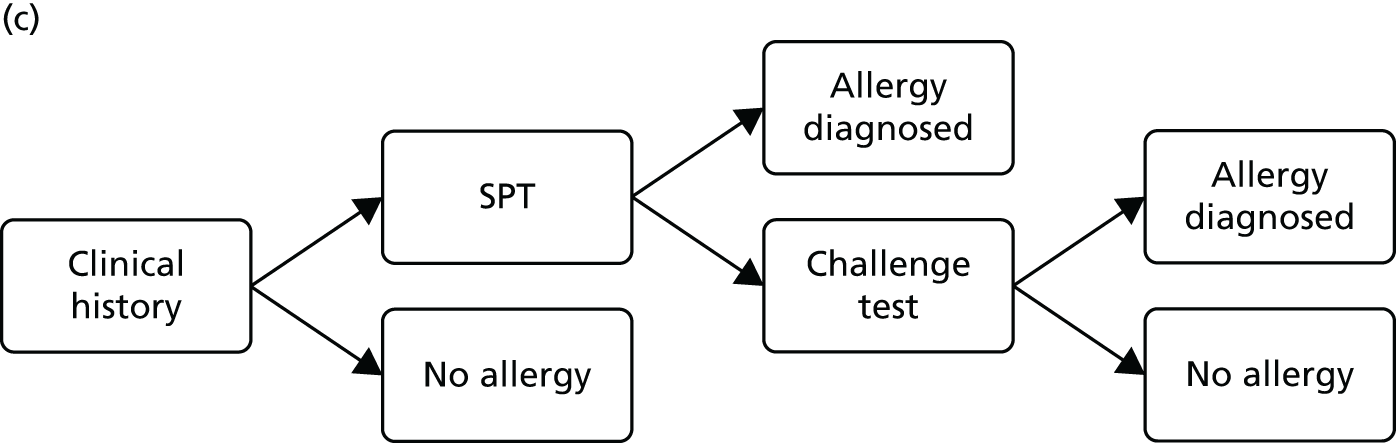
When considering patients with difficult to manage allergic disease who have been referred for assessment in secondary or tertiary care settings, multiplex allergen testing is likely to occur as a first-line investigation (assuming that all of the allergens of interest are on the array). Its role would be to identify which allergens a patient is sensitive to. Any allergens identified would have to be confirmed by SPT or OFC. The potential advantage of the array is that it can simultaneously test for homologous proteins or cross-sensitive proteins and therefore can aid the clinician in tailoring which confirmatory tests are required. For example, if the test is negative for particular proteins this might rule out the need for OFC. It is likely that multiplex allergen testing would replace single IgE testing, although some single IgE testing might still be required, for example if the array does not test for all suspected allergens. Figure 7 provides an overview of potential diagnostic pathways including multiplex allergen testing. In some pathways (see Figure 7a and b) it is assumed, based on clinical opinion (e-mail from Paul Turner, Imperial College London and St Mary’s Hospital, London, 15 July 2015, personal communication), that single IgE testing will always be performed before multiplex allergen testing (if single IgE testing is applicable). However, this might not always be the case, as multiplex allergen testing may also be performed instead of single IgE testing (see Figure 7a and d). The most important point is that multiplex allergen testing would be likely to reduce the number of single IgE tests, by ruling out particular allergens, thereby reducing the need for OFC.
FIGURE 7.
Potential diagnostic pathways including multiplex allergen testing. In these pathways it is assumed that no further testing will be performed if IgE-mediated allergic response can be ruled out as an explanation for the observed symptoms. In all other cases it is assumed that further testing will be performed.


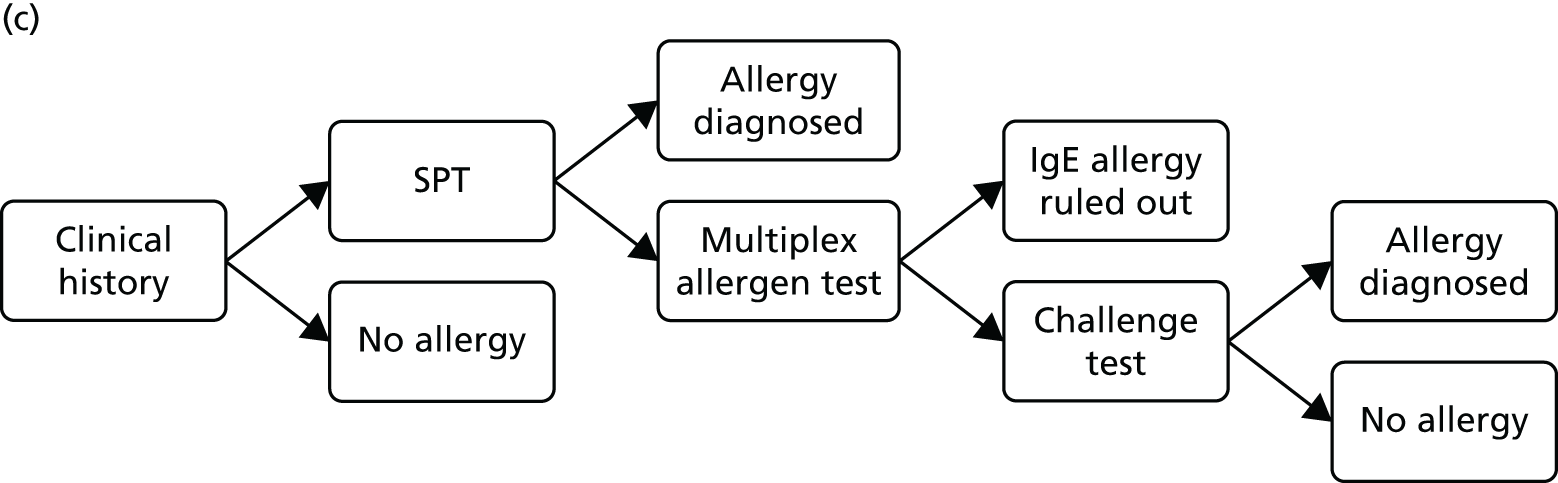
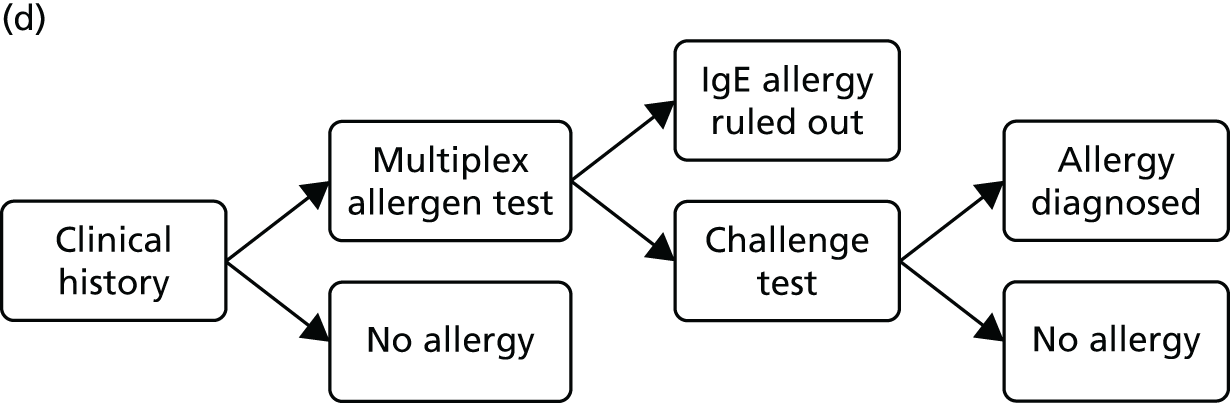
Model structure
This section describes a model structure that could potentially be used to assess the cost-effectiveness of multiplex allergen testing compared with current clinical practice for people with difficult to manage allergic disease in secondary or tertiary care settings. Three comparators would be evaluated in the economic model:
-
ImmunoCAP ISAC testing
-
Microtest testing
-
current (standard) diagnostic pathway.
The health-economic model would potentially consist of a decision tree and a state-transition (i.e. Markov) model. The decision tree can be used to model the short-term outcomes, based on test results and the accompanying treatment decision. These outcomes consist of ‘at risk of allergic reaction (treated)’, ‘not at risk of allergic reaction (treated)’, ‘at risk of allergic reaction (untreated)’ and ‘not at risk of allergic reaction (untreated)’. Moreover, potential AEs of testing can be considered in the decision tree. The decision tree is shown in Figure 8.
FIGURE 8.
Potential decision tree for the diagnostic pathway. a, Standard diagnostic pathway may consist of SPTs, single IgE testing and/or food challenge (see Figure 6); b, multiplex allergen testing might be performed in addition to the standard diagnostic pathway or instead of (part of) the standard diagnostic pathway (see Figure 7); c, treatment may consist of immunotherapy and/or symptom management (i.e. antihistamines and/or avoidance of the allergen) and is likely to lower the likelihood and/or severity of an allergic reaction. 86
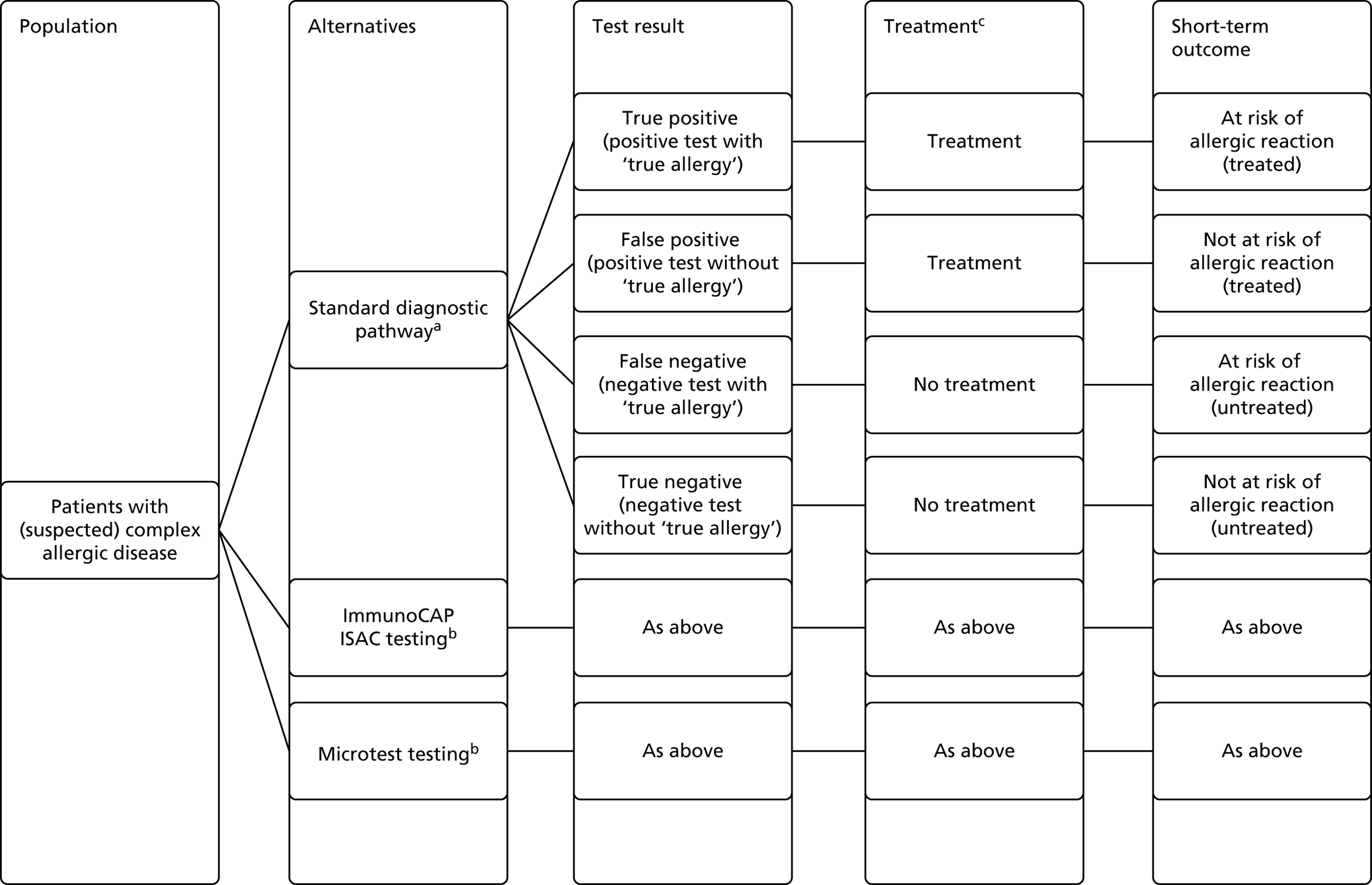
The long-term consequences in terms of costs and QALYs can be estimated using a state-transition cohort model (Figure 9) with a lifetime time horizon. The initial health state in the state-transition model is determined by the short-term outcome from the decision tree. The following health states are included in the state-transition model:
-
at risk of allergic reaction
-
not at risk of allergic reaction/remission
-
allergic reaction (experienced during cycle)
-
death (either background mortality or death due to an allergic reaction).
FIGURE 9.
Potential state-transition model structure. a, Different types and severities of allergic reactions can be separately included in the model. †Death.
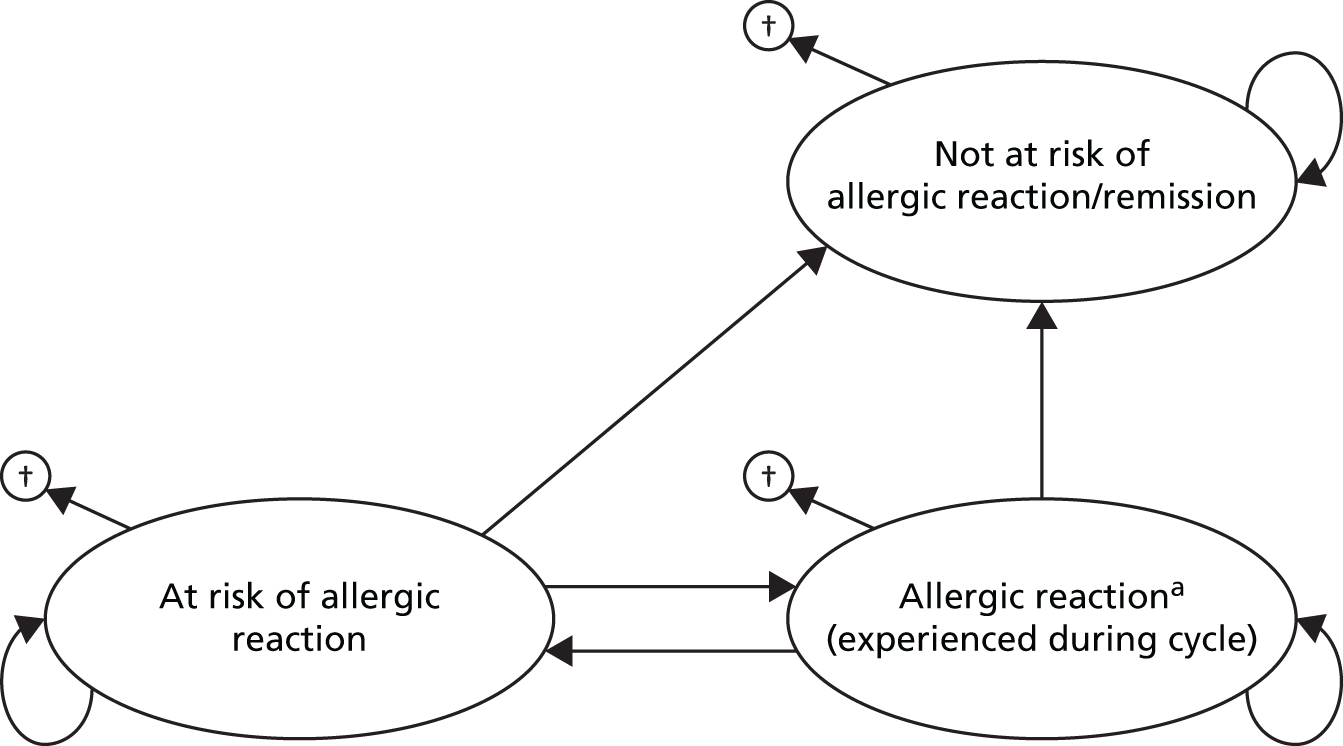
Different types and severities of allergic reactions can be included in the model separately. Given the diversity of allergy reactions, which depend on the type of allergy, separate models would ideally be developed for separate populations, for example those suspected of having clinical reactivity to an inhaled versus an ingested allergen.
Model parameters
Decision tree
To inform the decision tree for the diagnostic pathway the following parameters are required:
-
proportion of patients who receive a particular test (i.e. SPT, single IgE test, multiplex allergen test and/or OFC test) as well as the number of SPTs and/or single IgE tests per patient
-
accuracy of the diagnostic pathways (i.e. proportion of true positives, false positives, false negatives and true negatives as a result of the combined diagnostic performance of SPT, single IgE and/or multiplex testing)
-
the treatment decision.
The proportions of patients receiving a particular test and the number of tests per patient are unclear for both the standard diagnostic pathway and the diagnostic pathway including multiplex allergen testing. To alleviate this issue, a survey was sent to clinicians to inform these parameters (see Appendix 6 for the survey). However, no valid responses were received for multiplex allergen testing; the only respondent who indicated having any experience with ImmunoCAP ISAC responded that the number of OFC tests used was too few to comment on. Hence it was not possible to use the survey results in the cost analyses. Moreover, as described in the systematic review (see Effects on management, treatment and diagnostic classification of adding multiplex allergen testing to the diagnostic work-up of people with difficult to manage allergic disease, above), full information on the accuracy of the diagnostic pathways is not available. Finally, information on how treatment decisions relate to the diagnostic pathways is not available. Although two studies examined changes in SIT prescriptions32,38 following the addition of multiplex allergen testing results to standard diagnostic work-up, the results of these studies were not consistent: one study38 described an increase in SIT prescriptions following multiplex allergen testing, whereas the other study32 described a decrease in SIT prescriptions following multiplex allergen testing (see Table 5 for more details).
State-transition model
To inform the long-term state-transition model, the following parameters would be required (all conditional on the test result):
-
probability of allergic reactions (might be multiple allergic reactions and population specific)
-
probability of remission, and
-
probability of dying.
No long-term consequences for multiplex allergen testing were identified in the systematic review (see Chapter 3, Overview of included studies).
Health-state utilities
The evidence on utility values for allergic conditions in the UK population was limited. For food allergies, no utility values were found. For seasonal allergic rhinoconjunctivitis, EuroQol VAS scores from Pitt et al. 68 or EQ-5D scores from a European study63–65 could be taken. Stephens et al. 75 used standard gamble to obtain utility values for atopic eczema in UK children. Only in the study by Stephens et al. 75 were utilities reported per degree of severity of the allergic conditions (see Tables 10 and 11). Utility values for complications of allergies, such as anaphylactic shock, could not be found in the literature, apart from the assumption made by Armstrong et al. 79 that the impact of anaphylactic shock on QoL was equal to zero utility for a duration of 9 days at maximum.
Resource use and costs
To estimate the costs of the individual tests, a detailed cost calculation (see Appendix 7) was performed considering test costs, capital costs (if applicable), service and maintenance costs and personnel costs for performing and interpreting the tests. The results of the detailed test cost calculation are presented in Table 12. For ImmunoCAP ISAC and Microtest testing, the minimum and maximum prices were calculated and subsequently averaged. For ImmunoCAP ISAC testing, the main differences between the minimum and maximum prices can be attributed to the difference in time (between 5 and 60 minutes) that was needed to interpret the test results. This also holds true for Microtest testing, although the range was smaller (between 5 and 10 minutes). Additionally, for Microtest testing it is assumed that the test sample would be sent to the Microtest DX laboratory, where the test would be performed (companies preferred and most conservative scenario), whereas for ImmunoCAP ISAC testing it is assumed that the test would be performed at the service provider laboratory. Hence, for ImmunoCAP ISAC testing capital costs are included while for Microtest testing it is assumed that these costs would be included in the test costs (see Appendix 7). Capital costs are annuitised using a cost discount rate of 3.5%.
| £ per patient tested | Sources | |
|---|---|---|
| SPT | 62.28 | NICE 2011,86 Curtis88 |
| IgE test | 136.37 | NICE 2011,86 Curtis88 |
| OFC test | 570.00 | NICE 2011,86 Department of Health (NHS reference costs 2013–1489) |
| ImmunoCAP ISAC | 219.51 | Information submitted to NICE by Thermo Fisher Scientific, Curtis88 |
| Microtest | 156.85 | Information submitted to NICE by Microtest DX, Curtis88 |
Additional costs that would be considered in a long-term cost (-effectiveness) analysis may include the costs of SIT, health-state costs for being at risk of allergic reaction, and health-state costs for having experienced an allergic reaction. These costs are likely to be very specific for the population to be considered. Moreover, different types of SIT might be provided within a specific population (see, for example, the study by Sastre et al. 32). Hence the specific type(s) of SIT prescribed and the SIT duration would be required to calculate these costs (see, for example, the study by Meadows et al. 80 for a calculation of the immunotherapy costs for rhinitis).
Cost analyses
In this section we report on a cost comparison of three diagnostic strategies: with ImmunoCAP ISAC versus with Microtest versus the standard diagnostic pathway without multiplex allergen testing.
Given that the proportion of patients receiving single IgE and OFC tests in addition to ImmunoCAP ISAC or Microtest is unclear, the cost analyses are performed using two-way threshold analysis for these parameters. 90 Specifically, in pairwise comparisons of two test strategies, the minimal reduction (i.e. threshold) in proportions of single IgE and OFC tests is identified that was needed for the most expensive test strategy to become cheaper than the alternative test strategy, assuming that everything else remains equal. Here, 100% for both tests was defined as all patients receiving eight single IgE tests on average and all patients receiving on average one OFC test (see Appendix 7). Therefore, for example, if it was assumed that the use of multiplex allergen testing would result in no single IgE testing then this would imply a 100% reduction in single IgE testing compared with the standard diagnostic pathway. Given that multiplex allergen testing is more costly than single IgE testing, threshold analysis could then show what percentage reduction in OFC tests would be required to give the multiplex allergen pathway the same cost as the standard diagnostic pathway. On the other hand, if it was instead assumed that there was no reduction in single IgE testing by use of multiplex allergen, then this would result in a different threshold for the percentage reduction in OFC tests required to give the multiplex allergen pathway the same cost as the standard diagnostic pathway.
As previously stated, in these analyses, it is assumed everything except the number of single IgE tests and the number of OFC tests remains equal; this includes the assumption that the proportion of patients receiving any SPT is equal for all test strategies. Although this assumption is debatable, it might be justified given that SPT is a simple, safe and quick test (providing results within 15–20 minutes) that is often the first-line investigation in allergy diagnostics. Moreover, one clinician (e-mail from Paul Turner, personal communication), with experience with ImmunoCAP ISAC testing, indicated that all patients would receive SPT when using ImmunoCAP ISAC.
Scenario analyses
Several scenario analyses were performed:
-
In the calculation of the base-case costs for ImmunoCAP ISAC it is assumed that the LuxScan 10k reader would be used only for ImmunoCAP ISAC testing (on average 386 tests per year). However, the LuxScan 10k reader might be used for other purposes. Therefore, in the first scenario analysis, it is assumed that the LuxScan 10k reader would be fully occupied for 253 days per year. This reduces the ImmunoCAP ISAC testing costs to £201.91 per patient tested.
-
The second scenario analysis considered a scenario wherein the Microtest test would be performed at the service provider laboratory instead of at the Microtest DX laboratory (as assumed in the base-case analysis). This scenario reduces the costs of Microtest testing to £149.37 per patient tested (see Appendix 8).
-
The third scenario analysis considered the impact of the number of allergens tested using single IgE testing (base-case value = eight allergens tested per person). 86 The number of allergens was set to 1 and 20, respectively.
-
The final scenario analysis considered a reduced OFC costs of £256.00, excluding the costs of implementing the food elimination diet.
Threshold analyses
For the situation where ImmunoCAP ISAC or Microtest are used as replacement test(s) for single IgE testing (rather than as an add-on), a threshold analysis was performed to examine the minimum number of allergens to be tested with single IgE tests in order for single IgE testing to be equally as expensive as, or more expensive than multiplex allergen testing, assuming that everything else remains equal. This analysis was also performed for SPT.
Results of cost analyses
The cost analyses consider the short-term test costs using two-way threshold analyses for the proportion of single IgE tests and the proportion of OFC tests. The base-case analysis indicated that in order for ImmunoCAP ISAC and Microtest to be cost-saving compared with the standard diagnostic pathway, the absolute proportion of OFC tests should be reduced by at least 15 and 4 percentage points, respectively (e.g. from 50% to 35% or from 50% to 46%, respectively), if there was a 100% reduction in single IgE tests (i.e. from 100% to 0%). On the other hand, if there is no reduction in the proportion of single IgE tests (assuming an average of eight per person), the reduction in OFC tests should be at least 39% and 28% for ImmunoCAP ISAC and Microtest, respectively. Moreover, for ImmunoCAP ISAC compared with Microtest, the proportion of OFC tests for ImmunoCAP ISAC should be reduced by at least 11% if there is no reduction in the proportion of single IgE tests. When assuming no reduction in the proportion of OFC tests, the proportion of patients receiving an average of eight single IgE tests for ImmunoCAP ISAC should be reduced by at least 44% (Figure 10).
FIGURE 10.
Results of two-way threshold analyses (base case). Combinations of percentage reductions in food challenge and single IgE testing above a line lead to lower costs, and below a line lead to higher costs.
Scenario analyses
-
The ImmunoCAP ISAC costs are reduced by £18 when assuming that the LuxScan 10k reader would be fully occupied. This resulted in a decrease in the proportions for ImmunoCAP ISAC needed to reduce in order to be cost-saving compared with the standard diagnostic pathway and Microtest (Figure 11).
-
The Microtest costs are reduced by £7 when assuming that the Microtest test would be performed at the service provider laboratory instead of at the Microtest DX laboratory. This resulted in a decrease in the proportions for Microtest tests needed to reduce in order to be cost-saving compared with the standard diagnostic pathway (Figure 12).
-
Assuming that the number of allergens tested using single IgE testing is ‘one’ decreases the impact of reducing the proportion of patients with single IgE tests, while assuming 20 allergens tested increases the impact of reducing the proportion of patients with single IgE tests (Figures 13 and 14).
-
Finally, decreasing the OFC costs to £256.00 substantially increases the reduction in OFC needed in order for multiplex allergen testing to be cost-saving (Figure 15).
FIGURE 11.
Results of two-way threshold analyses (assuming that the LuxScan 10k reader would be fully occupied). Combinations of percentage reductions in food challenge and IgE testing above a line lead to lower costs, and below a line lead to higher costs.
FIGURE 12.
Results of two-way threshold analyses (assuming that the Microtest test would be performed at the service provider lab). Combinations of percentage reductions in food challenge and IgE testing above a line lead to lower costs, and below a line lead to higher costs.

FIGURE 13.
Results of two-way threshold analyses (assuming one allergy tested during single IgE test). Combinations of percentage reductions in food challenge and IgE testing above a line lead to lower costs, and below a line lead to higher costs.
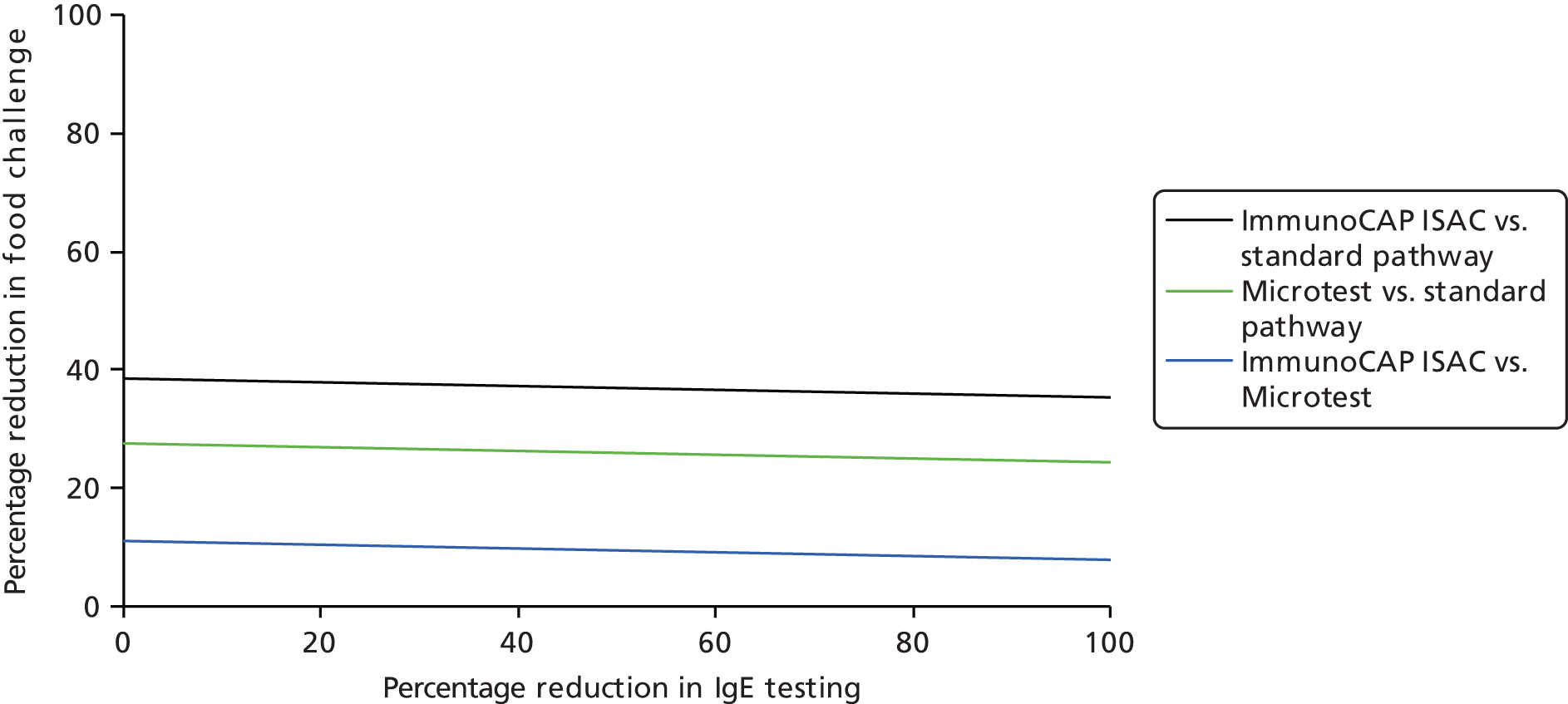
FIGURE 14.
Results of two-way threshold analyses (assuming 20 allergens tested during single IgE test). Combinations of percentage reductions in food challenge and IgE testing above a line lead to lower costs, and below a line lead to higher costs.

FIGURE 15.
Results of two-way threshold analyses (assuming OFC costs of £256.00). Combinations of percentage reductions in food challenge and IgE testing above a line lead to lower costs, and below a line lead to higher costs.
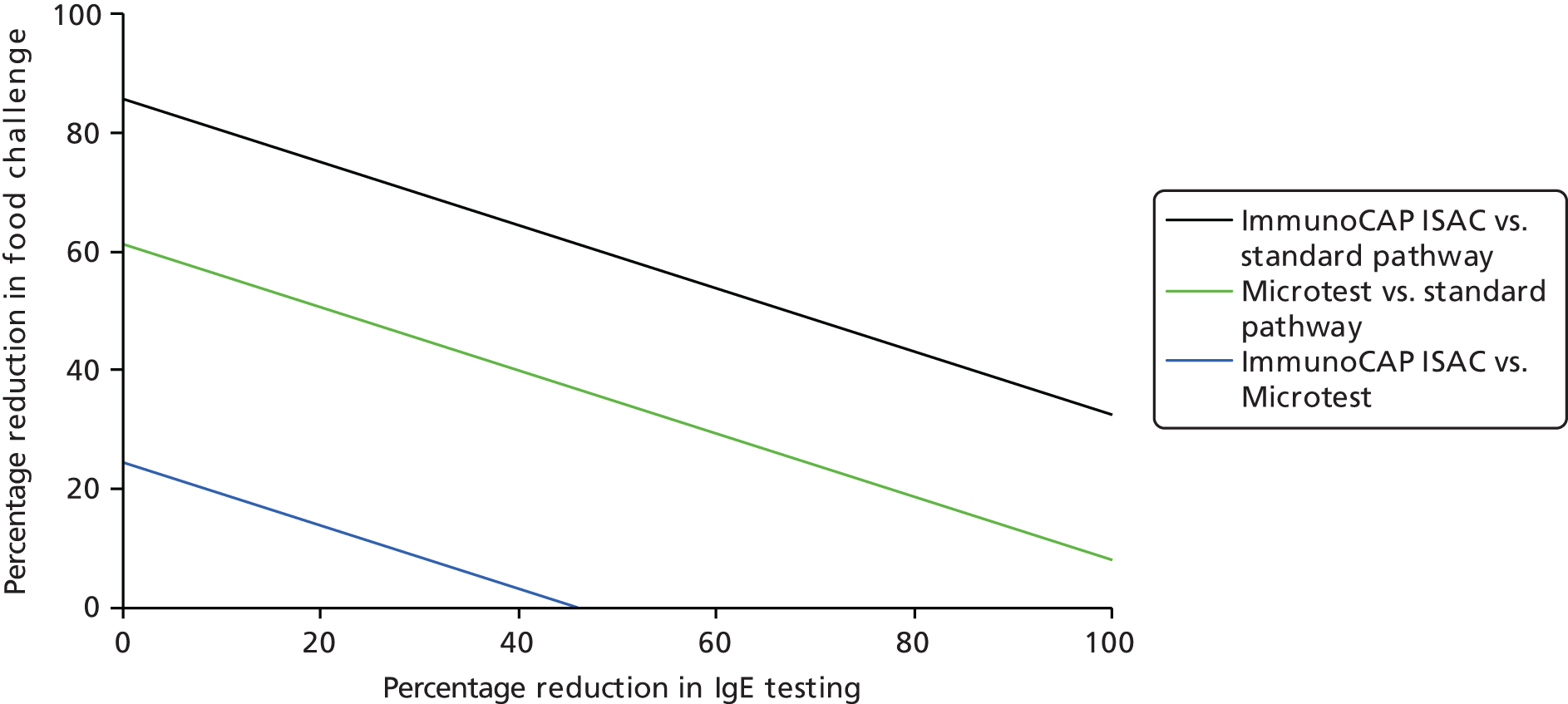
Threshold analyses
In these analyses, it is assumed that there is no reduction in OFC testing with multiplex testing. The minimum numbers of allergens tested using single IgE tests were 13 and 10 in order for the standard pathway to be as expensive as or more expensive than the ImmunoCAP ISAC and Microtest pathways, respectively. This means that, if multiplex testing replaced single IgE testing, then it would have to replace at least 13 or 10 tests to be cost-saving. For SPT these numbers were 39 and 27, respectively.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
The results of the very limited number of available studies provide some indication that the addition of multiplex allergen testing (ImmunoCAP ISAC) to standard diagnostic work-up can change clinicians’ views on the diagnosis, management and treatment of patients; no data were available for Microtest. There was some indication that the use of ImmunoCAP ISAC testing may guide decisions on the discontinuation of restrictive diets, the content of SIT prescriptions, and whether or not patients should receive SIT. However, importantly, none of the studies that we identified reported any information on clinical outcomes subsequent to changes in treatment or management based on ImmunoCAP ISAC. There was some evidence that ImmunoCAP ISAC may be useful for discriminating allergens that are structurally similar and are recognised by the same IgE antibody (cross-immunoreactive), and this may be useful for identifying the cause of food allergies. A UK-based study on the use of ImmunoCAP ISAC to investigate idiopathic anaphylaxis indicated that the addition of ImmunoCAP ISAC to standard diagnostic work-up may identify a potentially causative agent in previously undiagnosed patients. 39 However, it should be noted that the addition of ImmunoCAP ISAC also resulted in the identification of large numbers of sensitisations that were not considered to be associated with the anaphylaxis (i.e. large numbers of clinically false-positive test results).
The diagnostic performance of ImmunoCAP ISAC in comparison with other tests (single IgE and SPT) varied considerably between studies, according to the allergens investigated and the way in which ISAC testing was applied. In general, individual ISAC components tended to have high specificity, but low sensitivity, relative to whole-allergen single IgE tests or SPTs for the prediction of allergic response. The relatively low sensitivities of individual ISAC components are likely to be indicative of the proportions of patients in whom each component is associated with the observed allergic response. Conversely, a high specificity is indicative of a strong association between ISAC positivity for the individual component and an allergic response to whole allergen. When ISAC was used to measure the same component as single IgE testing or to measure multiple components (homologous proteins) with a positive test defined as any component positive, it appeared that equivalent sensitivities could be achieved without corresponding loss of specificity. As noted above, the ability of ImmunoCAP ISAC to discriminate between allergens that are structurally similar and are recognised by the same IgE antibody (cross-immunoreactive) may represent clinically useful additional information. Therefore, if the focused use of groups of ISAC components can achieve equivalent sensitivity and specificity to that of single IgE testing, ISAC testing may be preferred.
The clinical effects of adding multiplex allergen testing to the investigation of people with difficult to manage allergic disease have yet to be adequately investigated; in particular, the clinical consequences of changes to diagnosis or treatment, and the frequency and relevance of clinically false-positive sensitisations has been under investigated.
Cost-effectiveness
The initial aim of this assessment was to compare the cost-effectiveness of multiplex allergen testing with current clinical practice for people with difficult to manage allergic disease in secondary or tertiary care settings. However, the lack of data on the clinical consequences of multiplex allergen testing rendered the development of a long-term economic model uninformative for health policy decision-making. Therefore, instead of developing a long-term cost-effectiveness model, this assessment aimed to inform research decisions and support future model-based economic evaluations. For this purpose, relevant cost-effectiveness analyses and available health-state utility studies were identified and reviewed. Also, the current clinical diagnostic pathway, as well as the potential place for multiplex allergen testing, were examined, and a concept model structure was developed. Finally, a survey was performed to retrieve the number of patients receiving each test, test costs were calculated and cost analyses were performed to examine the short-term costs of the test strategies.
All four identified cost-effectiveness studies33,34,54–60 (all abstracts) showed an increased effectiveness when using ImmunoCAP ISAC and three54–60 out of four studies also showed cost savings when using ImmunoCAP ISAC. However, the methods and assumptions used in these assessments are largely unclear, severely hampering the assessment of the validity of the results. In addition, the credibility of these assessments was questioned as fundamental inputs of their models were based on expert opinion or inaccessible references, or no references were provided. Therefore, these findings should be interpreted with extreme caution.
The evidence on utility values for allergic conditions in the UK population was limited. For food allergies no utility values were found, whereas UK utility values were available for seasonal allergic rhinoconjunctivitis and atopic eczema in children.
Test costs for ImmunoCAP ISAC and Microtest were estimated to be £219.51 and £156.85, respectively. For SPT, single IgE and the food challenge test these were £62.29, £136.37 and £570.00, respectively. Detailed cost analyses were performed to estimate the short-term cost of diagnostic pathways with and without multiplex allergen testing. As the place of multiplex allergen testing in the diagnostic pathway and the proportions of patients receiving a particular test are unclear, and the survey did not provide the required results, different scenario and threshold analyses were performed. The results of these analyses depend on precisely the effect of multiplex testing on the need for single IgE, SPT and OFC testing. For example, if multiplex testing replaced single IgE testing (assuming eight tests per person) then a 15% or 4% reduction in OFC would be required for ImmunoCAP ISAC and Microtest, respectively, to be cost-saving. However, if there was no reduction in OFC testing, then the number of single IgE tests or SPTs per patient that needed replacing would have to be at least 39% or 28% for ImmunoCAP ISAC and Microtest, respectively, to be cost-saving.
Strengths and limitations of assessment
Clinical effectiveness
Extensive literature searches were conducted in an attempt to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trials registers and conference abstracts to identify unpublished studies. Despite this, we were unable to identify any studies that reported clinical outcomes and available data were generally very sparse.
The possibility of publication bias cannot be ruled out. Owing to the small number of included studies and between-study clinical heterogeneity, we were unable to perform any meta-analyses or to undertake a formal assessment of publication bias. However, our search strategy included a variety of routes to identify unpublished studies and resulted in the inclusion of a number of conference abstracts.
Clear inclusion criteria were specified in the registered protocol for this review (PROSPERO registration no. CRD42015019739). One change was made to the published protocol, expanding the inclusion criteria to allow inclusion of studies that reported direct comparisons of diagnostic accuracy between single IgE testing and multiplex allergen testing, using SPTs or allergen challenge tests as the reference standard, but did not report details of any participants for whom multiplex allergen testing provided additional information (details provided in Chapter 3; see Inclusion and exclusion criteria). These studies were included with the aim of providing some indication of the performance of multiplex allergen testing, compared with current single IgE antibody testing practice, for predicting clinical response; studies that reported only the accuracy of multiplex allergen testing, without a comparison to current testing practice, were therefore not included. In addition, we have provided specific reasons for exclusion for all of the studies that were considered potentially relevant at initial citation screening and were subsequently excluded on assessment of the full publication (see Appendix 3). The eligibility of studies for inclusion is therefore transparent.
The review process followed recommended methods to minimise the potential for error and/or bias;21 studies were independently screened for inclusion by two reviewers, and data extraction and quality assessment were done by one reviewer and checked by a second (MW and SL). Any disagreements were resolved by consensus.
Studies included in this review were assessed for risk of bias, where possible using published validated tools [the QUADAS-2 tool25 and CASP cohort risk-of-bias tool (www.casp-uk.net/)]. The review included a number of observational studies, which used a ‘before-and-after’ type study design to assess the effects of adding information from multiplex allergen testing to the standard diagnostic work-up in the same group of participants. We are not aware of any published risk-of-bias tool that is appropriate for the assessment of this type of study. A review-specific tool was therefore designed by the authors (MW, SL and NA) to allow the methodological quality of these studies to be systematically assessed and described. This tool focuses on elements of study design that we considered relevant to this specific study type, and is based upon the structure of the QUADAS-2 tool. The results of the risk-of-bias assessment are reported, in full, for all included studies in Appendix 4 and are summarised in Chapter 3 (see Study quality). Studies were generally of unclear quality due to limitations in reporting, with full publications lacking for many studies (reported as conference abstracts only). None of the studies included in this review can be considered to have low risk of bias; all studies were rated as ‘high’ or ‘unclear’ risk of bias on at least one domain of the relevant tool. The main ‘high’ risk-of-bias areas identified were participant selection (inappropriate exclusions) and application of testing procedures (variation in testing procedures between study participants and within-study optimisation of the diagnostic threshold).
An important potential advantage of ImmunoCAP ISAC and other multicomponent arrays is their ability to differentiate between allergens that have similar structures; these homologous allergens maybe recognised by the same antibody (cross-reactivity) or homologous allergens may cause the same allergic response (cross-sensitisation). For example, a blood sample that shows immunoreactivity to Bet v1 may indicate that a patient is sensitised to birch pollen or they may be sensitised to one of many protein homologues (proteins of similar molecular structure to Bet v1), or they may be sensitised to both. Bet v1 is a PR10 protein that has several homologues or protein family members. On the ISAC 112 chip there are nine Bet v1 homologues: rCor a1.0401 (hazelnut), rGly m4 (soybean), rAra h8 (peanut), rAct d8 (kiwi), rApi g1 (celery), rMal d1 (apple), rPru p1 (peach), rAln g1 (alder) and rCor a1.0101 (hazel). Therefore, ISAC 112 has the potential to discriminate between immunoreactivity to Bet v1 and nine homologues, a process that would take much longer by single allergen (single IgE) testing. According to the ImmunoCAP ISAC 112 technical brochure91 there are seven other protein families represented on the chip; therefore, its potential to provide information regarding cross-immunoreactivity of homologous proteins is apparent. However, data showing the effects of providing additional information of this type were very limited. It is important to note that ISAC multiplex testing on its own can differentiate between the immunoreactivity of a given allergen and a homologue only if that homologue is present on the array chip. Several reports raised the fact that not all useful components were present on the array. 37,39,40 These reports were carried out on earlier versions of ISAC and some of the suggested components now appear on ISAC 112. Further research maybe needed to target specific conditions and interpretation of data should always bear in mind what components are not on the array as well as what components are on the array.
Predominantly, the accuracy studies included in this review compared the performance of whole allergens (single IgE) to the ability of allergen components (ImmunoCAP ISAC) to detect specific IgE. Although both are aiming to identify the presence of specific antibodies in a patient serum sample, this is more likely to occur if using the whole allergen than part of the allergen. Classically, the interaction between an antibody and an antigen (allergen) occurs through very specific binding sites on both molecules. Therefore, the selected allergen component(s) may or may not contain this binding site. Furthermore, multiple antibodies may have been produced to one allergen each using different binding sites. The use of whole allergens is likely to give very different results to using a component and this may explain some of the discrepancies seen between the accuracy studies.
Overall, only one of the diagnostic studies compared like with like. Only one study compared the ability of the same component (rGLy m4) to detect specific antibodies using single IgE testing and ImmunoCAP ISAC. Interestingly, the two methods reported different sensitivities and specificities, indicating that they perform differently. This was not unexpected as the performance of the single IgE test will be maximised to give the best result for the single allergen of interest, whereas ImmunoCAP ISAC is developed to give the best results for a range of allergens. Therefore, the effect of non-specific binding within these two systems on diagnostic accuracy is unknown.
Studies often included patients with a clinical history of an immediate reaction to an allergen, indicating that these patients were likely to have had an IgE-mediated event, and excluded patients with delayed reactions possibly caused by mechanisms other than IgE. This must be borne in mind when evaluating the clinical performance of tests based on single IgE measurement, in that the research populations may be unrepresentative of those for whom the test would be used in practice. Nevertheless, it is expected that these tests will be used only on patients who are strongly suspected of an IgE-mediated reaction.
Finally, the studies included in this systematic review may have limited applicability to the specified population of interest (people with complex or difficult to manage allergies who are being assessed in UK secondary or tertiary health-care settings). Studies that did not specify that they included participants with difficult to manage allergic disease, or described inclusion criteria that could be considered consistent with this classification (e.g. polysensitised patients), were classified as having ‘high’ concerns regarding applicability. Studies that were conducted in non-UK settings and which assessed allergens considered unlikely to be relevant to UK populations (e.g. aeroallergens associated with Mediterranean countries) were also classified as having ‘high’ concerns regarding applicability. Only two39,40 of the studies included in the review were rated as having ‘low’ concerns with respect to both of these issues. None of the comparative accuracy studies was conducted in populations likely to be representative of people with difficult to manage allergic disease and two43,45 of these studies explicitly excluded people with complex allergies.
The studies identified by our systematic review did not provide sufficient evidence to adequately assess the clinical effectiveness of adding multiplex allergen testing to the investigation of people with difficult to manage allergic disease, in secondary or tertiary UK health-care settings. However, we believe that our comprehensive assessment of the limited available evidence has highlighted key areas in which data are lacking and provides a useful framework to guide the development of research recommendations.
Cost-effectiveness
This is the first time that a model structure including diagnostic pathways has been attempted to examine the place of multiplex testing in the UK. Our cost analysis is also the first to assess the short-term costs of multiplex allergen testing in the UK. For this purpose a detailed cost analysis was performed, and multiple scenario and threshold analyses were conducted. Extensive literature searches were conducted to provide an overview of available cost-effectiveness analyses and available health-state utilities. Additional searching in terms of reference checking and extensive hand searching was performed to maximise retrieval of relevant studies (including conference abstracts). Clear inclusion criteria were specified for selecting relevant studies. Relevant papers were summarised and the quality of cost-effectiveness analyses was assessed. Authors were contacted to provide more details if needed.
The main limitations of this assessment are the lack of information of the place of multiplex testing in the care pathway and any effectiveness evidence. There is therefore an inability to incorporate long-term costs and consequences (i.e. life-years and QALYs), particularly long-term outcomes conditional on the short-term test outcomes. As illustrated by previous assessments for the UK,80,82 it is possible to estimate long-term outcomes for treatments of allergic conditions (not conditional on a test result). Nevertheless, these assessments had severe limitations including that they required extensive assumptions (including mapping of utility values). 80
Uncertainties
Clinical effectiveness
The potential role of multiplex allergen testing in the care pathway of patients with complex allergic disease remains unclear. As described in the objectives for this assessment, multiplex allergen testing could be added to existing standard diagnostic work-up or could be used to replace some or all of the single IgE tests that would otherwise be used in some patients.
In order to adequately assess the effectiveness of multiplex allergen testing as an add-on test, studies would be required that compare the management of patients based on standard diagnostic work-up to management based on standard diagnostic work-up with the addition of multiplex allergen testing and which provide information on subsequent clinical outcomes. Although it might be suggested that RCTs represent the ‘gold standard’ for this comparison, other study designs may provide relevant information. In particular, earlier stage, exploratory studies can be important in determining whether RCTs or other large-scale comparative studies are justified and in informing the design of such studies. The seven ‘diagnostic before-and-after’ studies included in this assessment show participating clinicians changing various aspects of their judgement about a given group of patients when they have access to the results of ImmunoCAP ISAC testing. The findings of these studies indicate that multiplex allergen testing may provide information, additional to that obtained from standard diagnostic work-up, which can affect clinicians’ decision-making. However, the studies did not clearly report the extent to which the changes in clinicians’ judgement resulted in implementation of changes to the care of patients. Further, if additional information provided by multiplex allergen testing results in changes to the care of patients, it is important to collect information on subsequent clinical outcomes and to compare these outcomes with those seen in patients whose care has been based on standard diagnostic work-up. Such comparisons are necessary to assess whether care decisions based on information that included the results of multiplex allergen testing ultimately result in benefit or detriment to patients, or have no significant effect.
One study indicated that ImmunoCAP ISAC should be used before single IgE testing. This relates to the ability of the microarray to analyse 56 allergens and provide data to help identify allergens that are cross-sensitive or those that are cross-immunoreactive. This has been applied to complex food allergy, for which ImmunoCAP ISAC can help determine to which food allergens a patient is sensitised; in particular, it is able to determine which homologous allergens give rise to the sensitivity observed in a single IgE test. When several food allergens are suspected ImmunoCAP ISAC testing can allow the clinician to quickly determine and reduce the number of confirmatory oral challenges required. In the absence of information on clinical outcomes, it may therefore be useful to obtain information on how the addition of multiple allergen testing to the standard diagnostic work-up of people with difficult to manage allergic disease affects the overall testing burden (e.g. the number of single IgE tests and/or confirmatory challenge tests used) or other resource-use outcomes (e.g. the number of subsequent consultations with health-care professionals). We did not identify any studies that reported resource-use outcomes.
This assessment also includes eight studies that report information on the accuracy of various components and combinations of components on the ImmunoCAP ISAC chip compared with the accuracy of other testing options (single IgE or SPT) to predict allergic response (as defined by SPT or OFC). Studies of this type can determine whether multiplex allergen testing provides similar diagnostic information to that provided by single IgE or other testing options, when used in the same group of patients; comparable performance may be considered indicative of the potential of multiplex allergen testing to replace other tests without significant adverse diagnostic consequences (missed diagnoses or false positives). However, none of the comparative accuracy studies identified was conducted in populations with difficult to manage allergic disease. Studies therefore evaluated the diagnostic performance of single components or small groups of components on the ImmunoCAP ISAC chip that were relevant to the investigation of specific allergies (e.g. Gal d1, Gal d2 and Gal d3 for hen’s egg allergy). The focused use of the ImmunoCAP ISAC chip is likely to result in low numbers of false positives and high specificity estimates (i.e. sensitisations detected are likely to be associated with observed allergic response). These studies have limited applicability to the investigation of people with difficult to manage allergic disease, for whom there is greater diagnostic uncertainty and for whom it might be expected that all or a greater proportion of the components of the microchip might be used. If multiplex allergen testing is applied in this way, it might be expected that greater numbers of false positives would be generated (i.e. more sensitisations that are not associated with observed allergic response would be identified). Some evidence of this can be seen from the results of Heaps 2014,39 described in Chapter 3 (see Effects on management, treatment and diagnostic classification of adding multiplex allergen testing to the diagnostic work-up of people with difficult to manage allergic disease), which, as well as identifying sensitisations thought to be associated with anaphylaxis in some patients, identified large numbers of sensitisations that were not considered to be clinically relevant. In addition, two studies92,93 that did not meet the inclusion criteria of this systematic review reported data comparing rates of sensitisation to various allergen groups relevant to plant-food allergy in allergic and tolerant individuals. Both studies were conducted in Spain. The first study included 123 children with food allergy, of whom 55 were classified as peanut allergic and 68 as peanut tolerant (SPT and single IgE) and used ImmunoCAP ISAC 103 to assess sensitisation to a range of allergenic components. 92 There were no significant differences between peanut-allergic and peanut-tolerant children in the rates of sensitisation to pathogenesis-related protein family PR-10 allergens (Ara h8, Act d8, Cor a1, Gly m4, Mal d1, Pru p1), profilins (Bet v2, Ole e2, Hev b8, Mer a1, Phl p12), some lipid transfer proteins (LTPs) (Par j2, Pru p3), cross-reactive carbohydrate determinant Ana c2, or pollens (Ole e1, Phl p1). 92 The second study included 130 children with plant-food allergy and LTP sensitisation and found that sensitisation to a particular plant-food LTP, as determined by ImmunoCAP ISAC 112, was not always associated with clinical symptoms of allergy to that plant food: 69% (40/58) and 63% (17/27) of peach- and walnut-tolerant children were sensitised to Pru p3 and Jug r3, respectively; 60% (21/35) of children without seed/nut allergy were sensitised to storage proteins. 93 The potential of multiplex allergen testing to detect clinically false-positive sensitisations has not yet been adequately investigated and the long-term relevance of such sensitisations is unknown. However, the limited available data indicate a need for care in the application and interpretation of multiplex allergen testing.
Finally, we did not identify any studies of multiplex allergen testing using Microtest that met the inclusion criteria for this assessment. The manufacturer provided unpublished data on the concordance between test methods (Microtest, ImmunoCAP single IgE, ImmunoCAP ISAC and SPT). These data are summarised in Appendix 5, for information only.
Cost-effectiveness
The credibility of available cost-effectiveness studies in the literature can be questioned given the use of expert opinion for fundamental model inputs. Moreover, (UK) health-state utility values for allergic conditions are scarce (e.g. utilities for food allergy and for test AEs are lacking). However, the main source of uncertainty regarding the cost-effectiveness of multiplex allergen testing compared with current clinical practice for people with difficult to manage allergic disease was the lack of data on long-term clinical consequences conditional on test results of a diagnostic pathway including multiplex allergen testing.
The place of multiplex allergen testing in the diagnostic pathway and the proportions of patients receiving a particular test was unclear (for both the current diagnostic pathway and the diagnostic pathway including multiplex allergen testing). Therefore, speculative two-way threshold analyses were performed in this assessment to consider the diagnostic pathway costs only. These analyses required assumptions regarding all key parameters relating to the pathway, including the proportions/numbers per patient of single IgE and OFC tests and SPT. For example, it does seem likely that multiplex testing, by ruling out some allergens, might avoid confirmatory testing with OFC or SPT. However, SPT is a simple, safe and quick test (providing results within 15–20 minutes) and it is often the first-line investigation in allergy. In addition, one clinician (e-mail from Paul Turner, personal communication), with experience with ImmunoCAP ISAC testing, indicated that all patients would receive SPT when using ImmunoCAP ISAC. Hence, it might be that multiplex allergen testing would not reduce the number of SPT. Finally, whether single IgE might be used as an add-on to multiplex allergen testing or would be replaced by multiplex testing remains uncertain, particularly given the limited experience with multiplex allergen testing in the UK.
Chapter 6 Conclusions
Implications for service provision
No recommendations for service provision can be made based on the analyses included in this report. The clinical effectiveness and cost-effectiveness of using multiplex allergen testing in the investigation of people with difficult to manage allergic disease have yet to be adequately investigated. In particular, the clinical consequences of changes to diagnosis or treatment and the frequency and relevance of clinically false-positive sensitisations have been under investigated. From the limited evidence available it appears that the most likely role of multiplex allergen testing would be to replace some or all single IgE testing. The ability of multiplex testing to simultaneously identify multiple antibodies in the serum samples, combined with its ability to identify which homologous allergens are cross-immunoreactive, means that these tests have the potential to provide a lot of information in a single step. Although confirmatory testing (SPT or OFC) is still likely to be required, multiplex testing could be used to tailor confirmatory testing to the individual patient and thus reduce the overall testing burden; no studies were identified that assessed overall testing burden. It should be noted that all of the evidence identified related to one test (ImmunoCAP ISAC) and conclusions on the potential utility of multiplex allergen testing may not be generalisible to other products.
Suggested research priorities
There remains considerable uncertainty about the possible role of multiplex allergen testing in the investigation of people with difficult to manage allergic disease in the UK. The formulation of a consensus-based protocol for the use of multiplex allergen testing may represent a useful starting point for future research. A prospective study would then be needed to investigate the clinical effectiveness of the proposed protocol. The preferred design would be a RCT comparing diagnostic pathways with and without multiplex allergen testing. Alternatively, an observational study to compare outcomes in centres using multiplex allergen testing to those using diagnostic pathways without multiplex allergen testing may also be a useful approach. Such an approach would, however, require careful consideration of between-centre differences in patient care pathways (other than the use of multiplex allergen testing). Outcomes measured could include:
-
Short-term outcomes:
-
diagnostic performance, including discrimination between allergens responsible for allergic reactions and those that are cross-immunoreactive, and false-positive rate (i.e. the number of sensitisations identified that are not associated with allergic response) when the full panels of multiplex allergen testing devices are used in people with difficult to manage allergic disease
-
treatments or management decisions (including type and duration and the use and extent of restriction diets)
-
overall testing burden (i.e. the total number of tests, including multiplex allergen testing, single IgE testing, SPTs and OFC tests, required to reach a diagnosis and formulate a treatment/management plan)
-
any AEs associated with testing.
-
-
Long-term outcomes:
-
incidence and severity of allergic reactions
-
mortality
-
service use (e.g. repeat presentations with allergy symptoms requiring further investigation and/or treatment).
-
If different possible methods of multiplex allergen testing are being considered, then direct head-to-head comparisons would be needed.
Acknowledgements
The authors acknowledge the clinical advice and expert opinion provided by Dr Isabel Skypala, Consultant Allergy Dietitian & Clinical Lead for Food Allergy at Royal Brompton & Harefield NHS Foundation Trust; Dr Paul Turner, Clinical Senior Lecturer, Paediatric Allergy & Immunology, Imperial College London and St Mary’s Hospital, London; Ms Roisin Fitzsimons, Allergy Nurse Consultant, Guys and St Thomas’ NHS Foundation Trust; Dr Anthony Rowbottom, Consultant Immunologist, Lancashire Teaching Hospital NHS Trust; and Dr Michael Ardern-Jones, Senior Lecturer, University of Southampton, and Consultant Dermatologist, University Hospital Southampton NHS Foundation trust. We would also like to thank the lay members of the NICE Diagnostics Advisory Committee and Assessment Sub-group for providing input on the patients’ perspective at key stages of the assessment process.
This is a systematic review and therefore all extracted data are included in the report. Further information can be obtained from the corresponding author.
Contributions of authors
Marie Westwood and Shona Lang planned and performed the systematic review and interpretation of evidence.
Bram Ramaekers planned and performed the cost-effectiveness analyses and interpreted results.
Nigel Armstrong contributed to planning and interpretation of cost-effectiveness analyses, acquisition of input data and conducted model peer review.
Caro Noake and Shelley de Kock devised and performed the literature searches and provided information support to the project.
Manuela Joore, Johan Severens and Jos Kleijnen provided senior advice and support to the systematic review and cost-effectiveness analyses, respectively.
All parties were involved in drafting and/or commenting on the report.
Data sharing statement
This is a systematic review; therefore, there are no additional data to share. Further information can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Allergy Statistics. Sidcup: Allergy UK; 2014.
- Hippisley-Cox J. A Description of the Fourth version of the QRESEARCH Database: an analysis using QRESEARCH for the Department of Health. Nottingham: Department of Health; 2007.
- Hippisley-Cox J, Jumbu G, Fenty J, Holland R, Porter A, Heaps M. Primary Care Epidemiology of Allergic Disorders: Analysis Using QRESEARCH Database 2001–6: Final Report to the Information Centre and Department of Health. Nottingham: Department of Health; 2007.
- Simpson CR, Newton J, Hippisley-Cox J, Sheikh A. Trends in the epidemiology and prescribing of medication for eczema in England. J R Soc Med 2009;102:108-17. http://dx.doi.org/10.1258/jrsm.2009.080211.
- Ghouri N, Hippisley-Cox J, Newton J, Sheikh A. Trends in the epidemiology and prescribing of medication for allergic rhinitis in England. J R Soc Med 2008;101:466-72. http://dx.doi.org/10.1258/jrsm.2008.080096.
- Simpson CR, Newton J, Hippisley-Cox J, Sheikh A. Incidence and prevalence of multiple allergic disorders recorded in a national primary care database. J R Soc Med 2008;101:558-63. http://dx.doi.org/10.1258/jrsm.2008.080196.
- Food Allergy in Children and Young People: Diagnosis and Assessment of Food Allergy in Children and Young People in Primary Care and Community setTings. London: NICE; 2011.
- Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report – Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 2006;117:391-7. http://dx.doi.org/10.1016/j.jaci.2005.12.1303.
- NHS Accident and Emergency Attendances in England 2013–14: Tables. Leeds: HSCIC; 2015.
- González-Pérez A, Aponte Z, Vidaurre CF, Rodríguez LA. Anaphylaxis epidemiology in patients with and patients without asthma: a United Kingdom database review. J Allergy Clin Immunol 2010;125:1098-104. http://dx.doi.org/10.1016/j.jaci.2010.02.009.
- Anaphylaxis: Assessment to Confirm an Anaphylactic Episode and the Decision to Refer After Emergency Treatment for a Suspected Anaphylactic Episode. London: NICE; 2011.
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy 2000;30:1144-50. http://dx.doi.org/10.1046/j.1365-2222.2000.00864.x.
- Turner PJ, Gowland MH, Sharma V, Ierodiakonou D, Harper N, Garcez T, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012. J Allergy Clin Immunol 2015;135:956-63. http://dx.doi.org/10.1016/j.jaci.2014.10.021.
- Atopic Eczema in Under 12s. London: NICE; 2013.
- Atopic Eczema in Under 12s: Diagnosis and Management. London: NICE; 2007.
- Allergy: The Unmet Need: A Blueprint for Better Patient Care. London: RCP; 2003.
- Walker SM, Durham SR, Till SJ, Roberts G, Corrigan CJ, Leech SC, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy 2011;41:1177-200. http://dx.doi.org/10.1111/j.1365-2222.2011.03794.x.
- Allergy UK. Stolen Lives – The Allergy Report: the Impact of Allergies on People’s Lives in the UK Today. Sidcup: Allergy UK; 2003.
- Allergy UK. The Disturbing Impact of Skin Allergy and Sensitivity in the UK. Sidcup: Allergy UK; 2013.
- Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy 2010;65:933-45. http://dx.doi.org/10.1111/j.1398-9995.2010.02342.x.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2009.
- Diagnostics Assessment Programme Manual. Manchester: NICE; 2011.
- CADTH Peer Review Checklist for Search Strategies. Ottawa, ON: CADTH; 2013.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- 12 Questions to Help You Make Sense of a Cohort Study. Oxford: CASP; 2013.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. http://dx.doi.org/10.1016/j.jclinepi.2005.02.022.
- Harbord RM, Whiting P, Sterne JA, Egger M, Deeks JJ, Shang A, et al. An empirical comparison of methods for meta-analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol 2008;61:1095-103. http://dx.doi.org/10.1016/j.jclinepi.2007.09.013.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. http://dx.doi.org/10.1093/biostatistics/kxl004.
- Landivar Encalada M, Andregnette Roscigno V, Ruiz Garcia M, Hernandez Garcia De La Barrera E, Sastre Dominguez J. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex area of pollen sensitization (Madrid, Spain). Proceedings of the American Academy of Allergy, Asthma and Immunology (AAAAI), 2012 Annual Meeting, 2–6 March 2012, Orlando, FL, USA. J Allergy Clin Immunol 2012;129. http://dx.doi.org/10.1016/j.jaci.2011.12.061.
- Landivar Encalada M, Ruiz Garcia M, Andregnette Roscigno M, Hernandez Garcia de la Barrera E, Sastre Dominguez J. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex area of pollen sensitisation (Madrid, Spain). Proceedings of the European Academy of Allergy and Clinical Immunology, 31st Congress, 16–20 June 2012, Geneva, Switzerland. Allergy 2012;67:30-1.
- Sastre J, Landivar ME, Ruiz-García M, Andregnette-Rosigno MV, Mahillo I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy 2012;67:709-11. http://dx.doi.org/10.1111/j.1398-9995.2012.02808.x.
- Hermansson LL, Pensamo E, Korhonen K, Rantanen S, Isoaho R, Savolainen J. Health economic benefit of including component resolved diagnostics (CRD) (Immunocap ISAC) in in vitro diagnostic (IVD) algorithm in prospective trial with suspected food allergic school children in Finland. Proceedings of the International Society For Pharmacoeconomics and Outcomes Research, 19th Annual International Meeting, 31 May–4 June 2014, Montreal, QC, Canada. Value Health 2014;17. http://dx.doi.org/10.1016/j.jval.2014.03.1027.
- Hermansson LL, Pensamo E, Korhonen K, Rantanen S, Isoaho R, Savolainen J. Health economic benefit of including component resolved diagnostics (ImmunoCAP ISAC) in in vitro diagnostics algorithm in a prospective trial with suspected food allergic school children in Finland. Proceedings of the European Academy of Allergy and Clinical Immunology, 33rd Congress, 7–11 June 2014, Copenhagen, Denmark. Allergy 2014;69.
- Heaps A, Selwood C, El-Shanawany T, Moody M, Unsworth J, Deacock S, et al. The assessment of an immuno solid-phase allergen chip in the investigation of idiopathic anaphylaxis. Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI), 29th Congress, 5–9 June 2010, London, UK. Allergy 2010;65.
- Gay-Crosier F, Dayer E, Schmid-Grendelmeier P. Allergen-specific IgE levels and IgE/IgG4-ratios measured by microarray technique (ISAC) reflect the clinical response to subcutaneous immunotherapy (SIT). Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI), 29th Congress, 5–9 June 2010, London, UK. Allergy 2010;65.
- Luengo O, Labrador M, Guilarte M, Garriga T, Sala A, Cardona V. Structured assessment of component resolved diagnosis using a immunoassay platform for multiplex measurement of sIgE in multi-sensitised allergic patients. Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI), 29th Congress, 5–9 June 2010, London, UK. Allergy 2010;65.
- Passalacqua G, Melioli G, Bonifazi F, Bonini S, Maggi E, Senna G, et al. The additional values of microarray allergen assay in the management of polysensitized patients with respiratory allergy. Allergy 2013;68:1029-33. http://dx.doi.org/10.1111/all.12194.
- Heaps A, Carter S, Selwood C, Moody M, Unsworth J, Deacock S, et al. The utility of the ISAC allergen array in the investigation of idiopathic anaphylaxis. Clin Exp Immunol 2014;177:483-90. http://dx.doi.org/10.1111/cei.12334.
- Noimark L, Harnik E. ISAC allows greater understanding of the food allergy profile in children with moderate to severe eczema. Proceedings of the British Society for Allergy and Clinical Immunology, 2012 Annual Meeting, 2–4 July 2012, Nottingham, UK. Clin Exp Allergy 2012;42:1848-9.
- De Swert LF, Gadisseur R, Sjölander S, Raes M, Leus J, Van Hoeyveld E. Secondary soy allergy in children with birch pollen allergy may cause both chronic and acute symptoms. Pediatr Allergy Immunol 2012;23:117-23. http://dx.doi.org/10.1111/j.1399-3038.2011.01218.x.
- Alessandri C, Zennaro D, Scala E, Ferrara R, Bernardi ML, Santoro M, et al. Ovomucoid (Gal d 1) specific IgE detected by microarray system predict tolerability to boiled hen’s egg and an increased risk to progress to multiple environmental allergen sensitisation. Clin Exp Allergy 2012;42:441-50. http://dx.doi.org/10.1111/j.1365-2222.2011.03915.x.
- Cabrera-Freitag P, Goikoetxea MJ, Beorlegui C, Gamboa P, Gastaminza G, Fernandez-Benitez M, et al. Can component-based microarray replace fluorescent enzimoimmunoassay in the diagnosis of grass and cypress pollen allergy?. Clin Exp Allergy 2011;41:1440-6. http://dx.doi.org/10.1111/j.1365-2222.2011.03818.x.
- D’Urbano LE, Pellegrino K, Artesani MC, Donnanno S, Luciano R, Riccardi C, et al. Performance of a component-based allergen-microarray in the diagnosis of cow’s milk and hen’s egg allergy. Clin Exp Allergy 2010;40:1561-70. http://dx.doi.org/10.1111/j.1365-2222.2010.03568.x.
- Wöhrl S, Vigl K, Zehetmayer S, Hiller R, Jarisch R, Prinz M, et al. The performance of a component-based allergen-microarray in clinical practice. Allergy 2006;61:633-9. http://dx.doi.org/10.1111/j.1398-9995.2006.01078.x.
- Sokolova A, Costa AC, Barbosa MP, Trindade JC, Bento L, Pereira-Santos MC. [Immuno solid-phase allergen chip microarray for evaluation of cow’s milk proteins’ sensitisation profile]. Revista Portuguesa De Imunoalergologia 2009;17:325-42.
- Albarini M, Fiocchi A, Dahdah L, Melioli G, Mazzina O, Veglia F, et al. Food allergy and anaphylaxis-2061: clinical symptoms and molecular characterization of hazelnut allergy in Italian children. Proceedings of the 2nd WAO International Scientific Conference (WISC 2012), 6–9 December 2012, Hyderabad, India. World Allergy Organ 2013;6.
- Cabrera-Freitag P, Sanz M, Goikoetxea M, Javaloyes G, Berroa F, Ferrer M. Diagnostic reliability of the component-based allergen-microarray in grass, cypress and olive pollens allergy. Proceedings of the American Academy of Allergy, Asthma and Immunology, 2011 AAAAI Annual Meeting, 18–22 March 2011, San Francisco, CA, USA. J Allergy Clin Immunol 2011;127. http://dx.doi.org/10.1016/j.jaci.2010.12.480.
- Ott H, Baron JM, Heise R, Ocklenburg C, Stanzel S, Merk HF, et al. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy 2008;63:1521-8. http://dx.doi.org/10.1111/j.1398-9995.2008.01748.x.
- Abe H, Nukaga M, Niitsuma T, Odawara M. Allergen analysis by component-resolved diagnostics (CRD) in patients with oral allergy syndrome. J Tokyo Med Univ 2012;70:196-204.
- Stringari G, Tripodi S, Caffarelli C, Dondi A, Asero R, Di Rienzo Businco A, et al. The effect of component-resolved diagnosis on specific immunotherapy prescription in children with hay fever. J Allergy Clin Immunol 2014;134:75-81. http://dx.doi.org/10.1016/j.jaci.2014.01.042.
- Passalacqua G, Melioli G, Bonifazi F, Bonini S, Maggi E, Senna G, et al. The additional values of microarray allergen assay in the management of polysensitised patients with respiratory allergy. Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22 June 2013, Milan, Italy. Allergy 2013;68.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods For The Economic Evaluation Of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Hermansson LL, Mascialino B, Glaumann S, Borres M, Hubben G, Nilsson C. Is molecular allergology cost-effective and cost saving in children with suspected peanut allergy compared to double blind placebo controlled food challenge (DBPCFC) and skin prick test in US, Europe and Asia?. J Allergy Clin Immunol 2013;131. http://dx.doi.org/10.1016/j.jaci.2012.12.1530.
- Hermansson LL, Glaumann S, Borres M, Elenius M, Mascialino B, Hubben GA, et al. Cost-effectiveness of molecular IgE in vitro diagnostics (IVD) in children suspected with peanut allergy compared to double blind placebo controlled food challenge (DBPCFC) in EU, USA and Japan. Proceedings of the International Society for Pharmacoeconomics and Outcomes Research, 17th Annual International Meeting, ISPOR 2012, 2–6 June 2012, Washington, DC, USA. Value Health 2012;15. http://dx.doi.org/10.1016/j.jval.2012.03.373.
- Hermansson LL, Glaumann S, Mascialino B, Wang LL, Nilsson C. Cost-utility of molecular IgE in vitro diagnostics (IVD) in children suspected with peanut allergy compared to most used diagnostics in selected Asian markets. Proceedings of the International Society for Pharmacoeconomics and Outcomes Research, 5th Asia-Pacific Conference, 2–4 September 2012, Taipei, Taiwan. Value Health 2012;15. http://dx.doi.org/10.1016/j.jval.2012.08.223.
- Glaumann S, Hermansson LL, Mascialino B, Hubben G, Borres M, Nilsson C. Is molecular allergology cost-effective and cost saving in children with suspected peanut allergy compared to double blind placebo controlled food challenge (DBPCFC), open oral food challenge and skin prick test in Sweden? Proceedings of the Food Allergy and Anaphylaxis Meeting (FAAM 2013), 7–9 February 2013, Nice, France. Clin Transl Allergy 2013;3. http://dx.doi.org/10.1186/2045-7022-3-S3-P124.
- Mascialino B, Hermansson LL, Sastre J. Is the usage of molecular allergology diagnostics for allergen-specific immunotherapy indication in complex pollen areas cost-effective? Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22 June 2013, Milan, Italy. Allergy 2013;68:131-2.
- Mascialino B, Hermansson LL, Sastre J. The role of molecular allergology in allergen-specific immunotherapy adherence and patient quality of life in a complex pollen area: a simulation model [abstract 717]. Proceedings of the American Academy of Allergy, Asthma and Immunology (AAAAI 2013), 2013 Annual Meeting, 22–6 Feb 2013, San Antonio, TX, USA. J Allergy Clin Immunol 2013;131. http://dx.doi.org/10.1016/j.jaci.2012.12.1384.
- Hermansson LL, Mascialino B, Sastre J. Can molecular allergology improve allergen-specific immunotherapy adherence and patient quality of life in a complex pollen area? Proceedings of the International Society for Pharmacoeconomics and Outcomes Research, 15th Annual European Congress, 3–7 November 2012, Berlin, Germany. Value Health 2012;15. http://dx.doi.org/10.1016/j.jval.2012.08.913.
- Rodriguez-Ferran L, Cortes N, Cilveti R, Molina Francesc J, Rivera L, Olmo M. Screening respiratory allergy in children in primary care: which test should we use? Proceedings of the European Academy of Allergy and Clinical Immunology, 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66:198-9.
- Remenschneider AK, Scangas G, Meier JC, Gray ST, Holbrook EH, Gliklich RE, et al. EQ-5D-derived health utility values in patients undergoing surgery for chronic rhinosinusitis. Laryngoscope 2015;125:1056-61. http://dx.doi.org/10.1002/lary.25054.
- Poole CD, Bannister CA, Andreasen JN, Andersen JS, Currie CJ. Estimation of health-related utility (EQ-5D index) in subjects with seasonal allergic rhinoconjunctivitis to evaluate health gain associated with sublingual grass allergen immunotherapy. Health Qual Life Outcomes 2014;12. http://dx.doi.org/10.1186/1477-7525-12-99.
- Canonica GW, Poulsen PB, Vestenbaek U. Cost-effectiveness of GRAZAX for prevention of grass pollen induced rhinoconjunctivitis in Southern Europe. Respir Med 2007;101:1885-94. http://dx.doi.org/10.1016/j.rmed.2007.05.003.
- Bachert C, Vestenbaek U, Christensen J, Griffiths UK, Poulsen PB. Cost-effectiveness of grass allergen tablet (GRAZAX) for the prevention of seasonal grass pollen induced rhinoconjunctivitis: a Northern European perspective. Clin Exp Allergy 2007;37:772-9. http://dx.doi.org/10.1111/j.1365-2222.2007.02706.x.
- Petersen KD, Kronborg C, Larsen JN, Dahl R, Gyrd-Hansen D. Patient related outcomes in a real life prospective follow up study: allergen immunotherapy increase quality of life and reduce sick days. World Allergy Organ J 2013;6. http://dx.doi.org/10.1186/1939-4551-6-15.
- Moberg C, Alderling M, Meding B. Hand eczema and quality of life: a population-based study. Br J Dermatol 2009;161:397-403. http://dx.doi.org/10.1111/j.1365-2133.2009.09099.x.
- Pitt AD, Smith AF, Lindsell L, Voon LW, Rose PW, Bron AJ. Economic and quality-of-life impact of seasonal allergic conjunctivitis in Oxfordshire. Ophthalmic Epidemiol 2004;11:17-33. http://dx.doi.org/10.1076/opep.11.1.17.26437.
- Smith AF, Pitt AD, Rodruiguez AE, Alio JL, Marti N, Teus M, et al. The economic and quality of life impact of seasonal allergic conjunctivitis in a Spanish setting. Ophthalmic Epidemiol 2005;12:233-42. http://dx.doi.org/10.1080/09286580590967781.
- Wasserfallen JB, Gold K, Schulman KA, Baraniuk JN. Item responsiveness of a rhinitis and asthma symptom score during a pollen season. J Asthma 1999;36:459-65. http://dx.doi.org/10.3109/02770909909087288.
- Egert-Schmidt AM, Henrichs T, Fielenbach T, Thum-Oltmer S. Subcutaneous allergen immunotherapy with high-dose hypoallergenic pollen preparations improves quality of life and is effective and safe in real life. Allergy 2014;69.
- Petersen KD, Kronborg C, Gyrd-Hansen D, Dahl R, Larsen JN, Linneberg A. Characteristics of patients receiving allergy vaccination: to which extent do socio-economic factors play a role?. Eur J Public Health 2011;21:323-8. http://dx.doi.org/10.1093/eurpub/ckq063.
- Covaciu C, Bergström A, Lind T, Svartengren M, Kull I. Childhood allergies affect health-related quality of life. J Asthma 2013;50:522-8. http://dx.doi.org/10.3109/02770903.2013.789057.
- Mittmann N, Trakas K, Risebrough N, Liu BA. Utility scores for chronic conditions in a community-dwelling population. PharmacoEconomics 1999;15:369-76. http://dx.doi.org/10.2165/00019053-199915040-00004.
- Stevens KJ, Brazier JE, McKenna SP, Doward LC, Cork MJ. The development of a preference-based measure of health in children with atopic dermatitis. Br J Dermatol 2005;153:372-7. http://dx.doi.org/10.1111/j.1365-2133.2005.06736.x.
- Friedman JY, Reed SD, Weinfurt KP, Kahler KH, Walter EB, Schulman KA. Parents’ reported preference scores for childhood atopic dermatitis disease states. BMC Pediatr 2004;4. http://dx.doi.org/10.1186/1471-2431-4-21.
- Lundberg L, Johannesson M, Silverdahl M, Hermansson C, Lindberg M. Quality of life, health-state utilities and willingness to pay in patients with psoriasis and atopic eczema. Br J Dermatol 1999;141:1067-75. http://dx.doi.org/10.1046/j.1365-2133.1999.03207.x.
- Pickard AS, Tawk R, Shaw JW. The effect of chronic conditions on stated preferences for health. Eur J Health Econ 2013;14:697-702. http://dx.doi.org/10.1007/s10198-012-0421-8.
- Armstrong N, Wolff R, van Mastrigt G, Martinez N, Hernandez AV, Misso K, et al. A systematic review and cost-effectiveness analysis of specialist services and adrenaline auto-injectors in anaphylaxis. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17170.
- Meadows A, Kaambwa B, Novielli N, Huissoon A, Fry-Smith A, Meads C, et al. A systematic review and economic evaluation of subcutaneous and sublingual allergen immunotherapy in adults and children with seasonal allergic rhinitis. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17270.
- Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy 1991;21:77-83. http://dx.doi.org/10.1111/j.1365-2222.1991.tb00807.x.
- Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al. The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9290.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-16. http://dx.doi.org/10.1111/j.1365-2230.1994.tb01167.x.
- Caffarelli C, Dondi A, Povesi Dascola C, Ricci G. Skin prick test to foods in childhood atopic eczema: pros and cons. Ital J Pediatr 2013;39. http://dx.doi.org/10.1186/1824-7288-39-48.
- NHS Choices . Allergies – Diagnosis 2014. www.nhs.uk/Conditions/Allergies/Pages/Diagnosis.aspx (accessed 5 August 2015).
- Food Allergy in Children and Young People: Costing Report. London: NICE; 2011.
- Mehl A, Niggemann B, Keil T, Wahn U, Beyer K. Skin prick test and specific serum IgE in the diagnostic evaluation of suspected cow’s milk and hen’s egg allergy in children: does one replace the other?. Clin Exp Allergy 2012;42:1266-72. http://dx.doi.org/10.1111/j.1365-2222.2012.04046.x.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: University of Kent; 2014.
- NHS Reference Costs 2013 to 2014. London: DH; 2015.
- Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health 2012;15:835-42. http://dx.doi.org/10.1016/j.jval.2012.04.014.
- Frontline Biochip Technology – Features and Benefits in Allergy Diagnostics. Uppsala: Thermo Scientific; 2012.
- Pedrosa M, Boyano-Martinez T, Garcia-Ara MC, Caballero T, Quirce S. Peanut seed storage proteins are responsible for clinical reactivity in Spanish peanut-allergic children. Pediatr Allergy Immunol 2012;23:654-9. http://dx.doi.org/10.1111/j.1399-3038.2012.01337.x.
- Pascal M, Vazquez-Ortiz M, Folque MM, Jimenez-Feijoo R, Lozano J, Dominguez O, et al. Asymptomatic LTP sensitisation is common in plant-food allergic children from the Northeast of Spain. Allergol Immunopathol 2016;44:351-8. http://dx.doi.org/10.1016/j.aller.2015.10.003.
- Sanz M, Cabrera-Freitag P, Goikoetxea M, Fernandez-Benitez M, Gastaminza G, Javaloyes G, et al. Sensitivity and specificity for three mediterranean pollen allergen components in ISAC microarray CRD103 technology. Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI), 29th Congress, 5–9 June 2010, London, UK. Allergy 2010;65.
- Ackerbauer D, Bublin M, Radauer C, Varga EM, Hafner C, Ebner C, et al. Component-resolved IgE profiles in Austrian patients with a convincing history of peanut allergy. Int Arch Allergy Immunol 2015;166:13-24. http://dx.doi.org/10.1159/000371422.
- Acosta Rivera M, Alonso Diaz De Durana M, Moro Moro M, Rosado Ingelmo A, Tejedor Alonso M, Gajate Fernandez P, et al. Component resolved diagnosis in children with nut allergy. Proceedings of the European Academy of Allergy and Clinical Immunology, 31st Congress, 16–20 June 2012, Geneva, Switzerland. Allergy 2012;67.
- Alessandri C, Zennaro D, Ferrara R, Bernardi M, Santoro M, Scala E, et al. Predicting tolerability to boiled egg by means of hen’s egg allergen IgE detection by ISAC. Proceedings of the European Academy of Allergy and Clinical Immunology, 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66.
- Alonso MD, Goikoetxea MJ, Martinez-Aranguren R, Feo F, Gomez F, Moya C, et al. Prevalence of nut allergy in Spain and diagnostic capacity of specific IgE by microarray. Proceedings of the European Academy of Allergy and Clinical Immunology, 33rd Congress, 7–11 June 2014, Copenhagen, Denmark. Allergy 2014;69.
- Alvarado MI, De La Torre F, Jimeno L, Rivas B, Lazaro MJ, Barber D. Sensitisation profiles and clinical relevance of food allergy in patients sensitised to profilin. Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22 June 2013, Milan, Italy. Allergy 2013;68.
- Antonicelli L, Massaccesi C, Braschi MC, Cinti B, Bilò MB, Bonifazi F. Component resolved diagnosis in real life: the risk assessment of food allergy using microarray-based immunoassay. Eur Ann Allergy Clin Immunol 2014;46:30-4.
- Araujo L, Thom C, Neto HJC, Palazzo P, Liso M, Mari A, et al. Skin prick test reactivity compared to serum specific IgE by ISAC in patients with rhinitis. Proceedings of the 22nd World Allergy Congress, 4–8 November 2011, Cancun, Mexico. World Allergy Organiza 2012;5. http://dx.doi.org/10.1097/01.wox.0000412135.43307.21.
- Asero R, Bellotto E, Ghiani A, Aina R, Villalta D, Citterio S. Concomitant sensitization to ragweed and mugwort pollen: who is who in clinical allergy?. Ann Allergy Asthma Immunol 2014;113:307-13. http://dx.doi.org/10.1016/j.anai.2014.06.009.
- Bauermeister K, Ballmer-Weber BK, Bublin M, Fritsche P, Hanschmann KM, Hoffmann-Sommergruber K, et al. Assessment of component-resolved in vitro diagnosis of celeriac allergy. J Allergy Clin Immunol 2009;124:1273-81. http://dx.doi.org/10.1016/j.jaci.2009.07.033.
- Berneder M, Bublin M, Hoffmann-Sommergruber K, Hawranek T, Lang R. Allergen chip diagnosis for soy-allergic patients: Gly m 4 as a marker for severe food-allergic reactions to soy. Int Arch Allergy Immunol 2013;161:229-33. http://dx.doi.org/10.1159/000345970.
- Blankestijn MA, Klemans RJB, Otten HG, Rockmann H, Bruijnzeel-Koomen CAFM, Knol EF, et al. Evaluation of patients with food allergy with the help of the ImmunoCAP ISAC. Ned Tijdschrift Dermatol Venereol 2014;24:259-63.
- Bokanovic D, Aberer W, Hemmer W, Heinemann A, Komericki P, Scheffel J, et al. Determination of sIgE to rPhl p 1 is sufficient to diagnose grass pollen allergy. Allergy 2013;68:1403-9. http://dx.doi.org/10.1111/all.12263.
- Bonini S, Bonifazi F, Maggi E, Mascialino B, Melioli G, Mus-sap M, et al. Allergen component resolved diagnostics in multi-sensitized patients with respiratory symptoms. Proceedings of the American Academy of Allergy, Asthma and Immunology (AAAAI), 2010 Annual Meeting, 26 February–2 March 2010, New Orleans, LA, USA. J Allergy Clin Immunol 2010;125. http://dx.doi.org/10.1016/j.jaci.2009.12.536.
- Bonini S, Bonifazi F, Maggi E, Mascialino B, Melioli G, Mussap M, et al. Array-based molecular diagnostics in multi-sensitised adults with respiratory symptoms. Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI), 29th Congress, 5–9 June 2010, London, UK. Allergy 2010;65.
- Bonini M, Marcomini L, Gramiccioni C, Tranquilli C, Melioli G, Canonica GW, et al. Microarray evaluation of specific IgE to allergen components in elite athletes. Allergy 2012;67:1557-64. http://dx.doi.org/10.1111/all.12029.
- Brans R, Sauer I, Czaja K, Pfützner W, Merk HF. Microarray-based detection of specific IgE against recombinant ω-5-gliadin in suspected wheat-dependent exercise-induced anaphylaxis. Eur J Dermatol 2012;22:358-62.
- Cabrera-Freitag P, Goikoetxea MJ, Gamboa PM, Martínez-Aranguren R, Beorlegui C, Fernández J, et al. A study of the variability of the in vitro component-based microarray ISAC CDR 103 technique. J Investig Allergol Clin Immunol 2011;21:414-15.
- Caimmi S, Caimmi D, Marseglia A, Labo E, Bulzomi P, Giunta V, et al. Microarray test and peanut allergy. Proceedings of the European Academy of Allergy and Clinical Immunology, 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66.
- Caimmi S, Caimmi D, Cattaneo F, Artusio L, Brambilla I, Giunta V, et al. The use of microarray in the diagnosis of allergy in pediatrics. Proceedings of the European Academy of Allergy and Clinical Immunology, 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66:332-3.
- Caimmi S, De Amici M, Trovamala V, Caimmi D, Testa G, Marseglia A, et al. Usefulness of molecular diagnosis. Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22 June 2013, Milan, Italy. Allergy 2013;68:311-12.
- Carter S, Heaps A, Boswijk K, Jolles S, Kaminski E. Identification of clinically relevant allergens using the Phadia ISAC microarray in patients with idiopathic anaphylaxis. Proceedings of the British Society for Allergy and Clinical Immunology, 2012 Annual Meeting, 2–4 July 2012, Nottingham, UK. Clin Exp Allergy 2012;42:1829-30.
- Cavagni G, D’Urbano L, Donnanno S, Trimarco G, Misirocchi E, Artesani M, et al. Performance of a component-based allergen microarray in children with cow’s milk and egg allergy. Proceedings of the American Academy of Allergy, Asthma and Immunology (AAAAI), 2009 Annual Meeting, 13–17 March 2009, Washington, DC, USA. J Allergy Clin Immunol 2009;123. http://dx.doi.org/10.1016/j.jaci.2008.12.1059.
- Cavagni G, D’Urbano L, Pellegrino K, Luciano R, Mancini S, Riccardi C, et al. Utility in clinical practice of a component-based allergen-microarray in food allergy practice. Proceedings of the American College of Allergy, Asthma and Immunology, 2010 Annual Meeting, 11–16 November 2010, Phoenix, Arizona, USA. Ann Allergy Asthma Immunol 2010;105.
- Chambel M, Paiva M, Prates S, Loureiro V, Leiria Pinto P. Polysensitisation to pollens analyzed and reinterpreted with the microarray technique. Proceedings of the European Academy of Allergy and Clinical Immunology, 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66.
- Chambel M, Paiva M, Prates S, Loureiro V, Pinto PL. Polysensitization to pollens analyzed and re-interpreted with immunoCAP ISAC. Revista Portuguesa De Imunoalergologia 2012;20:211-19.
- Charalambous M, Teoh L, Makwana N. Is component-resolved diagnostics and ImmunoCAP ISAC assay useful in the management of paediatric allergy patients in a tertiary clinic? Proceedings of the European Academy of Allergy and Clinical Immunology, 33rd Congress, 7–11 June 2014, Copenhagen, Denmark. Allergy 2014;69:176-7.
- Chevallier M, Chabbert A, Juchet A, Didier A. Evaluation of the ISAC biopuce in children with suspected food allergy. Rev Fr Allergol 2013;53:405-10. http://dx.doi.org/10.1016/j.reval.2013.03.005.
- Chia IP, Yap GC, Lee BW, Van Bever H, Pei-Shi LS, Gerez IFA, et al. Food allergy and anaphylaxis-2044: component resolved allergen sensitzation profiles in shrimp allergy in the tropics. Proceedings of the World Allergy Organization, 2nd WAO International Scientific Conference (WISC 2012), 6–9 December 2012, Hyderabad, India. World Allergy Organ J 2013;6. http://dx.doi.org/10.1186/1939-4551-6-S1-P129.
- Cho JH, Suh JD, Kim JK, Hong SC, Park IH, Lee HM. Correlation between skin-prick testing, individual specific IgE tests, and a multiallergen IgE assay for allergy detection in patients with chronic rhinitis. Am J Rhinol Allergy 2014;28:388-91. http://dx.doi.org/10.2500/ajra.2014.28.4074.
- Choi JS, Roh JY, Lee JR. Clinical availability of component-resolved diagnosis using microarray technology in atopic dermatitis. Ann Dermatol 2014;26:437-46. http://dx.doi.org/10.5021/ad.2014.26.4.437.
- Choi J, Lee J, Kim J, Yoon C, Ryu H, Baek J, et al. Diagnostic efficacy of microarray-based specific IgE assay in atopic dermatitis. Proceedings of the Korean Dermatological Association, 3rd Eastern Asia Dermatology Congress, 24–6 Sept 2014, Jeju, South Korea. J Dermatol 2014;41.
- De Amici M, Trovamala V, Caimmi S, Licari A, Marseglia A, Testa G, et al. Clinical comparison between previous and new ISAC test kit. Proceedings of the European Academy of Allergy and Clinical Immunology, 33rd Congress, 7–11 June 2014, Copenhagen, Denmark. Allergy 2014;69.
- De Boer D, Bons J, Huisinga D, Michels-Mengels V, Menheere P. Analytical validation of immuno solid-phase allergen chip; limit of detection vs limit of reporting. Proceedings of the European Academy of Allergy and Clinical Immunology, 31st Congress, 16–20 June 2012, Geneva, Switzerland. Allergy 2012;67.
- De Boer D, Bons JAP, Nieuwhof CM, Menheere P. Component resolved diagnosis: recording of discrepancies between single and multiplex IgE analysis. Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22–26 June 2013, Milan, Italy. Allergy 2013;68:305-6.
- De Knop KJ, Verweij MM, Grimmelikhuijsen M, Philipse E, Hagendorens MM, Bridts CH, et al. Age-related sensitization profiles for hazelnut (Corylus avellana) in a birch-endemic region. Pediatr Allergy Immunol 2011;22:e139-49. http://dx.doi.org/10.1111/j.1399-3038.2011.01112.x.
- Doyen V, Valsamis J, Corazza F, Michel O. Omalizumab did not modify component resolved diagnosis in severe asthma. Proceedings of the European Academy of Allergy and Clinical Immunology, 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66.
- Ebo DG, Hagendorens MM, De Knop KJ, Verweij MM, Bridts CH, De Clerck LS, et al. Component-resolved diagnosis from latex allergy by microarray. Clin Exp Allergy 2010;40:348-58. http://dx.doi.org/10.1111/j.1365-2222.2009.03370.x.
- Ebo DG, Bridts CH, Verweij MM, De Knop KJ, Hagendorens MM, De Clerck LS, et al. Sensitization profiles in birch pollen-allergic patients with and without oral allergy syndrome to apple: lessons from multiplexed component-resolved allergy diagnosis. Clin Exp Allergy 2010;40:339-47. http://dx.doi.org/10.1111/j.1365-2222.2009.03345.x.
- Eller E, Bindslev-Jensen C. Clinical value of component-resolved diagnostics in peanut-allergic patients. Allergy 2013;68:190-4. http://dx.doi.org/10.1111/all.12075.
- Fernandez J, Flores E, Goicoetxea M, Cabrera P, Gonzalez P, Cervera L. Comparison of molecular allergens by two methods in allergy patients of a Mediterranean area. Proceedings of the European Academy of Allergy and Clinical Immunology, 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66.
- Flores E, Soriano V, Esteban A, Gonzalez P, Cueva B, Fernandez FJ. Comparison of specific IgE and molecular allergens in patients of the Mediterranean area. Proceedings of the European Academy of Allergy and Clinical Immunology, 33rd Congress, 7–11 June 2014, Copenhagen, Denmark. Allergy 2014;69.
- Fung I, Kim JS, Spergel JM. Microarray component testing in association with IgE mediated food allergy: a retrospective analysis assessing performance of multiple versus individual components. Proceedings of the American College of Allergy, Asthma and Immunology, 2012 Annual Meeting, 8–13 November 2012, Anaheim, CA, USA. Ann Allergy Asthma Immunol 2012;109.
- Gadisseur R, Chapelle J, Cavalier E. A new diagnostic tool for in vitro allergy. Proceedings of the European Academy of Allergy and Clinical Immunology, 28th Congress, 6–10 June 2009, Warszawa, Poland. Allergy 2009;64:30-1.
- Gadisseur R, Chapelle JP, Cavalier E. A new tool in the field of in-vitro diagnosis of allergy: preliminary results in the comparison of ImmunoCAP © 250 with the ImmunoCAP © ISAC. Clin Chem Lab Med 2011;49:277-80. http://dx.doi.org/10.1515/CCLM.2011.052.
- Gadisseur R, Dezfoulian B, Chapelle J, Cavalier E. Allergen microarrays and their utility in routine clinical practice. Proceedings of the European Academy of Allergy and Clinical Immunology, 31st Congress, 16–20 June 2012, Geneva, Switzerland. Allergy 2012;67:31-2.
- Garriga Baraut T, Olga L, Labrador M, Palazzo P, Guilarte M, Sala A, et al. RECORD study: recombinant allergens in diagnostic resolution in poly-pollen sensitised patients from Barcelona. Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI), 29th Congress, 5–9 June 2010, London, UK. Allergy 2010;65.
- Goikoetxea MJ, Sanz ML, Garcia BE, Mayorga C, Longo N, Gamboa PM, et al. Recommendations for the use of in vitro methods to detect specific immunoglobulin E: are they comparable?. J Investig Allergol Clin Immunol 2013;23:448-54.
- Goikoetxea MJ, D’Amelio C, Martínez R, Alonso MD, Alvarado MI, Bartra J, et al. Performance of component resolved microarray diagnosis in nuts allergy. Presented at Food Allergy and Anaphylaxis Meeting 2014, 9–11 October 2014, Dublin, Ireland. Clin Transl Allergy 2015;5. http://dx.doi.org/10.1186/2045-7022-5-S3-P33.
- Hoffmann HJ, Schmid J, Wurtzen PA, Dahl R. Increased allergen-specific IgG4 levels are linked to suppressed IgE titers when analysed by ISAC but not by immunocap. Presented at 5th International Symposium on Molecular Allergology (ISMA 2013), 6–7 December 2013, Vienna, Austria. Clin Transl Allergy 2014;4. http://dx.doi.org/10.1186/2045-7022-4-S2-O20.
- Hong X, Caruso DM, Birne JS, Schanta JL, Kumar R, Liu X, et al. Measurement of IgE antibodies to peanut component proteins distinguishes clinical peanut allergy from asymptomatic sensitization. Proceedings of the American Academy of Allergy, Asthma and Immunology (AAAAI), AAAAI Annual Meeting 2011, 18–22 March 2011, San Francisco, CA, USA. J Allergy Clin Immunol 2011;127. http://dx.doi.org/10.1016/j.jaci.2010.12.724.
- Hong X, Caruso D, Kumar R, Liu R, Liu X, Wang G, et al. IgE, but not IgG4, antibodies to Ara h 2 distinguish peanut allergy from asymptomatic peanut sensitization. Allergy 2012;67:1538-46. http://dx.doi.org/10.1111/all.12047.
- Javaloyes G, Goikoetxea MJ, García Núñez I, Sanz ML, Blanca M, Scheurer S, et al. Performance of different in vitro techniques in the molecular diagnosis of peanut allergy. J Investig Allergol Clin Immunol 2012;22:508-13.
- Jung JH. Utility of component-based allergen chip (ImmunoCAP ISAC) in diagnosis of allergic rhinitis. Presented at 22nd World Allergy Congress, 4–8 November 2011, Cancun, Mexico. World Allergy Organ J 2012;5:S196-7.
- Kattan JD, Gimenez G, Lieberman J, Sampson HA. The use of the ISAC microarray platform in food allergic. Proceedings of the American Academy of Allergy, Asthma and Immunology, 2013 Annual Meeting (AAAAI 2013), 22–6 February 2013, San Antonio, TX, USA. J Allergy Clin Immunol 2013;131.
- Kattan JD, Gimenez G, Lieberman JA, Sampson HA. The use of the ISAC microarray platform in food allergic patients. Proceedings of the American Academy of Allergy Asthma and Immunology (AAAAI), Annual Meeting, 28 February–4 March 2014, San Diego, CA, USA. J Allergy Clin Immunol 2014;133. http://dx.doi.org/10.1016/j.jaci.2013.12.407.
- Kim ST, Jung JH, Kang IG. Comparison of component-resolved diagnosis by using allergen microarray with the conventional tests in allergic rhinitis patients. Proceedings of the American Academy of Allergy, Asthma and Immunology, 2015 Annual Meeting (AAAAI 2015), 20–24 February 2015, Houston, TX, USA. J Allergy Clin Immunol 2015;135. http://dx.doi.org/10.1016/j.jaci.2014.12.1896.
- Klemans RJ, Liu X, Knulst AC, Knol MJ, Gmelig-Meyling F, Borst E, et al. IgE binding to peanut components by four different techniques: Ara h 2 is the most relevant in peanut allergic children and adults. Clin Exp Allergy 2013;43:967-74. http://dx.doi.org/10.1111/cea.12136.
- Klemans RJ, Knol EF, Bruijnzeel-Koomen CA, Knulst AC. The diagnostic accuracy of specific IgE to Ara h 6 in adults is as good as Ara h 2. Allergy 2014;69:1112-14. http://dx.doi.org/10.1111/all.12424.
- Konradsen J, Nordlund B, Winkler A, Baldracchini F, Mazzoleni G, Önell A, et al. Evaluation of Microtest allergy system compared to three established diagnostic methods. Allergy 2015;70.
- Lee JM, Koo NH, Jeong K, Jeon SA, Lee SY. Clinical and immunological characteristics in children with walnut allergy. Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22 June 2013, Milan, Italy. Allergy 2013;68.
- Liso M, Scala E, Palazzo P, Santoro M, Zennaro D, Ferrara R, et al. Total IgE detected on a microarray-based system using spotted omalizumab as anti-IgE. Proceedings of the European Academy of Allergy and Clinical Immunology, 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66.
- Liu X, Knulst A, Knol M, Gmelig-Meyling F, Borst E, Pasmans S, et al. Component-resolved diagnostics with Ara h 2 predictive of peanut allergy in children and adults. Proceedings of the European Academy of Allergy and Clinical Immunology, 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66:77-8.
- Lizaso MT, García BE, Tabar AI, Lasa E, Echechipía S, Alvarez MJ, et al. Comparison of conventional and component-resolved diagnostics by two different methods (Advia-Centaur/Microarray-ISAC) in pollen allergy. Ann Allergy Asthma Immunol 2011;107:35-41. http://dx.doi.org/10.1016/j.anai.2011.03.017.
- Lohman MJ, Chapman MD, King EM. Simultaneous detection of total and allergen-specific immunoglobulin-E in human plasma using multiplex array technology. Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22 June 2013, Milan, Italy. Allergy 2013;68.
- Luengo O, Labrador M, Guilarte M, Garriga T, Sala A, Cardona V. Component resolved diagnosis using an immunoassay platform for multiplex measurement of sIgE in multi-sensitised allergic patients. Proceedings of the American Academy of Allergy, Asthma and Immunology, 2011 AAAAI Annual Meeting, 18–22 March 2011, San Francisco, CA, USA. J Allergy Clin Immunol 2011;127.
- Mailhol C, Apoil PA, Montaigne E, Schwartz C, Blancher A, Didier A. Plant food allergy exploration in adults: micro arrayed-based component-resolved diagnosis. Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22 June 2013, Milan, Italy. Allergy 2013;68.
- Martinez-Aranguren RM, Lizaso MT, Goikoetxea MJ, Garcia BE, Cabrera-Freitag P, Trellez O, et al. Reproducibility analysis of multiplex IgE analysis. Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22 June 2013, Milan, Italy. Allergy 2013;68:306-7.
- Martinez-Aranguren RM, Goikoetxea MJ, Terrados S, Garcia BE, Blanca-Lopez N, Parra A, et al. Agreement between skin tests and specific IgE detection by components through conventional technique and microarray against panallergens and cross reactive carbohydrate determinants. Proceedings of the European Academy of Allergy and Clinical Immunology, 33rd Congress, 7–11 June 2014, Copenhagen, Denmark. Allergy 2014;69:7-8.
- Martínez-Aranguren RM, Gamboa PM, García-Lirio E, Asturias J, Goikoetxea MJ, Sanz ML. In vivo and in vitro testing with rAni s 1 can facilitate diagnosis of Anisakis simplex allergy. J Investig Allergol Clin Immunol 2014;24:431-8.
- Martínez-Aranguren R, Lizaso MT, Goikoetxea MJ, García BE, Cabrera-Freitag P, Trellez O, et al. Is the determination of specific IgE against components using ISAC 112 a reproducible technique?. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0088394.
- Melioli G, Bonifazi F, Bonini S, Maggi E, Mussap M, Passalacqua G, et al. The ImmunoCAP ISAC molecular allergology approach in adult multi-sensitized Italian patients with respiratory symptoms. Clin Biochem 2011;44:1005-11. http://dx.doi.org/10.1016/j.clinbiochem.2011.05.007.
- Confidential Information Has Been Removed n.d.
- Murng SHK, Egner W, Shrimpton A, Sargur R, Cowley D, Lees K. A study of ISAC (Immuno Solid-phase Allergen Chip) assay performance and interpretation. Proceedings of the British Society for Allergy and Clinical Immunology, 2011 Annual Meeting (BSACI 2011), 11–13 July 2011, Nottingham, UK. Clin Exp Allergy 2011;41.
- Murng SHK, Egner W, Shrimpton A, Sargur R. Validation of immunoCAP ISAC assay performance. Proceedings of the British Society for Immunology, Annual Congress 2011, 2–8 December 2011, Liverpool, UK. Immunology 2011;135.
- Nanda MK, Assa’ad AH, Khoury J, Lierl MB. What is the role of component IgE analysis by immunocap and microarray compared to food-specific IgE in peanut and egg allergy? Proceedings of the American Academy of Allergy, Asthma and Immunology, 2015 Annual Meeting (AAAAI 2015), 20–4 February 2015, Houston, TX, USA. J Allergy Clin Immunol 2015;135. http://dx.doi.org/10.1016/j.jaci.2014.12.1032.
- Nicaise-Rolland P, Aubier M, Collin E, Grootenboer-Mignot S, Neukirch C, Chollet-Martin S. Microarray-based IgE detection improves the diagnosis of allergic asthma. Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI), 29th Congress, 5–9 June 2010, London, UK. Allergy 2010;65:95-6.
- Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol 2010;125:191-7.e1.
- Nystrand M, Ghasemzadeh N, Harlin A, Elfverson G, Onell A, Kober A. Evaluation of a new version of a microarray immunoassay for detection of allergen component specific IgE antibodies. Proceedings of the European Academy of Allergy and Clinical Immunology, 31st Congress, 16–20 June 2012, Geneva, Switzerland. Allergy 2012;67.
- Olivieri M, Pahr S, Palazzo P, Biscardo C, Malerba G, Xumerle L, et al. IgE profiling using new wheat-derived allergens and ISAC 103 microarray in bakers with occupational allergic respiratory disease. Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI), 29th Congress, 5–9 June 2010, London, UK. Allergy 2010;65:57-8.
- Olivieri M, Biscardo CA, Palazzo P, Pahr S, Malerba G, Ferrara R, et al. Wheat IgE profiling and wheat IgE levels in bakers with allergic occupational phenotypes. Occup Environ Med 2013;70:617-22. http://dx.doi.org/10.1136/oemed-2012-101112.
- Onell A, Hjälle L, Borres MP. Exploring the temporal development of childhood IgE profiles to allergen components. Clin Transl Allergy 2012;2. http://dx.doi.org/10.1186/2045-7022-2-24.
- Ott H, Fölster-Holst R, Merk HF, Baron JM. Allergen microarrays: a novel tool for high-resolution IgE profiling in adults with atopic dermatitis. Eur J Dermatol 2010;20:54-61.
- Paes M, Carvalho F, Trindade M, Pereira Barbosa M, Pereira Santos M. Peach allergy: sensitisation profile by component resolved diagnosis microarray. Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI), 29th Congress, 5–9 June 2010, London, UK. Allergy 2010;65.
- Palomba A, Maccari M, Baldracchini F, Mazzoleni G, Crisanti A, Marcucci F. External Evaluation of Microtest Performance. PDF provided by company. Microtest Dx Technical Bulletin 2013;1:1-13.
- Palomba A, Maccari M, Baldracchini F, Mazzoleni G, Crisanti A, Marcucci F. Evaluation of a new microarray based system for allergy testing. Proceedings of the European Academy of Allergy and Clinical Immunology, 33rd Congress, 7–11 June 2014, Copenhagen, Denmark. Allergy 2014;69.
- Patelis A, Gunnbjörnsdottir M, Malinovschi A, Matsson P, Onell A, Högman M, et al. Population-based study of multiplexed IgE sensitization in relation to asthma, exhaled nitric oxide, and bronchial responsiveness. J Allergy Clin Immunol 2012;130:397-402. http://dx.doi.org/10.1016/j.jaci.2012.03.046.
- Pomponi D, Di Zenzo G, Zennaro D, Calabresi V, Eming R, Zuzzi S, et al. Detection of IgG and IgE reactivity to BP180 using the ISAC® microarray system. Br J Dermatol 2013;168:1205-14. http://dx.doi.org/10.1111/bjd.12161.
- Raulf BV. Are the results of component-resolved allergy diagnosis able to influence the prescription of specific immunotherapy in children with hay fever?. Allergologie 2014;37:419-21. http://dx.doi.org/10.5414/ALX01694.
- Röckmann H, van Geel MJ, Knulst AC, Huiskes J, Bruijnzeel-Koomen CA, de Bruin-Weller MS. Food allergen sensitization pattern in adults in relation to severity of atopic dermatitis. Clin Transl Allergy 2014;4. http://dx.doi.org/10.1186/2045-7022-4-9.
- Romano A, Scala E, Rumi G, Gaeta F, Caruso C, Alonzi C, et al. Lipid transfer proteins: the most frequent sensitizer in Italian subjects with food-dependent exercise-induced anaphylaxis. Clin Exp Allergy 2012;42:1643-53. http://dx.doi.org/10.1111/cea.12011.
- Sanchez-Lopez J, Pascal M, Gomez-Casado C, Munoz-Cano R, Rueda M, Vilella R, et al. Sensitisation to Act d 2 in patients allergic to Alternaria alternata: an epiphenomenon without clinical significance? Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22 June 2013, Milan, Italy. Allergy 2013;68.
- Santos ACS, Bardini GA, Santos KS, Barbosa MCR, Santos ABR, Arruda LK. IgE cross-reactivity to tropomyosins among patients with asthma and/or rhinitis from Brazil: analysis by component-based Allergen microarray. Proceedings of the American Academy of Allergy, Asthma and Immunology, 2011 AAAAI Annual Meeting, 18–22 March 2011, San Francisco, CA, USA. J Allergy Clin Immunol 2011;127.
- Santosa A, Andiappan AK, Rotzschke O, Wong HC, Chang A, Bigliardi-Qi M, et al. Evaluation of the applicability of the Immuno-solid-phase allergen chip (ISAC) assay in atopic patients in Singapore. Clin Transl Allergy 2015;5. http://dx.doi.org/10.1186/s13601-015-0053-z.
- Sastre J, Rodriguez F, Campo P, Laffont E, Marin A, Alonso-Diaz MD. Molecular diagnosis may help to predict adverse reactions to immunotherapy in Spanish grass allergic patients. Proceedings of the European Academy of Allergy and Clinical Immunology, 33rd Congress, 7–11 June 2014, Copenhagen, Denmark. Allergy 2014;69.
- Scala E, Alessandri C, Bernardi M, Ferrara R, Zennaro D, Palazzo P, et al. The lesson from Anisakis simplex sensitisation: the need for a molecular approach. Proceedings of the European Academy of Allergy and Clinical Immunology, 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66:388-9.
- Scala E, Alessandri C, Palazzo P, Pomponi D, Liso M, Bernardi M, et al. Allergenic molecule-based microarray-based allergy diagnosis: the second ‘real life’ study on a cohort of 23559 subjects. Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI), 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66.
- Schuler S, Ferrari G, Schmid-Grendelmeier P, Harr T. Microarray-based component-resolved diagnosis of latex allergy: isolated IgE-mediated sensitization to latexprofilin Hev b8 may act as confounder. Clin Transl Allergy 2013;3. http://dx.doi.org/10.1186/2045-7022-3-11.
- Seyfarth F, Hipler UC, Schliemann S, Elsner P. Detection of latex sensitizations using CAST, Western Blot, ImmunoCAP (R), and ISAC (R). Proceedings of der Deutschen Dermatologischen Gesellschaft, 46. Tagung Vereinigung Deutschsprachiger Dermatologen e. V, 30 March–2 April 2011, Dresden, Germany. J Dtsch Dermatol Ges 2011;9.
- Seyfarth F, Schliemann S, Wiegand C, Hipler U, Elsner P. Diagnosing latex sensitizations with recombinant latex allergens: the Immunosolid-phase allergen chip (ISAC). Proceedings of the Arbeitsgemeinschaft Dermatologische Forschung, 38th Annual Meeting, ADF 2011, 17–19 February 2011, Tuebingen, Germany. Exp Dermatol 2011;20.
- Seyfarth F, Schliemann S, Wiegand C, Hipler UC, Elsner P. Diagnostic value of the ISAC® allergy chip in detecting latex sensitizations. Int Arch Occup Environ Health 2014;87:775-81. http://dx.doi.org/10.1007/s00420-013-0921-6.
- Shibata R, Murakami Y, Odajima H. IgE antibodies to ara h 2 and ara h 6 by immunocap ISAC distinguish peanut anaphylaxis children from asymptomatic peanut sensitization. Proceedings of the American Academy of Allergy, Asthma and Immunology, 2014 Annual Meeting (AAAAI 2014), 28 February–4 March 2014, San Diego, CA, USA. J Allergy Clin Immunol 2014;133. http://dx.doi.org/10.1016/j.jaci.2013.12.408.
- Sousa N, Almeida E, Machado D, Rodrigues F, Carrapatoso I, Faria E, et al. Comparison between skin prick tests and ImmunoCAP ISACR in the determination of sensitisation to aeroallergens. Proceedings of the European Academy of Allergy and Clinical Immunology, 28th Congress, 6–10 June 2009, Warsaw, Poland. Allergy 2009;64.
- Tolkki L, Alanko K, Petman L, Skydtsgaard MB, Gronager PM, Seppala U, et al. Clinical and IgE profiling of birch (Betula verrucosa) allergic individuals suffering from allergic reactions to raw fruits and vegetables. Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22 June 2013, Milan, Italy. Allergy 2013;68.
- Tolkki L, Alanko K, Petman L, Skydtsgaard MB, Milvang PG, Seppälä U, et al. Clinical characterization and IgE profiling of birch (Betula verrucosa)-allergic individuals suffering from allergic reactions to raw fruits and vegetables. J Allergy Clin Immunol Pract 2013;1:623-31. http://dx.doi.org/10.1016/j.jaip.2013.07.010.
- Tripathi A, Workman LJ, Cho CB, Clark AS, Hamilton RG, Platts-Mills TA, et al. Investigation of eosinophilic esophagitis in adults using skin testing, ImmunoCAP, and ImmunoCAP ISAC IgE quantitation. Ann Allergy Asthma Immunol 2012;109.
- Tripathi A, Workman LJ, Commins S, Barnes B, Hamilton RG, Platts-Mills TAE, et al. Challenges with measurement of IgE antibodies to minor components in food allergy: eosinophilic esophagitis, peanut allergy, and delayed anaphylaxis to mammalian meat. Proceedings of the American Academy of Allergy, Asthma and Immunology, 2014 Annual Meeting (AAAAI 2014), 28 February–4 March 2014, San Diego, CA, USA. J Allergy Clin Immunol 2014;133. http://dx.doi.org/10.1016/j.jaci.2013.12.922.
- Tripathi A, Workman LJ, Commins SP, Hamilton R, Erwin EA, Platts-Mills TA. Challenges with measurement of IgE antibodies in eosinophilic esophagitis, peanut allergy, and mammalian meat allergy. Proceedings of the American College of Allergy, Asthma and Immunology, 2014 Annual Meeting, 6–10 November 2014, Atlanta, GA, USA. Ann Allergy Asthma Immunol 2014;113.
- Uriarte SA, Sastre J. Molecular diagnosis in dog allergy. Proceedings of the European Academy of Allergy and Clinical Immunology and World Allergy Organization, World Allergy and Asthma Congress 2013, 22 June 2013, Milan, Italy. Allergy 2013;68.
- Vitte J, Agabriel C, Liabeuf V, Cleach I, Grob JJ, Sarles JJ, et al. Sequential allergen microarray testing during the follow-up of allergic patients. Proceedings of the European Academy of Allergy and Clinical Immunology (EAACI), 5th International Symposium on Molecular Allergology (ISMA 2013), 6–7 December 2013, Vienna, Austria. Clin Transl Allergy 2014;4. http://dx.doi.org/10.1186/2045-7022-4-S2-P48.
- Weimann A, Suer W, Vetter K, Schnell S, Busekow A, Probst C, et al. Component-resolved multiparameter assays for the diagnosis of birch pollen and grass pollen allergy. Proceedings of the European Academy of Allergy and Clinical Immunology, 30th Congress, 11–15 June 2011, Istanbul, Turkey. Allergy 2011;66.
- Confidential Information Has Been Removed n.d.
- Mohamad Yadzir ZH, Misnan R, Abdullah N, Bakhtiar F, Leecyous B, Murad S. Component-resolved diagnosis (CRD): is it worth it? Frequency and differentiation in rhinitis patients with mite reactivity. Iran J Allergy Asthma Immunol 2014;13:240-6.
- Young S, North J, Karanam S, Makwana N, Noorani S. The utility of ISAC (immuno solid-phase allergen chip) in identifying clinically relevant IgE reactivity in patients with atopic dermatitis. Proceedings of the British Society for Allergy and Clinical Immunology, 2012 Annual Meeting, 2–4 July 2012, Nottingham, UK. Clin Exp Allergy 2012;42.
Appendix 1 Literature search strategies
Clinical effectiveness searches
EMBASE (via OvidSP): 1974–14 April 2015
Searched: 16 April 2015
-
allergy rapid test/ (334)
-
(ImmunoCAP or Immuno-CAP or Thermo Scientific).af. (2136)
-
ISAC.ti,ab,ot. (497)
-
(Immuno$ adj3 solid$ adj3 phase$ adj3 allerg$ adj3 chip$).af. (49)
-
(compon$ adj3 resolv$ adj3 diagnos$).af. (545)
-
(multi adj3 compon$ adj3 assay$).af. (14)
-
23$ allerg$.ti,ab,ot,hw. (52)
-
26$ allerg$.ti,ab,ot,hw. (52)
-
103$ allerg$.ti,ab,ot,hw. (35)
-
112$ allerg$.ti,ab,ot,hw. (21)
-
(Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”).af. (807)
-
or/1-11 (3743)
-
exp microarray analysis/ or (microarray$ or micro array$ or nanoarray$).ti,ab,ot,hw. (138,557)
-
(multiplex adj3 (test$ or assay$)).ti,ab,ot,hw. (5972)
-
or/13-14 (144,221)
-
exp hypersensitivity/ or (allerg$ or anaphyla$ or hypersensiti$ or hyper-sensiti$ or poly-sensiti$ or polysensiti$ or paucisensiti$).ti,ab,ot,hw. (572,729)
-
15 and 16 (2744)
-
12 or 17 (6131)
-
limit 18 to yr=“2005-Current” (5244)
MEDLINE (via OvidSP): 1946–week 2 April 2015
Searched: 16 April 2015
-
(ImmunoCAP or Immuno-CAP or Thermo Scientific).af. (465)
-
ISAC.ti,ab,ot. (116)
-
(Immuno$ adj3 solid$ adj3 phase$ adj3 allerg$ adj3 chip$).af. (9)
-
(compon$ adj3 resolv$ adj3 diagnos$).af. (223)
-
(multi adj3 compon$ adj3 assay$).af. (11)
-
23$ allerg$.ti,ab,ot,hw. (33)
-
26$ allerg$.ti,ab,ot,hw. (34)
-
103$ allerg$.ti,ab,ot,hw. (9)
-
112$ allerg$.ti,ab,ot,hw. (5)
-
(Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”).af. (520)
-
or/1-10 (1327)
-
exp Microarray Analysis/ or (microarray$ or micro array$ or nanoarray$).ti,ab,ot,hw. (103,970)
-
(multiplex adj3 (test$ or assay$)).ti,ab,ot,hw. (3489)
-
or/12-13 (107,253)
-
exp Hypersensitivity/ or (allerg$ or anaphyla$ or hypersensiti$ or hyper-sensiti$ or poly-sensiti$ or polysensiti$ or paucisensiti$).ti,ab,ot,hw. (373,147)
-
14 and 15 (1420)
-
11 or 16 (2648)
-
limit 17 to yr=“2005-Current” (1955)
MEDLINE In-Process Citations (via OvidSP): up to 15 April 2015; MEDLINE Daily Update (via OvidSP): up to 15 April 2015
Searched: 16 April 2015
-
(ImmunoCAP or Immuno-CAP or Thermo Scientific).af. (97)
-
ISAC.ti,ab,ot. (37)
-
(Immuno$ adj3 solid$ adj3 phase$ adj3 allerg$ adj3 chip$).af. (4)
-
(compon$ adj3 resolv$ adj3 diagnos$).af. (31)
-
(multi adj3 compon$ adj3 assay$).af. (0)
-
23$ allerg$.ti,ab,ot,hw. (2)
-
26$ allerg$.ti,ab,ot,hw. (2)
-
103$ allerg$.ti,ab,ot,hw. (2)
-
112$ allerg$.ti,ab,ot,hw. (1)
-
(Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”).af. (22)
-
or/1-10 (173)
-
exp Microarray Analysis/ or (microarray$ or micro array$ or nanoarray$).ti,ab,ot,hw. (7177)
-
(multiplex adj3 (test$ or assay$)).ti,ab,ot,hw. (423)
-
or/12-13 (7590)
-
exp Hypersensitivity/ or (allerg$ or anaphyla$ or hypersensiti$ or hyper-sensiti$ or poly-sensiti$ or polysensiti$ or paucisensiti$).ti,ab,ot,hw. (11,416)
-
14 and 15 (80)
-
11 or 16 (237)
NLM PubMed (internet): up to 22 April 2015
Searched: 22 April 2015
#6 Search (#4 and #5) (375)
#5 Search ((pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb])) (1,919,020)
#4 Search (#1 or #2 or #3) (2631)
#3 Search ((Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”)) (528)
#2 Search ISAC (373)
#1 Search (ImmunoCAP or Immuno-CAP or Thermo Scientific) (1776)
CDSR (via Wiley): 2015/April/Iss4; DARE (via Wiley): 2015/January/Iss1; CENTRAL (via Wiley): 2015/March/Iss3; HTA database (via Wiley): 2015/January/Iss1
Searched: 16 April 2015
#1 (ImmunoCAP or Immuno-CAP or Thermo Scientific) (48)
#2 ISAC (20)
#3 (Immuno* near/3 solid* adj3 phase* near/3 allerg* near/3 chip*) (0)
#4 (compon* near/3 resolv* near/3 diagnos*) (5)
#5 (multi near/3 compon* near/3 assay*) (0)
#6 23* near/1 allerg* (46)
#7 26* near/1 allerg* (7)
#8 103* near/1 allerg* (2)
#9 112* near/1 allerg* (1)
#10 (Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”) (56)
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 (175)
#12 MeSH descriptor: [Microarray Analysis] explode all trees (275)
#13 (microarray* or micro array* or nanoarray*) (575)
#14 (multiplex near/3 (test* or assay*)) (65)
#15 #12 or #13 or #14 (747)
#16 MeSH descriptor: [Hypersensitivity] explode all trees (15,709)
#17 (allerg* or anaphyla* or hypersensiti* or hyper-sensiti* or poly-sensiti* or polysensiti* or paucisensiti*) (25,363)
#18 #16 or #17 (32,535)
#19 #15 and #18 (39)
#20 #11 or #19 (210)
#21 #20 Publication Year from 2005 to 2015 (147)
-
CDSR search retrieved 41 records
-
DARE search retrieved 0 records*
-
Central search retrieved 104 records
-
HTA search retrieved 0 records.
*Please note: Records ceased to be added to the DARE resource on 31 March 2015; this search was for archival material only.
Science Citation Index Expanded (Web of Knowledge) 1970–21 April 2015; CPCI-S (via Web of Knowledge) 1990–21 April 2015
Searched: 23 April 2015
Indexes=SCI-EXPANDED, CPCI-S Timespan=2005–2015
#13 (2626) #12 OR #7
#12 (1312) #11 AND #10
#11 (108,808) TS=(allerg* or anaphyla* or hypersensiti* or hyper-sensiti* or poly-sensiti* or polysensiti* or paucisensiti*)
#10 (105,467) #9 OR #8
#9 (5844) TS=(multiplex NEAR/3 (test* or assay*))
#8 (100,025) TS=(microarray* or micro array* or nanoarray*)
#7 (1450) #6 OR #5 OR #4 OR #3 OR #2 OR #1
#6 (149) TS=(Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”)
#5 (11) TS=(multi NEAR/3 compon* NEAR/3 assay*)
#4 (368) TS=(compon* NEAR/3 resolv* NEAR/3 diagnos*)
#3 (16) TS=(Immuno* NEAR/3 solid* NEAR/3 phase* NEAR/3 allerg* NEAR/3 chip*)
#2 (454) TS=(ISAC)
#1 (581) TS=(ImmunoCAP or Immuno-CAP or “Thermo Scientific”)
BIOSIS Previews (via Web of Knowledge): 1956–21 April 2015
Searched: 23 April 2015
Indexes=BIOSIS Previews Timespan=2005–2015
#7 (1282) #6 OR #5 OR #4 OR #3 OR #2 OR #1
#6 (81) TS=(Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”)
#5 (9) TS=(multi NEAR/3 compon* NEAR/3 assay*)
#4 (202) TS=(compon* NEAR/3 resolv* NEAR/3 diagnos*)
#3 (22) TS=(Immuno* NEAR/3 solid* NEAR/3 phase* NEAR/3 allerg* NEAR/3 chip*)
#2 (230) TS=(ISAC)
#1 (914) TS=(ImmunoCAP or Immuno-CAP or “Thermo Scientific”)
LILACS: 1982–22 April 2015
http://regional.bvsalud.org/php/index.php?lang=en
Searched: 22 April 2015
| Terms | Records |
|---|---|
| ImmunoCAP or “Immuno-CAP” or “Thermo Scientific” or ISAC or Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX” | 156 |
| Total | 156 |
NIHR HTA programme (internet): up to 23 April 2015
Searched: 23 April 2015
Browsed with the following terms.
| Terms | Records |
|---|---|
| ImmunoCAP | 0 |
| Immuno-CAP | 0 |
| Thermo Scientific | 0 |
| ISAC | 0 |
| Allerwatch | 0 |
| ComforTen | 0 |
| MultiTest | 0 |
| True test | 0 |
| Microtest DX | 0 |
| Micro Test DX | 0 |
| Total | 0 |
FDA (internet): up to 23 April 2015
Searched: 23 April 2015
Searched Medical Devices.
| Search terms | Records |
|---|---|
| ImmunoCAP OR Immuno-CAP OR Thermo Scientific | 15 |
| ISAC | 13 |
| Allerwatch OR ComforTen OR MultiTest OR true test OR Microtest DX OR Micro Test DX | 16 |
| Total | 44 |
OpenGrey: up to 22 April 2015
Searched: 22 April 2015
| Search terms | Records |
|---|---|
| ImmunoCAP OR “Immuno-CAP” OR “Thermo Scientific” | 3 |
| ISAC | 8 |
| Allerwatch OR ComforTen OR “MultiTest” OR “true test” OR “Microtest DX” OR “Micro Test DX” | 4 |
| Total | 15 |
ClinicalTrials.gov (internet): up to 22 April 2015
http://clinicaltrials.gov/ct2/search/advanced
Searched: 22 April 2015
Advanced search option – search terms box.
| Search terms | Records |
|---|---|
| ImmunoCAP OR Immuno-CAP OR Thermo Scientific | 27 |
| ISAC | 5 |
| Allerwatch OR ComforTen OR “MultiTest” OR “true test” OR “Microtest DX” OR “Micro Test DX” | 8 |
| Total | 40 |
ISRCTN Registry (internet): up to 22 April 2015
Searched: 22 April 2015
Advanced search – Text Search.
| Search terms | Records |
|---|---|
| ImmunoCAP OR “Immuno-CAP” OR “Thermo Scientific” | 2 |
| ISAC | 1 |
| Allerwatch OR ComforTen OR “MultiTest” OR “true test” OR “Microtest DX” OR “Micro Test DX” | 1 |
| Total | 4 |
WHO ICTRP (internet): up to 22 April 2015
Searched: 22 April 2015
Advanced search option.
| Title | Intervention | Records | |
|---|---|---|---|
| ImmunoCAP OR Immuno-CAP OR Thermo Scientific | OR | ImmunoCAP OR Immuno-CAP OR Thermo Scientific | 1 |
| ISAC | OR | ISAC | 0 |
| Allerwatch OR ComforTen OR MultiTest OR true test OR Microtest DX OR Micro Test DX | OR | Allerwatch OR ComforTen OR MultiTest OR true test OR Microtest DX OR Micro Test DX | 13 |
| Total | 14 | ||
Conference searches
AAAAI Annual Meeting
Searched: 19 May 2015
Searched for last 5 years.
Used “search within this issue” option and exported results for articles only:
2015: www.jacionline.org/issue/S0091-6749%2814%29X0003-5
2014: www.jacionline.org/issue/S0091-6749%2813%29X0015-6
2013: www.jacionline.org/issue/S0091-6749%2813%29X0013-2
2012: www.jacionline.org/issue/S0091-6749%2812%29X0002-2
2011: www.jacionline.org/issue/S0091-6749%2811%29X0002-7
| Search terms | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| ImmunoCAP ISAC | 8 | 2 | 8 | 7 | 4 |
| Immuno-CAP ISAC | 5 | 6 | 9 | 6 | 3 |
| Microtest DX | 0 | 0 | 0 | 0 | 0 |
| Micro Test DX | 0 | 0 | 0 | 0 | 0 |
| Total | 13 | 8 | 17 | 13 | 7 |
| Total before deduplication | 58 | ||||
| Total after deduplication | 52 | ||||
EAACI
Searched: 19 May 2015
2015: www.professionalabstracts.com/eaaci2015/programme-eaaci2015.pdf
2014: www.sessionplan.com/eaaci2014/
2013: http://onlinelibrary.wiley.com/doi/10.1111/all.2013.68.issue-s97/issuetoc
2012: http://onlinelibrary.wiley.com/doi/10.1111/all.2012.67.issue-s96/issuetoc
2011: http://onlinelibrary.wiley.com/doi/10.1111/all.2011.66.issue-s94/issuetoc
| Search terms | 2011 | 2012 | 2013 | Search terms | 2014 | 2015 (June 2015) |
|---|---|---|---|---|---|---|
| ImmunoCAP | 78 | 38 | 104 | ImmunoCAP ISAC | 18 | NA |
| Immuno-CAP | 6 | 7 | 8 | Immuno-CAP ISAC | 0 | |
| Microtest | 0 | 0 | Microtest | 1 | ||
| Micro Test | 0 | 0 | Micro Test | 0 | ||
| Total | 84 | 45 | 112 | 19 | NA | |
| Total | 260 | |||||
BSACI
Searched: 19 May 2015
2015: NA – conference had not yet taken place at time of searching
2014: http://onlinelibrary.wiley.com/doi/10.1111/cea.12456/epdf
2013: http://onlinelibrary.wiley.com/doi/10.1111/cea.12197/epdf
2012: http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2222.2012.012033.x/epdf
2011: http://onlinelibrary.wiley.com/wol1/doi/10.1111/j.1365-2222.2011.03897.x/abstract
Please note: Unable to access 2011 online without payment.
| Search terms | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| ImmunoCAP | NA | 2 | 4 | 7 | NA |
| Immuno-CAP | 1 | 0 | 0 | ||
| Microtest | 0 | 0 | 0 | ||
| Micro Test | 0 | 0 | 0 | ||
| Total | 3 | 4 | 7 | ||
| Total | 14 | ||||
FAAM
Searched: 19 May 2015
Searched for last 5 years.
Used ‘Control F’ to search within saved PDFs.
2015: NA – conference not yet taken place at time of search
2014: www.ctajournal.com/supplements/5/S3
2013: www.ctajournal.com/supplements/3/S3
2012: NA – No record on website of having run in that year
2011: www.ctajournal.com/supplements/1/S1
| Search terms | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| ImmunoCAP ISAC | 3 | NA | 5 | 3 | NA |
| Immuno-CAP ISAC | 0 | 0 | 0 | ||
| Microtest DX | 0 | 0 | 0 | ||
| Micro Test DX | 0 | 0 | 0 | ||
| Total | 3 | 5 | 3 | ||
| Total before deduplication | 11 | ||||
ISMA
Searched: 20 May 2015
Searched for last 5 years.
Used ‘Control F’ to search within saved PDFs.
2015: Sixth conference had not yet taken place at time of search
2014: NA
2013: www.ctajournal.com/supplements/4/S2/all (5th conference)
2012: NA
2011: NA (Fourth conference 2010)
| Search terms | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| ImmunoCAP ISAC | NA | NA | 5 | NA | Not yet run |
| Immuno-CAP ISAC | 0 | ||||
| Microtest DX | 0 | ||||
| Micro Test DX | 0 | ||||
| Total | 5 | ||||
| Total | 5 | ||||
AAD Meeting
Searched: 26 May 2015
Searched for last 5 years.
2015: http://onlinedigitalpublishing.com/publication/?m=20143&l=1
2014: http://onlinedigitalpublishing.com/publication/?i=199001
2013: http://onlinedigitalpublishing.com/publication/?i=146375
2012: https://s3.amazonaws.com/aad-meetings/AM12_Program.pdf
2011: www.nxtbook.com/nxtbooks/aad/annualmeeting2011/#/0
| Search terms | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| ImmunoCAP | 0 | 0 | 0 | 0 | 0 |
| Immuno-CAP | 0 | 0 | 0 | 0 | 0 |
| Microtest DX | 0 | 0 | 0 | 0 | 0 |
| Micro Test DX | 0 | 0 | 0 | 0 | 0 |
| Total | 0 | 0 | 0 | 0 | 0 |
| Total before deduplication | 0 | ||||
BAD
Searched: 26 May 2015
Searched for last 5 years.
Used “Search within this issue”.
2015: Conference had not yet taken place at time of search
2014: http://onlinelibrary.wiley.com/doi/10.1111/bjd.2014.171.issue-s1/issuetoc
2013: http://onlinelibrary.wiley.com/doi/10.1111/bjd.2013.169.issue-s1/issuetoc
2012: http://onlinelibrary.wiley.com/doi/10.1111/bjd.2012.167.issue-s1/issuetoc
2011: http://onlinelibrary.wiley.com/doi/10.1111/bjd.2011.165.issue-s1/issuetoc
| Search terms | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| ImmunoCAP | 0 | 0 | 0 | 0 | |
| Immuno-CAP | 0 | 0 | 0 | 0 | |
| Microtest DX | 0 | 0 | 0 | 0 | |
| Micro Test DX | 0 | 0 | 0 | 0 | |
| Total | 0 | 0 | 0 | 0 | |
| Total before deduplication | 0 | ||||
Cost-effectiveness
EMBASE (via OvidSP): 1974–20 May 2015
Searched: 21 May 2015
-
allergy rapid test/ (381)
-
(ImmunoCAP or Immuno-CAP or Thermo Scientific or phadia).af. (2718)
-
ISAC.ti,ab,ot. (517)
-
(Immuno$ adj3 solid$ adj3 phase$ adj3 allerg$ adj3 chip$).af. (50)
-
(compon$ adj3 resolv$ adj3 diagnos$).af. (567)
-
(multi adj3 compon$ adj3 assay$).af. (14)
-
23$ allerg$.ti,ab,ot,hw. (52)
-
26$ allerg$.ti,ab,ot,hw. (53)
-
103$ allerg$.ti,ab,ot,hw. (35)
-
112$ allerg$.ti,ab,ot,hw. (22)
-
(Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”).af. (810)
-
or/1-11 (4290)
-
exp microarray analysis/ or (microarray$ or micro array$ or nanoarray$).ti,ab,ot,hw. (140,563)
-
(multiplex adj3 (test$ or assay$)).ti,ab,ot,hw. (6050)
-
or/13-14 (146,303)
-
exp hypersensitivity/ or (allerg$ or anaphyla$ or hypersensiti$ or hyper-sensiti$ or poly-sensiti$ or polysensiti$ or paucisensiti$).ti,ab,ot,hw. (578,447)
-
15 and 16 (2794)
-
12 or 17 (6704)
-
health-economics/ (34,457)
-
exp economic-evaluation/ (226,212)
-
exp health-care-cost/ (217,693)
-
exp pharmacoeconomics/ (173,447)
-
or/19-22 (506,020)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (670,947)
-
(expenditure$ not energy).ti,ab. (26,222)
-
(value adj2 money).ti,ab. (1530)
-
budget$.ti,ab. (26,297)
-
or/24-27 (696,870)
-
23 or 28 (978,855)
-
letter.pt. (887,374)
-
editorial.pt. (477,247)
-
note.pt. (599,443)
-
or/30-32 (1964064)
-
29 not 33 (886,862)
-
(metabolic adj cost).ti,ab. (989)
-
((energy or oxygen) adj cost).ti,ab. (3348)
-
((energy or oxygen) adj expenditure).ti,ab. (22,162)
-
or/35-37 (25,637)
-
34 not 38 (881,415)
-
18 and 39 (197)
Economics terms based on Costs filter
Centre for Reviews and Dissemination. Search strategies: NHS EED EMBASE using OvidSP (economics filter) (internet). York: CRD; 2014 (accessed 2 June 2014).
Available from www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedembase
MEDLINE (via OvidSP): 1946–week 3 May 2015
Searched: 21 May 2015
-
(ImmunoCAP or Immuno-CAP or Thermo Scientific).af. (471)
-
ISAC.ti,ab,ot. (116)
-
(Immuno$ adj3 solid$ adj3 phase$ adj3 allerg$ adj3 chip$).af. (9)
-
(compon$ adj3 resolv$ adj3 diagnos$).af. (225)
-
(multi adj3 compon$ adj3 assay$).af. (11)
-
23$ allerg$.ti,ab,ot,hw. (33)
-
26$ allerg$.ti,ab,ot,hw. (34)
-
103$ allerg$.ti,ab,ot,hw. (9)
-
112$ allerg$.ti,ab,ot,hw. (5)
-
(Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”).af. (520)
-
or/1-10 (1335)
-
exp Microarray Analysis/ or (microarray$ or micro array$ or nanoarray$).ti,ab,ot,hw. (105,216)
-
(multiplex adj3 (test$ or assay$)).ti,ab,ot,hw. (3540)
-
or/12-13 (108,548)
-
exp Hypersensitivity/ or (allerg$ or anaphyla$ or hypersensiti$ or hyper-sensiti$ or poly-sensiti$ or polysensiti$ or paucisensiti$).ti,ab,ot,hw. (375,235)
-
14 and 15 (1439)
-
11 or 16 (2675)
-
economics/ (26,620)
-
exp “costs and cost analysis”/ (187,805)
-
economics, dental/ (1859)
-
exp “economics, hospital”/ (20,266)
-
economics, medical/ (8615)
-
economics, nursing/ (3914)
-
economics, pharmaceutical/ (2572)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (445,087)
-
(expenditure$ not energy).ti,ab. (18,132)
-
(value adj1 money).ti,ab. (24)
-
budget$.ti,ab. (17,825)
-
or/18-28 (571,847)
-
((energy or oxygen) adj cost).ti,ab. (2735)
-
(metabolic adj cost).ti,ab. (818)
-
((energy or oxygen) adj expenditure).ti,ab. (16,789)
-
or/30-32 (19,615)
-
29 not 33 (567,494)
-
letter.pt. (847,644)
-
editorial.pt. (356,900)
-
historical article.pt. (316,205)
-
or/35-37 (1,505,328)
-
34 not 38 (538,417)
-
17 and 39 (67)
Costs filter
Centre for Reviews and Dissemination. NHS EED Economics Filter: MEDLINE (via Ovid) monthly search (internet). York: CRD; 2010 (cited 28 September 2010).
Available from: www.york.ac.uk/inst/crd/intertasc/nhs_eed_strategies.html
MEDLINE In-Process & Other Non-Indexed Citations (via OvidSP): up to 20 May 2015; MEDLINE Daily Update (via OvidSP): up to 20 May 2015
Searched: 21 May 2015
-
(ImmunoCAP or Immuno-CAP or Thermo Scientific).af. (95)
-
ISAC.ti,ab,ot. (39)
-
(Immuno$ adj3 solid$ adj3 phase$ adj3 allerg$ adj3 chip$).af. (4)
-
(compon$ adj3 resolv$ adj3 diagnos$).af. (33)
-
(multi adj3 compon$ adj3 assay$).af. (0)
-
23$ allerg$.ti,ab,ot,hw. (2)
-
26$ allerg$.ti,ab,ot,hw. (2)
-
103$ allerg$.ti,ab,ot,hw. (2)
-
112$ allerg$.ti,ab,ot,hw. (1)
-
(Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”).af. (24)
-
or/1-10 (176)
-
exp Microarray Analysis/ or (microarray$ or micro array$ or nanoarray$).ti,ab,ot,hw. (7501)
-
(multiplex adj3 (test$ or assay$)).ti,ab,ot,hw. (453)
-
or/12-13 (7944)
-
exp Hypersensitivity/ or (allerg$ or anaphyla$ or hypersensiti$ or hyper-sensiti$ or poly-sensiti$ or polysensiti$ or paucisensiti$).ti,ab,ot,hw. (11,612)
-
14 and 15 (89)
-
11 or 16 (248)
-
economics/ (0)
-
exp “costs and cost analysis”/ (128)
-
economics, dental/ (0)
-
exp “economics, hospital”/ (8)
-
economics, medical/ (1)
-
economics, nursing/ (1)
-
economics, pharmaceutical/ (0)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (60,839)
-
(expenditure$ not energy).ti,ab. (1828)
-
(value adj1 money).ti,ab. (3)
-
budget$.ti,ab. (2518)
-
or/18-28 (63,427)
-
((energy or oxygen) adj cost).ti,ab. (312)
-
(metabolic adj cost).ti,ab. (101)
-
((energy or oxygen) adj expenditure).ti,ab. (1452)
-
or/30-32 (1820)
-
29 not 33 (62,936)
-
letter.pt. (32,097)
-
editorial.pt. (21,356)
-
historical article.pt. (131)
-
or/35-37 (53,569)
-
34 not 38 (62,298)
-
17 and 39 (8)
Costs filter
Centre for Reviews and Dissemination. NHS EED Economics Filter: MEDLINE (via Ovid) monthly search (internet). York: CRD; 2010 (cited 28 September 2010).
Available from www.york.ac.uk/inst/crd/intertasc/nhs_eed_strategies.html
NHS EED (via Wiley): 2015/April/Iss2
Searched: 21 May 2015
#1 (ImmunoCAP or Immuno-CAP or Thermo Scientific) (48)
#2 ISAC (20)
#3 (Immuno* near/3 solid* adj3 phase* near/3 allerg* near/3 chip*) (0)
#4 (compon* near/3 resolv* near/3 diagnos*) (5)
#5 (multi near/3 compon* near/3 assay*) (0)
#6 23* near/1 allerg* (46)
#7 26* near/1 allerg* (7)
#8 103* near/1 allerg* (2)
#9 112* near/1 allerg* (1)
#10 (Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”) (56)
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 (175)
#12 MeSH descriptor: [Microarray Analysis] explode all trees 280)
#13 (microarray* or micro array* or nanoarray*) (586)
#14 (multiplex near/3 (test* or assay*)) (66)
#15 #12 or #13 or #14 (761)
#16 MeSH descriptor: [Hypersensitivity] explode all trees (15,764)
#17 (allerg* or anaphyla* or hypersensiti* or hyper-sensiti* or poly-sensiti* or polysensiti* or paucisensiti*) (25,504)
#18 #16 or #17 (32,705)
#19 #15 and #18 (39)
#20 #11 or #19 (210)
NHS EED search retrieved 1 record.
*Please note: records ceased to be added to the NHS EED resource on 31 March 2015; this search was for archival material only.
EconLit (via EBSCOhost): 1886–21 May 2015
Searched: 21 May 2015
S13 S7 OR S12 (18)
S12 S10 AND S11 (0)
S11 TX(allerg* or anaphyla* or hypersensiti* or hyper-sensiti* or poly-sensiti* or polysensiti* or paucisensiti*) (49)
S10 S8 OR S9 (103)
S9 TX(multiplex N3 (test* or assay*)) (1)
S8 TX(microarray* or micro array* or nanoarray*) (102)
S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 (18)
S6 TX (Allerwatch or ComforTen or “MultiTest” or “true test” or “Microtest DX” or “Micro Test DX”) (13)
S5 TX (multi N3 compon* N3 assay*) (0)
S4 TX (compon* N3 resolv* N3 diagnos*) (0)
S3 TX (Immuno* N3 solid* N3 phase* N3 allerg* N3 chip*) (0)
S2 TX (ISAC) (5)
S1 TX (ImmunoCAP or Immuno-CAP or “Thermo Scientific”) (0)
Research Papers in Economics (RePEc) (internet): up to 26 May 2015
Searched: 26 May 2015
IDEAS search interface
#1 (ImmunoCAP | Immuno-CAP | “Thermo Scientific”) (Results 1)
#2 (Allerwatch | ComforTen | “MultiTest” | “true test” | “Microtest DX” | “Micro Test DX”) (Results 17)
RePEc search retrieved 18 records.
Health-related quality of life/utilities searches
EMBASE (via OvidSP): 1974–29 June 2015
Searched: 30 June 2015
-
(food$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (27,518)
-
((Cereal$ or wheat or rye or barley or oat or oats or rice or maize or spelt or buckwheat or soybean or millet or sorghum or corn or Kamut) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (2562)
-
((Gluten or (glutenin adj3 gliadin) or prolamins) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1408)
-
((Dairy or milk or yog?urt$ or cream or butter$ or cheese$ or ice cream$ or kefir) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (6786)
-
((Casein or whey or lactose) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (4128)
-
(Egg$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (2858)
-
((Peanut$ or arachid or Arachis hypog?ea or groundnut) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (3239)
-
((ground or earth or monkey) adj3 nut$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1)
-
(arachis oil adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (0)
-
((Nut or nuts or hazelnut$ or cashew$ or walnut$ or pecan$ or almond$ or Macadamia$ or pistachio$ or chestnut$ or coconut$ or Candlenut) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1338)
-
((Brazil or pine or hickory or betel) adj3 nut$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (91)
-
((Crustacea$ or Mollusc$ or mollusk$ or shellfish or seafood or sea food or Lobster or crab$ or shrimp$ or prawn$ or squid$ or oyster$ or crayfish or cuttlefish or abalone or limpet$ or mussel$ or scallop$ or clam or clams or whelk$ or scampi or octopus or langoustine$ or cockle$ or winkles or krill) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1046)
-
((Fish or finfish or sole or mackerel$ or hake or whiting or dab or plaice or Anchov$ or Catfish or Eel$ or Haddock or Halibut or Sardine$ or Scad or Snapper or Tilapia or Trout or shark or swordfish or cod or tuna or herring$ or flounder or mullet or salmon or kipper$ or caviar or seer or mackerel or tilefish or Pollock or whitebait or flatfish or john dory or carp) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1799)
-
(Fish adj3 roe adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (7)
-
((Surimi or sashimi or sushi or cerviche or gravlax) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (2)
-
((Snail$ or frog$) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (221)
-
((Sulfites or sulphites) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (55)
-
(((sulphiting adj2 agents) or (sulphur adj2 dioxide)) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (13)
-
((Lentil$ or chickpea$ or pea or peas or garbanzo or bengal gram or chana or channa or leblebi) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (132)
-
((poppy or sunflower or cotton or flax) adj3 seed$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (59)
-
((fruit or fruits or vegetable$ or legume$ or kiwi or melon or banana$ or Carrot$ or apple$ or tomato$ or Apricot$ or pepper$ or Cabbage$ or Celery or Celeriac or Cherry or cherries or Courgette$ or zucchini or Aubergine$ or Dates or Fig or figs or plum or plums or garlic$ or grape$ or Lettuce$ or Lychee$ or Mango$ or Peach$ or Pear or pears or Pineapple$ or Pomegranate or Potato$ or Pumpkin$ or Strawberry or strawberries or Turnip$ or Avocado$ or Persimmon) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (2435)
-
((Acerola or Aniseed or Camomile or Castor bean$ or Cocoa or linseed or Lupin$ or Lupine or Sesame or soy$) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1227)
-
((Condiment$ or spice$ or Mustard$) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$, or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (230)
-
or/1-23 (42,706)
-
exp food allergy/ (24,867)
-
exp food allergen/ (4698)
-
or/25-26 (26,399)
-
24 or 27 (42,706)
-
exp Food/ (721,540)
-
food additive/ (8744)
-
crab meat/ or crab/ or crayfish/ or lobster/ or shrimp/ or mollusc/ or fish/ or fish meat/ (111,052)
-
or/29-31 (822,648)
-
exp allergen/ (54,384)
-
exp hypersensitivity/ (498,826)
-
anaphylaxis/ (34,246)
-
anaphylactic shock/ (4751)
-
(allerg$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$).ti,ab,ot,hw. (479,818)
-
or/33-37 (649,918)
-
32 and 38 (35,162)
-
28 or 39 (58,974)
-
pollen allergy/ or hay fever/ (14,584)
-
((Pollen or seasonal) adj3 (allerg$ or anaphyla$ or hypersensiti$ or hyper-sensiti$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (13,145)
-
(hayfever or hay fever or pollinosis or pollinoses).ti,ab,ot,hw. (11,916)
-
or/41-43 (21,333)
-
rhinitis/ or rhiniti$.ti,ab,ot,hw. (47,359)
-
(season$ or spring or summer or pollen$ or grass$ or birch or ragweed or tree$ or weed$ or mugwort or willow or alder).ti,ab,ot,hw. (397,576)
-
45 and 46 (9392)
-
44 or 47 (24,956)
-
40 or 48 (79,964)
-
quality adjusted life year/ or quality of life index/ (16,036)
-
Short Form 12/ or Short Form 20/ or Short Form 36/ or Short Form 8/ (16,212)
-
“International Classification of Functioning, Disability and Health”/ or “ferrans and powers quality of life index”/ or “gastrointestinal quality of life index”/ (1859)
-
(sf36 or sf 36 or sf-36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,ot. (26,380)
-
(sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,ot. (1612)
-
(sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,ot. (5102)
-
(sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab,ot. (855)
-
(sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,ot. (352)
-
(sf8 or sf 8 or sf-8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight).ti,ab,ot. (492)
-
“health related quality of life”.ti,ab,ot. (34,378)
-
(Quality adjusted life or Quality-adjusted-life).ti,ab,ot. (10,293)
-
“assessment of quality of life”.ti,ab,ot. (1910)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab,ot. (8735)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab,ot. (17,838)
-
(hye or hyes).ti,ab,ot. (98)
-
health$ year$ equivalent$.ti,ab,ot. (39)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab,ot. (2295)
-
(quality time or qwb or “quality of well being” or “quality of wellbeing” or “index of wellbeing” or index of well being).ti,ab,ot,hw. (837)
-
(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”).ti,ab,ot. (2463)
-
(QALY$ or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL or qald$ or qale$ or qtime$ or AQoL$).ti,ab,ot. (13,192)
-
(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble$ or “willingness to pay”).ti,ab,ot. (6245)
-
15d.ti,ab,ot. (1802)
-
(HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab,ot. (340)
-
(utilit$ adj3 (“quality of life” or valu$ or scor$ or measur$ or health or life or estimat$ or elicit$ or disease$)).ti,ab,ot. (11,429)
-
(utilities or disutili$).ti,ab,ot. (7194)
-
or/50-74 (108,471)
-
letter.pt. (896,144)
-
editorial.pt. (482,372)
-
note.pt. (606,238)
-
or/76-78 (1,984,754)
-
75 not 79 (105,171)
-
49 and 80 (433)
Health-related quality of life free-text terms based on:
-
Figure 4 Common free-text terms for electronic database searching for HSUVs in Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: the identification, review and synthesis of health-state utility values from the literature (internet), 2011 (accessed 18 August 2011). Available from: www.nicedsu.org.uk.
MEDLINE (via OvidSP): 1946–week 3 June 2015
Searched: 30 June 2015
-
(food$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (16,959)
-
((Cereal$ or wheat or rye or barley or oat or oats or rice or maize or spelt or buckwheat or soybean or millet or sorghum or corn or Kamut) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1605)
-
((Gluten or (glutenin adj3 gliadin) or prolamins) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (876)
-
((Dairy or milk or yog?urt$ or cream or butter$ or cheese$ or ice cream$ or kefir) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (3865)
-
((Casein or whey or lactose) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (3292)
-
(Egg$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1416)
-
((Peanut$ or arachid or Arachis hypog?ea or groundnut) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1419)
-
((ground or earth or monkey) adj3 nut$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (0)
-
(arachis oil adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (0)
-
((Nut or nuts or hazelnut$ or cashew$ or walnut$ or pecan$ or almond$ or Macadamia$ or pistachio$ or chestnut$ or coconut$ or Candlenut) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (643)
-
((Brazil or pine or hickory or betel) adj3 nut$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (63)
-
((Crustacea$ or Mollusc$ or mollusk$ or shellfish or seafood or sea food or Lobster or crab$ or shrimp$ or prawn$ or squid$ or oyster$ or crayfish or cuttlefish or abalone or limpet$ or mussel$ or scallop$ or clam or clams or whelk$ or scampi or octopus or langoustine$ or cockle$ or winkles or krill) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (636)
-
((Fish or finfish or sole or mackerel$ or hake or whiting or dab or plaice or Anchov$ or Catfish or Eel$ or Haddock or Halibut or Sardine$ or Scad or Snapper or Tilapia or Trout or shark or swordfish or cod or tuna or herring$ or flounder or mullet or salmon or kipper$ or caviar or seer or mackerel or tilefish or Pollock or whitebait or flatfish or john dory or carp) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1154)
-
(Fish adj3 roe adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (2)
-
((Surimi or sashimi or sushi or cerviche or gravlax) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (2)
-
((Snail$ or frog$) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (173)
-
((Sulfites or sulphites) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (36)
-
(((sulphiting adj2 agents) or (sulphur adj2 dioxide)) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (3)
-
((Lentil$ or chickpea$ or pea or peas or garbanzo or bengal gram or chana or channa or leblebi) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (84)
-
((poppy or sunflower or cotton or flax) adj3 seed$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (27)
-
((fruit or fruits or vegetable$ or legume$ or kiwi or melon or banana$ or Carrot$ or apple$ or tomato$ or Apricot$ or pepper$ or Cabbage$ or Celery or Celeriac or Cherry or cherries or Courgette$ or zucchini or Aubergine$ or Dates or Fig or figs or plum or plums or garlic$ or grape$ or Lettuce$ or Lychee$ or Mango$ or Peach$ or Pear or pears or Pineapple$ or Pomegranate or Potato$ or Pumpkin$ or Strawberry or strawberries or Turnip$ or Avocado$ or Persimmon) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1449)
-
((Acerola or Aniseed or Camomile or Castor bean$ or Cocoa or linseed or Lupin$ or Lupine or Sesame or soy$) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (743)
-
((Condiment$ or spice$ or Mustard$) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$, or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (166)
-
or/1-23 (27,183)
-
exp Food Hypersensitivity/ (15,608)
-
exp Food/ (1,100,276)
-
exp Food Additives/ (239,057)
-
or/26-27 (1,101,303)
-
allergens/ or exp Hypersensitivity/ (288,187)
-
(allerg$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$).ti,ab,ot,hw. (324,188)
-
or/29-30 (442,055)
-
28 and 31 (28,934)
-
24 or 25 or 32 (45,050)
-
Rhinitis, Allergic, Seasonal/ (12,580)
-
((Pollen or seasonal) adj3 (allerg$ or anaphyla$ or hypersensiti$ or hyper-sensiti$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (15,942)
-
(hayfever or hay fever or pollinosis or pollinoses).ti,ab,ot,hw. (3989)
-
(rhiniti$ adj3 (season$ or spring or summer or pollen$ or grass$ or birch or ragweed or tree$ or weed$ or mugwort or willow or alder)).ti,ab,ot,hw. (12,979)
-
or/34-37 (17,192)
-
33 or 38 (60,503)
-
quality-adjusted life years/ or quality of life/ (133,433)
-
(sf36 or sf 36 or sf-36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,ot. (16,140)
-
(sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,ot. (1038)
-
(sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,ot. (2869)
-
(sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab,ot. (460)
-
(sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,ot. (336)
-
(sf8 or sf 8 or sf-8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight).ti,ab,ot. (265)
-
“health related quality of life”.ti,ab,ot. (22,368)
-
(Quality adjusted life or Quality-adjusted-life).ti,ab,ot. (6465)
-
“assessment of quality of life”.ti,ab,ot. (1174)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab,ot. (4266)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab,ot. (10,537)
-
(hye or hyes).ti,ab,ot. (54)
-
health$ year$ equivalent$.ti,ab,ot. (38)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab,ot. (901)
-
(quality time or qwb or quality of well being or “quality of wellbeing” or “index of wellbeing” or “index of well being”).ti,ab,ot,hw. (617)
-
(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”).ti,ab,ot. (1788)
-
(QALY$ or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL or qald$ or qale$ or qtime$ or AQoL$).ti,ab,ot. (7212)
-
(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble$ or “willingness to pay”).ti,ab,ot. (3886)
-
15d.ti,ab,ot. (1173)
-
(HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab,ot. (238)
-
(utilit$ adj3 (“quality of life” or valu$ or scor$ or measur$ or health or life or estimat$ or elicit$ or disease$)).ti,ab,ot. (6934)
-
(utilities or disutili$).ti,ab,ot. (4124)
-
or/40-62 (158,071)
-
letter.pt. (854,881)
-
editorial.pt. (359,484)
-
historical article.pt. (317,628)
-
or/64-66 (1,516,490)
-
63 not 67 (150,867)
-
39 and 68 (739)
Health-related quality of life free-text terms based on:
-
Figure 4 Common free-text terms for electronic database searching for HSUVs in Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: the identification, review and synthesis of health state utility values from the literature (internet), 2011 (accessed 18 August 2011). Available from: www.nicedsu.org.uk.
MEDLINE In-Process & Other Non-Indexed Citations (via OvidSP): up to 29 June 2015; MEDLINE Daily Update (via OvidSP): up to 29 June 2015
Searched: 30 June 2015
-
(food$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1007)
-
((Cereal$ or wheat or rye or barley or oat or oats or rice or maize or spelt or buckwheat or soybean or millet or sorghum or corn or Kamut) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (204)
-
((Gluten or (glutenin adj3 gliadin) or prolamins) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (115)
-
((Dairy or milk or yog?urt$ or cream or butter$ or cheese$ or ice cream$ or kefir) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (229)
-
((Casein or whey or lactose) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (76)
-
(Egg$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (126)
-
((Peanut$ or arachid or Arachis hypog?ea or groundnut) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (155)
-
((ground or earth or monkey) adj3 nut$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (0)
-
(arachis oil adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (0)
-
((Nut or nuts or hazelnut$ or cashew$ or walnut$ or pecan$ or almond$ or Macadamia$ or pistachio$ or chestnut$ or coconut$ or Candlenut) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (51)
-
((Brazil or pine or hickory or betel) adj3 nut$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (5)
-
((Crustacea$ or Mollusc$ or mollusk$ or shellfish or seafood or sea food or Lobster or crab$ or shrimp$ or prawn$ or squid$ or oyster$ or crayfish or cuttlefish or abalone or limpet$ or mussel$ or scallop$ or clam or clams or whelk$ or scampi or octopus or langoustine$ or cockle$ or winkles or krill) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (44)
-
((Fish or finfish or sole or mackerel$ or hake or whiting or dab or plaice or Anchov$ or Catfish or Eel$ or Haddock or Halibut or Sardine$ or Scad or Snapper or Tilapia or Trout or shark or swordfish or cod or tuna or herring$ or flounder or mullet or salmon or kipper$ or caviar or seer or mackerel or tilefish or Pollock or whitebait or flatfish or john dory or carp) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (83)
-
(Fish adj3 roe adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1)
-
((Surimi or sashimi or sushi or cerviche or gravlax) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1)
-
((Snail$ or frog$) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (4)
-
((Sulfites or sulphites) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (0)
-
(((sulphiting adj2 agents) or (sulphur adj2 dioxide)) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (1)
-
((Lentil$ or chickpea$ or pea or peas or garbanzo or bengal gram or chana or channa or leblebi) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (9)
-
((poppy or sunflower or cotton or flax) adj3 seed$ adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (3)
-
((fruit or fruits or vegetable$ or legume$ or kiwi or melon or banana$ or Carrot$ or apple$ or tomato$ or Apricot$ or pepper$ or Cabbage$ or Celery or Celeriac or Cherry or cherries or Courgette$ or zucchini or Aubergine$ or Dates or Fig or figs or plum or plums or garlic$ or grape$ or Lettuce$ or Lychee$ or Mango$ or Peach$ or Pear or pears or Pineapple$ or Pomegranate or Potato$ or Pumpkin$ or Strawberry or strawberries or Turnip$ or Avocado$ or Persimmon) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (130)
-
((Acerola or Aniseed or Camomile or Castor bean$ or Cocoa or linseed or Lupin$ or Lupine or Sesame or soy$) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (65)
-
((Condiment$ or spice$ or Mustard$) adj4 (allerg$ or sensitivit$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$, or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (8)
-
or/1-23 (1826)
-
exp Food Hypersensitivity/ (16)
-
exp Food/ (1220)
-
exp Food Additives/ (177)
-
or/26-27 (1220)
-
allergens/ or exp Hypersensitivity/ (201)
-
(allerg$ or hypersensitiv$ or hyper-sensiti$ or intolerance$ or adverse reaction$ or anaphyla$ or pseudoanaphyla$ or poly-sensiti$ or polysensiti$ or paucisensiti$).ti,ab,ot,hw. (15,177)
-
or/29-30 (15,275)
-
28 and 31 (28)
-
24 or 25 or 32 (1845)
-
Rhinitis, Allergic, Seasonal/ (1)
-
((Pollen or seasonal) adj3 (allerg$ or anaphyla$ or hypersensiti$ or hyper-sensiti$ or poly-sensiti$ or polysensiti$ or paucisensiti$)).ti,ab,ot,hw. (324)
-
(hayfever or hay fever or pollinosis or pollinoses).ti,ab,ot,hw. (194)
-
(rhiniti$ adj3 (season$ or spring or summer or pollen$ or grass$ or birch or ragweed or tree$ or weed$ or mugwort or willow or alder)).ti,ab,ot,hw. (97)
-
or/34-37 (502)
-
33 or 38 (2301)
-
quality-adjusted life years/ or quality of life/ (282)
-
(sf36 or sf 36 or sf-36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,ot. (1709)
-
(sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,ot. (448)
-
(sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,ot. (418)
-
(sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab,ot. (50)
-
(sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,ot. (14)
-
(sf8 or sf 8 or sf-8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight).ti,ab,ot. (39)
-
“health related quality of life”.ti,ab,ot. (3142)
-
(Quality adjusted life or Quality-adjusted-life).ti,ab,ot. (927)
-
“assessment of quality of life”.ti,ab,ot. (121)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab,ot. (723)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab,ot. (1442)
-
(hye or hyes).ti,ab,ot. (2)
-
health$ year$ equivalent$.ti,ab,ot. (1)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab,ot. (109)
-
(quality time or qwb or quality of well being or “quality of wellbeing” or “index of wellbeing” or “index of well being”).ti,ab,ot,hw. (48)
-
(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”).ti,ab,ot. (331)
-
(QALY$ or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL or qald$ or qale$ or qtime$ or AQoL$).ti,ab,ot. (1076)
-
(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble$ or “willingness to pay”).ti,ab,ot. (541)
-
15d.ti,ab,ot. (116)
-
(HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab,ot. (21)
-
(utilit$ adj3 (“quality of life” or valu$ or scor$ or measur$ or health or life or estimat$ or elicit$ or disease$)).ti,ab,ot. (832)
-
(utilities or disutili$).ti,ab,ot. (552)
-
or/40-62 (8337)
-
letter.pt. (30,812)
-
editorial.pt. (22,136)
-
historical article.pt. (227)
-
or/64-66 (53,144)
-
63 not 67 (8298)
-
39 and 68 (20)
Health-related quality of life free-text terms based on:
-
Figure 4 Common free-text terms for electronic database searching for HSUVs in Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: the identification, review and synthesis of health state utility values from the literature (internet), 2011 (accessed 18 August 2011). Available from: www.nicedsu.org.uk.
NHS EED (via Wiley): 2015/April/Iss2
Searched: 1 July 2015
#1 food* near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (1044)
#2 (Cereal* or wheat or rye or barley or oat or oats or rice or maize or spelt or buckwheat or soybean or millet or sorghum or corn or Kamut) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (103)
#3 (Gluten or glutenin or prolamins) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (24)
#4 (Dairy or milk or yog*urt* or cream or butter* or cheese* or ice cream* or kefir) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (532)
#5 (Casein or whey or lactose) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (278)
#6 Egg* near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (266)
#7 (Peanut* or arachid or “Arachis hypog*ea” or groundnut) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (150)
#8 “arachis oil” near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (1)
#9 (Nut or nuts or hazelnut* or cashew* or walnut* or pecan* or almond* or Macadamia* or pistachio* or chestnut* or coconut* or Candlenut) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (55)
#10 (Crustacea* or Mollusc* or mollusk* or shellfish or seafood or “sea food” or Lobster or crab* or shrimp* or prawn* or squid* or oyster* or crayfish or cuttlefish or abalone or limpet* or mussel* or scallop* or clam or clams or whelk* or scampi or octopus or langoustine* or cockle* or winkles or krill) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (22)
#11 (Fish or finfish or sole or mackerel* or hake or whiting or dab or plaice or Anchov* or Catfish or Eel* or Haddock or Halibut or Sardine* or Scad or Snapper or Tilapia or Trout or shark or swordfish or cod or tuna or herring* or flounder or mullet or salmon or kipper* or caviar or seer or mackerel or tilefish or Pollock or whitebait or flatfish or “john dory” or carp) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (89)
#12 roe near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (1)
#13 (Surimi or sashimi or sushi or cerviche or gravlax) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (0)
#14 (Snail* or frog*) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (0)
#15 (Sulfites or sulphites) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (6)
#16 (sulphating or sulphur) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (5)
#17 (Lentil* or chickpea* or pea or peas or garbanzo or “bengal gram” or chana or channa or leblebi) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (8)
#18 (poppy or sunflower or cotton or flax) near/3 seed* near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (0)
#19 (fruit or fruits or vegetable* or legume* or kiwi or melon or banana* or Carrot* or apple* or tomato* or Apricot* or pepper* or Cabbage* or Celery or Celeriac or Cherry or cherries or Courgette* or zucchini or Aubergine* or Dates or Fig or figs or plum or plums or garlic* or grape* or Lettuce* or Lychee* or Mango* or Peach* or Pear or pears or Pineapple* or Pomegranate or Potato* or Pumpkin* or Strawberry or strawberries or Turnip* or Avocado* or Persimmon) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (101)
#20 (Acerola or Aniseed or Camomile or “Castor bean*” or Cocoa or linseed or Lupin* or Lupine or Sesame or soy*) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti*) (83)
#21 (Condiment* or spice* or Mustard*) near/4 (allerg* or sensitivit* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla*, or “poly-sensiti*” or polysensiti* or paucisensiti*) (8)
#22 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 (2007)
#23 MeSH descriptor: [Food Hypersensitivity] explode all trees (618)
#24 MeSH descriptor: [Food] explode all trees (22,580)
#25 MeSH descriptor: [Food Additives] explode all trees (545)
#26 #24 or #25 (22,598)
#27 MeSH descriptor: [Allergens] explode all trees (1554)
#28 MeSH descriptor: [Hypersensitivity] explode all trees (15,778)
#29 allerg* or hypersensitiv* or “hyper-sensiti*” or intolerance* or “adverse reaction*” or anaphyla* or pseudoanaphyla* or “poly-sensiti*” or polysensiti* or paucisensiti* (36,164)
#30 #27 or #28 or #29 (43,237)
#31 #26 and #30 (1384)
#32 #22 or #23 or #31 (2743)
#33 MeSH descriptor: [Rhinitis, Allergic, Seasonal] explode all trees 1611)
#34 (Pollen or seasonal) near/3 (allerg* or anaphyla* or hypersensiti* or “hyper-sensiti*” or “poly-sensiti*” or polysensiti* or paucisensiti*) (3657)
#35 hayfever or “hay fever” or pollinosis or pollinoses (951)
#36 rhiniti* near/3 (season* or spring or summer or pollen* or grass* or birch or ragweed or tree* or weed* or mugwort or willow or alder) (2962)
#37 #33 or #34 or #35 or #36 (4095)
#38 #32 or #37 (6656)
#39 MeSH descriptor: [Quality-Adjusted Life Years] this term only (3930)
#40 MeSH descriptor: [Quality of Life] this term only (15,292)
#41 sf36 or sf 36 or “sf-36” or “short form 36” or “shortform 36” or “sf thirtysix” or “sf thirty six” or “shortform thirtysix” or “shortform thirty six” or “short form thirty six” or “short form thirtysix” or “short form thirty six” (6646)
#42 sf6 or “sf 6” or sf-6 or “short form 6” or “shortform 6” or “sf six” or sfsix or “shortform six” or “short form six” (137)
#43 sf12 or “sf 12” or “sf-12” or “short form 12” or “shortform 12” or “sf twelve” or sftwelve or “shortform twelve” or “short form twelve” (975)
#44 sf6D or “sf 6D” or “sf-6D” or “short form 6D” or “shortform 6D” or “sf six D” or sfsixD or “shortform six D” or “short form six D” (186)
#45 sf20 or “sf 20” or “sf-20” or “short form 20” or “shortform 20” or “sf twenty” or sftwenty or “shortform twenty” or “short form twenty” (74)
#46 sf8 or “sf 8” or “sf-8” or “short form 8” or “shortform 8” or “sf eight” or sfeight or “shortform eight” or “short form eight” (58)
#47 “health related quality of life” (6922)
#48 “Quality adjusted life” or “Quality-adjusted-life” (6557)
#49 “assessment of quality of life” (318)
#50 euroqol or “euro qol” or eq5d or “eq 5d” (2744)
#51 hql or hrql or hqol or “h qol” or hrqol or “hr qol” (2599)
#52 hye or hyes (53)
#53 “health* year* equivalent*” (5)
#54 hui or hui1 or hui2 or hui3 or hui4 or “hui-4” or “hui-1” or “hui-2” or “hui-3” (1262)
#55 “quality time” or qwb or “quality of well being” or “quality of wellbeing” or “index of wellbeing” or “index of well being” (231)
#56 “Disability adjusted life” or “Disability-adjusted life” or “health adjusted life” or “health-adjusted life” or “years of healthy life” or “healthy years equivalent” or “years of potential life lost” or “years of health life lost” (350)
#57 QALY* or DALY* or HALY* or YHL or HYES or YPLL or YHLL or qald* or qale* or qtime* or AQoL* (5218)
#58 timetradeoff or “time tradeoff” or “time trade-off” or “time trade off” or TTO or “Standard gamble*” or “willingness to pay” (1916)
#59 15d (107)
#60 HSUV* or “health state* value*” or “health state* preference*” or HSPV* (83)
#61 utilit* near/3 (“quality of life” or valu* or scor* or measur* or health or life or estimat* or elicit* or disease*) (4632)
#62 utilities or disutili* (1656)
#63 #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 (33,724)
#64 #38 and #63 (393)
NHS EED search retrieved 34 records.*
*Please note: Records ceased to be added to the NHS EED resource on 31 March 2015, this search was for archival material only.
CEA Registry (internet): up to 1 July 2015
https://research.tufts-nemc.org/cear4/SearchingtheCEARegistry/SearchtheCEARegistry.aspx
Searched: 1 July 2015
Basic search for ‘articles’.
| Search term | Hits |
|---|---|
| Allergy | 16 |
| Allergies | 0 |
| Allergens | 0 |
| Allergic | 20 |
| Intolerance | 6 |
| Intolerances | 0 |
| Hypersensitivity | 4 |
| Hypersensitivities | 0 |
| Hyper-sensitivity | 0 |
| Hyper-sensitivities | 0 |
| Anaphylaxis | 4 |
| Anaphylactic | 1 |
| Pollen | 5 |
| Rhinitis | 9 |
| Hay fever | 0 |
| Hayfever | 0 |
| pollinosis | 0 |
| pollinoses | 0 |
| Total | 65 (including duplicates) |
PROQOLID (internet): up to 1 July 2015
Searched: 1 July 2015
Basic search
| Search term | Hits |
|---|---|
| Allergy | 0 |
| Allergies | 0 |
| Allergens | 0 |
| Allergic | 0 |
| Intolerance | 0 |
| Intolerances | 0 |
| Hypersensitivity | 0 |
| Hypersensitivities | 0 |
| Hyper-sensitivity | 0 |
| Hyper-sensitivities | 0 |
| Anaphylaxis | 0 |
| Anaphylactic | 0 |
| Pollen | 0 |
| Rhinitis | 9 |
| Hay fever | 0 |
| Hayfever | 0 |
| pollinosis | 0 |
| pollinoses | 0 |
| Total | 9 |
Appendix 2 Data extraction tables
A Baseline details (studies of change to management, treatment or diagnostic classification)
| Study details | Selection criteria | Participant details (allergic) | Participant details (healthy controls) |
|---|---|---|---|
| Gay-Crosier 201036 Country: NR Funding: NR Study design: Observational before-and-after study Recruitment: Participants undergoing subcutaneous immunotherapy. No further details reported No. of participants: 9 |
Inclusion criteria: Participants undergoing subcutaneous immunotherapy Exclusion criteria: None reported |
n: 9 Mean age, years (SD): NR Male (%): NR Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with gastrointestinal symptoms (%): NR n with respiratory symptoms (%): NR n with urticaria (%): NR n with atopic eczema (%): NR n with anaphylaxis (%): NR |
NA |
| Heaps 2014,39 201035 Country: UK Funding: Reagents and consumables provided by Thermo Fisher Scientific Study design: Prospective, observational before-and-after study Recruitment: Participants recruited from five specialist allergy centres. No further details reported No. of participants: 110 |
Inclusion criteria: Adult patients diagnosed with idiopathic anaphylaxis Exclusion criteria: None reported |
n: 110 Mean age, years (range): 42 (20–76) Male (%): 37 (33.6) Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with gastrointestinal symptoms (%): NR n with respiratory symptoms (%): NR n with urticaria (%): NR n with atopic eczema (%): NR n with anaphylaxis (%): 110 (100) |
NA |
| Hermansson 201433,34 Country: Finland Funding: NR Study design: Prospective, observational Recruitment: Database of 2317 primary school children used to identify 199 children who were on restrictive diets, of whom 85 agreed to participate in the study and were still classified as allergic following nurse interview No. of participants: 85 |
Inclusion criteria: Children who were on a restrictive diet in school catering Exclusion criteria: Coeliac disease |
n: 85 Mean age, years (range): NR Male (%): NR Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with gastrointestinal symptoms (%): NR n with respiratory symptoms (%): NR n with urticaria (%): NR n with atopic eczema (%): NR n with anaphylaxis (%): NR |
NA |
| Luengo 201037 Country: Spain, ‘Mediterranean population’ Funding: NR Study design: observational before-and-after study Recruitment: No details reported No. of participants: 55 |
Inclusion criteria: Well characterised, multisensitised, allergic patients Exclusion criteria: None reported |
n: 55 Mean age, years (range): NR Male (%): NR Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with gastrointestinal symptoms (%): NR n with respiratory symptoms (%): NR n with urticaria (%): NR n with atopic eczema (%): NR n with anaphylaxis (%): NR |
NA |
| Noimark 201240 Country: UK Funding: NR Study design: Case series, abstract only, no details reported Recruitment: Selected participants from a specialist allergy centre. No further details reported No. of participants: 12 |
Inclusion criteria: Children with moderate to severe eczema and multiple food allergies Exclusion criteria: None reported |
n: 12 Mean age, years (range): NR Male (%): NR Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with gastrointestinal symptoms (%): NR n with respiratory symptoms (%): NR n with urticaria (%): NR n with atopic eczema (%): 12 (100) n with anaphylaxis (%): NR |
NA |
| Passalacqua 201338 Country: Italy Funding: Phadia AB/Thermo Fisher Scientific Study design: Prospective, observational before-and-after study Recruitment: Participants recruited from six allergy centres. No further details reported No. of participants: 409 (318 allergy patients and 91 healthy controls) |
Inclusion criteria: Patients referred for respiratory allergic diseases who had at least two positive SPTs. Controls had negative SPTs Exclusion criteria: None reported |
n: 318 Mean age, years (range): 37 (12–78) Male (%): 148 (46.5) Median duration of allergy, years (range): NR n with oral allergy syndrome (%): 51 (16) n with gastrointestinal symptoms (%): 14 (4.4) n with respiratory symptoms (%): 318 (100) n with urticaria (%): 36 (11.3) n with atopic eczema (%): 4 (1.3) n with anaphylaxis (%): 6 (1.9) |
n: 91 Mean age, years (range): 40 (15–83) Male (%): 19 (20.9) Median duration of allergy, years (range): NA n with oral allergy syndrome (%): 0 (0) n with gastrointestinal symptoms (%): 0 (0) n with respiratory symptoms (%): 0 (0) n with urticaria (%): 0 (0) n with atopic eczema (%): 0 (0) n with anaphylaxis (%): 0 (0) |
| Sastre 201230–32,59 Country: Spain Funding: CIBER de Enfermedades Respiratorias and Instituto de Salud Carlos III of the Ministry of Science and Information, Spain Study design: Observational before-and-after study Recruitment: Participants attending an outpatient allergy clinic. No further details reported No. of participants: 141 |
Inclusion criteria: Patients with allergic rhinoconjunctivitis and/or asthma who were sensitised to pollen, with or without concomitant food allergy Exclusion criteria: None reported |
n: 141 Mean age, years (SD): 31 (13.6) Male (%): 58 (41) Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with gastrointestinal symptoms (%): NR n with respiratory symptoms (%): 141 (100) n with urticaria (%): NR n with atopic eczema (%): NR n with anaphylaxis (%): NR |
NA |
B Baseline accuracy study details (diagnostic case–control)
| Study details | Selection criteria | Participant details (allergic) | Participant details (tolerant) |
|---|---|---|---|
| Alessandri 201142 Country: Italy Funding: Italian Ministry of Health, Programma Ricerca Corrente 2008–2010 Recruitment: January 2008 to September 2010 No. of participants: 68 |
Inclusion criteria: Children referred for suspected hen’s egg allergy (based on history of reactions after ingestion and positive SPT or IgE to hen’s egg white extracts). All patients were following a hen’s egg elimination diet Exclusion criteria: Steroid treatment Recruitment site: Centre for Molecular Allergology, IDI-IRCCS, Rome, Italy |
n: 19 Median age, years (range): 4.3 (NR) Male (%): 15 (79) Median duration of allergy, years (range): NR n with atopic eczema (%): NR n with oral allergy syndrome (%): 3 (16)a n with asthma (%): 7 (37)a n with anaphylaxis (%): 1 (5) n with gastritis/vomiting (%): 11(58) n with skin symptom (%): NR n with respiratory symptoms (%): NR |
n:
n with oral allergy syndrome (%):n with asthma (%):n with anaphylaxis (%):n with gastritis/vomiting (%):n with atopic eczema (%): NR n with skin symptom (%): NR n with respiratory symptoms (%): NR |
| Cabrera-Freitag 2011,43 2010,94 201148 Country: Spain Funding: Spanish Society of Allergy and Clinical Immunology Foundation; Spanish Research Network on Adverse Reactions to Allergens and Drugs Recruitment: March 2008 to May 2009 No. of participants: 173 |
Inclusion criteria: Allergic patients had (1) an allergen-specific history (rhinitis, rhinoconjunctivitis and/or bronchial asthma) during the season of pollinisation of grass pollen and/or cypress pollen and (2) a positive SPT to the corresponding pollen, P. pratense and/or C. arizonica. Controls had no pollen allergen-specific history and had negative SPT to the corresponding pollen Exclusion criteria: Patients showing clinical history during the season of pollinisation of grass pollen and showing SPT-positive to grass pollen and to other pollen that pollinated in the same season (i.e. olive) Recruitment site: Clínica Universidad de Navarra, Pamplona, Spain |
n with oral allergy syndrome (%): NR n with asthma (%):
n with gastritis/vomiting (%): NR n with atopic eczema (%): NR n with skin symptom (%): NR n with respiratory symptoms (%): NR |
Mean age, years (25th–75th percentile):
n with oral allergy syndrome (%): NR n with asthma (%):
n with gastritis/vomiting (%): NR n with atopic eczema (%): NR n with skin symptom (%): NR n with respiratory symptoms (%): NR |
| De Swert 201241 Country: Belgium Funding: NR Recruitment: NR No. of participants: 15 |
Inclusion criteria: Subjects with birch pollen allergy (typical allergic symptoms during the birch pollen season in combination with a positive IgE response to birch or rBet v1), suspected of also being soy allergic Exclusion criteria: NR Recruitment site: Outpatient allergy clinic, Paediatric Department, University Hospital Gasthuisberg, Leuven, Belgium |
n: 8 Median age, years (range): 10.3 (4.8–15.6) Male (%): NR Median duration of allergy, years (range): tree 3.7 (1–9) n with oral allergy syndrome (%): 7 (88) n with asthma (%): NR n with atopic eczema (%): NR n with anaphylaxis (%): NR n with gastritis/vomiting (%): NR n with skin symptom (%): NR n with respiratory symptoms (%): NR |
n: 7 Median age, years (range): 10.1 (4.7–16) Male (%): NR Median duration of allergy, years (range): tree 3.5 (1–10) n with oral allergy syndrome (%): 4 (57) n with asthma (%): NR n with atopic eczema (%): NR n with anaphylaxis (%): NR n with gastritis/vomiting (%): NR n with skin symptom (%): NR n with respiratory symptoms (%): NR |
| Sokolova 200946 Country: Portugal Funding: Phadia, Portugal Recruitment: NR No. of participants: 41 |
Inclusion criteria: Patients from Food Allergy Outpatient Clinic who, at the time of diagnosis, had a clinical picture compatible with IgE-mediated CMPA, documented by SPT and positive specific IgE (> 0.35 kU/l) to whole milk and/or its protein fractions (α-LA, β-LG and casein). Control group consisted of four atopic individuals with no history of CMPA and who ingested cow’s milk daily Exclusion criteria: NR Recruitment site: Food Allergy Outpatient Clinic, Centro Hospitalar Lisboa Norte, Lisbon, Portugal |
n: 17 Mean age, years (range): 9.25 (2–19) Male (%): 10 (58.5) Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with asthma (%): NR n with anaphylaxis (%): NR n with gastritis/vomiting (%): NR n with atopic eczema (%): 2 (12) n with skin symptom (%): NR n with respiratory symptoms (%): NR |
n: 20 Control definition: Negative OFC Mean age, years (range): 6.15 (2–22) Male (%): 7 (65) Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with asthma (%): NR n with anaphylaxis (%): NR n with gastritis/vomiting (%): NR n with atopic eczema (%): 6 (30) n with skin symptom (%): NR n with respiratory symptoms (%): NR |
C Baseline accuracy study details (diagnostic cohort studies)
| Study details | Selection criteria | Participant details |
|---|---|---|
| Albarini 201347 Country: NR Funding: NR Recruitment: April 2007 to May 2012 No. of participants: 35 |
Inclusion criteria: Children with immediate reaction to hazelnut ingestion Exclusion criteria: NR Recruitment site: NR |
n: 35 Median age, years (range): 8.3 (2.2–14.2) Male (%): 26 (74) Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with asthma (%): NR n with anaphylaxis (%): NR n with gastritis/vomiting (%): NR n with atopic eczema (%): NR n with skin symptom (%): NR n with respiratory symptoms (%): NR |
| D’Urbano 201044 Country: Italy Funding: IRCCS Children’s Hospital Bambino Gesù Recruitment: NR No. of participants: 104 (58 cow’s milk and 46 hen’s egg) |
Inclusion criteria: Infants and children referred for evaluation of suspected IgE-mediated food hypersensitivity (history related to cow’s milk or hen’s egg consumption, of severe and/or immediate reactions) Exclusion criteria: Atopic eczema as the only indication for suspected allergy Recruitment site: Department of Paediatric Medicine–Allergy Unit, IRCCS Children’s Hospital Bambino Gesù, Rome, Italy |
n: 104 Median age, years (range): 4.9 (0.7–15.1) Male (%): 62 (60) Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with asthma (%): NR n with atopic eczema (%): NR n with skin symptom (%): 44 (42) n with respiratory symptoms (%): 6 (96) n with gastrointestinal symptoms (%): 33 (32) n with anaphylaxis (%): 4 (4) |
| Ott 200849 Country: Germany Funding: START programme of the Medical Faculty of the Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen Recruitment: NR No. of participants: 130 |
Inclusion criteria: Children referred for evaluation of suspected IgE-mediated food hypersensitivity Exclusion criteria: NR Recruitment site: Charité Allergy Center, Universitätsmedizin Berlin, Germany |
n: 130 Median age, months (range): NR [total = 14 (5–150)] Male (%): 70 (54) Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with asthma (%): NR n with atopic eczema (%): NR n with anaphylaxis (%): NR n with vomiting (%): 23 (16) n with skin symptom (%): NR n with respiratory symptoms (%): NR |
| Wohrl 200645 Country: Austria Funding: ISAC and IgE were performed in the laboratories of VBC-GENOMICS, Vienna, Austria Recruitment: September to October 2004 No. of participants: 120 patients with allergic rhinitis |
Inclusion criteria: Adults at the end of the pollen season Exclusion criteria: Total serum IgE > 1000 kU/l (to minimise non-specific binding in the ImmunoCAP system) Recruitment site: Allergy Outpatient Clinic of the Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna, and at a private outpatient allergy clinic FAZ–Floridsdorf Allergy Center, Vienna |
n: 120 Mean age, years (SD): 35.9 (14.4) Male (%): 50 (42) Median duration of allergy, years (range): NR n with oral allergy syndrome (%): NR n with asthma (%): NR n with atopic eczema (%): NR n with skin symptom (%): NR n with respiratory symptoms (%): NR n with gastrointestinal symptoms (%): NR n with anaphylaxis (%): NR |
D Index test and comparator details (studies of change to management, treatment or diagnostic classification)
| Study details | Index test details | Standard care details |
|---|---|---|
| Heaps 201439 | Version: ImmunoCAP ISAC 103 Manufacturer: Phadia/Thermo Fisher Scientific, Milan, Italy Method: ‘According to the manufacturer’s instructions.’ Slides were scanned using a GenePix 4000B microarray scanner (Molecular Devices, Sunnyvale, CA, USA). Image analysis was performed using the Microarray Image Analyser (MIA: Thermo Fisher Scientific/Phadia, Uppsala, Sweden). All new positive ISAC results were retested for confirmation Allergens (components) assessed: NR Definition of a positive result: ‘According to the manufacturer’s instructions’ ISU > 0.3 Positive control: NR Negative control: NR |
Method: SPT and clinical history, and sIgE and MCT SPT: NR sIgE and MCT: FEIA auto-analyser, ImmunoCAP 250 platform (Thermo Fisher Scientific/Phadia AB, Uppsala, Sweden), ‘according to the manufacturer’s instructions’ |
| Hermansson 201433,34 | Version: ImmunoCAP ISAC 112 Manufacturer: NR Method: NR Allergens (components) assessed: NR Definition of a positive result: NR Positive control: NR Negative control: NR |
Method: Clinical history/parental report, and sIgE sIgE: RAST |
| Luengo 201037 | Version: ImmunoCAP ISAC 103 Manufacturer: NR Method: NR Allergens (components) assessed: NR Definition of a positive result: NR Positive control: NR Negative control: NR |
Method: SPT and sIgE SPT: NR sIgE: NR |
| Noimark 201240 | Version: ImmunoCAP ISAC unspecified version Manufacturer: NR Method: NR Allergens (components) assessed: NR Definition of a positive result: NR Positive control: NR Negative control: NR |
Method: SPT and/or sIgE SPT: NR sIgE: NR |
| Passalacqua 201338 | Version: ImmunoCAP ISAC 103 Manufacturer: Thermo Fisher Scientific, Milan, Italy Method: According to the manufacturer’s instructions. Slides were read automatically using a Laser Scan Confocal microarray reader (LuxScan 10K/A, CapitalBio, Beijing, China) Allergens (components) assessed: NR Definition of a positive result: ≥ 0.35 ISU Positive control: NR Negative control: NR |
Method: SPT and clinical history, with sIgE as required SPT: Standard panel of commercial extractive preparations (ALK-Abelló, Milan, Italy), including mites, grass, olive, Parietaria, birch, cypress, ragweed, mugwort, cat and dog dander, Alternaria and Aspergillus. A positive result was defined as a weal reaction of ≥ 3 mm in diameter. 1% histamine was used as a positive control and diluent as a negative control sIgE: Commercial immunoenzymatic method (Thermo Fisher Scientific/Phadia AB, Uppsala, Sweden). A positive result was defined as > 0.35 kU/l |
| Sastre 201232 | Version: ImmunoCAP ISAC 96 Manufacturer: Phadia, Sweden Method: ‘According to the manufacturer’s instructions’ Allergens (components) assessed: Olive (Ole 1); cypress (Cup s 1); plane (pla a1, Pla a2); grass (Phl p1, phl p5); cynodon (Cyn d1) Definition of a positive result: ‘According to the manufacturer’s instructions’ Positive control: NR Negative control: NR |
Method: SPT and clinical history, taking into consideration the time of year of respiratory symptoms and European Academy of Allergy and Clinical Immunology guidelines SPT: Standard panel of commercial inhalants (ALK-Abelló, Madrid, Spain), including Olea e, Platanus a, Cupressus a, grass mix, Cynodon d, Phragmites c, Artemisia v, Salsola k, and Plantago l. A positive result was defined as a weal reaction ≥ 3 mm more than negative control. Histamine (10 mg/ml) was used as a positive control and glycerol–saline solution as a negative control |
E Index test and reference standard details (accuracy studies)
| Study details | Index test details | Reference standard details: oral challenge | ||
|---|---|---|---|---|
| ImmunoCAP/Microtest | Specific IgE tests | SPT | ||
| Albarini 201347 | Version: ImmunoCAP ISAC unspecified version Manufacturer: NR Method: NR Definition of positive result: NR Positive control: NR Negative control: NR |
Version: ImmunoCAP Manufacturer: NR Method: NR Definition of positive result: > 0.35 kUI/l Positive control: NR Negative control: NR |
Method: NR Allergen: NR Positive result: Mean weal diameter > 3 mm Positive control: NR Negative control: NR |
DBPCFC |
| Alessandri 201142 | Version: ImmunoCAP ISAC 103 Manufacturer: Phadia AB, Uppsala, Sweden Method: ‘According to the manufacturer’s instructions’ Definition of a positive result: Thresholds for each allergen/component derived from ROC analyses, but not reported Positive control: NR Negative control: NR |
Version: ImmunoCAP Manufacturer: Phadia AB, Uppsala, Sweden Method: ‘According to the manufacturer’s instructions’ Definition of a positive result: Thresholds for each allergen/component derived from ROC analyses, but not reported Positive control: NR Negative control: NR |
Method: Performed in duplicate on the volar surface of the forearm by the same investigator, following European Academy of Allergy and Clinical Immunology recommendations, using 1-mm-tipped lancets. Weal reactions were recorded after 15 minutes, by outlining with pen onto paper sheets, which were scanned to digitally measure areas Allergen: Commercial extracts (Allergopharma, Reinbek, Germany) and freshly prepared egg reagents Positive result: Mean weal diameter of ≥ 7 mm Positive control: Histamine diphosphate (10 mg/ml) Negative control: Glycerol–saline solution |
Double-blind placebo-controlled hen’s egg challenges were carried out using commercially available eggs Boiled egg: Administering an initial dose of 0.1 ml, and, in case of no reactions in the next 20 minutes, by progressively increasing the egg amount by a factor 5 (0.5, 2, 10 and 50 ml) up to the ingestion of one egg (approximately 6 g) Patients tolerating boiled egg were then challenged with raw egg in a similar way |
| De Swert 201241 | Version: ImmunoCAP ISAC unspecified version Manufacturer: Phadia AB, Uppsala, Sweden Method: ‘According to the manufacturer’s instructions’ Definition of a positive result: ≥ 0.3 ISU, ≥ 1.0 ISU for rGly m4 Positive control: NR Negative control: NR |
Version: ImmunoCAP FEIA Manufacturer: Phadia AB, Uppsala, Sweden Method: ‘According to the manufacturer’s instructions’ Definition of a positive result: ≥ 0.10 kU/l, ≥ 17.6 kU/I for rGly m4 Positive control: NR Negative control: NR |
Method: Performed using a microlance. Weal reactions were recorded after 15 minutes; orthogonal diameters were measured and mean diameters were calculated Allergen: 1/10 w/v dilution of soy flour (Sojameel, Biofresh, Genk, Belgium) Positive result: Mean weal diameter ≥ 3 mm, (cut-off point ≥ 7 mm rGly m4) Positive control: Histamine diphosphate (1 mg/ml) Negative control: Coca solution in 50% glycerol |
Subjects were on a soy-free diet for at least 8 weeks Challenge performed with Alpro soya natural drink; one drop of soy drink at the inner side of the lower lip If no reaction occurred within 15 minutes, increasing doses of 1, 2, 5, 10, 20, 40 and 80 ml of soy drink were given at 20-minute intervals, until appearance of objective allergic symptoms (or until 158 ml) If no symptoms after 2 hours, the parents were asked to give the child daily volumes of 120 ml of soy drink in the next 2 weeks, while continuing their diet otherwise unchanged Re-evaluation was provided after 2 weeks or earlier if required |
| D’Urbano 201044 | Version: ImmunoCAP ISAC 89 Manufacturer: Phadia AB, Uppsala, Sweden Method: ‘according to the manufacturer’s instructions’ Definition of positive result: NR Positive control: NR Negative control: NR |
Version: ImmunoCAP Manufacturer: Phadia AB, Uppsala, Sweden Method: ‘according to the manufacturer’s instructions’ Definition of positive result: NR Positive control: NR Negative control: NR |
Method: The response was read 15 min after puncture and results were expressed as the mean weal diameter (mm) Allergen: Natural food and commercial natural extracts to milk, α-lactalbumin, β-lactoglobulin, casein, egg white, egg yolk (Lofarma, Milan, Italy) Positive result: Mean weal diameter of > 3 mm with erythema Positive control: Histamine hydrochloride Negative control: Sodium chloride (0.9%) |
Performed in an open fashion The material was pasteurised cow’s milk and cooked egg (boiled for 10 minutes) or raw egg in the case of negative result to cooked egg When the patient tolerated the first dose, the subsequent doses were given every 15 minutes until objective symptoms developed or when the entire dose was ingested (equivalent to one egg; or up to 250 ml of milk) Positive result was scored if anaphylactic shock or two or more of the following objective clinical reactions were noted: bronchial asthma, lips/periorbital oedema, urticaria/angioedema, rhinitis, conjunctivitis, diarrhoea and repetitive vomiting |
| Ott 200849 | Version: ImmunoCAP ISAC 51 Manufacturer: VBC Genomics Bioscience Research, Vienna, Austria Method: ‘According to the manufacturer’s instructions’ Definition of positive result: Cut-off points used for analyses derived from ROC analyses Positive control: NR Negative control: NR |
Version: UniCAP® Manufacturer: Phadia AB, Uppsala, Sweden Method: ‘According to the manufacturer’s instructions’ Definition of positive result: > 0.35 kU/l (derived from ROC analyses) Positive control: NR Negative control: NR |
Method: One drop of either milk or native hen’s egg was applied to the patient’s forearm with 1-mm single-peak lancets (ALK, Copenhagen, Denmark) Allergen: Fresh cow’s milk (3.5% fat) or native hen’s egg (whisked white of egg and yolk). Definition of positive result: Mean weal diameter > 3 mm, or greater than negative control. Cut-off points used for analyses derived from ROC analyses Positive control: Histamine hydrochloride (1%) Negative control: Saline |
OFCs with either cow’s milk and/or hen’s egg The food challenges were scored as positive by a paediatric allergologist if one or more of the following objective clinical reactions were noted: urticaria, flushing, pruritus, angioedema, exacerbation of AE, vomiting, diarrhoea, stridor or other respiratory symptoms |
| Study details | Index test details | Reference standard details | |
|---|---|---|---|
| ImmunoCAP®/Microtest | Specific IgE tests | Oral challenge | |
| Sokolova 200946 | Version: ImmunoCAP ISAC NR Manufacturer: VBC Genomics Bioscience Research, Vienna, Austria Method: ‘According to the manufacturer’s instructions’ Definition of positive result: Cut-off points NR Positive control: NR Negative control: NR |
Version: UniCAP Manufacturer: Phadia, Uppsala, Sweden Method: ‘According to the manufacturer’s instructions’ Definition of positive result: > 0.35 kU/l Positive control: NR Negative control: NR |
Patients complaining of anaphylaxis after accidental ingestion of milk or its derivatives were considered persistent A diagnosis of persistent CMPA was confirmed in the remaining patients via a positive oral challenge test, performed following current recommendations. The initial dose administered was 0.1 ml with posterior duplication of the doses and administration at 30-minute intervals. It was considered positive if cutaneous (urticaria/angioedema), respiratory or gastrointestinal (vomiting, diarrhoea) symptoms occurred. A negative open oral challenge to cow’s milk was defined as a cumulative dose of 200 ml The control group consisted of four atopic individuals with no history of CMPA and who ingested cow’s milk daily |
| Study details | Index test details | Reference standard details: SPT + allergen-specific history | |
|---|---|---|---|
| ImmunoCAP/Microtest | Specific IgE tests | ||
| Cabrera-Freitag 201143 | Version: ImmunoCAP ISAC 103 Manufacturer: Phadia, Uppsala, Sweden Method: ‘According to the manufacturer’s instructions’ Definition of positive result: ≥ 0.3 ISU. Thresholds for each allergen/component derived from ROC analyses, but not clearly reported Positive control: NR Negative control: NR |
Version: ImmunoCAP FEIA Manufacturer: Phadia, Uppsala, Sweden Method: ‘According to the manufacturer’s instructions’ Definition of positive result: > 0.35 kU/l. Thresholds for each allergen/component derived from ROC analyses, but not clearly reported Positive control: NR Negative control: NR |
Method: 1-mm-tip lancet (ALK-Abelló) on the volar side of the forearm. Read after 20 minutes. Performed by the same experienced nurses Allergen: Commercial, natural extracts (ALK-Abelló, Madrid, Spain) Definition of positive result: Mean weal diameter of > 3 mm Positive control: Histamine hydrochloride (10 mg/ml) Negative control: Sodium chloride (0.9%) Allergen history: Rhinoconjunctivitis and/or bronchial asthma Controls had no pollen allergen-specific history and had negative SPT to the corresponding pollen |
| Wohrl 200645 | Version: ImmunoCAP ISAC CRD 50 Manufacturer: Genomics Bioscience Research, Vienna, Austria Method: ‘According to the manufacturer’s instructions’. Slides were scanned in an Affymetrix 428 microarray scanner (Affymetrix, Santa Clara, CA, USA). Images were analysed using the GenePix image analysis software (version 3.0.6.89; Axon Instruments, Union City, CA, USA) Definition of a positive result: Thresholds for each allergen/component derived from ROC analyses, but not reported Positive control: NR Negative control: NR |
Version: ImmunoCAP Manufacturer: Phadia, Uppsala, Sweden Method: ‘According to the manufacturer’s instructions’ Definition of a positive result: Thresholds for each allergen/component derived from ROC analyses, but not reported Positive control: NR Negative control: NR |
Method: SPTs were read after 20 minutes. Weals and flares were pen-marked, transferred to a paper with transparent adhesive tape and analysed with an investigator-independent system calculating the weal size in mm2 Allergen: Commercial extracts (HAL Allergie GmbH, Germany, and ALK, Hørsholm, Denmark) Positive result: Mean weal area of ≥ 7 mm2 or > 3 mm diameter Positive control: Histamine hydrochloride (ALK) Negative control: Sodium chloride (0.9%) Allergen history: Obtained in all subjects using a questionnaire that gave special regard to the clinical relevance of the sensitisation to each allergen (e.g. clinical relevance of the sensitisation to birch pollen was affirmed by asking for an oral allergy syndrome to apple and other Rosaceae fruits) All subjects without allergen-specific history (atopics) and those with additional negative SPTs (non-allergic) served as controls |
Appendix 3 Table of excluded studies with rationale
| Study details | Allergy type | Secondary or tertiary care? | Intervention | Reference | Outcome | Reason for exclusion and comments |
|---|---|---|---|---|---|---|
| Ackerbauer 201595 | Food | Yes | ImmunoCAP ISAC 112 | Other | Concordance only | No relevant outcomes Correlation of specific serum IgE levels measured by single ImmunoCAP sIgE and ImmunoCAP ISAC 112, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Acosta Rivera 201296 | Food | Yes | ImmunoCAP ISAC 103 | SPT | No relevant outcomes | Insufficient information for accuracy |
| Alessandri 201197 | Food | Yes | ImmunoCAP ISAC 103 | OFC | No relevant outcomes | Insufficient information for accuracy |
| Alonso 201498 | Food | ImmunoCAP ISAC 112 | Other | No relevant outcomes | Method of diagnosis not adequately reported; insufficient information for accuracy | |
| Alvarado 201399 | Food | Yes | ImmunoCAP ISAC other | NA | No relevant outcomes | No relevant outcomes |
| Antonicelli 2014100 | Food | Unclear | Other | Other | No relevant outcomes | Microarray and reference standard not specified and no relevant outcomes |
| Araujo 2012101 | Pollen | Unclear | ImmunoCAP ISAC other | Other | No relevant outcomes | Unspecified version ISAC, diagnosis according to Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines; insufficient information for accuracy |
| Asero 2014102 | Pollen | Unclear | Other | NA | No relevant outcomes | Not ISAC or Microtest |
| Bauermeister 2009103 | Food | Unclear | Other | NA | No relevant outcomes | ImmunoCAP® 250 – not ISAC or Microtest |
| Berneder 2013104 | Food | Unclear | ImmunoCAP ISAC 112 | NA | Concordance only | No relevant outcomes Correlation of specific serum IgE levels measured by single ImmunoCAP sIgE and ImmunoCAP ISAC 112, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Blankestijn 2014105 | Yes | ImmunoCAP ISAC other | NA | No relevant outcomes | Unspecified version ISAC, prevalence only | |
| Bokanovic 2013106 | Pollen | Yes | ImmunoCAP ISAC 103 | SPT | Accuracy only | Comparative accuracy: whole panel ISAC vs. sIgE vs. inhaled challenge. Some patients tested with ISAC 112. However, sIgE were reported as measured with ImmunoCAP component assays and ISAC, and accuracy data appeared to relate to the component measured rather than the test (i.e. included participants could have been tested using either method) |
| Bonini 2010107 | Other | Unclear | ImmunoCAP ISAC 103 | SPT | No relevant outcomes | Insufficient information for accuracy |
| Bonini 2010108 | Other | Unclear | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Bonini 2012109 | Other | Yes | ImmunoCAP ISAC 103 | NA | Concordance only | No relevant outcomes Correlation of specific serum IgE levels measured by single ImmunoCAP sIgE and ImmunoCAP ISAC 103, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Brans 2012110 | Food | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | Concordance only, FEIA vs. single IgE derived from ISAC Comparison of levels of sIgE against omega-5-gliadin, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Cabrera-Freitag 2011111 | Yes | ImmunoCAP ISAC 103 | NA | No relevant outcomes | Technical reproducibility data only | |
| Caimmi 2011112 | Food | ImmunoCAP ISAC other | SPT | No relevant outcomes | Unspecified version ISAC, insufficient information for accuracy or concordance | |
| Caimmi 2011113 | Other | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | Unspecified version ISAC, insufficient information for concordance or accuracy |
| Caimmi 2013114 | Other | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | No relevant outcomes |
| Carter 2012115 | Other | Yes | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Cavagni 2009116 | Food | Unclear | ImmunoCAP ISAC 89 | OFC | No relevant outcomes | Insufficient information for accuracy |
| Cavagni 2010117 | Food | Unclear | ImmunoCAP ISAC 89 | OFC | No relevant outcomes | Insufficient information for accuracy |
| Chambel 2011118 | Pollen | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | Unspecified version ISAC; insufficient information for accuracy or concordance |
| Chambel 2012119 | Pollen | Yes | ImmunoCAP ISAC 103 | SPT | No relevant outcomes | Insufficient information for accuracy; includes only patients with a positive reference standard diagnosis |
| Charalambous 2014120 | Other | Yes | ImmunoCAP ISAC other | NA | No relevant outcomes | Exclude ‘Audit’ of clinical records information on patients previously tested with ISAC; no details of test results or their effects on decision-making |
| Chevallier 2013121 | Food | Yes | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Chia 2013122 | Food | Unclear | Other | Other challenge test | No relevant outcomes | Intervention was unspecified version ISAC or ImmunoCAP; not enough detail for accuracy data |
| Cho 2014123 | Pollen | Yes | Other | NA | Concordance only | No relevant outcomes Comparison of positive rates between SPT, AdvanSure and ImmunoCAP sIgE |
| Choi 2014124 | Other | Yes | ImmunoCAP ISAC other | SPTs | No relevant outcomes | Unspecified version ISAC; insufficient information for patient level accuracy |
| Choi 2014125 | Other | Unclear | ImmunoCAP ISAC other | SPTs | No relevant outcomes | Unspecified version ISAC; insufficient details for accuracy |
| De Amici 2014126 | Other | Unclear | ImmunoCAP ISAC 112 | NA | No relevant outcomes | Comparison with ISAC 103, outcome was described as ‘diagnostic utility’ and ‘therapeutic utility’ scores, but no further details were reported |
| De Boer 2012127 | Other | Unclear | ImmunoCAP ISAC 103 | NA | No relevant outcomes | Technical reproducibility and limit of detection only, using a single pooled QC sample. Intra-assay and inter-assay variation |
| De Boer 2013128 | Other | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | No relevant outcomes |
| De Boer 2013128 | Food | Yes | ImmunoCAP ISAC 112 | NA | No relevant outcomes | No relevant outcomes |
| De Knop 2011129 | Food | Yes | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Doyen 2011130 | Unclear | ImmunoCAP ISAC 103 | NA | No relevant outcomes | Interference of omalizunab with ISAC and ImmunoCAP | |
| Ebo 2010131 | Latex | Yes | ImmunoCAP ISAC 103 | SPT | No relevant outcomes | Insufficient information for accuracy |
| Ebo 2010132 | Pollen | Yes | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Eller 2013133 | Food | Yes | Other | SPT | Accuracy only | ImmuoCAP sIgE only |
| Fernandez 2011134 | Pollen | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | Unspecified version ISAC, insufficient information for concordance |
| Flores 2014135 | Pollen | Yes | ImmunoCAP ISAC other | NA | No relevant outcomes | No relevant outcomes |
| Fung 2012136 | Food | Unclear | ImmunoCAP ISAC other | Other | No relevant outcomes | No relevant outcomes |
| Gadisseur 2009137 | Other | Unclear | ImmunoCAP ISAC 103 | NA | No relevant outcomes | Insufficient information for concordance or accuracy |
| Gadisseur 2011138 | Other | Unclear | ImmunoCAP ISAC 103 | NA | Concordance only | No relevant outcomes Reports agreement on allergen source between specific serum IgE levels measured by single ImmunoCAP sIgE and ImmunoCAP ISAC 103, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Gadisseur 2011138 | Other | Yes | ImmunoCAP ISAC 103 | NA | Concordance only | No relevant outcomes Reports agreement between sIgE and ImmunoCAP ISAC 103, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Gadisseur 2012139 | Food | Unclear | ImmunoCAP ISAC 112 | NA | No relevant outcomes | Refers to additional information provided by ISAC, but no details given |
| Garriga Baraut 2010140 | Pollen | Unclear | ImmunoCAP ISAC other | SPT | No relevant outcomes | Unspecified version ISAC; insufficient information for accuracy |
| Goikoetxea 2013141 | No | Other | NA | No relevant outcomes | Review article | |
| Goikoetxea 2015142 | Yes | ImmunoCAP ISAC 112 | NA | No relevant outcomes | No relevant outcomes | |
| Hermansson 201260 | Pollen | ImmunoCAP ISAC other | Other | Economics only | No relevant outcomes | |
| Hoffmann 2014143 | Pollen | Unclear | Other | NA | No relevant outcomes | Intervention was SIT: use of ISAC and IgE/IgG4 to measure response to SIT |
| Hong 2011144 | Food | Unclear | ImmunoCAP ISAC 103 | Other | No relevant outcomes | Insufficient information for accuracy |
| Hong 2012145 | Food | Yes | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Javaloyes 2012146 | Food | Yes | ImmunoCAP ISAC 103 | Other | No relevant outcomes | No relevant outcomes |
| Jung 2012147 | Pollen | Unclear | ImmunoCAP ISAC other | SPT | No relevant outcomes | Insufficient information for accuracy or concordance and unspecified version of ISAC |
| Kattan 2013148 | Food | Yes | ImmunoCAP ISAC 112 | OFC | No relevant outcomes | Insufficient information for accuracy |
| Kattan 2014149 | Food | Yes | ImmunoCAP ISAC 112 | OFC | Accuracy only | Accuracy of components of ISAC for peanut allergy only (no comparative data) |
| Kim 2015150 | Pollen | Unclear | ImmunoCAP ISAC other | SPT | No relevant outcomes | Insufficient detail on accuracy data |
| Klemans 2013151 | Food | Yes | ImmunoCAP ISAC 103 | SPT | No relevant outcomes | No relevant outcomes, incomplete accuracy data |
| Klemans 2014152 | Food | Yes | ImmunoCAP ISAC 112 | OFC | Accuracy only | ISAC only, no comparative accuracy data |
| Konradsen 2015153 | Other | Unclear | Microtest | NA | Concordance only | No relevant outcomes Correlation of specific serum IgE levels measured by Microtest, single ImmunoCAP sIgE and ImmunoCAP ISAC 112, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the three methods |
| Lee 2013154 | Food | Unclear | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Liso 2011155 | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | Experimental ISAC with omalizumab added and no relevant outcomes | |
| Liu 2011156 | Food | Unclear | ImmunoCAP ISAC other | OFC | Accuracy only | Accuracy reported for the allergen component (measured by a variety of methods). Data reported only for ISAC; no comparative accuracy data |
| Lizaso 2011157 | Pollen | Yes | ImmunoCAP ISAC 89 | NA | Concordance only | No relevant outcomes Reports percentage agreement between single ImmunoCAP sIgE, ImmunoCAP ISAC 89, and an Avida-Centaur component resolved assay, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Lohman 2013158 | Other | NA | No relevant outcomes | Not ISAC, development of a microarray | ||
| Luengo 2011159 | Food | Unclear | ImmunoCAP ISAC 103 | NA | No relevant outcomes | Insufficient outcome information |
| Mailhol 2013160 | Unclear | ImmunoCAP ISAC 112 | NA | No relevant outcomes | No relevant outcomes | |
| Martinez-Aranguren 2013161 | Unclear | ImmunoCAP ISAC 112 | NA | No relevant outcomes | Technical reproducibility only, wrong comparator | |
| Martinez-Aranguren 2014162 | Other | Unclear | ImmunoCAP ISAC 112 | NA | Concordance only | No relevant outcomes Reports agreement on sensitisations between single ImmunoCAP sIgE, ImmunoCAP ISAC112 and SPT, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Martinez-Aranguren 2014163 | Food | Yes | ImmunoCAP ISAC 112 | SPT | Accuracy only | ISAC only, no comparative accuracy |
| Martinez-Aranguren 2014164 | Other | Yes | ImmunoCAP ISAC 112 | NA | No relevant outcomes | Technical data, assay reproducibility only |
| Mascialino 201359 | Pollen | Yes | ImmunoCAP ISAC other | NA | Economics only | No relevant outcomes |
| Mascialino 201358 | Pollen | Economics only | No relevant outcomes | |||
| Melioli 2011165 | Pollen | Yes | ImmunoCAP ISAC 103 | NA | Concordance only | No relevant outcomes Reports percentage positive agreement between single ImmunoCAP sIgE and ImmunoCAP ISAC 103, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Microtest DX 2014166 | Other | Unclear | Microtest | NA | Concordance only | No relevant outcomes Correlation of specific serum IgE levels measured by Microtest, single ImmunoCAP sIgE and ImmunoCAP ISAC 112, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the three methods |
| Murng 2011167 | Other | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | Insufficient details of outcomes reported, unspecified version ISAC |
| Murng 2011168 | Food | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | Insufficient outcome information |
| Nanda 2015169 | Food | Unclear | Other | NA | Concordance only | No relevant outcomes Correlation of specific serum IgE levels measured by single ImmunoCAP sIgE and an unspecified version of ImmunoCAP ISAC, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Nicaise-Rolland 2010170 | Unclear | ImmunoCAP ISAC 103 | Other | No relevant outcomes | Insufficient information for accuracy | |
| Nicolaou 2010171 | Food | Other | NA | No relevant outcomes | Not ISAC or Microtest | |
| Nicolaou 2010171 | Food | Yes | Other | NA | No relevant outcomes | Not ISAC or Microtest |
| Nystrand 2012172 | Unclear | ImmunoCAP ISAC 112 | NA | No relevant outcomes | Technical reproducibility and limit of detection only | |
| Olivieri 2010 173 | Food | Unclear | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Olivieri 2013174 | Food | Yes | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Onell 2012175 | Yes | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes | |
| Ott 2010176 | Other | Unclear | Other | NA | No relevant outcomes | No relevant outcomes, unspecified microarray |
| Paes 2010177 | Food | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | No relevant outcomes |
| Palomba 2013178 | Other | Unclear | Microtest | NA | Concordance only | No relevant outcomes Correlation of specific serum IgE levels measured by Microtest and single ImmunoCAP sIgE, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Palomba 2014179 | Other | Unclear | Microtest | NA | No relevant outcomes | Concordance between Microtest and an unspecified commercially available system, insufficient detail reported |
| Pascal 201693 | Food | Yes | ImmunoCAP ISAC 112 | SPT, sIgE | Accuracy only | No comparison results for sIgE or SPT |
| Passalacqua 201352 | Other | Unclear | Other | NA | Treatment change | Insufficient outcome details, but abstract refers to treatment change |
| Patelis 2012180 | Pollen | Unclear | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Pedrosa 201292 | Food | Yes | ImmunoCAP ISAC 103 | Other | Accuracy only | Accuracy of various components on ISAC only (no comparison with other index tests, e.g. sIgE or SPT) |
| Pomponi 2013181 | Yes | ImmunoCAP ISAC 103 | NA | No relevant outcomes | Autoimmune disease | |
| Raulf 2014182 | No | Other | NA | No relevant outcomes | Review article | |
| Röckmann 2014183 | Food | Yes | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Rodriguez-Ferran 201161 | Unclear | Other | NA | No relevant outcomes | Review article | |
| Romano 2012184 | Food | Unclear | Other | Other | No relevant outcomes | No relevant outcomes |
| Sanchez-Lopez 2013185 | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | Unclear whether results were derived from an unspecified version of ISAC or UniCAP sIgE | |
| Santos 2011186 | Pollen | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | No relevant outcomes |
| Santosa 2015187 | Other | Yes | ImmunoCAP ISAC 112 | NA | No relevant outcomes | No relevant outcomes |
| Sanz 201094 | Pollen | Unclear | ImmunoCAP ISAC 103 | SPT | Accuracy only | Accuracy of various components on ISAC only (no comparison with other index tests, e.g. sIgE or SPT) |
| Sastre 2014188 | Pollen | Yes | ImmunoCAP ISAC other | NA | No relevant outcomes | No relevant outcomes |
| Scala 2011189 | Food | Unclear | ImmunoCAP ISAC 103 | Other | No relevant outcomes | Method of diagnosis not specified, insufficient information for accuracy |
| Scala 2011190 | Other | Unclear | ImmunoCAP ISAC 103 | NA | No relevant outcomes | No relevant outcomes |
| Schuler 2013191 | Latex | Yes | Other | SPT | No relevant outcomes | Intervention either ImmunoCAP sIgE or ISAC |
| Seyfarth 2011192 | Latex | Unclear | ImmunoCAP ISAC 103 | Other | No relevant outcomes | Partial accuracy only, no reference standard defined, non-clinical spiked sample |
| Seyfarth 2011192 | Latex | Unclear | ImmunoCAP ISAC other | Other | No relevant outcomes | Partial accuracy only, no reference standard defined, non-clinical spiked sample |
| Seyfarth 2011193 | Latex | Unclear | ImmunoCAP ISAC 103 | Other | No relevant outcomes | Partial accuracy only, no reference standard defined, non-clinical spiked sample |
| Seyfarth 2014194 | Latex | Yes | ImmunoCAP ISAC 103 | SPT | No relevant outcomes | Partial accuracy only, non-clinical spiked sample |
| Shibata 2014195 | Food | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | No relevant outcomes |
| Sousa 2009196 | Pollen | Unclear | ImmunoCAP ISAC other | SPT | Accuracy only | ISAC only, no comparative accuracy |
| Stringari 201451 | Pollen | Yes | Other | NA | Clinical | Not ISAC or Microtest, ImmunoCAP FEIA |
| Tolkki 2013197 | Food | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | No relevant outcomes |
| Tolkki 2013198 | Food | Yes | ImmunoCAP ISAC other | NA | No relevant outcomes | No relevant outcomes |
| Tripathi 2012199 | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | Eosinophilic esophagitis and no relevant outcomes | |
| Tripathi 2014200 | Food | Unclear | ImmunoCAP ISAC 112 | NA | No relevant outcomes | Eosinophilic esophagitis and no relevant outcomes |
| Tripathi 2014201 | Food | ImmunoCAP ISAC 112 | NA | No relevant outcomes | Mixed population | |
| Uriarte 2013202 | Other | Unclear | Other | NA | No relevant outcomes | Intervention was sIgE ImmunoCAP or ISAC |
| Vitte 2014203 | Other | Unclear | ImmunoCAP ISAC other | NA | No relevant outcomes | No relevant outcomes |
| Weimann 2011204 | Unclear | ImmunoCAP ISAC other | Other | Concordance only | No relevant outcomes Correlation of specific serum IgE levels measured by single ImmunoCAP sIgE and an unspecified version of ImmunoCAP ISAC, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
|
| Williams205 | Other | Yes | Microtest | Other | Concordance only | No relevant outcomes Correlation of specific serum IgE levels measured by Microtest and single ImmunoCAP sIgE, but insufficient data to compare diagnostic performance (e.g. sensitivity and specificity) of the two methods |
| Yadzir 2014206 | Other | Yes | ImmunoCAP ISAC 112 | SPT | No relevant outcomes | Insufficient information for accuracy |
| Young 2012207 | Other | Yes | ImmunoCAP ISAC 112 | NA | No relevant outcomes | No data |
Appendix 4 Risk-of-bias assessments
A Review-specific assessments of ‘diagnostic before-and-after’ studies
Study ID: Heaps 201435,39
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Patients with idiopathic anaphylaxis (cause could not be established by standard diagnostic work-up) recruited from five specialist allergy centres). No further details reported. No exclusion criteria reported | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): The cause of anaphylaxis could not be established using standard diagnostic work-up (including clinical history, SPT, single IgE). The study was conducted in the UK | |
| Do the included patients match the review question? | CONCERN: LOW |
DOMAIN 2: INDEX TEST(S)
A. Risk of bias
| Describe how the index test was conducted and interpreted: ISAC used ‘according to the manufacturer’s instructions’ | |
| Was the index test method, including threshold, prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
DOMAIN 3: COMPARATOR
A. Risk of bias
| Describe the comparator and how it was conducted and interpreted: Standard care, including clinical history, SPT and single IgE. ISAC testing was conducted after routine diagnostic work-up | |
| Was the diagnostic assessment, based on the comparator, made without knowledge of the results of the index test? | Yes |
| Could the comparator, its conduct or its interpretation have introduced bias? | RISK: LOW |
B. Applicability
| Is there concern that the comparator does not match current standard care, as defined in the review question? | CONCERN: LOW |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test or comparator: Three patients did not receive MCT testing (part of standard work-up). All patients were included in the analysis Describe the time interval and any interventions between index and comparator: Not reported |
|
| Was there an appropriate interval between index test and comparator? | Unclear |
| Did patients receive the same comparator/standard care? | No |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Study ID: Luengo 201037
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Abstract only, no details reported | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Population described as ‘well characterised, multi-sensitised’ and ‘Mediterranean’ | |
| Do the included patients match the review question? | CONCERN: UNCLEAR |
DOMAIN 2: INDEX TEST
A. Risk of bias
| Describe how the index test was conducted and interpreted: Abstract only, no details reported | |
| Was the index test method, including threshold, prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: UNCLEAR |
DOMAIN 3: COMPARATOR
A. Risk of bias
| Describe the comparator and how it was conducted and interpreted: SPT and single IgE, abstract only, no further details reported | |
| Was the diagnostic assessment, based on the comparator, made without knowledge of the results of the index test? | Unclear |
| Could the comparator, its conduct or its interpretation have introduced bias? | RISK: UNCLEAR |
B. Applicability
| Is there concern that the comparator does not match current standard care, as defined in the review question? | CONCERN: LOW |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test, or comparator: All participants appear to have received ISAC and standard diagnostic work-up Describe the time interval and any interventions between index and comparator: Not reported |
|
| Was there an appropriate interval between index test and comparator? | Unclear |
| Did patients receive the same comparator/standard care? | Unclear |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: UNCLEAR |
Study ID: Noimark 201240
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Abstract only, no details reported | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Children with moderate to severe atopic eczema and multiple food allergies. The study was conducted in a UK secondary care allergy clinic | |
| Do the included patients match the review question? | CONCERN: LOW |
DOMAIN 2: INDEX TEST
A. Risk of bias
| Describe how the index test was conducted and interpreted: Abstract only, no details reported | |
| Was the index test method, including threshold, prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: UNCLEAR |
DOMAIN 3: COMPARATOR
A. Risk of bias
| Describe the comparator and how it was conducted and interpreted: SPT and/or single IgE, abstract only, no further details reported | |
| Was the diagnostic assessment, based on the comparator, made without knowledge of the results of the index test? | Unclear |
| Could the comparator, its conduct or its interpretation have introduced bias? | RISK: UNCLEAR |
B. Applicability
| Is there concern that the comparator does not match current standard care, as defined in the review question? | CONCERN: LOW |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test, or comparator: All participants appear to have received ISAC and standard diagnostic work-up Describe the time interval and any interventions between index and comparator: ISAC was performed ‘alongside’ SPT and single IgE |
|
| Was there an appropriate interval between index test and comparator? | Yes |
| Did patients receive the same comparator/standard care? | Unclear |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Study ID: Passalacqua 201338
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Patients with respiratory allergic disease prospectively recruited from six allergy centres. No further details reported. No exclusion criteria were reported | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Polysensitised patients (at least two positive SPTs). However, the study was conducted in Italy, where there is likely to be a different pattern of pollen sensitisations to that observed in the UK | |
| Do the included patients match the review question? | CONCERN: HIGH |
DOMAIN 2: INDEX TEST
A. Risk of bias
| Describe how the index test was conducted and interpreted: ISAC 103, using manufacturer’s recommended threshold | |
| Was the index test method, including threshold, prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
DOMAIN 3: COMPARATOR
A. Risk of bias
| Describe the comparator and how it was conducted and interpreted: Standard care: SPT and clinical history, with single IgE(s) as required. Diagnosis and treatment plans were formulated based on standard care without ISAC. Decisions were then reviewed with ISAC results available. It was not clear how many participants received single IgE testing as part of standard care | |
| Was the diagnostic assessment, based on the comparator, made without knowledge of the results of the index test? | Yes |
| Could the comparator, its conduct or its interpretation have introduced bias? | RISK: LOW |
B. Applicability
| Is there concern that the comparator does not match current standard care, as defined in the review question? | CONCERN: UNCLEAR |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test, or comparator: All participants appear to have received both ISAC and standard care. Standard care could include single IgE, but it was not clear in how many participants single IgE assay(s) were performed Describe the time interval and any interventions between index and comparator: Not reported |
|
| Was there an appropriate interval between index test and comparator? | Unclear |
| Did patients receive the same comparator/standard care? | No |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: HIGH |
Study ID: Sastre 201230–32
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Patients with allergic rhinoconjunctivitis and/or asthma who were sensitised to pollen, with or without concomitant food allergy. Patients were attending an allergy outpatient clinic. No further details reported. No exclusion criteria were reported | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Patients sensitised to pollen, with or without concomitant food allergy. The study does not specify polysensitised patients or difficult to manage allergic disease. The study was conducted in Spain, where there is likely to be a different pattern of pollen sensitisations to that observed in the UK | |
| Do the included patients match the review question? | CONCERN: HIGH |
DOMAIN 2: INDEX TEST
A. Risk Of bias
| Describe how the index test was conducted and interpreted: ISAC 96, used according to the manufacturer’s instructions | |
| Was the index test method, including threshold, prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
DOMAIN 3: COMPARATOR
A. Risk of bias
| Describe the comparator and how it was conducted and interpreted: Standard care: SIT prescriptions were formulated based on clinical history, current guidance and SPT results, without knowledge of ISAC results, then reformulated with access to ISAC results. Standard care did not appear to have included single IgE testing | |
| Was the diagnostic assessment, based on the comparator, made without knowledge of the results of the index test? | Yes |
| Could the comparator, its conduct or its interpretation have introduced bias? | RISK: LOW |
B. Applicability
| Is there concern that the comparator does not match current standard care, as defined in the review question? | CONCERN: UNCLEAR |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test or comparator: All participants appear to have received both ISAC and standard care Describe the time interval and any interventions between index and comparator: Not reported |
|
| Was there an appropriate interval between index test and comparator? | Unclear |
| Did patients receive the same comparator/standard care? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
B Critical Appraisal Skills Programme Cohort tool assessment
| Gay-Crosier 201036 | ||
|---|---|---|
| (a) Are the results of the study valid? |
|
Yes ‘We compared the clinical responses to SIT with allergen specific IgE levels measured by Immuno-CAP and by a new microarray-based assay’ |
|
Unclear Reported as an abstract |
|
|
Unclear Reported as an abstract. Subcutaneous immunotherapy but no further details |
|
|
Unclear Reported as an abstract. Allergen-specific IgE and IgG4 levels were also measured before and after SIT, both by ImmunoCAP single IgE and by ISAC assays |
|
|
Unclear | |
|
No | |
|
Unclear Reported as an abstract |
|
|
Yes Follow-up was 3 years |
|
| (b) What are the results? |
|
Results clearly reported in relevant section |
|
Unclear; no variation reported | |
|
Unclear; too little information provided to be clear | |
| (c) Will the results help locally? |
|
It is unclear what the specific population was; therefore, it is unclear if the results apply to patients with complex allergy |
|
Unclear | |
|
Unclear at present, although conclusions are that ‘allergen specific IgE levels and even more the specific IgE/IgG4 ratio measured by a microarray assay (ISAC) is significantly related in this study, to the clinical outcome of SIT’ | |
C QUADAS-2 assessments of comparative accuracy studies
Study ID: Albarini 201347
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Not reported. Children reported with immediate reactions to hazelnut ingestion | |
| Was a consecutive or random sample of patients enrolled? Was a case–control design avoided? |
Unclear Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Patients were allergic to hazelnuts (response to ingestion). Patients were given an oral challenge and tests were compared between those who were tolerant and those who were allergic. Did not specify patients with ‘difficult to manage’ allergic disease or similar classification and only one allergy source was being investigated | |
| Do the included patients match the review question? | CONCERN: HIGH |
DOMAIN 2: INDEX TEST
A. Risk of bias
| Describe how the index test and any comparator tests were conducted and interpreted: The article was reported as an abstract with little methodological detail. The methods for ImmunoCAP ISAC (cut-off points: NR) and ImmunoCAP (positive result: ≥ 0.35 kU/l) were not provided. SPT method was not reported (positive result: mean weal diameter ≥ 3 mm) | |
| Were the index test results interpreted without knowledge of the results of the reference standard? If a threshold was used, was it prespecified? |
Unclear Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: UNCLEAR |
B. Applicability
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: UNCLEAR |
DOMAIN 3: REFERENCE STANDARD
A. Risk of bias
| Describe the reference standard and how it was conducted and interpreted: DBPCFC, no further details | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: LOW |
B. Applicability
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: LOW |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test, comparator(s) and/or reference standard or who were excluded from the 2 × 2 table(s): It appears that all patients were included in the analysis Describe the time interval and any interventions between index, comparator(s) and reference standard: No time intervals were reported |
|
| Was there an appropriate interval between index test and reference standard? | Unclear |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: UNCLEAR |
Study ID: Alessandri 201142
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Not described | |
| Was a consecutive or random sample of patients enrolled? Was a case–control design avoided? |
Unclear Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Patients had suspected hen’s egg allergy (history of reactions after ingestion and positive SPT or IgE to hen’s egg white extracts). Patients were given a hen’s egg oral challenge and tests were compared between those who were tolerant, partially tolerant (allergic to raw egg but not boiled) and those who were allergic. Did not specify patients with ‘difficult to manage’ allergic disease or similar classification and only one allergy source was being investigated. The study was not conducted in the UK | |
| Do the included patients match the review question? | CONCERN: HIGH |
DOMAIN 2: INDEX TEST
Risk of bias
| Describe how the index test and any comparator tests were conducted and interpreted: ImmunoCAP ISAC (positive result: NR) and ImmunoCAP FEIA (positive result: NR) performed according to the manufacturer’s instructions. Standard SPT was performed (positive result: mean weal diameter ≥ 7 mm). All thresholds were optimised based on ROC analyses | |
| Were the index test results interpreted without knowledge of the results of the reference standard? If a threshold was used, was it prespecified? |
Unclear No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: HIGH |
B. Applicability
| Is there concern that the index test, its conduct or interpretation differ from the review question? | CONCERN: LOW |
DOMAIN 3: REFERENCE STANDARD
A. Risk of bias
| Describe the reference standard and how it was conducted and interpreted: Oral challenge performed with boiled or raw hen’s egg. Increased egg amount until there was a reaction (or one egg or 6 g ingested) | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Unclear |
B. Applicability
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: LOW |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test, comparator(s) and/or reference standard or who were excluded from the 2 × 2 table(s): Unclear if all patients received all index tests (applying reported sensitivity and specificity estimates to the total numbers of participants who were positive to each of the reference standards does not result in whole numbers for 2 × 2 data). All patients received OFC Describe the time interval and any interventions between index, comparator(s) and reference standard: Timings were not reported; no other interventions were reported |
|
| Was there an appropriate interval between index test and reference standard? | Unclear |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | No |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: HIGH |
Study ID: Cabrera-Freitag 201143
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Not reported. Patients from the Clínica Universidad de Navarra in Pamplona, Spain. Patients with allergies to pollen other than grass or cypress were excluded, thereby excluding complex patients | |
| Was a consecutive or random sample of patients enrolled? Was a case–control design avoided? |
Unclear No |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: HIGH |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Patients were allergic to grass and cypress pollen (clinical history and SPTs). Control patients were negative to history and SPT. Both populations were examined by ISAC 103 or single IgE for components specific to grass and cypress pollen. Did not specify patients with ‘difficult to manage’ allergic disease or similar classification and only two allergy sources were being investigated. The study was not conducted in the UK | |
| Do the included patients match the review question? | CONCERN: HIGH |
DOMAIN 2: INDEX TEST
Risk of bias
| Describe how the index test and any comparator tests were conducted and interpreted: The method for ImmunoCAP ISAC (cut-off points: reported) and ImmunoCAP (cut-off points: reported) were described as per the manufacturer’s methods | |
| Were the index test results interpreted without knowledge of the results of the reference standard? If a threshold was used, was it prespecified? |
Unclear No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: HIGH |
B. Applicability
| Is there concern that the target condition as defined by the index test does not match the review question? | CONCERN: LOW |
DOMAIN 3: REFERENCE STANDARD
A. Risk of bias
| Describe the reference standard and how it was conducted and interpreted: SPT (grass and cypress pollen) and clinical history (rhinoconjunctivitis and/or bronchial asthma) | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: LOW |
B. Applicability
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: LOW |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test, comparator(s) and/or reference standard or who were excluded from the 2 × 2 table(s): All patients appear to have been included in the analysis Describe the time interval and any interventions between index, comparator(s) and reference standard: No time intervals were reported |
|
| Was there an appropriate interval between index test and reference standard? | Unclear |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: UNCLEAR |
Study ID: De Swert 201241
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Unclear, ‘we selected subjects with birch pollen allergy who were suspected of also being soy allergic’ | |
| Was a consecutive or random sample of patients enrolled? Was a case–control design avoided? |
Unclear No |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: HIGH |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Patients had been diagnosed with birch pollen allergy (clinical history and positive IgE response to birch or rBet v1). Patients were given a soy oral challenge and tests were compared between those who were tolerant and those who were allergic. Did not specify patients with ‘difficult to manage’ allergic disease or similar classification and only one allergy source was being investigated. The study was not conducted in the UK | |
| Do the included patients match the review question? | CONCERN: HIGH |
DOMAIN 2: INDEX TEST
A. Risk of bias
| Describe how the index test and any comparator tests were conducted and interpreted: The methods section described ImmunoCAP ISAC (positive result: ≥ 0.3 ISU) and ImmunoCAP FEIA (positive result: ≥ 0.1 kU/l) performed according to the manufacturer’s instructions. Standard SPT performed (positive result: mean weal diameter ≥ 3 mm). However, the results section reported different cut-off values for all three tests, suggesting that these may have been optimised for the study population | |
| Were the index test results interpreted without knowledge of the results of the reference standard? If a threshold was used, was it prespecified? |
Unclear No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: HIGH |
B. Applicability
| Is there concern that the index test, its conduct or interpretation differ from the review question? | CONCERN: LOW |
DOMAIN 3: REFERENCE STANDARD
A. Risk of bias
| Describe the reference standard and how it was conducted and interpreted: Oral challenge performed with Alpro soya natural drink. Increased volumes of drink until there was a reaction (or until 158 ml) | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: LOW |
B. Applicability
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: LOW |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test, comparator(s) and/or reference standard or who were excluded from the 2 × 2 table(s): SPT results for one patient and ISAC results for three patients were not included in the analysis Describe the time interval and any interventions between index, comparator(s) and reference standard: Timings were not reported, no other interventions were reported |
|
| Was there an appropriate interval between index test and reference standard? | Unclear |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: HIGH |
Study ID: D’Urbano 201044
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Not described | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: HIGH |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Patients had suspected hen’s egg or cow’s milk allergy (history of reactions after ingestion). Patients were given an OFC and tests were compared between those who were tolerant and those who were allergic. Did not specify patients with ‘difficult to manage’ allergic disease or similar classification and only one allergy source was being investigated. The study was not conducted in the UK | |
| Do the included patients match the review question? | CONCERN: HIGH |
DOMAIN 2: INDEX TEST
A. Risk of bias
| Describe how the index test and any comparator tests were conducted and interpreted: ImmunoCAP ISAC (positive result: NR) and ImmunoCAP FEIA (positive result: NR) performed according to the manufacturer’s instructions. Standard SPT performed (positive result: mean weal diameter of ≥ 3 mm). Thresholds for single IgE and ISAC were derived from ROC analysis | |
| Were the index test results interpreted without knowledge of the results of the reference standard? If a threshold was used, was it prespecified? |
Unclear No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: HIGH |
B. Applicability
| Is there concern that the index test, its conduct or interpretation differ from the review question? | CONCERN: LOW |
DOMAIN 3: REFERENCE STANDARD
A. Risk of bias
| Describe the reference standard and how it was conducted and interpreted: Oral challenge performed with pasteurised cow’s milk, boiled or raw hen’s egg. Increased egg amount until there was a reaction (or one egg or 6 g ingested or 250 ml milk) | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Unclear |
B. Applicability
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: LOW |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test, comparator(s) and/or reference standard or who were excluded from the 2 × 2 table(s): All patients appeared to have received both index tests (separate testing for patients with suspected cow’s milk and hen’s egg allergy). All patients received OFC. All patients appeared to have been included in the analyses (separate for patients with suspected cow’s milk and hen’s egg allergy) Describe the time interval and any interventions between index, comparator(s) and reference standard: Timings were not reported, no other interventions were reported |
|
| Was there an appropriate interval between index test and reference standard? | Unclear |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Study ID: Ott 200849
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Retrospective, but no further methodology | |
| Was a consecutive or random sample of patients enrolled? Was a case–control design avoided? |
Unclear Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Patients had suspected IgE-mediated food hypersensitivity. Patients were given a milk or hen’s egg oral challenge and tests were compared between those who were tolerant and those who were allergic. Did not specify patients with ‘difficult to manage’ allergic disease or similar classification and only one allergy source was being investigated. The study was not conducted in the UK | |
| Do the included patients match the review question? | CONCERN: HIGH |
DOMAIN 2: INDEX TEST
A. Risk of bias
| Describe how the index test and any comparator tests were conducted and interpreted: ImmunoCAP ISAC (positive result: NR) and UniCAP (positive result: > 0.35 kU/l) performed according to the manufacturer’s instructions. Standard SPT performed (positive result: mean weal diameter ≥ 3 mm or greater than negative control). Thresholds were derived from ROC analyses for all index tests | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: HIGH |
B. Applicability
| Is there concern that the index test, its conduct or interpretation differ from the review question? | CONCERN: LOW |
DOMAIN 3: REFERENCE STANDARD
A. Risk of bias
| Describe the reference standard and how it was conducted and interpreted: Oral challenge performed with milk or hen egg | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Unclear |
B. Applicability
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: LOW |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test, comparator(s) and/or reference standard or who were excluded from the 2 × 2 table(s): Unclear if all patients received all index tests. All patients received OFC (applied separately for suspected cow’s milk and suspected hen’s egg allergy) Describe the time interval and any interventions between index, comparator(s) and reference standard: Timings were not reported, no other interventions were reported |
|
| Was there an appropriate interval between index test and reference standard? | Unclear |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: UNCLEAR |
Study ID: Sokolova 200946
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Not reported. Patients from the food allergy clinic | |
| Was a consecutive or random sample of patients enrolled? Was a case–control design avoided? |
Unclear No |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: HIGH |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Patients were allergic to cow’s milk protein (SPTs and positive specific IgE). Patients were given an oral milk challenge and those who were positive [cutaneous (urticaria/angioedema), respiratory or gastrointestinal (vomiting, diarrhoea) symptoms] vs. tolerant (no symptoms to 200 ml milk) or were controls (no history of allergy and drank milk) were examined by ISAC or UNICAP. Did not specify patients with ‘difficult to manage’ allergic disease or similar classification and only one allergy source was being investigated. The study was not conducted in the UK | |
| Do the included patients match the review question? | CONCERN: HIGH |
DOMAIN 2: INDEX TEST
A. Risk of bias
| Describe how the index test and any comparator tests were conducted and interpreted: The method for ImmunoCAP ISAC (cut-off points: NR) and ImmunoCAP (cut-off points: NR) were described as per the manufacturer’s methods. Cut-off points were not reported | |
| Were the index test results interpreted without knowledge of the results of the reference standard? If a threshold was used, was it prespecified? |
Unclear Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Unclear |
B. Applicability
| Is there concern that the target condition as defined by the index test does not match the review question? | CONCERN: LOW |
DOMAIN 3: REFERENCE STANDARD
A. Risk of bias
| Describe the reference standard and how it was conducted and interpreted: OFC was described | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: LOW |
B. Applicability
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: LOW |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test, comparator(s) and/or reference standard or who were excluded from the 2 × 2 table(s): All patients appear to have been included in the analysis. Patients reporting anaphylaxis after accidental ingestion and controls with no history of CMPA who ingested cow’s milk daily did not receive OFC testing Describe the time interval and any interventions between index, comparator(s) and reference standard: No time intervals were reported. |
|
| Was there an appropriate interval between index test and reference standard? | Unclear |
| Did all patients receive a reference standard? | No |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: UNCLEAR |
Study ID: Wohrl 200645
DOMAIN 1: PATIENT SELECTION
A. Risk of bias
| Describe methods of patient selection: Not reported. Adults at the end of the pollen season from two allergy clinics. Patients with total serum IgE of > 1000 kU/l were excluded to minimise non-specific binding in the ImmunoCAP system. The system should be able to cope with the full range of patients | |
| Was a consecutive or random sample of patients enrolled? Was a case–control design avoided? |
Unclear No |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: HIGH |
B. Applicability
| Describe included patients (prior testing, presentation, intended use of index test and setting): Patients were allergic to one or more aeroallergens (house mite, cat, birch pollen, grass pollen or mugwort pollen). Patients were given a SPT and a history was taken. ISAC and single IgE test were used to distinguish between those who were allergic (positive SPT and history) vs. non-allergic (negative SPT and atopic/negative history). Did not specify patients with ‘difficult to manage’ allergic disease or similar classification and only five allergy sources were being investigated. The study was not conducted in the UK | |
| Do the included patients match the review question? | CONCERN: HIGH |
DOMAIN 2: INDEX TEST
Risk of bias
| Describe how the index test and any comparator tests were conducted and interpreted: The method for ImmunoCAP ISAC (cut-off points: NR) was provided but not for ImmunoCAP (cut-off points: NR). Cut-off points were calculated from ROC curves but were not described for individual components | |
| Were the index test results interpreted without knowledge of the results of the reference standard? If a threshold was used, was it prespecified? |
Unclear No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: HIGH |
B. Applicability
| Is there concern that the target condition as defined by the index test does not match the review question? | CONCERN: LOW |
DOMAIN 3: REFERENCE STANDARD
A. Risk of bias
| Describe the reference standard and how it was conducted and interpreted: SPT method was reported (positive result: mean weal diameter of ≥ 3 mm) and details of clinical history were partially reported | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: LOW |
B. Applicability
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: LOW |
DOMAIN 4: FLOW AND TIMING
A. Risk of bias
| Describe any patients who did not receive the index test, comparator(s) and/or reference standard or who were excluded from the 2 × 2 table(s): All patients appear to have been included in the analysis Describe the time interval and any interventions between index, comparator(s) and reference standard: No time intervals were reported |
|
| Was there an appropriate interval between index test and reference standard? | Unclear |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: UNCLEAR |
Appendix 5 Between-system concordance data provided by Microtest Matrices Ltd
Confidential information has been removed.
Appendix 6 Survey to inform the number of patients receiving each test
-
Do you have experience with ImmunoCAP ISAC testing? Yes/No
Although some of the questions below are related to ImmunoCAP ISAC testing, it would still be greatly appreciated if you could answer as many of the questions as possible.
For questions 2 and 3, consider patients where the primary presentation is inhalation allergy for whom you would consider testing with ImmunoCAP ISAC.
-
With current standard care:
-
– What is the number of IgE tests that you would order? (average, range)
-
– What is the proportion of patients that would get a food challenge test? (average, range)
-
– What is the proportion of patients that would get a skin prick test? (average, range)
-
– In those patients, what is the number of allergens tested (using skin prick testing)? (average, range).
-
-
With ISAC:For questions 4 and 5, consider patients where the primary presentation is food allergy for whom you would consider testing with ImmunoCAP ISAC.
-
– For what proportion of patients would you order further IgE tests? (average, range)
-
– In those patients, what is the number of IgE tests that you would order? (average, range)
-
– What is the proportion of patients that would get a food challenge test? (average, range)
-
– What is the proportion of patients that would get a skin prick test? (average, range)
-
– In those patients, what is the number of allergens tested (using skin prick testing)? (average, range).
-
-
With current standard care:
-
– What is the number of IgE tests that you would order? (average, range)
-
– What is the proportion of patients that would get a food challenge test? (average, range)
-
– What is the proportion of patients that would get a skin prick test? (average, range)
-
– In those patients, what is the number of allergens tested (using skin prick testing)? (average, range).
-
-
With ISAC:
-
– For what proportion of patients would you order further IgE tests? (average, range)
-
– In those patients, what is the number of IgE tests that you would order? (average, range)
-
– What is the proportion of patients that would get a food challenge test? (average, range)
-
– What is the proportion of patients that would get a skin prick test? (average, range)
-
– In those patients, what is the number of allergens tested (using skin prick testing)? (average, range).
-
Appendix 7 Cost calculation for individual tests
| SPT costs | Total | Cost per test | Costs per patient tested | Source |
|---|---|---|---|---|
| Vial costs (includes 80 drops, so can be used for 80 tests) | £17.00 | £0.21 | £1.70 | NICE 201186 |
| Control costs (includes 2 × 80 drops, so can be used for 160 tests) | £12.00 | £0.08 | £0.60 | NICE 201186 |
| Lancet costs (200 pack) | £12.00 | £0.06 | £0.48 | NICE 201186 |
| Capital costs | ||||
| None | ||||
| Other costs (service, maintenance) | ||||
| None | ||||
| Personnel costs to perform and interpret test | ||||
| Personnel time to interpret test results (hours per test): GP | 0.02 | NICE 201186 | ||
| Personnel costs for interpreting test results (per hour): GP | £234.00 | £4.88 | £39.00 | Curtis 201488 |
| Personnel time to perform tests (hours per tests): nurse | 0.06 | NICE 201186 | ||
| Personnel costs to perform one batch of 4 tests (per hour): nurse | £41.00 | £2.56 | £20.50 | Curtis 201488 |
| No. of tests | ||||
| No. of allergens tested per person | 8 | NICE 201186 | ||
| Total costs | £62.28 | |||
| IgE test costs | Total | Cost per test | Costs per patient tested | Source |
|---|---|---|---|---|
| Test costs per allergy | £12.00 | £12.00 | £96.00 | NICE 201186 |
| Capital costs | ||||
| None | ||||
| Other costs (service, maintenance) | ||||
| None | ||||
| Personnel costs to perform and interpret test | ||||
| Personnel time to interpret test results (hours per test): GP | 0.02 | NICE 201186 | ||
| Personnel costs for interpreting test results (per hour): GP | £234.00 | £4.88 | £39.00 | Curtis 201488 |
| Personnel time to perform tests (hours per patient): nurse | 0.03 | NICE 201186 | ||
| Personnel costs to perform one batch of four tests (per hour): nurse | £41.00 | £1.37 | Curtis 201488 | |
| No. of tests | ||||
| No. of allergens tested per person | 8 | NICE 201186 | ||
| Total costs | £136.37 | |||
| OFC test costs | Total | Cost per test | Costs per patient tested | Source |
|---|---|---|---|---|
| Hospital appointment to implement the food elimination diet | £314.00 | £314.00 | Department of Health 2013–1489 | |
| Hospital appointment to carry out OFC for diagnosis | £256.00 | £256.00 | Department of Health 2013–1489 | |
| Total costs | £570.00 |
| ImmunoCAP ISAC – minimum | Total | Per year (annuitised) | Costs per test (annuitised) | Costs per patient tested (annuitised) | Source |
|---|---|---|---|---|---|
| Test costs | |||||
| Costs per ImmunoCAP ISAC 112 IgE kit | £2500.00 | £125.00 | £125.00 | Information submitted to NICE by Thermo Fisher Scientific | |
| Wash solution costs | £18.50 | £0.34 | £0.34 | ||
| Capital costs | |||||
| Costs of the LuxScan 10k reader | £28,999.00 | £2411.73 | £6.25 | £6.25 | Information submitted to NICE by Thermo Fisher Scientific |
| Resale value | £0.00 | ||||
| Life time of LuxScan 10k reader (years) | 10 | ||||
| Cost of ImmunoCAP starter kit | £500.00 | £41.58 | £0.11 | £0.11 | |
| Resale value | £0.00 | Assumption | |||
| Life time of ImmunoCAP starter kit (years) | 10 | Assumption | |||
| Other costs (service, maintenance) | |||||
| Single flat fee to cover all eventualities (0–4 kits per month) | £2000.00 | £0.00 | £0.00 | Information submitted to NICE by Thermo Fisher Scientific | |
| Single flat fee to cover all eventualities (4–6 kits per month) | £4000.00 | £10.36 | £10.36 | ||
| Personnel costs to perform/process and interpret test | |||||
| Personnel time to interpret test results (hours per test) | 0.08 | Information submitted to NICE by Thermo Fisher Scientific | |||
| Personnel costs for interpreting test results (per hour): immunologista | £140.00 | £11.67 | £11.67 | Curtis 201488 | |
| Personnel time to perform one batch of 4 tests (hours per kit) | 0.05 | Information submitted to NICE by Thermo Fisher Scientific | |||
| Personnel costs to perform one batch of 4 tests (per hour): biomedical scientistb | £55.16 | £0.69 | £0.69 | Curtis 201488 | |
| No. of tests | |||||
| No. of kits per year | 97 | Information submitted to NICE by Thermo Fisher Scientific | |||
| No. of tests per kit | 4 | ||||
| Total costs | £154.41 | ||||
| ImmunoCAP ISAC – minimum | Total | Per year (annuitised) | Costs per test (annuitised) | Costs per patient tested (annuitised) | Source |
|---|---|---|---|---|---|
| Test costs | |||||
| Costs per ImmunoCAP ISAC 112 IgE kit | £2500.00 | £125.00 | £125.00 | Information submitted to NICE by Thermo Fisher Scientific | |
| Wash solution costs | £18.50 | £0.34 | £0.34 | ||
| Capital costs | |||||
| Costs of the LuxScan 10k reader | £28,999.00 | £3114.65 | £8.07 | £8.07 | Information submitted to NICE by Thermo Fisher Scientific |
| Resale value | £0.00 | ||||
| Life time of LuxScan 10k reader (years) | 8 | ||||
| Cost of ImmunoCAP starter kit | £500.00 | £53.70 | £0.14 | £0.14 | |
| Resale value | £0.00 | Assumption | |||
| Life time of ImmunoCAP starter kit (years) | 8 | Assumption | |||
| Other costs (service, maintenance) | |||||
| Single flat fee to cover all eventualities (0–4 kits per month) | £2000.00 | £0.00 | £0.00 | Information submitted to NICE by Thermo Fisher Scientific | |
| Single flat fee to cover all eventualities (4–6 kits per month) | £4000.00 | £10.36 | £10.36 | ||
| Personnel costs to perform/process and interpret test | |||||
| Personnel time to interpret test results (hours per test) | 1.00 | Information submitted to NICE by Thermo Fisher Scientific | |||
| Personnel costs for interpreting test results (per hour): immunologista | £140.00 | £140.00 | £140.00 | Curtis 201488 | |
| Personnel time to perform one batch of 4 tests (hours per kit) | 0.05 | Information submitted to NICE by Thermo Fisher Scientific | |||
| Personnel costs to perform one batch of 4 tests (per hour): biomedical scientistb | £55.16 | £0.69 | £0.69 | Curtis 201488 | |
| No. of tests | |||||
| No. of kits per year | 97 | Information submitted to NICE by Thermo Fisher Scientific | |||
| No. of test per kit | 4 | ||||
| Total costs | £284.60 | ||||
| Microtesta – minimum | Total | Costs per test | Costs per patient tested | Source |
|---|---|---|---|---|
| Test costs | ||||
| Cost of allergy reagents (can be used for 1–5 tests) | £1.00 | £1.00 | Information submitted to NICE by Microtest DX | |
| Shipping costs | £7.70 | £7.70 | ||
| Sample handling fee | £20.00 | £20.00 | ||
| Costs of allergy biochip | £100.00 | £100.00 | ||
| Capital costs | ||||
| None | £0.00 | £0.00 | ||
| Other costs (service, maintenance) | ||||
| None | £0.00 | £0.00 | Information submitted to NICE by Microtest DX | |
| Personnel costs to perform and interpret test | ||||
| Personnel time to interpret test results (hours per test) | 0.08 | Information submitted to NICE by Microtest DX | ||
| Personnel costs for interpreting test results (per hour): immunologistb | £140.00 | £11.67 | £11.67 | Curtis 201488 |
| Total costs | £140.37 | |||
| Microtesta – maximum | Total | Costs per test | Costs per patient tested | Source |
|---|---|---|---|---|
| Test costs | ||||
| Cost of allergy reagents (can be used for 1–5 tests) | £5.00 | £5.00 | Information submitted to NICE by Microtest DX | |
| Shipping costs | £15.00 | £15.00 | ||
| Sample handling fee | £30.00 | £30.00 | ||
| Costs of allergy biochip | £100.00 | £100.00 | ||
| Capital costs | ||||
| None | £0.00 | £0.00 | ||
| Other costs (service, maintenance) | ||||
| None | £0.00 | £0.00 | Information submitted to NICE by Microtest DX | |
| Personnel costs to perform and interpret test | ||||
| Personnel time to interpret test results (hours per test) | 0.17 | Information submitted to NICE by Microtest DX | ||
| Personnel costs for interpreting test results (per hour): immunologistb | £140.00 | £23.33 | £23.33 | Curtis 201488 |
| Total costs | £173.33 | |||
Appendix 8 Cost calculation for Microtest (performed at service provider laboratory)
| Microtesta – minimum | Total | Per year (annuitised) | Costs per test (annuitised) | Costs per patient tested (annuitised) | Source |
|---|---|---|---|---|---|
| Test costs | |||||
| Cost of allergy reagents (can be used for 1–5 tests) | £1.00 | £1.00 | Information submitted to NICE by Microtest DX | ||
| Costs of allergy biochip | £100.00 | £100.00 | |||
| Capital costs | |||||
| Microtest instrument | Confidential information has been removed | £6321.07 | £16.38 | £16.38 | Information submitted to NICE by Microtest DX |
| Resale value | £0.00 | ||||
| Life time of Microtest instrument (years) | 5 | ||||
| Other costs (service, maintenance) | |||||
| Personnel time for quality control | 7.00 | Information submitted to NICE by Microtest DX | |||
| Personnel costs for quality control | £55.16 | £1.00 | £1.00 | Curtis 201488 | |
| Personnel costs to perform and interpret test | |||||
| Personnel time to interpret test results (hours per test) | 0.08 | Information submitted to NICE by Microtest DX | |||
| Personnel costs for interpreting test results (per hour): immunologistb | £140.00 | £11.67 | £11.67 | Curtis 201488 | |
| Personnel time to perform one test (hours per test) | 0.17 | Information submitted to NICE by Microtest DX | |||
| Personnel costs to perform one batch of 4 tests (per hour): biomedical scientistc | £55.16 | £9.19 | £9.19 | Curtis 201488 | |
| No. of tests | |||||
| No. of tests per year | 386 | Assumption | |||
| Total costs | £139.24 | ||||
| Microtesta – minimum | Total | Per year (annuitised) | Costs per test (annuitised) | Costs per patient tested (annuitised) | Source |
|---|---|---|---|---|---|
| Test costs | |||||
| Cost of allergy reagents (can be used for 1–5 tests) | £5.00 | £5.00 | Information submitted to NICE by Microtest DX | ||
| Costs of allergy biochip | £100.00 | £100.00 | |||
| Capital costs | |||||
| Microtest instrument | £35,000.00 | £6,321.07 | £16.38 | £16.38 | Information submitted to NICE by Microtest DX |
| Resale value | £0.00 | ||||
| Life time of Microtest instrument (years) | 5 | ||||
| Other costs (service, maintenance) | |||||
| Personnel time for quality control | 7.00 | Information submitted to NICE by Microtest DX | |||
| Personnel costs for quality control | £55.16 | £1.00 | £1.00 | Curtis 201488 | |
| Personnel costs to perform and interpret test | |||||
| Personnel time to interpret test results (hours per test) | 0.17 | Information submitted to NICE by Microtest DX | |||
| Personnel costs for interpreting test results (per hour): immunologistb | £140.00 | £23.33 | £23.33 | Curtis 201488 | |
| Personnel time to perform one test (hours per test) | 0.25 | Information submitted to NICE by Microtest DX | |||
| Personnel costs to perform one batch of 4 tests (per hour): biomedical scientistc | £55.16 | £13.79 | £13.79 | Curtis 201488 | |
| No. of tests | |||||
| No. of tests per year | 386 | Assumption | |||
| Total costs | £159.50 | ||||
Appendix 9 Guidance relevant to difficult to manage allergic disease
National Institute for Health and Care Excellence guidance
Atopic Eczema in Children. NICE Quality Standards, QS44, September 2014.
Omalizumab for Treating Severe Persistent Allergic Asthma (review of technology appraisal guidance 133 and 201). NICE technology appraisals, TA278, April 2013.
Atopic Eczema in Children. NICE Pathway, August 2012.
Pharmalgen for the Treatment of Bee and Wasp Venom Allergy. NICE technology appraisals, TA246, February 2012.
Anaphylaxis. NICE Pathway, December 2011.
Anaphylaxis: Assessment to Confirm an Anaphylactic Episode and the Decision to Refer after Emergency Treatment for a Suspected Anaphylactic Episode. NICE Clinical Guidelines, CG134, December 2011.
Food Allergy in Children and Young People. NICE Pathway, December 2011.
Food Allergy in Children and Young People: Diagnosis and Assessment of Food Allergy in Children and Young People in Primary Care and Community Settings. NICE Clinical Guidelines, CG116, February 2011.
Alitretinoin for the Treatment of Severe Chronic Hand Eczema. NICE technology appraisals, TA177, August 2009.
Inhaled Corticosteroids for the Treatment Of Chronic Asthma in Adults and in Children aged 12 years and over. NICE technology appraisals, TA131, November 2007.
Atopic Eczema in Children: Management of Atopic Eczema in Children from Birth up to the age of 12 years. NICE Clinical Guidelines, CG57, December 2007.
Inhaled Corticosteroids for the Treatment of Chronic Asthma in Children under the age of 12 years. NICE technology appraisals, TA131, November 2007.
Tacrolimus and Pimecrolimus for Atopic Eczema. NICE technology appraisals, TA82, August 2004.
Frequency of Application of Topical Corticosteroids for Atopic Eczema. NICE technology appraisals, TA81, August 2004.
Guidance from other agencies
Patient UK (June 2014) Skin Prick Allergy Test.
BUPA (May 2014) Managing Your Allergies.
NHS Choices (April 2014) Allergies.
NHS Choices (April 2014) Food allergy.
Map of Medicine (April 2014) Anaphylaxis – Suspected.
British Society for Allergy & Clinical Immunology (April 2014) Guideline for the Diagnosis and Management of Cow’s Milk Allergy.
Anaphylaxis Campaign (February 2014) Latex Allergy: The Facts.
Anaphylaxis Campaign (February 2014) Food Allergens in Non-food Items.
World Allergy Organization (October 2013) A WAO – ARIA – GA2LEN Consensus Document on Molecular-based Allergy Diagnostics.
Children’s Acute Transport Service (June 2013) Anaphylaxis/Latex Allergy.
Anaphylaxis Campaign (April 2013) Celery Allergy: The Facts.
Patient UK (December 2012) Anaphylaxis.
Patient UK (May 2012) Food Allergy and Intolerance.
British Society for Allergy & Clinical Immunology (March 2012) Standards for Paediatric Allergy Services in Secondary Care.
Patient UK (March 2012) Allergy – General Overview.
Patient UK (March 2012) Nut Allergy.
Patient UK (March 2012) House Dust Mite and Pet Allergy.
Patient UK professional reference (February 2012) Anaphylaxis and its Treatment.
Royal College of Paediatrics and Child Health (November 2011) Care Pathway for Food Allergy in Children: an Evidence and Consensus Based National Approach.
Patient UK professional reference (November 2011) Food Allergy and Food Intolerance.
World Allergy Organization (February 2011) Guidelines for the Assessment and Management of Anaphylaxis.
Quality, Innovation, Productivity and Prevention (QIPP) (October 2011) Pharmacological Interventions to Prevent Allergic and Febrile Non-haemolytic Transfusion Reactions.
British Society for Allergy & Clinical Immunology (August 2011) Diagnosis and Management of Hymenoptera Venom Allergy.
British Society for Allergy & Clinical Immunology (July 2011) Immunotherapy for Allergic Rhinitis.
Scottish Intercollegiate Guidelines Network (SIGN) (March 2011) Management of Atopic Eczema in Primary Care.
Royal College of Paediatrics and Child Health (2011) Care Pathway for Latex Allergy.
Royal College of Paediatrics and Child Health (2011) Care Pathway for Venom Allergy.
Royal College of Paediatrics and Child Health (2011) Care Pathway for Drug Allergy.
Royal College of Paediatrics and Child Health (2011) Care Pathway for Urticaria, Angio-odema or Mastocytosis.
Royal College of Paediatrics and Child Health (2011) Care Pathway for Eczema.
Royal College of Paediatrics and Child Health (2011) Care Pathway for Asthma and/or Rhinitis.
Royal College of Paediatrics and Child Health (2011) Care Pathway for Food Allergy.
Royal College of Paediatrics and Child Health (2011) Care Pathway for Anaphylaxis.
British Society for Allergy & Clinical Immunology (June 2010) Guidelines for the Management of Egg Allergy.
British Society for Allergy & Clinical Immunology (December 2008) Guidelines for the Management of Drug Allergy.
Scottish Intercollegiate Guidelines Netwrok (SIGN) (July 2008 updated April 2014) Antibiotic Prophylaxis in Surgery.
Royal College of Physicians of London (May 2008) Latex Allergy: Occupational Aspects of Management.
Royal College of Physicians of London (March 2008) Latex Allergy Guideline.
Joint Royal Colleges Ambulance Liaison Committee (2007) Anaphylaxis and Allergic Reactions in Children.
Joint Royal Colleges Ambulance Liaison Committee (2007) Anaphylaxis and Allergic Reactions in Adults.
Glossary
- Allergen
- A substance that causes an allergic reaction.
- Antibody
- A large Y-shaped protein, also known as an immunoglobulin, which is used by the immune system to identify pathogens.
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Cross-immunoreactive
- An antibody which can interact or bind with more than one antigen.
- Cross-sensitisation
- The process of producing a specific IgE antibody from one of several homologous allergens.
- False negative
- Incorrect negative test result – number of diseased persons with a negative test result.
- False positive
- Incorrect positive test result – number of non-diseased persons with a positive test result.
- Homologous allergens
- Allergen molecules with very similar molecular structures.
- Immunoglobulin E
- A type of antibody that is found in mammals and which mediates allergic responses.
- Immunoreactive
- Interaction between an allergen and an antibody.
- Index test
- The test whose performance is being evaluated.
- Meta-analysis
- Statistical techniques used to combine the results of two or more studies and obtain a combined estimate of effect.
- Opportunity costs
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Publication bias
- Bias arising from the preferential publication of studies with statistically significant results.
- Quality-adjusted life-year
- A measure of health gain, used in economic evaluations, in which survival duration is weighted or adjusted by the patient’s quality of life during the survival period.
- Quality of life
- An individual’s emotional, social and physical well-being and their ability to perform the ordinary tasks of living.
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity which result from varying the diagnostic threshold.
- Reference standard
- The best currently available method for diagnosing the target condition. The index test is compared against this to allow calculation of estimates of accuracy.
- Sensitisation
- The process of producing a specific immunoglobulin E antibody from exposure to a specific allergen.
- Sensitivity
- Proportion of people with the target disorder who have a positive test result.
- Specificity
- Proportion of people without the target disorder who have a negative test result.
- True negative
- Correct negative test result – number of non-diseased persons with a negative test result.
- True positive
- Correct positive test result – number of diseased persons with a positive test result.
List of abbreviations
- AAAAI
- American Academy of Allergy, Asthma and Immunology
- AAD
- American Academy of Dermatology
- AE
- adverse event
- BAD
- British Association of Dermatologists
- BSACI
- British Society for Allergy & Clinical Immunology
- CASP
- Critical Appraisal Skills Programme
- CDSR
- Cochrane Database of Systematic Reviews
- CEA
- Cost-Effectiveness Analysis
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CPCI-S
- Conference Proceedings Citation Index – Science
- CRD
- Centre for Reviews and Dissemination
- DARE
- Database of Abstracts of Reviews of Effects
- DBPCFC
- double-blind placebo-controlled food challenge
- EAACI
- European Academy of Allergy and Clinical Immunology
- EQ-5D
- European Quality of Life-5 Dimensions
- FAAM
- Food Allergy and Anaphylaxis Meeting
- FDA
- US Food and Drug Administration
- HDM
- house dust mite
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICTRP
- International Clinical Trials Registry Platform
- IgE
- immunoglobulin E
- ISAC
- Immuno Solid-phase Allergen Chip
- ISMA
- International Symposium on Molecular Allergology
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ISU
- International Standard Unit
- ISU-E
- International Standard Unit for immunoglobulin E
- LILACS
- Latin American and Caribbean Health Sciences
- LTP
- lipid transfer protein
- MCT
- mast cell tryptase
- NA
- not applicable
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NLM
- National Library of Medicine
- NR
- not reported
- OFC
- oral food challenge
- PROQOLID
- Patient-Reported Outcome and Quality Of Life Instruments Database
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- RQLQ
- Rhinoconjunctivitis Quality of Life Questionnaire
- SCI
- Science Citation Index
- sIgE
- single specific IgE
- SIT
- allergen-specific immunotherapy
- SPT
- skin prick test
- VAS
- visual analogue scale
- WHO
- World Health Organization
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.