Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/60/43. The contractual start date was in September 2009. The draft report began editorial review in May 2015 and was accepted for publication in October 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Professor Priebe is on the Health Technology Assessment programme Mental, Psychological and Occupational Health Panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Priebe et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Non-adherence to medication and treatment programmes by people with mental health problems is a common problem,1–4 associated with a range of negative outcomes. In the case of schizophrenia, non-adherence is believed to increase the probability of relapse and subsequent rehospitalisation and contribute to significantly higher health-care costs. Non-adherence is defined as the ‘extent to which a person coincides with medical or health advice’5 or ‘the degree of conformity between treatment behaviour and treatment standards’. 6
Non-adherence and the effect on the individual
The range of negative outcomes for non-adherence with treatment can, in some cases, be severe. It has been suggested that non-compliance with treatment is a feature of at least one-quarter of suicides and homicides by people with mental health problems7 and has been found to be related to an increased potential for assault and dangerous behaviours, particularly during periods of psychosis. 1
Non-adherence and health-care costs
Aside from the effect of non-adherence on the individual, the health-care costs of patients with schizophrenia who do not adhere to medication are reportedly higher than the costs for those who do adhere. 8 It has been estimated that non-adherence accounts for 40% of rehospitalisation costs for patients with schizophrenia in the 2 years after their discharge from inpatient treatment. 9 Knapp and colleagues10 make the argument that given that between 25% and 80% of patients at some point in their treatment fail to take their medication correctly,11 total system-wide costs are likely to be substantial. These authors also found that patients who failed to adhere to their medication regimen were over one and a half times more likely than patients who did adhere to it to report use of inpatient services. Non-adherence can increase external service costs by a factor of three.
Interventions to reduce non-adherence in psychosis patients
Several strategies to help reduce non-adherence for psychotic patients have been developed in recent years. Newer atypical antipsychotic medications, which generally have different side effects, may make adherence easier to achieve and maintain; however, non-adherence can still be substantial, even with newer drugs. 12 Psychoeducation, which aims to inform patients about their condition, as well as family therapy have been found to be largely unsuccessful in promoting adherence. 13–16 In fact, for some patients, increasing their knowledge of their illness, medication and its side effects, may be disturbing and may reduce adherence. 17 Other interventions such as telephone prompting and compliance therapy have been found to have, at best, a very small effect. 18 Adherence therapy, a client-centred approach, which aims to improve adherence with medication by using a cognitive–behavioural approach, is specifically not recommended for people with schizophrenia, as it has been found to have no clear benefits for patients. 19,20
Currently, there is no consistent evidence for any intervention to significantly improve medication adherence in non-adherent patients with psychotic disorders. 3,18 It is against this background that financial incentives have been considered for increasing adherence to medication in non-adherent patients.
Financial incentive research
A great deal of research exists within general health studies which tend to support the effectiveness of financial incentives in shaping and improving behaviour. A systematic review found that 10 out of 11 randomised controlled studies21–31 using financial incentives described positive results for antituberculosis drugs, dental care, weight reduction, cocaine dependence and antihypertensive treatment. 32 The review found that a non-financial method of increasing compliance achieved a better result in only one study. 21
Carey and Carey33 tested the hypothesis that attendance at a day treatment programme could be increased by offering small incentives to patients with mental illness and substance abuse and found a modest increase in attendance over time. In 2006, Post and colleagues34 examined the attendance of 50 low-income African Americans with depression at therapy appointments without incentives for 12 weeks, followed by tracking 12 weeks during which US$10 payments were given at regular appointments. They found patients had better adherence when payments were attached to appointments. Roll and colleagues35 investigated the feasibility of using monetary reinforcement to promote abstinence from cigarette smoking in adults with schizophrenia. Abstinence was significantly greater when receiving money than when not.
Tidey36 conducted a review of studies evaluating incentive-based treatments for promoting tobacco and other drug abstinence, treatment attendance, medication use and increased physical activity. They concluded that given the medical and psychosocial costs of tobacco and other drug use, treatment non-compliance and physical inactivity and the efficacy of incentive-based treatments for improving these behaviours, incentive-based treatments should be further developed and integrated into behavioural treatment programmes for people with serious mental illness.
While these studies all show the benefits of financial incentives within mental health research, none of these studies investigated financial incentives and adherence to medication.
Evidence for financial incentives in mental health care
In psychiatric care, there are few well-designed studies that investigate how financial incentives are used to improve medication adherence. Tentative evidence does exist suggesting that financial incentives are effective at increasing adherence. A systematic review by Burton and colleagues37 focusing on mental health treatments found that the use of incentives was effective in encouraging attendance at therapeutic sessions and adherence to substance abuse programmes. In half of the included studies, the improvement was maintained even after the incentive had finished. There are, however, serious methodological problems with these studies. Only four of them were randomised controlled trials. Only one was conducted outside the USA, which means there is practically no evidence on the effectiveness of incentives within the unique setting of the NHS community psychiatric care system.
Two small sample observational studies have provided anecdotal evidence for the effectiveness of financial incentives for adherence to antipsychotic medication. A small study by Claassen and colleagues38 explored the practice of administering direct financial incentives to improve adherence in assertive outreach teams (AOTs) in England. Financial incentives were offered to five assertive outreach patients in east London. One patient declined the offer. Of the four in the scheme, three patients were not hospitalised after the introduction of the financial incentives. All four patients on the scheme were able to retain their independent accommodation and had fewer problems with their neighbours and the police than before. Only one patient on the scheme asked for the incentive to be increased. This was declined and the patient remained on the scheme. A further observational study was conducted by Staring and colleagues39 in the Netherlands, in which five patients with schizophrenia under an assertive community treatment team were offered financial incentives over the course of 1 year. All five patients’ adherence increased to 100% and only one patient was readmitted to hospital over the course of the year. Two patients in the study asked to receive their medication before their due date, but no problems arose when these requests were denied. While these results suggest financial incentives are effective, such results are anecdotal and few generalised conclusions can be drawn because of the lack of trial data.
Research within behavioural economics have found that financial incentives can lead to changes, or ‘spillovers’ in other behaviours not intended to be the target of the incentives; such as ‘crowding in’ or ‘promoting spillovers’, for which an increase in behaviour target by the incentives also leads to increases in other behaviours, or ‘crowding out’, whereby the likelihood of a behaviour being performed is worse as a result of being offered the incentives, as any intrinsic motivation has been removed. 40,41
The ethics of financial incentives
While there is little evidence for the effectiveness of financial incentives, there also is the question whether or not they are ethical. This is a contentious issue and has been debated widely with financial incentives being named as the key to improving health outcomes by those for incentives, and by critics as a form of bribery and rewarding people for unhealthy behaviour. Critics of financial incentives have argued that they are a form of coercion, which remove freedom of choice and autonomy from the patient who is forced to act out of financial need rather than personal motivation. Shaw42 has for instance argued that financial incentives place health professionals in a coercive role and can lead to the loss of a patient’s personal dignity and privacy.
Burns43 explored this argument, stating that the current debate on financial incentives uses oversimplified generalisations of concepts such as ‘autonomy’ and ‘coercion’, arguing that negotiation between the health professional and patient is a constant reality within mental health care. Financial incentives, rather than being an unethical manipulation of vulnerable patients, in fact offer a new model of respectful and mutual exchange. Ashcroft44 has argued also that too hasty a dismissal of incentives overlooks their potential benefits and may rely too heavily on a naive conception of the individual and their freedom of choice to accept or decline the incentive.
Surveys of clinicians have shown a general distrust towards financial incentives. An online survey conducted separately in the USA and the UK found that financial incentives were judged less acceptable and to be less fair than medical interventions (such as a weekly pill or injection). 45 There was a similarity in negative attitudes in both the UK and the USA. Claassen46 conducted a similar study in which team managers of AOTs were asked about their opinion of financial incentives. A total of 53 out of 70 managers mentioned concerns and expressed a negative attitude towards giving money for adherence to long-term antipsychotic injectable (LAI) medication also known as depot injections. Claassen’s paper makes a number of recommendations including receiving informed consent from the patient, an operational policy for the use of incentives, and randomised controlled trials as well as qualitative studies to evaluate their impact.
A focus group study by Priebe and colleagues4 among different stakeholder groups in the UK identified a number of issues or concerns relating to the use of financial incentives in improving adherence to antipsychotic medication. These concerns fell under four main themes:
-
Wider concerns: the value of antipsychotic medication, whether or not other services may suffer financially if health-care budgets were spent on incentives, how the incentives would be spent by patients and whether or not there were government motives underlying the use of incentives.
-
Problems requiring policies: the practicalities involved in implementing the incentives and how they can be incorporated as part of a clinician’s toolkit.
-
Inherent dilemmas: whether or not offering financial incentives are coercive or whether or not they are fair on others not receiving incentives.
-
Challenges for evidence and experience: whether or not the incentives would perversely incentivise patients to become intentionally non-adherent to qualify for the incentives or whether or not the incentives would affect the therapeutic relationship.
Despite controversial discussions in most groups, all talked about the importance of establishing solid evidence on whether or not financial incentives are effective and emphasised the need for systematic research on the issue. Overall, the debate over the ethics of using financial incentives would be greatly enhanced by more knowledge on whether or not incentives in fact work and how they are experienced by patients and clinicians.
Justification for the current study
In the light of the lack of effective methods to improve adherence to antipsychotic medication, anecdotal evidence on the effectiveness of financial incentives for medication adherence and the views of stakeholders, there is a need to provide systematic research evidence over whether or not the use of financial incentives would be effective in improving adherence to antipsychotic medication. The Financial Incentives for Adherence to Treatment (FIAT) study therefore aimed to:
-
examine the clinical effectiveness and cost-effectiveness of offering financial incentives to patients with psychotic disorders who demonstrate poor adherence to LAI medication (i.e. adherence ≤ 75%)
-
examine the views and experiences of both patients and clinicians with offering financial incentives to improve adherence to LAI medication to inform the concerns raised by focus groups4
-
evaluate the long-term outcomes of financial incentives on adherence and other outcomes; two follow-up studies were conducted at 6 and 24 months after the end of the intervention.
Chapter 2 Methods
This chapter describes the design and conduct of the FIAT study, including its cost-effectiveness and qualitative examinations. Sections of this chapter have been adapted from Priebe and colleagues47,48 and Henderson and colleagues49 and reproduced from Highton-Williamson and colleagues. 50
Design
The FIAT study was a cluster randomised controlled trial. The trial protocol is accessible in the public domain. 48 The study tested the hypothesis that offering financial incentives to patients who agree to their treatment but who have difficulties adhering sufficiently to it (i.e. have adherence ≤ 75%) would lead to improvements in their adherence.
The teams recruited were randomly allocated to an intervention group or a control group. Initially AOTs were approached; however, owing to slow recruitment, the trial was extended to include Community Mental Health Teams (CMHTs) and recovery teams (a new term for similar teams that was introduced in some services during the study period). The allocation of teams and not individuals to treatment conditions was designed to prevent contamination of practice within teams. A 1 : 1 allocation ratio was used to randomly assign mental health treatment teams in the community to either the intervention or control arm.
Eligible patients within the team were given a £15 incentive for every LAI medication received in the intervention arm or treatment as usual in which eligible patients within the team received no financial or any other incentive for taking their LAI medication in the control arm.
Setting
The study was co-ordinated through the Unit for Social and Community Psychiatry at Queen Mary University of London. There were three study sites from which recruitment and data collection were organised, namely Queen Mary University of London, the University of Oxford and the University of Liverpool.
Eligibility criteria
Inclusion criteria
The inclusion criteria for AOTs and CMHTs were that they care for patients with psychotic disorders and had patients who had problems adhering to LAI medication. The exclusion criteria were a lack of willingness to participate and an already existing practice of giving financial incentives to patients.
For the patients in the teams, the inclusion criteria were:
-
being cared for by an AOT or a CMHT for at least 4 months
-
aged between 18 and 65 years
-
capacity to give informed consent to participate in the study and actual written informed consent
-
established diagnosis of schizophrenia, schizoaffective psychosis or bipolar illness according to International Classification of Diseases, Tenth Edition51
-
being prescribed LAI medication
-
poor adherence to antipsychotic medication, that is, having received ≤ 75% of prescribed LAI medication, over the 4 months prior to screening
-
failure of all other methods available to the team to ensure adherence to medication.
Exclusion criteria
Exclusion criteria were:
-
an intellectual disability
-
poor command of English so that clinical communication and discussion of agreements is impaired.
Participant withdrawal criteria
No formal withdrawal procedures were defined for the study. Patients were informed while giving consent that they were free to withdraw their consent and discontinue their involvement in the study at any stage they wished. Research assistants (RAs) attempted to collect outcome data for all participants.
Intervention
Control group
Patients in teams allocated to the control arm received treatment as usual with no financial or other incentive for taking their medication. The type, frequency and dosage of the medication and other interventions were not affected by participation in this study.
Intervention group
Patients in the teams that were allocated to the intervention were offered a financial incentive for each LAI medication they received for a 12-month period, which was received either at the CMHT or in the patient’s home. Patients received £15 per injection. The total sum that a patient could receive could not exceed £60 for 1 month, as the maximum number of injections is four per month. The administering clinician gave the money in cash directly after the injection and patients signed a receipt on receiving the incentive.
There are several reasons why the sum of £15 was chosen as the incentive payment:
-
A fixed sum per injection simplifies the practice and makes it transparent for all clinicians and patients involved.
-
The sum of £15 is in line with the successful pilot study run in east London. 46
-
The sum is below the £20 per week limit, which would interfere with patients’ disability benefits. Most patients eligible for the study receive Disability Living Allowance, Income Support with Disability Premium or Incapacity Benefit. In all of these cases, patients are not entitled to have a separate income of > £20 without having their benefits reduced. That includes therapeutic earnings and income made through research participation.
-
£15 per injection is intended to be an incentive, helping motivate otherwise ambivalent patients. It is important to limit the total sum to a maximum of £60 per month so that patients do not become financially dependent on the additional income. The money was intended to provide an incentive but not lead to financial dependence on the scheme.
Implementation of screening and recruitment procedure
Patients were recruited from CMHTs and AOTs across England and Wales between March 2010 and November 2011. As stated in the published protocol48 the intention was to recruit from only AOTs, but during the recruitment process this was expanded to include CMHTs. Since the publication of the original protocol the landscape of community mental teams providing mental health care had changed. In various NHS trusts, AOTs had been decommissioned and CMHTs had taken over the original function of AOTs. Focusing the study exclusively on AOTs would have introduced a substantial bias in the study.
An inclusion criterion was originally set at a maximum adherence of 50% in the 4 months prior to screening. We changed this to 75% adherence for the following reasons:
-
We had many discussions with AOTs when presenting the study. These discussions showed that the clinical problem of poor adherence begins at a much higher percentage threshold than the originally envisaged 50%. AOTs follow up patients so intensively and assertively that patients commonly receive almost all medication, although there may be delays. In this context missing 25% reflects a significant failure in achieving treatment adherence.
-
Patients treated by AOTs are at particular risk of relapse with consequences for other types of clinical risks (e.g. self-harm or danger to others). AOTs (unlike ordinary CMHTs) therefore respond with immediate and drastic interventions (e.g. voluntary or involuntary hospital admission) when a patient’s adherence drops < 75%. When the study protocol was written, Community Treatment Orders had not been implemented and were envisaged by the Department of Health to be used for a maximum of 400 patients in England; since then they have changed clinical practice in AOTs. As a result, there are very few patients with a much lower adherence than 75% over several months (based on the data of 11 AOTs including teams in deprived inner city areas). If financial incentives are to be tested as an intervention that is relevant to clinical practice, patients who would realistically be offered financial incentives and who have adherence levels between 50% and 75% over a few months should be included.
-
The very few patients with adherence levels < 50% are usually those with whom AOTs have failed to establish regular or any contact at all. Such patients were unlikely to be recruited to a research study. If financial incentives are to be implemented, it would be very difficult to offer the incentives to patients with very little contact with the AOT. The patients who can realistically be offered financial incentives would have some more regular contact and commonly have adherence levels > 50% (although still < 75%).
A standardised screening procedure was used across all three study sites. A total of 540 mental health teams were approached (387 CMHTs and 153 AOTs). Teams approached were all based within reasonable distance of study sites so that frequent travel to each team was practical and achievable.
Research assistants made contact with team managers of CMHTs and AOTs through letters and telephone calls. Teams that expressed an interest were visited by researchers to check the team’s eligibility and to discuss the study further. Clinicians and managers in all teams received a structured presentation addressing the research background, the design of the trial and the ethical and practical implications of the study. Written informed consent was obtained from team managers and/or consultant psychiatrists from teams that agreed to take part.
All eligible patients in participating teams were approached by a clinician. If they agreed to take part in the study they were contacted by a researcher (or a Clinical Studies Officer from the Mental Health Research Network) in order to obtain informed consent and complete the quality-of-life and satisfaction with treatment questionnaire. After this initial contact with patients, there was no requirement for further contact with patients in either group throughout the 12-month intervention period. This non-intrusive procedure was meant to minimise the number of non-consenting patients and avoid a selection bias as far as possible. Only if patients volunteered to be contacted at the end of the trial did a researcher attempt such contact to ask the 11 questions on quality of life and satisfaction with treatment.
All participants provided signed written informed consent before taking part in the trial. Once written informed consent had been received from all eligible patients at the team, the team was randomised to either the intervention or the control arm and was informed of their allocation by a RA.
Issues encountered during patient recruitment
The patient recruitment was slower than foreseen because of a number of issues.
-
Owing to efficient work of the AOTs, few patients were found to have missed ≥ 25% depot injections in the period prior to screening. This issue might have been prevented by carrying out a pilot study, rather than merely relying on clinicians’ estimations.
-
Obtaining research and development approvals in participating trusts took longer than originally estimated, in a few cases even months. The procedure was made difficult by different trusts following different procedures in issuing the research and development approval and researchers’ letters of access.
-
In some teams, researchers were not allowed to screen for eligible patients or access medical records, despite having obtained the letter of access. A member of stuff would have to be asked to carry this out, which, again, contributed to difficulties in timely recruitment and data collection.
-
Obtaining AOTs’ and CMHTs’ consent for participation in the study proved to be a lengthy and rather complicated procedure. This required booking an initial 30- to 60-minute slot in a team meeting to present the research study and answer teams’ concerns regarding financial incentives. Thereafter, the team would meet without researchers and discuss any issues openly before agreeing or rejecting to participate in the study. Often, teams had to be followed up by researchers several times, and obtaining a team’s decision could take several months.
-
As patients recruited in the study were difficult to engage, they often found it problematic to attend appointments with the research team. This would result in the team’s repeated attempts to see patients before the informed consent was obtained. Reminding patients by sending out letters and telephone calls proved to be helpful to some extent.
-
The teams recruited to the study were from across England and Wales, which required a substantial amount of travelling from the research team, along with the associated costs and time.
Sample size
It was initially assumed three patients in each AOT and CMHT would provide data, that cluster size would vary little and that the intracluster correlation coefficient (ICC) of adherence would be 0.05. To detect a shift in mean adherence from 65% to 85% [standard deviation (SD) of 30% at baseline] with 90% power at the 5% significance level, it was calculated that 47 patients were required per group. This was then inflated to 68 patients per group (four patients in each of 17 clusters) to allow for clustering and drop out, that is a total of 136 participants from approximately 68 teams.
During the trial it became clear that average cluster size was smaller with a mean of only two individuals per cluster, but cluster sizes were more variable. In addition, the SD of the baseline adherence of patients was smaller than that assumed in the original sample size calculation. The sample size calculation was reviewed in the light of these changes. However, when all the changes in the inputs to the calculation were considered, the sample size required was virtually unchanged and so no changes were made. It was therefore planned to recruit 136 participants in total from approximately 68 teams.
Randomisation
Randomisation was carried out at the Pragmatic Clinical Trials Unit at Queen Mary University of London by a senior statistician who had no other involvement with the trial. The statistician was entirely independent of patient recruitment. Cluster randomisation was carried out with AOTs and CMHTs allocated as the clusters. Teams were stratified according to their national Mini Mental State Examination (MINI) score, high or low;52 the MINI score measures aggregate social isolation, poverty, unemployment, permanent sickness and temporary and insecure housing. It was assumed that teams in areas with higher deprivation would have more eligible patients, and potentially more challenging ones. A sequence of allocations in each stratum was generated using the ‘ralloc’ command in Stata (versions 10.1 and 12; StataCorp LP, College Station, TX, USA). 53 Sixty group allocations were generated for each stratum arranged in blocks of random length (2, 4 or 6) with a randomisation ratio of 1 : 1.
Allocation concealment mechanism
All clusters were identified and recruited prior to randomisation to minimise selection bias. Randomisation of clusters took place only once all participating patients identified from each CMHT had been recruited.
Blinding
It would have been impossible to blind participants and the clinicians delivering the financial incentives to the intervention group. The primary outcome (percentage of prescribed LAI medication taken) and secondary outcomes, with the exception of the clinical global improvement, were obtained objectively from the medical records and should therefore not be influenced by lack of blinding. The trial statistician, responsible for analysing the data became unblinded shortly after receiving the data, on learning that two patients withdrew from the trial as soon as their teams were randomised.
Safety evaluation
The trial intervention was non-medical with no expected serious adverse reactions. It was anticipated that financial incentives would improve adherence to antipsychotic medication. However, any serious adverse events were recorded as follows:
-
death
-
hospitalisation as a result of non-adherence
-
any patients based at teams allocated to the intervention group who were not involved in the study asking to receive incentives or threatening to stop receiving LAI medication unless included in the incentive scheme.
Ethical opinion and research governance
The study received a favourable opinion by the Ealing and West London Research Ethics Committee on 13 July 2009, Research Ethics Committee reference number 09/H0710/35. Research and development approval was obtained from each participating NHS trust. The trial was conducted in accordance with legislation from the Research Governance Framework for Health and Social Care. 54 A Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC) were established and involved throughout the design and implementation of the FIAT trial.
Patient and public involvement
Patients and members of the public were involved in the design and management of the FIAT study as members of the User Research Advisory Group of East London NHS Foundation Trust and the FIAT TSC. This led to specification of the practice for offering incentives, such as offering incentives for each depot received rather than on a monthly basis as originally foreseen; limiting patients’ monthly income from financial incentives to £60 so that patients’ benefits were not put at risk; and or the ways of disseminating the study’s findings to ensure they reach service users. Patients’ involvement was also reflected in conducting qualitative interviews with patients involved in the intervention arm of the trial.
Outcome measures
Primary and secondary outcome measures were collected at baseline (up to 12 months prior to randomisation) and at the end of the 12-month intervention, with all but clinical global improvement, subjective quality of life and treatment satisfaction also assessed at the 6-month follow-up.
Primary outcome
The primary outcome was the percentage of prescribed LAI medication taken in the community within the 12-month trial period. In calculating the primary outcome, periods of hospitalisation were discounted. Their inclusion would likely inflate adherence under the assumption that patients cannot avoid taking their LAI medication while in hospital. Adherence during imprisonment is less certain, as patients may have the right to refuse their medication. Periods of hospitalisation or imprisonment were therefore excluded, except if the period out of the community was less than the duration of one LAI medication treatment cycle, assuming that patients absent for relatively short periods had a high chance of receiving their prescribed LAI medication in the community.
Secondary outcomes
The secondary outcomes were as follows:
-
The percentage of patients achieving at least 95% adherence, calculated as a dichotomous outcome from the percentage of prescribed LAI medication actually received by the patient during the 12-month study period, again based on electronic and paper records of patients’ receipt of LAI medication.
-
Time ‘slippage’ of taking LAI medication, which is defined as the percentage of the time interval following the day of the prescribed LAI medication that has elapsed before the LAI medication is taken.
-
Patients’ clinical global improvement at the end of the 12-month study period. This was rated by asking the consultant psychiatrist or other clinician overseeing the care of each patient to complete the clinical improvement component of the Clinical Global Impression (CGI) scale. 55 This single-item observer-rated scale asks clinicians to rate the extent of their patient’s improvement with a score ranging from 1 (very much improved) to 7 (very much worse). The score was then dichotomised to give a binary outcome of ‘improved’ or ‘no change, or worse’. CGI scale global improvement scores have been shown to correlate highly with change in depression scores during the course of antidepressant treatment56,57 and discriminate treatment responders from non-responders,58 indicating good convergent validity.
-
Patients’ subjective quality of life, which was assessed at the end of the 12-month study period using the DIALOG scale. 59 The scale consists of 11 items asking patients to rate their satisfaction with eight life domains and three treatment aspects, one of which is medication, on a scale ranging from 1 (lowest satisfaction) to 7 (highest satisfaction). The subjective quality-of-life subscale has been shown to have good psychometric properties60 including high internal consistency (Cronbach’s alpha = 0.71), a meaningful factor structure and good convergent validity with the Manchester Short Assessment of Quality of Life61 (r = 0.95) and divergent validity with the Positive and Negative Symptoms Scale62 (r = –0.37). The treatment satisfaction subscale has been shown to have acceptable internal consistency (Cronbach’s alpha = 0.57) and to have good convergent validity with the Client Satisfaction Questionnaire63 and divergent validity with the Positive and Negative Symptoms Scale. 62
-
Satisfaction with medication.
-
The number of involuntary and voluntary hospital admissions.
-
Adverse events:
-
attempted and completed suicides
-
incidences of physical violence
-
police arrests.
-
-
Use and costs of inpatient care, outpatient and community mental health care and other health services during the 12-month treatment period, including the cost of the intervention, and in the subsequent 6-month post-intervention period.
Patient interviews
To explore the immediate and long-term impact of receiving the incentives, interviews were conducted at two time points, that is, at the end of the intervention and then 24 months after the end of the intervention. To be interviewed, patients had to receive financial incentives at least once, have a good command of the English language and have the capacity to consent to interview. Patients were not interviewed if they had not received any incentives throughout the intervention, spent the majority of time of the intervention period out of the community (e.g. hospitalised or imprisoned) or did not have the capacity to consent to interview. As the aim of the interviews at the end of the intervention was to explore patients’ views and experiences with the intervention, only those patients who completed the intervention within the previous 4 months were interviewed. No time requirement was used for the follow-up interviews.
An interview schedule consisting of open-ended questions was used at both time points. Questions posed at the end of the intervention included patients’ opinions about receiving financial incentives; whether or not receiving financial incentives affected their relationship with the clinician; whether or not the incentives changed the frequency of patients’ LAI medication appointments; how patients used the money; how they experienced the incentives having stopped; and how that affected them. Questions at the 24-month follow-up interviews included exploring the long-term impact of having received financial incentives. These included long-term changes in attitudes to treatment, relationship with family and friends and attitudes to receiving financial incentives.
At both time points, probing questions were employed for further development or clarification of a topic or a point. All participants were interviewed individually and the interviews were transcribed verbatim by an independent professional; the transcripts were checked for accuracy and any identifiable information was removed.
Clinician interviews
Semistructured interviews were carried out with the clinicians of patients in the intervention arm of the FIAT study. Interviews with clinicians were conducted at four time points to determine the immediate and long-term impact of offering the incentives, that is, 6 and 12 months into the intervention, and 6 months and 24 months after the end of the intervention. Clinicians were eligible for interview if they were involved in the patient’s care throughout the intervention period (e.g. team manager, consultant psychiatrist, care co-ordinator or nurse in LAI medication clinic). However, for interviews at 6 and 24 months’ follow-up, a number of clinicians or patients had transferred to other teams after the end of the intervention; therefore, it was not possible to interview all of the clinicians interviewed during the intervention period. To overcome this, other clinicians not originally interviewed but who were involved in the patient’s care during the intervention, or who had been involved since the intervention finished but knew the patient well enough during the trial itself to be able to answer questions, were interviewed.
An interview schedule with a mixture of yes/no questions and room for further unstructured responses was used at all time points. For interviews at 6 and 12 months into the intervention, questions included how clinicians thought patients spent the money, whether or not patients asked for more money or more frequent LAI medication appointments, the experiences of patients outside the trial (e.g. asked for incentives or become non-adherent to qualify) and the effect of the incentives on both the patient outcome and the interaction with the team. For interviews at the 6- and 24-month follow-ups, questions included whether or not clinicians continued using the incentives with patients within the trial or any new patients, whether or not patients within or outside the trial had asked for the incentives again or had become non-adherent to qualify, the long-term impact of offering/discontinuing the incentives on patient outcomes and interaction with the team, their opinions of the incentives before and after the intervention and the mechanisms underlying how the incentives worked. All responses recorded were written in shorthand.
Settings for data collection and interviews
Primary and secondary outcome data were collected from electronic and paper patient records and medication charts kept at the CMHTs and AOTs. Data on subjective quality of life and medication satisfaction were collected in person through interviews with patients either in their home or at their mental health team base. Qualitative interviews with patients were conducted either within their homes or with their mental health team base. Clinician interviews were conducted over the telephone, by e-mail or in person by a RA.
Time period for recruitment and data collection
The different stages of the study are presented in Figure 1. On the day of screening, adherence data for the previous 12 months were collected. Other relevant data were collected for the 12 months prior to the date of randomisation. Taken together, these data are referred to as ‘baseline’ data. The intervention period began 7 days after randomisation. Subsequent data collection was carried out 372 days after randomisation (referred to as ‘end of intervention’ data and covering the 12 months of the intervention), and then again 6 months after the end of the intervention (referred to as the 6-month follow-up).
FIGURE 1.
Timeline of data collection in the different stages of the study.
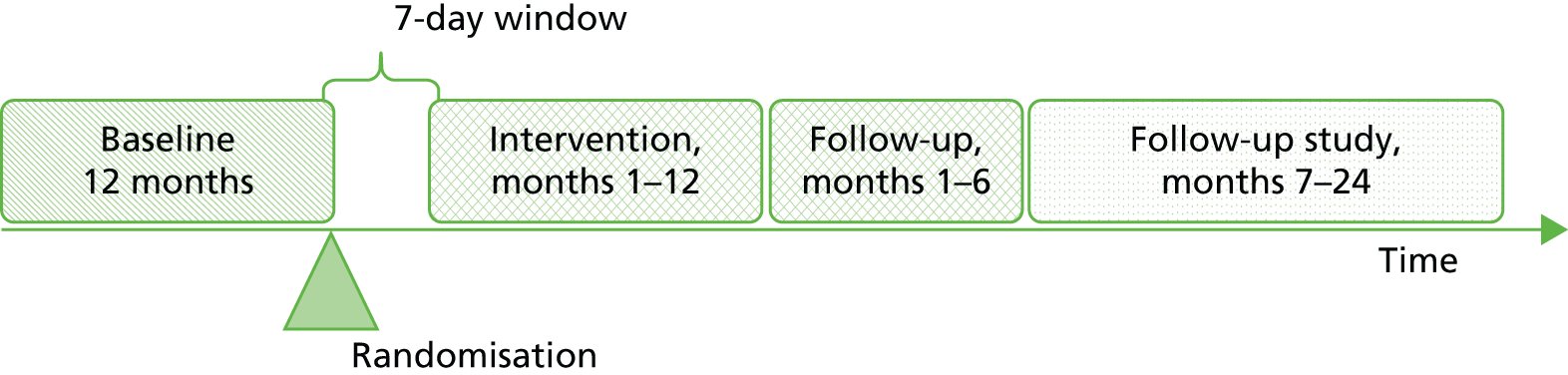
Recruitment took place between March 2010 and November 2011. Baseline data were collected from October 2011 (when the study recruitment period was due to finish). The end of intervention for teams took place between March 2011 and November 2012 and the 6-month follow-up period ran from September 2011 (i.e. 6 months after March 2011) to May 2013. Data from both periods were collected between May 2012 and May 2013.
Amendments to the study following commencement
All amendments to the study were carried out following consultation with the Research Ethics Committee. Three amendments were made to the original study protocol. The first was confirmed by the Ealing and West London Research Ethics Committee on 24 February 2010. The changes to the original protocol are as follows:
-
The first amendment involved the widening of recruitment to include not only AOTs but also CMHTs. Since the publication of the original study protocol, the landscape of CMHTs providing mental health care in the NHS had changed. In a number of services, AOTs had been decommissioned and CMHTs had taken over their function. During recruitment it became apparent that focusing the study exclusively on AOTs would have introduced a substantial bias to the study, as areas providing care for the same patients in CMHTs would have been excluded. The approval allowed for the recruitment of participants from both teams.
-
The amendment also allowed for a change of participant inclusion criterion. Originally, participants needed to have ≤ 50% adherence to be recruited to the study. This was changed to 75% for reasons explained in Implementation of screening and recruitment procedure.
-
The amendment also rectified an inconsistency between the patient consent form and the patient information sheet. On the information sheet patients were told that filling in any questionnaire was entirely optional. The original version of the consent form did not mention the term ‘optional’, which was corrected.
On the 22 October 2011, a minor amendment to the original study protocol was received from the National Research Ethics Service (NRES) Committee London – Harrow, North East London NHS Foundation Trust. The following amendment was made:
-
Participants were offered the opportunity to participate in a short semistructured interview on their experience of the trial to be offered at the same time as the quality-of-life questionnaire at the end of the intervention. The amendment outlined that patients were to be offered £20 for participating in the interview, which would last no longer than half an hour. The interview was in a semistructured format, covering the patients’ ideas about and experiences of the trial and how it impacted on them individually.
The study was granted a 10-month, time only, cost-neutral extension by the Health Technology Assessment programme on the 30 August 2011. The prolonged recruitment period meant that data collection was delayed. The extension of 10 months therefore allowed for the successful collection and analysis of data. Permission was sought from the NRES Committee London – Harrow to extend and collect data for the study until 30 September 2013. This request was granted by the committee on the 17 October 2011.
Follow-on study
The FIAT study was granted a 19-month extension in November 2012 by the Health Technology Assessment programme to investigate the impact of the intervention in the longer term. This extension included following up teams and patients for a further 18 months after the 6-month follow-up (i.e. between months 7 and 24 after the end of the intervention; this period is referred to as the 24-month follow-up). Data for the primary outcome as originally defined and secondary outcomes including the percentage of patients with adherence at least 95%, hospitalisations and adverse events were collected at 24 months post intervention, that is, between May 2013 and November 2014.
The extension allowed the opportunity to investigate the long-term impact of the incentives from the perspective of both patients and clinicians. We aimed to interview the clinicians of patients allocated to the intervention group to determine their experiences of implementation if financial incentives were continued and to explore the longer-term impact on patient outcomes, the therapeutic relationship and any consequences for other patients as a result of the incentives being continued or discontinued. We also planned to conduct in-depth interviews with 30 patients allocated to the intervention group to address how the incentives influenced their adherence, whether or not improvements in adherence had any impact on their outcomes, how patients experienced the role of incentives in their care and their opinions on the use of financial incentives.
Permission was sought from the NRES Committee London – Harrow in February 2013, which did not consider the follow-up study to be a substantial amendment and informed us that the follow-up could be implemented without the need for a full committee procedure.
Statistical analyses
Each outcome was analysed using all available cases, following the intention-to-treat principle, that is, analysing the patients in the groups to which their teams were randomised. Patients were included in any particular analysis where that outcome was measured or computed both at baseline and the end of the intervention, and at 6 months after the end of the intervention. At a TSC meeting held on 21 January 2013, it was decided that one additional analysis would be undertaken, for the primary outcome only, from which patients from the following categories were excluded:
-
those with diagnoses excluded by the inclusion criteria
-
those who were found to have been adherent (i.e. > 75%) during the 4-month screening period.
Owing to the logistics of patient recruitment, there was usually a substantial gap between patient screening and team randomisation. As periods spent out of the community were recorded for the 12 months before randomisation (rather than screening), any baseline LAI medication card data older than this were ignored for the purpose of calculating baseline adherence, as otherwise such absences could not be handled correctly because they were missing data. Treatment cycle (weekly, fortnightly, every 3 weeks or every 4 weeks) determines the number of LAI medications prescribed. The denominator of the adherence outcome also factored in number of LAI medications prescribed according to treatment cycle, periods out of the community longer than a treatment cycle, changes in LAI medication treatment cycle and periods only on oral medication.
Issues arising from long-term antipsychotic injectable medication card data
The baseline adherence data were asynchronous with all other baseline data that were collected for the year before team randomisation. However, the LAI medication data were collected for 365 days back from the screening date. This means, when there was a gap between screening and randomisation (mean gap was 61 days, SD 45 days), the earliest LAI medication data collected during the screening period did not coincide with the rest of the baseline data. This was problematic for calculating adherence, as it takes into account periods out of the community greater than one LAI medication treatment cycle. If hospitalisations or imprisonments were unknown for the early part of the screening period, they could not be handled correctly. This issue was addressed by delaying the start of the baseline period to 365 days prior to the point of randomisation for patients with gaps. This was to ensure that the baseline period, although shorter than 1 year, would be contemporaneous with the other baseline data. Figure 2 shows an illustration of the problem with the gaps in LAI medication data and the solution.
FIGURE 2.
Illustration of the gap problem and solution. E, start of the period in which eligibility was assessed, that is 122 days (4 months) prior to the screening date; g, gap between the screening date and the date the team was randomised; I0, intervention start date, which is fixed at 7 days after the randomisation date; I1, intervention end date, which is fixed at 365 days after the intervention start date; R, team randomisation date; S0, screening date, that is the patient’s adherence was initially assessed on this date; S-1, screening date – 365 days.

Defining the primary outcome
The primary outcome was adherence to LAI medication defined as the percentage of prescribed LAI medication actually taken during a 12-month period. This was defined as the following:
The analysis population, which is available cases following intention-to-treat principles, for the primary analysis of the primary outcome was defined as all randomised patients, in the group to which they were randomised subject to having one continuous period of at least 4 months in the community in both baseline and intervention periods for which depot data were available. Patients who had died or moved away were included providing they met these criteria and had not withdrawn/been withdrawn from the study. Those who had withdrawn from the study or had been withdrawn were excluded.
Definitions:
-
In the community implies neither in hospital (either psychiatric or general hospital admission) nor in prison.
-
Periods out of the community that span less than one cycle are treated as in community.
The treatment cycle possibilities are defined as follows:
-
1/52: weekly
-
2/52: fortnightly
-
3/52: every 3 weeks
-
4/52: every 4 weeks.
The periods of analysis were:
-
Baseline: the period from 365 days prior to team randomisation date to screening date.
-
Intervention: the period from the intervention start date (i.e. period from team randomisation date + 7 days) to the end of intervention (i.e. intervention start date + 365 days).
-
Six-month follow-up: defined as 1 day after the end of intervention date + 183 days (covering the 6 months after the end of the intervention).
-
Twenty-four-month follow-up: this time period was defined as 24 months after the end of the intervention; however, this also included the 6-month follow-up period. Therefore, this analysis period only included 18 months after the 6-month follow-up, which was defined as 1 day after the end of the 6-month follow-up date + 548 days (covering month 7 to month 24 after the end of the intervention).
Primary outcome
The effect of the intervention on percentage adherence was estimated using a linear mixed-effects regression model with a random effect for mental health team, adjusting for baseline adherence, MINI score category (low vs. high) and average number of weeks between prescribed LAI medication during the baseline period. The main analysis used data from all individuals who had at least 4 months of continuous data during both the baseline and the intervention periods, on an intention-to-treat basis whereby those who did not receive any incentive as intended were also included in the analysis. A per-protocol analysis was also conducted in order to identify the effect of the incentive on adherence in those who received the intervention, using the same model specification as described in Defining the primary outcome. Finally, a sensitivity analysis without adjustment for baseline adherence was conducted, although still adjusting for MINI score category and average number of weeks between prescribed LAI medication during the baseline period, and with a random effect for clinical treatment team.
The effect of the intervention on the primary outcome at the 24-month follow-up was modelled using a simple linear regression model including only a fixed effect for treatment group. The ICC for adherence across mental health teams was negative (–0.05); therefore, including a random effect would have biased the standard error (SE) of the treatment effect upwards. Sensitivity analyses were conducted, with treatment group being the only fixed-effects variable.
Secondary outcomes
Models assessing the effect of the intervention on the secondary outcomes were adjusted for MINI score category and average number of weeks between prescribed LAI medication, and had a random effect for clinical treatment team.
The effect of the intervention on the binary secondary outcomes was modelled using mixed-effects logistic regression (i.e. achieving vs. not achieving at least 95% adherence and improved vs. unchanged or worse on the CGI scale). For each of these models the baseline value of the relevant outcomes was included as a covariate in addition to those described, with the exception of the analysis of clinical improvement, as a baseline assessment with the CGI scale was not conducted.
The effect of the intervention on the continuous secondary outcome satisfaction with treatment was estimated using a linear mixed-effects model, while subjective quality of life was estimated using a random-effects model fitted by generalised least squares, since the mixed model did not converge. In addition to the covariates described, both models adjusted for the baseline score on the outcomes. Secondary outcomes in which low frequencies were expected, that is, hospital admissions and adverse events, were summarised descriptively only.
Economic evaluation: cost-effectiveness analysis
The cost-effectiveness analyses were conducted from a NHS perspective. The study was powered to detect a 20% improvement in adherence; therefore, for the purposes of the economic analysis, the outcome measure was defined as the incremental cost to achieve a 20% increase in adherence to prescribed LAI medication taken over the 12-month intervention period. The incremental cost of achieving ‘good’ adherence (achieving at least 95% adherence to prescribed LAI medication) over the intervention period was also calculated. The modelling approach was also applied to the secondary outcomes of subjective quality of life and clinical improvement (treated as a binary variable as in the clinical analyses).
Service use and costs
Service use considered in the economic evaluation included inpatient, outpatient and community mental health services, general hospital and primary care services, prescribed oral medications and prescribed and received depot medications. The cost-effectiveness analyses examined costs and outcomes at the end of the 12-month intervention period. In addition, health-care resource use over the 6 months after the end of the intervention was collected for descriptive analysis only. All data on service use were collected by the study RAs via the case report form (CRF), from a combination of electronic and paper health records.
Unit costs
Established unit costs (for the 2010–11 year) from national representative and other published sources64–67 were used to estimate the costs of direct health care. Costs of a contact in any setting (office/service and home/community settings) were calculated, as opposed to the duration of contact with a mental health professional, as little duration data were available, drawing on unit costs taken from the NHS reference costs for England. 65 Oral and LAI medication costs were calculated using the prescription cost analyses. 68 The cost of the intervention itself was calculated as the total number of £15 incentive payments given over the study period, while contacts with nurses, including nurses giving LAI medication, were tracked as part of the data collection via the CRF. Feedback from researchers working with the participating teams indicated no other resources were consumed in providing the financial incentives. The number of incentive payments was variable, depending on the number of LAI medication appointments attended by patients. Unit costs are shown in Table 1.
| Resource item | Unit cost, range (£, 2010–11) | Unit of measurement |
|---|---|---|
| Hospital use | ||
| Mental health inpatient service use | ||
| Mental health outpatient attendances (A&E, day and outpatient appointments) | 97–185 | Per attendance65 |
| Mental health inpatient bed-days | 327–633 | Per day65 |
| Mental health residential and hospital alternativesa | 92–279 | Per day64,66,67, (Dr B Barrett, King’s College London, 25 February 2013, personal communication) |
| General hospital inpatient service use | ||
| General hospital all outpatient attendances (A&E and outpatients) | 111–117 | Per attendance65 |
| General hospital inpatient bed-days | 424 | Per day65 |
| Community and primary health services | ||
| Family support worker | 46 | Hour64 |
| Vocational worker | 53 | Per contact64 |
| Substance abuse worker | 116 | Per contact65 |
| Counsellor | 60 | Per consult64 |
| CMHT contactb | 126 | Per contact65 |
| AOT contactb | 121 | Per contact65 |
| GP home visit | 82 | Per visit64 |
| GP surgery | 25 | Per visit64 |
| Medicationsc | Various | Standard quantity units68 |
Missing data
Cases treated as missing for the adherence outcome were treated the same way in the economic analysis. Cases were also counted as missing when all hospital or community service utilisation data were missing. The economic analyses drew on complete cases only.
A gap in the LAI medication data arose between randomisation and screening, necessitating imputation of LAI medication costs between the dates of screening and entry into the trial. So that the costs of the LAI medication would be in step with other costs, the period between the 12 months pre screening and the 12 months pre randomisation and the gap between the dates of the last LAI medication cycle and randomisation were calculated. The extra LAI medication cycles occurring in the gap were imputed by last observation carried forward. LAI medication unit costs were attached to the LAI medication units occurring over the pre-randomisation period and to the imputed LAI medication units in the gap, if the gap was as long as or longer than one LAI medication cycle. The total LAI medication cycle costs (including any imputed costs) were then adjusted by applying the proportion of prescribed LAI medication that had been taken over prior 12 months to screening.
Cost-effectiveness analyses
The financial incentive intervention should be considered cost-effective if it is more effective and less costly than the treatment as usual received by the control group or if it is more effective and more costly than treatment as usual and the purchaser is willing to pay the extra cost in order to gain the associated benefit. If the financial incentive strategy is more effective but also more costly, the additional cost per additional benefit produced [the incremental cost-effectiveness ratio (ICER)] would have to be less than the purchaser is willing to pay.
Incremental outcomes and costs were estimated through multilevel multivariate regression models, adjusting for the following covariates: intervention allocation, baseline measure of outcome (except in the case of the CGI scale, measured at end of intervention only), total costs in the pre-baseline year, high/low MINI score category and average LAI medication treatment cycle over the baseline period. The average number of weeks between prescribed LAI medication was controlled for, as some patients’ treatment cycles changed over the period. The modelling allowed costs and outcomes to be correlated both within and between clusters, with random effects for the participating teams as clusters.
Error terms for costs and outcome equations were assumed to be normally distributed. The coefficients on the allocation term in the costs and effects equations (giving the cost and outcome differences, respectively, between groups) were used to derive net monetary benefit values over a range of willingness to pay (£0–30,000) for the additional benefit associated with the intervention. The 95% confidence intervals (CIs) for the ICER were calculated from the model estimates using Fieller’s method. 69 Cost-effectiveness acceptability curves were constructed from the regression results, depicting the probability of the ICER being less than each willingness-to-pay value in the range.
Sensitivity analyses were also employed to examine the impact of altering key assumptions on the cost-effectiveness of achieving both adherence outcomes. The first analyses explored the impact of when patients ‘did not attend’ (DNA) an appointment, either through failing to turn up to an appointment or by not being in during a community visit (planned or unplanned). No data on the duration of contact for DNAs were available, and thus DNAs were excluded from the main analysis. However, it is possible that DNAs could consume a substantial amount of health professionals’ time, and to account for this, DNA costs were incorporated into total costs in a sensitivity analysis, assigning these visits the same unit costs as actual contacts with patients. Another sensitivity analysis explored the impact of the varying level of skill-mix of mental health professionals within teams. As a result of using national reference costs for contacts with professionals from CMHTs and AOTs, the costs may not reflect the variability in skill-mix within these teams or the actual duration of contact that patients had with particular mental health professionals. Therefore, to explore the impact of over- or underestimating these costs and their contribution to the ICER, unit costs of AOT and CMHT contacts were varied by 25%, 50% and 150%.
Qualitative analyses
Patient interviews
The analysis of the patient interviews followed a thematic analytical approach as described by Braun and Clarke. 70 Transcripts from the interviews conducted at the end of the intervention were subject to initial coding by researchers (Alexandra Forrest, Hana Pavlickova and Nicola O’Connell). Based on these codes, a provisional framework was devised capturing the similarities, differences and initial themes. The framework allowed for the addition of codes throughout the process. Data were then independently recoded by the researchers into this framework, which was continually refined and recoded reflecting the iterative discussion.
The interviews conducted 24 months after the end of the intervention were analysed in a similar fashion, with researchers performing initial coding of the transcripts (conducted by Adam Ziecik, Hana Pavlickova and Katherine Moran). An initial coding framework was drawn up based on these codes and recoded in line with this framework. However, throughout the analysis, the research team felt that the codes and themes arising were similar to those of the interviews conducted at the end of the intervention and, following extensive discussions, a decision was made to merge together the interviews conducted at both time points for analysis. The interviews from both time points were revisited, previous coding frameworks discarded and a new coding framework was devised capturing the best fit of the data at both time points. The data were recoded into this framework, which was continually refined and recoded into. This process occurred until all identified themes were internally homogenous and externally heterogeneous.
The research team comprising research psychologists and an academic and clinical psychiatrist were involved in conducting the iterative process of analysis and interpretation. All team members had been involved in implementing the FIAT trial at different points. Throughout the analysis at both time points, at least 60% of the data were coded by two or more researchers to establish inter-rater reliability. All data were imported, analysed and managed using NVivo (version 10; QSR International, Warrington, UK) qualitative analysis software.
Clinician interviews
The clinician interviews were analysed at two different time points, that is interviews conducted at 6 and 12 months of the intervention were analysed together for experiences during the intervention period and interviews conducted at 6 and 24 months after the end of the intervention were analysed together for experiences after the end of the intervention.
Electronic interview transcripts from all time points were then imported and analysed using the NVivo software (version 10) for qualitative data analysis.
Interviews at months 6 and 12 of the intervention were analysed based on a per-patient as opposed to per-clinician or per-interview approach. This was to minimise the possibility of falsely inflating the frequency of theme endorsement. To achieve this, the data on each patient across both time points was collapsed to gain an overall picture of clinician experiences with each patient. The yes/no responses were descriptively analysed whilst the more qualitative unstructured responses were thematically coded within NVivo. As the interview questions were formatted with the anticipation of yes/no responses, they were required to be topic specific (i.e. ‘did offering financial incentives influence the quality of the therapeutic relationship?’ and ‘did offering financial incentives have any other influence on treatment of the patient e.g. attending of the day hospital, contacts with the [general practitioner] etc.?’). Therefore, the analysis of the detailed responses was inductively driven, with themes arising directly from the very nature of the question (e.g. ‘therapeutic relationship’ or ‘other management’). During the original stages of coding, the research team worked together to discuss the application of a preliminary coding framework to each interview. Once a coding framework had been provisionally developed, the first and second authors independently coded the interviews. The authors then collaborated further to refine the emergent coding framework, by either collapsing or expanding codes to encompass emergent themes. Once the second stage framework had been agreed upon, the researchers continued to code independently. This was completed until the authors believed the themes to be internally homogenous and externally heterogeneous. Inter-rater reliability was established by researchers working together on the refinement of the coding framework at the two collaborative stages. 50
Reproduced from Highton-Williamson and colleagues50 under Creative Commons CC BY-NC-ND 3.0
The interviews at 6 and 24 months after the end of the intervention were analysed in a similar fashion. Data were collapsed into one time point and analysed on a per-patient approach. However, questions relating the clinician opinions on the use of financial incentives were analysed using a per-clinician approach to avoid artificially inflating any numbers. All yes/no responses were analysed descriptively and the unstructured answers were thematically coded within NVivo. Prior to coding, a coding framework was developed by researchers with the themes resembling the nature of the questions (e.g. responses for ‘did discontinuation of financial incentives have an impact on the quality of the therapeutic relationship?’ were coded into ‘therapeutic relationship’). After initial coding, researchers collaborated with each other to discuss and refine the themes from the analysis; this process was continued until all themes were believed to be internally homogenous and externally heterogeneous.
Data from the clinician interviews at 6 and 12 months during of the intervention were collated, coded and analysed by Elizabeth Highton-Williamson, Kirsten Barnicot and Tarrannum Kareen. Data from the clinician interviews at month 6 and month 24 after the end of the intervention were collated, coded and analysed by Katherine Moran and Adam Ziecik. Both teams of researchers were regularly supervised by SP. The research team consisted of two psychologists and one clinical and one clinical/academic psychiatrist for the interviews at month 6 and month 12 of the intervention, and two psychologists and one clinical/academic psychiatrist for the interviews at 6 and 24 months after the end of the intervention.
Chapter 3 Results
This chapter reports the results of the intervention period, the 6- and 24-month follow-up and the cost-effectiveness analysis. Finally, findings from patient and clinician interviews carried out during and after the intervention period are also reported. Sections of this chapter have been adapted from Priebe and colleagues47 and Henderson and colleagues,49 and reproduced from Highton-Williamson and colleagues50 under Creative Commons CC BY-NC-ND 3.0.
Study recruitment
In total 540 teams were approached, 184 (34%) of which consented to meet with the RAs to receive further information about the study. From these meetings, 73 (40%) teams were recruited, with 141 consenting patients, and randomised between 16 April 2010 and 15 November 2011. The number of AOTs, CMHTs and recovery teams (a new term for similar teams that was introduced in some services during the study period) that were recruited can be found in Table 2. Ninety-three consenting teams had no eligible patients, and a further 18 teams did not yield any patients for recruitment. The patient flow through the study and at the 6- and 24-month follow-up is reported in the Consolidated Standards of Reporting Trials diagram (Figure 3).
| Type of team recruited | Intervention (N = 37) | Control (N = 36) | Totals (N = 73) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Assertive outreach | 14 | 38 | 10 | 28 | 24 | 33 |
| Community mental health | 22 | 60 | 26 | 72 | 48 | 66 |
| Recovery | 1 | 2 | 0 | 0 | 1 | 1 |
FIGURE 3.
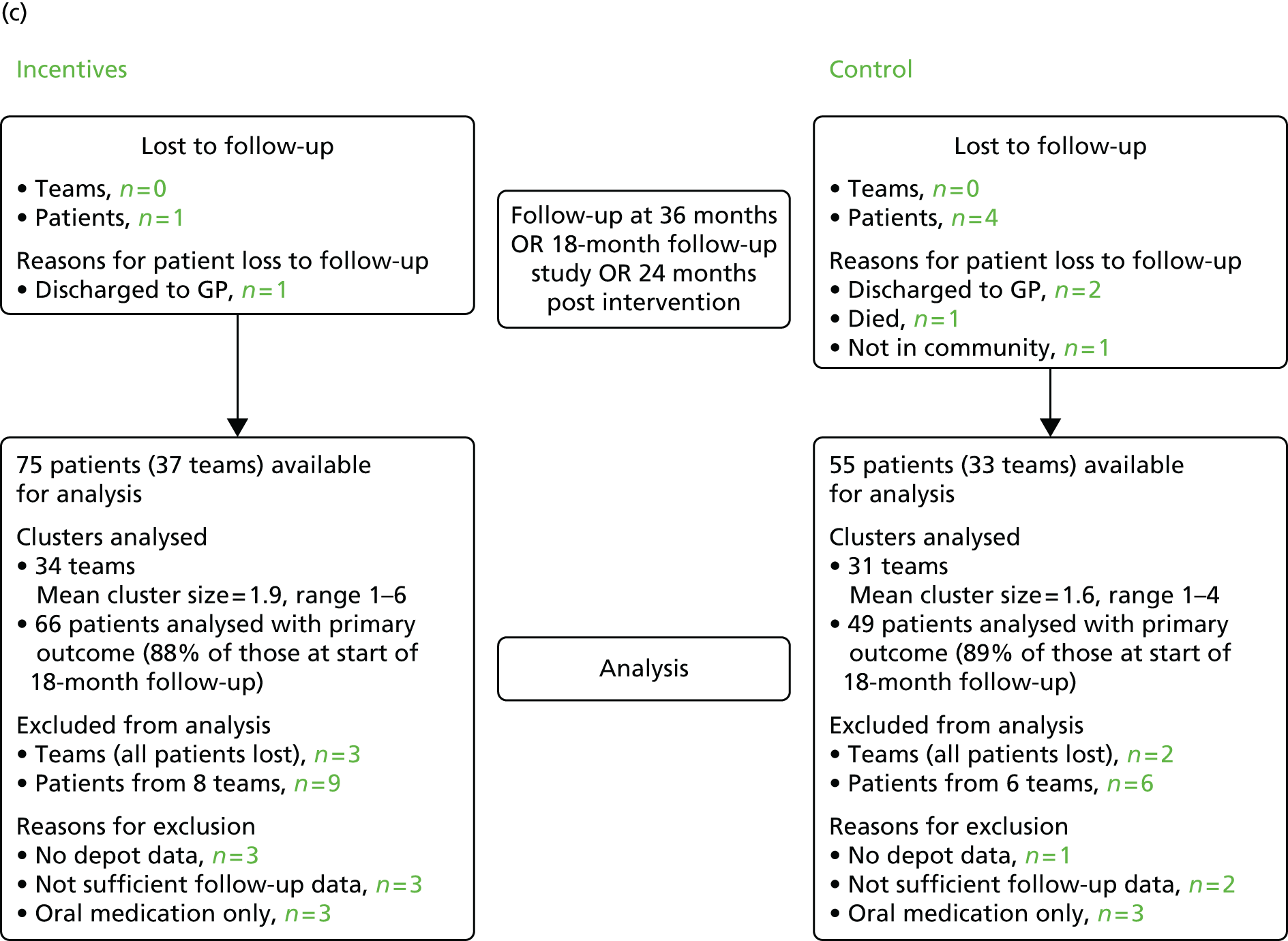
Consolidated Standards of Reporting Trials diagram showing participant flow at baseline, end of the intervention, 6 and 24 months’ follow-up. GP, general practitioner. Source: adapted from Priebe and colleagues. 47 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/.
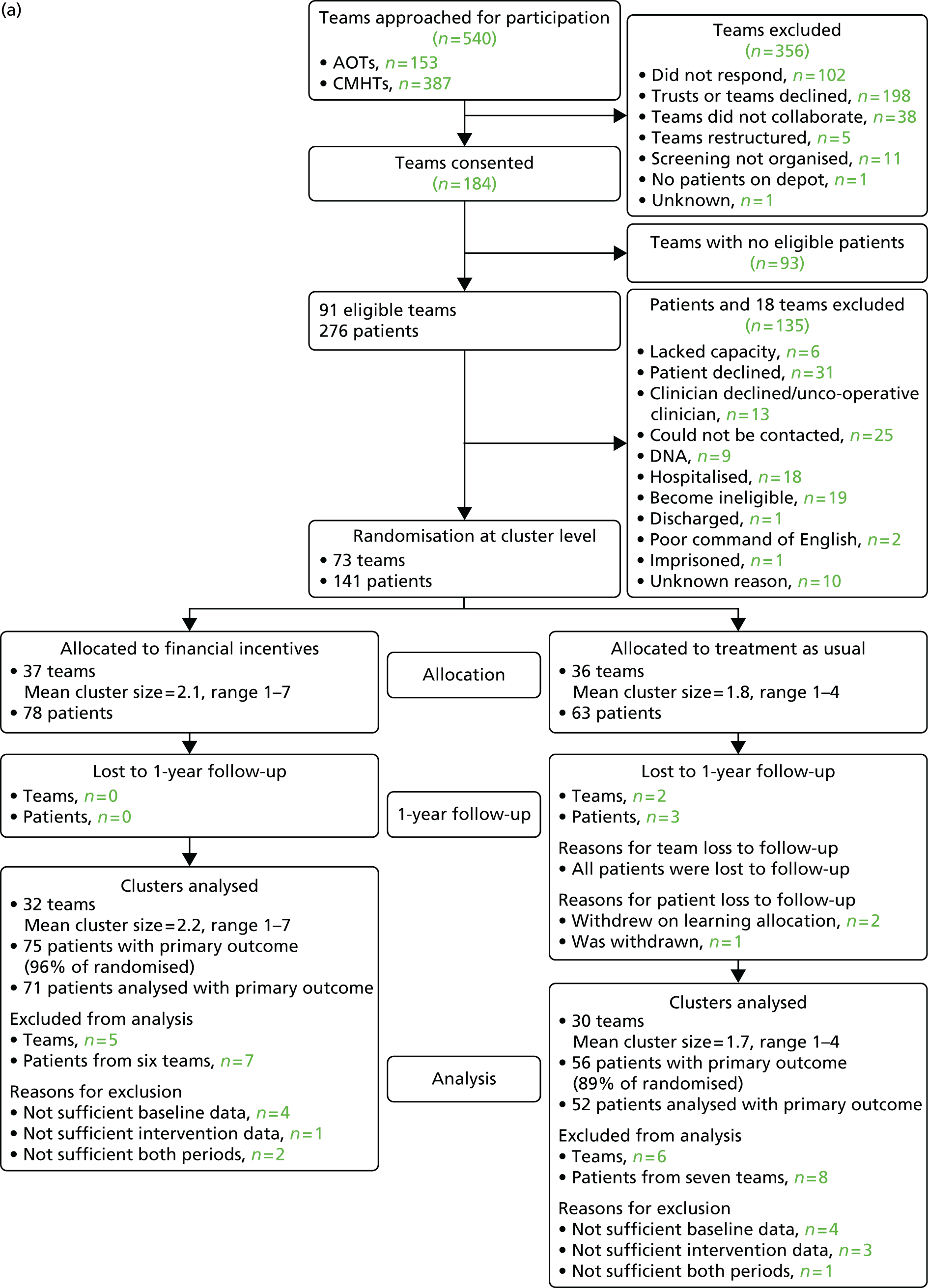
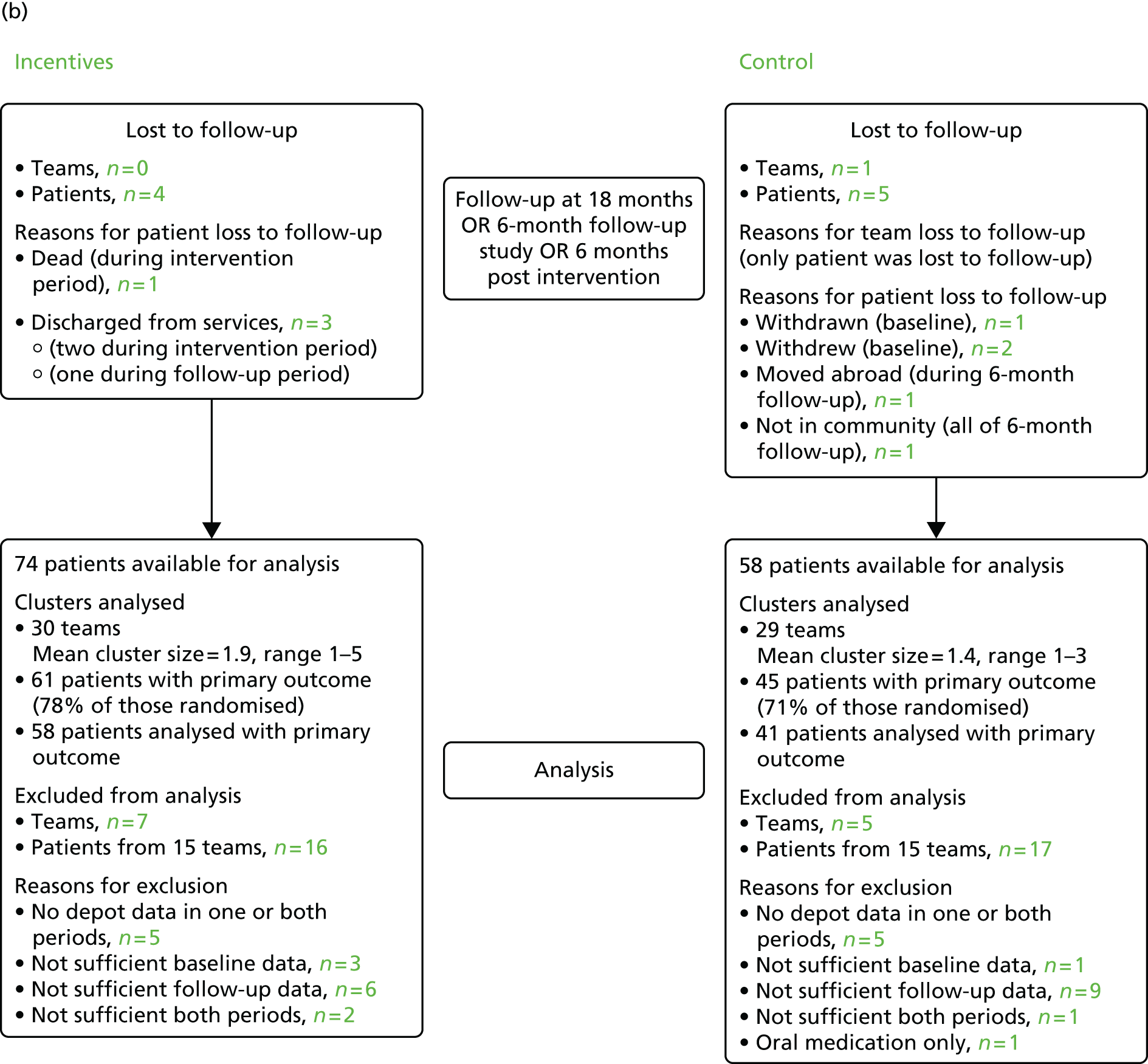
Of the 141 patients who were randomised, nine were lost to follow-up by the end of the 18-month intervention and follow-up period. Of the 132 remaining, the primary outcome could be defined for 106. However, only 99 had the primary outcome defined at both baseline and the end of the 6-month follow-up. Therefore, from the total of 132 patients total, 33 patients were excluded because they had no, or insufficient, depot data during baseline or 6-month follow-up, or both periods.
At 24 months post intervention, 131 patients remained out of the 132: an additional five patients were lost from the 6-month follow-up, while four patients who were lost to the 6-month follow-up returned to the study [i.e. two patients, who had moved away, and two who had been out of the community or discharged to their general practitioner (GP)]. The primary outcome could be defined for 116 patients. Therefore, in total, 15 patients were excluded because they had no, or not sufficient, depot data during the 18-month follow-up period.
Participant follow-up
Data were available for 138 of the 141 patients at the end of the intervention: two patients withdrew and one was withdrawn directly after randomisation to the control group. One intervention patient died during the intervention period of natural causes. Data for this patient until death were used in the study. All four patients were lost to the 6-month follow-up (i.e. leaving 137 patients available). An additional five patients were also lost at the 6-month follow-up: three patients in the intervention group were discharged from services, one patient in the control group moved abroad and another was no longer in the community (i.e. hospitalised or imprisoned). This meant that 132 patients remained at the 6-month follow-up.
At the 24-month follow-up, 131 patients remained. Three patients, one in the intervention group and two in the control group, were discharged from services, one patient died during the follow-up period and one patient was no longer in the community (both in the control group). However, four patients who were lost to the 6-month follow-up returned to the study: two patients who had moved away and two who had been out of the community or discharged to their GP.
During baseline data collection it was found that one patient recruited to the control group had not been prescribed LAI medication at the time of recruitment and was therefore deemed ineligible. In total, four patients did not meet inclusion criteria for diagnosis but were included in the analysis.
The original screening information on adherence in the previous 4 months was taken from a range of sources. When it was later checked against the records, seven patients in the intervention and four in the control group were found to be adherent. These patients were included in the primary analysis but, together with the four with excluded diagnoses, were removed for the per-protocol analysis. Table 3 outlines the available data in calculating the primary outcome at baseline and end of intervention.
| Participant flow | Intervention | Control | Total | |||
|---|---|---|---|---|---|---|
| n of patients (teams) | % | n of patients (teams) | % | n of patients (teams) | % | |
| Randomised | 78 (36) | 100 | 63 (37) | 100 | 141 (73) | 100 |
| Withdrew | 0 | 0 | 2 (2) | 3 | 2 (2) | 1 |
| Was withdrawn | 0 | 0 | 1 (1) | 2 | 1 (1) | 1 |
| Potentially available for analysis | 78 | 100 | 60a | 95 | 138 | 98 |
| Non-qualifying diagnosisa | 2 | 3 | 2 | 3 | 4 | 3 |
| Was adherent at baseline | 7 | 9 | 4 | 7 | 11 | 8 |
| Died during interventiona | 1 | 2 | 0 | 0 | 1 | 1 |
| Was discharged from servicea | 2 | 3 | 0 | 0 | 2 | 1 |
| Moved during study perioda | 2 | 3 | 4 | 7 | 5 | 4 |
| Has a completed baseline CRF | 78 | 100 | 60 | 100 | 138 | 100 |
| Has a completed intervention CRF | 78 | 100 | 58 | 97 | 136 | 99 |
| No baseline LAI medication data | 1 | 1 | 3 | 5 | 4 | 3 |
| < 4 months’ eligible LAI medication data during baseline | 5 | 6 | 2 | 3 | 7 | 5 |
| No intervention period LAI medication data | 3 | 4 | 3 | 5 | 6 | 4 |
| < 4 months’ eligible LAI medication data during intervention | 0 | 0 | 2 | 3 | 2 | 1 |
| Primary analysis population (available cases) | 71 (32) | 91 | 51 (29) | 85 | 122 | 88 |
Participant baseline characteristics
During the intervention period, 35 intervention group teams with 75 patients and 30 control group teams with 55 patients provided primary outcome data. Of those, 32 teams with 71 patients from the intervention group and 29 teams with 51 patients from the control group had sufficient data on adherence during the baseline and intervention periods and were included in the primary analysis. Sociodemographic baseline characteristics are described in Table 4 and clinical characteristics of the baseline group are described in Table 5.
| Sociodemographic characteristics | Missing data | Total (N = 141) | Intervention group (N = 78) | Control group (N = 63) | |||
|---|---|---|---|---|---|---|---|
| Mean or n | SD or % | Mean or n | SD or % | Mean or n | SD or % | ||
| Demographics | |||||||
| Age (years) | 0 | 43.7 | 9.8 | 44.4 | 9.6 | 42.7 | 10.2 |
| Male | 0 | 105 | 74% | 59 | 76% | 46 | 73% |
| Years of education | 29 | 11.0 | 1.6 | 10.9 | 1.7 | 11.2 | 1.5 |
| Ethnicity | 3 | ||||||
| White | 83 | 60% | 49 | 63% | 34 | 57% | |
| Black | 31 | 22% | 17 | 22% | 14 | 23% | |
| Asian | 9 | 7% | 5 | 6% | 4 | 7% | |
| Mixed and other | 15 | 11% | 7 | 9% | 8 | 13% | |
| Living situation | |||||||
| Married/cohabiting | 3 | 18 | 13% | 8 | 10% | 10 | 16% |
| Independent accommodation | 4 | 102 | 74% | 53 | 68% | 49 | 83% |
| Living alone | 20 | 75 | 62% | 41 | 62% | 34 | 62% |
| Paid employment (any) | 3 | 4 | 3% | 3 | 4% | 1 | 2% |
| Receiving benefits | 7 | 134 | 99% | 76 | 99% | 58 | 100% |
| Clinical characteristics and history | Missing data | Total (N = 141) | Incentives (N = 78) | Control (N = 63) | |||
|---|---|---|---|---|---|---|---|
| Mean or n | SD or % | Mean or n | SD or % | Mean or n | SD or % | ||
| Clinical diagnosis (according to International Classification of Diseases, Tenth Edition51) | 0 | ||||||
| Schizophrenia (F20.0–F20.9) | 113 | 80% | 61 | 78% | 52 | 82% | |
| Schizoaffective disorders (F25.0–F25.9) | 17 | 12% | 9 | 12% | 8 | 12% | |
| Bipolar disorder (F30.0) | 7 | 5% | 6 | 8% | 1 | 2% | |
| Other psychosis (F33.3) | 3 | 2% | 2 | 2% | 1 | 2% | |
| Other diagnosis (F60.0–F69.0) | 1 | < 1% | 0 | 0% | 1 | 2% | |
| Clinical history | |||||||
| Duration of illness (years) | 14 | 17.8 | 8.5 | 18.2 | 8.6 | 17.3 | 8.5 |
| Number of psychiatric hospitalisations in the last 2 years | 4 | 0.8 | 2.2 | 0.9 | 2.7 | 0.6 | 1.4 |
| One or more hospital admissions in past year | 3 | 32 | 23% | 20 | 26% | 12 | 20% |
| Recreational drug use during baseline | 5 | 104 | 76% | 57 | 74% | 47 | 80% |
| Criminal convictions during baseline | 4 | 2 | 1% | 1 | 1% | 1 | 2% |
| Imprisonment during baseline | 3 | 4 | 3% | 1 | 1% | 3 | 5% |
| CTO at time of randomisation | 4 | 7 | 5% | 3 | 4% | 4 | 7% |
Primary outcome
Primary outcome result
The unadjusted difference in adherence, using data from all 131 patients with primary outcome data was 14% (85% in the intervention group vs. 71% in the control group). Adherence to medication was significantly higher in the intervention group than in the control group [adjusted difference in means (β) 11.5%, 95% CI 3.9% to 19.0%; p = 0.003], representing on average 11.5% greater adherence in the incentives group after adjustment for covariates. See Table 6 for the primary outcome results.
| All patients | Intervention | Control | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (%) | SD (%) | Minimum (%) | 25th centile (%) | Median (%) | 75th centile (%) | Maximum (%) | n | Mean (%) | SD (%) | Minimum (%) | 25th centile (%) | Median (%) | 75th centile (%) | Maximum (%) | |
| Baseline | 72 | 69 | 16 | 32 | 58 | 68 | 81 | 100 | 55 | 67 | 16 | 12 | 56 | 67 | 78 | 99 |
| Intervention | 75 | 85 | 15 | 38 | 79 | 91 | 97 | 100 | 56 | 71 | 22 | 5 | 63 | 75 | 85 | 100 |
Table 7 shows only those patients with at least 4 months’ LAI medication data at baseline and follow-up. Table 8 shows the distribution of cluster size for clusters in which at least one patient had data at baseline and at the intervention period and Table 9 shows the summary statistics for LAI medication data cohort.
| Assessment period | Intervention | Control | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (%) | SD (%) | Minimum (%) | 25th centile (%) | Median (%) | 75th centile (%) | Maximum (%) | n | Mean (%) | SD (%) | Minimum (%) | 25th centile (%) | Median (%) | 75th centile (%) | Maximum (%) | |
| Baseline | 71 | 69 | 16 | 32 | 58 | 69 | 81 | 100 | 52 | 67 | 16 | 12 | 55 | 67 | 78 | 96 |
| Intervention | 71 | 85 | 16 | 38 | 79 | 91 | 97 | 100 | 52 | 72 | 21 | 5 | 67 | 75 | 84 | 100 |
| Cluster size | Intervention (N = 32) | Control (N = 30) | Total (N = 62) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| 1 | 13 | 41 | 17 | 56 | 30 | 48 |
| 2 | 11 | 34 | 6 | 20 | 17 | 28 |
| 3 | 3 | 9 | 5 | 17 | 8 | 13 |
| 4 | 1 | 3 | 2 | 7 | 3 | 5 |
| 5 | 2 | 6 | 0 | 0 | 2 | 4 |
| 6 | 1 | 3 | 0 | 0 | 1 | 2 |
| 7 | 1 | 3 | 0 | 0 | 1 | 2 |
| Cluster size | Intervention | Control | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Range | n | Mean | SD | Range | |
| Teams | 32 | 2.2 | 1.6 | 1–7 | 30 | 1.7 | 1.0 | 1– 4 |
Sensitivity analyses
The analysis excluding those with incorrect diagnoses or > 75% adherence in the 4 months prior to screening (adjusted difference in means 10.7%, 95% CI 3.0% to 18.5%; p = 0.006), and the sensitivity analysis without adjustment for baseline adherence (adjusted difference in means 11.6%, 95% CI 3.7% to 19·5%; p = 0.004) gave similar results. Excluding all patients with > 75% adherence in the previous 12 months gave an effect estimate of 15.7% (95% CI 5.7% to 25.6%; p = 0.002), and restricting the analysis to only patients with a diagnosis of schizophrenia gave an estimate of 10.7% (95% CI 3.0% to 18.5%; p = 0.006).
Secondary outcomes
The effect of financial incentives on the secondary outcomes is shown in Table 10. The percentage of patients achieving at least 95% adherence during the intervention period was significantly higher in the intervention than in the control group, adjusted odds ratio (OR) 8.21 (95% CI 2.00 to 33.67; p = 0.003).
| Secondary outcomes | Period | Intervention, N = 78 | Control, N = 60a | Type of effect estimate | Adjusted effect estimatec (intervention vs. control) | p-value | ICCd | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n b | Number of teams analysed | Number (%) or mean (SD) | n b | Number of teams analysed | Number (%) or mean (SD) | ||||||||
| Achieving at least 95% adherence vs. not | Baseline | 71 | 32 | 4 | 6% | 52 | 30 | 1 | 2% | – | – | – | – |
| 12-month study period | 71 | 32 | 20 | 28% | 52 | 30 | 3 | 6% | OR | 8.21 (2.00 to 33.67)e | 0.003 | 0.04 | |
| Slippage | Baseline | 71 | 32 | 46% | 29% | 52 | 30 | 43% | 28% | Difference in means | –19.5% (–29.8% to –9.3%) | < 0.001 | 0.19 |
| 12-month study period | 71 | 32 | 14% | 20% | 52 | 30 | 35% | 31% | – | – | – | – | |
| Clinical improvement (on CGI scale yes vs. no) | Baseline | – | – | – | – | – | – | – | – | – | – | – | – |
| 12-month study period | 57 | 29 | 33 | 58% | 44 | 26 | 18 | 41% | OR | 2.73 (0.64 to 11.59)e | 0.174 | 0.44 | |
| Subjective quality of life (DIALOG) | Baseline | 38 | 29 | 4.3 | 0.9 | 22 | 21 | 5.0 | 1.2 | – | – | – | – |
| 12-month study period | 38 | 29 | 5.4 | 0.8 | 22 | 21 | 4.8 | 1.1 | Difference in means | 0.71 (0.26 to 1.15)f | 0.002 | < 0.0001 | |
| Medication satisfaction | Baseline | 38 | 21 | 5.0 | 1.3 | 22 | 16 | 5.1 | 1.3 | – | – | – | – |
| 12-month study period | 38 | 21 | 5.7 | 1.5 | 22 | 16 | 5.2 | 1.4 | Difference in means | 0.26 (–0.74 to 1.27)g | 0.610 | 0.51 | |
| At least one psychiatric hospital admission | Baseline | 78 | 37 | 18 | 23% | 59 | 34 | 16 | 27% | – | – | – | – |
| 12-month study period | 78 | 37 | 15 | 19% | 59 | 34 | 14 | 24% | OR | 0.71 (0.29 to 1.73)e | 0.446 | < 0.0001 | |
| At least one suicide attempt vs. none | Baseline | 77 | – | 9 | 12% | 58 | – | 7 | 12% | – | – | – | – |
| 12-month study period | 77 | – | 8 | 10% | 58 | – | 4 | 7% | – | – | – | – | |
| At least one violent incident vs. none | Baseline | 77 | – | 15 | 19% | 59 | – | 8 | 14% | – | – | – | – |
| 12-month study period | 77 | – | 10 | 13% | 59 | – | 7 | 12% | – | – | – | – | |
| At least one police arrest vs. none | Baseline | 76 | – | 13 | 17% | 58 | – | 12 | 21% | – | – | – | – |
| 12-month study period | 76 | – | 10 | 13% | 58 | – | 10 | 17% | – | – | – | – | |
Patients in the intervention group were found to rate their quality of life significantly higher (β = 0.71, 95% CI 0.26 to 1.15; p = 0·002), and also showed significantly less time slippage (β = –19.5%, 95% CI –29.8% to –9.3%; p < 0.001). Differences on clinician-rated clinical improvement were not statistically significant, although the odds of being rated as improved were 2.73 times greater in the intervention group (95% CI 0.64 to 11.59; p = 0.174) than in the control group. There were no differences in medication satisfaction between the groups. Number of hospitalisations and adverse events were low in both groups and did not show substantial differences.
Six-month follow-up outcomes
Fewer patients were assessed at the 6-month follow-up (1–6 months after the end of intervention), with adherence calculated on 106 of the 132 patients available at the 6-month follow-up. Alongside the six patients lost to follow-up, adherence data were missing for a number of reasons including refusal, patients being taken off LAI medication and switched to oral medication, poor record keeping by clinicians or insufficient adherence data (< 4 months’ worth). While these data were therefore missing from the analysis, they could be accounted for and thus were not missing at random.
Adherence in the intervention group was 71% and 78% in the control group. The difference in adherence between both groups was not statistically significant [adjusted difference in means (β) = –7.4%, 95% CI –17.0% to 2.1%; p = 0.175]. The percentage of patients achieving at least 95% adherence during the follow-up was not significantly different between groups (adjusted OR = 0.42, 95% CI 0.11 to 1.61; p = 0.205), neither was the time slippage of taking LAI medication [adjusted difference in means (β) = 6.1%, 95% CI –5.3% to 17.5%; p = 0.293]. The number of hospitalisations and adverse events at the 6-month follow-up were low in both groups and did not show substantial differences.
The effect of financial incentives on the primary and secondary outcomes at the 6-month follow-up can be found in Table 11.
| Primary and secondary outcomes | Period | Intervention group | Control group | Type of effect estimate | Adjusted effect estimate (intervention vs. control) | p-value | ICC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of teams analysed | n,a 74b/75c | Number (%) | Number of teams analysed | n,a 58b/56c | Number (%) | ||||||||
| Primary outcome | |||||||||||||
| Adherence to depot medication | Baseline | 30 | 58 | 69% | 16% | 29 | 41 | 68% | 17% | ||||
| 6-month follow-up | 30 | 58 | 70% | 24% | 29 | 41 | 77% | 19% | Difference in meansd | –7.4% (–17.0% to 2.1%) | 0.127 | 0.175 | |
| Secondary outcomes | |||||||||||||
| Achieving at least 95% adherence vs. not | Baseline | 30 | 58 | 3 | 5% | 29 | 41 | 1 | 2% | ||||
| 6-month follow-up | 30 | 66 | 5 | 8% | 29 | 54 | 9 | 17% | ORb | 0.42 (0.11 to 1.61) | 0.205 | < 0.001 | |
| Slippage | Baseline | 29 | 55 | 48 | 29% | 29 | 40 | 43 | 28% | ||||
| 6-month follow-up | 29 | 55 | 27 | 29% | 29 | 40 | 20 | 25% | Difference in meansd | 6.1% (–5.3% to 17.5%) | 0.293 | 0.23 | |
| At least one psychiatric hospital admission | Baseline | 37 | 74 | 14 | 19% | 33 | 58 | 10 | 17% | ||||
| 6-month follow-up | 37 | 74 | 15 | 20% | 33 | 58 | 8 | 14% | |||||
| At least one suicide attempt vs. none | Baseline | 37 | 74 | 9 | 12% | 33 | 58 | 7 | 12% | ||||
| 6-month follow-up | 37 | 73 | 3 | 4% | 33 | 58 | 3 | 5% | |||||
| At least one violent incident vs. none | Baseline | 37 | 74 | 15 | 20% | 33 | 58 | 10 | 17% | ||||
| 6-month follow-up | 37 | 73 | 4 | 6% | 33 | 58 | 3 | 5% | |||||
| At least one police arrest vs. none | Baseline | 37 | 74 | 13 | 18% | 33 | 58 | 9 | 16% | ||||
| 6-month follow-up | 37 | 73 | 6 | 8% | 33 | 58 | 3 | 5% | |||||
Sensitivity analyses
Adherence in the intervention group was somewhat lower than the control group but failed to reach statistical significance. Sensitivity analyses led to the same conclusion.
During the 6-month follow-up there was evidence of patients refusing medication. For the purposes of analysis, these patients were given an adherence level of 0%. When including patients who refused in the analysis, data were available for 112 patients; adherence in the intervention group was not significantly lower than the control group (β = –4.9%, 95% CI –14.3% to 4.6%; p = 0.312). When excluding refusers, data were available for 108 patients. Again, there was no significant difference in adherence between both groups (β = –6.2%, 95% CI –13.1% to 0.1%; p = 0.078).
A number of patients had less than the required 4 months’ worth of adherence data. Adherence data for all patients, regardless of whether or not they had at least 4 months’ worth and including patients refusing medication was available for 127 patients; adherence in the intervention group was not significantly lower than in the control group (β = –5.4%; 95% CI –15.0% to 4.2%; p = 0.274). In other cases, adherence data were not available as some patients had been taken off LAI medication. In these instances patients were assumed to achieve 100% adherence. Adherence data for all patients with any adherence data, and including those assumed to have 100% adherence, were available for 130 patients. No significant differences in adherence was found between groups (β = –5.0%; 95% CI –14.7% to 4.7%; p = 0.316).
In some cases, adherence data were missing over the course of the follow-up which could not be accounted for. Single imputation was performed with last observation carried forward. As a result, data were available for 134 patients; adherence in the intervention group was not significantly lower than in the control group (β = –3.6%, 95% CI –13.3% to 6.2%; p = 0.475).
Twenty-four-month follow-up outcomes
Primary outcome data were available for 116 patients. Adherence in the intervention group was 68%, compared with 74% in the control group. Medication adherence between the two groups at 24-month follow-up was not significantly different (β = –5.7%, 95% CI –13.1% to 1.7%; p = 0.130). A graphical representation of adherence in the intervention and the control groups throughout the intervention and follow-up periods is shown in Figure 4.
FIGURE 4.
Adherence by treatment group throughout the intervention and follow-up periods.
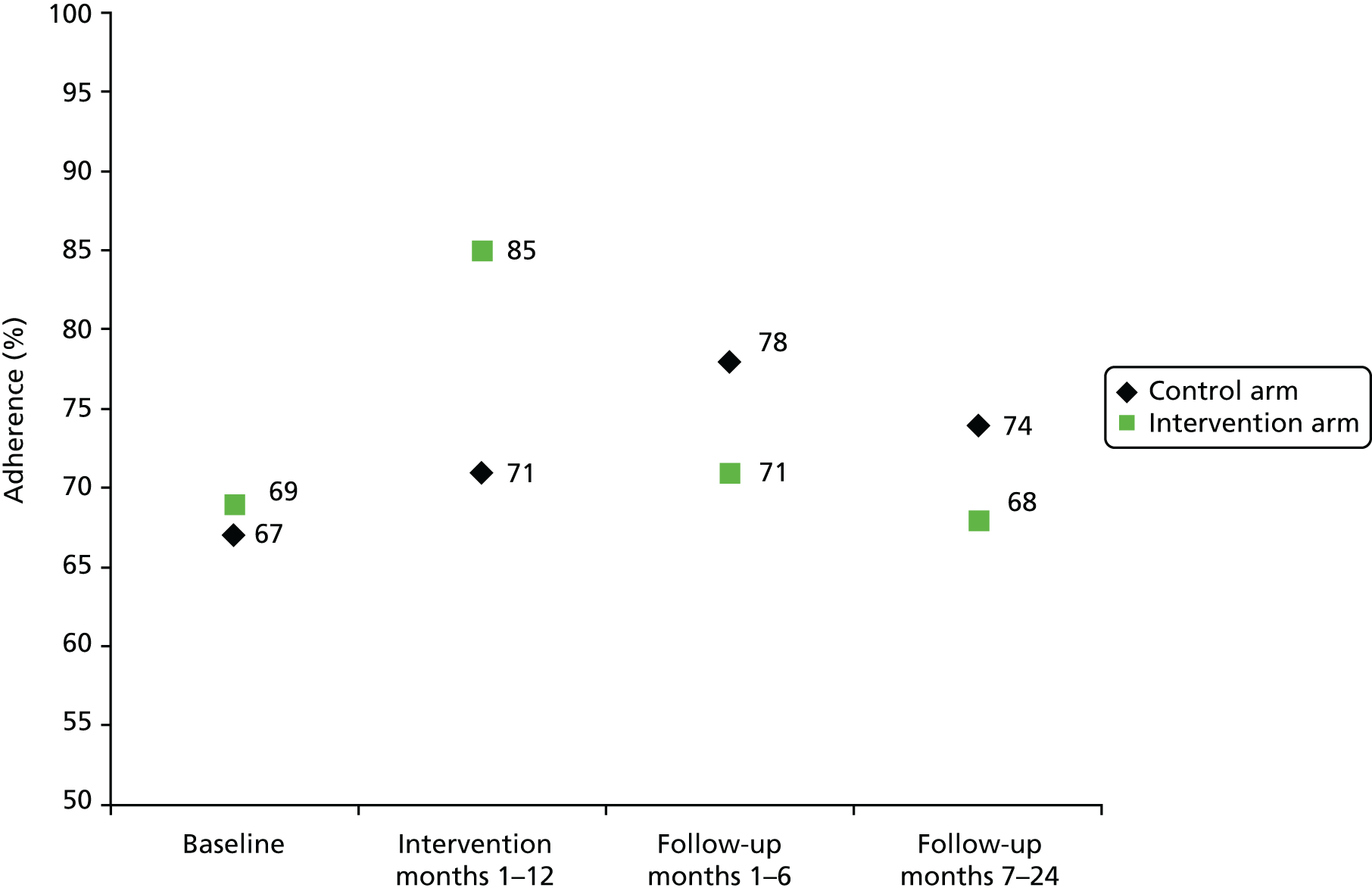
There were a higher number of hospital admissions in the intervention group than in the control group, but no differences in the number of adverse events.
Sensitivity analyses
At the 24-month follow-up, there were cases of patients being were discharged back to primary care services (i.e. under the care of GPs) which is normal practice for patients no longer in need of intensive input from secondary services. Adherence in these cases was therefore assumed to be 100%. Data were available for 119 patients; adherence in the intervention group was not significantly different compared with the control group (β = –6.2%, 95% CI –13.6% to 1.1%; p = 0.097). There was also evidence of patients refusing medication and being switched to alternative medication as a result. Adherence for refusers was assumed to be 0%. Data were available for 124 patients; adherence in the intervention group was not significantly lower than in the control group (β = –6.4%, 95% CI –15.0% to 2.1%; p = 0.14).
The descriptive statistics of adherence between the two groups at the 24-month follow-up can be found in Table 12. The results of the main analysis and sensitivity analysis for the primary outcome are shown in Table 13. Other secondary outcomes (arrests, incidents of violence, suicide attempts and hospitalisations) are shown in Tables 14 and 15.
| Incentives | Control | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (%) | SD (%) | Minimum (%) | 25th centile (%) | Median (%) | 75th centile (%) | Maximum (%) | n | Mean (%) | SD (%) | Minimum (%) | 25th centile (%) | Median (%) | 75th centile (%) | Maximum (%) | |
| Adherence | 66 | 68 | 21 | 27 | 51 | 71 | 87 | 100 | 50 | 74 | 19 | 15 | 66 | 77 | 89 | 100 |
| Analysis population | n | Difference in mean adherence (%) | 95% CI (%) | p-value |
|---|---|---|---|---|
| Main analysis: all participants with ≥ 4 months’ depot dataa | 116b | –5.7 | –13.1 to 1.7 | 0.130 |
| All participants as above, setting adherence to 100% for those discharged to GPa | 119 | –6.2 | –13.6 to 1.1 | 0.097 |
| All participants as above but setting adherence to 0% for refusersc | 124 | –6.4 | –15.0 – 2.1 | 0.142 |
| Untoward incidents | Intervention (N = 75) | Control (N = 58) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Arrested | 14 | 19 | 10 | 17 |
| Violent act | 11 | 15 | 7 | 12 |
| Suicide attempt | 5 | 7 | 3 | 5 |
| Intervention (N = 77) | Control (N = 60) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Psychiatric admission | 24 | 31 | 10 | 17 |
Serious adverse events
Known serious adverse events are as follows:
-
One patient was unhappy with being allocated to the control condition and refused all LAI medication as a result. This patient was subsequently hospitalised because of non-compliance with medication.
-
One patient outside of the trial refused all LAI medication after knowing that another participant (in the trial and allocated to the intervention group) was receiving financial incentives for their medication. This patient was subsequently placed on a Community Treatment Order and was reported to have died a few weeks after this incident. However, it was decided that this death was unrelated to the FIAT trial itself.
Cost-effectiveness
A summary of the resources used by patients in each group can be found in Tables 16 and 17. There were few notable differences in resource use between groups over the intervention period. Patients in the intervention group experienced fewer days in a psychiatric inpatient bed and more days in a general hospital inpatient bed than patients in the control group. Of the patients with admissions to psychiatric wards, patients in the intervention group had shorter stays on average than patients in the control group (27 days for 16 participants vs. 30.4 days for 14 participants), a difference of 3.4 days (95% CI –30.7 to 23.8 days). However, none of these differences was statistically significant.
| Resource item | Intervention (n = 78) | Control (n = 59) | Raw difference (95% CI) | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Mental health inpatient service use | |||||
| Mental health inpatient admissions | 0.37 | 0.10 | 0.4 | 0.11 | –0.03 (–0.33 to 0.26) |
| Mental health outpatient attendances (including A&E and day services) | 0.29 | 0.12 | 0.11 | 0.05 | 0.18 (–0.11 to 0.47) |
| Mental health inpatient bed-days | 8.49 | 2.63 | 9.98 | 4.14 | –1.49 (–10.76 to 7.77) |
| General hospital inpatient service use | |||||
| General hospital inpatient admissions | 0.04 | 0.02 | 0.02 | 0.02 | 0.02 (–0.04 to 0.08) |
| General hospital outpatient attendances (including A&E) | 0.47 | 0.29 | 0.18 | 0.06 | 0.29 (–0.4 to 0.98) |
| General hospital inpatient bed-days | 0.47 | 0.42 | 0.02 | 0.02 | 0.46 (–0.51 to 1.42) |
| Community health services | |||||
| Service settings, mental health professionals | |||||
| Mental health nurse/community psychiatric nurse | 16.23 | 1.58 | 11.33 | 1.64 | 5.21 (0.96 to 9.47)a |
| Occupational therapist | 0.3 | 0.15 | 0.15 | 0.11 | –0.23 (–1.2 to 0.74) |
| Psychiatrist | 1.58 | 0.26 | 0.61 | 0.19 | –0.2 (–1.05 to 0.64) |
| Social worker | 1.3 | 0.84 | 2.49 | 0.86 | 0.93 (–1.02 to 2.89) |
| Mental health support worker | 1.84 | 0.91 | 1.69 | 0.99 | 0.93 (–1.34 to 3.2) |
| Psychologist | 0.23 | 0.15 | 0.02 | 0.02 | –0.12 (–0.8 to 0.57) |
| Family support worker | 0 | 0 | 0.04 | 0.03 | 0 |
| Vocational worker | 0 | 0 | 0 | 0 | –0.04 (–0.1 to 0.03) |
| Substance abuse worker | 0.49 | 0.20 | 0 | 0 | 0.43 (–0.04 to 0.91) |
| All contacts in service settings | 21.96 | 2.46 | 16.16 | 2.36 | 7.03 (0.41 to 13.65)a |
| Community settings, mental health professionals | |||||
| Mental health nurse/community psychiatric nurse | 11.01 | 1.46 | 0.15 | 0.11 | –0.32 (–4.68 to 4.04) |
| Occupational therapist | 0.5 | 0.24 | 0.61 | 0.19 | 0.35 (–0.24 to 0.95) |
| Psychiatrist | 0.58 | 0.16 | 2.49 | 0.86 | –0.03 (–0.51 to 0.46) |
| Social worker | 2.05 | 0.88 | 1.69 | 0.99 | –0.44 (–2.94 to 2.06) |
| Mental health support worker | 3.58 | 1.2 | 0.02 | 0.02 | 1.89 (–1.34 to 5.12) |
| Psychologist | 0.01 | 0.01 | 0.04 | 0.03 | 0 (–0.05 to 0.04) |
| Family support worker | 0 | 0 | 0 | 0 | –0.04 (–0.08 to 0.01) |
| Vocational worker | 0 | 0 | 0 | 0 | 0 |
| Substance abuse worker | 0.11 | 0.08 | 16.16 | 2.36 | 0.11 (–0.07 to 0.28) |
| All contacts in community settings | 17.85 | 2.72 | 11.33 | 1.64 | 1.69 (–5.69 to 9.07) |
| Contacts with CMHT and AOT in any setting | |||||
| CMHT contactsb | 35.11 | 2.86 | 26.9 | 4.10 | 8.21 (–1.42 to 17.84) |
| AOT contactsc | 50.3 | 6.74 | 40.53 | 5.85 | 9.77 (–8.68 to 28.22) |
| Primary care | |||||
| GP (home) | 0.03 | 0.02 | 0 | 0 | 0.03 (–0.02 to 0.07) |
| GP (surgery) | 2.14 | 2.03 | 0.4 | 0.20 | 1.74 (–2.93 to 6.4) |
| Counsellor (service setting) | 0 | 0 | 0.07 | 0.05 | –0.07 (–0.16 to 0.01) |
| Counsellor (community setting) | 0 | 0 | 0 | 0 | 0 |
| Resource item | Intervention (n = 78) | Control (n = 59) | Raw difference (95% CI) | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Mental health nurse/community psychiatric nurse | 1.86 | 0.4 | 3.23 | 0.52 | –1.36 (–2.65 to 0.08)a |
| Occupational therapist | 0.07 | 0.07 | 0.13 | 0.13 | –0.06 (–0.33 to 0.21) |
| Psychiatrist | 0.22 | 0.08 | 0.13 | 0.04 | 0.09 (–0.11 to 0.29) |
| Social worker | 0.22 | 0.15 | 0.73 | 0.44 | –0.51 (–1.34 to 0.32) |
| Mental health support worker | 0.69 | 0.47 | 0.11 | 0.06 | 0.58 (–0.51 to 1.67) |
| Psychologist | 0.08 | 0.08 | 0.07 | 0.05 | 0.01 (–0.2 to 0.21) |
| Family support worker | 0 | 0 | 0 | 0 | 0 |
| Vocational worker | 0 | 0 | 0 | 0 | 0 |
| Substance abuse worker | 0.03 | 0.03 | 0 | 0 | 0.03 (–0.04 to 0.09) |
The majority of patients were treated by CMHTs over the study period (40 control and 54 intervention participants were seen by 26 and 24 teams, respectively); a smaller number were under the care of AOTs (9 and 13 teams seeing 17 control and 20 intervention participants, respectively). Intervention patients had significantly more contacts than control patients with community mental health nurses in service settings (5.2 more contacts, 95% CI 0.96 to 9.5 more contacts; p = 0.02). This finding appears consistent with the significantly greater number of LAI medications received by intervention participants over the period [20.2 intervention vs. 14.9 control, a difference of 5.2 (95% CI 2.8 to 7.6; p < 0.001)]. DNAs associated with community mental health nurses in any setting were also fewer in the intervention group [1.9 intervention vs. 3.2 control (difference: –1.4, 95% CI –2.6 to –0.1; p = 0.04)].
Most patients received relatively low-cost medication throughout the intervention period, although a substantial minority in both groups received risperidone (Risperdal Consta®, Janssen) LAI medication. The proportions within drug category did not generally differ substantially by group; however, somewhat larger proportions of intervention than control patients received flupenthixol (Depixol, Lundbeck) LAI medication on a 2-week treatment cycle in the intervention periods (21.2% vs. 12.3%, respectively). A larger proportion of control patients than intervention patients received LAI medication on a 4-week treatment cycle [18/56 (32%) vs. 13/75 (17%), respectively]. A summary of the LAI medication, treatment cycles and costs of each medication can be found in Table 18.
| Patients’ LAI medication treatment cycle | British National Formulary chemical name | Intervention group (N = 75) | Control group (N = 56) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | Average cost of an LAI medication per cycle (£) | n | % | Average cost of an LAI medication per cycle (£) | n | % | Average cost of an LAI medication per cycle (£) | ||
| 1 | Flupentixol decanoate | 2 | 2.4 | 5.12 | 2 | 3.1 | 2.58 | 4 | 2.7 | 3.85 |
| 1 | Pipotiazine palmitate | 1 | 1.2 | 26.65 | 0 | 0 | – | 1 | 0.7 | 26.55 |
| 1 | Zuclopenthixol decanoate | 1 | 1.2 | 5.78 | 1 | 1.5 | 3.77 | 2 | 1.3 | 4.77 |
| 2 | Flupentixol decanoate | 18 | 21.2 | 8.37 | 8 | 12.3 | 4.69 | 26 | 17.3 | 7.23 |
| 2 | Fluphenazine decanoate | 5 | 5.9 | 31.00 | 2 | 3.1 | 3.31 | 7 | 4.7 | 23.09 |
| 2 | Haloperidol decanoate | 1 | 1.2 | 8.88 | 1 | 1.5 | 5.59 | 2 | 1.3 | 7.24 |
| 2 | Missing medication name | 1 | 1.2 | 58.98 | 1 | 1.5 | 58.98 | 2 | 1.3 | 58.98 |
| 2 | Paliperidone | 0 | 0 | – | 2 | 3.1 | 307.94 | 2 | 1.3 | 307.94 |
| 2 | Pipotiazine palmitate | 2 | 2.4 | 34.79 | 3 | 4.6 | 39.65 | 5 | 3.3 | 37.71 |
| 2 | Risperidone | 22 | 25.9 | 112.54 | 12 | 18.5 | 134.55 | 34 | 22.7 | 120.31 |
| 2 | Zuclopenthixol acetate | 1 | 1.2 | 23.93 | 0 | 0 | – | 1 | 0.7 | 23.93 |
| 2 | Zuclopenthixol decanoate | 8 | 9.4 | 5.52 | 9 | 13.8 | 4.11 | 17 | 11.3 | 4.77 |
| 3 | Flupentixol decanoate | 3 | 3.5 | 5.66 | 0 | 0 | – | 3 | 2.0 | 5.66 |
| 3 | Missing medication name | 2 | 2.3 | 20.48 | 0 | 0 | – | 2 | 1.3 | 20.48 |
| 3 | Pipotiazine palmitate | 2 | 2.4 | 26.65 | 3 | 4.6 | 42.94 | 5 | 3.3 | 36.42 |
| 3 | Zuclopenthixol decanoate | 1 | 1.2 | 5.78 | 2 | 3.1 | 4.77 | 3 | 2.0 | 5.11 |
| 4 | Flupentixol decanoate | 4 | 4.7 | 9.76 | 4 | 6.2 | 6.05 | 8 | 5.3 | 7.91 |
| 4 | Fluphenazine decanoate | 2 | 2.4 | 9.20 | 3 | 4.5 | 13.61 | 5 | 3.3 | 11.84 |
| 4 | Haloperidol | 0 | 0 | – | 1 | 1.5 | 3.18 | 1 | 0.7 | 3.18 |
| 4 | Haloperidol decanoate | 0 | 0 | – | 1 | 1.5 | 17.76 | 1 | 0.7 | 17.76 |
| 4 | Paliperidone | 0 | 0 | – | 1 | 1.5 | 367.85 | 1 | 0.7 | 367.85 |
| 4 | Pipotiazine palmitate | 7 | 8.2 | 32.91 | 5 | 7.7 | 36.72 | 12 | 8.0 | 34.50 |
| 4 | Zuclopenthixol decanoate | 2 | 2.3 | 2.17 | 4 | 6.2 | 4.95 | 6 | 4.0 | 4.02 |
Community mental health service costs were significantly higher in the intervention than in the control group. The median number of financial incentives given over the study period was 21 (mean 20.2, interquartile range 8); the average cost of the incentive itself was £303 (SE £12). Total costs of intervention patients, including the cost of providing the financial incentive, were somewhat higher than those of control patients (£9350 vs. £8651), a difference of £699 (95% CI –£3535 to £4932). Community mental health services made up almost half (48%), hospital costs more than one-third (37%) and medication costs 12% of the total costs across the follow-up sample for which data were available for all cost categories. The average cost of the incentive itself made up only a small proportion (2%) of total intervention group costs. A summary of total costs between groups including costs from the sensitivity analyses can be found in Table 19.
| Cost category | Intervention | Control | Raw mean difference (95% CI) | ||
|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | ||
| Baseline | (N = 78) | (N = 60) | |||
| Total mental health hospital costs | 78 | 3342 (1173) | 60 | 4048 (1686) | –706 (–4648 to 3236) |
| Total general hospital costs | 78 | 262 (207) | 60 | 252 (219) | 10 (–592 to 611) |
| Total primary care costs | 75 | 47 (36) | 57 | 8 (3) | 39 (–43 to 121) |
| Total community mental health care costs | 78 | 5041 (443) | 59 | 3644 (375) | 1397 (201 to 2594)a |
| Total LAI medication costs | 72 | 714 (108) | 55 | 861 (174) | –147 (–535 to 241) |
| Total oral medication costs | 76 | 479 (149) | 56 | 313 (129) | 166 (–242 to 574) |
| Total costsb | 72 | 8058 (1024) | 54 | 9274 (1993) | –1217 (–5355 to 2921) |
| Total costs, including cost of DNA contacts | 72 | 10,088 (1059) | 54 | 10,511 (2004) | –423 (–4622 to 3777) |
| End of intervention | (N = 78) | (N = 59) | |||
| Total mental health hospital costs | 78 | 3407 (1101) | 59 | 5105 (1787) | –1698 (–5661 to 2266) |
| Total general hospital costs | 76 | 254 (181) | 57 | 27 (13) | 227 (–188 to 642) |
| Total primary care costs | 74 | 48 (43) | 57 | 14 (6) | 34 (–63 to 130) |
| Total community mental health care costs | 74 | 4964 (353) | 57 | 3859 (426) | 1105 (17 to 2192)a |
| Total LAI medication costs | 75 | 787 (132) | 56 | 759 (188) | 28 (–413 to 470) |
| Total oral medication costs | 76 | 364 (76) | 57 | 216 (68) | 149 (–61 to 358) |
| Total costs including financial incentive costsb | 71 | 9350 (1189) | 54 | 8651 (1890) | 699 (–3535 to 4932) |
| Financial incentives intervention costs | 75 | 303 (12) | 56 | 0 | 303 (277 to 329)c |
| Total costs excluding financial incentive costs | 71 | 9043 (1189) | 54 | 8651 (1890) | 392 (–3842 to 4625) |
| Total costs excluding oral medications and financial incentives | 71 | 8721 (1191) | 54 | 8476 (1855) | 245 (–3944 to 4433) |
| Total costs excluding medications and financial incentives | 74 | 8680 (1324) | 57 | 9050 (2030) | –370 (–4987 to 4248) |
| Sensitivity analyses | |||||
| Total including DNA contact costs | 71 | 10,162 (1221) | 54 | 9610 (1881) | 552 (–3715 to 4819) |
| Total, varying unit cost of contacts with CMHT/AOT | |||||
| at 25% | 71 | 5991 (1098) | 54 | 6118 (1769) | –127 (–4069 to 3814) |
| at 50% | 71 | 7381 (1131) | 54 | 7282 (1801) | 99 (–3931 to 4129) |
| at 150% | 71 | 12,943 (1337) | 54 | 11,938 (1982) | 1005 (–3567 to 5576) |
From the multilevel multivariate regressions, there was an adherence difference of 12.2% (95% CI 4.6% to 19.8%). The proportion of participants achieving adherence of at least 95% over the treatment period was 26.5% (95% CI 11.7% to 41.2%) higher in the intervention group than in the control group. The adjusted cost difference between groups was £598 (95% CI –£4533 to £5730; p = 0.818) on the continuous adherence outcome and £780 (95% CI –£4419 to £5979; p = 0.767) on the binary adherence outcome.
The ICER, or incremental cost for an increase in adherence to LAI medication of 20%, was £982 (95% CI –£8020 to £14,000), and the probability that the incentive treatment was cost-effective on this measure exceeded 97.5% at willingness-to-pay values over £14,000 (the upper confidence limit for the ICER). 69 The incremental cost of achieving good adherence was £2950 and the probability of cost-effectiveness was over 97.5% at willingness-to-pay values for this outcome over £27,800.
In terms of data available for the analyses of the relationship of costs and the outcomes of clinical improvement and subjective quality of life (see Table 19), data were missing in a number of cases (18% of the control group and 19% of the intervention group for clinical improvement; 39% of the control group and 21% of the intervention group for subjective quality of life). Quality-of-life scores indicated slightly higher satisfaction in the intervention group (a difference of 0.698; p = 0.003). The proportion of patients who clinically improved was higher in the intervention group, although this did not reach statistical significance (a difference of 12.5%; p = 0.320). ICERs were not calculated because of the level of missing data, which affected costs to the extent of reversing the direction of difference between groups.
The results of the cost-effectiveness and sensitivity analyses can be found in Table 20 and the cost-effectiveness acceptability curves for both adherence outcomes can be found in Figures 5 and 6.
| Costs and outcomes | Intervention (n = 68) | Control (n = 49) | Difference (95% CI) or ICERa | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Costs prior to baseline (£) (raw) | 7780 | 976 | 9755 | 2184 | –1974 (–6292 to 2344) |
| Costs over intervention period (£) (raw) | 9212 | 1234 | 9309 | 2061 | –97 (–4600 to 4406) |
| Achieving 20% adherence | |||||
| Proportion adherent (raw) | 85.8 | 14.3 | 71.6 | 21.7 | 14.2 (7.6 to 20.8)b |
| Proportion adherent (adjusted) | 85.6 | 2.9 | 73.4 | 3 | 12.2 (4.6 to 19.8)c |
| Costs over study period (£) (adjusted) | 9681 | 1740 | 9083 | 1931 | 598 (–4533 to 5730) |
| ICER (£) (20% increase adherence) | 982 (–8020 to 14 000)d | ||||
| Achieving at least 95% adherence | |||||
| Adherence ≥ 95% (raw) | 29.4 | 45.9 | 6.1 | 24.2 | 23.3 (9 to 37.5)c |
| Proportion adherent (adjusted) | 31.3 | 10.3 | 4.9 | 10.9 | 26.5 (11.7 to 41.2)b |
| Costs over study period (£) (adjusted) | 9724 | 1766 | 8944 | 1954 | 780 (–4419 to 5979) |
| ICER (£) (achievement ‘good’ adherence) | 2950 (–19,400 to 27,800)d | ||||
| Sensitivity | |||||
| Including costs of DNAs | |||||
| Costs over study period (£) (raw) | 10,290 | 1288 | 10,410 | 2052 | –120 (–4694 to 4454) |
| Proportion adherent (adjusted) | 85.6 | 2.9 | 73.4 | 2.9 | 12.2 (4.7 to 19.8)c |
| Costs over study period (£) (adjusted) | 10,486 | 1755 | 9755 | 2184 | 432 (–4747 to 5611) |
| ICER (£) (20% increase adherence) | 9309 | ||||
| Unit costs: at 25% of estimate | |||||
| Costs over study period (£) (raw) | 6271 | 1169 | 71.6 | 21.7 | –559 (–4803 to 3686) |
| Proportion adherent (adjusted) | 85.7 | 2.9 | 73.4 | 3 | 12.4 (4.8 to 20)c |
| Costs over study period (£) (adjusted) | 6480 | 1577 | 9083 | 1931 | 90 (–4593 to 4774) |
| ICER (£) (20% increase adherence) | 706 (–8300 to 13,540) | ||||
| Unit costs: at 50% of estimate | |||||
| Costs over study period (£) (raw) | 8023 | 1968 | 7611 | 1202 | –412 (–4748 to 3923) |
| Proportion adherent (adjusted) | 73.3 | 2.9 | 85.7 | 2.8 | 12.3 (4.8 to 19.9)c |
| Costs over study period (£) (adjusted) | 7369 | 1833 | 7663 | 1635 | 294 (–4555 to 5144) |
| ICER (£) (20% increase adherence) | 476 (–7900 to 12,120) | ||||
| Unit costs: at 150% of estimate | |||||
| Costs over study period (£) (raw) | 12,797 | 2158 | 12,970 | 1398 | 172 (–4705 to 5050) |
| Proportion adherent (adjusted) | 73.5 | 3 | 85.6 | 2.9 | 12.1 (4.5 to 19.7)c |
| Costs over study period (£) (adjusted) | 11,325 | 2075 | 12,397 | 1874 | 1072 (–4448 to 6592) |
| ICER (£) (20% increase adherence) | 8023 | 1968 | 1770 (–7880 to 16,380) | ||
FIGURE 5.
Cost-effectiveness acceptability curve and willingness-to-pay values to achieve 20% adherence. Source: reproduced from Henderson and colleagues49 © 2015 Henderson et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY-NC 4.0 licence), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. See: http://creativecommons.org/licenses/by/4.0/.

FIGURE 6.
Cost-effectiveness acceptability curve and willingness-to-pay values to achieve at least 95% adherence. Source: reproduced from Henderson and colleagues49 © 2015 Henderson et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY-NC 4.0 licence), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. See: http://creativecommons.org/licenses/by/4.0/.

Six-month follow-up
Resource use over the 6-month follow-up (in cases where data were also available at baseline) is shown in Table 21.
| Resource item | Control (n = 43) | Intervention (n = 60) | Raw difference (95% CI) | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Mental health inpatient service use | |||||
| Mental health inpatient admissions | 0.14 | 0.08 | 0.31 | 0.09 | 0.17 (–0.08 to 0.43) |
| Mental health outpatient attendances (including A&E, day services) | 0.07 | 0.05 | 0.10 | 0.06 | 0.03 (–0.13 to 0.19) |
| Mental health inpatient bed-days | 0.63 | 0.50 | 3.67 | 1.40 | 3.04 (–0.36 to 6.45) |
| General hospital inpatient service use | |||||
| General hospital inpatient admissions | 0.00 | 0.00 | 0.03 | 0.02 | 0.03 (–0.02 to 0.09) |
| General hospital outpatient attendances (including A&E) | 0.02 | 0.02 | 0.18 | 0.08 | 0.16 (–0.03 to 0.34) |
| General hospital inpatient bed-days | 0.00 | 0.00 | 0.13 | 0.12 | 0.13 (–0.14 to 0.4) |
| Community health services | |||||
| Service settings, mental health workers | |||||
| Mental health nurse/community psychiatric nurse | 6.02 | 0.76 | 6.49 | 0.71 | 0.47 (–1.63 to 2.57) |
| Occupational therapist | 0.00 | 0.00 | 0.05 | 0.03 | 0.05 (–0.02 to 0.12) |
| Psychiatrist | 1.12 | 0.29 | 0.98 | 0.15 | –0.13 (–0.74 to 0.47) |
| Social worker | 0.77 | 0.26 | 0.46 | 0.25 | –0.31 (–1.05 to 0.43) |
| Mental health support worker | 1.19 | 0.80 | 0.54 | 0.41 | –0.65 (–2.29 to 1) |
| Psychologist | 0.00 | 0.00 | 0.03 | 0.03 | 0.03 (–0.04 to 0.11) |
| Family support worker | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Vocational worker | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Substance abuse worker | 0.00 | 0.00 | 0.03 | 0.03 | 0.03 (–0.04 to 0.11) |
| All contacts in service settings | 9.09 | 1.27 | 8.59 | 1.04 | –0.50 (–3.74 to 2.73) |
| Community settings, mental health workers | |||||
| Mental health nurse/community psychiatric nurse | 5.95 | 1.25 | 6.33 | 1.07 | 0.37 (–2.9 to 3.65) |
| Occupational therapist | 0.02 | 0.02 | 0.11 | 0.08 | 0.09 (–0.11 to 0.3) |
| Psychiatrist | 0.33 | 0.13 | 0.43 | 0.13 | 0.1 (–0.27 to 0.47) |
| Social worker | 1.95 | 0.85 | 1.43 | 0.58 | –0.53 (–2.5 to 1.44) |
| Mental health support worker | 2.37 | 1.72 | 1.33 | 0.53 | –1.04 (–4.17 to 2.08) |
| Psychologist | 0.00 | 0.00 | 0.03 | 0.03 | 0.03 (–0.04 to 0.11) |
| Family support worker | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Vocational worker | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Substance abuse worker | 0.00 | 0.00 | 0.03 | 0.03 | 0.03 (–0.04 to 0.11) |
| All contacts in community settings | 10.63 | 2.70 | 9.69 | 1.93 | –0.94 (–7.35 to 5.47) |
| CMHT and AOT contacts in any setting | |||||
| CMHT contactsa | 16.47 | 3.23 | 13.38 | 1.40 | –3.09 (–9.27 to 3.09) |
| AOT contactsb | 27.23 | 5.30 | 36.08 | 6.80 | 8.85 (–8.95 to 26.64) |
| Primary care | |||||
| GP (home) | 0.02 | 0.02 | 0.00 | 0.00 | –0.02 (–0.06 to 0.02) |
| GP (surgery) | 0.19 | 0.07 | 0.26 | 0.10 | 0.08 (–0.18 to 0.33) |
| Counsellor (service setting) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Counsellor (community setting) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Medications | |||||
| Number of depot medications taken | 9.40 | 0.54 | 9.35 | 0.51 | –0.05 (–1.55 to 1.44) |
| Number of oral medicationsc | 0.83 | 0.18 | 0.85 | 0.15 | 0.02 (–0.45 to 0.49) |
There were few differences in resource use between groups in the 6 months after the end of the intervention. The mean number of visits from community psychiatric nurses in service settings were marginally higher in the intervention group (6.5 visits) than the control group (6.0 visits) (95% CI –1.6 to 2.6 visits), and in community settings (intervention 6.3 visits, control 6 visits, 95% CI –2.9 to 3.6 visits). The number of visits from mental health support workers in both community and service settings and the number of DNA appointments for community psychiatric nurses and psychiatrists were somewhat lower in the intervention group. While the number of inpatient admissions between groups did not differ significantly, the mean number of days spent in psychiatric hospital care was somewhat higher in the intervention group (3.7 days) than the control group (0.6 days) (95% CI –0.4 to 6.5 days).
Patient experiences of financial incentives
Recruitment
Out of the potential 78 participants, 26 patients consented to be interviewed at the end of the intervention and 32 patients consented to be interviewed at the 24-month follow-up, with 11 patients interviewed at both time points. At the end of the intervention, reasons for not participating included ineligibility (n = 23), not being contacted as sample size had been reached (n = 14), deceased (n = 1), not being contactable (n = 7) or refused to take part (n = 7). Out of the 26 participants who consented to be interviewed, one refused to be interviewed with a recording device and was therefore not included in analysis.
Reasons for not participating in an interview at the 24-month follow-up included ineligibility (n = 8), the sample size had been reached (n = 12), refused to participate (n = 8), having deceased (n = 4), not being contactable (n = 10) and failing to attend an arranged interview (n = 4). Of the 32 patients interviewed, one patient was interviewed despite having received no incentives and was removed from the final analysis.
Patient characteristics
Patient characteristics for 45 patients whose interviews were analysed are reported in Table 22. Twenty-one interviews were carried out on the premises of mental health teams and four in patients’ homes at the end of the intervention, and 25 interviews took place on the premises of clinical teams and six were interviewed in patients’ own homes at the 24-month follow-up. On average, patients were interviewed 5 weeks (between 1 and 12 weeks) and 27 months (between 19 and 40 months) after the end of intervention and at the 24-month follow-up, respectively.
| Sociodemographic and clinical characteristics | Mean (%) |
|---|---|
| Sex | |
| Female | 12 (27) |
| Male | 33 (73) |
| Age | |
| Years | 46.5 (range 23–69) |
| Ethnicity | |
| White British | 26 (58) |
| Turkish British | 2 (5) |
| Black British | 2 (5) |
| White other | 4 (8) |
| Black other | 8 (18) |
| Asian | 3 (6) |
| Clinical diagnosis | |
| Schizophrenia | 33 (73) |
| Bipolar disorder | 7 (16) |
| Schizoaffective disorder | 5 (11) |
Results
The analysis identified five general themes: ‘structuring the day of the appointment’, ‘relationship with clinicians’, ‘extra expenditure’, ‘personal dilemmas’ and ‘impact once incentives had stopped’. The themes were similar at the two time points, and those patients who were interviewed twice expressed consistent views at both time points.
Structuring the day of the appointment
The most prominent theme concerned the structure of the day of the appointment, and how it impacted on LAI medication adherence. Patients mentioned the difficulty in establishing a daily structure (such as general forgetting, not being bothered or boredom) before being offered financial incentives. Patients reported being more motivated to complete basic daily tasks as a result of the intervention and, consequently, becoming more active and organised on the days of the appointment.
It wasn’t so much ain’t liking having it, it was remembering to have it all the time.
Patient 147, male
Yeah I really enjoy it [incentive]. It gives me some sort of structure (. . .) I need some sort of structure for my day time so I don’t just lie in bed.
Patient 343, male
I used to be bored like but it got me up. I realise that it’s important to get my injection. Now I’ve realised that.
Patient 211, male
A number of patients talked about having developed a routine structure around attending their appointments. Some hoped that this would be sustained once financial incentives have been discontinued, while others were less optimistic.
I fell into a routine of going for my injection even though it wasn’t about the money at the end.
Patient 127, female
I can’t say that I’m going to be 100% sure that I’m going to remember to come here on time.
Patient 147, male
Relationship with clinicians
Some patients stated that before the study they had felt apathetic in regards to their LAI medication treatment. They were often lonely, and relationships with their clinician had been poor. This led to feelings of being disrespected and devalued. Patients reported that financial incentives improved the relationship in two ways. First, receiving £15 from the clinician was perceived as a sign of being valued. Second, the higher frequency of attended appointments nurtured a better relationship and mutual feelings of trust.
Well Monday’s not alright because I’ve already got plans for that Monday you know so it all seems to be on their terms and not anyone else’s.
Patient 127, female
I was lonely and I just thought I’m not going to have it, it doesn’t matter ‘cos I’m just sitting in my room.
Patient 342, female
And about receiving the money, how did you see it?
Oh yeah, yeah. I saw it as being rewarded sort of for coming on time and being respected for coming on time. I found it rewarding.
Patient 147, male
She can rely on me (. . .) She’ll know that I’ll come for it and that – she doesn’t have to chase me like before.
Patient 342, female
Finally, two further aspects of improved relationships were reported, that is feeling happier in themselves, or having a greater sense of worth which facilitated contacts with others, and the more frequent opportunities for meeting with other people. These included clinicians and other patients at the community teams, but also other casual social contacts during travelling to and from the appointments or going for a cake and coffee afterwards.
It helps out, you know, it makes you more confident, just more relaxed. You know you go and get your injection on time.
Patient 338, male
It’s a bit like a social event now coming here whereas before it was like oh I’ve got to go for my depot. Now I actually enjoy coming and you meet people who come for their depot and you talk to them.
Patient 126, male
But I don’t mind, it gets me out of the house to go there [clinic], a bit of therapy, to go out, to get used to going out.
Patient 322, male
However, this change did not apply to all participants. Some stated their relationships with clinicians had always been good and others expressed that the relationship remained as poor as before. Other patients did not recall any relationship with an individual clinician, and subsequently did not experience any change.
Yeah he’s a friend as well as a nurse, which is good. (. . .) He’s not just there to do his job and leave me alone. He kind of acknowledges me after the injection as well.
And has that always been the case? Have you always got on well with him?
Yeah always got on well with [community psychiatric nurse name]. Always.
Patient 126, male
And you said that the relationship that you have with the [hospital’s name] and your [community psychiatric nurse name] is not terribly good.
No I don’t think it is.
OK. Has it always been like that?
Yeah it’s always been like that.
Patient 127, female
Extra expenditure
Patients described the incentives as a reward since it meant an extra expenditure, beyond what they would otherwise have been able to afford. They reported to have used the money for practical daily needs (e.g. paying for bills or buying lunches for their children) and for small treats. A minority, however, said that they had spent the money on alcohol or gambling.
The money meant sugar in my tea, money to buy something, money meant a lot because on a Monday I am skint really. It might be a little but it’s a lot to somebody like me and somebody on benefits. It’s a lot.
Patient 149, male
That’s my bit of money for myself. So I keep that little bit of money for myself.
Patient 144, male
Oh I spent it on self-medicating myself. Drink. (. . .) When I drink it makes me calm.
Patient 120, male
Personal dilemmas
Some patients stated personal dilemmas about their decision to accept the incentives. These dilemmas included a feeling of unease or guilt for being paid for doing something for one’s own health, being negatively judged by the clinicians for accepting the incentives or feeling obliged or forced into taking their medication despite their dislike for it or belief that their medication was not helpful. Some patients said that the practice was unfair to those who adhered to their medication but were not offered any incentives. Some suggested that the incentives should be given to all patients, whereas others preferred that the incentives should be offered only to specific groups of patients.
It did make me feel uncomfortable yeah, yeah (. . .) like I was bribing people or that I was getting something that I shouldn’t be getting but I still took it. Yeah but I did feel uncomfortable yeah especially when it was in a brown envelope and you have to sign for it.
Patient 116, female
I thought that it’s grateful of them but the man who gave out the injection he said he didn’t agree with this money and see no point in it, but . . .
What did you think of that?
I didn’t say anything to him.
Patient 150, female
I mean it seemed like a joke to be paid to have medication when it shouldn’t be a problem like that. That shouldn’t be the issue.
Patient 154, female
I think you should just take the medication, if you’re getting the money or not, take the medication, you shouldn’t have money persuading you to take it.
Patient 343, male
I felt it would be unfair (. . .) if he is on a depot and I say well I have been chosen to get £15 every time I have my depot. How would you feel? You would feel like a mug.
Patient 149, male
Impact once the incentives stopped
In the FIAT trial, financial incentives were offered to patients for a 12-month period. To our knowledge, no team continued with the practice after the 12-month period. Most patients stated that the discontinuation of incentives had no real impact on their lives, and that they quickly adapted to the fact the incentives had been stopped. Some patients reported that they had known all along that the money would be available for only a 12-month period and were therefore prepared for it. However, some disappointment, missing the money or financial problems were also reported. Finally, some patients said their improved LAI medication adherence was sustained after incentives have ended, while others felt they were less motivated and their adherence was deteriorating as a result.
Because maybe £15 comes in handy but if you don’t get it you don’t get it so get on with your life.
Patient 356, female
Because I knew the £15 I was able to get food because I don’t get a lot of money (. . .) But now since I don’t have it I’m eating less and I’m getting skinnier and I’m getting more ill.
Patient 341, male
It’s better for me to take medication and it’s not because I want the money. It’s because for me to get well and the money I was getting.
Patient 105, male
But now I haven’t got the incentive to have it so I don’t have it anymore. I haven’t had it for 3 months now.
Patient 101, male
Clinicians’ experiences of financial incentives
Clinician interviews at 6 months and 12 months of the intervention
Fifty-nine clinicians were interviewed with regards to the effect of the incentives on 73 of the 78 patients allocated to receive incentives during the FIAT trial. Twenty clinicians were interviewed about more than one patient – and for 19 patients, a different clinician was interviewed at each timepoint. The number of clinicians interviewed and the number of patients for whom interviews were obtained at each time point are presented in Table 23.
| Clinicians | Patients | ||||
|---|---|---|---|---|---|
| N of Team Managers Interviewed | N of Care-coordinators interviewed | N of Community Psychiatric Nurses interviewed | Total N of interviews | N for whom a clinician interview was obtained | |
| Mid-Point (6 months) | 4 | 26 | 16 | 46 | 73 |
| End-Point (12 months) | 3 | 27 | 16 | 46 | 68 |
What did clinicians report about how patients spent the money?
The most common ways in which clinicians reported patients spending the money were on food (n = 24 patients), alcohol (n = 21), illicit drugs (n = 17), household goods (n = 11), hobbies (n = 10) and tobacco (n = 10).
Did clinicians report patients asking for more money or more frequent depot injections?
Clinicians reported 6 patients across 5 teams having asked for the incentive to be increased above £15, but clinicians said this was resolved quickly and did not cause problems for the remainder of the trial. Six patients were reported to have asked to receive their depot more frequently; an additional 12 patients were reported to have requested to have their depot days in advance of the due date, or to have turned up for it a few days early. Clinicians stated that when these requests (sometimes delivered in a ‘joking’ manner) were refused there were no implications for the patient’s treatment.
Did clinicians report other patients in the team asking to receive payment for depot injections – or becoming non-adherent to their medication in order to qualify?
Twenty-two patients not in the trial across 10 teams were reported as asking to be paid for their depot and/or asking why they were not being paid. Two patients were reported to have missed their depot as a consequence, whilst another patient was reported to have threatened not to take his depot. These problems were reported as being short-lived and rapidly resolved.
How did clinicians experience the effect of financial incentives on their patient’s interaction with the team?
The clinicians of 65 patients reported qualitative information on how incentives had positively or negatively influenced the interaction with the team. Clinicians for the remaining 8 patients reported no effect of the incentives on patients’ interaction with the team.
Positive effects on clinical management and relationships
Positive effects on clinical management
For 53 patients, clinicians reported that the financial incentives had led to improvements in their ability to effectively manage their patient’s care. The most obvious benefit, reported in the case of 47 patients, was that their attendance of depot appointments improved:
The patient has become compliant and turns up for their medication religiously
Patient 1, clinician 1
It made a lot of difference to this patient - he is wavering and the only thing keeping him on depot is the money
Patient 2, clinician 2
Twelve patients were reported as making extra efforts to ensure they received the depot on time (and hence received the incentive):
He will call up to speak to the team and check his depot due date when before involvement in the study he would never use a phone.
Patient 11, clinician 3
The clinicians of 32 patients reported that the incentives had had knock-on effects in terms of other aspects of clinical management. These benefits included allowing the clinicians to spend less time chasing the patient (7 patients), increased contact facilitating monitoring of the patient’s health (17 patients), increased engagement with the team (16 patients), and increased attendance of other meetings such as with the psychiatrist, drug and alcohol services, or CPA reviews (10 patients):
The incentives have made it easier for me to know where he is – I used to have to chase and he wouldn’t answer his phone . . . The financial incentive ensured the client would be there for his appointment.
Patient 4, clinician 5
Care coordinators need to have contact to be aware of issues with the client. The financial incentive eased this process as the client was forthcoming for their depot, therefore we were having face to face contact regularly.
Patient 5, clinician 6
. . . Since the study and during it he has been happily involved with the service, whereas his involvement fluctuated before
Patient 6, clinician 7
He is a lot more proactive about coming to the centre and engaging with the team than he used to be . . . he has agreed to see a psychiatrist for the first time in a couple of years
Patient 8, clinician 9
He is engaging well with the substance misuse team
Patient 9, clinician 10
Positive effects on relationships
For 21 patients, clinicians linked the incentive to improvements in their relationships with their patients. These improvements included increased trust and communication:
Before the start of this trial, the patient was very suspicious of the team and very guarded. This has dramatically changed since being involved in the study. The patient has come to trust the team more
Patient 10, clinician 3
Some clinicians linked the improved relationship to the increased contact which the incentive had generated:
The involvement in the study has given the team more access to patient. He engages a lot more with the team and the relationship has built up hugely
Patient 11, clinician 3
Others linked this to the positive effect of depot medication on the patient’s presentation:
He is very pleasant to work with because he is taking his regular depot – I have more positive contact with him. He is friendlier, more appropriate and there is less non-crisis contact than there had been before . . .
Patient 12, clinician 11
. . . I think the relationship would have grown anyway as the level of trust increased, however the incentive may have helped with this development as it meant that the client was getting the depot on a regular basis and his symptoms subsided, which allowed our relationship to be given a chance
Patient 4, clinician 5
Some emphasised that the improvement in their relationship made clinical management easier by facilitating constructive communication with the client:
The patient comes to the CMHT more often – before I couldn’t talk to her, but now I get to talk more with her about her plans for the future.
Patient 14, clinician 12
The linking with other services has increased. For example, the patient now asks for the team to be present when meeting his probation officer and lets the team know more about what is going on with the other services the patient is involved with.
Patient 10, clinician 3
Negative effects on clinical management and relationships
The clinicians of 19 patients reported negative effects of the incentives on the patient’s interaction with the team.
Negative effects on clinical management
The clinicians of 5 patients reported negative effects on their ability to manage their patient’s care. In two cases this was because they find the time and effort involved in providing the incentive was problematic:
I felt a bit of extra pressure – I don’t expect to be the banker. Sometimes I’d go to administer a depot and forget the money – but because the client expected it, I would have to return with the money, taking more time out of my day
Patient 16, clinician 14
In another three cases, this was because the patients disengaged with treatment, which the clinicians attributed to spending the incentive on drugs and alcohol:
The patient has been spending the money – presumably – on drugs and has consequently been discharged from the CMHT due to lack of engagement
Patient 17, clinician 15
Negative effects on the therapeutic relationship
The clinicians of 17 patients reported a negative effect of incentives on the therapeutic relationship. The most commonly reported type of negative impact (reported for 10 patients) was a monetarisation of the relationship:
The patient viewed receiving the depot as his ’pay day’ and there is no longer a rapport between myself and the patient.
Patient 18, clinician 16
The relationship has become generally more focused on money, not the interaction. I feel he is only interested in the money, not actually in interacting with staff . . .
Patient 19, clinician 17
In 5 cases, patients were reported to have become aggressive if the money was not delivered promptly:
The patient would get angry if the money wasn’t there and would become slightly threatening if he did not receive it.
Patient 20, clinician 18
For one patient management of this aggression required increased staff manpower:
The study used up more man-power as a second person had to come to give the financial incentive to the patient because of the aggression experienced by the CPN
Patient 21, clinician 19
What direct and indirect effects of financial incentives did clinicians perceive on patients’ health outcomes?
The clinicians of 42 patients reported qualitative information on how they perceived the effects of financial incentives on patients’ outcomes. This incorporated health and social outcomes such as symptoms, wellbeing, social functioning, insight and relationships with friends and family. The clinicians of the remaining 31 patients did not report any effect.
Positive Effect on Patient Outcomes
A positive effect was reported for 33 patients. This theme was further broken down into 3 sub-themes: Improved mental well-being; Improved social functioning; Insight.
Improved mental well-being
The clinicians of 15 patients reported that the incentives had had a positive effect on their patient’s well-being, including an improvement in mental health and reduction in symptoms (11 patients) and a reduction in drug and alcohol use (3 patients):
His mental health and presentation is better and he is less psychotic
Patient 22, clinician 20
He has been stepped down from CPA because he is doing well
Patient 23, clinician 21
He has had a major illness due to alcohol but has now stopped drinking.
Patient 24, clinician 22
The clinicians of 5 patients specifically linked the improvement in mental health to increased depot adherence:
He’s a long-term drug user but his depot reduces the psychotic effects of these drugs, and now he is coming for his depot on time, in the last 6 months we are noticing an improvement in his mental state
Patient 9, clinician 10
. . . The patient now attends regularly for their depot and has had no relapses which has helped the team
Patient 25, clinician 23
Improved insight
Relatedly, the clinicians of 12 patients reported that their patients had improved insight into their illness and into the beneficial effects of medication on their mental health:
The patient is gaining more of an insight into his illness and is recognising his symptoms, his dialogue has improved and he is more involved with his treatment plan. He says he is ’hoping to move forward.
Patient 10, clinician 3
The client has acknowledged his year of stability as a result of taking his medication regularly – he sees the benefit of the medication and being well is the incentive to take it. The financial incentive has helped with this but is not the main driver
Patient 6, clinician 7
Improved social functioning
The clinicians of 16 patients reported a positive effect of the incentives on the patient’s social functioning. The most common improvements were reported as the regular medication and contact having a stabilising effect on the patient’s lifestyle and improved family relationships:
He leads a very chaotic life and the money is providing him with more of a routine. He is including the money in his budget now.
Patient 28, clinician 27
He uses less illicit substances as he was treated better and the team had less contact from the police. He now has a less chaotic lifestyle… there are less problems with his family and the police.
Patient 29, clinician 28
Being on the study has helped because the regular contact and medication has given him stability and a sense of satisfaction with his lifestyle, and his relationships are more stable
Patient 6, clinician 7
The patient has a new relationship with a partner and getting more regular medication has helped a lot. I believe this is due to the FIAT money.
Patient 25, clinician 29
Other reported improvements included a more stable housing situation, finding a job, improved budgeting ability and improved self-care:
The money motivated him to attend his depot injection . . . His self-care and hygiene has improved massively . . . He continues to engage well with the home office about immigration and helps out at the hostel/accommodation in cooking.
Patient 30, clinician 31
He is now working as a waiter and talking about moving out of parents’ home and getting a place for himself.
Patient 31, clinician 8
Negative Effects on Patient Outcomes
The clinicians of 15 patients reported a negative effect of incentives on patient outcomes. These experiences all concerned negative effects of the payment itself, and were sub-divided into two sub-themes: Increased drug and alcohol use, and Other adverse effects of payment.
Increased drug and alcohol use
The clinicians of 8 patients said that the incentive was used to pay for drug or alcohol and therefore led to patients increasing their substance misuse:
The patient was in hospital twice since I took over. The financial incentive has helped improve our relationship but has also meant he can spend money on drugs.
Patient 32, clinician 33
The financial incentives have been beneficial with regard to adherence. However, in terms of impact on his lifestyle, the incentives are not beneficial as this has not improved. There is generally no change in his lifestyle, just increased substance misuse…. He has increased his alcohol and drug (crack) use . . .
Participant 33, clinician 4
He already had a lot of money due to DLA and income support so the FIAT money was pocket money for alcohol . . . he became less cooperative with the depot during the trial due to an increase in the use of alcohol . . .
Participant 34, clinician 44
He was hospitalised due to an increase in alcohol use. I believe that FIAT was a contributing factor in his hospitalisation due to the extra cash that he had. However I understand that the patient would probably have got access to alcohol somehow regardless of the FIAT study
Participant 35, clinician 45
Other adverse effects of payment
Other adverse effects of payment were reported by the clinicians of 8 patients. These included becoming dependent on the money, becoming secretive about the money, and being vulnerable to being taken advantage of by others because of the money:
He came every 2 weeks and there was never any need to remind him to appear, in fact the he would often come one or two hours early. Because of this I believe he is desperate for the money and has become dependent on it.
Patient 36, clinician 45
The money is hidden from his family and spent on beer . . . He makes a point of hiding it from his wife . . . However, this has not increased his drinking.
Patient 37, clinician 30
He started getting ‘hangers on’ i.e. people who took advantage of him and started camping out and drinking in his flat. They turned up when he got his incentive.
Patient 34, clinician 46
Link Between Themes
Clinicians endorsed different combinations of themes for each patient as depicted in Table 24. This shows that overall, for 77% of patients a positive effect on management and/or outcomes was reported, whilst for 33% a negative effect on management and/or outcomes was reported. These figures overlap because for some patients both positive and negative effects were reported.
| Effects on patient interaction with the team | Total | |||||
|---|---|---|---|---|---|---|
| Only positive effect reported | Only negative effect reported | Both positive and negative effects reported | No effect reported | |||
| Effects on patient health and social outcomes | Only positive effect reported, n | 23 | 2 | 1 | 1 | 27 |
| Only negative effect reported, n | 1 | 5 | 3 | 0 | 9 | |
| Both positive and negative effects reported, n | 4 | 0 | 2 | 0 | 6 | |
| No effect reported, n | 18 | 5 | 1 | 7 | 31 | |
| Total | 46 | 12 | 7 | 8 | 73 | |
Clinician interviews at 6 months and 24 months after the end of the intervention
Fifty-seven clinicians were interviewed for 59 of the 78 patients, with interviews for 25 of the 78 patients conducted at both time points; however, in some cases the same clinician was not interviewed. The clinicians for 19 patients were not interviewed because of clinician unavailability or because the clinician and/or patient had transferred to different teams since the end of the intervention.
Continuation of incentives
No clinicians reported that they continued to use the incentives with patients involved in the trial nor did they start using the incentives with any new patients within their team. Financial constraints or lack of funding was the most commonly reported reason for not continuing with the incentives. This was closely followed by issues with implementation (unaware that they were able to continue or practical issues) and a small number of clinicians felt that the incentives were not effective and thus saw no rationale behind continuing them.
Did patients ask for the incentive to be continued?
Fifteen patients were reported to have asked for the incentives to be continued but no major problems occurred when these requests were denied. Reports for the clinicians of seven patients who were interviewed at both time points gave different answers between the two time points and data for two patients were missing. Seven patients were reported to have become intentionally non-adherent to qualify for being offered the incentives again. Other patients were reported to show their disappointment over the incentives discontinuing and expressed that they missed the money. Again, reports for the clinicians of four patients interviewed at both time points differed and for two patients were missing.
Long-term consequences of the incentives being discontinued
Patient outcomes
The clinicians of 28 patients reported that there were no problems once the incentives were discontinued: adherence and mental health continued to remain stable even after the incentives had stopped. However, the clinicians of 20 patients reported problems as a result of the incentives stopping. These were largely concerned with the patient’s adherence fluctuating or refusing medication, and as a result their mental health deteriorated. In a minority of cases, this led to relapse, rehospitalisation or an increase in drug and/or alcohol use.
Responses for the clinicians of 10 patients interviewed at both time points differed and for one patient, a response was missing.
Therapeutic relationship
The majority of clinicians interviewed felt that the incentives stopping had no impact on their relationship with patients, as was the case for 36 patients; the clinicians continued to remain engaged and maintain a good relationship with their patients, some felt that communication within the team and with the patient had improved, and in some cases patients were more proactive about in their efforts to receive their LAI medication. Nevertheless, around one-third of clinicians interviewed felt that their relationship with their patients had deteriorated as a result of the incentives stopping, primarily that patients became disengaged with services/clinicians.
Responses for the clinicians of 11 patients interviewed at both time points differed and a response was missing for one patient.
Consequences for other patients
The majority of clinicians interviewed reported that no patients outside of the trial asked to be offered the incentives. However, six clinicians estimated that around 22 patients outside of the trial did ask to receive the incentives. In one case, a patient was reported to have been intentionally non-adherent to qualify to be offered the incentives.
Clinician opinions of financial incentives
Before the intervention
When asked retrospectively, 40 clinicians believed that the use of financial incentives were a good idea, primarily because they would be an effective method to improve adherence and would help patients in a number of ways (e.g. financially). A number of clinicians also felt that financial incentives would be a good idea but that they would be suitable for only certain patients.
Fourteen clinicians believed that prior to the intervention financial incentives were not a good idea, which was largely a result of ethical concerns or questions over their effectiveness. Some clinicians believed that financial incentives may be seen as coercive or a form of bribery, and that they would negatively reinforce good adherence, would negatively impact on the therapeutic relationship or would be used to fund drug/alcohol habits.
The opinions of three clinicians were missing.
After the intervention
After the intervention, 37 clinicians believed the incentives were a good idea and reported a number of advantages over their use. These include the incentives being an effective method to improve adherence and prevent relapse, being less costly than hospitalisation and allowing patients to develop an insight into the importance of medication. Other clinicians felt that they made clinical management of their patient easier and created more opportunities to communicate with their patients.
Other clinicians felt that financial incentives were an effective method, but felt that other forms of financial incentives may be more appropriate as opposed to money itself, for example through the use of vouchers or by redirecting their state benefits so that their entitlement to benefits was dependent on their adherence. Some also said that while they agree with using financial incentives these would be suitable for only certain patients.
Fourteen clinicians believed even after the trial that financial incentives were not a good idea because of ethical concerns, practical issues and questions over their effectiveness. Ethical concerns included questions over whether or not offering incentives is morally right, whether or not offering incentives would be unfair to others, whether or not the money would be spent on drugs/alcohol and whether or not patients may be reluctant to report side effects from medication if they are receiving the money for them. Some clinicians noted practical issues, including feeling that providing the incentives was more effort than it was worth. Other clinicians felt that financial incentives were not an effective method in improving adherence.
The opinions of four clinicians were missing.
Would clinicians consider using financial incentives again?
When asked if they would consider using financial incentives again, 40 of the 57 clinicians said that they would consider using them in the future, whereas 12 would not. One clinician interviewed for a patient at both time points gave different answers at both time points (i.e. said ‘no’ at the 6-month follow-up, but ‘yes’ at the 24-month follow-up), and the answers for four clinicians were missing.
Changes in clinicians’ opinions of financial incentives
The frequency counts characterising changes in clinicians’ opinions of financial incentives before and after the intervention are shown in Table 25. Please note that opinions of four clinicians who were interviewed about more than one patient were mixed, that is, their opinions differed in the interviews. The answers of four clinicians were missing. Thus, changes of opinions are presented for a total of 49 clinicians.
| After | Total | |||
|---|---|---|---|---|
| Yes | No | |||
| Before | Yes | 28 | 7 | 35 |
| No | 4 | 10 | 14 | |
| Total | 32 | 17 | 49 | |
The majority of the 49 clinicians had a favourable opinion over the use of incentives before and after the intervention, whereas one-fifth of clinicians had a negative opinion throughout. There were a greater number of clinicians who were positive about the incentives prior to the intervention but changed their opinion afterwards, compared with those who were negative prior to the intervention but then gave a more positive opinion afterwards; however, the differences between these two groups were marginal.
Chapter 4 Discussion
Sections in this chapter have been adapted from Priebe and colleagues. 47
Main findings
Offering financial incentives is an effective method for improving adherence among patients with psychotic disorders who demonstrate poor adherence to LAI medication. In this study, the average baseline adherence of 67% remained largely unchanged in the control group (an increase of 4%), whereas adherence in the intervention group increased substantially to 85%. Alongside this, 21 patients in the intervention group achieved adherence of at least 95%, compared with three in the control group. Patients in the intervention group also reported a significantly better subjective quality of life. Once the incentives were discontinued adherence levels did not appear to be maintained, with adherence in the intervention falling back to baseline levels (60% at the 6-month follow-up and 68% at the 24-month follow-up). Adherence in the control group was higher than the intervention group at both follow-up points; however, this difference was not statistically significant.
The total cost per patient receiving the incentives is not significantly higher than treatment as usual. The difference in costs between both groups was £598, which may take into account the increase in the number of LAI medications received owing to improved adherence and the contacts with nurses administering them. Even after the incentives were discontinued, the cost of the intervention group remained higher than the control group (mean difference of £1406); however, this appeared to be as a result of patients in the intervention group having longer mental health inpatient admissions than patients in the control group.
The majority of patients and clinicians felt that the incentives were a positive experience and reported numerous benefits from them. For patients, the incentives acted as a reminder or reward for their LAI medication and the money that they received helped towards the costs of living, alleviating the financial hardship experienced by those receiving state benefits. A large number of clinicians maintained the view that financial incentives were a good idea before and after the study and reported the benefits of the incentives on clinical management through improved adherence, contact, patient monitoring, communication and trust. Clinicians also reported improvements in insight, mental health and social functioning.
Negative experiences with the incentives differed between patients and their clinicians. One-third of patients felt uncomfortable with the idea of being offered financial incentives or raised ethical concerns over accepting them. On the other hand, clinicians who had negative experiences reported problems in patient management as a result of the incentives such as increased drug and alcohol use, and the monetisation of the therapeutic relationship, as well as disagreeing with using incentives on ethical grounds.
In the majority of cases, the incentives stopping had little impact from the perspective of both patients and clinicians. However, a small number of both clinicians and patients reported problems with adherence and thus deterioration in mental health and financial problems as a result of no longer receiving the incentives.
Strengths and limitations
One of the strengths of this study was that it involved a large number of mental health teams across the UK, from areas of differing social deprivation. Outcome data were available for the majority of patients, 131 out of 141 (93%), and statistically analysed for 123 patients (87%) at the end of the intervention. The estimated treatment effect was also robust to a variety of sensitivity analyses at the end of intervention, and at the 6- and 24-month follow-up. A substantial number of patients and clinicians were interviewed about their experiences both during the intervention and at the follow-up periods. Patient interviews were available for 45 of the 78 patients (58%) allocated to the intervention group; clinicians were interviewed for 73 of the 78 patients (94%) during the intervention period and for 59 of the 78 patients (76%) were interviewed during the follow-up periods.
Nevertheless, the study does have some weaknesses. There were instances of protocol violations, including of patients in the trial who did not have a diagnosis of schizophrenia or who had > 75% adherence at baseline and were still included in the trial. The study was not blinded. It was impossible to blind patients and clinicians to whether or not patients received financial incentives. It can be argued that the majority of outcomes were collected from electronic databases, and therefore were objective, and that researchers had no knowledge of patients’ allocation. However, systematic efforts could have been made to ensure that researchers were blinded. Particularly, blinding should have been ensured when collecting patients’ ratings of subjective quality of life and assessments of patients’ clinical improvement rated by their clinicians. Future research should seek to address this limitation in their design to keep the scientific rigour as sound as possible.
Furthermore, all teams who participated in the trial consented to the practice of offering financial incentives. Whether or not the incentives would be effective among teams who did not choose to participate in the study is unclear, for example if negative attitudes towards using incentives may have influenced any outcomes.
The study was powered to detect a statistically significant difference in adherence, the primary outcome. However, it was not necessarily powered to identify significant differences in secondary outcomes. While the intervention group still had a significantly better subjective quality of life, the advantage of the intervention group in global clinical improvement, despite being marked, did not reach statistical significance. The sample was clearly too small to test differences in rehospitalisation and other adverse events that are rather rare.
While study retention was high during the intervention period, there was attrition at both follow-up periods for a number of reasons. However, the reasons for unavailability were accounted for (e.g. discharge, refusing medication, hospitalisation and imprisonment) and thus the data are not ‘missing at random’. Furthermore, a number of clinicians were lost at follow-up periods for the clinician interviews and the large majority of interviews were conducted at 6 months only; therefore, the longer-term impact of the incentives may not be generalisable beyond the first 6 months.
The study was not fully powered to address costs, and the full extent of patient costs from a broader societal perspective is unknown. However, the data collected for the effectiveness analysis did not indicate differences in such societally relevant outcomes such as police arrests or taking up education/training courses. Data on service use were collected from patient records within NHS trusts and any service contact with NHS services outside of these trusts or with primary care services is unclear and may be dependent on local information-sharing policies and practice. Furthermore, costs did not reflect the duration of contact with mental health staff or the skill level (and thus cost) of each staff member.
The qualitative interviews and analyses also demonstrate weaknesses. Patients were selected from a convenience sample; therefore, there may be differences in the characteristics of those who consented to interview compared with those who declined. While the majority of experiences were positive, the sampling method may have excluded those who may have had more negative experiences. For example, those patients whose adherence and mental health had deteriorated once the incentives had stopped and may not have had the motivation or capacity to agree to an interview. Moreover, all researchers involved in the data collection and analysis were also part of the FIAT research team. This might have introduced a bias in the way the interviews were conducted or in the analysis of the material.
With regard to the clinician interviews, the way the questions were structured may have biased how the responses were interpreted. There was little opportunity for further exploration if a clinician answered ‘no’ to a question; therefore, if there was no change in a particular outcome (e.g. therapeutic relationship) it was difficult to understand why. The interviews themselves may, therefore, have been more receptive in finding changes in behaviour during the intervention and follow-up periods. There is also the possibility that the answers clinicians gave were merely speculative. For example, one clinician reported the same outcome for five patients during the intervention period, which may have led to some data being conflated. How clinicians believed patients spent their money was also speculative and any links that were made between the incentives and changes in patient management or health may be subjective and not reflect reality. Therefore, making causal attributions between the incentives and any changes must be taken with caution. The interview schedules at the 6- and 24-month follow-ups differed slightly, with some questions into the same aspect being worded differently at both time points and some questions being asked at one time point only. This has two implications: first, the differences in the phrasing of the question may be interpreted in dissimilar ways by clinicians and thus the responses given may have been different; and second, the answers given to some questions reflect one time point only and cannot be taken as a general answer for the long-term effects of the incentives stopping.
Comparisons against the literature
This is the first randomised controlled trial testing the clinical effectiveness and cost-effectiveness of financial incentives in improving adherence to antipsychotic medication. This adds to and extends the findings of previous research on the use of incentives influencing health behaviours in patients with mental illness, and small observational studies in which financial incentives were offered in secondary mental health settings.
Despite the effectiveness of the incentives, levels of adherence were not maintained and fell back to baseline once the incentives stopped. This finding is in line with studies included in a systematic review by Burton and colleagues37 into the use of financial incentives in health behaviours among mental health populations, which found that health behaviours had fallen back to baseline levels once the incentives had stopped. However, around half of the studies included in the review found that health behaviours were maintained even after the discontinuation of the incentives. However, such follow-up periods were shorter than even the 6-month follow-up in this study; therefore, it is difficult to know whether or not the longer-term effect of the incentives on health behaviour in these studies would be similar to that found in the FIAT study.
There is some evidence to suggest that behaviours influenced by financial incentives may fall below baseline levels once the incentives have been discontinued, so-called ‘crowding out’. The findings of this study have found no support for this, which suggests that there may be no major adverse impact for patients once the incentives have stopped and which is also substantiated by the majority of reports from patients and clinicians involved in the study.
The results found in this study can also answer some of the issues and concerns raised by stakeholders in a focus group study by Priebe and colleagues. 4
Primarily, this study answers the concern of whether or not the incentives would perversely incentivise patients to become intentionally non-adherent to qualify for the incentives or whether or not the incentives would affect the therapeutic relationship or ‘challenges for evidence and experience’: financial incentives were found to be an effective method in improving antipsychotic medication adherence and there was little evidence that the practice perversely incentivised patients to become intentionally non-adherent. The majority of clinicians felt that there was no effect on the therapeutic relationship.
Concerning the value of antipsychotic medication, whether or not other services may suffer financially if health-care budgets were spent on incentives, how the incentives would be spent by patients and whether or not there were government motives underlying the use of incentives or ‘wider concerns’
The incentives were spent in a variety of ways and patients enjoyed using the incentives towards some form of rewarding expenditure but, for some, this included the occasional expenditure on drugs and/or alcohol. Furthermore, offering financial incentives has been shown to be no more expensive that routine care.
Concerning the practicalities involved in implementing the incentives and how they can be incorporated as part of a clinician’s toolkit or ‘problems requiring policies’
Despite the effectiveness and a defined structure of how to provide the incentives in routine clinical practice, financial incentives have not been implemented since the study ended and thus have not become part of the clinician’s toolkit.
Concerning whether or not offering financial incentives are coercive or whether or not they are fair on others not receiving incentives or ‘inherent dilemmas’
The findings from this study overall cannot answer these questions in their entirety, as this was not the original research question, although there is some evidence from the patient interviews that some felt the practice of financial incentives may be coercive or unfair on others.
Chapter 5 Conclusions
Sections in this chapter have been adapted from Priebe and colleagues. 47
Implications for health care
Financial incentives are an effective method in improving adherence. They are more effective in patients with psychosis than any other method that has so far been subjected to a similarly rigorous evaluation in a randomised controlled trial. The improvement was substantial, although practically complete adherence was achieved in only 28% of patients receiving the incentives.
Community Mental Health Teams and AOTs aim to follow up patients proactively in the community who show poor adherence to medication and pose risks to themselves or others. In this study, on average, we did not recruit more than two patients from each team who agreed to participate. The number of patients with poor adherence is low and reflects the effectiveness of such teams in achieving adherence among this clinical population. If the incentives scheme was to be implemented nationally, the number of patients who will qualify for the scheme is difficult to predict as eligibility criteria for research purposes may differ from clinical practice. If eligibility in clinical settings matched the criterion as set in this study, it is likely that mental health teams will have one or two patients under the scheme at any time. With this in mind, the incentives may be offered to fewer than 1800 patients in the NHS in England, which is a small proportion of patients under secondary mental health care.
It is also important to note that offering financial incentives was part of a research trial and lasted for a 12-month period only; both patients and mental health staff were aware of this at the time of consent and therefore the eventuality of the incentives being discontinued could have been prepared for. If financial incentives are to be offered in routine clinical practice, the length of time that they would be provided for and the method of discontinuation is difficult to answer as this may vary across teams.
Incentives are effective for as long as they are offered but there is no long-term benefit. Once the incentives stop, adherence in patients who had received the incentives tends to return towards baseline levels, and there is no significant difference to those patients who remained without incentives all the time. On the other hand, there is no evidence of a substantial negative effect either. Patient and clinician experiences are largely positive, both when the incentives are offered and afterwards. However, it should be considered that a minority of patients may feel uneasy about some aspects of receiving financial incentives and about the consequences of stopping them at some stage.
In summary, one may conclude that the study provided substantial evidence justifying the use of financial incentives to improve poor adherence to antipsychotic maintenance medication in routine care. However, in the absence of any evidence of who is likely to benefit from being offered financial incentives and who is not, clinicians may want to consider carefully on a case-by-case basis who should receive them.
Recommendations for future research
This study looked at the effectiveness of offering financial incentives for LAI medication. Whether or not offering financial incentives of variable value or employing more elaborate payment schedules (e.g. with a reset component, previously shown superior to basic payment schedules) would be more effective in improving LAI medication adherence or indeed have long-term effects remains unclear and may be addressed by future research. Moreover, incentives were offered to patients of a lower socioeconomic background and future research should consider whether or not offering incentives to those with a more favourable background would be effective. In this study, we focused on an intervention with a fixed time scale of 1 year. In clinical practice, however, shorter or, in particular, longer periods may be considered, and longer-term studies should evaluate how such different use may impact on adherence, patient experiences and costs. Finally, the precise mechanisms of the effect of financial incentives are still poorly understood. The qualitative evaluation in this study suggested that the effect goes beyond the mere monetary value of the incentives. Identifying the psychological processes involved may help to utilise them more effectively to improve therapeutic relationships, with or without offering financial incentives.
In addition, many patients with psychosis may be prescribed only oral antipsychotic medication and future research could consider whether or not offering financial incentives would improve adherence in medication in this form. Finally, the use of financial incentives may be utilised to promote other health-related behaviours such as healthy lifestyle, sport activities and attendance at psychosocial intervention.
Implications for practice
-
Clinicians may consider offering financial incentives as an effective method to improve adherence to LAI medication in difficult-to-engage patients with psychotic disorders.
-
However, once financial incentives are discontinued, it is likely that patients will return to their original pattern of adherence.
-
So far the optimal duration for the administration of financial incentives is unclear.
-
Clinicians may expect patient experiences with financial incentives to be predominantly positive, although both positive and negative attitudes can coexist.
-
Careful consideration should be given to offering financial incentives to patients with substance abuse problems, and those who might have objections to the financial incentive practice.
Acknowledgements
The FIAT research team would like thank:
-
Sandra Eldridge who was involved in the study design, provided ongoing supervision and designed the statistical analysis plan.
-
All of the NHS trusts that agreed to participate in the trial: Oxford Health NHS Foundation Trust; Birmingham and Solihull Mental Health Foundation Trust; Leicestershire Partnership NHS Trust; Northamptonshire NHS Foundation Trust; Coventry and Warwickshire Partnership NHS Trust; Worcestershire Health and Care NHS Trust; Southern Health NHS Foundation; Avon and Wiltshire Mental Health Partnership NHS Trust; Surrey and Borders Partnership NHS Trust; South West London and St George’s Mental Health NHS Trust; Somerset Partnership NHS Trust; North East London NHS Foundation Trust; Barnet, Enfield and Haringey Mental Health NHS Trust; Camden and Islington NHS Foundation Trust; West London Mental Health NHS Trust; Central and North West London NHS Foundation Trust; South London and the Maudsley NHS Foundation Trust; East London NHS Foundation Trust; North Essex Partnership NHS Foundation Trust; 5 Boroughs Partnership NHS Trust; Lincolnshire Partnership NHS Trust; Lincolnshire Partnership NHS Trust; Besti Cadwaladr University Health Board Trust; Greater Manchester West Mental Health NHS Foundation Trust; Mersey Care NHS Trust; Tees, Esk & Wear Valley NHS Foundation Trust; Manchester Mental Health and Social Care Trust; Cumbria Partnership NHS Foundation Trust; and Pennine Care NHS Foundation Trust.
-
All mental health staff and patients involved with the study, and to those who agreed to participate in the interviews.
-
Members of the TSC and the DMEC for their involvement throughout. Members of the TSC were Swaran Singh (chairperson, Professor of Social and Community Psychiatry), Katy Axford (carer), Michael King (head of a clinical trials unit), Alan Simpson (Professor of Collaborative Mental Health) and Sharon O’Neill (service user representative). Members of the DMEC included Soren Holm (chairperson in Bioethics), Iris Gibson (psychiatrist) and Ranjit Lall (statistician).
Contributions of authors
Stefan Priebe was the chief investigator for the project and was involved in all aspects of the design, management and study implementation. In addition, he is the guarantor for the study.
Stephen A Bremner designed and conducted the statistical analysis plan and provided ongoing supervision.
Christoph Lauber was a principal investigator for the University of Liverpool site and was involved in study design and study management in addition to implementation in the University of Liverpool site.
Catherine Henderson was involved in the study design and conducted the cost-effectiveness analysis.
Tom Burns was the principal investigator for the University of Oxford site and was involved in study design and study management in addition to implementation in the Oxford University site.
All authors approved the final version of the manuscript.
Financial Incentives for Adherence to Treatment research team
Katherine Moran (RA) also contributed to the writing of the final report.
Trial managers were Claudia Ashton, Cornelia Geyer, Nicola O’Connell, Hana Pavlickova and Ksenija Yeeles. RAs were Kerri Bailey, Alexandra Forrest, Kirsten Barnicot, Elizabeth Highton-Williamson, Lauren Kelley, Helen Morley, Sarah Watkins and Adam Zięcik.
Permissions
The methods, results, discussion and conclusions of this report are paraphrased from sources published elsewhere. Aspects of the methods have been taken from the trial protocol which is accessible in the public domain,2 the main trial results have been taken from a paper published by Priebe and colleagues,47 results on the cost-effectiveness have been taken from a work published by Henderson and colleagues,49 the methods and results of the 6- and 24-month follow-up data from a manuscript in submission, the methods and results for patient experiences of the study have been paraphrased from a manuscript in submission, the methods and results for the clinicians’ experiences has been taken from Highton-Williamson and colleagues. 50 The discussion and conclusions have been paraphrased from all of the above sources.
All sources of adapted or reproduced material have been published as open access and reproduction has been permitted. The full trial protocol is accessible in the public domain. 2
Publications
Henderson C, Knapp M, Yeeles K, Bremner S, Eldridge S, David A, et al. Cost-effectiveness of financial incentives to promote adherence to depot antipsychotic medication: economic evaluation of a cluster-randomised controlled trial. PLOS ONE 2015;10:e0138816.
Priebe S, Burton A, Ashby D, Ashcroft R, Burns T, David A, et al. Financial incentives to improve adherence to anti-psychotic maintenance medication in non-adherent patients – a cluster randomised controlled trial (FIAT). BMC Psychiatry 2009;9:61.
Highton-Williamson E, Barnicot K, Kareem T, Priebe S. Offering financial incentives to increase adherence to antipsychotic medication: the clinician experience. J Clin Psychopharmacol 2015;35:120–7.
Priebe S, Burton A, Ashby D, Ashcroft R, Burns T, David A, et al. Financial incentives to improve adherence to anti-psychotic maintenance medication in non-adherent patients – a cluster randomised controlled trial (FIAT). [published online ahead of print 30 September 2009]. BMC Psychiatry 2009;9:61.
Moran K, Priebe S. Better quality of life in patients offered financial incentives for taking anti-psychotic medication: Linked to improved adherence or more money? [published online ahead of print 5 February 2016]. Qual Life Res 2016:1–6.
Priebe S, Bremner SA, Pavlickova H. Discontinuing financial incentives for adherence to antipsychotic depot medication: long-term outcomes of a cluster randomised controlled trial. BMJ Open 2016;6:e011673.
Pavlickova H, Bremner SA, Priebe S. The effect of financial incentives on adherence to antipsychotic depot medication: does it change over time? J Clin Psychiatry 2015;76:e1029–34.
Data sharing statement
Anonymised data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Fenton SW, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull 1997;23:637-51. http://dx.doi.org/10.1093/schbul/23.4.637.
- Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry 2002;63:892-909. http://dx.doi.org/10.4088/JCP.v63n1007.
- Nose M, Barbui C, Gray R, Tansella M. Clinical interventions for treatment of non-adherence in psychosis: meta-analysis. Br J Psychiatry 2003;183:197-206. http://dx.doi.org/10.1192/bjp.183.3.197.
- Priebe S, Sinclair J, Burton A, Marougka S, Larsen J, Firn M, et al. Acceptability of offering financial incentives to achieve medication adherence in patients with severe mental illness: a focus group study. J Med Ethics 2010;36:463-8. http://dx.doi.org/10.1136/jme.2009.035071.
- Haynes R, Haynes R, Sackett D, Taylor D. Compliance in Health Care. Baltimore, MD: John Hopkins University Press; 1979.
- Gaebel W. Towards the improvement of compliance: the significant psychoeducation and new antipsychotic drugs. Int Clin Psychopharmacol 1997;12:37-42. http://dx.doi.org/10.1097/00004850-199702001-00006.
- Appleby L. Safer services: conclusions from the report of the National Confidential Inquiry. Adv Psychiatr Treat 2000;6:5-15. http://dx.doi.org/10.1192/apt.6.1.5.
- Gilmer TP, Dolder CR, Lacro JP, Folsom DP, Lindamer L, Garcia P, et al. Adherence to treatment with antipsychotic medication and heath care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry 2004;161:692-9. http://dx.doi.org/10.1176/appi.ajp.161.4.692.
- Weiden P, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull 1995;21:419-29. http://dx.doi.org/10.1093/schbul/21.3.419.
- Knapp M, King D, Pugner K, Lapuerta P. Non-adherence to antipsychotic medication regimens: associations with resource use and costs. Br J Psychiatry 2004;184:509-16. http://dx.doi.org/10.1192/bjp.184.6.509.
- Conley R, Kelly D. Management of treatment resistence in schizophrenia. Biol Psychiatry 2001;50:898-911. http://dx.doi.org/10.1016/S0006-3223(01)01271-9.
- Dolder CR, Lacro JP, Dunn LB, Jeste DV. Antipsychotic medication adherence: is there a difference between typical and atypical agents?. Am J Psychiatry 2002;159:103-8. http://dx.doi.org/10.1176/appi.ajp.159.1.103.
- Macpherson R, Jerrom B, Hughes A. A controlled study of education about drug treatment in schizophrenia. Br J Psychiatry 1996;168:709-17. http://dx.doi.org/10.1192/bjp.168.6.709.
- Streicker SK, Amdur M, Dincin J. Educating patients about psychiatric medications: failure to enhance compliance. Psychosoc Rehabil J 1986;4:15-28. http://dx.doi.org/10.1037/h0099155.
- Azrin NH, Teichner G. Evaluation of an instructional program for improving medication compliance for chronically mentally ill out patients. Behav Res Ther 1998;36:849-61. http://dx.doi.org/10.1016/S0005-7967(98)00036-9.
- Guimon L, Eguiluz I, Bulbena A. Group pharmacotherapy in schizophrenics: attitudinal and clincial changes. Euro J Psychiatry 1993;7:147-54.
- Perkins RE, Repper JM. Compliance or informed consent. Br J Ment Health 1999;2:117-29.
- McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions – scientific review. JAMA 2002;288:2868-79. http://dx.doi.org/10.1001/jama.288.22.2868.
- Gray R, Leese M, Bindman J, Becker T, Burti L, David A, et al. Adherence therapy for people with schizophrenia European multicentre randomised controlled trial. Br J Psychiatry 2006;189:508-14. http://dx.doi.org/10.1192/bjp.bp.105.019489.
- Schizophrenia. Core Interventions in the Treatment and Management of Schizophrenia in Primary and Secondary Care (update). London: NICE; 2009.
- Parrish JM, Charlop, MH, Fenton LR. Use of a stated waiting list contingency and reward opportunity to increase appointment keeping in an outpatient pediatric psychology clinic. J Pediatr Psychol 1986;11:81-9. http://dx.doi.org/10.1093/jpepsy/11.1.81.
- Morisky DE, Malotte CK, Choi P, Davidson P, Rigler S, Sugland B, et al. A patient education program to improve adherence rates with antituberculosis drug regimens. Health Educ Q 1990;17:253-67. http://dx.doi.org/10.1177/109019819001700303.
- Pilote L, Tulsky JP, Zolopa AR, Hahn JA, Schecter GF, Moss AR. Tuberculosis prophylaxis in the homeless. Arch Intern Med 1996;156:161-5. http://dx.doi.org/10.1001/archinte.1996.00440020063008.
- Reiss ML, Bailey JS. Visiting the dentist: a behavioral community analysis of participation in a dental health screening and referral program. J Appl Behav Anal 1982;15:352-62. http://dx.doi.org/10.1901/jaba.1982.15-353.
- Reiss ML, Piotrowski WD, Bailey JS. Behavioral community psycology: encouraging low-income parents to seek dental care for their children. J Appl Behav Anal 1976;9:387-97. http://dx.doi.org/10.1901/jaba.1976.9-387.
- Smith PB, Weinman ML, Johnson TC, Wait RB. Incentives and their influence on appointment compliance in a teenage family-planning clinic. J Adolesc Health Care 1990;11:445-8. http://dx.doi.org/10.1016/0197-0070(90)90093-H.
- Steven-Simon C, O’Connor P, Bassford K. Incentives enhance postpartum compliance among adolescent prenatal patients. J Adolesc Health 1994;15:396-9. http://dx.doi.org/10.1016/1054-139X(94)90263-1.
- Higgins ST, Budney AJ, Bickle WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioural treatment of cocaine dependence. Arch Gen Psychiatry 1994;51:568-76. http://dx.doi.org/10.1001/archpsyc.1994.03950070060011.
- Shepard DS, Foster SB, Statson WB, Solomon HS, McArdle PJ, Gallagher S. Cost Effectiveness of Interventions to Improve Compliance with Anti-hypertensive Therapy. Washington, DC: National Conference of High Blood Pressure Control; 1979.
- Yorkley JM, Glenwick DS. Increasing the immunization of preschool children; an evaluation of applied community interventions. J Appl Behav Anal 1984;17:313-25. http://dx.doi.org/10.1901/jaba.1984.17-313.
- Jeffery RW, Wing RR, Thorson C, Burton LR, Raether C, Harvey J, et al. Strengthening behavioural interventions for weight loss: a randomized trial of food provision and monetary incentives. J Consult Clin Psychol 1993;61:1038-45. http://dx.doi.org/10.1037/0022-006X.61.6.1038.
- Giuffrida A, Torgerson DJ. Should we pay the patient? Review of financial incentives to enhance patient compliance. Br Med J 1997;315:703-7. http://dx.doi.org/10.1136/bmj.315.7110.703.
- Carey KB, Carey MP. Enhancing the treatment attendance of mentally ill chemical abusers. J Behav Ther Exp Psychiatry 1990;21:205-9. http://dx.doi.org/10.1016/0005-7916(90)90008-9.
- Post EP, Cruz M, Harman J. Incentive payments for attendance at appointments for depression among low-income African Americans. Psychiatr Serv 2006;57:414-16. http://dx.doi.org/10.1176/appi.ps.57.3.414.
- Roll JM, Higgins ST, Steingard S, McGinley M. Use of monetary reinforcemnet to reduce the cigarette smoking of persons with schizophrenia. Exp Clin Psychopharmacol 1998;6:157-61. http://dx.doi.org/10.1037/1064-1297.6.2.157.
- Tidey JW. Using incentives to reduce substance use and other health risk behaviours among people with serious mental illness. Prev Med 2012;55:S54-60. http://dx.doi.org/10.1016/j.ypmed.2011.11.010.
- Burton A, Marougka S, Priebe S. Do financial incentives increase treatment adherence in people with severe mental illness? A systematic review. Epidemiol Psichiatr Soc 2010;19:233-42.
- Claassen D, Fakhoury WK, Ford R, Priebe S. Money for medication: financial incentives to improve medication adherence in assertive outreach. Psychiatric Bulletin 2007;31:4-7. http://dx.doi.org/10.1192/pb.31.1.4.
- Staring AB, Mulder CL, Priebe S. Financial incentives to improve adherence to medication in five patients with schizophrenia in the Netherlands. Psychopharmacol Bull 2010;43:5-10.
- Gneezy U, Meier S, Rey-Biel R. When and why incentives (don’t) work to modify behavior. J Econ Perspect 2011;25:1-21. http://dx.doi.org/10.1257/jep.25.4.191.
- Dolan P, Galizzi MM. Like ripples on a pond: behavioral spillovers and their implications for research and policy. J Econ Psychol 2015;47:1-16. http://dx.doi.org/10.1016/j.joep.2014.12.003.
- Shaw J. Is it acceptable for people to be paid to adhere to medication? No. BMJ 2007;335. http://dx.doi.org/10.1136/bmj.39286.422639.BE.
- Burns T. Is it acceptable for people to be paid to adhere to medication? Yes. BMJ 2007;335. http://dx.doi.org/10.1136/bmj.39286.399514.BE.
- Ashcroft RE. Personal financial incentives in health promotion: where do they fit in an ethic of autonomy?. Health Expect 2011;14:191-200. http://dx.doi.org/10.1111/j.1369-7625.2011.00664.x.
- Promberger M, Brown RC, Ashcroft RE, Marteau TM. Acceptability of financial incentives to improve health outcomes in UK and US samples. J Med Ethics 2011;37:682-7. http://dx.doi.org/10.1136/jme.2010.039347.
- Claassen D. Financial incentives for antipsychotic depot medication: ethical issues. J Med Ethics 2007;33:189-93. http://dx.doi.org/10.1136/jme.2006.016188.
- Priebe S, Yeeles K, Bremner S, Lauber C, Eldridge S, Ashby D, et al. Effectiveness of financial incentives to improve adherence to maintenance treatment with antipsychotics: cluster randomised controlled trial. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f5847.
- Priebe S, Burton A, Ashby D, Ashcroft R, Burns T, David A, et al. Financial incentives to improve adherence to anti-psychotic maintenance medication in non-adherent patients – a cluster randomised controlled trial (FIAT). BMC Psychiatry 2009;9. http://dx.doi.org/10.1186/1471-244X-9-61.
- Henderson C, Knapp M, Yeeles K, Bremner S, Eldridge S, David A, et al. Cost-effectiveness of financial incentives to promote adherence to depot antipsychotic medication: economic evaluation of a cluster-randomised controlled trial. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0138816.
- Highton-Williamson E, Barnicot K, Kareem T, Priebe S. Offering financial incentives to increase adherence to antipsychotic medication: the clinician experience. J Clin Psychopharmacol 2015;35. http://dx.doi.org/10.1097/JCP.0000000000000276.
- International Classification of Diseases. Geneva: WHO; 2010.
- Glover GR, Leese M, McCrone P. More severe mental illness is more concentrated in deprived areas. Br J Psychiatry 1999;175:544-8. http://dx.doi.org/10.1192/bjp.175.6.544.
- Ryan P. Random allocation of treatments in blocks. Stata Tech Bull 1998;41:43-6.
- Research Governance Framework for Health and Social Care. London: Department of Health; 2005.
- Guy W. ECDEU Assessment Manuel for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976.
- Fieve RR, Goodnick PJ, Peselow ED, Barouche F, Schlegel A. Pattern analysis of antidepressant response to fluoxetine. J Clin Psychiatr 1986;47:560-2.
- Sato TL, Turnbull C, Davidson JRT, Madakasira S. Depressive illness and placebo response. Int J Psychiatry Med 1984;14:171-9. http://dx.doi.org/10.2190/KQMK-9TC4-6FVA-4JW4.
- Guelfi JD. Clinical research in psychopharmacology: new standards for drug development. An application to antidepressants. Psychiat Psychobiol 1990;5:289-94.
- Priebe S, McCabe R, Bullenkamp J. Structured patient-clinicial communication and one-year outcome in community mental health care: a cluster randomised controlled trial. Br J Psychiatry 2007;191:420-6. http://dx.doi.org/10.1192/bjp.bp.107.036939.
- Priebe S, Golden E, McCabe R, Reininghaus U. Patient-reported outcome data generated in a clinical intervention in community mental health care – psychometric properties. BMC Psychiatry 2012;12. http://dx.doi.org/10.1186/1471-244X-12-113.
- Priebe S, Huxley P, Knight S, Evans S. Application and results of the Manchester Short Assessment of Quality of Life (MANSA). Int J Soc Psychiatry 1999;45:7-12. http://dx.doi.org/10.1177/002076409904500102.
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale for schizophrenia. Schizophr Bull 1987;13:261-76. http://dx.doi.org/10.1093/schbul/13.2.261.
- Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann 1982;5:233-7. http://dx.doi.org/10.1016/0149-7189(82)90074-X.
- Curtis L. Unit Costs for Health and Social Care. Canterbury: Personal Social Services Research Unit; 2011.
- NHS Reference Costs 2010–2011. London: Department of Health; 2012.
- Byford S, Sharac J, Lloyd-Evans B, Gilburt H, Osborn DPJ, Leese M, et al. Alternatives to standard acute in-patient care in England: readmissions, service use and cost after discharge. Br J Psychiatry 2010;197:S20-25. http://dx.doi.org/10.1192/bjp.bp.110.081067.
- Slade M, Byford S, Barrett B, Lloyd-Evans B, Gilburt H, Osborn DP, et al. Alternatives to standard acute in-patient care in England: short-term clinical outcomes and cost-effectiveness. Br J Psychiatry 2010;53:S14-19. http://dx.doi.org/10.1192/bjp.bp.110.081059.
- Prescription Cost Analysis – England, 2010. London: Health and Social Care Information Centre; 2011.
- Glick H. Economic Evaluation in Clinical Trials. Oxford, NY: Oxford University Press; 2007.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2009.
Appendix 1 Case report form: end of intervention
Appendix 2 Clinician interview
Appendix 3 Patient interview: end of intervention
Interview question guide
Introductory remarks
-
Hello. I would like to start by thanking you for coming and taking part in this research on financial incentives. We really appreciate your involvement. Just to let you know, there are no right or wrong answers to any of these questions and you do not have to answer any questions that you do not wish to answer and you can leave the interview at any time you want.
Establishing rapport
-
First experiences with mental health: So, to start off I would just like to ask you a few questions about your first experiences with mental health services. When did you first come into contact with mental health services? What effect did this have on you at the time, for instance, did it affect your day to day life?
-
Family and peer support: Okay, so can I ask you what effect this had on you in terms of your family and friends or your children? Were they supportive at the time? Did you feel excluded or discriminated in your local community or did you get support at the time?
Health and identity
-
Health: Now, just moving on to some broader questions, I just want to talk to you about your health in general. How healthy do you feel at the moment? Both in terms of your physical and psychological health. Do you feel that you have much control over both your physical and psychological health? What things in your life do you think would improve your health in general? Do you think health is something that is easy to change in general or is it something that you can’t do much about? (Maybe some probing is needed here like “Could you explain that a bit further?”).
-
Identity: Okay, so again, I’d just like to ask you a really broad question. How would you define yourself? If you had to describe yourself to someone else what would be the most important things to mention? In other words, what do you see as the most important thing in defining who you are?
Depot and medication
-
Medication and health care experiences: Okay, so now I just want to talk to you about your health care experiences so far. I’m really interested to know about how you feel about the service that you receive. Do you have a good relationship with the team and your care co-ordinator? What are your experiences with taking medication? What are the psychological and physical effects of taking the medication, if any at all?
-
Experience with FIAT: Okay, that’s great. So now I’d like to talk to you about being part of the FIAT study. What were your initial thoughts when you were asked to take part in the study? Was it something you wanted to do? Why did you want to/or not want to? Did you talk to friends or family about it and what was their opinion in you taking part? Did getting the money when you were given the medication affect your relationship with your care co-ordinator, or not at all? If so, in what way did it affect the relationship? Did your experience with receiving £15 for your depot change over the year that you participated in the study? If so, how so?
-
Financial issues: During the trial, did you feel that you wanted or needed more money than the £15 that was given? In general, what did you use the money for? Did you feel at any point during the 12 months that you were becoming dependent or reliant on getting the money from your care co-ordinator? How have you coped since the 12 months have ended and you haven’t received any more money? Has this been something that has been easy or difficult to adapt to?
-
Post-intervention experiences: What happened in the aftermath of the study, when you didn’t receive £15 for getting your depot? Was this something that was difficult or easy to adapt to? Can you explain why? Are you still receiving your medication? Do you feel that you have benefited positively or negatively from being part of the study? Can you explain why? Has being part of the study affected how much control you feel that you have now over your health and health care, or not at all. (That last question is quite a leading question, so might be best left out)
-
Ethical issues: Did you feel in any way that you had to take part in this study or that you had to continue with the study when you did not want to? If so, can you explain a bit further for me? Speaking in general, do you think there are any ethical issues with conducting a study like this, where patients receive money when they get their depot medication, if any at all? Can you explain a bit further?
Wrapping-up questions
-
Quality of life: Okay, so we are now coming towards the end of the interview. I just have one or two more questions for you. In general, how would you describe your quality of life to me, as it is now, for example in terms of stress, work, family and taking your medication?
-
Future plans: What are your plans for the future? Has being part of the trial affected your plans for the future, if at all? Do you intend to keep taking your medication, or is it something that you plan to stop in the future?
Closing remarks
-
Well, I have come to the end of my questions. Is there anything that you have said that you would like to withdraw or is there anything that you would like to add further? Anything that might be important to you, for example, that I might have missed? Okay, well, once again, thank you for taking part in the study. If you have any questions or queries about taking part please talk to your care co-ordinator and I can be in touch. We really appreciate your time and participation.
End of interview.
Appendix 4 Patient interview: 24-month follow-up
Interview question guide
Introductory remarks
Thank you: Hello. I would like to start by thanking you for coming and taking part in this research on financial incentives. We really appreciate your involvement.
Financial incentives interview: I would now like to ask you about your experience with financial incentives.
Recording and confidentiality: There are no right or wrong answers to any of these questions and you do not have to answer any questions that you do not wish to answer and you can leave the interview at any time you want.
Also, if you don’t mind, I will record the interview, but all you say here will remain fully confidential.
Experience with FIAT
How the incentives influenced adherence to treatment?
-
About a year and a half ago you were getting £15 every time you had your depot. How did you feel about that?
-
And how do you feel now the money has stopped?
-
Before you started receiving the money, how did you feel about taking your medication?
-
Do you think receiving money influenced you taking your medication?
-
Do you think receiving the money affected your attitude to having your depot?
-
Now that the money has stopped, has that had any influence on you taking your medication?
How the possibility improved adherence affected outcomes?
-
Did receiving money for taking your medication have an effect on your life in any way?
-
Now that the money has stopped, have any of those things you described changed?
-
Did receiving money for medication affect your mental health?
-
Now that the money has stopped, have any of those things you described changed?
-
Did receiving money for medication affect how often you went into hospital?
-
Now that the money has stopped, have any of those things you described changed?
-
Did receiving money for medication affect your relationship with your family or friends?
-
Now that the money has stopped, have any of those things you described changed?
Effects on their attitude to treatment
-
Did receiving money for taking your medication affect your relationship with your care co-ordinator or clinician, or not at all?
-
Now that the money has stopped, have any of those things you described changed?
-
Did receiving money for taking your medication affect your attitude to mental health services?
-
Now that the money has stopped, have any of those things you described changed?
How patients experienced the long-term practice and its role in the overall treatment received in community mental health care?
-
What is your opinion about receiving financial incentives to take medication?
-
Did your experience with receiving £15 for your depot change over the year that you participated in the study?
-
In theory, do you think that paying patients to take their medication should happen again in your team?
-
Should it be rolled out nationwide?
-
Should it just be for patients who miss a lot of depot appointments – or should it be for all patients?
Closing remarks
Well, I have come to the end of my questions. Is there anything that you have said that you would like to withdraw or is there anything that you would like to add further?
Anything that might be important to you, for example, that I might have missed? Thank you for taking part in the study. If you have any questions or queries about taking part please talk to your care co-ordinator and I can be in touch.
We really appreciate your time and participation.
End of interview.
Appendix 5 End-of-study information
Dear FIAT research participant,
A few years ago, you took part in a research project where you were offered £15 for your depot injection, called the FIAT research project (‘financial incentives for adherence to treatment’). Thank you again for taking part; now the research has finished, we thought we would let you know what we found out.
The research was carried out with patients who missed a lot of their depot appointments. We wanted to see whether paying people a small amount for each depot injection could reduce the number of missed depot appointments. Patients in some mental health teams across the country were given £15 every time they had their depot, for one year. Patients in other mental health teams continued to receive their depot as normal without being paid. We found that the £15 helped patients turn up on time for their depot appointments, compared to patients not receiving the money. However, when the money stopped after one year, we found that people started to miss their depot appointments again.
We also invited patients to take part in an interview to talk about how they felt about receiving the money. Many people felt that the money helped them in many ways such as being a reminder for their depot appointment, or they could use the money to help out with the costs of everyday living. Some people talked about how felt the money was a reward for them having their depot on time and used the money to treat themselves to something. On the other hand, some people did not feel comfortable with receiving money for their medication. Once the money had stopped, most people felt that this did not affect them but some did feel that their attendance to depot appointments and their mental health had got worse.
We also interviewed mental health staff involved in the study to find out what they thought about using the £15. Many thought that using money was a good idea and felt that the money helped patients turn up to more depots, and they noticed that their patient’s mental health and their relationship with them had improved also. On the other hand, some staff felt that the money was a bad idea because it didn’t work, or that their relationship with patients became all about the money. Some staff believed that patients were spending the money on drugs/alcohol which made their mental health worse and working with them more difficult.
Overall, offering money did help people turn up to depot appointments more often but this does not last once the money stops. Many patients or staff felt that the money was a good idea but some felt uncomfortable with the money or thought that it was not a good idea. Offering small amounts of money to help people turn up on time for their depot injections may not work for everyone.
If you have any more questions about the research, please call XXXXXXXX and ask to speak to XXXXXXX.
We hope that you enjoyed being part of the research project. We very much enjoyed having you on board and are grateful for your participation!
Best wishes
The FIAT team
List of abbreviations
- AOT
- assertive outreach team
- CGI
- Clinical Global Impression
- CI
- confidence interval
- CMHT
- Community Mental Health Team
- CRF
- case report form
- DMEC
- Data Monitoring and Ethics Committee
- DNA
- did not attend
- FIAT
- Financial Incentives for Adherence to Treatment
- GP
- general practitioner
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- LAI
- long-term antipsychotic injectable
- MINI
- Mini Mental State Examination
- NRES
- National Research Ethics Service
- OR
- odds ratio
- RA
- research assistant
- SD
- standard deviation
- SE
- standard error
- TSC
- Trial Steering Committee