Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/14/19. The contractual start date was in October 2009. The draft report began editorial review in October 2015 and was accepted for publication in April 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Martin Tickle reports provision of free toothpaste and toothbrushes from Colgate-Palmolive for the trial. Seamus Killough was chairperson of the Northern Ireland Council of the British Dental Association throughout this trial.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Tickle et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Dental caries is the most common disease of childhood. Globally, the prevalence of dental caries varies in different parts of the world; it also varies within countries or regions. The World Health Organization estimates that the disease affects 60–90% of school children and the majority of adults. 1,2
Serial cross-sectional surveys in UK have shown that dental caries in the primary (milk) teeth of 5-year-old children is falling but, compared with other diseases of childhood, prevalence of caries remains high. The Child Dental Health Survey 2013. Report 2: Dental Disease and Damage in Children England, Wales and Northern Ireland. 3 reported that 31% of children living in England, Wales or Northern Ireland had ‘obvious decay experience’. In Northern Ireland, where this trial was conducted, 40% of 5-year-olds had obvious decay experience in their primary teeth in 2013. The severity of dental caries in the population is assessed using the decayed, missing, filled teeth in primary dentition (dmft – by convention denoted in lower case for the primary dentition) index, which provides the mean number of teeth per child affected by dental caries (decayed, missing because of extraction or filled). In 2013, the mean number of primary teeth with obvious decay experience (dmft) in 5-year-old children living in England, Wales and Northern Ireland was 0.9 per child and, for children with the disease, the mean number of teeth affected was 3.0 per child. 3
The disease has a significant impact on the lives of children and their families, with pain and extraction being common sequelae. 4 Very young children often have difficulty complying with dental treatment, making the management of their care difficult; consequently, young children with tooth decay often have a general anaesthetic (GA) to have carious teeth extracted. Tooth decay was the most common reason for hospital admissions in children aged 5–9 years in 2012–13. 5 Owing to its high prevalence, the management of this disease is very costly to the NHS, even though it is largely preventable.
The literature demonstrates a strong and consistent relationship between caries in 5-year-old children and deprivation. 6 As disease levels have fallen in the population over the last 40 years, the disease has become increasingly concentrated in the most disadvantaged communities. Unfortunately, there is an inverse relationship between deprivation and utilisation of dental services; children living in the most disadvantaged communities, with greatest need, are less likely to attend the dentist and complete a course of treatment than children living in more affluent areas. 7
Caries is a chronic, non-communicable disease determined by the social, cultural and economic environment a child grows up in. This environment influences behaviours such as diet, toothbrushing and dental visiting, which affect a child’s risk of developing the disease. National policies5,8 have taken an integrated approach to tackling the disease with complementary interventions delivered at a population (such as water fluoridation), community (such as school-based prevention programmes) and individual level (through preventative care provided in dental practices).
This trial estimates the costs and effects of a composite preventative intervention delivered in general dental practice to children who were aged 2–3 years at recruitment. It focuses on primary prevention: to prevent caries (cavitation of teeth) starting and its damaging sequelae from arising. Targeting very young children fits with the desire to instil healthy lifestyle behaviours from a young age. The trial is complementary to a number of other parallel National Institute for Health Research (NIHR)-funded studies commissioned to evaluate different caries preventative and treatment interventions delivered to children in different settings. These include an evaluation of the cost-effectiveness of water fluoridation in preventing caries in children,9 a comparison of the effectiveness of fluoride varnish and fissure sealants to prevent caries in the school setting10 and the Filling Children’s Teeth: Indicated Or Not? (FiCTION) trial,11 which compares different treatment regimens for managing carious primary teeth.
The setting for the trial is NHS general dental practices, where > 90% of NHS dental resources are consumed. Internationally, there is a consensus that dental services need to be designed to primarily focus on prevention of dental disease rather than its treatment. 12 In the UK, new NHS dental contracts that are designed to support and incentivise dentists to concentrate on prevention and quality are planned in England, Wales and Northern Ireland. In England, Delivering Better Oral Health: An Evidence-Based Toolkit for Prevention (DBOH)13 has been distributed to all NHS dental practices. First published in 2007 and now in its third edition, this national guidance aims to support dental practices to provide high-quality care and advice to prevent dental disease. DBOH provides advice about preventing caries in young children. However, most of the evidence for the fluoride-based interventions recommended is based on explanatory trials rather than pragmatic trials and, as a result, there is little understanding of the effectiveness of the interventions recommended by DBOH in ‘real-life’ NHS dental practices. A pragmatic evaluation of the prevention interventions, taking into account adherence of general dental practitioners (GDPs) and the target population to the interventions, is required. There is also very little information on the costs associated with preventative interventions delivered in general dental practice, which is important given the current cost pressures on the NHS. 14 Delivering a national preventative programme through general dental practice is potentially a costly method of improving dental health because of the high salary costs involved. 15 This trial therefore seeks to inform policy and clinical practice on the costs and effects of a composite preventative intervention provided to young children that is reflected in national guidelines distributed to all dental practices in England.
Scientific background
Epidemiology of caries in young children
Although dental caries is a preventable disease, it is a persistent international public health problem. A 2009 review16 of the available epidemiological data on caries from a number of countries suggested that the prevalence of dental caries was increasing and that the increases are concentrated in lower socioeconomic groups, new immigrants and children.
In the UK, national child dental health surveys are undertaken every 10 years and have been carried out since 1973, although Northern Ireland only started participating in the programme from 1983 and Scotland did not participate in the latest survey published in 2013. The Children’s Dental Health in the UK National Survey 200317 reported that 43% of 5-year-olds had tooth decay. The dental examination undertaken in the 2003 survey was an assessment of the ‘obvious decay experience’ of children’s teeth, defined as teeth that, at the time of the examination, had decay into dentine (including teeth that were filled in the past but which needed further treatment), were filled or were missing because of decay. Prevalence varied from 41% in England to 52% in Wales and 61% in Northern Ireland (data for Scotland were not reported). The 2003 survey showed that little had changed since the 1993 National Survey,18 which reported a prevalence of 45%.
The Children’s Dental Health Survey 2013. Report 2: Dental Disease and Damage in Children England, Wales and Northern Ireland3 results were released in 2015. The diagnostic protocol for the survey was changed from that of 2003 to enable dental caries to be measured across a range of detection thresholds. The rationale for this change was to reflect the way in which the detection and management of tooth decay has evolved towards more preventive approaches to care, rather than just providing treatment for disease.
There were also changes in the consent process; in previous surveys, negative (opt-out) parental consent was obtained for the dental examination of children. However, in 2006 the Department of Health in England produced guidance that required positive written consent from parents for the dental examination of young children participating in epidemiological surveys. As a result, the consent procedures used in the 2013 survey for dental examinations of 5-year-olds required written positive (opt-in) consent to be collected from parents. Children could also opt out on the day of the examination. Dental caries is closely associated with deprivation,6 but providing consent for school-based surveys is also associated with deprivation19 and, therefore, is likely to result in an under-representation of those children with the most severe dental caries. The 2013 survey reports ‘obvious decay experience’ which includes untreated caries that has progressed into dentine and caries that has previously been subject to restorative treatment (fillings) or tooth extraction. This categorisation includes both cavitated and ‘visual’ decay into dentine, the latter term describing caries lesions in which dentinal decay can be visualised through the enamel but without frank cavitation.
In the 2003 survey, using an opt-out approach, 88% of Northern Irish children selected received a dental examination. However, even with the opt-in approach used in the 2013 survey 79% of Northern Irish children selected received a dental examination. The changes to the diagnostic protocol and consent procedures meant that caries data for 5-year-olds reported in the 2013 survey were not directly comparable to those of previous surveys and, consequently, trends in caries among 5-year-old children were not presented in the 2013 survey (i.e. cross-sectional data for 5-year-olds were presented in isolation). Nevertheless, there is reason to believe that caries levels in 5-year-olds in Northern Ireland fell substantially between the 2003 and 2013 surveys. In 2003, in Northern Ireland, 61% of 5-year-olds had obvious decay experience. In 2013, with slightly revised diagnostic criteria and a changed consent process, 40% of 5-year-olds had obvious decay experience in their primary teeth.
Since the early 1980s, the NHS in England and Wales has funded a programme of local child dental health surveys complementary to the national child dental health surveys. These surveys of 5-year-old children are undertaken more frequently than the national surveys and involve much larger numbers of participants, which allows for statistics to be reported at lower levels of geography. Data from NHS surveys showed a significant reduction in caries prevalence in 5-year-olds from 2005/6 to 2007/8. 20 However, this reduction could be attributed to a change in the parental consent procedure between the survey in 2005/6, that used negative (opt-out) consent, and a requirement for positive (opt-in) consent in the 2007/8 survey. If consent was influenced by socioeconomic status, poor children with higher levels of disease might have been less likely to participate in the survey than their more affluent peers. 19 A further survey of 5-year-olds was published in 201321 using the same opt-in consent process. Comparing the results for England, prevalence fell from 30.9% in 2007/8 to 27.9% in 2011/12. However, we cannot assume that disease risk of children for whom consent was not provided was the same in both surveys. Nevertheless, these data suggest that disease levels across the UK are falling.
The UK has an enviable library of epidemiological data sets describing trends in prevalence and severity of dental caries in various population subgroups over the last 40 years. All of these data sets are cross-sectional and there are few prospective studies available to provide an understanding of how the disease behaves longitudinally. A prospective cohort study,22 published in 2008, followed 739 children aged 3–6 years attending 50 dental practices in the north-west of England over a 3-year period. This study demonstrated a stark difference between children who present with and without the disease at their first visit to the dentist. Over the study period, 25% of children who were initially caries free developed caries active; by contrast, 72% of those with the disease at their initial visit developed further cavities. No matter what age a child developed the disease, it progressed at the same rapid rate. An important finding of this study was that more ‘cases’ (children with caries active) arose from the initially caries-free population (n = 155, 21% of the total study population) than from those who presented with the disease at their first visit to the dentist (n = 118, 16% of the total study population). The study also reported that restoration (filling) of primary teeth made no difference to the trajectory of the disease. This is an important finding, as it points to the failure of secondary prevention (restoration) and demonstrates the importance of primary prevention in general dental practice.
Relationship with deprivation
A strong association between caries active in young children and deprivation has been reported consistently over the last 20 years in different countries. Inequalities in caries prevalence and experience have been demonstrated by poverty, race and ethnicity. In the USA, the National Center for Health Statistics reported findings of the National Health and Nutrition Examination Survey, 2009–2010, in 2012. 23 Among children aged 3–5 years, the prevalence of untreated caries was significantly higher in non-Hispanic black children (19.3%) and in Hispanic children (19.8%) than in non-Hispanic white children (11.3%). In this same age group, the percentage of untreated dental caries was significantly higher in children living at or below the federal poverty level (25.1%) than in children living above the poverty level (10.5%).
In the UK, both geographical and social gradients in caries prevalence and experience have been consistently observed. 24 The NHS 2011/12 survey21 reported a prevalence of 21% in the south-east of England (excluding London), compared with a prevalence of 35% in the north-west of England. The same survey reported a strong correlation between caries and deprivation score at lower tier local authority level. The Children’s Dental Health Survey 2013. Report 2: Dental Disease and Damage in Children England, Wales and Northern Ireland3 reported that 21% of 5-year-olds who were eligible for free school meals had severe or extensive tooth decay, compared with 11% of children who were not eligible for free school meals. In Northern Ireland, the percentage of 5-year-old children with severe or extensive dental decay showed a marked social gradient (Table 1): 38% of children in the most deprived quintile of deprivation [categorised using the 2010 Northern Ireland Multiple Deprivation Measure (MDM) quintiles25] were affected, compared with 10% in the least deprived quintile.
| Quintile | Decay prevalence (%) |
|---|---|
| 1 (most deprived) | 38 |
| 2 | 25 |
| 3 | 14 |
| 4 | 14 |
| 5 (least deprived) | 10 |
Impact of the disease
Once young children develop caries in their primary teeth, pain and extraction are common outcomes. A prospective cohort study conducted in the UK followed a population of 3- to 6-year-olds over a 3-year period. 4 Approximately one in five children with caries active presented with dental pain at an unscheduled visit at the dentist, compared with only 1 in 100 children who were caries free. In children with caries active, 1 in 10 had a primary molar tooth extracted each year. Dental extraction is the most common reason why young children have a GA. In 2013–14, in England, approximately 46,500 children and adolescents under 19 years of age were admitted to hospital with a primary diagnosis of dental caries. Most admissions were in the 5- to 9-year-old age group, among which group admissions showed a 14% increase between 2010–11 and 2013–14, from 22,574 to 25,812. The second highest number of admissions in 2013–14 were for tonsillitis, with approximately 11,500 cases, making dental caries the most common reason for children aged between 5 and 9 years being admitted to hospital. 27
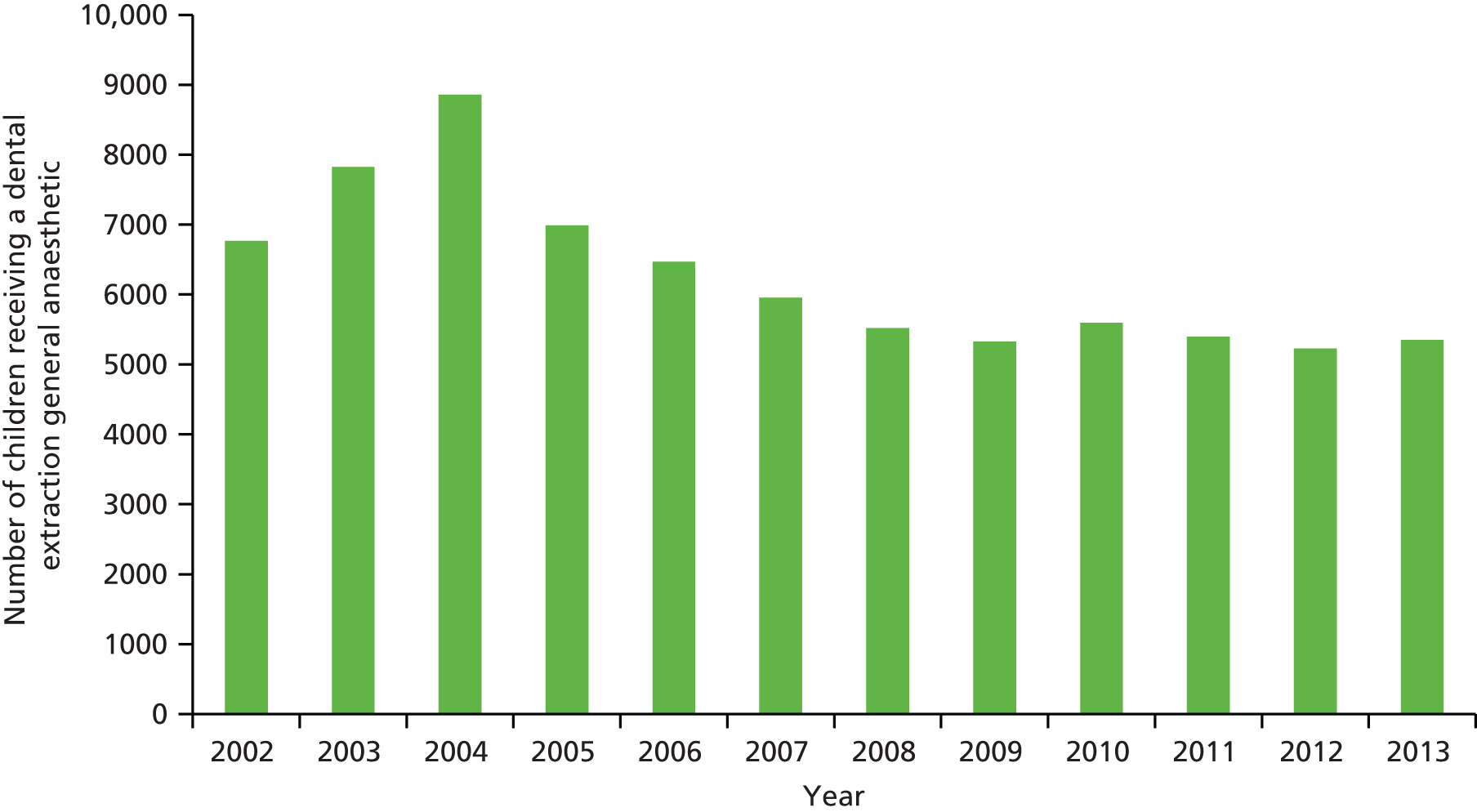
In Northern Ireland, the significant reduction in dental disease identified between the 2003 and 2013 national surveys has been reflected in a reduction in the number of GAs for dental extractions. Data on the annual number of primary teeth extracted under GA in Northern Ireland provided by the Business Services Organisation of Northern Ireland are presented in Figure 1. From a peak of 33,686 teeth extracted under GA in 2004, the number fell to 22,056 in 2013. Figure 2 shows a similar decline in the number of children who had dental extractions under GA in Northern Ireland, from a peak of 8856 in 2004 to 5351 in 2013.
FIGURE 1.
The number of primary teeth extracted under GA in Northern Ireland 2002–13 (provided by Business Services Organisation of Northern Ireland, Business Services Organisation, Belfast, UK).
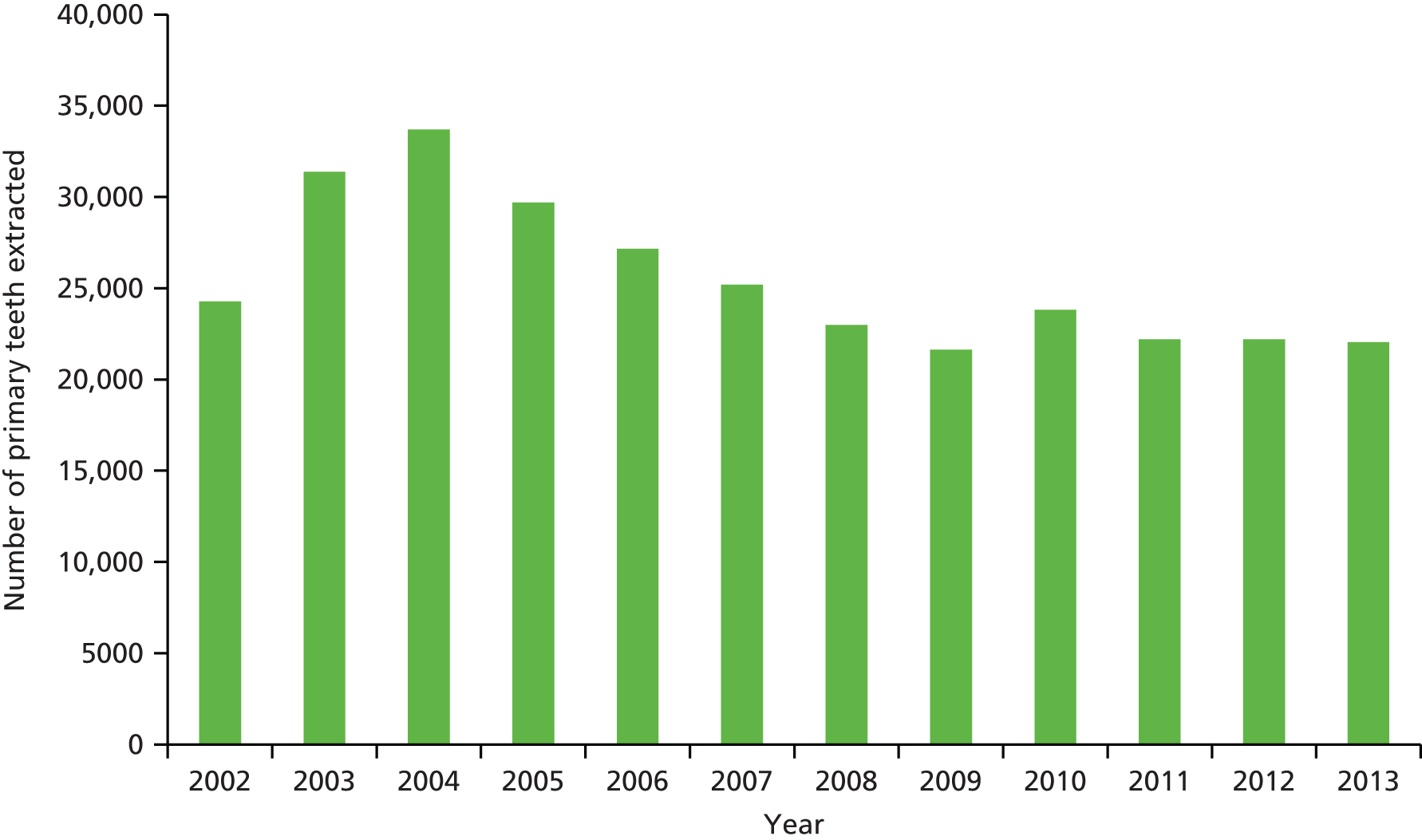
FIGURE 2.
Total number of children receiving a dental extraction GA in Northern Ireland 2002–13 (provided by Business Services Organisation of Northern Ireland, Business Services Organisation, Belfast, UK).
Extractions performed under GA have a negative impact on young children and their families,28 and there is a strong association between a history of dental extraction at a young age and the development of dental anxiety,29 which can continue to affect individuals in later life. 30
Children who develop caries active in early childhood are likely to have a high risk of the disease into adolescence. 31 In the Children’s Dental Health Survey 2013. Report 2: Dental Disease and Damage in Children England, Wales and Northern Ireland,3 15-year-old children were asked about the impact of oral disease on their daily lives. More than half (54%) of 15-year-olds who had severe or extensive decay had at least one problem resulting from oral health that affected their daily lives during the previous 3 months, compared with 44% of those with no severe or extensive decay. The problems most likely to be reported were embarrassment when smiling or laughing and difficulties with eating and cleaning their teeth.
Parents of 15-year-olds participating in the survey were asked whether or not the health of their child’s teeth and mouth had affected their family life during the past 6 months. Just over one-third (35%) of parents reported negative impacts resulting from oral health problems. The most frequent impacts were a parent having to take time off work (23%); the child needing more attention (15%); the parent feeling stressed or anxious (13%); and the parent feeling guilty (11%). Some 37% of parents of 15-year-olds with severe or extensive decay experience reported that their child had received a GA in the past as part of dental treatment, compared with 8% of parents whose children did not have severe disease.
Dental caries in young children also has a significant impact on NHS costs. It was estimated in 2014 that the NHS spends about £3.4B per year in England on primary dental care services. 32 The Health & Social Care Information Centre33 reported that in England 29.9 million patients were seen in the 24-month period ending June 2014, which included 7.9 million (26.4%) children. The same report identified that children received 10.6 million courses of treatment in 2013/14, just over one-quarter of the total number of courses of treatment provided for all patients (39.7 million). This activity consumes roughly one-quarter of the NHS dental budget, and is devoted primarily to the management of dental caries. These costs do not include GAs, as they are provided in hospital at a cost of about £700–800 per case.
Different types of NHS contracts with different in-built financial incentives are in place in the four home nations. In England and Wales a cost and volume contract, paying contract holders to deliver an agreed amount of activity for an agreed price using units of dental activity (UDAs) as the contract currency, has been in place since 2006. In both Northern Ireland and Scotland, patients are registered with a dentist and the majority of fees paid to dentists for the care of children is through capitation payments. In Scotland, the average cost to the general dental service (GDS) of treating a child during 2013/14 was £66. The total GDS spend on child dental care for the year ending March 2014 was over £68M; this total was made up of approximately 40% for items of service and 60% for capitation. 34
Table 2 shows that in 2013, in Northern Ireland, where the trial was conducted, 73.2% of children were registered with a GDS dentist. Registration has been increasing annually since 2010. Table 2 shows that there is a very steep increase in registration between the age groups of 0–2 years (29.3% registered) and 3–5 years (74.8% registered).
| Year | Age band (years) (%) | Total (%) | ||||
|---|---|---|---|---|---|---|
| 0–2 | 3–5 | 6–8 | 9–12 | 13–17 | ||
| 2010 | 22.2 | 64.4 | 73.8 | 72.5 | 70.2 | 60.6 |
| 2011 | 31.8 | 69.0 | 79.2 | 79.3 | 77.1 | 69.3 |
| 2012 | 28.1 | 74.5 | 84.0 | 84.5 | 83.3 | 72.2 |
| 2013 | 29.3 | 74.8 | 84.6 | 85.1 | 83.3 | 73.2 |
Table 3 summarises the costs of NHS dental care provided by the GDS in 2013/14 and 2014/15. The total costs in 2014/15 were £17.3M, with approximately 75% of costs made up of capitation payments.
| Cost | Time period | |
|---|---|---|
| 2013/14 | 2014/15 | |
| Item of service fees (£M) | 6.4 | 4.3 |
| Capitation fees (£M) | 13.0 | 13.0 |
| Totala | 19.4 | 17.3 |
Evidence base for interventions to prevent caries
Technologies designed to prevent caries fall into three broad categories designed to:
-
affect the dynamic balance of demineralisation and remineralisation at the tooth surface to favour remineralisation primarily through the use of fluoride
-
decrease the volume, and frequency of consumption, of refined carbohydrates
-
seal the surface of the tooth to insulate it from acid attack.
Evidence base for fluoride interventions
The large reduction in population levels of caries witnessed over the last 40 years has been attributed largely to increased exposure to fluoride. 35 Fluoride can be delivered using a number of different vehicles.
Water fluoridation
Fluoride occurs naturally in all domestic water supplies, usually at very low levels, but in many parts of the world it occurs naturally at concentrations high enough to prevent caries. The caries prevention effects of fluoride were discovered by comparing caries rates in areas where the water supply naturally contains high levels of fluoride with caries rates in areas with low levels of fluoride in the water. 36 A systematic review of water fluoridation in 200037 estimated that a 15% absolute difference in the proportion of caries-free children could be expected between fluoridated and non-fluoridated populations. It has been estimated that this equates to a difference of around 40% in caries increment. 38 More recently, a Cochrane review of water fluoridation has been published. 39 The included studies suggest that water fluoridation results in a 35% reduction in decayed, missing or filled primary teeth and a 26% reduction in decayed, missing or filled permanent teeth. However, the review team queried the applicability of these findings to current populations, as the majority of the studies included in the review were conducted before fluoride toothpastes and other preventative measures were widely introduced. Iheozor-Ejiofor et al. 39 reported that over 97% of the 155 studies included in the review were at a considerable risk of bias and concluded that the evidence for the effectiveness of water fluoridation is limited because of the considerable risk of bias within the studies and substantial between-study variation. In the UK, the NIHR has commissioned an evaluation of the cost, and effects, of water fluoridation in the contemporary context. 9 However, water fluoridation is not technically, economically or politically feasible in many areas of the UK, so other delivery vehicles, such as professionally applied fluoride-containing varnish and fluoride-containing toothpaste, feature prominently in DBOH. 13
Fluoride-containing toothpaste
Fluoride-containing toothpaste is cited as the technology that is responsible for the significant decline in dental caries since its introduction in the early 1970s. 35 A Cochrane review40 of fluoride-containing toothpaste use in children aged 5–16 years reported clear evidence that fluoride-containing toothpastes are efficacious in preventing caries in permanent teeth, but there was little information concerning its effectiveness in the primary dentition, or the incidence of adverse effects associated with its use. A Cochrane systematic review41 examined the effectiveness of any fluoride-containing agent (gel, varnish, mouth rinse) combined with toothpaste and reported a mean number of decayed, missing or filled tooth surfaces in the permanent dentition (DMFS) pooled preventative fraction (i.e. the difference in caries increments between the treatment and control groups expressed as a percentage of the increment in the control group) of 10% [95% confidence interval (CI) 2% to 17%; p = 0.01] in favour of a combined regimen over toothpaste alone, but the statistically significant difference in favour of the combined use of fluoride-containing varnish and toothpaste accrued from a very small trial with a high risk of bias. A third Cochrane review42 compared different concentrations of fluoride-containing toothpaste for preventing tooth decay in children and adolescents. This review included 79 trials on 73,000 children and confirmed the benefit of fluoride-containing toothpaste in preventing dental caries, but only for fluoride concentrations of 1000 parts per million (p.p.m.) and above.
Fluoride-containing varnish
A Cochrane systematic review of fluoride-containing varnish, which was first published in 2002,43 included nine randomised controlled trials and reported a pooled dmfs-prevented fraction estimate of 33% (95% CI 19% to 48%; p < 0.0001). A second systematic review44 of fluoride-containing varnish used different selection criteria and identified only three trials examining primary teeth and concluded that the evidence was inconclusive because of the poor quality of the studies. The updated Cochrane review of fluoride-containing varnish was published in 201345 and suggested that fluoride-containing varnish is efficacious. The pooled dmfs-prevented fraction estimate was 37% (95% CI 24% to 51%; p < 0.0001) for the 10 trials that contributed data for the primary tooth surfaces meta-analysis. The quality of the evidence was assessed as moderate, as it included mainly high risk of bias studies, with considerable heterogeneity. There was little information on cost-effectiveness, and the authors, despite the large number of trials identified, reported that ‘there is still a paucity of evidence from high quality randomised trials assessing the effectiveness of fluoride varnishes for the prevention of caries in children’. 45 The authors recommended that future trials collect data on potential side effects and acceptability of this technology. One of the recommendations for future research was that composite interventions using more than one fluoride delivery method (such as the one under test in this trial) need to be evaluated in new trials. Composite fluoride interventions reflect how fluoride is used in ‘real life’, that is, from multiple sources such as fluoride-containing varnish delivered by health-care professionals and fluoride-containing toothpaste consumed in the home.
Fluorosis
Dental fluorosis is a cosmetic defect affecting the teeth that is associated with ingestion of excessive fluoride in infancy, as the permanent teeth are developing. Fluorosis is usually manifested as diffuse, white patches on the teeth, but can present as severe mottling of the teeth with brown staining. Research shows that fluorosis risk is related to an elevated fluoride intake for all of the first 3 years of life,46 but that the first 2 years of life are the period with greatest risk. 47 A Cochrane review48 that assessed fluorosis risk associated with use of fluoride-containing toothpaste in early childhood included 25 studies of different designs. There was weak, unreliable evidence that brushing a child’s teeth with a toothpaste containing fluoride, before the age of 12 months, may be associated with an increased risk of developing mild fluorosis. There was stronger evidence that higher levels of fluoride (≥ 1000 p.p.m) in toothpaste is associated with an increased risk of fluorosis when given to children aged > 5–6 years. However, the authors concluded that more evidence with low risk of bias is needed. They advocated that future trials testing the caries preventative effect of fluorides in young children should have an adequate follow-up period to assess fluorosis risk.
Evidence base for restriction of sugar consumption
Recently there has been a broad public health movement to restrict the consumption of sugar. 49 Dental public health specialists have advocated a common risk factor approach to prevent conditions that have common determinants, as a more efficient and effective strategy than multiple disease-specific strategies. 5 Childhood caries and obesity are often cited as two chronic non-communicable diseases that have common determinants (consumption of sugar) and could be tackled through this common risk approach. However, although average sugar consumption and obesity prevalence have increased over the past decades,49 caries prevalence has decreased. 20 There is uncertainty about the relationship between the two diseases,50 with marked heterogeneity between studies making comparison difficult. Interventions to reduce sugar consumption involve lobbying for changes in regulation, legislation or taxation at a population-level intervention. However, health-care professionals also have a role to play in trying to change an individual’s dietary behaviour and reduce the volume and, particularly important for caries, the frequency of sugar consumption. Lingström et al. 51 published a systematic review in 2003 to evaluate the effectiveness of dietary changes to prevent dental caries and reported a lack of studies that could demonstrate an effect of health education/advisory interventions to reduce sugar intake/frequency on caries increment. In 2012, a Cochrane review52 of one-to-one dietary interventions delivered in a dental setting aimed at changing dietary behaviour was published. Five studies met the criteria for inclusion in the review and, of these, only one study evaluated a single intervention designed to prevent dental caries. 53 The authors of the review concluded there was little evidence that one-to-one dietary interventions delivered in a dental setting are effective in preventing dental caries.
Evidence base for fissure sealants
Caries in the permanent teeth of children are now primarily found in the pits and fissures of molar teeth. Fissure sealants are applied by a dentist or dental care professional and adhere to the surface of the teeth forming a hard coating, which covers up the vulnerable pits and fissures, thereby preventing bacteria and food ingressing into these pits and fissures and causing decay. There is good evidence that fissure sealants are effective in preventing caries in the permanent molar teeth of high-risk children. 54 However, they are primarily used to prevent caries in permanent teeth and are not advocated in national guidance for caries prevention in primary teeth. 13,55
Dental visiting and prevention
There is a strong association between dental visiting and caries in young children. Those children who attend the dentist regularly and asymptomatically are more likely to have lower levels of caries than peers who are irregular, symptomatic attenders. 7 A Cochrane review56 examined the effect of altering the recall interval for dental check-ups and oral health and health-care system costs. The review looked at different recall intervals for different types of dental check-up: (1) clinical examination only; (2) clinical examination plus scale and polish; (3) clinical examination plus preventative advice; and (4) clinical examination plus preventative advice plus scale and polish. The review included only one study57 of 185 children (aged 3–5-years) and young adults (aged 16–20 years) attending a public dental clinic in Norway. Participants were randomly chosen to have a clinical examination every 12 months or every 24 months and were followed up for 24 months. For 3- to 5-year-olds, there was a non-significant difference in mean dmfs increment of –0.90 (95% CI –1.96 to 0.16) in favour of a 12-month recall. The study was judged to be of very low quality and the authors could not make any conclusions about whether or not extending the time between dental check-ups reduces or increases the risk of tooth decay and/or costs.
The reasons for the association between dental caries and dental visiting patterns remain unclear. There is no evidence to demonstrate that this relationship is directly attributable to interventions provided by dental services, or if regular dental visiting is a marker for a set of caries risk-reducing behaviours adopted by parents of young children within the home, such as restricting sugar consumption and frequent use of fluoride-containing toothpaste. However, if part of the national strategy for caries reduction is to deliver evidence-based prevention within dental settings, then sufficient numbers of children have to attend the dentist and attend at the recommended frequency if the benefits are to be realised at both the individual and population levels.
Overall, the child dental health surveys3,17 suggest there has been little change in dental visiting patterns across England, Wales and Northern Ireland between 2003 and 2013. Dental registrations of children in Northern Ireland have increased in this period, but this appears to be a result of an increase in the registration interval from 15 months to 24 months rather than a change in attendance behaviour. According to parental responses in the national surveys, approximately 9 out of 10 children across all age groups, and countries, attended for a regular check-up in both 2003 and 2013. There has also been little change in the reported age of the first visit to the dentist since 2003, with around one-third of 5- to 8-year-old children having visited the dentist by the age of 2 years in 2013. Despite high-profile media coverage about problems in accessing NHS dentistry, more than 8 out of 10 parents, in both 2003 and 2013, reported that they had never experienced any difficulty finding an NHS dentist for their child. So although visiting patterns have remained stable, there has been a decline in disease, suggesting that a change in age of attendance and the volume and frequency of attendance are not responsible for the fall in caries. However, the quality and effectiveness of the preventative care delivered at dental visits may have improved. Research that informed the rationale and design of this study suggested that the preventative care provided by GDPs was ineffective and inequitable58 and that dentists were ill equipped in terms of their knowledge59 and how they present information to their patients60 to provide an effective preventative service. In response to the concerns about the quality of prevention in practice, the Department of Health in England developed and distributed DBOH13 to every dental practice in England. The advice for prevention of caries in children aged 0–6 years provided by the third edition of DBOH is replicated in Table 4, and the classification used to grade the evidence is provided in Table 5.
| Advice to be given | Professional intervention |
|---|---|
| Children aged up to 3 years | |
| Breastfeeding provides the best nutrition for babies I | |
| From 6 months of age infants should be introduced to drinking from a free-flow cup, and from age 1 year feeding from a bottle should be discouraged III | |
| Sugar should not be added to weaning foods or drinks V | |
| Parents/carers should brush or supervise toothbrushing I | |
| As soon as teeth erupt in the mouth, brush them twice daily with a fluoridated toothpaste I | |
| Brush last thing at night and on one other occasion III | |
| Use fluoridated toothpaste containing no less than 1000 p.p.m. fluoride I | |
| It is good practice to use only a smear of toothpaste | |
| The frequency and amount of sugary food and drinks should be reduced III, I | |
| Sugar-free medicines should be recommended III | |
| All children aged 3–6 years | |
| Brush at least twice daily, with a fluoridated toothpaste I | Apply a fluoride-containing varnish to teeth two times a year I (2.2% NaF–) (I) |
| Brush last thing at night and at least on one other occasion III | |
| Brushing should be supervised by a parent/carer I | |
| Use fluoridated toothpaste containing more than 1000 p.p.m. of fluoride I | |
| Use only a pea-size amount (good practice) | |
| Spit out after brushing and do not rinse, to maintain fluoride concentration levels III | |
| The frequency and amount of sugary food and drinks should be reduced (III, I) | |
| Sugar-free medicines should be recommended III | |
| Children aged 0–6 years giving concern (e.g. those likely to develop caries, those with special needs). All advice as above plus | |
| Use fluoridated toothpaste containing 1350–1500 p.p.m. fluoride I | Apply fluoride varnish to teeth two or more times a year (2.2% NaF–) I |
| Use only a smear or pea-size amount (good practice) | Reduce recall interval V |
| Investigate diet and assist adoption of good dietary practice in line with the Eatwell Plate I | |
| When medication is given frequently or long-term request that it is sugar free, or used to minimise cariogenic effects (good practice) | (It is good practice) when medication is given frequently or long term, liaise with medical practitioner to request that it is sugar free or used to minimise cariogenic effects |
| Grade | Strength of evidence |
|---|---|
| I | Strong evidence from at least one systematic review of multiple well-designed randomised control trial(s) |
| II | Strong evidence from at least one properly designed randomised control trial of appropriate size |
| III | Evidence from well-designed trials without randomisation, single-group pre-post, cohort, time series of matched case-control studies |
| IV | Evidence from well-designed non-experimental studies from more than one centre or research group |
| V | Opinions of respected authorities, based on clinical evidence, descriptive studies or reports of expert committees |
The guidance recommends prevention for all children including those who present caries free. The levels of evidence for each intervention are provided; however, this evidence is based largely on explanatory studies and there is an implicit assumption in the guidance that providing advice, or a professional intervention, will replicate the effects of explanatory studies. The effectiveness of these interventions relies on changing parents’ and health professionals’ behaviour in a sustainable way. The translation of advice to a change in behaviour is tenuous for both patients61 and health-care professionals. 62 A large amount of policy and commissioning effort has been put into DBOH, and there is a concern that this effort may not result in significant improvement at population or practice levels. DBOH is currently not an NHS contractual obligation for dentists in England; however, the Health & Social Care Information Centre63 reported that in 2014/15 children’s courses of treatment that included fluoride-containing varnish application rose 24.6% to 3.4 million from the previous year. The total number of courses of treatment provided to children in 2014/15 was 11 million, 70% of which were for band 1 (check-ups) alone. So although provision of fluoride-containing varnish has been increasing, only a relatively small proportion of courses of treatment provided to children involve reported application of fluoride-containing varnish.
There is also a concern that DBOH could increase inequalities in caries levels between the rich and poor in society, as children from the most deprived backgrounds, with the greatest risk of dental caries, are less likely to attend the dentist from an early age, and are less likely to attend on a regular basis, than children from more affluent backgrounds. The Children’s Dental Health Survey 2013 Report 1: Attitudes, Behaviours and Children’s Dental Health England, Wales and Northern Ireland, 201364 recorded that around 90% of parents reported that their 5-year-old child attends the dentist on a regular basis. Table 6 compares parentally reported visiting patterns in the different countries.
| Country | Pattern of attendance (%) | ||
|---|---|---|---|
| For a check-up | Only when have trouble with teeth | Never been to the dentist | |
| Northern Ireland | 90 | 4 | 4 |
| Wales | 92 | 3 | 5 |
| England | 89 | 4 | 7 |
Although these statistics may be subject to response bias, it seems that dental services can reach the majority of the child population. Other interventions are required to reach the children who do not attend the dentist on a regular basis. If prevention in practice is seen as a key means of improving population health in young children, we need to understand the cost-effectiveness of interventions recommended by DBOH. Estimating the cost-effectiveness of this approach to prevention is particularly important in the current financial climate of the NHS, as prevention delivered in dental practice is a potentially expensive option as a result of high staff costs. 15
Explanation of rationale for undertaking the study
Situation in 2008
The planning for this trial took place in 2008 and the application was approved in 2009. From the inception of the trial to its completion, policy has progressed with the intent to have prevention as the principal focus of NHS primary care dental services. More significantly, national epidemiological surveys show that there have been reductions in the prevalence and severity of the disease in the population of young children over the last 10 years, particularly in Northern Ireland where the fall in disease in 5-year-old children has been marked. Therefore, the trial has been conducted against a background of falling population disease levels. However, the NHS contractual arrangements under which dentists in Northern Ireland operate have remained stable during the period of the trial. During the conduct of the trial, policy-makers in all four home nations have sought to elicit a change in emphasis of NHS dental services to focus primarily on prevention rather than treatment. New NHS dental contracts with a system of remuneration based largely on capitation, aimed at supporting prevention, are being evaluated in England, Wales and Northern Ireland.
Caries in 5-year-olds: a priority
Prevention of dental caries in young children is a policy priority across the UK. The Primary Dental Care Strategy for Northern Ireland,65 published in 2006, placed a strong emphasis on prevention of caries in general practice and the subsequent An Oral Health Strategy for Northern Ireland,66 in 2007, sets targets for reduction in the caries levels of 5-year-olds. In England, tooth decay in children aged 5 years is an indicator in the Public Health Outcomes Framework67 and caries prevention in young children figures prominently in Public Health England’s document, Local Authorities Improving Oral Health: Commissioning Better Oral Health for Children and Young People. An Evidence-Informed Toolkit for Local Authorities. 5
In Scotland, Route Map to the 2020 Vision for Health and Social Care in Scotland68 identified preventative measures on alcohol, tobacco, dental health, physical activity and early detection of cancer as a particular focus. The priority for prevention of dental caries in young children is reflected in a national HEAT (Health Improvement, Efficiency, Access to treatment, Treatment) target: ‘At least 60% of 3 and 4 year old children in each Scottish Index of Multiple Deprivation (SIMD) quintile to receive at least two applications of fluoride varnish (FV) per year by March 2014’. 69 This forms part of the Quality Measurement Framework to measure progress of the National Quality Strategy to realise the 2020 vision. A national approach to prevention in Scotland started in 2011 with the establishment of the Childsmile programme, designed to improve the oral health of children in Scotland and reduce social inequalities both in dental health and access to dental services. There are a number of elements to the programme.
-
Childsmile Core programme: every child is provided with a dental pack containing a toothbrush and a tube of toothpaste containing 1000 p.p.m. fluoride on at least six occasions by the age of 5 years. Children also receive a free-flow feeder cup by 1 year of age. In addition, every 3- to 4-year-old child attending nursery (whether it is a local authority, voluntary or private nursery) is offered free, daily, supervised toothbrushing.
-
Childsmile practice: a network of Dental Health Support Workers facilitate regular attendance at dental practices of children from the age of 6 months to receive preventative care including twice-yearly fluoride-containing varnish applications from 2 years of age.
-
Childsmile Nursery and School: educational establishments with the highest proportion of children living in the most-deprived local quintile, as defined by the Scottish Index of Multiple Deprivation, are targeted for provision of additional twice-yearly fluoride-containing varnish applications within the nursery and school setting.
Childsmile is a composite national programme. It is an evaluation programme, which has largely been confined to assessment of process. 70 Serial cross-sectional studies71 in Glasgow, an early adopter of the Childsmile programme, show reductions in dental caries in 3-year-old children, but without a control population causal inference is difficult and no data have been published on the cost-effectiveness of the programme.
In Wales, Together for Health: A National Oral Health Plan for Wales (2013–18)72 was published in 2013. A central element of the plan is ‘Designed to Smile’,73 and this national programme has two main elements:
-
A preventative programme for nursery/primary school children: this involves the delivery of school-/nursery-based toothbrushing and fluoride-containing varnish programmes for children aged 3–5 years, helping establish good habits from an early age. In addition, children aged 6–11 years will receive a fissure sealant programme as well as preventative advice on how to look after their oral health.
-
A preventative programme for children from birth to 3 years of age. The aim of this programme is to give good consistent advice to parents, to provide toothbrushes and toothpaste, and to encourage going to the dentist.
Similar to the situation in Scotland, evaluation has been hampered by the lack of a counterfactual and monitoring reports have focused largely on process evaluation. 74
In Northern Ireland, funding has been provided to the community dental services (CDSs) to run fluoride-containing toothpaste schemes, which started in 2005. There are three categories of scheme, with children aged 3–5 years (inclusive) being the targeted age group:
-
postal schemes: fluoride toothpaste is posted out in a pack, along with a toothbrush and instructions on use, to children from deprived families
-
supervised tooth brushing schemes: children in pre-school settings were overseen by staff in a daily brushing routine with fluoride toothpaste
-
pre-school distribution schemes: children attending pre-schools in selected areas receive toothbrushes, toothpaste and instructions at school for home use.
In total, across all three types of scheme, approximately 22,000 children were/are involved each year, which equates to about one-third of all children in Northern Ireland in the 3- to 5-year-old cohort. A personal correspondence with the dental lead for the NHS in Northern Ireland confirmed that this programme is now well established and has involved consistent numbers of children each year from 2005 to 2015 (Mr Michael Donaldson, Consultant in Dental Public Health. Health and Social Care Board of Northern Ireland, 2015, personal communication).
Therefore, prevention of dental caries in early childhood is a priority for all four home nations. In each country evidence-based preventative programmes using fluoride-containing varnish and fluoride-containing toothpaste are delivered in various settings, including general dental practice. However, there have been no pragmatic trials that investigate health and cost outcomes of these interventions. Serial cross-sectional surveys show reductions in disease and are encouraging, but the evidence produced is weak with a high risk of bias. This illustrates the need to undertake high-quality pragmatic trials to establish the cost-effectiveness of these interventions, particularly at a time when the NHS across the UK is seeking to invest in prevention by redesigning dental services.
Redesigning NHS dental services to support prevention
Internationally there has been a consensus that dental services should be reoriented to prioritise prevention. The Liverpool Declaration: Promoting Oral Health in the 21st Century75 was produced by the eighth World Congress on Preventive Dentistry, organised jointly by the International Association for Dental Research, the World Health Organization, the European Association of Dental Public Health and the British Association for the Study of Community Dentistry. The declaration called for a number of areas to be strengthened by 2020, including ‘countries should ensure access to primary oral health care with emphasis on prevention and health promotion’.
In England and Wales, locally commissioned NHS dental contracts were introduced in April 2006. One of the reasons the English Department of Health changed the NHS dental contract at that time was to encourage prevention. However, the 2006 contract in England was heavily criticised by the dental profession and NHS managers76 and by the House of Commons Health Select Committee77 for offering little incentive for dentists to provide preventative care. Indeed, one of the recommendations of the House of Commons Health Select Committee report was that ‘the Department of Health undertake research to determine the extent to which the provision of preventive advice is being given and its cost-effectiveness’.
In England piloting of new contracts started in 2011, based on a registration and capitation remuneration model with additional financial incentives for quality. Central to the pilot contract was an information technology-supported system of care pathways, based on the outcomes of a standardised risk assessment with a strong focus on prevention. The pilots have not been subject to a robust academic evaluation testing a priori hypotheses. The reports of the pilots have been mainly descriptive in nature78 and the evaluation of the standardised oral health assessment, which forms the cornerstone of the new contract, was limited to professional and social acceptability. The ability of the associated risk algorithms to correctly classify patients and predict future disease development was not tested, only a count of the number of additional appointments made for those considered to be at most risk was reported. During the evaluation, changes were made to the data collected, and there were concerns about the quality of the information being generated, for example only a small sample (n = 10) of the 70 pilot practices were chosen to record tooth-level data.
The Department of Health in England developed a Dental Quality and Outcomes Framework (DQOF),79 which was planned to be included in the pilots. The DQOF includes caries in 5-year-old children as a quality indicator: ‘Decayed teeth (dt) aged 5 years old and under, reduction in number of carious teeth/child’. 79 The rationale for use of this indicator was to ‘monitor the primary dental care team’s adoption of evidenced informed preventative advice and intervention and their impact on oral health’. 79 However, the DQOF was not included in the pilots of the new English NHS dental contract and is also untested. This makes conclusions about the impact of the English pilots difficult to infer. In January 2015, the Department of Health announced dental contact reform prototypes,80 acknowledging the frustrations with the pace of reform and reiterated a commitment to prevention. The remuneration mechanism for prototypes is to be based on a blend of capitation, activity and quality. Remuneration will be based on minimum activity and capitation targets, adjusted for performance against the DQOF. The timescales for the start and completion of prototypes are unclear and the phrase ‘evolution not revolution’ was mentioned.
In Wales, piloting of a new dental contract started in 2011. The National Oral Health Plan for Wales72 referenced the fact that the Department of Health was testing a new prevention-oriented GDS contract. Two pilots were selected, one basing payment on capitation and quality to incentivise practices to maintain and increase patient numbers and to promote prevention. The second was a children and young people’s pilot for 0- to 17-year-olds, which aimed to incentivise preventative care for 0- to 17-year-olds and was designed to complement ‘Designed to Smile’. The pilots were completed on 31 March 2015 and at the time of writing the evaluation has yet to be published. 81
In Northern Ireland, the Department of Health Social Services and Public Safety and the Health & Social Care Board of Northern Ireland have started pilots for a new dental contract. The first wave of pilots to test a potential capitation-based model for primary dental care started on 13 November 2014 and has paved the way for a larger intake of practices in wave 2, which commenced in August 2015. The wave 2 pilot will run for a period of 1 year and the practices involved will switch from the current fee-for-service system of remuneration to capitation and then back to fee for service at the end of the year. This change in the contract, like those in England and Wales, is designed to support prevention. The evaluation of the new contract pilots in Northern Ireland will be subject to an independent evaluation funded by a NIHR project grant [URL: www.nets.nihr.ac.uk/projects/hsdr/141912 (accessed 26 August 2016)].
This summary demonstrates that prevention of caries in the primary dentition of young children is a public health priority for the four home nations. In all four nations there is activity to reorientate dental services to focus on prevention. The Children’s Dental Health Survey 2013 Report 1: Attitudes, Behaviours and Children’s Dental Health England, Wales and Northern Ireland, 201364 reported that 90% of 5-year-olds in England, Wales and Northern Ireland had visited a dentist in the last 12 months. Therefore, interventions delivered in dental practice, in which over 90% of NHS dental resource resides, should be able to reach a high proportion of the child population. Although there is national guidance13,55,82 on providing evidence-based preventative care, primarily using fluoride interventions, to young children in general practice, the guidance is based on evidence that has some significant gaps, particularly how evidence from largely explanatory trials performs in the real world, taking into account compliance of practitioners and patients. There is also little understanding of the economics of prevention delivered in a practice setting. Dentists are highly paid health-care professionals; the Health & Social Care Information Centre15 reported that the average NHS earnings for providing-performer dentists in England and Wales was £114,100 in 2012/13 and the average for performer-only dentists was £60,800. There have been attempts to use skill mix to reduce staff costs for prevention, a good example being use of the extended duties dental nurse in the Scottish Childsmile programme, but these programmes are staff intensive and may not be as cost-effective as community-based prevention programmes such as water fluoridation or distributed fluoride toothpaste programmes in reducing population disease levels. There is a need to understand the costs and effects of preventative programmes delivered in general practice through well-designed and adequately funded, pragmatic, randomised controlled trials, particularly in the context of falling population disease levels.
Once young children develop caries active in their primary teeth, they are very likely to experience pain or have an extraction over a 3-year period. 4 In addition, the majority of preventative care in dental practice is directed to children who initially present caries free, and it is believed that the majority of 5-year-old children who have the disease emerge from this population. 22 As adverse outcomes are common in children once they develop the disease and the majority of NHS resources are directed to those who initially present caries free to keep them in this state, the rationale for this trial was to develop the evidence base for preventative care delivered in general dental practice, with a focus on primary prevention and attempting to keep children caries free.
Dentists cannot prevent caries from starting in children who at their first visit already have the disease. These children with the disease should be considered as a separate population from those who are caries free; their dental care needs are quite different and are complicated by the effects of restorative treatment. Another Health Technology Assessment programme-funded trial is investigating the management of active disease in this population. 11
If the technologies tested in this trial are shown to be effective at preventing caries and/or reducing costs it will reassure policy-makers that the investment in prevention and the reorientation of dental services is justified. If the intervention is shown not to be a cost-effective use of resources, policy-makers may wish to consider the merits of community-based prevention interventions.
Objectives of the trial
Aim
The aim of the study was to measure the effects and costs of a composite fluoride intervention designed to prevent caries in young children attending dental services.
Objectives
The objectives of the study were to compare in children, aged 2–3 years who were caries free at baseline, the effectiveness of a varnish containing 22,600 p.p.m. fluoride, a toothpaste containing 1450 p.p.m. fluoride and standardised health education, provided twice a year in general dental practice, as a ‘preventative package’ versus standardised health education provided twice a year alone in:
-
reducing the conversion of children from caries-free to caries-active states in the primary dentition
-
reducing the number of carious surfaces (caries into dentine) in the primary dentition in children who convert from caries-free to caries-active states
-
reducing the number of episodes of pain and/or extraction of primary teeth.
The cost-effectiveness of the preventative package relative to standardised health education alone was also evaluated.
Chapter 2 Methods
Trial design
This was a pragmatic, two-arm, parallel-group, randomised controlled trial with an allocation ratio of 1 : 1. The trial was classified as a clinical trial of an investigative medicinal product (CTIMP) and was authorised by the Medicines and Healthcare products Regulatory Agency.
Patient and public involvement (PPI) played a major role in shaping the design and management of the trials, and the interpretation of our findings. We were supported by the Health & Social Care Research & Development Division through their PPI programme to connect with local groups and organisations in Northern Ireland. In addition, we had a PPI group made up of parents with young children, who met on a regular basis with the research team and provided advice and input at key stages of the research project.
Changes to trial design after trial commencement
A number of changes were made to the original protocol, which was originally published in 2011. 83 The Greater Manchester Central Research Ethics Committee had oversight of the trial. The committee provided a favourable ethical opinion on 8 July 2009 (Research Ethics Committee reference number 09/H1008/93), and all of the changes made to the protocol were approved by the Greater Manchester Central Research Ethics Committee.
In the original protocol we stated that practices would be selected to participate in the trial according to the following criteria:
-
willingness to participate in the study
-
access to a suitable population of children
-
availability of suitable premises and equipment to host recruitment and baseline assessment activities
-
agreement to comply with the protocol and good clinical practice (GCP) requirements of the trial.
A complication affecting the selection of practices occurred because of a change in research governance arrangements in Northern Ireland in 2010. The new arrangements meant that CDS dentists, who were responsible for conducting baseline and outcome examinations, were confined to working in the geographical area covered by their trust. We therefore had to select practices based on:
-
Practice size. The total number of children aged 2–3 years registered with the practice was used to determine the practice size. This information was provided by the Business Services Organisation of Northern Ireland to ensure there was access to sufficient numbers of eligible children attending the practice and sufficient space to accommodate the needs of the trial.
-
Practice location. Owing to restrictions placed on the geographical boundaries within which the CDS dentists could work, we selected practices to ensure that the CDS dentists in each trust had a similar, and manageable, number of practices to visit during the recruitment and follow-up phases of the trial.
We changed the measure of socioeconomic status used in the analyses. In the original protocol we proposed that we would use the following measures of socioeconomic status: dental charge exemption status of the child’s parents; eligibility for free school meals; or the Northern Ireland MDM 2005. 84 The Northern Ireland MDM was updated in 2010 and we elected to use this contemporary measure in our analyses. 25
During the follow-up period we provided a £25 gift voucher to recompense parents for the expense and inconvenience incurred in bringing the child for the final outcome assessment.
Participants: including eligibility criteria
The trial participants were children aged 2–3 years who attended GDS practices in Northern Ireland. There was a two-stage process of assessing and recruiting participants.
Stage 1: recruiting practices
A flyer was sent out to all practices across Northern Ireland to participate in the trial. An open meeting available to all practices in Northern Ireland hosted by the Chief Dental Officer was initially held to explain the aims of the trial and what would be expected of practices if they participated in the trial. This was followed by a working meeting of representatives of all of the local dental committees in Northern Ireland to discuss the practical aspects of the trial, for example how to minimise disruption and what would be a fair financial package to reimburse practices for disruption and loss of earnings as a result of hosting the trial. A reimbursement package for practices was agreed with the Health & Social Care Board of Northern Ireland to cover the additional costs to practices from participating in the trial. This included:
-
a £1000 initial payment to each dentist participating in the trial to cover out-of-pocket expenses for time taken for GCP- and trial-specific training and earnings lost because of the recruitment process
-
a £25 fee for each visit to provide the intervention and complete the case report form (CRF) as per protocol.
Practices were selected based on their size (registered population of 2- to 3-year-old children), location (to provide a similar and management number of practices for each CDS examining team in each NHS trust area), willingness to participate in the study and an agreement to comply with the protocol and GCP requirements of the trial.
Stage 2: recruiting participants
Participants were children aged 2–3 years who attend the selected GDS practices.
Children were eligible to participate in the study if they fulfilled the following criteria.
Inclusion criteria
-
Children aged 2–3 years.
-
Attending selected GDS practices.
-
Person with parental responsibility for the child signs a consent form.
Exclusion criteria
-
Children with caries into the dentine.
-
A past history of fillings or extractions because of caries.
-
Children with fissure sealants on primary molar teeth.
-
Children with a history of severe allergic reactions requiring hospitalisation.
-
Children already participating in any other investigative medicinal product study at recruitment.
Families usually attend the dentist as a unit; therefore, a rule was needed to guide the participation of siblings in the trial if more than one sibling was eligible. It was decided that the youngest eligible sibling in a family would be randomised and all other eligible siblings would be excluded from the study but receive their NHS dental care in the usual way.
Study settings
The study took place in 22 NHS general dental practices across Northern Ireland, UK. A map of the province (Figure 3) identifies the location of each practice participating in the study.
FIGURE 3.
Location of the 22 general dental practices that participated in the Northern Ireland Caries Prevention In Practice trial. Reproduced from Land and Property Services data with the permission of the Controller of Her Majesty’s Stationery Office, © Crown copyright and database rights. Departmental Memorandum of Understanding 2015.

Interventions
Intervention group
The intervention was a composite fluoride intervention comprising two elements:
-
A fluoride-containing varnish (at a fluoride concentration of 22,600 p.p.m.) in the form of Duraphat® (Colgate-Palmolive Ltd, Guildford, UK), provided in its normal commercial packaging. Duraphat (used off label) is classed by the Medicines and Healthcare products Regulatory Agency as an investigative medicinal product and, therefore, its use in this CTIMP had to comply with relevant UK regulations. 85 A participating dentist applied the fluoride-containing varnish to all of the dried primary teeth of the children at two visits to the dental surgery each year, at approximately 6-monthly intervals (± 4 weeks). One drop of varnish was applied to the primary teeth in each arch (two drops in total) using a standardised brush applicator. After application, parents were advised not to brush their children’s teeth for 24 hours.
-
Participating dentists and their staff were trained to apply the varnish in accordance with the product brochure and practices were provided with an illustrated fluoride-containing varnish application guide describing the process of application. The UK summary of product characteristics86 was also made available to the dentists. The varnish was dispensed by the pharmacy department at the Belfast Health and Social Care (HSC) Trust. The temperature of the varnish was monitored during distribution and storage using maximum and minimum thermometers to ensure the varnish used in the trial complied with the guidance in the product brochure.
-
The second element of the fluoride intervention comprised a free toothbrush and a free 50-ml tube of toothpaste containing 1450 p.p.m. fluoride. This element was provided to intervention group children twice a year along with the fluoride-containing varnish. The toothpaste was Colgate® Cavity Protection (Colgate-Palmolive Ltd, Guildford, UK), which was provided in its normal commercial packaging. Parents of participating children under 3 years of age were advised to use a smear of toothpaste, and those whose children were over 3 years were advised to use a pea-sized blob of toothpaste when brushing their teeth. Photographs of a smear and a pea-size blob were included in a standardised dental health education sheet (see Appendix 1). It was stressed to parents that an adult must supervise the child when they brushed their teeth.
Control group
Parents of children allocated to the control group were invited to bring their children for a dental check-up at 6-monthly intervals. At these visits the children received the same standardised dental health education as the children in the intervention group. The control group children did not receive any professionally applied or NHS service-provided fluoride interventions.
The trial visits were integrated into the usual 6-monthly dental check-up appointment of all children in both intervention and control groups over the 3-year follow-up period of the trial.
The date of visits for both intervention and control groups, and the date of each application of fluoride varnish, were recorded for each participant in the intervention group by the dentist (local investigator) on the CRF. The CRF identified the batch number of fluoride varnish used for each application. Empty or expired tubes of varnish were collected and retained. Participants who did not attend for their check-up appointments were sent out reminder letters.
Randomisation and blinding (sequence generation, type, allocation concealment mechanism, randomisation implementation and blinding)
The practices identified potentially eligible children from their practice databases, based on their age and treatment history. For those practices without a computer, the Business Services Organisation provided the practice with a list of registered children between the ages of 2 and 3 years.
An invitation letter was sent to parents of identified children asking if they would like their child to participate in the trial. The invitation included a trial information sheet, which explained the study to parents. An appointment to attend a dedicated assessment session in the child’s practice was included in the invitation pack. The child’s dentist or the external CDS dentists (who completed the baseline clinical examinations) obtained parental consent for the child to take part in the trial. Baseline assessment was undertaken after consent had been obtained for each child but prior to randomisation. A specific randomisation schedule was prepared by the clinical trials unit (CTU) for each practice, using randomised permutated blocks. The block lengths varied to ensure that the CDS examiners undertaking baseline assessments were blind to patient allocation. Children who met the eligibility criteria and for whom written, informed consent had been provided by a person with parental responsibility were enrolled onto the trial. Randomisation was undertaken centrally by the CTU on a dedicated trial telephone line. The CTU verified the child’s eligibility criteria and provided the local investigator with confirmation of the treatment allocation via fax (to provide a paper record of the allocation) and assigned a unique participant information number to each child.
Outcomes (primary and secondary outcomes how and when they were assessed)
Clinical examinations for caries at baseline and outcome at 3 years were performed by trained and calibrated examiners, who were dentists employed by the CDS. Calibration took place on at least 15 4- to 6-year-old children in a primary school setting prior to baseline and prior to and halfway through the outcome examination period. Examiners were trained and calibrated against the ‘beacon’ examiner for the NHS Child Dental Health Survey in the north-west of England. All of the examiners examined each child twice and intra- and inter-examiner agreements for recording carious teeth were assessed using the kappa statistic. Intra- and inter-examiner agreements for recording caries status at tooth level must have exceeded a kappa score of 0.70 or further training was provided and the calibration exercise repeated until acceptable levels of agreement were achieved. The results of baseline and outcome calibrations were made available to the joint sponsors, the Trial Steering Group and Independent Data Monitoring and Ethics Committee.
Outcome examiners were blinded to the treatment allocation and the same diagnostic protocol was used throughout the study.
Primary outcome measures (measured at 3 years)
-
Conversion of caries-free children to caries-active (caries into dentine) children. The diagnostic threshold for caries used in the trial was caries into dentine. We used the same diagnostic protocol and same examination processes and procedures that are used in the NHS child dental health surveys. 20,21 The clinical examination processes and procedures and the caries data collection form used in the CRF is included in Appendix 2. For the purposes of this study the term ‘caries free’ was used to denote a child whom the examiners (trained and calibrated to the diagnostic protocol used in the trial) judged to have no carious lesions into dentine. The term ‘caries active’ was used to denote a child that had at least one tooth with caries into dentine. Caries in the enamel were not assessed or recorded.
Secondary outcome measures (measured at 3 years)
-
Number of carious surfaces (caries into dentine in primary teeth) that develop in children who convert from caries free to caries active.
-
Number of teeth extracted in children who are caries active.
-
Number of episodes of pain.
-
Costs of dental care plus other health-care costs, as well as parental costs incurred as a result of visits to the dentist over the 3-year follow-up period.
-
Adverse reactions (ARs) and serious adverse events (SAEs).
Table 7 identifies the variables used in the statistical analyses and how these were measured and collected.
| Variable | Collected from | When |
|---|---|---|
| Age (years) | From date of examination and patient’s date of birth | Baseline |
| Gender (male/female) | Pretreatment medical history/physical examination form | Baseline |
| MDM (quintiles) | Patient’s postcode in dental records and verified at date of examination | Baseline |
| Practice (n = 22) | Pretreatment medical history/physical examination form | Baseline |
| Caries or not: tooth assessment | Caries data recording form. Each of the 20 teeth indicated in the chart were considered separately. For each tooth if one of the following surface codes has been entered on the chart, then the child was not caries free: 1, 2, 3, 4, 5, 6, R, N, C | Baseline |
| Third year follow-up examinations | ||
| Child caries free or not | This outcome was measured at 3 years by CDS dentists visiting the practices who were blind to the intervention. These dentists were trained and calibrated on diagnosis of caries and will examine all the primary teeth of the participants and record the data on a chart using the surface codes listed on the caries data recording form. Children were classified as caries free if all the surfaces were scored either sound (code 0), extracted for orthodontic reasons (code 7), unerupted (code 8), sealed surface reason unknown (code $) or trauma (code T). All other children were classified as having caries active | Baseline |
| Third year follow-up examinations | ||
| Number of carious surfaces that develop in children who convert from caries free to caries active | This was calculated by adding the number of codes 1, 2, 3, 4, 5, 6, R, N and C in children who were not caries free. If an incisor tooth was missing (code 8) it was scored as sound unless there was evidence from forms completed at each visit by the site research assistants on the site clinical record form that there was caries active in that tooth | Baseline |
| Third year follow-up examinations | ||
| Number of episodes of pain | Calculated from the report forms completed at each visit by the site research assistants on the site clinical record form. Also from section 1, question 1, in the parental questionnaire | At each visit during the 3 years’ follow-up |
| Number of episodes of toothache | Calculated from the report forms completed at each visit by the site research assistants. Also from section 1, questions 1 and 9 in parental questionnaire (see Appendix 3) | At each visit during the 3 years’ follow-up |
| ARs | Reporting of all ARs | Ongoing collection depending on severity |
| The market costs of varnish, toothpaste and toothbrushes | By reference to the providing manufacturer | At baseline |
| Time taken to deliver intervention and other dental treatments | Calculated from the report forms completed at each visit by the site research assistants (section 2 of the CRF). Validated by an observed time-and-motion study conducted in a sample of practices. The questionnaire identified any other dental activity to which the child received for services other than trial sites. Delivery time was monetised by reference to implicit average NHS dental pay rates provided by the CSA (see Appendix 4, Table 33) | At each visit during the 3 years’ follow-up |
| Measurement of non-health-care costs: reported total time taken to accompany the child for a dental visit, and time off work, plus distance travelled | Measured from data collected via the parental questionnaire. Travel costs were monetised using the AA reference costs per mile. Time costs were monetised using average earnings in Northern Ireland | At each visit during the 3 years’ follow-up |
All children who converted from caries free to caries active received dental treatment, for example fillings or extractions, in the usual way as prescribed by their dentist. All children who converted from caries free to caries active continued to receive the trial interventions (both intervention and control groups) for the duration of the trial.
Changes to outcomes after trial commencement
There were no changes made to the outcome measure used after commencement of the trial.
Sample size
The principal outcome measure is conversion from a caries-free state to caries-active state in the primary dentition. The sample size is therefore based on measuring an absolute difference between the intervention and control groups in the proportion of children who are free of the disease at 3 years. In the sample size calculation, we expected to see an absolute difference in the proportion of children with caries after 3 years of 0.1 between intervention and control groups. This expectation was based on the findings of a public health trial of toothpaste containing 1450 p.p.m. of fluoride, on preschool children in the north-west of England,87 which reported 0.08 absolute difference in the proportion of children with caries active between the intervention and control groups. In this proposal, as fluoride-containing toothpaste was supplemented with biannual applications of fluoride-containing varnish, we expected to see a larger effect size and, therefore, a 0.1 absolute difference in proportions.
The best data on the event rate for the practice-based population in Northern Ireland came from the Business Services Organisation database rather than epidemiological studies on other populations. Business Services Organisation data collected in 2008, at the time of planning the study, showed that 75% of 2- and 3-year-olds in Northern Ireland who were registered with a dentist were caries free at first attendance. Over a 3-year period, this reduced to 40% of 5- to 7-year-old children being caries free. Therefore, a further 35% of children were expected to develop caries active over a 3-year period. Based on these data and selecting caries-free children for inclusion in the trial, it was estimated that 47% would develop caries active over the 3 years. A two-group chi-squared test with a 0.05 two-sided significance level would have 90% power to detect the difference between a proportion of 0.47 and a proportion of 0.37 [odds ratio (OR) of 0.662], if the sample size in each group is 510.
We assumed that 2% of children would be excluded because of a history of severe allergic reaction and a further 1% for other reasons. We also assumed that 75% of children approached would be caries free at eligibility assessment, a 70% parental consent rate and a 15% dropout rate over the 3-year study period. Using these assumptions we estimated we would need to invite at least 2356 children to take part in the study and recruit 1200 children, to ensure we had sufficient power at the end of the trial.
Statistical methods including methods for additional analyses (subgroups, adjusted analyses and sensitivity analyses)
All statistical analyses were performed using Stata version 14 (StataCorp LP, College Station, TX, USA), with an intention-to-treat approach using a two-sided 5% significance level.
Primary outcome
The primary analysis compared the proportion of children in the two groups who converted from caries free to caries active over the 3 years using a binary logistic regression model (‘logistic’ procedure in Stata). The primary analysis was adjusted for age and socioeconomic status (as measured by MDM 2010 quintiles). 25 The assumption of logit of outcome changing linearly with each unit change in age was tested by categorising age into equal intervals and rerunning the model checking for linearity. The likelihood ratio goodness-of-fit test was used to indicate model appropriateness and the Wald statistic was used to test the significance of the difference between groups.
We also report two other analyses: first, an unadjusted analysis and, second, an analysis adjusting for practice as well as age and MDM 2010 quintile. The latter analysis was a secondary analysis, as we did not consider the clinical placement of the varnish (or distribution of toothpaste) to be practice specific. This analysis used the Huber–White approach within Stata [vce(cluster)] to deal with potential practice clustering effects (also known as sandwich estimator and robust estimator of variance). This technique relaxes the assumption of independence of the observations and can produce the ‘correct’ standard errors even if the observations are correlated.
We undertook a subgroup analysis for children from deprived/non-deprived areas. This dichotomy was achieved by comparing children in the two most-deprived quintiles of the MDM 2010, based on their home postcode, with those categorised into the three least deprived quintiles of deprivation. This was formally investigated by means of an ‘interaction test’ of the null hypothesis: that the relative efficacy of the two interventions was the same in children in deprived and non-deprived areas. It should be noted, however, that the trial was not formally powered to detect socioeconomic status interaction effects; consequently, we expected to observe an interaction as being statistically significant only if this was very large.
Secondary outcomes
Number of carious surfaces in patients with caries active
The number of carious tooth surfaces was calculated for each patient who had converted to being caries active. Assumptions of normally distributed data were assessed and, when necessary, log-transformations or other analysis methods were used. The groups were compared using a multiple linear regression model, adjusting for the same covariates as the primary analysis (age and MDM). Appropriate descriptive statistics were undertaken by group.
Number of teeth extracted
The number of teeth extracted for each patient who had converted to being caries active was compared between treatment groups using a negative binomial model (if appropriate), adjusting for the same covariates as the primary analyses (age and MDM). Appropriate descriptive statistics were undertaken by group.
Number of episodes of pain
The number of episodes of pain for each patient was compared between treatment groups using a negative binomial model, adjusting for age, MDM and for whether or not the child was caries active at the primary analyses (age and MDM). Appropriate descriptive statistics were undertaken by group.
Economic analysis
The economic analysis compared the mean cumulative costs per child incurred over the 3-year period in each arm of the trial and related these to primary and secondary outcomes achieved over the same period. NHS costs were subdivided into those related to the intervention (intervention group only), those associated with other oral health care provided by dentists (check-ups, fillings, pulpectomies, extractions, etc.] and those associated with care provided by other health service professionals [general practitioner (GP) visits, inpatient and outpatient episodes, etc.].
All intervention data were gathered using the site CRF, a paper record, completed at each of the 6-monthly scheduled visits. Unit costs associated with each activity are set out in Appendix 4, Table 33, together with the source from which these were obtained. For the intervention group, direct intervention costs comprised toothpaste and toothbrushes, fluoride-containing varnish and the delivery time involved in applying fluoride-containing varnish, as well as a dental check-up during the course of which the varnish was applied. In the control group, the visit to the dentist at the 6-month interval was treated as a check-up for cost purposes. Details of other dental activity provided during the course of scheduled visits by dentists, whether to the intervention or control group, were also gathered using the CRF completed at the 6-monthly scheduled visits. Unit costs associated the various types of activity undertaken are set out in Appendix 4, Table 33, together with the source from which these were derived, which in the majority of cases was the Statement of Dental Remuneration (SDR) 2014/15. 88 Dentists in Northern Ireland are reimbursed for publicly funded care on a fee-for-service basis, with reimbursement levels detailed in the SDR. The SDR contains much more specific data on which to base activity costs, with approximately 450 individual cost codes existing, than the broader UDAs operated in England. Moreover, as the SDR reflects the actual potential earnings available from publicly funded dentistry in Northern Ireland, it provides a superior estimate of the opportunity costs to them than the UDA system.
In respect of other health-care services, data were collected using a questionnaire given to parents at their scheduled 6-monthly visits. This collected details on GP visits, inpatient days, outpatient visits and accident and emergency (A&E) service visits in the preceding 6 months. Again, cumulative costs over the 3 years were examined. Unit costs in respect of these services were taken from standard UK references in the absence of more suitable data in Northern Ireland. Details are provided in Appendix 4, Table 33.
When participants undertook visits in addition to the 6-monthly scheduled visits, denoted in the study as unscheduled visits to the trial dentist, details on activity undertaken were captured in the CRF and unit costs applied in an identical fashion to that in respect of scheduled visits. When participants undertook unscheduled visits to other dentists, details on activity undertaken were captured in the parental questionnaire completed at 6-monthly intervals and unit costs were applied again in an identical fashion as in respect of scheduled visits to trial dentists.
Activity was aggregated over the course of the 3 years of the study and multiplied by the relevant unit cost to produce an estimate of health-care costs. Health-care costs were examined in total and under separate subheadings related to the intervention, non-intervention activity provided by trial dentists, non-intervention activity provided by other dentists, activity provided by GDPs, hospital inpatient units, outpatient units and A&E units. Direct health-care costs (intervention) and indirect health-care costs (non-intervention-related health care both dentistry and other) were also examined. Given the relatively short duration of the study, 3 years, neither costs nor outcomes were discounted.
Imputations
When activity was recorded or if it was explicitly recorded that none took place (i.e. a zero was returned), these values were used in analyses. When a value was not returned in the CRF or parental questionnaire, imputations were made. When the intervention was not observed to have taken place in the CRF (i.e. a missing value existed) at a particular time point, no intervention was assumed to have been provided other than the posting of toothbrushes and toothpaste. A zero cost was applied in respect of delivery time, but not in respect of these other elements of the intervention. In respect of activity provided by trial dentists during scheduled visits, related to fillings of primary or permanent teeth, pulpectomies and extractions involving a local anaesthetic, missing values in the CRF were replaced at the mean for the group at that time point for the activity concerned. (The assumption was that such activity may have occurred but simply been unobserved.) An examination of data captured under the heading ‘other activities’ (provided by dentists) revealed that this largely consisted of fissure sealant application or check-ups/advice. When ‘other activity’ was recorded a unit cost for fissure sealant was applied, and again when missing values arose, imputations at the mean for the group concerned and time point concerned applied. (An examination of the unit cost for fissure sealants and check-ups shows that these are virtually identical, as seen in Appendix 4, Table 33.)
In respect of GP visits, missing data were again imputed at the mean of the group for the time point concerned, as was the case in respect of A&E department visits. In respect of inpatient and outpatient days, which were rare occurrences, and when data were heavily skewed, imputations were based on the median of the group (which was zero); the median was thought to provide a more robust measure of central tendency in these cases.
In respect of unscheduled visits whether to the trial or other dentists, when activity was not observed (missing values) activity was assumed not to have taken place and a zero value recorded. Similar to inpatient and outpatient visits, unscheduled visits were relatively rare. Extractions under GA were captured in the CRF in free-text format and when free text explicitly referenced the use of GA for extractions, a cost was estimated (see Appendix 4, Table 33).
As part of the sensitivity analyses, costs incurred by parents were included in addition to those incurred by the health service, these costs related to travel and time taken off work. In respect of travel, the parental questionnaire captured details of distance travelled to the trial and other dentists. Although this was captured at six time points, the time point at which the data were most complete was the first, that is, it had fewest missing values. When scheduled or unscheduled visits were observed to occur the distance (there and back) for the first visit was used. When missing values existed, these were imputed at the mean for the group. Distance was monetised using the cost per mile for a typical car journey using Automobile Association (AA) estimates. (see Appendix 4, Table 33). Although some parents used public transport or walked what were short distances to the dentist, most drove and car costs were used to impute travel costs; therefore, all travel costs were monetised using the same unit cost.
Time off work was captured at six time points for visits to trial and other dentists. Here missing values were imputed as zeros on the premise that individuals who have taken time off work would remember and report this. The value of time was estimated at the average gross wage rate for Northern Ireland, as reported in Appendix 4, Table 33.
As with distance, journey times (there and back) were most complete at the first scheduled visit. It was therefore used for all observed subsequent scheduled and unscheduled visits to the trial dentist. The group mean was used to impute those parents for whom data on journey time were missing. In respect of other dentists, the time point at which data were most complete was the last. It was therefore used to impute journey time for all other visits when these were reported to occur, but data on journey time were missing. When visits to other dentists were not explicitly reported to have occurred, journey time was assumed to be zero. As the cost of journey times would have been included in the cost of time off work, no attempt was made to monetise this to avoid double counting. Parental costs were then the sum of time off work and travel costs over the duration of the study.
The economic analysis compared the total cost to the NHS for care in each of the two arms of the trial, in accordance with the levels of effectiveness for each of the two arms. In additional analyses, parental costs were added to those falling on the NHS.
We calculated the costs associated with the additional effects produced in the intervention group. A multiple linear regression model was fitted to the individual costs per child with group, age and socioeconomic status (measured by MDM 2010) as covariates. The model was estimated with robust standard errors. Ordinary least squares (OLS) estimates did not provide a good fit to the cost data. A generalised linear model was estimated with a log-link function and gamma distribution as an alternative. This model, which is commonly used in the analysis of cost data, was found to provide a superior fit to the data. This generated an assessment of the additional level of investment required to achieve the measured benefit. Incremental cost-effectiveness ratios (ICERs) were calculated to provide an estimate of the mean cost per additional unit of effectiveness produced by the intervention. A series of sensitivity analyses were undertaken. These included a re-estimation of cost-effectiveness when parental costs (travel and time off work) were included in costs; an analysis that examined cost-effectiveness based on measured delivery time as opposed to time reported by dentists (this used data captured during a time-and-motion study of 38 children treated with topical fluoride during the study); an analysis in which fluoride was assumed to have been applied by a dental nurse rather than a dentist; an analysis in which fluoride was assumed to have been applied by a dental hygienist rather than a dentist; and an analysis in which costs were examined solely from the perspective of a dental care service (i.e. ignoring parental costs or costs to other parts of the health service).
The ICERs were estimated following a bootstrapping exercise in which sample data were used to construct a sampling distribution of mean costs, effects, incremental costs and effects and ICERs. Net monetary benefits (NMBs) were also calculated. In the absence of an estimated threshold willingness to pay for the various measures of effect, a threshold of £1000 was selected for each. Cost-effectiveness acceptability curves (CEACs) were generated in respect of each outcome to examine uncertainty around the threshold. One thousand bootstrapped samples were generated. Although the primary analysis focused on the incremental health-care costs divided by the proportion caries free, additional analyses examined mean cost per carious surface avoided and per episode of pain avoided. Separate analyses examined health-care costs and health-care plus parental costs, as well as costs based on observed delivery time, dental nurse-based and hygienist-based application of fluoride and adopting a perspective of a dental service.
Mean ICERs are presented together with CIs. CIs were constructed by rank ordering the bootstrapped ICERs and identifying the 2.5th percentile and 97.5th percentile values. To examine uncertainty in the value of the ICER as a result of sampling variation, as noted, CEACs were also constructed. Issues around the modelling of uncertainty using ICERs are well documented in the literature. NMB estimates with bootstrapped CIs were also generated and used to interpret findings.
Adverse reactions
The protocol specified all adverse events (AEs) would be described and that we would compare AEs between the intervention and control groups, taking into account the same covariates as the primary analyses (age, MDM, etc.). However, because AEs were potentially so numerous and the majority unconnected with the intervention, on the advice of our Independent Data Monitoring and Ethics Committee we specified that only SAEs or ARs related to the fluoride-containing varnish would be monitored and reported. The number of patients with one or more SAEs or ARs related to the fluoride-containing varnish was summarised descriptively by treatment group.
Chapter 3 Results
Recruitment and randomisation
Between April 2010 and September 2010, there were 366 general dental practices in Northern Ireland providing care for NHS patients. All dental practices in Northern Ireland were sent a letter inviting them to participate in the trial, and 78 practices responded to express an interest in participating in the trial. From these 78 practices, 22 practices were selected based on size, location and willingness to participate. The 22 dental practices invited 2455 of their 2- to 3-year-old patients to attend for a screening examination to determine their eligibility to take part in the trial (Figure 4). Children attending were screened by 12 CDS dentists to ensure that they were caries free and eligible. Approximately half attended, and were eligible to be enrolled in the trial, with 624 children being randomised to each of the study arms. The numbers randomised (n = 1248) therefore exceeded the planned sample size of 1200 children. The reasons that children were not enrolled in the study are given in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram (see Figure 4) and fall into five categories: CDS assessor refused, parent withheld consent, did not attend screening, ineligible (e.g. unco-operative) and other reasons.
FIGURE 4.
The CONSORT flow chart. a, Not randomised (n = 1207, 49.2%). CDS assessor refusal (n = 36); parent withheld consent (n = 138); did not attend (n = 758); ineligible [n = 158; caries (n = 85), age (n = 35), allergies (n = 22), hospitalisation (n = 10), adverse medical history (n = 2), other trial (n = 2), history of lactose intolerance (n = 1) and not known (n = 1)]; other reasons [n = 117; patient would not co-operate (n = 64), sibling recruited (n = 16), appointment cancelled (n = 15), parent absent (n = 11), already on trial (n = 3), language barrier (n = 3), family migrating (n = 2), patient sick (n = 2) and family left owing to appointment (n = 1)]. b, Timings and reasons for withdrawals given in Tables 8 and 9. c, One child attended, but did not have caries examination.

The final clinical outcome assessments were undertaken 36 months later by 12 CDS dentists examining 1096 children, 549 in the intervention and 547 in the control arms, also greater than the sample size required to detect the difference specified in the protocol of 510 per group. Overall, 87.8% of children randomised were examined (estimated in the protocol to be 85%). Ninety-one children withdrew from the trial during the study and a further 60 children did not attend the outcome assessment (28 in the intervention arm and 32 in the control arm), so were lost to follow-up at this point. One child in the intervention group attended, but there was no caries charting done. The numbers of withdrawals are shown over time for each study group in Table 8 and the reasons in Table 9. Table 8 also shows how many children attended or failed to attend their dentist for each 6-monthly appointment during the trial. Both the numbers of withdrawals and failures to attend were similar between the different time periods and between the two study groups. The majority of withdrawals were initiated by dentists as a result of failure to attend successive appointments (see Table 9).
| Visit (months) | Group, n | |||||
|---|---|---|---|---|---|---|
| Intervention (n = 624) | Control (n = 624) | |||||
| Attended | DNA | Withdrawn | Attended | DNA | Withdrawn | |
| 6 | 602 | 16 | 6 | 591 | 26 | 7 |
| 12 | 579 | 28 | 11 | 578 | 31 | 8 |
| 18 | 554 | 46 | 7 | 553 | 51 | 5 |
| 24 | 550 | 43 | 7 | 553 | 42 | 9 |
| 30 | 542 | 41 | 10 | 540 | 45 | 10 |
| 36 (caries examination) | 550a | 28a | 5 | 547 | 32 | 6 |
| Total | 46 | 45 | ||||
| Reason | Group, n (%) | |
|---|---|---|
| Intervention (n = 46) | Control (n = 45) | |
| Dentist withdrew child as a result of failure to attend (n = 38) and child was unco-operative (n = 1) | 22 (48) | 17 (38) |
| Moved to another practice | 14 (30) | 15 (33) |
| Moved out of area | 5 (11) | 5 (11) |
| Enrolled in error (caries at baseline, sibling in study, wrong age) | 1 (2) | 2 (4) |
| Child did not want to participate | 1 (2) | 0 (0) |
| Parent withdrew child | 3 (7) | 5 (11) |
| Referred to CDS | 0 (0) | 1 (2) |
The reasons why dentists were withdrawing children resulting from failure to attend was that they were following local practice policies on non-attendance. This was picked up at an early stage and these local policies were stopped for trial children. The dentists with whom these children were registered recorded no reason for the withdrawal. However, there does not seem to be any bias in the numbers withdrawn by study group.
Data on the number of varnish applications and the number of study visits for participants who were examined at the 36-month outcome assessment are shown in Table 10. The number of visits is greater than the number of varnish applications for children in the intervention group as occasionally, although a child attended, the dentist was unable to deliver the fluoride-containing varnish. It can be seen that 87% of children in the intervention group and 85% of the children in the control group attended all the 6-monthly visits to the practice (p = 0.77). All of the children attended at least once.
| Summary measures | Group | ||
|---|---|---|---|
| Intervention (n = 549) | Control (n = 547) | ||
| Visits | Varnish applications | Visits | |
| Mean (SD) | 5.8 (0.56) | 5.8 (0.62) | 5.8 (0.57) |
| Median (minimum, maximum) | 6 (1, 6) | 6 (1, 6) | 6 (2, 6) |
| Number having six visits (%) | 476 (86.7) | 470 (85.6) | 462 (84.5) |
| Number receiving no visits | 0 | 0 | 0 |
| Missing | 0 | 0 | 0 |
The recruitment took place over a 14-month period from May 2011 to June 2012, during which time 1248 participants were steadily recruited to the Northern Ireland Caries Prevention In Practice (NIC-PIP) trial from 22 dental practices in Northern Ireland (Figure 5). The numbers of children recruited and randomised in each practice are shown in Table 11. The 36-month outcome assessments were carried out between April 2014 and June 2015, and the numbers examined for each practice are shown in Table 12. None of the practices dropped out of the trial. The percentage of the randomised children examined at the outcome assessment varies across practices from 72.4% to 100% in the intervention group and from 61.5% to 100% in the control group.
FIGURE 5.
Recruitment of the trial participants over time.
| Practice | Group, n (%) | Total recruited, n (%) | |
|---|---|---|---|
| Intervention | Control group | ||
| 11 | 120 (19.2) | 120 (19.2) | 240 (19.2) |
| 12 | 18 (2.9) | 18 (2.9) | 36 (2.9) |
| 13 | 20 (3.2) | 21 (3.4) | 41 (3.3) |
| 15 | 14 (2.2) | 14 (2.2) | 28 (2.2) |
| 16 | 16 (2.6) | 15 (2.4) | 31 (2.5) |
| 17 | 14 (2.2) | 13 (2.1) | 27 (2.2) |
| 18 | 44 (7.1) | 44 (7.1) | 88 (7.1) |
| 19 | 31 (5.0) | 32 (5.1) | 63 (5.1) |
| 20 | 14 (2.2) | 14 (2.2) | 28 (2.2) |
| 21 | 26 (4.2) | 26 (4.2) | 52 (4.23) |
| 22 | 12 (1.9) | 11 (1.8) | 23 (1.8) |
| 23 | 20 (3.2) | 20 (3.2) | 40 (3.2) |
| 24 | 22 (3.5) | 23 (3.7) | 45 (3.6) |
| 25 | 52 (8.3) | 52 (8.3) | 104 (8.3) |
| 26 | 21 (3.4) | 22 (3.5) | 43 (3.5) |
| 27 | 12 (1.9) | 13 (2.1) | 25 (2.0) |
| 29 | 21 (3.4) | 21 (3.4) | 42 (3.4) |
| 30 | 14 (2.2) | 13 (2.1) | 27 (2.2) |
| 31 | 58 (9.3) | 57 (9.1) | 115 (9.2) |
| 32 | 9 (1.4) | 9 (1.4) | 18 (1.4) |
| 33 | 32 (5.1) | 32 (5.1) | 64 (5.1) |
| 34 | 34 (5.5) | 34 (5.5) | 68 (5.5) |
| Total | 624 (100) | 624 (100) | 1248 (100) |
| Practice | Group, n (% from baseline) | Total examined, n (% from baseline) | |
|---|---|---|---|
| Intervention | Control group | ||
| 11 | 107 (89.2) | 109 (90.8) | 216 (90.0) |
| 12 | 16 (88.9) | 17 (94.4) | 33 (91.7) |
| 13 | 19 (95.0) | 20 (95.2) | 39 (95.1) |
| 15 | 14 (100.0) | 14 (100.0) | 28 (100.0) |
| 16 | 13 (81.3) | 10 (66.7) | 23 (74.2) |
| 17 | 13 (92.9) | 13 (100.0) | 26 (96.3) |
| 18 | 40 (90.9) | 40 (90.0) | 80 (90.9) |
| 19 | 29 (93.5) | 28 (87.5) | 57 (90.5) |
| 20 | 12 (85.7) | 13 (92.9) | 25 (89.3) |
| 21 | 19 (73.1) | 16 (61.5) | 35 (67.3) |
| 22 | 11 (91.7) | 11 (100.0) | 22 (95.7) |
| 23 | 20 (100.0) | 20 (100.0) | 40 (100.0) |
| 24 | 20 (90.9) | 20 (87.0) | 40 (88.9) |
| 25 | 47 (90.4) | 50 (96.2) | 97 (93.3) |
| 26 | 17 (81.0) | 21 (95.5) | 38 (88.4) |
| 27 | 11 (91.7) | 10 (76.9) | 21 (84.0) |
| 29 | 18 (85.7) | 19 (90.5) | 37 (88.1) |
| 30 | 12 (85.7) | 12 (92.3) | 24 (88.9) |
| 31 | 42 (72.4) | 43 (75.4) | 85 (73.9) |
| 32 | 7 (77.8) | 7 (77.8) | 14 (77.8) |
| 33 | 28 (87.5) | 26 (81.3) | 54 (84.4) |
| 34 | 34 (100.0) | 28 (82.4) | 62 (91.2) |
| Total | 549 (88.0) | 547 (87.7) | 1096 (87.8) |
Baseline data
The baseline demographic data for all children randomised are shown in Table 13. It can be seen that there is good balance between the two study groups for gender, age and MDM quintiles. Two MDM quintile values are missing, as the postcodes were not on the MDM 2010 databases. These were coded as the middle quintile in the analysis. Children aged between 2 and 3 years at baseline were recruited and the mean age was 3.1 years in both study groups. Table 14 shows the same data for children who were examined at the 36-month outcome examination. It can be seen that the numbers of children examined at 36 months were evenly balanced between groups, and had similar age and MDM quintile profiles to all the children at baseline. At the 36-month outcome assessment children in both groups had a mean age of 6.0 years.
| Demographic variables | Group | Total (n = 1248) | |
|---|---|---|---|
| Intervention (n = 624) | Control (n = 624) | ||
| Gender, n (%) | |||
| Male | 283 (45.4) | 296 (47.4) | 597 (46.4) |
| Female | 341 (54.7) | 328 (52.6) | 669 (53.6) |
| Age (years) | |||
| Mean (SD) | 3.1 (0.53) | 3.1 (0.53) | 3.1 (0.53) |
| Median (minimum, maximum) | 3.1 (2.0, 4.0) | 3.0 (2.0, 4.0) | 3.1 (2.0, 4.0) |
| Missing | 0 | 0 | 0 |
| MDM, n (%) | |||
| Quintile 1 (most deprived) | 88 (14.1) | 106 (17.0) | 194 (15.6) |
| Quintile 2 | 141 (22.6) | 134 (21.5) | 275 (22.1) |
| Quintile 3 | 172 (27.6) | 155 (24.9) | 327 (26.4) |
| Quintile 4 | 148 (23.8) | 155 (24.9) | 303 (24.3) |
| Quintile 5 (least deprived) | 74 (11.9) | 73 (11.7) | 147 (11.8) |
| Missing | 1 | 1 | 2 |
| Demographic variables | Group | Total (n = 1029) | |
|---|---|---|---|
| Intervention (n = 549) | Control (n = 547) | ||
| Gender, n (%) | |||
| Male | 242 (44.1) | 265 (48.5) | 507 (46.3) |
| Female | 307 (55.9) | 282 (51.6) | 589 (53.7) |
| Age (years) | |||
| Mean (SD) | 3.1 (0.52) | 3.1 (0.53) | 3.1 (0.53) |
| Median (minimum, maximum) | 3.1 (2.0, 4.0) | 3.0 (2.0, 4.0) | 3.1 (2.0, 4.0) |
| Missing | 0 | 0 | 0 |
| MDM, n (%) | |||
| Quintile 1 (most deprived) | 79 (14.4) | 95 (17.4) | 174 (15.9) |
| Quintile 2 | 116 (21.2) | 117 (21.4) | 233 (21.3) |
| Quintile 3 | 153 (27.9) | 136 (24.9) | 289 (26.4) |
| Quintile 4 | 136 (24.8) | 135 (24.7) | 271 (24.8) |
| Quintile 5 (least deprived) | 64 (11.7) | 63 (11.5) | 127 (11.6) |
| Missing | 1a (< 0.1) | 1a (< 0.1) | 2 (< 0.1) |
Outcomes of calibration of community dental service examiners
Three training sessions were held to calibrate the caries assessors with a gold standard-experienced caries epidemiologist, prior to the baseline screening, before the outcome assessment and at the mid-point of outcome assessments. At baseline, one of the 12 examiners failed to achieve a kappa value of > 0.7 for teeth and did not take part in any of the caries assessments (Table 15). Some additional examiners to those involved in the baseline assessments were involved in the outcome assessments, and all achieved the required standard.
| Examination and date | Range of inter-examiner assessments | |
|---|---|---|
| 12 dentists; 25 children; compared with gold standard | 12 dentists; 23–25 children | |
| Kappa | Kappa | |
| Baseline (5–6 October 2010) | ||
| Teeth (n = 500) | 0.681a to 0.907 | 0.855 to 0.955 |
| Surfaces (n = 2200) | 0.509 to 0.911 | 0.864 to 0.955 |
| Prior to outcome assessment (25–27 February 2014) | ||
| Teeth (n = 500) | 0.738 to 0.897 | 0.832 to 0.947 |
| Surfaces (n = 2200) | 0.793 to 0.902 | 0.829 to 0.955 |
| During outcome assessment (11–13 November 2014) | ||
| Teeth (n = 500) | 0.865 to 0.967 | 0.832 to 0.947 |
| Surfaces (n = 2200) | 0.633 to 0.928 | 0.902 to 0.968 |
Disease outcomes and estimation
Primary outcome: conversion from caries-free to caries-active
The caries data for three binary outcomes are shown in Table 16. The primary outcome, the percentage of children who converted to caries active over the trial, was 34% in the intervention group and 39% in the control group. The primary analysis for estimating differences between caries-free and caries-active children with respect to study group, fitting a logistic model adjusted for age and socioeconomic status measured by MDM 2010 quintiles, was not statistically significant (OR 0.81, 95% CI 0.64 to 1.04; p = 0.11) (Table 17). This analysis was repeated categorising age into equal intervals. The likelihood ratio test indicated no difference between the fit of the two models [likelihood ratio chi-squared (3 degrees of freedom) = 3.84; p = 0.28].
| Binary outcome | Group, n (%) | Total n (%) | Difference (control – intervention) in percentages (95% CI) | |
|---|---|---|---|---|
| Intervention | Control | |||
| All children | (n = 549) | (n = 547) | (n = 1096) | |
| Number of children becoming caries active | 187 (34.1) | 213 (38.9) | 400 (36.5) | 4.9 (–0.8 to 10.6) |
| Number of children with toothache | 106 (19.3) | 120 (21.9) | 226 (20.6) | 2.6 (–2.2 to 7.4) |
| Children who developed caries | (n = 187) | (n = 213) | (n = 400) | |
| Number of children who had teeth extracted | 21 (11.2) | 28 (13.1) | 49 (12.3) | 1.9 (–4.5 to 8.3) |
| Number of children with toothache | 69 (36.9) | 95 (44.6) | 164 (41.0) | 7.7 (–1.9 to 17.3) |
| Children who remained caries free | (n = 362) | (n = 334) | (n = 696) | |
| Number of children with toothache | 37 (10.2) | 25 (7.5) | 62 (8.9) | –2.7 (–6.9 to 1.5) |
| Independent variables | OR | Standard error | 95% CI for OR | p-value |
|---|---|---|---|---|
| Intervention-to-control ratio | 0.81 | 0.10 | 0.64 to 1.04 | 0.11 |
| Age | 1.49 | 0.18 | 1.17 to 1.89 | 0.001 |
| MDMa | ||||
| Quintile 2 | 0.76 | 0.16 | 0.51 to 1.14 | 0.19 |
| Quintile 3 | 0.73 | 0.14 | 0.49 to 1.07 | 0.10 |
| Quintile 4 | 0.61 | 0.12 | 0.41 to 0.91 | 0.015 |
| Quintile 5 | 0.46 | 0.12 | 0.28 to 0.76 | 0.002 |
The unadjusted model was similar to the estimate for the adjusted model with an OR of 0.81 (95% CI 0.63 to 1.04; p = 0.09), as was the adjusted model including the clustering of the dental practices, with an OR of 0.81 (95% CI 0.64 to 1.04; p = 0.10). Results for this model are shown in Appendix 5, Table 45.
It can be seen in Table 17 that the proportion of caries-active children was lower in the least deprived MDM quintiles. We undertook a subgroup analysis for children in deprived/affluent areas, as described in Statistical methods including methods for additional analyses. The ‘interaction’ test proved to be non-significant (p = 0.36), although this test does have low power (see Appendix 5, Table 40).
Secondary outcome: decayed, missing, filled tooth surfaces in primary dentition in children with caries active
Data for the discrete variables are presented in Table 18 only for children who were caries active. The secondary outcome was the number of carious surfaces in children who converted to being caries active. The mean number of carious surfaces among the 187 caries-active children in the intervention group was 7.2, compared with 9.6 among the 213 caries-active children in the control group. The adjusted multiple linear regression analysis indicates that this difference is statistically significant with a mean difference of –2.29 carious surfaces (95% CI –3.96 to –0.63 carious surfaces; p = 0.007) (Table 19).
| Discrete variable | Group, mean (SD) | Mean difference (95% CI) | |
|---|---|---|---|
| Intervention (n = 187) | Control (n = 213) | ||
| Mean number of carious surfaces in caries-active children (dmfs) | 7.18 (7.99) | 9.61 (8.75) | –2.43 (–4.08 to –0.77) |
| Mean number of teeth extracted in caries-active children (mt) | 0.45 (1.43) | 0.46 (1.44) | 0.001 (–0.28 to 0.28) |
| Mean number of episodes of pain in caries-active children | 0.85 (1.41) | 1.08 (1.60) | –0.23 (–0.53 to 0.07) |
| Independent variables | Coefficient | Standard error | 95% CI for coefficient | p-value |
|---|---|---|---|---|
| Mean difference (intervention – control) | –2.29 | 0.85 | –3.96 to –0.63 | 0.007 |
| Age | –0.01 | 0.82 | –1.62 to 1.60 | 0.99 |
| MDMa | ||||
| Quintile 2 | –0.17 | 1.31 | –2.75 to 2.40 | 0.89 |
| Quintile 3 | –1.20 | 1.26 | –3.67 to 1.28 | 0.34 |
| Quintile 4 | –0.94 | 1.30 | –3.50 to 1.62 | 0.47 |
| Quintile 5 | –4.04 | 1.71 | –7.41 to –0.67 | 0.02 |
Secondary outcome: number of extracted teeth in caries-active children
In the intervention group, 11.2% of caries-active children had teeth extracted over the 3-year period, compared with 13.1% of caries-active children in the control group (see Table 16), the mean percentage difference being 1.9% (95% CI –4.5% to 8.3%). The mean number of extracted teeth was 0.45 in the intervention group and 0.46 in the control group (see Table 18). A logistic regression model adjusted for age and MDM quintile was not statistically significant (OR 0.84, 95% CI 0.45 to 1.54; p = 0.56) (see Appendix 5, Table 47). The negative binomial model for the number of extracted teeth, which indicated significant overdispersion, was also not statistically significant (regression coefficient –0.03, 95% CI –0.88 to 0.82; p = 0.95) (see Appendix 5, Table 48).
Secondary outcome: number of episodes of pain
There were differences in the proportion of children with pain and the mean number of episodes per child between children who were or were not of caries-active status. The regression models therefore included caries status at follow-up as a covariate. There was no difference in the number of episodes of pain or proportion of children with toothache between the study groups over the 36 months (OR 0.95, 95% CI 0.69 to 1.30; p = 0.74) (see Table 16 and Appendix 5, Table 49). Forty-one per cent of caries-active children had toothache, compared with 9% of children who were caries free; this difference was statistically significant (OR 7.1, 95% CI 5.1 to 9.9; p < 0.001).
As it was difficult to determine single discrete episodes of pain (which went up to 17 episodes), this was capped for each child at a maximum of six over the 36-month period (this affected the scores of eight children). Among caries-active children, the mean number of episodes of pain was 0.85 in the intervention group compared with 1.08 in the control group. For all children, the negative binomial model, adjusted for caries status, for the number of episodes of pain, which indicated significant overdispersion, was also not statistically significant (regression coefficient –0.03, 95% CI –0.32 to 0.25; p = 0.81) (see Appendix 5, Table 50). There was a significant difference in the proportion of children with toothache between those who became caries active (164/400, 41.0%) and those who remained caries free (62/696, 8.9%) (OR 7.1, 95% CI 5.1 to 9.9; p < 0.0001).
Although not identified as outcomes for this study in the protocol, data for other caries indices such as dmft are presented for all children and those caries active in Appendix 5, Table 53. These data may be helpful in comparing the results from this trial with other studies. There was a statistically significant difference (p = 0.0013) in dmft index between the groups when all children are compared; mean dmft index was 1.15 [standard deviation (SD) 2.18] in the intervention group and 1.64 (SD 2.71) in the control group, indicating a relative reduction (prevented fraction) in disease of 30%.
Ancillary analyses of disease
The numbers and percentages of children who become caries active are shown for each MDM quintile and each group in Table 20. It can be seen that there was a large difference between the most deprived and least deprived quintiles, with, overall, 44% of children in the most deprived quintile being caries active, compared with 28% in the least deprived quintile. The mean number of tooth surfaces affected by caries is presented in Table 21 for these children and shows a reduction from 9.6 in the most deprived quintile to 5.3 in the least deprived quintile, a 45% reduction, with marked differences between the study groups.
| MDM | Group), n/N (%) | Total (n = 1096), n/N (%) | |
|---|---|---|---|
| Intervention (n = 549) | Control (n = 547) | ||
| Quintile 1a | 30/79 (38.0) | 47/95 (49.5) | 77/174 (44.3) |
| Quintile 2 | 41/116 (35.3) | 48/117 (41.0) | 89/233 (38.2) |
| Quintile 3 | 60/154 (39.0) | 49/137 (35.8) | 109/291 (37.5) |
| Quintile 4 | 38/136 (27.9) | 52/135 (38.5) | 90/271 (33.2) |
| Quintile 5 | 18/64 (28.1) | 17/63 (27.0) | 35/127 (27.6) |
| MDM | Group | Total (n = 400) | ||||
|---|---|---|---|---|---|---|
| Intervention (n = 187) | Control (n = 213) | |||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Quintile 1a | 30 | 7.93 (10.14) | 47 | 10.64 (8.79) | 77 | 9.58 (9.37) |
| Quintile 2 | 41 | 7.80 (7.37) | 48 | 10.48 (8.59) | 89 | 9.25 (8.12) |
| Quintile 3 | 60 | 7.35 (7.53) | 49 | 8.84 (9.05) | 109 | 8.02 (8.24) |
| Quintile 4 | 38 | 6.53 (8.70) | 52 | 10.06 (9.21) | 90 | 8.57 (9.12) |
| Quintile 5 | 18 | 5.33 (5.18) | 17 | 5.18 (5.45) | 35 | 5.26 (5.23) |
Adverse events and reactions
Once causality had been determined, only ARs, SAEs, serious ARs and suspected unexpected serious ARs defined in the protocol and summarised in Table 22 were recorded on the CRF.
| AEs to be reported | Criteria for reporting |
|---|---|
| AR |
|
| Unexpected AR | |
| SAEs |
|
| Serious ARs | |
| Suspected unexpected serious ARs |
Eighty-two of the 1248 children who were randomised experienced a total of 100 SAEs, 45 (7.2%) in the intervention group and 37 (5.9%) in the control group. The reasons for these are shown in Table 23. Eighty-five were considered to be unrelated, and the remainder unlikely to be related (10 in the intervention group and five in the control group). No serious ARs or suspected unexpected serious ARs were reported.
| SAEs category | Group, n | Total, n | |
|---|---|---|---|
| Intervention | Control | ||
| Cardiac disorders | 4 | 1 | 5 |
| Gastrointestinal disorders | 4 | 5 | 9 |
| General disorders and administration site conditions | 5 | 7 | 12 |
| Infections and infestations | 13 | 9 | 22 |
| Metabolism and nutrition disorders | 1 | 0 | 1 |
| Musculoskeletal and connective tissue disorders | 7 | 4 | 11 |
| Renal and urinary disorders | 1 | 0 | 1 |
| Respiratory, thoracic and mediastinal disorders | 10 | 12 | 22 |
| Skin and subcutaneous tissue disorders | 1 | 1 | 2 |
| Surgical and medical procedures | 9 | 6 | 15 |
| Total | 55 | 45 | 100 |
A logistic regression model for a child having a SAE or not, estimating the difference between the study groups and adjusted for age and MDM quintile, was not statistically significant (OR 1.23, 95% CI 0.79 to 1.94; p = 0.36) (see Appendix 5, Table 51). The negative binomial model for the number of SAEs, which indicated significant overdispersion, was also not statistically significant (regression coefficient 0.19, 95% CI –0.27 to 0.65; p = 0.42) (see Appendix 5, Table 52).
A further 10 children in the intervention group had ARs/unexpected ARs of a minor nature that were considered to be related to the treatment (four gastrointestinal disorders, five general disorders and administration site conditions, and one skin and subcutaneous tissue disorder).
Economic results
Table 24 presents details on intervention costs and the costs associated with scheduled check-ups among the control group. The largest elements of cost in the intervention group are seen to be time taken to apply fluoride (delivery time), which in this analysis is based on time taken as reported by the dentist. The SDs in respect of brushes and toothpaste as well as postage are seen to be zero, reflecting the assumption that all those who remained in the study received these regardless of whether or not they attended a scheduled check-up.
| Activity/consumable | Group | |||||
|---|---|---|---|---|---|---|
| Intervention (n = 549) | Control (n = 547) | |||||
| Mean cost (£) | SD (£) | Skewness | Mean cost (£) | SD (£) | Skewness | |
| Brushes and toothpaste | 2.40 | 0 | – | 0 | 0 | – |
| Delivery time | 94.81 | 26.44 | 0.88 | 0 | 0 | – |
| Fluoride varnish | 4.36 | 0.42 | –3.86 | 0 | 0 | – |
| Postage including time | 5.70 | 0 | – | 0 | 0 | – |
| Cost of check-up at which fluoride varnish is applied | 48.48 | 4.64 | –3.86 | 0 | 0 | – |
| Intervention | 155.74 | 28.75 | 0.37 | 48.21a | 4.76a | –2.94a |
In Table 25, indirect health-care costs are reported together with differences in these between treatment and control groups under a number of separate headings. Statistically significant differences are noted, for example in respect of a number of cost elements related to dental care, the control group having higher costs associated with fillings and extractions. In respect of other health-care costs, GP use and the various aspects of hospital use, no statistically significant differences in cost are evident.
| Activity | Group | Mean difference in n | Mean difference in cost (£) | |||
|---|---|---|---|---|---|---|
| Intervention | Control | |||||
| Mean number | Mean cost (£) | Mean number | Mean cost (£) | |||
| Dental | ||||||
| Non-intervention-related visits to trial dentist | 1.88 | 29.11 | 2.38 | 32.03 | 0.50 | 2.92 |
| Non-intervention-related visits to other dentists | 0.09 | 0.79 | 0.11 | 0.93 | 0.02 | 0.14 |
| Number of fillings | 0.68 | 6.01 | 1.02 | 9.08 | 0.34* | 3.06* |
| Number of extractions with injections | 0.01 | 0.09 | 0.12 | 0.95 | 0.10* | 0.86* |
| Number of extractions with GA | 0.016 | 12.97 | 0.015 | 11.57 | –0.002 | –1.40 |
| Number of other procedures | 1.19 | 10.06 | 1.25 | 10.49 | 0.05 | 0.43 |
| Non-dental | ||||||
| Number of GP visits | 9.04 | 416.01 | 8.75 | 402.65 | 0.29 | –13.36 |
| Number of outpatient visits | 0.70 | 131.51 | 0.40 | 76.01 | 0.29 | –55.49 |
| Number of inpatient days | 0.34 | 109.60 | 0.21 | 69.94 | –0.12 | –39.65 |
| Number of A&E visits | 1.17 | 144.76 | 1.17 | 145.14 | 0.00 | 0.38 |
In Table 26, costs incurred by parents associated with the consumption of dental care are reported, again with between-group differences. No statistically significant differences in cost were found between the two groups over the 3-year period of the study under the various headings presented.
| Resources consumed in association with dental care | Group | Mean difference | |
|---|---|---|---|
| Intervention | Control | ||
| Average travel time to trial dentist | 166.57 | 169.28 | 2.71 |
| Average travel time to other dentistsa | 1.27 | 2.29 | 1.02 |
| Average distance to trial dentist | 32.25 | 34.20 | 1.96 |
| Average distance to other dentistsa | 0.65 | 0.87 | 0.22 |
| Average time taken off work to visit trial dentist | 139.19 | 137.24 | –1.95 |
| Average time taken off work to visit other dentists | 4.34 | 5.65 | 1.30 |
In Table 27, total direct health-care (i.e. intervention) costs, total indirect health-care costs (other dental care, as well as care provided by GDPs and hospitals) and total parental costs are presented together with between-group differences in these. All costs are based on the cumulative use of services over the course of the 3 years of the study and expressed on a per-person basis. Also shown are total health-care costs (intervention and other health care) and total costs (total health care plus parental) as well as between-group differences in these. As can be seen, in respect of direct costs, total health-care costs and total costs, statistically significant between-group differences in cost are evident. In each case the intervention group is seen to have higher costs, although, as is evident from a comparison of Table 27 with previous tables, this is largely related to the cost of the intervention.
| Costs (£) | Group | Mean difference | |
|---|---|---|---|
| Intervention | Control | ||
| Average total direct health service costsa | 155.74 | 48.21 | –107.53b |
| Average total indirect health service costc | 831.79 | 726.76 | –105.03 |
| Average total parental costd | 39.78 | 40.72 | 0.94 |
| Average total health-care costs (sum of rows 1 and 2) | 987.53 | 774.97 | –212.56b |
| Average total coste | 1027.31 | 815.69 | –211.62b |
In Table 28, the results of an OLS regression examining the relationship between total costs, age, gender, socioeconomic status and group are shown. The model is seen to have limited explanatory power and only two of the covariates are seen to be statistically significant: gender and membership of the intervention group. In Table 29, the results of a generalised linear model are reported. The latter was found to provide a superior fit to the data than a simple OLS model. In terms of predictors, gender and group membership remained statistically significant. Females and those in the intervention group had higher costs than males and those in the control group.
| Independent variable | Coefficient | p-value |
|---|---|---|
| Age (at outcome) | –39.2326 | 0.39 |
| Gender (female) | 99.7099 | 0.05 |
| SES Q1 (most deprived) | –20.4995 | 0.78 |
| SES Q2 | 64.1385 | 0.45 |
| SES Q3 | 52.4055 | 0.59 |
| SES Q4 (least deprived) | –59.8999 | 0.35 |
| Group (intervention) | 206.0822 | < 0.01 |
| Constant | 852.7936 | < 0.01 |
| R 2 | 0.02 | |
| F-statistic | F(6,1089) = 2.64 | p = 0.01 |
| Independent variable | Coefficient | p-value |
|---|---|---|
| Age (at outcome) | –0.0431 | 0.36 |
| Gender (female) | 0.1068 | 0.04 |
| SES Q1 (most deprived) | –0.0047 | 0.82 |
| SES Q2 | 0.0820 | 0.49 |
| SES Q3 | 0.0577 | 0.56 |
| SES Q4 (least deprived) | –0.0547 | 0.50 |
| Group (intervention) | 0.2310 | < 0.01 |
| Constant | 6.7337 | < 0.01 |
| Diagnostics | ||
| AIC | 15.5569 | |
| BIC | –7356.09 |
A generalised linear model was fitted to the data with a log-link function and a gamma distribution for the mean–variance relationship.
A generalised linear model with identity link and Gaussian distribution was used to reproduce the estimates in Table 28. Akaike information criterion and Bayesian information criterion statistics were captured. These were 16.4197 and 8.55e × 108. The model in Table 29 is clearly superior. This in essence confirmed the estimated relationships between total costs and the regressors.
In Table 30, a series of ICERs are reported together with associated CIs. ICERs are reported for three outcome measures: caries-free status, carious surfaces and episodes of pain. In each case the negative ICER should be interpreted as the additional cost per outcome avoided. For example, in respect of caries-free status, it costs approximately £2093 to prevent one person converting to caries active; in respect of carious surfaces it costs approximately £251 to prevent the development of one carious surface. As is evident from Table 30, statistically significant results were obtained only in respect of carious surfaces. That is, and reflecting the results of the clinical analysis, the intervention was not found to produce a significant benefit despite incurring additional costs in respect of caries-free status and episodes of pain.
| Costs (£) | Mean | 95% CI |
|---|---|---|
| Mean difference in health service costs/mean difference in proportion caries free | –2092.59 | –30,100.40 to 27,921.80 |
| Mean difference in health service cost/mean difference in number of carious surfaces | –250.58 | –454.39 to –79.52 |
| Mean difference in health service cost/mean difference in number of episodes of pain | –259.07 | –14,664.00 to 14,941.60 |
| Mean difference in total costs/mean difference in proportion caries free | –2070.51 | –29,477.20 to 27,876.60 |
| Mean difference in total cost/mean difference in number of carious surfaces | –248.80 | –456.69 to –78.70 |
| Mean difference in total cost/mean difference in number of episodes of pain | –263.96 | –14,529.80 to 14,560.70 |
Note that the ICERs and CIs are based on 1000 bootstrapped samples used to construct the sampling distribution for costs and outcomes. As these are bootstrapped they will not (other than by chance) replicate the sample values. ICERs are based on each bootstrapped average incremental cost being divided by each bootstrapped incremental benefit. The CI is then constructed by rank ordering the ICERs and identifying those at the 2.5th and 97.5th percentiles.
To avoid issues associated with modelling uncertainty in ICERs, a series of NMB estimates relating outcomes to cost were also calculated (Table 31). Each is predicated on a threshold of £1000, that is, the assumption that society would be willing to pay £1000 for a 1-unit increase in the outcome in question.
| Effect | Mean | 95% CI |
|---|---|---|
| NMB per caries-free person | –£165.06 | –£291.04 to –£44.79 |
| NMB per carious surface avoided | £1063.81 | £298.08 to £1854.62 |
| NMB per episode of pain avoided | –£114.13 | –£280.45 to £36.46 |
Note that these are based on bootstrapped NMBs in which a threshold of £1000 is assumed for each outcome. As with Table 30, a positive NMB was found in respect of carious surfaces only. Here an NMB of £1063 was found. This suggests that if society was willing to pay £1000 to avoid a carious surface, the invention would deliver a NMB of approximately £1064 when the intervention and other costs associated with its generation are taken into consideration. Although the 95% CI around this value is positive, it is quite wide-ranging, from approximately £298 to £1855.
Cost-effectiveness planes for each outcome and CEACs in respect of each outcome are also reported (see Appendix 4, Figures 6–11). The appearance of the former in the north-east quadrant of the diagram (i.e. higher cost, negative outcome), simply reflects the structure of the outcome, that is, caries status, carious surfaces or episodes of pain avoided.
In respect of the CEACs, it is unclear what value society may place on each of the outcomes modelled. Over the range of thresholds examined, that the likelihood of the intervention being deemed to be cost-effective is highest in respect of carious surfaces avoided is entirely consistent with the results of previous analyses.
In Appendix 4, Tables 34–38, the results of a series of sensitivity analyses are reported. Appendix 4, Table 34, compares the measured time that dentists took to apply fluoride with the reported time taken. As can be seen, dentists consistently overestimated delivery time and adjusting for this markedly reduced intervention costs. As can be seen from Tables 30 and 31, however, this did not have a material effect on cost-effectiveness or on NMB. The cost-effectiveness ratio remained significant only in respect of carious surfaces, dropping to £150 (see Appendix 4, Table 37) from roughly £251, whereas the NMB rose to approximately £1119 (see Appendix 4, Table 38) from approximately £1064.
Similar results were obtained in respect of other sensitivity analyses based on using nurses and dental hygienists to apply fluoride and limiting the focus of the analyses solely to dental costs. That is, the reduction in staff costs (and exclusion of other health-care costs) improved the cost-effectiveness ratio and NMB calculation, but the intervention remained potentially cost-effective only in respect of reducing carious surfaces, and then only dependent on the willingness-to-pay threshold used.
Chapter 4 Discussion
This is the first large-scale CTIMP that has been successfully completed in UK general dental practices. The trial investigated the effects and costs of a composite dental caries ‘preventative package’ in general dental practice. As the NHS seeks to reform dental contracts to support a preventative approach to the care of patients, there is a need to understand if this policy direction benefits patients and reduces costs. Therefore, this trial provides timely and valuable information to inform and support parents, clinicians, policy-makers and NHS commissioners. In addition to providing information about the effects of the intervention on caries and its impact on health-care costs, the trial provides contemporary information about the longitudinal behaviour of dental caries in young children and the consequences of the disease. This descriptive information makes a valuable contribution to our understanding of the dental caries, as there are surprisingly few longitudinal data available on this, the most common disease of childhood.
The trial hit all of its recruitment and retention targets: outcome data at follow-up were available for 88% of children who were randomised. Only one small practice had a retention rate of < 70%. Parents and children in both arms of the trial exhibited high levels of adherence to the protocol: approximately 85% of children attended every 6 months for 3 years and a mean of 5.8 varnish applications were provided to children in the intervention group. The strict monitoring requirements of a CTIMP ensured high levels of treatment fidelity to the protocol in both intervention and control groups.
Despite the large proportion of children who attended every 6 months, 34% of children in the intervention group and 39% in the control group converted from caries-free to caries-active status over the 3-year period of the trial. This high level of disease in the trial population is reflected in the 40% prevalence of obvious decay experience in Northern Ireland 5-year-olds reported in the Children’s Dental Health Survey 2013. Report 2: Dental Disease and Damage in Children England, Wales and Northern Ireland. 3 The differences in age groups between the trial and child dental health survey population, and the under-representation of the most and least socially disadvantaged groups in the trial population (see Generalisability) when compared with the general population may explain the small observed difference between the trial population and child dental health survey population.
The high level of adherence demonstrates that parents were well motivated to attend the dentist. In addition to the fluoride intervention, both groups in the trial received the same caries prevention advice, based on the guidance in DBOH, it was therefore disappointing that over one-third of children in each group developed the disease. The failure to prevent disease in over one-third of participants, suggests that the motivation to attend the dentist on a regular basis did not translate to the adoption of other risk-reducing behaviours in the home setting, such as frequent use of fluoride-containing toothpaste and limiting the amount and frequency of sugar consumption. The limitations of health education and lack of strong evidence for one-to-one dietary interventions in producing sustained behaviour change have been documented. 52,89 Owing to ethical and pragmatic constraints, the trial did not have an arm in which children received no intervention, so we cannot infer that preventative care provided in general dental practice is ineffective. Instead we compared the costs and effects of an enhanced prevention regime with a proxy for ‘usual practice’. The results from this trial suggests that ‘enhanced’ prevention using a composite fluoride intervention provided by health-care professionals, delivered in two, approximately 15- to 20-minute encounters each year, are limited in their effectiveness to prevent caries.
The consequences of a child developing caries are starkly demonstrated by our results. From a situation of having no caries active at baseline, 20% of children in the trial population reported one or more episodes of toothache and 5% had an extraction within 3 years. These figures include the whole-trial population in the denominator. If we consider only those children who developed the disease, 36.9% in the intervention group and 44.6% in the control reported one or more episodes of toothache during the trial. Tickle et al. 4 conducted a prospective cohort study in 50 dental practices in the north-west of England and reported that approximately one in five children aged 3–6 years with caries active had an unscheduled dental visit because of dental pain each year. The large proportion of children in the trial who, once they developed caries, experienced pain is a consistent finding with this earlier descriptive study. This strong likelihood of pain and extraction following the development of the disease justifies the primary prevention rationale for the NIC-PIP trial, which is attempting to keep children in a caries-free state.
The use of the dmft/DMFT and dmfs/DMFS indices and the preventative fraction have long been used in dentistry as outcome measures for commercial studies of toothpaste efficacy. They are aggregate scores and include both caries-active and caries-free populations. These aggregate scores are not particularly helpful to assess the future caries risk of individuals and populations. This trial corroborates the findings of previous longitudinal studies in general practice4,22 and demonstrated that the transition from caries-free to caries-active states represents both a clinical and policy watershed, as this ‘conversion’ results in a significant risk of adverse outcomes that have a negative effect on patients and their families and have significant cost implications for the NHS.
Although there was a 5% difference in caries prevalence (primary outcome) between the groups after 3 years, in favour of the intervention group, this was not statistically significant. The trial was powered to detect a difference of 10%, with a projected 37% prevalence at outcome in the intervention group and an expected 47% prevalence in the control group. The 47% expected prevalence in the control group was based on epidemiological data available from the Children’s Dental Health in the UK 200317 when the study was designed, at that time the population prevalence of caries in 5-year-olds in Northern Ireland was 61%. However, during the trial there was a very large [in the order of a 20% absolute reduction3] fall in population levels of disease among 5-year olds in Northern Ireland. The effect size we estimated was based on data available from the 2002 Cochrane fluoride-containing varnish systematic review43 (subsequently updated in 201345) and a large toothpaste distribution trial. 87 The estimated effect size was based on the assumption that there would be an additive effect of fluoride-containing varnish and fluoride-containing toothpaste over a 3-year period, although at that time (and at the time of publication) there was very little information on the effects of composite fluoride interventions. An assessment of what would be a clinically significant effect also influenced the estimated effect size used to produce the sample size calculation. If we could demonstrate a 10% reduction in caries active, a reduction of this magnitude would encourage a change in clinical practice and justify the assumptions and costs of a policy to invest in prevention in practice. We performed a post hoc evaluation of the power of the study by recalculation of the sample size based on the 39% prevalence of caries active in the control group compared with 29% prevalence in the intervention group, applying the a priori effect size of a 10% difference between groups that we stipulated in the published protocol. The resultant sample size needed to demonstrate the predetermined effect size was less than the number of participants examined at follow-up (470 vs. 510 per group). The study was therefore not underpowered to detect the original effect size of 10%. One could argue that we set an ambitiously high effect size; however, we found a statistically significant difference in dmfs index between caries-active children in intervention and control groups, demonstrating that the true clinical effect of the intervention on the primary outcome measure is likely to be small and of questionable clinical and public health value. This interpretation of the findings also assumes that the high levels of adherence we achieved in the trial can be replicated in the average dental practice.
More sensitive measures of caries, dmfs and dmft showed statistically significant differences between the groups in favour of the intervention group. Over the 3 years of the intervention decreased caries by two surfaces per child converting to caries active, or by 0.8 teeth per child converting to caries active. These represented reductions of 25% and 19% compared with the control group. If all children are included the denominator then for the dmfs index the use of the intervention over 3 years represents a 34% reduction in disease, and for dmft index, a 30% reduction. When we consider only the children who developed the disease, the difference was 2.4 dmfs (25%), which was 0.8 dmft (19%) in favour of the intervention group. Using the dmfs index, the reduction in disease we report is consistent with the effect size reported in a recent Cochrane systematic review of fluoride-containing varnish. 45 The primary outcome measure for the review was the prevented fraction, that is, the difference in caries increments between the treatment and control groups expressed as a percentage of the increment in the control group; therefore, a relative percentage difference. The review reported a pooled dmfs-prevented fraction of 37% (95% CI 24% to 51%; p < 0.0001) for the 10 trials that contributed data for primary tooth surfaces.
A 2003 Cochrane review40 of the effectiveness of fluoride-containing toothpaste could not produce estimates of the effects of fluoride-containing toothpastes on caries increment in primary teeth or tooth surfaces, as there were no contributing data available in the selected trials. Another Cochrane review published in 2004 compared combinations of topical fluoride (toothpastes, mouth rinses, gels, varnishes) with single topical fluoride for preventing dental caries in children and adolescents. 41 There were few trials available to assess the effects of composite fluoride interventions on the primary dentition and no meta analyses were presented. The review cited one trial90 that compared the effects of fluoride-containing varnish combined with toothpaste, versus toothpaste alone on caries increment in the primary dentition. No statistically significant differences in caries increment during the 2 experimental years were found between the groups. Neither of these reviews has been updated since publication. The results of our trial are consistent with the outcomes of the fluoride-containing varnish systematic review, but without comparable data it is difficult to say that combining the two fluoride treatments had an additive effect.
The Cochrane fluoride-containing varnish review reported that there was little information concerning possible adverse effects or acceptability of treatment in previous trials. We monitored all SAEs and only ARs that had a possible causal relationship to the fluoride-containing varnish. There was a small number of ARs with a possible link to the varnish; all of these were minor in nature and self-limiting, which suggests that fluoride-containing varnish in this young age group is safe. There is a potential increased risk of fluorosis,48 which will only be apparent when the permanent incisor teeth erupt at 8–9 years of age and, therefore, assessment of this outcome is outside the scope of the trial reported here.
There was a strong relationship between deprivation and caries, as demonstrated by a mean dmfs index score of 9.6 among children living in areas in the most-deprived quintile compare to a mean dmfs index score of 5.3 among children in the least-deprived quintile, when calculated for children with caries. This strong relationship between deprivation and dmfs was also apparent when all children were included in the analysis. This relationship was expected, given the well-documented association between caries and deprivation in the literature. 6 Table 13 does not show a smooth, linear gradient in disease severity across the socioeconomic quintiles, in either intervention or control groups. This could be a result of the use of an area-based measure of deprivation and unobserved heterogeneity within the area-based measure; the ecological fallacy. We did not find any interaction between socioeconomic status and group for making the conversion from caries free to caries active. We therefore cannot deduce that children from more disadvantaged backgrounds are more likely to benefit from the intervention than their more affluent peers; however, this analysis lacked power.
Other studies on dental practice populations have reported a non-significant relationship between dental caries and socioeconomic status in young children. 91 Irrespective of their socioeconomic status, the majority of these children attended the dentist regularly, every 6 months and, therefore, exhibited different oral health-related behaviour than children in the same socioeconomic quintiles in the whole population. Children in the most-disadvantaged quintile were under-represented (see Table 32) in the trial population when compared with the whole population of Northern Ireland. This most disadvantaged quintile in the whole population will have the highest risk of disease and are most likely to have infrequent dental visiting patterns. 7 As the children in the trial were predominantly regular attenders they will not have the same caries risk as children in the same socioeconomic quintile in the whole population.
The application of fluoride-containing varnish, according to national statistics, is at a much lower level than we achieved in the trial. In 2014/15 in England, 32.1% of all children attending NHS primary care dental services had fluoride-containing varnish applied to their teeth. 63 Therefore, the effect size we report is probably greater than those currently achievable in dental practices in England for a number of reasons. First, a much larger proportion of children attended regularly and received fluoride-containing varnish in the trial than is evident in the general population in England. The children who consented to participate in the trial, as in any trial,92 are more likely to adhere to treatment regimes than children in the general population. As the trial was a CTIMP there was close monitoring of fidelity to delivery of the intervention as per protocol. Fidelity was also reinforced by additional payments to the dentists of £25 per visit of each child to cover the NHS costs of the dentists supporting the trial. Studies demonstrate that dentists’ behaviour is rapidly and significantly influenced by contractual and financial factors. 93 The nature of the trial population, the tight monitoring and financial incentives used in the trial are likely to have contributed to the high levels of adherence we achieved, which are unlikely to be replicated in the general population. The intervention also included provision of toothpaste containing 1450 p.p.m. of fluoride and a free toothbrush each time they attended as a ‘prompt’ for families to adopt and use fluoride-containing toothpaste in an optimal manner. This element of the intervention is not routinely provided to children attending NHS general dental practices and so this is another factor that would contribute to a larger effect size we found in the trial, than would be achievable in English dental practices that follow the guidance in DBOH. 13
Different aspects of the study’s findings may be emphasised by different stakeholder groups. The results illustrate the difference between statistical significance and clinical importance. A 34% reduction in disease sounds impressive, but it is a 34% reduction of a small mean number of carious teeth surfaces per child (a difference of 1.3 dmfs between the intervention and control groups). This reduction was statistically significant but it may not be a clinically worthwhile reduction or significant from a policy and commissioning perspective, and may not in itself warrant or justify the policy of focusing dental resources to pay dental practices to concentrate on prevention.
Given the small but statistically significant difference in dmfs the economic analyses are important. From a policy perspective identifying the costs and effects of potentially investing in prevention, at a time when there are significant and rising financial pressures on the NHS, are crucial. Prevention of disease, particularly in early years, can take time to provide a return on investment. The costs of care provision in the intervention group were statistically significantly greater than the costs for the control group over the 3-year period. For total direct dental service costs per child (see Table 27) there was a mean difference over the 3-years of the study of £107.53 (£155.74 intervention, £48.21 control; p < 0.05). When all health-care costs were compared the intervention group’s mean cost per child was £212.56 more that the control group (£987.53 intervention, £774.97 control; p < 0.05) over the 3 years of the trial.
The ICERs are based on each bootstrapped mean incremental cost per child being divided by each bootstrapped mean incremental effect per child. The mean additional costs per carious tooth surface avoided over the 3 years of the trial was calculated at £250.58. This was statistically significant but this value lacked precision, the 95% CIs were wide (£454.39 to £79.52) primarily because of the small effect size. NMB was calculated based on bootstrapped NMB for which an arbitrary threshold of £1000 was the assumed as the mean value of carious tooth surfaces avoided. The NMB per carious surface avoided was £1063.81 (95% CI £298.08 to £1854.62). The NMBs per caries-free child and per episode of pain avoided were not statistically significant (see Table 31).
It is important to realise that the costs included in our analyses do not include a specific financial incentive paid to dentists to provide preventative care or the cost of performance management to oversee the delivery of prevention. The £25 payment (NHS support costs) for each visit of each child and the trial monitoring infrastructure and processes required of a CTIMP could be considered as surrogates for these two costs. However, these costs were not included in the health economic analyses. If the intervention tested was to be translated into a NHS service, consideration would need to be given to the costs of incentivising practices to deliver it reliably and also the costs of monitoring if the adherence rates we report in the trial are to be achieved. This would increase the costs of the intervention significantly. However, other factors could reduce the costs of the intervention. The costs of prevention delivered in practice could be reduced through use of role substitution by employing appropriately trained health-care professionals, such as extended duties dental nurses, to provide preventative care. However, in the sensitivity analyses we conducted, substituting the salary costs of dentist for dental hygienists and dental nurses showed that although the mean direct dental costs of using dental nurses (£74.57) and dental hygienists (£106.37) to deliver the intervention were lower than costs of using dentists (£155.74), this was based on the assumption that time required to deliver the intervention was the same for each type of provider. The effect on the mean total costs of the intervention over the 3 years was minimal and there was no material effect on cost-effectiveness, as reflected in the ICER or NMB. This is perhaps unsurprising because the cost of the intervention alone was comparatively small.
Our findings only capture and summarise the costs and effects over a 3-year period and do not reflect the effects and costs incurred throughout later childhood and into adolescence, or indeed over the whole of the life course. This is very difficult to quantify; we do not know if the effect we report would increase between the groups as the children get older as a result of a 3-year long intervention delivered at this very young age. We also do not know if a larger effect would be found if the intervention were to be provided for a longer time period, extended throughout childhood.
Policy-makers and commissioners also need to consider the potential impact on health inequalities, because practice-based interventions do not reach a large proportion of children in the most disadvantaged localities, as is evident from the socioeconomic differences between the registered and the general population in Figure 6. The children with greatest risk of developing the disease are least likely to attend and hence receive the benefits of treatment, resulting in increased health inequalities.
In 2014/15 in England, 7.7 million band 1 (check-up, with no treatment) courses of treatment were provided to children (aged 0–18 years), making up 70% of all courses of treatment provided for children. 63 Vital signs data provided by the NHS Business Services Authority demonstrated that 13.5% of all payment claims were for a child reattending within 3 months of their initial appointment and 55.4% were for the same child reattending within 6 to 9 months. 94 The national average fee dentists receive for a band 1 (check-up) course of treatment is £25. 95 Therefore, the activity and costs to the NHS in England alone of repeat check-ups for children is substantial. According to Health & Social Care Information Centre data in 2014/15, only 32.1% of children received an application of fluoride-containing varnish. 63
In Scotland, the Information Services Department published data on HEAT target H9 (i.e. fluoride varnishing for 3- and 4-year-olds), the national target is at least 60% of 3- and 4-year-old children in each Scottish Index of Multiple Deprivation quintile to receive at least two applications of fluoride-containing varnish per year by March 2014. The Childsmile programme is the means by which the target will be delivered, and the target covers fluoride-containing varnish applications carried out in nurseries, schools and dental practices. In 2013/14, 33.3% of 3-year-olds and 35.6% of 4-year-olds (34.5% of combined 3- and 4-year-olds) received two or more fluoride-containing varnish treatments. 96
If the proportion of children receiving fluoride-containing varnish is to be increased to the adherence rates in our trial, the NHS may need to provide additional investment or shift resources from other areas to incentivise practices to provide this type of care. The results of this study suggest that expanding this enhanced preventative care is unlikely to have a large impact on increasing the number of children who are caries free, over and above usual practice currently delivered by dentists.
Other community-based interventions may provide better outcomes for lower costs by reaching disadvantaged groups that attend the dentist infrequently and have a high risk of developing the disease. The cost-effectiveness of water fluoridation has been recently assessed in Canada97 and Australia,98 and both studies suggested significant cost savings can be made as a result of water fluoridation, especially if a large population is covered by a scheme. We have a poor understanding of the cost-effectiveness of this public health intervention for the UK. A NIHR-funded study,9 currently in progress, will provide valuable contemporary information on the costs and effects of water fluoridation in the UK. Distributed fluoride-containing toothpaste programmes through the post have been shown to be effective in a trial conducted in the north-west of England. 87 The same study analysed the costs of the intervention and in 2003 reported that the mean cost per carious tooth avoided was £80.83, the mean cost per child of avoiding conversion from caries-free to caries-active states was £424.38 and the mean cost per extraction avoided was £679.01. 99 These cost-effectiveness estimates compare favourably with those we have identified for the practice-based intervention evaluated in the trial. A recent systematic review100 of the economic evaluation of caries prevention programmes included a total of 63 studies, evaluating dental sealants, water fluoridation and mixed interventions. The review could not make firm conclusions, because of the heterogeneity of the studies and limited information provided on adjustments for discounting and inadequate sensitivity analyses. It is therefore difficult to compare the cost-effectiveness of the intervention we evaluated in the trial with alternative prevention strategies. Value for money decisions are a matter for policy-makers and politicians, but the results of this trial should perhaps give them pause for thought in relation to the need for investing in enhanced practice-based preventative measures such as incentivising the expansion of fluoride-containing varnish application.
This trial raises questions about expanding investment in a practice-based approach to preventing dental caries. The intervention we tested probably represents the maximum that is pragmatically and financially possible in general dental practice in terms of prevention offered to children who present free of the disease. Although we found a small, statistically significant difference in the number of tooth surfaces affected by decay between the groups, perhaps the most startling finding is that over one-third of children in the intervention group developed the disease. Dentistry has had fluoride as its ‘magic bullet’ and the large fall in population levels of disease over the last 40 years have been largely attributed to fluoride-containing toothpaste. 35 These significant improvements are now levelling off in England3,21 and one could hypothesise that we are reaching the limits of what professionally applied fluoride can achieve in dental practice populations. There are still opportunities for targeted community-based fluoride programmes to reduce health inequalities and such programmes may represent better investments for policy-makers than practice-based interventions. The Department of Health in Northern Ireland commissioned a qualitative study that ran in parallel to the trial, which included interviews with the parents of the children participating in both arms of the trial and also parents of children in the same age group not involved in the trial. The most striking finding emerging from this study was the ubiquitous presence of sugar in children’s lives and how most parents found it very difficult to restrict the volume and frequency of sugar consumption by their children. Perhaps now is the time for a change of focus in the battle against caries to concentrate on sugar reduction and develop and test behavioural, environmental and fiscal interventions to reduce sugar consumption in a common risk approach to improving health.
Limitations
This was an ambitious trial; to undertake a CTIMP in a community setting, delivered through research-naive, small dental practices was a significant risk. However, we exceeded our recruitment target, retained all practices in the trial and achieved a retention rate of 88% over a 3-year follow-up period.
We could not demonstrate a statistically significant difference in the primary outcome measure. The caries active prevalence in the control group at the end of the 3-year follow-up period was 39%; lower than expected in the protocol (47%). However, a post hoc evaluation of the power of the study showed that this was probably because of the effect size of the intervention being less than we stipulated a priori. The use of the composite intervention showed a 5% reduction in caries-active children; the 10% difference was inside the 95% CI for the primary outcome (–1% to 11%). The large fall (in the order of a 20% absolute reduction, see discussion in Chapter 1, Epidemiology of caries in young children) in population caries over the time the trial was conducted had no effect on the power of the study, but an overestimation of the additive impact of fluoride-containing varnish and fluoride-containing toothpaste in the effect size included the protocol was a factor. We could have set the effect size at 5% at the start of the study; this would have significantly increased the number of participants required and also the costs but would not have meaningfully altered our conclusions. We chose a 10% effect size primarily as this was an effect size that would be important from both clinical and policy perspectives. We did show that the intervention had a statistically significant effect on dmfs, similar to that reported by the Cochrane systematic review of fluoride-containing varnish. 45 However, our results (over one-third of children developed the disease in the intervention group and over 40% who developed the disease reported pain) would suggest that this intervention, currently supported by policy and national guidance will have limited impact on the development of caries into dentine in young children.
The trial also suffered from the same limitation of all trials; external validity is discussed in the section Generalisability, but one limitation of the study is that a deeper understanding of the reasons for the findings cannot be provided by a trial design. Moreover, a single trial alone cannot provide a comprehensive understanding of how to address all of the multifactorial elements that contribute to an individual’s risk of developing caries. The intervention we tested was composite; the professionally applied fluoride-containing varnish and provision of the fluoride-containing toothpaste and toothbrush were designed to trigger and sustain caries risk-reducing behaviours in the home. To understand how the intervention affected behaviour in the home requires additional qualitative research. The team conducted a parallel mixed-methods study to provide a deeper understanding of the impact of the trial on children’s and parental behaviour in the home in both the intervention and control groups. This study was not funded by the Health Technology Assessment programme of the NIHR, but by the Northern Ireland Department of Health, Social Services and Public Safety. The early results of this mixed-methods study have been discussed above, but the full study will be published in a separate peer-reviewed journal. Additional trials are required of different caries preventions at different levels (individual, practice, community and population) and of different types of intervention (behavioural, social, economics and legislative) to increase our understanding of how to combat this disease.
The trial follow-up period was 3 years; our conclusions are therefore constrained by the time-limited measurement of outcomes and impact. We cannot say if a greater proportion of children would remain caries free if the intervention were to be provided throughout childhood and adolescence (sustained prevention is advocated by DBOH13) or what effect this would have on cost-effectiveness and value for money. Similarly, without long-term follow-up of the trial population, we cannot say what the long-term preventative effects, if any, the intervention would have if provision is confined to a 3-year period in early childhood. One could hypothesise that the intervention could have a lasting effect, as it is provided during early childhood and seeks to instil behaviour which should reduce caries risk over a lifetime, as a consequence this behaviour change would result in a widening of differences between intervention and control groups in late childhood and adolescence.
The primary outcome measure used was children converting from a caries-free state to a caries-active state. We recognise there are many possible points along the development of caries lesions that diagnosis can be undertaken. We did not measure enamel caries for a number of reasons. At a single time point (at baseline or follow-up) it is not possible to distinguish between an arrested enamel lesion (scarring) and an active enamel lesion; nor is it possible to distinguish between an enamel lesion that will progress to cavitation and one that will not progress. In contrast, caries into dentine have definite clinical and cost consequences, and we felt this hard end point was appropriate for this pragmatic trial. We followed national diagnostic criteria of caries used in routinely undertaken NHS epidemiological surveys. Assessing enamel caries involves a more complex and time-consuming clinical examination. This would have increased costs, provided a higher risk of diagnostic error and more importantly would not have been practical to undertake in a large population of children who were aged 2–3 years at baseline.
The trial was also limited by the costs and time required of large pragmatic trials to provide useful information for decision-makers. Over the 7- to 8-year period from design to completion of the trial there was a very large reduction in caries in the primary teeth within the population of young children in Northern Ireland, but the trial was locked into its procedures and methods via its protocol. Similar to all trials, this lack of agility and an ability to revise and adapt to changing circumstances is a limitation. Observational studies and point-of-care trials using electronic health records could play an important role in producing information in a more timely manner.
The study could not refer to a commonly used threshold mean willingness to pay for effects against which to assess the value for money of the intervention in respect of carious surfaces avoided. This limitation was imposed on the study by our inability to directly assess the utility gain (if any) of children associated with caries prevention. We could not in consequence assess value for money relative to thresholds frequently reported in the literature. Moreover, it is unlikely that mean willingness to pay is constant for any gain in effectiveness, given that differences in the scale of an intervention will have different opportunity costs. This also limits our ability to draw firm conclusions on whether or not the gains observed here might be considered to offer sufficient value for money to warrant the investment required by policy-makers.
Generalisability
One of the acknowledged limitations of all randomised controlled trials is external validity. 101 However, all research is subject to limitations in generalisability because of the requirement to obtain informed consent and the necessary limitations of the geographical, cultural and social environment in which the research is conducted.
Table 32 compares the socioeconomic profile of the trial population with that of the 2- to 3-year-old population, who were registered with a GDS dentist in Northern Ireland, and the whole population of 2- to 3-year-olds in Northern Ireland at the time of the baseline examination. This shows that the registered population had a greater proportion of children in the least-disadvantaged quintile and a lower proportion of children in the most-deprived quintile compared with the total population. This socioeconomic difference between the registered population and whole population was expected and reflects the relationship between socioeconomic status and dental visiting patterns reported by the Children’s Dental Health Survey 2013. Report 2: Dental Disease and Damage in Children England, Wales and Northern Ireland. 3
| Quintiles of deprivation | Total population of 2- to 3-year-olds in Northern Ireland (%) | Population of 2- to 3-year-olds registered with a dentist in Northern Ireland (%) | Baseline population of NIC-PIP trial (children aged 2–3 years between 6 May 2011 and 11 June 2012) (%) |
|---|---|---|---|
| Most deprived | 21.4 | 16.9 | 15.5 |
| 20–40% | 21.2 | 19.2 | 22.0 |
| 40–60% | 21.2 | 22 | 26.4 |
| 60–80% | 19.8 | 22 | 24.3 |
| Least deprived | 16.1 | 18.7 | 11.8 |
The NIC-PIP trial population had a smaller proportion of children in the most-deprived and most-affluent quintiles when compared with the registered population and the whole population of children in Northern Ireland and over-representation in the middle three quintiles compared with the registered and general populations. However, the trial population was not confined primarily to one or two socioeconomic groupings, with good representation across all socioeconomic quintiles. This socioeconomic spread of the trial population reflects the varied practice recruitment strategy adopted by the trial team. The socioeconomic distribution of the NIC-PIP trial population is more likely to be representative of children who are regular dental attenders, which is different from the registered population. From a policy evaluation perspective this can be seen as strength rather than a limitation of the study, as this was the population guidance in DBOH is targeted at.
Interpretation balancing benefits with harms and costs and considering other relevant evidence
Over 3 years the patient benefits were small and the costs per child were significant. The analysis of SAEs and ARs demonstrated that the fluoride-containing varnish was safe, even used in this young age group. There was insufficient power to detect a statistically significant difference in the primary outcome measure. However, the significant reductions in dmfs and dmft show that there is a real benefit and the intervention seems to have shifted the distribution of disease in the population to the left. The 5% difference in caries-free children is therefore likely to be a real difference, but at this level of power the statistical significance and precision of this difference cannot be determined. The costs of providing this preventative intervention outweighed any savings in treatment over the 3-year follow-up period. This intervention delivered in general dental practice is unlikely to produce a cost saving for the NHS. A key finding is that, even with this approach to evidence-based ‘enhanced’ prevention, over one-third of children still developed the disease. This fact, allied to the uncertainty that we can keep children caries free with interventions delivered in practice, plus the high costs of prevention in practice (which do not include financial incentives for dentists), we feel do not make a convincing argument for policy-makers and NHS commissioners to invest in this technology. Other interventions delivered in other settings are more likely to deliver greater benefits for lower costs.
Chapter 5 Conclusions
Key findings related to objectives
Aim
To measure the effects and costs of a composite fluoride intervention designed to prevent caries in young children attending dental services.
Objectives
To compare, in children aged between 2 and 3 years who were caries free at baseline, the effectiveness of a varnish containing 22,600 p.p.m. of fluoride, a toothpaste containing 1450 p.p.m. of fluoride and standardised health education, provided twice a year in general dental practice, as a ‘preventative package’ compared with standardised health education provided twice a year alone in:
-
reducing the conversion of children from caries-free to caries-active states in the primary dentition
-
reducing the number of carious surfaces (caries into dentine) in the primary dentition in children who convert from caries-free to caries-active states
-
reducing the number of episodes of pain and/or extraction of primary teeth.
The cost-effectiveness of the preventative package relative to standardised health education alone was also evaluated.
Objective 1
We did not demonstrate that the intervention prevented the conversion of children from caries-free to caries-active states.
Objective 2
We found a statistically significant difference between the intervention and control groups in the mean number of carious surfaces (caries into dentine) in children who converted from caries-free to caries-active states. Children who received the preventative package had on average 2.43 (95% CI –0.77 to 4.08) fewer tooth surfaces affected by decay than children in the control group.
Objective 3
We did not demonstrate that the intervention prevented episodes of pain or extraction of primary teeth.
Objective 4
The intervention was unlikely to be cost-effective in terms of either keeping children caries free or avoiding episodes of pain. The mean cost per carious surface avoided after 3 years was estimated at £251, with a wide 95% CI (£79.52 to £454.39).
Implications for clinical care
Fluoride-containing varnish is not a ‘magic bullet’ for practice-based populations. Although the intervention produced a 34% (relative) reduction in dmfs, this effect is clinically negligible, a point underlined by the fact that 34% of children in the intervention group, the majority of whom closely adhered to the intervention, still developed the disease. This finding demonstrates the limitations of the impact health-care professionals can have in preventing disease. The intervention we tested probably represents what is pragmatically and financially possible in busy NHS dental practices, but it was more expensive than routine care and had minimal impact. Clinicians need to recognise these limitations but should continue to provide advice based on up-to-date best evidence.
Implications for policy
The trial compared the effects of an enhanced preventative package based on the interventions advocated in Delivering Better Oral Health: An Evidence-based Toolkit for Prevention13 with a surrogate of routine care (advice only). The trial suggests that for very young children it is unlikely that enhanced prevention over and above the advice dentists currently provide to their patients will produce major improvements in dental health and may not be a wise investment of public monies.
This also has implications for a new NHS dental contract that seeks to reward practices for producing improvements in prevention based on a quality outcomes framework. The Department of Health in England has developed a DQOF. 79 The DQOF includes caries in 5-year-old children as a quality indicator: ‘Decayed teeth (dt) aged 5 years old and under, reduction in number of carious teeth/child’. 79 The rationale for use of this indicator was to ‘monitor the primary dental care team’s adoption of evidenced informed preventative advice and intervention and their impact on oral health’. 79 Irrespective of issues of shifting denominators in a dynamic practice population, the outcomes of this study suggest that it is unlikely that dental practices will be able to directly produce a significant change in the mean number of decayed teeth in their practice population. The findings of this trial suggest that this indicator in the DQOF may require further consideration.
However, there is considerable uncertainty about what people or the state might be willing to pay for an avoided carious surface or for a child remaining free of the disease. Our calculations of NMB society would need to value a carious surface avoided approximately 55 times the cost of restoring the surface (based on the £8.30 NHS costs of a filling in Northern Ireland88) before the intervention was deemed to be value for money. This difference may be more than society is willing to pay, but we accept that we do not know if this is the case. Moreover, there is uncertainty about how long the benefit we report would be sustained for, that is, how long an avoided carious surface remains avoided.
There are a number of key questions for policy-makers:
-
How can children be kept caries free?
-
How can the disease process be stopped once it starts?
-
What would the state or society be willing to pay on average to keep children caries free or to arrest the disease process once it has started?
The first question is largely a public health question; the second is more of a clinical issue, as those with the disease require clinical treatment. Policy-makers need to consider how best to spend resources to have the maximum impact for each pound spent and consider the relative costs and effects of community- and practice-based prevention programmes. Answers to the third question are required to start to compare the value for money of different interventions to achieve the goals in questions 1 and 2.
One issue that may need careful consideration is that the NHS may be reaching the limits of what fluoride can do to prevent dental caries in a dental practice setting in the face of rising sugar consumption. 102 Since 1991, government advice is that no more than 10% of a person’s average total energy intake should come from non-milk extrinsic sugars. Public Health England’s National Diet and Nutrition Survey Results from Years 1, 2, 3 and 4 (Combined) of the Rolling Programme (2008/2009–2011/2012)102 reported that all age groups consume well in excess of the 10% guideline. Children consumed the most non-milk extrinsic sugars: intake for 4- to 10-year-olds was 14.7% of total energy and for 11- to 18-year-olds was 15.4%.
Public Health England’s strategy for sugar control49 puts behaviour change as its main focus for intervention. Changing behaviour is notoriously difficult,52,89 at least without a restriction of the availability of sugar in the environment. Legislative, regulatory and fiscal interventions have a stronger evidence base for changing behaviour than interventions applied to individuals. However, the political and logistical difficulties of restricting sugar consumption at a societal level are well recognised.
Recommendations for future research
This study has produced important information that will influence future research in caries prevention. In this section of the report we set out our recommendations for research in order of priority.
-
A better epidemiological understanding of the longitudinal development of caries is required. A large amount of public health resource is spent on local serial cross-sectional surveys of caries. These surveys produce limited information to inform public health strategies to prevent caries. More longitudinal studies in different populations would greatly improve our understanding of the development of the disease, the impact of the disease and associated risk factors.
-
Further qualitative research is required to provide a much deeper understanding of parents’ and children’s behaviour in the home setting in relation to caries risk and how these behaviours change as the child matures. These behaviours relate to toothbrushing behaviour and behaviours associated with diet, in particular sugar consumption. This research is critical if we are to develop interventions to change behaviour concerning sugar consumption.
-
The significant reductions in caries at the population level seen over the last 10 years in Northern Ireland have been anecdotally attributed to community-based fluoride-containing toothpaste interventions. Robust evaluation, including randomised controlled trials, of community-based interventions including different methods of fluoride delivery and interventions which influence diet, particularly sugar consumption, are needed to provide high-quality evidence of their costs and effects in different contexts and their impact on health inequalities. In 2016, the government announced the intention to introduce a soft drinks industry levy across the UK. This initiative could have a significant impact on caries in young children; a high-quality research evaluation is needed to quantify its effect.
-
Research is required to help clinicians and parents to arrest the disease once caries into dentine has developed. We need to develop and test behavioural interventions designed to reduce the volume and frequency of sugar consumption and support the optimal use of fluoride.
-
We need to better understand how much the state and society are willing to pay to keep children free of the disease and to arrest the disease once it has started in order to determine whether or not the most cost-effective intervention represents value for money.
-
Long-term follow-up of the trial population to ascertain if the difference in caries between intervention and control groups: remains static; increases in favour of the intervention group; or if the small improvements seen in the intervention group are lost during later childhood. Long-term follow-up would also enable assessment of the effects of the intervention on the risk of fluorosis.
Acknowledgements
The practice principals and staff of the 22 dental practices involved in the trial; CDS dentists who undertook the baseline and outcome examinations; the HSC Board of Northern Ireland; the five HSC Trusts of Northern Ireland (Belfast HSC Trust, South Eastern HSC Trust, Western HSC Trust, Southern HSC Trust and Northern HSC Trust); the Department of Health, Social Services and Public Safety, former Chief Dental Officer Donncha O’Carolan; Simon Reid Chief Dental Officer; members of the Trial Steering Group chaired by Professor Donald Burden; members of the Independent Data Monitoring and Ethics Committee chaired by Professor Jan Clarkson and latterly by Professor Ivor Chestnutt; the staff of The Northern Ireland CTU including statistician Andrea Bryan; the PPI group chaired by Carolyn Slee; Ruth Floate, the Consumer co-ordinator for the Cochrane Oral Health Group (see Plain English summary); and Tom Goodwin and Helen Draper for bringing the report together.
Contributions of authors
Martin Tickle (Chief Investigator, grant holder) contributed to the grant application and the trial design, provided leadership on all aspects of the conduct of the trial and contributed to the preparation of the final report.
Ciaran O’Neill contributed to the grant application and the trial design, provided leadership on health economics aspects of the trial design and analysis, and contributed to the preparation of the final report.
Michael Donaldson (Principal Investigator) contributed to the grant application and the trial design, main point of contact with HSC Board, chaired the trial operational management committee and contributed to the preparation of the final report.
Stephen Birch contributed to the design of health economic elements of the trial, provided a contribution to analysis and interpretation of cost-effectiveness data. Contributed to the preparation of the final report.
Solveig Noble contributed to the grant application and the trial design, was a member of the trial operational management committee and provided overall management of the CDS involvement in the trial. Managed trial operational budget of Northern HSC Trust.
Seamus Killough contributed to the grant application and the trial design, member of the trial operational management committee. Played a key role in recruitment and liaison with dental practices in the trial. Contributed to the preparation of the final report.
Lynn Murphy contributed to the grant application and the trial design, and was a member of the trial operational management committee. Oversaw and managed CTU support for the trial.
Margaret Greer (Trial Manager) contributed to the trial design and was a member of the trial operational management committee. PPI management lead, prepared study protocol and obtained permissions. Managed trial fieldwork. Contributed to the preparation of the final report.
Julie Brodison (Associate Trial Manager) contributed to the trial design, was a member of the trial operational management committee. Supported the trial PPI group. Managed trial fieldwork. Contributed to the preparation of the final report.
Rejina Verghis (Statistician at Central Trials Unit) oversaw data files for release to the study statistician.
Helen V Worthington (Trial Statistician) contributed to the grant application and the trial design, provided leadership on all aspects of trial data collection and analysis and contributed to the preparation of the final report.
Data sharing statement
Data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ 2005;83:661-9.
- World Health Organization . Oral Health Information Systems: Oral Health Surveillance 2011. www.who.int/oral_health/action/information/surveillance/en/ (accessed 14 July 2016).
- Pitts N, Chadwick B, Anderson T. Children’s Dental Health Survey 2013. Report 2: Dental Disease and Damage in Children England, Wales and Northern Ireland. Leeds: Health & Social Care Information Centre; 2015.
- Tickle M, Blinkhorn AS, Milsom KM. The occurrence of dental pain and extractions over a 3-year period in a cohort of children aged 3–6 years. J Public Health Dent 2008;68:63-9. http://dx.doi.org/10.1111/j.1752-7325.2007.00048.x.
- Local Authorities Improving Oral Health: Commissioning Better Oral Health for Children and Young People. An Evidence-Informed Toolkit for Local Authorities. London: Public Health England; 2014.
- Schwendicke F, Dörfer CE, Schlattmann P, Foster Page L, Thomson WM, Paris S. Socioeconomic inequality and caries: a systematic review and meta-analysis. J Dent Res 2015;94:10-8. http://dx.doi.org/10.1177/0022034514557546.
- Milsom KM, Threlfall AG, Blinkhorn AS, Kearney-Mitchell PI, Buchanan KM, Tickle M. The effectiveness of school dental screening: dental attendance and treatment of those screened positive. Br Dent J 2006;200:687-90. http://dx.doi.org/10.1038/sj.bdj.4813724.
- Childsmile . Improving the Oral Health of Children in Scotland n.d. www.child-smile.org.uk (accessed 14 July 2016).
- Goodwin M, Emsley R, Kelly M, Rooney E, Sutton M, Tickle M, et al. The CATFISH study protocol: an evaluation of a water fluoridation scheme. BMC Oral Health 2016;16. http://dx.doi.org/10.1186/s12903-016-0169-0.
- Chestnutt IG, Chadwick BL, Hutchings S, Playle R, Pickles T, Lisles C, et al. Protocol for ‘Seal or Varnish?’ (SoV) trial: a randomised controlled trial to measure the relative cost and effectiveness of pit and fissure sealants and fluoride varnish in preventing dental decay. BMC Oral Health 2012;12. http://dx.doi.org/10.1186/1472-6831-12-51.
- Innes NP, Clarkson JE, Speed C, Douglas GV, Maguire A. FiCTION Trial Collaboration. The FiCTION dental trial protocol – filling children's teeth: indicated or not?. BMC Oral Health 2013;13. http://dx.doi.org/10.1186/1472-6831-13-25.
- Birch S, Bridgman C, Brocklehurst P, Ellwood R, Gomez J, Helgeson M, et al. Prevention in practice – a summary. BMC Oral Health 2015;15. http://dx.doi.org/10.1186/1472-6831-15-S1-S12.
- Delivering Better Oral Health: An Evidence-Based Toolkit for Prevention. London: Public Health England; 2014.
- Appleby J, Thompson J, Jabbal J. Quarterly Monitoring Report. London: The King’s Fund; 2015.
- Dental Earnings and Expenses, England and Wales – 2009–10. Leeds: Health & Social Care Information Centre; 2011.
- Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. Am J Dent 2009;22:3-8.
- Lader D, Chadwick B, Chestnutt I, Hawker R, Morris J, Nuttall N, et al. Children’s Dental Health in the UK National Survey 2003. London: Office for National Statistics; 2005.
- O’Brien M. Children’s Dental Health in the United Kingdom, 1993. London: Office of Population Censuses and Surveys Social Survey Division; 1994.
- White DA, Morris AJ, Hill KB, Bradnock G. Consent and school-based surveys. Br Dent J 2007;202:715-17. http://dx.doi.org/10.1038/bdj.2007.532.
- Pitts NB, Boyles J, Nugent ZJ, Thomas N, Pine CM. The dental caries experience of 5-year-old children in Great Britain (2005/6). Surveys co-ordinated by the British Association for the study of community dentistry. Community Dent Health 2007;24:59-63.
- Programme for England: Oral Health Survey of Five-Year-Old Children 2012. A report on the Prevalence and Severity of Dental Decay. London: Public Health England; 2013.
- Milsom KM, Blinkhorn AS, Tickle M. The incidence of dental caries in the primary molar teeth of young children receiving National Health Service funded dental care in practices in the North West of England. Br Dent J 2008;205. http://dx.doi.org/10.1038/sj.bdj.2008.582.
- Dye BA, Li X, Thornton-Evans G. Oral Health Disparities as Determined by Selected Healthy People. 2020 Oral Health Objectives for the United States, 2009–10. National Health and Nutrition Examination Survey, Data Brief Number 104. Hyattsville, MD: National Center for Health Statistics; 2012.
- Tickle M. The 80 : 20 phenomenon: help or hindrance to planning caries prevention programmes?. Community Dent Health 2002;19:39-42.
- Northern Ireland Multiple Deprivation Measure; 2010. Belfast: Northern Ireland Statistics & Research Agency; 2010.
- Ravaghi V, Hill K, Ryan R, Dennes M. Children’s Dental Health Survey 2013. Country Specific Report: Northern Ireland 2015. www.hscic.gov.uk/catalogue/PUB17137/CDHS2013-Northern-Ireland-Report.pdf (accessed August 2015).
- The State of Children’s Oral Health in England. London: Royal College of Surgeons; 2015.
- Bridgman CM, Ashby D, Holloway PJ. An investigation of the effects on children of tooth extraction under general anaesthesia in general dental practice. Br Dent J 1999;186:245-7.
- Tickle M, Jones C, Buchannan K, Milsom KM, Blinkhorn AS, Humphris GM. A prospective study of dental anxiety in a cohort of children followed from 5 to 9 years of age. Int J Paediatr Dent 2009;19:225-32. http://dx.doi.org/10.1111/j.1365-263X.2009.00976.x.
- Locker D, Liddell A, Dempster L, Shapiro D. Age of onset of dental anxiety. J Dent Res 1999;78:790-6. http://dx.doi.org/10.1177/00220345990780031201.
- Li Y, Wang W. Predicting caries in permanent teeth from caries in primary teeth: an eight-year cohort study. J Dent Res 2002;81:561-6. http://dx.doi.org/10.1177/154405910208100812.
- Improving Dental Care and Oral Health – A Call To Action. London: NHS England; 2014.
- NHS Dental Statistics for England: 2014–15. First Quarterly Report. Leeds: Health & Social Care Information Centre; 2014.
- Dental Statistics – NHS Treatment and Fees Statistics up to Financial Year 2013/14. Edinburgh: Information Services Division Scotland; n.d.
- Petersson GH, Bratthall D. The caries decline: a review of reviews. Eur J Oral Sci 1996;104:436-43. http://dx.doi.org/10.1111/j.1600-0722.1996.tb00110.x.
- Dean HT, Gies WJ. Fluorine in Dental Public Health. New York, NY: New York Institute of Clinical Oral Pathology; 1945.
- McDonagh MS, Whiting PF, Wilson PM, Sutton AJ, Chestnutt I, Cooper J, et al. Systematic review of water fluoridation. BMJ 2000;321:855-9. http://dx.doi.org/10.1136/bmj.321.7265.855.
- Worthington H, Clarkson J. The evidence base for topical fluorides. Community Dent Health 2003;20:74-6.
- Iheozor-Ejiofor Z, Worthington HV, Walsh T, O’Malley L, Clarkson JE, Macey R, et al. Water fluoridation for the prevention of dental caries. Cochrane Database Syst Rev 2015;6. http://dx.doi.org/10.1002/14651858.cd010856.pub2.
- Marinho VCC, Higgins JPT, Logan S, Sheiham A. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2003;1. http://dx.doi.org/10.1002/14651858.cd002278.
- Marinho VCC, Higgins JPT, Sheiham A, Logan S. Combinations of topical fluoride (toothpastes, mouth rinses, gels, varnishes) versus single topical fluoride for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2004;1.
- Walsh T, Worthington HV, Glenny AM, Appelbe P, Marinho VC, Shi X. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd007868.pub2.
- Marinho VC, Higgins JP, Logan S, Sheiham A. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2002;3. http://dx.doi.org/10.1002/14651858.cd002279.
- Petersson LG, Twetman S, Dahlgren H, Norlund A, Holm AK, Nordenram G, et al. Professional fluoride varnish treatment for caries control: a systematic review of clinical trials. Acta Odontol Scand 2004;62:170-6. http://dx.doi.org/10.1080/00016350410006392.
- Marinho VC, Worthington HV, Walsh T, Clarkson JE. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2013;7. http://dx.doi.org/10.1002/14651858.cd002279.pub2.
- Hong L, Levy SM, Warren JJ, Broffitt B, Cavanaugh J. Fluoride intake levels in relation to fluorosis development in permanent maxillary central incisors and first molars. Caries Res 2006;40:494-500. http://dx.doi.org/10.1159/000095648.
- Hong L, Levy SM, Broffitt B, Warren JJ, Kanellis MJ, Wefel JS, et al. Timing of fluoride intake in relation to development of fluorosis on maxillary central incisors. Community Dent Oral Epidemiol 2006;34:299-30. http://dx.doi.org/10.1111/j.1600-0528.2006.00281.x.
- Wong MC, Glenny AM, Tsang BW, Lo EC, Worthington HV, Marinho VC. Topical fluoride as a cause of dental fluorosis in children. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd007693.pub2.
- Sugar Reduction: Responding to the Challenge. Public Health England discussion paper. London: Public Health England; 2014.
- The Relationship Between Dental Caries and Obesity in Children: An Evidence Summary. London: Public Health England; 2015.
- Lingström P, Holm AK, Mejàre I, Twetman S, Söder B, Norlund A, et al. Dietary factors in the prevention of dental caries: a systematic review. Acta Odontol Scand 2003;61:331-40. http://dx.doi.org/10.1080/00016350310007798.
- Harris R, Gamboa A, Dailey Y, Ashcroft A. One-to-one dietary interventions undertaken in a dental setting to change dietary behaviour. Cochrane Database Syst Rev 2012;3. http://dx.doi.org/10.1002/14651858.cd006540.pub2.
- Wennerholm K, Birkhed D, Emilson CG. Effects of sugar restriction on Streptococcus mutans and Streptococcus sobrinus in saliva and dental plaque. Caries Res 1995;29:54-61. http://dx.doi.org/10.1159/000262041.
- Ahovuo-Saloranta A, Forss H, Walsh T, Hiiri A, Nordblad A, Mäkelä M, et al. Sealants for preventing dental decay in the permanent teeth. Cochrane Database Syst Rev 2013;3. http://dx.doi.org/10.1002/14651858.cd001830.pub4.
- SIGN 138 Dental Interventions to Prevent Caries in Children: A National Clinical Guideline. Edinburgh: SIGN; 2014.
- Riley P, Worthington HV, Clarkson JE, Beirne PV. Recall intervals for oral health in primary care patients. Cochrane Database of Syst Rev 2013;12. http://dx.doi.org/10.1002/14651858.cd004346.pub4.
- Wang N, Marstrander P, Holst D, Ovrum L, Dahle T. Extending recall intervals – effect on resource consumption and dental health. Community Dent Oral Epidemiol 1992;20:122-4. http://dx.doi.org/10.1111/j.1600-0528.1992.tb01544.x.
- Tickle M, Milsom KM, King D, Blinkhorn AS. The influences on preventive care provided to children who frequently attend the UK General Dental Service. Br Dent J 2003;194:329-32. http://dx.doi.org/10.1038/sj.bdj.4809947.
- Threlfall AG, Milsom KM, Hunt CM, Tickle M, Blinkhorn AS. Exploring the content of the advice provided by general dental practitioners to help prevent caries in young children. Br Dent J 2007;202. http://dx.doi.org/10.1038/bdj.2007.46.
- Threlfall AG, Hunt CM, Milsom KM, Tickle M, Blinkhorn AS. Exploring factors that influence general dental practitioners when providing advice to help prevent caries in children. Br Dent J 2007;202. http://dx.doi.org/10.1038/bdj.2007.143.
- Kay EJ, Locker D. Is dental health education effective? A systematic review of current evidence. Community Dent Oral Epidemiol 1996;24:231-5. http://dx.doi.org/10.1111/j.1600-0528.1996.tb00850.x.
- Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care 2001;39:II2-45. http://dx.doi.org/10.1097/00005650-200108002-00002.
- NHS Dental Statistics England 2014/15. Leeds: Health & Social Care Information Centre; 2015.
- Tsakos G, Hill K, Chadwick B, Anderson T. Children’s Dental Health Survey 2013 Report 1: Attitudes, Behaviours and Children’s Dental Health England, Wales and Northern Ireland, 2013. Leeds: Health & Social Care Information Centre; 2015.
- Primary Dental Care Strategy for Northern Ireland. Belfast: Department of Health, Social Services and Public Safety; 2006.
- An Oral Health Strategy for Northern Ireland. Belfast: Department of Health, Social Services and Public Safety; 2007.
- Healthy Lives, Healthy People: Improving Outcomes and Supporting Transparency. London: Public Health England; n.d.
- Route Map to the 2020 Vision for Health and Social Care in Scotland. Edinburgh: NHS Scotland; 2013.
- HEAT Targets Due for Delivery in 2013/14: Summary of Performance. Edinburgh: NHS Scotland; 2015.
- Childsmile . Evaluation Reports n.d. http://child-smile.org.uk/professionals/research-and-evaluation/evaluation-reports.aspx (accessed 14 July 2016).
- McMahon AD, Blair Y, McCall DR, Macpherson LM. Reductions in dental decay in 3-year old children in Greater Glasgow and Clyde: repeated population inspection studies over four years. BMC Oral Health 2011;11. http://dx.doi.org/10.1186/1472-6831-11-29.
- Together for Health: A National Oral Health Plan for Wales (2013–18). Cardiff: Welsh Government; 2013.
- Designed to Smile – A National Child Oral Health Improvement Programme. Welsh Health Circular. Cardiff: Welsh Government; 2008.
- Designed to Smile Monitoring Report – December 2014. Cardiff: Welsh Government; 2014.
- The Liverpool Declaration: Promoting Oral Health in the 21st Century. Geneva: WHO; 2005.
- Milsom KM, Threlfall A, Pine K, Tickle M, Blinkhorn AS, Kearney-Mitchell P. The introduction of the new dental contract in England - a baseline qualitative assessment. Br Dent J 2008;204:59-62. http://dx.doi.org/10.1038/bdj.2008.1.
- Dental Services Fifth Report of Session 2007–08. London: The Stationery Office; 2008.
- NHS Dental Contract Pilots – Learning after First Two Years of Piloting. The Second Report from the Dental Contract Pilots Evidence and Learning Reference Group. London: Department of Health; 2014.
- A Dental Quality and Outcomes Framework. London: Department of Health; 2011.
- English Dental Prototypes Overview. London: Department of Health; 2015.
- Welsh Dental Pilot Programme. Cardiff: Welsh Government; 2015.
- Oral Health: Approaches for Local Authorities and their Partners to Improve the Oral Health of their Communities. NICE guidelines. London: NICE; 2014.
- Tickle M, Milsom KM, Donaldson M, Killough S, O’Neill C, Crealey G, et al. Protocol for Northern Ireland Caries Prevention in Practice Trial (NIC-PIP) trial: a randomised controlled trial to measure the effects and costs of a dental caries prevention regime for young children attending primary care dental services. BMC Oral Health 2011;11. http://dx.doi.org/10.1186/1472-6831-11-27.
- Northern Ireland Multiple Deprivation Measure, 2005. Belfast: Northern Ireland Statistics & Research Agency; 2005.
- The Medicines for Human Use (Clinical Trials) Regulations 2004. London: The Stationery Office; 2004.
- Duraphat 50 mg/ml Dental Suspension. London: MHRA; 2014.
- Davies GM, Worthington HV, Ellwood RP, Bentley EM, Blinkhorn AS, Taylor GO, et al. A randomised controlled trial of the effectiveness of providing free fluoride toothpaste from the age of 12 months on reducing caries in 5-6 year old children. Community Dent Health 2002;19:131-6.
- Statement of Dental Remuneration 2014/15. Belfast: Northern Ireland Health & Social Care Board; n.d.
- Behaviour Change: the Principles for Effective Interventions. NICE guidelines. London: NICE; 2007.
- Petersson LG, Koch G, Rasmusson CG, Stanke H. Effect on caries of different fluoride prophylactic programs in preschool children. A two year clinical study. Swed Dent J 1985;9:97-104.
- Tickle M, Milsom K, Blinkhorn A. Inequalities in the dental treatment provided to children: an example from the UK. Community Dent Oral Epidemiol 2002;30:335-41. http://dx.doi.org/10.1034/j.1600-0528.2002.00058.x.
- van Onzenoort HA, Menger FE, Neef C, Verberk WJ, Kroon AA, de Leeuw PW, et al. Participation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort study. Hypertension 2011;58:573-8. http://dx.doi.org/10.1161/HYPERTENSIONAHA.111.171074.
- Tickle M, McDonald R, Franklin J, Aggarwal VR, Milsom K, Reeves D. Paying for the wrong kind of performance? Financial incentives and behaviour changes in National Health Service dentistry 1992-2009. Community Dent Oral Epidemiol 2011;39:465-73. http://dx.doi.org/10.1111/j.1600-0528.2011.00622.x.
- NHS Business Services Authority . Vital Signs Report Year End 2014 15 n.d. www.nhsbsa.nhs.uk/DentalServices/5314.aspx (accessed 14 July 2016).
- Dental Policies and Procedures. Dental Assurance Framework (General) Tier 2 – Single Contract. London: NHS England; 2013.
- Dental Statistics – HEAT Target H9: Fluoride Varnishing for 3 and 4 Year Olds. (Data as at 31 March 2014). Edinburgh: Information Service Division; 2015.
- Tchouaket E, Brousselle A, Fansi A, Dionne PA, Bertrand E, Fortin C. The economic value of Quebec’s water fluoridation program. Z Gesundh Wiss 2013;21:523-33. http://dx.doi.org/10.1007/s10389-013-0578-3.
- Cobiac LJ, Vos T. Cost-effectiveness of extending the coverage of water supply fluoridation for the prevention of dental caries in Australia. Community Dent Oral Epidemiol 2012;40:369-76. http://dx.doi.org/10.1111/j.1600-0528.2012.00684.x.
- Davies GM, Worthington HV, Ellwood RP, Blinkhorn AS, Taylor GO, Davies RM, et al. An assessment of the cost effectiveness of a postal toothpaste programme to prevent caries among five-year-old children in the North West of England. Community Dent Health 2003;20:207-10.
- Mariño RJ, Khan AR, Morgan M. Systematic review of publications on economic evaluations of caries prevention programs. Caries Res 2013;47:265-72. http://dx.doi.org/10.1159/000346917.
- Cochrane AL. Effectiveness and Efficiency: Random Reflections on Health Services. London: Nuffield Provincial Hospitals Trust; 1972.
- National Diet and Nutrition Survey Results from Years 1, 2, 3 and 4 (Combined) of the Rolling Programme (2008/2009–2011/2012). London: Public Health England; 2014.
- Health and Social Care Information Centre . Dental Earnings and Expenses: 2012 2013 Initial Analysis 2014. www.hscic.gov.uk/catalogue/PUB14920/dent-earn-expe-2012-13-init-rep.pdf (accessed August 2015).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU: University of Kent; 2014.
- Royal Mail . Our Prices: Your Handy Guide to Our UK and International Parcel and Letter Services Prices 2014. www.royalmail.com/sites/default/files/RM_OurPrices_Mar2014.pdf (accessed August 2015).
- Department of Enterprise, Trade and Investment . Northern Ireland Annual Survey of Hours and Earnings November 2014 n.d. www.detini.gov.uk/index/what-we-do/deti-stats-index/labour_market_statistics/stats-hours-and-earnings/ashe_tables.htm (accessed August 2015).
- HSC Business Services Organisation . Statement of Dental Remuneration 2014–2015 2014. www.hscbusiness.hscni.net/pdf/STATEMENT_OF_DENTAL_REMUNERATION_2014_–15.pdf (accessed August 2015).
- UK NHS Reference Costs 2013–14. London: DH; 2015.
- AA . AA Motoring Costs 2014 n.d. www.theaa.com/resources/Documents/pdf/motoring-advice/running-costs/petrol2014.pdf (accessed August 2015).
- British Dental Association . DCP Pay n.d. www.bda.org/dentists/policy-campaigns/research/workforce-finance/dcps/dcp-pay-surveys (accessed August 2015).
Appendix 1 Evidence-based, standardised parental advice sheet
Appendix 2 The Northern Ireland Caries Prevention In Practice trial caries data recording form and clinical examination processes and procedures
Appendix 3 The Northern Ireland Caries Prevention In Practice trial questionnaire for parents
Appendix 4 Additional health economic analyses
| Cost item | Cost (£) | Reference |
|---|---|---|
| Brushes and toothpaste per visit | 0.4 | Professor Martin Tickle, University of Manchester, 2014, personal communication |
| Duraphat per visit | 0.75 | Professor Martin Tickle, University of Manchester, 2014, personal communication |
| Delivery time by dentist per minute | 1.50 | Northern Ireland dental survey of adental earnings103 and bPSSRU times 2014104 |
| Postage per visit | 0.62, post, 0.33 time | Assuming a letter with stamps purchased online105 and average Northern Ireland hourly earnings with 2 minutes to post (Northern Ireland Annual Survey of Hours and Earnings November 2014106) |
| Filled surfaces per filling | 8.9 | Statement of Dental Remuneration 2014–2015 (1401)107 |
| Extractions with LA per extraction | 8.24 | Statement of Dental Remuneration 2014–2015 (2101)107 |
| Extractions with GA per extraction | 791 | Mrs Solveig Noble, Clinical Director of Community Dental Services, Northern Health and Social Care Trust, 2014, personal communication |
| Pulpectomy per pulpectomy | 8.60 | Statement of Dental Remuneration 2014–2015 4403107 |
| Advice/check-up per consultation | 8.34 | Statement of Dental Remuneration 2014–2015 0101107 |
| Polish per consultation | 13.23 | Statement of Dental Remuneration 2014–2015 1001107 |
| Other (check-up) per consultation | 8.34 | Statement of Dental Remuneration 2014–2015 0101107 |
| GP per consultation | 46 | PSSRU, assuming 11.7 minutes104 |
| Outpatient per episode | 189 | PSSRU, paediatric outpatient104 |
| Inpatient per night | 327 | UK NHS reference costs charge per day based on excess bed-day charge for elective inpatient108 |
| A&E per episode | 124 | UK NHS Reference Costs 2013–14 108 |
| Travel time | 0.167 per minute | c Northern Ireland Annual Survey of Hours and Earnings November 2014 106 |
| Time off work | 0.167 per minute | c Northern Ireland Annual Survey of Hours and Earnings November 2014 106 |
| Travel | 0.4804 per mile | AA total standing and running costs, assuming 15,000 miles per year, petrol car109 |
| Delivery time by dental nurse per minute | 0.22 | British Dental Association estimate of trained dental nurse earnings of £8.93 per hour.110 Adjusted by earnings-to-expenses ratio for dentists of 55.4%104 |
| Delivery time by dental hygienist per minute | 0.72 | British Dental Association estimate of trained dental hygienist earnings of £27.76 per hour.110 Adjusted by earnings-to-expenses ratio for dentists of 55.4%104 |
Figure 6 denotes the cost-effectiveness plane based on bootstrapped ICERs, together with the sample estimate. The numerator is incremental health-care costs and the denominator is incremental caries. The vertical axis shows incremental costs (the intervention group cost more) and the horizontal axis shows incremental caries (the intervention group has less caries). In standard terminology, the intervention results in a health gain, but has an associated cost.
FIGURE 6.
Cost-effectiveness plane difference in total health-care costs/difference in dmfs.
Figure 7 denotes the cost-effectiveness plane based on bootstrapped ICERs, together with the sample estimate. The numerator is incremental health-care costs and the denominator is incremental caries-free status. The vertical axis shows incremental costs (the intervention group cost more) and the horizontal axis shows the incremental proportion caries free (the intervention group have more caries-free people). In standard terminology, the intervention results in a health gain, but has an associated cost. Note, however, with reference to Table 30, the ICER is not significantly different from zero.
FIGURE 7.
Cost-effectiveness plane: difference in total health-care costs/difference in proportion caries free.
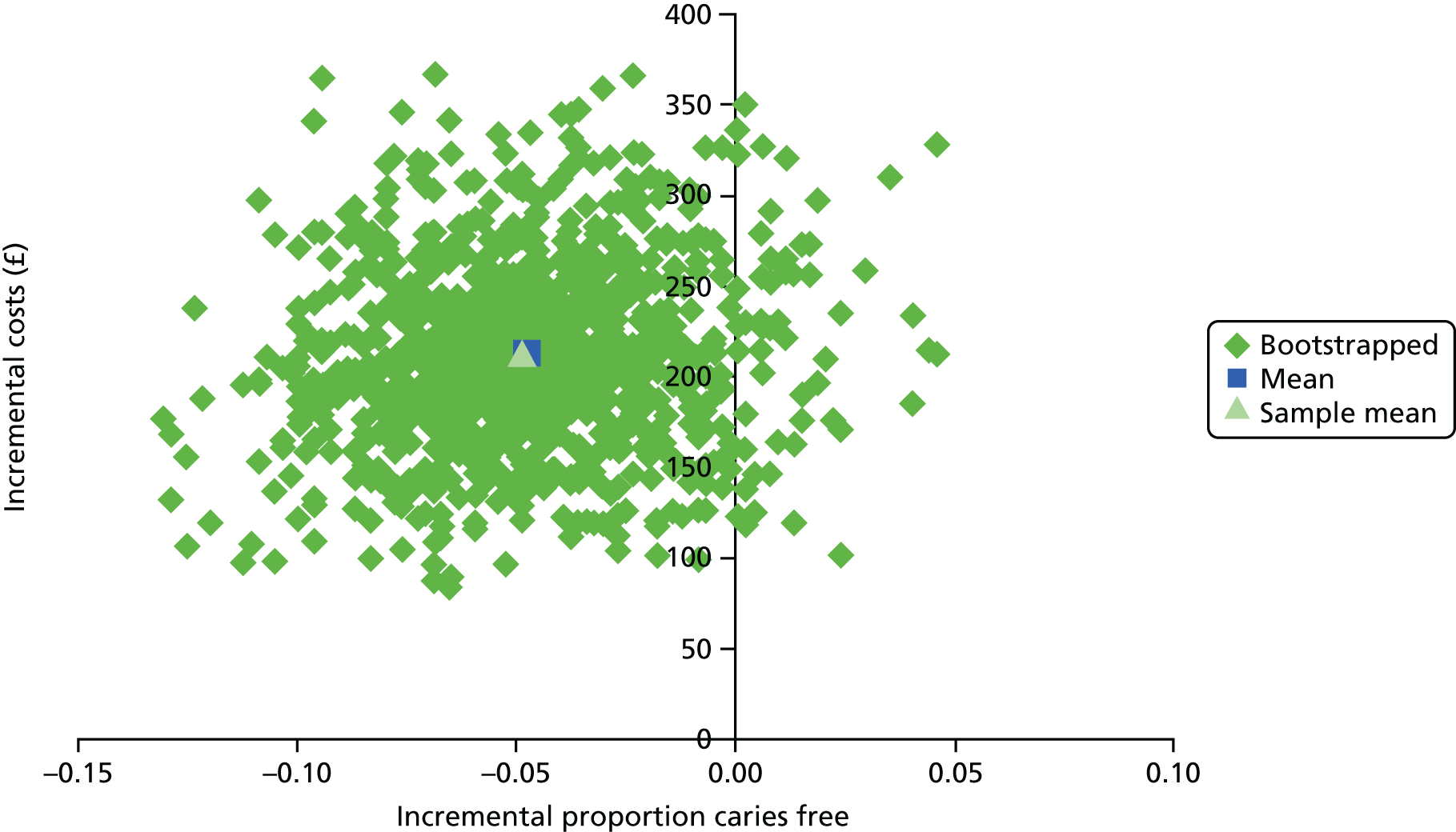
Figure 8 denotes the cost-effectiveness plane based on bootstrapped ICERs, together with the sample estimate. The numerator is incremental health-care costs and the denominator is incremental episodes of pain. The vertical axis shows incremental costs (the intervention group cost more) and the horizontal axis shows incremental proportion caries free (the intervention group have fewer episodes of pain). In standard terminology, the intervention results in a health gain, but has an associated cost. Note, however, with reference to Table 30, the ICER is not significantly different from zero.
FIGURE 8.
Cost-effectiveness plane: difference in total health-care costs/difference in episodes of pain.

FIGURE 9.
Caries free. The CEAC shows the probability of the intervention being deemed value for money at various hypothecated willingness-to-pay thresholds.
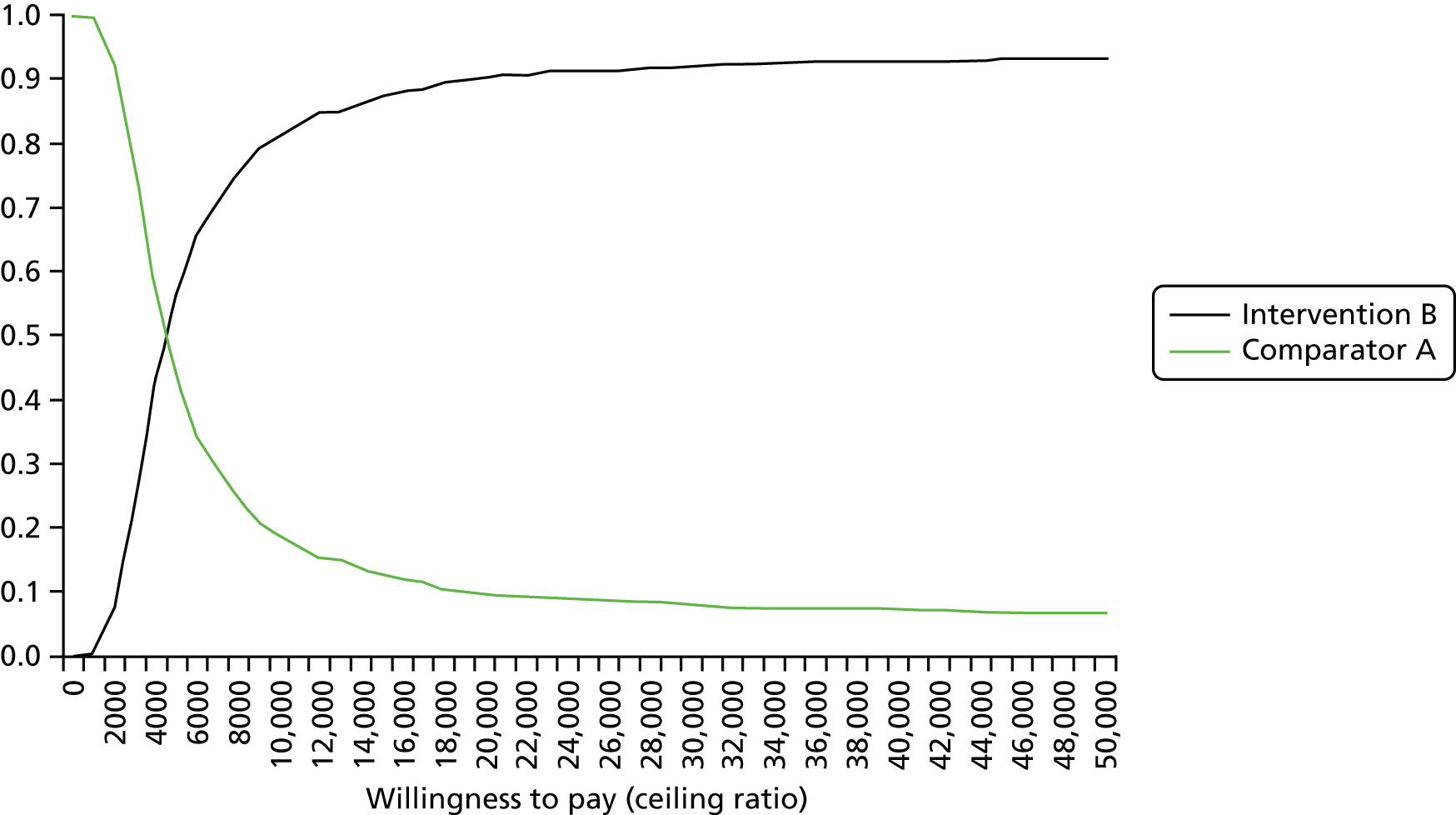
FIGURE 10.
Carious surfaces. The CEAC shows the probability of the intervention being deemed value for money at various hypothecated willingness-to-pay thresholds.
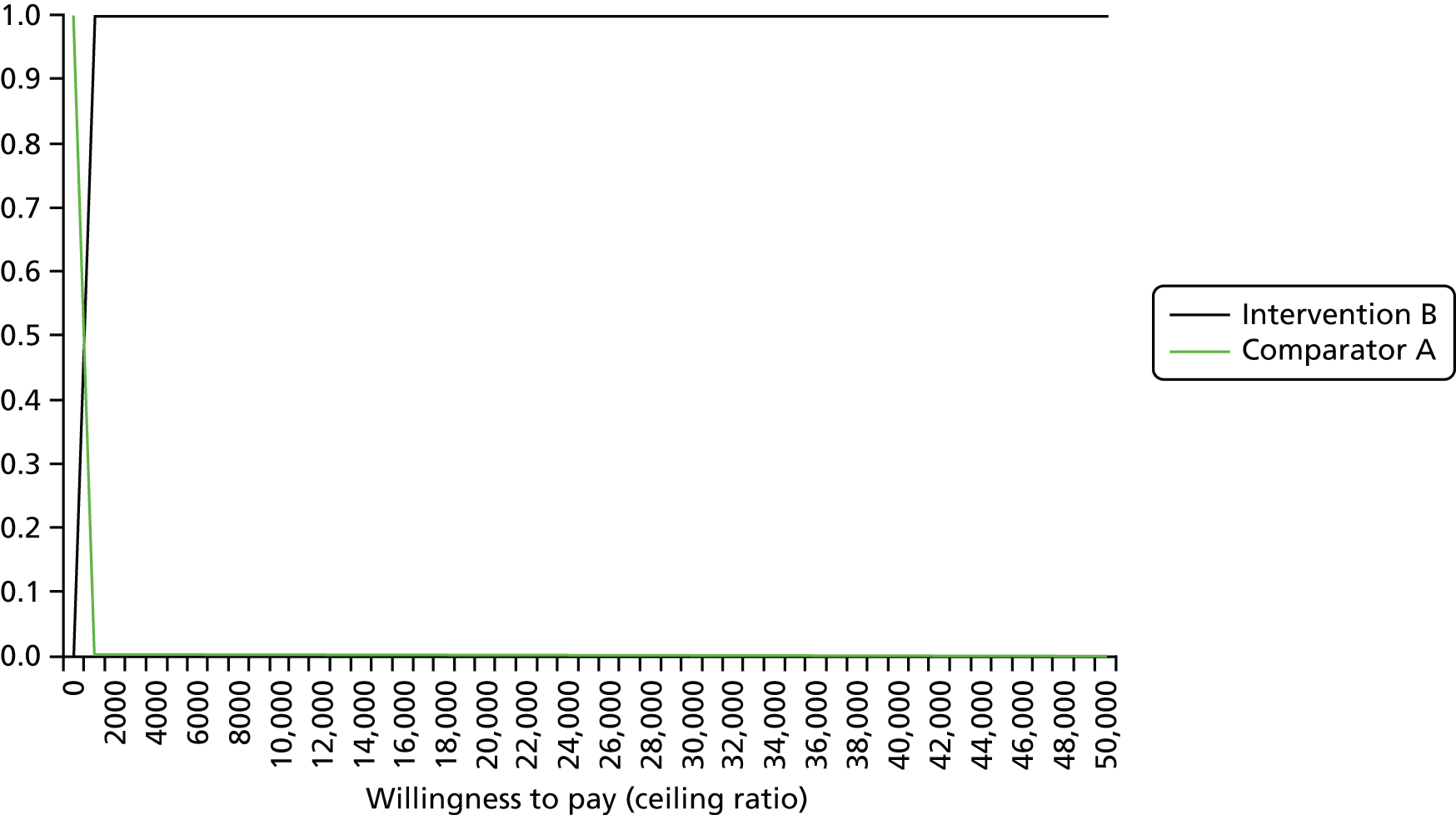
FIGURE 11.
Episodes of pain. The CEAC shows the probability of the intervention being deemed value for money at various hypothecated willingness-to-pay thresholds.

| Summary measures | Time (minutes) to provide the intervention | |
|---|---|---|
| Reported | Measured | |
| Mean | 10.03 | 6.15 |
| Median | 10.00 | 5.37 |
| Skewness | 0.93 | 1.22 |
| SD | 3.59 | 3.71 |
| n | 38 | 38 |
| Activity/consumable | Group | |||||
|---|---|---|---|---|---|---|
| Intervention (n = 549) | Control (n = 547) | |||||
| Mean cost (£) | SD (£) | Skewness | Mean cost (£) | SD (£) | Skewness | |
| Brushes and toothpaste | 2.40 | 0 | – | 0 | 0 | – |
| Delivery time | 51.84 | 14.46 | 0.88 | 0 | 0 | – |
| Fluoride varnish | 4.36 | 0.42 | –3.86 | 0 | 0 | – |
| Postage including time | 5.70 | 0 | – | 0 | 0 | – |
| Cost of check-up at which fluoride varnish is applied | 48.48 | 4.64 | –3.86 | 0 | 0 | – |
| Intervention | 112.77 | 17.03 | –0.14 | 48.21a | 4.76a | –2.94a |
| Costs | Group | Mean difference | |
|---|---|---|---|
| Intervention | Control | ||
| Average total direct health service costsa | 112.77 | 48.21 | –64.56* |
| Average total indirect health service costb | 831.79 | 726.76 | –105.03 |
| Average total parental costc | 39.78 | 40.72 | 0.94 |
| Average total health-care costs (sum of rows 1 and 2) | 944.56 | 774.97 | –169.59* |
| Average total costd | 984.34 | 815.69 | –168.65* |
| Costs (£) | Mean | 95% CI |
|---|---|---|
| Mean difference in health service costs/mean difference in proportion caries free | –2514.37 | –24,313.00 to 17,617.10 |
| Mean difference in health service cost/mean difference in number of carious surfaces | –150.25 | –338.87 to –50.32 |
| Mean difference in health service cost/mean difference in number of episodes of pain | –2030.53 | –13,031.80 to 7989.71 |
| Mean difference in total costs/mean difference in proportion caries free | –2494.17 | –24,586.60 to 17,856.90 |
| Mean difference in total cost/mean difference in number of carious surfaces | –149.48 | –337.06 to –50.06 |
| Mean difference in total cost/mean difference in number of episodes of pain | –2017.56 | –13,130.80 to 7912.04 |
| Effect | Mean | 95% CI |
|---|---|---|
| NMB per caries-free person | –120.70 | –243.92 to –4.47 |
| NMB per carious surface avoided | 1118.95 | 399.16 to 1863.53 |
| NMB per episode of pain avoided | –64.63 | –238.40 to 94.97 |
Appendix 5 Additional analyses of caries, pain and extraction outcomes
| Examiner | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.921 (0.028) | 0.885 (0.036) | 0.756 (0.047) | 0.864 (0.038) | 0.767 (0.045) | 0.832 (0.041) | 0.843 (0.039) | 0.808 (0.042) | 0.767 (0.045) | 0.808 (0.042) | 0.800 (0.043) | 0.593 (0.061) | 0.838 (0.041) |
| 2 | 0.955 (0.023) | 0.783 (0.044) | 0.869 (0.037) | 0.792 (0.042) | 0.857 (0.037) | 0.868 (0.036) | 0.853 (0.037) | 0.792 (0.042) | 0.805 (0.042) | 0.853 (0.037) | 0.694 (0.054) | 0.906 (0.031) | |
| 3 | 0.920 (0.026) | 0.766 (0.046) | 0.810 (0.039) | 0.816 (0.040) | 0.845 (0.037) | 0.813 (0.040) | 0.828 (0.037) | 0.805 (0.040) | 0.831 (0.038) | 0.688 (0.052) | 0.803 (0.042) | ||
| 4 | 0.935 (0.027) | 0.795 (0.042) | 0.819 (0.041) | 0.831 (0.040) | 0.816 (0.041) | 0.795 (0.042) | 0.808 (0.041) | 0.836 (0.039) | 0.457 (0.061) | 0.866 (0.036) | |||
| 5 | 0.908 (0.027) | 0.859 (0.035) | 0.867 (0.034) | 0.905 (0.028) | 0.907 (0.028) | 0.879 (0.032) | 0.914 (0.027) | 0.679 (0.051) | 0.845 (0.037) | ||||
| 6 | 0.917 (0.027) | 0.897 (0.031) | 0.918 (0.027) | 0.859 (0.035) | 0.855 (0.035) | 0.899 (0.030) | 0.678 (0.053) | 0.894 (0.032) | |||||
| 7 | 0.916 (0.028) | 0.909 (0.028) | 0.868 (0.033) | 0.847 (0.036) | 0.873 (0.033) | 0.691 (0.052) | 0.904 (0.030) | ||||||
| 8 | 0.931 (0.024) | 0.906 (0.028) | 0.903 (0.029) | 0.912 (0.028) | 0.681 (0.052) | 0.907 (0.029) | |||||||
| 9 | 0.941 (0.022) | 0.881 (0.033) | 0.923 (0.025) | 0.721 (0.048) | 0.847 (0.036) | ||||||||
| 10 | 0.870 (0.033) | 0.886 (0.031) | 0.636 (0.054) | 0.824 (0.039) | |||||||||
| 11 | 0.929 (0.025) | 0.681 (0.054) | 0.681 (0.054) | ||||||||||
| 12 | 0.855 (0.038) | 0.855 (0.038) | |||||||||||
| 0.921 (0.028) |
| Examiner | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.954 (0.014) | 0.842 (0.025) | 0.680 (0.032) | 0.796 (0.027) | 0.889 (0.024) | 0.778 (0.027) | 0.756 (0.028) | 0.776 (0.027) | 0.850 (0.024) | 0.802 (0.027) | 0.797 (0.027) | 0.450 (0.044) | 0.763 (0.028) |
| 2 | 0.955 (0.013) | 0.764 (0.027) | 0.881 (0.020) | 0.837 (0.024) | 0.873 (0.020) | 0.863 (0.021) | 0.890 (0.019) | 0.867 (0.021) | 0.850 (0.022) | 0.880 (0.020) | 0.556 (0.040) | 0.886 (0.019) | |
| 3 | 0.878 (0.019) | 0.774 (0.026) | 0.730 (0.029) | 0.782 (0.025) | 0.806 (0.023) | 0.781 (0.025) | 0.766 (0.027) | 0.772 (0.026) | 0.768 (0.026) | 0.487 (0.038) | 0.801 (0.024) | ||
| 4 | 0.902 (0.015) | 0.787 (0.026) | 0.878 (0.019) | 0.869 (0.020) | 0.857 (0.021) | 0.819 (0.024) | 0.831 (0.023) | 0.872 (0.020) | 0.466 (0.040) | 0.891 (0.018) | |||
| 5 | 0.930 (0.016) | 0.805 (0.025) | 0.771 (0.026) | 0.823 (0.024) | 0.931 (0.016) | 0.849 (0.023) | 0.831 (0.024) | 0.525 (0.040) | 0.784 (0.026) | ||||
| 6 | 0.924 (0.015 | 0.916 (0.016) | 0.905 (0.017) | 0.833 (0.023) | 0.850 (0.021) | 0.889 (0.018) | 0.533 (0.038) | 0.889 (0.018) | |||||
| 7 | 0.869 (0.017) | 0.901 (0.017) | 0.812 (0.024) | 0.841 (0.022) | 0.868 (0.020) | 0.527 (0.037) | 0.904 (0.017) | ||||||
| 8 | 0.899 (0.017) | 0.863 (0.021) | 0.873 (0.020) | 0.887 (0.019) | 0.527 (0.037) | 0.911 (0.016) | |||||||
| 9 | 0.950 (0.013) | 0.891 (0.019) | 0.872 (0.020) | 0.591 (0.038) | 0.826 (0.023) | ||||||||
| 10 | 0.890 (0.019) | 0.836 (0.021) | 0.514 (0.039) | 0.831 (0.023) | |||||||||
| 11 | 0.897 (0.018) | 0.563 (0.038) | 0.864 (0.020) | ||||||||||
| 12 | 0.864 (0.027) | 0.509 (0.038) | |||||||||||
| 13 |
| Examiner | 1a | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.942 (0.022) | 0.834 (0.036) | 0.803 (0.039) | 0.849 (0.035) | 0.849 (0.034) | 0.738 (0.041) | 0.897 (0.028) | 0.785 (0.038) | 0.792 (0.039) | 0.862 (0.032) | 0.845 (0.035) | 0.851 (0.032) | 0.811 (0.036) |
| 2 | 0.942 (0.023) | 0.894 (0.030) | 0.873 (0.033) | 0.854 (0.034) | 0.768 (0.040) | 0.856 (0.034) | 0.804 (0.038) | 0.794 (0.040) | 0.803 (0.039) | 0.903 (0.029) | 0.794 (0.039) | 0.799 (0.039) | |
| 3 | 0.846 (0.036) | 0.857 (0.035) | 0.822 (0.038) | 0.771 (0.040) | 0.808 (0.039) | 0.790 (0.039) | 0.796 (0.039) | 0.822 (0.037) | 0.853 (0.035) | 0.781 (0.040) | 0.817 (0.035) | ||
| 4 | 0.897 (0.032) | 0.870 (0.0332) | 0.735 (0.043) | 0.872 (0.032) | 0.770 (0.041) | 0.791 (0.040) | 0.834 (0.036) | 0.867 (0.034) | 0.776 (0.040) | 0.813 (0.038) | |||
| 5 | 0.947 (0.021) | 0.784 (0.038) | 0.886 (0.030) | 0.848 (0.033) | 0.873 (0.031) | 0.850 (0.033) | 0.849 (0.035) | 0.825 (0.035) | 0.814 (0.037) | ||||
| 6 | 0.832 (0.035) | 0.741 (0.041) | 0.783 (0.037) | 0.717 (0.043) | 0.726 (0.042) | 0.779 (0.039) | 0.789 (0.037) | 0.751 (0.040) | |||||
| 7 | 0.909 (0.027) | 0.805 (0.037) | 0.812 (0.037) | 0.852 (0.033) | 0.867 (0.033) | 0.841 (0.034) | 0.862 (0.032) | ||||||
| 8 | 0.848 (0.033) | 0.823 (0.035) | 0.831 (0.034) | 0.814 (0.036) | 0.822 (0.034) | 0.798 (0.037) | |||||||
| 9 | 0.891 (0.030) | 0.855 (0.032) | 0.789 (0.040) | 0.771 (0.039) | 0.808 (0.037) | ||||||||
| 10 | 0.893 (0.029) | 0.831 (0.036) | 0.823 (0.035) | 0.858 (0.032) | |||||||||
| 11 | 0.907 (0.029) | 0.852 (0.033) | 0.826 (0.036) | ||||||||||
| 12 | 0.919 (0.025) | 0.819 (0.035) | |||||||||||
| 13 | 0.920 (0.025) |
| Examiner | 1a | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.947 (0.013) | 0.891 (0.018) | 0.827 (0.023) | 0.863 (0.020) | 0.902 (0.017) | 0.819 (0.022) | 0.874 (0.018) | 0.793 (0.022) | 0.831 (0.022) | 0.894 (0.017) | 0.864 (0.019) | 0.851 (0.020) | 0.881(0.018) |
| 2 | 0.919 (0.016) | 0.857 (0.021) | 0.886 (0.018) | 0.890 (0.018) | 0.817 (0.022) | 0.868 (0.019) | 0.807 (0.022) | 0.810 (0.024) | 0.858 (0.020) | 0.904 (0.016) | 0.834 (0.021) | 0.881(0.018) | |
| 3 | 0.889 (0.019) | 0.809 (0.024) | 0.856 (0.021) | 0.790 (0.025) | 0.771 (0.025) | 0.737 (0.025) | 0.805 (0.025) | 0.837 (0.022) | 0.801 (0.024) | 0.756 (0.025) | 0.824 (0.023) | ||
| 4 | 0.925 (0.016) | 0.856 (0.020) | 0.787 (0.023) | 0.858 (0.019) | 0.819 (0.021) | 0.801 (0.024) | 0.843 (0.021) | 0.883 (0.018) | 0.846 (0.020) | 0.848 (0.020) | |||
| 5 | 0.955 (0.012) | 0.838 (0.021) | 0.861 (0.019) | 0.790 (0.023) | 0.884 (0.019) | 0.899 (0.017) | 0.857 (0.020) | 0.838 (0.021) | 0.892 (0.018) | ||||
| 6 | 0.870 (0.019 | 0.805 (0.022) | 0.786 (0.022) | 0.795 (0.025) | 0.822 (0.021) | 0.816 (0.021) | 0.801 (0.022) | 0.821(0.021) | |||||
| 7 | 0.909 (0.016) | 0.830 (0.020) | 0.776 (0.024) | 0.833 (0.021) | 0.876 (0.018) | 0.857 (0.019) | 0.864 (0.019) | ||||||
| 8 | 0.875 (0.017) | 0.752 (0.024) | 0.796 (0.022) | 0.857 (0.019) | 0.840 (0.019) | 0.795 (0.022) | |||||||
| 9 | 0.903 (0.018) | 0.858 (0.020) | 0.798 (0.024) | 0.766 (0.024) | 0.839 (0.021) | ||||||||
| 10 | 0.911 (0.016) | 0.851 (0.020) | 0.827 (0.021) | 0.895 (0.017) | |||||||||
| 11 | 0.915 (0.018) | 0.859 (0.019) | 0.866 (0.019) | ||||||||||
| 12 | 0.829 (0.014) | 0.832 (0.021) | |||||||||||
| 13 | 0.937 (0.014) |
| Examiner | 1a | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.942 (0.022) | 0.865 (0.034) | 0.923 (0.025) | 0.942 (0.022) | 0.915 (0.027) | 0.941 (0.022) | 0.915 (0.027) | 0.889 (0.030) | 0.951 (0.020) | 0.932 (0.024) | 0.909 (0.027) | 0.942 (0.022) | 0.967 (0.016) |
| 2 | 0.942 (0.023) | 0.924 (0.026) | 0.873 (0.033) | 0.897 (0.031) | 0.888 (0.032) | 0.856 (0.036) | 0.868 (0.035) | 0.865 (0.034) | 0.897 (0.031) | 0.855 (0.035) | 0.890 (0.031) | 0.847 (0.036) | |
| 3 | 0.846 (0.036) | 0.914 (0.027) | 0.902 (0.029) | 0.929 (0.025) | 0.920 (0.026) | 0.910 (0.028) | 0.906 (0.028) | 0.920 (0.026) | 0.897 (0.029) | 0.948 (0.021) | 0.906 (0.028) | ||
| 4 | 0.897 (0.032) | 0.923 (0.025) | 0.915 (0.027) | 0.859 (0.030) | 0.897 (0.029) | 0.959 (0.018) | 0.940 (0.022) | 0.917 (0.026) | 0.950 (0.020) | 0.926 (0.025) | |||
| 5 | 0.947 (0.021) | 0.901 (0.029) | 0.859 (0.035) | 0.867 (0.034) | 0.915 (0.027) | 0.929 (0.025) | 0.872 (0.032) | 0.905 (0.028) | 0.898 (0.029) | ||||
| 6 | 0.832 (0.035) | 0.921 (0.026) | 0.859 (0.035) | 0.924 (0.025) | 0.921 (0.026) | 0.932 (0.024) | 0.914 (0.027) | 0.924 (0.025) | |||||
| 7 | 0.909 (0.027) | 0.902 (0.029) | 0.881 (0.031) | 0.894 (0.030) | 0.889 (0.030) | 0.922 (0.028) | 0.915 (0.027) | ||||||
| 8 | 0.848 (0.033) | 0.889 (0.030) | 0.884 (0.032) | 0.945 (0.036) | 0.930 (0.024) | 0.872 (0.032) | |||||||
| 9 | 0.891 (0.030) | 0.932 (0.024) | 0.909 (0.027) | 0.942 (0.022) | 0.918 (0.026) | ||||||||
| 10 | 0.893 (0.029) | 0.889 (0.030) | 0.922 (0.026) | 0.898 (0.029) | |||||||||
| 11 | 0.907 (0.029) | 0.898 (0.029) | 0.909 (0.027) | ||||||||||
| 12 | 0.919 (0.025) | 0.908 (0.029) | |||||||||||
| 13 | 0.920 (0.025) |
| Examiner | 1a | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.951 (0.012) | 0.854 (0.021) | 0.879 (0.019) | 0.633 (0.034) | 0.877 (0.021) | 0.911 (0.016) | 0.898 (0.017) | 0.862 (0.020) | 0.864 (0.021) | 0.895 (0.018) | 0.878 (0.019) | 0.894 (0.017) | 0.928 (0.014) |
| 2 | 0.924 (0.015) | 0.882 (0.020) | 0.591 (0.037) | 0.866 (0.021) | 0.876 (0.020) | 0.870 (0.020) | 0.880 (0.019) | 0.825 (0.024) | 0.858 (0.021) | 0.868 (0.020) | 0.863 (0.020) | 0.858 (0.021) | |
| 3 | 0.925 (0.016) | 0.631 (0.036) | 0.907 (0.018) | 0.910 (0.017) | 0.883 (0.019) | 0.855 (0.021) | 0.894 (0.019) | 0.920 (0.016) | 0.849 (0.022) | 0.869 (0.020) | 0.871 (0.020) | ||
| 4 | 0.909 (0.021) | 0.622 (0.036) | 0.612 (0.036) | 0.605 (0.035) | 0.621 (0.034) | 0.614 (0.037) | 0.636 (0.036) | 0.608 (0.035) | 0.641 (0.034) | 0.626 (0.034) | |||
| 5 | 0.944 (0.014) | 0.887 (0.019) | 0.855 (0.021) | 0.833 (0.023) | 0.884 (0.020) | 0.911 (0.017) | 0.834 (0.022) | 0.854 (0.021) | 0.882 (0.019) | ||||
| 6 | 0.924 (0.015) | 0.915 (0.016) | 0.844 (0.022) | 0.854 (0.022) | 0.907 (0.017) | 0.888 (0.018) | 0.877 (0.019) | 0.904 (0.017) | |||||
| 7 | 0.940 (0.013) | 0.881 (0.019) | 0.821 (0.024) | 0.880 (0.019) | 0.876 (0.019) | 0.894 (0.018) | 0.927 (0.015) | ||||||
| 8 | 0.905 (0.017) | 0.813 (0.024) | 0.839 (0.022) | 0.880 (0.019) | 0.910 (0.016) | 0.870 (0.019) | |||||||
| 9 | 0.931 (0.016) | 0.891 (0.0190) | 0.814 (0.024) | 0.834 (0.023) | 0.849 (0.022) | ||||||||
| 10 | 0.956 (0.012) | 0.840 (0.022) | 0.866 (0.020) | 0.875 (0.020) | |||||||||
| 11 | 0.902 (0.017) | 0.917 (0.016) | 0.877 (0.019) | ||||||||||
| 12 | 0.968 (0.010) | 0.889 (0.018) | |||||||||||
| 13 | 0.941 (0.013) |
| Independent variables | OR | Robust standard error | 95% CI for OR | p-value |
|---|---|---|---|---|
| Preventative package: standard care | 0.81 | 0.10 | 0.64 to 1.04 | 0.103 |
| Age | 1.49 | 0.15 | 1.22 to 1.80 | < 0.001 |
| MDM | ||||
| Quintile 2a | 0.76 | 0.14 | 0.54 to 1.08 | 0.13 |
| Quintile 3 | 0.73 | 0.21 | 0.41 to 1.28 | 0.27 |
| Quintile 4 | 0.61 | 0.13 | 0.40 to 0.94 | 0.026 |
| Quintile 5 | 0.46 | 0.13 | 0.27 to 0.80 | 0.006 |
| Independent variables | OR | Standard error | 95% CI for OR | p-value |
|---|---|---|---|---|
| Intervention: control | 0.90 | 0.14 | 0.65 to 1.23 | 0.50 |
| Deprivation (quintiles 1 and 2 vs. quintiles 3, 4 and 5) | 1.90 | 0.77 | 0.86 to 4.19 | 0.11 |
| Interaction (group × deprivation) | 0.79 | 0.20 | 0.47 to 1.31 | 0.36 |
| Independent variables | OR | Standard error | 95% CI for OR | p-value |
|---|---|---|---|---|
| Intervention: control | 0.84 | 0.26 | 0.45 to 1.54 | 0.56 |
| Age | 1.22 | 0.37 | 0.68 to 2.22 | 0.50 |
| MDM | ||||
| Quintile 2a | 1.29 | 0.60 | 0.52 to 3.22 | 0.58 |
| Quintile 3 | 1.03 | 0.48 | 0.42 to 2.58 | 0.94 |
| Quintile 4 | 1.04 | 0.50 | 0.411 to 2.68 | 0.93 |
| Quintile 5 | 0.72 | 0.50 | 0.18 to 2.83 | 0.63 |
| Independent variables | Regression coefficients | Standard error | 95% CI | p-value |
|---|---|---|---|---|
| Intervention: control | –0.03 | 0.43 | –0.88 to 0.82 | 0.95 |
| Age | 0.08 | 0.46 | –0.81 to 0.98 | 0.86 |
| MDM | ||||
| Quintilea | 0.16 | 0.68 | –1.18 to 1.50 | 0.82 |
| Quintile 3 | 0.16 | 0.68 | –1.17 to 1.49 | 0.82 |
| Quintile 4 | 0.32 | 0.71 | –1.08 to 1.72 | 0.65 |
| Quintile 5 | –0.72 | 0.96 | –2.61 to 1.16 | 0.45 |
| Independent variables | OR | Standard error | 95% CI for OR | p-value |
|---|---|---|---|---|
| Intervention: control | 0.95 | 0.15 | 0.69 to 1.30 | 0.74 |
| Caries status | 6.92 | 1.17 | 4.97 to 9.65 | < 0.0001 |
| Age | 1.14 | 0.18 | 0.84 to 1.55 | 0.39 |
| MDM | ||||
| Quintile 2a | 0.92 | 0.23 | 0.56 to 1.51 | 0.75 |
| Quintile 3 | 0.64 | 0.16 | 0.39 to 1.04 | 0.07 |
| Quintile 4 | 0.85 | 0.21 | 0.52 to 1.38 | 0.51 |
| Quintile 5 | 0.63 | 0.21 | 0.33 to 1.20 | 0.16 |
| Independent variables | Regression coefficients | Standard error | 95% CI | p-value |
|---|---|---|---|---|
| Intervention: control | –0.03 | 0.15 | –0.32 to 0.25 | 0.81 |
| Caries status | 2.18 | 0.16 | 1.87 to 2.48 | < 0.001 |
| Age | 0.20 | 0.14 | –0.07 to 0.46 | 0.15 |
| MDM | ||||
| Quintile 2a | –0.14 | 0.22 | –0.58 to 0.30 | 0.53 |
| Quintile 3 | –0.41 | 0.22 | –0.84 to 0.02 | 0.06 |
| Quintile 4 | –0.25 | 0.22 | –0.68 to 0.18 | 0.25 |
| Quintile 5 | –0.30 | 0.28 | –0.86 to 0.26 | 0.30 |
| Independent variables | OR | Standard error | 95% CI for OR | p-value |
|---|---|---|---|---|
| Intervention: control | 1.23 | 0.28 | 0.79 to 1.94 | 0.36 |
| Age | 0.79 | 0.17 | 0.52 to 1.21 | 0.29 |
| MDM | ||||
| Quintile 2a | 1.07 | 0.41 | 0.50 to 2.28 | 0.87 |
| Quintile 3 | 1.04 | 0.39 | 0.50 to 2.17 | 0.91 |
| Quintile 4 | 0.91 | 0.35 | 0.42 to 1.94 | 0.80 |
| Quintile 5 | 1.61 | 0.66 | 0.72 to 3.59 | 0.25 |
| Independent variables | Regression coefficients | Standard error | 95% CI | p-value |
|---|---|---|---|---|
| Intervention: control | 0.19 | 0.24 | –0.27 to 0.65 | 0.42 |
| Age | –0.20 | 0.23 | –0.64 to 0.25 | 0.39 |
| MDM | ||||
| Quintile 2a | 0.23 | 0.39 | –0.54 to 0.99 | 0.56 |
| Quintile 3 | 0.09 | 0.39 | –0.67 to 0.85 | 0.81 |
| Quintile 4 | –0.07 | 0.40 | –0.86 to 0.71 | 0.85 |
| Quintile 5 | 0.36 | 0.44 | –0.50 to 1.23 | 0.41 |
| Caries indices | Group, mean (SD) | p-value from chi-squared test/t-test | |
|---|---|---|---|
| Intervention | Control | ||
| All children (n = 1096) | |||
| dmfs | 2.45 (5.77) | 3.74 (7.19) | 0.0010 |
| dfs | 1.67 (0.16) | 2.86 (0.23) | < 0.0001 |
| ds | 1.35 (0.14) | 2.27 (0.20) | 0.0002 |
| fs | 0.41 (0.06) | 0.79 (0.10) | 0.0012 |
| dmft | 1.15 (2.18) | 1.64 (2.71) | 0.0013 |
| dft | 0.98 (1.92) | 1.43 (2.50) | 0.0006 |
| Children with caries active (n = 400) | |||
| dmft | 3.39 (2.54) | 4.20 (2.88) | 0.0003 |
| dft (children with caries active) | 2.74 (2.17) | 3.69 (2.79) | 0.0015 |
List of abbreviations
- A&E
- accident and emergency
- AA
- Automobile Association
- AE
- adverse event
- AR
- adverse reaction
- CDS
- community dental service
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CTIMP
- clinical trial of an investigative medicinal product
- CTU
- clinical trials unit
- DBOH
- Delivering Better Oral Health: An Evidence-Based Toolkit for Prevention
- dmfs
- decayed, missing, filled tooth surfaces in primary dentition
- DMFS
- decayed, missing, filled tooth surfaces in permanent dentition
- dmft
- decayed, missing, filled teeth in primary dentition
- DMFT
- decayed, missing, filled teeth in permanent dentition
- DQOF
- Dental Quality and Outcomes Framework
- GA
- general anaesthetic
- GCP
- good clinical practice
- GDP
- general dental practitioner
- GDS
- general dental service
- GP
- general practitioner
- HEAT
- Health Improvement, Efficiency, Access to treatment, Treatment target
- HSC
- Health and Social Care
- ICER
- incremental cost-effectiveness ratio
- MDM
- Multiple Deprivation Measure
- NIC-PIP
- Northern Ireland Caries Prevention In Practice
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OLS
- ordinary least squares
- OR
- odds ratio
- PPI
- patient and public involvement
- p.p.m.
- parts per million
- SAE
- serious adverse event
- SD
- standard deviation
- SDR
- Statement of Dental Remuneration
- UDA
- unit of dental activity