Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/60/49. The contractual start date was in September 2009. The draft report began editorial review in October 2014 and was accepted for publication in June 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jayant S Vaidya has received a research grant from Photoelectron Corp. (1996–9) and from Carl Zeiss for supporting data management at the University of Dundee (Dundee, UK) and has subsequently received honoraria. Jayant S Vaidya also has a patent for the use of the word TARGIT for TARGeted Intraoperative radioTherapy. Frederik Wenz has received a research grant from Carl Zeiss for supporting radiobiological research. Frederik Wenz also has patents for US 8,724,775B2, US 2013/058460 A, PCT/EP2011/057518, DE/18.12.09/DEA10200905877 and DE/17.12.09/DEA10200905058581, all issues to Wenz/Zeiss. Chris Brew-Graves, Ingrid Potyka and Norman R Williams report that the Clinical Trials Group was paid an unrestricted grant from 1 November 2001 to 31 October 2010. Michael Baum was on the scientific advisory board of Carl Zeiss and was paid monthly consultancy fees until 2010. In addition, Jayant S Vaidya, Frederik Wenz, Max Bulsara, Jeffrey S Tobias, David J Joseph, Christobel Saunders and Michael Baum report that Carl Zeiss sponsors most of the travel and accommodation for meetings of the International Steering Committee and Data Monitoring Committee and, when necessary, for conferences where a presentation about targeted intraoperative radiotherapy is being made for all authors.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Vaidya et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Parts of the text in this report, with the exceptions of Chapters 4–6 and 11, have been based on Vaidya et al. 1,2
Research objectives
The TARGeted Intraoperative radioTherapy Alone (TARGIT-A) trial is a pragmatic trial that compares two treatment policies in patients with early breast cancer who have undergone local excision of a good-prognosis tumour. The conventional policy is that each patient receives a radical course of whole-breast external beam radiotherapy (EBRT) according to local treatment guidelines. The experimental policy is to give TARGeted Intraoperative radioTherapy (TARGIT) in a single dose, recognising that some patients randomised to this treatment, because of unfavourable features found subsequently in the pathological examination of the excised lesion, will need to have EBRT in addition (without the boost, which has been provided by the targeted dose). A nested study allows randomisation of patients to TARGIT or EBRT after pathological examination of the removed lesion. Patients in this randomisation stratum who are allocated to TARGIT will require a second surgical procedure for administration of the radiation. Individual trial centres may opt to use either or both of these strata for entry, although we encourage using the method in which a single procedure is undertaken, as this is expected to be the norm if the trial is successful. The main objective of the trial, regardless of the stratum followed, is to compare local tumour control between the two policies. The secondary outcomes include toxicity and survival.
Background
Breast cancer is the most common cancer in women. In the UK, there were 49,564 new cases of breast cancer diagnosed in 2010 and 11,556 deaths. The majority of these cases are treated with breast-conserving surgery followed by whole-breast EBRT, rather than radical mastectomy. Several randomised clinical trials have reported that this method is safe and effective. 3–6 However, EBRT is a long process, typically taking 3–7 weeks of daily radiotherapy; this commitment is at best inconvenient and often exhausting and entirely impractical, particularly for elderly women or those whose closest radiotherapy centre is far from their home. 7 In such cases, women can be forced to choose to undergo a mastectomy for a cancer that could have been treated without losing the breast. Additionally, the prevalence of the disease is so high that even side effects or complications that affect only a small proportion of patients have a large absolute burden.
Although the complete omission of radiotherapy increases the risk of local recurrence,8–11 the necessity of delivering radiation to the entire breast has been challenged. 12–14 In total, 90% of early breast recurrences occur at the site of the original primary tumour, regardless of margin involvement or adjuvant radiotherapy. 13,15,16 This is perhaps surprising given evidence from three-dimensional whole-organ analyses of mastectomy specimens, > 60% of which host additional cancer foci, with 80% of these foci found remote from the index quadrant. 17 It appears that these cancers in other quadrants lie dormant, rarely manifesting clinically, and recurrences develop in the tissues immediately surrounding the primary tumour. This could be because of a tumour-promoting effect of the microenvironment of the surgical wound,18 or the presence of morphologically normal cells in the peritumoural tissues, which may already be on the path of malignant progression, as evidenced by their loss of heterozygosity. 19,20 This suggests that local control could be achieved through the targeted irradiation of the tissues in the immediate vicinity of the primary tumour. 12–14
In the early 1990s the Christie hospital trial tested index quadrant irradiation with encouraging results;20,21 these results helped us identify two types of breast cancer unsuitable for such an approach: lobular cancers and those with an extensive intraductal component (EIC). However, the latter factor may lose its importance if tumour-free margins are well clear.
To test the hypothesis that localised radiotherapy might be sufficient, we needed to have an elegant means to provide such radiation. In early 1996 we received a fortuitous enquiry from the Photoelectron Corporation (Lexington, MA, USA) asking whether we would be interested in developing a radiotherapy device for breast cancer. From October 1996 to July 1998 we developed the device and the operative technique22 and treated the first patient on 2 July 1998 in Middlesex Hospital, University College London (UCL), London, with what we called TARGIT (TARGeted Intraoperative radioTherapy),23,24 in which low-energy X-rays are delivered to the tumour bed directly – a single dose of radiotherapy given at the time of the initial lumpectomy to replace several weeks of treatment, travel and stress.
These X-rays are delivered using the INTRABEAM® system, originally developed in collaboration with the Photoelectron Corporation, USA, and now manufactured by Carl Zeiss (Oberkochen, Germany). The INTRABEAM uses an electron beam to generate a point source of low-energy X-rays (50 kV maximum) at the tip of a 3.2-mm diameter tube. A personalised spherical applicator is then used to accurately target the tissues of the tumour bed for 20–35 minutes. The physics and dosimetry of this device have been well studied;25,26 the applicator delivers a uniform 20 Gy to the surface of the tumour bed and, as X-rays attenuate rapidly, spares lower tissues by delivering to them a much lower dose.
The TARGIT-A trial was set up to test whether or not this technique could be used instead of EBRT in the majority of early breast cancers; our primary outcome was local control, with secondary outcomes including mortality and radiation toxicity.
To test this widely we designed the trial with a pragmatic approach; patients were randomised to the TARGIT arm or EBRT arm but, if a patient in the TARGIT arm was found after surgery to have an unexpectedly higher risk of recurrence, protocol dictated that EBRT also be delivered. We expected this to be necessary in around 15% of cases. We had also ascertained that the combination of TARGIT and EBRT is safe, as tested in a 25-patient pilot study in UCL, London. 22,27
In fact, giving TARGIT at the time of surgery as a tumour bed boost followed by subsequent EBRT has been found to result in a very low 5-year recurrence rate of 1.73%,27,28 with favourable toxicity and cosmetic outcome results;29–32 furthermore, mathematical models of TARGIT have recently suggested that it could be superior in terms of local control to conventional radiotherapy. 33,34 This could be because of the radiotherapy limiting breast cancer cell proliferation normally caused by the trauma of surgery. 17
The biological, economical and technical advantages of TARGIT distinguish this trial from other trials that have since been launched with a similar aim to irradiate only the index quadrant, such as the ELectron IntraOperative radiotherapy (ELIOT) trial in Italy,35 the National Surgical Adjuvant Breast and Bowel Project (NSABP) in the USA [see www.nsabp.pitt.edu/B-39.asp (accessed 14 July 2016)] and the Intensity Modulated and Partial Organ RadioTherapy – low (IMPORT-low) trial in the UK [see www.icr.ac.uk/our-research/our-research-centres/clinical-trials-and-statistics-unit/clinical-trials/import_low (accessed 14 July 2016)].
Thus, the TARGIT-A trial was an investigator-initiated trial that was launched in March 2000. 36 At the time of our successful funding application to the Health Technology Assessment (HTA) programme it was already recruiting from 21 centres (increased from 16 in the previous 12 months) and 1301 patients had already been recruited. The original accrual goal of 2232 was achieved in April 2010. In July 2010, when we reported in The Lancet the initial results for local control and early complications as a fast-track publication,1 the 4-year Kaplan–Meier estimate of local recurrence in the conserved breast was 1.20% [95% confidence interval (CI) 0.53% to 2.71%] for those randomised to TARGIT and 0.95% (95% CI 0.39% to 2.31%) for those randomised to EBRT. A second analysis was planned after a further 2 years of follow-up. We continued randomisation until June 2012 to allow accrual in sub-protocols while the data matured further and closed the trial after accruing the planned 1200 additional patients (1219 accrued, total n = 3451) (see Appendix 2). We have recently published2 the updated analyses and 5-year estimates for local control and the first analysis of overall survival and whether or not the timing of TARGIT in relation to lumpectomy made a difference to the outcome.
Implications of this trial
The TARGIT technique could potentially save time, money and breasts:
-
Time – for the patient and the health system.
-
Money – we estimate that TARGIT could save around £30 million per year in the UK alone. 37 It is worth noting that, as EBRT is not needed in around half of the patients in the trial, the trial itself is also likely to be cost neutral or profitable to hospitals.
-
Breasts – TARGIT should allow more women to choose breast-conserving surgical options over a mastectomy.
-
Insight – the immediate effect of radiotherapy on human tissues in vivo can be studied, perhaps yielding important insight into the mechanism of action of radiotherapy.
-
Toxicity – the TARGIT technique does not irradiate vital nearby organs such as the heart or lungs and this may reduce deaths from radiation toxicity.
Chapter 2 Methods
The TARGIT-A trial was a pragmatic, prospective, international, multicentre, randomised Phase III trial that compared TARGIT with the conventional policy of whole-breast EBRT.
Patient selection
Women were eligible for randomisation if they were aged ≥ 45 years with an invasive breast tumour (T1 and small T2 ≤ 3.5 cm, N0–1, M0, as confirmed by cytology or histology) and conventional examination regarded them suitable for treatment with wide local excision.
A magnetic resonance imaging (MRI) scan was not necessary for inclusion and only 5.6% (n = 192) of patients went through this procedure.
All patients gave written informed consent and the protocol was approved by the appropriate regulatory and ethics authorities for each centre before enrolment could begin.
Inclusion criteria
All patients aged ≥ 45 years with operable invasive breast cancer [tumour, nodes, metastasis (TNM) – T1 and small T2 ≤ 3.5 cm, N0–1, M0], confirmed by cytological or histological examination, who were suitable for breast-conserving surgery were eligible. The tumour needed to be clinically suitable for breast conservation on conventional imaging. A MRI scan was not required. Individual centres could restrict entry to a more exactly defined subset of patients, in which case only patients with these characteristics could be entered by that particular centre. For example, centres could at the outset decide to recruit only women aged > 50 years or even only women aged > 65 years. Such treatment policies were predefined in writing and approved by the International Steering Committee (ISC). Patients needed to be available for regular follow-up (according to local policies) for at least 10 years.
Exclusion criteria
-
More than one obvious cancer in the same breast as diagnosed by clinical examination, mammography or ultrasonography (MRI not required).
-
Bilateral breast cancer at the time of diagnosis.
-
Ipsilateral breast had a previous cancer and/or irradiation.
-
Patients known to have BRCA gene mutations but testing for gene mutations was not required.
-
Lobular cancer or EIC (in EIC ≥ 25% of the tumour is intraductal) on core biopsy or initial pathology (if performed).
-
Patients undergoing primary medical treatment (hormones or chemotherapy) as initial treatment with neoadjuvant intent of reducing tumour size were excluded; those given short-duration (up to 4 weeks) systemic therapy could be included.
-
Patients presenting with gross nodal disease, considered to be clinically malignant or proven cytologically or by scanning. In general, four or more positive nodes or extranodal spread meant that a patient was not suitable for TARGIT alone and should receive EBRT as well. However, individual centres could decide that anything more than micrometastasis should receive EBRT.
-
Patients with any severe concomitant disease that may limit their life expectancy. Previous history of malignant disease did not preclude entry if the expectation of relapse-free survival at 10 years was ≥ 90%.
-
Any factor included as an exclusion criterion in the local centre’s treatment policy. This was particularly relevant to patients entered into the postpathology stratum.
-
No more than 30 days elapsed between last breast cancer surgery (not axillary) and entry into the trial for patients in the postpathology stratum.
Each patient was given time to consider participation and ask questions before giving consent by signing the patient consent form and randomisation occurred only after fully informed consent was freely given.
The treatments in each of the arms are described in detail in the protocol, which is published in full at www.nets.nihr.ac.uk/projects/hta/076049 (accessed 14 July 2016). Patients in both arms underwent a similar primary surgical procedure. The patients in the conventional arm underwent standard EBRT (according to the centres’ predefined local policy). Patients in the experimental arm underwent TARGIT as described previously in detail. 22,24,36 The papers, presentations and video related to TARGIT are available at www.targit.org.uk (accessed 29 June 2016) and a video demonstrating the operative technique is available at http://goo.gl/iuF9ZR (accessed 29 June 2016).
Trial design
The TARGIT-A trial was a pragmatic, randomised clinical trial to directly compare the outcome, primarily in terms of local control and secondarily in terms of toxicity and mortality, of two approaches to adjuvant radiotherapy: TARGIT within a risk-adapted approach and conventional EBRT. Nested within the pragmatic trial was a more selective stratum that was employed in some centres in some or all patients as per a pre-declared policy. Eligible patients were enrolled once they received information and gave their consent. The flowchart in Figure 1 shows the trial design.
FIGURE 1.
Flow chart outlining TARGIT-A recruitment. Reproduced with permission from Vaidya et al. 2 Copyright © Vaidya et al. Open Access article distributed under the terms of CC BY-NC-ND.

Protocol amendment
In the initial trial design, randomisation to TARGIT or EBRT was carried out before lumpectomy (prepathology). However, the trial was also firmly rooted in the principles of pragmatism to test a new approach (single-dose TARGIT to the tumour bed followed by EBRT in patients with unforeseen adverse factors). Therefore, when some of the centres planning to join the trial requested us to allow them to give intraoperative radiotherapy (TARGIT IORT) as a second procedure by reopening the wound, we permitted this. This decision facilitated a more stringent selection of patients (tumour pathology was available, hence postpathology) and was logistically easier, allowing enrolment of patients from neighbouring centres who had already had the lumpectomy. We therefore made a protocol amendment on 22 September 2004, obtained ethics approval and added this postpathology stratum to the trial, along with a completely separate randomisation table for such patients.
We specified that postpathology patients should be randomised within 30 days after lumpectomy. If allocated to TARGIT, patients in the prepathology stratum received it concurrently, immediately after surgical excision under the same anaesthesia; patients in the postpathology stratum received it as a subsequent procedure. We planned a separate analysis of the two strata (prepathology vs. postpathology). The rationale for stratification according to the scheduling of radiotherapy was that randomisation to the trial after full pathology had become available might theoretically allow better case selection. Conversely, treatment given at the time of initial lumpectomy could have a greater effectiveness because of its immediacy. Furthermore, the degree of accuracy of placement of the radiotherapy applicator for giving TARGIT by reopening the cavity might be quite different from that achieved at the time of original lumpectomy.
The randomisation process
All randomisations were performed by staff at the Trial Operations Group in London, except for those in Australia (performed in Perth because of the large time difference). Details have been described previously32 and are as follows. The randomisation schedules were generated centrally by computer (securely kept in trial centres in Perth, Australia, for Australian centres and London, UK, for all other centres). Requests for randomisation were sent by telephone or fax to the trials office (Perth or London), where a trained member of staff checked patient eligibility. Treatment was allocated from a preprinted randomisation schedule available to authorised staff only and not to any clinician or investigators. Written confirmation of randomisation was sent by fax to the study site. Neither patients nor investigators or their teams were masked to treatment given after randomisation. Individual sites were unblinded to treatment given to their own patients, but they were not given access to these data for other sites. Confidential unblinded reports for the Data Monitoring Committee (DMC), and blinded reports for the ISC were produced by the trial statistician. Unblinded analyses were carried out according to a pre-specified statistical analysis plan.
Patients were randomly assigned in a 1 : 1 ratio to receive a risk-adapted approach using single-dose TARGIT or EBRT as per standard schedules over several weeks, with blocks stratified by site and by proposed timing of delivery of TARGIT (prepathology and postpathology strata; Figure 2).
FIGURE 2.
Schematic diagram showing the two strata according to timing of randomisation in relation to the initial tumour excision.

A risk-adapted approach meant that if the final pathology report showed unpredicted pre-specified adverse features, then addition of EBRT to TARGIT was recommended, in which case TARGIT served as the tumour bed boost. The core protocol defined three such features within the experimental group, the presence of which resulted in a recommendation to supplement TARGIT with EBRT: tumour-free margin < 1 mm, extensive in situ component or unexpected invasive lobular carcinoma. Pragmatically, individual centres could pre-specify more than these core factors, such as close margins (e.g. 1–10 mm) or other adverse prognostic factors (e.g. several positive nodes, extensive lymphovascular invasion) in a treatment policy document before starting recruitment. Therefore, the trial was a comparison of two policies –conventional whole-breast radiotherapy for all patients compared with individualised risk-adapted therapy in which a proportion of patients who received TARGIT were also given EBRT if they were shown to have adverse tumour factors. This situation was expected in 15% of cases and was incorporated into the power calculations. All analyses were by intention to treat (ITT).
Randomisation was stratified, first, according to participating site. Patients were then randomised into the trial in one of three strata.
Thirty-three centres in 11 countries participated in the trial. Data from individual patients were collected by each centre and sent to the central office using standard case report forms (CRFs).
Stratum 1: prepathology entry
Eligible patients’ consent was sought and randomisation was carried out prior to the surgical removal of the tumour. Postoperatively, some patients were found to have characteristics that militated against a single intraoperative dose of radiotherapy (e.g. lobular carcinoma, positive margins at first excision, extensive lymphovascular invasion, multiple involved axillary nodes). In these cases (as per each centre’s pre-specified treatment policy), patients were recommended to have a full course of EBRT, without the tumour bed boost. Grade 3 cancers were not necessarily excluded. This histology was considered in combination with other factors such as presence of lymphovascular invasion, as specified in each centre’s policy document. For example, a patient with a screen-detected 1.5-cm grade 3 oestrogen receptor (ER)-positive tumour would have been eligible and been randomised. However, in rare circumstances she may postoperatively have been found to have extensive lymphovascular invasion and multiple positive lymph nodes. This would mean that if she had received TARGIT she would have needed to have EBRT as well, while remaining in the TARGIT arm of the study. The intraoperatively delivered radiotherapy replaced the boost in this instance. The two arms were compared on the basis of the policy to provide local tumour control, accepting that some (about 15%) of the patients in the TARGIT arm would also receive EBRT. This design tested the ‘real-world’ policy as it was the most likely way that TARGIT would be implemented in the future if proven to be non-inferior and/or less toxic in the trial.
Stratum 2: postpathology entry
Eligible patients were randomised for entry to the trial after they had had their cancer removed with a lumpectomy. If allocated to the TARGIT arm they had further surgery to reopen the wound, with TARGIT delivered to the tumour bed. This stratum was added for logistical reasons, particularly in some centres. Furthermore, this stratum allowed easier operating theatre logistics and a more stringent case selection, although it required a reoperation to reopen the wound to provide TARGIT as a delayed procedure. This was a disadvantage for patients in this stratum, although reopening the wound could have been carried out under local anaesthetic. 38 However, it allowed for the entry of patients who had already received surgery at an outlying centre, a common practice in countries with a large land mass and large rural populations.
Stratum 3: previous contralateral breast cancer
Most patients who require treatment for a metachronous breast cancer are excluded from entry to trials of local treatment. However, the second cancer is usually treated in a manner similar to the first one and intraoperative treatment may be particularly suitable. This trial assessed this stratum only for ipsilateral local control and data were censored for other end-point assessments.
It is important to recognise that the trial was pragmatic and tested two policies rather than two techniques. In other words, the trial did not test TARGIT compared with EBRT but a pragmatic policy of TARGIT (in 100%) ± EBRT (in approximately 15%) compared with EBRT (in 100%), that is, the novel approach was to use TARGIT in potentially eligible patients and add EBRT if an unexpectedly higher risk was found postoperatively. From our Phase I/II studies we had established that the TARGIT ± EBRT protocol was safe22,23 and very effective. 27,28,39
Planned interventions
Prior to patient recruitment
Prior to entry of any patients, each centre registered with the ISC and completed a treatment policy document that defined the categories of patients to be entered (e.g. patients aged > 50 years, all N0) together with some details of treatment policy (e.g. fractionation and dose of conventional radiotherapy to be used). Any change to practice during the course of the trial had to be notified to the ISC in writing prior to implementation. This was to enable the ISC to audit the patients entered and to confirm that treatment remained true to the core protocol.
Only clinical centres with the INTRABEAM or those that were able to refer patients to such a centre could enter the trial. Centres with newly acquired equipment were required to consult the TARGIT trial operations office prior to entering patients into the trial. Confirmation of the quality control of the system set-up had to be received at the operations office before randomisation could begin.
Before entering any patients into the trial, centres were expected to submit data for each X-ray source probe and applicator set in use. Each centre was responsible for measuring data for the probe and applicator set and submitted the data supplied by the manufacturer for comparison with measured data together with a copy of the letter of acceptance supplied by Carl Zeiss.
In addition, a minimum of five ‘pilot’ cases (non-randomised patients) were performed in every new centre followed by an audit by a member of the ISC (or an appointed delegate).
Surgery
All patients had wide local excision of the primary tumour following appropriate clinical work-up. No special assessments prior to randomisation were required, although mammography and ultrasound were recommended to try to exclude multifocal disease and to determine as accurately as possible the size of the tumour.
Surgery was carried out according to usual local practice. Complete macroscopic excision of the tumour was required. The aim of the local excision was to achieve the widest margin of excision while maintaining a good cosmetic outcome. The final histological margin needed to be ≥ 1 mm clear of all invasive and in situ disease.
For superficial tumours an ellipse of overlying skin was normally excised. The depth of resection depended on the position of the tumour within the breast and the size of the breast, but in most instances extended to the pectoral fascia.
The protocol specified that in all patients, but especially in those women with impalpable tumours in whom preoperative localisation had been performed, the specimen needed to be well orientated with sutures or clips according to local protocols and to be radiographed intraoperatively. The specimen radiograph was examined in theatre to ensure complete excision of the lesion and to help with the assessment of adequacy of the margins. Further tissue had to be taken (and marked) from a margin if the radiographic abnormality had extended near the margin.
Either a standard sentinel node biopsy or at least level II axillary node clearance had to be performed in all patients. The protocol specified that similar surgical techniques should be employed in all patients regardless of randomisation, wound closure should be performed meticulously (air and water tight) as described22–24 and sutures (if non-absorbable) should remain in place for 14 days. In our pilot study of 300 patients27,28,39 we had found that, with the meticulous wound closure specified, wound healing was not a problem whether TARGIT was delivered during the primary surgery or as a secondary procedure.
Prepathology stratum
In this stratum, patients were randomised before the tumour was removed. If randomised to receive TARGIT, it was given during the initial removal of the cancer. The lumpectomy itself was carried out in the same way as usual. Following surgery, detailed histopathological examination of the specimen was discussed in the multidisciplinary team meeting and a decision was made whether whole-breast EBRT needed to be added or not.
Patients with adverse prognostic factors
If final pathology showed involved or close margins (evidence of invasive or in situ tumour at, or within 1 mm of, an excision margin), re-excision was strongly recommended. In some cases this necessitated a mastectomy. For patients who had already received TARGIT, such re-excision to clear margins was recommended to be followed by EBRT, excluding the tumour bed boost.
Additional EBRT was also recommended for patients with other markers linked with a higher risk of relapse in the breast, especially in the other quadrants (e.g. lobular cancer, EIC).
In 2008 we recommended that every patient receive a preoperative diagnosis with a core biopsy. When the trial started in 2000, tissue diagnosis of cancer was provided only by fine-needle aspiration cytology (FNAC) and so lobular carcinoma or extensive ductal carcinoma in situ (DCIS) was not identified preoperatively. However, almost all centres now perform a core biopsy and exclude these patients, as recommended in the protocol (but not compulsory, within the pragmatic spirit of the trial). Therefore, our original recommendation remained that patients found on pathological examination of the operation specimen to have either invasive lobular cancer or EIC (or other adverse criteria such as extensive lymphovascular invasion and node involvement, as defined by the local centre) receive EBRT as these patients are at a higher risk of developing recurrence in the ipsilateral breast at a site other than that of the excised primary tumour. For those patients randomised to intraoperative radiation this was in addition to the treatment that they had already received and it was recommended that any boost was omitted. These patients remained in the trial. However, most of these patients (e.g. those with lobular carcinoma) would not have been included in the first instance if a preoperative core biopsy had been performed.
Alternatively, a mastectomy could be performed if it was deemed necessary, based on the final histopathology, multidisciplinary team meeting and joint consultation between the patient and the clinician, irrespective of the arm of randomisation. This had to be recorded on the patient’s trial CRF; the patient remained in the trial and continued to be followed according to the protocol.
Postpathology stratum
Patients randomised in this stratum had their cancer removed as a lumpectomy or excision biopsy and the histopathological examination completed, preferably with clear margins confirmed, before being randomised. If no other adverse pathological features were present, patients with involved margins could be randomised provided that repeat excision to clear margins was performed prior to radiotherapy (TARGIT or EBRT). If after delivering TARGIT it was found that the re-excision margin was also involved, these patients received EBRT in addition, as repeated positive margins is a poor prognostic factor. 40
The procedure for delivering TARGIT was the same as in the prepathology stratum. In this group of patients, antibiotics were given for 3 days (rather than just one pre-incision dose as used in the prepathology stratum) and special care was taken to ensure that the wound closure was meticulous and air and water tight and that sutures were not removed for at least 2 weeks. If an absorbable suture was used it was specified to be at least 3-0 in thickness and to not be absorbable within 2 weeks (not ‘rapide’) and Steri-Strips™ (3M, St Paul, MN, USA) were to be used and left in situ for 2 weeks.
Contralateral stratum
Patients were managed as appropriate in the pre- or postpathology stratum.
Pathological examination
Data from pathological examinations was recorded on the appropriate data collection forms. We recommended that the minimum data as requested on the case record forms were recorded.
Radiotherapy
Targeted intraoperative radiotherapy
Intraoperative radiotherapy was delivered either in the operating theatre immediately after the removal of the tumour or as a subsequent procedure, a short time later.
The concept and the TARGIT technique, which was pioneered by investigators at UCL, London,22–24 allows the patient to potentially receive all of the required radiation in a single fraction before she awakes from surgery22–24,34,37,41–48 (Figure 3). The INTRABEAM device provides a point source of 50-kV energy X-rays at the centre of a spherical applicator. The appropriately sized (1.5–5 cm diameter) applicator is placed in the tumour bed using meticulous surgical technique including a carefully inserted purse-string suture that ensures that breast tissues at risk of local recurrence receive the prescribed dose while skin and deeper structures are protected. Radiation is delivered to the tumour bed over 20–45 minutes. The surface of the tumour bed typically receives 20 Gy, which attenuates to 5–7 Gy at a depth of 1 cm.
FIGURE 3.
The TARGIT technique: (a) The INTRABEAM device; (b) a schematic diagram showing how the spherical applicator fits into the tumour bed; (c) IORT being delivered in the operating theatre; and (d) a close-up of the spherical applicator in the tumour bed. Reproduced with permission from Vaidya et al. 2 Copyright © Vaidya et al. Open Access article distributed under the terms of CC BY-NC-ND.


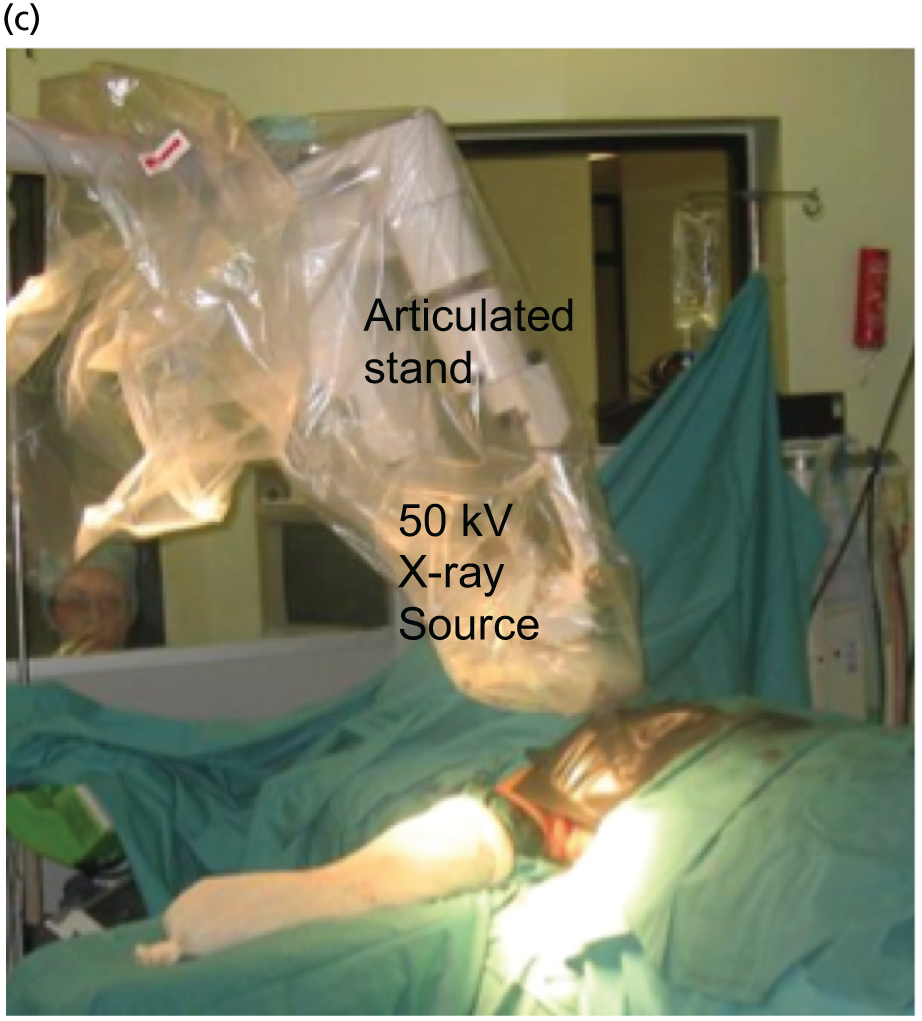
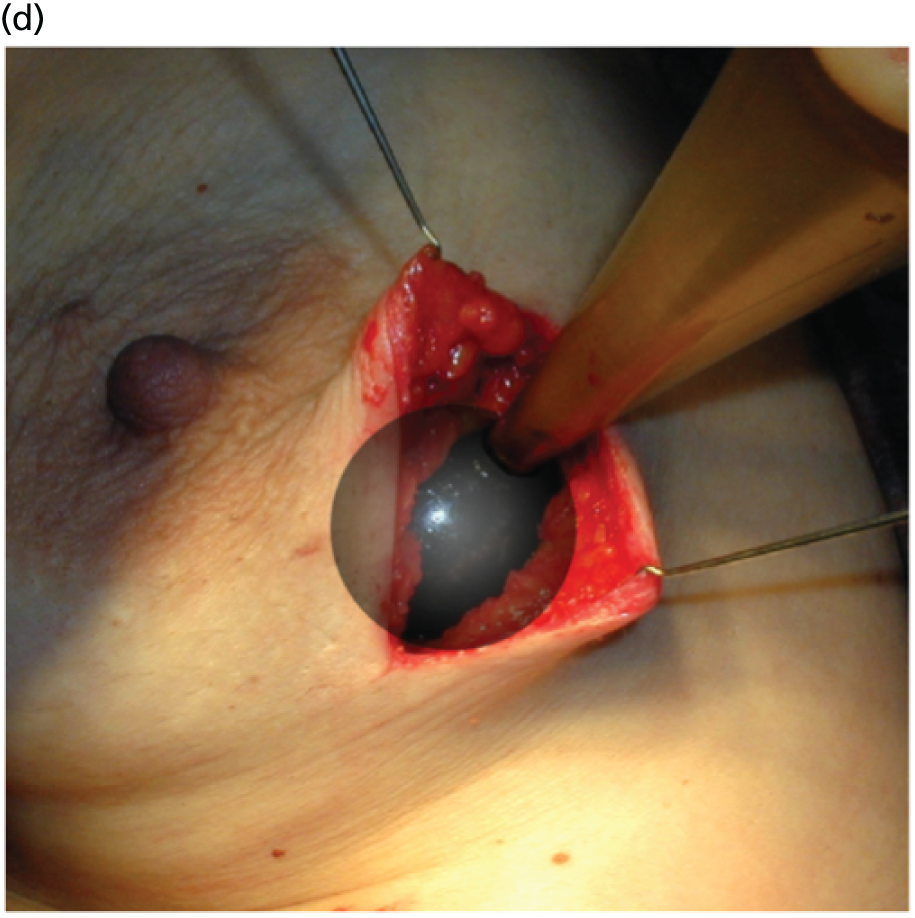
The procedure has been described previously. 22–24 The papers, presentations (see Appendix 3) and video related to TARGIT are available at www.targit.org.uk (accessed 29 June 2016) and a video demonstrating the operative technique is available at http://goo.gl/iuF9ZR (accessed 29 June 2016).
All patients should receive a prophylactic dose of antibiotic just before skin incision. The device and the arm of the stand are wrapped in a sterile clear plastic cover. The individual applicators are sterilised prior to the theatre session. The size of the sphere is determined at surgery by the surgeon and/or the radiation oncologist. An appropriately sized INTRABEAM sphere fits comfortably without tension in the surrounding tissue so that the skin and subcutaneous tissues can be gathered with a purse-string suture over the sphere. Any other technique to assist this apposition may also be used. The surgeon and radiation oncologist should choose the largest possible suitable applicator to ensure that the highest dose is delivered to the tumour bed tissue.
It is essential that complete haemostasis is achieved before insertion of the applicator sphere, because even a small ooze of blood can distort the cavity around the sphere and significantly change the target dose. The applicator sphere is inserted into the surgical cavity and a deep surgical purse-string suture is inserted in the breast tissues to bring together the target breast tissue so that it applies well to the surface of the INTRABEAM applicator sphere and holds it in place during treatment. The skin, but not the breast tissue, should usually be everted and held away from the delivery device by surgical sutures to prevent direct contact with the sphere. One patient in the pilot series did develop an area of skin necrosis. It is important to keep the skin at a distance of at least 1 cm from the applicator.
If necessary, protective caps (made from tungsten-impregnated rubber and available from Carl Zeiss) may be fashioned by the surgeon to protect deep or superficial structures. If the deep margin of excision is such that the left anterior descending branch of the coronary artery could receive a significant radiation dose, then the surface of the applicator sphere should be covered with a protective cap at the chest wall. However, in most patients the normal thickness of the chest wall (muscle and rib cage) provides adequate shielding and such a protective cap is not required. Sometimes the superficial skin flap may require protection with a 0.5-cm thick, cut piece of wet gauze. Care must be taken, however, not to inadvertently shield the areas of tissue that require radiation treatment. The shaft does not emit radiation so wet gauze should not be placed between the shaft and the skin. The anterior surface of the tumour bed should be a relatively thick skin flap so that it can receive radiation from inside without the applicator being too close to the dermis or if this skin is too thin it can be excised as a skin ellipse to get clear margins.
Radiation protection shielding material should also be used to cover the would around the radiation device; this significantly reduces the dose to the operating theatre staff to very low levels.
The protocol allowed for two dose prescriptions, each of which was equivalent to 20 Gy at the surface of the applicator. Each participating site decided on one method and used this procedure for all patients for the duration of the trial.
-
Alternative A. A dose of 20 Gy at the surface of the applicator (in water) was prescribed by the radiation oncologist and delivered to the breast tissue. This takes approximately 30 minutes, depending on the size of the applicator.
-
Alternative B. A dose of 6 Gy at 1 cm (in water) was prescribed by the radiation oncologist and delivered to the breast tissue. This also takes approximately 30 minutes, depending on the size of the applicator. This dose is equivalent to 5 Gy at a 1-cm depth for adipose tissue.
Using prescription A ensured that larger tumours received a slightly higher dose in the tumour bed (> 6 Gy at 1 cm and beyond). Previous versions of the TARGIT protocol recommended a dose of 5 Gy at 10 mm in adipose tissue, which is equivalent to 6 Gy at 10 mm in water. Rules in some countries such as Germany dictate that the prescription should always be at the highest dose delivered. Therefore, we adopted alternative A but at the same time, as the dose at the surface cannot be physically measured (but rather estimated), we kept the option of using alternative B, prescribing the dose at 1 cm, because the dose delivered by both approaches will typically be very similar. With the publication of further research40,45 it was later recommended that 20 Gy at the applicator surface should be adopted by all new sites. Such a dose prescription is arguably more logical as it ensured that tumour beds from larger tumours received a higher dose than small tumours.
During the radiation treatment, the anaesthetist, clinician and physicist could remain in the room. To avoid unnecessary exposure we recommended that as many people as possible vacated the operating theatre and those remaining either wore a lead apron or remained behind a shielded screen. No modifications to the operating theatre were required.
After completion of radiation, the conforming stitches are removed. Strict haemostasis needs to be obtained following the removal of the INTRABEAM device. The skin is sutured meticulously to achieve a water-tight closure and a good cosmetic result. If non-absorbable sutures are used they are left in situ for 14 days and, if absorbable sutures are used, Steri-Strips covering the entire wound are left in place for 14 days.
Conventional radiotherapy: the control arm
Planning protocols for conventional radiotherapy varied from centre to centre but for each centre a written policy was required. All patients randomised to receive conventional radiotherapy within this trial were treated in accordance with this policy. Dosage was applied only to the breast; axillary, supraclavicular and internal mammary nodes were not generally to be irradiated by discrete fields. Patients with previously irradiated adjacent fields, for example those with previous contralateral breast cancer, needed to have the radiotherapy fields modified according to local policies. The announcement of the Standardisation of Breast Radiotherapy (START) trial49 results increased the popularity of a 3- to 4-week schedule in the UK (but not elsewhere in the world). With the pragmatic nature of the trial and with individual centre stratification, it was possible to incorporate these changes in practice into the trial without jeopardising its statistical or scientific integrity. Although it could have altered the economic analysis to some extent, TARGIT would still maintain its potential advantages in terms of avoiding geographical and temporal misses and reducing the duration from 15–20 postoperative fractions to a single intraoperative fraction of radiotherapy while also retaining its promise of significantly improving the accessibility to breast-conserving surgery in remote areas around the world.
Adjuvant systemic therapy
Following completion of TARGIT, patients were recommended appropriate adjuvant therapy according to local practice or trial protocols. For all trial patients, the sequencing of these other therapies was not governed by this protocol, but careful consideration needed to be given for patients randomised to TARGIT but needing EBRT and adjuvant chemotherapy. The policy for such treatments needed to be declared in advance in the treatment policy document. It was recommended that even the postpathology TARGIT was delivered before beginning chemotherapy.
Follow-up and notification of recurrence, adverse events and death
Patients were followed up according to local guidelines, but at least at 6-monthly intervals for the first 5 years and annually thereafter until at least 10 years. At each visit patients were offered a physical examination and asked whether or not they had experienced any adverse events. We recommended that mammography of the ipsilateral breast was carried out annually and of the contralateral breast at least every 3 years. However, usually, annual mammography was performed on both breasts. Any other examination was at the discretion of the local clinician.
Adverse events
Details of management of adverse events, definitions of suspected serious adverse reactions and suspected unexpected serious adverse reactions and the reporting requirements are provided in the protocol. The ISC and the DMC reviewed data on adverse events and complications. Acute and late radiation morbidity was graded according to the Radiation Therapy Oncology Group (RTOG) criteria and ‘pain due to radiation’ according to the common toxicity criteria. 50 These were the only expected adverse events but other adverse events were also recorded.
Proposed outcome measures
Patient assessments were scheduled at entry, 3 months and 6 months; thereafter, they were scheduled every 6 months up to 5 years and then yearly for up to 10 years.
Local tumour control was defined as no recurrent tumour (defined as invasive or in situ breast cancer) in the conserved breast. The primary outcome measure was the absolute difference in local recurrence in the conserved breast between the TARGIT and the EBRT groups. Power calculations were based on this outcome measure for an absolute non-inferiority margin of 2.5% (as detailed in section 9 of the protocol) and the original recruitment goal was 2232 patients in total.
Patients were regularly monitored as per the individual centre’s policy, provided that this met the minimum criteria for follow-up, that is, 6-monthly for the first 5 years and then yearly until 10 years after randomisation. Recurrence was confirmed by at least cytology and preferably core biopsy.
Overall survival was the time interval between randomisation and death. The secondary outcomes were toxicity and overall survival, including breast cancer deaths and non-breast-cancer deaths. An independent senior clinician, masked to randomisation, reviewed the available data and ascertained the cause of death in all cases. If breast cancer was present at the time of death, the death was presumed to be from breast cancer. We pre-specified a formal analysis for deaths from cardiovascular causes and deaths from other cancers.
Disease-free survival was a global parameter that assessed the health of the patient with regard to the disease in question. Disease-free survival was calculated as the time interval between randomisation and relapse or death and local-recurrence-free survival between randomisation and local recurrence or death.
Local toxicity and morbidity were recorded as adverse events related to the primary treatment of the breast cancer. The expected toxicities of acute skin reaction, wound infection, wound breakdown, late skin reaction (i.e. after 90 days) and pain from radiation were graded according to RTOG criteria, the LENT-SOMA (Late Effects in Normal Tissues – Subjective, Objective, Management and Analytic) scales or common toxicity criteria. 50 Any other toxicity was recorded and graded according to standard clinical criteria. The data were recorded on the complications form, which contained a pre-specified checklist: haematoma, seroma, wound infection, skin breakdown, delayed wound healing, RTOG (version 2.0) toxicity grade 3 or 4 for dermatitis, telangiectasia, pain in the irradiated field or other. We analysed seroma needing more than three aspirations, wound infections needing intravenous antibiotics, any complication needing surgical intervention or RTOG toxicity grade > 2. Skin breakdown or delayed wound healing or RTOG toxicity grade > 2 were classified as major toxicity.
To compare the extent of local surgery we analysed the specimen weight, margin status and reoperation for margins. No changes were made to trial outcomes after commencement of the trial.
Funding for analysis of cosmesis, patient satisfaction, quality of life, patient preference and cardiac toxicity is being sought and is not covered in this report.
We carried out exploratory analyses for regional recurrence (axilla plus supraclavicular), locoregional recurrence (local plus regional), distant recurrence, any other recurrence (regional, contralateral breast and distant recurrence) and all recurrence (local recurrence in the conserved breast and any other recurrence).
Statistical considerations
Proposed sample size
The main objective of the trial was to determine whether or not the use of IORT gave rates of local control that were not inferior to those obtained using EBRT.
This was a non-inferiority trial with a one-sided design and we selected a clinically meaningful non-inferiority margin, δ0 > 0. We were interested in testing H0: δ = δ0 compared with H1: δ < δ0 using a one-sided level-α test with power 1 − β to reject H0 when δ = 0.
We defined the power calculations for this trial using absolute values for local breast relapse. Estimates were obtained from published data. The Oxford Overview of radiotherapy in breast cancer showed a baseline rate of about 7.8% at about 10 years. 6 However, most of these trials began in or before 1985 and the outcomes have much improved since then such that many papers are reporting much lower rates with conventional radiotherapy. Hence, we had originally considered a baseline relapse rate of 6% at 5 years and therefore for a power of 80% to detect an absolute increase or decrease in relapse rate of 2.5% (the non-inferiority margin) we would need 2232 patients. If the absolute rate of recurrence is lower, for example 4%, as may be expected from recently announced trial results,49,51–53 slightly fewer (n = 2153) patients would allow an 80% chance of detecting a difference of 2.1% (the non-inferiority margin). Although the odds ratio detectable for an even lower (than 4%) background recurrence rate may not be as low, the absolute difference in recurrence rates would be very low (< 2%) and clinically acceptable. We therefore maintained the original accrual goal of a total of 2232 patients to have adequate power to meet the primary objective. It is possible that the final recurrence rate is low and the numbers accrued will give substantially more than adequate power.
The sample size was calculated for the main end point – local recurrence – for the whole patient population. At the time of the funding application to HTA in 2007–8, 70% of patients were in the single-procedure stratum and 25% in the two-procedure stratum, with < 5% in the contralateral stratum. The main (ITT) analysis was planned to be performed on the whole population and stratified analyses in the two main strata. We expected to yield a meaningful answer even in the smaller postpathology (two-procedure) stratum, in which we initially expected to have about 600 very-low-risk patients. The reasoning for this is as follows. The background recurrence rate in this stratum was expected to be very low, possibly as low as 1–2%. As we can clinically accept the original absolute non-inferiority margin of 2.5%, the nominal statistical hazard is high (e.g. 1.2% vs. 3.7%). Therefore, the statistical power (one-sided log-rank, 80% power, 95% confidence) of these 600 patients would be adequate for a meaningful result.
This rationale for power analysis also holds true if we have a significantly lower overall recurrence rate, in which case the study will have more than adequate power to confidently dismiss a clinically significant difference in local recurrence rate for establishing non-inferiority.
We alluded to this concept in our original application and were interested to see that it has been used in the recently published results of the START trial. 49
Therefore, once we had recruited > 800 patients with > 3 years of follow up, we planned to perform a futility analysis, which would be able to inform us whether or not the difference between the two groups will ever reach a clinically significant level.
Early stopping of the trial because of demonstration of superiority, or non-inferiority, or for significant safety reasons was within the remit of the DMC.
Our plan was to undertake one formal interim analysis for efficacy when half the expected number of events had been reached using an O’Brien–Fleming rule with a stringent p-value of < 0.001. Thus, stopping early for futility would follow an analysis which demonstrates that the 99.9% CIs fall outside the lower range defined for equivalence (i.e. the upper end of the 99.9% CI of 5-year local recurrence-free survival rate is < 91.5%).
If, however, any of the following were demonstrated at p < 0.01 the DMC would consider recommending early stopping:
-
a significant increase in grade 4 skin or rib fractures (as a sign of radionecrosis)
-
delays in wound healing, which after detailed review by the DMC as to time course and severity were considered clinically significant.
Early stopping could be applied to specific strata, with other strata allowed to continue recruitment. In addition, early stopping would be applied depending on the results of the futility analysis. However, by that time, most of the target recruitment would have already occurred.
When the original sample size of 2232 was calculated, we based our estimate of the 5-year local recurrence rate of 6% on the literature available in 1999. 6,54 We chose the non-inferiority margin as an absolute difference of 2.5% because this seemed clinically acceptable to physicians and patients. However, during the past decade recurrence rates have substantially reduced. The recurrence rate in the control group of our trial was 0.95% at 3 years. It would be logical to extrapolate the 5-year local recurrence rate to 1.5%, which is not unexpected. For example, in the UK START trial,55 patients had a worse prognosis (e.g. 36% had a tumour size of > 2 cm vs. 14% in TARGIT-A and 22% were node positive vs. 17% in TARGIT-A) and were treated a few years before the patients in our trial. In the START trial the recurrence rate at 5 years was 2.3%. Therefore, the estimate of 1.5% for our trial is not unrealistic.
Statistical analysis
The major end point was the incidence of local recurrence in the conserved breast. This was compared on an ITT basis (i.e. all randomised patients were analysed) and the log-rank test was used. This test allows for the hazards to be non-linear. This was performed once the baseline data had been compared to test the randomisation and to define whether or not any stratified analyses were required. In addition, ratios of radiological lesion size to clinical and pathological size, and ratios of specimen weights in the two arms of the trial, were compared to ensure that the extent of the surgical procedure was similar in both groups. The baseline comparisons between the two groups were carried out using the chi-square test for categorical variables and an independent two-sample t-test for all continuous variables. Statistical significance was defined at the p < 0.05 level in the first analysis and at the p < 0.01 level in the second analysis. We planned to use Kaplan–Meier curves and proportional hazard regression models to account for time to event and censoring of the data. Standard tests for non-inferiority were performed with the margin of non-inferiority set at 2.5% absolute difference in local recurrence at 5 years. We analysed the non-inferiority statistic by calculating the difference in binomial proportions of local recurrences in the conserved breast between the two randomised groups (TARGIT vs. EBRT).
All reports of local and regional recurrence and death were checked (before the data were unblinded, thus masking the randomised allocation), to ensure that they were correct.
To assess stability over time, we also calculated this statistic for the mature cohort (n = 2232), reported in 2010, and for the earliest cohort (excluding the last 4 years of enrolment; n = 1222), who had a median follow up of 5 years. We calculated the z-score and Pnon-inferiority using established methods53–55 for the whole cohort and the two pre-specified strata – prepathology and postpathology.
Early complications were reported in 20101 and complications arising > 6 months after randomisation were reported in 2014. 2
To address the issue of follow-up, we charted the absolute differences in the 5-year Kaplan–Meier estimates of local recurrence in the conserved breast and overall mortality for patients with prepathology randomisation in the whole trial along with those for the mature cohort reported in 2010, who had a longer follow-up (median 3 years 8 months, maximum 12 years) and the earliest cohort.
A patient was deemed to have adequate follow-up if they had at least 5 years of follow-up or if they were seen within the year before database lock. Patients were censored when they were last seen or withdrawn from the trial. The database (customised Microsoft Access® 1999 onwards; Microsoft Corporation, Redmond, WA, USA) as validated on 29 June 2012 was used for this analysis, with 1 June 2012 as a reference date. SAS (version 9.3; SAS Institute Inc., Cary, NC, USA), Microsoft Excel® 2011, Stata (version 12.0; StataCorp LP, College Station, TX, USA) and IBM SPSS Statistics (version 20.0; IBM Corporation, Armonk, NY, USA) were used for data compilation, validation and analysis. Kaplan–Meier graphs were displayed as recommended by Pocock et al. 56 and a log-rank test was used to compare the difference between the survival function and to obtain p-values (significance level set at p < 0.01 for local recurrence and p < 0.05 for survival).
The 2010 analysis was prompted by the DMC at the March 2010 ISC meeting. The DMC felt that the data needed to be made public. The results of the first analyses were therefore submitted to an American Society of Clinical Oncology (ASCO) meeting and to The Lancet. The Lancet editors chose to put the manuscript through their fast-track rigorous review process and published it online1 on the same day as the ASCO presentation on 5 June 2010.
In 2010 it was also recommended by the DMC that the next analysis should be performed after 2 more years, in 2012. The final 2012 analysis was presented to the ISC and DMC in September 2012 and submitted with their recommendation to the San Antonio Breast Cancer Symposium (SABCS) as a late-breaking abstract, where it was accepted and presented on 6 December 2012. The manuscript was submitted to The Lancet in 2013 and published online in November 2013 and in print in February 2014. 2
Planned subgroup analysis
Although most patients recruited in the trial were good-prognosis patients, there was a substantial number of ‘high-risk’ cases, for example approximately 500 cases were node positive, or grade 3, because of the broad inclusion criteria. When the TARGIT approach is applied to normal clinical practice, patient selection will be crucial in ensuring that the results of day-to-day practice reflect the results obtained in the clinical trial.
Evidence from randomised trials and laboratory research suggested that progesterone receptor (PgR) status may be a predictive factor for local recurrence. The 2011 Oxford overview3 had found for the first time that the hormone receptor status of the tumour predicted benefit from radiotherapy. The proportional reduction in recurrence as a result of radiotherapy was higher in patients with ER-positive tumours (nearly two-thirds reduction from 22% to 8.7%) than in patients with ER-negative tumours (about one-third reduction from 43.8% to 28.9%). Furthermore, molecular analysis of tumours in the ELIOT study presented at the European Breast Cancer Conference EBCC-8 in March 2011 and subsequently published35 suggested that IORT was less effective in patients with non-luminal A (hormone receptor-negative) tumours. Evidence from Prat et al. 57 suggested that tumours that are PgR negative [even though ER positive and human epidermal growth factor receptor-2 (HER2) negative] should not be classified as luminal A as they may represent a more aggressive tumour type [IMProving care And Knowledge through Translational research (IMPAKT) meeting in Brussels, May 2012, and published later57]. Furthermore, three additional studies from Australia and the USA58–60 have suggested that PgR status is an independent predictor of local recurrence following radiotherapy, perhaps because PgR status is associated with more infiltrating and irregular margins and would warrant a wider radiation field. Hence, we considered testing whether or not PgR status was a predictive factor for local recurrence. Another reason for this was that most patients in the trial were ER positive (< 10% were ER negative) and there were very few events overall; therefore, an effect of ER status on local recurrence would not be discernible. On the other hand, PgR positivity is an expression of a functional ER receptor and, as not all ER-positive cases are PgR positive, many more cases would be PgR negative and this would make the analysis by PgR status more meaningful.
In view of these findings, we hypothesised that hormone sensitivity might be predictive of response to TARGIT and therefore, before the data were unblinded for this analysis, we planned to analyse whether or not the response to radiotherapy in the TARGIT-A trial was dependent on hormone receptor responsiveness, using PgR status as a marker.
We also tested whether or not patient age and certain other tumour factors, as defined below, were predictive of response or prognostic of outcome. This analysis of patient and tumour factors may help select patients in whom partial breast irradiation (PBI) approaches such as TaRGIT achieve the best results. These analyses may help refine current guidelines that were mainly based on a presumed risk of recurrence rather than effectiveness of radiation, for which only randomised evidence can be relied on.
A substantial number (18%) in each stratum were PgR negative (554 among all patients and 535 among those who had breast-conserving therapy). PgR status was unknown in only 88 patients among the 3104 patients in whom ER status was known.
As described above, we hypothesised that PgR negativity, as a surrogate marker of functional ER status, might be a predictor of poor response to radiotherapy. Analyses were performed by plotting Kaplan–Meier curves and tested for significance using the log-rank test and using a p-value of 0.05 as the boundary for statistical significance.
-
We first analysed whether or not PgR status affected the primary and secondary end points of local recurrence and survival (deaths from breast cancer and deaths from other causes) in the whole trial population.
-
As per the updated main results, TARGIT should ideally be used concurrently with the primary excision of the tumour, that is, as used in the prepathology stratum. Therefore, within the prepathology stratum we analysed the difference in local control and survival (deaths from breast cancer and deaths from other causes) between the TARGIT arm and the EBRT arm in PgR-negative and PgR-positive cases.
-
We also subsequently analysed in all patients whether or not certain patient or tumour characteristics (randomisation arm, timing of randomisation/delivery of TARGIT, age, tumour grade, presence of DCIS, margin positivity, ER, PgR and HER2 status, whether screen detected or not, presence of lymphovascular invasion, node positivity) influenced the effect of radiotherapy using the Cox proportional hazards model.
Ethical arrangements
This trial was set up in 1999–2000 and recruitment started in March 2000 at UCL. UCL has been the sponsor of this trial and the original ethical approval was obtained from the University College London Hospitals (UCLH) Ethics Committee (reference number 99/0307). The other UK centres required only local ethics committee approval as we had already obtained national Multicentre Research Ethics Committee (MREC) approval. These centres started recruitment in 2005 (Ninewells, Hospital, Dundee), 2008 (Royal Free Hospital, London, and Royal Hampshire County Hospital, Winchester), 2009 (Whittington Hospital and Guy’s Hospital, London) and 2012 (Hospital of St John and St Elizabeth, London). Other centres were sponsored by local institutions and governed by local policies and institutional review boards/ethics committees. The non-UK sites were responsible for obtaining approvals locally, with a copy of the final approval letter sent to the Trials Operations Office.
The ethical issues were as follows. In terms of risks and benefits there would be no additional risks for EBRT patients as they received standard care. For patients randomised to TARGIT there was a small chance of an increased risk of local recurrence and of a poorer cosmetic outcome because of the high local dose of treatment. On the other hand, the cosmetic outcome might be better because the whole breast was not subjected to radiotherapy and local recurrence might be lower because radiotherapy was targeted to the correct area without a ‘geographical miss’ and was given immediately after surgery without any delay, that is, before adjuvant chemotherapy, whereas EBRT was normally given after the chemotherapy course was completed. Furthermore, localisation of radiotherapy to the high-risk areas spared radiation damage to the nearby heart and lung, which has contributed to significant morbidly and mortality in previous clinical trials of EBRT. Should recurrence occur in a patient who had received TARGIT, there was the possibility of further surgery and EBRT without a mastectomy, which is otherwise a common treatment for recurrence.
All trial participants were informed of these potential risks and benefits in the patient information sheet, which received MREC approval. Every participant was given sufficient time – at least 24 hours, but usually several days – to consider the trial before signing the consent form. The trial data were held on secure, restricted-access computers and were to be retained for at least a 10-year follow-up period.
In our experience, the main ethical issue was the obvious convenience and logical correctness of the experimental treatment, leading to a demand from some patients that they be given the experimental treatment without randomisation. We dealt with this issue by explaining that the experimental treatment was still unproven to be equivalent to the standard treatment and that the new way of giving radiotherapy was therefore available only in the context of the randomised trial. Once this was explained clearly, patients were happy to take part in the trial.
Research governance
As this was an international multicentre trial it was agreed that each centre would take responsibility for the collection and management of its own data. Randomisation was performed centrally through the TARGIT trial operations office, which was also responsible for the administration of the trial database. Data could be added by site staff over the internet via a secure website. Access to the entire data set was restricted to designated trial staff and the trial statistician. Regular electronic reports were produced and passed to each centre for audit and checking purposes. This not only allowed each centre to have full responsibility for its own data, but also ensured that there was adequate back-up and central auditing of the data. Standard operating procedures were in place for any data management or clinical queries.
The project was managed by a four-tiered approach: quarterly meetings of the ISC; biannual meetings of the DMC; fortnightly meetings of the Trial Operations Group; and weekly meetings of operational staff in the Trial Operations Group. The trial operations office was based at UCL and the finances were managed by the university finance office, which ensured propriety.
Quality control
Each new centre underwent structured training on using the IORT equipment, which was organised by the Clinical Trials Group and involved the co-applicants on the grant applications. After training, the new sites had to perform at least five pilot cases, which were reviewed by a member of the ISC, and only after a satisfactory appraisal could patients be randomised into the trial.
Quality control of the radiation delivered with TARGIT was monitored in each individual case by the physicist who calibrated and controlled the equipment in the operating room.
The trial was initiated on a purely academic basis. The device used for TARGIT was manufactured by Carl Zeiss, who provided limited funding mainly by means of reimbursement of some of the costs of the DMC and ISC meetings. Carl Zeiss had some representation on the ISC but had no executive powers and played no part in the design and conduct of the trial or the analysis or publication of the data.
One member of the DMC was a consumer representative, a well-known patient’s advocate who had been through the experience of breast-conserving surgery and postoperative radiotherapy herself.
Patient and public engagement
Breast cancer survivors sat on the ISC and DMC. They were involved in all discussions and decisions about the trial design and management, as well as writing of the manuscripts and dissemination of data.
There has been widespread media coverage about the trial, which has been reported on national and international television and radio and in daily newspapers and specialist newspapers, including the BBC, ITV, several channels in the USA and China, online channels, The Times, The Daily Mail, The Telegraph, The Independent, The New York Times, The Los Angeles Times, The Times of India, The Wall Street Journal, TIME magazine and Reader’s Digest.
The data from the TARGIT-A trial have been presented at a myriad of conferences – national and international – as well as to physician and patient groups (see Appendix 3).
The TARGIT websites [see www.targit.org.uk, http://facebook.com/targittrials (accessed 4 July 2016)] and the Chief Investigator’s Twitter (www.twitter.com; Twitter, Inc., San Francisco, CA, USA) account (@jsvaidya) generate relatively significant traffic and are another means of public engagement.

The TARGIT treatment is already standard of care in over 250 centres in several countries in Europe (e.g. Germany) and in several centres in the USA and has been recommended by the Medical Services Advisory Committee [the National Institute for Health and Care Excellence (NICE) equivalent in Australia] for NHS funding in Australia and was included in the Australian government’s health budget from 2015 onwards. It was given provisional approval by NICE in the UK for NHS funding in July 2014 and, when asked by the BMJ why there was a delay in final approval, NICE61 stated that:
While this NICE appraisal is ongoing, intrabeam radiotherapy can continue to be offered to NHS patients who need it. Until NICE publishes its final guidance, decisions on whether or not to fund specific treatments are the responsibility of local NHS bodies.
Project timetable and milestones
At the time of the grant submission in 2009 we already had a high and increasing recruitment rate. We had annually recruited 220, 315, 359 and 408* patients in the years 2005–8, respectively (*based on 204 in the first 6 months of 2008) and the total stood at 1301. At this rate, we expected to reach the protocol-specified accrual goal of 2232 patients by late 2010. We reached this goal in April 2010. However, recruitment was continued until June 2012 with 3451 total patients being recruited, as described earlier (see Appendix 2).
Chapter 3 Main results
Patient population
The first patient was randomised on 24 March 2000 and the trial recruited 3451 patients from 33 centres in 11 countries to 25 June 2012. Of these, 1721 patients were randomly allocated to TARGIT and 1730 to EBRT. Two-thirds of patients (n = 2298) were randomised before lumpectomy (prepathology) and a third (n = 1153) were randomised after lumpectomy (postpathology). As per protocol, of those who received TARGIT, 15.2% (239/1571) received both TARGIT and EBRT [21.6% (219/1012) in the prepathology stratum and 3.6% (20/559) in the postpathology stratum].
Participant flow is shown in the Consolidated Standards of Reporting Trials (CONSORT) diagrams in Figures 4 and 5. The risk-adapted design is shown in the trial profile, for example of the 1140 patients allocated TARGIT in the prepathology stratum, 219 received TARGIT and EBRT as per protocol because they were shown to have characteristics of high-risk disease postoperatively. There was no significant difference between prepathology and postpathology in the timing of delivery of EBRT (p = 0.58).
FIGURE 4.
Consolidated Standards of Reporting Trials (CONSORT) diagram. a, Protocol deviations: 78/1721 (4.5%) patients allocated to TARGIT received EBRT and 12/1730 (0.7%) patients allocated to EBRT received TARGIT; b, 239/1721 (13.8%) patients allocated to TARGIT received EBRT after TARGIT as per protocol and 239/1571 (15.2%) patients who received TARGIT received EBRT after TARGIT (as per treatment received). Reproduced with permission from Vaidya et al. 2 Copyright © Vaidya et al. Open Access article distributed under the terms of CC BY-NC-ND.
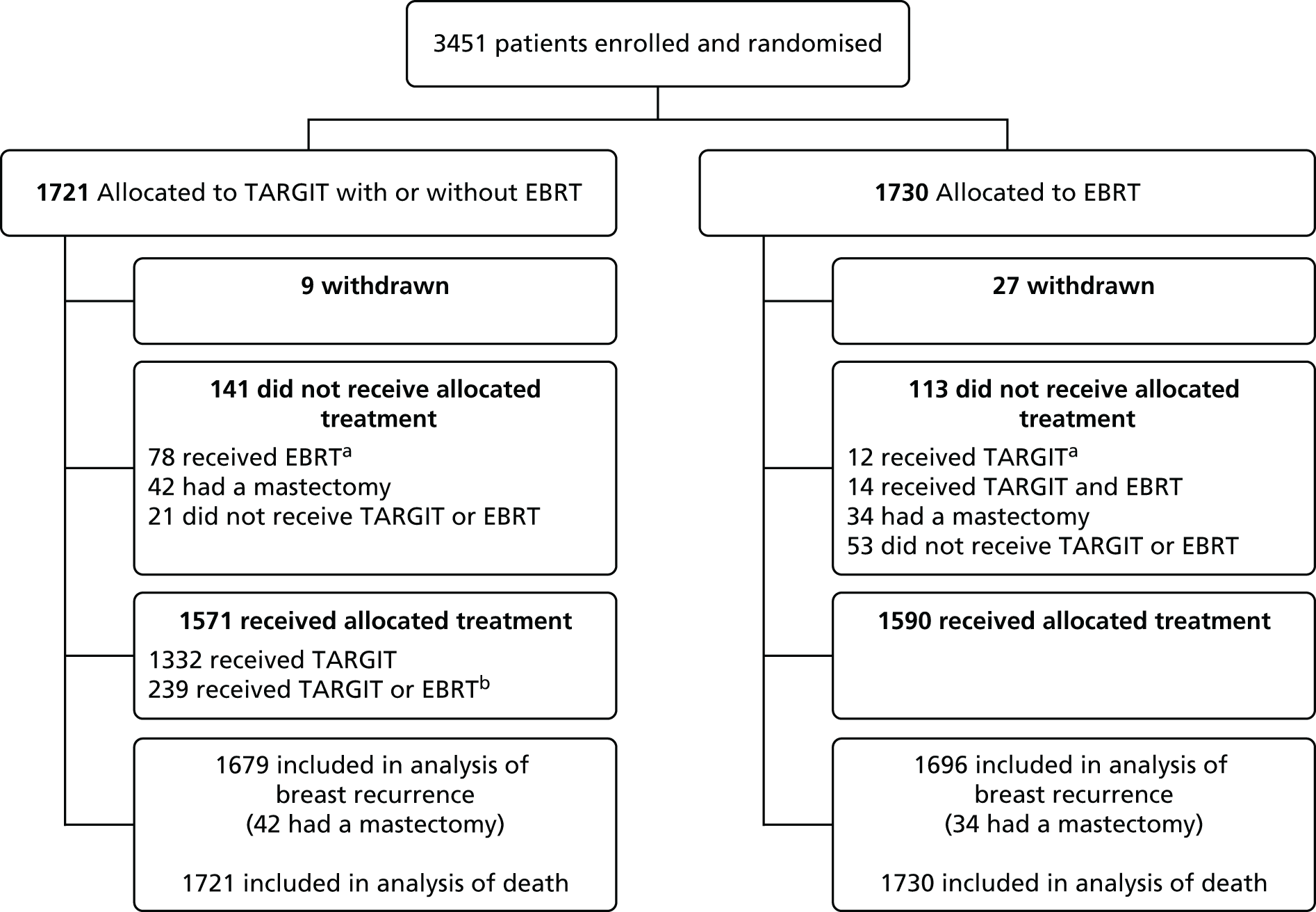
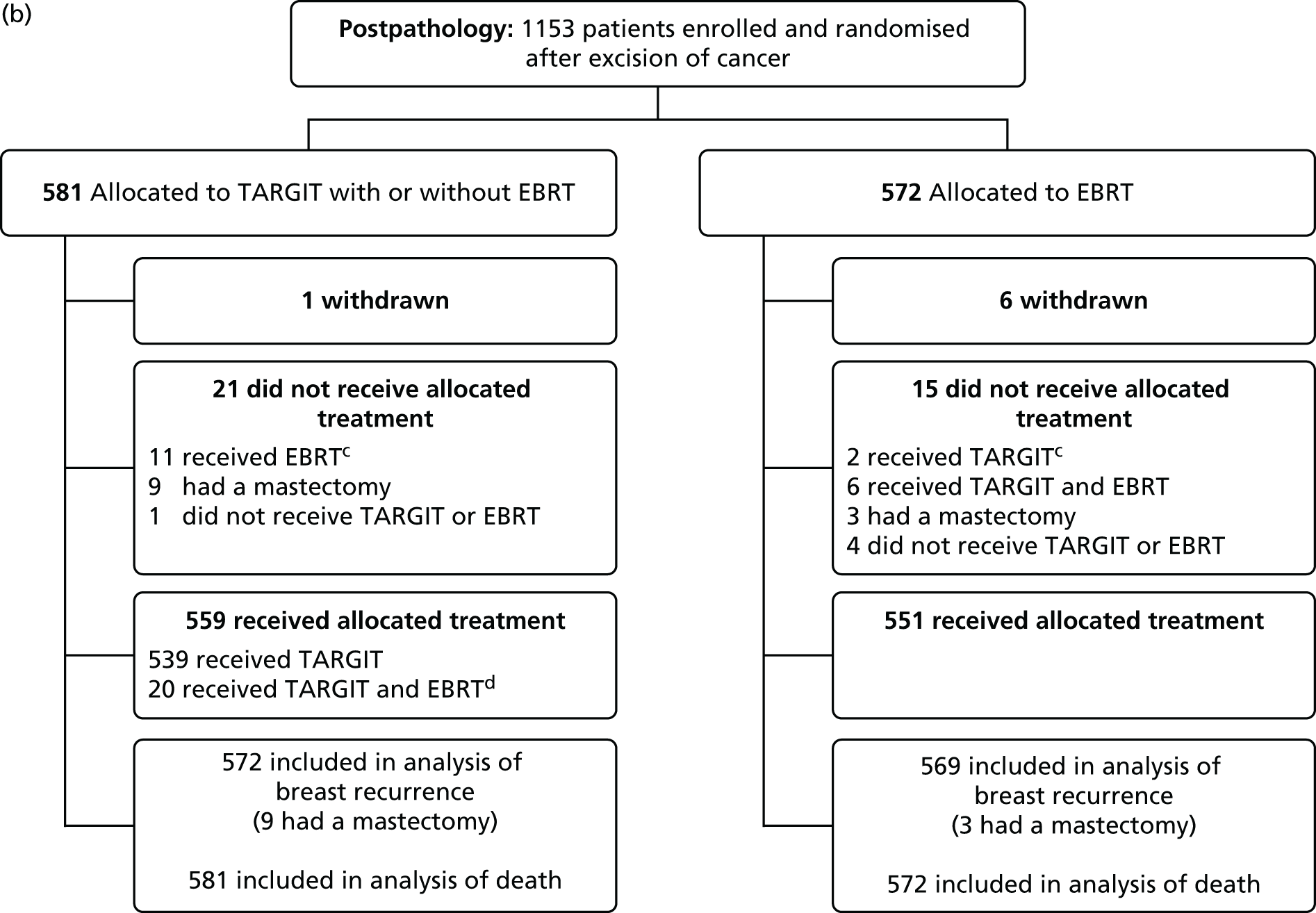
FIGURE 5.
Consolidated Standards of Reporting Trials (CONSORT) diagram for the (a) prepathology; and (b) postpathology strata. a, Prepathology: protocol deviations: 67/1140 (5.9%) patients allocated to TARGIT received EBRT and 10/1158 (0.9%) patients allocated to EBRT received TARGIT; b, prepathology: 219/1140 (19.2%) patients allocated to TARGIT received EBRT after TARGIT as per protocol; c, postpathology: protocol deviations: 11/581 (1.9%) patients allocated to TARGIT received EBRT and 2/572 (0.3%) patients allocated to EBRT received TARGIT; d, postpathology: 20/581 (3.4%) patients allocated to TARGIT received EBRT after TARGIT as per protocol. Reproduced with permission from Vaidya et al. 2 Copyright © Vaidya et al. Open Access article distributed under the terms of CC BY-NC-ND.

Patient and tumour characteristics are provided in Table 1. Most cancers were small and of good prognosis [87% (2685/3082) up to 2 cm, 85% (2573/3032) grade 1 or 2, 84% (2610/3112) node negative, 93% (2874/3093) ER positive, 82% (2462/3016) PgR positive and 69% detected by screening (2102/3063)]. The numbers of patients from each centre and each country are provided in Tables 2 and 3, respectively.
| Characteristic | TARGIT (n = 1721) | EBRT (n = 1730) | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | |||||
| ≤ 50 | 150 | 9 | 122 | 7 | p = 0.274 |
| 51–60 | 527 | 31 | 548 | 32 | |
| 61–70 | 781 | 45 | 807 | 47 | |
| > 70 | 263 | 15 | 253 | 15 | |
| Pathological tumour size (cm) | |||||
| ≤ 1 | 611 | 39 | 597 | 39 | p = 0.273 |
| 1.1–2 | 751 | 48 | 726 | 48 | |
| > 2 | 190 | 12 | 207 | 14 | |
| Unknown | 169 | 10 | 200 | 12 | |
| Grade | |||||
| 1 | 538 | 35 | 558 | 37 | p = 0.394 |
| 2 | 757 | 50 | 720 | 48 | |
| 3 | 232 | 15 | 227 | 15 | |
| Unknown | 194 | 11 | 225 | 13 | |
| Lymphovascular invasion | |||||
| Absent | 1348 | 87 | 1343 | 88 | p = 0.224 |
| Present | 194 | 13 | 178 | 12 | |
| Unknown | 179 | 10 | 209 | 12 | |
| Nodes involved | |||||
| 0 | 1307 | 83 | 1303 | 85 | p = 0.091 |
| 1–3 | 219 | 14 | 211 | 14 | |
| > 3 | 43 | 3 | 29 | 2 | |
| Unknown | 152 | 9 | 187 | 11 | |
| ER status | |||||
| ER +ve | 1441 | 92 | 1433 | 94 | p = 0.090 |
| ER –ve | 120 | 8 | 99 | 7 | |
| Unknown | 160 | 9 | 198 | 12 | |
| PgR status | |||||
| PgR +ve | 1232 | 81 | 1230 | 82 | p = 0.179 |
| PgR –ve | 289 | 19 | 265 | 18 | |
| Unknown | 200 | 12 | 235 | 14 | |
| HER 2 receptor | |||||
| Positive | 170 | 11 | 178 | 12 | p = 0.585 |
| Negative | 1329 | 89 | 1309 | 88 | |
| Unknown | 222 | 13 | 243 | 14 | |
FIGURE 6.
Bar chart summarising the data in Table 1. It shows that although most patients had a good prognosis, there were a substantial number of patients who had a high-risk disease: nearly 3000 patients were < 70 years and between 400 and 550 patients had tumours > 2 cm, or grade 3 or node positive.

| Centre name (in order of first patient recruited) | Number of patients |
|---|---|
| UCL, London, UK | 189 |
| Sir Charles Gairdner Hospital, Perth, WA, Australia | 385 |
| Centro di Riferimento Oncologico, Aviano, Italy | 309 |
| Ninewells Hospital, Dundee, UK | 270 |
| University of California, San Francisco, CA, USA | 110 |
| Universitätsmedizin Mannheim, Universität Heidelberg, Mannheim, Germany | 186 |
| Frauenklinik vom Roten Kreuz, Munich, Germany | 257 |
| Sankt Gertrauden-Krankenhaus, Berlin, Germany | 54 |
| Universität Frankfurt am Main, Frankfurt, Germany | 44 |
| University of Southern California, Los Angeles, CA, USA | 75 |
| Ospedale San Giuseppe di Empoli, Empoli, Italy | 57 |
| Medical University of Lublin, Lublin, Poland | 42 |
| University of Nebraska Medical Center, Omaha, NE, USA | 18 |
| Peter MacCallum Cancer Centre, Melbourne, VIC, Australia | 9 |
| Ludwig Maximilians Universität, Munich, Germany | 100 |
| Herlev/Rigs Hospitals, Copenhagen, Denmark | 514 |
| Royal Free/Whittington Hospitals, London, UK | 115 |
| Lafayette Surgical Clinic, Lafayette, IN, USA | 12 |
| Princess Margaret Hospital, Toronto, ON, Canada | 24 |
| Sentara Surgery Specialists, Hampton, VA, USA | 11 |
| Universitätsklinikum des Saarlandes, Homburg, Germany | 65 |
| Brust-Zentrum Seefeld, Zurich, Switzerland | 59 |
| Royal Hampshire County Hospital, Winchester, UK | 115 |
| St Olav’s University Hospital, Trondheim, Norway | 111 |
| Universitäts Spital Zurich, Zurich, Switzerland | 39 |
| Guy’s Hospital, London, UK | 22 |
| Vassar Brothers Medical Center, Poughkeepsie, New York, NY, USA | 36 |
| St John’s Riverside Health Hospital, Dobbs Ferry, New York, NY, USA | 4 |
| Medizinische Hochschule Hannover, Germany | 28 |
| Centre René Gauducheau, Nantes, France | 72 |
| Instituto Oncologico Veneto, Padova, Italy | 110 |
| Hospital of St John and St Elizabeth, London, UK | 3 |
| Institut Bergonié, Bordeaux, France | 6 |
| Country | Number of cases |
|---|---|
| UK | 714 |
| Australia | 394 |
| Italy | 476 |
| Germany | 734 |
| USA | 266 |
| Poland | 42 |
| Denmark | 514 |
| Canada | 24 |
| Switzerland | 98 |
| Norway | 111 |
| France | 78 |
| Total | 3451 |
Follow-up
In total, 93.7% (3234/3451) of patients were seen within the year before data lock or had at least 5 years of follow-up (Figure 7). The whole cohort of 3451 patients had a median follow-up of 2 years and 5 months [interquartile range (IQR) 12–52 months], 2020 patients had a median follow-up of 4 years and 1222 patients had a median follow-up of 5 years. The mature cohort of 2232 patients, which was originally reported in 2010, had a median follow-up of 3 years and 7 months (IQR 30–61 months). Figure 8 shows that a large number of patients, even in the prepathology stratum, had a long median follow-up time.
FIGURE 7.
Completeness of follow-up. Reproduced with permission from Vaidya et al. 2 Copyright © Vaidya et al. Open Access article distributed under the terms of CC BY-NC-ND.

FIGURE 8.
Patients and their length of follow-up. Each vertical green line represents one patient and the location of the green line on the x-axis represents the date of randomisation. The height of the green line represents the follow-up duration.

Extent of surgery
The analysis of amount of tissue excised was presented in the 2010 paper in The Lancet1 and included the first 2232 patients. Table 4 shows that the median amount of tissue excised during breast-conserving surgery was similar in the two groups, as was the proportion of first excisions with free margins. The difference in re-excision rate was not significant between the two main strata [prepathology 119 (7.8%) vs. postpathology 63 (9.0%); p = 0.31] or between the two randomised groups (p = 0.07).
| TARGIT | EBRT | |
|---|---|---|
| Specimen weight (g)a | 45.5 (28–72) | 47 (29–76) |
| Margins at first excision | ||
| Free | 970/1072 (90.49) | 968/1073 (90.21) |
| DCIS only | 46/1072 (4.29) | 43/1073 (4.01) |
| Invasive | 56/1072 (5.22) | 62/1073 (5.78) |
| Unknown | 41/1113 (3.68) | 46/1119 (4.11) |
| Re-excision for margins | ||
| Prepathology stratum | 52/766 (6.79) | 65/768 (8.72) |
| Postpathology stratum | 27/347 (7.78) | 36/351 (10.26) |
| Total | 79/1113 (7.1) | 106/1119 (9.2) |
Complications and local toxicity
All complications were reported in the 2010 paper in The Lancet1 and complications after 6 months were reported in the 2014 paper in The Lancet. 2 The number of patients with any complication (Table 5) was similar between the groups. Table 6 shows the five clinically significant complications reported, three of which had a similar rate in the two groups. Wound seroma needing more than three aspirations were more frequent in the TARGIT group than in the EBRT group (2.1% vs. 0.8%) whereas a RTOG score of 3 or 4 was more frequent in the EBRT group than in the TARGIT group (2.1% vs. 0.5%). The total rate of major toxicities was similar in the two groups.
| Number of complications per patient | TARGIT | EBRT | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| 0 | 917 | 82.39 | 946 | 84.54 | |
| 1 | 151 | 13.57 | 139 | 12.42 | |
| 2 | 29 | 2.61 | 27 | 2.41 | |
| 3 | 11 | 0.99 | 5 | 0.45 | |
| 4 | 3 | 0.27 | 0 | 0.00 | |
| 5 | 2 | 0.18 | 0 | 0.00 | |
| 6 | 0 | 0.00 | 3 | 0.27 | |
| All complications | 196 | 17.61 | 174 | 15.46 | chisq 1.74, p = 0.19 |
| Total cases | 1113 | 1119 | |||
| Complication | TARGIT | EBRT | Statistical significance | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| a | Hematoma requiring surgical evacuation | 11 | 0.99 | 7 | 0.63 | chisq 0.92, p = 0.34 |
| b | Seroma requiring more than three aspirations | 23 | 2.07 | 9 | 0.80 | chisq 6.29, p = 0.012 |
| c | Infection requiring intravenous antibiotics or surgical intervention | 20 | 1.89 | 14 | 1.25 | chisq 1.11, p = 0.29 |
| d | Skin breakdown or delayed wound healinga | 31 | 2.79 | 21 | 1.88 | chisq 2.02, p = 0.16 |
| e | RTOG toxicity grade 3 or 4 (nil grade 4) | 6 | 0.54 | 23 | 2.06 | chisq 10.0, p = 0.002 |
| f | Major toxicity (d + e) | 37 | 3.32 | 44 | 3.93 | chisq 0.59, p = 0.44 |
In the 2014 analysis of complications 6 months after randomisation,2 we noted no significant difference in any protocol-defined wound-related complication (Table 7). There were fewer grade 3 or 4 radiotherapy-related skin complications with TARGIT than with EBRT (4/1721 vs. 13/1730, p = 0.029).
| Complication | Total number of patients | TARGIT vs. EBRT |
|---|---|---|
| Haematoma/seroma requiring more than three aspirations or surgery | 3445 | 4 vs. 2 |
| Wound infection requiring intravenous antibiotics or surgery | 3431 | 12 vs. 9 |
| Skin breakdown/delayed wound healing | 3443 | 3 vs. 5 |
| Total | 19 vs. 16 (p = 0.599) |
Local recurrence and mortality
Table 8 shows the results of the test of non-inferiority in terms of local recurrence in the conserved breast. TARGIT was non-inferior to EBRT for the whole trial (Pnon-inferiority = 0.00000012) and for the prepathology stratum (Pnon-inferiority = 0.0000000013), but not for the postpathology stratum (Pnon-inferiority = 0.06640).
| Cohort | Number of patients: total number (TARGIT vs. EBRT patients with conserved breast)b | Median follow-up | Number of events (TARGIT vs. EBRT) | Difference in the binomial proportionsc of local recurrenceb in the conserved breast, (TARGIT minus EBRT), absolute difference (90% CI) (%) | z-score | Pnon-inferiority for the non-inferiority margin of 2.5% |
|---|---|---|---|---|---|---|
| Whole trial | All patients n = 3451 (1679 vs. 1696) | 2 years 5 months | 23 vs. 11 | 0.72 (0.2 to 1.3) | –5.168 | 0.00000012 |
| Mature cohort n = 2232 (1078 vs. 1093) | 3 years 7 months | 22 vs. 10 | 1.13 (0.3 to 2.0) | –2.652 | 0.004 | |
| Earliest cohort n = 1222 (593 vs. 592) | 5 years | 15 vs. 8 | 1.14 (–0.1 to 2.4) | –1.75 | 0.040 | |
| Prepathology (TARGIT given concurrent with lumpectomy) | All patients n = 2298 (1107 vs. 1127) | 2 years 4 months | 10 vs. 6 | 0.37 (–0.2 to 1.0) | –5.954 | 0.0000000013 |
| Mature cohort n = 1450 (689 vs. 710) | 3 years 8 months | 9 vs. 5 | 0.6 (–0.3 to 1.5) | –3.552 | 0.00019 | |
| Earliest cohort n = 817 (n = 398 vs. 405) | 5 years | 6 vs. 3 | 0.76 (–0.4 to 2.0) | –2.36 | 0.00914 | |
| Postpathology (TARGIT given as a delayed procedure by reopening the wound) | All patients n = 1153 (581 vs. 572) | 2 years 4 months | 13 vs. 5 | 1.39 (0.2 to 2.6) | –1.503 | 0.06640 |
| Mature cohort n = 782 (389 vs. 383) | 3 years 7 months | 13 vs. 5 | 2.04 (0.3 to 3.8) | –0.429 | 0.33390 | |
| Earliest cohort n = 405 (195 vs. 187) | 5 years | 9 vs. 5 | 1.8 (–1.2 to 4.8) | –0.382 | 0.35108 |
For the first 1222 patients randomised in the trial the median follow-up was 5 years and the test for non-inferiority found that TARGIT was non-inferior to EBRT in terms of local recurrence in the conserved breast for both strata together (Pnon-inferiority = 0.040) and for the prepathology stratum (Pnon-inferiority = 0.00914), but not for the postpathology stratum (Pnon-inferiority = 0.35108).
For all 3451 patients, the 5-year estimated risk for local recurrence in the conserved breast (with deaths censored) was 3.3% (95% CI 2.1% to 5.1%) for TARGIT and 1.3% (95% CI 0.7% to 2.5%) for EBRT; the difference was not statistically significant (log-rank p-value 0.04, which was above the pre-specified limit of 0.01) (Table 9 and Figure 9). Half of the patients with local recurrence (17/34) were treated with a mastectomy and there was no significant difference between TARGIT and EBRT in the need for mastectomy for recurrence (p = 0.271).
FIGURE 9.
Kaplan–Meier plots for patients who had breast-conserving therapy (a) for local recurrence in the conserved breast; (b) for regional recurrence (axillary and supraclavicular); and (c) for deaths in all patients. Regional recurrence was an exploratory outcome. Three of the 14 regional recurrences had breast recurrence as well (n = 1 TARGIT and n = 2 EBRT). Reproduced with permission from Vaidya et al. 2 Copyright © Vaidya et al. Open Access article distributed under the terms of CC BY-NC-ND.
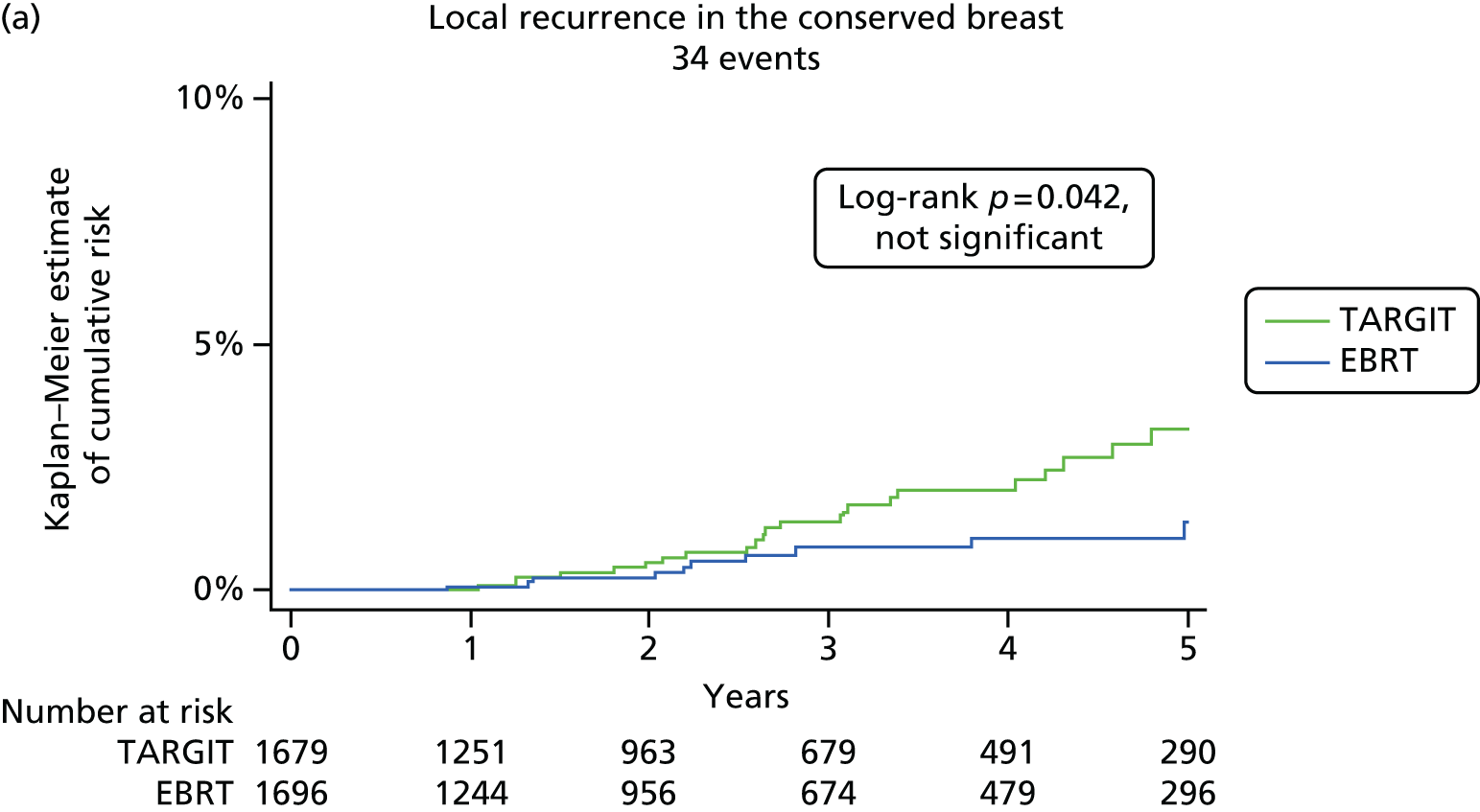
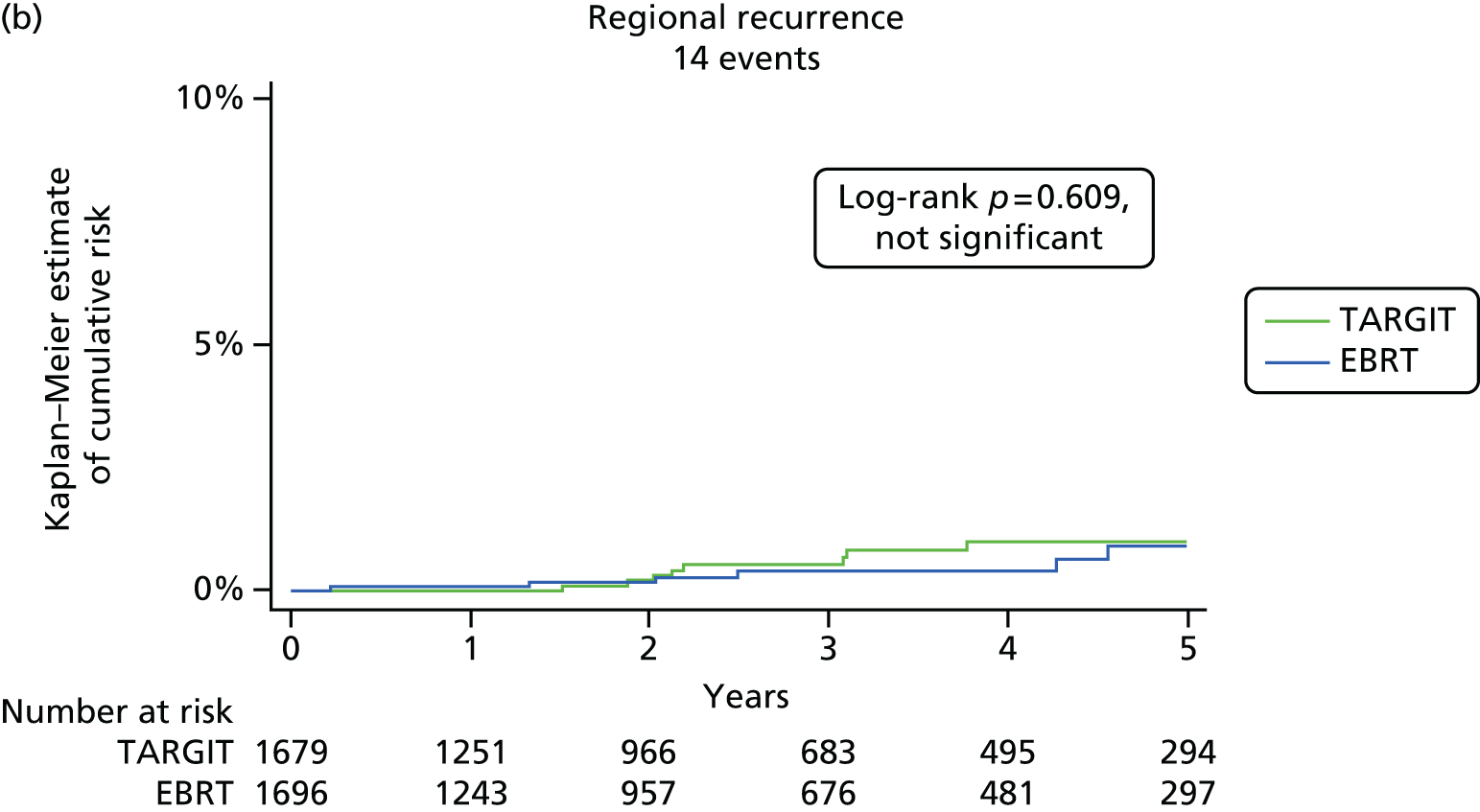

Breast cancer mortality was much the same in the two groups [5-year rate 2.6% (95% CI 1.5% to 4.3%) for TARGIT vs. 1.9% (95% CI 1.1% to 3.2%) for EBRT; p = 0.56] but there were significantly fewer non-breast-cancer deaths in the TARGIT group than in the EBRT group [1.4% (95% CI 0.8% to 2.5%) vs. 3.5% (95% CI 2.3% to 5.2%); p = 0.0086], attributable to fewer deaths from cardiovascular causes and other cancers (Table 10 and Figure 10). Overall, mortality at 5 years for TARGIT was numerically 1.4% lower than for EBRT [TARGIT 3.9% (95% CI 2.7% to 5.8%) vs. EBRT 5.3% (95% CI 3.9% to 7.3%); p = 0.099]. Overall, in absolute terms, there were 12 additional local recurrences but 14 fewer deaths in the TARGIT group (see Table 9).
| Cohort | n | Outcome | Events; 5-year cumulative risk (95% CI) (%) | Absolute difference (TARGIT minus EBRT) | |
|---|---|---|---|---|---|
| TARGIT | EBRT | Difference in K–M point estimate at 5 years (%) | |||
| All patients | 3375 | Local recurrence in the conserved breast | 23 | 11 | 12 |
| 3.3 (2.1 to 5.1) | 1.3 (0.7 to 2.5) | +2.0 | |||
| 3451 | Death | 37 | 51 | –14 | |
| 3.9 (2.7 to 5.8) | 5.3 (3.9 to 7.3) | –1.4 | |||
| Prepathology (TARGIT given concurrently with lumpectomy) | 2234 | Local recurrence in the conserved breast | 10 | 6 | 4 |
| 2.1 (1.1 to 4.2) | 1.1 (0.5 to 2.5) | +1.0 | |||
| 2298 | Death | 29 | 42 | –13 | |
| 4.6 (1.8 to 6.0) | 6.9 (4.3 to 9.6) | –2.3 | |||
| Postpathology (TARGIT given as a delayed second procedure) | 1141 | Local recurrence in the conserved breast | 13 | 5 | 8 |
| 5.4 (3.0 to 9.7) | 1.7 (0.6 to 4.9) | +3.7 | |||
| 1153 | Death | 8 | 9 | –1 | |
| 2.8 (1.3 to 5.9) | 2.3 (1 to 5.2) | +0.5 | |||
| Causes of death other than breast cancer | TARGIT | EBRT |
|---|---|---|
| Other cancers | 8 | 16 |
| Cardiovascular causes | ||
| Cardiac included a ‘sudden death at home’ in EBRT group | 2 | 8 |
| Stroke | 0 | 2 |
| Ischaemic bowel | 0 | 1 |
| Other causes: TARGIT: 2 diabetes, 1 renal failure, 1 liver failure, 1 sepsis, 1 Alzheimer’s disease, 1 unknown. EBRT: 1 myelopathy, 1 perforated bowel, 1 pneumonia, 1 old age and 4 unknown | 7 | 8 |
| Total | 17 | 35 |
| 5-year risk = 1.4% vs. 3.5% | ||
| Log-rank p = 0.0086 | ||
FIGURE 10.
Kaplan–Meier plots for (a) breast cancer deaths; and (b) non-breast-cancer deaths. Reproduced with permission from Vaidya et al. 2 Copyright © Vaidya et al. Open Access article distributed under the terms of CC BY-NC-ND.


Analysis limited to the mature cohort, first reported in 2010 (n = 2232, median follow-up of 3 years 7 months), in which most events had occurred (32 of 34 local recurrences and 85 of 88 deaths), yielded much the same results.
The effect of timing of randomisation and delivery of radiotherapy in relation to lumpectomy: the prepathology and postpathology strata
Figure 11 shows the Kaplan–Meier graphs for the prepathology and postpathology strata.
FIGURE 11.
Primary and secondary outcomes for the two strata defined by timing of randomisation and delivery of TARGIT: (a and c) prepathology (n = 2298) (randomised before lumpectomy and TARGIT given concurrently with lumpectomy); (b and d) postpathology (n = 1153) (randomised after lumpectomy and TARGIT given by reopening the wound). Kaplan–Meier plots for local recurrence in the conserved breast in patients who had breast-conserving surgery (a and b) and for deaths in all patients (c and d). Reproduced with permission from Vaidya et al. 2 Copyright © Vaidya et al. Open Access article distributed under the terms of CC BY-NC-ND.


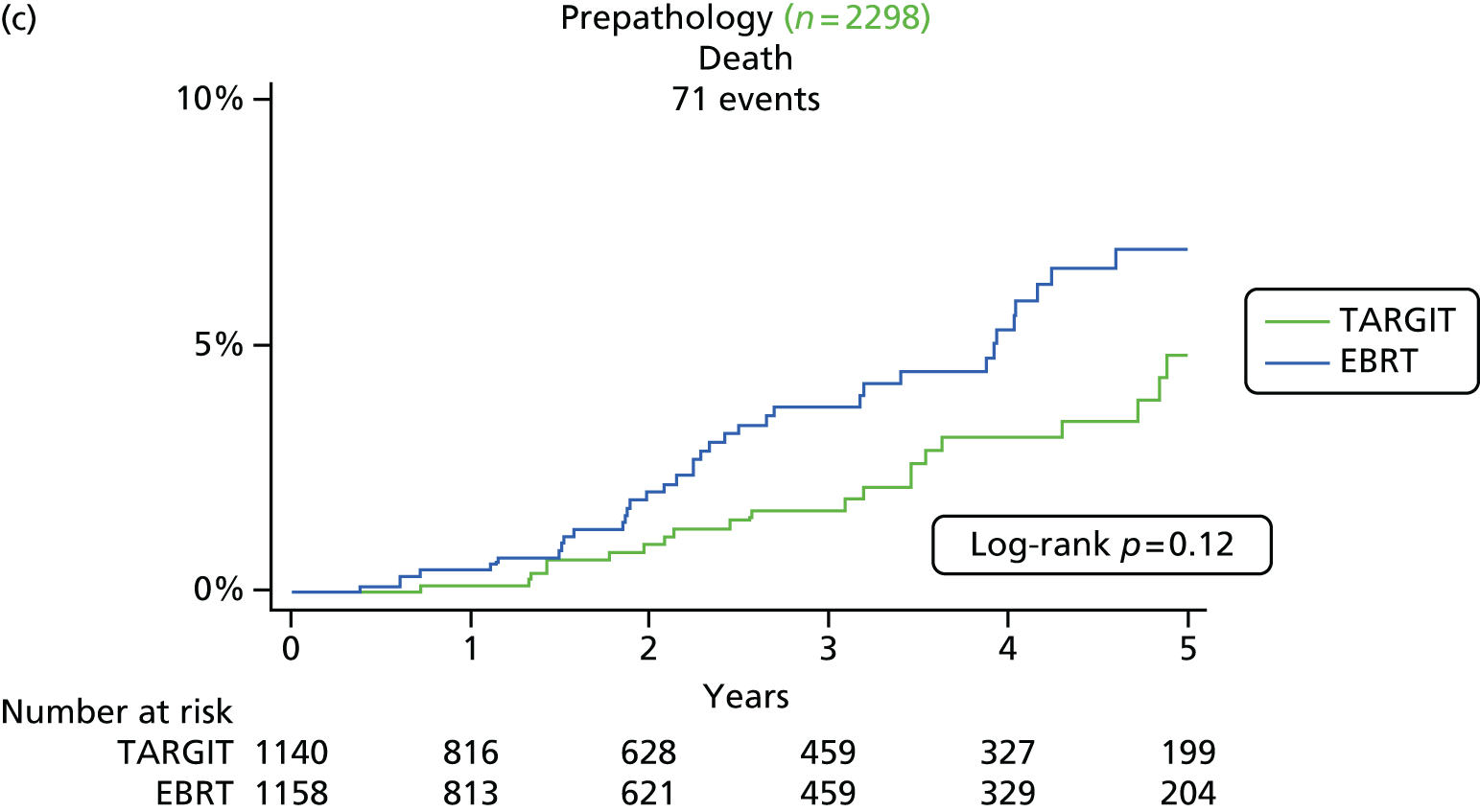
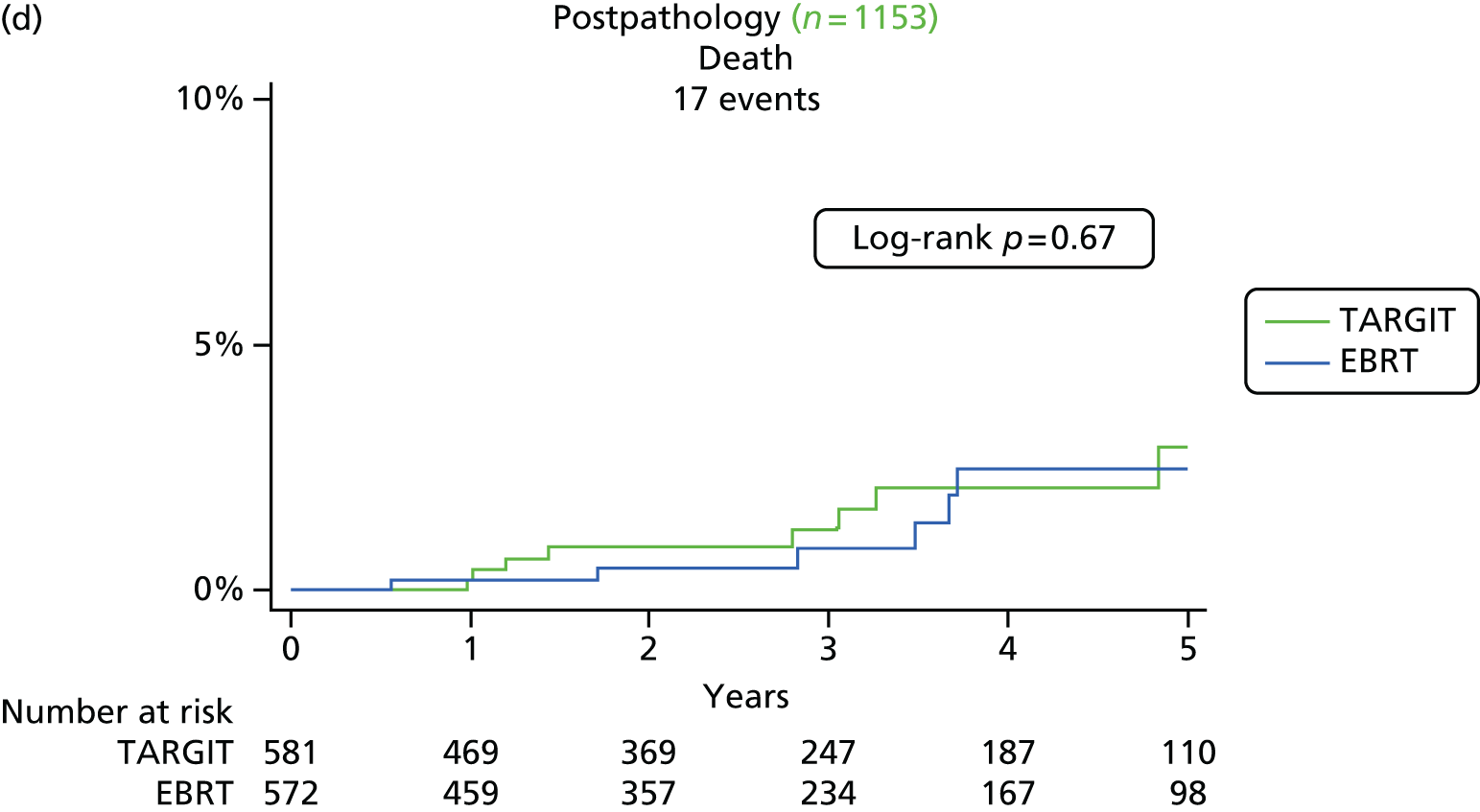
In the prepathology stratum (n = 2298), that is, when TARGIT was delivered during the initial lumpectomy, the risk of local recurrence in the conserved breast was much the same for TARGIT as for EBRT [TARGIT 2.1% (95% CI 1.1% to 4.2%) vs. EBRT 1.1% (95% CI 0.5% to 2.5%); p = 0.31]. In total, 17 patients died from breast cancer in the TARGIT group and 15 patients died from breast cancer in the EBRT group [TARGIT 3.3% (95% CI 1.9% to 5.8%) vs. EBRT 2.7% (1.5% to 4.6%); p = 0.72]; the equivalent figures for non-breast-cancer mortality were 12 and 27, respectively [TARGIT 1.3% (95% CI 0.7% to 2.8%) vs. EBRT 4.4% (95% CI 2.8% to 6.9%); p = 0.016]. Thus, in absolute terms, there were four additional local recurrences but 13 fewer deaths in the prepathology stratum.
In the postpathology stratum (n = 1153), that is, when TARGIT was delivered as a delayed procedure by reopening the lumpectomy cavity, the difference between the two groups in local recurrence in the conserved breast was larger than 2.5% [TARGIT 5.4% (95% CI 3.0% to 9.7%) vs. EBRT 1.7% (95% CI 0.6% to 4.9%); p = 0.069]. In total, three patients died from breast cancer in the TARGIT group and one patient died from breast cancer in the EBRT group [TARGIT 1.2% (95% CI 0.4% to 4.2%) vs. EBRT 0.5% (95% CI 0.1% to 3.5%); p = 0.35]; the equivalent figures for non-breast-cancer mortality were five and eight, respectively [TARGIT 1.58% (95% CI 0.62% to 3.97%) vs. 1.76% (95% CI 0.7% to 4.4%); p = 0.32]. Thus, in absolute terms, there were eight additional local recurrences and one less death in the postpathology stratum.
Figures 12–14 show more detailed results for the prepathology stratum. Figure 12 shows the primary (local recurrence in the conserved breast) and secondary (deaths) outcomes. Figure 13 shows the differences in 5-year estimates for these outcomes for the whole cohort, the mature cohort and the earliest cohort. This figure demonstrates the stability of the results with longer follow-up and the trade-offs between the two outcomes. Figure 14 shows the 10-year Kaplan–Meier graph for the prepathology stratum, which shows that the small difference in local recurrence does not increase with longer follow-up.
FIGURE 12.
Primary and secondary outcomes for the prepathology stratum (n = 2298). Kaplan–Meier plots for (a) local recurrence in the conserved breast in patients who had breast-conserving surgery; (b) breast cancer deaths in all patients; and (c) non-breast-cancer deaths in all patients. Reproduced with permission from Vaidya et al. 2 Copyright © Vaidya et al. Open Access article distributed under the terms of CC BY-NC-ND.
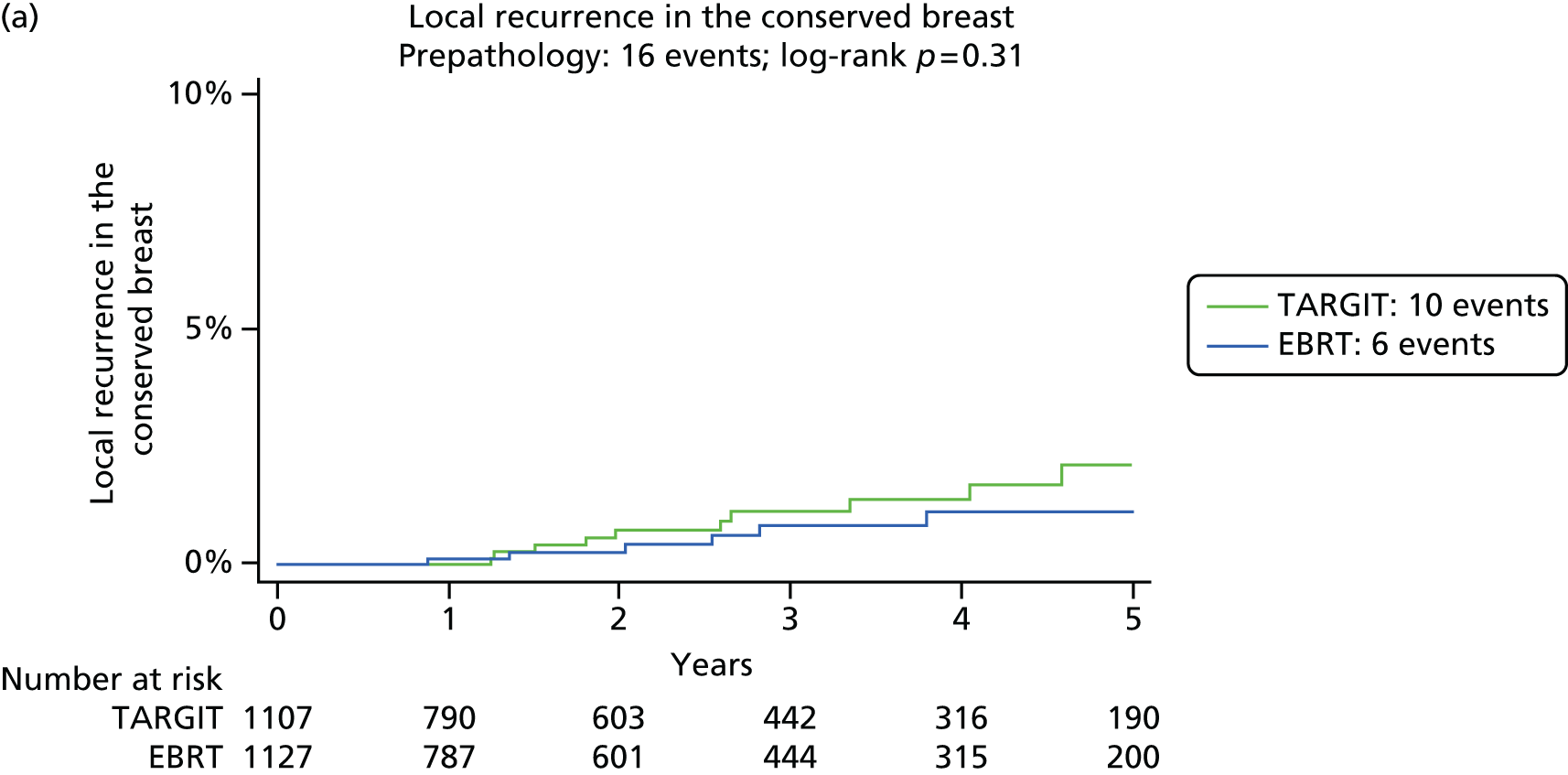
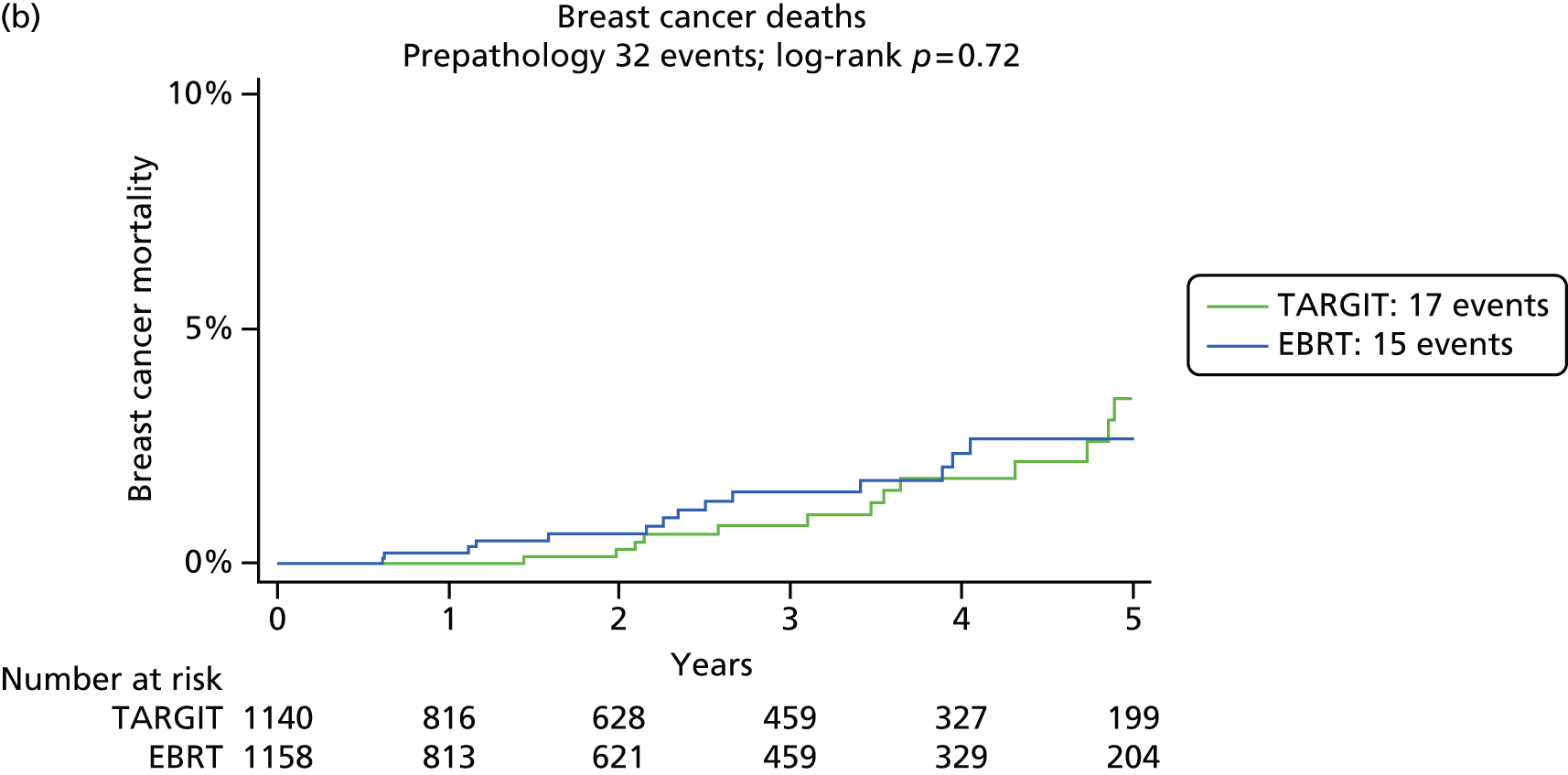
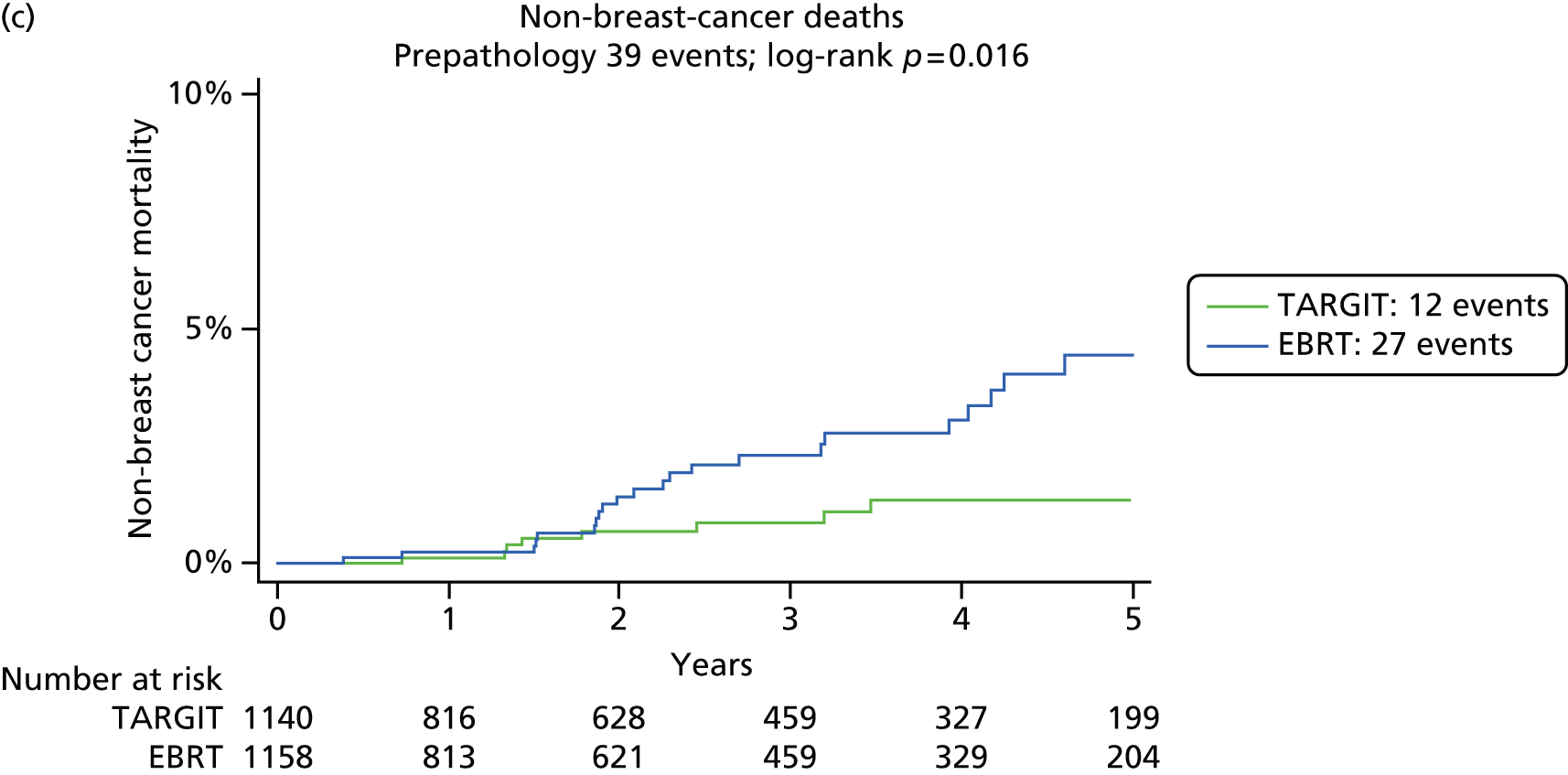
FIGURE 13.
Absolute differences in 5-year Kaplan–Meier estimates of local recurrence in the conserved breast and overall mortality (TARGIT – EBRT) for the prepathology stratum in the whole cohort, the mature cohort and the earliest cohort. All patients in the earlier cohorts are included in later cohorts. Median follow-up for the whole cohort was 2 years 4 months, median follow-up for the mature cohort was 3 years 8 months and median follow-up for the earliest cohort was 5 years. Reproduced with permission from Vaidya et al. 2 Copyright © Vaidya et al. Open Access article distributed under the terms of CC BY-NC-ND.

FIGURE 14.
Prepathology stratum: Kaplan–Meier plot of local recurrence in the conserved breast up to 12 years.

One possible concern might be that the addition of EBRT was different between the two strata. If prespecified unsuspected adverse factors were found when the full pathology report was available, whole-breast radiotherapy was recommended. This was more common in the prepathology stratum than in the postpathology stratum (21% vs. 3%). To assess whether or not more frequent use of additional EBRT could be the cause of better outcomes in the prepathology stratum, we performed an exploratory non-randomised comparison of tumour characteristics and primary and secondary outcomes between prepathology and postpathology patients who received TARGIT alone.
Patients in the super-selected postpathology stratum had a much better prognosis than patients in the prepathology stratum, as seen from the tumour size, tumour grade, lymph node status and breast cancer survival (Table 11). Despite this, the 5-year local control in prepathology cases who received TARGIT alone appears to be better than that in the postpathology cases who received TARGIT alone (2.7% vs. 5.9%) and similar to that in the whole prepathology stratum (2.1%).
| Variable | Prepathology patients who received TARGIT alone (n = 793) | Prepathology patients who received TARGIT + EBRT (n = 219) | Postpathology patients who received TARGIT alone (n = 539) |
|---|---|---|---|
| Tumour size (cm) | |||
| ≤ 1 | 36% (256/703) | 24% (47/199) | 52% (269/515) |
| 1–2 | 52% (366/703) | 49% (98/199) | 43% (223/515) |
| > 2 | 12% (81/703) | 27% (54/199) | 5% (23/515) |
| Grade | |||
| 1 | 27% (188/707) | 19% (38/200) | 59% (285/486) |
| 2 | 54% (380/707) | 60.5% (121/200) | 38% (182/486) |
| 3 | 20% (139/707) | 20.5% (41/200) | 4% (19/486) |
| Lymph node positivity | |||
| Node negative | 85% (604/712) | 63% (126/201) | 95% (492/519) |
| 1–3 nodes involved | 13% (94/712) | 29% (59/201) | 5% (27/519) |
| > 3 nodes involved | 2% (14/712) | 8% (16/201) | 0 (0/519) |
| 5-year local recurrence in the conserved breast (95% CI) | 2.7 (1.3 to 5.5) | 0.9 (0.1 to 6.1) | 5.9 (3.3 to 10.5) |
| 5-year risk of breast cancer death (95% CI) | 1.8 (0.7 to 4.6) | 8.0 (3.5 to 17.5) | 0.6 (0.2 to 2.5) |
| 5-year risk of death from non-breast-cancer causes (95% CI) | 1.9 (0.9 to 4.0) | 0 | 1.5 (0.6 to 4.3) |
These data suggest that TARGIT during lumpectomy is effective and that the accurate and timely application of radiation to the fresh tumour bed avoiding spatial and temporal miss rather than the addition of EBRT in higher-risk cases is responsible for the better results in the prepathology stratum.
Analysis of the earliest cohort of over 800 patients randomised in the first 8 years in the prepathology stratum of the TARGIT-A trial, and who have a median follow-up of 5 years
We paid special attention to the earliest cohort of over 800 patients randomised in the first 8 years in the prepathology stratum of the TARGIT-A trial. This cohort had a median follow-up of 5.01 years. As well as being a conventional yardstick, this 5-year point happens to be considerably in excess of the known peak hazard of local recurrence. In addition, the effect of radiotherapy on local recurrence is limited to the first 5 years. From the results of the main analysis it was determined that giving TARGIT during lumpectomy should be the preferred option and hence we analysed these earliest patients.
This section provides details of the analysis for this cohort of patients who were randomised in the first 8 years of the TARGIT-A trial, that is, from 2000 to 2008. All patients randomised in this period in the prepathology stratum were included. These patients happen to have the longest follow-up and have not been ‘cherry-picked’.
In total, there were 817 patients in the prepathology stratum of the earliest cohort. For local recurrence in the conserved breast, TARGIT was non-inferior to EBRT (Pnon-inferiority = 0.0091). The Kaplan–Meier plots are shown in Figure 15.
FIGURE 15.
Kaplan–Meier plots for the earliest cohort of patients randomised in the first 8 years of the trial: (a) local recurrence in the conserved breast; (b) breast cancer deaths; and (c) non-breast-cancer deaths.



The 5-year estimated risk was not statistically different between groups for local recurrence [TARGIT 1.8% (95% CI 0.84% to 4.2%) vs. EBRT 0.84% (95% CI 0.3% to 2.6%); p = 0.32] or deaths from breast cancer [TARGIT 3.9% (95% CI 2.3% to 6.7%) vs. EBRT 3.0% (95% CI 1.7% to 5.4%); p = 0.34]. However, there were significantly fewer deaths from causes other than breast cancer with TARGIT [1.9% (95% CI 0.9 to 3.9)] than with EBRT [5.1% (95% CI 3.2% to 8.0%); p = 0.04.]
It should be noted that the number needed to prove non-inferiority was calculated to be 5851 and, therefore, this earliest cohort of 817 patients had enough power to draw reliable conclusions.
From this analysis it is clear that, if the trial had been stopped after 8 years of recruitment, there would have been an adequate number of patients for testing the hypothesis with a median follow-up of 5 years. The patients who were recruited more recently should not be perceived as weakening the result because the Kaplan–Meier analysis as well as the cohort analysis for non-inferiority (see Table 8) takes it all into account.
Disease-free survival and survival without local recurrence
For the whole trial cohort and the prepathology stratum, disease-free survival was similar between the groups (p = 0.78 and p = 0.69 respectively), as shown in Figures 16 and 17, respectively.
FIGURE 16.
Ten-year disease-free survival for all patients in the trial.

FIGURE 17.
Ten-year disease-free survival for patients in the prepathology stratum.
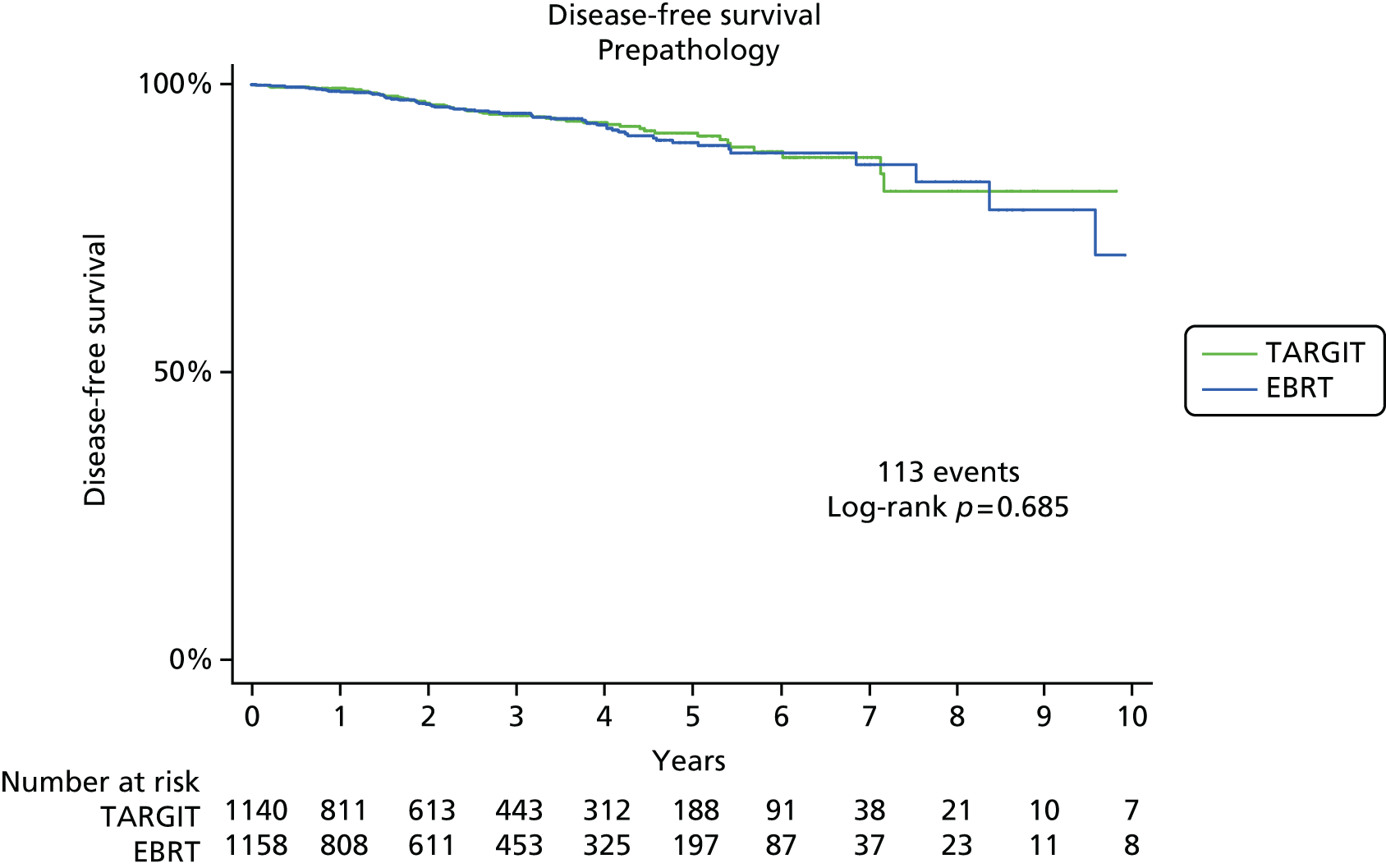
The Kaplan–Meier estimates for disease-free survival for the whole trial were similar for TARGIT and EBRT (log-rank p = 0.78). Five-year disease-free survival was 90.5% (95% CI 88.1% to 92.5%) for TARGIT and 91.0% (95% CI 88.6% to 92.98%) for EBRT and 10-year disease-free survival was 77.7% (95% CI 65.5% to 86.8%) for TARGIT and 71.5% (95% CI 49.8% to 85.1%) for EBRT.
The Kaplan–Meier estimates for disease-free survival for the prepathology stratum, in which TARGIT was given during surgical removal of the cancer, were not statistically different for TARGIT and EBRT (log-rank p = 0.69): 5-year disease-free survival was 91.6% (95% CI 88.7% to 93.8%) for TARGIT and 90.1% (95% CI 86.8% to 92.6%) for EBRT and 10-year disease-free survival was 81.3% (95% CI 71% to 88%) for TARGIT and 71.2% (95% CI 49% to 85%) for EBRT.
Finally, survival without local recurrence (in which deaths are not censored, in line with recommendations by the US Food and Drug Administration62 and elsewhere63) was not statistically different between the two arms [TARGIT vs. EBRT, all patients: 93.1% (95% CI 90.8% to 94.9%) vs. 93.8% (95% CI 91.7% to 95.4%), p = 0.81; prepathology: 93.9% (95% CI 90.9% to 95.9%) vs. (95% CI 89.7% to 94.6%), p = 0.35] (Figure 18). These results are shown in the form of a pictogram (Figure 19) that can be used during discussion between the clinician and the patient before breast-conserving (lumpectomy) surgery for breast cancer. It explains the outcome for patients treated with TARGIT IORT compared with the outcome for those receiving EBRT, based on randomised evidence.
FIGURE 18.
Kaplan–Meier plot: true representation of how patients with breast cancer would fare in the first 5 years following treatment with TARGIT during lumpectomy or EBRT with respect to local control. Censoring is carried out at the point of last follow-up or withdrawal. For any patient, the chance of being alive without local recurrence can be read off this plot. Five-year survival without local recurrence is 93.9% (95% CI 90.9% to 95.9%) for TARGIT and 92.5% (95% CI 89.7% to 94.6%) for EBRT (p = 0.35). Reproduced from Vaidya et al. 64 © 2015 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/ licenses/by-nc-nd/4.0/).

FIGURE 19.
A pictogram to help patients and doctors make a shared well-informed decision. Adapted with permission from Vaidya et al. 64

Thus, the clinical effectiveness with TARGIT was at least as good as that with EBRT.
Chapter 4 Planned subgroup analysis
Background
The results presented in the previous chapter suggest that the prepathology approach, in which TARGIT is delivered under the same anaesthetic concurrently with the first excision of breast cancer rather than at a later date by reopening the wound (i.e. postpathology), would currently lend itself to be the preferred option of delivery of TARGIT. In this prepathology stratum TARGIT achieved statistically similar breast cancer control to EBRT and maintained a statistically significant lower mortality rate from other causes than EBRT.
Although most patients recruited to the trial were good-prognosis patients, there was a substantial number of ‘high-risk’ cases, for example approximately 500 cases were node positive, or grade 3, or larger than 2 cm, because of the broad inclusion criteria. When the TARGIT approach is applied to normal clinical practice, patient selection will be crucial in ensuring that the results of day-to-day practice reflect the results obtained in the clinical trial.
Shortly before the statistical analysis plan was finalised on 14 June 2012, new data suggesting that hormone sensitivity, particularly PgR status, may be an important factor in determining outcome from radiotherapy were presented. Details of the rationale are described in Chapter 2 (see Planned subgroup analysis). Therefore, before the trial database was unblinded for this analysis, we hypothesised that PgR negativity, as a surrogate marker of functional ER status, might be a predictor of the effectiveness of radiotherapy.
This analysis of the randomised data may help refine current guidelines for selecting patients for PBI approaches, which are mainly based on a presumed risk of recurrence rather than the effectiveness of radiation, for which only randomised evidence can be relied on.
Methods
We performed an analysis with respect to PgR status for the primary and secondary end points of local recurrence and survival (deaths from breast cancer and deaths from other causes) in the whole trial population. Given the main results, which suggested that TARGIT should preferably be used concurrently with the primary excision of the tumour, that is, as used in the prepathology stratum, we also analysed within the prepathology stratum the difference in local control and survival (deaths from breast cancer and deaths from other causes) between TARGIT and EBRT in PgR-negative and PgR-positive cases.
Analyses were performed by plotting Kaplan–Meier curves and testing for significance using the log-rank test; a p-value of 0.05 was used as the boundary for statistical significance.
We subsequently analysed in all patients whether certain patient or tumour characteristics (timing of randomisation/delivery of TARGIT, age, whether screen detected or not, tumour grade, presence of DCIS, margin positivity, ER, PgR and HER2 status, lymphovascular invasion, node positivity) influenced the effect of radiotherapy using the Cox proportional hazards model.
Fitting a separate regression model is equivalent to fitting an interaction of the treatment arm with all of the independent risk factors in the model and also assumes that the variance in both treatment groups is similar. We also fitted a full model with a treatment by PgR status interaction.
For visual representation of the non-inferiority results, we created a forest plot for the non-inferiority test for each of these subgroups. In this plot we also added two groups of the prepathology stratum based on a median follow-up of < 5 years and ≥ 5 years.
Results
There were 2298 patients in the prepathology stratum in whom randomisation occurred before lumpectomy and TARGIT was delivered concurrently with lumpectomy a median of 23 days after initial diagnostic needle biopsy and a median of 7 days after randomisation. The postpathology stratum consisted of those patients who had already had their tumour excised up to 30 days before randomisation (n = 1153). When allocated TARGIT, it was delivered as a second surgical procedure by reopening the wound (median 62 days after initial diagnostic needle biopsy, 37 days after the initial surgery and 17 days after randomisation).
A substantial number (18%) in each stratum were PgR negative (554 among all patients and 535 among those who had breast-conserving therapy). PgR status was unknown in only 77 patients among the 3093 patients in whom ER status was known. In total, 99.5% (2449/2462) of PgR-positive cases were also ER positive and 85.2% (2449/2874) of all ER-positive cases were also PgR positive. ER status was known in 89.6% (3093/3451) of cases, of whom PgR status was known in 97.5% (3016/3093).
These results have been presented at the European Cancer Congress 2013. 65
Effect of progesterone receptor status
Figure 20 shows the Kaplan–Meier plots, 5-year risks with CIs and significance levels for all patients, that is, both prepathology and postpathology strata combined.
FIGURE 20.
Local recurrence (a and b) and overall mortality (c and d) for the pre-specified subgroups defined by PgR status.



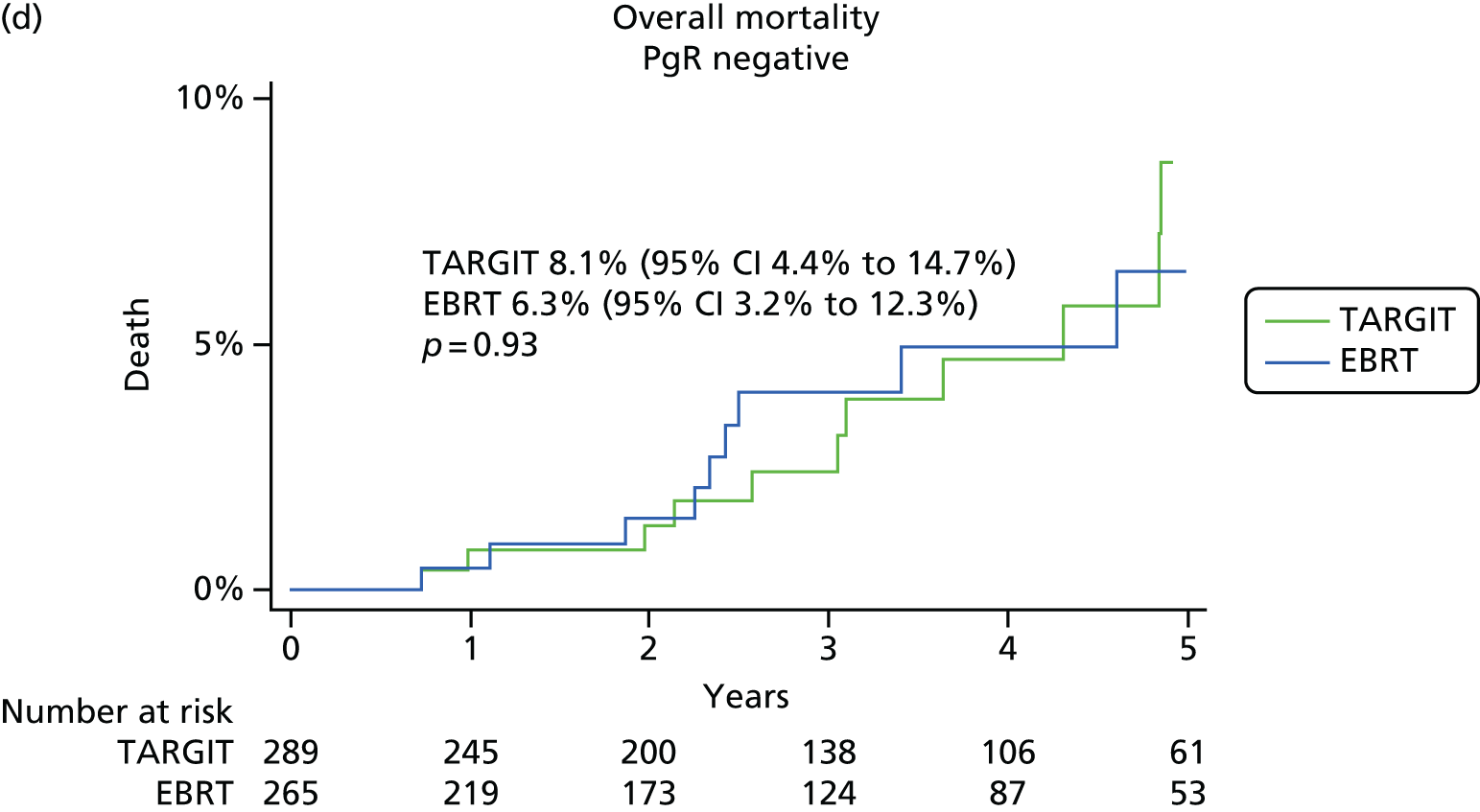
Among patients who had PgR-positive tumours, there was no significant difference in the primary outcome of local recurrence in the conserved breast at 5 years between TARGIT and EBRT [13/1204 vs. 10/1203, 5-year risk 2.3% (95% CI 1.3% to 4.3%) vs. 1.5% (95% CI 0.75% to 3.0%); p = 0.51] and there were significantly fewer deaths with TARGIT than with EBRT [22/1232 vs. 37/1230, 5-year risk 2.7% (95% CI 1.6% to 4.5%) vs. 4.9% (95% CI 3.3% to 7.1%); p = 0.0487].
Among patients who had PgR-negative tumours, local recurrence in the conserved breast was higher in the TARGIT arm than in the EBRT arm [9/276 vs. 1/259, 5-year risk 7.0% (95% CI 3.5% to 13.6%) vs. 0.5% (95% CI 0.1% to 3.7%); p = 0.017]. Overall mortality was similar between the groups [13/289 vs. 11/265, 5-year risk 8.1% (95% CI 4.4% to 14.7%) vs. 6.3% (95% CI 3.2% to 12.3%)].
Effect of progesterone receptor status in the prepathology stratum when targeted intraoperative radiotherapy was given concurrently with lumpectomy
Figure 21 shows the Kaplan–Meier plots, 5-year risks with CIs and significance levels for the prepathology stratum. Figure 22 shows the local recurrence and overall mortality in cohorts of patients with increasing follow-up periods. This demonstrates that the results remain stable with longer follow-up periods, including in the earliest cohort of 636 patients with a median follow-up of 5 years.
FIGURE 21.
Effect of PgR status on primary and secondary outcomes in the prepathology stratum (TARGIT given concurrently with lumpectomy): (a)–(c) PgR positive; (d)–(f) PgR negative. (a) Local recurrence in the conserved breast – events: TARGIT n = 4, EBRT n = 5; (b) deaths from breast cancer – events: TARGIT n = 8, EBRT n = 9; (c) deaths from other causes – events: TARGIT n = 10, EBRT n = 22; (d) local recurrence in the conserved breast – events: TARGIT n = 5, EBRT n = 1; (e) deaths from breast cancer – events: TARGIT n = 8, EBRT n = 5; and (f) deaths from other causes – events: TARGIT n = 2, EBRT n = 5.
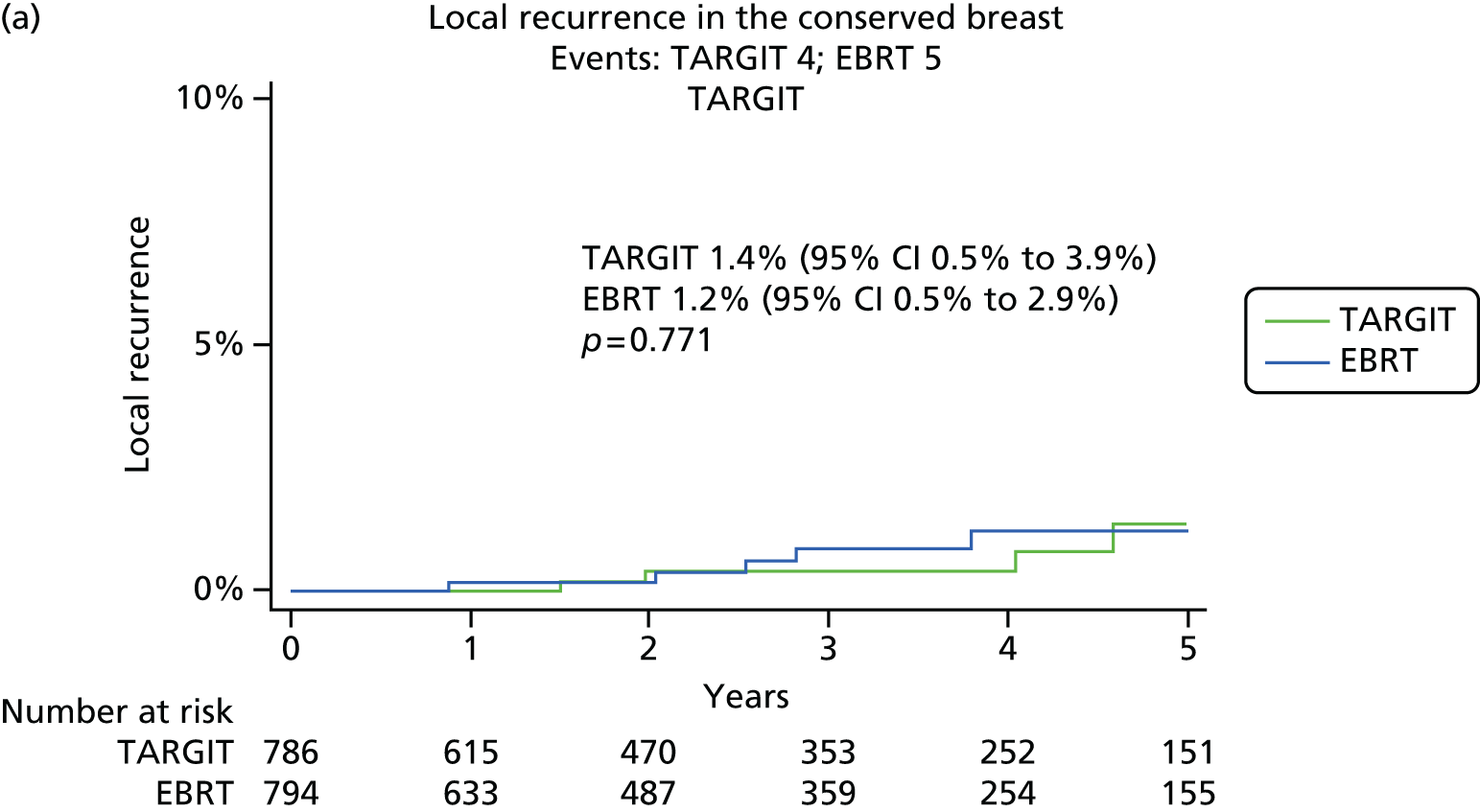


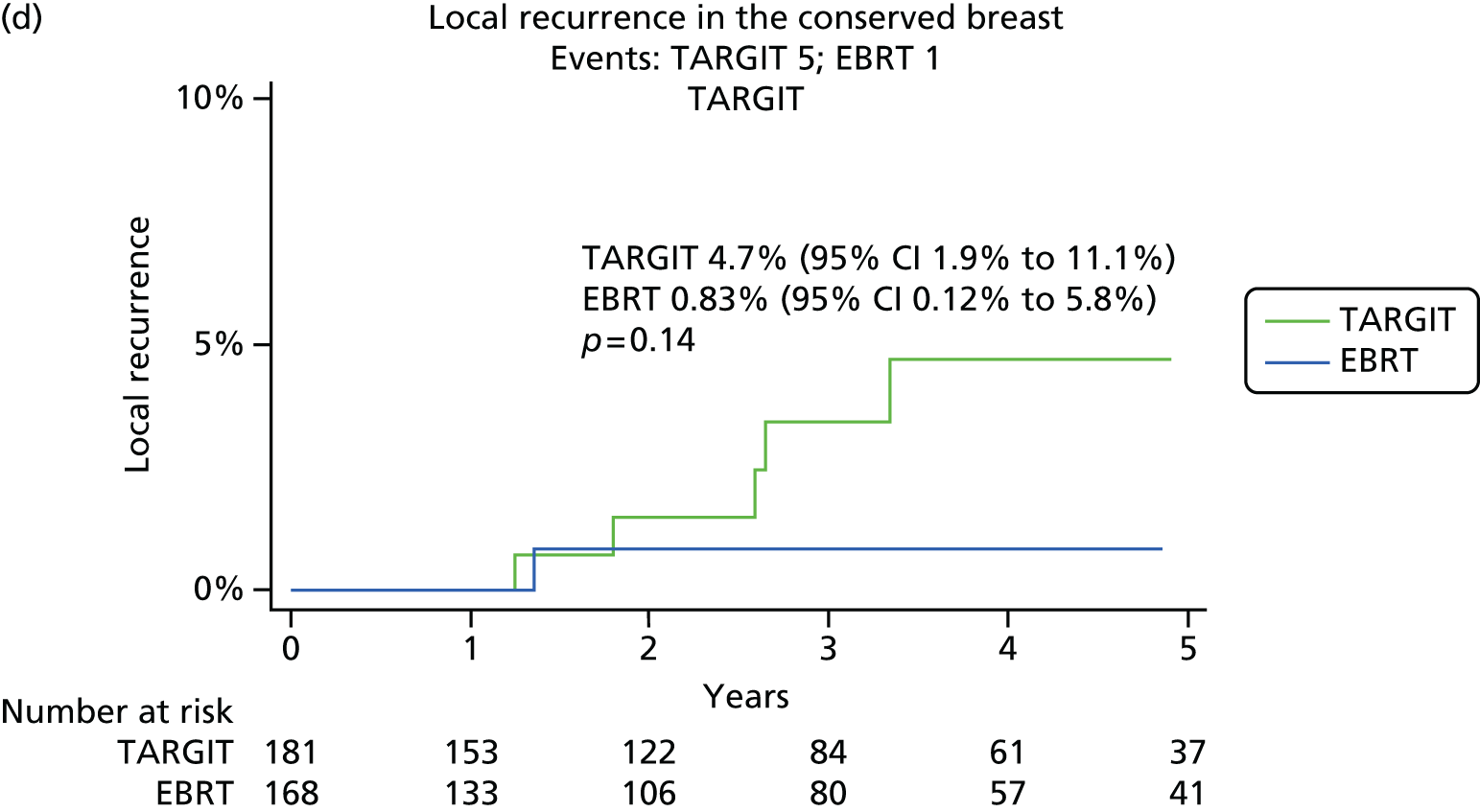
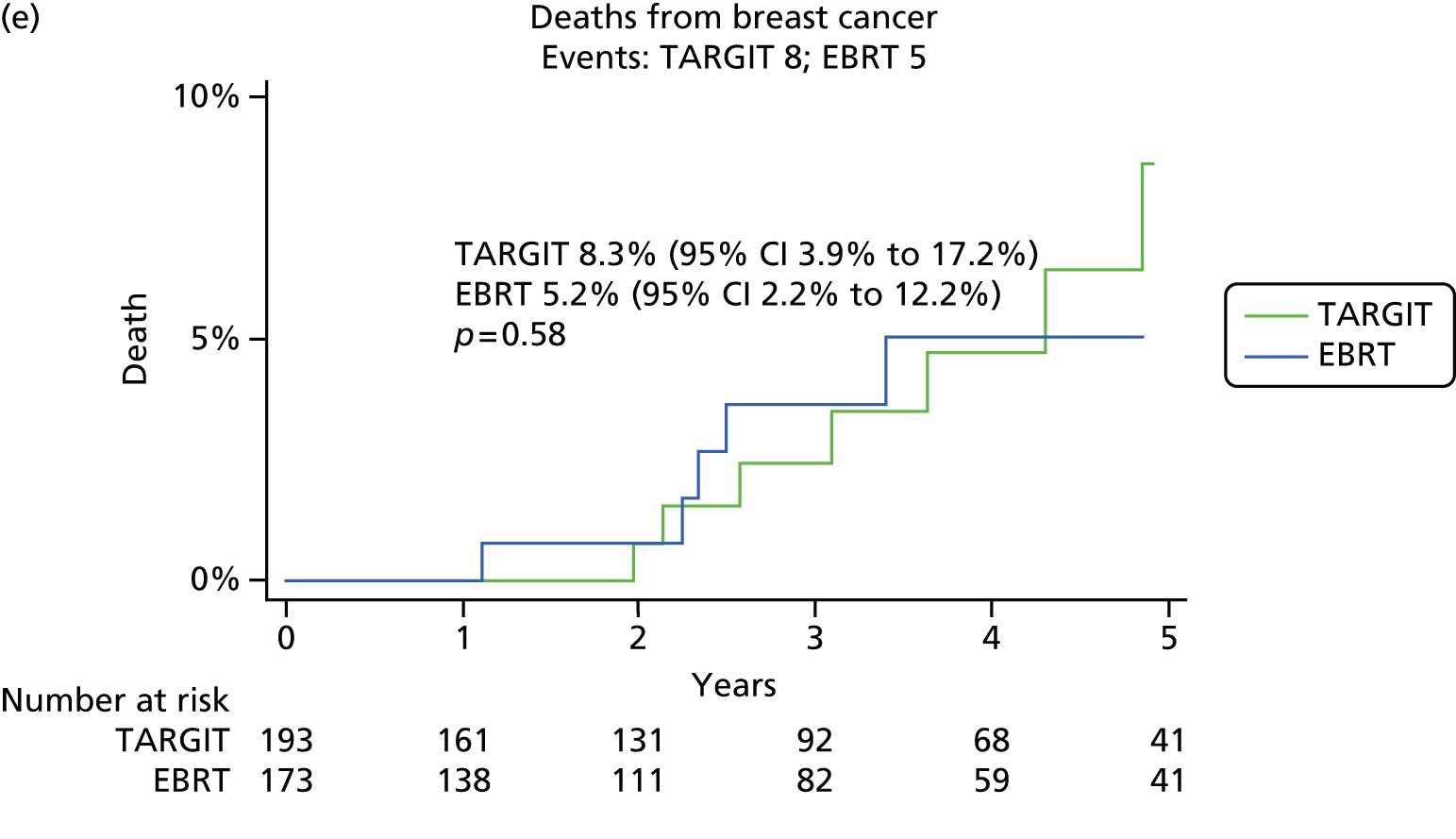

FIGURE 22.
Local recurrence in the conserved breast and overall mortality for PgR-positive cases in the prepathology stratum.

When TARGIT was given concurrently in patients who had PgR-positive tumours (n = 1625), local recurrence in the conserved breast [1.4% (95% CI 0.5% to 3.9%) vs. 1.2% (95% CI 0.5% to 2.9%)] and breast cancer mortality [1.78% (95% CI 0.7% to 4.4%) vs. 1.98% (95% CI 0.94% to 4.2%)] were similar for TARGIT and EBRT and mortality from other causes was significantly lower by 2.9% in the TARGIT arm [1.6% (95% CI 0.74% to 3.4%) vs. 4.5% (95% CI 2.8% to 7.3%); p = 0.04], leading to a 3.1% reduction in overall mortality [3.3% (95% CI 1.8% to 6.0%) vs. 6.4% (95% CI 4.3% to 9.6%); p = 0.083].
For patients with PgR-negative tumours (n = 366), local recurrence in the conserved breast was numerically, but not statistically, higher in the TARGIT arm than in the EBRT arm. There was no difference between the two arms in breast cancer mortality, non-breast-cancer mortality or overall mortality.
Thus, when TARGIT is delivered at the time of primary surgery, the chance of remaining free from local recurrence at 5 years possibly drops from 99% to 98% (p = 0.31), but the chance of staying alive possibly increases from 93.1% to 95.4% (p = 0.12). The p-value expresses the probability of the difference we found being observed if there was no real difference between the two treatments. Alternatively, one can say that there is no difference in local recurrence or mortality, with a large benefit of having the treatment in one sitting.
Cox model
The following factors were entered into the Cox model for the primary outcome of local recurrence in the conserved breast: randomisation arm, timing of randomisation/delivery of TARGIT, age, whether screen detected or not, tumour grade, presence of DCIS, margin positivity, ER, PgR and HER2 status, lymphovascular invasion and node positivity.
We found that age, whether screen detected or not, tumour grade, ER status, HER2 status, lymphovascular invasion and nodal status did not appear to have any influence on local recurrence, whereas timing of randomisation and delivery of TARGIT [hazard ratio (HR) for delayed TARGIT 2.26, 95% CI 1.07 to 4.78; p = 0.033] were significant predictors of local recurrence. PgR status was a significant predictor of local recurrence overall (HR for PgR negative 2.46, 95% CI 1.11 to 5.47; p = 0.027) and in the TARGIT arm (HR for PgR negative 3.14, 95% CI 1.29 to 7.61; p = 0.011). Margin status was significant overall (HR for positive first margin 2.68, 95% CI 1.08 to 6.68; p = 0.034) and in the EBRT arm (HR for positive first margin 5.17, 95% CI 1.50 to 17.8; p = 0.009), but not in the TARGIT arm. Fitting a separate regression model is equivalent to fitting an interaction of treatment arm with all of the independent risk factors in the model and also assumes that the variance in both treatment groups is similar. We also fitted a full model with a treatment by PgR status interaction, giving an overall Wald statistic p-value of 0.08.
For those patients who received only TARGIT during lumpectomy, multivariate analysis showed that only PgR status (HR for PgR negative 5.57, 95% CI 1.23 to 25.21; p = 0.026) remained significant. Other factors, such as younger age, grade (grade 3), tumour size > 2 cm or node positivity, did not influence the difference between the two randomised arms: the 5-year rates of local recurrence in the conserved breast were as follows: for 263 patients with age ≤ 50 years [events 3/145 vs. 3/118, TARGIT 2.9% (95% CI 0.94% to 8.64%) vs. EBRT 6.2% (95% CI 1.99% to 18.47%); p = 0.70]; for 436 grade 3 cancers [events 2/20 vs. 2/216, TARGIT 1.5% (95% CI 0.37% to 5.87%) vs. EBRT 1.7% (95% CI 0.42% to 6.45%); p = 0.98]; for the 363 patients with tumour ≥ 2 cm [events 1/173 vs. 1/190, TARGIT 0.75% (95% CI 0.1% to 5.2%) vs. EBRT 0.86% (95% CI 0.1% to 6.0%); p = 0.95]; for the 472 node positive patients [events 1/245 vs. 2/227, TARGIT 0.72% (95% CI 0.1% to 5.0%) vs. EBRT 1.3% (95% CI 0.3% to 5.2%); p = 0.56].
Forest plot
The forest plot in Figure 23 shows that TARGIT was non-inferior to EBRT in every subgroup apart from the ER-negative and PgR-negative subgroups.
FIGURE 23.
Forest plot showing the non-inferiority statistic for each of the subgroups. The green shaded area is the non-inferiority margin and each of the green squares is the local recurrence rate in terms of binomial proportions along with the 90% CI. From this plot it appears that only ER and PgR status affects whether or not there is a difference between TARGIT and EBRT. LVI, lymphovascular invasion.

Discussion
This planned subgroup analysis found that hormone receptor status is the only factor that influences the outcome of patients treated with TARGIT versus EBRT. In patients with PgR-positive tumours (almost all of whom were ER positive), there was no statistically significant difference between TARGIT and EBRT in terms of local control and overall survival was better by 2.2% with TARGIT than EBRT. The only factor that predicted a less favourable outcome for patients in the TARGIT arm was negative hormone receptor status. Other factors such as younger age, higher grade (grade 3), tumour size > 2 cm or node positivity changed the local recurrence rate equally in the two randomised arms. There were a substantial number of such patients in the trial and TARGIT was found to be as effective as EBRT in patients with these higher-risk factors.
Chapter 5 Other analyses
Other quadrant recurrences: confirmation of the original hypothesis tested in the TARGIT-A trial – cancer foci in other quadrants do not need treatment with whole-breast radiotherapy
Background
In 1996 we reported that 63% of specimens of mastectomy performed for a unifocal cancer harbour other cancer foci, with 80% of these foci in other quadrants. 13 In contrast, local recurrence after a lumpectomy occurs mainly at the site of the original tumour. Therefore, we hypothesised that the cancer foci in other quadrants remain dormant even in the absence of radiation treatment to the whole breast. This academic insight led us to develop the TARGIT technique and the INTRABEAM device. In the TARGIT-A randomised trial (n = 3451) we compared risk-adapted TARGIT vs. whole-breast radiotherapy. 2
Method
Randomisation occurred either before surgery (prepathology stratum; TARGIT given during lumpectomy) or after surgery (TARGIT given as a delayed procedure). In the prepathology stratum we assessed the number of local recurrences that occurred in other quadrants in the patients who received only TARGIT and in the patients who received EBRT. We also estimated the number of cancer foci in quadrants other than that with the original tumour.
Results
In total, 94.4% of cases in the TARGIT-A trial did not undergo a preoperative MRI scan. A total of 793 patients in the prepathology stratum randomised to TARGIT had only TARGIT as their radiotherapy. With 2098 women-years of follow-up, there were nine recurrences in the conserved breast. The 5-year local recurrence rate in those who received TARGIT alone was 2.7% (95% CI 1.3% to 5.5%), which was not different from that in the whole prepathology cohort randomised to TARGIT [2.1% (95% CI 1.1% to 4.2%)]. In these 793 patients, one would expect 63% (i.e. 500) to have additional foci of cancer in their breasts and 80% of these (i.e. 400) should be in quadrants other than the index quadrant. In reality, after 2098 women-years of follow-up, seven patients had recurrence in the scar, six had new contralateral cancers and two had cancers growing in other quadrants, implying that the remaining 398 foci had remained dormant. Among 935 patients who received whole-breast radiotherapy the same number of cancers (n = 2) grew in other quadrants and there were five new contralateral cancers. In the randomised comparison, there were two local recurrences in other quadrants in each of the randomised arms of the trial.
Conclusion
Cancer foci in breast that are away from the site of the primary tumour remain dormant and behave no differently from those in the contralateral breast. They also appear to be unaffected by whole-breast radiotherapy. This analysis provides level 1 evidence supporting PBI.
Axillary recurrence: omitting whole-breast radiotherapy did not increase axillary recurrence in the TARGIT-A trial
These data were presented at the St Gallen Symposium in March 2013 (poster presentation)66 and at the American Society for Radiation Oncology (ASTRO) Annual Meeting in September 2013 (oral presentation). 67
Background
The Z–11 trial68 found that, even when axillary clearance is not performed after finding one or two positive sentinel nodes, it does not affect local control, despite 23% of patients in the axillary clearance (control) arm (and, therefore, those in the experimental arm as well) having positive nodes. However, every patient in this trial received whole-breast radiotherapy and it has been suggested that inadvertent non-therapeutic irradiation of the lower axilla that occurs with tangential fields of conventional whole-breast radiotherapy could be responsible for controlling the growth of the minimal residual axillary disease. Therefore, questions are being raised whether following the concept of sentinel node biopsy or not dissecting the axilla after one to two positive nodes is applicable to patients receiving PBI.
Methods
We compared the risk of axillary recurrence in patients with negative sentinel node biopsy and those with one to two positive nodes as per treatment received – whether they received EBRT or TARGIT only within the updated TARGIT-A trial. 2 It is important to note that this was a comparison as per treatment received rather than as per randomised allocation (Figure 24).
FIGURE 24.
Comparison of the two non-randomised groups for the outcome of local recurrence, with or without EBRT.

Results
Overall, 3375 patients underwent breast-conserving surgery. In total, 1222 patients had a median follow-up of 5 years and all patients had a median follow-up of 2 years 5 months, giving 9491 women-years of follow-up.
The trial patients had a generally good prognosis but a substantial number (> 1200) were aged ≤ 60 years and > 500 were node positive and/or grade 3.
In total, 91% of patients had a sentinel node biopsy, 90% of whom had < 10 nodes removed if this was negative. Eleven patients had axillary recurrence, one of whom had previously undergone axillary clearance with the other 10 having a negative sentinel node biopsy. The risk of axillary recurrence was similar whether the patients received EBRT [6/1762, 5-year risk 0.82% (95% CI 0.34% to 2.02%)] or not [5/1613, 5-year risk 0.68% (95% CI 0.28% to 1.6%); HR 0.84 (95% CI 0.26 to 2.74); p = 0.8]. The results were similar if only patients with one or two positive axillary nodes were included (EBRT 1/255 vs. no EBRT 0/127) (Figures 25 and 26).
FIGURE 25.
Numbers of patients and axillary recurrences in the EBRT and no EBRT groups (non-randomised comparison).

FIGURE 26.
Kaplan–Meier plot for axillary recurrence in patients who were given EBRT and patients who were not given EBRT.
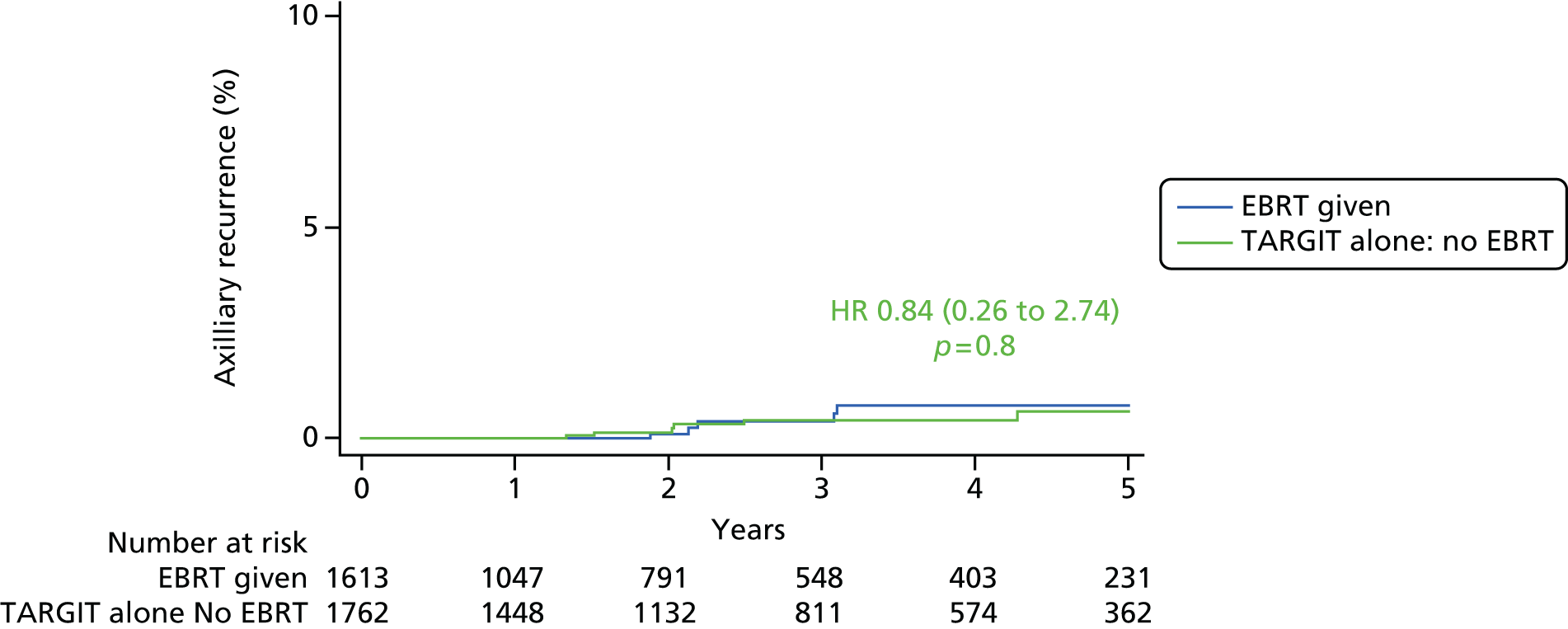
Conclusion
Omitting whole-breast radiotherapy after a sentinel node biopsy in this good-prognosis population was not associated with an increased axillary recurrence rate.
Reduction in non-breast-cancer mortality as a beneficial effect of targeted intraoperative radiotherapy
Background
The TARGIT-A randomised trial tested the outcomes of breast cancer treatment between traditional EBRT given over several weeks versus a risk-adapted approach using single-dose TARGIT. In the prepathology stratum TARGIT was given concurrently with lumpectomy. If on postoperative histopathological examination of the operative specimen any unsuspected high-risk factors were found, EBRT was added in 15–20% of cases. The main results showed that for the for local control, compared with EBRT, TARGIT achieved non-inferior local control in all cases and identical local control when given concurrently with lumpectomy in ER-positive, PgR-positive cases. Interestingly, the patients in the TARGIT arm had a significantly lower risk of dying from non-breast-cancer causes such as heart attacks and other cancers. We investigated whether or not this difference could be fully explained by the avoidance of the effects of EBRT on the heart and other organs in the TARGIT arm.
These data were first presented at the March 2013 St Gallen Symposium (poster presentation) and in September 2013 at the ASTRO Annual Meeting (poster presentation). 69,70
Methods
-
We compared cardiac deaths for left- and right-sided breast cancer.
-
We estimated cardiac deaths based on age, sex and follow-up.
-
We compared survival between the two randomised arms in the prepathology stratum, but limited to those who had received EBRT. Therefore, the control arm included patients who were allocated EBRT and the experimental arm included patients who were allocated TARGIT and received EBRT in addition; because both groups received EBRT, any difference that was found between the groups would be attributable to TARGIT (Figure 27).
FIGURE 27.
Non-randomised comparison between those who received TARGIT + EBRT and those who received EBRT for deaths from non-breast-cancer causes: (a) schema of the analysed cohorts of patients; and (b) Kaplan–Meier plot depicting deaths from causes other than breast cancer in prepathology patients who received EBRT – non-randomised comparison.
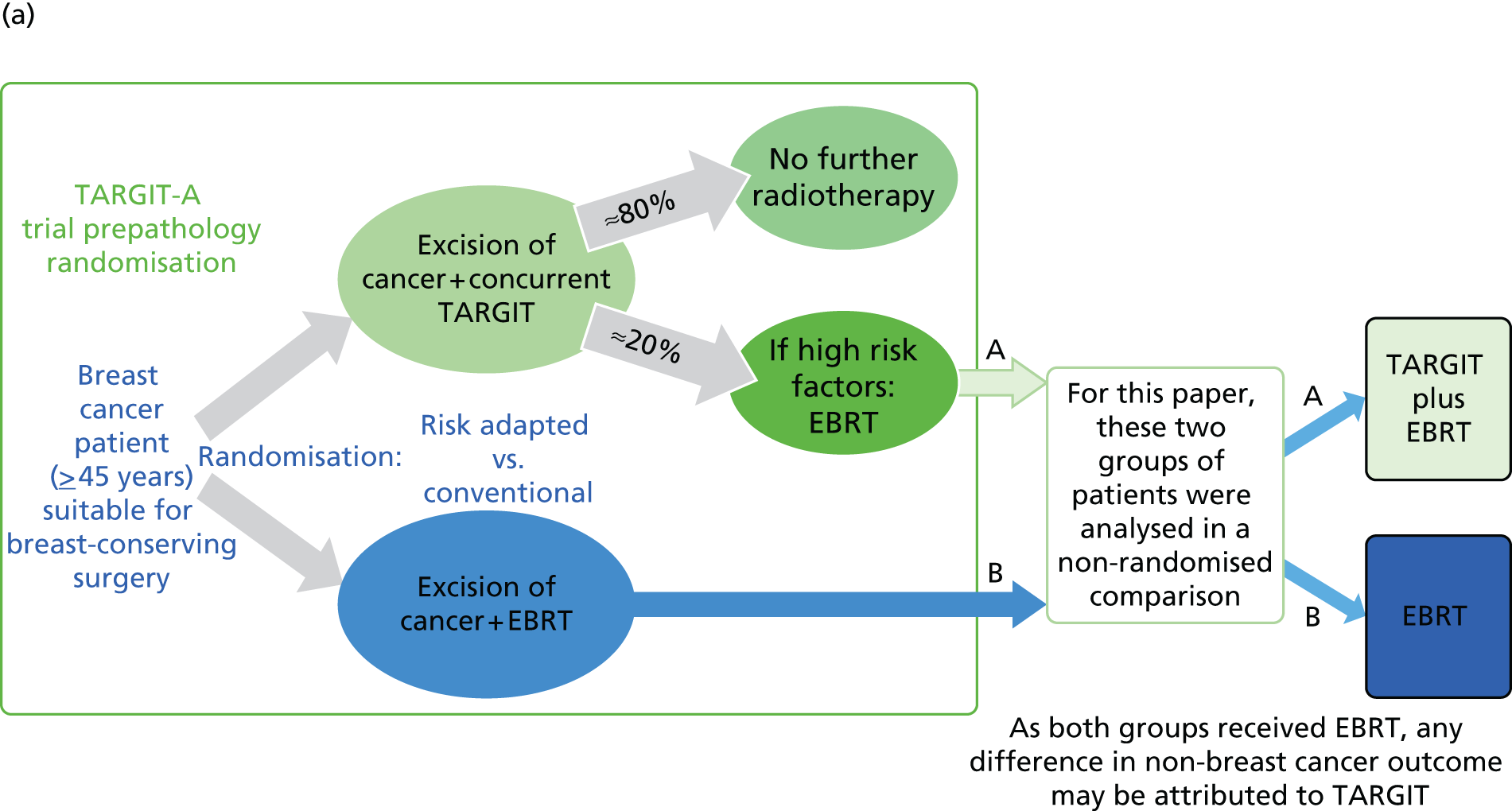
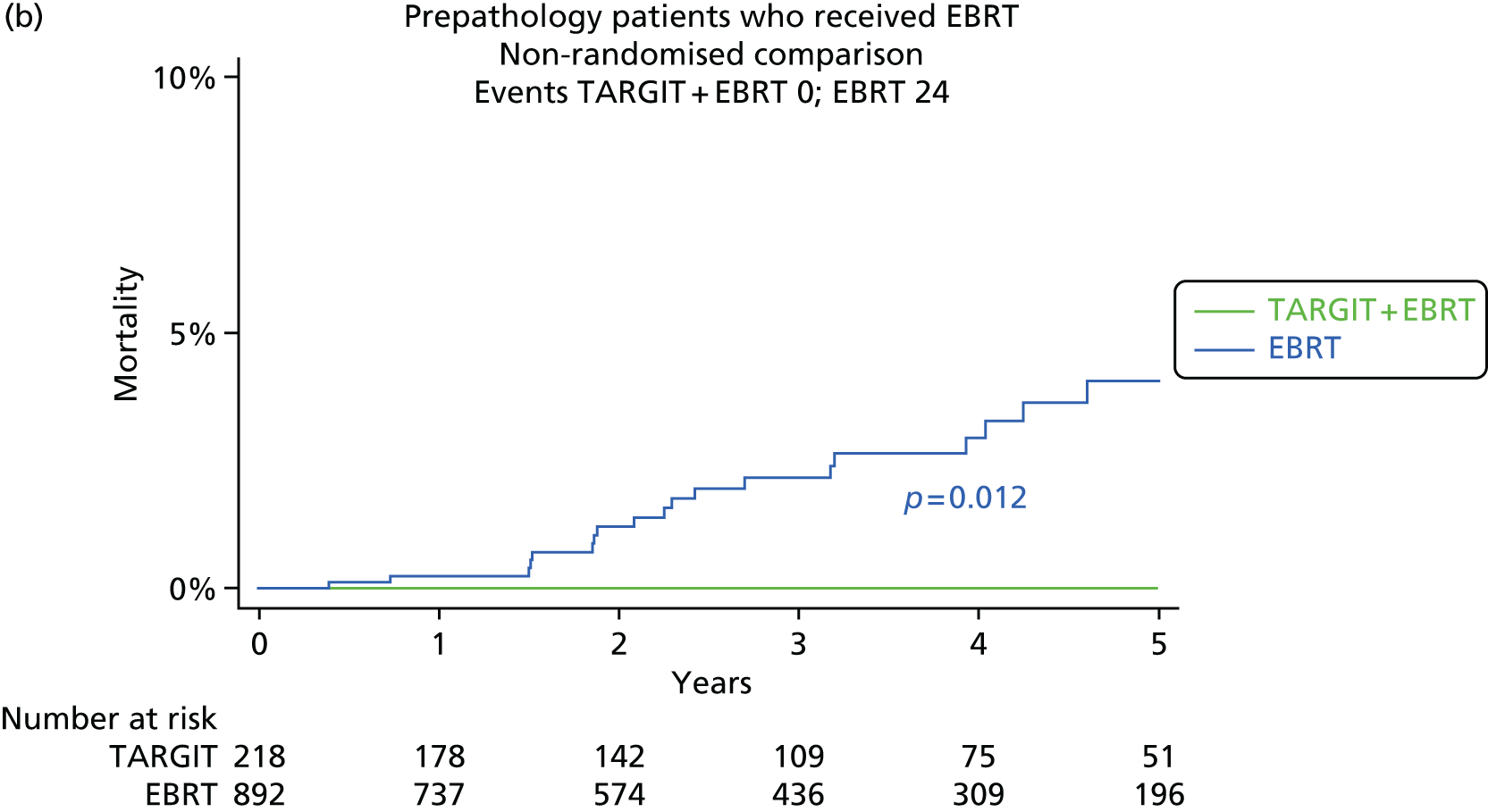
Results
In the whole trial there was a highly significant reduction in non-breast-cancer mortality in the TARGIT arm (HR 0.47; p = 0.0086):
-
There were two cardiac deaths in the TARGIT arm and eight in the EBRT arm, with similar numbers in each arm for left- and right-sided breast cancer (1/775 vs. 4/795 for left; 1/776 vs. 4/746 right).
-
Among the 1730 patients randomised to receive EBRT, the number of cardiac deaths (n = 8) was not higher than the 12 estimated based on age, sex and follow-up period.
-
There were no deaths from non-breast-cancer causes in the TARGIT + EBRT group compared with 24 in the EBRT group. Thus, there were significantly fewer deaths in those patients who received TARGIT (0/218 vs. 24/892; log-rank p = 0.012) (see Figure 27).
Conclusion
One might expect irradiation for left-sided cancers to result in higher cardiac toxic effects. However, the ratio of cardiac risk of left- to right-sided cancers is small (1.34 according to Darby et al. 71). Furthermore, Darby et al. 71 recorded no significant effect of laterality on cardiac toxic effects per Gy. With modern radiotherapy designed to reduce the cardiac dose, the absolute difference in cardiac deaths between those with left-sided breast cancer and those with right-sided breast cancer is likely to be even lower and undetectable with few events. Note that an absence of an effect of laterality on cardiac deaths cannot be used to imply that there is no cardiac toxicity, because the difference in the effect between the two sides is not likely to be large. Finally, with eight cardiac deaths a ratio of 1.34 would not be detectable (e.g. it would need to be distributed as 3.5 vs. 4.7, with even 3 vs. 5 already being a ratio of 1.7). Therefore, an absence of a difference between the left and the right sides should not be interpreted as an absence of cardiac toxicity.
A risk similar to that in the age-matched population should be interpreted with the knowledge that patients in clinical trials are generally in better general health than the normal population and we should therefore have expected a lower risk of cardiac mortality in the trial population.
The most interesting finding was that there were significantly fewer deaths among patients who were allocated to TARGIT + EBRT compared with those allocated to EBRT. In this comparison the only difference between the two groups was the delivery of TARGIT in one group, because both groups received EBRT, albeit with a slightly higher dose – the boost – in the EBRT group. It suggests that the difference in non-breast-cancer mortality could arise from an increase in mortality from EBRT toxicity, as one would think conventionally, as well as a reduction in mortality because of a potential beneficial effect of TARGIT. The latter is a radically new hypothesis that the local effect of TARGIT on the tumour bed by inhibiting cancer-stimulating cytokines17 may spill over to reduce the systemic inflammatory response to trauma and have significant long-term systemic beneficial effects that might be protective against cardiac and cancer mortality (Figure 28). This remarkable finding, albeit a non-randomised comparison, could potentially have far-reaching implications. It is fortuitous that it will be tested in the HTA programme-funded TARGeted Intraoperative radioTherapy Boost (TARGIT-B) randomised trial (ISRCTN43138042), which will compare TARGIT boost with EBRT boost, with both randomised arms receiving whole-breast radiotherapy (Figure 29).
FIGURE 28.
Targeted intraoperative radiotherapy abrogates the tumour-stimulating effect of surgical wound fluid. AgRP, Agouti-related protein; EGFR, epidermal growth factor receptor; FAS/TNFRSF6, FAS receptor or tumour necrosis factor receptor superfamily member 6; FGF-4, fibroblast growth factor 4; Flt-3, Fms-like tyrosine kinase 3; G-CSF, granulocyte colony-stimulating factor; GRO, growth-regulating oncogene; HGF, hepatocyte growth factor; IGFBP-6, insulin-like growth factor-binding protein 6; IL, interleukin; MCP-1/-2, monocyte chemoattractant protein 1/2; Mip-1a/1d, macrophage inflammatory protein 1a/1d; PDGF-BB, platelet-derived growth factor BB; RANTES, regulated on activation, normal T cell expressed and secreted; sTNFR-I/-II, soluble tumour necrosis factor receptor I/II; Tie-1/-2, tyrosine kinase containing immunoglobulin and epidermal growth factor homology domain-1 and 2; uPAR, urokinase-type plasminogen activator receptor; VEGF-R3, vascular endothelial growth factor receptor 3.

FIGURE 29.
Could TARGIT be protective against heart attacks and deaths from other cancers? This hypothesis will be tested in the TARGIT-B trial.
Adequacy of 1 mm as the threshold for a negative margin: the policy of adding external beam radiotherapy to targeted intraoperative radiotherapy when the tumour-free margin is < 1 mm (rather than 10 mm) is appropriate – data from the TARGIT-A trial
Background
In the TARGIT-A trial protocol,36 a positive margin leads to a recommendation of additional EBRT after TARGIT. The core protocol defined 1 mm as an adequate tumour-free margin but individual centres were allowed to pre-specify a wider margin. In all German centres EBRT was recommended after TARGIT if the margin was < 10 mm. We analysed the impact of such a decision on treatment received and local recurrence.
Methods
All German cases were planned to be treated in the prepathology stratum, that is, randomisation before excision of the tumour and TARGIT delivered during lumpectomy.
Therefore, we analysed in the prepathology stratum the proportion of cases in whom additional EBRT was actually given after TARGIT, as well as the primary (local recurrence in the conserved breast) and secondary (breast cancer and non-breast-cancer mortality) outcomes. We compared these values for the German cohort with the equivalent values for the rest of the trial population in a post hoc exploratory non-randomised analysis.
Results
Additional EBRT was given in nearly twice the number of patients in the TARGIT arm in the German centres as in the TARGIT arm in the rest of the trial population [31.3% (96/306) vs. 17.4% (123/706)]. However, the 5-year local recurrence rate in the German cohort was the same as in rest of the trial population [German 2.6% (95% CI 0.87% to 7.8%) vs. rest of the trial population 1.9% (95% CI 0.81% to 4.5%)]. Furthermore, there was no significant difference between the German cohort and rest of the trial population in breast cancer mortality [2.5% (95% CI 0.64% to 9.7%) vs. 3.3% (95% CI 1.7% to 6.4%)] or non-breast-cancer mortality [0.78% (95% CI 0.11% to 5.4%) vs. 1.7% (0.8% to 3.7%)].
Conclusion
The greater use of additional EBRT as seen in the German cohort prompted by the higher limit of a 10-mm tumour-free margin may not be necessary and a policy of adding EBRT after TARGIT when the margin is < 1 mm appears to be appropriate.
Chapter 6 Cost–utility analysis of external beam radiotherapy compared with targeted intraoperative radiotherapy in breast cancer
Background
There is limited evidence about the cost-effectiveness of TARGIT. Picot et al. 72 recently undertook a systematic review of published economic evaluations and found two primary studies. 73,74 Both were modelling studies using aggregate data from the TARGIT-A trial supplemented with data from other sources. Alvarado et al. 73 found that TARGIT was less costly and produced more quality-adjusted life-years (QALYs) than EBRT and concluded that TARGIT was the dominant strategy. Based on the results of a cost-minimisation analysis,72 TARGIT was associated with substantial cost savings compared with whole-breast irradiation delivered using three-dimensional conformal radiotherapy or accelerated PBI delivered with intensity-modulated radiotherapy. Both studies were based in the USA and because of differences in treatment practices and patients the results are unlikely to be applicable to the UK.
Picot et al. 72 undertook a UK-based cost–utility analysis of TARGIT using data from the TARGIT-A trial supplemented with data from other sources. They found that TARGIT was less costly than EBRT and also less effective, producing fewer QALYs. This is more relevant than the studies by Alvarado et al. 73 and Shah et al. 74 because it is a UK-based study, but it is a modelling study using aggregate data from the TARGIT-A trial. Hence, we undertook a cost–utility analysis of TARGIT compared with EBRT using patient-level data from the TARGIT-A trial.
Methods
Patients
The analysis was based on costs and outcomes for the 817 patients randomised in the ‘earliest cohort’ in the prepathology stratum of the TARGIT-A trial. Several issues were considered when deciding which cohort of patients to include in the cost–utility analysis:
-
We did not include the postpathology stratum from the earliest cohort because the results in this group were less favourable than those of the prepathology stratum. Hence, it is highly unlikely that TARGIT would be adopted in clinical practice for this group. As patients from this stratum were not included in the analysis, the results cannot be applied to this group.
-
The number of participants needed to prove non-inferiority was calculated to be 585 and therefore the earliest cohort of 817 patients had enough power to draw reliable conclusions.
-
The earliest cohort was randomised between 2000 and 2008 and the average follow-up was 5 years, permitting a reasonable follow-up period without a large number of missing data. The complete prepathology stratum from TARGIT-A consisted of 2298 patients, with an average follow-up of 2 years 4 months. Hence, by including the full cohort we would have substantially increased the proportion of missing data in the sample if we wanted to use a 5-year time horizon or we would have had to use a shorter time horizon.
We therefore balanced the number of patients in the whole cohort compared with the number in the earliest cohort against the duration of follow-up in the two cohorts against the fact that the earliest cohort had enough statistical power to draw reliable conclusions and decided to base our analysis on the 817 patients randomised in the earliest cohort of the TARGIT-A trial in the prepathology stratum. In this cohort, as in the mature cohort in the prepathology stratum and all patients in the prepathology stratum, TARGIT was non-inferior to EBRT with respect to local recurrence and the 5-year estimated risks of local recurrence were not statistically different between the treatment groups.
Overview of the cost–utility analysis
We undertook a cost–utility analysis to compare the costs and outcomes associated with TARGIT compared with EBRT in the prepathology stratum of the TARGIT-A trial. The outcome measure was QALYs, which combine length of life and quality of life, consistent with NICE guidelines. 75 Cost-effectiveness was expressed as incremental net monetary benefits. 75 The analysis took a UK NHS and personal social services (PSS) perspective. 75 Resource use data were included from all participating centres and UK unit costs were applied. Costs are presented in 2013/14 UK pounds. The time horizon was 5 years, reflecting the average follow-up in the earliest cohort in the prepathology stratum of the TARGIT-A trial. Extrapolation beyond the end of the trial using decision-analytical modelling was not undertaken because the within-trial analysis found no evidence of significant differences in QALYs between the groups. This probably reflects the main finding from the TARGIT-A trial that TARGIT was non-inferior to EBRT with regard to local recurrence. Although there was some evidence of differences in costs, these differences were accrued during the first year, with no evidence of significant differences in costs beyond the first year. Hence, the 5-year time horizon was long enough to reflect all important differences in costs or outcomes between the two treatments. An annual discount rate of 3.5% was applied to costs and outcomes. 75
Resource use and costs
Cost components
We calculated the costs incurred by every patient during the 5-year time horizon using resource use and event data collected prospectively in the trial. The following costs were included: TARGIT, EBRT, index procedure, additional procedures, chemotherapy, mastectomy, complications, recurrence-free survival, local recurrence, distant recurrence, breast cancer deaths and non-breast-cancer deaths. Unit costs were obtained from published sources72,76–80 (Table 12), inflated when appropriate82 and multiplied by resource use. Annual costs were calculated for every patient for each year of the 5-year time horizon. These were discounted and summed across all 5 years to calculate total costs per patient over the whole period.
| Cost item | Unit costa | Notes/source |
|---|---|---|
| TARGIT | £1882 per patient | Picot et al.72 Base-case value calculated assuming 126 procedures performed per year and a 10-year lifetime of the INTRABEAM device |
| EBRT | £123 per fraction | Department of Health76 – Currency code SC23Z. Outpatients |
| £769 for planning meeting | Department of Health76 – Currency code SC52Z. Outpatients | |
| EBRT boost | £126 per fraction | Department of Health76 – Currency code SC23Z. Outpatients |
| £769 for planning meeting | Department of Health76 – Currency code SC52Z. Outpatients | |
| NHS patient transport | £68 per return journey | Department of Health77 – Currency code PTS. Outpatients |
| Index procedure | £1128 per procedure | Department of Health76 – Currency code JA09G. Combined day case/ordinary elective spell |
| Overnight stay related to index procedure | £227 per additional overnight stay | Department of Health76 – Currency code JA09G. Long stay for days exceeding time point |
| Mastectomy with breast reconstruction | £6504 per procedure | Department of Health76 – Currency code JA16Z. Combined day case/ordinary elective spell |
| Additional procedures (excision of positive margins, axillary dissection or clearance) | £1128 per procedure | Department of Health76 – Currency code JA09G. Combined day case/ordinary elective spell |
| Chemotherapy | £2087 per course of treatment | NICE78 – docetaxel infusion every 21 days (six cycles). Department of Health76 – Currency code first attendance SB14Z, subsequent attendance SB15Z. Outpatient |
| Complications | ||
| Surgical evacuation of haematoma | £362 per procedure | Department of Health76 – Currency Code JA12C. Day case |
| Aspiration for seroma | £397 per complication | Department of Health76 – treatment function 370, consultant led WF01B for first and WF01A for second and third aspirations. Only applied each time three aspirations of seroma noted. Outpatient |
| Wound infection requiring oral antibiotics | £2.60 per course of treatment | British National Formulary79 – treatment using flucloxacillin 500 mg, £2.60 for a 28-tablet pack (1 week) |
| Wound infection requiring intravenous antibiotics | £362 per course of treatment | Department of Health76 – Currency code JA12C. Day case |
| Skin breakdown/delayed wound healing | £29 per course of treatment | British National Formulary79 – soft non-woven dressing impregnated with Intrasite® (Smith & Nephew, London, UK) gel, 10 cm × 10 cm, £1.70 (2 weeks) = £23.80 plus flucloxacillin 500 mg, £2.60 for a 28-tablet pack (2 weeks) = £5.20 |
| RTOG toxicity grade 3 | £5 per course of treatment | British National Formulary79 – aqueous cream 500 g |
| RTOG toxicity grade 4 | £23.80 per course of treatment | British National Formulary79 – soft non-woven dressing impregnated with Intrasite® gel, 10 cm × 10 cm, £1.70 (2 weeks) = £23.80 |
| Pain in the irradiated field | £3 per course of treatment | British National Formulary79 – paracetamol 500 mg, 100-tablet pack |
| Events | ||
| Recurrence free | £1057 per year | Hind et al.81 – one oncologist visit per year for 5 years, one mammogram per year for 5 years, 5 years of anstrozole/tamoxifen (70 : 30) hormonal therapy |
| Local recurrence | Mean £4956 (SD £3953) per recurrence | Mean (SD) from patient-level costing |
| Distant recurrence | £1040 per month | Hind et al.81 – monthly cost of supportive care for metastatic breast cancer |
| Breast cancer death | £3659 per death | Hind et al.81 – cost of death from breast cancer |
| Non-breast-cancer death | £3659 per death | Hind et al.81 – assumed to be the same as cost of death from breast cancer |
Targeted intraoperative radiotherapy
A fixed cost per patient was assumed for TARGIT, based on recently published calculations by Picot et al. 72 This cost includes one-off capital costs and annual maintenance costs associated with the INTRABEAM device; one-off, annual and per-treatment costs requiring additional staff resources; the cost of consumables required for each use of the device; and the cost of additional operating theatre time for each use of the device. The capital and one-off costs were annualised using a device lifetime of 10 years. These costs and the annual costs were assigned to individual treatments assuming that each device was used to undertake 126 procedures per year. On this basis Picot et al. 72 calculate the unit cost per patient to be £1882 (2013/14 prices), which is the value that we used in our analysis for the base case. This was varied in sensitivity analysis.
External beam radiotherapy
Patient-level data were collected in the TARGIT-A trial on the number of fractions of EBRT received by each patient. A proportion of patients randomised to TARGIT also received EBRT and these were also included in the analysis. The mean [standard deviation (SD)] number of fractions given to patients in the trial who received EBRT was 23 (5). This is higher than current recommendations stating that 15 fractions are required to complete a course of treatment for patients with early invasive breast cancer after breast-conserving surgery or mastectomy. 78 In our base case we therefore assumed that all patients in the TARGIT-A trial who received EBRT received a fixed number of 15 fractions. We applied a unit cost per fraction plus a one-off cost for a planning meeting (see Table 12). In sensitivity analyses we estimated cost-effectiveness based on the actual number of fractions of EBRT received in the trial.
Standard treatment of breast cancer includes an EBRT boost as part of the course of whole-breast radiotherapy; however, it is sometimes omitted in patients at a lower risk of local recurrence. 83–85 In the TARGIT-A trial, patient-level data were also recorded on whether or not patients received an EBRT boost and if so the number of fractions received. These were included in our base case. We applied a unit cost per fraction plus a one-off cost for an additional planning meeting (see Table 12). In sensitivity analysis we estimated cost-effectiveness assuming no EBRT boost.
External beam radiotherapy requires several trips to hospital for treatment, incurring time and travel costs for patients and their families. Our analysis was undertaken from a NHS and PSS perspective and so we did not include these costs. However, some patients use NHS patient transport to travel to hospital for EBRT, which is a cost incurred by the NHS. We were unable to find any pre-existing evidence on the proportion of EBRT patients who use NHS patient transport and so we undertook a short survey at two sites. The first site was Great Western Hospital in Swindon, where patients receiving EBRT typically travel to radiotherapy centres at the John Radcliffe Hospital, Oxford, the Royal United Hospital, Bath, or Cheltenham General Hospital for treatment. The second was Princess Alexandra Hospital, Harlow, where patients typically travel to North Middlesex Hospital, Enfield, for treatment. Patients were asked to indicate their method of transport to the radiotherapy centre, with possible responses being by car, by hospital transport or by public transport. We received 37 responses (17 from patients at Great Western Hospital and 20 from patients at Princess Alexandra Hospital), with five (13.5%) patients reporting using hospital transport. In our base case we therefore assumed that 13.5% of patients receiving EBRT use NHS patient transport over the course of their treatment and applied a unit cost per return journey (see Table 12). We varied the proportion of patients using NHS patient transport to travel to hospital for EBRT in sensitivity analysis.
Other cost components
The cost of the index procedure included the cost of the lumpectomy procedure itself plus the cost of any associated hospital stay, which was recorded in the trial. Any additional procedures related to excision of margins or axillary dissection and/or clearance were recorded, as well as whether or not the patient received chemotherapy and had a mastectomy. For the index procedure, additional procedures and mastectomies, unit costs based on NHS reference costs76 were applied. Costs for a course of chemotherapy were based on current treatment recommendations. 78
Data were recorded in the trial on the number of the following complications: haematoma requiring surgical evacuation; seroma requiring three or more aspirations; infection requiring oral or intravenous antibiotics or surgical intervention; skin breakdown or delayed wound healing; and RTOG toxicity of grade 3 or 4. Details were recorded on how each individual complication was treated and these were costed separately and included in the analysis (see Table 12).
We included the costs of remaining recurrence free, local recurrence, distant recurrence, breast cancer death and non-breast-cancer death. Unit costs were taken from a published source81 and applied to patient-level data from the trial. Treatments for local recurrence were recorded in the trial and were costed on an individual patient basis. Treatments for local recurrence included mastectomy, TARGIT, EBRT, hormone therapy and chemotherapy. The mean (SD) cost per patient of local recurrence was £4956 (£3953; see Table 12).
Utilities and quality-adjusted life-years
The outcome measure in our cost–utility analysis was QALYs, which combine length of life and quality of life, the latter being measured by utility scores. A utility score of 1 represents full health and a score of 0 denotes death; negative values represent states worse than death.
Utility data were not collected in the TARGIT-A trial. Patient-level data on the timing of events were collected and for every patient we created a data set describing the health state that they were in during every day of the 5-year time horizon. Utility values from published sources were then applied to each health state. These were used to construct five 1-year utility profiles for every patient covering the 5-year time horizon. QALYs for every patient for each year were calculated as the area under the utility profile for that year. These were discounted and summed across all 5 years to calculate QALYs per patient over the whole period.
The health states included in the cost–utility analysis were recurrence free, local recurrence, distant recurrence, breast cancer death and non-breast-cancer death. A review of the Cost-Effectiveness Analysis Registry86 was undertaken using the search term ‘breast cancer’ to identify studies reporting relevant utility scores and 1291 results (utility scores) were identified. Picot et al. 72 recently undertook an extensive literature search of studies providing utility values for such patients and identified nine suitable studies. The criteria for the values that they selected in their analysis were that they would ideally be based on EQ-5D scores, would ideally have been derived from UK patients and these patients would ideally reflect the younger age range of patients in the TARGIT-A trial. The values that they selected, from studies by Turnbull et al. 87 and Lidgren et al. ,88 were as follows:
-
recurrence free in first year: 0.7728
-
recurrence free after first year: 0.8112
-
local recurrence: 0.8112
-
recurrence free after local recurrence: 0.8112
-
distant recurrence: 0.658.
We used these values in our base case. The values imply that the utility associated with local recurrence is the same as the utility associated with being recurrence free after the first year and the utility associated with being recurrence free after local recurrence. We undertook a sensitivity analysis using values from an alternative study by Hayman et al. ,89,90 which have been used in previous studies, as follows:
-
recurrence free: 0.92
-
local recurrence: 0.87
-
recurrence free after local recurrence: 0.92
-
distant recurrence: 0.70.
Patients who died in the TARGIT-A trial (either from breast cancer or from other causes) were assigned a utility value of 0 at their date of death until the end of the 5-year time horizon.
In the cost–utility analysis we did not incorporate utility losses associated with additional procedures, chemotherapy, mastectomy or complications. Given the low incidence of these events, that they were evenly distributed between treatment groups and that the time period affected is likely to be short, this is unlikely to affect the QALYs associated with each treatment group. We also did not include any utility losses associated with EBRT. Therefore, this would make our estimates more conservative because such an omission would work against TARGIT.
Missing data
There were some missing data on patient follow-up, meaning that for some patients we did not know whether or not they had experienced events. This affected both the total costs incurred by each patient and the total QALYs. Multiple imputation was used to impute missing data separately for costs in years 1–5, total costs, QALYs in years 1–5 and total QALYs. The following variables were included in the imputation models as additional explanatory variables: cost of EBRT, cost of the index procedure, cost of additional procedures, cost of chemotherapy, cost of mastectomy, whether or not the patient had each type of complication, age at randomisation, tumour size in millimetres at randomisation, ER status at randomisation, PgR status at randomisation, contralateral cancer or not, whether the cancer was screen detected or not, study centre, year of randomisation and treatment allocation. We used multivariate normal regression to impute missing values and generated 20 imputed data sets. We repeated the multiple imputation several times using different random number seeds to investigate whether or not the conclusions of the analysis changed.
Statistical methods
Mean costs, outcomes and net monetary benefits were compared between all patients randomly assigned to EBRT and TARGIT, irrespective of which treatment was administered and whether or not patients received additional therapies of either type. We calculated differences in mean costs and QALYs and incremental net monetary benefits between groups. Net monetary benefits for EBRT and TARGiT were calculated as the mean QALYs per patient multiplied by the maximum willingness to pay for a QALY minus the mean cost per patient. Incremental net monetary benefits were calculated as the difference in mean QALYs per patient with TARGIT compared with EBRT multiplied by the maximum willingness to pay for a QALY minus the difference in mean costs per patient. We used the cost-effectiveness threshold range recommended by NICE of £20,000–30,00075 as the lower and upper limits of the maximum willingness to pay for a QALY. If the incremental net monetary benefit is positive (negative) then TARGIT (EBRT) is preferred on cost-effectiveness grounds. The QALYs gained and incremental costs were adjusted for age at randomisation, tumour size in millimetres at randomisation, ER status at randomisation, PgR status at randomisation, contralateral cancer or not, whether the cancer was screen detected or not, study centre and year of randomisation. For each of the 20 imputed data sets we ran 1000 bootstrap replications and combined the results using equations described by Briggs et al. 91 to calculate standard errors (SEs) around mean values accounting for uncertainty in the imputed values, the skewed nature of the cost data and utility values and sampling variation. SEs were used to calculate 95% CIs around point estimates. A similar analytical approach has been used previously. 92
Sensitivity analyses
We undertook deterministic sensitivity analyses to evaluate the impact of uncertainty in the following components. In each case the changes made were applied one at a time to the base case.
-
No adjustment for age at randomisation, tumour size in millimetres at randomisation, ER status at randomisation, PgR status at randomisation, contralateral cancer or not, whether the cancer was screen detected or not, study centre and year of randomisation.
-
Complete case analysis without imputing missing values.
-
Complete case analysis without imputing missing values plus with no adjustment for age at randomisation, tumour size in millimetres at randomisation, ER status at randomisation, PgR status at randomisation, contralateral cancer or not, whether the cancer was screen detected or not, study centre and year of randomisation.
-
EBRT costs based on number of fractions received in the trial [mean (SD) number of fractions administered per patient who received EBRT in the trial was 23 (5)].
-
No EBRT boost.
-
Costs of EBRT per fraction of £101 and £154, based on the lower and upper values of the IQR of the NHS reference costs. 76
-
Costs of TARGIT of £1300, £1500, £1700, £1900, £2100, £2300, £2500 and £2700. The value of £1300 corresponds to the minimum value in Picot et al. ,72 in which the capital and one-off costs were annualised using a device lifetime of 10 years and these costs and the annual costs were assigned to individual treatments assuming that each device was used to undertake 631 procedures per year. The value of £2500 corresponds to the maximum value in Picot et al. ,72 with a device lifetime of 5 years and 100 procedures per year.
-
Percentage of patients using NHS transport for EBRT of 0% (no patients use NHS transport) and 30%.
-
Health states valued using utilities from Hayman et al. 89,90
A cost-effectiveness acceptability curve93 showing the probability that TARGIT was cost-effective compared with EBRT at a range of values for the maximum willingness to pay for a QALY was generated based on the proportion of the bootstrap replications across all 20 imputed data sets with positive incremental net monetary benefits. 94 The probability that TARGIT was cost-effective at a maximum willingness to pay for a QALY of £20,000 and £30,000 was reported, based on the proportion of bootstrap replications with positive incremental net monetary benefits at these values.
Results
Resource use and costs
In total, 15.2% of patients randomised to TARGIT also received EBRT (Table 13). We assumed that every patient receiving EBRT received 15 fractions. In total, 38% of patients randomised to EBRT also received an EBRT boost [mean (SD) 5 (2) fractions]. We assumed that 13.5% of all EBRT patients used NHS transport to travel to hospital for their radiotherapy treatment. The mean (median) number of nights in hospital for the initial procedure was 4 (3) for both TARGIT and EBRT patients. A total of 19% of EBRT patients received additional procedures, compared with 12% of TARGIT patients. In total, 20% of EBRT patients received chemotherapy and 4% had a mastectomy; for TARGIT the figures were 23% and 3%, respectively. The incidence of complications was low in both treatment groups. The number of events for TARGIT and EBRT were local recurrences (6 vs. 3), distant recurrences (21 vs. 18), breast cancer deaths (13 vs. 11) and non-breast-cancer deaths (7 vs. 18).
| Resource | EBRT (n = 416) | TARGIT (n = 401) |
|---|---|---|
| EBRT, % | 100 | 15.2 |
| Fractions of EBRT, n | 15 | 15 |
| EBRT boost, % | 38 | 0 |
| Boost fractions, mean (SD) | 5 (2) | – |
| NHS transport for EBRT, % | 13.5 | 13.5 |
| Index procedure, number of nights | ||
| Mean (SD) | 4 (4) | 4 (4) |
| Median (IQR) | 3 (1–6) | 3 (1–6) |
| Additional procedures, n (%) | ||
| 0 | 338 (81) | 353 (88) |
| 1 | 72 (17) | 46 (11) |
| 2 | 5 (1) | 1 (0) |
| 3 | 1 (0) | 1 (0) |
| Chemotherapy, n (%) | 84 (20) | 93 (23) |
| Mastectomy, n (%) | 17 (4) | 13 (3) |
| Complications, n (%) | ||
| Haematoma requiring surgical evacuation or seroma needing three or more aspirations | 1 (0) | 1 (0) |
| Infection requiring oral or intravenous antibiotics or surgical intervention | 5 (1) | 5 (1) |
| Skin breakdown or delayed wound healing, number (%) | 3 (1) | 2 (0) |
| RTOG toxicity grade 3 or 4 | 9 (2) | 2 (0) |
| Events, n | ||
| Local recurrence | 3 | 6 |
| Distant recurrence | 18 | 21 |
| Breast cancer death | 11 | 13 |
| Non breast cancer death | 18 | 7 |
Accounting for missing data using multiple imputation, mean total costs per patient (95% CI) were £11,840 (£11,422 to £12,259) in the EBRT group (n = 416) and £11,404 (£10,800 to £12,008) in the TARGIT group (n = 401; Table 14). The mean radiotherapy cost per patient (summing the cost of TARGIT plus EBRT plus EBRT boost plus NHS transport for EBRT) was £3373 in the EBRT group and £2307 in the TARGIT group. Other costs were similar for EBRT and TARGIT. Values were similar for complete cases (Table 15).
| Variable | EBRT (n = 416) | TARGIT (n = 401) | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Costs (£)a | ||||
| TARGIT | 0 | b | 1882 | b |
| EBRT | 2659 | b | 405 | b |
| EBRT boost | 557 | 487 to 628 | 0 | b |
| NHS transport for EBRT | 157 | 154 to 160 | 21 | b |
| Total EBRT | 3373 | 3300 to 3447 | 425 | b |
| Total EBRT plus TARGIT | 3373 | 3300 to 3447 | 2307 | b |
| Index operation | 2069 | 1986 to 2153 | 2101 | 2009 to 2193 |
| Additional procedures | 230 | 182 to 279 | 143 | 103 to 184 |
| Chemotherapy | 421 | 341 to 502 | 484 | 397 to 571 |
| Mastectomy | 266 | 142 to 390 | 211 | 98 to 324 |
| Costs associated with health statusc | ||||
| Year 1 | 1099 | 1063 to 1135 | 1124 | 1052 to 1196 |
| Year 2 | 1176 | 1073 to 1278 | 1254 | 1123 to 1384 |
| Year 3 | 1126 | 1014 to 1237 | 1265 | 1108 to 1423 |
| Year 4 | 1059 | 945 to 1173 | 1269 | 1099 to 1438 |
| Year 5 | 1020 | 879 to 1161 | 1246 | 1072 to 1420 |
| Total costs | 11,840 | 11,422 to 12,259 | 11,404 | 10,800 to 12,008 |
| QALYs | ||||
| Year 1 | 0.810 | 0.808 to 0.8120 | 0.811 | 0.810 to 0.811 |
| Year 2 | 0.774 | 0.766 to 0.781 | 0.777 | 0.772 to 0.782 |
| Year 3 | 0.728 | 0.714 to 0.742 | 0.738 | 0.727 to 0.749 |
| Year 4 | 0.695 | 0.679 to 0.710 | 0.704 | 0.691 to 0.717 |
| Year 5 | 0.657 | 0.640 to 0.674 | 0.674 | 0.660 to 0.689 |
| Total QALYs | 3.663 | 3.614 to 3.713 | 3.704 | 3.664 to 3.744 |
| Net monetary benefits (£) | ||||
| £20,000 | 61,426 | 60,299 to 62,544 | 62,678 | 61,542 to 63,762 |
| £30,000 | 98,059 | 96,470 to 99,644 | 99,720 | 98,228 to 101,147 |
| Variable | EBRT | TARGIT | ||||
|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | |
| Costs (£)a | ||||||
| TARGIT | 0 | 0 | 416 | 1882 | 0 | 401 |
| EBRT | 2659 | 0 | 416 | 405 | 0 | 401 |
| EBRT boost | 557 | 733 | 416 | 0 | 0 | 401 |
| NHS transport for EBRT | 157 | 28 | 416 | 21 | 0 | 401 |
| Total EBRT | 3373 | 760 | 416 | 425 | 0 | 401 |
| Total EBRT plus TARGIT | 3373 | 760 | 416 | 2307 | 0 | 401 |
| Index operation | 2069 | 865 | 416 | 2101 | 937 | 401 |
| Additional procedures | 230 | 506 | 416 | 143 | 409 | 401 |
| Chemotherapy | 421 | 839 | 416 | 484 | 882 | 401 |
| Mastectomy | 266 | 1289 | 416 | 211 | 1153 | 401 |
| Costs associated with health statusb | ||||||
| Year 1 | 1099 | 347 | 406 | 1125 | 730 | 393 |
| Year 2 | 1184 | 1040 | 395 | 1261 | 1325 | 388 |
| Year 3 | 1129 | 1130 | 387 | 1264 | 1600 | 382 |
| Year 4 | 1052 | 1113 | 360 | 1279 | 1771 | 354 |
| Year 5 | 1057 | 1301 | 266 | 1260 | 1916 | 233 |
| Total costs | 11,956 | 4656 | 266 | 11,789 | 7301 | 233 |
| QALYs | ||||||
| Year 1 | 0.810 | 0.019 | 406 | 0.811 | 0.006 | 393 |
| Year 2 | 0.773 | 0.078 | 395 | 0.777 | 0.052 | 388 |
| Year 3 | 0.726 | 0.143 | 387 | 0.737 | 0.110 | 382 |
| Year 4 | 0.689 | 0.167 | 360 | 0.700 | 0.136 | 354 |
| Year 5 | 0.631 | 0.214 | 266 | 0.651 | 0.183 | 231 |
| Total QALYs | 3.593 | 0.620 | 266 | 3.642 | 0.518 | 231 |
Quality-adjusted life-years
Accounting for missing data using multiple imputation, mean QALYs per year were similar for the two groups and there was a decline over time. Mean QALYs per patient (95% CI) fell from 0.810 (0.808 to 0.812) in the EBRT group in year 1 to 0.657 (0.640 to 0.674) in year 5. In the TARGIT group the values were 0.811 (0.810 to 0.811) and 0.674 (0.660 to 0.689), respectively. Mean total QALYs per patient over the 5-year period were 3.663 (3.614 to 3.713) in the EBRT group and 3.704 (3.664 to 3.744) in the TARGIT group (see Table 14). QALYs were similar for complete cases (see Table 15).
Cost–utility analysis
Accounting for missing data using multiple imputation, the mean net monetary benefits for EBRT and TARGIT were £61,426 (95% CI £60,299 to £62,544) and £62,678 (95% CI £61,542 to £63,762) at a maximum willingness to pay for a QALY of £20,000 and £98,059 (95% CI £96,470 to £99,644) and £99,720 (95% CI £98,228 to £101,147) at a maximum willingness to pay for a QALY of £30,000 (see Table 14).
In the base-case analysis TARGIT was less costly than EBRT (mean incremental cost –£685) and produced slightly more QALYs than EBRT (mean QALYs gained 0.034; Table 16). The difference in costs between the two groups was statistically significant (mean incremental cost for TARGIT vs. EBRT –£685, 95% CI –£1131 to –£63) but the difference in QALYs was not (mean QALYs gained 0.034, 95% CI –0.026 to 0.095). The incremental net monetary benefit for TARGIT compared with EBRT was positive indicating that TARGIT was cost-effective: at a maximum willingness to pay for a QALY of £20,000 or £30,000 the mean incremental net monetary benefit was £1363 and £1730 (see Table 16). The incremental net monetary benefit was not significantly different from zero at a maximum willingness to pay for a QALY of £20,000 (mean £1363, 95% CI –£66 to £2838) or £30,000 (mean £1730, 95% CI –£284 to £3740). However, the incremental net monetary benefit for TARGIT compared with EBRT was borderline significantly different from zero: at a maximum willingness to pay for a QALY of £20,000 the 90% CI was £175 to £2818 and at £30,000 it was £38 to £3746. In a hypothesis test, this would indicate that against a null hypothesis the incremental net monetary benefit equals zero; the p-value for rejecting the null hypothesis would be between 0.05 and 0.1.
| Parameter | Mean | 95% CI |
|---|---|---|
| Incremental costs (£),a TARGIT vs. EBRT | –685 | –1341 to –63 |
| QALYs gained, TARGIT vs. EBRT | 0.034 | –0.026 to 0.095 |
| Incremental net monetary benefits (£), TARGIT vs. EBRT | ||
| £20,000 | 1363 | –66 to 2838 |
| £30,000 | 1703 | –284 to 3740 |
| Probability TARGIT cost-effective | p-value | |
| £20,000 | 0.965 | |
| £30,000 | 0.950 | |
We repeated the analysis several times using alternative versions of the multiple imputation process using different random number seeds to investigate whether or not the conclusions of the analysis changed; in every case the results were qualitatively the same.
Sensitivity analyses
In all but one of the scenarios tested in the deterministic sensitivity analysis TARGIT was less costly than EBRT (Table 17). The exception was when the cost of TARGIT was £2700 per patient, which is higher than the maximum value in Picot et al. 72 (£2500). The costs were statistically significantly lower for TARGIT compared with EBRT (the 95% CI did not cross zero) when EBRT costs were based on the number of fractions received in the trial, the unit cost per fraction of EBRT was £154 (the upper quartile unit cost in the NHS reference costs76), the cost of TARGIT was ≤ £1900 per patient and the alternative utility values were used.
| Analysis | QALYs gained | Incremental costs (£)a | Incremental net monetary benefits (£) | Probability TARGIT cost-effective | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | |||||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | £20,000 | £30,000 | |
| Base caseb | 0.034 | –0.026 to 0.095 | –685 | –1341 to –63 | 1363 | –66 to 2838 | 1703 | –284 to 3740 | 0.965 | 0.950 |
| Unadjustedc | 0.041 | –0.022 to 0.102 | –436 | –1185 to 323 | 1253 | –335 to 2796 | 1661 | –502 to 3762 | 0.941 | 0.937 |
| Complete case analysis adjustedd | 0.029 | –0.072 to 0.125 | –583 | –1549 to 315 | 1159 | –1199 to 3498 | 1446 | –1860 to 4691 | 0.834 | 0.803 |
| Complete case analysis unadjustede | 0.049 | –0.052 to 0.149 | –167 | –1272 to 926 | 1138 | –1367 to 3650 | 1623 | –1818 to 5069 | 0.817 | 0.824 |
| EBRT costs based on number of fractions received in the trialf | 0.034 | –0.029 to 0.095 | –1377 | –2026 to –771 | 2055 | 562 to 3569 | 2394 | 313 to 4485 | 0.998 | 0.987 |
| No EBRT boost | 0.034 | –0.028 to 0.095 | –90 | –733 to 519 | 766 | –676 to 2226 | 1104 | –913 to 3132 | 0.787 | 0.829 |
| Costs of EBRT per fraction (£) | ||||||||||
| 101 | 0.034 | –0.026 to 0.095 | –366 | –1009 to 271 | 1041 | –387 to 2504 | 1379 | –607 to 3413 | 0.926 | 0.917 |
| 154 | 0.034 | –0.028 to 0.093 | –1042 | –1664 to –443 | 1715 | 267 to 3149 | 2052 | 28 to 4043 | 0.989 | 0.978 |
| Costs of TARGIT (£) | ||||||||||
| 1300 | 0.034 | –0.032 to 0.096 | –1267 | –1896 to –680 | 1940 | 420 to 3452 | 2276 | 141 to 4378 | 0.996 | 0.986 |
| 1500 | 0.034 | –0.029 to 0.097 | –1064 | –1705 to –444 | 1738 | 247 to 3261 | 2075 | –7 to 4194 | 0.994 | 0.980 |
| 1700 | 0.034 | –0.026 to 0.095 | –869 | –1516 to –281 | 1544 | 150 to 3018 | 1881 | –72 to 3924 | 0.979 | 0.963 |
| 1900 | 0.034 | –0.025 to 0.092 | –667 | –1304 to –73 | 1342 | –59 to 2765 | 1679 | –271 to 3642 | 0.968 | 0.954 |
| 2100 | 0.034 | –0.029 to 0.094 | – 471 | –1130 to 146 | 1147 | –336 to 2623 | 1486 | –584 to 3524 | 0.936 | 0.920 |
| 2300 | 0.034 | –0.025 to 0.094 | – 264 | –910 to 329 | 944 | –433 to 2395 | 1284 | –638 to 3292 | 0.909 | 0.904 |
| 2500 | 0.034 | –0.027 to 0.096 | – 65 | –702 to 515 | 740 | –695 to 2264 | 1078 | –927 to 3186 | 0.855 | 0.863 |
| 2700 | 0.034 | –0.023 to 0.092 | 135 | –496 to 748 | 540 | –840 to 1966 | 877 | –1032 to 2847 | 0.785 | 0.818 |
| Patients using NHS transport for EBRT (%) | ||||||||||
| 0 | 0.034 | –0.028 to 0.096 | –546 | –1205 to 52 | 1220 | –202 to 2728 | 1557 | –437 to 3649 | 0.951 | 0.935 |
| 30 | 0.034 | –0.025 to 0.097 | –852 | –1484 to –262 | 1530 | 176 to 3000 | 1868 | –34 to 3924 | 0.990 | 0.981 |
| Health statuses valued using utilities from Hayman et al.89,90 | 0.033 | –0.040 to 0.104 | –686 | –1338 to –67 | 1347 | –367 to 3051 | 1678 | –737 to 4061 | 0.912 | 0.903 |
In every case the QALYs gained were small, positive and non-significant. Note that these were unlikely to change given that the parameters varied in the deterministic sensitivity analysis were mainly cost parameters.
In all cases tested the incremental net monetary benefits for TARGIT compared with EBRT were positive at a maximum willingness to pay for a QALY of £20,000 and £30,000. The incremental net monetary benefits were significantly greater than zero (the 95% CI did not cross zero) when EBRT costs were based on the number of fractions received in the trial, the unit cost per fraction of EBRT was £154 and the cost of TARGIT was ≤ £1700 per patient at a maximum willingness to pay for a QALY of £20,000 or ≤ £1300 per patient at a maximum willingness to pay for a QALY of £30,000.
The probability that EBRT is cost-effective is equal to 1 minus the probability that TARGIT is cost-effective at each value of the maximum willingness to pay for a QALY. The cost-effectiveness acceptability curve shows that, at a maximum willingness to pay for a QALY of £20,000 (£30,000), the probability that TARGIT was cost-effective was 0.965 (0.950) in the base case (Figure 30 and see Table 17). In the deterministic sensitivity analyses the probability that TARGIT was cost-effective at a maximum willingness to pay for a QALY of £20,000 was > 0.75 in every case. At a maximum willingness to pay for a QALY of £30,000 the probability that TARGIT was cost-effective was > 0.80 in every case.
FIGURE 30.
Cost-effectiveness acceptability curve showing the probability that TARGIT is cost-effective compared with EBRT at different values of the maximum willingness to pay for a QALY.

Potential budget impact
The cost savings per patient found in our base case could translate into cost savings per year for the NHS if TARGIT was carried our routinely instead of EBRT in eligible patients. The latest available evidence suggests that in 2011 there were 49,936 new cases of breast cancer in the UK. 95 Figures from Germany96 based on 1108 new cases of breast cancer treated at a single centre between 2003 and 2009 suggest that 258 patients (23.3% cases) would have met the eligibility criteria for participation in the TARGIT-C trial (ClinicalTrials.gov NCT02290782),97 which has similar but more restrictive inclusion and exclusion criteria than the TARGIT-A trial (e.g. age ≥ 50 years rather than ≥ 45 years, tumour size ≤ 2 cm rather than ≤ 3.5 cm). This conservatively suggests that around 49,936 × 23.3% = 11,600 patients may be eligible for TARGIT in the UK each year. Applying the cost saving per patient in our base case to this estimate suggests that the NHS might save around 11,600 × –£685 = £8 million a year.
Figures from France98 based on two cohorts of patients between 1980 and 2008 indicate that, across a combined total of 12,025 patients receiving breast-conserving surgery, 5545 patients (46%) would have been eligible for TARGIT according to the eligibility criteria of the TARGIT-A trial. Approximately 58% of newly diagnosed patients with breast cancer in the UK undergo lumpectomy. 99 Therefore, according to these figures, around 49,936 × 58% × 46% = 13,300 patients may be eligible for TARGIT in the UK each year. Applying the cost saving per patient in our base case to this estimate suggests that the NHS might save around 13,300 × £685 = £9.1 million a year.
Combined, these calculations suggest that if TARGIT was carried our routinely instead of EBRT in eligible patients the potential cost savings to the NHS would be around £8–9.1 million each year.
Discussion
Summary
We undertook a cost–utility analysis comparing TARGIT versus EBRT in the prepathology stratum of the earliest cohort of the TARGIT-A trial. In our base case TARGIT was statistically significantly less costly than EBRT, produced similar QALYs, had a positive incremental net monetary benefit that was borderline statistically significantly different from zero and had a probability of > 90% of being cost-effective. Although there appears to be some uncertainty about the statistical significance of the differences in costs and whether or not the incremental net monetary benefit is different from zero, the appears to be little uncertainty in the point estimates, based on deterministic and probabilistic sensitivity analyses.
Comparison with other studies
Alvarado et al. 73 found that TARGIT dominated EBRT (was less costly and more effective) in that it resulted in a QALY gain of 0.00026 compared with EBRT and cost US$5191 less. Based on their analysis using TARGIT-A trial data, Shah et al. 74 reported that use of TARGIT was associated with cost savings of US$3.6–4.3 million per 1000 patients compared with EBRT. Neither study reported CIs around the point estimates and so it is unclear if the QALYs gained or cost savings were significantly different from zero. Our results are qualitatively similar to those of Alvarado et al. 73 in that based on the point estimates in our base case we also found a cost saving for TARGIT and a small QALY gain compared with EBRT. Our findings are also qualitatively similar to those of Shah et al. 74 in that we also found a cost saving with TARGIT compared with EBRT. However, given that both studies were US based it is difficult to draw close comparisons.
Picot et al. 72 found that TARGIT produced a small cost saving compared with EBRT and a small QALY loss; the authors’ conclusion was that EBRT was associated with more QALYs than TARGIT at a broadly similar overall cost. The point estimates of the costs saved per QALY lost were < £20,000, indicating that TARGIT was not cost-effective (in cases in which an intervention is less costly and less effective than the comparator then for it to be cost-effective the incremental cost-effectiveness ratio must lie above the threshold value). CIs around the cost and outcome differences and the incremental cost-effectiveness measures were not reported and so it is difficult to make a full comparison of the findings. Other than the use of patient-level data, the main differences between the study by Picot et al. 72 and the present study were the time horizon and the range of costs included. Picot et al. 72 modelled costs and outcomes using a time horizon of 40 years, whereas the time horizon in the present study was 5 years based on the average length of follow-up in the trial. We did not extrapolate beyond the end of the trial because the within-trial analysis found no evidence of significant differences in QALYs between the groups and, although there was some evidence of differences in costs, these differences were all accrued during the first year. In terms of costs included, there were several differences between the present study and that by Picot et al. 72 During the radiotherapy treatment period the present study included the cost of EBRT boost and NHS transport costs for EBRT, which were not included in the study by Picot et al. 72 More generally, the total cost per patient in the study by Picot et al. 72 over the 40-year period, based on the costs included in the analysis, was around £2300 in both groups. In our study the total cost per patient over the 5-year period, based on the costs included in the analysis, was around £11,600 in both groups, suggesting large differences in the range of cost components included.
Strengths and limitations
The main strength of our analysis is that it is based on a large international multicentre randomised trial with detailed information on resource use and events for a median follow-up period of 5 years.
There are several limitations. First, the time horizon was 5 years. Extrapolation beyond the end of the trial using decision-analytical modelling was not undertaken because the within-trial analysis found no evidence of significant differences in QALYs between groups during the 5-year period. This probably reflects the main finding from the TARGIT-A trial that TARGIT was non-inferior to EBRT with regard to local recurrence. Although there was some evidence of differences in costs these differences were all accrued during the first year; there was no evidence of significant differences in costs beyond the first year. Hence, the 5-year time horizon was long enough to reflect all important differences in costs or outcomes between the two treatments. Although local recurrence (and other events) are likely to continue to occur over a patient’s lifetime, the evidence from the TARGIT-A trial is that TARGIT is non-inferior to EBRT. Hence, taking a longer time horizon is unlikely to have affected the results of the incremental analyses.
Second, utility data were not collected in the TARGIT-A trial. We therefore applied utility values from published sources to the health states experienced by patients in the trial. The utility values that we applied may not reflect the values of patients in the study. Given the relatively small number of events, and that the numbers of events were largely not different between the two groups, the QALY differences between the two groups may not be expected to change much with alternative utility values. This is borne out by our sensitivity analysis, which showed that the results did not change appreciably when we used alternative values. We did not incorporate utility losses associated with additional procedures, chemotherapy, mastectomy or complications in our analysis. Given the low incidence of these events, that they were evenly distributed between treatment groups and that the time period affected is likely to be short this is unlikely to affect the QALYs associated with each treatment group. We also did not include any utility losses associated with EBRT. Therefore, this would make our estimates more conservative because such an omission would work against TARGIT.
Third, the dose of EBRT administered to patients in the TARGIT-A trial does not reflect current UK treatment guidelines. This reflects the multinational nature of the trial, plus that it began recruiting patients in 2000 when treatment recommendations were different. We accounted for this in our base case by assuming that all patients in the TARGIT-A trial who received EBRT received a fixed number of 15 fractions.
Fourth, the analysis took a NHS/PSS perspective on costs. A wider perspective (e.g. societal) could have been taken to measure costs, including impacts on the rest of society, patients, families and businesses. If a wider perspective was taken this should include the additional costs borne by patients and families in terms of time and travel costs associated with additional radiotherapy visits for EBRT compared with TARGIT. If these costs were included it is likely that the cost savings attributable to TARGIT would be greater than demonstrated. Taking the example of the transport costs, we used the figure of 13.5% for the proportion of patients for whom the NHS paid for transport for radiotherapy visits for EBRT. Assuming that the same cost is paid out of pocket by the remaining patients, the difference in costs between TARGIT and EBRT would be increased by £877 to £1562 per patient, taking the total saving to the UK national economy to between 11,600 × £1562 = £18.1 million and 13,400 × £1562 = £20.9 million each year. These are crude estimates and further research to evaluate the wider impacts of TARGIT, including on other costs to the rest of society, would be useful.
Chapter 7 Discussion
Summary of findings
Targeted intraoperative radiotherapy is risk-adapted one-shot radiotherapy to peritumoural tissues, designed to be completed during the lumpectomy procedure for removal of cancer, whereas EBRT is given over a 3- to 6-week course of daily treatments at the radiotherapy centre, a few weeks after lumpectomy. The TARGIT-A trial found that there was no significant difference in the 5-year local recurrence rate between TARGIT and EBRT. Non-inferiority was established overall and in the prepathology stratum but not in the postpathology stratum. There was no difference in 5-year breast cancer mortality between these two treatments but 5-year mortality from causes other than breast cancer was significantly lower in the TARGIT arm than in the EBRT arm. Subgroup analysis found that only hormone receptor status had any impact on the effectiveness of TARGIT. TARGIT was more cost effective than EBRT. As the trial was conducted in 33 different centres in 11 countries in North America, Europe and Australia, we believe that the findings would be generalisable to the eligible patient population of at least these continents. There are 250 centres in five continents now using TARGIT IORT as a part of standard treatment for suitable patients with breast cancer and about 20,000 patients have been treated as of October 2015.
Non-inferiority for local recurrence
Our primary outcome was non-inferiority in terms of local recurrence. We tested for non-inferiority of the TARGIT technique for local control within a risk-adapted design. When we designed the trial in 1999, we set the margin of non-inferiority to be an absolute difference in the local recurrence rate of 2.5%. This is a stringent and relatively conservative margin compared with that in contemporary studies. For example, the only other trial of IORT – the ELIOT trial35 – was set up with the assumption that a difference of 7.5% in local recurrence between the experimental and the control groups would result in the two treatments being considered as equivalent. The Cancer and Leukemia Group B (CALGB) study11 found that there was a 7% difference in local recurrence when radiotherapy was omitted in low-risk (T1N0) patients aged > 70 years and concluded that this difference was not significant. Furthermore, two patient preference studies conducted in parallel with the main trial have also corroborated that, given the convenience of a single treatment session, the median increase in local recurrence that patients would be ready to accept is 2.5%. 100,101 Most importantly, analyses by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) indicate that an absolute increase in local recurrence of < 10% does not result in any increase in mortality and therefore we did not feel that there were any ethical concerns in setting up this study. 4–6 We were also certain that the trial was well powered to detect a difference of ≥ 2.5% and therefore that if the results showed non-inferiority they could be relied on. In fact, we provided detailed calculations of how, in view of the recurrence rates seen in the trial, 585 patients would have provided sufficient power for the trial.
Although non-inferiority trials are becoming more common, especially when cure rates are high and a reduction in treatment toxicity without a loss of efficacy is desirable, the concept of non-inferiority can be difficult to grasp. The TARGIT-A trial was such a non-inferiority trial, which meant that, even if there was a statistically significant difference between the groups, as long as it was less than the pre-specified value (2.5% in the TARGIT-A trial), the two groups would be judged to be non-inferior to each other. In these circumstances, less expensive, less toxic or more convenient treatment becomes the preferred choice (Figure 31).
FIGURE 31.
The meaning of non-inferiority: 10 examples of different scenarios that might occur in a randomised trial testing non-inferiority between two treatments. The dots represent the absolute difference and the lines the CI. The blue circle includes two of the trial results: on the left is the TARGIT prepathology stratum (difference 0.37%) and on the right is the earliest cohort of the whole trial (n = 1222), which had a median follow-up of 5 years (difference 1.13%) (see Table 8). 2,102
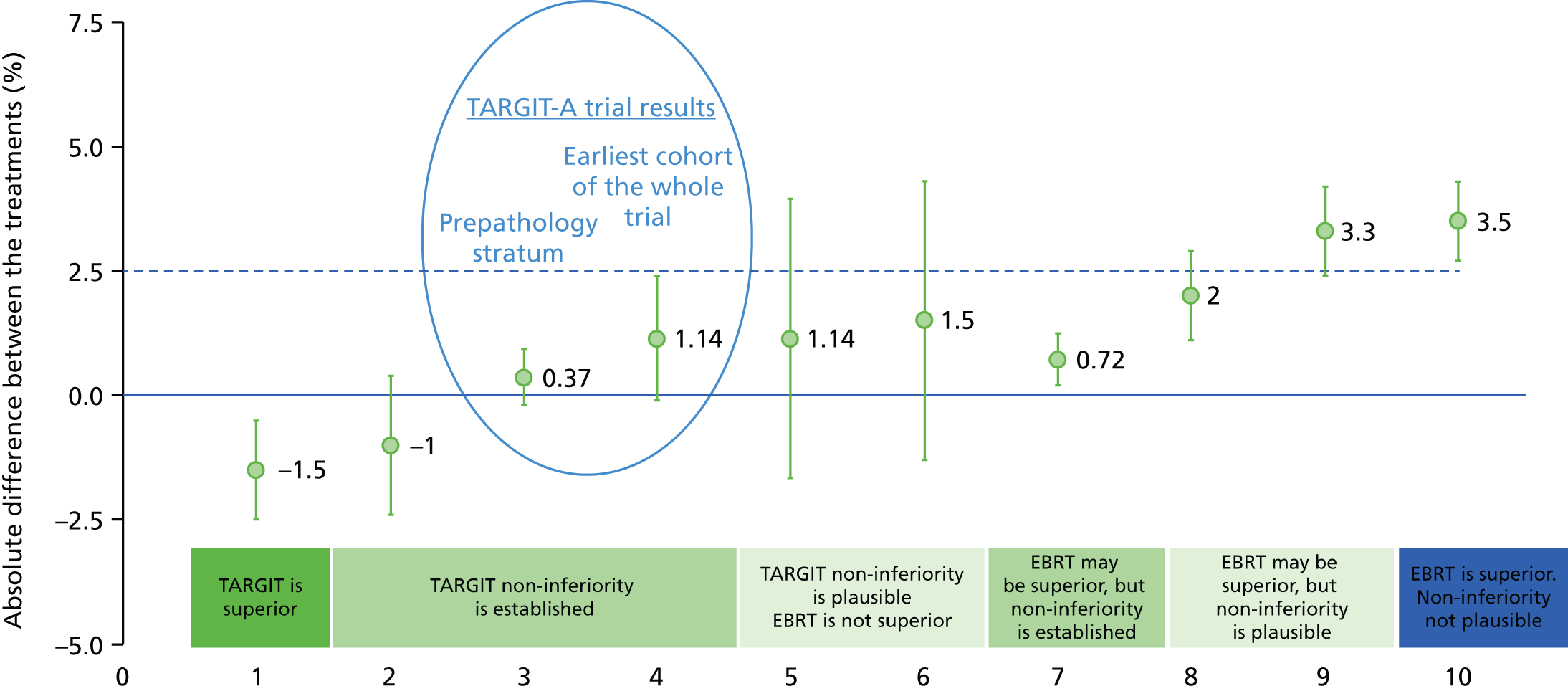
The pre-specified non-inferiority boundary of a 2.5% absolute difference in local recurrence is very conservative and has been validated in patient preference studies. 101,103,104 It is much smaller than the 7.5% margin in the ELIOT trial105 and lower than the difference considered ‘acceptable’ by the CALGB (5% difference)11 and Post-operative Radiotherapy In Minimum-risk Elderly (PRIME) II (3% difference)106 studies. At this boundary, TARGIT was non-inferior to EBRT (Pnon-inferiority < 0.00001). Within the trial of 3451 patients, the first 1222 patients had a median follow-up of 5-years; the safety, efficacy and non-inferiority results of these 1222 patients were similar to those seen in all patients (see Table 8). 2
We found that, when all patients were analysed together, the risk of local recurrence for TARGIT was non-inferior to that for EBRT (5-year risk: TARGIT 3.3% vs. EBRT 1.3%). We had pre-specified that the significant p-value for difference for the log-rank test would be < 0.01 for local recurrence, as this was the second such analysis. Therefore, the 2% absolute difference was not only within the non-inferiority margin but also, as the p-value was 0.04, strictly speaking, it was not statistically significant.
We used the standard method of using binomial proportions to calculate the non-inferiority statistic, rather than using single 5-year point estimates, which do not represent absolute events and can lead to erroneous conclusions, especially when the event rate is low. The standard method that we used makes a comprehensive assessment of the whole follow-up period, which stretches from the day of randomisation to the longest follow-up of more than 12 years and includes all events in that period. To address the issue of follow-up, we repeated the analysis by restricting the analysis to cohorts randomised early in the trial, including the earliest 1222 patients; these patients had a median follow-up of 5 years. The multiple comparisons issue does not apply here as we have not chosen one of several comparisons to make our conclusions but rather used all three comparisons to draw an informed conclusion.
When analysed according to the timing of randomisation/delivery of TARGIT, we found that, whereas the trial as a whole as well as the prepathology stratum confirmed the non-inferiority of TARGIT, the difference in local recurrence in the postpathology stratum was > 2.5%. Therefore, we have recommended that TARGIT should be used during the initial lumpectomy, as in the prepathology stratum (note that this is a stratum not a subgroup), in which the difference between the two treatments was undoubtedly not statistically significant (p = 0.31). Finally, survival without local recurrence (in which deaths are not censored, in line with recommendations by the US Food and Drug Administration62 and elsewhere63) was not statistically different between the two arms (see Figures 18 and 19).
Toxicity
There was no increased wound-related toxicity with TARGIT and there was reduced radiotherapy-related skin toxicity. This was not negated by the EBRT boost delivered to 15% of the TARGIT arm patients; toxicity was the same whether such an EBRT boost was applied to TARGIT or EBRT patients. 107
There were significantly fewer grade 3 or 4 radiotherapy-related complications in the TARGIT arm; however, one should note that the incidence of these complications was very low in both arms of the trial. This, and the low local recurrence rate reported, is a testament to the high-quality radiotherapy given in the EBRT arm.
Studies conducted parallel to the TARGIT-A trial in individual centres have shown that TARGIT delivers a better cosmetic outcome,108 lower short-term and long-term skin toxicity and an overall better quality of life. 107,109,110
Mortality
Although the number of breast cancer deaths did not differ significantly between groups, there were significantly fewer deaths from other causes in the TARGIT arm of the trial (number of deaths from causes other than breast cancer: TARGIT 17, EBRT 35). In particular, there were far fewer deaths from cancers other than breast cancer (TARGIT 8 vs. 16) or from cardiovascular disease (EBRT 2 vs. 11).
This early decrease in cardiovascular deaths was somewhat surprising as it was previously believed that any EBRT-related increase in cardiovascular disease would not be apparent until 7–10 years after radiation. 3 On the other hand, given the size of the trial, it is unlikely that there was a significant imbalance in baseline comorbidities and, statistically, the probability of observing the difference we observed if, in reality, there was no real difference in non-breast-cancer mortality between the randomised arms, is very low (p = 0.0086). The difference is also unlikely to be caused by poor EBRT delivery, given the low rates of local recurrence (1.2%) and grade 3/4 radiotherapy toxicity (2.1%) among patients receiving EBRT. These results therefore deserve a search for an explanation, rather than dismissal.
Indeed, a large recent study from a group from Oxford, UK, published in the New England Journal of Medicine, has shown that EBRT toxicity appears within the first 5 years. 71 The authors found an increase in cardiac mortality of 16.3% per gray in the first 5 years, 15.5% in the second 5 years, 1.2% in the years 10–19 and 8.2% after 20 years. As the effect appears to be the largest in the first 10 years – more than twice that of the average – the yardstick should be based on the period of follow-up and it would be wrong to use the average value of a 7.4% increase in cardiac mortality per gray as a yardstick. The higher increase in the first 5 and 10 years is consistent with our findings as they fall within each others’ 95% CIs.
It is possible that the TARGIT-A trial may have uncovered a very small increase in non-breast-cancer mortality associated with EBRT that has previously been masked by breast cancer deaths. The 5-year risk of breast cancer mortality was 2.2% in TARGIT-A, compared with, for instance, 30% in the Cancer Research Campaign (CRC)1 trial111 (Figure 32). The absolute difference in non-breast-cancer mortality in the TARGIT-A trial was just 2.1% (TARGIT 1.4% vs. EBRT 3.5%) and approximately one-third of this increase (0.6%) was from cardiac causes.
FIGURE 32.
(a) The high breast cancer mortality rate (70% at 5 years) in older trials such as the CRC trials could have masked the deaths from causes other than breast cancer in the early years, which were revealed only when the breast cancer mortality lines started flattening. (b) In the TARGIT-A trial, however, the breast cancer mortality rate was very low (2.1%), which would allow the early, albeit small in absolute terms, increase in non-breast-cancer mortality to be unmasked. Part (a) is reproduced from Postoperative radiotherapy and late mortality: evidence from the Cancer Research Campaign trial for early breast cancer, Haybittle JL, Brinkley D, Houghton J, et al. , vol. 298, pp. 1611–14,111 with permission from BMJ Publishing Group Ltd.
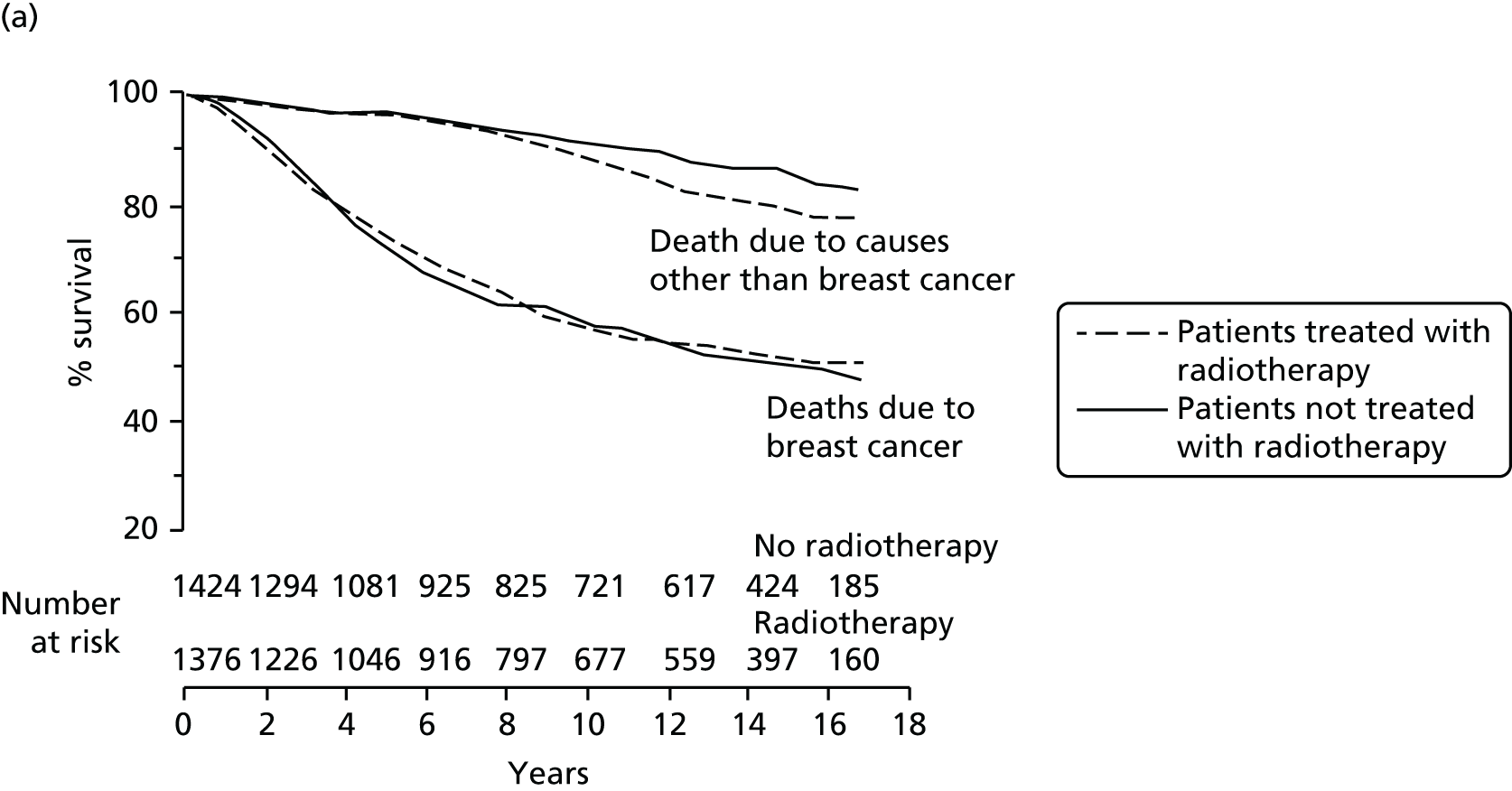

As can be seen in Figure 32, the difference in deaths from other causes starts becoming apparent only when the deaths from breast cancer start levelling off. The slope of the curve denoting breast cancer deaths at 10 years in the CRC1 trial is similar to that seen in the TARGIT-A trial from the beginning, which has probably allowed us to see the small difference in non-breast-cancer morality between those who received some irradiation to the heart and other organs and those who did not. This idea may well explain the recently published results of a meta-analysis of randomised trials comparing PBI and whole-breast irradiation. 112 The meta-analysis demonstrates a reduction in non-breast-cancer and overall mortality with PBI compared with whole-breast irradiation.
Alternatively, we believe that TARGIT may in fact be protective against deaths from cardiovascular causes and other cancers,69,70,113 perhaps through a reduction of the systemic inflammatory response that potentiates cardiovascular diseases or cancers; this, in combination with the reduced radiation toxicity, may be the cause of the difference that we have observed here. The first hints of this may be found in the finding of a significantly lower non-breast-cancer mortality hazard in patients who received TARGIT + EBRT than in those receiving EBRT alone. It is worth keeping in mind, however, that this comparison was non-randomised and the numbers were small.
Further research into the potential positive effects of TARGIT given alongside EBRT is currently under way in the TARGIT-B trial, which is looking at the impact of TARGIT given as a radiotherapy boost.
Strengths and limitations
Trial design: experimental treatment = a risk-adapted approach
The TARGIT-A trial was a pragmatic trial that tested two radiotherapy policies for non-inferiority in local recurrence after breast-conserving surgery: risk-adapted radiotherapy and conventional standard radiotherapy given daily over several weeks.
Following breast-conserving surgery, the control arm received EBRT over several weeks according to local treatment guidelines. This conventional treatment does not normally vary with tumour characteristics and everyone receives the same dose of whole-breast radiation, although there could be variation between centres. There could also be some variation in terms of delivery or omission of the tumour bed boost depending on the age of the patient.
The experimental arm, on the other hand, aimed to deliver all radiotherapy in a single dose in the majority of patients, using the TARGIT technique and the INTRABEAM device. TARGIT was given either at the time of lumpectomy (the prepathology stratum, 2298 patients) or as a second procedure several days after the initial lumpectomy, by reopening the wound (the postpathology stratum, 1153 patients).
The risk-adapted design meant that, if after TARGIT adverse features were found in the final histopathological specimen, the trial protocol mandated the addition of EBRT to the rest of the breast (excluding a tumour bed boost). Individual centres decided on the set of histological adverse features before randomisation began, in addition to the factors stipulated in the core protocol: the presence of positive margins, evidence of an invasive lobular carcinoma or an EIC.
Such additional EBRT was needed in 15% of cases, as per our original estimations. This proportion was higher in the prepathology stratum, with one-fifth of the patients receiving TARGIT at the time of their primary surgery also being given EBRT because of adverse prognostic factors as determined in the multidisciplinary discussion of the full histopathology after surgery. This adaptation of the treatment based on risk should ensure that the experimental group in our clinical trial reflects how TARGIT would be delivered in the real world; meanwhile, the patients in the EBRT arm provided a good control group, representing how the treatment is currently given, according to local guidelines. The trial should therefore tell us how the outcome for patients would change (or not) if TARGIT were to be introduced into routine clinical practice. We believe that this is the only method of accurately reflecting practice in the real world, without sacrificing statistical validity; it may be disappointing for the women who needed supplemental EBRT but it is worth remembering that TARGIT allows the large majority of patients to avoid several weeks of EBRT.
We believe that this type of pragmatic non-inferiority design is a strength of the trial, although at the same time it could make it more difficult for purists to interpret the results. This is quite contrary to how patients and the lay public interpret the results – they have consistently felt that the small statistically non-significant difference in local recurrence was really not a concern, particularly compared with the great benefit and convenience of patients being able to have all of their treatment in one sitting100,101,103,104,114,115 [see http://goo.gl/j3jyM1, http://goo.gl/x6Sk80, http://goo.gl/j8xwHZ, www.bbc.co.uk/programmes/p01lhjm2, www.telegraph.co.uk/women/womens-health/10439775/A-revolution-in-breast-cancer-therapy.html, www.bbc.co.uk/news/health-28485504, http://goo.gl/dJQKAF, www.dailymail.co.uk/health/article-1378267/Me-operation-Targeted-intraoperative-radiotherapy.html and www.wsj.com/articles/alternative-way-to-treat-early-stage-breast-cancer-with-radiation-1440448587 (accessed 5 July 2016)]. The observed reduction in non-breast-cancer mortality was an added advantage.
Accrual, power and follow-up duration
The current analysis represents mature data from a large number of patients. Fifteen years ago our original power calculations expected a background local recurrence rate of 6% and, if we accept a non-inferiority margin of 2.5%, the sample size required to achieve 80% power and 95% confidence would then have been 2232 patients. The local recurrence rate measured in the EBRT group of the TARGIT-A trial at 5 years was in fact just 1.5% and, given this lower value, we needed only 585 patients to achieve 80% power and 95% confidence (Table 18). 1,116 It has been well documented that, in the first 5 years after treatment, breast cancer patients face the highest risk of local recurrence. The peak hazard of local recurrence is between 2 and 3 years after surgery, as seen in many studies, including the Oxford overview4,117,118 (Figure 33).
| Scenario | Background recurrence rate (%) | Background + non-inferiority margin of 2.5% = recurrence rate in the experimental arm (%) | Total sample size required for 80% power and 95% confidence | Number of patients with median follow-up of 5 years in the TARGIT-A trial at the last data lock |
|---|---|---|---|---|
| Expected at the time of trial set-up in 1999 | 6 | 8.5 | 2232 | 1222 |
| Expected following recent publications (e.g. START trial)49 | 3 | 5.5 | 1151 | |
| Control arm (EBRT group) of the TARGIT trial in 2010 | 1.5 | 4 | 585 |
FIGURE 33.
Two large studies118,119 demonstrating that the risk of local recurrence is mainly in the first few years after surgery and flattens after 5 years. (a) Reprinted from Cancer Epidemiology, Biomarkers and Prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 2012, vol. 21, Cheng L, Swartz MD, Zhao H, et al. , Hazard of recurrence among women after primary breast cancer treatment – a 10 year follow up using data from SEER-Medicare,118 with permission from AACR. (b) Reproduced from N Engl J Med, Fisher B, Anderson S, Bryant J, et al. , Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer, vol. 347, pp. 1233–41. 119 Copyright © 2002 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
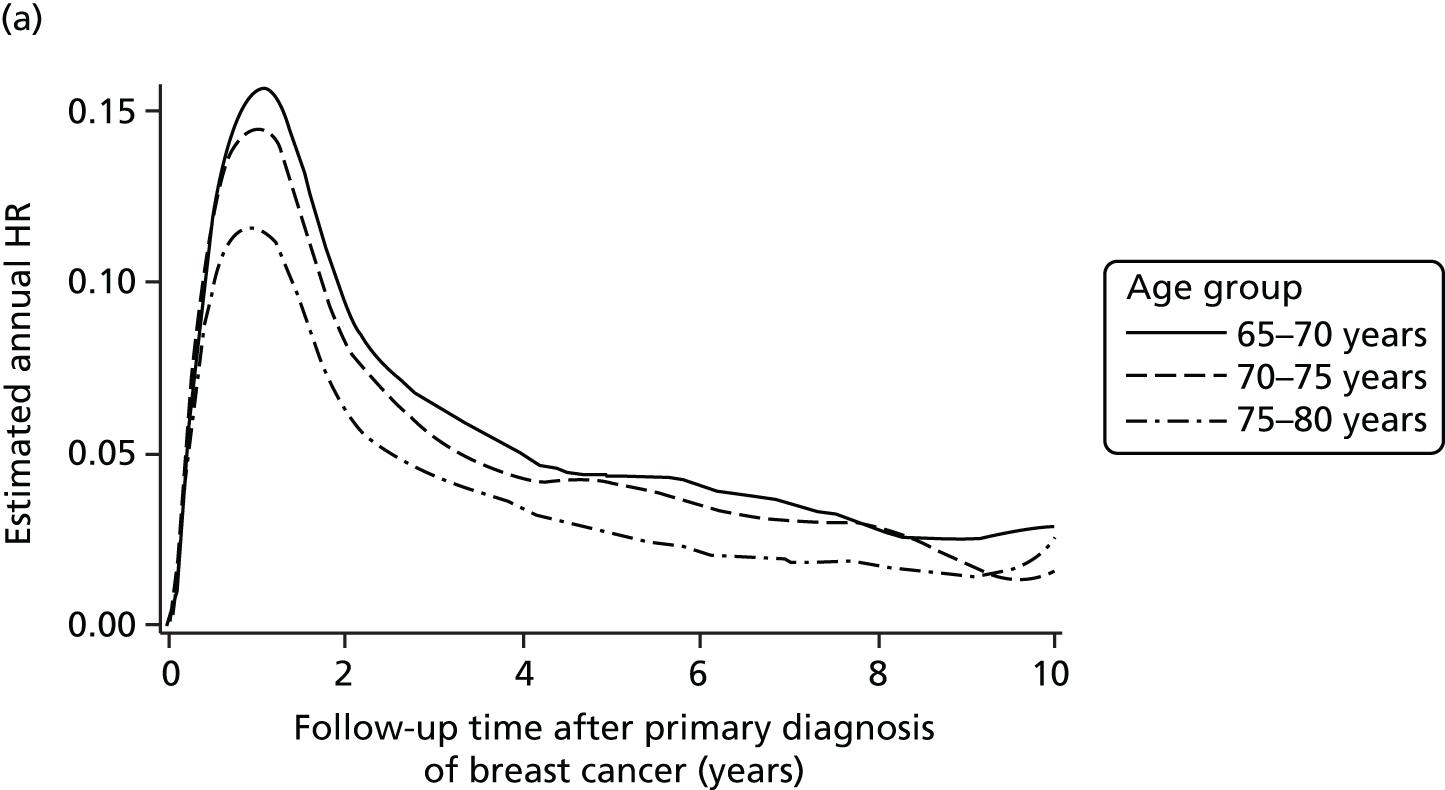
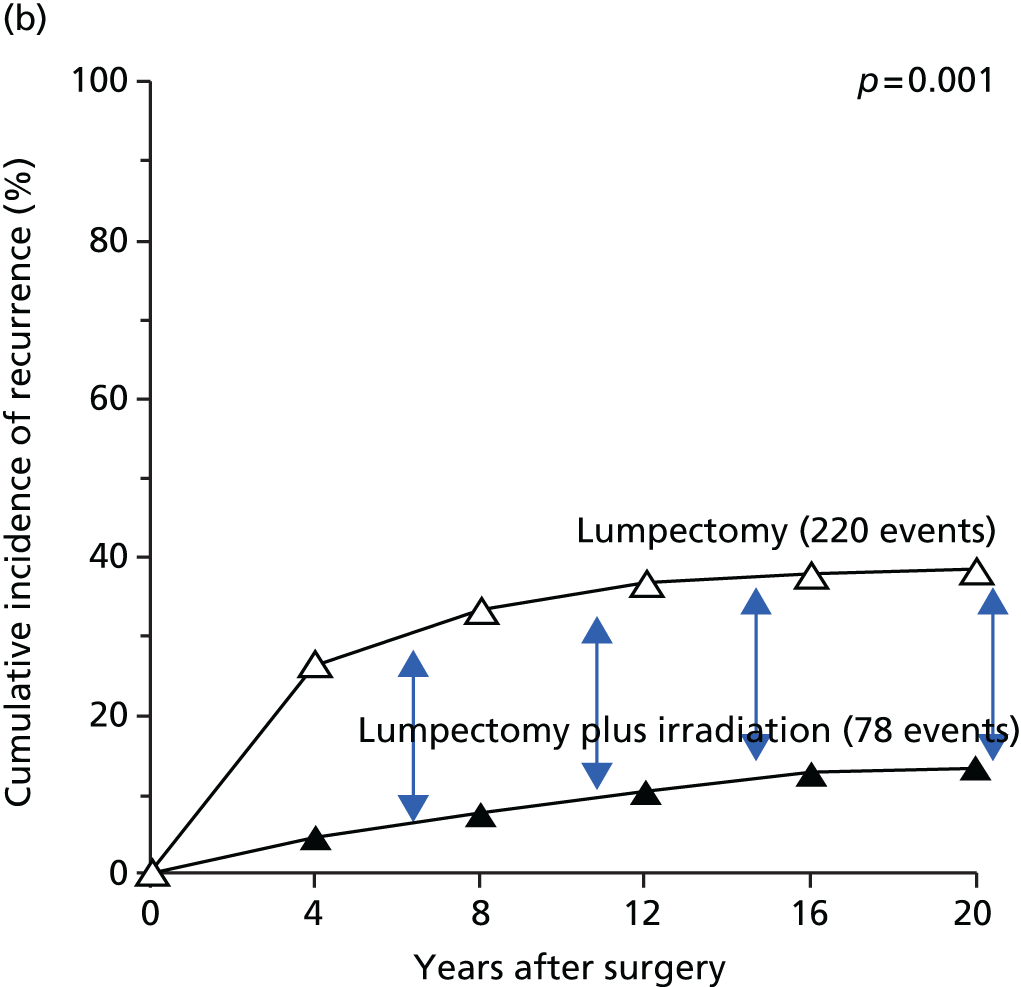
Esserman et al. 120 have recently applied a decision-analytic framework to conclude that the median follow-up of the TARGIT-A trial is sufficient to strongly recommend wide adoption.
Indeed, a large recent study with a 20-year follow up, conducted by Wickberg et al. ,121 has shown that, whereas radiotherapy decreases the number of recurrences in the first 5 years, it ceases to have any effect after the first 5 years; thus the rate of local recurrence after the first 5 years following radiotherapy is similar to that in patients who received only lumpectomy. This is best demonstrated in Figure 34, which depicts how the lines representing local recurrence are parallel after 5 years and most of the hazard of not taking radiotherapy is in the first 5 years. 122
FIGURE 34.
A large Swedish study121,122 with a long follow-up demonstrates that, although the local recurrence risk persists even after 5 years, the effect of radiotherapy on reducing that risk is seen only in the early years. This is seen most clearly in (c), with the risk reduction from radiation being mainly in the first 5 years, with very little effect thereafter. Part (c) is drawn using data from Wickberg et al. 122 (a) Local recurrence with and without radiotherapy over 25 years;121 and (b) hazard of not taking radiotherapy is mainly in the first 5 years;122 and (c) hazard EBRT vs. no- EBRT. 122 (a) Reprinted with permission from Wickberg et al. , J Clin Oncol, Vol. 32, Issue 8, 2014: 791–7. 121 © (2014) American Society of Clinical Oncology. All rights reserved; and (b) reprinted with permission from Wickberg et al. , J Clin Oncol, Vol. 32, Issue 29, 2014: 3340. 122 © (2014) American Society of Clinical Oncology. All rights reserved.
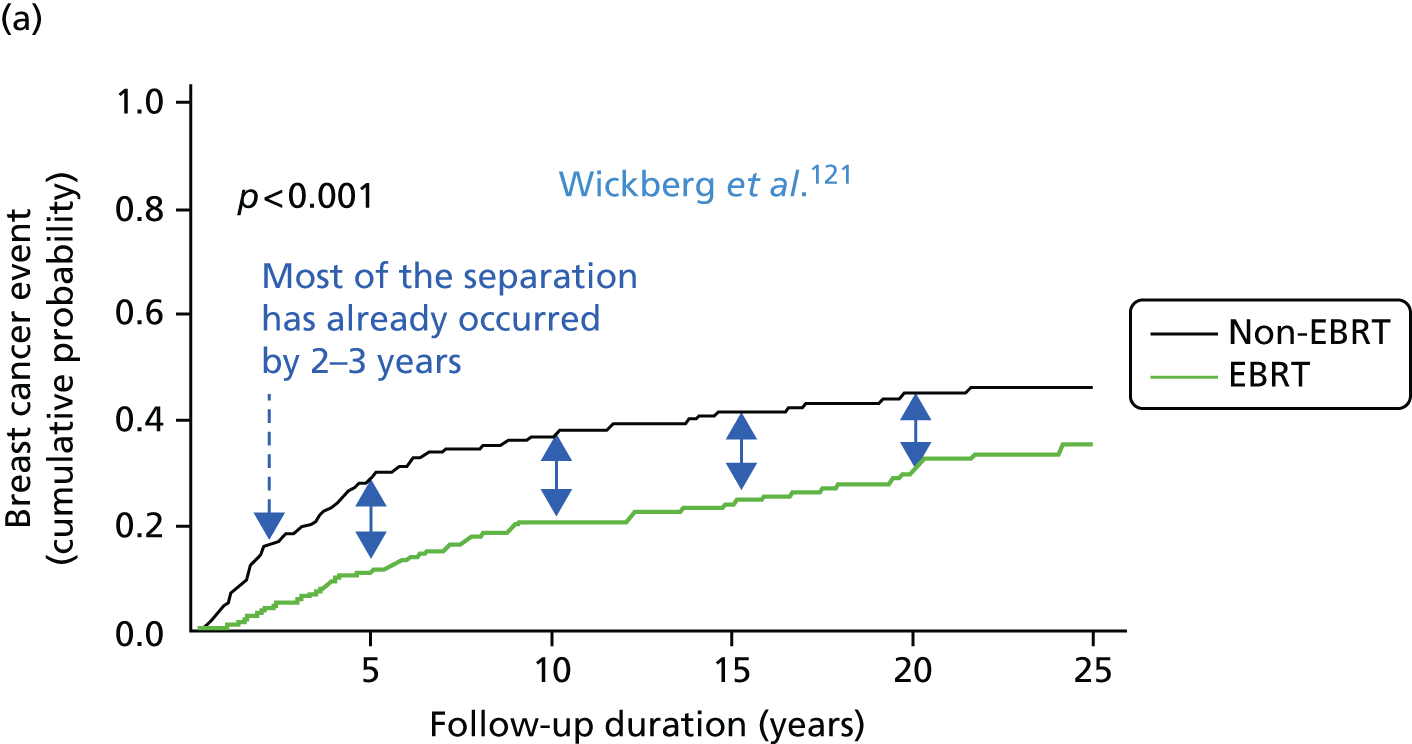

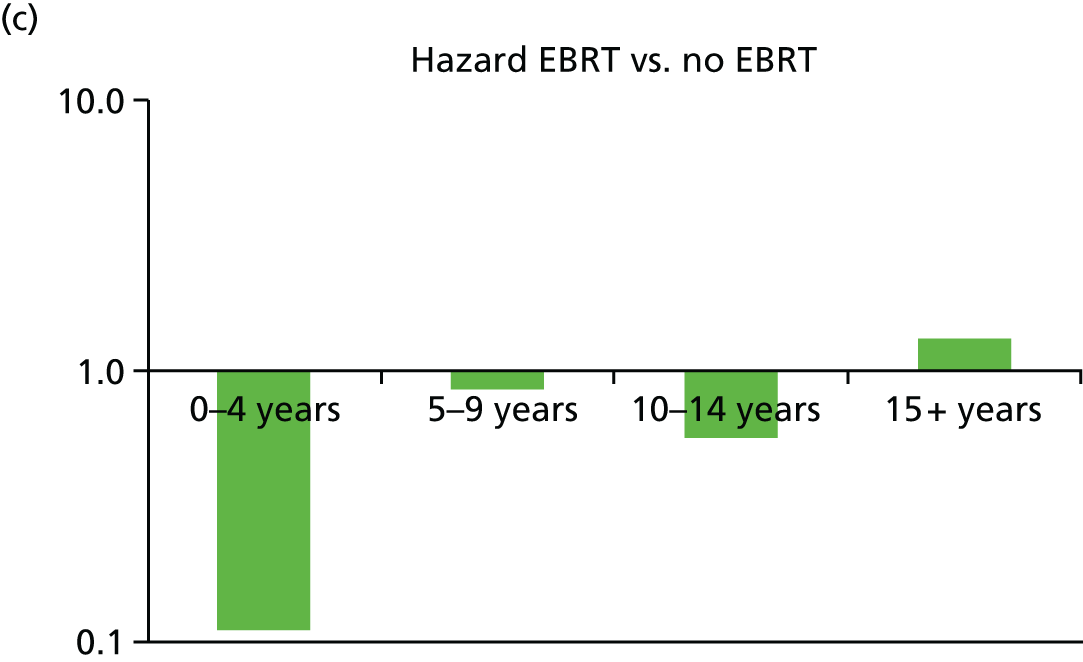
The first patient in the TARGIT-A trial was randomised on 25 March 2000 and the last patient > 12 years later on 25 June 2012. This report presents data from all of the patients randomised in this period: 3451 women in 33 centres across 11 countries. Of these 3451 patients, the first 1222 (called the earliest cohort in our 2014 paper), recruited in the first 8 years of the trial, have a median follow-up of 5 years and the first 2232 patients, originally deemed necessary for our power calculations (called the mature cohort in our 2014 paper2), have a median follow-up of close to 4 years.
Figure 35a plots each patient’s follow-up according to when they were recruited; one can see that the rate of accrual was slow initially and increased over the last few years of the trial, giving us a misleadingly lower total median follow-up. This is already accounted for in our statistical tests: Kaplan–Meier curves and the log-rank test. These give accurate calculations for risk at 5 years for the entire trial. We have also conducted our main analyses for patients randomised in the first 8 years of the trial, as these patients have a median follow-up of 5 years. The results for these 817 patients in the prepathology stratum (more than the 585 needed for statistical power) matched our overall results.
FIGURE 35.
The accrual and follow-up duration of patients in the prepathology stratum. (a) Each vertical green line represents one patient and the location of the green line on the x-axis represents the date of randomisation. The height of the green line represents the follow-up duration. The blue line represents the cumulative number of patients accrued in the trial (during 2001–2 the line was flat with no accrual because of the chief investigator working in a different centre); and (b) average accrual per month through the 12 years of the trial.
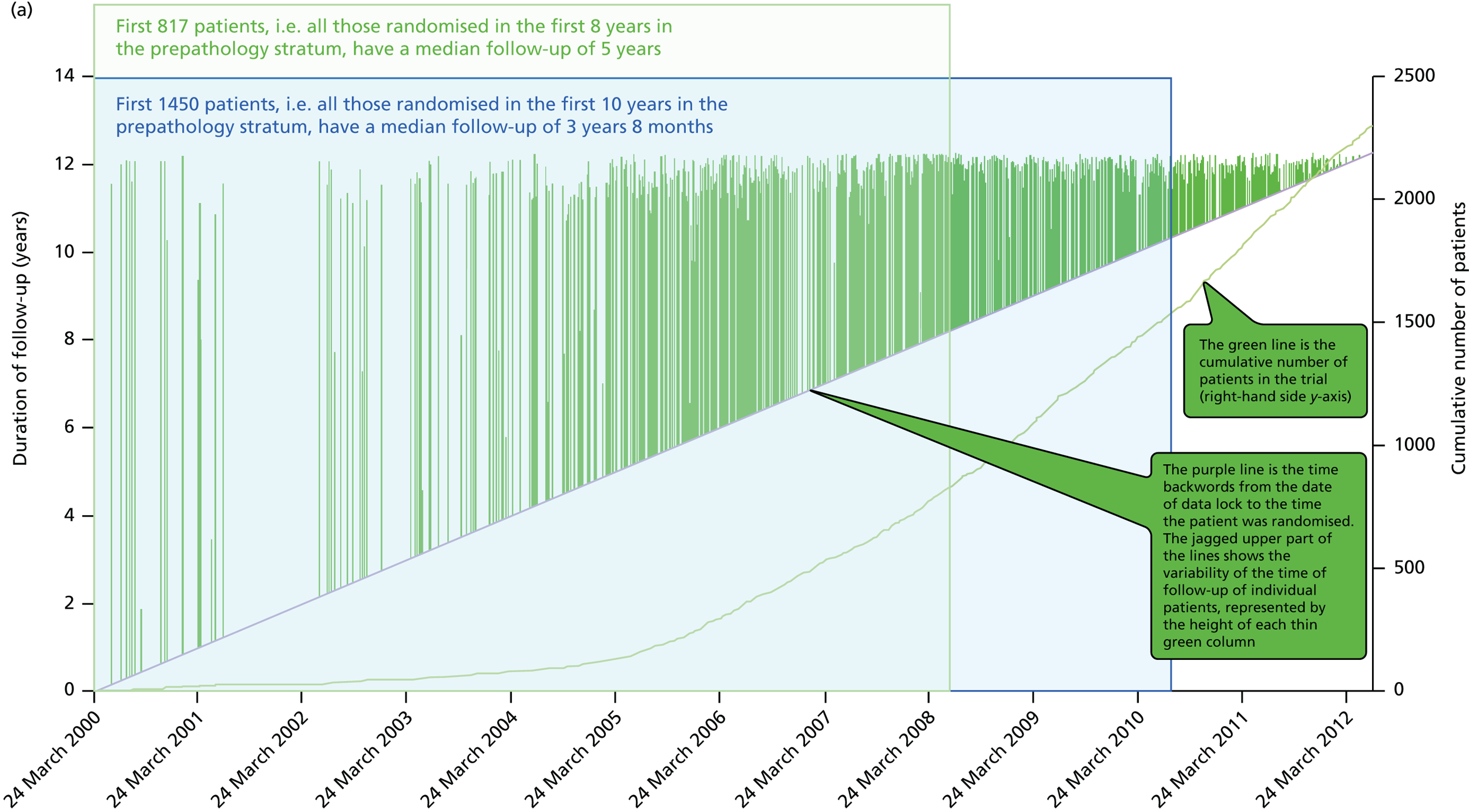
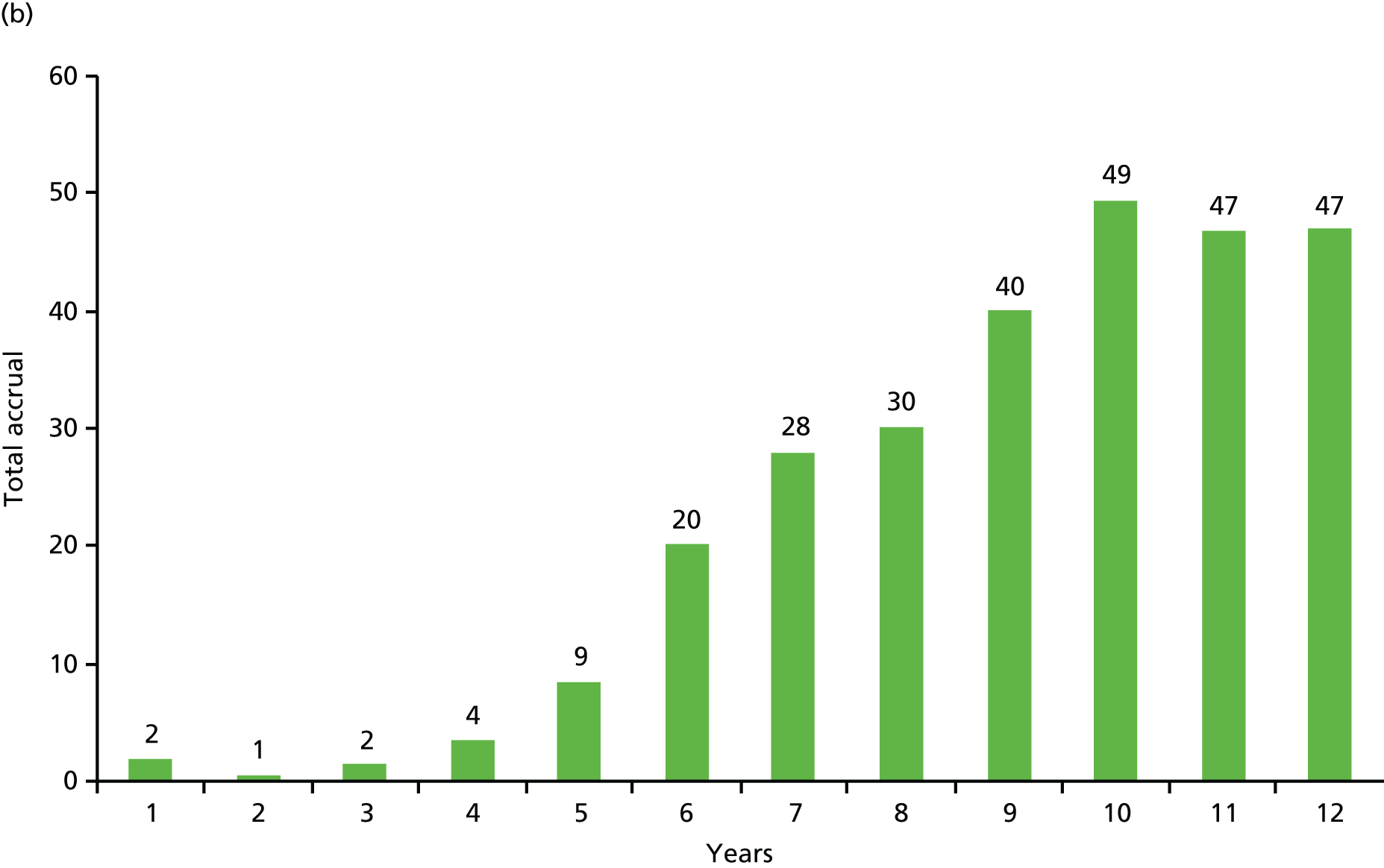
We therefore believe that the trial is sufficiently powered to demonstrate that TARGIT is non-inferior, particularly in the prepathology stratum, that is, when TARGIT is given simultaneously with the initial lumpectomy, which is the approach that we believe is preferable, based on current data, to using TARGIT as a second procedure by reopening the initial lumpectomy wound.
Two strata, two distinct treatments
The original trial design set out that patients should be randomised before the initial lumpectomy and, if allocated to TARGIT, this should be delivered at the time of surgery. When the Australian centre joined, they found it logistically difficult to give TARGIT during the first procedure. They suggested that they would like to give TARGIT as a second procedure by reopening the wound. This required us to add a new stratum with its own randomisation table: the postpathology stratum. The new stratum allowed patients to be randomised after lumpectomy. Although this allowed more stringent case selection, and simplified the logistics of arranging the theatre slot and staffing for each case, it also necessitated the reopening of the surgical wound. Thus, the difference between the postpathology stratum and the original prepathology stratum was in the timing of randomisation in relation to removal of the cancer either as a lumpectomy or an excision biopsy. The two strata differed in two major aspects: (1) in the postpathology stratum case selection could be more stringent as the tumour had already been examined and (2) in the postpathology stratum the experimental intervention was practically very different from that in the prepathology stratum: radiation was delivered to a reopened wound, with an accuracy that may not be as good as when it is delivered immediately after lumpectomy, and it was delivered to tissues that were fibrous scar rather than a pliable fresh wound.
A separate analysis was planned for these two strata of patients from the outset.
Randomisation in the TARGIT trial was carried out before lumpectomy in 2298 patients, or after the excision of the cancer in 1153 patients, in the prepathology and postpathology strata, respectively, whose treatment, selection and ultimately, the outcomes differed.
Having two strata allowed more centres to participate and we believed that the more stringent criteria would give us a nested cohort with a better cancer prognosis in whom the results might be even better. However, we did not realise that the immediacy may be so important and that irradiating the scarred tumour bed may not be as effective as giving radiation to the fresh tumour bed. Therefore, although the better case selection might have allowed for a better outcome, the different mode of delivery of radiation may have jeopardised local control, albeit by a small amount.
When analysed together, local recurrence with TARGIT was non-inferior to EBRT and there was a trend towards lower mortality with TARGIT. There was no significant difference in deaths from breast cancer but there was a significant reduction in the number of deaths from cardiovascular causes and other cancers in the TARGIT arm compared with the EBRT arm.
Analysis of the prepathology stratum also found similar results: non-inferiority for local recurrence, no significant difference in breast cancer mortality and a significant reduction in non-breast-cancer mortality.
However, in the postpathology stratum, the difference in local recurrence between TARGIT and EBRT was more than the pre-specified 2.5% non-inferiority margin. There was no difference in mortality. This relatively reduced efficacy of postpathology delivery of TARGIT may be attributable to the need to reopen the surgical wound (increasing trauma), the reduced accuracy in placement of the applicator, the radiation being given to scar tissue or missing a critical temporal window in radiation delivery; the median time between the primary surgery and TARGIT delivery in the postpathology stratum was 37 days.
Statistical tests comparing the two strata were not planned, as such comparisons would be non-randomised. Nevertheless, we believe that the difference observed is the result of the difference in TARGIT timing, as the two EBRT groups were similar in breast cancer recurrence (1.1% prepathology vs. 1.7% postpathology; p = 0.49) and non-breast-cancer mortality.
The postpathology stratum has therefore highlighted the importance of temporal precision in the delivery of radiation using this technique; the results from the postpathology stratum are not favourable, in terms of a higher local recurrence but, reassuringly, there was no decrease in mortality.
When we assessed whether or not more frequent use of additional EBRT could be the cause of better outcomes in the prepathology stratum (21% vs. 3% in postpathology stratum), we found that patients in the super-selected postpathology stratum had a much better prognosis than those in the prepathology stratum, as expected. Despite this worse prognosis, the 5-year local control in prepathology cases who received TARGIT alone appears to be better – not worse – than those in the postpathology stratum who received TARGIT alone (2.7% vs. 5.9%, respectively) and similar to that in the whole prepathology stratum (2.1%). These data suggest that delivery of TARGIT IORT at the time of initial lumpectomy leads to the accurate and timely application of radiation to a well-vascularised, undisturbed, fresh tumour bed that ensures temporal and spatial accuracy rather than that the addition of EBRT in higher-risk cases is responsible for the favourable results in the large prepathology stratum.
Furthermore, it has been reported that surgical wounds promote tumorigenesis,123 possibly via a T-cell-dependent mechanism. 124 Delivered at the time of surgery, TARGIT has been shown to reduce the motility of the wound fluid, thereby perhaps reducing recurrence through a beneficial effect on the tumour microenvironment, that is, it abrogates the stimulation of motility, proliferation and invasiveness. 17,125
Recent translational work126,127 has identified increased levels of microRNA-223 when TARGIT is delivered to the fresh surgical wound, which reduces the malignant potential of cancerous cells. TARGIT appears to act through an epidermal growth factor receptor (EGFR) pathway. A possible mechanism is shown in Figure 36. These data suggest that giving TARGIT at the time of lumpectomy would be preferable to giving it as a second procedure.
FIGURE 36.
Possible molecular biological explanation of the effectiveness of TARGIT during lumpectomy particularly in PgR positive cases. miRNA, microRNA.
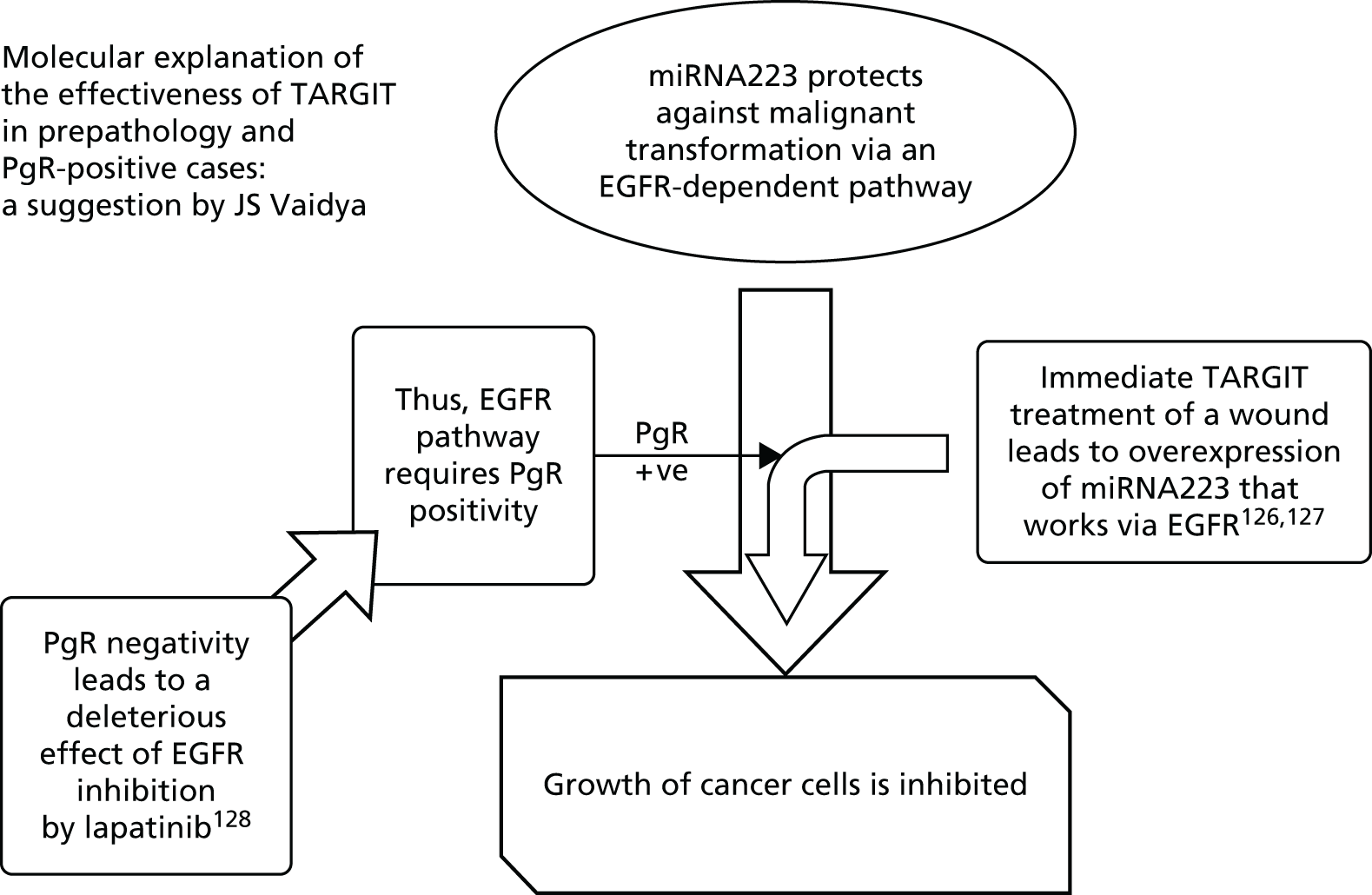
Risk-adapted approach
About one-fifth of the patients receiving TARGIT at the time of their first surgery were also given EBRT; this was indicated if pathology conducted after lumpectomy showed any adverse prognostic factors as determined in the treatment policy document and the multidisciplinary discussion of the full histopathology after surgery. Overall, that is when postpathology stratum is included, 15% of patients randomised to the risk-adapted TARGIT arm were given both TARGIT and EBRT.
We believe that a design in which the experimental arm was a risk-adapted approach was the best method of accurately reflecting practice in the real world, without sacrificing statistical validity; it may be disappointing for the women who needed supplemental EBRT but it is worth remembering that TARGIT allows the large majority of patients (80–85%) to avoid several weeks of EBRT.
Is ‘no radiotherapy’ an option? Only if one is prepared to take the risk of local recurrence
Sceptics have suggested that these 15% of patients who received both TARGIT and EBRT were indeed those very patients who would otherwise have recurred without radiation, but who did not do so because they received EBRT. The remaining 85% of patients, so the sceptics argue, were therefore those who would never have recurred even without radiation and TARGIT did not really prevent any recurrences, only the added EBRT did. If this were true, the trial guidelines detailing when EBRT was to be added have extraordinarily managed to identify all patients who might recur, based on tumour characteristics alone! Obviously, such an algorithm for case selection is currently impossible.
Although this would be fortunate, it is clearly not the case. Other studies testing the benefits of EBRT over no radiotherapy have enrolled patients whose tumour characteristics were far better than those in the TARGIT arm who did not receive EBRT have found that entirely omitting radiotherapy results in a significantly higher recurrence rate. 11,106,129 Additionally, as the postpathology stratum usually did not need additional EBRT, as this stratum was randomised after lumpectomy and pathology, these patients provided an internal control. As seen in Table 11, the tumour characteristics in this postpathology stratum were much better than those in the prepathology TARGIT arm who did not receive EBRT, but recurrence was much higher, again indicating that TARGIT, delivered at the correct time, is particularly good at avoiding local recurrence. 5,130
A recently published CALGB randomised clinical trial,11 which has addressed this even in the very best prognosis patients (age >70 years, T1N0M0, ER positive), found that giving ‘no radiotherapy’ leads to a significantly higher local recurrence rate (9%, i.e. one in every 11, vs. just 2% with radiotherapy). In a more recently reported PRIME II trial106 with super-selected patients with an extremely good prognosis (much better than that of the TARGIT-A trial patients), there was a statistically significant reduction in disease-free survival when radiotherapy was omitted and local recurrence without radiotherapy was 4.1%. Even in these highly selected good-prognosis patients, the subgroup of patients who were ER positive had a local recurrence rate of 3.2% without compared with 0.8% with radiotherapy, which is relatively much higher than that achieved by TARGIT during lumpectomy in ER-positive, PgR-positive cancers (1.4% with TARGIT vs. 1.2% with EBRT). If such patients were treated by TARGIT IORT initially, they could avoid the need for EBRT with its associated risks, inconvenience and costs as well as the increased risk of recurrence associated with completely withholding radiotherapy (see Table 20).
Other trials
Several other systems of delivering a targeted dose of radiation to the affected area of the breast have been trialled; these have been reviewed elsewhere. 112,131 It is worth keeping in mind that, of these, the TARGIT-A trial alone took a truly risk-adjusted approach. A recent meta-analysis of the TARGIT-A trial and the GEC-ESTRO (Groupe Européen de Curiethérapie ESTRO) trial of PBI with interstitial wires has found that the local control with PBI is non-inferior to whole-breast irradiation and there is a reduction in non-breast-cancer mortality. 132 Importantly, the overall survival benefit of using TARGIT IORT instead of traditional EBRT has been confirmed in the meta-analysis of all randomised trials of PBI versus whole-breast irradiation. 112
The ELIOT trial,35,133 which recently published results indicating poorer outcomes with IORT using the NOVAC-7 (New Radiant Technology) device than with EBRT,35 was the only other randomised trial testing intraoperative partial breast radiotherapy. Their technique used 4- to 12-MeV electrons to deliver 21 Gy over 3–5 minutes; this seems comparable to TARGIT’s 50 kV over 20–30 minutes to deliver 20 Gy to the tumour bed surface, a dose that attenuates to 5–7 Gy at a 1-cm depth. However, the preparation for delivery is fundamentally different. Whereas the TARGIT technique delivers radiotherapy from within the breast, with the radiation source sitting in the tumour bed – where the tumour was before its excision – and radiation given directly to the well-vascularised tissues of the tumour bed, in the ELIOT trial radiotherapy was delivered from outside the body; this therefore requires the extensive mobilisation of the mammary gland, insertion of a prepectoral lead shield and the apposition of the edges of the tumour bed. Such dissection could in theory lead to relative ischaemia, at least in the short term, of the edges of the mobilised breast gland, the very tissues that are being immediately irradiated. The higher (albeit low in absolute terms) recurrence rate seen in the ELIOT trial might therefore be because of the lower oxygen saturation of the tissues being irradiated as it is well known that tissue ischaemia reduces the effectiveness of radiation. 134–141 In addition, theoretically, some at-risk tissue such as the retracted skin and subcutaneous breast tissue and the prepectoral tissue behind the shield would not receive any radiation while exposing more raw surface to tumour seeding.
Health economics
A cost–utility analysis comparing TARGIT during lumpectomy with EBRT (prepathology stratum) was carried out. The base case showed that TARGIT was statistically significantly less costly than EBRT, produced similar QALYs, had a positive incremental net monetary benefit that was borderline statistically significantly different from zero and had a probability of > 90% of being cost-effective.
In our base case we assumed that 15.2% of patients randomised to TARGIT also received EBRT. Although standard practice during most of the period of the TARGIT-A trial was a 6-week course of EBRT, we used 15 as the number of EBRT fractions delivered because that is now standard practice in the UK. In the trial, 38% of patients randomised to EBRT also received an EBRT boost; we used this proportion for the base case as it is expected to be the norm today as well. We used costs from Picot et al. 72 of £1882.00 for TARGIT and £2659 for 15 fractions of EBRT.
In the base-case analysis TARGIT was less costly than EBRT (mean incremental cost –£685) and produced slightly more QALYs than EBRT (mean QALYs gained 0.034; see Table 16). The difference in costs between the two groups was statistically significant (mean incremental cost for TARGIT vs. EBRT –£685, 95% CI –£1131 to –£63) but the difference in QALYs was not (mean QALYs gained 0.034, 95% CI –0.026 to 0.095).
Based on the findings of this cost–utility analysis, there could be a potential budget saving to the NHS if TARGIT was carried our routinely instead of EBRT in eligible patients. The potential cost savings to the NHS would be around £8–9.1 million each year.
Taking a wider perspective (e.g. societal) to measure costs resulted in even greater potential cost savings. For example, with regard to transport costs, we assumed that 13.5% of EBRT patients used NHS transport. Assuming that the same cost is paid out-of-pocket by the remaining EBRT patients, the difference between TARGIT and EBRT would increase to £1562 per patient, taking the total saving to the UK national economy to between £18.1 million and £20.9 million each year.
The main strength of our analysis is that it was based on a large international multicentre randomised trial with detailed information on resource use and events for a median follow-up period of 5 years.
Limitations include the fact that the time horizon was 5 years. Extrapolation beyond the end of the trial using decision-analytic modelling was not undertaken because the within-trial analysis found no evidence of significant differences in QALYs between groups during the 5-year period. Although there was some evidence of a difference in costs, these differences were all accrued during the first year; there was no evidence of significant differences in costs beyond the first year. Hence, the 5-year time horizon was long enough to reflect all important differences in costs or outcomes between the two treatments. Although local recurrence (and other events) are likely to continue to occur over a patient’s lifetime, the evidence from the TARGIT-A trial is that TARGIT is non-inferior to EBRT; hence, taking a longer time horizon is unlikely to have affected the results of the incremental analyses.
Utility data were not collected in the TARGIT-A trial. We therefore applied utility values from published sources to the health states experienced by patients in the trial. Given the relatively small number of events, and that the number of events was largely not different between the two groups, the QALY differences between the two groups may not be expected to change much with alternative utility values. This was borne out by our sensitivity analysis, which showed that the results did not change appreciably when alternative values were used. We did not incorporate utility losses in our analysis associated with additional procedures, chemotherapy, mastectomy or complications. Given the low incidence of these events, that they were evenly distributed between treatment groups and that the time period affected is likely to be short this is unlikely to affect the QALYs associated with each treatment group.
Our estimates of cost savings from the use of TARGIT are likely to be conservative because, first, we did not include any utility losses associated with EBRT and, second, the analysis took a NHS/PSS perspective on costs. A wider perspective (e.g. societal and environmental costs such as carbon footprint) could have been taken to measure costs, including impacts on the rest of society, patients, families and businesses. If these costs were included it is likely that the cost savings attributable to TARGIT would be greater than demonstrated. Finally, we used the current standard dose used for EBRT (3 weeks rather than the 5–6 weeks planned for in the TARGIT-A trial).
Case selection
The results of analysis of the tumour and patient characteristics appear to predict which patients would be best suitable for TARGIT. This section discusses the findings of the subgroup analysis of tumour and patient factors that had an effect on the outcomes. As the number of events is small, some interactions may have been missed and this should be taken into consideration when interpreting the results.
Hormone sensitivity
We used PgR status as a surrogate marker for a functionally active ER receptor and found that PgR status appears to predict response to radiotherapy. Patients whose tumours were less hormone sensitive, as shown by a negative PgR status, had a significantly higher risk of local recurrence overall and in the TARGIT arm.
Interestingly, however, no such difference was observed in the EBRT arm of our trial. This could be because of the small number of events in the EBRT arm (n = 11) or because of the nature of PgR-negative tumours: the more infiltrative margins57–59 should perhaps most appropriately be irradiated by the wide and uniform field of EBRT. The smaller volume of tissue irradiated with partial breast radiotherapies could result in a failure to wholly irradiate the cancerous cells. Furthermore, the EGFR pathway by which TARGIT increases mi223 levels and therefore reduces tumour growth is PgR dependent; in ER-positive, PgR-positive patients, this may contribute to the clinical effectiveness of appropriately timed TARGIT given during lumpectomy, but with a hormone-insensitive tumour this mechanism of local recurrence reduction would not be in place. This may explain why, even though among PgR-positive cases (n = 2462) there was no significant difference between the TARGIT and the EBRT arms (2.3% vs. 1.5%; p = 0.51), in the PgR-negative cases (n = 554) local recurrence was significantly higher in the TARGIT arm (7.0% vs. 0.5%; p = 0.017).
It is possible that with more events the TARGIT and EBRT local recurrences for PgR-negative cases may equalise and that the current finding is only a mere chance finding. However, the decision to analyse by PgR status was made before unblinding of the database rather than while analysing multiple other factors. The plan for this analysis was prompted by results from other studies, for example molecular analysis of the tumours in the ELIOT trial35 had revealed IORT to be less effective with hormone receptor-negative (non-luminal A) tumours and evidence from Perou’s group (Prat et al. 57) presented in 2012 suggested that PgR-negative tumours in particular should not be classified as luminal A at all. Furthermore, statistically, both separate models and an interaction model produced statistically similar results for PgR status, hence minimising the probability that it is a chance finding.
Margin status
We found that margin status was a predictive factor for local recurrence in the EBRT arm (HR 5.17) and overall (HR 2.68). However, margin status did not significantly affect recurrence in the TARGIT arm; although this could be a chance finding (although the p-value = 0.034), it could also be because of the temporal and spatial accuracy of radiotherapy delivery that TARGIT facilitates. It is worth remembering that, although in both arms of the trial > 90% of patients with initial positive margins did undergo re-excision, only 25% of re-excision specimens had tumour present on histology, perhaps indicating inaccurate removal. Alternatively, the proliferation of the remaining tumour cells until the start of EBRT may be responsible for the local recurrence and delay does not occur when TARGIT is given immediately after lumpectomy under the same anaesthetic, so margin positivity did not come out as a factor predicting outcome after TARGIT IORT.
Forest plot
The forest plot in Figure 23 clearly shows how it is mainly ER and PgR receptor status that affected the difference between the two arms. Other factors such as age, grade, tumour size or nodal status did not have an effect; for example, TARGIT was as effective as EBRT in younger patients or those with larger or grade 3 or node-positive tumours.
Suggested modification to American SocieTy for Radiation Oncology and European SocieTy for Radiotherapy & Oncology criteria
The results of the trial suggest that effective cancer control, a reduction in toxicity and improved patient quality of life can be achieved by switching suitable patients to TARGIT, delivered at the time of surgery.
The suitability criteria for PBI that have been suggested by ASTRO142 and the European SocieTy for Radiotherapy & Oncology (ESTRO)143 might be refined based on the TARGIT-A trial results. The ASTRO and ESTRO guidelines would normally exclude many of the patients with ‘adverse’ factors who were in fact eligible for the TARGIT-A trial’s risk-adapted approach, and in the TARGIT-A trial it was found that these ‘adverse’ factors do not predict a worse outcome with TARGIT compared with EBRT. One exception is hormone sensitivity, with TARGIT appearing to work best for patients who are ER and PgR positive.
Table 19 shows how the suitability criteria for PBI may be altered using the data from the TARGIT-A trial.
| Patient and tumour characteristics | Suitable for PBI based on ASTRO consensus | Suitable for PBI based on ESTRO consensus | Eligibility and treatment as per TARGIT-A trial protocol |
|---|---|---|---|
| Age | ≥ 60 years | > 50 years | ≥ 45 years |
| Tumour size | ≤ 2 cm | ≤ 3 cm | ≤ 3.5 cm |
| Grade | Any | Any | Any |
| Histology | Invasive ductal | Invasive ductal | Invasive ductal |
| Invasive lobular | Not allowed | Not allowed | Not allowed/add EBRT if found later |
| Pure DCIS | Not allowed | Not allowed | Not allowed |
| ER status | Positive | Any | Positive had better results |
| PgR status | Any | Any | Positive had better results; results might be better if additional EBRT if PgR negative |
| HER2 status | Any | Any | Any |
| Positive margins | Not allowed | Not allowed | Additional EBRT |
| Positive nodes | Not Allowed | Not allowed | Additional EBRT, particularly if multiple positive nodes |
| Extensive lymphovascular invasion | Not allowed | Not allowed | Additional EBRT |
Chapter 8 Conclusions
Using a large multicentre randomised trial design, we have falsified the hypothesis that omission of radiation to the whole breast will significantly increase local breast cancer recurrence in quadrants away from the original cancer. We found that a risk-adapted approach using TARGIT delivered as a single dose during lumpectomy, and focused to the tissues immediately surrounding a small breast cancer, was non-inferior to several weeks of conventional whole-breast EBRT in controlling breast cancer recurrence or breast cancer death. We also found that with such focused irradiation that avoids other organs there is also some reduction in mortality from causes other than breast cancer.
Although welcomed by patients and many clinicians, the results of this trial have been subjected to a storm of criticism, which some have suggested144 could arise from an undeclared but readily available conflict of interest. We have responded to and refuted these challenges to the interpretation of our data. 64 The details of this response as well as several additional analyses are included in Chapter 11. Even if we take the worst case scenario based on a misunderstanding of the non-inferiority metric, then we are left with a 1–2%, non-significant excess of local recurrences in the experimental arm of the study, with no concomitant increase in cause-specific or all-cause mortality. We suggest that given a choice women happily opt for TARGIT to avoid several weeks of daily attendance at the radiotherapy centre or, in resource-poor parts of the world, the threat of a mastectomy. 22,100,101,103,104,114,115
From a patient’s perspective, the most important benefit of TARGIT for a woman with breast cancer is that it allows her to complete her entire local treatment (lumpectomy and radiation therapy) at the time of her operation, with lower toxicity. Therefore, the use of TARGIT in relatively mobile patients in the most developed and well-resourced communities would avoid the inconvenience and cost of this prolonged daily radiation course whereas others, who may have been obliged to choose a mastectomy, can avail themselves of breast-conserving therapy. Furthermore, the survival benefit by avoiding non-breast-cancer deaths in such patients with relatively good prognosis breast cancer has been confirmed in a recent meta-analysis. 112
From a health economic perspective, in the base-case analysis TARGIT was less costly than EBRT (mean incremental cost –£685) and produced slightly more QALYs than EBRT (mean QALYs gained 0.034). TARGIT had a positive incremental net monetary benefit that was borderline statistically significantly different from zero and had a probability of > 90% of being cost-effective. If TARGIT were given instead of EBRT in suitable patients, it might potentially reduce costs to health-care providers by £8–9.1 million each year. This does not include environmental, patient and societal costs. Taking into account the travel cost alone increases the total saving to the UK national economy to between £18.1 million and £20.9 million each year.
If our risk-adapted, pragmatic approach tested in the TARGIT-A trial is to be applied in the real world, patient selection will be of importance – those who are aged ≥ 45 years with a small (≤ 3.5 cm) invasive ductal carcinoma, bearing in mind that the addition of one positive tumour factor (PgR- or ER-positive status) appears to predict local control with TARGIT during lumpectomy that is nearly identical to that seen with conventional whole-breast EBRT. The patients who do not fulfil these criteria or for whom clinicians feel that TARGIT alone is not suitable would usually fulfil the eligibility criteria for participation in the HTA-funded TARGIT-B randomised trial, which is evaluating whether or not TARGIT boost is superior to EBRT boost when given in addition to whole-breast radiation.
Chapter 9 Implication for health care and practice
Over 250 breast cancer teams worldwide now use TARGIT, including 60 in the USA (e.g. Cleveland Clinic, OH; University of California San Francisco and University of Southern California; Cornell University, NY; Georgetown University, Washington, DC; Advocate Health Care hospitals in Chicago, IL; Moffitt Cancer Center, FL; Cancer Treatment Centers of America), about two-thirds of breast cancer centres in Germany (n = 60) and several centres in the rest of Europe. The busiest centre has treated > 500 cases.
Worldwide adoption: a survey of 125 of the 250 centres using the TARGIT IORT technique has reported that 11,740 patients have been treated until October 2015. 145 Thus, an estimate of more than 20,000 patients have already undergone this treatment in centres of excellence in the world. 146 It is considered as a standard option in several countries with advanced health-care systems, such as Germany (60 centres), Italy and the USA (60 centres).
In Australia, the Medical Services Advisory Committee, a body similar to NICE, has approved TARGIT for NHS funding based on the published evidence and the treatment made available from September 2015.
Thus, this idea and resulting research have revolutionised the treatment paradigm from radical radiotherapy to localised radiotherapy.
Impact on patients and their families: improvement in length and quality of life at a reduced cost
Many women are obliged to choose a mastectomy when they are not able to take the prolonged postoperative course of radiotherapy because of geography, time or money constraints. Many receive suboptimal treatment. With TARGIT, such women can have a lumpectomy and preserve their breast. Even among those who have a lumpectomy, TARGIT causes less pain, higher levels of patient satisfaction and higher quality-of-life scores than conventional radiotherapy. 107 There is a significant improvement in a woman’s cancer journey when the whole of local treatment is completed at the time of the cancer operation, rather than having a daily 3- to 6-week commute to the cancer hospital. TARGIT patients also have half the risk of dying from heart disease or other cancers than those undergoing EBRT. From the numbers in the trial (13 fewer deaths among 1140 patients who were randomised to receive TARGIT at the time of lumpectomy), one can extrapolate that, of 20,000 patients, 228 such deaths have already been prevented. In communities in which patients have to pay for their treatment, TARGIT is a fraction of the cost of conventional radiotherapy, leading to more equitable availability of treatment.
Impact on routine treatment guidelines/recommendations for patients in the community
The TARGIT-A trial was the first proof of principle of ‘partial breast irradiation’ and other methods of giving PBI have proliferated. TARGIT, either as a tumour bed boost or as definitive treatment, as well as other methods of PBI are now included in guidelines issued by the European Society of Breast Cancer Specialists147 and the European Society of Medical Oncology (which are also endorsed by the Japanese Society of Medical Oncology)148 and in German national guidelines. 149
In March 2011, at the biennial international St Gallen Consensus Conference, 52 world experts voted in favour of using intraoperative radiation in selected patients. At this time, the only level 1 randomised evidence was from the TARGIT-A trial. 150 In December 2012, the newsletter at the largest breast cancer conference at St Gallen featured our late-breaking paper. 151 Our research has also attracted significant media attention both in scientific periodicals and in the lay press (copies of articles without URLs are available on request). 152–167
National Institute for Health and Care Excellence is currently consulting on the use of TARGIT in routine practice and gave a provisional recommendation for its funding in the NHS in July 2014 [see www.nice.org.uk/guidance/GID-TAG353/documents/breast-cancer-early-intrabeam-radiotherapy-system-appraisal-consultation-document (accessed 6 July 2016)]. Since then they have requested further information, which has been supplied (see details in Chapter 11). 168 The Marmot committee on screening has suggested that, in patients whose cancers are found only on mammographic screening, if TARGIT is used rather than EBRT this would minimise side effects because of the overdiagnosis and overtreatment that is known to occur with mammographic screening. 169 We have also demonstrated that TARGIT is a method for avoiding the cardiac toxicity of EBRT. 170
Impact on health-care delivery and budget
Breast cancer constitutes one-third of the workload of a typical radiotherapy department and in many areas there can be long waiting lists. When a significant proportion of this time is freed up by using TARGIT in the operating theatre, it can be used to treat other cancers in a timely manner. A recently published paper originating from several centres in France98 concluded that > 50% of patients with early breast cancer would be suitable for TARGIT.
One estimate is that the 20,000 patients who had TARGIT during their lumpectomy would have required 20,000 × 20 = 400,000 radiotherapy sessions rather than the single session in the operating theatre if they were to have lumpectomy and conventional radiotherapy. Assuming that TARGIT takes the time equivalent of four routine radiotherapy sessions, this is a saving of 400,000 – 80,000 = 320,000 radiotherapy sessions to date. Assuming that each session costs a conservative £200, the saving of 320,000 sessions has already saved £64 million worldwide. It is estimated that adoption of TARGIT in the UK will potentially save £8–9.1 million per year. A North American model has predicted this amount to be US$280 million in the USA. 171
Chapter 10 Recommendations for research
This large international randomised clinical trial has challenged the existing dogma and has opened a door to a new way of thinking about adjuvant radiotherapy, particularly for breast cancer.
Future research should focus on assessing the biological mechanisms of action of this type of radiation given in this manner, to the fresh tumour bed. Of particular interest is the way in which it appears to alter the tumour microenvironment.
Addition of EBRT to TARGIT appears to have interesting effects. These need to be studied in further detail and we hope that the clinical data from the TARGIT-B trial will be helpful in this regard. Funding for translational research would allow for biological pathways to be discovered and may even lead to the development of drugs that could replace the need for radiation.
The effect that we have observed on non-breast-cancer mortality is interesting and needs to be investigated further with an open mind; if the mechanism is not simply the avoidance of EBRT toxicity, it may well open several new doors.
For the health economic analyses, further research is required to measure directly the impact of TARGIT on patient utility values. In addition, future analyses should take a wider (e.g. societal) perspective when measuring costs.
With regard to further work on the TARGIT-A trial population, including continuing to collect follow-up information for 10 years on every patient, details are provided in Appendix 4.
Chapter 11 Further analysis as requested by the National Institute for Health and Care Excellence
Introduction
National Institute for Health and Care Excellence is currently appraising the TARGIT technique for adoption in the NHS.
On 27 July 2014, NICE issued a press release and published draft guidance on the use of INTRABEAM radiotherapy, giving a draft recommendation for the use of TARGIT in the NHS (see Appendix 5).
Following this announcement widespread positive media attention was given to the research and its results and how it could improve the care of patients with breast cancer. However, there was an unprecedented level of opposition to the recommendations, mainly from a small group of individuals in the radiation oncology community. This led to a prolonged delay in the final recommendations, which were due to be announced in August 2014.
From July 2014, the TARGIT investigators, as part of the response to NICE, collaborated with various departments in the Department of Health to create tables that show how the use of TARGIT in the NHS would be coded. The detailed tables of these codes, prepared in collaboration with the national cancer registries and the national coding teams, are provided in Appendix 6.
In the course of communications with NICE, NICE requested further analysis. It requested that we should use the dataset that was used for the recent Lancet publications without re-unblinding of the data.
Such analysis was performed and submitted to NICE in March 2015. The TARGIT investigators also offered to provide the raw data but were told that NICE were satisfied with the analysis and did not wish to see the raw data.
This chapter provides the details of this new analysis.
Further information requested by the National Institute for Health and Care Excellence
National Institute for Health and Care Excellence requested further information from the authors in August 2014. These were initially supplied in a meeting in December 2014 and the following further information was supplied on 10 March 2015 in a face-to-face meeting. At this meeting it was clarified that we had initially supplied the integrated difference between the Kaplan–Meier curves but that NICE had requested that we also supply the difference in the values for the 5-year Kaplan–Meier estimates as well as the 95% CIs. It was agreed that the NICE committee recognised that:
-
The right-hand end of the Kaplan–Meier curve is the one with the most uncertainty and with the widest CIs.
-
In the presence of censoring the Kaplan–Meier point estimate at a particular time point, for example 5 years are not a simple binomial proportions, and treating them as a binomial proportion introduces a bias resulting in a wider CI. Therefore, it is inappropriate to apply the simple formula normally used to calculate the SE (and CI) of a difference between binomial proportions, namely SE12+SE22 to calculate differences between such point estimates.
-
These values, that is, 5-year point estimates, should not be used to calculate the CI of the difference or for testing non-inferiority.
-
These values are being requested by NICE mainly for completion and will not be wrongly used to assess non-inferiority.
The rest of this chapter includes all of the graphs and statistics requested by the committee, as of the data lock on 25 June 2012, in addition to the blinded analysis including new events until 1 October 2014.
In response to the recommendation in the draft recommendation, we have also included a diagram to help patients make a shared decision along with their consultant.
Executive summary of the analysis provided to the National Institute for Health and Care Excellence
-
This document provides all of the additional analyses requested by NICE. Following the initial request, NICE and the TARGIT-A trial investigators met on 9 December 2014. The TARGIT-A investigators gave a response on 18 December. NICE responded on 16 February 2015, which was followed by a meeting on 3 March to discuss and confirm that all of the required analyses had been included.
-
It is well established that the peak hazard of recurrence is in the first 2–3 years. Most importantly, the effect of radiotherapy on local recurrence is limited to the first 5 years, with most of the radiotherapy effect being seen in the first 2–3 years.
-
The results in the TARGIT-A trial were obtained despite the fact that the eligibility criteria and cases in the trial were not limited to very-low-risk cases (between 450 and 550 patients had a grade 3 tumour or a tumour size > 2 cm or node-positive disease).
-
With over 1200 patients with a median follow-up of 5 years, there were very few events for local recurrence (n = 34), suggesting that both treatments work very well; on the other hand, the number of events for local recurrence-free survival was substantial (n = 120) and similar with TARGIT and EBRT, giving sufficient data to change clinical practice.
-
The Kaplan–Meier curves for local recurrence, survival without local recurrence, overall survival, breast cancer survival and disease-free survival all demonstrated that the lines representing TARGIT and EBRT with their 95% CI overlap each other.
-
On the other hand, the 95% CIs for the TARGIT and EBRT curves for non-breast-cancer survival do not overlap, demonstrating the previously published statistically significant difference.
-
Absolute difference between Kaplan–Meier curves:
-
the appropriate method of calculating the difference between Kaplan–Meier curves was by using the integrated difference of the two survival functions to quantify the difference between the Kaplan–Meier curves
-
for the primary outcome of local recurrence, the difference between the Kaplan–Meier curves for TARGIT and EBRT from 0 to 5 years was as follows:
-
for the whole trial 0.62% (95% CI 0.007% to 1.24%)
-
for the prepathology stratum 0.3% (95% CI –0.4% to 1.03%).
-
-
-
Data from further follow up: as of October 2014, the number of patients with a minimum follow-up of 5 years in the whole trial was 1116. For the prepathology stratum this number was 776, with 15 new events of local recurrence in addition to the previous 16. We were told that unplanned unblinding for new analysis was not necessary. Therefore, we have remained blind to the apportioning of the new events and have provided a blinded analysis. In the most plausible hypothetical scenario, weighted against TARGIT the new local recurrences might be distributed as TARGIT 10, EBRT 5. In this case, the difference between the binomial proportions of the two arms would be 0.83 (90% CI 0.0 to 1.6, 95% CI –0.1 to 1.8; Pnon-inferiority = 0.00038) and TARGIT would remain non-inferior to EBRT.
-
There was no significant difference between TARGIT during lumpectomy and EBRT by conventional log-rank test (p = 0.31) and TARGIT was non-inferior to EBRT using the standard test for non-inferiority (Pnon-inferiority < 0.00001) and the 5-year survival without local recurrence was 93.9% (95% CI 90.9% to 95.9%) for TARGIT and 92.5% (95% CI 89.7% to 94.6%) for EBRT (p = 0.35).
-
Mortality cannot be ignored when a difference is generated within a randomised trial (TARGIT 29 vs. EBRT 42 deaths), particularly when the number of events is several times higher than the number of local recurrence (71 deaths vs. 16 local recurrences) and death is clearly an important outcome. If a statistically significant difference is found at the time of analysis initial ‘power’ calculations are not relevant.
-
Cosmetic outcome and quality of life have been shown to be better with TARGIT than with EBRT as per the published randomised data from the TARGIT-A trial.
-
In conclusion, using three quite separate statistical methodologies, the risk-adapted approach using single-dose TARGIT IORT given during lumpectomy is found to provide breast cancer control that is not inferior to that seen with several weeks of conventional radiotherapy; it also provides a more cost-effective treatment that is more convenient for patients.
-
Figure 37 provides a pictogram to help patients and doctors make a shared well-informed decision.
FIGURE 37.
A pictogram to help patients and doctors to make a shared, well-informed decision.
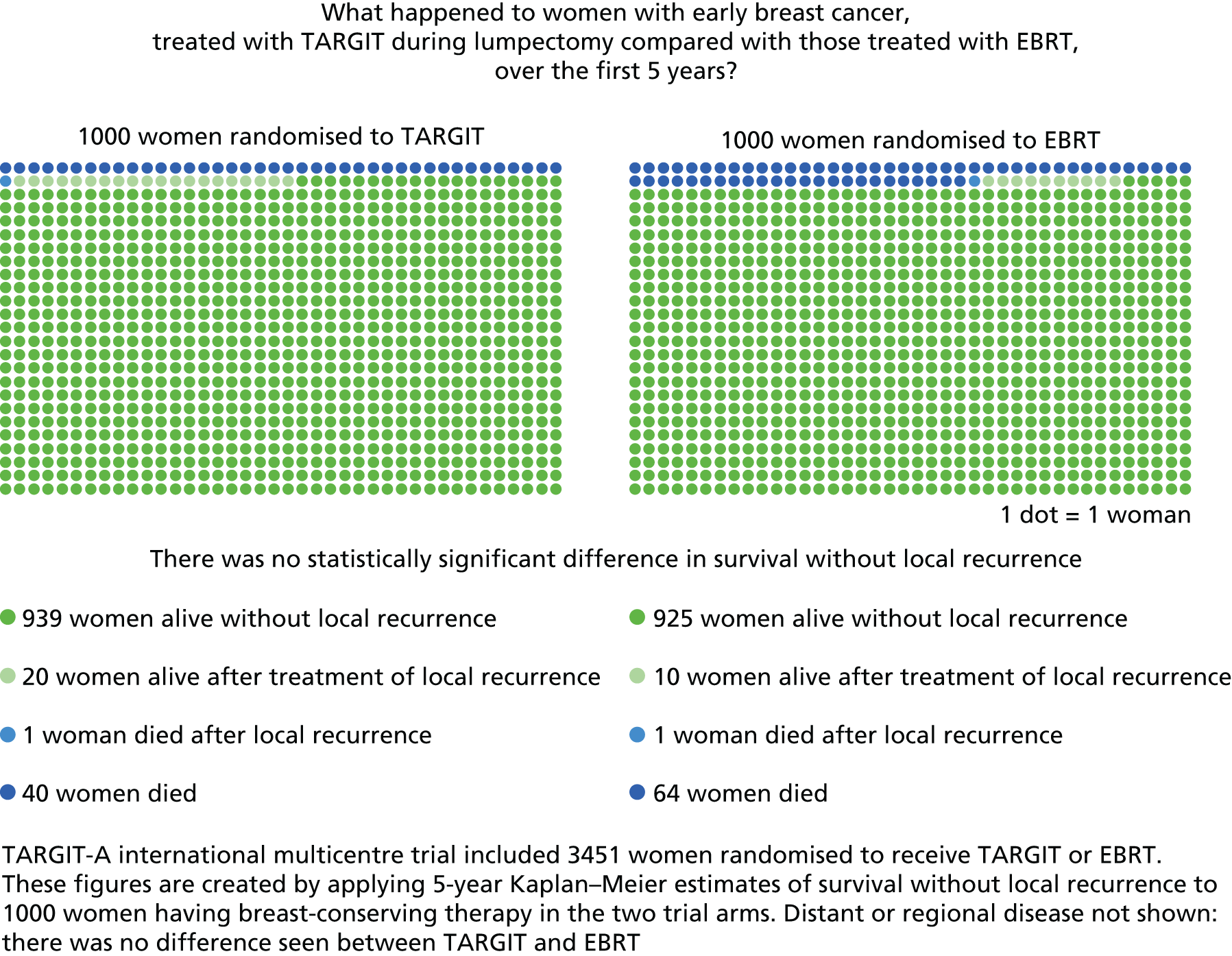
Important considerations about the use of radiotherapy for breast cancer
Breast cancer surgery has evolved from being very radical (e.g. radical mastectomy and axillary clearance) to being more individualised and precise (lumpectomy and sentinel node biopsy).
Even though randomised evidence supported less radical treatment, there was strong initial opposition to its adoption only a few decades ago. The TARGIT-A trial has provided evidence that radiotherapy also does not need to be radical and should be more precisely and individually optimised. The opposition to its adoption appears to be similar to that shown by strong proponents of radical mastectomy towards those who wanted to spare women this mutilating operation. Some important and relevant points about the natural history of breast cancer have been elucidated via the results of several randomised controlled trials:
-
The peak hazard of recurrence is in the first 2–3 years and, more importantly, the effect of radiotherapy on local recurrence is limited to the first 5 years, with most of the radiotherapy effect being seen in the first 2–3 years. 4,119,121,122 This is clearly seen from the figures below. Thus, local recurrence occurring between 5 and 25 years is no more frequent in non-irradiated patients than in those who have received radiotherapy. 4,119,121,122
[Parts of Figures 33 and Figures 34 that were included in the correspondence are reproduced here for ease of reading.] These figures demonstrate how the reduction in local recurrence by radiotherapy occurs only in the first 5 years, with most of the effect already seen in the first 2–3 years. Figure 33b (top figure) is the Kaplan–Meier plot from the landmark NSABP B06 trial by Fisher et al. 119 of radiotherapy compared with no radiotherapy after lumpectomy. Figure 34a (middle figure) is the Kaplan–Meier plot taken from the Swedish trial by Wickberg et al. 121 of radiotherapy compared with no radiotherapy after lumpectomy with a 25-year follow-up. Figures 34b and c (bottom figures) are also based on the data from the same Swedish trial by Wickberg et al. 122
Therefore, the available follow-up in the TARGIT-A trial provides enough information to enable its use in clinical practice.
-
When the reduction in local recurrence because of radiotherapy is < 10%, there is no discernible benefit for survival from breast cancer.
-
The detrimental effect of conventional whole-breast radiotherapy on non-breast-cancer deaths (e.g. from cardiac disease/other cancers) becomes more important when deaths from breast cancer are few and this effect starts in the first few years.
-
It has been suggested that TARGIT treatment is as good as ‘no radiotherapy’. Table 20 provides the results of randomised controlled trials testing the effect of completely omitting radiotherapy. As can be seen clearly in the table, one in every 17–25 of even the most stringently selected low-risk patients would have a local recurrence if radiotherapy were omitted. 11,106,129 On the other hand, when TARGIT is given during lumpectomy local recurrence is rare – one in 48, which reduces to one in 71 when just a single selection criterion (ER positive) is applied.
-
Importantly, TARGIT-A trial eligibility was not limited to ‘good prognosis’ cases. In fact, 85% of patients in the TARGIT-A trial were aged < 70 years and there were a large number of patients in each of the adverse prognostic groups such as node positive (n = 502), ER or PgR negative (n = 554), grade 3 (n = 459) or tumour size > 2 cm (n = 397); > 60% of cases in the TARGIT-A trial would be considered ‘unsuitable’ or ‘cautionary’ by the ASTRO criteria for PBI;172 and only 17.5% of patients in the TARGIT-A trial prepathology stratum would have been eligible for the PRIME II trial – all others (82.5%) had ‘worse’ prognosis cancers. The results in the trial were obtained despite the fact that the eligibility criteria and cases in the TARGIT-A trial were not limited to very-low-risk cases.
-
In a non-inferiority trial, if the difference between the treatments being tested (and its upper confidence limit) is less than a preset non-inferiority margin the treatments are considered non-inferior, even if the difference is ‘statistically significant’ using a log-rank test. The pre-specified non-inferiority boundary of a 2.5% absolute difference in local recurrence is very conservative and has been validated in patient preference studies. 101,103,104 At this boundary, TARGIT is non-inferior to EBRT (Pnon-inferiority < 0.00001) (Figure 38). Furthermore, we have recommended that TARGIT should be used during the initial lumpectomy, as in the prepathology stratum (note that this is a stratum not a subgroup), when the difference between the two treatments was undoubtedly not statistically significant (p = 0.31).
FIGURE 38.
The meaning of non-inferiority: 10 examples of different scenarios that might occur in a randomised trial testing non-inferiority between two treatments. The dots represent the absolute difference and lines the CIs. The blue circle includes two of the trial results: on the left is the TARGIT prepathology stratum (difference 0.37%) and on the right is the earliest cohort of the whole trial (n = 1222), who have a median follow-up of 5 years (difference 1.14%) (see table 3 in Vaidya et al. 2). 2,102

Whole-study population: local recurrence
-
Local recurrence:
-
The absolute number of local recurrence events (n) (Table 21).
-
A Kaplan–Meier analysis including all patients from TARGIT-A for each treatment group showing the cumulative risk of local recurrence over time using the latest available follow-up data. The requested figures for this survival analysis are given below, with or without censor lines and 95% CI curves (Figures 39–43). Figure 44 shows the 5-year survival without local recurrence. The numbers of patients at risk of local recurrence in each treatment group at yearly intervals are reported below the plot.
-
The absolute difference in the Kaplan–Meier estimate of the 5-year risk of local recurrence between treatment groups and the 95% CI around that difference.
-
In the paper,2 we reported the 90% CI of the difference in binomial proportions, not of the difference in Kaplan–Meier estimates (for non-inferiority testing the convention is to use the 90% CI rather than the 95% CI105,173–175). The difference between the binomial proportions of local recurrence for TARGIT and EBRT was 0.72% (90% CI 0.15% to 1.30%) (95% CI 0.05% to 1.40%).
-
In the presence of censoring, the Kaplan–Meier point estimate at a particular time point, for example 5 years, is not a simple binomial proportion. Therefore, it is inappropriate to apply the simple formula normally used to calculate the SE (and CI) of a difference between binomial proportions, namely SE12+SE22, to calculate differences between such point estimates.
-
Furthermore, when looking at Kaplan–Meier curves, the right-hand end of the curve is the one with the most uncertainty and with the widest CIs. These values, that is, 5-year point estimates, should not be used to calculate the CI of the difference or for testing non-inferiority.
-
-
National Institute for Health and Care Excellence agreed that the differences in 5-year Kaplan–Meier estimates are not to be used for assessing non-inferiority, but are provided for completion. For local recurrence the difference is 2% (95% CI –0.14% to 4.14%) (90% CI 0.18% to 3.82%). For survival without local recurrence the difference is 0.64% (95% CI –2.09% to 3.37%) (90% CI –1.69% to 2.97%).
The appropriate method for calculating the difference between Kaplan–Meier curves is by using the integrated difference of the two survival functions to quantify the difference between the Kaplan–Meier curves. 176 The difference between the Kaplan–Meier curves for TARGIT and EBRT from 0 to 5 years is 0.62% (95% CI 0.007% to 1.24%).
Whole-study population: survival
-
Survival (whole trial).
-
The absolute number of deaths (n) (Table 22).
-
The number of patients with different causes of death (n) (Table 23).
-
Kaplan–Meier analysis of mortality:
A Kaplan–Meier analysis including all patients from TARGIT-A for each treatment group showing the cumulative risk of death over time using the latest available follow-up data. The two requested figures for this survival analysis are given below, with or without 95% CI curves (Figures 44 and 45).
-
The absolute difference in the Kaplan–Meier estimate of the 5-year risk of overall mortality between treatment groups (TARGIT and EBRT) in the whole-study population and the 95% CI around that difference. Note that the caveats mentioned in Whole-study population: local recurrence about using 5-year estimates to calculate the difference between treatments and its CI should be read before using these figures. The difference between 5-year Kaplan–Meier estimates is –1.38% (95% CI –3.67% to 0.91%) (90% CI –3.3% to 0.57%). Using the integrated difference of the two survival functions to quantify the difference between the Kaplan–Meier curves,176 the difference between the Kaplan–Meier curves for TARGIT and EBRT from 0 to 5 years is –0.85% (95% CI –1.75% to 0.04%).
-
-
Breast cancer mortality (whole trial).
-
The absolute number of breast cancer deaths (n) (Table 24).
-
Kaplan–Meier analysis of breast cancer deaths.
A Kaplan–Meier analysis including all patients from TARGIT-A for each treatment group showing the cumulative risk of breast-cancer death over time using the latest available follow-up data. The two requested figures for this survival analysis are given below, with or without 95% CI curves (Figures 46 and 47).
-
The absolute difference in the Kaplan–Meier estimate of the 5-year risk of breast cancer mortality between treatment groups (TARGIT and EBRT) in the whole-study population and the 95% CI around that difference. Note that the caveats mentioned in Whole-study population: local recurrence about using 5-year estimates to calculate the difference between treatments and its CI should be read before using these figures. The difference between 5-year Kaplan–Meier estimates is 0.67% (95% CI –1.01% to 2.35%) (90% CI –0.76% to 2.10%). Using the integrated difference of the two survival functions to quantify the difference between the Kaplan–Meier curves,176 the difference between the Kaplan–Meier curves for TARGIT and EBRT from 0 to 5 years is 0.15% (95% CI –0.71% to 0.42%).
-
-
Non-breast-cancer mortality (whole trial).
-
The absolute number of non-breast-cancer deaths (n) (Table 25).
-
Kaplan–Meier analysis of non-breast-cancer mortality:
A Kaplan–Meier analysis including all patients from TARGIT-A for each treatment group showing the cumulative risk of non-breast-cancer death over time using the latest available follow-up data. The two requested figures for this survival analysis are given below, with or without 95% CI curves (Figures 48 and 49).
-
The absolute difference in the Kaplan–Meier estimate of the 5-year risk of non-breast-cancer mortality between treatment groups (TARGIT and EBRT) in the whole-study population and the 95% CI around that difference. Note that the caveats mentioned in Whole-study population: local recurrence about using 5-year estimates to calculate the difference between treatments and its CI should be read before using these figures. The difference between 5-year Kaplan–Meier estimates is –2.08% (95% CI –3.70% to –0.46%) (90% CI –3.46% to –0.70%). Using the integrated difference of the two survival functions to quantify the difference between the Kaplan–Meier curves,176 the difference between the Kaplan–Meier curves for TARGIT and EBRT from 0 to 5 years is –0.72 (95% CI –1.42 to –0.02).
-
-
Tabulation of the number of patients with at least 5 years of follow-up data (Table 26).
Prepathology stratum: local recurrence
-
Local recurrence.
-
The absolute number of local recurrence events (n) (Table 27).
-
A Kaplan–Meier analysis including all patients in the prepathology stratum of the TARGIT-A trial for each treatment group showing the cumulative risk of local recurrence over time using the latest available follow-up data. The requested figures for this survival analysis are given below, with or without censor lines and 95% CI curves (Figures 50–53). Figure 54 shows the 5-year survival without local recurrence. The numbers of patients at risk of local recurrence in each treatment group at yearly intervals are reported below the plot.
-
The absolute difference in the Kaplan–Meier estimate of the 5-year risk of local recurrence between treatment groups and the 95% CI around that difference.
-
In the paper,2 we reported the 90% CI of the difference in binomial proportions, not of the difference in Kaplan–Meier estimates (for non-inferiority testing the convention is to use the 90% CI rather than the 95% CI105,173–175).
-
The difference between binomial proportions of local recurrence for TARGIT and EBRT was 0.37% (90% CI –0.23% to 0.97%) (95% CI –0.33% to 1.07%).
-
In the presence of censoring, the Kaplan–Meier point estimate at a particular time point, for example 5 years, is not a simple binomial proportion. Therefore, it is inappropriate to apply the simple formula normally used to calculate the SE (and CI) of a difference between binomial proportions, namely SE12+SE22, to calculate differences between such point estimates.
-
Furthermore, when looking at Kaplan–Meier curves, the right-hand end of the curve is the one with the most uncertainty and with the widest CIs. These values, that is, 5-year point estimates, should not be used to calculate the CI of the difference or for testing non-inferiority.
-
-
National Institute for Health and Care Excellence agreed that the differences in 5-year Kaplan–Meier estimates are not to be used for assessing non-inferiority, but are provided for completion. For local recurrence the difference is 1% (95% CI –0.68% to 2.68%) (90% CI –0.43% to 2.43%). For survival without local recurrence the difference is –1.32% (95% CI –4.74% to 2.10%) (90% CI –4.24% to 1.60%).
If the difference in Kaplan–Meier estimates is being used to assess the difference between treatments (despite the concerns about using these), then although the upper confidence limit of the 95% CI of the difference in local recurrence is 2.68%, the absolute difference in overall mortality between TARGIT and EBRT is –2.33% (95% CI –5.48% to 0.82%), favouring TARGIT. The lower confidence limit of the 95% CI for overall mortality is –5.48 and this is a very much larger value than for local recurrence and is in the opposite direction – for a much more important outcome. It would mean that, although at worst the difference in local recurrence might be 2.68% favouring EBRT, the difference in overall mortality might be 5.48% favouring TARGIT. One cannot consider one and ignore the other value.
In addition, while discussing the actual difference between recurrence rates (not for non-inferiority determination), both the 95% confidence limits need to be looked at, not just one. If the upper 95% confidence limit is considered, that is, 2.68%, as the worst-case scenario, then the lower 95% confidence limit (–0.68%) should also be considered as it has exactly the same chance of occurring.
The chance of being alive without local recurrence is the most relevant statistic (there cannot really be a local recurrence if the patient is no longer alive). For this statistic, the two treatments have very similar outcomes; the number of events is also large and similar in the two arms [TARGIT 57 vs. EBRT 59 for the whole trial (n = 3451) and TARGIT 36 vs. EBRT 45 for the prepathology stratum (n = 2298): the number of events without excluding those who had a mastectomy were 120 (59 vs. 61) in the whole trial and 85 (TARGIT 38 vs. EBRT 47) in the prepathology stratum].
The appropriate method of calculating the difference between Kaplan–Meier curves is by using the integrated difference of the two survival functions to quantify the difference between the Kaplan–Meier curves. 176 The difference between the Kaplan–Meier curves for TARGIT and EBRT from 0 to 5 years is 0.3% (95% CI –0.4% to 1.03%).
Prepathology stratum: survival
-
The absolute number of deaths (n) (Table 28).
-
The number of patients with different causes of death (n) (Table 29).
-
Kaplan–Meier analysis of survival:
A Kaplan–Meier analysis including all patients in the prepathology stratum of the TARGIT-A trial for each treatment group showing the cumulative risk of death over time using the latest available follow-up data. The two requested figures for this survival analysis are given below, with or without 95% CI curves (Figures 55 and 56).
-
The absolute difference in the Kaplan–Meier estimate of the 5-year risk of overall mortality between treatment groups (TARGIT and EBRT) in the whole-study population and the 95% CI around that difference. Note that the caveats mentioned in Whole-study population: local recurrence about using 5-year estimates to calculate the difference between treatments and its CI should be read before using these figures. The difference between the 5-year Kaplan–Meier estimates is –2.33% (95% CI –5.48% to 0.82%) (90% CI –5.02% to 0.36%). Using the integrated difference of the two survival functions to quantify the difference between the Kaplan–Meier curves,176 we found that the difference between the Kaplan–Meier curves for TARGIT and EBRT from 0 to 5 years is –1.43% (95% CI –2.66% to –0.2%).
-
Breast cancer mortality (prepathology stratum).
-
The absolute number of breast cancer deaths (n) (Table 30).
-
Kaplan–Meier analysis of survival:
A Kaplan–Meier analysis including all patients in the prepathology stratum of the TARGIT-A trial for each treatment group showing the cumulative risk of breast cancer death over time using the latest available follow-up data. The two requested figures for this survival analysis are given below, with or without 95% CI curves (Figures 57 and 58).
-
The absolute difference in the Kaplan–Meier estimate of the 5-year risk of breast cancer mortality between treatment groups (TARGIT and EBRT) in the whole-study population and the 95% CI around that difference. Note that the caveats mentioned in Whole-study population: local recurrence about using 5-year estimates to calculate the difference between treatments and its CI should be read before using these figures. The difference between the 5-year Kaplan–Meier estimates is 0.66% (95% CI –1.72% to 3.04%) (90% CI –1.36% to 2.68%). Using the integrated difference of the two survival functions to quantify the difference between the Kaplan–Meier curves,176 we found that the difference between the Kaplan–Meier curves for TARGIT and EBRT from 0 to 5 years is –0.34% (95% CI –1.17% to 0.49%).
-
-
Non-breast-cancer mortality (prepathology stratum).
-
The absolute number of non-breast-cancer deaths (n) (Table 31).
-
Kaplan–Meier analysis of survival:
A Kaplan–Meier analysis including all patients in the prepathology stratum of the TARGIT-A trial for each treatment group showing the cumulative risk of non-breast-cancer death over time using the latest available follow-up data. The two requested figures for this survival analysis are given below, with or without 95% CI curves (Figures 59 and 60).
-
The absolute difference in the Kaplan–Meier estimate of the 5-year risk of non-breast-cancer mortality between treatment groups (TARGIT and EBRT) in the whole-study population and the 95% CI around that difference. Note that the caveats mentioned in Whole-study population: local recurrence about using 5-year estimates to calculate the difference between treatments and its CI should be read before using these figures. The difference between the 5-year Kaplan–Meier estimates is –3.07% (95% CI –5.25% to 0.89%) (90% CI –4.93% to 1.21%). Using the integrated difference of the two survival functions to quantify the difference between the Kaplan–Meier curves,176 we found that the difference between the Kaplan–Meier curves for TARGIT and EBRT from 0 to 5 years is –1.11% (95% CI –2.05% to –0.17%).
-
-
Tabulation of the number of patients with at least 5 years of follow-up data (Table 32).
New analysis
-
During the meeting of 8 December 2014, we presented an additional analysis of the data published in The Lancet,2 namely that the disease-free survival of the patients in the TARGIT and EBRT arms is identical for the whole trial (p = 0.78) and for the prepathology stratum (p = 0.68), with the Kaplan–Meier curves overlapping each other (Figure 61).
FIGURE 61.
Ten-year Kaplan–Meier estimates for disease-free survival for (a) all patients; and (b) the prepathology stratum [TARGIT 91.6% (95% CI 88.7% to 93.8%) vs. EBRT 90.1% (95% CI 86.8% to 92.6%) at 5 years and TARGIT 81.3% (95% CI 71% to 88%) vs. EBRT 71.2% (95% CI 49% to 85%) at 10 years].


-
As it was made clear to us that the NICE committee did not expect us to unblind the trial at this moment, we presented new updated data resulting from increased follow-up as of 1 October 2014, using the total number of events without unblinding the trial.
-
Further follow up: at the time of this analysis, the median follow-up was 4 years, which means that a very large number of patients (n = 1725) had at least 4 years of follow-up or longer. This number is large compared with the majority of breast cancer trials.
-
New events: with additional follow-up since the last data lock in June 2012, there was a total of 15 new local recurrences in the prepathology stratum. This was in addition to the 16 already reported in The Lancet,2 out of a total number of 2298 patients.
-
Remaining blind to the randomisation arm, we presented two hypothetical scenarios – one worst-case scenario and one less extreme but still weighted against TARGIT.
-
For the 15 total new events of local recurrence, rather than an even split of seven compared with eight or eight compared with seven, remaining blind to the randomisation arm we modelled the events as in Tables 33 and 34.
-
For death, the secondary end point, there were 28 new events and these would need to have occurred in a ratio of 20 in the TARGIT arm to 8 in the EBRT arm to equalise the total number of deaths between the two treatments (Table 35). As the initial observation was 29 deaths in the TARGIT arm and 42 in the EBRT arm, the probability of such a drastic reversal is low (p = 0.008) and so the difference in deaths is likely to remain in favour of TARGIT.
Although NICE clarified that mortality was not the remit of this committee, we are compelled to emphasise the fact to your attention because it arose during the discussion. It is important to recognise that, when a study actually finds a difference, the question of power is not relevant any more. Then, the probability of observing the difference favouring TARGIT that was observed, if there was no real difference between the two groups is given by the p-value, which in the case of the TARGIT-A trial was 0.099 for all deaths and 0.0086 for deaths from causes other than breast cancer. We believe we cannot ignore these randomised data, particularly when deaths were more frequent than recurrences (n = 88 vs. n = 34) and non-breast-cancer deaths (n = 52) were more frequent than breast cancer deaths (n = 36) or local recurrences (n = 34).
-
We were asked by the NICE committee whether or not we would offer INTRABEAM to patients in the control arm of the TARGIT-A trial who have already received EBRT if NICE gives a positive response. In fact, the surgery and radiation treatment of patients in the TARGIT-A trial (whether INTRABEAM or whole-breast radiotherapy) has already been long completed (the trial closed in June 2012). Therefore, all those who received EBRT in the TARGIT-A trial cannot and will not be offered an operation to reopen their wound and give INTRABEAM.
-
Please note that, as published in The Lancet,2 there is no significant difference between TARGIT and EBRT by conventional log-rank test (p = 0.31) and TARGIT was non-inferior to EBRT using the standard test for non-inferiority (Pnon-inferiority < 0.00001).
The TARGIT-A trial investigators offered to supply the raw data as long as all of the governance, consent, custody, data access and security issues were looked after appropriately. NICE declined, saying that it was not necessary to supply the raw data because all of the analysis requested by the committee had been satisfactorily supplied.
Table 36 shows the results of the four different methods of analyses and the raw numbers, which essentially show very similar results.
| Outcome | Whole trial | Prepathology stratum: TARGIT simultaneous with lumpectomy | Interpretation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All 3451 patients | First 1222 patients (median follow-up 5 years) | All 2298 patients | First 817 patients (median follow-up 5 years) | ||||||
| TARGIT | EBRT | TARGIT | EBRT | TARGIT | EBRT | TARGIT | EBRT | ||
| Raw numbers | 1721 | 1730 | 613 | 609 | 1140 | 1158 | 401 | 416 | Most patients have done very well |
| Local recurrence | 23 | 11 | 15 | 8 | 10 | 6 | 6 | 3 | At a median follow-up of 2 years 5 months:
Prepathology: 4 more local recurrences and 13 less deaths |
| Local recurrence-free survival | 57 | 59 | 45 | 47 | 36 | 45 | 31 | 35 | |
| Disease-free survival | 89 | 84 | 66 | 64 | 54 | 59 | 45 | 45 | |
| Breast cancer mortality | 20 | 16 | 19 | 13 | 17 | 15 | 17 | 12 | |
| Non-breast-cancer mortality | 17 | 35 | 14 | 29 | 12 | 27 | 11 | 23 | |
| Overall mortality | 37 | 51 | 33 | 42 | 29 | 42 | 28 | 35 | |
| Whole trial | Prepathology stratum: TARGIT simultaneous with lumpectomy | ||||||||
| TARGIT | EBRT | Statistical significance | TARGIT | EBRT | Statistical significance | ||||
| Method 1: standard Kaplan–Meier plots, 5-year point estimates (95% CI) and log-rank test | |||||||||
| Local recurrence | 3.3% (2.1% to 5.1%) | 1.3% (0.7% to 2.5%) | p = 0.04 (not significant) | 2.1% (1.1% to 4.2%) | 1.1% (0.5% to 2.5%) | p = 0.31 (not significant) | There is no statistically significant difference between TARGIT and EBRT, for the whole trial as well as for the prepathology stratum, for all of these outcomes. There is a trend for a slightly higher local recurrence rate overall, but not in the prepathology stratum | ||
| Local recurrence-free survival | 93.1% (90.8% to 94.9%) | 93.8% (91.7% to 95.4%) | p = 0.81 (not significant) | 93.9% (90.9% to 95.9%) | 92.5% (89.7% to 94.6%) | p = 0.35 (not significant) | |||
| Disease-free survival | 90.5% (88.1% to 92.5%) | 91.0% (88.6% to 93.0%) | p = 0.78 (not significant) | 91.6% (88.7% to 93.8%) | 90.1% (86.8% to 92.6%) | p = 0.68 (not significant) | |||
| Breast cancer mortality | 2.6% (1.5% to 4.3%) | 1.9% (1.1% to 3.2%) | p = 0.56 (not significant) | 3.3% (1.9% to 5.8%) | 2.7% (1.5% to 4.6%) | p = 0.72 (not significant) | |||
| Non-breast-cancer mortality | 1.4% (0.8% to 2.5%) | 3.5% (2.3% to 5.2%) | p = 0.0086 (significant) | 1.3% (0.7% to 2.8%) | 4.4% (2.8% to 6.9%) | p = 0.016 (significant) | There is a statistically significant difference in non-breast-cancer mortality | ||
| Overall mortality | 3.9% (2.7% to 5.8%) | 5.3% (3.9% to 7.3%) | p = 0.099 (not significant) | 4.6% (1.8% to 6.0%) | 6.9% (4.3% to 9.6%) | p = 0.12 (not significant) | There is a trend for improved survival overall with TARGIT | ||
| Absolute difference | Significance | Absolute difference | Significance | ||||||
| Method 2: absolute difference in binomial proportions (90% CI) | |||||||||
| Local recurrence – all patients (median follow-up 2 years 5 months) | 0.72% (0.2% to 1.3%) | Pnon-inferiority < 0.0001 | 0.37% (–0.2% to 1.0%) | Pnon-inferiority < 0.0001 | Non-inferiority is established at a median follow-up of 2 years and 5 months, for the whole trial and the prepathology stratum | ||||
| Local recurrence – those randomised in the first 8 years (median follow-up 5 years) | (n = 1222) 1.14% (–0.1% to 2.4%) | Pnon-inferiority = 0.04 | 0.76% (–0.4% to 2.0%) | Pnon-inferiority = 0.0091 | Non-inferiority is established at a median follow-up of 5 years, for the whole trial and the prepathology stratum | ||||
| Whole trial | Prepathology stratum: TARGIT given simultaneous with lumpectomy | ||||||||
| Difference in the 5-year point estimate | Statistical significance | Difference in the 5-year point estimate | Statistical significance | ||||||
| Method 3: absolute difference in 5-year point estimates (95% and 90% CIs) | |||||||||
| Local recurrence | 2% (95% CI –0.14% to 4.14%) (90% CI 0.18% to 3.82%) | p = 0.04 (not significant) | 1% (95% CI –0.68% to 2.68%) (90% CI –0.43% to 2.43%) | p = 0.31 (not significant) | The difference between TARGIT and EBRT for local recurrence and breast cancer mortality is not statistically significant. The confidence limits of a point estimate are not a valid measure for testing non-inferiority, as it is only a point estimate. The NICE committee agreed (meeting in March 2015) that the right-hand end of a Kaplan–Meier curve is the one with the most uncertainty and with the widest CIs. In the presence of censoring, the Kaplan–Meier point estimates at a particular time point, e.g. 5 years, are not simple binomial proportions and treating them as such introduces a bias, resulting in a wider CI. Therefore, it is inappropriate to apply the simple formula normally used to calculate the SE (and CI) of a difference between binomial proportions, namely SE12+SE22 to calculate differences between such point estimates. These values, i.e. 5-year point estimates, should not be used to calculate the CI of the difference or for testing non-inferiority. These values are being shown mainly for completion and will not be wrongly used to assess non-inferiority | ||||
| Local recurrence-free survival | 0.64% (95% CI –2.09% to 3.37%) (90% CI –1.69% to 2.97%) | p = 0.81 (not significant) | –1.32% (95% CI –4.74% to 2.10%) (90% CI –4.24% to 1.60%) | p = 0.35 (not significant) | |||||
| Breast cancer mortality | 0.67% (95% CI –1.01% to 2.35%) (90% CI –0.76% to 2.10%) | p = 0.56 (not significant) | 0.66% (95% CI –1.72% to 3.04%) (90% CI –1.36% to 2.68%) | p = 0.72 (not significant) | |||||
| Non-breast-cancer mortality | –2.08% (95% CI –3.70% to –0.46%) (90% CI –3.46% to –0.70%) | p = 0.0086 (significant) | –3.07% (95% CI –5.25% to 0.89%) (90% CI –4.93% to 1.21%) | p = 0.016 (significant) | There is a statistically significant difference in non-breast-cancer mortality. The CI of the 5-year point estimate is a poor indicator of the difference between the two Kaplan–Meier curves across the whole 5-year period | ||||
| Overall mortality | –1.38% (95% CI –3.67% to 0.91%) (90% CI –3.3% to 0.57%) | p = 0.099 (not significant) | –2.33% (95% CI –5.48% to 0.82%) (90% CI –5.02% to 0.36%) | p = 0.12 (not significant) | There is a trend for reduced mortality with TARGIT | ||||
| Whole trial | Prepathology stratum: TARGIT given simultaneous with lumpectomy | ||||||||
| Method 4: the integrated difference between the two survival functions to quantify the difference between the Kaplan–Meier curves for TARGIT and EBRT from 0 to 5 years (95% CI) | |||||||||
| Local recurrence | 0.62% (0.007% to 1.24%) | 0.3% (–0.4% to 1.03%) | The local recurrence difference between TARGIT and EBRT is < 2.5% and the upper 95% confidence limit of the difference over the first 5 years is 1.24% for the whole trial and 1.03% for the prepathology stratum. For mortality, the 95% CI of the difference between TARGIT and EBRT straddles zero and is more negative than positive. For overall mortality, the lower 95% confidence limit is –1.75% overall and –2.66% (> 2.5% potential survival benefit from TARGIT) for the prepathology stratum | ||||||
| Breast cancer mortality | 0.15% (–0.71% to 0.42%) | –0.34% (–1.17% to 0.49%) | |||||||
| Non-breast-cancer mortality | –0.72 (–1.42% to –0.02%) | –1.11% (–2.05% to –0.17%) | |||||||
| Overall mortality | –0.85% (–1.75% to 0.04%) | –1.43% (–2.66% to –0.2%) | |||||||
In conclusion, using four quite separate statistical methodologies, the TARGIT-A trial has demonstrated that the risk-adapted approach using single-dose TARGIT IORT given during lumpectomy provides breast cancer control that is not inferior to several weeks of conventional radiotherapy.
Acknowledgement
We thank Dr Hajime Uno PhD (Department of Biostatistics, Harvard University, Boston, MA, USA) for providing the software code and for independently verifying our results and our interpretation.
Acknowledgements
The trial was initiated by an academic insight and collaboration with industry was solely for the development of the device. The manufacturers of the INTRABEAM device (Carl Zeiss) did not have any part in the concept, design or management of the trial or in data analysis, data interpretation or writing of the report. The study was sponsored by University College London Hospitals (UCLH)/UCL Comprehensive Biomedical Research Centre. Funding was provided by UCLH Charities, the National Institute for Health Research (NIHR) HTA programme, Ninewells Cancer Campaign, the National Health and Medical Research Council and the German Federal Ministry of Education and Research (BMBF) (grant FKZ 01ZP0508). The infrastructure of the trial operations office in London, UK, was supported by core funding from the CRC [now Cancer Research UK (CRUK)] when the trial was initiated. We thank Michael D O’Shea (Woodward Informatics, Chilton, UK) for database development, Stephen Ebbs (Croydon Health Services, Croydon, UK) for independently assessing the cause of death, Andrew Lee (Ninewells Hospital, Dundee, UK) for help with data collection, Carol Roach for help with copy editing of the report and several other contributors who have now left the individual centres. Travel and accommodation costs for meetings of the ISC and DMC were provided by Carl Zeiss. Funding for the TARGIT trials operations office was provided by the NIHR HTA programme. Individual centres were self-financed. We thank all of the patients who kindly participated in the trial. Manuscript preparation was helped by the trial operations staff and their respective families.
In accordance with the UCL records management policy, the research findings will need to be stored by UCL as sponsor for 20 years after the research has finished, based on recommendations made in the Medical Research Council’s Ethics Series on Good Clinical Practice. This is deemed a sufficient period of time to allow for audit and inspections by regulatory authorities. The UCL Records Office provides a service to UCL staff and maintains archived records in a safe and secure off-site location. All activities were conducted in accordance with the Data Protection Act 1988177 and UCL data protection policy. Access to the data is strongly regulated and permission to access the data is treated on a case-by-case basis. The Records Office allows for records to be catalogued onto an online database and assists in recalling archives on demand.
The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author and the trial statistician had full access to all the data in the study and all authors were responsible for the decision to submit for publication.
TARGIT-A team
Trial operations staff: London, UK – Michael Baum (Director), Jayant S Vaidya (Scientific Director), Norman R Williams, Ingrid Potyka, Chris Brew-Graves, Cindy Li, Cinzia Baldini and Mortez Ali. Previously: Joan Houghton, Christina Lennon, Olive Murphy and Alexandra Sherley; Mannheim, Germany – Anke Keller; Perth, WA, Australia – Tammy Corica, Celeste Lopez and Eva Vosikova.
Individual centre teams (in order of the date of randomisation of the first patient): University College London Hospital, London, UK – JS Vaidya, JS Tobias, M Baum, M Keshtgar, G Blackman, C Brew-Graves, M Douek, D D’Souza, M Falzon, E Harrison, C Kocjan, S Lakhani, M Metaxas, P Mulvey, SV Naidu, G Petralia, R Sainsbury, C Saunders, C Stacey, I Taylor and N Williams; Universitätsmedizin Mannheim, Universität Heidelberg, Germany – Frederik Wenz, E Blank, M Bohrer, S Clausen, B Hermann, R Hildenbrand, Anke Keller, Uta Kraus-Tiefenbacher, B Kuepper, A Marx, F Melchert, D Neumann, F Schneider, V Steil, M Suetterlin and M Trunk; Sir Charles Gairdner Hospital, Perth, WA, Australia – Christobel Saunders, David J Joseph, Sean Bydder, Sheryl Campbell, Joe Clarke, Tammy Corica, Annette Haworth, Rosemary Hedges, Robin Kane, Peter Lanzon, Celeste Lopez, Mandy Taylor, Eva Vosikova, Claire Haworth, Katayun Mohammadi, Alberta Abreu, Nik Zeps, Diana Hastrich, David Ingram, Lee Jackson, Neill Kling, Margaret Latham, David Minchin, Annemarie Naylor, David Oliver and Rosemary Wilkinson; Centro di Riferimento Oncologico, Aviano, Italy – Samuele Massarut, M Arcicasa, E Bidoli, E Cadiani, E Capra, M Oliva, T Perin, S Reccanello, M Roncadin, G Sartor, G Tabaro, M Trovo, R Volpe Mario Mileto, Erica Piccoli and Antonella Spada; Ninewells Hospital, Dundee, UK – M Adams, DJA Adamson, K Armoogum, J Bosch, DC Brown, JA Dewar, S Edwards, J Gardner, A Gunning, M Hawkes, LB Jordan, A Lee, J Lindsay, G Little, C Mackay, AJ Munro, J Parry, CA Purdie, MM Reis, AM Thompson, JS Vaidya, V Walker and RAB Wood; University of California, San Francisco Medical Center, San Francisco, CA, USA – Laura Esserman, Michael Alvarado, Alfred Au, Alison Bevan, Jay Connolly, Cheryl Ewing, Clark Fisher, Shelley Hwang, K Lane, Christina Minami, Michelle Oboite, Cathy Park, Jean Pouliot, Theadora Sakata, Aron Mohan, Brittany Harrison, Albert Chan and Mitchell Hayes; Frauenklinik vom Roten Kreuz, Munich, Germany – Wolfgang Eiermann, Beyhan Ataseven, C Becker, B Hoegel, P Kneschaurek, A Lackermeier, M Molls, Carsten Nieder, Steffi Pigorsch, J Rauch, Barbara Röper, Sabine Schill, Brigitte Werner and Christopher Wolf; University of Southern California, Los Angeles, CA, USA – Dennis R Holmes, Melvin Astrahan, Carryl Dubois, Jacqueline Majors, Sylvia Villegas Mendez, Afshin Rashtian, Ronald Rivera, Howard Silberman, Melvin Silverstein, Rashida Soni, Oscar E Streeter Jr, Lina Wang, Heather Macdonald, Stephen Sener and America Casillas; Ospedale San Giuseppe di Empoli, Empoli, Italy – Gianmaria Fiorentini, Carli Ballola Adele, Rafaella Barca, Mauro Biancalani, Giampaolo Biti, Enrico Cellai, Antonella Compagnucci, Claudio Caponi, Vito Maria Fontanarosa, Roberta Ghezzi, Alessandro Ghirelli, Gloria Giustarini, Barbara Grilli Leonulli, Francesca Littori, Maurizio Pertici, Visna Petrina, Paola Raffaele, Francesca Righi, Serenella Russo, Michele de Simone, Gina Turrisi and Giuditta Zipoli; Sankt Gertrauden-Krankenhaus, Berlin, Germany – Jens-Uwe Blohmer, Petra Feyer, J Gross, G Jautzke, K Luebbert, Michaela Platzer, Joerg Preussler, D Puppe and Esther Wiedemann; Peter MacCallum Cancer Centre, Melbourne, VIC, Australia – Michael Henderson, David Blakey, Boon Chua, Ram Das, Roslyn Drummond, Annette Haworth, Penny Fogg, Stephen Fox, Jodi Lynch, Jane O’Brien, Catherine Poliness, Ann-Marie Power, David Speakman, Tina Thorpe and Melanie Walker; Ludwig Maximilians Universität, Munich, Germany – Wolfgang Janni, Ulrich Andergassen, C Balka, Darius Dian, Sylvia Dondl, Klaus Friese, Julia Jueckstock, Thomas Kirchner, Klaus Krimmel, Doris Mayr, Susanne Reinhard, Dr Schaffer, Christian Schindlbeck, Harald Sommer and Justus Well; Universität Frankfurt am Main, Frankfurt, Germany – M Kaufmann, H Boettcher, J Moog, Achim Rody, Claus Rödel, S Schopohl, Christian Weiss, Inge Fraunholz, Ulla Ramm, Martin-Leo Hansmann and R Strohmeier; Herlev/Rigs Hospitals, Copenhagen, Denmark – Henrik Flyger, Eva Balslev, Niels Bentzon, Paul Geertsen, Helle Holtveg, Claus Kamby, Niels Kroman, Faisal Mahmood, Fritz Rank, Birgitte Bruun Rasmussen, Lone Gry Schäfer, Peter Michael Vestlev, Vera Timmermans Wielenga and Eva Wilken; Medical University of Lublin, Lublin, Poland – Wojciech P Polkowski, Malgorzata Jankiewicz, Andrzej Kurylcio, Maria Mazurkiewicz, Jerzy Mielko, Krzysztof Paprota, Barbara Pawlowska-Wakowicz, Urszula Radwanska, Jaroslaw Romanek, Zofia Siezieniewska, Magdalena Skorzewska, Andrzej Stanislawek, Monika Lewicka, Jadwiga Sierocinska-Sawa, Edyta Matajek, Rafael Smyk, Andrzej Bedinski, Bogumila Cislo and Bogumila Cisel; Royal Free Hospital, London, UK – Mohammed Keshtgar, Katherine Pigott, Tim Davidson, Jayant S Vaidya, Debasis Ghosh, Alison Jones, Jawad Keshtgar, Samia Shah, Katia Pasciuti, Neil Dancer, Kashmira Metha, Benjamin Earner, Stephan Duck and David Woolf; Whittington Hospital, London, UK – Jayant S Vaidya, Jeffrey S Tobias, Alan Wilson, Mohammed Keshtgar, Peng Tan, Debashis Ghosh, Glen Blackman, Renata Rowicka, Veronica Conteh, Su Ramachandra, Lucy Harbin, R Chaudhuri, Ros Crooks, Francesca Peters, Tom Connors, Alison Jones, Mulyati Mohamed, Tim Crook, Vivienne Maidens, Sylvia Grieve Lotta Jonsson and Ciara McNulty; Lafayette Surgical Clinic, Lafayette, IN, USA – Thomas L Summer, Mario Contreras, Paul M DesRosiers, Irene Gordon, Kazumi Chino, Bedatri Sinha, Cindy McDowell, Mike Ringer, Tammy Spurlock and Lisa Ramsey; Sentara Surgery Specialists, Hampton, VA, USA – Richard A Hoefer, Mary Berry, Donna Forrest, Arvind Mahatma, Michael Miller, Song Kang, Gregory J Lavalle, Deborah A Martinez and Sandra Piggee; Uniklinikum des Saarlandes, Homburg, Germany – Erich Solomayer, K Abel, S Baum, Rainer Allgayer, R M Bohle, Mustafa Deryal, J Fleckenstein, R Grobholz, Jeanett Koehn, Anja Martin-Riedheimer, Marcus Niewald, Markus Promnik, Christian Ruebe and W Schmidt; Princess Margaret Cancer Centre, Toronto, ON, Canada – David McCready, Akbar Beiki-Ardakani, John Cho, Susan Done, Jamie Escallon, Anthony W Fyles, Wilfred Levin, Alex Vitkin and Marie Vranic; Royal Hampshire County Hospital, Winchester, UK – Dick Rainsbury, Claire Birch, Lyn Booth, Caroline Cross, Alan Gately, Virginia Hall, Kevin Harris, Siobhan Laws, Sanjay Raj, Balvinder Shoker, Virginia Straker and Jennifer Wilson; Brust Zentrum Seefeld, Zurich, Switzerland – Christopher Rageth, Uwe Gneveckow, Elisabeth Grob, Guenther Gruber, Baerbel Papassotiropoulos, Barbara Tausch, C Tausch, Zsuszanna Varga and Iris Vergin; Universitäts Spital Zurich, Zurich, Switzerland – Claudia Hutzli, Yvonne Burgstaller, Rosemary Caduff, Daniel Fink, Guntram Kunz, Claudia Linsenmeier, Yousef Najafi, Natalie Gabriel, Cornelia Betschart, Eleftherios Samartzis, Ana-Maria Schmidt, Tino Streller, Z Varga, Madeleine Wick, Cornelia Leo, Zsuzsanna Varga and Leila Kocan; St Olav’s Hospital, Trondheim, Norway – Steinar Lundgren, Anne Brit Abusland, Marianne Brekke, Dagrun Danielsen, Hans E Fjosne, Jomar Frengen, Kristen Helset, Jarle Karlsen, Petter Osthus, Hanne-Brit Grong Smethurst and Trond Strickert; University of Nebraska Medical Center, Omaha, NE, USA – James Edney, Aaron Sasson, Debra Spence, Robert Thompson, William W West and Sumin Zhou; Guy’s and St Thomas’ Hospital, London, UK – Michael Douek, Sarah Aldridge, Ashutosh Kothari, Nick Beechey-Newman, Charles Deehan, Ian Fentiman, Hisham Hamed, Sarah Harris, Hardeep Johal, Sarah Pinder, Arnie Purushotham, Vernie Ramalingam and Chris Stacey; Vassar Brothers Medical Center, Poughkeepsie, NY, USA – Angela Keleher, Eileen Abate, Nicole Cappillino, Laszlo Csury, Edward Farhangi, Anne Kim, Sutini Ngadiman, Dimitrios Papadopoulos, Dan Pavord, P Hank Schmidt, Camilo Torres and Erika Mednick; Ashikari Clinic, New York Medical College, New York, USA – P Kelemen, Andrew Ashikari, Ulrich Hermato, Helen Li, Demetrious Makrides, Mike Malamed, Wanda Rivera, Yadita Samnarain, Alfred Tinger, Raphael Yankelevich and Yasmin Yusuf; Medizinische Hochschule Hannover, Germany – Tjoung-Won Park-Simon, Peter Hillemans, Ursula Hille, Michael Bremer, Frank Bruns, Frank Rudolf, Hans Grudtke, Jorg Fruhauf, HH Kreipe, Florian Laenger and Adelheid Klein; Centre René Gauducheau, Nantes, France – Magali Le Blanc-Onfroy, Maud Aumont, Francois Dravet, Magali Dejode, Albert Lisbona, Delphine Loussouarn, Christine Sagan, Nicolas Rougé and Stephanie Gaudaine-Josset; Instituto Oncologico Veneto, Padova, Italy – Michele Pignataro, Fernando Bozza, Raffaello Grigoletto, Silvia Michieletto, Stefano Valente, Tania Saibene, Franco Berti, Ornella Lora, Marta Paiusco, Sonia Reccanello, Davide Canonico, Enrico Orvieto, Marcello Lo Mele and Liliana Spangaro; Hospital of St John and St Elizabeth, London, UK – Katharine Pigott, Punita Vyas, Catherine O’Connor, Donna Gibbs, Simon Stevens, Ashley Richmond, Tabasom Ghaus, Thomas Ashford, Deborah Waters and Mohammed Keshtgar; Institut Bergonie, Bordeaux, France – Marion Fournier, Christine Tunon De Lara, Christelle Breton-Callu, Philippe Lagarde, Sarah Belhomme, Gaetan MacGrogan, Beatrice Gonzalves and Mickael Antoine.
Contributions of others
This section acknowledges those who contributed but were not involved with writing of this particular report. Dr Samuele Massarut, Professor Mo Keshtgar, Dr Henrik Flyger and Dr Michael Alvarado contributed to training and accreditation of centres, patient accrual and treatment, data collection, and writing of the report. Tammy Corica and Professor Laura Esserman contributed to trial design, patient accrual, patient treatment, data interpretation, and writing of the report. Professor Herik Flyger, Professor Wolfgang Eiermann, Professor Mo Keshtgar, Dr Mario Roncadin and Professor Marc Sutterlin contributed to patient accrual, patient treament, data collection and data interpretation. Dr Henrik Flyger, Dr Samuele Massarut, Professor Wolfgang Eiermann, Professor Mo Keshtgar, Professor John Dewar, Dr Elena Sperk, Professor Mark Sutterlin, Dr Mario Roncadin, Dr Helle M R Holtveg, Mr Douglas Brown and Dr Steffi Pigorsch contributed to setting up their centres, patient accrual, treatment, data collection, and approval of the report. Marinos Metaxas was involved with design and preclinical tests of the INTRABEAM system. Marinos Metaxas was involved with continuing quality assurance and dosimetry analysis and data collection and approved the report. Dr Mary Falzon was involved with primary diagnosis and subsequent assessment of the histology from University College London Hospitals. April Matthews reviewed the data generated from the trial and their interpretation and approved the report.
JSV, MBa, and JST were responsible for trial concept, trial design, trial management, data interpretation, and writing of the report. FW, DJJ, JST, MBa, and JSV contributed to trial concept, trial design, trial management, training and accreditation of centres, patient accrual and treatment, data collection, data interpretation, and writing of the report. JSV, FW, JST, MBa, Professor Mo Keshtgar, Dr Samuele Massarut, Dr Henrik Flyger and Dr Michael Alvarado contributed to training and accreditation of centres, patient accrual and treatment, data collection, and writing of the report. JSV and NRW designed the statistical analysis plan and contributed to statistical analysis, trial co-ordination, data collection, data interpretation, and writing of the report. MBu and JSV did the statistical analyses and contributed to data interpretation and writing of the report. CS, Tammy Corica, and Professor Laura Esserman contributed to trial design, patient accrual, patient treatment, data interpretation, and writing of the report. Professor Herik Flyger, Professor Wolfgang Eiermann, Professor Mo Keshtgar, Dr Mario Roncadin, and Professor Marc Sutterlin contributed to patient accrual, patient treatment, data collection, and data interpretation. Dr Henrik Flyger, Dr Samuele Massarut, Professor Wolfgang Eiermann, Professor Mo Keshtgar, Professor John Dewar, Dr Elena Sperk, Professor Mark Sutterlin, Dr Mario Roncadin, Dr Helle M R Holtveg, Mr Douglas Brown, Dr Steffi Pigorsch contributed to setting up their centres, patient accrual, treatment, data collection, and approval of the report. JSV and Marinos Metaxas were involved with design and preclinical tests of the Intrabeam system. CB-G and IP contributed training, trial co-ordination, trial management, data collection, and writing of the report. Marinos Metaxas was involved with continuing quality assurance and dosimetry analysis, data collection, and approved the report. Dr Mary Falzon was involved with primary diagnosis and subsequent assessment of the histology from University College London Hospitals. April Matthews reviewed the data generated from the trial and their interpretation and approved the report. All authors apart from Dr Henrik Flyger, Dr Mario Roncadin, Dr Steffi Pigorsch, Mr Douglas Brown and Dr Helle M R Holtveg attended the International Steering Comittee meetings as members. The authors take full responsibility for the report. The initial draft was written by JSV, MBu and MBa, then revised following comments from all other authors. All authors attended the ISC meetings as members.
Contributions of authors
Jayant S Vaidya (Professor of Surgery and Oncology) contributed to the trial concept, the trial design, patient recruitment and treatment, follow-up, data collection, site recruitment, training and accreditation of sites, trial management, the statistical analysis plan, data analysis and interpretation of the data. He wrote the initial draft and made subsequent changes as per comments from other authors, reviewers and editors and approved the final version.
Frederik Wenz (Professor of Radiation Oncology) contributed to the trial concept, the trial design, patient recruitment and treatment, follow-up, data collection, site recruitment, training and accreditation of sites, trial management, interpretation of the data, writing of the manuscript and approved the final version of the manuscript.
Max Bulsara (Professor of Biostatistics) contributed to the statistical analysis plan, performed the statistical analysis of the data, interpretation of the data, writing of the manuscript and approved the final version of the manuscript.
Jeffrey S Tobias (Professor of Cancer Medicine) contributed to the trial concept, the trial design, patient recruitment and treatment, follow-up, data collection, site recruitment, training and accreditation of sites, trial management, interpretation of the data, writing of the manuscript and approved the final version of the manuscript.
David J Joseph (Professor of Radiation Oncology) contributed to the trial concept, the trial design, patient recruitment and treatment, follow-up, data collection, site recruitment, training and accreditation of sites, trial management, interpretation of the data, writing of the manuscript and approved the final version of the manuscript.
Christobel Saunders (Professor of Surgery) contributed to the trial design, patient recruitment and treatment, follow-up, data collection, site recruitment, training and accreditation of sites, trial management, interpretation of the data, writing of the manuscript and approved the final version of the manuscript.
Chris Brew-Graves (Deputy Director, Operations) contributed to data collection, trial management, interpretation of the data, the health economics sections, writing of the manuscript and approved the final version of the manuscript.
Ingrid Potyka (Senior Clinical Operations Manager) contributed to data collection and validation, trial management, interpretation of the data, writing of the manuscript and approved the final version of the manuscript.
Stephen Morris (Professor of Health Economics) contributed to the health economic sections and approved the final version of the manuscript.
Hrisheekesh J Vaidya (Medical Student, University of Oxford and Imperial College) reworded the introduction and discussion sections, contributed to the writing of rest of the document and approved the final version of the manuscript.
Norman R Williams (Deputy Director, Quality and Governance) contributed to data collection, site recruitment, trial management, the statistical analysis plan, data analysis, interpretation of the data, writing of the manuscript and approved the final version of the manuscript.
Michael Baum (Professor of Surgery) contributed to the trial concept, the trial design, patient recruitment and treatment, follow-up, data collection, site recruitment, training and accreditation of sites, trial management and interpretation of the data and approved the final version of the manuscript.
A large proportion of the content of this report is taken from the protocol and peer-reviewed publications by the authors and other colleagues, as acknowledged within those publications and above.
Publications
The main publications are in bold.
Coombs NJ, Coombs JM, Vaidya UJ, Singer J, Bulsara M, Tobias JS, et al. Environmental and social benefits of the targeted intraoperative radiotherapy for breast cancer: data from UK TARGIT-A trial centres and two UK NHS hospitals offering TARGIT IORT. BMJ Open 2016;6:e010703.
Vaidya JS, Bulsara M, Wenz F, Coombs N, Singer J, Ebbs S, et al. Reduced mortality with partial breast irradiation for early breast cancer – a meta-analysis of randomised trials. Int J Radiat Oncol Biol Phys 2016;96:259–65.
Corica T, Nowak AK, Saunders C, Bulsara M, Taylor M, Vaidya JS, et al. Cosmesis and breast-related quality of life outcomes after intraoperative radiation therapy for early breast cancer: a substudy of the TARGIT-A trial. Int J Radiat Oncol Biol Phys 2016;96:55–64.
Vaidya JS, Bulsara M, Wenz F, Joseph D, Saunders C, Massarut S, et al. In regard to Hepel and Wazer. Int J Radiat Oncol Biol Phys 2015;92:953–4.
Vaidya JS, Bulsara M, Wenz F, Joseph D, Saunders C, Massarut S, et al. Pride, prejudice, or science – attitudes towards the results of the TARGIT-A trial of targeted intraoperative radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys 2015;92:491–7.
Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph D, Baum M. Radiotherapy for breast cancer, the TARGIT-A trial – authors’ reply. Lancet 2014;383:1719–20.
Esserman LJ, Alvarado MD, Howe RJ, Mohan AJ, Harrison B, Park C, et al. Application of a decision analytic framework for adoption of clinical trial results: are the data regarding TARGIT-A IORT ready for prime time? Breast Cancer Res Treat 2014;144:371–8.
Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603–13.
Corica T, Joseph D, Saunders C, Bulsara M, Nowak AK. Intraoperative radiotherapy for early breast cancer: do health professionals choose convenience or risk? Radiat Oncol 2014;9:33.
Alvarado MD, Conolly J, Park C, Sakata T, Mohan AJ, Harrison BL, et al. Patient preferences regarding intraoperative versus external beam radiotherapy following breast-conserving surgery. Breast Cancer Res Treat 2014;143:135–40.
Vaidya JS, Bulsara M, Wenz F. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med 2013;368:2526–7.
Keshtgar MR, Williams NR, Bulsara M, Saunders C, Flyger H, Cardoso JS, et al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat 2013;140:519–25.
Welzel G, Boch A, Sperk E, Hofmann F, Kraus-Tiefenbacher U, Gerhardt A, et al. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: results from the randomized Phase III trial TARGIT-A. Radiat Oncol 2013;8:9.
Engel D, Schnitzer A, Brade J, Blank E, Wenz F, Suetterlin M, et al. Are mammographic changes in the tumor bed more pronounced after intraoperative radiotherapy for breast cancer? Subgroup analysis from a randomized trial (TARGIT-A). Breast J 2013;19:92–5.
Deneve JL, Hoefer RA, Harris EE, Laronga C. Accelerated partial breast irradiation: a review and description of an early North American surgical experience with the intrabeam delivery system. Cancer Control 2012;19:295–308.
Clausen S, Schneider F, Jahnke L, Fleckenstein J, Hesser J, Glatting G, Wenz F. A Monte Carlo based source model for dose calculation of endovaginal TARGIT brachytherapy with INTRABEAM and a cylindrical applicator. Z Med Phys 2012;22:197–204.
Sperk E, Welzel G, Keller A, Kraus-Tiefenbacher U, Gerhardt A, Sütterlin M, Wenz F. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized Phase III trial TARGIT A. Breast Cancer Res Treat 2012;135:253–60.
Neumaier C, Elena S, Grit W, Yasser AM, Uta KT, Anke K, et al. TARGIT-E(lderly) – prospective Phase II study of intraoperative radiotherapy (IORT) in elderly patients with small breast cancer. BMC Cancer 2012;12:171.
Avanzo M, Rink A, Dassie A, Massarut S, Roncadin M, Borsatti E, Capra E. In vivo dosimetry with radiochromic films in low-voltage intraoperative radiotherapy of the breast. Med Phys 2012;39:2359–68.
Eaton DJ, Best B, Brew-Graves C, Duck S, Ghaus T, Gonzalez R, et al. In vivo dosimetry for single-fraction targeted intraoperative radiotherapy (TARGIT) for breast cancer. Int J Radiat Oncol Biol Phys 2012;82:e819–24.
Andersen KG, Gärtner R, Kroman N, Flyger H, Kehlet H. Persistent pain after targeted intraoperative radiotherapy (TARGIT) or external breast radiotherapy for breast cancer: a randomized trial. Breast 2012;21:46–9.
Vaidya JS, Baum M, Tobias JS, Wenz F, Massarut S, Keshtgar M, et al. Long-term results of targeted intraoperative radiotherapy (Targit) boost during breast-conserving surgery. Int J Radiat Oncol Biol Phys 2011;81:1091–7.
Keshtgar MR, Vaidya JS, Tobias JS, Wenz F, Joseph D, Stacey C, et al. Targeted intraoperative radiotherapy for breast cancer in patients in whom external beam radiation is not possible. Int J Radiat Oncol Biol Phys 2011;80:31–8.
Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91–102.
Sautter-Bihl ML, Sedlmayer F, Budach W, Dunst J, Engenhart-Cabillic R, Fietkau R, et al. Intraoperative radiotherapy as accelerated partial breast irradiation for early breast cancer: beware of one-stop shops? Strahlenther Onkol 2010;186:651–7.
Herskind C, Griebel J, Kraus-Tiefenbacher U, Wenz F. Sphere of equivalence – a novel target volume concept for intraoperative radiotherapy using low-energy X rays. Int J Radiat Oncol Biol Phys 2008;72:1575–81.
Baum M, Vaidya JS. Targeted intra-operative radiotherapy – TARGIT for early breast cancer. Ann N Y Acad Sci 2008;1138:132–5.
Belletti B, Vaidya JS, D’Andrea S, Entschladen F, Roncadin M, Lovat F, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res 2008;14:1325–32.
Holmes DR, Baum M, Joseph D. The TARGIT trial: targeted intraoperative radiation therapy versus conventional postoperative whole-breast radiotherapy after breast-conserving surgery for the management of early-stage invasive breast cancer (a trial update). Am J Surg 2007;194:507–10.
Vaidya JS. Partial breast irradiation using targeted intraoperative radiotherapy (TARGIT). Nat Clin Pract Oncol 2007;4:384–5.
Enderling H, Chaplain MA, Anderson AR, Vaidya JS. A mathematical model of breast cancer development, local treatment and recurrence. J Theor Biol 2007;246:245–59.
Vaidya JS, Baum M, Tobias JS, Massarut S, Wenz F, Murphy O, et al. Targeted intraoperative radiotherapy (TARGIT) yields very low recurrence rates when given as a boost. Int J Radiat Oncol Biol Phys 2006;66:1335–8.
Enderling H, Anderson AR, Chaplain MA, Munro AJ, Vaidya JS. Mathematical modelling of radiotherapy strategies for early breast cancer. J Theor Biol 2006;241:158–71.
Vaidya JS, Walton L, Dewar J. Single dose targeted intraoperative radiotherapy (TARGIT) for breast cancer can be delivered as a second procedure under local anaesthetic. World J Surg Oncol 2006;4:2.
Vaidya JS, Tobias JS, Baum M, Wenz F, Kraus-Tiefenbacher U, D’souza D, et al. TARGeted Intraoperative radiotherapy (TARGIT): an innovative approach to partial-breast irradiation. Semin Radiat Oncol 2005;15:84–91.
Tobias JS, Vaidya JS, Keshtgar M, D’Souza DP, Baum M. Reducing radiotherapy dose in early breast cancer: the concept of conformal intraoperative brachytherapy. Br J Radiol 2004;77:279–84.
Vaidya JS. A Novel Approach for Local Treatment of Early Breast Cancer . PhD thesis. London: University of London; 2002. URL: www.ucl.ac.uk/~rmhkjsv/papers/thesis.htm (accessed 23 July 2016).
Vaidya JS, Baum M, Tobias JS, Morgan S, D’Souza D. The novel technique of delivering targeted intraoperative radiotherapy (TARGIT) for early breast cancer. Eur J Surg Oncol 2002;28:447–54.
Vaidya JS, Baum M, Tobias JS, D’Souza DP, Naidu SV, Morgan S, et al. Targeted intra-operative radiotherapy (TARGIT): an innovative method of treatment for early breast cancer. Ann Oncol 2001;12:1075–80.
Vaidya JS, Baum M. Clinical and biological implications of the Milan breast conservation trials. Eur J Cancer 1998;34:1143–4.
Baum M, Vaidya JS, Mittra I. Multicentricity and recurrence of breast cancer. Lancet 1997;349:208.
Vaidya JS, Vyas JJ, Chinoy RF, Merchant N, Sharma OP, Mittra I. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer 1996;74:820–4.
Vaidya JS, Vyas JJ, Mittra I, Chinoy RF. Multicentricity and its influence on conservative breast cancer treatment strategy. Hong Kong International Cancer Congress, 1995. Abstract 44.4.
Data sharing statement
All requests for data sharing will adhere to the UCL Surgical & Interventional Trials Unit (SITU) data sharing agreement policy. UCL Medical School is supportive of data sharing and will endeavour to assist in requests for data sharing. These data will be held at UCL on secure servers and will not be released to any third parties until the final study papers (not this publication) have been published. All requests for access to the data will be formally requested through the use of a SITU data request form which will state the purpose, analysis and publication plans together with the named collaborators. All requests are dealt with on a case by case basis by SITU and the Trial Steering committee. All requests will be logged and those successful will have a data transfer agreement which will specify appropriate acknowledgement of the TARGIT Trialists’ Group, the sponsor, and funders.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91-102. http://dx.doi.org/10.1016/S0140-6736(10)60837-9.
- Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. http://dx.doi.org/10.1016/S0140-6736(13)61950-9.
- Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. http://dx.doi.org/10.1016/S0140-6736(11)61629-2.
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. http://dx.doi.org/10.1016/S0140-6736(05)67887-7.
- Early Breast Cancer Trialists’ Collaborative Group . Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet 2000;355:1757-70. http://dx.doi.org/10.1016/S0140-6736(00)02263-7.
- Early Breast Cancer Trialists’ Collaborative Group . Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med 1995;333:1444-55. http://dx.doi.org/10.1056/NEJM199511303332202.
- Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key CR. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst 2000;92:269-71. http://dx.doi.org/10.1093/jnci/92.3.269.
- Fisher B, Bryant J, Dignam JJ, Wickerham DL, Mamounas EP, Fisher ER, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol 2002;20:4141-9. http://dx.doi.org/10.1200/JCO.2002.11.101.
- Fyles AW, McCready DR, Manchul LA, Trudeau ME, Merante P, Pintilie M, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 2004;351:963-70. http://dx.doi.org/10.1056/NEJMoa040595.
- Potter R, Gnant M, Kwasny W, Tausch C, Handl-Zeller L, Pakisch B, et al. Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int J Radiat Oncol Biol Phys 2007;68:334-40. http://dx.doi.org/10.1016/j.ijrobp.2006.12.045.
- Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 2013;31:2382-7. http://dx.doi.org/10.1200/JCO.2012.45.2615.
- Vaidya JS, Vyas JJ, Mittra I, Chinoy RF. Multicentricity and its influence on conservative breast cancer treatment strategy. Hongkong International Cancer Congress 1995.
- Vaidya JS, Vyas JJ, Chinoy RF, Merchant N, Sharma OP, Mittra I. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer 1996;74:820-4. http://dx.doi.org/10.1038/bjc.1996.442.
- Baum M, Vaidya JS, Mittra I. Multicentricity and recurrence of breast cancer. Lancet 1997;349. http://dx.doi.org/10.1016/S0140-6736(05)60950-6.
- Fisher ER, Anderson S, Redmond C, Fisher B. Ipsilateral breast tumor recurrence and survival following lumpectomy and irradiation: pathological findings from NSABP protocol B-06. Semin Surg Oncol 1992;8:161-6.
- Vaidya JS, Baum M. Clinical and biological implications of the Milan breast conservation trials. Eur J Cancer 1998;34:1143-4.
- Belletti B, Vaidya JS, D’Andrea S, Entschladen F, Roncadin M, Lovat F, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res 2008;14:1325-32. http://dx.doi.org/10.1158/1078-0432.CCR-07-4453.
- Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science 1996;274:2057-9. http://dx.doi.org/10.1126/science.274.5295.2057.
- Fukino K, Shen L, Patocs A, Mutter GL, Eng C. Genomic instability within tumor stroma and clinicopathological characteristics of sporadic primary invasive breast carcinoma. JAMA 2007;297:2103-11. http://dx.doi.org/10.1001/jama.297.19.2103.
- Ribeiro GG, Dunn G, Swindell R, Harris M, Banerjee SS. Conservation of the breast using two different radiotherapy techniques: interim report of a clinical trial. Clin Oncol 1990;2:27-34. http://dx.doi.org/10.1016/S0936-6555(05)80215-8.
- Ribeiro GG, Magee B, Swindell R, Harris M, Banerjee SS. The Christie Hospital breast conservation trial: an update at 8 years from inception. Clin Oncol (R Coll Radiol) 1993;5:278-83. http://dx.doi.org/10.1016/S0936-6555(05)80900-8.
- Vaidya JS. A Novel Approach for Local Treatment of Early Breast Cancer 2002. www.ucl.ac.uk/~rmhkjsv/papers/thesis.htm (accessed 15 July 2016).
- Vaidya JS, Baum M, Tobias JS, D’Souza DP, Naidu SV, Morgan S, et al. Targeted intra-operative radiotherapy (TARGIT): an innovative method of treatment for early breast cancer. Ann Oncol 2001;12:1075-80. http://dx.doi.org/10.1023/A:1011609401132.
- Vaidya JS, Baum M, Tobias JS, Morgan S, D’Souza D. The novel technique of delivering targeted intraoperative radiotherapy (TARGIT) for early breast cancer. Eur J Surg Oncol 2002;28:447-54. http://dx.doi.org/10.1053/ejso.2002.1275.
- Douglas RM, Beatty J, Gall K, Valenzuela RF, Biggs P, Okunieff P, et al. Dosimetric results from a feasibility study of a novel radiosurgical source for irradiation of intracranial metastases. Int J Radiat Oncol Biol Phys 1996;36:443-50. http://dx.doi.org/10.1016/S0360-3016(96)00293-3.
- Cosgrove GR, Hochberg FH, Zervas NT, Pardo FS, Valenzuela RF, Chapman P. Interstitial irradiation of brain tumors, using a miniature radiosurgery device: initial experience. Neurosurgery 1997;40:518-23.
- Vaidya JS, Baum M, Tobias JS, Massarut S, Wenz F, Murphy O, et al. Targeted intraoperative radiotherapy (TARGIT) yields very low recurrence rates when given as a boost. Int J Radiat Oncol Biol Phys 2006;66:1335-8. http://dx.doi.org/10.1016/j.ijrobp.2006.07.1378.
- Vaidya JS, Baum M, Tobias JS, Wenz F, Massarut S, Keshtgar M, et al. Long-term results of targeted intraoperative radiotherapy (TARGIT) boost during breast-conserving surgery. Int J Radiat Oncol Biol Phys 2011;81:1091-7. http://dx.doi.org/10.1016/j.ijrobp.2010.07.1996.
- Joseph DJ, Bydder S, Jackson LR, Corica T, Hastrich DJ, Oliver DJ, et al. Prospective trial of intraoperative radiation treatment for breast cancer. ANZ J Surg 2004;74:1043-8. http://dx.doi.org/10.1111/j.1445-1433.2004.03264.x.
- Vaidya JS, Wilson AJ, Houghton J, Tobias JS, Joseph D, Wenz F, et al. Cosmetic outcome after targeted intraoperative radiotherapy (TARGIT) for early breast cancer. Breast Cancer Res Treat 2003;82.
- Kraus-Tiefenbacher U, Bauer L, Kehrer T, Hermann B, Melchert F, Wenz F. Intraoperative radiotherapy (IORT) as a boost in patients with early-stage breast cancer – acute toxicity. Onkologie 2006;29:77-82. http://dx.doi.org/10.1159/000091160.
- Kraus-Tiefenbacher U, Bauer L, Scheda A, Fleckenstein K, Keller A, Herskind C, et al. Long-term toxicity of an intraoperative radiotherapy boost using low energy X-rays during breast-conserving surgery. Int J Radiat Oncol Biol Phys 2006;66:377-81. http://dx.doi.org/10.1016/j.ijrobp.2006.05.042.
- Enderling H, Anderson AR, Chaplain MA, Munro AJ, Vaidya JS. Mathematical modelling of radiotherapy strategies for early breast cancer. J Theor Biol 2006;241:158-71. http://dx.doi.org/10.1016/j.jtbi.2005.11.015.
- Enderling H, Chaplain MA, Anderson AR, Vaidya JS. A mathematical model of breast cancer development, local treatment and recurrence. J Theor Biol 2007;246:245-59. http://dx.doi.org/10.1016/j.jtbi.2006.12.010.
- Veronesi U, Orecchia R, Maisonneuve P, Viale G, Rotmensz N, Sangalli C, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. http://dx.doi.org/10.1016/S1470-2045(13)70497-2.
- Vaidya JS, Baum M, Tobias JS, Houghton J. Targeted intraoperative radiothearpy (TARGIT) – trial protocol. Lancet 1999. www.thelancet.com/protocol-reviews/99PRT-47 (accessed 15 July 2016).
- Vaidya JS, Tobias JS, Baum M, Keshtgar M, Joseph D, Wenz F, et al. Intraoperative radiotherapy for breast cancer. Lancet Oncol 2004;5:165-73. http://dx.doi.org/10.1016/S1470-2045(04)01412-3.
- Vaidya JS, Walton L, Dewar J. Single dose targeted intraoperative radiotherapy (TARGIT) for breast cancer can be delivered as a second procedure under local anaesthetic. World J Surg Oncol 2006;4. http://dx.doi.org/10.1186/1477-7819-4-2.
- Vaidya JS, Baum M, Tobias JS, Massarut S, Wenz FK, Hilaris B, et al. Efficacy of targeted intraoperative radiotherapy (Targit) boost after breast conserving surgery: updated results. J Clin Oncol 2008;26.
- Menes TS, Tartter PI, Bleiweiss I, Godbold JH, Estabrook A, Smith SR. The consequence of multiple re-excisions to obtain clear lumpectomy margins in breast cancer patients. Ann Surg Oncol 2005;12:881-5. http://dx.doi.org/10.1245/ASO.2005.03.021.
- Tobias JS, Vaidya JS, Keshtgar M, D’Souza DP, Baum M. Reducing radiotherapy dose in early breast cancer: the concept of conformal intraoperative brachytherapy. Br J Radiol 2004;77:279-84. http://dx.doi.org/10.1259/bjr/17186381.
- Herskind C, Steil V, Kraus-Tiefenbacher U, Wenz F. Radiobiological aspects of intraoperative radiotherapy (IORT) with isotropic low-energy X rays for early-stage breast cancer. Radiat Res 2005;163:208-15. http://dx.doi.org/10.1667/RR3292.
- Vaidya JS. Partial breast irradiation using targeted intraoperative radiotherapy (TARGIT). Nat Clin Pract Oncol 2007;4:384-5. http://dx.doi.org/10.1038/ncponc0850.
- Baum M, Vaidya JS. Targeted intra-operative radiotherapy – TARGIT for early breast cancer. Ann N Y Acad Sci 2008;1138:132-5. http://dx.doi.org/10.1196/annals.1414.019.
- Herskind C, Griebel J, Kraus-Tiefenbacher U, Wenz F. Sphere of equivalence – a novel target volume concept for intraoperative radiotherapy using low-energy X rays. Int J Radiat Oncol Biol Phys 2008;72:1575-81. http://dx.doi.org/10.1016/j.ijrobp.2008.08.009.
- Enderling H, Vaidya JS, Bellomo N, Tezduyor TE. Selected Topics in Cancer Modeling: Genesis, Evolution, Immune Competition and Therapy. Boston, MA: Springer; 2008.
- Herskind C, Wenz F. Radiobiological comparison of hypofractionated accelerated partial-breast irradiation (APBI) and single-dose intraoperative radiotherapy (IORT) with 50-kV X-rays. Strahlenther Onkol 2010;186:444-51. http://dx.doi.org/10.1007/s00066-010-2147-9.
- Herskind C, Schalla S, Hahn EW, Höver KH, Wenz F. Influence of different dose rates on cell recovery and RBE at different spatial positions during protracted conformal radiotherapy. Radiat Prot Dosimetry 2006;122:498-505. http://dx.doi.org/10.1093/rpd/ncl480.
- Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;371:1098-107. http://dx.doi.org/10.1016/S0140-6736(08)60348-7.
- National Cancer Institute . National Cancer Institute Common Toxicity Criteria: Radiation Related Adverse Events and Appendix IV; RTOG EORTC Late Radiation Morbidity Scoring Scheme 1999. http://ctep.cancer.gov/forms/CTCv20_4–30-992.pdf (accessed 14 June 2002).
- Dewar JA, Haviland JS, Agrawal RK, Bliss JM, Hopwood P, Magee B, et al. Hypofractionation for early breast cancer: first results of the UK standardisation of breast radiotherapy (START) trials. J Clin Oncol 2007;25.
- Polgár C, Fodor J, Major T, Németh G, Lövey K, Orosz Z, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma – 5-year results of a randomized trial. Int J Radiat Oncol Biol Phys 2007;69:694-702. http://dx.doi.org/10.1016/j.ijrobp.2007.04.022.
- Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513-20. http://dx.doi.org/10.1056/NEJMoa0906260.
- Clark RM, McCulloch PB, Levine MN, Lipa M, Wilkinson RH, Mahoney LJ, et al. Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst 1992;84:683-9. http://dx.doi.org/10.1093/jnci/84.9.683.
- Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, et al. START Trialists’ Group . The UK Standardisation of Breast Radiotherapy (START) trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 2008;9:331-41. http://dx.doi.org/10.1016/S1470-2045(08)70077-9.
- Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet 2002;359:1686-9. http://dx.doi.org/10.1016/S0140-6736(02)08594-X.
- Prat A, Cheang MC, Martín M, Carrasco E, Caballero R, Tyldesley S, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically-defined luminal A breast cancer. Ann Oncol 2012;23:ii17-18.
- Nguyen BT, Deb S, Fox S, Hill P, Collins M, Chua BH. A prospective pathologic study to define the clinical target volume for partial breast radiation therapy in women with early breast cancer. Int J Radiat Oncol Biol Phys 2012;84:1116-22. http://dx.doi.org/10.1016/j.ijrobp.2012.02.038.
- Cannon DM, McHaffie DR, Patel RR, Adkison JB, Das RK, Anderson BD, et al. Locoregional recurrence following accelerated partial breast irradiation for early-stage invasive breast cancer: significance of estrogen receptor status and other pathological variables. Ann Surg Oncol 2013;20:3446-52. http://dx.doi.org/10.1245/s10434-013-3015-5.
- Shah C, Wilkinson JB, Lyden M, Beitsch P, Vicini FA. Predictors of local recurrence following accelerated partial breast irradiation: a pooled analysis. Int J Radiat Oncol Biol Phys 2012;82:e825-30. http://dx.doi.org/10.1016/j.ijrobp.2011.11.042.
- Hawkes N. Start of cheaper technique for breast cancer is delayed in UK despite adoption elsewhere. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h2874.
- Guidance for Industry: Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. Rockville, MD: FDA, US Government; 2007.
- Lamont EB, Herndon JE, Weeks JC, Henderson IC, Earle CC, Schilsky RL, et al. Measuring disease-free survival and cancer relapse using Medicare claims from CALGB breast cancer trial participants (companion to 9344). J Natl Cancer Inst 2006;98:1335-8. http://dx.doi.org/10.1093/jnci/djj363.
- Vaidya JS, Bulsara M, Wenz F, Joseph D, Saunders C, Massarut S, et al. Pride, Prejudice, or Science – attitudes towards the results of the TARGIT-A trial of targeted intraoperative radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys 2015;92:491-7. http://dx.doi.org/10.1016/j.ijrobp.2015.03.022.
- Vaidya JS, Wenz F, Bulsara M, Tobias JS, Massarut M, Williams N, et al. Case selection for targeted intraoperative radiotherapy (TARGIT). Eur J Cancer 2013;49.
- Vaidya JS, Bulsara M, Wenz F, Massarut S, Joseph D, Tobias J, et al. Omitting whole breast radiotherapy does not increase axillary recurrence – data from TARGIT-A trial. Breast 2013.
- Vaidya JS, Wenz F, Bulsara M, Massarut S, Tobias J, Williams N, et al. Omitting whole breast radiation therapy did not increase axillary recurrence in the TARGIT-A Trial. Int J Radiat Oncol Biol Phys 2013;87. http://dx.doi.org/10.1016/j.ijrobp.2013.06.025.
- Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. http://dx.doi.org/10.1001/jama.2011.90.
- Vaidya JS, Bulsara M, Wenz F, Massarut S, Joseph D, Tobias J, et al. The lower non-breast cancer mortality with TARGIT in the TARGIT-A trial could be a systemic effect of TARGIT on tumor microenvironment. Int J Radiat Oncol Biol Phys 2013;87. http://dx.doi.org/10.1016/j.ijrobp.2013.06.623.
- Vaidya JS, Bulsara M, Wenz F, Massarut M, Joseph D, Tobias JS, et al. Fewer non-breast cancer deaths in the TARGIT-A trial. Systemic benefit of TARGIT or lack of EBRT toxicity?. Breast 2013;22.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. http://dx.doi.org/10.1056/NEJMoa1209825.
- Picot J, Copley V, Colquitt JL, Kalita N, Hartwell D, Bryant J. The clinical and cost effectiveness of the INTRABEAM® Photon Radiotherapy System for the adjuvant treatment of early breast cancer. Health Technol Assess 2014;19.
- Alvarado MD, Mohan AJ, Esserman LJ, Park CC, Harrison BL, Howe RJ, et al. Cost-effectiveness analysis of intraoperative radiation therapy for early-stage breast cancer. Ann Surg Oncol 2013;20:2873-80. http://dx.doi.org/10.1245/s10434-013-2997-3.
- Shah C, Badiyan S, Khwaja S, Shah H, Chitalia A, Nanavati A, et al. Evaluating radiotherapy options in breast cancer: does intraoperative radiotherapy represent the most cost-efficacious option?. Clin Breast Cancer 2014;14:141-6. http://dx.doi.org/10.1016/j.clbc.2013.10.005.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Department of Health . National Schedule of Reference Costs 2013–14: The Main Schedule n.d. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed 18 July 2015).
- Department of Health . NHS Reference Costs 2009–10, Appendix NSRC01: NHS Trust Reference Cost Schedules n.d. www.gov.uk/government/publications/nhs-reference-costs-2009–10 (accessed 18 July 2015).
- NICE . Early and Locally Advanced Breast Cancer: Diagnosis and Treatment. n.d. www.nice.org.uk/guidance/cg80/chapter/1-recommendations (accessed 18 July 2015).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A. Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11400.
- Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L. Hormonal therapies for early breast cancer: systematic review and economic evaluation. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11260.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den BW, Fourquet A, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol 2007;25:3259-65. http://dx.doi.org/10.1200/JCO.2007.11.4991.
- Poortmans PM, Collette L, Bartelink H, Struikmans H, Van den Bogaert WF, Fourquet A, et al. The addition of a boost dose on the primary tumour bed after lumpectomy in breast conserving treatment for breast cancer. A summary of the results of EORTC 22881-10882 ‘boost versus no boost’ trial. Cancer Radiother 2008;12:565-70. http://dx.doi.org/10.1016/j.canrad.2008.07.014.
- Jones HA, Antonini N, Hart AA, Peterse JL, Horiot JC, Collin F, et al. Impact of pathological characteristics on local relapse after breast-conserving therapy: a subgroup analysis of the EORTC boost versus no boost trial. J Clin Oncol 2009;27:4939-47. http://dx.doi.org/10.1200/JCO.2008.21.5764.
- Cost-Effectiveness Analysis Registry n.d. https://research.tufts-nemc.org/cear4/SearchingtheCEARegistry/SearchtheCEARegistry.aspx (accessed 19 July 2015).
- Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P, et al. Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE). Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14010.
- Lidgren M, Wilking N, Jönsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res 2007;16:1073-81. http://dx.doi.org/10.1007/s11136-007-9202-8.
- Hayman JA, Fairclough DL, Harris JR, Weeks JC. Patient preferences concerning the trade-off between the risks and benefits of routine radiation therapy after conservative surgery for early-stage breast cancer. J Clin Oncol 1997;15:1252-60.
- Hayman JA, Hillner BE, Harris JR, Weeks JC. Cost-effectiveness of routine radiation therapy following conservative surgery for early-stage breast cancer. J Clin Oncol 1998;16:1022-9.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing . . . presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. http://dx.doi.org/10.1002/hec.766.
- Petrou S, Dakin H, Abangma G, Benge S, Williamson I. Cost–utility analysis of topical intranasal steroids for otitis media with effusion based on evidence from the GNOME trial. Value Health 2010;13:543-51. http://dx.doi.org/10.1111/j.1524-4733.2010.00711.x.
- Briggs AH, Gray AM. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess 1999;3.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:S68-80. http://dx.doi.org/10.1177/0272989X98018002S09.
- Cancer Research UK . Breast Cancer Incidence Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/incidence-invasive (accessed 19 July 2015).
- Sperk E, Astor D, Keller A, Welzel G, Gerhardt A, Tuschy B, et al. A cohort analysis to identify eligible patients for intraoperative radiotherapy (IORT) of early breast cancer. Radiat Oncol 2014;9. http://dx.doi.org/10.1186/1748-717X-9-154.
- ClinicalTrials.gov . TARGIT-C (Consolidaton) Prospective Phase IV Study of IORT in Patients With Small Breast Cancer (TARGIT-C) n.d. https://clinicaltrials.gov/ct2/show/NCT02290782 (accessed 19 July 2015).
- Ziouèche-Mottet A, Houvenaeghel G, Classe JM, Garbay JR, Giard S, Charitansky H, et al. Eligibility criteria for intraoperative radiotherapy for breast cancer: study employing 12,025 patients treated in two cohorts. BMC Cancer 2014;14. http://dx.doi.org/10.1186/1471-2407-14-868.
- Locker GY, Sainsbury JR, Cuzick J. ATAC Trialist’ Group . Breast surgery in the ‘Arimidex, Tamoxifen Alone or in Combination’ (ATAC) trial: American women are more likely than women from the United Kingdom to undergo mastectomy. Cancer 2004;101:735-40. http://dx.doi.org/10.1002/cncr.20435.
- Alvarado M, Connolly J, Oboite M, Moore D, Park C, Esserman L. Patient preference for choosing intra-operative or external-beam radiotherapy following breast conservation. Eur J Cancer Suppl 2010;8. http://dx.doi.org/10.1016/S1359-6349(10)70260-3.
- Corica T, Nowak A, Saunders C, Bulsara M, Joseph D. Patient preferences for adjuvant radiotherapy in early breast cancer – an Australian sub-study of the international TARGIT trial. Eur J Cancer 2012;48. http://dx.doi.org/10.1016/S0959-8049(12)70547-6.
- Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph D, Baum M. Radiotherapy for breast cancer, the TARGIT-A trial – authors’ reply. Lancet 2014;383:1719-20. http://dx.doi.org/10.1016/S0140-6736(14)60830-8.
- Alvarado MD, Conolly J, Park C, Sakata T, Mohan AJ, Harrison BL, et al. Patient preferences regarding intraoperative versus external beam radiotherapy following breast-conserving surgery. Breast Cancer Res Treat 2014;143:135-40. http://dx.doi.org/10.1007/s10549-013-2782-9.
- Corica T, Joseph D, Saunders C, Bulsara M, Nowak AK. Intraoperative radiotherapy for early breast cancer: do health professionals choose convenience or risk?. Radiat Oncol 2014;9. http://dx.doi.org/10.1186/1748-717X-9-33.
- Tunes da Silva G, Logan BR, Klein JP. Methods for equivalence and noninferiority testing. Biol Blood Marrow Transplant 2009;15:120-7. http://dx.doi.org/10.1016/j.bbmt.2008.10.004.
- Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM. PRIME II investigators . Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266-73. http://dx.doi.org/10.1016/S1470-2045(14)71221-5.
- Welzel G, Boch A, Sperk E, Hofmann F, Kraus-Tiefenbacher U, Gerhardt A, et al. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: results from the randomized phase III trial TARGIT-A. Radiat Oncol 2013;8. http://dx.doi.org/10.1186/1748-717X-8-9.
- Keshtgar MR, Williams NR, Bulsara M, Saunders C, Flyger H, Cardoso JS, et al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat 2013;140:519-25. http://dx.doi.org/10.1007/s10549-013-2641-8.
- Sperk E, Welzel G, Keller A, Kraus-Tiefenbacher U, Gerhardt A, Sütterlin M, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Breast Cancer Res Treat 2012;135:253-60. http://dx.doi.org/10.1007/s10549-012-2168-4.
- Corica T, Nowak AK, Saunders C, Bulsara M, Taylor M, Vaidya JS, et al. Cosmesis and breast-related quality of life outcomes after intraoperative radiation therapy for early breast cancer: a substudy of the TARGIT-A trial. Int J Radiat Oncol Biol Phys 2016;96:55-64. http://dx.doi.org/10.1016/j.ijrobp.2016.04.024.
- Haybittle JL, Brinkley D, Houghton J, A’Hern RP, Baum M. Postoperative radiotherapy and late mortality: evidence from the Cancer Research Campaign trial for early breast cancer. BMJ 1989;298:1611-14. http://dx.doi.org/10.1136/bmj.298.6688.1611.
- Vaidya JS, Bulsara M, Wenz F, Coombs N, Singer J, Ebbs S, et al. Reduced mortality with partial breast irradiation for early breast cancer – a meta-analysis of randomised trials. Int J Radiat Oncol Biol Phys 2016;96:259-65.
- Vaidya JS, Bulsara M, Wenz F. Ischemic heart disease and breast cancer radiotherapy. N Engl J Med 2013;27368:2526-7.
- Joseph D, Nowak A, Corica T, Saunders C, Herbert C, Bulsara M, et al. Patient preferences for adjuvant radiotherapy in early breast cancer – an Australian sub-study of the pilot TARGIT study. Eur J Surg Oncol 2006;32:79-80. http://dx.doi.org/10.1016/S0748-7983(06)70699-0.
- Joseph D, Nowak A, Corica T, Saunders C, Herbert C, Bulsara M, et al. Patient Preferences for Adjuvant Radiotherapy in Early Breast Cancer: An Australian Sub-Study of the Pilot TARGIT Study n.d.
- Armitage P, Berry G. Statistical Methods in Medical Research. Oxford, Boston, MA: Blackwell Science Publications; 1994.
- Retsky MW, Demicheli R, Hrushesky WJ, Baum M, Gukas ID. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS 2008;116:730-41. http://dx.doi.org/10.1111/j.1600-0463.2008.00990.x.
- Cheng L, Swartz MD, Zhao H, Kapadia AS, Lai D, Rowan PJ, et al. Hazard of recurrence among women after primary breast cancer treatment – a 10 year follow up using data from SEER-Medicare. Cancer Epidemiol Biomarkers Prev 2012;21:800-9. http://dx.doi.org/10.1158/1055-9965.EPI-11-1089.
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. http://dx.doi.org/10.1056/NEJMoa022152.
- Esserman LJ, Alvarado MD, Howe RJ, Mohan AJ, Harrison B, Park C, et al. Application of a decision analytic framework for adoption of clinical trial results: are the data regarding TARGIT-A IORT ready for prime time?. Breast Cancer Res Treat 2014;144:371-8. http://dx.doi.org/10.1007/s10549-014-2881-2.
- Wickberg A, Holmberg L, Adami HO, Magnuson A, Villman K, Liljegren G. Sector resection with or without postoperative radiotherapy for stage I breast cancer: 20-year results of a randomized trial. J Clin Oncol 2014;32:791-7. http://dx.doi.org/10.1200/JCO.2013.50.6600.
- Wickberg A, Holmberg L, Adami HO, Magnuson A, Villman K, Liljegren G, et al. J Clin Oncol 2014;32. http://dx.doi.org/10.1200/JCO.2014.56.8493.
- Ben-Baruch A. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res 2003;5:31-6. http://dx.doi.org/10.1186/bcr554.
- Stuelten CH, Barbul A, Busch JI, Sutton E, Katz R, Sato M, et al. Acute wounds accelerate tumorigenesis by a T cell-dependent mechanism. Cancer Res 2008;68:7278-82. http://dx.doi.org/10.1158/0008-5472.CAN-08-1842.
- Vaidya JS, Baldassarre G, Massarut S. Beneficial effects of intraoperative radiotherapy on tumor microenvironment could improve outcomes (Comment on Int J Radiat Oncol Biol Phys 2008;72:1575–81). Int J Radiat Oncol Biol Phys 2009;74. http://dx.doi.org/10.1016/j.ijrobp.2009.02.041.
- Fabris L, Berton S, Massarut M, D’Andrea S, Roncadin M, Perin T, et al. Radiotherapy-induced miR expression influences the formation of local recurrence in breast cancer. Cancer Res 2012;72.
- Fabris L, Berton S, Citron F, D’Andrea S, Segatto I, Nicoloso MS, et al. Radiotherapy-induced miR-223 prevents relapse of breast cancer by targeting the EGF pathway. Oncogene 2016;35:4914-26. http://dx.doi.org/10.1038/onc.2016.23.
- Finn RS, Press MF, Dering J, Arbushites M, Koehler M, Oliva C, et al. Estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor expression and benefit from lapatinib in a randomized trial of paclitaxel with lapatinib or placebo as first-line treatment in HER2-negative or unknown metastatic breast cancer. J Clin Oncol 2009;27:3908-15. http://dx.doi.org/10.1200/JCO.2008.18.1925.
- Blamey RW, Bates T, Chetty U, Duffy SW, Ellis IO, George D, et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer 2013;49:2294-302. http://dx.doi.org/10.1016/j.ejca.2013.02.031.
- Early-Breast-Cancer-Trialists’-Collaborative-Group . Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2006;366:2087-106.
- Offersen BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol 2009;90:1-13. http://dx.doi.org/10.1016/j.radonc.2008.08.005.
- Vaidya JS, Bulsara M, Wenz F, Tobias JS, Joseph D, Baum M. Partial breast irradiation and the GEC-ESTRO trial. Lancet 2016;387. http://dx.doi.org/10.1016/S0140-6736(16)30255-0.
- Veronesi U, Orecchia R, Luini A, Galimberti V, Gatti G, Intra M, et al. Full dose intraoperative radiotherapy with electrons (ELIOT) during breast conserving surgery - experience with 1246 cases. Ecancermedicine 2008;2. http://dx.doi.org/10.3332/ecms.2008.65.
- Hockel M, Schlenger K, Hockel S, Aral B, Schaffer U, Vaupel P. Tumor hypoxia in pelvic recurrences of cervical cancer. Int J Cancer 1998;79:365-9. http://dx.doi.org/10.1002/(SICI)1097-0215(19980821)79:4<365::AID-IJC10>3.0.CO;2-4.
- Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 1997;38:285-9. http://dx.doi.org/10.1016/S0360-3016(97)00101-6.
- Nordsmark M, Hoyer M, Keller J, Nielsen OS, Jensen OM, Overgaard J. The relationship between tumor oxygenation and cell proliferation in human soft tissue sarcomas. Int J Radiat Oncol Biol Phys 1996;35:701-8. http://dx.doi.org/10.1016/0360-3016(96)00132-0.
- Carlson DJ, Stewart RD, Semenenko VA. Effects of oxygen on intrinsic radiation sensitivity: a test of the relationship between aerobic and hypoxic linear-quadratic (LQ) model parameters. Medical Physics 2006;33. http://dx.doi.org/10.1118/1.2229427.
- Movsas B, Chapman JD, Hanlon AL, Horwitz EM, Greenberg RE, Stobbe C, et al. Hypoxic prostate/muscle pO2 ratio predicts for biochemical failure in patients with prostate cancer: preliminary findings. Urology 2002;60:634-9. http://dx.doi.org/10.1016/S0090-4295(02)01858-7.
- Knocke TH, Weitmann HD, Feldmann HJ, Selzer E, Potter R. Intratumoral pO2-measurements as predictive assay in the treatment of carcinoma of the uterine cervix. Radiother Oncol 1999;53:99-104. http://dx.doi.org/10.1016/S0167-8140(99)00139-5.
- Fyles AW, Milosevic M, Wong R, Kavanagh MC, Pintilie M, Sun A, et al. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol 1998;48:149-56. http://dx.doi.org/10.1016/S0167-8140(98)00044-9.
- Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol 1996;41:31-9. http://dx.doi.org/10.1016/S0167-8140(96)01811-7.
- Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009;74:987-1001. http://dx.doi.org/10.1016/j.ijrobp.2009.02.031.
- Polgar C, Limbergen EV, Potter R, Kovacs G, Polo A, Lyczek J, et al. GEC-ESTRO Recommendations: patient selection for accelerated partial-breats irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiat Oncol 2010;94:264-73. http://dx.doi.org/10.1016/j.radonc.2010.01.014.
- Zietman A. Letters regarding the TARGIT-A trial: the editor’s introduction. Int J Radiat Oncol Biol Phys 2015;92:951-2. http://dx.doi.org/10.1016/j.ijrobp.2015.05.048.
- Vaidya JS. Worldwide Adoption of TARGeted Intraoperative RadioTherapy TARGIT IORT for Breast Cancer n.d. http://jayantvaidya.org/breast-cancer-surgeon/worldwide-adoption-of- targeted-intraoperative-radiotherapy-targit-iort-for-breast-cancer/ (accessed 15 April 2016).
- Bernstein M. Intraoperative radiation therapy for breast cancer: a patient’s view. Lancet 2016;387:1904-5. http://dx.doi.org/10.1016/S0140-6736(16)30415-9.
- Biganzoli L, Wildiers H, Oakman C, Marotti L, Loibl S, Kunkler I, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol 2012;13:e148-60. http://dx.doi.org/10.1016/S1470-2045(11)70383-7.
- Senkus E, Kyriakides S, Penault-Llorca F. behalf of the ESMO Guidelines Working Group . Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;26:v8-30. http://dx.doi.org/10.1093/annonc/mdt284.
- AGO Breast Committee . Updated German Guidelines: The 2015 Update of Guidelines of the Association of Gynecological Oncology (AGO), an Autonomous Community of the German Society of Gynecology and Obstetrics (DGGG) and the German Cancer Society n.d. www.ago-online.de/fileadmin/downloads/leitlinien/mamma/maerz2015/en/2015E_Updated_Guidelines.pdf (accessed 15 July 2016).
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Panel members . Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47. http://dx.doi.org/10.1093/annonc/mdr304.
- San Antonio Breast Cancer Symposium programme committee . 2012 San Antonio Breast Cancer Symposium highlights: TARGIT vs conventional radiotherapy: 10 years on. Newsletter 2012;3.
- Schmidt C. Early breast cancer: single dose of radiation during surgery gains support. JNCI 2010;102:1304-9. http://dx.doi.org/10.1093/jnci/djq351.
- Partial breast irradiation safe for some women with invasive breast cancer . NCI News Bulletin 2010;7.
- Helwick C, Goodman A, Cavallo J. Research roundup from San Antonio: intraoperative radiotherapy vs. external-beam radiotherapy. ASCO Post 2013;4.
- BBC News . One-Shot Radiotherapy ‘Success Against Breast Cancer 2010. http://bbc.in/p3SCnh (accessed 6 July 2016).
- Laurance J. Dramatic advance in treatment of breast cancer. The Independent 2010. http://ind.pn/9teYZP (accessed 6 July 2016).
- Sinha K. Soon, one-shot radiotherapy for breast cancer?. The Times of India 2010. http://bit.ly/15DATUo (accessed 6 July 2016).
- Pollack A. Findings may alter care for early breast cancer. The New York Times 2010.
- Thomas M, Maugh II. New Treatment for breast cancer: one-shot radiation found effective with breast cancer. Los Angeles Times 2010.
- Jourdan T. Me and my operation: targeted intraoperative radiotherapy blasted my breast tumour. Daily Mail 2011. http://dailym.ai/fqgOaR (accessed 6 July 2016).
- Bernstein M. A revolution in breast cancer therapy. The Daily Telegraph 2013. http://goo.gl/uioHC8 (accessed 6 July 2016).
- Hope J. One-stop breast cancer treatment: radiation breakthrough will help thousands. Daily Mail 2013. http://goo.gl/C5nbkg (accessed 6 July 2016).
- Radiotherapy after surgery could aid breast cancer recovery . Herald Scotland 2013. http://goo.gl/nBdcwF (accessed 6 July 2016).
- Pilkington D. Me and my operation: the one-stop breast cancer op that spares women weeks of radiotherapy. Daily Mail 2013. http://goo.gl/HQ74aK (accessed 6 July 2016).
- TARGIT Breast Cancer Therapy 2013. www.bbc.co.uk/programmes/p01lhjm2 (accessed 6 July 2016).
- The Lancet 2013. http://download.thelancet.com/pb/assets/raw/Lancet/stories/audio/lancet/2013/11november.mp3 (accessed 23 July 2016).
- TARGIT for Breast Cancer Using Intrabeam n.d. http://youtu.be/heVPwtXoTOg (accessed 6 July 2016).
- NICE Appraisal ‘Intrabeam Targeted Intraoperative Radiotherapy for the Treatment of Early or Locally Advanced Breast Cancer’ [ID618] To Appraise the Clinical and Cost-Effectiveness of the INTRABEAM Photon Radiosurgery System for the Adjuvant Treatment of Early or Locally Advanced Breast Cancer During Surgical Removal of the Tumour n.d. www.nice.org.uk/news/press-and-media/nice-to-recommend-new-breast-cancer-radiotherapy-treatment-alongside-further-research (accessed 18 August 2016).
- Marmot M, Altman G, Cameron DA, Dewar JA, Thompson SG, Wilcox M. BMJ. 2013.
- Vaidya JS, Bulsara M, Wenz F. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med 2013;368:2526-7.
- Alvarado M, Ozanne E, Mohan A, Esserman L. Cost-effectiveness of intraoperative radiation therapy for breast conservation. J Clin Oncol 2011;29. http://dx.doi.org/10.1016/s0960-9776(11)70206-5.
- Vaidya JS, Benson JR, Tobias JS, Benson JR, Gui GPH, Tuttle T. Early Breast Cancer: From Screening to Multidisciplinary Management. London: CRC Press, Taylor Francis Group; 2013.
- Smits PC, Hofma S, Togni M, Vázquez N, Valdés M, Voudris V, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet 2013;381:651-60. http://dx.doi.org/10.1016/S0140-6736(12)61852-2.
- Cuzick J. Radiotherapy for breast cancer, the TARGIT-A trial. Lancet 2014;383. http://dx.doi.org/10.1016/S0140-6736(14)60825-4.
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. Group C . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594-604. http://dx.doi.org/10.1001/jama.2012.87802.
- Zhao L, Tian L, Uno H, Solomon SD, Pfeffer MA, Schindler JS, et al. Utilizing the integrated difference of two survival functions to quantify the treatment contrast for designing, monitoring, and analyzing a comparative clinical study. Clinical Trials 2012;9:570-7. http://dx.doi.org/10.1177/1740774512455464.
- Data Protection Act 1988. London: The Stationery Office; 1988.
- Leventhal H, Weinman J, Leventhal EA, Phillips LA. Health psychology: the search for pathways between behavior and health. Annu Rev Psychol 2008;59:477-505. http://dx.doi.org/10.1146/annurev.psych.59.103006.093643.
Appendix 1 International Steering Committee and Data Monitoring Committee
| Meeting | Date | Venue |
|---|---|---|
| 01 | March 2002 | Barcelona |
| 02 | November 2002 | Frankfurt |
| 03 | March 2003 | St Gallen |
| 04 | December 2003 | San Antonio |
| 05 | March 2004 | Hamburg |
| 06 | June 2004 | New Orleans |
| 07 | December 2004 | San Antonio |
| 08 | January 2005 | St Gallen |
| 09 | May 2005 | Orlando |
| 10 | October 2005 | Paris |
| 11 | March 2006 | Nice |
| 12 | November 2006 | Venice |
| 13 | March 2007 | St Gallen |
| 14 | November 2007 | Frankfurt |
| 15 | April 2008 | Berlin |
| 16 | September 2008 | London |
| 17 | March 2009 | St Gallen |
| 18 | September 2009 | Berlin |
| 19 | December 2009 | Teleconference |
| 20 | March 2010 | Barcelona |
| 21 | November 2010 | London |
| 22 | March 2011 | St Gallen |
| 23 | September 2011 | Frankfurt |
| 24 | March 2012 | Vienna |
| 25 | July 2012 | London |
| 26 | September 2012 | London |
| 27 | March 2014 | London |
International Steering Committee members
Jayant S Vaidya, Frederik Wenz, Max Bulsara, Jeffrey S Tobias, David J Joseph (co-chairperson), Mohammed Keshtgar, Henrik L Flyger, Samuele Massarut, Michael Alvarado, Christobel Saunders, Wolfgang Eiermann, Marinos Metaxas, Elena Sperk, Marc Sütterlin, Laura Esserman, Alastair Thompson, John A Dewar, Mary Falzon, Eleanor Harris, Frank Melchert, Albert Schmidt, Olive Murphy, Joan Houghton, Oscar Streeter, Michael Osborne, Chuck Vecoli, Uta Kraus-Tiefenbacher, April Matthews, Tammy Corica, Norman R Williams and Michael Baum (co-chairperson).
Data Monitoring Committee members
Jack Cuzick, Hazel Thornton and Alan Rodger.
Contrary to the statement by Jack Cuzick published in the correspondence columns of The Lancet on 17 May 2014, he did not resign. This was corrected on the Lancet website on 10 April 2015.
The remit of the DMC was completed when the trial finished recruitment in June 2012. The committee was thanked and dissolved on 24 May 2013.
Appendix 2 Accrual
FIGURE 62.
Final accrual graph for the TARGIT trial, 28 June 2012 (n = 3451 patients).
FIGURE 63.
Accrual by continent for the TARGIT trial.
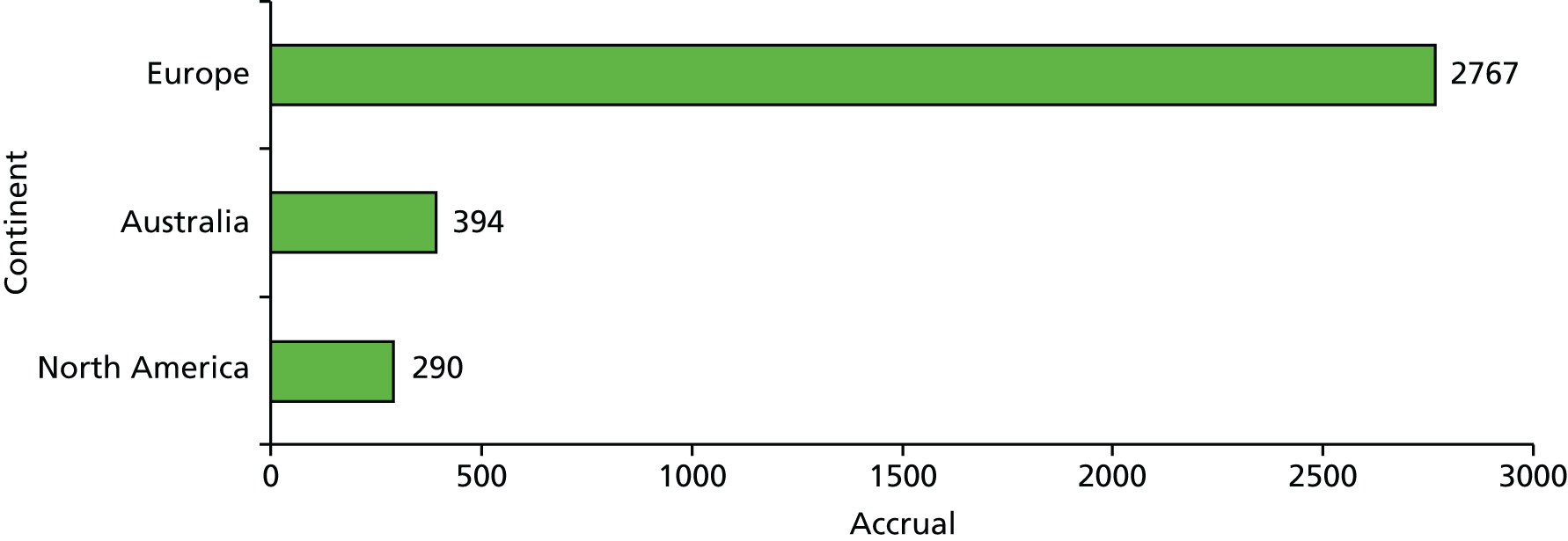
FIGURE 64.
Accrual by country for the TARGIT trial.

FIGURE 65.
Accrual in the UK for the TARGIT trial. GST, Guy’s and St Thomas’ Hospital; HJE, Hospital of St Johns and St Elizabeth; RFH, Royal Free Hospital.
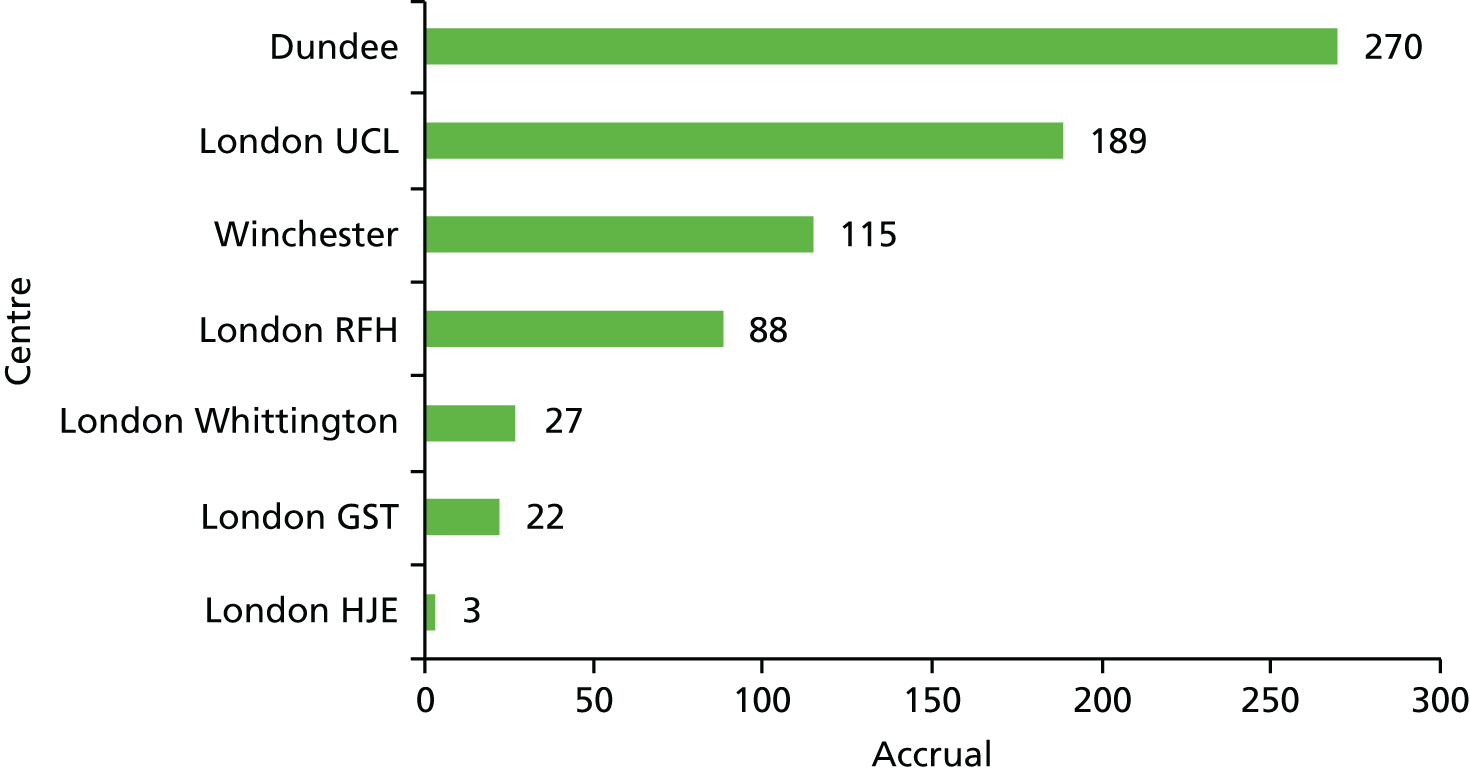
Appendix 3 List of presentations
The TARGIT website [see www.targit.org.uk (accessed 7 July 2016)] has a wealth of information about the TARGIT technique and the TARGIT trials.
| Date | Invited presentation title and meeting |
|---|---|
| 2 March 1997 | Multicentricity of breast cancer: new findings and their clinical and biological implications (15 minutes) |
| 1st Annual Meeting of the Indian Breast Group at Tata Memorial Hospital, Mumbai, India | |
| 2 March 1998 | Local recurrence has nothing to do with residual disease (20 minutes) |
| Biennial Presidential Conference of the British Oncological Association, Royal Society of Medicine, London, UK | |
| 19 September 1998 | Radiosurgery: a novel method of treatment of early breast cancer (15 minutes) |
| International Meeting on Whole-Body Stereotactic Radiotherapy, Edinburgh, UK | |
| 13 November 1998 | Radiosurgery: a novel method of treatment of early breast cancer (15 minutes) |
| North London Cancer Network Breast Cancer Tumour Board Away Day, Royal Society of Arts, London, UK | |
| 18 February 1999 | Novel radiotherapy techniques at the Middlesex hospital (45 minutes) |
| Meyerstein Institute of Oncology Seminar, London, UK | |
| 22 June 1999 | Radiosurgery: an innovative approach to management of early breast cancer (45 minutes) |
| Surgical Forum, Department of Surgery, Norfolk and Norwich University Hospital, Norwich, UK | |
| 7 September 1999 | Radiosurgery: an innovative approach to local treatment of breast cancer (1 hour) |
| Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA | |
| 8 September 1999 | Radiosurgery: an innovative approach to local treatment of breast cancer (1 hour) |
| Cleveland Clinic, Cleveland, OH, USA | |
| 13 September 1999 | Radiosurgery: an innovative approach to local treatment of breast cancer (1 hour) |
| Our Lady of Mercy Medical School, New York, NY, USA | |
| 18 February 2000 | Breast conservative therapy – novel approaches – targeted Intraoperative radiotherapy (20 minutes) |
| Breast Cancer in the New Millennium, Nagpur, India | |
| 1–2 December 2000 | Rethinking cancer research – discussion |
| ‘Blue-Skying’ the Future of Cancer Research, Cambridge, UK | |
| 9 December 2000 | TARGIT – a novel approach to local treatment of breast cancer (30 minutes) |
| 23rd Annual San Antonio Breast Cancer Conference (Satellite Symposium), San Antonio, TX, USA | |
| 31 January 2001 | Targeted intraoperative radiotherapy for breast cancer – a randomised trial (20 minutes) |
| International Conference of Radiation Oncology (ICRO) (Satellite Symposium), Melbourne, VIC Australia | |
| 27 February 2002 | Intraoperative radiotherapy for breast cancer (20 minutes) |
| Breakthrough Breast Cancer, London, UK | |
| 8 March 2002 | Intraoperative radiotherapy for breast cancer – the UK experience |
| 8th Annual Clinical Oncology Symposium: Current Concepts and Developments in Intraoperative Radiation Oncology, New York, NY, USA | |
| 20 March 2002 | Targeted intraoperative radiotherapy – rationale, technique and results (30 minutes) |
| 3rd European Breast Cancer Conference, Barcelona, Spain | |
| 17 May 2002 | Targeted Intraoperative radiotherapy – rationale, technique and results |
| Congress of the Portuguese Society of Radiology and Radiotherapy, Algarve, Portugal | |
| 22 May 2002 | Targeted intraoperative radiotherapy for early breast cancer – a randomised trial |
| American Brachytherapy Society meeting, Orlando, FL, USA | |
| 5 June 2002 | Targeted intraoperative radiotherapy – a randomised trial (30 minutes) |
| 4th Milan Breast Cancer Conference, Milan, Italy | |
| 17 June 2002 | TARGIT |
| 5th meeting of UK clinical triallists, Birmingham, UK | |
| 25 September 2002 | Supervision of first two cases of intraoperative radiotherapy for breast cancer using INTRABEAM and lecture (1 hour) |
| CRO (National Cancer Institute), Aviano, Italy | |
| 5 November 2002 | Intraoperative radiotherapy for breast cancer |
| Symposium on Novel Treatments for Breast Cancer, Empoli, Italy | |
| 13 March 2003 | Update on cosmetic outcome of TARGIT |
| 8th International Conference: Primary Therapy of Early Breast Cancer (Satellite Symposium on Intraoperative Radiotherapy), St Gallen, Switzerland | |
| 6 June 2003 | TARGIT – targeted Intraoperative radiotherapy for breast cancer (30 minutes) |
| 5th Milan Breast Cancer Conference, Milan, Italy | |
| 19 August 2003 | Targeted intraoperative radiotherapy, its rationale, technique and trials (45 minutes) |
| MD Anderson Cancer Center, Houston, TX, USA | |
| 4 December 2003 | Intraoperative radiotherapy for breast cancer (TARGIT) (30 minutes) – webcast [PowerPoint® (Microsoft Corporation, Redmond, WA, USA)] |
| 26th Annual Breast Cancer Conference, San Antonio, TX, USA | |
| 9 December 2003 | The novel approach of targeted intraoperative radiotherapy for breast cancer (45 minutes) |
| Memorial Sloan Kettering Cancer Center, New York, NY, USA | |
| 9 December 2003 | The novel approach of targeted intraoperative radiotherapy for breast cancer (45 minutes) |
| New York Presbyterian Hospital, Cornell Institute, New York, NY, USA | |
| 3 February 2004 | Intraoperative radiotherapy for breast cancer (TARGIT) |
| Ninewells Hospital and Medical School, University of Dundee, Dundee, UK | |
| 18 Jun 2004 | Updated results of TARGIT (15 minutes) |
| 6th Milan Breast Cancer Conference, Milan, Italy | |
| 19 November 2004 | Intraoperative radiotherapy for breast cancer |
| Annual Meeting of the Scottish Radiological Society, Dundee, UK | |
| 26 November 2004 | Intraoperative radiotherapy for breast cancer |
| Focus on Innovative and Locoregional Treatments – VII Congresso Nazionale SITILO (Societa’ Italiana di Terapie Integrate Locoregionali in Oncologia), Florence, Italy | |
| 7 December 2004 | TARGIT meeting with surgeons and radiation oncologists |
| New Orleans, LA, USA | |
| 21 December 2004 | The international TARGIT trial |
| 1st Workshop on the Health Technology Assessment Programme for Italy (Programma Ricerca e Innovazione Emilia-Romagna – 1° Workshop – La radiotherapia intraoperatoria nel tumore della mammella), Bologna, Italy | |
| 25 February 2005 | Targeted intraoperative radiotherapy trial |
| Scottish Cancer Triallists Meeting, St Andrews, UK | |
| 1 November 2005 | Debate on partial breast irradiation in selected patients and accurate targeting of boost in others: rationale for partial breast irradiation in selected patients (15 minutes) |
| 13th European Cancer Conference (ECCO-13), Paris, France | |
| 17 December 2005 | Intraoperative radiotherapy for breast cancer (20 minutes) |
| 3rd European Conference on Plastic and Reconstructive Surgery of the Breast, Milan, Italy | |
| 10 March 2006 | Targeted intraoperative radiotherapy trial |
| Breast Cancer Triallists Meeting, Dundee, UK | |
| 24 June 2006 | The indications for TARGIT (20 minutes) |
| 8th Milan Breast Cancer Conference, Milan, Italy | |
| 14 March 2007 | Targeted intraoperative radiotherapy – rationale, technique and clinical trials (30 minutes) |
| 10th International Conference on Primary Therapy of Early Breast Cancer (Satellite Symposium), St Gallen, Switzerland | |
| 18 April 2007 | Breast conserving surgery – TARGIT for the 21st century and related translational research (40 minutes) |
| Cambridge International Conference on Breast Screening, Cambridge, UK | |
| 15 September 2007 | Cutting edge techniques at the forefront of breast surgery (40 minutes) |
| Delegates: about 100 surgeons from 17 countries (14–16 September 2007), Conrad Dublin, Dublin, Ireland | |
| 19 September 2007 | The TARGIT trials – alone and as a boost (1 hour) |
| Nottingham International Breast Cancer Conference (Satellite Symposium), Nottingham, UK | |
| 6 December 2007 | Round table discussion between cancer specialists in the UK along with the Minister for Health, the Cancer Czar, Chairman of the National Cancer Research Network (NCRN) and the All Party Chairman On Cancer Care |
| House of Commons, London, UK | |
| 20 December 2007 | Breast conserving surgery – TARGIT for the 21st century (1 hour) |
| Goa Medical College Staff Society Meeting, Goa, India | |
| 12 April 2008 | Individualised treatment for breast cancer: TARGIT for the 21st century |
| Indian Breast Cancer Initiative, New Delhi, India | |
| 12 April 2008 | Demonstration of operative technique: targeted intraoperative radiotherapy for breast cancer |
| Indian Breast Cancer Initiative, New Delhi, India | |
| 29 May 2008 | An update of the novel approach to local treatment of breast cancer using intraoperative radiotherapy |
| Radiation Medicine Program, RMP Special Lecture, Radiation Physics Department, University Health Network, Princess Margaret Hospital, Toronto, ON, Canada | |
| 2 June 2008 | TARGIT Satellite Meeting (30 minutes) |
| ASCO Satellite, Chicago, IL, USA | |
| 27 June 2008 | TARGIT German user group meeting (30 minutes) |
| University of Mannheim, Mannheim, Germany | |
| 13 January 2009 | The TARGIT trial |
| Institute Curie, Paris, France | |
| 16 January 2009 | The TARGIT trial |
| Sheffield Cancer Network, Sheffield, UK | |
| 12 March 2009 | Targeted intraoperative radiotherapy – update of the randomised trial (20 minutes) |
| 11th International Conference on Primary Therapy of Early Breast Cancer (Satellite Symposium), St Gallen, Switzerland | |
| 8 May 2009 | Individualising breast cancer treatment – TARGIT for the 21st century |
| Lincoln, UK | |
| 11 June 2009 | TARGIT trial – progress |
| UK Breast Intergroup, Birmingham, UK | |
| 3 September 2009 | Targeted intraoperative radiotherapy – the clinical trials |
| Copenhagen, Denmark | |
| 23 October 2009 | Targeted Intraoperative radiotherapy |
| All India Institute of Medical Sciences, Army Medical Referral and Research Centre, New Delhi, India | |
| 29 October 2009 | Targeted intraoperative radiotherapy |
| Tata Memorial Hospital, Mumbai, India | |
| 30 October 2009 | Demonstration of two surgical operations with the TARGIT procedure |
| Indo-American Cancer Centre and Research Institute, Hyderabad, India | |
| 7 November 2009 | Innovations and progress in breast cancer treatment (unable to take up) |
| Innaugural conference, Institute of Breast Cancer, Saifee Hospital, Mumbai India | |
| 25 March 2010 | TARGIT trials: a 15-year evolution |
| TARGIT Satellite Meeting, European Breast Cancer Conference, Barcelona, Spain | |
| 30 April 2010 | Intraoperative radiotherapy (TARGIT trials) (plenary) |
| American Brachytherapy Society Meeting, Atlanta, GA, USA | |
| 6 June 2010 | First results of the TARGIT-A trial: safety and efficacy of targeted intraoperative radiotherapy vs. external beam radiotherapy results with a 10-year maximum follow up |
| TARGIT Symposium at the ASCO Meeting, Chicago, IL, USA | |
| 9 June 2010 | First results of the TARGIT-A trial: safety and efficacy of targeted intraoperative radiotherapy vs. external beam radiotherapy results with a 10-year maximum follow up |
| University of Louisville, KY, USA and University of Indianapolis, IN, USA | |
| 10 June 2010 | First results of the TARGIT-A trial: safety and efficacy of targeted intraoperative radiotherapy vs. external beam radiotherapy results with a 10-year maximum follow up |
| Cleveland Clinic, Cleveland, OH, USA | |
| 18 June 2010 | First results of the TARGIT-A trial: safety and efficacy of targeted intraoperative radiotherapy vs. external beam radiotherapy results with a 10-year maximum follow up |
| TARGIT Symposium, Milan Breast Cancer Conference, Milan, Italy | |
| 16 September 2010 | Targit-A trial: the results at the end of 10 years maximum follow up and TARGIT-B trial: targeted intra-operative radiotherapy boost – a randomised trial for young and high risk patients including those after post-neoadjuvant systemic therapy lumpectomy (30 minutes) |
| European Society of Surgical Oncology, Bordeaux, France | |
| 16 September 2010 | Targit-A trial- the results at the end of 10 years maximum follow up (15 minutes) [plenary – given via Skype™ (Microsoft Corporation, Redmond, WA, USA)] |
| British Breast Cancer Research Conference, Nottingham, UK | |
| 16 September 2010 | Workshop on New Technologies – Intraoperative Radiotherapy (30 minutes) (could not take up) |
| British Breast Cancer Research Conference, Nottingham, UK | |
| 22 September 2010 | TARGIT for the 21st century (30 minutes) |
| Annual South West England breast screening education day, Taunton Racecourse, Taunton, Exeter | |
| 23 September 2010 | TARGIT-A trial results |
| Weekly meetings of the Institute of Womens’ Health, University College London, London, UK | |
| 7 October 2010 | TARGIT-A trial results – lecture followed by panel discussion (25 minutes) |
| University College London Partners and North London Cancer Network Breast Cancer Away Day, RIBA, London, UK | |
| 12 November 2010 | TARGIT-A trial results, TARGIT-Boost trial and implications for India |
| Tata Memorial Hospital, Mumbai, India, and Raheja Hospital, Mumbai, India | |
| 13 November 2010 | Plenary: TARGIT-A trial and TARGIT-Boost trial and implications for India; Panel discussion: screening for breast cancer in India |
| New India Cancer Charity Initiative Brinker Awardee Symposium, New Delhi, India | |
| 14 November 2010 | TARGIT-A trial results, TARGIT-Boost trial and implications for India |
| Apollo Hospital, New Delhi, India | |
| 26 February 2011 | The evolution of TARGIT |
| National Undergraduate Medical Students Conference, University College London, London, UK | |
| 17 March 2011 | TARGIT-A trial – 1 year on |
| St Gallen Breast Cancer Conference (Satellite Symposium), Switzerland | |
| 29 April 2011 | Intraoperative radiotherapy (plenary) |
| American Society of Breast Surgeons, Washington, DC, USA | |
| 7 May 2011 | Oxford style debate on intraoperative radiotherapy as a boost (plenary debate) |
| International Society of Intraoperative Radiotherapy (ISIORT) and ESTRO, London | |
| 12/13 May 2011 | Demonstration of technique of TARGIT; TARGIT-A trial results and TARGIT-B trial |
| University of Padua Medical School, Padua, Italy | |
| 17 June 2011 | TARGIT-A and TARGIT-Boost trials and implications for India |
| Medicity Hospital, New Delhi, India | |
| 18 June 2011 | TARGIT-A and TARGIT-Boost trials and implications for India |
| Association of Radiation Oncologists in India (AROI) (Tamilnadu and Pondicherry Chapter), Chennai, India | |
| 19 June 2011 | TARGIT-A and TARGIT-Boost trials and implications for India |
| Bangalore Oncology Group Meeting, Bangalore, India | |
| 30 June 2011 | TARGIT-A trial results |
| 2nd UK National Breast Cancer Meeting, King’s Fund, London, UK | |
| 1 July 2011 | TARGIT trials and training day |
| User group and training meeting, University of Mannheim, Mannheim, Germany | |
| 28 August 2011 | Main speaker, Swedish Surgical Society’s Annual Surgical Week |
| Karolinska Institute, Sweden | |
| 3 September 2011 | Keynote address (30 minutes): Original research: evolution of TARGIT; Screening, MRI, and skin sparing mastectomy (15 minutes) |
| Breast Cancer CME and Symposium, 1st West Zone CME Programme, Association of Breast Surgeons of India, Pune, India | |
| 7 November 2011 | Targeted intraoperative radiotherapy for breast cancer (TARGIT) (plenary) |
| British Association of Surgical Oncology – Association of Cancer Surgeons (BASO-ACS) Scientific Conference, Royal College of Surgeons of England, London [see www.baso.org/conferences/past-conferences/baso-∼-acs-scientific-conference-nov-2011.aspx (accessed 18 July 2016)] | |
| 29 December 2011 | Keynote address: TARGIT – intraoperative radiotherapy to the breast (unable to take up) |
| 71st Annual Conference of the Association of Surgeons of India (ASICON), Kochi, India | |
| 23 February 2012 | New models of radiotherapy. Cutting-edge cancer treatments: expense and expectation? |
| An Independent Cancer Patients’ Voice workshop, CRUK London Research Institute, London, UK | |
| 27 February 2012 | TARGIT trials update |
| Breast Tumour Away Day, North London Cancer Network, London, UK | |
| 15 March 2012 | Intraoperative radiotherapy vs. whole breast radiotherapy (plenary – given via Skype) |
| 9th Annual Miami Breast Cancer Conference, Miami, FL, USA | |
| 18 May 2012 | IORT – who, when and how: TARGIT for India (plenary – remotely with audience participation) |
| 1st Annual Conference of the Association of Breast Surgeons of India International Congress, Hyderabad, India | |
| 9 June 2012 | Update on targeted radiotherapy |
| 9th Düsseldorf Breast Cancer Conference, Düsseldorf, Germany | |
| 13 June 2012 | Expert advisor, Medical Technologies Advisory Committee meeting |
| NICE, London, UK | |
| 29 June 2012 | TARGIT trials – surgical aspects and trial update |
| University of Mannheim, Mannheim, Germany | |
| 12 August 2012 | Felicitation and lifetime achievement award lecture |
| Centenary Celebrations of the Gomant Vidya Niketan, Goa, India | |
| 13 September 2012 | Invited speaker: intraoperative radiotherapy: results from the randomised trial |
| ASCO Breast Symposium, San Francisco, CA, USA | |
| 10/11 October 2012 | Visiting Professorship, Morris Deutsch Memorial Lectureship Dinner and Grand Rounds |
| Johns Hopkins Hospital, Baltimore, MD, USA | |
| 19 October 2012 | TARGIT-A and TARGIT-B trials |
| Master’s course – seminar on intraoperative radiotherapy, Madrid, Spain | |
| 30 November 2012, will present Remotely | Surgery in breast cancer: does ‘less mean more?’ |
| Patient First – Joint International Conference, Sri Balaji Vidhyapeeth, Pondicherry, India, and the Royal College of Physicians and Surgeons, Glasgow, UK | |
| 2 February 2013 | Main speaker: TARGIT trials in breast cancer |
| New York Metropolitan Breast Cancer Group (NYMBCG) 40th Annual Symposium, New York Academy of Medicine, NY, USA | |
| 13/14 April 2013 | Guest plenary lecture: Breast conservation: emerging trends and future directions |
| Rajiv Gandhi Cancer Institute and Research Centre, New Delhi, India | |
| 13 June 2013 | TARGIT – update *Highlight* |
| 7th INTRABEAM user meeting, Mannheim, Germany | |
| 19 September 2013 | TARGIT trials |
| Clinicians from University of Miami, Miami, FL, USA | |
| 20 September 2013 | TARGIT trials |
| Ashikari Breast Center, Dobbs Ferry, NY, USA | |
| 21 September 2013 | TARGIT trials |
| Satellite meeting at ASTRO and Emory University, Atlanta, GA, USA | |
| 2 October 2013 | Controversies in management of breast cancer: the evolution of the TARGIT treatment |
| Divisional Away Day, University College London, London, UK | |
| 4 October 2013 | TARGIT trials |
| Journées Franco-Brésiliennes de Cancérologie Mammaire: Mise à Jour sur le Cancer du Sein – Updating on Breast Cancer, Strasbourg, France | |
| 24 October 2013 | Intraoperative technique – one stop shopping – what could be better? (25 minutes) |
| American Brachytherapy Society School of Breast Brachytherapy, Las Vegas, NV, USA | |
| 25 October 2013 | Debate – IORT vs. APBI (1 hour) |
| American Brachytherapy Society School of Breast Brachytherapy, Las Vegas, NV, USA | |
| 9 January 2014 | Developing evidence for new devices in clinical practice with randomised trials |
| UCL French Embassy Science and Technology Workshop, Nanomaterials for Biomedical Applications, London, UK | |
| 7 February 2014 | Results of the TARGIT-A trial |
| 4th International Breast Disease Centres Congress, Paris, France | |
| 6 May 2014 | The TARGIT-A and TARGIT-B trials (45 minutes) |
| Sírio Libanês Hospital, São Paulo, Brazil | |
| 7 May 2014 | The TARGIT-A and TARGIT-B trials (30 minutes) |
| Brazilian National Cancer Hospital, with remote connection to the Cancer Hospital in Fortaleza and Oswaldo Cruz Hospital in Recife, Brazil | |
| 8 May 2014 | The TARGIT-A and TARGIT-B trials (30 minutes, plenary) |
| 2nd International Breast Symposium, Salvador, Brazil | |
| 11 October 2014 | The TARGIT-A and TARGIT-B trials |
| International Conference on Advances in Breast Cancer, Goa, India | |
| 31 October 2014 | The TARGIT-A and TARGIT-B trials |
| ESSO-34 European Society of Surgical Oncology and British Association of Surgical Oncology Joint Conference, Liverpool UK | |
| 12 November 2014 | The TARGIT-A and TARGIT-B trials |
| Breast Educational Day, Oxford, UK | |
| 9 December 2014 | The TARGIT-A trial results |
| NICE, London, UK | |
| 10 February 2015 | Oxford-style debate: the TARGIT-A trial results |
| Royal College of Radiologists, London, UK | |
| 27 April 2015 | The TARGIT-A trial results – it is safe to not irradiate rest of the breast |
| ESTRO Forum, Barcelona, Spain | |
| 7 May 2015 | The TARGIT-A and TARGIT-B trials |
| Mount Carmel Hospital West, Columbus OH, USA | |
| 7 May 2015 | The TARGIT-A and TARGIT-B trials |
| William Beaumont Hospital, Michigan OH, USA | |
| 9 May 2015 | The TARGIT-A and TARGIT-B trials |
| Advances in Brachytherapy Symposium, Weill Cornell Medical Center, New York, NY, USA | |
| 16 May 2015 | The TARGIT-A and TARGIT-B trials [via FaceTime (Apple Inc., Cupertino, CA, USA)] |
| 4th MAYMET SENATURK Meeting on Value Based Quality in Breast Care, Kusadasi, Aydin, Turkey | |
| 9–10 July 2015 | Keynote lecture on the TARGIT trials, chairing of sessions |
| 9th Intrabeam System User Meeting, Manheimm, Germany | |
| 26 August 2015 | Expert opinion (no presentation) |
| INTRABEAM appraisal by NICE, London, UK |
Late-breaking proffered papers
| Date | Presentation title and meeting |
|---|---|
| 1 November 2010 | Targeted intraoperative radiotherapy (TARGIT) vs. whole breast external beam radiotherapy (EBRT) by ASTRO risk categories: late-breaking abstract (oral presentation in the plenary hall) |
| 52nd Annual meeting of ASTRO, San Diego, CA, USA | |
| 6 December 2012 | Targeted intraoperative radiotherapy for early breast cancer: TARGIT-A trial – updated analysis of local recurrence and first analysis of survival (oral presentation in the plenary hall) |
| 35th San Antonio Breast Cancer Symposium, San Antonio, TX, USA |
Appendix 4 Future work
The grant applicants believe that there is much further work required and are in the process of applying for funding to carry out some or all of the following:
-
work package 1: continue to gather efficacy and safety data to year 10, all centres
-
work package 2: gather death and new primary cancer data from UK patients through the Office for National Statistics
-
work package 3: lymphoedema
-
work package 4: health economics
-
work package 5: patient preference
-
work package 6: quality of life
-
work package 7: cardiopulmonary effects.
Work package 1: continue to gather efficacy, safety and follow-up data to year 10, all centres, using the current case report forms as per protocol
Aim and rationale
The latest analysis of the TARGIT-A trial includes a very large number of patients (n = 1222) with a median follow-up of 5 years and our analysis suggests that the results remain stable for cohorts of patients with increasing periods of follow-up up to 5 years. However, for the whole trial, the median follow-up is 2.6 years and one of the barriers to widespread adaptations of the new treatment appears to be the perception that we should undertake 5-year follow-up of all of the patients in the trial. This will mean that a substantial number will also have 10-year follow-up, a milestone that is now considered essential in many trials. Importantly, follow-up up to 10 years was stipulated in the original protocol.
Work package 1 will deliver this.
Primary and secondary objectives
To evaluate in the longer term the primary outcome of local recurrence in the conserved breast and secondary outcomes of the main trial, including complications/side effects and breast cancer and non-breast-cancer survival.
Work package 2: collect death and new primary cancer data from UK patients through the Office for National Statistics
Collection of death and new primary cancer data from UK patients through the Office for National Statistics will help improve the completeness of the data and will support work package 1.
Work Package 3: lymphoedema [Joint Evaluation With Emphasis on Lymphoedema and Shoulder (JEWELS)]
This work package addresses the Joint Evaluation With Emphasis on Lymphoedema and Shoulder (JEWELS) of TARGIT and EBRT post treatment. It will involve a postal questionnaire assessment of quality of life and shoulder and arm morbidity in patients randomised within the TARGIT trial, which compares intraoperative and conventional EBRT as part of the treatment of women with early breast cancer.
Aim and rationale
This work package is designed to investigate whether the two methods of radiotherapy utilised in the TARGIT trial are associated with a longer-term difference in quality of life, shoulder and arm function or lymphoedema.
Primary and secondary objectives
To evaluate the longer-term side effects (particularly arm and shoulder function and lymphoedema) and quality of life in patients randomised within the TARGIT trial to one of two methods of radiotherapy after surgical treatment of early breast cancer.
Work package 4: health economics
This work package will be a longitudinal cross-sectional study addressing the health economics aspects of a randomised controlled trial to compare TARGIT with conventional postoperative radiotherapy after breast conservative surgery for women with early-stage breast cancer.
Primary and secondary objectives
To evaluate the costs and benefits experienced by patients in the trial using the following tools:
-
European Quality of Life-5 Dimensions five-level questionnaire (EQ-5D 5L)
-
cost data:
-
capital costs associated with the TARGIT equipment (completed)
-
NHS contacts for receipt of radiotherapy (completed)
-
NHS contacts for treating the side effects of radiotherapy
-
costs incurred by patients and families.
-
Work package 5: patient preferences for adjuvant radiotherapy in early breast cancer (TARGIT-A trial)
Detailed description
This will be a cross-sectional analytical study to determine what increased risk of local recurrence women who have completed postoperative radiotherapy for early breast cancer are prepared to accept in return for the increased convenience and altered toxicity profile of TARGIT and the factors that influence these preferences, to guide women and their doctors in making choices about radiotherapy after surgery for early breast cancer.
This work package is based on a study successfully run in Sir Charles Gairdner Hospital, Perth, WA, Australia. It will address the patient preference subprotocol of TARGIT-A.
Aim and rationale
Patient preferences for adjuvant radiotherapy in early breast cancer, a substudy of the TARGIT-A trial.
Work package 6: quality of life
The aim of this package is to assess the impact of IORT compared with conventional radiotherapy on early-stage breast cancer patients’ quality of life, psychosocial well-being and satisfaction with treatment. In addition, the role of moderating/mediating variables such as treatment cognitions and treatment effects will be examined according to the model developed by Leventhal et al. 178
In this work package the primary end point will be quality of life and the secondary end points will be treatment satisfaction, mood, symptoms, fatigue and treatment impact.
Work package 7: cardiopulmonary effects
Aim and rationale
The conventional treatment of early breast cancer involves surgical excision of the tumour, surgery to the axillary lymph nodes and EBRT to the conserved breast tissue. Although this is an effective treatment with a low rate of local recurrence of cancer, recent developments have explored alternatives to EBRT.
The TARGIT trial studied the use of single-dose IORT compared with conventional radiotherapy. The trial has demonstrated equivalence with both study arms having a low local recurrence rate and acceptable acute toxicity.
This work package is designed to investigate whether the two methods of radiotherapy utilised in the TARGIT trial have adversely affected the heart.
Primary and secondary objectives
To evaluate the cardiac and respiratory outcomes in patients randomised within the TARGIT trial to one of two methods of radiotherapy after surgical treatment of early breast cancer.
Appendix 5 National Institute for Health and Care Excellence draft recommendation and adoption
National Institute for Health and Care Excellence is currently appraising the TARGIT technique for adoption in the NHS. The status of this appraisal is available at www.nice.org.uk/guidance/indevelopment/gid-tag353 (accessed 7 July 2016).
National Institute for Health and Care Excellence press release
On 27 July 2014, NICE issued the following press release. This text has been reproduced with permission from NICE from www.nice.org.uk/news/press-and-media/nice-to-recommend-new-breast-cancer-radiotherapy-treatment-alongside-further-research. 168
NICE to recommend new breast cancer radiotherapy treatment alongside further research
An innovative new type of radiotherapy for breast cancer is set to be recommended for NHS patients in England under carefully controlled circumstances.
National Institute for Health and Care Excellence has published draft guidance on the use of intrabeam radiotherapy as a treatment option for people with early breast cancer [see Explanation of terms point 1]. It says that the radiotherapy treatment should be recommended for NHS funding provided patients are properly informed about its pros and cons and that further data are collected.
Professor Carole Longson, director of health technology evaluation at NICE, said the treatment has the potential to be a much more efficient form of radiotherapy:’ Unlike regular radiotherapy, with the Intrabeam Radiotherapy System only one dose is required.
‘This single dose is given at the same time as surgery, eliminating the need for numerous hospital visits. Regular radiotherapy typically requires numerous doses over a 3 week period – although some people may receive it for longer – and is performed weeks or months after surgery or chemotherapy.
‘The Appraisal Committee concluded that whilst current evidence was not extensive, this type of radiotherapy was more convenient for patients and can improve a person’s quality of life.’
Just over 41,500 women and 300 men in England are diagnosed with breast cancer every year [see Explanation of terms point 2]. Figures suggest that about 86% of them – 35,970 people each year – will potentially have early breast cancer [see Explanation of terms point 3].
The draft guidance, published for consultation, says that intrabeam radiotherapy should be offered to NHS patients as long as doctors:
-
explain the full range of treatment options available to patients, and their associated risks and benefits. This is to allow patients to make an informed decision about whether to choose Intrabeam or conventional radiotherapy.
-
enter details about all of their breast cancer patients having treatment with the Intrabeam Radiotherapy System onto a national register [see Explanation of terms point 4].
-
audit, review and document clinical outcomes locally and consider the relationship between outcomes and patients’ characteristics.
-
‘It’s still a new treatment,’ Professor Longson explained.’ So far, only 6 centres in the UK have used the Intrabeam Radiotherapy System to treat early breast cancer.
‘Because it is still relatively new it is only right to recommend its use in a carefully controlled way. This will ensure patients are fully aware of the risks and benefits before choosing which treatment to have and allow doctors to gather more information about the treatment.’
The institute’s public consultation runs until Friday 15 August 2014. Final guidance is expected to be published in November 2014. Until then, local NHS bodies are expected to make their own funding decisions for new treatments.
Explanation of terms
-
Early stage breast cancer is when the tumour is confined to the breast area and has not spread beyond the lymph nodes to other parts of the body.
-
These data were provided to Cancer Research UK by the Office for National Statistics on request, July 2013. Further information can be found on the CRUK website: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/breast/.
-
Early invasive breast cancer is defined as cancer that is confined to the breast and ipsilateral axillary lymph nodes. This is usually classified as stage I and II breast cancer. Data on around 17,800 women diagnosed with breast cancer in the East of England in 2006–2009 shows that, of the 92% of cancers for which a stage was recorded, 41% were stage I, 45% stage II, 9% stage III and 5% stage IV. Source: Lyratzopoulos G, Abel GA, Barbiere JM, et al. Variation in advanced stage at diagnosis of lung and female breast cancer in an English region 2006–2009. Br J Cancer 2012. 106(6):1068–75: http://www.ncbi.nlm.nih.gov/pubmed/22382691. For more information about the stages of breast cancer, please see: http://www.cancerresearchuk.org/cancer-help/type/breast-cancer/treatment/number-stages-of-breast-cancer.
-
The data and clinical outcomes to be entered into the national register include:
-
histology of the cancer and patients’ characteristics (including type, size, side of tumour, grade, lymph node status, oestrogen receptor status, progesterone receptor status, human epidermal growth factor receptor 2 status and age of the patient)
-
local recurrence
-
– treatment received after local recurrence
-
– development of metastatic disease
-
– disease-free survival
-
– overall survival
-
– adverse effects of treatment
-
-
health-related quality of life (including EQ-5D)
-
About the guidance
-
The draft guidance for Intrabeam (IORT) for early breast cancer will be available from the NICE website at http://www.nice.org.uk/Guidance/InDevelopment/GID-TAG353 from Friday 25 July 2014. Embargoed copies of the draft guidance are available from the NICE press office on request.
-
The Appraisal Committee concluded that the evidence did not conclusively show that intrabeam radiotherapy is as effective as conventional radiotherapy (called External Beam Radiotherapy – EBRT) at preventing local breast cancer recurrence (when the cancer returns to the same breast). However, the committee acknowledged that the recurrence rates reported in the clinical trial could be considered low in absolute terms. Based on the evidence available so far, it agreed that these were not out of line compared with current recurrence rates with EBRT in the NHS.
-
The committee also acknowledged that for some people, the benefits of avoiding the need for post-surgical radiotherapy would outweigh the consideration that much less is known about the long term outcomes of Intrabeam treatment compared with conventional radiotherapy.
-
The independent Appraisal Committee considered that it was not possible to declare a most plausible incremental cost-effectiveness ratio (ICER) for intrabeam compared with EBRT. This was because of a high degree of uncertainty in the cost-effectiveness analysis. However, the committee concluded that intrabeam was associated with slightly lower costs and fewer QALYs (Quality-adjusted Life-years) than EBRT.
The committee noted that there were also several benefits highlighted by the patient expert and clinical specialists in terms length of treatment and improving patients’ quality of life, which could not be captured in the QALY calculation.
-
According to the manufacturer, the cost of an intrabeam radiotherapy machine is £435,000 (excluding VAT). The manufacturer estimated that device maintenance and servicing costs per year are in the region of £35,000.
-
When published, this guidance will apply to England only.
Draft guidance
On 25 July 2014 the UK NICE issued draft guidance recommending that TARGIT with Intrabeam should be made available in the NHS for suitable patients. ‘The Intrabeam Radiotherapy System is recommended as an option for adjuvant treatment of early invasive breast cancer during breast conserving surgical removal of the tumour, only if clinicians:
-
fully explain the treatment options available to patients, including their associated risks and benefits, so that patients can make an informed choice about their treatment. Clinicians should ensure that patients understand that less is known about the long-term outcomes of treatment with the Intrabeam Radiotherapy System than with conventional external beam radiotherapy and that the rate of local recurrence with Intrabeam could be higher than with external beam radiotherapy and
-
enter details about all patients having treatment with the Intrabeam Radiotherapy System for adjuvant treatment of early invasive breast cancer onto a national register. They should audit, review and document clinical outcomes (see section 6) locally and consider the relationship between outcomes and patients’ characteristics.
There were two further NICE appraisal public meetings in August 2014 following which NICE requested further information from the investigators and these were supplied (as per Chapter 11).
The last meeting of NICE was held in August 2015 and the November 2015 statement is as follows: the Committee decided that further work by the Institute would be required before it could issue a recommendation to the NHS. The Committee appreciated the advantages of Intrabeam, especially for people who are keen to avoid having external beam radiotherapy, but was also concerned about the remaining uncertainties in the evidence, and the difficulties these present for both patients and clinicians in making an informed choice about the best treatment. The technology appraisals team is exploring options for evidence development that could be used to support the use of Intrabeam intraoperative radiotherapy in the NHS. Consequently, the timeline for the development of the provisional guidance has been extended.
NICE have also stated that ‘While this NICE appraisal is ongoing, intrabeam radiotherapy can continue to be offered to NHS patients who need it. Until NICE publishes its final guidance, decisions on whether or not to fund specific treatments are the responsibility of local NHS bodies’. 58
Appendix 6 Method of data collection for patients treated with TARGIT using the INTRABEAM device as per National Institute for Health and Care Excellence draft guidance
This appendix provides details about how data from patients treated with TARGIT using the INTRABEAM device are entered onto the existing national cancer registry (National Cancer Registration Service; NCRS) and how slight modifications will ensure that these data could simply and easily be collected without any additional costs. This method was sent to NICE shortly after the draft guidance appeared.
The method was compiled and sent to NICE by Professor Jayant S Vaidya in collaboration with:
-
the clinical coding team at UCLH (Robert Gray, Clinical Coding Auditor, UCLH NHS Foundation Trust, Moorfields NHS FT, Base: UCLH Clinical Coding Department, London, UK).
-
the NCRS, Public Health England (Claire Beattie, Registration Manager; Sally Vernon, Head of Quality and Analysis, Eastern Office, Public Health England, Cambridge, UK).
-
the Knowledge and Intelligence Team, Public Health England [John Broggio, West Midlands, Senior Public Health Cancer Analyst (Cancer); Catherine Lagord, Breast Cancer Audit Project Manager].
-
the National Cancer Intelligence Network, Public Health England (Sean McPhail, Senior Information Analyst, Wellington House, London, UK).
From the National Cancer Registration Service
The NCRS collects data on all invasive, in situ and uncertain malignancies in England (and also benign tumours of the brain). This includes both invasive and in situ breast cancer data.
The NCRS aims to collect all Cancer Outcomes and Services Dataset (COSD) data items [see www.ncin.org.uk/collecting_and_using_data/data_collection/cancer_outcomes_and_services_dataset_cosd_latest_downloads (accessed 7 July 2016)]. However, COSD was introduced only in 2013 and data completeness is not guaranteed from all trusts yet.
The NCRS does not explicitly collect data on TARGIT treatment. However, such treatment is simple to identify by selecting cases that have both surgery and radiotherapy coded (see From the coding team) on the same date or by looking in the National Radiotherapy Dataset (RTDS).
Of the data items on your list, the NCRS historically collects and has good data completeness on NHS number, histology, size, side, grade, stage (including lymph node status) and age of the patient. The NCRS also collects ER, PR and HER2 receptor status. The NCRS receives feeds from the Office for National Statistics and so has good data on time of overall survival.
The Cancer Outcomes and Services Dataset mandates that data items should allow the measurement of local recurrence, metastatic disease and disease-free survival. However, these data have not been collected well historically and there would be more data quality issues around them.
The NCRS does not directly collect adverse effects of treatment. However, we do get linked hospital admission data, which may show reasons for admission that are an adverse effect of treatment.
The NCRS does not directly collect health-related quality of life (including EQ-5D) data for all patients. When these data have been collected as part of another NHS survey (such as the Cancer Patient Experience Survey or Patient Reported Outcomes Measures), they may be held by the cancer registry. These data could also be collected as part of local audit by individual trusts.
As a test, the Public Health England Knowledge and Intelligence Team searched for patients with both breast surgery and radiotherapy treatment on the same day. They found that 146 patients had the specific combination of codes representing the TARGIT procedure. This number is very similar to the number expected in the trial for that period. This gives reassurance that the current coding system works. Further standardisation as detailed below would make the process even more robust.
I can confirm that the codes that you have agreed with your clinical coders for capturing IORT should enable cancer registry staff to recognise and record this treatment. We would ensure that our staff were aware of this.
From the coding team
Currently, this procedure is being coded in the major centres as the following:
-
X65.2 – Preparation for intracavitary radiotherapy
-
X68.2 – Delivery of a fraction of intracavitary radiotherapy
-
Y35.4 – Introduction of radioactive substance for brachytherapy
-
Z15 – Breast.
In this manner all cases treated with TARGIT will be captured as a matter of routine.
We would like to ensure that it is coded uniformly, capturing all stages, and this document could be used by NICE to advise all hospitals treating such patients as the standard operating procedure.
The procedure is broken down into three sections as follows.
1. Radiotherapy preparation for targeted intraoperative radiotherapy using the INTRABEAM device
-
X68.2 – Preparation for intracavitary brachytherapy (as currently used).
This is because the treatment involves irradiation of tissues immediately next to the applicator within a surgically prepared cavity (tumour bed) following wide local excision of breast cancer. The correct size of the applicator is chosen by measuring the size of the cavity.
As there is no specific code in the book at present, this code is chosen because it best approximates the TARGIT procedure. However, the cavity that is prepared is different from the physiological cavities that this code is usually meant for (e.g. thoracic, abdominal, pelvic, oral cavity).
In the next edition of OPCS-4, it would be preferable to describe this new surgical technique with a new code, for example X68.4 – Preparation for giving targeted intraoperative radiotherapy using INTRABEAM. Until then, X68.2 should be used.
2. Surgical preparation for targeted intraoperative radiotherapy using the INTRABEAM device (including insertion of the applicator)
The codes that we would suggest would be:
-
B37.8 – Other specified other operations on breast, plus
-
Y258 – Other specified suture of organ not otherwise classified (NOC), plus
-
Y02.8 – Other specified placement of prosthesis in organ NOC.
This is because, after wide local excision of breast cancer, the tumour bed needs to be prepared. First, complete haemostasis needs to be achieved as small amounts of oozing blood can affect the treatment. Then, a carefully placed purse-string suture that is taken in such a manner that the tissues closely appose the spherical applicator without bringing the dermis/skin or the ribs too close to the applicator surface. The applicator is attached to the X-ray source, which is covered with a sterile drape, and is then placed within the tumour bed. The purse string is then tightened. After delivery of the radiation is complete, the purse string is cut and the applicator is removed and the tumour bed inspected. The breast is then closed in the usual manner.
In the next edition of the OPCS-4, it would be preferable to describe this new surgical technique with new codes:
-
B37.6 – Preparation of tumour bed for giving targeted intraoperative radiotherapy (TARGIT) with INTRABEAM, plus
-
Y02.3 – Placement of INTRABEAM applicator for giving targeted intraoperative radiotherapy (TARGIT).
Until then, B37.8 plus Y25.8 plus Y02.8 should be used.
3. Delivery of targeted intraoperative radiotherapy using the INTRABEAM device
The codes should be:
-
X65.2 – Delivery of a fraction of intracavitary radiotherapy (as currently used), plus
-
Y35.8 – Other specified introduction of removable radioactive material into organ NOC, plus
-
Y89.8 – Other specified brachytherapy, plus
-
Z15.– – Breast (as currently used).
This is because, after the applicator is placed in the tumour bed, single-dose radiation is delivered to the tumour bed. This takes between 15 and 35 minutes depending on the size of the applicator/tumour bed. The radiation source is X-rays, which are emitted only after the radiation is switched on. Once the radiation has been delivered, the radiation is switched off.
First, the word ‘fraction’ in X65.2 might imply that this treatment is part of a course of several fractions (5–15); however, TARGIT using INTRABEAM is always given as a single dose and therefore, ideally, a new code should be introduced in the next edition of the coding manual, for example X69.1 – Delivery of single dose of targeted intraoperative radiotherapy (TARGIT) using INTRABEAM.
Second, this is not strictly a ‘radioactive material’ as specified in Y35.8; however, as there is no code to describe the introduction of a X-ray source into an organ and, in reality, the source does emit radiation when switched on, this code should be used until a new code is introduced in the next edition of the OPCS-4 to describe the procedure. This could be achieved by removing the word ‘radioactive’ from the three category heading at Y35, for example Y35.5 – Introduction of low-energy/low-dose rate X-ray source – INTRABEAM – into breast.
Third, the additional code of Y89.8 should be used to specify that this is a brachytherapy (internal radiotherapy) that is neither high dose nor pulsed dose until a new code is introduced in the update of the OPCS-4, for example Y89.3 – Single low-dose brachytherapy treatment with INTRABEAM.
Until then the X65.2 plus Y35.8 plus Y89.8 plus Z15.– codes should be used.
Ideally, there should be a single new code for the procedure of ‘Wide local excision of breast cancer + TARGIT-IORT’ because the latter cannot be given without initially removing the tumour. This will allow easier coding the classification.
Codes for targeted intraoperative radiotherapy
| Current code | Description (explanation) |
|---|---|
| X68.2 | Preparation for intracavitary brachytherapy (radiotherapy preparation) |
| B37.8 | Other specified other operations on breast (surgical preparation of the tumour bed) |
| Y25.8 | Other specified suture of organ NOC (insertion of the purse-string suture) |
| Y02.8 | Other specified placement of prosthesis in organ NOC (insertion of INTRABEAM applicator) |
| X65.2 | Delivery of a fraction of intracavitary radiotherapy (delivery of TARGIT IORT with INTRABEAM) |
| Y35.8 | Other specified introduction of removable radioactive material into organ NOC (insertion of INTRABEAM X-ray source in the applicator) |
| Y89.8 | Other specified brachytherapy (single rather than multiple brachytherapy treatments) |
| Z15.– | Breast (to indicate that this is a breast operation) |
| New code | Description (explanation) |
|---|---|
| X68.4 | Preparation for giving targeted intraoperative radiotherapy using INTRABEAM |
| B37.6 | Preparation of tumour bed for giving targeted intraoperative radiotherapy (TARGIT) with INTRABEAM |
| Y02.3 | Placement of INTRABEAM applicator for giving targeted intraoperative radiotherapy (TARGIT) |
| X69.1 | Delivery of single dose of targeted intraoperative radiotherapy (TARGIT) using INTRABEAM |
| Y35.5 | Introduction of low-energy/low-dose rate X-ray source – INTRABEAM – into breast |
| Y89.3 | Single low-dose brachytherapy treatment with INTRABEAM |
| Z15.– | Breast (to indicate that this is a breast operation) |
Highlights
- Rationale based on biology and natural history
- An academic insight led to the development of the new radiotherapy device and surgical operation and scientific and academic enthusiasm enabled recruitment of 3451 patients in 33 centres in 11 countries.
- Non-inferiority
- For patients with breast cancer carefully selected based on the TARGIT-A trial results, TARGIT within a risk-adapted approach, particularly when given concurrent with lumpectomy, is as effective as and safer than several weeks of postoperative EBRT.
- Non-breast-cancer mortality
- In the randomised TARGIT-A trial, there were fewer deaths from causes other than breast cancer in patients allocated to TARGIT.
- Evidence to support new trials
- The trial provides several new findings that support future trials (e.g. the TARGIT-B trial and at least seven other work packages).
- Wide dissemination
- The trial results have been published in a high-impact journal that is open access and the results have been widely disseminated through many publications and media attention.
- Worldwide acceptance
- The St Gallen panel of experts, which meets every 2 years to recommend breast cancer treatment, has broadly accepted the new treatment. About 20,000 women have been treated with the TARGIT technique worldwide. The technique is currently being assessed by the National Institute for Health and Care Excellence for adoption in the UK.
List of abbreviations
- ASCO
- American Society of Clinical Oncology
- ASTRO
- American Society for Radiation Oncology
- CALGB
- Cancer and Leukemia Group B
- CI
- confidence interval
- CRC
- Cancer Research Campaign
- CRF
- case report form
- CRUK
- Cancer Research UK
- DCIS
- ductal carcinoma in situ
- DMC
- Data Monitoring Committee
- EBRT
- external beam radiotherapy
- EGFR
- epidermal growth factor receptor
- EIC
- extensive intraductal component
- ELIOT
- ELectron IntraOperative radiotherapy
- ER
- oestrogen receptor
- ESTRO
- European SocieTy for Radiotherapy & Oncology
- HER2
- human epidermal growth factor receptor-2
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- IORT
- intraoperative radiotherapy
- IQR
- interquartile range
- ISC
- International Steering Committee
- ITT
- intention to treat
- MREC
- Multicentre Research Ethics Committee
- MRI
- magnetic resonance imaging
- NCRS
- National Cancer Registration Service
- NICE
- National Institute for Health and Care Excellence
- NOC
- not otherwise classified
- NSABP
- National Surgical Adjuvant Breast and Bowel Project
- PBI
- partial breast irradiation
- PgR
- progesterone receptor
- PRIME
- Post-operative Radiotherapy In Minimum-risk Elderly
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- RTOG
- Radiation Therapy Oncology Group
- SD
- standard deviation
- SE
- standard error
- SITU
- Surgical & Interventional Trials Unit
- START
- Standardisation of Breast Radiotherapy
- TARGIT
- TARGeted Intraoperative radioTherapy
- TARGIT-A
- TARGeted Intraoperative radioTherapy Alone
- TARGIT-B
- TARGeted Intraoperative radioTherapy Boost
- TNM
- tumour, nodes, metastasis
- UCL
- University College London
- UCLH
- University College London Hospitals